BodyMedia 908901PROD2 Physiological monitoring device User Manual Summary operating manual
BodyMedia, Inc. Physiological monitoring device Summary operating manual
Summary operating manual

body monitoring system
Instructions For Use
SenseWear® software
SenseWear® Professional software
SenseWear® armband
and data collection

Introduction ........................................................2
Important Information About the SenseWear® armband ............................3
Retrieving armband Data to Your Computer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
Viewing and Analyzing the Data ..........................................13
Viewing and Editing Health Indicators . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
Advanced Graphing of the Data ..........................................18
Annotating the Data ..................................................20
Creating Reports ....................................................22
Exporting Data .....................................................24
Application Preferences ...............................................24
Using the Software to Congure an armband and display ..........................28
Features of the SenseWear® armband .......................................32
Important Troubleshooting Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 43
NOTE:
Read these instructions and the Warnings and Cautions on pages 2-6 before using the
Body Monitoring System.
TABLE OF CONTENTS
2
This manual will instruct you in the use of the
SenseWear® Body Monitoring System™. It covers
the routine operations and frequently-used
features of the SenseWear® software,
SenseWear® Professional software and the
SenseWear® armband.
If you have not done so already, please refer
to the Getting Started Guide included in the
System box to install the SenseWear software or
SenseWear Professional software and congure the
armband. After completing the initial set-up
(Step 3), you may proceed with this manual.
IMPORTANT
To run the SenseWear software or
SenseWear Professional software,
the minimum requirements for your
PC are Pentium III or higher, with at
least 256 MB of RAM, Windows 2000
SP3 or later/XP/Vista and a USB
port for the armband connection.
For the SenseWear Professional you
will need an additional USB port to
accommodate the license key.
Intended Use
The SenseWear® armband can be used as a monitor for applications such as: nutritional diagnos-
tics, metabolic diseases, pediatrics, pulmonary and cardiac studies, geriatrics, internal medi-
cine, occupational medicine, neurology, psychiatrics, sleep screening, and in general anywhere
it is necessary to monitor caloric and energy consumption, movement, physical activity, quality
of life, lifestyle, behavior and/or stress.
Important Information About the SenseWear® armband
Introduction
WARNINGS
This product complies with the general requirements for a safe medical device under applicable
directives. However, this product alone is not meant to substitute for proper medical diagnosis,
care, or treatment. Clinicians should not make drastic changes to a user’s lifestyle based solely
on data from the armband. The Body Monitoring System has been clinically validated for sub-
jects between 7 and 65 years of age who are engaged in resting, ambulatory, stationary biking,
motoring and weight-lifting activities, etc. Due to metabolic variations, subjects who are 1) out-
3
WARNINGS
side this age range or 2) engaged in alternate or obscure activities may see decreased accuracy
in the data. Any decisions based on the data from this device should be made only by medical or
paramedic personnel and should consider the condition and lifestyle of the subject tested. The
SenseWear® armband should not be used for life critical applications; improper usage may result
in harm or even death to the wearer.
This product is non-debrillation proof.
Do not get the device close to other devices that can cause electromagnetic interferences of
any nature.
EQUIPMENT not suitable for use in the presence of a FLAMMABLE ANAESTHETIC MIXTURE WITH
AIR or WITH OXYGEN OR NITROUS OXIDE.
Please be sure to verify equipment is connected and used compliant to UL1950.
Medical electrical equipment needs special precautions regarding EMC and needs to be installed
and put into service according to the EMC information provided on pages 7-10. Portable and
mobile RF communications equipment can affect medical electrical equipment.
The equipment or system should not be used adjacent to or stacked with other equipment and
if adjacent or stacked use is necessary, the equipment or system should be observed to verify
normal operation in the conguration in which it will be used.
The SenseWear® wireless communicator should not be used in airplanes, hospitals or locations
where cellular telephones or electronic devices are prohibited.
Keep the SenseWear® armband and wireless communicator out of reach of children. Both prod-
ucts contain smaller, removable parts which can become chocking hazards.
Important Information About the SenseWear® armband
4
WARNINGS
Important Information About the SenseWear® armband
Wear comfortably
Be careful not to over-tighten the armband while on your arm. If, at any time, you feel constric-
tion or loss of circulation, simply loosen the adjustable strap and re-fasten it to a more comfort-
able setting.
Be sure that both your arm and the sensors on the back of the armband are cleaned daily. To
clean the sensors, wipe with a soft, damp cloth. If you develop a rash where the armband comes
in contact with your skin, discontinue use and consult your physician before continuing regular
use of the armband. The design of the armband involved many materials experts, physicians,
and suppliers who are familiar with wearable materials and products. Each material was chosen
for its precedent in other skin contact products or has been independently approved for skin
contact. However, everyone’s skin is different and wearers with very sensitive skin may experi-
ence irritation or redness after wearing the armband. If this occurs, discontinue use and consult
your physician. If you have known metals allergies, you should consult your physician prior to
wearing. Do not wear armband when open sores are present
Check armband for sharp edges or damage before each use.
There have been reports of scratches/cuts associated with the Velcro tab of the strap. Also,
excessively hot armbands, such as those left in a car in the summer, may cause skin burns if they
are not allowed to cool before use.
When the armband is on the arm, DO NOT connect it to the USB cable.
Users with sensitive skin should avoid wearing the armband excessively. If you have sensitive
skin, remove the armband 1 hour for every 24 hours of wear time to reduce potential for skin
irritation.
5
Water resistance
DO NOT IMMERSE THE ARMBAND IN WATER. The monitor is not designed to be used underwater or
to come in continuous contact with water. To prevent a shock hazard, never use the armband in
water environments (e.g., in the shower, swimming pool, or rain). IPX0 classied.
Ordinary Protection, not protected against ingress to moisture.
Batteries
Batteries may explode or leak and can cause burn injury if recharged, disposed of in re, or dis-
assembled. Do not remove the battery label. Dispose of properly. For further information about
the disposal of the battery, please follow manufacturer’s instructions.
Batteries may present a choking hazard for small children.
Remove battery if armband will not be used for over 30 days.
Handling
Though the SenseWear® armband was designed for wearability and long-term use, it is a sensi-
tive monitoring device. Rough handling can break internal components. Never drop or shock the
armband and always store it in a safe place when not in use.
Avoid exposing the armband to extreme temperatures, direct sunlight, moisture, sand, dust, or
mechanical shock.
To prevent possible damage to the USB cable, grasp the plug end when disconnecting the USB
cable. Replace the cable if it becomes frayed.
WARNINGS
Important Information About the SenseWear® armband
CAUTIONS
6
CAUTIONS
Important Information About the SenseWear® armband
Dispose of device in accordance with local, state, federal, or country specic regulations.
Maintenance
Do not attempt to open the armband yourself. It contains no user-serviceable parts. Refer all
servicing to qualied Service Personnel. Opening the armband yourself will void the warranty.
Changes or modications to this equipment not expressly approved by the party responsible for
compliance could void the user’s authority to operate the equipment.
If the armband is dropped, ensure that it is working properly and not physically damaged before
relying on readings.
Cleaning
Always clean and dry the armband after vigorous sweating activities or when it becomes notice-
ably moist or dirty. Failure to keep the armband clean, or improper cleaning, may irritate the skin
and affect the sensor performance.
Moisten a soft cloth or towel with mild disinfectant soap and water. Wipe and dry the skin-touch-
ing side of the Armband. Never use solvents to clean the armband, only for disinfecting (see be-
low). The adjustable strap should be hand-washed with mild soap and warm water, then air-dried.
Machine drying may affect the performance and lifespan of the strap.
Disinfecting
Wipe back of armband with soft cloth dampened with 70% isopropyl alcohol. Allow armband to dry
for 5-10 minutes before wearing DO NOT STERILIZE THIS UNIT.
7
Flicker
IEC 6100-3-3
N/A
Harmonics
IEC 6100-3-2
N/A The 908901PROD2 is suitable for use in all establishments,
including domestic, and those directly connected to the public
low-voltage power supply network that supplies buildings
used for domestic purposes.
RF Emissions
CISPR 11
Class B, Group 1 The 908901PROD2 uses RF energy only for its inernal function.
Therefore, its RF emissions are very low and are not likely to
cause any interference in nearby electronic equipment.
The 908901PROD2 (SenseWear® armband) is intended for use in the electromagnetic environment specified below. The
customer or user of the 908901PROD2 should ensure that it is used in such an environment.
Guidance and Manufacturer’s Declaration - Emissions
Emissions Test Compliance Electromagnetic Environment - Guidance
Important Information About the SenseWear® armband
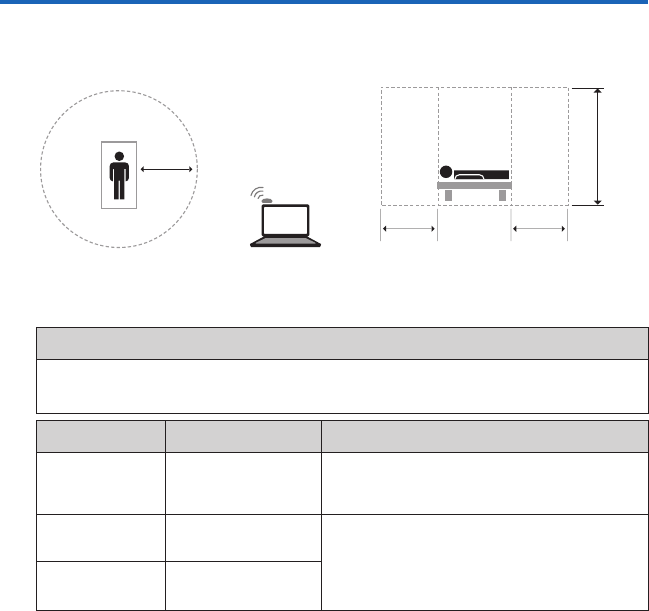
Computer and Wireless Communicator
1.5m
Diagram not to scale.
1.5m 1.5m
2.5m
Patient Environment
8
3A/m Power frequency magnetic fields should be that
of a typical commercial or hospital environment.
Power
Frequency 50/60Hz
Magnetic Field
IEC 61000-4-8
3A/m
N/A Mains power quality should be that of a typical
commercial or hospital environment. If the
user of the 908901PROD2 requires continued
operation during power mains interruptions, it is
recommended that 908901PROD2 be powered
from an uniterruptible power supply or battery.
Voltage Dips/
Dropout
IEC 61000-4-11
>95% Dip for
0.5 Cycles
60% Dip for 5 Cycles
30% Dip for 25 Cycles
>95% Dip for 5
Seconds
N/ASurge
IEC 61000-4-11
±1kV Differential
±2kV Common
Mains power quality should be that of a typical
commercial or hospital environment.
N/AEFT
IEC 61000-4-4
±2kV Mains
±1kV I/Os
±6kV Contact
±8kV Air
Floors should be wood, concrete, or ceramic
tile. If floors are synthetic, the r/h should be at
least 30%.
ESD
IEC 61000-4-2
±6kV Contact
±8kV Air
The 908901PROD2 (SenseWear® armband) is intended for use in the electromagnetic environment specified below. The
customer or user of the 908901PROD2 should ensure that it is used in such an environment.
Guidance and Manufacturer’s Declaration - Immunity
Immunity Test IEC 60601
Test Level
Compliance
Level
Electromagnetic
Environment - Guidance
Important Information About the SenseWear® armband
9
Important Information About the SenseWear® armband
3 Vrms
150 kHz to
80 MHz
3 V/m
80 MHz to
2.5 GHz
3 Vrms
150 kHz to
80 MHz
3 V/m
80MHz to
2.5 GHz
Portable and mobile communications equipment
should be separated from 908901PROD2 by no
less than the distances calculated/listed below:
D=(3.5/V1)(Sqrt P)
D=(3.5/E1)(Sqrt P)
80 to 800 MHz
D=(7/EI)(Sqrt P)
800 MHz to 2.5 GHz
Where P is the max power in watts and D is the
recommended separation distance in meters.
FIeld strengths from fixed transmitters, as deter-
mined by an electromagnetic site survey, should
be less than the compliance levels (V1 and E1).
Interference may occur in the vicinity of equip-
ment containing a transmitter.
•
•
•
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
The 908901PROD2 (SenseWear® armband) is intended for use in the electromagnetic environment specified below. The
customer or user of the 908901PROD2 should ensure that it is used in such an environment.
Guidance and Manufacturer’s Declaration - Emissions
IEC 60601
Test Level
Compliance
Level
Electromagnetic
Environment - Guidance
Immunity Test
10
Important Information About the SenseWear® armband
0.1 0.3689 0.3689 0.7378
The 908901PROD2 (SenseWear® armband) is intended for use in the electromagnetic environment specified below.
The customer or user of the 908901PROD2 can help prevent electromagnetic interference by maintaining a minimum
distance between portable and mobile RF Communications Equipment and the 908901PROD2 as recommended below,
according to the maximum output power of the communications equipment.
Recommended Separations Distances for the 908901PROD2
0.01 0.1166 0.1166 0.2333
Separation (m)
150kHz to 80MHz
D=(3.5/V1)(Sqrt P)
Separation (m)
80 to 800MHz
D=(3.5/V1)(Sqrt P)
Separation (m)
800MHz to 2.5GHz
D=(7/E1)(Sqrt P)
Max Output Power
(Watts)
1 1.1666 1.1666 2.3333
10 3.6893 3.6893 7.3786
100 11.6666 11.6666 23.3333
11
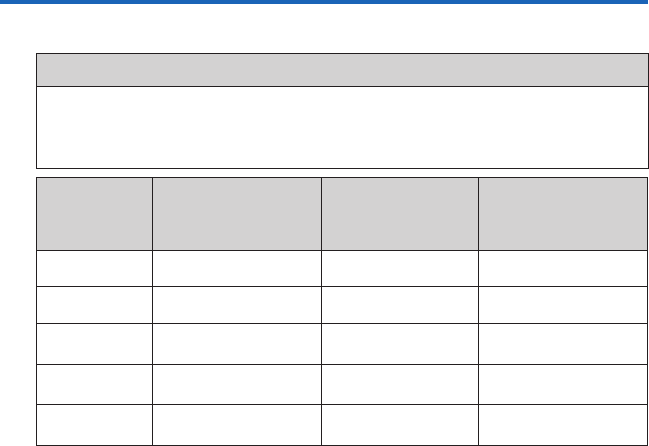
Retrieving armband Data to Your Computer
To start the program, double-click on the SenseWear® software or SenseWear® Professional*
software icon on your desktop. The main screen (gure 1) will appear as shown. There are three
buttons at the top under the menu bar: Retrieve Armband Data, View & Annotate Armband
Data and Congure Armband. Retrieve Armband Data will already be selected.
gure 1
Connect the USB cable to the armband and to
one of your computer’s USB ports. Then click
Retrieve to transfer data from the armband
to your PC.
A window will pop up (gure 2), requesting
you to enter a le name. Enter a name and
click Continue. The data le will be saved in
your default directory.
If you want to change the location of your
default directory, click Browse and select a
new destination directory.
*SenseWear Professional software requires the
License Key to be inserted into a USB port.
gure 2
TIP
When naming your les use numbers
rather than names to protect your
patients’ identities. For example,
Patient1234.
IMPORTANT
After the armband data has been
successfully retrieved and saved to
a data le, the data is cleared from
the armband. If you want the data to
remain on the armband then unselect
the Clear armband for next use…
checkbox before clicking Continue.
12
Retrieving armband Data to Your Computer
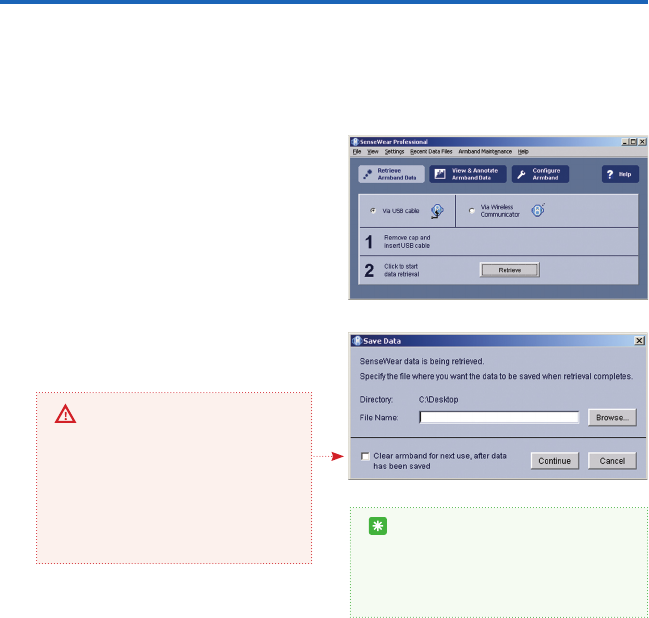
If the armband was not congured prior to
wearing, you will receive a pop up window
(gure 3) after the data has been retrieved.
The window will prompt you to enter the
wearer’s Subject Info and other important
information needed to derive accurate lifestyle
information. The subject information MUST be
entered to ensure that the software can ac-
curately analyze the raw data collected by the
armband. (see Getting Started Guide, Step 2,
Conguration).
The software lets you know that you have
successfully retrieved the data by a pop up
message that appears on your screen (gure
4). The newly retrieved data will be displayed
automatically.
gure 3
TIP
A new AAA battery can collect up to 14 days of continuous data. Keep in mind that the bat-
tery life is reduced under colder conditions. To avoid losing data or interrupting a longer
continuous data collection, pay particular attention to the battery level after retrieval
(gure 4). As a rule of thumb, 50% battery life will last about 7 days and 25% battery life
will last approximately 3 days. See page 32 for more information about battery life and
how to check the level from the armband.
gure 4
13
Viewing & Analyzing the Data
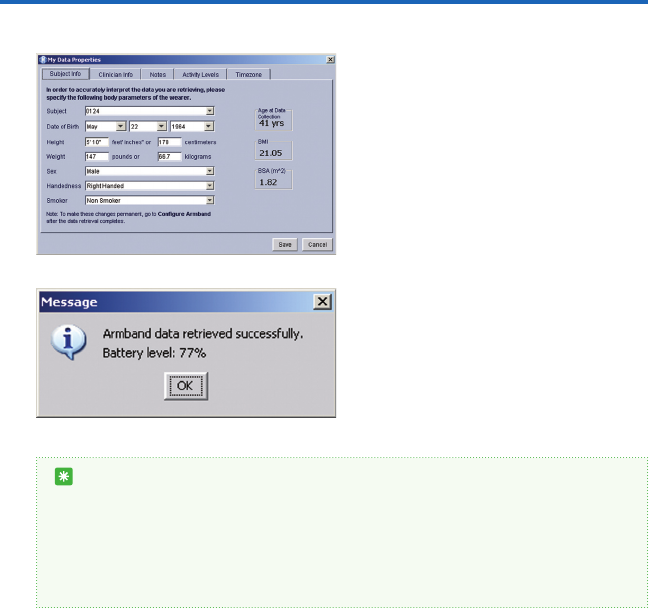
If you have just retrieved data from the
armband, then the data le will already be
displayed. If you would like to view a previ-
ously saved data le, go to File on your menu
bar and select Open Data File. A window
(gure 5) will open up to your default direc-
tory. Double click on the data le you wish
to open.
gure 5
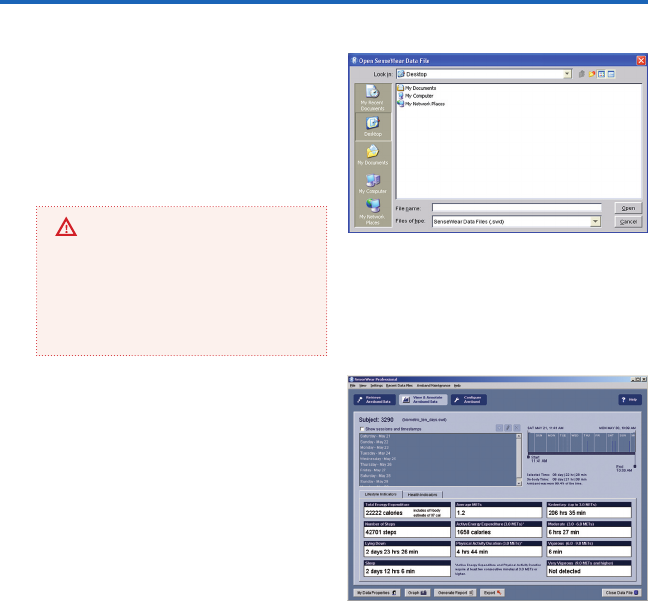
On the View & Annotate Armband Data
screen (gure 6), the total duration of the
le and on-body data collection times is
indicated on the interactive time selector
(gure 7) on the upper right of your screen.
The bottom half of the screen is a summary
of the Lifestyle Indicators and Health Indica-
tors. By default, the Lifestyle Indicators tab
will be selected, displaying a summary of the
derived armband data for the entire duration
of the collected data le. Clicking on the
Health Indicators tab will display a graph of
any available biometric data (blood glucose,
blood pressure, or weight) retrieved from the
armband.
IMPORTANT
SenseWear® software and SenseWear®
Professional software v. 6.0 are de-
signed to work with SenseWear Pro2 and
Pro3 armbands. The software does not
support earlier armbands or data les
by earlier armbands.
gure 6
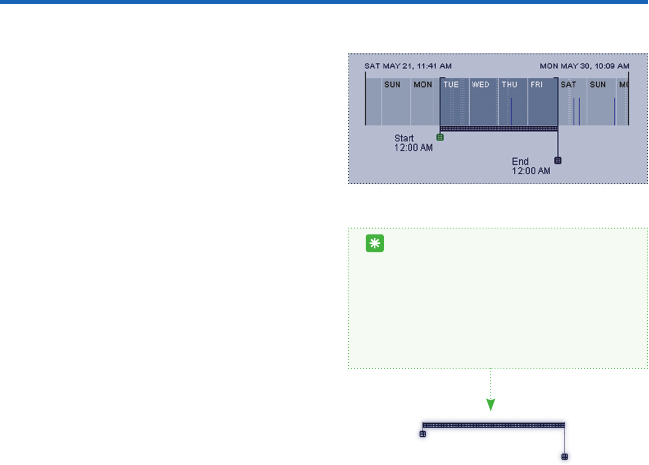
14
Viewing & Analyzing the Data
The date and time located to the left and
right just above the selector indicate the
total duration of the data le. Inside the
rectangle itself, the beginning of each day
(midnight) within this duration is marked
by the date and a thin vertical white line.
Solid-colored areas show periods when the
armband was on-body and collecting data.
Thin horizontal grey lines show periods when
the armband was off-body and not collect-
ing data. The shorter dark blue vertical lines
indicate that the Timestamp/Status button
was pressed.
You can highlight different time periods with-
in the entire duration of the collected data
with the time selector. To select only one
day from the time selector, click anywhere
on the day you wish to view. The selector
will automatically select that day (midnight
to midnight). To select multiple complete
days click on the rst day you would like to
view and shift-click on the nal day you want
displayed.
gure 7
Start
12:00 AM
End
12:00 AM
TIP
To select only one day from the interac-
tive time selector (Figure 7), simply
position your mouse on top of the day
you wish to view and click. You can then
drag the selected area to move this 24
hour time period.
To select start and end times, drag the time selectors below the horizontal box. You can then
move the selected time period by dragging the bar between the widgets. All values for these
selected times will automatically update on the right.
Click the Show Sessions and Timestamps checkbox under the Subject ID on the upper left of
your screen for a chronological list of all on-body sessions and Timestamps. The list will appear
in the window below the Subject ID. Clicking on a specic day or time on the list will also high-
light that item in the interactive time selector.
30
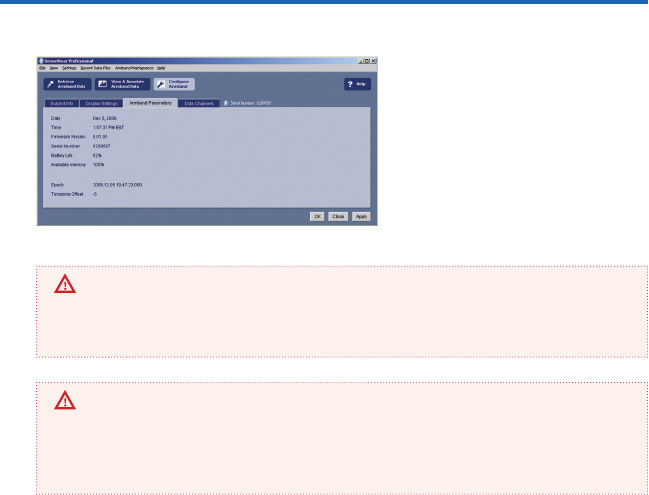
The armband Parameters tab (gure 27)
allows you to verify the date and time of
the armband clock. It also allows you to
check the hardware version, serial num-
ber, battery level, and memory capacity.
Using the Software to Configure an armband and display
gure 27
IMPORTANT
If the armband clock is not synchronized with your PC clock, a message may suggest that
you synchronize the clocks. If your PC clock time is the correct time, we recommend doing
this by clicking the Apply button.
IMPORTANT
If using the optional SenseWear® display, be sure to have your PC clock set with the same
time zone of the location you will be wearing the display. Because data is displayed in 24
hour increments, this will ensure the accuracy of your data. Please refer the SenseWear®
display Operating Manual for proper usage.
31
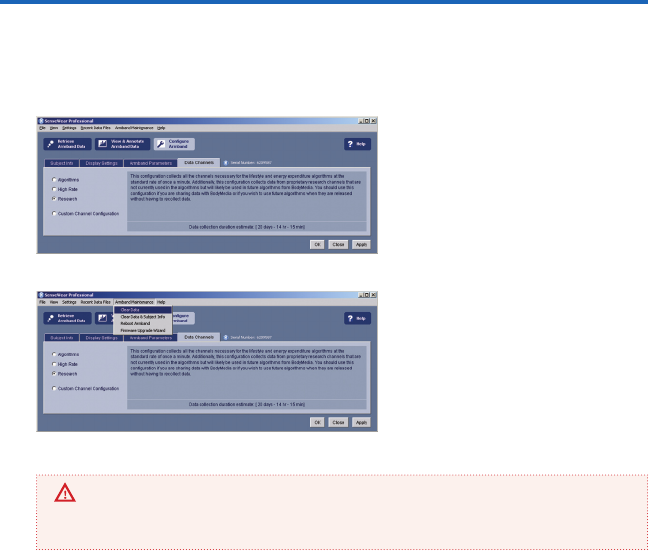
The SenseWear® Professional software includes the Data Channels tab (gure 22) feature.
This tab lets you congure the data channels to be collected and the sampling rates used
by each channel.
If you are not familiar with the low-level
physiological parameters the device
collects, we strongly recommend not
changing this section.
gure 28
Using the Software to Configure an armband and display
If you need to clear memory from an
armband, select Armband Maintenance
(gure 23) from the menu bar at the top
of your screen and select the appropriate
command from the list.
gure 29
IMPORTANT
Do not select Reboot Armband unless instructed to do so by a BodyMedia representative or
Technical Support Specialist. Unnecessary reboots of the device can result in data loss.
SenseWear® Professional software
32
Battery
The armband is powered by one AAA battery. During continuous use (24/7), it will last approxi-
mately 14 days.
To check the status of the battery, remove the armband and press the Timestamp/Status button.
The light above the word “battery” will turn on as follows:
Green (solid) = More than 24 hours of battery life remains.
Amber (ashing) = Less than 24 hours of battery life remains.
Red (ashing) = Battery life is very low and the armband will not collect data. Change the
battery before continuing use.
If you are wearing the armband, a subtle vibration and sound will alert you when there is less
than 24 hours of battery life remaining. When the battery is too low to operate, the alert will
become more urgent.
In addition to the battery status light on the armband, you can also view how much battery life
is left every time you retrieve your data (Figure 4).
•
•
•
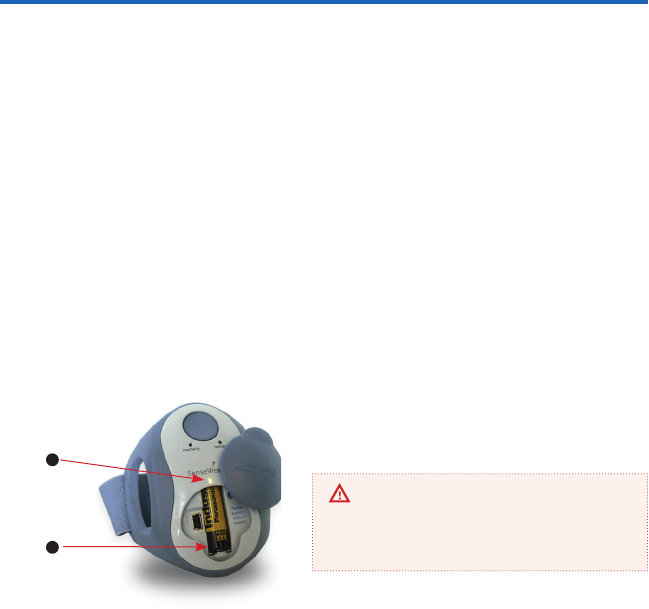
To replace the battery, lift and rotate the top hatch
counterclockwise. Remove the drained AAA battery
and replace it with a new AAA battery immediately.
Ensure proper disposal of batteries.
Features of the SenseWear® armband
+
–
WARNING
Once you remove the AAA battery, you have 30
seconds to replace it with the new one. Failure
to do so may lead to loss of data.
33
Memory
Under default congurations, the armband has approximately 10 days of data collection mem-
ory. To check the memory status, remove the armband and press the Timestamp/Status button.
The light above the word “memory” will turn on as follows:
Green (solid) = More than 24 hours of memory remains.
Amber (ashing) = Less than 24 hours of memory life remains.
Red (ashing) = Available memory is low and the armband will not collect data. Retrieve
your data before continuing use.
If you are wearing the armband, a subtle vibration and sound will alert you when there is less
than 24 hours of memory life remaining. When the memory is full, the alert will become more
urgent.
Wearing your armband
The SenseWear® armband is designed to be worn on the back of the upper right arm (the tri-
ceps), touching the skin.
Make sure that your upper right arm is clean and dry. You should not wear any lotion
or body oil where the armband will come in contact with your skin.
Slide the armband onto the back of your upper right arm with the SenseWear logo
facing up.
Adjust the strap so that it ts on your arm comfortably, then secure the oval pull-tab.
Flex the arm a few times to make sure that the strap is neither too tight nor too loose.
It should be snug, but comfortable. You do not need to adjust the strap again in the
future; just slide it on and off.
•
•
•
1.
2.
3.
Features of the SenseWear® armband
WARNING
Be careful not to overtighten the armband. If, at any time, you feel constriction or loss
of circulation, simply loosen the adjustable strap and refasten it to a more
comfortable setting.
34
Features of the SenseWear® armband
Threading your adjustable strap
Your SenseWear® armband comes with two identical adjustable straps. One strap comes at-
tached to the armband and the other is a spare to be used if you’re cleaning the rst one. See
the Cleaning section (page 6) of this manual for information on cleaning your adjustable strap.
Follow these steps to thread your adjustable strap into your armband.
Hold the armband upright with the sensors facing you. Take the square end of the adjust-
able strap with the two Velcro® pads and thread it through the narrow, vertical slot in the
wing. The Velcro® pads should be facing you, with the edge of the wing in between the
two pads.
Fold over the end of the strap and press the Velcro® pads together.
Take the other end of the adjustable strap with the oval pull tab and thread it through
the narrow, vertical slot in the other wing. The Velcro® side of the oval pull tab should be
facing you.
Pull the strap through the slot in the wing and press the oval pull tab against the adjust-
able band. Create a space that is approximately the size of the circumference of your
upper arm.
When completed, the square end of the strap should be on the inside of the band and the
oval pull tab should be on the outside of band. Use the oval pull tab to adjust the size of
the strap while on your arm.
1.
2.
3.
4.
5.
Two AAA Batteries (Part No.###)
WARNING
Do not use unapproved accessories with the armband.

35
Features of the SenseWear® armband
SenseWear® armband - Part No.100156 (US), 100153 (Europe-Italian),
100154 (Europe-English).
Wearable body monitor with multi-sensor array collects data directly off skin
of the wearer’s arm.
SenseWear® wireless communicator - Part No.100155 (US) and 100158 (Europe)
Directly connects to computer to wirelessly retrieve data off of armband.
USB Cable - Part No.100108 (US/Europe-English) and 100128 (Europe-Italian)
Connects wireless communicator or armband to computer.
SenseWear® armband Strap - Part No.100114 (Sm), 100115 (Med), 100116 (L)
Spare strap for armband.
Two AAA Batteries
•
•
•
•
A
B
C
D
E
BA
C
E
D

36
Features of the SenseWear® armband
Armband features
Gathers raw physiological data, including movement, heat ux, skin
temperature, near body temperature, and galvanic skin response.
Battery lasts approximately 14 days when worn continuously.
Stores approximately 10 days of continuous physiological and lifestyle data with default
conguration.
Product specications
Sensors:
Accelerometer (2-axis)
Heat Flux
Skin Temperature
Near Body Temperature
Galvanic Skin Response
Materials:
Monitor: ABS, urethane, FDA approved co-polyester, hypo-allergenic grade
stainless steel
Wireless Station: ABS
Adjustable Strap: Nylon, polyester, polyisoprene (no latex content)
Battery type: 1 AAA battery
Battery power: about 14 days under continuous use (24/7)
Memory capacity: about 10 days under continuous use (24/7) with default congurations.
Monitor size (without wings): (l) 85.3mm x (w) 53.4mm x (h) 19.5mm; [(l) 3.4 in x (w) 2.1
in x (h) 0.8 in]
Monitor weight (with adjustable strap): 2.8 oz (79 g)
Water resistance: splash-resistant
Operating temperature/humidity: 0° C to +45° C (32° F to 113° F)/100% RH
non-condensing
Storage temperature/humidity: 0° C to +45° C (32° F to 113° F)/100% RH non-condensing
Design and specications are subject to change without notice.
•
•
•
•
–
–
–
–
–
•
–
–
–
•
•
•
•
•
•
•
•

37
Features of the SenseWear® armband
Sensor Accuracy
Accelerometer
(2-axis)
Absolute range is +/- 2.00g
The minimum resolution is 0.01g
A two-standard-deviation range of +/-0.05g, up to 1.00g on
longitudinal axis
A two-standard-deviation range of +/-12.00% of expected value
otherwise on the longitudinal axis
A two-standard-deviation range of +/-0.06g up to 1.00g on the
transverse axis
A two-standard-deviation range of +/-12.00% of expected value
otherwise on transverse axis.
•
•
•
•
•
•
Heat Flux Range is from 0.00 W/m2 to 300.00W/m2
A minimum resolution of 1.00W/m2
A two-standard-deviation range of +/-10.00W/m2 at heat ux less
than 50W/m2
A two-standard-deviation range of +/-35.00% of expected value
otherwise
•
•
•
•
Galvanic Skin
Response
Range is from 56KW to 20MW (50.00 nSiemens – 17.00 uSiemens)
A two-standard-deviation range of +/- 7.00 nSiemen up to 233.34
nSiemens reading
A two-standard-deviation range of +/- 3.00% of expected value
otherwise
•
•
•
Skin Temperature Range is from 20.00ºC to 40.00ºC
A minimum resolution of 0.05ºC
A 2 standard deviation range of +/- 0.80°C across the
temperature range
•
•
•

38
Features of the SenseWear® armband
Follow operating instructions.
Caution
TYPE B APPLIED PART
The Waste Electrical and Electronic Equipment Regulations indicates
separate collection for electrical and electronic equipment.
Tested to applicable safety standards.
Identication code of Notied Body involved: 0051.
Classication of the device, as per 93/42 directives : IIa (rule 10)
Certication procedure : 93/42/EEC, Annex VI, VII.
Identication code of Notied Body involved: 0051
Transmit Power Class 8 - Less than 10mW output power
Duty Cycle Class 4 - permitted to operate at 100% duty cycle
Receiver Class 3 - Standard reliable SRD communication media

39
Features of the SenseWear® armband
FCC statement
NOTE: This equipment has been tested and found to comply with the limits for a Class B digital
device, pursuant to Part 15 of the FCC Rules. These limits are designed to provide reasonable
protection against harmful interference in a residential installation. This equipment generates,
uses, and can radiate radio frequency energy and, if not installed and used in accordance with
the instructions, may cause harmful interference to radio communications. However, there is
no guarantee that interference will not occur in a particular installation. If this equipment does
cause harmful interference to radio or television reception, which can be determined by turning
the equipment off and on, the user is encouraged to try to correct the interference by one or
more of the following measures:
Reorient or relocate the receiving antenna.
Increase the separation between the equipment and receiver.
Connect the equipment into an outlet on a circuit separate from
the receiver.
Consult the dealer or an experienced radio/TV technician for help.
CAUTION: Changes or modications to this equipment not expressly approved by the party
responsible for compliance could void the user’s authority to operate the equipment.
FCC 47CFR 15C TCB - 47 CFR Part 15 Subpart C Intentional Radiator
Certication Test
FCC 47CFR 15B clA - 47 CFR Part 15 Subpart B Unintentional Radiators Class A Verication
UL 60601-1 - UL Standard for Safety Medical Electrical Equipment, Part 1:
General Requirements for Safety First Edition
CENELEC EN 60601-1-2 - 2001 - Medical Electrical Equipment Part 1-2: General Requirements for
Safety - Collateral Standard: Electromagnetic Compatibility - Requirements and Tests IEC 60601-
1-2: 2001
•
•
•
•
•

40
Features of the SenseWear® armband
CENELEC EN 60601-1-1 - Medical Electrical Equipment - Part 1: General Requirements for Safety
- Collateral Standard: Safety Requirements for Medical Electrical Systems.
CAN/CSA-C22.2 No.606.1-M90
ETSI EN 301 489-1 - Electromagnetic Compatibility and Radio Spectrum Matters (ERM); Elec-
troMagnetic Compatibility (EMC) Standard for Radio Equipment and Services; Part 1: Common
Technical Requirements V1.3.1
ETSI EN 301 489-3 - (Draft) Electromagnetic Compat. and Radio Spectrum Matters (ERM); Har-
monized EN for ElectroMag. Compatibility (EMC) of Radio Comms. Equip. & Srvs.; Pt. 3: Specic
Conditions for Short-Range Devices (SRD) Operating on Freqs Between 9 KHz and 40 GHz V1.3.1
ETSI EN 300 440-1 V1.3.1 (2001-07) Electromagnetic compatibility and Radio spectrum Matters
(ERM);Short range devices; Radio equipment to be used in the 1 GHz to 40 GHz frequency range