Contec Medical Systems PM10 Portable ECG Monitor User Manual
Contec Medical Systems Co., Ltd. Portable ECG Monitor Users Manual
Users Manual
Contec Medical Systems Co., Ltd.
PM10
Portable ECG Monitor
I
Contents
Foreword ............................................................................................................................................ II
Chapter 1 Notice ................................................................................................................................. 1
1.1 Generic notice ....................................................................................................................... 1
1.2 Measurement notice .............................................................................................................. 1
1.3 Safety notice .......................................................................................................................... 1
1.4 EMC notice ........................................................................................................................... 1
Chapter 2 Introduction ........................................................................................................................ 2
2.1 Characteristic ........................................................................................................................ 2
2.2 Application ............................................................................................................................ 2
Chapter 3 Primary Technical Orders ................................................................................................... 3
3.1 Normal work environment .................................................................................................... 3
3.2 Basic parameters ................................................................................................................... 3
Chapter 4 Operation Directions .......................................................................................................... 4
4.1 How to use ............................................................................................................................ 4
4.2 Menu operations .................................................................................................................... 4
4.3 Sync software operations ...................................................................................................... 6
Chapter 5 Trouble Shooting and Solution ........................................................................................... 7
Chapter 6 Maintenance&Transportation&Storage .............................................................................. 7
6.1 Cleaning and disinfecting ...................................................................................................... 7
6.2 Maintenance .......................................................................................................................... 7
6.3 Transportation and storage .................................................................................................... 8
Chapter 7 The Explanation of Symbols .............................................................................................. 8
Chapter 8 Packing List ........................................................................................................................ 8

II
Foreword
Thank you very much for purchasing the PM10 Portable ECG Monitor.
This user manual introduces detail product information about its character, requirement,
structure, performance, specification, appropriate methods of transportation, installation, usage,
operation, repair, maintenance and storage, and safety measures of how to protect the operator and
product. Please read details in the following chapters.
Please read the user manual carefully before using the product and strictly follow its
regulations to operate. The user manual indicates the operations that users need to pay much
attention to, that may lead to abnormality, or may danger to the device or human body during using.
Our company will not response the security, reliability and performance for any abnormality or
device and human body damage caused by not following this user manual to use, maintain and
store, nor provide free service for any situations above.
We apologize for the content in the manual is subject to change according to product upgrades
without notice.
The product is reusable as a medical instrument.
Warning:
The product is not a examination device applied in clinical medicine, and its results can not
serve as the basis for diagnosis, but can be used as a reference for patient to take further
medical treatment and reference for doctor to diagnose.
The reliability depends on whether users are following the operation and maintenance in the
user manual or not.
All servicing and future upgrade to the device must be carried out by personnel trained and
authorized by our company, and using the original fittings for maintenance.
This user manual contains proprietary information, which is protected by copyright. All rights
reserved. Reproduction, adaption or translation, for any part of the manual without prior written
permission, is prohibited.
Our company takes the responsibilities as follows:
1. To provide qualified products according to enterprise standard for users;
2. To provide services of installation, debugging and training according to the contract;
3. To provide one year warranty and product maintenance after warranty period according to
the contract;
4. To respond user’s requests in time.
1
Chapter 1 Notice
1.1 Generic notice
1) Do not use the device in locations subject to high temperatures or humidity. Use in the
temperature within 5 to 40˚C and humidity within 25% to 80%RH.
2) Do not wash the device with water.
3) Do not use or store the device in the following ambient conditions:
Near fires or open flames
Locations exposed to strong vibration
Locations exposed to strong electromagnetic fields
4) Do not disinfect the device in autoclave or gas sterilizer.
5) Such as skin allergies or skin damage, do not use this device.
6) The device service lift is 3 years. Do not throw away the device and accessories when
they can't work. If the device needs to dispose, it should meet the local laws and
regulations requirement.
7) lay responsible organization must contact its local authorities to determine the proper
method of disposal of potentially bio hazardous parts and accessories.
1.2 Measurement notice
1) If your skin is dry, wipe them with disinfectant alcohol or electric salve to strengthen the
electric capability
2) You are better to comfortably sit, draw yourself up, begin to measure when the waveform
level off.
3) When measuring, the finger and chest electrodes should touch your skin exactly, roundly
and well.
1.3 Safety notice
1) No sampling in the battery-charging.
2) Lay the device in shady and cool environment when you are not going to use it for a long
period of time, and electrify per three months.
3) Do not use the device in the environment placed inflammables objects, such as anesthetic.
1.4 EMC notice
1) Please note the effect from EMC when using the device, because it can be influenced by
portable or movable high electromagnetic compatibility RF devices.

2
2) This equipment needs to be installed and put into service in accordance with the
information provided in the ACCOMPANYING DOCUMENTS.
3) Wireless communications equipment can affect ME EQUIPMENT and should be kept at least
a distance d away from the equipment. The distance d is calculated by the
MANUFACTURER from the 800 MHz to 2,5 GHz column of Table 5 or Table 6 of IEC
60601-1-2:2007.
Chapter 2 Introduction
The portable ECG monitor is designed for family and individual users. It is a good helper for
family members to prevent from cardiovascular disease, for it can monitoring patients ECG
anytime at anyplace with easy operation. The device can record, analysis and display user’s ECG
waveform, capturing the pathological ECG waveform when user happen to heart attack or other
unpleasant symptoms. The ECG monitor can be conducted not limited in the hospital, which saves
money from the physical check-up for users. After connected with a computer, users can print their
ECG waveform, providing data reference for doctors.
2.1 Characteristic
1) Handsome shape, handy operation, convenient tote.
2) Monitor and record real-time ECG waveform and HR anytime and anywhere.
3) Built-in large capability rechargeable lithium battery, continuously sample 200 ECG
waveform after charged once.
4) The applied part is the electrode
2.2 Application
1) Occasion: family, medical clinic and hospital. The device can't be used as a general
electrocardiogram for clinical examination.
2) Object: people under high pressure and workload for long time, heart disease patients,
middle aged and aged people, sub-health people
3) Purpose: The device is only used for ECG monitoring and data storage. It is NOT a
therapy equipment. Operation method is simple and less requirement for the operating
personnel.

3
Chapter 3 Primary Technical Orders
3.1 Normal work environment
1) Operation environment
Temperature: +5 ~+40℃℃
Relative humidity: 25%~80%
Atmospheric pressure:70kPa~106kPa
Power supply: built-in rechargeable lithium battery, voltage: 3.7V
2) Transportation and storage environment
Temperature: -40℃~+55℃
Relative humidity: ≤95%
Atmospheric pressure:50kPa~106kPa
3.2 Basic parameters
1) Calibration voltage: 1mV±5%
2) Standard sensitivity: 10mm/mV±5%
3) Amplitude frequency characteristic: standard: 10Hz; 1Hz~20Hz; (+0.4dB,-3dB)
4) Noise level: ≤30μV
5) CMRR: ≥60dB
6) Scanning speed: 25mm/s±5%
7) Sampling rate: 250 dots/s
8) HR measurement range: 30bpm~300bpm, error: ±1bpm or 1%
9) Battery Voltage: DC 3.7V 530mAh
10) Type of protection against electric shock: Internal power device
11) Degree of protection against electric shock: Type BF applied part
12) Degree of waterproof: IP22
4
Chapter 4 Operation Directions
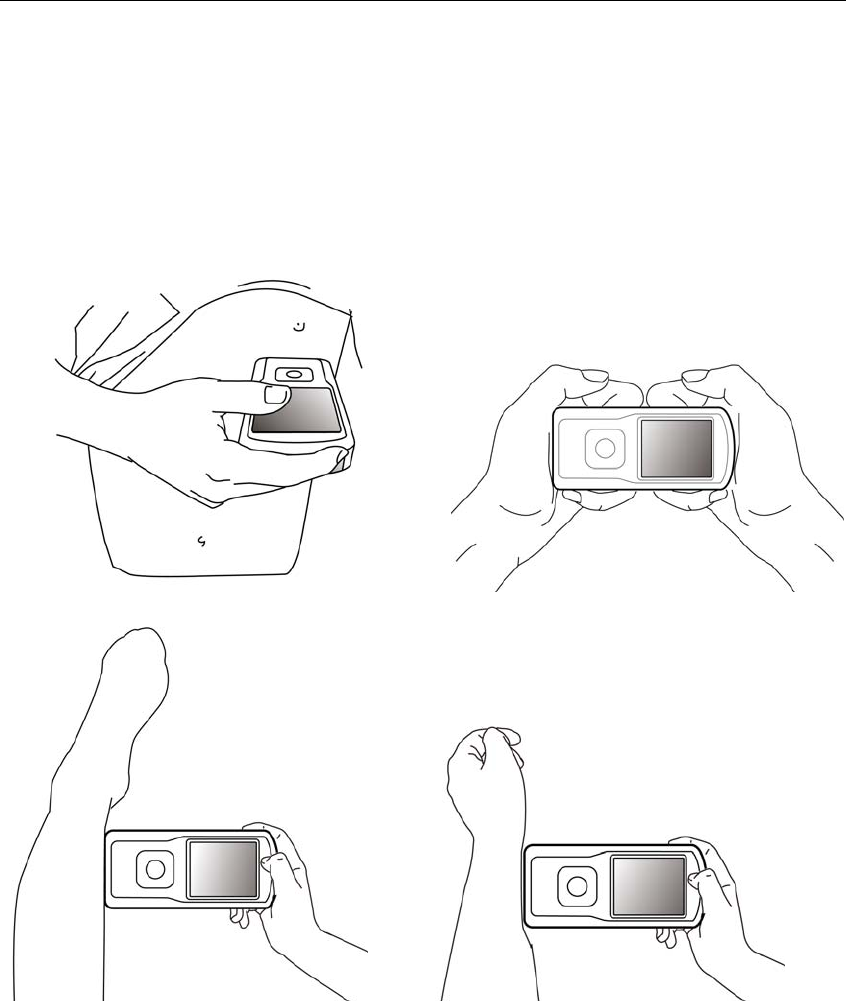
4.1 How to use
There are several measurement methods as shown in the following pictures.
4.2 Menu operations
1) Start-up
Long press the on/off key for 2 seconds, you will hear a beep sound and see the screen lighting.
The device will keep level off when not measuring.
2) Start measurement
After start-up, the device will enter into pre-sample interface. Please use the correct
measurement method as guided, the ECG waveform will displayed on the screen, as shown in
Figure 4.1.
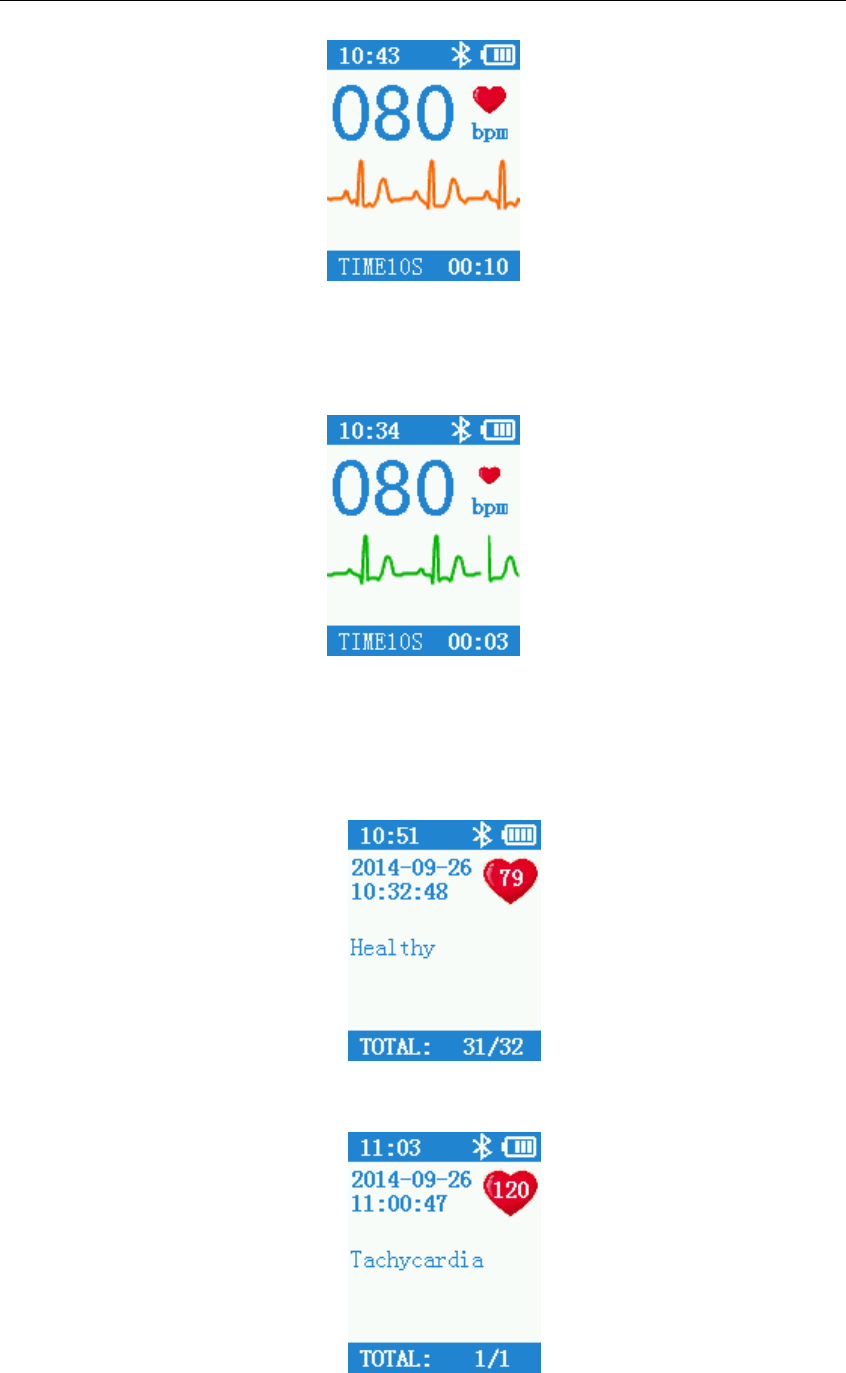
5
Figure 4.1 Pre-sample Interface
When the waveform becomes stable, the device will start formal sampling automatically, the
color of waveform turns to green, sample time countdown on the bottom right corner begins until
finished one time sample. See Figure 4.2:
Figure 4.2 Formal Sample Interface
The device will enter into case review interface after completed sampling. Case review
interface displays the sampling start time, heart rate and diagnosis. The diagnosis including
normal and different kinds of heart rate abnormal, please refer to Figure 4.3 and Figure 4.4.
Figure 4.3 Case Review Interface (Normal)
Figure 4.4 Case Review Interface
6
When the device enters into case review interface, it will display the latest sampled case.
Click the button to review other cases information. The device can store 99 pieces of cases at
most. If reaches to the limit, new stored case will cover the original case, the one that stored at
the earliest, piece by piece.
The device will automatically turn to sampling interface to continue if the user holding
the electrode at both ends again when the device is under the case review interface.
3) Battery Operation Notes
4) Li-battery
Battery Voltage: DC 3.7V 530mAh. The device can continuously work for more than 4
hours when battery is completely charged.The cycle life of the battery up to 300 times.
Two method for charging:
(a) Connect the device with a computer by using Micro USB cable, charging completed
after about 2~4 hours.
(b) Use a Micro USB to connect the device with a power adapter (output current>
500mA, 5V), charging completed after about 2 hours.
(c) The connected power supply shall be in compliance with IEC 60950-1standard.
Battery display
(d)
5) Auto power off
The device will automatically shut down after no operations within 1 minutes.
4.3 Sync software operations
Users can operate in the PC synchronous software according to necessary, which including
sample mode and time setting, upload case, case review, measurement, print, etc. Please refer to
operation directions of PC synchronous software for details.
4.4 accessories list
No. Description Quantity
1 Host 1
2 USB cable 1
No. Indicator Description
a full power
b capacity : 3/4
c capacity: 1/2
d capacity : 1/4
e
Using battery, low power, it is recommended to recharge the
battery.The device will automatically shut down.
7
Chapter 5 Trouble Shooting and Solution
If the device has a problem account, please look up the following sheet for solutions first, if
not included in the following issues and you can not solve either, please contact with the customer
service.
Problem Cause Solution
Start-up failure after long press
the on/off key The batteries are worn out. Please recharge the batteries.
Automatically shut down in
using process The batteries are worn out. Please recharge the batteries.
The noise is too big or the
waveform is random in ECG
sample process.
Your skin is dry. Wipe them with disinfectant
alcohol or electric salve
There is unwanted
movement in sample process
Please comfortably sit, draw
yourself up to carry on sample
The sample environment has
strong electromagnetic
noise.
Please close interference
source or resample in no
strong electromagnetic noise
environment.
Chapter 6 Maintenance&Transportation&Storage
6.1 Cleaning and disinfecting
Turn off the device before cleaning. Medical alcohol is available for the device disinfection,
then air dry. Or just wipe it with a dry and clean cloth for cleaning. Do not allow any liquid to enter
the device.
If for single patient use, the cleaning frequency is before use.
If for multiple patients use, the cleaning frequency should be between use. The disinfection
frequency is 1time/week.
6.2 Maintenance
1) Non-maintenance personnel designated by our company, do not open the instrument case
so as to avoid damage to internal components.
2) Any equipment maintenance and upgrades must be carried by the professionals who are
trained and authorized of the company.
3) Prevent any liquid from seeping into the device as it will affect the safety and
performance of the device.
4) The device should avoid the use of violent shaking or impact.
5) Do not place objects on the device. This could damage the touch screen.
8
6.3 Transportation and storage
1) The device transportation adopts general transportation means or follows the contract
requirements. Avoid violent shock, vibration, rain and snow splash during the process of
transportation.
2) Store the packaged device in an environment with temperature -40℃~+55℃, relative
humidity no more than 95%, atmospheric pressure 500hPa~1060hPa, no corrosion gas and
well-ventilated room.
Chapter 7 The Explanation of Symbols
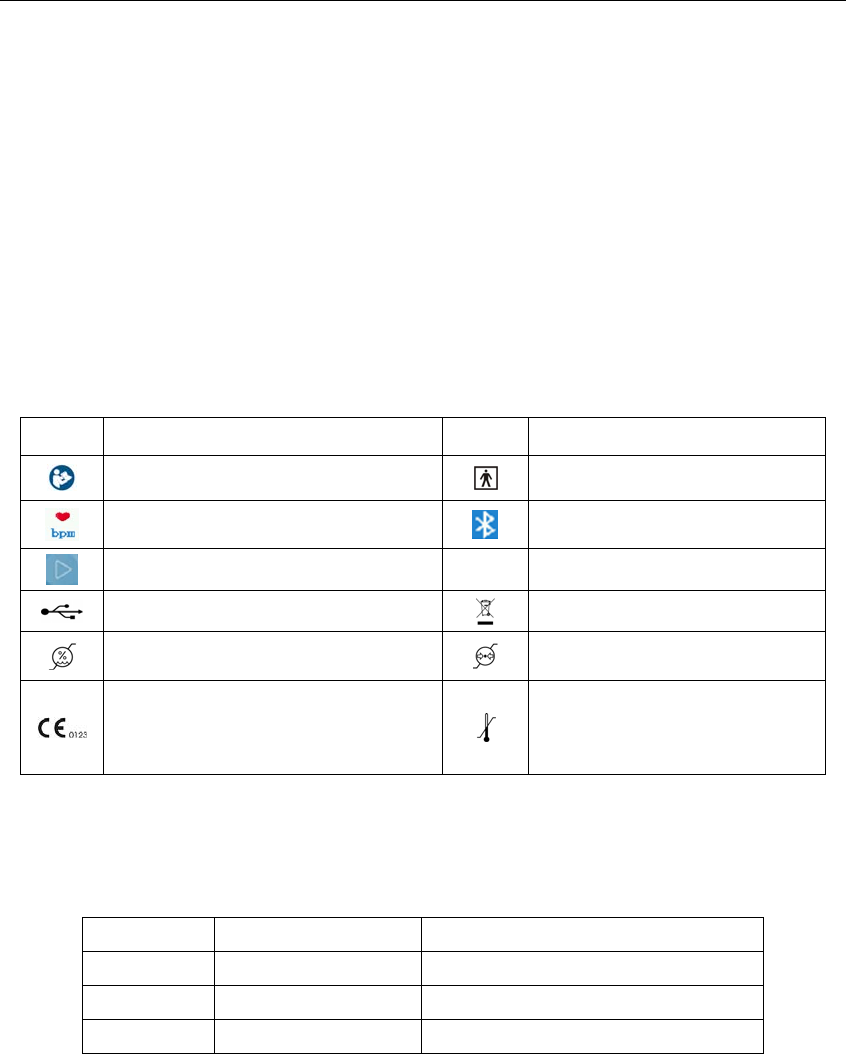
Signal Description Signal Description
Refer to instruction manual/booklet Type BF
Pulse rate (bpm) Bluetooth
power button/function button IP22 International Protection
USB RQO\IRUEDWWHU\FKDUJLQJ WEEE (2002/96/EC)
Humidity limitation Atmosperic pressure limitation
This item is compliant with Medical
Device Directive 93/42/EEC of June
14, 1993, a directive of the European
Economic Community.
Temperature limitation
Chapter 8 Packing List
No. Description Quantity
1 Host 1
2 USB cable 1
3 User Manual 1
1RWH

FCC WARNING
This device complies with part 15 of the FCC rules. Operation is subject to the following two conditions:
(1) this device may not cause harmful interference, and
(2) this device must accept any interference received, includinginterference that may cause undesired operation.
Changes or modifications not expressly approved by the party responsible for compliance could void the user’s authority
to operate the equipment.
NOTE: This equipment has been tested and found to comply with the limits for a Class B digital device, pursuant to part
15 of the FCC Rules. These limits are designed to provide reasonable protection against harmful interference in a
residential installation. This equipment generates uses and can radiate radio frequency energy and, if not installed and
used in accordance with the instructions, may cause harmful interference to radio communications. However, there is no
guarantee that interference will not occur in a particular installation. If this equipment does cause harmful interference to
radio or television reception, which can be determined by turning the equipment off and on, the user is encouraged to try
to correct the interference by one or more of the following measures:
- Reorient or relocate the receiving antenna.
- Increase the separation between the equipment and receiver.
-Connect the equipment into an outlet on a circuit different from that to which the receiver is connected.
-Consult the dealer or an experienced radio/TV technician for help
Contec Medical Systems Co., Ltd.
A
ddress:No.112 Qinhuang West Street, Economic &Technical Development Zone,
Qinhuangdao, Hebei Province, PEOPLE’S REPUBLIC OF CHINA
Tel: 0086-335-8015430
Fax: 0086-335-8015588
Technical support: 0086-335-8015431
E-mail: cms@contecmed.com.cn
contec88@gmail.com
Website: http://www.contecmed.com
EC REPRESENTATIVE
Shanghai International Holding Corp. GmbH(Europe)
A
ddress: Eiffestrasse 80,20537,Hamburg,Germany
Tel: +49-40-2513175
Fax: +49-40-255726
E-mail: shholding@hotmail.com
File No.: CMS2.782.350ESS/0.91
Ver si o n : 0 . 0