BIOTRONIK SE and KG SAFESYNC SafeSync Module User Manual 380184 D GA SafeSyncModule en 2011 06 15
BIOTRONIK SE & Co. KG SafeSync Module 380184 D GA SafeSyncModule en 2011 06 15
15_SafeSync UserMan

Revision: D (2011-04-26)
11-D-xx
© by BIOTRONIK SE & Co. KG
Alle Rechte vorbehalten.
Technische Änderungen vorbehalten
BIOTRONIK SE & o K
Woermannkehre 1
12359 Berln ermany
Tel +49 (0) 30 68905-0
Fax +49 (0) 30 6852804
salesbotronkcom
wwwbotronkcom
SafeSync Module
Erweterungsmodul fr Programmergerte zur drahtlosen Kommunkaton
Cardiac Rhythm Management
External Devices
Gebrauchsanweisung
380184--D_GA_SafeSyncModule_de_Cover_2011-04-xx.indd 1-2 23.05.2011 12:28:48

Table of Contents 3
Table of Contents
Table of Contents
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
About the Device . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
About this Technical Manual. . . . . . . . . . . . . . . . . . . . . . 8
Safety During Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
Intended Medical Use . . . . . . . . . . . . . . . . . . . . . . . . . . 10
Required Expertise . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
Residual Risk . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
General Safety Instructions. . . . . . . . . . . . . . . . . . . . . . 13
Electromagnetic Interference. . . . . . . . . . . . . . . . . . . . 14
Operating Conditions . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
Maintenance, Care and Disposal . . . . . . . . . . . . . . . . . 18
Startup. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
Device Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 22
Transportation and Setup . . . . . . . . . . . . . . . . . . . . . . . 23
Connections and Cables . . . . . . . . . . . . . . . . . . . . . . . . 24
Unit Handling . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
Appendix . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33
Technical Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 34
Scope of Delivery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 37
Electromagnetic Compatibility in Compliance
with EN 60601-1-2:2007 . . . . . . . . . . . . . . . . . . . . . . . . 38
Legend for the Label . . . . . . . . . . . . . . . . . . . . . . . . . . . 42
Symbols on the Device. . . . . . . . . . . . . . . . . . . . . . . . . . 44
Directories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 45
List of Keywords. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 46

4Table of Contents
6Introduction
About the Device
General descrip-
tion
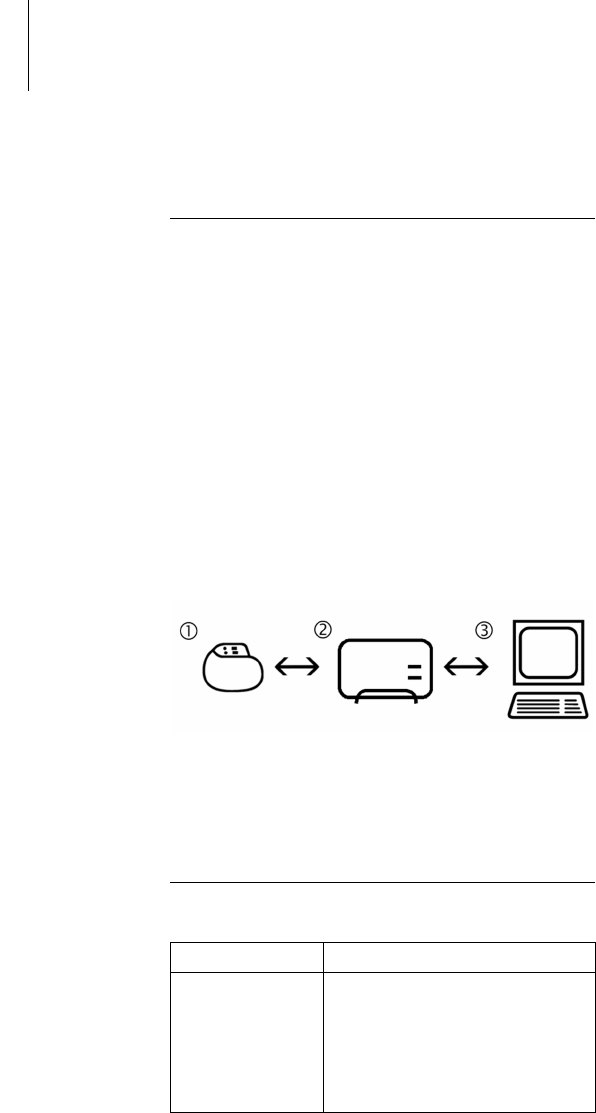
The SafeSync Module can be connected to the ICS
3000 and Renamic programmers and permits:
A wandless telemetry connection (SafeSync RF
telemetry) between the programmer and devices
with the BIOTRONIK SafeSync function and
Optional communication with networks via the
cellular phone network or WLAN (depending on the
software version of the programmer).
Devices with the BIOTRONIK SafeSync function are
equipped with a special transmitter and receiver (1).
This sends all the relevant information to the
SafeSync Module (2), which then forwards the infor-
mation to the programmer (3). The device also
receives all information that the programmer
forwards to the SafeSync Module for transmission.
Fig. 1: SafeSync function principle
It is used during the implantation procedure and
follow-up of implantable pacemakers and ICDs
(implantable cardioverter-defibrillators) with the
BIOTRONIK SafeSync function.
Primary function The device extends the programming devices of
BIOTRONIK to include the following functions:
Function Purpose
BIOTRONIK
SafeSync function
Wandless telemetry connection
(SafeSync RF telemetry) for
interrogating, testing and
programming pacemakers and
ICDs with the BIOTRONIK
SafeSync function
Introduction 7
Other functions
(depending on the
software version of
the programmer)
The device extends the programming devices of
BIOTRONIK to include the following functions:
Function Purpose
Data transfer Exporting the follow-up data in
hospital or private practice
networks
Update function Downloading the latest,
approved software version for
the programmer from
BIOTRONIK

8Introduction
About this Technical Manual
Objective This technical manual provides the user with all the
safety information required to use the device.
The following topics are covered in this manual:
Device startup
Target group This technical manual is intended for physicians and
trained medical personnel who are familiar with the
following:
The use of implantable pulse generators and ICDs
The risks and possible complications associated
with using these systems
Additional requirements include:
Medical knowledge:
- Basic medical knowledge of the therapy applied
- Training in the handling and programming of
implantable pulse generators and ICDs
Technical knowledge:
- Ability to work with a PC
- Ability to use software-controlled medical
devices
Other technical
manuals
To ensure the safe and correct use of the device, you
must follow these additional instructions:
The technical manual for the programmer
Technical software manual for programming the
intended implantable pulse generator / ICD
Technical manual for the intended implantable
pulse generator / ICD
Safety During Use 9
2 Safety During Use
Safety Duri ng Use2380184-DDoc-cl assECM--SafeSync Mo dule
What's in this
chapter?
This chapter contains the following topics:
Topic Page
Intended Medical Use 10
Required Expertise 11
Residual Risk 12
General Safety Instructions 13
Electromagnetic Interference 14
Operating Conditions 16
Maintenance, Care and Disposal 18

10 Safety During Use
Intended Medical Use
Intended medical
use
During implantation or follow-up, the SafeSync
Module establishes telemetry between a device with
BIOTRONIK SafeSync function and the ICS 3000 or
Renamic programmer.
Thus the programmer is able to perform the following
without a programming head:
Conduct sensing, pacing threshold and impedance
tests
Interrogate data of the implanted device such as
program parameters, recorded statistical data and
episodes, as well as real-time IEGMs
Display, printout, save and export data of the
implanted device for analysis and reporting
purposes
Transferring parameters to the device

Safety During Use 11
Required Expertise
Required expertise The programmer is intended for use by physicians and
trained medical staff. Along with their basic medical
knowledge, a detailed knowledge of cardiac electro-
therapy is also required. Only qualified medical
specialists with knowledge of cardiac electrotherapy
can properly operate the device.
German medical
device ordinance
This ordinance only applies in the Federal Republic of
Germany. However, we recommend that customers in
other countries comply with this ordinance as well.
According to section 2, § 5, operation and use:
'The user may operate a (...) listed medical product
only after the manufacturer or the authorized agent
who acts on behalf of the manufacturer has
performed the following requirements:
1. Checked the functionality of this medical product
at the location where the device will be used.
2. Trained the staff appointed by the user to
correctly handle, use and operate the medical
product. This training must include handling, using
and operating the product in conjunction with other
medical products, implements and accessories in
accordance with the technical manual, as well as
any applicable safety-related information and
maintenance instructions.
(...)
(3) Proof of a functional test have been performed as
stated in Paragraph 1 Item 1, and the training record
of the staff appointed by the user, discussed in Para-
graph 1 Item 2, are to be documented.'
12 Safety During Use
Residual Risk
Risk analysis The risk analysis carried out by the manufacturer's
Risk Management Team has determined that the
residual risk is as low as reasonably possible.
It is a prerequisite that the programmer has been
serviced and inspected according to the manufac-
turer's specifications by qualified medical staff and in
compliance with the safety-relevant instructions in
this technical manual.

Safety During Use 13
General Safety Instructions
Technical manual Only use the programmer in accordance with this
technical manual.
Risks of improper
handling
Disregarding the safety instructions can endanger the
patient, the staff and the equipment.
The following dangers may arise in the event of
improper use:
Failure of important device functions
Danger to persons due to electrical effects
Changes not
permitted
Only the manufacturer or a party expressly authorized
by BIOTRONIK may perform corrective maintenance,
enhancements or modifications to the device.
Replacement parts
and accessories
To ensure safety compliance, use only original
replacement parts and accessories authorized by
BIOTRONIK. Using any other parts voids the manufac-
turer's liability for any consequences, guarantee and
warranty.
Defects Do not use defective or damaged devices.
Liquids Never use a damp or wet device.
Protect the device from the accidental ingression
of fluids (e.g. infusion fluids).
Electrostatic
potentials
Ensure that electrostatic potentials between medical
staff and patients are balanced. Before handling the
device, the electrostatic potential between the doctor
or medical staff and the patient must be balanced by
touching the patient at a point as far away from the
leads as possible.
Note: Failure to observe the safety precautions voids
all damage claims and manufacturer liability.

14 Safety During Use
Electromagnetic Interference
Possible electro-
magnetic interfer-
ence
The programmer is protected from disturbances
resulting from electromagnetic irradiation, electro-
static discharges and other sources. Simultaneously,
the emitted interference has been reduced to a
minimum. Thus the programmer conforms to the
requirements of EN 60601-1-2 (in its valid form at the
time of delivery).
However, strong electromagnetic interferences that
occur in the close vicinity of electrical motors, power
cables, PCs, monitors, or other – possibly defective –
electrical devices may compromise the function of the
programmer in certain cases.
This kind of device malfunction should be considered
if the following is observed:
The device switches on by itself.
The unit passes on incorrect intrinsic events, which
are displayed on the ECG, IEGM or marker channel
(artifacts) of the programmer and monitoring
device.
The device displays other inexplicable functions.
Correct operation of the device can be restored with
the following:
Switch off the malfunctioning electronic device.
Remove the source of interference from the device.
Switch the programmer on and off or cut off the
electrical connection between the device and the
source of interference if this is possible without
causing any danger.
If the interference continues, contact BIOTRONIK
immediately.
Note: If accessories other than those specified by
BIOTRONIK are used, increased interference or
lower resistance to interference can be expected.

Safety During Use 15
EMI test Telemetry between the SafeSync Module and the
implanted device can be impaired by electromagnetic
interference (EMI). This can be observed when it
becomes difficult or even impossible to interrogate or
program the implanted device. Using the EMI test
(refer to device software help), the source of the elec-
tromagnetic interference can be located and then
turned off.
Note: If accessories specified by BIOTRONIK are
used on other devices, increased interference or
lower resistance to interference can be expected.
Note: Portable radio communication devices can
interfere with the programmer functioning.
16 Safety During Use
Operating Conditions
Storage and
transportation
If the packaging is damaged, please contact
BIOTRONIK immediately. Do not put the device into
operation.
Installation site Only operate the device in rooms that fulfill the
following conditions:
No danger of explosion
Suitable for medical purposes
Place the unit on a flat, dry surface so that the patient
cannot touch it. The unit should be placed so that it
cannot slide – even with the cables connected.
Power supply The unit is powered via the programmer's USB cable.
Cable and plug
connections
Replace any cable that shows even slight damage.
Lay all cables within the measuring apparatus in
such a way that they pose no danger of tripping over
them and that any tensile forces that may occur can
be safely buffered.
As a general rule, cables should only be connected
or disconnected when the unit is switched off,
unless expressly permitted in the corresponding
section of this technical manual.
Ensure that the contacts of all connections and
plugs are clean. Soiled contacts can lead to signal
distortions, and thus to false diagnoses.
Do not touch any connections such as USB ports or
interfaces for modules and the patient at the same
time.
!
!
CAUTION
Functional impairment due to external damage
Mechanical impact, for example dropping the unit -
even from a height of over 5 cm if unpackaged - can
permanently impair the function of the system.
Do not use the device if it shows visible damage.
Contact BIOTRONIK for testing and, if necessary,
repair of the equipment.

Safety During Use 17
Ensure that there is no condensation on the plugs
or in the connector ports. If condensation is
present, dry it before use.
Do not force plugs into the connector ports and
when disconnecting the plugs, do not pull on the
cable.
18 Safety During Use
Maintenance, Care and Disposal
The following regulations are valid for the device.
Cleaning and
disinfecting
Use soft, lint-free cloths.
Clean the housing with a damp cloth and mild soap
solution or 70% isopropanol.
Disinfect with alcohol or aldehyde-based agents
such as Aerodesin 2000, Fugaten spray,
Lysoformin 2000 or Aldasan 2000.
Visually inspect the connections: make sure that
the contacts for all connections and cables are
clean and free of any type of dirt.
To disinfect the patient cable and patient adapter,
use a mixture of 70% isopropanol and 30% water or
Lysoformin 3000: Allow it to take effect for 15
minutes at 2% concentration.
Do not use the unit for about 1 hour after cleaning
and disinfecting.
Sterilization The device cannot be sterilized.
!
!
WARNING
Exposure to fluids may result in fatal injury
Before cleaning and disinfecting the device surface:
Disconnect all USB cables!
!
!
CAUTION
Danger of explosion if exposed to cleaning and
disinfecting agents
Let cleaning and disinfection agents evaporate
before operating the device.
!
!
CAUTION
May be damaged by cleaning agents
Strong and abrasive cleaning agents and other
organic solvents, such as ether or benzine, corrode
the surface of the device and must not be used.

Safety During Use 19
Test before each
use
A short test of the device and the approved acces-
sories should be performed prior to each use. This
test consists of the following visual inspections and
a simple functional test:
- Inspect the housing for mechanical damage,
dents, loose parts, cracks, etc.
- Inspect cables and connection areas to ensure
proper insulation, no breaks, etc.
- Inspect the labeling for legibility
- Simple electrical function test: by connecting
the unit, an internal function test will be
conducted automatically
- If no error message appears, then no errors
were found and the device can be used
Inspection The inspection consists of the regular safety inspec-
tion according to medical device standards. This
ensures the safety of the device.
The inspection must be performed
- After use in conjunction with high-frequency
surgical instruments or defibrillators,
- If malfunctions are suspected,
-Once a year.
This inspection can be performed by BIOTRONIK.
The inspection should conform with the manufac-
turer specifications. These are available upon
request. The specifications list all necessary test
steps and the necessary equipment.
Disposal This device contains materials that must be
correctly disposed of in accordance with environ-
mental protection regulations. The European
Directive 2002/96/EC regarding waste electrical
and electronic equipment (WEEE) applies.
The symbol on the label – a crossed out garbage
can – indicates that the device must be disposed of
in accordance with the WEEE directive. The black
bar indicates that the device was sold after the
national implementation of the WEEE directive was
enforced in your country.
Return devices that are no longer used to
BIOTRONIK.
20 Safety During Use
Disposal of cables
Non-contaminated cables must be disposed of in
accordance with the European Directive 2002/96/EC
regarding waste electrical and electronic equipment
(WEEE).
Note: Cables to be disposed of due to contact with
blood must be disposed of as medical waste, in
accordance with environmental regulations.

22 Startup
Device Overview
Front view
Fig. 2: View of the unit from the front
Explanation of
items
Explanation of the individual items:
Back view
Fig. 3: View of the unit from the back
Explanation of
items
Explanation of the individual items:
1
2
Item Designation / description
1 Status indicator for SafeSync RF telem-
etry
2 Status indicator for WLAN or mobile
connection
3
4
Item Designation / description
3 Port for a USB printer or flash memory
stick (functional only when using the
ICS 3000)
4 Mini USB port to connect to the
programmer.
Startup 23
Transportation and Setup
Transporting the
device
The unit can be transported in the included
carrying case.
Setting up the
device
Place the device on a flat dry surface. Make sure
that the unit cannot slip even with the cables
connected and cannot be touched by the patient.
The physician must not touch any connections such
as USB ports or interfaces for modules and the
patient at the same time.
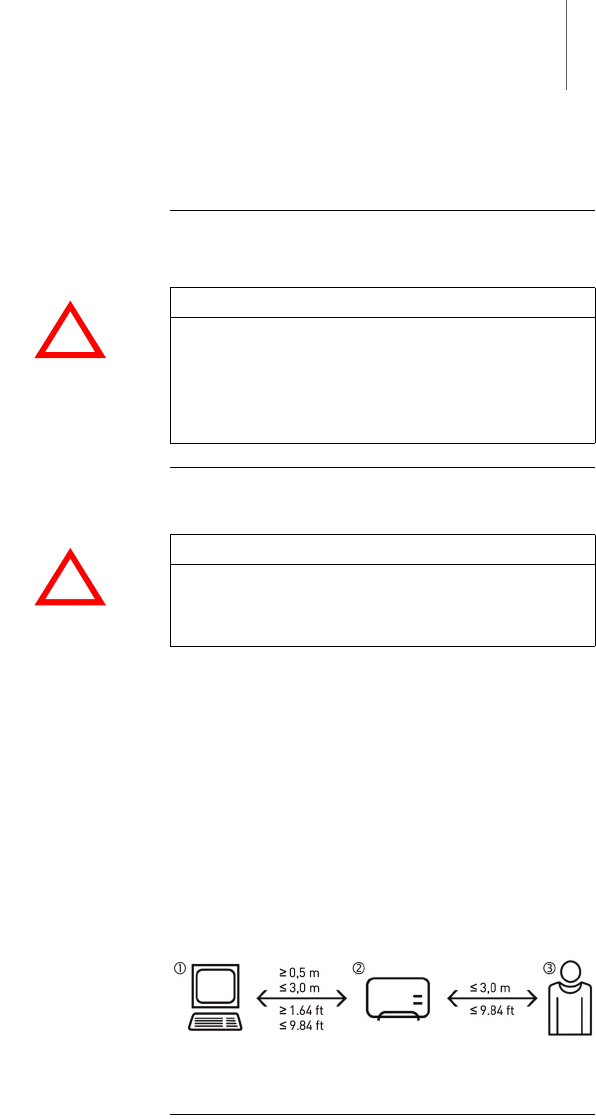
Make sure that the distance between the SafeSync
Module (2) and the patient (3) does not exceed 3 m
and that the patient cannot touch the SafeSync
Module (2).
Make sure that the distance between the SafeSync
Module (2) and the programmer (1) is at least
50 cm and does not exceed 3 m.
Fig. 4: Distance of the SafeSync Module from the
programmer and from the patient
!
!
WARNING
Danger to the user
Danger of tripping over connected cables during
device transport.
Prior to transporting the unit, remove the
attached cables and store them carefully.
!
!
WARNING
Danger to the patient
The device is not sterile and cannot be sterilized.
Do not set up the unit in a sterile area.
24 Startup
Connections and Cables
Basic notes for
cables and
connections
!
!
WARNING
Danger to patient by damaged cables
Damaged cables are limited in functionality and
pose a danger to patients.
Do not use damaged cables.
!
!
WARNING
Danger to patient from allergic reactions
If the cable comes into contact with open wounds, it
can cause allergic reactions.
Prevent cables from coming into contact with
open wounds and the patient's skin.
!
!
WARNING
Danger from loss of function
Damp cables have limited functionality and pose a
danger to patients.
Do not use damp cables.
Note:
Cables to be disposed of due to contact with blood
must be disposed of as medical waste in accor-
dance with environmental regulations.
Do not force the plugs into the ports. When
disconnecting plugs, do not pull on the cable.
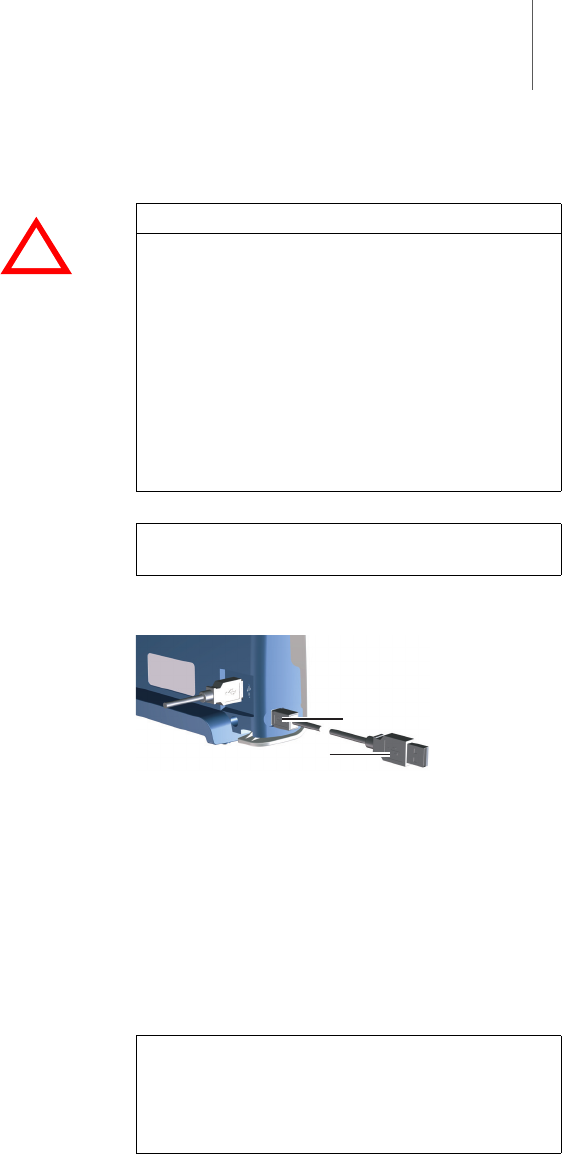
Startup 25
Connect
The mini USB port for connecting to the programmer
is located on the left side of the device.
Fig. 5: Mini USB connector on the SafeSync Module
Connect the mini USB connector (5) of the USB
cable to the SafeSync Module and the USB
connector (6) to the programmer.
If the programmer is switched on, the unit performs a
self-test and the LEDs all light up yellow.
After successful completion of the self-test, the
LEDs all light up green.
If the self-test is not successful, the LEDs flash
yellow.
!
!
WARNING
Risk to the patient caused by interference with or
termination of the ECG display.
Connecting or disconnecting the SafeSync Module
from the programmer can result in interference with
or termination of the ECG display.
Do not connect the unit to a programmer during
follow-up.
Do not disconnect the unit from the programmer
during follow-up.
Do not remove the Operation Module from the ICS
3000 during follow-up.
Note: The SafeSync Module can be connected when
the programmer is switched on.
Note:
You cannot connect more than 1 SafeSync Module
to a single programmer.
You cannot connect a SafeSync Module to another
SafeSync Module.
5
6
26 Startup
Connection of USB
devices
Only the following compatible devices can be
connected to the unit's USB port:
USB flash memory sticks with USB 2.0 and older
USB standards
Printers with USB 2.0 and older USB standard
(battery-powered or mains-operated)
!
!
WARNING
Risk to the patient caused by interference with or
termination of the ECG display.
Connecting or disconnecting USB devices to the
SafeSync Module can result in interference with or
termination of the ECG display
Do not connect any USB devices to the SafeSync
Module during follow-up.
Do not disconnect any USB devices from the
SafeSync Module during follow-up.
!
!
CAUTION
Damage to the USB port caused by connecting non-
compatible USB devices.
Connecting non-compatible USB devices can
damage the USB port.
Only connect the USB devices listed below.
Note:
Units that derive their power from the USB port
may not require more than 100 mA.
The USB port can be disconnected and recon-
nected while the device is still active.
Startup 27
!
!
WARNING
Danger to the user when connecting non-
conforming USB accessories.
Leakage currents can cause injuries to the skin or
cause an arrhythmia.
When using in combination with other devices, do
not use portable multiple socket outlets, but
connect all devices to fixed outlets in the same
electrical circuit used for medical purposes.
!
!
CAUTION
Risk of exceeding the leakage currents when
connecting external devices with their own power
supply or an electrically conductive connection to
other devices.
Only connect devices that comply with IEC
standard 60601-1:2005 or IEC 60950.
Line-powered devices must comply with the
standard IEC 60601-1:2005 or must be connected
to the USB port via an isolating separator (IEC
60601-1:2005 paragraph 16.5) with a dielectric
strength of at least 1.5 kV (e.g. an isolating USB
hub model UISOHUB4 by B&B electronics).
Place devices that do not adhere to the IEC 60601-
1:2005 standard at least 1.5 m away from the
patient.
Before initial commissioning, check and
document all device combinations according to
IEC 60601-1:2005 paragraph 16.6 for observance
of leakage currents.
Perform this inspection at least once per year
according to the legal requirements.
28 Startup
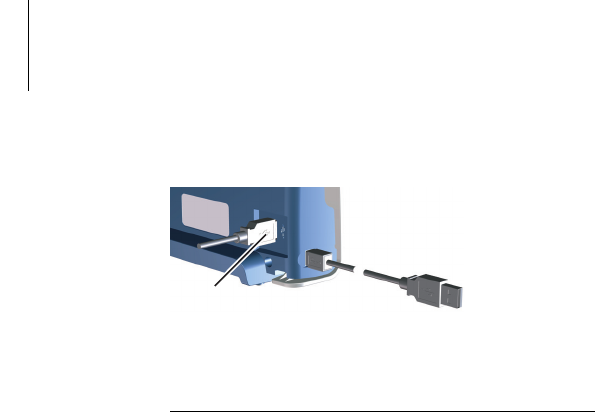
An ECG port is located on the back left of the unit.
Fig. 6: Position of the USB port
Connect the respective USB device (7) to the USB
port.
7
Startup 29
Unit Handling
Indicators during
operation
The LEDs provide information on the status of the
SafeSync Module or the respective function.
Establishing
SafeSync RF
telemetry
The telemetry connection is controlled using the
programmer software.
The software is installed on the drive of the
programmer by BIOTRONIK employees.
You can use the programmer's user interface to
activate or disable the SafeSync function independent
of the WLAN and cellular function. Both features can
be activated, but you can not use both simultaneously.
Using the user interface, you can either establish
SafeSync RF telemetry with a device or set up commu-
nication with a network (depending on the software
version of the programmer).
Establishment of the SafeSync RF telemetry depends
on the respective device and the suitable location of
the SafeSync Module.
To establish SafeSync RF telemetry, proceed as
described in the device's technical manual.
Position the SafeSync Module using the user inter-
face and the programmer's online help.
LED behavior Status
Both LEDs are
constantly yellow
Self-test is being performed or
the firmware of the SafeSync
Module is being updated
The respective
LED flashes
yellow
Self-test not passed; function is
not available
The respective
LED is constantly
green
The function is technically ready
for use (but may be turned off in
the programmer software)
The respective
LED flashes green
The function is active and data is
being exchanged with a device
or network
30 Startup
Interrogating and
programming the
device
The BIOTRONIK devices can communicate bidirec-
tionally with the programmer. Once the telemetry
connection has been established via the SafeSync
Module, the program data and all data stored in the
device can be transferred to the programmer.
Depending on the implanted device, a large number of
adjustable parameter sets are available. These
parameter sets are combined and saved in the
program that is currently active. The programmer
detects obvious program errors and requires these to
be corrected before the program is transferred to the
device.
The following programs can be transmitted:
Permanent program
A permanent program is a program that is
programmed in the pacemaker and which
performs pacing permanently without a telemetry
connection.
!
!
WARNING
Risk to the patient caused by termination of the ECG
display.
Removing the Operation Module on the ICS 3000
causes termination of the ECG display, which may
pose a risk to the patient.
Do not remove the Operation Module from the ICS
3000 during follow-up.
!
!
WARNING
Risk to the patient due to higher power consump-
tion in the device.
The SafeSync RF telemetry requires more energy
and decreases the device's longevity.
Only establish SafeSync RF telemetry if neces-
sary.
Check the device's battery capacity at regular
intervals (refer to the online help for the
programmer).
Startup 31
Temporary program
A temporary program is a program that the pace-
maker uses to provide temporary pacing as long as
the telemetry connection exists.
Safe program
A safe program is a device-specific program used
for safety pacing with high energy in either VVI or
SSI mode.
Establishing a
WLAN or mobile
connection
(depending on the
programmer's
software version)
You can use the programmer's user interface to
activate or disable the WLAN or cellular function inde-
pendent of the SafeSync function. Both features can
be activated, but you can not use both simultaneously.
Using the user interface, you can either establish
SafeSync RF telemetry with a device or set up commu-
nication with a network (depending on the software
version of the programmer).
Establishing the WLAN or mobile connection depends
on the respective network.
To set up the WLAN or mobile connection, proceed
as described in the programmer's online help.
Note: Use of a temporary program can be stopped at
any time and the permanent program of the
implanted device can be automatically reactivated
with the following:
Disconnect the telemetry connection using the
programmer's user interface.
Or:
Switch the programmer off.

32 Startup
34 Appendix
Technical Data
Physical
characteristics
General
classification
Longevity
Ambient conditions
Category Design
Dimensions (W x D x H) 203 x 136.5 x 80 mm
Weight approx. 450 g
Housing material PC/ABS
Category Design
Classification AIMD according to direc-
tive 90/385/EEC
Safety class IIb
Protection rating IP 30
Operating mode Continuous operation
Category Design
Longevity 6 years
Category Design
Temperature range for
operation
+10°C ... +40°C
Temperature range for
storage
0°C ... +50°C
Relative humidity 30% ... 75%, no
condensation
Atmospheric pressure 700 ... 1060 hPa
Operation at altitudes Up to 3000 m
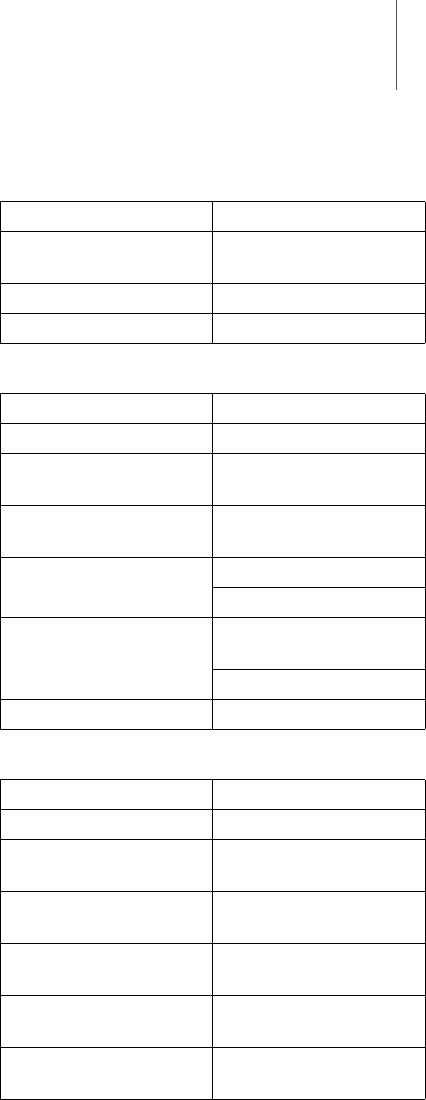
Appendix 35
MICS
GSM module
UMTS module
Category Design
Frequency band 9 channels
402 – 405 MHz
Bandwidth 300 kHz
Modulation FSK
Category Design
Model G24L or G24
Type GSM/GPRS quadband
Motorola
GSM frequency 850 MHz, 900 MHz,
1800 MHz, 1900 MHz
Max. power of transmis-
sion
2 W, 850/900 MHz
1 W, 1800/1900 MHz
Max. bandwidth
(Downlink)
GPRS (G24L/G24):
85.6 kbps
EGPRS (G24): 270 kbps
GPRS Multislot class 10
Category Design
Model H24
Type Motorola, 4 band GSM +
3 band UMTS
UMTS frequencies 850 MHz, 1900 MHz,
2100 MHz
GSM frequencies 850 MHz, 900 MHz,
1800 MHz, 1900 MHz
Max. UMTS transmission
power
0.25 W
Max. GSM power of
transmission
2 W, 850/900 MHz1 W,
1800/1900 MHz
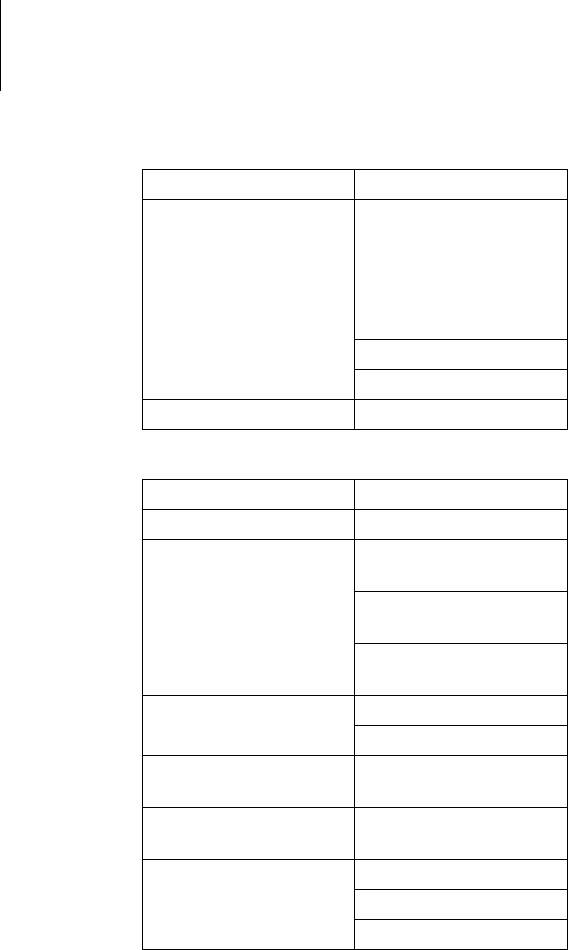
36 Appendix
WLAN module
Max. bandwidth
(Downlink)
UMTS:
7.2 Mbps
UE CAT [1-8], 11, 12
supported
Compressed mode
(3GPP TS25.212)
GPRS: 80 kbps
EGPRS: 236 kbps
GPRS Multislot class 12
Category Design
Category Design
Model WiReach BK
Transmission
frequencies
Europe: 2.412
GHz to 2.472 GHz
USA: 2.412 GHz to 2.462
GHz
Japan: 2.412
GHz to 2.484 GHz
Typ. transmission power 250 mA @ 16 dbm
235 mA @ 12 dbm
Reports WEP, WPA, WPA2,
HTTPS
Standards IEEE 802.11b, IEEE
802.11g
Channels Europe: 13 channels
USA: 11 channels
Japan: 14 channels
Appendix 37
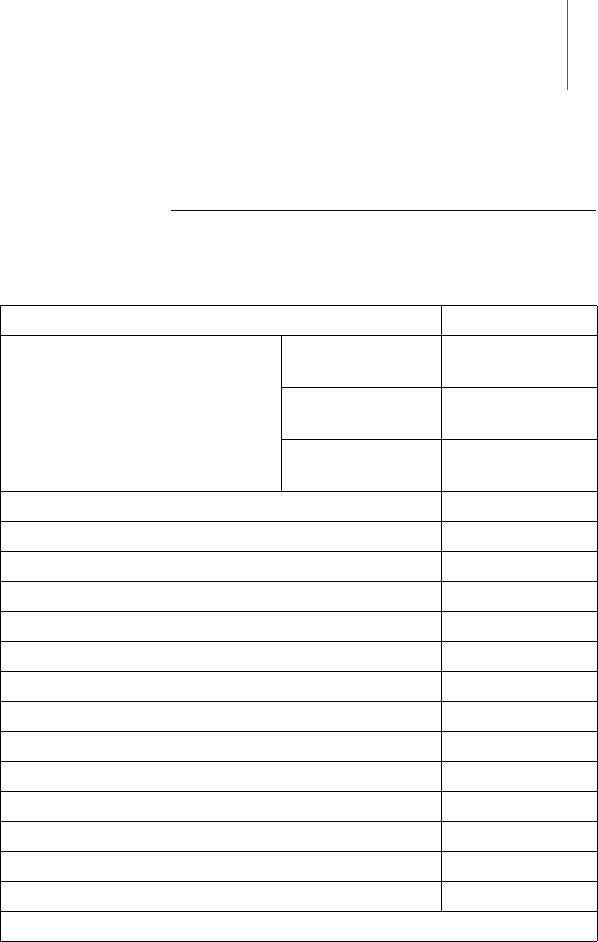
Scope of Delivery
Scope of Delivery SafeSync Module (order no.: 37xxxx)
Item designation Amount
SafeSync Module (single device) WLAN module* Customer-
specific
GSM module* Customer-
specific
UMTS module* Customer-
specific
USB cable 1
Case 1
Protective cover 1
Multilingual technical manual (de, en, es, fr, it) Country-specific
Multilingual technical manual (pl, tr) Country-specific
Technical manual ZH (printed) Country-specific
Quick reference guide DE (printed) Country-specific
Quick reference guide EN (printed) Country-specific
Quick reference guide ES (printed) Country-specific
Quick reference guide FR (printed) Country-specific
Quick reference guide IT (printed) Country-specific
Quick reference guide PL (printed) Country-specific
Quick reference guide TR (printed) Country-specific
Quick reference guide ZH (printed) Country-specific
*Not available in all countries
38 Appendix
Electromagnetic Compatibility in Compliance with
EN 60601-1-2:2007
As the user, you must ensure that the device is
operated in a suitable electromagnetic environ-
ment.
The following guidelines may not be applicable in
all cases. The propagation of electromagnetic
values is, for example, affected by the absorption
and reflection of structures, objects and people.
This data is for your personal information. There
should be at least 20 cm distance between the
device and the SafeSync Module to avoid interfer-
ence with the device caused by the electromagnetic
fields emitted by the SafeSync Module.
Electromagnetic
Emissions (Table 1)
Units with the warning sign “Transmitter
with non-ionizing radiation at designated
frequency” must not be operated in the envi-
ronment of the device due to potential inter-
ference.
Measuring the
emitted interfer-
ence
Compliance Guidelines for the electromagnetic
environment
RF interference
according to
CISPR 11
Group 1 The device uses RF energy exclusively
for its own function. Therefore, the RF
interference emitted is very low and
not likely to cause any interference in
nearby electronic equipment.
RF interference
according to
CISPR 11
Class B The device is suitable for use in all
establishments. This includes resi-
dences and facilities directly
connected to the public power supply
network that supplies buildings used
for domestic purposes.
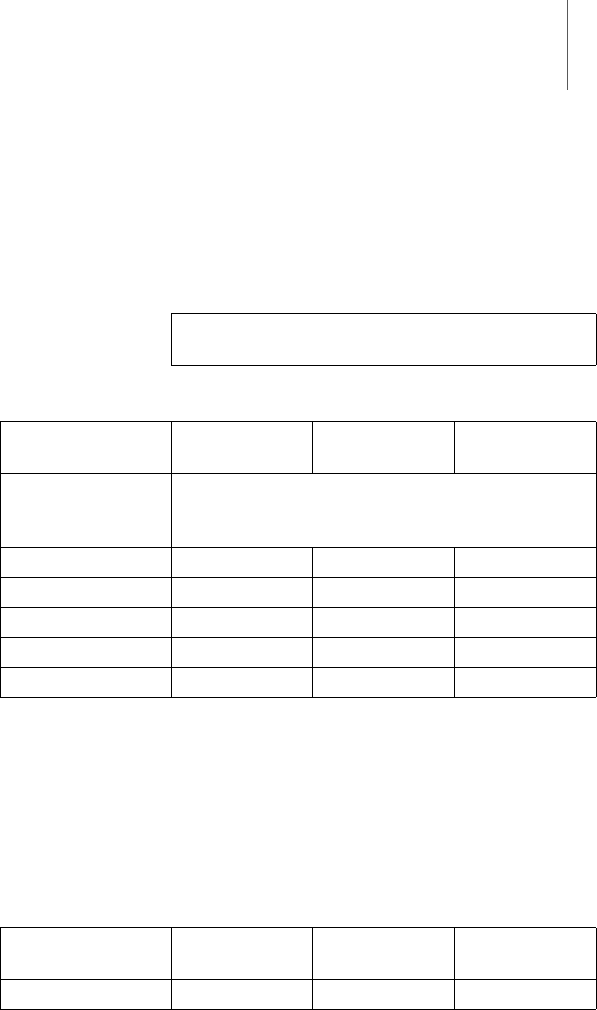
Appendix 39
Recommended
safety distances
(Table 6)
Safety distances help prevent interference if you
maintain a minimum distance between transmit-
ters such as mobile RF telecommunication devices
and the Renamic programmer. The necessary
distance depends on the respective power output of
the transmitter.
For transmitters whose maximum output power is
not indicated in the table, the recommended safety
distance [d] can be calculated in meters using an
equation that is suitable for the respective trans-
mission frequency range. P is the maximum output
power of the transmitter in watts [W] according to
the specification of the transmitter's manufac-
turer.
Resistance to elec-
tromagnetic inter-
ference
(tables 2 and 4)
When the measured field strength exceeds the
specified compliance level at the operating location
of the Renamic device, observe the device in order
to determine whether it is functioning properly.
Note: At 80 MHz and at 800 MHz, the higher
frequency range applies.
Transmission
frequency
150 kHz to
80 MHz
80 MHz to
800 MHz
800 MHz to
2.5 GHz
Maximum output
power of the trans-
mitter [W]
Safety distance [m]
0.01 0.12 0.12 0.24
0.1 0.37 0.37 0.74
1 1.17 1.17 2.34
10 3.70 3.70 7.40
100 11.7 11.7 23.4
Transmission
frequency
150 kHz to 80 M
Hz
80 MHz to
800 MHz
800 MHz to
2.5 GHz
Equation d = 1.17 P d = 1.17 P d = 2.34 P
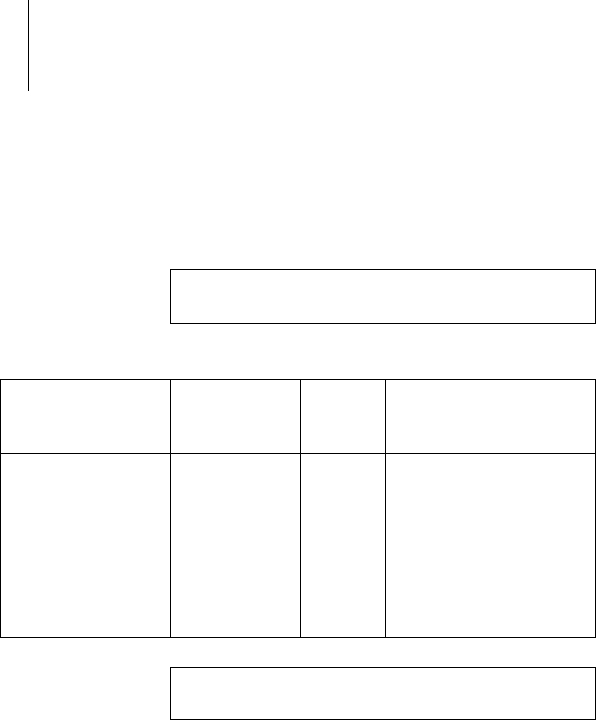
40 Appendix
If abnormal performance is observed, change the
orientation or the location of the device. In the
frequency range of 150 kHz to 80 MHz, ensure that
field strengths are lower than 3 V/m.
Note: UT is the mains alternating voltage before
applying the test levels.
Test of resistance
to interference
Test level
according to
IEC 60601-1-2
Compli-
ance
Guidelines for the elec-
tromagnetic environ-
ment
Electrostatic
discharge (ESD)
according to
IEC 61000-4-2
6kV contact
discharge
8 kV air
discharge
Same as
test level
Operate the devices
on floors made of
wood, concrete, or
ceramic tile. If the
floor is covered with
synthetic material,
the relative humidity
must be at least 30%.
Note: At 80 MHz and at 800 MHz, the higher
frequency range applies.
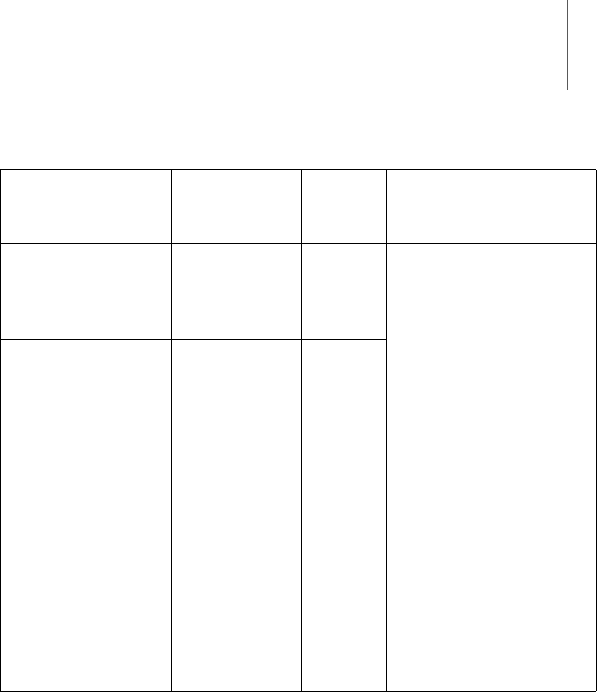
Appendix 41
Testing resistance
to interference
Test level
according to
IEC 60601-1-2
Compli-
ance
Guidelines for the elec-
tromagnetic environ-
ment
Conducted RF
interferences
according to
IEC 61000-4-6
3V
eff 3V Maintain safety
distance of mobile
radio equipment to
the Renamic
programmer; see
table 6.
The field strength of
stationary transmit-
ting devices must be
measured on site and
must be lower than
the compliance level
at all frequencies:
consider conducting a
study of the site.
The field strength
must be lower than
3 V/m over the
frequency range of
150 kHz to 80 MHz.
Radiated RF inter-
ferences according
to IEC 61000-4-3
3 V/m 80 MHz
to 2.5 GHz
3V/m
42 Appendix
Legend for the Label
The label icons symbolize the following:
Manufacturing date
BIOTRONIK order number
Serial number
Temperature limit for storage
Air pressure limit for storage
Humidity limit for storage
Non-sterile
Consult the instructions for use
Contents
Do not use if packaging is damaged
European approval mark
Caution: Federal (U.S.A.) law
restricts this product to sale by, or
on the order of, a physician.
NON
STERILE
Appendix 43
Device contains materials that
must be correctly disposed of in
accordance with environmental
protection regulations.
European Directive 2002/96/EC
regarding waste electrical and
electronic equipment (WEEE)
applies.
Return devices that are no longer
used to BIOTRONIK.
SafeSync Module
44 Appendix
Symbols on the Device
Symbols on the
front side
The symbols mean the following:
Symbols on the
back
The symbols mean the following:
Symbols on the left
side
The symbols mean the following:
SafeSync RF telemetry
WLAN or mobile connection
USB port
Consult the instructions for use
USB port

46 Directories
List of Keywords
C
Characteristics, 34
Cleaning, 18
Connect, 25
Connection of external devices
USB devices, 26
D
Damage, 16
Disinfecting, 18
Disposal, 19
Disposal of cables, 20
E
Electromagnetic compatibility, 38
Electromagnetic emissions, 38
Electromagnetic interference, 14
Test, 15
Electrostatic potentials, 13
Establishing a WLAN or mobile connection, 31
Establishing SafeSync RF telemetry, 29
Expertise, required, 11
I
Indicators during operation, 29
Installation site, 16
Intended use, medical, 10
Interrogating and programming the device, 30
M
Maintenance, 18
Maintenance, inspection, 19
Maintenance, test before each use, 19
R
Recommended safety distances, 39
Resistance to electromagnetic interference, 39
Risks, 12

48 Directories