Clinical Manual Of Pain Management In Psychiatry (Concise Guides) 114 Raphael J. Leo 1585622753 A
User Manual: manual pdf -FilePursuit
Open the PDF directly: View PDF ![]() .
.
Page Count: 284 [warning: Documents this large are best viewed by clicking the View PDF Link!]
- Contents
- List of Tables
- List of Figures
- Preface
- 1 Introduction
- 2 Sensory Pathways of Pain and Acute Versus Chronic Pain
- Pain-Relaying Pathways and Mechanisms
- Role of the Autonomic Nervous System in Pain
- Pain-Modulating Processes Within the Nervous System
- Acute Versus Chronic Pain
- Classifications of Acute and Chronic Pain
- Categories of Chronic Pain
- "Patienthood" as a Psychosocial State: The Patient With Simple Versus Chronic Pain
- Multiaxial Pain Classification
- Key Points
- References
- 3 Evaluation of the Pain Patient
- 4 Common Psychiatric Comorbidities and Psychiatric Differential Diagnosis of the Pain Patient
- 5 Pharmacology of Pain
- Opiate Analgesics
- Tramadol
- Nonopiate Analgesics
- Cyclooxygenase-2 Inhibitors
- Antidepressants
- Anticonvulsant Drugs
- Considering Treatment Options: Antidepressant, Anticonvulsant, or Both?
- Antihistamines
- Benzodiazepines and Anxiolytics
- Triptans
- Stimulants
- Neuroleptics
- Mexiletine
- Alpha[sub(2)]-Adrenergic Agonists
- N-Methyl-D-Aspartate Antagonists
- Corticosteroids
- Muscle Antispasmodics
- Topical Agents
- Herbal Agents
- Cannabinoids
- Placebo Effects
- Key Points
- References
- 6 Psychotherapy
- 7 Special Techniques in Pain Management
- Acupuncture
- Transcutaneous Electrical Nerve Stimulation
- Prolotherapy
- Botulinum Toxin (Botox) Injection
- Anesthesia at the Level of the Spinal Cord
- Regional Neural Blockade
- Neurosurgical Techniques
- Repetitive Transcranial Magnetic Stimulation
- Role of the Psychiatrist in Pain Management Related to Interventions
- Key Points
- References
- 8 Common Pain Disorders
- 9 Special Populations
- 10 Forensic Issues Pertaining to Pain
- Index

Clinical Manual of
Pain Management
in Psychiatry
This page intentionally left blank

Washington, DC
London, England
Clinical Manual of
Pain Management
in Psychiatry
Raphael J. Leo, M.D.
Associate Professor, State University of New York at Buffalo
School of Medicine and Biomedical Sciences;
Department of Psychiatry and
Center for Comprehensive Multidisciplinary Pain Management
Erie County Medical Center
Buffalo, New York
Note: The authors have worked to ensure that all information in this book is accurate
at the time of publication and consistent with general psychiatric and medical standards,
and that information concerning drug dosages, schedules, and routes of administration
is accurate at the time of publication and consistent with standards set by the U.S.
Food and Drug Administration and the general medical community. As medical
research and practice continue to advance, however, therapeutic standards may change.
Moreover, specific situations may require a specific therapeutic response not included
in this book. For these reasons and because human and mechanical errors sometimes
occur, we recommend that readers follow the advice of physicians directly involved in
their care or the care of a member of their family.
Books published by American Psychiatric Publishing, Inc., represent the views and
opinions of the individual authors and do not necessarily represent the policies and
opinions of APPI or the American Psychiatric Association.
If you would like to buy between 25 and 99 copies of this or any other APPI title,
you are eligible for a 20% discount; please contact APPI Customer Service at
appi@psych.org or 800-368-5777. If you wish to buy 100 or more copies of the same
title, please e-mail us at bulksales@psych.org for a price quote.
Copyright © 2007 American Psychiatric Publishing, Inc.
ALL RIGHTS RESERVED
Manufactured in the United States of America on acid-free paper
1110090807 54321
First Edition
Typeset in Adobe’s Formata and AGaramond.
American Psychiatric Publishing, Inc.
1000 Wilson Boulevard, Arlington, VA 22209-3901
www.appi.org
Library of Congress Cataloging-in-Publication Data
Leo, Raphael J., 1962–
Clinical manual of pain management in psychiatry / by Raphael J. Leo.—1st ed.
p. ; cm.
Includes bibliographical references and index.
ISBN 978-1-58562-275-7 (pbk. : alk. paper)
1. Pain—Treatment—Handbooks, manuals, etc. 2. Pain—Psychological aspects—
Handbooks, manuals, etc. 3. Psychotherapy—Handbooks, manuals, etc. 4. Chronic
pain—Handbooks, manuals, etc. I. Title.
[DNLM: 1. Pain—therapy. 2. Chronic Disease—psychology. 3. Pain—psychology.
WL 704 L576m 2007]
RB127.L3966 2007
616′.0472—dc22
2007018819
British Library Cataloguing in Publication Data
A CIP record is available from the British Library.
Contents
List of Tables . . . . . . . . . . . . . . . . . . . . . . . . . . . . ix
List of Figures . . . . . . . . . . . . . . . . . . . . . . . . . . . xiii
Preface . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . xv
1Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Origins and Development of Pain Management . . . . . . .2
Interdisciplinary Pain Medicine . . . . . . . . . . . . . . . . . . . . .3
Traditional Medical Models of Pain Management
Versus the Current Biopsychosocial Paradigm . . . . . . .4
Role of Psychiatrists in Interdisciplinary
Pain Medicine . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5
Key Points . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9
References. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9
2Sensory Pathways of Pain and Acute
Versus Chronic Pain . . . . . . . . . . . . . . . . . . . . . . . 11
Pain-Relaying Pathways and Mechanisms . . . . . . . . . . .12
Role of the Autonomic Nervous System in Pain . . . . . .18
Pain-Modulating Processes
Within the Nervous System . . . . . . . . . . . . . . . . . . . . . .19
Acute Versus Chronic Pain. . . . . . . . . . . . . . . . . . . . . . . .25
Classifications of Acute and Chronic Pain . . . . . . . . . . .25
Categories of Chronic Pain. . . . . . . . . . . . . . . . . . . . . . . .27
“Patienthood” as a Psychosocial State:
The Patient With Simple Versus Chronic Pain . . . . . . .27
Multiaxial Pain Classification . . . . . . . . . . . . . . . . . . . . . .30
Key Points . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .31
References. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .31
3Evaluation of the Pain Patient . . . . . . . . . . . . . . 35
Conducting an Interview . . . . . . . . . . . . . . . . . . . . . . . . .37
Obtaining the Pain History. . . . . . . . . . . . . . . . . . . . . . . .38
Evaluation of Treatment Suitability . . . . . . . . . . . . . . . . .47
Pain Assessment Instruments . . . . . . . . . . . . . . . . . . . . .47
Key Points . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .60
References. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .60
4Common Psychiatric Comorbidities and
Psychiatric Differential Diagnosis
of the Pain Patient . . . . . . . . . . . . . . . . . . . . . . . . 63
Pain Disorder. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .64
Depression. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .70
Anxiety . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .72
Sleep Disorders. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .74
Substance-Related Disorders. . . . . . . . . . . . . . . . . . . . . .75
Personality Disorders . . . . . . . . . . . . . . . . . . . . . . . . . . . .77
Schizophrenia . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .78
Key Points . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .79
References. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .80
5Pharmacology of Pain . . . . . . . . . . . . . . . . . . . . . 83
Opiate Analgesics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .83
Tramadol . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .94
Nonopiate Analgesics. . . . . . . . . . . . . . . . . . . . . . . . . . . .95
Cyclooxygenase-2 Inhibitors . . . . . . . . . . . . . . . . . . . . . .96
Antidepressants. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .97
Anticonvulsant Drugs . . . . . . . . . . . . . . . . . . . . . . . . . . .104
Considering Treatment Options: Antidepressant,
Anticonvulsant, or Both? . . . . . . . . . . . . . . . . . . . . . . .106
Antihistamines . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .110
Benzodiazepines and Anxiolytics . . . . . . . . . . . . . . . . .111
Triptans . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .112
Stimulants . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .113
Neuroleptics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .114
Mexiletine . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .114
Alpha2-Adrenergic Agonists . . . . . . . . . . . . . . . . . . . . . .115
N-Methyl-D-Aspartate Antagonists. . . . . . . . . . . . . . . . .115
Corticosteroids . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .116
Muscle Antispasmodics . . . . . . . . . . . . . . . . . . . . . . . . .117
Topical Agents. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .119
Herbal Agents . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .120
Cannabinoids . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .120
Placebo Effects . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .122
Key Points . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .122
References. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .124
6Psychotherapy . . . . . . . . . . . . . . . . . . . . . . . . . . 131
Resistance to Psychotherapy . . . . . . . . . . . . . . . . . . . . .133
Factors to Be Addressed in Psychotherapy . . . . . . . . .134
Behavior Therapy . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .140
Cognitive-Behavioral Therapy . . . . . . . . . . . . . . . . . . . .143
Supportive Therapy. . . . . . . . . . . . . . . . . . . . . . . . . . . . .147
Marital, Couples, and Family Therapy. . . . . . . . . . . . . .148
Group Therapy . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .150
Adjunctive Interventions. . . . . . . . . . . . . . . . . . . . . . . . .151
Vocational Rehabilitation. . . . . . . . . . . . . . . . . . . . . . . .156
Key Points . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .157
References. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .158
7Special Techniques in Pain Management . . . .161
Acupuncture . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .162
Transcutaneous Electrical Nerve Stimulation. . . . . . . .164
Prolotherapy . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .164
Botulinum Toxin (Botox) Injection . . . . . . . . . . . . . . . .164
Anesthesia at the Level of the Spinal Cord . . . . . . . . .165
Regional Neural Blockade . . . . . . . . . . . . . . . . . . . . . . .166
Neurosurgical Techniques . . . . . . . . . . . . . . . . . . . . . . .170
Repetitive Transcranial Magnetic Stimulation . . . . . . .172
Role of the Psychiatrist in Pain Management
Related to Interventions. . . . . . . . . . . . . . . . . . . . . . . .173
Key Points . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .177
References. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .177
8Common Pain Disorders . . . . . . . . . . . . . . . . . . 179
Headache. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .179
Back Pain. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .186
Nonarticular Pain Disorders. . . . . . . . . . . . . . . . . . . . . .188
Osteoarthritis and Rheumatoid Arthritis. . . . . . . . . . . .194
Neuropathic Pain . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .197
Sympathetically Mediated Pain: Complex Regional
Pain Syndromes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .200
Phantom Limb Pain . . . . . . . . . . . . . . . . . . . . . . . . . . . .202
Cancer and HIV . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .204
Key Points . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .207
References. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .208
9Special Populations . . . . . . . . . . . . . . . . . . . . . . 213
Pediatric Patients . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .213
Geriatric Patients. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .216
Pregnancy . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .219
Cultural Issues. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .220
Substance-Dependent and Substance-Abusing Patients. . .222
Patients With Terminal Conditions . . . . . . . . . . . . . . . .224
Key Points . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .229
References. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .229
10 Forensic Issues Pertaining to Pain . . . . . . . . . . 233
Litigation and Pain . . . . . . . . . . . . . . . . . . . . . . . . . . . . .233
Medication Diversion . . . . . . . . . . . . . . . . . . . . . . . . . . .234
Opiate Adulteration and Misuse . . . . . . . . . . . . . . . . . .236
Legal Issues Related to Opioid Prescribing . . . . . . . . .237
Patient Contracts. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .238
Drug Testing in Clinical Practice . . . . . . . . . . . . . . . . . .240
State and National Prescribing Data Banks . . . . . . . . .240
Confidentiality . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .242
Disability Compensation . . . . . . . . . . . . . . . . . . . . . . . .244
Key Points . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .247
References. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .248
Index . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 251
List of Tables
Table 1–1 Traditional versus biopsychosocial models of pain . . 6
Table 1–2 Role of psychiatrists in pain management
(biopsychosocial approach) . . . . . . . . . . . . . . . . . . . . 8
Table 2–1 Sensory neural fiber types of first-order neurons. . . 14
Table 2–2 Mediators of pain processing and transmission. . . . 20
Table 2–3 Monoamine neurotransmission involved in
descending pain inhibition . . . . . . . . . . . . . . . . . . . . 22
Table 2–4 Causes of chronic pain . . . . . . . . . . . . . . . . . . . . . . . 23
Table 2–5 Features distinguishing acute and chronic pain . . . . 26
Table 2–6 Categories of chronic pain. . . . . . . . . . . . . . . . . . . . . 28
Table 2–7 Common problems encountered by patients with
chronic pain . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
Table 2–8 Simple versus complex chronic pain . . . . . . . . . . . . 30
Table 3–1 Obtaining a pain history. . . . . . . . . . . . . . . . . . . . . . . 38
Table 3–2 Psychiatric disorders accompanying acute and
chronic pain . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 39
Table 3–3 Problematic cognitive patterns in pain . . . . . . . . . . . 43
Table 3–4 Factors that suggest poor surgical outcome for
pain disorders. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 48
Table 3–5 Psychometric scales used in assessing chronic
pain. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 49
Table 3–6 Pain assessment instruments for acute and
chronic pain . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 50
Table 3–7 Short form of the McGill Pain Questionnaire . . . . . . 55
Table 3–8 Uses of the Minnesota Multiphasic Personality
Inventory for patients with chronic pain . . . . . . . . . . 58
Table 4–1 DSM-IV-TR diagnostic criteria for pain disorder . . . . 65
Table 4–2 Limitations of the DSM-IV-TR nosology of
pain disorder . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 66
Table 4–3 Psychiatric disorders in which pain can be a
prominent complaint . . . . . . . . . . . . . . . . . . . . . . . . . 68
Table 4–4 Factors that raise suspicion of malingering. . . . . . . . 69
Table 4–5 Physiologic substrates common to pain and
depression . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 71
Table 4–6 Distinguishing features of pseudoaddiction and
addiction. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 77
Table 5–1 Opiate dosing and use . . . . . . . . . . . . . . . . . . . . . . . 84
Table 5–2 Medication suggestions for pain in the adult
patient. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 86
Table 5–3 Opioid therapy guidelines for chronic
nonmalignant pain. . . . . . . . . . . . . . . . . . . . . . . . . . . 87
Table 5–4 Dosing guidelines. . . . . . . . . . . . . . . . . . . . . . . . . . . . 91
Table 5–5 Analgesic effects of antidepressants . . . . . . . . . . . . 100
Table 5–6 Antidepressants used in chronic pain. . . . . . . . . . . 101
Table 5–7 Tricyclic antidepressant side effects . . . . . . . . . . . . 102
Table 5–8 Uses of anticonvulsants in various pain
conditions. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 104
Table 5–9 Anticonvulsant mechanisms of action
relevant to pain . . . . . . . . . . . . . . . . . . . . . . . . . . . . 105
Table 5–10 Side effects of anticonvulsants . . . . . . . . . . . . . . . . 107
Table 5–11 Number-needed-to-treat (NNT) values obtained
from two meta-analyses assessing antidepressant
and anticonvulsant efficacy in neuropathy . . . . . . . 109
Table 5–12 Triptans available for abortive migraine
treatment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 112
Table 5–13 Side effects of corticosteroid use in pain . . . . . . . . 117
Table 5–14 Dosing of muscle relaxants . . . . . . . . . . . . . . . . . . . 118
Table 5–15 Herbal agents used for pain . . . . . . . . . . . . . . . . . . 121
Table 6–1 Components of pain and associated
psychotherapeutic interventions . . . . . . . . . . . . . . . 132
Table 6–2 Psychotherapy interventions employed to
facilitate access of emotions . . . . . . . . . . . . . . . . . . 136
Table 6–3 Reasons why emotions are poorly identified
and regulated . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 137
Table 6–4 Early psychodynamic conceptualizations of
pain. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 137
Table 6–5 Steps of behavior therapy . . . . . . . . . . . . . . . . . . . . 141
Table 6–6 Script for deep breathing exercises. . . . . . . . . . . . . 154
Table 6–7 Techniques of hypnosis. . . . . . . . . . . . . . . . . . . . . . 156
Table 7–1 Treatment interventions for management of
acute and chronic pain conditions . . . . . . . . . . . . . 162
Table 7–2 Complications and contraindications of
subarachnoid and epidural analgesia . . . . . . . . . . . 167
Table 7–3 Risks of continuous epidural or subarachnoid
anesthetic infusions . . . . . . . . . . . . . . . . . . . . . . . . . 167
Table 7–4 Modalities of regional nerve blockade . . . . . . . . . . 169
Table 7–5 Types of and uses for autonomic nerve blocks . . . 170
Table 7–6 Neurosurgical interventions for use in pain
management . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 171
Table 7–7 Role of psychiatrists in the care of patients
undergoing interventional pain management . . . . 173
Table 7–8 Psychiatrists’ guide for assessing patient
decision-making capacity. . . . . . . . . . . . . . . . . . . . . 175
Table 8–1 Evaluation of the headache patient . . . . . . . . . . . . 180
Table 8–2 Treatment of headache . . . . . . . . . . . . . . . . . . . . . . 182
Table 8–3 Factors influencing likelihood of response to
psychotherapeutic measures in patients with
tension headache . . . . . . . . . . . . . . . . . . . . . . . . . . 183
Table 8–4 Common auras associated with migraine
headache . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 184
Table 8–5 Common causes of back pain . . . . . . . . . . . . . . . . 187
Table 8–6 Treatment options for back pain. . . . . . . . . . . . . . . 189
Table 8–7 Diagnostic criteria for fibromyalgia . . . . . . . . . . . . . 190
Table 8–8 Features associated with fibromyalgia . . . . . . . . . . 191
Table 8–9 Summary of treatment approaches for
fibromyalgia based on strength of evidence
in the available literature . . . . . . . . . . . . . . . . . . . . . 193
Table 8–10 Pharmacologic treatment strategies for
comorbidities of fibromyalgia . . . . . . . . . . . . . . . . . 194
Table 8–11 Treatment options for patients with
osteoarthritis. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 195
Table 8–12 Treatment options for patients with
rheumatoid arthritis . . . . . . . . . . . . . . . . . . . . . . . . . 196
Table 8–13 Types of neuropathic pain. . . . . . . . . . . . . . . . . . . . 199
Table 8–14 Treatment strategies for patients with
neuropathic pain . . . . . . . . . . . . . . . . . . . . . . . . . . . 200
Table 8–15 Treatment of patients with complex
regional pain syndrome. . . . . . . . . . . . . . . . . . . . . . 202
Table 8–16 Common pain-related disorders associated
with HIV/AIDS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 205
Table 8–17 Management of cancer pain and pain
associated with HIV and AIDS, based on
pain ratings. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 206
Table 9–1 Behavior parameters warranting consideration
to assess discomfort . . . . . . . . . . . . . . . . . . . . . . . . 218
Table 9–2 Barriers to pain management in minority
patients. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 221
Table 9–3 Pain management in patients with substance
abuse/dependence . . . . . . . . . . . . . . . . . . . . . . . . 223
Table 9–4 Medicare requirements for hospice care . . . . . . . . 227
Table 9–5 Approaches to dealing with spiritual issues . . . . . . 229
Table 10–1 Items to be factored into a patient contract
for use with controlled substances . . . . . . . . . . . . . 239
Table 10–2 Advantages of urine drug testing in
clinical pain management practice . . . . . . . . . . . . . 241
Table 10–3 Purposes of the National All Schedules
Prescription Electronic Reporting Act
database system . . . . . . . . . . . . . . . . . . . . . . . . . . . 242
Table 10–4 Limitations considered by Social Security
Administration adjudicators . . . . . . . . . . . . . . . . . . . 245
List of Figures
Figure 2–1 Dimensions of pain and the biopsychosocial
model . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
Figure 2–2 The affective-motivational pathway and
the sensory-discriminative pathway . . . . . . . . . . . . . 17
Figure 3–1 Components of the pain history . . . . . . . . . . . . . . . . 36
Figure 3–2 Single-dimension pain assessment instruments . . . 51
Figure 3–3 Example of a pain diary format . . . . . . . . . . . . . . . . . 54
Figure 3–4 Common Minnesota Multiphasic Personality
Inventory–2 profiles in chronic pain . . . . . . . . . . . . . 59
Figure 4–1 The anxiety–pain relationship . . . . . . . . . . . . . . . . . . 73
Figure 5–1 Influence of nonsteroidal anti-inflammatory drugs
(NSAIDs) and cyclooxygenase-2 (COX-2) inhibitors
on thromboxane and prostacyclin effects. . . . . . . . . 98
Figure 5–2 Equation for calculating number-needed-to-treat
(NNT) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 108
Figure 6–1 Modalities involved in cognitive-behavioral
therapy . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 145
This page intentionally left blank
xv
Preface
The text of this manual, an update of the Concise Guide to Pain Management
for Psychiatrists, was written in recognition of the significant advances in, and
dynamism within, the field of pain medicine. Frequently, references are made to
the importance of multidisciplinary, comprehensive treatment of the patient
with pain. Nonetheless, the role of the psychiatrist and mental health practi-
tioner in pain management continues to be the focus in this text. The con-
tributory role of the psychiatrist in evaluation and assessment, pharmacologic
management, psychotherapeutic interventions, and comprehensive treatment
planning is emphasized. While interventional approaches to pain manage-
ment are discussed herein, the importance of examining psychological variables
that can limit outcomes and that may preclude undertaking aggressive, inter-
ventional approaches is elaborated on.
Information has been evolving regarding the biological substrates underly-
ing both pain and psychiatric comorbidities (e.g., depression). Such knowledge
expands the care and management of patients afflicted with pain and common
psychiatric comorbidities. Particularly extensive revisions have been made in
the sections describing the use of psychiatric and other adjunctive medications
in pain management. While there is ample discussion of the role of opiates and
weak analgesics in pain management, concerns frequently arise pertaining to
long-term adverse effects, dependence, and behaviors resembling addiction (i.e.,
pseudoaddiction). Discussion of these issues has been expanded in the current
edition. Effort is directed at delineating the utility of adjunctive treatments,
including evolving data on the use of antidepressants and related medications,
in the care of patients with chronic pain.

xvi Clinical Manual of Pain Management in Psychiatry
An overview of chronic pain conditions is presented, with an update of
trends in treatment approaches that have been developed since publication of
the Concise Guide edition. Finally, emerging legal and forensic issues pertinent
to pain medicine have been updated in this text.
The positive comments that were received about the Concise Guide edi-
tion of the book were gratifying. It is this author’s hope that the manual is still
more useful to those psychiatrists and mental health practitioners who wish to
devote their skills and expertise to the care of patients with pain.
Disclosure of competing interests: The author has no competing interests or
conflicts to declare.

1
1
Introduction
Pain is one of the most ubiquitous health problems in the United States. Es-
timates suggest that 10%–20% of Americans have some form of chronic pain
condition (Gatchel and Okifuji 2006). The impact of pain is far-reaching, ad-
versely affecting vocational endeavors and contributing significantly to dis-
ability (Andersson 1999; White and Gordon 1982). The economic impact of
chronic pain is enormous when one considers health care costs (estimated at
over $70 billion annually) as well as the costs of absenteeism, reduced produc-
tivity, and disability compensation (estimated at over $150 billion annually)
(Gatchel and Okifuji 2006). In addition, pain interferes with individuals’ ac-
tivities, interests, and relationships and limits the enjoyment of life.
Significant losses can accompany chronic pain (e.g., losses of income and
autonomy). Patients may experience guilt, blaming themselves for their in-
ability to overcome or master pain. In the home, the patient’s role, and conse-
quently the roles of others, may require modification, leading perhaps to strained
relationships.
Pain is a common complaint among patients seeking medical attention.
Despite the pervasiveness of pain and its multiple ramifications, the manage-
ment of pain has often been elusive to clinicians. This is particularly true when

2Clinical Manual of Pain Management in Psychiatry
one considers the diversity of pain disorders that lack clear identifiable etiol-
ogies or when pain exceeds expectations given the underlying medical condi-
tion. Complex pain in a patient (i.e., pain that has not responded to common
pain medications and interventions) can present an enormous burden to med-
ical services.
Increasing attention has been directed to the issues of pain management,
mobilized in part by the efforts of organizations that educate the public about
pain management, such as the Compassion in Dying Federation, and by the stan-
dards of the Joint Commission on Accreditation of Healthcare Organizations
(JCAHO). In addition, pain management has received increased attention
from the medical community as a result of societal demands for more effective
and comprehensive treatment. JCAHO requires that physicians consider pain
as the fifth vital sign and that pain severity be documented by means of a stan-
dardized pain scale and be appropriately managed. Pain management also has
received greater political and legal attention. Congress passed a provision, sub-
sequently signed into law by President Clinton, declaring the decade 2001–
2010 the “decade of pain control and research.” From a medicolegal standpoint,
physicians have been disciplined by state regulatory boards after review of med-
ical records suggested negligence based on inadequate pain treatment (Albert
2001; Charatan 1999). Cumulatively, each of the aforementioned influences
increases the external pressure, ensuring that physicians become current in ef-
fective pain management.
Origins and Development of Pain Management
Pain management first emerged as a subspecialty of anesthesiology. The need
for the development of the science underlying pain and the skills for its treat-
ment prompted efforts to refine training strategies and provide specialized pain
clinics. Since the first pain clinic’s appearance in 1951, approximately 3,800
pain clinics, programs, and solo practices have been established in the United
States. This expansion points to the enormous need for specialized settings
and specialists to work toward the amelioration of pain.
However, it became readily apparent that the knowledge and skills re-
quired of pain practitioners far exceeded those skills available to traditional
anesthesiology training models. In addition to physical discomfort, patients
with pain experience marked emotional distress. Emotional factors (e.g., depres-

Introduction 3
sion and anxiety) not only emerge as a consequence of pain but also can con-
tribute to pain, thereby exacerbating and maintaining it. Psychological factors
can likewise interfere with treatment adherence and efficacy. Patients’ frustra-
tion caused by ongoing pain, the effects on functioning, and the impact on fam-
ilies and relationships can contribute significantly to psychiatric morbidity. As
a result of these factors, leaders in pain management training declared tradi-
tional medical models of pain to be too shortsighted and required a modifi-
cation in traditional training conceptualizations (Loeser 2001).
Interdisciplinary Pain Medicine
Pain medicine has emerged as a medical subspecialty in its own right. Although
not yet universally accepted, the term pain medicine is intended to encompass
the principles of pain management and embody an interdisciplinary approach.
Currently, specialists in pain medicine view pain as a distinct multifactorial ill-
ness (Gallagher 1999). Thus, no one discipline embodies the skills and mastery
required to address pain. Rather, management and treatment of pain require
the joint efforts of multiple clinical specialties, each of which can contribute to
the effective treatment of pain. Hence, the skills of the anesthesiology expert can
be complemented by the rehabilitation skills of the physiatrist and the psycho-
therapeutic and psychopharmacologic skills of the psychiatrist.
In 1998, the American Board of Psychiatry and Neurology (ABPN) and
the American Board of Physical Medicine and Rehabilitation joined the Amer-
ican Board of Anesthesiology in recognizing pain management as an interdis-
ciplinary subspecialty. Regardless of the primary discipline, pain medicine sub-
specialists need to understand the anatomy and physiology of pain perception,
the psychological factors modifying the pain experience, and the basic princi-
ples of pain management.
Eligibility for the certifying examination in pain medicine requires that
the applicant possess a medical license. The applicant must also be board certified
in one of the aforementioned disciplines and, as of 2006, must have completed
residency training in pain medicine approved by the Accreditation Council for
Graduate Medical Education (ACGME).
Currently, the ACGME is refining fellowship training in pain medicine to
reflect multiple disciplines. Toward this end, efforts are currently under way to
specify the training experiences, didactics, and rotations in anesthesiology as

4Clinical Manual of Pain Management in Psychiatry
well as psychiatry, neurology, and physical medicine and rehabilitation. Pa-
rameters for specialty tracks in pain medicine for each of these disciplines may
also become available.
ABPN diplomates were first eligible to sit for the subspecialty certification
examination in 2000. Subspecialty certification is appropriate for psychiatrists
whose practices are largely devoted to pain management. However, given the
pervasiveness of pain complaints, even the general psychiatrist will encounter
pain management issues among his or her patients, and the general psychia-
trist should also have the training and experience to recognize and treat basic
psychiatric issues associated with pain. This book provides a concise guide to
the psychiatric aspects of the management of pain.
Traditional Medical Models of Pain Management
Versus the Current Biopsychosocial Paradigm
In traditional medical models of pain management, pain is seen as a signal of
underlying disease or a pathophysiologic state. The physician is proactive in
undertaking pharmacologic and other treatment interventions to treat the un-
derlying disease state or relieve pain. The focus is on the disorder rather than
on the person with the disorder. Consequently, the physician enlisted to treat
the patient with pain first makes a determination as to the etiology of the pain.
In ambiguous cases, there is often an exhaustive search for biomedical causes
and treatment. When such efforts fail, the patient is dismissed as being “untreat-
able,” and he or she is often left to persist in pain, with no improvement in func-
tional adaptations.
In these traditional models there is a dualistic notion of pain, dividing it
by organic versus psychogenic causes. Thus, there is a tendency to attribute to
psychic factors any pain process in which the physical causes cannot be fully
delineated. Similarly, if the pain complaints seem disproportionate to the un-
derlying disease or if the pain fails to respond to treatment as expected, there
is a belief that psychological processes underlie the pain. For many patients
with chronic pain, such dualistic notions are inadequate (Boissevain and McCain
1991; Lynch 1992).
Frustrated by an inability to account for or to explain the cause of a pa-
tient’s pain or by a feeling of futility or defeat when faced with a patient who
persistently experiences pain despite the clinician’s best efforts, the clinician may

Introduction 5
cease to take the patient’s complaints seriously. For such patients, psychiatry
becomes the treatment of last resort, prompted by resignation that the pain is
psychic rather than somatic. Pain patients do not often think of themselves as
needing to see a psychiatrist. Physicians are accustomed to hearing such pa-
tients ask, “Why do I have to see a psychiatrist?” The implication that the pain
could be psychogenic can contribute to patients’ distress. They may perceive
that their doctors have given up on them, that their pain complaints are no
longer taken seriously, or that they are being blamed for their persistent pain de-
spite treatment (Gamsa 1994). (See Table 1–1 for a summary of the traditional
pain model.)
Current conceptualizations of pain medicine adopt a biopsychosocial per-
spective (Engel 1977) (see Table 1–1). This model contends that the health sta-
tus of individuals with chronic illnesses, the course of the illness, and the out-
come of treatment are influenced by the interaction of biological, psychological,
and social factors. The model provides a useful paradigm in which to view
chronic pain states. The focus is on the rehabilitation and reclamation of the pain
patient in the context of the pivotal doctor–patient relationship. Pain is viewed
not exclusively as a signal of disease but as an experience with biological, psycho-
logical, and social derivatives. The treatment hinges on patient participation, and
the physician serves as a guide, teacher, and interventionist to facilitate the reha-
bilitation process. The goal, therefore, is not necessarily a cure, because in many
cases pain can be a chronic or even lifelong process. The biopsychosocial para-
digm addresses relief from pain while addressing the impact of the pain condi-
tion on other aspects of one’s functioning, relationships, vocational adaptations,
and emotional well-being. The patient’s emotional experiences, beliefs, and ex-
pectations can determine the outcome of treatment and are fully emphasized as
the focus of treatment intervention. The biopsychosocial perspective pursues
and examines psychological and social facets of the patient’s pain experience
without discounting the pain based on the presence of such facets. The goal is to
identify and rectify any impediments to recovery and rehabilitation.
Role of Psychiatrists in Interdisciplinary
Pain Medicine
Comprehensive pain treatment programs involving interdisciplinary, multi-
modal treatment approaches are often required in treating complex and dis-

6Clinical Manual of Pain Management in Psychiatry
Table 1–1.
Traditional versus biopsychosocial models of pain
Traditional pain model Biopsychosocial model
View of pain As an illness As an experience
Determinants of pain Disease Biological, social, and psychological factors
Responsibility for
treatment
Physician Patient
Role of clinician Expert on pain relief Educator, motivator, physician-healer
Role of patient Passive Proactive
Goal(s) of treatment Cure or pain relief Increased function
Improved quality of life
Restored or improved relationships
Methods Pharmacologic Educational
Technical Motivational
Interpersonal
Psychological
Pharmacologic
Technical
Focus of attention Somatic complaints
Pain as corresponding to pathology;
if pain does not correspond, it is
not real
Reciprocal relationship between somatic complaints and emotion,
psychological processes, and interpersonal functions
Disregard for patient’s beliefs related to
pain
Regard for patient’s beliefs related to pain
Focus on cause of pain Focus on widespread impact of pain on life

Introduction 7
abling pain conditions. Encompassing specialists in the fields of anesthesiology,
neurology, psychiatry, psychology, and rehabilitation medicine, such programs
are directed at implementing measures whereby the patient gains mastery over
pain and refines cognitive styles and coping strategies (Jensen et al. 1994,
2001). Such comprehensive treatment programs are effective in producing
symptomatic pain relief, reducing affective distress, and improving adaptive
functioning and quality of life (Flor et al. 1992; Jensen et al. 2001; Skevington
et al. 2001). The duration of effects appears to be sustained over time, reducing
disability and health care utilization (Flor et al. 1992). Comprehensive pain treat-
ment programs have emerged as the most efficacious and cost-effective means of
addressing chronic pain, even among the most recalcitrant patients (Gatchel and
Okifuji 2006).
Unfortunately, attrition rates can be quite high (Jensen et al. 1994). Fac-
tors predisposing to attrition include a long history of pretreatment pain, de-
pendence on medications, multiple prior surgeries related to pain, and perceived
lack of social support for maintaining participation in treatment (King and
Snow 1989; Maruta et al. 1979).
Given that a significant number of psychosocial stressors and psycholog-
ical comorbidities complicate the experience of chronic pain, there is wide ac-
ceptance of the necessity of psychiatric and other mental health practitioners
in the comprehensive assessment and treatment of patients with pain. General
psychiatric training renders the psychiatrist particularly well suited for the treat-
ment of pain (Leo et al. 2003). Traditionally, psychiatrists view patients ho-
listically and adopt a biopsychosocial perspective. Psychiatrists are trained in
communication skills and are familiar with an array of pharmacologic agents
that can reduce pain.
The psychiatrist enlisted to care for the pain patient can perform a variety of
functions pivotal to the biopsychosocial approach (Table 1–2). Psychiatrists
may choose the roles they wish to assume in pain treatment as defined by their
skills, training, and expertise and by the collaborative efforts of other clinicians.
Thus, psychiatrists might serve a role in diagnosing and managing discrete psy-
chiatric disorders that accompany pain or interfere with treatment. Psychiatrists
might be involved in facilitating the patient’s adaptation after trauma or injury
resulting in pain (e.g., motor vehicle crashes, work-related injuries), in interven-
tions to treat pain (e.g., after an amputation), or in fostering improved quality of
life, including social and vocational factors. Naturally, the psychiatrist can be in-
8Clinical Manual of Pain Management in Psychiatry
volved in facilitating communication between the patient and clinicians with
whom the patient interacts. (In some circumstances, the patient’s perceptions of
how he or she is being treated, believed, or construed may adversely affect ther-
apeutic alliances and compromise the pain treatment team.)
Psychiatrists may be involved in the direct assessment and treatment of
pain, in coordinating referrals to other pain specialists, and in all facets of the
treatment plan. Conversely, they may respond to referrals from specialists in
other disciplines to complement existing treatment strategies in which psycho-
logical factors are thought to be complicating recovery.
Psychiatrists can offer pharmacologic interventions for pain. They also can
address emotional and cognitive sequelae of pain or its treatment and factors
interfering with treatment (e.g., treatment adherence). The patient with chronic
pain can become dependent on opiate analgesics, requiring psychiatric inter-
vention. In some cases, the care of the pain patient may be entirely delegated
to the psychiatrist.
Physicians seek psychiatric intervention to help in treating patients who
have acute pain or painful terminal disorders, especially to address the psy-
chological consequences and psychiatric comorbidities. More frequently, phy-
sicians seek consultation for patients with chronic pain with whom they are
frustrated by lack of treatment response. Often, such patients are pejoratively
labeled as noncompliant, uncooperative, attention seeking, medication seek-
ing, or malingering (Gallagher 1999). Deciphering the relative contribution
of biological and psychological variables to pain complaints requires evalua-
tion by a psychiatrist with skills in the biopsychosocial assessment of pain. The
Table 1–2.
Role of psychiatrists in pain management
(biopsychosocial approach)
Assess pain.
Assess intervening variables that affect pain.
Prognosticate (consider factors that might influence pain, treatment compliance, and
effects of treatment).
Determine problem areas for the patient.
Establish a treatment approach.
Delineate goals of treatment.
Reassess treatment efficacy.
Make modifications in the treatment plan as necessary.

Introduction 9
patient’s frustration caused by ongoing pain, the effects on functioning, and
the impact on relationships contributes to significant psychiatric morbidity. It
is not surprising, therefore, that the presence of chronic pain is a significant
risk factor for suicide (Fishbain 1999). Thus, there is a great demand for psy-
chiatrists to assist with pain management.
Key Points
• Pain is a ubiquitous health problem, the impact of which is far-reaching.
On a societal level, the economic burdens are enormous. For the individual,
pain adversely affects vocational endeavors, activities, interests, and rela-
tionships and limits enjoyment of life.
• From a biopsychosocial perspective, pain is viewed as an experience with bi-
ological, psychological, and social derivatives. This model provides a useful
paradigm in which the focus is on bringing about rehabilitation of the pain
patient and reclamation of his or her life, addressing symptomatic pain relief,
reducing affective distress, improving adaptive functioning and quality of life,
and thereby improving interpersonal and emotional well-being.
• Comprehensive pain treatment programs involving interdisciplinary, multi-
modal treatment approaches are often required for complex and disabling
pain conditions.
• Interdisciplinary treatment programs involve the coordination and collab-
orative endeavors of a number of health care providers, with the goals of al-
leviating symptoms, enhancing patient independence, improving functional
adaptation, addressing the psychological comorbidities accompanying pain,
and improving psychosocial functioning.
• Within the biopsychosocial perspective, there are several pivotal roles that
the psychiatrist can perform in the assessment, treatment, and ongoing man-
agement of the patient with pain.
References
Albert T: Doctor guilty of elder abuse for undertreating pain. Am Med News, July 23,
2001, pp 1, 4
Andersson GBJ: Epidemiological features of chronic low-back pain. Lancet 354:581–
585, 1999

10 Clinical Manual of Pain Management in Psychiatry
Boissevain MD, McCain GA: Toward an integrated understanding of fibromyalgia
syndrome, I: medical and pathophysiologic aspects. Pain 45:227–238, 1991
Charatan F: Doctor disciplined for “grossly undertreating” pain. BMJ 319:728, 1999
Engel GL: The need for a new medical model: a challenge for biomedicine. Science
196:129–136, 1977
Fishbain DA: The association of chronic pain and suicide. Semin Clin Neuropsychiatry
4:221–227, 1999
Flor H, Fydrich T, Turk DC: Efficacy of multidisciplinary pain treatment centers: a
meta-analytic review. Pain 49:221–230, 1992
Gallagher RM: Treatment planning in pain medicine: integrating medical, physical
and behavioral therapies. Med Clin North Am 83:823–849, 1999
Gamsa A: The role of psychological factors in chronic pain, II: a critical appraisal. Pain
57:17–29, 1994
Gatchel RJ, Okifuji A: Evidence-based scientific data documenting the treatment and
cost-effectiveness of comprehensive pain programs for chronic nonmalignant
pain. J Pain 7:779–793, 2006
Jensen MP, Turner JA, Romano JM: Correlates of improvement in multidisciplinary
treatment of chronic pain. J Consult Clin Psychol 62:172–179, 1994
Jensen MP, Turner JA, Romano JM: Changes in beliefs, catastrophizing, and coping
are associated with improvement in multidisciplinary pain treatment. J Consult
Clin Psychol 69:655–662, 2001
King SA, Snow BR: Factors for predicting premature termination from a multidisci-
plinary inpatient chronic pain program. Pain 39:281–287, 1989
Leo RJ, Pristach CA, Streltzer J: Incorporating pain management training into the
psychiatry residency curriculum. Acad Psychiatry 27:1–11, 2003
Loeser JD: Multidisciplinary pain programs, in Bonica’s Management of Pain, 5th
Edition. Edited by Loeser JD, Butler SH, Chapman CR, et al. Philadelphia, PA,
Lippincott Williams & Wilkins, 2001, pp 255–264
Lynch M: Psychological aspects of reflex sympathetic dystrophy: a review of the adult
and pediatric literature. Pain 49:337–347, 1992
Maruta T, Swanson DW, Swenson WM: Chronic pain: which patients may a pain-
management program help? Pain 7:321–329, 1979
Skevington SM, Carse MS, Williams AC: Validation of the WHOQOL-100: pain
management improves quality of life for chronic pain patients. Clin J Pain 17:264–
275, 2001
White AA 3rd, Gordon SL: Synopsis: workshop on idiopathic low-back pain. Spine
7:141–149, 1982

11
2
Sensory Pathways of Pain and
Acute Versus Chronic Pain
Pain is a multidimensional concept (Loeser 1982), with biological, psycho-
logical, and social components (Figure 2–1). First, there is a sensory compo-
nent of the pain experience—an unpleasant sensation detected in the body
and processed in the central nervous system (CNS). This process, referred to
as nociception, relies on the transfer of information about the sensory experi-
ence from receptors in the periphery through nerves to the spinal cord and on
to the brain.
Second, there is an assessment of the unpleasant sensory experience. This as-
sessment involves a cognitive awareness of the sensation arising from the periph-
ery that the person labels as pain. Not all sensations are painful; some can be
construed as unusual, uncomfortable, or irritating but not painful. Thus, the
common pins-and-needles sensation in one’s foot might be described as the foot
“falling asleep” but not as pain. Similarly, one may experience an itch, a twitch,
or other noxious sensations (e.g., burning, throbbing) that are labeled as painful.
Third, there is a perception and cognitive appraisal that the discomfort is
associated with suffering. This subsumes not only the sensation and awareness
12 Clinical Manual of Pain Management in Psychiatry
of pain but also the reactions to the experience (e.g., distress, dysphoria, anx-
iety, hopelessness). The experience of pain may connote various experiences for
the individual, including many unpleasant emotional states.
The final dimension of pain consists of the pain behaviors displayed by
the patient in response to the unpleasant experience. Here the person conveys
to others how much distress he or she is experiencing. These behaviors can be
verbal (e.g., “Wow, this really hurts!”), paraverbal (e.g., moaning), or nonverbal
(e.g., guarding an affected limb, splinting, wearing a neck brace, taking med-
ication, reclining).
The neurophysiologic substrates of the pain experience are discussed in
this chapter. The first part of the chapter comprises a discussion of the sensory
pathways and mechanisms. The second part focuses on distinctions between
acute and chronic pain.
Pain-Relaying Pathways and Mechanisms
In their most basic aspects, the pain relay pathways involve three sets of
neurons. First-order neurons relay noxious information from the periphery or
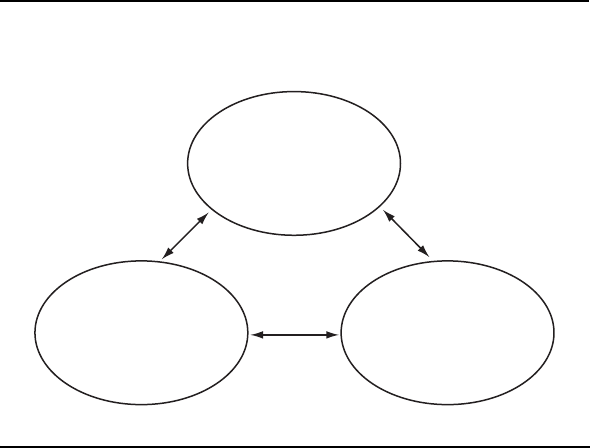
Figure 2–1.
Dimensions of pain and the biopsychosocial model.
Biological
Nociception
Psychological
Cognitive appraisal
and suffering
Social
Pain behavior

Sensory Pathways of Pain and Acute Versus Chronic Pain 13
viscera to the spinal cord. Second-order neurons relay sensory information
from the spinal cord to the thalamus. Third-order neurons arising from the
thalamus send information to higher brain regions where complex processing
of the pain experience occurs.
First-Order Neurons: Relay to the Spinal Cord
The detection of pain requires that information regarding injury, trauma, and
noxious stimulation be detected by a transducer. Transducers serve the pur-
pose of taking information (e.g., changes in temperature, chemical irritation,
pressure) from some location on the body surface, muscles, or internal organs
and converting it into neurochemical information that is interpretable by the
brain. The transducers for pain include the free nerve endings of the first-order
neurons in the pain pathway (i.e., the Aδ and C neurons). These neurons have
long dendrites with fine terminal arborizations present in the skin, muscle,
connective tissue, joints, bone, and internal organs. When these neurons are
stimulated, action potentials extend from the dendrites to the cell body (in the
dorsal root ganglion) and from the axon to the spinal cord.
Information relayed about other nonnoxious sensory modalities (e.g.,
pressure, touch, proprioception) is detected by other types of sensory trans-
ducers. These include pacinian corpuscles, Meissner’s corpuscles, and other
transducers. When sufficiently stimulated, these receptors in turn pass infor-
mation along another set of first-order neurons, the Aβ neurons.
First-order neurons differ in their diameter, myelination, and, therefore,
electrical conduction rates (Table 2–1). Miscalculating the intended align-
ment of a hammer to the intended target (i.e., the head of the nail), one can
quickly appreciate the pain relay process once the swift blow of the hammer
makes inadvertent contact with the fingers of the hand holding the nail in
place. First, Aβ fibers, responding to low-threshold touch receptors, relay in-
formation about the blunt object making contact with the hand. Simultaneously
(when the stimulus is disconcertingly high, as in this example), high-threshold
mechanoreceptors and heat receptors are activated. This information is con-
ducted quickly and robustly, via Aδ fibers, immediately after the injury. Aδ fi-
ber information is well localized, sharp, pricking, and pulsating but very short-
lived. Shortly thereafter, C fibers relay information from the injured hand—
information that is less definitive or localized than that of the Aδ fibers (Bes-
son and Chaouch 1987). These C fibers mediate the slowly emerging, more
14 Clinical Manual of Pain Management in Psychiatry
persistent, dull, aching, and burning pain that is experienced. All three types
of fibers relay information to the dorsal horn of the spinal cord, where they
synapse directly, or indirectly through interneurons, on the second-order neu-
rons involved in the nociceptive pathway (Byers and Bonica 2001).
Viscera are also supplied by C fibers and Aδ fibers. These are activated by
inflammatory processes, ischemia, disease, rapid distention, and contraction.
These events trigger free nerve endings to transduce the noxious information
into electrical information that is eventually transmitted to the dorsal horn.
Pain from the face is relayed to the CNS through the fifth cranial (i.e., the
trigeminal) nerve. Noxious information from the face is relayed via dendrites of
one of the three branches of the trigeminal nerve, passes through to the trigem-
inal ganglion (containing the cell bodies of the trigeminal nerves), and passes
to the second-order neurons in the pons and medulla. In a pattern that par-
allels that of spinal cord pathways, information is ultimately relayed to the
thalamus and higher brain structures (Dubner and Bennett 1983). The face
and mouth have a high density of pain transducers and pain fibers and are thus
exquisitely sensitive to stimulation. Similarly, the representation of the face and
mouth in the somatosensory cortex of the brain is extensive, suggesting that
processing and encoding of sensory information from the face and mouth is
quite elaborate.
Dorsal Horn Anatomy
The gray matter of the spinal cord is classified by histological characteristics
into 10 layers or laminae (referred to as Rexed layers). The dorsal horn contains
6 of these. Those layers that are essential in the pain processing pathways in-
clude laminae I, II, and V, primarily where Aδ and C fibers terminate (Terman
and Bonica 2001). Cells within the dorsal horn include those that are nociceptive
Table 2–1.
Sensory neural fiber types of first-order neurons
AβAδC
Diameter Large (5–15 µm) Intermediate
(1–4 µm)
Thin (0.5–1.5 µm)
Myelination Yes Ye s No
Conduction rate Fast (30–70 m/s) Fast (12–30 m/s) Slow (0.5–2.0 m/s)
Sensory
information
Cutaneous touch
and pressure
Well localized
Sharp pain
Poorly localized
Dull, aching pain

Sensory Pathways of Pain and Acute Versus Chronic Pain 15
specific (i.e., respond to noxious stimulation). Other cells respond only to innoc-
uous stimuli, whereas still others, referred to as wide dynamic range (WDR) cells,
respond to noxious and innocuous stimuli but discharge at a higher frequency
to noxious stimuli.
The substantia gelatinosa, contained within lamina II, has a significant
role in modulating pain. This substance involves small interneurons that serve
as control switches or gates through which sensory information from the
periphery is either enhanced or depressed. Certain of these interneurons (i.e.,
islet cells) are inhibitory, whereas others (i.e., stalked cells) are excitatory. The
substantia gelatinosa receives extensive serotonergic and noradrenergic input
from nerve fibers emanating from higher brain centers, which likewise influ-
ences the gating process.
When one experiences pain from a joint, massage of the overlying skin
may reduce some of that discomfort. This phenomenon, referred to as counter-
irritation, is attributed to the gating mechanism of the substantia gelatinosa.
Massaging, by stimulating the tactile receptors of the skin, activates Aβ fibers.
These fibers in turn activate interneurons within the substantia gelatinosa that
serve to inhibit pain-mediating pathways (Melzack and Wall 1965; Wall
1980). Counterirritation has been offered as an explanation to account for why
certain therapeutic modalities can be effective in mitigating pain—for exam-
ple, use of liniment or transcutaneous electrical nerve stimulation units.
Lamina V contains WDR cells with large receptive fields (i.e., they receive
extensive inputs from multiple sources). For example, Aδ and C fibers arising
from visceral structures enter lamina V. The WDR cells receiving the neural
input from visceral organs simultaneously receive input from other sites. This
process is the basis for referred pain (i.e., the interpretation of unpleasant sen-
sory input arising from viscera as emanating from peripheral sites) (Pomeranz
et al. 1968). Thus, hypoxic injury to the heart is perceived as pain in the left
arm, because the afferents from the heart synapse on those lamina V cells of
the left lower cervical and upper thoracic segments. The brain interprets the
stimulation of those cells as pain originating in the left upper chest and arm.
Second-Order Neurons: The Spinothalamic Tract
The axons arising from neurons making up the spinothalamic tract emanate
from the entire gray matter of the spinal cord. Most of these fibers cross to the
other side of the spinal cord through the ventral commissure in the midline

16 Clinical Manual of Pain Management in Psychiatry
and ascend in the anterolateral aspect of the white matter (myelinated) portion
of the spinal column. These fibers ascend without interruption through the
spinal column and brain stem and terminate in the contralateral thalamus
(Besson and Chaouch 1987; Dennis and Melzack 1977). However, a small
number of fibers project to the ipsilateral thalamus. These pain relay pathways
diverge into the two pathways within the spinothalamic system: the neo-
spinothalamic (sensory-discriminative) and the paleospinothalamic (affective-
motivational) pathways (Figure 2–2). The latter is considered to be a phylo-
genetically older pathway.
Third-Order Neurons: The Thalamus and Beyond
The thalamus is the primary relay station for sensory information from the
spinal cord to the cortex (Chudler and Bonica 2001; Rome and Rome 2000).
The second-order neurons from the neospinothalamic tract terminate in the
lateral aspect of the ventral posterior nucleus (VPN). Third-order neurons
from the VPN relay information to the somatosensory cortex (parietal lobe).
Through this pathway, discriminative aspects of pain, localization of the pain,
and coordinated motor responses to the pain are possible (Rome and Rome
2000).
Information is relayed simultaneously from the paleospinothalamic path-
way through a parallel pathway to the reticular formation, medial thalamus,
hypothalamus, and prefrontal cortex (Giesler et al. 1994). The nociceptive
system thereby influences affect, attention, cognition, and memory that relate
to painful sensory information (Chudler and Bonica 2001). A stress reaction
develops to noxious sensation, involving the hypothalamic-pituitary axis and
autonomic nervous system (Giesler et al. 1994). Ultimately, this information
is relayed to both cortices. As a result, the affective quality and coloring of the
pain experience are possible. In addition, affective influences mediated by lim-
bic involvement are likely to modulate pain. Thus, one’s surprise (e.g., “I can’t
believe I hit my hand with the hammer!”), alarm (e.g., “I didn’t see that com-
ing!”), anger (e.g., “That was so stupid!”—along with use of expletives), and
fear (e.g., “My hand is damaged for good!”) are likely to shape the experience
of pain. Mood states (e.g., a predisposition to anxiety or depression) can shape
the cognitive strategies one employs to deal with the pain, one’s sense of effi-
cacy in dealing with the injury, and one’s expectations for the future. The relay
of pain information to the bilateral cortices adds a cognitive component to the
Sensory Pathways of Pain and Acute Versus Chronic Pain 17
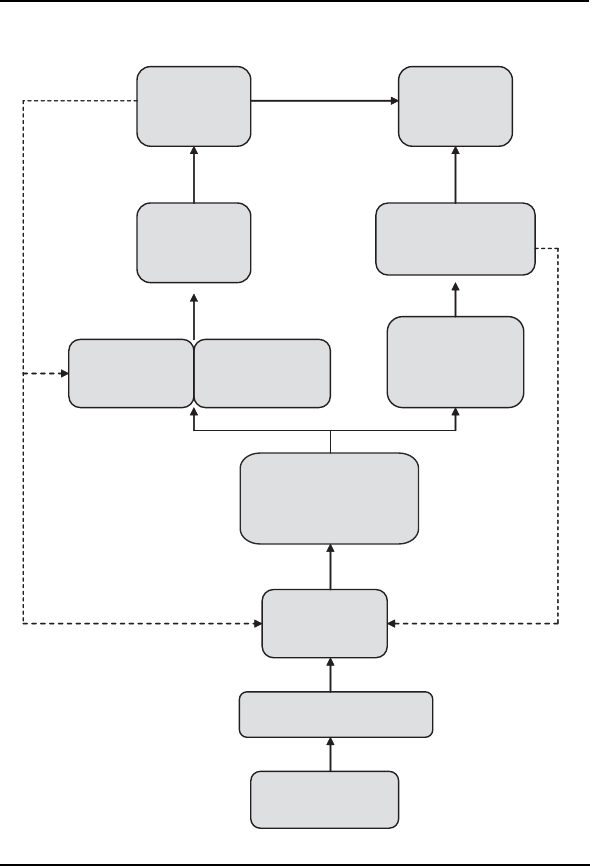
Figure 2–2.
The affective-motivational pathway (left) and the sensory-
discriminative pathway (right).
Note. Dashed line=inhibitory pathways; solid line=pain-facilitating pathways.
Aδ and C fibers
Noxious
stimulation
Dorsal
horn
Medial
thalamus
Somatosensory
cortex
Autonomic
nervous
system
Ventral
posterior
thalamus
Reticular
formation
Limbic
system
Frontal
cortex
Spinothalamic
tract

18 Clinical Manual of Pain Management in Psychiatry
pain experience. Thus, the mutual influences of mood, cognition, expectation,
and pain are mediated.
The frontal cortex mediates the cognitive processes underlying pain. These
processes involve the identification and evaluation of, and decision making
pertaining to, the noxious sensory information input from the periphery. Hence,
immediate short-term problem solving can be undertaken (e.g., ignoring the
hammering, preferring instead to tend to the injured hand). Other cognitive
processes—such as one’s expectations along with the attributions, beliefs, and
meanings ascribed to the painful experience—are likewise derived from cortical
processes and influence both the pain experience and the decision making
around the pain. In addition, memory of the painful experience is established
and encoded for further referral and to guide subsequent behaviors—for ex-
ample, how (and whether) one tries carpentry again.
Role of the Autonomic Nervous System
in Pain
The autonomic nervous system plays a significant role in pain. Signals of
threat and danger are relayed to the hypothalamus. From there, specifically
the posterior portion, information is relayed to the spinal cord (i.e., the tho-
racic and lumbar regions) by sympathetic neural pathways (McMahon 1991).
Ultimately, fibers from the thoracolumbar regions innervate a number of end-
organs producing activation (i.e., a state of heightened arousal). Those end-
organ activities that are necessary for fight-or-flight responses are promoted,
whereas those that are not necessary are suppressed. Thus, the information
signaling threat or danger results in elevated heart rate and blood pressure, in-
creased oxygen use, sweating, dilation of pupils, and increased glycogen utili-
zation within muscles. Other organ functions (e.g., peristalsis) are inhibited.
The effects of sympathetic nervous system activity are relatively brief in
duration because of the rapid release and degradation of norepinephrine and
acetylcholine released in the mediation of the fight-or-flight response. More
sustained reactions to stressors or threat are mediated by neuroendocrine ef-
fects, a product of adrenal medulla activation. Sympathetic nervous system
activation of the adrenal gland results in release of norepinephrine and epi-
nephrine, mimicking sympathetic activity.

Sensory Pathways of Pain and Acute Versus Chronic Pain 19
Pain-Modulating Processes
Within the Nervous System
The pain transmission pathways that have been presented thus far are simplis-
tic. In reality, a number of processes can influence pain transmission from the
periphery through the entire CNS. These mechanisms involve complex and
dynamic interactions among various neurotransmitters, their receptors, and
other pain-reducing and pain-augmenting processes. These are discussed briefly
in the following sections.
Neurochemicals in Pain Processing
A number of neurotransmitters and chemical substrates are involved in pain
transmission; several are listed in Table 2–2. For example, in the periphery, tis-
sue injury results in the activation of a number of cellular processes that release
chemical compounds that can activate free nerve endings for pain transmis-
sion (Snyder 1980), such as acetylcholine, bradykinin, histamine, potassium
ion, and serotonin (Levine et al. 1993). Additional agents that are active within
the CNS are also listed in Table 2–2. Some of these substances have a pain-
promoting role, whereas others have a pain inhibitory role. Many of these sub-
stances are the targets of influence when analgesics are employed (e.g., anti-
inflammatory agents and antidepressants), as is described further in Chapter 5,
“Pharmacology of Pain,” of this book.
Endogenous Opiates
The endogenous opiates consist of β-endorphin, enkephalins, and dynor-
phins (Sewell and Lee 1980). These are abundantly distributed throughout
the CNS, thereby modulating pain transmission. Enkephalins are endoge-
nous opiates found in the interneurons of the substantia gelatinosa that me-
diate the effects of inhibitory interneurons within the dorsal horn. Binding to
opioid receptors, enkephalins can inhibit the release of substance P from noci-
ceptors. In fact, intraspinal application of opiates (e.g., morphine) is thought
to influence the enkephalin receptors, thereby mitigating pain transmission
from the spinal cord. Cells producing β-endorphin arise from the hypothalamus
and are thought to exert their influence within the limbic system and mid-
brain.

20 Clinical Manual of Pain Management in Psychiatry
Pain-Reducing Pathways
Several structures serve to diminish the pain sensory information coming into
the CNS. Intuitively, pain-modulating mechanisms prevent the organism
from being overcome by unbridled pain, thereby allowing the organism an
opportunity to “escape” and tend to the injury. The four regions in the CNS
that can function to reduce pain sensation and control pain awareness are
1) the cortex and limbic structures, 2) the midbrain (the periaqueductal gray
[PAG]), 3) the rostral ventromedial medulla (RVM), and 4) the spinal dorsal
horn (Besson and Chaouch 1987; Terman and Bonica 2001). The gating
mechanism of the substantia gelatinosa within the dorsal horn was described
earlier in this chapter. The cortex and reticular formation can influence atten-
tion, arousal, expectations of pain, and psychological factors that can in turn
influence pain experiences. The specific mechanisms by which these structures
influence pain have yet to be clarified.
Table 2–2.
Mediators of pain processing and transmission
Pain promoting Pain inhibiting
Peripheral nervous system Acetylcholine
Adenosine
Bradykinin
Cytokines
Glutamate
Histamine
K+
Prostaglandins (E series)
Serotonin
Substance P
Endogenous opiates
Central nervous system Cholecystokinin
Glutamate
Serotonin
Norepinephrine
Substance P
Endogenous opiates
β-Endorphin
Endorphins
Dynorphins
Serotonin
Norepinephrine
Neurotensin
Source. Adapted from Terman GW, Bonica JJ: “Spinal Mechanisms and Their Modulation,” in
Bonica’s Management of Pain, 3rd Edition. Edited by Loeser JD, Butler SH, Chapman CR, et al.
Philadelphia, PA, Lippincott Williams & Wilkins, 2001, pp. 73–152.

Sensory Pathways of Pain and Acute Versus Chronic Pain 21
The PAG is a midbrain structure with an abundance of opiate receptors.
Neural connections extend from the PAG to neighboring serotonergic structures
(e.g., the RVM) and noradrenergic structures (e.g., the dorsolateral ponto-
mesencephalic tegmentum [DLPT]). The pain-modulating influences of the
PAG are conducted predominately, if not exclusively, through the RVM. The
RVM in turn projects onto the cells of the dorsal horn in laminae I, II, and V
(Terman and Bonica 2001). Analgesia results if morphine is injected into the
PAG, RVM, or amygdala. In µ-opiate receptor–deficient mice, morphine in-
jection into the PAG, RVM, and amygdala is completely ineffective in mitigat-
ing pain. The µ-receptor, therefore, is responsible for the supraspinal analgesia
produced by opiates, mediated through the PAG and RVM.
Additionally, axons of supraspinal nuclei extend down the spinal cord,
synapse within the dorsal horn, and release monoamines that influence pain
transmission (see Table 2–3). Essentially all of the serotonin-containing neu-
rons in the dorsal horn originate from the RVM and the raphe nuclei. Thus,
for example, stimulation of the RVM evokes serotonin release with resultant
analgesia, an effect that can be blocked by concomitant serotonin antagonist
(e.g., methysergide) administration. The analgesic effects of serotonin are
thought to be mediated largely by 5-HT1A receptors (Terman and Bonica
2001).
As with serotonin, norepinephrine released by axons extending from the
locus coeruleus and DLPT and terminating within the dorsal horn can inhibit
pain transmission (see Table 2–3). The analgesic effect of norepinephrine is
thought to be mediated by activity at the α2-receptor. Support for this is
suggested by the fact that α2-receptor agonists (e.g., clonidine) can produce
analgesia. In addition, norepinephrine appears to be essential for opioid-
induced analgesia; blockade of norepinephrine (e.g., by phentolamine) re-
duces the effects of systemically applied opioids. However, serotonin and
norepinephrine have both nociceptive and antinociceptive effects depend-
ing on the specific receptor subtypes and the neural circuitry activated (see
Table 2–3).
Opiate Receptors and Descending Inhibition of
Pain Pathways
There are four classes of opiate receptors recognized to date (Terenius 1985).
The µ-receptor is responsible for supraspinal analgesia. It also produces eu-
22 Clinical Manual of Pain Management in Psychiatry
phoria from opiate use and is responsible for physical dependence. Physical
effects of opiates (e.g., hypotension, decreased respirations, hypothermia, pru-
ritus, and decreased gastrointestinal motility) are all attributed to µ-receptor ac-
tivation from opiate use.
Analgesia on a spinal level is a result of the opiate activation of the κ-recep-
tor. Activation results in pupil constriction and sedation. The δ-receptors also
produce analgesia and are activated by endogenous opiates. The role of the σ-
receptor is more controversial. It produces no analgesia but is responsible for
the dysphoria, and possibly hallucinations, associated with opiate use. Because
of these disparate effects, compared with other opiate receptors, there is con-
troversy about whether the σ-receptor is actually an opiate receptor.
The µ-receptor produces more analgesia than the other types of receptors.
It is also responsible for changes in respiratory activity, gastrointestinal motility,
and sphincter tone produced by opiates. The µ-receptor is abundantly present
in the dorsal horn, medulla, and the PAG. It is also responsible for pain mod-
ulation at peripheral nerve endings. Its abundance in the myenteric plexus is
likely responsible for the effects of the opiates on gastrointestinal motility and
sphincter tone.
Table 2–3.
Monoamine neurotransmission involved in
descending pain inhibition
Effect on pain transmission
Neurotransmitter and sources Receptors Inhibitory Promoting
Serotonin
Rostral ventromedial medulla
Raphe nuclei
5-HT1A
5-HT2
5-HT1B
5-HT3
+
+
+
+
+
+
Norepinephrine
Locus coeruleus
DLPT
α2
α1
+
+
Note. Serotonin and norepinephrine have both nociceptive (pain promoting) and antinocicep-
tive (pain inhibiting) effects depending on the specific receptor subtypes and the neural circuitry
activated. DLPT =dorsolateral pontomesencephalic tegmentum.
Source. Adapted from Terman GW, Bonica JJ: “Spinal Mechanisms and Their Modulation,” in
Bonica’s Management of Pain, 3rd Edition. Edited by Loeser JD, Butler SH, Chapman CR, et al.
Philadelphia, PA, Lippincott Williams & Wilkins, 2001, pp. 73–152.

Sensory Pathways of Pain and Acute Versus Chronic Pain 23
Pain-Augmenting Mechanisms and the
Emergence of Chronic Pain
When acute pain is inadequately treated, there is an increased risk of emer-
gence of chronic pain. Several mechanisms are postulated to play a role in the
development of chronic and enduring pain (see Table 2–4).
Ongoing abnormalities in peripheral tissues, with resultant inflammation,
can result in activation of nociceptive pathways, rendering pain chronic. In
such cases, treatment is best directed at the inflammatory mechanisms (e.g.,
aspirin or nonsteroidal anti-inflammatory agents). Peripheral nerves may be-
come dysfunctional due to injury or disease (e.g., diabetes, infection, toxin
exposure). Damaged neurons may fire spontaneously. Nociceptive fibers fir-
ing in this way are perceived in the CNS as signaling pain, yet in the peripheral
tissues there may be no current injury. In such cases, antidepressants and anti-
convulsants might be the most helpful treatment.
Trauma and injury can produce reflex motor activity in the vicinity of the
injury, producing spasm. This process may initially serve a protective function
in acute pain states, but in chronic pain states it can lead to aggravated muscle
tension that exacerbates painful states (Zimmerman 1979).
The dorsal horn can become sensitized by a number of mechanisms that
can potentiate chronic pain. Changes that occur within the dorsal horn may
account for the maintenance of pain sensation that loses its relevance in its
ability to signal danger. This sensitization appears to be related to changes me-
diated by CNS neurotransmitters, especially the excitatory neurotransmitter
glutamate. With repeated stimulation (e.g., in poorly treated acute pain or in
Table 2–4.
Causes of chronic pain
Ongoing activation of pain pathways from the periphery can cause pain
to become chronic.
Peripheral nerves may be dysfunctional.
Motor reflexes may potentiate pain.
Dorsal horn can become sensitized.
Sympathetic nervous system can become a major contributor to
ongoing pain.
Cortical and limbic activity can contribute to pain.

24 Clinical Manual of Pain Management in Psychiatry
re-injury), glutamate activity can expand to include other receptors, including
N-methyl-D-aspartate (NMDA) receptors. With expansion of glutamate
activity, a series of intracellular processes occur that result in the heightened
activation of dorsal horn cells, referred to as wind up. Such processes become
difficult to interrupt from a therapeutic standpoint; however, NMDA recep-
tor blockers (e.g., ketamine) can be helpful.
Ongoing NMDA activation can result in cell death. Death of neurons leaves
areas of deafferentation in the spinal cord pathways. As a result, nearby sen-
sory neurons often sprout collaterals into the deafferentated area to replace the
synaptic connections lost after cell death. This replacement results in an inner-
vation of pain pathways that correspond to the injured areas stimulated or ac-
tivated by nearby undamaged areas.
The sympathetic nervous system can become a major contributor to on-
going pain (Zimmerman 1979). Trauma and injury trigger a sympathetic re-
sponse that can effectively alter the neurochemical milieu of nociceptors in the
periphery, along with causing changes in the microcirculation. This situation
can alter the sensitivity of peripheral pain receptors, thereby augmenting pain
sensitivity.
Pain can be maintained—despite lack of injury or even after effective heal-
ing—by the actions of the sympathetic nervous system. In such cases, pro-
tracted painful conditions can arise, such as reflex sympathetic dystrophy and
complex regional pain disorder. Some of these disorders can be alleviated by
blockade of sympathetic activity (i.e., sympatholysis); however, not all re-
spond to sympatholytic techniques. Treatment for such disorders can include
nerve blocks, sympathetic nerve blocks, and psychotropic medications (e.g.,
antidepressants and anticonvulsants).
Commensurate alterations in the thalamus and somatosensory cortex can
occur after peripheral nerve injury. Thus, even after an amputation, the area of
the somatosensory cortex corresponding to the amputated limb increases.
Other cortical and limbic events can contribute to pain. The experience of pain
can be shaped and influenced by the diffuse interconnections of the pain path-
ways with limbic and cortical pathways. In such cases, psychoactive medica-
tions, psychotherapy, and adjunctive therapeutic approaches such as relaxation
training may be helpful modes of treatment.

Sensory Pathways of Pain and Acute Versus Chronic Pain 25
Acute Versus Chronic Pain
The sensation of pain serves an adaptive function. Specifically, nociceptive
pathways serve to alarm the organism that some damage or injury has been
sustained and that efforts may need to be directed at tending to the injury and
avoiding further injury. Consider a disorder such as Hansen’s disease, also known
as leprosy, which is characterized by dysfunction in pain-mediating pathways.
Persons infected with Mycobacterium leprae, the agent that causes the disease,
have deficits in pain perception. On the surface, this seems quite desirable;
however, over the course of the illness, these patients sustain marked deficits in
self-care and are exposed to hazards brought on by physical injury and infec-
tion. Death can result from the lack of appropriate awareness of the bodily
warning mechanisms that would otherwise motivate treatment.
Problems arise when pain takes on a life of its own. Certainly, in medical
conditions in which there is ongoing tissue damage—arthritis, for example—
pain may likewise be ongoing. One might question the utility of such pain, be-
cause one certainly is aware of the trauma that warrants medical attention. On the
other hand, and perhaps more troubling, there are situations in which pain
persists despite healing (e.g., postherpetic neuralgia) or situations in which
pathologic processes emerge within the CNS or the peripheral nervous system
(or both) to produce aberrant activity that is interpreted and experienced as
pain, as in Dejerine-Roussy syndrome (a pain syndrome related to thalamic
stroke).
Classifications of Acute and Chronic Pain
Pain is classified in several ways, including the familiar categories of acute and
chronic. For example, pain can be classified based on its temporal aspects, its
etiology and its associated features from differing sources, and its functional
significance (see Table 2–5). Acute pain has been customarily defined as pain
that is less than 6 months in duration. Chronic pain is defined as pain persist-
ing beyond 6 months (Crue 1983). There are always problems with arbitrary
definitions such as these. For example, it becomes difficult to classify some
painful conditions (e.g., migraine or osteoarthritis) based on temporal aspects.
Migraine is a recurrent painful disorder that can persist for years, but the spe-
cific episodes of pain are relatively short-lived. Osteoarthritis, on the other hand,
26 Clinical Manual of Pain Management in Psychiatry
is a chronic, progressive medical condition that is accompanied by a mixture
of acute and chronic pain components. Acute pain can be precipitated by new
injury, whereas chronic pain features can arise from prior injuries and sensitiza-
tion of peripheral nervous system involvement.
Generally, acute pain is considered to be pain that serves self-protective
functions. The value of the alarm functions of pain brought on by the inad-
vertent slamming of one’s thumb with a hammer is obvious. Such pain, it is
hoped, is discrete and mobilizes the person to take measures to minimize pain
and prevent further injury. Conversely, chronic pain is considered to have lost
such meaningful aspects. One is hard-pressed to arrive at any adaptive func-
tion gleaned from chronic neuropathic pains or fibromyalgia.
Acute pain arises from tissue injury, trauma, or inflammation. Chronic pain
extends beyond the period of healing and can be brought on by pathophysi-
ologic processes within the nervous system. Some pain states can be mediated
by the ongoing barrage of peripheral pain sensors (i.e., nociceptors). The patho-
logic firing of peripheral or CNS pathways that mediate pain can also trigger
chronic pain.
Another distinction between acute and chronic pain states is based on the
presence of psychological and psychiatric conditions that accompany or ag-
gravate the pain. The pain brought on by fracture or another traumatic injury
is not necessarily accompanied by the personality changes and psychiatric dis-
turbances that can accompany chronic pain states. Psychological sequelae of
acute pain are likely to be discrete and obvious.
Table 2–5.
Features distinguishing acute and chronic pain
Acute pain Chronic pain
Duration <6 months >6 months
Cause Tissue damage, injury,
inflammation
Pathophysiologic processes
in the peripheral or
CNS pathways
Psychogenic factors
Biological utility Yes No
Psychological factors
contributing
No Yes
Note. CNS=central nervous system.

Sensory Pathways of Pain and Acute Versus Chronic Pain 27
The long duration and pervasive effects of chronic pain states likely have an
impact on a person’s functioning. Naturally, pain can have profound effects on
social, interpersonal, and emotional functioning. By virtue of the long-term
course, there may be changes in mood, thought patterns, perceptions, and per-
sonality that accompany the pain. One’s life experiences and ability to adapt to
ongoing demands and stress are affected. Therefore, it is incomprehensible to
address chronic pain without considering psychological and social functioning.
Categories of Chronic Pain
Chronic pain has been categorized as nociceptive, neuropathic, or psycho-
genic (Table 2–6). These classifications differ with respect to their character-
istics and responsiveness to varying types of treatment interventions (Leo and
Singh 2002; Portenoy 1989). For example, nociceptive pain responds to anti-
inflammatory agents and opiate analgesics, whereas neuropathic pain responds
to antidepressants, anticonvulsants, and opiates.
“Patienthood” as a Psychosocial State:
The Patient With Simple Versus Chronic Pain
An array of factors can become central to the life experiences of the patient with
chronic pain (Table 2–7). As a result of these factors, a number of emotional and
psychological sequelae are associated with the chronic pain state. Physicians have
long noted a puzzling discrepancy between physical disease status and progres-
sion and the patient’s subjective experiences (Weisberg and Clavel 1999). Some
patients with severe disease present few complaints and report less disability and
emotional distress. Yet some others with little documented disease report severe
symptoms and experience marked distress and disability.
Despite the pervasiveness of chronic pain, most individuals with chronic
pain can nonetheless maintain basic functioning, work, and interests. They
are able to work with their clinicians and other care providers and can respond
with some relief to medications or interventions. At times, psychotherapeutic
interventions may be required to address mood disturbances, stress, and cop-
ing. This cluster of patients is sometimes referred to as having simple chronic
pain. Small proportions of patients with chronic pain are entirely debilitated
by the pain and are sometimes referred to as having complex chronic pain (see

28 Clinical Manual of Pain Management in Psychiatry
Table 2–6.
Categories of chronic pain
Source Localization Features Examples Effective medication
Nociceptive: somatic Damage to tissue,
soft tissue, or bone;
inflammation;
trauma
Well localized Aching, sharp Pain of arthritis,
cancer
Aspirin, NSAIDs,
COX-2 inhibitors,
opiates
Nociceptive: visceral Injury or damage to
visceral structures,
organs
Referred pain, fairly
well localized
Aching, sharp Pain of angina, kidney
stones, appendicitis
Opiates, other
analgesics
Neuropathic Damage to nerve
tissue, either
peripheral or CNS
Nerve distributions,
poorly localized
with CNS sources
Paresthetic, numb,
burning, pins-
and-needles
Postherpetic neuralgia,
trigeminal neuralgia
Antidepressants,
anticonvulsants
Psychogenic No clear underlying
cause; psycho-
logical distress
Poorly localized Vague, sweeping Somatization disorder Psychotropic
medications,
psychotherapy
Note. CNS=central nervous system; COX-2= cyclooxygenase-2; NSAID= nonsteroidal anti-inflammatory drug.
Source. Reprinted from Leo RJ, Singh A: “Pain Management in the Elderly: Use of Psychopharmacologic Agents.” Annals of Long-Term Care: Clinical
Care and Aging 10:37–45, 2002. Used with permission.

Sensory Pathways of Pain and Acute Versus Chronic Pain 29
Table 2–8 for a comparison of the two categories). In this subset, patients have
a notable preoccupation with pain. For these persons, life revolves around the
pain. Activities are forestalled, and work is not pursued. The patients may be
thrust into positions of marked dependency on others. Several, perhaps all, as-
pects of their lives are made contingent on pain experiences or are put off be-
cause the patients fear their pain might get worse (Sternbach 1974). For such
persons, being a patient is a primary psychosocial state. Life experiences be-
come centered on doctors’ visits. If these visits are unsatisfying, patients may
develop a history of “doctor shopping.” They may seek invasive and diagnos-
tic procedures to confirm the existence of the pain or alleviate their distress.
Such patients may display increasing preoccupation with medication use and,
possibly, abuse. Numerous psychological factors beset the patient with complex
pain, many of which can exacerbate and maintain pain (Weisberg and Clavel
1999).
Table 2–7.
Common problems encountered by patients with
chronic pain
Medical Problems with access to appropriate care
Difficulties in establishing a working relationship with
practitioner skilled in pain management
Psychological Comorbid mood disturbances
Physical The pain itself
Deconditioning resulting from inactivity
Medical complications from use of multiple medications
Vocatio nal Job loss
Restrictions from usual types of job activities
Financial Financial problems arising from job loss, loss of medical coverage,
or the cost of medical care
Legal Litigation related to injuries, workers’ compensation, or disability
issues
Family Pain’s interference with customary role of the patient, causing
others within the family to adopt new roles
Limited reserves of energy and time left for other family members’
needs (e.g., children’s needs, their activities, their schoolwork,
etc.) because so much is taken up with pain and the pain patient
Sociocultural Pain’s interference with patient’s ability to engage in customary
activities and maintain social ties, resulting in significant losses
in the patient’s social support network
30 Clinical Manual of Pain Management in Psychiatry
Patients with simple chronic pain may do well with a single pain specialist,
with referral as needed to services provided by practitioners in other specialties
(e.g., psychiatrists, therapists). Patients with complex pain, on the other hand,
can overwhelm a single practitioner. Clearly, such patients need a multidisci-
plinary approach to their pain, involving the coordinated joint efforts of prac-
titioners in medical, surgical, physical, neurologic, or psychiatric services.
Multiaxial Pain Classification
The International Association for the Study of Pain (1986) has advocated a
multiaxial classification of chronic pain, comparable to that used in psychiat-
ric diagnosis. The intention behind the classification system is to standardize
diagnosis and facilitate research endeavors in pain treatment. Axis I refers to
the body region that is the source of pain (e.g., lower back); Axis II refers to the
systemic source whose abnormal functioning produces pain (e.g., neurologic
system); Axis III characterizes the pattern of the pain and its temporal char-
acteristics (e.g., 6 months of continuous pain that radiates to the lower ex-
tremity); Axis IV refers to the patient’s rating of pain intensity and pain
duration (e.g., severe); and Axis V refers to the etiology (e.g., intervertebral
disk rupture). This classification approach may be useful if it is systematized
Table 2–8.
Simple versus complex chronic pain
Simple pain Complex pain
Pain is clearly defined. Multiple pain complaints are present.
Patient is easily enlisted into treatment. Difficulty can be encountered enlisting
patient into treatment.
Patient’s support systems are stable. Patient has unstable social systems.
Comorbid psychological factors are
easily defined.
Severe complicating psychological factors
can be present.
Patient’s pain shows some response to
medications and treatment.
Patient’s pain shows poor response to
medications and treatment.
Patient may require short-term
psychotherapy or psychological
interventions.
Patient may require multidisciplinary
treatment approaches, including
psychiatric treatment.
Litigation is not central to the patient’s
presentation.
Litigation is apt to be central to the patient’s
presentation.

Sensory Pathways of Pain and Acute Versus Chronic Pain 31
and employed uniformly. However, the multiaxial system has not yet been re-
quired by insurers or universally accepted in clinical circles.
Key Points
• Consistent with a biopsychosocial perspective, pain is multidimensional,
with biological (nociceptive and sensory), psychological (cognitive ap-
praisal and suffering), and social (overt behaviors and communicated dis-
tress) components.
• The neurophysiologic substrates of the pain experience can be broken down
into those elements of pain transmission emanating from peripheral, spi-
nal, and supraspinal processes.
• The mechanisms involved in pain processing within the central nervous sys-
tem are complex, influenced by the dynamic interaction of neurotransmitters,
their receptors, and pain-augmenting and pain-inhibiting neural circuits.
• Awareness of the diverse array of neurophysiologic processes underlying
the experience of pain can form the basis for appreciating various modes of
intervention for pain treatment. Overlap between neurophysiologic pro-
cesses underlying pain and related conditions (e.g., depression) may account
for the high rates of comorbidity between them.
• Differences between acute and chronic pain have largely been based on the
duration of pain. It may be more pertinent to distinguish patients with pain
by the extent to which their lives are adversely affected by their symptoms.
Thus, in the case of patients with complex pain, one’s life becomes centered
on pain and disability; being a patient becomes a primary psychosocial state.
Multidisciplinary treatment approaches become essential not only to ad-
dress symptom relief but also to mitigate related mood disturbances, im-
prove adaptive functioning, and reduce the life dissatisfaction that accompa-
nies such complex pain states.
References
Besson JM, Chaouch A: Peripheral and spinal mechanisms of nociception. Physiol Rev
67:67–186, 1987
Byers MR, Bonica JJ: Peripheral pain mechanisms and nociceptor plasticity, in Bonica’s
Management of Pain, 3rd Edition. Edited by Loeser JD, Butler SH, Chapman
CR, et al. Philadelphia, PA, Lippincott Williams & Wilkins, 2001, pp 26–72

32 Clinical Manual of Pain Management in Psychiatry
Chudler EH, Bonica JJ: Supraspinal mechanisms of pain and nociception, in Bonica’s
Management of Pain, 3rd Edition. Edited by Loeser JD, Butler SH, Chapman
CR, et al. Philadelphia, PA, Lippincott Williams & Wilkins, 2001, pp 153–179
Crue BL: Neurophysiology and taxonomy of pain, in Management of Patients With Chronic
Pain. Edited by Brena SF, Chapman SL. New York, Spectrum, 1983, pp 21–31
Dennis SG, Melzack R: Pain-signaling systems in the dorsal and ventral spinal cord.
Pain 4:97–132, 1977
Dubner R, Bennett GJ: Spinal and trigeminal mechanisms of nociception. Annu Rev
Neurosci 6:381–418, 1983
Giesler GJ, Katter JT, Dado RJ: Direct spinal pathways to the limbic system for noci-
ceptive information. Trends Neurosci 17:244–250, 1994
International Association for the Study of Pain, Subcommittee on Taxonomy: Classi-
fication of chronic pain: descriptions of chronic pain syndromes and definitions
of pain terms. Pain Suppl 3:S1–S226, 1986
Leo RJ, Singh A: Pain management in the elderly: use of psychopharmacologic agents.
Annals of Long-Term Care: Clinical Care and Aging 10:37–45, 2002
Levine JD, Fields HL, Basbaum AI: Peptides and the primary afferent nociceptor.
J Neurosci 13:2273–2286, 1993
Loeser JD: Concepts of pain, in Chronic Low Back Pain. Edited by Stanton-Hicks M,
Boas R. New York, Raven, 1982, pp 145–148
McMahon SB: Mechanisms of sympathetic pain. Br Med Bull 47:584–600, 1991
Melzack R, Wall PD: Pain mechanisms: a new theory. Science 150:971–979, 1965
Pomeranz B, Wall PD, Weber WV: Cord cells responding to fine myelinated afferents
from viscera, muscle, and skin. J Physiol 199:511–532, 1968
Portenoy RK: Mechanisms of clinical pain: observations and speculations. Neurol Clin
7:205–230, 1989
Rome H, Rome J: Limbically augmented pain syndrome (LAPS): kindling, corticolim-
bic sensitization, and the convergence of affective and sensory symptoms in chronic
pain disorders. Pain Med 1:7–23, 2000
Sewell RDE, Lee RL: Opiate receptors, endorphins and drug therapy. Postgrad Med J
56:25–30, 1980
Snyder SH: Brain peptides as neurotransmitters. Science 209:976–983, 1980
Sternbach RA: Pain Patients: Traits and Treatment. New York, Academic Press, 1974
Terenius L: Families of opioid peptides and classes of opioid receptors, in Advances in
Pain Research and Therapy, Vol 9. Edited by Fields HL, Dubner R, Cervero F.
New York, Raven, 1985, pp 463–477
Terman GW, Bonica JJ: Spinal mechanisms and their modulation, in Bonica’s Man-
agement of Pain, 3rd Edition. Edited by Loeser JD, Butler SH, Chapman CR, et al.
Philadelphia, PA, Lippincott Williams & Wilkins, 2001, pp 73–152

Sensory Pathways of Pain and Acute Versus Chronic Pain 33
Wall PD: The role of substantia gelatinosa as a gate control. Res Publ Assoc Res Nerv
Ment Dis 58:205–231, 1980
Weisberg MB, Clavel AL Jr: Why is chronic pain so difficult to treat? Psychological
considerations from simple to complex care. Postgrad Med 106:141–142, 145–
148, 157–160, 163–164, 1999
Zimmerman M: Peripheral and central nervous mechanisms of nociception, pain and
pain therapy: facts and hypotheses, in Advances in Pain Research and Therapy.
Edited by Bonica JJ, Liebeskind JC, Albe-Fessard DG. New York, Raven, 1979,
pp 3–32
This page intentionally left blank

35
3
Evaluation of the Pain Patient
Comprehensive assessment of the pain patient involves judicious use of his-
tory, physical examination, pain assessments, diagnostic testing, and psy-
chological testing. In this chapter I focus on the elements of comprehensive
history gathering. Given that pain is a subjective experience, a number of mea-
surement indices have been employed as part of the assessment that allow for
grading the quantity, extent, or severity of the pain. The elements of the his-
tory are the focus of the first portion of this chapter, and pain assessments are
discussed in the second portion.
The neural processing of pain is quite complex, involving more than just
sensory processes. In fact, pain processing involves neurologic substrates com-
mon to emotion and cognition (i.e., limbic and cortical systems). The experi-
ence of pain and the appreciation of, and reactions to, pain information involve
cognitive and emotional factors. The behaviors displayed by the pain patient
can likewise have an impact on social and adaptive functioning. It follows,
then, that pain assessment can be quite complex, involving somatic and psy-
chological factors (cognitive, emotional, and motivational), as well as adaptive
and social functioning (Figure 3–1).
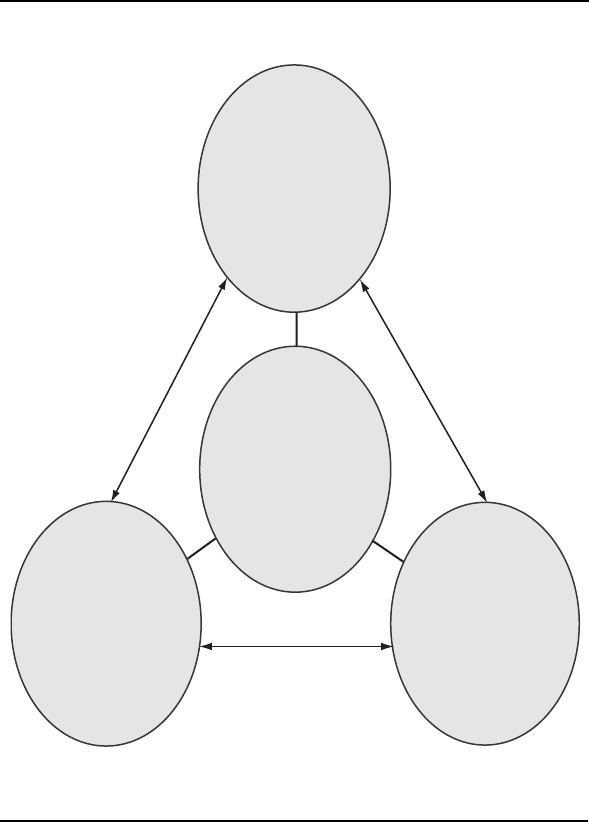
36 Clinical Manual of Pain Management in Psychiatry
Figure 3–1.
Components of the pain history.
Psychological
Mood and affect
Cognitive content
and processes
Coping repertoire
Psychiatric illness
Lethality
assessment
Social
Impact on relationships
Capacity for intimacy,
mutuality, sexuality
Activities of daily living
Vocational
Somatic
Onset/Duration
Location
Quality
Intensity
Associated features
Aggravating factors
Alleviating factors
PAIN

Evaluation of the Pain Patient 37
Conducting an Interview
During the course of verbal evaluation, the patient should be encouraged to
speak freely. Open-ended lines of inquiry should be employed to prompt the
patient to elaborate (e.g., “Tell me more about the pain” or “How has the pain
affected your lifestyle?”). Very specific lines of inquiry (e.g., “Is the pain ag-
gravated by movement?”) often restrict the free elaboration of the patient, can
prematurely limit the patient’s willingness to provide information, and can
confine the patient’s reporting of history to the examiner’s persuasion and bi-
ases. Open-ended lines of inquiry communicate that the patient is listened to,
whereas a litany of questions can suggest that the patient’s impressions and ex-
periences of pain are of less importance.
Occasional paraphrased recapitulation of the patient’s report allows the
interviewer to clarify what was conveyed. If aspects of the history are missing,
the practitioner can quickly insert clarifying inquiries (e.g., “Let me see if I got
this right. You indicated that you have been experiencing dull, aching pain in
your lower back. It seems to radiate down your right leg, and it makes you feel
unsteady when you walk. Is that right?”). If the paraphrasing is confirmed, the
practitioner might consider redirecting and clarifying questions (e.g., “I am
still unclear about something. What seems to bring on the pain?”). With pa-
tients who have difficulty articulating characteristics of the pain, encouraging
the use of metaphor could facilitate capturing the features of the pain experience
(Fishman and Loscalzo 1987).
Patients appear for psychiatric evaluation with varying agendas. For ex-
ample, a patient might seek out psychiatric assessment at the recommendation
of a clinician who is concerned about the patient’s adherence to treatment or
about psychological issues that might be contributing to or exacerbating pain.
On the other hand, the patient might have an agenda that is quite different
from the clinician’s agenda (e.g., the impact of pain on relationships, employ-
ment, and quality of life). The open-ended line of inquiry allows for exposure
and examination of the more covert aspects of the patient’s reasons for seeking
evaluation or treatment. Ascertaining the patient’s agenda can be helpful in
guiding the interview and arriving at a reasonable treatment plan that reflects
the patient’s needs. An example: A patient sought help on the recommenda-
tion of her physician, who presumed that depression was complicating her
chronic back pain. During the evaluation, it became apparent that she had con-

38 Clinical Manual of Pain Management in Psychiatry
cerns about marital issues arising from role modifications within the home as
a result of her pain (e.g., “My husband doesn’t understand my pain”). It seemed
that more direct attention to facilitating effective patterns of communication,
problem solving, and coping within the marriage would be required in order
to mitigate both the depression and the back pain.
Obtaining the Pain History
Somatic Component
Essential to the diagnosis of pain disorders is information about the somatic
components of the pain: its duration, course, intensity, and precipitating and
mitigating factors. The more detailed this history, the more likely it is that the
psychiatrist will arrive at an understanding of the pain features amenable to
treatment. In addition, clarity of these features allows the clinician to determine
whether the interventions and treatments used are effective, because one
should be able to detect changes and improvements in some or all of these pa-
rameters. Table 3–1 summarizes the somatic components to be obtained in
the pain history (Morgan and Engel 1969).
Table 3–1.
Obtaining a pain history
Onset When did the pain begin?
Location Where is the pain located? Does it radiate? If so, where?
What factors influence the radiation?
Quality What is the pain like?
Quantity How intense is the pain?
Duration and
chronology
How long has the pain been going on? What has the course
of your pain been like? Is it getting better? Worse?
Setting Under what circumstances does the pain occur?
Aggravating and
alleviating factors
What factors aggravate the pain? What brings about relief
or attenuation of pain?
Associated features What other symptoms are associated with the pain?
How does it affect your sleep? Your appetite?
Your energy level?
Source. Adapted from Morgan ML, Engel GL: The Clinical Approach to the Patient. Philadelphia,
PA, WB Saunders, 1969.
Evaluation of the Pain Patient 39
Chronic disorders of any sort, by virtue of their occurrence over time, will
produce changes in a person’s emotional and psychological state, influence
adaptive functioning, and affect social roles and relationships. Thus, evalua-
tion of such patients naturally prompts inquiry into the factors that predispose,
activate, and perpetuate the pain and disability. These factors may possibly in-
volve psychosocial stressors. Examination of the factors that render treatment
ineffective is also warranted. Once all of these factors are evaluated and ad-
dressed, appropriate treatment, rehabilitation, and reclamation of the patient
will be possible (Aronoff et al. 2000). Having established the history of pain
complaints, the physician can direct attention to the psychological and social
correlates of the pain.
Psychological Component
Certainly, any focus on somatic concerns should prompt the psychiatrist to
consider psychiatric comorbidities in which pain or other related somatic
concerns might be a feature or focus (Fishbain 1999a). Careful inquiry will
need to be made into common psychiatric comorbidities associated with pain
(Table 3–2).
The psychiatrist should inquire into the relationship of emotional and psy-
chological states to subjective pain complaints and exacerbations. Inquiry
should be conducted in a manner that does not trigger defensiveness on the part
of the patient. Often, patients have become accustomed to being rejected by
Table 3–2.
Psychiatric disorders accompanying acute and
chronic pain
Anxiety disorders
Delirium
Depression
Sexual dysfunction
Sleep disorders
Somatoform disorders
Conversion disorder
Hypochondriasis
Pain disorder
Somatization disorder
Substance abuse or dependence

40 Clinical Manual of Pain Management in Psychiatry
physicians who use traditional medical approaches and have felt as though their
pain complaints have been dismissed as being all in their head. Consequently,
patients with chronic pain can be exquisitely sensitive to inquiry that suggests
even the most remote aspects of psychological and emotional dysfunction. They
may well fear that attention could be directed away from the physical aspects of
treatment. Nonetheless, they could respond favorably to the comprehensiveness
of an assessment—including the possible emotional and psychological effects of
chronic pain—and the prospect that treatment directed at the psychological fac-
tors accompanying pain might also have pain-mitigating effects.
The essential components of the psychological variables related to pain are
summarized in Figure 3–1. These items are interrelated, so there is consider-
able overlap in a person’s moods, cognitive appraisals, and coping strategies.
Thus, flexibility is required in assessment of each of these components.
Mood and Affect
Careful examination of mood states can begin with inquiry into the patient’s
pervasive mood over recent weeks and whether this represents a change from
his or her baseline mood. Concerned primarily about illness, disability, func-
tioning, and treatment, the patient may well dismiss his or her own mood or
affective states as being natural reactions to the pain state. Consequently, inquiry
might be directed at how other people in the patient’s life have commented on
the patient’s recent mood and whether they have construed the recent mood as
a distinct change from the patient’s usual mood.
Inquiry into mood and affective states is likely to reveal the emotional fac-
tors related to the pain experiences among patients with chronic pain. Among
inpatients with chronic pain, pain ratings were linearly correlated with anger,
fear (or anxiety), and depression. On the other hand, the presence of joy, in-
terest, and surprise were predictive of lower pain states. Other emotional states
(e.g., shame, disgust, contempt) were only weakly correlated with pain com-
plaints (Fernandez and Milburn 1994).
It might be possible to determine in the interview that certain affective
states are temporally related to pain levels. Discrete, situational emotions are
important determinants of pain ratings (Gaskin et al. 1992). Thus, for exam-
ple, it could become apparent that certain emotional states precede pain,
whereas other emotional states arise after the experience of pain. Exacerbations
of pain could possibly predispose a person to certain affective states (e.g., dys-

Evaluation of the Pain Patient 41
phoria, anxiety, anger) (Huyser and Parker 1999). On the other hand, the
presence of affective states (e.g., anger) could possibly result in exacerbations
of pain. Thus, dysphoria can arise as a reaction to the pain experience, as a re-
sult of the lack of satisfactory treatment, in response to the sequelae of pain, or
as an independent disorder (i.e., depression) warranting treatment. It might
be helpful to discern whether such relationships exist, because these relation-
ships can have implications for possible treatment interventions.
Anger can be an important component of the experience of the patient
with chronic pain (Wade et al. 1990). The presence of anger in and of itself is
not a problem. Rather, problems arise when there is a conflict around the ex-
pression of anger, there is difficulty around the expression of anger, or there are
high levels of hostility. In these circumstances, there is likely to be activation of
the autonomic nervous system and endocrine systems (e.g., increased cortisol
levels) and other physiologic effects of anger (Fernandez and Turk 1995). Pa-
tients incapable of diffusing anger or channeling it into appropriate avenues
are prone to resentment, suspicion, mistrust, and heightened levels of arousal.
Likewise, inability to modulate unpleasant emotional states is likely to heighten
levels of pain (Kinder and Curtiss 1988).
Anxiety disorders are accompanied by an increased incidence of somatic
symptoms and pain complaints (Beidel et al. 1991). Anxiety can lower pain
thresholds and predispose one to heightened somatic concerns (Barsky et al.
1988).
More important than the mere presence of a particular affective state are the
ways in which unpleasant emotional states are managed. Thus, inquiry into
coping strategies would be warranted (see also section “Coping Strategies” later
in this chapter). Naturally, the repertoire of strategies one employs to comfort
oneself and the effectiveness of those strategies would be of interest.
Cognitive Patterns
The psychiatrist needs to examine the patient’s cognitions about pain—that
is, the beliefs held by the patient about the meaning of the pain, expectations
about future pain, and interpretation of the impact the pain has on his or her
life, functioning, and relationships. Cognitive appraisal of pain depends on
the individual’s perspective on the consequences of pain on his or her well-
being, the importance he or she assigns to the pain, and his or her view of the
measures available to cope with the pain and its ramifications.

42 Clinical Manual of Pain Management in Psychiatry
Questions arise as to the role the pain plays in the patient’s life. This aspect
of inquiry might naturally follow from discussions of the medical conditions
underlying the pain. The topic can be introduced by asking patients what they
were told about the cause of the pain and what their reactions were to these ex-
planations. For example, for some patients the explanations offered by clini-
cians are reassuring; for others, they are met with incredulity. This line of in-
quiry can reveal the sorts of preoccupations and concerns about the pain that
might not have been overtly expressed to other clinicians. For example, in pa-
tients with cancer, this line of inquiry might unveil thoughts the patients have
about fears that the cancer is spreading or that it now renders them “terminal.”
It also can reveal misconceptions about the pain or distortions of what has
been disclosed to them and, therefore, a need for clarification and education.
When there is no clear etiology for the pain, the interviewer can gain insight
into the patient’s disease conviction. Disease conviction refers to the extent to
which patients maintain that they are ill, how much they are bothered by symp-
toms, and the extent to which they would accept the reassurances of the physi-
cian. Disease conviction is notably present among depressed patients with so-
matic concerns (Pilowsky 1975).
Attention needs to be directed to listening for the distorted cognitive pat-
terns and styles that can be manifested by the patient with pain (Table 3–3).
These patterns and styles may be influenced by one’s prevailing emotional
state. On the other hand, such cognitive patterns can influence one’s emo-
tional state. Thus, the relationships are likely to be reciprocal. Such cognitive
styles are likely to reduce self-efficacy, hamper development of effective cop-
ing, drain one’s support systems, accentuate unpleasant emotional states (e.g.,
anger, anxiety, depression), and exacerbate pain. The presence of such pat-
terns, therefore, could signal the need for psychotherapeutic interventions.
In a recent interview, a distressed patient reported, “The pain—it’s always
there. It ruins my entire life. There is absolutely nothing that gives me relief.”
The patterns reflected in these statements signal the presence of catastrophizing
(“It ruins my entire life”), overgeneralizing (“It’s always there”), and helplessness
(“Absolutely nothing that gives me relief”). Recognition of these features might
prompt further inquiry into similar beliefs about other aspects of the patient’s
life. Using this line of inquiry, the evaluator can determine how pervasive these
patterns of beliefs are, how rigidly they are maintained, and how malleable the
person is in terms of alternative ways of looking at his or her condition.

Evaluation of the Pain Patient 43
Coping Strategies
Identification of problematic emotions and cognitive patterns should signal a
need for inquiry into the coping strategies used by the individual to self-
soothe, self-comfort, reduce distress, and modulate unpleasant states. Inquiry
should again be open-ended (e.g., “When you get to feeling [or thinking] this
way, how do you cope?”). Attentive listening to active and passive strategies is
required. Some patients have a propensity to retreat and withdraw from other
Table 3–3.
Problematic cognitive patterns in pain
Catastrophizing Tendency to view and expect the worst (e.g., “I am doomed
to have pain and misery forever!”)
Helplessness Belief that nothing that one does matters, that there is no
benefit despite one’s best efforts (e.g., “My doctor says that
I should exercise to improve my osteoarthritis. I know it
won’t help!”)
Help-rejecting Rejection of the efforts of well-meaning others as a means
of expressing anger, securing ongoing support or attention,
or even manipulating others (e.g., “I had problems with
the last four medicines you gave me.”)
Labeling Ascribing a behavior of a person to a characteristic or
nature of the person; the patient who is disappointed
about the ineffectiveness of a medication might need
to discount the qualifications of the clinician
(e.g., “The medication the doctor gave me didn’t
help. What a quack!”)
Magnification Exaggeration of the significance of a negative event (e.g.,
“My pain got worse at work yesterday. I had to leave an
hour early. I might as well come to grips with the fact that
I am totally disabled!”)
Overgeneralization Expanding one adverse event or setback to many or all
aspects of one’s life (e.g., “If this medication doesn’t help
me, nothing will!”)
Personalization Interpretation that an event or situation is indicative of
something about oneself (e.g., “Because of the pain,
I am a worthless failure!”)
Selective abstraction Propensity to attend selectively to negative aspects of
one’s life while ignoring satisfying and rewarding aspects
(e.g., “Everything that happens in my life is bad!”)

44 Clinical Manual of Pain Management in Psychiatry
people (thus affecting social functioning). Other patients might have a need
to enlist and secure the support of others (an approach that can be adaptive or
maladaptive). Still other patients have a tendency to engage in passive coping
strategies (e.g., hoping for relief, praying, sleeping). For still others, there might
be a tendency to distract themselves with other activities, engage in self-state-
ments that can produce relief, and so forth (Rosenstiel and Keefe 1983).
Some patients are apt to focus on somatic complaints and pain, as op-
posed to dealing with the distress and emotional responses incurred by having
a painful condition. In addition, somatic amplification might be invoked when
emotionally laden distress is difficult for patients to tolerate or to address di-
rectly. Thus, some patients tend instead to focus on, embellish, or magnify so-
matic complaints and concerns so as to enlist the support of others and to com-
municate to others their level of distress. Others might cope with unpleasant
emotions and cognitive patterns by self-medicating with analgesics or even by
abusing substances.
Coping strategies that involve the support of other people may be healthy
or they may be overdemanding and exasperating to members of the patient’s
social support network. Patients’ cognitive processes can serve to put up bar-
riers to addressing and dealing with problem areas in their lives. Statements
such as “This pain will be the ruin of me! It will never get better, no matter
what they tell me to do!” might actually serve to foster passivity and buffer the
patient from taking any responsibility in the rehabilitation process.
It is critical to assess lethality. Patients with chronic pain and unremitting
or terminal illnesses are particularly prone to despair. The risk of suicide is in-
creased among persons with medical illnesses in which there is distress over
disfigurement (Work Group on Suicidal Behaviors 2003), pain (Chochinov et
al. 1995; Fishbain 1999b; Fishbain et al. 1991), comorbid mood disorders
(Work Group on Suicidal Behaviors 2003), substance abuse (Borges et al. 2000),
severe functional impairments (Waern et al. 2002), or increased perceived lev-
els of disability. It is imperative to carefully inquire into thoughts of despair,
hopelessness, suicidal ideas and suicide intent, and whether plans are present.
In ascertaining the severity of these issues, it is important to determine the fol-
lowing: Is there a history of prior suicide attempt? If so, did the attempt occur
within recent months? Is there a family history of suicide? What support sys-
tems are in place to ensure the patient’s safety? Does the patient make use of
those available supports?

Evaluation of the Pain Patient 45
Social and Adaptational Component
The patient’s social history can be especially important in understanding the
profound impact of pain (see Figure 3–1). Another important feature to con-
sider is the patient’s adaptive functioning. Inquiry should be focused on what
the patient is able to do and what activities are avoided because of the pain. A
thorough evaluation of the following factors is indicated: the patient’s day-to-
day activities and interests, loss of (or decline in) activities due to the pain, the
patient’s occupation, how work is affected, how the patient is supported (if
not by work), concerns over the accessibility and cost of medical care, whether
litigation related to the cause of pain is pending, and whether applications for
disability are under review. Inquiry into the patient’s general life satisfaction is
critical (e.g., how free time is spent, pursuit of interests, how the patient comforts
himself or herself, how the patient manages pleasant and unpleasant emotions).
The interviewer needs to listen for elements that suggest the patient assumes
an invalid role in all or most aspects of his or her life and assess the function
that role serves for the patient.
Especially pertinent is the identification of significant persons in the patient’s
life and how the pain has influenced relationships with those persons. For exam-
ple, pain patients and their significant others can experience loss in intimacy and
sexual dissatisfaction because of the impact of pain on sexual functioning.
Inquiry into how pain is communicated to others, the expected responses,
the responses generated and from whom, and how the patient perceives those
responses is germane. Inquiry should be directed to how the patient’s pain in-
fluences the behaviors of others. To avoid defensiveness on the part of the pa-
tient, the examiner should avoid implying that such influence on others is
intentional or manipulative. However, the interviewer should recognize the
possibility that such influences may be unconsciously driven. Given that in-
terpersonal relationships are bidirectional, it is equally important to ascertain
the extent to which the patient’s adaptation in the context of pain may be
shaped or reinforced by the responses of others in his or her life (Turk and
Okifuji 2002).
Careful histories of alcohol and drug use are imperative. Abused agents
can include medications prescribed for the patient’s use (e.g., opiate analge-
sics), and patients may become defensive if they fear that analgesics provided
to them—even if not fully effective in eradicating pain—might be withdrawn
or withheld. Patients might require assurance that this line of inquiry is part of

46 Clinical Manual of Pain Management in Psychiatry
a comprehensive approach. This inquiry is also important in determining what
types of medical and pharmacologic approaches best suit the patients’ needs.
Longitudinal Approach
A historical perspective on the pain history can be quite helpful in under-
standing the evolution of the present pain experience. Thus, it can be useful to
evaluate the patient’s early pain complaints, history of medical interventions,
and response to treatment. There can be indications of the quality of previous
doctor–patient interactions; how proactive or passive a role the patient took;
and the patient’s history of adherence to medical treatment, including physical
or occupational therapy, medications, diet, exercise, and other components.
Earlier history can constitute a backdrop against which the current pain ex-
periences can be evaluated. Previous experiences can influence the patient’s
expectations of current treatment, his or her current doctor–patient relation-
ships, and the patient’s participation and follow-through with treatment.
Other longitudinal aspects include developmental life experiences. The
presence of early childhood illnesses (e.g., diabetes) can have a profound im-
pact on family interactions and the shaping of early relationships. These ex-
periences can shape current relationships as well. Family history of medical ill-
nesses, particularly those involving pain, and how these were experienced by
the family—and by the patient in particular—can reveal patterns of pain be-
haviors with which the patient grew up and that influence current experiences
(Fishman and Loscalzo 1987). These patterns may have an impact on how the
patient currently manages his or her pain. Degree of impairment and disability
arising from painful medical or surgical conditions can also have been “learned”
by the patient in such early experiences. Responses of family members to treat-
ment endeavors might have shaped the patient’s expectations and beliefs about
the utility and effectiveness of pain interventions, whether or not those early
experiences bore directly on the type of pain (or the source of pain) the patient
experiences in his or her own current medical condition.
Other developmental factors may have an impact on a person’s current so-
cial and adaptive functioning. For example, a history of sexual abuse is present
in a number of patients with chronic pelvic pain, abdominal pain, and even
disorders such as fibromyalgia (Gross et al. 1980). Accessing this information
can relay significant history about the dynamics within the home, how crises
were managed, what support systems were available to the patient while grow-

Evaluation of the Pain Patient 47
ing up, and whether the patient has a support system in place to actually sup-
port, nurture, and protect him or her. This inquiry should be conducted in a
sensitive and respectful manner. Patients may well be reluctant to confide such
information, fearing that it could reflect adversely on them. They may feel a
need or a desire to protect family members despite the abuse itself or the family’s
failure to intervene on their behalf even in the face of evidence or suggestion of
abuse. Patients may need to be informed that such experiences can influence
how they approach their world, can affect the patients’ expectations regarding
the reliability and goodwill of treating sources (authority figures), and have
been related to certain chronic pain disorders.
Evaluation of Treatment Suitability
At times, psychiatric evaluation is requested to assess the factors that contrib-
ute barriers to effective pain treatment. Evaluation can determine whether
psychopharmacologic treatment, psychotherapeutic modalities, or both should
be employed. Sometimes psychiatric evaluation is requested when psycholog-
ical factors are mediating the pain experience or when psychiatric disorders
more completely explain the origin of the pain. Psychiatric evaluation can be
conducted to assess the patient’s suitability for other interventions (e.g., sur-
gery). Table 3–4 lists the factors that predict a poor surgical outcome for pain
disorders. Once such factors are addressed in psychiatric treatment, however,
a patient could possibly be considered for surgical interventions (see also sec-
tion “Role of the Psychiatrist in Pain Management Related to Interventions”
in Chapter 7, “Special Techniques in Pain Management,” of this book).
Pain Assessment Instruments
Pain assessment tests and scales are useful adjuncts to the evaluation of the
pain patient. These instruments allow the examiner to ascertain the severity
and intensity of the pain experience. A number of scales are available, and the
selection of the assessment instrument to be used is determined in part by the
characteristics of the pain and the elements gleaned from the interview (see
Table 3–5 for a list of psychometric scales used in assessing chronic pain). In
acute pain states, certain types of assessments are desirable that are of limited
utility in assessing chronic pain states (see Table 3–6 for a summary of the

48 Clinical Manual of Pain Management in Psychiatry
instruments used in acute vs. chronic pain). On the other hand, multidimen-
sional pain assessments and complex behavioral assessments can be invoked
when complex interactions between pain states and psychosocial factors are
implicated in mediating pain.
Patients with acute pain (e.g., postoperative pain) are preoccupied with the
situational characteristics of the pain. The pain is expected to be time limited.
Conversely, for those with chronic pain, their day-to-day, interpersonal, aca-
demic, and vocational functioning are overshadowed by the pain experience.
Therefore, assessments used in acute pain settings will be fundamentally dif-
ferent from those used in chronic pain. Measures for acute pain, summarized in
Table 3–6, focus on the experience and intensity of pain and assess responsive-
ness to treatment interventions. Chronic pain assessments focus on broader as-
pects of patients’ pain experiences, functioning, and psychological adaptation.
The pain assessments described in the following subsections serve a num-
ber of functions, including assessment of pain intensity, quality, and duration.
In addition, such scales can assist the physician in making treatment selections
and assessing the efficacy of treatment.
Table 3–4.
Factors that suggest poor surgical outcome for
pain disorders
Emotional factors
Anger
Anxiety
Depression
Cognitive factors
Catastrophizing
Perception of loss of control
Vocational factors
Financial settlement or pending litigation
Job dissatisfaction
Workers’ compensation
Social factors
Marital dissatisfaction
Historical factors
History of physical abuse
History of sexual abuse
Prior psychological treatment
Substance abuse or dependence

Evaluation of the Pain Patient 49
Single-Dimension Scales
The most commonly used pain scales involve single-dimension ratings of pain
intensity. Such scales are appealing because of their ease of administration and
interpretation. Patients also find them easy to complete (e.g., requiring little
in the way of time commitment or concentration). However, single-dimension
scales have been criticized for oversimplifying pain ratings and for ignoring
factors that contribute to or exacerbate the pain experience (e.g., emotional
and cognitive factors).
Verbal Descriptor Scale
A verbal descriptor scale (VDS) requires that a patient rate the pain experienced
according to one of five to seven verbal descriptors. Only limited types of re-
sponses are permitted. Such scales can be used in clinical and experimental set-
tings. Of course, use of a VDS assumes that the patient has intact verbal skills
(i.e., reading and comprehension). Thus, these measures may not be useful for
patients who have significant language barriers or cognitive impairments or for
Table 3–5.
Psychometric scales used in assessing chronic pain
Coping Strategies
Questionnaire
Assesses the coping strategies in patient’s repertoire
to deal with chronic pain. May predict the level of
activity, physical impairment, and psychological
functioning associated with pain.
Fear-Avoidance Beliefs
Questionnaire
Assesses beliefs characterized by danger, threat, or
harm associated with pain. The degree to which
patients assign threat to activities can limit their
participation in, and lead to avoidance of,
activities related to work.
McGill Pain Questionnaire Assesses the features of pain severity and intensity.
Allows patients to qualify pain in emotional,
cognitive-evaluative, and sensory terms.
Minnesota Multiphasic
Personality Inventory
Provides personality profile and pathologic
assessment of patient with chronic pain.
Pain Interference Indices Assess the impact of chronic pain states on various
aspects of a person’s activity, functioning, and role
responsibilities.
Multidimensional Pain
Inventory
Assesses patient’s appraisal of pain, its impact on his
or her functioning, and the patient’s perceived
responses of others to his or her pain.
50 Clinical Manual of Pain Management in Psychiatry
young children. The scoring of a VDS correlates with pain ratings of other scales
(e.g., a visual analog scale [VAS]). The VDS, included in Figure 3–2, ignores the
emotional, cognitive, and behavioral components of pain.
Numeric Rating Scale
A numeric rating scale (NRS) is often used to measure pain experience and in-
tensity. Patients are asked to rate their pain on an 11-point scale, from 0 (no
pain) to 10 (worst pain). A variation of this scale shows a rating of pain from
0 to 100, with similar anchors (Jensen et al. 1986). These scales are reliable and
correlate with other simple assessment measures. Use of an NRS requires that
the patient have intact language and cognitive skills. One drawback of NRSs
is that the patient’s rating of pain (i.e., the number selected) has no intrinsic
meaning. Thus, if a patient rates his pain as a 5, this rating cannot be assumed
to be one-half that of another patient who rates her pain as a 10. Furthermore,
the transition from one rating (e.g., from 5 to 4) might not mean the same,
even within the same patient, as a transition at another point in the scale (e.g.,
from 9 to 8).
Visual Analog Scale
An extension of NRSs, the Visual Analogue Scale consists of a 10-cm line with
anchors at 0 and 10 or verbal anchors (see Figure 3–2). The patient is asked to
draw an X along the line that best denotes his or her level of pain. The VAS has
been used in clinical as well as experimental settings. The denoted pain levels for
an individual can be compared over time to quantify levels of pain worsening or
improving. These serial comparisons, unlike those employed with repeated use
of NRSs, are proportional. However, the use of the VAS for clinical compari-
Table 3–6.
Pain assessment instruments for acute and chronic pain
Acute pain Recurrent/chronic pain
Visual analog scale or numeric
rating scale
Visual analog scale, McGill Pain
Questionnaire
Medication use Medication use
Observer rating Observer rating
Pain diary
Multidimensional Pain Inventory
Psychological measures
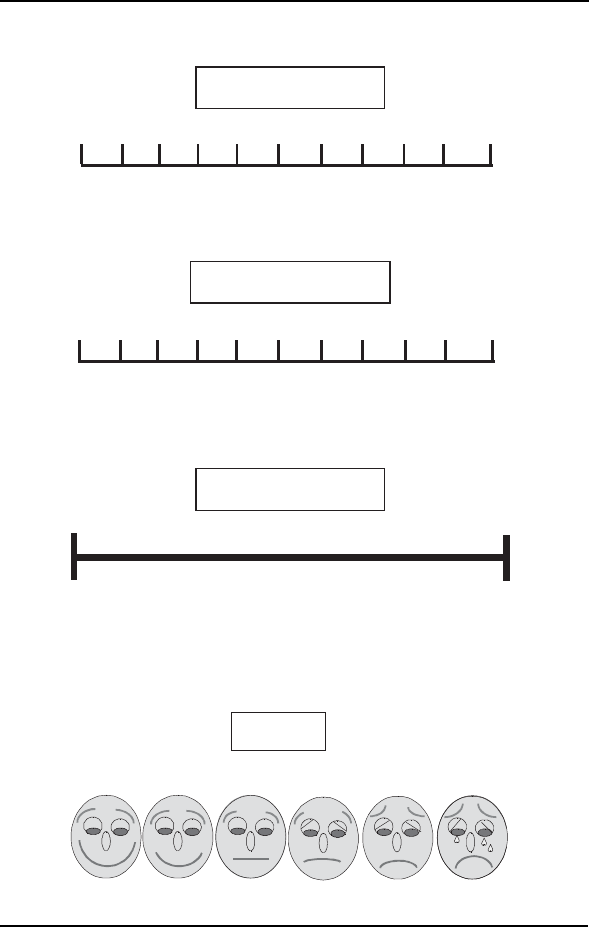
Evaluation of the Pain Patient 51
Figure 3–2.
Single-dimension pain assessment instruments.
No
pain Mild
pain Moderate
pain Severe
pain Very
severe
pain
Worst
pain
possible
Verbal descriptor scale
012 345678910
No
pain
Moderate
pain
Worst
pain
Numeric rating scale
Visual analog scale
No
pain
Pain as
bad as it
could be
Pain as
bad as
it could be
Faces scale
Faces scale

52 Clinical Manual of Pain Management in Psychiatry
sons has been questioned (Carlsson 1983). A VAS might not be suitable for el-
derly patients or patients who have difficulty understanding its abstract
concept. In such cases, pain can be erroneously denoted, and the faulty values
can impede the appropriate characterization of the pain and its treatment.
Faces Scale
With the faces scale, the patient is asked to rate pain intensity according to
printed facial expressions conveying varying amounts of distress (see Figure 3–2).
This is an easily understood rating instrument and one that has appeal for chil-
dren. The faces scale allows the examiner to bypass issues related to language
barriers (Frank et al. 1982).
Behavioral Measures
Behavioral measures are instruments that assess pain in a manner vastly dif-
ferent from the simple rating scales. Although some professionals in the field
have questioned the utility and reliability of observer ratings of pain behavior,
these ratings are often used by clinicians and nursing staff. Thus, when some-
one complains of distress, the clinician often examines the overt behavior of
the patient to look for corroborating behaviors that substantiate the allegation
of pain. The clinician might look for evidence of distress, facial grimacing,
wincing or frowning, guarding of an affected area, limping, splinting, muscle
tension, and similar behaviors. The problem with observer ratings is that the
results are contingent upon the skill of the observer in detecting pain symp-
toms. However, observer ratings are also subject to a great deal of bias. For in-
stance, observing a patient with chronic pain who is able to smile or make puns
periodically might lead one to dismiss the patient’s allegations of pain because
of the bias that such behaviors would be entirely inconsistent with pain.
Medication Use
Reviews of the patient’s use of medications can be an indicator of the patient’s
pain experience and severity. It is useful to assess the patient’s adherence to
treatment, appropriateness of medication use, and excess medication use. Pain
patients can be unreliable about medication use and can significantly under-
estimate the extent of their opioid use (Ready et al. 1982). Thus, the support
of collateral informants (e.g., from a spouse or other family member) to pro-
vide information about the use of medications can be helpful.

Evaluation of the Pain Patient 53
Pain Diary
A pain diary can be as simple or as detailed as the clinician deems necessary
(see Figure 3–3 for an example). Categories that might be included in a pain
diary include the date and time; the pain rating; the situation; the patient’s
emotions, behavior, and thoughts; and the response of others to the patient’s
pain. Pain diaries can be very useful, revealing patterns of pain intensity, ex-
acerbations of pain, and mitigating factors. They can likewise uncover varying
psychological states temporally related to episodes of pain or pain relief. This
information can be particularly useful when patients are resistant to the idea
that psychological states could be related to the pain experience. Also, diaries can
be a source of information about the extent to which patients engage in activ-
ities (e.g., reclining, pursuing their interests).
Completion of pain diaries can be quite time-consuming for patients. For
the diary to be maximally effective, the patient must be committed to main-
taining it. Completion of the entire diary for a week in the hour preceding the
doctor’s appointment limits the utility of the diary, because the entries will be
based on the patient’s memory and recollections. Instead, the utility of the diary
is best derived when the patient maintains the diary reliably and consistently
during the periods of study. However, the more complex the diary, the less in-
clined the patient might be to reliably make entries, because the task can seem
overwhelming. In addition, clinicians need to devote some time to review the
diary contents to look for trends in the pain ratings and associated temporal
features.
Multidimensional Pain Scales
McGill Pain Questionnaire
In the McGill Pain Questionnaire (MPQ; Melzack 1975), a verbal rating scale
commonly used to characterize pain, subjects are asked to select verbal de-
scriptors for their pain among sets of categories of descriptors. A long form
and short form are available for use. The long form contains 20 sets of cate-
gories. The first 10 sets of descriptors refer to the sensory-discriminative aspects
of the pain. Sets 11–15 contain items that characterize the affective-emotional
components of the pain. Set 16 contains descriptors that correspond to the
evaluative components of pain, and the remaining sets (17–20) contain mis-
cellaneous items. The short form of the MPQ (see Table 3–7) contains 15 items.
54 Clinical Manual of Pain Management in Psychiatry
Date/Time
Pain
rating Situation Mood/Affect Activity Thoughts
Response
of others
Figure 3–3.
Example of a pain diary format.

Evaluation of the Pain Patient 55
The patient is asked to rank the extent to which each item corresponds to the
intensity of his or her pain (Melzack 1987). The short form offers the advantage
of being easier to complete, and the scoring correlates with results obtained in
the longer form. The MPQ has been translated into a number of different lan-
guages for patients for whom language barriers impede the reading or under-
standing of the English version.
Pain Interference
Any assessment of pain level—or of pain relief resulting from treatment—is
less meaningful without a consideration of the patient’s perception of the ex-
tent to which pain impairs activity and social functioning. Thus, any reduc-
tions in pain ratings that suggest an intervention is successful mean little
without commensurate changes in the patient’s psychological and social well-
being and changes in adaptive functions. Examples of such instruments include
the Pain Disability Index (Pollard 1984) and the Oswestry Disability Question-
Table 3–7.
Short form of the McGill Pain Questionnaire
None Mild Moderate Severe
Throbbing 0)_____ 1)_____ 2)_____ 3)_____
Shooting 0)_____ 1)_____ 2)_____ 3)_____
Stabbing 0)_____ 1)_____ 2)_____ 3)_____
Sharp 0)_____ 1)_____ 2)_____ 3)_____
Cramping 0)_____ 1)_____ 2)_____ 3)_____
Gnawing 0)_____ 1)_____ 2)_____ 3)_____
Hot–burning 0)_____ 1)_____ 2)_____ 3)_____
Aching 0)_____ 1)_____ 2)_____ 3)_____
Heavy 0)_____ 1)_____ 2)_____ 3)_____
Ten der 0)_____ 1)_____ 2)_____ 3)_____
Splitting 0)_____ 1)_____ 2)_____ 3)_____
Tiring–exhausting 0)_____ 1)_____ 2)_____ 3)_____
Sickening 0)_____ 1)_____ 2)_____ 3)_____
Fearful 0)_____ 1)_____ 2)_____ 3)_____
Punishing–cruel 0)_____ 1)_____ 2)_____ 3)_____
Source. Reprinted from Pain, Volume 30, Melzack R: “The Short-Form McGill Pain Question-
naire,” pp. 191–197, 1987. Copyright 1984, with permission from Dr. R. Melzack, McGill Uni-
versity and the International Association for the Study of Pain.

56 Clinical Manual of Pain Management in Psychiatry
naire (Fairbank et al. 1980). Factors included in such indices include ratings of
ability to perform activities of daily living and other customary role responsi-
bilities. These issues can be the basis for modifications in pain treatment and
can also be the focus of psychotherapeutic endeavors.
Psychological Assessments
Multidimensional Pain Inventory
The Multidimensional Pain Inventory (MPI) (formerly referred to as the West
Haven–Yale Multidimensional Pain Inventory; Kerns et al. 1985) is a 52-item
inventory developed for the assessment of a patient’s idiosyncratic appraisals
of chronic pain. The instrument relies on components of the cognitive-behav-
ioral approach to help understand and conceptualize pain. The instrument is
used to examine a person’s perceptions, appraisals, and emotions and behav-
iors associated with pain. Coping strategies used by the individual patient are
also assessed, as are the patient’s reactions to the responses of others to pain
complaints. Not only does the MPI enable the examiner to understand the pa-
tient’s view of his or her own pain, but it can also serve as a basis for the de-
velopment of treatment interventions (e.g., to be used in psychotherapy).
Response patterns can reveal patient profiles that might become a focus of
clinical attention. A dysfunctional profile reveals high levels of perceived pain,
life interference from the pain, low levels of perceived life control, and subjective
distress. An interpersonally distressed profile is likewise characterized by high
levels of perceived pain and life interference, and patients with this profile per-
ceive themselves as having low levels of social support. Last, an adaptive pro-
file is one in which the patient perceives high levels of self-control, along with
low levels of perceived pain and perceived life interference from the pain. The
profiles summarized here can have predictive value in terms of treatment ap-
proaches and treatment outcomes (Bradley and McKendree-Smith 2001).
Fear-Avoidance Beliefs Questionnaire
The Fear-Avoidance Beliefs Questionnaire (FABQ; Waddell et al. 1993) is a
16-item instrument that assesses the beliefs and fears a patient associates with
back pain. Each item is ranked along a 7-point Likert scale that ranges from
“strongly agree” to “strongly disagree.” The patient’s beliefs and fears can have
an impact on his or her range and extent of activity. The FABQ assesses fears
the patient has about eliciting pain through behaviors required at work and in

Evaluation of the Pain Patient 57
general activity. The higher the level of fear, the higher the level of the patient’s
perceived disability.
Coping Strategies Questionnaire
The Coping Strategies Questionnaire (Rosenstiel and Keefe 1983) is useful in
assessing active (e.g., diverting one’s attention, increasing the level of activity)
and passive (e.g., praying, hoping, ignoring pain) coping strategies used by
patients dealing with chronic pain. The instrument measures the extent to
which maladaptive strategies (e.g., catastrophizing) or more adaptive strate-
gies (e.g., reinterpreting the meaning of the pain and using coping self-state-
ments) are employed. Thus, the scale can illustrate those strategies that are
effective and that, therefore, should be maximized when dealing with pain. In
addition, those strategies that are ineffective and maladaptive can be the focus
of therapeutic interventions to foster modification of those strategies.
Minnesota Multiphasic Personality Inventory
The Minnesota Multiphasic Personality Inventory (MMPI; Hathaway et al.
1989) has been used extensively as an assessment tool for a variety of psycho-
logical disturbances. It is also among the most widely used assessment instru-
ments in pain syndromes. The MMPI consists of 566 statements requiring
true or false responses. The MMPI comprises 10 standard clinical scales as-
sessing psychopathologic states, 3 validity scales, and 4 additional scales eval-
uating ego strength and other factors. Consultation with a psychologist trained
in the administration and interpretation of the MMPI can be very helpful in
the use of this instrument.
The MMPI’s strength is its usefulness in identifying psychological factors
that warrant clinical attention (e.g., drawing attention to those characteristics
that might present barriers to treatment and that ultimately could require psy-
chotherapeutic intervention) (see Table 3–8). The three scales that have the
most relevance to patients with pain are Hypochondriasis (Scale 1), Depres-
sion (Scale 2), and Hysteria (Scale 3). High scores on Scale 1 suggest that patients,
when emotionally distressed, symptomatically channel the distress into somatic
complaints. Scale 2 may be an indicator of general distress, but elevated ratings
on this scale can suggest a possible depressive disorder. Elevations on Scale 2 may
suggest one is unhappy, pessimistic, and self-deprecating. Patients who score
high on Scale 3 are characterologically prone to react by developing physical
58 Clinical Manual of Pain Management in Psychiatry
symptoms when confronted with stress or uncomfortable emotions. Scales 1
and 3 are often related (Trimboli and Kilgore 1983).
The two most common patterns noted among patients with chronic pain
are the conversion V and the neurotic triad (see Figure 3–4). In the conversion V
pattern, elevated ratings on Scales 1 and 3, relative to that on Scale 2, form a val-
ley or Vshape when represented on an MMPI graphic profile. Despite use of
the term conversion, it was never maintained that the pain complaints character-
ized features of a conversion disorder. Rather, persons with this profile endorse
somatic concerns, develop physical complaints in the face of stress, often deny
depressive symptoms, and often lack insight into their emotional states (Trim-
boli and Kilgore 1983). By contrast, those with the neurotic triad pattern (i.e.,
with elevations on each of the three scales) have somatic preoccupations and neu-
rovegetative symptoms of depression and are often demanding and complaining.
MMPI profiles may change as a patient makes the transition from acute to
chronic pain, suggesting commensurate changes in the patient’s psychological
state over time. The MMPI patterns are modifiable with successful treatment
(Naliboff et al. 1988).
Finally, although some empirical work has suggested that the MMPI can be
useful in predicting response outcome to treatment interventions (e.g., surgery),
its use has not uniformly demonstrated predictive value in this regard (Block
et al. 2003). One argument against the interpretation of chronic pain profiles
rests with the contention that the items of the MMPI have intrinsic bias—that
the Hypochondriasis, Hysteria, and Depression scale items would be endorsed
Table 3–8.
Uses of the Minnesota Multiphasic Personality
Inventory for patients with chronic pain
Can be useful for
Identifying personality traits/temperament
Clarifying emotional characteristics
Clarifying psychological functioning of the patient
Determining whether psychological factors play a significant role in the pain
Should not be used
For determining “real” versus functional (i.e., “psychogenic”) pain
As a stand-alone prescreening assessment of surgical or other treatment
interventions
For predicting response outcomes for surgical or other treatment interventions
Evaluation of the Pain Patient 59
Figure 3–4.
Common Minnesota Multiphasic Personality Inventory–2 profiles in chronic pain.
Note. In the first graph, the conversion V pattern is depicted by elevations in scales 1 (Hypochondriasis), 2 (Depression), and 3
(Hysteria), but with depression scores less than those of the other two scales creating a valley. In the second graph, the neurotic triad
pattern is depicted by elevations in all three scales.
L, Lie Scale; F, Infrequency Scale; K, Suppressor Scale; 1, Hypochondriasis; 2, Depression; 3, Hysteria; 4, Psychopathic Deviance;
5, Masculinity–Femininity; 6, Paranoia; 7, Psychasthenia; 8, Schizophrenia; 9, Hypomania; 10, Social Introversion.
Conversion V pattern
L F K 1 2 3 4 5 6 7 8 9 10
110
100
90
80
70
60
50
L F K 1 2 3 4 5 6 7 8 9 10
Neurotic triad pattern
40
30
20
10
0
110
100
90
80
70
60
50
40
30
20
10
0

60 Clinical Manual of Pain Management in Psychiatry
simply by virtue of the fact that the person is in pain (Smythe 1984). The en-
dorsed items might simply replicate the patient’s pain complaints and might not
fundamentally characterize a personality prototype of the patient with chronic
pain, nor would they necessarily predict treatment response.
Key Points
• Pain assessment needs to be individualized, comprehensive, measurable,
and documented sufficiently such that all providers involved in the pa-
tient’s care have a clear understanding of the patient’s pain experiences.
• On a basic level, the clinician needs to characterize the type and intensity of
pain. In addition, comprehensive pain assessment requires evaluation of
the cognitive, emotional, and social factors influencing the pain experience.
Identification of such influences of the pain experience can identify addi-
tional areas warranting treatment.
• Psychological factors influencing the pain experience can include distur-
bances in mood and affect, cognitive appraisals, and coping strategies.
• Social factors influencing the pain experience can include adaptive func-
tioning, the impact of pain on interpersonal relationships, access to medical
care, financial and litigation issues, and substance abuse or dependence.
• Standardized instruments allow for quantifiable assessments that, in many
cases, are reproducible and reliable. Several single-dimension pain assess-
ment instruments are available that assist with the quantification of pain
severity and intensity. Use of multidimensional assessments and psycho-
logical instruments can enhance the information gathered from the clinical
interview, revealing emotional, cognitive, and subsyndromal psychological
factors contributing to the pain experience.
• Together, physical, psychological, and social factors can predispose one to
pain, precipitate pain, augment or mitigate pain, and influence one’s reac-
tions to pain.
References
Aronoff GM, Tota-Faucette M, Phillips L, et al: Are pain disorder and somatization
disorder valid diagnostic entities? Curr Rev Pain 4:309–312, 2000

Evaluation of the Pain Patient 61
Barsky AJ, Geringer E, Wool CA: A cognitive-educational treatment for hypochondri-
asis. Gen Hosp Psychiatry 10:322–327, 1988
Beidel DC, Christ MAG, Long PJ: Somatic complaints in anxious children. J Abnorm
Child Psychol 19:659–670, 1991
Block AR, Gatchel RJ, Deardorff WW, et al: The Psychology of Spine Surgery. Wash-
ington, DC, American Psychological Association, 2003
Borges G, Walters EE, Kessler RC: Association of substance use, abuse and dependence
with subsequent suicidal behavior. Am J Epidemiol 151:781–789, 2000
Bradley LA, McKendree-Smith NL: Assessment of psychological status using interviews
and self-report instruments, in Handbook of Pain Assessment, 2nd Edition. Edited
by Turk DC, Melzack R. New York, Guilford, 2001, pp 292–319
Carlsson AM: Assessment of chronic pain, I: aspects of the reliability and validity of
the visual analogue scale. Pain 16:87–101, 1983
Chochinov HM, Wilson KG, Enns M, et al: Desire for death in the terminally ill. Am
J Psychiatry 152:1185–1191, 1995
Fairbank JC, Couper J, Davies JB, et al: The Oswestry low back pain disability ques-
tionnaire. Physiotherapy 66:271–273, 1980
Fernandez E, Milburn TW: Sensory and affective predictors of overall pain and emo-
tions associated with affective pain. Clin J Pain 10:3–9, 1994
Fernandez E, Turk DC: The scope and significance of anger in the experience of chronic
pain. Pain 61:165–175, 1995
Fishbain DA: Approaches to treatment decisions for psychiatric comorbidity in
the management of the chronic pain patient. Med Clin North Am 83:737–760,
1999a
Fishbain DA: The association of chronic pain and suicide. Semin Clin Neuropsychiatry
4:221–227, 1999b
Fishbain DA, Goldberg M, Rosomoff RS, et al: Completed suicide in chronic pain.
Clin J Pain 7:29–36, 1991
Fishman B, Loscalzo M: Cognitive-behavioral interventions in management of cancer
pain: principles and applications. Med Clin North Am 71:271–287, 1987
Frank AJM, Moll JMH, Hort JF: A comparison of three ways of measuring pain.
Rheumatol Rehabil 21:211–217, 1982
Gaskin ME, Greene AF, Robinson ME, et al: Negative affect and the experience of
chronic pain. J Psychosom Res 36:707–713, 1992
Gross RJ, Doerr H, Caldirola D, et al: Borderline syndrome and incest in chronic pelvic
pain patients. Int J Psychiatry Med 10:79–96, 1980
Hathaway SR, McKinley JC, Butcher JN, et al: Minnesota Multiphasic Personality
Inventory–2: Manual for Administration. Minneapolis, University of Minnesota
Press, 1989

62 Clinical Manual of Pain Management in Psychiatry
Huyser BA, Parker JC: Negative affect and pain in arthritis. Rheum Dis Clin North Am
25:105–121, 1999
Jensen MP, Karoly P, Braver S: The measurement of clinical pain intensity: a compari-
son of six methods. Pain 27:117–126, 1986
Kerns RD, Turk DC, Rudy TE: The West Haven–Yale Multidimensional Pain Inven-
tory (WHYMPI). Pain 23:345–356, 1985
Kinder BN, Curtiss G: Assessment of anxiety, depression and anger in chronic pain
patients: conceptual and methodological issues, in Advances in Personality As-
sessment. Edited by Speilberger CD, Butcher JN. Hillsdale, NJ, Erlbaum, 1988,
pp 161–174
Melzack R: The McGill Pain Questionnaire: major properties and scoring methods.
Pain 1:277–299, 1975
Melzack R: The Short-Form McGill Pain Questionnaire. Pain 30:191–197, 1987
Morgan ML, Engel GL: The Clinical Approach to the Patient. Philadelphia, PA, WB
Saunders, 1969
Naliboff BD, McCreary CP, McArthur DL, et al: MMPI changes following behavioral
treatment of chronic low back pain. Pain 35:271–277, 1988
Pilowsky I: Dimensions of abnormal illness behaviour. Aust N Z J Psychiatry 9:141–147,
1975
Pollard CA: Preliminary validity study of the Pain Disability Index. Percept Mot Skills
59:974, 1984
Ready LB, Sarkis E, Turner JA: Self-reported vs. actual use of medications in chronic
pain patients. Pain 12:285–294, 1982
Rosenstiel AK, Keefe FJ: The use of coping strategies in chronic low back pain patients:
relationship to patient characteristics and current adjustment. Pain 17:33–44, 1983
Smythe HA: Problems with the MMPI. J Rheumatol 11:417–418, 1984
Trimboli FK, Kilgore RB: A psychodynamic approach to MMPI interpretation. J Pers
Assess 47:614–626, 1983
Turk DC, Okifuji A: Psychological factors in chronic pain: evolution and revolution.
J Consult Clin Psychol 70:678–690, 2002
Waddell G, Newton M, Henderson I, et al: A Fear-Avoidance Beliefs Questionnaire
(FABQ) and the role of fear-avoidance beliefs in chronic low back pain and dis-
ability. Pain 52:157–168, 1993
Wade JB, Price DD, Hamer RM, et al: An emotional component analysis of chronic
pain. Pain 40:303–310, 1990
Waern M, Rubenowitz E, Runeson B, et al: Burden of illness and suicide in elderly
people: case-control study. BMJ 324:1355–1357, 2002
Work Group on Suicidal Behaviors: Practice guideline for the assessment and treatment
of patients with suicidal behaviors. Am J Psychiatry 160 (11 suppl):1–60, 2003

63
4
Common Psychiatric
Comorbidities and Psychiatric
Differential Diagnosis
of the Pain Patient
Chronic pain rarely presents alone. Patients with chronic pain tend to have
multiple comorbidities that warrant medical attention. The psychiatrist en-
listed to care for the patient with chronic pain must consider an extensive
psychiatric differential diagnosis. The psychiatric diagnosis can be carefully
extracted on the basis of the patient’s symptoms, the notable signs during eval-
uation and interview, the longitudinal course, and supporting evidence pro-
vided by laboratory investigations and physical examination. By building on
the biopsychosocial approach to pain management outlined in the previous
chapter, a thorough psychiatric assessment can enhance understanding of the
psychological conditions mediating the pain experience and raise awareness of
psychiatric comorbidities that require appropriate treatment.

64 Clinical Manual of Pain Management in Psychiatry
Pain Disorder
Pain disorder requires that pain be a primary symptom and be severe enough
to warrant clinical attention. Marked disability might be alleged, and the pa-
tient’s life becomes centered on the pain.
Earlier versions of the Diagnostic and Statistical Manual of Mental Disor-
ders (DSM) required that clinicians infer whether psychological underpin-
nings or conflicts precipitated pain complaints. Thus, if it was evident from
physical examination and diagnostic evaluation that a physical cause could not
fully account for the pain, psychiatric labels were invoked that reflected the
psychological origins of the pain—for example, psychogenic pain disorder
from DSM-III (American Psychiatric Association 1980) and somatoform pain
disorder from DSM-III-R (American Psychiatric Association 1987).
In DSM-IV (American Psychiatric Association 1994) there was a transition
in the thinking underlying the diagnosis of pain disorder. The terms somatoform
and psychogenic were dropped. There was no longer a requirement for exclu-
sion of a physical cause for the pain, and the primacy of psychological factors
(i.e., conflicts, defenses, and emotional states) underlying and accounting for
pain was de-emphasized. DSM-IV-TR (American Psychiatric Association
2000) leaves open the possibility that psychological factors can contribute to
the pain experience by precipitating, exacerbating, or maintaining pain, but
they do not necessarily have to fully account for the pain. This approach is
more consistent with current views of the interrelationships between pain and
psychological factors. The DSM-IV-TR diagnostic criteria for pain disorder
are presented in Table 4–1.
Pain disorder can be associated with a general medical condition, psycho-
logical factors, or both. Pain disorder associated with a general medical con-
dition is recorded solely on Axis III, because psychological factors are thought
to have minimal or no involvement in the pain experience. When psycholog-
ical factors are implicated and are believed to have a significant contributory
role in the pain, one of the two other types of pain disorder would be coded on
Axis I. However, questions arise as to whether the subtypes represent clinically
useful subclassifications. (For example, Aigner and Bach [1999] determined
that patients with pain who were categorized into subtypes with psychological
versus psychological and general medical features could not be distinguished
in terms of pain severity or disability measures.)

Psychiatric Comorbidities and Psychiatric Differential Diagnosis 65
Table 4–1.
DSM-IV-TR diagnostic criteria for pain disorder
A. Pain in one or more anatomical sites is the predominant focus of the clinical
presentation and is of sufficient severity to warrant clinical attention.
B. The pain causes clinically significant distress or impairment in social,
occupational, or other important areas of functioning.
C. Psychological factors are judged to have an important role in the onset, severity,
exacerbation, or maintenance of the pain.
D. The symptom or deficit is not intentionally produced or feigned (as in factitious
disorder or malingering).
E. The pain is not better accounted for by a mood, anxiety, or psychotic disorder
and does not meet criteria for dyspareunia.
Code as follows:
307.80 Pain Disorder Associated With Psychological Factors:
psychological factors are judged to have the major role in the onset, severity,
exacerbation, or maintenance of the pain. (If a general medical condition is
present, it does not have a major role in the onset, severity, exacerbation, or
maintenance of the pain.) This type of pain disorder is not diagnosed if criteria
are also met for somatization disorder.
Specify if:
Acute: duration of less than 6 months
Chronic: duration of 6 months or longer
307.89 Pain Disorder Associated With Both Psychological Factors and a
General Medical Condition: both psychological factors and a general
medical condition are judged to have important roles in the onset, severity,
exacerbation, or maintenance of the pain. The associated general medical
condition or anatomical site of the pain (see below) is coded on Axis III.
Specify if:
Acute: duration of less than 6 months
Chronic: duration of 6 months or longer
Note: The following is not considered to be a mental disorder and is included here
to facilitate differential diagnosis.
Pain Disorder Associated With a General Medical Condition: a general medical
condition has a major role in the onset, severity, exacerbation, or maintenance of the
pain. (If psychological factors are present, they are not judged to have a major role
in the onset, severity, exacerbation, or maintenance of the pain.) The diagnostic code
for the pain is selected based on the associated general medical condition if one has
been established (see Appendix G) or on the anatomical location of the pain if the
underlying general medical condition is not yet clearly established—for example, low
back (724.2), sciatic (724.3), pelvic (625.9), headache (784.0), facial (784.0), chest
(786.50), joint (719.40), bone (733.90), abdominal (789.0), breast (611.71), renal
(788.0), ear (388.70), eye (379.91), throat (784.1), tooth (525.9), and urinary (788.0).
Source. Reprinted with permission from American Psychiatric Association 2000.
66 Clinical Manual of Pain Management in Psychiatry
Although improved over previous versions, there are several limitations of
the DSM-IV-TR taxonomy (see Table 4–2). In contrast to criteria for many
other psychiatric disorders, the criteria for pain disorder often are perceived as
insufficiently defined, lacking a checklist of symptoms that collectively delin-
eate the syndrome. Thus, for example, there are no guidelines allowing one to
ascertain whether pain is “not better accounted for” by a mood disorder (Sul-
livan 2000); in fact, it can be quite difficult to make this determination given
the high comorbidity of mood disturbances with pain, as discussed in the sec-
tion “Depression”). Similarly, an inference is still required on the part of the
clinician to determine whether and to what extent psychological factors are in-
volved in the patient’s plight.
By being grouped under the rubric of somatoform disorders, pain disorder
may still connote the implied mind-body dualism of other somatoform dis-
orders (i.e., somatic preoccupation occurring in the absence of, or in excess of,
what would be expected, given objective findings). As a result, the nosology of
pain disorder is likely to be misunderstood by nonpsychiatric clinicians (Mayou
et al. 2003); the potential pejorative implication may be that the patient is dis-
ingenuous, exaggerating, or faking. Ironically, the intent of redefining pain
disorder in DSM-IV-TR was to overcome this archaic dualism.
Pain Disorder Versus Other Somatoform Disorders
Discrepancies arise among diagnosticians when it comes to the psychiatric dif-
ferential diagnosis accompanying pain. In one review of patients with chronic
pain, somatoform disorders were diagnosed in 16%–53% of the patients (Dwor-
Table 4–2.
Limitations of the DSM-IV-TR nosology of pain disorder
Criteria are not sufficiently operationalized; specifically, there are no guidelines that
allow one to determine
Whether psychological factors “have an important role” in pain (criterion C).
When pain is “not better accounted for” by a mood disorder (criterion E).
Whether, and to what extent, psychological factors are involved in the patient’s
pain.
The nosology of pain disorder is likely to be misunderstood by nonpsychiatric
clinicians.
The characteristics of pain patients can overlap considerably with those of patients
with other somatoform disorders.

Psychiatric Comorbidities and Psychiatric Differential Diagnosis 67
kin and Caligor 1988). In another series, approximately 40% of 283 patients with
chronic pain received a diagnosis of conversion disorder (Fishbain et al. 1986).
Part of the discrepancy may be due to the diagnostic criteria employed—that
is, some diagnoses may have been based on earlier DSM versions in which dis-
tinctions among the psychiatric conditions might have been less clear. In ad-
dition, sampling bias (i.e., studying patients from very distinct subsets of the
population) may have contributed to the diagnostic inconsistencies (Fishbain
1999).
Any pain complaint would not necessarily invoke a diagnosis of pain dis-
order; rather, it must be considered among a number of other psychiatric
disorders in which pain can be a prominent complaint (see Table 4–3). Distin-
guishing pain disorder from other psychiatric disorders is pertinent to determin-
ing appropriate pharmacologic and psychotherapeutic treatment. Nonethe-
less, the characteristics of pain patients can overlap considerably with those of
patients with other somatoform disorders, obscuring distinction (Hiller et al.
2000). For example, certain pain disorders—central pain states and fibromyal-
gia, for example—can mimic somatoform disorders. It is possible that a medical
condition that is as yet unrecognized can lead, by default, to the erroneous
conclusion that the pain associated with that condition is psychogenic. Pa-
tients with persisting pain, particularly pain with no clear etiology, might, as
would the person with hypochondriasis, insist on finding a cause. This insis-
tence might be grounded in the effort to convince the clinician of the legiti-
macy of the pain complaints and the need for treatment.
Somatic Amplification and Its Function
In some patients with pain, like patients with other somatoform disorders,
amplification of somatic symptoms (including pain) may be a manifestation
of psychological distress. For some persons, this amplification might reflect a
strategy to cope with or manage psychological distress (Lipowski 1988). For
others, somatic amplification may result from the distress associated with un-
pleasant affective states such as depression or anxiety. These emotional states
may lead to heightened self-consciousness and a propensity to attend to bodily
signals that might otherwise be ignored. Influenced by the distress of the pre-
vailing emotional state combined with cognitive distortions (e.g., catastro-
phizing and magnification), as described in Chapter 3 (“Evaluation of the Pain

68 Clinical Manual of Pain Management in Psychiatry
Patient”; see Table 3–3), the individual is likely to misinterpret the bodily sen-
sations as signaling a significant health- or pain-related disorder.
Somatic amplification can serve a number of psychological functions for
the patient. For some patients, it can serve to secure and enlist the support of
others. For other patients, it can serve to distance others or allow expression of
discontent or anger or avoidance of interpersonal conflicts. In addition, some
patients may derive benefits from the somatic amplification, such as avoidance
of unpleasant tasks and responsibilities.
Somatic amplification and the focus on somatic symptoms can serve to
mask other psychological symptoms. Depression in a patient presenting with
somatic concerns is quite common. For example, for some patients, there
might be the wish to deny depressive symptoms entirely, because to acknowl-
edge them may incur the stigma of a psychiatric disorder or imply something
about their character (e.g., weakness in the face of adversity). Still, for other
patients, the propensity to present with somatic complaints may be based on
Table 4–3.
Psychiatric disorders in which pain can be a prominent
complaint
Pain disorder
Pain complaints the focus of clinical attention
Associated psychological features (can be present or absent)
Somatization disorder
At least four pain complaints
Other somatic concerns (genitourinary or gastrointestinal)
Pseudoneurologic disturbance
Hypochondriasis
Preoccupation with fears of having a serious medical condition
Based on a misinterpretation of bodily sensations, which can include discomfort
or pain
Conversion disorder
Loss of voluntary motor function or sensory function other than pain
Dyspareunia
Pain arising exclusively during intercourse
Factitious disorder
Deliberate deceit or feigning of symptoms (can include complaints of pain)
No obvious secondary gain
Psychiatric Comorbidities and Psychiatric Differential Diagnosis 69
the belief that somatic concerns will be taken more seriously than will emo-
tional factors (Lipowski 1990).
Pain and Deceit
Both factitious disorder and malingering involve the voluntary production or
exaggeration of symptoms. Pain can be invoked with either condition. Al-
though deceit is essential for both factitious disorder and malingering, the
motives for the two conditions differ. In factitious disorder, the motive is to
assume the “sick role”—that is, to gain something from the association with
medical treatment; the motive does not include a concrete secondary gain.
Malingerers, on the other hand, derive a clear secondary gain that could be un-
derstandable from the person’s environmental circumstances. Examples of sec-
ondary gains include securing financial gain (e.g., from litigation or disability
compensation), obtaining a haven (e.g., in the case of one who is homeless), and
acquiring psychoactive substances (e.g., opiates). Factors that suggest possible
malingering are summarized in Table 4–4.
Ailments claimed by patients seeking opiate analgesics typically include
those not readily verifiable (e.g., renal colic, tic douloureux, sickle cell crisis).
Thus, the patient might allege severe flank pain and provide a urine sample
into which a drop of blood was deliberately placed. In the busy emergency de-
partment, such complaints can be met with a tendency to placate the patient
with provision of the sought-out narcotic.
Table 4–4.
Factors that raise suspicion of malingering
Patient presentation to medical attention has a medicolegal context.
Marked discrepancy exists between patient’s claims of disability or distress and
objective findings.
Patient’s reports may be vague, may be loaded with overgeneralizations, or may seem
rehearsed. Patient history may fall apart when examiner’s questions are reframed.
Patient’s responses to questions may be cryptic, and there is a tendency to hedge
statements.
Once patient’s objective is achieved, symptoms seem to take on less significance.
Patient rejects all forms of treatment that do not include psychoactive medications.
Patient exhibits lack of cooperation with medical diagnostic interventions.
Patient shows poor compliance with treatment interventions.
Antisocial personality disorder is present.

70 Clinical Manual of Pain Management in Psychiatry
Depression
Among patients with chronic pain, depression prevalence rates were estimated
at 30%–54%, much higher than in the general population (Banks and Kerns
1996). A number of studies have demonstrated that the severity of depressive
symptoms predicted the number and severity of pain complaints (Dworkin et al.
1990; Faucett 1994). The causal relationships between depression and pain
are unclear. Most data suggest that pain predisposes patients to depression
(Fishbain et al. 1997) (i.e., developing as a consequence of pain [Gamsa 1990]
or medical treatment [Massie and Holland 1990]). Alternatively, depression
may precede the pain and may be related to its maintenance. In two longitudinal
studies, depression predicted subsequent development of painful musculo-
skeletal disorders (Forseth et al. 1999; Leino and Magni 1993). The biopsycho-
social approach to pain assessment recognizes that the relationship between pain
and emotional disturbances (e.g., depression) is likely to be reciprocal.
Physiologic substrates common to both depression and pain are summa-
rized in Table 4–5. Conceivably, a preexisting vulnerability (e.g., recurrent
stress, preexisting depression) may increase the risk of developing chronic pain
(diathesis) through modification of catecholamines and substance P and cyto-
kine activity (Dersh et al. 2002). A perpetuating cycle is potentially set in motion
such that with protracted pain and resultant increases in cytokine and substance
P activity, the patient is predisposed to depression. The depression in turn can
augment pain through cytokine activity, substance P activity, diminished opioid
receptor responsiveness, and so on.
Patients with chronic pain are often plagued with distress related to unre-
mitting pain, life changes, modifications in lifestyle and adaptive functioning,
loss of rewarding activities, and a decline in social reinforcements. The poten-
tial pitfall is that clinicians might assume the depression is expected given the
pain and overlook a potentially treatable disorder. Conversely, depression may
be assumed to be present when physical examination and diagnostic interven-
tions fail to account for the severity of the pain or if treatment measures are
unsuccessful in mitigating pain. In such cases, the clinician can be misled into
ignoring the pain complaints in the attempt to address the presumed depres-
sion. The patient may well construe this lack of attention to the pain complaints
as devaluing the pain, causing confrontations to arise and possibly thwarting
treatment measures.
Psychiatric Comorbidities and Psychiatric Differential Diagnosis 71
Table 4–5.
Physiologic substrates common to pain and depression
Physiologic
substrate Pain Depression Relationship
Catecholamines Rostral ventromedial medulla
(5-HT) and dorsolateral
pontine tegmentum (NE)
inhibit pain transmission from
spinal dorsal horn.a
Locus coeruleus (NE) and raphe
nucleus (5-HT) project to limbic
system and other brain areas.b
Decreased catecholamines increase
pain and depression.
Substance P Binding to neurokinin receptors
centrally and peripherally
increases pain transmission.c
Central substance P activity is
increased in animal paradigms of
stress; inhibits activity of locus
coeruleus and raphe nuclei.d
Increased central substance P activity
increases pain and depression.
Opioid receptor Modulate pain transmission
centrally and peripherally.
Present in limbic structures;
deactivation of µ-receptors is
associated with dysphoria.e
Decreased activity can augment pain
and depression.
Cytokines Proinflammatory agents that
promote pain.
Administration of interferon-alpha
to patients induces depression.fIncreased activity augments pain and
depression.
Note. 5-HT=serotonin; NE=norepinephrine.
aFields and Basbaum 1999.
bBlier and Abbott 2001.
cDoyle and Hunt 1999.
dMantyh 2002; Santarelli et al. 2002.
eKennedy et al. 2006.
fCapuron et al. 2002.
Source. Reprinted from Leo RJ: “Chronic Pain and Comorbid Depression.” Current Treatment Options in Neurology 7:403–412, 2005. Copyright 2005,
Current Science, Inc. Used with permission.

72 Clinical Manual of Pain Management in Psychiatry
In assessing depression, the clinician should keep in mind that neuroveg-
etative symptoms such as sleep disturbances, appetite changes, and fatigue can
overlap considerably with symptoms of medical and surgical conditions asso-
ciated with pain. Thus, reliance on these features may lead to the erroneous di-
agnosis of a depressive disorder.
For effective diagnosis of depression in the medically ill, it may be best for
the clinician to focus on the psychological symptoms of depression, which are un-
likely to be influenced by medical conditions or their complications. Thus, loss
of interests of any kind; lack of mood reactivity; lack of concentration; inde-
cisiveness; and preoccupation with guilt, worthlessness, death, dying, suicide,
despair, and hopelessness are more suggestive of an underlying depression
(Cavanaugh 1984). Similarly, items in diagnostic scales—for example, the Beck
Depression Inventory (Beck and Steer 1987)—can overlap considerably with
medical symptoms, exaggerating depression severity in certain pain patients.
Anxiety
There is much speculation as to how anxiety can influence pain. For example,
pain can heighten anxiety, which in turn can result in muscle strain and spasm.
If the strain and spasm are severe enough, localized ischemia and muscle cell
damage can arise, resulting in the release of pain-producing substances that
then precipitate further pain. Heightened responses of lower back muscles, as
shown by electromyography (EMG), were observed among patients discuss-
ing personal anxiety-provoking stressors (Flor et al. 1985). Similarly, height-
ened tension in frontal muscles, associated with personal distress, was noted
among patients with tension headaches. This anxiety–pain relationship makes
intuitive sense but has not been consistently borne out in evaluations using
EMG. One reason for this is that although EMG can reflect activity in superfi-
cial muscles, it may not measure the recordings of deeper muscles, where pain
may actually originate. Also, a time lag is expected between the experience of
distress and the production of muscle contraction and release of pain-producing
substances (Flor and Turk 1989). If electromyographic recordings are not mea-
sured for a sufficient duration to account for this lag time, it is conceivable that
negative results would be yielded.
A general model of the anxiety–pain relationship is depicted in Figure 4–1.
In this model, fear and apprehension precipitated by pain experiences may lead
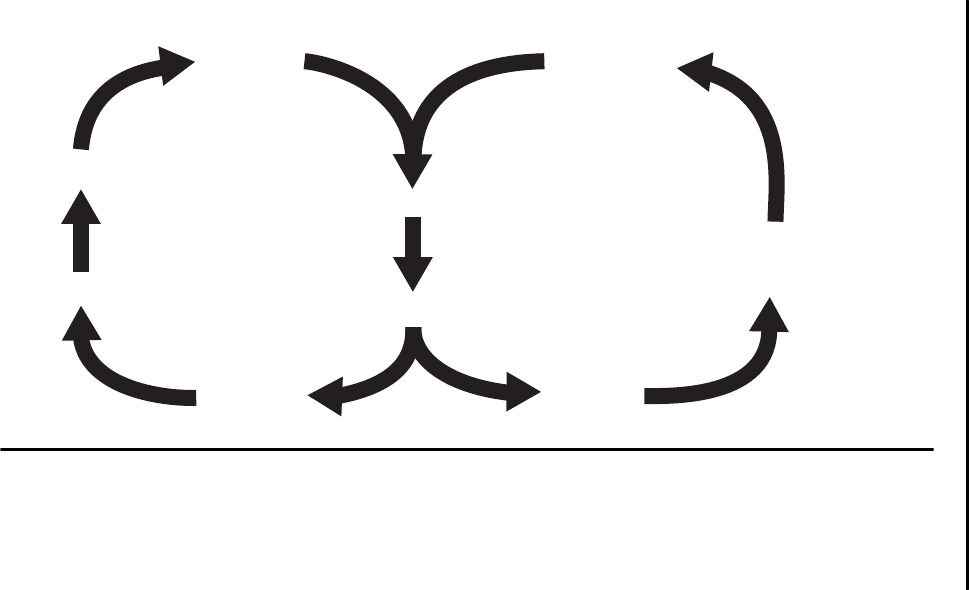
Psychiatric Comorbidities and Psychiatric Differential Diagnosis 73
Figure 4–1.
The anxiety–pain relationship.
Pain
Fear
Avoidance
of activity
Inactivity
Deconditioning
Weakness
Somatic
focus
Impaired
cognitive
functioning
Misattribution
and
magnification

74 Clinical Manual of Pain Management in Psychiatry
to heightened somatic vigilance and concern. Anxiety over these experiences
may give rise to cognitive distortions of physical symptoms, whose meaning
may become distorted, magnified, and even amplified, leading to muscle ten-
sion and heightened pain. In addition, anxiety over precipitating pain can lead
to restriction of movement and avoidance of physical therapy. This situation
can further exacerbate pain by contributing to deconditioning and muscle weak-
ness. Then, once activity is undertaken, one may be vulnerable to re-injury
and pain (Asmundson et al. 1999).
Anxiety is common among patients with chronic pain. Anticipatory anx-
iety (e.g., preoperative anxiety) predicts increased rates of postoperative pain
reporting and distress (Thomas et al. 1998). However, other researchers have
questioned the impact of anxiety on pain states (Arntz et al. 1991). Anxiety
disorders are accompanied by an increased incidence of somatic symptoms and
pain complaints (Beidel et al. 1991). Anxious patients may experience higher
than expected pain levels, mediated by limbic system activation. Limbic sys-
tem activity during anxiety may nullify the pain-inhibiting processes that might
normally emerge from the midbrain and descending inhibitory pathways (Mer-
skey 1989), thereby intensifying the pain experience.
Patients with posttraumatic stress disorder (PTSD) often present with mul-
tiple physical ailments and complaints (McFarlane 1986). PTSD may accom-
pany and perhaps exacerbate pain associated with traumatic injuries, warranting
treatment. Patients with PTSD are prone to somatization, affective dysregu-
lation, and dissociation. These patients lack the ability to differentiate be-
tween relevant and irrelevant information, predisposing them to focus atten-
tion on and misinterpret somatic experiences (van der Kolk et al. 1996).
Terminal cancer patients and others with chronic pain may experience
existential anxiety, whereby a person questions the quality and meaning of life.
Distress arises as one reflects on unaccomplished goals, time remaining to
achieve goals, the meaning of one’s life, one’s legacy, and so on. The interrup-
tion of ho ped-for life achievements can lead to marked dysph oria, anxiety, and
narcissistic injury, requiring psychotherapy.
Sleep Disorders
Sleep disturbances frequently accompany pain. Patients often experience longer
sleep onset latencies, frequent nighttime awakenings, and overall shorter dura-

Psychiatric Comorbidities and Psychiatric Differential Diagnosis 75
tion of sleep (Morin et al. 1998). Such disturbances are likely to result from hav-
ing a painful condition (e.g., rheumatoid arthritis, fibromyalgia, back pain) and
would therefore be categorized as a sleep disorder due to a general medical con-
dition according to DSM-IV-TR diagnostic criteria. However, it should be
noted that sleep disturbances can reciprocally exacerbate pain severity. For ex-
ample, deficits in Stage IV sleep, even among healthy individuals, may increase
rates of reported muscle tenderness, aching, and stiffness (Moldofsky and Scaris-
brick 1976). When these deficits are corrected, the painful symptoms often dis-
appear.
Other factors (e.g., comorbid depression) may also contribute to sleep dis-
turbances among patients with pain. Abnormalities in the sleep patterns of
patients with fibromyalgia have been noted to likewise contribute to sleep dis-
turbances. Specifically, alpha-delta sleep—in which intrusion of low-voltage,
rapid alpha waves occurs amid higher voltage, slow waves characteristic of
delta sleep—has been associated with fatigue and the perception that the sleep
is nonrestorative (see also section “Fibromyalgia” in Chapter 8, “Common Pain
Disorders,” of this book). Additionally, patients with chronic pain (e.g., neu-
ropathic conditions) may experience sleep difficulties secondary to restless
legs syndrome (Yunus and Aldag 1996). Restless legs syndrome is character-
ized by tingling, paresthesias, and cramping (often of the lower extremities)
that arises when one reclines but is transiently alleviated by movement. This
need for movement interferes with sleep. Recognition of and differentiating
among the possible etiologies for sleep disturbances of patients with pain is
critical, as treatment approaches are partly defined by underlying causes. For
example, an antidepressant may be warranted in cases of depression, whereas
a dopamine agonist or anticonvulsant may be warranted to mitigate sleep dif-
ficulties arising from restless legs syndrome.
Substance-Related Disorders
The prevalence of substance dependence among patients with chronic pain is
variable, ranging from 3.2% to 18.9% (Fishbain et al. 1992). The differences in
the rates reported appear to be a result of the diagnostic criteria for dependence
employed, the settings in which samples were gathered, and the types of chronic
pain conditions examined. Although one study suggested that rates of sub-
stance dependence among patients with chronic pain and a comparable group of

76 Clinical Manual of Pain Management in Psychiatry
(nonpain) patients attending the same medical clinic did not differ significantly
(Brown et al. 1996), further systematic investigation is warranted as to whether
rates of substance dependence among patients with chronic pain differ from
those in the general population.
Fear of drug addiction is often offered as an explanation of why clinicians
have difficulty managing patients’ pain. Such concerns are particularly height-
ened when treatment of chronic, nonmalignant pain is required. Patients with
somatoform pain disorders may be more prone to dependence on psycho-
active substances than patients with more serious physical illnesses (Streltzer et
al. 2000). However, some studies indicate that among patients with chronic
pain and substance use disorders, the substance use disorder preceded the on-
set of the pain disorder (Brown et al. 1996).
One feature of substance dependence suggestive of a pathologic state is
the desire for acquisition of such substances (e.g., opiates) for something other
than just pain relief (i.e., for psychological relief). Behaviors suggestive of
substance abuse or dependence include problematic behaviors such as solic-
itation of medications from multiple sources, forgery of prescriptions, theft
of medications, and acquisition of psychoactive substances from illegal
sources.
Clinicians can be apt to misinterpret some behaviors of pain patients as
being indicative of an underlying substance-related disorder. Referred to as
pseudoaddiction, such behaviors are likely to arise when patients are under-
treated (i.e., with inadequate dosage or inappropriately lengthy spacing of
doses) with analgesics (Weissman and Haddox 1989). Deciphering between
addiction and pseudoaddiction (see Table 4–6) can be somewhat difficult,
however, and has been a source of controversy, in part because often no direct
correlation can be found between objective medical findings or assessments
and the degree of subjective pain complaints (Streltzer 2001; Streltzer et al.
2000). To acquire more analgesics, pseudoaddicted patients, like patients with
substance dependence, may manipulate clinicians, embellish symptoms, and
use up prescribed medications in excess of the intended rates. However, the
agenda of pseudoaddicted patients, unlike the agenda of those with addiction,
is based on a desire for pain relief. For pseudoaddicted patients, optimizing
the analgesic treatment will eliminate future manipulative behaviors, whereas
for addicted individuals it likely will not. Use of analgesics should enhance
treatment adherence (e.g., participation in physical therapy) and improve adap-

Psychiatric Comorbidities and Psychiatric Differential Diagnosis 77
tive functioning. If functioning improves once the analgesics are optimized,
the presumption is that the behavior is indeed a reflection of an underlying
pseudoaddiction. On the other hand, for the addicted patient, increased ac-
cess to analgesics such as opiates will impair functioning.
Personality Disorders
Common personality disorders associated with chronic pain include histri-
onic, dependent, paranoid, and borderline types (Fishbain 1999). Personality
disorders refers to long-standing, pervasive patterns of behavior, including pat-
terns of relating to others, managing emotions, and viewing the world (reality
testing). Diagnosis of such Axis II disorders should facilitate treatment, for ex-
ample, by identifying those features of the personality that may interfere with
treatment. Thus, the identification of a personality disorder should heighten
Table 4–6.
Distinguishing features of pseudoaddiction and
addiction
Pseudoaddiction Addiction
Dose escalation Stops once pain
is controlled
Continues
Goal Pain relief Euphoria/intoxication
Signs of intoxication No Yes
Concern over side effects Yes No
Follows other treatment
suggestions
Yes No
May invoke manipulative
measures
Yes Yes
Feigns symptoms No Yes
Withdrawal with abrupt
cessation
Yes Yes
Effect of provision of
additional analgesic
Improved
functioning
Worsened
functioning
Source. Adapted from Fishbain DA: “Chronic Pain and Addiction,” in Weiner’s Pain Manage-
ment: A Practical Guide for Clinicians, 7th Edition. Edited by Boswell MV, Cole BE. Boca Raton,
FL, CRC Taylor & Francis Press, 2006, p. 128. Copyright 2006, Routledge/Taylor & Francis Group.
Used with permission.

78 Clinical Manual of Pain Management in Psychiatry
the clinician’s awareness of the patient’s needs, the meanings of the illness to
the patient, and the patient’s coping repertoire (Weisberg and Keefe 1997).
Unfortunately, for the patient with complex pain, the identification of Axis II
disorders may be employed pejoratively to imply that the patient is difficult to
treat and does little to facilitate or complement treatment.
One problem that frequently arises is whether the behaviors observed
(those suggesting a personality disorder) are reflective of a long-standing pat-
tern of functioning or whether they arise from the chronic pain experience.
The patient may have a biological and environmental or developmental pre-
disposition toward the expression of a particular personality trait characteristic
of a disorder. Under ordinary circumstances, the predisposition might never be
fully actualized. However, in a person who is under the stress of a chronic pain
state—with its ensuing social, occupational, financial, and legal ramifications—
such traits may be heightened and expressed in a manner that, on the surface,
might suggest a personality disorder.
Schizophrenia
The literature on pain among patients with schizophrenia has suggested that
these patients demonstrate an insensitivity or indifference to pain associated
with serious medical conditions. A number of experimental paradigms have
demonstrated that patients with schizophrenia tend to have reduced pain sen-
sitivities and higher thresholds for pain compared with patients who do not have
schizophrenia (Dworkin 1994). Reduced pain sensitivity was found to be a fa-
milial trait present among healthy relatives (i.e., without schizophrenia) of pa-
tients with schizophrenia (Hooley and Delgado 2001).
A number of mechanisms for these findings have been postulated. Higher
pain thresholds have been correlated with higher levels of endogenous opioids
in the cerebrospinal fluid in patients with schizophrenia as compared with those
without schizophrenia. These endogenous opioids might mitigate awareness
of and sensitivity to pain. In addition, alteration of cortical evoked potentials
during noxious stimulation has been demonstrated, suggesting changes in the
perception and processing of painful sensory information. Finally, distur-
bances in affective (limbic) systems accounting for the negative symptoms of
schizophrenia may likewise interfere with the processing of pain and reactions
to pain (Dworkin 1994).

Psychiatric Comorbidities and Psychiatric Differential Diagnosis 79
Preliminary genetic studies have revealed that allelic variants of the δ-opi-
ate receptor prevail among people with schizophrenia. The δ-receptor is nor-
mally responsive to endogenous opiates; however, the allelic variants observed
among people with schizophrenia could be less sensitive to endogenous opi-
ates (Xu et al. 2002). Thus, a feedback mechanism accounting for increased
endogenous opiates in the cerebrospinal fluid might be explained. Further in-
vestigations are required to clarify the pathophysiology of pain insensitivity in
schizophrenia.
Key Points
• To effectively manage most patients with chronic pain, clinicians must
identify, diagnose, and treat common psychiatric comorbidities.
• The diagnosis of pain disorder assumes that pain is the primary symptom
warranting clinical attention; the diagnostic taxonomy contained within
DSM-IV-TR recognizes subtypes in the classification of pain disorder based
on whether psychological factors contribute significantly to the pain expe-
rience.
• Critics of the diagnostic taxonomy of pain disorder in DSM-IV-TR
contend that the criteria are insufficiently operationalized, failing to dis-
tinguish pain disorder from other psychiatric conditions, and that the no-
sology still retains vestiges of mind-body dualism.
• There are several common psychiatric comorbidities accompanying and
complicating the experience of pain that warrant clinical attention and that
can be the focus of psychiatric treatment. These include depression, anxiety,
sleep disorders, somatoform disorders, substance-related disorders, and per-
sonality disorders.
• Expanding knowledge of the pathophysiology of pain processing and psy-
chiatric disorders (e.g., depression) is beginning to unveil common neuro-
logic substrates that may account for the high rates of co-occurrence of such
conditions.
• Treating pain symptomatically without addressing psychiatric comorbidi-
ties is inadequate for the management of the patient, in part because it ne-
glects potential sources of suffering and disability and potential impedi-
ments to the rehabilitation process.

80 Clinical Manual of Pain Management in Psychiatry
References
Aigner M, Bach M: Clinical utility of DSM-IV pain disorder. Compr Psychiatry 40:353–
357, 1999
American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disor-
ders, 3rd Edition. Washington, DC, American Psychiatric Association, 1980
American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders,
3rd Edition, Revised. Washington, DC, American Psychiatric Association, 1987
American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disor-
ders, 4th Edition. Washington, DC, American Psychiatric Association, 1994
American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders,
4th Edition, Text Revision. Washington, DC, American Psychiatric Association, 2000
Arntz A, Dreessen L, Merckelbach H: Attention, not anxiety, influences pain. Behav
Res Ther 29:41–50, 1991
Asmundson GJG, Norton PJ, Norton GR: Beyond pain: the role of fear and avoidance
in chronicity. Clin Psychol Rev 19:97–119, 1999
Banks SM, Kerns RD: Explaining high rates of depression in chronic pain: a diathesis-
stress framework. Psychol Bull 119:95–110, 1996
Beck AT, Steer RA: Beck Depression Inventory Manual. San Antonio, TX, Psycholog-
ical Corporation, 1987
Beidel DC, Christ MAG, Long PJ: Somatic complaints in anxious children. J Abnorm
Child Psychol 19:659–670, 1991
Blier P, Abbott FV: Putative mechanisms of action of antidepressant drugs in affective
and anxiety disorders and pain. J Psychiatry Neurosci 26:37–43, 2001
Brown RL, Patterson JJ, Rounds LA, et al: Substance abuse among patients with chronic
back pain. J Fam Pract 43:152–160, 1996
Capuron L, Gumnick JF, Musselman DL, et al: Neurobehavioral effects of interferon-
alpha in cancer patients: phenomenology and paroxetine responsiveness of symp-
tom dimensions. Neuropsychopharmacology 26:643–652, 2002
Cavanaugh SVA: Diagnosing depression in the hospitalized patient with chronic med-
ical illness. J Clin Psychiatry 45:13–16, 1984
Dersh J, Polatin PB, Gatchel RJ: Chronic pain and psychopathology: research findings
and theoretical considerations. Psychosom Med 64:773–786, 2002
Doyle CA, Hunt SP: Substance P receptor (neurokinin-1)–expressing neurons in lamina
I of the spinal cord encode for the intensity of noxious stimulation: a c-Fos study
in rat. Neuroscience 89:17–28, 1999
Dworkin RH: Pain insensitivity in schizophrenia: a neglected phenomenon and some
implications. Schizophr Bull 20:235–248, 1994
Dworkin RH, Caligor E: Psychiatric diagnosis and chronic pain: DSM-III-R and be-
yond. J Pain Symptom Manage 3:87–98, 1988

Psychiatric Comorbidities and Psychiatric Differential Diagnosis 81
Dworkin SF, von Korff M, LeResche L: Multiple pains and psychiatric disturbance:
an epidemiologic investigation. Arch Gen Psychiatry 47:239–244, 1990
Faucett JA: Depression in painful chronic disorders: the role of pain and conflict about
pain. J Pain Symptom Manage 9:520–526, 1994
Fields HL, Basbaum AI: Central nervous system mechanisms of pain modulation, in
Textbook of Pain, 4th Edition. Edited by Wall PD, Melzack R. Edinburgh, Scot-
land, Churchill Livingstone, 1999, pp 309–329
Fishbain DA: Approaches to treatment decisions for psychiatric comorbidity in the
management of the chronic pain patient. Med Clin North Am 83:737–760, 1999
Fishbain DA: Chronic pain and addiction, in Weiner’s Pain Management: A Practical
Guide for Clinicians, 7th Edition. Edited by Boswell MV, Cole BE. Boca Raton,
FL, CRC Taylor & Francis Press, 2006, pp 117–139
Fishbain DA, Goldberg M, Meagher BR, et al: Male and female chronic pain patients
categorized by DSM-III psychiatric diagnostic criteria. Pain 26:181–197, 1986
Fishbain DA, Rosomoff HL, Rosomoff RS: Drug abuse, dependence, and addiction
in chronic pain patients. Clin J Pain 8:77–85, 1992
Fishbain DA, Cutler R, Rosomoff HL, et al: Chronic pain–associated depression: ante-
cedent or consequence of chronic pain? A review. Clin J Pain 13:116–137, 1997
Flor H, Turk DC: Psychophysiology of chronic pain: do chronic pain patients exhibit
symptom-specific psychophysiologic responses? Psychol Bull 105:215–259, 1989
Flor H, Turk DC, Birbaumer N: Assessment of stress-related psychophysiological re-
actions in chronic back pain patients. J Consult Clin Psychol 53:354–364, 1985
Forseth KO, Husby G, Gran IT, et al: Prognostic factors for the development of fibro-
myalgia in women with self-reported musculoskeletal pain: a prospective study.
J Rheumatol 26:2458–2467, 1999
Gamsa A: Is emotional disturbance a precipitator or a consequence of chronic pain?
Pain 42:183–195, 1990
Hiller W, Heuser J, Fichter MM: The DSM-IV nosology of chronic pain: a comparison
of pain disorder and multiple somatization syndrome. Eur J Pain 4:45–55, 2000
Hooley JM, Delgado ML: Pain insensitivity in the relatives of schizophrenic patients.
Schizophr Res 47:265–273, 2001
Kennedy SE, Koeppe RA, Young EA, et al: Dysregulation of endogenous opioid emotion
regulation circuitry in major depression in women. Arch Gen Psychiatry 63:1199–
1208, 2006
Leino P, Magni G: Depressive and distress symptoms as predictors of low back pain,
neck-shoulder pain, and other musculoskeletal morbidity: a 10-year follow-up of
metal industry employees. Pain 53:89–94, 1993
Leo RJ: Chronic pain and comorbid depression. Curr Treat Options Neurol 7:403–
412, 2005

82 Clinical Manual of Pain Management in Psychiatry
Lipowski ZJ: Somatization: the concept and its clinical application. Am J Psychiatry
145:1358–1368, 1988
Lipowski ZJ: Somatization and depression. Psychosomatics 31:13–21, 1990
Mantyh PW: Neurobiology of substance P and the NK1 receptor. J Clin Psychiatry 63
(suppl 11):6–10, 2002
Massie MJ, Holland JC: Depression and the cancer patient. J Clin Psychiatry 51
(suppl 7):12–17, 1990
Mayou R, Levenson J, Sharpe M: Somatoform disorders in DSM-V. Psychosomatics
44:449–451, 2003
McFarlane AC: Posttraumatic morbidity of a disaster: a study of cases presenting for
psychiatric treatment. J Nerv Ment Dis 174:4–14, 1986
Merskey H: Psychiatry and chronic pain. Can J Psychiatry 34:329–336, 1989
Moldofsky H, Scarisbrick P: Introduction of neurasthenic musculoskeletal pain syn-
drome by selective sleep stage deprivation. Psychosom Med 38:35–44, 1976
Morin CM, Gibson D, Wade J: Self-reported sleep and mood disturbance in chronic
pain patients. Clin J Pain 14:311–314, 1998
Santarelli L, Gobbi G, Blier P, et al: Behavioral and physiologic effects of genetic or
pharmacologic inactivation of the substance P receptor (NK1). J Clin Psychiatry
63 (suppl 11):11–17, 2002
Streltzer J: Pain management in the opioid-dependent patient. Curr Psychiatry Rep 3:489–
496, 2001
Streltzer J, Eliashof BA, Kline AE, et al: Chronic pain disorder following physical
injury. Psychosomatics 41:227–234, 2000
Sullivan MD: DSM-IV pain disorder: a case against the diagnosis. Int Rev Psychiatry
12:91–98, 2000
Thomas T, Robinson C, Champion D, et al: Prediction and assessment of the severity
of post-operative pain and of satisfaction with management. Pain 75:177–185, 1998
van der Kolk BA, Pelcovitz D, Roth S, et al: Dissociation, somatization, and affect dysreg-
ulation: the complexity of adaptation to trauma. Am J Psychiatry 153:83–93, 1996
Weisberg JN, Keefe FJ: Personality disorders in the chronic pain population: basic
concepts, empirical findings, and clinical implications. Pain Forum 6:1–9, 1997
Weissman DE, Haddox JD: Opioid pseudoaddiction: an iatrogenic syndrome. Pain
36:363–366, 1989
Xu J, Leo RJ, DiMartino S, et al: Linkage studies of delta opioid receptor gene and
pain insensitivity in schizophrenia. Paper presented at the annual meeting of the
American Psychiatric Association, Research Colloquium for Junior Investigators,
Philadelphia, PA, May 2002
Yunus MB, Aldag JC: Restless legs syndrome and leg cramps in fibromyalgia syndrome:
a controlled study. BMJ 312:1339, 1996

83
5
Pharmacology of Pain
Familiarity with the range of medications employed in the treatment of pain
is essential. In this chapter I focus on the utility of such agents and the disor-
ders in which they may be employed, as well as dosing and side effects.
Opiate Analgesics
Opiates have long been the mainstay of treatment in acute pain states and ter-
minal disorders. However, long-term use in chronic, nonmalignant states has
raised significant concerns for many clinicians about predisposing patients to
functional impairments, dependence, and poor treatment response (Buckley
et al. 1986; Finlayson et al. 1986; McNairy et al. 1984). Knowledge of opiate
activity in the management of pain is essential. Familiarity with the dosing
and duration of effects is required, as well as a knowledge of the ceiling effects
(i.e., the maximal dose beyond which no further analgesic effect would be ob-
tained) (Inturrisi 1984) (see Table 5–1).

84 Clinical Manual of Pain Management in Psychiatry
Table 5–1.
Opiate dosing and use
Equianalgesic
dose, mg
Agent Oral Parenteral
Starting dose,
mg
Duration,
hours
Affinity for
µ-receptor
Ceiling
effect
Morphine-like agents
Morphine 30 10 15–30 (oral) 4–5 +++ No
Codeine 200 120 30–60 (oral) 4–6 +Yes
Hydromorphone 7.5 1.5 4–8 (oral) 4–5 +++ No
Meperidine 300 75 50–100a (oral) 2–5 ++ No
Oxycodone 20 NA 15–30 (oral) 4–6b++ No
Methadone 20 10 5–10 (oral) 3–8 +++ No
Levorphanol 4 2 2–4 (oral) 4–5 +++ No
Fentanylc—0.1 0.1 (iv) 4–6 ++++ No
Partial agonist
Buprenorphine —0.4 0.3–0.6 (iv) 4–6 Partial Yes

Pharmacology of Pain 85
Agonist-antagonists
Nalbuphine —10 10–20 (iv) 3–6 Antagonist Yes
Butorphanol — 2 0.5–2 (iv) 3–4 Antagonist Yes
Dezocine —10 5–20 (iv) 3–6 Antagonist Yes
Pentazocine —30 30–60 (iv) 3–4 Antagonist Yes
Note. Dash (—) indicates not available in oral form. iv= intravenous; NA=not applicable.
aHigher starting doses may be required for moderate to severe pain.
bSustained-release form available.
cFentanyl transdermal patch not listed here.
Source. Bouckoms 1988; Cassem 1989; Jaffe and Martin 1990; Wise and Rundell 2005.
Table 5–1.
Opiate dosing and use (continued)
Equianalgesic
dose, mg
Agent Oral Parenteral
Starting dose,
mg
Duration,
hours
Affinity for
µ-receptor
Ceiling
effect

86 Clinical Manual of Pain Management in Psychiatry
Guidelines for Use
The affinity of the opiates for the µ-receptors within the central nervous sys-
tem (CNS) determines their effectiveness in mitigating pain. Certain opiates,
such as codeine and propoxyphene, are considered to be weak opiates, useful
in mild to moderate pain. For more severe pain, stronger opiates, such as mor-
phine, are required. (General recommendations for mild, moderate, and se-
vere pain are summarized in Table 5–2.)
Morphine is the agent against which all other opioids tend to be compared.
Fentanyl is a very potent analgesic that has greater affinity for the µ-receptor
than has morphine. The doses recommended in Tables 5–1 and 5–2 are of-
fered as a guideline; the optimal analgesic dose may vary widely among patients.
Opiate rotation—that is, sequential trials of analgesic—may be required to iden-
Table 5–2.
Medication suggestions for pain in the adult patient
Pain level Medication suggestions
Mild (score of 1–3) Acetaminophen, COX-2 inhibitor, NSAIDs,
salicylates
Moderate (score of 4–6) Codeine 30 mg with acetaminophen
Hydrocodone 5 mg with acetaminophen
Oxycodone 5 mg with acetylsalicylic acid
(aspirin) or acetaminophen
Tramadol 50 mg
Tramadol 37.5 mg/acetaminophen 325 mg
Severe (score of 7–10) Codeine 30–60 mg po q 3–4 hours
Fentanyl (transdermal) 25 µg/hour q 3 days
Hydrocodone 5–10 mg po q 4–6 hours
Hydromorphone 4–8 mg po q 3–4 hours
Methadone 5–10 mg po q 6–12 hours
Morphine 15–30 mg po q 3–4 hours (immediate
release or oral suspension)
Oxycodone 10 mg po q 4–6 hours
Note. COX-2= cyclooxygenase-2; NSAID=nonsteroidal anti-inflammatory drug. Suggestions
here assume that pain scores are based on use of a numeric rating scale (see Chapter 3, “Evalua-
tion of the Pain Patient,” of this manual). In recommending these doses, it is assumed that patients
are opiate naive; patients with prior opioid treatment use or opiate (e.g., heroin) dependence may
require higher starting doses for optimal pain relief. Use morphine cautiously in patients with re-
nal disease; hydromorphone may be preferred in such cases. Use acetaminophen cautiously; the
maximum dose is 4,000 mg per 24 hours.

Pharmacology of Pain 87
tify the opiate that optimally addresses pain while offering the most favorably
tolerated side effects. Equianalgesic doses of opiates must be a consideration. Se-
lection of an opiate may be based on several factors (Table 5–3), including po-
tential for medication side effects, drug interactions, complications related to
medical comorbidities, and selection of optimal routes of medication adminis-
tration (summarized later in this section).
Side Effects
The patient’s prior experiences (efficacy of a drug or its untoward effects)
might influence the clinician’s analgesic choice. Affinity for the µ-receptor also
determines the severity of the side effects encountered with opioids, influenc-
ing respiratory rate, gastrointestinal (GI) motility, sphincter tone, and so forth.
Table 5–3.
Opioid therapy guidelines for chronic nonmalignant
pain
Consider opiates after other reasonable alternatives have failed.
Consider contraindications for opiate use.
Prescription of opiates should be managed by a single practitioner.
Select opiates based on the following:
Ease of administration (oral, transdermal, other)
Cost
Duration of analgesic effect
Tolerability of side effects
Potential risk of drug interactions
Administer opiates around the clock, instead of as needed.
Adjust or increase opiate dose gradually, contingent on the following:
Degree of pain relief
Degree of functional improvement
Side-effect profiles
Address side effects.
Formalize treatment and document well.
Consider use of patient contract.
Consider making opiate therapy contingent on participation in other aspects of
treatment (e.g., physical therapy, occupational therapy, psychotherapy).
Follow up weekly to monthly until patient is stable; once the patient is stabilized,
less frequent follow-up may be possible.

88 Clinical Manual of Pain Management in Psychiatry
Side effects of opiate analgesics may include constipation, nausea, vomiting, ex-
cess sedation, pruritus, and respiratory depression. Laxatives may be used for
constipation, and stimulants may be used for excessive sedation. Pruritus may
be addressed with concomitant use of antihistamines (e.g., hydroxyzine). Res-
piratory depression is rare in patients who receive chronic opiate treatment, but
it may arise with excessive doses and in opiate-naive patients. Reversal of res-
piratory depression may require the use of low doses of an opiate antagonist (e.g.,
naloxone). If opiate antagonists are used judiciously, it is possible to avoid pre-
cipitating pain or significant withdrawal symptoms.
To avoid some of the side effects associated with morphine-like opioids, a
mixed agonist-antagonist is sometimes prescribed. The agonist-antagonist class
of opioids consists of pentazocine, butorphanol, nalbuphine, and dezocine.
These agents are agonists at κ-receptors but antagonists at µ-receptors, and they
have less influence on the µ-receptors of the GI tract, producing less nausea,
vomiting, and constipation than pure opiate agonists. Because these agents have
antagonist properties, employing higher doses may actually depress analgesia.
Ceiling effects limit the utility of such agents among certain patients.
Drug Interactions
Choice of opiate analgesic might be determined by other concurrently admin-
istered medications. Toxic reactions occur when meperidine is administered
to patients taking monoamine oxidase inhibitors (MAOIs). When these med-
ications are coadministered, a serotonin syndrome may result, producing dia-
phoresis, rigidity, hyperreflexia, hypertension or hypotension, tachycardia,
delirium, seizures, hyperthermia, and occasionally death (Gratz and Simpson
1993; Sternbach 1991). More selective MAOIs—for example, selegiline, a
monoamine oxidase B inhibitor used to treat parkinsonism and depression
(e.g., selegiline transdermal)—may also interact with narcotic analgesics to
produce toxic reactions. To avoid toxicity, the MAOI should be discontinued
approximately 2 weeks before anticipated opiate initiation. When this is not
possible, codeine, oxycodone, and morphine might be safer alternatives. Ad-
ditionally, because the analgesic effects of the opiates may be potentiated by
the interference of the hepatic metabolism caused by the MAOIs, the doses of
opiates should be substantially lower than usual.
The analgesic effects of the opioids may be augmented by coadministra-
tion of several other psychotropic medications, including fluoxetine, tricyclic

Pharmacology of Pain 89
antidepressants (TCAs; e.g., amitriptyline, imipramine, clomipramine), and an-
tipsychotics (e.g., promethazine, chlorpromazine, haloperidol) (Keeri-Szanto
1974; Maltbie et al. 1979; Stambaugh and Wainer 1981). To avoid untoward
effects, lower opiate doses may be required if the opiate is coadministered with
these agents. Opioids (e.g., propoxyphene) may inhibit the metabolism of TCAs
(e.g., doxepin), leading to marked sedation and anticholinergic and α-adren-
ergic effects (Abernethy et al. 1982). Cigarette smoking diminishes the effective-
ness of opiate analgesics (e.g., propoxyphene, pentazocine) by potentiating their
metabolism.
Medical Conditions
Concurrent medical conditions might influence opiate selection. Among
patients with renal failure, metabolites of selected opiates (i.e., morphine,
meperidine, and propoxyphene) may produce significant untoward effects.
The metabolites of these agents, which depend on renal clearance, may accu-
mulate, producing prolonged sedation. In the case of meperidine, seizures and
CNS toxicity may arise, whereas with propoxyphene, cardiac conduction ab-
normalities may develop. Doses of these agents must be reduced significantly
in patients with renal compromise. Alternatively, hydromorphone lacks me-
tabolites with significant activity and is a safer choice.
Route of Administration
The selected route of administration of the analgesic may be influenced by
several factors, including ease of administration, degree of desired patient con-
trol, and tolerability of or preference for the administration route. The oral
route of administration is convenient, flexible, and nonintrusive. The draw-
back of this route is that for most opiates, 1.5–2 hours must be allowed for
peak drug effects. If patients have difficulty swallowing pills, liquid formula-
tions are available. Some agents (e.g., hydromorphone) may be crushed and
added to liquids for ingestion. For patients who are too incapacitated to swallow
oral medications, alternative routes (e.g., transmucosal, transdermal, intramus-
cular, subcutaneous, intravenous, intraspinal) would have to be explored. Oral
transmucosal fentanyl citrate is available. This formulation allows for rapid
absorption of fentanyl for rapid pain relief (in 5–10 minutes) and is useful for
patients with breakthrough pain despite optimal analgesic treatment. The peak
analgesic effect with intramuscular administration has a faster onset but also

90 Clinical Manual of Pain Management in Psychiatry
dissipates faster than with oral administration; however, intramuscular ad-
ministration is intrusive and painful. Repeated intramuscular opioid admin-
istration may lead to abscess formation and muscle fibrosis. Subcutaneous ad-
ministration is an alternative to intramuscular injections and may be better
tolerated. Intravenous administration of opiates is less intrusive, convenient,
and quick acting. One variant of the intravenous infusion is patient-controlled
analgesia (PCA), whereby the patient self-administers the drug and regulates
administration (Table 5–4). When patients control the rate of opiate adminis-
tration, they often require smaller doses of opiate than when they have to rely on
dosing prescribed on an as-needed basis.
Transdermal fentanyl is slowly released through a reservoir, transfers
through subcutaneous tissue, accumulates in subcutaneous fat tissue, and slowly
diffuses into the bloodstream. A slow concentration gradient develops be-
tween the fat and neighboring blood vessels. Normally, fentanyl has a 3-hour
half-life, but with the slow release from the subcutaneous reservoir, the effec-
tive half-life increases to 17 hours. Because of the delay in movement to the
bloodstream, transdermal fentanyl will not have immediate analgesic effects for
approximately the first 24 hours. Consequently, during this time, supplemen-
tal analgesics are required. Once the effectiveness of the fentanyl patch has been
appreciated, the supplemental analgesics may be discontinued. The patch needs
to be replaced every 3 days. Both PCA and transdermal administration offer
the advantage of reduced demand on staff time for administering as-needed
opiates.
In some cases, switching from one route to another may require signifi-
cant dose modifications. For example, oral meperidine has approximately one-
fourth the analgesic efficacy of similar parenteral doses. Thus, when a switch
from intramuscular to oral meperidine is made, four times the parenteral dose is
generally required.
Continuous pain, present most of the day, requires that analgesics be ad-
ministered around the clock as opposed to being administered as needed (Por-
tenoy and Hagen 1990). When medications are dispensed as needed, higher
doses of the opiate may be required to relieve pain. Furthermore, a longer pe-
riod of time might elapse before sufficient relief is attained. Scheduled anal-
gesics might avoid both of these difficulties.
When treated with short-acting agents (see Table 5–1), patients may ex-
perience recurrence of pain at the end of the dosing interval, when levels of the

Pharmacology of Pain 91
analgesic agent are reduced. Such effects, including interference with sleep,
may be difficult for patients to tolerate. Longer-acting agents, therefore, are pre-
ferred. With these agents, pain recrudescence at the end of the dosing interval is
less likely to occur. Agents such as levorphanol have long durations of action,
as compared with morphine, and might be useful in this regard. Longer-acting
Table 5–4.
Dosing guidelines
1. Converting from conventional immediate-release oral morphine (or equivalent)
to controlled-release morphine
a. Calculate the total amount of conventional immediate-release oral morphine
(or equivalent) used in a 24-hour period.
b. Divide this amount by 3.
c. The resulting amount is the dose of controlled-release morphine to be
administered three times daily.
2. Converting from oral morphine (or equivalent) to transdermal fentanyl
a. Calculate the total amount of oral morphine (or equivalent) employed in a
24-hour period.
b. Divide total oral morphine dose by 3.
c. Select the nearest patch strength in µg/hour (i.e., 25, 50, 75, or 100 µg/hour).
d. Continue to administer the scheduled oral morphine for the first
12–24 hours after the first patch is in place.
3. Converting from oxycodone to extended-release oxycontin
a. Calculate the total amount of oral oxycodone employed in a 24-hour
period.
b. Divide the total oxycodone dose by 2.
c. The resulting amount is the dose of extended-release oxycontin to be
administered twice daily.
4. PCA specifications
a. Administer loading dose, generally 1–2 mg of morphine (or equivalent).
b. Decide the hourly rate of the opiate that will be administered continuously
(e.g., 1–2 mg morphine [or equivalent] hourly).
The patient-requested rate needs to be determined. The PCA has lockout
periods, during which no opiate will be administered even if the patient presses
the button to administer the medication. The lockout period is generally
determined to be 6–12 minutes. The patient will be able to self-administer
1–2 mg morphine (or equivalent) when the button is pressed beyond the
preestablished lockout period.
Note. PCA=patient-controlled analgesia.

92 Clinical Manual of Pain Management in Psychiatry
formulations of other agents (e.g., controlled-released morphine, controlled-
release oxycodone, transdermal fentanyl) are now available for sustained anal-
gesia (see Table 5–4). Such formulations allow for more gradual release through
the GI tract and may be dosed at 6- and 12-hour intervals. Crushing of the
slow-release formulations (e.g., to be administered via nasogastric tube) will re-
sult in release of all of the opioid and eliminates any long-acting effects.
As with transdermal fentanyl, long-acting formulations do not produce
immediate relief. Thus, supplementation with short-acting agents may be re-
quired until the analgesic effects of the long-acting agent are appreciated.
Agents With Multiple Uses
Methadone has been used primarily for opioid detoxification and maintenance
treatment of individuals with opioid dependence. Its utility in such situations
is that it has a long half-life and binds preferentially to opiate receptors. Meth-
adone has analgesic ability as well. Its analgesic effects are short-lived, however;
thus, more frequent dosing is required. For patients already receiving metha-
done maintenance therapy, their usual doses may be divided to optimize pain
relief. Patients requiring methadone maintenance acquire the drug on an out-
patient basis by appearing at the methadone clinics daily. If a patient were to
simultaneously require pain management—that is, more frequent dosing with
smaller doses—the patient would have to appear at the clinic several times daily,
which could prove to be logistically difficult. A federal license is required to
prescribe methadone for detoxification or maintenance treatment related to
opioids but is not required for purposes of pain management.
Methadone is available in two isomeric forms, the d- and l-isomers. Al-
though the d-isomer does not bind to the opiate receptor, it does bind to N-
methyl-D-aspartate (NMDA) receptors. It is thought that this action may
exert an analgesic effect independent of µ-receptor binding. Thus, the metha-
done is likely to be more potent than expected by conventional opiate phar-
macokinetics. When switching patients from one opiate to methadone, some
clinicians advocate a reduction of as much as 75%–90% in the dose because of
the unexpectedly high potency of the methadone.
Buprenorphine is a partial agonist that binds preferentially to µ-receptors
and has antagonist activities at the κ-receptors. Its effects (e.g., respiratory de-
pression) are not easily reversed by antagonists (e.g., naloxone). It is adminis-
tered intravenously for pain control in moderate to severe pain. There is an an-

Pharmacology of Pain 93
algesic ceiling effect; thus, for pain persisting beyond optimal dosing, the
clinician may have to explore alternatives. Buprenorphine is approved by the
U.S. Food and Drug Administration (FDA) for the treatment of opioid de-
pendence. It is administered sublingually for this purpose, either alone or in
combination with naloxone.
Tolerance, Dependence, and Pseudoaddiction
Tolerance may emerge with the long-term use of opiate analgesics—that is, higher
doses are required to achieve the same level of analgesia previously achieved at
lower doses. Because of this propensity, opiate rotation has been advocated. To
rotate from one opiate analgesic to another, refer to the equianalgesic doses
shown in Table 5–1. Because the cross-tolerance among opioids is incomplete,
the equianalgesic dose of the newer agent is reduced by 25%–50% of its usual
prescribed dose. Alternatively, supplementation with adjunctive agents (e.g.,
nonopioid analgesics) may augment the analgesic effect of the opiate without
requiring significant opiate dose increments.
If a patient presents with increases in pain despite analgesic use, it is inap-
propriate to assume that this increase reflects tolerance without first examining
the patient and determining if another health condition has emerged that war-
rants attention. It is possible that disease progression or a secondary illness or
physical process is emerging, aggravating the pain despite analgesic use and re-
quiring alternative treatment interventions (Portenoy 1996).
A common misconception is that the development of tolerance might
signal dependence. Physical dependence is probable with the long-term use of
opiate analgesics. It is likely present if the patient develops signs of opiate
withdrawal when the dosage is suddenly reduced or the agent is abruptly dis-
continued. Signs may include lacrimation, rhinorrhea, gooseflesh, irritability
and anxiety, nausea, vomiting, abdominal cramping, lower extremity cramp-
ing, and other features. If the opioid needs to be discontinued after long-term
use, the process should be undertaken gradually to avoid the unnecessary dis-
comfort that accompanies abrupt withdrawal. Because of their antagonist prop-
erties, the clinician must be cautious about administering agonist-antagonists
to patients with opiate dependence or to those previously treated with full ag-
onist opiates. Agonist-antagonists may likewise precipitate withdrawal, add-
ing significant distress to the pain already experienced. Alternatively, metha-
done may be employed to successfully detoxify the patient while minimizing

94 Clinical Manual of Pain Management in Psychiatry
withdrawal symptoms. Use of adjunctive agents (e.g., transdermal clonidine)
may also reduce some of the autonomic symptoms that may accompany with-
drawal.
Patients who are inadequately treated with opiate analgesics may display
behaviors (i.e., pseudoaddiction) that on the surface appear to be manipulative
and characteristic of drug seeking (Weissman and Haddox 1989). These be-
haviors generally cease once opioids are dosed appropriately to mitigate pain.
However, distinguishing substance dependence from pseudoaddiction may be
difficult (see the section “Substance-Related Disorders” in Chapter 4, “Common
Psychiatric Comorbidities and Psychiatric Differential Diagnosis of the Pain Pa-
tient,” of this book).
Clinicians may be uncomfortable about prescribing opioids for the long
term, especially in cases of chronic nonmalignant pain, because of concerns about
federal regulations and the risks of substance abuse. To ease their concern, they
may establish a patient contract (see also Table 10–1 in Chapter 10, “Forensic
Issues Pertaining to Pain,”of this book).
Tramadol
Tramadol is unique in its pharmacologic effects. It has opiate effects (affinity
for the µ-receptor is approximately one-tenth that of codeine) and influences
other neurotransmitter pathways (i.e., it inhibits the reuptake of norepineph-
rine [NE] and serotonin [5-HT]). Both NE and 5-HT are thought to inhibit
the influence of pain-mediating neurotransmitters (i.e., substance P) within the
dorsal horn of the spinal cord. A newer variant combining the anti-inflamma-
tory effects of acetaminophen (325 mg) with tramadol (37.5 mg) (i.e., Ultracet),
as well as a once-daily tramadol extended-release formulation, is available.
Tramadol use has been associated with seizures, although the risk is low;
the risk may, however, increase with concomitant antidepressant (e.g., bupro-
pion) use. In addition, serotonin syndrome may result from combinations of
tramadol with selective serotonin reuptake inhibitors (SSRIs).
The abuse and dependence liability of tramadol was once thought to be
quite low. However, several reports have emerged that indicate that tramadol
is an agent on which patients may become quite dependent. In one case, a pa-
tient had been using such dramatic daily amounts that inpatient methadone
detoxification was required (Leo et al. 2000).

Pharmacology of Pain 95
Nonopiate Analgesics
Acetylsalicylic acid (aspirin), other salicylates, acetaminophen, nonsteroidal
anti-inflammatory drugs (NSAIDs), and cyclooxygenase-2 (COX-2) inhibi-
tors are useful for treating acute and chronic pain. These nonopioid agents act
by interfering with prostaglandin synthesis and disrupting pain by reducing
inflammation at peripheral sites. (However, the mechanism of the analgesia
produced by acetaminophen is unclear.) Some of these are also useful as anti-
pyretic agents. The efficacy of these agents is limited by a ceiling effect, and their
usefulness is limited by the risk of side effects.
Aspirin produces irreversible platelet aggregation, a concern if surgery is
anticipated in the near future. Other salicylates (e.g., choline magnesium tri-
salicylate, salsalate) have fewer GI side effects and less effect on bleeding time.
Reye’s syndrome is associated with aspirin use in children who have a viral ill-
ness (varicella). Aspirin hypersensitivity is of two types: one involving respira-
tory reactions (observed in patients with rhinitis, asthma, or nasal polyps), and
the second involving rapid development of urticaria, angioedema, hypotension,
shock, or syncope.
Acetaminophen does not produce GI distress, nor does it produce platelet
inhibition. However, when combined with warfarin, it may predispose pa-
tients to marked anticoagulation and bleeding. Severe hepatotoxicity may
arise with excessive use of acetaminophen and with concurrent use of alcohol
and acetaminophen. Patients should therefore restrict their alcohol use when
taking acetaminophen.
NSAIDs have efficacy comparable to that of the salicylates and are viable
alternatives for persons with hypersensitivities to aspirin. However, there are
occasional cross-sensitivities to NSAIDs in aspirin-sensitive individuals. Some
NSAIDs have significantly more analgesic efficacy than salicylates (e.g.,
parenteral ketorolac has analgesic efficacy comparable to that of 6–12 mg of
morphine). NSAIDs are nonselective inhibitors of the cyclooxygenase enzyme
responsible for the prostaglandin synthesis in peripheral tissues that results in
inflammatory pain. Because of their lack of selectivity, NSAIDs have inhibi-
tory effects on the COX-1 enzyme, interfering with prostaglandins that me-
diate beneficial physiologic effects in the GI tract and renal tubules.
The most problematic side effects of NSAID use are gastric irritation and
ulceration, bleeding due to reduced platelet aggregation, and renal dysfunc-

96 Clinical Manual of Pain Management in Psychiatry
tion. Unlike aspirin, NSAIDs do not produce irreversible platelet inhibition.
Patients at risk for GI disturbances are those of advanced age, those with his-
tories of prior ulcers, and those simultaneously receiving steroids. To reduce
the risk of GI complications, the lowest doses possible should be used. Use
of misoprostol or omeprazole may be efficacious in preventing ulcers. Ranit-
idine and cimetidine are not useful in preventing ulceration, but they may be
employed to treat the gastric ulcers produced by NSAID use. NSAIDs may re-
duce renal blood flow and glomerular filtration. As a consequence, there is
increased water and electrolyte reabsorption in the proximal tubule, pre-
disposing vulnerable persons to increased blood pressure and congestive heart
failure. Other toxic effects of NSAIDs include hepatotoxicity and acute renal
failure.
The use of NSAIDs has been associated with the emergence of psychiatric
disturbances (e.g., depression, paranoia) among older patients. Although the
mechanisms underlying this association are unclear, blockade of prostaglandin
synthesis may be related to alterations in modulation of 5-HT and dopamine
neurotransmitter systems within the CNS. The influence of NSAIDs on psy-
chiatric symptoms is suggested by the close temporal relationship between
drug initiation and onset of symptoms; this effect may occur in individuals
with and without preexisting psychiatric illness (Browning 1996; Jiang and
Chang 1999). Concomitant use of NSAIDs and lithium may lead to lithium
toxicity. Aspirin, acetaminophen, and perhaps the NSAID sulindac may be
safely employed in patients concomitantly treated with lithium (Ragheb and
Powell 1986).
Cyclooxygenase-2 Inhibitors
COX-2 inhibitors, because of their selectivity, are less likely than NSAIDs to
produce any adverse GI or renal effects. Only celecoxib is currently available for
use in the United States; rofecoxib and valdecoxib were removed from the mar-
ket because of adverse effects. Celecoxib (100 mg twice daily) has been useful in
reducing pain associated with osteoarthritis; a dosage of 200 mg twice daily has
been employed to manage rheumatoid arthritis and sickle cell pain.
Concerns have been raised regarding the cardiovascular safety of the COX-2
inhibitors. The alleged mechanism underlying the risk for adverse cardiovas-
cular events is thought to be related to the prothrombotic effects of the COX-2

Pharmacology of Pain 97
inhibitors. Specifically, by interfering with the COX-2 enzyme, these agents
interfere with prostacyclin formation, which confers beneficial vascular ef-
fects. Because these agents do not block the COX-1 enzyme, the formation of
thromboxane A2 goes unchecked (see Figure 5–1). The latter confers prothrom-
botic effects, predisposing patients to potential risks of myocardial infarction,
ischemic stroke, pulmonary embolism, and so on.
As a result, use of COX-2 inhibitors should be avoided in patients with
risk factors for cardiovascular events (e.g., hypertension, hyperlipidemia, diabe-
tes mellitus, cigarette smoking). Use is contraindicated in patients with conges-
tive heart failure, ischemic heart disease, cerebrovascular disease, or severe
peripheral arterial disease, as well as patients with sensitivities to COX-2 inhib-
itors or allergies to sulfonamides or NSAIDs. Concomitant use of aspirin, al-
though it reduces risk of cardiovascular complications, may increase GI com-
plications that would otherwise have been preventable with use of the COX-2
inhibitor.
Antidepressants
Antidepressants have long been advocated for the treatment of several chronic
pain disorders. Pain reduction with use of antidepressants has been demonstrated
among nondepressed pain patients. Among depressed pain patients, antide-
pressants may produce analgesia faster and at doses far lower than those required
for antidepressant effects.
The pain-mitigating effects of antidepressants remain a subject of intensive
investigation and are thought to involve a number of supraspinal, spinal, and
peripheral processes (see Table 5–5). Analgesia produced by antidepressants is
thought to be primarily mediated by enhancement of the inhibitory neurotrans-
mitters (e.g., NE and 5-HT) present within descending pain-mediating path-
ways. In the spinal cord, the synthesis and release of pain-promoting neuro-
transmitters (e.g., glutamate) are reduced by these agents. In addition, certain
antidepressants may augment opiate effects within the spinal cord. Morphine
analgesia is potentiated by amitriptyline, imipramine, clomipramine, fluoxe-
tine, sertraline, and nefazodone (Larson and Takemori 1977; Lee and Spencer
1980; Pick et al. 1992; Taiwo et al. 1985). On the other hand, within the brain,
the antidepressants reduce the extent of limbic output, which might otherwise
contribute to depression and anxiety that exacerbate underlying pain.
98 Clinical Manual of Pain Management in Psychiatry
Figure 5–1.
Influence of nonsteroidal anti-inflammatory drugs (NSAIDs)
and cyclooxygenase-2 (COX-2) inhibitors on thromboxane and prostacyclin
effects.
(A) NSAIDs, by blocking thromboxane and prostacyclin effects simultaneously, keep
both prothrombotic and antithrombotic effects in balance, without significantly alter-
ing the patients’ risk of cardiovascular events. (B) COX-2 inhibitors, by blocking prosta-
cyclin, leave the thromboxane effects unabated, predisposing patients to vasoconstric-
tion and platelet aggregation, increasing the risk of cardiovascular events.
A
B
COX-1
Thromboxane effects
Vasoconstriction
↑Platelet aggregation
COX-2
Prostacyclin effects
Vasodilation
↓Platelet aggregation
COX-1
Thromboxane effects
Vasoconstriction
↑Platelet aggregation
COX-2
Prostacyclin effects
Vasodilation
↓Platelet aggregation
COX-2
inhibitors
NSAIDs

Pharmacology of Pain 99
Tricyclic Antidepressants
The bulk of the evidence pertaining to the use of antidepressants in pain states
has been directed at the utility of TCAs (Table 5–6). Neuropathic and idiopathic
pains may respond better to TCA use than do nociceptive pains. However, these
agents may be effective in nociceptive pain states when there is a comorbid de-
pression or anxiety that complicates the disorder. The efficacy of TCAs appears to
be related to the reuptake inhibition of NE and 5-HT. TCAs with a broad spec-
trum of activity may be more effective in pain reduction than those with neu-
rotransmitter-specific effects. Thus, amitriptyline and imipramine appear to be
more effective than desipramine or clomipramine (Max et al. 1992).
Generally speaking, TCAs are initiated at a dose of 10–25 mg at bedtime.
Analgesia appears to be dose dependent. The dose may be increased slowly (e.g.,
increased in increments of 10–25 mg every 3–7 days) until analgesia or intoler-
able side effects occur. Specific analgesic serum levels for the TCAs are unknown.
However, if a dosage of 150 mg/day is achieved without significant pain relief,
assessment of serum levels may be prudent. This assessment may help to as-
certain whether there is poor adherence to the medication regimen, poor ab-
sorption, rapid metabolism of the TCA, or all of these factors. If the patient is
adherent to the regimen but, because of metabolic factors, has seemingly low se-
rum levels, the dosage of the TCA may be increased beyond 150 mg/day if the
side effects are not prohibitive. Serum levels should be checked to assess for toxic
ranges, particularly for nortriptyline. However, if side effects preclude dose ad-
vancement, alternatives need to be considered.
Unfortunately, the adverse effects of the TCAs may limit their utility
(Table 5–7). Amitriptyline and imipramine have more troublesome side ef-
fects than the secondary-amine TCAs (e.g., nortriptyline and desipramine). TCAs
are contraindicated in patients with closed-angle glaucoma, recent myocardial in-
farction, cardiac arrhythmias, poorly controlled seizures, or severe benign pros-
tatic hypertrophy.
Serotonin-Norepinephrine Reuptake Inhibitors
The serotonin-norepinephrine reuptake inhibitors (SNRIs) (i.e., venlafaxine
and duloxetine) have demonstrated utility as analgesic agents, and they bypass
several of the untoward effects commonly associated with the TCAs. Both agents
have been demonstrated to have pain-mitigating effects in randomized con-
100 Clinical Manual of Pain Management in Psychiatry
Table 5–5.
Analgesic effects of antidepressants
Site of action Mechanism Effect
SupraspinalaDescending inhibitory fibers
from raphe nucleus and
locus coeruleus
Increased 5-HT and NE Enhanced pain modulation through
heightened activity of descending
fibers extending to the dorsal horn
Spinal Pain-inhibitory interneuronsbStimulation of α1-adrenergic
receptors
Increased GABA and glycine release,
inhibiting pain transmission
Pain-promoting interneuronscStimulation of α2-adrenergic
receptors
Decreased glutamate release,
preventing further pain augmentation
Peripheral Peripheral neuronsdVoltage-gated sodium channel
blockade
Decreased firing of neurons, reduced
input to the dorsal horn
Inflammatione5-HT2 antagonism Reduced pain in formalin models of
pain
Note. 5-HT=serotonin; GABA=γ-aminobutyric acid; NE=norepinephrine.
aSindrup and Jensen 1999; Fields and Basbaum 1999.
bBaba et al. 2000.
cKawasaki et al. 2003.
dGerner et al. 2001.
eAbbott et al. 1997.

Pharmacology of Pain 101
trolled trials of patients with neuropathy (Goldstein et al. 2005; Rowbotham et
al. 2004; Sindrup et al. 2003) or fibromyalgia (Arnold et al. 2004; Zijlstra et al.
2002), with and without comorbid depression. Duloxetine has received FDA
approval for treatment of diabetic neuropathy. Simultaneous NE and 5-HT in-
fluences are achieved at low doses with duloxetine; a dosage as low as 20 mg/day
may be sufficient (Goldstein et al. 2005). For venlafaxine, unlike duloxetine, the
5-HT effects predominate at low doses. It may be necessary to dose at an anti-
depressant level to achieve pain-mitigating effects, (Zijlstra et al. 2002). Adverse
effects of SNRIs may include nausea, dry mouth, nervousness, constipation, and
somnolence. Venlafaxine may be associated with weight loss and elevations in
diastolic blood pressure. If TCAs are not tolerated by the pain patient, these
agents may prove to be workable alternatives.
Selective Serotonin Reuptake Inhibitors
SSRIs offer the advantages of greater tolerability of side effects and relative safety
in overdose as compared with TCAs. However, the literature on SSRI effective-
ness is limited by the small sample sizes and small dosage ranges employed in the
Table 5–6.
Antidepressants used in chronic pain
Tricyclic antidepressants
Neuropathy
Diabetic type
Postherpetic
Poststroke
Nondiabetic peripheral
Headache
Ten si on
Migraine
Rheumatologic disorders
Fibromyalgia
Osteoarthritis
Rheumatoid arthritis
Other
Atypical facial pain
Phantom limb pain
Complex regional pain
syndrome
Monoamine oxidase
inhibitors
Peripheral neuropathy
Migraine headache
Selective serotonin
reuptake inhibitors
Diabetic neuropathy
Tension headache
Serotonin-norepinephrine
reuptake inhibitors
Fibromyalgia
Chronic headache
Diabetic neuropathy
Peripheral neuropathy
Bupropion
Peripheral neuropathy
Tr a zo d o n e
Diabetic neuropathy
Nefazodone
Headache
Te n s i o n
Migraine
Mirtazapine
Chronic daily tension
headache
Fibromyalgia
102 Clinical Manual of Pain Management in Psychiatry
published studies (Ansari 2000). SSRIs found to be effective for one type of
pain do not necessarily have efficacy in other types (e.g., fluoxetine may be
useful for chronic daily tension headaches but might not be effective for dia-
betic neuropathy) (Diamond and Freitag 1989; Saper et al. 1994). Similarly,
the effectiveness of one SSRI cannot be generalized to other SSRIs (i.e., if one
SSRI is effective for a certain type of pain, this does not mean that other SSRIs
will show similar efficacy). For example, paroxetine appears to be effective for
neuropathic pain, but fluoxetine does not (Max et al. 1992; Sindrup et al.
1990, 1991).
There is some question whether the reduced efficacy of SSRIs as com-
pared with TCAs is related to the 5-HT selectivity of SSRIs. As noted in the
section on TCAs, those TCAs with broader spectra of neurotransmitter activ-
ity tend to be more effective than those with more selective 5-HT activity. In
one study, fluoxetine was less effective than amitriptyline and desipramine and
was no better than placebo (Max et al. 1992).
Doses of SSRIs are increased slowly as tolerated and as warranted by the
need for analgesic, antidepressant, or anxiolytic efficacy. Side effects associated
with their use include nausea, diarrhea, insomnia or sedation, tremors, and sex-
ual dysfunction.
Other Antidepressants
Bupropion is an antidepressant with a broad spectrum of activity of neuro-
transmitters, including NE, 5-HT, and dopamine, and has displayed some prom-
Table 5–7.
Tricyclic antidepressant side effects
Anticholinergic
Dry mouth
Constipation
Memory impairments
Blurred vision
Urinary retention
Delirium
Antihistamine
Sedation
Weight gain
α-Adrenergic
Orthostatic hypotension
Cardiac
Palpitations
Sweating
Ta c h y c a r d i a
Prolonged Q-T interval
Neurologic
Myoclonus
Tardive dyskinesia
Parkinsonism
Seizures

Pharmacology of Pain 103
ise with respect to efficacy in neuropathic pain (Semenchuk et al. 2001) (see
Table 5–6). Bupropion lacks significant cardiac or anticholinergic side effects
and has fewer risks of drug interactions; however, there is a risk of seizure as-
sociated with higher doses.
Mirtazapine may be helpful in mitigating symptoms associated with fibro-
myalgia (Samborski et al. 2004) and prophylaxis of chronic daily tension head-
ache (Bendtsen and Jensen 2004). Researchers in two studies—one involving
patients with neuropathy and the other involving chronic headache patients—
found pain-mitigating effects with nefazodone. Patients with migraine or ten-
sion headache experienced marked reductions in the frequency, severity, and
duration of recurrent headache when treated with daily nefazodone (Goodnick
et al. 2000; Saper et al. 2001). Nefazodone use has been linked with hepatic dys-
function, and its use should be avoided in patients concurrently taking med-
ications with potential hepatotoxic effects (e.g., acetaminophen). Additional
clinical trials investigating the roles of these antidepressants are warranted.
Trazodone appears to be minimally, but not conclusively, efficacious in
pain. Although two double-blind studies demonstrated efficacy of trazodone
in diabetic neuropathy and pain resulting from nerve deafferentation (Khurana
1983; Ventafridda et al. 1988), the effects on patients with headache, fibro-
myalgia, rheumatoid arthritis, or chronic low back pain seemed less promising
(Ansari 2000). Trazodone has been employed for its sedative properties. Some
patients, particularly those taking opiates or other sedating agents, may find
the sedative effects too incapacitating. The analgesic properties of trazodone
appear to be independent of its sedative effects (Ansari 2000). Caution con-
cerning other potential adverse effects associated with trazodone use (e.g.,
orthostasis, priapism) is warranted; doses should be kept to a minimum and
increased only gradually to reduce the risk of incurring adverse effects.
The MAOI phenelzine has been found to be effective in the treatment of
selected pain disorders. However, MAOI use has been limited by medication
side effects, the need for a tyramine-free diet, and the potential risk of drug in-
teractions (e.g., serotonin syndrome arising from coadministration with me-
peridine).
Although not currently available in the United States, emerging antide-
pressant therapies potentially useful for pain include milnacipran, an SNRI;
and reboxetine, an NE reuptake inhibitor (Krell et al. 2005; Leo and Brooks
2006; Vitton et al. 2004). Milnacipran has demonstrated efficacy in the relief
104 Clinical Manual of Pain Management in Psychiatry
of pain associated with fibromyalgia; reboxetine has been used in case reports
of patients with musculoskeletal pain and fibromyalgia.
Anticonvulsant Drugs
Anticonvulsant drugs (ACDs) historically have demonstrated efficacy in neuro-
pathic pain, including trigeminal neuralgia and phantom limb pain (McQuay
et al. 1995), as well as migraine (Pappagallo 2003; Snow et al. 2002) (Table 5–8).
The mechanisms underlying analgesia produced by ACDs are varied (e.g.,
slowing the peripheral nerve conduction of primary afferent fibers and thereby
dampening the painful sensory information relayed to the CNS, modulating
sodium and/or calcium channel activity) (see Table 5–9). In addition, ACDs
also function to inhibit the production of pain-promoting neurotransmitters
(Swerdlow 1984).
Gabapentin has received FDA approval for use in the treatment of neuro-
pathic pain. Gabapentin offers numerous advantages over other available ACDs.
It is unlikely to produce serious side effects associated with the use of other
anticonvulsants (e.g., hyponatremia associated with carbamazepine, hepatic
effects associated with valproate). Patients taking gabapentin do not require
Table 5–8.
Uses of anticonvulsants in various pain conditions
Carbamazepine
Trigeminal neuralgia
Neuropathy
Gabapentin
Neuropathy
Atypical facial pain
Reflex sympathetic dystrophy
Central pain
Migraine prophylaxis
Divalproex sodium
Migraine prophylaxis
Neuropathy
Lamotrigine
Trigeminal neuralgia
Peripheral neuropathy
Central neuropathy
Oxcarbazepine
Trigeminal neuralgia
Tiagabine
Diabetic polyneuropathy
Peripheral neuropathy
Phantom limb pain
Pregabalin
Postherpetic neuralgia
Diabetic neuropathy
Phenytoin
Trigeminal neuralgia
Diabetic neuropathy
To p i r a m a t e
Neuropathy
Migraine prophylaxis

Pharmacology of Pain 105
serum drug, hematologic, electrolyte, or hepatic enzyme monitoring, as is of-
ten required with other ACDs. The most common adverse events reported
with its use are somnolence, dizziness, ataxia, tremor, fatigue, and nystagmus.
Gabapentin is excreted unchanged from the kidneys; plasma clearance is
proportional to creatinine clearance (McLean 1994). Dose reductions are re-
quired for patients with compromised renal functioning and those who require
dialysis.
Pregabalin has been FDA approved for both postherpetic neuralgia and
diabetic neuropathy. Pregabalin is pharmacologically similar to gabapentin,
and like gabapentin, it is a structural analog of γ-aminobutyric acid (GABA),
although the exact mechanism underlying its analgesic efficacy remains unclear.
Both agents are thought to influence glutamate release through alterations of
voltage-gated calcium channels (Frampton and Scott 2004). Pregabalin is pri-
marily eliminated by renal excretion; dose modifications are required in pa-
tients with impaired renal function.
Table 5–9.
Anticonvulsant mechanisms of action relevant to pain
Drug
Decrease in
Na channel
activity
Increase in
CNS GABA
activity
Modulation
of Ca2+
channels
Reduction of
EAA activity
Carbamazepine +
Phenytoin +
Valproate + + +
Gabapentin + + + (?)
Lamotrigine + +
Oxcarbazepine +
Topiramate + + +
Pregabalin + +
Tiagabine +
Zonisamide + +
Note. Ca2+=calcium; CNS=central nervous system; EAA=excitatory amino acid; GABA=γ-
aminobutyric acid; Na= sodium.
Source. Adapted from Leo RJ: “Treatment Considerations in Neuropathic Pain.” Current Treat-
ment Options in Neurology 8:389–400, 2006. Copyright 2006, Current Science, Inc.; Massie MJ
(ed.): Pain: What Psychiatrists Need to Know (Review of Psychiatry Series, Vol 19; Oldham JM
and Riba MB, series eds.). Washington, DC, American Psychiatric Press, 2000, p. 37. Copyright
2000, American Psychiatric Press.

106 Clinical Manual of Pain Management in Psychiatry
Carbamazepine is FDA approved for use in trigeminal neuralgia; divalproex
sodium and topiramate have both been indicated for migraine prophylaxis.
Along with phenytoin, carbamazepine and valproate have been shown to be
efficacious in chronic neuropathic pain (Galer 1995; Maciewicz et al. 1985;
Swerdlow 1984).
Emerging evidence suggests the potential analgesic roles of newer ACDs (i.e.,
lamotrigine, oxcarbazepine, tiagabine, topiramate, and zonisamide) (Galer 1995;
Khoromi et al. 2005; Novak et al. 2001; Pappagallo 2003), which offer greater
tolerability than some of the older ACDs. Although these agents demonstrate
some promise with regard to potential utility in neuropathic states (Remillard
1994; Solaro et al. 2001; Zakrzewska et al. 1997), their utility and safety among
pain patients require further investigation. These newer agents may offer better
tolerability over other ACDs (e.g., carbamazepine) and may be useful for pa-
tients with intractable pain or pain that is poorly responsive to other agents.
As with most psychopharmacologic agents, low initial doses of ACDs
should be employed and the doses increased gradually while monitoring for any
intolerance or adverse events. Pain-mitigating doses are comparable with those
employed for anticonvulsant efficacy. Common adverse effects of ACDs are seda-
tion, fatigue, and GI and motor complications. Table 5–10 lists selected ACDs
and their common side effects (Leo and Narendran 1999; Swerdlow 1984).
Considering Treatment Options: Antidepressant,
Anticonvulsant, or Both?
Because several ACDs have received FDA approval for use in selected pain
states, it is perhaps an inclination of many pain practitioners to prescribe an
ACD when confronting the pain associated with neuropathy. However, given
the efficacy of antidepressants in a number of chronic pain conditions, it is
nonetheless important to consider when to use antidepressants and/or ACDs
when treating a particular patient. Considerations of medication selection in-
clude tolerability of side effects and safety of use of particular medications in
the context of the patient’s comorbid medical conditions (Leo 2006).
It is often difficult to elicit sufficient funding from pharmaceutical com-
panies to conduct head-to-head comparisons of various agents in pain treat-
ment. One means of bypassing this is to compare analgesic efficacy of differ-
ent agents across several studies, using the number-needed-to-treat (NNT)

Pharmacology of Pain 107
formula. As shown in the equation in Figure 5–2, the NNT is calculated
by considering the proportion of patients who achieve at least 50% relief with
the use of an analgesic agent and taking from that value those achieving at least
50% relief with placebo. The difference is then divided into 1. The value of
the NNT assigned is an indicator of usefulness, with 1 being ideal and higher
numbers reflecting less efficacy. Stated another way, the NNT provides an es-
timate of the number of patients who need to be given a medication to obtain
one patient who achieves at least 50% relief.
The NNT was calculated for antidepressants and anticonvulsants in two
meta-analyses of multiple placebo-controlled studies involving patients with
neuropathy (Collins et al. 2000; Sindrup and Jensen 1999) (see Table 5–11).
Table 5–10.
Side effects of anticonvulsants
Carbamazepine
Sedation
Nystagmus, diplopia, ataxia
Nausea, vomiting
Myoclonus
Elevated liver enzymes
Hyponatremia, SIADH
Rash
Leukopenia, neutropenia
Aplastic anemia
Gabapentin
Sedation
Dizziness, ataxia
Fatigue
Nystagmus, diplopia
Headache
Tr e m o r
Nausea, vomiting
Lamotrigine
Dizziness
Ataxia, diplopia, blurred vision
Sedation
Headache
Vomiting
Rash, Stevens-Johnson syndrome
Valproate
Nausea, heartburn, indigestion
Vomiting, diarrhea
Sedation
Weight gain
Tr e mo r
Rash
Hair loss
Thrombocytopenia
Hepatotoxicity
Pancreatitis
Phenytoin
Nausea/vomiting
Ataxia
Diplopia
Hirsutism
Gingival hyperplasia
Confusion
Note. SIADH=syndrome of inappropriate antidiuretic hormone.
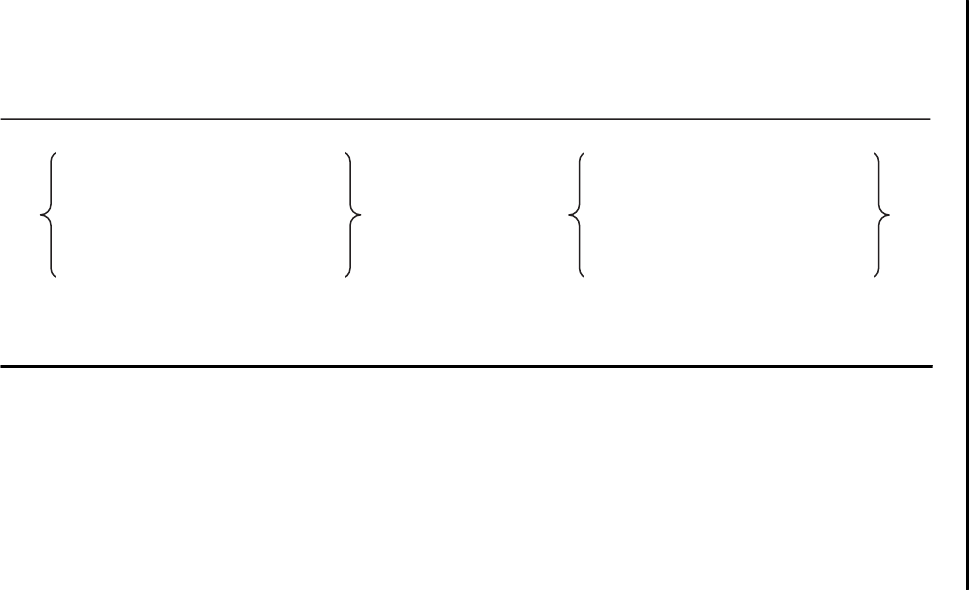
108 Clinical Manual of Pain Management in Psychiatry
Figure 5–2.
Equation for calculating number-needed-to-treat (NNT).
Source. McQuay HJ, Moore RA: “Methods of Therapeutic Trials,” in Te x t b o o k o f Pa i n , 4th Edition. Edited by Wall PD, Melzack R.
Edinburgh, Churchill Livingstone, 1999, p. 1128.
(n achieving relief with
analgesic)
------------------------------------
Total N receiving analgesic
(n achieving relief with
placebo)
------------------------------------
Total N receiving placebo
1
–
Pharmacology of Pain 109
Essentially, for both antidepressants and anticonvulsants, three patients would
need to be treated in order for one of them to achieve 50% pain relief. TCAs
tended to fare better than SSRIs and, to some extent, anticonvulsants. However,
adverse effects were more common with antidepressant use, particularly TCAs,
than with anticonvulsant use (Collins et al. 2000).
Selection of an antidepressant might be based on the fact that several of these
agents have direct pain-mitigating effects, independent of effects on mood.
However, for the patient with comorbid depression and/or anxiety that may
accompany and exacerbate pain, selection of an antidepressant might be most
promising to address pain and mood disturbances simultaneously. In addi-
tion, antidepressants might be a consideration when there is a desire to simul-
taneously address sleep and appetite disturbances accompanying painful con-
ditions.
ACDs have mood-stabilizing effects and are useful for pain patients with
psychiatric comorbidities, such as bipolar disorder, schizoaffective disorder,
and impulsivity arising from dementia (Leo and Narendran 1999). Thus, patients
with mood disturbances, impulsivity, and unpredictable aggression along
with coexistent chronic pain may be ideal candidates for ACD selection.
Table 5–11.
Number-needed-to-treat (NNT) values obtained
from two meta-analyses assessing antidepressant and
anticonvulsant efficacy in neuropathy
Collins et al. 2000 Sindrup and Jensen 1999
Medication NNT Medication NNT
Diabetic
neuropathy
Anticonvulsants
Antidepressants
2.7
3.4
TCAs
Carbamazepine
Gabapentin
SSRIs
2.4
3.3
3.7
6.7
Postherpetic
neuralgia
Antidepressants
Anticonvulsants
2.1
3.2
TCAs
Gabapentin
2.3
3.2
Note. TCA =tricyclic antidepressant; SSRI=selective serotonin reuptake inhibitor.
Source. Data from Collins SL, Moore RA, McQuay HJ, et al.: “Antidepressants and Anticon-
vulsants for Diabetic Neuropathy and Postherpetic Neuralgia: A Quantitative Systematic Review.”
Journal of Pain Symptom Management 20:449–458, 2000; Sindrup SH, Jensen TS: “Efficacy of
Pharmacological Treatments of Neuropathic Pain: An Update and Effect Related to Mechanism
of Drug Action.” Pain 83:389–400, 1999.

110 Clinical Manual of Pain Management in Psychiatry
Because of the differences in the pain-relieving effects of ACDs as com-
pared with antidepressants, it is plausible that ACDs would be workable al-
ternatives for patients with persisting pain despite optimal antidepressant use
or patients for whom antidepressant use has proved intolerable. Alternatively,
simultaneous administration of antidepressants and ACDs could be used, be-
cause the analgesic mechanisms of these classes of agents complement each
other. When antidepressants and ACDs are coadministered, lower doses of the
antidepressant, the ACD, or both are possible, perhaps allowing analgesia to
occur while circumventing the higher doses of either agent that predispose a
person to adverse effects.
The presence of certain comorbidities may preclude the use of an antide-
pressant or ACD. Heart block, arrhythmias, and severe cardiac disease pro-
hibit use of TCAs. In the event of renal dysfunction, doses of venlafaxine, car-
bamazepine, oxcarbazepine, gabapentin, pregabalin, and topiramate would
need to be reduced, and if the renal dysfunction is severe enough, use of these
agents may be precluded. For patients with hepatic disease, doses of carbam-
azepine, oxcarbazepine, and lamotrigine should be reduced. TCAs may con-
ceivably exacerbate encephalopathy associated with hepatic disease.
Antihistamines
Histamines have been implicated in a number of pain states (e.g., headache
and inflammatory pains) because of their role in facilitating inflammatory
processes (e.g., prostaglandin production). Antihistamine effects, therefore,
could be expected to reduce pain mediated by inflammatory processes. Fur-
thermore, antihistamine effects appear to augment opiate receptor binding of
opioid analgesics (Rumore and Schlichting 1986). Thus, antihistamines might
be seen both as augmenting agents to further the effects of other analgesics
(e.g., opiates) and as solitary agents on their own. Used alone, antihistamines
such as diphenhydramine and hydroxyzine appear to have a ceiling effect. Ad-
ditionally, these agents may be particularly useful in patients, given their sed-
ative, antiemetic, and anxiolytic properties. Antihistamines are fairly well
tolerated, with few respiratory or GI side effects. These agents may be sedat-
ing, however, and may intensify appetite; therefore, they may be problematic in
patients for whom excess weight might exacerbate current pain disorders (e.g.,
obese patients with low back pain) and in those with musculoskeletal pains for

Pharmacology of Pain 111
whom weight loss might reduce discomfort and deconditioning. An additional
concern is that confusion and delirium may arise from use of antihistamines.
Antihistamines may need to be initiated at low doses, with dose increases as
tolerated and warranted to address pain, sleep, and so forth. For example, hy-
droxyzine may be initiated at a dosage of 25 mg/day, and the dosage may be
increased slowly as tolerated.
Benzodiazepines and Anxiolytics
Benzodiazepines have been employed acutely to mitigate pain arising from
muscle spasm (e.g., after spinal cord injury). The presumption is that patients
with marked anxiety are prone to heightened muscle tension, which may ex-
acerbate musculoskeletal pain. Other uses for benzodiazepines have included
treatment of restless legs syndrome, tension headache, and neuropathy (Bar-
tusch et al. 1996; Bouckoms and Litman 1985; Dellemijn and Fields 1994).
Clonazepam, a long-acting benzodiazepine, might be effective in patients with
neuropathic pain in which allodynia (painful sensations elicited by normally
nonnoxious stimuli, such as a bedsheet pulled up along the legs) appears to be
a prominent feature (Bouckoms and Litman 1985).
Protracted benzodiazepine use among patients with chronic pain is con-
troversial in that benzodiazepines are GABA agonists and as such may influ-
ence 5-HT neurotransmitter release, attenuating opioid analgesia (Nemmani
and Mogil 2003), with the potential for increasing pain sensitivity. In addi-
tion, protracted benzodiazepine use may be counterproductive. In a study of
patients with chronic pain referred to a tertiary pain center, regression analysis
revealed that long-term benzodiazepine use predicted low activity levels, high
utilization of ambulatory medical services, and high disability levels (Ciccone
et al. 2000). Use of benzodiazepines must be undertaken cautiously, because
these agents may contribute to excessive sedation, gait instability, and memory
impairments (Fishbain et al. 1992; King and Strain 1990).
Buspirone is an anxiolytic agent that differs from benzodiazepines, medi-
ating an antianxiety effect through the 5-HT1A receptor. One study reported
that patients with neuropathic pain tended not to respond favorably to bus-
pirone treatment (Kishore-Kumar et al. 1989). However, in patients with se-
vere anxiety that may aggravate pain experiences, a trial of buspirone to miti-
gate anxiety may be warranted.
112 Clinical Manual of Pain Management in Psychiatry
Triptans
The triptans are 5-HT1B/1D receptor agonists used for acute migraine treat-
ment (see Table 5–12). The triptans are effective in aborting migraine through
influences on cranial vasoconstriction, peripheral neural inhibition, and inhi-
bition of trigeminocervical (second-order) neurons. Ideally, these agents should
be employed early in the onset of migraine for greatest effect (Goadsby et al.
2002).
The side effects of triptans may include paresthesias, dizziness, flushing,
neck stiffness, and warm sensations extending over the head, neck, and chest.
Angina, chest pain and pressure, and myocardial ischemia are possible because
of the triptans’ potential for coronary vasoconstriction. Triptans should be avoided
in patients with uncontrolled hypertension, coronary artery disease, Prinzmetal’s
angina, cerebrovascular disorders, or peripheral vascular disease (Goadsby et
al. 2002; Matchar et al. 2002).
Table 5–12.
Triptans available for abortive migraine treatment
Duration of effect Agent Formulation(s) Dosing
Long acting Eletriptan Oral 20–40 mga
Frovatriptan Oral 2.5 mgb
Naratriptan Oral 1–2.5 mgc
Short acting Almotriptan Oral 6.25–12.5 mga
Rizatriptan Oral
Rapid dissolving
5–10 mga
5–10 mga
Sumatriptan Oral
Nasal spray
Subcutaneous
injection
25–50 mga
5–20 mga
6 mgd
Zolmitriptan Oral
Nasal spray
Rapid dissolving
2.5–5 mga
5 mga
2.5–5 mga
Note. Generally no more than two doses should be employed in 24 hours, and no more often
than 2 days per week.
aMay repeat after 2 hours.
bMay repeat after 6–8 hours.
cMay repeat after 4 hours.
dMay repeat after 1 hour.

Pharmacology of Pain 113
Sumatriptan and other triptans may be taken concurrently with seroto-
nergic antidepressants; however, there have been a few case reports of seroto-
nin syndrome arising from such combinations (Mathew et al. 1996; Putnam
et al. 1999). Use of MAOIs may impede the metabolism of sumatriptan and
rizatriptan (McEvoy 2006).
Stimulants
Stimulants may have analgesic effects when combined with opioids (Forrest et
al. 1977; Laska et al. 1984). Dextroamphetamine (5–15 mg two or three times
daily) or methylphenidate (5–15 mg two to four times daily) has been used to
augment opiate analgesia. These drugs have also been used to reduce the se-
dation, dysphoria, and cognitive inefficiency that may accompany opiate use.
Both pemoline (up to 75 mg/day) and caffeine (65 mg two or three times
daily) have likewise been used for such purposes. However, use of stimulants
may be limited by intervening adverse effects, including overstimulation (e.g.,
anxiety, insomnia, paranoia), appetite suppression (problematic in patients
who are undernourished and cachectic), and confusion. Additionally, in per-
sons who are predisposed to motor abnormalities, tics and other dyskinetic
movements may be exacerbated. When these drugs are taken in overdose, an
extreme form of these adverse effects may occur, resulting potentially in hyper-
tension, arrhythmias, seizures, hallucinations (including formication), delir-
ium, and death. Contraindications for stimulant use include glaucoma,
poorly controlled hypertension, arrhythmias and cardiovascular disorders, an-
orexia, seizure disorders, and hyperthyroidism. Pemoline has been removed
from the market at the urging of the FDA because of concerns about liver dys-
function and hepatotoxicity associated with its use.
The use of stimulants has been limited because of fears regarding abuse and
addiction. Caution is advised in patients with current or preexisting substance
use disorders, especially prior stimulant abuse (e.g., cocaine). Both dextroam-
phetamine and methylphenidate are Schedule II medications under federal
regulatory control. Physicians may be reluctant to make use of these agents be-
cause they fear possible misinterpretation by government agencies and puni-
tive measures that may be taken when these agents are prescribed.
Modafinil recently has been studied for its utility in addressing opioid-
induced sedation and postoperative fatigue related to general anesthesia use

114 Clinical Manual of Pain Management in Psychiatry
(Larijani et al. 2004; Reissig and Rybarczyk 2005). Generally, 200–400 mg
daily may be required for such purposes. Lower doses (e.g., 100 mg daily) are
recommended in patients with hepatic impairments. Adverse effects are sim-
ilar to those associated with other sympathomimetic agents.
Neuroleptics
There have been a limited number of studies demonstrating the efficacy of
various neuroleptics (e.g., fluphenazine) in chronic pain states. These agents
have been found to be useful in certain cases of neuropathic pain (Gomez-Perez
et al. 1985). In animal models, clozapine and risperidone were found to have an-
algesic effects, but olanzapine had only a modest antinociceptive influence
(Schreiber et al. 1997, 1999). In two small clinical case series, olanzapine was ef-
fective in reducing the severity ratings of recurrent migrane and tension head-
ache refractory to other interventions (Silberstein et al. 2000) and cancer pain
(Fishbain et al. 2004). In another small series, olanzapine was useful in miti-
gating cancer pain and reducing opiate requirements among these patients
(Khojainova et al. 2002). One antipsychotic, methotrimeprazine, has demon-
strated analgesic activity comparable to low-dose morphine (Beaver et al.
1966). Methotrimeprazine is available in Canada and Europe but not in the
United States. It appears that the limited data on the efficacy of neuroleptics,
the abundance of other agents to choose from, and the side effects of the neu-
roleptics would warrant avoiding these agents. The risks of neuroleptic use
(e.g., extrapyramidal side effects, tardive dyskinesia) appear to far outweigh any
analgesic efficacy. Use of neuroleptics should probably be confined to the pa-
tient who has delirium and psychosis, although the potential role of atypical
antipsychotics in refractory conditions may warrant further investigation (Fish-
bain et al. 2004).
Mexiletine
Mexiletine is one of many antiarrhythmics that may be used for pain; it has
the advantage of being administered orally. The risk is that patients may ex-
perience untoward cardiac effects (i.e., the drug may influence cardiac rhythms).
Patients with heart block may be particularly vulnerable to adverse cardiac ef-
fects brought on by mexiletine. Mexiletine has been used to treat neuropathic

Pharmacology of Pain 115
pain. The medication is initiated at a dosage of 150 mg/day given in divided
doses, and the dosage is increased gradually (approximately every 3–7 days) to
a maximum of 1,200 mg/day, given in divided doses.
Alpha2-Adrenergic Agonists
α2-Adrenergic agonists (e.g., clonidine) are available for epidural administra-
tion for neuropathic pain (Eisenach et al. 1995). Hypotension and bradycar-
dia may result from use of these agents, but the risk is reduced if low doses are
used. Adverse effects include hemodynamic instability. There is a risk of re-
bound hypertension with acute withdrawal of therapy. Topical clonidine gel is
being developed for use in neuropathic pain states. Tizanidine, another α2-
adrenergic agonist, is used for muscle relaxation.
N-Methyl-D-Aspartate Antagonists
Although the mechanisms are complex and have yet to be fully elucidated, re-
search implicates the excitatory neurotransmitter glutamate in the develop-
ment and maintenance of chronic pain. Some evidence suggests that NMDA
antagonists (i.e., dextromethorphan, ketamine, memantine, and amantadine)
may therefore have a role in mitigating chronic pain, including neuropathy,
chronic phantom pain, and fibromyalgia, and pain associated with spinal cord
injury (Fisher et al. 2000; Sang et al. 2002). However, the analgesic effects
in various trials have been inconsistent (Eisenberg et al. 1998; Enarson et al.
1999).
The side effects associated with the NMDA antagonists include sedation,
dry mouth, headache, and constipation; in some cases these effects may be
prohibitively severe, limiting usefulness (e.g., ketamine may produce disso-
ciation, hallucinations, frightening nightmares, and delirium) (Eide et al.
1994). Ketamine at a dosage of 50–60 mg four to six times daily (taken in
juice or oral suspension) may produce pain relief without incurring these se-
rious effects. Ketamine’s hallucinogenic properties have rendered it popular
with drug abusers (Dillon et al. 2003). Long-term use of ketamine is not
currently advocated, partly because the long-term effects are unknown. It
may result in long-term cognitive changes, hepatic dysfunction, and gastric
ulcers.

116 Clinical Manual of Pain Management in Psychiatry
Widely available as an over-the-counter medication, dextromethorphan is
notable for its cough-suppressing effects. It is a low-affinity NMDA receptor
antagonist that, when combined with opiate analgesics, may enhance opiate
analgesia and reduce opiate tolerance (Elliott et al. 1994). Analgesic effects
require dosing at rates substantially higher than those for antitussive effects.
Dextromethorphan augments 5-HT in the CNS. Consequently, combination
with serotonergic antidepressants (e.g., SSRIs) may predispose a patient to se-
rotonin syndrome.
At a dosage of 200–300 mg orally per day, amantadine may have several
untoward effects associated with other NMDA antagonists (e.g., nervousness,
depression, concentration disturbances, sleep disturbances). Memantine may
be better tolerated than other NMDA antagonists and, given its utility among
patients with dementia, may be particularly ideal in patients with dementia
and complicating pain conditions.
Methadone, too, has NMDA antagonist properties. One isomer of meth-
adone acts by blocking NMDA receptors (Shimoyama et al. 1997), which may
contribute to the fact that methadone maintains analgesic efficacy as com-
pared with other opioids and, therefore, might not require opiate rotations
(Gorman et al. 1997; see sections “Agents With Multiple Uses” and “Toler-
ance, Dependence, and Pseudoaddiction” in this chapter).
Corticosteroids
Corticosteroids have been used in pain states associated with chronic medical
conditions (e.g., inflammatory pain, irritable bowel, spinal cord compression,
headache caused by increased intracranial pressure). The corticosteroids have
also been used to treat cancer-related pain, especially in patients who have
bone metastases, hepatic or biliary involvement from tumor, or epidural me-
tastases with spinal cord compression. Side effects associated with cortico-
steroid use are summarized in Table 5–13. The benefits of steroid use must be
weighed against the risks associated with long-term use. When administered
intramuscularly, intrathecally, or epidurally, corticosteroids may be used at
substantially lower doses than when administered orally. The lower doses may
help to reduce potential systemic adverse effects.
Corticosteroids inhibit the release of phospholipase A, necessary for the
conversion of arachidonic acid to leukotrienes and prostaglandins. The latter

Pharmacology of Pain 117
are responsible for inflammatory pains; they also produce reduced ectopic firing
of neurons (e.g., after amputation), producing phantom limb pain. Cortico-
steroids inhibit activity of C fibers but not of other sensory fibers (e.g., Aβ fi-
bers). Each of the corticosteroids produces equivalent analgesia (Bruera et al.
1985; Ettinger and Portenoy 1988). Selection of one corticosteroid over an-
other may be based on the tolerability and the desire to avoid serious miner-
alocorticoid effects (e.g., such as those seen with dexamethasone). Patients in
whom steroid use might be contraindicated include those with severe cardiac
compromise, a predisposition to congestive heart failure, severe osteoporosis,
diabetes mellitus, or electrolyte disturbances.
Muscle Antispasmodics
Muscle antispasmodics include true muscle relaxants (e.g., baclofen, dantro-
lene) along with agents in which the mechanism of action is unclear (e.g., car-
isoprodol, cyclobenzaprine, methocarbamol, orphenadrine). Some of these
agents may suppress polysynaptic reflexes and thereby reduce pain, but they
do not influence skeletal muscles per se.
Generally, antispasmodic agents are to be used for acute pain arising from
muscle strain or injury (see Table 5–14). Baclofen might be indicated for more
chronic pain arising from muscle spasticity (e.g., after stroke or severe spinal
cord injury). The utility of these agents for long-term use is unclear. There may
be abuse potential associated with carisoprodol and methocarbamol. Cariso-
prodol is metabolized to meprobamate; therefore, ongoing use might pos-
sibly lead to meprobamate dependence. Abrupt discontinuation of carisoprodol
may produce mild withdrawal, including abdominal cramps, insomnia, nausea,
Table 5–13.
Side effects of corticosteroid use in pain
Immune suppression (increased infection risk, Candida infection)
Myopathy
Sodium imbalance–electrolyte disturbance
Fluid overload (congestive heart failure, peripheral edema, pulmonary edema)
Glucose intolerance (iatrogenic diabetes, worsening of preexisting diabetes mellitus)
Gastrointestinal disturbances (bleeding, peritonitis)
Skin breakdown
Neuropsychiatric disturbances (mood, perceptual, and cognitive disturbances)
118 Clinical Manual of Pain Management in Psychiatry
headache, and anxiety. Carisoprodol is contraindicated in patients with acute
intermittent porphyria. Severe psychiatric disturbances, such as psychotic
depression, have been reported in association with baclofen use (Sommer and
Petrides 1992); delirium may result from its abrupt discontinuation (Leo and
Baer 2005).
Side effects often associated with muscle antispasmodics include somno-
lence and anticholinergic effects. Patients should be advised that such agents
may impede their ability to operate heavy machinery or to drive a vehicle. Com-
bination with other sedative agents (i.e., alcohol, sedative-hypnotics, benzo-
diazepines, barbiturates) may produce additive sedative effects. Chlorzoxa-
zone has been associated with hepatotoxicity and might therefore be an unwise
selection as a muscle relaxant for long-term use. This risk may be increased if
the drug is combined with other agents with hepatotoxic effects (e.g., acetamino-
phen or nefazodone).
Baclofen is an agonist of GABAB receptors. It may also suppress the release
of excitatory amino acids such as glutamate and inhibit substance P. Baclofen
has been used primarily for spasticity, but it also may be helpful in reducing
neuropathic pains (e.g., of trigeminal neuralgia) and other neuropathies. The
routes of administration are oral and intrathecal.
Tizanidine is an α2-adrenergic agonist (like clonidine) that may function
to reduce muscle spasticity by decreasing the spinal excitatory amino acid re-
lease, which may have an impact on muscle contractility.
Cyclobenzaprine is used in treating fibromyalgia. It has a tricyclic chemical
structure and is accompanied by anticholinergic side effects. Cyclobenzaprine
Table 5–14.
Dosing of muscle relaxants
Agent Dosage recommendation
Baclofen 5 mg tid, up to 40–80 mg/day
Carisoprodol 350 mg qid
Chlorzoxazone 250–750 mg tid to qid
Cyclobenzaprine 10 mg tid, up to a maximum of 60 mg/day
Orphenadrine 100 mg bid
Methocarbamol 1,500 mg qid for 72 hours, then 1,000 mg qid
Diazepam 2–10 mg tid to qid
Tizanidine 2–8 mg tid to qid

Pharmacology of Pain 119
is contraindicated in persons with ischemic heart disease, congestive heart fail-
ure, cardiac arrhythmias, or heart block. It is extremely lethal in overdose, and
caution must be undertaken when prescribing this agent to persons with risk
factors for suicide. Cyclobenzaprine must never be used in conjunction with
MAOIs because of risks of toxic reactions, which include hyperthermia.
Topical Agents
Topical agents allow for pain relief through direct application of analgesics to
the skin, presumably at the direct source of pain, thereby bypassing significant
systemic effects.
Capsaicin
Derived from red chili peppers, capsaicin has efficacy in reducing arthritic
pain and some neuropathic pain states. The mechanism for pain relief is
thought to be derived from depletion of substance P at sites of pain. Alterna-
tively, counterirritation mechanisms (see Chapter 2, “Sensory Pathways of Pain
and Acute Versus Chronic Pain,” of this book) may be involved. Some patients
find the burning sensations at the site of application to be too uncomfortable,
precluding use.
Lidocaine Patch
The lidocaine patch functions to ameliorate pain when the patch is applied to
intact skin surfaces affected by pain related to neuropathy or myofascial or
osteoarthritis-related pain. The local anesthetic is thought to mitigate pain by
influencing voltage-sensitive sodium channels to stabilize neural membranes.
The patch provides approximately 12 hours of relief, requiring reapplication
of additional patches for refractory and recurrent pain. The patch is generally
well tolerated, but irritation and erythema may occur at the site of application.
Eutectic Mixture of Local Anesthetics
A eutectic mixture of local anesthetics (EMLA) is a solution consisting of two
local anesthetics (lidocaine and prilocaine). This topically applied anesthetic
agent has marked analgesic properties (Kapelushnik et al. 1990). It is custom-
arily employed in conditions involving skin surgery, such as curettage of skin

120 Clinical Manual of Pain Management in Psychiatry
lesions, split-thickness graft harvesting, collagen implants, removal of warts and
port-wine stains, and so forth. Application of EMLA may cause localized vas-
oconstriction of skin. In addition, methemoglobinemia and hypoxia may also
arise as a response to prilocaine contained in the EMLA solution. Edema and
erythema may develop as well, particularly when EMLA is applied to diseased
tissues or skin.
Herbal Agents
Use of herbal agents has become increasingly popular in the treatment of pain
(Kaufman et al. 2002); agents used for a variety of pain complaints are listed
in Table 5–15. Often framed as “natural remedies,” herbal agents are often be-
lieved to be safe and devoid of adverse effects and therefore may go unreported.
It is important to make inquiries into the patient’s use of herbs, because these
may be associated with significant adverse effects and drug interactions when
unknowingly combined with prescribed medications (Ernst 2000).
Cannabinoids
Animal models suggest that cannabinoids have potent antinociceptive effects
(Fox et al. 2001). Analgesic effects are thought to be mediated by binding to
cannabinoid receptors within the CNS (CB1 receptor) and in the periphery
(CB2 receptor). Additionally, cannabinoids are also capable of influencing
serotonergic and dopaminergic activity and endogenous opiates, thereby in-
fluencing pain transmission. Cannabinoids have been advocated for use in mi-
graine prophylaxis and neuropathy and for anti-inflammatory purposes. For
example, dronabinol (10 mg/day) was effective in reducing spontaneous pain
associated with multiple sclerosis and was more effective than placebo in a
small study (Svendsen et al. 2004). Side effects were common, unfortunately,
and included fatigue, dizziness, headache, muscle ache, and potential mood
disturbances (e.g., anxiety, mania). Several oral cannabinoids are currently un-
der development for anti-inflammatory and neuropathic pain conditions; de-
velopment of agents with specificity for the peripheral CB2 receptor may
provide sufficient analgesia while avoiding untoward effects associated with
central (CB1) receptor activity (e.g., fatigue, dizziness).

Pharmacology of Pain 121
Table 5–15.
Herbal agents used for pain
Herb Use Comment
Black cohash Menstrual pain Can produce adverse gastrointestinal (GI) effects; and
abdominal, headache, and joint pain; can increase intensity
of contraceptives
Chamomile Anti-inflammatory Acts as emetic; may interfere with anticoagulants
Evening primrose
oil
Anti-inflammatory; rheumatoid arthritis,
migraine
May trigger temporal lobe epilepsy; unsafe in patients taking
phenothiazine antipsychotics
Feverfew Anti-inflammatory; migraine Interacts with antithrombotic agents
Ginkgo biloba Claudication-related pain Can produce GI distress, headache
Goldenseal Anti-inflammatory Can produce seizure, B vitamin deficiencies; induces abortion
in pregnancy
Kava Antispasmodic Can potentiate sedation from barbiturates and alcohol
To pi c a l St . Jo hn’s
wort
Anti-inflammatory GI distress; can produce serotonin syndrome if combined with
selective serotonin reuptake inhibitors (uncertain if this is a
risk with topical applications)
Valerian Migraine headache GI distress, insomnia; interferes with anticonvulsants and
anxiolytics

122 Clinical Manual of Pain Management in Psychiatry
Placebo Effects
The physiologic aspects of pain are inextricably linked with cognitive and
emotional aspects. Thus, it is not uncommon to find that many patients with
pain may derive relief from placebos (i.e., agents or interventions believed to
be inefficacious). In experimental paradigms, placebo effects of pain relief
were encountered in 15%–58% of patients (Turner et al. 1994). Factors me-
diating the placebo effect include patient expectations and hopes for relief.
Placebo effects are expected to be enhanced by the conviction of the clinician
of the expected relief as well as the costs of the intervention. It is possible that
administration of a placebo may reduce patient anxiety and distress, thereby
mitigating pain awareness. On the other hand, placebo administration has at
times been associated with increases in endogenous opiate release within the
CNS, thereby mitigating pain.
Caution is advised not to ascribe any influence to a treatment that is nothing
more than the natural course and variability intrinsic to a disease state. Thus, ex-
perimental approaches assessing the efficacy of a treatment will naturally re-
quire a placebo arm and perhaps a wait-list control group to tease out those
changes that occur over time. In addition, the clinician is cautioned about dis-
missing the complaints of a patient who appears to respond positively to a pla-
cebo. The positive response does not inherently imply that the allegations of
pain are psychologically based.
Key Points
• Opiates have been the mainstay of treatment of acute and terminal pain
states. Use in chronic, nonmalignant pain states may be indicated, but for
many clinicians, such use raises concerns regarding dependence.
• Factors influencing choice of an opiate analgesic include pain severity, side-
effect profiles, the possibilities of drug interactions, and concerns related to
use of opiates in patients with medical comorbidities.
• Analgesic agents employed in mild pain include acetaminophen, NSAIDs,
and celecoxib. Safety of the use of such agents must be considered before
they are employed for the long term.

Pharmacology of Pain 123
• Adjuvant analgesic agents employed for chronic pain include several psy-
choactive agents, such as antidepressants, anticonvulsants, antihistamines,
benzodiazepines, stimulants, and, in selected refractory cases, neuroleptics.
• Antidepressants have direct pain-mitigating effects apart from influences
on mood. Among the antidepressants, those with simultaneous NE and
5-HT influences appear to have greater analgesic utility as compared with
agents with primarily single-neurotransmitter influences. Antidepressants
can produce analgesia faster and at doses far lower than those required for an-
tidepressant effects. The pain-mitigating effects of antidepressants remain a
subject of intensive investigation and are thought to involve a number of
supraspinal, spinal, and peripheral processes.
• On the basis of two meta-analyses of multiple placebo-controlled studies
involving patients with neuropathy, the number-needed-to-treat calcula-
tions derived for antidepressants and anticonvulsants are comparable; how-
ever, adverse effects were more common with antidepressants, particularly
TCAs, than with anticonvulsants. Selection of an antidepressant versus an
anticonvulsant might theoretically be based on the presence of mood dis-
orders and other psychiatric comorbidities accompanying pain amenable
to medications of a particular class, the potential for drug interactions, and the
presence of medical conditions that may contraindicate use of agents of a
particular class.
• Several analgesic agents have been associated with potential for psychiatric
sequelae—for example, opiates and benzodiazepines (depression, delir-
ium, cognitive impairments, withdrawal after sudden cessation), NSAIDs
(depression), tramadol (delirium secondary to seizures), stimulants (anxi-
ety, decreased appetite), ketamine and dextromethorphan (hallucinations,
psychosis), triptans (serotonin syndrome if combined with serotonergic
antidepressants), baclofen (delirium secondary to abrupt withdrawal), and
cannabinoids (anxiety, mania).
• Herbal agents have become increasingly popular in treating a variety of
pain complaints; however, caution is advised because of concerns over ad-
verse effects and potential drug interactions when herbs are combined with
prescribed medications.
• Analgesic agents with abuse or addiction potential or diversion appeal
include opiates, benzodiazepines, tramadol, stimulants, selected NMDA
antagonists, and selected muscle relaxants.

124 Clinical Manual of Pain Management in Psychiatry
References
Abbott FV, Hong Y, Blier P: Persisting sensitization of the behavioural response to
formalin-induced injury through activation of serotonin2A receptors. Neuro-
science 77:575–584, 1997
Abernethy DR, Greenblatt DJ, Steel K, et al: Impairment of hepatic drug oxidation
by propoxyphene. Ann Intern Med 97:223–224, 1982
Ansari A: The efficacy of newer antidepressants in the treatment of chronic pain: a
review of current literature. Harv Rev Psychiatry 7:257–277, 2000
Arnold LM, Lu Y, Crofford LJ, et al: A double-blind, multicenter trial comparing
duloxetine with placebo in the treatment of fibromyalgia patients with or without
major depressive disorder. Arthritis Rheum 50:2974–2984, 2004
Baba H, Shimoji K, Yoshimura M: Norepinephrine facilitates inhibitory transmission
in substantia gelatinosa of adult rat spinal cord (part 1): effects on axon terminals
of GABAergic and glycinergic neurons. Anesthesiology 92:473–484, 2000
Bartusch SL, Sanders BJ, D’Alessio JG, et al: Clonazepam for the treatment of lanci-
nating phantom limb pain. Clin J Pain 12:59–62, 1996
Beaver WT, Wallenstein SL, Houde RW, et al: A comparison of the analgesic effects
of methotrimeprazine and morphine in patients with cancer. Clin Pharmacol Ther
7:436–466, 1966
Bendtsen L, Jensen R: Mirtazapine is effective in the prophylactic treatment of chronic
tension-type headache. Neurology 62:1706–1711, 2004
Bouckoms A: Psychiatric aspects of pain in the critically ill, in Problems in Critical
Care. Edited by Wise MG. Philadelphia, PA, JB Lippincott, 1988
Bouckoms AJ, Litman RE: Clonazepam in the treatment of neuralgic pain syndrome.
Psychosomatics 26:933–936, 1985
Browning CH: Nonsteroidal anti-inflammatory drugs and severe psychiatric side ef-
fects. Int J Psychiatry Med 26:25–34, 1996
Bruer a E, Roca E, Cedaro L , et al: Action of oral me thylprednisolon e in termi nal cancer
patients: a prospective randomized double-blind study. Cancer Treat Rep 69:751–
754, 1985
Buckley FP, Sizemore WA, Charlton JE: Medication management in patients with
chronic nonmalignant pain: a review of the use of a drug withdrawal protocol.
Pain 26:153–165, 1986
Cassem NH: Pain (Current Topics in Medicine, Vol. 2), in Scientific American Medicine.
Edited by Rubenstein E, Federman DD. New York, Scientific American, 1989
Ciccone DS, Just N, Bandilla EB, et al: Psychological correlates of opioid use in patients
with chronic nonmalignant pain: a preliminary test of the downhill spiral hypoth-
esis. J Pain Symptom Manage 20:180–192, 2000

Pharmacology of Pain 125
Collins SL, Moore RA, McQuay HJ, et al: Antidepressants and anticonvulsants for
diabetic neuropathy and postherpetic neuralgia: a quantitative systematic review.
J Pain Symptom Manage 20:449–458, 2000
Dellemijn PL, Fields HL: Do benzodiazepines have a role in chronic pain management?
Pain 57:137–152, 1994
Diamond S, Freitag FG: The use of fluoxetine in the treatment of headache. Clin J
Pain 5:200–201, 1989
Dillon P, Copeland J, Jansen K: Patterns of use and harms associated with non-medical
ketamine use. Drug Alcohol Depend 69:23–28, 2003
Eide PK, Jorum E, Stubhaug A, et al: Relief of post-herpetic neuralgia with the N-
methyl-aspartic acid receptor antagonist ketamine: a double-blind cross-over com-
parison with morphine and placebo. Pain 58:347–354, 1994
Eisenach JC, DuPen SL, Dubois M, et al: Epidural clonidine analgesia for intractable
cancer pain. Pain 61:391–399, 1995
Eisenberg E, Kleiser A, Dotort A, et al: The NMDA (N-methyl-D-aspartate) receptor
antagonist memantine in the treatment of postherpetic neuralgia: a double-blind,
placebo-controlled study. Eur J Pain 2:321–327, 1998
Elliott K, Hynansky A, Inturrisi CE: Dextromethorphan attenuates and reverses anal-
gesic tolerance to morphine. Pain 59:361–368, 1994
Enarson MC, Hays H, Woodroffe MA: Clinical experience with oral ketamine. J Pain
Symptom Manage 17:384–386, 1999
Ernst E: Herb–drug interactions: potentially important but woefully under-researched.
Eur J Clin Pharmacol 56:523–524, 2000
Ettinger AB, Portenoy RK: The use of corticosteroids in the treatment of symptoms
associated with cancer. J Pain Symptom Manage 3:99–103, 1988
Fields HL, Basbaum AI: Central nervous system mechanisms of pain modulation, in
Textbook of Pain, 4th Edition. Edited by Wall PD, Melzack R. Edinburgh, Scot-
land, Churchill Livingstone, 1999, pp 309–329
Finlayson RE, Maruta T, Morse RM, et al: Substance dependence and chronic pain:
profile of 50 patients treated in an alcohol and drug dependence unit. Pain 26:167–
174, 1986
Fishbain DA, Rosomoff HL, Rosomoff RS: Detoxification of nonopiate drugs in the chronic
pain setting and clonidine opiate detoxification. Clin J Pain 8:191–203, 1992
Fishbain DA, Cutler RB, Lewis J, et al: Do the second-generation “atypical neuroleptics”
have analgesic properties? A structured evidence-based review. Pain Med 5:359–
365, 2004
Fisher K, Coderre TJ, Hagen NA: Targeting the N-methyl-D-aspartate receptor for
chronic pain management: preclinical animal studies, recent clinical experience
and future research directions. J Pain Symptom Manage 20:358–373, 2000

126 Clinical Manual of Pain Management in Psychiatry
Forrest WH, Brown BW, Brown CR, et al: Dextroamphetamine with morphine for
the treatment of postoperative pain. N Engl J Med 296:712–715, 1977
Fox A, Kesingland A, Gentry C, et al: The role of central and peripheral cannabinoid1
receptors in the antihyperalgesic activity of cannabinoids in a model of neuro-
pathic pain. Pain 92:91–100, 2001
Frampton JE, Scott LJ: Pregabalin in the treatment of painful diabetic neuropathy.
Drugs 64:2813–2820, 2004
Galer BS: Neuropathic pain of peripheral origin: advances in pharmacologic treatment.
Neurology 45 (suppl 9):17–25, 1995
Gerner P, Mujtaba M, Sinnott CJ, et al: Amitriptyline versus bupivacaine in rat sciatic
nerve blockade. Anesthesiology 94:661–667, 2001
Goadsby PJ, Lipton RB, Ferrari MD: Drug therapy: migraine—current understanding
and treatment. N Engl J Med 346:257–270, 2002
Goldstein DJ, Lu Y, Detke MJ, et al: Duloxetine vs. placebo in patients with painful
diabetic neuropathy. Pain 116:109–118, 2005
Gomez-Perez FJ, Rull JA, Dies H, et al: Nortriptyline and fluphenazine in the symp-
tomatic treatment of diabetic neuropathy: a double-blind cross-over study. Pain
23:395–400, 1985
Goodnick PJ, Breakstone K, Khumar A, et al: Nefazodone in diabetic neuropathy: re-
sponse and biology. Psychosom Med 62:599–600, 2000
Gorman AL, Elliott KJ, Inturrisi CE: The d- and l-isomers of methadone bind to the
noncompetitive site on the N-methyl-D-aspartate (NMDA) receptor in rat fore-
brain and spinal cord. Neurosci Lett 223:5–8, 1997
Gratz SS, Simpson GM: MAOI–narcotic interactions (letter). J Clin Psychiatry 54:439, 1993
Inturrisi CE: Role of opioid analgesics. Am J Med 77(suppl):27–37, 1984
Jaffe JH, Martin WR: Opioid analgesics and antagonists, in The Pharmacological Basis
of Therapeutics. Edited by Gilman AG, Rall TW, Nies AS, et al. New York,
Pergamon, 1990
Jiang HK, Chang DM: Non-steroidal anti-inflammatory drugs with adverse psychi-
atric reactions: five case reports. Clin Rheumatol 18:339–345, 1999
Kapelushnik J, Koren G, Solh H, et al: Evaluating the efficacy of EMLA in alleviating
pain associated with lumbar puncture; comparison of open and double-blinded
protocols in children. Pain 42:31–34, 1990
Kaufman DW, Kelly JP, Rosenberg L, et al: Recent patterns of medication use in the
ambulatory adult population of the United States—the Slone Survey. JAMA 287:337–
344, 2002
Kawasaki Y, Kumamoto E, Furue H, et al: α2 adrenoceptor-mediated presynaptic
inhibition of primary afferent glutamatergic transmission in rat substantia gelat-
inosa neurons. Anesthesiology 98:682–689, 2003

Pharmacology of Pain 127
Keeri-Szanto M: The mode of action of promethazine in potentiating narcotic drugs.
Br J Anaesth 46:918–924, 1974
Khojainova N, Santiago-Palma J, Kornick C, et al: Olanzapine in the management of
cancer pain. J Pain Symptom Manage 23:346–350, 2002
Khoromi S, Patsalides A, Parada S, et al: Topiramate in chronic lumbar radicular pain.
J Pain 6:829–836, 2005
Khurana RC: Treatment of painful diabetic neuropathy with trazodone (letter). JAMA
250:1392, 1983
King SA, Strain J: Benzodiazepine use by chronic pain patients. Clin J Pain 6:143–147, 1990
Kishore-Kumar R, Schafer SC, Lawlor BA, et al: Single doses of the serotonin agonists
buspirone and m-chlorophenylpiperazine do not relieve neuropathic pain. Pain
37:223–227, 1989
Krell HV, Leuchter AF, Cook IA, et al: Evaluation of reboxetine, a noradrenergic anti-
depressant, for the treatment of fibromyalgia and chronic low back pain. Psycho-
somatics 46:379–384, 2005
Larijani GE, Goldberg ME, Hojat M, et al: Modafinil improves recovery after general
anesthesia. Anesth Analg 98:976–981, 2004
Larson AA, Takemori AE: Effect of fluoxetine hydrochloride (Lilly 110140), a specific
inhibitor of serotonin uptake, on morphine analgesia and the development of
tolerance. Life Sci 21:1807–1812, 1977
Laska EM, Sunshine A, Mueller F, et al: Caffeine as an analgesic adjuvant. JAMA 251:1711–
1718, 1984
Lee RL, Spencer PSJ: Effect of tricyclic antidepressants on analgesic activity in labora-
tory animals. Postgrad Med J 56 (suppl 1):19–24, 1980
Leo RJ: Treatment considerations in neuropathic pain. Curr Treat Options Neurol
8:389–400, 2006
Leo RJ: Pain disorders, in The American Psychiatric Publishing Textbook of Clinical
Psychiatry, 5th Edition. Edited by Hales RE, Yudofsky SC, Gabbard GO. Wash-
ington, DC, American Psychiatric Publishing, 2008
Leo RJ, Baer D: Delirium associated with baclofen withdrawal: a review of common
presentations and management strategies. Psychosomatics 46:503–507, 2005
Leo RJ, Brooks VL: Clinical potential of milnacipran, a serotonin and norepinephrine
reuptake inhibitor, in pain. Curr Opin Investig Drugs 7:637–642, 2006
Leo RJ, Narendran R: Anticonvulsant use in the treatment of bipolar disorder: a primer
for primary care physicians. Prim Care Companion J Clin Psychiatry 1:74–84, 1999
Leo RJ, Narendran R, DeGuiseppe B: Methadone detoxification of tramadol depen-
dence. J Subst Abuse Treat 19:297–299, 2000
Maciewicz R, Bouckoms A, Martin JB: Drug therapy of neuropathic pain. Clin J Pain
1:39–49, 1985

128 Clinical Manual of Pain Management in Psychiatry
Maltbie AA, Cavenar JO, Sullivan JL, et al: Analgesia and haloperidol: a hypothesis.
J Clin Psychiatry 40:323–326, 1979
Massie MJ (ed): Pain: What Psychiatrists Need to Know (Review of Psychiatry Series,
Vol 19; Oldham JM and Riba MB, series eds). Washington, DC, American Psy-
chiatric Press, 2000
Matchar DB, Young WB, Rosenberg JH, et al: Evidence-based guidelines for migraine
headache in the primary care setting: pharmacologic management of acute attacks.
2002. Available at: http://www.aan.com/professionals/practice/pdfs/gl0087.pdf.
Accessed February 26, 2007.
Mathew NT, Tietjen GE, Lucker C: Serotonin syndrome complicating migraine phar-
macotherapy. Cephalalgia 16:323–327, 1996
Max MB, Lynch SA, Muir J, et al: Effects of desipramine, amitriptyline, and fluoxetine
on pain in diabetic neuropathy. N Engl J Med 326:1250–1256, 1992
McEvoy G (ed): American Hospital Formulary Service (AHFS) Drug Information
2006. Bethesda, MD, American Society of Health-System Pharmacists, 2006
McLean M: Clinical pharmacokinetics of gabapentin. Neurology 44 (suppl 5):17–22, 1994
McNairy SL, Maruta T, Ivnik RJ, et al: Prescription medication dependence and neuro-
psychologic function. Pain 18:169–177, 1984
McQuay H, Carroll D, Jadad AR, et al: Anticonvulsant drugs for management of pain:
a systematic review. BMJ 311:1047–1052, 1995
McQuay HJ, Moore RA: Methods of therapeutic trials, in Textbook of Pain, 4th Edi-
tion. Edited by Wall PD, Melzack R. Edinburgh, Churchill Livingstone, 1999,
pp 1125–1138
Nemmani KVS, Mogil JS: Serotonin-GABA interactions in the modulation of mu-
and kappa-opioid analgesia. Neuropharmacology 44:304–310, 2003
Novak V, Kanard R, Kissel JT, et al: Treatment of painful sensory neuropathy with
tiagabine: a pilot study. Clin Auton Res 11:357–361, 2001
Pappagallo M: Newer antiepileptic drugs: possible uses in the treatment of neuropathic
pain and migraine. Clin Ther 25:2506–2538, 2003
Pick CG, Paul D, Eison MS, et al: Potentiation of opioid analgesia by the antidepressant
nefazodone. Eur J Pharmacol 211:375–381, 1992
Portenoy RK: Opioid therapy for chronic nonmalignant pain: a review of the critical
issues. J Pain Symptom Manage 11:203–217, 1996
Portenoy RK, Hagen NA: Breakthrough pain: definition, prevalence and characteris-
tics. Pain 41:273–281, 1990
Putnam GP, O’Quinn S, Bolden-Watson CP, et al: Migraine polypharmacy and the toler-
ability of sumatriptan: a large-scale, prospective study. Cephalalgia 19:668–675, 1999
Ragheb MA, Powell AL: Failure of sulindac to increase serum lithium levels. J Clin
Psychiatry 47:33–34, 1986

Pharmacology of Pain 129
Reissig JE, Rybarczyk AM: Pharmacologic treatment of opioid-induced sedation in
chronic pain. Ann Pharmacother 39:727–731, 2005
Remillard G: Oxcarbazepine and intractable trigeminal neuralgia. Epilepsia 35:528–
529, 1994
Rowbotham MC, Goli V, Kunz NR, et al: Venlafaxine extended release in the treatment
of painful diabetic neuropathy: a double-blind, placebo-controlled study. Pain
110:697–706, 2004
Rumore MM, Schlichting DA: Clinical efficacy of antihistamines as analgesics. Pain
25:7–22, 1986
Samborski W, Lezanska-Szpera M, Rybakowski JK: Open trial of mirtazapine in pa-
tients with fibromyalgia. Pharmacopsychiatry 37:168–170, 2004
Sang CN, Booher S, Gilron I, et al: Dextromethorphan and memantine in painful
diabetic neuropathy and postherpetic neuralgia: efficacy and dose-response trials.
Anesthesiology 96:1053–1061, 2002
Saper JR, Silberstein SD, Lake AE, et al: Double-blind trial of fluoxetine: chronic daily
headache and migraine. Headache 34:497–502, 1994
Saper JR, Lake AE, Tepper SJ: Nefazodone for chronic daily headache prophylaxis: an
open-label study. Headache 41:465–474, 2001
Schreiber S, Backer MM, Weizman R, et al: Augmentation of opioid induced anti-
nociception by the atypical antipsychotic drug risperidone in mice. Neurosci Lett
228:25–28, 1997
Schreiber S, Getslev V, Backer MM, et al: The atypical neuroleptics clozapine and
olanzapine differ regarding their antinociceptive mechanisms and potency. Phar-
macol Biochem Behav 64:75–80, 1999
Semenchuk MR, Sherman S, Davis B: Double-blind, randomized trial of bupropion
SR for the treatment of neuropathic pain. Neurology 57:1583–1588, 2001
Shimoyama N, Shimoyama M, Elliott KJ, et al: D-Methadone is antinociceptive in the
rat formalin test. J Pharmacol Exp Ther 283:648–652, 1997
Silberstein SD, Young WB, Hopkins MM, et al: Olanzapine in the treatment of re-
fractory migraine and chronic daily headache. Cephalalgia 20:382–383, 2000
Sindrup SH, Jensen TS: Efficacy of pharmacological treatments of neuropathic pain:
an update and effect related to mechanism of drug action. Pain 83:389–400, 1999
Sindrup SH, Gram LF, Brosen K, et al: The selective serotonin reuptake inhibitor
paroxetine is effective in the treatment of diabetic neuropathy symptoms. Pain
42:135–144, 1990
Sindrup SH, Grodum E, Gram LF, et al: Concentration–response relationship in par-
oxetine treatment of diabetic neuropathy symptoms: a patient-blinded dose-
escalation study. Ther Drug Monit 13:408–414, 1991

130 Clinical Manual of Pain Management in Psychiatry
Sindrup SH, Bach FW, Madsen C, et al: Venlafaxine versus imipramine in painful
polyneuropathy: a randomized, controlled trial. Neurology 60:1284–1289, 2003
Snow V, Weiss K, Wall EM, et al: Pharmacologic management of acute attacks of
migraine and prevention of migraine headache. Ann Intern Med 137:840–849,
2002
Solaro C, Uccelli MM, Brichetto G, et al: Topiramate relieves idiopathic and symp-
tomatic trigeminal neuralgia. J Pain Symptom Manage 21:367–368, 2001
Sommer BR, Petrides G: A case of baclofen-induced psychotic depression. J Clin Psy-
chiatry 53:211–212, 1992
Stambaugh JE, Wainer IW: Drug interaction: meperidine and chlorpromazine, a toxic
combination. J Clin Pharmacol 21:140–146, 1981
Sternbach H: The serotonin syndrome. Am J Psychiatry 148:705–713, 1991
Svendsen KB, Jensen TS, Bach FW: Does the cannabinoid dronabinol reduce central
pain in multiple sclerosis? Randomised double blind placebo controlled crossover
trial. BMJ 329:253–260, 2004
Swerdlow M: Anticonvulsant drugs and chronic pain. Clin Neuropharmacol 7:51–82,
1984
Taiwo YO, Fabian A, Pazoles CJ, et al: Potentiation of morphine antinociception by
monoamine reuptake inhibitors in the rat spinal cord. Pain 21:329–337, 1985
Turner JA, Deyo RA, Loeser JD, et al: The importance of placebo effects in pain
treatment and research. JAMA 271:1609–1614, 1994
Ventafridda V, Caraceni A, Saita L, et al: Trazodone for deafferentation pain: compar-
ison with amitriptyline. Psychopharmacology (Berl) 95 (suppl):S44–S49, 1988
Vitton O, Gendreau M, Gendreau J, et al: A double-blind placebo-controlled trial of
milnacipran in the treatment of fibromyalgia. Hum Psychopharmacol Clin Exp
19 (suppl 1):S27–S35, 2004
Weissman DE, Haddox JD: Opioid pseudoaddiction: an iatrogenic syndrome. Pain
36:363–366, 1989
Wise MG, Rundell JR: Pain and analgesics, in Clinical Manual of Psychosomatic Med-
icine: A Guide to Consultation-Liaison Psychiatry. Washington, DC, American
Psychiatric Publishing, 2005, pp 228–229
Zakrzewska JM, Chaudhry Z, Nurmikko TJ, et al: Lamotrigine (Lamictal) in refractory
trigeminal neuralgia: results from a double-blind placebo controlled crossover
trial. Pain 73:223–230, 1997
Zijlstra TR, Barendregt PJ, van de Laar MAF: Venlafaxine in fibromyalgia: results of
a randomized, placebo-controlled, double-blind trial (abstract). Arthritis Rheum
46 (suppl 9):S105, 2002

131
6
Psychotherapy
Given that pain is a multidimensional construct, a number of psychother-
apeutic and adjunctive techniques may be employed to address the biological,
psychological, and social features associated with and contributing to pain
(Table 6–1). These interventions are not mutually exclusive but complement
each other to effectively address a particular patient’s needs and produce re-
lief—that is, reducing sensory components of pain (e.g., by relaxation and hyp-
nosis) or reducing the emotional and psychosocial distress that may accom-
pany pain.
The psychotherapies differ with regard to their approach, perspectives,
and goals. Behavior therapy is based on learning principles and holds that the
patient engages in behavior(s) maintained by environmental contingencies.
To modify behavior, the proactive therapist, in conjunction with others in the
patient’s life, modifies those contingencies to facilitate desired behaviors or ex-
tinguish problematic ones.
By contrast, psychodynamically oriented therapies are based on the perspec-
tive that the patient’s behaviors are grounded in earlier developmental ex-
periences. To understand these experiences, the therapist relies on exploration of
defenses, transference, and resistance (Lakoff 1983). The patient is active, pro-
132 Clinical Manual of Pain Management in Psychiatry
ducing the material that makes up the focus of the therapeutic interventions. The
therapist integrates and interprets the material brought forth in therapy, present-
ing it for the patient’s assimilation and use. Ideally, the patient gains insight into
the origins of these behaviors and then can make determinations of how to read-
just current patterns of behavior to more satisfactorily meet his or her needs.
If behavior therapy and psychodynamically oriented therapies are consid-
ered to be the extreme poles of a continuum of psychotherapeutic approaches,
supportive psychotherapy and cognitive-behavioral therapy (CBT) would lie
somewhere along the middle. With these therapies there is no attempt to re-
late current behavior patterns to early developmental experiences. In support-
ive therapy, positive transferences are encouraged and attempts are made to
bolster adaptive defenses. In contrast, CBT focuses on the patient’s belief sys-
tems that are temporally related to problematic behaviors and that, when mod-
ified, bring about behavior change.
Selection of therapeutic approach depends on the patient’s needs and de-
sired goals, the resources available, and the therapist’s training, skills, and pref-
erences. Thus, if a patient presents with excessive and incapacitating use of
opiate analgesics, excessive reclining, and deficiencies in self-care, the behav-
ioral approach might be entertained. However, this approach would probably
be abandoned if the therapist could not enlist the support of others with whom
the patient resided to maintain the behavioral contingencies that would result
in modifications of maladaptive behaviors. Dynamically oriented therapies are
Table 6–1.
Components of pain and associated
psychotherapeutic interventions
Pain component Psychotherapeutic intervention
Biological Relaxation training
Biofeedback
Psychological
Cognitive Cognitive-behavioral therapy
Hypnosis
Affective Dynamically oriented therapy
Supportive psychotherapy
Social Behavior therapy
Marital, couples, and family therapies
Vocational training

Psychotherapy 133
designed to bring about fundamental personality changes and address relation-
ship difficulties, but these therapies might well be abandoned if the patient is
not psychologically minded or is in a terminal condition in which life expect-
ancy could prove to be prohibitive. CBT could be more desirable in such sit-
uations, but it would be abandoned if the patient could not modify cognitive
strategies encompassing his or her worldview and, similarly, coping strategies
because of intervening cognitive disorders.
Resistance to Psychotherapy
Among patients with complex pain, there can be several challenges to the
prospect of psychotherapy. Such patients have a history of medical disap-
pointments and could well be frustrated with health care providers. As a con-
sequence, these patients might believe there is no relief on the horizon. They
could be angry, suspicious, and defensive, and they may possibly reject psycho-
logical interventions (Stieg et al. 1999).
To have the patient align with the therapist and learn the work of psycho-
therapy, the therapist needs to establish an atmosphere of safety. The therapist
needs to facilitate an understanding of the processes involved in the therapy by
educating the patient about the role of therapy and what the patient can come
to expect from the work of therapy in addressing pain-related issues. A partic-
ular mechanism might involve emphasizing the empowerment that may be
derived from therapy. Although the variables in an individual’s life—and in par-
ticular, pain—are not all entirely under his or her direct control, patients may be
able to effect changes—within the range of their abilities—and take control of
their lives. The therapist is instrumental in forging the therapeutic alliance
and a working relationship.
For some patients, being proactive in effecting life changes can be a stir-
ring and stimulating prospect, empowering them to take charge of their lives,
pain, quality of life, and destinies. For others, this prospect can be frightening
and one to be avoided at all cost. The therapist needs to address such ambiv-
alences in the context of the early work in therapy.
Some patients are more amenable to effecting life changes than others.
Such changes may include making efforts to reduce medication use or misuse,
increasing physical activity, increasing social participation, returning to pro-
ductive activity, undertaking weight loss, or addressing nicotine, alcohol, or sub-

134 Clinical Manual of Pain Management in Psychiatry
stance abuse cessation. Patient readiness for the rehabilitation changes required
in chronic pain can be conceived of in several stages (Prochaska and DiClemente
1983). Some persons are not at all ready to effect life changes or see no need
for lifestyle modifications (i.e., are in the precontemplation stage). Others
might be ambivalent about making such changes but feel that they lack insight
into how to go about making them (the contemplation stage). Patients in the
contemplation stage may benefit from education about and exploration of the
impact of pain on current life patterns. Following the contemplation stage is the
preparation stage, in which a patient begins to make efforts to effect change;
then come the action and maintenance stages, in which the patient, with the
assistance of the therapist and others in his or her life, employs strategies to ef-
fect changes and maintains the modifications in behavior while avoiding lapses
to less effective coping and behavioral strategies.
Resistance to therapy may be overcome by engaging the patient with chronic
pain. First, the therapist must accept the pain at face value rather than try
to ascertain its validity or establish its somatic origins. The therapist’s focus
should be on the patient’s subjective experience of pain and the impact of that
pain on the patient’s life. Second, education of the patient about the bridge
between psychological factors and pain might be required. Finally, the thera-
pist may enlist the patient by emphasizing that the goals of therapy can em-
power the patient by facilitating growth, coping, adaptation, and so forth.
Factors to Be Addressed in Psychotherapy
Regardless of the psychotherapeutic approach undertaken, there are certain
essential psychological components of the pain experience that are likely to be
the focus of therapy. Some of these might be more central to a particular psy-
chotherapeutic approach than others.
Affect
Chronic pain is associated with a wide range of psychosocial problems, includ-
ing strained relationships, alienation from others, problems with depression and
anger, and loss experiences (e.g., bodily integrity, self-efficacy). The psycho-
logical experiences of patients with chronic pain may include significant mood
disturbances. These in turn may have an impact on thought patterns and be-
lief systems, all of which may profoundly influence the pain experience and

Psychotherapy 135
the extent to which patients adapt to their condition, adhere to treatment, and
participate in the work of rehabilitation.
Little can be done to facilitate rehabilitation and restoration of functioning
unless comorbid mood disorders are addressed and treated. Given that the neu-
rophysiology and neuroanatomy of pain processing pathways overlap with those
of affective processing and experience, pain perception and mood states (e.g., de-
pression and anxiety) have mutually reciprocal relationships. Thus, affective
states may influence pain perception, pain reporting, and pain-related behaviors.
For example, the severity of a patient’s depressive symptoms has been shown to
predict the number and severity of pain complaints (Hawley and Wolfe 1988).
Depression is a significant predictor of average daily pain (Affleck et al. 1991).
The experience and expression of anger may have an impact on chronic
pain. Higher levels of anger (as well as depression and anxiety) are found among
patients with chronic low back pain than are found among asymptomatic con-
trol subjects (Feuerstein 1986). Poorly managed anger adversely affects pain lev-
els. In one study, patients with chronic tension headache differed from control
subjects in their experience and expression of anger (Hatch et al. 1991). Head-
ache patients were prone to hostility (i.e., feelings of resentment, suspicion,
and mistrust), anger arousal (i.e., perceiving situations as annoying or frustrat-
ing and aroused to anger frequently), and anger suppression (i.e., being more
likely to suppress angry feelings once aroused). However, once overtly angry,
headache patients tended to expend less control over the expression of anger
than control subjects. Taken together, these studies suggest that modulation
of anger and hostility might be a major determinant of the experience of cer-
tain chronic pain conditions (Burns 1997).
In the context of psychotherapy, a patient’s unpleasant emotions may be
focused on to reduce distress. Emotions—including anger—contain informa-
tion value. The patient can become empowered by using the information
gleaned from these emotions. So, for anger, the emotion might serve as a sig-
nal that one’s rights have been violated, one’s needs are not being met, an in-
justice has been done, or one is compromising oneself. Recognizing this, pa-
tients may possibly expend less energy suppressing anger and instead engage
in measures such as determining how their needs are not being met and what
action to take. The experience of anger is likely to contribute to physical dis-
comfort when there is a conflict around the expression of anger and there are
high levels of hostility.

136 Clinical Manual of Pain Management in Psychiatry
Psychotherapy may be particularly useful in assisting patients with pain to
manage unpleasant emotional states. The basic approach is outlined in Table
6–2. However, the approaches differ. Psychodynamically oriented approaches
might consider how emotions were dealt with and managed earlier in devel-
opment. The strategy invoked is to demonstrate how such approaches, when
employed in adulthood, are ineffective in producing growth. CBT, on the other
hand, is more focused on here-and-now experiences, problem solving, and de-
velopment of effective coping strategies.
Factors that contribute to impairments in regulating emotions are outlined
in Table 6–3. Certain disorders—for example, posttraumatic stress disorder
and dissociative and somatoform disorders—are characterized by an inability
to access emotions, leading to affective blunting, somatic amplification, or
both. Even among pain patients without comorbid Axis I disorders, there may
be a propensity to employ defenses (e.g., isolation of affect and alexithymia)
that shield them from intolerable emotions (Beutler et al. 1986).
Defenses
Early psychodynamic conceptualizations of pain emphasized that the symptoms
of pain serve a function (see Table 6–4), such as allowing one to cope with un-
pleasant emotions, allowing one to enlist the support of others, or serving as a way
to expiate guilt. Such conceptualizations have been difficult to corroborate em-
pirically (Weisberg and Keefe 1999) but nonetheless illustrate that conflicts and
defenses against those conflicts may have an impact on the experience of pain.
Table 6–2.
Psychotherapy interventions employed to facilitate
access of emotions
Help patients to
Identify and label feelings.
Recognize affect as a signal.
Identify the precipitant for the feelings.
Express feelings with words instead of actions (e.g., substance abuse,
excessive narcotic use, suicide gestures).
Reduce anhedonia.
Take better care of themselves.
Determine what can be done with unpleasant feelings (e.g., use judicious expression
of emotions, take constructive action).
Psychotherapy 137
Defenses serve to reduce the access of intolerable affective states or im-
pulses from awareness. For example, if a person cannot tolerate an unpleasant
emotion (e.g., anger), he or she might project that emotion onto others (e.g.,
the therapist), leading to potential disruptions in relationships (e.g., the doc-
tor–patient relationship). Primitive defenses might also include projective
identification, whereby the patient enlists the object onto whom he or she has
projected to act out the patient’s aggressive impulses. Such strategies may po-
tentially undermine relationships and exasperate available support systems.
Additional defenses accompanying chronic pain include denial, reaction for-
mation, and repression (Tauschke et al. 1990).
Table 6–3.
Reasons why emotions are poorly identified and
regulated
Patient fears that if emotions are expressed
He or she will be abandoned.
The emotions (experienced as intense states) might lead to some catastrophic
result (e.g., rage-filled reaction).
Patient has not learned that emotions can be expressed appropriately.
Patient has not had good role models for the effective expression of emotion
(e.g., had parent who had tantrums).
Patient is unable to access emotions because of posttraumatic stress disorder,
dissociative disorders, personality disorders, or substance abuse disorders.
Patient is using emotional defenses (e.g., isolation of affect, alexithymia).
Table 6–4.
Early psychodynamic conceptualizations of pain
Proponent Concept
Freud (1893) Psychological distress is expressed through somatic complaints.
Chronic pain is similar to mourning.
Szasz (1957) Pain serves a symbolic function for emotions that are difficult to
tolerate and therefore remain unexpressed.
Pain diverts attention away from the emotion and underlying
conflict.
Pain provides one with a basis for seeking assistance from others.
Engel (1959) Pain serves to
Absolve one from guilt.
Distract one from aggressive impulses.
Rationalize failure and justify one’s persistent perception of
being defeated.

138 Clinical Manual of Pain Management in Psychiatry
Patients with chronic pain may have deficits in self-regulation brought on by
difficulties in managing affect and behavior. For example, alexithymia, present
among those who demonstrate asymbolic and concrete thinking, is characterized
by the patient’s inability to identify and communicate feeling states (Krystal
1982). Just as often, the person’s feelings are vague, ill defined, and confusing.
These factors may lead to impairments in self-regulation that could bring about
generalized states of distress and acting out in ways that undermine treatment,
disturb relationships, and exacerbate life problems. For example, intense feelings
may be temporarily dissipated in several ways: by focusing on pain (instead of the
unpleasant emotion), through use of analgesics that can produce changes in one’s
emotional states (e.g., opiates), and through abuse of substances.
In examining the therapist–patient relationship, the therapist can be aware
of the defenses employed and can redirect the patient’s attention to the prob-
lematic emotions that may be underlying the defense. As with addressing un-
pleasant emotional states, the goals of therapy may be to assist the patient with
identifying the utility of his or her defenses, replacing destructive or primitive
defenses, and substituting healthier defenses (e.g., humor, sublimation).
A number of higher-level defenses may be employed in the management of
unpleasant emotional states. Of these, humor can be quite effective. Patients
who are humorless are prone to being overwhelmed by the ordinary vicissitudes
of life. Similarly, loss of humor among those enlisted to assist in the care of the
patient might signal impending burnout. In experimental paradigms, individ-
uals exposed to painful stimuli had greater pain tolerability when exposed to
laughter-inducing tapes than persons who used distracting mental tasks (e.g.,
calculating mental arithmetic) or those who were not given any instruction
about strategies to use with regard to tolerating pain (Cogan et al. 1987).
A question arises as to whether laughter is the same as humor. Laughter is
really a reaction to a laughter-provoking event, whereas humor is a defense
employed to diffuse the emotional valence of one’s actions. Humor is a mech-
anism of distancing oneself from life stressors, examining one’s own actions,
and so forth. Nonetheless, the utility of humor with regard to pain has been long
advocated.
Belief Systems and Cognitive Distortions
One’s beliefs can have an impact on pain, treatment, and response to treat-
ment. Belief systems (or schemata) are of three major types. One type includes

Psychotherapy 139
belief systems that are broad and encompass aspects and beliefs about one’s
life, world, relationships, and so forth. The second type comprises those belief
systems that are considered to be more stable, influencing one’s relationships,
work ethic, and manner of relating to others—these would be commensurate
with personality characteristics. The third type includes belief systems that are
more or less specific to the pain experience. The stable beliefs affecting person-
ality style as well as those pertaining specifically to pain are likely to influence
coping and adaptation to the entire pain experience. They can be evaluated by
various assessment techniques (DeGood and Tait 2001) and might become
some of the grist for the mill in psychotherapeutic interventions. These beliefs
can affect coping strategies and are likely to be of relevance in interventions
such as cognitive-behavioral, dynamic, and supportive therapies. A person
with a grin-and-bear-it schema would approach pain brought on by physical
therapy differently from the person who views pain as reflecting a serious
pathologic state. For the latter patient, activity might be construed as danger-
ous. A person who believes that pain is equivalent to disability may be more
inclined to neglect usual responsibilities than one who does not.
Expectations and beliefs about treatment are likewise likely to have an im-
pact on a person’s response to pain and on a patient’s treatment adherence.
Thus, the patient’s perception of his or her role (e.g., proactive, informed, and
instrumental versus passive, uninformed, and dependent on the physician’s
interventions) will likely affect treatment adherence and willingness to explore
possible treatment interventions. Similarly, the patient might have expectations
about the role of the physician and others involved in the multidisciplinary
treatment of pain. A patient’s expectation might be that to be effective, a treat-
ment must entirely alleviate pain and discomfort. In reality, however, a more
plausible approach might be to expect some relief along with improved adap-
tation and quality of life. For some patients, such improvements can fall short
of expectations. Some patients see the role of treatment to be exclusively med-
ically based, whereas others might be amenable to recognizing that psycho-
logical variables can, and often do, reduce pain and improve life quality. Sim-
ilarly, expectations of treatment outcome are likely to have an impact on one’s
expectations of recovery, rehabilitation, and restoration of function.
Belief systems can arise from one’s early developmental experiences, earlier
life experiences, earlier experiences with the medical community (either direct

140 Clinical Manual of Pain Management in Psychiatry
or indirect through the medical encounters shared with others who were ill),
and relationships with others. Beliefs shape expectations, not only about re-
covery and rehabilitation but also about what one can expect out of life in gen-
eral (e.g., shaping one’s sense of future hope versus helplessness). Such belief
systems influence how one views oneself, shaping one’s sense of self-efficacy,
autonomy, and self-esteem (Seligman 1990).
In addition to belief systems, the patient’s cognitive styles and propensity
toward cognitive distortions (see also Chapter 3, “Evaluation of the Pain Pa-
tient,” of this book) are likely to reduce self-efficacy, hamper development of
effective coping, drain the patient’s support systems, accentuate unpleasant
emotional states (e.g., anger, anxiety, depression), and exacerbate pain. For exam-
ple, catastrophizing (i.e., the tendency to view and expect the worst in response
to pain) has been seen as a cognitive approach that may predispose one to
heightened pain (Sullivan et al. 1998). Cognitive distortions such as this can
be determined, or at least influenced, by mood states (e.g., depression, anxi-
ety). Because depression often coexists with unremitting pain, it becomes dif-
ficult to determine whether catastrophizing is a result of pain and predisposes
one to depression, or whether pain and comorbid depression result in a ten-
dency to catastrophize.
Catastrophizing may consist of three distinct cognitive distortions: rumi-
nation, magnification, and helplessness (Sullivan et al. 1995). The clinician’s
identification of these distortions, ascertained by careful clinical inquiry (see
also Chapter 3 of this book) and perhaps by use of assessment scales, could
be pivotal to understanding the psychological, emotional, and other disabling
aspects of pain. The goal of cognitive-behavioral strategies is to address these
distortions. When these distortions are altered, the patient may more effec-
tively cope, may experience less emotional distress, and may overcome some
of the disabling aspects of the pain.
Behavior Therapy
The goal of behavior therapy is to mitigate excessive problematic pain-associ-
ated behaviors (e.g., excessive medication usage, limping) and increase those
adaptive behaviors occurring infrequently or not at all (e.g., walking, exercise,
self-care, work) (Sanders 2003). The steps involved in behavior therapy are
outlined in Table 6–5. As originally described, behavior therapy was conducted
with patients with chronic pain on an inpatient basis (Fordyce et al. 1982).

Psychotherapy 141
The therapy was highly structured by the therapist and treating staff. In outpa-
tient settings, however, behavior therapy is obviously less well controlled. The
assistance of others is needed to ensure that environmental contingencies are
systematically applied at home or in other relevant settings.
Behavior therapy is predicated on the recognition that the events (conse-
quences) following a behavior will influence the extent to which that behavior
is likely to recur. Simply put, behaviors that are followed by pleasant or desir-
able consequences (reinforcements) are likely to be repeated in the future,
whereas those followed by negative consequences are not likely to recur. There
are two types of reinforcement: positive and negative. Positive reinforcement
increases the likelihood of a target (i.e., desired) behavior by providing a pos-
itive, pleasant, or desired consequence. On the other hand, negative reinforce-
ment increases the likelihood of a target (desired) behavior by removing an
unpleasant consequence. Behavior change is brought about by active modifi-
Table 6–5.
Steps of behavior therapy
Step 1: Define problem behaviors (operants) that warrant attention
(e.g., medication use, excessive reclining, avoidance of activities).
Step 2: Determine the relationship between operants and environmental
consequences (i.e., identify reinforcers and the temporal contingencies that exist
to maintain these).
Step 3: Assess whether the link between operants and reinforcers is modifiable
(i.e., identify whether reinforcers can be modified so that they become contingent
on desired [adaptive] behaviors, identify how frequently reinforcers should be
applied after desired behaviors occur, identify what quotas might need to
be established, identify those persons who should be involved in the contingency
management process [e.g., spouse, significant other]).
Step 4: Establish how the systematic disruption of problem behaviors and
consequences can be conducted (i.e., how to extinguish undesired behaviors [which
reinforcers should be withheld and when]).
Step 5: Establish transferability to home and work (i.e., consider to what extent
the contingencies can be translated into the home, work, or any setting in which it
becomes necessary to maintain these desired behaviors; perform follow-up
assessments; determine if contingencies need to be modified in other settings;
determine if the newly learned behaviors have been extinguished and whether these
can be reinstated).
Source. Adapted from Fordyce WE, Roberts AH, Sternbach RA: “The Behavioral Management
of Chronic Pain: A Response to Critics.” Pain 22:113–125, 1985. Copyright 1985, International
Association for the Study of Pain. Used with permission.

142 Clinical Manual of Pain Management in Psychiatry
cation of the consequences that follow the patient’s behavior. Reinforcement
can include something given to the person—for example, attention, praise, or
the chance to do something the patient finds rewarding (e.g., rest, reading,
watching a favorite television program). A contingency might be established
that if the patient walks 200 feet, the patient is rewarded with the opportunity
to watch a favored 30-minute television program. Quotas might be set for greater
distances over time, upon which reinforcement would be contingent (Fordyce
et al. 1985).
Undesirable behaviors are those that interfere with adaptive functioning
(e.g., excessive medication use; avoidance of rehabilitative measures, leading
to generalized deconditioning). Undesirable behaviors can be extinguished by
modifying the consequences. When the reinforcers that normally follow these
behaviors are withheld, the behavior is less likely to recur. Alternatively, aver-
sive consequences (e.g., punishment) can be applied following the behavior
and are likely to reduce the likelihood of recurrence. In behavior therapy, pun-
ishments are not employed to modify problematic pain-related behaviors.
There are several drawbacks to the use of punishment (e.g., the patient en-
gages in these behaviors when the “punisher” is not around to notice). Conse-
quently, withholding of consequences that would customarily reinforce unde-
sirable behaviors is the preferred approach.
Critics of behavior therapy argue that the behavioral modifications might
not be sustained. Setbacks may occur once the desired outcome is achieved or
the incentive is gone. Alternative reinforcers (e.g., praise, attention) may need
to be set in place to maintain the behaviors. The behavioral changes can dis-
sipate in settings (e.g., home, work) in which reinforcement patterns are less
systematic or consistent. In addition, despite the changes acquired in behavior
therapy, the patient may nonetheless experience pain—what is modified is the
overt behavior, not the perception of pain.
Critics also argue that the behavioral approach is simplistic—specifically,
the idea that the person engages in behavior because he or she comes to expect
a particular outcome. Thus, behavior therapy fails to factor in those qualities
of being human that influence and dictate behavior. For example, expectation,
anticipation, thinking, planning, and remembering can also influence behav-
ior and mediate pain-related behavior and perception (Seligman 1990).
As an illustration, passivity and inactivity can be particularly problematic
in a variety of pain states (e.g., low back pain, arthritis). This lack of activity

Psychotherapy 143
can result in generalized deconditioning, muscle weakness, and reduced en-
durance—all of which can exacerbate pain once an effort is undertaken. From
a behavioral perspective, such passivity might be reinforced by others (e.g., a
spouse who engages in solicitous behaviors and tends to the patient’s needs
once the inactivity is noticed). An alternative explanation might attribute the
inactivity or restricted activity to expectations the patient has that activity will
exacerbate pain. Thus, the patient is avoiding the prospect of pain, a factor
that may be more pivotal in determining the inactivity. Yet another explana-
tion suggests that passivity arises from the patient’s belief that what he or she
does is pointless (i.e., learned helplessness), so why bother to walk, exercise,
and so forth. Thus, the cognitive-behavioral approach has been invoked to ad-
dress problematic behaviors by taking into account the cognitive aspects of the
patient that might dictate such behaviors.
Cognitive-Behavioral Therapy
CBT addresses the correction of distorted thinking processes and the devel-
opment of strategies (coping) with which to deal effectively with pain, its
effects, and psychosocial stressors. CBT has been applied to a number of differ-
ent chronic pain problems, including low back pain, headache, fibromyalgia,
osteoarthritis, rheumatoid arthritis, and temporomandibular joint disorders
(Turner and Chapman 1982a). Empirical evidence suggests that CBT is as ef-
fective in facilitating psychological adjustment and reducing reported pain levels
as standard medical treatment conditions (Compas et al. 1998; Keefe et al.
1992; Morley et al. 1999). However, when return to work was assessed among
patients with low back pain specifically, CBT was found to be no more effec-
tive than control situations (Scheer et al. 1997).
CBT focuses on internal appraisals of pain and disability by examining and
addressing the cognitions, emotions, and behaviors associated with pain and
pain-related activities. Conducted in about 8–12 fifty-minute sessions, the ther-
apy is structured, with clear agendas set by the patient and therapist focusing on
prominent areas of concern for the patient. The therapist is directive, guiding
the use of homework treatments, outlining exercises, and assessing the efficacy
of the modalities employed, yet this role remains flexible, with the patient’s in-
put guiding any shifts undertaken in the therapy. CBT assumes a collaborative
effort between the patient and therapist (Fishman and Loscalzo 1987).

144 Clinical Manual of Pain Management in Psychiatry
The model for understanding the approach of CBT is illustrated in Figure
6–1. In this model, disturbed emotional and behavioral responses are a direct
function of specific maladaptive cognitions (i.e., one’s beliefs, expectations, and
thought processes). These in turn lead to an array of physiologic changes that
can precipitate or exacerbate pain. CBT emphasizes modification of maladap-
tive cognitions and beliefs (schemata) and the development of effective coping
strategies. CBT provides patients with direction and instruction to reappraise
thoughts and events occurring in their life experiences. Faulty appraisals and
misattributions are reframed and replaced with those that are reality-based
(i.e., less irrational). The presumption, then, is that there will be less physiologic
arousal occurring within the patient. This diminished arousal in turn would
be less apt to accentuate the individual’s pain experiences.
In CBT, it is recognized that the patient often cannot control or avoid dis-
tressing life events. However, the patient can almost always exert some control
over how much distress, suffering, and life disruption those events produce.
This control can be achieved by altering one’s perceptions of these events, their
significance, and their meaning, and by altering one’s coping. Unlike in other
psychotherapeutic approaches, external contingencies of reward, internal dis-
positions, and acquired developmental processes are de-emphasized.
The patients with the best long-term results are those who apply CBT
skills learned in session to real-life situations. In these sessions, homework is
required. This homework entails keeping a diary (much like the pain diary de-
scribed in Chapter 3 of this book), in which the patient records his or her lev-
els of pain in different situations, along with feelings and thoughts occurring
at the time. Homework assignments, if completed properly, help the patient
and therapist identify those situations, moods, feelings, and thought processes
associated with pain. This process then highlights the points of interventions
to work on in therapy (i.e., which cognitions require restructuring and when
to employ coping strategies).
Cognitive restructuring, an interactive process involving the Socratic method,
is used to teach patients to identify and modify maladaptive, negatively dis-
torted thoughts that may lead to negative feelings, such as depression, anxiety,
and anger. The patient is encouraged to examine irrational, self-defeating
thoughts and discriminate between these and more rational alternatives. For
patients with pain, emotional reactions to pain can be greatly influenced by
thoughts. Coping skills training is aimed at helping patients develop a reper-
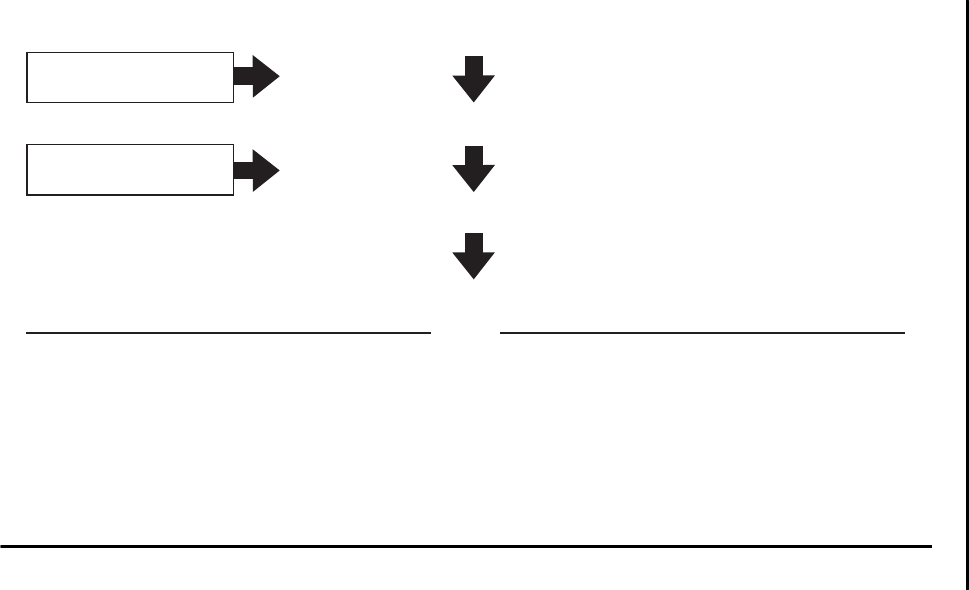
Psychotherapy 145
Figure 6–1.
Modalities involved in cognitive-behavioral therapy.
Disturbed emotional and behavioral responses
Physiologic changes
Pain
Steps involved in
Cognitive restructuring: Coping skills training:
Examine one’s thoughts. Examine one’s existing coping skills.
Test the “accuracy” of the thoughts. Determine the usefulness of existing coping
strategies.
Identify those thoughts that are maladaptive.
Replace the maladaptive thoughts with
those that are reality-based.
Identify those strategies that are maladaptive.
Replace the maladaptive coping strategies with
reality-based, adaptive alternatives.
Cognitive restructuring
Coping skills training
Maladaptive cognitions
Steps involved in

146 Clinical Manual of Pain Management in Psychiatry
toire of skills for managing pain and stress and providing patients with a gen-
eral set of problem-solving or coping skills that can be used in a wide range of
situations that induce pain.
Coping refers to the strategies used by the person to deal with the pain and
life stressors (Weickgenant et al. 1993). The strategies employed are based on
how the person appraises a given situation or life event. Components of ap-
praisal include the value or significance the person assigns to the event, the
perception of the impact of the event, and the assessment of the resources the
person believes himself or herself to have with which to deal with the event.
The diversity and range of coping strategies employed might signal that
the patient’s repertoire of dealing with pain and its effects on his or her life re-
quires modification. Ineffective coping may possibly be associated with psy-
chiatric disturbances such as depression. For example, depressed patients with
low back pain were found to be restricted in the range and types of coping strat-
egies they used when compared with nondepressed patients with low back pain
(Weickgenant et al. 1993). The therapist helps the patient develop a broader
range of effective coping strategies by examining existing coping strategies, de-
termining their effectiveness, and facilitating the development of a broader
range of strategies. Different strategies may need to be employed in different
settings, and patients may need assistance in deciphering which strategies would
be most effective.
Active coping can involve developing problem-solving strategies (e.g.,
problem-focused coping, social support seeking). By contrast, passive coping
involves internal self-statements that the patient learns to say to him- or her-
self to facilitate coping and might include wishful thinking, self-blame, and
avoidance. For example, depressed patients with low back pain were inclined
to be less productive and to employ passive coping strategies when dealing
with life stressors, whereas nondepressed patients with low back pain em-
ployed active coping strategies. However, the latter group did employ passive
coping strategies when attempting to deal with the pain experience (Weick-
genant et al. 1993). Coping then may be either problem focused or emotion
focused.
To help patients modify coping strategies, the therapist should identify
currently employed strategies, assess the utility of these strategies (whether
they facilitate the patient’s relief), and point out, develop, and refine alterna-
tives. Thus, the patient who tends to employ passive strategies, such as self-

Psychotherapy 147
blame and avoidance, might be encouraged to develop strategies that are more
active and self-soothing.
CBT has the advantage of broad applicability in a number of situations. It
is a relatively low-cost intervention and appears to be cost-effective. On the
other hand, CBT has the disadvantage of requiring sustained active patient
participation. Also, therapists need to have specialized training in CBT in or-
der to use it effectively.
Supportive Therapy
Much of what clinicians do can be considered under the auspices of support-
ive psychotherapy. For the patient with chronic or complex pain, complete
pain relief might not be possible; supportive therapy might then be a viable
approach. The therapist should undertake a warm, reflective, and empathic
approach to reduce patient distress and reassure the patient that he or she is
understood and that the magnitude of his or her plight is appreciated. Feed-
back should be given, providing patients with the outcome and findings of
physical and laboratory assessments, pain assessments, and psychometric test-
ing. Rather than telling the patient that “nothing can be done,” the therapist
should emphasize that modification and improvement in functioning are es-
sentially the patient’s responsibility. This emphasis can enhance the patient’s
sense of personal control and self-efficacy, which is critical for overcoming the
tendency of the patient to succumb to powerlessness and helplessness. Ther-
apists may offer a menu of concrete advice for strategies to reduce discomfort,
improve sleep, address medication use, and develop pain-modulating inter-
ventions (e.g., relaxation training). Such a menu fosters the notion of the patient’s
responsibility and autonomy by allowing the patient to select those aspects of
treatment that are most appealing, thus possibly facilitating patient compli-
ance (Miller and Sanchez 1994).
Ongoing follow-up with supportive therapy is required. The interventions
described above will be most successful when subsequent meetings with the
patient allow for repeated interventions. In addition, setbacks may be the basis
for determining what modifications in medications and other treatment in-
terventions might be required. Follow-up reinforces the notion that the pa-
tient and physician or clinician are working in concert to effectively mitigate
pain and optimize adaptive functioning while restoring pleasure and balance

148 Clinical Manual of Pain Management in Psychiatry
and bolstering relationships in the patient’s life. With this reinforcement, the
patient can avoid the potential pitfall of viewing himself or herself as passive
and helpless in the treatment process.
Marital, Couples, and Family Therapy
Pain is often myopically viewed as an individual patient’s problem. Painful dis-
orders have an impact on a broader spectrum of persons, including the patient’s
partner and family, along with friendships and work relationships. Conversely,
social factors may contribute to the onset, exacerbation, maintenance, and course
of the pain disorder.
Significant changes in the functioning and activity of the family unit can arise
as a result of having a member with pain. These changes can include alterations in
the role responsibilities within the home in order to assume those that the pain
patient is no longer able to perform. Such role modifications may be emotionally
laden, with some members feeling burdened and the patient experiencing loss of
self-efficacy or feeling as though he or she is viewed as incapable. There also may
be resulting shifts of influence and decision making within the home.
Several stressors may affect the patient’s relationships. One such stressor
may be related to financial issues, such as lost employment, treatment costs,
and inadequate medical coverage. The patient and partner (along with other
family members) may disagree about the feasibility of return to work or explo-
ration of other vocational pursuits.
Another stressor may involve the reactions of others to the patient’s pain. The
reactions of persons within the home can be quite varied, including solicitous re-
sponses, withdrawal, and indifference. The solicitous spouse or significant other
of the patient might inadvertently reinforce pain complaints and related pain be-
haviors (e.g., grimacing, limping, sighing) by eagerly accommodating the patient
when such behaviors are expressed. Some studies have demonstrated that the de-
gree of solicitous behavior on the part of one’s partner is an important factor in
determining or predicting pain reports (Block et al. 1980).
Patients also vary in their response to others’ reactions. They may harbor
expectations of how the partner (or others within the family) should respond
to their pain complaints and pain-related behaviors. They may view such re-
sponses negatively (e.g., as demeaning or indicative of the patient’s “invalid”
status), or they may view them as supportive and helpful.

Psychotherapy 149
Strained communication patterns within the couple or family may be an-
other stressor. Direct discussion of the patient’s behavior may be difficult. The
pain may serve as a means of avoiding discussion about particular topics, which
leads to unresolved conflicts and emotional difficulties. Sexual dysfunction and
a decline in intimacy from prepain periods may add to the burden of the patient
and his or her partner as well. Formal sex therapy may be needed, especially
when such difficulties foreshadow more deleterious effects on the relationship.
Another potential stressor is that normal interests (e.g., in outside hobbies
and activities) may no longer be available to the patient, partner, or other fam-
ily members. Social support networks that would normally be relied on might
be less accessible. The patient and his or her primary caregivers may have little
emotional reserve remaining to support and care for others within the family.
As a result of any or all of these factors, the partner–patient dyad and fam-
ily relationships as a whole can experience marked strain as a result of the pain
disorder. Thus, marital and family therapy can be useful to address these is-
sues, mitigating the strain experienced by the patient and others in the pa-
tient’s life (Romano and Schmaling 2001). Cohesive family relationships and
a supportive, mutual, and intimate relationship with a partner can be a buffer
against depression for the pain patient. In addition, pain patients in such re-
lationships tend to respond best to treatment and have lower levels of disabil-
ity (Kerns and Haythornthwaite 1988; Kerns and Turk 1984).
Despite the difficulties that can afflict couples in which pain is prominent,
such couples typically remain together for long periods of time. One possible
explanation is that the pain serves the role of preserving homeostasis within a
troubled relationship. External pressures (e.g., how others would interpret di-
vorce or separation when one partner is so ill) may also influence why some
couples remain together. Financial factors can prove to be prohibitive for one
or both parties, especially when disability interferes with work functioning
and financial support. Substantial financial gains could be hoped for through
pending litigation, which might mean that the well partner would want to hold
out for a piece of the expected financial gain. Interestingly, partners may also
come to endorse significant pain problems of their own as well. Often, there is
overlap of this pain in location and quality with the pain reported by the pa-
tient (Sholevar and Perkel 1990).
Assessment scales and questionnaires can augment the information gleaned
from clinical interview. For example, the Multidimensional Pain Inventory,

150 Clinical Manual of Pain Management in Psychiatry
mentioned in Chapter 3 (“Evaluation of the Pain Patient”) of this book, has a
portion devoted to analyzing the partner’s responses to pain. Other assessment
instruments that assess relationship satisfaction and the family environment are
available as well. Such instruments can be quite helpful when attempting to ad-
dress issues arising in the social milieu of the pain patient (Romano and Schmal-
ing 2001).
Group Therapy
One of the most devastating effects of chronic pain can be the isolation it pro-
duces for the patient. Group therapy, including self-help groups, can serve to
diffuse the sense of isolation experienced by the pain patient. These groups al-
low for the mutual sharing of experiences, provide an opportunity to learn
from the experiences of others, foster education about treatment strategies,
and foster coping strategies. The shared experiences of persons in the group
can offer the patient a sense of being fully understood, often without the re-
quirement of having to explain his or her experiences or emotions to others
who do not share the same level of physical pain.
Some groups are exclusive—that is, organized around particular disorders
(e.g., groups for persons with fibromyalgia, arthritis, or cancer pain). Others can
be inclusive—broader and open to persons with recurrent or chronic pain of
various sorts. Some groups are open to family members and significant others as
well.
The purposes of group therapy include reducing feelings of isolation (i.e.,
universalization) and providing a forum in which mutual support, information
exchange, advice, modeling of effective coping, and abreaction of emotional ex-
periences are possible. The structure of the group sets the stage for and dictates the
focus and perspective of the participants. For the group to be effective, the rules of
the group process need to be delineated early. If patients are allowed to ventilate
distress and can respond to the support and advice offered by peers, attendance in
therapy sessions can be a source of inspiration and empowerment. A special ben-
efit arising from group process is abreaction, whereby repressed, emotionally laden
experiences are brought to conscious awareness (i.e., reexperienced) and in the
process, insight is gained. Some members of the group can inspire others with
hope or the means to effectively address emotional-psychological consequences
associated with the pain experience (Keefe et al. 1996).

Psychotherapy 151
Psychotherapeutic goals can be organized around exploring individual
psychodynamics and bringing about personality change. Self-help groups, on
the other hand, have vastly different goals—for example, fostering emotional
well-being—and focus on coping with specific life problems. Groups vary as
to whether they are organized around a particular philosophy or approach.
For example, some groups have actually adapted the 12-step program origi-
nated within Alcoholics Anonymous, substituting pain for alcohol (Back and
Neck Injury 2002). The goals of such programs are to view pain as something
one might not be able to fully control or remove. Thus, adaptations in a per-
son’s life might be required to overcome the many obstacles in life, relation-
ships, and spiritual life.
Adjunctive Interventions
Adjunctive interventions are not forms of therapy but are nonetheless helpful
techniques that can be undertaken with the pain patient. These techniques
can complement other medical treatments and psychotherapies.
Biofeedback
Biofeedback refers to a procedure in which physical parameters (e.g., muscle ten-
sion) are continuously monitored and fed back to the patient, who then at-
tempts to alter the physical parameter. For example, an individual attempting
to regulate and modify the degree of muscle tension in forehead muscles would
have electromyographic electrodes placed on the forehead. The electrical sig-
nals from these electrodes would be relayed to a monitor and presented in any
of a number of formats (e.g., visual, auditory). The patient, attending to the sig-
nal, would then use the information presented to develop strategies to reduce
muscle tension. The principle relies on the idea that the patient uses the feed-
back signal as an indicator of the degree of physical activity that can be mod-
ified. In pain management, the idea is that the physical parameter measured
and ideally altered is somehow linked to the genesis of pain (Turk et al. 1979;
Turner and Chapman 1982b).
Various biofeedback procedures, focusing on selected physiologic parame-
ters for selected pain states, may be employed. Thus, biofeedback from elec-
tromyography assists the patient in learning to reduce muscle tension; the levels
of measured muscle tension are signaled back to the patient for modification.

152 Clinical Manual of Pain Management in Psychiatry
This technique is useful in tension headache, temporomandibular joint dis-
orders, fibromyalgia, and other myofascial pain disorders. Thermal biofeed-
back monitors skin temperature to give the patient an indicator of the degree
of peripheral vasodilation. The cooler the skin, the greater the vascular constric-
tion; this reflects the amount of prevailing sympathetic activity. By increasing
the skin temperature, one is able to suppress the extent of sympathetic activity.
This approach has been used in the treatment of migraine and sympathetically
maintained pain. Electroencephalography has also been linked with biofeed-
back. Brain electrical activity is fed back to the patient and is subject to mod-
ification by the patient. The goal is to achieve states of alpha rhythm brain ac-
tivity that reflect a state of relaxation. Customarily, electroencephalographic
biofeedback is quite cumbersome and is not regularly used in pain treatment
settings.
The technology involved in biofeedback has enhanced the opportunities
and approaches available in pain management. Eventually, with practice, the pa-
tient learns to attend to physical cues that signal that relaxation, deep breathing,
and changes in cognitive strategies are required (e.g., sensing one’s own height-
ened muscle tension). The patient is then able to invoke those strategies to
reduce the tension without requiring the use of the biofeedback equipment.
However, some practitioners question the utility of biofeedback and the ne-
cessity of all the cumbersome technology, arguing that other strategies (e.g.,
relaxation training) are equally efficacious (Silver and Blanchard 1978).
Relaxation and Imagery Training
Relaxation and imagery (R&I) has been employed in both acute and chronic
pain and has been successfully implemented in the treatment of tension head-
ache, migraine headache, temporomandibular joint pain, chronic back pain,
and myofascial pain syndrome (Turner and Chapman 1982a). Progressive
muscle relaxation (PMR) is the most common approach used. In PMR, the
patient is instructed to tense muscles in a region of the body or a limb and
then subsequently relax those muscles. The tension and subsequent relaxation
of muscle groups is conducted in a logical and sequential manner, generally
from head to foot or the reverse. For some patients (e.g., those with myofascial
pain and fibromyalgia), the process of tensing sequential muscle groups could
prove to be too difficult or fatiguing. Consequently, alternative measures can
be employed (e.g., guided imagery or deep breathing exercises).

Psychotherapy 153
Autogenic training is similar to PMR in that the patient focuses on se-
quential and progressive relaxation of parts of the body, but the step of tensing
muscles in those regions is not required. Instead, the patient is asked to focus
on sensations of heaviness and warmth extending throughout the body.
Guided imagery involves talking the patient through vivid images that are
particularly comforting and relaxing. Just as imagining the taste and aroma of
a craved meal or an erotic thought can induce a dramatic constellation of phys-
iologic responses, so too, it is thought, can guided imagery modify physiologic
reactions to pain. The patient is asked to imagine a place that he or she finds
most relaxing in order to set the stage for being in an emotional state that would
be inconsistent with anxiety or tension. The patient is then guided to vividly
recapture as much of the imagined place as possible—the sights, the sounds,
smells, tastes, and physical sensations and feelings. Guided imagery has been
employed successfully in postsurgical and chronic pain conditions (Daake and
Gueldner 1989; Mannix et al. 1999).
Deep breathing exercises involve the direction of deep breathing in a man-
ner that potentiates a deepened relaxation. A script for deep breathing exer-
cises is provided in Table 6–6. The patient is instructed in deep breathing so
that the technique can be used at home. The technique can be used in a matter
of few seconds or over a span of several minutes (McCaffery and Beebe 1999).
R&I offers several clinical advantages. It is easy for clinicians to learn how
to implement R&I, and it is easily learned by patients as well. Unlike biofeed-
back, R&I requires no specialized equipment. At most, patients might require
a tape or compact disc player to practice R&I; thus it is a low-cost interven-
tion. Patients may be more receptive to R&I compared with other interven-
tions, such as hypnosis, about which there may be misconceptions.
R&I has several disadvantages. It is not a sufficient treatment for pain
states. Adverse effects may arise, including muscle cramping and fatigue, par-
ticularly with the use of PMR. Lack of patient practice at home limits its use-
fulness. In addition, certain patients might find it difficult to gain any benefit
from R&I. Highly distressed or distractible patients might have marked dif-
ficulty concentrating on the techniques required. In addition, patients dis-
tracted by intense, severe pain might find that they are unable to benefit from
R&I. Some religious patients and those who are rigid or concrete in their
thinking might be resistant to R&I, fearing that it invokes practices contrary
to their spiritual experiences or cognitive styles. (This resistance can generally
154 Clinical Manual of Pain Management in Psychiatry
be overcome with thoughtful patient education and reassurances.) Skeptical pa-
tients might not construe any benefit to the technique. In some cases, the ap-
proach of biofeedback might be more convincing, because the patient receives
feedback about biological parameters that improve in response to relaxation
techniques.
In a review of nine studies examining the use of R&I alone in pain reduc-
tion, only four studies showed a significant difference among pain outcome
measures between pretreatment and posttreatment (Carroll and Seers 1998).
In only three studies was a significant improvement found when R&I, as com-
pared with a wait-list control condition, was used. This analysis suggests that
although R&I can have some efficacy in mitigating pain experiences, it prob-
ably is not sufficient as a sole intervention. Compared with alternative treat-
ments, behavior therapy was found to be more effective than R&I, and in another
study, biofeedback was found to yield better long-term results than R&I when
patients at 6-month follow-up.
Hypnosis
Hypnosis has been recognized as a legitimate form of medical treatment when
administered by an appropriately trained therapist. It is a self-induced state
Table 6–6.
Script for deep breathing exercises
Have the patient assume a comfortable position in a quiet place where there will be
no interruptions. Ask the patient to imagine that he or she is doing this exercise in
a place that is calming and relaxing. Then, instruct the patient as follows:
1. Breathe in slowly and deeply.
2. As you breathe out slowly, feel yourself beginning to relax as tension begins to
leave your body.
3. Now, breathe in and out regularly and slowly. Constrict your abdominal muscles,
and feel your chest wall expand as your abdomen compresses inward. Feel your
ribs expand, hold this, then slowly release. As you release the air, feel your body
relax more and more. Imagine tension leaving your body with each exhalation.
4. As you breathe in, slowly count to three. As you breathe out, slowly count to
three. Each time you breathe out, repeat a word to yourself—a word such as
“peace” or “relax.”
5. Repeat steps 3 and 4 as needed for up to about 20 minutes.
6. End with a slow deep breath. As you breathe out, say to yourself, “I feel relaxed.”

Psychotherapy 155
brought on by the patient with the assistance of the hypnotist. The mecha-
nism of pain relief brought on by hypnosis is unclear. Questions arise as to
whether it is the pain (unpleasant sensations) that is removed or, rather, the re-
actions to normally painful stimuli. For example, Esdaile, a surgeon in Bengal,
India, reported success in using hypnosis for patients in more than 300 oper-
ations in the early 1800s. Although there was a report of significant success us-
ing hypnosis, it was also reported that many patients were not entirely pain
free. For example, some patients “moved and moaned” or awoke in the midst
of the operation and “cried out.” Afterward, however, they appeared to be am-
nestic for the surgery and the pain. In other acute pain settings, patients reacted
as if they had pain but subsequently could not recall the painful experience (Bar-
ber 1963; Orne 1976).
Current conceptualizations view hypnosis as a form of focused attention that
is useful in managing acute and chronic pain states, including headache, fibro-
myalgia, back pain, trigeminal neuralgia, arthritis, phantom limb pain, and can-
cer pain. Analgesia produced in response to hypnosis was thought to be brought
on by modification of attention control systems (i.e., anterior frontal cortex)
within the brain. By invoking the techniques summarized in Table 6–7, the pa-
tient develops strategies to not attend to painful signals arising from the thalamus
and other pain-mediating subcortical brain structures (Crawford et al. 1998).
Analgesia produced through hypnosis is an active process involving the
patient’s full cooperation. Imaging technology has supported that the process
of hypnosis produces signal changes in areas of the brain concerned with sen-
sation and perception (i.e., the sensory cortex and thalamus) and areas of the
brain where sensory information is integrated (Raz and Shapiro 2002).
Hypnotic responsiveness varies considerably from person to person but
appears to be a stable trait. A number of scales are available to assess respon-
siveness to hypnosis (Kurtz and Strube 1996). These scales test the patient’s abil-
ity to respond to a variety of hypnotic suggestions. The most important factor
determining response to hypnosis appears to be the patient’s imaginative ab-
sorption, on which the skillful hypnotist is able to capitalize (Smyser and Baron
1993). Pain reduction is predicted by imaginative absorption.
Because some patients are less susceptible to hypnosis than others, hyp-
nosis might not be a suitable intervention for every patient with complex pain.
Hypnosis requires active psychological engagement with the patient in order
to be effective; thus, barriers previously mentioned for R&I can likewise in-
terfere with hypnosis.

156 Clinical Manual of Pain Management in Psychiatry
Vocational Rehabilitation
Patients with pain can experience significant losses, including the loss of work.
Beyond the obvious resultant loss of income, and perhaps medical coverage,
the loss of work can imply several other losses for the patient depending on the
meaning of work for that person. For many persons, work is a source of self-
identity, power, influence, and a social network. Loss of work, therefore, can
mean the feared loss of control and a feared loss of usefulness.
Psychiatrists can facilitate pursuit of vocational training, which may be
helpful in helping the patient transition back to the workplace. If the patient is
not returning to his or her prior work, vocational rehabilitation may serve an
Table 6–7.
Techniques of hypnosis
Dissociation The patient is taught to experience another state, place,
or time, such as in a vivid daydream, so as to
dissociate from the awareness of pain.
Time disorientation The patient is taught to distort the perception of time
(e.g., experiencing a recurrent painful sensation as
rapidly passing).
Substitution of sensations The patient is taught to substitute the unpleasant
sensation with another that is more tolerable or
less incapacitating. The technique is most effective
if the substituted sensation is not entirely pleasant
(e.g., substituting a stabbing pain for a pinching
pain).
Displacement The patient is taught to move the painful sensation to
another area of the body. For example, if a pain is
experienced in the patient’s dominant hand,
interfering with work, the patient can learn to
displace the pain to the small finger of the other
hand—and thereby be less adversely affected by the
pain.
Reinterpretation The patient is taught to alter the meaning of the pain
to an interpretation that is better tolerated. The pain
encountered after a burn, for example, can be
reinterpreted as the efforts of the body to restore
healing, to channel soothing fluids to bathe and clean
up damaged cells.

Psychotherapy 157
instructional role, helping the patient develop skills that will be suitable for
other kinds of work. Vocational rehabilitation also may be helpful in fostering
the patient’s independence, autonomy, and self-efficacy and may assist the pa-
tient particularly when there is financial need, ineligibility for disability or
compensation, and the need for insurance and medical coverage. Vocational
rehabilitation can help the patient go beyond the boundaries of his or her ill-
ness. Many state agencies provide vocational and educational services for per-
sons with disabilities that can help patients adapt to a work setting. Within such
programs, the patient’s interests, skills, aptitudes, limitations imposed by
physical and psychiatric conditions, and employment needs are assessed. On
the basis of these assessments, work training and supervision are provided, with
eventual job placement being the ultimate goal.
Key Points
• Effective pain management requires multidimensional approaches. Psy-
chotherapeutic interventions are often necessary for managing pain as well
as its psychiatric comorbidities.
• Psychotherapeutic measures can be useful in the comprehensive manage-
ment of pain to address comorbid psychiatric conditions and subsyndro-
mal psychological states interfering with rehabilitation, and in reducing the
adversity produced by the pain itself.
• Psychotherapeutic techniques employed in multidimensional approaches
to pain can include the following: supportive therapy, behavior therapy,
cognitive-behavioral therapy, dynamic therapy, family/couples therapy,
and group therapy.
• In clinical practice, psychotherapists are likely to invoke elements of the
varied psychotherapeutic approaches to address the patient’s needs.
• Adjuvant approaches, used alone or in combination with psychotherapeu-
tic interventions, include relaxation and imagery training, biofeedback,
hypnosis, and vocational rehabilitation.
• Special needs may arise in the palliative care setting, with attention di-
rected to assisting patients with issues arising from existential concerns and
spiritual issues.

158 Clinical Manual of Pain Management in Psychiatry
References
Affleck G, Tennen H, Urrows S, et al: Individual differences in the day-to-day experi-
ence of chronic pain: a prospective daily study of rheumatoid arthritis patients.
Health Psychol 10:419–426, 1991
Back and Neck Injury/Chronic Pain: Chronic Pain Anonymous. Available at: http://
www.backandneck.miningco.com/library/blchair.html. Accessed June 1, 2002.
Barber TX: The effects of “hypnosis” on pain. Psychosom Med 25:303–333, 1963
Beutler LE, Engle D, Oro-Beutler ME, et al: Inability to express intense affect: a com-
mon link between depression and pain? J Consult Clin Psychol 54:752–759, 1986
Block A, Kremer E, Gaylor M: Behavioral treatment of chronic pain: the spouse as a
discriminative cue for pain behavior. Pain 9:243–252, 1980
Burns JW: Anger management style and hostility: predicting symptom-specific physiolog-
ical reactivity among chronic low back pain patients. J Behav Med 20:505–522, 1997
Carroll D, Seers K: Relaxation for the relief of chronic pain: a systematic review. J Adv
Nurs 27:476–487, 1998
Cogan R, Cogan D, Waltz W, et al: Effects of laughter and relaxation on discomfort
thresholds. J Behav Med 10:139–144, 1987
Compas BE, Haaga DAF, Keefe FJ, et al: Sampling of empirically supported psycho-
logical treatments from health psychology: smoking, chronic pain, cancer, and
bulimia nervosa. J Consult Clin Psychol 66:89–112, 1998
Crawford H, Knebel T, Vendemia JMC: The nature of hypnotic analgesia: neurophys-
iological foundation and evidence. Contemporary Hypnosis 15:22–34, 1998
Daake DR, Gueldner SH: Imagery instruction and the control of postsurgical pain.
Appl Nurs Res 2:114–120, 1989
DeGood DE, Tait RC: Assessment of pain beliefs and pain coping, in Handbook of
Pain Assessment, 2nd Edition. Edited by Turk DC, Melzack R. New York, Guil-
ford, 2001, pp 320–345
Feuerstein M: Ambulatory monitoring of paraspinal skeletal muscle, autonomic and
mood–pain interaction in chronic low back pain. Paper presented at the 7th annual
meeting of the Society of Behavioral Medicine, San Francisco, CA, March 5–8, 1986
Fishman B, Loscalzo M: Cognitive-behavioral interventions in management of cancer
pain: principles and applications. Med Clin North Am 71:271–287, 1987
Fordyce WE, Shelton JL, Dundore DE: The modification of avoidance learning pain
behaviors. J Behav Med 5:405–414, 1982
Fordyce WE, Roberts AH, Sternbach RA: The behavioral management of chronic pain:
a response to critics. Pain 22:113–125, 1985
Hatch JP, Schoenfeld LS, Boutros NN, et al: Anger and hostility in tension-type head-
ache. Headache 31:302–304, 1991

Psychotherapy 159
Hawley DJ, Wolfe F: Anxiety and depression in patients with rheumatoid arthritis: a
prospective study of 400 patients. J Rheumatol 15:932–941, 1988
Keefe FJ, Dunsmore J, Burnett R: Behavioral and cognitive-behavioral approaches to
chronic pain: recent advances and future directions. J Consult Clin Psychol 60:528–
536, 1992
Keefe FJ, Baupre PM, Gil KM: Group therapy for patients with chronic pain, in Psy-
chosocial Approaches to Pain Management: A Practitioner’s Handbook. Edited
by Gatchel RJ, Turk DC. New York, Guilford, 1996, pp 259–282
Kerns RD, Haythornthwaite J: Depression among chronic pain patients: cognitive-
behavioral analysis and effects on rehabilitation outcome. J Consult Clin Psychol
56:870–876, 1988
Kerns RD, Turk DC: Depression and chronic pain: the mediating role of the spouse.
J Marriage Fam 46:845–852, 1984
Krystal H: Alexithymia and the effectiveness of psychoanalytic treatment. Int J Psy-
choanal Psychother 9:353–388, 1982
Kurtz RM, Strube MJ: Multiple susceptibility testing: is it helpful? Am J Clin Hypn
38:172–184, 1996
Lakoff R: Interpretive psychotherapy with chronic pain patients. Can J Psychiatry 28:650–
653, 1983
Mannix LK, Chandurkar RS, Rybicki LA, et al: Effect of guided imagery on quality of
life for patients with chronic tension-type headache. Headache 39:326–334, 1999
McCaffery M, Beebe A: Pain: Clinical Manual for Nursing Practice, 2nd Edition. St Louis,
MO, CV Mosby, 1999
Miller WR, Sanchez VC: Motivating young adults for treatment and lifestyle change,
in Alcohol Use and Misuse by Young Adults. Edited by Howard G, Nathan PE.
Notre Dame, IN, University of Notre Dame Press, 1994, pp 55–82
Morley S, Eccleston C, Williams A: Systematic review and meta-analysis of randomized
controlled trials of cognitive behaviour therapy and behaviour therapy for chronic
pain in adults, excluding headache. Pain 80:1–13, 1999
Orne MT: Mechanisms of hypnotic pain control, in Advances in Pain Research and Therapy,
Vol 1. Edited by Bonica JJ, Albe-Fessard D. New York, Raven, 1976, pp 717–726
Prochaska JO, DiClemente CC: Stages and processes of self-change of smoking: toward
an integrative model of change. J Consult Clin Psychol 51:390–395, 1983
Raz A, Shapiro T: Hypnosis and neuroscience: a cross talk between clinical and cognitive
research. Arch Gen Psychiatry 59:85–90, 2002
Romano JM, Schmaling KB: Assessment of couples and families with chronic pain, in
Handbook of Pain Assessment, 2nd Edition. Edited by Turk DC, Melzack R.
New York, Guilford, 2001, pp 346–361

160 Clinical Manual of Pain Management in Psychiatry
Sanders SH: Operant therapy with pain patients: evidence for its effectiveness, in Sem-
inars in Pain Medicine, Vol 1. Edited by Lebovits AH. Philadelphia, PA, WB
Saunders, 2003, pp 90–98
Scheer SJ, Watanabe TK, Radack KL: Randomized controlled trials in industrial low
back pain, Part 3: subacute/chronic pain interventions. Arch Phys Med Rehabil
78:414–423, 1997
Seligman MEP: Learned Optimism. New York, Pocket Books, 1990
Sholevar GP, Perkel R: Family systems intervention and physical illness. Gen Hosp
Psychiatry 12:363–372, 1990
Silver BV, Blanchard EB: Biofeedback and relaxation training in the treatment of psy-
chophysiological disorders: or are the machines really necessary? J Behav Med 1:217–
239, 1978
Smyser CH, Baron DA: Hypnotizability, absorption, and subscales of the Dissociative
Experiences Scale in a nonclinical population. Dissociation: Progress in the Dis-
sociative Disorders 6:42–46, 1993
Stieg RL, Lippe P, Shepard TA: Roadblocks to effective pain treatment. Med Clin
North Am 83:809–821, 1999
Sullivan MJL, Bishop S, Pivik J: The pain catastrophizing scale: development and
validation. Psychol Assess 7:524–532, 1995
Sullivan MJL, Stanish W, Waite H, et al: Catastrophizing, pain, and disability in patients
with soft-tissue injuries. Pain 77:253–260, 1998
Tauschke E, Merskey H, Helmes E: Psychological defence mechanisms in patients with
pain. Pain 40:161–170, 1990
Turk DC, Meichenbaum DH, Berman WH: Application of biofeedback for the reg-
ulation of pain: a critical review. Psychol Bull 86:1322–1338, 1979
Turner JA, Chapman CR: Psychological interventions for chronic pain: a critical review, I:
relaxation training and biofeedback. Pain 12:1–21, 1982a
Turner JA, Chapman CR: Psychological interventions for chronic pain: a critical review, II:
operant conditioning, hypnosis, and cognitive-behavioral therapy. Pain 12:23–46,
1982b
Weickgenant AL, Slater MA, Patterson TL, et al: Coping activities in chronic low back
pain: relationship with depression. Pain 53:95–103, 1993
Weisberg JN, Keefe FJ: Personality, individual differences, and psychopathology in
chronic pain, in Psychosocial Factors in Pain. Edited by Gatchel RJ, Turk DC.
New York, Guilford, 1999, pp 56–73

161
7
Special Techniques in
Pain Management
There are a number of therapeutic modalities and interventions available for
management of acute and chronic pain (Loeser et al. 2001). The appropriate-
ness of an intervention depends in part on the etiology and nature of the pa-
tient’s pain, the potential risks of the procedure, and the likelihood of bene-
ficial effects. Generally, conservative modalities are employed first. Certain
procedures (e.g., acupuncture, physical therapy) carry relatively fewer risks
than more aggressive interventions (e.g., neurosurgical interventions). However,
if less invasive measures fail, one may advance in terms of the number of con-
current modalities applied and eventually perhaps to those interventions that
carry more risk (see Table 7–1).
It should be noted that highly invasive procedures, although useful, are
not a panacea. For example, back pain patients randomly assigned to lumbar
fusion surgery or noninvasive educational/exercise programs did not differ
significantly at 1-year follow-up on measures of perceived disability, subjective
pain ratings, use of analgesics, and general life satisfaction (Brox et al. 2003).
162 Clinical Manual of Pain Management in Psychiatry
Similarly, comprehensive interdisciplinary treatment programs (largely based
on cognitive-behavioral therapy combined with exercise therapy) produced
effects that were comparable to those of lumbar spine fusion surgery at 2-year
follow-up (Fairbank et al. 2005). Although measures of disability were re-
duced slightly more among the surgically treated patients, there were no signif-
icant differences between the two groups of patients with regard to posttreat-
ment depression or perceived physical and mental health. These data highlight
the beneficial impact of psychologically based therapies (see also Chapter 6,
“Psychotherapy,” of this book) combined with relatively noninvasive proce-
dures in the long-term management of patients with chronic pain. In fact,
such rehabilitative endeavors are likely to be more cost-effective than highly
invasive (and riskier) interventions (Rivero-Arias et al. 2005).
A brief overview of some of these interventions is presented in this chap-
ter. I also discuss the role of psychiatrists in the care of patients who are pur-
suing specialized treatment techniques.
Acupuncture
Acupuncture is among the oldest forms of pain intervention, dating as far
back as 2600 BCE. Its use has been applied to patients with acute and chronic
pain, but the mechanism of action remains unclear. Acupuncture has been a
Table 7–1.
Treatment interventions for management of acute and
chronic pain conditions
Least invasive Moderately invasive Most invasive
Physical therapy Botox injection Neurostimulation
Massage Trigger point injection Implantable drug pumps
Chiropractic
manipulation
Peripheral nerve blocks Neuroablation/neurolysis
Transcutaneous electrical
nerve stimulation
Epidural/intrathecal
blocks
Surgical interventions
Acupuncture Thermal procedures
Transcranial magnetic
stimulationaProlotherapy
aPreliminary evidence only.

Special Techniques in Pain Management 163
source of marked controversy in Western medicine because of the lack of clear
understanding of its physiologic effects. One reason for this lack of under-
standing is that the points of stimulation in acupuncture are derived from an-
cient Chinese beliefs about disease processes and are frequently not related to
known nervous system pathways.
Nonetheless, anecdotal evidence does support that the technique of acu-
puncture, if performed properly, can produce significant physiologic changes,
some of which have pain-mitigating effects (Brown et al. 1974; Han and Tere-
nius 1982; Mayer et al. 1977). These changes can include increasing endorphin
levels within the central nervous system (CNS) (naloxone antagonizes the ef-
fectiveness of acupuncture); augmenting other neurotransmitters, including
dopamine, serotonin, and norepinephrine (use of serotonin-blocking agents
can reduce analgesic effects produced by acupuncture, whereas agents such as
clomipramine may possibly augment acupuncture-induced analgesia); alter-
ation of electroencephalographic and cortical evoked potentials; and other po-
tentially beneficial physiologic effects (e.g., vasodilation). Acupuncture has been
employed in a number of pain states, including headache, musculoskeletal
pains, fibromyalgia, arthritis, bursitis, and synovitis.
Needles (20–100 gauge) are inserted at varying angles through the skin, cor-
responding to the areas that can mediate pain in various parts of the body. Once
the needles are in place, stimulation at the insertion sites is produced by manual
manipulation of the needles (e.g., rotated in place, oscillated, or raised up and
down with a twirling motion) or through electrical stimulation. Electrical stim-
ulation is less labor intensive and allows for more homogenous and consistent
stimulation than manual manipulation techniques.
Fainting, seizures, infection, and pneumothorax (if needles are inserted
into the trunk) are some of the potential complications. Needles can break af-
ter insertion, requiring surgical excision. When electrical currents are applied
to the needle, localized skin burns can result. Patients receiving anticoagulants
may be prone to bleeding and hemorrhage. Patients with rheumatic heart dis-
ease can develop bacterial endocarditis as a complication of acupuncture. Be-
cause of the changes in the vasculature arising from acupuncture, hypovolemic
patients might be particularly prone to syncope. Acupuncture has led to spon-
taneous abortion in the first 3 months of pregnancy and thus should be avoided
in the first trimester.

164 Clinical Manual of Pain Management in Psychiatry
Transcutaneous Electrical Nerve Stimulation
Transcutaneous electrical nerve stimulation (TENS) is a modality employed to
reduce pain in an array of acute and chronic pain states, including labor pain,
postoperative pain, neuropathic pain, and musculoskeletal pain (Loeser et al.
1975; Tyler et al. 1982). When TENS is used in such settings, patients require less
in the way of analgesics (e.g., opiates) and, depending on the circumstances, re-
port increased activity, less interference with work, and improved functional abil-
ities. A TENS unit consists of a battery and electrodes. The electrodes are placed
along the surface of the skin overlying painful areas. The battery generates elec-
trical currents of approximately 100 milliamperes; the rate and width of the pulses
are modifiable by the patient. The efficacy of TENS has been supported by evi-
dence of counterirritation techniques (see also Chapter 2, “Sensory Pathways of
Pain and Acute Versus Chronic Pain,” of this book), whereby stimulation of Aβ
fibers inhibits the pain-promoting activities within the substantia gelatinosa.
Prolotherapy
Prolotherapy is a long-described intervention whereby pain of musculoskele-
tal origin is treated by injection of a dextrose/lidocaine solution into connec-
tive tissue sites that are thought to be related to the genesis and perpetuation of
pain. Prolotherapy has been used in the treatment of discogenic low back
pain; chronic cervical, thoracic, or lumbar pain; osteoarthritis; and failed back
surgery (postspinal surgery) pain (Linetsky et al. 2006). Contraindications in-
clude treatment of patients with pain related to neoplastic processes and
patients requiring large quantities of narcotic analgesics before and after treat-
ment. Prolotherapy may be employed in conjunction with other procedures
(e.g., peripheral nerve blocks).
Botulinum Toxin (Botox) Injection
The neurotoxins produced by Clostridium botulinum exert physiologic effects by
inhibiting release of acetylcholine from nerve terminals. These effects account for
the utility of botulinum injections in alleviating blepharospasm or facial spasm
but not necessarily pain. Analgesic effects of botulinum toxin type A may in-
volve other neurotransmitter influences, including inhibition of glutamate and

Special Techniques in Pain Management 165
substance P. Botulinum toxin (both A and B types) has been invoked in the
treatment of cervical dystonia, migraine headache, tension headache, temporo-
mandibular joint disorders, and chronic back pain (Argoff 2005). Multiple series
of injections may be required to achieve maximal analgesia. Contraindications
include pregnancy, concurrent aminoglycoside antibiotic use (e.g., gentamicin,
tobramycin), myasthenia gravis, Eaton-Lambert syndrome, and known sensi-
tivity to toxins. Clinical resistance brought on by development of antibodies to
toxins may reduce clinical efficacy after repeated administrations.
Anesthesia at the Level of the Spinal Cord
Anesthesia at the level of the spinal cord involves application of anesthetics to in-
fluence spinal or sympathetic nerves, or both, to produce pain relief. Epidural
anesthesia involves application of the anesthetic outside the dura mater—the
dura mater is not punctured (at least not intentionally). Epidural anesthesia has
become the cornerstone of acute pain management (e.g., operative and post-
operative pain). Intrathecal (subarachnoid) application of anesthetics involves
inserting a needle through the dura mater and applying the anesthetic into the
cerebrospinal fluid. Both epidural and intrathecal analgesia are invoked in the
management of patients with chronic pain in whom pharmacologic interven-
tions produce intolerable side effects or are insufficient to control pain.
The subarachnoid administration of anesthetics allows for more direct ac-
cess of anesthetics to pain-relaying and sympathetic nerves. On the other hand,
the epidural space contains areolar (fat) tissue, epidural veins, and lymphatic tis-
sue, into which the anesthetic will diffuse and be absorbed. Thus, epidural ad-
ministration is an indirect means of providing blockade of pain-sensitive nerves
entering the spinal cord. Because of the distance traveled by the anesthetic agent
and absorption of the agent by the fat layer surrounding the dura, anesthetics
introduced in this manner take a longer time to exert a full effect. In addition,
less intense sensory (and motor) blockade results from epidural injection com-
pared with subarachnoid injection. Consequently, in achieving anesthesia
through the epidural technique, generally more anesthetic is required than is the
case for subarachnoid injections. The dura mater is not penetrated or broken
through, so post–spinal-injection headache and meningitis are less likely to oc-
cur with epidural injection. Epidural anesthesia is also less likely to produce car-
diovascular effects that are observed with subarachnoid injections.

166 Clinical Manual of Pain Management in Psychiatry
Neural influences occur in a sequential manner after the anesthetic is in-
troduced. Because fibers are small and unmyelinated and are more susceptible
to the effects of the anesthetic agents than are the larger myelinated fibers,
sympathetic nerves are blocked first. This is followed by involvement of sen-
sory fibers and subsequently motor nerves. Motor fibers tend to be deeper in
the nerves, and, thus, greater diffusion of the anesthetic is required to influ-
ence motor blockade.
Complications associated with subarachnoid and epidural injections are
summarized in Table 7–2. Puncture of the dura mater during an intended epi-
dural injection, referred to as “wet tap,” can produce significant headache. Af-
ter subarachnoid injection, analgesia and paralysis may be produced at levels
higher than intended (i.e., high spinal). In this way, sympathetic innervation
to vital organs arising from thoracic spinal segments can be inadvertently sup-
pressed, resulting in compromised cardiac output and ventilatory mechanics.
Contraindications for both techniques are also outlined in Table 7–2 (Barash
et al. 2001).
Multiple agents can be administered via the subarachnoid and epidural
techniques, including opiates, steroids, α2-adrenergic agonists (e.g., cloni-
dine), and muscle relaxants (e.g., baclofen). Continuous opiate analgesia may
be applied through implantable drug delivery systems inserted through the
epidural or subarachnoid route. These systems are often employed in patients
with cancer pain and are being increasingly explored in chronic, nonmalig-
nant pain. Risks associated with continuous implantable infusions are sum-
marized in Table 7–3.
Regional Neural Blockade
Regional neural blockade (RNB) encompasses a number of techniques whereby
nociceptive inputs of nerve fibers are interrupted (e.g., peripheral or sympa-
thetic nerve blockade). The purpose of RNB is multifaceted. RNB can serve a
diagnostic function, helping to decipher peripheral nerve pain from sympa-
thetically mediated pain. Some procedures may be invoked to provide pro-
phylaxis of future pain after surgical procedures (e.g., to minimize phantom
limb pain postamputation). In addition, RNB techniques may be invoked to
provide therapeutic pain relief in selected disorders, especially in those chronic
pain disorders involving sympathetically mediated pain and complex regional

Special Techniques in Pain Management 167
pain syndrome. At times, RNB is employed to determine the suitability of
other more permanent interventions (e.g., neural destruction techniques).
The types of RNB techniques and the ways in which these are employed, as
well as complications and indications, have been summarized extensively else-
where (Barash et al. 2001).
Table 7–2.
Complications and contraindications of subarachnoid
and epidural analgesia
Complications Contraindications
Subarachnoid
Hypotension
Post–spinal-injection headache
High spinala
Nausea, vomiting
Urinary retention
Backache
Epidural
Unintentional injection of anesthetic
Into subarachnoid space
Into vascular space
“Wet tap”
Local anesthetic overdose
Hypotension
Spinal cord trauma
Epidural hematoma
Absolute
Lack of patient consent
Allergy or sensitivities to local anesthetics
Increased intracranial pressure
Infection at the potential puncture site
Relative
Hypovolemia
Coagulopathy
Sepsis
Progressive neurologic diseases
Chronic back pain
aAnalgesia and paralysis produced at levels higher than intended.
Table 7–3.
Risks of continuous epidural or subarachnoid
anesthetic infusions
Catheter problems
Moving
Kinking
Breaking
Disconnection
Occlusion (by fibrosis in epidural placement site)
Infection
Local infection
Epidural abscess
Meningitis

168 Clinical Manual of Pain Management in Psychiatry
Peripheral Nerve Blockade
When pain is refractory to pharmacologic treatment, peripheral nerve block-
ade may be an option (Raj 1996). Somatic nerve blocks are used in patients
with intractable pain, generally from cancerous invasion of parts of the body,
including the nervous system. At times, these blocks are employed in periph-
eral nerve pain, sciatica, and carpal tunnel syndrome. In addition, peripheral
nerve blocks are employed to provide analgesia during localized surgery so that
the patient can avoid general anesthesia.
Nerve blocks can include anesthetic and ablative modalities (see Table 7–4).
In the case of anesthetic blockade, introduction of local anesthetics (lidocaine or
bupivacaine) allows for interruption of pain transmission, producing effec-
tive, albeit temporary, pain relief. The activity of thin and unmyelinated nerve
fibers (e.g., Aδ and C fibers) is particularly prone to inhibition, requiring min-
imal doses of anesthetic agents. If higher doses are used, it is possible to inhibit
larger myelinated fibers, such as motor neurons. Pain interruption might be of
sufficient duration to allow the patient to become more proactive with phys-
ical therapy, which in turn might set the stage for further health improvements
and rehabilitation. Ablative techniques involve application of ethanol or phenol
or other interventions (see Table 7–4) that result in permanent destruction of
the nerve. Neural destruction is never attempted unless local application of an-
esthetics has first been tried. If local anesthetics fail to produce pain relief, it is
unlikely that neural destruction will be effective in mitigating pain.
Anesthetic infusions can produce untoward effects (e.g., hypotension,
localized numbness, muscle weakness, infection, bleeding). Such measures
should never be attempted unless resuscitation equipment is readily available.
Sympathetic Nerve Blockade
As with peripheral nerve blockade, a number of comparable measures may be
undertaken to reduce pain that is sympathetically mediated. Complex pain syn-
dromes involving the arm, head, and neck might benefit from a stellate gan-
glion block. Similarly, diffuse pain in the lower extremities can benefit from a
lumbar sympathetic block. Visceral pain syndromes (e.g., pain arising from
the pancreas and other upper abdominal organs) can be mitigated by celiac
plexus block; pain originating from pelvic organs can be reduced with hypo-
gastric plexus block (Eisenberg et al. 1995). Such measures may not provide

Special Techniques in Pain Management 169
Table 7–4.
Modalities of regional nerve blockade
Type of intervention Purpose Examples
Anesthetic blockade Use of local anesthetics to interrupt nociceptive
input from peripheral nerves
Interscalene, supraclavicular and infraclavicular,
axillary, sciatic nerve, and lumbar plexus blocks
Neuroablative blockade Use of chemical, thermal, cryogenic, or surgical
modalities applied to induce intentional nerve
injury to reduce pain transmission from the
periphery; often impermanent, lasting 3–6
months
Peripheral nerve ablation for focal pain related to
cancer invasion; sympathetic nerve ablation for
visceral, retroperitoneal, and complex regional
pain syndrome related to cancer

170 Clinical Manual of Pain Management in Psychiatry
complete pain relief. However, even if only partial relief is achieved, this may
be sufficient to allow the patient to lower the amount of opiate analgesics re-
quired, thereby reducing the risks of untoward effects.
Sympathetic nerve blockades are employed in conditions of unremitting
pain arising from the viscera, generally induced by cancer. In some cases, the
use of opiate analgesics and adjuvants proves to be unsatisfactory or intolera-
ble. Types of and uses for autonomic nerve blocks are summarized in Table 7–5.
Neurosurgical Techniques
Neurosurgical techniques consist of anatomic, augmentative (i.e., neural stim-
ulation), and ablative techniques (see Table 7–6). Anatomic interventions are
relatively common interventions that correct defects (e.g., disc herniations)
Table 7–5.
Types of and uses for autonomic nerve blocks
Type of nerve block Uses
Celiac plexus block Pain arising from viscera, secondary to cancer
Pancreas
Alimentary tract (esophagus through transverse
colon)
Hypogastric plexus block Pelvic, vaginal, scrotal pain
Buttock, inguinal pain
Lumbar sympathetic block Complex regional pain syndrome
Neuropathic pain (e.g., postherpetic neuralgia)
Vascular insufficiency
Sphenopalatine ganglion
block
Frontal headache
Cluster headache
Migraine headache
Stellate ganglion block Complex regional pain syndrome
Postherpetic neuralgia
Intractable angina pectoris
Vascular insufficiency (e.g., Raynaud disease,
scleroderma)
Superior mesenteric
ganglion block
Pain arising from viscera, secondary to cancer
Bladder, kidney, ureters
Distal colon, rectum
Prostate, testicle
Uterus

Special Techniques in Pain Management 171
that produce neurologic deficits and pain. Presumably, correction of the ana-
tomic defect will reduce pain and restore neurologic functioning (e.g., weak-
ness due to nerve impingement).
Augmentative, or stimulation, techniques are employed to suppress the
activity of nerves relaying painful information. Inhibition is rather dramatic,
such that when high-frequency, low-amplitude stimulation is applied, dorsal
horn cell activity is inhibited (Meyerson and Linderoth 2001). Stimulation
techniques have been used in chronic, intractable pain affecting the limbs or
the trunk, including conditions such as phantom limb pain, arachnoiditis, neu-
ropathy, and complex regional pain syndrome.
Table 7–6.
Neurosurgical interventions for use in pain
management
Type of
intervention Purpose Examples
Anatomic Reduce compression of nerve
fibers; reduce pain and
improve neurologic effects
Laminectomy
Discectomy
Microvascular decompression
Augmentative Stimulate the neural axis to
reduce pain; can be costly
but is relatively safe and
reversible
Spinal nerve stimulation
Peripheral nerve stimulation
Intracranial stimulation
Ablative Generally, interventions of
last resort, predominantly
patients with terminal
conditions
Peripheral Denervate an area of the
body
Sympathectomy
Neurectomy
Ganglionectomies
Rhizotomy of cranial nerves
Spinal Destroy pain input; relief is
often time limited; there can
be neurologic complications
Dorsal root entry zone
lesioning
Cordotomy
Myelotomy
Brain Disrupt widespread pain
transmission intracranially;
effects are often
short-term
Thalamotomy
Cingulotomy

172 Clinical Manual of Pain Management in Psychiatry
Neural stimulation techniques may be employed within the CNS (i.e.,
through implantable devices inserted within the spinal column to stimulate the
dorsal horn) or peripherally. The peripheral technique is employed to treat neu-
rogenic pain in the limbs; a generator and stimulation electrodes are placed be-
neath the skin, and the electrodes are placed in proximity to the peripheral nerve.
Because spinal cord stimulation is such a complex procedure, this approach
is reserved for those patients whose pain has been refractory to other interven-
tions or for whom other interventions prove intolerable. Stimulation is not
allowable in patients who have a demand cardiac pacemaker, who require mag-
netic resonance imaging in the immediate future, or who have ongoing sub-
stance dependence. In addition, because of the invasiveness of this procedure,
there must be a clearly identified basis for the nature of the pain.
In general, ablative techniques are invoked for severe, refractory pain con-
ditions, particularly in patients with cancer-related pain. Ablative techniques
involve removal or destruction of nerve centers or other components of pain
pathways, at the level of peripheral or cranial nerves (e.g., trigeminal ganglion
ablation), spinal cord, or brain.
Repetitive Transcranial Magnetic Stimulation
An emerging therapy, transcranial magnetic stimulation (TMS), has been dem-
onstrated to be useful in treating a number of conditions involving disturbances
in neural circuitry (i.e., depression, seizures, and other neurologic conditions).
TMS is a technology whereby an electrical current is passed through an insu-
lated circular or figure-eight coil to produce a magnetic pulse. When the coil
is applied to the head, the magnetic pulse is capable of passing uninterrupted
through the skin and skull, and ultimately to the cortex. When a series of
pulses repeated at equal intervals are generated, referred to as repetitive TMS
(rTMS), it is possible to modify neuronal activity locally in focal regions of the
cortex and at distant (i.e., subcortical) sites (Wassermann and Lisanby 2001).
Literature has emerged that suggests that TMS may have potential utility in
treating neuropathic pain. In a review of studies assessing the influence of rTMS
in neuropathic pain, it was noted that rTMS was able to produce pain relief but
often analgesia was brief (Leo and Latif 2007). The influence of rTMS appears to
be best when high-frequency stimulation is applied and when the site of stimula-
tion is focused over the motor cortex (as compared with other cortical sites).
Special Techniques in Pain Management 173
rTMS is a noninvasive strategy with a benign side-effect profile; it is easy
to administer. Although adverse effects are minimal, its practical application
and efficacy for pain treatment have yet to be determined by further empirical
investigation.
Role of the Psychiatrist in Pain Management
Related to Interventions
There are several aspects to the psychiatrist’s role in the care of patients un-
dergoing any of the aforementioned interventions. By spanning preinter-
vention to postintervention (see Table 7–7), the psychiatrist can be helpful in
ensuring that the patient is prepared for the intervention and continues to par-
ticipate in functional restoration measures postintervention.
Table 7–7.
Role of psychiatrists in the care of patients undergoing
interventional pain management
Preintervention role Assess capacity to give informed consent.
Assist patients in
Acquiring information necessary to make informed
decision regarding interventions.
Developing realistic expectations of likely outcomes
derived from varied interventions.
Screen patients for conditions
That preclude undergoing interventions, particularly
those that are highly invasive and/or risky.
That may interfere with yielding optimal benefits
from interventions.
Provide treatment to remediate psychiatric comorbidities
that may (if untreated) preclude, or limit efficacy of,
interventions.
Postintervention role Facilitate patient participation in other facets of the
rehabilitation process.
Assist patients with managing pain, activity limitations,
disappointments arising after an intervention is
pursued.

174 Clinical Manual of Pain Management in Psychiatry
Preintervention Role
Psychiatrists may be enlisted to assess the mental and cognitive abilities of the
patient to give consent to undergo a procedure, particularly if the procedure is
highly invasive. Unlike competency, which requires a judicial determination as
to the person’s possession of the requisite natural or legal qualifications to en-
gage in an endeavor, capacity is determined by the physician (often, although
not exclusively, by a psychiatrist). A capacity assessment essentially determines
the validity of a patient’s decision to undergo or forgo a particular proposed
treatment. Table 7–8 summarizes a reasonable guide for the physician assessing
a patient’s decision-making capacity (Leo 1999). With highly invasive proce-
dures, one must carefully weigh the risks of the proposed procedure against the
potential benefits. The psychiatrist must ensure that the patient has sufficient
cognitive and psychological skills to understand the illness, the proposed in-
tervention, its risks and benefits, and the likely long-term outcomes of a proce-
dure. Inquiries should be directed to the patient, according to the guidelines,
and the patient’s responses should be systematically recorded in the medical
record, preferably as quotations. Failure of the patient to understand, appreciate,
and form rational decisions would suggest that the patient does not have the ca-
pacity to make reasoned decisions regarding the proposed medical treatment.
If a person lacks the capacity to make treatment decisions, it may be incum-
bent on the consulting psychiatrist to remedy barriers to capacity. In some cases,
disturbances in the doctor–patient relationship (i.e., ineffective communica-
tion) might be the sole basis for the lack of capacity. The psychiatrist may be en-
listed to clarify and rectify such communication difficulties. In other cases, a
psychiatric disturbance (e.g., delirium, psychosis, a mood disorder) may be im-
peding the patient’s capacity to give informed consent, but once the condition is
rectified (e.g., through use of medication, therapy) the patient is able to do so.
If the patient lacks capacity and the problem is not remediable, the treating
clinician (other than a psychiatrist) may need to invoke substituted consent. Al-
though states vary as to who may be appointed as a substitute, this role often falls
to spouses, relatives, ethics boards, and so forth. In some situations, a judicial de-
cision of incompetence is required. If so deemed, the patient would have a court-
appointed guardian assigned to make medical decisions on his or her behalf.
Despite possession of a knowledge base suggesting that they possess req-
uisite skills to make decisions, some patients may, nonetheless, harbor overly
optimistic and unrealistic expectations of the outcome of an intervention. In
Special Techniques in Pain Management 175
reality, there are no guarantees of the success of interventions undertaken on
behalf of the patient with chronic pain. Sometimes, patients present with per-
manent, irreversible damage, such that the pain will not be resolved entirely
no matter how effective the intervention is purported to be. It may be pru-
dent, therefore, for psychiatrists to work with the patient in establishing real-
istic expectations regarding selected interventions and treatment goals. It may
become necessary to ascertain the patient’s preparedness for the risks or unto-
ward effects potentially associated with an intervention.
Psychiatrists may be enlisted to assess the patient’s suitability for various
interventions (e.g., identifying whether psychiatric conditions would interfere
with outcome) (Trief et al. 2000). The extent of involvement of a psychiatrist
in determining patient suitability depends in part on the severity/aggressive-
ness of the intervention. The least invasive procedures may not warrant psy-
chiatric prescreening, whereas more invasive procedures (e.g., neurosurgical
interventions) would warrant such evaluation (Nelson et al. 1996). The pres-
ence of major psychiatric disturbances (e.g., psychosis, major mood disorders,
somatoform disorders, suicidality) is likely to predict poor response to surgical
interventions. Patients with ambiguous conditions (i.e., inconsistent physical
findings, poorly defined pain, or pain without neurologic correlates) are not
likely to have successful results with highly invasive interventions (Block et al.
2003). Significant cognitive deficits contraindicate such interventions as well,
because the cognitively impaired patient is not likely to adhere to treatment
Table 7–8.
Psychiatrists’ guide for assessing patient decision-
making capacity
The patient must demonstrate an understanding of each of the following:
1. Current medical condition
2. Natural course of his or her current medical condition
3. Proposed treatment intervention
4. Potential risks and benefits of the proposed treatment intervention
5. Consequences of refusing the proposed treatment intervention
6. Presence of any viable alternatives to the proposed treatment intervention
7. Potential risks and benefits of alternative treatments
Source. Adapted from Leo RJ: “Competency and the Capacity to Make Treatment Decisions:
A Primer for Primary Care Physicians.” Primary Care Companion to the Journal of Clinical Psychi-
atry 1:131–141, 1999. Copyright 1999, Physicians Postgraduate Press. Used with permission.

176 Clinical Manual of Pain Management in Psychiatry
and may lack the wherewithal to mobilize medical assistance if complications
arise. Similarly, refractory substance abuse, deception, and threats to treating
sources that drastic measures will be undertaken if the interventions are not
undertaken may preclude pursuit of invasive interventions (see also the section
“Evaluation of Treatment Suitability” in Chapter 3, “Evaluation of the Pain
Patient,” of this book).
Other factors that may raise concerns about patient suitability for various
interventions may include a history of treatment nonadherence, use of large
amounts of narcotics and/or anxiolytics (raising concerns regarding substance
dependence), and concerns over behaviors suggesting secondary gain (e.g., on-
going litigation issues) (Block et al. 2003). Careful consideration of such factors
might warrant that invasive procedures be delayed until corrective measures can
be put in place (e.g., treatment of comorbid depression or substance depen-
dence). Similarly, psychiatrists may be instrumental in eliciting patient adher-
ence with other related facets to the patient’s treatment. It may be particularly
helpful to engage the patient in weight reduction measures, smoking cessation,
participation in exercise programs, and relaxation training, before attempts are
made at intervening with more aggressive interventions. When undertaken, such
endeavors convey that the patient is taking on a proactive role in his or her re-
habilitation. Patient refusal with such measures may be an indication that ag-
gressive interventions may need to be delayed or perhaps withheld altogether.
Postintervention Role
It may become necessary to assist patients who are disappointed over unachieved
outcomes, or less than optimal results, after an intervention has been under-
taken. Pain relief may be only partial, and there may be residual physical activity
limitations that persist after an intervention, necessitating psychiatric follow-
up. Strong reactions such as alarm (“Something has gone wrong!”), disap-
pointment (“I was hoping I would be better.”), and futility (“Nothing will
help.”) may undermine rehabilitative treatment measures and any potentially
achievable gains. Adjunctive treatment modalities such as hypnosis, relaxation
and imagery, guided imagery, and biofeedback may be helpful in mitigating
pain perception and distress in such cases (see also Chapter 6 in this book).
Psychotherapeutic measures may be helpful in assisting patients with reframing
such reactions to mitigate any potential undermining of rehabilitative endeav-
ors. Ongoing collaborative endeavors between the psychiatrist and physical and

Special Techniques in Pain Management 177
occupational therapists, along with other treating sources, may be essential to
assist the patient in overcoming psychological barriers that may impede func-
tional restoration measures postintervention.
Key Points
• Several interventions are available for management of acute and chronic
pain, ranging from those that carry few risks to those that are more risky and
invasive.
• Interventions warranting consideration for pain management include
acupuncture, transcutaneous electrical nerve stimulation, prolotherapy,
botulinum toxin injection, epidural and spinal analgesia, nerve blocks, and
neurosurgical techniques.
• Patient selection for various interventions is pivotal in achieving efficacy
and positive outcomes. Most chronic pain patients respond best when such
procedures are part of multidisciplinary treatment approaches encompass-
ing physical therapy, psychotherapy, and vocational rehabilitation.
• Psychiatrists may be involved in assessing the patient’s capacity to provide
informed consent for and his or her suitability to undergo interventions
(particularly those that are highly invasive). It may also be necessary to as-
sist patients in developing realistic expectations regarding likely treatment
goals and outcomes.
• Psychiatrists may be enlisted postintervention to assist patients with over-
coming psychological barriers that may impede rehabilitative measures.
References
Argoff CE: Botulinum toxin injections, in Pain Medicine and Management: Just the Facts.
Edited by Wallace MS, Staats PS. New York, McGraw-Hill, 2005, pp 266–272
Barash PG, Cullen BF, Stoelting RK (eds): Clinical Anesthesia, 4th Edition. Philadel-
phia, PA, Lippincott Williams & Wilkins, 2001
Block AR, Gatchel RJ, Deardorff WW, et al: The Psychology of Spine Surgery, 1st
Edition. Washington, DC, American Psychological Association, 2003
Brown ML, Ulett GA, Stern JA: Acupuncture loci: techniques for location. Am J Chin
Med (Gard City N Y) 2:67–74, 1974
Brox JI, Sorenson R, Friis PT, et al: Randomized clinical trial of lumbar instrumental
fusion and cognitive intervention and exercises in patients with chronic low back
pain and disc degeneration. Spine 28:1913–1921, 2003

178 Clinical Manual of Pain Management in Psychiatry
Eisenberg E, Carr DB, Chalmers TC: Neurolytic celiac plexus block for treatment of
cancer pain: a meta-analysis. Anesth Analg 80:290–295, 1995
Fairbank J, Frost H, Wilson-MacDonald J, et al: Randomised controlled trial to com-
pare surgical stabilisation of the lumbar spine with an intensive rehabilitation
programme for patients with chronic low back pain: the MRC spine stabilization
trial. BMJ 330:1233–1238, 2005
Han JS, Terenius L: Neurochemical basis of acupuncture analgesia. Annu Rev Phar-
macol Toxicol 22:193–220, 1982
Leo RJ: Competency and the capacity to make treatment decisions: a primer for primary
care physicians. Prim Care Companion J Clin Psychiatry 1:131–141, 1999
Leo RJ, Latif T: Repetitive transcranial magnetic stimulation (rTMS) in acute and
chronic neuropathic pain: a review. J Pain 8:453–459, 2007
Linetsky FS, Derby R, Miguel R, et al: Pain management with regenerative injection therapy,
in Weiner’s Pain Management: A Practical Guide for Clinicians, 7th Edition. Edited
by Boswell MV, Cole BE. Boca Raton, FL, Taylor & Francis Group, 2006, pp 939–965
Loeser JD, Black RG, Christman A: Relief of pain by transcutaneous stimulation.
J Neurosurg 42:308–314, 1975
Loeser JD, Butler SH, Chapman CR, et al (eds): Bonica’s Management of Pain, 3rd
Edition. Philadelphia, PA, Lippincott Williams & Wilkins, 2001
Mayer DJ, Price DD, Rafii A: Antagonism of acupuncture analgesia in man by the
narcotic antagonist naloxone. Brain Res 121:368–372, 1977
Meyerson BA, Linderoth B: Spinal cord stimulation, in Bonica’s Management of Pain,
3rd Edition. Edited by Loeser JD, Butler SH, Chapman CR, et al. Philadelphia,
PA, Lippincott Williams & Wilkins, 2001, pp 1857–1876
Nelson DV, Kennington M, Novy DM, et al: Psychological selection criteria for im-
plantable spinal cord stimulators. Pain Forum 5:93–103, 1996
Raj PP: Peripheral nerve blocks, in Pain Management: A Comprehensive Review. Edited
by Raj PP. St Louis, MO, Mosby, 1996, pp 200–226
Rivero-Arias O, Campbell H, Gray A, et al: Surgical stabilization of the spine compared
with a programme of intensive rehabilitation for the management of patients with
chronic low back pain: cost utility analysis based on a randomized controlled trial.
BMJ 330:1239–1243, 2005
Trief PM, Grant W, Fredrickson B: A prospective study of psychological predictors of
lumbar surgery outcome. Spine 25:2616–2621, 2000
Tyler E, Caldwell C, Ghia JN: Transcutaneous electrical nerve stimulation: an alterna-
tive approach to the management of postoperative pain. Anesth Analg 61:449–
456, 1982
Wassermann EM, Lisanby SH: Therapeutic application of repetitive transcranial mag-
netic stimulation: a review. Clin Neurophysiol 112:1367–1377, 2001

179
8
Common Pain Disorders
Headache
Headache is among the most prevalent of pain conditions, estimated to ac-
count for 10 million office visits annually in the United States (Collins 1986).
It is the most common reason for lost worker productivity (Stewart et al. 2003).
Evaluation of the patient with headache, and development of treatment strat-
egies, relie primarily on the history, supplemented by physical examination
and judicious use of diagnostic testing when indicated by history and physical
findings (see Table 8–1). Three common benign headache conditions are dis-
cussed briefly below.
Tension Headache
Tension headache is very common, affecting as many as 70% of men and 85%
of women annually. Tension headache can also occur among persons with mi-
graine headache—often confounding diagnostic features—but is not any more
prevalent among persons with migraine headache. Classically, the features of
tension headache involve a pressing and tightening quality that is bilateral and
often described as bandlike or surrounding the head (Welch 2001). The in-

180 Clinical Manual of Pain Management in Psychiatry
Table 8–1.
Evaluation of the headache patient
History
Characteristic features Onset
Te m p o ral p a t t er n s
Duration
Quality of pain
Location
Change in course of one’s customary headache
Associated features
Aggravating factors
Mitigating factors
Factors suggesting
secondary headache
Environmental factors
Head trauma/posttrauma
Infection
Postlumbar puncture
Prior pattern of analgesic use (e.g., medication
overuse and rebound)
Worrisome symptoms/
features
Fever
Weight loss
Altered consciousness
Stiff neck
Weakness, gait disturbance
Abrupt, “split-second” onset
Onset after age 50 years
Other conditions (e.g., HIV, cancer)
Physical examination Blood pressure
Fundoscopic examination
Temporal artery tenderness
Kernig’s/Brudzinski’s sign
Neurologic examination
Diagnostic testing Sedimentation rate
Head computed tomography with/without
contrast
Head magnetic resonance imaging
Lumbar puncture
Magnetic resonance venogram (e.g., suspected
sagittal sinus thrombosis)

Common Pain Disorders 181
tensity of tension headache can vary from mild to moderate in severity. Al-
though tension headache can inhibit activities, it does not prohibit them.
Tension headache is rarely aggravated by routine activities such as walking and
climbing stairs. Generally, tension headache is not accompanied by nausea,
vomiting, or photophobia or phonophobia.
Tension headache is thought to arise from peripheral changes (i.e., myo-
fascial pain sensitivity and increased pericranial muscle activity). It can be
brought on by physical and psychological stress as well as poor posture and er-
gonomic conditions. Emotional factors can contribute to the frequency and
severity of headache. It is speculated that limbic system activity invoked dur-
ing emotional distress might both contribute to muscle contraction and in-
hibit endogenous antinociceptive pathways; however, the exact process remains
unclear (Holm et al. 1986).
Treatment of acute episodes of tension headache includes use of non-
steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen, ketorolac,
ketoprofen, and naproxen (see Table 8–2). There does not appear to be any
indication for muscle relaxants or analgesics containing opiates. Prophylaxis of
tension headache is probably facilitated most by tricyclic antidepressants
(TCAs). A TCA can be used for 4 months. If the frequency of headache is re-
duced significantly, the TCA can be tapered and discontinued. There might
be a need to reinstate the TCA for longer-term use if the headaches recur.
In a recent study, nefazodone was effective in treating patients with chronic
daily headache. However, patients with comorbid depression had better re-
sults from nefazodone than did those who were not depressed (Saper et al. 2001).
Other researchers have reported that patients with chronic daily headache had
reduced symptoms with amitriptyline, nortriptyline, or stress management.
No information was provided about which patients with headache were more
apt to benefit from these measures (Holroyd et al. 2001). Serotonin-selective
antidepressants, mirtazapine, and venlafaxine might also be efficacious in
chronic headache (Ansari 2000; Bendtsen and Jensen 2004; Diamond 1995).
Effective psychotherapeutic modalities include biofeedback training and
relaxation training (see Chapter 6, “Psychotherapy,” of this book). Stress man-
agement therapy and cognitive-behavioral therapy (CBT) may also be helpful
in reducing stress levels, but they are probably most effective when combined
with relaxation or biofeedback training (Holroyd et al. 1977). Characteristics
of patients who are likely to improve with the use of psychotherapeutic treat-

182 Clinical Manual of Pain Management in Psychiatry
ment are summarized in Table 8–3. Of these factors, the most striking is that
those patients in relaxation training who show positive responses by the fourth
session (as measured by muscle level reactivity on electromyogram [EMG]) are
likely to have an excellent response to relaxation or biofeedback training (Bogaards
and ter Kuile 1994). Uncontrolled studies have revealed that dental complica-
tions may predispose some persons to tension headache, and these persons may
Table 8–2.
Treatment of headache
Abortive Prophylactic
Tension headache
NSAIDs Antidepressants
Acetaminophen Tricyclic antidepressants
Aspirin SSRIs (e.g., fluoxetine)
Mirtazapine
Nefazodone
Migraine headache
Triptans (e.g., sumatriptan,
zolmitriptan, naratriptan)
β-Blockers (e.g., propranolol, timolol)
Calcium channel blockers (e.g., verapamil)
Ergotamines Methysergide
NSAIDs Ergotamines
Butorphanol Tricyclic antidepressants
Opiates
Caffeine
Anticonvulsants (e.g., divalproex, gabapentin,
topiramate)
Antiemetics Clonidine
Botulinum toxin injection
Cluster headache
Oxygen inhalation Lithium
Ergotamines Divalproex sodium/topiramate
Tr i p t a ns Calcium channel blockers (e.g., verapamil)
Ergotamines
Methysergide
Steroids
Note. NSAID=nonsteroidal anti-inflammatory drug; SSRI= selective serotonin reuptake in-
hibitor.

Common Pain Disorders 183
respond favorably to occlusal adjustments and splints and therapeutic mastica-
tory exercises. Patients with chronic daily headache are often unable to obtain
much benefit from psychotherapy interventions. For such patients, the combi-
nation of psychotherapy (e.g., CBT) and the judicious use of pharmacologic
interventions is likely to yield better clinical improvements than those achieved
with either treatment alone (Lake 2001; Lipchik and Nash 2002).
Migraine Headache
Migraine headache occurs less frequently than tension headache, affecting ap-
proximately 6% of men and 15%–18% of women. Hormonal influences might
account for these gender differences, because the rates of migraine among pre-
pubescent boys and girls are equal (Silberstein 1992). Classically, migraine
headache can last approximately 4–72 hours. Generally, these headaches are
unilateral, with a pulsating quality. The intensity of migraine headache is moder-
ate to severe (i.e., inhibits and prohibits activities). Migraine headache is often
aggravated by routine activities (e.g., walking, climbing stairs). It is often accom-
panied by photophobia or phonophobia, nausea, and vomiting (Welch 2001).
Migraine headache can be preceded by an aura. Migraine headache with-
out aura is approximately twice as frequent as that with aura. However, both
can occur in the same patient. The variability in the features of migraine head-
ache, even in the same person among different episodes, can complicate the di-
agnosis. Aura precedes migraine headache and develops gradually over several
minutes; it is a transient neurologic symptom and generally does not last beyond
60 minutes. The aura is followed by the headache, generally within 60 minutes.
At times, migraine headache can be preceded by more than one aura, occur-
Table 8–3.
Factors influencing likelihood of response to
psychotherapeutic measures in patients with tension headache
Young age of patient
Shorter duration of headache, compared with chronic headache
Relaxation training that produces at least 50% reduction of activity as shown on
EMG by the fourth session (predictive of an excellent response)
Lower scores on psychometric measures of depression
Patient’s requiring minimal analgesia
Note. EMG=electromyogram.

184 Clinical Manual of Pain Management in Psychiatry
ring in succession. Auras must be distinguished from other neurologic symp-
toms that can signal other disorders (e.g., seizure, transient ischemic attacks).
The commonly encountered auras are listed in Table 8–4. Given that these
symptoms can mimic symptoms of other disorders and can trigger marked pa-
tient and physician distress, psychiatric evaluation of the patient might be re-
quested. Careful assessment of the patient is warranted to differentiate symp-
toms of migraine (e.g., visual or auditory hallucinations) from those of
psychiatric disorders such as schizophrenia or delirium. Differentiating these
features from functional psychiatric disorders is possible by referring to the pa-
tient’s longitudinal history, family history, and presence of cognitive and other
neurologic symptoms. The transience of auras and their temporal relationship
to headache and other migraine features suggest migraine headache instead of
functional psychiatric disorders. The clinician is cautioned against hastily ini-
tiating antipsychotic medications and thus failing to appropriately evaluate
and treat the migraine condition. Of most concern is that other serious condi-
tions (e.g., transient ischemic attacks) may be dismissed as reflecting func-
Table 8–4.
Common auras associated with migraine headache
Visual
Flashing lights
Fortification spectra
Scotomata
Hemianopsia
Visual hallucinations
Auditory
Abnormal auditory sensations (e.g., tinnitus)
Auditory hallucinations
Decreased hearing
Olfactory
Abnormal olfactory sensations
Olfactory hallucinations
Sensory
Paresthesia
Vertigo
Motor
Weakness

Common Pain Disorders 185
tional psychiatric disturbances, and workup and appropriate treatment (e.g.,
with clopidogrel) might be overlooked.
Common precipitants of migraine headache include alcohol use, emotional
distress, and menstruation. It is interesting to note that migraine headache fre-
quency appears to decline during pregnancy and menopause, supporting the
notion that hormonal factors have an impact on migraine headache. However,
hormone therapy during menopause can prolong the migraine condition even
after menopause (Epstein et al. 1975). Certain psychiatric disorders (e.g.,
childhood somnambulism) may possibly predict adulthood migraine head-
ache. Other common psychiatric comorbidities among migraineurs include
depression, anxiety disorders (e.g., generalized anxiety and panic disorder), and
bipolar disorder (particularly bipolar II disorder) (Breslau et al. 2003; Fasmer
2001; Zwart et al. 2003).
Treatment of migraine headache depends on the frequency and severity of
the headaches, the presence of neurologic symptoms, and the impact of poten-
tial interventions on the patient’s lifestyle. Headaches occurring once per month
or less might require only abortive therapies, whereas those of greater frequency
may possibly require prophylactic interventions (see Table 8–2). Agents cus-
tomarily employed for abortive therapy include isometheptene mucate (a sym-
pathomimetic amine used to constrict dilated cranial and cerebral arterioles), er-
gotamines, triptans, NSAIDs, and, in refractory cases, intravenous lidocaine.
Isometheptene is fairly well tolerated, producing fewer gastrointestinal side
effects that often accompany other interventions. NSAIDs might be poorly tol-
erated, because they can aggravate the nausea and vomiting that often accom-
pany migraine. Sumatriptan has high efficacy and quick onset when adminis-
tered subcutaneously but works more slowly when administered by intranasal or
oral routes. However, the effectiveness of subcutaneous sumatriptan is brief,
often necessitating a repeated dose. Generally, sumatriptan, rizatriptan, and zol-
mitriptan are rapidly effective but short-acting agents. If a longer duration of an-
algesia is required, a longer-acting triptan (e.g., eletriptan, frovatriptan, nara-
triptan), can be employed (Goadsby 1998; Goadsby et al. 2002).
Prophylactic headache treatment strategies are listed in Table 8–2. The
pharmacologic agents listed are intended to reduce the morbidity and disabil-
ity associated with recurrent migraine headache. Agents with moderate to high
efficacy with minimal side effects include amitriptyline, divalproex sodium,
gabapentin, propranolol, and timolol (Silberstein 2000).

186 Clinical Manual of Pain Management in Psychiatry
Among psychotherapeutic interventions, relaxation training, biofeedback,
and CBT are all somewhat effective in the prophylaxis of migraine headache
(Campbell et al. 2002). However, no one modality has been demonstrated to
be any more efficacious in migraine headache than any other. Certainly, inte-
grated or combination modalities might prove to be effective for some patients
(Lake 2001).
Cluster Headache
Cluster headache is significantly less common than either tension headache or
migraine headache. Gender is a factor in cluster headache, with a marked prev-
alence among males. Cluster headache begins in one’s 30s or 40s. The pain oc-
curs in a series of attacks spanning weeks to months, with pain-free intervals of
months to years. During the active period, headache can occur daily or every
other day. Typically, the pain is unilateral, located in the ocular, frontal, and/or
temporal areas (Welch 2001). The headache can last anywhere from 30 minutes
to 2 hours, and the pain can be severe and excruciating, often arising 2–3
hours after retiring or resulting in early morning awakening. Associated fea-
tures include conjunctival injection, lacrimation, stuffiness of the nose on the
affected side, rhinorrhea, and ipsilateral ptosis and miosis.
Alcohol use and increased altitude are possible precipitants of cluster head-
ache. Treatment (see Table 8–2) includes oxygen as the primary abortive agent,
applied at 10 L/minute via nasal cannula. Prophylactic interventions can in-
clude ergotamines, calcium channel blockers, divalproex, methysergide, and
lithium carbonate. Although lithium (600–900 mg daily) has been employed in
the prophylaxis of chronic cluster headache, a double-blind, placebo-controlled
trial found that lithium was not effective for prophylaxis of episodic cluster
headache (Steiner et al. 1997). In such cases, calcium channel blockers or cor-
ticosteroids may be better alternatives. Note that the combination of lithium
and NSAIDs would be deleterious, raising the risk of severe lithium toxicity.
Clearly, avoidance of alcohol can constitute a prophylactic intervention.
Back Pain
Back pain has become one of the most common, and simultaneously one of
the most elusive, chronic pain disorders. Estimates suggest that United States
health care costs expended for low back pain approximate $50 billion annu-

Common Pain Disorders 187
ally. Without a doubt, back pain results in significant morbidity and consti-
tutes the leading cause of long-term disability in the United States (Atlas and
Deyo 2001).
Clinicians treating patients with back pain often are confronted with a per-
plexing differential diagnosis. Common causes of back pain include those
listed in Table 8–5. Back pain may emanate from injury in a number of body
areas (e.g., the vertebrae, facet joints, nerve roots, muscle, connective tissue).
In 60% of back pain patients, there is often no direct relationship between
physical findings discovered on physical examination or diagnostic testing
and the patient’s perceived level of pain, disability, and psychological distress
(Reesor and Craig 1988). Patients whose back pain appears disproportionate
to the level of pathology noted on examination have a marked propensity to-
ward depression and maladaptive cognitive patterns compared with those with
clear pathology for their pain. Such patients are particularly prone to catas-
trophizing as a maladaptive cognitive approach.
Patients with chronic back pain often feel frustrated by their lack of relief
and may feel helpless and sometimes hopeless. Negative attitudes and beliefs
that these patients have that might contribute to affective associations with
the pain include a sense of loss over aspects of one’s life resulting from the pain.
The medical system becomes a fundamental aspect of one’s life, which can be
perplexing and troubling to patients, who often become frustrated with inef-
Table 8–5.
Common causes of back pain
Intervertebral disc rupture or herniation
Meningeal irritation
Infection
Tu m or
Bleeding
Arachnoiditis
Tr a um a
Prior back surgery
Facet injury
Lumbar ligament injury
Neoplasm
Spinal cord involvement (extremely dangerous)
Cauda equina syndrome (extremely dangerous)

188 Clinical Manual of Pain Management in Psychiatry
fective treatment strategies and extensive diagnostic testing. At times, physi-
cians may struggle with the lack of clear identifiable causes of the pain or may
become frustrated with the inability to arrive at a reasonable treatment strat-
egy. This struggle possibly may be communicated to the patient, who might
perceive the physician’s approach as an endorsement of not taking the patient
seriously (Walker et al. 1999).
Patients with back pain have demonstrated marked back muscle tension
on EMG when discussing situations that were personally distressing, com-
pared with other patients with chronic pain or healthy control subjects. Ad-
ditionally, patients with back pain have demonstrated a protracted latency to
recover or resume normal back muscle tone on EMG after cessation of the dis-
cussion (Flor et al. 1985). Distress caused or exacerbated by such discussions
contributes to physiologic changes that potentially aggravate back pain.
Exercise is a fundamental aspect of treatment of patients with back pain.
Patients who are encouraged by their physicians to remain active and resume
their usual activity levels will have reduced disability and will return to work
sooner (Waddell et al. 1997). The longer patients are out of work, the harder it
is for them to return (McGill 1968). Surgical intervention might be required
for structural and mechanical causes (e.g., disk herniation, spinal nerve en-
croachment). Nerve blocks, epidural anesthetics, or spinal anesthetics might
also provide relief (see Chapter 7, “Special Techniques in Pain Management,”
in this volume), particularly if back pain is accompanied by radicular pain.
Medications to consider for treatment of back pain are summarized in
Table 8–6. Antidepressants do not appear to have any analgesic effect greater
than placebo in patients with chronic back pain (Turner and Denny 1993). How-
ever, they could be useful for patients with comorbid depression or anxiety
disorders that complicate the patients’ coping, adaptation, and rehabilitation
process (Deyo and Weinstein 2001). Brief courses of CBT have been effica-
cious in reducing perceived disability and facilitating return to work in patients
with chronic low back pain (Turner 1996).
Nonarticular Pain Disorders
Myofascial Pain
Myofascial pain refers to a syndrome in which the patient complains of pain in
nonarticular regions of the body that originates from muscle and is precipitated

Common Pain Disorders 189
by muscle trigger points. The nature of the pain can simulate other disorders
(e.g., trigger points in trapezius and cervical muscles can produce headache pain,
and those in the paravertebral muscles can produce low back pain). Identifi-
cation of trigger points is essential in diagnosing myofascial pain.
Trigger points are taut bands, palpable in muscles, that are approximately
1 cm or more across and that can roll beneath or between the fingers. Taut bands
of this kind can be present both in symptomatic pain–generating muscles and
in nonpainful muscles. The bands are tender, producing severe radiating pain
on palpation. When stimulated, the taut bands contract briskly, producing
a twitch. There may be commensurate restriction in range of motion of the
limb containing the affected muscles. Pathologic investigations have not con-
sistently demonstrated specific pathologic changes associated with trigger
points.
Treatment of trigger points is possible with physiotherapy, specifically
stretching and passive manipulation. Heat therapy or cryotherapy (i.e., appli-
cation of cold) or application of transcutaneous electrical nerve stimulation
(TENS) may also be a useful adjunct to treatment.
Fibromyalgia
Fibromyalgia, by contrast, requires the presence of multiple tender points for
diagnosis. These points are located on both sides of the body, above and below
the waist. Failure to meet the requisite number and distribution of tender points
Table 8–6.
Treatment options for back pain
Exercise
Flexibility exercises
Range-of-motion exercises
Aerobic exercises
Muscle strengthening exercises (e.g., of abdominal muscles)
Return to activity
Medications
Nonsteroidal anti-inflammatory drugs, acetaminophen
Tramadol, opioid analgesics
Antidepressants (primarily for mood disturbances)
Other interventions
Surgery
Spinal cord stimulation
190 Clinical Manual of Pain Management in Psychiatry
required for fibromyalgia leads to a diagnosis of myofascial pain syndrome.
Controversy surrounds the diagnosis of fibromyalgia. The American College
of Rheumatology (ACR) criteria for the diagnosis of fibromyalgia are listed in
Table 8–7 (Wolfe et al. 1990). Some researchers and clinicians consider the di-
agnostic criteria to be too restrictive, requiring identification of a specific num-
ber and location of tender points to establish the diagnosis. Yet many patients
experience tender points in other regions of the body that are not included in
the “acceptable” locations defined by the ACR. However, some physicians have
considered fibromyalgia to be no more than a rheumatologic rubric for so-
matoform disorders (Hadler 1997).
The prevalence of fibromyalgia varies depending on the populations un-
der study. In general medical clinics, the rate of fibromyalgia is approximately
5%–10%, whereas in rheumatology practices, the rate is approximately 15%.
Estimates of fibromyalgia in the general population are approximately 2%.
The prevalence appears to increase with age and is higher among women (3%)
than men (0.5%).
Table 8–7.
Diagnostic criteria for fibromyalgia
The pain is widespread and of 3 months’ duration.
Pain consists of axial pain, on both the right and left sides of the body, and above
and below the waist. Pain must be present in at least three segments of the body.
There must be at least 11 tender points (of the critical 18) on digital examination
(approximate force of 4 kg):
Insertion of the suboccipital muscles into the occiput
Upper border of the midportion of the trapezius
Muscle attachments to the medial scapular border
Anterior aspects of the C5 and C7 intertransverse spaces
Second rib space at the costochondral junctions (approximately 3 cm from the
sternal border)
Muscle insertions 2 cm distal to the lateral epicondyle
Upper outer quadrant of the gluteal muscle
Muscle attachments posterior to the greater trochanter
Medial fat pad proximal to the knee joint
Source. Adapted from Wolfe F, Smythe HA, Yunus MB, et al.: “The American College of Rheuma-
tology 1990 Criteria for the Classification of Fibromyalgia: Report of the Multicenter Criteria Com-
mittee.” Arthritis and Rheumatism 33:171, 1990. Copyright 1990 John Wiley & Sons. Used with
permission.
Common Pain Disorders 191
Patients with fibromyalgia suffer immensely; additional features of the dis-
order are summarized in Table 8–8. In addition to muscle pain, some patients
may experience joint pain and stiffness. Fatigue is prominent as a result of the
deconditioning that results from chronic pain and the patient’s attempts to
reduce pain by rest. Other factors causing or contributing to fatigue include
poor sleep, depression, and accompanying endocrine abnormalities (i.e., ab-
normalities in the hypothalamic-pituitary axis).
Sleep disturbances are characterized by disordered, poorly restorative sleep.
Patients with fibromyalgia may be characteristically light sleepers, with a pro-
pensity to frequent arousals precipitated by noise, disruptions in the environ-
ment, or psychological distress. Some patients with fibromyalgia have alpha
rhythm intrusions into Stage III and Stage IV sleep (Cohen et al. 2000). Dur-
ing these stages, there are normally slow-wave patterns (on electroencephalo-
gram), and it is a time when restorative functions within the body are under-
taken. Alpha rhythms signal a heightened arousal and suggest easy arousability
and a diminution of normal restorative functions. Alpha rhythm intrusions
are not exclusive to fibromyalgia but may occur in a number of other chronic
pain disorders.
Sleep disturbances can also arise from restless legs syndrome (i.e., unusual
sensations such as tingling, itching, or cramping that occur in the lower ex-
tremities with reclining but that are alleviated with leg movement, stretching,
or walking). Although movement provides relief, it interferes with sleep. Rest-
less legs disturbances may occur in as many as 30% of fibromyalgia patients.
Table 8–8.
Features associated with fibromyalgia
Pain
Fatigue
Disordered sleep
Cognitive dysfunction
Dizziness
Psychological distress
Restless legs syndrome
Irritable bowel syndrome
Irritable bladder
Cold intolerance
Neurally mediated hypotension

192 Clinical Manual of Pain Management in Psychiatry
Irritable bowel symptoms (constipation, diarrhea, or alternating consti-
pation and diarrhea) along with abdominal pain and distention can occur in
patients with fibromyalgia. Similarly, irritable bladder (dysuria, urgency, fre-
quency in the absence of urinary tract infection or cystitis) may occur.
Paradoxical hypotensive reactions may arise resulting from catecholamine
surges. Venous pooling reduces ventricular filling, resulting in lowered blood
pressure. The heart rate is normally increased to compensate for the reduced
pressure. During catecholamine surges, ventricular contractions increase so
much that the ventricles have insufficient time to fill. Vagal reflexes are initi-
ated, precipitated by ventricular mechanoreceptors, leading to syncope or near-
syncope experiences.
Patients with fibromyalgia commonly experience psychological distress in
the form of depression and anxiety. Depression is common in patients with
fibromyalgia, but the rate of fibromyalgia is not necessarily higher among de-
pressed patients. Other common disorders accompanying fibromyalgia include
somatization disorders and pain disorders. High rates of childhood sexual abuse
are seen in patients with fibromyalgia. Such experiences might contribute to dis-
turbances in coping strategies commonly encountered in these patients.
Many studies have assessed the efficacy of various treatment approaches
for patients with fibromyalgia. Many of these studies, however, are limited by
short durations of follow-up of treatment efficacy, inadequate blinding, and
small sample sizes. A summary of treatment measures available for patients with
fibromyalgia, based on the more empirically rigorous investigations available to
date, is listed in Table 8–9 (Goldenberg et al. 2004). Exercise is probably the
most effective treatment. A variety of exercises can be undertaken to enhance
muscle tone and reduce deconditioning, and aerobic activity can improve sleep
and reduce cold intolerance. However, high-impact activities (e.g., jogging)
may exacerbate pain, and thus low-impact exercises (e.g., elliptical exercise
machines) might be better tolerated. Treatment of pain may be attempted
with the use of antidepressants, which may also reduce comorbid depression.
Low doses of antidepressant are generally required for pain, whereas the usual
antidepressant doses might be required for comorbid mood or anxiety disorders.
Some clinicians advocate the use of tramadol, and opiate analgesics might be re-
quired, at least temporarily. Treatment options for associated features of fibro-
myalgia (e.g., restless legs syndrome) are listed in Table 8–10.

Common Pain Disorders 193
A multidisciplinary approach to pain management is advocated for pa-
tients with fibromyalgia. Involvement of psychiatrists, rheumatologists, and
physiatrists might best lead to a comprehensive treatment approach. Group
therapy can be very helpful for these patients. The context of such groups may
reduce the sense of isolation that can occur in patients with a debilitating dis-
order, may foster group problem solving, and allows for the sharing of effec-
tive treatment strategies among peers. Demands on medical resources could
be reduced by patients’ participation in such groups.
Table 8–9.
Summary of treatment approaches for fibromyalgia
based on strength of evidence in the available literature
Strength of evidence Pharmacologic Nonpharmacologic
Strong Amitriptyline
Cyclobenzaprine
Cardiovascular exercise
CBT
Group educational sessions
Moderate Tramadol±acetaminophen
Fluoxetine
Duloxetine
Venlafaxine
Milnacipran
Pregabalin
Strength training
Acupuncture
Biofeedback
Hypnosis
Weak Growth hormone
5-Hydroxytryptamine
S-Adenosylmethionine
Massage
Ultrasound
Chiropractic therapy
No evidence Opioids
NSAIDs
Benzodiazepines
Thyroid hormone
Calcitonin
Dehydroepiandrosterone
Magnesium
Tender point injections
Note. Strength of evidence for treatment efficacy: strong=positive results from a meta-analysis or
from more than one randomized controlled trial (RCT); moderate=positive results from one RCT,
largely positive results from multiple RCTs, or consistently positive results from multiple non-
RCTs; weak=positive results from descriptive and case studies, inconsistent results from RCTs, or
both. CBT=cognitive-behavioral therapy; NSAID=nonsteroidal anti-inflammatory drug.
Source. Adapted with permission from Goldenberg DL, Burckhardt C, Crofford L: “Management
of Fibromyalgia Syndrome.” Journal of the American Medical Association 292:2388–2395, 2004.
Copyright 2004, American Medical Association.

194 Clinical Manual of Pain Management in Psychiatry
Osteoarthritis and Rheumatoid Arthritis
In addition to the nonarticular rheumatologic conditions discussed previously, a
number of articular disorders warrant the attention of the pain specialist. Among
these, osteoarthritis and rheumatoid arthritis are among the most common. In
the United States, as many as 40 million persons are affected by arthritis and
musculoskeletal conditions.
Osteoarthritis (degenerative joint disease) is the most common form of ar-
thritis and is the most prevalent articular disease affecting elderly persons. The
condition results from destruction of joint cartilage by chondrocytes and af-
fects multiple joints, including the distal interphalangeal joints, the proximal
interphalangeal joints, spine, hip, and knees, but rarely wrist, shoulder, or
metacarpal-phalangeal joints. Weight-bearing joints are most apt to be af-
fected. Osteoarthritis may arise from primary joint dysfunction, involving the
synovial capsule, or it can arise from secondary processes (e.g., prior injury or joint
trauma). Symptoms of osteoarthritis can be very distressing and may include
pain, joint stiffness (especially after inactivity), swelling, deformity, and ulti-
mate loss of function. Because of joint degeneration, osteoarthritis causes sig-
nificant morbidity, accounting for substantial work disability in persons over
age 50 years.
Treatment endeavors should include weight loss, exercise (especially of the
knee joints), and analgesics. Weight loss becomes difficult for many patients, par-
Table 8–10.
Pharmacologic treatment strategies for
comorbidities of fibromyalgia
Anxiolytics (for anxiety)
Antidepressants (for pain, comorbid depression)
Dopamine agonists (e.g., ropinirole, pramipexole, carbidopa-levodopa 10/100 at
bedtime [first choice for restless legs syndrome]); clonazepam 0.5–1.0 mg at bedtime
is an alternative for restless legs syndrome
Serotonin (5-HT4) agonists (e.g., tegaserod [for constipation-type irritable bowel])
Serotonin (5-HT3) antagonists (e.g., alosetron [for diarrhea-type irritable bowel])
Antispasmodics (e.g., Donnatal or dicyclomine [for irritable bowel syndrome] or
oxybutynin [for irritable bladder])
Fludrocortisone (for neurally mediated hypotension)
Sedative-hypnotics

Common Pain Disorders 195
ticularly because patients tend to minimize activity levels so as to avoid pain.
Thus, they run the risk of becoming deconditioned and less capable of sus-
taining aerobic activity and physical fitness. Pain relief can be brought forth by
any of the NSAIDs. Celecoxib might be better tolerated because of its reduced
propensity to interfere with gastric and renal functioning (see also Chapter 5,
“Pharmacology of Pain,” of this book for adverse effects associated with
NSAIDs and cyclooxygenase-2 inhibitors). These and other medications to
employ in the treatment of osteoarthritis are summarized in Table 8–11.
Rheumatoid arthritis, on the other hand, is an inflammatory process af-
fecting the synovium of articular joints and producing a number of systemic
manifestations. Rheumatoid arthritis affects approximately 1%–2% of the pop-
ulation, with about two to three times as many women affected as men. The
joints affected by rheumatoid arthritis include the hands, wrists, elbows, ankles,
Table 8–11.
Treatment options for patients with osteoarthritis
Medication
Celecoxib
Nonsteroidal anti-inflammatory drugs
Steroids
Topical agents
Capsaicin
Methyl salicylate creams
Intra-articular injections
Glucocorticoids
Hyaluronic acid
Nonpharmacologic interventions
Physical therapy
Weight loss
Surgical management
Arthroplasty
Arthroscopic removal of loose bodies
Correction of anatomic defects (spondylosis, spinal stenosis)
Emerging trends
Exogenous growth factors (to stimulate chondrocyte proliferation)
Transplantation of healthy chondrocytes, engineered to maximally produce
growth factors

196 Clinical Manual of Pain Management in Psychiatry
feet, and cervical spine. The joints may be swollen and tender, and over time
there occur significant deformities of the hands, including ulnar deviation and
the swan-neck and boutonniere deformities of the fingers. Patients with rheu-
matoid arthritis experience a number of systemic problems arising from the
inflammatory processes of the disease, including subcutaneous nodules, anemia,
vasculitic processes, entrapment neuropathy, interstitial nephritis, and effu-
sions (pericardial and pleural). Treatment endeavors directed at patients with
rheumatoid arthritis are listed in Table 8–12.
Because there is so much loss, disability, and discomfort accompanying ar-
thritic conditions, it is not surprising that significant mood disturbances can
accompany the disorder. Depression appears to be the most prevalent psycho-
logical disturbance accompanying osteoarthritis and rheumatoid arthritis. Pain
severity among patients with arthritis was found to be correlated with the pres-
ence of depression—higher among those who were depressed, compared with
Table 8–12.
Treatment options for patients with rheumatoid
arthritis
Anti-inflammatory agents
Nonsteroidal anti-inflammatory drugs
Celecoxib
Corticosteroids
Disease modifying antirheumatic drugs
Leflunomide
Soluble interleukin-1 receptor therapy
Anakinra
Tumor necrosis factor inhibitors
Etanercept
Infliximab
Methotrexate
Hydroxychloroquine
Sulfasalazine
Intramuscular gold
Cytotoxic agents
Cyclosporin A
Azathioprine
Cyclophosphamide

Common Pain Disorders 197
nondepressed patients or those with only a remote history of depression (Frank
et al. 1988). The relationship between pain severity and mood disturbances is
not exclusive to depression, however. Ratings of pain severity have also been
associated with other unpleasant emotional states, such as anger and anxiety
(Huyser and Parker 1999). In addition, functional impairments and perceived
disability associated with arthritic conditions are likewise related to these
emotional states. Depression and anxiety may interfere with treatment adher-
ence (e.g., lack of participation in an exercise program and weight loss). Con-
sequently, psychopharmacologic agents, although not directly analgesic in ar-
thritic conditions, may indirectly reduce emotional distress and perceived
functional impairments and thereby reduce perceived pain severity and facil-
itate treatment.
Psychological variables may mediate levels of pain and disability as well.
Cognitive approaches—including a propensity to catastrophize, overgeneral-
ize, or selectively abstract—appear to be related to perceived levels of distress
and disability associated with arthritic conditions (Smith et al. 1988). Patients
with passive coping strategies (e.g., wishful thinking, self-blame) have been
found to have poorer functional abilities than those with more active, prob-
lem-solving approaches (Young 1992). Thus, psychotherapies (e.g., CBT)
might be particularly advantageous in improving functional adaptations in pa-
tients with arthritic conditions by addressing cognitive distortions and foster-
ing improved coping strategies.
Neuropathic Pain
Neuropathic pain is often confusing, in part because the causes of neuropathic
pain are often unclear. In addition, treatment of neuropathic pain can be diffi-
cult, and the pain can progress to the point of causing complete disability.
Consequently, patients and physicians alike can be frustrated with neuropathic
pain, being overwhelmed by its effects and disgruntled with its relatively refrac-
tory quality.
Neuropathic pain is associated with features of unusual sensations in an
area of the body that intensify over the course of the day and over time. Typ-
ically, patients describe the pain as constant burning or electric in quality in a
body area that was previously injured but that demonstrates no ongoing dam-
age (Rowbotham 1995). The pain often emanates from areas of the body that

198 Clinical Manual of Pain Management in Psychiatry
on physical examination demonstrate sensory loss. The condition is charac-
terized by allodynia (i.e., pain produced by normally nonnoxious stimulation)
and hyperpathia (i.e., exaggerated pain response to a noxious stimulus). At
times, physical signs commensurate with sympathetic nervous system involve-
ment are present.
Neuropathic pain can arise from a number of sources (see Table 8–13). Aδ
and C fibers are neurons responsible for mediation of pain (see Chapter 2,
“Sensory Pathways of Pain and Acute Versus Chronic Pain,” of this book). Pro-
cesses that may aggravate the pain-relaying Aδ and C fibers include nerve im-
pingement (e.g., carpal tunnel syndrome, tumor impingement on brachial or
lumbar plexus, disk herniation compressing adjacent nerve roots). Trigeminal
neuralgia is attributed to localized compression of the trigeminal nerve by
neighboring vascular structures. For these conditions, nerve conduction stud-
ies might reveal delays in the conduction velocities between the affected (pain-
ful) side and the unaffected side. The EMG might also reveal concomitant weak-
ness when the motor components of the nerve are adversely affected. Computed
tomography or magnetic resonance imaging may detect the affected nerves.
Treatment is directed at relieving the underlying compression or nerve irrita-
tion (e.g., decompression of the median nerve, use of anticancer drugs, radi-
ation therapy, splinting the affected limb, use of corticosteroids). Nerves that
are damaged by illness or trauma can lead to firing of neurons at ectopic sites
in a way that relays pain. For example, large-diameter fibers (A fibers) are dam-
aged in disease states such as diabetes mellitus; when this happens, C fibers fire
unabatedly, relaying chronic pain in the distribution of the affected nerve.
Peripheral nociceptors relay information from the skin, joints, muscles,
bone, organs, and so forth. The fibers synapse on second-order neurons within
the spinal cord. These, in turn, relay information to third-order neurons
within the thalamus. Damage to peripheral fibers can actually lead to sponta-
neous firing in second- or third-order neurons in the pain-mediating process.
This firing may be perceived as painful. Central nervous system lesions (e.g.,
stroke, arteriovascular malformations) may also produce pain in the pain trans-
mission pathway.
Treatment strategies employed for neuropathic states are summarized in
Table 8–14 (see also Chapter 5 of this book). The mainstays of treatment in-
clude TCAs, anticonvulsants, antiarrhythmics, and topical agents (Argoff et

Common Pain Disorders 199
al. 2006; Galer 1995; Leo 2006). Treatment should be initiated early in the
course of illness for optimal results (e.g., when amitriptyline is initiated within
3 months of developing the rash of herpes zoster infection, patients are less
likely to develop the complications of postherpetic neuralgia) (Bowsher
1997). Restriction of and delays in the efficacy of TCAs in producing analge-
sia would be expected if they are administered after significant peripheral and
central pathophysiologic mechanisms have set in. There are emerging data
suggesting that anticonvulsants (e.g., pregabalin, gabapentin) may likewise
have a preemptive analgesic role (Dahl et al. 2004). Other treatment interven-
tions that may mitigate the pain experience include TENS, biofeedback, re-
laxation training, and hypnosis.
Table 8–13.
Sources of neuropathic pain
Anatomic conditions causing neuropathy
Entrapment neuropathy (carpal tunnel syndrome, ulnar entrapment, others)
Trigeminal neuralgia
Medical conditions causing neuropathy
Diabetic neuropathy
HIV-related neuropathy
Malignancy
Postherpetic neuralgia
Rheumatologic conditions producing neuropathy (rheumatoid arthritis,
Sjögren’s syndrome, systemic lupus erythematosus)
Toxin-induced neuropathy
Alcohol
Arsenic
Cisplatin
Dideoxynucleoside
Paclitaxel
Vincristine
Guillain-Barré syndrome
Fabry’s disease
Vasculitic neuropathy
Amyloid neuropathy
Idiopathic distal small-fiber neuropathy

200 Clinical Manual of Pain Management in Psychiatry
Sympathetically Mediated Pain: Complex
Regional Pain Syndromes
Complex regional pain syndromes (CRPSs) comprise two types of disorders:
CRPS I (previously referred to as reflex sympathetic dystrophy) and CRPS II
(previously referred to as causalgia). CRPS I results from some inciting event,
often a traumatic, infectious, or vascular event within soft tissue, whereas
CRPS II arises from direct nerve injury (Bonica 1973). In either case, the in-
citing event induces a series of responses that are mediated by the sympathetic
Table 8–14.
Treatment strategies for patients with neuropathic
pain
Anticonvulsants Gabapentin 900–3,600 mg/day
Pregabalin 300–600 mg/day
Carbamazepine 600–1,200 mg/day
Divalproex sodium 750–2,500 mg/day
Clonazepam 0.5–3 mg/day
Opiates Methadone 5–100 mg/day
Oxycodone controlled-release 10–60 mg q 12 hours
Tramadol 50–400 mg/day
Antidepressants Duloxetine 60–120 mg/day
Amitriptyline 25–200 mg/day
Nortriptyline 25–150 mg/day
Desipramine 25–200 mg/day
Other medications Mexiletine 450–900 mg/day
Dexamethasone 6–100 mg/day
Topical capsaicin
Topical lidocaine (5% patch)
Other interventions Transcutaneous electrical nerve stimulation
Sympathetic nerve blocks
Epidural and intrathecal nerve blocks
Nerve ablation
Cordotomy, rhizotomy
Spinal stimulation
Hypnosis, biofeedback, relaxation training

Common Pain Disorders 201
nervous system. If these conditions are untreated or unabated, the sympathetic
nervous system responses produce marked trophic changes in a limb.
The most prominent feature of CRPS is pain, often throbbing (for CRPS I)
or burning (for CRPS II). The pain is usually constant, but paroxysms of pain
can occur, ranging from mild to severe. Initially, the pain is often located at the
site of injury, but it can extend through the entire affected limb. Over time,
there can be extension of pain to other limbs unaffected by the initial trauma
or injury. The affected limb is often exquisitely sensitive to ambient temper-
ature, touch, or stimulation associated with movement. Thus, the patient will
tend to guard the painful limb and withdraw from physical examination that
could raise the risk of pain from touch or direct manipulation. In addition,
there are a number of changes in temperature, skin, muscle tone, vascular sys-
tem, and bone that occur as the course of the disorder progresses. Classic fea-
tures of CRPS include changes in blood flow within, along with resultant tem-
perature changes in, the affected limb as compared with other limbs; edema;
and trophic skin changes, along with changes in the musculature and changes
in bone scan activity and density. The rate of progression of the changes associ-
ated with CRPS varies from person to person.
Several investigations may be employed to assess the features and staging
of CRPS. For example, skin temperature depends on the extent of cutaneous
blood flow, which is under the direct influence of sympathetic activity. In early
stages of CRPS, thermography may reveal increased temperature in the af-
fected limb compared with the contralateral limb or other limbs of the body. In
later stages, skin blood flow and temperature are reduced. Bone scanning by
scintigraphy may reveal increased activity throughout the bones of the affected
limb. Bone density measurements may reveal progressive changes in the bone
of the affected limb over time.
Treatment approaches for CRPS include pharmacologic treatment (see
Table 8–15). A number of invasive techniques may be employed as well, given
the relatively refractory nature of such syndromes to pharmacologic ap-
proaches. Nerve blocks may be employed to disrupt ongoing sympathetic
nervous system activity in the affected limb. Surgical interventions to disrupt
sympathetic nervous system activity may be employed (sympathectomy) for
patients who are unable to sustain extended relief from repeated nerve blocks.
Affective disorders are common among patients with CRPS—for exam-
ple, more than 60% of patients meet criteria for major depression (Galer et al.

202 Clinical Manual of Pain Management in Psychiatry
2001). Substance abuse disorders also are common among this population.
There is controversy about whether premorbid psychological disturbances
predispose one to CRPS. In any event, psychiatric treatment for these con-
ditions is warranted. Biofeedback and relaxation training can reduce distress
and foster adaptive strategies with which to deal with pain (Barowsky et al.
1987).
Phantom Limb Pain
After amputation, it is common for patients to experience sensations of the re-
moved limb, just as though it were present (phantom sensations). In some
cases, these sensations progress to distressing painful sensations (phantom
pain) perceived to be emanating from the amputated limb.
Although phantom limb sensations are almost universal following ampu-
tation, approximately 85%–97% of amputees experience some form of pain.
Sensations are almost immediate, whereas pain might not emerge for some
Table 8–15.
Treatment of patients with complex regional pain
syndrome
Medications
Anticonvulsants (carbamazepine, phenytoin, gabapentin, pregabalin)
Antidepressants (tricyclic antidepressants)
Baclofen (oral/intrathecal)
Calcium channel blockers
Clonidine (oral/intrathecal)
Corticosteroids (chronic use is not recommended)
Lidocaine patch
Opioids
Phenoxybenzamine
Prazosin
Propranolol
Local nerve block
Regional sympathetic block (cervical, lumbar)
Sympathectomy
Spinal cord stimulation (implantable device)
Physical therapy

Common Pain Disorders 203
time after amputation, typically from 1 month to 1 year. Pain is often charac-
terized as burning, cramping, or aching. Other features (e.g., crushing, squeez-
ing, twisting, pins and needles, grinding) are also described (Jensen et al.
1985). Pain may be exacerbated by physical stimulation and emotional factors
(e.g., depression). The characteristics of phantom limb pain can vary widely in
terms of the quality of sensory experiences and impact on quality of life. Thus,
in the mildest forms of phantom limb pain, patients may experience mild, in-
termittent paresthesias that do not interfere with normal activity or sleep; in
extreme forms, paresthesias may be constant and very uncomfortable, inter-
fering with activity and sleep.
The course of phantom limb pain is variable, with up to 56% of patients
reporting resolution or improvement in symptoms over time. Pain emerging
over time warrants investigation of possible causes producing pain (e.g., in-
fection at the site of amputation, scar tissue, neuroma formation). The cause
of phantom pain is unclear. Peripheral processes (i.e., spontaneous discharges
emanating from severed nerves containing Aδ and C fibers) brought on by
scar tissue in the amputated limb are thought to be a possible basis of this pain.
Spontaneous discharges such as these may be inhibited by infusions of lidocaine
but could be exacerbated by irritation of the stump (e.g., tapping) or cold ex-
posure. Alternatively, central processes (i.e., reverberating neural circuits within
the central nervous system) have also been implicated as the basis for phantom
limb pain.
Specific psychological correlates of persons experiencing phantom limb
pain are lacking. However, some data tend to suggest that among those expe-
riencing this form of pain, there is a tendency to be rigid, inflexible, and self-
reliant; to suppress emotions (thus depriving one of the propensity to grieve
the lost limb); and to cope by using denial as a primary defense. It is theorized
that phantom limb pain possibly may arise from psychopathologic interpre-
tations of phantom limb sensations (Parkes 1973).
Treatment for patients with phantom limb pain may include antidepres-
sants and anticonvulsants (Hord 1996). However, controlled studies on the
efficacy of such agents are generally lacking; efficacy has been based mostly on
anecdotal reports. Another group of agents, β-blockers, may also reduce phan-
tom limb pain and may be considered as alternatives to, or used in conjunction
with, antidepressants and anticonvulsants. Opiates tend not to be effective for
long-term use in phantom limb pain.

204 Clinical Manual of Pain Management in Psychiatry
Surgical interventions have been employed in the past for phantom limb
pain but, because of their limited utility, have largely been abandoned (e.g.,
thalamotomy, sympathectomy, cordotomy). Neurolytic and sympathetic blocks
might be useful in selected patients. Epidural anesthesia administered for
3 days prior to the amputation may reduce the severity of postoperative phan-
tom limb pain (Bach et al. 1988). Psychological therapies (e.g., relaxation
training) possibly may help to reduce the distress associated with the pain and
help to reduce the pain sensations. Psychotherapy might be required to help
the patient mourn the loss of the limb, address concerns over physical appear-
ance or disfigurement, and facilitate adaptation to the use of prostheses and
making the changes necessary to modify his or her usual activities.
Cancer and HIV
Pain complicates the clinical picture of approximately 50% of patients with met-
astatic cancer (Ahles et al. 1983). The nature of the pain—its location, intensity,
radiation, quality, and other characteristics—may vary depending on the type
of cancer, its progression, and the treatment measures undertaken to treat it.
Among patients with HIV/AIDS, prevalence rates for pain vary from 30%
to 97%. The variability in prevalence rates of pain associated with HIV is in
part attributable to the progression of the disease and the clinical settings in
which patient evaluations were conducted (Breitbart and Patt 1994). Com-
mon pain-related disorders associated with HIV/AIDS (Lebovits et al. 1989)
are summarized in Table 8–16.
Pain ratings for patients with cancer are higher among those with comor-
bid psychiatric conditions than in those without such conditions (Massie and
Holland 1987). Common psychiatric comorbidity includes adjustment dis-
orders, depression, anxiety, and delirium.
Effective pain treatment is obviously contingent on the nature and source
of pain (Benedetti et al. 2000). Thus, opioids might be required for severe
bone pain due to metastases, fractures, or both; antidepressants, anticonvul-
sants, or both may be employed in neuropathic types of pains. Pharmacologic
approaches to pain management (see Table 8–17) need to be customized to
the patient’s needs, factoring pain severity, impact on functioning, and toler-
ability of side effects. Anti-inflammatory agents are required for mild pain,
but opioids might be required for moderate to severe pain states. Dosing sched-

Common Pain Disorders 205
ules should be simple to maximize adherence and should be administered in
the least invasive manner. Persistent pain requires around-the-clock dosing of
analgesics. Certainly psychiatric interventions, including pharmacotherapy
and psychotherapy, are prudent in conditions in which there is psychiatric
comorbidity. Adjunctive techniques (e.g., hypnosis, relaxation training, bio-
Table 8–16.
Common pain-related disorders associated with
HIV/AIDS
Nociceptive
Cutaneous
Kaposi sarcoma
Oral cavity pain
Candidiasis
Aphthous ulcers
Herpes simplex virus and cytomegalovirus infection
Visceral
Ulcerative esophagitis
Gastritis
Pancreatitis and biliary tract disorders
Tu m or
Infection
Deep somatic
Arthralgias
Back pain
Myopathies
Headache
Meningitis
Encephalitis
Iatrogenic causes (e.g., zidovudine)
Neuropathic
Mononeuropathy
Guillain-Barré syndrome
Mononeuropathy multiplex
Polyneuropathy
Herpes simplex (postherpetic neuralgia)
Antiretroviral toxicity–associated neuropathy

206 Clinical Manual of Pain Management in Psychiatry
feedback, deep breathing exercises) might facilitate mitigation of pain and ac-
companying psychological distress. However, the presence of an underlying
delirium would certainly limit the ability of the patient to obtain any benefit
from psychotherapeutic interventions. In such cases, resolution of the under-
lying medical condition, the addition of antipsychotics, or both, might be re-
quired to reduce the interference of any psychological interventions by a de-
lirium.
The attributions assigned to the pain by the cancer patient may be the ba-
sis for a great deal of psychological distress. It is not uncommon for the patient
to ascribe ominous interpretations to the presence of the pain, which in turn
Table 8–17.
Management of cancer pain and pain associated with
HIV and AIDS, based on pain ratings
Mild (1–3) Consider nonsteroidal anti-inflammatory drug,
acetaminophen
If ineffective, augment with opiate
If ineffective, administer short-acting opiate
Moderate (4–6) Consider short-acting opiate
Consider augmentation of opiates with psychotropics
Address side effects of opiates (gastrointestinal side effects,
sedation, delirium)
Severe (7–10) Consider rapid dose increases of short-acting opiate
5–10 mg morphine (or equivalent)
Reassess after 1 hour
Double the dose if pain is unchanged; reassess after
1hour
Repeat dose if pain level is halved; reassess
after 1 hour
If improved (pain is less than 50% of original level),
reassess after 1 hour
Give the effective dose administered after 4 hours, then
give this dose every 4 hours around the clock
Consider augmentation of opiates with psychotropics
Address side effects of opiates (gastrointestinal side effects,
sedation, delirium)
Note. Pain ratings are based on a numeric rating scale of 1–10.

Common Pain Disorders 207
could exacerbate the pain experience. However, such attributions that pain could
signal disease progression might not be entirely unrealistic (Ahles et al. 1983).
Other sources of distress may include immediate concrete needs (e.g., financial
needs, housing, transportation), family or interpersonal concerns, changes in
one’s autonomy and independence, need for assistance with activities of daily
living, and spiritual concerns (e.g., afterlife issues, making amends, mending in-
terpersonal conflicts). Such issues, once identified, might require the joint ef-
forts of the psychiatrist, psychologist, social worker, and pastoral counselor for
effective management (Holland 1999).
Key Points
• For many chronic, nonmalignant pain conditions, the goals of treatment
include symptomatic pain control, improvement in function, and reduc-
tion of disability, along with management of comorbidities (e.g., sleep dis-
turbances, fatigue, mood disturbances).
• Primary headache disorders are highly prevalent conditions. Successful
management requires accurate diagnosis, recognition of appropriate treat-
ment modalities available, and management of common comorbidities.
• Back pain is a common cause of pain and disability. For many patients with
nonemergent, nonspecific back pain, treatment directed at reassurance, ex-
ercise, use of weak analgesics, and psychotherapeutic interventions may be
all that is necessary.
• Fibromyalgia is characterized by widespread nonarticular pain. It is often
associated with fatigue, sleep disturbances, restless legs syndrome, irritable
bowel symptoms, and hypotension. A multidisciplinary approach employ-
ing prudent pharmacologic interventions, graded exercise programs, pa-
tient education, and cognitive-behavioral therapy can address symptoms
and improve functioning.
• Treatment of articular disorders (e.g., osteoarthritis, rheumatoid arthritis)
necessitates pharmacologic interventions, physical/occupational therapies,
ambulatory/assistive devices, and, in some cases, surgical interventions.
Psychological comorbidities can contribute to distress by magnifying pain,
enhancing perceived disability, and undermining treatment and rehabili-
tative interventions and may warrant intervention.
• Although neuropathic pain can arise from an array of etiologies, several ef-
fective treatment modalities are available that mitigate peripheral and central

208 Clinical Manual of Pain Management in Psychiatry
nervous system processes that enhance pain. The mainstays of treatment in-
clude tricyclic antidepressants, anticonvulsants, antiarrhythmics, and topical
agents.
• Pain related to cancer and HIV is multifactorial—that is, due to the primary
illness and comorbid illnesses and conditions, and due to the treatment
itself—warranting aggressive treatment. Such pain can be magnified by
psychological and spiritual distress.
References
Ahles TA, Blanchard EB, Ruckdeschel JC: The multidimensional nature of cancer-
related pain. Pain 17:277–288, 1983
Ansari A: The efficacy of newer antidepressants in the treatment of chronic pain: a
review of current literature. Harv Rev Psychiatry 7:257–277, 2000
Argoff CE, Backonja MM, Belgrade MJ, et al: Consensus guidelines: treatment plan-
ning and options. Mayo Clin Proc 81 (suppl 4):12–25, 2006
Atlas SJ, Deyo RA: Evaluating and managing acute low back pain in the primary care
setting. J Gen Intern Med 16:120–131, 2001
Bach S, Noreng MF, Tjellden NU: Phantom limb pain in amputees during the first
12 months following limb amputation, after preoperative lumbar epidural block-
ade. Pain 33:297–301, 1988
Barowsky EI, Zweig JB, Moskowitz J: Thermal biofeedback in the treatment of symptoms
associated with reflex sympathetic dystrophy. J Child Neurol 2:229–232, 1987
Bendtsen L, Jensen R: Mirtazapine is effective in the prophylactic treatment of chronic
tension-type headache. Neurology 62:1706–1711, 2004
Benedetti C, Brock C, Cleeland C, et al: NCCN practice guidelines for cancer pain.
Oncology 14:135–150, 2000
Bogaards MC, ter Kuile MM: Treatment of recurrent tension headache: a meta-analytic
review. Clin J Pain 10:174–190, 1994
Bonica JJ: Causalgia and other reflex sympathetic dystrophies. Postgrad Med 53:143–
148, 1973
Bowsher D: The effects of pre-emptive treatment of postherpetic neuralgia with ami-
triptyline: a randomized, double-blind, placebo-controlled trial. J Pain Symptom
Manage 13:327–331, 1997
Breitbart W, Patt RB: Pain management in the patient with AIDS. Hem/Onc Annals: The
Journal of Continuing Education in Hematology and Oncology 2:391–399, 1994
Breslau N, Lipton RB, Stewart WF, et al: Comorbidity of migraine and depression:
investigating potential etiology and prognosis. Neurology 60:1308–1312, 2003

Common Pain Disorders 209
Campbell JK, Penzien DB, Wall EM: Evidence-based guidelines for migraine headache:
behavioral and physical treatments. The U.S. Headache Consortium. Available at:
http://www.aan.com/professionals/practice/pdfs/gL0089.pdf. Accessed June 2002.
Cohen MJM, Menefee LA, Doghramji K, et al: Sleep in chronic pain: problems and
treatments. Int Rev Psychiatry 12:115–127, 2000
Collins JG: Prevalence of selected chronic conditions, United States, 1979–1981. Data
from the National Health Survey. Vital Health Stat 10:1–66, 1986
Dahl JB, Mathiesen O, Moiniche S: “Protective premedication”: an option with gaba-
pentin and related drugs? A review of gabapentin and pregabalin in the treatment
of post-operative pain. Acta Anaesthesiol Scand 48:1130–1136, 2004
Deyo RA, Weinstein JN: Low back pain. N Engl J Med 344:363–370, 2001
Diamond S: Efficacy and safety profile of venlafaxine in chronic headache. Headache
Quarterly, Current Treatment and Research 6:212–214, 1995
Epstein MT, Hockaday JM, Hockaday TDR: Migraine and reproductive hormones
throughout the menstrual cycle. Lancet 1:543–548, 1975
Fasmer OB: The prevalence of migraine in patients with bipolar and unipolar affective
disorders. Cephalalgia 21:894–899, 2001
Flor H, Turk DC, Birbaumer N: Assessment of stress-related psychophysiological re-
actions in chronic back pain patients. J Consult Clin Psychol 53:354–364, 1985
Frank RG, Beck NC, Parker JC, et al: Depression in rheumatoid arthritis. J Rheumatol
15:920–925, 1988
Galer BS: Neuropathic pain of peripheral origin: advances in pharmacologic treatment.
Neurology 45 (suppl 9):17–25, 1995
Galer BS, Schwartz L, Allen RJ: Complex regional pain syndromes, type I: reflex sym-
pathetic dystrophy, and type II: causalgia, in Bonica’s Management of Pain, 3rd
Edition. Edited by Loeser JD, Butler SH, Chapman CR, et al. Philadelphia, PA,
Lippincott Williams & Wilkins, 2001, pp 388–411
Goadsby PJ: Serotonin 5-HT1B/1D receptor agonists in migraine: comparative phar-
macology and its therapeutic implications. CNS Drugs 10:271–286, 1998
Goadsby PJ, Lipton RB, Ferrari MD: Migraine: current understanding and treatment.
N Engl J Med 346:257–270, 2002
Goldenberg DL, Burckhardt C, Crofford L: Management of fibromyalgia syndrome.
JAMA 292:2388–2395, 2004
Hadler NM: Fibromyalgia, chronic fatigue, and other iatrogenic diagnostic algorithms.
Postgrad Med 102:161–177, 1997
Holland JC: Update: NCCN practice guidelines for the management of psychosocial
distress. Oncology 13:459–507, 1999
Holm JE, Holroyd KA, Hursey KG, et al: The role of stress in recurrent tension head-
ache. Headache 26:160–167, 1986

210 Clinical Manual of Pain Management in Psychiatry
Holroyd KA, Andrasik F, Westbrook T: Cognitive control of tension headache. Cognit
Ther Res 1:121–133, 1977
Holroyd KA, O’Donnell FJ, Stensland M, et al: Management of chronic tension-type
headache with tricyclic antidepressant medication, stress management therapy,
and their combination. JAMA 285:2208–2215, 2001
Hord AH: Phantom pain, in Pain Management: A Comprehensive Review. Edited by
Raj PP. St Louis, MO, Mosby, 1996, pp 483–491
Huyser BA, Parker JC: Negative affect and pain in arthritis. Rheum Dis Clin North
Am 25:105–121, 1999
Jensen TS, Krebs B, Nielsen J, et al: Immediate and long-term phantom limb pain in
amputees: incidence, clinical characteristics and relationship to pre-amputation
limb pain. Pain 21:267–278, 1985
Lake AE 3rd: Behavioral and nonpharmacologic treatments of headache. Med Clin
North Am 85:1055–1075, 2001
Lebovits AH, Lefkowitz M, McCarthy D, et al: The prevalence and management of
pain in patients with AIDS: a review of 134 cases. Clin J Pain 5:245–248, 1989
Leo RJ: Treatment considerations in neuropathic pain. Curr Treat Options Neurol
8:389–400, 2006
Lipchik GL, Nash JM: Cognitive-behavioral issues in the treatment and management
of chronic daily headache. Curr Pain Headache Rep 6:473–479, 2002
Massie MJ, Holland JC: The cancer patient with pain: psychiatric complications and
their management. Med Clin North Am 71:243–258, 1987
McGill CM: Industrial back problems: a control program. J Occup Med 10:174–178,
1968
Parkes CM: Factors determining the persistence of phantom pain in the amputee.
J Psychosom Res 17:97–108, 1973
Reesor KA, Craig KD: Medically incongruent chronic back pain: physical limitations,
suffering, and ineffective coping. Pain 32:35–45, 1988
Rowbotham MC: Chronic pain: from theory to practical management. Neurology 45
(suppl 9):5–10, 1995
Saper JR, Lake AE, Tepper SJ: Nefazodone for chronic daily headache prophylaxis: an
open-label study. Headache 41:465–474, 2001
Silberstein SD: The role of sex hormones in headache. Neurology 42 (suppl 2):37–42,
1992
Silberstein SD: Practice parameter: evidence-based guidelines for migraine headache
(an evidence-based review): report of the Quality Standards Subcommittee of the
American Academy of Neurology. Neurology 55:754–762, 2000
Smith TW, Peck JR, Milano RA, et al: Cognitive distortion in rheumatoid arthritis:
relation to depression and disability. J Consult Clin Psychol 56:412–416, 1988

Common Pain Disorders 211
Steiner TJ, Hering R, Couturier EG, et al: Double-blind placebo-controlled trial of
lithium in episodic cluster headache. Cephalalgia 17:673–675, 1997
Stewart WF, Ricci JA, Chee E, et al: Lost productive time and cost due to common
pain conditions in the US workforce. JAMA 290:2443–2454, 2003
Turner JA: Educational and behavioral interventions for back pain in primary care.
Spine 21:2851–2857, 1996
Turner JA, Denny MC: Do antidepressant medications relieve chronic low back pain?
J Fam Pract 37:545–553, 1993
Waddell G, Feder G, Lewis M: Systematic reviews of bed rest and advice to stay active
for acute low back pain. Br J Gen Pract 47:647–652, 1997
Walker J, Holloway I, Sofaer B: In the system: the lived experience of chronic back
pain from the perspectives of those seeking help from pain clinics. Pain 80:621–
628, 1999
Welch KMA: Headache, in Bonica’s Management of Pain, 3rd Edition. Edited by
Loeser JD, Butler SH, Chapman CR, et al. Philadelphia, PA, Lippincott Williams
& Wilkins, 2001, pp 867–894
Wolfe F, Smythe HA, Yunus MB, et al: The American College of Rheumatology 1990
criteria for the classification of fibromyalgia: report of the Multicenter Criteria
Committee. Arthritis Rheum 33:160–172, 1990
Young LD: Psychological factors in rheumatoid arthritis. J Consult Clin Psychol 60:619–
627, 1992
Zwart JA, Dyb G, Hagen K, et al: Depression and anxiety disorders associated with
headache frequency: the Nord-Trondelag Health Study. Eur J Neurol 10:147–
152, 2003
This page intentionally left blank

213
9
Special Populations
Pain management of special populations, each with its own set of issues, can
be challenging. The recognition and treatment of pain in the very young and
the very old have been poor. There are fears, misconceptions, and stereotypes
that have an impact on pain management among pregnant patients, culturally
distinct groups, and persons with substance abuse histories. Unique issues also
arise among patients with terminal conditions and their families.
Pediatric Patients
Pain management among children has been abysmally poor. For example, the
level of analgesics provided to children postoperatively was markedly low
compared with the level provided to adults in comparable surgical interven-
tions (Beyer et al. 1983). Part of this poor pain management has been due to
misconceptions about pediatric pain, to inadequate pain assessments and
scales for use in young children, and to fears about the use of powerful anal-
gesics (e.g., opiates) among young patients.
The neonatal nervous system is well equipped to process nociceptive input
(Goldschneider et al. 2001). However, the communication of painful states is

214 Clinical Manual of Pain Management in Psychiatry
limited. Common chronic pain states in children include headache, recurrent
abdominal and chest pain, and those pain states associated with chronic ill-
nesses (e.g., diabetes, sickle cell anemia, hemophilia, juvenile rheumatoid ar-
thritis, cancer).
Pain and Childhood Developmental Phases
Any pain management approach needs to include the psychological, cogni-
tive, and emotional factors present at varying developmental phases appropri-
ate to children. Among infants, pain is responded to reflexively, with expression
of discomfort conveyed through crying. Facial expressions and crying patterns
appear to be distinct for pain as compared with other unpleasant states (e.g.,
hunger) (Sifford 1997). Among toddlers, words might be expressed for pain
(e.g., “ouch,” “boo-boo”). However, such children are likely to view pain in
terms of punishment (i.e., as an indicator of being “bad”). It is imperative that
provision of pain relief be undertaken in a way that avoids fostering such mis-
conceptions. At school age, in concrete operational ways, the child may assign
emotional terms to pain. Pain is no longer viewed as punishment, and the child
can assimilate cause-and-effect understanding of the pain. Differentiation
among varying pain intensities becomes possible at approximately ages 5–7
years. Later, among young adults, there is a sense of invulnerability (which
could account in part for the reckless behaviors that precipitate injuries war-
ranting pain treatment). Body image, peer relations, and school attendance
are central to their experiences. Thus, the presence of pain may threaten the
adolescent’s sense of invulnerability, separate him or her from peers, and lead
to concerns about self-image and self-esteem.
Pain Assessment Scales
Pain assessments for children and adolescents need to be appropriate to develop-
mental stage, and suitable assessment scales can be helpful. In infants and prever-
bal children, the emphasis of assessment scales is on observational methods—
including assessments of crying patterns, facial expressions, and physiologic
parameters (blood pressure, diaphoresis, heart rate, and respiratory rate). Among
verbal young children, one can add quantifiable pain assessments (e.g., the
“poker chip” tool, “oucher” scale, faces scale). Some instruments (e.g., a visual
analog scale and pain diaries) are appropriate for older children and adoles-
cents (Stevens 1997).

Special Populations 215
The poker chip tool involves use of concrete objects (e.g., poker chips) to
quantify or approximate pain ratings. The child is asked to rate the pain in-
tensity by choosing up to four poker chips, depicting “pieces” of hurt. This
method can be a particularly useful assessment index for younger children
(i.e., ages 4–8 years). One problem with this assessment tool is that the child
is unlikely to tease out how much “hurt” is related to pain per se and how
much is ascribable to the affective components (e.g., fear associated with the
pain).
The oucher scale is a variant of the faces scale, with six photographs of
children’s expressions in varying degrees of distress organized vertically along-
side a numeric scale ranging from 0 to 100. The ratings, however, can vary
depending on which anchors are employed. For example, if the lowest pain
rating is depicted with a smiling face as opposed to a photograph with a neu-
tral facial expression, different pain ratings might be endorsed. Thus, the ex-
aminer is cautioned to use the same scale consistently when comparing pain
ratings of a young child over time. There might be differences in pain ratings
depending on the ethnic backgrounds and genders depicted in the photographs
in the scale as well.
The faces scale (see also Chapter 3, “Evaluation of the Pain Patient,” of this
book) may be used with children to rate pain severity. The scale is fairly easily
understood at about age 6 years and thereafter. It allows one to bypass the po-
tential gender and ethnic biases that can confound pain ratings encountered
with the oucher scale.
Pain diaries may be useful for adolescents with pain. Younger children
would be expected to be incapable of completing a pain diary, but parents might
find such diaries useful. In the diary, pain ratings are ranked and descriptions
can be noted of ongoing environmental factors, extenuating circumstances,
prevailing mood states, thought patterns, and other factors. The diary can re-
veal temporal patterns between pain states and mood, thought processes, and
extenuating circumstances surrounding the adolescent’s pain. Diaries can be
useful in encouraging young adults to develop self-management strategies and
can foster increased mastery over one’s pain.
Analgesics (as outlined further in Chapter 5, “Pharmacology of Pain,” of this
book) need to be applied judiciously and dosed according to weight (Leith
and Weisman 1997) as well as the tolerability of side effects. Routes of admin-
istration need to be selected to provide the least invasiveness and distress in

216 Clinical Manual of Pain Management in Psychiatry
application of the analgesic. Very painful conditions (e.g., sickle cell crisis, mod-
erate to severe postoperative pain) may require opiate analgesics. These should
not be withheld because of fears of addiction.
Specialized analgesic techniques used with children include local anesthet-
ics and a eutectic mixture of local anesthetics (see Chapter 5 of this book). Pa-
tient-controlled anesthesia (PCA) or anesthesia controlled by the parent can
be employed for severe pain states. PCA might be best employed with children
who are at least age 6 years (Berde and Masek 1999). The advantage of PCA is
that the patient can control the amount of analgesic administered. Parent-
controlled analgesia raises the risk that misinterpretation of the child’s be-
haviors can result in too little or too much of an analgesic administered. Other
interventions might include physical therapy, occupational therapy, and psy-
chotherapeutic interventions. There is strong evidence suggesting that relax-
ation techniques and cognitive-behavioral therapy can be quite effective in
mitigating chronic pain in children and adolescents (Eccleston et al. 2002).
Geriatric Patients
Elderly persons are prone to multiple medical conditions predisposing them
to pain. Estimates suggest that rates of chronic pain among elderly persons are
twice those of younger individuals (Crook et al. 1984). The elderly are also
likely to experience pain from terminal medical conditions (Cleeland 1998).
Among community samples, 20%–50% of older persons reported chronic pain
(Crook et al. 1984), whereas among those in long-term-care settings, estimates
of chronic pain were substantially higher, approaching 45%–80% (Ferrell
1990; Roy and Thomas 1986; Won et al. 1999). Untreated pain interferes with
adaptive functioning, interpersonal functioning, and quality of life. Common
disorders contributing to chronic pain include arthritis, cancer, diabetic neu-
ropathy, herpes zoster, and osteoporosis (Ferrell 1991; Gallagher et al. 2000).
Underrecognition of Pain in Elderly Patients
Pain is often poorly recognized and poorly treated among the elderly (Seng-
staken and King 1993). Reasons include poor recognition of symptoms, inade-
quate time spent evaluating patients, and failure to inquire about pain symptoms.
Appetite and sleep disturbances, social withdrawal, reduced inclination to en-
gage in activities, and distress resulting from pain may be mislabeled as an emo-

Special Populations 217
tional disturbance (e.g., depression, anxiety). Ascertaining pain may be further
hindered by dementia. Inadequate trials of medications to relieve pain, con-
cerns regarding medication’s adverse effects, and addiction fears interfere with
effective treatment.
Misconceptions that pain sensitivity is reduced among the elderly may be
attributed to the unusually painless presentation of some elderly patients with
common illnesses. Much has yet to be learned about age-related physiologic
changes within the nociceptive pathway system. Although transmission via Aδ
and C neurons might be altered with age, the role such changes have in inter-
fering with the recognition or perception of pain remains unclear. Physiologic
changes in the nociceptive system occurring with age might not bear much clin-
ical significance (Gagliese et al. 1999).
On the other hand, there might be alterations in the affective (limbic) and
cognitive (cortical) processes that are factors in the processing and apprecia-
tion of pain. Still, elderly patients could have a reduced inclination to commu-
nicate or report pain, which might emanate from belief systems held by some
elderly persons (e.g., that pain is to be expected as one ages and therefore does
not warrant clinical attention). Further investigation is warranted to assess to
what extent such factors influence the perception and reporting of pain among
the elderly population.
Nonetheless, careful assessment of the older patient is necessary to effec-
tively address and treat pain. Some education of the patient might be required
to circumvent the problems of erroneous expectations interfering with the re-
porting of pain. Some elderly patients could be concerned that notifying phy-
sicians of their pain might invoke treatments that are invasive and painful,
that could complicate other health conditions, or that might involve hospi-
talization and, therefore, separation from family and other usual supports.
Standard assessment scales can be employed in an attempt to quantify the
degree of discomfort experienced (see Chapter 3 of this book). Some of these
might be easier than others for the elderly to use. Thus, for example, a visual
analog scale is harder for elderly patients to comprehend and reliably use than
are other assessment devices (e.g., numeric rating scales, faces scale, verbal
scales) (Herr and Mobily 1993). Among patients with cognitive impairments
that limit effective communication of pain and discomfort, it may be neces-
sary to rely on observation of behavioral changes; some helpful parameters are
outlined in Table 9–1.
218 Clinical Manual of Pain Management in Psychiatry
From a biopsychosocial perspective, it becomes imperative to understand
the psychological and social factors contributing to the patient’s plight and
pain. Among the elderly, the psychological issues that prevail include concerns
over life review and the need to have fulfilled life goals and to have made con-
tributions to the world.
Treatment Strategies
Older patients are often excluded from studies assessing various medication
effects in pain reduction. Often, an attempt is made in clinical trials to avoid
the influences of other medical conditions or drug interactions with coadmin-
istered medications. Thus, for example, most of the literature on employing
psychotropic agents in pain mitigation has largely focused on diverse patient
populations and not exclusively on the elderly.
The pharmacologic approaches discussed in Chapter 5 of this book are ap-
plicable to the geriatric patient with chronic pain. Opiates might be indicated in
acute pain, in chronic malignant pain, and in cases of chronic nonmalignant
pain. The elderly can be particularly sensitive to the adverse effects of anal-
gesic agents (e.g., sedation, confusion, constipation associated with opiates,
gastrointestinal effects associated with nonsteroidal anti-inflammatory drugs
[NSAIDs]) (American Geriatrics Society 1998). In some cases, particularly in
patients with neuropathic pain and those with psychological factors contributing
significantly to pain, a variety of psychotropic medications are available (Leo and
Table 9–1.
Behavior parameters warranting consideration to
assess discomfort
Parameter Signs
Breathing Labored, loud, gasping, rapid rate, hyperventilation
Vocalizations Groaning, moaning, muttering, rapid speech
Facial expression Tearful, sad, troubled, worried, fearful, alarmed, frowning,
scowling
Body language Tense, strained, fidgety, restless, rubbing or guarding of
affected body parts, aggression
Source. Adapted from Warden V, Hurley AC, Volicer L: “Development and Psychometric Eval-
uation of the Pain Assessment in Advanced Dementia (PAINAD) Scale.” Journal of the American
Medical Directors Association 4:9–15. Copyright 2003, American Medical Directors Association.
Used with permission.

Special Populations 219
Singh 2002). In general, the approach with the elderly patient is that initial doses
should be low and incremental increases should proceed slowly, guided by the
diminution of pain, improvement in function, and tolerability of side effects.
Some effects of pain (e.g., sleep disturbances, appetite suppression) arising
from ongoing pain might require attention. Hence, efforts should be directed
at the development of sleep hygiene strategies and the selective use of sedating
agents (i.e., eszopiclone, zolpidem, zaleplon, trazodone, and possibly benzo-
diazepines). Cyproheptadine and megestrol acetate have been employed in sit-
uations in which a pharmacologic intervention is selected to increase appetite.
Nonpharmacologic approaches ought to be considered in the treatment of
the elderly patient with pain (American Geriatrics Society 1998). These endeav-
ors—including exercise, transcutaneous electrical nerve stimulation (TENS),
acupuncture, massage, relaxation training, and psychotherapy, among others—
can supplement pharmacologic and other invasive treatment strategies.
Pregnancy
Because of concerns about fetal effects, the use of analgesics during pregnancy
has been restricted. Opioid treatment during pregnancy has been documented
in cases of women treated with methadone for opiate dependence and with
brief opioid requirements during labor. In such cases, the risks to the fetus are
outweighed by the potential risks arising from either continued heroin use or
complications arising in the delivery process. Nonetheless, concerns still arise
with regard to the use of analgesics for pregnant women who experience ma-
lignant or nonmalignant pain in the course of pregnancy. Long-term exposure
of the fetus to opiates is likely to produce the following effects in the newborn:
opiate dependence, acute withdrawal after delivery, and growth retardation.
Some patients might still be capable of achieving long-term relief of pain with
spinal or intrathecal administration of opiates while minimizing the infant’s
exposure to the opiate (Wen et al. 1996).
Among other analgesics, NSAIDs administered during pregnancy may
prolong labor, result in constriction of the ductus arteriosus in the fetus, and
produce renal and hematologic abnormalities in the newborn (Ostensen 1998).
Other agents (e.g., aspirin) have been invoked to treat complications in late
pregnancy (e.g., preeclampsia), although the risks to fetal growth and develop-
ment have been a concern.

220 Clinical Manual of Pain Management in Psychiatry
Use of adjuvant agents (e.g., antidepressants, anticonvulsants) carries with
it the risks of fetal complications (e.g., neural tube defects), increased spontane-
ous abortion rates, and anticholinergic effects in the newborn.
For women who are breastfeeding, acetaminophen and ibuprofen appear
to be safest among the nonopioid analgesics because of their high protein bind-
ing (Ebert 1997). Most opioids are safe for use during lactation, without harm
to the breastfeeding infant. The infant’s methadone exposure is determined by
the maternal dose and time of breastfeeding in relation to when peak drug effects
are obtained. Breastfeeding is permitted for women taking methadone at dos-
ages less than 20 mg/day and should occur at least 6–10 hours after the meth-
adone dose is received (Renehan 1989).
Cultural Issues
With clichés such as “no pain, no gain” and slogans such as “tough it out,” one can
only be impressed with popular notions about pain. Societal attitudes toward
pain may evoke reluctance to report pain and fear of stigma associated with pain
complaints.
Although there are no racial or ethnic differences in the ability to discriminate
pain (Zatzick and Dimsdale 1990), culture influences what meanings persons
derive from illness, suffering, and pain. The psychiatrist addressing pain pa-
tients must consider the impact cultural influences might have on the pain ex-
perience. Thus, in some black communities, Christian religious beliefs have a
profound impact on the understanding of pain. In such communities, suffer-
ing has a redeeming function, and efforts to avoid pain might constitute a
“failure of faith” (Crawley et al. 2000).
Cultural biases and stereotypes might impede effective pain treatment. Racial
and ethnic disparities in pain treatment have been observed in emergency set-
tings (Todd et al. 1993) as well as for cancer, postoperative, and low back pain
(Bonham 2001). A multicenter study assessing the adequacy of pain manage-
ment in cancer found that nonwhite patients with metastatic cancer pain were
three times more likely to be undermedicated as compared with white cancer
patients (Cleeland et al. 1994). Several factors have been identified that may
contribute to disparities in the treatment of pain among nonwhite patients
(Table 9–2).
Special Populations 221
The tendency to be expressive or unemotional about pain can be dictated
by a person’s ethnic, religious, and familial background. Language and cul-
tural impediments to pain treatment might be overcome through the use of
culturally sanctioned pain assessments. Some assessment instruments (e.g.,
McGill Pain Questionnaire; see Chapter 3 of this book) have been translated
into various languages for such purposes.
Economic disparities affecting certain subcultures might preclude medi-
cal follow-up with pain management. Patients living in financially disadvan-
taged areas might not have access to prescribed analgesics; pharmacies in such
areas might not stock analgesics because of fears regarding theft.
Although the prevailing cultural norm within the United States and that
of the legal system emphasize the importance of a person’s autonomy and the
primacy of the individual in decision making, this norm is not shared by many
other cultural communities. Thus, among other cultures, the primacy of the
family may be emphasized in the decision-making process (Kagawa-Singer and
Blackhall 2001). While exploring treatment interventions, the clinician may
have to consider varying approaches to decision making, factoring in the need
to include others in the patient’s life in the proposal of potential treatment
options.
Table 9–2.
Barriers to pain management in minority patients
Language
Less frequent follow-up care
Loss to follow-up
Poor arrangement of follow-up services
Poor access to follow-up services
Addiction concerns
Unavailability of analgesics in pharmacies in areas in which minorities live
(e.g., pharmacies may not stock opiates for fear of theft and violence)
Economic disadvantages (e.g., limited resources result in decisions based on
paying for analgesics versus paying for other necessities)
Inadequate assessment
Culturally inappropriate pain rating scales
Basing of pain assessments on the clinician’s perception of overt
pain behaviors

222 Clinical Manual of Pain Management in Psychiatry
Substance-Dependent and
Substance-Abusing Patients
The patient presenting with pain complaints who simultaneously has ongoing
substance abuse or dependence (or a prior history of either) can pose signifi-
cant challenges in terms of treatment options. Although effective pain man-
agement should never be withheld because of an abuse or addiction history,
effective treatment might require an array of pain-reducing approaches (e.g.,
use of adjunctive agents, agents with low abuse potential, physical and psy-
chological therapies, and patient participation in a concurrent substance abuse
treatment program). General principles for pain management of this group
are outlined in Table 9–3 (Prater et al. 2002; Savage 1998; Scimeca et al. 2000).
The patient’s detoxification from the substance(s) on which he or she is de-
pendent might be required before the initiation of treatment. An overly aggres-
sive detoxification can be particularly distressing for patients, perhaps result-
ing in the inclination to abandon treatment/detoxification. In addition, during
the course of detoxification, it could be imperative to undertake simultaneous
measures to mitigate pain; otherwise, the distress to which the patient is sub-
jected might be too great, and the patient may develop intense fears that his or
her pain will be unrecognized and inadequately treated.
Detoxification is required for the patient who is alcohol dependent. In
some cases, the substances abused might have appeal as a means of controlling
one’s psychological distress (e.g., cannabis and benzodiazepine abuse to ad-
dress underlying anxiety or ineffective coping). Hence, psychological inter-
ventions, along with prudent psychopharmacologic interventions for underly-
ing psychiatric disorders, might also be required to effect optimal pain control.
Patients who receive opiates over the long term for treatment of pain may
become addicted to the opiates. This issue is one that raises controversy. Some
authors suggest that opiate dependence does not accompany appropriate dos-
ing in pain patients (Portenoy and Foley 1986). Nonetheless, the issue can and
does arise—that patients who have been treated with analgesics can become
dependent on pain medications.
Iatrogenic Drug Dependence
As mentioned earlier, persons may become addicted to medications pre-
scribed by physicians to treat pain (e.g., opioids, benzodiazepines, stimulants).

Special Populations 223
Table 9–3.
Pain management in patients with substance abuse/
dependence
Address pain with
Pharmacologic strategies
Nonopioid analgesics are encouraged
If opioids are required,
Use those that produce less euphoria
Scheduled doses are preferred over “as needed”
Long-acting medications are preferred
Nonpharmacologic therapies
Physical/occupational therapy
Heat/cold therapies
Transcutaneous electrical nerve stimulation
Relaxation/hypnosis/biofeedback
Psychotherapy
Allay fears regarding inadequate pain treatment
Address psychiatric comorbidities
Depression
Anxiety
Sleep disorders
Monitor and treat substance withdrawal (e.g., alcohol, benzodiazepine, opiate)
Support measures to achieve/sustain addiction recovery
Address detoxification and rehabilitation needs for ongoing substance abuse
Assess adherence with substance abuse treatment programs
Consider making opioid analgesia contingent on verifiable participation in
addiction recovery treatment program
Assess for signs of relapse
Periodic, random urine toxicology screens
Frequent follow-up and monitoring
Assessment of overall functioning
Potential for misuse of extended-release formulations (e.g., injecting
extended-release medications intended for oral use)
Enlist family member or other trusted person to assist with dispensing medication
Consider use of a patient treatment contract (see Chapter 10)
Source. Adapted from Prater CD, Zylstra RG, Miller KE: “Successful Pain Management for the
Recovering Addicted Patient.” Primary Care Companion to the Journal of Clinical Psychiatry
4:125–131, 2002. Copyright 2002, Physicians Postgraduate Press. Used with permission.

224 Clinical Manual of Pain Management in Psychiatry
This dependence may occur in a variety of ways. The patient might seek out
multiple physicians to acquire such medications, or a single physician may be
involved. Patients might offer somatic complaints to acquire medications. The
prescription of medications might be construed by the patient as a sign of car-
ing and nurturance. The well-meaning physician might be highly esteemed and
valued by the patient and as a consequence might placate the patient’s requests
for additional medications.
The clinician might not anticipate the patient’s risk of dependence on the
medications. Often, clinicians are so preoccupied with the patient’s allegations
of pain that they fail to recognize that increasing levels of medications are be-
ing requested or that, despite the use of these medications, the patient’s func-
tioning does not appear to improve in adaptive areas. This should signal that
the medications are not effective or might be being consumed inappropriately
by the patient such that the misused medications interfere with the patient’s
adaptive function and rehabilitation.
In cases of iatrogenic drug dependence, the patient may require careful sys-
tematic detoxification from the medications on which he or she has become
dependent. The patient’s functioning might then improve. Behavioral measures
could be required to de-emphasize the focus on somatic complaints. Cogni-
tive-behavioral interventions might be required to address ineffective coping,
troublesome cognitive appraisals, and adverse emotions. It is these emotions
the patient has been trying to “treat” with the medications prescribed.
There are concerns that long-term use of opiates might actually interfere
with pain mitigating processes. A number of cellular mechanisms are being
explored to assess mechanisms underlying tolerance to analgesic efficacy (Fish-
bain et al. 1992; Streltzer 2001). It should be borne in mind that the opiate-
dependent patient requires substantially higher opiate doses to effectively
manage severe pain conditions.
Patients With Terminal Conditions
Patients with terminal medical conditions who have pain require compassionate
care. In addition to pain management, sensitivity to the patient’s perceptions of
and concerns regarding death is required. Patients with terminal conditions
require regular visitation, a fact frequently ignored by physicians, who often
focus on correctable disease or might subconsciously view a patient’s death as

Special Populations 225
their failure. Visiting, even when a patient avoids discussions about his or her
health or prognosis, conveys that the clinician is reliable, dependable, and avail-
able should the occasion (and need) arise to discuss more openly important is-
sues that surround a terminal condition.
Patients may require physical contact, appropriate to the context of the sit-
uation, to convey support and attentiveness. Attention to the content of what
the patient says is necessary, as is answering the patient’s questions in an honest
and respectful manner. Patients may inquire about the nature of their illnesses
and whether they are at terminal stages. By basing their responses on what is
known about the nature and course of the illnesses, physicians can be honest
without eradicating hope. Building false hopes is discouraged.
Some patients may find such disclosures to be overwhelming, which the
physician may assume is a form of denial. However, this assumption can be in-
sulting to the patient and perhaps intrusive, especially if a psychiatric consult-
ant is asked to evaluate the patient about this presumed denial. Respect for the
patient’s defenses, even denial, may well be required at such times. Aggres-
sively attempting to eradicate this defensive stance may serve only to distance
the patient, causing him or her to become more resistant to the clinician’s ef-
forts and care. On the other hand, patients may have fears about asking ques-
tions or may not know how to begin making inquiries. The physician might
inquire of the patient how much it is he or she wishes to know about the cur-
rent illness, thus facilitating dialogue on the matter.
Physicians also need to be sensitive about the extent to which the patient,
family, significant others, and medical staff are aware of the terminal nature of the
patient’s illness. Every effort should be made to facilitate communication be-
tween the patient and family (and significant others) about the prospect of
death—but, again, the issue should be gently and respectfully broached, not
forced. Reluctance on the part of the physician to openly discuss such matters
may come from fear that the family and patient will think the physician has given
up on effective care of the patient or from fears of discouraging or upsetting the
patient and family. Opening discussion among the patient and the patient’s fam-
ily could help reduce the isolation, loneliness, and even the dehumanizing fea-
tures that surround death for the patient. Family conflicts can emerge around the
time of death. Opening dialogue between family members at such times can re-
lieve their stress and help them begin the work of bereavement. Family meetings
may provide an opportunity for refocusing on the issues that matter.

226 Clinical Manual of Pain Management in Psychiatry
Physicians may be the target of anger from the patient, the family, or both.
Taking a defensive stance in such situations should be avoided. The patient
and family might need to ventilate their feelings about death and about the
meaning of the patient’s illness for them. The best strategy might be to articulate
the feelings and emotions underlying the anger, such as “This has been such a
long illness and a long struggle for everyone. It is impossible to imagine what
it feels like to be challenged with the prospect that our life activities are inter-
rupted prematurely, that our dreams will go unfulfilled.” or “It probably feels
as though I let you down.” Such approaches are likely to diffuse the tension of
the impending death and are less likely to escalate into a conflict, as could be
the case if the physician took a defensive stance. The doctor may be the safest
target of anger, especially if unresolved matters in the patient’s relationships are
too overwhelming to address directly in the final days.
The family and patient may need reassurance that pain will be maximally
controlled. The patient and others may be concerned that pain control will
make the patient unaware of his or her surroundings or otherwise incapaci-
tated. Modifying the patient’s pain-relief regimen according to his or her wishes
is desirable, whereas withholding effective pain treatment to appease the fam-
ily’s need to keep the patient alert is not. Family members may need to be re-
minded that severe pain will likely mitigate the sedating properties of opiates
and other analgesics. The family could also need to be reassured that the an-
algesics will not result in or hasten the patient’s death.
Hospice Care
Hospice care is a system of care for patients with terminal conditions and their
families. The goals of therapy within hospice care are to provide palliation
(i.e., pain management) but not cure and to ensure dignity of care without the
encumbrances of life support measures and invasive procedures.
Care for patients is often arranged in the home, an environment that is fa-
miliar and often more comfortable for the patient. Access to the patient by
family, friends, and customary supports is more feasible when the patient re-
mains at home. Service to the patient is coordinated among family, other mem-
bers of the patient’s usual support system, medical and mental health personnel,
clergy, and volunteers.
Generally, the cost of hospice care is far less than that for hospital care. How-
ever, higher costs are incurred when hospice care is provided in nursing homes

Special Populations 227
or hospitals or when patients are furnished with care in an established hospice
setting. Medicare coverage is provided for hospice care; however, there are lim-
itations to the coverage provided (see Table 9–4). Medicare provides a flat
amount for hospice services. Thus, funding provided for a patient expected to
live no more than 6 months is quite low, but if the patient lives beyond
6 months, the provision of hospice services continues without reimbursement,
and the hospice agency incurs a financial loss. As a result, hospice agencies
may be reluctant to accept patients whose illnesses are not inclined to result in
death within 6 months. Physicians may be inclined to erroneously withhold
an offer of hospice care to patients and their families, fearing that the antici-
pated time until death might exceed 6 months. Therefore, physicians may be
offering hospice care to patients too late in the course of the illness.
As more patients utilize hospice services, psychiatric involvement will be
ever increasing in hospice care. Many patients develop psychiatric symptoms
either as a consequence of the illnesses that prompted their admission or as
effects of treatment. Common psychiatric disturbances likely to be encoun-
tered include mood disturbances, anxiety, delirium, and dementia. A number of
psychiatric interventions and medications can be employed to reduce the dele-
terious effects of these psychiatric disturbances. Psychiatrists can be particu-
larly influential in ensuring that pain is adequately addressed in hospice pa-
tients.
Family members and friends may have emotional difficulties associated
with hospice care. These can include a sense of futility, anticipatory bereave-
ment, guilt (i.e., viewing hospice as a “giving up” on the patient or a with-
holding of necessary care), and despair. Psychiatrists can be helpful in facili-
tating the family’s adjustment to the hospice care transition and in preventing
emotional difficulties from impeding connections with the patient that might
be required in the time they have remaining. Psychiatrists can help the family
recognize that the focus has become one of ensuring improved quality of life
Table 9–4.
Medicare requirements for hospice care
Physician must certify that the patient’s condition is terminal (i.e., the patient’s life
expectancy is expected to be 6 months or less).
Patient must consent to hospice care in lieu of traditional hospital care.
Hospice care agency must be certified by Medicare in order to accept benefits from
the federal government.

228 Clinical Manual of Pain Management in Psychiatry
for the patient and can help them maximize the time they have available. Is-
sues concerning death and dying may need to be a focus of care for the patient
as well as his or her family and other loved ones.
Religious Issues and Spirituality
Issues of spirituality may be encountered when caring for the patient who has
a terminal condition. There may be uncertainties and confusion about treat-
ment decisions (e.g., advance directives), particularly if the patient has fears
about the moral implications of a particular treatment. Sometimes religious
inquiries can arise when there is a conflict with the physician about prognosis,
a distrust of the medical system, and/or a rejection of the physician’s expertise.
Some patients are distressed about the prognosis and therefore hope for some
miraculous intervention. After all, when the prognosis is grim, who would not
want a miracle? In situations in which there is persistent pain or severe unto-
ward effects of treatment, some patients may be concerned about punishment,
guilt, and prior transgressions.
Many physicians feel unskilled or uncomfortable addressing religious or
spiritual issues, often bypassing them to focus on somatic concerns, treatment
issues, or even the psychological underpinnings of the discussion (Lo et al.
2002). Yet avoidance of these topics may further increase the patient’s isola-
tion as he or she struggles with issues pertaining to such matters. This avoid-
ance may inadvertently jeopardize the therapeutic alliance.
Because of the diversity of concerns that can prompt issues of spirituality,
careful inquiry by the physician may expose the patient’s underlying concerns
or fears (see Table 9–5). While respecting the patient’s spiritual issues and di-
lemmas, the clinician should attempt to reflect religious issues back onto the
patient—for example, by inquiring, “Why do you ask?” or “I wonder if you
have concerns about what to do next?” Open-ended inquiry can lead to discus-
sions of matters that have an impact on treatment and on the doctor–patient re-
lationship. The clinician is cautioned against being drawn into the potential
pitfalls of religious and theological arguments, scriptural interpretations, and
so forth that can bypass the patient’s underlying concerns. It might be also be
prudent to elicit the support of others (e.g., clergy and pastoral counselors) when
confronted with moral issues.
Special Populations 229
Key Points
• Pain management approaches need to include psychological, cognitive,
and emotional factors pertinent to the needs of patients.
• It is imperative to attend to the unique issues pertinent to the assessment
and treatment of pain in special populations, reflective of the patient’s cul-
tural norms, age, and stage of development.
• Patients with the greatest barriers to adequate pain treatment include those
with known or suspected substance abuse and/or dependence. Such pa-
tients are at risk for undertreatment, particularly as clinicians may be fear-
ful of contributing to abuse and addiction.
• Pain management among individuals with abuse and addiction histories
requires relatively close monitoring, careful documentation of treatment
measures undertaken and outcomes, use of pain treatment contracts, care-
ful utilization of analgesics, and use of adjuvant agents and multimodal
treatments to reduce sole reliance on potentially addicting analgesic agents.
References
American Geriatrics Society: The management of chronic pain in older persons: AGS
Panel on Chronic Pain in Older Persons. J Am Geriatr Soc 46:635–651, 1998
Berde CB, Masek B: Pain in children, in Textbook of Pain, 4th Edition. Edited by Wall
PD, Melzack R. London, England, Churchill Livingstone, 1999, pp 1463–1477
Table 9–5.
Approaches to dealing with spiritual issues
Use open-ended inquiry.
Acknowledge the patient’s spiritual concerns.
Empathize with the patient.
Refocus spiritual questions back onto the patient.
Attempt to clarify whether the patient’s spiritual dilemmas concern
Decisions about further treatment interventions.
Decisions about advance directives.
The doctor–patient relationship.
Questions about the validity and utility of medical interventions.
Avoid reassuring the patient prematurely.
Avoid trying to solve the patient’s spiritual dilemmas for him or her.

230 Clinical Manual of Pain Management in Psychiatry
Beyer J, DeGood DE, Ashley LC, et al: Patterns of postoperative analgesic use with
adults and children following cardiac surgery. Pain 17:71–81, 1983
Bonham VL: Race, ethnicity, and pain treatment: striving to understand the causes
and solutions to the disparities in pain treatment. J Law Med Ethics 29:52–68,
2001
Cleeland CS: Undertreatment of cancer pain in elderly patients. JAMA 279:1914–
1915, 1998
Cleeland CS, Gonin R, Hatfield AK, et al: Pain and its treatment in outpatients with
metastatic cancer. N Engl J Med 330:592–596, 1994
Crawley L, Payne R, Bolden J, et al: Palliative and end-of-life care in the African Amer-
ican community. JAMA 284:2518–2521, 2000
Crook J, Rideout E, Browne G: The prevalence of pain complaints in a general pop-
ulation. Pain 18:299–314, 1984
Ebert AM: Use of nonnarcotic analgesics during breastfeeding. J Hum Lact 13:61–64,
1997
Eccleston C, Morley S, Williams A, et al: Systematic review of randomised controlled
trials of psychological therapy for chronic pain in children and adolescents, with
a subset meta-analysis of pain relief. Pain 99:157–165, 2002
Ferrell BA: Pain in the nursing home. J Am Geriatr Soc 38:409–414, 1990
Ferrell BA: Pain management in elderly people. J Am Geriatr Soc 39:64–73, 1991
Fishbain DA, Rosomoff HL, Rosomoff RS: Drug abuse, dependence, and addiction
in chronic pain patients. Clin J Pain 8:77–85, 1992
Gagliese L, Katz J, Melzack R: Pain in the elderly, in Textbook of Pain, 4th Edition.
Edited by Wall PD, Melzack R. London, England, Churchill Livingstone, 1999,
pp 991–1006
Gallagher RM, Verma S, Mossey J: Chronic pain: sources of late-life pain and risk
factors for disability. Geriatrics 55:40–47, 2000
Goldschneider KR, Mancuso TJ, Berde CB: Pain and its management in children, in
Bonica’s Management of Pain, 3rd Edition. Edited by Loeser JD, Butler SH,
Chapman CR, et al. Philadelphia, PA, Lippincott Williams & Wilkins, 2001,
pp 797–812
Herr KA, Mobily PR: Comparison of selected pain assessment tools for use with the
elderly. Appl Nurs Res 6:39–46, 1993
Kagawa-Singer M, Blackhall LJ: Negotiating cross-cultural issues at the end of life:
“You got to go where he lives.” JAMA 286:2993–3001, 2001
Leith PJ, Weisman SJ: Pharmacologic interventions for pain management in children.
Child Adolesc Psychiatr Clin N Am 6:797–815, 1997
Leo RJ, Singh A: Pain management in the elderly: use of psychopharmacologic agents.
Annals of Long-Term Care: Clinical Care and Aging 10:37–45, 2002

Special Populations 231
Lo B, Ruston D, Kates LW, et al: Discussing religious and spiritual issues at the end of
life. JAMA 287:749–754, 2002
Ostensen M: Nonsteroidal anti-inflammatory drugs during pregnancy. Scand J Rheu-
matol 107:128–132, 1998
Portenoy RK, Foley KM: Chronic use of opioid analgesics in non-malignant pain:
report of 38 cases. Pain 25:171–186, 1986
Prater CD, Zylstra RG, Miller KE: Successful pain management for the recovering
addicted patient. Prim Care Companion J Clin Psychiatry 4:125–131, 2002
Renehan BW: The galactopharmacopedia. Narcotic analgesics: use in the breastfeeding
woman. J Hum Lact 5:135–137, 1989
Roy R, Thomas M: A survey of chronic pain in an elderly population. Can Fam Phy-
sician 32:513–516, 1986
Savage S: Principles of pain treatment in the addicted patient, in Principles of Addiction
Medicine, 2nd Edition. Edited by Graham AW, Schultz TK, Wilford BB. Chevy
Chase, MD, American Society of Addiction Medicine, 1998, pp 919–944
Scimeca MM, Savage SR, Portenoy R, et al: Treatment of pain in methadone-main-
tained patients. Mt Sinai J Med 67:412–422, 2000
Sengstaken EA, King SA: The problems of pain and its detection among geriatric
nursing home residents. J Am Geriatr Soc 41:541–544, 1993
Sifford LA: Psychiatric assessment of the child with pain. Child Adolesc Psychiatr Clin
N Am 6:745–781, 1997
Stevens B: Pain assessment in children: birth through adolescence. Child Adolesc Psy-
chiatr Clin N Am 6:725–743, 1997
Streltzer J: Pain management in the opioid-dependent patient. Curr Psychiatry Rep
3:489–496, 2001
Todd KH, Samaroo N, Hoffman JR: Ethnicity as a risk factor for inadequate emergency
department analgesia. JAMA 269:1537–1539, 1993
Warden V, Hurley AC, Volicer L: Development and psychometric evaluation of the
Pain Assessment in Advanced Dementia (PAINAD) scale. J Am Med Dir Assoc
4:9–15, 2003
Wen YR, Hou WY, Chen YA, et al: Intrathecal morphine for neuropathic pain in a
pregnant cancer patient. J Formos Med Assoc 95:252–254, 1996
Won A, Lapane K, Gambassi G, et al: Correlates and management of nonmalignant
pain in the nursing home. J Am Geriatr Soc 47:936–942, 1999
Zatzick DF, Dimsdale JE: Cultural variations in response to painful stimuli. Psychosom
Med 52:544–557, 1990
This page intentionally left blank

233
10
Forensic Issues Pertaining to Pain
Litigation and Pain
Concerns naturally arise about the potential role of ongoing litigation and its
effect on the experience of pain, the rehabilitation process, and allegations of
disability. It is conceivable that the pursuit of litigation could have an impact
on the treatment and rehabilitation of a patient’s pain (Gatchel et al. 1995). It
has been reported that people with whiplash injuries who were in the midst of
litigation reported more pain than those who were no longer involved in liti-
gation (i.e., whose cases were settled or resolved) (Swartzman et al. 1996). How-
ever, there was no statistically significant difference between those who were in
the midst of litigation and those who were not with regard to employment sta-
tus, return to work, and functional adaptation. The clinician is cautioned
against making causal assumptions about the role of the litigation in influenc-
ing the patient’s agenda (Swartzman et al. 1996). It is possible that for some
patients, pain is exaggerated so as to increase the magnitude of the monetary
gain of a litigation claim. On the other hand, it is possible that the stress of the
litigation process leads to marked muscle tension, resulting in accentuation
and exaggeration of pain complaints. Alternatively, a third factor (e.g., fears of

234 Clinical Manual of Pain Management in Psychiatry
disability, long-term effects of pain, fears of future limitations in work capac-
ity, fears of unemployment and financial hardship) may lead to the simulta-
neous accentuation of pain complaints as well as the pursuit of litigation.
Careful attention to the types of concerns the patient has could be highly
informative in determining which of these interpretations is operating. Atten-
tive listening can lead to those interventions that allay the patient’s fears. One
of the biggest concerns that patients have is that their pain complaints might
not be well received or might be misinterpreted as a ploy to achieve secondary
gains. A clinician’s reassurance that the clinician is working with the patient to
address his or her pain, to improve functional adaptation, and to reduce dis-
ability while improving quality of life can help to alleviate such fears. The cli-
nician may need to discuss openly the issues and concerns that beset the patient
so that they no longer concern the patient and so they do not adversely affect the
doctor–patient relationship.
In addition, pain management has received increasing legal attention. A phy-
sician was found guilty of elder abuse based on the premise that he inadequately
treated a patient’s pain before the patient’s death (Albert 2001). In a similar ac-
tion, a physician was sanctioned by the Oregon Board of Medical Examiners
for negligence (i.e., the failure to meet the standard of care as it related to ad-
equate pain management) (Charatan 1999). As these cases illustrate, external
pressures are increasing to ensure that physicians are knowledgeable about ef-
fective pain management. Consultation with pain management specialists
would be warranted in particularly difficult cases.
Medication Diversion
The issues around medication diversion have acquired increasing media, pub-
lic, and legal attention, especially as related to pain medications. Diversion re-
fers to the misappropriation of prescribed medications, either by physicians or
by other persons who acquire medications from treating sources. Opiate an-
algesics of all sorts and varieties can be diverted for their abuse appeal. In the
past, agents such as butorphanol had abuse appeal; lately, concerns about di-
version have focused on oxycodone. The controlled-release formulation, when
crushed and taken into the body intranasally or intravenously, produces a sen-
sation of euphoria; in some communities its popularity has exceeded that of
crack cocaine and heroin (Tough 2001).

Forensic Issues Pertaining to Pain 235
From a drug regulatory standpoint, medication diversion is difficult to con-
trol, because medications are produced by a legitimate pharmaceutical company,
prescribed by doctors, and dispensed, presumably, to legitimate patients. There
are neither drug lords with whom to contend nor border patrol issues. Instead, the
culprits can include physicians (who, for example, acquire illicit drugs in exchange
for prescribing medications with diversion appeal), patients, and others associated
with legitimate patients who surreptitiously divert the medications meant for the
patient. The Internet has become an increasingly popular source of acquisition of
controlled substances as well (Forman et al. 2006). For a nominal fee, one’s claims
are reviewed by an online “physician,” who then arranges for delivery of con-
trolled substances to one through the mail. Again, from a drug regulatory stand-
point, these Internet sources are difficult to monitor and control.
Many psychoactive medications can be diverted. Clearly, the opiates are
particularly appealing for their euphoric effects, allowing the abuser to avoid
the encumbrances and risks associated with intravenous heroin abuse. On the
other hand, benzodiazepines, barbiturates, and psychostimulants have also been
diverted because of their appeal on the street.
Physicians may possibly be colluding with patients in the diversion process.
Physicians might be swept away by patient insinuations that if the physician
“really cared about” him or her, the medications would be prescribed. Ideali-
zation of the physician who complies with the patient’s demands and the phy-
sician’s need for such idealizations can result in the blurring of boundaries that
traps the physician into prescribing medications with diversion appeal. Over-
identification with the patient and the propensity to assume the mantle of
power and authority to alleviate the patient’s distress can lead to inappropriate
prescription of such medications. In addition, the physician’s inward denial
regarding reasonable treatment alternatives can interfere with stopping such
endeavors. Thus, for example, despite the ongoing pain and distress reported
by the patient, the physician ignores alternative strategies to address pain, in-
stead increasing the doses of the opiates (or other requested medications) to
appease the patient. The physician can have difficulty managing the hostile,
demanding patient, could be unable to set therapeutic limits, and might fear
the patient’s threats to stop treatment—all of which can perpetuate inappro-
priate prescribing practices and potential drug diversion.
If diversion is suspected, the physician is entitled to shore up reasonable
precautionary practices. Thus, a patient treatment contract (as described be-

236 Clinical Manual of Pain Management in Psychiatry
low) might be required. The details of the contract can specify that the patient
will have to comply with random checks of pill counts to continue in treat-
ment. Exhausting one’s prescribed medications shortly after receiving them
could suggest that the medications are being diverted to others. The patient
might need to be approached to clarify the reasons for the significantly lower
than expected pill counts and the whereabouts of that medication. If the drug
is insufficiently accounted for, a decision can be made to terminate the treat-
ment plan.
Undertaking legal measures once diversion is substantiated is a consider-
ation, but the clinician faces a risk of confidentiality breach under such circum-
stances. Legal consultation should be sought before any law enforcement reports
are filed. Consultation with colleagues and use of alternative treatment strat-
egies (e.g., use of medications with less abuse or diversion appeal, acupunc-
ture, transcutaneous electrical nerve stimulation units, physical or occupa-
tional therapies, massage) could be considered in managing any ongoing pain.
Opiate Adulteration and Misuse
Using an opiate in a manner that is unintended contributes to the abuse po-
tential of such agents and has increased the appeal of diversion of prescribed
medications (e.g., the aforementioned ingestion of crushed controlled-release
oxycodone for its euphoric effects). It was earlier thought that by antagonizing
µ-receptors, less abuse liability would be associated with the opioid agonist-
antagonists. Unfortunately, these expectations were not borne out. Thus, it
was discovered that some persons pulverize the medication pentazocine and in-
ject it intravenously for its euphoric effects. To counter this, the manufacturer
has added naloxone (an opiate antagonist) to the tablet. When taken orally, as in-
tended, the naloxone is poorly absorbed and, therefore, does not interfere
with the intended analgesic effects. However, if pulverized and injected, the
naloxone becomes fully effective and mitigates any euphoric effect produced
by the abuse of this medication. The same approach has been employed to re-
duce misuse (i.e., intravenous injection) of Suboxone (buprenorphine with
naloxone). Butorphanol has been made available as a nasal spray formulation
to facilitate ease of administration, particularly in patients with difficulty swal-
lowing. When combined with inhaled antihistamines, butorphanol can pro-
duce a euphoric effect similar to that of heroin.

Forensic Issues Pertaining to Pain 237
Concerted efforts on the part of clinicians in screening patients for prob-
lematic prescription and illicit substance use (e.g., Prescription Drug Use
Questionnaire) (Compton et al. 1998) may help to avoid contributing to an-
algesic misuse and adulteration. In addition, ongoing efforts on the part of the
pharmaceutical industry will be necessary to refine strategies that reduce op-
portunities for adulteration and misuse.
Legal Issues Related to Opioid Prescribing
The federal guidelines for prescribing opioids are defined in the Code of Fed-
eral Regulations (CFR), Controlled Substances Act (21, USC 802). An ex-
cerpt pertaining to the guidelines for clinicians is provided below:
A prescription for a controlled substance to be effective must be issued for a
legitimate medical purpose by an individual practitioner acting in the usual
course of his professional practice. The responsibility for the proper prescrib-
ing and dispensing of controlled substances is upon the prescribing practitioner,
but a corresponding responsibility rests with the pharmacist who fills the pre-
scription. An order purporting to be a prescription issued not in the usual
course of professional treatment or in legitimate and authorized research is
not a prescription and the person knowingly filling such a purported prescrip-
tion, as well as the person issuing it, shall be subject to the penalties provided
for violations of the law. (Title 21, CFR 8, 1306.04[a])
The purpose of the Controlled Substances Act is to impart that clinicians,
pharmacists, and the patient share responsibilities regarding the regulation of
controlled substances. Thus, for the clinician, Schedule II substances should
be prescribed only in the context of an ongoing clinician-patient relationship.
The clinician should be comfortable that the patient is indeed capable of uti-
lizing the prescribed substances responsibly and that there is a verifiable med-
ical condition that warrants implementing such treatments. Establishing the
veracity of the medical condition necessitates a careful history, patient assess-
ment and physical examination, review of medical records, appropriate labo-
ratory investigation, and patient follow-up. Patient monitoring is essential to
corroborate responsible use of the prescribed agents and to establish the effi-
cacy of the prescribed agents in terms of pain relief, improvement of adaptive
functioning, and overall well-being. There should be a corresponding docu-
mentation in the medical record of the above matters, with regular follow-up

238 Clinical Manual of Pain Management in Psychiatry
of patterns of use, efficacy (as suggested by pain scale ratings and functional as-
sessments), any issues pertaining to substance abuse concerns, and justification
of continued prescribing of controlled substances, if continued prescribing is
necessary.
Pharmacists, likewise, are responsible to ensure that the prescriptions are
legitimate, to determine the purpose of the medication, and to verify that the
person to whom the medication is dispensed is the intended recipient. If an-
other is acting on behalf of the patient, it may be prudent to ensure that the
individual is indeed acting as the patient’s representative and to secure gov-
ernment issued photo identification (included in the patient’s record). Such
measures may mitigate concerns regarding lost or stolen prescriptions.
It is important to recognize that patients are entrusted with the prescribed
medications and are likewise imbued with responsibilities according to the
CFR. The patient should be apprised that he or she is not to share controlled
medications with others, that medications are to be kept locked up in a secure
setting, and that if medications are used up prematurely (e.g., using more than
20% of the medication early), the patient must contact the physician to discuss
adjustment of the dosing regimen so as to anticipate future prescription needs.
In this way, concerns around securing emergency refills and additional med-
ications before the next scheduled visit for the clinician and pharmacist can be
allayed and addressed in the treatment relationship.
Patient Contracts
Clinicians may be uncomfortable about prescribing opioids (or other con-
trolled substances) for the long term, especially in chronic nonmalignant pain.
The discomfort is often related to concerns about federal regulations, substance
abuse, addiction, and diversion. Use of a treatment contract (see Table 10–1) for
patients receiving chronic opioid treatment has been advocated by medical
and legal experts (Burchman and Pagel 1995), and may help to reinforce ad-
herence with the guidelines offered in the CFR. The contract assumes that the
clinician and patient are involved in a mutual endeavor and outlines explicitly
the rights and responsibilities of both parties. In this way, both the clinician and
the patient are clear about the treatment and are protected on issues pertaining
to the use of controlled substances. The contract provides for the appropriate
disclosure of these issues and the parameters for treatment—including the use

Forensic Issues Pertaining to Pain 239
and acquisition of analgesics and other treatment responsibilities. The patient
is made aware of his or her responsibilities with regard to use of the medica-
tion; other responsibilities regarding participation in nonpharmacologic facets
of treatment (e.g., physical therapy, smoking cessation, avoidance of alcohol,
weight loss, psychotherapy, substance abuse treatment) can also be delineated.
The patient is informed that violation of the conditions of the contract can al-
low the physician to terminate the use of controlled substances. The contract
can specify limitations regarding the acquisition of controlled substances (e.g.,
that prescriptions will not be provided for “running out early,” prescription loss,
or spilled or misplaced medications) and can state that prescriptions should not
be acquired from other clinicians, emergency departments, or after-hours fa-
Table 10–1.
Items to be factored into a patient contract for use
with controlled substances
Physician responsibilities
Disclose that the substances prescribed are controlled by local, state, and federal agencies.
Specify that prescriptions for controlled substances will be made only during regular
office hours—not at night, on weekends, on holidays, and so forth.
Specify that unknown risks may be associated with long-term use of controlled
substances and that the patient will be kept apprised of any advances in the field
that call attention to such risks.
Disclose information regarding potential development of tolerance and physical
dependence.
Specify the grounds for termination of prescription of controlled substances.
Patient responsibilities
Prevent loss, misplacement, or theft of controlled substances, understanding that
controlled substances will not be readily replaced.
Be present in person to pick up prescriptions for controlled substances.
Take the medication in the dose and at the intervals prescribed.
Keep track of the amount of medication remaining.
Comply with random urine or blood testing to document the proper use of
medications and confirm adherence.
Comply with the laws of the state regarding use of medications and operation of
motorized vehicles.
Work with the physician in developing better health habits.
Do not divert or dispense controlled substances to others.

240 Clinical Manual of Pain Management in Psychiatry
cilities. A copy of the contract, signed by the clinician and the patient, can be
given to the patient; the original should be filed in the patient’s chart. In this
way, the contract can serve as a basis for the overall treatment plan.
Drug Testing in Clinical Practice
Drug testing is increasingly utilized in the clinical setting, particularly because
it can assist with complex medical and legal aspects of pain management care.
Although drug testing can create a level of discomfort for clinician and patient
alike, recommendations for urine drug testing may offer several advantages to
the care of patients, especially those with chronic, nonmalignant pain, requir-
ing long-term opioids and other controlled substances for pain relief (Heit
and Gourlay 2004). (Some of these advantages are outlined in Table 10–2.) It
should be recognized that with open disclosure and dialogue regarding the
uses of drug testing within the scope of the clinician–patient treatment rela-
tionship, some of the discomfort associated with the stigma of drug testing
can be allayed. It is imperative, however, that appropriate documentation and
diagnostic information be provided to ensure accuracy of the testing and ap-
propriately secure patient confidentiality. Clinicians also need to be aware that
there are several mechanisms by which individuals can attempt to undermine
testing. These can include provision of substitute urine from another person,
adulteration of the urine sample so as to conceal substances, and dilution of
the sample, necessitating that certain standardized procedural measures be im-
plemented in securing that samples are appropriately obtained and handled
for testing. On the other hand, the advantage of urine testing can be a factor
that leads to the development of mutual trust between clinician and patient,
bolstering the therapeutic alliance.
State and National Prescribing Data Banks
Because of concerns regarding abuse and diversion of prescription medica-
tions, President Bush signed the National All Schedules Prescription Elec-
tronic Reporting Act into law on August 11, 2005. It is hoped that sweeping
changes will occur across the country as a result, such that by 2010 all states
will have to establish drug monitoring programs. The purpose of the moni-

Forensic Issues Pertaining to Pain 241
toring program is to establish a data bank that allows physicians and pharma-
cists to access patient records, track the prescription of controlled substances,
and avoid duplicate prescribing of controlled substances. (The purposes of the
database system are outlined in Table 10–3.)
Additionally, clinicians will be held accountable for updating such systems
and tracking medications prescribed by other treating sources through the
data bank. In this way, the clinicians will be expected to review the controlled
substances that have already been prescribed, avoid overprescribing, and mon-
itor for potential untoward reactions and drug interactions. Unfortunately,
such measures can impose a greater burden on clinicians with regard to track-
ing patient treatment. Further, there may be greater liability for clinicians for
Table 10–2.
Advantages of urine drug testing in clinical pain
management practice
Urine drug testing provides
A baseline identification of drug use on admission
Corroboration of prescription drug adherence
Identification of medications provided by other treatment providers and verification
of patient’s history
Identification of other disease states that can complicate treatment planning
(e.g., kidney disease, diabetes)a
Identification of illicit substance use
Confirmation of or refuting of concerning behaviors manifested by the patient
(e.g., intoxicated appearances, pill count discrepancies, requests for early refills)
Delineation of the need for substance abuse treatment
A barrier to diverting controlled substances
Proof of complying with federal regulatory standards that the clinician is monitoring
controlled substances prescribed
An objective therapeutic regimen for reports to insurance providers, workers’
compensation, medical boards, and other regulatory agencies
Reduced mistrust on the part of the clinician regarding the patient’s presentation and
behavior
aWhen combined with macroscopic urinalysis.
Source. Adapted from Fornari FA, Siwicki DM, Bauer GB: “Urine Drug Testing and Monitoring
in Pain Management.” Practical Pain Management 6:12–14, 2006. Copyright 2006, PPM Com-
munications. Used with permission.

242 Clinical Manual of Pain Management in Psychiatry
untoward effects from prescribing, especially when the information in the
data bank will be at one’s disposal.
Naturally, with such a system, it is possible to access information about
what medications the patient has been prescribed, by whom, and why. Con-
cerns over issues regarding confidentiality are likely to be raised from such en-
deavors. Nonetheless, when the system is fully implemented, and several states
have already begun to initiate such data bank efforts, patients may need to be
apprised of the disclosure of such information, its accessibility, and implica-
tions for their care.
Confidentiality
Patient information can be released only with the authorization of the patient
or under proper legal compulsion. For the release authorization to be valid, the
patient must be informed of the purposes of the release and of the recipient(s) to
whom the information will be sent. At times, the patient must be informed
of the extent of the information released and its contents. Of course, to au-
thorize release of information, the patient must be competent to give consent
for the release (American Psychiatric Association Committee on Confidenti-
ality 1987).
Table 10–3.
Purposes of the National All Schedules Prescription
Electronic Reporting Act database system
As a national database, allows for the tracking of
Patient prescription use
Prescribing patterns of medical practitioners
Prescribing patterns according to geographical regions
Prescribing rates and patterns of controlled medications
As a clinician access database, allows for the
Tracking of your patient’s prescriptions
Assessment and evaluation of efficacy of prior and current medications prescribed
Continuous apprisal of medications prescribed to the patient by other treatment
providers
Ascertainment of whether controlled substances are obtained from multiple
providers and pharmacies concurrently
Monitoring for the potential of drug interactions

Forensic Issues Pertaining to Pain 243
Clinicians must always ensure that the contents of the patient’s medical
record are kept confidential. In the era of managed care and the sweeping de-
mands of insurance companies, reimbursement is often tied to the review of the
medical record (Hartmann 2001). Physicians often find that their business
interests (e.g., reimbursement) are at odds with patient protection. Patients
need to be apprised that the contents of the medical record might be requested
for reimbursement to be possible. If request for this information is refused, the
insurance company might not make payments for the cost of the services pro-
vided. It may be possible in some cases to withhold, without adversely influ-
encing reimbursement, selected aspects of the record that the patient finds too
difficult to divulge.
In addition, relevant, easily identifiable information pertaining to a specific
patient’s case cannot be divulged for educational, training, or publication pur-
poses. If the content of a patient’s history, presentation, or similar information is
to be used for such purposes, every effort should be made to remove any identi-
fying information that would allow the intended audience to identify the source.
The courts might require that physicians divulge the confidential infor-
mation contained in a medical record. Legal consultation might be required to
assist the clinician in determining the extent of the information that needs to
be disclosed. At times, the full content of the record is not required, and par-
ticularly personal matters might be best avoided in the legal forum to protect
the patient’s privacy.
To protect a patient (or another person) from imminent danger, it might
be required that the clinician reveal confidential information (Tara s o f f v.
Regents of the University of California 1976). Thus, if a patient threatens self-
harm, has a realistic plan, is at risk for lethality, and is not working with the
clinician in any way to ensure his or her safety, the clinician might have no re-
course but to divulge aspects of the history to potential sources of help who
might be able to mobilize the patient’s safety (e.g., family, friends, police, crisis
services). The clinician must have good cause to suspect that the patient (or
another person) is at risk of imminent danger and that other alternatives are
not available to remediate the situation. Again, the extent of the information
revealed could be limited to only those aspects that will affect the decision
making at hand and ensure the safety of the patient and others. Clinicians
must be familiar with state laws that regulate such disclosures and with limi-
tations of the confidentiality regulations.

244 Clinical Manual of Pain Management in Psychiatry
Disability Compensation
Disability Versus Impairment
Adisability is a putative deficit in functioning, implying a lack of ability to
perform activities required for work. It is rather subjective (i.e., is a perception
of restriction of function). On the other hand, an impairment is an abnormal-
ity in psychological or physiologic functioning, verifiable by a clinician and
based on observable findings (e.g., physical and mental status examination,
laboratory findings). The determination of eligibility for disability awards can
be quite complex, and the standards vary depending on the agency from which
the award is sought. The basis for the claim involves a review of evidence gath-
ered from a number of medical and, in some cases, nonmedical sources.
Workers’ Compensation
Workers’ compensation is a system o f laws ensuring that compensation awards
are provided for persons who sustain injuries during the course of work; it
does not litigate fault or negligence (Crook 1994). The goal of workers’ compen-
sation is to provide injured employees with money to cover the costs of med-
ical expenses and provide some portion of lost wages. A person who is injured
while on duty is not entitled to intangible compensation (e.g., for pain and
suffering). Critical to the award is the nature of the alleged injury and its re-
lationship to the work setting. A compensable physical or mental injury must be
reasonably connected to the work. The injury cannot arise solely from the
worker’s own health risks (e.g., coronary artery disease brought on by years of
cigarette smoking). Similarly, preexisting illnesses are not compensable. How-
ever, preexisting illnesses can predispose a person to complications arising from
work injuries. In such cases, the injury and its treatment are compensable. To
qualify as compensable, the injury must be accidental and cannot be deliber-
ately self-inflicted.
Critical to workers’ compensation is the establishment that the impair-
ment interferes with the performance of prior work. Prior work includes those
jobs that are suitable to, or related to, one’s training and qualifications. De-
terminations of awards are contingent on the anticipated prognosis and the
impact of the injury on one’s functional capacity. Functional capacity refers to
one’s abilities to perform activities of daily living, manage one’s finances, main-
tain relationships, and so forth.
Forensic Issues Pertaining to Pain 245
Social Security Disability
Unlike workers’ compensation awards, Social Security disability awards are
based on the finding that one’s physical or mental impairment interferes with
the ability to perform any job in the national economy, not just one’s former
job (see Table 10–4).
The impairment cannot be transient but must be present for a continuous
period of at least 12 months (Social Security Administration 2001). Accord-
ing to the Social Security Administration (SSA), to be eligible for disability the
impairment must preclude working to meet a minimum standard of financial
income. Essential to the assessment of claims of pain disabilities is the assess-
ment of one’s functional capacity (Enelow and Leo 2002) (including activities
of daily living and ability to lift, carry, push, pull, sit, stand, walk, manipulate,
see, hear, understand, remember, concentrate, and follow simple instruc-
tions). In addition to financial compensation, recipients can become eligible
for medical insurance (i.e., Medicaid or Medicare) (Institute of Medicine 1987;
Leo and Del Regno 2002).
Veterans Affairs Benefits
The U.S. Department of Veterans Affairs (VA) offers a disability program for
disabled American veterans. Unlike the SSA, no means test is required, and
unlike the SSA and workers’ compensation systems, there is no requirement
that the individual demonstrate an inability to work. Compensation through
the VA is of two types: service-connected benefits (i.e., for disabilities acquired
in the course of military service) and non-service-connected benefits (i.e., for
disabilities acquired after military service and precluding ability to work at a
Table 10–4.
Limitations considered by Social Security
Administration adjudicators
Physical limitations Mental limitations
Exertional Understanding and memory deficits
Postural Concentration deficits
Manipulative Deficits in social interaction
Visual Deficits in adaptation
Communicative Severe emotional disturbances
Environmental Psychosis

246 Clinical Manual of Pain Management in Psychiatry
substantial level) (Institute of Medicine 1987). In order to be compensable, the
alleged mental and/or physical disability must be validated by medical sources
and must be expected to last indefinitely. For a person to qualify for benefits,
a clinician must provide medical documentation that substantiates the allega-
tion of a disorder that is grounded in anatomic or physiologic evidence and
appropriate clinical signs and symptoms. In addition to financial awards, ben-
eficiaries are eligible to receive medical care provided through the VA health
system, including physical rehabilitation, prosthetic devices (if required), and
vocational rehabilitation services.
Disability and the Doctor–Patient Relationship
Several issues need to be addressed with the patient who intends to file a claim
for disability benefits (Mischoulon 2002), because the outcome of the disabil-
ity determination can have an impact on the doctor–patient relationship. Am-
bivalence may arise in response to a favorable decision. Although allowance of
a disability award means access to resources of financial support and medical
insurance, it might also stir up feelings of dependency, inadequacy, and loss
of self-sufficiency in the patient. On the other hand, an unfavorable decision
might be interpreted negatively (e.g., a withholding of needed resources). Such
feelings can be directed at the treating physician. Discussions between the phy-
sician and patient before the claim is submitted could possibly diffuse these po-
tential reactions and prevent them from impeding the treatment alliance.
It would be prudent for the physician to review with the patient the process
by which disability determinations are made, emphasizing that the physician
does not make the decision about disability eligibility but that the decision rests
with adjudicators (and possibly judges and courts if appeals are undertaken). In
addition, patients should be apprised that the physician is but one source con-
tacted by disability adjudicators for clinical information and that clinical infor-
mation is considered in light of information provided from other sources.
It is imperative that a signed release of information be obtained before any
information is disclosed to any agency determining disability eligibility. The
limits of confidentiality need to be disclosed to the patient (American Psy-
chiatric Association Committee on Confidentiality 1987). Addressing this
matter directly avoids the potential for confrontations regarding disclosure of
sensitive information after it has been provided to disability or compensation
reviewers.

Forensic Issues Pertaining to Pain 247
The nature of the disability program intrinsically creates incentives for
claimants to maximize monetary gains by emphasizing functional limitations
and overstating the severity of illnesses (Fontana and Rosenheck 1998). The
person with mild symptoms who dramatizes the severity of his or her disabil-
ity can trigger marked countertransference reactions in the clinician (Heiman
and Shanfield 1978). Physicians might harbor resentment and other feelings at
being pulled into the position of rewarding idleness, inactivity, and dependency.
Inattention to countertransference reactions can lead the physician to under-
estimate the severity of the claimant’s symptoms, distance himself or herself
from the patient, and thereby undermine any attempts at rehabilitation.
Clinicians could have concerns about the unstructured time patients have
once disability benefits are awarded. There could be concerns that treatment
endeavors (e.g., development of autonomy and self-sufficiency) might be un-
dermined. Among the factors that adversely influence return to work after a
disabling injury is the length of time spent on disability compensation (Chat-
field 1998). Other factors include current litigation, significant depression and
psychiatric comorbidity, substance abuse, and lack of personal satisfaction with
work (Chatfield 1998).
Directly addressing such concerns with the patient could be meaningful
and therapeutic (i.e., laying the foundation for vocational rehabilitation and
work preparatory skills). In addition, the time available off work might allow for
pursuit of more intensive treatment, including psychotherapy, group therapy,
and day treatment. In this way, the pursuit of disability benefits does not be-
come an end in itself but a means to improve the rehabilitation of the patient.
Key Points
• Ongoing litigation issues and pursuit of disability claims may contribute to
the experience of pain (e.g., predisposing patients to exaggeration of per-
ceived disability and reinforcing patients’ overt pain behaviors).
• Increasingly, legal action has been undertaken on behalf of patients for
whom pain has been inadequately treated. Such trends reflect the societal
standards regarding the importance of pain management and the premium
placed on having clinicians in virtually all medical specialties appropriately
trained in pain management treatment strategies.

248 Clinical Manual of Pain Management in Psychiatry
• Preventing drug abuse is an important societal concern. Standards put
forth by the Code of Federal Regulations attempt to codify responsibilities
of all parties involved in the prescription, dispensing, and use of analgesics
and potentially addicting medications. Additional measures (e.g., the Na-
tional All Schedules Prescription Electronic Reporting Act) are being im-
plemented in order to reduce the likelihood of inordinate prescription of
controlled substances and to minimize the risks of diversion and misap-
propriation of controlled substances.
• Clinicians are often at the forefront of ensuring that controlled substances
are prescribed for legitimate medical purposes. Vigilance for possible adul-
teration or abuse of such substances, or for controlled substance misap-
propriation and diversion, is warranted. Regular medical follow-up, docu-
mentation of treatment response and functional assessments, periodic pill
counts, and judicious use of drug testing in clinical practice may be help-
ful.
• Use of a treatment contract may be helpful in establishing goals of treat-
ment and stipulating conditions upon which continued treatment will be
based.
• Determination of eligibility for disability awards can be complex; the stan-
dards vary depending upon the entitlement program from which the award
is sought (i.e., workers’ compensation, Veterans’ Affairs, or Social Security
Disability Insurance or Supplemental Security Income). Clinicians need to
be cognizant of the potential pitfalls to the doctor–patient relationship that
may arise from pursuit of disability claims. Addressing such impediments
in the therapeutic context is necessary so as to mitigate potential barriers to
rehabilitative and treatment endeavors.
References
Albert T: Doctor guilty of elder abuse for undertreating pain. Am Med News, July 23,
2001, pp 1, 4
American Psychiatric Association Committee on Confidentiality: Guidelines on con-
fidentiality. Am J Psychiatry 144:1522–1526, 1987
Burchman SL, Pagel PS: Implementation of a formal treatment agreement for outpa-
tient management of chronic nonmalignant pain with opioid analgesics. J Pain
Symptom Manage 10:556–563, 1995

Forensic Issues Pertaining to Pain 249
Charatan F: Doctor disciplined for “grossly undertreating” pain. BMJ 319:728, 1999
Chatfield JW: Symptom magnification: an overview from clinical practice, in Pain
Management: A Practical Guide for Clinicians, 5th Edition, Vol 2. Edited by
Weiner RS. Boca Raton, FL, St Lucie Press, 1998, pp 737–742
Compton P, Darakjian J, Miotto K: Screening for addiction in patients with chronic
pain and “problematic” substance use: evaluation of a pilot assessment tool. J Pain
Symptom Manage 16:355–363, 1998
Controlled Substances Act, Title 21, Code of Federal Regulations CFR 8, 1306.04(a),
(USC 802)
Crook PL: Worker’s compensation, in Handbook of Pain Management, 2nd Edition.
Edited by Tollison CD, Satterthwaite JR, Tollison JW. Baltimore, MD, Williams
& Wilkins, 1994, pp 722–731
Enelow AJ, Leo RJ: Evaluation of the vocational factors impacting on psychiatric dis-
ability. Psychiatr Ann 32:293–297, 2002
Fontana A, Rosenheck R: Effects of compensation-seeking on treatment outcomes
among veterans with posttraumatic stress disorder. J Nerv Ment Dis 186:223–
230, 1998
Forman RF, Woody GE, McLellan T, et al: The availability of web sites offering to sell
opioid medications without prescriptions. Am J Psychiatry 163:1233–1238, 2006
Fornari FA, Siwicki DM, Bauer GB: Urine drug testing and monitoring in pain man-
agement. Practical Pain Management 6:12–14, 2006
Gatchel RJ, Polatin PB, Mayer TG: The dominant role of psychosocial risk factors in
the development of chronic low back pain disability. Spine 20:2702–2709, 1995
Hartmann L: Confidentiality, in Ethics Primer of the American Psychiatric Association.
Edited by the American Psychiatric Association Ethics Committee. Washington,
DC, American Psychiatric Association, 2001, pp 39–44
Heiman EM, Shanfield SB: Psychiatric disability assessment: clarification of problems.
Compr Psychiatry 19:449–454, 1978
Heit HA, Gourlay DL: Urine drug testing in pain medicine. J Pain Symptom Manage
27:260–267, 2004
Institute of Medicine Committee on Pain, Disability, and Chronic Illness Behavior:
Disability determination and the role of pain, in Pain and Disability: Clinical,
Behavioral, and Public Policy Perspectives. Edited by Osterweis M, Kleinman A,
Mechanic D. Washington, DC, National Academy Press, 1987, pp 37–65
Leo RJ, Del Regno P: Social Security claims of psychiatric disability: elements of case
adjudication and the role of primary care physicians. Prim Care Companion J Clin
Psychiatry 3:255–262, 2002
Mischoulon D: Potential pitfalls to the therapeutic relationship arising from disability
claims. Psychiatr Ann 32:299–302, 2002

250 Clinical Manual of Pain Management in Psychiatry
Social Security Administration: Disability evaluation under Social Security (SSA Publ
No 64–039). Baltimore, MD, Social Security Administration, January 2001
Swartzman LC, Teasell RW, Shapiro AP, et al: The effect of litigation status on adjust-
ment to whiplash injury. Spine 21:53–58, 1996
Tarasoff v Regents of the University of California, 17 Cal 3d 425, 551 P2d 334, 131
Cal Rptr 14 (1976)
Tough P: The OxyContin underground. The New York Times Magazine, July 29,
2001, pp 50–63
251
Index
Page numbers printed in boldface type refer to tables or figures.
Ablative neurosurgical interventions,
171, 172
Aβ neurons, 13, 14, 15
Abreaction, and group therapy, 150
Accreditation Council for Graduate
Medical Education (ACGME),
3–4
Acetaminophen
breastfeeding and, 220
cancer and HIV/AIDS pain, 206
headache and, 182
medication suggestions for adult
patients and, 86
side effects and drug interactions of,
95
tramadol and, 94
Acetylcholine, 18, 19
Acupuncture, 162–163, 193
Acute pain, 25–27
Adaptive functioning, and assessment
of chronic pain, 45. See also
Functional capacity
Addiction, 76, 77. See also
Pseudoaddiction; Substance abuse
and substance use disorders
Aδ fibers, 13–14
Adenosine, 20
Adjunctive interventions, and
psychotherapy, 151–155
Administration, of opiate analgesics,
89–92
Adolescents, and pain management,
214, 215
Adrenal gland, 18
Affect, and psychotherapy, 134–136.
See also Affective states; Anger;
Mood
Affective disorders, and complex
regional pain syndromes,
201–202. See also Depression
Affective-motivational pathway, 16, 17
Affective states, and assessment of pain,
40–41. See also Affect
Agonist-antagonist opioids, 85, 88
AIDS. See HIV/AIDS
Alcohol and alcohol abuse, 45–46, 95,
186. See also Substance abuse and
substance use disorders
Alcoholics Anonymous, 151
Alexithymia, 138
Almotriptan, 112
Alpha2-adrenergic agonists, 115

252 Clinical Manual of Pain Management in Psychiatry
Altitude, and cluster headache, 186
Amantadine, 116
American Board of Anesthesiology, 3
American Board of Physical Medicine
and Rehabilitation, 3
American Board of Psychiatry and
Neurology (ABPN), 3, 4
American College of Rheumatology
(ACR), 190
Amitriptyline, 99, 185
Amputation, and phantom limb pain,
202–204
Amyloid neuropathy, 199
Anatomic conditions, and neuropathic
pain, 199
Anatomic neurosurgical interventions,
171
Anesthesia, at level of spinal cord,
165–166
Anger
assessment of chronic pain and, 41
psychotherapy and, 135
terminal conditions and, 226
Anticipatory anxiety, 74
Anticonvulsant drugs
combination of with
antidepressants, 106–110
headache and, 182
neuropathic pain and, 199, 200
phantom limb pain and, 203
pharmacology of pain and, 104–106
side effects of, 106, 107
Antidepressants. See also Tr i c y c l i c
antidepressants
back pain and, 188
combination of with
anticonvulsants, 106–110
fibromyalgia and, 192, 194
headache and, 182
neuropathic pain and, 200
phantom limb pain and, 203
pharmacology of pain and, 97,
99–104
Antiemetics, 182
Antihistamines, 110–111
Antipsychotics, 89, 206. See also
Neuroleptics
Antiretroviral toxicity–associated
neuropathy, 205
Anxiety and anxiety disorders
assessment of chronic pain and, 41
buspirone and, 111
differential diagnosis of, 72–74
fibromyalgia and, 192
Anxiolytics, 111
Arachnoiditis, 187
Arthralgias, 205
Aspirin, 95, 97, 182.See also Salicylates
Assessment. See also Assessment scales;
Competency; Diagnosis;
Differential diagnosis; Evaluation
components of patient history and,
36
conduct of interview for, 37–38
methods for obtaining patient
history for, 38–47
treatment suitability and, 47, 48
Assessment scales
assessment of chronic pain and,
47–60
children and, 214–216
cultural issues and, 221
family therapy and, 149–150
geriatric patients and, 217
Auditory aura, and migraine, 184
Augmentative neurosurgical
interventions, 171–172
Aura, and migraines, 183–184

Index 253
Autogenic training, 153
Autonomic nerve blocks, 170
Autonomic nervous system, and
sensory pathways for pain, 18
Back pain, 161–162, 186–188, 189,
205
Baclofen, 117, 118
Barbiturates, 235
Beck Depression Inventory, 72
Behavioral measures, for assessment of
pain, 52–53
Behavior parameters, for pain in elderly,
218
Behavior therapy, 132, 140–143
Belief systems, and psychotherapy,
138–140. See also Religious issues
Benzodiazepines, 111, 235
Beta-blockers, 182, 203
Beta-endorphin, 19, 20
Biofeedback, 132, 151–152, 181, 182,
193
Biopsychosocial model, of pain
management, 4–5, 6, 7–8, 12,
218
Black cohosh, 121
Bone density measurements, 201
Botulinum toxin (Botox) injection,
162, 164–165, 182
Bradykinin, 19
Brain. See Midbrain
Breastfeeding, and pharmacology, 220
Buprenorphine, 84, 92–93, 236
Bupropion, 101, 102–103
Buspirone, 111
Butorphanol, 85, 182, 236
Caffeine, 113, 182
Calcium channel blockers, 182, 186
Cancer, 116, 166, 204, 206–207
Cannabinoids, 120
Capacity assessment, 174, 175. See also
Functional capacity
Capsaicin, 119
Carbamazepine, 104, 105, 106, 107,
109, 110
Cardiovascular safety, of COX-2
inhibitors, 96–97, 98
Caregivers, and family therapy,
149
Carisoprodol, 117–118
Catastrophizing, 43, 140
Catecholamines, 70, 71, 192
Cauda equina syndrome, 187
Celecoxib, 96, 195
Celiac plexus block, 170
Cell death, and pain-augmenting
mechanisms, 24
Central nervous system. See also
Autonomic nervous system;
Peripheral nervous system;
Sensory pathways; Sympathetic
nervous system
neural stimulation techniques,
172
pain-modulating processes within,
19–24
Certification, and pain medicine as
subspecialty, 3–4
C fibers, 13–14
Chamomile, 121
Children. See also Developmental
factors
aspirin use in, 95
pain management for, 213–216
Chiropractic manipulation, 162
Chlorzoxazone, 118
Cholecystokinin, 20

254 Clinical Manual of Pain Management in Psychiatry
Chronic pain. See also Pain disorders
acute pain vs., 25, 26
categories of, 27, 28
causes of, 23
classifications of, 25–27
common problems of patients with,
29
definition of, 25
depression and, 70–72
Minnesota Multiphasic Personality
Inventory and, 58
multiple comorbidities and, 63
opiate therapy guidelines, 87
pain-augmenting mechanisms and
emergence of, 23–24
“patienthood” as psychosocial state
and, 27, 29–30
personality disorders associated
with, 77
simple pain vs., 30
substance-related disorders and, 75–77
Cigarette smoking, and opiate
analgesics, 89
Classifications, of pain, 25–27, 30–31
Clomipramine, 99
Clonazepam, 111
Clonidine, 115, 182
Clostridium botulinum, 164
Clozapine, 114
Cluster headache, 182, 186
Codeine, 84, 86
Cognitive-behavioral therapy (CBT),
132, 136, 143–147, 181, 188
Cognitive issues
assessment of chronic pain and,
41–42, 43
patient suitability for interventions
and, 175–176
psychotherapy and, 140, 144–146
Communication, and couples or family
therapy, 149
Comorbidity. See also Medical
conditions; Psychiatric disorders
anticonvulsants and, 109, 110
migraine headache and, 185
psychotherapy for mood disorders
and, 135
substance abuse and, 223
Compassion in Dying Federation, 2
Competency, assessment of, 174
Complex regional pain syndromes
(CRPSs), 24, 200–202
Comprehensive pain treatment
programs, 5, 7
Computed tomography, and
neuropathic pain, 198
Confidentiality, as legal issue, 242–243,
246
Consent, and competency assessments,
174
Consultation, and role of psychiatrists
in interdisciplinary pain medicine,
8–9
Control, and cognitive-behavioral
therapy, 144
Controlled Substances Act, 237–238
Conversion disorder, 67, 68
Conversion pattern, 58, 59
Coping strategies
assessment of chronic pain and,
43–44
belief systems and, 139
cognitive-behavioral therapy and,
144–147
Coping Strategies Questionnaire, 49, 57
Cortex, of central nervous system, 20
Corticosteroids, 116–117, 186. See also
Steroids

Index 255
Cost. See also Financial issues
economic impact of chronic pain, 1
health care for back pain and,
186–187
of hospice care, 226–227
Counterirritation techniques, 15, 164
Countertransference, and disability
compensation, 247
Couples therapy, 132, 148–150
COX-2 inhibitors, 86, 96–97, 98
Cross-sensitivities, to NSAIDs in
aspirin-sensitive individuals, 95
Cross-tolerance, of opiate analgesics, 93
Cultural issues, in pain management,
220–221
Cutaneous pain, in HIV/AIDS, 205
Cyclobenzaprine, 118–119
Cyclooxygenase-2 inhibitors. See
COX-2 inhibitors
Cyproheptadine, 219
Cytokines, 20, 70, 71
Data banks, state and national,
240–242
Death, and terminal conditions, 225
Deceit, and pain, 69
Decision making, and cultural issues,
221. See also Competency
Deep breathing exercises, 153, 154
Defenses
psychotherapy and, 136–138
terminal conditions and, 225
Dejerine-Roussy syndrome, 25
Delirium, and HIV/AIDS, 206
δ-receptors, 22, 79
Denial, and terminal conditions,
225
Dental complications, and tension
headache, 182–183
Dependence, and opiate analgesics,
93–94. See also Substance abuse
and substance use disorders;
Substance dependence
Depression
complex regional pain syndromes
and, 201–202
differential diagnosis of, 70–72
fibromyalgia and, 192
headache and, 181
osteoarthritis and rheumatoid
arthritis, 196–197
as predictor of pain, 135
psychotherapy and, 140
somatic amplification in, 68
Desipramine, 99
Detoxification, and substance abuse,
222, 223, 224
Developmental factors. See also
Adolescents; Children
assessment of chronic pain and,
46–47
belief systems and, 139–140
pain management for children and,
214
Dextroamphetamine, 113
Dextromethorphan, 116
Dezocine, 85
Diabetic neuropathy, 109
Diagnosis, of fibromyalgia, 190.
See also Assessment;
Differential diagnosis
Diagnostic testing, and headache, 180
Diazepam, 118
Differential diagnosis. See also
Diagnosis
of anxiety, 72–74
of back pain, 187
of depression, 70–72

256 Clinical Manual of Pain Management in Psychiatry
Differential diagnosis (continued)
of migraine headache, 184
of pain disorder, 64–69
of personality disorders, 77–78
of schizophrenia, 78–79
of sleep disorders, 74–75
of substance-related disorders,
75–77
Diphenhydramine, 110
Disability compensation, and forensic
issues, 244–247
Disease conviction, 42
Displacement, and hypnosis, 156
Dissociation, and hypnosis, 156
Dissociative disorders, 136
Divalproex sodium, 104, 106, 182,
185, 186
Doctor–patient relationship, and
disability compensation, 246–247
“Doctor shopping,” 29
Dorsal horn. See Spinal cord
Dorsolateral pontomesencephalic
tegmentum (DLPT), 21, 22
Dosages. See also Pharmacology
of amantadine, 116
of anticonvulsant drugs, 106
of antihistamines, 111
of muscle relaxants, 118
of opiate analgesics, 84–85, 91
of tricyclic antidepressants, 99
of triptans, 112
Dronabinol, 120
Drug interactions, and opiate
analgesics, 88–89
Drug testing, in clinical practice, 240,
241
DSM-IV-TR
diagnosis of pain disorders and, 64,
65–66
sleep disorder due to a general
medical condition in, 75
Duloxetine, 101
Dyspareunia, 68
Dysphoria, 41
Economics. See Cost
Elderly
abuse of, 234
NSAIDs and psychiatric
disturbances in, 96
osteoarthritis and, 194
pain management for, 216–219,
234
Eletriptan, 112
Electroencephalography, and
biofeedback, 152
Electromyography (EMG)
anxiety–pain relationship and,
72
back pain and, 188
biofeedback and, 151–152
neuropathic pain and, 198
Emotions, and psychotherapy, 135,
136, 137. See also Anger; Mood
Encephalitis, 205
Endogenous opiates, 19, 20
Enkephalins, 19, 20
Epidural/intrathecal blocks, 162,
165–166, 167, 204. See also
Nerve block
Epinephrine, and sympathetic nervous
system activity, 18
Ergotamines, 182, 185, 186
Eutectic mixture of local anesthetics,
119–120
Evaluation, of headache patient, 180.
See also Assessment
Evening primrose oil, 121

Index 257
Exercise
back pain and, 188, 189
fibromyalgia and, 192, 193
Existential anxiety, 74
Expectations, and psychotherapy, 139,
148
Fabry's disease, 199
Face, and pain-relaying sensory
pathways, 14
Faces scale, 51, 52, 215
Facet injury, and back pain, 187
Factitious disorder, 68, 69
Family. See also Family therapy;
Interpersonal relationships;
Support networks
cultural issues and, 221
terminal conditions and, 225,
227–228
Family therapy, 132, 148–150
Fatigue, and fibromyalgia, 191
Fear-Avoidance Beliefs Questionnaire,
49, 56–57
Fentanyl, 84, 86, 89, 90, 91
Feverfew, 121
Fibromyalgia, 75, 103, 104, 189–193,
194
Fight-or-flight response, 18
Financial issues. See also Cost
cultural issues and, 221
family therapy and, 148, 149
Fluoxetine, 88–89, 102
Follow-up, and supportive therapy,
147–148
Forensic issues
confidentiality and, 242–243
disability compensation and,
244–247
drug testing and, 240, 241
litigation and, 233–234
medication diversion and, 234–236
opiate adulteration and misuse,
236–237
opioid prescribing and, 237–238
patients contracts and, 235–236,
238–240
state and national prescribing data
banks, 240–242
Freud, Sigmund, 137
Frovatriptan, 112
Functional capacity, and workers’
compensation, 244
Gabapentin, 104–105, 107, 109, 110,
185
Gastritis, 205
Gender, and headaches, 183, 186
Genetic studies, of schizophrenia, 79
Geriatric patients. See Elderly
Ginkgo biloba, 121
Glutamate, 20
Goldenseal, 121
Group therapy, 150–151, 193
Guided imagery, 153
Guidelines, for opiate analgesics,
86–93
Guillain-Barré syndrome, 199, 205
Hansen’s disease, 25
Headache, 135, 179–186, 205. See also
Migraine
Health care. See also Medicine;
Physicians
common problems of patients with
chronic pain, 29
confidentiality as legal issue in,
242–243
Helplessness, 43

258 Clinical Manual of Pain Management in Psychiatry
Hepatic disease, and use of antidepres-
sants or anticonvulsants, 110
Hepatoxicity, of acetaminophen, 95
Herbal agents, and pharmacology of
pain, 120, 121
Herpes simplex, 205
Histamine, 19
HIV/AIDS, and treatment of pain,
204–206
Homework, and cognitive-behavioral
therapy, 144
Hormonal factors, and migraine, 183,
185
Hospice care, 226–228
Humor, and psychotherapy, 138
Hydrocodone, 86
Hydromorphone, 84, 86, 89
Hydroxyzine, 110, 111
Hypnosis, 132, 154–155, 156, 193
Hypochondriasis, 67, 68
Hypogastric plexus block, 170
Hypotensive reactions, in fibromyalgia,
192
Iatrogenic causes, of pain, 205
Ibuprofen, 220
Idiopathic distal small-fiber
neuropathy, 199
Idiopathic pain, and tricyclic
antidepressants, 99
Imipramine, 99
Impairment, and disability, 244
Implantable drug pumps, 162
Inactivity, and behavior therapy,
142–143
Infants, and expression of pain, 214
Inflammation, and chronic pain, 23
International Association for the Study
of Pain, 30
Internet, and medication diversion, 235
Interpersonal relationships, and
assessment of chronic pain, 45.
See also Family
Intervertebral disc rupture or
herniation, 187
Interviews, for assessment of pain,
37–38
Intramuscular administration, of opiate
analgesics, 89–90
Intravenous administration, of opiate
analgesics, 90
Irritable bowel syndrome, 192
Isometheptene, 185
Joint Commission on Accreditation of
Healthcare Organizations
(JCAHO), 2
Kaposi sarcoma, 205
κ-receptors, 22
Kava, 121
Ketamine, 115
Labeling, 43
Lamina V, 15
Lamotrigine, 104, 105, 106, 107, 110
Language, and cultural issues, 220–221
Laughter, and humor, 138
Legal issues. See Competency; Forensic
issues
Levorphanol, 84
Lidocaine patch, 119
Life changes, and psychotherapy,
133–134
Limbic system activation, and anxiety–
pain relationship, 74
Lithium, 96, 182, 186
Litigation, and forensic issues, 233–234
Locus coeruleus, 22

Index 259
Longitudinal approach, to assessment
of chronic pain, 46–47
Lumbar ligament injury, 187
Lumbar sympathetic block, 170
Magnetic resonance imaging, and
neuropathic pain, 198
Magnification, 43
Malingering, 69
Managed care, 243
Marital therapy, 132, 148–150
Massage and massaging, 15, 162
McGill Pain Questionnaire (MPQ), 49,
50, 53, 55
Medical conditions
antidepressants or anticonvulsants
and, 110
diagnosis of depression and, 72
neuropathic pain and, 199
opiate analgesics and, 89
pain disorder associated with, 64, 65
sleep disorder due to, 75
terminal conditions and, 224–228
Medical model, of pain management,
4–5, 6
Medical records, and confidentiality,
242–243
Medicare, 227
Medication diversion, 234–236
Medication use, reviews of, 50, 52
Medicine, pain management as
subspecialty of, 3–4. See also
Health care; Medical conditions;
Medical records; Physicians
Megestrol acetate, 219
Meningeal irritation, 187
Meningitis, 205
Meperidine, 84, 88, 89, 90
Meprobamate, 117
Methadone, 84, 86, 92, 93–94, 116, 220
Methocarbamol, 118
Methotrimeprazine, 114
Methylphenidate, 113
Methylsergide, 182, 186
Mexiletine, 114–115
Midbrain, and pain-reducing pathways,
20–21
Migraine, 112, 182, 183–188
Milnacipran, 103–104
Mind-body dualism, 66
Minnesota Multiphasic Personality
Inventory, 49, 57–60
Mirtazapine, 101, 103, 182
Modafinil, 113–114
Monoamine oxidase inhibitors
(MAOI), 88, 101, 103, 113
Mononeuropathy, 205
Mood. See also Affect; Emotions
anticonvulsants and, 109
assessment of pain and, 40–41
Mood disorders, and psychotherapy,
135
Morphine, 84, 86, 91, 206
Motor aura, and migraine, 184
Multiaxial pain classification, 30–31
Multidimensional Pain Inventory, 49,
50, 56, 150
Multidimensional pain scales, 53,
55–56
µ-receptor, 21–22
Muscle antispasmodics, and
pharmacology of pain, 117–119
Mycobacterium leprae, 25
Myofascial pain, 188–189
Myopathies, 205
Nalbuphine, 85
Naloxone, 236

260 Clinical Manual of Pain Management in Psychiatry
Naratriptan, 112
National All Schedules Prescription
Electronic Reporting Act,
240–242
Nefazodone, 101, 103, 181, 182
Negative reinforcement, 141
Negligence, as legal issue, 234
Neoplasm, 187
Neospinothalamic pathway, 16, 17
Nerve block, 201, 202. See also
Epidural/intrathecal blocks
Neuroablation/neurolysis, 162, 166–
170
Neurochemicals, in pain processing, 19,
20
Neuroleptics, and pharmacology of
pain, 114
Neurons, and pain-relaying sensory
pathways, 13–14, 15–16, 18
Neuropathic pain
categories of chronic pain and, 27, 28
definition of, 27
HIV/AIDS and, 205
transcranial magnetic stimulation,
172
treatment of, 197–199, 200
tricyclic antidepressants and, 99
Neurostimulation, 162
Neurosurgical techniques, 170–172
Neurotensin, 20
Neurotic triad pattern, 58, 59
N-methyl-D-aspartate (NMDA)
antagonists, 115–116
N-methyl-D-aspartate (NMDA)
receptor, 24
Nociception, 11
Nociceptive pain
categories of chronic pain and, 27, 28
definition of, 27
HIV/AIDS and, 205
tricyclic antidepressants and, 99
Nonarticular pain disorders, 188–193
Nonopiate analgesics, 95–96
Nonsteroidal anti-inflammatory drugs.
See also Acetaminophen; Aspirin
efficacy and side effects of, 95–96,
98
headaches and, 181, 182, 185
medication suggestions for adult
patients and, 86
osteoarthritis and, 195
pain associated with cancer or HIV/
AIDS, 206
pregnancy and, 219
Norepinephrine, 18, 20, 21, 22
Nortriptyline, 99
Number-needed-to-treat (NNT)
formula, 106–109
Numeric rating scale (NRS), 50, 51
Olanzapine, 114
Olfactory aura, and migraine, 184
Open-ended inquiry, 37, 228
Opiate analgesics
adulteration and misuse of as
forensic issue, 236–237
anesthesia at level of spinal cord
and, 166
cancer pain and, 206
factitious disorder and malingering,
69
fibromyalgia and, 192
headache and, 182
HIV/AIDS associated pain and, 206
legal issues in prescribing of,
237–238
medication diversion and, 234, 235
neuropathic pain and, 200

Index 261
pharmacology of pain and, 83,
84–85, 86–94
pregnancy and lactation, 219, 220
rotation of, 86–87
substance abuse and, 222, 223, 224
Opioid receptors, 21–22, 71
Oral cavity pain, 205
Oregon Board of Medical Examiners, 234
Orphenadrine, 118
Osteoarthritis, 194–197
Oucher scale, for pain assessment, 215
Overgeneralization, and cognitive
patterns, 43
Owestry Disability Questionnaire, 55–56
Oxacarbazepine, 104, 105, 106, 110
Oxycodone, 84, 86, 91, 234
Oxycontin, 91
Oxygen inhalation, for headache, 182,
186
Pain diaries, 50, 53, 54, 215
Pain Disability Index, 55
Pain disorders. See also Acute pain;
Chronic pain; Neuropathic pain;
Nociceptive pain; Pain and pain
management
back pain, 186–188
cancer and, 204, 206–207
complex regional pain syndromes,
200–202
differential diagnosis of, 64–69
DSM-IV-TR diagnostic criteria for,
64, 65–66
headache, 135, 179–86
HIV/AIDS and, 204–206
nonarticular forms of, 188–193
osteoarthritis and rheumatoid
arthritis, 194–197
phantom limb pain, 202–204
Pain interference scales, 49, 55–56
Pain and pain management. See also
Acute pain; Assessment; Chronic
pain; Forensic issues; Neuropathic
pain; Nociceptive pain; Pain
disorders; Pharmacology;
Psychotherapy; Special
populations; Special techniques
autonomic nervous system and,
18
classifications of, 25–27, 30–31
increasing attention to, 2
medical vs. biopsychosocial models
of, 4–5, 6
as medical subspecialty, 3–4
as multidimensional concept,
11–12
origins and development of
management systems, 2–3
pain-modulating processes within
central nervous system, 19–24
“patienthood” as psychosocial state
and, 27, 29–30
role of psychiatrists in
interdisciplinary pain
medicine, 5, 7–9
sensory pathways and mechanisms
of, 12–18
social and economic impact of, 1
Pain-reducing pathways, 20–21
Paleospinothalamic pathway, 16, 17
Pancreatitis, 205
Paraphrased recapitulation, of patient
history, 37
Parents, and pain management for
children, 216
Paroxetine, 102
Passivity, and behavioral therapy,
142–143

262 Clinical Manual of Pain Management in Psychiatry
Patient, and assessment of suitability for
interventional pain management,
175–176. See also Doctor–patient
relationship; Patient history;
Patient treatment contract;
Special populations; Therapist–
patient relationship
Patient-controlled analgesia (PCA), 90,
91, 216
Patient history, and assessment, 36, 38–47
“Patienthood,” as psychosocial state,
27, 29–30
Patient treatment contract, 235–236,
238–240
Pemoline, 113
Pentazocine, 85, 236
Periaqueductal gray (PAG), 20–21
Peripheral nerve block, 162, 168
Peripheral nervous system, role of in
pain processing and transmission,
20, 198
Peripheral technique, of
neurostimulation, 172
Personality disorders, differential
diagnosis of, 77–78
Personalization, 43
Phantom limb pain, 202–204
Pharmacists, and legal issues, 238
Pharmacology. See also Administration;
Dosages; Drug interactions;
Prescriptions; Side effects; specific
medications
alpha2-adrenergic agonists and, 115
anticonvulsant drugs and, 104–110,
182, 199, 200, 203
antidepressants and, 88–89, 97,
99–110, 181, 182, 188, 192,
194, 199, 200, 203
antihistamines and, 110–111
anxiolytics and, 111
back pain and, 189
benzodiazepines and, 111, 235
cannabinoids and, 120
children and, 215–216
combination of antidepressants and
anticonvulsants, 106–110
complex regional pain syndromes
and, 201, 202
corticosteroids and, 116–117,
186
fibromyalgia and, 193, 194
geriatric patients and, 218–219
headache and, 182
herbal agents and, 120, 121
HIV/AIDS and, 204–206
medication suggestions for adult
patient, 86
mexiletine and, 114–115
muscle antispasmodics and,
117–119
neuroleptics and, 89, 114, 206
neuropathic pain and, 200
nonopiate analgesics and, 95–96
opiate analgesics and, 69, 83–94,
166, 182, 192, 200, 206, 219,
220, 222, 223, 224, 234–238
osteoarthritis and, 195
placebo effects and, 122
pregnancy and, 219–220
rheumatoid arthritis and, 196
stimulants and, 113–114
substance abuse and, 222, 223, 224
topical agents and, 119–120
tramadol and, 94, 192
Phenelzine, 103
Phenytoin, 104, 105, 107

Index 263
Physical examination, and headache,
180
Physical therapy, 162
Physicians. See also Health care;
Medicine
disability compensation and,
246–247
“doctor shopping” by patients with
chronic pain, 29
medication diversion and, 235
patient contracts and, 239
Physiotherapy, for trigger points, 189
Placebo effects, and pharmacology of
pain, 122
Poker chip tool, for pain assessment,
215
Polyneuropathy, 205
Positive reinforcement, 141
Postherpetic neuralgia, 109, 205
Postintervention role, of psychiatrist in
pain management, 173, 176–177
Posttraumatic stress disorder (PTSD),
74, 136
Potassium ion, 19
Pregabalin, 104, 105, 110
Pregnancy, and management of pain,
219–220
Preintervention role, of psychiatrist in
pain management, 173, 174–176
Prescriptions, and legal issues,
237–238, 240–242
Prior work, and workers’ compensation,
244
Problem-solving, and cognitive-
behavioral therapy, 146
Progressive muscle relaxation (PMR),
152, 153
Projective identification, 137
Prolotherapy, 162, 164
Propoxyphene, 89
Propranolol, 185
Prostaglandins, 20
Pseudoaddiction, 76–77, 94
Psychiatric disorders. See also
Comorbidity; specific disorders
assessment and, 39
pain as prominent complaint in, 68
Psychiatrists. See also Therapist–patient
relationship
role of in interdisciplinary pain
medicine, 5, 7–9
special techniques in pain
management and, 173–177
Psychodynamic approaches, to
psychotherapy, 132–133, 136,
137
Psychogenic pain, 27, 28
Psychological assessment instruments,
56–60
Psychological component, of pain
history, 36, 39–44
Psychometric scales, 49
Psychostimulants, 235
Psychotherapy
adjunctive interventions and,
151–155
arthritic conditions and, 197
behavior therapy and, 132, 140–
143
cognitive-behavioral therapy and,
132, 143–147, 181, 188, 193
components of pain and, 132
factors to be addressed in, 134–140
group therapy and, 150–151, 193
headache and, 181–182
marital, couples, and family therapy
and, 132, 148–150
migraine and, 186

264 Clinical Manual of Pain Management in Psychiatry
Psychotherapy (continued)
phantom limb pain and, 204
postintervention and, 176
resistance to, 133–134
selection of therapeutic approach
and, 131–133
supportive therapy and, 147–148
vocational rehabilitation and,
156–157
Punishments, and behavior therapy,
142
Racial issues, in pain treatment, 220
Raphe nuclei, 21, 22
Rating scales. See Assessment scales
Reboxetine, 103–104
Referrals, and role of psychiatrist in
interdisciplinary pain medicine, 8
Reflex sympathetic dystrophy, 24
Regional neural blockade (RNB),
166–170
Reinforcements, and behavior therapy,
141–142
Reinterpretation, and hypnosis, 156
Relapse, and substance abuse, 223
Relaxation and imagery training, 132,
152–154, 181, 182
Religious issues, and terminal
conditions, 228, 229. See also
Belief systems
Renal dysfunction or failure, 89, 110
Repetitive transcranial magnetic
stimulation, 172–173
Resistance, to psychotherapy, 133–134
Respiratory depression, and opiate
analgesics, 88
Restless legs syndrome, 75, 191
Rexed layers, 14
Reye's syndrome, 95
Rheumatoid arthritis, 194–197
Risperidone, 114
Rizatriptan, 112, 185
Rofecoxib, 96
Rostral ventromedial medulla (RVM),
20–21, 22
St. John’s wort, 121
Salicylates, 86, 95. See also Aspirin
School-age children, and pain
management, 214
Secondary headache, 180
Selective abstraction, 43
Selective serotonin reuptake inhibitors
(SSRIs), 94, 101–102, 182
Self-help groups, 150, 151
Self-protective functions, of acute pain,
26
Self-regulation, and psychotherapy, 138
Sensory aura, and migraine, 184
Sensory pathways, and neurochemistry
of pain, 12–24
Serotonin, 19, 21, 22
Serotonin-norepinephrine reuptake
inhibitors (SNRIs), 99, 101
Sex therapy, 149
“Sick role,” and factitious disorder, 69
Side effects, of medications. See also
Cardiovascular safety
of anticonvulsant drugs, 106, 107
of antihistamines, 110–111
of cannabinoids, 120
of corticosteroids, 116, 117
of muscle antispasmodics, 118
of NMDA antagonists, 115
of opiate analgesics, 87–88
of selective-norephinephrine
reuptake inhibitors, 101
of selective serotonin reuptake
inhibitors, 102

Index 265
of stimulants, 113
of tricyclic antidepressants, 99, 102
of triptans, 112
Single-dimension scales, of pain
intensity, 49–52
Sleep disorders and disturbances,
74–75, 191, 219
Social and adaptational component, of
pain history, 36, 45–46
Social security, and disability awards, 245
Somatic amplification, 67–69
Somatic component, of pain history,
36, 38–39
Somatic nerve blocks, 168
Somatic pain, and HIV/AIDS, 205
Somatization disorder, 68
Somatoform disorders, 66–67, 136
Special populations, and pain
management
children and, 213–216
cultural issues and, 220–221
geriatric patients and, 216–219
pregnancy and, 219–220
substance abuse and, 222–224
terminal conditions and, 224–228
Special techniques
acupuncture, 162–163
botox injection, 162, 164–165
chiropractic manipulation, 162
epidural/intrathecal blocks, 162,
165–166, 167
implantable drug pumps, 162
massage, 162
neuroablation/neurolysis, 162,
166–170
neurostimulation, 162
neurosurgical techniques, 170–172
peripheral nerve blocks, 162, 168
physical therapy, 162
prolotherapy, 162, 164
role of psychiatrist in, 173–177
surgical interventions, 162
thermal procedures, 162
transcranial magnetic stimulation,
162, 172–173
transcutaneous electrical nerve
stimulation, 162, 164
trigger point injection, 162
Sphenopalatine ganglion block, 170
Spinal cord. See also Nerve blocks
anatomy of dorsal horn and, 14–15
back pain and, 187
dorsal horn and chronic pain,
20–21, 23–24
pain-relaying sensory pathways and,
13–14
Spinal cord stimulation, 172
Spinothalamic tract, 15–16
Spirituality, and terminal conditions,
228, 229. See also Belief systems
State laws, on confidentiality and
disclosure of medical records, 243
Stellate ganglion block, 170
Steroids, 182. See also Corticosteroids
Stimulants, 113–114
Stimulation techniques, and
neurosurgery, 171–172
Stress management therapy, 181
Subarachnoid analgesia, 165, 166, 167
Subcutaneous administration, of opiate
analgesics, 90
Subspecialty, and pain medicine, 3–4
Substance abuse and substance use
disorders. See also Alcohol and
alcohol abuse; Substance
dependence
assessment of chronic pain and,
45–46

266 Clinical Manual of Pain Management in Psychiatry
Substance abuse and substance use
disorders (continued)
complex regional pain syndromes
and, 202
differential diagnosis of, 75–77
opiate analgesics and, 94
special issues in pain management
and, 222–224
stimulants and, 113
Substance dependence, 76, 93–94, 222,
224. See also Dependence;
Substance abuse and substance use
disorders
Substance P, 20, 70, 71
Substantia gelatinosa, 15
Substitution of sensations, and
hypnosis, 156
Suicide
chronic pain as risk factor for, 9, 44
cyclobenzaprine and, 119
Sumatriptan, 112, 113, 185
Superior mesenteric ganglion block,
170
Supportive psychotherapy, 132,
147–148
Support networks, and assessment of
chronic pain, 46–47. See also
Family; Interpersonal relationships
Surgical outcome, and assessment of
chronic pain, 47, 48
Surgical interventions. See also
Neurosurgical techniques
back pain and, 161–162, 188, 189
complex regional pain syndromes
and, 201, 202,
for management of acute and
chronic pain, 162
phantom limb pain and, 204
Sympathectomy, 201, 202
Sympathetically mediated pain,
200–202
Sympathetic nerve blockade, 168, 170
Sympathetic nervous system, and pain-
augmenting mechanisms, 24
Tension headache, 179, 181–183
Terminal conditions, and pain
management, 224–228
Thalamus, and pain-relaying sensory
pathways, 16, 18
Therapist–patient relationship, and
psychotherapy, 133, 138
Thermal procedures, 152, 162, 201
Thermography, and complex regional
pain syndromes, 201
Tiagabine, 104, 105, 106
Time disorientation, and hypnosis,
156
Timolol, 185
Tizanidine, 115, 118
Tolerance, and opiate analgesics, 93. See
also Substance dependence
Topical agents, and pharmacology of
pain, 119–120
Topir amate , 104, 105, 106, 110, 182
Toxin-induced neuropathy, 199
Tramadol, 94, 192
Transcranial magnetic stimulation
(TMS), 162, 172–173
Transcutaneous electrical nerve
stimulation, 162, 164
Transducers, for pain, 13, 14
Trauma and injury
back pain and, 187
causes of chronic pain and, 23
Trazodone, 101, 103
Treatment suitability, evaluation of
patient for, 47, 48

Index 267
Tricyclic antidepressants (TCAs)
headache and, 181, 182
medical comorbidities and, 110
neuropathic pain and, 199
opiate analgesics and, 88–89
pharmacology of pain and, 99, 101,
102
side effects of, 99, 102, 109
Trigeminal neuralgia, 198
Trigger points, 162, 189
Triptans, 112–113, 182, 185
12-step program, 151
Ulcerative esophagitis, 205
Underrecognition, of pain in elderly
patients, 216–218
Urine drug testing, 241
Valdecoxib, 96
Valerian, 121
Valproate, 105, 106, 107
Vasculitic neuropathy, 199
Venlafaxine, 110
Ventral posterior nucleus (VPN), 16
Verbal descriptor scale (VDS), 49–50,
51
Veterans Affairs benefits, 245–246
Visceral pain, in HIV/AIDS, 205
Visual analog scale, 50, 51 52
Visual aura, and migraine, 184
Vocational rehabilitation, 132,
156–157
Warfarin, 95
Weight loss, and osteoarthritis,
194–195
Wide dynamic range (WDR) cells, 15
Withdrawal, from substances of abuse,
223
Workers’ compensation, 244
Young children, and expression of pain,
214
Zolmitriptan, 112, 185
Zonisamide, 105, 106