Stryker Endoscopy XFC An integrated arthroscopy resection console. User Manual Cover Page
Stryker Endoscopy An integrated arthroscopy resection console. Cover Page
Contents
- 1. User Manual
- 2. User Manual Compliance Statement
User Manual
Stryker Endoscopy
5900 Optical Court, San Jose, CA 95138
APPROVALS DATE TITLE
DRAWN BY:
A. Infanger 05/15/09
ORIGINATOR
C. Bennett 05/19/09
Crossfire Console
User Guide (English)
SIZE REV. DOCUMENT NO. SHEET OF
A E 1000-401-036 1 48
REVISIONS REVISIONS
LTR ECN NO. DATE APPROVED LTR ECN NO. DATE APPROVED
A ECO116602 02/17/09 R. Cardenas
B 45458 03/05/09 R. Cardenas
C 45524 03/12/09 C. Bennett
D ECO30971 04/22/09 C. Bennett
E ECO31369 05/19/09 C. Bennett
F
G
H
J
K
L
M
N
P
R
T
U
V
W
Y
Printing Instructions
1. Print 5.5 w × 8.5 h
2. Black and white
3. Front and back
4. Select binding and paper weight based on cost
Global Source
Archive this document as a user guide (not a service or maintenance
manual).
Languages
This document is translated into the following languages:
1. English

Crossfire™ Console
REF 0475000000
User Guide
Contents
Warnings and Cautions ..............................................................5
Symbol Glossary ............................................................................9
Product Description and Intended Use ..........................11
Indications/Contraindications ..........................................................12
Package Contents ...........................................................................12
Available Accessories ......................................................................12
The Crossre™ Console ..................................................................13
Setup and Interconnection ....................................................15
Electromagnetic Compatibility .........................................................15
Connections .....................................................................................20
Powering the Console On and Off ...................................................22
Operation .........................................................................................23
The Crossre™ Interface .................................................................24
Adjusting User and System Settings ...............................................25
Reading the LCD Screen .................................................................27
Selecting Between RF and Shaver Modes ......................................30
Adjusting Power and Speed Settings ..............................................30
Using the Handpiece .......................................................................31
Using the Footswitch .......................................................................32
Audible Feedback ............................................................................36
Troubleshooting ...........................................................................37
Cleaning and Maintenance ....................................................39
Cleaning ...........................................................................................39
Disposal ...........................................................................................40
Technical Specifications .........................................................41
Generator Output .............................................................................42

EN-5
Warnings and Cautions
Please read this manual and follow its instructions carefully. e words
warning, caution, and note carry special meanings and should be carefully
reviewed:
Warning Warnings indicate risks to the safety of the patient or user.
Failure to follow warnings may result in injury to the patient or
user.
Caution Cautions indicate risks to the equipment. Failure to follow
cautions may result in product damage.
Note Notes provide special information to clarify instructions or
present additional useful information.
To avoid potential serious injury to the user and the patient and/or damage to
this device, the user must obey the following warnings. e warranty is void if
any of these warnings is disregarded.
1. Federal law (USA) restricts this device to use by, or on order of, a
physician.
2. Attempt no internal repairs or adjustments not specically detailed in
this operating manual. Refer any readjustments, modications, and/or
repairs to Stryker Endoscopy or its authorized representatives.
3. Pay close attention to the care and cleaning instructions in this manual.
Failure to follow these instructions may result in product damage.
4. Install this device in an operating room that complies with all applicable
IEC, CEC, and NEC requirements for safety of electrical devices.
5. DO NOT use the Crossre™ system on patients with cardiac
pacemakers or other electronic device implants. Doing so could lead to
electromagnetic interference and possible death.
Fire/Explosion Warnings
1. DO NOT use this device in the presence of ammable anaesthetics,
other ammable gases or objects, near ammable uids such as skin
prepping agents and tinctures, or oxidizing agents. Observe appropriate
re precautions at all times.
2. DO NOT use this device in oxygen-enriched atmospheres, nitrous
oxide (NO) atmospheres, or in the presence of other oxidizing agents,
to prevent risk of explosion. Ensure that oxygen connections are not
leaking.
EN-6
3. Electrosurgical components, such as the probe, may remain hot
following activation. Keep all electrosurgical equipment away from
ammable materials to avoid combustion.
4. To prevent the risk of re, DO NOT replace console fuses. If it is
suspected that fuses are damaged, return console to Stryker for repair.
Prior to Surgery
1. e operator of the Crossre™ system should be a qualied physician,
having complete knowledge of the use of this equipment and awareness
of the risks associated with arthroscopic and electrosurgical procedures.
2. e operator of the Crossre™ system should be experienced in
arthroscopic and electrosurgical practices and techniques.
3. e operator of the Crossre™ system should read this manual
thoroughly and be familiar with its contents prior to operating the
equipment.
4. e operator of the Crossre™ system should be sure that the system
functions as outlined in this manual prior to a surgical procedure. e
Crossre™ system was fully tested at the factory before shipment.
5. Crossre™ system components are designed to be used together as a
system. Use only the appropriate footswitch, handpiece, and disposable
attachments described in this manual.
6. Carefully unpack the unit and ensure that all components are accounted
for and remain undamaged from shipment. Inspect the handpiece cable
for any damage to insulation. If damage to any component is detected,
refer to the “Service and Claims” section of this manual.
7. Ensure the proper connection of the primary power cord of the
Crossre™ System to a grounded receptacle. To prevent risk of electric
shock DO NOT use extension cords or adapter plugs.
8. DO NOT wrap the handpiece cable around metal objects, or the
induction of hazardous currents may result.
9. Position the cables to avoid contact with the patient, electrodes, cables,
and any other electrical leads which provide paths for high frequency
current.
10. Position the console so the fan directs the ow of air away from the
patient.
11. When the Crossre™ system and physiological monitoring equipment
are used simultaneously on a patient, position any monitoring electrodes
as far as possible from the surgical electrodes. Monitoring equipment
using high frequency, current-limiting devices is recommended. Needle
monitoring electrodes are NOT recommended.
EN-7
12. Smoke generated during electrosurgical procedures may be harmful to
surgical personnel. Take appropriate precautions by wearing surgical
masks or other means of protection.
During Surgery
1. DO NOT use the Crossre™ system with non-conductive irrigants (e.g.
sterile water, air, gas, glycine, etc.). Use only conductive irrigants such as
saline or Ringer’s lactate in order for the system to function properly.
2. DO NOT allow the patient to come into contact with grounded metal
objects or objects that have an appreciable capacitance to the earth, such
as a surgical table frame or instrument table, to prevent risk of shock.
e use of antistatic sheeting is recommended for this purpose.
3. DO NOT activate the Crossre™ system for prolonged lengths of time
when the attachment is not in contact with tissue. Doing so may lead to
unintentional damage to surrounding tissue.
4. When the Crossre™ system is activated, the conducted and radiated
electrical elds may interfere with other electrical medical equipment.
Provide as much possible distance between the console and other
electronic medical equipment.
5. Select the lowest output power required to prevent patient injury.
6. Maintain the active electrode in the eld of view at all times to avoid
tissue damage.
7. Remove the handpiece and disposable attachments from the surgical
site and place them away from metallic objects when not in use.
Attachments should be separated from other electrosurgical equipment
to avoid inadvertent electrical coupling between devices. Inadvertent
activation may cause user/patient injury and/or product damage.
8. Keep the ends of the handpiece cable connectors, footswitch cable
connectors, and console receptacles away from all uids.
9. DO NOT activate the Crossre™ system until the probe is properly
positioned in the patient.
10. Ensure that the probe tip, including the return electrode, is completely
surrounded by irrigant solution during use.
11. Keep the activation indication lights and speaker in eld of view and
hearing at all times during activation. e light and sound are important
safety features.
12. DO NOT touch the attachment to metal objects, such as an endoscope
or metal cannula, while activating the handpiece. Damage to the
attachments or other devices may result.
13. DO NOT obstruct the fan (located near the rear of the console).
EN-8
14. Failure of the system may result in an unintended increase in output
power.
15. During use, operators should wear standard surgical gloves to help
reduce the risk of electric shock.
After Surgery
1. DO NOT attempt to reuse or resterilize any product labeled “Single-
Use,” as this may lead to equipment malfunction, patient/user injury,
and/or cross contamination.
2. DO NOT use ammable agents for cleaning and disinfection of the
Crossre™ console, handpiece, or footswitch.
3. DO NOT remove the cover of the console as this could cause electric
shock and product damage.
4. Attempt no internal repairs or adjustments, unless specied otherwise
in this manual. Units requiring repair should be returned to Stryker.
5. Disconnect the Crossre™ system from the electrical output when
inspecting fuses.
EN-9
Symbol Glossary
is device and its labeling contain symbols that provide important information
for the safe and proper use of the device. ese symbols are dened below.
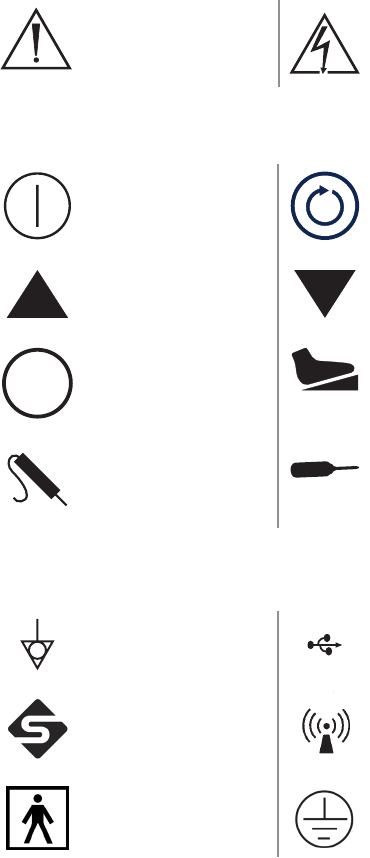
Warning Symbols
Warning/Caution:
See instructions for
use
Hazardous voltage present
Front Console Symbols
Power Select
Up Down
MENU Menu Footswitch
Probe Handpiece
Rear Console Symbols
Equipotentiality USB
Stryker rewire Emits RF radiation
Type BF rated Protective ground earth
EN-10
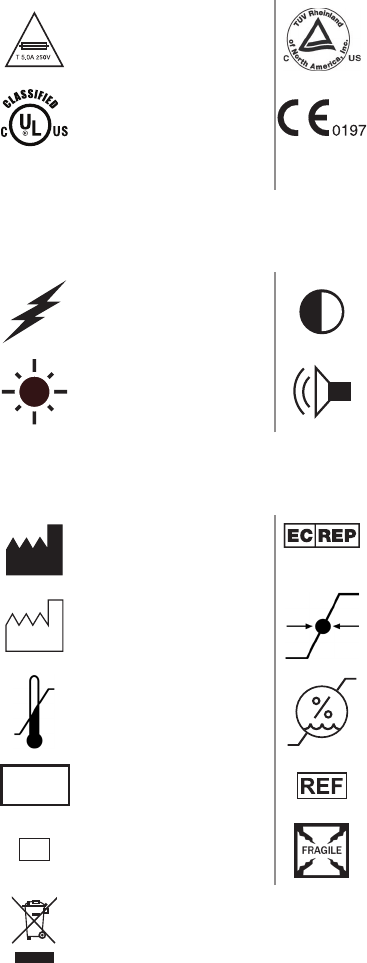
Fuse rating Compliant to CSA C22.2 No.
601.1-M90, and UL 601-1
UL classied
Fullls requirements of the
European Medical Device
Directive 93/42/EEC
LCD Symbols
Electrosurgical unit Contrast
Brightness Sound
Packaging/Labeling Symbols
Legal manufacturer Authorized representative in
Europe
Date of manufacture Atmospheric pressure range
Ambient temperature
range Relative humidity range
LOT
Lot number
Product number
SN
Serial number Fragile
is product contains electrical waste or electronic equipment. It
must not be disposed of as unsorted municipal waste and must be
collected separately.
EN-11
Product Description and Intended Use
e Crossre™ Integrated Arthroscopy System is a combination powered
shaver system/electrosurgical generator, intended for use in arthroscopic and
orthopedic procedures.
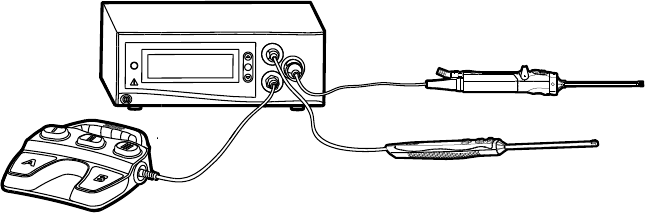
Illustrated below, the Crossre™ system consists of the following components:
2
1
3
4
1. Crossre™ Console
(featured in this
manual)
• Actsasaconnectionhubforthevarious
components of the Crossre™ system
• Powersamotorizedshaverhandpiecefor
the mechanical cutting and debridement of
bone and so tissue
• Generatesbipolarradiofrequency(RF)
energy for the electrosurgical cutting and
coagulation of tissue
• Providesacentraluserinterfacefor
operating the Crossre™ system
2. Disposable RF probe Enables RF cutting and coagulation
3. Powered shaver
handpiece
(and disposable
attachments)
Enables arthroscopic cutting and debridement
4. Crossre™ Footswitch Provides remote, foot control of the powered
shaver handpiece and RF probe
EN-12
Indications/Contraindications
e Crossre™ system is indicated for use in orthopedic and arthroscopic
procedures for the knee, shoulder, ankle, elbow, wrist, and hip. e Crossre™
System provides abrasion, resection, debridement, and removal of bone and so
tissue through its shaver blade, and the ablation and coagulation of so tissue, as
well as hemostasis of blood vessels, through its electrosurgical probe.
Examples of uses of the product include resection, ablation, and coagulation of
torn knee cartilage, subacromial decompression, and resection of synovial tissue
in other joints.
e electrosurgical probe is contraindicated for use in procedures where a
nonconductive irrigant is used or with patients having cardiac pacemakers or
other electronic implants.
Package Contents
Carefully unpack the Crossre™ console and inspect each of the following
components. Report any damaged components to Stryker.
(1) Crossre™ console
(1) Hospital-grade power cord (0105-003-001)
(1) User guide
Available Accessories
e Crossre™ system is compatible with the following accessories:
0279-xxx-xxx SERFAS™ Energy family of electrosurgical probes
0375-708-500 Formula® 180 Handpiece
0375-704-500 Formula® Handpiece (with buttons)
0375-701-500 Formula® Handpiece (without buttons)
0275-601-500 Small-Joint Shaver Handpiece
0277-200-100 iSWITCH™ Universal Wireless Footswitch Receiver
0277-100-100 iSWITCH™ Universal Wireless Footswitch
6000-001-020 Stryker rewire cable
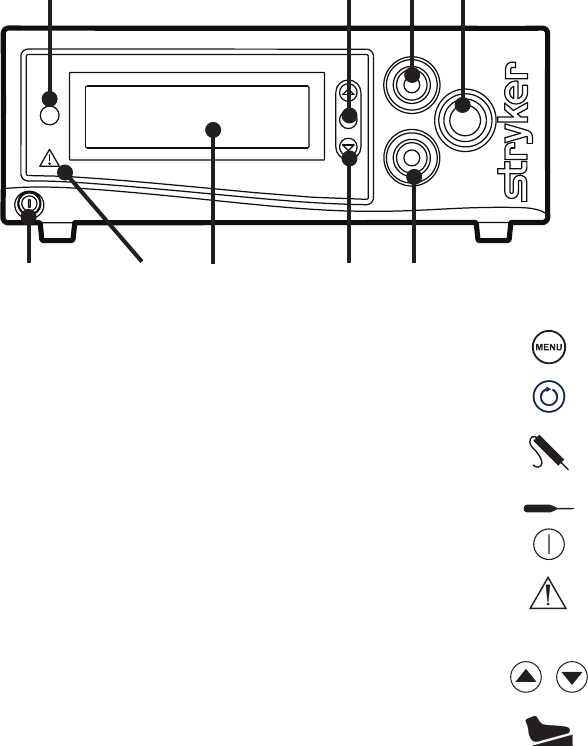
EN-13
The Crossfire™ Console
e Crossre™ console is the connection hub for the components of the
Crossre™ system. It generates RF energy, powers motorized shavers, and
provides user controls and system feedback.
Front Panel
e front console panel features ports for connecting handpieces, controls for
adjusting handpiece settings, and an LCD screen to provide system feedback.
1 2
5 6 7 8 9
3 4
1. Menu Selects menu items
2. Select Selects which device displays on
the LCD screen.
3. RF connector SERFAS Energy probe
4. Handpiece connector Powered shaver handpiece
5. Power Powers the console on and o
6. Error indicator Shines red to indicate errors (error
details appear in the LCD)
7. LCD screen Provides system feedback
8. Adjust Adjusts options for connected
devices
9. Footswitch connector Crossre™ Footswitch
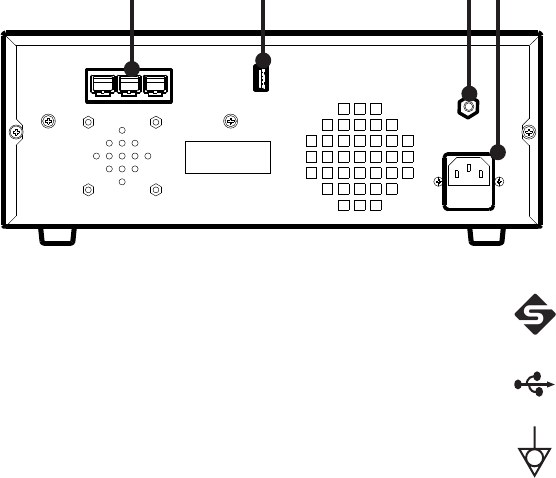
EN-14
Rear Panel
e rear panel provides ports for connecting the console to other Stryker
equipment.
9 10 11 12
9. Firewire Connectors Enables connection to other Stryker
Firewire devices, such as the iSWITCH
Universal Wireless Footswitch
10. USB Drive Enables uploading of preset user
settings
11. Equipotential
Ground Plug
—
12. AC Power Inlet —

EN-15
Setup and Interconnection
Stryker Endoscopy considers instructional training an integral part of the
Crossre™ system. Your Stryker Endoscopy sales representative will perform at
least one inservice at your convenience to help you set up your equipment and
instruct you and your sta on its operation and maintenance. Please contact
your local Stryker Endoscopy representative to schedule an in-service aer your
equipment has arrived.
Electromagnetic Compatibility
Like other electrical medical equipment, the Crossre™ System requires special
precautions to ensure electromagnetic compatibility with other electrical medical
devices. To ensure electromagnetic compatibility (EMC), the Crossre™ System
must be installed and operated according to the EMC information provided in
this manual.
e Crossre™ System has been designed and tested to comply with IEC 60601-
1-2:2001 requirements for EMC with other devices.
Warning is equipment is intended for use by health care
professionals only. is equipment may cause radio
interference or may disrupt the operation of nearby
equipment. It may be necessary to take mitigation
measures, such as reorienting or relocating the equipment
or shielding the location.
Portable and mobile RF communications equipment
can aect the normal function of the Crossre™ System
even if such equipment meets the applicable emissions
requirements.
e Crossre™ System was not tested for immunity to
electromagnetic disturbances.
Do not use cables or accessories other than those provided
with the Crossre™ System, as this may result in increased
electromagnetic emissions or decreased immunity to such
emissions.
EN-16
If the Crossre™ System is used adjacent to or stacked with
other equipment, observe and verify normal operation
of the Crossre™ System in the conguration in which
it will be used prior to using it in a surgical procedure
as interference may occur. Consult the tables below for
guidance in placing the Crossre™ System.
When the Crossre™ System is interconnected with other
medical electrical equipment, leakage currents may be
additive. To minimize total patient leakage current, any
Type BF applied part should be used together with other
Type BF applied parts. Ensure all systems are installed
according to the requirements of IEC 60601-1-1.
e separable AC power cord is provided as a means of
emergency shutdown and disconnection from the power
source. Do not position the console in a way that is dicult
to disconnect the AC power cord.
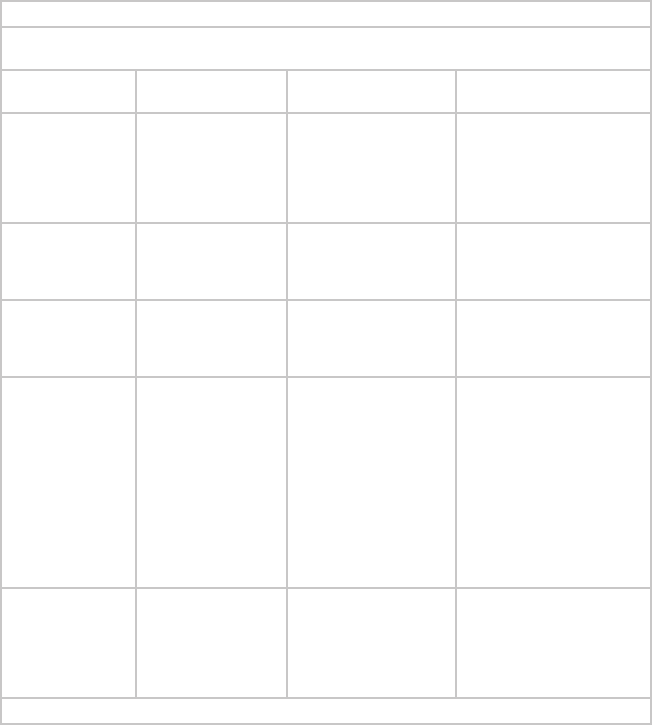
Guidance and Manufacturer’s Declaration: Electromagnetic Emissions
The Crossre™ System is intended for use in the electromagnetic environment specied below. The
customer or the user of Crossre™ System should ensure that it is used in such an environment.
Emissions Test Compliance Electromagnetic Environment
- Guidance
RF emissions
CISPR11
Group 1 The Crossre™ System must emit
electromagnetic energy in order to
perform its intended function. Nearby
electronic equipment may be affected.
RF emissions
CISPR11
Class A Crossre™ System is suitable for
use in all establishments other than
domestic and those directly connected
to the public low-voltage power supply
network that supplies buildings used
for domestic purposes.
Harmonic emissions
IEC 61000-3-2
Class A
Voltage Fluctuations/
icker emissions
IEC 61000-3-3
Complies
EN-17
Guidance and Manufacturer’s Declaration: Electromagnetic Immunity
The Crossre™ System is intended for use in the electromagnetic environment specied below. The
customer or the user of Crossre™ System should ensure that it is used in such an environment
Immunity Test IEC 60601 Test
Level
Compliance Level Electromagnetic
Environment: Guidance
Electrostatic
Discharge (ESD)
IEC 61000-4-2
±6kV contact
±8kV air
±2,4,6kV contact
±2,4,8kV air
Floors should be wood,
concrete, or ceramic tile.
If oors are covered with
synthetic material, the
relative humidity should be
at least 30%.
Electrical fast
transient/burst
IEC 61000-4-4
±2kV for power
supply lines
±1kV for input/output
lines
±2kV for power supply
lines
±1kV for input/output
lines
Mains power quality
should be that of a typical
commercial or hospital
environment.
Surge
IEC 61000-4-5
±1kV differential
mode
±2kV common mode
±0.5, 1kV differential
mode
±1, 2kV common mode
Mains power quality
should be that of a typical
commercial or hospital
environment.
Voltage dips, short
interruptions and
voltage variations
on power supply
input lines
IEC 61000-4-11
<5% Ut (>95% dip in
Ut) for 0.5 cycle
40% Ut (60% dip in
Ut) for 5 cycles
70% Ut (30% dip in
Ut) for 25 cycles
<5% Ut (>95% dip in
Ut) for 5 sec.
<5% Ut (>95% dip in Ut)
for 0.5 cycle
40% Ut (60% dip in Ut)
for 5 cycles
70% Ut (30% dip in Ut)
for 25 cycles
<5% Ut (>95% dip in Ut)
for 5 sec.
Mains power quality
should be that of a typical
commercial or hospital
environment. If the user of
Crossre™ System requires
continued operation during
power mains interruptions,
it is recommended that
Crossre™ System
be powered from an
uninterruptible power supply
or a battery.
Power frequency
(50/60Hz)
magnetic eld
IEC 61000-4-8
3 A/m N/A Power-frequency magnetic
elds should be at levels
characteristic of a typical
location in a typical
commercial or hospital
environment.
NOTE: Ut is the a.c. mains voltage prior to application of the test level.

EN-18
Guidance and Manufacturer’s Declaration: Electromagnetic Immunity
Crossre™ System is intended for use in the electromagnetic environment specied below. The
customer or the user of Crossre™ System should ensure that it is used in such an environment.
Immunity Test IEC 60601 Test
Level
Compliance Level Electromagnetic Environment:
Guidance
Portable and mobile RF
communications equipment should
be used no closer to any part of
the Crossre™ system, including
its cables, than the recommended
separation distance calculated
from the equation applicable to the
frequency of the transmitter.
Recommended Separation Distance
d = 1.17 √P
Conducted RF
IEC 61000-4-6
3 Vrms
150 kHz to 80
MHz
3 V d = 1.17 √P
80 MHz to 800 MHz
Radiated RF
IEC 61000-4-3
3 V/m
80MHz to 2.5 GHz
3 V/m d = 2.33 √P
80 MHz to 2.5 GHz
where P is the maximum output
power rating of the transmitter
in watts (W) according to the
transmitter manufacturer and d is the
recommended separation distance in
meters (m).
Field strengths from xed RF
transmitters, as determined by an
electromagnetic site survey (a), should
be less than the compliance level in
each frequency range(b).
Interference may occur in the vicinity
of equipment marked with the
following symbol:
NOTE 1: At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reection from structures, objects, and people.
(a) Field strengths from xed transmitters, such as base stations for radio (cellular/cordless)
telephones and land mobile radios, amateur radio, AM and FM radio broadcast, and TV broadcast,
cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due
to xed RF transmitters, an electromagnetic site survey should be considered. If the measured
eld strength in the location in which the Crossre™ System is used exceeds the applicable RF
compliance level above, the Crossre™ System should be observed to verify normal operation. If
abnormal performance is observed, additional measures may be necessary, such as reorienting or
relocating the Crossre™ System.
(b) Over the frequency range 150 kHz to 80 MHz, eld strengths should be less than 3 V/m.
EN-19
Recommended Separation Distances Between Portable and Mobile RF Communications
Equipment and the Crossre™ System
The Crossre™ System is intended for use in an electromagnetic environment in which radiated RF
disturbances are controlled. The user of the Crossre™ System can help prevent electromagnetic
interference by maintaining a minimum distance between portable and mobile RF communications
equipment (transmitters) and the Crossre™ System as recommended below, according to the
maximum output power of the communications equipment.
Rated maximum
output power (W) of
transmitter
Separation distance (m) according to frequency of transmitter
150 kHz to 80 MHz 80 MHz to 800 MHz 800 MHz to 2.5 GHz
0.01 0.12 0.12 0.23
0.1 0.37 0.37 0.74
1 1.17 1.17 3.70
10 3.70 2.33 7.37
100 11.70 11.70 23.30
For transmitters rated at a maximum output power not listed above, the recommended separation distance (d)
in meters (m) can be estimated using the equation applicable to the frequency of the transmitter, where P is the
maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and
reection from structures, objects, and people.

EN-20
Connections
Warning Be sure that no liquid is present between connections to the
console and the handpiece. Connection of wet accessories
may lead to electric shock or electrical short.
To avoid the risk of electric shock, this equipment must
only be connected to a supply mains with protective earth.
Use only hospital-grade power cables. Using other cables may result in
increased RF emissions or decreased immunity from such emissions.
Only the handpieces and disposable attachments are suitable for use in the
patient environment. e console and footswitch are not sterile devices and
should not enter the sterile eld.
e Crossre™ System is compatible only with the Stryker handpieces and
footswitches listed in this manual. Do not connect any equipment not
specied in this manual, as unexpected results or serious injury will occur.
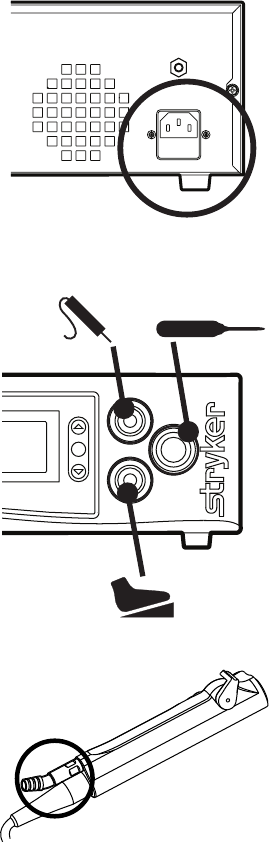
EN-21
1. Place the console on a sturdy platform, such as a Stryker cart.
• Selectalocationaccordingtotherecommendationsinthe
preceding EMC tables.
• Leavefourinchesofspacearoundallsidesforconvectioncooling.
2. Connect the AC power.
3. Connect the handpieces and
footswitch.
4. Connect suction tubing (for all
suction-capable devices).
EN-22
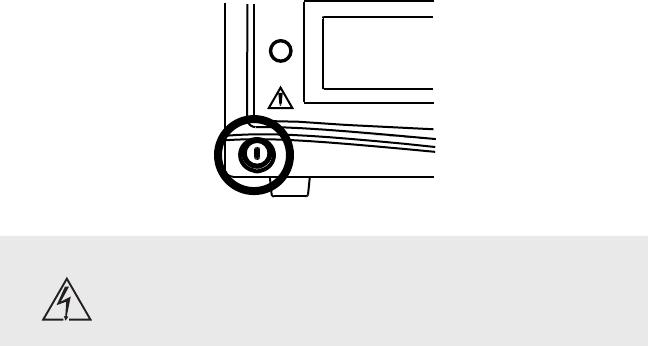
Powering the Console On and Off
Press the power button to power the console on and o. e button will shine
green when the console is on.
Warning Should emergency shutdown become necessary, power o
the console as described above. As an added safety measure,
the console can be separated from the AC power mains by
detaching the AC power cord from either end.

EN-23
Operation
e Crossre™ interface displays system status, enables you to choose between RF
and shaver modes, and enables you to adjust power and speed settings.
Activating the actual handpieces is performed through controls on the handpiece
and on the Crossre™ Footswitch.
Warning e Crossre™ system is intended for use only by licensed
medical professionals, properly trained in the use of
electrosurgical equipment and techniques. e Crossre™
system generates potentially hazardous levels of energy that
can result in injury or even death if improperly used.
Before using the Crossre™ system in an actual procedure,
verify that each component is installed and functioning
properly. Improper connection may cause arcing or
malfunction of the handpiece or console, which can result
in injury, unintended surgical eect, or product damage.
During use, operators should wear standard surgical gloves
to help reduce the risk of electric shock.
EN-24
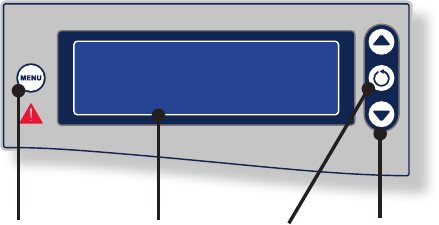
The Crossfire™ Interface
1 3 42
Control Description See
1. Menu The Menu button sets user and
system settings.
“Adjusting User and
System Settings”
2. LCD screen e LCD screen displays system
status, error codes, mode of
operation, cutting speed, and power
levels.
“Reading the LCD
Screen”
3. Select e Select button toggles between
RF and Shaver controls. e selected
device can then be controlled using
the Crossre™ interface.
“Selecting Between
RF and Shaver
Modes”
4. Adjust e Adjust buttons increase/decrease
speed and power settings for the
selected device.
“Adjusting Power
and Speed Settings”
EN-25
Adjusting User and System Settings
User Preference Settings
User preferences, such as power and cutting speeds and button assignments for
the handpiece and footswitch, can be adjusted through the Crossre™ interface.
Select from the default settings provided with the console, or contact your
Stryker representative to customize your own.
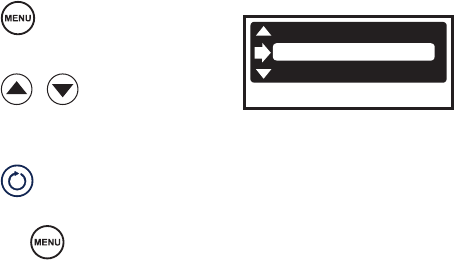
1. Press . DEFAULT
SMITH SHLDR
SMITH KNEE
2. Press to select a
default setting.
3. Press to conrm
selection and exit.
Or, press to cancel
selection.
Note: User preference settings will not take eect unless a disposable attachment
is connected to the shaver.
Note: When using small-joint handpieces, system defaults will take eect (see
“Default Shaver Controls”). No user preferences can be applied.
EN-26
System Settings
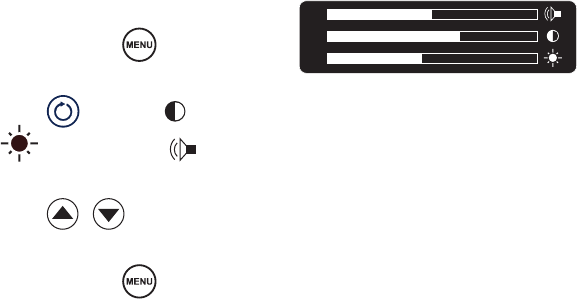
System settings, such as screen brightness, contrast, and system sound can be
adjusted through the Crossre™ interface.
1. Press and hold .
2. Press to choose (contrast),
(brightness), or (sound).
3. Press to adjust.
4. Press and hold to exit.
(Note: A short press will display
the current version of the console
software.)
EN-27
Reading the LCD Screen
e LCD screen displays the devices that are connected to the console and their
current status.
RF Mode
In RF mode, the LCD will show:
11
SERFAS
1
2
3
54
1. Footswitch status Crossre footswitch connected
iSwitch footswitch connected
not connected
2. Mode cut mode
coagulation mode
3. Force modulation force modulation activated
force modulation not activated
4. Probe name (name)
5. Power (#) power setting
EN-28
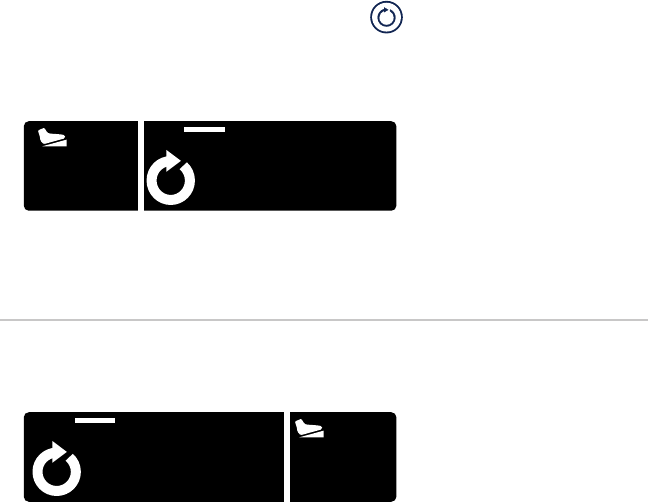
Shaver Mode
In shaver mode, the LCD will show:
VAR
F
9000
MC DISP NAME
1 2
3 5
4
1. Footswitch status Crossre footswitch connected
iSWITCH footswitch connected
not connected
2. Button response 1
TOUCH one touch
(pressing the shaver button once will
activate the shaver to a default speed;
pressing again will stop it)
FIXED xed
(pressing the shaver button at any
pressure will result in a constant speed)
VARvariable (for small-joint handpieces)
(shaver speed will vary, depending
on the pressure applied to the shaver
button)
3. Direction
F
forward
R
reverse
oscillate
4. Cutter name (name)
5. Speed (#) rotations per minute
EN-29
Dual Mode
In dual mode, the LCD will show the status of both devices. e shaver status
will always appear to the right, except during adjustments to RF settings.
To select between RF and shaver modes, press .
F
9000
FIXED
11
SERFASMCDISP NAME
Dual mode, normal
screen.
F
9000
FIXED 11
SERFASMC DISP NAME
Dual mode, adjustments
being made to RF power
settings
e screen will revert to
normal aer 5 seconds of
inactivity.

EN-30
Selecting Between RF and Shaver Modes
To select the appropriate mode, do one of the following:
• Press on the Crossre interface. e interface will toggle
between modes.
• Pressthemodebuttononthefootswitch.
• Pressanybuttononthedesiredhandpiece.
Adjusting Power and Speed Settings
Use the buttons on the console to manually adjust the power or speed
setting for the active handpiece.
Note: Forward and reverse settings are adjusted independent of each other.
Adjusting settings in one mode will not aect the other.
Note: In RF mode, power can also be adjusted with the buttons on the handpiece
or footswitch.
Note: To switch between and in RF mode, use the buttons on the
handpiece or footswitch.
Note: In shaver mode, the console uses radio frequency identication () to
automatically detect which type of disposable attachment is connected to the
handpiece. Upon recognition, the console adjusts to an optimal preset cutting
speed, direction, and power.
EN-31
Using the Handpiece
Warning During use, the RF and shaver handpieces generate
electronic noise that may interfere with EKG readings.
Before responding to any erratic EKG readings, rst power
down the system to ensure the readings are not the result of
system noise.
RF handpieces are intended for single use only and should
not be reprocessed or reused.
Shaver handpieces are provided nonsterile and must
be cleaned and sterilized prior to each use, according to
the reprocessing instructions provided in the handpiece
manual.
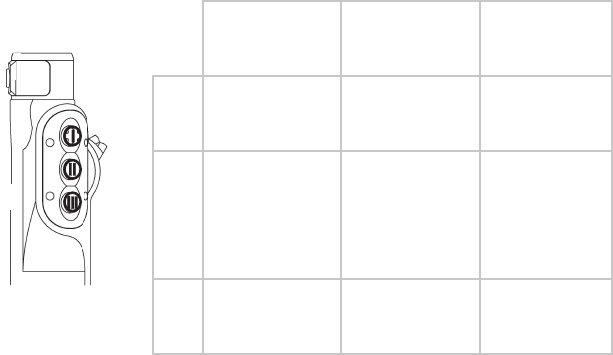
Each handpiece has its own set of controls.
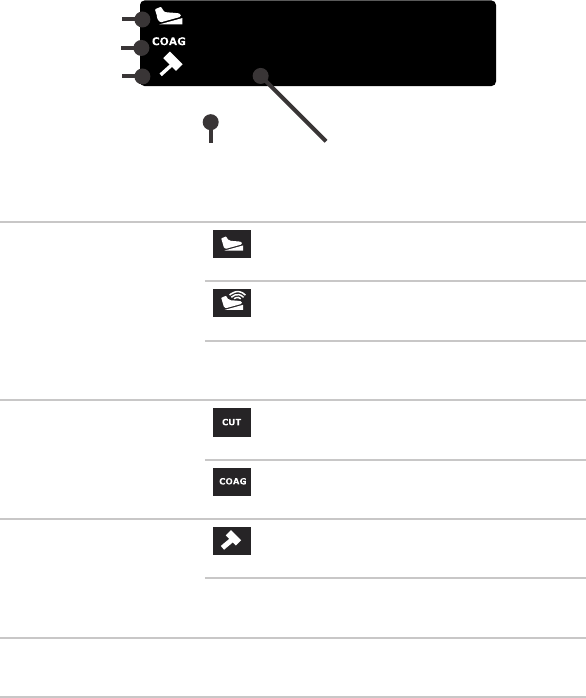
RF Hand Controls
1
2
3
1. Adjust CUT power level
2. Activate CUT
3. ActivateCOAG
EN-32
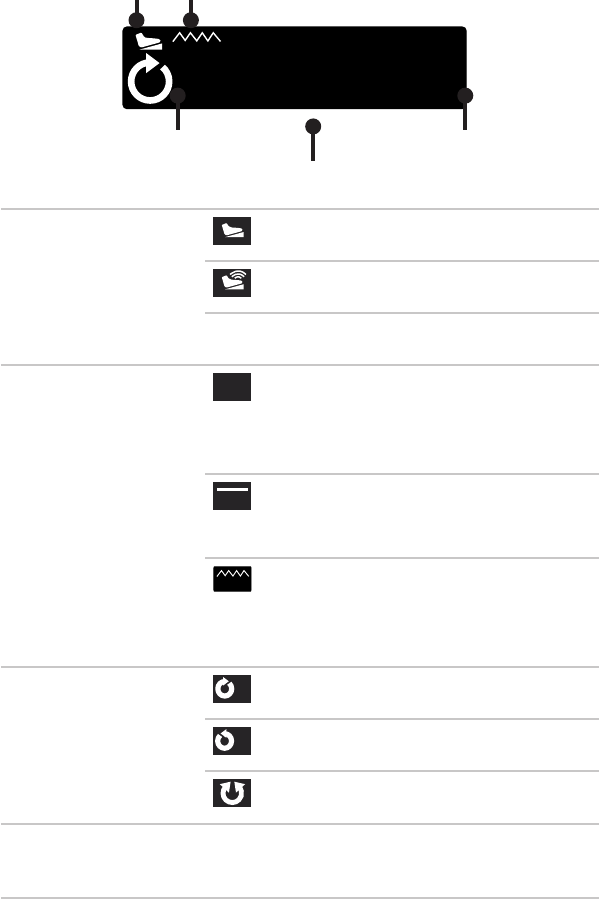
Shaver Hand Controls
1
2
3
Default 1 Default 2 /
None
Default 3
1. Oscillate
(one-touch)
Activate Oscillate
(one-touch)
2. Forward
(one-touch)
Select Mode:
Oscillate
or Forward
/Reverse
Jog
3. Reverse
(one-touch)
Forward/
Reverse
Forward
(one-touch)
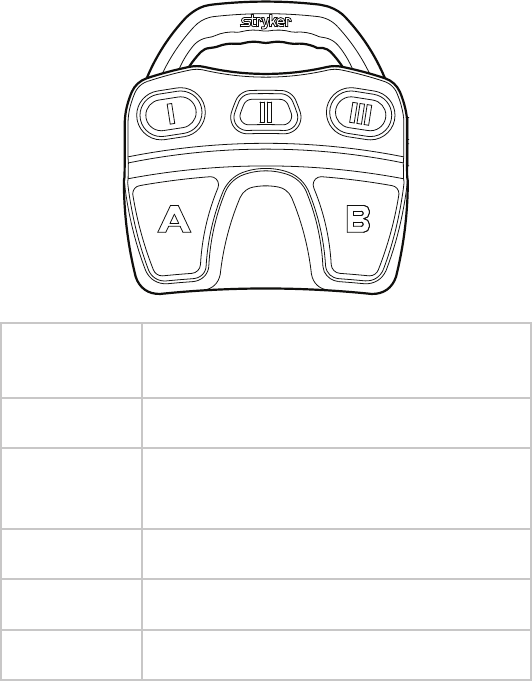
Using the Footswitch
e RF and shaver handpieces can also be controlled by the Crossre™
Footswitch. e default footswitch controls are shown below. To customize
button assignments, contact your Stryker representative.
Note: To keep the footswitch clean during use, Stryker recommends using
disposable bags (P/N 0277-500-100). Contact your local Stryker representative
for ordering information.
EN-33
Default RF Controls
Button Function
(controls are the same for defaults 1, 2, and 3)
I Decrease Cut Level
II Select Handpiece:
RF or Shaver
III Increase Cut Level
ACut
BCoag
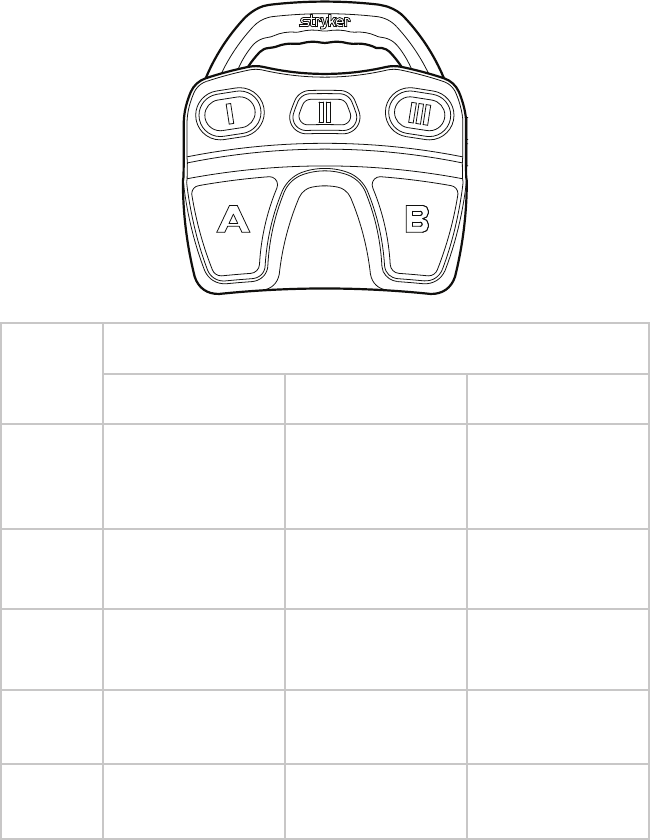
EN-34
Default Shaver Controls
Button Function
Default 1 Default 2 / None Default 3
I Jog Select Mode:
Oscillate or
Forward/Reverse
Select Mode:
Oscillate or
Forward/Reverse
II Select Handpiece:
RF or Shaver
Select Handpiece:
RF or Shaver
Select Handpiece:
RF or Shaver
III Select Direction:
Forward or Reverse
Select Speed:
High or Low
Select Speed:
High or Low
A Oscillate (xed) Oscillate/Reverse
(variable)
Oscillate/Reverse
(xed)
B Forward/Reverse
(variable)
Oscillate/Forward
(variable)
Oscillate/Forward
(xed)
Note: When using small-joint handpieces, only Default 2 settings are available.
No other defaults or user preferences can be applied.
EN-35
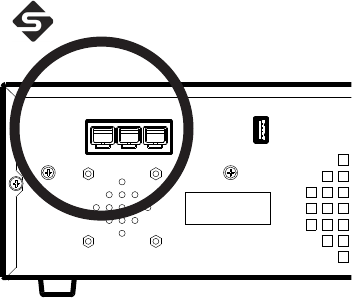
Using the iSWITCH™ Wireless Footswitch
e Crossre™ system can be used with the iSWITCH Wireless Footswitch
System.
1. Connect the Crossre™ console to the iSWITCH™ console using one of
the Firewire connection ports on each console.
2. Consult the iSWITCH™ Operating and Maintenance Manual
(P/N 1000-400-700) for further operation instructions.
EN-36
Audible Feedback
e Crossre™ Console will provide audible feedback for the following events:
Event Signal
During system startup
System self-test Two-second beep
Handpiece connected Single beep
Disposable attachment connected Single beep
Footswitch detected Single beep
RF probe detected Single beep
During use
RFCOAGmodeactive Continuous tone (low)
RF CUT mode active Continuous tone (high)
System error ree beeps
Force modulation on / o Single beep
Shaver reverse mode activated Five short beeps
Toggle (from footswitch) between RF
and Shaver
e console will say, “Shaver” or
“SERFAS”
EN-37
Troubleshooting
Problem Possible Solution
Console A hardware fault is
detected
• Turnthepoweroandonagain.
• Iftheproblempersists,contacta
Stryker representative or return the
console for repair.
e AC voltage is
incorrect
• Turnthepoweroffandonagain.
• Iftheproblempersists,contacta
Stryker representative or return the
console for repair.
A soware default
is detected
• Turnthepoweroffandonagain.
• Iftheproblempersists,contacta
Stryker representative or return the
console for repair.
e system does
not power on
• Checkthepowercordtoensureit
is properly connected.
• Checktoensurethecordis
connected to a grounded outlet.
e electrical
interference is
sporadic
• Powerdownallelectrical
equipment not in use.
• Increasedistanceofotherelectrical
equipment.
• Connecttheunitandother
equipment into dierent outlets.
e generator
temperature is too
high
Ensure that there is proper airow
around the unit.
A power-on self
test error has
occurred
• Turnthepoweroandonagain.
• Iftheproblempersists,contacta
Stryker representative or return the
console for repair.
Hand-
piece
e temperature is
higher than normal
Allow the unit to cool before
restarting.
e unit has
reached its
recommended
service interval
Contact your Stryker
representative.

EN-38
Disposable
Attachments
RF probe is not
ready
Check the connection to the
console.
RF probe is expired Replace probe.
RF probe
identication is
invalid
Replace probe.
RF probe
communication
error
• Checktheconnectiontothe
console.
• Ifnecessary,replaceprobe.
Exceeded time
usage
Replace probe
RF power is too
high
• Checktheprobefordamage.
• Ifnecessary,replaceprobe.
RF voltage is too
high
• Checktheprobefordamage.
• Ifnecessary,replaceprobe.
RF current is too
high
• Checktheprobefordamage.
• Ifnecessary,replaceprobe.
RF delivery
has exceeded
continuous limit
Clear error and continue
Low impedance
detected
• Checktheprobefordamage.
• Ifnecessary,replaceprobe.
Footswitch A wireless
footswitch is
detected
Disconnect the wired footswitch.
e footswitch icon
does not appear
• Ensuretheunitisconnected.
• Ensurethatthereisnodamageto
the cable or connector.
Note: If a disturbance occurs on the video monitor, the user should ensure that
the probe cable is not near any other instrument cables.

EN-39
Cleaning and Maintenance
Cleaning
Console
Should the console need cleaning, wipe it down with a sterile cloth and mild
cleaning solution. If needed, wipe the console with a disinfectant.
Warning To avoid electric shock and potentially fatal injury, unplug
the Crossre™ console from the electrical outlet before
cleaning.
Do not sterilize the console or immerse it in any liquid.
Doing so will damage the unit.
Do not clean the console with alcohol, solvents, or cleaning
solutions that contain ammonia. Doing so will damage the
unit.
Footswitch
Consult the footswitch user guide for cleaning and reprocessing instructions.
RF Handpiece
RF handpieces are intended for single use only and should not be cleaned,
sterilized, or reused.
Shaver Handpiece
Consult the appropriate user guide for cleaning and reprocessing instructions.
Disposable attachments are intended for single use only and should not be
cleaned, sterilized, or reused.
Maintenance
e Crossre™ console requires no preventative or periodic maintenance.
However, Stryker recommends you reboot the system daily for best performance.
EN-40
Disposal
is product contains electrical waste or electronic equipment. It
must not be disposed of as unsorted municipal waste and must
be collected separately in accordance with applicable national
or institutional related policies relating to obsolete electronic
equipment.
Dispose of any system accessories according to normal institutional practice
relating to potentially contaminated items.
EN-41
Technical Specifications
Stryker Endoscopy reserves the right to make improvements to the product(s)
described herein. Product(s), therefore, may not agree in detail to the published
design or specications. All specications are subject to change without notice.
Please contact the local Stryker Endoscopy distributor or call your local Stryker
Endoscopy sales representative or agent for information on changes and new
products.
Dimensions
Size: 16.9" L × 12.5" H × 4.5" W
Weight: 20 lbs
Environmental Specifications
Operating temperature: 5 – 40°C
Operating humidity: 30 – 95% RH
Shipping temperature: -18 – 60°C
Shipping humidity: 15 – 90% RH
System Input Power Requirements
Voltage: 100-240 VAC @ 50/60Hz, 6 – 10 A
Inlet Fuse: 15 A, 250V
Electrical Specifications
Motor output max speed: 12000 RPM
Motor duty cycle: Continuous operation
RF output waveform: 200 kHz ± 1%, square wave,
Crest factor <1.5 @ 200 ohms

EN-42
Classifications
Warning is equipment is not suitable for use in the presence of a
ammable anesthetic mixture with air, oxygen, or nitrous
oxide.
• ClassIequipment
• TypeBFappliedpart
• Degreeofprotectionagainstharmfulingressofwater
• Generator:IEC60601-2-2:Requirementperclause44.3
• Probe:IEC60601-2-2:Requirementperclause44.6
• Footswitch:IEC60601-2-2:Requirementperclause44.6,
IPX7 Water-tight Equipment
Approvals
Complies with medical safety standards:
• IEC60601-1:1998+A1:1991+A2:1995
• AS3200.1.0:1998
• IEC60601-1-2:2001
• IEC60601-2-2:2006
• UL60601-1:2003
• CSAC22.2No.601-1-M90
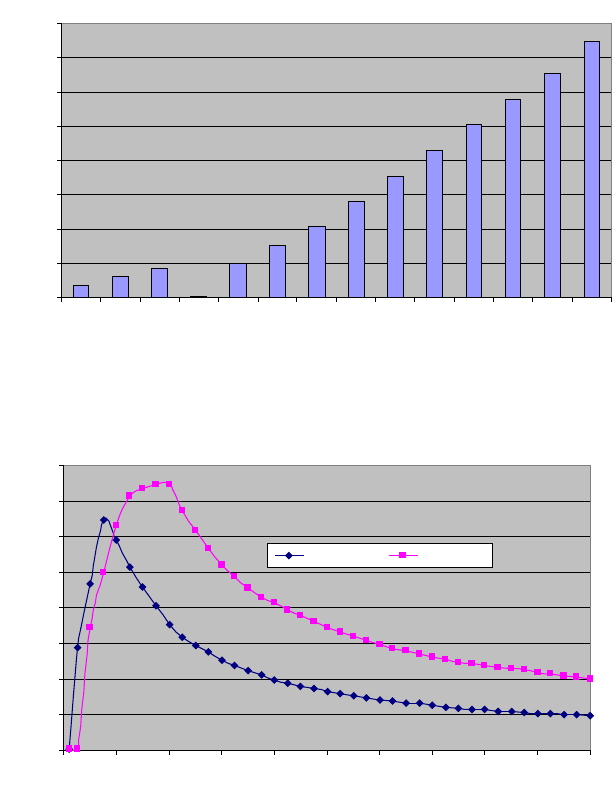
Generator Output
Output power at each set point with specied load resistance (per IEC 60601-2-2,
sub clause 6.8.3) is given in the graphs below.
EN-43
Output Power versus Setting at 200ohms Resistive Load
Output Power versus Setting at 200 Ohm Load
0
50
100
150
200
250
300
350
400
Coag
1
Coag
2
Coag
3
1234567 8910 11
Cut Level
Power (W)
Output Power (CUT) versus Load Resistance
Output Power (Cut) versus Load Resistance
0
50
100
150
200
250
300
350
400
0100 200 300 400 500 600 700 800 900 1000
Load Resistance (ohms)
(Power (W)
Half Setting Full Setting
EN-44
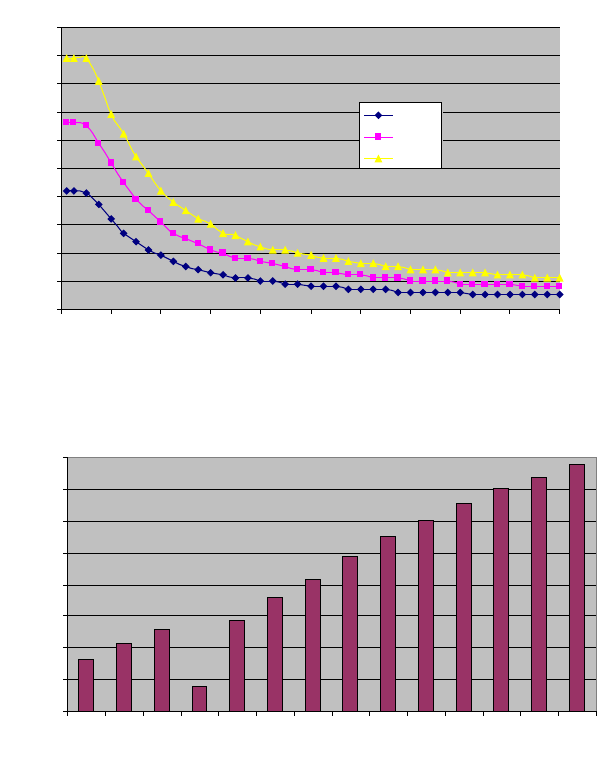
Output Power (COAG) versus Load Resistance
Output Power (Coag) versus Load Resistance
0
10
20
30
40
50
60
70
80
90
100
0100 200 300 400 500 600 700 800 900 1000
Load Resistance (Ohms)
Power (W)
Coag 1
Coag 2
Coag 3
Maximum Open Circuit Voltage versus Set Point
Maximum Output Voltage (RMS) versus Setting
0
50
100
150
200
250
300
350
400
Coag
1
Coag
2
Coag
3
12345678910 11
Cut Level
Voltage (Vrms)

Stryker Endoscopy
5900 Optical Court
San Jose, CA 95138 USA
1-408-754-2000, 1-800-624-4422
www.stryker.com
European Representative:
Regulatory Manager, Stryker France
ZACSatolasGreenPusignan
Av.DeSatolasGreen
69881 MEYZIEU Cedex, France
1000-401-036 Rev E
2009/05