Users Manual

Kanso™ Sound Processor
User Guide
DRAFT ONLY FOR CLINICAL TESTING

II KANSO™ SOUND PROCESSOR
DRAFT ONLY FOR CLINICAL TESTING
This guide is intended for Cochlear implant recipients and
their carers using the Cochlear™ Nucleus® Kanso™ Sound
Processor (model number CP950).
The processor works with your implant to transfer sound
to your ear. It is a self-contained unit that contains the
processing unit, microphones, magnet and batteries.
You can control your processor by pressing the button, as
shown in this guide.
You can also use a Cochlear Nucleus CR210 Remote
Control or Cochlear Nucleus CR230 Remote Assistant.
They also provide extra troubleshooting functions. For
more information, please see your remote’s user guide.
NOTES
• Refer to the Cautions and Warnings sections for safety
advice relating to the use of the Kanso Sound Processor,
batteries and components.
• Please also refer to your Important Information document for
essential advice that applies to Cochlear implant systems.
Symbols used in this guide
NOTE
Important information or advice.
TIP
Time saving hint.
CAUTION (no harm)
Special care to be taken to ensure safety and
effectiveness. Could cause damage to equipment.
WARNING (harmful)
Potential safety hazards and serious adverse reactions.
Could cause harm to person.
USER GUIDE 1
DRAFT ONLY FOR CLINICAL TESTING
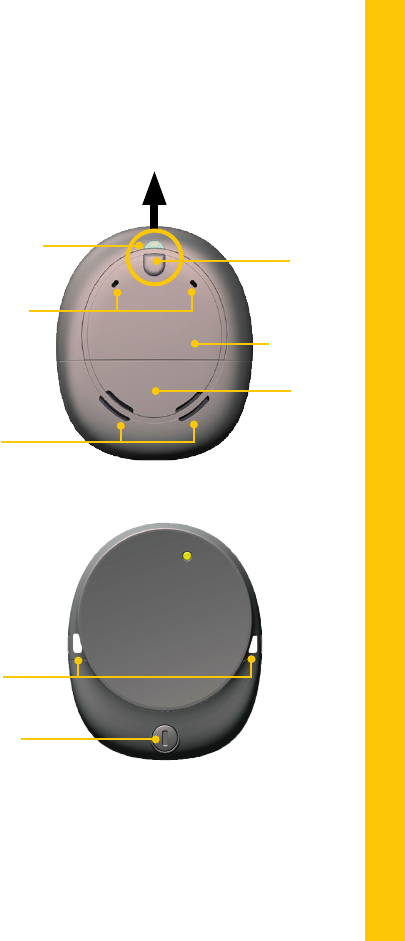
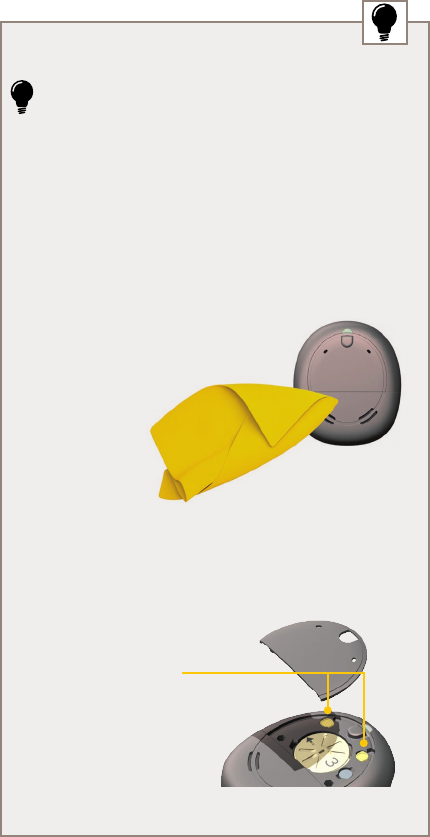
Microphone
ports
Indicator light
ABOUT
Front cover
Control
button
Battery
cover
Kanso™ Sound Processor
Air slots
Back
Battery cover
lock
Safety line
attachment points
Front
THIS WAY UP
2 KANSO™ SOUND PROCESSOR
DRAFT ONLY FOR CLINICAL TESTING
Contents
Power
Batteries . . . . . . . . . . . . . . . . . . . . . . . . . 4
Battery life . . . . . . . . . . . . . . . . . . . . . . . . 4
Lock/unlock the battery cover . . . . . . . . . . . . . 5
Change the batteries . . . . . . . . . . . . . . . . . . 6
Use
Turn on and off . . . . . . . . . . . . . . . . . . . . . 8
Pair with remotes . . . . . . . . . . . . . . . . . . . . 9
Change program . . . . . . . . . . . . . . . . . . . 10
Change volume and sensitivity. . . . . . . . . . . . 11
Use audio sources . . . . . . . . . . . . . . . . . . 12
Telecoil . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
Wireless Accessories. . . . . . . . . . . . . . . . . . . . 12
Wear
Wear your processor . . . . . . . . . . . . . . . . . 14
For users with two implants . . . . . . . . . . . . . 15
Attach a SoftWear pad . . . . . . . . . . . . . . . . 16
Change the magnet . . . . . . . . . . . . . . . . . . 18
Attach the Safety Line . . . . . . . . . . . . . . . . 20
Sport and exercise . . . . . . . . . . . . . . . . . . 22
Travel . . . . . . . . . . . . . . . . . . . . . . . . . 23
USER GUIDE 3
DRAFT ONLY FOR CLINICAL TESTING
Care
Storage . . . . . . . . . . . . . . . . . . . . . . . . 25
Regular care . . . . . . . . . . . . . . . . . . . . . . 24
Change microphone protectors . . . . . . . . . . . 26
Water, sand and dirt . . . . . . . . . . . . . . . . . 29
Lights and Beeps
Lights . . . . . . . . . . . . . . . . . . . . . . . . . 30
Beeps . . . . . . . . . . . . . . . . . . . . . . . . . 32
Troubleshoot . . . . . . . . . . . . . . . 34
Cautions . . . . . . . . . . . . . . . . . . . 39
Warnings
For parents and carers . . . . . . . . . . . . . . . . 40
Processors and parts . . . . . . . . . . . . . . . . . 41
Batteries . . . . . . . . . . . . . . . . . . . . . . . . 44
Medical treatments . . . . . . . . . . . . . . . . . 45
Other information . . . . . . . . . 48

4 KANSO™ SOUND PROCESSOR
DRAFT ONLY FOR CLINICAL TESTING
Batteries
For everyday use, the Kanso Sound Processor uses two high
power p675 (PR44) zinc air disposable batteries designed for
Cochlear implant use
NOTE
You will need to use other battery types only when you are
using the Aqua+ for Kanso accessory. Please see its user guide
for details.
Battery life
Batteries should be replaced as needed just as you would
with any other electronic device. Battery life varies
according to the programs used each day, your implant type
and the thickness of skin covering your implant.
Your Kanso Sound Processor has been designed to provide
the majority of users with a battery life of more than 16
hours for typical use with zinc air batteries. However this will
vary depending on your system settings and hearing
situations.
To help you get the longest life from the batteries, your
sound processor will turn off two minutes after you take it
off your implant.
USER GUIDE 5
DRAFT ONLY FOR CLINICAL TESTING
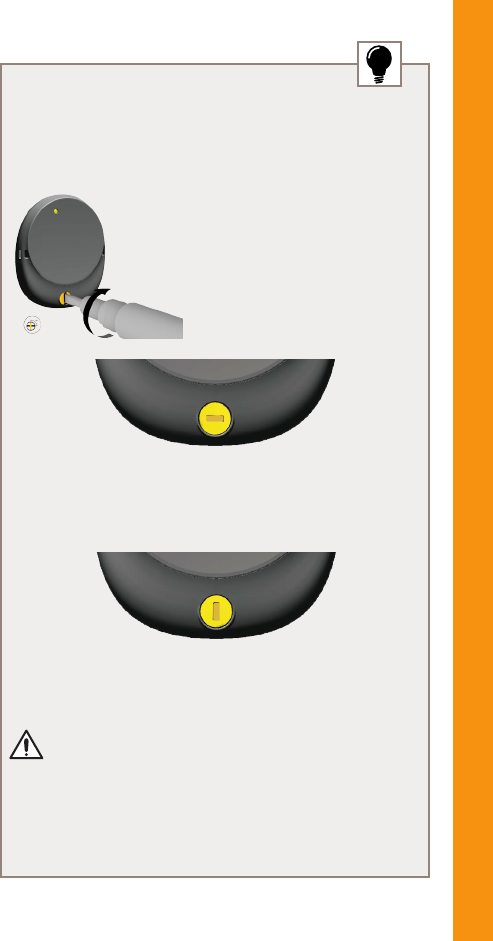
Lock/unlock the battery cover
The battery cover has a tamper resistant lock to help
prevent children opening the battery cover.
1. To lock, turn the locking
screw clockwise with the
battery cover locking tool
until it is horizontal.
LOCKED
2. To unlock, turn the locking screw anticlockwise until it
is vertical.
UNLOCKED
CAUTION
Always check the locking screw is unlocked before
attaching or removing the battery cover.
POWER
6 KANSO™ SOUND PROCESSOR
DRAFT ONLY FOR CLINICAL TESTING
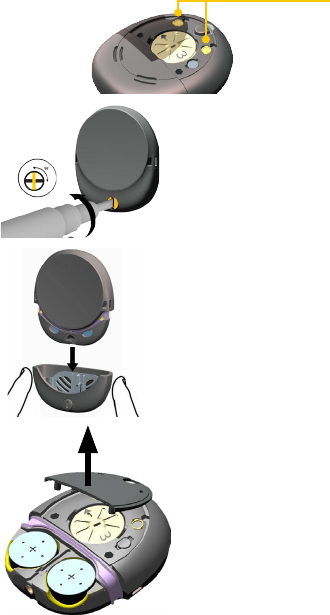
Change the batteries
1. If the battery cover is
locked, turn the lock
screw anticlockwise to
unlock it.
2. Remove the battery
cover.
Use your fingers on the
sides to pull off the
cover.
3. Push down on each battery with your thumb in the
cutout section in the side. The batteries pop up. Pull the
batteries out.

USER GUIDE 7
DRAFT ONLY FOR CLINICAL TESTING
POWER
4. Remove the new batteries
from the packet, and let
them stand for a few
seconds.
See Batteries on page 4.
5. Insert the batteries into the
battery holder with the side
with holes on it (positive
terminal) facing out.
6. Replace the battery cover.
Lock the cover if required.
Your processor will
automatically turn on.
NOTE
If you do not put your sound processor on your implant, it
will turn off automatically after two minutes.
8 KANSO™ SOUND PROCESSOR
DRAFT ONLY FOR CLINICAL TESTING
Turn on and off
1. Press the button
to turn on.
2. To turn off,
press and hold
the button
until the light
is a steady orange.
NOTE
Your sound processor will also turn off automatically after
being off your implant for two minutes.

USER GUIDE 9
DRAFT ONLY FOR CLINICAL TESTING
Pair with remotes
You need to pair your sound processor to your CR210
Remote Control or CR230 Remote Assistant to use their
control and monitoring functions.
Please see your remote’s user guide for details.
USE
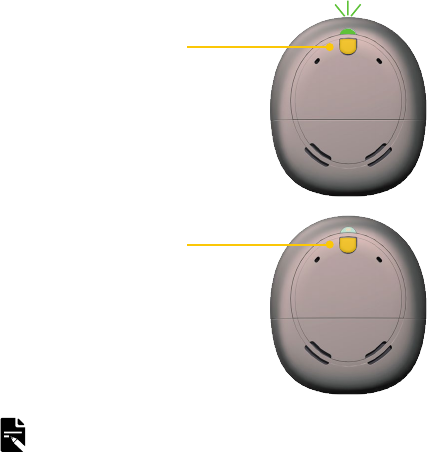
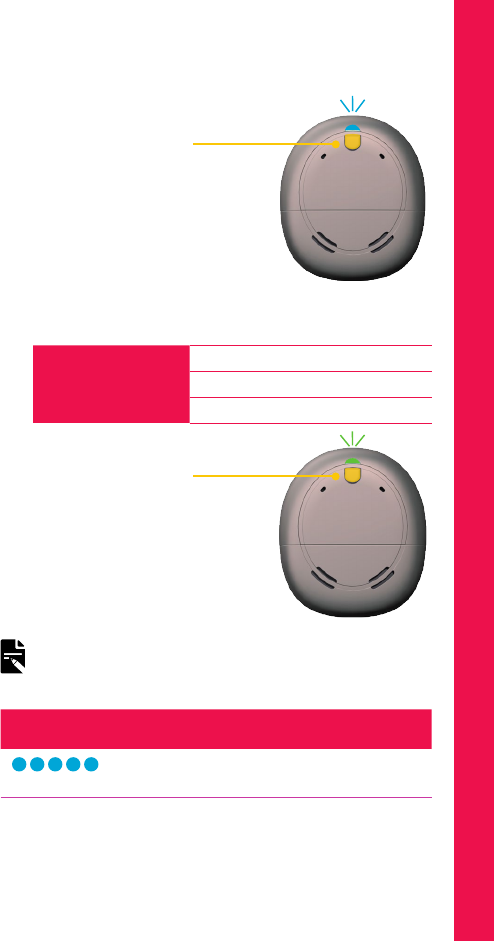
INDICATOR LIGHTS WHAT IT MEANS
Green flashes
Turning on processor.
The number of flashes indicates the
number of the current program.
Orange flashes
Processor is off the implant.
…
Quick green flashes
Processor flashes while receiving
sound from microphones (Child mode
only).

10 KANSO™ SOUND PROCESSOR
DRAFT ONLY FOR CLINICAL TESTING
Change program
You can choose between programs to change the way your
sound processor deals with sound, e.g. in noisy or quiet
places. Usually two programs are all you need, but your
clinician can give you up to four programs.
1. Press the button
to switch
between programs.
INDICATOR LIGHT WHAT IT MEANS
Green flashes
Changing the program (Child mode
only).
The number of flashes indicates the
number of the current program.
NOTE
If your clinician has enabled SCAN, your sound processor can
automatically select the best program for you.
USER GUIDE 11
DRAFT ONLY FOR CLINICAL TESTING
USE
Change volume and sensitivity
If set up by your clinician, you can control the levels of
volume or sensitivity (if available) using your CR210
Remote Control or CR230 Remote Assistant.
Please see your remote’s user guide for details.
NOTE
You need to pair your sound processor with your remote first.
See your remote’s user guide for details.

12 KANSO™ SOUND PROCESSOR
DRAFT ONLY FOR CLINICAL TESTING
Use audio sources
Your processor can receive sound from external audio
sources.
Telecoil
Telecoil enables you to listen to room hearing loops.
NOTE
Telecoil is not recommended for phone use with the Kanso
Sound Processor. We recommend you use the Cochlear
Wireless Phone Clip.
Wireless Accessories
Cochlear Wireless Accessories can wirelessly stream sound
to your processor:
• Audio sources gives you access to a Mini Microphone
or TV Streamer
• You use the buttons on the Phone Clip to control phone
calls.
NOTES
• You first need to pair your Wireless Accessories with your
sound processor.
• For more details, see the Wireless Accessories User Guide.
Cycling through audio sources
All your available audio sources are accessed by this
button-press. Your processor cycles through the audio
sources in order, with each button press activating the next
available audio source:
1. Telecoil
2. First paired Wireless Accessory
3. Next paired Wireless Accessory...
USER GUIDE 13
DRAFT ONLY FOR CLINICAL TESTING
USE
1. Press and hold the
button (about 3
seconds) to activate
audio sources.
2. Press and hold the button again (about 3 seconds) if you
need to cycle to another audio source:
FIRST PRESS Telecoil (if enabled)
SECOND PRESS First paired Wireless Accessory
THIRD PRESS... Next paired Wireless Accessory...
3. Press the button to
turn audio off.
NOTE
You can also use your remote to control audio sources. See its
user guide for details.
INDICATOR LIGHTS WHAT IT MEANS
…
Quick blue flashes
Processor flashes while receiving sound
from an audio source (Child mode only).
14 KANSO™ SOUND PROCESSOR
DRAFT ONLY FOR CLINICAL TESTING
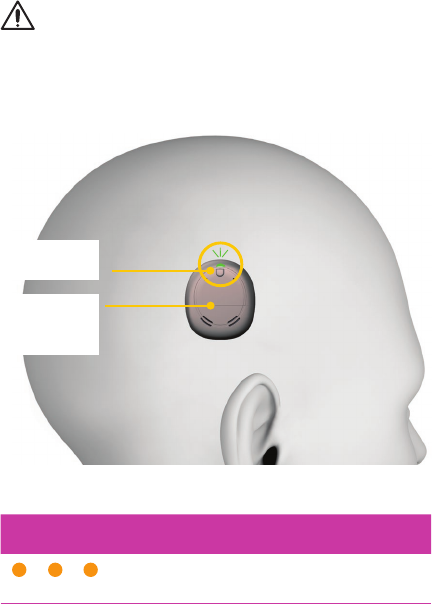
Wear your processor
Place the processor on your implant with the button/light
facing up and battery compartment facing down.
CAUTION
It is important to position your processor correctly to get the
best performance, and so it does not fall off the implant.
Button/light
facing up
Battery
compartment
facing down
INDICATOR LIGHTS WHAT IT MEANS
Flash of orange every second
Processor flashes while coil is off
(or connected to the wrong implant).
USER GUIDE 15
DRAFT ONLY FOR CLINICAL TESTING
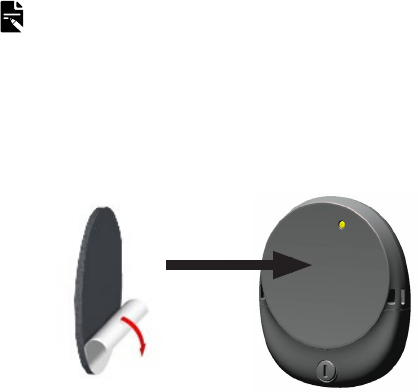
For users with two implants
Ask your clinician to give you coloured stickers (red for
right, blue for left) to make identifying left and right
processors easier.
CAUTION
If you have two implants, you must use the correct sound
processor for each implant.
NOTE
Your sound processor will recognise the implant’s ID, so it will
not work on the wrong implant.
WEAR
16 KANSO™ SOUND PROCESSOR
DRAFT ONLY FOR CLINICAL TESTING
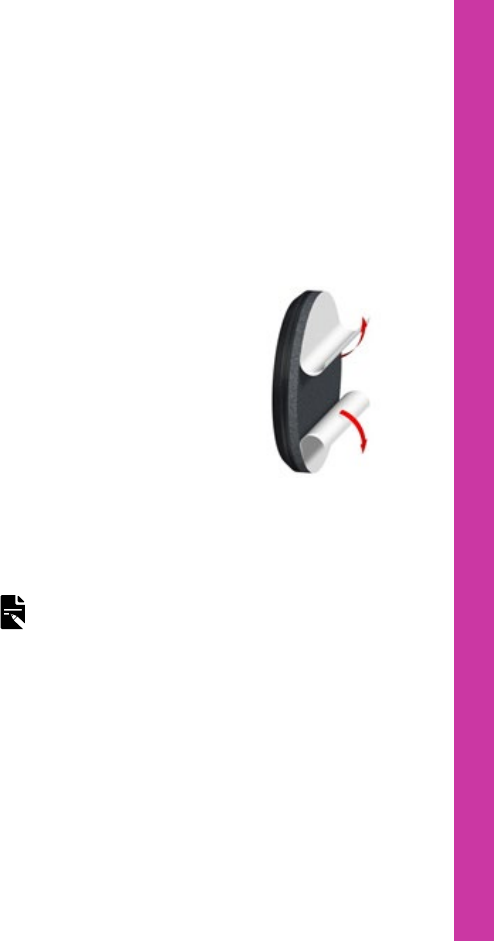
Attach a SoftWear pad
The Cochlear SoftWear™ pad is optional. If you experience
discomfort when wearing your processor, you can attach
this adhesive pad to the back of your processor.
NOTE
You may need to change to a stronger magnet after attaching
a SoftWear pad.
1. Peel off the single
backing strip on the
adhesive side of the pad.
2. Attach the pad to the
back of the processor –
press down firmly.
USER GUIDE 17
DRAFT ONLY FOR CLINICAL TESTING
3. Peel off the two semicircle
backing covers on the
cushion side of the pad.
4. Wear your processor as
usual.
NOTE
The SoftWear pad may affect your sound processor’s
performance. If you notice any change, contact your clinician.
WEAR
18 KANSO™ SOUND PROCESSOR
DRAFT ONLY FOR CLINICAL TESTING
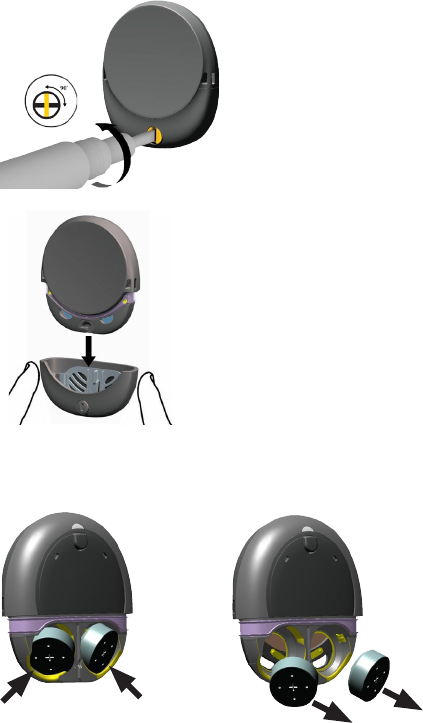
Change the magnet
If your sound processor’s magnet is too weak the processor
may fall off, or if it is too strong it may cause discomfort.
You can adjust the magnet strength by replacing it with a
magnet of a different strength from ½ (weakest) to
6 (strongest).
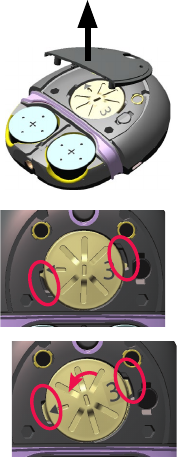
1. If the battery cover is locked, turn the lock screw
anticlockwise to unlock it.
2. Remove the battery cover.
Use your fingers on the sides to pull off the cover.
3. Use your thumbnail to
remove the magnet cover.
4. Press down with your finger
and turn the magnet
anticlockwise until the arrow
and side tabs line up with the
square notches in the
processor case.
USER GUIDE 19
DRAFT ONLY FOR CLINICAL TESTING
WEAR
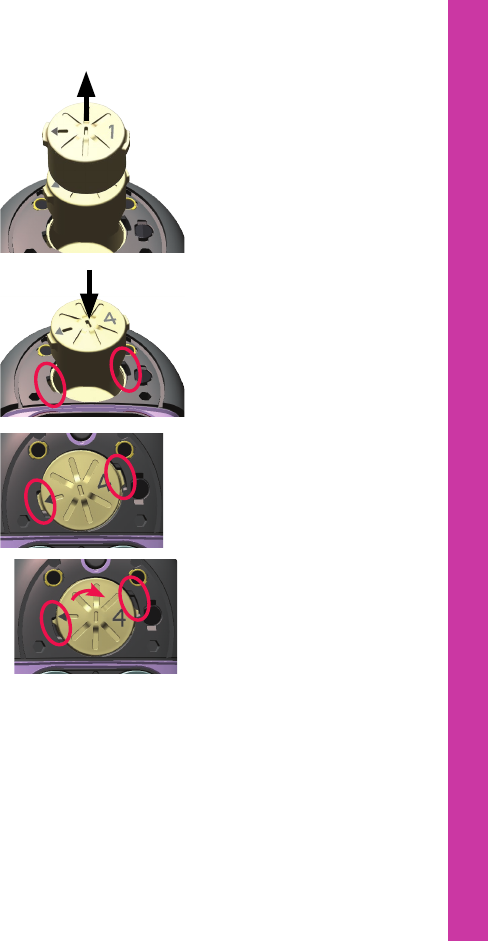
5. Use another magnet to pull
the magnet from the
processor.
6. Insert the new magnet in
the processor, with the side
tabs in the square notches
in the processor case.
7. Press down with your finger
and turn the magnet
clockwise to lock the tab on
the magnet under the
processor’s case.
8. Replace the magnet cover.
9. Replace the battery cover and lock it if required.

20 KANSO™ SOUND PROCESSOR
DRAFT ONLY FOR CLINICAL TESTING
Attach the Safety Line
To reduce the risk of losing your processor, you can attach
a Cochlear Nucleus Safety Line that clips onto your
clothing.
WARNING
Retention lines longer than the Safety Line (standard length)
are not recommended for use by children as they may present
a risk of strangulation.
1. Pinch the loop on the
end of the line between
your finger and thumb.
2. Pass the loop through
the attachment hole in
the sound processor
from front to back.
TIP
Use the attachment
hole that will be at the
rear of the processor
when it is on your
head.

USER GUIDE 21
DRAFT ONLY FOR CLINICAL TESTING
WEAR
3. Pass the clip through
the loop and pull the
line tight.
4. Open the clip by lifting
the lever.
TIP
Make sure you LIFT the
tab to OPEN the clip.
5. Place the clip on your
clothing and press
down to close.
22 KANSO™ SOUND PROCESSOR
DRAFT ONLY FOR CLINICAL TESTING
Sport and exercise
TIP
Always ensure the battery cover is locked when you
exercise or play sport.
1. Use accessories such as the Safety Line or a headband
to help hold your processor in place when you play
sport or exercise.
2. After exercise, wipe your processor with a soft cloth to
remove sweat or grime.
3. Then check your microphone protectors for dirt.
See Change microphone protectors on page 26.
Microphone
protectors

USER GUIDE 23
DRAFT ONLY FOR CLINICAL TESTING
Travel
NOTE
Visit www.cochlear.com/clinic-finder to find the nearest clinic
in places you are travelling.
• Take a printout from your clinician of your most recent
program in case you need help with your processor.
• If you have a backup sound processor, check that it is
programmed correctly and take it with you.
• It’s okay to move through metal detectors and full body
scanners with your sound processor on. To avoid any
possible buzzing sounds in your ear, turn off the telecoil.
• Ask your clinician for a Patient Identification Card. In
the unlikely event that your implant sets off a metal
detector the ID card will help explain that you have an
implanted medical device.
• If you need to remove your sound processor as you
move through airport security, place it in a case in your
hand luggage.
• Your sound processor will not interfere with a plane’s
navigation system so you won’t need to turn it off
during takeoff and landing. If you use a remote control
for your processor, switch it off before takeoff as it
transmits high frequency radio waves when switched
on.
WEAR

24 KANSO™ SOUND PROCESSOR
DRAFT ONLY FOR CLINICAL TESTING
Regular care
CAUTIONS
• Do not use cleaning agents or alcohol to clean your
processor.
• Turn your processor off before cleaning or performing
maintenance.
Every day
• Check all parts and any accessories you use (e.g.
SoftWear pad, Safety Line) for dirt and moisture. Wipe
the processor with a soft dry cloth.
• Keep your processor free from moisture by drying it
every night in your dry aid kit.
• Check the microphone protectors for signs of dirt or
grime and replace if needed. See Change microphone
protectors on page 26.
Every month
• Remove batteries and check for signs of dirt or grime.
Wipe the contacts with a soft dry cloth.
• Replace a SoftWear pad (if used) if it is worn or
damaged, or has accumulated dirt or moisture that
cannot be wiped off. If you have any problem with
comfort, that is not helped by changing the SoftWear
pad, contact your clinician. See Attach a SoftWear pad
on page 16.
• Check if the Safety Line (if used) is showing signs of
wear. Replace as needed. See Attach the Safety Line on
page 20.

USER GUIDE 25
DRAFT ONLY FOR CLINICAL TESTING
Every two months
• Replace the dry brick in your dry aid kit.
Every three months
• Replace the microphone protectors – this is very
important for the quality of sound. See Change
microphone protectors on page 26.
Storage
Dry aid kit
Store your processor at night
in the dry aid kit provided by
Cochlear. Store the processor
fully assembled for 8 hours
for optimal drying effect.
Storage case
For long term storage,
remove the batteries and
store so they do not touch
each other. Storage cases are
available from Cochlear.
CARE
26 KANSO™ SOUND PROCESSOR
DRAFT ONLY FOR CLINICAL TESTING
Change microphone protectors
Replace your microphone protectors every three months,
or if they look dirty or you notice any loss in sound quality.
Always replace both microphone protectors at the same
time.
Step 1: Remove microphone protectors
Microphone
protectors
1. If the battery cover is locked,
turn the lock screw
anticlockwise to unlock it.
2. Remove the battery cover.
Use your fingers on the sides
to pull off the cover.
3. Use your thumbnail to
remove the sound
processor’s front cover.

USER GUIDE 27
DRAFT ONLY FOR CLINICAL TESTING
CARE
4. Firmly push the tip of the
removal tool into the
middle of the microphone
protector.
5. Firmly push, and then
turn the tool 90°
clockwise.
6. Lift out the used
microphone protector.
7. Pull the used microphone
protector from the tool
and discard.
8. Repeat steps 4 to 7 to
remove the other
microphone protector.

28 KANSO™ SOUND PROCESSOR
DRAFT ONLY FOR CLINICAL TESTING
Step 2: Insert new microphone protectors
1. Pull the Kanso Microphone Protector Applicator out of its
sleeve.
2. With the microphone
protectors facing down, lay
the applicator over the
processor, with protectors
over the microphones.
3. Press the microphone
protectors down with your
finger.
4. Remove the applicator
carefully, peeling it upwards
from the side.
5. Replace the front cover.
6. Replace the battery cover. Lock the cover if required.

USER GUIDE 29
DRAFT ONLY FOR CLINICAL TESTING
Water, sand and dirt
Your processor is protected against failure from dust
penetration or splashing water (IP54 rated).
However, it is still a precision electronic device so you
should take the following precautions.
If your processor ever gets wet,
dry it with a soft cloth.
Then remove the batteries, dry
them and the contacts with a
soft cloth, and replace them.
Replace the microphone
protectors and place your
processor in the dry aid kit
provided by Cochlear for 8 hours.
See Change the batteries on page 6.
See Change microphone protectors on
page 26.
If sand or dirt ever enter the
processor, remove it by carefully
brushing all indents and holes in
the processor’s casing.
CARE
30 KANSO™ SOUND PROCESSOR
DRAFT ONLY FOR CLINICAL TESTING
Lights
Your clinician can set up your processor to show some or
all of the following light indications.
Turning on and off
LIGHT WHAT IT MEANS
…
Quick green flashes
Processor flashes while receiving sound
from microphones (Child mode only).
Quick green flashes
Turning on and changing programs.
Number of flashes indicates the number
of the current program.
Long flash of orange
Turning off processor.
Alerts
LIGHT WHAT IT MEANS
…
Flash of orange
every second
Processor flashes while it is off your head
(or connected to the wrong implant).
Orange flashes
Processor batteries are low.
Change batteries.
Steady orange
Fault. Contact your clinician.
Stays on until the issue is resolved.
USER GUIDE 31
DRAFT ONLY FOR CLINICAL TESTING
Audio sources
LIGHT WHAT IT MEANS
Quick blue flash
Processor flashes when pairing to Wireless
Accessory is successful.
…
Quick blue flashes
Processor flashes while receiving audio from
an audio source (Child mode only).
LIGHTS AND BEEPS
32 KANSO™ SOUND PROCESSOR
DRAFT ONLY FOR CLINICAL TESTING
Beeps
Your clinician can set up your processor so you can hear
the following beeps. The beeps are only audible to the
recipient.
Turning on and off
BEEP WHAT IT MEANS
Short high beeps
Changing the program. The number of
beeps indicates the number of the
selected program.
Short high beep
Changing volume or sensitivity level (if
available).
Short high then short
low beep
When changing volume or sensitivity,
indicates upper or lower limit of volume/
sensitivity reached.
Wireless Accessories
BEEP WHAT IT MEANS
3-tone chime
Connecting with Wireless Accessory to
begin streaming audio.
Short beep
When stopping streaming.
Telecoil
BEEP WHAT IT MEANS
Long high beep
Switching between using the
microphones and the telecoil.

USER GUIDE 33
DRAFT ONLY FOR CLINICAL TESTING
Alerts
BEEP WHAT IT MEANS
2 Short low beeps
Processor batteries are low.
Replace batteries.
Short low beeps for 4 seconds
Batteries are empty and
processor is turning off.
Replace batteries.
4 long low beeps over 4 seconds
General fault.
Consult your clinician.
Adjusting bass and treble *
BEEP WHAT IT MEANS
Loud medium beep
Adjusting master volume
level.
Loud long high beep
Adjusting treble level.
Loud long low beep
Adjusting bass level.
* If available, Remote Assistant only
LIGHTS AND BEEPS
34 KANSO™ SOUND PROCESSOR
DRAFT ONLY FOR CLINICAL TESTING
Troubleshoot
Contact your clinician if you have any concerns regarding
the operation or safety of your sound processor.
PROBLEM RESOLUTION
Processor will not
turn on/button
will not respond
1. Try turning the processor on again. See
Turn on and off on page 8.
2. Replace the batteries. See Change the
batteries on page 6.
3. If you have two implants, check that you
are wearing the correct sound processor
on each implant.
4. If the problem continues, contact your
clinician.
The processor
switches off
1. This is normal operation, as the processor
automatically switches off when not
connected to the implant for more than
two minutes.
2. Replace the batteries. See Change the
batteries on page 6.
The processor will
not turn off
1. Remove the batteries from the
processing unit. See Change the batteries
on page 6.
You want to
perform a regular
check on your
processor
See Regular care on page 24.

USER GUIDE 35
DRAFT ONLY FOR CLINICAL TESTING
PROBLEM RESOLUTION
You are not sure
what processor
beeps or light
flashes mean
See Lights on page 30 and Beeps on page
32.
You want to
confirm your
processor is
receiving sound
1. Check the light on the top of the
processor (if enabled). See Lights on
page 30.
2. If you use a CR230 Remote Assistant,
check the sound meter on the status
screen.
3. If the problem continues, contact your
clinician.
The processor
becomes hot
1. Remove the processor from your head
immediately and contact your clinician.
You experience
tightness,
discomfort or
develop a skin
irritation at your
implant site
1. Try using an adhesive SoftWear pad. See
Attach a SoftWear pad on page 16.
2. If you are using a retention aid, such as a
headband, this may be placing pressure
on your processor. Adjust your retention
aid, or try a different aid.
3. Your processor magnet may be too
strong. Ask your clinician to change to a
weaker magnet (and use a retention aid
such as the Safety Line if required). See
Change the magnet on page 18.
4. If the problem continues, contact your
clinician.
TROUBLESHOOT

36 KANSO™ SOUND PROCESSOR
DRAFT ONLY FOR CLINICAL TESTING
PROBLEM RESOLUTION
You do not hear
sound or sound is
intermittent
1. Try a different program. See Change
program on page 10.
2. Replace the batteries. See Change the
batteries on page 6.
3. Make sure the sound processor is
properly oriented on your head, see Wear
your processor on page 14.
4. If the problem continues, contact your
clinician.
You do not hear
sound from a
Wireless
Accessory
1. Check that the Wireless Accessory is
charged and turned on.
2. Check that the Wireless Accessory is
paired with your processor.
3. Check the volume of the Wireless
Accessory.
4. If you use a CR230 Remote Assistant,
use the Streaming menu to check the
connection to the accessory.
5. If you use a CR230 Remote Assistant,
check and adjust the accessory/
microphone mixing ratio.
6. If available, try a different processor.
7. For more troubleshooting, see the
Wireless Accessory User Guide.

USER GUIDE 37
DRAFT ONLY FOR CLINICAL TESTING
PROBLEM RESOLUTION
You hear
intermittent
sound, a buzzing
sound or distorted
speech
1. Check for sources of interference such as
radio and TV transmission towers (within
approximately 1.6 km or 1 mile),
shopping centres, airport security
systems and mobile phones.
2. Try moving away from any source of
magnetic or electronic interference.
3. If the problem continues, contact your
clinician.
Sound is too loud
or uncomfortable
1. Try a different program. See Change
program on page 10.
2. If you use a CR210 Remote Control, turn
down the volume.
3. If you have two sound processors (one
for each side), ensure you have them on
the correct side.
4. If the problem continues, Remove your
external equipment immediately (sound
processor, etc) and contact your clinician.
Sound is too quiet
or muffled
1. Try a different program. See Change
program on page 10.
2. If you use a CR210 Remote Control, turn
up the volume.
3. Try changing the microphone protectors.
See Change microphone protectors on
page 26.
4. If the problem continues, contact your
clinician.
TROUBLESHOOT

38 KANSO™ SOUND PROCESSOR
DRAFT ONLY FOR CLINICAL TESTING
PROBLEM RESOLUTION
The processor gets
wet
1. Dry the processor with a soft cloth,
change the microphone protectors
and place it in the dry aid kit provided
by Cochlear for 8 hours. See Water,
sand and dirt on page 29
Batteries are not
lasting as long as
usual
1. Clean the battery contacts carefully
without bending them. Use the
cleaning brush, then wipe the
processor with a soft cloth.
2. If you are using a non-recommended
retention aid that covers your sound
processor, replace it with an aid
recommended by Cochlear.
3. Check that you are using the
recommended batteries. See Batteries
on page 4.
4. Don’t forget to let new batteries
stand for a few seconds before
putting them in the sound processor.
5. If the problem continues, contact
your clinician.

USER GUIDE 39
DRAFT ONLY FOR CLINICAL TESTING
Cautions
CAUTIONS
• Young children who are developing motor skills are at
greater risk of an impact to the head from a hard object
(e.g. table or chair). Impact to the sound processor may
cause damage to the processor or its parts. Impact to the
head in the area of the Cochlear implant could damage it
and result in its failure.
• Most patients can benefit from electrical stimulation levels
that are considered safe, based on animal experimental
data. The long-term effects of such stimulation in humans
are unknown.
40 KANSO™ SOUND PROCESSOR
DRAFT ONLY FOR CLINICAL TESTING
Warnings
For parents and carers
• Removable parts of the system (e.g. microphone
protectors, batteries, magnets, battery cover, Safety Line)
can be lost or may be a choking or strangulation hazard.
Keep out of reach of children or lock the tamper-proof
screw on the processor cover.
• Keep the dry brick from the dry aid kit away from small
children. Swallowing this material can cause serious
internal injuries.
• Carers must routinely check the device for signs of
overheating and for signs of discomfort or skin irritation at
the implant site. Remove the processor immediately if
there is any discomfort or pain (e.g. if device becomes hot,
or sound is uncomfortably loud) and inform clinician.
• Carers must monitor for signs of discomfort or skin
irritation if a retention aid (e.g. headband) is used that
applies pressure to the sound processor. Remove the aid
immediately if there is any discomfort or pain, and inform
clinician.
• Dispose of used batteries promptly and carefully, in
accordance with local regulations. Keep away from
children.
• Do not allow children to replace batteries without adult
supervision.

USER GUIDE 41
DRAFT ONLY FOR CLINICAL TESTING
WARNINGS
Processors and parts
• Each processor is programmed specifically for each
implant. Never wear another person’s processor or lend
yours to another person.
• Use your Cochlear implant system only with approved
devices and accessories.
• If you experience a significant change in performance,
remove your processor and contact your clinician.
• Your processor and other parts of the system contain
complex electronic parts. These parts are durable but must
be treated with care.
• No modification of this equipment is allowed. Warranty
will be void if modified.
• If you experience tightness or pain at the implant site, or
develop significant skin irritation, stop using your sound
processor and contact your clinician.
• Do not apply continued pressure to the processor when in
contact with the skin (e.g. sleeping while lying on
processor, or using tight fitting headwear).
• Do not push the volume too high for comfort in case a
loud noise occurs nearby.
42 KANSO™ SOUND PROCESSOR
DRAFT ONLY FOR CLINICAL TESTING
• Do not place the processor or parts in any household
devices (e.g. microwave oven, dryer).
• Do not use a dry aid kit that has an Ultra Violet C (UVC)
lamp (e.g. do not use the Freedom™ Dry and Store).
• The magnetic attachment of your sound processor to your
implant may be affected by other magnetic sources.
• Store spare magnets safely and away from cards that may
have a magnetic strip (e.g. credit cards, bus tickets).
• Your device contains magnets that should be kept away
from life supporting devices (e.g. cardiac pacemakers and
ICDs (implantable cardioverter defibrillators) and magnetic
ventricular shunts), as the magnets may affect the function
of these devices. Keep your processor at least 15 cm (6 in)
from such devices. Contact the manufacturer of the
specific device to find out more.
• Your sound processor and remote control radiate
electromagnetic energy that may interfere with life
supporting devices (e.g. cardiac pacemakers and ICDs).
Keep your processor and remote control at least 15 cm (6
in) from such devices. Contact the manufacturer of the
specific device to find out more.
• Do not place the device or accessories inside any part of
your body (e.g. nose, mouth).

USER GUIDE 43
DRAFT ONLY FOR CLINICAL TESTING
WARNINGS
• Seek medical advice before entering any environment that
may adversely affect the operation of your Cochlear
implant, including areas protected by a warning notice
preventing entry by patients fitted with a pacemaker.
• Some types of digital mobile telephones (e.g. Global
System for Mobile communications (GSM) as used in
some countries), may interfere with the operation of your
external equipment. You may hear distorted sound when
close, 1-4 m (~3-12 ft), to a digital mobile telephone in
use.
• For Cochlear Nucleus cochlear implant recipients only, the
maximum diving depth is 40 m (~131 ft). Seek medical
advice before diving to ensure you do not have any
conditions that might make diving contraindicated (e.g.
middle ear infection). When wearing a mask, avoid
pressure over the implant site.
• Before activities that create electrostatic discharge (e.g.
playing on plastic slides), remove your processor. In rare
cases, discharge of static electricity can damage your
Cochlear implant’s electrical components or corrupt the
processor’s program. If static electricity is present (e.g.
when putting on clothes over your head, or getting out of
a car), before the Cochlear implant system touches any
object or person, you should touch something conductive
such as a metal door handle.
44 KANSO™ SOUND PROCESSOR
DRAFT ONLY FOR CLINICAL TESTING
Batteries
• Use only Cochlear supplied or recommended p675 zinc air
batteries for everyday use. Other batteries may only be
used with the Aqua+ for Kanso accessory. (see its user
guide for details).
• Insert batteries in the correct orientation.
• Do not mix disposable batteries that differ by
manufacturer, brand, type, age or previous usage.
• Do not short-circuit batteries (e.g. do not let terminals of
batteries contact each other, do not place batteries loose
in pockets, etc.).
• Do not disassemble, deform, immerse in water or dispose
of batteries in fire.
• Store unused batteries in original packaging, in a clean and
dry place.
• When processor is not in use, remove the batteries and
store separately in a clean and dry place.
• Wipe batteries with a clean dry cloth if they become dirty.
• Do not expose batteries to heat (e.g. never leave batteries
in sunlight, behind a window or in a car).
• Do not use damaged or deformed batteries. If skin or eyes
come into contact with battery fluid or liquid, wash out
with water and seek medical attention immediately.
• Never put batteries in mouth. If swallowed, contact your
physician or local poison information service.

USER GUIDE 45
DRAFT ONLY FOR CLINICAL TESTING
WARNINGS
Medical treatments
Magnetic resonance imaging (MRI)
MRI is contraindicated except under special
circumstances. Do not allow a patient with an
implant to be in a room where an MRI scanner is
located except under special circumstances.
Full MRI safety information is available at
www.cochlear.com/warnings or by calling your regional
Cochlear office (contact numbers available at the end of this
document).
If the patient is implanted with other implants, consult the
manufacturer’s instructions before performing MRI.

46 KANSO™ SOUND PROCESSOR
DRAFT ONLY FOR CLINICAL TESTING
Medical treatments generating induced currents, heat and
vibration
Having a cochlear implant means extra care must be taken
when receiving some medical treatments. Before starting
medical treatment, the information in this section should be
discussed with the recipient’s physician.
The sound processor must be removed before starting any of
the medical treatments listed in this section.
Some medical treatments generate induced currents that
may cause tissue damage or permanent damage to the
implant. Before initiating any of the following treatments
deactivate the device.
Warnings for specific treatments are provided below.
CONDITION WARNING
Diathermy Do not use therapeutic or medical diathermy
(thermopenetration) using electromagnetic
radiation (magnetic induction coils or
microwave). High currents induced into the
electrode lead can cause tissue damage to the
cochlea/brainstem or permanent damage to the
implant. Medical diathermy using ultrasound
may be used below the head and neck.
Electroconvulsive
therapy
Do not use electroconvulsive therapy on an
implant patient under any circumstances.
Electroconvulsive therapy can cause tissue
damage or damage to the implant

USER GUIDE 47
DRAFT ONLY FOR CLINICAL TESTING
WARNINGS
CONDITION WARNING
Electrosurgery Electrosurgical instruments can induce radio
frequency currents that could flow through the
electrode.
Monopolar electrosurgical instruments must not
be used on the head or neck of an implant
patient as induced currents could cause damage
to cochlear/neural tissues or permanent damage
to the implant.
When using bipolar electrosurgical instruments
on the head and neck of a patient, the cautery
electrodes must not contact the implant and
should be kept more than 1 cm (½ in.) from the
electrodes.
Ionising radiation
therapy
Do not use ionizing radiation therapy directly
over the implant. It may cause damage to the
implant.
Neurostimulation Do not use neurostimulation directly over the
implant. High currents induced into the electrode
lead can cause tissue damage to the cochlea/
brainstem or permanent damage to the implant.
Therapeutic
ultrasound
Do not use therapeutic levels of ultrasound
energy directly over the implant. It may
inadvertently concentrate the ultrasound field
and cause tissue damage or damage to the
implant.
48 KANSO™ SOUND PROCESSOR
DRAFT ONLY FOR CLINICAL TESTING
Physical configuration
The processing unit comprises:
• Two omni-directional microphones for receiving sound.
• An internal telecoil for receiving magnetic fields radiated
by room loops.
• Custom analogue and digital integrated circuits with
digital signal processing (DSP) and bi-directional
wireless communication capabilities.
• A tri-colour visual indication of processor function or
problem.
• One button allowing user control of key features.
The batteries provide power to the processor, which transfers
energy and data to the implant.
Materials
• Processing unit: polyamide
• Magnet casing is made of acrylonitrile butadiene styrene
(ABS).
Other information

USER GUIDE 49
DRAFT ONLY FOR CLINICAL TESTING
Batteries
Check the battery manufacturer’s recommended operating
conditions for disposable batteries used in your processor.
Wireless communication link
The remote control/remote assistant wireless communication
link operates in the 2.4 GHz ISM band using GFSK (Gaussian
frequency shift keying) on 5 channels. The link uses a
proprietary bi-directional communication protocol and
operates over a distance of up to 2 metres from the processor.
When interference is present, the wireless communication link
switches between the 5 channels to find a channel where the
interference least affects the operation of the link. The
remotes indicate via their displays when the processor is not
within operating distance, and when the link has been
interrupted due to interference (see the relevant remote user
guide for more information).
Environmental conditions
CONDITION MINIMUM MAXIMUM
Storage & transport temperature -10°C (14°F) +55°C (131°F)
Operating temperature +5°C (41°F) +40°C (104°F)
Operating relative humidity 0% RH 90% RH
Operating pressure 700 hPa 1030 hPa
OTHER INFORMATION

50 KANSO™ SOUND PROCESSOR
DRAFT ONLY FOR CLINICAL TESTING
Product dimensions (Typical values)
COMPONENT LENGTH WIDTH DEPTH
Kanso processing unit 40.9 mm 35.7 mm 11.3 mm
Product weight (Typical values)
COMPONENT WEIGHT
Kanso processing unit (no batteries or magnet) 8.3 g
Kanso processing unit (including M1 magnet) 11.6 g
Kanso processing unit (including M1 magnet
and two zinc air batteries)
13.8 g
Operating characteristics
CHARACTERISTIC VALUE/RANGE
Sound input frequency range 100 Hz to 8 kHz
Wireless technology Proprietary low power
bi-directional wireless link
RF frequency 2.4 GHz
Operating voltage 2.0 V to 3.1 V
Power consumption 20 mW to 60 mW
Button functions Turn processor on and off, turn audio
sources on and off, change program
Remote communication range Up to 2 m
Batteries Two PR44 (zinc air) button cell
batteries, 1.45V (nominal) each
Cochlear recommends p675 zinc air
batteries designed for Cochlear implant
use

USER GUIDE 51
DRAFT ONLY FOR CLINICAL TESTING
OTHER INFORMATION
Electromagnetic compatiblity (EMC)
Guidance and manufacturer’s declaration
The Nucleus range of sound processors, remote assistants and
remote controls are intended for use in the electromagnetic
environments specified in this document.
They have been tested and found to be in compliance as
shown. You should take care to use your equipment as
described.
Electromagnetic emissions
EMISSION TEST COMPLIANCE GUIDANCE
RF emissions
CISPR 11
Group 1 RF energy is only used for its
internal function. The RF
emissions are very low and
not likely to cause any
interference in nearby
electronic equipment
RF emissions
CISPR 11
Class B The device is suitable for use
in all establishments, including
domestic establishments and
those directly connected to
public low-voltage power
supply network that supplies
buildings used for domestic
purposes.
Harmonic emissions
IEC 61000-3-2
Not applicable
Voltage fluctuations/
flicker emissions IEC
61000-3-3

52 KANSO™ SOUND PROCESSOR
DRAFT ONLY FOR CLINICAL TESTING
Electromagnetic immunity
IMMUNITY TEST IEC 60601
TEST LEVEL
COMPLIANCE
LEVEL GUIDANCE
Electrostatic
discharge
IEC 61000-4-2
±6 kV contact
±8 kV air
±6 kV contact
±8 kV air
See Warnings and
Cautions sections
Electrical fast
transient/burst IEC
61000-4-4
Not applicable
Surge
IEC 61000-4-5
Voltage dips, short
interruptions and
voltage variations
on power supply
input lines IEC
61000-4-11
Power frequency
(50/60 Hz)
magnetic field IEC
61000-4-8
3 A/m 3 A/m Power frequency
magnetic fields be
at levels
characteristic of a
typical location in a
typical commercial
or hospital
environment
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
Not applicable
3 V/m 80 MHz
to 2.5 GHz
3 V/m See Warnings and
Cautions sections,
and “Guidance”
below

USER GUIDE 53
DRAFT ONLY FOR CLINICAL TESTING
OTHER INFORMATION
Guidance
Portable and mobile RF communications equipment should
be used no closer to any part of the devices, including cables,
than the recommended separation distance calculated from
the equation applicable to the frequency of the transmitter.
Recommended separation distance (d):
d = 1.2
P
80 MHz to 800 MHz
d = 2.3
P
800 MHz to 2.5 GHz
where P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter
manufacturer and d is the recommended separation distance
in metres (m). Field strengths from fixed RF transmitters, as
determined by an electromagnetic site surveya, should be less
than the compliance level in each frequency rangeb.
Interference may occur in the vicinity of equipment marked
with the following symbol:
NOTES
• At 80 MHz and 800 MHz, the higher frequency range
applies.
• These guidelines may not apply in all situations.
Electromagnetic propagation is affected by absorption and
reflection from structures, objects and people.
54 KANSO™ SOUND PROCESSOR
DRAFT ONLY FOR CLINICAL TESTING
Explanatory notes:
a. Field strengths from fixed transmitters, such as base
stations for radio (cellular/cordless) telephones and land
mobile radios, amateur radio, AM and FM radio broadcast
and TV broadcast cannot be predicted theoretically with
accuracy. To assess the electromagnetic environment due
to fixed RF transmitters, an electromagnetic site survey
should be considered. If the measured field strength in the
location in which the processor is used exceeds the
applicable RF compliance level above, the processor
should be observed to verify normal operation. If
abnormal performance is observed, additional measures
may be necessary, such as reorienting or relocating the
processor.
b. Over the frequency range 150 kHz to 80 MHz, field
strengths should be less than 3 V/m.
Recommended separation distances
Your processor is intended for use in an electromagnetic
environment where the radiated RF disturbances are
controlled.
To prevent electromagnetic interference, maintain a
minimum distance between the portable and mobile RF
communications equipment (transmitters) and the device as
recommended below, according to the maximum output
power of the communications equipment.

USER GUIDE 55
DRAFT ONLY FOR CLINICAL TESTING
OTHER INFORMATION
RATED
MAXIMUM
OUTPUT
POWER OF
TRANSMITTER
W
SEPARATION DISTANCE ACCORDING TO
FREQUENCY OF TRANSMITTER M
150 KHZ TO
80 MHZ
D = 1.2
P
80 MHZ TO
800 MHZ
D = 1.2
P
800 MHZ TO
2.5 GHZ
D = 2.3
P
0.01 Not applicable 0.12 0.23
0.1 0.38 0.73
1 1.2 2.3
10 3.8 7.3
100 12 23
For transmitters rated at a maximum output power not listed
above, the recommended separation distance d in metres (m)
can be estimated using the equation applicable to the
frequency of the transmitter, where P is the maximum output
power rating of the transmitter in watts (W) according to the
transmitter manufacturer.
NOTE
• At 80 MHz and 800 MHz, the separation distance for the
higher frequency range applies.
• These guidelines may not apply in all situations.
Electromagnetic propagation is affected by absorption and
reflection from structures, objects and people.
56 KANSO™ SOUND PROCESSOR
DRAFT ONLY FOR CLINICAL TESTING
Equipment classification
Your sound processor is internally powered equipment Type B
applied part as described in the international standard IEC
60601-1:2012, Medical Electrical Equipment – Part 1: General
Requirements for Basic Safety and Essential Performance.
FCC (Federal Communications Commission)
and Canadian IC compliance
This device complies with part 15 of the FCC Rules and with
RSS-210 of Industry Canada. Operation is subject to the
following two conditions:
• This device may not cause harmful interference.
• This device must accept any interference received,
including interference that may cause undesired operation.
Changes or modifications made to this equipment not
expressly approved by Cochlear Limited may void the FCC
authorisation to operate this equipment.
This equipment has been tested and found to comply with the
limits for a Class B digital device, pursuant to Part 15 of the
FCC Rules. These limits are designed to provide reasonable
protection against harmful interference in a residential
installation.
This equipment generates, uses and can radiate radio
frequency energy and, if not installed and used in accordance
with the instructions, may cause harmful interference to radio
communications. However, there is no guarantee that
interference will not occur in a particular installation.

USER GUIDE 57
DRAFT ONLY FOR CLINICAL TESTING
OTHER INFORMATION
If this equipment does cause harmful interference to radio or
television reception, which can be determined by turning the
equipment off and on, the user is encouraged to try to correct
the interference by one or more of the following measures:
• Reorient or relocate the receiving antenna.
• Increase the separation between the equipment and
receiver.
• Connect the equipment into an outlet or a circuit different
from that to which the receiver is connected.
• Consult the dealer or an experienced radio/TV technician
for help.
FCC ID: WTO-CP950
IC ID: 8039A-CP950
Certification and applied standards
The Kanso Sound Processor fulfils the essential requirements
listed in Annex 1 of the EC directive 90/385/EEC on Active
Implantable Medical Devices as per the conformity
assessment procedure in Annex 2.
The year in which authorisation to affix the CE mark was
granted was 2016.
The Kanso Sound Processor also fulfils the essential
requirements listed in the EC directive 1999/5/EC on Radio
and Terminal Telecommunication Equipment as per the
conformity assessment procedure in Annex IV.
58 KANSO™ SOUND PROCESSOR
DRAFT ONLY FOR CLINICAL TESTING
Environmental protection
Your sound processor contains electronic components subject
to the Directive 2002/96/EC on waste electrical and
electronic equipment.
Help protect the environment by not disposing of your sound
processor or batteries with your unsorted household waste.
Please recycle your sound processor according to your local
regulations.
Privacy and the collection of personal
information
During the process of receiving a Cochlear device, personal
information about the user/recipient or their parent, guardian,
carer and hearing health professional will be collected for use
by Cochlear and others involved in care with regard to the
device.
For more information please read Cochlear’s Privacy Policy on
www.cochlear.com or request a copy from Cochlear at the
address nearest you.
USER GUIDE 59
DRAFT ONLY FOR CLINICAL TESTING
OTHER INFORMATION
Labelling symbols
The following symbols may appear on your processor or
remote components and/or packaging:
Refer to instruction manual
Specific warnings or precautions associated with the device,
which are not otherwise found on the label
Manufacturer
Authorised representative in the European Community
Catalogue number
Serial number
Batch code
Date of manufacture
Temperature limits
CE registration mark with notified body number
Radio compliance certification for Australia and New
Zealand
R
202
LSB001
Radio compliance certification for Japan
KCC-CRM-
COH-CP900
Radio compliance certification for Korea
By prescription

60 KANSO™ SOUND PROCESSOR
DRAFT ONLY FOR CLINICAL TESTING
Recyclable material
Dispose of electrical components in accordance with your
local regulations
Type B applied part
Ingress Protection Rating
• Protected against failure from dust penetration
• Protected against failure from splashing with water
Legal statement
The statements made in this guide are believed to be true and
correct as of the date of publication. However, specifications
are subject to change without notice.
© Cochlear Limited 2016

Notes
...........................................................................................
...........................................................................................
...........................................................................................
...........................................................................................
...........................................................................................
...........................................................................................
...........................................................................................
...........................................................................................
...........................................................................................
...........................................................................................
...........................................................................................
...........................................................................................
...........................................................................................
...........................................................................................
...........................................................................................
...........................................................................................
...........................................................................................
...........................................................................................
...........................................................................................
...........................................................................................
...........................................................................................
...........................................................................................
...........................................................................................
...........................................................................................
...........................................................................................
...........................................................................................

552811 ISS1 OCT15
ACE, Advance Off-Stylet, AOS, AutoNRT, Autosensitivity, Beam, Button, Carina, Cochlear,
コクレア, Codacs, Contour, Contour Advance, Custom Sound, ESPrit, Freedom,
Hear now. And always, Hybrid, inHear, Invisible Hearing, MET, MP3000, myCochlear, NRT,
Nucleus, 科利耳, Off-Stylet, SmartSound, Softip, SoftWear, SPrint, the elliptical logo and
Whisper are either trademarks or registered trademarks of Cochlear Limited. Ardium, Baha,
Baha Divino, Baha Intenso, Baha PureSound, Baha SoftWear, BCDrive, DermaLock, Vistafix
and WindShield are either trademarks or registered trademarks of Cochlear Bone Anchored
Solutions AB. Bluetooth is a registered trademark of Bluetooth SIG.
© Cochlear Limited 2016
Cochlear Ltd
(ABN 96 002 618 073) 1 University Avenue, Macquarie University, NSW 2109, Australia
Tel: +61 2 9428 6555 Fax: +61 2 9428 6352
Cochlear Ltd
(ABN 96 002 618 073) 14 Mars Road, Lane Cove, NSW 2066, Australia
Tel: +61 2 9428 6555 Fax: +61 2 9428 6352
Cochlear Americas
13059 E Peakview Avenue, Centennial, CO 80111, USA
Tel: +1 303 790 9010 Fax: +1 303 792 9025
Cochlear Canada Inc
2500-120 Adelaide Street West, Toronto, ON M5H 1T1, Canada
Tel: +1 416 972 5082 Fax: +1 416 972 5083
Cochlear AG
EMEA Headquarters, Peter Merian-Weg 4, 4052 Basel, Switzerland
Tel: +41 61 205 8204 Fax: +41 61 205 8205
Cochlear Deutschland GmbH & Co. KG
Karl-Wiechert-Allee 76A, 30625 Hannover, Germany
Tel: +49 511 542 770 Fax: +49 511 542 7770
Cochlear Europe Ltd 6 Dashwood Lang Road, Bourne Business Park, Addlestone, Surrey KT15 2HJ, United Kingdom
Tel: +44 1932 26 3400 Fax: +44 1932 26 3426
Cochlear Benelux
NV Schaliënhoevedreef 20 i, B-2800 Mechelen, Belgium
Tel: +32 15 79 55 11 Fax: +32 15 79 55 70
Cochlear France S.A.S.
135 Route de Saint-Simon, 31100 Toulouse, France
Tel: +33 5 34 63 85 85 (International) or 0805 200 016 (National) Fax: +33 5 34 63 85 80
Cochlear Italia
S.r.l. Via Larga 33, 40138 Bologna, Italy
Tel: +39 051 601 53 11 Fax: +39 051 39 20 62
Cochlear Nordic AB
Konstruktionsvägen 14, 435 33 Mölnlycke, Sweden
Tel +46 31 335 14 61 Fax +46 31 335 14 60
Cochlear Tıbbi Cihazlar ve Sağlık Hizmetleri Ltd. Şti.
Çubuklu Mah. Boğaziçi Cad., Boğaziçi Plaza No: 6/1, Kavacık, TR-34805 Beykoz-Istanbul, Turkey
Tel: +90 216 538 5900 Fax: +90 216 538 5919
Cochlear (HK) Limited
Room 1204, 12/F, CRE Building, No 303 Hennessy Road, Wanchai, Hong Kong SAR
Tel: +852 2530 5773 Fax: +852 2530 5183
Cochlear Korea Ltd
1st floor, Cheongwon building, 828-5, Yuksam dong, Kangnam gu, Seoul, Korea
Tel: +82 2 533 4663 Fax: +82 2 533 8408
Cochlear Medical Device (Beijing) Co., Ltd
Unit 2208 Gemdale Tower B, 91 Jianguo Road, Chaoyang District, Beijing 100022, P.R. China
Tel: +86 10 5909 7800 Fax: +86 10 5909 7900
Cochlear Medical Device Company India Pvt. Ltd.
Ground Floor, Platina Building, Plot No C-59, G-Block, Bandra Kurla Complex, Bandra (E), Mumbai – 400 051, India
Tel: +91 22 6112 1111 Fax: +91 22 6112 1100
株式会社日本コクレア
(Nihon Cochlear Co Ltd)〒 113-0033 東京都文京区本郷2-3-7 お茶の水元町ビル
Tel: +81 3 3817 0241 Fax: +81 3 3817 0245
Cochlear Middle East FZ-LLC
Dubai Healthcare City, Al Razi Building 64, Block A, Ground Floor, Offices IR1 and IR2, Dubai, United Arab Emirates
Tel: +971 4 818 4400 Fax: +971 4 361 8925
Cochlear Latinoamérica S.A.
International Business Park, Building 3835, Office 103, Panama Pacifico, Panama
Tel: +507 830 6220 Fax: +507 830 6218
Cochlear NZ Limited
Level 4, Takapuna Towers, 19-21 Como St, Takapuna, Auckland 0622, New Zealand
Tel: + 64 9 914 1983 Fax: +61 2 8002 2800
www.cochlear.com