44630 9789241502085 Eng
User Manual: 44630
Open the PDF directly: View PDF ![]() .
.
Page Count: 52
PHARMACEUTICALS
IN DRINKING-WATER
PHARMACEUTICALS
IN DRINKING-WATER


PHARMACEUTICALS
IN DRINKING-WATER

WHO Library Cataloguing-in-Publication Data
Pharmaceuticals in drinking-water.
1.Water pollutants, Chemical. 2.Pharmaceutical preparations. 3.Water purification. 4.Potable water. I.World Health Organization.
ISBN 978 92 4 150208 5 (NLM classification: WA 30.5)
© World Health Organization 2012
All rights reserved. Publications of the World Health Organization are available on the WHO web site (www.who.int) or can be purchased
from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791
4857; e-mail: bookorders@who.int).
Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be
addressed to WHO Press through the WHO web site (http://www.who.int/about/licensing/copyright_form/en/index.html).
The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on
the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning
the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full
agreement.
The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However,
the published material is being distributed without warranty of any kind, either expressed or implied. The responsibility for the interpretation
and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use.
Design by paprika-annecy.com
Printed in France
Contents
List of acronyms and abbreviations vi
Acknowledgements vii
Executive summary viii
1. Occurrence of pharmaceuticals in water 1
1.1 Advances in analytical and detection methods 3
1.2 Occurrence of pharmaceuticals in surface water 4
1.3 Occurrence of pharmaceuticals in drinking-water 6
1.4 Conclusion 6
2. Human health risk assessment for pharmaceuticals in
drinking-water 7
2.1 Introduction 8
2.2 Assessing risks associated with pharmaceuticals in drinking-water 8
2.3 Applying the MTD approach: a Drinking Water Inspectorate study 10
2.4 Applying the ADI approach 12
2.4.1 Awwa Research Foundation study 12
2.4.2 Australian Guidelines for Water Recycling 13
2.5 Conclusion 13
3. Treatment technologies for removal of pharmaceuticals
from water 15
3.1 Introduction 16
3.2 Removal of pharmaceuticals by wastewater treatment processes 16
3.3 Removal of pharmaceuticals by drinking-water treatment processes 18
3.4 Conclusion 20
4. Preventing pharmaceuticals in drinking-water 23
4.1 Improved regulations and guidance on pharmaceutical waste
management 24
4.2 Pharmaceutical take-back programmes 25
4.3 Raising consumer awareness 26
4.4 Conclusion 26
5. Conclusions, recommendations and knowledge gaps 27
5.1 Conclusions 28
5.2 Recommendations 28
5.3 Knowledge gaps and future research 29
References 30

vi
List of acronyms and abbreviations
ADI acceptable daily intake
DWEL drinking-water equivalent level
EDC endocrine disrupting chemical
FAO Food and Agriculture Organization of the United Nations
GAC granular activated carbon
GC gas chromatography
LC liquid chromatography
LOAEL lowest-observed-adverse-effect level
LOQ limit of quantification
MF microfiltration
MOE margin of exposure
MS mass spectrometry
MS/MS tandem mass spectrometry
MTD minimum therapeutic dose
nd not detected
NF nanofiltration
NOAEL no-observed-adverse-effect level
NSAID non-steroidal anti-inflammatory drug
PAC powdered activated carbon
PoD point of departure
PUB Public Utilities Board (Singapore)
RO reverse osmosis
SF sand filtration
TDI tolerable daily intake
UF ultrafiltration
USA United States of America
USEPA United States Environmental Protection Agency
UV ultraviolet
WHO World Health Organization
WSH Water, Sanitation, Hygiene and Health unit (WHO)
vi

vii
The World Health Organization (WHO) wishes to express its appreciation to all those who contributed to the preparation and development of this document
through the provision of their time, expertise and experience.
WHO thanks the United States Environmental Protection Agency (USEPA) and Public Utilities Board (PUB) Singapore for their financial and technical support in
developing this guidance to address an emerging issue for drinking-water.
WHO acknowledges the contributions of the members of the Working Group on Pharmaceuticals in Drinking-water, who provided important technical inputs for
WHO’s consideration in the development of this document. The working group members are:
• Dr Joe Cotruvo, Independent Consultant, Joseph Cotruvo and Associates, United States of America (USA)
• Dr Mary Couper, formerly Quality Assurance and Safety: Medicines, WHO, Switzerland
• Dr David Cunliffe, Department of Health, Environmental Health Service, Australia
• Mr John Fawell, Independent Consultant, England
• Ms Michèle Giddings, Water, Air and Climate Change Bureau, Health Canada, Canada
• Dr Edward Ohanian, USEPA, USA
• Professor Choon Nam Ong, National University of Singapore, Singapore
• Dr Hans Sanderson, Danish National Environmental Research Institute, Aarhus University, Denmark
• Dr Dai Simizaki, National Institute of Public Health, Japan
• Professor Giampaolo Velo, University of Verona, Italy
Special appreciation is extended to Mr John Fawell, independent consultant, England, who provided valuable time and technical expertise in the development
of this document. Appreciation also goes to Dr Emma Goslan, Cranfield University, England, who contributed technical inputs to the chapter on the efficacy of
removal of pharmaceuticals during wastewater and drinking-water treatment.
The development and production of this document were coordinated and managed by staff of the Water, Sanitation, Hygiene and Health (WSH) unit of WHO,
including Mr Robert Bos (Coordinator, WSH), Mr Bruce Gordon and Mr Chee-Keong Chew (technical officers). Ms Carolyn Vickers and Dr Angelika Tritscher,
WHO Headquarters, provided valuable inputs related to chemical risk assessments.
The professional editing services of Ms Marla Sheffer of Ottawa, Canada, and the secretarial support provided by Ms Penny Ward are also gratefully
acknowledged.
Acknowledgements

viii
Background
In the last decade, traces of pharmaceuticals, typically at levels in the nanograms
to low micrograms per litre range, have been reported in the water cycle,
including surface waters, wastewater, groundwater and, to a lesser extent,
drinking-water. Advances in analytical technology have been a key factor
driving their increased detection. Their presence in water, even at these very
low concentrations, has raised concerns among stakeholders, such as drinking-
water regulators, governments, water suppliers and the public, regarding the
potential risks to human health from exposure to traces of pharmaceuticals via
drinking-water.
Following requests from several Member States for information regarding
the potential health impacts of residual concentrations of pharmaceuticals in
drinking-water, this issue was added to the work plan of the World Health
Organization (WHO) Drinking-water Quality Committee in 2005. It was
proposed that a working group of experts be assembled to undertake a rapid
review of the state of the science of pharmaceuticals in drinking-water and
develop guidance and recommendations in a report and fact sheet.
A WHO working group that comprised experts in toxicology, water chemistry,
water quality and health, water treatment, pharmacology, and drinking-water
regulation and policy was formed in 2009. Consultations were held in 2009
and 2010 with the Drinking-water Quality Committee and additional experts
to review and summarize the available scientific knowledge and evidence.
A literature review was a key source of evidence. This examined the fate and
occurrence of pharmaceuticals in water, exposure to pharmaceuticals in drinking-
water, assessment of the human health risk associated with pharmaceuticals
in drinking-water, removal of pharmaceuticals during wastewater and drinking-
water treatment, and preventive management measures to reduce potential
exposure to pharmaceuticals in drinking-water.
This report, originally published in 2011
1, contains the key findings and
recommendations of the working group and consultations with experts in the
Drinking Water Quality Committee. It aims to provide practical guidance and
recommendations for managing the emerging concern about pharmaceuticals in
drinking-water, taking into consideration the evidence from the literature review.
1 This publication is the professionally designed version of the technical document, WHO/HSE/
WSH/11.05. This version supercedes the 2011 version.
More importantly, it emphasizes the need to prioritize this emerging issue in the
overall context of water safety management, which includes microbial and other
chemical risks that may threaten the safety of drinking-water.
Scope
This report focuses primarily on reviewing the risks to human health associated
with exposure to trace concentrations of pharmaceuticals in drinking-water. It
does not discuss the potential impacts on aquatic ecosystems or the broader
physical environment.
Occurrence of pharmaceuticals in water
Pharmaceuticals are synthetic or natural chemicals that can be found in
prescription medicines, over-the-counter therapeutic drugs and veterinary
drugs. Pharmaceuticals contain active ingredients that have been designed
to have pharmacological effects and confer significant benefits to society.
The occurrence of pharmaceuticals in the environment and the water cycle
at trace levels (in the range of nanograms to low micrograms per litre) has
been widely discussed and published in literature in the past decade. The
increase in detection is largely attributable to the advances in analytical
techniques and instrumentation. Many surveys and studies have confirmed
the presence of pharmaceuticals in municipal wastewater and effluents,
and these have been identified as a major source of pharmaceuticals in
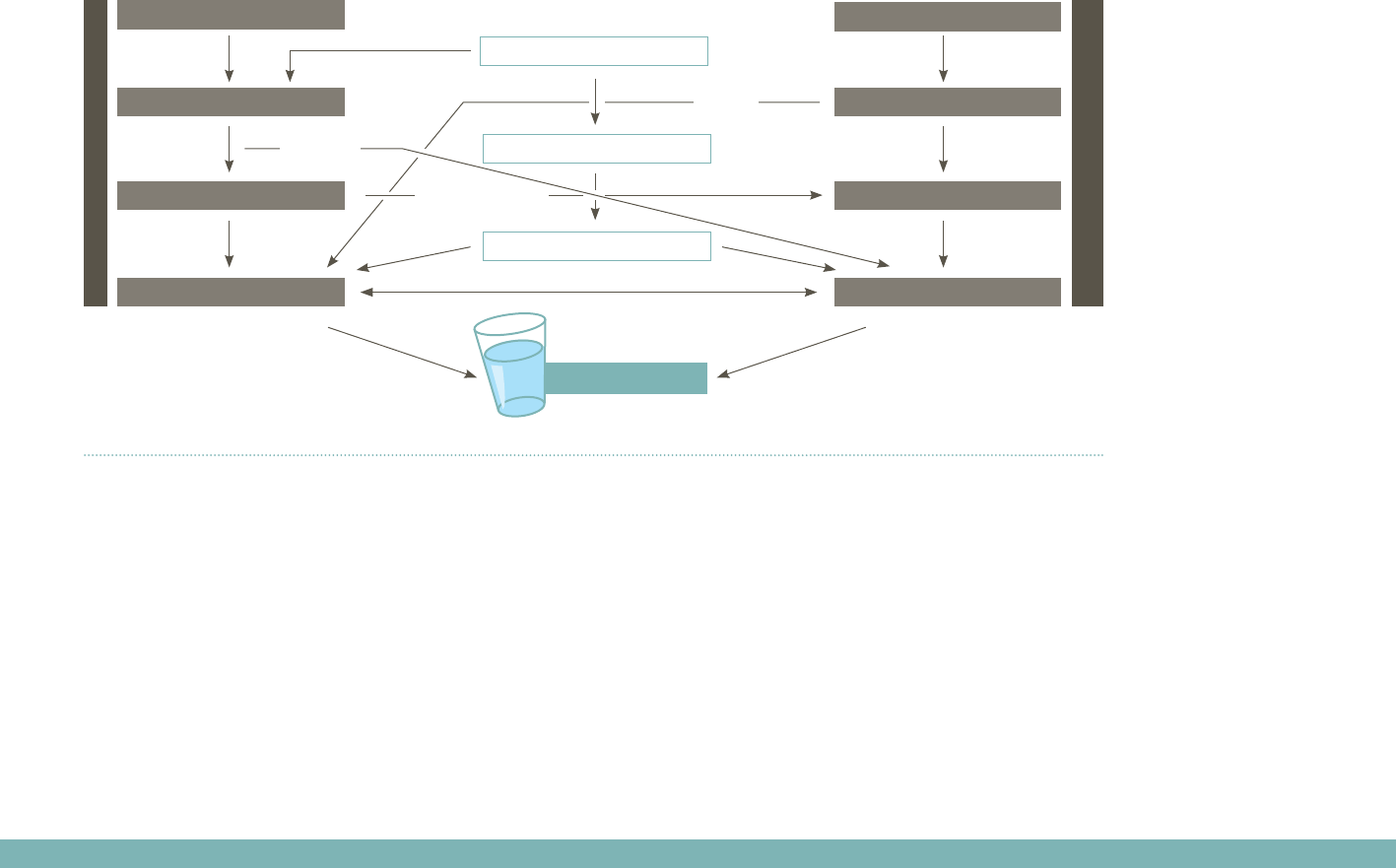
drinking-water (Figure ES1).
Routine monitoring programmes to test drinking-water for pharmaceuticals
have not been implemented, as is the case for regulated chemical and
microbial parameters. Generally, data on the occurrence of pharmaceuticals
in drinking-water have resulted from ad hoc surveys or targeted research
projects and investigations. Available studies have reported that concentrations
of pharmaceuticals in surface waters, groundwater and partially treated
water are typically less than 0.1 µg/l (or 100 ng/l), and concentrations in
treated water are generally below 0.05 µg/l (or 50 ng/l).
More systematic studies will help to further our understanding of the transport,
occurrence and fate of pharmaceuticals in the environment, especially
drinking-water sources. Standardization of protocols for sampling and
analysing pharmaceuticals would help to facilitate the comparison of data.
Executive summary
ix
Human health risk assessment for
pharmaceuticals in drinking-water
Pharmaceuticals are normally governed by stringent regulatory processes and
require rigorous preclinical and clinical studies to assess their efficacy and
safety before commercialization. Therefore, pharmaceuticals are generally
better characterized than other environmental contaminants.
This report reviews human health risk assessments of pharmaceuticals in
drinking-water conducted in the United Kingdom, Australia and the United
States of America (USA). The approaches of acceptable daily intake
(ADI) or minimum therapeutic dose (MTD) were adopted as the point of
departure (PoD) in these studies to assess potential risks to human health
through exposure to pharmaceuticals in drinking-water. Margins of exposure
(MOEs) were derived by comparing measured or modelled exposure levels
in drinking-water with a reference exposure concentration, which was usually
the ADI or MTD or sometimes a drinking-water equivalent level (DWEL). A
judgement of safety could then be based on the magnitude of this MOE for
the pharmaceutical under consideration. In other words, screening values to
determine whether further action is warranted could be derived from the ADI
or the MTD, with uncertainty factors applied as appropriate.
Analysis of the results indicated that appreciable adverse health impacts
to humans are very unlikely from exposure to the trace concentrations
of pharmaceuticals that could potentially be found in drinking-water.
Figure ES1: Fate and transport of pharmaceuticals in the environment (adapted from Ternes, 1998)
Note: STP is sewage treatment plant
Human drugs
Veterinarydrugs•Feedadditives
Drinking-water
excretion
disposal
excretion
sewage
waste
(liquid) manure
STP
landfill site
soil
surface water groundwater
(runoff)
(digested sludge)
(leakages)

x
Concentrations of pharmaceuticals in drinking-water are generally more than
1000-fold below the MTD, which is the lowest clinically active dosage. The
findings from these three case-studies are in line with the evidence published
over the past decade, which suggests that appreciable risks to health arising
from exposure to trace levels of pharmaceuticals in drinking-water are
extremely unlikely.
Treatment technologies for removal of
pharmaceuticals from drinking-water
Having established that raw sewage and wastewater effluents are a major
source of pharmaceuticals found in surface waters and drinking-water, it is
important to consider and characterize the efficiency of processes for the
removal of pharmaceuticals during wastewater and drinking-water treatment.
Most of the research has been conducted at the laboratory scale or at full
scale in developed countries, including the USA, Japan, the Republic of
Korea and countries in Europe.
Even though wastewater and drinking-water treatment processes are not
designed specifically to remove pharmaceuticals, they may do so to
varying degrees. Pharmaceuticals are not “unusual” chemicals; their removal
efficiencies during wastewater and drinking-water treatment are dependent
on their physical and chemical properties. In cases where regulations require
controls to mitigate risks from exposure to pesticides, treatment barriers may
already be optimized to remove pharmaceuticals.
Conventional wastewater treatment facilities generally have activated sludge
processes or other forms of biological treatment such as biofiltration. These
processes have demonstrated varying removal rates for pharmaceuticals,
ranging from less than 20% to greater than 90%. The efficiency of these
processes for the removal of pharmaceuticals varies within and between
studies and is dependent on operational configuration of the wastewater
treatment facility. Factors influencing removal include sludge age, activated
sludge tank temperature and hydraulic retention time. Comparatively,
advanced wastewater treatment processes, such as reverse osmosis,
ozonation and advanced oxidation technologies, can achieve higher removal
rates for pharmaceuticals.
Studies on conventional drinking-water treatment processes have shown
that coagulation is largely ineffective in removing pharmaceuticals. Free
chlorine is able to remove up to approximately 50% of the pharmaceuticals
investigated, whereas chloramines have lower removal efficiency.
Compounds that showed high removal by free chlorine but low removal
by chloramines include antibiotics, such as sulfamethoxazole, trimethroprim
and erythromycin.
Advanced water treatment processes, such as ozonation, advanced
oxidation, activated carbon and membranes (e.g. nanofiltration, reverse
osmosis), are able to achieve higher removal rates (above 99%) for targeted
pharmaceutical compounds in various studies in the published literature.
Advanced and costly water treatment technology will not be able to
completely remove all pharmaceuticals to concentrations less than the
detection limits of the most sensitive analytical procedures at all times.
Therefore, it is imperative that the toxicological relevance of various
compounds be considered in the context of appreciable risks to human
health. An informed risk assessment is essential before scarce resources are
allocated to upgrade or invest in additional advanced treatment processes
to reduce trace concentrations of pharmaceuticals in drinking-water.
Preventing pharmaceuticals in drinking-
water
Conventional drinking-water quality monitoring that focuses on end-product
testing is resource intensive in terms of capital investment and human
resources. Coupled with an expanding list of chemical contaminants in
drinking-water and water sources that may be of insignificant health concern,
an overemphasis on end-product monitoring and the upgrading of treatment
infrastructure is not a sustainable, optimal use of limited resources.
As outlined in the WHO Guidelines for Drinking-water Quality, the water
safety plan approach is “the most effective means of consistently ensuring
the safety of a drinking-water supply … through the use of a comprehensive
risk assessment and risk management approach that encompasses all
steps in the water supply from catchment to consumer”. Water safety plans
highlight the importance of considering risk assessment and risk management
comprehensively from source to tap and adopting preventive measures to
address the source of risks.
Adapting the water safety plan approach to the context of pharmaceuticals
in drinking-water means that preventing pharmaceuticals from entering the
water supply cycle during their production, consumption (i.e. excretion) and

xi
disposal is a pragmatic and effective means of risk management. Preventive
measures need to be applied as close as possible to the source of the risk
and hazard.
Inappropriate disposal practices, such as flushing unwanted or excess
drugs down toilets and sinks and discarding them into household waste, are
common and may be the main contributors to pharmaceuticals in wastewater
and other environmental media, such as surface waters and landfill leachate.
Preventive measures, such as policies promoting or regulations governing
disposal practices at concentrated point sources (e.g. health-care and
veterinary facilities), can reduce the amount of pharmaceutical waste entering
water bodies. In addition, take-back programmes, guidance and enhanced
consumer education will support efforts for the proper disposal of medicines
and reduce the impact of pharmaceuticals entering our water sources.
Conclusions
Published literature and national studies have shown that concentrations of
pharmaceuticals in surface water and groundwater sources impacted by
wastewater discharges are typically less than 0.1 µg/l (or 100 ng/l), and
concentrations in treated drinking-water are usually well below 0.05 µg/l (or
50 ng/l). There are few comprehensive, systematic studies on the occurrence
of pharmaceuticals in drinking-water. Limited data on the occurrence of
pharmaceuticals in drinking-water are a challenge in assessing potential
human health risks from exposure to trace concentrations of pharmaceuticals
in drinking-water.
Several approaches to screen and prioritize pharmaceuticals have been
published in peer-reviewed literature. These approaches usually apply the
principles of the PoD to derive an MOE between the reported worst-case
exposure and the MTD, the ADI or sometimes the DWEL.
Targeted investigations conducted in the United Kingdom, the USA and
Australia found that pharmaceuticals are largely present in drinking-water
at concentrations several orders of magnitude (more than 1000-fold) below
the MTD and largely below the calculated ADIs and DWELs. The substantial
margins of safety for individual compounds suggest that appreciable adverse
impacts on human health are very unlikely at current levels of exposure in
drinking-water.
From a treatment perspective, pharmaceuticals are not unusual organic
chemicals, and treatment removal rates depend on the physical and
chemical properties of the compounds. Conventional treatment processes
with chlorination (free chlorine) can remove about 50% of these compounds,
whereas advanced treatment processes, such as ozonation, advanced
oxidation, activated carbon and membranes (e.g. reverse osmosis,
nanofiltration), can achieve higher removal rates; reverse osmosis, for
example, can remove more than 99% of large pharmaceutical molecules.
Recommendations
Trace quantities of pharmaceuticals in drinking-water are very unlikely to pose
risks to human health because of the substantial MOE or margin of safety
between the concentrations detected and the concentrations likely to evoke
a pharmacological effect.
Concerns over pharmaceuticals should not divert the attention and valuable
resources of water suppliers and regulators from the various bacterial, viral
and protozoan waterborne pathogens and other chemical priorities, such as
lead and arsenic.
The current levels of exposure to pharmaceuticals in drinking-water also
suggest that the development of formal guideline values for pharmaceuticals
in the WHO Guidelines for Drinking-water Quality is unwarranted.
Routine monitoring of pharmaceuticals in water sources and drinking-
water at the national level and the installation of specialized drinking-
water treatment infrastructure to reduce the very low concentrations of
pharmaceuticals in drinking-water are not currently deemed necessary
given the limited additional health benefits. However, where specific
circumstances, such as a catchment survey, indicate a potential for elevated
concentrations of pharmaceuticals in the water cycle (surface water,
groundwater, wastewater effluent and drinking-water), relevant stakeholders
could undertake targeted, well-designed and quality-controlled investigative
studies to obtain more information to assess potential health risks arising
from exposure through drinking-water. If necessary, screening values could
be developed and an assessment of the need for treatment enhancement
could also be considered within the context of other risks and priorities using
the water safety plan.
Human exposure to pharmaceuticals through drinking-water can be
reduced through a combination of preventive measures, such as take-back
programmes, regulations, public guidance and consumer education to
encourage the proper disposal of unwanted pharmaceuticals and minimize
the introduction of pharmaceuticals into the environment.

xii
Enhanced risk communication to the public and public education efforts on
water quality issues from the human health standpoint will help the public
to better understand this issue relative to other hazards, such as pathogenic
microbial risks. This means conveying the risks of exposure to very low
concentrations of pharmaceuticals in drinking-water to the public using plain
language.
Knowledge gaps and future research
Although current published risk assessments indicate that trace concentrations
of pharmaceuticals in drinking-water are very unlikely to pose risks to human
health, knowledge gaps exist in terms of assessing risks associated with long-
term exposure to low concentrations of pharmaceuticals and the combined
effects of mixtures of pharmaceuticals.
Future research in these areas may be beneficial to better characterize
potential health risks from long-term, low-level exposure to pharmaceuticals,
particularly for sensitive subpopulations.
One of the key challenges in estimating exposures to pharmaceuticals
in drinking-water and assessing the potential risks to human health is the
limited occurrence data for such a diverse group of human and veterinary
pharmaceuticals. Implementing monitoring programmes is resource intensive
in terms of costs, human resources and infrastructure, and there is also a lack of
standardized sampling and analysis protocols to support monitoring studies.
Future research should focus on filling these knowledge gaps, including by
providing support to practitioners through the development of cost-effective
methods and protocols for prioritizing pharmaceuticals within the context of
an overall risk assessment for all drinking-water hazards.
Noting that pharmaceuticals in drinking-water are an emerging issue, WHO
will continue to review relevant scientific evidence and, where necessary,
update the guidance provided in this report.

1Occurrence of
pharmaceuticals in water
2
1. Occurrence of pharmaceuticals in water
Pharmaceuticals are synthetic or natural chemicals that can be found in
prescription medicines, over-the-counter therapeutic drugs and veterinary drugs,
and they contain active ingredients that evoke pharmacological effects and
confer significant benefits to society. The ubiquitous use of pharmaceuticals
in human and veterinary medical practices, aquaculture and agricultural
products has led to the continual release of a wide array of pharmaceutical
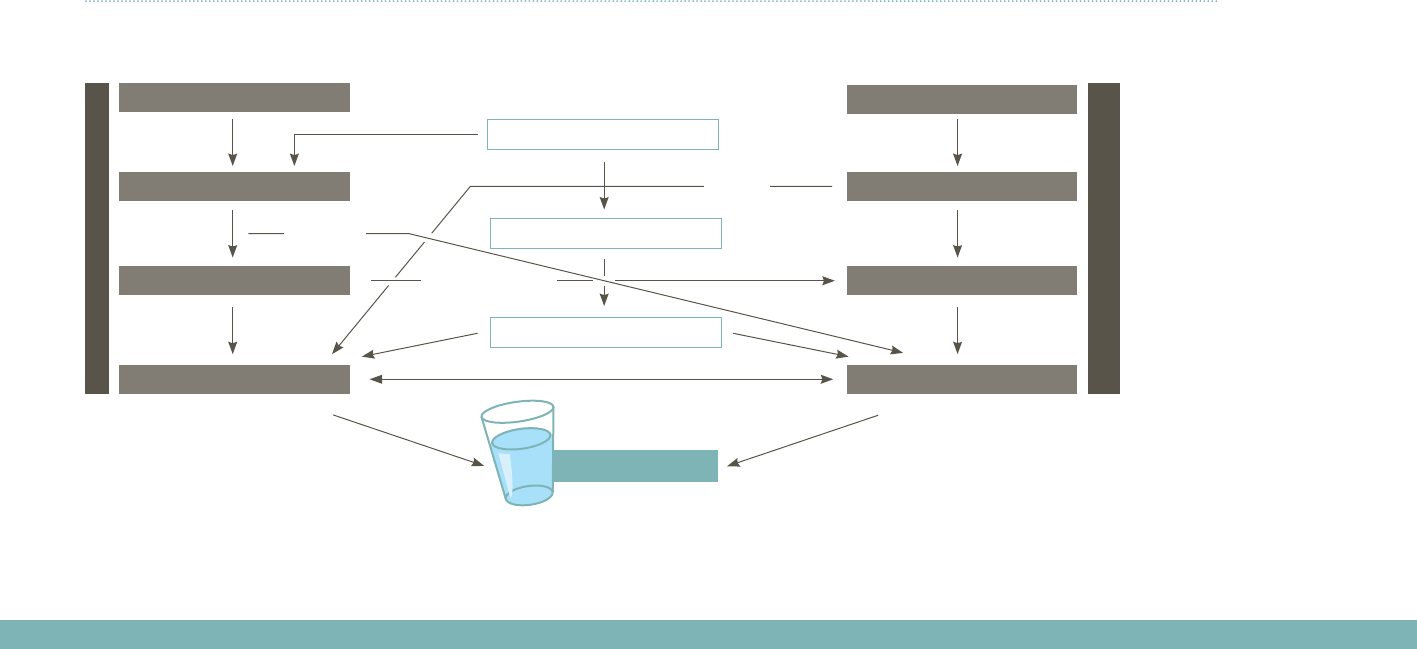
chemicals into our environment. As illustrated in Figure 1, pharmaceuticals
enter the environment through many routes, including human or animal excreta,
wastewater effluent, treated sewage sludge, industrial waste, medical waste
from health-care and veterinary facilities, landfill leachate and biosolids.
Pharmaceuticals and their metabolites undergo natural attenuation by
adsorption, dilution or degradation in the environment, depending on their
hydrophobicity and biodegradability and on the temperature. Therefore,
pharmaceuticals in water sources and drinking-water are often present at
trace concentrations, as these compounds would have undergone metabolism
and removal through natural processes and, if applicable, wastewater and
drinking-water treatment processes.
Figure 1: Fate of pharmaceuticals in the environment (adapted from Ternes, 1998)
Human drugs
Veterinarydrugs•Feedadditives
Drinking-water
excretion
disposal
excretion
sewage
waste
(liquid) manure
STP
landfill site
soil
surface water groundwater
(runoff)
(digested sludge)
(leakages)
Note: STP is sewage treatment plant
3
1.1 Advances in analytical and
detection methods
The increase in reported detections of very low concentrations of
pharmaceuticals in various environmental matrices, including the water cycle
(e.g. surface water, groundwater, treated wastewater effluent and drinking-
water), is mainly attributable to technological advances in the sensitivity and
accuracy of detection equipment and analytical methods. Gas chromatography
with mass spectrometry (GC-MS) or tandem mass spectrometry (GC-MS/MS)
and liquid chromatography with mass spectrometry (LC-MS) or tandem mass
spectrometry (LC-MS/MS)2 are advanced methods that are able to determine
target compounds to the nanogram per litre level and are commonly applied
for the detection of pharmaceutical compounds in water and wastewater. The
selection of methods is dependent on the physical and chemical properties of
the target compound. LC-MS/MS analysis is more suitable for measuring target
compounds that are more polar and highly soluble in water, whereas GC-MS/
MS is better for more volatile target compounds. Figure 2 provides examples
of pharmaceuticals in water and wastewater that can be detected using these
advanced analytical methods (Fatta et al., 2007).
Whereas improved detection and analytical capabilities will allow us to
learn more about the fate and occurrence of pharmaceutical chemicals in the
environment, including the water cycle, it is important to recognize that detection
of these compounds does not directly correlate to human health risks that could
be verified by available human risk assessment methods. In addition, there is
currently no standardized practice or protocol for the sampling and analytical
determination of pharmaceuticals in water or any other environmental media
that ensures the comparability and quality of the data generated.
2 GC-MS/MS and LC-MS/MS are referred to as GC-MS2 and LC-MS2, respectively, in Figure 2.
Figure 2: An illustration of analytical methods applied to detect pharmaceuticals in
water and wastewater (adapted from Fatta et al., 2007)
GC-MS or GC-MS2
without derivatization
Aspirin
Codeine
Cyclophosphamide
Galaxolide
Pentoxyfylline
Tonalide
Triclosan
LC-MS or LC-MS2
17α-Ethinylestradiol
17β-Estradiol
Acetyl-sulfamethoxazole
Amidotrizoic acid
Aminopyrine
Amoxycillin
Anhydro-erythromycin
Atenolol
Betaxolol
Bisoprolol
Chloramphenicol
Chlortetracycline
Ciprofloxacin
Clarithromycin
Clenbuterol
Cloxacillin
Cyclophosphamide
Dapsone
Demethyl diazepam
Dextropropoxyphene
Dicloxacillin
Doxycycline
Estrone
Hydrochlorothiazide
Iopamidol
Lofepramine
Metronidazole
Ofloxacin
Enalapril
Furazolidone
Ifosfamide
Ketorolac
Methicillin
Nafcillin
Erythromycin
Furosemide
Iomeprol
Lincomycin
Methotrexate
Norfloxacin
Oleandomycin
Simvastatin
Sotalol
Omeprazole
Oxacillin
Oxytetracycline
Penicillin G
Penicillin V
Pindolol
Piroxicam
Ranitidine
Ronidazole
Spiramycin
Sulfacetamide
Sulfadiazine
Sulfadimethoxine
Sulfadimidine
Sulfaguanidine
Sulfamethazine
Sulfapyridine
Sulfasalazine
Sulfathiazine
Tamoxifen
Terbutaline
Tetracycline
Tilmicosin
Trimethoprim
Tylosin
Virginiamycin
GC-MS or GC-MS2
after derivatization
Aspirin
Etofibrate
Etofyllinclofibrat
Flurbiprofen
Ketoprofen
Nadolol
Tolfenamic acid
Carbamazepine
Diazepam
Ibuprofen
Paracetamol
Phenazone Bezafibrate
Clofibrate
Diclofenac
Fenofibrate
Fenoprofen
Gemfibrozil
Indomethacine
Iopromide
Mefenamic acid
Metoprolol
Naproxen
Propranolol
Propyphenazone
Roxithromycin
Salbutamol
Sulfamethoxazole

4
1.2 Occurrence of pharmaceuticals in
surface water
Scientists demonstrated the presence of pharmaceuticals in the environment
more than 30 years ago, with studies in the United States of America (USA)
in the 1970s that reported the presence of heart medications, pain relievers
and birth control medications in wastewater (Tabak & Bunch, 1970; Garrison,
Pope & Allen, 1976; Hignite & Azarnoff, 1977). The most cited reference in
the peer-reviewed literature on the occurrence of pharmaceuticals in surface
waters is the survey by the United States Geological Survey, in which more
than 50 pharmaceuticals in 139 streams across 30 states in the USA were
investigated during 1999 and 2000 (Kolpin et al., 2002).
Many peer-reviewed and published studies have shown that the primary
sources of pharmaceuticals entering surface water are from excretion and
bathing through treated or untreated municipal wastewater effluent discharges
into receiving surface water bodies (Buser, Muller & Theobald, 1998; Ternes,
1998; Buser, Poiger & Muller, 1999; Daughton & Ternes, 1999; Daughton,
2001; Heberer et al., 2001; Heberer, Reddersen & Mechlinski, 2002; Kolpin
et al., 2002) and improper disposal of pharmaceutical waste and excess
medication by consumers and health-care and veterinary facilities into sewers
and drains. Table 1 illustrates several classes of pharmaceuticals found in
wastewater influent in a study conducted by the Drinking Water Inspectorate
in the United Kingdom.
A monitoring programme in the United Kingdom focused on 12 pharmaceutical
compounds or their metabolites in surface waters (Ashton, Hilton & Thomas,
2004). The results showed that a range of pharmaceuticals from different
therapeutic classes were present in both effluents from sewage treatment works
and receiving waters in England. The values reported were within the same
range as those reported in continental Europe and the USA, where more
extensive monitoring has been conducted. Results in the published literature
for studies conducted in the USA and Europe also suggest that usage data
are positively associated with concentrations of pharmaceuticals measured in
effluent and in surface water bodies receiving the treated effluent. Tables 2
and 3 show additional illustrative examples of pharmaceuticals that have been
found in the United Kingdom and other European countries, respectively.
Table 1. Excretion rates of unmetabolized active ingredients for
selected pharmaceuticals
Compound Pharmaceutical
product group
Parent
compound
excreted (%)
Reference
Amoxycillin Antibiotic 60 Bound & Voulvoulis (2005)
Atenolol Beta blocker 90 Bound & Voulvoulis (2005)
Bezafibrate Lipid regulator 50 Bound & Voulvoulis (2005)
Carbamazepine Antiepileptic 3Bound & Voulvoulis (2005)
Cetirizine Antihistamine 50 Bound & Voulvoulis (2005)
Clofibric acid Active metabolite 6 Alder et al. (2006)
Diclofenac Anti-inflammatory 15 Alder et al. (2006)
Erythromycin Antibiotic 25 Bound & Voulvoulis (2005)
Felbamate Antiepileptic 40–50 Bound & Voulvoulis (2005)
Ibuprofen Analgesic 10 Bound & Voulvoulis (2005)
Source: DWI (2007)

5
Table 2. Measured concentrations of selected pharmaceuticals in the aquatic environment in the United Kingdom
Compound Median (maximum) concentration (ng/l) References
Sewage treatment works effluent Stream or river waters
Bleomycin 11 (19 ) nd (17) Aherne, Hardcastle & Nield (1990)
Clotrimazole 14 (27) 21 (34) Roberts & Thomas (2006)
—7 (22) Thomas & Hilton (2004)
Diclofenac 424 (2349) < LOQ (568) Ashton, Hilton & Thomas (2004)
289 (598) < LOQ Roberts & Thomas (2006)
— < LOQ (195) Thomas & Hilton (2004)
Dextropropoxyphene 195 (585) 58 (682) Ashton, Hilton & Thomas (2004)
37 (64) 12 ( 98) Roberts & Thomas (2006)
Erythromycin — < LOQ (80) Thomas & Hilton (2004)
< LOQ (1842) < LOQ (1022) Ashton, Hilton & Thomas (2004)
Fluoxetine 202 (290) 5 (70) Roberts & Thomas (2006)
7. 6 – 5 2 .9 2–43.7 Boucard & Gravell (2006)
Ibuprofen 3086 (27 256) 826 (5044) Ashton, Hilton & Thomas (2004)
2972 (4239) 297 (2370) Roberts & Thomas (2006)
—48 (930) Thomas & Hilton (2004)
Mefenamic acid 133 (14 40 ) 62 (366) Ashton, Hilton & Thomas (2004)
340 (396) < LOQ Roberts & Thomas (2006)
— < LOQ (196) Thomas & Hilton (2004)
Norfluoxetine 5.2–30.7 4.5–83.0 Boucard & Gravell (2006)
Paracetamol < 20 —Roberts & Thomas (2006)
—555 Bound & Voulvoulis (2006)
Propanolol 76 (284) 29 (215) Ashton, Hilton & Thomas (2004)
304 (373) 61 (107) Roberts & Thomas (2006)
— < LOQ (56) Thomas & Hilton (2004)
Sulfamethoxazole < LOQ (132) < LOQ Ashton, Hilton & Thomas (2004)
Tamoxifen < LOQ (42) < LOQ Ashton, Hilton & Thomas (2004)
Tetracycline —~1000 Watts et al. (1983)
Theophylline —~1000 Watts et al. (1983)
Trimethoprim 70 (1288 ) < LOQ (42) Ashton, Hilton & Thomas (2004)
271 (322) 9 (19) Roberts & Thomas (2006)
—7 (569) Thomas & Hilton (2004)
LOQ, limit of quantification; nd, not detected (below the detection limit)
Source: DWI (2007)

6
Table 3. Concentrations of selected pharmaceuticals found in European surface waters
Compound Median (maximum) concentrations (ng/l)
Austria Finland France Germany Switzerland
Bezafibrate 20 (160) 5 (25) 102 (430) 350 ( 310 0 ) —
Carbamazepine 75 (294) 70 (370) 78 (800) 2 5 (110 ) 30 –150
Diclofenac 20 (64) 15 (40 ) 18 (41) 150 (120 0 ) 20 –150
Ibuprofen nd 10 (65) 23 (120 ) 70 (530) nd (150)
Iopromide 91 ( 211 ) —7 (17) 100 (910) —
Roxithromycin nd —9 (37) < LOQ (560) —
Sulfamethoxazole
and —25 (133) 30 (480) —
LOQ, limit of quantification; nd, not detected (below the detection limit)
a Includes the human metabolite N4-acetyl-sulfamethoxazole.
Source: Ternes et al. (2005)
1.3 Occurrence of pharmaceuticals in
drinking-water
Most countries (if any) do not have monitoring programmes to routinely test for
pharmaceuticals in drinking-water owing to practical difficulties, such as high costs and
lack of availability of routine analytical technologies and laboratory infrastructure to
detect a diverse range of pharmaceuticals and their metabolites. As a result, the majority
of the occurrence data for pharmaceuticals in drinking-water and surface waters come
from targeted research projects, targeted investigations and ad hoc surveys, most of
which were designed to develop, test and fine-tune detection and analytical methods.
Nevertheless, they did provide an initial indication of the presence of pharmaceuticals
in the environment.
Studies in the USA have detected very low levels of pharmaceuticals in finished
drinking-water. The highest concentration reported was 40 ng/l for meprobamate
(Benotti et al., 2009). Studies have also found several pharmaceuticals in tap water at
concentrations ranging from nanograms to low micrograms per litre in several countries
in Europe, including Germany, the Netherlands and Italy (Huerta-Fontela, Galceran &
Ventura, 2011). Two separate studies in Germany (Reddersen, Heberer & Dünnbier,
2002; Zühlke et al., 2004) found phenazone and propylphenazone (an analgesic and
an antipyretic drug, respectively) in Berlin drinking-water, with the highest concentration
being 400 ng/l for phenazone. This high value was largely attributed to groundwater,
used as a drinking-water source, contaminated with sewage (Jones, Lester & Voulvoulis,
2005). In the Netherlands, traces of antibiotics, antiepileptics and beta blockers were
detected in the drinking-water supply at concentrations below 100 ng/l, with most
concentrations below 50 ng/l (Mons, Hoogenboom & Noij, 2003).
To date, between 15 and 25 pharmaceuticals have been detected in treated drinking-
water worldwide, as reported in the peer-reviewed scientific literature (Jones, Lester
& Voulvoulis, 2005; Benotti et al., 2009). More pharmaceutical compounds have
been detected in untreated water sources, such as wastewater, surface waters
and groundwaters (Focazio et al., 2008) in the water cycle, largely attributable to
pharmaceuticals of very high usage, including antihyperlipidaemic compounds and
non-steroidal anti-inflammatory drugs (NSAIDs).
1.4 Conclusion
The occurrence of pharmaceuticals in the environment, including the water cycle, at
concentrations ranging from nanograms to low micrograms per litre has been widely
discussed and published in the literature in the past decade (Heberer, Schmidt-Bäumler
& Stan, 1998; Zuccato et al., 2000; Heberer et al., 2001, 2004; Stackelberg et al.,
2004, 2007; Zühlke et al., 2004; Jones, Lester & Voulvoulis, 2005; Vieno, Tuhkanen
& Kronberg, 2005; Loraine & Pettigrove, 2006; Snyder et al., 2006; Vanderford &
Snyder, 2006; Loos et al., 2007; Pérez & Barceló, 2007; Togola & Budzinski, 2008;
Mompelat, Le Bot & Thomas, 2009).
The published literature and national studies have shown that concentrations of
pharmaceuticals in surface water and groundwater sources impacted by wastewater
discharges are typically less than 0.1 µg/l (or 100 ng/l), and concentrations in treated
drinking-water are usually well below 0.05 µg/l (or 50 ng/l).
There are few comprehensive, systematic monitoring studies on pharmaceuticals in
drinking-water, and limited occurrence data are a challenge in assessing potential
human health risks from exposure to trace concentrations of pharmaceuticals in drinking-
water. In addition, there is no standardized protocol for the sampling and analytical
determination of pharmaceuticals. More systematic studies, using comparable methods,
will help further research on the transport, occurrence and fate of these compounds in
various environmental media, and standardization of protocols for their sampling and
analytical determination would help to facilitate the comparison of data.

Human health risk
assessment for pharmaceuticals
in drinking-water
2
Human health risk
assessment for pharmaceuticals
in drinking-water

8
2. Human health risk assessment for pharmaceuticals
in drinking-water
2.1 Introduction
Regulatory approval processes for pharmaceuticals require thorough
assessments to demonstrate the efficacy and safety of active compounds. These
assessments determine the margin of safety associated with human consumption
and take into account the risk–benefit equation. Those pharmaceuticals that
are most widely used, particularly those approved for over-the-counter sales,
require the most stringent assessment and require a substantial margin of safety.
Most of the pharmaceuticals that are likely to be found in water fall into the
high usage category, because it is those substances that will be present in
the greatest quantity. The assessments for approval for particular uses cover
a series of preclinical, clinical and sometimes mechanistic studies and are
usually performed at doses close to the intended therapeutic dose. For those
substances that will be widely used, some studies are also conducted at doses
well above those anticipated. Because of these stringent regulatory approval
processes, pharmaceuticals will be better characterized and controlled than
most environmental contaminants.
Concern has been raised, however, because exposure to pharmaceuticals
through drinking-water is an unintended and involuntary exposure over
potentially long periods of time. Moreover, there are few scientific risk
assessments of exposure to low levels of pharmaceuticals, both as individual
species or as mixtures, in drinking-water.
2.2 Assessing risks associated with
pharmaceuticals in drinking-water
Chemical risk assessment methods for substances found in food and drinking-
water involve establishing an acceptable daily intake (ADI) or tolerable daily
intake (TDI) based on a variety of calculations (e.g. from extrapolations,
applications of uncertainty factors) applied to a selected point of departure
(PoD) from the toxicological and epidemiological database. A common and
widely accepted PoD is that concentration at which no adverse effects are
detected, which is the no-observed-adverse-effect level (NOAEL), or, less
optimally, the lowest concentration at which adverse effects are detected,
which is the lowest-observed-adverse-effect level (LOAEL), in combination
with an additional uncertainty factor. The PoD may also be derived through a
benchmark dose based on statistical evaluation of the dose–response curve of
the critical study (FAO/WHO, 2009).
Health risks from pharmaceuticals in water have been most frequently assessed
using the minimum therapeutic dose (MTD, the lowest concentration that evokes
a desired therapeutic effect among target populations) as the PoD (DWI, 2007;
Bull et al., 2011). This is due to practical reasons, including the lack of readily
available toxicological data in the public domain that would be necessary
to derive a NOAEL/LOAEL or benchmark dose. The MTD is usually a dose
below those concentrations where, in rare instances, unacceptable adverse
or toxic effects are observed. Therefore, the use of the MTD as a PoD for risk
assessment would often result in the development of conservative screening
values (reference concentrations used to determine whether further action is
warranted, as described below).
The application of the MTD to inform the derivation of screening values does
present certain limitations. The MTD is determined by controlled studies in
specific preselected populations, which may not be based on the sensitivities
of vulnerable subpopulations that would not normally be given the drug. In
addition, in specific cases, such as with cytotoxic cancer treatment drugs,
the MTD may be at a concentration above which toxic effects are observed.
Notwithstanding this, especially in cases where the margins of exposure
(MOEs) are substantial, use of the MTD could be considered a pragmatic and
sensible method to broadly assess and screen risks.
The main challenges in assessing risks include the limited occurrence
data available for pharmaceuticals in drinking-water, the diverse range
of pharmaceuticals in use, the wide variation in the use of individual
pharmaceuticals between countries, the limited number of data in the public
domain and technical limitations relating to assessing risks from chronic
exposure to low doses of pharmaceuticals and mixtures. Nonetheless, several
publicly available approaches (USEPA, 2008b) have been used for screening
and prioritizing pharmaceuticals for assessing the potential risks to human

9
health from exposure to low concentrations of pharmaceuticals in drinking-
water. These reports (DWI, 2007; USEPA, 2008b; Bull et al., 2011) have
been subject to scrutiny and peer review. These studies have used the MTD
as the PoD for the risk assessment, with subsequent application of uncertainty
factors to derive screening values and margins of safety against which to assess
the potential risk.
These screening values are values against which to judge the likelihood that a
particular substance could be of concern at the concentrations observed and so
warrant further, more detailed investigation. Screening values are also used to
identify those substances from a long list that are the most important and should
be considered more closely. As indicated above, there are two approaches
that have been used. An ADI or TDI is an amount that can be ingested daily
for an extended period, generally a lifetime, without significant risk to health.
The large uncertainty factors frequently involved in establishing an ADI or TDI
generally serve to provide assurance that exposure exceeding the ADI or TDI
for shorter periods, or sometimes for longer periods if the exceedance is small,
is unlikely to have any deleterious effect. However, any exceedance of the
ADI or TDI needs to be evaluated on a case-by-case basis, as it is very much
dependent on the substance and its toxicological profile.
ADIs are typically set by determining the dose at which no adverse effect is
observed (the NOAEL) or, less optimally, the lowest level at which an adverse
effect is observed (the LOAEL). In both cases, uncertainty factors are applied
to reflect uncertainties in extrapolation from experimental animals to humans,
in the likely variation within the exposed population or important gaps in the
database, to derive the ADI. These uncertainty factors are based on expert
judgement, but there is a considerable body of experience in their use. Data
from well-conducted studies, where a clear dose–response relationship has
been demonstrated, are preferred, typically using experimental animal models;
however, where suitable data on human populations are available, these
would normally be preferred. The approaches used in developing guideline
or screening values for chemicals in drinking-water are described in chapter 8
of the WHO Guidelines for Drinking-water Quality (WHO, 2011). Using
an ADI to determine a suitable level for drinking-water requires assumptions
to be made regarding body weight, as an ADI is usually presented as an
intake per kilogram of body weight. WHO uses a value of 60 kg for an adult
and assumes consumption of 2 litres of drinking-water per day. Usually for
substances for which an ADI is derived, exposure can also be from food and
air, and so a proportion of the ADI is allocated to drinking-water to allow for
exposure from other sources. In the case of pharmaceuticals, exposure from
other sources is negligible, and so the allocation can be high, even 100%.
For individuals taking the pharmaceutical for medical purposes, the additional
amount from drinking-water is so small as to make no difference.
The MTD, or the lowest clinically effective dose, is usually equivalent to the
lowest dose prescribed or recommended and takes into account the number of
doses in a day. These values are derived from an assessment of the balance
between efficacy and safety. The approach used to derive a screening value for
drinking-water is to divide the MTD by a factor that would provide reasonable
assurance that effects, either pharmacological or toxic, would be extremely
unlikely. The derivation of this factor is based on expert judgement, as are the
uncertainty factors used in the derivation of the ADI. The use of the MTD as a
starting point for assessing potential risks of pharmaceuticals to human health
or for deriving guideline values has been applied by Schwab et al. (2005) in a
human health risk assessment of pharmaceuticals in surface waters in the USA
and by Versteegh, van der Aa & Dijkman (2007), Webb et al. (2003), van der
Aa et al. (2009) and Bull et al. (2011). DWI (2007) also used the MTD as the
basis for assessing the risk from pharmaceuticals in drinking-water.
The screening values developed are then used as reference points against which
the results of monitoring can be judged. In some cases, because monitoring
data are so limited, modelling has been used to develop worst-case estimates
of potential exposure through water. The screening values are then used as the
criteria to support decision-making when a chemical is detected in source water
or drinking-water. If the concentration of a particular pharmaceutical exceeds
the screening value, then further evaluations of the toxicity and occurrence of
the pharmaceutical compound might be warranted. On the other hand, if the
concentration is below the screening value, this strongly suggests that adverse
health impacts should not be expected.

10
2.3 Applying the MTD approach: a
Drinking Water Inspectorate study3
The Drinking Water Inspectorate for England and Wales commissioned a
comprehensive desk-based review of current knowledge on and estimation of
potential levels of 396 pharmaceuticals and 11 illegal drugs in drinking-water
in the United Kingdom based on specific demographic and usage data on
active pharmaceutical ingredients and using modelled concentrations based
on actual catchments. The DWI (2007) approach was to determine an MOE
for each pharmaceutical by comparing the MTD with the theoretical maximum
intake from drinking-water.
The modelled concentrations from drinking-water intake were based on two
methods: 1) a deterministic method that resulted in estimates of worst-case
concentrations in drinking-water and 2) a probabilistic method that resulted in
more realistic estimates of the concentrations in drinking-water. Pharmaceuticals
considered were first evaluated using the deterministic method; for those
24 compounds that had the lowest MOEs, further evaluation was done using
the probabilistic method.
The health end-point used in this review was the MTD. Owing to insufficient
data, an MTD value of 10 mg per day was used for topically applied
pharmaceuticals and a conservative MTD value of 1 mg per day was used for
pharmaceuticals for which there were no data, including illegal drugs. For the
DWI (2007) evaluation, an uncertainty factor of 1000 was applied for all the
compounds as a precautionary value to extrapolate below the level at which
effects might be seen. The resultant screening values were used for determining
the priority substances for further examination by probabilistic modelling. This
additional uncertainty factor, which is widely accepted as a precautionary step
by the medical profession, also provides an additional reassurance with regard
to exposure of infants and young children.
The MOE for each of the targeted pharmaceuticals was derived by comparing
the maximum estimated concentrations in drinking-water with the MTD. The
results allow an assessment of the significance of individual pharmaceuticals
through drinking-water exposure.
3 This section is based on DWI (2007).
From the worst-case deterministic modelling, only 10 substances showed an
MOE less than 1000, of which 4 were illegal drugs, with highly precautionary
values for the lowest active dose. In only one case was the exposure ratio less
than 100, and this was a unique case, as a combined total for all NSAIDs
was used, but compared against the lowest individual MTD for any of the
NSAIDs in the group. The results therefore suggested that even in this worst-
case situation, there is no significant health risk from intake of pharmaceuticals
via drinking-water.
When probabilistic modelling was used to obtain a more realistic estimate
of concentrations in drinking-water, the estimated concentrations of all but
one substance were significantly lower. The MOEs for all substances were
significantly greater than 1000, and only tetrahydrocannabinol and oseltamivir
carboxylate had an MOE less than 1000 (Table 4).
The DWI (2007) study led to the conclusion that a majority of the
pharmaceuticals had MOEs greater than 1000, suggesting a substantial
margin of safety against potential adverse health impacts from exposure to
trace concentrations of pharmaceuticals in drinking-water.
11
Table 4. Probabilistic modelling data for the top 24 drugs from worst-case deterministic modelling
Drug name Mean PECdw (µg/l) MTD (mg) MOE Comments
Total NSAIDs 2.74 7. 5 2 737 Combination of 19 anti-inflammatory drugs
Cannabis (tetrahydrocannabinol) 1.377 1726 Illegal drug
Oseltamivir carboxylate (Tamiflu active metabolite) 107 52 486 Used under pandemic conditions
LSD 0.097 110 309 Illegal drug
Cocaine (methylbenzoylecgonine) 0.029 134 483 Illegal drug
Aminophylline 0.15 16 667 Smooth muscle relaxant
Beclometasone 0.005 0.05 10 000 Anti-asthmatic
Zidovudine 0.057 0.5 8 772 Antiviral
Ecstasy 0.487 12 053 Illegal drug
Acamprosate 0.435 12 299 Alcoholism treatment
Total statins 1.27 53 937 Cholesterol reduction
Nitroglycerine 0.035 4 0.15 4 234 Vasodilator
Heroin (diamorphine) 0.004 49 1222 717 Illegal drug
Simvastatin 1.18 54 227 Cholesterol reduction
Codeine 0.015 7 20 1 277 139 Narcotic analgesic
Ramipril 0.153 1.25 8 177 Diuretic
Lisinopril 0.396 2.5 6 316 Angiotensin converting enzyme inhibitor
Methadone 0.082 2 112 173 Opioid agonist
Furosemide 1.74 20 11 5 0 7 Diuretic
Amphetamine 0.017 4 157 405 Illegal drug
Norethisterone 0.023 6 0.35 14 824 Progesterone derivative
Doxazosin 0.0 06 81 1146 843 Alpha blocker
Bendroflumethiazide 0.275 2.5 9 094 Diuretic
Cyclosporin 0.000 8 22 500 000 Immunosuppression
LSD, lysergic acid diethylamide; PECdw, predicted concentration in drinking-water
Source: DWI (2007)

12
2.4 Applying the ADI approach
2.4.1 Awwa Research Foundation study4
The Awwa Research Foundation commissioned a study to provide critical
information regarding the occurrence of and risk assessment for pharmaceuticals
and potential endocrine disrupting chemicals (EDCs) in drinking-water. The
study examined 62 chemicals, including 20 pharmaceuticals and active
metabolites, 26 potential EDCs, 5 steroid hormones and 11 phytoestrogens
(natural estrogens from plants). The health value applied in this study was the
ADI, and a conservative approach was taken in the process of developing the
ADI values, as illustrated in Table 5.
In this study, the ADIs were converted to drinking-water equivalent levels
(DWELs) in micrograms per litre (or parts per billion) based on assumptions of
a 70 kg body weight in adults and consumption of 2 litres per day.
Even with the use of advanced and highly sensitive analytical procedures (with
reporting limits in the nanograms per litre or parts per trillion range), none of the
pharmaceuticals tested in this study were detected in finished drinking-water
above the calculated health risk thresholds. Adopting a conservative worst-
case scenario approach, the maximum detected concentrations in finished and
piped drinking-water were used to calculate DWELs for each of the target
pharmaceuticals. It was found that none of the pharmaceuticals detected in
drinking-water exceeded their corresponding ADI.
The minimum margin of safety or MOE for each compound tested was
calculated by dividing the DWEL by the maximum detected water concentration.
According to United States Environmental Protection Agency (USEPA) policy,
compounds with MOEs greater than 100 would generally indicate a low level
of concern. Table 6 contains the calculated MOEs for some of the compounds
that were detected in drinking-water; these were orders of magnitude above
100, suggesting a low level of concern.
4 This section is based on Snyder et al. (2008).
Table 5. Principles for deriving ADIs for compounds considered
in this study
Category of analytes Derivation of ADIs
Compounds that are not
carcinogenic
Dividing the highest dose at
which an effect was not observed
(NOAEL) or the lowest dose at
which an effect was observed
(LOAEL) in animal or human toxicity
studies by uncertainty factors
to account for extrapolation to
potentially sensitive populations
Compounds with positive evidence
of carcinogenicity in high-dose
animal studies and data on tumour
incidence per dose level
A linear extrapolation model was
used to predict the tumorigenic
response at low dose level
Carcinogenic compounds with
reported evidence in animal studies,
but no available tumour incidence
data
A safe dose corresponding to a
cancer risk of one in a million was
estimated
Table 6. MOEs calculated for compounds considered in the Awwa
Research Foundation study
Compound MOE
Atenolol 2 700
Diazepam 110 000
Fluoxetine 41 000
Meprobamate 6 000
Norfluoxetine 44 000
Sulfamethoxazole 6 000 000
Triclosan 2 200 000

13
2.4.2 Australian Guidelines for Water
Recycling5
The Australian Guidelines for Water Recycling were developed to serve as an
authoritative reference for using recycled wastewater to augment drinking-water
supplies. These guidelines were established to protect against microbial and
chemical risks, including pharmaceuticals. The pharmaceuticals considered
were categorized into two groups: those used solely for humans and those
used for agricultural and veterinary purposes.
For veterinary pharmaceuticals, the health end-point is determined based
on ADIs established for pharmaceuticals used for agricultural and veterinary
purposes by organizations such as the Joint FAO/WHO Expert Committee
on Food Additives, the Australian Therapeutic Goods Administration and the
European Medicines Agency.
For human pharmaceuticals, the health end-point was a surrogate ADI, which
was derived by dividing the lowest daily therapeutic dose by safety factors
ranging from 1000 to 10 000. The use of the lowest daily therapeutic dose as
a starting point for deriving guideline values or assessing risk has been adopted
by others (Webb et al., 2003; Schwab et al., 2005; DWI, 2007; Versteegh,
van der Aa & Dijkman, 2007; Bull et al., 2011). With respect to pharmaceutical
metabolites in source waters, it was considered that the activity of metabolites
is generally lower than that of the parent compound, and application of safety
factors in the range of 1000–10 000 should provide a safety buffer that is
sufficiently conservative.
For most pharmaceuticals, a safety factor of 1000 was applied to the lowest
daily therapeutic dose; it consists of a 10-fold factor for sensitive humans,
a 10-fold factor for infants and children and a 10-fold factor for the lowest
therapeutic dose not being a no-effect level. In addition, a factor of 10 was
added for cytotoxic drugs as a result of the higher toxicity associated with
these compounds and for hormonally active steroids, which are active at very
low concentrations and for which there is a high public perception of adverse
effects.
In applying the guidelines, the calculated guideline values for the
pharmaceuticals were compared with the highest concentrations measured in
secondary treated effluent to derive the MOEs. Most of the calculated MOEs
are more than 1000; given that this does not take into account reductions
achieved by treatment processes, it is unlikely that pharmaceutical chemicals
will be present at levels approaching the recommended guideline values or
cause any adverse impacts on human health.
5 This section is based on NRMMC, EPHC & NHMRC (2008).
2.5 Conclusion
Risk assessments from the United Kingdom, the USA and Australia have applied
the ADI or the MTD approaches, in conjunction with uncertainty factors, to
derive screening values for pharmaceuticals in drinking-water. Analysis of the
results indicated that adverse human health impacts are very unlikely from
exposure to the trace concentrations of pharmaceuticals that could potentially
be found in treated drinking-water. Available data have shown that for
those substances that have been detected, the concentrations are more than
1000-fold less than the MTD, which is the lowest clinically active dosage.
These findings are in line with other studies over the past decade that also
supported the conclusion that discernible risks to health arising from trace levels
of pharmaceuticals in drinking-water are extremely unlikely (e.g. Christensen,
1998; Schulman et al., 2002; Webb et al., 2003; Jones, Lester & Voulvoulis,
2005; Bercu et al., 2008; Snyder, 2010).
Given the low likelihood of human health risk, it is not considered necessary
to implement routine monitoring programmes that are resource intensive and
detract from other drinking-water concerns that are more important and more
acute, particularly the threat of waterborne pathogens. However, where specific
circumstances indicate a potential for elevated concentrations, screening values
and targeted investigative monitoring could be considered.
Future research could consider investigating the robustness and feasibility
of adapting the concept of the threshold of toxicological concern, which
is currently more widely used for food additives and contaminants, as an
alternative screening-level risk assessment, rather than developing values
for each substance individually (Kroes et al., 2004). Research could also
look into improvement to risk assessment methodology to address concerns
related to pharmaceutical mixtures and the effects of chronic, low-level
exposure to pharmaceutical, including exposure of sensitive subpopulations,
such as pregnant women and patients with particular diseases and medical
treatments (Rowney, Johnson & Williams, 2009). The WHO Framework for Risk
Assessment of Combined Exposure to Multiple Chemicals (Meek et al., 2011)
could be utilized to further consider the issue of mixtures.


3Treatment technologies for
removal of pharmaceuticals
from water

16
3. Treatment technologies for removal of
pharmaceuticals from water
3.1 Introduction
Many studies have reported the presence of pharmaceuticals in effluents
from wastewater treatment facilities (Ternes, 1998; Andreozzi et al., 2003;
Miao et al., 2004; Paxéus, 2004; Castiglioni et al., 2006; Vieno, Tuhkanen
& Kronberg, 2007) and identified these effluents as the main conveyors of
pharmaceuticals and their metabolites into receiving water sources, such as
rivers, lakes, reservoirs and groundwater aquifers, that are used for drinking-
water supply (Heberer, 2002; Ternes & Joss, 2006; Xu et al., 2007; Zhang,
Geissen & Gal, 2008; Huerta-Fontela, Galceran & Ventura, 2011).
The presence of trace concentrations of pharmaceuticals in the water cycle,
typically in the nanogram to low microgram per litre range, has raised questions
concerning the efficacy of drinking-water and wastewater treatment processes
in removing pharmaceuticals. The majority of research studies on treatment
efficacy have been conducted in Europe and the USA, with some studies
conducted in developed countries in Asia (Lee et al., 2008; Simazaki et al.,
2008; Van De Steene, Stove & Lambert, 2010; Huerta-Fontela, Galceran
& Ventura, 2011). In addition, there are more studies that focus on removal
efficacies at laboratory scale or by single treatment processes rather than at
full scale, especially for drinking-water treatment processes.
This chapter provides an overview of the removal of pharmaceuticals by
conventional and advanced wastewater and drinking-water treatment
processes based on the published literature.
3.2 Removal of pharmaceuticals by
wastewater treatment processes
Conventional wastewater treatment facilities typically have biological
degradation using the activated sludge process, whereas advanced facilities
have tertiary treatment processes, such as reverse osmosis, ozonation and
advanced oxidation technologies. Pharmaceuticals are a diverse group of
chemicals, with varying physical and chemical properties (Jelic et al., 2011).
Treatment efficacy depends on these physical and chemical characteristics
(e.g. hydrophobicity), their reactivity towards different treatment processes
and process control, such as solids retention time, temperature and hydraulic
retention time. For example, the majority of pharmaceuticals are relatively
hydrophobic and therefore less effectively removed by sorption to sludge
(Vieno, Tuhkanen & Kronberg, 2007). Treatment removal efficiency could
therefore vary significantly between different treatment facilities or at different
time periods within the same treatment facility (Vieno, Tuhkanen & Kronberg,
2007).
Table 7 collates the results of several studies to illustrate the removal rates that
can be expected by different wastewater treatment processes. These are based
on observations of treatment processes ranging from single unit processes to
full-scale wastewater treatment facilities found in the various studies.
Table 7 demonstrates that conventional wastewater treatment facilities with
activated sludge processes can achieve higher removal efficiency than
simple biological filters. Removal rates for pharmaceuticals can vary and
could sometimes be limited (Kasprzyk-Hordern, Dinsdale & Guwy, 2009),
depending on such factors as sludge age (DWI, 2007), activated sludge tank
temperature and hydraulic retention time (Wick et al., 2009; Gabet-Giraud et
al., 2010).
Advanced wastewater treatment processes, such as ozonation, membrane
treatment and advanced oxidation, can generally achieve higher removal rates
(up to 100%) for pharmaceuticals compared with conventional processes.
For example, another bench-scale study showed that advanced oxidation
processes can achieve up to 100% removal for diclofenac (Klavarioti,
Mantzavinos & Kassinos, 2009).
Prediction of removal rates for wastewater treatment processes is possible
for pharmaceuticals with very similar chemical structures. However, practical
difficulties do exist in predicting removal rates between different wastewater
treatment facilities, as highly variable removal rates are obtained for beta
blockers, depending on the wastewater treatment facility under consideration.
For example, the beta blockers betaxolol, bisprolol, carazolol and metprolol
are significantly removed by activated sludge processes, with reported removal
rates varying from 65% to about 90% (Ternes, 1998; Gabet-Giraud et al.,
2010), whereas low removal rates of less than 20% and approximately 32%
are reported for soltalol and propranolol, respectively, in other studies (Bendz
et al., 2005; Gabet-Giraud et al., 2010).
17
Table 7. Conventional and advanced wastewater treatment processes and their expected range of removal efficiency for pharmaceuticals
Treatment process Removal range (%) Water source Areas studied Reference
> Conventional wastewater treatment processes
Activated sludge 11– 9 9 Raw sewage Australia Watkinson, Murby & Costanzo (2007)
7–100 Primary settled sewage Europe, Japan DWI (2007)
< 20–80 Primary settled sewage France Gabet-Giraud et al. (2010)
−193–86
aPrimary settled sewage Europe Vieno, Tuhkanen & Kronberg (2007)
8–98 Not specified Brazil, Europe, Japan Ziylan & Ince (2011)
Biological filtration 6–71 Primary settled sewage Europe DWI (2007)
Primary settling 3–45 Not specified Brazil, Europe, Japan Ziylan & Ince (2011)
Coagulation, filtration and settling 5–36 Not specified
Sand filtration 0–99 Activated sludge effluent
> Advanced wastewater treatment processes
Ozonation 1–99 Activated sludge effluent Brazil, Europe, Japan Ziylan & Ince (2011)
86–100 Secondary effluent France Gabet-Giraud et al. (2010)
Ozonation/ultrasound and sonocatalysis 23–45 Not specified Europe, India, Japan,
Turkey, USA
Ziylan & Ince (2011)
Ozonation and catalytic ozonation > 9–100
UV irradiation 29 Not specified Brazil, Europe, Japan Ziylan & Ince (2011)
Photolysis (UV/hydrogen peroxide) 52–100 Not specified Europe, India, Japan,
Turkey, USA
Ziylan & Ince (2011)
Dark and light Fenton 80 –100
UV/TiO2> 95
Biomembrane 23–99 Treated effluent Brazil, Europe, Japan Ziylan & Ince (2011)
Microfiltration and reverse osmosis 91–100 Secondary treated
effluent
Australia Watkinson, Murby & Costanzo (2007)
Reverse osmosis 62–97 Secondary treated
effluent
France Gabet-Giraud et al. (2010)
Ultrasound 24–100 Not specified Europe, India, Japan,
Turkey, USA
Ziylan & Ince (2011)
UV, ultraviolet
a The removal of some pharmaceuticals appears to be negative. This has been attributed to the way in which removal is calculated, without hydraulic retention time being considered. This
means that the effluent sample does not directly correspond to the influent sample. In the case of carbamazepine, the increase observed was consistent, and the most probable cause was
reported to be conversion of carbamazepine glucuronides and other conjugated metabolites to the parent compound by enzymatic processes in the treatment plant (Ternes et al., 1999; Vieno,
Tuhkanen & Kronberg, 2007).

18
3.3 Removal of pharmaceuticals by
drinking-water treatment processes
Treated effluents from wastewater treatment facilities that have an impact on
receiving water bodies constitute the main source of pharmaceuticals in surface
waters, which could be used for drinking-water supply (Rahman, Yanful &
Jasim, 2009). Other possible pathways of pharmaceuticals to drinking-water
sources include leaching of pharmaceuticals to groundwater (Gomes & Lester,
2003) from sources such as leaking sewage systems and pipes.
None of the wide range of drinking-water treatment processes available have
been designed specifically to remove pharmaceuticals that may be present
in source waters. Nonetheless, removal of pharmaceuticals during drinking-
water treatment is largely dependent on their physical and chemical properties,
and treatment processes can therefore achieve some level of removal. For
example, biodegradation on slow sand filters and/or sorption to particles
removed by coagulation may help reduce the levels of some pharmaceuticals
present in drinking-water sources; granular activated carbon (GAC) and
powdered activated carbon (PAC) are increasingly adopted in drinking-
water treatment to remove pesticides and improve taste and odour, and these
processes may remove some pharmaceuticals by sorption (or biodegradation
on GAC). Groundwater sources that are used for drinking-water typically have
low particulate matter and organic matter content. Therefore, drinking-water
treatment is mostly single-stage disinfection, without multiple treatment barriers.
Table 8 summarizes the findings in various published studies on the removal
efficiencies of conventional and advanced water treatment processes for
pharmaceuticals in drinking-water. The majority of these studies focused
on bench-scale removal by spiking water samples with target compounds,
subjecting these samples to treatment and measuring the resulting
concentrations. However, some full-scale studies at drinking-water treatment
facilities have been carried out.
Bench-scale studies using both alum and ferric chloride as coagulants
for natural water or pure water samples spiked with pharmaceutical target
compounds showed that coagulation (with or without chemical softening) is
largely ineffective in removing pharmaceutical target compounds (Westerhoff
et al., 2005; Yoon et al., 2006; Snyder et al., 2007). An Awwa Research
Foundation project also concluded that coagulation was largely ineffective for
pharmaceutical removal in bench-scale, pilot-scale and full-scale investigations
(Khiari, 2007).
Chlorination and ozonation can achieve higher removal rates, with efficacy
a function of chemical structure and treatment conditions, such as pH and
oxidant dose (Zwiener & Frimmel, 2000; Adams et al., 2002; Huber et al.,
2003, 2005; Snyder et al., 2003; Ternes et al., 2003; Pinkston & Sedlak,
2004; Kim et al., 2007). In some studies, free chlorine was found to oxidize
approximately half of the pharmaceuticals investigated, but chloramine was
comparatively less efficient. Antibiotics such as sulfamethoxazole, trimethroprim
and erythromycin are among the compounds that showed high removal by
free chlorine (Khiari, 2007). Advanced oxidation processes using ozone with
hydrogen peroxide greatly improve oxidation and are frequently applied in
wastewater recycling processes for indirect potable reuse to convert recalcitrant
organic chemicals.
PAC and GAC can achieve high removal of pharmaceutical target compounds,
especially hydrophobic compounds. Removal efficacy is a function of contact
time, organic loading, chemical structure, solubility and carbon type (Ternes
et al., 2002; Yoon et al., 2003; Snyder et al., 2006). Iopromide, ibuprofen,
meprobamate, sulfamethoxazole and diclofenac were some of the compounds
found to be most resistant to activated carbon removal (Khiari, 2007).
Membrane treatment is highly effective in removing chemicals from water, and
removal efficacy is a function of physical and chemical properties, such as
molecular weight, hydrophobicity, polarity, chemical nature and pore size of
the membranes. Some studies (Yoon et al., 2006; Khiari, 2007) suggested
that nanofiltration (NF) can achieve better removal rates for most target
compounds than ultrafiltration (UF)/microfiltration (MF) membranes as a result
of both hydrophobic adsorption and size exclusion. Higher molecular weight
substances would be removed by size exclusion, especially by NF membranes.
Reverse osmosis (RO) was highly effective, despite trace quantities of some
target compounds breaching RO membranes. However, a double-pass RO
system was reported to remove all target compounds to below detection limits
(Khiari, 2007).
Ultraviolet (UV) irradiation at typical disinfection dosages was ineffective
for removing most target compounds, even though it can achieve more
than 50% removal of sulfamethoxazole (antibiotic), triclosan (antimicrobial)
and diclofenac (NSAID). However, a combination of higher-dose UV
(400 mJ/cm2 and higher) with hydrogen peroxide (3 mg/l and above)
removed most target compounds (Rosenfeldt & Linden, 2004; Khiari, 2007).
19
Table 8. Drinking-water treatment processes and their expected range of removal of pharmaceuticals
Treatment process Removal range (%) Scale Country studied (no.
of compounds) Reference
RO > 99 Pilot Germany (6) Heberer, Reddersen & Mechlinski (2002)
RO1 70 – 91 Bench Japan (6) Kimura et al. (2004)
RO2 10–85 Bench
UV/H2O23 – > 95 Bench USA (2) Rosenfeldt & Linden (2004)
Coag 24–72 Bench USA (49) Westerhoff et al. (2005)
PAC (20 mg/l) > 80 Bench
PAC (1 mg/l) 40 –75 Bench
CI225–75 Bench
O35–95 Bench
O333–100 Bench Germany (9) McDowell et al. (2005)
ClO20–100 Bench G e r m a n y (11) Huber et al. (2005)
NF1 > 98 Bench Australia (3) Nghiem, Schäfer & Elimelech (2005)
NF2 > 80 Bench
UF < 30 Bench USA (27) Yoon et al. (2006)
NF 30–90 Bench
Coag < 5–30 Bench Finland (5) Vieno, Tuhkanen & Kronberg (2006)
Cl220 –100 Bench Japan (9) Simazaki et al. (2008)
PAC > 98 Bench
Coag < 15 Bench
Constructed wetlands 28–60 Pilot Singapore (4) Zhang et al. (2011)
Aeration/SF 25 – > 95 Full Germany (5) Reddersen, Heberer & Dünnbier (2002)
O3/Coag/Sed/Cl2100 Full USA (2) Boyd et al. (2003)
PAC/Coag/Sed 0Full USA (1)
Cl2100 Full USA (1)
Coag 0Full Republic of Korea (6) Kim et al. (2007)
UF 0Full
GAC 100 Full
20
Treatment process Removal range (%) Scale Country studied (no.
of compounds) Reference
NF 30 – > 90 Full Sp ain (12) Radjenović et al. (2008)
RO 45 – > 90 Full
Disinfection 2–97 Full France (7)aANSES (2011)
Physical and chemical 31– 94 Full
O3 + AC 47–97 Full
Membranes 6–68 Full
Pre-Cl20 – > 99 Full Spain (35) Huerta-Fontela, Galceran & Ventura (2011)
Coag/Floc/SF < 30–100 Full
O35 – > 99 Full
GAC 55 – > 75 Full
Cl214–100 Full
AC, activated carbon; Cl2, chlorine; ClO2, chlorine dioxide; Coag, coagulation; Floc, flocculation; GAC, granular activated carbon; H2O2, hydrogen peroxide; NF, nanofiltration; O3,
ozonation; PAC, powdered activated carbon; RO, reverse osmosis; Sed, sedimentation; SF, sand filtration; UF, ultrafiltration; UV, ultraviolet
a Note that this was a national study incorporating 78 instances of pharmaceutical removal.
.
Conclusion
This chapter has provided an overview of the removal of pharmaceuticals
by conventional and advanced wastewater and drinking-water treatment
processes based on the published literature.
Conventional wastewater treatment typically consists of activated sludge
processes. Biological treatment, such as activated sludge and biofiltration,
has demonstrated significant removal rates for pharmaceuticals that are
biodegradable or readily bind to particles (Ternes et al., 1999; Joss et al.,
2005; Kim et al., 2007). However, removal rates for pharmaceuticals can
vary within and between studies (Kasprzyk-Hordern, Dinsdale & Guwy, 2009;
Wick et al., 2009), depending on such factors as sludge age (DWI, 2007),
activated sludge tank temperature and hydraulic retention time. For example,
diclofenac removal in the activated sludge process ranges from 21% to 50%,
but this can be optimized by operating the process at a sludge age of eight
days or more (Ziylan & Ince, 2011).
Advanced wastewater treatment processes that comprise membranes,
advanced oxidation technologies, etc. have shown higher removal efficiencies
for pharmaceuticals (e.g. advanced oxidation processes can achieve up to
100% removal for diclofenac) (Klavarioti, Mantzavinos & Kassinos, 2009).
However, conventional treatment is generally sufficient to meet regulatory
requirements, and capital-intensive advanced treatment processes are not
commonly adopted for wastewater treatment (Spellman, 2010).
With respect to conventional drinking-water treatment, bench-scale studies
showed that coagulation (with or without chemical softening) is largely
ineffective in removing pharmaceuticals (Westerhoff et al., 2005; Yoon et al.,
2006; Snyder et al., 2007). Free chlorine was found to oxidize approximately
half of the pharmaceuticals investigated, and chloramine was less efficient.
Antibiotics such as sulfamethoxazole, trimethroprim and erythromycin are
among the compounds that showed high removal by free chlorine (Khiari,
2007).
Table 8. (continued)

21
Advanced water treatment processes such as ozonation, advanced oxidation,
activated carbon and membrane processes (nanofiltration, reverse osmosis)
were demonstrated to achieve higher removal rates (above 99%) for targeted
pharmaceutical compounds in various published literature studies. However,
advanced oxidation processes can lead to incomplete degradation products,
such as metabolites, and future research could consider the value and feasibility
of studying the formation and impact of these metabolites (Celiz, Tso & Aga,
2009).
For drinking-water sources that are contaminated with pesticides, advanced
treatment may already be in place to meet regulations. In such cases, removal
of pharmaceuticals during treatment may already be optimized.
Most importantly, it is prudent to note that advanced and costly water
treatment technology will not be able to completely remove all micropollutants
to concentrations below the detection limits of the most sensitive analytical
procedures at all times. Therefore, it is imperative to consider the toxicological
relevance of various compounds in the context of appreciable risks to human
health. Increased or rapidly changing exposure arising from specific local
circumstances (e.g. a significant increase in the concentration of pharmaceuticals
in surface waters impacted by wastewater discharge) should be investigated.
An informed risk assessment considering the above principles is essential
before allocating scarce resources to upgrade or invest in additional advanced
treatment processes to reduce trace concentrations of pharmaceuticals in
drinking-water.
In view of the substantial margin of safety for consumption of very low
concentrations of pharmaceuticals in drinking-water (Chapter 2 in this report),
concerns over pharmaceuticals should not divert the attention and resources of
water suppliers and regulators from other chemical and pathogenic microbial
priorities. For example, although the government in Australia has issued
proposed guideline values for 84 pharmaceuticals for water reuse schemes,
microbial pathogens remain their overriding priority in water reuse (NRMMC,
EPHC & NHMRC, 2008).


Preventing pharmaceuticals
in drinking-water
4

24
4. Preventing pharmaceuticals in drinking-water
Conventional drinking-water quality monitoring that places emphasis
on end-product testing is very resource intensive in terms of capital
investment and human resources. With an expanding list of chemical
contaminants detected in drinking-water and water sources that may
be of insignificant health concern, an overemphasis on end-product
monitoring and the upgrading of treatment infrastructure is clearly not
sustainable or an optimal use of limited resources.
Chapter 4 in the fourth edition of the WHO Guidelines for Drinking-
water Quality states that the water safety plan is “the most effective
means of consistently ensuring the safety of a drinking-water
supply… through the use of a comprehensive risk assessment and
risk management approach that encompasses all steps in the water
supply from catchment to consumer” (WHO, 2011). The key principles
of water safety plans underline the importance of looking at risk
assessment and risk management across the entire water cycle starting
at source. Adapting this full life cycle approach to pharmaceuticals in
drinking-water means that preventing pharmaceuticals from entering
the environment during their production, consumption and disposal is a
pragmatic and effective means of risk management.
Inappropriate disposal practices, such as flushing unwanted or excess
drugs down toilets and sinks and discarding them in household waste,
are common and often a significant contributor of pharmaceuticals
present in wastewater and other environmental media (e.g. surface
waters and landfill leachate). A survey from Germany’s Management
Strategies for Pharmaceutical Residues in Drinking Water research
programme showed that consumers discarded 23% of liquid
pharmaceuticals prescribed and 7% of tablets. While some went into
household trash, the equivalent amount of pharmaceuticals that was
flushed away is approximately 364 tons every year (Lubick, 2010).
Another survey of households in the United Kingdom in 2003 found that
63% of unwanted pharmaceuticals were discarded in household waste
and 11.5% were flushed down sinks or toilets (Bound & Voulvoulis,
2005). Similarly, proper and well-managed disposal practices at
concentrated point sources such as health-care and veterinary facilities
will help mitigate the entry of pharmaceuticals into our environment.
Currently, tighter rules and regulations apply to controlled substances
and cytotoxic drugs than for other pharmaceuticals. Despite this,
disposal to sewers is not precluded (USEPA, 2008a). Disposal of non-
controlled substances tends to be more variable and is often developed
on a local, jurisdictional or regional basis. A scan of the current
literature, which is not exhaustive, revealed a few broadly categorized
preventive measures in Australia, Canada, the USA and European
countries that could potentially reduce the entry of pharmaceuticals into
our environment. These measures are described below.
4.1 Improved regulations and
guidance on pharmaceutical waste
management
All health-care facilities should have policies and procedures in place
for the correct management of pharmaceutical waste. In Australia,
the Environmental Protection Authority and the National Health and
Medical Research Council had guidelines on the management of
waste generated in health-care facilities. The National Health and
Medical Research Council stated that, where possible, pharmaceutical
waste should be incinerated and should not be sent to landfills or
discharged to sewers (NHMRC, 1999). Licensed waste disposal
companies collected all clinical and pharmaceutical waste for disposal
in authorized waste disposal facilities.
In the USA, frequently used pharmaceuticals, such as epinephrine,
warfarin and selected chemotherapeutic agents, are regulated as

25
hazardous waste under the Resource Conservation and Recovery
Act. Failure to comply with the regulations under this Act through
improper management and disposal of waste can potentially constitute
serious violations and incur heavy penalties. To guide stakeholders on
acceptable disposal practices, the USEPA supported the development of
Managing Pharmaceutical Waste: A 10-Step Blueprint for Health Care
Facilities in the United States, which recommends a stepwise approach
to help health-care facilities develop and implement a comprehensive
pharmaceutical hazardous waste management programme. This
blueprint adopts the best practices in waste minimization to meet
regulatory compliance for pharmaceutical waste disposal and
safeguard human health and the environment in a cost-effective manner
(Pines & Smith, 2006).
To this end, the USEPA (2010b) has also drafted a guidance document,
Best Management Practices for Unused Pharmaceuticals at Health Care
Facilities, to advise health-care and veterinary facilities on reducing
pharmaceutical waste, on pharmaceutical waste management and
on application of disposal regulations. The aim is to help reduce the
amount of pharmaceuticals that are discharged to water bodies.
4.2 Pharmaceutical take-back
programmes
To augment regulations, take-back programmes have been established
by government and private organizations in several countries to reduce
the amount of drugs entering our environment (Daughton, 2003,
2004; Glassmeyer et al., 2009; Teleosis Institute, 2009). A survey of
households in the United Kingdom in 2003 showed that 22% of excess
pharmaceuticals were returned to pharmacists; although take-back
programmes were effective, further improvement is needed (Bound &
Voulvoulis, 2005).
These programmes can be of different scales, ranging from small one-day
collection events to regular and systematic regional collection, ongoing
return of unused and excess medicines to participating pharmacies and
mail-back programmes where excess medicines are returned in prepaid
packs to government-supervised mailboxes (SCBWMI, 2005). Several
household hazardous waste collection programmes have also added
pharmaceuticals to the list over the years (Glassmeyer et al., 2009).
In Australia, the Commonwealth Department of Health & Ageing
Services provided funds to establish a system for the collection and
disposal of unwanted medicines, known as the Return Unwanted
Medicines (RUM) Project. Estimates from RUM showed that in 2010–
2011, more than 34 tonnes of unwanted medicines on average were
collected monthly by community pharmacies across Australia and
subsequently incinerated according to guidelines (RUM, 2011).
In the USA, many scheduled pharmaceutical collection events facilitate
prudent disposal of unwanted medications at the regional level, such
as the successful “Great Lakes Earth Day Challenge”, which collected
4.5 million pills for safe disposal. The USEPA has also awarded grants
to support take-back of non-controlled, unused medicines at pharmacies
and mail-back of unused medicines with appropriate involvement of
law enforcement (USEPA, 2010a). Other mechanisms to reduce the
entry of pharmaceuticals into the environment include establishing
best management practices for handling solid wastes and minimizing
discharge from landfills.
Canada has formal stewardship programmes for household
pharmaceutical waste at the provincial level or in cities that provide
convenient options for consumers to return pharmaceuticals to community
pharmacies for safe disposal.

26
Europe has widespread standardized take-back programmes. In the
2010 report Pharmaceuticals in the Environment: Results of an EEA
Workshop, the European Environment Agency (EEA, 2010) stated that
most countries in Europe collect unused drugs separately from household
waste, usually at pharmacies (a handful also have separate collection
sites alongside pharmacies). The national systems are operated and
funded by the pharmaceuticals industry, retail pharmacies or the
public sector. The operation of the take-back programmes may be the
responsibility of the retail pharmacies or of public or private waste
contractors (Teleosis Institute, 2009).
4.3 Raising consumer awareness
Consumers are accustomed to disposing of unwanted and expired
medicines through household waste and sewers. Such improper
disposal practices release pharmaceuticals into our environment,
wastewater and water sources. There is therefore a need to raise
public awareness and encourage consumers to adopt proper disposal
practices for unwanted pharmaceuticals. In Australia, the RUM Project
focuses on raising consumer awareness to inform consumers of the
appropriate option for drug disposal (RUM, 2010). In addition to
regulations under New York’s Drug Management and Disposal Act, the
New York State Department of Environmental Conservation publishes
posters for all pharmacies and retail stores that sell drugs to advise
consumers on the proper storage and disposal of unwanted medication
(DEC, 2010). Consumers can then serve as environmental stewards to
reduce water pollution.
4.4 Conclusion
Appropriate regulations governing disposal practices at point sources of
hazards, widespread take-back programmes, guidance and enhanced
consumer education will support efforts for the proper disposal of
unwanted and excess medicines and reduce the environmental impact
of pharmaceuticals entering our environment, including water sources.
As most pharmaceuticals enter the water cycle through wastewater
discharges or from poorly controlled manufacturing or production
facilities that are primarily associated with generic medicines, the
discharge of untreated or poorly treated wastewater to water bodies
used as drinking-water sources should be strongly discouraged.

5Conclusions,
recommendations
and knowledge gaps

28
5. Conclusions, recommendations and knowledge gaps
Pharmaceuticals are synthetic or natural chemicals that can be found in
prescription medicines, over-the-counter therapeutic drugs and veterinary drugs.
They contain active ingredients that are designed to achieve pharmacological
effects and confer significant benefits to society. Pharmaceuticals are primarily
introduced into the environment via human excretion, sewage effluent, improper
drug disposal, agricultural runoff, and livestock and veterinary waste. The
ubiquitous use of pharmaceuticals in various settings has resulted in a continuous
discharge of pharmaceuticals and metabolites into the environment, leading to
their “pseudo-persistence” in the environment. Significant advancements in the
sensitivity of detection and analytical technologies and methods have made it
possible to detect very low concentrations of pharmaceuticals in the range of
nanograms to low micrograms per litre in the water cycle. As pharmaceuticals
contain active ingredients that are designed to achieve specific pharmacological
effects based on their biological reactivity and biochemical properties, their
presence at trace concentrations in the water cycle has generated concerns
among various stakeholders, including governments, regulators and the public,
over potential risks to human health through very low level exposure via
drinking-water.
5.1 Conclusions
Targeted investigative studies conducted in the United Kingdom, the USA and
Australia have shown that concentrations of pharmaceuticals in surface water
and groundwater sources impacted by wastewater discharges are typically
less than 0.1 µg/l (or 100 ng/l). Detection in treated drinking-water is rare;
if pharmaceuticals are present, their concentrations are usually well below
0.05 µg/l (or 50 ng/l). There are, however, very few systematic monitoring
programmes or comprehensive, systematic studies on the occurrence of
pharmaceuticals in drinking-water, and limited occurrence data present one
of the key challenges in assessing the potential risks associated with trace
concentrations of pharmaceuticals in drinking-water.
Nonetheless, several approaches to screen and prioritize pharmaceuticals have
been published in the peer-reviewed literature. MTDs, ADIs and sometimes the
DWELs have been used as reference values by which to derive a margin of
safety between these and the reported or predicted worst-case exposure in
drinking-water.
Targeted investigations conducted in the above-mentioned countries found
that traces of pharmaceuticals in drinking-water are largely present at several
orders of magnitude (more than 1000-fold) below the lowest therapeutic dose
and largely below the calculated ADIs. The substantial margins of safety for
individual compounds suggest that appreciable adverse impacts on human
health are very unlikely at current levels of exposure in drinking-water.
From a treatment perspective, pharmaceuticals are not unusual organic
chemicals, and treatment removal rates are reasonably predictable based
upon the physical and chemical properties of the compounds. Conventional
treatment processes with coagulation, filtration and chlorination can remove
about 50% of these compounds, whereas advanced treatment, such as
ozonation, advanced oxidation, activated carbon and membrane processes
(e.g. reverse osmosis, nanofiltration), can achieve higher removal rates; reverse
osmosis, for example, can remove more than 99% of large pharmaceutical
molecules.
5.2 Recommendations
The substantial margin of safety for consumption of very low concentrations of
pharmaceuticals in drinking-water suggests that appreciable adverse impacts on
human health are very unlikely. As such, concerns over pharmaceuticals should
not divert attention and valuable resources of water suppliers and regulators
from other priorities, such as pathogenic microbial water quality issues. The
low risk to human health from current levels of exposure in drinking-water
suggests that development of formal guideline values for pharmaceuticals in the
WHO Guidelines for Drinking-water Quality and the installation of specialized
treatment processes to reduce trace concentrations of pharmaceuticals are not
warranted.
Routine monitoring programmes for pharmaceuticals in water sources and
drinking-water and additional or specialized drinking-water treatment to reduce
very low concentrations of pharmaceuticals in drinking-water are not deemed
necessary due to the limited public health benefits. However, where local
circumstances, such as a catchment survey, indicate a potential for elevated
levels of pharmaceuticals in the water cycle (surface water, groundwater,
wastewater effluent and drinking-water), relevant stakeholders could undertake

29
targeted, well-designed and quality-controlled investigative studies to obtain
more information with which to assess the potential health risks arising from
exposure through drinking-water. If necessary, screening values could be
developed based on the MTD or the ADI approaches, and an assessment
of the need for treatment enhancement could also be considered within the
context of other risks and priorities using water safety plans.
Reduction of human exposure to pharmaceuticals through drinking-water can
be achieved through a combination of preventive measures, such as take-
back programmes, regulations, public guidance and consumer education to
encourage the proper disposal of unwanted pharmaceuticals and minimize
the introduction of pharmaceuticals into the environment. It is also imperative
to enhance public communication and education on water quality issues from
the human health standpoint. For example, conveying to the public the potential
health risks from exposure to very low concentrations of pharmaceuticals in
drinking-water will help them to better understand this issue relative to other
hazards, such as waterborne pathogenic microorganisms. However, in the
long term, improvement of wastewater treatment to more efficiently remove
a range of organic substances that are seen as emerging contaminants of
concern would provide a more sustainable and comprehensive solution in
preventing their entry into the water environment.
5.3 Knowledge gaps and future
research
Although current risk assessments indicate that very low concentrations of
pharmaceuticals in drinking-water are very unlikely to pose any risks to human
health, there are knowledge gaps in terms of assessing the risks associated
with long-term, low-level exposures to pharmaceuticals and possible combined
effects of chemical mixtures, including pharmaceuticals. Future research
investigating the possible additive or synergistic effects of mixtures would be
beneficial for an accurate exposure assessment to determine whether there are
any potential risks to human health, taking into account sensitive subpopulations.
One of the key challenges in estimating exposures to pharmaceuticals in
drinking-water and assessing the potential risks to human health is the limited
occurrence data for the diverse group of human and veterinary pharmaceuticals
in use today. Implementing monitoring programmes is resource intensive in
terms of costs, human resources and infrastructure, and there is also a lack of
standardized sampling and analysis protocols to support monitoring studies.
As such, future research looking into cost-effective methods to prioritize
pharmaceuticals within the context of an overall risk assessment will benefit our
appreciation of low levels of pharmaceuticals in drinking-water from a human
health perspective.

30
References
Adams C et al. (2002).
Removal of antibiotics from surface and distilled water in conventional water
treatment processes. Journal of Environmental Engineering, 128:253–260.
Aherne GW, Hardcastle A, Nield AH (1990).
Cytotoxic drugs and the aquatic environment: estimation of bleomycin in river and
water samples. Journal of Pharmacy and Pharmacology, 42:741–742 [cited in
DWI, 2007].
Alder AC et al. (2006).
Consumption and occurrence. In: Ternes TA, Joss A, eds. Human pharmaceuticals,
hormones and fragrances: the challenge of micropollutants in urban water
management. London, IWA Publishing [cited in DWI, 2007].
Andreozzi R et al. (2003).
Ozonation and H2O2/UV treatment of clofibric acid in water: a kinetic investigation.
Journal of Hazardous Materials, B103:233–246.
ANSES (2011).
Campagne nationale d’occurrence des résidus de médicaments dans les eaux
destinées à la consommation humaine. Ressources en eaux brutes et eaux traitées.
Nancy, Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement
et du travail, Laboratoire d’Hydrologie de Nancy.
Ashton D, Hilton M, Thomas KV (2004).
Investigating the environmental transport of human pharmaceuticals to streams in the
United Kingdom. Science of the Total Environment, 333:167–184 [cited in DWI,
2007].
Bendz D et al. (2005).
Occurrence and fate of pharmaceutically active compounds in the environment, a
case study: Hoje River in Sweden. Journal of Hazardous Materials, 122:195 –204.
Benotti MJ et al. (2009).
Pharmaceuticals and endocrine disrupting compounds in U.S. drinking water.
Environmental Science & Technology, 43 (3):597–603.
Bercu JP et al. (2008).
Human health risk assessment for three neuropharmaceutical compounds in surface
waters. Regulatory Toxicology and Pharmacology, 50:420–427.
Boucard T, Gravell A (2006).
Personal communication: Concentrations of fluoxetine and norfluoxetine in UK
sewage effluents and river waters. United Kingdom Environment Agency [cited in
DWI, 2007].
Bound JP, Voulvoulis N (2005).
Household disposal of pharmaceuticals as a pathway for aquatic contamination in
the United Kingdom. Environmental Health Perspectives, 113 :17 0 5 –1711.
Bound JP, Voulvoulis N (2006).
Predicted and measured concentrations for selected pharmaceuticals in UK rivers:
implications for risk assessment. Water Research, 40:2885–2892 [cited in DWI,
2007].
Boyd GR et al. (2003).
Pharmaceuticals and personal care products (PPCPs) in surface water and treated
waters of Louisiana, USA and Ontario, Canada. Science of the Total Environment,
311:13 5 –14 9.
Bull RJ et al. (2011).
Therapeutic dose as the point of departure in assessing potential health hazards
from drugs in drinking water and recycled municipal wastewater. Regulatory
Toxicology and Pharmacology, 60:1–19.
Buser HR, Muller MD, Theobald N (1998).
Occurrence of the pharmaceutical drug clofibric acid and the herbicide mecoprop
in various Swiss lakes and in the North Sea. Environmental Science & Technology,
32(1):188–192.
Buser HR, Poiger T, Muller MD (1999).
Occurrence and environmental behavior of the chiral pharmaceutical drug ibuprofen
in surface waters and in wastewater. Environmental Science & Technology,
33(15):2529–2535.
Castiglioni S et al. (2006).
Removal of pharmaceuticals in sewage treatment plants in Italy. Environmental
Science & Technology, 40:357–363.
Celiz MD, Tso J, Aga DS (2009).
Pharmaceutical metabolites in the environment: analytical challenges and ecological
risks. Environmental Toxicology and Chemistry, 28(12):2473–2484.

31
Christensen FM (1998).
Pharmaceuticals in the environment—a human risk? Regulatory Toxicology and
Pharmacology, 28:212–221.
Daughton CG (2001).
Pharmaceuticals and personal care products in the environment: overarching issues
and overview. ACS Symposium Series, 791:2–38.
Daughton CG (2003).
Cradle-to-cradle stewardship of drugs for minimizing their environmental disposition
while promoting human health. II. Drug disposal, waste reduction and future
directions. Environmental Health Perspectives, 111: 7 75 –78 5.
Daughton CG (2004).
PPCPs in the environment: future research—beginning with the end always in mind.
In: Kümmerer K, ed. Pharmaceuticals in the environment, 2nd ed. Springer, pp.
463–495.
Daughton CG, Ternes TA (1999).
Pharmaceuticals and personal care products in the environment: agents of subtle
change? Environmental Health Perspectives, 107(Suppl. 6):907–938.
DEC (2010).
Drugs in New York’s waters: how drugs get into our waters and why DEC
is concerned. New York State Department of Environmental Conservation
(http://www.dec.ny.gov/chemical/45083.html).
DWI (2007).
Desk based review of current knowledge on pharmaceuticals in drinking water and
estimation of potential levels. Final report prepared by Watts and Crane Associates
for Drinking Water Inspectorate, Department for Environment, Food and Rural Affairs
(Defra Project Code: CSA 7184/WT02046/DWI70/2/213; http://dwi.defra.
gov.uk/research/completed-research/reports/dwi70-2-213.pdf).
EEA (2010).
Pharmaceuticals in the environment: results of an EEA workshop. Copenhagen,
European Environment Agency (EEA Technical Report No. 1; http://www.eea.europa.
eu/publications/pharmaceuticals-in-the-environment-result-of-an-eea-workshop).
FAO/WHO (2009).
Principles and methods for the risk assessment of chemicals in food. Rome, Food
and Agriculture Organization of the United Nations; Geneva, World Health
Organization (Environmental Health Criteria 240).
Fatta D et al. (2007).
Analytical methods for tracing pharmaceutical residues in water and wastewater.
Trends in Analytical Chemistry, 26 (6):515–533.
Focazio MJ et al. (2008).
A national reconnaissance for pharmaceuticals and other organic wastewater
contaminants in the United States—II) Untreated drinking water sources. Science of
the Total Environment, 402:201–216.
Gabet-Giraud V et al. (2010).
Occurrence and removal of estrogens and beta blockers by various processes in
wastewater treatment plants. Science of the Total Environment, 408:4257–4269.
Garrison AW, Pope JD, Allen FR (1976).
GC/MS analysis of organic compounds in domestic wastewaters. In: Keith LH, ed.
Identification and analysis of organic pollutants in water. Ann Arbor, MI, Ann Arbor
Science Publishers Inc., pp. 517–556.
Glassmeyer ST et al. (2009).
Disposal practices for unwanted residential medications in the United States.
Environment International, 35:566–572.
Gomes RL, Lester JN (2003).
Endocrine disrupters in drinking water and water reuse. In: Birkett JW, Lester JN, eds.
Endocrine disrupters in wastewater and sludge treatment processes. Boca Raton, FL,
CRC Press, pp. 219–266.
Heberer T (2002).
Occurrence, fate, and removal of pharmaceutical residues in the aquatic
environment: a review of recent research data. Toxicology Letters, 131 : 5 –17.
Heberer T, Reddersen K, Mechlinski A (2002).
From municipal sewage to drinking water: fate and removal of pharmaceutical
residues in the aquatic environment in urban areas. Water Science & Technology
,
46:81–88.
Heberer T, Schmidt-Bäumler K, Stan HJ (1998).
Occurrence and distribution of organic contaminants in the aquatic system in
Berlin. Part II: Substituted phenols in Berlin surface water. Acta Hydrochimica et
Hydrobiologica, 26:272–278.

32
Heberer T et al. (2001).
Occurrence of pharmaceutical residues in sewage, river, ground, and drinking
water in Greece and Berlin (Germany). In: Daughton C, Jones-Lepp T, eds.
Pharmaceuticals and personal care products in the environment; scientific and
regulatory issues. Washington, DC, American Chemical Society.
Heberer T et al. (2004).
Field studies on the fate and transport of pharmaceutical residues in bank filtration.
Ground Water Monitoring and Remediation, 2 4 : 7 0 –7 7.
Hignite C, Azarnoff DL (1977).
Drugs and drug metabolites as environmental contaminants: chlorophenoxyisobutyrate
and salicylic acid in sewage water effluent. Life Sciences, 20 (2):337–341.
Huber MM et al. (2003).
Oxidation of pharmaceuticals during ozonation and advanced oxidation processes.
Environmental Science & Technology, 37:1016–1024.
Huber MM et al. (2005).
Oxidation of pharmaceuticals during water treatment with chlorine dioxide. Water
Research, 39:3607–3617.
Huerta-Fontela M, Galceran MT, Ventura F (2011).
Occurrence and removal of pharmaceuticals and hormones through drinking water
treatment. Water Research, 45:1432–1442.
Jelic A et al. (2011).
Occurrence, partition and removal of pharmaceuticals in sewage water and sludge
during wastewater treatment. Water Research, 4 5 :116 5 –1176 .
Jones OA, Lester JN, Voulvoulis N (2005).
Pharmaceuticals: a threat to drinking water? Trends in Biotechnology, 23 (4):163–
16 7.
Joss A et al. (2005).
Removal of pharmaceuticals and fragrances in biological wastewater treatment.
Water Research, 39:3139 –3152.
Kasprzyk-Hordern B, Dinsdale RM, Guwy AJ (2009).
The removal of pharmaceuticals, personal care products, endocrine disruptors and
illicit drugs during wastewater treatment and its impact on the quality of receiving
waters. Water Research, 43:363–380.
Khiari D (2007).
Endocrine disruptors, pharmaceuticals, and personal care products in drinking
water: an overview of AwwaRF research to date. Denver, CO, Awwa Research
Foundation.
Kim SD et al. (2007).
Occurrence and removal of pharmaceuticals and endocrine disruptors in South
Korean surface, drinking, and waste waters. Water Research, 41:1013–1021.
Kimura K et al. (2004).
Rejection of neutral endocrine disrupting compounds (EDCs) and pharmaceutical
active compounds (PhACs) by RO membranes. Journal of Membrane Science,
245(1–2):71–78.
Klavarioti M, Mantzavinos D, Kassinos D (2009).
Removal of residual pharmaceuticals from aqueous systems by advanced oxidation
processes. Environment International, 3 5 : 4 0 2 – 417.
Kolpin DW et al. (2002).
Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S.
streams, 1999–2000: a national reconnaissance. Environmental Science &
Technology, 36:1202–1211.
Kroes R et al. (2004).
Structure-based thresholds of toxicological concern (TTC): guidance for application
to substances present at low levels in the diet. Food and Chemical Toxicology,
42:65–83.
Lee S et al. (2008).
Efficient removals of tris(2-chloroethyl) phosphate (TCEP) and perchlorate using NF
membrane filtrations. Desalination, 221:234–237.
Loos R et al. (2007).
Polar herbicides, pharmaceutical products, perfluorooctanesulfonate (PFOS),
perfluorooctanoate (PFOA), and nonylphenol and its carboxylates and ethoxylates
in surface and tap waters around Lake Maggiore in Northern Italy. Analytical and
Bioanalytical Chemistry, 387:1469–1478.
Loraine GA, Pettigrove ME (2006).
Seasonal variations in concentrations of pharmaceuticals and personal care
products in drinking water and reclaimed wastewater in Southern California.
Environmental Science & Technology, 40:687–695.
Lubick N (2010).
Drugs in the environment: do pharmaceutical take-back programs make a
difference? Environmental Health Perspectives, 118 ( 5 ) : A 211– A 214 .
McDowell DC et al. (2005).
Ozonation of carbamazepine in drinking water: identification and kinetic study of
major oxidation products. Environmental Science & Technology, 39:8014–8022.
Meek ME et al. (2011).
Risk assessment of combined exposure to multiple chemicals: a WHO/IPCS
framework. Regulatory Toxicology and Pharmacology, 60:S1–S14.
Miao X-S et al. (2004).
Occurrence of antimicrobials in the final effluent of wastewater treatment plants in
Canada. Environmental Science & Technology, 38(13):3533–3541.

33
Mompelat S, Le Bot B, Thomas O (2009).
Occurrence and fate of pharmaceutical products and by-products, from resource to
drinking water. Environment International, 35:803–814.
Mons MN, Hoogenboom AC, Noij THM (2003).
Pharmaceuticals and drinking water supply in the Netherlands. Nieuwegein, Kiwa
Water Research (Kiwa Report No. BTO 2003.040).
Nghiem LD, Schäfer AI, Elimelech M (2005).
Pharmaceutical retention mechanisms by nanofiltration membranes. Environmental
Science & Technology, 39 (19):7698–7705.
NHMRC (1999).
National guidelines for waste management in the health industry. Canberra,
National Health and Medical Research Council (http://www.nhmrc.gov.au/_files_
nhmrc/file/publications/synopses/withdrawn/eh11.pdf).
NRMMC, EPHC, NHMRC (2008).
Australian guidelines for water recycling: managing health and environmental risks
(PHASE 2). Augmentation of drinking water supplies. Natural Resource Management
Ministerial Council, Environment Protection and Heritage Council and National
Health and Medical Research Council (http://www.qwc.qld.gov.au/prw/pdf/
agwr-augmentation-drinking-water-supplies-08-05.pdf).
Paxéus N (2004).
Removal of selected non-steroidal anti-inflammatory drugs (NSAIDs), gemfibrozil,
carbamazepine, β-blockers, trimethoprin and triclosan in conventional wastewater
treatment plants in five EU countries and their discharge to the aquatic environment.
Water Science & Technology, 50(5):253–260.
Pérez S, Barceló D (2007).
Application of advanced MS techniques to analysis and identification of human
and microbial metabolites of pharmaceuticals in the aquatic environment. Trends in
Analytical Chemistry, 26(6):494–514.
Pines E, Smith C (2006).
Managing pharmaceutical waste: a 10-step blueprint for health care facilities in
the United States. Hospitals for a Healthy Environment (http://www.premierinc.
com/quality-safety/tools-services/safety/topics/pharma-waste/downloads/h2e-
pharma-blueprint-04-15-06.pdf).
Pinkston KE, Sedlak DL (2004).
Transformation of aromatic ether- and amine-containing pharmaceuticals during
chlorine disinfection. Environmental Science & Technology, 38:4019–4025.
Radjenović J et al. (2008).
Rejection of pharmaceuticals in nanofiltration and reverse osmosis membrane
drinking water treatment. Water Research, 42(14):3601–3610.
Rahman MF, Yanful EK, Jasim SY (2009).
Occurrences of endocrine disrupting compounds and pharmaceuticals in the
aquatic environment and their removal from drinking water: challenges in the context
of the developing world. Desalination, 248:578–585.
Reddersen K, Heberer T, Dünnbier U (2002).
Identification and significance of phenazone drugs and their metabolites in ground-
and drinking water. Chemosphere, 49:539–544.
Roberts PH, Thomas KV (2006).
The occurrence of selected pharmaceuticals in wastewater effluent and surface
waters of the lower Tyne catchment. Science of the Total Environment, 356:143–
153 [cited in DWI, 2007].
Rosenfeldt EJ, Linden KG (2004).
Degradation of endocrine disrupting chemicals bisphenol A, ethinyl estradiol and
estradiol during UV photolysis and advanced oxidation processes. Environmental
Science & Technology, 38:5476–5486.
Rowney NC, Johnson AC, Williams RJ (2009).
Cytotoxic drugs in drinking water: a prediction and risk assessment exercise for the
Thames catchment in the United Kingdom. Environmental Toxicology and Chemistry,
28(12):2733–2743.
RUM (2010).
Consumer awareness. Cheltenham, The National Return and Disposal of Unwanted
Medicine Limited, Returning Unwanted Medicine Project (http://www.returnmed.
com.au/consumer-awareness).
RUM (2011).
Collection 2010–2011. Cheltenham, The National Return and Disposal of Unwanted
Medicine Limited, Returning Unwanted Medicine Project (http://www.returnmed.
com.au/collections).
SCBWMI (2005).
Discussion paper on pharmaceutical disposal to sewer systems. White paper
prepared by the Emerging Contaminants Working Group of the Santa Clara Basin
Watershed Management Initiative (http://www.scbwmi.org/PDFs/WMI_Pharm_
White_Paper_FinalMarch05.pdf).
Schulman LJ et al. (2002).
A human health risk assessment of pharmaceuticals in the aquatic environment.
Human and Ecological Risk Assessment, 8:657–680.
Schwab RW et al. (2005).
Human pharmaceuticals in US surface waters: a human health risk assessment.
Regulatory Toxicology and Pharmacology, 42:296 –312.
Simazaki D et al. (2008).
Removal of selected pharmaceuticals by chlorination, coagulation–sedimentation
and powdered activated carbon treatment. Water Science & Technology,
58 ( 5 ) :112 9 –113 5.
34
Snyder SA (2010).
Occurrence of pharmaceuticals in U.S drinking water. ACS Symposium Series,
1048:69–80.
Snyder SA et al. (2003).
Pharmaceuticals, personal care products and endocrine disruptors in water:
implications for the water industry. Environmental Engineering Science, 20:449–
469.
Snyder SA et al. (2006).
Role of membranes and activated carbon in the removal of endocrine disruptors
and pharmaceuticals. Desalination, 202:156–181.
Snyder SA et al. (2007).
Removal of EDCs and pharmaceuticals in drinking water and reuse treatment
processes. Denver, CO, Awwa Research Foundation.
Snyder SA et al. (2008).
Toxicological relevance of EDCs and pharmaceuticals in drinking water. Denver,
CO, Awwa Research Foundation.
Spellman FR (2010).
Spellman’s standard handbook for wastewater operators. Volume III. Advanced
level, 2nd ed. CRC Press.
Stackelberg PE et al. (2004).
Persistence of pharmaceutical compounds and other organic wastewater
contaminants in a conventional drinking-water treatment plant. Science of the Total
Environment, 329:99–113.
Stackelberg PE et al. (2007).
Efficiency of conventional drinking-water-treatment processes in removal of
pharmaceuticals and other organic compounds. Science of the Total Environment,
377(2–3):255–272.
Tabak HH, Bunch RL (1970).
Steroid hormones as water pollutants. I. Metabolism of natural and synthetic
ovulation-inhibiting hormones by microorganisms of activated sludge and primary
settled sewage. Developments in Industrial Microbiology, 11:367–376.
Teleosis Institute (2009).
Medicine take back locations. Berkeley, CA, Teleosis Institute (http://www.teleosis.
org/gpp-locations.php).
Ternes T (1998).
Occurrence of drugs in German sewage treatment plants and rivers. Water
Research, 32:3245–3260.
Ternes TA, Joss A, eds (2006).
Human pharmaceuticals, hormones and fragrances: the challenge of micropollutants
in urban water management. London, IWA Publishing.
Ternes TA et al. (1999).
Behavior and occurrence of estrogens in municipal sewage treatment plants—1.
Investigations in Germany, Canada and Brazil. Science of the Total Environment,
225:81–90.
Ternes TA et al. (2002).
Removal of pharmaceuticals during drinking water treatment. Environmental Science
& Technology, 36:3855–3863.
Ternes TA et al. (2003).
Ozonation: a tool for removal of pharmaceuticals, contrast media and musk
fragrances from wastewater? Water Research, 37:1976–1982.
Ternes TA et al. (2005).
Assessment of technologies for the removal of pharmaceuticals and personal care
products in sewage and drinking water to improve the indirect potable water reuse.
POSEIDON project detailed report (EU Contract No. EVK1-CT-2000-00047).
Thomas KV, Hilton MJ (2004).
The occurrence of selected human pharmaceutical compounds in UK estuaries.
Marine Pollution Bulletin, 49:436–444 [cited in DWI, 2007].
Togola A, Budzinski H (2008).
Multi-residue analysis of pharmaceutical compounds in aqueous samples. Journal of
Chromatography A, 1177:150 –158.
USEPA (2008a).
Health services industry study: management and disposal of unused pharmaceuticals
(interim technical report). Washington, DC, United States Environmental Protection
Agency (EPA-821-R-08-013).
USEPA (2008b).
Approaches to screening for risk from pharmaceuticals in drinking-water and
prioritization for further evaluation. Washington, DC, United States Environmental
Protection Agency (Contract No. C-07-021, WA-B-02, Task 6 under the supervision
of Dr O. Conerly).
USEPA (2010a).
Report released on pharmaceutical mail-back pilot program funded by EPA: Agency
provided $150,000 grant to University of Maine’s Center on Aging to undertake
study. Washington, DC, United States Environmental Protection Agency (h t t p : //
yosemite.epa.gov/opa/admpress.nsf/d0cf6618525a9efb85257359003fb69d
/97c00306844616bb852577270052ab58!OpenDocument).
USEPA (2010b).
Guidance document: Best management practices for unused pharmaceuticals at
health care facilities. Draft. Washington, DC, United States Environmental Protection
Agency (EPA-821-R-10-006; http://water.epa.gov/scitech/wastetech/guide/upload/
unuseddraft.pdf).

35
van der Aa NGFM et al. (2009).
Drugs of abuse and tranquilizers in Dutch surface waters, drinking water and
wastewater. Results of screening monitoring 2009. Bilthoven, National Institute for
Public Health and the Environment (RIVM Report No. 703719064/2010).
Vanderford BJ, Snyder SA (2006).
Analysis of pharmaceuticals in water by isotope dilution liquid chromatography/
tandem mass spectrometry. Environmental Science & Technology, 40:7312–7320.
Van De Steene JC, Stove CP, Lambert WE (2010).
A field study on 8 pharmaceuticals and 1 pesticide in Belgium: removal rates in
waste water treatment plants and occurrence in surface water. Science of the Total
Environment, 408:3448–3453.
Versteegh JFM, van der Aa NGFM, Dijkman E (2007).
Pharmaceuticals in drinking water and drinking water sources. Results of the
monitoring program 2005/2006. Bilthoven, National Institute for Public Health and
the Environment, pp. 1–53 (RIVM Report No. 703719016/2007).
Vieno NM, Tuhkanen T, Kronberg L (2005).
Seasonal variation in the occurrence of pharmaceuticals in effluents from a sewage
treatment plant and in the recipient water. Environmental Science & Technology,
39:8220–8226.
Vieno N, Tuhkanen T, Kronberg L (2006).
Removal of pharmaceuticals in drinking water treatment: effect of chemical
coagulation. Environmental Technology, 27:183–192.
Vieno N, Tuhkanen T, Kronberg L (2007).
Elimination of pharmaceuticals in sewage treatment plants in Finland. Water
Research, 41:1001–1012.
Watkinson AJ, Murby EJ, Costanzo SD (2007).
Removal of antibiotics in conventional and advanced wastewater treatment:
implications for environmental discharge and wastewater recycling. Water Research,
41(18):416 4–4176.
Watts CD et al. (1983).
Identification of non-volatile organics in water using field desorption mass
spectrometry and high performance liquid chromatography. In: Angeletti G, Bjørseth
A, eds. Analysis of organic micropollutants in water. Dordrecht, D. Reidel Publishing
Co., pp. 120–131 [cited in DWI, 2007].
Webb S et al. (2003).
Indirect human exposure to pharmaceuticals via drinking water. Toxicology Letters,
14 2 :15 7 –16 7.
Westerhoff P et al. (2005).
Fate of endocrine disruptor, pharmaceutical, and personal care product chemicals
during simulated drinking water treatment processes. Environmental Science &
Technology, 39(17):6649–6663.
WHO (2011).
Guidelines for drinking-water quality, 4th ed. Geneva, World Health Organization.
Wick A et al. (2009).
Fate of beta blockers and psycho-active drugs in conventional wastewater treatment.
Water Research, 43(4):1060–1074.
Xu W-H et al. (2007).
Determination of selected antibiotics in the Victoria Harbour and the Pearl River,
South China using high-performance liquid chromatography–electrospray ionization
tandem mass spectrometry. Environmental Pollution, 145(3):672–679.
Yoon Y et al. (2003).
HPLC-fluorescence detection and adsorption of bisphenol A, 17β-estradiol,
and 17α-ethynyl estradiol on powdered activated carbon. Water Research,
37(14):3530–3537.
Yoon Y et al. (2006).
Nanofiltration and ultrafiltration of endocrine disrupting compounds, pharmaceuticals
and personal care products. Journal of Membrane Science, 270:88–100.
Zhang DQ et al. (2011).
Removal of pharmaceutical compounds in tropical constructed wetlands. Ecological
Engineering, 37(3):460–464.
Zhang YJ, Geissen SU, Gal C (2008).
Carbamazepine and diclofenac: removal in wastewater treatment plants and
occurrence in water bodies. Chemosphere, 73 ( 8 ) :1151–1161.
Ziylan A, Ince NH (2011).
The occurrence and fate of anti-inflammatory and analgesic pharmaceuticals
in sewage and fresh water: treatability by conventional and non-conventional
processes. Journal of Hazardous Materials, 187(1–3):24–36.
Zuccato E et al. (2000).
Presence of therapeutic drugs in the environment. Lancet, 355(9217):1789–1790.
Zühlke S et al. (2004).
Detection and identification of phenazone-type drugs and their microbial
metabolites in ground and drinking water applying solid-phase extraction and gas
chromatography with mass spectrometric detection. Journal of Chromatography A,
1050:201–209.
Zwiener C, Frimmel FH (2000).
Oxidative treatment of pharmaceuticals in water. Water Research, 34 ( 6 ):1881–
1885.

Reports of trace concentrations of pharmaceuticals
in the water cycle have raised concerns among
various stakeholders, such as drinking-water
regulators, governments, water suppliers and the
public, over potential human health risks from
exposure to very low levels of pharmaceuticals in
drinking-water.
This technical report aims to provide practical
guidance and recommendations in managing
concerns over pharmaceuticals in drinking-water,
taking into consideration the available scientific
knowledge and evidence. It emphasizes the
importance to prioritize this emerging issue in the
overall context of water safety management, which
includes microbial and other chemical risks that may
threaten the safety of drinking-water.
ISBN 978 92 4 150208 5