Boston Scientific Neuromodulation PSC1110W Precision SCS System Implantable Pulse Generator User Manual II Physician Trial Kit Insert
Boston Scientific Neuromodulation Corporation Precision SCS System Implantable Pulse Generator II Physician Trial Kit Insert
Contents
- 1. Patient Handbook
- 2. Patient Trial Guide
- 3. Physicians Implant Manual
- 4. Physician Trial Kit Insert
- 5. Lead Manual
Physician Trial Kit Insert
Physician Trial Kit
Copyright 2004, Advanced Bionics® Corporation. All Rights Reserved.
Part No. MP9055214 Rev. A
Physician Trial Kit Model SC-7005-02
Trial Stimulator Model SC-5110
Remote Control Model SC-5210
CAUTION:
Federal law restricts this device to sale, distribution
and use by or on the order of a physician.
The Advanced Bionics® Trial Stimulator is intended
to be used during a screening trial to assess the
efficacy of spinal cord stimulation in the treatment of
patients with chronic pain of the trunk or limbs. The
Trial Stimulator communicates with, and is
controlled by, both a patient programmer (the
Remote Control) and a clinician programmer via a
wireless link.
The Remote Control unit is a hand-held device for
patient control of both the external Trial Stimulator
and the permanent, implantable pulse generator
(IPG) models of the Precision™ SCS device.
Following set up of the Remote Control by the
clinician, the Trial Stimulator is able to respond to
patient control of:
• stimulation on/off functions
• stimulation amplitude and other (optional)
parameters
• stimulation delivery areas
• individualized stimulation therapy programs.
Patient use instructions are outlined in the Patient
Trial Handbook.
CAUTION:
• To avoid unexpected stimulation results, never
make connections while the Trial Stimulator is
on.
• The battery used in the Trial Stimulator may
present a risk of fire or chemical burn if mis-
treated. Do not disassemble, heat above 100ºC
(212ºF), or incinerate.
• Handle the devices with care. Although dura-
bility testing has been performed to ensure
quality manufacturing and performance, drop-
ping the devices on hard surfaces or in water,
or other destructive handling, can cause per-
manent damage.
• Avoid all sources of water that can come into
contact with the devices.
• Do not expose the devices to extreme tempera-
tures. Store between 5 – 40 ºC (41 – 104 ºF).
• Do not dispose of the devices in fire.
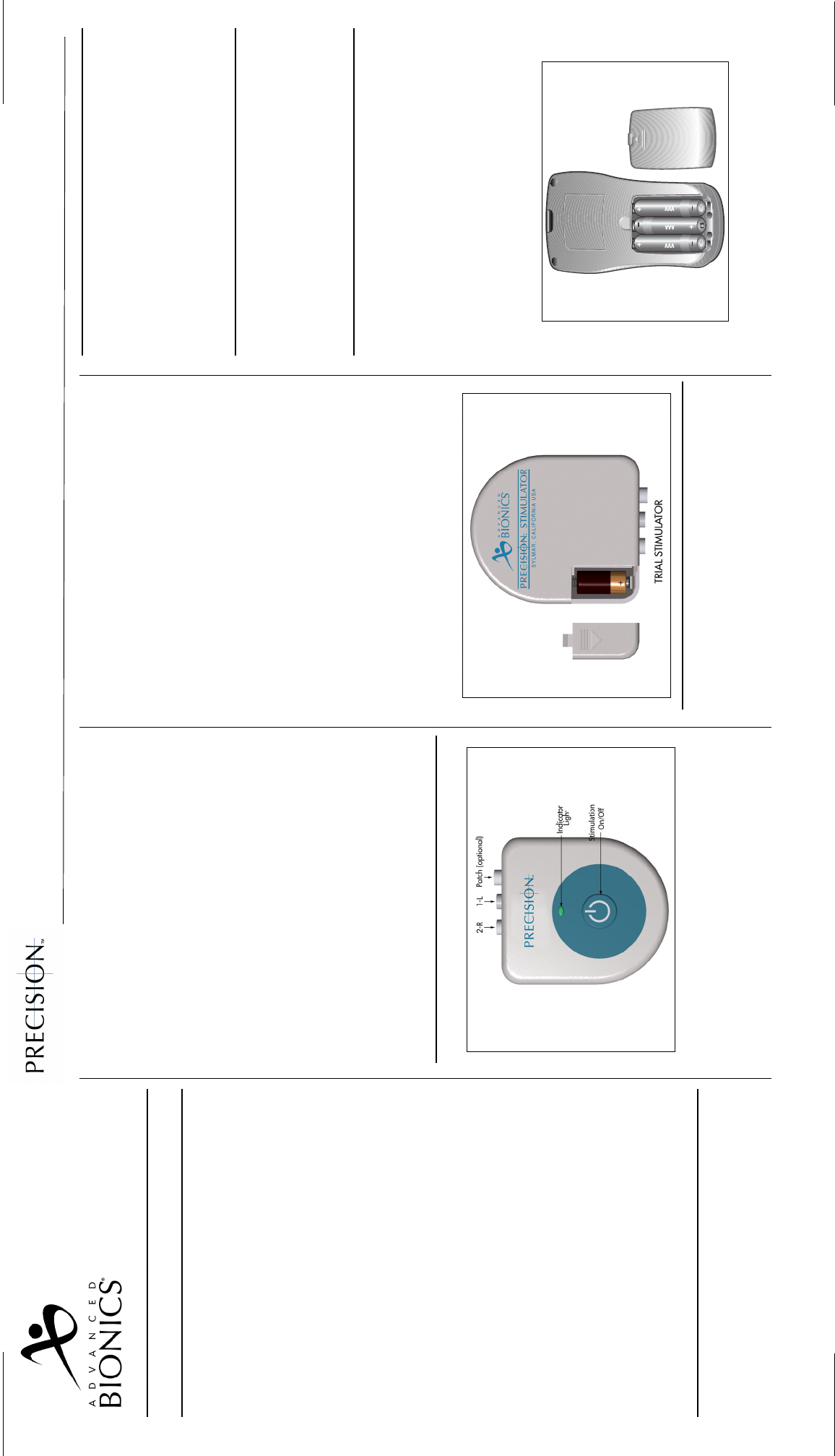
Trial Stimulator
General Instructions
1. Ensure that the Trial Stimulator is equipped with
a new 6-volt battery before conducting intraop-
erative stimulation testing, and for each new
trial. (A battery is included in the Patient Trial
Kit.)
2. Before connecting the OR Cable for intraopera-
tive testing, ensure that the Trial Stimulator is
off (no indicator light). Turn the stimulator on
for testing, then return it to off before discon-
necting the test cables.
3. When fitting the patient with the Trial Stimula-
tor, ensure that the OR Cables are properly con-
nected and labelled. Superior (upper or left)
leads connect to socket 1-L. Inferior (lower or
right) leads connect to socket 2-R. If only a sin-
gle lead is being used, connect it to 1-L.
4. Before releasing the patient, ensure that he/she
understands:
• Basic control of the Trial Stimulator using the
Remote Control, and that stimulation may be
turned on and off with the E button on either
unit.
• How to disconnect and reconnect the OR
Cable(s)
with the power off.
• How to replace the Trial Stimulator battery.
(See illustration below)
NOTE: Immediately following a Trial Stimulator
battery replacement, the linked Remote Control
will display an error/reset message. Press D to
reset the stimulator .
Remote Control
Battery Information
The Remote Control uses three AAA batteries. The
device will issue warnings when its batteries are low
and when the Trial Stimulator battery needs
replacement. Instruct patients to pay close attention
to all battery messages.
NOTE: The first time it comes on following a
battery replacement, the Remote Control will
reload information from the implanted or exter-
nal stimulator. The remote must be within com-
munication range (two feet) of the stimulator
during this period.
Battery Replacement
1. On the rear of the remote, push in slightly and
slide the battery compartment cover down.
2. Remove the old batteries.
3. Place the new batteries in the slots by matching
the positive (+) and negative (-) battery ends
with the + / - markings in the compartment.
4. Align the cover on the case and slide the cover
into position util it snaps closed.
DRAFT
Physician Trial Kit Model SC 7005-02
Copyright 2004, Advanced Bionics® Corporation. All Rights Reserved.
Part No. MP9055214 Rev. A
General Instructions
1. To turn stimulation on or off, press the E but-
ton.
If the Remote Control is in sleep/idle mode and
you press any button other than E, the remote
will display a “Keys Locked” message. You must
press Dto unlock the “function” buttons when
reactivating the Remote Control from sleep
mode.
2. To increase or decrease the stimulation level of
all programmed therapy areas, press either S or
T.
3. To change the stimulation level of just one area:
a) From the level screen, press Cas many times
as necessary to find the desired area by name or
number.
b) Press S or T to make the change.
4. To activate a stimulation program:
a) Press D from the level screen.
b) From the program screen press D as many
times as necessary to highlight the desired pro-
gram.
c) Press S to activate the selected program.
Once the program is running, you may change
the stimulation level by pressing S or T.
To save the new level in a program, return to the
program screen, select the intended program
number, and press T.
5. To access patient options:
Rate
1. Press and hold Cfrom the level screen to acti-
vate the Rate Area 1 screen.
2. Press C(normal press) as necessary to cycle to
the area you want to adjust, then press S or T
to increase or decrease the Rate.
Pulse Width
1. From the level screen press and hold Cto access
the Rate Area 1 screen.
2. From
any
Rate screen, press and hold Cagain to
access the Width Area 1 screen.
3. Press C(normal press) as necessary to cycle to
the desired area.
4. Press S or T to increase or decrease Pulse
Width.
To save a Rate or Pulse Width change--
1. Press Dfrom the level screen.
2. From the program screen press Das necessary to
cycle to the active program.
3. With the program highlighted, press T to save
changes.
Restore
1. Press and hold Dto access the Restore screen.
2. Press Dagain as necessary to highlight the pro-
gram you want to restore, then press S.
3. Respond to the confirmation screen to complete
the operation.
Maintenance
The Trial Stimulator and Remote Control may be
cleaned with a cloth lightly dampened with water or
a mild household cleaner. Do not use abrasive
cleansers on the devices, and do not sterilize them.
Technical Service
If you have questions concerning the Remote Control
or the Trial Stimulator, please contact Advanced
Bionics’ Technical Service Department:
• Phone: (866) 566-8913
• Fax: (661) 362-1503
• Address: Advanced Bionics® Corporation
Pain Management Division
25129 Rye Canyon Loop
Valencia, CA 91355
Limited Warranty
Advanced Bionics® warrants to the purchaser that the Precision™
Trial Stimulator and Remote Control are free from defects in
workmanship and materials for a period of one (1) year from the
date of purchase.
A Stimulator or Remote Control unit that fails to function within
normal tolerances within one (1) year from the date of purchase is
covered under this Limited Warranty. The liability of Advanced
Bionics® under this warranty shall be limited to: (a) replacement
with a functionally equivalent component; or (b) full credit equal
to the original purchase price to be applied towards the purchase
of a replacement device. Product claims under Advanced Bionics®
Limited Warranty are subject to the following conditions and
limitations:
The product registration card must be completed and returned to
Advanced Bionics® within 30 days of receipt of product in order
to obtain warranty rights.
The component failure must be confirmed by Advanced Bionics®.
This warranty specifically excludes defects or malfunctions caused
by: (a) fire, floods, lightning, natural disasters, water damage and
other calamities commonly defined as “Acts of God”; (b) accident,
misuse, abuse, negligence, failure to operate the system and its
components in accordance with manufacturer’s instructions; (c)
unauthorized attempts to repair, maintain, or modify the
equipment; or (d) attachment of any equipment not supplied by
Advanced Bionics® without prior approval.
The decision as to product replacement or credit shall be made
solely at the discretion of Advanced Bionics®. For a replacement
component, the warranty will run only to the end of the warranty
period for the original component that was replaced.
This warranty is in lieu of any other warranty, expressed or
implied, including any warranty of merchantability or fitness for
intended use. Except as expressly provided by this Limited
Warranty, Advanced Bionics® shall not be responsible or liable for
any direct, consequential or incidental damages caused by device
malfunction, failure or defect, whether the claim is based on
warranty, contract, tort or otherwise.