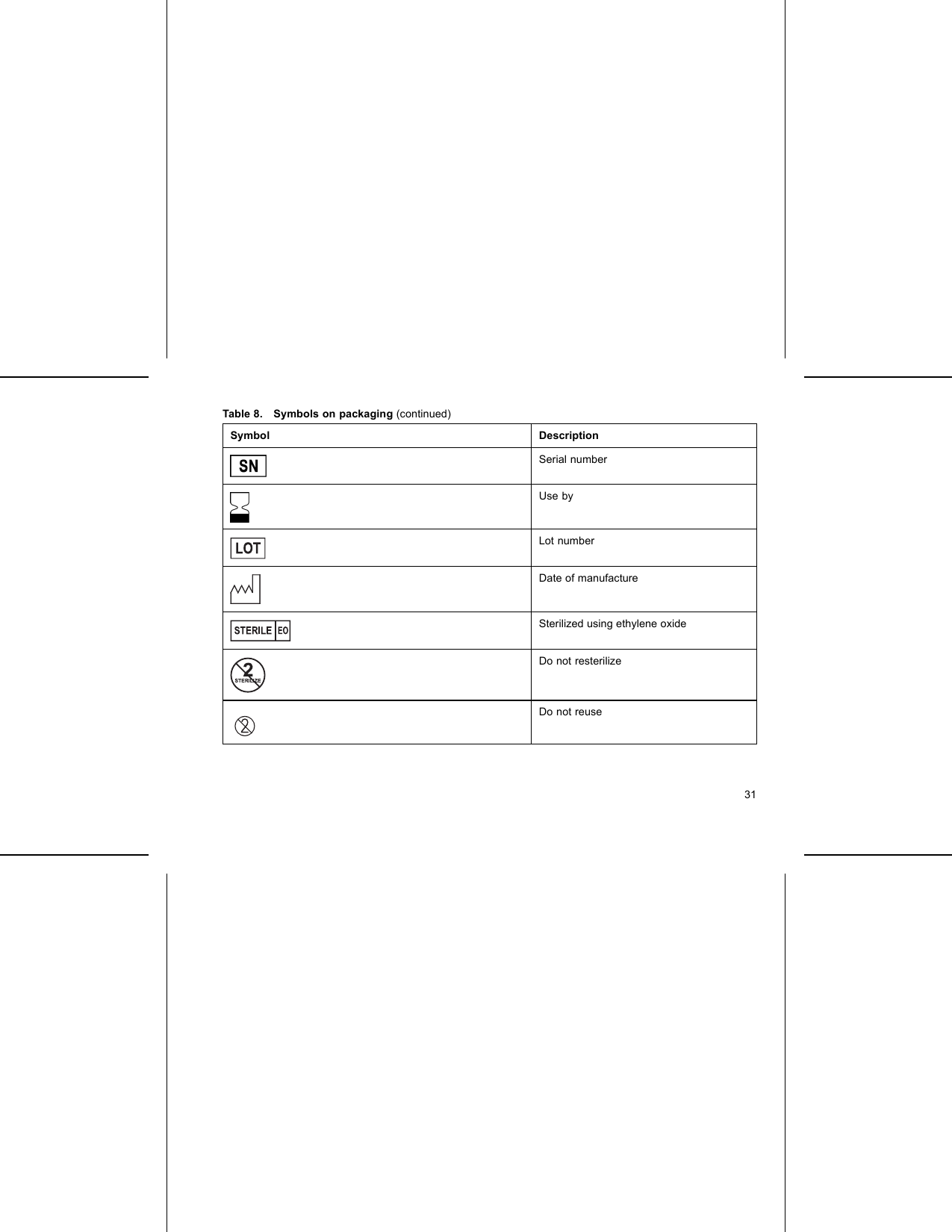
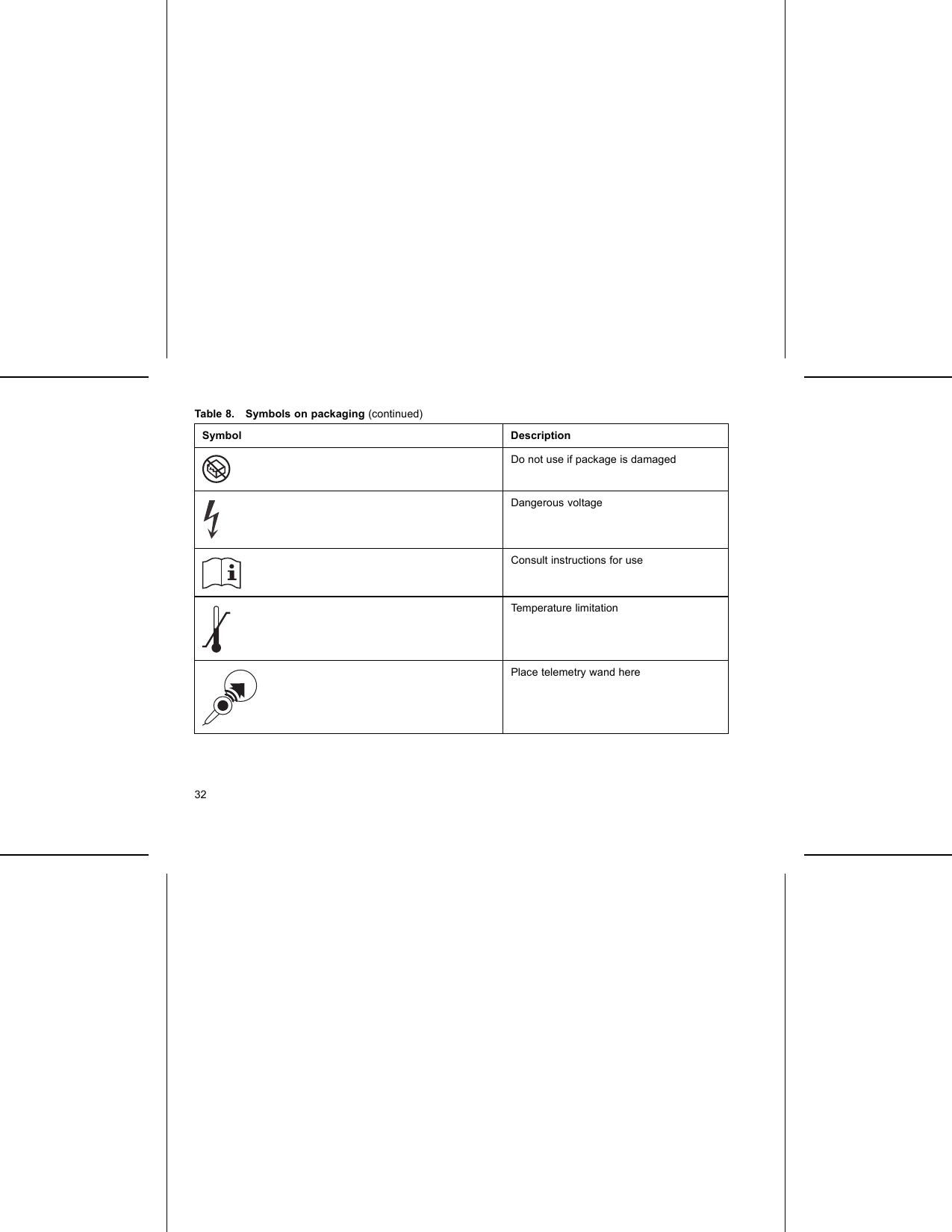
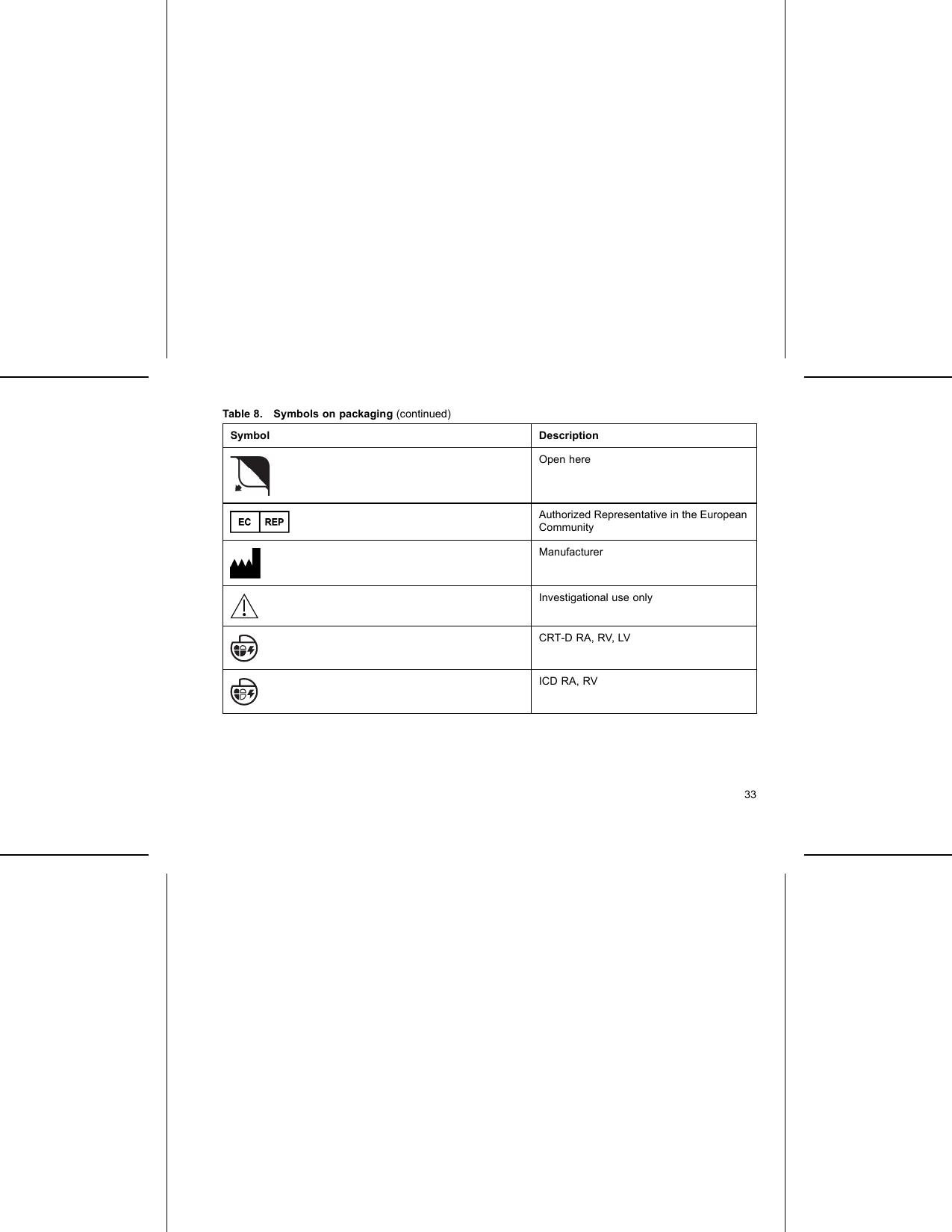
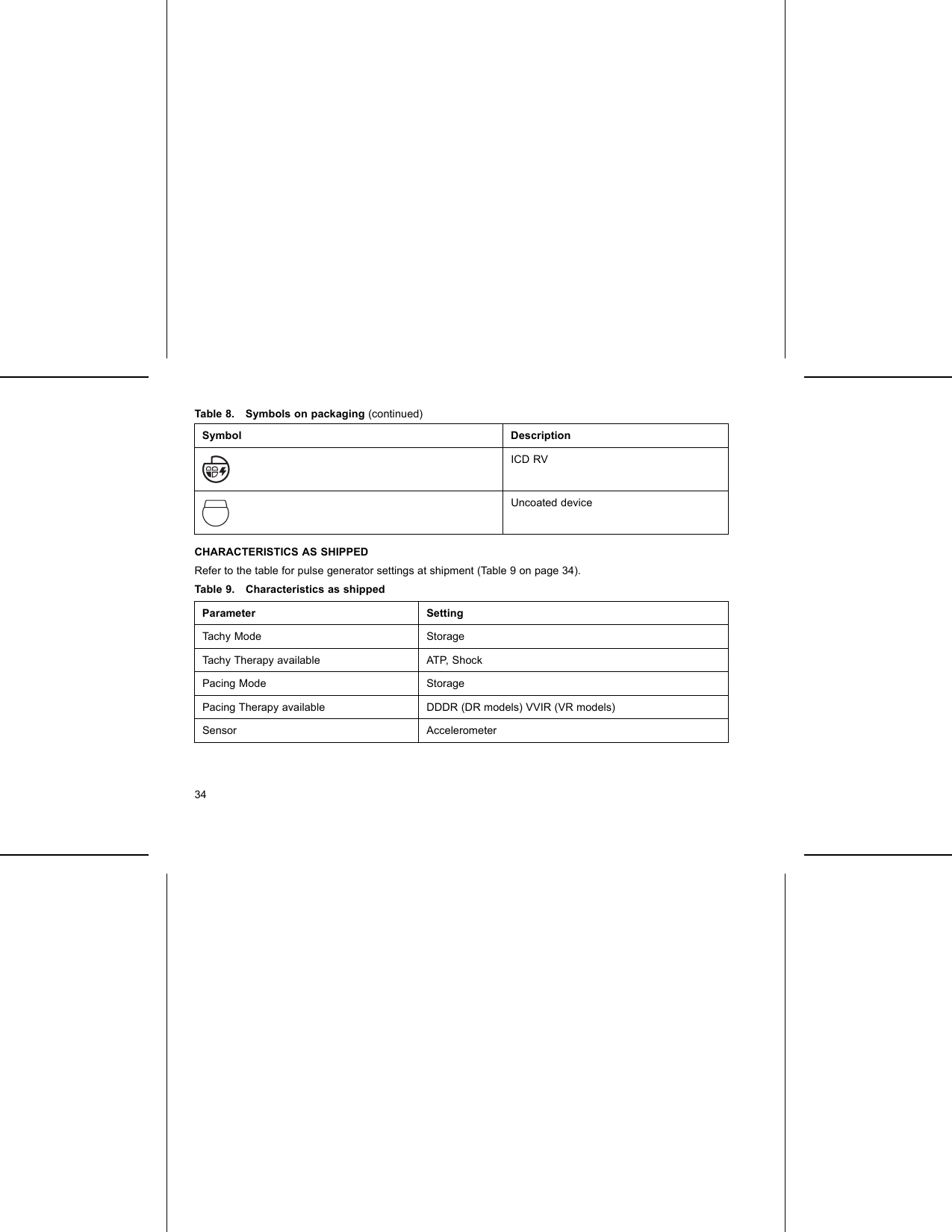
Boston Scientific CRMG17912 G168,G160,G166,G161,G164,D032,D030,D033,D031,D162,D160,D163,D161,G158,G150,G156,G151, G154,D022,D020,D023,D021,D152,D150,D153,D151,G148,G140,G146,G141,D012,D010,D013,D011, D142,D140,D143,D141,G058,G050,G056,G051,D001,D000,D003,D002,D052,D050,D053,D05 User Manual Updated 2
Boston Scientific Corporation G168,G160,G166,G161,G164,D032,D030,D033,D031,D162,D160,D163,D161,G158,G150,G156,G151, G154,D022,D020,D023,D021,D152,D150,D153,D151,G148,G140,G146,G141,D012,D010,D013,D011, D142,D140,D143,D141,G058,G050,G056,G051,D001,D000,D003,D002,D052,D050,D053,D05 Updated 2
Updated user manual 2























![• Chronic nerve damage• Component failure• Conductor coil fracture•Death• Elevated thresholds•Erosion• Excessive fibrotic tissue growth• Extracardiac stimulation (muscle/nerve stimulation)• Failure to convert an induced arrhythmia• Fluid accumulation• Foreign body rejection phenomena• Formation of hematomas or seromas• Heart block• Heart failure following chronic RV apical pacing• Inability to defibrillate or pace• Inappropriate therapy (e.g., shocks and antitachycardia pacing [ATP] where applicable, pacing)• Incisional pain• Incomplete lead connection with pulse generator• Infection including endocarditis• Insulating myocardium during defibrillation with internal or external paddles• Lead dislodgment• Lead fracture• Lead insulation breakage or abrasion24](https://usermanual.wiki/Boston-Scientific/CRMG17912.Updated-user-manual-2/User-Guide-2033790-Page-28.png)

![X-RAY IDENTIFIERThe pulse generator has an identifier that is visible on x-ray film or under fluoroscopy. This identifier providesnoninvasive confirmation of the manufacturer and consists of the following:• The letters, BSC, to identify Boston Scientific as the manufacturer• The number, 140, to identify the Model 2868 PRM software application needed to communicate with thepulse generatorThe x-ray identifier is embedded in the header of the device. For a left side pectoral implant, the identifier willbe visible by x-ray or fluorography at the approximate location shown (Figure 1 on page 36).123[1] X-Ray Identifier [2] Header [3] Pulse Generator CaseFigure 1. X-ray identifierFor information on identifying the device via the PRM, refer to the PRM Operator’s Manual.36](https://usermanual.wiki/Boston-Scientific/CRMG17912.Updated-user-manual-2/User-Guide-2033790-Page-40.png)





![435DF4-LLHHIS-1 BIRVRA12[1] RA: White [2] RV: Red [3] RA (-) [4] Suture Holes [5] RV (-)Figure 2. Lead connections and setscrew locations, RA: IS-1, RV: DF4-LLHH32DF4-LLHH RV1[1] RV: Red [2] RV (-) [3] Suture HolesFigure 3. Lead connections and setscrew locations, RV: DF4-LLHH45](https://usermanual.wiki/Boston-Scientific/CRMG17912.Updated-user-manual-2/User-Guide-2033790-Page-49.png)
![95678IS-1 BI RV–+RADF-1DF-1IS-1 BI1 24 3[1] Defib(-): Red[2]Defib (+): Blue [3] RA: White [4] RV: White [5] Defib(+)[6]Defib (-) [7] RA (-) [8]RV (-) [9] Suture HoleFigure 4. Lead connections and setscrew locations, RA: IS-1, RV: IS-1/DF-146](https://usermanual.wiki/Boston-Scientific/CRMG17912.Updated-user-manual-2/User-Guide-2033790-Page-50.png)
![7456IS-1 BI RV–+DF-1DF-1123[1] Defib(-):Red[2]Defib(+): Blue[3]RV:White[4]Defib(+)[5]Defib (-) [6] RV (-) [7] Suture HoleFigure 5. Lead connections and setscrew locations, RV: IS-1/DF-1NOTE: The pulse generator case is used as a defibrillating electrode unless the pulse generator has beenprogrammed to the Distal Coil to Proximal Coil (or “Cold Can”) Shock Vector.IMPLANTING THE PULSE GENERATORImplant the pulse generator by performing the following steps in the sequence provided. Some patientsmay require pacing therapies immediately upon connecting the leads to the pulse generator. In such cases,consider programming the pulse generator before or in parallel with implanting the lead system and formingthe implantation pocket.Step A: Check EquipmentIt is recommended that instrumentation for cardiac monitoring, defibrillation, and lead signal measurementshould be available during the implant procedure. This includes the PRM system with its related accessories47](https://usermanual.wiki/Boston-Scientific/CRMG17912.Updated-user-manual-2/User-Guide-2033790-Page-51.png)















![20°–30°[1] Clockwise rotation to free setscrews stuck in the retracted position [2] Counterclockwise rotation to freesetscrews stuck in the extended positionFigure 7. Rotating the torque wrench to loosen a stuck setscrewFOLLOW UP TESTINGIt is recommended that device functions be evaluated with periodic follow-up testing by trained personnel.Follow up guidance below will enable thorough review of device performance and associated patient healthstatus throughout the life of the device.WARNING: Ensure that an external defibrillator and medical personnel skilled in CPR are present duringpost-implant device testing should the patient require external rescue.65](https://usermanual.wiki/Boston-Scientific/CRMG17912.Updated-user-manual-2/User-Guide-2033790-Page-69.png)