Andon Health BP5 Spread Spectrum Transmitter User Manual 140100 0614 GB
Andon Health Co., Ltd Spread Spectrum Transmitter 140100 0614 GB
Manual
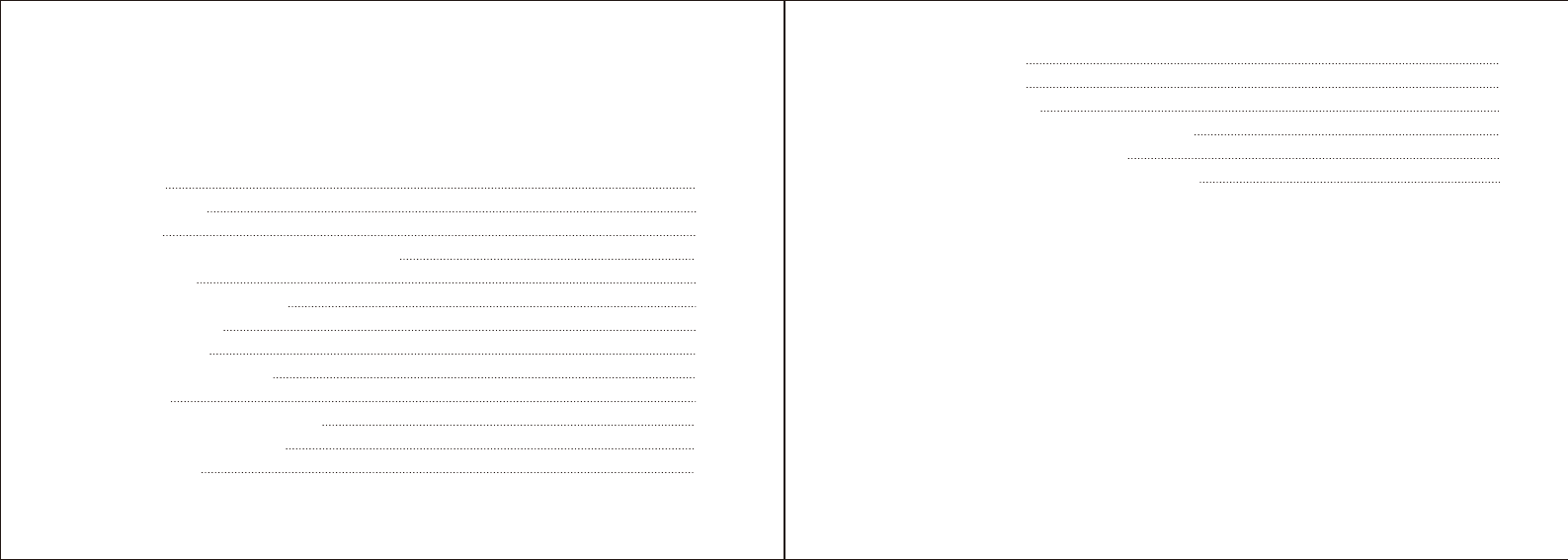
Wireless Blood Pressure Monitor (BP5)
OWNER’S MANUAL
iHealthTM
Table of Contents
INTRODUCTION
PACKAGE CONTENTS
INTENDED USE
BLOOD PRESSURE CLASSIFICATION FOR ADULTS
CONTRAINDICATION
PARTS AND DISPLAY INDICATORS
SET UP REQUIREMENTS
SET UP PROCEDURES
MEASUREMENT PROCEDURES
SPECIFICATIONS
GENERAL SAFETY AND PRECAUTIONS
BATTERY HANDLING AND USAGE
TROUBLESHOOTING
1
1
1
1
2
2
2
3
4
6
7
8
9
CARE AND MAINTENANCE
WARRANTY INFORMATION
EXPLANATION OF SYMBOLS
IMPORTANT INFORMATION REQUIRED BY THE FCC
OTHER STANDARDS AND COMPLIANCES
ELECTROMAGNETIC COMPATIBILITY INFORMATION
10
11
11
12
13
14
INTRODUCTION
Thank you for selecting the iHealth Wireless Blood Pressure Monitor. The iHealth Wireless Blood
Pressure Monitor is a fully automatic arm cuff blood pressure monitor that uses the oscillometric
principle to measure your blood pressure and pulse rate. The monitor works
with your iOS devices to test, track and share vital blood pressure data.
• 1 iHealth Wireless Blood Pressure Monitor
• 1 Owner’s Manual
• 1 Quick Start Guide
• 1 Charging Cable
• 1 Travel Bag
PACKAGE CONTENTS
INTENDED USE
It is not recommended for people with serious arrhythmia to use this Wireless Blood Pressure
Monitor.
Note: This chart is not intended to provide a basis for any type of emergency condition or
diagnosis based on the color scheme; this chart only depicts different classifications of blood
pressure. Consult your physician for proper interpretation of blood pressure results.
CONTRAINDICATION
The iHealth Wireless Blood Pressure Monitor (Electronic Sphygmomanometer) is intended for use
in a professional setting or at home and is a non-invasive blood pressure measurement system. It
is designed to measure the systolic and diastolic blood pressures and pulse rate of an adult
individual by using a technique in which an inflatable cuff is wrapped around the upper arm. The
measurement range of the cuff circumference is 8.6” to18.9”(22cm-48cm).
The World Health Organization (WHO) has created the following guide for assessing high blood
pressure (without regard to age or gender). Please note that other factors (e.g. diabetes, obesity,
smoking, etc.) also need to be considered. Consult with your physician for accurate assessment.
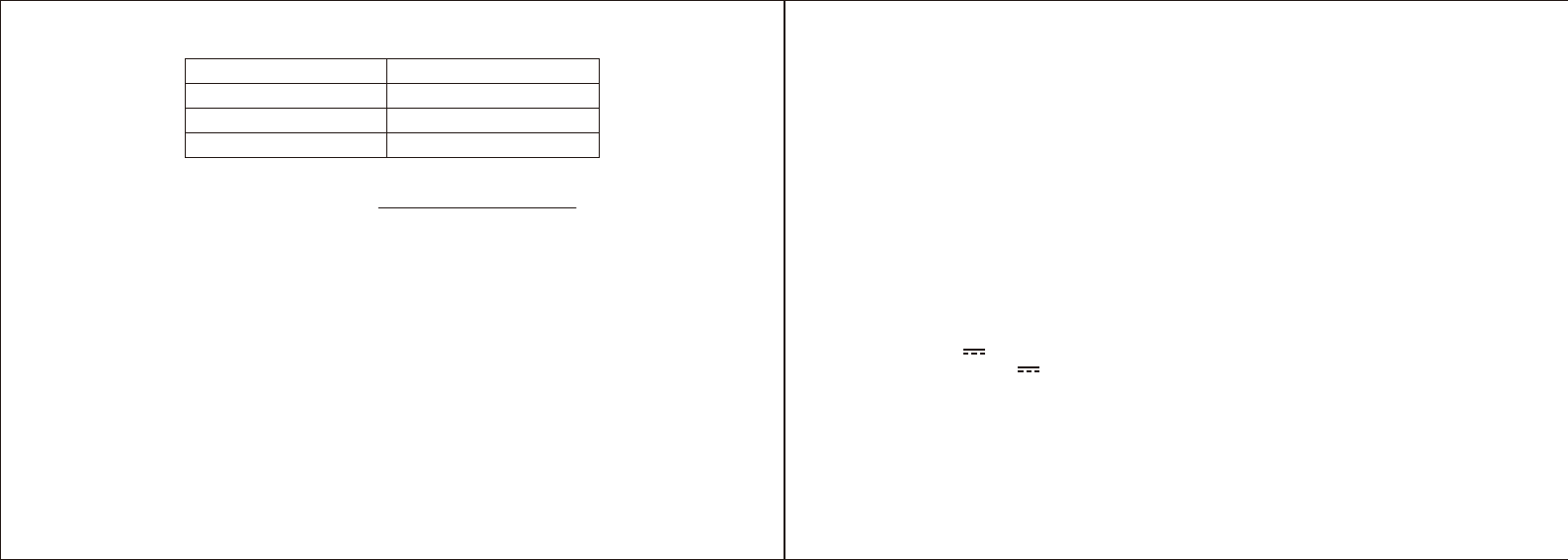
BLOOD PRESSURE CLASSIFICATION FOR ADULTS
160
180
Systolic
(mmHg)
Diastolic
(mmHg)
140
130
120
80 85 90 100 110
Severe Hypertension
Moderate Hypertension
Mild Hypertension
High-normal BP
Normal BP
Classification of blood pressure for adults
BLOOD PRESSURE
CLASSIFICATION
SBP
mmHg
DBP
mmHg
COLOR
INDICATOR
Optimal <120 <80 GREEN
Normal 120-129 80-84 GREEN
High-normal 130-139 85-89
Grade 1 Hypertension 140-159 90-99
GREEN
Grade 2 Hypertension 160-179 100-109
YELLOW
Grade 3 Hypertension RED
ORANGE
≥180 ≥100
WHO/ISH Definitions and Classification of Blood Pressure Levels
1 2
!
The iHealth Wireless Blood Pressure Monitor is designed to be used with the following iPod touch,
iPhone and iPad models:
iPod touch (4th generation)
iPod touch (3rd generation)
iPhone 4S
iPhone 4
iPhone 3GS
iPad (3rd generation)
iPad 2
iPad
The iOS version of these devices should be V4.0 or higher.
SET UP REQUIREMENTS
PARTS AND DISPLAY INDICATORS
3 4
Bluetooth indicator Status indicator
START/STOP button
Cuff
SET UP PROCEDURES
Note: Please repeat step 1 when you switch an iOS device.
Download the Free iHealth APP
Prior to first use, download and install the iHealth App from the App Store. Use
keyword search terms “iHealth”, “BP5” or ”BPM”.
• Apply the cuff or press the START/STOP button,
the Bluetooth Indicator Light will begin flashing.
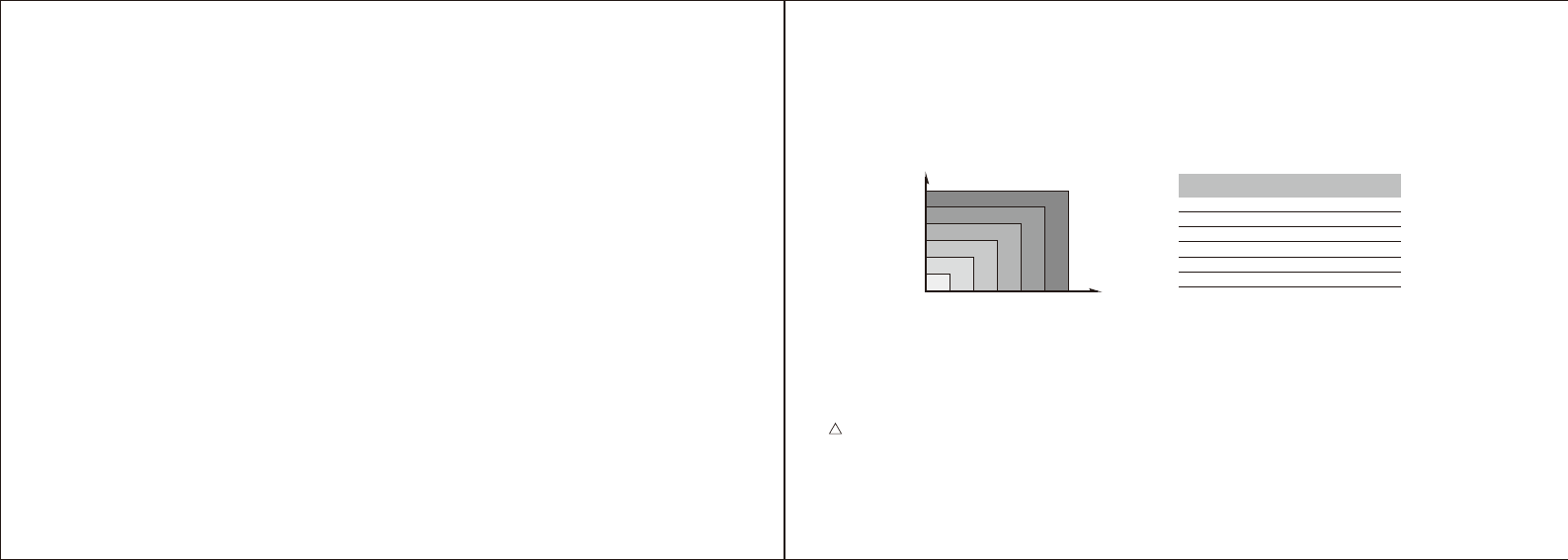
• Turn Bluetooth “On” under the “Settings” Menu
(Settings->General->Bluetooth->On)
• Wait until the model name printed on the monitor,
(i.e. "BP5 xxxxxx") and "Not Paired" appear in the
Bluetooth Menu, and select the model name “BP5
xxxxxx” to pair and connect. The Bluetooth
Indicator Light will remain steady upon successful
connection. When using the monitor for the first
time, it may take up to 30 seconds for your iOS
device to detect the Bluetooth signal.
Connect to iOS Device Via Bluetooth
• Each subsequent time you use the monitor "Not Connected" will be displayed next to “BP5
xxxxxx” in the Bluetooth Menu. Tap to re-establish the connection.
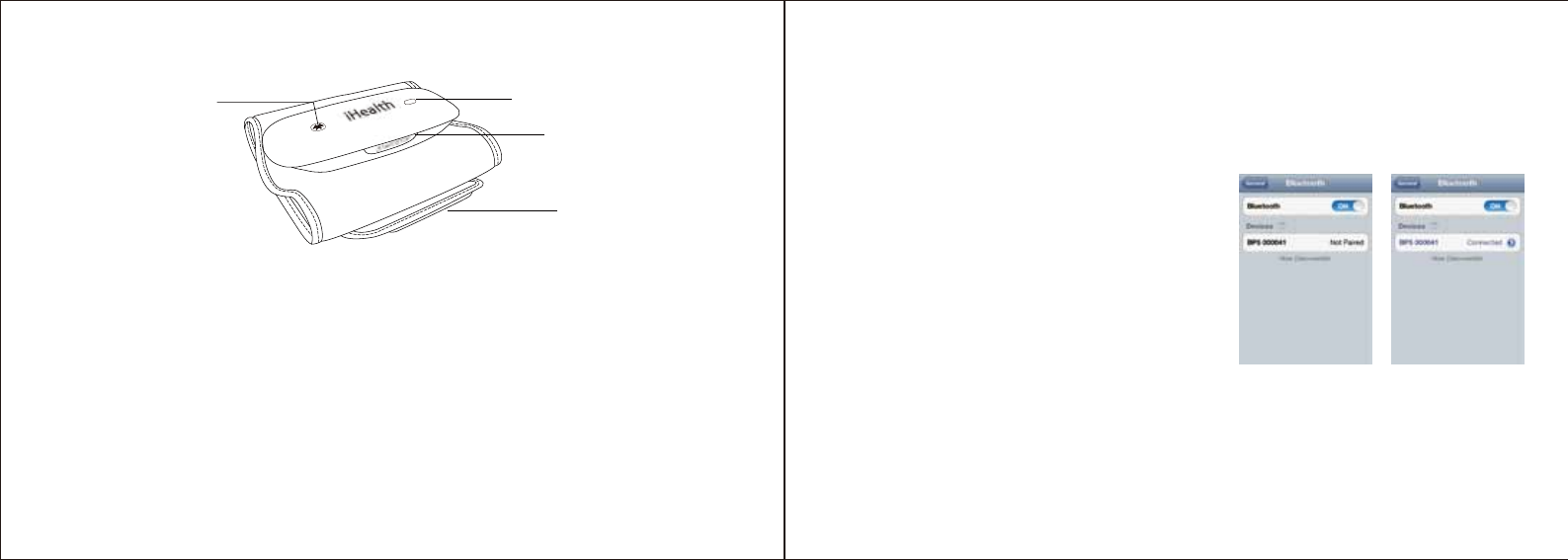
Apply the Cuff
a. Pull the cuff end through the metal loop, positioning it outward
(away from your body).
Body Posture
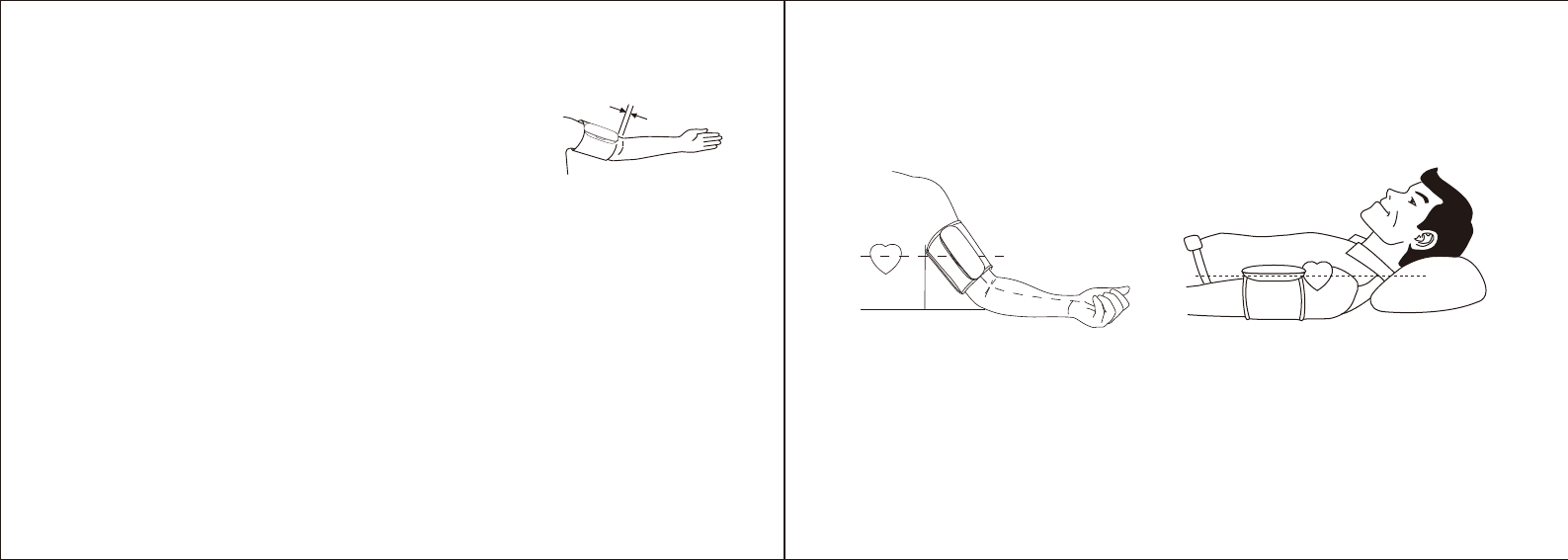
Sitting During Measurement
a. Be seated with your feet flat on the floor without crossing your legs.
b. Place your hand palm-side up in front of you on a flat surface such as a desk or table.
c. The middle of the cuff should be at the level of the right atrium of your heart.
MEASUREMENT PROCEDURES
5 6
b. Place a bare arm through the cuff and position the cuff
1/2”(1-2cm) above the elbow joint.
c. Tighten the cuff and close it by pulling it towards your
body, securing it closed with the Velcro fastener.
d. While seated, place your hand palm-side up in front of you on a flat surface such as a desk
or table. Position the monitor in the middle of your arm aligned with your middle finger.
Remember to:
1. Make sure that the appropriate cuff size is used; refer to the cuff circumference range in
“SPECIFICATIONS”.
2. Measure on the same arm each time.
3. Stay still during measurement. Do not move your arm, body, or the monitor.
4. Stay still and calm for one to one and half minutes before taking a blood pressure
measurement.
5. Keep the cuff clean. Cleaning the cuff after every 200 times of usage is recommended.
If the cuff becomes dirty, clean it with a moistened cloth. Do not rinse the monitor or cuff
with running water.
Lying Down During Measurement
a. Lie on your back.
b. Place your arm straight along your side with your hand palm-side up.
c. The cuff should be placed at the same level as your heart.
Note: Blood pressure can be affected by the position of the cuff and your physiologic condition.
During measurement, press the “START/STOP” button for 2 seconds to turn off the monitor
manually.
1/2”(1-2cm)
ON/OFF
i
H
e
a
l
t
h
e. The cuff should fit comfortably, yet snugly around your arm. You should be able to insert one
finger between your arm and the cuff.
Note: Please consult a health care professional for interpretation of blood pressure
measurements.
Important: Please consult a healthcare professional for interpretation of blood pressure
measurements.
1. Product name: Wireless Blood Pressure Monitor
2. Model: BP5
3. Classification: Internally powered, Type BF applied part, IPX0, No AP or APG, Continuous
operation
4. Machine size: approx. 5.7” x 2.3” x 1.2”(145mm × 58mm × 30mm)
5. Cuff circumference: 8.6”-16.5”(22cm-42cm), 16.5”-18.9”(42cm-48cm) (XL size sold
separately)
6. Weight: approx. 4.8oz (135g) (excluding cuff)
7. Measuring method: Oscillometric method, automatic inflation and measurement
8. Memory volume: 120 times with time and date stamp (off-line measurement only)
9. Power: DC: 5V 1.0A,
Battery: 1*3.7V Li-ion 400mAh
10. Measurement range:
Cuff pressure: 0-300 mmHg
Systolic: 60-260 mmHg
Diastolic: 40-199 mmHg
Pulse rate: 40-180 beats/minute
SPECIFICATIONS
Operation Instructions With iOS Device
For detailed operating instructions, please visit http://www.ihealthlabs.com.
During measurement, press the “START/STOP” button to stop measurement, press the
“START/STOP” button for 2 seconds to turn off the monitor manually.
Measuring without an iOS Device
Offline measurement is enabled automatically at your first online measurement. To start offline
measurement when disconnected from the iOS device, turn on the monitor by pressing the
“START/STOP” button. The status indicator light will be green and the monitor will begin measure-
ment. If measured successfully, the measurement will be stored in the iHealth Wireless Blood
Pressure Monitor and the status indicator light will flash green. The reading is not visible until the
next iOS connection. The measurement will be uploaded to the device automatically
7 8
Monitor Status Bluetooth Indicator
Waiting to connect Flashing blue light
Measuring Steady blue light
Measurement completed Gradually extinguishing light
upon the next iOS connection. Otherwise the status indicator will be red for a moment then
change to flashing green. The monitor will turn off automatically after 2 minutes of
non-operation. Alternatively, you can keep on pressing the “START/STOP” button for 2
seconds to turn off the monitor manually.
During measurement, press the “START/STOP” button to stop the measurement process.
Note: These specifications are subject to change without notice.
1. Read all of the information in the Owner’s Manual and other provided instructions before
operating the unit.
2. Consult your physician for any of the following situations:
a) The application of the cuff over a wound or inflamedarea.
b) The application of the cuff on any limb with intravas cular access or therapy, or an arterio-
venous (A-V) shunt.
c) The application of the cuff on the arm on the side of a mastectomy.
d) Simultaneous use with other medical monitoring equipment on the same limb.
e) The blood circulation of the user needs to be checked.
GENERAL SAFETY AND PRECAUTIONS
9 10
3. This Wireless Blood Pressure Monitor is designed for adults and should never be used on
infants, young children, pregnant or pre-eclamptic patients. Consult your physician before
use on children.
4. Do not use this product in a moving vehicle as this may result in inaccurate measurements.
5. Blood pressure measurements determined by this product are equivalent to those obtained
by professional healthcare practitioners using the cuff/stethoscope auscultation method
within the limits prescribed by the American National Standard, Electronic or Automated
Sphygmomanometer.
6. Information regarding potential electromagnetic or other interference between the blood
pressure monitor and other devices together with advice regarding avoidance of such
interference, please see ELECTROMAGNETIC COMPATIBILITY INFORMATION. It is
suggested that the blood pressure monitor be kept 10 meters away from other wireless
devices, such as WLAN unit, cell phone, microwave oven, etc.
7. It is recommended that the iOS device be set in Airplane mode during measurement to
avoid strong magnetism interference. If a call comes in during the measurement, the
measurement process will be terminated automatically.
8. If Irregular Heartbeat (IHB) is detected during the measurement procedure, the IHB symbol
will be displayed. Under this condition, the Wireless Blood Pressure Monitor can keep
functioning, but the results may be inaccurate. Please consult your physician for accurate
assessment.
There are 2 conditions under which the signal of IHB will be displayed:
a) The coefficient of variation (CV) of pulse period >25%.
b) The difference of adjacent pulse period ≥0.14s and the number of such pulse takes more
than 53 percent of the total number of pulses.
9. Please do not use any other cuff other than that supplied by the manufacturer as this may
!
18. Battery life: more than 80 measurements on a full charge
19. The blood pressure measurement system includes accessories: pump, valve, cuff, and sensor
11. Accuracy:
Pressure: ±3 mmHg
Pulse rate: ±5%
12. Wireless communication: Bluetooth V3.0 + EDR Class 2 SPP
Frequency Band: 2.402-2.480 GHz
13. Environmental temperature for operation: 5°C~40°C(41°F~104°F)
14. Environmental humidity for operation: ≤90%RH
15. Environmental temperature for storage and transport: -20°C~55°C(-4°F~131°F)
16. Environmental humidity for storage and transport: ≤95%RH
17. Environmental pressure: 80kPa-105kPa
Do not change the battery. If the battery can no longer be charged, please contact Customer
Service.
• When charging is needed, please connect the monitor to a power source. The monitor can
work normally while charging.
• When the monitor is connected to an iPod touch, iPhone, or iPad, the battery volume will be
displayed on the iPod touch, iPhone, or iPad screen. If the power is less than 25%, please
BATTERY HANDLING AND USAGE
11
10. This product might not meet its performance specifications if stored or used outside the
specified temperature and humidity ranges.
11. Please do not share the cuff with any infectious person to avoid cross-infection.
12. This product should not be used as a USB device.
13. This product is verified by auscultatory method. It is recommended that you check Annex
B of ANSI/AAMI SP-10:2002+A1:2003+A2:2006 for verification method details if needed.
14. If the determined blood pressure (systolic or diastolic) is outside the rated range specified
in SPECIFICATIONS, the app will immediately display a technical alarm on screen. In
this case, consult a physician or ensure that proper measurement procedures are followed.
The technical alarm is preset in the factory and cannot be adjusted or inactivated. This
technical alarm is assigned as low priority according to IEC 60601-1-8.The technical alarm
is non-latching and does not need to be reset.
15. A medical AC adapter with an output of DC 5.0V and complies with IEC 60601-1/UL
60601-1 and IEC 60601-1-2/EN 60601-1-2 is suitable for this monitor, such as
ASP5-05010002JU (input: 100-240V, 50/60Hz, 200mA; output: DC 5V, 1.0A). Please note
that the monitor jack size is USB mini B.
!
!
!
result in measurement errors and a biocompatible hazard.
Note: The battery has limited charge cycles and may eventually need to replaced by an iHealth
service provider. Battery life and charge cycles vary by use and settings.
12
the battery. The monitor will not work until the battery has enough power.
• When you charge the monitor, the LED will display with different colors indicating the charging
status. See the table below for details.
• It is suggested that you charge the battery when the battery is less than 25%. Overcharging the
battery may reduce its lifetime.
!
!
!
!
!
Lithium battery replacement by inadequately trained personnel could result in a hazard such as
a fire or explosion.
Do not plug or unplug the power cord into the electrical outlet with wet hands.
If the AC adapter is abnormal, please change the adapter.
Do not pull out the adapter when you are using the monitor.
Do not use any other type of AC adapter as it may harm the monitor.
The monitor, cable, battery and cuff must be disposed of according to local regulations at the
end of their usage.
Monitor Status Status Indicator
Charging Flashing green light
Fully charged Steady green light
Low battery Flashing red light (for a few seconds)
Abnormal state Steady red light
13 14
1. Do not drop this monitor or subject it to strong impact.
2. Avoid high temperature and direct sunlight. Do not immerse the monitor in water as this will
result in damage to the monitor.
3. If this monitor is stored near freezing temperatures, allow it to acclimate to room temperature
before use.
4. Do not attempt to disassemble this monitor.
5. If the monitor is not used for a long time, please sure to fully charge it every month.
6. It is recommended that product performance be checked every 2 years or after each repair.
Please contact the Customer Service.
CARE AND MAINTENANCE
TROUBLESHOOTING
!
!
!
PROBLEM POSSIBLE CAUSE SOLUTION
Battery is less than 20% Charge the batteryLow Battery
Blood pressure is outside of measurement range Retest, make sure your blood pressure is within
measurement range
Arm or monitor was moved during test Retest, make sure not to move your arm or the monitor
The cuff does not inflate properly or pressure
falls quickly during test
Review the cuff application instructions and retest
Irregular heartbeat (arrhythmia) It is inappropriate for people with serious arrhythmia to
use this monitor. Check with physician
The cuff was not properly
applied
Review the cuff application instructions and retest
Display reads
“ERROR”
The cuff position was not correct or it was not
properly tightened
Review the cuff application instructions and retest
Incorrect operation or strong electromagnetic
interference
Press the START/STOP button about 10 seconds to reset
the device, relaunch app, and reconnect the iOS device
to the monitor
No response
Body posture was not correct during testing Review body posture instructions and retest
Speaking, moving arm or body, being angry,
excited or nervous during test
Retest when calm; avoid speaking or movement
during the test
Bluetooth connection unsuccessful, monitor is
abnormal, or strong electromagnetic
interference is present
Reset iOS device. Reset monitor by pressing the
START/STOP button about 10s. Make sure the monitor
and iOS device are away from other electrical
equipment. Please see GENERAL SAFETY AND
PRECAUTIONS.
Display reads
an abnormal
result
Bluetooth
connection
unstable
7. No monitor component needs to be maintained by the user. The circuit diagrams, component
part lists, descriptions, calibration instructions, or other information which will assist the user’s
appropriately qualified technical personnel to repair those parts of the equipment which are
designated for repair can be supplied.
8. Clean the monitor with a dry, soft cloth or a moistened and well wrung soft cloth using water,
diluted disinfectant alcohol, or diluted detergent.
9. The monitor can maintain the safety and performance characteristics for a minimum of 10,000
measurements or three years of usage.
10. The battery can maintain the performance characteristics for a minimum of 300 charge cycles.
Battery replacement should only be performed by a qualified iHealth technician. To will void
your warranty and possibly damage your unit.
11. Cuff replacement should only be performed by a qualified iHealth technician. To do otherwise
will possibly damage your unit.
15 16
The iHealth Wireless Blood Pressure Monitor is warranted to be free from defects in materials
and workmanship within one year from the date of purchase when used in accordance with the
provided instructions. The warranty extends only to the end user. We will, at our option, repair
or replace without charge the iHealth Wireless Blood Pressure Monitor covered by the
warranty. Repair or replacement is our only responsibility and your only remedy under the
warranty.
WARRANTY INFORMATION
EXPLANATION OF SYMBOLS
iHealth is a trademark of iHealth Lab Inc.
“Made for iPod”, “Made for iPhone”, and “Made for iPad” mean that an electronic accessory has
been designed to connect specifically to iPod, iPhone, or iPad, respectively, and has been
certified by the developer to meet Apple performance standards. Apple is not responsible for the
operation of this device or its compliance with safety and regulatory standards. Please note that
the use of this accessory with iPod, iPhone, or iPad may affect wireless performance.
Symbol for "WARNING"
!
Symbol for "EUROPEAN REPRESENTATIVE"
EC REP
Symbol for "COMPILES WITH MDD93
/42/EEC REQUIREMENTS"
Symbol for
"
MANUFACTURER
"
Symbol for "SERIAL NUMBER"
SN
Symbol for "ENVIRONMENT PROTECTION – Waste electrical products should
not be disposed of with household waste. Please recycle where facilities exist.
Check with your local authority or retailer for recycling advice"
Symbol for "THE OPERATION GUIDE MUST BE READ"
The sign background color: blue The sign graphical symbol: white
Symbol for "KEEP DRY"
Symbol for "TYPE BF APPLIED PARTS" (cuff only)
Symbol for "THIS DEVICE COMPLIES WITH
PART 15 OF THE FCC RULES"
12. It is recommended that if the cuff is used, for example, in a hospital or a clinic, it be
disinfected twice a week. Wipe the inner side (the side that contacts skin) of the cuff with a
soft cloth lightly moistened with Ethyl alcohol (75-90%). Then air dry the cuff.
iPad, iPhone, and iPod touch are trademarks of Apple Inc., registered in the U.S. and other
countries.
17 18
IMPORTANT INFORMATION REQUIRED BY THE FCC
This device complies with Part 15 of the FCC Rules. Its operation is subject to the following two
conditions:
(1) This device may not cause harmful interference, and
(2) this device must accept any interference received, including interference that may cause
undesired operation.
Changes or modifications not expressly approved by iHealth Lab Inc. would void the user’s
authority to operate the product.
Lotus Global Co., Ltd.
15 Alexandra Road, London UK, NW8 0DP
Tel: +0044-20-75868010 Fax: +0044-20-79006187
EC REP
ANDON HEALTH CO., LTD.
No. 3 Jinping Street, YaAn Road, Nankai District,
Tianjin 300190, China. Tel: 86-22-60526161
Manufactured for iHealth Lab Inc.
719 N. Shoreline Blvd., Mountain View, CA 94043, USA 1-855-816-7705
www.ihealthlabs.com
The Bluetooth® word mark and logos are registered trademarks owned by Bluetooth SIG, Inc.
and any use of such marks by iHealth Lab Inc. is under license. Other trademarks and trade
names are those of their respective owners.
Note: This product has been tested and found to comply with the limits for a Class B digital device,
pursuant to Part 15 of the FCC Rules. These limits are designed to provide reasonable protection
against harmful interference in a residential installation. This product generates, uses, and can
radiate radio frequency energy and, if not installed and used in accordance with the instructions,
may cause harmful interference to radio communications. However, there is no guarantee that
interference will not occur in a particular installation. If this product does cause harmful interference
to radio or television reception, which can be determined by turning the equipment off and on, the
user is encouraged to try to correct the interference by one or more of the following measures:
—Reorient or relocate the receiving antenna.
—Increase the separation between the equipment and receiver.
—Connect the equipment into an outlet on a circuit different from that to which the receiver is
connected.
—Consult the dealer or an experienced radio/TV technician for help.
This product complies with Industry Canada. IC: RSS-210
This product is approved in accordance to R&TTE directive transmitter.
IC NOTICE
This device complies with Industry Canada licence-exempt RSS standard(s). Operation is subject
to the following two conditions: (1) this device may not cause interference, and
(2) this device must accept any interference, including interference that may cause undesired
operation of the device.
19 20
OTHER STANDARDS AND COMPLIANCES ELECTROMAGNETIC COMPATIBILITY INFORMATION
The Wireless Blood Pressure Monitor corresponds to the following standards:
IEC 60601-1:2006 (Medical electrical equipment – Part 1: General requirements for safety);
IEC 60601-1-2:2007 (Medical electrical equipment – Part 1: General requirements for safety;
Collateral Standard-Electromagnetic compatibility - Requirements and tests);
EN 1060-1: 1995 + A1: 2002 + A2: 2009 (Non-invasive sphygmomanometers - Part 1: General
requirements);
EN 1060-3: 1997 + A1: 2005 + A2: 2009 (Non-invasive sphygmomanometers - Part 3: Supplemen-
tary requirements for electro-mechanical blood pressure measuring systems);
ANSI/AAMI SP-10:2002+A1:2003+A2:2006;
IEC 80601-2-30:2009+Cor.2010/EN 80601-2-30:2010(Medical electrical equipment –Part 2-30:
Particular requirements for the basic safety and essential performance of automated non-invasive
sphygmomanometers).
For all ME EQUIPMENT and ME SYSTEMS
Emissions test
Compliance
Electromagnetic environment - guidance
RF emissions
CISPR 11
RF emissions
CISPR 11
Harmonic
emissions
IEC 61000-3-2
Voltage fluctuations/
flicker emissions
IEC 61000-3-3
Group 1
Class B
Class A
Complies
The Wireless Blood Pressure Monitor uses RF energy only for its internal function.
Therefore, its RF emissions are very low and are not likely to cause any interference in
nearby electronic equipment.
The Wireless Blood Pressure Monitor is suitable for use in all establishments,
including domestic establishments and those directly connected to the public
low-voltage power supply network that supplies buildings used for domestic
purposes.
The Wireless Blood Pressure Monitor is intended for use in the electromagnetic environment specified below.
The customer or the user of the Wireless Blood Pressure Monitor should assure that it is used in such an environment.
Guidance and manufacture’s declaration - electromagnetic emissions
Table 1
21 22
Guidance and manufacturer’s declaration - electromagnetic immunity
The Wireless Blood Pressure Monitor is intended for use in the electromagnetic environment specified below. The customer or the user
of the Wireless Blood Pressure Monitor should assure that it is used in such an environment.
For ME EQUIPMENT and ME SYSTEMS that are not LIFE-SUPPORTING
IMMUNITY test IEC 60601test level Compliance level Electromagnetic environment - guidance
Conducted RF
IEC 61000-4-6
3 Vrms 150
kHz to 80 MHz
3 V
3 V/m
Portable and mobile RF communications equipment should be
used no closer to any part of the BP5, including cables, than the
recommended separation distance calculated from the
equation applicable to the frequency of the transmitter.
Recommended separation distance:
Radiated RF
IEC 61000-4-3
3 V/m 80 MHz
to 2.5 GHz
Pd 2.1=
Pd 2.1=
80 MHz to 800 MHz
Pd 3.2=
800 MHz to 2.5 GHz
Where P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter
manufacturer and d is the recommended separation
distance in meters (m).
Guidance and manufacturer’s declaration - electromagnetic immunity
The Wireless Blood Pressure Monitor is intended for use in the electromagnetic environment specified below. The customer
or the user of the Wireless Blood Pressure Monitor should assure that it is used in such an environment.
IMMUNITY test Electromagnetic environment - guidance
Electrostatic
discharge (ESD)
IEC 61000-4-2
± 6 kV contact
± 8 kV air
± 6 kV contact
± 8 kV air
Floors should be wood, concrete or ceramic tile. If floors
are covered with synthetic material, the relative humidity
should be at least 30 %.
Electrical fast
transient/burst
IEC 61000-4-4
± 2 kV for power
supply lines
± 2 kV for power
supply lines
Mains power quality should be that of a typical
commercial or hospital environment.
Surge
IEC 61000-4-5
± 1 kV line(s) to
line(s)
± 2 kV line(s) to earth
± 1 kV line(s) to
line(s)
± 2 kV line(s) to earth
Mains power quality should be that of a typical
commercial or hospital environment.
Voltage dips, short
interruptions and
voltage variations
on power supply
input lines
IEC 61000-4-11
<5 % UT
(>95 % dip in UT)
for 0.5 cycle
40 % UT
(60 % dip in UT)
for 5 cycles
70 % UT
(30 % dip in UT)
for 25 cycles
<5 % UT
(>95 % dip in UT) for 5 s
<5 % UT
(>95 % dip in UT)
for 0.5 cycle
40 % UT
(60 % dip in UT)
for 5 cycles
70 % UT
(30 % dip in UT)
for 25 cycles
<5 % UT
(>95 % dip in UT) for 5 s
Mains power quality should be that of a typical
commercial or hospital environment. If the user of
theBP5 requires continued operation during power
mains interruptions, it is recommended that theBP5 be
powered from an uninterruptible power supply or a
battery.
NOTE: UT is the a.c. mains voltage prior to application of the test level.
Power frequency
(50/60 Hz)
magnetic field
IEC 61000-4-8
3 A/m 3 A/m Power frequency magnetic fields should be at levels
characteristic of a typical location in a typical
commercial or hospital environment.
IEC 60601test level Compliance level
For all ME EQUIPMENT and ME SYSTEMS
Table 2
Table 3
23 24
Field strengths from fixed RF transmitters, as determined by
an electromagnetic site survey,a should be less than the
compliance level in each frequency range.b
Interference may occur in the vicinity of equipment
marked with the following symbol:
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from
structures, objects and people.
a) Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur
radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic
environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the
location in which the BP5 is used exceeds the applicable RF compliance level above, the BP5 should be observed to verify normal
operation. If abnormal performance is observed, additional measures may be necessary, such as re-orienting or relocating the BP5.
b) Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
For ME EQUIPMENT and ME SYSTEMS that are not LIFE-SUPPORTING
Pd 2.1=Pd 2.1=Pd 3.2=
Recommended separation distances between
portable and mobile RF communications equipment and the Wireless Blood Pressure Monitor
The Wireless Blood Pressure Monitor is intended for use in an electromagnetic environment in which radiated RF disturbances are
controlled. The customer or the user of the Wireless Blood Pressure Monitor can help prevent electromagnetic interference by
maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the Wireless Blood
Pressure Monitor as recommended below, according to the maximum output power of the communications equipment.
Rated maximum output
power of transmitter
W
Separation distance according to frequency of transmitter
m
150 kHz to 80 MHz 80 MHz to 800 MHz 800 MHz to 2,5 GHz
0.12 0.230.120.01
0.38 0.730.380.1
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in meters (m) can be
determined using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from
structures, objects and people.
1.2 2.31.21
3.8 7.33.810
12 2312100
Table 4