Cardionet 1014 C5 Ambulatory ECG Monitoring System User Manual FCC Part 15
Cardionet C5 Ambulatory ECG Monitoring System FCC Part 15
Manual

3998 FAU Blvd. Suite 310 Boca Raton, FL 33431 Tel: 561-961-5585 Fax: 561-961-5587
Certification Exhibit
FCC ID: QBI-1014
FCC Rule Part: 15.247
ACS Project: 16-2030
Manufacturer: Cardionet
Model(s): 100-0025-01, 900-0604-00
User Manual

CARDIONET MCOT
TM
MANUAL
MOBILE CARDIAC OUTPATIENT TELEMETRY TM

Mobile Cardiac Outpatient Telemetry™
Better Detection. Better Repor
tin
g.
V
alidated by Clinic
al Data.
We’d know about it.

CARDIONET LIMITED WARRANTY
CardioNet products are warranted to be free from manufacturing and material defects for a
period of one (1) year from the date of shipment from CardioNet to the original purchaser.
This warranty does not apply to any product which CardioNet determines has been modied
or damaged by the customer. Excluded from this warranty are expendable supply items
including, but not limited to, electrodes, lead wires, and batteries. Except for the express
warranties stated above, CardioNet disclaims all warranties including implied warranties
of merchantability and tness. The stated express warranties are in lieu of all obligations of
liabilities on the part of CardioNet for damages, including but not limited to, special indirect
or consequential, arising out of or in connection with the use or performance of CardioNet
products. Any action for breach of warranty shall be commenced within one (1) year of said
breach or be forever barred. Any repairs made to the product, which are not covered by
the warranty, shall be billed to the customer. Device is to be serviced by Factory Authorized
Technicians only. Do not attempt to repair, modify, or service the CardioNet CN1006 MCOT
system. Do not attempt to open or tamper with MCOT System. Opening the case will void
product warranty.
CARDIONET MCOT TM MANUALCARDIONET MCOT TM MANUAL
MOBILE CARDIAC OUTPATIENT TELEMETRY
TM
TABLE OF CONTENTS
01. OVERVIEW....................................................................................... 1
02. INDICATIONS FOR USE.................................................................. 2
03. PRECAUTIONS................................................................................ 4
04. CAUTIONS....................................................................................... 5
05. WARNINGS...................................................................................... 6
06. MEDICAL PRACTICE INSTRUCTIONS........................................... 7
07. KIT CONTENTS................................................................................ 8
08. MONITOR AND SENSOR................................................................ 9
09. ATTACHING THE ELECTRODES & SENSOR TO YOUR SKIN........ 10
10. GETTING STARTED WITH MONITORING...................................... 12
11. RECORDING SYMPTOMS............................................................... 13
12. CHARGING THE CARDIONET MONITOR...................................... 14
13. RECEIVING TEXT MESSAGES......................................................... 15
14. SKIN CARE & REPLACING ELECTRODES...................................... 16
15. SHOWERING, BATHING AND SWIMMING.................................... 17
16. TROUBLESHOOTING....................................................................... 18
17. RETURNING THE UNIT.................................................................... 19
18. EQUIPMENT SYMBOLS................................................................... 20
19. SPECIFICATIONS.............................................................................. 21

CARDIONET MCOT TM MANUAL 1
CARDIONET MCOT TM MANUAL
01.
OVERVIEW
The CardioNet Mobile Cardiac Outpatient Telemetry TM (MCOT TM) system is an ambulatory
ECG Monitor with the capability to detect cardiac arrhythmias and transmit ECG data to a
staed Monitoring center.
The subject device is comprised of two (2) main components: 1) a patient-worn Sensor, and
2) a Monitor
A Sensor acquires the ECG signal from the patient’s body and transmits the signal to a
PDA sized Monitor where the data is stored and analyzed by an automated arrhythmia
detection algorithm residing in the Monitor. When events are detected by the analysis
algorithm or when indicated by the patient pressing the Record Symptom button on the
Monitor, the Monitor will transmit the data to the Monitoring Center. Data is uploaded
to the Monitoring Center via Cellular RF modem transmission. The data is received and
reviewed by trained technicians at the Monitoring Center.
Caution: U.S. federal law restricts this device to sale by or on the order of a physician.
CARDIONET MCOT TM MANUAL
2
MOBILE CARDIAC OUTPATIENT TELEMETRY
TM
02.
INDICATIONS FOR USE
1. Patients who have a demonstrated need for cardiac monitoring. These may include but are
not limited to patients who require monitoring for: a) non-life threatening arrhythmias such
as supraventricular tachycardias (e.g. atrial brillation, atrial utter, PACs, PSVT) and ventricular
ectopy; b) evaluation of bradyarrhythmias and intermittent bundle branch block, including after
cardiovascular surgery and myocardial infarction; and c) arrhythmias associated with co-morbid
conditions such as hyperthyroidism or chronic lung disease
2. Patients with symptoms that may be due to cardiac arrhythmias. These may include but are not
limited to symptoms such as: a) dizziness or lightheadedness; b) syncope of unknown etiology
in which arrhythmias are suspected or need to be excluded; and c) dyspnea (shortness of breath).
3. Patients with palpitations with or without known arrhythmias to obtain correlation of rhythm
with symptoms.
4. Patients who require monitoring of antiarrhythmic therapy: a) Monitoring of therapeutic and
potential proarrhythmic eects of membrane active drugs, b) Monitoring of eect of drugs to
control ventricular rate in various atrial arrhythmias (e.g. atrial brillation).
5. Patients recovering from cardiac surgery who are indicated for outpatient arrhythmia monitoring.
6. Patients with diagnosed sleep disordered breathing including sleep apnea (obstructive, central)
to evaluate possible nocturnal arrhythmias.
7. Patients requiring arrhythmia evaluation of etiology of stroke or transient cerebral ischemia,
possibly secondary to atrial brillation or atrial utter.
8. Patients requiring measurement, analysis and reporting of QT interval, excluding patients with
a documented history of sustained atrial brillation or atrial utter.
9. Patient who require monitoring for potential arrhythmias based on risk factors (e.g. atrial brillation).
10. Patients requiring measurement of ST segment changes. The device is not intended to
sound any alarms for ST segment changes.

CARDIONET MCOT TM MANUAL 3
CONTRAINDICATIONS:
1. Patients with potentially life-threatening arrhythmias who require inpatient monitoring.
2.Patients who the attending physician thinks should be hospitalized.
3. This device should not be used for monitoring of QT interval during the initiation of
antiarrhythmic therapy, where in-hospital monitoring is required by the labeling of that drug.
4. The device does not replace the QT interval measurement by a trained observer using
diagnostic 12-lead ECG in a clinical environment. This device is not intended to sound any
alarms for QT interval changes.
5. The device does not annotate QT interval for QRS durations >160 ms or for T wave amplitudes
≤5% of the peak QRS amplitude.
FOR USE ON ADULT AND PEDIATRIC PATIENTS ONLY
The CardioNet MCOT System is intended for use on adults and children. It is not intended to be
used on infants weighing less than 10kg (22 lbs).

CARDIONET MCOT TM MANUAL
4
MOBILE CARDIAC OUTPATIENT TELEMETRY
TM
03.
PRECAUTIONS
A. DISPOSE OF BATTERIES PROPERLY
Observe all local laws for the disposal of alkaline batteries.
B. WHEN NOT IN USE, REMOVE SENSOR BATTERY
Do not leave the battery in the Sensor when it is not in use.
C. AVOID ELECTROMAGNETIC INTERFERENCE
For the best recording results, you should avoid close proximity to heavy equipment or other
sources of electromagnetic interference such as electric blankets, heating pads, water beds, etc.
D. POTENTIAL FOR ELECTROMAGNETIC INTERFERENCE
There is a potential for electromagnetic interference to other devices while using
the CardioNet Service.
E. USE WITH IMPLANTED PACEMAKERS AND ICDs ( DEFIBRILLATORS)
If you have an implanted pacemaker or defibrillator (ICD), the manufacturer may have
recommended you take certain precautions when using a cellular phone. Since the CardioNet
Monitor contains a cellular phone, you should take the same precautions when carrying and
using the Monitor. In general, most manufacturers recommend the following:
• You should keep a distance of at least six inches (15 cm) between the cellular phone
and a pacemaker or debrillator.
• You should hold the cellular phone on the opposite side of the body from the
pacemaker or debrillator.
• Do not carry a cellular phone in a breast pocket or on a belt if that would place
the phone within six inches of the pacemaker or debrillator.
• You should refer to the manufacturer’s information for guidance regarding
your pacemaker or ICD and interference issues.

CARDIONET MCOT TM MANUAL 5
04.
CAUTIONS
A. POWER DOWN MONITOR AND SENSOR BEFORE SHOWERING
Power down the Monitor, remove the Sensor, and remove battery from Sensor
before showering. The CardioNet Sensor is water resistant, not waterproof.
B. DO NOT GET THE MONITOR AND SENSOR WET
Make sure the Monitor and Sensor stay dry at all times.
C. CLEANING
Use a soft cloth to clean the equipment.
D. LIMITATIONS OF COVERAGE
CardioNet’s ability to obtain information regarding a cardiac event and to contact you
or your physician in a timely manner is limited by a number of factors including:
•Transmission of information about a cardiac event to CardioNet‘s Monitoring
Center is potentially limited by the availability of cellular phone coverage.
•There is an inherent time delay from the time that an event is detected to when the
events are analyzed and confirmed by a Certified Cardiac Technician (CCT).
•There is an inherent time delay from when the event is analyzed and confirmed by
the CCT to when CardioNet is able to make contact with you or your physician.
• If you or your physician are not accessible by telephone, CardioNet will not
succeed in making contact with you or your physician.
CARDIONET MCOT TM MANUAL
6
MOBILE CARDIAC OUTPATIENT TELEMETRY
TM
05.
WARNINGS
A. NOT AN APNEA MONITOR
The CardioNet Monitor is not to be used as an apnea monitor.
B. NOT AN EMERGENCY RESPONSE SERVICE
CardioNet is not an emergency response service. If you experience any symptoms that
concern you, seek medical help.
C. DO NOT TAMPER WITH DEVICE
There are no serviceable parts in the CardioNet MCOT TM System components.
Removing the cover of any component may alter device performance.
D. DO NOT TAMPER WITH MONITOR BATTERY
The Monitor battery can present a fire or chemical burn hazard if mistreated.
Do not disassemble, heat, incinerate, or recharge using any device other than the
CardioNet supplied power cord.
E. USE ONLY CARDIONET POWER CORD IN SINGULAR OUTLET
Do not use any power cord other than the one provided in the CardioNet service kit. A
multiple portable socket outlet or extension cord should not be used with the
equipment.
F. DO NOT USE NEAR FLAMMABLE ANESTHETIC
Units are not to be used in the presence of flammable anesthetic.

CARDIONET MCOT TM MANUAL 7
06.
MEDICAL PRACTICE INSTRUCTIONS
DEPLOYING THE DEVICE
1. Receive and store Monitor and Sensor sets in a secure area at practice.
2. To provide devices for a new prescription, retrieve a Monitor and Sensor from available inventory.
3. Turn ON Monitor and insert the battery in Sensor.
Note: If there is already a battery in the Sensor, remove it and reinsert it.
4. Go to https://access.cardionet.com and go to Enrollment screen. Click on ‘MCOT FFS Enrollment’ and it
will display Prescription/Order information to be lled out.
5. Enter Patient information and prescription information. From the on-screen list, select the serial
numbers of the Monitor and Sensor chosen in step (2).
6. Click ‘Activate Prescription’.
7. The Monitor screen will display message ‘PAIRING’. Once pairing is completed, click on Continue.
8. Choose the language and click on Continue.
9. Once Activation is completed, the Monitor will display message showing paired Sensor number
for verication.
10. Once the Monitor-Sensor verication is conrmed, the device state is updated as Activated/Unavailable.
11. Once the pair is Activated, the set is ready to be sent to the patient.
12. Turn OFF Monitor and remove battery from Sensor; put the Monitor and Sensor in the kit.
RECEIPT OF DEVICE
1. Receive the device set sent by patient. Unpack it and disinfect it.
2. Turn ON Monitor and insert the battery in Sensor.
3. Go to https://access.cardionet.com webpage and go to prescriptions under Device Maintenance tab.
4. Click on ‘Deactivate’. This will reset the device including purging of old data and un-pairing of Monitor
and Sensor.
5. If the device is returned back for replacement, click on ‘Replace’ and follow the process of deployment
per above.
6. Once the device set is Deactivated, the screen will show status of Monitor and Sensor as ‘Available’.
7. Turn OFF the Monitor and remove the Sensor battery.
8. Shelve the device in secure area.
CARDIONET MCOT TM MANUAL
8
MOBILE CARDIAC OUTPATIENT TELEMETRY
TM
5
07.
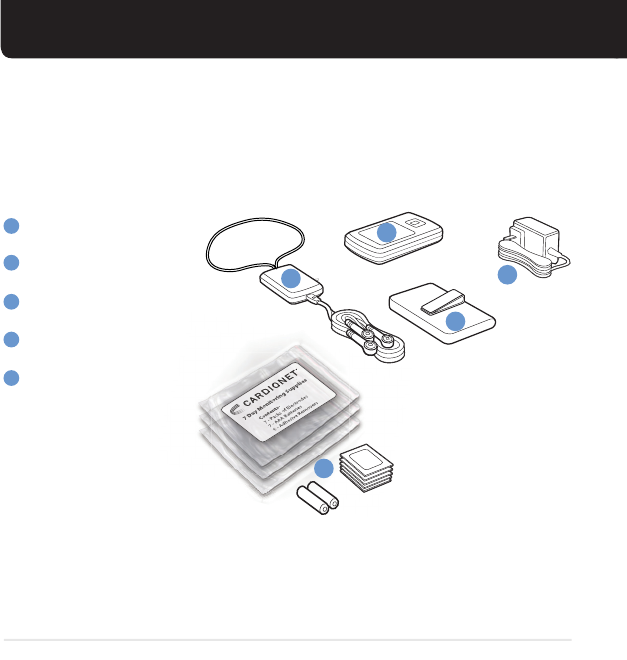
KIT CONTENTS
SENSOR
MONITOR
POWER CORD
MONITOR CASE
ELECTRODES, BATTERIES,
& ADHESIVE REMOVER
2
1
3
4
5
13
4
2
CARDIONET MCOT TM MANUAL 9
08.
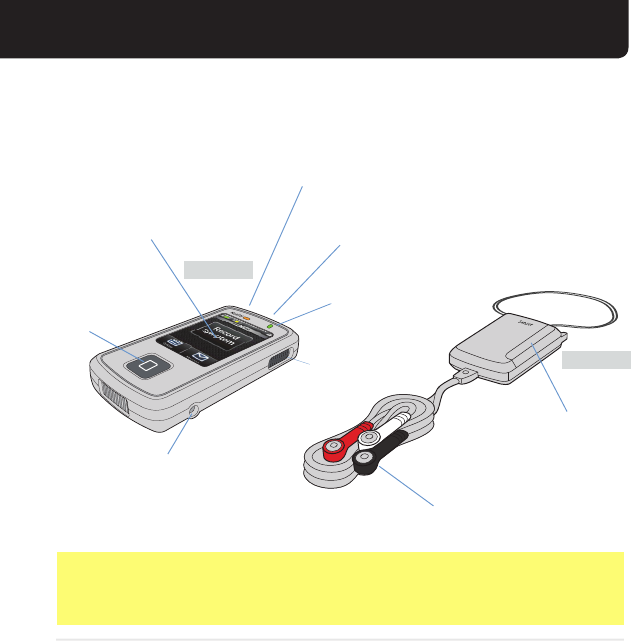
MONITOR AND SENSOR
TOUCH SCREEN
To operate monitor, touch the
screen where indicated. Monitor is
still functioning when dark (Screen
will go dark after 1 minute). STATUS LIGHT
Intermittent Green = Communication with sensor
Red = Read the screen for instructions
CHARGING LIGHT (when charging)
Orange = Charging
Green = Fully Charged
CHARGING PORT
Plug power cord end here to
charge the monitor.
WAKE BUTTON
If you want to view
the screen, press
the Wake button.
CELL STRENGTH
INDICATOR
Slide to power monitor
On (White)/Off (Black)
BATTERY DOOR
To access AAA-size battery.
LEADS
Each wire will snap to
an electrode that will be
attached to your body.
Your monitor should remain with you at all times. Although your monitor detects
and transmits arrhythmias automatically, it is important to use the monitor’s Record
Symptom button to notify CardioNet and your doctor when you are feeling a symptom.
MONITOR
SENSOR
POWER SWITCH
CARDIONET MCOT TM MANUAL
10
MOBILE CARDIAC OUTPATIENT TELEMETRY
TM
09.
ATTACHING THE ELECTRODES
TO YOUR SKIN
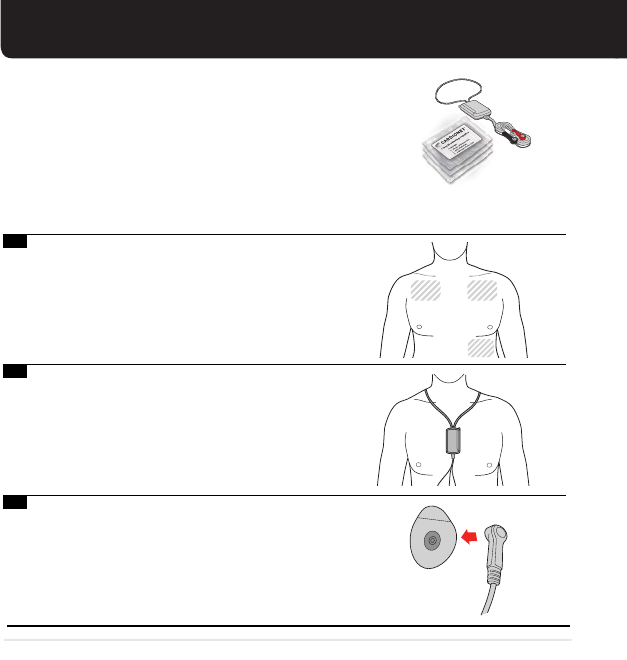
Electrodes are placed in the shaded areas; wash
and dry these areas. Do not use powder or
lotion. If you have chest hair, shave these areas.
Remove sensor and neck strap from box. Place the
cloth strap over your head around your neck.
3.
Tear open electrode pack and remove 3 electrodes.
Snap the lead (wires) end onto the electrodes.
Note: Please do not use the adhesive remover wipes prior to placing electrodes on your skin.
These should only be used to remove adhesive from your skin after removing electrodes if needed.
1.
2.
CARDIONET MCOT TM MANUAL 11
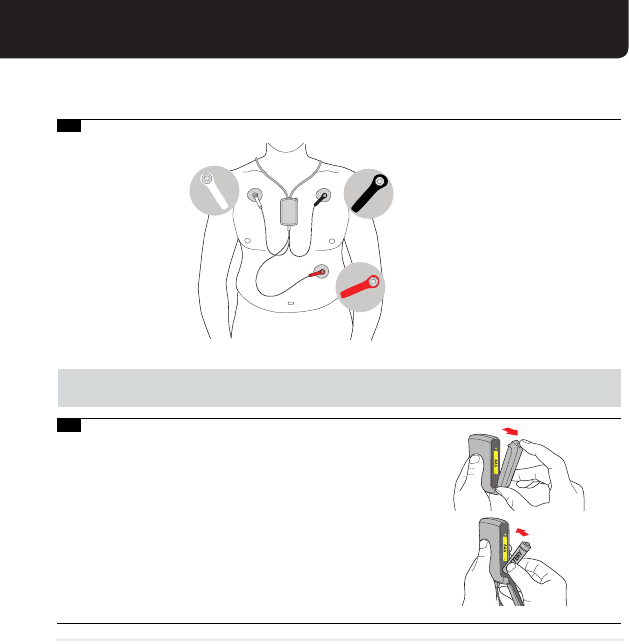
Open the door of your sensor. Place a AAA battery from
the CardioNet MCOT Kit into the sensor as shown below.
Use the AAA battery image on the inside of the sensor to
ensure that the plus (+) and minus ( - ) ends of the battery
are properly oriented. If you have inserted the battery
correctly, you will hear a chime. Close the sensor door.
Note: You will need to change your sensor battery every day.
Peel the adhesive
backing o the
White electrode.
Place the electrode
about 3 ngers
width below your
right collar bone.
WHITE
BLACK
RED
Peel the adhesive backing o
the Black electrode. Place the
electrode about 3 ngers width
below your left collar bone.
Peel the adhesive backing o the
Red electrode. Place the electrode
on the lower left side of your rib
cage, in line with the electrode
under your left collar bone.
White (Right)
Black (Left)
Red (Left Side)
4.
5.
You should now have all three wires attached to the electrodes in the positions shown.
Wait 15 minutes before proceeding to step 5.
CARDIONET MCOT TM MANUAL
12
MOBILE CARDIAC OUTPATIENT TELEMETRY
TM
10.
GETTING STARTED WITH MONITORING
1. The monitor should be powered on (green light above the screen). If it isn’t,
please turn it on using the power switch on the right side of the monitor.
2. If the monitor is on, and the screen is dark, touch the Wake button (black
button, with white square on it) to light up the screen.
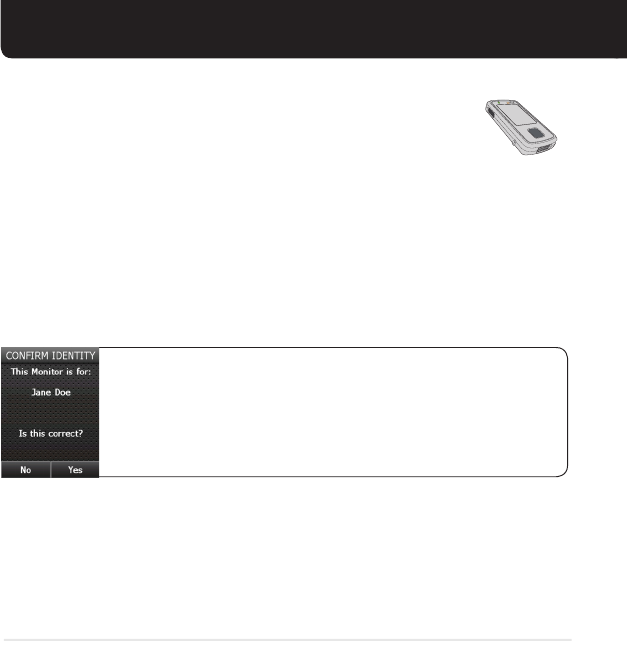
3. Confirm identity.
Please verify that your name is correct. If correct, press Yes, and read
the monitor screens that follow. If incorrect, including spelling, press
No. Turn off the monitor, take off the sensor and remove the battery,
and call your physician.

CARDIONET MCOT TM MANUAL 13
11.
RECORDING SYMPTOMS
1
Touch Record
Symptom to proceed.
2
Yes to proceed.
No to return to
the Main Menu
3
Touch any symptoms
you are feeling. Touch
Next for additional
symptom options.
4
Touch any
additional symptoms
you are feeling.
Touch Next to
proceed.
5
Touch your current
activity level.
Touch Done to
proceed.
Please remember to keep
your monitor with you at
all times so that you may
record any symptoms
you feel.
It is important to use the
monitor to record any
symptoms you may feel.
When you feel a symptom,
press Record Symptom
and follow the instructions.
Data will be transmitted to
CardioNet automatically.
There is no need to call to
conrm receipt.
CardioNet MCOTTM is not
an emergency response
service. If at any time you
experience a symptom
that you feel is a medical
emergency, you should
immediately dial 911 for
medical assistance.
Confirmation message
received that your
symptom was recorded.
6

CARDIONET MCOT TM MANUAL
14
MOBILE CARDIAC OUTPATIENT TELEMETRY
TM
12.
CHARGING THE CARDIONET MCOT™ MONITOR
It is important to charge the monitor
every night. It may take up to 4 hours
to fully charge the monitor.
DIRECT POWER CORD CHARGING
Take the power cord with you if you plan
to be away from home all day. To charge
the monitor, plug the power cord into
the hole on the side of the monitor.
Look for the orange light on the top
left of the monitor. The “Charge
Monitor” alarm will sound when the
battery is critically low.
CHECKING BATTERY POWER
Locate the battery power gauges in the
top left of the monitor screen. The
picture on the left represents the
monitor battery level (M) and the
picture on the right represents the
sensor battery level (S). Touch this
picture to display current battery levels.
Direct Power Cord Charging
Battery
Strength
Indicator

CARDIONET MCOT TM MANUAL 15
13.
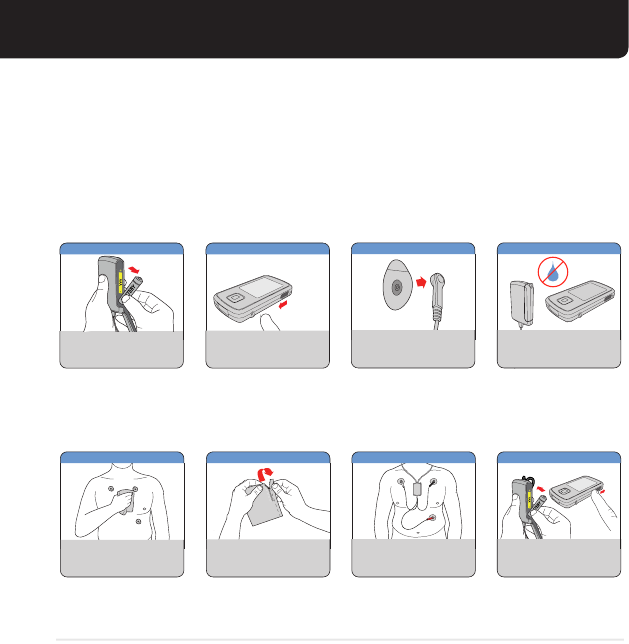
RECEIVING TEXT MESSAGES
1 New Message
ABOUT TEXT MESSAGES
CardioNet may send you text messages during your monitoring. If you hear an alert
and see the New Message statement on the screen, you have a new text message.
Touch the Messages button to read the message.
Follow the instructions on the screen. Press OK when you are nished.
CARDIONET MCOT TM MANUAL
16
MOBILE CARDIAC OUTPATIENT TELEMETRY
TM
14.
SKIN CARE & REPLACING ELECTRODES
+
Change your electrodes every other day.
When removing the electrodes, never pull
them off quickly. Use soap and water and
gently lift the old electrodes from your
skin.
If necessary, use the adhesive remover
wipes to take the excess adhesive off
your skin. Wash and dry the areas
thoroughly before putting on new
electrodes.
When you replace your electrodes, do not
put new electrodes in the same locations
each time. It is very important that you
move them from the original locations to
protect your skin. Please refer to the
suggested alternate locations in the
illustration.
SOAP
CARDIONET MCOT TM MANUAL 17
15.
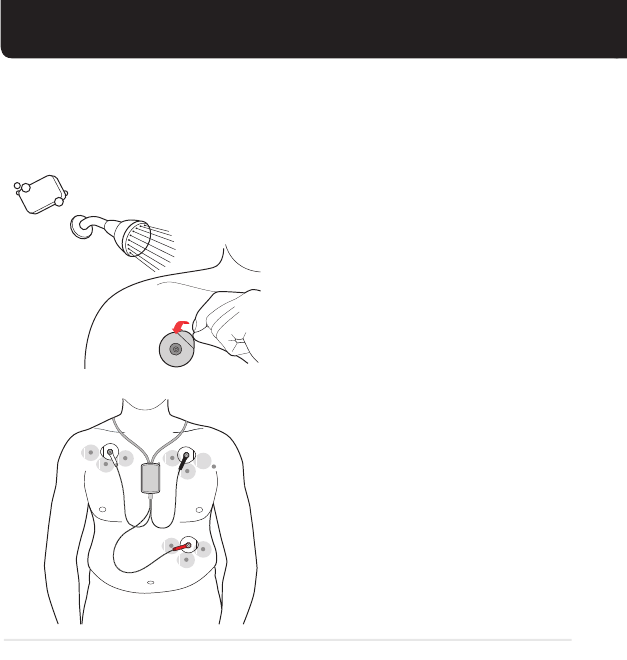
SHOWERING, BATHING AND SWIMMING
BEFORE showering, bathing or other water activities:
AFTER showering, bathing or other water activities:
Snap the lead wires to the
electrodes. White on RIGHT,
Black on LEFT, Red on RIB.
Put the battery in the sensor.
Turn on the monitor.
Dry the electrodes. If they are loose or moving
around, put on new electrodes
and wait 10-15 minutes before
following the next steps.
1 2
Unsnap the lead wires
from the electrodes.
Keep the monitor and sensor
out of the bathroom and
away from water and steam.
3 4
Remove the sensor
battery.
Turn o the monitor.
123
Note: The electrodes are water resistant. You may wear them for showering and bathing.
3 4
BATTER Y
BATTERY
CARDIONET MCOT TM MANUAL
18
MOBILE CARDIAC OUTPATIENT TELEMETRY
TM
16.
TROUBLESHOOTING
Monitor Battery Low
Blank Monitor Screen
Lead O
Ensure that you recharge the battery every day by correctly by plugging the power cord
directly into the side of the monitor. A full recharge takes approximately 4 hours.
The orange light shown on page 18 will be lit if the Monitor is charging. The monitor
battery life can vary day-to-day depending upon several factors, including cell
communication and cell coverage. This may require you to charge the battery sooner
on some days than on other days. Refer to page 18 for more information.
Ensure that the monitor is on by conrming that the light on the top of the Monitor is
green. If you press the wake button, the monitor screen should light up. If it does not,
the monitor may be low on power. You should recharge the monitor.
Check that the lead is snapped rmly onto the electrode. Press on the electrode to ensure
it is rmly adhered to the skin. If you have just changed your electrodes, press Silence on
your Monitor screen. Your electrodes may take up to 15 minutes to properly adhere.
Irritated or Reddened Skin An allergic reaction to the adhesive or gel on the electrodes is possible and can cause
irritated skin. If you experience irritation, worse than minor itching, call your physician.
No Communication 1. Your Monitor and Sensor may not be close enough to communicate. Move the Monitor
closer to the sensor.
2. Check to make sure the sensor battery has been inserted correctly with the proper
(+) ( - ) orientation.
3. Replace the Sensor battery if alarm continues after correcting battery orientation.
Problem Possible Solution

CARDIONET MCOT TM MANUAL 19
17.
RETURNING THE UNIT
RETURN THE PACKED KIT BACK TO YOUR PHYSICIAN'S OFFICE
AS INSTRUCTED BY YOUR PHYSICIAN.
ABOUT DEACTIVATION MESSAGE
When your monitoring is complete, a message will
appear on your Monitor. It is your responsibility to
return the kit back as soon as possible so that other
patients can benet using this valuable service and
to avoid being charged for the equipment.
Please follow these steps when you receive the
message that your monitoring is completed
• Take o the Sensor. Remove Sensor battery.
• Turn o the Monitor using the Power switch
on the side of the Monitor.
• Place everything back in the kit properly
so that the device is not damaged.

CARDIONET MCOT TM MANUAL
20
MOBILE CARDIAC OUTPATIENT TELEMETRY
TM
18.
EQUIPMENT SYMBOLS
Type BF Applied Part
Attention:
Consult accompanying documents.
Non-Ionizing Radiation Transmitter
SYMBOL DESCRIPTION
Manufacturer: Braemar Mfg, LLC
1285 Corporate Center Drive
Suite 150
Eagan, MN, 55121
USA
Phone: 1-800-327-2719
Fax: 1-651-286-8629
E-mail: sales-braemar@gobio.com
Web: http://www.GoBio.com
Contact Braemar Mfg for further
technical information.
MANUFACTURER NAME AND ADDRESS

CARDIONET MCOT TM MANUAL 21
19.
SPECIFICATIONS
PHYSICAL
Sensor 3 inches x 1.9 inches x 0.7 inches; Weight: 3.0 oz. with battery
Monitor 4.7 inches x 2.6 inches x 0.9 inches; Weight: 6.0 oz.
Display 2.27 inches x 1.7 inches; Touch screen: color
FUNCTIONAL
Sample Rate 250 samples per second
ECG Resolution 12 bits
Dynamic range of ECG +/- 5 mV
Bandwidth 0.1 to 40 HZ Channels 2
Battery Life: Monitor 10 hrs (with cleared memory & fully recharged battery)
Battery Life: Sensor 24 hrs (1 AAA Alkaline)
Leakage Current Less than .1 µ A Electrodes
TRANSMISSION
Sensor to Monitor 900 MHz ISM band RF transmission, digital error corrected.
Minimum 150 foot range. Retransmission if data is corrupted.
Monitor to Center CDMA (PCS and cellular) wireless, digital error corrected.
OPERATING CONDITIONS
Operating Temperature- 0 - 45oC
Operating Humidity 10% - 95% noncondensing
Storage Temperature -20 - 65oC noncondensing
CARDIONET MCOT TM MANUAL
22
MOBILE CARDIAC OUTPATIENT TELEMETRY
TM
CONNECTIONS
Monitor Power in (15V, 1.2A max)
WALL ADAPTOR
Power In: 100 – 240 VAC; Power Out: 15V, 1.0A; or 15V, 1.67A
STANDARDS COMPLIANCE
Monitor EN60601-1; AAMI EC-38; FCC Part 15
Sensor EN60601-1; AAMI EC-38; FCC Part 15
AECG Equipment Type I
IN HOME REQUIREMENTS
1. Cellular / PCS wireless coverage suitable for data transmission
2. AC powered outlet
FCC COMPLIANCE
This device complies with part 15 of the FCC Rules. Operation is subject to the following
two conditions: (1) This device may not cause harmful interference and, (2) This device must accept
interference received including interference that may cause undesired operation.
FCC ID
Sensor ISM QBI-1011
Monitor ISM QBI-1014
Monitor Contains:
FCC ID: Q2331308
FCC ID: RI7CE910-DUAL
FCC RULES PART 15
The Model CN1006 has been tested and complies with the limits for Part 15 of the FCC Rules for a class
B digital device. These limits are designed to provide reasonable protection against harmful
interference when the equipment is operated in a residential environment. This equipment generates,
uses, and can radiate radio frequency energy and, if not installed and used in accordance with the
instruction manual, can cause harmful interference to radio communications.

CARDIONET MCOT TM MANUAL 23
However, there is no guarantee that interference will not occur in a particular installation. If this
equipment does cause harmful interference to radio or television reception, which can be determined
by turning the equipment off and on, the user is encouraged to try to correct the interference by one or
more of the following measures:
•Reorient or relocate the receiving antenna.
•Increase the separation between the equipment and receiver.
•Connect the equipment into an outlet on a circuit different from that to which the receiver is
•connected.
•Consult the dealer or an experienced radio/TV technician for help.
CHANGES OR MODIFICATIONS NOT EXPRESSLY APPROVED BY CARDIONET COULD VOID THE USER’S
AUTHORITY TO OPERATE THE EQUIPMENT
Model CN1006 Monitor can be linked to any Bluetooth compatible receiver.
SERVICE
In the event of equipment malfunction, all repairs should be performed by CardioNet or an authorized
agent. It is the responsibility of users requiring service to report the need for service to CardioNet or to
one of our authorized agents. Service can be facilitated through our office at:
CardioNet 1000 Cedar Hollow Road, Suite 102, Malvern, PA 19355. Tel #1 888-312-2328.
CARDIONET MCOT TM MANUAL
24
MOBILE CARDIAC OUTPATIENT TELEMETRY TM
FCC RADIO FREQUENCY EXPOSURE INFORMATION
In August 1996, the Federal Communications Commission (FCC) of the United States, with its action in Report
and Order FCC 96-326, adopted an updated safety standard for human exposure to radio frequency (RF)
electromagnetic energy emitted by FCC regulated transmitters. Those guidelines are consistent with the
safety
standard previously set by both U.S. and international standards bodies. The design of this device
complies with the FCC guidelines and these international standards. Use only the supplied antenna.
Unauthorized
antennas, damaged antennas, modifications, or attachments could impair call quality, damage
the device, or
result in violation of FCC regulations. Please contact CardioNet if damage to the unit is
apparent.
BODY-WORN OPERATION
This device was tested and was found to comply with the FCC exposure requirements. The device was
also tested and found to comply with SAR (Specific Absorption Rate) testing. For more information
about RF exposure, please visit the FCC website at www.fcc.gov.
ELECTRODES
Conductive parts of Electrodes and associated connectors, including NEUTRAL ELECTRODE, should not
contact other conductive parts including earth.
For questions on electrodes, contact:
S&W Healthcare – www.swhealthcare.com or 1-800-843-1201
Kendall – www.tycohealthcare.com or 1-800-962-9888
Kendall 233 and S&W electrodes have been tested for up to 72 hr. wear
Vermed – http://www.vermed.com/ or 1-800-245-4025

CARDIONET MCOT TM MANUAL 25
220-0126-01 Rev E
1000 Cedar Hollow Road
Suite 102
Malvern, PA 19355
Manufactured by
Braemar Inc.
1285 Corporate Center Drive
Suite 150
Eagan, MN 55121