TROGARZO Dosing Guide
User Manual:
Open the PDF directly: View PDF ![]() .
.
Page Count: 4

TROGARZO™ is a trademark of TaiMed Biologics Inc.,
under license to Theratechnologies Inc.
© 2018 Theratechnologies Inc. All rights reserved. 344-01-11/17
IMPORTANT SAFETY INFORMATION
Warnings and Precautions
• Immune Reconstitution Inflammatory Syndrome
(IRIS) has been reported in 1 patient treated
with TROGARZO™ in combination with other
antiretrovirals. During the initial phase of
combination antiretroviral therapies, patients
whose immune systems respond may develop
an inflammatory response to indolent or
residual opportunistic infections, which may
necessitate further evaluation and treatment.
Adverse Reactions
• The most common adverse reactions (reported
in ≥5.0% of patients) were diarrhea (8%),
dizziness (8%), nausea (5%) and rash (5%).
• Most (90%) of the adverse reactions reported
were mild or moderate in severity. Two subjects
experienced severe adverse reactions: 1 subject
had a severe rash and 1 subject developed IRIS
manifested as an exacerbation of progressive
multifocal leukoencephalopathy.
Use in Specific Populations
• Pregnancy: No adequate human data
are available to establish whether or not
TROGARZO™ poses a risk to pregnancy
outcomes. Monoclonal antibodies, such
as ibalizumab-uiyk, are transported across
the placenta as pregnancy progresses;
therefore, ibalizumab-uiyk has the potential
to be transmitted from the mother to the
developing fetus.
• Lactation: No data are available regarding
the presence of TROGARZO™ in human
milk, the eects on the breastfed child, or
the eects on milk production. Because of
the potential for HIV-1 transmission, instruct
mothers not to breastfeed if they are
receiving TROGARZO™.
To report suspected adverse reactions, contact
THERA patient support™ (1-833-238-4372) or
the FDA (1-800-FDA-1088 or fda.gov/medwatch).
Please see the enclosed full Prescribing
Information for TROGARZO™.
For more information,
talk to your
Theratechnologies
Representative or call
at 1-833-238-4372.
*If the patient does not experience any infusion-related adverse reactions.
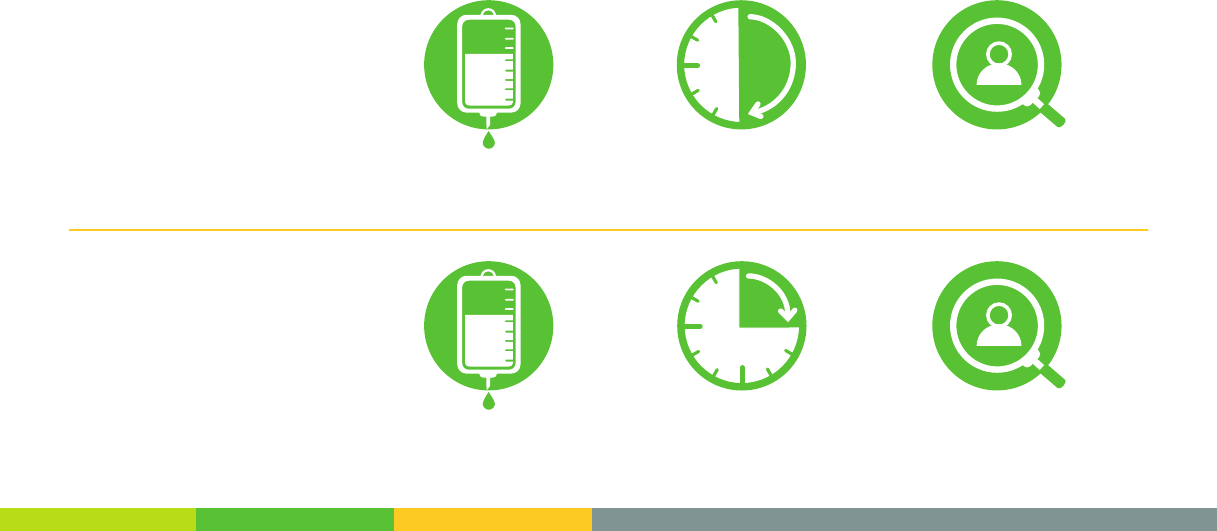
Administration1
Loading Dose
2,000 mg 30 min
infusion 1 hr
observation
800 mg 15 min
observation*
Maintenance Doses
15 min
infusion*
Reference: 1. TROGARZO™ Prescribing Information. Theratechnologies Inc.
TROGARZO™
(ibalizumab-uiyk) Injection
Dosing and Administration
TROGARZO™, in combination with other antiretroviral(s), is indicated for the
treatment of human immunodeficiency virus type 1 (HIV-1) infection in heavily
treatment-experienced adults with multi-drug resistant HIV-1 infection failing
their current antiretroviral regimen.

TROGARZO™ is administered by intravenous (IV)
infusion as:
• A single loading dose of 2,000 mg
• Maintenance doses of 800 mg every 2 weeks
If a scheduled maintenance dose is missed by 3 or more days,
a loading dose should be administered as early as possible.
Resume maintenance doses every 2 weeks thereafter.
• TROGARZO™ is used in combination
with other antiretroviral(s).
• Dose modifications of TROGARZO™
are not required when administered
with any other antiretrovirals
or concomitant treatments.
• Drug-drug interactions are not
expected, based on TROGARZO™’s
mechanism of action and
target-mediated drug disposition.
TROGARZO™ (ibalizumab-uiyk) Injection
Dosing1
TROGARZO™ is a trademark of TaiMed Biologics Inc.,
under license to Theratechnologies Inc.
© 2018 Theratechnologies Inc. All rights reserved. 344-01-11/17
IMPORTANT SAFETY INFORMATION
Warnings and Precautions
• Immune Reconstitution Inflammatory Syndrome
(IRIS) has been reported in 1 patient treated
with TROGARZO™ in combination with other
antiretrovirals. During the initial phase of
combination antiretroviral therapies, patients
whose immune systems respond may develop
an inflammatory response to indolent or
residual opportunistic infections, which may
necessitate further evaluation and treatment.
Adverse Reactions
• The most common adverse reactions (reported
in ≥5.0% of patients) were diarrhea (8%),
dizziness (8%), nausea (5%) and rash (5%).
• Most (90%) of the adverse reactions reported
were mild or moderate in severity. Two subjects
experienced severe adverse reactions: 1 subject
had a severe rash and 1 subject developed IRIS
manifested as an exacerbation of progressive
multifocal leukoencephalopathy.
Use in Specific Populations
• Pregnancy: No adequate human data
are available to establish whether or not
TROGARZO™ poses a risk to pregnancy
outcomes. Monoclonal antibodies, such
as ibalizumab-uiyk, are transported across
the placenta as pregnancy progresses;
therefore, ibalizumab-uiyk has the potential
to be transmitted from the mother to the
developing fetus.
• Lactation: No data are available regarding
the presence of TROGARZO™ in human
milk, the eects on the breastfed child, or
the eects on milk production. Because of
the potential for HIV-1 transmission, instruct
mothers not to breastfeed if they are
receiving TROGARZO™.
To report suspected adverse reactions, contact
THERA patient support™ (1-833-238-4372) or
the FDA (1-800-FDA-1088 or fda.gov/medwatch).
Please see the enclosed full Prescribing
Information for TROGARZO™.
For more information,
talk to your
Theratechnologies
Representative or call
at 1-833-238-4372.
*If the patient does not experience any infusion-related adverse reactions.
Administration1
Loading Dose
2,000 mg 30 min
infusion 1 hr
observation
800 mg 15 min
observation*
Maintenance Doses
15 min
infusion*
Reference: 1. TROGARZO™ Prescribing Information. Theratechnologies Inc.
TROGARZO™
(ibalizumab-uiyk) Injection
Dosing and Administration
TROGARZO™, in combination with other antiretroviral(s), is indicated for the
treatment of human immunodeficiency virus type 1 (HIV-1) infection in heavily
treatment-experienced adults with multi-drug resistant HIV-1 infection failing
their current antiretroviral regimen.
TROGARZO™ is a trademark of TaiMed Biologics Inc.,
under license to Theratechnologies Inc.
© 2018 Theratechnologies Inc. All rights reserved. 344-01-11/17
IMPORTANT SAFETY INFORMATION
Warnings and Precautions
• Immune Reconstitution Inflammatory Syndrome
(IRIS) has been reported in 1 patient treated
with TROGARZO™ in combination with other
antiretrovirals. During the initial phase of
combination antiretroviral therapies, patients
whose immune systems respond may develop
an inflammatory response to indolent or
residual opportunistic infections, which may
necessitate further evaluation and treatment.
Adverse Reactions
• The most common adverse reactions (reported
in ≥5.0% of patients) were diarrhea (8%),
dizziness (8%), nausea (5%) and rash (5%).
• Most (90%) of the adverse reactions reported
were mild or moderate in severity. Two subjects
experienced severe adverse reactions: 1 subject
had a severe rash and 1 subject developed IRIS
manifested as an exacerbation of progressive
multifocal leukoencephalopathy.
Use in Specific Populations
• Pregnancy: No adequate human data
are available to establish whether or not
TROGARZO™ poses a risk to pregnancy
outcomes. Monoclonal antibodies, such
as ibalizumab-uiyk, are transported across
the placenta as pregnancy progresses;
therefore, ibalizumab-uiyk has the potential
to be transmitted from the mother to the
developing fetus.
• Lactation: No data are available regarding
the presence of TROGARZO™ in human
milk, the eects on the breastfed child, or
the eects on milk production. Because of
the potential for HIV-1 transmission, instruct
mothers not to breastfeed if they are
receiving TROGARZO™.
To report suspected adverse reactions, contact
THERA patient support™ (1-833-238-4372) or
the FDA (1-800-FDA-1088 or fda.gov/medwatch).
Please see the enclosed full Prescribing
Information for TROGARZO™.
For more information,
talk to your
Theratechnologies
Representative or call
at 1-833-238-4372.
*If the patient does not experience any infusion-related adverse reactions.
Administration1
Loading Dose
2,000 mg 30 min
infusion 1 hr
observation
800 mg 15 min
observation*
Maintenance Doses
15 min
infusion*
Reference: 1. TROGARZO™ Prescribing Information. Theratechnologies Inc.
TROGARZO™
(ibalizumab-uiyk) Injection
Dosing and Administration
TROGARZO™, in combination with other antiretroviral(s), is indicated for the
treatment of human immunodeficiency virus type 1 (HIV-1) infection in heavily
treatment-experienced adults with multi-drug resistant HIV-1 infection failing
their current antiretroviral regimen.