Biosafety Laboratory Manual Biosafety7
User Manual: Laboratory Biosafety Manual Troubleshoot Laboratory Biosafety |
Open the PDF directly: View PDF ![]() .
.
Page Count: 186 [warning: Documents this large are best viewed by clicking the View PDF Link!]

World Health Organization
Geneva
2004
Laboratory biosafety manual
Third edition
WHO Library Cataloguing-in-Publication Data
World Health Organization.
Laboratory biosafety manual. – 3rd ed.
1.Containment of biohazards - methods 2.Laboratories - standards 3.Laboratory
infection - prevention and control 4.Manuals I.Title.
ISBN 92 4 154650 6 (LC/NLM classification: QY 25) WHO/CDS/CSR/LYO/2004.11
© World Health Organization 2004
All rights reserved. Publications of the World Health Organization can be obtained from Marketing and
Dissemination, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22
791 2476; fax: +41 22 791 4857; email: bookorders@who.int). Requests for permission to reproduce or
translate WHO publications – whether for sale or for noncommercial distribution – should be addressed
to Publications, at the above address (fax: +41 22 791 4806; email: permissions@who.int).
The designations employed and the presentation of the material in this publication do not imply the
expression of any opinion whatsoever on the part of the World Health Organization concerning the legal
status of any country, territory, city or area or of its authorities, or concerning the delimitation of its
frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not
yet be full agreement.
The mention of specific companies or of certain manufacturers’ products does not imply that they are
endorsed or recommended by the World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished
by initial capital letters.
The World Health Organization does not warrant that the information contained in this publication is
complete and correct and shall not be liable for any damages incurred as a result of its use.
Designed by minimum graphics
Printed in Malta
This publication was supported by Grant/Cooperative Agreement Number U50/CCU012445-08
from the Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA. Its contents are
solely the responsibility of the authors and do not necessarily represent the official views of the
CDC.

Contents
• iii •
Foreword vii
Acknowledgements viii
1. General principles 1
Introduction 1
PART I. Biosafety guidelines 5
2. Microbiological risk assessment 7
Specimens for which there is limited information 8
Risk assessment and genetically modified microorganisms 8
3. Basic laboratories – Biosafety Levels 1 and 2 9
Code of practice 9
Laboratory design and facilities 12
Laboratory equipment 14
Health and medical surveillance 16
Training 16
Waste handling 17
Chemical, fire, electrical, radiation and equipment safety 19
4. The containment laboratory – Biosafety Level 3 20
Code of practice 20
Laboratory design and facilities 21
Laboratory equipment 22
Health and medical surveillance 22
5. The maximum containment laboratory – Biosafety Level 4 25
Code of practice 25
Laboratory design and facilities 25
6. Laboratory animal facilities 28
Animal facility – Biosafety Level 1 29
Animal facility – Biosafety Level 2 29
Animal facility – Biosafety Level 3 30
Animal facility – Biosafety Level 4 31
Invertebrates 32
7. Guidelines for laboratory/facility commissioning 33
8. Guidelines for laboratory/facility certification 36

PART II. Laboratory biosecurity 45
9. Laboratory biosecurity concepts 47
PART III. Laboratory equipment 49
10. Biological safety cabinets 51
Class I biological safety cabinet 51
Class II biological safety cabinets 53
Class III biological safety cabinet 56
Biological safety cabinet air connections 56
Selection of a biological safety cabinet 57
Using biological safety cabinets in the laboratory 57
11. Safety equipment 61
Negative-pressure flexible-film isolators 61
Pipetting aids 63
Homogenizers, shakers, blenders and sonicators 63
Disposable transfer loops 64
Microincinerators 64
Personal protective equipment and clothing 64
PART IV. Good microbiological techniques 67
12. Laboratory techniques 69
Safe handling of specimens in the laboratory 69
Use of pipettes and pipetting aids 70
Avoiding the dispersal of infectious materials 70
Use of biological safety cabinets 70
Avoiding ingestion of infectious materials and contact with skin and eyes 71
Avoiding injection of infectious materials 71
Separation of serum 72
Use of centrifuges 72
Use of homogenizers, shakers, blenders and sonicators 73
Use of tissue grinders 73
Care and use of refrigerators and freezers 73
Opening of ampoules containing lyophilized infectious materials 74
Storage of ampoules containing infectious materials 74
Standard precautions with blood and other body fluids, tissues and excreta 74
Precautions with materials that may contain prions 76
13. Contingency plans and emergency procedures 78
Contingency plan 78
Emergency procedures for microbiological laboratories 79
14. Disinfection and sterilization 82
Definitions 82
Cleaning laboratory materials 83
• iv •
LABORATORY BIOSAFETY MANUAL

Chemical germicides 83
Local environmental decontamination 88
Decontamination of biological safety cabinets 89
Hand-washing/hand decontamination 90
Heat disinfection and sterilization 90
Incineration 92
Disposal 93
15. Introduction to the transport of infectious substances 94
International transport regulations 94
The basic triple packaging system 95
Spill clean-up procedure 95
PART V. Introduction to biotechnology 99
16. Biosafety and recombinant DNA technology 101
Biosafety considerations for biological expression systems 102
Biosafety considerations for expression vectors 102
Viral vectors for gene transfer 102
Transgenic and “knock-out” animals 102
Transgenic plants 103
Risk assessments for genetically modified organisms 103
Further considerations 104
PART VI. Chemical, fire and electrical safety 105
17. Hazardous chemicals 107
Routes of exposure 107
Storage of chemicals 107
General rules regarding chemical incompatibilities 107
Toxic effects of chemicals 107
Explosive chemicals 108
Chemical spills 108
Compressed and liquefied gases 109
18. Additional laboratory hazards 110
Fire hazards 110
Electrical hazards 111
Noise 111
Ionizing radiation 111
PART VII. Safety organization and training 115
19. The biosafety officer and biosafety committee 117
Biosafety officer 117
Biosafety committee 118
• v •
CONTENTS

20. Safety for support staff 119
Engineering and building maintenance services 119
Cleaning (domestic) services 119
21. Training programmes 120
PART VIII. Safety checklist 123
22. Safety checklist 125
Laboratory premises 125
Storage facilities 125
Sanitation and staff facilities 126
Heating and ventilation 126
Lighting 126
Services 126
Laboratory biosecurity 127
Fire prevention and fire protection 127
Flammable liquid storage 128
Compressed and liquefied gases 128
Electrical hazards 128
Personal protection 129
Health and safety of staff 129
Laboratory equipment 130
Infectious materials 130
Chemicals and radioactive substances 130
PART IX. References, annexes and index 133
References 135
Annex 1 First aid 138
Annex 2 Immunization of staff 139
Annex 3 WHO Biosafety Collaborating Centres 140
Annex 4 Equipment safety 141
Equipment that may create a hazard 141
Annex 5 Chemicals: hazards and precautions 145
Index 170
• vi •
LABORATORY BIOSAFETY MANUAL
Foreword
• vii •
The World Health Organization (WHO) has long recognized that safety and, in
particular, biological safety are important international issues. WHO published the
first edition of the Laboratory biosafety manual in 1983. The manual encouraged
countries to accept and implement basic concepts in biological safety and to develop
national codes of practice for the safe handling of pathogenic microorganisms in
laboratories within their geographical borders. Since 1983, many countries have used
the expert guidance provided in the manual to develop such codes of practice. A second
edition of the manual was published in 1993.
WHO continues to provide international leadership in biosafety through this third
edition of the manual by addressing biological safety and security issues facing us in
the current millennium. The third edition stresses throughout the importance of
personal responsibility. New chapters have been added on risk assessment, safe use of
recombinant DNA technology and transport of infectious materials. Recent world
events have revealed new threats to public health through deliberate misuse and release
of microbiological agents and toxins. The third edition therefore also introduces
biosecurity concepts – the protection of microbiological assets from theft, loss or
diversion, which could lead to the inappropriate use of these agents to cause public
health harm. This edition also includes safety information from the 1997 WHO
publication Safety in health-care laboratories (1).
The third edition of the WHO Laboratory biosafety manual is a helpful reference
and guide to nations that accept the challenge to develop and establish national codes
of practice for securing microbiological assets, yet ensuring their availability for clinical,
research and epidemiological purposes.
Dr A. Asamoa-Baah
Assistant Director-General
Communicable Diseases
World Health Organization
Geneva, Switzerland
Acknowledgements
• viii •
The development of this third edition of the Laboratory biosafety manual has been
made possible through the contributions of the following, whose expertise is gratefully
acknowledged:
Dr W. Emmett Barkley, Howard Hughes Medical Institute, Chevy Chase, MD, USA
Dr Murray L. Cohen, Centers for Disease Control and Prevention, Atlanta, GA, USA
(retired)
Dr Ingegerd Kallings, Swedish Institute of Infectious Disease Control, Stockholm,
Sweden
Ms Mary Ellen Kennedy, Consultant in Biosafety, Ashton, Ontario, Canada
Ms Margery Kennett, Victorian Infectious Diseases Reference Laboratory, North Mel-
bourne, Australia (retired)
Dr Richard Knudsen, Office of Health and Safety, Centers for Disease Control and
Prevention, Atlanta, GA, USA
Dr Nicoletta Previsani, Biosafety programme, World Health Organization, Geneva,
Switzerland
Dr Jonathan Richmond, Office of Health and Safety, Centers for Disease Control and
Prevention, Atlanta, GA, USA (retired)
Dr Syed A. Sattar, Faculty of Medicine, University of Ottawa, Ottawa, Ontario, Canada
Dr Deborah E. Wilson, Division of Occupational Health and Safety, Office of Research
Services, National Institutes of Health, Department of Health and Human Serv-
ices, Washington, DC, USA
Dr Riccardo Wittek, Institute of Animal Biology, University of Lausanne, Lausanne,
Switzerland
The assistance of the following is also gratefully acknowledged:
Ms Maureen Best, Office of Laboratory Security, Health Canada, Ottawa, Canada
Dr Mike Catton, Victorian Infectious Diseases Reference Laboratory, North Melbourne,
Australia
Dr Shanna Nesby, Office of Health and Safety, Centers for Disease Control and Pre-
vention, Atlanta, GA, USA
Dr Stefan Wagener, Canadian Science Centre for Human and Animal Health, Winni-
peg, Canada
The writers and reviewers also wish to acknowledge the original contributions of the
many professionals whose work was embodied in the first and second editions of the
Laboratory biosafety manual and in the 1997 WHO publication Safety in health-care
laboratories (1).

• 1 •
1. General principles
Introduction
Throughout this manual, references are made to the relative hazards of infective
microorganisms by risk group (WHO Risk Groups 1, 2, 3 and 4). This risk group
classification is to be used for laboratory work only. Table 1 describes the risk groups.
Table 1. Classification of infective microorganisms by risk group
Risk Group 1
(no or low individual and community risk)
A microorganism that is unlikely to cause human or animal disease.
Risk Group 2
(moderate individual risk, low community risk)
A pathogen that can cause human or animal disease but is unlikely to be a serious hazard to
laboratory workers, the community, livestock or the environment. Laboratory exposures may
cause serious infection, but effective treatment and preventive measures are available and the
risk of spread of infection is limited.
Risk Group 3
(high individual risk, low community risk)
A pathogen that usually causes serious human or animal disease but does not ordinarily spread
from one infected individual to another. Effective treatment and preventive measures are available.
Risk Group 4
(high individual and community risk)
A pathogen that usually causes serious human or animal disease and that can be readily
transmitted from one individual to another, directly or indirectly. Effective treatment and preventive
measures are not usually available.
Laboratory facilities are designated as basic – Biosafety Level 1, basic – Biosafety Level 2,
containment – Biosafety Level 3, and maximum containment – Biosafety Level 4.
Biosafety level designations are based on a composite of the design features,
construction, containment facilities, equipment, practices and operational procedures
required for working with agents from the various risk groups. Table 2 relates but
does not “equate” risk groups to the biosafety level of laboratories designed to work
with organisms in each risk group.
Countries (regions) should draw up a national (regional) classification of
microorganisms, by risk group, taking into account:

• 2 •
LABORATORY BIOSAFETY MANUAL
1. Pathogenicity of the organism.
2. Mode of transmission and host range of the organism. These may be influenced
by existing levels of immunity in the local population, density and movement of
the host population, presence of appropriate vectors, and standards of environ-
mental hygiene.
3. Local availability of effective preventive measures. These may include: prophylaxis
by immunization or administration of antisera (passive immunization); sanitary
measures, e.g. food and water hygiene; control of animal reservoirs or arthropod
vectors.
4. Local availability of effective treatment. This includes passive immunization,
postexposure vaccination and use of antimicrobials, antivirals and chemo-
therapeutic agents, and should take into consideration the possibility of the
emergence of drug-resistant strains.
The assignment of an agent to a biosafety level for laboratory work must be based on
a risk assessment. Such an assessment will take the risk group as well as other factors
into consideration in establishing the appropriate biosafety level. For example, an agent
that is assigned to Risk Group 2 may generally require Biosafety Level 2 facilities,
equipment, practices and procedures for safe conduct of work. However, if particular
experiments require the generation of high-concentration aerosols, then Biosafety
Table 2. Relation of risk groups to biosafety levels, practices and equipment
RISK BIOSAFETY LABORATORY LABORATORY SAFETY
GROUP LEVEL TYPE PRACTICES EQUIPMENT
1Basic – Basic teaching, GMT None; open bench
Biosafety research work
Level 1
2Basic – Primary health GMT plus protective Open bench plus BSC
Biosafety services; diagnostic clothing, biohazard for potential aerosols
Level 2 services, research sign
3Containment – Special diagnostic As Level 2 plus BSC and/or other
Biosafety services, research special clothing, primary devices for all
Level 3 controlled access, activities
directional airflow
4Maximum Dangerous pathogen As Level 3 plus Class III BSC, or
containment – units airlock entry, shower positive pressure suits
Biosafety exit, special waste in conjunction with
Level 4 disposal Class II BSCs, double-
ended autoclave
(through the wall),
filtered air
BSC, biological safety cabinet; GMT, good microbiological techniques (see Part IV of this manual)

• 3 •
Level 3 may be more appropriate to provide the necessary degree of safety, since it
ensures superior containment of aerosols in the laboratory workplace. The biosafety
level assigned for the specific work to be done is therefore driven by professional
judgement based on a risk assessment, rather than by automatic assignment of a
laboratory biosafety level according to the particular risk group designation of the
pathogenic agent to be used (see Chapter 2).
Table 3 summarizes the facility requirements at the four biosafety levels.
Table 3. Summary of biosafety level requirements
BIOSAFETY LEVEL
1234
Isolationa of laboratory No No Yes Yes
Room sealable for decontamination No No Yes Yes
Ventilation:
—inward airflow No Desirable Yes Yes
—controlled ventilating system No Desirable Yes Yes
—HEPA-filtered air exhaust No No Yes/NobYes
Double-door entry No No Yes Yes
Airlock No No No Yes
Airlock with shower No No No Yes
Anteroom No No Yes —
Anteroom with shower No No Yes/NocNo
Effluent treatment No No Yes/NocYes
Autoclave:
—on site No Desirable Yes Yes
—in laboratory room No No Desirable Yes
—double-ended No No Desirable Yes
Biological safety cabinets No Desirable Yes Yes
Personnel safety monitoring capabilitydNo No Desirable Yes
aEnvironmental and functional isolation from general traffic.
bDependent on location of exhaust (see Chapter 4).
cDependent on agent(s) used in the laboratory.
dFor example, window, closed-circuit television, two-way communication.
Thus, the assignment of a biosafety level takes into consideration the organism
(pathogenic agent) used, the facilities available, and the equipment practices and
procedures required to conduct work safely in the laboratory.
1. GENERAL PRINCIPLES


PART I
Biosafety guidelines
2. Microbiological
risk assessment
• 7 •
The backbone of the practice of biosafety is risk assessment. While there are many
tools available to assist in the assessment of risk for a given procedure or experiment,
the most important component is professional judgement. Risk assessments should
be performed by the individuals most familiar with the specific characteristics of the
organisms being considered for use, the equipment and procedures to be employed,
animal models that may be used, and the containment equipment and facilities
available. The laboratory director or principal investigator is responsible for ensuring
that adequate and timely risk assessments are performed, and for working closely with
the institution’s safety committee and biosafety personnel to ensure that appropriate
equipment and facilities are available to support the work being considered. Once
performed, risk assessments should be reviewed routinely and revised when necessary,
taking into consideration the acquisition of new data having a bearing on the degree
of risk and other relevant new information from the scientific literature.
One of the most helpful tools available for performing a microbiological risk assess-
ment is the listing of risk groups for microbiological agents (see Chapter 1). However,
simple reference to the risk grouping for a particular agent is insufficient in the conduct
of a risk assessment. Other factors that should be considered, as appropriate, include:
1. Pathogenicity of the agent and infectious dose
2. Potential outcome of exposure
3. Natural route of infection
4. Other routes of infection, resulting from laboratory manipulations (parenteral,
airborne, ingestion)
5. Stability of the agent in the environment
6. Concentration of the agent and volume of concentrated material to be manipulated
7. Presence of a suitable host (human or animal)
8. Information available from animal studies and reports of laboratory-acquired
infections or clinical reports
9. Laboratory activity planned (sonication, aerosolization, centrifugation, etc.)
10. Any genetic manipulation of the organism that may extend the host range of the
agent or alter the agent’s sensitivity to known, effective treatment regimens (see
Chapter 16)
11. Local availability of effective prophylaxis or therapeutic interventions.
• 8 •
LABORATORY BIOSAFETY MANUAL
On the basis of the information ascertained during the risk assessment, a biosafety
level can be assigned to the planned work, appropriate personal protective equipment
selected, and standard operating procedures (SOPs) incorporating other safety
interventions developed to ensure the safest possible conduct of the work.
Specimens for which there is limited information
The risk assessment procedure described above works well when there is adequate
information available. However, there are situations when the information is
insufficient to perform an appropriate risk assessment, for example, with clinical
specimens or epidemiological samples collected in the field. In these cases, it is prudent
to take a cautious approach to specimen manipulation.
1. Standard precautions (2) should always be followed, and barrier protections applied
(gloves, gowns, eye protection), whenever samples are obtained from patients.
2. Basic containment – Biosafety Level 2 practices and procedures should be the
minimum requirement for handling specimens.
3. Transport of specimens should follow national and/or international rules and
regulations.
Some information may be available to assist in determining the risk of handling these
specimens:
1. Medical data on the patient
2. Epidemiological data (morbidity and mortality data, suspected route of trans-
mission, other outbreak investigation data)
3. Information on the geographical origin of the specimen.
In the case of outbreaks of disease of unknown etiology, appropriate ad hoc guidelines
may be generated and posted by national competent authorities and/or WHO on the
Wor ld Wide Web (as was the case during the 2003 emergence of the severe acute
respiratory syndrome (SARS)) to indicate how specimens should be consigned for
shipment and the biosafety level at which they should be analysed.
Risk assessment and genetically modified microorganisms
A detailed discussion of risk assessment and genetically modified organisms (GMOs)
is provided in Chapter 16.
• 9 •
3. Basic laboratories –
Biosafety Levels 1 and 2
For the purposes of this manual, the guidance and recommendations given as minimum
requirements pertaining to laboratories of all biosafety levels are directed at
microorganisms in Risk Groups 1–4. Although some of the precautions may appear
to be unnecessary for some organisms in Risk Group 1, they are desirable for training
purposes to promote good (i.e. safe) microbiological techniques (GMT).
Diagnostic and health-care laboratories (public health, clinical or hospital-based)
must all be designed for Biosafety Level 2 or above. As no laboratory has complete
control over the specimens it receives, laboratory workers may be exposed to organisms
in higher risk groups than anticipated. This possibility must be recognized in the
development of safety plans and policies. In some countries, accreditation of clinical
laboratories is required. Globally, standard precautions (2) should always be adopted
and practised.
The guidelines for basic laboratories – Biosafety Levels 1 and 2 presented here are
comprehensive and detailed, as they are fundamental to laboratories of all biosafety
levels. The guidelines for containment laboratories – Biosafety Level 3 and maximum
containment laboratories – Biosafety Level 4 that follow (Chapters 4 and 5) are
modifications of and additions to these guidelines, designed for work with the more
dangerous (hazardous) pathogens.
Code of practice
This code is a listing of the most essential laboratory practices and procedures that are
basic to GMT. In many laboratories and national laboratory programmes, this code
may be used to develop written practices and procedures for safe laboratory operations.
Each laboratory should adopt a safety or operations manual that identifies known
and potential hazards, and specifies practices and procedures to eliminate or minimize
such hazards. GMT are fundamental to laboratory safety. Specialized laboratory
equipment is a supplement to but can never replace appropriate procedures. The most
important concepts are listed below.
Access
1. The international biohazard warning symbol and sign (Figure 1) must be displayed
on the doors of the rooms where microorganisms of Risk Group 2 or higher risk
groups are handled.

• 10 •
LABORATORY BIOSAFETY MANUAL
2. Only authorized persons should be allowed to enter the laboratory working areas.
3. Laboratory doors should be kept closed.
4. Children should not be authorized or allowed to enter laboratory working areas.
5. Access to animal houses should be specially authorized.
6. No animals should be admitted other than those involved in the work of the
laboratory.
Personal protection
1. Laboratory coveralls, gowns or uniforms must be worn at all times for work in the
laboratory.
2. Appropriate gloves must be worn for all procedures that may involve direct or
accidental contact with blood, body fluids and other potentially infectious materials
or infected animals. After use, gloves should be removed aseptically and hands
must then be washed.
3. Personnel must wash their hands after handling infectious materials and animals,
and before they leave the laboratory working areas.
Figure 1. Biohazard warning sign for laboratory doors
BIOHAZARD
ADMITTANCE TO AUTHORIZED PERSONNEL ONLY
Biosafety Level: _________________________________
Responsible Investigator: _________________________
In case of emergency call: ________________________
Daytime phone: __________Home phone: ___________
Authorization for entrance must be obtained from
the Responsible Investigator named above.
WHO 04.64
• 11 •
4. Safety glasses, face shields (visors) or other protective devices must be worn when
it is necessary to protect the eyes and face from splashes, impacting objects and
sources of artificial ultraviolet radiation.
5. It is prohibited to wear protective laboratory clothing outside the laboratory, e.g.
in canteens, coffee rooms, offices, libraries, staff rooms and toilets.
6. Open-toed footwear must not be worn in laboratories.
7. Eating, drinking, smoking, applying cosmetics and handling contact lenses is
prohibited in the laboratory working areas.
8. Storing human foods or drinks anywhere in the laboratory working areas is
prohibited.
9. Protective laboratory clothing that has been used in the laboratory must not be
stored in the same lockers or cupboards as street clothing.
Procedures
1. Pipetting by mouth must be strictly forbidden.
2. Materials must not be placed in the mouth. Labels must not be licked.
3. All technical procedures should be performed in a way that minimizes the formation
of aerosols and droplets.
4. The use of hypodermic needles and syringes should be limited. They must not be
used as substitutes for pipetting devices or for any purpose other than parenteral
injection or aspiration of fluids from laboratory animals.
5. All spills, accidents and overt or potential exposures to infectious materials must
be reported to the laboratory supervisor. A written record of such accidents and
incidents should be maintained.
6. A written procedure for the clean-up of all spills must be developed and followed.
7. Contaminated liquids must be decontaminated (chemically or physically) before
discharge to the sanitary sewer. An effluent treatment system may be required,
depending on the risk assessment for the agent(s) being handled.
8. Written documents that are expected to be removed from the laboratory need to
be protected from contamination while in the laboratory.
Laboratory working areas
1. The laboratory should be kept neat, clean and free of materials that are not pertinent
to the work.
2. Work surfaces must be decontaminated after any spill of potentially dangerous
material and at the end of the working day.
3. All contaminated materials, specimens and cultures must be decontaminated before
disposal or cleaning for reuse.
4. Packing and transportation must follow applicable national and/or international
regulations.
5. When windows can be opened, they should be fitted with arthropod-proof screens.
3. BASIC LABORATORIES – BIOSAFETY LEVELS 1 AND 2
• 12 •
LABORATORY BIOSAFETY MANUAL
Biosafety management
1. It is the responsibility of the laboratory director (the person who has immediate
responsibility for the laboratory) to ensure the development and adoption of a
biosafety management plan and a safety or operations manual.
2. The laboratory supervisor (reporting to the laboratory director) should ensure
that regular training in laboratory safety is provided.
3. Personnel should be advised of special hazards, and required to read the safety or
operations manual and follow standard practices and procedures. The laboratory
supervisor should make sure that all personnel understand these. A copy of the
safety or operations manual should be available in the laboratory.
4. There should be an arthropod and rodent control programme.
5. Appropriate medical evaluation, surveillance and treatment should be provided
for all personnel in case of need, and adequate medical records should be
maintained.
Laboratory design and facilities
In designing a laboratory and assigning certain types of work to it, special attention
should be paid to conditions that are known to pose safety problems. These include:
1. Formation of aerosols
2. Work with large volumes and/or high concentrations of microorganisms
3. Overcrowding and too much equipment
4. Infestation with rodents and arthropods
5. Unauthorized entrance
6. Workflow: use of specific samples and reagents.
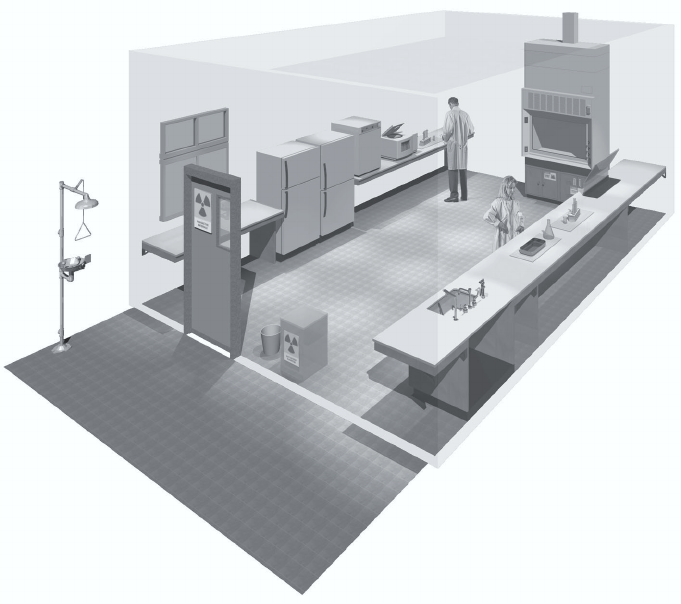
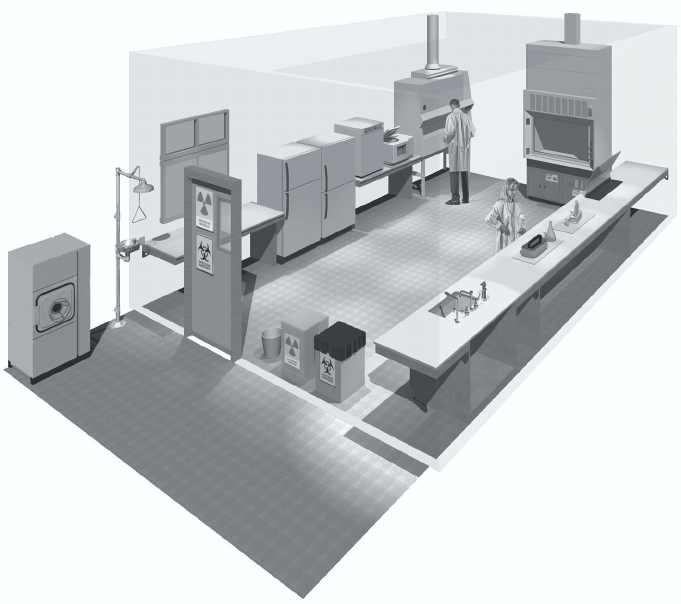
Examples of laboratory designs for Biosafety Levels 1 and 2 are shown in Figures 2
and 3, respectively.
Design features
1. Ample space must be provided for the safe conduct of laboratory work and for
cleaning and maintenance.
2. Walls, ceilings and floors should be smooth, easy to clean, impermeable to liquids
and resistant to the chemicals and disinfectants normally used in the laboratory.
Floors should be slip-resistant.
3. Bench tops should be impervious to water and resistant to disinfectants, acids,
alkalis, organic solvents and moderate heat.
4. Illumination should be adequate for all activities. Undesirable reflections and glare
should be avoided.
5. Laboratory furniture should be sturdy. Open spaces between and under benches,
cabinets and equipment should be accessible for cleaning.
6. Storage space must be adequate to hold supplies for immediate use and thus prevent
clutter on bench tops and in aisles. Additional long-term storage space, conveniently
located outside the laboratory working areas, should also be provided.
• 13 •
7. Space and facilities should be provided for the safe handling and storage of solvents,
radioactive materials, and compressed and liquefied gases.
8. Facilities for storing outer garments and personal items should be provided outside
the laboratory working areas.
9. Facilities for eating and drinking and for rest should be provided outside the
laboratory working areas.
10. Hand-washing basins, with running water if possible, should be provided in each
laboratory room, preferably near the exit door.
11. Doors should have vision panels, appropriate fire ratings, and preferably be self-
closing.
12. At Biosafety Level 2, an autoclave or other means of decontamination should be
available in appropriate proximity to the laboratory.
13. Safety systems should cover fire, electrical emergencies, emergency shower and
eyewash facilities.
14. First-aid areas or rooms suitably equipped and readily accessible should be available
(see Annex 1).
3. BASIC LABORATORIES – BIOSAFETY LEVELS 1 AND 2
Figure 2. A typical Biosafety Level 1 laboratory
(graphics kindly provided by CUH2A, Princeton, NJ, USA)
• 14 •
LABORATORY BIOSAFETY MANUAL
15. In the planning of new facilities, consideration should be given to the provision of
mechanical ventilation systems that provide an inward flow of air without
recirculation. If there is no mechanical ventilation, windows should be able to be
opened and should be fitted with arthropod-proof screens.
16. A dependable supply of good quality water is essential. There should be no cross-
connections between sources of laboratory and drinking-water supplies. An anti-
backflow device should be fitted to protect the public water system.
17. There should be a reliable and adequate electricity supply and emergency lighting
to permit safe exit. A stand-by generator is desirable for the support of essential
equipment, such as incubators, biological safety cabinets, freezers, etc., and for the
ventilation of animal cages.
18. There should be a reliable and adequate supply of gas. Good maintenance of the
installation is mandatory.
19. Laboratories and animal houses are occasionally the targets of vandals. Physical
and fire security must be considered. Strong doors, screened windows and restricted
issue of keys are compulsory. Other measures should be considered and applied,
as appropriate, to augment security (see Chapter 9).
Laboratory equipment
Together with good procedures and practices, the use of safety equipment will help to
reduce risks when dealing with biosafety hazards. This section deals with basic
principles related to equipment suitable for laboratories of all biosafety levels.
Requirements for laboratory equipment pertinent to higher biosafety levels are dealt
with in the relevant chapters.
The laboratory director should, after consultation with the biosafety officer and
safety committee (if designated), ensure that adequate equipment is provided and
that it is used properly. Equipment should be selected to take account of certain general
principles, i.e. it should be:
1. Designed to prevent or limit contact between the operator and the infectious
material
2. Constructed of materials that are impermeable to liquids, resistant to corrosion
and meet structural requirements
3. Fabricated to be free of burrs, sharp edges and unguarded moving parts
4. Designed, constructed and installed to facilitate simple operation and provide for
ease of maintenance, cleaning, decontamination and certification testing; glassware
and other breakable materials should be avoided, whenever possible.
Detailed performance and construction specifications may need to be consulted to
ensure that the equipment possesses the necessary safety features (see also Chapters
10 and 11).
• 15 •
3. BASIC LABORATORIES – BIOSAFETY LEVELS 1 AND 2
Figure 3. A typical Biosafety Level 2 laboratory
(graphics kindly provided by CUH2A, Princeton, NJ, USA). Procedures likely to generate
aerosols are performed within a biological safety cabinet. Doors are kept closed and
are posted with appropriate hazard signs. Potentially contaminated wastes are separated
from the general waste stream.
Essential biosafety equipment
1. Pipetting aids – to avoid mouth pipetting. Many different designs are available.
2. Biological safety cabinets, to be used whenever:
—infectious materials are handled; such materials may be centrifuged in the open
laboratory if sealed centrifuge safety cups are used and if they are loaded and
unloaded in a biological safety cabinet
—there is an increased risk of airborne infection
—procedures with a high potential for producing aerosols are used; these may
include centrifugation, grinding, blending, vigorous shaking or mixing, sonic
disruption, opening of containers of infectious materials whose internal pressure
may be different from the ambient pressure, intranasal inoculation of animals,
and harvesting of infectious tissues from animals and eggs.
3. Plastic disposable transfer loops. Alternatively, electric transfer loop incinerators
may be used inside the biological safety cabinet to reduce aerosol production.
• 16 •
LABORATORY BIOSAFETY MANUAL
4. Screw-capped tubes and bottles.
5. Autoclaves or other appropriate means to decontaminate infectious materials.
6. Plastic disposable Pasteur pipettes, whenever available, to avoid glass.
7. Equipment such as autoclaves and biological safety cabinets must be validated with
appropriate methods before being taken into use. Recertification should take place
at regular intervals, according to the manufacturer’s instructions (see Chapter 7).
Health and medical surveillance
The employing authority, through the laboratory director, is responsible for ensuring
that there is adequate surveillance of the health of laboratory personnel. The objective
of such surveillance is to monitor for occupationally acquired diseases. Appropriate
activities to achieve these objectives are:
1. Provision of active or passive immunization where indicated (see Annex 2)
2. Facilitation of the early detection of laboratory-acquired infections
3. Exclusion of highly susceptible individuals (e.g. pregnant women or immuno-
compromised individuals) from highly hazardous laboratory work
4. Provision of effective personal protective equipment and procedures.
Guidelines for the surveillance of laboratory workers handling microorganisms
at Biosafety Level 1
Historical evidence indicates that the microorganisms handled at this level are unlikely
to cause human disease or animal disease of veterinary importance. Ideally, however,
all laboratory workers should undergo a pre-employment health check at which their
medical history is recorded. Prompt reporting of illnesses or laboratory accidents is
desirable and all staff members should be made aware of the importance of maintaining
GMT.
Guidelines for the surveillance of laboratory workers handling microorganisms
at Biosafety Level 2
1. A pre-employment or preplacement health check is necessary. The person’s medical
history should be recorded and a targeted occupational health assessment
performed.
2. Records of illness and absence should be kept by the laboratory management.
3. Women of childbearing age should be made aware of the risk to an unborn child
of occupational exposure to certain microorganisms, e.g. rubella virus. The precise
steps taken to protect the fetus will vary, depending on the microorganisms to
which the women may be exposed.
Training
Human error and poor technique can compromise the best of safeguards to protect
the laboratory worker. Thus, a safety-conscious staff, well informed about the
recognition and control of laboratory hazards, is key to the prevention of laboratory-
• 17 •
acquired infections, incidents and accidents. For this reason, continuous in-service
training in safety measures is essential. An effective safety programme begins with the
laboratory managers, who should ensure that safe laboratory practices and procedures
are integrated into the basic training of employees. Training in safety measures should
be an integral part of new employees’ introduction to the laboratory. Employees should
be introduced to the code of practice and to local guidelines, including the safety or
operations manual. Measures to assure that employees have read and understood the
guidelines, such as signature pages, should be adopted. Laboratory supervisors play
the key role in training their immediate staff in good laboratory techniques. The
biosafety officer can assist in training and with the development of training aids and
documentation (see also Chapter 21).
Staff training should always include information on safe methods for highly
hazardous procedures that are commonly encountered by all laboratory personnel
and which involve:
1. Inhalation risks (i.e. aerosol production) when using loops, streaking agar plates,
pipetting, making smears, opening cultures, taking blood/serum samples,
centrifuging, etc.
2. Ingestion risks when handling specimens, smears and cultures
3. Risks of percutaneous exposures when using syringes and needles
4. Bites and scratches when handling animals
5. Handling of blood and other potentially hazardous pathological materials
6. Decontamination and disposal of infectious material.
Waste handling
Waste is anything that is to be discarded.
In laboratories, decontamination of wastes and their ultimate disposal are closely
interrelated. In terms of daily use, few if any contaminated materials will require actual
removal from the laboratory or destruction. Most glassware, instruments and
laboratory clothing will be reused or recycled. The overriding principle is that all
infectious materials should be decontaminated, autoclaved or incinerated within the
laboratory.
The principal questions to be asked before discharge of any objects or materials
from laboratories that deal with potentially infectious microorganisms or animal tissues
are:
1. Have the objects or materials been effectively decontaminated or disinfected by an
approved procedure?
2. If not, have they been packaged in an approved manner for immediate on-site
incineration or transfer to another facility with incineration capacity?
3. Does the disposal of the decontaminated objects or materials involve any additional
potential hazards, biological or otherwise, to those who carry out the immediate
disposal procedures or who might come into contact with discarded items outside
the facility?
3. BASIC LABORATORIES – BIOSAFETY LEVELS 1 AND 2
• 18 •
LABORATORY BIOSAFETY MANUAL
Decontamination
Steam autoclaving is the preferred method for all decontamination processes. Materials
for decontamination and disposal should be placed in containers, e.g. autoclavable
plastic bags, that are colour-coded according to whether the contents are to be
autoclaved and/or incinerated. Alternative methods may be envisaged only if they
remove and/or kill microorganisms (for more details see Chapter 14).
Handling and disposal procedures for contaminated materials and wastes
An identification and separation system for infectious materials and their containers
should be adopted. National and international regulations must be followed. Categories
should include:
1. Non-contaminated (non-infectious) waste that can be reused or recycled or
disposed of as general, “household” waste
2. Contaminated (infectious) “sharps” – hypodermic needles, scalpels, knives and
broken glass; these should always be collected in puncture-proof containers fitted
with covers and treated as infectious
3. Contaminated material for decontamination by autoclaving and thereafter washing
and reuse or recycling
4. Contaminated material for autoclaving and disposal
5. Contaminated material for direct incineration.
Sharps
After use, hypodermic needles should not be recapped, clipped or removed from
disposable syringes. The complete assembly should be placed in a sharps disposal
container. Disposable syringes, used alone or with needles, should be placed in sharps
disposal containers and incinerated, with prior autoclaving if required.
Sharps disposal containers must be puncture-proof/-resistant and must not be filled
to capacity. When they are three-quarters full they should be placed in “infectious
waste” containers and incinerated, with prior autoclaving if laboratory practice requires
it. Sharps disposal containers must not be discarded in landfills.
Contaminated (potentially infectious) materials for autoclaving and reuse
No precleaning should be attempted of any contaminated (potentially infectious)
materials to be autoclaved and reused. Any necessary cleaning or repair must be done
only after autoclaving or disinfection.
Contaminated (potentially infectious) materials for disposal
Apart from sharps, which are dealt with above, all contaminated (potentially infectious)
materials should be autoclaved in leakproof containers, e.g. autoclavable, colour-coded
plastic bags, before disposal. After autoclaving, the material may be placed in transfer
containers for transport to the incinerator. If possible, materials deriving from health-
care activities should not be discarded in landfills even after decontamination. If an
• 19 •
incinerator is available on the laboratory site, autoclaving may be omitted: the
contaminated waste should be placed in designated containers (e.g. colour-coded bags)
and transported directly to the incinerator. Reusable transfer containers should be
leakproof and have tight-fitting covers. They should be disinfected and cleaned before
they are returned to the laboratory for further use.
Discard containers, pans or jars, preferably unbreakable (e.g. plastic), should be
placed at every work station. When disinfectants are used, waste materials should
remain in intimate contact with the disinfectant (i.e. not protected by air bubbles) for
the appropriate time, according to the disinfectant used (see Chapter 14). The discard
containers should be decontaminated and washed before reuse.
Incineration of contaminated waste must meet with the approval of the public health
and air pollution authorities, as well as that of the laboratory biosafety officer (see
section on Incineration in Chapter 14).
Chemical, fire, electrical, radiation and equipment safety
A breakdown in the containment of pathogenic organisms may be the indirect result
of chemical, fire, electrical or radiation accidents. It is therefore essential to maintain
high standards of safety in these fields in any microbiological laboratory. Statutory
rules and regulations for each of these will normally be laid down by the competent
national or local authority, whose assistance should be sought if necessary. Chemical,
fire, electrical and radiation hazards are considered in greater detail in Part VI of this
manual (Chapters 17 and 18).
Additional information regarding safety equipment is presented in Chapter 11.
3. BASIC LABORATORIES – BIOSAFETY LEVELS 1 AND 2
4. The containment laboratory –
Biosafety Level 3
The containment laboratory – Biosafety Level 3 is designed and provided for work
with Risk Group 3 microorganisms and with large volumes or high concentrations of
Risk Group 2 microorganisms that pose an increased risk of aerosol spread. Biosafety
Level 3 containment requires the strengthening of the operational and safety pro-
grammes over and above those for basic laboratories – Biosafety Levels 1 and 2 (set
out in Chapter 3).
The guidelines given in this chapter are presented in the form of additions to those
for basic laboratories – Biosafety Levels 1 and 2, which must therefore be applied before
those specific for the containment laboratory – Biosafety Level 3. The major additions
and changes are in:
1. Code of practice
2. Laboratory design and facilities
3. Health and medical surveillance.
Laboratories in this category should be registered or listed with the national or other
appropriate health authorities.
Code of practice
The code of practice for basic laboratories – Biosafety Levels 1 and 2 applies except
where modified as follows.
1. The international biohazard warning symbol and sign (see Figure 1) displayed on
laboratory access doors must identify the biosafety level and the name of the
laboratory supervisor who controls access, and indicate any special conditions for
entry into the area, e.g. immunization.
2. Laboratory protective clothing must be of the type with solid-front or wrap-around
gowns, scrub suits, coveralls, head covering and, where appropriate, shoe covers or
dedicated shoes. Front-buttoned standard laboratory coats are unsuitable, as are
sleeves that do not fully cover the forearms. Laboratory protective clothing must
not be worn outside the laboratory, and it must be decontaminated before it is
laundered. The removal of street clothing and change into dedicated laboratory
clothing may be warranted when working with certain agents (e.g. agricultural or
zoonotic agents).
• 20 •
3. Open manipulations of all potentially infectious material must be conducted within
a biological safety cabinet or other primary containment device (see also Chapter 10).
4. Respiratory protective equipment may be necessary for some laboratory procedures
or working with animals infected with certain pathogens (see Chapter 11).
Laboratory design and facilities
The laboratory design and facilities for basic laboratories – Biosafety Levels 1 and 2
apply except where modified as follows:
1. The laboratory must be separated from the areas that are open to unrestricted
traffic flow within the building. Additional separation may be achieved by placing
the laboratory at the blind end of a corridor, or constructing a partition and door
or access through an anteroom (e.g. a double-door entry or basic laboratory –
Biosafety Level 2), describing a specific area designed to maintain the pressure
differential between the laboratory and its adjacent space. The anteroom should
have facilities for separating clean and dirty clothing and a shower may also be
necessary.
2. Anteroom doors may be self-closing and interlocking so that only one door is
open at a time. A break-through panel may be provided for emergency exit use.
3. Surfaces of walls, floors and ceilings should be water-resistant and easy to clean.
Openings through these surfaces (e.g. for service pipes) should be sealed to facilitate
decontamination of the room(s).
4. The laboratory room must be sealable for decontamination. Air-ducting systems
must be constructed to permit gaseous decontamination.
5. Windows must be closed, sealed and break-resistant.
6. A hand-washing station with hands-free controls should be provided near each
exit door.
7. There must be a controlled ventilation system that maintains a directional airflow
into the laboratory room. A visual monitoring device with or without alarm(s)
should be installed so that staff can at all times ensure that proper directional
airflow into the laboratory room is maintained.
8. The building ventilation system must be so constructed that air from the contain-
ment laboratory – Biosafety Level 3 is not recirculated to other areas within the
building. Air may be high-efficiency particulate air (HEPA) filtered, reconditioned
and recirculated within that laboratory. When exhaust air from the laboratory (other
than from biological safety cabinets) is discharged to the outside of the building, it
must be dispersed away from occupied buildings and air intakes. Depending on
the agents in use, this air may be discharged through HEPA filters. A heating,
ventilation and air-conditioning (HVAC) control system may be installed to prevent
sustained positive pressurization of the laboratory. Consideration should be given
to the installation of audible or clearly visible alarms to notify personnel of HVAC
system failure.
4. THE CONTAINMENT LABORATORY – BIOSAFETY LEVEL 3
• 21 •
• 22 •
LABORATORY BIOSAFETY MANUAL
9. All HEPA filters must be installed in a manner that permits gaseous
decontamination and testing.
10. Biological safety cabinets should be sited away from walking areas and out of cross-
currents from doors and ventilation systems (see Chapter 10).
11. The exhaust air from Class I or Class II biological safety cabinets (see Chapter 10),
which will have been passed through HEPA filters, must be discharged in such a
way as to avoid interference with the air balance of the cabinet or the building
exhaust system.
12. An autoclave for the decontamination of contaminated waste material should be
available in the containment laboratory. If infectious waste has to be removed from
the containment laboratory for decontamination and disposal, it must be
transported in sealed, unbreakable and leakproof containers according to national
or international regulations, as appropriate.
13. Backflow-precaution devices must be fitted to the water supply. Vacuum lines should
be protected with liquid disinfectant traps and HEPA filters, or their equivalent.
Alternative vacuum pumps should also be properly protected with traps and filters.
14. The containment laboratory – Biosafety Level 3 facility design and operational
procedures should be documented.
An example of laboratory design for Biosafety Level 3 is shown in Figure 4.
Laboratory equipment
The principles for the selection of laboratory equipment, including biological safety
cabinets (see Chapter 10) are the same as for the basic laboratory – Biosafety Level 2.
However, at Biosafety Level 3, manipulation of all potentially infectious material must
be conducted within a biological safety cabinet or other primary containment device.
Consideration should be given to equipment such as centrifuges, which will need
additional containment accessories, for example, safety buckets or containment rotors.
Some centrifuges and other equipment, such as cell-sorting instruments for use with
infected cells, may need additional local exhaust ventilation with HEPA filtration for
efficient containment.
Health and medical surveillance
The objectives of health and medical surveillance programmes for basic laboratories –
Biosafety Levels 1 and 2 also apply to containment laboratories – Biosafety Level 3,
except where modified as follows:
1. Medical examination of all laboratory personnel who work in containment
laboratories – Biosafety Level 3 is mandatory. This should include recording of a
detailed medical history and an occupationally-targeted physical examination.
2. After a satisfactory clinical assessment, the examinee may be provided with a medical
contact card (e.g. as shown in Figure 5) stating that he or she is employed in a
facility with a containment laboratory – Biosafety Level 3. This card should include
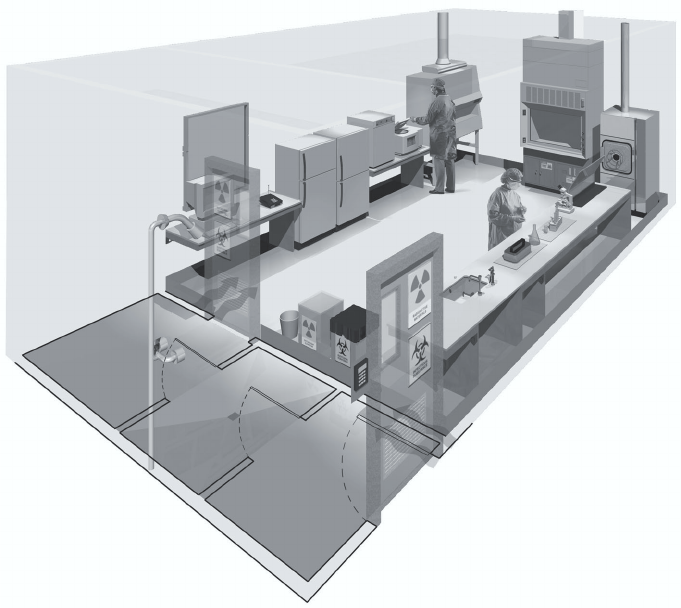
• 23 •
4. THE CONTAINMENT LABORATORY – BIOSAFETY LEVEL 3
Figure 4. A typical Biosafety Level 3 laboratory
(graphics kindly provided by CUH2A, Princeton, NJ, USA). The laboratory is separated
from general traffic flow and accessed through an anteroom (double door entry or
basic laboratory – Biosafety Level 2) or an airlock. An autoclave is available within the
facility for decontamination of wastes prior to disposal. A sink with hands-free operation
is available. Inward directional airflow is established and all work with infectious
materials is conducted within a biological safety cabinet.
a picture of the card holder, be wallet-sized, and always be carried by the holder.
The name(s) of the contact persons to be entered will need to be agreed locally but
might include the laboratory director, medical adviser and/or biosafety officer.
• 24 •
LABORATORY BIOSAFETY MANUAL
A. Front of card
ILLNESS SURVEILLANCE NOTICE
Name
TO THE EMPLOYEE
Keep this card in your possession. In case of unexplained febrile illness,
present the card to your physician and notify one of the following in the order
listed.
Dr Tel (Work):
Tel (Home):
Dr Tel (Work):
Tel (Home):
B. Back of card
TO THE PHYSICIAN
The holder of this card works in an area at
in which pathogenic viruses, rickettsia, bacteria, protozoa or helminths are
present. In the event of an unexplained febrile illness, please call the employer
for information on agents to which this employee may have been exposed.
Name of laboratory:
Address:
Tel:
Figure 5. Suggested format for medical contact card
Card holder’s
picture

• 25 •
5. The maximum containment
laboratory – Biosafety Level 4
The maximum containment laboratory – Biosafety Level 4 is designed for work with
Risk Group 4 microorganisms. Before such a laboratory is constructed and put into
operation, intensive consultations should be held with institutions that have had
experience of operating a similar facility. Operational maximum containment
laboratories – Biosafety Level 4 should be under the control of national or other
appropriate health authorities. The following information is intended only as
introductory material. Entities working to pursue development of a Biosafety Level 4
laboratory should contact the WHO Biosafety programme for additional information.1
Code of practice
The code of practice for Biosafety Level 3 applies except where modified as follows:
1. The two-person rule should apply, whereby no individual ever works alone. This is
particularly important if working in a Biosafety Level 4 suit facility.
2. A complete change of clothing and shoes is required prior to entering and upon
exiting the laboratory.
3. Personnel must be trained in emergency extraction procedures in the event of
personnel injury or illness.
4. A method of communication for routine and emergency contacts must be
established between personnel working within the maximum containment
laboratory – Biosafety Level 4 and support personnel outside the laboratory.
Laboratory design and facilities
The features of a containment laboratory – Biosafety Level 3 also apply to a maximum
containment laboratory – Biosafety Level 4 with the addition of the following.
1. Primary containment. An efficient primary containment system must be in place,
consisting of one or a combination of the following.
—Class III cabinet laboratory. Passage through a minimum of two doors prior to
entering the rooms containing the Class III biological safety cabinet(s) (cabinet
room) is required. In this laboratory configuration the Class III biological safety
1Biosafety programme, Department of Communicable Disease Surveillance and Response, World Health
Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (http://www.who.int/csr/).
• 26 •
LABORATORY BIOSAFETY MANUAL
cabinet provides the primary containment. A personnel shower with inner and
outer changing rooms is necessary. Supplies and materials that are not brought
into the cabinet room through the changing area are introduced through a
double-door autoclave or fumigation chamber. Once the outer door is securely
closed, staff inside the laboratory can open the inner door to retrieve the
materials. The doors of the autoclave or fumigation chamber are interlocked
in such a way that the outer door cannot open unless the autoclave has been
operated through a sterilization cycle or the fumigation chamber has been
decontaminated (see Chapter 10).
—Suit laboratory. A protective suit laboratory with self-contained breathing
apparatus differs significantly in design and facility requirements from a
Biosafety Level 4 laboratory with Class III biological safety cabinets. The rooms
in the protective suit laboratory are arranged so as to direct personnel through
the changing and decontamination areas prior to entering areas where infectious
materials are manipulated. A suit decontamination shower must be provided
and used by personnel leaving the containment laboratory area. A separate
personnel shower with inner and outer changing rooms is also provided.
Personnel who enter the suit area are required to don a one-piece, positively
pressurized, HEPA-filtered, supplied-air suit. Air to the suit must be provided
by a system that has a 100% redundant capability with an independent source
of air, for use in the event of an emergency. Entry into the suit laboratory is
through an airlock fitted with airtight doors. An appropriate warning system
for personnel working in the suit laboratory must be provided for use in the
event of mechanical system or air failure (see Chapter 10).
2. Controlled access. The maximum containment laboratory – Biosafety Level 4 must
be located in a separate building or in a clearly delineated zone within a secure
building. Entry and exit of personnel and supplies must be through an airlock or
pass-through system. On entering, personnel must put on a complete change of
clothing; before leaving, they should shower before putting on their street clothing.
3. Controlled air system. Negative pressure must be maintained in the facility. Both
supply and exhaust air must be HEPA-filtered. There are significant differences in
the ventilating systems of the Class III cabinet laboratory and suit laboratory:
—Class III cabinet laboratory. The supply air to the Class III biological safety
cabinet(s) may be drawn from within the room through a HEPA filter mounted
on the cabinet or supplied directly through the supply air system. Exhaust air
from the Class III biological safety cabinet must pass through two HEPA filters
prior to release outdoors. The cabinet must be operated at negative pressure to
the surrounding laboratory at all times. A dedicated non-recirculating
ventilating system for the cabinet laboratory is required.
—Suit laboratory. Dedicated room air supply and exhaust systems are required.
The supply and exhaust components of the ventilating system are balanced to
provide directional airflow within the suit area from the area of least hazard to
• 27 •
the area(s) of greatest potential hazard. Redundant exhaust fans are required
to ensure that the facility remains under negative pressure at all times. The
differential pressures within the suit laboratory and between the suit laboratory
and adjacent areas must be monitored. Airflow in the supply and exhaust
components of the ventilating system must be monitored, and an appropriate
system of controls must be used to prevent pressurization of the suit laboratory.
HEPA-filtered supply air must be provided to the suit area, decontamination
shower and decontamination airlocks or chambers. Exhaust air from the suit
laboratory must be passed through a series of two HEPA filters prior to release
outdoors. Alternatively, after double HEPA filtration, exhaust air may be
recirculated, but only within the suit laboratory. Under no circumstances shall
the exhaust air from the Biosafety Level 4 suit laboratory be recirculated to
other areas. Extreme caution must be exercised if recirculation of air within
the suit laboratory is elected. Consideration must be given to the types of
research conducted, equipment, chemicals and other materials used in the suit
laboratory, as well as animal species that may be involved in the research.
All HEPA filters need to be tested and certified annually. The HEPA filter housings
are designed to allow for in situ decontamination of the filter prior to removal.
Alternatively, the filter can be removed in a sealed, gas-tight primary container for
subsequent decontamination and/or destruction by incineration.
4. Decontamination of effluents. All effluents from the suit area, decontamination
chamber, decontamination shower, or Class III biological safety cabinet must be
decontaminated before final discharge. Heat treatment is the preferred method.
Effluents may also require correction to a neutral pH prior to discharge. Water
from the personnel shower and toilet may be discharged directly to the sanitary
sewer without treatment.
5. Sterilization of waste and materials. A double-door, pass-through autoclave must
be available in the laboratory area. Other methods of decontamination must be
available for equipment and items that cannot withstand steam sterilization.
6. Airlock entry ports for specimens, materials and animals must be provided.
7. Emergency power and dedicated power supply line(s) must be provided.
8. Containment drain(s) must be installed.
Because of the great complexity of the engineering, design and construction of Biosafety
Level 4 facilities, in either cabinet or suit configuration, schematic representations of
such facilities have not been included.
Because of the great complexity of the work in the Biosafety Level 4 laboratory, a
separate detailed work manual should be developed and tested in training exercises.
In addition, an emergency programme must be devised (see Chapter 13). In the
preparation of this programme, active cooperation with national and local health
authorities should be established. Other emergency services, e.g. fire, police and
designated receiving hospitals, should also be involved.
5. THE MAXIMUM CONTAINMENT LABORATORY – BIOSAFETY LEVEL 4

6. Laboratory animal facilities
Those who use animals for experimental and diagnostic purposes have a moral
obligation to take every care to avoid causing them unnecessary pain or suffering. The
animals must be provided with comfortable, hygienic housing and adequate wholesome
food and water. At the end of the experiment they must be dealt with in a humane
manner.
For security reasons, the animal house should be an independent, detached unit. If
it adjoins a laboratory, the design should provide for its isolation from the public
parts of the laboratory should such need arise, and for its decontamination and
disinfestation.
Table 4. Animal facility containment levels: summary of practices and safety
equipment
RISK GROUP CONTAINMENT LEVEL LABORATORY PRACTICES AND SAFETY EQUIPMENT
1ABSL-1 Limited access, protective clothing and gloves.
2ABSL-2 ABSL-1 practices plus: hazard warning signs. Class I
or II BSCs for activities that produce aerosols.
Decontamination of waste and cages before washing.
3ABSL-3 ABSL-2 practices plus: controlled access. BSCs and
special protective clothing for all activities.
4ABSL-4 ABSL-3 plus: strictly limited access. Clothing change
before entering. Class III BSCs or positive pressure
suits. Shower on exit. Decontamination of all wastes
before removal from facility.
ABSL, animal facility Biosafety Level; BSCs, biological safety cabinets
Animal facilities, like laboratories, may be designated according to a risk assessment
and the risk group of the microorganisms under investigation, as Animal facility
Biosafety Level 1, 2, 3 or 4.
With respect to agents to be used in the animal laboratory, factors for consideration
include:
1. The normal route of transmission
• 28 •
2. The volumes and concentrations to be used
3. The route of inoculation
4. Whether and by what route these agents may be excreted.
With respect to animals to be used in the animal laboratory, factors for consideration
include:
1. The nature of the animals, i.e. their aggressiveness and tendency to bite and scratch
2. Their natural ecto- and endoparasites
3. The zoonotic diseases to which they are susceptible
4. The possible dissemination of allergens.
As with laboratories, the requirements for design features, equipment and precautions
increase in stringency according to the animal biosafety level. These are described
below and summarized in Table 4. These guidelines are additive, so that each higher
level incorporates the standards of the lower levels.
Animal facility – Biosafety Level 1
This is suitable for the maintenance of most stock animals after quarantine (except
nonhuman primates, regarding which national authorities should be consulted), and
for animals that are deliberately inoculated with agents in Risk Group 1. GMT are
required. The animal facility director must establish policies, procedures and protocols
for all operations, and for access to the vivarium. An appropriate medical surveillance
programme for the staff must be instituted. A safety or operations manual must be
prepared and adopted.
Animal facility – Biosafety Level 2
This is suitable for work with animals that are deliberately inoculated with micro-
organisms in Risk Group 2. The following safety precautions apply:
1. All the requirements for animal facilities – Biosafety Level 1 must be met.
2. Biohazard warning signs (see Figure 1) should be posted on doors and other
appropriate places.
3. The facility must be designed for easy cleaning and housekeeping.
4. Doors must open inwards and be self-closing.
5. Heating, ventilation and lighting must be adequate.
6. If mechanical ventilation is provided, the airflow must be inwards. Exhaust air is
discharged to the outside and should not be recirculated to any part of the building.
7. Access must be restricted to authorized persons.
8. No animals should be admitted other than those for experimental use.
9. There should be an arthropod and rodent control programme.
10. Windows, if present, must be secure, resistant to breakage and, if able to be opened,
must be fitted with arthropod-proof screens.
11. After use, work surfaces must be decontaminated with effective disinfectants (see
Chapter 14).
6. LABORATORY ANIMAL FACILITIES
• 29 •
• 30 •
LABORATORY BIOSAFETY MANUAL
12. Biological safety cabinets (Classes I or II) or isolator cages with dedicated air supplies
and HEPA-filtered exhaust air must be provided for work that may involve the
generation of aerosols.
13. An autoclave must be available on site or in appropriate proximity to the animal
facility.
14. Animal bedding materials must be removed in a manner that minimizes the
generation of aerosols and dust.
15. All waste materials and bedding must be decontaminated before disposal.
16. Use of sharp instruments should be restricted whenever possible. Sharps should
always be collected in puncture-proof/-resistant containers fitted with covers and
treated as infectious.
17. Material for autoclaving or incineration must be transported safely, in closed
containers.
18. Animal cages must be decontaminated after use.
19. Animal carcasses should be incinerated.
20. Protective clothing and equipment must be worn in the facility, and removed on
leaving.
21. Hand-washing facilities must be provided. Staff must wash their hands before
leaving the animal facility.
22. All injuries, however minor, must be treated appropriately, reported and recorded.
23. Eating, drinking, smoking and application of cosmetics must be forbidden in the
facility.
24. All personnel must receive appropriate training.
Animal facility – Biosafety Level 3
This is suitable for work with animals that are deliberately inoculated with agents in
Risk Group 3, or when otherwise indicated by a risk assessment. All systems, practices
and procedures need to be reviewed and recertified annually. The following safety
precautions apply:
1. All the requirements for animal facilities – Biosafety Levels 1 and 2 must be met.
2. Access must be strictly controlled.
3. The facility must be separated from other laboratory and animal house areas by a
room with a double-door entrance forming an anteroom.
4. Hand-washing facilities must be provided in the anteroom.
5. Showers should be provided in the anteroom.
6. There must be mechanical ventilation to ensure a continuous airflow through all
the rooms. Exhaust air must pass through HEPA filters before being discharged to
the atmosphere without recirculation. The system must be designed to prevent
accidental reverse flow and positive pressurization in any part of the animal house.
7. An autoclave must be available at a location convenient for the animal house where
the biohazard is contained. Infectious waste should be autoclaved before it is moved
to other areas of the facility.
• 31 •
8. An incinerator should be readily available on site or alternative arrangements should
be made with the authorities concerned.
9. Animals infected with Risk Group 3 microorganisms must be housed in cages in
isolators or rooms with ventilation exhausts placed behind the cages.
10. Bedding should be as dust-free as possible.
11. All protective clothing must be decontaminated before it is laundered.
12. Windows must be closed and sealed, and resistant to breakage.
13. Immunization of staff, as appropriate, should be offered.
Animal facility – Biosafety Level 4
Work in this facility will normally be linked with that in the maximum containment
laboratory – Biosafety Level 4, and national and local rules and regulations must be
harmonized to apply to both. If work is to be done in a suit laboratory, additional
practices and procedures must be used over and above those described here (see
Chapter 5).
1. All the requirements for animal facilities – Biosafety Levels 1, 2 and 3 must be met.
2. Access must be strictly controlled; only staff designated by the director of the
establishment should have authority to enter.
3. Individuals must not work alone: the two-person rule must apply.
4. Personnel must have received the highest possible level of training as microbiologists
and be familiar with the hazards involved in their work and with the necessary
precautions.
5. Housing areas for animals infected with Risk Group 4 agents must maintain the
criteria for containment described and applied for maximum containment
laboratories – Biosafety Level 4.
6. The facility must be entered by an airlock anteroom, the clean side of which must
be separated from the restricted side by changing and showering facilities.
7. Staff must remove street clothing when entering and put on special, protective
clothing. After work they must remove the protective clothing for autoclaving, and
shower before leaving.
8. The facility must be ventilated by a HEPA-filtered exhaust system designed to ensure
a negative pressure (inward directional airflow).
9. The ventilation system must be designed to prevent reverse flow and positive-
pressurization.
10. A double-ended autoclave with the clean end in a room outside the containment
rooms must be provided for exchange of materials.
11. A pass-through airlock with the clean end in a room outside the containment rooms
must be provided for exchange of non-autoclavable materials.
12. All manipulations with animals infected with Risk Group 4 agents must take place
under maximum containment – Biosafety Level 4 conditions.
13. All animals must be housed in isolators.
14. All animal bedding and waste must be autoclaved before removal from the facility.
6. LABORATORY ANIMAL FACILITIES
• 32 •
LABORATORY BIOSAFETY MANUAL
15. There must be medical supervision of staff.
Invertebrates
As with vertebrates, the animal facility biosafety level will be determined by the risk
groups of the agents under investigation or when otherwise indicated by a risk
assessment. The following additional precautions are necessary with certain arthropods,
particularly with flying insects:
1. Separate rooms should be provided for infected and noninfected invertebrates.
2. The rooms should be capable of being sealed for fumigation.
3. Insecticide sprays should be readily available.
4. “Chilling” facilities should be provided to reduce, where necessary, the activity of
invertebrates.
5. Access should be through an anteroom containing insect traps and with arthropod-
proof screens on the doors.
6. All exhaust ventilation ducts and openable windows should be fitted with
arthropod-proof screens.
7. Waste traps on sinks and sluices should not be allowed to dry out.
8. All waste should be decontaminated by autoclaving, as some invertebrates are not
killed by all disinfectants.
9. A check should be kept on the numbers of larval and adult forms of flying, crawling
and jumping arthropods.
10. Containers for ticks and mites should stand in trays of oil.
11. Infected or potentially infected flying insects must be contained in double-netted
cages.
12. Infected or potentially infected arthropods must be handled in biological safety
cabinets or isolators.
13. Infected or potentially infected arthropods may be manipulated on cooling trays.
For further information see references (3–6).
• 33 •
7. Guidelines for
laboratory/facility
commissioning
Laboratory/facility commissioning may be defined as the systematic review and
documentation process signifying that specified laboratory structural components,
systems and/or system components have been installed, inspected, functionally tested
and verified to meet national or international standards, as appropriate. The respective
building system’s design criteria and design function establish these requirements. In
other words, laboratories designated as Biosafety Levels 1–4 will have different and
increasingly complex commissioning requirements. Geographical and climatic
conditions, such as geological fault lines or extreme heat, cold or humidity may also
affect the laboratory design and therefore the commissioning requirements. Upon the
completion of the commissioning process, the pertinent structural components and
support systems will have been subjected to the various operating conditions and failure
modes that can be reasonably expected, and will have been approved.
The commissioning process and acceptance criteria should be established early,
preferably during the programming phase of the construction or renovation project.
By acknowledging the commissioning process early in the project, architects, engineers,
safety and health personnel and ultimately the laboratory occupants understand the
performance requirements of the specific laboratory and set uniform expectations for
laboratory and/or facility performance. The commissioning process provides the
institution and the surrounding community with a greater degree of confidence that
the structural, electrical, mechanical and plumbing systems, containment and
decontamination systems, and security and alarm systems will operate as designed, to
assure containment of any potentially dangerous microorganisms being worked with
in a particular laboratory or animal facility.
Commissioning activities generally begin during the programming phase of the
project and proceed through the construction and subsequent warranty period for
the laboratory/facility. Warranty periods should generally extend for one year following
occupancy. It is recommended that a commissioning agent is retained who is
independent of the architectural, engineering and construction firms involved in the
design and construction. The commissioning agent serves as an advocate for the
institution constructing or renovating the laboratory and should be considered as a
member of the design team; involvement of the agent in the early programming phase
of the project is essential. In some cases, the institution may act as its own
commissioning agent. In the case of more complex laboratory facilities (Biosafety
• 34 •
LABORATORY BIOSAFETY MANUAL
Levels 3 or 4), the institution may wish to retain an outside commissioning agent who
has demonstrated experience and success in the commissioning of complex biosafety
laboratory and animal facilities. When an independent commissioning agent is used,
the institution should still be a member of the commissioning team. It is recommended
that, in addition to the commissioning agent, the institution’s Safety Officer, Project
Officer, Programme Manager and a representative of the Operations and Maintenance
staff are also part of the team.
The following is a list of laboratory systems and components that may be included
in a commissioning plan for functional testing, depending on the containment level
of the facility being renovated or constructed. The list is not exhaustive. Obviously,
the actual commissioning plan will reflect the complexity of the laboratory being
planned.
1. Building automation systems including links to remote monitoring and control
sites
2. Electronic surveillance and detection systems
3. Electronic security locks and proximity device readers
4. Heating, ventilation (supply and exhaust) and air-conditioning (HVAC) systems
5. High-efficiency particulate air (HEPA) filtration systems
6. HEPA decontamination systems
7. HVAC and exhaust air system controls and control interlocks
8. Airtight isolation dampers
9. Laboratory refrigeration systems
10. Boilers and steam systems
11. Fire detection, suppression and alarm systems
12. Domestic water backflow prevention devices
13. Processed water systems (i.e. reverse osmosis, distilled water)
14. Liquid effluent treatment and neutralization systems
15. Plumbing drain primer systems
16. Chemical decontaminant systems
17. Medical laboratory gas systems
18. Breathing air systems
19. Service and instrument air systems
20. Cascading pressure differential verification of laboratories and support areas
21. Local area network (LAN) and computer data systems
22. Normal power systems
23. Emergency power systems
24. Uninterruptible power systems
25. Emergency lighting systems
26. Lighting fixture penetration seals
27. Electrical and mechanical penetration seals
28. Telephone systems
• 35 •
29. Airlock door control interlocks
30. Airtight door seals
31. Window and vision-panel penetration seals
32. Barrier pass-through penetration
33. Structural integrity verification: concrete floors, walls and ceilings
34. Barrier coating verification: floors, walls and ceilings
35. Biosafety Level 4 containment envelope pressurization and isolation functions
36. Biological safety cabinets
37. Autoclaves
38. Liquid nitrogen system and alarms
39. Water detection systems (e.g. in case of flooding inside containment zone)
40. Decontamination shower and chemical additive systems
41. Cage-wash and neutralization systems
42. Waste management.
7. GUIDELINES FOR LABORATORY/FACILITY COMMISSIONING
8. Guidelines for
laboratory/facility
certification
Laboratories are complex and dynamic environments. Today’s biomedical research
and clinical laboratories must be able to adapt quickly to continuously increasing public
health needs and pressures. An example of this is the need for laboratories to adjust
priorities to meet the challenges of emerging or re-emerging infectious diseases. In
order to assure that adaptation and maintenance are undertaken promptly and in an
appropriate and safe manner, all biological research and clinical laboratories should
be regularly certified. Laboratory certification helps to ensure that:
1. Proper engineering controls are being used and are functioning adequately as
designed
2. Appropriate site and protocol specific administrative controls are in place
3. Personal protective equipment is appropriate for the tasks being performed
4. Decontamination of waste and materials has been adequately considered and proper
waste management procedures are in place
5. Proper procedures for general laboratory safety, including physical, electrical and
chemical safety are in place.
Laboratory certification differs from laboratory commissioning activities (Chapter 7)
in several important ways. Laboratory certification is the systematic examination of
all safety features and processes within the laboratory (engineering controls, personal
protective equipment and administrative controls). Biosafety practices and procedures
are also examined. Laboratory certification is an on-going quality and safety assurance
activity that should take place on a regular basis.
Adequately trained safety and health or biosafety professionals may conduct
laboratory certification activities. Institutions may employ personnel having the
appropriate skill-set required for conducting audits, surveys or inspections (these terms
are used interchangeably) associated with the certification process. However,
institutions may consider engaging or be required to engage a third party to provide
these services.
Biomedical research and clinical laboratory facilities may develop audit, survey or
inspection tools to help ensure consistency in the certification process. These tools
should be flexible enough to allow for the physical and procedural differences between
laboratories necessitated by the type of work being conducted, while at the same time
providing a consistent approach within the institution. Care must be taken to ensure
• 36 •

that these tools are used only by appropriately trained personnel, and that they are not
used as a substitute for a sound professional biosafety assessment. Examples of such
tools are provided in Tables 5–7.
Findings of the audit, survey or inspection should be discussed with laboratory
personnel and management. Within the laboratory, an individual should be identified
and made responsible for ensuring that corrective actions are taken for all deficiencies
identified during the audit process. Certification of the laboratory should not be
completed, and the laboratory should not be declared functional, until deficiencies
have been adequately addressed.
The complexity of Biosafety Level 4 laboratory operations goes beyond the scope
of this manual. For details and further information, please contact the WHO Biosafety
programme1 (see also Annex 3).
8. GUIDELINES FOR LABORATORY/FACILITY CERTIFICATION
1WHO Biosafety programme, Department of Communicable Disease Surveillance and Response, World
Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (http://www.who.int/csr/).
• 37 •

• 38 •
LABORATORY BIOSAFETY MANUAL
Table 5. Basic Laboratory – Biosafety Level 1: laboratory safety survey
Location Date
Person in charge of laboratory
CHECKED ITEM (ENTER DATE OF CHECK) YES NO N/A COMMENTS
Laboratory
Proper signage: ultraviolet light, laser,
radioactive material, etc. ........................
Appropriate biosafety guidelines available
and followed ...........................................
Laboratory equipment properly labelled
(biohazardous, radioactive, toxic, etc.) ..
Laboratory design
Designed for easy cleaning .........................
Room ultraviolet lights on interlock switch
All shelves secured .....................................
Bench-tops waterproof and resistant to
acids, alkali, organic solvents and heat .
Adequate illumination provided ..................
Adequate storage space available and
appropriately used .................................
Gas cylinders
All cylinders secured ...................................
Caps on reserve cylinders ...........................
Asphyxiating and hazardous gases only in
ventilated rooms ....................................
Excess or empty cylinders present .............
Chemicals
Flammables stored in flammable storage
cabinet ....................................................
Peroxide formers double-dated (received
and opened) ...........................................
Chemicals properly segregated...................
Hazardous chemicals stored above eye
level ........................................................
Chemicals stored on the floor .....................
Chemical containers left open .....................
All solutions properly labelled.....................
Mercury thermometers in use ....................
Refrigerators/freezers/cold rooms
Food for human consumption present .......
Flammables in explosion-proof/-safe units
Labelled externally if containing
carcinogens, radioactivity and/or
biohazards ..............................................
Cold-room has emergency release .............
Biosafety Level:
Attach the appropriate
Biosafety Level Survey
Form

• 39 •
CHECKED ITEM (ENTER DATE OF CHECK) YES NO N/A COMMENTS
Electrical equipment
Extension cords present .............................
Outlets earthed/grounded and with
proper polarity .......................................
Connections by sinks, under showers,
etc. .........................................................
Equipment with frayed or damaged
wiring .....................................................
Overloaded outlets or electrical strips ........
Power strips mounted off the floor .............
Proper fuses in conduits .............................
Electrical outlets near water sources meet
local codes .............................................
Earths/grounds present on electrical cords
Portable space heaters ................................
Personal protective equipment
Eyewash available in laboratory ..................
Safety shower available ..............................
Personal protective equipment available
(gloves, gowns, goggles, etc.) ...............
Occupants properly attired .........................
Laboratory coats, gowns, smocks, gloves
and other personal protective clothing
not worn outside the laboratory .............
Personal protective equipment available
for cryogenic storage .............................
Waste management
Evidence of improper waste disposal .........
Wastes segregated in proper containers ....
Chemical waste containers tagged, labelled,
dated and kept closed ............................
Chemical waste containers appropriately
handled and stored ................................
Sharps containers used and disposed of
properly ..................................................
No trash on floor .........................................
Waste disposal procedures posted in
laboratory ...............................................
Occupational health and safety programmes available
Hazard communication ...............................
Respiratory protection ................................
Hearing conservation ..................................
Formaldehyde monitoring ...........................
Ethylene oxide monitoring ..........................
Anaesthetic gas monitoring ........................
8. GUIDELINES FOR LABORATORY/FACILITY CERTIFICATION

• 40 •
LABORATORY BIOSAFETY MANUAL
CHECKED ITEM (ENTER DATE OF CHECK) YES NO N/A COMMENTS
General engineering controls
Laboratory airflow is negative to general
occupancy, corridor and office areas .....
Cup sinks or drains acting as vents ............
Sink available for hand-washing .................
Exposed machine parts (pulleys, gears) .....
Vacuum line has filters and traps on
laboratory benches ................................
Backflow hazards to water supply ..............
Distilled water systems in good condition ..
Active and effective arthropod and rodent
control programme ................................
General practices and procedures
Food for human consumption stored
outside the laboratory ............................
Microwave oven(s) clearly labelled “No
Food Preparation, Laboratory Use Only”
Eating, drinking, smoking and/or applying
of cosmetics occurring in the laboratory
Pressurized glass containers taped or
shielded (i.e. vacuum traps) ..................
Mouth pipetting prohibited .........................
Mechanical pipetting devices available
and used .................................................
Protective laboratory clothing stored
separately from street clothing ..............
General laboratory housekeeping
Glass containers stored on the floor ...........
Trip hazards evident ....................................
Clean absorbent pads on work surfaces .....
Broken glassware handled by mechanical
means (brush and dustpan, tongs, etc.)
Fire protection
Sprinkler heads free and unobstructed .......
Open penetrations in walls, ceiling, floor, etc.
Wiring or tubing through door openings ....
Minimum passage width of 1 m in laboratory
Storage observed on ductwork or light fixtures
Excess combustibles stored in laboratory ..
Heated constant temperature baths
Equipped with low water level and
overheat shutoff .....................................
Constructed of noncombustible materials ..
Safety surveyor’s signature: Date survey completed:

• 41 •
Table 6. Basic laboratory – Biosafety Level 2: laboratory safety survey.
This form is used in conjunction with the Biosafety Level 1 laboratory safety
survey form
Location Date
Person in charge of laboratory
CHECKED ITEM (ENTER DATE OF CHECK) YES NO N/A COMMENTS
Biological safety cabinet (BSC) Date:
Certification within last year .......................
BSC surface wiped down with appropriate .Location:
disinfectant at beginning and end of
each procedure ...................................... Brand:
Front grill and exhaust filter unobstructed..
Open flames used inside cabinet ................ Type:
Vacuum lines have in-line filters and
disinfectant traps in use ......................... Serial no.:
BSC compromised by room air or location
BSC used when there is potential for
creating aerosols ....................................
Laboratory
Access limited and restricted to authorized
personnel ...............................................
Entry limited to personnel advised of all
potential hazards ....................................
Biohazard sign posted on laboratory door
as appropriate ........................................
•Information on sign accurate and
current ..............................................
•Sign legible and not defaced .............
All doors closed ..........................................
Decontamination
Decontaminant specific to the organism(s)
in use......................................................
All spills and accidents involving infectious
materials reported to the laboratory
supervisor ..............................................
Appropriate decontaminant used during
spill clean-ups ........................................
Work surfaces decontaminated before and
after each procedure, daily and after
spills .......................................................
Handling of contaminated waste
Infectious waste containers properly used ....
Containers not overfilled .............................
Containers properly labelled and closed .....
Culture stocks and other regulated waste
properly decontaminated before disposal
8. GUIDELINES FOR LABORATORY/FACILITY CERTIFICATION

• 42 •
LABORATORY BIOSAFETY MANUAL
CHECKED ITEM (ENTER DATE OF CHECK) YES NO N/A COMMENTS
Materials decontaminated outside the
laboratory transported in closed,
durable, leakproof containers according
to local rules and regulations .................
Mixed waste biologically decontaminated
prior to disposal as chemical or
radiological waste ..................................
Personal protection
Laboratory personnel reminded of
appropriate immunizations/tests for
agents handled .......................................
Appropriate medical services contacted for
medical evaluations, surveillance and
treatment of occupational exposures .....
Gloves worn when handling infectious
material or contaminated equipment .....
Face protection provided when working
outside the BSC with infectious material
Hands washed after removing gloves, after
working with infectious agents, before
leaving the laboratory ............................
Antimicrobial agent available for immediate
first aid ...................................................
Practices
BSC used when potential for creating
infectious aerosols/splashes exists .......
Biosafety manual prepared and adopted ....
Personnel read, review and follow the
instructions on practices and procedures,
including safety or operations manual
(required for all personnel annually)......
Procedures performed so as to minimize
aerosols/splashes ..................................
Needle-locking syringes/single-use needle-
syringe units used with infectious agents
Centrifuge cups and rotors opened only in
a BSC......................................................
Infectious specimens transported outside
a BSC in approved containers following
approved transport regulations..............
Facility
Hand-washing sink available near
laboratory exit ........................................
Safety surveyor’s signature: Date survey completed:

• 43 •
Table 7. Containment laboratory – Biosafety Level 3: laboratory safety survey.
This form is used in conjunction with the Bioafety Level 1 and Biosafety
Level 2 laboratory safety survey forms
Location Date
Person in charge of laboratory
CHECKED ITEM (ENTER DATE OF CHECK) YES NO N/A COMMENTS
Facility
Laboratory separated from unrestricted
traffic flow in building ............................
Access to laboratory through an anteroom
with self-closing doors ..........................
All penetrations in laboratory sealed or
sealable for decontamination .................
Room exhaust air single-pass and
exhausted away from occupied areas ....
Controlled ventilation system to monitor
directional airflow available ....................
Personal protection
Closed-front gowns worn in laboratory ......
Protective laboratory clothing worn only in
laboratory areas .....................................
Hand-washing sink foot, elbow or
automatically controlled .........................
Hand protection
Double gloves worn when handling infectious
material, potentially contaminated
equipment and work surfaces ................
Respiratory protection
Respiratory protection worn by all personnel
in the laboratory when aerosols are not
safely contained in a BSC .......................
Practices
Mucous membrane protection provided
when working with infectious material
outside a BSC .........................................
Personnel advised of special hazards
associated with the agent(s) ..................
Personnel required to read and follow all
instructions on practices and procedures,
including safety or operations manual ...
Personnel receive annual updates/additional
training for procedural changes .............
All contaminated waste autoclaved prior to
disposal ..................................................
Safety surveyor’s signature: Date survey completed:
8. GUIDELINES FOR LABORATORY/FACILITY CERTIFICATION


PART II
Laboratory biosecurity
9. Laboratory biosecurity concepts
• 47 •
The Laboratory biosafety manual has in the past focused on traditional biosafety
guidance for laboratories. The manual emphasizes the use of good microbiological
work practices, appropriate containment equipment, proper facility design, operation
and maintenance, and administrative considerations to minimize the risk of worker
injury or illness. In following these recommendations, the risk to the environment
and surrounding community-at-large is also minimized. It has now become necessary
to expand this traditional approach to biosafety through the introduction of laboratory
biosecurity measures. Global events in the recent past have highlighted the need to
protect laboratories and the materials they contain from being intentionally
compromised in ways that may harm people, livestock, agriculture or the environment.
Before the laboratory biosecurity needs of a facility can be defined, however, it is
important to understand the distinction between “laboratory biosafety” and “laboratory
biosecurity”.
“Laboratory biosafety” is the term used to describe the containment principles,
technologies and practices that are implemented to prevent unintentional exposure
to pathogens and toxins, or their accidental release. “Laboratory biosecurity” refers to
institutional and personal security measures designed to prevent the loss, theft, misuse,
diversion or intentional release of pathogens and toxins.
Effective biosafety practices are the very foundation of laboratory biosecurity
activities. Through risk assessments, performed as an integral part of an institution’s
biosafety programme, information is gathered regarding the type of organisms
available, their physical location, the personnel who require access to them, and the
identification of those responsible for them. This information can be used to assess
whether an institution possesses biological materials that are attractive to those who
may wish to use them improperly. National standards should be developed that
recognize and address the ongoing responsibility of countries and institutions to protect
specimens, pathogens and toxins from misuse.
A specific laboratory biosecurity programme must be prepared and implemented
for each facility according to the requirements of the facility, the type of laboratory
work conducted, and the local conditions. Consequently, laboratory biosecurity
activities should be representative of the institution’s various needs and should include
input from scientific directors, principal investigators, biosafety officers, laboratory

• 48 •
LABORATORY BIOSAFETY MANUAL
scientific staff, maintenance staff, administrators, information technology staff, and
law enforcement agencies and security staff if appropriate.
Laboratory biosecurity measures should be based on a comprehensive programme
of accountability for pathogens and toxins that includes an updated inventory with
storage location, identification of personnel with access, description of use,
documentation of internal and external transfers within and between facilities, and
any inactivation and/or disposal of the materials. Likewise, an institutional laboratory
biosecurity protocol should be established for identifying, reporting, investigating and
remediating breaches in laboratory biosecurity, including discrepancies in inventory
results. The involvement and roles and responsibilities of public health and security
authorities in the event of a security infraction must be clearly defined.
Laboratory biosecurity training, distinct from laboratory biosafety training, should
be provided to all personnel. Such training should help personnel understand the
need for protection of such materials and the rationale for the specific biosecurity
measures, and should include a review of relevant national standards and institution-
specific procedures. Procedures describing the security roles and responsibilities of
personnel in the event of a security infraction should also be presented during training.
The professional and ethical suitability for working with dangerous pathogens of
all personnel who have regular authorized access to sensitive materials is also central
to effective laboratory biosecurity activities.
In summary, security precautions should become a routine part of laboratory work,
just as have aseptic techniques and other safe microbiological practices. Laboratory
biosecurity measures should not hinder the efficient sharing of reference materials,
clinical and epidemiological specimens and related information necessary for clinical
or public health investigations. Competent security management should not unduly
interfere with the day-to-day activities of scientific personnel or be an impediment to
conducting research. Legitimate access to important research and clinical materials
must be preserved. Assessment of the suitability of personnel, security-specific training
and rigorous adherence to pathogen protection procedures are reasonable means of
enhancing laboratory biosecurity. All such efforts must be established and maintained
through regular risk and threat assessments, and regular review and updating of
procedures. Checks for compliance with these procedures, with clear instructions on
roles, responsibilities and remedial actions, should be integral to laboratory biosecurity
programmes and national standards for laboratory biosecurity.

PART III
Laboratory equipment
10. Biological safety cabinets
Biological safety cabinets (BSCs) are designed to protect the operator, the laboratory
environment and work materials from exposure to infectious aerosols and splashes
that may be generated when manipulating materials containing infectious agents, such
as primary cultures, stocks and diagnostic specimens. Aerosol particles are created by
any activity that imparts energy into a liquid or semiliquid material, such as shaking,
pouring, stirring or dropping liquid onto a surface or into another liquid. Other
laboratory activities, such as streaking agar plates, inoculating cell culture flasks with
a pipette, using a multichannel pipette to dispense liquid suspensions of infectious
agents into microculture plates, homogenizing and vortexing infectious materials, and
centrifugation of infectious liquids, or working with animals, can generate infectious
aerosols. Aerosol particles of less than 5 µm in diameter and small droplets of 5–100 µm
in diameter are not visible to the naked eye. The laboratory worker is generally not
aware that such particles are being generated and may be inhaled or may cross-
contaminate work surface materials. BSCs, when properly used, have been shown to
be highly effective in reducing laboratory-acquired infections and cross-contaminations
of cultures due to aerosol exposures. BSCs also protect the environment.
Over the years the basic design of BSCs has undergone several modifications. A
major change was the addition of a high-efficiency particulate air (HEPA) filter to the
exhaust system. The HEPA filter traps 99.97% of particles of 0.3 µm in diameter and
99.99% of particles of greater or smaller size. This enables the HEPA filter to effectively
trap all known infectious agents and ensure that only microbe-free exhaust air is
discharged from the cabinet. A second design modification was to direct HEPA-filtered
air over the work surface, providing protection of work surface materials from
contamination. This feature is often referred to as product protection. These basic
design concepts have led to the evolution of three classes of BSCs. The type of protection
provided by each is set out in Table 8.
Note. Horizontal and vertical outflow cabinets (“clean-air work stations”) are not
biological safety cabinets and should not be used as such.
Class I biological safety cabinet
Figure 6 provides a schematic diagram of a Class I BSC. Room air is drawn in through
the front opening at a minimum velocity of 0.38 m/s, it passes over the work surface
and is discharged from the cabinet through the exhaust duct. The directional flow of
• 51 •
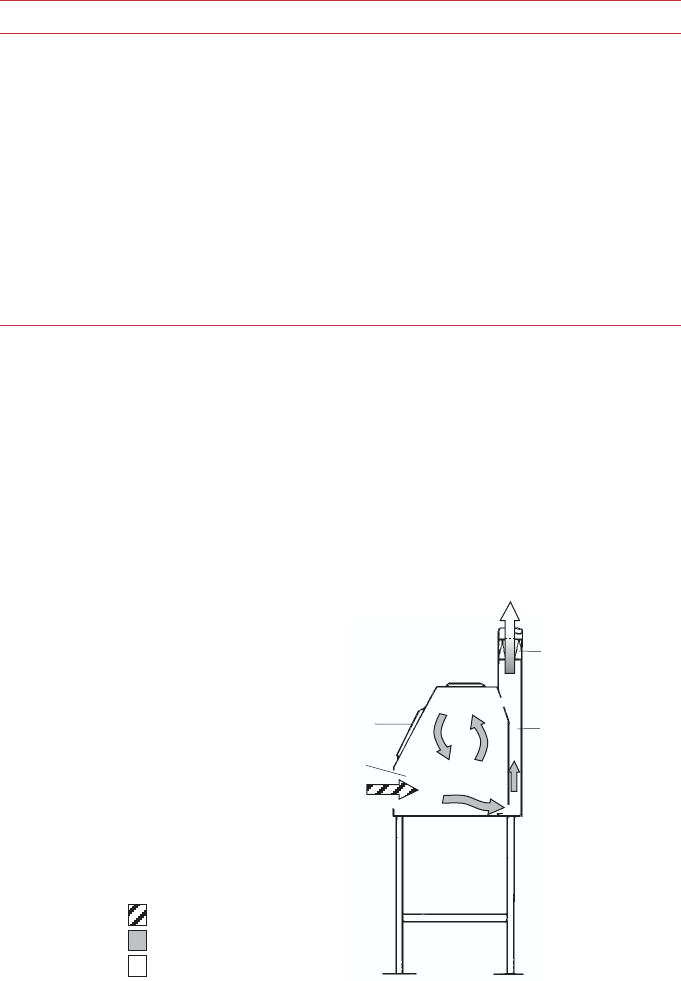
• 52 •
LABORATORY BIOSAFETY MANUAL
air whisks aerosol particles that may be generated on the work surface away from the
laboratory worker and into the exhaust duct. The front opening allows the operator’s
arms to reach the work surface inside the cabinet while he or she observes the work
surface through a glass window. The window can also be fully raised to provide access
to the work surface for cleaning or other purposes.
The air from the cabinet is exhausted through a HEPA filter: (a) into the laboratory
Table 8. Selection of a biological safety cabinet (BSC), by type of protection needed
TYPE OF PROTECTION BSC SELECTION
Personnel protection, microorganisms Class I, Class II, Class III
in Risk Groups 1–3
Personnel protection, microorganisms Class III
in Risk Group 4, glove-box laboratory
Personnel protection, microorganisms Class I, Class II
in Risk Group 4, suit laboratory
Product protection Class II, Class III only if laminar flow included
Volatile radionuclide/chemical protection, Class IIB1, Class IIA2 vented to the outside
minute amounts
Volatile radionuclide/chemical protection Class I, Class IIB2, Class III
Figure 6. Schematic diagram of a Class I biological safety cabinet.
A, front opening; B, sash; C, exhaust HEPA filter; D, exhaust plenum.
room air
potentially contaminated air
HEPA-filtered air
WHO 02.135
side view
A
D
C
B
• 53 •
10. BIOLOGICAL SAFETY CABINETS
and then to the outside of the building through the building exhaust; (b) to the outside
through the building exhaust; or (c) directly to the outside. The HEPA filter may be
located in the exhaust plenum of the BSC or in the building exhaust. Some Class I
BSCs are equipped with an integral exhaust fan, whereas others rely on the exhaust
fan in the building exhaust system.
The Class I BSC was the first recognized BSC and, because of its simple design, is
still in wide use throughout the world. It has the advantage of providing personnel
and environmental protection and can also be used for work with radionuclides and
volatile toxic chemicals. Because unsterilized room air is drawn over the work surface
through the front opening, it is not considered to provide consistently reliable product
protection.
Class II biological safety cabinets
As the use of cell and tissue cultures for the propagation of viruses and other purposes
grew, it was no longer considered satisfactory for unsterilized room air to pass over
the work surface. The Class II BSC was designed not only to provide personnel
protection but also to protect work surface materials from contaminated room air.
Class II BSCs, of which there are four types (A1, A2, B1 and B2), differ from Class I
BSCs by allowing only air from a HEPA-filtered (sterile) supply to flow over the work
surface. The Class II BSC can be used for working with infectious agents in Risk
Groups 2 and 3. Class II BSCs can be used for working with infectious agents in Risk
Group 4 when positive-pressure suits are used.
Class II type A1 biological safety cabinet
The Class II type A1 BSC is shown in Figure 7. An internal fan draws room air (supply
air) into the cabinet through the front opening and into the front intake grill. The
inflow velocity of this air should be at least 0.38 m/s at the face of the front opening.
The supply air then passes through a supply HEPA filter before flowing downwards
over the work surface. As the air flows downwards it “splits” about 6–18 cm from the
work surface, one half of the downwards flowing air passing through the front exhaust
grill, and the other half passing through the rear exhaust grill. Any aerosol particles
generated at the work surface are immediately captured in this downward airflow and
passed through the front or rear exhaust grills, thereby providing the highest level of
product protection. The air is then discharged through the rear plenum into the space
between the supply and exhaust filters located at the top of the cabinet. Owing to the
relative size of these filters, about 70% of the air recirculates through the supply HEPA
filter back into the work zone; the remaining 30% passes through the exhaust filter
into the room or to the outside.
Air from the Class IIA1 BSC exhaust can be recirculated to the room or discharged
to the outside of the building through a thimble connection to a dedicated duct or
through the building exhaust system.
Recirculating the exhaust air to the room has the advantage of lowering building
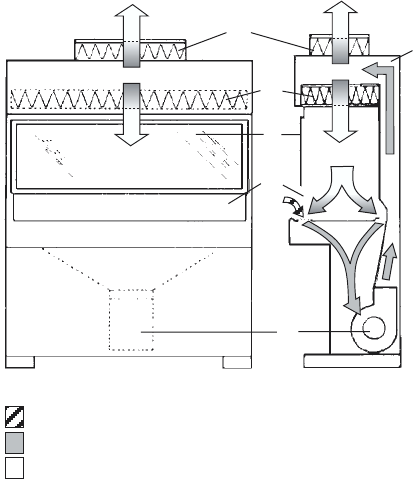
• 54 •
LABORATORY BIOSAFETY MANUAL
fuel costs because heated and/or cooled air is not being passed to the outside
environment. A connection to a ducted exhaust system also allows some BSCs to be
used for work with volatile radionuclides and volatile toxic chemicals (Table 8).
Class II type A2 vented to the outside, B1 and B2 biological safety cabinets
Class IIA2 vented to the outside, IIB1 (Figure 8) and IIB2 BSCs are variations of the
type IIA1. Their characteristics, along with those of Class I and Class III BSCs, are
indicated in Table 9. Each variation allows the BSC to be used for specialized purposes
(see Table 8). These BSCs differ from one another in several aspects: the air intake
velocity through the front opening; the amount of air recirculated over the work surface
and exhausted from the cabinet; the exhaust system, which determines whether air
from the cabinet is exhausted to the room, or to the outside, through a dedicated
exhaust system or through the building exhaust; and the pressure arrangements
(whether cabinets have biologically contaminated ducts and plenums under negative
pressure, or have biological contaminated ducts and plenums surrounded by negative-
pressure ducts and plenums).
Complete descriptions of the various Class IIA and IIB BSCs can be obtained from
references (7) and (8), and from manufacturers’ brochures.
Figure 7. Schematic representation of a Class IIA1 biological safety cabinet.
A, front opening; B, sash; C, exhaust HEPA filter; D, rear plenum; E, supply HEPA filter;
F, blower.
WHO 02.137
front view side view
room air
potentially contaminated air
HEPA-filtered air
B
A
E
C
D
F

• 55 •
10. BIOLOGICAL SAFETY CABINETS
Figure 8. Schematic diagram of a Class IIB1 biological safety cabinet.
A, front opening; B, sash: C, exhaust HEPA filter; D, supply HEPA filter; E, negative-
pressure exhaust plenum; F, blower; G, HEPA filter for supply air. Connection of the
cabinet exhaust to the building exhaust air system is required.
Table 9. Differences between Class I, II and III biological safety cabinets (BSCs)
BSC FACE VELOCITY (m/s) AIRFLOW (%) EXHAUST SYSTEM
RECIRCULATED EXHAUSTED
Class Ia0.36 0 100 Hard duct
Class IIA1 0.38–0.51 70 30 Exhaust to room or thimble
connection
Class IIA2 0.51 70 30 Exhaust to room or thimble
vented to the connection
outsidea
Class IIB1a0.51 30 70 Hard duct
Class IIB2a0.51 0 100 Hard duct
Class IIIaNA 0 100 Hard duct
NA, not applicable.
aAll biologically contaminated ducts are under negative pressure or are surrounded by negative pressure
ducts and plenums.
WHO 02.138
front view side view
room air
potentially contaminated air
HEPA-filtered air
B
A
F
C
G
F
E
D

• 56 •
LABORATORY BIOSAFETY MANUAL
Class III biological safety cabinet
This type (Figure 9) provides the highest level of personnel protection and is used for
Risk Group 4 agents. All penetrations are sealed “gas tight”. Supply air is HEPA-filtered
and exhaust air passes through two HEPA filters. Airflow is maintained by a dedicated
exhaust system exterior to the cabinet, which keeps the cabinet interior under negative
pressure (about 124.5 Pa). Access to the work surface is by means of heavy duty rubber
gloves, which are attached to ports in the cabinet. The Class III BSC should have an
attached pass-through box that can be sterilized and is equipped with a HEPA-filtered
exhaust. The Class III cabinet may be connected to a double-door autoclave used to
decontaminate all materials entering or exiting the cabinet. Several glove boxes can be
joined together to extend the work surface. Class III BSCs are suitable for work in
Biosafety Level 3 and 4 laboratories.
Biological safety cabinet air connections
A “thimble” or “canopy hood” is designed for use with Class IIA1 and IIA2 vented to
the outside BSCs. The thimble fits over the cabinet exhaust housing, sucking the cabinet
exhaust air into the building exhaust ducts. A small opening, usually 2.5 cm in diameter,
Figure 9. Schematic representation of a Class III biological safety cabinet (glove box).
A, glove ports for arm-length gloves; B, sash; C, double-exhaust HEPA filters;
D, supply HEPA filter; E, double-ended autoclave or pass-through box; F, chemical
dunk tank. Connection of the cabinet exhaust to an independent building exhaust air
system is required.
WHO 02.136
room air
potentially contaminated air
HEPA-filtered air
front view side view
B
A
E
CDC
F
• 57 •
is maintained between the thimble and the cabinet exhaust housing. This small opening
enables room air to be sucked into the building exhaust system as well. The building
exhaust capacity must be sufficient to capture both room air and the cabinet exhaust.
The thimble must be removable or be designed to allow for operational testing of the
cabinet. Generally, the performance of a thimble-connected BSC is not affected much
by fluctuations in the airflow of the building
Class IIB1 and IIB2 BSCs are hard-ducted, i.e. firmly connected without any
openings, to the building exhaust system or, preferably, to a dedicated exhaust duct
system. The building exhaust system must be precisely matched to the airflow
requirements specified by the manufacturer for both volume and static pressure.
Certification of hard-duct connected BSCs is more time-consuming than that for BSCs
that recirculate air to the room or which are thimble-connected.
Selection of a biological safety cabinet
A BSC should be selected primarily in accordance with the type of protection needed:
product protection; personnel protection against Risk Group 1–4 microorganisms;
personnel protection against exposure to radionuclides and volatile toxic chemicals;
or a combination of these. Table 8 shows which BSCs are recommended for each type
of protection.
Volatile or toxic chemicals should not be used in BSCs that recirculate exhaust air
to the room, i.e. Class I BSCs that are not ducted to building exhaust systems, or
Class IIA1 or Class IIA2 cabinets. Class IIB1 BSCs are acceptable for work with minute
amounts of volatile chemicals and radionuclides. A Class IIB2 BSC, also called a total
exhaust cabinet, is necessary when significant amounts of radionuclides and volatile
chemicals are expected to be used.
Using biological safety cabinets in the laboratory
Location
The velocity of air flowing through the front opening into a BSC is about 0.45 m/s. At
this velocity the integrity of the directional air inflow is fragile and can be easily
disrupted by air currents generated by people walking close to the BSC, open windows,
air supply registers, and opening and shutting doors. Ideally, BSCs should be situated
in a location remote from traffic and potentially disturbing air currents. Whenever
possible a 30-cm clearance should be provided behind and on each side of the cabinet
to allow easy access for maintenance. A clearance of 30–35 cm above the cabinet may
be required to provide for accurate air velocity measurement across the exhaust filter
and for exhaust filter changes.
Operators
If BSCs are not used properly, their protective benefits may be greatly diminished.
Operators need to be careful to maintain the integrity of the front opening air inflow
when moving their arms into and out of cabinets. Arms should be moved in and out
10. BIOLOGICAL SAFETY CABINETS
• 58 •
LABORATORY BIOSAFETY MANUAL
slowly, perpendicular to the front opening. Manipulations of materials within BSCs
should be delayed for about 1 min after placing hands and arms inside to allow the
cabinet to adjust and to “air sweep” the surface of the hands and arms. The number of
movements across the front opening should also be minimized by placing all necessary
items into the cabinet before beginning manipulations.
Material placement
The front intake grill of Class II BSCs must not be blocked with paper, equipment or
other items. Materials to be placed inside the cabinet should be surface-decontaminated
with 70% alcohol. Work may be performed on disinfectant-soaked absorbent towels
to capture splatters and splashes. All materials should be placed as far back in the
cabinet, towards the rear edge of the work surface, as practical without blocking the
rear grill. Aerosol-generating equipment (e.g. mixers, centrifuges, etc.) should be placed
towards the rear of the cabinet. Bulky items, such as biohazard bags, discard pipette
trays and suction collection flasks should be placed to one side of the interior of the
cabinet. Active work should flow from clean to contaminated areas across the work
surface.
The autoclavable biohazard collection bag and pipette collection tray should not
be placed outside the cabinet. The frequent in-and-out movement needed to use these
containers is disruptive to the integrity of the cabinet’s air barrier, and can compromise
both personnel and product protection.
Operation and maintenance
Most BSCs are designed to permit operation 24 h/day, and investigators find that
continuous operation helps to control the levels of dust and particulate materials in
the laboratory. Class IIA1 and IIA2 BSCs exhausting to the room or connected by
thimble connections to dedicated exhaust ducts can be turned off when not in use.
Other types such as IIB1 and IIB2 BSCs, which have hard-duct installations, must
have airflow through them at all times to help maintain room air balance. Cabinets
should be turned on at least 5 min before beginning work and after completion of
work to allow the cabinet to “purge”, i.e. to allow time for contaminated air to be
removed from the cabinet environment.
All repairs made on BSCs should be made by a qualified technician. Any malfunction
in the operation of the BSC should be reported and repaired before the BSC is used
again.
Ultraviolet lights
Ultraviolet lights are not required in BSCs. If they are used, they must be cleaned
weekly to remove any dust and dirt that may block the germicidal effectiveness of the
light. Ultraviolet light intensity should be checked when the cabinet is recertified to
ensure that light emission is appropriate. Ultraviolet lights must be turned off while
the room is occupied, to protect eyes and skin from inadvertent exposure.
• 59 •
Open flames
Open flames should be avoided in the near microbe-free environment created inside
the BSC. They disrupt the airflow patterns and can be dangerous when volatile,
flammable substances are also used. To sterilize bacteriological loops, microburners
or electric “furnaces” are available and are preferable to open flames.
Spills
A copy of the laboratory’s protocol for handling spills should be posted, read and
understood by everyone who uses the laboratory. When a spill of biohazardous material
occurs within a BSC, clean-up should begin immediately, while the cabinet continues
to operate. An effective disinfectant should be used and applied in a manner that
minimizes the generation of aerosols. All materials that come into contact with the
spilled agent should be disinfected and/or autoclaved.
Certification
The functional operation and integrity of each BSC should be certified to national or
international performance standards at the time of installation and regularly thereafter
by qualified technicians, according to the manufacturer’s instructions. Evaluation of
the effectiveness of cabinet containment should include tests for cabinet integrity, HEPA
filter leaks, downflow velocity profile, face velocity, negative pressure/ventilation rate,
air-flow smoke pattern, and alarms and interlocks. Optional tests for electrical leaks,
lighting intensity, ultraviolet light intensity, noise level and vibration may also be
conducted. Special training, skills and equipment are required to perform these tests
and it is highly recommended that they are undertaken by a qualified professional.
Cleaning and disinfection
All items within BSCs, including equipment, should be surface-decontaminated and
removed from the cabinet when work is completed, since residual culture media may
provide an opportunity for microbial growth.
The interior surfaces of BSCs should be decontaminated before and after each use.
The work surfaces and interior walls should be wiped with a disinfectant that will kill
any microorganisms that might be found inside the cabinet. At the end of the work
day, the final surface decontamination should include a wipe-down of the work surface,
the sides, back and interior of the glass. A solution of bleach or 70% alcohol should be
used where effective for target organisms. A second wiping with sterile water is needed
when a corrosive disinfectant, such as bleach, is used.
It is recommended that the cabinet is left running. If not, it should be run for 5 min
in order to purge the atmosphere inside before it is switched off.
Decontamination
BSCs must be decontaminated before filter changes and before being moved. The most
common decontamination method is by fumigation with formaldehyde gas. BSC
decontamination should be performed by a qualified professional.
10. BIOLOGICAL SAFETY CABINETS
• 60 •
LABORATORY BIOSAFETY MANUAL
Personal protective equipment
Personal protective clothing should be worn whenever using a BSC. Laboratory coats
are acceptable for work being performed at Biosafety Levels 1 and 2. A solid front,
back-closing laboratory gown provides better protection and should be used at
Biosafety Levels 3 and 4 (except for suit laboratories). Gloves should be pulled over
the wrists of the gown rather than worn inside. Elasticized sleeves can be worn to
protect the investigator’s wrists. Masks and safety glasses may be required for some
procedures.
Alarms
BSCs can be equipped with one of two kinds of alarm. Sash alarms are found only on
cabinets with sliding sashes. The alarm signifies that the operator has moved the sash
to an improper position. Corrective action for this type of alarm is returning the sash
to the proper position. Airflow alarms indicate a disruption in the cabinet’s normal
airflow pattern. This represents an immediate danger to the operator or product. When
an airflow alarm sounds, work should cease immediately and the laboratory supervisor
should be notified. Manufacturers’ instruction manuals should provide further details.
Training in the use of BSCs should cover this aspect.
Supplementary information
Selecting the correct type of BSC, installing it, using it properly and annually certifying
its operation are complex processes. It is highly recommended that they proceed under
the supervision of a well-trained and experienced biosafety professional. The
professional should be highly familiar with the relevant literature listed in the References
section, and should have been trained on all aspects of BSCs. Operators should receive
formal training in the operation and use of BSCs.
For further information see references (5) and (7–16), and Chapter 11.
• 61 •
11. Safety equipment
As aerosols are important sources of infection, care should be taken to reduce the
extent of their formation and dispersion. Hazardous aerosols can be generated by
many laboratory operations, e.g. blending, mixing, grinding, shaking, stirring,
sonicating and centrifuging of infectious materials. Even when safe equipment is used,
it is best to carry out these operations in an approved biological safety cabinet whenever
possible. Biological safety cabinets and their use and testing are discussed in Chapter 10.
The use of safety equipment is no assurance of protection unless the operator is trained
and uses proper techniques. Equipment should be tested regularly to ensure its
continued safe performance.
Table 10 provides a checklist of safety equipment designed to eliminate or reduce
certain hazards and briefly outlines the safety features. Further details of much of this
equipment are given in subsequent pages. Additional information on its proper use is
provided in Chapter 12.
Information on equipment and operations that may create hazards is presented in
Annex 4.
Negative-pressure flexible-film isolators
The negative-pressure flexible-film isolator is a self-contained primary containment
device that provides maximum protection against hazardous biological materials. It
may be mounted on a mobile stand. The workspace is totally enclosed in a transparent
polyvinylchloride (PVC) envelope suspended from a steel framework. The isolator is
maintained at an internal pressure lower than atmospheric pressure. Inlet air is passed
through one HEPA filter and outlet air is passed through two HEPA filters, thus
obviating the need to duct exhaust air outside the building. The isolator may be fitted
with an incubator, microscope and other laboratory equipment, such as centrifuges,
animal cages, heat blocks, etc. Material is introduced and removed from the isolator
through supply and sample ports without compromising microbiological security.
Manipulations are performed using gloved sleeves incorporating disposable gloves. A
manometer is installed to monitor envelope pressure.
Flexible-film isolators are used to manipulate high-risk organisms (Risk Groups 3
or 4) in field work where it is not feasible or appropriate to install or maintain
conventional biological safety cabinets.

• 62 •
LABORATORY BIOSAFETY MANUAL
Table 10. Biosafety equipment
EQUIPMENT HAZARD CORRECTED SAFETY FEATURES
Biological safety
cabinet
— Class I Aerosol and spatter • Minimum inward airflow (face
velocity) at work access opening.
Adequate filtration of exhaust air.
•Does not provide product protection
— Class II Aerosol and spatter • Minimum inward airflow (face
velocity) at work access opening.
Adequate filtration of exhaust air
•Provides product protection
— Class III Aerosol and spatter • Maximum containment
•Provides product protection if
laminar flow air is included
Negative pressure Aerosol and spatter • Maximum containment
flexible-film isolator
Spatter shield Spatter of chemicals • Forms screen between operator and
work
Pipetting aids Hazards from pipetting by • Ease of use
mouth, e.g. ingestion of • Controls contamination of suction
pathogens, inhalation of end of pipette, protecting pipetting
aerosols produced by mouth aid, user and vacuum line
suction on pipette, blowing • Can be sterilized
out of liquid or dripping from • Controls leakage from pipette tip
pipette, contamination of
suction end of pipette
Loop microinciner- Spatter from transfer loops • Shielded in open-ended glass or
ators, disposable ceramic tube. Heated by gas or
loops electricity
•Disposable, no heating necessary
Leakproof vessels Aerosols, spillage and • Leakproof construction with lid or
for collection and leakage cover
transport of • Durable
infectious materials • Autoclavable
for sterilization
within a facility
Sharps disposal Puncture wounds • Autoclavable
containers • Robust, puncture-proof
Transport containers Release of microorganisms • Robust
between laboratories, • Watertight primary and secondary
institutions containers to contain spills
•Absorbent material to contain spills

• 63 •
Pipetting aids
A pipetting aid must always be used for pipetting procedures. Mouth pipetting must
be strictly forbidden.
The importance of pipetting aids cannot be overemphasized. The most common
hazards associated with pipetting procedures are the result of mouth suction. Oral
aspiration and ingestion of hazardous materials have been responsible for many
laboratory-associated infections.
Pathogens can also be transferred to the mouth if a contaminated finger is placed
on the suction end of a pipette. A lesser known hazard of mouth pipetting is the
inhalation of aerosols caused by suction. The cotton plug is not an efficient microbial
filter at negative or positive pressure, and particles may be sucked through it. Violent
suction may be applied when the plug is tightly packed, resulting in the aspiration of
plug, aerosol and even liquid. The ingestion of pathogens is prevented by the use of
pipetting aids.
Aerosols can also be generated when a liquid is dropped from a pipette on to a
work surface, when cultures are mixed by alternate sucking and blowing, and when
the last drop is blown out of a pipette. The inhalation of aerosols unavoidably generated
during pipetting operations can be prevented by working in a biological safety cabinet.
Pipetting aids should be selected with care. Their design and use should not create
an additional infectious hazard and they should be easy to sterilize and clean. Plugged
(aerosol-resistant) pipette tips should be used when manipulating microorganisms
and cell cultures.
Pipettes with cracked or chipped suction ends should not be used as they damage
the seating seals of pipetting aids and so create a hazard.
Homogenizers, shakers, blenders and sonicators
Domestic (kitchen) homogenizers are not sealed and release aerosols. Only equipment
designed for laboratory use should be used. Their construction minimizes or prevents
11. SAFETY EQUIPMENT
EQUIPMENT HAZARD CORRECTED SAFETY FEATURES
Autoclaves, manual Infectious material (made • Approved design
or automatic safe for disposal or reuse) • Effective heat sterilization
Screw-capped bottles Aerosols and spillage • Effective containment
Vacuum line Contamination of laboratory • Cartridge-type filter prevents
protection vacuum system with passage of aerosols (particle size
aerosols and overflow fluids 0.45 µm)
•Overflow flask contains appropriate
disinfectant. Rubber bulb may be
used to close off vacuum auto
matically when storage flask is full
•Entire unit autoclavable
• 64 •
LABORATORY BIOSAFETY MANUAL
such release. Stomachers, which are now available for use with large and small volumes,
may also produce aerosols.
Homogenizers used for Risk Group 3 microorganisms should always be loaded
and reopened in biological safety cabinets.
Sonicators may release aerosols. They should be operated in biological safety cabinets
or covered with shields during use. The shields and outsides of sonicators should be
decontaminated after use.
Disposable transfer loops
The advantage of disposable transfer loops is that they do not have to be sterilized and
can therefore be used in biological safety cabinets where Bunsen burners and
microincinerators would disturb the airflow. These loops should be placed in
disinfectant after use and discarded as contaminated waste (see Chapter 3).
Microincinerators
Gas- and electrically-heated microincinerators have borosilicate glass or ceramic shields
that minimize the spatter and dispersal of infected material when transfer loops are
sterilized. However, microincinerators can disturb the airflow and should therefore be
placed towards the back of the work surface in biological safety cabinets.
Personal protective equipment and clothing
Personal protective equipment and clothing may act as a barrier to minimize the risk
of exposure to aerosols, splashes and accidental inoculation. The clothing and
equipment selected is dependent on the nature of the work performed. Protective
clothing should be worn when working in the laboratory. Before leaving the laboratory,
protective clothing should be removed, and hands should be washed. Table 11
summarizes some personal protective equipment used in laboratories and the
protection afforded.
Laboratory coats, gowns, coveralls, aprons
Laboratory coats should preferably be fully buttoned. However, long-sleeved, back-
opening gowns or coveralls give better protection than laboratory coats and are
preferred in microbiology laboratories and when working at the biological safety
cabinet. Aprons may be worn over laboratory coats or gowns where necessary to give
further protection against spillage of chemicals or biological materials such as blood
or culture fluids. Laundering services should be provided at/near the facility.
Laboratory coats, gowns, coveralls, or aprons should not be worn outside the
laboratory areas.
Goggles, safety spectacles, face shields
The choice of equipment to protect the eyes and face from splashes and impacting
objects will depend on the activity performed. Prescription or plain eye glasses can be

• 65 •
11. SAFETY EQUIPMENT
Table 11. Personal protective equipment
EQUIPMENT HAZARD CORRECTED SAFETY FEATURES
Laboratory coats, Contamination of clothing • Back opening
gowns, coveralls • Cover street clothing
Plastic aprons Contamination of clothing • Waterproof
Footwear Impact and splash • Closed-toe
Goggles Impact and splash • Impact-resistant lenses (must be optically
correct or worn over corrective eye glasses)
•Side shields
Safety spectacles Impact • Impact-resistant lenses (must be optically
correct)
•Side shields
Face shields Impact and splash • Shield entire face
•Easily removable in case of accident
Respirators Inhalation of aerosols • Designs available include single-use
disposable; full-face or half-face air
purifying; full-face or hooded powered air
purifying (PAPR); and supplied air
respirators
Gloves Direct contact with • Disposable microbiologically approved latex,
microorganisms vinyl or nitrile
•Hand protection
Cuts • Mesh
manufactured with special frames that allow lenses to be placed in frame from the
front, using shatterproof material either curved or fitted with side shields (safety
glasses). Safety spectacles do not provide for adequate splash protection even when
side shields are worn with them. Goggles for splash and impact protection should be
worn over normal prescription eye glasses and contact lenses (which do not provide
protection against biological or chemical hazards). Face shields (visors) are made of
shatterproof plastic, fit over the face and are held in place by head straps or caps.
Goggles, safety spectacles, or face shields should not be worn outside the laboratory
areas.
Respirators
Respiratory protection may be used when carrying out high-hazard procedures (e.g.
cleaning up a spill of infectious material). The choice of respirator will depend on the
type of hazard(s). Respirators are available with interchangeable filters for protection
against gases, vapours, particulates and microorganisms. It is imperative that the filter
is fitted in the correct type of respirator. To achieve optimal protection, respirators

should be individually fitted to the operator’s face and tested. Fully self-contained
respirators with an integral air supply provide full protection. Advice should be sought
from a suitably qualified person, e.g. an occupational hygienist, for selection of the
correct respirator. Surgical type masks are designed solely for patient protection and
do not provide respiratory protection to workers. Some single-use disposable
respirators (ISO 13.340.30) have been designed for protection against exposures to
biological agents.
Respirators should not be worn outside the laboratory areas.
Gloves
Contamination of hands may occur when laboratory procedures are performed. Hands
are also vulnerable to “sharps” injuries. Disposable microbiologically approved latex,
vinyl or nitrile surgical-type gloves are used widely for general laboratory work, and
for handling infectious agents and blood and body fluids. Reusable gloves may also be
used but attention must be given to their correct washing, removal, cleaning and
disinfection.
Gloves should be removed and hands thoroughly washed after handling infectious
materials, working in a biological safety cabinet and before leaving the laboratory.
Used disposable gloves should be discarded with infected laboratory wastes.
Allergic reactions such as dermatitis and immediate hypersensitivity have been
reported in laboratory and other workers wearing latex gloves, particularly those with
powder. Alternatives to powdered latex gloves should be available.
Stainless steel mesh gloves should be worn when there is a potential exposure to
sharp instruments e.g. during postmortem examinations. Such gloves protect against
slicing motion but do not protect against puncture injury.
Gloves should not be worn outside the laboratory areas.
For further information see references (12), (17) and (18).
• 66 •
LABORATORY BIOSAFETY MANUAL

PART IV
Good microbiological
techniques
12. Laboratory techniques
• 69 •
Human error, poor laboratory techniques and misuse of equipment cause the majority
of laboratory injuries and work-related infections. This chapter provides a compendium
of technical methods that are designed to avoid or minimize the most commonly
reported problems of this nature.
Safe handling of specimens in the laboratory
Improper collection, transport and handling of specimens in the laboratory carry a
risk of infection to the personnel involved.
Specimen containers
Specimen containers may be of glass or preferably plastic. They should be robust and
should not leak when the cap or stopper is correctly applied. No material should remain
on the outside of the container. Containers should be correctly labelled to facilitate
identification. Specimen request or specification forms should not be wrapped around
the containers but placed in separate, preferably waterproof envelopes.
Transport of specimens within the facility
To avoid accidental leakage or spillage, secondary containers, such as boxes, should be
used, fitted with racks so that the specimen containers remain upright. The secondary
containers may be of metal or plastic, should be autoclavable or resistant to the action
of chemical disinfectants, and the seal should preferably have a gasket. They should be
regularly decontaminated.
Receipt of specimens
Laboratories that receive large numbers of specimens should designate a particular
room or area for this purpose.
Opening packages
Personnel who receive and unpack specimens should be aware of the potential health
hazards involved, and should be trained to adopt standard precautions (2), particularly
when dealing with broken or leaking containers. Primary specimen containers should
be opened in a biological safety cabinet. Disinfectants should be available.
• 70 •
LABORATORY BIOSAFETY MANUAL
Use of pipettes and pipetting aids
1. A pipetting aid must always be used. Pipetting by mouth must be prohibited.
2. All pipettes should have cotton plugs to reduce contamination of pipetting devices.
3. Air should never be blown through a liquid containing infectious agents.
4. Infectious materials should not be mixed by alternate suction and expulsion through
a pipette.
5. Liquids should not be forcibly expelled from pipettes.
6. Mark-to-mark pipettes are preferable to other types as they do not require expulsion
of the last drop.
7. Contaminated pipettes should be completely submerged in a suitable disinfectant
contained in an unbreakable container. They should be left in the disinfectant for
the appropriate length of time before disposal.
8. A discard container for pipettes should be placed within the biological safety cabinet,
not outside it.
9. Syringes fitted with hypodermic needles must not be used for pipetting.
10. Devices for opening septum-capped bottles that allow pipettes to be used and avoid
the use of hypodermic needles and syringes should be used.
11. To avoid dispersion of infectious material dropped from a pipette, an absorbent
material should be placed on the working surface; this should be disposed of as
infectious waste after use.
Avoiding the dispersal of infectious materials
1. In order to avoid the premature shedding of their loads, microbiological transfer
loops should have a diameter of 2–3 mm and be completely closed. The shanks
should be not more than 6 cm in length to minimize vibration.
2. The risk of spatter of infectious material in an open Bunsen burner flame should
be avoided by using an enclosed electric microincinerator to sterilize transfer loops.
Disposable transfer loops, which do not need to be resterilized, are preferable.
3. Care should be taken when drying sputum samples, to avoid creating aerosols.
4. Discarded specimens and cultures for autoclaving and/or disposal should be placed
in leakproof containers, e.g. laboratory discard bags. Tops should be secured (e.g.
with autoclave tape) prior to disposal into waste containers.
5. Working areas must be decontaminated with a suitable disinfectant at the end of
each work period.
For further information see reference (12).
Use of biological safety cabinets
1. The use and limitations of biological safety cabinets should be explained to all
potential users (see Chapter 10), with reference to national standards and relevant
literature. Written protocols or safety or operations manuals should be issued to
staff. In particular, it must be made clear that the cabinet will not protect the
operator from spillage, breakage or poor technique.
• 71 •
2. The cabinet must not be used unless it is working properly.
3. The glass viewing panel must not be opened when the cabinet is in use.
4. Apparatus and materials in the cabinet must be kept to a minimum. Air circulation
at the rear plenum must not be blocked.
5. Bunsen burners must not be used in the cabinet. The heat produced will distort
the airflow and may damage the filters. An electric microincinerator is permissible
but sterile disposable transfer loops are better.
6. All work must be carried out in the middle or rear part of the working surface and
be visible through the viewing panel.
7. Traffic behind the operator should be minimized.
8. The operator should not disturb the airflow by repeated removal and reintroduction
of his or her arms.
9. Air grills must not be blocked with notes, pipettes or other materials, as this will
disrupt the airflow causing potential contamination of the material and exposure
of the operator.
10. The surface of the biological safety cabinet should be wiped using an appropriate
disinfectant after work is completed and at the end of the day.
11. The cabinet fan should be run for at least 5 min before beginning work and after
completion of work in the cabinet.
12. Paperwork should never be placed inside biological safety cabinets.
For further information about biological safety cabinets see Chapter 10.
Avoiding ingestion of infectious materials and contact with skin and eyes
1. Large particles and droplets (> 5 µm in diameter) released during microbiological
manipulations settle rapidly on bench surfaces and on the hands of the operator.
Disposable gloves should be worn. Laboratory workers should avoid touching their
mouth, eyes and face.
2. Food and drink must not be consumed or stored in the laboratory.
3. No articles should be placed in the mouth – pens, pencils, chewing gum – in the
laboratory.
4. Cosmetics should not be applied in the laboratory.
5. The face, eyes and mouth should be shielded or otherwise protected during any
operation that may result in the splashing of potentially infectious materials.
Avoiding injection of infectious materials
1. Accidental inoculation resulting from injury with broken or chipped glassware
can be avoided through careful practices and procedures. Glassware should be
replaced with plastic ware whenever possible.
2. Accidental injection may result from sharps injuries e.g. with hypodermic needles
(needle-sticks), glass Pasteur pipettes, or broken glass.
3. Needle-stick injuries can be reduced by: (a) minimizing the use of syringes and
needles (e.g. simple devices are available for opening septum-stoppered bottles so
12. LABORATORY TECHNIQUES
• 72 •
LABORATORY BIOSAFETY MANUAL
that pipettes can be used instead of syringes and needles; or (b) using engineered
sharp safety devices when syringes and needles are necessary.
4. Needles should never be recapped. Disposable articles should be discarded into
puncture-proof/puncture-resistant containers fitted with covers.
5. Plastic Pasteur pipettes should replace those made of glass.
Separation of serum
1. Only properly trained staff should be employed for this work.
2. Gloves and eye and mucous membrane protection should be worn.
3. Splashes and aerosols can only be avoided or minimized by good laboratory
technique. Blood and serum should be pipetted carefully, not poured. Pipetting by
mouth must be forbidden.
4. After use, pipettes should be completely submerged in suitable disinfectant. They
should remain in the disinfectant for the appropriate time before disposal or
washing and sterilization for reuse.
5. Discarded specimen tubes containing blood clots, etc. (with caps replaced) should
be placed in suitable leakproof containers for autoclaving and/or incineration.
6. Suitable disinfectants should be available for clean-up of splashes and spillages
(see Chapter 14).
Use of centrifuges
1. Satisfactory mechanical performance is a prerequisite of microbiological safety in
the use of laboratory centrifuges.
2. Centrifuges should be operated according to the manufacturer’s instructions.
3. Centrifuges should be placed at such a level that workers can see into the bowl to
place trunnions and buckets correctly.
4. Centrifuge tubes and specimen containers for use in the centrifuge should be made
of thick-walled glass or preferably of plastic and should be inspected for defects
before use.
5. Tubes and specimen containers should always be securely capped (screw-capped
if possible) for centrifugation.
6. The buckets must be loaded, equilibrated, sealed and opened in a biological safety
cabinet.
7. Buckets and trunnions should be paired by weight and, with tubes in place, correctly
balanced.
8. The amount of space that should be left between the level of the fluid and the rim
of the centrifuge tube should be given in manufacturer’s instructions.
9. Distilled water or alcohol (propanol, 70%) should be used for balancing empty
buckets. Saline or hypochlorite solutions should not be used as they corrode metals.
10. Sealable centrifuge buckets (safety cups) must be used for microorganisms in Risk
Groups 3 and 4.
11. When using angle-head centrifuge rotors, care must be taken to ensure that the
tube is not overloaded as it might leak.
• 73 •
12. The interior of the centrifuge bowl should be inspected daily for staining or soiling
at the level of the rotor. If staining or soiling are evident then the centrifugation
protocols should be re-evaluated.
13. Centrifuge rotors and buckets should be inspected daily for signs of corrosion and
for hair-line cracks.
14. Buckets, rotors and centrifuge bowls should be decontaminated after each use.
15. After use, buckets should be stored in an inverted position to drain the balancing
fluid.
16. Infectious airborne particles may be ejected when centrifuges are used. These
particles travel at speeds too high to be retained by the cabinet airflow if the
centrifuge is placed in a traditional open-fronted Class I or Class II biological safety
cabinet. Enclosing centrifuges in Class III safety cabinets prevents emitted aerosols
from dispersing widely. However, good centrifuge technique and securely capped
tubes offer adequate protection against infectious aerosols and dispersed particles.
Use of homogenizers, shakers, blenders and sonicators
1. Domestic (kitchen) homogenizers should not be used in laboratories as they may
leak or release aerosols. Laboratory blenders and stomachers are safer.
2. Caps and cups or bottles should be in good condition and free from flaws or
distortion. Caps should be well-fitting and gaskets should be in good condition.
3. Pressure builds up in the vessel during the operation of homogenizers, shakers
and sonicators. Aerosols containing infectious materials may escape from between
the cap and the vessel. Plastic, in particular, polytetrafluoroethylene (PTFE) vessels
are recommended because glass may break, releasing infectious material and
possibly wounding the operator.
4. When in use, homogenizers, shakers and sonicators should be covered by a strong
transparent plastic casing. This should be disinfected after use. Where possible, these
machines should be operated, under their plastic covers, in a biological safety cabinet.
5. At the end of the operation the containers should be opened in a biological safety
cabinet.
6. Hearing protection should be provided for people using sonicators.
Use of tissue grinders
1. Glass grinders should be held in absorbent material in a gloved hand. Plastic (PTFE)
grinders are safer.
2. Tissue grinders should be operated and opened in a biological safety cabinet.
Care and use of refrigerators and freezers
1. Refrigerators, deep-freezers and solid carbon dioxide (dry-ice) chests should be
defrosted and cleaned periodically, and any ampoules, tubes, etc. that have broken
during storage removed. Face protection and heavy duty rubber gloves should be
worn during cleaning. After cleaning, the inner surfaces of the cabinet should be
disinfected.
12. LABORATORY TECHNIQUES
• 74 •
LABORATORY BIOSAFETY MANUAL
2. All containers stored in refrigerators, etc. should be clearly labelled with the scientific
name of the contents, the date stored and the name of the individual who stored
them. Unlabelled and obsolete materials should be autoclaved and discarded.
3. An inventory must be maintained of the freezer’s contents.
4. Flammable solutions must not be stored in a refrigerator unless it is explosion-
proof. Notices to this effect should be placed on refrigerator doors.
Opening of ampoules containing lyophilized infectious materials
Care should be taken when ampoules of freeze-dried materials are opened, as the
contents may be under reduced pressure and the sudden inrush of air may disperse
some of the materials into the atmosphere. Ampoules should always be opened in a
biological safety cabinet. The following procedures are recommended for opening
ampoules.
1. First decontaminate the outer surface of the ampoule.
2. Make a file mark on the tube near to the middle of the cotton or cellulose plug, if
present.
3. Hold the ampoule in alcohol-soaked cotton to protect hands before breaking it at
a file scratch.
4. Remove the top gently and treat as contaminated material.
5. If the plug is still above the contents of the ampoule, remove it with sterile forceps.
6. Add liquid for resuspension slowly to the ampoule to avoid frothing.
Storage of ampoules containing infectious materials
Ampoules containing infectious materials should never be immersed in liquid nitrogen
because cracked or imperfectly sealed ampoules may break or explode on removal. If
very low temperatures are required, ampoules should be stored only in the gaseous
phase above the liquid nitrogen. Otherwise, infectious materials should be stored in
mechanical deep-freeze cabinets or on dry ice. Laboratory workers should wear eye
and hand protection when removing ampoules from cold storage.
The outer surfaces of ampoules stored in these ways should be disinfected when
the ampoules are removed from storage.
Standard precautions with blood and other body fluids, tissues and excreta
Standard precautions (which include “universal precautions” (19)) are designed to
reduce the risk of transmission of microorganisms from both recognized and
unrecognized sources of infection (2).
Collection, labelling and transport of specimens
1. Standard precautions (2) should always be followed; gloves should be worn for all
procedures.
2. Blood should be collected from patients and animals by trained staff.
3. For phlebotomies, conventional needle and syringe systems should be replaced by
• 75 •
single-use safety vacuum devices that allow the collection of blood directly into
stoppered transport and/or culture tubes, automatically disabling the needle after
use.
4. The tubes should be placed in adequate containers for transport to the laboratory
(see Chapter 15 for transport requirements) and within the laboratory facility (see
section on Transport of specimens within the facility in this chapter). Request forms
should be placed in separate waterproof bags or envelopes.
5. Reception staff should not open these bags.
Opening specimen tubes and sampling contents
1. Specimen tubes should be opened in a biological safety cabinet.
2. Gloves must be worn. Eye and mucous membrane protection is also recommended
(goggles or face shields).
3. Protective clothing should be supplemented with a plastic apron.
4. The stopper should be grasped through a piece of paper or gauze to prevent
splashing.
Glass and “sharps”
1. Plastics should replace glass wherever possible. Only laboratory grade (borosilicate)
glass should be used, and any article that is chipped or cracked should be discarded.
2. Hypodermic needles must not be used as pipettes (see also section on Avoiding
injection of infectious materials in this chapter).
Films and smears for microscopy
Fixing and staining of blood, sputum and faecal samples for microscopy do not
necessarily kill all organisms or viruses on the smears. These items should be handled
with forceps, stored appropriately, and decontaminated and/or autoclaved before
disposal.
Automated equipment (sonicators, vortex mixers)
1. Equipment should be of the closed type to avoid dispersion of droplets and aerosols.
2. Effluents should be collected in closed vessels for further autoclaving and/or
disposal.
3. Equipment should be disinfected at the end of each session, following manu-
facturers’ instructions.
Tissues
1. Formalin fixatives should be used.
2. Frozen sectioning should be avoided. When necessary, the cryostat should be
shielded and the operator should wear a safety face shield. For decontamination,
the temperature of the instrument should be raised to at least 20 °C.
12. LABORATORY TECHNIQUES
• 76 •
LABORATORY BIOSAFETY MANUAL
Decontamination
Hypochlorites and high-level disinfectants are recommended for decontamination.
Freshly prepared hypochlorite solutions should contain available chlorine at 1 g/l for
general use and 5 g/l for blood spillages. Glutaraldehyde may be used for decontami-
nating surfaces (see Chapter 14).
Precautions with materials that may contain prions
Prions (also referred to as “slow viruses”) are associated with the transmissible spongi-
form encephalopathies (TSEs), notably Creutzfeldt-Jakob disease (CJD; including the
new variant form), Gerstmann-Sträussler-Scheinker syndrome, fatal familial insomnia
and kuru in humans; scrapie in sheep and goats; bovine spongiform encephalopathy
(BSE) in cattle; and other transmissible encephalopathies of deer, elk and mink.
Although CJD has been transmitted to humans, there appear to be no proven cases of
laboratory-associated infections with any of these agents. Nevertheless, it is prudent
to observe certain precautions in the handling of material from infected or potentially
infected humans and animals.
The selection of a biosafety level for work with materials associated with TSEs will
depend on the nature of the agent and the samples to be studied, and should be
undertaken in consultation with national authorities. The highest concentrations of
prions are found in central nervous system tissue. Animal studies suggest that it is
likely that high concentrations of prions are also found in the spleen, thymus, lymph
nodes and lung. Recent studies indicate that prions in lingual and skeletal muscle
tissue may also present a potential infection risk (20–23).
As complete inactivation of prions is difficult to achieve, it is important to stress
the use of disposable instruments whenever possible, and to use a disposable protective
covering for the work surface of the biological safety cabinet.
The main precaution to be taken is to avoid ingestion of contaminated materials or
puncture of the laboratory worker’s skin. The following additional precautions should
be taken, as the agents are not killed by the normal processes of laboratory disinfection
and sterilization.
1. The use of dedicated equipment, i.e. equipment not shared with other laboratories,
is highly recommended.
2. Disposable laboratory protective clothing (gowns and aprons) and gloves must be
worn (steel mesh gloves between rubber gloves for pathologists).
3. Use of disposable plastic ware, which can be treated and discarded as dry waste, is
highly recommended.
4. Tissue processors should not be used because of the problems of disinfection. Jars
and beakers (plastic) should be used instead.
5. All manipulations must be conducted in biological safety cabinets.
6. Great care should be exercised to avoid aerosol production, ingestion, and cuts
and punctures of the skin.
• 77 •
7. Formalin-fixed tissues should be regarded as still infectious, even after prolonged
exposure to formalin.
8. Histological samples containing prions are substantially inactivated after exposure
to 96% formic acid for 1 h (24), (25).
9. Bench waste, including disposable gloves, gowns and aprons, should be autoclaved
using a porous load steam sterilizer at 134–137 °C for a single cycle of 18 min, or
six successive cycles of 3 min each, followed by incineration.
10. Non-disposable instruments, including steel mesh gloves, must be collected for
decontamination.
11. Infectious liquid waste contaminated with prions should be treated with sodium
hypochlorite containing available chlorine at 20 g/l (2%) (final concentration) for
1h.
12. Paraformaldehyde vaporization procedures do not diminish prion titres and prions
are resistant to ultraviolet irradiation. However, the cabinets must continue to be
decontaminated by standard methods (i.e. formaldehyde gas) to inactivate other
agents that may be present.
13. Prion-contaminated biological safety cabinets and other surfaces can be
decontaminated with sodium hypochlorite containing available chlorine at 20 g/l
(2%) for 1 h.
14. High-efficiency particulate air (HEPA) filters should be incinerated at a minimum
temperature of 1000 °C after removal. Recommended additional steps prior to
incineration include:
a. spraying of the exposed face of the filter with lacquer hairspray prior to removal,
b. “bagging” of filters during removal, and
c. removal of the HEPA filter from the working chamber so that the inaccessible
plenum of the cabinet is not contaminated.
15. Instruments should be soaked in sodium hypochlorite containing available chlorine
at 20 g/l (2%) for 1 h and then rinsed well in water before autoclaving.
16. Instruments that cannot be autoclaved can be cleaned by repeated wetting with
sodium hypochlorite containing available chlorine at 20 g/l (2%) over a 1-h period.
Appropriate washing to remove residual sodium hypochlorite is required.
For further information on the handling of unconventional agents see references (12),
(26) and (27).
12. LABORATORY TECHNIQUES
13. Contingency plans and
emergency procedures
Every laboratory that works with infective microorganisms should institute safety
precautions appropriate to the hazard of the organisms and the animals being handled.
A written contingency plan for dealing with laboratory and animal facility accidents
is a necessity in any facility that works with or stores Risk Group 3 or 4 microorganisms
(containment laboratory – Biosafety Level 3 and maximum containment laboratory –
Biosafety Level 4). National and/or local health authorities should be involved in the
development of the emergency preparedness plan.
Contingency plan
The contingency plan should provide operational procedures for:
1. Precautions against natural disasters, e.g. fire, flood, earthquake and explosion
2. Biohazard risk assessment
3. Incident-exposure management and decontamination
4. Emergency evacuation of people and animals from the premises
5. Emergency medical treatment of exposed and injured persons
6. Medical surveillance of exposed persons
7. Clinical management of exposed persons
8. Epidemiological investigation
9. Post-incident continuation of operations.
In the development of this plan the following items should be considered for inclusion:
1. Identification of high-risk organisms
2. Location of high-risk areas, e.g. laboratories, storage areas, animal facilities
3. Identification of at-risk personnel and populations
4. Identification of responsible personnel and their duties, e.g. biosafety officer, safety
personnel, local health authority, clinicians, microbiologists, veterinarians,
epidemiologists, and fire and police services
5. Lists of treatment and isolation facilities that can receive exposed or infected persons
6. Transport of exposed or infected persons
7. Lists of sources of immune serum, vaccines, drugs, special equipment and supplies
8. Provision of emergency equipment, e.g. protective clothing, disinfectants, chemical
and biological spill kits, decontamination equipment and supplies.
• 78 •
Emergency procedures for microbiological laboratories
Puncture wounds, cuts and abrasions
The affected individual should remove protective clothing, wash the hands and any
affected area(s), apply an appropriate skin disinfectant, and seek medical attention as
necessary. The cause of the wound and the organisms involved should be reported,
and appropriate and complete medical records kept.
Ingestion of potentially infectious material
Protective clothing should be removed and medical attention sought. Identification
of the material ingested and circumstances of the incident should be reported, and
appropriate and complete medical records kept.
Potentially infectious aerosol release (outside a biological safety cabinet)
All persons should immediately vacate the affected area and any exposed persons should
be referred for medical advice. The laboratory supervisor and the biosafety officer
should be informed at once. No one should enter the room for an appropriate amount
of time (e.g. 1 h), to allow aerosols to be carried away and heavier particles to settle. If
the laboratory does not have a central air exhaust system, entrance should be delayed
(e.g. for 24 h).
Signs should be posted indicating that entry is forbidden. After the appropriate
time, decontamination should proceed, supervised by the biosafety officer. Appropriate
protective clothing and respiratory protection should be worn.
Broken containers and spilled infectious substances
Broken containers contaminated with infectious substances and spilled infectious
substances should be covered with a cloth or paper towels. Disinfectant should then
be poured over these and left for the appropriate amount of time. The cloth or paper
towels and the broken material can then be cleared away; glass fragments should be
handled with forceps. The contaminated area should then be swabbed with disinfectant.
If dustpans are used to clear away the broken material, they should be autoclaved or
placed in an effective disinfectant. Cloths, paper towels and swabs used for cleaning
up should be placed in a contaminated-waste container. Gloves should be worn for all
these procedures.
If laboratory forms or other printed or written matter are contaminated, the
information should be copied onto another form and the original discarded into the
contaminated-waste container.
Breakage of tubes containing potentially infectious material in centrifuges
not having sealable buckets
If a breakage occurs or is suspected while the machine is running, the motor should be
switched off and the machine left closed (e.g. for 30 min) to allow settling. If a breakage
is discovered after the machine has stopped, the lid should be replaced immediately
13. CONTINGENCY PLANS AND EMERGENCY PROCEDURES
• 79 •
• 80 •
LABORATORY BIOSAFETY MANUAL
and left closed (e.g. for 30 min). In both instances, the biosafety officer should be
informed.
Strong (e.g. thick rubber) gloves, covered if necessary with suitable disposable gloves,
should be worn for all subsequent operations. Forceps, or cotton held in the forceps,
should be used to retrieve glass debris.
All broken tubes, glass fragments, buckets, trunnions and the rotor should be placed
in a noncorrosive disinfectant known to be active against the organisms concerned
(see Chapter 14). Unbroken, capped tubes may be placed in disinfectant in a separate
container and recovered.
The centrifuge bowl should be swabbed with the same disinfectant, at the appropriate
dilution, and then swabbed again, washed with water and dried. All materials used in
the clean-up should be treated as infectious waste.
Breakage of tubes inside sealable buckets (safety cups)
All sealed centrifuge buckets should be loaded and unloaded in a biological safety
cabinet. If breakage is suspected within the safety cup, the safety cap should be loosened
and the bucket autoclaved. Alternatively, the safety cup may be chemically disinfected.
Fire and natural disasters
Fire and other services should be involved in the development of emergency
preparedness plans. They should be told in advance which rooms contain potentially
infectious materials. It is beneficial to arrange for these services to visit the laboratory
to become acquainted with its layout and contents.
After a natural disaster, local or national emergency services should be warned of
the potential hazards within and/or near laboratory buildings. They should enter only
when accompanied by a trained laboratory worker. Infectious materials should be
collected in leakproof boxes or strong disposable bags.
Salvage or final disposal should be determined by biosafety staff on the basis of
local ordinances.
Emergency services: whom to contact
The telephone numbers and addresses of the following should be prominently displayed
in the facility:
1. The institution or laboratory itself (the address and location may not be known in
detail by the caller or the services called)
2. Director of the institution or laboratory
3. Laboratory supervisor
4. Biosafety officer
5. Fire services
6. Hospitals/ambulance services/medical staff (names of individual clinics,
departments, and/or medical staff, if possible)
• 81 •
7. Police
8. Medical officer
9. Responsible technician
10. Water, gas and electricity services.
Emergency equipment
The following emergency equipment must be available:
1. First-aid kit, including universal and special antidotes
2. Appropriate fire extinguishers, fire blankets
The following are also suggested but may be varied according to local circumstances:
1. Full protective clothing (one-piece coveralls, gloves and head covering – for
incidents involving microorganisms in Risk Groups 3 and 4)
2. Full-face respirators with appropriate chemical and particulate filter canisters
3. Room disinfection apparatus, e.g. sprays and formaldehyde vaporizers
4. Stretcher
5. Tools, e.g. hammers, axes, spanners, screwdrivers, ladders, ropes
6. Hazard area demarcation equipment and notices.
For further information see references (12) and (28).
13. CONTINGENCY PLANS AND EMERGENCY PROCEDURES
14. Disinfection and sterilization
A basic knowledge of disinfection and sterilization is crucial for biosafety in the
laboratory. Since heavily soiled items cannot promptly be disinfected or sterilized, it is
equally important to understand the fundamentals of cleaning prior to disinfection
(precleaning). In this regard, the following general principles apply to all known classes
of microbial pathogens.
Specific decontamination requirements will depend on the type of experimental
work and the nature of the infectious agent(s) being handled. The generic information
given here can be used to develop both standardized and more specific procedures to
deal with biohazard(s) involved in a particular laboratory.
Contact times for disinfectants are specific for each material and manufacturer.
Therefore, all recommendations for use of disinfectants should follow manufacturers’
specifications.
Definitions
Many different terms are used for disinfection and sterilization. The following are
among the more common in biosafety:
Antimicrobial – An agent that kills microorganisms or suppresses their growth and
multiplication.
Antiseptic – A substance that inhibits the growth and development of microorganisms
without necessarily killing them. Antiseptics are usually applied to body surfaces.
Biocide – A general term for any agent that kills organisms.
Chemical germicide – A chemical or a mixture of chemicals used to kill micro-
organisms.
Decontamination – Any process for removing and/or killing microorganisms. The
same term is also used for removing or neutralizing hazardous chemicals and radio-
active materials.
Disinfectant – A chemical or mixture of chemicals used to kill microorganisms, but
not necessarily spores. Disinfectants are usually applied to inanimate surfaces or
objects.
Disinfection – A physical or chemical means of killing microorganisms, but not
necessarily spores.
Microbicide – A chemical or mixture of chemicals that kills microorganisms. The
term is often used in place of “biocide”, “chemical germicide” or “antimicrobial”.
• 82 •
Sporocide – A chemical or mixture of chemicals used to kill microorganisms and
spores.
Sterilization – A process that kills and/or removes all classes of microorganisms and
spores.
Cleaning laboratory materials
Cleaning is the removal of dirt, organic matter and stains. Cleaning includes brushing,
vacuuming, dry dusting, washing or damp mopping with water containing a soap or
detergent. Dirt, soil and organic matter can shield microorganisms and can interfere
with the killing action of decontaminants (antiseptics, chemical germicides and
disinfectants).
Precleaning is essential to achieve proper disinfection or sterilization. Many germi-
cidal products claim activity only on precleaned items. Precleaning must be carried
out with care to avoid exposure to infectious agents.
Materials chemically compatible with the germicides to be applied later must be
used. It is quite common to use the same chemical germicide for precleaning and
disinfection.
Chemical germicides
Many types of chemicals can be used as disinfectants and/or antiseptics. As there is an
ever-increasing number and variety of commercial products, formulations must be
carefully selected for specific needs.
The germicidal activity of many chemicals is faster and better at higher temperatures.
At the same time, higher temperatures can accelerate their evaporation and also degrade
them. Particular care is needed in the use and storage of such chemicals in tropical
regions, where their shelf-life may be reduced because of high ambient temperatures.
Many germicides can be harmful to humans or the environment. They should be
selected, stored, handled, used and disposed of with care, following manufacturers’
instructions. For personal safety, gloves, aprons and eye protection are recommended
when preparing dilutions of chemical germicides.
Chemical germicides are generally not required for regular cleaning of floors, walls,
equipment and furniture. However, their use may be appropriate in certain cases of
outbreak control.
Proper use of chemical germicides will contribute to workplace safety while reducing
the risk from infectious agents. As far as possible, the number of germicidal chemicals
to be used should be limited for economic reasons, inventory control and to limit
environmental pollution.
Commonly used classes of chemical germicides are described below, with generic
information on their applications and safety profiles. Unless otherwise indicated, the
germicide concentrations are given in weight/volume (w/v). Table 12 summarizes the
recommended dilutions of chlorine-releasing compounds.
14. DISINFECTION AND STERILIZATION
• 83 •

• 84 •
LABORATORY BIOSAFETY MANUAL
Chlorine (sodium hypochlorite)
Chlorine, a fast-acting oxidant, is a widely available and broad-spectrum chemical
germicide. It is normally sold as bleach, an aqueous solution of sodium hypochlorite
(NaOCl), which can be diluted with water to provide various concentrations of available
chlorine.
Chlorine, especially as bleach, is highly alkaline and can be corrosive to metal. Its
activity is considerably reduced by organic matter (protein). Storage of stock or working
solutions of bleach in open containers, particularly at high temperatures, releases
chlorine gas thus weakening their germicidal potential. The frequency with which
working solutions of bleach should be changed depends on their starting strength, the
type (e.g. with or without a lid) and size of their containers, the frequency and nature
of use, and ambient conditions. As a general guide, solutions receiving materials with
high levels of organic matter several times a day should be changed at least daily, while
those with less frequent use may last for as long as a week.
A general all-purpose laboratory disinfectant should have a concentration of 1 g/l
available chlorine. A stronger solution, containing 5 g/l available chlorine, is recom-
mended for dealing with biohazardous spillage and in the presence of large amounts
of organic matter. Sodium hypochlorite solutions, as domestic bleach, contain 50 g/l
available chlorine and should therefore be diluted 1:50 or 1:10 to obtain final concen-
trations of 1 g/l and 5 g/l, respectively. Industrial solutions of bleach have a sodium
hypochlorite concentration of nearly 120 g/l and must be diluted accordingly to obtain
the levels indicated above.
Granules or tablets of calcium hypochlorite (Ca(ClO)2) generally contain about
70% available chlorine. Solutions prepared with granules or tablets, containing 1.4 g/l and
7.0 g/l, will then contain 1.0 g/l and 5 g/l available chlorine, respectively.
Bleach is not recommended as an antiseptic, but may be used as a general-purpose
Table 12. Recommended dilutions of chlorine-releasing compounds
“CLEAN” CONDITIONSa“DIRTY” CONDITIONSb
Available chlorine required 0.1% (1 g/l) 0.5% (5 g/l)
Sodium hypochlorite solution (5% available chlorine) 20 ml/l 100 ml/l
Calcium hypochlorite (70% available chlorine) 1.4 g/l 7.0 g/l
Sodium dichloroisocyanurate powder 1.7 g/l 8.5 g/l
(60% available chlorine)
Sodium dichloroisocyanurate tablets 1 tablet 4 tablets
(1.5 g available chlorine per tablet) per litre per litre
Chloramine (25% available chlorine)c20 g/l 20 g/l
aAfter removal of bulk material.
bFor flooding, e.g. on blood or before removal of bulk material.
cSee text.
• 85 •
14. DISINFECTION AND STERILIZATION
disinfectant and for soaking contaminated metal-free materials. In emergencies, bleach
can also be used to disinfect water for drinking, with a final concentration of 1–2 mg/l
available chlorine.
Chlorine gas is highly toxic. Bleach must therefore be stored and used in well-
ventilated areas only. Also, bleach must not be mixed with acids to prevent the rapid
release of chlorine gas. Many by-products of chlorine can be harmful to humans and
the environment, so that indiscriminate use of chlorine-based disinfectants, in
particular bleach, should be avoided.
Sodium dichloroisocyanurate
Sodium dichloroisocyanurate (NaDCC) in powder form contains 60% available
chlorine. Solutions prepared with NaDCC powder at 1.7 g/l and 8.5 g/l will contain
1g/l or 5 g/l available chlorine, respectively. Tablets of NaDCC generally contain the
equivalent of 1.5 g available chlorine per tablet. One or four tablets dissolved in 1 l of
water will give approximately the required concentrations of 1 g/l or 5 g/l, respectively.
NaDCC as powder or tablets is easy and safe to store. Solid NaDCC can be applied on
spills of blood or other biohazardous liquids and left for at least 10 min before removal.
Further cleaning of the affected area can then take place.
Chloramines
Chloramines are available as powders containing about 25% available chlorine.
Chloramines release chlorine at a slower rate than hypochlorites. Higher initial
concentrations are therefore required for efficiencies equivalent to those of
hypochlorites. On the other hand, chloramine solutions are not inactivated by organic
matter to the same extent as hypochlorite solutions, and concentrations of 20 g/l are
recommended for both “clean” and “dirty” situations.
Chloramine solutions are virtually odour-free. However, items soaked in them must
be thoroughly rinsed to remove any residue of the bulking agents added to chloramine-
T (sodium tosylchloramide) powders.
Chlorine dioxide
Chlorine dioxide (ClO2) is a strong and fast-acting germicide, disinfectant agent and
oxidizer, often reported to be active at concentrations levels lower than those needed
by chlorine as bleach. Chlorine dioxide is unstable as a gas and will undergo
decomposition into chlorine gas (Cl2), oxygen gas (O2), giving off heat. However,
chlorine dioxide is soluble in water and stable in an aqueous solution. Chlorine dioxide
can be obtained in two ways: (1) on-site generation by mixing of two separate
components, hydrochloric acid (HCl) and sodium chlorite (NaClO2); and (2) ordering
its stabilized form, which is then activated on-site when required.
Of the oxidizing biocides, chlorine dioxide is the most selective oxidant. Ozone and
chlorine are much more reactive than chlorine dioxide, and they will be consumed by
most organic compounds. Chlorine dioxide, however, reacts only with reduced sulfur
• 86 •
LABORATORY BIOSAFETY MANUAL
compounds, secondary and tertiary amines, and some other highly reduced and reactive
organic compounds. A more stable residue can therefore be achieved with chlorine
dioxide at much lower doses than when using either chlorine or ozone. Generated
properly, chlorine dioxide can be used more effectively than ozone or chlorine in cases
of higher organic loading because of its selectivity.
Formaldehyde
Formaldehyde (HCHO) is a gas that kills all microorganisms and spores at temperatures
above 20 °C. However, it is not active against prions.
Formaldehyde is relatively slow-acting and needs a relative humidity level of about
70%. It is marketed as the solid polymer, paraformaldehyde, in flakes or tablets, or as
formalin, a solution of the gas in water of about 370 g/l (37%), containing methanol
(100 ml/l) as a stabilizer. Both formulations are heated to liberate the gas, which is
used for decontamination and disinfection of enclosed volumes such as safety cabinets
and rooms (see section on Local environmental decontamination in this chapter).
Formaldehyde (5% formalin in water) may be used as a liquid disinfectant.
Formaldehyde is a suspected carcinogen. It is a dangerous, irritant gas that has a
pungent smell and its fumes can irritate eyes and mucous membranes. It must therefore
be stored and used in a fume-hood or well-ventilated area. National chemical safety
regulations must be followed.
Glutaraldehyde
Like formaldehyde, glutaraldehyde (OHC(CH2)3CHO) is also active against vegetative
bacteria, spores, fungi and lipid- and nonlipid-containing viruses. It is non-corrosive
and faster acting than formaldehyde. However, it takes several hours to kill bacterial
spores.
Glutaraldehyde is generally supplied as a solution with a concentration of about
20 g/l (2%) and some products may need to be “activated” (made alkaline) before use
by the addition of a bicarbonate compound supplied with the product. The activated
solution can be reused for 1–4 weeks depending on the formulation and type and
frequency of its use. Dipsticks supplied with some products give only a rough indication
of the levels of active glutaraldehyde available in solutions under use. Glutaraldehyde
solutions should be discarded if they become turbid.
Glutaraldehyde is toxic and an irritant to skin and mucous membranes, and contact
with it must be avoided. It must be used in a fume-hood or in well-ventilated areas. It
is not recommended as a spray or solution for the decontamination of environmental
surfaces. National chemical safety regulations must be followed.
Phenolic compounds
Phenolic compounds, a broad group of agents, were among the earliest germicides.
However, more recent safety concerns restrict their use. They are active against veg-
etative bacteria and lipid-containing viruses and, when properly formulated, also show
• 87 •
activity against mycobacteria. They are not active against spores and their activity
against nonlipid viruses is variable. Many phenolic products are used for the decon-
tamination of environmental surfaces, and some (e.g. triclosan and chloroxylenol)
are among the more commonly used antiseptics.
Triclosan is common in products for hand-washing. It is active mainly against
vegetative bacteria and safe for skin and mucous membranes. However, in laboratory-
based studies, bacteria made resistant to low concentrations of triclosan also show
resistance to certain types of antibiotics. The significance of this finding in the field
remains unknown.
Some phenolic compounds are sensitive to and may be inactivated by water hardness
and therefore must be diluted with distilled or deionized water.
Phenolic compounds are not recommended for use on food contact surfaces and
in areas with young children. They may be absorbed by rubber and can also penetrate
the skin. National chemical safety regulations must be followed.
Quaternary ammonium compounds
Many types of quaternary ammonium compounds are used as mixtures and often in
combination with other germicides, such as alcohols. They have good activity against
some vegetative bacteria and lipid-containing viruses. Certain types (e.g. benzalkonium
chloride) are used as antiseptics.
The germicidal activity of certain types of quaternary ammonium compounds is
considerably reduced by organic matter, water hardness and anionic detergents. Care
is therefore needed in selecting agents for precleaning when quaternary ammonium
compounds are to be used for disinfection. Potentially harmful bacteria can grow in
quaternary ammonium compound solutions. Owing to low biodegradability, these
compounds may also accumulate in the environment.
Alcohols
Ethanol (ethyl alcohol, C2H5OH) and 2-propanol (isopropyl alcohol, (CH3)2CHOH)
have similar disinfectant properties. They are active against vegetative bacteria, fungi
and lipid-containing viruses but not against spores. Their action on nonlipid viruses
is variable. For highest effectiveness they should be used at concentrations of
approximately 70% (v/v) in water: higher or lower concentrations may not be as
germicidal. A major advantage of aqueous solutions of alcohols is that they do not
leave any residue on treated items.
Mixtures with other agents are more effective than alcohol alone, e.g. 70% (v/v)
alcohol with 100 g/l formaldehyde, and alcohol containing 2 g/l available chlorine. A
70% (v/v) aqueous solution of ethanol can be used on skin, work surfaces of laboratory
benches and biosafety cabinets, and to soak small pieces of surgical instruments. Since
ethanol can dry the skin, it is often mixed with emollients. Alcohol-based hand-rubs
are recommended for the decontamination of lightly soiled hands in situations where
proper hand-washing is inconvenient or not possible. However, it must be remembered
14. DISINFECTION AND STERILIZATION
• 88 •
LABORATORY BIOSAFETY MANUAL
that ethanol is ineffective against spores and may not kill all types of nonlipid viruses.
Alcohols are volatile and flammable and must not be used near open flames. Working
solutions should be stored in proper containers to avoid the evaporation of alcohols.
Alcohols may harden rubber and dissolve certain types of glue. Proper inventory and
storage of ethanol in the laboratory is very important to avoid its use for purposes
other than disinfection. Bottles with alcohol-containing solutions must be clearly
labelled to avoid autoclaving.
Iodine and iodophors
The action of these disinfectants is similar to that of chlorine, although they may be
slightly less inhibited by organic matter. Iodine can stain fabrics and environmental
surfaces and is generally unsuitable for use as a disinfectant. On the other hand,
iodophors and tinctures of iodine are good antiseptics. Polyvidone-iodine is a reliable
and safe surgical scrub and preoperative skin antiseptic. Antiseptics based on iodine
are generally unsuitable for use on medical/dental devices. Iodine should not be used
on aluminium or copper.
Iodine can be toxic. Organic iodine-based products must be stored at 4–10 °C to
avoid the growth of potentially harmful bacteria in them.
Hydrogen peroxide and peracids
Like chlorine, hydrogen peroxide (H2O2) and peracids are strong oxidants and can be
potent broad-spectrum germicides. They are also safer than chlorine to humans and
the environment.
Hydrogen peroxide is supplied either as a ready-to-use 3% solution or as a 30%
aqueous solution to be diluted to 5–10 times its volume with sterilized water. However,
such 3–6% solutions of hydrogen peroxide alone are relatively slow and limited as
germicides. Products now available have other ingredients to stabilize the hydrogen
peroxide content, to accelerate its germicidal action and to make it less corrosive.
Hydrogen peroxide can be used for the decontamination of work surfaces of
laboratory benches and biosafety cabinets, and stronger solutions may be suitable for
disinfecting heat-sensitive medical/dental devices. The use of vaporized hydrogen
peroxide or peracetic acid (CH3COOOH) for the decontamination of heat-sensitive
medical/surgical devices requires specialized equipment.
Hydrogen peroxide and peracids can be corrosive to metals such as aluminium,
copper, brass and zinc, and can also decolorize fabrics, hair, skin and mucous
membranes. Articles treated with them must be thoroughly rinsed before contact with
eyes and mucous membranes. They should always be stored away from heat and
protected from light.
Local environmental decontamination
Decontamination of the laboratory space, its furniture and its equipment requires a
combination of liquid and gaseous disinfectants. Surfaces can be decontaminated using
• 89 •
a solution of sodium hypochlorite (NaOCl); a solution containing 1 g/l available
chlorine may be suitable for general environmental sanitation, but stronger solutions
(5 g/l) are recommended when dealing with high-risk situations. For environmental
decontamination, formulated solutions containing 3% hydrogen peroxide (H2O2) make
suitable substitutes for bleach solutions.
Rooms and equipment can be decontaminated by fumigation with formaldehyde
gas generated by heating paraformaldehyde or boiling formalin. This is a highly
dangerous process that requires specially trained personnel. All openings in the room
(i.e. windows, doors, etc.) should be sealed with masking tape or similar before the gas
is generated. Fumigation should be conducted at an ambient temperature of at least
21 °C and a relative humidity of 70%. (See also section on Decontamination of
biological safety cabinets in this chapter.)
After fumigation the area must be ventilated thoroughly before personnel are allowed
to enter. Appropriate respirators must be worn by anyone entering the room before it
has been ventilated. Gaseous ammonium bicarbonate can be used to neutralize the
formaldehyde.
Fumigation of smaller spaces with hydrogen peroxide vapour is also effective but
requires specialized equipment to generate the vapour.
Decontamination of biological safety cabinets
To decontaminate Class I and Class II cabinets, equipment that independently generates,
circulates and neutralizes formaldehyde gas is available. Alternatively, the appropriate
amount of paraformaldehyde (final concentration of 0.8% paraformaldehyde in air)
should be placed in a frying pan on an electric hot plate. Another frying pan, containing
10% more ammonium bicarbonate than paraformaldehyde, on a second hot plate is
also placed inside the cabinet. The hot plate leads are plugged in outside the cabinet,
so that operation of the pans can be controlled from the outside by plugging and
unplugging the hot plates as necessary. If the relative humidity is below 70%, an open
container of hot water should also be placed inside the cabinet before the front closure
is sealed in place with strong tape (e.g. duct tape). Heavy gauge plastic sheeting is
taped over the front opening and exhaust port to make sure that the gas cannot seep
into the room. Penetration of the electric leads passing through the front closure must
also be sealed with duct tape.
The plate for the paraformaldehyde pan is plugged in. It is unplugged when all the
paraformaldehyde has vaporized. The cabinet is left undisturbed for at least 6 h. The
plate for the second pan is then plugged in and the ammonium bicarbonate is allowed
to vaporize. This plate is then unplugged and the cabinet blower is switched on for
two intervals of approximately 2 s each to allow the ammonium bicarbonate gas to
circulate. The cabinet should be left undisturbed for 30 min before the front closure
(or plastic sheeting) and the exhaust port sheeting are removed. The cabinet surfaces
should be wiped down to remove residues before use.
14. DISINFECTION AND STERILIZATION
• 90 •
LABORATORY BIOSAFETY MANUAL
Hand-washing/hand decontamination
Whenever possible, suitable gloves should be worn when handling biohazardous
materials. However, this does not replace the need for regular and proper hand-washing
by laboratory personnel. Hands must be washed after handling biohazardous materials
and animals, and before leaving the laboratory.
In most situations, thorough washing of hands with ordinary soap and water is
sufficient to decontaminate them, but the use of germicidal soaps is recommended in
high-risk situations. Hands should be thoroughly lathered with soap, using friction,
for at least 10 s, rinsed in clean water and dried using a clean paper or cloth towel (if
available, warm-air hand-dryers may be used).
Foot- or elbow-operated faucets are recommended. Where not fitted, a paper/cloth
towel should be used to turn off the faucet handles to avoid recontaminating washed
hands.
As mentioned above, alcohol-based hand-rubs may be used to decontaminate lightly
soiled hands when proper hand-washing is not available.
Heat disinfection and sterilization
Heat is the most common among the physical agents used for the decontamination of
pathogens. “Dry” heat, which is totally non-corrosive, is used to process many items
of laboratory ware which can withstand temperatures of 160 °C or higher for 2–4 h.
Burning or incineration (see below) is also a form of dry heat. “Moist” heat is most
effective when used in the form of autoclaving.
Boiling does not necessarily kill all microorganisms and/or pathogens, but it may
be used as the minimum processing for disinfection where other methods (chemical
disinfection or decontamination, autoclaving) are not applicable or available.
Sterilized items must be handled and stored such that they remain uncontaminated
until used.
Autoclaving
Saturated steam under pressure (autoclaving) is the most effective and reliable means
of sterilizing laboratory materials. For most purposes, the following cycles will ensure
sterilization of correctly loaded autoclaves:
1. 3 min holding time at 134 °C
2. 10 min holding time at 126 °C
3. 15 min holding time at 121 °C
4. 25 min holding time at 115 °C.
Examples of different autoclaves include the following.
Gravity displacement autoclaves. Figure 10 shows the general construction of a gravity-
displacement autoclave. Steam enters the chamber under pressure and displaces the
heavier air downwards and through the valve in the chamber drain, fitted with a HEPA
filter.
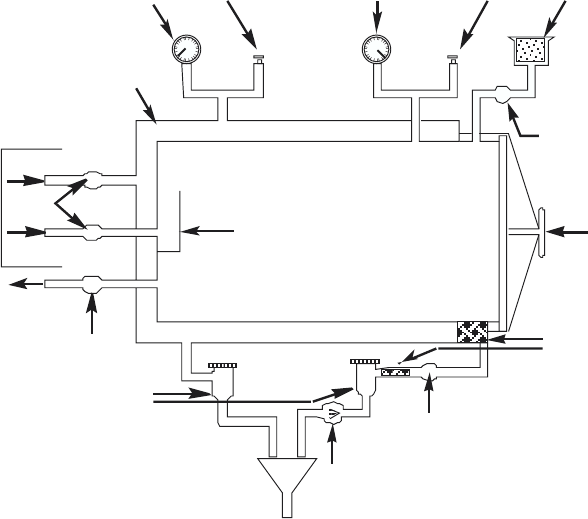
• 91 •
Pre-vacuum autoclaves. These machines allow the removal of air from the chamber before
steam is admitted. The exhaust air is evacuated through a valve fitted with a HEPA
filter. At the end of the cycle, the steam is automatically exhausted. These autoclaves
can operate at 134 °C and the sterilization cycle can therefore be reduced to 3 min. They
are ideal for porous loads, but cannot be used to process liquids because of the vacuum.
Fuel-heated pressure cooker autoclaves. These should be used only if a gravity displace-
ment autoclave is not available. They are loaded from the top and heated by gas,
electricity or other types of fuels. Steam is generated by heating water in the base of
the vessel, and air is displaced upwards through a relief vent. When all the air has been
removed, the valve on the relief vent is closed and the heat reduced. The pressure and
temperature rise until the safety valve operates at a preset level. This is the start of the
holding time. At the end of the cycle the heat is turned off and the temperature allowed
to fall to 80 °C or below before the lid is opened.
Loading autoclaves
Materials should be loosely packed in the chamber for easy steam penetration and air
removal. Bags should allow the steam to reach their contents.
14. DISINFECTION AND STERILIZATION
Figure 10. Gravity displacement autoclave
pressure
gauge
baffle
to jacket
valve
steam supply
to chamber
Valve
to vacuum pump
or steam ejector
near-to-steam traps
strainers
non-return valve
door
cotton wool
filter
valve
safety
valve
combined pressure
and vacuum gauge
safety
valve
chamber
valve
Jacket
WHO 02.139
• 92 •
LABORATORY BIOSAFETY MANUAL
Precautions in the use of autoclaves
The following rules can minimize the hazards inherent in operating pressurized vessels.
1. Responsibility for operation and routine care should be assigned to trained
individuals.
2. A preventive maintenance programme should include regular inspection of the
chamber, door seals and all gauges and controls by qualified personnel.
3. The steam should be saturated and free from chemicals (e.g. corrosion inhibitors)
that could contaminate the items being sterilized.
4. All materials to be autoclaved should be in containers that allow ready removal of
air and permit good heat penetration; the chamber should be loosely packed so
that steam will reach the load evenly.
5. For autoclaves without an interlocking safety device that prevents the door being
opened when the chamber is pressurized, the main steam valve should be closed
and the temperature allowed to fall below 80 °C before the door is opened.
6. Slow exhaust settings should be used when autoclaving liquids, as they may boil
over when removed due to superheating.
7. Operators should wear suitable gloves and visors for protection when opening the
autoclave, even when the temperature has fallen below 80 °C.
8. In any routine monitoring of autoclave performance, biological indicators or
thermocouples should be placed at the centre of each load. Regular monitoring
with thermocouples and recording devices in a “worst case” load is highly desirable
to determine proper operating cycles.
9. The drain screen filter of the chamber (if available) should be removed and cleaned
daily.
10. Care should be taken to ensure that the relief valves of pressure cooker autoclaves
do not become blocked by paper, etc. in the load.
Incineration
Incineration is useful for disposing of animal carcasses as well as anatomical and other
laboratory waste, with or without prior decontamination (see Chapter 3). Incineration
of infectious materials is an alternative to autoclaving only if the incinerator is under
laboratory control.
Proper incineration requires an efficient means of temperature control and a
secondary burning chamber. Many incinerators, especially those with a single
combustion chamber, are unsatisfactory for dealing with infectious materials, animal
carcasses and plastics. Such materials may not be completely destroyed and the effluent
from the chimney may pollute the atmosphere with microorganisms, toxic chemicals
and smoke. However, there are many satisfactory configurations for combustion
chambers. Ideally the temperature in the primary chamber should be at least 800 °C
and that in the secondary chamber at least 1000 °C.
Materials for incineration, even with prior decontamination, should be transported
• 93 •
to the incinerator in bags, preferably plastic. Incinerator attendants should receive
proper instructions about loading and temperature control. It should also be noted
that the efficient operation of an incinerator depends heavily on the right mix of
materials in the waste being treated.
There are ongoing concerns regarding the possible negative environmental effects
of existing or proposed incinerators, and efforts continue to make incinerators more
environmentally friendly and energy-efficient.
Disposal
The disposal of laboratory and medical waste is subject to various regional, national
and international regulations, and the latest versions of such relevant documents must
be consulted before designing and implementing a programme for handling,
transportation and disposal of biohazardous waste. In general, ash from incinerators
may be handled as normal domestic waste and removed by local authorities. Autoclaved
waste may be disposed of by off-site incineration or in licensed landfill sites (see
Chapter 3).
For further information see references (13) and (29–39).
14. DISINFECTION AND STERILIZATION
15. Introduction to the transport
of infectious substances
Transport of infectious and potentially infectious materials is subject to strict national
and international regulations. These regulations describe the proper use of packaging
materials, as well as other shipping requirements.
Laboratory personnel must ship infectious substances according to applicable
transport regulations. Compliance with the rules will:
1. Reduce the likelihood that packages will be damaged and leak, and thereby
2. Reduce the exposures resulting in possible infections
3. Improve the efficiency of package delivery.
International transport regulations
The regulations for the transport of infectious materials (by any mode of transport)
are based upon the United Nations Model Regulations on the Transport of Dangerous
Goods (40). These recommendations are developed by the United Nations Committee
of Experts on the Transport of Dangerous Goods (UNCETDG). To become legally
binding, the United Nations Model Regulations have to be introduced into national
regulations and international modal regulations by the competent authorities (e.g.
the Technical Instructions for the Safe Transport of Dangerous Goods by Air (41) of the
International Civil Aviation Organization (ICAO) for air transport and the European
Agreement concerning the International Carriage of Dangerous Goods by Road (ADR)
(42).
The International Air Transport Association (IATA) issues Infectious Substances
Shipping Guidelines (43) every year. IATA guidelines must follow ICAO’s Technical
Instructions as a minimal standard, but may impose additional restrictions. IATA
guidelines must be followed if a shipment is carried by members of IATA.
Since the United Nations Model Regulations on the Transport of Dangerous Goods is
a dynamic set of recommendations subject to biennial amendments, the reader is
referred to the latest issuances of national and international modal regulations for
applicable regulatory texts.
WHO serves in an advisory capacity to UNCETDG. Major changes to the transport
regulations pertaining to the transport of infectious substances were introduced into
the 13th edition (2003) of the United Nations Model Regulations (40). Guidance on
the background to adopted amendments is available from WHO (44).
• 94 •
International modal regulations are not intended to supersede any local or national
requirements. However, in situations where national requirements do not exist,
international modal regulations should be followed.
It is important to note that international transport of infectious substances is also
dependent on and subject to national import/export regulations.
The basic triple packaging system
The triple packaging system, the choice for the transport of infectious and potentially
infectious substances, is exemplified in Figure 11. This packaging system consists of
three layers: the primary receptacle, the secondary packaging and the outer packaging.
The primary receptacle containing the specimen must be watertight, leakproof and
appropriately labelled as to content. The primary receptacle is wrapped in enough
absorbent material to absorb all fluid in case of breakage or leakage.
A second watertight, leakproof packaging is used to enclose and protect the primary
receptacle(s). Several wrapped primary receptacles may be placed in a single secondary
packaging. Volume and/or weight limits for packaged infectious substances are included
in certain regulatory texts.
The third layer protects the secondary packaging from physical damage while in
transit. Specimen data forms, letters and other types of information that identify or
describe the specimen and identify the shipper and receiver, and any other
documentation required, must also be provided according to latest regulations.
The United Nations Model Regulations prescribe the use of two different triple
packaging systems. The basic triple packaging system applies for the transport of a
variety of infectious substances; however, high-risk organisms must be shipped
according to more stringent requirements. For further details about the use of the
different packagings according to the materials to be transported, it is advisable to
consult national and/or international modal regulations for applicable regulatory texts.
Spill clean-up procedure
In the event of a spill of infectious or potentially infectious material, the following
spill clean-up procedure should be used.
1. Wear gloves and protective clothing, including face and eye protection if indicated.
2. Cover the spill with cloth or paper towels to contain it.
3. Pour an appropriate disinfectant over the paper towels and the immediately
surrounding area (generally, 5% bleach solutions are appropriate; but for spills on
aircraft, quaternary ammonium disinfectants should be used).
4. Apply disinfectant concentrically beginning at the outer margin of the spill area,
working toward the centre.
5. After the appropriate amount of time (e.g. 30 min), clear away the materials. If
there is broken glass or other sharps involved, use a dustpan or a piece of stiff
cardboard to collect the material and deposit it into a puncture-resistant container
for disposal.
15. INTRODUCTION TO THE TRANSPORT OF INFECTIOUS SUBSTANCES
• 95 •
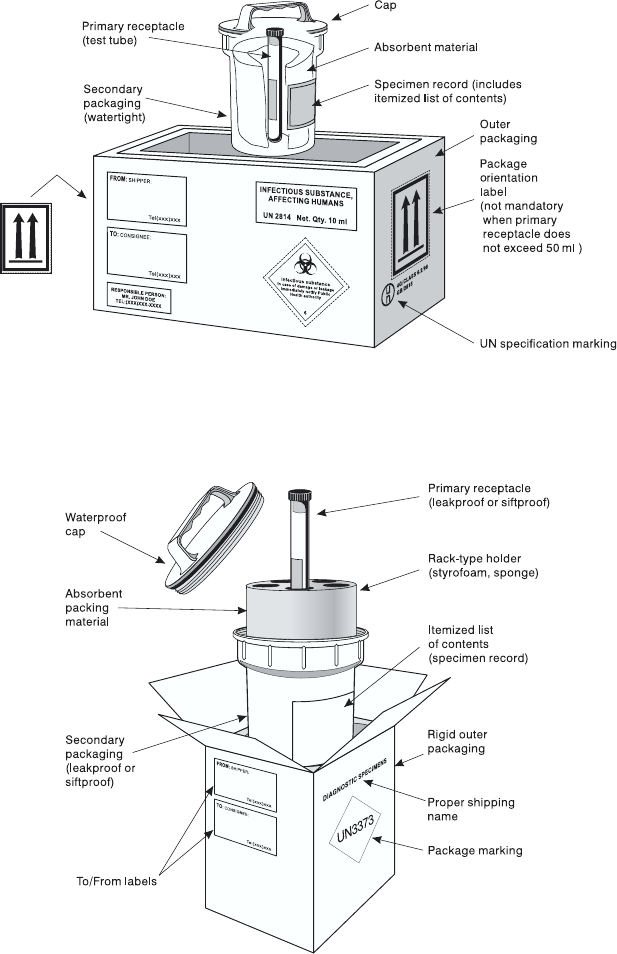
• 96 •
LABORATORY BIOSAFETY MANUAL
Figure 11. Examples of triple packaging systems
(graphics kindly provided by IATA, Montreal, Canada)
Packing and labelling of Category A infectious substances
Packing and labelling of Category B infectious substances
• 97 •
6. Clean and disinfect the area of the spillage (if necessary, repeat steps 2–5).
7. Dispose of contaminated materials into a leakproof, puncture-resistant waste
disposal container.
8. After successful disinfection, inform the competent authority that the site has now
been decontaminated
15. INTRODUCTION TO THE TRANSPORT OF INFECTIOUS SUBSTANCES


PART V
Introduction to
biotechnology
16. Biosafety and recombinant
DNA technology
• 101 •
Recombinant DNA technology involves combining genetic material from different
sources thereby creating genetically modified organisms (GMOs) that may have never
existed in nature before. Initially there was concern among molecular biologists that
such organisms might have unpredictable and undesirable properties that could
represent a biohazard if they escaped from the laboratory. This concern became the
focus of a scientific conference held in Asilomar, CA, USA, in 1975 (45). At that meeting,
safety issues were discussed and the first guidelines for recombinant DNA technology
were proposed. The subsequent 25+ years of research experience have demonstrated
that genetic engineering may be conducted in a safe manner when an appropriate risk
assessment is performed and adequate safety measures are used.
Recombinant DNA technology or genetic engineering was first used to clone DNA
segments in bacterial hosts in order to overexpress specific gene products for further
studies. Recombinant DNA molecules have also been used to create GMOs such as
transgenic and “knock-out” animals and transgenic plants.
Recombinant DNA technology has already had an enormous impact on biology
and medicine, and will probably have an even greater influence now that the nucleotide
sequence of the entire human genome is available. Tens of thousands of genes of yet
unknown functions will be studied using recombinant DNA technology. Gene therapy
may become a routine treatment for certain diseases, and new vectors for gene transfer
are likely to be devised using genetic engineering techniques. Also, transgenic plants
produced by recombinant DNA technology may play an increasingly important role
in modern agriculture.
Experiments involving the construction or use of GMOs should be conducted after
performing a biosafety risk assessment. The pathogenic properties and any potential
hazards associated with such organisms may be novel and not well-characterized. The
properties of the donor organism, the nature of the DNA sequences that will be
transferred, the properties of the recipient organism, and the properties of the
environment should be evaluated. These factors should help determine the biosafety
level that is required for the safe handling of the resulting GMO, and identify the
biological and physical containment systems that should be used.
• 102 •
LABORATORY BIOSAFETY MANUAL
Biosafety considerations for biological expression systems
Biological expression systems consist of vectors and host cells. A number of criteria
must be satisfied to make them effective and safe to use. An example of such a biological
expression system is plasmid pUC18. Frequently used as a cloning vector in
combination with Escherichia coli K12 cells, the pUC18 plasmid has been entirely
sequenced. All genes required for expression in other bacteria have been deleted from
its precursor plasmid pBR322. E. coli K12 is a non-pathogenic strain that cannot
permanently colonize the gut of healthy humans or animals. Routine genetic
engineering experiments can safely be performed in E. coli K12/pUC18 at Biosafety
Level 1, provided the inserted foreign DNA expression products do not require higher
biosafety levels.
Biosafety considerations for expression vectors
Higher biosafety levels may be required when:
1. The expression of DNA sequences derived from pathogenic organisms may increase
the virulence of the GMO
2. Inserted DNA sequences are not well characterized, e.g. during preparation of
genomic DNA libraries from pathogenic microorganisms
3. Gene products have potential pharmacological activity
4. Gene products code for toxins.
Viral vectors for gene transfer
Viral vectors, e.g. adenovirus vectors, are used for the transfer of genes to other cells.
Such vectors lack certain virus replication genes and are propagated in cell lines that
complement the defect.
Stocks of such vectors may be contaminated with replication-competent viruses,
generated by rare spontaneous recombination events in the propagating cell lines, or
may derive from insufficient purification. These vectors should be handled at the same
biosafety level as the parent adenovirus from which they are derived.
Transgenic and “knock-out” animals
Animals carrying foreign genetic material (transgenic animals) should be handled in
containment levels appropriate to the characteristics of the products of the foreign
genes. Animals with targeted deletions of specific genes (“knock-out” animals) do not
generally present particular biological hazards.
Examples of transgenic animals include animals expressing receptors for viruses
normally unable to infect that species. If such animals escaped from the laboratory
and transmitted the transgene to the wild animal population, an animal reservoir for
that particular virus could theoretically be generated.
This possibility has been discussed for poliovirus and is particularly relevant in the
context of poliomyelitis eradication. Transgenic mice expressing the human poliovirus
• 103 •
receptor generated in different laboratories were susceptible to poliovirus infection
by various inoculation routes and the resulting disease was clinically and histo-
pathologically similar to human poliomyelitis. However, the mouse model differs from
humans in that alimentary tract replication of orally administered poliovirus is either
inefficient or does not occur. It is therefore very unlikely that escape of such transgenic
mice to the wild would result in the establishment of a new animal reservoir for
poliovirus. Nevertheless, this example indicates that, for each new line of transgenic
animal, detailed studies should be conducted to determine the routes by which the
animals can be infected, the inoculum size required for infection, and the extent of
virus shedding by the infected animals. In addition, all measures should be taken to
assure strict containment of receptor transgenic mice.
Transgenic plants
Transgenic plants expressing genes that confer tolerance to herbicides or resistance to
insects are currently a matter of considerable controversy in many parts of the world.
The discussions focus on the food-safety of such plants, and on the long-term ecological
consequences of their cultivation.
Transgenic plants expressing genes of animal or human origin are used to develop
medicinal and nutritional products. A risk assessment should determine the appropriate
biosafety level for the production of these plants.
Risk assessments for genetically modified organisms
Risk assessments for work with GMOs should consider the characteristics of donor
and recipient/host organisms.
Examples of characteristics for consideration include the following.
Hazards arising directly from the inserted gene (donor organism)
Assessment is necessary in situations where the product of the inserted gene has known
biologically or pharmacologically active properties that may give rise to harm, for
example:
1. Toxins
2. Cytokines
3. Hormones
4. Gene expression regulators
5. Virulence factors or enhancers
6. Oncogenic gene sequences
7. Antibiotic resistance
8. Allergens.
The consideration of such cases should include an estimation of the level of expression
required to achieve biological or pharmacological activity.
16. BIOSAFETY AND RECOMBINANT DNA TECHNOLOGY

Hazards associated with the recipient/host
1. Susceptibility of the host
2. Pathogenicity of the host strain, including virulence, infectivity and toxin
production
3. Modification of the host range
4. Recipient immune status
5. Consequences of exposure.
Hazards arising from the alteration of existing pathogenic traits
Many modifications do not involve genes whose products are inherently harmful, but
adverse effects may arise as the result of alteration of existing non-pathogenic or
pathogenic traits. Modification of normal genes may alter pathogenicity. In an attempt
to identify these potential hazards, the following points may be considered (the list is
not exhaustive).
1. Is there an increase in infectivity or pathogenicity?
2. Could any disabling mutation within the recipient be overcome as a result of the
insertion of the foreign gene?
3. Does the foreign gene encode a pathogenicity determinant from another organism?
4. If the foreign DNA does include a pathogenicity determinant, is it foreseeable that
this gene could contribute to the pathogenicity of the GMO?
5. Is treatment available?
6. Will the susceptibility of the GMO to antibiotics or other forms of therapy be
affected as a consequence of the genetic modification?
7. Is eradication of the GMO achievable?
Further considerations
The use of whole animals or plants for experimental purposes also requires careful
consideration. Investigators must comply with the regulations, restrictions and
requirements for the conduct of work with GMOs in host countries and institutions.
Countries may have national authorities that establish guidelines for work with
GMOs, and may help scientists classify their work at the appropriate biosafety level. In
some cases classification may differ between countries, or countries may decide to
classify work at a lower or higher level when new information on a particular vector/
host system becomes available.
Risk assessment is a dynamic process that takes into account new developments
and the progress of science. The performance of appropriate risk assessments will
assure that the benefits of recombinant DNA technology remain available to
humankind in the years to come.
For further information see references (17) and (46–48).
• 104 •
LABORATORY BIOSAFETY MANUAL

PART VI
Chemical, fire and
electrical safety
17. Hazardous chemicals
• 107 •
Workers in microbiological laboratories are not only exposed to pathogenic
microorganisms, but also to chemical hazards. It is important that they have proper
knowledge of the toxic effects of these chemicals, the routes of exposure and the hazards
that may be associated with handling and storage (see Annex 5). Material safety data
sheets or other chemical hazard information are available from chemical manufacturers
and/or suppliers. These should be accessible in laboratories where these chemicals are
used, e.g. as part of a safety or operations manual.
Routes of exposure
Exposure to hazardous chemicals may occur by:
1. Inhalation
2. Contact
3. Ingestion
4. Needle-sticks
5. Through broken skin.
Storage of chemicals
Only amounts of chemicals necessary for daily use should be stored in the laboratory.
Bulk stocks should be kept in specially designated rooms or buildings.
Chemicals should not be stored in alphabetical order.
General rules regarding chemical incompatibilities
To avoid fire and/or explosions, substances in the left-hand column of Table 13 should
be stored and handled so that they cannot come into contact with the corresponding
substances in the right-hand column of the table.
Toxic effects of chemicals
Some chemicals adversely affect the health of those who handle them or inhale their
vapours. Apart from overt poisons, a number of chemicals are known to have various
toxic effects. The respiratory system, blood, lungs, liver, kidneys and the gastrointestinal
system, as well as other organs and tissues may be adversely affected or seriously
damaged. Some chemicals are known to be carcinogenic or teratogenic.

• 108 •
LABORATORY BIOSAFETY MANUAL
Table 13. General rules for chemical incompatibilities
SUBSTANCE CATEGORY INCOMPATIBLE SUBSTANCES
Alkali metals, e.g. sodium, potassium, Carbon dioxide, chlorinated hydrocarbons, water
caesium and lithium
Halogens Ammonia, acetylene, hydrocarbons
Acetic acid, hydrogen sulfide, aniline, Oxidizing agents, e.g. chromic acid, nitric acid,
hydrocarbons, sulfuric acid peroxides, permanganates
Some solvent vapours are toxic when inhaled. Apart from the more serious effects
noted above, exposure may result in impairments that show no immediate discernible
effects on health, but can include lack of coordination, drowsiness and similar
symptoms, leading to an increased proneness to accidents.
Prolonged or repeated exposure to the liquid phase of many organic solvents can
result in skin damage. This may be due to a defatting effect, but allergic and corrosive
symptoms may also arise.
For detailed information on the toxic effects of chemicals see Annex 5.
Explosive chemicals
Azides, often used in antibacterial solutions, should not be allowed to come into contact
with copper or lead (e.g. in waste pipes and plumbing), as they may explode violently
when subjected even to a mild impact.
Ethers that have aged and dried to crystals are extremely unstable, and potentially
explosive.
Perchloric acid, if allowed to dry on woodwork, brickwork or fabric, will explode
and cause a fire on impact.
Picric acid and picrates are detonated by heat and impact.
Chemical spills
Most manufacturers of laboratory chemicals issue charts describing methods for
dealing with spills. Spillage charts and spillage kits are also available commercially.
Appropriate charts should be displayed in a prominent position in the laboratory. The
following equipment should also be provided:
1. Chemical spill kits
2. Protective clothing, e.g. heavy-duty rubber gloves, overshoes or rubber boots,
respirators
3. Scoops and dustpans
4. Forceps for picking up broken glass
5. Mops, cloths and paper towels
6. Buckets

• 109 •
7. Soda ash (sodium carbonate, Na2CO3) or sodium bicarbonate (NaHCO3) for
neutralizing acids and corrosive chemicals
8. Sand (to cover alkali spills)
9. Non-flammable detergent.
The following actions should be taken in the event of a significant chemical spill.
1. Notify the appropriate safety officer.
2. Evacuate non-essential personnel from the area.
3. Attend to persons who may have been contaminated.
4. If the spilled material is flammable, extinguish all open flames, turn off gas in the
room and adjacent areas, open windows (if possible), and switch off electrical
equipment that may spark.
5. Avoid breathing vapour from spilled material.
6. Establish exhaust ventilation if it is safe to do so.
7. Secure the necessary items (see above) to clean up the spill.
Compressed and liquefied gases
Information regarding storage of compressed and liquefied gases is given in Table 14.
Table 14. Storage of compressed and liquefied gases
CONTAINER STORAGE INFORMATION
Compressed gas cylinders and • Should be securely fixed (e.g. chained) to the wall
liquefied gas containersa,b or a solid bench so that they are not inadvertently
dislodged.
•Must be transported with their caps in place and
supported on trolleys.
•Should be stored in bulk in an appropriate facility at
some distance from the laboratory. This area should
be locked and appropriately identified.
•Should not be placed near radiators, open flames
other heat sources, sparking electrical equipment,
or in direct sunlight.
Small, single-use gas cylindersa,b •Must not be incinerated.
aThe main high-pressure valve should be turned off when the equipment is not in use and when the room
is unoccupied.
bRooms where flammable gas cylinders are used and/or stored should be identified by warning notices on
the doors.
For further information see references (1) and (49–51), and Annex 5.
17. HAZARDOUS CHEMICALS
18. Additional laboratory hazards
Laboratory personnel may confront hazards posed by forms of energy including fire,
electricity, radiation and noise. Basic information about each of these is presented in
this chapter.
Fire hazards
Close cooperation between safety officers and local fire prevention officers is essential.
Apart from chemical hazards, the effects of fire on the possible dissemination of
infectious material must be considered. This may determine whether it is best to
extinguish or contain the fire.
The assistance of local fire prevention officers in the training of laboratory staff in
fire prevention, immediate action in case of fire and the use of fire-fighting equipment
is desirable.
Fire warnings, instructions and escape routes should be displayed prominently in
each room and in corridors and hallways.
Common causes of fires in laboratories are:
1. Electrical circuit overloading
2. Poor electrical maintenance, e.g. poor and perished insulation on cables
3. Excessively long gas tubing or long electrical leads
4. Equipment unnecessarily left switched on
5. Equipment that was not designed for a laboratory environment
6. Open flames
7. Deteriorated gas tubing
8. Improper handling and storage of flammable or explosive materials
9. Improper segregation of incompatible chemicals
10. Sparking equipment near flammable substances and vapours
11. Improper or inadequate ventilation.
Fire-fighting equipment should be placed near room doors and at strategic points in
corridors and hallways. This equipment may include hoses, buckets (of water or sand)
and a fire extinguisher. Fire extinguishers should be regularly inspected and maintained,
and their shelf-life kept up to date. Specific types and uses of fire extinguishers are
shown in Table 15.
• 110 •

Table 15. Types and uses of fire extinguishers
TYPE USE FOR DO NOT USE FOR
Water Paper, wood, fabric Electrical fires, flammable liquids,
burning metals
Carbon dioxide (CO2)Flammable liquids and gases, Alkali metals, paper
extinguisher gases electrical fires
Dry powder Flammable liquids and gases, Reusable equipment and
alkali metals, electrical fires instruments, as residues are very
difficult to remove
Foam Flammable liquids Electrical fires
For further information see reference (49).
Electrical hazards
It is essential that all electrical installations and equipment are inspected and tested
regularly, including earthing/grounding systems.
Circuit-breakers and earth-fault-interrupters should be installed in appropriate
laboratory electrical circuits. Circuit-breakers do not protect people; they are intended
to protect wiring from being overloaded with electrical current and hence to prevent
fires. Earth-fault-interrupters are intended to protect people from electric shock.
All laboratory electrical equipment should be earthed/grounded, preferably through
three-prong plugs.
All laboratory electrical equipment and wiring should conform to national electrical
safety standards and codes.
Noise
The effect of excessive noise is insidious over time. Some types of laboratory equipment,
such as certain laser systems, as well as facilities where animals are housed, can produce
significant noise exposure to workers. Noise measurement surveys can be conducted
to determine the noise hazard. Where warranted by data, engineering controls such as
enclosures or barriers around noisy equipment or between noisy areas and other work
areas, can be considered. Where noise levels cannot be abated and where laboratory
personnel routinely experience excessive exposures, a hearing conservation programme
that includes the use of hearing protection while working in hazardous noise and a
medical monitoring programme to determine the effect of noise on the workers should
be instituted.
Ionizing radiation
Radiological protection is concerned with protecting humans against the harmful
effects of ionizing radiation, which include:
18. ADDITIONAL LABORATORY HAZARDS
• 111 •
• 112 •
LABORATORY BIOSAFETY MANUAL
1. Somatic effects, e.g. clinical symptoms observable in exposed individuals. Somatic
effects include radiation-induced cancers, e.g. leukaemia and bone, lung and skin
cancers, the onset of which may occur many years after irradiation. Less severe
somatic effects include minor skin damage, hair loss, blood deficiencies, gastro-
intestinal damage and cataract formation.
2. Hereditary effects, e.g. symptoms observed in the descendants of exposed indi-
viduals. The hereditary effects of radiation exposure to the gonads include chromo-
some damage or gene mutation. Irradiation of the germ cells in the gonads in high
doses can also cause cell death, resulting in impaired fertility in both sexes or
menstrual changes in women. Exposure of the developing fetus, particularly in
weeks 8–15 of pregnancy, may increase the risk of congenital malformations, mental
impairment or radiation-induced cancers in later life.
Principles of ionizing radiation protection
To limit the harmful effects of ionizing radiation, the use of radioisotopes should be
controlled and should comply with relevant national standards. Protection from
radiation is managed on the basis of four principles:
1. Minimizing the time of radiation exposure
2. Maximizing the distance from the radiation source
3. Shielding the radiation source
4. Substituting the use of radionuclides with non-radiometric techniques.
Protection activities include the following.
1. Time. The time of exposure experienced during manipulations of radioactive
material can be reduced by:
—Practising new and unfamiliar techniques without using the radionuclide until
the techniques are mastered
—Working with radionuclides in a deliberate and timely manner without rushing
—Ensuring that all radioactive sources are returned to storage immediately after
use
—Removing radioactive waste from the laboratory at frequent intervals
—Spending as little time as possible in the radiation area or laboratory
—Exercising effective time management and planning of laboratory manipulations
involving radioactive material.
The less time spent in a radiation field, the smaller the received personal dose, as
described in the equation:
Dose = Dose rate
x
time
2. Distance. The dose rate for most γ- and X-radiation varies as the inverse square of
the distance from a point source:
Dose rate = Constant
x
1/Distance2
• 113 •
Doubling the distance from a radiation source will result in reducing the exposure
by one-fourth over the same period of time. Various devices and mechanical aids
are used to increase the distance between the operator and the radiation source,
e.g. long-handled tongs, forceps, clamps and remote pipetting aids. Note that a
small increase in distance can result in significant decrease in the dose rate.
3. Shielding. Radiation energy-absorbing or attenuating shields placed between the
source and the operator or other occupants of the laboratory will help limit their
exposure. The choice and thickness of any shielding material depends on the
penetrating ability (type and energy) of the radiation. A barrier of acrylic, wood
or lightweight metal, thickness 1.3–1.5 cm, provides shielding against high-energy
β particles, whereas high-density lead is needed to shield against high energy γ-
and X-radiation.
4. Substitution. Radionuclide-based materials should not be used when other
techniques are available. If substitution is not possible, then the radionuclide with
the least penetrating power or energy should be used.
Safe practices for work with radionuclides
Rules for working with radioactive substances should include considerations in four
areas:
1. Radiation area
2. Work-bench area
3. Radioactive waste area
4. Records and emergency response.
Some of the most important rules include the following:
1. Radiation area
—Use radioactive substances only in dedicated areas.
—Allow the presence of essential staff only.
—Use personal protective equipment, including laboratory coats, safety spectacles
and disposable gloves.
—Monitor personal radiation exposures.
Laboratories where radionuclides are used should be designed to simplify contain-
ment, cleaning and decontamination. The radionuclide work area should be located
in a small room adjoining the main laboratory, or in a dedicated area within the
laboratory away from other activities. Signs displaying the international radiation
hazard symbol should be posted at the entrance to the radiation area (Figure 12).
2. Work-bench area
—Use spill trays lined with disposable absorbent materials.
—Limit radionuclide quantities.
—Shield radiation sources in the radiation, work bench and radioactive waste
areas.
18. ADDITIONAL LABORATORY HAZARDS

—Mark radiation containers with the radiation symbol, including radionuclide
identity, activity and assay date.
—Use radiation meters to monitor working areas, protective clothing and hands
after completion of work.
—Use appropriately shielded transport containers.
3. Radioactive waste area
—Remove radioactive waste frequently from the working area.
—Maintain accurate records of use and disposal of radioactive materials.
—Screen dosimetry records for materials exceeding the dose limits.
—Establish and regularly exercise emergency response plans.
—In emergencies, assist injured persons first.
—Clean contaminated areas thoroughly.
—Request assistance from the safety office, if available.
—Write and keep incident reports.
Figure 12. International radiation
hazard symbol
• 114 •
LABORATORY BIOSAFETY MANUAL

PART VII
Safety organization
and training
19. The biosafety officer and
biosafety committee
• 117 •
It is essential that each laboratory organization has a comprehensive safety policy, a
safety manual, and supporting programmes for their implementation. The
responsibility for this normally rests with the director or head of the institute or
laboratory, who may delegate certain duties to a biosafety officer or other appropriate
personnel.
Laboratory safety is also the responsibility of all supervisors and laboratory
employees, and individual workers are responsible for their own safety and that of
their colleagues. Employees are expected to perform their work safely and should report
any unsafe acts, conditions or incidents to their supervisor. Periodic safety audits by
internal or external personnel are desirable.
Biosafety officer
Wherever possible a biosafety officer should be appointed to ensure that biosafety
policies and programmes are followed consistently throughout the laboratory. The
biosafety officer executes these duties on behalf of the head of the institute or laboratory.
In small units, the biosafety officer may be a microbiologist or a member of the technical
staff, who may perform these duties on a defined part-time basis. Whatever the degree
of involvement in biosafety, the person designated should possess the professional
competence necessary to suggest, review and approve specific activities that follow
appropriate biocontainment and biosafety procedures. The biosafety officer should
apply relevant national and international rules, regulations and guidelines, as well as
assist the laboratory in developing standard operating procedures. The person
appointed must have a technical background in microbiology, biochemistry and basic
physical and biological sciences. Knowledge of laboratory and clinical practices and
safety, including containment equipment, and engineering principles relevant to the
design, operation and maintenance of facilities is highly desirable. The biosafety officer
should also be able to communicate effectively with administrative, technical and
support personnel.
The activities of the biosafety officer should include the following:
1. Biosafety, biosecurity and technical compliance consultations.
2. Periodic internal biosafety audits on technical methods, procedures and protocols,
biological agents, materials and equipment.
• 118 •
LABORATORY BIOSAFETY MANUAL
3. Discussions of violation of biosafety protocols or procedures with the appropriate
persons.
4. Verification that all staff have received appropriate biosafety training.
5. Provision of continuing education in biosafety.
6. Investigation of incidents involving the possible escape of potentially infectious or
toxic material, and reporting of findings and recommendations to the laboratory
director and biosafety committee.
7. Coordination with medical staff regarding possible laboratory-acquired infections.
8. Ensuring appropriate decontamination following spills or other incidents involving
infectious material(s).
9. Ensuring proper waste management.
10. Ensuring appropriate decontamination of any apparatus prior to repair or servicing.
11. Maintaining awareness of community attitudes regarding health and environmental
considerations.
12. Establishment of appropriate procedures for import/export of pathogenic material
to/from the laboratory, according to national regulations.
13. Reviewing the biosafety aspects of all plans, protocols and operating procedures
for research work involving infectious agents prior to the implementation of these
activities.
14. Institution of a system to deal with emergencies.
Biosafety committee
A biosafety committee should be constituted to develop institutional biosafety policies
and codes of practice. The biosafety committee should also review research protocols
for work involving infectious agents, animal use, recombinant DNA and genetically
modified materials. Other functions of the committee may include risk assessments,
formulation of new safety policies and arbitration in disputes over safety matters.
The membership of the biosafety committee should reflect the diverse occupational
areas of the organization as well as its scientific expertise. The composition of a basic
biosafety committee may include:
1. Biosafety officer(s)
2. Scientists
3. Medical personnel
4. Veterinarian(s) (if work with animals is conducted)
5. Representatives of technical staff
6. Representatives of laboratory management.
The biosafety committee should seek advice from different departmental and specialist
safety officers (e.g. with expertise in radiation protection, industrial safety, fire
prevention, etc.) and may at times require assistance from independent experts in
various associated fields, local authorities and national regulatory bodies. Community
members may also be helpful if there is a particularly contentious or sensitive protocol
under discussion.
• 119 •
20. Safety for support staff
The safe and optimum operation of a laboratory is dependent to a great extent on the
support staff, and it is essential that such personnel are given appropriate safety training.
Engineering and building maintenance services
Skilled engineers and craftsmen who maintain and repair the structure, facilities and
equipment, should have some knowledge of the nature of the work of the laboratory,
and of safety regulations and procedures.
Testing of equipment after servicing, e.g. testing the efficiency of biological safety
cabinets after new filters have been fitted, may be carried out by or under supervision
of the biosafety officer.
Laboratories or institutions that do not have internal engineering and maintenance
services should establish good relationships with local service providers to familiarize
them with the equipment and work of the laboratory.
Engineering and maintenance staff should only enter Biosafety Level 3 or Biosafety
Level 4 laboratories with clearance and supervision by the biosafety officer and/or the
laboratory supervisor.
Cleaning (domestic) services
Biosafety Level 3 and Biosafety Level 4 laboratories should be cleaned by the laboratory
staff. Cleaning personnel should only enter Biosafety Level 3 or Biosafety Level 4
laboratories with clearance and supervision by the biosafety officer and/or the
laboratory supervisor.
21. Training programmes
A continuous, on-the-job safety training programme is essential to maintain safety
awareness among laboratory and support staff. Laboratory supervisors, with the
assistance of the biosafety officer and other resource persons, play the key role in staff
training. The effectiveness of biosafety training, indeed all safety and health training,
depends on management commitment, motivational factors, adequate initial job
training, good communications, and ultimately the organization’s goals and objectives.
The following are critical elements for an effective biosafety training programme.
1. Needs assessment. This process includes defining the tasks involved, the order of
importance (in terms of frequency, criticality, complexity) and details of the steps
necessary to accomplish them.
2. Establishing training objectives. These are observable behaviours that the trainee
is expected to demonstrate, on the job, after the training. Objectives may acknow-
ledge the conditions under which certain activities or behaviours are performed
and the required level of proficiency.
3. Specifying training content and media. Content is the knowledge or skill that the
trainee must master to be able to meet the behavioural objectives. Those individuals
who know the job and its demands best usually define the content of the biosafety
training programme. Other approaches used may focus on the products of problem-
solving exercises or the design of learning measures to correct mistakes people
have made in using a skill. It is not clear that one teaching method (lectures, televised
instruction, computer-aided instruction, interactive video, etc.) is superior to
another. Much depends on specific training needs, the make-up of the trainee group,
etc.
4. Accounting for individual learning differences. Effective training must take into
account the characteristics or attributes of the trainees. Individuals and groups
may differ in aptitude, literacy, culture, spoken language and pre-training skill levels.
How the training programme is viewed by trainees in terms of improving their job
performance or personal safety may dictate the approach used. Some individuals
are more visual or “hands-on” learners; others learn well from written materials.
Any special needs of employees must also be addressed, such as course adaptation
for those with hearing impairments. In addition to taking account of these elements,
it is recommended that the developers of any safety training programme become
acquainted with the principles of adult learning.
• 120 •
5. Specifying learning conditions. The instructional event (e.g. training course,
videotape, written materials, etc.) should not conflict with, inhibit or be unrelated
to mastery of the skill or topic being taught. For example, if the intent of the in-
struction is to develop capabilities in problem-solving techniques, the instructional
approach should stress thinking/reasoning approaches rather than rote memori-
zation. The instruction provided should require productive behaviour and/or ap-
propriate feedback (positive/accurate/credible). In addition, instructional events
that provide opportunities for practice under conditions similar to that of the job
will enhance the transfer of the skill to the actual job.
6. Training evaluation. This provides information that helps to determine whether
the instruction has had the intended effect. Training evaluations generally take
four forms:
—measuring the trainees’ reaction to the instruction provided
—measuring the trainees’ recollection and/or performance
—assessing behavioural change on the job
—measuring tangible results in terms of the organization’s objectives or goals.
The most complete evaluation of a training effort involves assessments for each of
the four areas. The least efficient method of evaluation is to consider only the
trainees’ reactions to the instruction as this may bear little relationship to the extent
of actual learning. It should not be used as the sole measurement of training
effectiveness.
7. Training revision. Training evaluations rarely indicate that a training programme
is a complete success or failure because multiple criteria are used to measure results.
Usually the data indicate a better understanding, retention or application of some
parts of the course material as compared with others. Variation or gaps in knowledge
or the desired competencies resulting from the training effort may reflect the need
to consider more training time, alternative instructional techniques or more capable
instructors.
WHO provides various tools for microbiological safety training.
21. TRAINING PROGRAMMES
• 121 •


PART VIII
Safety checklist
22. Safety checklist
• 125 •
This checklist is intended to assist in assessments of microbiological laboratory safety
and security status of biomedical laboratories.
Laboratory premises
1. Have guidelines for commissioning and certification been considered for facility
construction or post-construction evaluations?
2. Do the premises meet national and local building requirements, including those
relating to natural disaster precautions if necessary?
3. Are the premises generally uncluttered and free from obstructions?
4. Are the premises clean?
5. Are there any structural defects in floors?
6. Are floors and stairs uniform and slip-resistant?
7. Is the working space adequate for safe operation?
8. Are the circulation spaces and corridors adequate for the movement of people and
large equipment?
9. Are the benches, furniture and fittings in good condition?
10. Are bench surfaces resistant to solvents and corrosive chemicals?
11. Is there a hand-washing sink in each laboratory room?
12. Are the premises constructed and maintained to prevent entry and harbourage of
rodents and arthropods?
13. Are all exposed steam and hot water pipes insulated or guarded to protect personnel?
14. Is an independent power support unit provided in case of power breakdown?
15. Can access to laboratory areas be restricted to authorized personnel?
16. Has a risk assessment been performed to ensure that appropriate equipment and
facilities are available to support the work being considered?
Storage facilities
1. Are storage facilities, shelves, etc. arranged so that stores are secure against sliding,
collapse or falls?
2. Are storage facilities kept free from accumulations of rubbish, unwanted materials
and objects that present hazards from tripping, fire, explosion and harbourage of
pests?
3. Are freezers and storage areas lockable?
• 126 •
LABORATORY BIOSAFETY MANUAL
Sanitation and staff facilities
1. Are the premises maintained in a clean, orderly and sanitary condition?
2. Is drinking-water available?
3. Are clean and adequate toilet (WC) and washing facilities provided separately for
male and female staff?
4. Are hot and cold water, soap and towels provided?
5. Are separate changing rooms provided for male and female staff?
6. Is there accommodation (e.g. lockers) for street clothing for individual members
of the staff?
7. Is there a staff room for lunch, etc.?
8. Are noise levels acceptable?
9. Is there an adequate organization for the collection and disposal of general
household rubbish?
Heating and ventilation
1. Is there a comfortable working temperature?
2. Are blinds fitted to windows that are exposed to full sunlight?
3. Is the ventilation adequate, e.g. at least six changes of air per hour, especially in
rooms that have mechanical ventilation?
4. Are there HEPA filters in the ventilation system?
5. Does mechanical ventilation compromise airflows in and around biological safety
cabinets and fume cupboards?
Lighting
1. Is the general illumination adequate (e.g. 300–400 lx)?
2. Is task (local) lighting provided at work benches?
3. Are all areas well-lit, with no dark or ill-lit corners in rooms and corridors?
4. Are fluorescent lights parallel to the benches?
5. Are fluorescent lights colour-balanced?
Services
1. Is each laboratory room provided with enough sinks, water, electricity and gas
outlets for safe working?
2. Is there an adequate inspection and maintenance programme for fuses, lights, cables,
pipes, etc.?
3. Are faults corrected within a reasonable time?
4. Are internal engineering and maintenance services available, with skilled engineers
and craftsmen who also have some knowledge of the nature of the work of the
laboratory?
5. Is the access of engineering and maintenance personnel to various laboratory areas
controlled and documented?
• 127 •
6. If no internal engineering and maintenance services are available, have local
engineers and builders been contacted and familiarized with the equipment and
work of the laboratory?
7. Are cleaning services available?
8. Is the access of cleaning personnel to various laboratory areas controlled and
documented?
9. Are information technology services available and secured?
Laboratory biosecurity
1. Has a qualitative risk assessment been performed to define risks that a security
system should protect against?
2. Have acceptable risks and incidence response planning parameters been defined?
3. Is the whole building securely locked when unoccupied?
4. Are doors and windows break-proof?
5. Are rooms containing hazardous materials and expensive equipment locked when
unoccupied?
6. Is access to such rooms, equipment and materials appropriately controlled and
documented?
Fire prevention and fire protection
1. Is there a fire alarm system?
2. Are the fire doors in good order?
3. Is the fire detection system in good working order and regularly tested?
4. Are fire alarm stations accessible?
5. Are all exits marked by proper, illuminated signs?
6. Is access to exits marked where the routes to them are not immediately visible?
7. Are all exits unobstructed by decorations, furniture and equipment, and unlocked
when the building is occupied?
8. Is access to exits arranged so that it is not necessary to pass through a high-hazard
area to escape?
9. Do all exits lead to an open space?
10. Are corridors, aisles and circulation areas clear and unobstructed for movement of
staff and fire-fighting equipment?
11. Is all fire-fighting equipment and apparatus easily identified by an appropriate
colour code?
12. Are portable fire extinguishers maintained fully charged and in working order,
and kept in designated places at all times?
13. Are laboratory rooms with potential fire hazards equipped with appropriate
extinguishers and/or fire blankets for emergency use?
14. If flammable liquids and gases are used in any room, is the mechanical ventilation
sufficient to remove vapours before they reach a hazardous concentration?
15. Are personnel trained to respond to fire emergencies?
22. SAFETY CHECKLIST
• 128 •
LABORATORY BIOSAFETY MANUAL
Flammable liquid storage
1. Is the storage facility for bulk flammable liquids separated from the main building?
2. Is it clearly labelled as a fire-risk area?
3. Does it have a gravity or mechanical exhaust ventilation system that is separate
from the main building system?
4. Are the switches for lighting sealed or placed outside the building?
5. Are the light fittings inside sealed to protect against ignition of vapours by sparking?
6. Are flammable liquids stored in proper, ventilated containers that are made of
non-combustible materials?
7. Are the contents of all containers correctly described on the labels?
8. Are appropriate fire extinguishers and/or fire blankets placed outside but near to
the flammable liquid store?
9. Are “No smoking” signs clearly displayed inside and outside the flammable liquid
store?
10. Are only minimum amounts of flammable substances stored in laboratory rooms?
11. Are they stored in properly constructed flammable storage cabinets?
12. Are these cabinets adequately labelled with “Flammable liquid – Fire hazard” signs?
13. Are personnel trained to properly use and transport flammable liquids?
Compressed and liquefied gases
1. Is each portable gas container legibly marked with its contents and correctly colour-
coded?
2. Are compressed-gas cylinders and their high-pressure and reduction valves regularly
inspected?
3. Are reduction valves regularly maintained?
4. Is a pressure-relief device connected when a cylinder is in use?
5. Are protection caps in place when cylinders are not in use or are being transported?
6. Are all compressed gas cylinders secured so that they cannot fall, especially in the
event of natural disaster?
7. Are cylinders and liquid petroleum gas tanks kept away from sources of heat?
8. Are personnel trained to properly use and transport compressed and liquefied gases?
Electrical hazards
1. Are all new electrical installations and all replacements, modifications or repairs
made and maintained in accordance with a national electrical safety code?
2. Does the interior wiring have an earthed/grounded conductor (i.e. a three-wire
system)?
3. Are circuit-breakers and earth-fault interrupters fitted to all laboratory circuits?
4. Do all electrical appliances have testing laboratory approval?
5. Are the flexible connecting cables of all equipment as short as practicable, in good
condition, and not frayed, damaged or spliced?
6. Is each electric socket outlet used for only one appliance (no adapters to be used)?
• 129 •
Personal protection
1. Is protective clothing of approved design and fabric provided for all staff for normal
work, e.g. gowns, coveralls, aprons, gloves?
2. Is additional protective clothing provided for work with hazardous chemicals and
radioactive and carcinogenic substances, e.g. rubber aprons and gloves for chemicals
and for dealing with spillages; heat-resistant gloves for unloading autoclaves and
ovens?
3. Are safety glasses, goggles and shields (visors) provided?
4. Are there eye-wash stations?
5. Are there emergency showers (drench facilities)?
6. Is radiation protection in accordance with national and international standards,
including provision of dosimeters?
7. Are respirators available, regularly cleaned, disinfected, inspected and stored in a
clean and sanitary condition?
8. Are appropriate filters provided for the correct types of respirators, e.g. HEPA filters
for microorganisms, appropriate filters for gases or particulates?
9. Are respirators fit-tested?
Health and safety of staff
1. Is there an occupational health service?
2. Are first-aid boxes provided at strategic locations?
3. Are qualified first-aiders available?
4. Are such first-aiders trained to deal with emergencies peculiar to the laboratory,
e.g. contact with corrosive chemicals, accidental ingestion of poisons and infectious
materials?
5. Are non-laboratory workers, e.g. domestic and clerical staff, instructed on the
potential hazards of the laboratory and the material it handles?
6. Are notices prominently posted giving clear information about the location of
first-aiders, telephone numbers of emergency services, etc.?
7. Are women of childbearing age warned of the consequences of work with certain
microorganisms, carcinogens, mutagens and teratogens?
8. Are women of childbearing age told that if they are, or suspect that they are,
pregnant they should inform the appropriate member of the medical/scientific
staff so that alternative working arrangements may be made for them if necessary?
9. Is there an immunization programme relevant to the work of the laboratory?
10. Are skin tests and/or radiological facilities available for staff who work with
tuberculous materials or other materials requiring such measures?
11. Are proper records maintained of illnesses and accidents?
12. Are warning and accident prevention signs used to minimize work hazards?
13. Are personnel trained to follow appropriate biosafety practices?
14. Are laboratory staff encouraged to report potential exposures?
22. SAFETY CHECKLIST
• 130 •
LABORATORY BIOSAFETY MANUAL
Laboratory equipment
1. Is all equipment certified safe for use?
2. Are procedures available for decontaminating equipment prior to maintenance?
3. Are biological safety cabinets and fume cupboards regularly tested and serviced?
4. Are autoclaves and other pressure vessels regularly inspected?
5. Are centrifuge buckets and rotors regularly inspected?
6. Are HEPA filters regularly changed?
7. Are pipettes used instead of hypodermic needles?
8. Is cracked and chipped glassware always discarded and not reused?
9. Are there safe receptacles for broken glass?
10. Are plastics used instead of glass where feasible?
11. Are sharps disposal containers available and being used?
Infectious materials
1. Are specimens received in a safe condition?
2. Are records kept of incoming materials?
3. Are specimens unpacked in biological safety cabinets with care and attention to
possible breakage and leakage?
4. Are gloves and other protective clothing worn for unpacking specimens?
5. Are personnel trained to ship infectious substances according to current national
and/or international regulations?
6. Are work benches kept clean and tidy?
7. Are discarded infectious materials removed daily or more often and disposed of
safely?
8. Are all members of the staff aware of procedures for dealing with breakage and
spillage of cultures and infectious materials?
9. Is the performance of sterilizers checked by the appropriate chemical, physical and
biological indicators?
10. Is there a procedure for decontaminating centrifuges regularly?
11. Are sealed buckets provided for centrifuges?
12. Are appropriate disinfectants being used? Are they used correctly?
13. Is there special training for staff who work in containment laboratories – Biosafety
Level 3 and maximum containment laboratories – Biosafety Level 4?
Chemicals and radioactive substances
1. Are incompatible chemicals effectively separated when stored or handled?
2. Are all chemicals correctly labelled with names and warnings?
3. Are chemical hazard warning charts prominently displayed?
4. Are spill kits provided?
5. Are staff trained to deal with spills?
6. Are flammable substances correctly and safely stored in minimal amounts in
approved cabinets?
• 131 •
7. Are bottle carriers provided?
8. Is a radiation protection officer or appropriate reference manual available for
consultation?
9. Are staff appropriately trained to safely work with radioactive materials?
10. Are proper records of stocks and use of radioactive substances maintained?
11. Are radioactivity screens provided?
12. Are personal radiation exposures monitored?
22. SAFETY CHECKLIST


PART IX
References, annexes
and index
References
• 135 •
1. Safety in health-care laboratories. Geneva, World Health Organization, 1997, (http://
whqlibdoc.who.int/hq/1997/WHO_LAB_97.1.pdf).
2. Garner JS, Hospital Infection Control Practices Advisory Committee. Guideline for isola-
tion precautions in hospitals. American Journal of Infection Control, 1996, 24:24–52, (http:/
/www.cdc.gov/ncidod/hip/isolat/isolat.htm).
3. Hunt GJ, Tabachnick WJ. Handling small arbovirus vectors safely during biosafety level 3
containment: Culicoides variipennis sonorensis (Diptera: Ceratopogonidae) and exotic blue-
tongue viruses. Journal of Medical Entomology, 1996, 33:271–277.
4. National Research Council. Occupational health and safety in the care and use of research
animals. Washington, DC, National Academy Press, 1997.
5. Richmond JY, Quimby F. Considerations for working safely with infectious disease agents
in research animals. In: Zak O, Sande MA, eds. Handbook of animal models of infection.
London, Academic Press, 1999:69–74.
6. Biosafety in microbiological and biomedical laboratories, 4th ed. Washington, DC, United
States Department of Health and Human Services/Centers for Disease Control and Pre-
vention/National Institutes of Health, 1999.
7. Class II (laminar flow) biohazard cabinetry. Ann Arbor, MI, National Sanitation Founda-
tion, 2002 (NSF/ANSI 49–2002).
8. Richmond JY, McKinney RW. Primary containment for biohazards: selection, installation
and use of biological safety cabinets, 2nd ed. Washington, DC, United States Department of
Health and Human Services/Centers for Disease Control and Prevention/National Insti-
tutes of Health, 2000.
9. Microbiological safety cabinets. Recommendations for information to be exchanged between
purchaser, vendor and installer and recommendations for installation. London, British Stand-
ards Institution, 1992 (Standard BS 5726–2:1992).
10. Microbiological safety cabinets. Recommendations for selection, use and maintenance. Lon-
don, British Standards Institution, 1992 (Standard BS 5726–4:1992).
11. Biological containment cabinets (Class I and II): installation and field testing. Toronto, Cana-
dian Standards Association, 1995 (Standard Z316.3–95 (R2000)).
12. Collins CH, Kennedy DA. Laboratory acquired infections: history, incidence, causes and pre-
vention, 4th ed. Oxford, Butterworth-Heinemann, 1999.
13. Health Canada. Laboratory biosafety manual, 2nd ed. Ottawa, Minister of Supply and Serv-
ices Canada, 1996.
14. Biological safety cabinets – biological safety cabinets (Class I) for personnel and environment
protection. Sydney, Standards Australia International, 1994 (Standard AS 2252.1–1994).
15. Biological safety cabinets – laminar flow biological safety cabinets (Class II) for personnel,
environment and product protection. Sydney, Standards Australia International, 1994 (Stand-
ard AS 2252.2–1994).
• 136 •
LABORATORY BIOSAFETY MANUAL
16. Standards Australia/Standards New Zealand. Biological safety cabinets – installation and
use. Sydney, Standards Australia International, 2000 (Standard AS/NZS 2647:2000).
17. Advisory Committee on Dangerous Pathogens. Guidance on the use, testing and mainte-
nance of laboratory and animal flexible film isolators. London, Health and Safety Executive,
1990.
18. Standards Australia/Standards New Zealand. Safety in laboratories – microbiological aspects
and containment facilities. Sydney, Standards Australia International, 2002 (Standard AS/
NZS 2243.3:2002).
19. Centers for Disease Control and Prevention. Recommendations for prevention of HIV trans-
mission in health-care settings. Morbidity and Mortality Weekly Report, 1987, 36 (Suppl.
2):1S–18S.
20. Bosque PJ et al. Prions in skeletal muscle. Proceedings of the National Academy of Sciences of
the United States of America, 2002, 99:3812–3817.
21. Bartz JC, Kincaid AE, Bessen RA. Rapid prion neuroinvasion following tongue infection.
Journal of Virology, 2003, 77:583–591.
22. Thomzig A et al. Widespread PrPSc accumulation in muscles of hamsters orally infected
with scrapie. EMBO Reports, 2003, 4:530–533.
23. Glatzel M et al. Extraneural pathologic prion protein in sporadic Creutzfeld-Jakob disease.
New England Journal of Medicine, 2003, 349:1812–1820.
24. Brown P, Wolff A, Gajdusek DC. A simple and effective method for inactivating virus infec-
tivity in formalin-fixed tissue samples from patients with Creutzfield-Jakob disease. Neu-
rology, 1990, 40:887–890.
25. Taylor DM et al. The effect of formic acid on BSE and scrapie infectivity in fixed and unfixed
brain-tissue. Veterinary Microbiology, 1997, 58:167–174.
26. Safar J et al. Prions. In: Richmond JY, McKinney RW, eds. Biosafety in microbiological and
biomedical laboratories, 4th ed. Washington, DC, United States Department of Health and
Human Services, 1999:134–143.
27. Bellinger-Kawahara C et al. Purified scrapie prions resist inactivation by UV irradiation.
Journal of Virology, 1987, 61:159–166.
28. Health Services Advisory Committee. Safe working and the prevention of infection in clinical
laboratories. London, HSE Books, 1991.
29. Russell AD, Hugo WB, Ayliffe GAJ. Disinfection, preservation and sterilization, 3rd ed. Ox-
ford, Blackwell Scientific, 1999.
30. Ascenzi JM. Handbook of disinfectants and antiseptics. New York, NY, Marcel Dekker, 1996.
31. Block SS. Disinfection, sterilization & preservation, 5th ed. Philadelphia, PA, Lippincott
Williams & Wilkins, 2001.
32. Rutala WA. APIC guideline for selection and use of disinfectants. 1994, 1995, and 1996
APIC Guidelines Committee. Association for Professionals in Infection Control and Epi-
demiology, INC. American Journal of Infection Control, 1996, 24:313–342.
33. Sattar SA, Springthorpe VS, Rochon M. A product based on accelerated and stabilized hy-
drogen peroxide: evidence for broad-spectrum germicidal activity. Canadian Journal of
Infection Control, 1998, 13:123–130.
34. Schneider PM. Emerging low temperature sterilization technologies. In: Rutala WA, eds.
Disinfection & sterilization in health care. Champlain, NY, Polyscience, 1997:79–92.
35. Springthorpe VS. New chemical germicides. In: Rutala WA, eds. Disinfection & sterilization
in health care. Champlain, NY, Polyscience, 1997:273–280.
• 137 •
36. Steelman VM. Activity of sterilization processes and disinfectants against prions. In: Rutala
WA, e ds. Disinfection & sterilization in health care. Champlain, NY, Polyscience, 1997:255–
271.
37. Taylor DM. Transmissible degenerative encephalopathies: inactivation of the unconven-
tional causal agents. In: Russell AD, Hugo WB, Ayliffe GAJ, eds. Disinfection, preservation
and sterilization, 3rd ed. Oxford, Blackwell Scientific, 1999:222–236.
38. Infection control guidelines for hand washing, cleaning, disinfection and sterilization in health
care, 2nd ed. Ottawa, Laboratory Centre for Disease Control, Health Canada, 1998.
39. Springthorpe VS, Sattar SA. Chemical disinfection of virus-contaminated surfaces. CRC
Critical Reviews in Environmental Control, 1990, 20:169–229.
40. Recommendations on the transport of dangerous goods, 13th revised edition, New York and
Geneva, United Nations, 2003, (http://www.unece.org/trans/danger/publi/unrec/rev13/
13files_e.html).
41. Technical instructions for the safe transport of dangerous goods by air, 2003–2004 Edition.
Montreal, International Civil Aviation Organization, 2002.
42. Economic Commission for Europe Inland Transport Committee. Restructured ADR appli-
cable as from 1 January 2003. New York and Geneva, United Nations, 2002, (http://
www.unece.org/trans/danger/publi/adr/adr2003/ContentsE.html).
43. Infectious substances shipping guidelines. Montreal, International Air Transport Association,
2003, (http://www.iata.org/ads/issg.htm).
44. Transport of Infectious Substances. Geneva, World Health Organization, 2004, (http://
www.who.int/csr/resources/publications/WHO_CDS_CSR_LYO_2004_9/en/).
45. Berg P et al. Asilomar conference on recombinant DNA molecules. Science, 1975, 188:991–
994.
46. European Council. Council Directive 98/81/EC of 26 October 1998 amending Directive
90/219/EEC on the contained use of genetically modified microorganisms. Official Jour-
nal, 1998, L330:13–31.
47. O’Malley BW Jr et al. Limitations of adenovirus-mediated interleukin-2 gene therapy for
oral cancer. Laryngoscope, 1999, 109:389–395.
48. World Health Organization. Maintenance and distribution of transgenic mice susceptible
to human viruses: memorandum from a WHO meeting. Bulletin of the World Health Or-
ganization, 1993, 71:497–502.
49. Furr AK. CRC handbook of laboratory safety, 5th ed. Boca Raton, FL, CRC Press, 2000.
50. Lenga RE. The Sigma-Aldrich Library of Chemical Safety Data, 2nd ed. Milwaukee, WI,
Aldrich Chemical Company, 1988.
51. Lewis RJ. Sax’s dangerous properties of industrial materials, 10th ed. Toronto, John Wiley
and Sons, 1999.
REFERENCES

ANNEX 1
First aid
First aid is the skilled application of accepted principles of medical treatment at the
time and place of an accident. It is the approved method of treating a casualty until he
or she is placed in the care of a doctor for definitive treatment of the injury.
The minimum first-aid equipment consists of a first-aid box, protective clothing
and safety equipment for the person rendering the first aid, and eye irrigation
equipment.
The first-aid box
The first-aid box should be constructed from materials that will keep the contents
dust- and damp-free. It should be kept in a prominent position and be easily recognized.
By international convention, the first-aid box is identified by a white cross on a green
background.
The first-aid box should contain:
1. Instruction sheet giving general guidance
2. Individually-wrapped sterile adhesive dressings in a variety of sizes
3. Sterile eye-pads with attachment bandages
4. Triangular bandages
5. Sterile wound coverings
6. Safety pins
7. A selection of sterile but unmedicated wound dressings
8. An authoritative first-aid manual, e.g. one issued by the International Red Cross.
Protective equipment for the person rendering first aid includes:
1. Mouthpiece for mouth-to-mouth resuscitation
2. Gloves and other barrier protections against blood exposures,1 and
3. Clean-up kit for blood spills (see Chapter 14 of the manual).
Eye irrigation equipment should also be readily available and staff trained in its correct
use.
1 Garner JS, Hospital Infection Control Practices Advisory Committee. Guideline for isolation precautions
in hospitals. American Journal of Infection Control, 1996, 24:24–52, (http://www.cdc.gov/ncidod/hip/
isolat/isolat.htm).
• 138 •
ANNEX 2
Immunization of staff
The risks of working with particular agents should be fully discussed with individual
researchers. The local availability, licensing state and utility of possible vaccines and/
or therapeutic drugs (e.g. antibiotic treatments) in case of exposure should be evaluated
before work with such agents is started. Some workers may have acquired immunity
from prior vaccination or infection.
If a particular vaccine or toxoid is locally licensed and available, it should be offered
after a risk assessment of possible exposure and a clinical health assessment of the
individual have been carried out.
Facilities for specific clinical case management following accidental infections should
also be available.
• 139 •
ANNEX 3
WHO Biosafety
Collaborating Centres
Information on the availability of training courses, aids and materials may be obtained
by writing to any of the following:
•Biosafety programme, Department of Communicable Disease Surveillance and
Response, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzer-
land (http://www.who.int/csr/).
•WHO Collaborating Centre for Biological Safety, Swedish Institute for Infectious
Disease Control, Nobels Väg 18, S-171 82 Solna, Sweden
(http://www.smittskyddsinstitutet.se/English/english.htm).
•WHO Collaborating Centre on Biosafety Technology and Consultative Services,
Office of Laboratory Security, Health Canada, 100 Colonnade Road, Loc.: 6201A,
Ottawa, Ontario, Canada K1A 0K9 (http://www.hc-sc.gc.ca/pphb-dgspsp/ols-bsl).
•WHO Collaborating Centre for Applied Biosafety Programmes and Training,
Office of Health and Safety, Centers for Disease Control and Prevention, 1600 Clifton
Road, Mailstop F05, Atlanta, GA 30333, USA (http://www.cdc.gov/).
•WHO Collaborating Centre for Applied Biosafety Programmes and Research,
Division of Occupational Health and Safety, Office of Research Services, National
Institutes of Health, Department of Health and Human Services, 13/3K04
13 South Drive MSC 5760, Bethesda, MD 20892-5760, USA (http://www.nih.gov/).
•WHO Collaborating Centre for Biosafety, Victorian Infectious Diseases Reference
Laboratory, 10 Wreckyn St, Nth Melbourne, Victoria 3051, Australia. Postal ad-
dress: Locked Bag 815, PO Carlton Sth, Victoria 3053, Australia (http://www.
vidrl.org.au/).
• 140 •

ANNEX 4
Equipment safety
Certain items of equipment may create microbiological hazards when they are used.
Other items are specifically designed to prevent or reduce biological hazards (see
Chapter 11 of the manual).
Equipment that may create a hazard
Table A4-1 lists equipment and operations that may create hazards and suggests how
such hazards may be eliminated or reduced.
Table A4-1. Equipment and operations that may create hazards
EQUIPMENT HAZARD HOW TO ELIMINATE OR REDUCE THE HAZARD
Hypodermic Accidental inoculation, • Do not recap or clip needles.
needles aerosol or spillage • Use a needle-locking type of syringe to
prevent separation of needle and syringe, or
use a disposable type where the needle is an
integral part of the syringe unit.
•Use good laboratory techniques, e.g.:
—fill the syringe carefully to minimize air
bubbles and frothing of inoculum
—avoid using syringes to mix infectious
liquids; if used, ensure that the tip of the
needle is held under the surface of the
fluid in the vessel and avoid excessive
force
—wrap the needle and stopper in a cotton
pledget moistened with an appropriate
disinfectant before withdrawing the needle
from a rubber-stoppered bottle
—expel excess liquid and air bubbles from
the syringe vertically into a cotton pledget
moistened with an appropriate disinfectant
or into a small bottle containing cotton.
•Use a biological safety cabinet for all
operations with infectious material.
• 141 •

• 142 •
LABORATORY BIOSAFETY MANUAL
EQUIPMENT HAZARD HOW TO ELIMINATE OR REDUCE THE HAZARD
•Restrain animals while they are being
inoculated. Use blunt needles or cannulas for
intranasal or oral inoculation. Use a biological
safety cabinet.
•Autoclave after use and ensure proper
disposal. If a disposable needle and syringe
unit is used, do not disassemble prior to
autoclaving.
Centrifuges Aerosols, splashing • Use sealable buckets (safety cups) or sealed
and tube breakage rotors. Open buckets or rotors after aerosols
have settled (30 min) or in a biological safety
cabinet.
Ultra-centrifuges Aerosols, splashing • Install a HEPA filter between centrifuge and
and tube breakage vacuum pump.
•Maintain a logbook of operating hours for
each rotor and a preventive maintenance
programme to reduce risk of mechanical
failure.
•Load and unload buckets or rotors in a
biological safety cabinet.
Anaerobic jars Explosion, dispersing • Ensure integrity of wire capsule around
infectious materials catalyst.
Desiccators Implosion, dispersing • Place in a stout wire cage.
glass fragments and
infectious materials
Homogenizer, Aerosols, leakage and • Operate and open equipment in a biological
tissue grinders container breakage safety cabinet.
•Use specially designed models that prevent
leakage from rotor bearings and O-ring
gaskets, or use a stomacher.
•Before opening the blender bowl, wait 30 min
to allow the aerosol cloud to settle.
Refrigerate to condense aerosols.
•If manual tissue grinders are used, hold tube
in a wad of absorbent material.
Sonicators, Aerosols, impaired • Operate and open equipment in a biological
ultrasonic hearing, dermatitis safety cabinet or sealed unit.
cleaners • Ensure insulation to protect against
subharmonics.
•Wear gloves to protect skin against chemical
effects of detergents.

• 143 •
EQUIPMENT HAZARD HOW TO ELIMINATE OR REDUCE THE HAZARD
Culture stirrers, Aerosols, splashing • Operate in a biological safety cabinet or
shakers, agitators and spillage specially designed primary containment.
•Use heavy-duty screw-capped culture flasks,
fitted with filter-protected outlets, if
necessary, and well secured.
Freeze-dryers Aerosols and direct • Use O-ring connectors to seal the unit
(lyophilizers) contact contamination throughout.
•Use air filters to protect vacuum lines.
•Use a satisfactory method of
decontamination, e.g. chemical.
•Provide an all-metal moisture trap and a
vapour condenser.
•Carefully inspect all glass vacuum vessels for
surface scratches. Use only glassware
designed for vacuum work.
Water baths Growth of micro- • Ensure regular cleaning and disinfection.
organisms. Sodium • Do not use sodium azide for preventing
azide forms explosive growth of organisms.
compounds with
some metals.
In addition to microbiological hazards, safety hazards associated with equipment
should also be anticipated and prevented. Table A4-2 lists examples of some of the
causes of accidents.
ANNEX 4. EQUIPMENT SAFETY

• 144 •
LABORATORY BIOSAFETY MANUAL
Table A4-2. Common causes of equipment-related accidents
ACCIDENT ACCIDENT CAUSE REDUCING OR ELIMINATING THE HAZARD
Faulty design or construction
Electrical fires in No over-temperature cut-out • Compliance with national
incubators standards.
Electrical shock Failure to provide reliable
earthing/grounding
Improper use
Centrifuge accident Failure to balance buckets • Train and supervise staff.
on swing-out rotors
Anaerobic incubator Use of incorrect gas • Train and supervise staff.
explosion
Improper adaptation
Explosion in domestic Improper transport of liquid • Use of specially designed
vacuum flask nitrogen equipment.
Explosion in domestic- Dangerous chemical not • Store low-flashpoint solvents
type refrigerator stored in spark-/explosion- and extracts only in spark-/
proof container, explosion-proof refrigerators or
e.g. diethyl ether with cabinets.
leaking screw cap
Lack of proper maintenance
Fire in flame Incorrect reassembly of • Train and supervise staff.
photometer components during
maintenance
• 145 •
ANNEX 5
Chemicals: hazards
and precautions
This annex lists the basic health and safety information, data and appropriate safety
precautions for a selected number of chemicals found commonly in health-care and
research laboratories. The list is not exhaustive and the absence of any particular
chemical does not imply that it is non-hazardous. All laboratory chemicals should be
treated with caution and in ways that will minimize exposure.
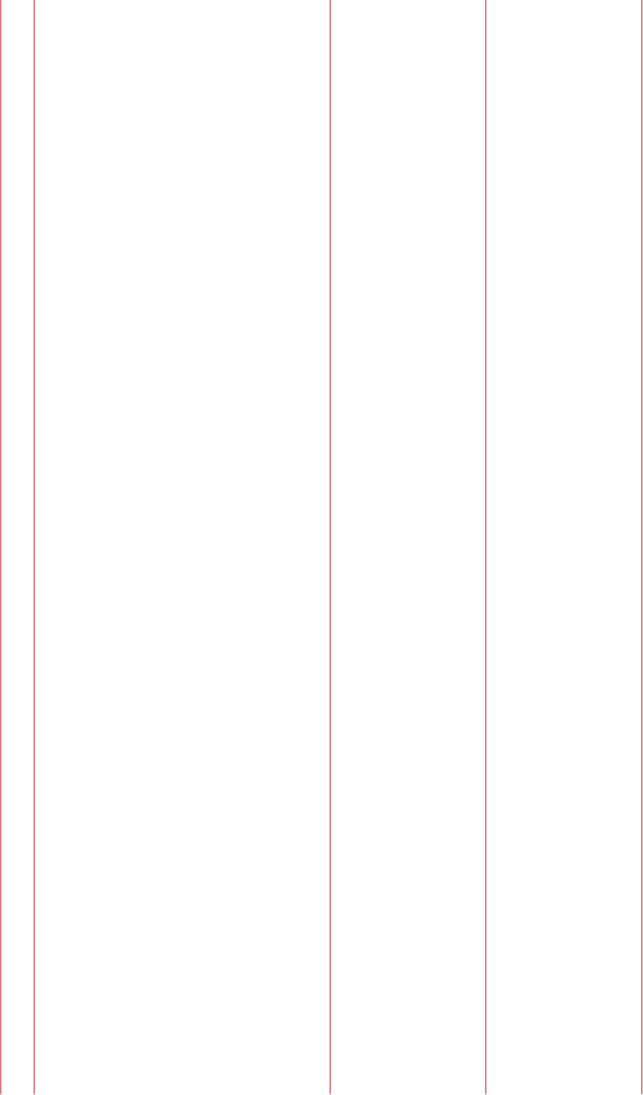
• 146 •
LABORATORY BIOSAFETY MANUAL
Table A5-1. Chemicals: hazards and precautions
CHEMICAL PHYSICAL PROPERTIES HEALTH HAZARDS FIRE HAZARDS SAFETY PRECAUTIONS INCOMPATIBLE CHEMICALS OTHER HAZARDS
Acetaldehyde Colourless liquid or Mild eye and Extremely flammable; No open flames, no Can form explosive
CH
3
CHO gas with a pungent, respiratory tract vapour/air mixtures sparks, no smoking, peroxides in contact
fruity odour; irritation. Effects on are explosive; no contact with hot with air. May polymerize
m.p. –121 °Cthe central nervous flash point –39 °Csurfaces. Store in under influence of acids,
b.p. 21 °C. system, respiratory flammable range tightly sealed alkaline materials, in
tract and kidneys. 4–57%. containers in areas the presence of trace
Possible carcinogen. separate from metals. A strong
oxidizers; store only if reducing agent, reacts
stabilized. Use in violently with oxidants,
exhaust cupboard or with various organic
with good ventilation. substances, halogens,
Wear rubber gloves, sulfuric acid and
safety goggles, and amines.
respiratory protection.
Acetic acid Colourless liquid with Corrosive; causes Flammable; Do not breathe fumes. Violent or explosive
CH
3
CO
2
Hpungent odour; severe burns; irritating flashpoint 40 °CIn case of contact with reaction with oxidizers.
m.p. 17 °Cvapour. Effects may flammable range eyes rinse immediately
b.p. 118 °C; be delayed. 5.4–16%. with water and seek
miscible with water.medical advice. Wear
nitrile gloves and eye
protection.
Acetic Colourless liquid with Severe irritation of Flammable; evolves No open flames, no Reacts violently with
anhydride a strong pungent, eyes and upper irritation or toxic sparks, no smoking. boiling water, steam,
(CH
3
CO)
2
Ovinegar-like odour; respiratory tract fumes or gases in a Prevent skin and eye strong oxidants, alcohols,
m.p. –73 °Cirritation; corrosive fire; contact. amines, strong bases and
b.p. 139 °C. action. Effects may be flashpoint 49 °Cmany other compounds.
delayed. explosive limits Attacks many metals
2.7–10.3%. in presence of water.

• 147 •
ANNEX 5. CHEMICALS: HAZARDS AND PRECAUTIONS
Acetone Colourless volatile Slight eye, nose and Highly flammable; Keep container in Reacts violently with Earth/ground large
CH
3
COCH
3
liquid with sweetish throat irritation. flashpoint –18 °Cwell-ventilated area; oxidizers (e.g. chromic containers and
odour; Inhalation may cause explosive limits keep away from and nitric acids) and vessels to prevent
m.p. –95 °C, dizziness, narcosis 2.2–12.8%. sources of ignition. chloroform in the static electricity.
b.p. 56 °C.; and coma. Do not breathe vapour. presence of base.
miscible with water.Use respiratory Incompatible with
protection; wear eye concentrated sulfuric
protection. and nitric acid mixtures.
Acetonitrile Colourless liquid with Respiratory, eye and Highly flammable; No open flames, no Reacts with aqueous
CH
3
CN an aromatic odour; skin irritation. flashpoint 12.8 °Csparks, no smoking, acids and bases,
m.p –46 °CExposure may result in explosive limits no contact with oxidants. producing toxic
b.p. 82 °C. convulsions 3.0–16%. Use only in areas free of fumes. Reacts with
unconsciousness, ignition sources. Store strong oxidants.
cyanide poisoning. in tightly sealed Attacks some forms
containers in areas of plastic, rubber and
separate from oxidizers. coatings. Decomposes
Work with exhaust on burning producing
ventilation. Avoid skin, hydrogen cyanide and
eye and mucous nitrogen oxides.
membrane contact. Use
respiratory protection and
rubber gloves.
Acetylene Colourless gas with Simple asphyxiant; Extremely flammable; For skin protection use Strong reducing agent;
HC≡CH a faint, ethereal or frostbite on skin flammable range cold-insulating gloves reacts violently with
garlic-like odour; contact. 2.5–100%. and safety goggles or oxidants and with
shipped under pressure, face shield. No open fluorine or chlorine
dissolved in acetone; flames, no sparks, no under influence of light.
m.p. –81 °Csmoking. Work with Reacts with copper,
sublimes at –84 °C. local exhaust ventilation,silver and mercury or
explosion-proof electrical their salts, forming shock-
equipment and lighting. sensitive compounds.
• 148 •
LABORATORY BIOSAFETY MANUAL
CHEMICAL PHYSICAL PROPERTIES HEALTH HAZARDS FIRE HAZARDS SAFETY PRECAUTIONS INCOMPATIBLE CHEMICALS OTHER HAZARDS
Acrolein Colourless or yellow Lacrimation. Severe Highly flammable; Prevent skin and eye Oxidizers, acids, alkalis,
CH
2
=CHCHO liquid with a piercing, respiratory irritation; flashpoint –26 °Ccontact. Work in fume ammonia, amines. Poly-
disagreeable odour; lung oedema at high explosive limits cupboard or with good merizes readily unless
m.p. –87 °Cexposure levels. 2.8–31%. ventilation. inhibited, usually with
b.p. 53 °C. Effects may be delayed. hydroquinone. May
form shock-sensitive
peroxides over time.
Ammonia Colourless liquid with Corrosive to eyes, As ammonia gas; Keep container tightly Reacts violently with
solutions pungent odour; for gas: respiratory system flammable range closed. In case of contact heavy metals such as
m.p. –78 °Cand skin on ingestion; 15–28%. with eyes, rinse mercury and their salts
b.p. –33 °C; lung oedema at high immediately and seek to form explosive
for 25% solution: levels of exposure to medical advice. Work in products.
m.p. –58 °Cgas or vapour. fume cupboard. Wear
b.p. 38 °C; rubber or plastic gloves and
miscible with water.chemical-grade goggles.
Aniline Colourless to brown, Cyanosis due to Combustible; Store in tightly sealed Strong oxidizers,
C
6
H
5
NH
2
oily liquid with an methaemoglobinaemia. flashpoint 70 °Ccontainers in areas strong acids.
aromatic amine-like Eye and skin irritation. explosive range separate from oxidizers.
odour; May be absorbed 1.2–11%. Prevent skin and eye
m.p. –6 °Cthrough the skin; contact. Work with local
b.p. 185 °C. repeated or prolonged exhaust ventilation or
exposure may cause respiratory protection,
sensitization. protective gloves, protective
clothing, face shield.
Auramine Yellow flakes or Harmful by ingestion, Avoid skin contact and Strong oxidizing agents.
4,4'-Carbono- powder; inhalation and skin inhalation of dust. Wear
imidoylbis m.p. 136 °C; contact. May cause rubber or plastic gloves
(N,N-dimethyl- insoluble in water. eye or skin irritation. and chemical-grade goggles.
benzenamine) Possible carcinogen. Work in fume cupboard or
wear dust respirator.
• 149 •
ANNEX 5. CHEMICALS: HAZARDS AND PRECAUTIONS
Benzene Colourless volatile Inhalation of vapour Highly flammable; Keep container in well- Can react violently
C
6
H
6
liquid with causes effects on flashpoint –11 °Cventilated area and away with oxidizers
characteristic central nervous flammable range from sources of ignition. including chromic acid,
aromatic odour; system resulting in 1.3–8%. Work in fume cupboardpotassium
m.p. 6 °Cvertigo and headache; or hood with adequate permanganate and
b.p. 80 °C. at high concentrations, ventilation. Wear eye liquid oxygen.
unconsciousness and protection and nitrile or
death. Risk of aplastic PVC gloves. Prevent
anaemia, leukaemia, formation of electrical
liver damage on charges by earthing/
prolonged or chronic grounding.
exposure. May be
absorbed through skin.
Benzidine Light yellow powder; May be absorbed Combustible, gives Avoid all exposure. Use is prohibited or
1,1'-Biphenyl- m.p. 128 °Cthrough skin. May off toxic fumes Wear eye and skin legally controlled in
4,4'-diamine b.p. 400 °Ccause bladder cancer.(gases) in a fire. protection. Work in fume many countries.
slightly soluble in Avoid all exposure. cupboard with exhaust
water but very soluble ventilation.
in acids and organic
solvents.
Bromine Dark reddish-brown Corrosive. Vapour is Not combustible but Use in closed system Strong oxidant, Attacks some
Br
2
fuming liquid with corrosive to eyes and enhances combustion and with ventilation. reacts violently with forms of plastic,
pungent odour; respiratory tract; of other substances. Wear protective gloves combustible and rubber and
m.p. –7.2 °Cinhalation may cause Many reactions may and clothing, safety reducing materials. coatings.
b.p. 58.8 °C. lung oedema and cause fire or goggles, face shield Reacts violently with
effects on central explosion. Heating or eye protection in aqueous ammonia,
nervous system. Eye will cause rise in combination with oxidants, metals,
contact can cause pressure with risk respiratory protection. organic compounds
blurred vision, redness, of burning. and phosphorus.
pain, severe tissue
burns.

• 150 •
LABORATORY BIOSAFETY MANUAL
CHEMICAL PHYSICAL PROPERTIES HEALTH HAZARDS FIRE HAZARDS SAFETY PRECAUTIONS INCOMPATIBLE CHEMICALS OTHER HAZARDS
Carbon dioxide Translucent white Risk of asphyxiation Wear protective Alkali metals, strong
(solid; “dry solid at –79 °C; in confined or poorly insulated gloves. Store bases.
ice”) CO
2
sublimes to gas at ventilated areas; contact only in ventilated room
ambient temperature. with solid “dry ice” or area in open
causes frostbite. container.
Carbon tetra- Colourless liquid with May be absorbed Not combustible. Avoid all contact. Work On contact with hot
chloride characteristic ether-through skin; may Gives off irritating with ventilation, local surfaces or flames,
CCl
4
like odour; cause dermatitis on or toxic fumes or exhaust or respiratory decomposes forming
m.p. –23 °Cprolonged exposure. gases in a fire. protection; use nitrile toxic and corrosive
b.p. 76.5 °C. Eye irritation. May gloves and protective fumes and gases
cause liver and kidney clothing, face shield or (hydrogen chloride,
damage and central eye protection in chlorine, phosgene).
nervous system combination with Reacts with some
disturbances resulting respiratory protection. metals such as
in headache, nausea, aluminium,
slight jaundice, loss of magnesium, zinc.
appetite and narcosis.
An animal carcinogen.
Chlorine Greenish-yellow gas Corrosive to eyes, skin Not combustible but Work with closed system Solution in water is a Attacks many
Cl
2
with pungent odour; and respiratory tract. enhances and ventilation. Wear strong acid, reacts metals in
m.p. –101 °CInhalation of gas may combustion of other cold-insulating gloves, violently with bases presence of water.
b.p. –34 °C. cause pneumonitis and substances. protective clothing, and many organic Attacks plastics,
lung oedema, resulting safety goggles or eye compounds, acetylene, rubber and
in reactive airways dys- protection in combination butadiene, benzene coatings.
function syndrome (RADS). with respiratory and other petroleum
Rapid evaporation of the protection. fractions, ammonia,
liquid may cause frostbite. hydrogen, sodium
High exposures may carbide, turpentine
result in death. Effects and finely divided
may be delayed; medical metals causing fire
observation indicated. and explosion hazard.
• 151 •
ANNEX 5. CHEMICALS: HAZARDS AND PRECAUTIONS
Chlorine Yellow to red gas or Severe irritation of Not combustible Work in closed system A strong oxidant; reacts
dioxide ClO
2
a red-brown liquid; eyes, skin and but enhances with ventilation. Wear violently with combustible
m.p –59 °Crespiratory tract; combustion of other protective gloves and and reducing materials.
b.p. 10 °C. inhalation of gas may substances; may clothing, safety goggles Reacts violently with
cause lung oedema. explode on heating, or eye protection in phosphorus, potassium
Effects may be delayed; on exposure to sun- combination with hydroxide, sulfur,
medical observation light or if subjected respiratory protection. ammonia, methane,
indicated. to shock and sparks. phosphine and hydrogen
sulfide.
Chloroform Colourless volatile Harmful by inhalation, Wear protective Strong bases; some When heated to
CHCl
3
liquid with ingestion and skin contact; clothing, nitrile gloves metals such as decomposition,
characteristic odour; skin irritation. May cause and eye protection. aluminium or forms phosgene
m.p. –63 °Ceffects on liver, kidneys Work in a fume magnesium, zinc gas.
b.p. 61 °C; and central nervous cupboard. powder; strong Attacks plastics,
slightly soluble in system resulting in oxidizers. rubber.
water. headache, nausea, slight
jaundice, loss of appetite,
narcosis. Prolonged or
chronic exposure causes
cancer in animals; sus-
pected human carcinogen.
Chromic acid Dark red odourless Irritation of eyes, skin Decomposes above Prevent skin and eye In aqueous solution is
CrO
3
flakes or powder often and respiratory 250 °C to chromic contact; avoid inhalation a strong acid which
Chromium VI used in aqueous system. Repeated or oxide and oxygen of fine dust and mist. reacts with bases and
oxide solutions; prolonged contact with with increased fire Work with ventilation, is corrosive. Strong
m.p. 197 °C. skin may cause derma- hazard. Many local exhaust or oxidant, reacts with
titis, chrome ulcers and reactions may cause respiratory protection. combustible, organic
skin sensitization. hazards. or other readily
Inhalation may cause oxidizable materials
asthma-like reactions. (paper, wood, sulfur,
May cause perforation of aluminium, plastics
nasal septum. Human etc.). Corrosive to
carcinogen. metals.
• 152 •
LABORATORY BIOSAFETY MANUAL
CHEMICAL PHYSICAL PROPERTIES HEALTH HAZARDS FIRE HAZARDS SAFETY PRECAUTIONS INCOMPATIBLE CHEMICALS OTHER HAZARDS
Copper Reddish, lustrous, Inhalation of copper Combustible. Work with local exhaust Shock-sensitive
Cu malleable, odourless fume may cause metal or repiratory protection, compounds are formed
solid; red powder, fume fever. protective gloves and with acetylenic
turns green on goggles. compounds, ethylene
exposure to moist air; oxides azides and
m.p. 1083 °Chydrogen peroxide.
b.p. 2567 °C. Reacts with strong
oxidants like chlorates,
bromates and iodates,
causing explosion hazard.
Cyanogen Colourless or white Severe eye, skin and Not combustible but Work in closed system Decomposes on heating
bromide crystals, with respiratory tract forms flammable gas with ventilation. Wear and on contact with
BrCN pungent odour; effects; inhalation of on heating. Gives off protective gloves and acids producing highly
m.p. 52 °Cvapour may cause lung irritating or toxic protective clothing, toxic and flammable
b.p. 61 °C. oedema which may fumes or gases in safety goggles face hydrogen cyanide and
result in convulsions, a fire. shield or eye protection corrosive hydrogen
unconsciousness, in combination with bromide. Reacts with
respiratory failure and respiratory protection. strong oxidants. Reacts
death. slowly with water and
moisture to produce
hydrogen bromide and
hydrogen cyanide.
Attacks many metals in
the presence of water.
Cytochalasin White powder; Toxic by ingestion, Avoid contact with eyes, Strong oxidizing agents.
(A–J) m.p. varies. inhalation or absorption skin, clothing; wear
through skin. May chemical-grade goggles
cause congenital fetal and rubber or plastic
malformation. gloves.
• 153 •
Diethyl ether Colourless highly Irritation of eyes and Extremely flammable; Keep container in well- Exposure to air and
C
2
H
5
OC
2
H
5
volatile liquid with respiratory tract . May flashpoint –45 °Cventilated area; keep light may result in
sweet characteristic affect central nervous flammable range away from sources of formation of explosive
odour; system causing 1.7–48%. ignition; earth/ground peroxides. Can react
m.p. –116 °Cdrowsiness and containers to prevent violently with oxidizers
b.p. 34 °C; unconsciousness. static electrical and halogens.
slightly soluble in Repeated inhalation discharges. Work in
water. may cause addiction. fume cupboard. Wear
nitrile gloves to prevent
defatting of skin.
Dimethylamine Colourless volatile Severe irritation of Extremely flammable; Keep away from sources Can react with oxidizers,
(CH
3
)
2
NH liquefied gas with eyes and respiratory flashpoint –26 °Cof ignition; in case of mercury.
pungent odour; system; inhalation flammable limits contact with eyes rinse
m.p. –93 °Cmay cause lung oedema. 2.8–14%. immediately and seek
b.p. 7 °C; Rapid evaporation may Solution highly medical advice. Work in
miscible with water.cause frostbite. flammable; fume cupboard. Wear
Solution is corrosive to flashpoint –18 °C. nitrile gloves and
eyes and skin. chemical-grade goggles.
2,4-Dinitro- Orange-red crystalline Irritation of skin and Keep moist to reduce Can react vigorously
phenyl-hydrazine powder; eyes. Harmful by explosion risk. Wear with oxidizers and
C
6
H
3
(NO
2
)
2
-m.p. 200 °C; ingestion, inhalation dust respirator, rubber reducers.
NHNH
2
slightly soluble in water.and skin contact. or plastic gloves and
1-Hydrazino- chemical-grade goggles.
2,4-dinitro-
benzene
ANNEX 5. CHEMICALS: HAZARDS AND PRECAUTIONS
• 154 •
LABORATORY BIOSAFETY MANUAL
CHEMICAL PHYSICAL PROPERTIES HEALTH HAZARDS FIRE HAZARDS SAFETY PRECAUTIONS INCOMPATIBLE CHEMICALS OTHER HAZARDS
Dioxane Colourless liquid, Irritation of eyes and Highly flammable; Work with ventilation, Can form explosive
C
4
H
8
O
2
with characteristic respiratory tract. May distant ignition local exhaust. No open peroxides. Reacts
Diethylene odour; affect central nervous possible; as a result flames, no sparks, no vigorously with strong
dioxide m.p. 12 °Csystem resulting in of flow, agitation, smoking, no contact oxidants and
b.p. 101 °C. headache, nausea, etc., electrostatic with strong oxidants or concentrated strong
cough, sore throat, charges can be hot surfaces. Do not use acids. Reacts
abdominal pain, generated. compressed air for filling, explosively with some
dizziness, drowsiness, discharging or handling; catalysts. Attacks many
vomiting, unconscious- use non-sparking tools. plastics.
ness. May be absorbed Wear protective gloves,
through skin. Kidney clothing, face shield or
and liver damage. eye protection, in
Probably a human combination with
carcinogen. respiratory protection.
Ethanol Colourless volatile Harmful if ingested. Highly flammable; Keep container tightly Reacts violently with
CH
3
CH
2
OH liquid with slight, Irritation of eyes. May flashpoint 12 °Cclosed; keep away from strong oxidizers.
characteristic odour; affect central nervous flammable limits ignition sources.
m.p. –117 °Csystem. 3–19%.
b.p. 79 °C;
miscible with water.
Ethanolamine Colourless non-volatile Corrosive to eyes, Flashpoint 85 °C. Wear rubber or plastic Reacts with strong
H
2
NCH
2
CH
2
OH viscous liquid with respiratory system gloves and eye oxidizers.
2-Amino- ammoniacal odour; and skin. May cause protection.
ethanol m.p 10 °Cskin sensitization.
b.p. 171 °C;
miscible with water.
• 155 •
Formaldehyde Colourless liquid with Severely irritation of Flashpoint 50 °C. Wear protective clothing Can react vigorously Concentrated
solution a pungent odour; eyes and skin, irritation such as plastic apron, with oxidizers, with formaldehyde
(37–41% b.p. 96 °C; of respiratory tract; pro- rubber or plastic gloves nitromethane to solutions become
formaldehyde miscible with water.longed exposure to the and chemical-grade produce explosive cloudy if stored
with 11–14% vapour may cause goggles. Work in fume products, with below 21 °C and
methanol) asthma-like symptoms, cupboard or well- hydrochloric acid to should be kept at
HCHO conjunctivitis, laryngitis, ventilated area. produce the potent 21–25 °C. Dilute
bronchitis or broncho- carcinogen
bis
solutions (1–5%)
pneumonia. May cause (chloromethyl) ether. and medium-
sensitization by skin strength solutions
contact. Possible risk (5–25%) retain
of irreversible health many of the
effects. Possible hazards of the
carcinogen. concentrated form.
Glutaraldehyde Colourless or pale Severe irritation of Work in fume cupboardCan react vigorously Often supplied in
OHC(CH
2
)
3
CHO yellow solution with eyes and upper or well-ventilated area. with oxidizers. aqueous solution
pungent odour; respiratory tract; Wear rubber or plastic at various concen-
m.p. –14 °Cprolonged inhalation gloves and eye trations with added
b.p. 189 °C; exposure or skin contact protection. stabilizer to
miscible with water.may cause sensitization. enhance stability.
Hydrochloric Colourless fuming Corrosive to eyes, Do not breathe fumes; Reacts violently with Releases highly
acid (10–37%) liquid with a pungent respiratory system use respiratory protection. bases (solids and toxic fumes in
HCl odour; and skin; repeated In case of contact with concentrated solutions), fires.
Hydrogen b.p. –121 °C; inhalation of vapour eyes, rinse immediately explosively with
chloride miscible with water.can cause chronic with water and seek solid potassium
bronchitis. medical advice; in case of permanganate. Gives
contact with skin, wash off toxic or explosive
immediately with plenty gases on contact with
of water. Work in fume many metals.
cupboard. Wear rubber
or plastic gloves and eye
protection (spectacles or
goggles).
ANNEX 5. CHEMICALS: HAZARDS AND PRECAUTIONS
• 156 •
LABORATORY BIOSAFETY MANUAL
CHEMICAL PHYSICAL PROPERTIES HEALTH HAZARDS FIRE HAZARDS SAFETY PRECAUTIONS INCOMPATIBLE CHEMICALS OTHER HAZARDS
Hydrogen Colourless liquid; Corrosive at high Oxidizing agent; In case of contact with Reacts vigorously with Can decompose
peroxide m.p. –39 °C (70%) concentration (60%), contact with skin, wash immediately a variety of chemical evolving oxygen,
H
2
O
2
b.p. 125 °C (70%); and at low combustible material with plenty of water.reagents including causing pressure
miscible with water,concentration (6%) can cause fire. Wear nitrile gloves and oxidizers and bases. rise in container.
supplied in aqueous if contact with skin is eye protection if Attacks most metals Store in dark, cool
solution at various prolonged. Dilute concentration or their salts, flammable place. Do not use
concentrations. solutions are irritating exceeds 20%. liquids and other metallic containers
to eyes, respiratory combustible materials or equipment, e.g.
system and skin. (paper, textiles), aniline brass, copper,
and nitromethane. iron.
Hydrogen Colourless gas with May cause effects on Extremely flammable; Work with ventilation, Strong oxidizers and Sense of smell
sulfide a strong odour of central nervous system explosive limits local exhaust. Wear strong nitric acid. becomes rapidly
H
2
Srotten eggs; resulting in headache, 4.3–46%. safety goggles or eye Attacks many metals fatigued and
b.p. –60 °Cdizziness, cough, sore protection in and plastics. cannot be relied
m.p. –85 °C. throat, nausea, laboured combination with on to warn of the
breathing, unconscious- respiratory protection. continuous
ness and death. Inhala- presence of the
tion may cause lung gas.
oedema. Redness, pain,
severe deep burns of eyes.
Iodine Bluish-black Irritation of eyes, Not combustible Do not breathe vapour; Reacts violently with
I
2
crystalline scales with respiratory system but enhances avoid contact with metals including
a characteristic odour; and skin. Repeated combustion of other eyes. Wear nitrile aluminium, potassium
m.p. 114 °Cexposure may cause substances. Many gloves. and sodium, and with
b.p. 184 °C; skin sensitization. reactions may cause ethanol/phosphorus
practically insoluble May have effect on fire or explosion. Gives mixtures, acetylene
in water. thyroid. off irritating or toxic and ammonia.
fumes (or gases) in a fire.
• 157 •
Mercury Heavy silvery liquid; May be absorbed Not combustible. Keep container tightly Acetylene, fulminic Store containers
Hg m.p. –39 °Cthrough skin. Gives off irritating or closed. Work in fume acid. Reacts with and use over catch-
(Quicksilver) b.p. 357 °C; Repeated exposure toxic fumes in a fire. cupboard or well- ammonia, azides and ment trays to
insoluble in water.may affect kidneys ventilated area. Prevent ethylene oxide to form contain spillage;
and central nervous spills. Observe strict explosive products. suck up spilt drop-
system, and may cause hygiene. Wear nitrile Reacts violently with lets into a small
vomiting, diarrhoea, gloves. bromine. Forms respiratory bottle
headache, nausea, amalgams with many fitted with a capillary
swollen gums, loose metals. connecting tube
teeth. and connected to
a pump; treat spilt
areas with zinc dust
to form an amalgam.
Methanol Colourless volatile Effects on central Highly flammable; Keep container tightly Can react vigorously
CH
3
OH liquid with nervous system result- flashpoint –16 °Cclosed; keep away from with oxidizers. Reactions
characteristic odour; ing in unconsciousness; flammable range ignition sources. Avoid with magnesium or
m.p. –98 °Cmucous membrane 7–37%. breathing vapour and bromine can be violent
b.p. 65 °C; irritation. Chronic contact with skin. Work and those with strong
miscible with water.exposure can cause in fume cupboard or well- oxidants or chloroform
damage to retina and ventilated area. Wear with sodium can be
optic nerve. Prolonged rubber or plastic gloves explosive.
skin contact may cause and eye protection.
dermatitis. May be
absorbed through skin.
Naphthylamine White to pink crystals Both forms very toxic Combustible. Avoid all exposure; wear Use is prohibited or
(alpha and beta) with characteristic odour; by inhalation, ingestion suitable protective legally controlled in
C
10
H
9
Nalpha: m.p. 50 °Cand skin contact. clothing. Work in fume many countries.
N
-phenyl-α-b.p. 301 °C; Human carcinogen cupboard or hood, or
naphthylamine beta: m.p. 113 °Ccausing bladder cancer.with exhaust ventilation.
and b.p. 306 °C; Experimental mutagen
N
-phenyl-β-poorly soluble in water and teratogen.
naphthylamine but hydrochloride is Absorbed through skin.
water-soluble.
ANNEX 5. CHEMICALS: HAZARDS AND PRECAUTIONS
• 158 •
LABORATORY BIOSAFETY MANUAL
CHEMICAL PHYSICAL PROPERTIES HEALTH HAZARDS FIRE HAZARDS SAFETY PRECAUTIONS INCOMPATIBLE CHEMICALS OTHER HAZARDS
Ninhydrin Pale yellow solid, Harmful by ingestion Flammable, Avoid inhalation of the Contact with skin
C
9
H
6
O
4
decomposes before and inhalation. Irritation combustible solid; spray or vapour and produces a
melting at 241 °C. of eyes, respiratory flashpoint 39 °C. contact with eyes. Wear persistent violet
Supplied in aerosol system and skin. rubber or plastic gloves stain.
spray cans as 0.5% Repeated exposure may and chemical-grade
solution in butanol; cause skin sensitization. goggles.
soluble in water.
Nitric acid Colourless or pale Corrosive; causes Oxidizer; contact Do not breathe vapour; Acetic acid, chromic Concentrated nitric
(50–70%) yellow fuming liquid; severe burns to eyes with combustible use respiratory protection. acid, hydrocyanic acid, acid is involved in
HNO
3
m.p. –42 °Cand skin. Inhalation of material may cause In case of contact with aniline, carbon, more dangerous
b.p. 83–121 °C; vapour may cause lung fire. Evolves toxic eyes, rinse immediately hydrogen sulfide, bases, reactions than any
miscible in water. oedema. fumes in a fire. and seek medical attention; metals and many other other chemical
in case of contact with substances. reagent.
skin, wash off immediately;
remove contaminated
clothing. Wear PVC gloves,
plastic apron and chemical-
grade goggles. Work in
fume cupboard.
Nitrobenzene Pale yellow oily liquid, Methaemoglobinanaemia Combustible; risk of Work with ventilation, On combustion forms
C
6
H
5
NO
2
with characteristic odour; with cyanosis, liver fire and explosion; local exhaust or corrosive fumes including
m.p. 6 °Cdamage; symptoms flashpoint 88 °C. respiratory protection. nitrogen oxides. Reacts
b.p. 211 °C. include blue lips or Wear protective violently with strong
fingernails, blue skin, gloves, protective oxidants and reducing
dizziness, nausea, clothing, safety goggles. agents, causing fire and
weakness, unconscious- explosion hazard. Attacks
ness. Absorbed through many plastics. Forms ex-
skin. plosive (thermally unstable)
substances or mixtures
with many organic and
inorganic compounds.
• 159 •
Osmium Pale yellow crystals with Very toxic by inhalation, Powerful oxidizing Keep container tightly
tetroxide pungent odour; ingestion and skin agent. Not combustible closed and in a well-
OsO
4
m.p. 40 °Ccontact, causing severe but enhances ventilated area. Work with
b.p. 130 °C; burns and irritation. combustion of other solid and solutions in fume
sublimes below boiling Vapour, solid and substances. cupboard or hood. Wear
point; soluble in water.solutions are corrosive chemical-grade goggles
to skin and respiratory and protective gloves. To
tract. Inhalation may make up solutions, add
cause lung oedema. unopened ampoule to
required volume of water,
stopper and shake to break
ampoule.
Oxalic acid Colourless crystals; Harmful if in contact Combustible. Gives Avoid contact with skin Oxidizing agents; also
HO
2
CCO
2
Hsoluble in water; with skin or if ingested. off irritating or toxic and eyes; wear eye silver and mercury
m.p. 190 °C, Dust irritates respiratory fumes (or gases) in a protection and gloves. and their compounds.
decomposes. tract and eyes. Solutions fire.
irritate eyes and may
cause skin burns.
Oxygen Colourless compressed At very high concen- Not combustible but No open flames, no A strong oxidant, reacts
O
2
gas; trations, irritation of enhances combustion sparks, no smoking, no with combustible and
m.p. –218.4 °Crespiratory tract. of other substances. contact with flammable reducing materials,
b.p. –183 °C. Heating will cause rise substances. causing fire and explosion
in pressure of container hazard. Reacts with oils,
with risk of bursting. greases, hydrogen, and
flammable liquids, solids
and gases.
ANNEX 5. CHEMICALS: HAZARDS AND PRECAUTIONS
• 160 •
LABORATORY BIOSAFETY MANUAL
CHEMICAL PHYSICAL PROPERTIES HEALTH HAZARDS FIRE HAZARDS SAFETY PRECAUTIONS INCOMPATIBLE CHEMICALS OTHER HAZARDS
Perchloric acid Colourless liquid; Corrosive; causes severe Powerful oxidizing Avoid breathing vapour Combustible materials Powerful oxidizing
HClO
4
miscible with water.burns to eyes and skin agent. Not combustible and other exposure; wear and reducing agents: agent; may form
and if ingested. Vapour but enhances protective clothing acetic anhydride, explosive products
is corrosive to eyes, skin, combustion of other including nitrile gloves, bismuth and its alloys, if in contact with
and respiratory tract. substances. eye and face protection. alcohol, metal, paper,many inorganic and
Inhalation of vapours With hot solutions work wood and other organic organic materials;
may cause lung in fume cupboard or hood. materials. contaminated wood-
oedema. en floors, benches,
etc. May explode on
percussion.
Phenol Colourless or pale Substance and vapours Flashpoint 80 °CDo not breathe vapour; Reacts with oxidants
C
6
H
5
OH pink crystals with are corrosive to eyes, flammable range use respiratory protection. causing fire and
characteristic odour; skin and respiratory 1.7–6%. Avoid eye and skin contact. explosion hazard.
m.p. 41 °Ctract causing severe Work in fume cupboard.
b.p. 182 °C; burns; absorbed through Wear nitrile gloves and
soluble in water. skin. Central nervous eye protection. In case of
system disturbance, contact with eyes, rinse
coma. Kidney and liver immediately with water
damage. Symptoms and seek medical advice;
include abdominal pain, in case of contact with
vomiting, diarrhoea, skin skin, remove any
irritation, eye pain. contaminated clothing
Prolonged contact with and swab the
dilute solutions may contaminated area with
cause dermatitis. glycerol, polyethylene
glycol 300 or a mixture
of liquid polyethylene
glycol (70%) and methyl-
ated spirit (30%) and then
flush with water.
• 161 •
Phosphoric Colourless viscous Corrosive; causes Attacks many metals In case of contact with
acid liquid or hygroscopic burns to the skin and producing hydrogen. eyes, rinse with water and
H
3
PO
4
white crystals; eyes. Gives off toxic fumes obtain medical advice.
m.p. 42 °Cin a fire. Wear nitrile gloves and
decomposes below eye protection.
boiling point at 213 °C;
soluble in water.
Phosphorus Hygroscopic white Corrosive to the eyes, Not combustible but Work with local exhaust Solution in water is a
pentoxide crystals or powder; skin, respiratory tract, enhances combustion protection. Wear strong acid; reacts
P
2
O
5
m.p. 340 °C, sublimation leading to sore throat, of other substances. protective gloves and violently with bases and
point 360 °C. cough, burning Many reactions may clothing, face shield, or is corrosive. Reacts
sensation, shortness of cause fire or explosion. eye protection in violently with perchloric
breath; skin burns, pain, Gives off irritating or combination with acid causing fire and
blisters, eye burns. toxic fumes (or gases) respiratory protection. explosion hazard. Reacts
Inhalation may cause in a fire. violently with water
lung oedema. Ingestion forming phosphoric acid.
may cause abdominal Attacks many metals in
cramps, burning presence of water.
sensation, diarrhoea,
sore throat, vomiting.
Picric acid Yellow crystals Toxic by ingestion, Explosive when dry. Keep moistened with Forms salts with many Yellow skin stains.
C
6
H
2
(NO
2
)
3
OH moistened with water inhalation or skin water at all times or use metals which are more
2,4,6- or dissolved in alcohol; contact. Ingestion may only in alcoholic solution. explosive than the acid
Trinitrophenol m.p. 122 °C; result in headache, itself. In contact with
slightly soluble in water.nausea. Irritation of concrete may form
eyes. calcium picrate, which is
a friction-sensitive
explosive. May react
vigorously with reducing
agents.
ANNEX 5. CHEMICALS: HAZARDS AND PRECAUTIONS
• 162 •
LABORATORY BIOSAFETY MANUAL
CHEMICAL PHYSICAL PROPERTIES HEALTH HAZARDS FIRE HAZARDS SAFETY PRECAUTIONS INCOMPATIBLE CHEMICALS OTHER HAZARDS
Potassium White flakes, powder,Corrosive to In case of contact with Reacts violently with Attacks some
hydroxide pellets or sticks; respiratory system, eyes, rinse immediately acids and with nitro- metals (aluminium,
KOH m.p. 360 °Ceyes and skin; with water and seek benzene and many zinc, tin) in the
b.p. 1320 °C; inhalation of dust medical advice; in case other detergents. presence of
very soluble in water.causes lung oedema. of contact with skin, wash Evolves large quantity moisture.
immediately; remove of heat when mixed
contaminated clothing. with water; store in a
Wear rubber or plastic well-sealed container.
gloves and eye protection
even for dilute solutions.
Potassium Purple crystals; Corrosive if swallowed Powerful oxidizing Wear protective clothing, Reacts violently or
permanganate m.p. 240 °Cor if dust is inhaled. agent; may ignite eye protection and explosively if mixed with
KMnO
4
(decomposes); Extreme irritation of combustible materials. particulate respirator if a wide variety of
readily soluble in water.eyes and respiratory dust is produced. inorganic and organic
tract, inhalation of dust compounds or powdered
may cause lung oedema. metals.
Potassium White deliquescent Toxic by ingestion and Wear protective
tellurite crystals; very soluble inhalation of dust. clothing.
K
2
TeO
3
in water. Irritation of skin and eyes.
Propan-2-ol Colourless liquid with Irritation of eyes and Highly flammable; Keep container tightly Can react vigorously 70–85% propan-2-
(CH
3
)
2
CHOH alcoholic odour; respiratory tract. May flashpoint 112 °Cclosed; keep away from with oxidizers to form ol in water used as
Isopropanol m.p. –89 °Caffect central nervous flammable range ignition sources. Work in unstable peroxides on a disinfectant spray
b.p 82 °C; system causing 2.3–12.7%. fume cupboard. Wear prolonged exposure to remains a
miscible with water.headache, dizziness, nitrile gloves and eye air and light. flammable hazard
nausea, vomiting and protection. and should not be
coma. used near ignition
sources.
• 163 •
Pyridine Colourless liquid with Affects central nervous Highly flammable; Work with ventilation, Reacts violently with
C
5
H
5
Ncharacteristic odour; system causing dizzi- flashpoint 20 °Clocal exhaust or strong oxidants and
m.p. 42 °Cness, headache, nausea, explosive limits, respiratory protection; strong acids.
b.p. 115 °C. shortness of breath, 1.8–12.4%. wear gloves and
unconsciousness. May Gives off irritating or protective clothing.
be absorbed through skin toxic fumes (or gases)
causing redness and in a fire. Vapour/
burning sensation. In- mixtures are explosive.
gestion causes abdominal
pain, diarrhoea, vomiting,
weakness. Repeated
exposure causes liver
and kidney effects.
Selenium Odourless solid in Irritation of skin and Combustible. Gives Prevent dispersion of Reacts violently with oxi-
Se various forms, dark eye. Inhalation of dust off irritating or toxic dust. Observe strict dants and strong acids.
red-brown to bluish- may cause lung oedema. fumes (or gases) in hygiene. Work with local Reacts with water at 50 °C
black amorphous solid Repeated exposure may a fire. exhaust. Wear protective forming flammable
or red transparent cause loss of nails, gloves, clothing, and hydrogen and selenious
crystals or metallic grey gastrointestinal effects. safety spectacles. acids. Reacts with incan-
to black crystals; descence on gentle
m.p. 170–217 °Cheating with phosphorus
b.p. 685 °C. and metals such as nickel,
potassium, platinum,
sodium and zinc.
Silver White metal, turns dark Inhalation of high Not combustible Work with local exhaust. Incompatible with
Ag on exposure to ozone, amounts of metallic except as powder. Wear protective gloves acetylene, ammonium
hydrogen sulfide or silver vapours may cause and safety spectacles or compounds, oxalic acid
sulfur; lung damage with eye protection in and tartaric acid.
m.p. 962 °Cpulmonary oedema. May combination with
b.p. 2212 °C. cause a grey-blue discol- respiratory protection for
oration of the eyes, nose, powder or fume.
throat and skin on long-
term or repeated
exposure (argyria).
ANNEX 5. CHEMICALS: HAZARDS AND PRECAUTIONS
• 164 •
LABORATORY BIOSAFETY MANUAL
CHEMICAL PHYSICAL PROPERTIES HEALTH HAZARDS FIRE HAZARDS SAFETY PRECAUTIONS INCOMPATIBLE CHEMICALS OTHER HAZARDS
Silver nitrate White crystals; May cause severe Not combustible but Prevent dispersion of Ammoniacal solutions
AgNO
3
m.p. 212 °Cirritation and burns to enhances combustion dust. Observe strict can precipitate explosive
b.p. 444 °C; eyes and skin. Corrosive of other substances. hygiene. Wear protective silver nitrite in the
soluble in water. by ingestion. May cause rubber or plastic gloves, presence of base or
a red-blue discoloration and face shield or eye glucose. Can form
of the skin on long-term protection in combination explosive products with
or repeated exposure with respiratory protection. ethanol and may cause
(argyria). In case of contact with explosive polymerization
eyes, rinse with water with acrylonitrile. May
and seek medical advice. cause ignition of explosion
if mixed with charcoal,
magnesium, phosphorus
or sulfur.
Sodium azide Colourless crystalline Very toxic by ingestion, Decomposes In case of contact with Explosive reactions with
N
3
Na solid; inhalation and skin explosively when skin, wash immediately.bromine, carbon disulfide
m.p. 300 °C; contact; may cause heated above its melt- Do not inhale dust. or chromyl chloride. Solid
soluble in water. burns. Dust and ing point. Gives off Wear rubber or plastic reacts with heavy metals
solution irritate eyes and toxic fumes when gloves and eye including copper, lead and
skin; may be absorbed heated; do not use protection. mercury to form explosive
through skin. water to extinguish metal azide salts. On
fires. contact with acid, develops
highly toxic and explosive
gas.
Sodium Colourless, white Toxic by ingestion and Wear protective clothing. Oxidizing agents.
biselenite crystalline powder; inhalation of dust;
NaHSeO
3
soluble in water. possible danger of
cumulative effects.
Experimental teratogen.
Prolonged skin contact
may cause dermatitis.
• 165 •
ANNEX 5. CHEMICALS: HAZARDS AND PRECAUTIONS
Sodium cyanide White crystalline Extremely toxic by May give off toxic Do not inhale dust; use Liberates extremely Treat spillage of
NaCN powder with almond ingestion, inhalation fumes in a fire. respiratory protection. toxic hydrogen cyanide solutions with
odour; and skin contact; Avoid eye and skin contact; (HCN) gas on contact bleaching powder
m.p. 563 °Cseverely irritating to in case of contact with with acids or with water (sodium hypo-
b.p. 1496 °C; eyes. May be absorbed skin, wash immediately containing dissolved chlorite) and leave
very soluble in water. through skin. Repeated with water and remove carbon dioxide. Can for 24 h. Sweep up
exposure may affect contaminated clothing. form explosive mixtures solid spills carefully
thyroid. Wear chemical-grade with nitrites. and add to water
goggles and rubber or containing
plastic gloves. Keep in a bleaching powder;
securely locked, ventilated leave for 24 h
store. before discarding.
Keep cyanide anti-
dote kit available in
the laboratory.
Sodium Colourless flakes, Solid and concentrated Not combustible. In case of contact with Evolves large quantity Store in well-sealed
hydroxide powder, pellets or solute. Inhalation of Contact with moisture eyes rinse immediately of heat when mixed with container in dry
NaOH sticks; dust causes damage to or water may generate and seek medical advice; water. Reacts vigorously place.
m.p. 318 °Crespiratory tract, lung sufficient heat to in case of contact with with chloroform-
b.p. 1390 °C; oedema. Corrosive by ignite combustible skin wash immediately methanol mixtures and
soluble in water. ingestion. Dilute substances. with water, remove with strong acids.
solutions irritating to contaminated clothing.
eyes or may cause severe Wear rubber or plastic
damage if eye contact is gloves and eye protection
prolonged. even with dilute solutions.

• 166 •
LABORATORY BIOSAFETY MANUAL
CHEMICAL PHYSICAL PROPERTIES HEALTH HAZARDS FIRE HAZARDS SAFETY PRECAUTIONS INCOMPATIBLE CHEMICALS OTHER HAZARDS
Sodium Colourless or pale Corrosive to eyes and Strong oxidant. May In case of contact with Liberates highly toxic Gradually loses
hypochlorite yellow solution with skin; corrosive by give off toxic fumes eyes, rinse immediately gas in contact with acids. chlorine during
solution chlorine odour; ingestion and to in a fire. with water and seek Can react vigorously storage; dilute
(10–14% miscible with water.respiratory tract; medical advice; in case of with combustible and solutions used as
available inhalation may cause contact with skin, wash reducing compounds. disinfectant rapidly
chlorine) lung oedema. Repeated immediately. Do not inhale May react with nitrogen deteriorate. Store
NaOCl exposure may cause vapour; use respiratory compounds to form away from acids in
skin sensitization. protection. Work in well- explosive N-chloro- a dark, cool, well-
ventilated area. Wear compounds; may react ventilated area.
rubber or plastic gloves violently with methanol.
and chemical-grade eye
protection.
Sulfuric acid Colourless, odourless Concentrated solution May give off toxic In case of contact with Is a powerful oxidizing Localized boiling
H
2
SO
4
viscous liquid; (15%) corrosive, causes fumes in a fire. Not eyes rinse immediately desiccant and reacts may occur if
m.p. 10°Csevere burns; mist and combustible. Many and seek medical advice; violently with many concentrated acid
b.p. (decomposes) vapour highly corrosive reactions may cause in case of contact with reagents including is added to water.
340 °C. by inhalation; dilute fire or explosion. skin wash immediately organic nitro compounds,
solutions irritating to Dilution with water remove contaminated potassium permanganate,
eyes and skin; cause generates heat and clothing. Wear nitrile alkali metals and per-
burns and dermatitis. spattering or boiling gloves, eye and face chlorates, combustible
may occur. Always protection. No contact materials, oxidizers,
add acid to water, with flammable amines, bases, water,
never add water to substances.excess heat and most
acid.metals.
• 167 •
ANNEX 5. CHEMICALS: HAZARDS AND PRECAUTIONS
Tetrahydrofuran Colourless liquid, with Central nervous system Highly flammable; Work with ventilation, Reacts violently with
C
4
H
8
Ocharacteristic odour; depressant causing may form explosive local exhaust or strong oxidants, strong
Diethylene oxide m.p. –108.5 °Cnarcosis. Eye, skin and peroxides; respiratory protection, bases and some metal
Tetramethylene b.p. 66 °C. respiratory irritation. flashpoint –14 °C. protective gloves, safety halide, causing fire and
oxide Water may be ineffect- spectacles. explosion hazard. Attacks
ive to fight fires some forms of plastics,
involving tetrahydro- rubber and coatings.
furan, but it can be Tetrahydrofuran may
used to cool fire- polymerize in the presence
exposed containers. of cationic initiators.
Refluxing with calcium
hydroxide can cause
explosions.
Thallium acetate White deliquescent Extremely toxic by Keep container tightly
TlC
2
H
3
O
2
crystals; ingestion with possible closed. Work in fume
m.p. 110 °C; cumulative effects. cupboard, hood or
very soluble in water.Affects nervous and with exhaust ventilation.
cardiovascular systems. Wear protective clothing
Harmful through eye and including dust respirator,
skin contact. chemical-grade goggles,
rubber or plastic gloves,
eye protection.
o
-Tolidine Colourless crystals; Harmful by contact with Combustible. Gives Avoid contact; wear eye Oxidizing agents.
(C
6
H
3
-(3CH
3
)- m.p. 131 °Cskin or ingestion. Dust off irritating or toxic protection and gloves.
(4NH
2
))
2
b.p. 200 °C; irritates respiratory tract fumes (or gases) in
3,3'-Dimethyl- poorly soluble in water.and eyes. Probably a a fire.
(1,1'-biphenyl)- human carcinogen.
4,4'-diamine
• 168 •
LABORATORY BIOSAFETY MANUAL
CHEMICAL PHYSICAL PROPERTIES HEALTH HAZARDS FIRE HAZARDS SAFETY PRECAUTIONS INCOMPATIBLE CHEMICALS OTHER HAZARDS
Toluene Colourless liquid with Central nervous Highly flammable; Keep container tightly Can react with strong
C
7
H
8
characteristic odour; system depressant. vapour may cause closed; keep away from acids, alkalis and
Methylbenzene m.p. –95 °CIrritation of eyes, flash fire; ignition sources; earth/ oxidizers.
b.p. 111 °C; mucous membranes, flashpoint 4 °C(ground) containers to
not miscible with water.skin. Repeated exposure flammable range prevent static electrical
may cause toxicity in 1.4–7%. discharge. Do not inhale
human reproduction or Extinguishing media vapour; use respiratory
development. for a small fire: dry protection. Work in fume
chemicals, carbon cupboard or well-ventilated
dioxide, foam, water area. Wear nitrile gloves.
fog or inert gas
(nitrogen).
Trichloroacetic White hygroscopic Corrosive; causes Not combustible. May Avoid contact with eyes Violent reaction with Store in a dry place.
acid crystals with pungent severe burns to eyes, give off toxic fumes and skin; wear rubber or copper/dimethyl Concentrated
CCl
3
COOH odour; skin, respiratory tract. in fire. plastic gloves and sulfoxide mixtures and aqueous solutions
m.p. 58 °Cchemical-grade goggles on contact with bases, may decompose
b.p. 197.5 °C; or face shield in strong oxidizing agents violently.
soluble in water, ethanol, combination with and metals such as iron,
diethylether. respiratory protection. In zinc, aluminium.
case of contact with eyes,
rinse immediately and
seek medical advice.
• 169 •
ANNEX 5. CHEMICALS: HAZARDS AND PRECAUTIONS
Trichloro- Colourless liquid, Irritation of eyes, skin; Combustible under Work with ventilation, On contact with hot
ethylene characteristic odour; prolonged exposure specific conditions. local exhaust. Wear surfaces or flames,
CHClCCl
2
m.p. –73 °Cmay cause dermatitis gloves, safety spectacles decomposes forming
b.p. 87 °C. and affect the central or other eye protection toxic and corrosive gases
nervous system resulting in combination with (phosgene, hydrogen
in loss of memory. May respiratory protection. chloride). Decomposes
affect liver and kidneys. on contact with strong
Probably a human alkali producing dichloro-
carcinogen. acetylene; reacts violently
with metal powders such
as aluminium, barium,
magnesium and titanium;
slowly decomposed by
light in the presence of
moisture, with formation
of hydrochloric acid.
Xylene (mixed Colourless liquid with May affect central Flammable liquid; Avoid contact with eyes. May contain
isomers) aromatic odour; nervous system flashpoint 27–32 °C. Wear nitrile gloves and ethylbenzene as an
C
6
H
4
(CH
3
)
2
m.p. –95 to –13 °Cresulting in headache, eye protection. Keep impurity.
Dimethyl- b.p. 136–145 °C; dizziness, fatigue and container tightly closed; Ethylbenzene is a
benzene insoluble in water. nausea. Liquid and keep away from ignition possible human
vapour irritate eyes, skin, sources. carcinogen.
mucous membranes,
respiratory tract. Harmful
if ingested. Prolonged
skin contact may defat
the skin. Non-specific
neurological impairment.
Exposure may enhance
hearing damage caused
by exposure to noise.
Animal tests suggest
toxicity to human repro-
duction or development.
Index
• 170 •
abrasions 79
access
animal facilities 29, 30, 31
laboratory 9–10, 21, 26
accidents 11
equipment-related 144
see also first aid; injuries; spills
acetaldehyde 146
acetic acid 146
acetic anhydride 146
acetone 147
acetonitrile 147
acetylene 147
acrolein 148
aerosols
activities generating 51
biological safety cabinets and 15, 51
pipetting hazards 63
release of potentially infectious 79
safety equipment and 61
air, exhaust see exhaust air
air filters see high-efficiency particulate air
(HEPA) filters
airflow, directional
alarms 21, 60
animal facilities 29, 30, 31
biological safety cabinets 53, 56
Biosafety Level 3: 21
Biosafety Level 4: 26–27
air systems
biological safety cabinets 51–52, 53–54,
56–57
protective suit 26
see also ventilation systems
airlocks 26, 27, 31
alarms 21, 60
alcohol-based hand rubs 87–88, 90
alcohols 87–88
allergy, latex 66
2-amino-ethanol 154
ammonia solutions 148
ammonium bicarbonate 89
ampoules of infectious materials
opening 74
storage 74
anaerobic jars/incubators 142, 144
aniline 148
animal facilities 10, 28–32
Biosafety Level 1: 29
Biosafety Level 2: 29–30
Biosafety Level 3: 30–31
Biosafety Level 4: 31–32
containment levels 28
invertebrates 32
animal facility Biosafety Levels (ABSL) 28
animals
disposal of carcasses 30
non-experimental 10, 29
transgenic and knock-out 102–103
anterooms 21, 30, 31
antimicrobial 82
antiseptics 82, 87, 88
aprons 64, 65
arthropods
control of verminous 12, 29
housing facilities 32
audit 36–37
auramine 148
autoclaves 63, 90–92
animal facilities 30, 31
availability 13, 16, 22, 27
double-door pass-through 26, 27
• 171 •
fuel-heated pressure cooker 91
gravity displacement 90, 91
loading 91
pre-vacuum 91
precautions for use 92
validation 16
autoclaving 18, 90–92
azides 108, 164
backflow prevention 14, 22
basic laboratory (Biosafety Levels 1 and 2)
1, 9–19
chemical, fire, electrical, radiation and
equipment safety 19
code of practice 9–12
design and facilities 12–14, 15
equipment 13–16
health and medical surveillance 16
safety survey forms 38–42
training 16–17
waste handling 17–19
bedding, animal 30, 31
bench tops 12
benzene 149
benzidine 149
biocide 82
biohazard warning sign 9, 10, 20, 29
biological expression systems 102
biological safety cabinets (BSCs) 51–60, 62
air connections 56–57
animal facilities 30
certification 59
class I 51–53, 55
class II 52–54, 55
type A1 53–54
types A2, B1 and B2 54, 55
class III 55, 56
laboratory 25–27
decontamination 59, 89
exhaust air see under exhaust air
location 22, 57
operation and maintenance 58
prion contamination 77
required use 15, 21, 22
safe use 57–60, 70–71
selection 52, 57
biosafety
management 12
versus laboratory biosecurity 47
Biosafety Collaborating Centres, WHO
140
biosafety committee 118
Biosafety Level 1: 1, 3, 9–19
animal facilities 29
laboratory design 12–14
laboratory safety survey form 38–40
health and medical surveillance 16
see also basic laboratory
Biosafety Level 2: 1, 3, 9–19
animal facilities 29–30
laboratory design 12–14, 15
laboratory safety survey form 41–42
health and medical surveillance 16
see also basic laboratory
Biosafety Level 3: 1, 3, 20–23
animal facilities 30–31
laboratory design 21–22
laboratory safety survey form 43
see also containment laboratory
Biosafety Level 4: 1, 3, 25–27
animal facilities 31–32
laboratory design 25–27
see also maximum containment
laboratory
biosafety levels 1
animal facility (ABSL) 28
facility requirements 3
microbiological risk groups and 1–3
biosafety officer 17, 117–118
biosecurity 47–48
1,1’-biphenyl-4,4’-diamine” 149
bleach (sodium hypochlorite) 84–85, 89,
166
blenders 63–64, 73
blood, standard precautions 74–76
body fluids, standard precautions 74–76
boiling 90
bottles, screw-capped 16, 63
bromine 149
BSCs see biological safety cabinets
building maintenance services 119
Bunsen burners 70, 71
INDEX
• 172 •
LABORATORY BIOSAFETY MANUAL
cages
animal 30, 31
flying insects 32
calcium hypochlorite 84
carbon dioxide, solid (dry ice) 150
carbon tetrachloride 150
4,4'-carbonoimidoylbis
(N,N-dimethylbenzenamine) 148
ceilings 12, 21
centrifuges 72–73, 142
breakage of tubes in 79–80
containment accessories 22
improper use 144
certification
biological safety cabinets 59
laboratory/facility 36–37
checklist, safety 125–131
chemical germicides 82, 83–88
chemicals (hazardous) 19, 107–109
biological safety cabinets 57
explosive 108, 144
incompatible, general rules 107, 108
routes of exposure 107
safety checklist 130–131
specific 145–169
spills 108–109
storage 107
toxic effects 107–108
children 10
chilling facilities, arthropods 32
chloramines 84, 85
chlorine 84–85, 150
chlorine dioxide 85–86, 151
chlorine-releasing compounds 84–85
chloroform 151
chromic acid 151
chromium VI oxide 151
circuit-breakers 111
class III cabinet laboratory 25–26
controlled air system 26–27
clean-air work stations 51
clean-up procedure, spills 95–97
cleaning
biological safety cabinets 59
domestic 119
laboratory materials 83
refrigerators and freezers 73
clothing, protective see personal protective
equipment/clothing
coats, laboratory 64, 65
codes of practice
Biosafety Level 1 and 2: 9–12
Biosafety Level 3: 20–21
Biosafety Level 4: 25
commissioning, laboratory/facility 33–35
commissioning agent 33–34
communication facilities 25
contact lenses 11
containers
broken 79
contaminated waste 18–19
leakproof 62
sharps disposal 18, 62
specimen 69, 75, 95
containment, primary
at Biosafety Level 4 25–26
containment laboratory (Biosafety Level 3)
1, 3, 20–23
code of practice 20–21
design and facilities 21–22, 23
equipment 22
health and medical surveillance 22–23,
24
safety survey form 43
containment levels, animal facility 28
see also biosafety levels
contaminated liquids/effluents 11, 27
contaminated materials see infectious
materials
contingency plans 78
copper 152
cosmetics 11
coveralls 64, 65
Creutzfeldt-Jakob disease (CJD) 76
culture stirrers, shakers, agitators 143
cuts 79
cyanogen bromide 152
cytochalasin 152
decontamination
biological safety cabinets 59, 89
blood/body fluids 76
• 173 •
definition 82
effluents 11, 27
hand 90
local environmental 88–89
prion-contaminated materials 77
waste materials 18, 22
see also cleaning; disinfection
desiccators 142
design, laboratory
Biosafety Level 1 and 2: 12–14, 15
Biosafety Level 3: 21–22, 23
Biosafety Level 4: 25–27
commissioning requirements and 33
diethyl ether 153
diethylene dioxide 154
diethylene oxide 167
3,3'-dimethyl-(1,1'-biphenyl)-4,4'-diamine
167
dimethylamine 153
dimethylbenzene 169
2,4-dinitrophenyl-hydrazine 153
dioxane 154
disasters, natural 78, 80
disinfectants 82, 83–88
disinfection 82–93
biological safety cabinets 59
chemical 83–88
cleaning prior to 82, 83
definition 82
heat 90–92
spills 95–97
waste materials 19
see also decontamination; sterilization
dispersal of infectious materials, avoiding
70
doors
animal facilities 29
laboratory 13, 21, 26
drains, containment 27
drinking 11, 13, 30
earth-fault-interrupters 111
eating 11, 13, 30, 71
effluents, contaminated 11, 27
electrical hazards 19, 111, 144
safety checklist 128
electricity supply 14, 27
emergencies 78–81
Biosafety level 4: 25, 27
contingency plans 78
laboratory procedures 79–81
emergency equipment 81
emergency services 80–81
engineering services 119
equipment
basic laboratory 14–16
containment laboratory 22
emergency 81
hazards 141–144
personal protective see personal
protective equipment/clothing
safety 19, 61–66
checklist 130
Escherichia coli K12 102
ethanol (ethyl alcohol) 87–88, 154
ethanolamine 154
ethers 108
excreta, standard precautions 74–76
exhaust air
animal facilities 30
biological safety cabinets 22, 26, 51, 52–
54, 56, 57
containment laboratory 21, 22
maximum containment laboratory 26–
27
explosive chemicals 108, 144
expression systems, biological 102
expression vectors 102
eye protection 11, 64–65, 71
face protection 11, 64–65
face shields (visors) 11, 65
facilities, laboratory
Biosafety Level 1 and 2: 12–14
Biosafety Level 3: 21–22, 23
Biosafety Level 4: 25–27
biosafety level designations 1
films, for microscopy 75
fire extinguishers 110–111
fires 19, 110–111
causes 110, 144
emergency procedures 80
INDEX
• 174 •
LABORATORY BIOSAFETY MANUAL
prevention and protection checklist
127
first aid 13, 138
first-aid box 138
flame photometer 144
flames, open 59, 70
flammable liquid storage 128
floors 12, 21
food 11
footwear 11, 20, 25, 65
formaldehyde 86, 89, 155
formalin 86, 89
freeze-dryers 143
freezers 73–74
fumigation 89
furniture, laboratory 12
gas(es)
compressed and liquefied 109, 128
supply to laboratory 14
gene transfer 101, 102
genetic engineering 101
genetically modified organisms (GMOs)
101–104
further considerations 104
risk assessments 103–104
types 102–103
germicides, chemical 82, 83–88
glass 75
handling broken 79, 80, 95
precautions in use 71, 75
gloves 10, 60, 65, 66
glutaraldehyde 86, 155
GMOs see genetically modified organisms
goggles 64–65
good microbiological technique (GMT)
9–12, 69–77
gowns 64, 65
hand decontamination 90
hand rubs, alcohol-based 87–88, 90
hand washing 10, 66, 90
animal facility staff 30
facilities 13, 21, 30
health and medical surveillance
basic laboratory 16
checklist 129
containment laboratory 22–23, 24
hearing protection 111
heat
disinfection and sterilization 90–92
dry 90
moist 90
heating, ventilation and air-conditioning
(HVAC) system 21
safety checklist 126
HEPA filters see high-efficiency particulate
air filters
high-efficiency particulate air (HEPA)
filters
animal facilities 30
biological safety cabinets 51, 53, 56
Biosafety Level 3: 21, 22
Biosafety Level 4: 26, 27
prion-contaminated 77
homogenizers 63–64, 73, 142
horizontal and vertical outflow cabinets 51
1-hydrazino-2,4-dinitrobenzene 153
hydrochloric acid 155
hydrogen chloride 155
peroxide 88, 89, 156
hydrogen sulfide 156
illumination see lighting
immunization, staff 139
incidents see accidents; spills
incineration 19, 92–93
incinerators 31, 92
infectious materials
autoclaving and reuse 18
avoiding dispersal 70
contact with skin and eyes 71
decontamination see decontamination
disposal 18–19, 22
ingestion 71, 79
safety checklist 130
spills 11, 79, 95–97
infective microorganisms, risk groups see
risk groups, microbiological
ingestion of infectious material 71, 79
injuries
animal facility staff 30
• 175 •
emergency procedures 79
prevention 71–72
inoculation, accidental 71–72
insects, flying 32
inspection, laboratory 36–37
International Air Transport Association
(IATA) 94
International Civil Aviation Organization
(ICAO) 94
international transport regulations 94–95
invertebrates 32
iodine 88, 156
iodophores 88
isolator cages 30, 31
isolators, negative-pressure flexible-film
61, 62
isopropanol (isopropyl alcohol) 87, 162
knock-out animals 102–103
labelling, specimen 74–75
laboratory
biosafety levels see biosafety levels
biosecurity 47–48
certification 36–37
commissioning 33–35
facilities see facilities, laboratory
premises, safety checklist 125
safety survey forms 38–43
services, safety checklist 126–127
techniques 69–77
working areas 11
see also basic laboratory; containment
laboratory; maximum containment
laboratory
laboratory director 12, 117
laboratory supervisors 12, 117
training role 17, 120
latex allergy 66
lighting 12, 14, 126
loops see transfer loops
lyophilized infectious materials, opening
ampoules 74
lyophilizers 143
maintenance personnel 119
maximum containment laboratory
(Biosafety Level 4) 1, 3, 25–27
code of practice 25
design and facilities 25–27
medical contact card 22–23, 24
medical surveillance see health and medical
surveillance
mercury 157
methanol 157
methylbenzene 168
microbicide 82–83
microbiological risk assessment see risk
assessment, microbiological
microincinerators 62, 64
microscopy, films and smears for 75
mites 32
naphthylamine 157
natural disasters 78, 80
needle-stick injuries, avoiding 71–72
needles, hypodermic 11, 75, 141–142
disposal 18
negative-pressure flexible-film isolators
61, 62
ninhydrin 158
nitric acid 158
nitrobenzene 158
noise 111
osmium tetroxide 159
outbreaks, diseases of unknown etiology 8
oxalic acid 159
oxygen 159
packaging systems 95, 96
paraformaldehyde 86, 89
peracids 88
perchloric acid 108, 160
personal protective equipment/clothing
64–66
animal facility 30, 31
basic laboratory 10–11
biological safety cabinet 60
checklist 129
containment laboratory 20–21
INDEX
• 176 •
LABORATORY BIOSAFETY MANUAL
maximum containment laboratory 25,
26
prions 77
personnel
biosafety management 12
biosecurity issues 47
facilities, safety checklist 126
health and medical surveillance see
health and medical surveillance
immunization 139
personal items/clothing 13
responsibility for own safety 117
support 119
training see training
phenol 160
phenolic compounds 86–87
N-phenyl-α-naphthylamine 157
N-phenyl-β-naphthylamine 157
phosphoric acid 161
phosphorus pentoxide 161
picric acid/picrates 108, 161
pipettes 16, 70
pipetting 70
aids 15, 62, 63, 70
mouth 11, 63
plants, transgenic 101, 103
plasmid pUC18 102
poliovirus-susceptible mice 102–103
potassium hydroxide 162
potassium permanganate 162
potassium tellurite 162
precleaning 82, 83
primary containment 25–26
primates, non-human 29
prions 76–77
product protection 51, 53
propan-2-ol (2-propanol) 87, 162
protective suit 26
see also suit laboratory
pyridine 163
quaternary ammonium compounds 87
radiation, ionizing 19, 111–114
harmful effects 111
principles of protection 112–113
safety checklist 130–131
work-bench area 113–114
radiation area 113
radiation hazard symbol 113, 114
radioactive waste 114
radionuclides
biological safety cabinets 57
safe working practices 112–113
substitution 113
recombinant DNA technology 101–104
refrigerators 73–74, 144
respirators (respiratory protective
equipment) 21, 65–66
rest facilities 13
risk assessment, microbiological 2, 7–8
animal facilities 28–29
genetically modified organisms 103–
104
risk groups, microbiological
basic laboratories 9
biosafety levels and 2–3
classification 1–2
rodent control 12, 29
safety checklist 125–131
safety equipment see equipment, safety
sanitation facilities 126
screens, arthropod-proof 32
screw-capped bottles 16, 63
security precautions 14, 127
selenium 163
serum, separation 72
shakers 63–64, 73
sharps 18
animal facilities 30
avoiding injuries 66, 71–72, 75
disposal containers 18, 62
shoes see footwear
showers 26, 30
silver 163
silver nitrate 164
skin
contact 71
puncture wounds, cuts and abrasions
79
see also injuries
• 177 •
smears, for microscopy 75
smoking 11, 30
soda ash (sodium carbonate) 109
sodium azide 164
sodium bicarbonate 109
sodium biselenite 164
sodium cyanide 165
sodium dichloroisocyanurate (NaDCC)
84, 85
sodium hydroxide 165
sodium hypochlorite (bleach) 84–85, 89,
166
sonicators 63–64, 73, 75, 142
spatter shield 62
specimens 69
collection 74–75
containers 69, 75, 95
labelling 74–75
with limited information 8
opening packages 69
opening tubes and sampling contents
75
receipt 69
standard precautions 74–75
transport 69, 74–75
triple packaging system 95, 96
spectacles, safety 64–65
spills
in biological safety cabinets 59
blood 76
chemical 108–109
infectious materials 11, 79, 95–97
sporocide 83
staff see personnel
standard precautions 74–76
sterilization 27, 82–93
cleaning prior to 82, 83
definition 83
heat 90–92
prion-contaminated materials 77
see also decontamination; disinfection
storage
ampoules of infectious materials 74
chemicals 108
compressed and liquefied gases 109,
128
facilities, checklist 125
flammable liquids 128
space, laboratory 12
suit, protective 26
suit laboratory 26
controlled air system 26–27
sulfuric acid 166
support staff 119
survey, laboratory safety 36–37
forms 38–43
syringes 11, 18
tetrahydrofuran 167
tetramethylene oxide 167
thallium acetate 167
ticks 32
tissue grinders 73, 142
tissues
prion-containing 77
standard precautions 75
o-tolidine 167
toluene 168
total exhaust cabinet 57
training 120–121
animal facility workers 30, 31
biological safety cabinet use 60
biosecurity 47
laboratory workers 16–17
transfer loops
disposable 15, 62, 64
microincinerators 62, 64
safe use 70
transgenic animals 102–103
transgenic plants 101, 103
transmissible spongiform encephalopathies
(TSEs) 76
transport 11, 94–97
infectious waste 18–19, 22
international regulations 94–95
specimens 69, 74–75
triple packaging system 95, 96
trichloroacetic acid 168
trichloroethylene 169
triclosan 87
2,4,6-trinitrophenol (picric acid) 108, 161
triple packaging systems 95, 96
INDEX
tubes
breakage in centrifuges 79–80
screw-capped 16
two-person rule 25, 31
ultra-centrifuges 142
ultrasonic cleaners 142
ultraviolet lights 58
United Nations Committee of Experts on
the Transport of Dangerous Goods
(UNCETDG) 94
universal precautions 74–76
vacuum flasks 144
vacuum lines 22, 63
validation, equipment 16
vectors 102
ventilation systems
animal facilities 29, 30, 31
basic laboratory 14
checklist 126
containment laboratory 21
maximum containment laboratory 26–
27
viral vectors 102
walls 12, 21
warranty periods, laboratory/facility 33
waste 17–19
animal facilities 30, 31
Biosafety Level 4: 27
decontamination 18, 22
disposal 18–19, 22, 93
invertebrate facilities 32
prion-contaminated 77
radioactive 114
water baths 143
water supply 14, 22
windows
animal facilities 29, 31
invertebrate facilities 32
laboratory 11, 14, 21
women of childbearing age 16, 129
work surfaces
animal facilities 29
laboratory 11, 12
working areas, laboratory 11
World Health Organization (WHO)
Biosafety Collaborating Centres 140
Biosafety programme 25
xylene 169
• 178 •
LABORATORY BIOSAFETY MANUAL