Cadenza
File info: application/pdf · 23 pages · 325.88KB
Cadenza
empty
Extracted Text
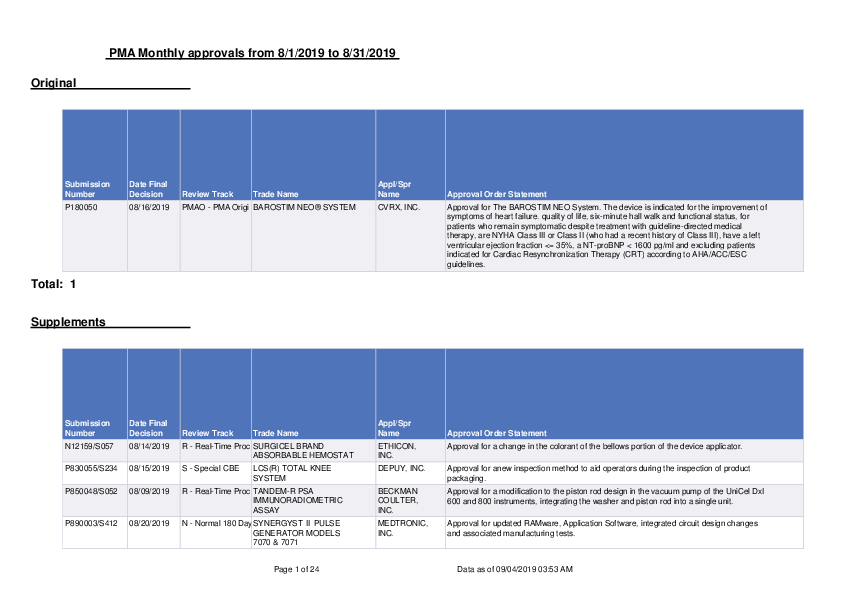
Original PMA Monthly approvals from 8/1/2019 to 8/31/2019 Submission Number P180050 Date Final Decision 08/16/2019 Review Track Trade Name PMAO - PMA Origi BAROSTIM NEO� SYSTEM Appl/Spr Name CVRX, INC. Total: 1 Approval Order Statement Approval for The BAROSTIM NEO System. The device is indicated for the improvement of symptoms of heart failure. quality of life, six-minute hall walk and functional status, for patients who remain symptomatic despite treatment with guideline-directed medical therapy, are NYHA Class III or Class II (who had a recent history of Class III), have a left ventricular ejection fraction <= 35%, a NT-proBNP < 1600 pg/ml and excluding patients indicated for Cardiac Resynchronization Therapy (CRT) according to AHA/ACC/ESC guidelines. Supplements Submission Number N12159/S057 P830055/S234 P850048/S052 P890003/S412 Date Final Decision 08/14/2019 08/15/2019 08/09/2019 08/20/2019 Review Track Trade Name R - Real-Time Proc SURGICEL BRAND ABSORBABLE HEMOSTAT S - Special CBE LCS(R) TOTAL KNEE SYSTEM R - Real-Time Proc TANDEM-R PSA IMMUNORADIOMETRIC ASSAY N - Normal 180 Day SYNERGYST II PULSE GENERATOR MODELS 7070 & 7071 Page 1 of 24 Appl/Spr Name ETHICON, INC. DEPUY, INC. BECKMAN COULTER, INC. MEDTRONIC, INC. Approval Order Statement Approval for a change in the colorant of the bellows portion of the device applicator. Approval for anew inspection method to aid operators during the inspection of product packaging. Approval for a modification to the piston rod design in the vacuum pump of the UniCel DxI 600 and 800 instruments, integrating the washer and piston rod into a single unit. Approval for updated RAMware, Application Software, integrated circuit design changes and associated manufacturing tests. Data as of 09/04/2019 03:53 AM Submission Number P960011/S032 P960016/S079 P960058/S131 Date Final Decision 08/05/2019 08/22/2019 08/30/2019 Review Track Trade Name R - Real-Time Proc BIOLON 1% SODIUM HYALURONATE VISCOELASTIC SURGICAL AID FLUID O - Normal 180 DayLIVEWIRE(R) CARDIAC ABLATION SYSTEM N - Normal 180 Day CLARION MULTISTRATEGY COCHLEAR IMPLANT P970038/S040 P980016/S701 08/09/2019 08/01/2019 R - Real-Time Proc TANDEM-R FREE PSA IMMUNORADIOMETRIC ASSAY/TANDEM-MP FREE PSA IMMUNOENZYMETRIC ASSAY Y - 135 Review Tra VIRTUSO/ENTRUST/ MAXIMO/INTRINSIC/ MARQUIS/IMPLANTABLE CARDIVERTER DEFIBRILLATORS P980035/S582 P980035/S594 P980035/S595 P980040/S100 P980041/S045 08/01/2019 08/20/2019 08/22/2019 08/29/2019 08/09/2019 Y - 135 Review Tra MEDTRONIC KAPPA 700/600 SERIES PULSE GENERATORS AND MODEL 9953 SOFTWARE N - Normal 180 Day MEDTRONIC KAPPA 700/600 SERIES PULSE GENERATORS AND MODEL 9953 SOFTWARE R - Real-Time Proc MEDTRONIC KAPPA 700/600 SERIES PULSE GENERATORS AND MODEL 9953 SOFTWARE Y - 135 Review Tra SENSAR SOFT ACRYLIC UV-LIGHT ABSORBING POSTERIOR CHAMBER INTRAOCULAR LENS R - Real-Time Proc ACCESS AFP IMMUNOASSAY SYSTEM Appl/Spr Name AMRING PHARMACEUT ICALS Approval Order Statement Approval for material changes to the tip cap and syringe tip cap system over-closure for BIOLON�. ST. JUDE MEDICAL ADVANCED BIONICS BECKMAN COULTER, INC. Approval for an alternate sterilization site at Midwest Sterilization Corporation, 1204 Lenco Avenue, Jackson, Missouri. Approval of the HiRes Fidelity 120 and HiRes Optima sound processing strategies for adults and pediatric patients 12 months and older. Approval of the ClearVoice and Front-End Processing features of StereoZoom, UltraZoom, SoundRelax, WindBlock, and EchoBlock for pediatric population 6 years and older. Approval for a modification to the piston rod design in the vacuum pump of the UniCel DxI 600 and 800 instruments, integrating the washer and piston rod into a single unit. MEDTRONIC CARDIAC RHYTHM DISEASE MANAGEMEN T MEDTRONIC INC. Approval for a change to a sub-tier raw material supplier for vanadium pentoxide used during battery cathode fabrication. Approval for a change to a sub-tier raw material supplier for vanadium pentoxide used during battery cathode fabrication. MEDTRONIC INC. Approval for updated RAMware, Application Software, integrated circuit design changes and associated manufacturing tests. MEDTRONIC INC. Approval for new coating treatment of the battery cathode current collectors in mediumrate batteries. JOHNSON & JOHNSON SURGICAL VISION, INC. BECKMAN COULTER, INC. Approval for adding an alternate supplier of the injection molded screw plunger and pushrod components for the Preloaded TECNIS� 1-Piece IOL, Model PCB00 and TECNIS� iTEC Preloaded Delivery System, Model PMB00. Approval for a modification to the piston rod design in the vacuum pump of the UniCel DxI 600 and 800 instruments, integrating the washer and piston rod into a single unit. Page 2 of 24 Data as of 09/04/2019 03:53 AM Submission Number P000025/S112 Date Final Decision 08/22/2019 Review Track Trade Name R - Real-Time Proc COMBI 40+ COCHLEAR IMPLANT SYSTEM P000037/S054 P000039/S067 P000046/S027 08/15/2019 08/22/2019 08/28/2019 O - Normal 180 DayON-X (R) PROSTHETIC HEART VALVE, MODEL ONXA O - Normal 180 DayTHE AMPLATZER(R) SEPTAL OCCLUDER (ASO) AND THE AMPLATZER EXCHANGE SYSTEM R - Real-Time Proc STAARVISC II P010015/S398 P010015/S407 P010015/S411 P010031/S661 P010031/S672 P020024/S057 08/01/2019 08/22/2019 08/23/2019 08/01/2019 08/23/2019 08/22/2019 Y - 135 Review Tra MEDTRONIC INSYNC(TM) BIVENTRICAL PACING SYSTEM R - Real-Time Proc MEDTRONIC INSYNC(TM) BIVENTRICAL PACING SYSTEM R - Real-Time Proc MEDTRONIC INSYNC(TM) BIVENTRICAL PACING SYSTEM Y - 135 Review Tra CONCERTO/INSYNC SENTRY/INSYNC MAXIMO IMPLANTABLE CARDIOVASCULAR DEFIBRILLATORS WITH CARDIAC RESYNCHICNIZATION R - Real-Time Proc CONCERTO/INSYNC SENTRY/INSYNC MAXIMO IMPLANTABLE CARDIOVASCULAR DEFIBRILLATORS WITH CARDIAC RESYNCHICNIZATION O - Normal 180 DayAMPLATZER DUCT OCCLUDER AND 180 DEGREE DELIVERY SYSTEM Appl/Spr Name MED-EL CORP. ON-X LIFE TECHNOLOGI ES, INC. ABBOTT MEDICAL Approval Order Statement Approval for the SONNET 2 and SONNET 2 EAS audio processors. The SONNET 2 and SONNET 2 EAS audio processors are updates to existing products (SONNET and SONNET EAS). Approval for the revised protocol for the Newly Enrolled On-X Post-Approval Study. Approval for an alternate sterilization site at Midwest Sterilization Corporation, 1204 Lenco Avenue, Jackson, Missouri. ANIKA THERAPEUTI CS, INC. MEDTRONIC INC. Approval for an extension of the shelf-life of Ophthalmic Viscoelastic� from 24 months to 36 months. Approval for a change to a sub-tier raw material supplier for vanadium pentoxide used during battery cathode fabrication. MEDTRONIC INC. Approval for a minor battery design change for Delta 26H3 or medium-rate (MR) batteries. MEDTRONIC INC. Approval for updated firmware and software for the EffectivCRT during AF feature. MEDTRONIC CARDIAC RHYTHM DISEASE MANAGEMEN T Approval for a change to a sub-tier raw material supplier for vanadium pentoxide used during battery cathode fabrication. MEDTRONIC CARDIAC RHYTHM DISEASE MANAGEMEN T Approval for updated firmware and software for the EffectivCRT during AF feature. ABBOTT MEDICAL Approval for an alternate sterilization site at Midwest Sterilization Corporation, 1204 Lenco Avenue, Jackson, Missouri. Page 3 of 24 Data as of 09/04/2019 03:53 AM Submission Number P030011/S073 P040013/S022 P040020/S087 Date Final Decision 08/15/2019 08/19/2019 08/26/2019 Review Track Trade Name S - Special CBE SYNCARDIA TEMPORARY CARDIO WEST TOTAL ARTIFICIAL HEART (TAH-T) O - Normal 180 DayGEM 21S (GROWTHFACTOR ENHANCED MATRIX P - Panel Track ACRYSOF RESTOR APODIZED DIFFRACTIVE OPTIC POSTERIOR CHAMBER IOL P040029/S006 P040040/S037 P050028/S077 08/07/2019 08/22/2019 08/13/2019 O - Normal 180 DayJSZ ORTHOKERATOLOGY (OPRIFOCON A) CONTACT LENSES FOR OVERNIGHT WEAR O - Normal 180 DayAMPLATZER MUSCULAR VSD OCCLUDER S - Special CBE COBAS TAQMAN HBV TEST P060019/S043 P060030/S078 08/16/2019 08/13/2019 Y - 135 Review Tra IBI THERAPY COOL PATH ABLATION CATHETER & IBI-1500T9 RF S - Special CBE COBAS AMPLIPREP/COBAS TAQMAN HCV TEST P060040/S072 08/12/2019 O - Normal 180 DayTHORATEC HEARTMATE II LEFT VENTRICULAR ASSIST SYSTEM Appl/Spr Name SYNCARDIA SYSTEMS, LLC LYNCH BIOLOGICS LLC ALCON RESEARCH, LTD. EUCLID SYSTEMS CORPORATIO N ABBOTT MEDICAL ROCHE MOLECULAR SYSTEMS, INC. IRVINE BIOMEDICAL, INC. ROCHE MOLECULAR SYSTEMS, INC. THORATEC CORP. Approval Order Statement Approval for the addition of a red key tag with caution statement on the key ring of the Companion 2 Driver key. Approval to change the supplier who fills the B-TCP cup component of the GEM21S device from A+ Secure Packaging to Alliance Contract Pharma. Approval for the AcrySof� IQ PanOptix� Trifocal Intraocular lens, which is indicated for primary implantation in the capsular bag in the posterior chamber of the eye for the visual correction of aphakia in adult patients, with less than 1 diopter of pre-existing corneal astigmatism, in whom a cataractous lens has been removed. The lens mitigates the effects of presbyopia by providing improved intermediate and near visual acuity, while maintaining comparable distance visual acuity with a reduced need for eyeglasses, compared to a monofocal IOL. Approval for the AcrySof� IQ PanOptix� Toric Trifocal Intraocular lens, which is indicated for primary implantation in the capsular bag in the posterior chamber of the eye for the visual correction of aphakia and the reduction of residual refractive astigmatism, in adult patients in whom a cataractous lens has been removed. The lens mitigates the effects of presbyopia by providing improved intermediate and near visual acuity, while maintaining comparable distance visual acuity with a reduced need for eyeglasses, compared to a monofocal IOL. Approval for new manufacturing site at Euclid Systems Corporation, 45472 Holiday Drive, Suite 7, Herndon, Virginia. Approval for an alternate sterilization site at Midwest Sterilization Corporation, 1204 Lenco Avenue, Jackson, Missouri. Approval for the addition of an instruction to the labeling to visually inspect reagent cassette and vial prior to use. Approval for changes in the finished device electrical safety test for the Cool Point Irrigation Pump. Approval for the addition of an instruction to the labeling to visually inspect reagent cassette and vial prior to use. Approval for a manufacturing site located at Sterigenics in Salt Lake City, Utah for ethylene oxide sterilization of the HeartMate 3 LVAS and HeartMate II LVAS components. Page 4 of 24 Data as of 09/04/2019 03:53 AM Submission Number P070026/S065 Date Final Decision 08/06/2019 Review Track Trade Name O - Normal 180 DayCERAMAX CERAMIC HIP SYSTEM P070026/S067 08/14/2019 S - Special CBE CERAMAX CERAMIC HIP SYSTEM P080006/S139 P090016/S028 08/27/2019 08/29/2019 O - Normal 180 DayMEDTRONIC ATTAIN ABILITY MODEL 4196 LEAD N - Normal 180 Day BELOTERO BALANCE P090026/S026 P100009/S034 08/09/2019 08/30/2019 R - Real-Time Proc ACCESS HYBRITECH P2PSA ON THE ACCESS IMMUNOASSAY SYSTEMS S - Special CBE MITRACLIP NT, NTR, XTR, G4 CLIP DELIVERY SYSTEM P100026/S070 08/01/2019 O - Normal 180 DayNEUROPACE RNS SYSTEM Appl/Spr Name DEPUY ORTHOPAEDI CS, INC. DEPUY ORTHOPAEDI CS, INC. MEDTRONIC INC. MERZ NORTH AMERICA, INC BECKMAN COULTER, INC. ABBOTT VASCULAR INC. NEUROPACE INC Approval Order Statement Approval for a manufacturing site located at DePuy (Ireland) , Loughbeg, Ringaskiddy Co. CORK, Ireland, for coating, machining, cleaning, and packaging of the Summit femoral stems. Approval for the addition of an updated packaging inspection. Approval for labeling updates to the clinical study summary for the post approval study. Approval for use of Belotero Balance with Integral Lidocaine for injection into the mid-todeep dermis for correction of moderate-to-severe facial wrinkles and folds such as nasolabial folds. Approval for a modification to the piston rod design in the vacuum pump of the UniCel DxI 600 and 800 instruments, integrating the washer and piston rod into a single unit. Approval for manufacturing process changes to reduce Clip Arm spread variability and to implement an inspection for final Clip Arm spread. Approval for the following protocol changes and associated changes to data collection and informed consent forms: P110015/S005 08/29/2019 Y - 135 Review Tra GASTRIC EMPTYING BREATH TEST (GEBT) P110016/S063 P110033/S042 08/22/2019 08/29/2019 O - Normal 180 DayTHERAPY COOL PATH DUO/ SAFIRE BLU DUO ABLATION CATHETER AND IBI 1500T9-CP V1.6 CARDIAC ABLATION GENERATOR N - Normal 180 Day JUVEDERM VOLUMA XC ADVANCED BREATH DIAGNOSTICS ST. JUDE MEDICAL, INC. (IRVINE BIOMEDICAL) 1) Increase the upper limit on the number of subjects that can be implanted per study site; 2) Simplify the text to remove references to activities that are not study-specific (i.e. are standard in the management of the RNS System); 3) Revise inclusion/exclusion criteria to only include study-specific criteria; 4) Add the option of phone appointments for specific follow-up time points; and 5) Minor clarifications and administrative changes. for the post-approval studies (PAS) protocol. Approval for changing the manufacturing site for the unit dose packaged 13C-Spirulina/egg mix that constitutes the diagnostic drug product in the Gastric Emptying Breath Test (GEBT). Approval for an alternate sterilization site at Midwest Sterilization Corporation, 1204 Lenco Avenue, Jackson, Missouri. ALLERGAN Approval for an update to the labeling for Juvederm Voluma XC to include the use of cannula. Page 5 of 24 Data as of 09/04/2019 03:53 AM Submission Number P110037/S048 P120005/S082 P120021/S012 P130008/S043 Date Final Decision 08/13/2019 08/20/2019 08/22/2019 08/06/2019 Review Track Trade Name S - Special CBE COBAS� AMPLIPREP/ COBAS� TAQMAN� CMV TEST (CAP/CTM CMV TEST) R - Real-Time Proc DEXCOM G4 PLATINUM CONTIUOUS GLUCOSE MONITORING SYSTEM O - Normal 180 DayAMPLATZER PFO OCCLUDER R - Real-Time Proc INSPIRE II UPPER AIRWAY STIMULATOR P130021/S058 08/16/2019 P - Panel Track MEDTRONIC COREVALVE SYSTEM P130022/S025 08/18/2019 R - Real-Time Proc NEVRO SENZA SPINAL CORD STIMULATION (SCS) SYSTEM P130026/S048 08/22/2019 O - Normal 180 DayTACTICATH QUARTZ SET P140029/S017 P140031/S085 08/13/2019 08/16/2019 R - Real-Time Proc RESTYLANE REFYNE, RESTYLANE DEFYNE P - Panel Track SAPIEN 3 TRANSCATHETER HEART VALVE AND ACCESSORIES P140031/S090 P140031/S091 08/15/2019 08/21/2019 S - Special CBE SAPIEN 3 TRANSCATHETER HEART VALVE AND ACCESSORIES R - Real-Time Proc SAPIEN 3 TRANSCATHETER HEART VALVE AND ACCESSORIES Appl/Spr Name ROCHE MOLECULAR SYSTEMS, INC. DEXCOM, INC. ABBOTT MEDICAL INSPIRE MEDICAL SYSTEMS MEDTRONIC COREVALVE LLC NEVRO CORPORATIO N ST. JUDE MEDICAL Q-MED AB Approval Order Statement Approval for the addition of an instruction to the labeling to visually inspect reagent cassette and vial prior to use. Approval for changes to improve drop impact resistance to the receiver component of the Dexcom G4 PLATINUM Continuous Glucose Monitoring System and Dexcom G5 Mobile Continuous Glucose Monitoring System. Approval for an alternate sterilization site at Midwest Sterilization Corporation, 1204 Lenco Avenue, Jackson, Missouri. Approval for proposed changes to the Inspire systems MRI Guidelines Manual. Approval for the Medtronic CoreValve Evolut R System and Medtronic CoreValve Evolut PRO System for expanding the indication to include patients at low risk for surgical aortic valve replacement. The devices are indicated for relief of aortic stenosis in patients with symptomatic heart disease due to severe native calcific aortic stenosis who are judged by a heart team, including a cardiac surgeon, to be appropriate for the transcatheter heart valve replacement therapy. Approval for a change in the approved packaging for the IPG (NIPG1500, NIPG2000), Lead Extension kits (MADP2008-25B M8, SADP2008-25B S8), and Lead Adapter kits (LEAD2008-25B, LEAD2008-35B, LEAD2008-60B) of your Senza Spinal Cord Stimulation (SCS) System. Approval for an alternate sterilization site at Midwest Sterilization Corporation, 1204 Lenco Avenue, Jackson, Missouri. Approval for reclassification of impurity C in lidocaine HCl. EDWARDS LIFESCIENCE S, LLC. EDWARDS LIFESCIENCE S, LLC. EDWARDS LIFESCIENCE S, LLC. Approval for the Edwards SAPIEN 3 Transcatheter Heart Valve System and Edwards SAPIEN 3 Ultra Transcatheter Heart Valve System for expanding the indication to include patients at low risk for surgical aortic valve replacement. The devices are indicated for relief of aortic stenosis in patients with symptomatic heart disease due to severe native calcific aortic stenosis who are judged by a heart team, including a cardiac surgeon, to be appropriate for the transcatheter heart valve replacement therapy. Approval for a revision to the Instructions for Use (IFU) for the Edwards SAPIEN 3 Ultra Transcatheter Heart Valve System with the Edwards SAPIEN 3 Ultra Delivery System to add a warning related to balloon burst during the valve deployment process. Approval for updates to the labeling of the SAPIEN 3 Ultra Transcatheter Heart Valve System regarding the option to use the Commander delivery system with the eSheath for the delivery and deployment of the SAPIEN 3 Ultra valve. Page 6 of 24 Data as of 09/04/2019 03:53 AM Submission Number P150005/S047 Date Final Decision 08/23/2019 Review Track Trade Name R - Real-Time Proc BLAZER OPEN-IRRIGATED ABLATION CATHETER P150031/S011 P150033/S050 P160001/S039 08/19/2019 08/01/2019 08/20/2019 N - Normal 180 Day VERCISE DEEP BRAIN STIMULATION (DBS) SYSTEM Y - 135 Review Tra MEDTRONIC MICRA TRANSCATHETER PACEMAKER SYSTEM R - Real-Time Proc OBALON BALLOON SYSTEM Appl/Spr Name BOSTON SCIENTIFIC CORP. BOSTON SCIENTIFIC CORP. MEDTRONIC INC. OBALON THERAPEUTI CS, INC. Approval Order Statement Approval for a material change to the proximal extension tubing of the BSC Open Irrigation Ablation Catheters Approval for MR Conditional labeling of the Vercise Gevia Deep Brain Stimulation (DBS) System. Approval for a change to a sub-tier raw material supplier for vanadium pentoxide used during battery cathode fabrication. Approval for updating the Touch Dispenser software to disable the touchscreen recalibration functionality. Page 7 of 24 Data as of 09/04/2019 03:53 AM P160001/S042 08/08/2019 O - Normal 180 DayOBALON BALLOON SYSTEM OBALON THERAPEUTI CS, INC. Approval of the revised protocol for the post-approval study protocol. The Obalon Balloon System Post-Approval Study is a prospective, open-label, single-arm study of the safety and effectiveness of the Obalon 6-month Balloon System, as an adjunct to weight loss for obese adults 22 years of age and older with a Body Mass Index (BMI) of 30 kg/m2 to 40 kg/m2. This is a 12-month follow-up study in which subjects will be treated during the first 6 months with placement (via swallow) of up to three Obalon Balloons in conjunction with a moderate intensity weight loss and behavioral modification program standardized throughout the sites, followed by observational evaluation for an additional 6 months after device removal. This study will include Obalon balloons contained in animal-based capsules (approved in original PMA) and plant-based hydroxy propyl methyl cellulose (HPMC) capsules (being approved under PMA S003). A total of 200 subjects will be enrolled at 10 to 15 sites in the United States; 180 evaluable subjects will be available at 6 months, including a minimum of 50 patients receiving animal-based gelatin capsules and a minimum of 50 patients receiving plant-based HPMC capsules. The primary endpoint is to evaluate the safety of Obalon by assessing the rate of deviceor procedure-related Serious Adverse Event(s) (SAEs) (composite safety endpoint). Where the SAE is defined as any AE that results in death or persistent/significant disability and/or incapacity, which may include emergency room visits, is life-threatening, requires inpatient hospitalization or prolongation of existing hospitalization, or requires medical/surgical intervention to prevent any of the above, through 6 months of treatment with the Obalon 6month Balloon System. The observed rate will be compared to a performance goal of 10% at 6 months assuming an expected 4.5% device or procedure related SAEs rate. The secondary effectiveness endpoint is comprised (1) the mean % Total Body Loss (%TBL) and (2) the proportion of subjects achieving at least -5% TBL through the first 6 months after the device is implanted. Additional endpoints include observational safety and effectiveness analyses including the percentage of subjects and frequency of individual Adverse Events (AEs) that are deviceor procedure-related, frequency and cause of early explantations, rates of gastric ulceration, esophageal tear, balloon deflation, means of other weight loss metrics such as % Excess Weight Loss (EWL), Weight Loss (WL) in pounds, and BMI change, percentage of subjects with at least 6%, 7%, 8%, 9%, and 10% TBL, percentage of subjects with at least 25% EWL, patient-reported outcomes assessing tolerability of device and/or quality of life, weight loss metrics by number of balloons placed, weight loss metrics by frequency of weight loss and behavioral modification program counseling. Descriptive analyses will be presented and stratified by capsule type. Follow-up assessments will be in office visits at Day 0, monthly during the first 6 months and at 12 months after initial implant. The Obalon 6-month Balloon System requires removal of all 3 balloons at the end of the 6-month period. Subjects will be followed for an additional 6-month period to ensure there are no Adverse Events as a result of balloon removal or residual events due to balloon use. Subjects with gastric ulcerations at the time of device explant will be followed with endoscopic evaluation every 8 weeks until the ulcer has visually resolved. Page 8 of 24 Data as of 09/04/2019 03:53 AM Submission Number P160022/S011 P160035/S005 P160048/S011 P160054/S019 P160055/S005 Date Final Decision 08/09/2019 08/05/2019 08/29/2019 08/12/2019 08/06/2019 Review Track Trade Name R - Real-Time Proc X SERIES�, R SERIES�, AED PRO�, AED 3� BLS PROFESSIONAL DEFIBRILLATORS, PROPADZ RADIOTRANSPARENT ELECTRODE, SUREPOWER BATTERY PACK, SUREPOWER II BATTERY PACK, AED PRO� NONRECHARGEABLE LITHIUM BATTERY PACK, AED 3 BATTERY PACK, SUREPOWER CHARGER, AND SUREPOWER SINGLE BAY CHARGER R - Real-Time Proc EXCOR PEDIATRIC VENTRICULAR ASSIST DEVICE N - Normal 180 Day EVERSENSE CONTINUOUS GLUCOSE MONITORING SYSTEM O - Normal 180 DayHEARTMATE 3 LEFT VENTRICULAR ASSIST SYSTEM R - Real-Time Proc LIGHT ADJUSTABLE LENS (LAL) AND LIGHT DELIVERY DEVICE (LDD) P170019/S009 08/21/2019 S - Special CBE FOUNDATIONONE CDX P170030/S003 P170036/S001 08/29/2019 08/02/2019 O - Normal 180 DayORSIRO SIROLIMUS ELUTING CORONARY STENT SYSTEM O - Normal 180 DayM6-C ARTIFICIAL CERVICAL DISC Appl/Spr Name ZOLL MEDICAL CORPORATIO N Approval Order Statement Approval for design changes to achieve compliance with EN 60601-1-2 (4th edition), PCB layout and parts changes, and minor mechanical changes to the speaker, printer, and display assemblies. BERLIN HEART INC. Approval for a change in the wiring system at the nurse call relay switch of the EXCOR IKUS driver. SENSEONICS, INCORPORAT ED THORATEC CORPORATIO N RXSIGHT, INC. FOUNDATION MEDICINE, INC. BIOTRONIK, INC Approval for a design change to the sensor component of the Eversense Continuous Glucose Monitoring System. Approval for a manufacturing site located at Sterigenics in Salt Lake City, Utah for ethylene oxide sterilization of the HeartMate 3 LVAS and HeartMate II LVAS components. Approval for the addition of a barcode scanner, internalization of the (optional) external LDD camera and update to the red reticle to enhance LAL visualization and alignment monitoring (called Align Assist), addition of an alternate supplier and model for the system computer and graphics card, and modification to LDD table legs to meet pinch point IEC 60601-1 testing requirements. Approval for changing the reporting of some microsatellite stable results to Microsatellite Status-Cannot be Determined and to add a disclaimer in your report where you report microsatellite status and in the limitations section of the report indicating Patients with Microsatellite Status-Cannot be Determined should be retested with an orthogonal (alternative) method. Approval for test protocols addressing the bench-top performance testing. SPINAL Approval of the protocol for the Post-Approval Study (PAS) protocol. KINETICS LLC Page 9 of 24 Data as of 09/04/2019 03:53 AM Submission Number P180002/S007 Date Final Decision 08/01/2019 Review Track Trade Name Y - 135 Review Tra ZEPHYR ENDOBRONCHIAL VALVE SYSTEM P180036/S002 08/13/2019 R - Real-Time Proc OPTIMIZER SMART SYSTEM Total: 69 30-Day Notice Appl/Spr Name PULMONX CORPORATIO N IMPULSE DYNAMICS (USA), INC. Approval Order Statement Approval to implement a revised process for the lot release testing sampling plan for Zephyr Endobronchial Valve System products. Approval for changes to the circuit components of the OPTIMIZER Smart IPG to improve manufacturability, robustness, and reliability. Submission Number N12159/S060 N12159/S061 Date Final Decision 08/02/2019 08/02/2019 Review Track Trade Name X - 30-Day Notice SURGICEL BRAND ABSORBABLE HEMOSTAT X - 30-Day Notice SURGICEL BRAND ABSORBABLE HEMOSTAT Appl/Spr Name ETHICON, INC. ETHICON, INC. N12159/S062 08/14/2019 N970012/S166 08/30/2019 P810032/S070 08/01/2019 P830055/S233 08/01/2019 X - 30-Day Notice X - 30-Day Notice X - 30-Day Notice SURGICEL BRAND ABSORBABLE HEMOSTAT AMS 700 INFLATABLE PENILE PROSTHESIS, AND AMS AMBICOR INFLATABLE PENILE PROSTHESIS MODELS B-13F (P-10) & B-1H (P-11) ETHICON, INC. BOSTON SCIENTIFIC CORP. ALCON LABORATORI ES X - 30-Day Notice LCS(R) TOTAL KNEE SYSTEM DEPUY, INC. P840001/S439 08/14/2019 X - 30-Day Notice ITREL(R) TOTALLY IMPLANTABLE SPINAL CORD STIM. SYS MEDTRONIC NEUROMODU LATION Approval Order Statement Installation of a duplicate dehumidifier unit for SURGICEL� Absorbable Hemostats manufactured at the Ethicon SARL, Neuchatel Switzerland site. Addition of a fourth pass of humidified intermediate fine fibers during the roller compactor/ sieving manufacturing process of SURGICEL Powder at the Ethicon, San Lorenzo, Puerto Rico facility. Process changes from manual to automated Foiling and Cartoning for SURGICEL Nu-Knit Absorbable Hemostat manufactured at the Ethicon SARL, Neuchatel Switzerland site. Change of the supplier for packaging trays for the Inflatable Penile Prosthesis and Artificial Urinary Sphincter devices. Addition of a 100% Ethylene Oxide (EO) sterilization chamber, integrated aeration cell, and supporting equipment at the Alcon Huntington manufacturing facility. Changes to the current processing of the ATTUNE Revision Tibial Sleeve Blanks at the DePuy vendor site (Tecomet [Symmetry Medical Manufacturing, Inc.]) in Lansing, Michigan. Use of newer test instrumentation for testing the electrolyte material and for updates to the test method used for the testing. Page 10 of 24 Data as of 09/04/2019 03:53 AM Submission Number P840001/S441 Date Final Decision 08/22/2019 P840060/S047 08/01/2019 P860004/S335 08/14/2019 P860004/S337 08/20/2019 P880087/S029 08/01/2019 P890017/S020 08/21/2019 P900033/S082 08/08/2019 P900033/S083 08/28/2019 P900033/S084 08/28/2019 P900033/S085 08/28/2019 Review Track Trade Name X - 30-Day Notice RESTORE, ITREL, SYNERGY AND INTELLIS SPINAL CORD STIMULATION SYSTEMS AND PISCES, SPECIFY, AND VECTRIS SPINAL CORD STIMULATION LEADS X - 30-Day Notice SM-1, CR-1, & GR-1 IOLS X - 30-Day Notice MEDTRONIC(R) SYNCHROMED(TM) PUMP & INFUSION SYSTEM X - 30-Day Notice MEDTRONIC(R) SYNCHROMED(TM) PUMP & INFUSION SYSTEM X - 30-Day Notice KELMAN MULTIFLEX 2 MODELS: MT3-MT7 & MT2U-MT7U X - 30-Day Notice PALMAZ BALLOON EXPANDABLE STENT X - 30-Day Notice INTEGRA DERMAL REGENERATION TEMPLATE X - 30-Day Notice INTEGRA DERMAL REGENERATION TEMPLATE AND INTEGRA MESHED DERMAL REGENERATION TEMPLATE X - 30-Day Notice INTEGRA DERMAL REGENERATION TEMPLATE, INTEGRA MESHED DERMAL REGENERATION TEMPLATE AND INTEGRA OMNIGRAFT DERMAL REGENERATION MATRIX X - 30-Day Notice INTEGRA DERMAL REGENERATION TEMPLATE, INTEGRA MESHED DERMAL REGENERATION TEMPLATE AND INTEGRA OMNIGRAFT DERMAL REGENERATION MATRIX Appl/Spr Name MEDTRONIC NEUROMODU LATION ALCON LABORATORI ES MEDTRONIC INC. MEDTRONIC INC. ALCON LABORATORI ES CORDIS CORP. INTEGRA LIFESCIENCE S CORP. INTEGRA LIFESCIENCE S CORP. INTEGRA LIFESCIENCE S CORP. INTEGRA LIFESCIENCE S CORP. Approval Order Statement Implementation of process controls for Soft Straight-Line Finish (SLF) cosmetic rework at Medtronic�s final device manufacturing facilities-Medtronic Puerto Rico Operations Company (MPROC), located in Juncos, Puerto Rico. Addition of a 100% Ethylene Oxide (EO) sterilization chamber, integrated aeration cell, and supporting equipment at the Alcon Huntington manufacturing facility. Use of newer test instrumentation for testing the electrolyte material and for updates to the test method used for the testing. Update the manufacturing process to plate the leads of the electrical components with lead/tin solder for the Medtronic SynchroMed II and Medtronic Implantable System for Remodulin. Addition of a 100% Ethylene Oxide (EO) sterilization chamber, integrated aeration cell, and supporting equipment at the Alcon Huntington manufacturing facility. Transfer Receiving Inspection and Final Release documentation review activities between internal sites. Reduce the sample size for Bacterial Endotoxin Testing for Integra products in conformance with ANSI/AAMI ST-072 standard. Change to the Water for Injection Pretreatment System for INTEGRA Dermal Regeneration Template and INTEGRA Meshed Dermal Regeneration Template. Implement new peristaltic pumps used in the manufacture of Integra Dermal Regeneration Template, Integra Meshed Dermal Regeneration Template and Integra Omnigraft Dermal Regeneration Matrix. Implement a change of designation of meshed collagen product as master product and alignment of MIDRT bioburden specification with other members in the same sterilization product family Page 11 of 24 Data as of 09/04/2019 03:53 AM Submission Number P910023/S417 P910056/S038 P910066/S030 P920047/S117 Date Final Decision 08/19/2019 08/06/2019 08/02/2019 08/23/2019 Review Track Trade Name X - 30-Day Notice CADENCE(R) TIERED THERAPY DEFIBRILLATION SYSTEM X - 30-Day Notice SOFLEX UV-ABSORBING SILICONE POSTERIOR CHAMBER INTRAOCULAR LENS X - 30-Day Notice ORTHOLOGIC (TM)1000 BONE GROWTH STIMULATOR X - 30-Day Notice P930014/S123 P930014/S125 P950005/S072 08/01/2019 08/19/2019 08/02/2019 X - 30-Day Notice ACRYSOF (R) UV ABSORBING INTRAOCULAR LENSES X - 30-Day Notice ACRYSOF (R) UV ABSORBING INTRAOCULAR LENSES X - 30-Day Notice CELSIUS CATHETER P960009/S354 P960009/S356 P960043/S106 P960058/S141 P960058/S142 P970004/S295 08/14/2019 08/22/2019 08/21/2019 08/23/2019 08/27/2019 08/14/2019 X - 30-Day Notice MEDTRONIC ACTIVA TREMOR CONTROL SYSTEM X - 30-Day Notice ACTIVA DEEP BRAIN STIMULATION THERAPY SYSTEM X - 30-Day Notice PROSTAR 9 FR. PERCUTANEOUS VASCULAR SURGICAL (PVS) SYSTEM X - 30-Day Notice CLARION MULTISTRATEGY COCHLEAR IMPLANT X - 30-Day Notice HIRESOLUTION BIONIC EAR SYSTEM X - 30-Day Notice MEDTRONIC INTERSTIM THERAPY SYSTEM FOR URINARY CONTROL Page 12 of 24 Appl/Spr Name ST. JUDE MEDICAL BAUSCH & LOMB, INC. DJO, LLC BOSTON SCIENTIFIC CORP. ALCON RESEARCH, LTD. ALCON RESEARCH, LTD. BIOSENSE WEBSTER, INC MEDTRONIC INC. MEDTRONIC INC. ABBOTT VASCULAR INC. ADVANCED BIONICS ADVANCED BIONICS MEDTRONIC NEUROMODU LATION Approval Order Statement Add an alternative solder paste dispensing method for hybrid circuit boards. Elimination of the In-Vitro Pyrogen Testing (IPT) at product release during the Finished Product Inspection of all models of the enVista Hydrophobic Acrylic lntraocular Lens (IOL). Proposing a process change which introduces an upgraded version of the Automated Calibration System (ACS) for the SpinaLogic, OL1000 Dual Coil, and OL1000 Single Coil (SC) (sizes 1, 2, 3, 4) Bone Growth Stimulator devices. Update Maestro 4000 Controller FW from version 5.14 to version 5.23 (market approved) in the field. Addition of a 100% Ethylene Oxide (EO) sterilization chamber, integrated aeration cell, and supporting equipment at the Alcon Huntington manufacturing facility. Use of coating solutions prepared at the manufacturing facility in West Virginia in the manufacture of UltraSert and AcrySert nozzle components. Alternate supplier for tip electrode components. Use of newer test instrumentation for testing the electrolyte material and for updates to the test method used for the testing. Implementation of process controls for Soft Straight-Line Finish (SLF) cosmetic rework at Medtronics final device manufacturing facilities-Medtronic Puerto Rico Operations Company (MPROC), located in Juncos, Puerto Rico. Alternate supplier for a packaging pouch. Alternative supplier for the Electrode Platinum Contact on the HiFocus Mid-Scala Electrode. Facility move for the testing of cochlear implant capacitors. Use of newer test instrumentation for testing the electrolyte material and for updates to the test method used for the testing. Data as of 09/04/2019 03:53 AM Submission Number P970004/S296 P970051/S189 P980003/S090 P980016/S716 P980016/S717 P980022/S208 Date Final Decision 08/22/2019 08/27/2019 08/23/2019 08/06/2019 08/30/2019 08/14/2019 Review Track Trade Name X - 30-Day Notice INTERSTIM THERAPY SYSTEM AND VERIFY EVALUATION SYSTEM (SNS URINARY) X - 30-Day Notice NUCLEUS 24 COCHLEAR IMPLANT SYSTEM X - 30-Day Notice MAESTRO 4000 CONTROLLER FIRMWARE Appl/Spr Name MEDTRONIC NEUROMODU LATION COCHLEAR AMERICAS BOSTON SCIENTIFIC CORP. X - 30-Day Notice VIRTUSO/ENTRUST/ MAXIMO/INTRINSIC/ MARQUIS/IMPLANTABLE CARDIVERTER DEFIBRILLATORS MEDTRONIC CARDIAC RHYTHM DISEASE MANAGEMEN T X - 30-Day Notice PROTECTA ICD, PROTECTA VR, MEDTRONIC XT ICD AND SECURA ICD, CARDIAC SECURA DR ICD RHYTHM DISEASE MANAGEMEN T X - 30-Day Notice CONTINUOUS GLUCOSE MONITORING SYSTEM MEDTRONIC MINIMED P980035/S602 P980035/S603 P980035/S604 08/06/2019 08/27/2019 08/30/2019 X - 30-Day Notice MEDTRONIC KAPPA 700/600 SERIES PULSE GENERATORS AND MODEL 9953 SOFTWARE MEDTRONIC INC. X - 30-Day Notice ADVISA DR IPG, ADVISA DR MRI MEDTRONIC IPG, ADVISA SR MRI IPG INC. X - 30-Day Notice ADVISA DR MRI AND ADVISA DR/DR MRI IPG MEDTRONIC INC. Approval Order Statement Implementation of process controls for Soft Straight-Line Finish (SLF) cosmetic rework at Medtronics final device manufacturing facilities-Medtronic Puerto Rico Operations Company (MPROC), located in Juncos, Puerto Rico. Addition of an automated punch and form tool for coil and hardball crimps. Update Maestro 4000 Controller FW from version 5.14 to version 5.23 (market approved) in the field. Implementation of process controls for Soft Straight-Line Finish (SLF) cosmetic work. Automated tester equipment conversion from LTX to SPEA. Automation of an inspection method at a contract manufacturer used during the packaging of components for the MiniLink REAL-Time transmitter, Guardian Link Transmitter, Guardian Link (3) Transmitter, Guardian Connect System, and iPro2 Recorder. The MiniLink REAL-Time transmitter is a component of the Minimed 530g System, Paradigm REAL-Time Revel System, and the Paradigm REAL-Time System. The Guardian Link Transmitter is a component of the Minimed 630G Insulin Pump System. The Guardian Link (3) Transmitter is a component of the MiniMed 670G System. The Guardian Link Transmitter is a component of the Medtronic MiniMed 630G Insulin Pump System. The Guardian Connect Transmitter is a component of the Guardian Connect System. The iPro2 Recorder is a component of the Ipro2 Continuous Glucose Monitoring System with Enlite Sensor. Implementation of process controls for Soft Straight-Line Finish (SLF) cosmetic work. Change the functional test sequence from the XYZ tester to the Titan Device Tester single axis test system. Automated tester equipment conversion from LTX to SPEA. Page 13 of 24 Data as of 09/04/2019 03:53 AM Submission Number P980040/S105 P980053/S017 P000006/S052 P000015/S039 P000029/S085 P000053/S106 P010001/S020 P010003/S036 P010014/S090 P010015/S416 P010015/S417 P010015/S418 P010019/S073 Date Final Decision 08/07/2019 08/29/2019 08/21/2019 08/27/2019 08/29/2019 08/30/2019 08/09/2019 08/14/2019 08/06/2019 08/06/2019 08/27/2019 08/30/2019 08/30/2019 Review Track X - 30-Day Notice X - 30-Day Notice X - 30-Day Notice X - 30-Day Notice X - 30-Day Notice X - 30-Day Notice X - 30-Day Notice X - 30-Day Notice X - 30-Day Notice X - 30-Day Notice X - 30-Day Notice X - 30-Day Notice X - 30-Day Notice Trade Name Appl/Spr Name SENSAR SOFT ACRYLIC UV-LIGHT ABSORBING POSTERIOR CHAMBER INTRAOCULAR LENS DURASPHERE INJECTABLE BULKING AGENT JOHNSON & JOHNSON SURGICAL VISION, INC. CARBON MEDICAL TECHNOLOGI ES, INC. TITAN INFLATABLE PENILE PROSTHESIS COLOPLAST CORP. Nucleus Auditory Brainstem Implant COCHLEAR System AMERICAS DEFLUX INJECTABLE GEL PALETTE LIFE SCIENCES AMS 800 ARTIFICIAL URINARY BOSTON SPHINCTER SCIENTIFIC CORP. CERAMIC TRANSCEND HIP ARTICULATION SYSTEM BIOGLUE SURGICAL ADHESIVE OXFORD(TM) MENISCAL UNICOMPARTMENTAL KNEE SYSTEM MEDTRONIC INSYNC(TM) BIVENTRICAL PACING SYSTEM CONSULTA CRT-P, SYNCRA CRT-P, VIVA CRT-P CONSULTA, SYNCA AND VIVA CRT-P LOTRAFILCON A AND B SOFT CONTACT LENSES CERAMTEC GMBH CRYOLIFE, INC. BIOMET MANUFACTUR ING CORP. MEDTRONIC INC. MEDTRONIC INC. MEDTRONIC INC. ALCON LABORATORI ES, INC. Approval Order Statement Alternate testing facility to evaluate bioburden for the TECNIS� 1-Piece IOL with TECNIS iTec Preloaded Delivery System Model PCB00 and TECNIS� iTec Preloaded Delivery System, Model PMB00. New vendor to supply the glucan powder for the subject device. Process change to the kink resistant tubing tests for the Titan Inflatable Penile Prosthesis. Addition of an automated punch and form tool for coil and hardball crimps. Replacement of a wall section with a door in the 3:125B clean room at the Q-Med manufacturing site. Change of the supplier for packaging trays for the Inflatable Penile Prosthesis and Artificial Urinary Sphincter devices. Addition of polishing machine used for polishing the intake and polishing the inner sphere of the ceramic inserts of the Transcend Hip Articulation System. Change to the in-process incoming inspection of pouched, irradiated BioGlue syringes. Site change for the Izod evaluation testing of the Ultra-High Molecular Weight Polyethylene (UHMWPE) tibial meniscal bearing components of the Oxford� Partial Knee System. Implementation of process controls for Soft Straight-Line Finish (SLF) cosmetic work. Change the functional test sequence from the XYZ tester to the Titan Device Tester single axis test system. Automated tester equipment conversion from LTX to SPEA. Qualification of a new X-Ray Photoelectron Spectrophotometer used for the measurement of lens plasma coating during in-process quality control inspection in the production of Alcon lotrafilcon A and lotrafilcon B soft contact lenses for daily and extended wear. Page 14 of 24 Data as of 09/04/2019 03:53 AM Submission Number P010030/S123 Date Final Decision 08/09/2019 P010031/S677 08/06/2019 P010031/S678 08/30/2019 P020025/S121 08/23/2019 P030031/S099 08/02/2019 P030052/S026 08/26/2019 P030054/S369 08/19/2019 P040002/S063 08/16/2019 P040014/S037 08/28/2019 P040020/S091 08/01/2019 P040036/S068 08/02/2019 Review Track Trade Name X - 30-Day Notice LIFEVEST WEARABLE DEFIBRILLATOR X - 30-Day Notice CONCERTO/INSYNC SENTRY/INSYNC MAXIMO IMPLANTABLE CARDIOVASCULAR DEFIBRILLATORS WITH CARDIAC RESYNCHICNIZATION X - 30-Day Notice CONSULTA, SYNCA AND VIVA CRT-P X - 30-Day Notice MAESTRO 4000 CONTROLLER FIRMWARE X - 30-Day Notice CELSIUS, EZ STEER, AND NAVISTAR THERMOCOOL CATHETER X - 30-Day Notice X - 30-Day Notice ST JUDE MEDICAL EPIC HF SYSTEM X - 30-Day Notice ENDOLOGIX POWERLINK SYSTEM X - 30-Day Notice THERAPY ABLATION , THERAPY 4MM THERMISTOR ABLATION CATHETER X - 30-Day Notice ACRYSOF RESTOR APODIZED DIFFRACTIVE OPTIC POSTERIOR CHAMBER IOL X - 30-Day Notice CELSIUS, EZ STEER, AND NAVISTAR THERMOCOOL CATHETER Appl/Spr Name ZOLL MANUFACTUR ING CORPORATIO N MEDTRONIC CARDIAC RHYTHM DISEASE MANAGEMEN T MEDTRONIC CARDIAC RHYTHM DISEASE MANAGEMEN T BOSTON SCIENTIFIC BIOSENSE WEBSTER, INC. ABBOTT MOLECULAR ST. JUDE MEDICAL ENDOLOGIX, INC. IRVINE BIOMEDICAL, INC. ALCON RESEARCH, LTD. BIOSENSE WEBSTER, INC. Approval Order Statement Automated test system for the LifeVest 4000 electrode belts and a minor test flow change. Implementation of process controls for Soft Straight-Line Finish (SLF) cosmetic work. Automated tester equipment conversion from LTX to SPEA. Update Maestro 4000 Controller FW from version 5.14 to version 5.23 (market approved) in the field. Alternate supplier for tip electrode components. Update to software. Add an alternative solder paste dispensing method for hybrid circuit boards. Modifications to the routine endotoxin (pyrogen) testing sampling plan for the AFX Endovascular AAA System. Change to the catheter shaft bonding process. Addition of a 100% Ethylene Oxide (EO) sterilization chamber, integrated aeration cell, and supporting equipment at the Alcon Huntington manufacturing facility. Alternate supplier for tip electrode components. Page 15 of 24 Data as of 09/04/2019 03:53 AM Submission Number P040042/S043 Date Final Decision 08/28/2019 P050018/S027 08/22/2019 P050042/S039 08/13/2019 P050047/S072 08/27/2019 P060037/S061 08/07/2019 P080004/S024 08/21/2019 P080011/S095 08/09/2019 P080012/S059 08/07/2019 P080025/S190 08/14/2019 P080025/S191 08/22/2019 P100014/S022 08/29/2019 P100026/S073 08/08/2019 Review Track Trade Name X - 30-Day Notice THERAPY ABLATION , THERAPY 4MM THERMISTOR ABLATION CATHETER Appl/Spr Name IRVINE BIOMEDICAL,I NC.(IBI) X - 30-Day Notice ANGIOSCULPT PTCA SCORING SPECTRANETI BALLOON CATHETER CS CORP. X - 30-Day Notice ARCHITECT ANTI-HCV ASSAY; ARCHITECT ANTIHCV CALIBRATOR; ARCHITECT ANTI-HCV CONTROL ABBOTT LABORATORI ES INC X - 30-Day Notice JUVEDERM INJECTABLE GEL ALLERGAN IMPLANTS (ULTRA, ULTRA XC AND ULTRA PLUS XC) X - 30-Day Notice NEXGEN LPS-FLEX MOBILE AND LPS-MOBILE BEARING KNEE SYSTEM ZIMMER, INC. X - 30-Day Notice HOYA ISPHERIC MODEL YA-60BB INTRAOCULAR LENS HOYA SURGICAL OPTICS, INC. X - 30-Day Notice BIOFINITY (COMFILCON A) COOPERVISIO N MANUFACTUR ING, LTD. X - 30-Day Notice PROMETRA PROGRAMMABLE INFUSION PUMP SYSTEM FLOWONIX MEDICAL, INC. X - 30-Day Notice X - 30-Day Notice X - 30-Day Notice X - 30-Day Notice MEDTRONIC INTERSTIM SACRAL NERVE STIMULATION THERAPY SYSTEM INTERSTIM THERAPY SYSTEM AND VERIFY EVALUATION SYSTEM (SNS BOWEL) DEFLUX INJECTABLE GEL NEUROPACE RNS SYSTEM MEDTRONIC NEUROMODU LATION MEDTRONIC NEUROMODU LATION PALETTE LIFE SCIENCES NEUROPACE INC Page 16 of 24 Approval Order Statement Change to the catheter shaft bonding process. Change in the final device labeling software. Manufacturing location change for a supplier of a critical raw material. Implement a new incoming warehouse for the storage of raw materials and packaging items for Juv�derm injectable gel implants in the Pringy II building. Removal of the cytotoxicity test requirement from the routine process monitoring of the final cleaning process for the NexGen� Complete Knee Solution, Legacy� Knee � Posterior Stabilized (LPS) and the LPS-Flex Mobile Bearing Knee femoral components, at the Shannon, Ireland facility. Modifications to the 1-piece IOL haptic inspection method and acceptance criteria. Software update to the wet Automated Inspection System (AIS) used for Biofinity (comfilcon A) soft (hydrophilic) extended wear contact lenses manufactured at the CooperVision Manufacturing, Ltd. facility in Hamble, United Kingdom. Alternate supplier for the bellows component of the Prometra II Programmable Infusion Pump System. Use of newer test instrumentation for testing the electrolyte material and for updates to the test method used for the testing. Implementation of process controls for Soft Straight-Line Finish (SLF) cosmetic rework at Medtronics final device manufacturing facilities-Medtronic Puerto Rico Operations Company (MPROC), located in Juncos, Puerto Rico. Replacement of a wall section with a door in the 3:125B clean room at the Q-Med manufacturing site. Modify the PXI Vader automated test equipment (ATE) software to improve yield of the components used to manufacture the RNS� Neurostimulator (model RNS320) during the custom packaged integrated circuit (Cassandra) and printed circuit assembly (PCA) testing. In addition, this submission includes an update to PXI Vader ATE software for final electrical test to support future product changes currently under development. Data as of 09/04/2019 03:53 AM Submission Number P100026/S074 Date Final Decision 08/26/2019 Review Track Trade Name X - 30-Day Notice NEUROPAC RNS SYSTEM Appl/Spr Name NEUROPAC E INC P100042/S025 08/27/2019 X - 30-Day Notice APTIMA HPV ASSAY P100047/S143 08/23/2019 X - 30-Day Notice HEARTWARE VENTRICULAR ASSIST SYSTEM GENPROBE INCORPOR AT ED MEDTRONI C Approval Order Statement Addition of an alternate laser welder, the IPG Laser Weld System, to perform laser welding on the RNS Neurostimulator during manufacturing operations at NeuroPace (455 N. Bernardo Ave. Mountain, California). Remove or modify QC testing for reagent labels. Component change to the battery pack assembly. P110010/S168 P110012/S019 P110013/S099 P110033/S048 P120007/S023 P120010/S132 08/20/2019 08/26/2019 08/12/2019 08/27/2019 08/27/2019 08/14/2019 X - 30-Day Notice PROMUS PREMIER EVEROLIMUS-ELUTING PLATINUM CHROMIUM CORONARY STENT SYSTEM BOSTON SCIENTIFIC CORP. MONORAIL (MR) AND OVER- THE-WIRE (OTW) AND PROMUS ELITE EVEROLIMUS-ELUTING PLATINUM CHROMIUM CORONARY STENT SYSTEM (MONORAIL) X - 30-Day Notice VYSIS ALK BREAK APART FISH ABBOTT PROBE KIT MOLECULAR, INC. X - 30-Day Notice RESOLUTE INTEGRITY MEDTRONIC CORONARY STENT SYSTEMS VASCULAR X - 30-Day Notice JUVEDERM INJECTABLE GEL IMPLANTS (ULTRA, ULTRA XC AND ULTRA PLUS XC) X - 30-Day Notice APTIMA HPV ASSAY ALLERGAN GEN-PROBE INCORPORAT ED X - 30-Day Notice MINIMED 530G SYSTEM MEDTRONIC INC. Addition of extrusion lines for manufacturing distal outer tubes. Update of software. Implementation of a new dishwasher and water purification system in the shared glassware cleaning area within the Medtronic Ireland manufacturing facility. Implement a new incoming warehouse for the storage of raw materials and packaging items for Juv�derm injectable gel implants in the Pringy II building. Remove or modify QC testing for reagent labels. Automation of an inspection method at a contract manufacturer used during the packaging of components for the MiniLink REAL-Time transmitter, Guardian Link Transmitter, Guardian Link (3) Transmitter, Guardian Connect System, and iPro2 Recorder. The MiniLink REAL-Time transmitter is a component of the Minimed 530g System, Paradigm REAL-Time Revel System, and the Paradigm REAL-Time System. The Guardian Link Transmitter is a component of the Minimed 630G Insulin Pump System. The Guardian Link (3) Transmitter is a component of the MiniMed 670G System. The Guardian Link Transmitter is a component of the Medtronic MiniMed 630G Insulin Pump System. The Guardian Connect Transmitter is a component of the Guardian Connect System. The iPro2 Recorder is a component of the Ipro2 Continuous Glucose Monitoring System with Enlite Sensor. Page 17 of 24 Data as of 09/04/2019 03:53 AM Submission Number P120010/S133 Date Final Decision 08/26/2019 P120014/S009 08/26/2019 P130001/S005 08/30/2019 P130021/S061 08/19/2019 P130021/S062 08/23/2019 P130028/S026 08/09/2019 P130028/S027 08/28/2019 P140018/S016 08/12/2019 P140030/S010 08/14/2019 P140032/S037 08/14/2019 P140032/S038 08/20/2019 P140032/S039 08/27/2019 P140033/S046 08/30/2019 Review Track Trade Name X - 30-Day Notice MINIMED 530G SYSTEM Appl/Spr Name MEDTRONIC INC. X - 30-Day Notice THXID BRAF ASSAY KIT X - 30-Day Notice EPI PROCOLON X - 30-Day Notice COREVALVE EVOLUT R SYSTEM AND COREVALVE EVOLUT PRO SYSTEM X - 30-Day Notice COREVALVE EVOLUT R SYSTEM AND COREVALVE EVOLUT PRO SYSTEM X - 30-Day Notice ALGOVITA SPINAL CORD STIMULATION SYSTEM X - 30-Day Notice ALGOVITA SPINAL CORD STIMULATION SYSTEM X - 30-Day Notice RESOLUTE INTEGRITY CORONARY STENT SYSTEMS X - 30-Day Notice ASTRON PERIPHERAL SELF-EXPANDING NITINOL STENT SYSTEM X - 30-Day Notice IMPLANTABLE SYSTEM FOR REMODULIN X - 30-Day Notice IMPLANTABLE SYSTEM FOR REMODULIN BIOMERIEUX, INC. EPIGENOMIC S AG MEDTRONIC COREVALVE LLC MEDTRONIC COREVALVE LLC NUVECTRA CORPORATIO N NUVECTRA CORPORATIO N MEDTRONIC VASCULAR INC BIOTRONIK, INC. MEDTRONIC, INC. MEDTRONIC, INC. X - 30-Day Notice IMPLANTABLE SYSTEM FOR REMODULIN X - 30-Day Notice TENDRIL MRI STEROID ELUTING CARDIAC LEADS MEDTRONIC, INC. ST. JUDE MEDICAL, INC. Approval Order Statement Addition of new sterilization equipment at two previously approved sterilization facilities for the sterilization of the Enlite and Guardian Sensor (3) continuous glucose monitoring sensors. The Enlite sensor is a component of the MiniMed 530G system, the MiniMed 630G system, the Paradigm Real-Time Revel system, and the iPro2 with Enlite sensor system. The Guardian Sensor (3) is a component of the MiniMed 630G system, the Guardian Connect system, and the MiniMed 670G system. Manufacturing site change for a raw material (critical component). Change of storage and distribution facility. Modification to the nitinol capsule frame used in the 18F and 20F delivery systems. Reduce the number of units required to be tested for routine Bacterial Endotoxin Testing (BET) for the EnVeo R and EnVeo PRO Delivery Catheter System and Loading System. Addition of alternative suppliers for the housing subcomponent and the spring/coil contact block subcomponent of the Algovita� Internal Pulse Generator (IPG). Implementation of an update to the injection molding process for the distal subassembly of the Algovita� 12-Electrode Percutaneous Leads, Trial Leads, and Lead Extensions. Implementation of a new dishwasher and water purification system in the shared glassware cleaning area within the Medtronic Ireland manufacturing facility. Automation of a stent expansion process and an ultrasonic cleaning process. Use of newer test instrumentation for testing the electrolyte material and for updates to the test method used for the testing. Update the manufacturing process to plate the leads of the electrical components with lead/tin solder for the Medtronic SynchroMed II and Medtronic Implantable System for Remodulin. Facility improvements with the goal of achieving the ISO 14644-1 Class 8 Cleanroom certification. Add an additional packaging integrity testing laboratory site. Page 18 of 24 Data as of 09/04/2019 03:53 AM Submission Number P150001/S072 Date Final Decision 08/14/2019 Review Track Trade Name X - 30-Day Notice MINIMED 630G SYSTEM WITH SMARTGUARD(TM) Appl/Spr Name MEDTRONIC MINIMED P150001/S073 08/26/2019 X - 30-Day Notice MINIMED 530G SYSTEM MEDTRONIC MINIMED P150002/S006 P150003/S051 P150003/S052 P150006/S004 08/15/2019 08/09/2019 08/20/2019 08/26/2019 X - 30-Day Notice INCRAFT(R) AAA STENT GRAFT SYSTEM CORDIS CORPORATIO N X - 30-Day Notice SYNERGY EVEROLIMUSELUTING PLATINUM CHROMIUM CORONARY STENT SYSTEM BOSTON SCIENTIFIC CORPORATIO N X - 30-Day Notice PROMUS PREMIER EVEROLIMUS-ELUTING PLATINUM CHROMIUM CORONARY STENT SYSTEM MONORAIL (MR) AND OVER- BOSTON SCIENTIFIC CORPORATIO N THE-WIRE (OTW) AND PROMUS ELITE EVEROLIMUS-ELUTING PLATINUM CHROMIUM CORONARY STENT SYSTEM (MONORAIL) X - 30-Day Notice VASORUM LTD Approval Order Statement Automation of an inspection method at a contract manufacturer used during the packaging of components for the MiniLink REAL-Time transmitter, Guardian Link Transmitter, Guardian Link (3) Transmitter, Guardian Connect System, and iPro2 Recorder. The MiniLink REAL-Time transmitter is a component of the Minimed 530g System, Paradigm REAL-Time Revel System, and the Paradigm REAL-Time System. The Guardian Link Transmitter is a component of the Minimed 630G Insulin Pump System. The Guardian Link (3) Transmitter is a component of the MiniMed 670G System. The Guardian Link Transmitter is a component of the Medtronic MiniMed 630G Insulin Pump System. The Guardian Connect Transmitter is a component of the Guardian Connect System. The iPro2 Recorder is a component of the Ipro2 Continuous Glucose Monitoring System with Enlite Sensor. Addition of new sterilization equipment at two previously approved sterilization facilities for the sterilization of the Enlite and Guardian Sensor (3) continuous glucose monitoring sensors. The Enlite sensor is a component of the MiniMed 530G system, the MiniMed 630G system, the Paradigm Real-Time Revel system, and the iPro2 with Enlite sensor system. The Guardian Sensor (3) is a component of the MiniMed 630G system, the Guardian Connect system, and the MiniMed 670G system. Relocate the stent manufacturing line to another location within the same building. Alternate process to automate top assembly process steps for the manufacture of stent delivery catheters. Addition of extrusion lines for manufacturing distal outer tubes. Expanded e-beam sterilization dose range. Page 19 of 24 Data as of 09/04/2019 03:53 AM Submission Number P150019/S057 Date Final Decision 08/14/2019 Review Track Trade Name X - 30-Day Notice PARADIGM REAL-TIME REVEL SYSTEM Appl/Spr Name MEDTRONIC MINIMED P150019/S058 08/26/2019 X - 30-Day Notice PARADIGM REAL-TIME REVEL MEDTRONIC SYSTEM MINIMED P150021/S043 P150021/S044 P150029/S030 08/13/2019 08/15/2019 08/14/2019 X - 30-Day Notice FREESTYLE LIBRE PRO FLASH GLUCOSE MONITORING SYSTEM X - 30-Day Notice FREESTYLE LIBRE PRO FLASH GLUCOSE MONITORING SYSTEM X - 30-Day Notice IPRO2 CGM SYSTEM WITH ENLITE SENSOR ABBOTT DIABETES CARE INC. ABBOTT DIABETES CARE INC. MEDTRONIC MINIMED P150029/S031 08/26/2019 X - 30-Day Notice MINIMED 530G SYSTEM MEDTRONIC MINIMED Approval Order Statement Automation of an inspection method at a contract manufacturer used during the packaging of components for the MiniLink REAL-Time transmitter, Guardian Link Transmitter, Guardian Link (3) Transmitter, Guardian Connect System, and iPro2 Recorder. The MiniLink REAL-Time transmitter is a component of the Minimed 530g System, Paradigm REAL-Time Revel System, and the Paradigm REAL-Time System. The Guardian Link Transmitter is a component of the Minimed 630G Insulin Pump System. The Guardian Link (3) Transmitter is a component of the MiniMed 670G System. The Guardian Link Transmitter is a component of the Medtronic MiniMed 630G Insulin Pump System. The Guardian Connect Transmitter is a component of the Guardian Connect System. The iPro2 Recorder is a component of the Ipro2 Continuous Glucose Monitoring System with Enlite Sensor. Addition of new sterilization equipment at two previously approved sterilization facilities for the sterilization of the Enlite and Guardian Sensor (3) continuous glucose monitoring sensors. The Enlite sensor is a component of the MiniMed 530G system, the MiniMed 630G system, the Paradigm Real-Time Revel system, and the iPro2 with Enlite sensor system. The Guardian Sensor (3) is a component of the MiniMed 630G system, the Guardian Connect system, and the MiniMed 670G system. Addition of manufacturing space at Abbott Diabetes Care for the sensor component manufacturing process. The sensor is a component of the FreeStyle Libre Pro Flash Glucose Monitoring System and Freestyle Libre Flash Glucose Monitoring System. Change to introduce an alternate supplier of a sensor component for the Libre sensor. The Libre sensor is a component of the Freestyle Libre Pro Flash Glucose Monitoring System and the Freestyle Libre 14 day Flash Glucose Monitoring System. Automation of an inspection method at a contract manufacturer used during the packaging of components for the MiniLink REAL-Time transmitter, Guardian Link Transmitter, Guardian Link (3) Transmitter, Guardian Connect System, and iPro2 Recorder. The MiniLink REAL-Time transmitter is a component of the Minimed 530g System, Paradigm REAL-Time Revel System, and the Paradigm REAL-Time System. The Guardian Link Transmitter is a component of the Minimed 630G Insulin Pump System. The Guardian Link (3) Transmitter is a component of the MiniMed 670G System. The Guardian Link Transmitter is a component of the Medtronic MiniMed 630G Insulin Pump System. The Guardian Connect Transmitter is a component of the Guardian Connect System. The iPro2 Recorder is a component of the Ipro2 Continuous Glucose Monitoring System with Enlite Sensor. Addition of new sterilization equipment at two previously approved sterilization facilities for the sterilization of the Enlite and Guardian Sensor (3) continuous glucose monitoring sensors. The Enlite sensor is a component of the MiniMed 530G system, the MiniMed 630G system, the Paradigm Real-Time Revel system, and the iPro2 with Enlite sensor system. The Guardian Sensor (3) is a component of the MiniMed 630G system, the Guardian Connect system, and the MiniMed 670G system. Page 20 of 24 Data as of 09/04/2019 03:53 AM Submission Number P160007/S024 Date Final Decision 08/14/2019 Review Track Trade Name X - 30-Day Notice GUARDIAN CONNECT SYSTEM P160007/S025 08/26/2019 X - 30-Day Notice MINIMED 530G SYSTEM P160017/S069 08/14/2019 X - 30-Day Notice MINIMED 670G SYSTEM P160017/S070 08/26/2019 X - 30-Day Notice MINIMED 530G SYSTEM Appl/Spr Name MEDTRONIC MINIMED MEDTRONIC MINIMED MEDTRONIC MINIMED, INC. MEDTRONIC MINIMED, INC. Approval Order Statement Automation of an inspection method at a contract manufacturer used during the packaging of components for the MiniLink REAL-Time transmitter, Guardian Link Transmitter, Guardian Link (3) Transmitter, Guardian Connect System, and iPro2 Recorder. The MiniLink REAL-Time transmitter is a component of the Minimed 530g System, Paradigm REAL-Time Revel System, and the Paradigm REAL-Time System. The Guardian Link Transmitter is a component of the Minimed 630G Insulin Pump System. The Guardian Link (3) Transmitter is a component of the MiniMed 670G System. The Guardian Link Transmitter is a component of the Medtronic MiniMed 630G Insulin Pump System. The Guardian Connect Transmitter is a component of the Guardian Connect System. The iPro2 Recorder is a component of the Ipro2 Continuous Glucose Monitoring System with Enlite Sensor. Addition of new sterilization equipment at two previously approved sterilization facilities for the sterilization of the Enlite and Guardian Sensor (3) continuous glucose monitoring sensors. The Enlite sensor is a component of the MiniMed 530G system, the MiniMed 630G system, the Paradigm Real-Time Revel system, and the iPro2 with Enlite sensor system. The Guardian Sensor (3) is a component of the MiniMed 630G system, the Guardian Connect system, and the MiniMed 670G system. Automation of an inspection method at a contract manufacturer used during the packaging of components for the MiniLink REAL-Time transmitter, Guardian Link Transmitter, Guardian Link (3) Transmitter, Guardian Connect System, and iPro2 Recorder. The MiniLink REAL-Time transmitter is a component of the Minimed 530g System, Paradigm REAL-Time Revel System, and the Paradigm REAL-Time System. The Guardian Link Transmitter is a component of the Minimed 630G Insulin Pump System. The Guardian Link (3) Transmitter is a component of the MiniMed 670G System. The Guardian Link Transmitter is a component of the Medtronic MiniMed 630G Insulin Pump System. The Guardian Connect Transmitter is a component of the Guardian Connect System. The iPro2 Recorder is a component of the Ipro2 Continuous Glucose Monitoring System with Enlite Sensor. Addition of new sterilization equipment at two previously approved sterilization facilities for the sterilization of the Enlite and Guardian Sensor (3) continuous glucose monitoring sensors. The Enlite sensor is a component of the MiniMed 530G system, the MiniMed 630G system, the Paradigm Real-Time Revel system, and the iPro2 with Enlite sensor system. The Guardian Sensor (3) is a component of the MiniMed 630G system, the Guardian Connect system, and the MiniMed 670G system. Page 21 of 24 Data as of 09/04/2019 03:53 AM Submission Number P160022/S012 P160025/S008 P160030/S036 P160030/S037 P160033/S004 P160034/S001 P160038/S013 P160043/S026 P160045/S016 Date Final Decision 08/22/2019 08/14/2019 08/13/2019 08/15/2019 08/20/2019 08/20/2019 08/08/2019 08/12/2019 08/19/2019 Review Track Trade Name Appl/Spr Name X - 30-Day Notice X SERIES�, R SERIES�, AED PRO�, AED 3� BLS PROFESSIONAL DEFIBRILLATORS, PROPADZ RADIOTRANSPARENT ELECTRODE, SUREPOWER BATTERY PACK, SUREPOWER II BATTERY PACK, AED PRO� NONRECHARGEABLE LITHIUM BATTERY PACK, AED 3 BATTERY PACK, SUREPOWER� CHARGER, AND SUREPOWER SINGLE BAY CHARGER ZOLL MEDICAL CORPORATIO N X - 30-Day Notice ASTRON PULSAR STENT SYSTEM, PULSAR-18 STENT SYSTEM BIOTRONIK, INC. X - 30-Day Notice FREESTYLE LIBRE FLASH GLUCOSE MONITORING SYSTEM ABBOTT DIABETES CARE INC. X - 30-Day Notice FREESTYLE LIBRE FLASH GLUCOSE MONITORING SYSTEM ABBOTT DIABETES CARE INC. X - 30-Day Notice POWERHEART� G3, G3 PLUS AND G5 AEDS CARDIAC SCIENCE CORPORATIO N X - 30-Day Notice POWERHEART� G3, G3 PLUS AND G5 AEDS CARDIAC SCIENCE CORPORATIO N X - 30-Day Notice PRAXIS EXTENDED RAS PANEL ILLUMINA, INC. X - 30-Day Notice RESOLUTE INTEGRITY MEDTRONIC CORONARY STENT SYSTEMS VASCULAR X - 30-Day Notice ONCOMINE DX TARGET TEST LIFE TECHNOLOGI ES CORPORATIO N Page 22 of 24 Approval Order Statement Addition of a conformal coating and hipot test to the analog board, and updates to the system level testing. Automation of a stent expansion process and an ultrasonic cleaning process. Addition of manufacturing space at Abbott Diabetes Care for the sensor component manufacturing process. The sensor is a component of the FreeStyle Libre Pro Flash Glucose Monitoring System and Freestyle Libre Flash Glucose Monitoring System. Change to introduce an alternate supplier of a sensor component for the Libre sensor. The Libre sensor is a component of the Freestyle Libre Pro Flash Glucose Monitoring System and the Freestyle Libre 14 day Flash Glucose Monitoring System. Update to the Smart Packing Software to automate a verification step. Update to the Smart Packing Software to automate a verification step. Changing the source of two raw materials. Implementation of a new dishwasher and water purification system in the shared glassware cleaning area within the Medtronic Ireland manufacturing facility. Relocation of a formulation room within your manufacturing facility. Data as of 09/04/2019 03:53 AM Submission Number P160048/S013 Date Final Decision 08/29/2019 Review Track Trade Name X - 30-Day Notice EVERSENSE CONTINUOUS GLUCOSE MONITORING SYSTEM Appl/Spr Name SENSEONICS, INCORPORAT ED Approval Order Statement New manufacturing site for the electronics assembly and encasement process for the sensor component of the Eversense continuous glucose monitoring system. P160054/S020 08/25/2019 P170008/S020 08/16/2019 P170035/S004 08/01/2019 P180025/S005 08/06/2019 P180029/S009 08/15/2019 P180029/S010 08/21/2019 Total: 129 X - 30-Day Notice HEARTMATE 3TM LEFT THORATEC VENTRICULAR ASSIST SYSTEM CORPORATIO N X - 30-Day Notice ELUNIR RIDAFOROLIMUS ELUTING CORONARY STENT SYSTEM MEDINOL, LTD. X - 30-Day Notice BAUSCH + LOMB ULTRA (SAMFILCON A) CONTACT LENSES BAUSCH AND LOMB, INC. X - 30-Day Notice MANTA VASCULAR CLOSURE DEVICE X - 30-Day Notice LOTUS EDGE VALVE SYSTEM X - 30-Day Notice LOTUS EDGE VALVE SYSTEM ESSENTIAL MEDICAL, INC. BOSTON SCIENTIFIC CORPORATIO N BOSTON SCIENTIFIC CORPORATIO N Add an alternate supplier for the Spline Pump Cover component. Modification of the supporting tools used for a Quality Control Leak test procedure for the EluNIR Ridaforolimus Eluting Coronary Stent System. Changing the in-process acceptance criteria for a component of the Ultra (samfilcon A) Visibility Tinted Contact Lens. Reduce the number of in-process inspections for pouch seal strength testing. Automation of a manual dimensional inspection. Change to bonding equipment used to bond the MLE tube to the distal end cap of the delivery system. Page 23 of 24 Data as of 09/04/2019 03:53 AM