CDAR2 Implementation Guide 2012JUL
CDAR2_Implementation_Guide_2012JUL
User Manual:
Open the PDF directly: View PDF ![]() .
.
Page Count: 581 [warning: Documents this large are best viewed by clicking the View PDF Link!]

CDAR2_IG_IHE_CONSOL_DSTU_R1.1_2012JUL
HL7 Implementation Guide for CDA® Release 2:
IHE Health Story Consolidation, DSTU Release 1.1
(US Realm)
Draft Standard for Trial Use
July 2012
Publication of this draft standard for trial use and comment has been approved by
Health Level Seven International (HL7). This draft standard is not an accredited
American National Standard. The comment period for use of this draft standard shall
end 24 months from the date of publication. Suggestions for revision should be
submitted at http://www.hl7.org/dstucomments/index.cfm.
Following this 24 month evaluation period, this draft standard, revised as necessary,
will be submitted to a normative ballot in preparation for approval by ANSI as an
American National Standard. Implementations of this draft standard shall be viable
throughout the normative ballot process and for up to six months after publication of
the relevant normative standard.
Copyright © 2012 Health Level Seven International ® ALL RIGHTS RESERVED. The reproduction
of this material in any form is strictly forbidden without the written permission of the publisher.
HL7 International and Health Level Seven are registered trademarks of Health Level Seven
International. Reg. U.S. Pat & TM Off.

Page 2 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
1 IMPORTANT NOTES:
HL7 licenses its standards and select IP free of charge. If you did not acquire a free license from HL7 for
this document, you are not authorized to access or make any use of it. To obtain a free license, please visit
http://www.HL7.org/implement/standards/index.cfm.
If you are the individual that obtained the license for this HL7 Standard, specification or other freely
licensed work (in each and every instance "Specified Material"), the following describes the permitted
uses of the Material.
A. HL7 INDIVIDUAL, STUDENT AND HEALTH PROFESSIONAL MEMBERS, who register and agree to
the terms of HL7’s license, are authorized, without additional charge, to read, and to use Specified Material
to develop and sell products and services that implement, but do not directly incorporate, the Specified
Material in whole or in part without paying license fees to HL7.
INDIVIDUAL, STUDENT AND HEALTH PROFESSIONAL MEMBERS wishing to incorporate additional
items of Special Material in whole or part, into products and services, or to enjoy additional authorizations
granted to HL7 ORGANIZATIONAL MEMBERS as noted below, must become ORGANIZATIONAL
MEMBERS of HL7.
B. HL7 ORGANIZATION MEMBERS, who register and agree to the terms of HL7's License, are authorized,
without additional charge, on a perpetual (except as provided for in the full license terms governing the
Material), non-exclusive and worldwide basis, the right to (a) download, copy (for internal purposes only) and
share this Material with your employees and consultants for study purposes, and (b) utilize the Material for
the purpose of developing, making, having made, using, marketing, importing, offering to sell or license, and
selling or licensing, and to otherwise distribute, Compliant Products, in all cases subject to the conditions set
forth in this Agreement and any relevant patent and other intellectual property rights of third parties (which
may include members of HL7). No other license, sublicense, or other rights of any kind are granted under
this Agreement.
C. NON-MEMBERS, who register and agree to the terms of HL7’s IP policy for Specified Material, are
authorized, without additional charge, to read and use the Specified Material for evaluating whether to
implement, or in implementing, the Specified Material, and to use Specified Material to develop and sell
products and services that implement, but do not directly incorporate, the Specified Material in whole or in
part.
NON-MEMBERS wishing to incorporate additional items of Specified Material in whole or part, into products
and services, or to enjoy the additional authorizations granted to HL7 ORGANIZATIONAL MEMBERS, as
noted above, must become ORGANIZATIONAL MEMBERS of HL7.
Please see http://www.HL7.org/legal/ippolicy.cfm for the full license terms governing the Material.
Ownership. Licensee agrees and acknowledges that HL7 owns all right, title, and interest, in and to the
Trademark. Licensee shall take no action contrary to, or inconsistent with, the foregoing.
Licensee agrees and acknowledges that HL7 may not own all right, title, and interest, in and to the
Materials and that the Materials may contain and/or reference intellectual property owned by third
parties (“Third Party IP”). Acceptance of these License Terms does not grant Licensee any rights
with respect to Third Party IP. Licensee alone is responsible for identifying and obtaining any
necessary licenses or authorizations to utilize Third Party IP in connection with the Materials or
otherwise. Any actions, claims or suits brought by a third party resulting from a breach of any Third
Party IP right by the Licensee remains the Licensee’s liability.
Following is a non-exhaustive list of third-party terminologies that may require a separate license:
Terminology
Owner/Contact
Current Procedures Terminology
(CPT) code set
American Medical Association
http://www.ama-assn.org/ama/pub/physician-
resources/solutions-managing-your-practice/coding-billing-
insurance/cpt/cpt-products-services/licensing.page?
SNOMED CT
International Healthcare Terminology Standards Developing
Organization (IHTSDO) http://www.ihtsdo.org/snomed-
ct/get-snomed-ct or info@ihtsdo.org
Logical Observation Identifiers
Names & Codes (LOINC)
Regenstrief Institute
International Classification of
Diseases (ICD) codes
World Health Organization (WHO)
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 3
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Primary
Editor/
Co-Chair:
Brett Marquard
Lantana Consulting Group
brett.marquard@lantanagroup.com
Co-Editor:
Kanwarpreet (KP) Sethi
Deloitte Consulting LLP
ksethi@deloitte.com
Co-Chair:
Calvin Beebe
Mayo Clinic
cbeebe@mayo.edu
Co-Editor:
George Benny Varghese
Deloitte Consulting LLP
gvarghese@deloitte.com
Co-Chair:
Austin Kreisler
SAIC Consultant to CDC/NHSN
duz1@cdc.gov
Co-Editor:
Corey Spears
McKesson
Corey.Spears@McKesson.com
Primary
Editor/
Co-Chair:
Robert H. Dolin, MD
Lantana Consulting Group
bob.dolin@lantanagroup.com
Co-Editor:
Michael Tyburski
Social Security Administration
michael.tyburski@ssa.gov
Co-Chair:
Grahame Grieve
Kestral Computing Pty Ltd
grahame@kestral.com.au
Co-Editor:
Kevin Coonan, MD
Deloitte Consulting LLP
kcoonan@deloitte.com
Co-Editor:
Liora Alschuler
Lantana Consulting Group
liora.alschuler@lantanagroup.com
Co-Editor:
Ryan Murphy
Tenino Tek
teninotek@gmail.com
Co-Editor:
Dave Carlson
U.S. Department of Veterans Affairs
David.Carlson@va.gov
Co-Editor:
Bob Yencha
Lantana Consulting Group
bob.yencha@lantanagroup.com
Co-Editor:
Keith W. Boone
GE Healthcare
keith.boone@ge.com
Co-Editor:
Zabrina Gonzaga
Lantana Consulting Group
zabrina.gonzaga@lantanagroup.com
Co-Editor:
Pete Gilbert
Covisint
peterngilbert@gmail.com
Co-Editor:
Jingdong Li
Lantana Consulting Group
jingdong.li@lantanagroup.com
Co-Editor:
Gaye Dolin
Lantana Consulting Group
gaye.dolin@lantanagroup.com
Co-Editor:
Rick Geimer
Lantana Consulting Group
rick.geimer@lantanagroup.com
Co-Editor:
Rich Kernan
Deloitte Consulting LLP
rkernan@deloitte.com
Co-Editor:
Sean McIlvenna
Lantana Consulting Group
sean.mcilvenna@lantanagroup.com
Co-Editor:
David Parker
Evolvent Technologies, Inc.
david.parker@evolvent.com
Co-Editor:
Sean Muir
U.S. Department of Veterans Affairs
Sean.Muir@va.gov
Co-Editor:
Jas Singh
Deloitte Consulting LLP
jassingh3@deloitte.com
Technical
Editor:
Susan Hardy
Lantana Consulting Group
susan.hardy@lantanagroup.com
Technical
Editor:
Diana Wright
Lantana Consulting Group
diana.wright@lantanagroup.com
Current Work Group also includes all those who participated in the ONC S&I Framework
See the full list of participants (approximately 140) here:
http://wiki.siframework.org/CDA+Harmonization+WG

Page 4 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Acknowledgments
This guide was produced and developed through the joint efforts of Health Level
Seven (HL7), Integrating the Healthcare Environment (IHE), the Health Story
Project, and the Office of the National Coordinator (ONC) within the US
Department of Health and Human Services (HSS).
The project was carried out within the ONC’s Standards and Interoperability
(S&I) Framework as the Clinical Document Architecture (CDA) Consolidation
Project with a number of goals, one of which is providing a set of harmonized
CDA templates for the US Realm.
The co-editors appreciate the support and sponsorship of the HL7 Structured
Documents Working Group (SDWG) and all the volunteers, staff and contractors
participating in the S&I Framework.
The conformance requirements included here for review were generated from two
model-driven tools: the Model-Driven Health Tools (MDHT)—developed as on
open source tool under the auspices of the Veterans Administration, IBM, and
the ONC—and the Trifolia Template Database (Tdb)—developed initially for the
Centers for Disease Control and Prevention (CDC) and released by Lantana
Consulting Group under an open source license.
This material contains content from SNOMED CT®
(http://www.ihtsdo.org/snomed-ct/). SNOMED CT is a registered trademark of
the International Health Terminology Standard Development Organisation
(IHTSDO).
This material contains content from LOINC® (http://loinc.org). The LOINC table,
LOINC codes, and LOINC panels and forms file are copyright © 1995-2012,
Regenstrief Institute, Inc. and the Logical Observation Identifiers Names and
Codes (LOINC) Committee and available at no cost under the license at
http://loinc.org/terms-of-use.

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 5
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table of Contents
1 INTRODUCTION ........................................................................................................... 30
1.1 Audience .............................................................................................................. 30
1.2 Purpose ................................................................................................................ 30
1.3 Scope ................................................................................................................... 31
1.4 Approach ............................................................................................................. 31
1.5 Organization of This Guide ................................................................................... 32
1.6 Use of Templates .................................................................................................. 32
1.6.1 Originator Responsibilities: General Case.......................................................... 33
1.6.2 Recipient Responsibilities: General Case ........................................................... 33
1.7 Levels of Constraint .............................................................................................. 33
1.8 Conformance Conventions Used in This Guide ...................................................... 34
1.8.1 Templates and Conformance Statements .......................................................... 34
1.8.2 Open and Closed Templates ............................................................................. 36
1.8.3 Conformance Verbs (Keywords) ......................................................................... 36
1.8.4 Cardinality ....................................................................................................... 37
1.8.5 Optional and Required with Cardinality ............................................................ 38
1.8.6 Vocabulary Conformance .................................................................................. 38
1.8.7 Containment Relationships .............................................................................. 39
1.8.8 Null Flavor ....................................................................................................... 40
1.8.9 Unknown Information ...................................................................................... 42
1.8.10 Data Types ....................................................................................................... 43
1.9 XML Conventions Used in This Guide ................................................................... 44
1.9.1 XPath Notation ................................................................................................. 44
1.9.2 XML Examples and Sample Documents ............................................................ 44
1.10 UML Diagrams ..................................................................................................... 45
1.11 Content of the Package ......................................................................................... 45
2 GENERAL HEADER TEMPLATE .................................................................................... 46
2.1 Document Type Codes .......................................................................................... 46
2.2 US Realm Header ................................................................................................. 46
2.2.1 RecordTarget .................................................................................................... 48
2.2.2 Author ............................................................................................................. 58
2.2.3 DataEnterer ..................................................................................................... 60
2.2.4 Informant ......................................................................................................... 62
2.2.5 Custodian ........................................................................................................ 63

Page 6 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
2.2.6 InformationRecipient ........................................................................................ 64
2.2.7 LegalAuthenticator ........................................................................................... 65
2.2.8 Authenticator ................................................................................................... 67
2.2.9 Participant (Support) ........................................................................................ 69
2.2.10 InFulfillmentOf ................................................................................................. 70
2.2.11 DocumentationOf/serviceEvent ........................................................................ 70
2.2.12 Authorization/consent ...................................................................................... 72
2.2.13 ComponentOf ................................................................................................... 73
2.3 US Realm Address (AD.US.FIELDED) .................................................................... 73
2.4 US Realm Date and Time (DT.US.FIELDED) .......................................................... 74
2.5 US Realm Date and Time (DTM.US.FIELDED) ....................................................... 75
2.6 US Realm Patient Name (PTN.US.FIELDED) .......................................................... 75
2.7 US Realm Person Name (PN.US.FIELDED) ............................................................. 77
2.8 Rendering Header Information for Human Presentation ......................................... 77
3 DOCUMENT-LEVEL TEMPLATES .................................................................................. 79
3.1 Continuity of Care Document (CCD)/HITSP C32 ................................................... 84
3.1.1 Header Constraints Specific to CCD .................................................................. 84
3.1.2 CCD Body Constraints ..................................................................................... 86
3.2 Consultation Note ................................................................................................. 96
3.2.1 Consultation Note Header Constraints .............................................................. 96
3.2.2 Consultation Note Body Constraints ............................................................... 103
3.3 Diagnostic Imaging Report .................................................................................. 112
3.3.1 DIR Header Constraints .................................................................................. 113
3.3.2 DIR Body Constraints ..................................................................................... 124
3.4 Discharge Summary ........................................................................................... 130
3.4.1 Discharge Summary Header Constraints ........................................................ 130
3.4.2 Discharge Summary Body Constraints ............................................................ 134
3.5 History and Physical (H&P) Note ......................................................................... 146
3.5.1 H&P Note Header Constraints ......................................................................... 147
3.5.2 H&P Note Body Constraints ............................................................................ 150
3.6 Operative Note .................................................................................................... 160
3.6.1 Operative Note Header Constraints ................................................................. 160
3.6.2 Operative Note Body Constraints .................................................................... 165
3.7 Procedure Note ................................................................................................... 169
3.7.1 Procedure Note Header Constraints ................................................................ 169
3.7.2 Procedure Note Body Constraints ................................................................... 177

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 7
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
3.8 Progress Note ...................................................................................................... 188
3.8.1 Progress Note Header Constraints .................................................................... 188
3.8.2 Progress Note Body Constraints ....................................................................... 192
3.9 Unstructured Document ...................................................................................... 197
3.9.1 Unstructured Document Header Constraints ................................................... 198
3.9.2 Unstructured Document Body Constraints ...................................................... 199
4 SECTION-LEVEL TEMPLATES...................................................................................... 203
4.1 Advance Directives Section 42348-3 ..................................................................... 210
4.2 Allergies Section 48765-2 .................................................................................... 212
4.3 Anesthesia Section 59774-0 ................................................................................. 214
4.4 Assessment and Plan Section 51847-2 ................................................................. 215
4.5 Assessment Section 51848-0 ............................................................................... 216
4.6 Chief Complaint and Reason for Visit Section 46239-0 ......................................... 217
4.7 Chief Complaint Section 10154-3 ......................................................................... 218
4.8 Complications Section 55109-3............................................................................ 219
4.9 DICOM Object Catalog Section - DCM 121181 ..................................................... 220
4.10 Discharge Diet Section 42344-2 ........................................................................... 222
4.11 Encounters Section 46240-8 ................................................................................ 222
4.12 Family History Section 10157-6 ........................................................................... 224
4.13 Findings Section (DIR) 18782-3 ........................................................................... 226
4.14 Functional Status Section 47420-5 ...................................................................... 227
4.15 General Status Section 10210-3 .......................................................................... 232
4.16 History of Past Illness Section 11348-0 ................................................................ 233
4.17 History of Present Illness Section 10164-2 ........................................................... 234
4.18 Hospital Admission Diagnosis Section 46241-6 .................................................... 235
4.19 Hospital Admission Medications Section 42346-7 (entries optional) ...................... 236
4.20 Hospital Consultations Section 18841-7 .............................................................. 237
4.21 Hospital Course Section 8648-8 ........................................................................... 237
4.22 Hospital Discharge Diagnosis Section 11535-2 ..................................................... 238
4.23 Hospital Discharge Instructions Section 8653-8 ................................................... 239
4.24 Hospital Discharge Medications Section 10183-2 ................................................. 240
4.25 Hospital Discharge Physical Section 10184-0 ....................................................... 242
4.26 Hospital Discharge Studies Summary Section 11493-4 ........................................ 243
4.27 Immunizations Section 11369-6 .......................................................................... 244
4.28 Instructions Section 69730-0 ............................................................................... 247
4.29 Interventions Section 62387-6 ............................................................................. 248

Page 8 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
4.30 Medical Equipment Section 46264-8 ................................................................... 249
4.31 Medical (General) History Section 11329-0 .......................................................... 250
4.32 Medications Administered Section 29549-3 ......................................................... 251
4.33 Medications Section 10160-0 .............................................................................. 252
4.34 Objective Section 61149-1 .................................................................................. 254
4.35 Operative Note Fluid Section 10216-0 ................................................................. 255
4.36 Operative Note Surgical Procedure Section 10223-6 ............................................ 256
4.37 Payers Section 48768-6 ...................................................................................... 257
4.38 Physical Exam Section 29545-1 .......................................................................... 259
4.39 Plan of Care Section 18776-5 .............................................................................. 260
4.40 Planned Procedure Section 59772-4 .................................................................... 262
4.41 Postoperative Diagnosis Section 10218-6 ............................................................ 263
4.42 Postprocedure Diagnosis Section 59769-0 ........................................................... 264
4.43 Preoperative Diagnosis Section 10219-4 .............................................................. 265
4.44 Problem Section 11450-4 .................................................................................... 266
4.45 Procedure Description Section 29554-3 ............................................................... 269
4.46 Procedure Disposition Section 59775-7 ............................................................... 270
4.47 Procedure Estimated Blood Loss Section 59770-8 ............................................... 270
4.48 Procedure Findings Section 59776-5 ................................................................... 271
4.49 Procedure Implants Section 59771-6 ................................................................... 272
4.50 Procedure Indications Section 59768-2 ............................................................... 273
4.51 Procedure Specimens Taken Section 59773-2...................................................... 274
4.52 Procedures Section 47519-4 ............................................................................... 275
4.53 Reason for Referral Section 42349-1 ................................................................... 278
4.54 Reason for Visit Section 29299-5 ........................................................................ 279
4.55 Results Section 30954-2 ..................................................................................... 280
4.56 Review of Systems Section 10187-3 ..................................................................... 282
4.57 Social History Section 29762-2 ........................................................................... 283
4.58 Subjective Section 61150-9 ................................................................................. 285
4.59 Surgical Drains Section 11537-8 ......................................................................... 286
4.60 Vital Signs Section 8716-3 .................................................................................. 287
5 ENTRY-LEVEL TEMPLATES ........................................................................................ 289
5.1 Admission Medication ......................................................................................... 289
5.2 Advance Directive Observation ............................................................................ 291
5.3 Age Observation ................................................................................................. 296
5.4 Allergy - Intolerance Observation ........................................................................ 298

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 9
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
5.5 Allergy Problem Act ............................................................................................. 306
5.6 Allergy Status Observation ................................................................................... 309
5.7 Assessment Scale Observation ............................................................................. 310
5.8 Assessment Scale Supporting Observation ........................................................... 314
5.9 Authorization Activity .......................................................................................... 315
5.10 Boundary Observation ......................................................................................... 317
5.11 Caregiver Characteristics ..................................................................................... 318
5.12 Code Observations ............................................................................................... 321
5.13 Cognitive Status Problem Observation.................................................................. 323
5.14 Cognitive Status Result Observation .................................................................... 328
5.15 Cognitive Status Result Organizer ........................................................................ 331
5.16 Comment Activity ................................................................................................ 333
5.17 Coverage Activity ................................................................................................. 336
5.18 Deceased Observation ......................................................................................... 337
5.19 Discharge Medication .......................................................................................... 339
5.20 Drug Vehicle ....................................................................................................... 341
5.21 Encounter Activities ............................................................................................ 343
5.22 Encounter Diagnosis ........................................................................................... 346
5.23 Estimated Date of Delivery ................................................................................... 348
5.24 Family History Death Observation ........................................................................ 349
5.25 Family History Observation .................................................................................. 351
5.26 Family History Organizer ..................................................................................... 356
5.27 Functional Status Problem Observation ............................................................... 360
5.28 Functional Status Result Observation .................................................................. 363
5.29 Functional Status Result Organizer ..................................................................... 366
5.30 Health Status Observation ................................................................................... 368
5.31 Highest Pressure Ulcer Stage ............................................................................... 371
5.32 Hospital Admission Diagnosis .............................................................................. 372
5.33 Hospital Discharge Diagnosis ............................................................................... 373
5.34 Immunization Activity .......................................................................................... 375
5.35 Immunization Medication Information .................................................................. 381
5.36 Immunization Refusal Reason .............................................................................. 384
5.37 Indication ............................................................................................................ 386
5.38 Instructions ........................................................................................................ 388
5.39 Medication Activity .............................................................................................. 390
5.40 Medication Dispense ............................................................................................ 399

Page 10 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
5.41 Medication Information ....................................................................................... 402
5.42 Medication Supply Order .................................................................................... 404
5.43 Medication Use – None Known (deprecated) ......................................................... 407
5.44 Non-Medicinal Supply Activity ............................................................................ 408
5.45 Number of Pressure Ulcers Observation .............................................................. 410
5.46 Plan of Care Activity Act ...................................................................................... 412
5.47 Plan of Care Activity Encounter ........................................................................... 413
5.48 Plan of Care Activity Observation ........................................................................ 414
5.49 Plan of Care Activity Procedure ........................................................................... 416
5.50 Plan of Care Activity Substance Administration ................................................... 417
5.51 Plan of Care Activity Supply ................................................................................ 418
5.52 Policy Activity ..................................................................................................... 419
5.53 Postprocedure Diagnosis ..................................................................................... 430
5.54 Precondition for Substance Administration .......................................................... 431
5.55 Pregnancy Observation ....................................................................................... 432
5.56 Preoperative Diagnosis ........................................................................................ 434
5.57 Pressure Ulcer Observation ................................................................................. 436
5.58 Problem Concern Act (Condition) ......................................................................... 444
5.59 Problem Observation .......................................................................................... 446
5.60 Problem Status ................................................................................................... 451
5.61 Procedure Activity Act ......................................................................................... 452
5.62 Procedure Activity Observation ........................................................................... 460
5.63 Procedure Activity Procedure .............................................................................. 466
5.64 Procedure Context .............................................................................................. 472
5.65 Product Instance ................................................................................................ 473
5.66 Purpose of Reference Observation ....................................................................... 475
5.67 Quantity Measurement Observation .................................................................... 476
5.68 Reaction Observation .......................................................................................... 480
5.69 Referenced Frames Observation .......................................................................... 483
5.70 Result Observation ............................................................................................. 484
5.71 Result Organizer ................................................................................................. 488
5.72 Series Act ........................................................................................................... 490
5.73 Service Delivery Location .................................................................................... 493
5.74 Severity Observation ........................................................................................... 495
5.75 Smoking Status Observation ............................................................................... 497
5.76 Social History Observation .................................................................................. 500

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 11
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
5.77 SOP Instance Observation ................................................................................... 502
5.78 Study Act ............................................................................................................ 505
5.79 Text Observation ................................................................................................. 507
5.80 Tobacco Use ........................................................................................................ 510
5.81 Vital Sign Observation ......................................................................................... 512
5.82 Vital Signs Organizer ........................................................................................... 515
6 REFERENCES ............................................................................................................. 518
APPENDIX A — ACRONYMS AND ABBREVIATIONS .......................................................... 520
APPENDIX B — CHANGES FROM PREVIOUS GUIDES ...................................................... 522
New and Updated Templates ............................................................................................ 522
Cardinality Changes ........................................................................................................ 523
Section Code Changes ..................................................................................................... 524
Conformance Verbs ......................................................................................................... 525
Template ID Changes ....................................................................................................... 527
Consolidated Entries........................................................................................................ 535
Changes Within Sections ................................................................................................. 539
APPENDIX C — TEMPLATE IDS IN THIS GUIDE ............................................................... 557
APPENDIX D — CODE SYSTEMS IN THIS GUIDE ............................................................. 563
APPENDIX E — VALUE SETS IN THIS GUIDE ................................................................... 565
APPENDIX F — SINGLE-VALUE BINDINGS IN THIS GUIDE .............................................. 568
APPENDIX G — EXTENSIONS TO CDA R2 ........................................................................ 569
APPENDIX H — XDS-SD AND US REALM CLINICAL DOCUMENT HEADER
COMPARISON .............................................................................................................. 571
APPENDIX I — MIME MULTIPART/RELATED MESSAGES ............................................... 573
MIME Multipart/Related Messages ................................................................................... 573
RFC-2557 MIME Encapsulation of Aggregate Documents, Such as HTML (MHTML) .......... 573
Referencing Supporting Files in Multipart/Related Messages ............................................ 573
Referencing Documents from Other Multiparts within the Same X12 Transactions ............ 574
APPENDIX J — ADDITIONAL PHYSICAL EXAMINATION SUBSECTIONS ........................... 575
APPENDIX K — ADDITIONAL EXAMPLES ......................................................................... 577
Names Examples ............................................................................................................. 577
Addresses Examples ........................................................................................................ 577
Time Examples ................................................................................................................ 578

Page 12 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
CD Examples .................................................................................................................. 578
APPENDIX L — LARGE UML DIAGRAMS ......................................................................... 580

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 13
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table of Figures
Figure 1: Constraints format example ........................................................................ 35
Figure 2: Constraints format – only one allowed......................................................... 37
Figure 3: Constraints format – only one like this allowed ........................................... 37
Figure 4: Binding to a single code .............................................................................. 38
Figure 5: XML expression of a single-code binding ..................................................... 39
Figure 6: Translation code example ........................................................................... 39
Figure 7: nullFlavor example ..................................................................................... 40
Figure 8: Attribute required....................................................................................... 41
Figure 9: Allowed nullFlavors when element is required (with xml examples) .............. 41
Figure 10: nullFlavor explicitly disallowed ................................................................. 41
Figure 11: Unknown medication example .................................................................. 42
Figure 12: Unknown medication use of anticoagulant drug example .......................... 43
Figure 13: No known medications example ................................................................ 43
Figure 14: XML document example ........................................................................... 44
Figure 15: XPath expression example ........................................................................ 44
Figure 16: ClinicalDocument example ....................................................................... 44
Figure 17: US Realm header example ........................................................................ 48
Figure 18: effectiveTime with time zone example ........................................................ 48
Figure 19: recordTarget example ............................................................................... 56
Figure 20: Person author example ............................................................................. 60
Figure 21: Device author example ............................................................................. 60
Figure 22: dataEnterer example ................................................................................ 62
Figure 23: Informant with assignedEntity example .................................................... 63
Figure 24: Custodian example ................................................................................... 64
Figure 25: informationRecipient example ................................................................... 65
Figure 26: legalAuthenticator example ....................................................................... 67
Figure 27: Authenticator example.............................................................................. 68
Figure 28: Participant example for a supporting person ............................................. 70
Figure 29: DocumentationOf example ........................................................................ 72
Figure 30: Procedure note consent example ............................................................... 73
Figure 31: CCD ClinicalDocument/templateId example ............................................. 84
Figure 32: CCD code example ................................................................................... 85
Figure 33: Consultation note ClinicalDocument/templateId example ......................... 97
Figure 34: Consultation note ClinicalDocument/code example ................................. 100

Page 14 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 35: Consultation note translation of local code example ................................ 100
Figure 36: Consultation note uncoordinated document type codes example .............. 101
Figure 37: Consultation note inFulfillmentOf example ............................................. 101
Figure 38: Consultation note componentOf example ................................................ 103
Figure 39: DIR ClinicalDocument/templateId example ............................................. 113
Figure 40: DIR ClinicalDocument/code example ...................................................... 115
Figure 41: DIR use of the translation element to include local codes for document type
....................................................................................................................... 115
Figure 42: DIR participant example ......................................................................... 116
Figure 43: DIR inFulfillmentOf example ................................................................... 117
Figure 44: DIR procedure context (CDA Header) illustration (non-normative) ............ 117
Figure 45: DIR documentationOf example................................................................ 118
Figure 46: DIR relatedDocument example ................................................................ 119
Figure 47: DIR componentOf example...................................................................... 121
Figure 48: Physician reading study performer example ............................................ 122
Figure 49: Physician of record participant example .................................................. 123
Figure 50: WADO reference using linkHtml example ................................................ 127
Figure 51: Fetus subject context example ................................................................ 128
Figure 52: Observer context example ....................................................................... 129
Figure 53: Discharge summary ClinicalDocument/templateId example .................... 130
Figure 54: Discharge summary ClinicalDocument/code example ............................ 131
Figure 55: Discharge summary componentOf example ............................................. 133
Figure 56: H&P ClinicalDocument/templateId example ............................................ 147
Figure 57: H&P ClinicalDocument/code example ................................................... 148
Figure 58: H&P use of translation to include local equivalents for document type ..... 148
Figure 59: H&P componentOf example .................................................................... 150
Figure 60: Operative note ClinicalDocument/templateId example ............................. 160
Figure 61: Operative note ClinicalDocument/code example ..................................... 162
Figure 62: Operative note serviceEvent example....................................................... 164
Figure 63: Operative note performer example ........................................................... 165
Figure 64: Procedure note ClinicalDocument/templateId category I example ............ 170
Figure 65: Procedure note ClinicalDocument/code example ..................................... 171
Figure 66: Procedure note serviceEvent example ...................................................... 176
Figure 67: Procedure note serviceEvent example with null value in width element .... 176
Figure 68: Procedure note performer example .......................................................... 177
Figure 69: Progress note ClinicalDocument/templateId example .............................. 188
Figure 70: Progress note ClinicalDocument/code example ...................................... 190

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 15
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 71: Progress note serviceEvent example ........................................................ 191
Figure 72: Progress note componentOf example ....................................................... 192
Figure 73: nonXMLBody example with embedded content ........................................ 200
Figure 74: nonXMLBody example with referenced content ....................................... 200
Figure 75: nonXMLBody example with compressed content ..................................... 200
Figure 76: Unique file reference example ................................................................. 202
Figure 77: Advance directives section UML diagram ................................................. 210
Figure 78: Advance directives section example ......................................................... 211
Figure 79: Allergies section UML diagram ................................................................ 212
Figure 80: Allergies section example ........................................................................ 213
Figure 81: Anesthesia section example .................................................................... 215
Figure 82: Assessment and plan section example .................................................... 216
Figure 83: Assessment section example ................................................................... 217
Figure 84: Chief complaint and reason for visit section example ............................... 218
Figure 85: Chief complaint section example ............................................................. 218
Figure 86: Complications section example ............................................................... 219
Figure 87: DICOM object catalog section example .................................................... 221
Figure 88: Discharge diet section example ............................................................... 222
Figure 89: Encounters section UML diagram ........................................................... 223
Figure 90: Encounters section example ................................................................... 224
Figure 91: Family history section UML diagram ....................................................... 225
Figure 92: Family history section example ............................................................... 225
Figure 93: Findings section example ........................................................................ 226
Figure 94: Functional status section UML diagram .................................................. 227
Figure 95: Functional status section example .......................................................... 230
Figure 96: General status section example .............................................................. 232
Figure 97: History of past illness section example .................................................... 233
Figure 98: History of present illness section example ............................................... 234
Figure 99: Hospital admission diagnosis section example......................................... 235
Figure 100: Hospital admission medications section example ................................... 236
Figure 101: Hospital consultations section example ................................................. 237
Figure 102: Hospital course section example ........................................................... 238
Figure 103: Hospital discharge diagnosis section example ........................................ 239
Figure 104: Hospital discharge instructions section example ................................... 240
Figure 105: Hospital discharge medications section example.................................... 242
Figure 106: Hospital discharge physical section example ......................................... 243

Page 16 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 107: Hospital discharge studies summary section example ............................ 244
Figure 108: Immunization section* UML diagram .................................................... 244
Figure 109: Immunization section example .............................................................. 246
Figure 110: Instructions section example ................................................................ 248
Figure 111: Interventions section example ............................................................... 249
Figure 112: Medical equipment section UML diagram .............................................. 249
Figure 113: Medical equipment section example ...................................................... 250
Figure 114: Medical (general) history section example .............................................. 251
Figure 115: Medications administered section example ............................................ 252
Figure 116: Medications section UML diagram ......................................................... 252
Figure 117: Medications section entries example ..................................................... 254
Figure 118: Objective section example ..................................................................... 255
Figure 119: Operative Note fluid section example ..................................................... 256
Figure 120: Operative Note surgical procedure section example ............................... 256
Figure 121: Payers section UML diagram ................................................................. 257
Figure 122: Payers section example ......................................................................... 258
Figure 123: Physical exam section example ............................................................. 260
Figure 124: Plan of care section UML diagram ......................................................... 260
Figure 125: Plan of care section example ................................................................. 261
Figure 126: Planned procedure section example ...................................................... 263
Figure 127: Postoperative diagnosis section example................................................ 264
Figure 128: Postprocedure diagnosis section example .............................................. 265
Figure 129: Preoperative diagnosis section example ................................................. 266
Figure 130: Problem section UML diagram............................................................... 266
Figure 131: Problem section example ...................................................................... 268
Figure 132: Pressure ulcer on a problem list example .............................................. 268
Figure 133: Procedure description section example .................................................. 269
Figure 134: Procedure disposition section example .................................................. 270
Figure 135: Procedure estimated blood loss section example .................................... 271
Figure 136: Procedure findings section example ....................................................... 272
Figure 137: Procedure implants section example ..................................................... 273
Figure 138: Procedure indications section example .................................................. 274
Figure 139: Procedure specimens taken section example ......................................... 275
Figure 140: Procedures section UML diagram .......................................................... 275
Figure 141: Procedures section example .................................................................. 277
Figure 142: Reason for referral section example ....................................................... 278

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 17
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 143: Reason for visit section example ........................................................... 279
Figure 144: Results section UML diagram................................................................ 280
Figure 145: Results section example ....................................................................... 282
Figure 146: Review of systems section example........................................................ 283
Figure 147: Social history section UML diagram ...................................................... 283
Figure 148: Social history section example .............................................................. 284
Figure 149: Subjective section example ................................................................... 285
Figure 150: Surgical drains section example ............................................................ 286
Figure 151: Vital signs section UML diagram ........................................................... 287
Figure 152: Vital signs section example ................................................................... 288
Figure 153: Admission medication entry example .................................................... 291
Figure 154: Advance directive observation example .................................................. 295
Figure 155: Age observation example ....................................................................... 298
Figure 156: Allergy - intolerance observation example .............................................. 305
Figure 157: Allergy problem act example ................................................................. 308
Figure 158: Allergy status observation example ....................................................... 310
Figure 159: Assessment scale observation example .................................................. 313
Figure 160: Assessment scale supporting observation example ................................ 315
Figure 161: Authorization activity example .............................................................. 317
Figure 162: Boundary observation example ............................................................. 318
Figure 163: Caregiver characteristics example with assertion ................................... 320
Figure 164: Caregiver characteristics example without assertion .............................. 320
Figure 165: Code observation example .................................................................... 323
Figure 166:Cognitive status problem observation example ....................................... 327
Figure 167: Cognitive status result observation example .......................................... 331
Figure 168 Cognitive status result organizer example .............................................. 333
Figure 169: Comment act example .......................................................................... 335
Figure 170: Coverage activity example ..................................................................... 337
Figure 171: Deceased observation example .............................................................. 339
Figure 172: Discharge medication entry example ..................................................... 341
Figure 173: Drug vehicle entry example ................................................................... 342
Figure 174: Encounter activities example ................................................................ 346
Figure 175: Encounter diagnosis act example .......................................................... 348
Figure 176: Estimated date of delivery example ....................................................... 349
Figure 177: Family history death observation example ............................................. 350
Figure 178: Family history observation scenario ...................................................... 353

Page 18 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 179: Family history observation example ...................................................... 354
Figure 180: Family history organizer example .......................................................... 359
Figure 181: Functional status problem observation example .................................... 363
Figure 182: Functional status result observation example ........................................ 366
Figure 183: Functional status result organizer example ........................................... 368
Figure 184: Health status observation example ........................................................ 370
Figure 185: Hospital admission diagnosis example .................................................. 373
Figure 186: Hospital discharge diagnosis act example .............................................. 375
Figure 187: Immunization activity example .............................................................. 381
Figure 188: Immunization medication information example ..................................... 384
Figure 189: Immunization refusal reason ................................................................ 385
Figure 190: Indication entry example ...................................................................... 387
Figure 191: Instructions entry example ................................................................... 389
Figure 192: Medication activity example .................................................................. 397
Figure 193: Medication dispense example ................................................................ 402
Figure 194: Medication information example ........................................................... 404
Figure 195: Medication supply order example .......................................................... 407
Figure 196: Medication use – none known example .................................................. 408
Figure 197: Non-medicinal supply activity example .................................................. 410
Figure 198: Number of pressure ulcers example ...................................................... 412
Figure 199: Plan of care activity act example ........................................................... 413
Figure 200: Plan of care activity encounter example ................................................. 414
Figure 201: Plan of care activity observation example .............................................. 416
Figure 202: Plan of care activity procedure example ................................................. 417
Figure 203: Plan of care activity substance administration example ......................... 418
Figure 204: Plan of care activity supply example ...................................................... 419
Figure 205: Policy activity example .......................................................................... 427
Figure 206: Postprocedure diagnosis example .......................................................... 431
Figure 207: Precondition for substance administration example ............................... 432
Figure 208: Pregnancy observation example ............................................................ 434
Figure 209: Preoperative diagnosis example ............................................................. 436
Figure 210: Pressure ulcer observation example ...................................................... 443
Figure 211: Problem concern act (condition) example ............................................... 445
Figure 212: Problem observation example ................................................................ 449
Figure 213: Problem observation with specific problem not observed ........................ 450
Figure 214: Problem observation for no known problems ......................................... 450

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 19
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 215: NullFlavor example ............................................................................... 450
Figure 216: Problem status example ........................................................................ 452
Figure 217: Procedure activity act example .............................................................. 459
Figure 218: Procedure activity observation example ................................................. 465
Figure 219: Procedure activity procedure example ................................................... 471
Figure 220: Procedure context template example ..................................................... 473
Figure 221: Product instance example ..................................................................... 474
Figure 222: Purpose of reference example ................................................................ 476
Figure 223: Quantity measurement observation example ......................................... 479
Figure 224: Reaction observation example ............................................................... 483
Figure 225: Referenced frames observation example ................................................ 484
Figure 226: Result observation example .................................................................. 487
Figure 227: No evaluation procedures (e.g., labs/x-rays) performed example ............ 488
Figure 228: Local code example ............................................................................... 488
Figure 229: Result organizer example ...................................................................... 490
Figure 230: Series act example ................................................................................ 492
Figure 231: Service delivery location example .......................................................... 494
Figure 232: Severity observation example ................................................................ 497
Figure 233: Smoking status observation example .................................................... 499
Figure 234: Unknown if ever smoked ....................................................................... 499
Figure 235: Social history observation template example ......................................... 502
Figure 236: SOP instance observation example ........................................................ 504
Figure 237: Study act example ................................................................................ 507
Figure 238: Text observation example...................................................................... 510
Figure 239: Tobacco use entry example ................................................................... 512
Figure 240: Vital sign observation example .............................................................. 515
Figure 241: Vital signs organizer example ................................................................ 517
Figure 242: Correct use of name example 1 ............................................................. 577
Figure 243: Incorrect use of name example 1 - whitespace ....................................... 577
Figure 244: Incorrect use of Patient name example 2 - no tags ................................. 577
Figure 245: Correct use telecom address example .................................................... 577
Figure 246: Correct use postal address example ...................................................... 577
Figure 247: Correct use of IVL_TS example .............................................................. 578
Figure 248: Correct use of TS with precision to minute example .............................. 578
Figure 249: Correct use of TS with time zone offset example .................................... 578
Figure 250: Incorrect use of IVL_TS example ........................................................... 578

Page 20 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 251: Incorrect use of TS - insufficient precision example ............................... 578
Figure 252: Incorrect use of TS when time zone offset required example ................... 578
Figure 253: Incorrect use of time zone offset - not enough precision example ........... 578
Figure 254: Correct use of CD with no code example................................................ 578
Figure 255: Incorrect use of CD with no code - missing nullFlavor attribute example 579
Figure 256: Immunizations section UML diagram (larger copy) ................................. 580
Figure 257: Functional Status section UML diagram (larger copy) ............................ 580
Figure 258: Medications section UML diagram (larger copy) ..................................... 580
Figure 259: Plan of care section UML diagram (larger copy) ...................................... 580
Table of Tables
Table 1: Content of the Package ................................................................................ 45
Table 2: Basic Confidentiality Kind Value Set............................................................. 47
Table 3: Language Value Set (excerpt) ........................................................................ 47
Table 4: Telecom Use (US Realm Header) Value Set .................................................... 52
Table 5: Administrative Gender (HL7) Value Set ......................................................... 52
Table 6: Marital Status Value Set .............................................................................. 53
Table 7: Religious Affiliation Value Set (excerpt) ......................................................... 53
Table 8: Race Value Set (excerpt) ............................................................................... 54
Table 9: Ethnicity Value Set ...................................................................................... 54
Table 10: Personal Relationship Role Type Value Set (excerpt) .................................... 54
Table 11: State Value Set (excerpt) ............................................................................ 55
Table 12: Postal Code Value Set (excerpt)................................................................... 55
Table 13: Country Value Set (excerpt) ........................................................................ 55
Table 14: Language Ability Value Set ......................................................................... 56
Table 15: Language Ability Proficiency Value Set........................................................ 56
Table 16: IND Role classCode Value Set ..................................................................... 69
Table 17: PostalAddressUse Value Set ....................................................................... 74
Table 18: EntityNameUse Value Set ........................................................................... 76
Table 19: EntityPersonNamePartQualifier Value Set ................................................... 77
Table 20: Document Types and Required/Optional Sections with Structured Body ..... 80
Table 21: Template Containment for a CCD ............................................................... 88
Table 22: Consultation Note LOINC Document Codes ................................................ 98
Table 23: Invalid Codes for Consultation Note .......................................................... 100
Table 24: Template Containment for a Consultation Note ......................................... 105

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 21
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 25: DIR LOINC Document Type Codes ............................................................ 114
Table 26: Template Containment for Constrained DIR Sections ................................ 124
Table 27: DIR Section Type Codes ........................................................................... 125
Table 28: Discharge summary LOINC Document Codes ........................................... 131
Table 29: HL7 Discharge Disposition Codes ............................................................. 133
Table 30: Template Containment for a Discharge Summary ..................................... 137
Table 31: H&P LOINC Document Type Codes ........................................................... 148
Table 32: Template Containment for an H&P Note ................................................... 152
Table 33: Surgical Operation Note LOINC Document Codes ..................................... 161
Table 34: Provider Type Value Set (excerpt) .............................................................. 164
Table 35: Procedure Codes from SNOMED CT ......................................................... 164
Table 36: Template Containment for an Operative Note ............................................ 167
Table 37: Procedure Note LOINC Document Type Codes ........................................... 171
Table 38: Participant Scenario ................................................................................. 172
Table 39: Healthcare Provider Taxonomy Value Set .................................................. 175
Table 40: Template Containment for a Procedure Note ............................................. 180
Table 41: Progress Note LOINC Document Codes ..................................................... 190
Table 42: Template Containment for a Progress Note ............................................... 194
Table 43: Supported File Formats Value Set (Unstructured Documents) ................... 200
Table 44: Sections and Required/Optional Document Types with Structured Body ... 204
Table 45: Advance Directives Section Contexts ........................................................ 210
Table 46: Allergies Section Contexts ........................................................................ 212
Table 47: Anesthesia Section Contexts .................................................................... 214
Table 48: Assessment and Plan Section Contexts ..................................................... 215
Table 49: Assessment Section Contexts ................................................................... 216
Table 50: Chief Complaint and Reason for Visit Section Contexts ............................. 217
Table 51: Chief Complaint Section Contexts ............................................................ 218
Table 52: Complications Section Contexts ............................................................... 219
Table 53: DICOM Object Catalog Section - DCM 121181 Contexts ........................... 220
Table 54: Discharge Diet Section Contexts ............................................................... 222
Table 55: Encounters Section Contexts ................................................................... 222
Table 56: Family History Section Contexts ............................................................... 224
Table 57: Findings Section Contexts ........................................................................ 226
Table 58: Functional Status Section Contexts .......................................................... 227
Table 59: Functional and Cognitive Status Problem Observation Examples .............. 228
Table 60: Functional and Cognitive Status Result Observation Examples ................. 229

Page 22 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 61: General Status Section Contexts .............................................................. 232
Table 62: History of Past Illness Section Contexts .................................................... 233
Table 63: History of Present Illness Section Contexts ............................................... 234
Table 64: Hospital Admission Diagnosis Section Contexts ........................................ 235
Table 65: Hospital Admission Medications Section Contexts ..................................... 236
Table 66: Hospital Consultations Section Contexts .................................................. 237
Table 67: Hospital Course Section Contexts ............................................................. 237
Table 68: Hospital Discharge Diagnosis Section Contexts ......................................... 238
Table 69: Hospital Discharge Instructions Section Contexts ..................................... 239
Table 70: Hospital Discharge Medications Section Contexts ..................................... 240
Table 71: Hospital Discharge Physical Section Contexts ........................................... 242
Table 72: Hospital Discharge Studies Summary Section Contexts ............................ 243
Table 73: Immunizations Section Contexts .............................................................. 244
Table 74: Interventions Section Contexts ................................................................. 247
Table 75: Interventions Section Contexts ................................................................. 248
Table 76: Medical Equipment Section Contexts ........................................................ 249
Table 77: Medical (General) History Section Contexts ............................................... 250
Table 78: Medications Administered Section Contexts .............................................. 251
Table 79: Medications Section Contexts ................................................................... 252
Table 80: Objective Section Contexts ....................................................................... 254
Table 81: Operative Note Fluids Section Contexts .................................................... 255
Table 82: Operative Note Surgical Procedure Section Contexts ................................. 256
Table 83: Payers Section Contexts ........................................................................... 257
Table 84: Physical Exam Section Contexts ............................................................... 259
Table 86: Plan of Care Section Contexts................................................................... 260
Table 87: Planned Procedure Section Contexts......................................................... 262
Table 88: Postoperative Diagnosis Section Contexts ................................................. 263
Table 89: Postprocedure Diagnosis Section Contexts ................................................ 264
Table 90: Preoperative Diagnosis Section Contexts ................................................... 265
Table 91: Problem Section Contexts ......................................................................... 266
Table 92: Procedure Description Section Contexts ................................................... 269
Table 93: Procedure Disposition Section Contexts .................................................... 270
Table 94: Procedure Estimated Blood Loss Section Contexts .................................... 270
Table 95: Procedure Findings Section Contexts ........................................................ 271
Table 96: Procedure Implants Section Contexts ....................................................... 272
Table 97: Procedure Indications Section Contexts .................................................... 273

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 23
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 98: Procedure Specimens Taken Section Contexts .......................................... 274
Table 99: Procedures Section Contexts .................................................................... 275
Table 100: Reason for Referral Section Contexts ...................................................... 278
Table 101: Reason for Visit Section Contexts ........................................................... 279
Table 102: Results Section Contexts ........................................................................ 280
Table 103: Review of Systems Section Contexts ....................................................... 282
Table 104: Social History Section Contexts .............................................................. 283
Table 105: Subjective Section Contexts ................................................................... 285
Table 106: Surgical Drains Section Contexts ........................................................... 286
Table 107: Vital Signs Section Contexts ................................................................... 287
Table 108: Admission Medication Contexts .............................................................. 289
Table 109: Admission Medication Constraints Overview ........................................... 290
Table 110: Advance Directive Observation Contexts ................................................. 291
Table 111: Advance Directive Observation Constraints Overview .............................. 292
Table 112: Advance Directive Type Code Value Set .................................................. 295
Table 113: Age Observation Contexts ...................................................................... 296
Table 114: Age Observation Constraints Overview ................................................... 297
Table 115: AgePQ_UCUM Value Set ......................................................................... 298
Table 116: Allergy - Intolerance Observation Contexts ............................................. 298
Table 117: Allergy - Intolerance Observation Constraints Overview .......................... 299
Table 118: Allergy/Adverse Event Type Value Set .................................................... 303
Table 119: Medication Brand Name Value Set (excerpt) ............................................ 303
Table 120: Medication Clinical Drug Value Set (excerpt) ........................................... 304
Table 121: Medication Drug Class Value Set (excerpt) .............................................. 304
Table 122: Ingredient Name Value Set (excerpt) ....................................................... 305
Table 123: Allergy Problem Act Contexts .................................................................. 306
Table 124: Allergy Problem Act Constraints Overview ............................................... 307
Table 125: ProblemAct statusCode Value Set ........................................................... 308
Table 126: Allergy Status Observation Contexts ....................................................... 309
Table 127: Allergy Status Observation Constraints Overview .................................... 309
Table 128: HITSP Problem Status Value Set ............................................................ 310
Table 129: Assessment Scale Observation Contexts ................................................. 310
Table 130: Assessment Scale Observation Constraints Overview .............................. 311
Table 131: Assessment Scale Supporting Observation Contexts ............................... 314
Table 132: Assessment Scale Supporting Observation Constraints Overview ............ 314
Table 133: Authorization Activity Contexts .............................................................. 315

Page 24 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 134: Authorization Activity Constraints Overview ........................................... 316
Table 135: Boundary Observation Contexts ............................................................. 317
Table 136: Boundary Observation Constraints Overview .......................................... 317
Table 137: Caregiver Characteristics Contexts ......................................................... 318
Table 138: Caregiver Characteristics Constraints Overview ...................................... 319
Table 139: Code Observations Contexts ................................................................... 321
Table 140: Code Observations Constraints Overview ................................................ 322
Table 141: Cognitive Status Problem Observation Contexts ...................................... 323
Table 142: Cognitive Status Problem Observation Constraints Overview ................... 324
Table 143: Problem type value set ........................................................................... 326
Table 144: Problem Value Set (excerpt) .................................................................... 326
Table 145: Cognitive Status Result Observation Contexts......................................... 328
Table 146: Cognitive Status Result Observation Constraints Overview ...................... 328
Table 147: Cognitive Status Result Organizer Contexts ............................................ 331
Table 148: Cognitive Status Result Organizer Constraints Overview ......................... 332
Table 149: Comment Activity Contexts .................................................................... 333
Table 150: Comment Activity Constraints Overview ................................................. 334
Table 151: Coverage Activity Contexts ..................................................................... 336
Table 152: Coverage Activity Constraints Overview .................................................. 336
Table 153: Deceased Observation Contexts .............................................................. 337
Table 154: Deceased Observation Constraints Overview ........................................... 338
Table 155: Discharge Medication Contexts ............................................................... 339
Table 156: Discharge Medication Constraints Overview ............................................ 340
Table 157: Drug Vehicle Contexts ............................................................................ 341
Table 158: Drug Vehicle Constraints Overview ......................................................... 342
Table 159: Encounter Activities Contexts ................................................................. 343
Table 160: Encounter Activities Constraints Overview .............................................. 343
Table 161: Encounter Type Value Set ...................................................................... 345
Table 162: Encounter Diagnosis Contexts................................................................ 346
Table 163: Encounter Diagnosis Constraints Overview ............................................. 347
Table 164: Estimated Date of Delivery Contexts ....................................................... 348
Table 165: Estimated Date of Delivery Constraints Overview .................................... 348
Table 166: Family History Death Observation Contexts ............................................ 349
Table 167: Family History Death Observation Constraints Overview ......................... 350
Table 168: Family History Observation Contexts ...................................................... 351
Table 169: Family History Observation Constraints Overview ................................... 351

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 25
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 170: Family History Organizer Contexts ......................................................... 356
Table 171: Family History Organizer Constraints Overview ...................................... 356
Table 172: Family History Related Subject Value Set (excerpt) ................................. 359
Table 173: Functional Status Problem Observation Contexts ................................... 360
Table 174: Functional Status Problem Observation Constraints Overview ................ 360
Table 175: Functional Status Result Observation Contexts ...................................... 363
Table 176: Functional Status Result Observation Constraints Overview ................... 364
Table 177: Functional Status Result Organizer Contexts .......................................... 366
Table 178: Functional Status Result Organizer Constraints Overview ....................... 367
Table 179: Health Status Observation Contexts ....................................................... 368
Table 180: Health Status Observation Constraints Overview .................................... 368
Table 181: HealthStatus Value Set .......................................................................... 369
Table 182: Highest Pressure Ulcer Stage Contexts ................................................... 371
Table 183: Highest Pressure Ulcer Stage Constraints Overview ................................ 371
Table 184: Hospital Admission Diagnosis Contexts .................................................. 372
Table 185: Hospital Admission Diagnosis Constraints Overview ............................... 372
Table 186: Hospital Discharge Diagnosis Contexts ................................................... 373
Table 187: Hospital Discharge Diagnosis Constraints Overview ................................ 374
Table 188: Immunization Activity Contexts .............................................................. 375
Table 189: Immunization Activity Constraints Overview ........................................... 376
Table 190: Immunization Medication Information Contexts ...................................... 381
Table 191: Immunization Medication Information Constraints Overview ................... 382
Table 192: Vaccine Administered (Hepatitis B) Value Set (excerpt) ............................ 383
Table 193: Immunization Refusal Reason Contexts .................................................. 384
Table 194: Immunization Refusal Reason Constraints Overview ............................... 384
Table 195: No Immunization Reason Value Set ........................................................ 385
Table 196: Indication Contexts ................................................................................ 386
Table 197: Indication Constraints Overview ............................................................. 386
Table 198: Instructions Contexts ............................................................................. 388
Table 199: Instructions Constraints Overview .......................................................... 388
Table 200: Patient Education Value Set ................................................................... 389
Table 201: Medication Activity Contexts .................................................................. 390
Table 202: Medication Activity Constraints Overview ............................................... 390
Table 203: MoodCodeEvnInt Value Set .................................................................... 395
Table 204: Medication Route FDA Value Set (excerpt) .............................................. 396
Table 205: Body Site Value Set (excerpt) .................................................................. 396

Page 26 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 206: Medication Product Form Value Set (excerpt) .......................................... 397
Table 207: Unit of Measure Value Set (excerpt) ........................................................ 397
Table 208: Medication Dispense Contexts ................................................................ 399
Table 209: Medication Dispense Constraints Overview ............................................. 400
Table 210: Medication Fill Status Value Set ............................................................. 401
Table 211: Medication Information Contexts ............................................................ 402
Table 212: Medication Information Constraints Overview ......................................... 403
Table 213: Medication Supply Order Contexts ......................................................... 404
Table 214: Medication Supply Order Constraints Overview ...................................... 405
Table 215: Non-Medicinal Supply Activity Contexts .................................................. 408
Table 216: Non-Medicinal Supply Activity Constraints Overview ............................... 409
Table 217: Number of Pressure Ulcers Observation Contexts ................................... 410
Table 218: Number of Pressure Ulcers Observation Constraints Overview................. 410
Table 219: Plan of Care Activity Act Contexts ........................................................... 412
Table 220: Plan of Care Activity Act Constraints Overview ........................................ 412
Table 221: Plan of Care moodCode (Act/Encounter/Procedure) ................................ 413
Table 222: Plan of Care Activity Encounter Contexts ................................................ 413
Table 223: Plan of Care Activity Encounter Constraints Overview ............................. 414
Table 224: Plan of Care Activity Observation Contexts ............................................. 414
Table 225: Plan of Care Activity Observation Constraints Overview .......................... 415
Table 226: Plan of Care moodCode (Observation) Value Set ...................................... 415
Table 227: Plan of Care Activity Procedure Contexts ................................................ 416
Table 228: Plan of Care Activity Procedure Constraints Overview ............................. 416
Table 229: Plan of Care Activity Substance Administration Contexts ........................ 417
Table 230: Plan of Care Activity Substance Administration Constraints Overview ..... 417
Table 231: Plan of Care moodCode (SubstanceAdministration/Supply) Value Set ..... 418
Table 232: Plan of Care Activity Supply Contexts ..................................................... 418
Table 233: Plan of Care Activity Supply Constraints Overview .................................. 419
Table 234: Policy Activity Contexts .......................................................................... 419
Table 235: Policy Activity Constraints Overview ....................................................... 420
Table 236: Health Insurance Type Value Set (excerpt) .............................................. 426
Table 237: Coverage Type Value Set ........................................................................ 427
Table 238: Financially Responsible Party Value Set (excerpt) .................................... 427
Table 239: Postprocedure Diagnosis Contexts .......................................................... 430
Table 240: Postprocedure Diagnosis Constraints Overview ....................................... 430
Table 241: Precondition for Substance Administration Contexts ............................... 431

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 27
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 242: Precondition for Substance Administration Constraints Overview ............ 431
Table 243: Pregnancy Observation Contexts ............................................................ 432
Table 244: Pregnancy Observation Constraints Overview ......................................... 432
Table 245: Preoperative Diagnosis Contexts ............................................................. 434
Table 246: Preoperative Diagnosis Constraints Overview .......................................... 435
Table 247: Pressure Ulcer Observation Contexts ...................................................... 436
Table 248: Pressure Ulcer Observation Constraints Overview ................................... 436
Table 249 Pressure Ulcer Stage Value Set ................................................................ 440
Table 250: Pressure Point Value Set ........................................................................ 441
Table 251: Target Site Qualifiers Value Set .............................................................. 442
Table 252: Problem Concern Act (Condition) Contexts .............................................. 444
Table 253: Problem Concern Act (Condition) Constraints Overview ........................... 444
Table 254: Problem Observation Contexts ............................................................... 446
Table 255: Problem Observation Constraints Overview............................................. 446
Table 256: Problem Status Contexts ........................................................................ 451
Table 257: Problem Status Constraints Overview ..................................................... 451
Table 258: Procedure Activity Act Contexts .............................................................. 452
Table 259: Procedure Activity Act Constraints Overview ........................................... 454
Table 260: Procedure Act Status Code Value Set ...................................................... 458
Table 261: Act Priority Value Set ............................................................................. 458
Table 262: Procedure Activity Observation Contexts ................................................ 460
Table 263: Procedure Activity Observation Constraints Overview.............................. 460
Table 264: Procedure Activity Procedure Contexts.................................................... 466
Table 265: Procedure Activity Procedure Constraints Overview ................................. 466
Table 266: Procedure Context Contexts ................................................................... 472
Table 267: Procedure Context Constraints Overview ................................................ 472
Table 268: Product Instance Contexts ..................................................................... 473
Table 269: Product Instance Constraints Overview .................................................. 474
Table 270: Purpose of Reference Observation Contexts ............................................ 475
Table 271: Purpose of Reference Observation Constraints Overview ......................... 475
Table 272: DICOM Purpose of Reference Value Set .................................................. 476
Table 273: Quantity Measurement Observation Contexts ......................................... 476
Table 274: Quantity Measurement Observation Constraints Overview ...................... 477
Table 275: DIR Quantity Measurement Type Value Set ............................................ 478
Table 276: DICOM Quantity Measurement Type Value Set ....................................... 479
Table 277: Reaction Observation Contexts ............................................................... 480

Page 28 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 278: Reaction Observation Constraints Overview ............................................ 480
Table 279: Referenced Frames Observation Constraints Overview ............................ 483
Table 280: Result Observation Contexts .................................................................. 484
Table 281: Result Observation Constraints Overview ............................................... 485
Table 282: Result Status Value Set .......................................................................... 487
Table 283: Result Organizer Contexts ...................................................................... 488
Table 284: Result Organizer Constraints Overview ................................................... 489
Table 285: Series Act Contexts ................................................................................ 490
Table 286: Series Act Constraints Overview ............................................................. 491
Table 287: Service Delivery Location Contexts ......................................................... 493
Table 288: Service Delivery Location Constraints Overview ...................................... 493
Table 289: HealthcareServiceLocation Value Set (excerpt) ........................................ 494
Table 290: Severity Observation Contexts ................................................................ 495
Table 291: Severity Observation Constraints Overview ............................................. 495
Table 292: Problem Severity Value Set ..................................................................... 496
Table 293: Smoking Status Observation Contexts .................................................... 497
Table 294: Smoking Status Observation Constraints Overview ................................. 498
Table 295: Smoking Status Value Set ...................................................................... 499
Table 296: Social History Observation Contexts ....................................................... 500
Table 297: Social History Observation Constraints Overview .................................... 500
Table 298: Social History Type Set Definition Value Set ........................................... 501
Table 299: SOP Instance Observation Contexts ........................................................ 502
Table 300: SOP Instance Observation Constraints Overview ..................................... 502
Table 301: Study Act Contexts ................................................................................ 505
Table 302: Study Act Constraints Overview ............................................................. 506
Table 303: Text Observation Contexts...................................................................... 507
Table 304: Text Observation Constraints Overview ................................................... 508
Table 305: Tobacco Use Observation Contexts ......................................................... 510
Table 306: Tobacco Use Constraints Overview ......................................................... 511
Table 307: Tobacco Use Value Set ........................................................................... 512
Table 308: Vital Sign Observation Contexts ............................................................. 512
Table 309: Vital Sign Observation Constraints Overview .......................................... 513
Table 310: Vital Sign Result Type Value Set ............................................................. 514
Table 311: Vital Signs Organizer Contexts ............................................................... 515
Table 312: Vital Signs Organizer Constraints Overview ............................................ 516
Table 313: Templates Added and Updated in May 2012 Ballot ................................. 522

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 29
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 314: H&P Cardinality Updates ....................................................................... 523
Table 315: Consultation Note Cardinality Updates ................................................... 523
Table 316: Discharge Summary Cardinality Updates ............................................... 524
Table 317: Surgical Operative Codes Mapping to Generic Procedure Codes............... 524
Table 318: Consolidated Conformance Verb Matrix .................................................. 525
Table 319: Section Template Change Tracking ......................................................... 527
Table 320: Entry Change Tracking Table ................................................................. 535
Table 321: Result Section Changes .......................................................................... 539
Table 322: Problems Section Changes ..................................................................... 540
Table 323: Vital Signs Section Changes ................................................................... 543
Table 324: Procedures Section Changes .................................................................. 545
Table 325: Medications Section Changes ................................................................. 548
Table 326: Template Ids Alphabetically by Template Type ........................................ 557
Table 327: Code Systems in This Guide ................................................................... 563
Table 328: Value Sets in This Guide ........................................................................ 565
Table 329: Single-Value Bindings in This Guide....................................................... 568
Table 330: Comparison of XDS-SD and Clinical Document Header .......................... 571
Page 30 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
2 INTRODUCTION
2.1 Audience
The audiences for this implementation guide are the architects and developers of
healthcare information technology (HIT) systems in the US Realm that exchange
patient clinical data. This includes those exchanges that comply to the Health
Information Technology for Economic and Clinical Health (HITECH) provisions of
the American Recovery And Reinvestment Act of 2009, the Final Rules for Stage
1 Meaningful Use, and the 45 CFR Part 170 – Health Information Technology:
Initial Set of Standards, Implementation Specifications, and Certification Criteria
for Electronic Health Record Technology; Final Rule.1
Business analysts and policy managers can also benefit from a basic
understanding of the use of Clinical Document Architecture (CDA) templates
across multiple implementation use cases.
2.2 Purpose
This guide contains a library of CDA templates, incorporating and harmonizing
previous efforts from Health Level Seven (HL7), Integrating the Healthcare
Enterprise (IHE), and Health Information Technology Standards Panel (HITSP). It
represents harmonization of the HL7 Health Story guides, HITSP C32, related
components of IHE Patient Care Coordination (IHE PCC), and Continuity of Care
(CCD), and it includes all required CDA templates in Final Rules for Stage 1
Meaningful Use and 45 CFR Part 170 – Health Information Technology: Initial
Set of Standards, Implementation Specifications, and Certification Criteria for
Electronic Health Record Technology; Final Rule.
This guide is a single source for implementing the following CDA documents (see
the References section for complete source listings):
Continuity of Care Document (CCD) (Release 1.1)
Consultation Notes (Release 1.1)
Discharge Summary (Release 1.1)
Imaging Integration, and DICOM Diagnostic Imaging Reports (DIR) (US
Realm - Release 1)
History and Physical (H&P) (Release 1.1)
Operative Note (Release 1.1)
Progress Note (Release 1.1)
Procedure Note (US Realm – Release 1)
Unstructured Documents (Release 1.1)
1 Many aspects of this guide were designed to meet the anticipated clinical document exchange
requirements of Stage 2 Meaningful Use. At the time of this publication, Stage 2 Meaningful Use
has not been published.

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 31
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
The release 1.1 documents supersede existing release 1 publications. Procedure
Note and DIR are designated as release 1 because this guide is the first US
Realm release of these standards. The existing, separate Procedure Note and DIR
universal-realm guides are still valid for outside the US.
2.3 Scope
This document is scoped by the content of the eight Health Story Guides, CCD,
and additional constraints from IHE and HITSP. New conformance rules were
not introduced unless an ambiguity or conflict existed among the standards.
All CDA templates required for Final Rules for Stage 1 Meaningful Use2 are
included in this guide. All CDA templates required for Health Story compliance
to the section level are included, as well, of course, as the Health Story reuse of
Stage 1 Meaningful Use templates.
This guide fully specifies a compliant CDA R2 document for each document
type.
Additional optional CDA elements, not included here, can be included and the
result will be compliant with the documents in this standard.
2.4 Approach
In the development of this specification, the Consolidation Project team reviewed
the eight existing HL7 Health Story guides, CCD, and the additional constraints
from IHE, HITSP and Stage 1 Meaningful Use.
The Consolidation Project team members completed the analysis by creating a
fully compliant CCD document, then layering in the additional HITSP, IHE and
Stage 1 Meaningful Use constraints. When a new constraint introduced an
issue, conflict or ambiguity, the item was flagged for review with the full
consolidation team. The full analysis covered the CDA Header, section-level and
entry-level requirements sufficient for Stage 1 Meaningful Use. The Project also
reviewed document and section-level requirements for the full set of document
types.
All major template changes are summarized in the Change Appendix
All involved in the Consolidation Project recognize the critical need for an
intrinsic tie between the human-readable conformance requirements, the
computable expression of those requirements, the production of validation test
suites and application interfaces to facilitate adoption. To that end, the analysis
performed by the volunteers and staff of the Consolidation Project was the
prelude to data entry into a set of model-based tools.
Conformance requirements and value set tables published here are output from
the Template Database (Tdb), an open-source application first developed for the
Centers for Disease Control and Prevention and in active use by the National
2 http://edocket.access.gpo.gov/2010/pdf/2010-17207.pdf
Many aspects of this guide were designed to meet the anticipated clinical document exchange
requirements of Stage 2 Meaningful Use, which had not been released when this guide was
published
Page 32 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Healthcare Safety Network3. The Tdb is the source for generation of platform-
independent validation rules as Schematron4 (compiled XPath). The Tdb is
available as the Trifolia Workbench (Consolidation Project Edition) on the HL7
website5.
The consolidation of templates developed across these organizations and their
publication in catalog form driven from model-based tools is a strong step
toward satisfying the full range of requirements for clinical information use and
reuse through templated CDA.
2.5 Organization of This Guide
This guide includes a set of CDA Templates and prescribes their use for a set of
specific document types. The main chapters are:
Chapter 2. General Header Template. This chapter defines a template for the
header constraints that apply across all of the consolidated document types.
Chapter 3. Document-Level Templates. This chapter defines each of the nine
document types. It defines header constraints specific to each and the section-
level templates (required and optional) for each.
Chapter 4. Section-Level Templates. This chapter defines the section templates
referenced within the document types described here. Sections are atomic units,
and can be reused by future specifications.
Chapter 5. Entry-Level Templates. This chapter defines entry-level templates,
called clinical statements. Machine processable data are sent in the entry
templates. The entry templates are referenced by one or more section templates.
Entry-level templates are always contained in section-level templates, and
section-level templates are always contained in a document.
Appendices. The Appendices include non-normative content to support
implementers. It includes a Change Appendix summary of previous and updated
templates.
2.6 Use of Templates
Template identifiers (templateId) are assigned at the document, section, and
entry level. When valued in an instance, the template identifier signals the
imposition of a set of template-defined constraints. The value of this attribute
(e.g. @root="2.16.840.1.113883.10.20.22.4.8") provides a unique identifier
for the template in question.
If a template is a specialization of another template, its first constraint indicates
the more general template. The general template is not always required. In all
cases where a more specific template conforms to a more general template,
3 http://www.lantanagroup.com/resources/tools/
4 http://www.schematron.com/
5 http://www.lantanagroup.com/newsroom/press-releases/trifolia-workbench/
You must be logged in as a member of HL7.org to access this resource:
http://www.hl7.org/login/singlesignon.cfm?next=/documentcenter/private/standards/cda/Trifo
lia_HL7_Consolidation_20110712-dist.zip

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 33
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
asserting the more specific template also implies conformance to the more
general template.
2.6.1 Originator Responsibilities: General Case
An originator can apply a templateId if there is a desire to assert conformance
with a particular template.
In the most general forms of CDA exchange, an originator need not apply a
templateId for every template that an object in an instance document conforms
to. The implementation guide (IG) shall assert whenever templateIds are
required for conformance.
2.6.2 Recipient Responsibilities: General Case
A recipient may reject an instance that does not contain a particular
templateId (e.g., a recipient looking to receive only Procedure Note documents
can reject an instance without the appropriate templateId).
A recipient may process objects in an instance document that do not contain a
templateId (e.g., a recipient can process entries that contain Observation acts
within a Problems section, even if the entries do not have templateIds).
2.7 Levels of Constraint
The CDA standard describes conformance requirements in terms of three
general levels corresponding to three different, incremental types of conformance
statements:
Level 1 requirements impose constraints upon the CDA Header. The body
of a Level 1 document may be XML or an alternate allowed format. If
XML, it must be CDA-conformant markup.
Level 2 requirements specify constraints at the section level of a CDA
XML document: most critically, the section code and the cardinality of
the sections themselves, whether optional or required.
Level 3 requirements specify constraints at the entry level within a
section. A specification is considered “Level 3” if it requires any entry-
level templates.
Note that these levels are rough indications of what a recipient can expect in
terms of machine-processable coding and content reuse. They do not reflect the
level or type of clinical content, and many additional levels of reusability could
be defined.
In this consolidated guide, Unstructured Documents, by definition, are Level 1.
Stage 1 Meaningful Use of CCD requires certain entries and is therefore a Level
3 requirement. The balance of the document types can be implemented at any
level.
In all cases, required clinical content must be present. For example, a CDA
Procedure Note carrying the templateId that asserts conformance with Level 1
may use a PDF (portable document format) or HTML (hypertext markup

Page 34 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
language) format for the body of the document that contains the required
clinical content. Conformance, in this case, to the clinical content requirements
could not be validated without human review.
The section libraries for each document type list the required and optional
sections.
2.8 Conformance Conventions Used in This Guide
2.8.1 Templates and Conformance Statements
Conformance statements within this implementation guide are presented as
constraints from a Template Database (Tdb). An algorithm converts constraints
recorded in a Templates Database to a printable presentation. Each constraint is
uniquely identified by an identifier at or near the end of the constraint (e.g.,
CONF:7345). These identifiers are persistent but not sequential.
Bracketed information following each template title indicates the template type
(section, observation, act, procedure, etc.), the templateId, and whether the
template is open or closed.
Each section and entry template in the guide includes a context table. The "Used
By" column indicates which documents or sections use this template, and the
"Contains Entries" column indicates any entries that the template uses. Each
entry template also includes a constraint overview table to summarize the
constraints following the table.
The following figure shows a typical template explanation presented in this
guide. The next sections describe specific aspects of conformance statements—
open vs. closed statements, conformance verbs, cardinality, vocabulary
conformance, containment relationships, and null flavors.
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 35
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 1: Constraints format example
Severity Observation
[observation: templateId 2.16.840.1.113883.10.20.22.4.8(open)]
Table xxx: Severity Observation Contexts
Used By:
Contains Entries:
Reaction Observation
Allergy - Intolerance Observation
This clinical statement represents the severity of the reaction to an agent. A
person may manifest many symptoms …
Table yyy: Severity Observation Contexts
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
Green
Severity
Observation
observation[templateId/@root = '2.16.840.1.113883.10.20.22.4.8']
@classCode
1..1
SHALL
7345
2.16.840.1.113883.5.6
(HL7ActClass) = OBS
@moodCode
1..1
SHALL
7346
2.16.840.1.113883.5.1001
(ActMood) = EVN
templateId
1..1
SHALL
SET<II>
7347
@root
1..1
SHALL
10525
2.16.840.1.113883.10.20.22.4.8
code
1..1
SHALL
CE
7349
2.16.840.1.113883.5.4 (ActCode) =
SEV
severity
FreeText
text
0..1
SHOULD
ED
7350
reference
/@value
0..1
SHOULD
7351
statusCode
1..1
SHALL
CS
7352
2.16.840.1.113883.5.14 (ActStatus)
= completed
severity
Coded
value
1..1
SHALL
CD
7356
2.16.840.1.113883.3.88.12.3221.6.8
(Problem Severity)
interpretation
Code
0..*
SHOULD
CE
9117
code
0..1
SHOULD
CE
9118
2.16.840.1.113883.1.11.78
(Observation Interpretation (HL7))
1. SHALL contain exactly one [1..1] @classCode="OBS" Observation
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6) (CONF:7345).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001) (CONF:7346).
3. SHALL contain exactly one [1..1] templateId (CONF:7347) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.8" (CONF:10525).

Page 36 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
4. SHALL contain exactly one [1..1] code with @xsi:type="CE"="SEV" Severity
Observation (CodeSystem: ActCode 2.16.840.1.113883.5.4)
(CONF:7349).
5. SHOULD contain zero or one [0..1] text (CONF:7350).
a. The text, if present, SHOULD contain zero or one [0..1]
reference/@value (CONF:7351).
i. This reference/@value SHALL begin with a '#' and SHALL point
to its corresponding narrative (using the approach defined in
CDA Release 2, section 4.3.5.1) (CONF:7378).
6. SHALL contain exactly one [1..1] statusCode="completed" Completed
(CodeSystem: ActStatus 2.16.840.1.113883.5.14) (CONF:7352).
7. SHALL contain exactly one [1..1] value with @xsi:type="CD", where the @code
SHALL be selected from ValueSet Problem Severity
2.16.840.1.113883.3.88.12.3221.6.8 DYNAMIC (CONF:7356).
8. SHOULD contain zero or more [0..*] interpretationCode (CONF:9117).
a. The interpretationCode, if present, SHOULD contain zero or one [0..1]
code with @xsi:type="CE", where the @code SHOULD be selected from
ValueSet Observation Interpretation (HL7)
2.16.840.1.113883.1.11.78 DYNAMIC (CONF:9118).
2.8.2 Open and Closed Templates
In open templates, all of the features of the CDA R2 base specification are
allowed except as constrained by the templates. By contrast, a closed template
specifies everything that is allowed and nothing further may be included.
Estimated Date of Delivery (templateId 2.16.840.1.113883.10.20.15.3.1) is
an example of a closed template in this guide.
Open templates allow HL7 implementers to develop additional structured
content not constrained within this guide. HL7 encourages implementers to
bring their use cases forward as candidate requirements to be formalized in a
subsequent version of the standard to maximize the use of shared semantics.
2.8.3 Conformance Verbs (Keywords)
The keywords SHALL, SHOULD, MAY, NEED NOT, SHOULD NOT, and SHALL NOT in this
document are to be interpreted as described in the HL7 Version 3 Publishing
Facilitator's Guide (http://www.hl7.org/v3ballot/html/help/pfg/pfg.htm):
SHALL: an absolute requirement
SHALL NOT: an absolute prohibition against inclusion
SHOULD/SHOULD NOT: best practice or recommendation. There may be
valid reasons to ignore an item, but the full implications must be
understood and carefully weighed before choosing a different course
MAY/NEED NOT: truly optional; can be included or omitted as the author
decides with no implications

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 37
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
The keyword "SHALL" allows the use of nullFlavor unless the requirement is on
an attribute or the use of nullFlavor is explicitly precluded.
The Consolidated Conformance Verb Matrix table represents a matrix of the
conformance verbs used across the standards reviewed for the consolidation
guide.
The subject of a conformance verb (keyword) in a top-level constraint is the
template itself; for example, the subject of CONF:5249 is the ClinicalDocument
element. In nested constraints, the subject is the element in the containing
constraint. Top-level constraints are those that begin with a number and are not
indented.
2.8.4 Cardinality
The cardinality indicator (0..1, 1..1, 1..*, etc.) specifies the allowable occurrences
within a document instance. The cardinality indicators are interpreted with the
following format “m…n” where m represents the least and n the most:
0..1 zero or one
1..1 exactly one
1..* at least one
0..* zero or more
1..n at least one and not more than n
When a constraint has subordinate clauses, the scope of the cardinality of the
parent constraint must be clear. In the next figure, the constraint says exactly
one participant is to be present. The subordinate constraint specifies some
additional characteristics of that participant.
Figure 2: Constraints format – only one allowed
1. SHALL contain exactly one [1..1] participant (CONF:2777).
a. This participant SHALL contain exactly one [1..1] @typeCode="LOC"
(CodeSystem: 2.16.840.1.113883.5.90 HL7ParticipationType)
(CONF:2230).
In the next figure, the constraint says only one participant “like this” is to be
present. Other participant elements are not precluded by this constraint.
Figure 3: Constraints format – only one like this allowed
1. SHALL contain exactly one [1..1] participant (CONF:2777) such that it
a. SHALL contain exactly one [1..1] @typeCode="LOC" (CodeSystem:
2.16.840.1.113883.5.90 HL7ParticipationType) (CONF:2230).

Page 38 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
2.8.5 Optional and Required with Cardinality
The terms optional and required describe the lower bound of cardinality as
follows:
Optional means that the number of allowable occurrences of an element may be
0; the cardinality will be expressed as [0..1] or [0..*] or similar. In these
cases, the element may not be present in the instance.
Required means that the number of allowable occurrences of an element must
be at least 1; the cardinality will be expressed as [m..n] where m >=1 and n
>=1 for example [1..1] or [1..*].. In these cases, the element must be
present in the instance. If an element is required, but is not known (and would
otherwise be omitted if it were optional), it must be represented by a nullFlavor.
2.8.6 Vocabulary Conformance
The templates in this document use terms from several code systems. These
vocabularies are defined in various supporting specifications and may be
maintained by other bodies, as is the case for the LOINC® and SNOMED CT®
vocabularies.
Note that value-set identifiers (e.g., ValueSet 2.16.840.1.113883.1.11.78
Observation Interpretation (HL7) DYNAMIC) do not appear in CDA
submissions; they tie the conformance requirements of an implementation guide
to the appropriate code system for validation.
Value-set bindings adhere to HL7 Vocabulary Working Group best practices,
and include both a conformance verb (SHALL, SHOULD, MAY, etc.) and an
indication of DYNAMIC vs. STATIC binding. Value-set constraints can be STATIC,
meaning that they are bound to a specified version of a value set, or DYNAMIC,
meaning that they are bound to the most current version of the value set. A
simplified constraint, used when the binding is to a single code, includes the
meaning of the code, as follows.
Figure 4: Binding to a single code
... SHALL contain exactly one [1..1] code (CONF:15407).
a. This code SHALL contain exactly one [1..1] @code="11450-4" Problem
List
(CodeSystem: LOINC 2.16.840.1.113883.6.1) (CONF:15408).
The notation conveys the actual code (11450-4), the code’s displayName
(Problem List), the OID of the codeSystem from which the code is drawn
(2.16.840.1.113883.6.1), and the codeSystemName (LOINC).
HL7 Data Types Release 1 requires the codeSystem attribute unless the
underlying data type is “Coded Simple” or “CS”, in which case it is prohibited.
The displayName and the codeSystemName are optional, but recommended, in
all cases.

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 39
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
The above example would be properly expressed as follows.
Figure 5: XML expression of a single-code binding
<code code="11450-4" codeSystem="2.16.840.1.113883.6.1"/>
<!-- or -->
<code code="11450-4" codeSystem="2.16.840.1.113883.6.1"
displayName="Problem List"
codeSystemName=”LOINC”/>
A full discussion of the representation of vocabulary is outside the scope of this
document; for more information, see the HL7 V3 Normative Edition 20106
sections on Abstract Data Types and XML Data Types R1.
There is a discrepancy in the implementation of translation code versus the
original code between HL7 Data Types R1 and the convention agreed upon for
this specification. The R1 data type requires the original code in the root. This
implementation guide specifies the standard code in the root, whether it is
original or a translation. This discrepancy is resolved in HL7 Data Types R2.
Figure 6: Translation code example
<code code='206525008’
displayName='neonatal necrotizing enterocolitis'
codeSystem='2.16.840.1.113883.6.96'
codeSystemName='SNOMED CT'>
<translation code='NEC-1'
displayName='necrotizing enterocolitis'
codeSystem='2.16.840.1.113883.19'/>
</code>
2.8.7 Containment Relationships
Containment constraints between a section and its entry are indirect in this
guide, meaning that where a section asserts containment of an entry, that entry
can either be a direct child or a further descendent of that section.
For example, in the following constraint:
1. SHALL contain at least one [1..*] entry (CONF:8647) such that it
a. SHALL contain exactly one [1..1] Advance Directive Observation
(templateId:2.16.840.1.113883.10.20.22.4.48) (CONF:8801).
the Advance Directive Observation can be a direct child of the section (i.e.,
section/entry/AdvanceDirectiveObservation) or a further descendent of
that section (i.e., section/entry/…/AdvanceDirectiveObservation). Either of
these are conformant.
6 HL7 Version 3 Interoperability Standards, Normative Edition 2010.
http://www.hl7.org/memonly/downloads/v3edition.cfm - V32010

Page 40 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
All other containment relationships are direct, for example:
1. SHALL contain exactly one [1..1]
templateId/@root="2.16.840.1.113883.10.20.22.2.21" (CONF:7928).
The templateId must be a direct child of the section (i.e., section/templateId).
2.8.8 Null Flavor
Information technology solutions store and manage data, but sometimes data
are not available: an item may be unknown, not relevant, or not computable or
measureable. In HL7, a flavor of null, or nullFlavor, describes the reason for
missing data.
For example, if a patient arrives at an Emergency Department unconscious and
with no identification, we would use a null flavor to represent the lack of
information. The patient’s birth date would be represented with a null flavor of
“NAV”, which is the code for “temporarily unavailable”. When the patient regains
consciousness or a relative arrives, we expect to know the patient’s birth date.
Figure 7: nullFlavor example
<birthTime nullFlavor=”NAV”/> <!--coding an unknown birthdate-->
Use null flavors for unknown, required, or optional attributes:
NI No information. This is the most general and default null flavor.
NA Not applicable. Known to have no proper value (e.g., last
menstrual period for a male).
UNK Unknown. A proper value is applicable, but is not known.
ASKU Asked, but not known. Information was sought, but not found
(e.g., the patient was asked but did not know).
NAV Temporarily unavailable. The information is not available, but
is expected to be available later.
NASK Not asked. The patient was not asked.
MSK There is information on this item available but it has not been
provided by the sender due to security, privacy, or other
reasons. There may be an alternate mechanism for gaining
access to this information.
This above list contains those null flavors that are commonly used in clinical
documents. For the full list and descriptions, see the nullFlavor vocabulary
domain in the CDA normative edition7.
Any SHALL conformance statement may use nullFlavor, unless the attribute is
required or the nullFlavor is explicitly disallowed. SHOULD and MAY
conformance statement may also use nullFlavor.
7 HL7 Clinical Document Architecture (CDA Release 2)
http://www.hl7.org/implement/standards/cda.cfm
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 41
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 8: Attribute required
1. SHALL contain exactly one [1..1] code (CONF:15407).
a. This code SHALL contain exactly one [1..1] @code="11450-4" Problem
List
(CodeSystem: LOINC 2.16.840.1.113883.6.1) (CONF:15408).
or
2. SHALL contain exactly one [1..1] effectiveTime/@value (CONF:5256).
Figure 9: Allowed nullFlavors when element is required (with xml examples)
1. SHALL contain at least one [1..*] id
2. SHALL contain exactly one [1..1] code
3. SHALL contain exactly one [1..1] effectiveTime
<entry>
<observation classCode="OBS" moodCode="EVN">
<id nullFlavor="NI"/>
<code nullFlavor="OTH">
<originalText>New Grading system</originalText>
</code>
<statusCode code="completed"/>
<effectiveTime nullFlavor="UNK"/>
<value xsi:type="CD" nullFlavor="NAV">
<originalText>Spiculated mass grade 5</originalText>
</value>
</observation>
</entry>
Figure 10: nullFlavor explicitly disallowed
1. SHALL contain exactly one [1..1] effectiveTime (CONF:5256).
a. SHALL NOT contain [0..0] nullFlavor (CONF:52580).

Page 42 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
2.8.9 Unknown Information
If a sender wants to state that a piece of information is unknown, the following
principles apply:
1. If the sender doesn’t know an attribute of an act, that attribute can be
null.
Figure 11: Unknown medication example
<entry>
<text>patient was given a medication but I do not know what it
was</text>
<substanceAdministration moodCode="EVN" classCode="SBADM">
<consumable>
<manufacturedProduct>
<manufacturedLabeledDrug>
<code nullFlavor="NI"/>
</manufacturedLabeledDrug>
</manufacturedProduct>
</consumable>
</substanceAdministration>
</entry>

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 43
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
2. If the sender doesn’t know if an act occurred, the nullFlavor is on the
act (detail could include specific allergy, drug, etc.).
Figure 12: Unknown medication use of anticoagulant drug example
<entry>
<substanceAdministration moodCode="EVN" classCode="SBADM"
nullFlavor="NI">
<text>I do not know whether or not patient received an anticoagulant
drug</text>
<consumable>
<manufacturedProduct>
<manufacturedLabeledDrug>
<code code="81839001" displayName="anticoagulant drug"
codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT"/>
</manufacturedLabeledDrug>
</manufacturedProduct>
</consumable>
</substanceAdministration>
</entry>
3. If the sender wants to state ‘no known’, a negationInd can be used on
the corresponding act (substanceAdministration, Procedure, etc.)
Figure 13: No known medications example
<entry>
<substanceAdministration moodCode="EVN" classCode="SBADM"
negationInd=”true”>
<text>No known medications</text>
<consumable>
<manufacturedProduct>
<manufacturedLabeledDrug>
<code code="410942007" displayName="drug or medication"
codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT"/>
</manufacturedLabeledDrug>
</manufacturedProduct>
</consumable>
</substanceAdministration>
</entry>
Previously CCD, IHE, and HITSP recommended using specific codes to assert no
known content, for example 160244002 No known allergies or 160245001 No
current problems or disability. Specific codes are still allowed; however,
use of these codes is not recommended.
2.8.10 Data Types
All data types used in a CDA document are described in the CDA R2 normative
edition8. All attributes of a data type are allowed unless explicitly prohibited by
this specification.
8 HL7 Clinical Document Architecture (CDA Release 2).
http://www.hl7.org/implement/standards/cda.cfm

Page 44 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
2.9 XML Conventions Used in This Guide
2.9.1 XPath Notation
Instead of the traditional dotted notation used by HL7 to represent Reference
Information Model (RIM) classes, this document uses XML Path Language
(XPath) notation9 in conformance statements and elsewhere to identify the
Extended Markup Language (XML) elements and attributes within the CDA
document instance to which various constraints are applied. The implicit
context of these expressions is the root of the document. This notation provides
a mechanism that will be familiar to developers for identifying parts of an XML
document.
XPath statements appear in this document in a monospace font.
XPath syntax selects nodes from an XML document using a path containing the
context of the node(s). The path is constructed from node names and attribute
names (prefixed by a ‘@’) and catenated with a ‘/’ symbol.
Figure 14: XML document example
<author>
<assignedAuthor>
...
<code codeSystem='2.16.840.1.113883.6.96' codeSystemName='SNOMED CT'
code='17561000' displayName='Cardiologist' />
...
</assignedAuthor>
</author>
In the above example, the code attribute of the code could be selected with the
XPath expression in the next figure.
Figure 15: XPath expression example
author/assignedAuthor/code/@code
2.9.2 XML Examples and Sample Documents
Extended Mark-up Language (XML) examples appear in figures in this document
in this monospace font. Portions of the XML content may be omitted from the
content for brevity, marked by an ellipsis (...) as shown in the example below.
Figure 16: ClinicalDocument example
<ClinicalDocument xmls="urn:h17-org:v3">
...
</ClinicalDocument>
Within the narrative, XML element (code, assignedAuthor, etc.) and attribute
(SNOMED CT, 17561000, etc.) names also appear in this monospace font.
9 http://www.w3.org/TR/xpath/

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 45
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
This publication package includes complete sample documents as listed in the
Content of the Package table below. These documents conform to the Level 1,
Level 2, and Level 3 constraints of this guide (see the Levels of Constraint
section).
2.10 UML Diagrams
Some sections may include a Unified Modeling Language (UML) class diagram to
provide further clarification. For example, a class diagram might describe the
generalization-specialization hierarchy of Act classes (see the Results section
UML Diagram figure.) The UML diagrams were output from the Model-Driven
Health Tools (MDHT) developed under the auspices of the Veterans
Administration and IBM with assistance from the ONC Standards &
Interoperability Framework10.
2.11 Content of the Package
The following files comprise the package:
Table 1: Content of the Package
Filename
Description
Standards
Applicability
CDAR2_IG_IHE_CONSOL_R1_U1_2012MAY
Implementation Guide
Normative
Consults.sample.xml
Consultation Note
Informative
DIR.sample.xml
Diagnostic Imaging Report
Informative
CCD.sample.xml
Continuity of Care
Document/C32
Informative
DS.sample.xml
Discharge Summary Report
Informative
HandP.sample.xml
History and Physical Report
Informative
OpNote.sample.xml
Operative Note
Informative
Procedure_Note.sample.xml
Procedure Note
Informative
Progress_Note.sample.xml
Progress Note
Informative
UD.sample.xml
Unstructured Document
Informative
cda.xsl
CDA stylesheet
Informative
Discharge_Summary_cda.xsl
Adds discharge disposition
to cda.xsl header
Informative
Consolidated CCD template hierarchy
Hierarchy of CCD sections
and entries
Informative
CDA_Schema_Files (folder)
Updated schema to validate
extensions to CDA R2
introduced in this guide
Informative
10 http://www.openhealthtools.org/charter/Charter-ModelingToolsForHealthcare.pdf

Page 46 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
3 GENERAL HEADER TEMPLATE
This template describes constraints that apply to the header for all documents
within the scope of this implementation guide. Header constraints specific to
each document type are described in the appropriate document-specific section
below.
3.1 Document Type Codes
CDA R2 states that LOINC is the preferred vocabulary for document type codes,
which specify the type of document being exchanged (e.g., History and Physical).
Each document type in this guide recommends a single preferred
clinicalDocument/code, with further specification provided by author or
performer, setting, or specialty.
3.2 US Realm Header
[ClinicalDocument: templateId
2.16.840.1.113883.10.20.22.1.1(open)]
1. SHALL contain exactly one [1..1] realmCode="US" (CONF:16791).
2. SHALL contain exactly one [1..1] typeId (CONF:5361).
a. This typeId SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.1.3" (CONF:5250).
b. This typeId SHALL contain exactly one [1..1]
@extension="POCD_HD000040" (CONF:5251).
3. SHALL contain exactly one [1..1] templateId (CONF:5252) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.1.1" (CONF:10036).
4. SHALL contain exactly one [1..1] id (CONF:5363).
a. This id SHALL be a globally unique identifier for the document
(CONF:9991).
5. SHALL contain exactly one [1..1] code (CONF:5253).
a. This code SHALL specify the particular kind of document (e.g. History
and Physical, Discharge Summary, Progress Note) (CONF:9992).
6. SHALL contain exactly one [1..1] title (CONF:5254).
a. Can either be a locally defined name or the display name
corresponding to clinicalDocument/code (CONF:5255).
7. SHALL contain exactly one [1..1] effectiveTime (CONF:5256).
a. The content SHALL be a conformant US Realm Date and Time
(DTM.US.FIELDED) (2.16.840.1.113883.10.20.22.5.4) (CONF:16865).
8. SHALL contain exactly one [1..1] confidentialityCode, which SHOULD be
selected from ValueSet HL7 BasicConfidentialityKind
2.16.840.1.113883.1.11.16926 STATIC 2010-04-21 (CONF:5259).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 47
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
9. SHALL contain exactly one [1..1] languageCode, which SHALL be selected from
ValueSet Language 2.16.840.1.113883.1.11.11526 DYNAMIC
(CONF:5372).
10. MAY contain zero or one [0..1] setId (CONF:5261).
a. If setId is present versionNumber SHALL be present (CONF:6380).11
11. MAY contain zero or one [0..1] versionNumber (CONF:5264).
a. If versionNumber is present setId SHALL be present (CONF:6387).12
Table 2: Basic Confidentiality Kind Value Set
Value Set: HL7 BasicConfidentialityKind 2.16.840.1.113883.1.11.16926 STATIC 2010-04-21
Code System(s):
Confidentiality Code 2.16.840.1.113883.5.25
Code
Code System
Print Name
N
Confidentiality Code
Normal
R
Confidentiality Code
Restricted
V
Confidentiality Code
Very Restricted
Table 3: Language Value Set (excerpt)
Value Set: Language 2.16.840.1.113883.1.11.11526 DYNAMIC
Code System(s):
Internet Society Language 2.16.840.1.113883.1.11.11526
Description:
A value set of codes defined by Internet RFC 4646 (replacing RFC 3066).
Please see ISO 639 language code set maintained by Library of Congress for
enumeration of language codes
http://www.ietf.org/rfc/rfc4646.txt
Code
Code System
Print Name
en
Internet Society Language
english
fr
Internet Society Language
french
ar
Internet Society Language
arabic
en-US
Internet Society Language
English, US
es-US
Internet Society Language
Spanish, US
…
11 From CDA Normative Web edition: 4.2.1.7 ClinicalDocument.setId - Represents an identifier
that is common across all document revisions and “Document Identification, Revisions, and
Addenda” under 4.2.3.1 ParentDocument
12 From CDA Normative Web edition: 4.2.1.8 ClinicalDocument.versionNumber An integer value
used to version successive replacement documents
Page 48 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 17: US Realm header example
<realmCode code="US"/>
<typeId root="2.16.840.1.113883.1.3" extension="POCD_HD000040"/>
<!-- US General Header Template -->
<templateId root="2.16.840.1.113883.10.20.22.1.1"/>
<!-- History and Physical Template -->
<templateId root="2.16.840.1.113883.10.20.22.1.3"/>
<id extension="999021" root="2.16.840.1.113883.19"/>
<code codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC" code="34117-2"
displayName="History and Physical Note"/>
<title>Good Health History & Physical</title>
<effectiveTime value="20050329171504+0500"/>
<confidentialityCode code="N" codeSystem="2.16.840.1.113883.5.25"/>
<languageCode code="en-US"
displayName="English, US"
codeSystem="2.16.840.1.113883.1.11.11526"
codeSystemName="Internet Society Language"/>
<setId extension="111199021" root="2.16.840.1.113883.19"/>
<versionNumber value="1"/>
Figure 18: effectiveTime with time zone example
<!-- the syntax is "YYYYMMDDHHMMSS.UUUU[+|-ZZzz]" where digits can be
omitted
the right side to express less precision. -->
<effectiveTime value=”201107061227-08”/>
<!-- July 6, 2011, 12:27, 8 hours before UTC -->
3.2.1 RecordTarget
The recordTarget records the patient whose health information is described by
the clinical document; each recordTarget must contain at least one
patientRole element.
12. SHALL contain at least one [1..*] recordTarget (CONF:5266).
a. Such recordTargets SHALL contain exactly one [1..1] patientRole
(CONF:5267).
i. This patientRole SHALL contain at least one [1..*] id
(CONF:5268).
ii. This patientRole SHALL contain at least one [1..*] addr
(CONF:5271).
1. The content of addr SHALL be a conformant US Realm
Address (AD.US.FIELDED)
(2.16.840.1.113883.10.20.22.5.2) (CONF:10412).
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 49
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
iii. This patientRole SHALL contain at least one [1..*] telecom
(CONF:5280).
1. Such telecoms SHOULD contain exactly one [1..1] @use,
which SHALL be selected from ValueSet Telecom Use
(US Realm Header)
2.16.840.1.113883.11.20.9.20 DYNAMIC
(CONF:5375).
3.2.1.1 Patient
iv. This patientRole SHALL contain exactly one [1..1] patient
(CONF:5283).
1. This patient SHALL contain exactly one [1..1] name
(CONF:5284).
a. The content of name SHALL be a conformant US
Realm Patient Name (PTN.US.FIELDED)
(2.16.840.1.113883.10.20.22.5.1)
(CONF:10411).
2. This patient SHALL contain exactly one [1..1]
administrativeGenderCode, which SHALL be selected
from ValueSet Administrative Gender (HL7 V3)
2.16.840.1.113883.1.11.1 DYNAMIC (CONF:6394).
3. This patient SHALL contain exactly one [1..1]
birthTime (CONF:5298).
a. SHALL be precise to year (CONF:5299).
b. SHOULD be precise to day (CONF:5300).
4. This patient SHOULD contain zero or one [0..1]
maritalStatusCode, which SHALL be selected from
ValueSet HL7 MaritalStatus
2.16.840.1.113883.1.11.12212 DYNAMIC
(CONF:5303).
5. This patient MAY contain zero or one [0..1]
religiousAffiliationCode, which SHALL be selected
from ValueSet HL7 Religious Affiliation
2.16.840.1.113883.1.11.19185 DYNAMIC
(CONF:5317).
6. This patient MAY contain zero or one [0..1] raceCode,
which SHALL be selected from ValueSet Race
2.16.840.1.113883.1.11.14914 DYNAMIC
(CONF:5322).
7. This patient MAY contain zero or more [0..*]
sdwg:raceCode, where the @code SHALL be selected
from ValueSet Race 2.16.840.1.113883.1.11.14914
DYNAMIC (CONF:7263).
8. This patient MAY contain zero or one [0..1]
ethnicGroupCode, which SHALL be selected from
ValueSet Ethnicity Value
Page 50 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
2.16.840.1.114222.4.11.837 DYNAMIC
(CONF:5323).
3.2.1.2 Guardian
9. This patient MAY contain zero or more [0..*] guardian
(CONF:5325).
a. The guardian, if present, SHOULD contain zero
or one [0..1] code, which SHALL be selected
from ValueSet Personal Relationship Role
Type 2.16.840.1.113883.1.11.19563
DYNAMIC (CONF:5326).
b. The guardian, if present, SHOULD contain zero
or more [0..*] addr (CONF:5359).
i. The content of addr SHALL be a
conformant US Realm Address
(AD.US.FIELDED)
(2.16.840.1.113883.10.20.22.5.2)
(CONF:10413).
c. The guardian, if present, MAY contain zero or
more [0..*] telecom (CONF:5382).
i. The telecom, if present, SHOULD contain
exactly one [1..1] @use, which SHALL be
selected from ValueSet Telecom Use
(US Realm Header)
2.16.840.1.113883.11.20.9.20
DYNAMIC (CONF:7993).
d. The guardian, if present, SHALL contain exactly
one [1..1] guardianPerson (CONF:5385).
i. This guardianPerson SHALL contain at
least one [1..*] name (CONF:5386).
1. The content of name SHALL be a
conformant US Realm Person Name
(PN.US.FIELDED)
(2.16.840.1.113883.10.20.22.5.1.1)
(CONF:10414).
3.2.1.3 Birthplace
10. This patient MAY contain zero or one [0..1] birthplace
(CONF:5395).
a. The birthplace, if present, SHALL contain
exactly one [1..1] place (CONF:5396).
i. This place SHALL contain exactly one
[1..1] addr (CONF:5397).
1. This addr SHOULD contain zero or
one [0..1] country, where the @code
SHALL be selected from ValueSet

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 51
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
CountryValueSet
2.16.840.1.113883.3.88.12.80.63
DYNAMIC (CONF:5404).
2. This addr MAY contain zero or
one [0..1] postalCode, where the @code
SHALL be selected from ValueSet
PostalCodeValueSet
2.16.840.1.113883.3.88.12.80.2
DYNAMIC (CONF:5403).
3. If country is US, this addr SHALL
contain exactly one [1..1] state, which
SHALL be selected from ValueSet
2.16.840.1.113883.3.88.12.80.1
StateValueSet DYNAMIC (CONF:5402).
3.2.1.4 LanguageCommunication
11. This patient SHOULD contain zero or more [0..*]
languageCommunication (CONF:5406).
a. The languageCommunication, if present, SHALL
contain exactly one [1..1] languageCode, which
SHALL be selected from ValueSet Language
2.16.840.1.113883.1.11.11526 DYNAMIC
(CONF:5407).
b. The languageCommunication, if present, MAY
contain zero or one [0..1] modeCode, which
SHALL be selected from ValueSet HL7
LanguageAbilityMode
2.16.840.1.113883.1.11.12249 DYNAMIC
(CONF:5409).
c. The languageCommunication, if present,
SHOULD contain zero or one [0..1]
proficiencyLevelCode, which SHALL be
selected from ValueSet
LanguageAbilityProficiency
2.16.840.1.113883.1.11.12199 DYNAMIC
(CONF:9965).
d. The languageCommunication, if present, MAY
contain zero or one [0..1] preferenceInd
(CONF:5414).
3.2.1.5 ProviderOrganization
v. This patientRole MAY contain zero or one [0..1]
providerOrganization (CONF:5416).
1. The providerOrganization, if present, SHALL contain at
least one [1..*] id (CONF:5417).
Page 52 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
a. Such ids SHOULD contain zero or one [0..1]
@root="2.16.840.1.113883.4.6" National
Provider Identifier (CONF:16820).
2. The providerOrganization, if present, SHALL contain at
least one [1..*] name (CONF:5419).
3. The providerOrganization, if present, SHALL contain at
least one [1..*] telecom (CONF:5420).
a. Such telecoms SHOULD contain exactly one
[1..1] @use, which SHALL be selected from
ValueSet Telecom Use (US Realm Header)
2.16.840.1.113883.11.20.9.20 DYNAMIC
(CONF:7994).
4. The providerOrganization, if present, SHALL contain at
least one [1..*] addr (CONF:5422).
a. The content of addr SHALL be a conformant US
Realm Address (AD.US.FIELDED)
(2.16.840.1.113883.10.20.22.5.2)
(CONF:10415).
3.2.1.6 RecordTarget Value Sets
Table 4: Telecom Use (US Realm Header) Value Set
Value Set: Telecom Use (US Realm Header) 2.16.840.1.113883.11.20.9.20 DYNAMIC
Code System(s):
AddressUse 2.16.840.1.113883.5.1119
Code
Code System
Print Name
HP
AddressUse
primary home
WP
AddressUse
work place
MC
AddressUse
mobile contact
HV
AddressUse
vacation home
Table 5: Administrative Gender (HL7) Value Set
Value Set: Administrative Gender (HL7 V3) 2.16.840.1.113883.1.11.1 DYNAMIC
Code System(s): AdministrativeGender 2.16.840.1.113883.5.1
Code
Code System
Print Name
F
AdministrativeGender
Female
M
AdministrativeGender
Male
UN
AdministrativeGender
Undifferentiated
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 53
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 6: Marital Status Value Set
Value Set: HL7 Marital Status 2.16.840.1.113883.1.11.12212 DYNAMIC
Code System(s):
MaritalStatus 2.16.840.1.113883.5.2
Code
Code System
Print Name
A
MaritalStatus
Annulled
D
MaritalStatus
Divorced
I
MaritalStatus
Interlocutory
L
MaritalStatus
Legally Separated
M
MaritalStatus
Married
P
MaritalStatus
Polygamous
S
MaritalStatus
Never Married
T
MaritalStatus
Domestic partner
W
MaritalStatus
Widowed
Table 7: Religious Affiliation Value Set (excerpt)
Value Set: HL7 Religious Affiliation 2.16.840.1.113883.1.11.19185 DYNAMIC
Code System(s):
ReligiousAffiliation 2.16.840.1.113883.5.1076
Description:
A value set of codes that reflect spiritual faith affiliation
http://www.hl7.org/memonly/downloads/v3edition.cfm#V32008
Code
Code System
Print Name
1026
ReligiousAffiliation
Judaism
1020
ReligiousAffiliation
Hinduism
1041
ReligiousAffiliation
Roman Catholic Church
…
Page 54 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 8: Race Value Set (excerpt)
Value Set: Race 2.16.840.1.113883.1.11.14914 DYNAMIC
Code System(s):
Race and Ethnicity - CDC 2.16.840.1.113883.6.238
Description:
A Value Set of codes for Classifying data based upon race.
Race is always reported at the discretion of the person for whom this attribute
is reported, and reporting must be completed according to Federal guidelines
for race reporting. Any code descending from the Race concept (1000-9) in
that terminology may be used in the exchange
http://phinvads.cdc.gov/vads/ViewCodeSystemConcept.action?oid=2.16.840.
1.113883.6.238&code=1000-9
Code
Code System
Print Name
1002-5
Race and Ethnicity- CDC
American Indian or Alaska Native
2028-9
Race and Ethnicity- CDC
Asian
2054-5
Race and Ethnicity- CDC
Black or African American
2076-8
Race and Ethnicity- CDC
Native Hawaiian or Other Pacific
Islander
2106-3
Race and Ethnicity- CDC
White
...
Table 9: Ethnicity Value Set
Value Set: Ethnicity Value Set 2.16.840.1.114222.4.11.837 DYNAMIC
Code System(s):
Race and Ethnicity - CDC 2.16.840.1.113883.6.238
Code
Code System
Print Name
2135-2
Race and Ethnicity Code Sets
Hispanic or Latino
2186-5
Race and Ethnicity Code Sets
Not Hispanic or Latino
Table 10: Personal Relationship Role Type Value Set (excerpt)
Value Set: Personal Relationship Role Type 2.16.840.1.113883.1.11.19563 DYNAMIC
Code System(s):
RoleCode 2.16.840.1.113883.5.111
Description:
A Personal Relationship records the role of a person in relation to another
person. This value set is to be used when recording the relationships between
different people who are not necessarily related by family ties, but also
includes family relationships.
http://www.hl7.org/memonly/downloads/v3edition.cfm#V32008
Code
Code System
Print Name
HUSB
RoleCode
husband
WIFE
RoleCode
wife
FRND
RoleCode
friend
SISINLAW
RoleCode
sister-in-law
…
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 55
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 11: State Value Set (excerpt)
Value Set: StateValueSet 2.16.840.1.113883.3.88.12.80.1 DYNAMIC
Code System(s):
FIPS 5-2 (State) 2.16.840.1.113883.6.92
Description:
Codes for the Identification of the States, the District of Columbia and the
Outlying Areas of the United States, and Associated Areas Publication # 5-2,
May, 1987
http://www.itl.nist.gov/fipspubs/fip5-2.htm
Code
Code System
Print Name
AL
FIPS 5-2 (State Alpha Codes)
Alabama
AK
FIPS 5-2 (State Alpha Codes)
Alaska
AZ
FIPS 5-2 (State Alpha Codes)
Arizona
AR
FIPS 5-2 (State Alpha Codes)
Arkansas
…
Table 12: Postal Code Value Set (excerpt)
Value Set: PostalCodeValueSet 2.16.840.1.113883.3.88.12.80.2 DYNAMIC
Code System(s):
US Postal Codes 2.16.840.1.113883.6.231
Description:
A value set of codes postal (ZIP) Code of an address in the United States.
http://zip4.usps.com/zip4/welcome.jsp
Code
Code System
Print Name
19009
US Postal Codes
Bryn Athyn, PA
92869-1736
US Postal Codes
Orange, CA
32830-8413
US Postal Codes
Lake Buena Vista, FL
…
Table 13: Country Value Set (excerpt)
Value Set: CountryValueSet 2.16.840.1.113883.3.88.12.80.63 DYNAMIC
Code System(s):
ISO 3166-1 Country Codes: 1.0.3166.1
Description:
A value set of codes for the representation of names of countries, territories
and areas of geographical interest.
Note: This table provides the ISO 3166-1 code elements available in the alpha-
2 code of ISO's country code standard
http://www.iso.org/iso/country_codes/iso_3166_code_lists.htm
Code
Code System
Print Name
AW
ISO 3166-1 Country Codes
Aruba
IL
ISO 3166-1 Country Codes
Israel
KZ
ISO 3166-1 Country Codes
Kazakhstan
US
ISO 3166-1 Country Codes
United States
…
Page 56 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 14: Language Ability Value Set
Value Set: HL7 LanguageAbilityMode 2.16.840.1.113883.1.11.12249 DYNAMIC
Code System(s):
LanguageAbilityMode 2.16.840.1.113883.5.60
Description:
A value representing the method of expression of the language.
Code
Code System
Print Name
ESGN
LanguageAbilityMode
Expressed signed
ESP
LanguageAbilityMode
Expressed spoken
EWR
LanguageAbilityMode
Expressed written
RSGN
LanguageAbilityMode
Received signed
RSP
LanguageAbilityMode
Received spoken
RWR
LanguageAbilityMode
Received written
Table 15: Language Ability Proficiency Value Set
Value Set: LanguageAbilityProficiency 2.16.840.1.113883.1.11.12199 DYNAMIC
Code System(s):
LanguageAbilityProficiency 2.16.840.1.113883.5.61
Description:
A value representing the level of proficiency in a language.
Code
Code System
Print Name
E
LanguageAbilityProficiency
Excellent
F
LanguageAbilityProficiency
Fair
G
LanguageAbilityProficiency
Good
P
LanguageAbilityProficiency
Poor
3.2.1.7 RecordTarget Example
Figure 19: recordTarget example
<recordTarget>
<patientRole>
<id extension="12345" root="2.16.840.1.113883.19"/>
<!-- Fake ID using HL7 example OID. -->
<id extension="111-00-1234" root="2.16.840.1.113883.4.1"/>
<!-- Fake Social Security Number using the actual SSN OID. -->
<addr use="HP">
<!-- HP is "primary home" from codeSystem 2.16.840.1.113883.5.1119 --
>
<streetAddressLine>17 Daws Rd.</streetAddressLine>
<city>Blue Bell</city>
<state>MA</state>
<postalCode>02368</postalCode>
<country>US</country>
<!-- US is "United States" from ISO 3166-1 Country Codes:
1.0.3166.1 -->
</addr>
<telecom value="tel:(781)555-1212" use="HP"/>
<!-- HP is "primary home" from AddressUse 2.16.840.1.113883.5.1119 --
>

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 57
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
<patient>
<name use="L">
<!-- L is "Legal" from EntityNameUse 2.16.840.1.113883.5.45 -->
<prefix>Mr.</prefix>
<given>Adam</given>
<given qualifier="CL">Frankie</given>
<!-- CL is "Call me" from EntityNamePartQualifier
2.16.840.1.113883.5.43 -->
<family>Everyman</family>
</name>
<administrativeGenderCode code="M"
codeSystem="2.16.840.1.113883.5.1" displayName="Male"/>
<birthTime value="19541125"/>
<maritalStatusCode code="M" displayName="Married"
codeSystem="2.16.840.1.113883.5.2"
codeSystemName="MaritalStatusCode"/>
<religiousAffiliationCode code="1013"
displayName="Christian (non-Catholic, non-specific)"
codeSystemName="Religious Affiliation "
codeSystem="2.16.840.1.113883.5.1076"/>
<raceCode code="2106-3" displayName="White"
codeSystem="2.16.840.1.113883.6.238"
codeSystemName="Race & Ethnicity - CDC"/>
<ethnicGroupCode code="2186-5"
displayName="Not Hispanic or Latino"
codeSystem="2.16.840.1.113883.6.238"
codeSystemName="Race & Ethnicity - CDC"/>
<guardian>
<code code="GRFTH" displayName="Grandfather"
codeSystem="2.16.840.1.113883.5.111"
codeSystemName="RoleCode"/>
<addr use="HP">
<streetAddressLine>17 Daws Rd.</streetAddressLine>
<city>Blue Bell</city>
<state>MA</state>
<postalCode>02368</postalCode>
<country>US</country>
</addr>
<telecom value="tel:(781)555-1212" use="HP"/>
<guardianPerson>
<name>
<given>Ralph</given>
<family>Relative</family>
</name>
</guardianPerson>
</guardian>
<birthplace>
<place>
<addr>
<state>MA</state>
<postalCode>02368</postalCode>
<country>US</country>
</addr>
</place>
</birthplace>
Page 58 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
<languageCommunication>
<languageCode code="fr-CN"/>
<modeCode code="RWR" displayName="Receive Written"
codeSystem="2.16.840.1.113883.5.60"
codeSystemName="LanguageAbilityMode"/>
<preferenceInd value="true"/>
</languageCommunication>
</patient>
<providerOrganization>
<id root="2.16.840.1.113883.19"/>
<name>Good Health Clinic</name>
<telecom use="WP" value="tel:(781)555-1212"/>
<addr>
<streetAddressLine>21 North Ave</streetAddressLine>
<city>Burlington</city>
<state>MA</state>
<postalCode>02368</postalCode>
<country>US</country>
</addr>
</providerOrganization>
</patientRole>
</recordTarget>
3.2.2 Author
The author element represents the creator of the clinical document. The author
may be a device, or a person.
13. SHALL contain at least one [1..*] author (CONF:5444).
a. Such authors SHALL contain exactly one [1..1] time (CONF:5445).
i. The content SHALL be a conformant US Realm Date and Time
(DTM.US.FIELDED) (2.16.840.1.113883.10.20.22.5.4)
(CONF:16866).
b. Such authors SHALL contain exactly one [1..1] assignedAuthor
(CONF:5448).
i. This assignedAuthor SHALL contain exactly one [1..1] id
(CONF:5449) such that it
1. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.4.6" National Provider
Identifier (CONF:16786).
ii. This assignedAuthor SHOULD contain zero or one [0..1] code
(CONF:16787).
1. The code, if present, SHOULD contain exactly one [1..1]
@code, which SHOULD be selected from ValueSet
Healthcare Provider Taxonomy (NUCC - HIPAA)
2.16.840.1.114222.4.11.1066 DYNAMIC
(CONF:16788).
iii. This assignedAuthor SHALL contain at least one [1..*] addr
(CONF:5452).
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 59
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
1. The content SHALL be a conformant US Realm Address
(AD.US.FIELDED) (2.16.840.1.113883.10.20.22.5.2)
(CONF:16871).
iv. This assignedAuthor SHALL contain at least one [1..*] telecom
(CONF:5428).
1. Such telecoms SHOULD contain exactly one [1..1] @use,
which SHALL be selected from ValueSet Telecom Use
(US Realm Header)
2.16.840.1.113883.11.20.9.20 DYNAMIC
(CONF:7995).
v. This assignedAuthor SHOULD contain zero or one [0..1]
assignedPerson (CONF:5430).
1. The assignedPerson, if present, SHALL contain at least
one [1..*] name (CONF:16789).
a. The content SHALL be a conformant US Realm
Person Name (PN.US.FIELDED)
(2.16.840.1.113883.10.20.22.5.1.1)
(CONF:16872).
vi. This assignedAuthor SHOULD contain zero or one [0..1]
assignedAuthoringDevice (CONF:16783).
1. The assignedAuthoringDevice, if present, SHALL
contain exactly one [1..1] manufacturerModelName
(CONF:16784).
2. The assignedAuthoringDevice, if present, SHALL
contain exactly one [1..1] softwareName
(CONF:16785).
vii. There SHALL be exactly one assignedAuthor/assignedPerson or
exactly one assignedAuthor/assignedAuthoringDevice
(CONF:16790).

Page 60 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 20: Person author example
<author>
<time value="20050329224411+0500"/>
<assignedAuthor>
<id extension="KP00017" root="2.16.840.1.113883.19.5"/>
<addr>
<streetAddressLine>21 North Ave.</streetAddressLine>
<city>Burlington</city>
<state>MA</state>
<postalCode>02368</postalCode>
<country>US</country>
</addr>
<telecom use="WP" value="tel:(555)555-1003"/>
<assignedPerson>
<name>
<given>Henry</given>
<family>Seven</family>
</name>
</assignedPerson>
</assignedAuthor>
</author>
Figure 21: Device author example
<author>
<time value="20050329224411+0500"/>
<assignedAuthor>
<id extension="KP00017dev" root="2.16.840.1.113883.19.5"/>
<addr>
<streetAddressLine>21 North Ave.</streetAddressLine>
<city>Burlington</city>
<state>MA</state>
<postalCode>02368</postalCode>
<country>US</country>
</addr>
<telecom use="WP" value="tel:(555)555-1003"/>
<assignedAuthoringDevice>
<manufacturerModelName>Good Health Medical
Device</manufacturerModelName >
<softwareName>Good Health Report Generator</softwareName >
</ assignedAuthoringDevice >
</assignedAuthor>
</author>
3.2.3 DataEnterer
The dataEnterer element represents the person who transferred the content,
written or dictated by someone else, into the clinical document. The guiding rule
of thumb is that an author provides the content found within the header or
body of the document, subject to their own interpretation, and the dataEnterer
adds that information to the electronic system. In other words, a dataEnterer

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 61
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
transfers information from one source to another (e.g., transcription from paper
form to electronic system).
14. MAY contain zero or one [0..1] dataEnterer (CONF:5441).
a. The dataEnterer, if present, SHALL contain exactly one [1..1]
assignedEntity (CONF:5442).
i. This assignedEntity SHALL contain at least one [1..*] id
(CONF:5443).
1. Such ids SHOULD contain zero or one [0..1]
@root="2.16.840.1.113883.4.6" National Provider
Identifier (CONF:16821).
ii. This assignedEntity SHALL contain at least one [1..*] addr
(CONF:5460).
1. The content of addr SHALL be a conformant US Realm
Address (AD.US.FIELDED)
(2.16.840.1.113883.10.20.22.5.2) (CONF:10417).
iii. This assignedEntity SHALL contain at least one [1..*] telecom
(CONF:5466).
1. Such telecoms SHOULD contain exactly one [1..1] @use,
which SHALL be selected from ValueSet Telecom Use
(US Realm Header)
2.16.840.1.113883.11.20.9.20 DYNAMIC
(CONF:7996).
iv. This assignedEntity SHALL contain exactly one [1..1]
assignedPerson (CONF:5469).
1. This assignedPerson SHALL contain at least one [1..*]
name (CONF:5470).
a. The content of name SHALL be a conformant US
Realm Person Name (PN.US.FIELDED)
(2.16.840.1.113883.10.20.22.5.1.1)
(CONF:10418).
v. This assignedEntity MAY contain zero or one [0..1] code which
SHOULD be selected from coding system NUCC Health Care
Provider Taxonomy 2.16.840.1.113883.6.101 (CONF:9944).

Page 62 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 22: dataEnterer example
<dataEnterer>
<assignedEntity>
<id root="2.16.840.1.113883.19.5" extension="43252"/>
<addr>
<streetAddressLine>21 North Ave.</streetAddressLine>
<city>Burlington</city>
<state>MA</state>
<postalCode>02368</postalCode>
<country>US</country>
</addr>
<telecom use="WP" value="tel:(555)555-1003"/>
<assignedPerson>
<name>
<given>Henry</given>
<family>Seven</family>
</name>
</assignedPerson>
</assignedEntity>
</dataEnterer>
3.2.4 Informant
The informant element describes the source of the information in a medical
document.
Assigned health care providers may be a source of information when a document
is created. (e.g., a nurse's aide who provides information about a recent
significant health care event that occurred within an acute care facility.) In these
cases, the assignedEntity element is used.
When the informant is a personal relation, that informant is represented in the
relatedEntity element. The code element of the relatedEntity describes the
relationship between the informant and the patient. The relationship between
the informant and the patient needs to be described to help the receiver of
the clinical document understand the information in the document.
15. MAY contain zero or more [0..*] informant (CONF:8001).
a. SHALL contain exactly one [1..1] assignedEntity OR exactly one [1..1]
relatedEntity (CONF:8002).
i. SHOULD contain at least one [1..*] addr (CONF:8220).
1. The content of addr SHALL be a conformant US Realm
Address (AD.US.FIELDED)
(2.16.840.1.113883.10.20.22.5.2) (CONF:10419).
ii. SHALL contain exactly one [1..1] assignedPerson OR exactly
one [1..1] relatedPerson (CONF:8221).
1. SHALL contain at least one [1..*] name (CONF:8222).
a. The content of name SHALL be a conformant US
Realm Person Name (PN.US.FIELDED)
(2.16.840.1.113883.10.20.22.5.1.1)
(CONF:10420).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 63
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
iii. Ii. This assignedEntity MAY contain zero or one [0..1] code
which SHOULD be selected from coding system NUCC Health
Care Provider Taxonomy 2.16.840.1.113883.6.101
(CONF:9947).
iv. SHOULD contain zero or more [0..*] id (CONF:9945).
1. If assignedEntity/id is a provider then this id, SHOULD
include zero or one [0..1] id where id/@root
="2.16.840.1.113883.4.6" National Provider Identifier
(CONF:9946).
Figure 23: Informant with assignedEntity example
<informant>
<assignedEntity>
<id extension="KP00017" root="2.16.840.1.113883.19.5"/>
<addr>
<streetAddressLine>21 North Ave.</streetAddressLine>
<city>Burlington</city>
<state>MA</state>
<postalCode>02368</postalCode>
<country>US</country>
</addr>
<telecom value="tel:(555)555-1003"/>
<assignedPerson>
<name>
<given>Henry</given>
<family>Seven</family>
</name>
</assignedPerson>
</assignedEntity>
</informant>
3.2.5 Custodian
The custodian element represents the organization that is in charge of
maintaining the document. The custodian is the steward that is entrusted with
the care of the document. Every CDA document has exactly one custodian. The
custodian participation satisfies the CDA definition of Stewardship. Because
CDA is an exchange standard and may not represent the original form of the
authenticated document (e.g., CDA could include scanned copy of original), the
custodian represents the steward of the original source document. The
custodian may be the document originator, a health information exchange, or
other responsible party.
16. SHALL contain exactly one [1..1] custodian (CONF:5519).
a. This custodian SHALL contain exactly one [1..1] assignedCustodian
(CONF:5520).
i. This assignedCustodian SHALL contain exactly one [1..1]
representedCustodianOrganization (CONF:5521).
1. This representedCustodianOrganization SHALL contain
at least one [1..*] id (CONF:5522).

Page 64 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
a. Such ids SHOULD contain zero or one [0..1]
@root="2.16.840.1.113883.4.6" National
Provider Identifier (CONF:16822).
2. This representedCustodianOrganization SHALL contain
exactly one [1..1] name (CONF:5524).
3. This representedCustodianOrganization SHALL contain
exactly one [1..1] telecom (CONF:5525).
a. This telecom SHOULD contain exactly one [1..1]
@use, which SHALL be selected from ValueSet
Telecom Use (US Realm Header)
2.16.840.1.113883.11.20.9.20 DYNAMIC
(CONF:7998).
4. This representedCustodianOrganization SHALL contain
at least one [1..*] addr (CONF:5559).
a. The content of addr SHALL be a conformant US
Realm Address (AD.US.FIELDED)
(2.16.840.1.113883.10.20.22.5.2)
(CONF:10421).
Figure 24: Custodian example
<custodian>
<assignedCustodian>
<representedCustodianOrganization>
<id root="2.16.840.1.113883.19.5"/>
<name>Good Health Clinic</name>
<telecom value="tel:(555)555-1212" use="WP"/>
<addr use="WP">
<streetAddressLine>17 Daws Rd.</streetAddressLine>
<city>Blue Bell</city>
<state>MA</state>
<postalCode>02368</postalCode>
<country>US</country>
</addr>
</representedCustodianOrganization>
</assignedCustodian>
</custodian>
3.2.6 InformationRecipient
The informationRecipient element records the intended recipient of the
information at the time the document is created. For example, in cases where
the intended recipient of the document is the patient's health chart, set the
receivedOrganization to be the scoping organization for that chart.
17. MAY contain zero or more [0..*] informationRecipient (CONF:5565).
a. The informationRecipient, if present, SHALL contain exactly one [1..1]
intendedRecipient (CONF:5566).
i. This intendedRecipient MAY contain zero or one [0..1]
informationRecipient (CONF:5567).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 65
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
1. The informationRecipient, if present, SHALL contain at
least one [1..*] name (CONF:5568).
a. The content of name SHALL be a conformant US
Realm Person Name (PN.US.FIELDED)
(2.16.840.1.113883.10.20.22.5.1.1)
(CONF:10427).
ii. This intendedRecipient MAY contain zero or one [0..1]
receivedOrganization (CONF:5577).
1. The receivedOrganization, if present, SHALL contain
exactly one [1..1] name (CONF:5578).
Figure 25: informationRecipient example
<informationRecipient>
<intendedRecipient>
<informationRecipient>
<name>
<given>Henry</given>
<family>Seven</family>
</name>
</informationRecipient>
<receivedOrganization>
<name>Good Health Clinic</name>
</receivedOrganization>
</intendedRecipient>
</informationRecipient>
3.2.7 LegalAuthenticator
The legalAuthenticator identifies the single person legally responsible for the
document and must be present if the document has been legally authenticated.
(Note that per the following section, there may also be one or more document
authenticators.)
Based on local practice, clinical documents may be released before legal
authentication. This implies that a clinical document that does not contain this
element has not been legally authenticated.
The act of legal authentication requires a certain privilege be granted to the legal
authenticator depending upon local policy. All clinical documents have the
potential for legal authentication, given the appropriate credentials.
Local policies MAY choose to delegate the function of legal authentication to a
device or system that generates the clinical document. In these cases, the legal
authenticator is a person accepting responsibility for the document, not the
generating device or system.
Note that the legal authenticator, if present, must be a person.
18. SHOULD contain zero or one [0..1] legalAuthenticator (CONF:5579).
a. The legalAuthenticator, if present, SHALL contain exactly one [1..1]
time (CONF:5580).
Page 66 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
i. The content SHALL be a conformant US Realm Date and Time
(DTM.US.FIELDED) (2.16.840.1.113883.10.20.22.5.4)
(CONF:16873).
b. The legalAuthenticator, if present, SHALL contain exactly one [1..1]
signatureCode (CONF:5583).
i. This signatureCode SHALL contain exactly one [1..1]
@code="S" (CodeSystem: Participationsignature
2.16.840.1.113883.5.89) (CONF:5584).
c. The legalAuthenticator, if present, SHALL contain exactly one [1..1]
assignedEntity (CONF:5585).
i. This assignedEntity SHALL contain at least one [1..*] id
(CONF:5586).
1. Such ids MAY contain zero or one [0..1]
@root="2.16.840.1.113883.4.6" National Provider
Identifier (CONF:16823).
ii. This assignedEntity MAY contain zero or one [0..1] code,
which SHOULD be selected from ValueSet Healthcare
Provider Taxonomy (NUCC - HIPAA)
2.16.840.1.114222.4.11.1066 (CONF:17000).
iii. This assignedEntity SHALL contain at least one [1..*] addr
(CONF:5589).
1. The content of addr SHALL be a conformant US Realm
Address (AD.US.FIELDED)
(2.16.840.1.113883.10.20.22.5.2) (CONF:10429).
iv. This assignedEntity SHALL contain at least one [1..*] telecom
(CONF:5595).
1. Such telecoms SHOULD contain exactly one [1..1] @use,
which SHALL be selected from ValueSet Telecom Use
(US Realm Header)
2.16.840.1.113883.11.20.9.20 DYNAMIC
(CONF:7999).
v. This assignedEntity SHALL contain exactly one [1..1]
assignedPerson (CONF:5597).
1. This assignedPerson SHALL contain at least one [1..*]
name (CONF:5598).
a. The content of name SHALL be a conformant US
Realm Person Name (PN.US.FIELDED)
(2.16.840.1.113883.10.20.22.5.1.1)
(CONF:10430).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 67
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 26: legalAuthenticator example
<legalAuthenticator>
<time value="20050329224411+0500"/>
<signatureCode code="S"/>
<assignedEntity>
<id extension="KP00017" root="2.16.840.1.113883.19"/>
<addr>
<streetAddressLine>21 North Ave.</streetAddressLine>
<city>Burlington</city>
<state>MA</state>
<postalCode>02368</postalCode>
<country>US</country>
</addr>
<telecom use="WP" value="tel:(555)555-1003"/>
<assignedPerson>
<name>
<given>Henry</given>
<family>Seven</family>
</name>
</assignedPerson>
</assignedEntity>
</legalAuthenticator>
3.2.8 Authenticator
The authenticator identifies a participant or participants who attested to the
accuracy of the information in the document.
19. MAY contain zero or more [0..*] authenticator (CONF:5607).
a. The authenticator, if present, SHALL contain exactly one [1..1] time
(CONF:5608).
i. The content SHALL be a conformant US Realm Date and Time
(DTM.US.FIELDED) (2.16.840.1.113883.10.20.22.5.4)
(CONF:16874).
b. The authenticator, if present, SHALL contain exactly one [1..1]
signatureCode (CONF:5610).
i. This signatureCode SHALL contain exactly one [1..1]
@code="S" (CodeSystem: Participationsignature
2.16.840.1.113883.5.89) (CONF:5611).
c. The authenticator, if present, SHALL contain exactly one [1..1]
assignedEntity (CONF:5612).
i. This assignedEntity SHALL contain at least one [1..*] id
(CONF:5613).
1. Such ids SHOULD contain zero or one [0..1]
@root="2.16.840.1.113883.4.6" National Provider
Identifier (CONF:16824).
ii. This assignedEntity MAY contain zero or one [0..1] code
(CONF:16825).
1. The code, if present, MAY contain zero or one [0..1]
@code, which SHOULD be selected from ValueSet
Page 68 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Healthcare Provider Taxonomy (NUCC - HIPAA)
2.16.840.1.114222.4.11.1066 (CONF:16826).
iii. This assignedEntity SHALL contain at least one [1..*] addr
(CONF:5616).
1. The content of addr SHALL be a conformant US Realm
Address (AD.US.FIELDED)
(2.16.840.1.113883.10.20.22.5.2) (CONF:10425).
iv. This assignedEntity SHALL contain at least one [1..*] telecom
(CONF:5622).
1. Such telecoms SHOULD contain exactly one [1..1] @use,
which SHALL be selected from ValueSet Telecom Use
(US Realm Header)
2.16.840.1.113883.11.20.9.20 DYNAMIC
(CONF:8000).
v. This assignedEntity SHALL contain exactly one [1..1]
assignedPerson (CONF:5624).
1. This assignedPerson SHALL contain at least one [1..*]
name (CONF:5625).
a. The content of name SHALL be a conformant US
Realm Person Name (PN.US.FIELDED)
(2.16.840.1.113883.10.20.22.5.1.1)
(CONF:10424).
Figure 27: Authenticator example
<authenticator>
<time value="20050329224411+0500"/>
<signatureCode code="S"/>
<assignedEntity>
<id extension="KP00017" root="2.16.840.1.113883.19"/>
<addr>
<streetAddressLine>21 North Ave.</streetAddressLine>
<city>Burlington</city>
<state>MA</state>
<postalCode>02368</postalCode>
<country>US</country>
</addr>
<telecom use="WP" value="tel:(555)555-1003"/>
<assignedPerson>
<name>
<given>Henry</given>
<family>Seven</family>
</name>
</assignedPerson>
</assignedEntity>
</authenticator>

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 69
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
3.2.9 Participant (Support)
The participant element identifies other supporting participants, including
parents, relatives, caregivers, insurance policyholders, guarantors, and other
participants related in some way to the patient.
A supporting person or organization is an individual or an organization with a
relationship to the patient. A supporting person who is playing multiple roles
would be recorded in multiple participants (e.g., emergency contact and next-of-
kin)
20. MAY contain zero or more [0..*] participant (CONF:10003).
a. The participant, if present, MAY contain zero or one [0..1] time
(CONF:10004).
b. Such participants, if present, SHALL have an associatedPerson or
scopingOrganization element under participant/associatedEntity
(CONF:10006).
c. Unless otherwise specified by the document specific header
constraints, when participant/@typeCode is IND,
associatedEntity/@classCode SHALL be selected from ValueSet
2.16.840.1.113883.11.20.9.33 INDRoleclassCodes STATIC 2011-09-30
(CONF:10007).
Table 16: IND Role classCode Value Set
Value Set: INDRoleclassCodes 2.16.840.1.113883.11.20.9.33 STATIC 2011-09-30
Code System(s):
RoleClass 2.16.840.1.113883.5.110
Code
Code System
Print Name
PRS
RoleClass
personal relationship
NOK
RoleClass
next of kin
CAREGIVER
RoleClass
caregiver
AGNT
RoleClass
agent
GUAR
RoleClass
guarantor
ECON
RoleClass
emergency contact

Page 70 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 28: Participant example for a supporting person
<participant typeCode='IND'>
<time xsi:type="IVL_TS">
<low value="19590101"/>
<high value="20111025"/>
</time>
<associatedEntity classCode='NOK'>
<code code='MTH' codeSystem='2.16.840.1.113883.5.111'/>
<addr>
<streetAddressLine>17 Daws Rd.</streetAddressLine>
<city>Blue Bell</city>
<state>MA</state>
<postalCode>02368</postalCode>
<country>US</country>
</addr>
<telecom value='tel:(555)555-2006' use='WP'/>
<associatedPerson>
<name>
<prefix>Mrs.</prefix>
<given>Martha</given>
<family>Mum</family>
</name>
</associatedPerson>
</associatedEntity>
</participant>
3.2.10 InFulfillmentOf
The inFulfillmentOf element represents orders that are fulfilled by this
document.
21. MAY contain zero or more [0..*] inFulfillmentOf (CONF:9952).
a. The inFulfillmentOf, if present, SHALL contain exactly one [1..1] order
(CONF:9953).
i. This order SHALL contain at least one [1..*] id (CONF:9954).
3.2.11 DocumentationOf/serviceEvent
A serviceEvent represents the main act, such as a colonoscopy or a cardiac
stress study, being documented. In a continuity of care document, CCD, the
serviceEvent is a provision of healthcare over a period of time. In a provision of
healthcare serviceEvent, the care providers, PCP or other longitudinal
providers, are recorded within the serviceEvent. If the document is about a
single encounter, the providers associated can be recorded in the
componentOf/encompassingEncounter.
22. MAY contain zero or more [0..*] documentationOf (CONF:14835).
a. The documentationOf, if present, SHALL contain exactly one [1..1]
serviceEvent (CONF:14836).
i. This serviceEvent SHALL contain exactly one [1..1]
effectiveTime (CONF:14837).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 71
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
1. This effectiveTime SHALL contain exactly one [1..1] low
(CONF:14838).
ii. This serviceEvent SHOULD contain zero or more [0..*]
performer (CONF:14839).
1. The performer, if present, SHALL contain exactly one
[1..1] @typeCode="PRF" Participation physical
performer (CodeSystem: HL7ParticipationType
2.16.840.1.113883.5.90) (CONF:14840).
a. The performer participant represents clinicians
who actually and principally carry out the
serviceEvent. In a transfer of care this
represents the healthcare providers involved in
the current or pertinent historical care of the
patient. Preferably, the patient’s key healthcare
care team members would be listed,
particularly their primary physician and any
active consulting physicians, therapists, and
counselors (CONF:16753).
2. The performer, if present, MAY contain zero or one
[0..1] functionCode (CONF:16818).
a. The functionCode, if present, SHOULD contain
zero or one [0..1] @codeSystem, which SHOULD
be selected from CodeSystem
participationFunction
(2.16.840.1.113883.5.88) (CONF:16819).
3. The performer, if present, SHALL contain exactly one
[1..1] assignedEntity (CONF:14841).
a. This assignedEntity SHALL contain at least one
[1..*] id (CONF:14846).
i. Such ids SHOULD contain zero or one
[0..1]
@root="2.16.840.1.113883.4.6"
National Provider Identifier
(CONF:14847).
b. This assignedEntity SHOULD contain zero or one
[0..1] code (CONF:14842).
i. The code, if present, SHALL contain
exactly one [1..1] @code, which SHOULD
be selected from CodeSystem
NUCCProviderTaxonomy
(2.16.840.1.113883.6.101)
(CONF:14843).

Page 72 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 29: DocumentationOf example
<documentationOf>
<serviceEvent classCode="ACT">
<id root="1.2.840.113619.2.62.994044785528.114289542805"/>
<id extension="123453"
root="1.2.840.113619.2.62.994044785528.26"/>
<code code="93041"
displayName="Rhythm ECG, one to three leads; tracing
only without interpretation and report"
codeSystem="2.16.840.1.113883.6.12"
codeSystemName="CPT4"/>
<effectiveTime value="20080813222400"/>
<performer typeCode="PRF">
<templateId root="2.16.840.1.113883.10.20.6.2.1"/>
<assignedEntity>
<id extension="121008" root="2.16.840.1.113883.19.5"/>
<code code="208D00000X "
codeSystem="2.16.840.1.113883.6.101"
codeSystemName="NUCC"
displayName="General Practice"/>
<addr nullFlavor="NI"/>
<telecom nullFlavor="NI"/>
<assignedPerson>
<name>
<given>Matthew</given>
<family>Care</family>
<suffix>MD</suffix>
</name>
</assignedPerson>
</assignedEntity>
</performer>
</serviceEvent>
</documentationOf>
3.2.12 Authorization/consent
The header can record information about the patient’s consent.
The type of consent (e.g., a consent to perform the related serviceEvent) is
conveyed in consent/code. Consents in the header have been finalized
(consent/statusCode must equal Completed) and should be on file. This
specification does not address how Privacy Consent’ is represented, but does not
preclude the inclusion of ‘Privacy Consent’.
23. MAY contain zero or more [0..*] authorization (CONF:16792) such that it
a. SHALL contain exactly one [1..1] consent (CONF:16793).
i. This consent MAY contain zero or more [0..*] id (CONF:16794).
ii. This consent MAY contain zero or one [0..1] code
(CONF:16795).
1. The type of consent (e.g., a consent to perform the
related serviceEvent) is conveyed in consent/code
(CONF:16796).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 73
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
iii. This consent SHALL contain exactly one [1..1] statusCode
(CONF:16797).
1. This statusCode SHALL contain exactly one [1..1]
@code="completed" Completed (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6)
(CONF:16798).
Figure 30: Procedure note consent example
<authorization typeCode="AUTH">
<consent classCode="CONS" moodCode="EVN">
<id root="629deb70-5306-11df-9879-0800200c9a66" />
<code codeSystem=" 2.16.840.1.113883.6.1" codeSystemName="LOINC"
code="64293-4" displayName="Procedure consent"/>
<statusCode code="completed"/>
</consent>
</authorization>
3.2.13 ComponentOf
The componentOf element contains the encompassing encounter for this
document. The encompassing encounter represents the setting of the clinical
encounter during which the document act(s) or ServiceEvent occurred.
In order to represent providers associated with a specific encounter, they are
recorded within the encompassingEncounter as participants.
In a CCD the encompassingEncounter may be used when documenting a
specific encounter and its participants. All relevant encounters in a CCD may be
listed in the encounters section.
24. MAY contain zero or one [0..1] componentOf (CONF:9955).
a. The componentOf, if present, SHALL contain exactly one [1..1]
encompassingEncounter (CONF:9956).
i. This encompassingEncounter SHALL contain at least one [1..*]
id (CONF:9959).
ii. This encompassingEncounter SHALL contain exactly one [1..1]
effectiveTime (CONF:9958).
3.3 US Realm Address (AD.US.FIELDED)
[addr: 2.16.840.1.113883.10.20.22.5.2(open)]
Reusable "address" template, designed for use in US Realm CDA Header.
1. SHOULD contain exactly one [1..1] @use, which SHALL be selected from
ValueSet PostalAddressUse 2.16.840.1.113883.1.11.10637 STATIC
2005-05-01 (CONF:7290).
2. SHOULD contain zero or one [0..1] country, where the @code SHALL be
selected from ValueSet CountryValueSet
2.16.840.1.113883.3.88.12.80.63 DYNAMIC (CONF:7295).
Page 74 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
3. SHOULD contain zero or one [0..1] state (ValueSet: StateValueSet
2.16.840.1.113883.3.88.12.80.1 DYNAMIC) (CONF:7293).
a. State is required if the country is US. If country is not specified, its
assumed to be US. If country is something other than US, the state
MAY be present but MAY be bound to different vocabularies
(CONF:10024).
4. SHALL contain exactly one [1..1] city (CONF:7292).
5. SHOULD contain zero or one [0..1] postalCode (ValueSet:
PostalCodeValueSet 2.16.840.1.113883.3.88.12.80.2 DYNAMIC)
(CONF:7294).
a. PostalCode is required if the country is US. If country is not specified,
its assumed to be US. If country is something other than US, the
postalCode MAY be present but MAY be bound to different
vocabularies (CONF:10025).
6. SHALL contain at least one and not more than 4 streetAddressLine
(CONF:7291).
7. SHALL NOT have mixed content except for white space13 (CONF:7296).
Table 17: PostalAddressUse Value Set
Value Set: PostalAddressUse 2.16.840.1.113883.1.11.10637 STATIC 2005-05-01
Code System(s):
AddressUse 2.16.840.1.113883.5.1119
Code
Code System
Print Name
BAD
AddressUse
bad address
DIR
AddressUse
direct
H
AddressUse
home address
HP
AddressUse
primary home
HV
AddressUse
vacation home
PHYS
AddressUse
physical visit address
PST
AddressUse
postal address
PUB
AddressUse
public
TMP
AddressUse
temporary
WP
AddressUse
work place
3.4 US Realm Date and Time (DT.US.FIELDED)
[effectiveTime: 2.16.840.1.113883.10.20.22.5.3(open)]
The US Realm Clinical Document Date and Time datatype flavor records date
and time information. If no time zone offset is provided, you can make no
assumption about time, unless you have made a local exchange agreement.
This data type uses the same rules as US Realm Date and Time
(DTM.US.FIELDED), but is used with the effectiveTime element.
13 For information on mixed content see Extensible Markup Language (XML)
(http://www.w3.org/TR/2008/REC-xml-20081126/#sec-mixed-content).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 75
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
1. SHALL be precise to the day (CONF:10078).
2. SHOULD be precise to the minute (CONF:10079).
3. MAY be precise to the second (CONF:10080).
4. If more precise than day, SHOULD include time-zone offset (CONF:10081).
3.5 US Realm Date and Time (DTM.US.FIELDED)
[time: 2.16.840.1.113883.10.20.22.5.4(open)]
The US Realm Clinical Document Date and Time datatype flavor records date
and time information. If no time zone offset is provided, you can make no
assumption about time, unless you have made a local exchange agreement.
This data type uses the same rules as US Realm Date and Time
(DT.US.FIELDED), but is used with the time element.
1. SHALL be precise to the day (CONF:10127).
2. SHOULD be precise to the minute (CONF:10128).
3. MAY be precise to the second (CONF:10129).
4. If more precise than day, SHOULD include time-zone offset (CONF:10130).
3.6 US Realm Patient Name (PTN.US.FIELDED)
[PN: templateId 2.16.840.1.113883.10.20.22.5.1 (open)]
The US Realm Patient Name datatype flavor is a set of reusable constraints that
can be used for the patient or any other person. It requires a first (given) and
last (family) name. If a patient or person has only one name part (e.g., patient
with first name only) place the name part in the field required by the
organization. Use the appropriate nullFlavor, "Not Applicable" (NA), in the
other field.
For information on mixed content see the Extensible Markup Language
reference (http://www.w3c.org/TR/2008/REC-xml-20081126/).
1. MAY contain zero or one [0..1] @use, which SHALL be selected from ValueSet
EntityNameUse 2.16.840.1.113883.1.11.15913 STATIC 2005-05-01
(CONF:7154).
2. SHALL contain exactly one [1..1] family (CONF:7159).
a. This family MAY contain zero or one [0..1] @qualifier, which SHALL
be selected from ValueSet EntityPersonNamePartQualifier
2.16.840.1.113883.11.20.9.26 STATIC 2011-09-30 (CONF:7160).
3. SHALL contain at least one [1..*] given (CONF:7157).
a. Such givens MAY contain zero or one [0..1] @qualifier, which SHALL
be selected from ValueSet EntityPersonNamePartQualifier
2.16.840.1.113883.11.20.9.26 STATIC 2011-09-30 (CONF:7158).
b. The second occurrence of given (given[2]) if provided, SHALL include
middle name or middle initial (CONF:7163).
4. MAY contain zero or more [0..*] prefix (CONF:7155).
a. The prefix, if present, MAY contain zero or one [0..1] @qualifier,
which SHALL be selected from ValueSet
Page 76 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
EntityPersonNamePartQualifier
2.16.840.1.113883.11.20.9.26 STATIC 2011-09-30 (CONF:7156).
5. MAY contain zero or one [0..1] suffix (CONF:7161).
a. The suffix, if present, MAY contain zero or one [0..1] @qualifier,
which SHALL be selected from ValueSet
EntityPersonNamePartQualifier
2.16.840.1.113883.11.20.9.26 STATIC 2011-09-30 (CONF:7162).
6. SHALL NOT have mixed content except for white space (CONF:7278).
Table 18: EntityNameUse Value Set
Value Set: EntityNameUse 2.16.840.1.113883.1.11.15913 STATIC 2005-05-01
Code System(s):
EntityNameUse 2.16.840.1.113883.5.45
Code
Code System
Print Name
A
EntityNameUse
Artist/Stage
ABC
EntityNameUse
Alphabetic
ASGN
EntityNameUse
Assigned
C
EntityNameUse
License
I
EntityNameUse
Indigenous/Tribal
IDE
EntityNameUse
Ideographic
L
EntityNameUse
Legal
P
EntityNameUse
Pseudonym
PHON
EntityNameUse
Phonetic
R
EntityNameUse
Religious
SNDX
EntityNameUse
Soundex
SRCH
EntityNameUse
Search
SYL
EntityNameUse
Syllabic
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 77
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 19: EntityPersonNamePartQualifier Value Set
Value Set: EntityPersonNamePartQualifier 2.16.840.1.113883.11.20.9.26 STATIC
2011-09-30
Code System(s):
EntityNamePartQualifier 2.16.840.1.113883.5.43
Code
Code System
Print Name
AC
EntityNamePartQualifier
academic
AD
EntityNamePartQualifier
adopted
BR
EntityNamePartQualifier
birth
CL
EntityNamePartQualifier
callme
IN
EntityNamePartQualifier
initial
NB
EntityNamePartQualifier
nobility
PR
EntityNamePartQualifier
professional
SP
EntityNamePartQualifier
spouse
TITLE
EntityNamePartQualifier
title
VV
EntityNamePartQualifier
voorvoegsel
3.7 US Realm Person Name (PN.US.FIELDED)
[name: 2.16.840.1.113883.10.20.22.5.1.1(open)]
The US Realm Clinical Document Person Name datatype flavor is a set of
reusable constraints that can be used for Persons.
1. SHALL contain exactly one [1..1] name (CONF:9368).
a. The content of name SHALL be either a conformant Patient Name
(PTN.US.FIELDED), or a string (CONF:9371).
b. The string SHALL NOT contain name parts (CONF:9372).
3.8 Rendering Header Information for Human Presentation
Metadata carried in the header may already be available for rendering from
electronic medical records (EMRs) or other sources external to the document;
therefore, there is no strict requirement to render directly from the document.
An example of this would be a doctor using an EMR that already contains the
patient’s name, date of birth, current address, and phone number. When a CDA
document is rendered within that EMR, those pieces of information may not
need to be displayed since they are already known and displayed within the
EMR’s user interface.
Good practice would recommend that the following be present whenever the
document is viewed:
Document title and document dates
Service and encounter types, and date ranges as appropriate
Names of all persons along with their roles, participations, participation
date ranges, identifiers, address, and telecommunications information

Page 78 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Names of selected organizations along with their roles, participations,
participation date ranges, identifiers, address, and telecommunications
information
Date of birth for recordTarget(s)
In Operative and Procedure Notes, the following information is typically
displayed in the electronic health record (EHR) and/or rendered directly in the
document:
The performers of the surgery or procedure, including any assistants
The surgery or procedure performed (serviceEvent)
The date of the surgery or procedure

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 79
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
4 DOCUMENT-LEVEL TEMPLATES
Document-level templates describe the purpose and rules for constructing a
conforming CDA document. Document templates include constraints on the
CDA header and refer to section-level templates. The Document Types and
Required/Optional Sections table lists the sections used by each document type.
Each document-level template contains the following information:
Scope and intended use of the document type
Description and explanatory narrative.
Template metadata (e.g., templateId, etc.)
Header constraints: this includes a reference to the US Realm Clinical
Document Header template and additional constraints specific to each
document type
Required and optional section-level templates
Page 80 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 20: Document Types and Required/Optional Sections with Structured Body
Document Type
Preferred LOINC
templateId
Required Sections
Optional Sections
CCD (Summarization of Episode
Note)
34133-9 (required)14
2.16.840.1.113883.10.20.22.1.2
Allergies
Medications
Problem List
Procedures15 (List of Surgeries)
(History of Procedures)
Results
Advance Directives
Encounters
Family History
Functional Status
Immunizations
Medical Equipment
Payers
Plan of Care
Social History
Vital Signs
Consultation Note
11488-4
2.16.840.1.113883.10.20.22.1.4
Assessment and
Plan/Assessment/Plan of
Care*
History of Present Illness
Physical Exam
Reason for Referral/Reason for
Visit16 **
Allergies
Chief Complaint **
Chief Complaint and Reason
for Visit **
Family History
General Status
History of Past Illness (Past
Medical History)
Immunizations
Medications
Problem List
Procedures (List of Surgeries)
(History of Procedures)
Results
Review of Systems
Social History
Vital Signs
14 CCD is the only document with a fixed clinicalDocument/code
15 Required only for inpatient settings
16 Either Reason for Referral or Reason for Visit must be present.
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 81
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Document Type
Preferred LOINC
templateId
Required Sections
Optional Sections
Diagnostic Imaging Report
18748-4
2.16.840.1.113883.10.20.22.1.5
DICOM Object Catalog
Findings (Radiology Study
Observation)
Addendum
Clinical Presentation
Complications
Conclusions
Current Imaging Procedure
Descriptions
Document Summary
Key Images
Medical (General) History
Prior Imaging Procedure
Descriptions
Radiology - Impression
Radiology Comparison Study -
Observation
Radiology Reason For Study
Radiology Study -
Recommendation
Requested Imaging Studies
Information
Discharge Summary (Discharge
Summarization Note)
18842-5
2.16.840.1.113883.10.20.22.1.8
Allergies
Hospital Course
Hospital Discharge Diagnosis
Hospital Discharge Medications
Plan of Care
Chief Complaint **
Chief Complaint and Reason
for Visit **
Discharge Diet
Family History
Functional Status
History of Past Illness (Past
Medical History)
History of Present Illness
Hospital Admissions Diagnosis
Hospital Consultations
Hospital Discharge
Instructions
Hospital Discharge Physical
Hospital Discharge Studies
Summary
Immunizations
Problem List
Procedures (List of Surgeries)
(History of Procedures)
Reason for Visit **
Review of Systems
Social History
Vital Signs
Page 82 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Document Type
Preferred LOINC
templateId
Required Sections
Optional Sections
History & Physical Note
34117-2
2.16.840.1.113883.10.20.22.1.3
Allergies
Assessment and
Plan/Assessment/Plan of
Care*
Chief Complaint **
Chief Complaint and Reason
for Visit **
Family History
General Status
History of Past Illness (Past
Medical History)
Medications
Physical Exam
Reason for Visit **
Results
Review of Systems
Social History
Vital Signs
History of Present Illness
Immunizations
Instructions
Problem List
Procedures (List of Surgeries)
(History of Procedures)
Operative Note (Surgical
Operation Note)
11504-8
2.16.840.1.113883.10.20.22.1.7
Anesthesia
Complications
Postoperative Diagnosis
Preoperative Diagnosis
Procedure Estimated Blood
Loss
Procedure Findings
Procedure Specimens Taken
Procedure Description
Procedure Implants
Operative Note Fluids
Operative Note Surgical
Procedure
Plan of Care
Planned Procedure
Procedure Disposition
Procedure Indications
Surgical Drains

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 83
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Document Type
Preferred LOINC
templateId
Required Sections
Optional Sections
Procedure Note
28570-0
2.16.840.1.113883.10.20.22.1.6
Assessment and
Plan/Assessment/Plan of
Care*
Complications
Postprocedure Diagnosis
Procedure Description
Procedure Indications
Allergies
Anesthesia
Chief Complaint **
Chief Complaint and Reason
for Visit **
Family History
History of Past Illness
History of Present Illness
Medical (General) History
Medications
Medications Administered
Physical Exam
Planned Procedure
Procedure Disposition
Procedure Estimated Blood
Loss
Procedure Findings
Procedure Implants
Procedure Specimens Taken
Procedures (List of Surgeries)
(History of Procedures)
Reason for Visit **
Review of Systems
Social History
Progress Note (Subsequent
Evaluation Note)
11506-3
2.16.840.1.113883.10.20.22.1.9
Assessment and
Plan/Assessment/Plan of
Care*
Allergies
Chief Complaint
Instructions
Interventions
Medications
Objective
Physical Exam
Problem List
Results
Review of Systems
Subjective
Vital Signs
Unstructured Document
Non-preferred
2.16.840.1.113883.10.20.21.1.1
0
N/A
N/A
* Wherever referenced, intent is that either “Assessment and Plan” is present or both
“Assessment” and “Plan of Care”. Only these combinations should be used.
** Wherever referenced, intent is that either Chief Complaint/Reason for Visit Section is
present or Chief Complaint Section and/or Reason for Visit unique Sections should be
present.

Page 84 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
4.1 Continuity of Care Document (CCD)/HITSP C32
[ClinicalDocument: templateId 2.16.840.1.113883.10.20.22.1.2(open)]
This section—Continuity of Care Document (CCD) Release 1.1—describes CDA
constraints in accordance with Stage 1 Meaningful Use. The CCD requirements
in this guide supersede CCD Release 1; in the near future, this guide could
supersede HITSP C3217.
The CCD is a core data set of the most relevant administrative, demographic,
and clinical information facts about a patient's healthcare, covering one or more
healthcare encounters. It provides a means for one healthcare practitioner,
system, or setting to aggregate all of the pertinent data about a patient and
forward it to another practitioner, system, or setting to support the continuity of
care. The primary use case for the CCD is to provide a snapshot in time
containing the pertinent clinical, demographic, and administrative data for a
specific patient18. More specific use cases, such as a Discharge Summary or
Progress Note, are available as alternative documents in this guide.
4.1.1 Header Constraints Specific to CCD
The Continuity of Care Document must conform to the US Realm Header. The
following sections include additional header constraints for conformant CCD.
1. SHALL contain exactly one [1..1] templateId (CONF:9441) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.1.1" (CONF:10037).
4.1.1.1 ClinicalDocument/templateId
Conformant documents must carry the document-level templateId asserting
conformance with specific constraints of CCD as well as the templateId for the
US Realm Clinical Document Header template.
2. SHALL contain exactly one [1..1] templateId (CONF:8450) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.1.2" (CONF:10038).
Figure 31: CCD ClinicalDocument/templateId example
<!-- indicates conformance with US Realm Clinical Document Header
template -->
<templateId root="2.16.840.1.113883.10.20.22.1.1"/>
<!-- conforms to CCD requirements -->
<templateId root='2.16.840.1.113883.10.20.22.1.2'/>
17 HITSP Summary Documents Using HL7 Continuity of Care Document (CCD) Component;
(HITSP/C32); Versions 2.1, 2.2, 2.3, 2.5; December 13, 2007 - July 8, 2009
18 CCD was initially scoped to reflect the ASTM E2369-05 Standard Specification for Continuity of
Care Record (CCR). The requirements specified here, comply with Stage 1 Meaningful Use.

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 85
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
4.1.1.2 ClinicalDocument/code
In accordance with the CDA specification, the ClinicalDocument/code element
must be present and specifies the type of the clinical document. CCD requires
the document type code 34133-9 "Summarization of Episode Note".
3. SHALL contain exactly one [1..1] code (CONF:17180).
a. This code SHALL contain exactly one [1..1] @code="34133-9"
Summarization of Episode Note (CodeSystem: LOINC
2.16.840.1.113883.6.1) (CONF:17181).
Figure 32: CCD code example
<code codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC" code="34133-9"
displayName="Summarization of Episode Note"/>
4.1.1.3 DocumentationOf/serviceEvent
The main activity being described by a CCD is the provision of healthcare over a
period of time. This is shown by setting the value of
ClinicalDocument/documentationOf/serviceEvent/@classCode to “PCPR”
(care provision) and indicating the duration over which care was provided in
ClinicalDocument/documentationOf/serviceEvent/effectiveTime.
Additional data from outside this duration may also be included if it is relevant
to care provided during that time range (e.g., reviewed during the stated time
range).
NOTE: Implementations originating a CCD should take care to discover what the
episode of care being summarized is. For example, when a patient fills out a
form providing relevant health history, the episode of care being documented
might be from birth to the present.
4. SHALL contain exactly one [1..1] documentationOf (CONF:8452).
a. This documentationOf SHALL contain exactly one [1..1] serviceEvent
(CONF:8480).
i. This serviceEvent SHALL contain exactly one [1..1]
@classCode="PCPR" Care Provision (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:8453).
ii. This serviceEvent SHALL contain exactly one [1..1]
effectiveTime (CONF:8481).
1. This effectiveTime SHALL contain exactly one [1..1] low
(CONF:8454).
2. This effectiveTime SHALL contain exactly one [1..1]
high (CONF:8455).
iii. This serviceEvent SHOULD contain zero or more [0..*]
performer (CONF:8482).
1. serviceEvent/performer represents the healthcare
providers involved in the current or pertinent historical
care of the patient. Preferably, the patient’s key

Page 86 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
healthcare providers would be listed, particularly their
primary physician and any active consulting
physicians, therapists, and counselors (CONF:10026).
2. Such performers SHALL contain exactly one [1..1]
@typeCode="PRF" Participation physical performer
(CodeSystem: HL7ParticipationType
2.16.840.1.113883.5.90) (CONF:8458).
3. Such performers MAY contain zero or more [0..1]
assignedEntity (CONF:8459).
a. This assignedEntity SHALL contain at least one
[1..*] id (CONF:8460).
i. SHOULD include zero or one [0..1] id
where id/@root
="2.16.840.1.113883.4.6" National
Provider Identifier (CONF:10027).
b. This assignedEntity MAY contain zero or one
[0..1] code (CONF:8461).
i. The code MAY be the NUCC Health Care
Provider Taxonomy (CodeSystem:
2.16.840.1.113883.6.101). (See
http://www.nucc.org) (CONF:8462).
4.1.1.4 Author
5. CCD SHALL contain at least one [1..*] author (CONF:9442)
a. SHALL contain exactly one [1..1] assignedAuthor (CONF:9443)
i. SHALL contain exactly one [1..1] assignedPerson or exactly one
[1..1] representedOrganization. (CONF:8456).
ii. If assignedAuthor has an associated representedOrganization
with no assignedPerson or assignedAuthoringDevice, then the
value for
"ClinicalDocument/author/assignedAuthor/id/@NullFlavor"
SHALL be "NA" "Not applicable" 2.16.840.1.113883.5.1008
NullFlavor STATIC. (CONF:8457).
4.1.2 CCD Body Constraints
The Continuity of Care Document supports both narrative sections and sections
requiring coded clinical statements. The required and optional sections are listed
in the Document Types and Required/Optional Sections table. The table below
the constraints shows all templates including entries within each section.
1. The component/structuredBody SHALL conform to the section constraints
below (CONF:9536).
a. SHALL contain exactly one [1..1] Allergies Section(entries
required) (templateId:2.16.840.1.113883.10.20.22.2.6.1)
(CONF:9445).
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 87
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
b. SHALL contain exactly one [1..1] Medications Section (entries
required) (templateId:2.16.840.1.113883.10.20.22.2.1.1)
(CONF:9447).
c. SHALL contain exactly one [1..1] Problem Section (entries
required) (templateId:2.16.840.1.113883.10.20.22.2.5.1)
(CONF:9449).
d. SHOULD contain exactly one [1..1] Procedures Section (entries
required) (templateId:2.16.840.1.113883.10.20.22.2.7.1)
(CONF:9451).
e. SHALL contain exactly one [1..1] Results Section (entries
required) (templateId:2.16.840.1.113883.10.20.22.2.3.1)
(CONF:9453).
f. MAY contain zero or one [0..1] Advance Directives Section
(entries optional)
(templateId:2.16.840.1.113883.10.20.22.2.21) (CONF:9455).
g. MAY contain zero or one [0..1] Encounters Section (entries
optional) (templateId:2.16.840.1.113883.10.20.22.2.22)
(CONF:9457).
h. MAY contain zero or one [0..1] Family History Section
(templateId:2.16.840.1.113883.10.20.22.2.15) (CONF:9459).
i. MAY contain zero or one [0..1] Functional Status Section
(templateId:2.16.840.1.113883.10.20.22.2.14) (CONF:9461).
j. MAY contain zero or one [0..1] Immunizations Section (entries
optional) (templateId:2.16.840.1.113883.10.20.22.2.2)
(CONF:9463).
k. MAY contain zero or one [0..1] Medical Equipment Section
(templateId:2.16.840.1.113883.10.20.22.2.23) (CONF:9466).
l. MAY contain zero or one [0..1] Payers Section
(templateId:2.16.840.1.113883.10.20.22.2.18) (CONF:9468).
m. MAY contain zero or one [0..1] Plan of Care Section
(templateId:2.16.840.1.113883.10.20.22.2.10) (CONF:9470).
n. MAY contain zero or one [0..1] Social History Section
(templateId:2.16.840.1.113883.10.20.22.2.17) (CONF:9472).
o. MAY contain zero or one [0..1] Vital Signs Section (entries
optional) (templateId:2.16.840.1.113883.10.20.22.2.4)
(CONF:9474).
Page 88 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
The following table shows relationships among the templates in the body of a
CCD.
Table 21: Template Containment for a CCD
Template Title
Template
Type
templateId
Continuity of Care Document (CCD)
document
2.16.840.1.113883.10.20.22.1.2
Advance Directives Section (entries
optional)
section
2.16.840.1.113883.10.20.22.2.21
Advance Directive Observation
entry
2.16.840.1.113883.10.20.22.4.48
Allergies Section (entries required)
section
2.16.840.1.113883.10.20.22.2.6.1
Allergy Problem Act
entry
2.16.840.1.113883.10.20.22.4.30
Allergy Observation
entry
2.16.840.1.113883.10.20.22.4.7
Allergy Status Observation
entry
2.16.840.1.113883.10.20.22.4.28
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 89
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Template Title
Template
Type
templateId
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Encounters Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.22
Encounter Activities
entry
2.16.840.1.113883.10.20.22.4.49
Encounter Diagnosis
entry
2.16.840.1.113883.10.20.22.4.80
Problem Observation
entry
2.16.840.1.113883.10.20.22.4.4
Age Observation
entry
2.16.840.1.113883.10.20.22.4.31
Health Status Observation
entry
2.16.840.1.113883.10.20.22.4.5
Problem Status
entry
2.16.840.1.113883.10.20.22.4.6
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Family History Section
section
2.16.840.1.113883.10.20.22.2.15
Family History Organizer
entry
2.16.840.1.113883.10.20.22.4.45
Family History Observation
entry
2.16.840.1.113883.10.20.22.4.46
Age Observation
entry
2.16.840.1.113883.10.20.22.4.31
Family History Death Observation
entry
2.16.840.1.113883.10.20.22.4.47
Functional Status Section
section
2.16.840.1.113883.10.20.22.2.14
Assessment Scale Observation
entry
2.16.840.1.113883.10.20.22.4.69
Caregiver Characteristics
entry
2.16.840.1.113883.10.20.22.4.72
Cognitive Status Problem Observation
entry
2.16.840.1.113883.10.20.22.4.73
Assessment Scale Observation
entry
2.16.840.1.113883.10.20.22.4.69
Caregiver Characteristics
entry
2.16.840.1.113883.10.20.22.4.72

Page 90 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Template Title
Template
Type
templateId
Non-Medicinal Supply Activity
entry
2.16.840.1.113883.10.20.22.4.50
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Cognitive Status Result Observation
entry
2.16.840.1.113883.10.20.22.4.74
Assessment Scale Observation
entry
2.16.840.1.113883.10.20.22.4.69
Caregiver Characteristics
entry
2.16.840.1.113883.10.20.22.4.72
Non-Medicinal Supply Activity
entry
2.16.840.1.113883.10.20.22.4.50
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Cognitive Status Result Organizer
entry
2.16.840.1.113883.10.20.22.4.75
Cognitive Status Result Observation
entry
2.16.840.1.113883.10.20.22.4.74
Assessment Scale Observation
entry
2.16.840.1.113883.10.20.22.4.69
Caregiver Characteristics
entry
2.16.840.1.113883.10.20.22.4.72
Non-Medicinal Supply Activity
entry
2.16.840.1.113883.10.20.22.4.50
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Functional Status Problem Observation
entry
2.16.840.1.113883.10.20.22.4.68
Assessment Scale Observation
entry
2.16.840.1.113883.10.20.22.4.69
Caregiver Characteristics
entry
2.16.840.1.113883.10.20.22.4.72
Non-Medicinal Supply Activity
entry
2.16.840.1.113883.10.20.22.4.50
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Functional Status Result Observation
entry
2.16.840.1.113883.10.20.22.4.67
Assessment Scale Observation
entry
2.16.840.1.113883.10.20.22.4.69
Caregiver Characteristics
entry
2.16.840.1.113883.10.20.22.4.72
Non-Medicinal Supply Activity
entry
2.16.840.1.113883.10.20.22.4.50
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Functional Status Result Organizer
entry
2.16.840.1.113883.10.20.22.4.66
Functional Status Result Observation
entry
2.16.840.1.113883.10.20.22.4.67
Assessment Scale Observation
entry
2.16.840.1.113883.10.20.22.4.69
Caregiver Characteristics
entry
2.16.840.1.113883.10.20.22.4.72
Non-Medicinal Supply Activity
entry
2.16.840.1.113883.10.20.22.4.50
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Non-Medicinal Supply Activity
entry
2.16.840.1.113883.10.20.22.4.50
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Immunizations Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.2
Immunization Activity
entry
2.16.840.1.113883.10.20.22.4.52
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Immunization Refusal Reason
entry
2.16.840.1.113883.10.20.22.4.53
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 91
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Template Title
Template
Type
templateId
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Page 92 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Template Title
Template
Type
templateId
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Medical Equipment Section
section
2.16.840.1.113883.10.20.22.2.23
Non-Medicinal Supply Activity
entry
2.16.840.1.113883.10.20.22.4.50
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Medications Section (entries required)
section
2.16.840.1.113883.10.20.22.2.1.1
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 93
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Template Title
Template
Type
templateId
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Payers Section
section
2.16.840.1.113883.10.20.22.2.18
Coverage Activity
entry
2.16.840.1.113883.10.20.22.4.60
Policy Activity
entry
2.16.840.1.113883.10.20.22.4.61
Plan of Care Section
section
2.16.840.1.113883.10.20.22.2.10
Plan of Care Activity Act
entry
2.16.840.1.113883.10.20.22.4.39
Plan of Care Activity Encounter
entry
2.16.840.1.113883.10.20.22.4.40
Plan of Care Activity Observation
entry
2.16.840.1.113883.10.20.22.4.44
Plan of Care Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.41
Plan of Care Activity Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.42
Plan of Care Activity Supply
entry
2.16.840.1.113883.10.20.22.4.43
Problem Section (entries required)
section
2.16.840.1.113883.10.20.22.2.5.1
Problem Concern Act (Condition)
entry
2.16.840.1.113883.10.20.22.4.3
Problem Observation
entry
2.16.840.1.113883.10.20.22.4.4
Age Observation
entry
2.16.840.1.113883.10.20.22.4.31
Health Status Observation
entry
2.16.840.1.113883.10.20.22.4.5
Problem Status
entry
2.16.840.1.113883.10.20.22.4.6
Procedures Section (entries required)
section
2.16.840.1.113883.10.20.22.2.7.1
Procedure Activity Act
entry
2.16.840.1.113883.10.20.22.4.12
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Page 94 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Template Title
Template
Type
templateId
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Procedure Activity Observation
entry
2.16.840.1.113883.10.20.22.4.13
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 95
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Template Title
Template
Type
templateId
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Results Section (entries required)
section
2.16.840.1.113883.10.20.22.2.3.1
Result Organizer
entry
2.16.840.1.113883.10.20.22.4.1

Page 96 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Template Title
Template
Type
templateId
Result Observation
entry
2.16.840.1.113883.10.20.22.4.2
Social History Section
section
2.16.840.1.113883.10.20.22.2.17
Pregnancy Observation
entry
2.16.840.1.113883.10.20.15.3.8
Estimated Date of Delivery
entry
2.16.840.1.113883.10.20.15.3.1
Smoking Status Observation
entry
2.16.840.1.113883.10.22.4.78
Social History Observation
entry
2.16.840.1.113883.10.20.22.4.38
Vital Signs Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.4
Vital Signs Organizer
entry
2.16.840.1.113883.10.20.22.4.26
Vital Sign Observation
entry
2.16.840.1.113883.10.20.22.4.27
4.2 Consultation Note
[ClinicalDocument: templateId 2.16.840.1.113883.10.20.22.1.4(open)]
For the purpose of this Implementation Guide, a consultation visit is defined by
the evaluation and management guidelines for a consultation established by the
Centers for Medicare and Medicaid Services (CMS). According to those
guidelines, a Consultation Note must be generated as a result of a physician or
nonphysician practitioner's (NPP) request for an opinion or advice from another
physician or NPP. Consultations must involve face-to-face time with the patient
or fall under guidelines for telemedicine visits.
A Consultation Note must be provided to the referring physician or NPP and
must include the reason for the referral, history of present illness, physical
examination, and decision-making component (Assessment and Plan).
An NPP is defined as any licensed medical professional as recognized by the
state in which the professional practices, including, but not limited to, physician
assistants, nurse practitioners, clinical nurse specialists, social workers,
registered dietitians, physical therapists, and speech therapists.
Reports on visits requested by a patient, family member, or other third party are
not covered by this specification. Second opinions, sometimes called
"confirmatory consultations," also are not covered here. Any question on use of
the Consultation Note defined here should be resolved by reference to CMS or
American Medical Association (AMA) guidelines.
4.2.1 Consultation Note Header Constraints
The Consultation Note must conform to the US Realm Clinical Document
Header. The following sections include additional header constraints for
conformant Consultation Notes.
1. SHALL contain exactly one [1..1] templateId (CONF:9477) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.1.1" (CONF:10039)

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 97
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
4.2.1.1 ClinicalDocument/templateId
Conformant documents must carry the document-level templateId asserting
conformance with specific constraints of a Consultation Note as well as the
templateId for the US Realm Clinical Document Header template.
2. SHALL contain exactly one [1..1] templateId (CONF:8375) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.1.4" (CONF:10040).
Figure 33: Consultation note ClinicalDocument/templateId example
<!-- indicates conformance with US Realm Clinical Document Header
template -->
<templateId root="2.16.840.1.113883.10.20.22.1.1"/>
<!-- conforms to a Consultation Note --><templateId
root=2.16.840.1.113883.10.20.22.1.4'/>
4.2.1.2 ClinicalDocument/code
The Consultation Note limits document type codes to those codes listed in the
Consultation Note LOINC Document Codes table (invalid codes are listed in a
separate table). Implementation may use translation elements to specify a local
code that is equivalent to a document type (see the Consultation Note
translation of local code figure).
The Consultation Note recommends use of a single document type code, 11488-
4 "Consultation Note", with further specification provided by author or
performer, setting, or specialty. The specialized codes in the Consultation Note
LOINC Document Codes table are pre-coordinated with the practice setting or
the training or professional level of the author. Use of these codes is not
recommended, as this duplicates information that may be present in the header.
When pre-coordinated codes are used, any coded values describing the author
or performer of the service act or the practice setting must be consistent with
the LOINC document type. For example, a Cardiology Consultation Note would
not be authored by an Obstetrician.
3. SHALL contain exactly one [1..1] code (CONF:17176).
a. This code SHALL contain exactly one [1..1] @code, which SHALL be
selected from ValueSet ConsultDocumentType
2.16.840.1.113883.11.20.9.31 DYNAMIC (CONF:17177).
Page 98 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 22: Consultation Note LOINC Document Codes
Value Set: ConsultDocumentType 2.16.840.1.113883.11.20.9.31 DYNAMIC
Code System: LOINC 2.16.840.1.113883.6.1
LOINC Code
Type of Service
(“Component”)
Setting
(“System”)
Specialty/ Training/
Professional Level
(“Method”)
Root Level Document Type Code
11488-4
Consultation Note
{Provider}
Specialized by Setting
34100-8
Consultation Note
Critical care unit
{Provider}
34104-0
Consultation Note
Hospital
{Provider}
51845-6
Consultation Note
Outpatient
{Provider}
51853-0
Consultation Note
Inpatient
{Provider}
51846-4
Consultation Note
Emergency
Dept.
{Provider}
Specialized by Setting and Specialty
34101-6
Consultation Note
Outpatient
General medicine
34749-2
Consultation Note
Outpatient
Anesthesia
34102-4
Consultation Note
Hospital
Psychiatry
Specialized by Specialty19
34099-2
Consultation Note
Cardiology
34756-7
Consultation Note
Dentistry
34758-3
Consultation Note
Dermatology
34760-9
Consultation Note
Diabetology
34879-7
Consultation Note
Endocrinology
34761-7
Consultation Note
Gastroenterology
34764-1
Consultation Note
General medicine
34771-6
Consultation Note
General surgery
34776-5
Consultation Note
Gerontology
34777-3
Consultation Note
Gynecology
34779-9
Consultation Note
Hematology+Oncology
34781-5
Consultation Note
Infectious disease
34783-1
Consultation Note
Kinesiotherapy
34785-6
Consultation Note
Mental health
34795-5
Consultation Note
Nephrology
34797-1
Consultation Note
Neurology
34798-9
Consultation Note
Neurosurgery
34800-3
Consultation Note
Nutrition+Dietetics
34803-7
Consultation Note
Occupational health
34855-7
Consultation Note
Occupational therapy
34805-2
Consultation Note
Oncology
19 Use of these codes is not recommended, as it duplicates information that may be present in the header
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 99
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Value Set: ConsultDocumentType 2.16.840.1.113883.11.20.9.31 DYNAMIC
Code System: LOINC 2.16.840.1.113883.6.1
LOINC Code
Type of Service
(“Component”)
Setting
(“System”)
Specialty/ Training/
Professional Level
(“Method”)
34807-8
Consultation Note
Ophthalmology
34810-2
Consultation Note
Optometry
34812-8
Consultation Note
Oromaxillofacial surgery
34814-4
Consultation Note
Orthopedics
34816-9
Consultation Note
Otorhinolaryngology
34820-1
Consultation Note
Pharmacy
34822-7
Consultation Note
Physical medicine and
rehabilitation
34824-3
Consultation Note
Physical therapy
34826-8
Consultation Note
Plastic surgery
34828-4
Consultation Note
Podiatry
34788-0
Consultation Note
Psychiatry
34791-4
Consultation Note
Psychology
34103-2
Consultation Note
Pulmonary
34831-8
Consultation Note
Radiation oncology
34833-4
Consultation Note
Recreational therapy
34835-9
Consultation Note
Rehabilitation
34837-5
Consultation Note
Respiratory therapy
34839-1
Consultation Note
Rheumatology
34841-7
Consultation Note
Social work
34845-8
Consultation Note
Speech
therapy+Audiology
34847-4
Consultation Note
Surgery
34849-0
Consultation Note
Thoracic surgery
34851-6
Consultation Note
Urology
34853-2
Consultation Note
Vascular surgery
Page 100 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 23: Invalid Codes for Consultation Note20
LOINC Code
Type of Service
(“Component”)
Setting
(“System”)
Specialty/ Training/
Professional Level
(“Method”)
18841-7
Hospital consultations
8647-0
Hospital consultations
(scale = nom)
33720-4
Blood bank consult
24611-6
Confirmatory consultation
note
Outpatient
{Provider}
47040-1
Confirmatory consultation
note
{Provider}
47041-9
Confirmatory consultation
note
Inpatient
{Provider}
28569-2
Subsequent evaluation note
Consulting physician
18763-3
Initial evaluation note
Consulting physician
Figure 34: Consultation note ClinicalDocument/code example
<code codeSystem='2.16.840.1.113883.6.1' codeSystemName='LOINC'
code='11488-4' displayName='CONSULTATION NOTE'/>
Figure 35: Consultation note translation of local code example
<code code='34761-7'
displayName='GASTROENTEROLOGY CONSULTATION NOTE'
codeSystem='2.16.840.1.113883.6.1'
codeSystemName='LOINC'>
<translation code='X-GICON'
displayName='GI CONSULTATION NOTE'
codeSystem='2.16.840.1.113883.19'/>
</code>
20 The Invalid Codes for Consultation Note are from the original Consultation Note DSTU.

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 101
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 36: Consultation note uncoordinated document type codes example
<ClinicalDocument xmlns='urn:hl7-org:v3'>
...
<code codeSystem='2.16.840.1.113883.6.1' codeSystemName='LOINC'
code='11488-4' displayName='CONSULTATION NOTE'/>
<title>Good Health Cardiology Consultation Note</title>
...
<author>
<functionCode codeSystem='2.16.840.1.113883.5.88'
codeSystemName='ParticipationFunction'
code='ATTPHYS' />
<assignedAuthor>
...
<code codeSystem='2.16.840.1.113883.6.96' codeSystemName='SNOMED
CT'
code='17561000' displayName='Cardiologist' />
...
</assignedAuthor>
</author>
...
<componentOf>
<encompassingEncounter>
...
<healthCareFacility>
<code codeSystem='2.16.840.1.113883.5.111'
codeSystemName='RoleCode'
code='HOSP' />
</healthCareFacility>
</encompassingEncounter>
</componentOf>
</ClinicalDocument>
4.2.1.3 InFulfillmentOf
The inFulfillmentOf element describes the prior orders that are fulfilled (in
whole or part) by the service events described in the Consultation Note. For
example, the prior order might be for the consultation being reported in the
Note.
4. SHALL contain at least one [1..*] inFulfillmentOf (CONF:8382).
a. This inFulfillmentOf SHOULD contain exactly one
[1..1] order (CONF:8385).
i. This order SHALL contain at least one [1..*] id (CONF:9102).
Figure 37: Consultation note inFulfillmentOf example
<inFulfillmentOf typeCode="FLFS">
<order classCode="ACT" moodCode="RQO">
<id root="2.16.840.1.113883.19" extension="12345-67890"/>
</order>
</inFulfillmentOf>
Page 102 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
4.2.1.4 ComponentOf
A Consultation Note is always associated with an encounter; the componentOf
element must be present and the encounter must be identified.
CDA R2 requires encompasingEncounter and the id element of the
encompassingEncounter is required to be present and represents the identifier
for the encounter.
The encounterParticipant elements may be present. If present, they represent
only those participants in the encounter, not necessarily the entire episode of
care (see related information under Participant above).
The responsibleParty element may be present. If present, it represents only
the party responsible for the encounter, not necessarily the entire episode of
care.
5. SHALL contain exactly one [1..1] componentOf (CONF:8386).
a. This componentOf SHALL contain exactly one [1..1]
encompassingEncounter (CONF:8387).
i. This encompassingEncounter SHALL contain exactly one [1..1]
id (CONF:8388).
ii. This encompassingEncounter SHALL contain exactly one [1..1]
effectiveTime (CONF:8389).
1. This effectiveTime SHALL contain exactly one [1..1] US
Realm Date and Time (DT.US.FIELDED)
(2.16.840.1.113883.10.20.22.5.3)
(CONF:10132).
iii. This encompassingEncounter MAY contain zero or one [0..1]
responsibleParty (CONF:8391).
1. The responsibleParty element records only the party
responsible for the encounter, not necessarily the
entire episode of care. (CONF:8393).
2. The responsibleParty element, if present, SHALL
contain an assignedEntity element which SHALL
contain an assignedPerson element, a
representedOrganization element, or both.
(CONF:8394).
iv. This encompassingEncounter MAY contain zero or more [0..*]
encounterParticipant (CONF:8392).
1. The encounterParticipant element, if present, records
only participants in the encounter, not necessarily in
the entire episode of care. (CONF:8395).
2. An encounterParticipant element, if present, SHALL
contain an assignedEntity element which SHALL
contain an assignedPerson element, a
representedOrganization element, or both.
(CONF:8396).
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 103
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 38: Consultation note componentOf example
<componentOf>
<encompassingEncounter>
<id extension='9937012' root='1.3.6.4.1.4.1.2835.12'/>
<effectiveTime value="20060828170821"/>
<code codeSystem='2.16.840.1.113883.6.12'
codeSystemName='CPT-4'
code='99213'
displayName='Evaluation and Management'/>
...
</encompassingEncounter>
</componentOf>
4.2.2 Consultation Note Body Constraints
The Consultation Note supports both narrative sections and sections requiring
coded clinical statements. The required and optional sections are listed in the
Document Types and Required/Optional Sections table. The table below the
constraints shows all templates including entries within each section.
1. SHALL contain exactly one [1..1] component (CONF:8397).
a. A Consultation Note can have either a structuredBody or a
nonXMLBody. (CONF:8398).
i. A Consultation Note can conform to CDA Level 1
(nonXMLBody), CDA Level 2 (structuredBody with sections
that contain a narrative block), or CDA Level 3
(structuredBody containing sections that contain a narrative
block and coded entries). In this template (templateId
2.16.840.1.113883.10.20.22.1.4), coded entries are optional.
(CONF:8399).
b. If structuredBody, the component/structuredBody SHALL conform to
the section constraints below. (CONF:9503).
i. SHALL include an Assessment and Plan Section, or an
Assessment Section and a Plan Section. (CONF:9501).
ii. SHALL NOT include an Assessment/Plan Section when an
Assessment Section and a Plan of Care Section are present.
(CONF:10028)
iii. MAY contain zero or one [0..1] Assessment Section
(templateId:2.16.840.1.113883.10.20.22.2.8)
(CONF:9487).
iv. MAY contain zero or one [0..1] Plan of Care Section
(templateId:2.16.840.1.113883.10.20.22.2.10)
(CONF:9489).
v. MAY contain zero or one [0..1] Assessment and Plan
Section (templateId:2.16.840.1.113883.10.20.22.2.9)
(CONF:9491).
Page 104 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
vi. SHALL contain exactly one [1..1] History of Present
Illness Section (templateId:
1.3.6.1.4.1.19376.1.5.3.1.3.4) (CONF:9493).
vii. SHOULD contain exactly one [1..1] Physical Exam Section
(templateId:2.16.840.1.113883.10.20.2.10)
(CONF:9495).
viii. SHALL include a Reason for Referral or Reason for Visit section
(CONF:9504).
ix. MAY contain zero or one [0..1] Reason for Referral
Section (templateId:1.3.6.1.4.1.19376.1.5.3.1.3.1)
(CONF:9498).
x. MAY contain zero or one [0..1] Reason for Visit Section
(templateId:2.16.840.1.113883.10.20.22.2.12)
(CONF:9500).
xi. MAY contain zero or one [0..1] Allergies Section (entries
optional)
(templateId:2.16.840.1.113883.10.20.22.2.6)
(CONF:9507).
xii. SHALL NOT include a combined Chief Complaint and Reason
for Visit Section with either a Chief Complaint Section or a
Reason for Visit Section. (CONF:10029).
xiii. MAY contain zero or one [0..1] Chief Complaint Section
(templateId:1.3.6.1.4.1.19376.1.5.3.1.1.13.2.1)
(CONF:9509).
xiv. MAY contain zero or one [0..1] Chief Complaint and Reason
for Visit Section
(templateId:2.16.840.1.113883.10.20.22.2.13)
(CONF:9511).
xv. MAY contain zero or one [0..1] Family History Section
(templateId:2.16.840.1.113883.10.20.22.2.15)
(CONF:9513).
xvi. MAY contain zero or one [0..1] General Status Section
(templateId:2.16.840.1.113883.10.20.2.5)
(CONF:9515).
xvii. MAY contain zero or one [0..1] History of Past Illness
Section
(templateId:2.16.840.1.113883.10.20.22.2.20)
(CONF:9517).
xviii. MAY contain zero or one [0..1] Immunizations Section
(entries optional)
(templateId:2.16.840.1.113883.10.20.22.2.2)
(CONF:9519).
xix. MAY contain zero or one [0..1] Medications Section
(entries optional)
(templateId:2.16.840.1.113883.10.20.22.2.1)
(CONF:9521).
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 105
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
xx. MAY contain zero or one [0..1] Problem Section (entries
optional)
(templateId:2.16.840.1.113883.10.20.22.2.5)
(CONF:9523).
xxi. MAY contain zero or one [0..1] Procedures Section
(entries optional)
(templateId:2.16.840.1.113883.10.20.22.2.7)
(CONF:9525).
xxii. MAY contain zero or one [0..1] Results Section (entries
optional)
(templateId:2.16.840.1.113883.10.20.22.2.3)
(CONF:9527).
xxiii. MAY contain zero or one [0..1] Review of Systems
Section (templateId:1.3.6.1.4.1.19376.1.5.3.1.3.18)
(CONF:9529).
xxiv. MAY contain zero or one [0..1] Social History Section
(templateId:2.16.840.1.113883.10.20.22.2.17)
(CONF:9531).
xxv. MAY contain zero or one [0..1] Vital Signs Section
(entries optional)
(templateId:2.16.840.1.113883.10.20.22.2.4)
(CONF:9533).
The following table shows relationships among the templates in the body of a
Consultation Note.
Table 24: Template Containment for a Consultation Note
Template Title
Template
Type
templateId
Consultation Note
document
2.16.840.1.113883.10.20.22.1.4
Allergies Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.6
Allergy Problem Act
entry
2.16.840.1.113883.10.20.22.4.30
Allergy - Intolerance Observation
entry
2.16.840.1.113883.10.20.22.4.7
Allergy Status Observation
entry
2.16.840.1.113883.10.20.22.4.28
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Page 106 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Template Title
Template
Type
templateId
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Assessment and Plan Section
section
2.16.840.1.113883.10.20.22.2.9
Plan of Care Activity Act
entry
2.16.840.1.113883.10.20.22.4.39
Assessment Section
section
2.16.840.1.113883.10.20.22.2.8

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 107
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Template Title
Template
Type
templateId
Chief Complaint and Reason for Visit
Section
section
2.16.840.1.113883.10.20.22.2.13
Chief Complaint Section
section
1.3.6.1.4.1.19376.1.5.3.1.1.13.2.1
Family History Section
section
2.16.840.1.113883.10.20.22.2.15
Family History Organizer
entry
2.16.840.1.113883.10.20.22.4.45
Family History Observation
entry
2.16.840.1.113883.10.20.22.4.46
Age Observation
entry
2.16.840.1.113883.10.20.22.4.31
Family History Death Observation
entry
2.16.840.1.113883.10.20.22.4.47
General Status Section
section
2.16.840.1.113883.10.20.2.5
History of Past Illness Section
section
2.16.840.1.113883.10.20.22.2.20
Problem Observation
entry
2.16.840.1.113883.10.20.22.4.4
Age Observation
entry
2.16.840.1.113883.10.20.22.4.31
Health Status Observation
entry
2.16.840.1.113883.10.20.22.4.5
Problem Status
entry
2.16.840.1.113883.10.20.22.4.6
History of Present Illness Section
section
1.3.6.1.4.1.19376.1.5.3.1.3.4
Immunizations Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.2
Immunization Activity
entry
2.16.840.1.113883.10.20.22.4.52
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Immunization Refusal Reason
entry
2.16.840.1.113883.10.20.22.4.53
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Page 108 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Template Title
Template
Type
templateId
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 109
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Template Title
Template
Type
templateId
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Medications Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.1
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Physical Exam Section
section
2.16.840.1.113883.10.20.2.10
Plan of Care Section
section
2.16.840.1.113883.10.20.22.2.10
Plan of Care Activity Act
entry
2.16.840.1.113883.10.20.22.4.39
Plan of Care Activity Encounter
entry
2.16.840.1.113883.10.20.22.4.40
Plan of Care Activity Observation
entry
2.16.840.1.113883.10.20.22.4.44
Plan of Care Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.41
Plan of Care Activity Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.42
Plan of Care Activity Supply
entry
2.16.840.1.113883.10.20.22.4.43
Problem Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.5

Page 110 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Template Title
Template
Type
templateId
Problem Concern Act (Condition)
entry
2.16.840.1.113883.10.20.22.4.3
Problem Observation
entry
2.16.840.1.113883.10.20.22.4.4
Age Observation
entry
2.16.840.1.113883.10.20.22.4.31
Health Status Observation
entry
2.16.840.1.113883.10.20.22.4.5
Problem Status
entry
2.16.840.1.113883.10.20.22.4.6
Procedures Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.7
Procedure Activity Act
entry
2.16.840.1.113883.10.20.22.4.12
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Procedure Activity Observation
entry
2.16.840.1.113883.10.20.22.4.13
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 111
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Template Title
Template
Type
templateId
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Page 112 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Template Title
Template
Type
templateId
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Reason for Referral Section
section
1.3.6.1.4.1.19376.1.5.3.1.3.1
Reason for Visit Section
section
2.16.840.1.113883.10.20.22.2.12
Results Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.3
Result Organizer
entry
2.16.840.1.113883.10.20.22.4.1
Result Observation
entry
2.16.840.1.113883.10.20.22.4.2
Review of Systems Section
section
1.3.6.1.4.1.19376.1.5.3.1.3.18
Social History Section
section
2.16.840.1.113883.10.20.22.2.17
Pregnancy Observation
entry
2.16.840.1.113883.10.20.15.3.8
Estimated Date of Delivery
entry
2.16.840.1.113883.10.20.15.3.1
Smoking Status Observation
entry
2.16.840.1.113883.10.22.4.78
Social History Observation
entry
2.16.840.1.113883.10.20.22.4.38
Vital Signs Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.4
Vital Signs Organizer
entry
2.16.840.1.113883.10.20.22.4.26
Vital Sign Observation
entry
2.16.840.1.113883.10.20.22.4.27
4.3 Diagnostic Imaging Report
[ClinicalDocument: templateId 2.16.840.1.113883.10.20.22.1.5(open)]
A Diagnostic Imaging Report (DIR) is a document that contains a consulting
specialist’s interpretation of image data. It conveys the interpretation to the
referring (ordering) physician and becomes part of the patient’s medical record.
It is for use in Radiology, Endoscopy, Cardiology, and other imaging specialties.
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 113
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
4.3.1 DIR Header Constraints
The DIR must conform to the US Realm Clinical Document Header. The
following sections include additional header constraints for conformant DIR
Notes.
1. SHALL contain exactly one [1..1] templateId (CONF:9405) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.1.1" (CONF:10041).
4.3.1.1 ClinicalDocument/templateId
Conformant documents must carry the document-level templateId asserting
conformance with specific constraints of a DIR as well as the templateId for the
U.S. Realm CDA Header Constraints template.
2. SHALL contain exactly one [1..1] templateId (CONF:8404) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.1.5" (CONF:10042).
Figure 39: DIR ClinicalDocument/templateId example
<!-- indicates conformance with US Realm Clinical Document Header
template -->
<templateId root="2.16.840.1.113883.10.20.22.1.1"/>
<!-- conforms to DIR requirements -->
<templateId root='2.16.840.1.113883.10.20.22.1.5'/>
4.3.1.2 ClinicalDocument/id
3. The ClinicalDocument/id/@root attribute SHALL be a syntactically correct
OID, and SHALL NOT be a UUID. (CONF:8405).
a. OIDs SHALL be represented in dotted decimal notation, where each
decimal number is either 0 or starts with a nonzero digit. More
formally, an OID SHALL be in the form ([0-2])(.([1-9][0-9]*|0))+
(CONF:8406).
b. OIDs SHALL be no more than 64 characters in length. (CONF:8407).
4.3.1.3 ClinicalDocument/code
Given that DIR documents may be transformed from established collections of
imaging reports already stored with their own type codes, there is no static set of
Document Type codes. The set of LOINC codes listed in the DIR LOINC
Document Type Codes table may be extended by additions to LOINC and
supplemented by local codes as translations.
The DIR document recommends use of a single document type code, 18748-4
"Diagnostic Imaging Report", with further specification provided by author
or performer, setting, or specialty. Some of these codes in the DIR LOINC
Document Type Codes table are pre-coordinated with either the imaging
modality, body part examined, or specific imaging method such as the view. Use
Page 114 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
of these codes is not recommended, as this duplicates information potentially
present with the header. When pre-coordinated codes are used, any coded
values describing the author or performer of the service act or the practice
setting must be consistent with the LOINC document type. This table is drawn
from LOINC Version 2.36, June 30, 2011, and consists of codes whose scale is
DOC and that refer to reports for diagnostic imaging procedures.
4. SHALL contain exactly one [1..1] code (CONF:14833).
a. This code SHOULD contain zero or one [0..1] @code, which SHOULD be
selected from ValueSet DIRDocumentTypeCode
2.16.840.1.113883.11.20.9.32 DYNAMIC (CONF:14834).
Table 25: DIR LOINC Document Type Codes
Value Set: DIRDocumentTypeCodes 2.16.840.1.113883.11.20.9.32 DYNAMIC
Code System: LOINC 2.16.840.1.113883.6.1
LOINC
Code
DIR
‘Modality’
Common
DIR
Display
Name
Type of
Service
‘Component’
Setting
‘System’
Specialty/
Training/
Professional Level
‘Method_Type’
Preferred Code
18748-4
Any
Diagnositic
Imaging
Report
Study Report
Diagnostic Imaging
Additional Codes
18747-6
Computed
Tomography
CT Report
Study
CT
18755-9
Magnetic
Resonance
Imaging
MRI Report
Study report
MRI
18760-9
Ultrasound
Ultrasound
Report
Study
US
18757-5
Nuclear
Medicine
Nuclear
Medicine
Report
Study report
RadNuc
18758-3
Positron
Emission
Tomography
PET Scan
Report
Study
Pet scan
18745-0
Cardiac
Radiography
/Fluoro-
scopy
Cardiac
Catheteriza
tion Report
Study report
Heart
Cardiac
catheterization
11522-0
Cardiac
Ultrasound
Echocardio
graphy
Report
Study report
Heart
Cardiac echo
18746-8
Colonoscopy
Colon-
oscopy
Report
Study report
Lower GI
tract
Colonoscopy
18751-8
Endoscopy
Endoscopy
Report
Study report
Upper GI
tract
Endoscopy

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 115
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Value Set: DIRDocumentTypeCodes 2.16.840.1.113883.11.20.9.32 DYNAMIC
Code System: LOINC 2.16.840.1.113883.6.1
LOINC
Code
DIR
‘Modality’
Common
DIR
Display
Name
Type of
Service
‘Component’
Setting
‘System’
Specialty/
Training/
Professional Level
‘Method_Type’
11525-3
Ultrasound
Obstetrical
Ultrasound
Report
Study report
Pelvis+Fe
tus
OB US
Figure 40: DIR ClinicalDocument/code example
<code code="18748-4"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="Diagnostic Imaging Report"/>
Figure 41: DIR use of the translation element to include local codes for document
type
<code code="18748-4"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="Diagnostic Imaging Report”>
<translation code='XRPEDS'
displayName='Pediatric Radiography Report'
codeSystem='2.16.840.1.123456.78.9'/>
</code>
4.3.1.4 InformationRecipient
5. SHALL NOT contain [0..0] informant (CONF:8410).
6. MAY contain zero or more [0..*] informationRecipient (CONF:8411).
a. The physician requesting the imaging procedure
(ClincalDocument/participant[@typeCode=REF]/associatedEntity), if
present, SHOULD also be recorded as an informationRecipient, unless
in the local setting another physician (such as the attending
physician for an inpatient) is known to be the appropriate recipient of
the report. (CONF:8412).
b. When no referring physician is present, as in the case of self-referred
screening examinations allowed by law, the intendedRecipient MAY be
absent. The intendedRecipient MAY also be the health chart of the
patient, in which case the receivedOrganization SHALL be the scoping
organization of that chart. (CONF:8413).
4.3.1.5 Participant
7. MAY contain zero or one [0..1] participant (CONF:8414) such that it

Page 116 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
a. If participant is present, the assignedEntity/assignedPerson element
SHALL be present and SHALL represent the physician requesting the
imaging procedure (the referring physician AssociatedEntity that is
the target of ClincalDocument/participant@typeCode=REF).
(CONF:8415).
i. This SHALL contain exactly one [1..1] US Realm Person Name
(PN.US.FIELDED) (2.16.840.1.113883.10.20.22.5)
(CONF:9406).
Figure 42: DIR participant example
<participant typeCode="REF">
<associatedEntity classCode="PROV">
<id nullFlavor="NI"/>
<addr nullFlavor="NI"/>
<telecom nullFlavor="NI"/>
<associatedPerson>
<name>
<given>Amanda</given>
<family>Assigned</family>
<suffix>MD</suffix>
</name>
</associatedPerson>
</associatedEntity>
</participant>
4.3.1.6 InFulfillmentOf
An inFulfillmentOf element represents the Placer Order that is either a group
of orders (modeled as PlacerGroup in the Placer Order RMIM of the Orders &
Observations domain) or a single order item (modeled as ObservationRequest
in the same RMIM). This optionality reflects two major approaches to the
grouping of procedures as implemented in the installed base of imaging
information systems. These approaches differ in their handling of grouped
procedures and how they are mapped to identifiers in the Digital Imaging and
Communications in Medicine (DICOM) image and structured reporting data.
The example of a CT examination covering chest, abdomen, and pelvis will be
used in the discussion below.
In the IHE Scheduled Workflow model, the Chest CT, Abdomen CT, and Pelvis
CT each represent a Requested Procedure, and all three procedures are grouped
under a single Filler Order. The Filler Order number maps directly to the
DICOM Accession Number in the DICOM imaging and report data.
A widely deployed alternative approach maps the requested procedure identifiers
directly to the DICOM Accession Number. The Requested Procedure ID in such
implementations may or may not be different from the Accession Number, but is
of little identifying importance because there is only one Requested Procedure
per Accession Number. There is no identifier that formally connects the
requested procedures ordered in this group.
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 117
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
In both cases, inFulfillmentOf/order/id is mapped to the DICOM Accession
Number in the imaging data.
Figure 43: DIR inFulfillmentOf example
<inFulfillmentOf>
<order>
<id extension="10523475" root="2.16.840.1.113883.19.4.27"/>
<!-- {root}.27= accession number list *-->
</order>
</inFulfillmentOf>
4.3.1.7 DocumentationOf
Each documentationOf/serviceEvent indicates an imaging procedure that the
provider describes and interprets in the content of the DIR. The main activity
being described by this document is the interpretation of the imaging procedure.
This is shown by setting the value of the @classCode attribute of the
serviceEvent element to ACT, and indicating the duration over which care was
provided in the effectiveTime element. Within each documentationOf
element, there is one serviceEvent element. This event is the unit imaging
procedure corresponding to a billable item. The type of imaging procedure may
be further described in the serviceEvent/code element. This guide makes no
specific recommendations about the vocabulary to use for describing this event.
Figure 44: DIR procedure context (CDA Header) illustration (non-normative)
0..* serviceEvent
typeCode*: <= DOC
documentationOf
ServiceEvent
classCode*: <= ACT
moodCode*: <= EVN
id: SET<II> [0..*]
code: CE CWE [0..1]
effectiveTime: IVL<TS> [0..1]
0..* assignedEntity
performer
typeCode*: <= x_ServiceEventPerformer
functionCode: CE CWE [0..1] <= ParticipationFunction
time: IVL<TS> [0..1]
0..1 assignedPerson
0..1 representedOrganization
AssignedEntity
ClinicalDocument
classCode*: <= DOCCLIN
moodCode*: <= EVN
id*: II [1..1]
code*: CE CWE [1..1] <= DocumentType
title*: ST [1..1]
effectiveTime*: TS [1..1]
confidentialityCode*: CE CWE [1..1] <=
x_BasicConfidentialityKind
languageCode: CS CNE [0..1] <= HumanLanguage
setId: II [0..1]
versionNumber: INT [0..1]
copyTime: TS [0..1] (Deprecated)
In IHE Scheduled Workflow environments, one serviceEvent/id element
contains the DICOM Study Instance UID from the Modality Worklist, and the
second serviceEvent/id element contains the DICOM Requested Procedure ID
from the Modality Worklist. These two ids are in a single serviceEvent.
The effectiveTime for the serviceEvent covers the duration of the imaging
procedure being reported. This event should have one or more performers, which
may participate at the same or different periods of time.
Service events map to DICOM Requested Procedures. That is,
documentationOf/serviceEvent/id is the ID of the Requested Procedure.
8. SHALL contain exactly one [1..1] documentationOf (CONF:8416) such that it

Page 118 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
a. SHALL contain exactly one [1..1] serviceEvent (CONF:8431).
i. This serviceEvent SHALL contain exactly one [1..1]
@classCode="ACT" (CodeSystem: HL7ActClass
2.16.840.1.113883.5.6) (CONF:8430).
ii. This serviceEvent SHOULD contain zero or more [0..*] id
(CONF:8418).
iii. This serviceEvent SHALL contain exactly one [1..1] code
(CONF:8419).
1. The value of serviceEvent/code SHALL NOT conflict with
the ClininicalDocument/code. When transforming
from DICOM SR documents that do not contain a
procedure code, an appropriate nullFlavor SHALL be
used on serviceEvent/code. (CONF:8420).
iv. This serviceEvent SHOULD contain zero or more [0..*]
Physician Reading Study Performer
(templateId:2.16.840.1.113883.10.20.6.2.1)
(CONF:8422).
Figure 45: DIR documentationOf example
<documentationOf>
<serviceEvent classCode="ACT">
<id root="1.2.840.113619.2.62.994044785528.114289542805"/>
<!-- study instance UID -->
<id extension="123453" root="1.2.840.113619.2.62.994044785528.26"/>
<!-- DICOM Requested Procedure ID -->
<code code="71020"
displayName="Radiologic examination, chest, two views,
frontal and lateral
codeSystem="2.16.840.1.113883.6.12"
codeSystemName="CPT4"/>
<effectiveTime value="20060823222400"/>
<performer typeCode="PRF">
<templateId root="2.16.840.1.113883.10.20.6.2.1"/>
<assignedEntity>
<id extension="121008" root="2.16.840.1.113883.19.5"/>
<code code="2085R0202X" codeSystem="2.16.840.1.113883.6.101"
codeSystemName="NUCC"
displayName="Diagnostic Radiology"/>
<addr nullFlavor="NI"/>
<telecom nullFlavor="NI"/>
<assignedPerson>
<name>
<given>Christine</given>
<family>Cure</family>
<suffix>MD</suffix>
</name>
</assignedPerson>
</assignedEntity>
</performer>
</serviceEvent>
</documentationOf>
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 119
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
4.3.1.8 RelatedDocument
A DIR may have three types of parent document:
A superseded version that the present document wholly replaces
(typeCode = RPLC). DIRs may go through stages of revision prior to
being legally authenticated. Such early stages may be drafts from
transcription, those created by residents, or other preliminary versions.
Policies not covered by this specification may govern requirements for
retention of such earlier versions. Except for forensic purposes, the
latest version in a chain of revisions represents the complete and
current report.
An original version that the present document appends (typeCode =
APND). When a DIR is legally authenticated, it can be amended by a
separate addendum document that references the original.
A source document from which the present document is transformed
(typeCode = XFRM). A DIR may be created by transformation from a
DICOM Structured Report (SR) document or from another DIR. An
example of the latter case is the creation of a derived document for
inclusion of imaging results in a clinical document.
9. MAY contain zero or one [0..1] relatedDocument (CONF:8432) such that it
a. When a Diagnostic Imaging Report has been transformed from a
DICOM SR document, relatedDocument/@typeCode SHALL be XFRM,
and relatedDocument/parentDocument/id SHALL contain the SOP
Instance UID of the original DICOM SR document. (CONF:8433).
10. The relatedDocument/id/@root attribute SHALL be a syntactically correct
OID, and SHALL NOT be a UUID. (CONF:10030).
a. OIDs SHALL be represented in dotted decimal notation, where each
decimal number is either 0 or starts with a nonzero digit. More
formally, an OID SHALL be in the form ([0-2])(.([1-9][0-9]*|0))+
(CONF:10031).
b. OIDs SHALL be no more than 64 characters in length. (CONF:10032).
Figure 46: DIR relatedDocument example
<!-- transformation of a DICOM SR -->
<relatedDocument typeCode="XFRM">
<parentDocument>
<id
root="1.2.840.113619.2.62.994044785528.20060823.200608232232322.9"/>
<!-- SOP Instance UID (0008,0018) of SR sample document-->
</parentDocument>
</relatedDocument>
4.3.1.9 ComponentOf
The id element of the encompassingEncounter represents the identifier for the
encounter. When the diagnostic imaging procedure is performed in the context
Page 120 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
of a hospital stay or an outpatient visit for which there is an Encounter Number,
that number should be present as the ID of the encompassingEncounter.
The effectiveTime represents the time interval or point in time in which the
encounter took place. The encompassing encounter might be that of the hospital
or office visit in which the diagnostic imaging procedure was performed. If the
effective time is unknown, a nullFlavor attribute can be used.
11. MAY contain zero or one [0..1] componentOf (CONF:8434).
a. This componentOf, if present, SHALL contain exactly one [1..1]
encompassingEncounter (CONF:8449).
i. This encompassingEncounter SHALL contain at least one [1..*]
id (CONF:8435).
1. In the case of transformed DICOM SR documents, an
appropriate null flavor MAY be used if the id is
unavailable. (CONF:8436).
ii. This encompassingEncounter SHALL contain exactly one [1..1]
effectiveTime (CONF:8437).
1. This effectiveTime SHALL contain exactly one [1..1] US
Realm Date and Time (DT.US.FIELDED)
(2.16.840.1.113883.10.20.22.5.3) (CONF:10133).
iii. This encompassingEncounter MAY contain zero or more [0..1]
responsibleParty (CONF:8438).
1. This responsibleParty, if present, SHALL contain exactly
one [1..1] assignedEntity (CONF:9407).
a. SHOULD contain zero or one [0..1]
assignedPerson OR contain zero or one [0..1]
representedOrganization (CONF:8439).
iv. This encompassingEncounter SHOULD contain zero or one
[0..1] Physician of Record Participant
(templateId:2.16.840.1.113883.10.20.6.2.2)
(CONF:8448).
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 121
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 47: DIR componentOf example
<componentOf>
<encompassingEncounter>
<id extension="9937012" root="1.3.6.4.1.4.1.2835.12"/>
<effectiveTime value="20060828170821"/>
<encounterParticipant typeCode="ATND">
<templateId root="2.16.840.1.113883.10.20.6.2.2"/>
<assignedEntity>
<id extension="4" root="2.16.840.1.113883.19"/>
<code code="208D00000X" codeSystem="2.16.840.1.113883.6.101"
codeSystemName="NUCC"
displayName="General Practice"/>
<addr nullFlavor="NI"/>
<telecom nullFlavor="NI"/>
<assignedPerson>
<name>
<prefix>Dr.</prefix>
<given>Fay </given>
<family>Family</family>
</name>
</assignedPerson>
</assignedEntity>
</encounterParticipant>
</encompassingEncounter>
</componentOf>
4.3.1.10 Physician Reading Study Performer
[performer: templateId 2.16.840.1.113883.10.20.6.2.1(open)]
This participant is the Physician Reading Study Performer defined in
documentationOf/serviceEvent and is usually different from the attending
physician. The reading physician interprets the images and evidence of the
study (DICOM Definition)
1. SHALL contain exactly one [1..1] @typeCode="PRF" Performer (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:8424).
2. SHALL contain exactly one [1..1]
templateId/@root="2.16.840.1.113883.10.20.6.2.1" (CONF:8423).
3. MAY contain zero or one [0..1] time (CONF:8425).
a. This time SHALL contain exactly one [1..1] US Realm Date and Time
(DTM.US.FIELDED) (2.16.840.1.113883.10.20.22.5.4)
(CONF:10134).
4. SHALL contain exactly one [1..1] assignedEntity (CONF:8426).
a. This assignedEntity SHALL contain at least one [1..*] id
(CONF:10033).
i. The id SHOULD include zero or one [0..1] id where id/@root
="2.16.840.1.113883.4.6" National Provider Identifier
(CONF:10034).
b. This assignedEntity SHALL contain exactly one [1..1] code
(CONF:8427).

Page 122 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
i. SHALL contain a valid DICOM personal identification code
sequence (@codeSystem is 1.2.840.10008.2.16.4) or an
appropriate national health care provider coding system (e.g.,
NUCC in the U.S., where @codeSystem is
2.16.840.1.113883.6.101). (CONF:8428).
c. Every assignedEntity element SHALL have at least one assignedPerson
or representedOrganization. (CONF:8429).
Figure 48: Physician reading study performer example
<performer typeCode="PRF">
<templateId root="2.16.840.1.113883.10.20.6.2.1"/>
<assignedEntity>
<id extension="111111111" root="2.16.840.1.113883.4.6"/>
<code code="2085R0202X"
codeSystem="2.16.840.1.113883.6.101"
codeSystemName="NUCC"
displayName="Diagnostic Radiology"/>
<addr nullFlavor="NI"/>
<telecom nullFlavor="NI"/>
<assignedPerson>
<name>
<given>Christine</given>
<family>Cure</family>
<suffix>MD</suffix>
</name>
</assignedPerson>
</assignedEntity>
</performer>
4.3.1.11 Physician of Record Participant
[encounterParticipant: templateId
2.16.840.1.113883.10.20.6.2.2(open)]
This encounterParticipant is the attending physician and is usually different
from the Physician Reading Study Performer defined in
documentationOf/serviceEvent.
1. SHALL contain exactly one [1..1] @typeCode="ATND" Attender (CodeSystem:
HL7ParticipationType 2.16.840.1.113883.5.90) (CONF:8881).
2. SHALL contain exactly one [1..1]
templateId/@root="2.16.840.1.113883.10.20.6.2.2" (CONF:8440).
3. SHALL contain exactly one [1..1] assignedEntity (CONF:8886).
a. This assignedEntity SHALL contain at least one [1..*] id (CONF:8887).
i. The id SHOULD include zero or one [0..1] id where id/@root
="2.16.840.1.113883.4.6" National Provider Identifier
(CONF:10035).
b. This assignedEntity SHALL contain exactly one [1..1] code
(CONF:8888).
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 123
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
i. SHALL contain a valid DICOM Organizational Role from
DICOM CID 745221 (Value Set
1.2.840.10008.6.1.516)(@codeSystem is
1.2.840.10008.2.16.4) or an appropriate national health
care provider coding system (e.g., NUCC in the U.S., where
@codeSystem is 2.16.840.1.113883.6.101) (CONF:8889).
c. This assignedEntity SHOULD contain zero or one [0..1] name
(CONF:8890).
Figure 49: Physician of record participant example
<encounterParticipant typeCode="ATND">
<templateId root="2.16.840.1.113883.10.20.6.2.2"/>
<assignedEntity>
<id extension="44444444" root="2.16.840.1.113883.4.6"/>
<code code="208D00000X"
codeSystem="2.16.840.1.113883.6.101"
codeSystemName="NUCC"
displayName="General Practice"/>
<addr nullFlavor="NI"/>
<telecom nullFlavor="NI"/>
<assignedPerson>
<name>
<prefix>Dr.</prefix>
<given>Fay</given>
<family>Family</family>
</name>
</assignedPerson>
</assignedEntity>
</encounterParticipant>
21 DICOM Part 16 (NEMA PS3.16), page 631 in the 2011 edition. See
ftp://medical.nema.org/medical/dicom/2011/11_16pu.pdf
Page 124 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
4.3.2 DIR Body Constraints
The DIR supports both narrative sections and sections requiring coded clinical
statements. The required and optional sections are listed in the Document Types
and Required/Optional Sections table.
The following table shows relationships among the constrained templates in the
body of a DIR report.
Table 26: Template Containment for Constrained DIR Sections
Template Title
Template
Type
templateId
Diagnostic Imaging Report
document
2.16.840.1.113883.10.20.22.1.5
Fetus Subject Context
section
2.16.840.1.113883.10.20.6.2.3
Findings Section (DIR)
section
2.16.840.1.113883.10.20.6.1.2
Observer Context
section
2.16.840.1.113883.10.20.6.2.4
Physician of Record Participant
unspecified
2.16.840.1.113883.10.20.6.2.2
Physician Reading Study Performer
unspecified
2.16.840.1.113883.10.20.6.2.1
Procedure Context
entry
2.16.840.1.113883.10.20.6.2.5
DICOM Object Catalog Section - DCM
121181
section
2.16.840.1.113883.10.20.6.1.1
Study Act
entry
2.16.840.1.113883.10.20.6.2.6
Series Act
entry
2.16.840.1.113883.10.20.22.4.63
SOP Instance Observation
entry
2.16.840.1.113883.10.20.6.2.8
Purpose of Reference Observation
entry
2.16.840.1.113883.10.20.6.2.9
Referenced Frames Observation
entry
2.16.840.1.113883.10.20.6.2.10
Boundary Observation
entry
2.16.840.1.113883.10.20.6.2.11
4.3.2.1 DIR Section Constraints
The Section Type codes used by DIR are described below in the DIR Section Type
Codes table. All section codes shown in this table describe narrative document
sections22. The column headings of this table are:
DCM Code:
The code of the section in DICOM (Context
Group CID 7001)
DCM Code Meaning:
The display name of the section in DICOM
(Context Group CID 7001)
LOINC Code:
The code of the section in LOINC
LOINC Component Name:
The display name of the section in LOINC
22 SCALE_TYP = 'NAR' in the LOINC tables.
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 125
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Use:
The use column indicates that a section in
a Diagnostic Imaging Report is:
R – required
C – conditionally required
O – optional
Table 27: DIR Section Type Codes
DICOM Code
DICOM Code Meaning
LOINC Code
LOINC Code Meaning
Use
121181
DICOM Object Catalog
N/A
N/A
C
121060
History
11329-0
HISTORY GENERAL
O
121062
Request
55115-0
REQUESTED IMAGING
STUDIES INFORMATION
O
121064
Current Procedure
Descriptions
55111-9
CURRENT IMAGING
PROCEDURE DESCRIPTIONS
O
121066
Prior Procedure
Descriptions
55114-3
PRIOR IMAGING PROCEDURE
DESCRIPTIONS
O
121068
Previous Findings
18834-2
RADIOLOGY COMPARISON
STUDY - OBSERVATION
O
121070
Findings (DIR)
18782-3
RADIOLOGY STUDY
OBSERVATION
R
121072
Impressions
19005-8
RADIOLOGY - IMPRESSION
O
121074
Recommendations
18783-1
RADIOLOGY STUDY -
RECOMMENDATION
O
121076
Conclusions
55110-1
CONCLUSIONS
O
121078
Addendum
55107-7
ADDENDUM
O
121109
Indications for
Procedure
18785-6
RADIOLOGY REASON FOR
STUDY
O
121110
Patient Presentation
55108-5
CLINICAL PRESENTATION
O
121113
Complications
55109-3
COMPLICATIONS
O
121111
Summary
55112-7
DOCUMENT SUMMARY
O
121180
Key Images
55113-5
KEY IMAGES
O
For Level 2 conformance, all section elements that are present in the Body of
the document must have a code and some nonblank text or one or more
subsections, even if the purpose of the text is only to indicate that information is
unknown.
There is no equivalent to section/title in DICOM SR, so for a CDA to SR
transformation, the section/code will be transferred and the title element
will be dropped.
1. SHALL contain exactly one [1..1] component (CONF:14907).
a. A Diagnostic Imaging Report can have either a structuredBody or a
nonXMLBody (CONF:14908).
i. A Diagnostic Imaging Report can conform to CDA Level 1
(nonXMLBody), CDA Level 2 (structuredBody with sections
that contain a narrative block), or CDA Level 3

Page 126 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
(structuredBody containing sections that contain a narrative
block and coded entries). In this template (templateId
2.16.840.1.113883.10.20.22.1.5), coded entries are optional
(CONF:14909).
b. If structuredBody, the component/structuredBody SHALL conform to
the section constraints below (CONF:14910).
i. The DICOM Object Catalog section (templateId
2.16.840.1.113883.10.20.6.1.1), if present, SHALL be the first
section in the document Body (CONF:9408).
ii. SHALL contain exactly one [1..1] Findings Section (DIR)
(templateId:2.16.840.1.113883.10.20.6.1.2)
(CONF:9484).
iii. SHOULD contain zero or one [0..1] DICOM Object Catalog
Section - DCM 121181
(templateId:2.16.840.1.113883.10.20.6.1.1)
(CONF:15141).
iv. With the exception of the DICOM Object Catalog (templateId
2.16.840.1.113883.10.20.6.1.1), all sections within the
Diagnostic Imaging Report content SHOULD contain a title
element (CONF:9409).
v. The section/code SHOULD be selected from LOINC or DICOM
for sections not listed in the DIR Section Type Codes table
(CONF:9410).
1. Descriptions for sections is under development in
DICOM in cooperation with the RSNA reporting
initiative (CONF:9423).
vi. All sections defined in the DIR Section Type Codes table SHALL
be top-level sections (CONF:9411).
vii. A section element SHALL have a code element, which SHALL
contain a LOINC code or DCM code for sections that have no
LOINC equivalent. This only applies to sections described in
the DIR Section Type Codes table (CONF:9412).
viii. Apart from the DICOM Object Catalog (templateId
2.16.840.1.113883.10.20.6.1.1), all other instances of section
SHALL contain at least one text element or one or more
component elements (CONF:9413).
ix. All text or component elements SHALL contain content. text
elements SHALL contain PCDATA or child elements, and
component elements SHALL contain child elements
(CONF:9414).
x. The text elements (and their children) MAY contain Web Access
to DICOM Persistent Object (WADO) references to DICOM
objects by including a linkHtml element where @href is a valid
WADO URL and the text content of linkHtml is the visible text
of the hyperlink (CONF:9415).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 127
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
xi. If clinical statements are present, the section/text SHALL
represent faithfully all such statements and MAY contain
additional text (CONF:9416).
xii. MAY contain zero or more [0..*] Procedure Context
(templateId:2.16.840.1.113883.10.20.6.2.5)
(CONF:9417).
1. If the service context of a section is different from the
value specified in documentationOf/serviceEvent, then
the section SHALL contain one or more entries
containing Procedure Context (templateId
2.16.840.1.113883.10.20.6.2.5), which will reset the
context for any clinical statements nested within those
elements (CONF:9418).
xiii. MAY contain zero or more [0..*] Fetus Subject Context
(templateId:2.16.840.1.113883.10.20.6.2.3)
(CONF:9419).
1. If the subject of a section is a fetus, the section SHALL
contain a subject element containing a Fetus Subject
Context (templateId 2.16.840.1.113883.10.20.6.2.3)
(CONF:9420).
xiv. MAY contain zero or more [0..*] Observer Context
(templateId:2.16.840.1.113883.10.20.6.2.4)
(CONF:9421).
1. : If the author of a section is different from the
author(s) listed in the Header, an author element
SHALL be present containing Observer Context
(templateId 2.16.840.1.113883.10.20.6.2.4)
(CONF:9422).
Figure 50: WADO reference using linkHtml example
<text>
...
<paragraph>
<caption>Source of Measurement</caption>
<linkHtml
href="http://www.example.org/wado?requestType=WADO&studyUID=1.2.840.1
13619.2.62.994044785528.114289542805&seriesUID=1.2.840.113619.2.62.99
4044785528.20060823223142485051&objectUID=1.2.840.113619.2.62.9940447
85528.20060823.200608232232322.3&contentType=application/dicom">Chest
_PA</linkHtml>
</paragraph>
...
</text>
4.3.2.2 Fetus Subject Context
[relatedSubject: templateId 2.16.840.1.113883.10.20.6.2.3(open)]
For reports on mothers and their fetus(es), information on a mother is mapped
to recordTarget, PatientRole, and Patient. Information on the fetus is mapped to

Page 128 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
subject, relatedSubject, and SubjectPerson at the CDA section level. Both
context information on the mother and fetus must be included in the document
if observations on fetus(es) are contained in the document.
1. SHALL contain exactly one [1..1] templateId (CONF:9189) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.6.2.3" (CONF:10535).
2. SHALL contain exactly one [1..1] code="121026" (CodeSystem: DCM
1.2.840.10008.2.16.4) (CONF:9190).
3. SHALL contain exactly one [1..1] subject (CONF:9191).
4. SHALL contain exactly one [1..1] name (CONF:9192).
a. The name element is used to store the DICOM fetus ID, typically a
pseudonym such as fetus_1 (CONF:9193).
Figure 51: Fetus subject context example
<relatedSubject>
<templateId root="2.16.840.1.113883.10.20.6.2.3"/>
<code code="121026"
codeSystem="1.2.840.10008.2.16.4"
displayName="Fetus"/>
<subject>
<name>fetus_1</name>
</subject>
</relatedSubject>
4.3.2.3 Observer Context
[assignedAuthor: templateId 2.16.840.1.113883.10.20.6.2.4(open)]
The Observer Context is used to override the author specified in the CDA
Header. It is valid as a direct child element of a section.
1. SHALL contain exactly one [1..1] templateId (CONF:9194) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.6.2.4" (CONF:10536).
2. SHALL contain exactly one [1..1] assignedAuthor (CONF:9195).
3. SHALL contain at least one [1..*] id (CONF:9196).
a. The id element contains the author's id or the DICOM device observer
UID (CONF:9197).
4. Either assignedPerson or assignedAuthoringDevice SHALL be present
(CONF:9198).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 129
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 52: Observer context example
<assignedAuthor>
<templateId root="2.16.840.1.113883.10.20.6.2.4"/>
<id extension="121008" root="2.16.840.1.113883.19.5"/>
<assignedPerson>
<name>
<given>Richard</given>
<family>Blitz</family>
<suffix>MD</suffix>
</name>
</assignedPerson>
</assignedAuthor>
4.3.2.4 DIR Clinical Statements
A Diagnostic Imaging Report may contain CDA entries that represent, in coded
form findings, image references, annotation, and numeric measurements based
on DICOM Basic Diagnostic Imaging Report (Template 2000) and Transcribed
Diagnostic Imaging Report (Template 2005). Most of the constraints for this
document have been inherited from the DICOM PS 3.20 “Transformation of
DICOM to and from HL7 Standards”.
This document type and the companion DICOM PS 3.20 “Transformation of
DICOM to and from HL7 Standards” guide further constrain the transformation
because image Spatial Coordinates region of interest (SCOORD) for linear, area,
and volume measurements are not encoded in the CDA document. If it is
desired to show images with such graphical annotations, the annotations should
be encoded in DICOM Softcopy Presentation State objects that reference the
image. Report applications that display referenced images and annotation
should retrieve a rendered image using a WADO reference, including the image
and Presentation State, or other DICOM retrieval and rendering methods. This
approach avoids the risks of errors in registering a region of interest annotation
with DICOM images.
DICOM Template 2000 defines imaging report documents that are comprised of
a number of optional sections, including those defined above in DIR Section
Type Codes Each section contains:
Text Observations (Text Elements in DICOM SR), optionally inferred from
Quantity Measurement Observation or Image references
Code Observations (Code Elements in DICOM SR), optionally inferred
from Quantity Measurement Observation or Image references
Quantity Measurement Observation (Numeric Elements in DICOM SR)
with a coded measurement type, optionally inferred from an image
reference
Service Object Pair (SOP) Instance Observations containing image
references
The number or order of the observations and image references in the above
bullet points are not constrained in a section.
Page 130 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
4.4 Discharge Summary
[ClinicalDocument: templateId 2.16.840.1.113883.10.20.22.1.8(open)]
The Discharge Summary is a document that is a synopsis of a patient's
admission to a hospital; it provides pertinent information for the continuation of
care following discharge. The Joint Commission requires the following
information to be included in the Discharge Summary23:
The reason for hospitalization
The procedures performed
The care, treatment, and services provided
The patient’s condition and disposition at discharge
Information provided to the patient and family
Provisions for follow-up care
4.4.1 Discharge Summary Header Constraints
The Discharge Summary must conform to the US Realm Clinical Document
Header. The following sections include additional header constraints for
conformant Discharge Summaries.
1. SHALL contain exactly one [1..1] templateId (CONF:9479) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.1.1" (CONF:10043).
4.4.1.1 ClinicalDocument/templateId
Conformant documents must carry the document-level templateId asserting
conformance with specific constraints of a Discharge Summary as well as the
templateId for the US Realm Clinical Document Header template.
2. SHALL contain exactly one [1..1] templateId (CONF:8463) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.1.8" (CONF:10044).
Figure 53: Discharge summary ClinicalDocument/templateId example
<!-- indicates conformance with Clinical Document Header Constraints -->
<templateId root="2.16.840.1.113883.10.20.3"/>
<!—indicates conformance to Discharge Summary -->
<templateId root="2.16.840.1.113883.10.20.22.1.8"/>
23 Joint Commission Requirements for Discharge Summary (JCAHO IM.6.10 EP7). See
http://www.jointcommission.org/NR/rdonlyres/C9298DD0-6726-4105-A007-
FE2C65F77075/0/CMS_New_Revised_HAP_FINAL_withScoring.pdf..
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 131
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
4.4.1.2 ClinicalDocument/code
The Discharge Summary LOINC Document Codes table shows the LOINC codes
suitable for Discharge Summary, as of publication of this implementation guide.
This is a dynamic value set meaning that these codes may be added to or
deprecated by LOINC.
The Discharge Summary recommends use of a single document type code,
18842-5 "Discharge Summarization Note", with further specification
provided by author or performer, setting, or specialty. Some of the LOINC codes
listed here pre-coordinate the practice setting or the training or professional
level of the author. Use of these codes is not recommended, as this duplicates
information that may be present in the header. If used, the pre-coordinated
codes must be consistent with the LOINC document type code.
3. SHALL contain exactly one [1..1] code (CONF:17178).
a. This code SHALL contain exactly one [1..1] @code, which SHALL be
selected from ValueSet DischargeSummaryDocumentTypeCode
2.16.840.1.113883.11.20.4.1 DYNAMIC (CONF:17179).
Table 28: Discharge summary LOINC Document Codes
Value Set: DischargeSummaryDocumentTypeCode 2.16.840.1.113883.11.20.4.1 DYNAMIC
Code System: LOINC 2.16.840.1.113883.6.1
LOINC Code
Type of Service
‘Component’
Setting
‘System’
Specialty/Training/Professional
Level ‘Method_Type’
Preferred Code
18842-5
Discharge summarization
note
{Setting}
{Provider}
Additional Codes
11490-0
Discharge summarization
note
{Setting}
Physician
28655-9
Discharge summarization
note
{Setting}
Attending physician
29761-4
Discharge summarization
note
{Setting}
Dentistry
34745-0
Discharge summarization
note
{Setting}
Nursing
34105-7
Discharge summarization
note
Hospital
{Provider}
34106-5
Discharge summarization
note
Hospital
Physician
Figure 54: Discharge summary ClinicalDocument/code example
<code codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC" code="18842-5"
displayName="DISCHARGE SUMMARIZATION NOTE"/>

Page 132 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
4.4.1.3 Participant
The participant element in the Discharge Summary header follows the General
Header Constraints for participants. Discharge Summary does not specify any
use for functionCode for participants. Local policies will determine how this
element should be used in implementations.
4.4.1.4 ComponentOf
The Discharge Summary is always associated with a Hospital Admission using
the encompassingEncounter element in the header.
The dischargeDispositionCode records the disposition of the patient at time
of discharge. Access to the National Uniform Billing Committee (NUBC) code
system requires a membership. The following conformance statement aligns with
HITSP C80 requirements.
The responsibleParty element represents only the party responsible for the
encounter, not necessarily the entire episode of care.
The encounterParticipant elements represent only those participants in the
encounter, not necessarily the entire episode of care.
The admission date is recorded in the
componnentOf/encompassingEncounter/
effectiveTime/low.
4. SHALL contain exactly one [1..1] componentOf (CONF:8471).
a. This componentOf SHALL contain exactly one [1..1]
encompassingEncounter (CONF:8472).
i. This encompassingEncounter SHALL contain exactly one [1..1]
effectiveTime/low (CONF:8473).
ii. This encompassingEncounter SHALL contain exactly one [1..1]
effectiveTime/high (CONF:8475).
iii. The dischargeDispositionCode SHALL be present where the
value of code SHOULD be selected from ValueSet NUBC UB-04
FL17-Patient Status 2.16.840.1.113883.3.88.12.80.33
DYNAMIC (http://www.nubc.org) (CONF:8476).
1. The dischargeDispositionCode, @displayName, or
NUBC UB-04 Print Name, SHALL be displayed when the
document is rendered. (CONF:8477).
iv. The responsibleParty element MAY be present. If present, the
responsibleParty/assignedEntity element SHALL have at least
one assignedPerson or representedOrganization element
present. (CONF:8479).
v. The encounterParticipant elements MAY be present. If present,
the encounterParticipant/assignedEntity element SHALL have
at least one assignedPerson or representedOrganization
element present. (CONF:8478).
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 133
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 29: HL7 Discharge Disposition Codes
Code System: HL7 Discharge Disposition 2.16.840.1.113883.12.112
Code
Print Name
01
Discharged to home or self care (routine discharge)
02
Discharged/transferred to another short-term general hospital for inpatient
care
03
Discharged/transferred to skilled nursing facility (SNF)
04
Discharged/transferred to an intermediate-care facility (ICF)
05
Discharged/transferred to another type of institution for inpatient care or
referred for outpatient services to another institution
06
Discharged/transferred to home under care of organized home health service
organization
07
Left against medical advice or discontinued care
08
Discharged/transferred to home under care of Home IV provider
09
Admitted as an inpatient to this hospital
10 …19
Discharge to be defined at state level, if necessary
20
Expired (i.e., dead)
21 ... 29
Expired to be defined at state level, if necessary
30
Still patient or expected to return for outpatient services (i.e., still a patient)
31 … 39
Still patient to be defined at state level, if necessary (i.e., still a patient)
40
Expired (i.e., died) at home
41
Expired (i.e., died) in a medical facility; e.g., hospital, SNF, ICF, or free-
standing hospice
42
Expired (i.e., died) - place unknown
Figure 55: Discharge summary componentOf example
<componentOf>
<encompassingEncounter>
<id extension="9937012" root="2.16.840.1.113883.19"/>
<effectiveTime>
<low value="20050329"/>
<high value="20050329"/>
</effectiveTime>
<dischargeDispositionCode code="01"
codeSystem="2.16.840.1.113883.12.112"
displayName="Routine Discharge"
codeSystemName="HL7 Discharge Disposition"/>
</encompassingEncounter>
</componentOf>
Page 134 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
4.4.2 Discharge Summary Body Constraints
The Discharge Summary supports both narrative sections and sections requiring
code clinical statements. The required and optional sections are listed in the
Document Types and Required/Optional Sections table. The table below the
constraints shows all templates including entries within each section.
1. SHALL contain exactly one [1..1] component (CONF:9539).
a. A Discharge Summary can have either a structuredBody or a
nonXMLBody. (CONF:9537).
i. A Discharge Summary can conform to CDA Level 1
(nonXMLBody), CDA Level 2 (structuredBody with sections
that contain a narrative block), or CDA Level 3
(structuredBody containing sections that contain a narrative
block and coded entries). In this template (templateId
2.16.840.1.113883.10.20.22.1.8), coded entries are optional.
(CONF:9538).
b. If structuredBody, the component/structuredBody SHALL conform to
the section constraints below. (CONF:9540).
i. SHALL contain exactly one [1..1] Allergies Section
(entries optional)
(templateId:2.16.840.1.113883.10.20.22.2.6)
(CONF:9542).
ii. SHALL contain exactly one [1..1] Hospital Course Section
(templateId:1.3.6.1.4.1.19376.1.5.3.1.3.5)
(CONF:9544).
iii. SHALL contain exactly one [1..1] Hospital Discharge
Diagnosis Section
(templateId:2.16.840.1.113883.10.20.22.2.24)
(CONF:9546).
iv. SHALL contain exactly one [1..1] Hospital Discharge
Medications Section (entries optional)
(templateId:2.16.840.1.113883.10.20.22.2.11)
(CONF:9548).
v. SHALL contain exactly one [1..1] Plan of Care Section
(templateId:2.16.840.1.113883.10.20.22.2.10)
(CONF:9550).
vi. SHALL NOT include a Chief Complaint and Reason for Visit
Section with either a Chief Complaint Section or a Reason for
Visit Section. (CONF:10055)
vii. MAY contain zero or one [0..1] Chief Complaint Section
(templateId:1.3.6.1.4.1.19376.1.5.3.1.1.13.2.1)
(CONF:9554).
viii. MAY contain zero or one [0..1] Chief Complaint and Reason
for Visit Section
(templateId:2.16.840.1.113883.10.20.22.2.13)
(CONF:9556).
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 135
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
ix. MAY contain zero or one [0..1] Discharge Diet Section
(templateId:1.3.6.1.4.1.19376.1.5.3.1.3.33)
(CONF:9558).
x. MAY contain zero or one [0..1] Family History Section
(templateId:2.16.840.1.113883.10.20.22.2.15)
(CONF:9560).
xi. MAY contain zero or one [0..1] Functional Status Section
(templateId:2.16.840.1.113883.10.20.22.2.14)
(CONF:9562).
xii. MAY contain zero or one [0..1] History of Past Illness
Section
(templateId:2.16.840.1.113883.10.20.22.2.20)
(CONF:9564).
xiii. MAY contain zero or one [0..1] History of Present Illness
Section (templateId: 1.3.6.1.4.1.19376.1.5.3.1.3.4)
(CONF:9566).
xiv. MAY contain zero or one [0..1] Hospital Admission
Diagnosis Section
(templateId:2.16.840.1.113883.10.20.22.2.43)
(CONF:9928).
xv. MAY contain zero or one [0..1] Hospital Admission
Medications Section (entries optional)
(2.16.840.1.113883.10.20.22.2.44) (CONF:10111).
xvi. MAY contain zero or one [0..1] Hospital Consultations
Section
(templateId:2.16.840.1.113883.10.20.22.2.42)
(CONF:9924).
xvii. MAY contain zero or one [0..1] Hospital Discharge
Instructions Section
(templateId:2.16.840.1.113883.10.20.22.2.41)
(CONF:9926).
xviii. MAY contain zero or one [0..1] Hospital Discharge
Physical Section
(templateId:1.3.6.1.4.1.19376.1.5.3.1.3.26)
(CONF:9568).
xix. MAY contain zero or one [0..1] Hospital Discharge Studies
Summary Section
(templateId:2.16.840.1.113883.10.20.22.2.16)
(CONF:9570).
xx. MAY contain zero or one [0..1] Immunizations Section
(entries optional)
(templateId:2.16.840.1.113883.10.20.22.2.2)
(CONF:9572).
xxi. MAY contain zero or one [0..1] Problem Section (entries
optional)
(templateId:2.16.840.1.113883.10.20.22.2.5)
(CONF:9574).

Page 136 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
xxii. MAY contain zero or one [0..1] Procedures Section
(entries optional)
(templateId:2.16.840.1.113883.10.20.22.2.7)
(CONF:9576).
xxiii. MAY contain zero or one [0..1] Reason for Visit Section
(templateId:2.16.840.1.113883.10.20.22.2.12)
(CONF:9578).
xxiv. MAY contain zero or one [0..1] Review of Systems
Section (templateId:1.3.6.1.4.1.19376.1.5.3.1.3.18)
(CONF:9580).
xxv. MAY contain zero or one [0..1] Social History Section
(templateId:2.16.840.1.113883.10.20.22.2.17)
(CONF:9582).
xxvi. MAY contain zero or one [0..1] Vital Signs Section
(entries optional)
(templateId:2.16.840.1.113883.10.20.22.2.4)
(CONF:9584).
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 137
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
The following table shows relationships among the templates in the body of a
Discharge Summary.
Table 30: Template Containment for a Discharge Summary
Template Title
Template
Type
templateId
Discharge Summary
document
2.16.840.1.113883.10.20.22.1.8
Allergies Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.6
Allergy Problem Act
entry
2.16.840.1.113883.10.20.22.4.30
Allergy - Intolerance Observation
entry
2.16.840.1.113883.10.20.22.4.7
Allergy Status Observation
entry
2.16.840.1.113883.10.20.22.4.28
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Page 138 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Template Title
Template
Type
templateId
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Chief Complaint and Reason for Visit
Section
section
2.16.840.1.113883.10.20.22.2.13
Chief Complaint Section
section
1.3.6.1.4.1.19376.1.5.3.1.1.13.2.1
Discharge Diet Section
section
1.3.6.1.4.1.19376.1.5.3.1.3.33
Family History Section
section
2.16.840.1.113883.10.20.22.2.15
Family History Organizer
entry
2.16.840.1.113883.10.20.22.4.45
Family History Observation
entry
2.16.840.1.113883.10.20.22.4.46
Age Observation
entry
2.16.840.1.113883.10.20.22.4.31
Family History Death Observation
entry
2.16.840.1.113883.10.20.22.4.47
Functional Status Section
section
2.16.840.1.113883.10.20.22.2.14
Assessment Scale Observation
entry
2.16.840.1.113883.10.20.22.4.69
Caregiver Characteristics
entry
2.16.840.1.113883.10.20.22.4.72
Cognitive Status Problem Observation
entry
2.16.840.1.113883.10.20.22.4.73
Assessment Scale Observation
entry
2.16.840.1.113883.10.20.22.4.69
Caregiver Characteristics
entry
2.16.840.1.113883.10.20.22.4.72
Non-Medicinal Supply Activity
entry
2.16.840.1.113883.10.20.22.4.50
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Cognitive Status Result Observation
entry
2.16.840.1.113883.10.20.22.4.74
Assessment Scale Observation
entry
2.16.840.1.113883.10.20.22.4.69
Caregiver Characteristics
entry
2.16.840.1.113883.10.20.22.4.72
Non-Medicinal Supply Activity
entry
2.16.840.1.113883.10.20.22.4.50
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Cognitive Status Result Organizer
entry
2.16.840.1.113883.10.20.22.4.75
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 139
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Template Title
Template
Type
templateId
Cognitive Status Result Observation
entry
2.16.840.1.113883.10.20.22.4.74
Assessment Scale Observation
entry
2.16.840.1.113883.10.20.22.4.69
Caregiver Characteristics
entry
2.16.840.1.113883.10.20.22.4.72
Non-Medicinal Supply Activity
entry
2.16.840.1.113883.10.20.22.4.50
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Functional Status Problem
Observation
entry
2.16.840.1.113883.10.20.22.4.68
Assessment Scale Observation
entry
2.16.840.1.113883.10.20.22.4.69
Caregiver Characteristics
entry
2.16.840.1.113883.10.20.22.4.72
Non-Medicinal Supply Activity
entry
2.16.840.1.113883.10.20.22.4.50
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Functional Status Result Observation
entry
2.16.840.1.113883.10.20.22.4.67
Assessment Scale Observation
entry
2.16.840.1.113883.10.20.22.4.69
Caregiver Characteristics
entry
2.16.840.1.113883.10.20.22.4.72
Non-Medicinal Supply Activity
entry
2.16.840.1.113883.10.20.22.4.50
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Functional Status Result Organizer
entry
2.16.840.1.113883.10.20.22.4.66
Functional Status Result
Observation
entry
2.16.840.1.113883.10.20.22.4.67
Assessment Scale Observation
entry
2.16.840.1.113883.10.20.22.4.69
Caregiver Characteristics
entry
2.16.840.1.113883.10.20.22.4.72
Non-Medicinal Supply Activity
entry
2.16.840.1.113883.10.20.22.4.50
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Non-Medicinal Supply Activity
entry
2.16.840.1.113883.10.20.22.4.50
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
History of Past Illness Section
section
2.16.840.1.113883.10.20.22.2.20
Problem Observation
entry
2.16.840.1.113883.10.20.22.4.4
Age Observation
entry
2.16.840.1.113883.10.20.22.4.31
Health Status Observation
entry
2.16.840.1.113883.10.20.22.4.5
Problem Status
entry
2.16.840.1.113883.10.20.22.4.6
History of Present Illness Section
section
1.3.6.1.4.1.19376.1.5.3.1.3.4
Hospital Admission Diagnosis Section
section
2.16.840.1.113883.10.20.22.2.43
Hospital Admission Diagnosis
entry
2.16.840.1.113883.10.20.22.4.34
Problem Observation
entry
2.16.840.1.113883.10.20.22.4.4
Age Observation
entry
2.16.840.1.113883.10.20.22.4.31
Health Status Observation
entry
2.16.840.1.113883.10.20.22.4.5
Problem Status
entry
2.16.840.1.113883.10.20.22.4.6
Hospital Admission Medications Section
(entries optional)
section
2.16.840.1.113883.10.20.22.2.44
Admission Medication
entry
2.16.840.1.113883.10.20.22.4.36

Page 140 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Template Title
Template
Type
templateId
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Hospital Consultations Section
section
2.16.840.1.113883.10.20.22.2.42
Hospital Course Section
section
1.3.6.1.4.1.19376.1.5.3.1.3.5
Hospital Discharge Diagnosis Section
section
2.16.840.1.113883.10.20.22.2.24
Hospital Discharge Diagnosis
entry
2.16.840.1.113883.10.20.22.4.33
Problem Observation
entry
2.16.840.1.113883.10.20.22.4.4
Age Observation
entry
2.16.840.1.113883.10.20.22.4.31
Health Status Observation
entry
2.16.840.1.113883.10.20.22.4.5
Problem Status
entry
2.16.840.1.113883.10.20.22.4.6
Hospital Discharge Instructions Section
section
2.16.840.1.113883.10.20.22.2.41
Hospital Discharge Medications Section
(entries optional)
section
2.16.840.1.113883.10.20.22.2.11
Discharge Medication
entry
2.16.840.1.113883.10.20.22.4.35
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 141
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Template Title
Template
Type
templateId
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Hospital Discharge Physical Section
section
1.3.6.1.4.1.19376.1.5.3.1.3.26
Hospital Discharge Studies Summary
Section
section
2.16.840.1.113883.10.20.22.2.16
Immunizations Section (entries
optional)
section
2.16.840.1.113883.10.20.22.2.2
Immunization Activity
entry
2.16.840.1.113883.10.20.22.4.52
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Immunization Refusal Reason
entry
2.16.840.1.113883.10.20.22.4.53
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54

Page 142 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Template Title
Template
Type
templateId
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 143
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Template Title
Template
Type
templateId
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Plan of Care Section
section
2.16.840.1.113883.10.20.22.2.10
Plan of Care Activity Act
entry
2.16.840.1.113883.10.20.22.4.39
Plan of Care Activity Encounter
entry
2.16.840.1.113883.10.20.22.4.40
Plan of Care Activity Observation
entry
2.16.840.1.113883.10.20.22.4.44
Plan of Care Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.41
Plan of Care Activity Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.42
Plan of Care Activity Supply
entry
2.16.840.1.113883.10.20.22.4.43
Problem Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.5
Problem Concern Act (Condition)
entry
2.16.840.1.113883.10.20.22.4.3
Problem Observation
entry
2.16.840.1.113883.10.20.22.4.4
Age Observation
entry
2.16.840.1.113883.10.20.22.4.31
Health Status Observation
entry
2.16.840.1.113883.10.20.22.4.5
Problem Status
entry
2.16.840.1.113883.10.20.22.4.6
Procedures Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.7
Procedure Activity Act
entry
2.16.840.1.113883.10.20.22.4.12
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24

Page 144 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Template Title
Template
Type
templateId
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Procedure Activity Observation
entry
2.16.840.1.113883.10.20.22.4.13
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 145
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Template Title
Template
Type
templateId
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
entry
2.16.840.1.113883.10.20.22.4.25

Page 146 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Template Title
Template
Type
templateId
Administration
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Reason for Visit Section
section
2.16.840.1.113883.10.20.22.2.12
Review of Systems Section
section
1.3.6.1.4.1.19376.1.5.3.1.3.18
Social History Section
section
2.16.840.1.113883.10.20.22.2.17
Pregnancy Observation
entry
2.16.840.1.113883.10.20.15.3.8
Estimated Date of Delivery
entry
2.16.840.1.113883.10.20.15.3.1
Smoking Status Observation
entry
2.16.840.1.113883.10.22.4.78
Social History Observation
entry
2.16.840.1.113883.10.20.22.4.38
Vital Signs Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.4
Vital Signs Organizer
entry
2.16.840.1.113883.10.20.22.4.26
Vital Sign Observation
entry
2.16.840.1.113883.10.20.22.4.27
4.5 History and Physical (H&P) Note
[ClinicalDocument: templateId 2.16.840.1.113883.10.20.22.1.3(open)]
A History and Physical (H&P) Note is a medical report that documents the
current and past conditions of the patient. It contains essential information that
helps determine an individual's health status.
The first portion of the report is a current collection of organized information
unique to an individual, typically supplied by the patient or their caregiver,
about the current medical problem or the reason for the patient encounter. This
information is followed by a description of any past or ongoing medical issues,
including current medications and allergies. Information is also obtained about
the patient's lifestyle, habits, and diseases among family members.
The next portion of the report contains information obtained by physically
examining the patient and gathering diagnostic information in the form of
laboratory tests, imaging, or other diagnostic procedures.
The report ends with the clinician's assessment of the patient's situation and the
intended plan to address those issues.
A History and Physical Examination is required upon hospital admission as well
as before operative procedures. An initial evaluation in an ambulatory setting is
often documented in the form of an H&P Note.

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 147
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
4.5.1 H&P Note Header Constraints
The H&P Note must conform to the US Realm Clinical Document Header. The
following sections include additional header constraints for conformant H&P
Notes.
1. SHALL contain exactly one [1..1] templateId(CONF:9968) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.1.1" (CONF:10045).
4.5.1.1 ClinicalDocument/templateId
Conformant documents must carry the document-level templateId asserting
conformance with specific constraints of a H&P Note as well as the templateId
for the US Realm Clinical Document Header template.
1. SHALL contain exactly one [1..1] templateId (CONF:8283) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.1.3" History and Physical
Note (CONF:10046).
Figure 56: H&P ClinicalDocument/templateId example
<!-- indicates conformance with US Realm Clinical Document Header
template -->
<templateId root="2.16.840.1.113883.10.20.22.1.1"/>
<!-- conforms to a H&P Note -->
<templateId root="2.16.840.1.113883.10.20.22.1.3"/>
4.5.1.2 ClinicalDocument/code
At publication time for this guide, H&P Note limits the
ClinicalDocument/code to those codes shown in the H&P LOINC Document
Type Codes table. Valid codes are those whose scale is DOC and whose type of
service is some variation of History and Physical.
The H&P Note recommends use of a single document type code, 34117-2
"History & Physical", with further specification provided by author or
performer, setting, or specialty. Some codes in the H&P LOINC Document Type
Codes table are pre-coordinated with the practice setting or the training or
professional level of the author. Use of these codes is not recommended, as this
duplicates information potentially present with the header. When pre-
coordinated codes are used, any coded values describing the author or
performer of the service act or the practice setting must be consistent with the
LOINC document type.
2. SHALL contain exactly one [1..1] code (CONF:17185).
a. This code SHALL contain exactly one [1..1] @code, which SHALL be
selected from ValueSet HPDocumentType
2.16.840.1.113883.1.11.20.22 DYNAMIC (CONF:17186).
Page 148 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 31: H&P LOINC Document Type Codes
Value Set: HPDocumentType 2.16.840.1.113883.1.11.20.22 DYNAMIC
Code System: LOINC 2.16.840.1.113883.6.1
LOINC Code
Type of Service
‘Component’
Setting
‘System’
Specialty/Training/
Professional Level
‘Method_Type’
Preferred Code
34117-2
History & Physical
Additional Codes
11492-6
History & Physical
Hospital
28626-0
History & Physical
Physician
34774-0
History & Physical
General surgery
34115-6
History & Physical
Hospital
Medical Student
34116-4
History & Physical
Nursing home
Physician
34095-0
Comprehensive History &
Physical
34096-8
Comprehensive History &
Physical
Nursing home
51849-8
Admission History & Physical
47039-3
Admission History & Physical
Inpatient
34763-3
Admission History & Physical
General medicine
34094-3
Admission History & Physical
Hospital
Cardiology
34138-8
Targeted History & Physical
Figure 57: H&P ClinicalDocument/code example
<code codeSystem='2.16.840.1.113883.6.1'
codeSystemName='LOINC'
code='34117-2'
displayName='HISTORY and PHYSICAL'/>
Figure 58: H&P use of translation to include local equivalents for document type
<code code='34117-2'
displayName='HISTORY and PHYSICAL'
codeSystem='2.16.840.1.113883.6.1'
codeSystemName='LOINC'>
<translation code='X-GISOE'
displayName='GI HISTORY and PHYSICAL'
codeSystem='2.16.840.1.113883.19'/>
</code>
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 149
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
4.5.1.3 Participant
The participant element in the H&P header follows the General Header
Constraints for participants. H&P Note does not specify any use for
functionCode for participants. Local policies will determine how this element
should be used in implementations.
4.5.1.4 InFulfillmentOf
inFulfillmentOf elements describe the prior orders that are fulfilled (in whole
or part) by the service events described in this document. For example, the prior
order might be a referral and the H&P Note may be in partial fulfillment of that
referral.
2. MAY contain zero or more [0..*] inFulfillmentOf (CONF:8336).
a. An inFulfillmentOf element records the prior orders that are fulfilled
(in whole or part) by the service events described in this document.
For example, the prior order might be a referral and this H&P Note
may be in partial fulfillment of that referral. (CONF:8337).
4.5.1.5 ComponentOf
The H&P Note is always associated with an encounter.
The effectiveTime represents the time interval or point in time in which the
encounter took place.
The encounterParticipant elements represent only those participants in the
encounter, not necessarily the entire episode of care.
The responsibleParty element represents only the party responsible for the
encounter, not necessarily the entire episode of care.
3. SHALL contain exactly one [1..1] componentOf (CONF:8338).
a. This componentOf SHALL contain exactly one [1..1]
encompassingEncounter (CONF:8339).
i. This encompassingEncounter SHALL contain exactly one [1..1]
id (CONF:8340).
ii. This encompassingEncounter SHALL contain exactly one [1..1]
effectiveTime (CONF:8341).
1. This effectiveTime SHALL contain exactly one [1..1] US
Realm Date and Time (DT.US.FIELDED)
(2.16.840.1.113883.10.20.22.5.3) (CONF:10135).
iii. This encompassingEncounter MAY contain zero or one [0..1]
location (CONF:8344).
iv. This encompassingEncounter MAY contain zero or one [0..1]
responsibleParty (CONF:8345).
1. The responsibleParty element records only the party
responsible for the encounter, not necessarily the
entire episode of care. (CONF:8347).
2. The responsibleParty element, if present, SHALL
contain an assignedEntity element, which SHALL

Page 150 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
contain an assignedPerson element, a
representedOrganization element, or both.
(CONF:8348).
v. This encompassingEncounter MAY contain zero or more [0..*]
encounterParticipant (CONF:8342).
1. The encounterParticipant element, if present, records
only participants in the encounter, not necessarily in
the entire episode of care. (CONF:8346).
2. An encounterParticipant element, if present, SHALL
contain an assignedEntity element, which SHALL
contain an assignedPerson element, a
representedOrganization element, or both.
(CONF:8343).
Figure 59: H&P componentOf example
<componentOf>
<encompassingEncounter>
<id extension='9937012' root='2.16.840.1.113883.19'/>
<code codeSystem='2.16.840.1.113883.6.12' codeSystemName='CPT-4'
code='99213' displayName='Evaluation and Management'/>
<effectiveTime>
<low value='20050329'/>
<high value='20050329'/>
</effectiveTime>
</encompassingEncounter>
</componentOf>
4.5.2 H&P Note Body Constraints
The H&P Note supports both narrative sections and sections requiring code
clinical statements. The required and optional sections are listed in the
Document Types and Required/Optional Sections table. The table below the
constraints shows all templates including entries within each section.
1. SHALL contain exactly one [1..1] component (CONF:8349).
a. A History and Physical document can have either a structuredBody
or a nonXMLBody. (CONF:8350).
i. A History and Physical document can conform to CDA Level 1
(nonXMLBody), CDA Level 2 (structuredBody with sections
that contain a narrative block), or CDA Level 3
(structuredBody containing sections that contain a narrative
block and coded entries). In this template (templateId
2.16.840.1.113883.10.20.22.1.3), coded entries are optional.
(CONF:8352).
b. If structuredBody, the component/structuredBody SHALL conform to
the section constraints below (CONF:9597).
i. This section SHALL contain exactly one [1..1] Allergies
Section (entries optional)

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 151
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
(templateId:2.16.840.1.113883.10.20.22.2.6)
(CONF:9602).
ii. SHALL include an Assessment and Plan Section, or an
Assessment Section and a Plan Section. (CONF:9986).
iii. SHALL NOT include an Assessment/Plan Section when an
Assessment Section and a Plan of Care Section are present.
(CONF:10056)
iv. MAY contain zero or one [0..1] Assessment Section
(templateId:2.16.840.1.113883.10.20.22.2.8)
(CONF:9605).
v. MAY contain zero or one [0..1] Plan of Care Section
(templateId:2.16.840.1.113883.10.20.22.2.10)
(CONF:9607).
vi. MAY contain zero or one [0..1] Assessment and Plan
Section (templateId:2.16.840.1.113883.10.20.22.2.9)
(CONF:9987).
vii. SHALL include a Chief Complaint and Reason for Visit Section,
Chief Complaint Section, or a Reason for Visit Section.
(CONF:9642).
viii. SHALL NOT include a Chief Complaint and Reason for Visit
Section with either a Chief Complaint Section or a Reason for
Visit Section. (CONF:10057)
ix. MAY contain zero or one [0..1] Chief Complaint Section
(templateId:1.3.6.1.4.1.19376.1.5.3.1.1.13.2.1)
(CONF:9611).
x. MAY contain zero or one [0..1] Chief Complaint and Reason
for Visit Section
(templateId:2.16.840.1.113883.10.20.22.2.13)
(CONF:9613).
xi. SHALL contain exactly one [1..1] Family History Section
(templateId:2.16.840.1.113883.10.20.22.2.15)
(CONF:9615).
xii. SHALL contain exactly one [1..1] General Status Section
(templateId:2.16.840.1.113883.10.20.2.5)
(CONF:9617).
xiii. SHALL contain exactly one [1..1] History of Past Illness
Section
(templateId:2.16.840.1.113883.10.20.22.2.20)
(CONF:9619).
xiv. SHALL contain exactly one [1..1] Medications Section
(entries optional)
(templateId:2.16.840.1.113883.10.20.22.2.1)
(CONF:9623).
xv. SHALL contain exactly one [1..1] Physical Exam Section
(templateId:2.16.840.1.113883.10.20.2.10)
(CONF:9625).
Page 152 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
xvi. SHALL contain exactly one [1..1] Reason for Visit Section
(templateId:2.16.840.1.113883.10.20.22.2.12)
(CONF:9627).
xvii. SHALL contain exactly one [1..1] Results Section (entries
optional)
(templateId:2.16.840.1.113883.10.20.22.2.3)
(CONF:9629).
xviii. SHALL contain exactly one [1..1] Review of Systems
Section (templateId:1.3.6.1.4.1.19376.1.5.3.1.3.18)
(CONF:9631).
xix. SHALL contain exactly one [1..1] Social History Section
(templateId:2.16.840.1.113883.10.20.22.2.17)
(CONF:9633).
xx. SHALL contain exactly one [1..1] Vital Signs Section
(entries optional)
(templateId:2.16.840.1.113883.10.20.22.2.4)
(CONF:9635).
xxi. SHOULD contain exactly one [1..1] History of Present
Illness Section (templateId:
1.3.6.1.4.1.19376.1.5.3.1.3.4) (CONF:9621).
xxii. MAY contain zero or one [0..1] Immunizations Section
(entries optional)
(templateId:2.16.840.1.113883.10.20.22.2.2)
(CONF:9637).
xxiii. MAY contain zero or one [0..1] Instructions Section
(templateId:2.16.840.1.113883.10.20.22.2.45)
(CONF:16807).
xxiv. MAY contain zero or one [0..1] Problem Section (entries
optional)
(templateId:2.16.840.1.113883.10.20.22.2.5)
(CONF:9639).
xxv. MAY contain zero or one [0..1] Procedures Section
(entries optional)
(templateId:2.16.840.1.113883.10.20.22.2.7)
(CONF:9641).
The following table shows relationships among the templates in the body of an
H&P Note.
Table 32: Template Containment for an H&P Note
Template Title
Template
Type
templateId
History and Physical
document
2.16.840.1.113883.10.20.22.1.3
Allergies Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.6
Allergy Problem Act
entry
2.16.840.1.113883.10.20.22.4.30
Allergy - Intolerance Observation
entry
2.16.840.1.113883.10.20.22.4.7
Allergy Status Observation
entry
2.16.840.1.113883.10.20.22.4.28

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 153
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Template Title
Template
Type
templateId
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
entry
2.16.840.1.113883.10.20.22.4.54
Page 154 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Template Title
Template
Type
templateId
Information
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Assessment and Plan Section
section
2.16.840.1.113883.10.20.22.2.9
Plan of Care Activity Act
entry
2.16.840.1.113883.10.20.22.4.39
Assessment Section
section
2.16.840.1.113883.10.20.22.2.8
Chief Complaint and Reason for Visit
Section
section
2.16.840.1.113883.10.20.22.2.13
Chief Complaint Section
section
1.3.6.1.4.1.19376.1.5.3.1.1.13.2.1
Family History Section
section
2.16.840.1.113883.10.20.22.2.15
Family History Organizer
entry
2.16.840.1.113883.10.20.22.4.45
Family History Observation
entry
2.16.840.1.113883.10.20.22.4.46
Age Observation
entry
2.16.840.1.113883.10.20.22.4.31
Family History Death Observation
entry
2.16.840.1.113883.10.20.22.4.47
General Status Section
section
2.16.840.1.113883.10.20.2.5
History of Past Illness Section
section
2.16.840.1.113883.10.20.22.2.20
Problem Observation
entry
2.16.840.1.113883.10.20.22.4.4
Age Observation
entry
2.16.840.1.113883.10.20.22.4.31
Health Status Observation
entry
2.16.840.1.113883.10.20.22.4.5
Problem Status
entry
2.16.840.1.113883.10.20.22.4.6
History of Present Illness Section
section
1.3.6.1.4.1.19376.1.5.3.1.3.4
Immunizations Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.2
Immunization Activity
entry
2.16.840.1.113883.10.20.22.4.52
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Immunization Refusal Reason
entry
2.16.840.1.113883.10.20.22.4.53
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 155
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Template Title
Template
Type
templateId
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Page 156 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Template Title
Template
Type
templateId
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Medications Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.1
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 157
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Template Title
Template
Type
templateId
Physical Exam Section
section
2.16.840.1.113883.10.20.2.10
Plan of Care Section
section
2.16.840.1.113883.10.20.22.2.10
Plan of Care Activity Act
entry
2.16.840.1.113883.10.20.22.4.39
Plan of Care Activity Encounter
entry
2.16.840.1.113883.10.20.22.4.40
Plan of Care Activity Observation
entry
2.16.840.1.113883.10.20.22.4.44
Plan of Care Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.41
Plan of Care Activity Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.42
Plan of Care Activity Supply
entry
2.16.840.1.113883.10.20.22.4.43
Problem Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.5
Problem Concern Act (Condition)
entry
2.16.840.1.113883.10.20.22.4.3
Problem Observation
entry
2.16.840.1.113883.10.20.22.4.4
Age Observation
entry
2.16.840.1.113883.10.20.22.4.31
Health Status Observation
entry
2.16.840.1.113883.10.20.22.4.5
Problem Status
entry
2.16.840.1.113883.10.20.22.4.6
Procedures Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.7
Procedure Activity Act
entry
2.16.840.1.113883.10.20.22.4.12
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Page 158 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Template Title
Template
Type
templateId
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Procedure Activity Observation
entry
2.16.840.1.113883.10.20.22.4.13
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 159
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Template Title
Template
Type
templateId
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Reason for Visit Section
section
2.16.840.1.113883.10.20.22.2.12
Results Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.3
Result Organizer
entry
2.16.840.1.113883.10.20.22.4.1
Result Observation
entry
2.16.840.1.113883.10.20.22.4.2
Review of Systems Section
section
1.3.6.1.4.1.19376.1.5.3.1.3.18
Social History Section
section
2.16.840.1.113883.10.20.22.2.17
Pregnancy Observation
entry
2.16.840.1.113883.10.20.15.3.8
Estimated Date of Delivery
entry
2.16.840.1.113883.10.20.15.3.1
Smoking Status Observation
entry
2.16.840.1.113883.10.22.4.78
Social History Observation
entry
2.16.840.1.113883.10.20.22.4.38
Vital Signs Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.4
Vital Signs Organizer
entry
2.16.840.1.113883.10.20.22.4.26
Vital Sign Observation
entry
2.16.840.1.113883.10.20.22.4.27
Page 160 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
4.6 Operative Note
[ClinicalDocument: templateId 2.16.840.1.113883.10.20.22.1.7(open)]
The Operative Note is a frequently used type of procedure note with specific
requirements set forth by regulatory agencies.
The Operative Note is created immediately following a surgical procedure and
records the pre- and post-surgical diagnosis, pertinent events of the procedure,
as well as the condition of the patient following the procedure. The report should
be sufficiently detailed to support the diagnoses, justify the treatment, document
the course of the procedure, and provide continuity of care.24
4.6.1 Operative Note Header Constraints
The Operative Note must conform to the US Realm Clinical Document Header.
The following sections include additional header constraints for conformant
Operative Notes.
1. SHALL contain exactly one [1..1] templateId (CONF:9914) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.1.1" (CONF:10047).
4.6.1.1 ClinicalDocument/templateId
Conformant documents must carry the document-level templateId asserting
conformance with specific constraints of an Operative Note as well as the
templateId for the US Realm Clinical Document Header template.
The following asserts conformance to an Operative Note.
2. SHALL contain exactly one [1..1] templateId (CONF:8483) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.1.7" (CONF:10048).
Figure 60: Operative note ClinicalDocument/templateId example
<!-- indicates conformance with US Realm Clinical Document Header
template -->
<templateId root="2.16.840.1.113883.10.20.22.1.1"/>
<!-- conforms to the Operative Note requirements -->
<templateId root='2.16.840.1.113883.10.20.22.1.7'/>
4.6.1.2 ClinicalDocument/code
The Surgical Operation Note LOINC Document Codes table shows the LOINC
codes suitable for Discharge Summary, as of publication of this implementation
guide. This is a dynamic value set meaning that these codes may be added to or
deprecated by LOINC.
24
http://www.jointcommission.org/mobile/standards_information/jcfaqdetails.aspx?Stan
dardsFAQId=215&StandardsFAQChapterId=13
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 161
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
The Operative Note recommends use of a single document type code, 11504-8
"Surgical Operation Note", with further specification provided by author or
performer, setting, or specialty. Some of the LOINC codes in the Surgical
Operation Note LOINC Document Codes table are pre-coordinated with the
practice setting or the training or professional level of the author. Use of pre-
coordinated codes is not recommended because of potential conflict with other
information in the header. When these codes are used, any coded values
describing the author or performer of the service act or the practice setting must
be consistent with the LOINC document type.
3. SHALL contain exactly one [1..1] code (CONF:17187).
a. This code SHALL contain exactly one [1..1] @code, which SHALL be
selected from ValueSet SurgicalOperationNoteDocumentTypeCode
2.16.840.1.113883.11.20.1.1 DYNAMIC (CONF:17188).
Table 33: Surgical Operation Note LOINC Document Codes
Value Set: SurgicalOperationNoteDocumentTypeCode 2.16.840.1.113883.11.20.1.1
DYNAMIC
Code System: LOINC 2.16.840.1.113883.6.1
LOINC
Code
Type of Service
‘Component’
Setting
‘System’
Specialty/Training/Professional
Level ‘Method_Type’
Preferred Code
11504-8
Surgical operation
note
{Setting}
{Provider}
Additional Codes
34137-0
Surgical operation
note
Outpatient
{Provider}
28583-3
Surgical operation
note
{Setting}
Dentistry
28624-5
Surgical operation
note
{Setting}
Podiatry
28573-4
Surgical operation
note
{Setting}
Physician
34877-1
Surgical operation
note
{Setting}
Urology
34874-8
Surgical operation
note
{Setting}
Surgery
34870-6
Surgical operation
note
{Setting}
Plastic surgery
34868-0
Surgical operation
note
{Setting}
Orthopedics
34818-5
Surgical operation
note
{Setting}
Otorhinolaryngology
The following code should not be used; it is a duplicate
34871-4
Surgical operation
note
{Setting}
Podiatry

Page 162 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 61: Operative note ClinicalDocument/code example
<code codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"code="11504-8"
displayName="SURGICAL OPERATION NOTE"/>
4.6.1.3 DocumentationOf
A serviceEvent represents the main act, such as a colonoscopy or an
appendectomy, being documented. A serviceEvent can further specialize the
act inherent in the ClinicalDocument/code, such as where the
ClinicalDocument/code is simply "Surgical Operation Note" and the procedure
is "Appendectomy." serviceEvent is required in the Operative Note and it must
be equivalent to or further specialize the value inherent in the
ClinicalDocument/code; it shall not conflict with the value inherent in the
ClinicalDocument/code, as such a conflict would create ambiguity.
serviceEvent/effectiveTime can be used to indicate the time the actual
event (as opposed to the encounter surrounding the event) took place.
If the date and the duration of the procedure is known,
serviceEvent/effectiveTime/low is used with a width element that
describes the duration; no high element is used. However, if only the date is
known, the date is placed in both the low and high elements.
4. SHALL contain at least one [1..*] documentationOf (CONF:8486).
a. Such documentationOf SHALL contain exactly one [1..1]
serviceEvent (CONF:8493).
i. The value of Clinical Document
/documentationOf/serviceEvent/code SHALL be from ICD9
CM Procedures (CodeSystem 2.16.840.1.113883.6.104), CPT-
4 (CodeSystem 2.16.840.1.113883.6.12), or values
descending from 71388002 (Procedure) from the SNOMED
CT (CodeSystem 2.16.840.1.113883.6.96) ValueSet
Procedure 2.16.840.1.113883.3.88.12.80.28 DYNAMIC.
(CONF:8487).
ii. This serviceEvent SHALL contain exactly one [1..1]
effectiveTime (CONF:8494).
1. This effectiveTime SHALL contain exactly one [1..1] US
Realm Date and Time (DT.US.FIELDED)
(2.16.840.1.113883.10.20.22.5.3)
(CONF:10136).
2. The serviceEvent/effectiveTime SHALL be present with
effectiveTime/low (CONF:8488).
3. If a width is not present, the
serviceEvent/effectiveTime SHALL include
effectiveTime/high. (CONF:10058)
4. When only the date and the length of the procedure
are known a width element SHALL be present and the

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 163
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
serviceEvent/effectiveTime/high SHALL not be
present. (CONF:10060).
The performer represents clinicians who actually and principally carry out the
serviceEvent. Typically, these are clinicians who have surgical privileges in
their institutions such as Surgeons, Obstetrician/Gynecologists, and Family
Practice Physicians. The performer may also be Nonphysician Providers (NPP)
who have surgical privileges. There may be more than one primary performer in
the case of complicated surgeries. There are occasionally co-surgeons. Usually
they will be billing separately and will each dictate their own notes. An example
may be spinal surgery , where a general surgeon and an orthopedic surgeon
both are present and billing off the same Current Procedural Terminology (CPT)
codes. Typically two Operative Notes are generated; however, each will list the
other as a co-surgeon.
iii. This serviceEvent SHALL contain exactly one [1..1] performer
(CONF:8489) such that it
1. SHALL contain exactly one [1..1] @typeCode="PPRF"
Primary performer (CodeSystem:
HL7ParticipationType 2.16.840.1.113883.5.90)
(CONF:8495).
2. SHALL contain exactly one [1..1] assignedEntity
(CONF:10917).
a. This assignedEntity SHALL contain exactly one
[1..1] code with @xsi:type="CE" (CONF:8490).
i. This code SHOULD contain exactly one
[1..1] @code, which SHOULD be selected
from ValueSet Provider Type
2.16.840.1.113883.3.88.12.3221.4
DYNAMIC (CONF:8491).
b. Any assistants SHALL be identified and SHALL be identified as
secondary performers (SPRF). (CONF:8512).
Page 164 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 34: Provider Type Value Set (excerpt)
Value Set: Provider Type 2.16.840.1.113883.3.88.12.3221.4 DYNAMIC
Code System(s):
NUCC Health Care Provider Taxonomy 2.16.840.1.113883.6.101
Description:
The Provider type vocabulary classifies providers according to the type of
license or accreditation they hold or the service they provide.
http://www.nucc.org/index.php?option=com_content&task=view
&id=14&Itemid=40
Code
Code System
Print Name
207L00000X
NUCC Health Care Provider
Taxonomy
Anesthesiology
207X00000X
NUCC Health Care Provider
Taxonomy
Orthopedic Surgery
207VG0400X
NUCC Health Care Provider
Taxonomy
Gynecology
…
Table 35: Procedure Codes from SNOMED CT
Value Set: Procedure 2.16.840.1.113883.3.88.12.80.28 DYNAMIC
Code System(s):
SNOMED CT 2.16.840.1.113883.6.96
Description:
SNOMED CT Procedure codes. Any code descending from 71388002
(Procedure) inclusive.
https://uts.nlm.nih.gov/snomedctBrowser.html (requires sign-up)
Code
Code System
Print Name
408816000
SNOMED CT
Artificial rupture of membranes
20050329
SNOMED CT
Laparoscopic Appendectomy
62013009
SNOMED CT
Ambulating patient
…
Figure 62: Operative note serviceEvent example
<serviceEvent classCode="PROC">
<code code="801460020"
codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT"
displayName="Laparoscopic Appendectomy"/>
<effectiveTime>
<low value="201003292240"/>
<width value="15" unit="m"/>
</effectiveTime>
...
</serviceEvent>

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 165
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 63: Operative note performer example
<performer typeCode="PPRF">
<assignedEntity>
<id extension="1" root="2.16.840.1.113883.19"/>
<code code="2086S0120X" codeSystem="2.16.840.1.113883.6.101"
codeSystemName="NUCC" displayName="Pediatric Surgeon"/>
<addr>
<streetAddressLine>1013 Healthcare Drive</streetAddressLine>
<city>Ann Arbor</city>
<state>MI</state>
<postalCode>99999</postalCode>
<country>US</country>
</addr>
<telecom value="tel:(555)555-1013"/>
<assignedPerson>
<name>
<prefix>Dr.</prefix>
<given>Carl</given>
<family>Cutter</family>
</name>
</assignedPerson>
</assignedEntity>
</performer>
4.6.2 Operative Note Body Constraints
The Operative Note supports both narrative sections and sections requiring code
clinical statements. The required and optional sections are listed in the
Document Types and Required/Optional Sections table. The table below the
constraints shows all templates including entries within each section.
1. SHALL contain exactly one [1..1] component (CONF:9585).
a. An Operative Note can have either a structuredBody or a
nonXMLBody (CONF:9586).
i. An Operative Note can conform to CDA Level 1 (nonXMLBody),
CDA Level 2 (structuredBody with sections that contain a
narrative block), or CDA Level 3 (structuredBody containing
sections that contain a narrative block and coded entries). In
this template (templateId 2.16.840.1.113883.10.20.22.1.7),
coded entries are optional. (CONF:9587).
b. If structuredBody, the component/structuredBody SHALL conform to
the section constraints below (CONF:9596).
i. SHALL contain exactly one [1..1] Anesthesia Section
(2.16.840.1.113883.10.20.22.2.25) (CONF:9883).
ii. SHALL contain exactly one [1..1] Complications Section
(2.16.840.1.113883.10.20.22.2.37) (CONF:9885).
iii. SHALL contain exactly one [1..1] Postoperative Diagnosis
Section (2.16.840.1.113883.10.20.22.2.35)
(CONF:9913).
Page 166 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
iv. SHALL contain exactly one [1..1] Preoperative Diagnosis
Section (2.16.840.1.113883.10.20.22.2.34)
(CONF:9888).
v. SHALL contain exactly one [1..1] Procedure Estimated
Blood Loss Section
(2.16.840.1.113883.10.20.18.2.9) (CONF:9890).
vi. SHALL contain exactly one [1..1] Procedure Findings
Section (2.16.840.1.113883.10.20.22.2.28)
(CONF:9892).
vii. SHALL contain exactly one [1..1] Procedure Specimens
Taken Section (2.16.840.1.113883.10.20.22.2.31)
(CONF:9894).
viii. SHALL contain exactly one [1..1] Procedure Description
Section (2.16.840.1.113883.10.20.22.2.27)
(CONF:9896).
ix. MAY contain zero or one [0..1] Procedure Implants Section
(2.16.840.1.113883.10.20.22.2.40) (CONF:9898).
x. MAY contain zero or one [0..1] Operative Note Fluids
Section (2.16.840.1.113883.10.20.7.12) (CONF:9900).
xi. MAY contain zero or one [0..1] Operative Note Surgical
Procedure Section (2.16.840.1.113883.10.20.7.14)
(CONF:9902).
xii. MAY contain zero or one [0..1] Plan of Care Section
(2.16.840.1.113883.10.20.22.2.10) (CONF:9904).
xiii. MAY contain zero or one [0..1] Planned Procedure Section
(2.16.840.1.113883.10.20.22.2.30) (CONF:9906).
xiv. MAY contain zero or one [0..1] Procedure Disposition
Section (2.16.840.1.113883.10.20.18.2.12)
(CONF:9908).
xv. MAY contain zero or one [0..1] Procedure Indications
Section (2.16.840.1.113883.10.20.22.2.29)
(CONF:9910).
xvi. MAY contain zero or one [0..1] Surgical Drains Section
(2.16.840.1.113883.10.20.7.13) (CONF:9912).
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 167
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
The following table shows relationships among the templates in the body of an
Operative Note.
Table 36: Template Containment for an Operative Note
Template Title
Template
Type
templateId
Operative Note
document
2.16.840.1.113883.10.20.22.1.7
Anesthesia Section
section
2.16.840.1.113883.10.20.22.2.25
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Page 168 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Template Title
Template
Type
templateId
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Complications Section
section
2.16.840.1.113883.10.20.22.2.37
Problem Observation
entry
2.16.840.1.113883.10.20.22.4.4
Age Observation
entry
2.16.840.1.113883.10.20.22.4.31
Health Status Observation
entry
2.16.840.1.113883.10.20.22.4.5
Problem Status
entry
2.16.840.1.113883.10.20.22.4.6
Operative Note Fluids Section
section
2.16.840.1.113883.10.20.7.12
Operative Note Surgical Procedure Section
section
2.16.840.1.113883.10.20.7.14
Plan of Care Section
section
2.16.840.1.113883.10.20.22.2.10
Plan of Care Activity Act
entry
2.16.840.1.113883.10.20.22.4.39
Plan of Care Activity Encounter
entry
2.16.840.1.113883.10.20.22.4.40
Plan of Care Activity Observation
entry
2.16.840.1.113883.10.20.22.4.44
Plan of Care Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.41
Plan of Care Activity Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.42
Plan of Care Activity Supply
entry
2.16.840.1.113883.10.20.22.4.43
Planned Procedure Section
section
2.16.840.1.113883.10.20.22.2.30
Plan of Care Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.41
Postoperative Diagnosis Section
section
2.16.840.1.113883.10.20.22.2.35
Preoperative Diagnosis Section
section
2.16.840.1.113883.10.20.22.2.34
Preoperative Diagnosis
entry
2.16.840.1.113883.10.20.22.4.65
Problem Observation
entry
2.16.840.1.113883.10.20.22.4.4
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 169
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Template Title
Template
Type
templateId
Age Observation
entry
2.16.840.1.113883.10.20.22.4.31
Health Status Observation
entry
2.16.840.1.113883.10.20.22.4.5
Problem Status
entry
2.16.840.1.113883.10.20.22.4.6
Procedure Description Section
section
2.16.840.1.113883.10.20.22.2.27
Procedure Disposition Section
section
2.16.840.1.113883.10.20.18.2.12
Procedure Estimated Blood Loss Section
section
2.16.840.1.113883.10.20.18.2.9
Procedure Findings Section
section
2.16.840.1.113883.10.20.22.2.28
Problem Observation
entry
2.16.840.1.113883.10.20.22.4.4
Age Observation
entry
2.16.840.1.113883.10.20.22.4.31
Health Status Observation
entry
2.16.840.1.113883.10.20.22.4.5
Problem Status
entry
2.16.840.1.113883.10.20.22.4.6
Procedure Implants Section
section
2.16.840.1.113883.10.20.22.2.40
Procedure Indications Section
section
2.16.840.1.113883.10.20.22.2.29
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Procedure Specimens Taken Section
section
2.16.840.1.113883.10.20.22.2.31
Surgical Drains Section
section
2.16.840.1.113883.10.20.7.13
4.7 Procedure Note
[ClinicalDocument: templateId 2.16.840.1.113883.10.20.22.1.6(open)]
Procedure Note is a broad term that encompasses many specific types of non-
operative procedures including interventional cardiology, interventional
radiology, gastrointestinal endoscopy, osteopathic manipulation, and many
other specialty fields. Procedure Notes are documents that are differentiated
from Operative Notes in that the procedures documented do not involve incision
or excision as the primary act.
The Procedure Note is created immediately following a non-operative procedure
and records the indications for the procedure and, when applicable, post-
procedure diagnosis, pertinent events of the procedure, and the patient’s
tolerance of the procedure. The document should be sufficiently detailed to
justify the procedure, describe the course of the procedure, and provide
continuity of care.
4.7.1 Procedure Note Header Constraints
The Procedure Note must conform to the US Realm Clinical Document Header.
The following sections include additional header constraints for conformant
Procedure Notes
1. SHALL contain exactly one [1..1] templateId/ (CONF:9969) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.1.1" (CONF:10049).
Page 170 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
4.7.1.1 ClinicalDocument/templateId
Conformant documents must carry the document-level templateId asserting
conformance with specific constraints of a Procedure Note as well as the
templateId for the US Realm Clinical Document Header template.
2. SHALL contain exactly one [1..1] templateId (CONF:8496) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.1.6" (CONF:10050).
Figure 64: Procedure note ClinicalDocument/templateId category I example
<!-- indicates conformance with US Realm Clinical Document Header
template -->
<templateId root="2.16.840.1.113883.10.20.22.1.1"/>
<templateId root= "2.16.840.1.113883.10.20.22.1.6"/>
<!-- conforms to the Procedure Note constraints -->
4.7.1.2 ClinicalDocument/code
The Procedure Note limits document type codes to those codes listed in the
LOINC Codes for Procedure Note Documents. The tables lists all codes having
the scale DOC (document) and a ‘component’ referring to a non-operative
procedure, whether or not the text string "Procedure" is present.
The Procedure Note recommends use of a single document type code, 28570-0
"Procedure Note", with further specification provided by author or performer,
setting, or specialty. Some of the LOINC codes in the LOINC Codes for Procedure
Note Documents table are pre-coordinated with the practice setting or the
training or professional level of the author. Use of pre-coordinated codes is not
recommended because of potential conflict with other information in the header.
When these codes are used, any coded values describing the author or
performer of the service act or the practice setting must be consistent with the
LOINC document type.
3. SHALL contain exactly one [1..1] code (CONF:17182).
a. This code SHALL contain exactly one [1..1] @code, which SHALL be
selected from ValueSet ProcedureNoteDocumentTypeCodes
2.16.840.1.113883.11.20.6.1 DYNAMIC (CONF:17183).
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 171
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 37: Procedure Note LOINC Document Type Codes
Value Set: ProcedureNoteDocumentTypeCodes 2.16.840.1.113883.11.20.6.1 DYNAMIC
Code System: LOINC 2.16.840.1.113883.6.1
LOINC
Code
Type of Service
‘Component’
Setting
‘System’
Specialty/Training/Professional Level
‘Method_Type’
Preferred Code
28570-0
Procedure note
{Setting}
{Provider}
Additional Codes
11505-5
Procedure note
{Setting}
Physician
18744-3
Study report
Respiratory
system
Bronchoscopy
18745-0
Study report
Heart
Cardiac catheterization
18746-8
Study report
Lower GI
tract
Colonoscopy
18751-8
Study report
Upper GI
tract
Endoscopy
18753-4
Study report
Lower GI
tract
Flexible sigmoidoscopy
18836-7
Procedure
Cardiac
stress study
*
28577-5
Procedure note
{Setting}
Dentistry
28625-2
Procedure note
{Setting}
Podiatry
29757-2
Study report
Cvx/Vag
Colposcopy
33721-2
Bone marrow biopsy
report
Bone mar
34121-4
Interventional
procedure note
{Setting}
34896-1
Interventional
procedure note
{Setting}
Cardiology
34899-5
Interventional
procedure note
{Setting}
Gastroenterology
47048-4
Diagnostic
interventional study
report
{Setting}
Interventional radiology
48807-2
Bone marrow
aspiration report
Bone mar
Figure 65: Procedure note ClinicalDocument/code example
<code codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
code="28570-0"
displayName="PROCEDURE NOTE"/>
Page 172 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
4.7.1.3 ComponentOf
4. SHOULD contain zero or one [0..1] componentOf/encompassingEncounter
(CONF:8499).
a. This componentOf/encompassingEncounter SHALL contain exactly
one [1..1] code (CONF:8501).
b. This componentOf/encompassingEncounter SHALL contain at least
one [1..*] location/healthCareFacility/id (CONF:8500).
c. This componentOf/encompassingEncounter MAY contain zero or one
[0..1] encounterParticipant (CONF:8502) such that it
i. SHALL contain exactly one [1..1] @typeCode="REF" Referrer
(CodeSystem: HL7ParticipationType
2.16.840.1.113883.5.90) (CONF:8503).
4.7.1.4 Generic Participant: Primary Care Provider
The participant element in the Procedure Note header follows the General
Header Constraints for participants. The Participant Scenarios table shows a
number of scenarios and the values for various participants.
5. MAY contain zero or more [0..*] participant (CONF:8504) such that it
a. SHALL contain exactly one [1..1] @typeCode="IND" Individual
(CodeSystem: HL7ParticipationType 2.16.840.1.113883.5.90)
(CONF:8505).
b. SHALL contain exactly one [1..1] functionCode="PCP" Primary Care
Physician (CodeSystem: participationFunction
2.16.840.1.113883.5.88) (CONF:8506).
c. SHALL contain exactly one [1..1]
associatedEntity/@classCode="PROV" Provider (CodeSystem:
HL7ParticipationType 2.16.840.1.113883.5.90) (CONF:8507).
i. This associatedEntity/@classCode SHALL contain exactly one
[1..1] associatedPerson (CONF:8508).
4.7.1.5 Participant Scenarios
Table 38: Participant Scenario
Scenario
Author
Custo-
dian
Data
Enterer
Encom-
passing
Encounter/
Encounter
Participant
Legal
Authen-
ticator
Parti-
cipant
Service
Event/
Performer
Colonoscopy Participant Scenario: A surgeon refers a patient to an endoscopist. A colonoscopy is
performed at an outpatient surgery center. The endoscopist inputs information into an EHR. The
outpatient surgery center EHR generates a Procedure Note to send to the Hospital EHR.
Endo-
scopic
CDA
Procedure
Note
Endo-
scopist
Out-
patient
surgery
center
None
Surgeon
[REF
(referrer)]
Endo-
scopist
None
Endoscopis
t
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 173
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Scenario
Author
Custo-
dian
Data
Enterer
Encom-
passing
Encounter/
Encounter
Participant
Legal
Authen-
ticator
Parti-
cipant
Service
Event/
Performer
Office Removal of Wart Participation Scenario: A wart is removed during an office visit. The PCP
dictates the procedure into the local transcription system. The transcription system generates a CDA
Procedure Note to the EHR.
CDA
Procedure
Note
PCP
PCP
office
Transcrip-
tionist
None
PCP
None
PCP
Dental Procedure Participation Scenario: Dentist extracts a tooth after the patient has a cleaning by
the hygenist. He enters the information into his Dental EHR.
Procedure
input to
EHR
Dentist
Dentist
office
Varies
None
Dentist
None
Dentist
Hygenist
Transjugular Intrahepatic Portosystemic Shunt (TIPS) Procedure (Interventional Radiology)
Participant Scenario: At a university hospital, a TIPS procedure is performed by the interventional
radiology fellow, with the help of an interventional radiology nurse, under the supervision of an
attending interventional radiologist. The radiology technician enters the data into the EMR. The patient
was referred to the university hospital by his oncologist. The patient is insured by Cigna.
Procedure
Note is
input in
EHR
Interven-
tional
radiology
fellow
Good
Health
Hospita
l
Interven-
tional
radiology
technician
REF
(referrer)
Oncologist
Attending
interven-
tional
radiolo-
gist
Cigna
Interven-
tional
radiology
fellow
Nurse
Attending
interven-
tional
radiologist
Lumbar Puncture (spinal tap) Procedure Participant Scenario: At a university hospital, a lumbar
puncture is performed by a medical student, with the help of an intern, under the supervisory authority
of an attending neurologist. The student performs the procedure and dictates the note. The note is
signed by the intern and attending. The patient has a family doctor that is not participating in the
procedure, did not refer the patient, and does not have privileges at the providing organization but is
recorded in the note.
Procedure
Note is
dictated
by the
medical
student
Medical
student
Good
Health
Hospita
l
Transcrip-
tionist
None
Neurology
attending
(Intern is
authen-
ticator)
Family
doctor
Medical
student
Intern
4.7.1.6 ServiceEvent
A serviceEvent is required in the Procedure Note to represent the main act,
such as a colonoscopy or a cardiac stress study, being documented. It must be
equivalent to or further specialize the value inherent in the
ClinicalDocument/@code (such as where the ClinicalDocument/@code is
simply "Procedure Note" and the procedure is "colonoscopy"), and it shall not

Page 174 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
conflict with the value inherent in the ClinicalDocument/@code, as such a
conflict would create ambiguity. A serviceEvent/effectiveTime element
indicates the time the actual event (as opposed to the encounter surrounding
the event) took place.
serviceEvent/effectiveTime may be represented two different ways in the
Procedure Note. For accuracy to the second, the best method is
effectiveTime/low together with effectiveTime/high. If a more general
time, such as minutes or hours, is acceptable OR if the duration is unknown, an
effectiveTime/low with a width element may be used. If the duration is
unknown, the appropriate HL7 null value such as "NI" or "NA" must be used for
the width element.
6. SHALL contain at least one [1..*] documentationOf (CONF:8510).
a. Such documentationOf SHALL contain exactly one [1..1]
serviceEvent (CONF:10061).
i. The value of Clinical Document
/documentationOf/serviceEvent/code SHALL be from ICD9 CM
Procedures (codeSystem 2.16.840.1.113883.6.104), CPT-4
(codeSystem 2.16.840.1.113883.6.12), or values descending
from 71388002 (Procedure) from the SNOMED CT
(codeSystem 2.16.840.1.113883.6.96) ValueSet Procedure
2.16.840.1.113883.3.88.12.80.28 DYNAMIC. (CONF:8511).
ii. This serviceEvent SHALL contain exactly one [1..1]
effectiveTime (CONF:10062).
1. This effectiveTime SHALL contain exactly one [1..1] US
Realm Date and Time (DT.US.FIELDED)
(2.16.840.1.113883.10.20.22.5.3) (CONF:10063)
2. The serviceEvent/effectiveTime SHALL be present with
effectiveTime/low (CONF:8513).
3. If a width is not present, the
serviceEvent/effectiveTime SHALL include
effectiveTime/high. (CONF:8514)
4. When only the date and the length of the procedure
are known a width element SHALL be present and the
serviceEvent/effectiveTime/high SHALL not be present.
(CONF:8515).
The performer participant represents clinicians who actually and principally
carry out the serviceEvent. Typically, these are clinicians who have the
appropriate privileges in their institutions such as gastroenterologists,
interventional radiologists, and family practice physicians. Performers may also
be non-physician providers (NPPs) who have other significant roles in the
procedure such as a radiology technician, dental assistant, or nurse.
iii. SHALL contain exactly one [1..1] performer (CONF:8520) such
that it
1. SHALL contain exactly one [1..1] @typeCode="PPRF"
Primary Performer (CodeSystem:
HL7ParticipationType 2.16.840.1.113883.5.90)
(CONF:8521).
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 175
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
2. SHALL contain exactly one [1..1] assignedEntity
(CONF:14911).
a. This assignedEntity SHOULD contain zero or one
[0..1] code (CONF:14912).
i. The code, if present, SHOULD contain
zero or one [0..1] @code, which SHALL be
selected from ValueSet Healthcare
Provider Taxonomy (NUCC - HIPAA)
2.16.840.1.114222.4.11.1066
DYNAMIC (CONF:14913).
iv. Any assistants SHALL be identified and SHALL be identified as
secondary performers (SPRF). (CONF:8524).
Table 39: Healthcare Provider Taxonomy Value Set
Value Set: Healthcare Provider Taxonomy (NUCC - HIPAA) 2.16.840.1.114222.4.11.1066
DYNAMIC
Code System(s):
NUCC Health Care Provider Taxonomy 2.16.840.1.113883.6.101
Code
Code System
Print Name
122300000X
NUCC Health Care Provider Taxonomy
Dentist
124Q00000X
NUCC Health Care Provider Taxonomy
Dental Hygienist
126800000X
NUCC Health Care Provider Taxonomy
Dental Assistant/Tech
133V00000X
NUCC Health Care Provider Taxonomy
Dietitian, Registered
146L00000X
NUCC Health Care Provider Taxonomy
EMT/Paramedic
163W00000X
NUCC Health Care Provider Taxonomy
Registered Nurse
163WI0500X
NUCC Health Care Provider Taxonomy
IVT Team Staff
163WI0600X
NUCC Health Care Provider Taxonomy
Infection Control Professional
163WX0106X
NUCC Health Care Provider Taxonomy
Occupational Health
Professional
164W00000X
NUCC Health Care Provider Taxonomy
Licensed Practical Nurse
167G00000X
NUCC Health Care Provider Taxonomy
Psychiatric Technician
183500000X
NUCC Health Care Provider Taxonomy
Pharmacist
207PE0004X
NUCC Health Care Provider Taxonomy
Other First Responder
227800000X
NUCC Health Care Provider Taxonomy
Respiratory Therapist/Tech
227900000X
NUCC Health Care Provider Taxonomy
Other Student
246QM0706X
NUCC Health Care Provider Taxonomy
Medical Technologist
246RP1900X
NUCC Health Care Provider Taxonomy
Phlebotomist/IV Team
247100000X
NUCC Health Care Provider Taxonomy
Radiologic Technologist
261QD0000X
NUCC Health Care Provider Taxonomy
Other Dental Worker
261QP2000X
NUCC Health Care Provider Taxonomy
Physical Therapist
261QR1100X
NUCC Health Care Provider Taxonomy
Researcher
332B00000X
NUCC Health Care Provider Taxonomy
Central Supply
363A00000X
NUCC Health Care Provider Taxonomy
Physician Assistant
363L00000X
NUCC Health Care Provider Taxonomy
Nurse Practitioner
Page 176 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Value Set: Healthcare Provider Taxonomy (NUCC - HIPAA) 2.16.840.1.114222.4.11.1066
DYNAMIC
Code System(s):
NUCC Health Care Provider Taxonomy 2.16.840.1.113883.6.101
Code
Code System
Print Name
364SC1501X
NUCC Health Care Provider Taxonomy
Public Health Worker
367500000X
NUCC Health Care Provider Taxonomy
Nurse Anesthetist
367A00000X
NUCC Health Care Provider Taxonomy
Nurse Midwife
3747A0650X
NUCC Health Care Provider Taxonomy
Attendant/orderly
376K00000X
NUCC Health Care Provider Taxonomy
Nursing Assistant
Figure 66: Procedure note serviceEvent example
<serviceEvent classCode="PROC">
<code code="118155006" codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT"
displayName="Gastrointestinal tract endoscopy"/>
<effectiveTime>
<low value="201003292240" />
<width value="15" unit="m"/>
</effectiveTime>
...
</serviceEvent>
Figure 67: Procedure note serviceEvent example with null value in width element
<serviceEvent classCode="PROC">
<code code="118155006" codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT"
displayName="Gastrointestinal tract endoscopy"/>
<effectiveTime>
<low value="201003292240" />
<width nullFlavor="NI"/>
</effectiveTime>
...
</serviceEvent>
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 177
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 68: Procedure note performer example
<performer typeCode="PPRF">
<assignedEntity>
<id extension="IO00017" root="2.16.840.1.113883.19.5" />
<code code="207RG0100X"
codeSystem="2.16.840.1.113883.6.96"
codeSystemName="NUCC"
displayName="Gastroenterologist" />
<addr>
<streetAddressLine>1001 Hospital Lane</streetAddressLine>
<city>Ann Arbor</city>
<state>MI</state>
<postalCode>99999</postalCode>
<country>US</country>
</addr>
<telecom value="tel:(999)555-1212" />
<assignedPerson>
<name>
<prefix>Dr.</prefix>
<given>Tony</given>
<family>Tum</family>
</name>
</assignedEntity>
</performer>
4.7.2 Procedure Note Body Constraints
The Procedure Note supports both narrative sections and sections requiring code
clinical statements. The required and optional sections are listed in the
Document Types and Required/Optional Sections table. The table below the
constraints shows all templates including entries within each section.
1. SHALL contain exactly one [1..1] component (CONF:9588).
a. A Procedure Note can have either a structuredBody or a
nonXMLBody (CONF:9589).
i. A Procedure Note can conform to CDA Level 1 (nonXMLBody),
CDA Level 2 (structuredBody with sections that contain a
narrative block), or CDA Level 3 (structuredBody containing
sections that contain a narrative block and coded entries). In
this template (templateId 2.16.840.1.113883.10.20.22.1.6),
coded entries are optional. (CONF:9590).
b. If structuredBody, the component/structuredBody SHALL conform to
the section constraints below (CONF:9595).
i. Each section SHALL have a title and the title SHALL NOT
be empty (CONF:9937).
ii. SHALL include an Assessment and Plan Section, or an
Assessment Section and a Plan Section. (CONF:9643).
iii. SHALL NOT include an Assessment/Plan Section when an
Assessment Section and a Plan of Care Section are present.
(CONF:10064)
Page 178 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
iv. MAY contain zero or one [0..1] Assessment Section
(templateId:2.16.840.1.113883.10.20.22.2.8)
(CONF:9645).
v. MAY contain zero or one [0..1] Plan of Care Section
(templateId:2.16.840.1.113883.10.20.22.2.10)
(CONF:9647).
vi. MAY contain zero or one [0..1] Assessment and Plan
Section (templateId:2.16.840.1.113883.10.20.22.2.9)
(CONF:9649).
vii. SHALL contain exactly one [1..1] Complications Section
(templateId:2.16.840.1.113883.10.20.22.2.37)
(CONF:9802).
viii. SHALL contain exactly one [1..1] Postprocedure Diagnosis
Section
(templateId:2.16.840.1.113883.10.20.22.2.36)
(CONF:9850).
ix. SHALL contain exactly one [1..1] Procedure Description
Section
(templateId:2.16.840.1.113883.10.20.22.2.27)
(CONF:9805).
x. SHALL contain exactly one [1..1] Procedure Indications
Section
(templateId:2.16.840.1.113883.10.20.22.2.29)
(CONF:9807).
xi. MAY contain zero or one [0..1] Allergies Section (entries
optional)
(templateId:2.16.840.1.113883.10.20.22.2.6)
(CONF:9809).
xii. MAY contain zero or one [0..1] Anesthesia Section
(templateId:2.16.840.1.113883.10.20.22.2.25)
(CONF:9811).
xiii. SHALL NOT include a Chief Complaint and Reason for Visit
Section with either a Chief Complaint Section or a Reason for
Visit Section. (CONF:10065)
xiv. MAY contain zero or one [0..1] Chief Complaint Section
(templateId:1.3.6.1.4.1.19376.1.5.3.1.1.13.2.1)
(CONF:9813).
xv. MAY contain zero or one [0..1] Chief Complaint and Reason
for Visit Section
(templateId:2.16.840.1.113883.10.20.22.2.13)
(CONF:9815).
xvi. MAY contain zero or one [0..1] Family History Section
(templateId:2.16.840.1.113883.10.20.22.2.15)
(CONF:9817).
xvii. MAY contain zero or one [0..1] History of Past Illness
Section

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 179
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
(templateId:2.16.840.1.113883.10.20.22.2.20)
(CONF:9819).
xviii. MAY contain zero or one [0..1] History of Present
Illness Section (templateId:
1.3.6.1.4.1.19376.1.5.3.1.3.4) (CONF:9821).
xix. MAY contain zero or one [0..1] Medical (General) History
Section
(templateId:2.16.840.1.113883.10.20.22.2.39)
(CONF:9823).
xx. MAY contain zero or one [0..1] Medications Section
(entries optional)
(templateId:2.16.840.1.113883.10.20.22.2.1)
(CONF:9825).
xxi. MAY contain zero or one [0..1] Medications Administered
Section
(templateId:2.16.840.1.113883.10.20.22.2.38)
(CONF:9827).
xxii. MAY contain zero or one [0..1] Physical Exam Section
(templateId:2.16.840.1.113883.10.20.2.10)
(CONF:9829).
xxiii. MAY contain zero or one [0..1] Planned Procedure
Section
(templateId:2.16.840.1.113883.10.20.22.2.30)
(CONF:9831).
xxiv. MAY contain zero or one [0..1] Procedure Disposition
Section
(templateId:2.16.840.1.113883.10.20.18.2.12)
(CONF:9833).
xxv. MAY contain zero or one [0..1] Procedure Estimated Blood
Loss Section
(templateId:2.16.840.1.113883.10.20.18.2.9)
(CONF:9835).
xxvi. MAY contain zero or one [0..1] Procedure Findings
Section
(templateId:2.16.840.1.113883.10.20.22.2.28)
(CONF:9837).
xxvii. MAY contain zero or one [0..1] Procedure Implants
Section
(templateId:2.16.840.1.113883.10.20.22.2.40)
(CONF:9839).
xxviii. MAY contain zero or one [0..1] Procedure Specimens
Taken Section
(templateId:2.16.840.1.113883.10.20.22.2.31)
(CONF:9841).
xxix. MAY contain zero or one [0..1] Procedures Section
(entries optional)
(templateId:2.16.840.1.113883.10.20.22.2.7)
(CONF:9843).
Page 180 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
xxx. MAY contain zero or one [0..1] Reason for Visit Section
(templateId:2.16.840.1.113883.10.20.22.2.12)
(CONF:9845).
xxxi. MAY contain zero or one [0..1] Review of Systems
Section (templateId:1.3.6.1.4.1.19376.1.5.3.1.3.18)
(CONF:9847).
xxxii. MAY contain zero or one [0..1] Social History Section
(templateId:2.16.840.1.113883.10.20.22.2.17)
(CONF:9849).
The following table shows relationships among the templates in the body of a
Procedure Note.
Table 40: Template Containment for a Procedure Note
Template Title
Template
Type
templateId
Procedure Note
document
2.16.840.1.113883.10.20.22.1.6
Allergies Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.6
Allergy Problem Act
entry
2.16.840.1.113883.10.20.22.4.30
Allergy - Intolerance Observation
entry
2.16.840.1.113883.10.20.22.4.7
Allergy Status Observation
entry
2.16.840.1.113883.10.20.22.4.28
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 181
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Template Title
Template
Type
templateId
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Anesthesia Section
section
2.16.840.1.113883.10.20.22.2.25
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54

Page 182 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Template Title
Template
Type
templateId
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Assessment and Plan Section
section
2.16.840.1.113883.10.20.22.2.9
Plan of Care Activity Act
entry
2.16.840.1.113883.10.20.22.4.39
Assessment Section
section
2.16.840.1.113883.10.20.22.2.8

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 183
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Template Title
Template
Type
templateId
Chief Complaint and Reason for Visit
Section
section
2.16.840.1.113883.10.20.22.2.13
Chief Complaint Section
section
1.3.6.1.4.1.19376.1.5.3.1.1.13.2.1
Complications Section
section
2.16.840.1.113883.10.20.22.2.37
Problem Observation
entry
2.16.840.1.113883.10.20.22.4.4
Age Observation
entry
2.16.840.1.113883.10.20.22.4.31
Health Status Observation
entry
2.16.840.1.113883.10.20.22.4.5
Problem Status
entry
2.16.840.1.113883.10.20.22.4.6
Family History Section
section
2.16.840.1.113883.10.20.22.2.15
Family History Organizer
entry
2.16.840.1.113883.10.20.22.4.45
Family History Observation
entry
2.16.840.1.113883.10.20.22.4.46
Age Observation
entry
2.16.840.1.113883.10.20.22.4.31
Family History Death Observation
entry
2.16.840.1.113883.10.20.22.4.47
History of Past Illness Section
section
2.16.840.1.113883.10.20.22.2.20
Problem Observation
entry
2.16.840.1.113883.10.20.22.4.4
Age Observation
entry
2.16.840.1.113883.10.20.22.4.31
Health Status Observation
entry
2.16.840.1.113883.10.20.22.4.5
Problem Status
entry
2.16.840.1.113883.10.20.22.4.6
History of Present Illness Section
section
1.3.6.1.4.1.19376.1.5.3.1.3.4
Medical (General) History Section
section
2.16.840.1.113883.10.20.22.2.39
Medications Administered Section
section
2.16.840.1.113883.10.20.22.2.38
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9

Page 184 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Template Title
Template
Type
templateId
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Medications Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.1
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Physical Exam Section
section
2.16.840.1.113883.10.20.2.10
Plan of Care Section
section
2.16.840.1.113883.10.20.22.2.10
Plan of Care Activity Act
entry
2.16.840.1.113883.10.20.22.4.39
Plan of Care Activity Encounter
entry
2.16.840.1.113883.10.20.22.4.40
Plan of Care Activity Observation
entry
2.16.840.1.113883.10.20.22.4.44
Plan of Care Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.41
Plan of Care Activity Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.42
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 185
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Template Title
Template
Type
templateId
Plan of Care Activity Supply
entry
2.16.840.1.113883.10.20.22.4.43
Planned Procedure Section
section
2.16.840.1.113883.10.20.22.2.30
Plan of Care Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.41
Postprocedure Diagnosis Section
section
2.16.840.1.113883.10.20.22.2.36
Postprocedure Diagnosis
entry
2.16.840.1.113883.10.20.22.4.51
Problem Observation
entry
2.16.840.1.113883.10.20.22.4.4
Age Observation
entry
2.16.840.1.113883.10.20.22.4.31
Health Status Observation
entry
2.16.840.1.113883.10.20.22.4.5
Problem Status
entry
2.16.840.1.113883.10.20.22.4.6
Procedure Description Section
section
2.16.840.1.113883.10.20.22.2.27
Procedure Disposition Section
section
2.16.840.1.113883.10.20.18.2.12
Procedure Estimated Blood Loss Section
section
2.16.840.1.113883.10.20.18.2.9
Procedure Findings Section
section
2.16.840.1.113883.10.20.22.2.28
Problem Observation
entry
2.16.840.1.113883.10.20.22.4.4
Age Observation
entry
2.16.840.1.113883.10.20.22.4.31
Health Status Observation
entry
2.16.840.1.113883.10.20.22.4.5
Problem Status
entry
2.16.840.1.113883.10.20.22.4.6
Procedure Implants Section
section
2.16.840.1.113883.10.20.22.2.40
Procedure Indications Section
section
2.16.840.1.113883.10.20.22.2.29
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Procedure Specimens Taken Section
section
2.16.840.1.113883.10.20.22.2.31
Procedures Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.7
Procedure Activity Act
entry
2.16.840.1.113883.10.20.22.4.12
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54

Page 186 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Template Title
Template
Type
templateId
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Procedure Activity Observation
entry
2.16.840.1.113883.10.20.22.4.13
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 187
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Template Title
Template
Type
templateId
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Reason for Visit Section
section
2.16.840.1.113883.10.20.22.2.12
Review of Systems Section
section
1.3.6.1.4.1.19376.1.5.3.1.3.18
Social History Section
section
2.16.840.1.113883.10.20.22.2.17
Pregnancy Observation
entry
2.16.840.1.113883.10.20.15.3.8
Estimated Date of Delivery
entry
2.16.840.1.113883.10.20.15.3.1
Smoking Status Observation
entry
2.16.840.1.113883.10.22.4.78
Social History Observation
entry
2.16.840.1.113883.10.20.22.4.38
Page 188 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
4.8 Progress Note
[ClinicalDocument: templateId 2.16.840.1.113883.10.20.22.1.9(open)]
A Progress Note documents a patient’s clinical status during a hospitalization or
outpatient visit; thus, it is associated with an encounter.
Taber’s25 medical dictionary defines a Progress Note as “An ongoing record of a
patient's illness and treatment. Physicians, nurses, consultants, and therapists
record their notes concerning the progress or lack of progress made by the
patient between the time of the previous note and the most recent note.”
Mosby’s26 medical dictionary defines a Progress Note as “Notes made by a nurse,
physician, social worker, physical therapist, and other health care professionals
that describe the patient's condition and the treatment given or planned.”
A Progress Note is not a re-evaluation note. A Progress Note is not intended to be
a Progress Report for Medicare. Medicare B Section 1833(e) defines the
requirements of a Medicare Progress Report.
4.8.1 Progress Note Header Constraints
The Progress Note must conform to the US Realm Clinical Document Header.
The following sections include additional header constraints for conformant
Progress Notes.
1. SHALL contain exactly one [1..1] templateId/ (CONF:9483) such that it
a. SHALL contain exactly one [1..1]
templateId/@root="2.16.840.1.113883.10.20.22.1.1"
(CONF:10051).
4.8.1.1 ClinicalDocument/templateId
Conformant documents must carry the document-level templateId asserting
conformance with specific constraints of a Progress Note as well as the
templateId for the US Realm Clinical Document Header template.
The following asserts conformance to a Progress Note.
2. SHALL contain exactly one [1..1] templateId (CONF:7588) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.1.9" (CONF:10052).
Figure 69: Progress note ClinicalDocument/templateId example
<!-- indicates conformance with US Realm Clinical Document Header
template -->
<templateId root="2.16.840.1.113883.10.20.22.1.1"/>
<!-- conforms to the Progress Note -->
<templateId root="2.16.840.1.113883.10.20.22.1.9"/>
25 Taber's Cyclopedic Medical Dictionary, 21st Edition, F.A. Davis Company.
http://www.tabers.com
26 Mosby's Medical Dictionary, 8th edition. © 2009, Elsevier.

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 189
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
4.8.1.2 ClinicalDocument/code
The Progress Note limits document type codes to those codes listed in the
Progress Note LOINC Document Codes, as of publication of this implementation
guide. This is a dynamic value set meaning that these codes may be added to or
deprecated by LOINC. The table lists all codes that have the scale DOC
(document) and a ‘component’ referring to “subsequent evaluation notes”.
The Progress Note recommends use of a single document type code, 11506-3
"Subsequent evaluation note", with further specification provided by author
or performer, setting, or specialty. Some of the LOINC codes in the Progress Note
LOINC Document Codes table are pre-coordinated with the practice setting or
the training or professional level of the author. Use of pre-coordinated codes is
not recommended because of potential conflict with other information in the
header. When these pre-coordinated codes are used, any coded values
describing the author or performer of the service act or the practice setting must
be consistent with the LOINC document type. Note: The LOINC display name
"Subsequent evaluation note" is equivalent to Progress Note.
3. SHALL contain exactly one [1..1] code (CONF:17189).
a. This code SHALL contain exactly one [1..1] @code, which SHALL be
selected from ValueSet ProgressNoteDocumentTypeCode
2.16.840.1.113883.11.20.8.1 DYNAMIC (CONF:17190).
Page 190 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 41: Progress Note LOINC Document Codes
Value Set: ProgressNoteDocumentTypeCode 2.16.840.1.113883.11.20.8.1 DYNAMIC
Code System: LOINC 2.16.840.1.113883.6.1
LOINC
Code
Type of Service
‘Component’
Setting ‘System’
Specialty/Training/
Professional Level
‘Method_Type’
Preferred Code
11506-3
Subsequent evaluation note
{Setting}
{Provider}
Additional Codes
18733-6
Subsequent evaluation note
{Setting}
Attending physician
18762-5
Subsequent evaluation note
{Setting}
Chiropractor
28569-2
Subsequent evaluation note
{Setting}
Consulting physician
28617-9
Subsequent evaluation note
{Setting}
Dentistry
34900-1
Subsequent evaluation note
{Setting}
General medicine
34904-3
Subsequent evaluation note
{Setting}
Mental health
18764-1
Subsequent evaluation note
{Setting}
Nurse practitioner
28623-7
Subsequent evaluation note
{Setting}
Nursing
11507-1
Subsequent evaluation note
{Setting}
Occupational therapy
11508-9
Subsequent evaluation note
{Setting}
Physical therapy
11509-7
Subsequent evaluation note
{Setting}
Podiatry
28627-8
Subsequent evaluation note
{Setting}
Psychiatry
11510-5
Subsequent evaluation note
{Setting}
Psychology
28656-7
Subsequent evaluation note
{Setting}
Social service
11512-1
Subsequent evaluation note
{Setting}
Speech therapy
34126-3
Subsequent evaluation note
Critical care unit
{Provider}
15507-7
Subsequent evaluation note
Emergency …
{Provider}
34129-7
Subsequent evaluation note
Home health
{Provider}
34125-5
Subsequent evaluation note
Home health care
Case manager
34130-5
Subsequent evaluation note
Hospital
{Provider}
34131-3
Subsequent evaluation note
Outpatient
{Provider}
34124-8
Subsequent evaluation note
Outpatient
Cardiology
34127-1
Subsequent evaluation note
Outpatient
Dental hygienist
34128-9
Subsequent evaluation note
Outpatient
Dentistry
34901-9
Subsequent evaluation note
Outpatient
General medicine
34132-1
Subsequent evaluation note
Outpatient
Pharmacy
Figure 70: Progress note ClinicalDocument/code example
<code codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC" code="11056-3"
displayName="Subsequent evaluation note"/>
<title>Progress Note</title>
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 191
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
4.8.1.3 DocumentationOf
A documentationOf can contain a serviceEvent to further specialize the act
inherent in the ClinicalDocument/code.
In a Progress Note, a serviceEvent can represent the event of writing the
Progress Note. The serviceEvent/effectiveTime is the time period the note
documents.
4. SHOULD contain zero or one [0..1] documentationOf (CONF:7603).
a. SHALL contain exactly one [1..1] serviceEvent/@classCode="PCPR"
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6) (CONF:7604).
i. SHALL contain exactly one [1..1] templateId (CONF:9480)
such that it
1. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.21.3.1"
(CONF:10068).
ii. SHOULD contain exactly one [1..1] effectiveTime
(CONF:9481).
1. The serviceEvent/effectiveTime SHALL contain exactly
one [1..1] US Realm Date and Time
(DT.US.FIELDED)
(2.16.840.1.113883.10.20.22.5.3) (CONF:10137)
2. The serviceEvent/effectiveTime element SHOULD be
present with effectiveTime/low element (CONF:9482).
3. If a width element is not present, the serviceEvent
SHALL include effectiveTime/high (CONF:10066).
Figure 71: Progress note serviceEvent example
<documentationOf>
<serviceEvent classCode="PCPR">
<templateId root="2.16.840.1.113883.10.20.21.3.1"/>
<effectiveTime>
<low value="200503291200"/>
<high value="200503291400"/>
</effectiveTime>
...
</serviceEvent>
</documentationOf>
4.8.1.4 ComponentOf
The Progress Note is always associated with an encounter by the
componentOf/encompassingEncounter element in the header.
The effectiveTime element for an encompassingEncounter represents the
time or time interval in which the encounter took place. A single encounter may
contain multiple Progress Notes; hence the effectiveTime elements for a
Progress Note (recorded in serviceEvent) and for an encounter (recorded in
encompassingEncounter) represent different time intervals.

Page 192 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
All visits take place at a specific location. When available, the location ID is
included in the encompassingEncounter/location/healthCareFacility/id
element.
5. SHALL contain exactly one [1..1] componentOf (CONF:7595).
a. This componentOf SHALL contain exactly one [1..1]
encompassingEncounter (CONF:7596).
i. This encompassingEncounter SHALL contain at least [1..*] id
(CONF:7597).
ii. This encompassingEncounter SHALL contain exactly one [1..1]
effectiveTime (CONF:7598).
1. This effectiveTime SHALL contain exactly one [1..1] US
Realm Date and Time (DT.US.FIELDED)
(2.16.840.1.113883.10.20.22.5.3)
(CONF:10138).
2. This effectiveTime SHALL contain exactly one [1..1] low
(CONF:7599).
iii. This encompassingEncounter SHALL contain exactly one [1..1]
location/healthCareFacility/id (CONF:7611).
Figure 72: Progress note componentOf example
<componentOf>
<encompassingEncounter>
<id extension="9937012" root="2.16.840.1.113883.19"/>
<effectiveTime>
<low value="20050329"/>
<high value="20050329"/>
</effectiveTime>
<location>
<healthCareFacility>
<id root="2.16.540.1.113883.19.2"/>
</healthCareFacility>
</location>
</encompassingEncounter>
</componentOf>
4.8.2 Progress Note Body Constraints
The Progress Note supports both narrative sections and sections requiring code
clinical statements. The sections are listed in the table below and in the
Document Types and Required/Optional Sections table. The table below the
constraints shows all templates including entries within each section.
1. SHALL contain exactly one [1..1] component (CONF:9591).
a. A Progress Note can have either a structuredBody or a nonXMLBody
(CONF:9592).
i. A Progress Note can conform to CDA Level 1 (nonXMLBody),
CDA Level 2 (structuredBody with sections that contain a
narrative block), or CDA Level 3 (structuredBody containing
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 193
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
sections that contain a narrative block and coded entries). In
this template (templateId 2.16.840.1.113883.10.20.22.1.9),
coded entries are optional (CONF:9593).
b. If structuredBody, the component/structuredBody SHALL conform to
the section constraints below (CONF:9594).
i. SHALL include an Assessment and Plan Section, or an
Assessment Section and a Plan Section. (CONF:8704).
ii. SHALL NOT include an Assessment/Plan Section when an
Assessment Section and a Plan of Care Section are present.
(CONF:10069)
iii. MAY contain zero or one [0..1] Assessment Section
(templateId:2.16.840.1.113883.10.20.22.2.8)
(CONF:8776).
iv. MAY contain zero or one [0..1] Plan of Care Section
(templateId:2.16.840.1.113883.10.20.22.2.10)
(CONF:8775).
MAY contain zero or one [0..1] Assessment and Plan
Section (templateId:2.16.840.1.113883.10.20.22.2.9)
(CONF:8774).
v. MAY contain zero or one [0..1] Allergies Section (entries
optional)
(templateId:2.16.840.1.113883.10.20.22.2.6)
(CONF:8773).
vi. MAY contain zero or one [0..1] Chief Complaint Section
(templateId:1.3.6.1.4.1.19376.1.5.3.1.1.13.2.1)
(CONF:8772).
vii. MAY contain zero or one [0..1] Instructions Section
(templateId:2.16.840.1.113883.10.20.22.2.45)
(CONF:16806).
viii. MAY contain zero or one [0..1] Interventions Section
(templateId:2.16.840.1.113883.10.20.21.2.3)
(CONF:8778).
ix. MAY contain zero or one [0..1] Medications Section
(entries optional)
(templateId:2.16.840.1.113883.10.20.22.2.1)
(CONF:8771).
x. MAY contain zero or one [0..1] Objective Section
(templateId:2.16.840.1.113883.10.20.21.2.1)
(CONF:8770).
xi. MAY contain zero or one [0..1] Physical Exam Section
(templateId:2.16.840.1.113883.10.20.2.10)
(CONF:8780).
xii. MAY contain zero or one [0..1] Problem Section (entries
optional)
(templateId:2.16.840.1.113883.10.20.22.2.5)
(CONF:8786).
Page 194 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
xiii. MAY contain zero or one [0..1] Results Section (entries
optional)
(templateId:2.16.840.1.113883.10.20.22.2.3)
(CONF:8782).
xiv. MAY contain zero or one [0..1] Review of Systems Section
(templateId:1.3.6.1.4.1.19376.1.5.3.1.3.18)
(CONF:8788).
xv. MAY contain zero or one [0..1] Subjective Section
(templateId:2.16.840.1.113883.10.20.21.2.2)
(CONF:8790).
xvi. MAY contain zero or one [0..1] Vital Signs Section
(entries optional)
(templateId:2.16.840.1.113883.10.20.22.2.4)
(CONF:8784).
The following table shows relationships among the templates in the body of a
Progress Note.
Table 42: Template Containment for a Progress Note
Template Title
Template
Type
templateId
Progress Note
document
2.16.840.1.113883.10.20.22.1.9
Allergies Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.6
Allergy Problem Act
entry
2.16.840.1.113883.10.20.22.4.30
Allergy - Intolerance Observation
entry
2.16.840.1.113883.10.20.22.4.7
Allergy Status Observation
entry
2.16.840.1.113883.10.20.22.4.28
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 195
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Template Title
Template
Type
templateId
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Assessment and Plan Section
section
2.16.840.1.113883.10.20.22.2.9
Plan of Care Activity Act
entry
2.16.840.1.113883.10.20.22.4.39
Assessment Section
section
2.16.840.1.113883.10.20.22.2.8
Chief Complaint Section
section
1.3.6.1.4.1.19376.1.5.3.1.1.13.2.1
Instructions Section
section
2.16.840.1.113883.10.20.21.2.45
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Interventions Section
section
2.16.840.1.113883.10.20.21.2.3
Medications Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.1
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Page 196 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Template Title
Template
Type
templateId
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication
Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Objective Section
section
2.16.840.1.113883.10.20.21.2.1
Physical Exam Section
section
2.16.840.1.113883.10.20.2.10
Plan of Care Section
section
2.16.840.1.113883.10.20.22.2.10
Plan of Care Activity Act
entry
2.16.840.1.113883.10.20.22.4.39
Plan of Care Activity Encounter
entry
2.16.840.1.113883.10.20.22.4.40
Plan of Care Activity Observation
entry
2.16.840.1.113883.10.20.22.4.44
Plan of Care Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.41
Plan of Care Activity Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.42
Plan of Care Activity Supply
entry
2.16.840.1.113883.10.20.22.4.43
Problem Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.5
Problem Concern Act (Condition)
entry
2.16.840.1.113883.10.20.22.4.3
Problem Observation
entry
2.16.840.1.113883.10.20.22.4.4
Age Observation
entry
2.16.840.1.113883.10.20.22.4.31
Health Status Observation
entry
2.16.840.1.113883.10.20.22.4.5
Problem Status
entry
2.16.840.1.113883.10.20.22.4.6
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 197
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Template Title
Template
Type
templateId
Results Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.3
Result Organizer
entry
2.16.840.1.113883.10.20.22.4.1
Result Observation
entry
2.16.840.1.113883.10.20.22.4.2
Review of Systems Section
section
1.3.6.1.4.1.19376.1.5.3.1.3.18
Subjective Section
section
2.16.840.1.113883.10.20.21.2.2
Vital Signs Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.4
Vital Signs Organizer
entry
2.16.840.1.113883.10.20.22.4.26
Vital Sign Observation
entry
2.16.840.1.113883.10.20.22.4.27
4.9 Unstructured Document
[ClinicalDocument: templateId 2.16.840.1.113883.10.20.21.1.10(open)]
An unstructured document is a document which is used when the patient
record is captured in an unstructured format that is encapsulated within an
image file or as unstructured text in an electronic file such as a word processing
or Portable Document Format (PDF) document.
There is a need to raise the level of interoperability for these documents to
provide full access to the longitudinal patient record across a continuum of care.
Until this gap is addressed, image and multi-media files will continue to be a
portion of the patient record that remains difficult to access and share with all
participants in a patient’s care. The Unstructured Document type addresses this
gap by providing consistent guidance on the use of CDA for such documents.
An Unstructured Document (UD) document type can (1) include unstructured
content, such as a graphic, directly in a text element with a mediaType
attribute, or (2) reference a single document file, such as a word-processing
document, using a text/reference element.
For guidance on how to handle multiple files, on the selection of media types for
this IG, and on the identification of external files, see the subsections which
follow the constraints below.
IHE’s XDS-SD (Cross-Transaction Specifications and Content Specifications,
Scanned Documents Module) profile addresses a similar, more restricted use
case, specifically for scanned documents or documents electronically created
from existing text sources, and limits content to PDF-A or text. This
Unstructured Documents implementation guide is applicable not only for
scanned documents in non-PDF formats, but also for clinical documents
produced through word processing applications, etc.
For conformance with both specifications, please review the appendix on XDS-
SD and US Realm Clinical Document Header Comparison and ensure that your

Page 198 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
documents at a minimum conform to all the SHALL constraints from either
specification27.
4.9.1 Unstructured Document Header Constraints
An Unstructured Document must conform to the US Realm Clinical Document
Header. The following sections include additional header constraints for
conformant Unstructured Documents.
1. SHALL contain exactly one [1..1] templateId (CONF:9970) such that it
a. SHALL contain exactly one [1..1]
templateId/@root="2.16.840.1.113883.10.20.22.1.1"
(CONF:10053).
4.9.1.1 ClinicalDocument/templateId
Conformant Unstructured Documents must carry the document-level
templateId asserting conformance with this guide.
2. SHALL contain exactly one [1..1] templateId (CONF:7710) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.1.10" (CONF:10054).
4.9.1.2 RecordTarget
The recordTarget element records the patient or patients whose health
information is recorded in the Unstructured Documents instance. The following
constraint is an addition to those in the US Realm Clinical Document Header.
3. SHALL contain exactly one [1..1] recordTarget/patientRole/id
(CONF:7643).
4.9.1.3 Author
The author represents the person who created the original document.
If the referenced document is a scan, the person who did the scan must be
recorded in dataEnterer.
The following constraints are in addition to those in the US Realm Clinical
Document Header.
4. SHALL contain exactly one [1..1] author/assignedAuthor (CONF:7640).
a. This author/assignedAuthor SHALL contain exactly one [1..1] addr
(CONF:7641).
b. This author/assignedAuthor SHALL contain exactly one [1..1] telecom
(CONF:7642).
27 Note that the Consolidation Project is providing a number of change requests to IHE. One of
those recommendations should be the elimination of these discrepancies so that the IHE profile is
a proper subset of this guide.

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 199
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
4.9.1.4 Custodian
The following constraints are in addition to those in the US Ream Header.
5. SHALL contain exactly one [1..1]
custodian/assignedCustodian/representedCustodianOrganization
(CONF:7645).
a. This
custodian/assignedCustodian/representedCustodianOrganization
SHALL contain exactly one [1..1] id (CONF:7648).
b. This
custodian/assignedCustodian/representedCustodianOrganization
SHALL contain exactly one [1..1] name (CONF:7649).
c. This
custodian/assignedCustodian/representedCustodianOrganization
SHALL contain exactly one [1..1] telecom (CONF:7650).
d. This
custodian/assignedCustodian/representedCustodianOrganization
SHALL contain exactly one [1..1] addr (CONF:7651).
4.9.2 Unstructured Document Body Constraints
An Unstructured Document must include a nonXMLBody component with a
single text element. The text element can reference an external file using a
reference element, or include unstructured content directly with a mediaType
attribute.
The nonXMLBody/text element also has a "compression" attribute that can be
used to indicate that the unstructured content was compressed before being
Base64Encoded. At a minimum, a compression value of "DF" for the deflate
compression algorithm (RFC 1951 [http://www.ietf.org/rfc/rfc1951.txt]) must
be supported although it is not required that content be compressed.
6. SHALL contain exactly one [1..1] component/nonXMLBody (CONF:7620).
a. This component/nonXMLBody SHALL contain exactly one [1..1] text
(CONF:7622).
i. The text element SHALL either contain a reference element
with a value attribute, or have a representation attribute
with the value of B64, a mediaType attribute, and contain
the media content. (CONF:7623).
1. The value of @mediaType, if present, SHALL be drawn
from the value set 2.16.840.1.113883.11.20.7.1
SupportedFileFormats STATIC 20100512.
(CONF:7624).
Page 200 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 43: Supported File Formats Value Set (Unstructured Documents)
Value Set: SupportedFileFormats 2.16.840.1.113883.11.20.7.1 STATIC 20100512
Word Processing/Narrative Formats
Code
MSWord
application/msword*
PDF
application/pdf
Plain Text
text/plain
RTF Text
text/rtf
HTML
text/html
Graphic Formats
Code
GIF Image
image/gif
TIF Image
image/tiff
JPEG Image
image/jpeg
PNG Image
image/png
* The developers explicitly excluded newer versions of MSWord because they are well-
formed, structured XML documents, which are not appropriate in an Unstructured
Document. MSWord versions after 2007 have media type:
application/vnd.openxmlformats-officedocument.wordprocessingml.document.
Figure 73: nonXMLBody example with embedded content
<component>
<nonXMLBody>
<text mediaType="text/rtf" representation="B64">e1xydGY...</text>
</nonXMLBody>
</component>
Figure 74: nonXMLBody example with referenced content
<component>
<nonXMLBody>
<text>
<reference value="UD_sample.pdf"/>
</text>
</nonXMLBody>
</component>
Figure 75: nonXMLBody example with compressed content
<component>
<nonXMLBody>
<text mediaType="text/rtf" representation="B64"
compression="DF">dhUhkasd437hbjfQS7…</text>
</nonXMLBody>
</component>

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 201
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
4.9.2.1 Multiple Files and File Packaging
If multiple files, such as several scanned files, constitute a single document,
options include: use a CDA document type that has a structuredBody, use a
multi-page/graphic file type such as PDF, or stitch the separate images into a
single image.
For guidance on how to package a CDA Unstructured Document together with
an unstructured document it references, see the MIME Multipart/Related
Messages appendix.
4.9.2.2 Media Types Supported
The Unstructured Document model does not support all possible file formats
and it excludes structured formats such as generic XML. The media types
supported are commonly used within a healthcare setting as part of the patient
record.
The CDA Data Types specification28 provides an extensible value set of MIME
(Multipurpose Internet Mail Extensions) media types that are supported by base
CDA. Exclusions from and extensions to that list are discussed below.
Media type exclusions. This guide restricts usage of media types listed in the
CDA Data Types specification. In the absence of a use case for a video format as
part of the patient record, video formats are not included. However, an
unstructured document can link to a video or other file format; for example, a
Microsoft Word file can contain a link to a video.
Media type extensions. Although the CDA Data Types specification indicates
that ‘application/msword’ should not be used, that format is very common in
use cases that apply to Unstructured Documents, and this guide allows it. The
usage applies only to documents in binary format; it is not appropriate for rich
text format (RTF) which has a separate MIME type, or the .docx format, which is
not currently recommended for use in an Unstructured Document.
Local policy. Some content formats—in particular, tagged-image file format
(TIFF)—entail further complexity. While this guide allows TIFF because it is in
common use, its variants introduce profound interoperability issues: local
implementations would establish policy to ensure appropriate interoperability.
Microsoft Word binary formats entail similar issues.
4.9.2.3 Identification of Referenced Files
The example code in this section and in the sample file use simple filenames
with relative paths because they are easy to read as examples. However, simple
filenames and relative paths can cause problems when files are moved among
systems.
The hazard to be avoided can be illustrated as follows: Suppose an
Unstructured Document that references a file "ekg.pdf" is transmitted to a
receiver who places that Unstructured Document in a directory that already
contains an Unstructured Document for another patient, which also references
28 http://www.hl7.org/v3ballot/html/infrastructure/datatypes/datatypes.htm

Page 202 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
a file "ekg.pdf". Now the patient header information for the transmitted
document is associated with the ekg.pdf of the previously-existing document.
Thus, the use of relative paths and simple filenames can pose a danger to
patient safety.
The alternative of providing an absolute URL (Uniform Resource Locator) will
fail if the URL is inaccessible; even within a single organization, machine
identifiers may be mapped differently at different locations.
Therefore this guide, while it cannot specify business practices, recommends
the use of unique names for referenced files.
One approach to generating a unique name is to construct it from the globally-
unique document id (root and extension) concatenated to a locally unique
reference for the external file. The following figure illustrates this technique
used with a CDA document that has an id root 2.16.840.1.113883.19 and
extension 999021.
Figure 76: Unique file reference example
<reference value="ref-2.16.840.1.113883.19-999021-ekg-1.pdf"/>

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 203
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
5 SECTION-LEVEL TEMPLATES
This section contains the section-level templates referenced by one or more of
the document types of this consolidated guide. These templates describe the
purpose of each section and the section-level constraints.
Section-level templates are always included in a document.
Each section-level template contains the following:
Template metadata (e.g., templateId, etc.)
Description and explanatory narrative
LOINC section code
Section title
Requirements for a text element
Entry-level template names and Ids for referenced templates (required
and optional)
Narrative Text
The text element within the section stores the narrative to be rendered, as
described in the CDA R2 specification29, and is referred to as the CDA narrative
block.
The content model of the CDA narrative block schema is hand crafted to meet
requirements of human readability and rendering. The schema is registered as a
MIME type (text/x-hl7-text+xml), which is the fixed media type for the text
element.
As noted in the CDA R2 specification, the document originator is responsible for
ensuring that the narrative block contains the complete, human readable,
attested content of the section. Structured entries support computer processing
and computation and are not a replacement for the attestable, human-readable
content of the CDA narrative block. The special case of structured entries with
an entry relationship of "DRIV" (is derived from) indicates to the receiving
application that the source of the narrative block is the structured entries, and
that the contents of the two are clinically equivalent.
As for all CDA documents—even when a report consisting entirely of structured
entries is transformed into CDA—the encoding application must ensure that the
authenticated content (narrative plus multimedia) is a faithful and complete
rendering of the clinical content of the structured source data. As a general
guideline, a generated narrative block should include the same human readable
content that would be available to users viewing that content in the originating
system. Although content formatting in the narrative block need not be identical
to that in the originating system, the narrative block should use elements from
the CDA narrative block schema to provide sufficient formatting to support
29 HL7 Clinical Document Architecture, Release 2.0.
http://www.hl7.org/v3ballot/html/infrastructure/cda/cda.htm
Page 204 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
human readability when rendered according to the rules defined in Section
Narrative Block (§ 4.3.5 ) of the CDA R2 specification.
By definition, a receiving application cannot assume that all clinical content in a
section (i.e., in the narrative block and multimedia) is contained in the
structured entries unless the entries in the section have an entry relationship of
"DRIV".
Additional specification information for the CDA narrative block can be found in
the CDA R2 specification in sections 1.2.1, 1.2.3, 1.3, 1.3.1, 1.3.2, 4.3.4.2, and
6.
Required and Optional Sections
The table on Sections and Required/Optional Document Types summarizes the
use and reuse of section-level templates across the document types. Note that
the constraints for the entry templates themselves are contained in the entry-
level templates section of this guide. The templates required for the Final Rules
on Stage 1 Meaningful Use are noted by an “R” in the last column of the table.
Table 44: Sections and Required/Optional Document Types with Structured Body
Section
Name
LOINC
templateId
Coded Entries Required
Coded Entries Optional
CCD
Consultation Note
Diagnostic Imaging Report
Discharge Summary
H&P Note
Operative Note
Procedure Note
Progress Note
Unstructured Document
Stage 1 Meaningful Use
Advance
Directives
42348-3
(no coded entries required)
2.16.840.1.113883.10.20.22.2.21
O
–
–
–
–
–
–
–
*
Addendum
55107-7
–
–
O
–
–
–
–
–
*
Allergies
48765-2
2.16.840.1.113883.10.20.22.2.6.1
2.16.840.1.113883.10.20.22.2.6
R
O
–
R
R
–
O
O
*
R
Anesthesia
59774-0
(no coded entries required)
2.16.840.1.113883.10.20.22.2.25
–
–
–
–
–
R
O
–
*
Assessment
**
51848-0
(no coded entries required)
2.16.840.1.113883.10.20.22.2.8
–
R
–
–
R
–
R
–
*
Assessment
and Plan**
51847-2
(no coded entries required)
2.16.840.1.113883.10.20.22.2.9
–
R
–
–
R
–
R
R
*
Chief
Complaint***
10154-3
1.3.6.1.4.1.19376.1.5.3.1.1.13.2.1
–
O
–
O
R
–
O
O
Chief
Complaint
and Reason
for Visit***
46239-0
(no coded entries required)
2.16.840.1.113883.10.20.22.2.13
–
R
–
O
R
–
O
–
*
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 205
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Section
Name
LOINC
templateId
Coded Entries Required
Coded Entries Optional
CCD
Consultation Note
Diagnostic Imaging Report
Discharge Summary
H&P Note
Operative Note
Procedure Note
Progress Note
Unstructured Document
Stage 1 Meaningful Use
Clinical
Presentation
55108-5
–
–
O
–
–
–
–
–
*
Complica-
tions
55109-3
(no coded entries required)
2.16.840.1.113883.10.20.22.2.37
–
–
O
–
–
R
R
–
*
Conclusions
55110-1
–
–
O
–
–
–
–
–
*
Current
Imaging
Procedure
Descriptions
55111-9
–
–
O
–
–
–
–
–
*
DICOM
Object
Catalog
121181
(DCM)
2.16.840.1.113883.10.20.6.1.1
–
–
R
–
–
–
–
–
–
Discharge
Diet
42344-2
(no coded entries required)
1.3.6.1.4.1.19376.1.5.3.1.3.33
–
–
–
O
–
–
–
–
*
Document
Summary
55112-7
–
–
O
–
–
–
–
–
*
Encounters
46240-8
(no coded entries required)
2.16.840.1.113883.10.20.22.2.22
O
–
–
–
–
–
–
–
*
Family
History
10157-6
—
2.16.840.1.113883.10.20.22.2.15
O
O
–
O
R
–
O
–
*
Findings
(Radiology
Study -
Observation)
18782-3
(no coded entries required)
2.16.840.1.113883.10.20.6.1.2
–
–
R
–
–
–
–
–
*
Functional
Status
47420-5
(no coded entries required)
2.16.840.1.113883.10.20.22.2.14
O
–
–
O
–
–
–
–
*
General
Status
10210-3
(no coded entries required)
2.16.840.1.113883.10.20.2.5
–
O
–
–
R
–
–
–
History of
Past Illness
(Past Medical
History)
11348-0
(no coded entries required)
2.16.840.1.113883.10.20.22.2.20
–
O
–
O
O
–
O
–
*
History of
Present
Illness
10164-2
(no coded entries required)
1.3.6.1.4.1.19376.1.5.3.1.3.4
–
R
–
O
O
–
O
–
*
Page 206 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Section
Name
LOINC
templateId
Coded Entries Required
Coded Entries Optional
CCD
Consultation Note
Diagnostic Imaging Report
Discharge Summary
H&P Note
Operative Note
Procedure Note
Progress Note
Unstructured Document
Stage 1 Meaningful Use
Hospital
Admission
Diagnosis
46241-6
(no coded entries required)
2.16.840.1.113883.10.20.22.2.43
–
–
–
O
–
–
–
–
–
Hospital
Consultation
18841-7
(no coded entries required)
2.16.840.1.113883.10.20.22.2.42
–
–
–
O
–
–
–
–
–
Hospital
Course
8648-8
(no coded entries required)
1.3.6.1.4.1.19376.1.5.3.1.3.5
–
–
–
R
–
–
–
–
*
Hospital
Discharge
Diagnosis
11535-2
(no coded entries required)
2.16.840.1.113883.10.20.22.2.24
–
–
–
R
–
–
–
–
*
Hospital
Discharge
Instructions
8653-8
(no coded entries required)
2.16.840.1.113883.10.20.22.2.41
–
–
–
O
–
–
–
–
–
Hospital
Discharge
Medications
10183-2
2.16.840.1.113883.10.20.22.2.11.1
2.16.840.1.113883.10.20.22.2.11
–
–
–
R
–
–
–
–
*
Hospital
Discharge
Physical
10184-0
(no coded entries required)
1.3.6.1.4.1.19376.1.5.3.1.3.26
–
–
–
O
–
–
–
–
*
Hospital
Discharge
Studies
Summary
11493-4
(no coded entries required)
2.16.840.1.113883.10.20.22.2.16
–
–
–
O
–
–
–
–
*
Immuniza-
tions
11369-6
2.16.840.1.113883.10.20.22.2.2.1
2.16.840.1.113883.10.20.22.2.2
O
O
–
O
O
–
–
–
*
Instructions
69730-0
(no coded entries required)
2.16.840.1.113883.10.20.22.2.45
–
–
–
–
O
–
–
O
*
Interventions
62387-6
(no coded entries required)
2.16.840.1.113883.10.20.21.2.3
–
–
–
–
–
–
–
O
*
Key Images
55113-5
–
–
O
–
–
–
–
–
*
Medical
Equipment
46264-8
(no coded entries required)
2.16.840.1.113883.10.20.22.2.23
O
–
–
–
–
–
–
–
*
Medical
(General)
History
11329-0
2.16.840.1.113883.10.20.22.2.39
–
–
O
–
–
–
O
–
*

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 207
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Section
Name
LOINC
templateId
Coded Entries Required
Coded Entries Optional
CCD
Consultation Note
Diagnostic Imaging Report
Discharge Summary
H&P Note
Operative Note
Procedure Note
Progress Note
Unstructured Document
Stage 1 Meaningful Use
Medications
10160-0
2.16.840.1.113883.10.20.22.2.1.1
2.16.840.1.113883.10.20.22.2.1
R
O
–
–
R
–
O
O
*
R
Medications
Administered
29549-3
(no coded entries required)
2.16.840.1.113883.10.20.22.2.38
–
–
–
–
–
–
O
–
*
Objective
61149-1
(no coded entries required)
2.16.840.1.113883.10.20.21.2.1
–
–
–
–
–
–
–
O
*
Operative
Note Fluids
10216-0
(no coded entries required)
2.16.840.1.113883.10.20.7.12
–
–
–
–
–
O
–
–
*
Operative
Note Surgical
Procedure
10223-6
(no coded entries required)
2.16.840.1.113883.10.20.7.14
–
–
–
–
–
O
–
–
*
Payers
48768-6
(no coded entries required)
2.16.840.1.113883.10.20.22.2.18
O
–
–
–
–
–
–
–
*
Physical
Exam
29545-1
(no coded entries required)
2.16.840.1.113883.10.20.2.10
–
R
–
–
R
–
O
O
*
Plan of
Care**
18776-5
(no coded entries required)
2.16.840.1.113883.10.20.22.2.10
O
R
–
R
R
O
R
–
*
Planned
Procedure
59772-4
(no coded entries required)
2.16.840.1.113883.10.20.22.2.30
–
–
–
–
–
O
O
–
*
Post-
operative
Diagnosis
10218-6
(no coded entries required)
2.16.840.1.113883.10.20.22.2.35
–
–
–
–
–
R
–
–
*
Post-
procedure
Diagnosis
59769-0
(no coded entries required)
2.16.840.1.113883.10.20.22.2.36
–
–
–
–
–
–
R
–
*
Preoperative
Diagnosis
10219-4
(no coded entries required)
2.16.840.1.113883.10.20.22.2.34
–
–
–
–
–
R
–
–
*
Prior Imaging
Procedure
Descriptions
55114-3
(no coded entries required)
–
–
O
–
–
–
–
–
*
Problem
11450-4
2.16.840.1.113883.10.20.22.2.5.1
2.16.840.1.113883.10.20.22.2.5
R
O
–
O
O
–
–
O
*
R
Procedure
Description
29554-3
(no coded entries required)
2.16.840.1.113883.10.20.22.2.27
–
–
–
–
–
R
R
–
*
Procedure
Disposition
59775-7
(no coded entries required)
2.16.840.1.113883.10.20.18.2.12
–
–
–
–
–
O
R
–
*
Page 208 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Section
Name
LOINC
templateId
Coded Entries Required
Coded Entries Optional
CCD
Consultation Note
Diagnostic Imaging Report
Discharge Summary
H&P Note
Operative Note
Procedure Note
Progress Note
Unstructured Document
Stage 1 Meaningful Use
Procedure
Estimated
Blood Loss
59770-8
(no coded entries required)
2.16.840.1.113883.10.20.18.2.9
–
–
–
–
–
R
O
–
*
Procedure
Findings
59776-5
(no coded entries required)
2.16.840.1.113883.10.20.22.2.28
–
–
–
–
–
R
O
–
*
Procedure
Implants
59771-6
(no coded entries required)
2.16.840.1.113883.10.20.22.2.40
–
–
–
–
–
–
O
*
Procedure
Indications
59768-2
(no coded entries required)
2.16.840.1.113883.10.20.22.2.29
–
–
–
–
–
O
R
–
*
Procedure
Specimens
Taken
59773-2
(no coded entries required)
2.16.840.1.113883.10.20.22.2.31
–
–
–
–
–
R
O
–
*
Procedures
List of
Surgeries
(History of
Procedures)
47519-4
2.16.840.1.113883.10.20.22.2.7.1
2.16.840.1.113883.10.20.22.2.7
O
O
–
O
O
–
O
–
*
R
30
Radiology
Comparison
Study –
Observation
18834-2
–
–
O
–
–
–
–
–
*
Radiology –
Impression
19005-8
–
–
O
–
–
–
–
–
*
Radiology
Study –
Recommenda
tions
18783-1
–
–
O
–
–
–
–
–
*
Radiology
Reason for
Study
18785-6
–
–
O
–
–
–
–
–
*
Reason for
Referral****
42349-1
(no coded entries required)
1.3.6.1.4.1.19376.1.5.3.1.3.1
–
R
–
–
–
–
–
–
*
Reason for
Visit***
29299-5
2.16.840.1.113883.10.20.22.2.12
–
R
–
O
R
–
O
–
–
Requested
Imaging
Studies
Information
55115-0
–
–
O
–
–
–
–
–
*
30 Required only for inpatient settings
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 209
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Section
Name
LOINC
templateId
Coded Entries Required
Coded Entries Optional
CCD
Consultation Note
Diagnostic Imaging Report
Discharge Summary
H&P Note
Operative Note
Procedure Note
Progress Note
Unstructured Document
Stage 1 Meaningful Use
Results
30954-2
2.16.840.1.113883.10.20.22.2.3.1
2.16.840.1.113883.10.20.22.2.3
R
O
–
–
R
–
–
O
*
R
Review of
Systems
10187-3
(no coded entries required)
1.3.6.1.4.1.19376.1.5.3.1.3.18
–
O
–
O
R
–
O
O
*
Social
History
29762-2
(no coded entries required)
2.16.840.1.113883.10.20.22.2.17
O
O
–
O
R
–
O
–
*
Subjective
61150-9
(no coded entries required)
2.16.840.1.113883.10.20.21.2.2
–
–
–
–
–
–
–
O
*
Surgical
Drains
11537-8
(no coded entries required)
2.16.840.1.113883.10.20.7.13
–
–
–
–
–
O
–
–
*
Vital Signs
8716-3
2.16.840.1.113883.10.20.22.2.4.1
2.16.840.1.113883.10.20.22.2.4
O
O
–
O
R
–
–
O
*
– not required or optional; these sections can be included if appropriate for the
document type
* content could be present and is unstructured
** wherever referenced, intent is that either “Assessment and Plan” is present or both
“Assessment” and “Plan of Care”. Only these combinations should be used
*** wherever referenced, intent is that either “Chief Complaint/Reason for Visit” is
present or “Chief Complaint”, and/or “Reason for Visit”. Only these combinations
should be used
****in Consultation Note, either “Reason for Referral”, “Reason for Visit”, or “Chief
Complaint/Reason for Visit” must be present
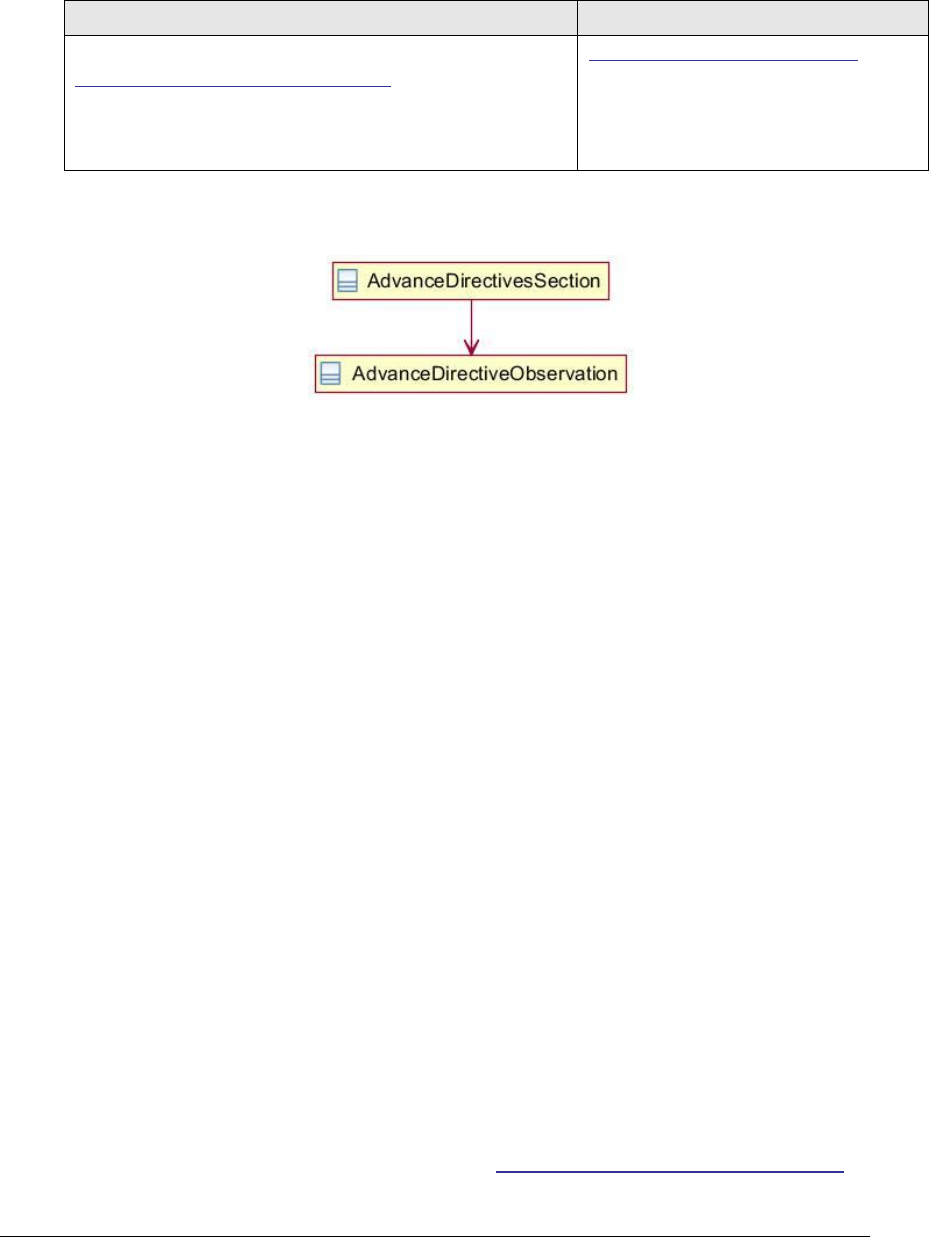
Page 210 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
5.1 Advance Directives Section 42348-3
Table 45: Advance Directives Section Contexts
Used By:
Contains Entries:
Coded entries optional:
Continuity of Care Document (CCD) (optional)
Coded entries required:
---
Advance Directive Observation
Figure 77: Advance directives section UML diagram
This section contains data defining the patient’s advance directives and any
reference to supporting documentation. The most recent and up-to-date
directives are required, if known, and should be listed in as much detail as
possible. This section contains data such as the existence of living wills,
healthcare proxies, and CPR and resuscitation status. If referenced documents
are available, they can be included in the CCD exchange package.
NOTE: The descriptions in this section differentiate between “advance directives”
and “advance directive documents”. The former are the directions whereas the
latter are legal documents containing those directions. Thus, an advance
directive might be “no cardiopulmonary resuscitation”, and this directive might
be stated in a legal advance directive document.
Advance Directives Section with Coded Entries Optional
[section: templateId 2.16.840.1.113883.10.20.22.2.21(open)]
The following constraints apply to an Advance Directive section in which entries
are not required.
1. SHALL contain exactly one [1..1] templateId (CONF:7928) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.21" (CONF:10376).
2. SHALL contain exactly one [1..1] code (CONF:15340).
a. This code SHALL contain exactly one [1..1] @code="42348-3" Advance
Directives (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:15342).
3. SHALL contain exactly one [1..1] title (CONF:7930).
4. SHALL contain exactly one [1..1] text (CONF:7931).
5. MAY contain zero or more [0..*] entry (CONF:7957) such that it
a. SHALL contain exactly one [1..1] Advance Directive Observation
(2.16.840.1.113883.10.20.22.4.48) (CONF:8800).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 211
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Advance Directives Section with Coded Entries Required
[section: templateId 2.16.840.1.113883.10.20.22.2.21.1(open)]
The following constraints apply to an Advance Directive section in which entries
are required.
1. Conforms to Advance Directives Section (entries optional)
template (2.16.840.1.113883.10.20.22.2.21).
2. SHALL contain exactly one [1..1] templateId (CONF:8643) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.21.1" (CONF:10377).
3. SHALL contain exactly one [1..1] code (CONF:15343).
a. This code SHALL contain exactly one [1..1] @code="42348-3" Advance
Directives (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:15344).
4. SHALL contain exactly one [1..1] title (CONF:8645).
5. SHALL contain exactly one [1..1] text (CONF:8646).
6. SHALL contain at least one [1..*] entry (CONF:8647) such that it
a. SHALL contain exactly one [1..1] Advance Directive Observation
(2.16.840.1.113883.10.20.22.4.48) (CONF:8801).
Figure 78: Advance directives section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.21.1"/>
<!-- Template with coded entries required. -->
<code code="42348-3" codeSystem="2.16.840.1.113883.6.1"/>
<title>Advance Directives</title>
<text>
...
</text>
<entry>
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.48"/>
...
</observation>
</entry>
</section>
Page 212 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
5.2 Allergies Section 48765-2
Table 46: Allergies Section Contexts
Used By:
Contains Entries:
Coded entries optional:
Progress Note (optional)
Consultation Note (optional)
Discharge Summary (required)
History and Physical (required)
Procedure Note (optional)
Coded entries required:
Continuity of Care Document (CCD) (required)
Allergy Problem Act
Figure 79: Allergies section UML diagram
This section lists and describes any medication allergies, adverse reactions,
idiosyncratic reactions, anaphylaxis/anaphylactoid reactions to food items, and
metabolic variations or adverse reactions/allergies to other substances (such as
latex, iodine, tape adhesives) used to assure the safety of health care delivery. At
a minimum, it should list currently active and any relevant historical allergies
and adverse reactions.
Allergies Section with Coded Entries Optional
[section: templateId 2.16.840.1.113883.10.20.22.2.6(open)]
The following constraints apply to an Allergies section in which entries are not
required.
1. SHALL contain exactly one [1..1] templateId (CONF:7800) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.6" (CONF:10378).
2. SHALL contain exactly one [1..1] code (CONF:15345).
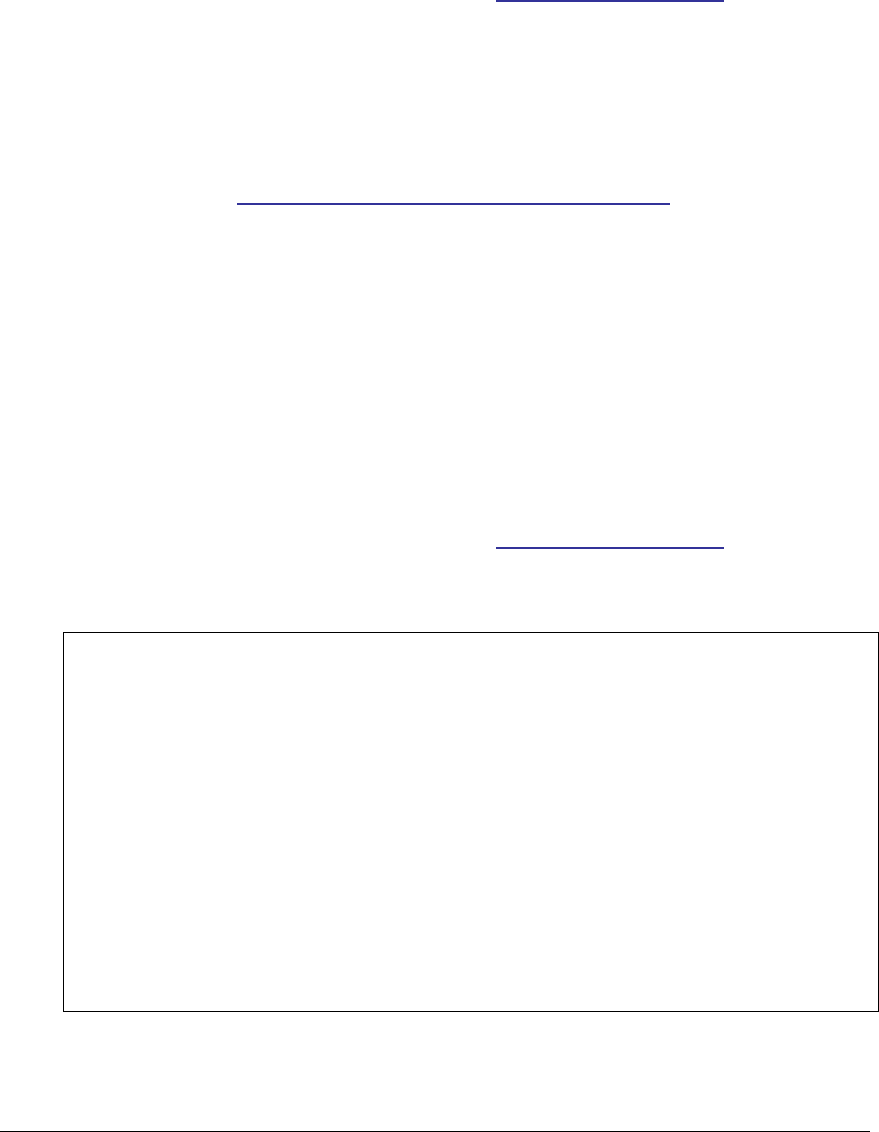
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 213
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
a. This code SHALL contain exactly one [1..1] @code="48765-2"
Allergies, adverse reactions, alerts (CodeSystem: LOINC
2.16.840.1.113883.6.1) (CONF:15346).
3. SHALL contain exactly one [1..1] title (CONF:7802).
4. SHALL contain exactly one [1..1] text (CONF:7803).
5. SHOULD contain zero or more [0..*] entry (CONF:7804) such that it
a. SHALL contain exactly one [1..1] Allergy Problem Act
(2.16.840.1.113883.10.20.22.4.30) (CONF:7805)
Allergies Section with Coded Entries Required
[section: templateId 2.16.840.1.113883.10.20.22.2.6.1(open)]
The following constraints apply to an Allergies section in which entries are
required.
1. Conforms to Allergies Section (entries optional) template
(2.16.840.1.113883.10.20.22.2.6).
2. SHALL contain exactly one [1..1] templateId (CONF:7527) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.6.1" (CONF:10379).
3. SHALL contain exactly one [1..1] code (CONF:15349).
a. This code SHALL contain exactly one [1..1] @code="48765-2"
Allergies, adverse reactions, alerts (CodeSystem: LOINC
2.16.840.1.113883.6.1) (CONF:15350).
4. SHALL contain exactly one [1..1] title (CONF:7534).
5. SHALL contain exactly one [1..1] text (CONF:7530).
6. SHALL contain at least one [1..*] entry (CONF:7531) such that it
a. SHALL contain exactly one [1..1] Allergy Problem Act
(2.16.840.1.113883.10.20.22.4.30) (CONF:7532).
Figure 80: Allergies section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.6"/>
<code code="48765-2"
displayName="Allergies, adverse reactions, alerts"
codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"/>
<title>Allergies</title>
<text>
...
</text>
<entry typeCode="DRIV">
<act classCode="ACT" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.30"/>
<!-- Allergy Problem Act template -->
...
</act>
</entry>
</section>
Page 214 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
5.3 Anesthesia Section 59774-0
[section: templateId 2.16.840.1.113883.10.20.22.2.25(open)]
Table 47: Anesthesia Section Contexts
Used By:
Contains Entries:
Procedure Note (optional)
Operative Note (required)
Medication Activity
Procedure Activity Procedure
The Anesthesia section briefly records the type of anesthesia (e.g., general or
local) and may state the actual agent used. This may or may not be a subsection
of the Procedure Description section. The full details of anesthesia are usually
found in a separate Anesthesia Note.
1. SHALL contain exactly one [1..1] templateId (CONF:8066) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.25" (CONF:10380).
2. SHALL contain exactly one [1..1] code (CONF:15351).
a. This code SHALL contain exactly one [1..1] @code="59774-0"
Anesthesia (CONF:15352).
3. SHALL contain exactly one [1..1] title (CONF:8068).
4. SHALL contain exactly one [1..1] text (CONF:8069).
5. MAY contain zero or more [0..*] entry (CONF:8092) such that it
a. SHALL contain exactly one [1..1] Procedure Activity Procedure
(2.16.840.1.113883.10.20.22.4.14) (CONF:8093).
6. MAY contain zero or more [0..*] entry (CONF:8094) such that it
a. SHALL contain exactly one [1..1] Medication Activity
(2.16.840.1.113883.10.20.22.4.16) (CONF:8095).
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 215
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 81: Anesthesia section example
<section>
<templateId root="2.16.840.1.113883.10.20.18.2.7"/>
<templateId root="2.16.840.1.113883.10.20.22.2.25"/>
<code code="59774-0"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="PROCEDURE ANESTHESIA"/>
<title>Procedure Anesthesia</title>
<text> Conscious sedation with propofol 200 mg IV </text>
<entry>
<procedure classCode="PROC" moodCode="EVN">
<!-- Procedure activity procedure template -->
<templateId root="2.16.840.1.113883.10.20.22.4.14"/>
...
</procedure>
</entry>
<entry>
<substanceAdministration classCode="SBADM" moodCode="EVN">
<!-- Medication activity template -->
<templateId root="2.16.840.1.113883.10.20.22.4.16"/>
...
</subtanceAdministration>
</entry>
</section>
5.4 Assessment and Plan Section 51847-2
[section: templateId 2.16.840.1.113883.10.20.22.2.9(open)]
Table 48: Assessment and Plan Section Contexts
Used By:
Contains Entries:
Progress Note (optional)
Consultation Note (optional)
Procedure Note (optional)
History and Physical (optional)
Plan of Care Activity Act
The Assessment and Plan sections may be combined or separated to meet local
policy requirements.
The Assessment and Plan section represents both the clinician’s conclusions
and working assumptions that will guide treatment of the patient (see
Assessment Section above) and pending orders, interventions, encounters,
services, and procedures for the patient (see Plan of Care Section below).
1. SHALL contain exactly one [1..1] templateId (CONF:7705) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.9" (CONF:10381).
2. SHALL contain exactly one [1..1] code (CONF:15353).
Page 216 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
a. This code SHALL contain exactly one [1..1] @code="51847-2"
Assessment and Plan (CONF:15354).
3. SHALL contain exactly one [1..1] text (CONF:7707).
4. MAY contain zero or more [0..*] entry (CONF:7708) such that it
a. SHALL contain exactly one [1..1] Plan of Care Activity Act
(2.16.840.1.113883.10.20.22.4.39) (CONF:8798).
Figure 82: Assessment and plan section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.9"/>
<code codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC" code="51847-2"
displayName="ASSESSMENT AND PLAN"/>
<title>ASSESSMENT AND PLAN</title>
<text>
...
</text>
<entry>
<act moodCode="RQO" classCode="ACT">
<templateId root="2.16.840.1.113883.10.20.22.4.39"/>
<!-- Plan of Care Activity Act -->
...
</act>
</entry>
</section>
5.5 Assessment Section 51848-0
[section: templateId 2.16.840.1.113883.10.20.22.2.8(open)]
Table 49: Assessment Section Contexts
Used By:
Contains Entries:
Progress Note (optional)
Consultation Note (optional)
History and Physical (optional)
Procedure Note (optional)
The Assessment section (also referred to as “impression” or “diagnoses” outside
of the context of CDA) represents the clinician's conclusions and working
assumptions that will guide treatment of the patient. The assessment may be a
list of specific disease entities or a narrative block.
1. SHALL contain exactly one [1..1] templateId (CONF:7711) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.8" (CONF:10382).
2. SHALL contain exactly one [1..1] code (CONF:14757).
a. This code SHALL contain exactly one [1..1] @code="51848-0"
Assessments (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:14758).
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 217
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
3. SHALL contain exactly one [1..1] title (CONF:16774).
4. SHALL contain exactly one [1..1] text (CONF:7713).
Figure 83: Assessment section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.8"/>
<code codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC" code="51848-0"
displayName="ASSESSMENTS"/>
<title>ASSESSMENTS</title>
<text>
...
</text>
</section>
5.6 Chief Complaint and Reason for Visit Section 46239-0
[section: templateId 2.16.840.1.113883.10.20.22.2.13(open)]
Table 50: Chief Complaint and Reason for Visit Section Contexts
Used By:
Contains Entries:
Consultation Note (optional)
Discharge Summary (optional)
History and Physical (optional)
Procedure Note (optional)
This section records the patient's chief complaint (the patient’s own description)
and/or the reason for the patient's visit (the provider’s description of the reason
for visit). Local policy determines whether the information is divided into two
sections or recorded in one section serving both purposes.
1. SHALL contain exactly one [1..1] templateId (CONF:7840) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.13" (CONF:10383).
2. SHALL contain exactly one [1..1] code (CONF:15449).
a. This code SHALL contain exactly one [1..1] @code="46239-0" Chief
Complaint and Reason for Visit (CodeSystem: LOINC
2.16.840.1.113883.6.1) (CONF:15450).
3. SHALL contain exactly one [1..1] title (CONF:7842).
4. SHALL contain exactly one [1..1] text (CONF:7843).
Page 218 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 84: Chief complaint and reason for visit section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.13"/>
<code code="46239-0"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="CHIEF COMPLAINT AND REASON FOR VISIT"/>
<title> CHIEF COMPLAINT</title>
<text>Back Pain</text>
</section>
5.7 Chief Complaint Section 10154-3
[section: templateId 1.3.6.1.4.1.19376.1.5.3.1.1.13.2.1(open)]
Table 51: Chief Complaint Section Contexts
Used By:
Contains Entries:
Progress Note (optional)
Consultation Note (optional)
Discharge Summary (optional)
History and Physical (optional)
Procedure Note (optional)
This section records the patient's chief complaint (the patient’s own description).
1. SHALL contain exactly one [1..1] templateId (CONF:7832) such that it
a. SHALL contain exactly one [1..1]
@root="1.3.6.1.4.1.19376.1.5.3.1.1.13.2.1" (CONF:10453).
2. SHALL contain exactly one [1..1] code (CONF:15451).
a. This code SHALL contain exactly one [1..1] @code="10154-3" Chief
Complaint (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:15452).
3. SHALL contain exactly one [1..1] title (CONF:7834).
4. SHALL contain exactly one [1..1] text (CONF:7835).
Figure 85: Chief complaint section example
<section>
<templateId root="1.3.6.1.4.1.19376.1.5.3.1.1.13.2.1"/>
<code code="10154-3"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="CHIEF COMPLAINT"/>
<title> CHIEF COMPLAINT</title>
<text>Back Pain</text>
</section>
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 219
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
5.8 Complications Section 55109-3
[section: templateId 2.16.840.1.113883.10.20.22.2.37(open)]
Table 52: Complications Section Contexts
Used By:
Contains Entries:
Procedure Note (required)
Operative Note (required)
Problem Observation
The Complications section records problems that occurred during the procedure
or other activity. The complications may have been known risks or
unanticipated problems.
1. SHALL contain exactly one [1..1] templateId (CONF:8174) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.37" (CONF:10384).
2. SHALL contain exactly one [1..1] code (CONF:15453).
a. This code SHALL contain exactly one [1..1] @code="55109-3"
Complications (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:15454).
3. SHALL contain exactly one [1..1] title (CONF:8176).
4. SHALL contain exactly one [1..1] text (CONF:8177).
5. There SHALL be a statement providing details of the complication(s) or it
SHALL explicitly state there were no complications. (CONF:8797).
6. MAY contain zero or more [0..*] entry (CONF:8795) such that it
a. SHALL contain exactly one [1..1] Problem Observation
(2.16.840.1.113883.10.20.22.4.4) (CONF:8796).
Figure 86: Complications section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.37"/>
<code code="55109-3" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="Complications"/>
<title>Complications</title>
<text>Asthmatic symptoms while under general anesthesia.</text>
<entry>
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.4"/>
<!-- Problem Observation -->
...
</observation>
</entry>
</section>

Page 220 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
5.9 DICOM Object Catalog Section - DCM 121181
[section: templateId 2.16.840.1.113883.10.20.6.1.1(open)]
Table 53: DICOM Object Catalog Section - DCM 121181 Contexts
Used By:
Contains Entries:
Diagnostic Imaging Report
Study Act
DICOM Object Catalog lists all referenced objects and their parent Series and
Studies, plus other DICOM attributes required for retrieving the objects.
DICOM Object Catalog sections are not intended for viewing and contain empty
section text.
9. SHALL contain exactly one [1..1] templateId (CONF:8525) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.6.1.1" (CONF:10454).
10. A DICOM Object Catalog SHALL be present if the document contains
references to DICOM Images. If present, it SHALL be the first section in the
document (CONF:8527).
11. SHALL contain exactly one [1..1] code (CONF:15456).
a. This code SHALL contain exactly one [1..1] @code="121181" Dicom
Object Catalog (CodeSystem: DCM 1.2.840.10008.2.16.4)
(CONF:15457).
12. SHALL contain at least one [1..*] entry (CONF:8530).
a. Such entries SHALL contain exactly one [1..1] Study Act
(templateId:2.16.840.1.113883.10.20.6.2.6) (CONF:15458).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 221
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 87: DICOM object catalog section example
<section classCode="DOCSECT" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.6.1.1"/>
<code code="121181" codeSystem="1.2.840.10008.2.16.4"
codeSystemName="DCM" displayName="DICOM Object Catalog"/>
<entry>
<!-- **** Study Act **** -->
<act classCode="ACT" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.6.2.6"/>
<id root="1.2.840.113619.2.62.994044785528.114289542805"/>
<code code="113014" codeSystem="1.2.840.10008.2.16.4"
codeSystemName="DCM" displayName="Study"/>
<!-- **** Series Act****-->
<entryRelationship typeCode="COMP">
<act classCode="ACT" moodCode="EVN">
<id
root="1.2.840.113619.2.62.994044785528.20060823223142485051"/>
<code code="113015" codeSystem="1.2.840.10008.2.16.4"
codeSystemName="DCM" displayName="Series">
...
</code>
<!-- **** SOP Instance UID *** -->
<!-- 2 References -->
<entryRelationship typeCode="COMP">
<observation classCode="DGIMG" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.6.2.8"/>
...
</observation>
</entryRelationship>
<entryRelationship typeCode="COMP">
<observation classCode="DGIMG" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.6.2.8"/>
...
</observation>
</entryRelationship>
</act>
</entryRelationship>
</act>
</entry>
</section>
Page 222 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
5.10 Discharge Diet Section 42344-2
[section: templateId 1.3.6.1.4.1.19376.1.5.3.1.3.33(open)]
Table 54: Discharge Diet Section Contexts
Used By:
Contains Entries:
Discharge Summary (optional)
This section records a narrative description of the expectations for diet and
nutrition, including nutrition prescription, proposals, goals, and order requests
for monitoring, tracking, or improving the nutritional status of the patient, used
in a discharge from a facility such as an emergency department, hospital, or
nursing home.
1. SHALL contain exactly one [1..1] templateId (CONF:7975) such that it
a. SHALL contain exactly one [1..1]
@root="1.3.6.1.4.1.19376.1.5.3.1.3.33" (CONF:10455).
2. SHALL contain exactly one [1..1] code (CONF:15459).
a. This code SHALL contain exactly one [1..1] @code="42344-2"
Discharge Diet (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:15460).
3. SHALL contain exactly one [1..1] title (CONF:7977).
4. SHALL contain exactly one [1..1] text (CONF:7978).
Figure 88: Discharge diet section example
<section>
<templateId root="1.3.6.1.4.1.19376.1.5.3.1.3.33"/>
<code code="42344-2"
displayName="DISCHARGE DIET"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"/>
<title>Discharge Diet</title>
<text> Low-fat, low-salt, cardiac diet </text>
</section>
5.11 Encounters Section 46240-8
Table 55: Encounters Section Contexts
Used By:
Contains Entries:
Coded entries optional:
Continuity of Care Document (CCD) (optional)
Coded entries required:
---
Encounter Activities

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 223
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 89: Encounters section UML diagram
This section lists and describes any healthcare encounters pertinent to the
patient’s current health status or historical health history. An Encounter is an
interaction, regardless of the setting, between a patient and a practitioner who is
vested with primary responsibility for diagnosing, evaluating, or treating the
patient’s condition. It may include visits, appointments, as well as non-face-to-
face interactions. It is also a contact between a patient and a practitioner who
has primary responsibility for assessing and treating the patient at a given
contact, exercising independent judgment. This section may contain all
encounters for the time period being summarized, but should include notable
encounters.
Encounters Section with Coded Entries Optional
[section: templateId 2.16.840.1.113883.10.20.22.2.22(open)]
The following constraints apply to an Encounters section in which entries are
not required.
1. SHALL contain exactly one [1..1] templateId (CONF:7940) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.22" (CONF:10386).
2. SHALL contain exactly one [1..1] code (CONF:15461).
a. This code SHALL contain exactly one [1..1] @code="46240-8"
Encounters (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:15462).
3. SHALL contain exactly one [1..1] title (CONF:7942).
4. SHALL contain exactly one [1..1] text (CONF:7943).
5. SHOULD contain zero or more [0..*] entry (CONF:7951) such that it
a. SHALL contain exactly one [1..1] Encounter Activities
(2.16.840.1.113883.10.20.22.4.49) (CONF:8802).
Encounters Section with Coded Entries Required
[section: templateId 2.16.840.1.113883.10.20.22.2.22.1(open)]
The following constraints apply to an Encounters section in which entries are
required.
1. Conforms to Encounters Section (entries optional) template
(2.16.840.1.113883.10.20.22.2.22).
2. SHALL contain exactly one [1..1] templateId (CONF:8705) such that it
Page 224 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.22.1" (CONF:10387).
3. SHALL contain exactly one [1..1] code (CONF:15466).
a. This code SHALL contain exactly one [1..1] @code="46240-8"
Encounters (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:15467).
4. SHALL contain exactly one [1..1] title (CONF:8707).
5. SHALL contain exactly one [1..1] text (CONF:8708).
6. SHALL contain at least one [1..*] entry (CONF:8709) such that it
a. SHALL contain exactly one [1..1] Encounter Activities
(2.16.840.1.113883.10.20.22.4.49) (CONF:8803).
Figure 90: Encounters section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.22"/>
<!-- Encounters Section - Entries optional -->
<code code="46240-8" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC" displayName="History of encounters"/>
<title>Encounters</title>
<text>
...
</text>
<entry typeCode="DRIV">
<encounter classCode="ENC" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.49"/>
<!-- Encounter Activities -->
...
</encounter>
</entry>
</section>
5.12 Family History Section 10157-6
[section: templateId 2.16.840.1.113883.10.20.22.2.15(open)]
Table 56: Family History Section Contexts
Used By:
Contains Entries:
Consultation Note (optional)
Discharge Summary (optional)
History and Physical (required)
Procedure Note (optional)
Continuity of Care Document (CCD) (optional)
Family History Organizer
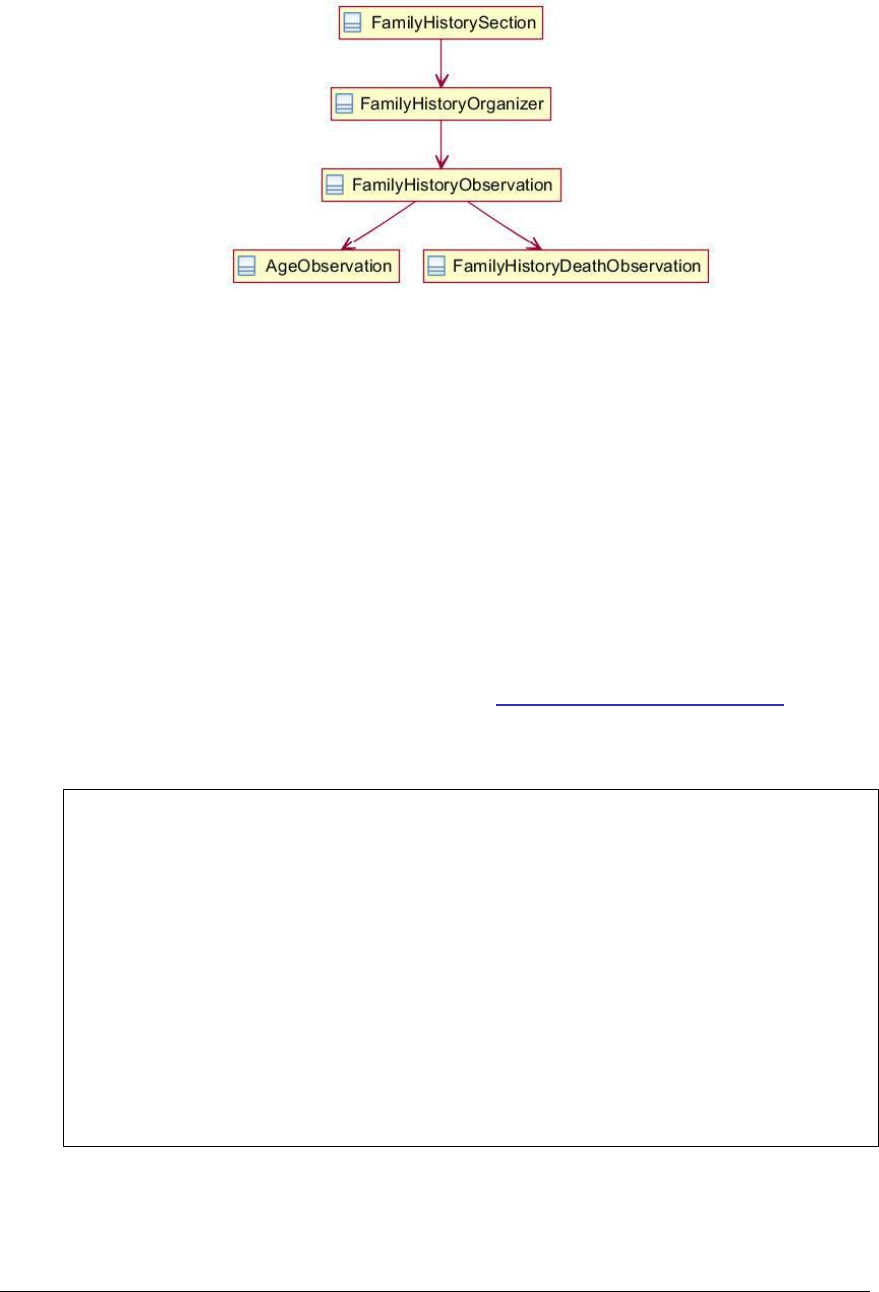
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 225
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 91: Family history section UML diagram
This section contains data defining the patient’s genetic relatives in terms of
possible or relevant health risk factors that have a potential impact on the
patient’s healthcare risk profile.
1. SHALL contain exactly one [1..1] templateId (CONF:7932) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.15" (CONF:10388).
2. SHALL contain exactly one [1..1] code (CONF:15469).
a. This code SHALL contain exactly one [1..1] @code="10157-6" Family
History (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:15470).
3. SHALL contain exactly one [1..1] title (CONF:7934).
4. SHALL contain exactly one [1..1] text (CONF:7935).
5. MAY contain zero or more [0..*] entry (CONF:7955) such that it
a. SHALL contain exactly one [1..1] Family History Organizer
(2.16.840.1.113883.10.20.22.4.45) (CONF:8799).
Figure 92: Family history section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.15"/>
<!-- Family history section template -->
<code code="10157-6" codeSystem="2.16.840.1.113883.6.1"/>
<title>Family history</title>
<text>
...
</text>
<entry typeCode="DRIV">
<organizer moodCode="EVN" classCode="CLUSTER">
<templateId root="2.16.840.1.113883.10.20.22.4.45"/>
<!-- Family history organizer template -->
...
</organizer>
</entry>
</section>

Page 226 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
5.13 Findings Section (DIR) 18782-3
[section: templateId 2.16.840.1.113883.10.20.6.1.2(open)]
Table 57: Findings Section Contexts
Used By:
Contains Entries:
Diagnostic Imaging Report (required)
The Findings section contains the main narrative body of the report. While not
an absolute requirement for transformed DICOM SR reports, it is suggested that
Diagnostic Imaging Reports authored in CDA follow Term Info guidelines31 for
the codes in the various observations and procedures recorded in this section.
1. SHALL contain exactly one [1..1] templateId (CONF:8531) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.6.1.2" (CONF:10456).
2. This section SHOULD contain only the direct observations in the report, with
topics such as Reason for Study, History, and Impression placed in separate
sections. However, in cases where the source of report content provides a
single block of text not separated into these sections, that text SHALL be
placed in the Findings section. (CONF:8532).
Figure 93: Findings section example
<section>
<templateId root="2.16.840.1.113883.10.20.6.1.2"/>
<code code="121070"
codeSystem="1.2.840.10008.2.16.4"
codeSystemName="DCM"
displayName="Findings"/>
<title>Findings</title>
<text>
<paragraph>
<caption>Finding</caption>
<content ID="Fndng2">The cardiomediastinum is . </content>
</paragraph>
<paragraph>
<caption>Diameter</caption>
<content ID="Diam2">45mm</content>
</paragraph>
...
</text>
<entry>
<templateId root="2.16.840.1.113883.10.20.6.2.12"/>
...
</entry>
</section>
31 http://www.hl7.org/special/committees/terminfo/index.cfm

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 227
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
5.14 Functional Status Section 47420-5
[section: templateId 2.16.840.1.113883.10.20.22.2.14(open)]
Table 58: Functional Status Section Contexts
Used By:
Contains Entries:
Discharge Summary (optional)
Continuity of Care Document (CCD) (optional)
Assessment Scale Observation
Caregiver Characteristics
Cognitive Status Problem Observation
Cognitive Status Result Observation
Cognitive Status Result Organizer
Functional Status Problem Observation
Functional Status Result Observation
Functional Status Result Organizer
Non-Medicinal Supply Activity
Highest Pressure Ulcer Stage
Number of Pressure Ulcers Observation
Pressure Ulcer Observation
Figure 94: Functional status section UML diagram
*The Large UML Diagrams appendix provides a larger version of this diagram
The Functional Status section describes the patient’s physical state, status of
functioning, and environmental status at the time the document was created.
A patient’s physical state may include information regarding the patient’s
physical findings as they relate to problems, including but not limited to:
Pressure Ulcers
Amputations
Heart murmur
Ostomies
A patient’s functional status may include information regarding the patient
relative to their general functional and cognitive ability, including:
Ambulatory ability
Mental status or competency
Activities of Daily Living (ADLs), including bathing, dressing, feeding,
grooming
Home or living situation having an effect on the health status of the
patient
Ability to care for self
Page 228 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Social activity, including issues with social cognition, participation with
friends and acquaintances other than family members
Occupation activity, including activities partly or directly related to
working, housework or volunteering, family and home responsibilities or
activities related to home and family
Communication ability, including issues with speech, writing or
cognition required for communication
Perception, including sight, hearing, taste, skin sensation, kinesthetic
sense, proprioception, or balance
A patient’s environmental status may include information regarding the patient’s
current exposures from their daily environment, including but not limited to:
Airborne hazards such as second-hand smoke, volatile organic
compounds, dust, or other allergens
Radiation
Safety hazards in home, such as throw rugs, poor lighting, lack of
railings/grab bars, etc.
Safety hazards at work, such as communicable diseases, excessive heat,
excessive noise, etc.
The patient's functional status may be expressed as a problem or as a result
observation. A functional or cognitive status problem observation describes a
patient’s problem, symptoms or condition. A functional or cognitive status result
observation may include observations resulting from an assessment scale,
evaluation or question and answer assessment.
Any deviation from normal function displayed by the patient and recorded in the
record should be included. Of particular interest are those limitations that
would interfere with self-care or the medical therapeutic process in any way. In
addition, a note of normal function, an improvement, or a change in functioning
status may be included.
Table 59: Functional and Cognitive Status Problem Observation Examples
Problem Observation
Functional Status
Cognitive Status
Problem/Condition/Symptom
Dysphagia
Dementia
Orthopnea
Chronic confusion
Shortness of Breath
Depressed mood
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 229
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 60: Functional and Cognitive Status Result Observation Examples
Result Observation
Functional Status
Cognitive Status
Frequency
Observation
Incontinency Frequency
Behavior Frequency
Assessment Scale or
Evaluation Result
Pain Scale
Brief Interview for Mental Status
Assessment
Question/Answer
Eating
Independent
Partial/Moderate
Assistance
Substantial Assistance
Dependent
Disorganized thinking
Behavior not present
Behavior continuously present
Behavior present, fluctuates
1. SHALL contain exactly one [1..1] templateId (CONF:7920) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.14" (CONF:10389).
2. SHALL contain exactly one [1..1] code (CONF:14578).
a. This code SHALL contain exactly one [1..1] @code="47420-5"
Functional Status (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:14579).
3. SHALL contain exactly one [1..1] title (CONF:7922).
4. SHALL contain exactly one [1..1] text (CONF:7923).
5. MAY contain zero or more [0..*] entry (CONF:14414) such that it
a. SHALL contain exactly one [1..1] Functional Status Result
Organizer (templateId:2.16.840.1.113883.10.20.22.4.66)
(CONF:14415).
6. MAY contain zero or more [0..*] entry (CONF:14416) such that it
a. SHALL contain exactly one [1..1] Cognitive Status Result
Organizer (templateId:2.16.840.1.113883.10.20.22.4.75)
(CONF:14417).
7. MAY contain zero or more [0..*] entry (CONF:14418) such that it
a. SHALL contain exactly one [1..1] Functional Status Result
Observation (templateId:2.16.840.1.113883.10.20.22.4.67)
(CONF:14419).
8. MAY contain zero or more [0..*] entry (CONF:14420) such that it
a. SHALL contain exactly one [1..1] Cognitive Status Result
Observation (templateId:2.16.840.1.113883.10.20.22.4.74)
(CONF:14421).
9. MAY contain zero or more [0..*] entry (CONF:14422) such that it
a. SHALL contain exactly one [1..1] Functional Status Problem
Observation (templateId:2.16.840.1.113883.10.20.22.4.68)
(CONF:14423).

Page 230 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
10. MAY contain zero or more [0..*] entry (CONF:14424) such that it
a. SHALL contain exactly one [1..1] Cognitive Status Problem
Observation (templateId:2.16.840.1.113883.10.20.22.4.73)
(CONF:14425).
11. MAY contain zero or more [0..*] entry (CONF:14426) such that it
a. SHALL contain exactly one [1..1] Caregiver Characteristics
(templateId:2.16.840.1.113883.10.20.22.4.72) (CONF:14427).
12. MAY contain zero or more [0..*] entry (CONF:14580) such that it
a. SHALL contain exactly one [1..1] Assessment Scale Observation
(templateId:2.16.840.1.113883.10.20.22.4.69) (CONF:14581).
13. MAY contain zero or more [0..*] entry (CONF:14582) such that it
a. SHALL contain exactly one [1..1] Non-Medicinal Supply Activity
(templateId:2.16.840.1.113883.10.20.22.4.50) (CONF:14583).
14. MAY contain zero or more [0..*] entry (CONF:16777) such that it
a. SHALL contain exactly one [1..1] Pressure Ulcer Observation
(templateId:2.16.840.1.113883.10.20.22.4.70) (CONF:16778).
15. MAY contain zero or more [0..*] entry (CONF:16779) such that it
a. SHALL contain exactly one [1..1] Number of Pressure Ulcers
Observation (templateId:2.16.840.1.113883.10.20.22.4.76)
(CONF:16780).
16. MAY contain zero or more [0..*] entry (CONF:16781) such that it
a. SHALL contain exactly one [1..1] Highest Pressure Ulcer Stage
(templateId:2.16.840.1.113883.10.20.22.4.77) (CONF:16782).
Figure 95: Functional status section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.14"/>
<!-- **** Functional status section template **** -->
<code code="47420-5" codeSystem="2.16.840.1.113883.6.1"/>
<title>Functional Status</title>
<text>
<table border="1" width="100%">
<thead>
<tr>
<th>Functional and Cognitive Assessment</th>
<th>March 23 to March 25, 2012</th>
<th>Condition Status</th>
</tr>
</thead>
<tbody>
<tr>
<td>Dependence on cane</td>
<td>1998</td>
<td>Active</td>
</tr>
<tr>
<td>Memory impairment</td>
<td>1999</td>
<td>Active</td>
</tr>
</tbody>

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 231
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
</table>
</text>
<entry typeCode="DRIV">
<templateId root="2.16.840.1.113883.10.20.22.4.67"/>
<!-- **** Functional Status Result Observation template **** -->
...
</entry>
<entry typeCode="DRIV">
<templateId root="2.16.840.1.113883.10.20.22.4.74"/>
<!-- **** Cognitive Status Result Observation template **** -->
...
</entry>
<entry typeCode="DRIV">
<templateId root="2.16.840.1.113883.10.20.22.4.68"/>
<!-- **** Functional Status Problem Observation template **** -->
...
</entry>
<entry typeCode="DRIV">
<templateId root="2.16.840.1.113883.10.20.22.4.73"/>
<!-- **** Cognitive Status Problem Observation template **** -->
...
</entry>
<entry typeCode="DRIV">
<templateId root="2.16.840.1.113883.10.20.22.4.66"/>
<!-- **** Functional Status Result Organizer template **** -->
...
</entry>
<entry typeCode="DRIV">
<templateId root="2.16.840.1.113883.10.20.22.4.75"/>
<!-- **** Cognitive Status Result Organizer template **** -->
...
</entry>
<entry typeCode="DRIV">
<templateId root="2.16.840.1. 113883.10.20.22.4.72"/>
<!-- **** Caregiver Characteristics template **** -->
...
</entry>
<entry typeCode="DRIV">
<templateId root="2.16.840.1. 113883.10.20.22.4.50"/>
<!-- **** Non-Medicinal Supply **** -->
...
</entry>
<entry typeCode="DRIV">
<templateId root="2.16.840.1.113883.10.20.22.4.69"/>
<!-- **** Assessment Scale template **** -->
...
</entry>
...
</section>

Page 232 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
5.15 General Status Section 10210-3
[section: templateId 2.16.840.1.113883.10.20.2.5(open)]
Table 61: General Status Section Contexts
Used By:
Contains Entries:
Consultation Note (optional)
History and Physical (required)
The General Status section describes general observations and readily
observable attributes of the patient, including affect and demeanor, apparent
age compared to actual age, gender, ethnicity, nutritional status based on
appearance, body build and habitus (e.g., muscular, cachectic, obese),
developmental or other deformities, gait and mobility, personal hygiene, evidence
of distress, and voice quality and speech.
1. SHALL contain exactly one [1..1] templateId (CONF:7985) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.2.5" (CONF:10457).
2. SHALL contain exactly one [1..1] code (CONF:15472).
a. This code SHALL contain exactly one [1..1] @code="10210-3" General
Status (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:15473).
3. SHALL contain exactly one [1..1] title (CONF:7987).
4. SHALL contain exactly one [1..1] text (CONF:7988).
Figure 96: General status section example
<section>
<templateId root="2.16.840.1.113883.10.20.2.5" />
<code code="10210-3"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="GENERAL STATUS" />
<title>GENERAL STATUS</title>
<text>
<paragraph>Alert and in good spirits, no acute distress.
</paragraph>
</text>
</section>

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 233
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
5.16 History of Past Illness Section 11348-0
[section: templateId 2.16.840.1.113883.10.20.22.2.20(open)]
Table 62: History of Past Illness Section Contexts
Used By:
Contains Entries:
Consultation Note (optional)
Discharge Summary (optional)
History and Physical (required)
Procedure Note (optional)
Problem Observation
This section describes the history related to the patient’s past complaints,
problems, or diagnoses. It records these details up until, and possibly pertinent
to, the patient’s current complaint or reason for seeking medical care.
1. SHALL contain exactly one [1..1] templateId (CONF:7828) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.20" (CONF:10390).
2. SHALL contain exactly one [1..1] code (CONF:15474).
a. This code SHALL contain exactly one [1..1] @code="11348-0" History
of Past Illness (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:15475).
3. SHALL contain exactly one [1..1] title (CONF:7830).
4. SHALL contain exactly one [1..1] text (CONF:7831).
5. MAY contain zero or more [0..*] entry (CONF:8791) such that it
a. SHALL contain exactly one [1..1] Problem Observation
(2.16.840.1.113883.10.20.22.4.4) (CONF:8792).
Figure 97: History of past illness section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.20"/>
<!-- ** History of Past Illness Section ** -->
<code codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC" code="11348-0"
displayName="HISTORY OF PAST ILLNESS"/>
<title>PAST MEDICAL HISTORY</title>
<text>
<paragraph>Patient has had ..... </paragraph>
</text>
<entry>
<!-- Sample With Problem Observation. -->
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.4"/>
<!-- Problem Observation -->
...
</observation>
</entry>
</section>

Page 234 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
5.17 History of Present Illness Section 10164-2
[section: templateId 1.3.6.1.4.1.19376.1.5.3.1.3.4(open)]
Table 63: History of Present Illness Section Contexts
Used By:
Contains Entries:
Consultation Note (required)
Discharge Summary (optional)
History and Physical (optional)
Procedure Note (optional)
The History of Present Illness section describes the history related to the reason
for the encounter. It contains the historical details leading up to and pertaining
to the patient’s current complaint or reason for seeking medical care.
1. SHALL contain exactly one [1..1] templateId (CONF:7848) such that it
a. SHALL contain exactly one [1..1] @root="
1.3.6.1.4.1.19376.1.5.3.1.3.4" (CONF:10458).
2. SHALL contain exactly one [1..1] code (CONF:15477).
a. This code SHALL contain exactly one [1..1] @code="10164-2" History
of Present Illness (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:15478).
3. SHALL contain exactly one [1..1] title (CONF:7850).
4. SHALL contain exactly one [1..1] text (CONF:7851).
Figure 98: History of present illness section example
<section>
<templateId root="1.3.6.1.4.1.19376.1.5.3.1.3.4"/>
<code codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"
code="10164-2"
displayName="HISTORY OF PRESENT ILLNESS"/>
<title>HISTORY OF PRESENT ILLNESS</title>
<text>
<paragraph>This patient was only recently discharged for a recurrent
GI bleed as described below.</paragraph>
<paragraph>He presented to the ER today c/o a dark stool yesterday
but a normal brown stool today. On exam he was hypotensive in the
80?s resolved after .... .... .... </paragraph>
<paragraph>Lab at discharge: Glucose 112, BUN 16, creatinine 1.1,
electrolytes normal. H. pylori antibody pending. Admission
hematocrit 16%, discharge hematocrit 29%. WBC 7300, platelet
count 256,000. Urinalysis normal. Urine culture: No growth. INR
1.1, PTT 40.</paragraph>
<paragraph>He was transfused with 6 units of packed red blood cells
with .... .... ....</paragraph>
<paragraph>GI evaluation 12 September: Colonoscopy showed single red
clot in .... .... ....</paragraph>
</text>
</section>
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 235
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
5.18 Hospital Admission Diagnosis Section 46241-6
[section: templateId 2.16.840.1.113883.10.20.22.2.43(open)]
Table 64: Hospital Admission Diagnosis Section Contexts
Used By:
Contains Entries:
Discharge Summary (optional)
Hospital Admission Diagnosis
The Hospital Admitting Diagnosis section contains a narrative description of the
primary reason for admission to a hospital facility. The section includes an
optional entry to record patient conditions.
1. SHALL contain exactly one [1..1] templateId (CONF:9930) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.43" (CONF:10391).
2. SHALL contain exactly one [1..1] code (CONF:15479).
a. This code SHALL contain exactly one [1..1] @code="46241-6" Hospital
Admission Diagnosis (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:15480).
3. SHALL contain exactly one [1..1] title (CONF:9932).
4. SHALL contain exactly one [1..1] text (CONF:9933).
5. SHOULD contain zero or one [0..1] entry (CONF:9934).
a. SHALL contain exactly one [1..1] Hospital Admission Diagnosis
(2.16.840.1.113883.10.20.22.4.34) (CONF:9935).
Figure 99: Hospital admission diagnosis section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.43"/>
<code code="46241-6" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="Hospital Admission Diagnosis"/>
<title>HOSPITAL ADMISSION DIAGNOSIS</title>
<text>Appendicitis</text>
<entry>
<act classCode="ACT" moodCode="EVN">
<!—Hospital Admission Diagnosis template -->
<templateId root="2.16.840.1.113883.10.20.22.4.34"/>
...
</entry>
</section>
Page 236 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
5.19 Hospital Admission Medications Section 42346-7 (entries
optional)
[section: templateId 2.16.840.1.113883.10.20.22.2.44 (open)]
Table 65: Hospital Admission Medications Section Contexts
Used By:
Contains Entries:
Discharge Summary (optional)
Admission Medication
The Hospital Admission Medications section defines the relevant medications
administered prior to admission to the facility. The currently active medications
must be listed.
1. SHALL contain exactly one [1..1] templateId (CONF:10098) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.44" (CONF:10392).
2. SHALL contain exactly one [1..1] code (CONF:15482).
a. This code SHALL contain exactly one [1..1] @code="42346-7"
Medications on Admission (CodeSystem: LOINC
2.16.840.1.113883.6.1) (CONF:15483).
3. SHALL contain exactly one [1..1] title (CONF:10100).
4. SHALL contain exactly one [1..1] text (CONF:10101).
5. SHOULD contain zero or more [0..*] entry (CONF:10102) such that it
a. SHALL contain exactly one [1..1] Admission Medication
(2.16.840.1.113883.10.20.22.4.36) (CONF:10110).
Figure 100: Hospital admission medications section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.44"/>
<code code="42346-7"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="ADMISSION MEDICATIONS"/>
<title>Hospital Admission Medications</title>
<text>
...
</text>
<entry typeCode="DRIV">
<act classCode="ACT" moodCode="EVN">
<!-- Admission Medication Entry -->
<templateId root="2.16.840.1.113883.10.20.22.4.36"/>
...
</act>
</entry>
...
</section>

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 237
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
5.20 Hospital Consultations Section 18841-7
[section: templateId 2.16.840.1.113883.10.20.22.2.42(open)]
Table 66: Hospital Consultations Section Contexts
Used By:
Contains Entries:
Discharge Summary (optional)
The Hospital Consultations section records consultations that occurred during
the admission.
1. SHALL contain exactly one [1..1] templateId (CONF:9915) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.42" (CONF:10393).
2. SHALL contain exactly one [1..1] code (CONF:15485).
a. This code SHALL contain exactly one [1..1] @code="18841-7" Hospital
Consultations Section (CodeSystem: LOINC
2.16.840.1.113883.6.1) (CONF:15486).
3. SHALL contain exactly one [1..1] title (CONF:9917).
4. SHALL contain exactly one [1..1] text (CONF:9918).
Figure 101: Hospital consultations section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.42"/>
<code code="18841-7" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="Hospital Consultations Section"/>
<title>HOSPITAL CONSULTATIONS</title>
<text>
<list listType="ordered">
<item>Gastroenterology</item>
<item>Cardiology</item>
<item>Dietitian</item>
</list>
</text>
</section>
5.21 Hospital Course Section 8648-8
[section: templateId 1.3.6.1.4.1.19376.1.5.3.1.3.5(open)]
Table 67: Hospital Course Section Contexts
Used By:
Contains Entries:
Discharge Summary (required)
The Hospital Course section describes the sequence of events from admission to
discharge in a hospital facility.
Page 238 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
1. SHALL contain exactly one [1..1] templateId (CONF:7852) such that it
a. SHALL contain exactly one [1..1]
@root="1.3.6.1.4.1.19376.1.5.3.1.3.5" (CONF:10459)
2. SHALL contain exactly one [1..1] code (CONF:15487).
a. This code SHALL contain exactly one [1..1] @code="8648-8" Hospital
Course (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:15488).
3. SHALL contain exactly one [1..1] title (CONF:7854)
4. SHALL contain exactly one [1..1] text (CONF:7855)
Figure 102: Hospital course section example
<section>
<templateId root="1.3.6.1.4.1.19376.1.5.3.1.3.5"/>
<code code="8648-8"
displayName="HOSPITAL COURSE"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"/>
<title>Hospital Course</title>
<text> The patient was admitted and started on Lovenox and
nitroglycerin paste. The patient had ... </text>
</section>
5.22 Hospital Discharge Diagnosis Section 11535-2
[section: templateId 2.16.840.1.113883.10.20.22.2.24(open)]
Table 68: Hospital Discharge Diagnosis Section Contexts
Used By:
Contains Entries:
Discharge Summary (required)
Hospital Discharge Diagnosis
The Hospital Discharge Diagnosis section describes the relevant problems or
diagnoses at the time of discharge that occurred during the hospitalization or
that need to be followed after hospitalization. This section includes an optional
entry to record patient conditions.
1. SHALL contain exactly one [1..1] templateId (CONF:7979) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.24" (CONF:10394).
2. SHALL contain exactly one [1..1] code (CONF:15355).
a. This code SHALL contain exactly one [1..1] @code="11535-2" Hospital
Discharge Diagnosis (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:15356).
3. SHALL contain exactly one [1..1] title (CONF:7981).
4. SHALL contain exactly one [1..1] text (CONF:7982).
5. SHOULD contain zero or one [0..1] entry (CONF:7983).
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 239
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
a. This entry, if present, SHALL contain exactly one [1..1] Hospital
Discharge Diagnosis
(templateId:2.16.840.1.113883.10.20.22.4.33) (CONF:7984).
Figure 103: Hospital discharge diagnosis section example
<section>
<!-- Discharge Summary Hospital Discharge Diagnosis Template Id -->
<templateId root="2.16.840.1.113883.10.20.22.2.24"/>
<id extension="9937012" root="2.16.840.1.113883.19"/>
<code code="11535-2" displayName="Hospital Discharge Diagnosis"
codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"/>
<title>Hospital Discharge Diagnosis</title>
<text>Diverticula of intestine</text>
<entry>
<act classCode="ACT" moodCode="EVN">
<!—Hospital discharge Diagnosis act -->
<templateId root="2.16.840.1.113883.10.20.22.4.33"/>
...
</act>
</entry>
</section>
5.23 Hospital Discharge Instructions Section 8653-8
[section: templateId 2.16.840.1.113883.10.20.22.2.41(open)]
Table 69: Hospital Discharge Instructions Section Contexts
Used By:
Contains Entries:
Discharge Summary (optional)
The Hospital Discharge Instructions section records instructions at discharge.
1. SHALL contain exactly one [1..1] templateId (CONF:9919) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.41" (CONF:10395).
2. SHALL contain exactly one [1..1] code (CONF:15357).
a. This code SHALL contain exactly one [1..1] @code="8653-8" Hospital
Discharge Instructions (CodeSystem: LOINC
2.16.840.1.113883.6.1) (CONF:15358).
3. SHALL contain exactly one [1..1] title (CONF:9921).
4. SHALL contain exactly one [1..1] text (CONF:9922).
Page 240 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 104: Hospital discharge instructions section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.41"/>
<code code="8653-8" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="HOSPITAL DISCHARGE INSTRUCTIONS"/>
<title>HOSPITAL DISCHARGE INSTRUCTIONS</title>
<text>
<list listType="ordered">
<item>Take all of your prescription medication as directed.</item>
<item>Make an appointment with your doctor to be seen two weeks
from the
date of your procedure.</item>
<item>You may feel slightly bloated after the procedure because of
air
that was introduced during the examination.</item>
<item>Call your physician if you notice:<br/>
Bleeding or black stools.<br/>
Abdominal pain.<br/>
Fever or chills.<br/>
Nausea or vomiting.<br/>
Any unusual pain or problem.<br/>
Pain or redness at the site where the intravenous needle was
placed.<br/>
</item>
<item>Do not drink alcohol for 24 hours. Alcohol amplifies the
effect of
the sedatives given.</item>
<item>Do not drive or operate machinery for 24 hours.</item>
</list>
</text>
</section>
5.24 Hospital Discharge Medications Section 10183-2
Table 70: Hospital Discharge Medications Section Contexts
Used By:
Contains Entries:
Coded entries optional:
Discharge Summary (required)
Coded entries required:
---
Discharge Medication
The Hospital Discharge Medications section defines the medications that the
patient is intended to take (or stop) after discharge. The currently active
medications must be listed. The section may also include a patient’s prescription
history and indicate the source of the medication list, for example, from a
pharmacy system versus from the patient.
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 241
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Hospital Discharge Medications Section with Coded Entries Optional
[section: templateId 2.16.840.1.113883.10.20.22.2.11(open)]
The following constraints apply to a Hospital Discharge Medications section in
which entries are not required.
1. SHALL contain exactly one [1..1] templateId (CONF:7816) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.11" (CONF:10396).
2. SHALL contain exactly one [1..1] code (CONF:15359).
a. This code SHALL contain exactly one [1..1] @code="10183-2" Hospital
Discharge Medications (CodeSystem: LOINC
2.16.840.1.113883.6.1) (CONF:15360).
3. SHALL contain exactly one [1..1] title (CONF:7818).
4. SHALL contain exactly one [1..1] text (CONF:7819).
5. SHOULD contain zero or more [0..*] entry (CONF:7820) such that it
a. SHALL contain exactly one [1..1] Discharge Medication
(2.16.840.1.113883.10.20.22.4.35) (CONF:7883).
Hospital Discharge Medications Section with Coded Entries Required
[section: templateId 2.16.840.1.113883.10.20.22.2.11.1(open)]
The following constraints apply to a Hospital Discharge Medications section in
which entries are required.
1. Conforms to Hospital Discharge Medications Section (entries
optional) template (2.16.840.1.113883.10.20.22.2.11).
2. SHALL contain exactly one [1..1] templateId (CONF:7822) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.11.1" (CONF:10397).
3. SHALL contain exactly one [1..1] code (CONF:15361).
a. This code SHALL contain exactly one [1..1] @code="10183-2" Hospital
Discharge Medications (CodeSystem: LOINC
2.16.840.1.113883.6.1) (CONF:15362).
4. SHALL contain exactly one [1..1] title (CONF:7824).
5. SHALL contain exactly one [1..1] text (CONF:7825).
6. SHALL contain at least one [1..*] entry (CONF:7826) such that it
a. SHALL contain exactly one [1..1] Discharge Medication
(2.16.840.1.113883.10.20.22.4.35) (CONF:7827).
Page 242 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 105: Hospital discharge medications section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.11"/>
<code code="10183-2"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName=" HOSPITAL DISCHARGE MEDICATIONS"/>
<title>Hospital Discharge Medications</title>
<text>
...
</text>
<entry typeCode="DRIV">
<act classCode="ACT" moodCode="EVN">
<!-- Discharge Medication Entry -->
<templateId root="2.16.840.1.113883.10.20.22.4.35"/>
...
</act>
</entry>
...
</section>
5.25 Hospital Discharge Physical Section 10184-0
[section: templateId 1.3.6.1.4.1.19376.1.5.3.1.3.26(open)]
Table 71: Hospital Discharge Physical Section Contexts
Used By:
Contains Entries:
Discharge Summary (optional)
The Hospital Discharge Physical section records a narrative description of the
patient’s physical findings.
1. SHALL contain exactly one [1..1] templateId (CONF:7971) such that it
a. SHALL contain exactly one [1..1]
@root="1.3.6.1.4.1.19376.1.5.3.1.3.26" (CONF:10460)
2. SHALL contain exactly one [1..1] code (CONF:15363).
a. This code SHALL contain exactly one [1..1] @code="10184-0" Hospital
Discharge Physical (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:15364).
3. SHALL contain exactly one [1..1] title (CONF:7973).
4. SHALL contain exactly one [1..1] text (CONF:7974).
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 243
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 106: Hospital discharge physical section example
<section>
<templateId root="1.3.6.1.4.1.19376.1.5.3.1.3.26"/>
<code code="10184-0"
displayName="HOSPITAL DISCHARGE PHYSICAL"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"/>
<title>Hospital Discharge Physical</title>
<text>GENERAL: Well-developed, slightly obese man. <br/>
NECK: Supple, with no jugular venous distension. <br/>
HEART: Intermittent tachycardia without murmurs or
gallops.<br/>
PULMONARY: Decreased breath sounds, but no clear-cut rales
or
wheezes. <br/>
EXTREMITIES: Free of edema.</text>
</section>
5.26 Hospital Discharge Studies Summary Section 11493-4
[section: templateId 2.16.840.1.113883.10.20.22.2.16(open)]
Table 72: Hospital Discharge Studies Summary Section Contexts
Used By:
Contains Entries:
Discharge Summary (optional)
This section records the results of observations generated by laboratories,
imaging procedures, and other procedures. The scope includes hematology,
chemistry, serology, virology, toxicology, microbiology, plain x-ray, ultrasound,
CT, MRI, angiography, echocardiography, nuclear medicine, pathology, and
procedure observations. This section often includes notable results such as
abnormal values or relevant trends, and could record all results for the period of
time being documented.
Laboratory results are typically generated by laboratories providing analytic
services in areas such as chemistry, hematology, serology, histology, cytology,
anatomic pathology, microbiology, and/or virology. These observations are based
on analysis of specimens obtained from the patient and submitted to the
laboratory.
Imaging results are typically generated by a clinician reviewing the output of an
imaging procedure, such as where a cardiologist reports the left ventricular
ejection fraction based on the review of an echocardiogram.
Procedure results are typically generated by a clinician wanting to provide more
granular information about component observations made during the
performance of a procedure, such as when a gastroenterologist reports the size
of a polyp observed during a colonoscopy.
Note that there are discrepancies between CCD and the lab domain model, such
as the effectiveTime in specimen collection.
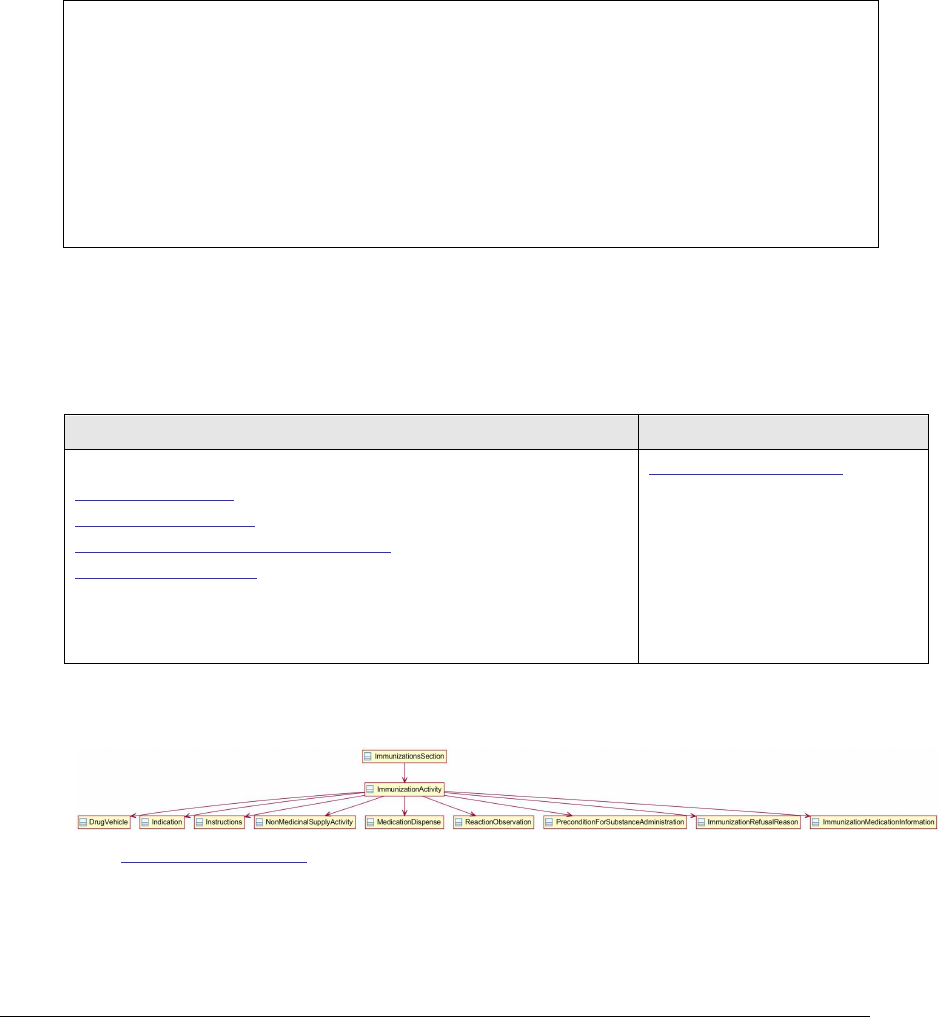
Page 244 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
1. SHALL contain exactly one [1..1] templateId (CONF:7910) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.16" (CONF:10398).
2. SHALL contain exactly one [1..1] code (CONF:15365).
a. This code SHALL contain exactly one [1..1] @code="11493-4" Hospital
Discharge Studies Summary (CodeSystem: LOINC
2.16.840.1.113883.6.1) (CONF:15366).
3. SHALL contain exactly one [1..1] title (CONF:7912).
4. SHALL contain exactly one [1..1] text (CONF:7913).
Figure 107: Hospital discharge studies summary section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.16"/>
<code code="11493-4"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="HOSPITAL DISCHARGE STUDIES SUMMARY"/>
<title>Hospital Discharge Studies Summary</title>
<text>
...
</text>
</section>
5.27 Immunizations Section 11369-6
Table 73: Immunizations Section Contexts
Used By:
Contains Entries:
Coded entries optional:
Consultation Note (optional)
Discharge Summary (optional)
Continuity of Care Document (CCD) (optional)
History and Physical (optional)
Coded entries required:
---
Immunization Activity
Figure 108: Immunization section* UML diagram
*The Large UML Diagrams appendix provides a larger version of this diagram
The Immunizations section defines a patient's current immunization status and
pertinent immunization history. The primary use case for the Immunization

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 245
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
section is to enable communication of a patient's immunization status. The
section should include current immunization status, and may contain the entire
immunization history that is relevant to the period of time being summarized.
Immunizations Section with Coded Entries Optional
[section: templateId 2.16.840.1.113883.10.20.22.2.2(open)]
The following constraints apply to an Immunization section in which entries are
not required.
1. SHALL contain exactly one [1..1] templateId (CONF:7965) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.2" (CONF:10399).
2. SHALL contain exactly one [1..1] code (CONF:15367).
a. This code SHALL contain exactly one [1..1] @code="11369-6"
Immunizations (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:15368).
3. SHALL contain exactly one [1..1] title (CONF:7967).
4. SHALL contain exactly one [1..1] text (CONF:7968).
5. SHOULD contain zero or more [0..*] entry (CONF:7969) such that it
a. SHALL contain exactly one [1..1] Immunization Activity
(2.16.840.1.113883.10.20.22.4.52) (CONF:7970).
Immunizations Section with Coded Entries Required
[section: templateId 2.16.840.1.113883.10.20.22.2.2.1(open)]
The following constraints apply to an Immunization section in which entries are
required.
1. Conforms to Immunizations Section (entries optional) template
(2.16.840.1.113883.10.20.22.2.2)
2. SHALL contain exactly one [1..1] templateId (CONF:9015) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.2.1" (CONF:10400)
3. SHALL contain exactly one [1..1] code (CONF:15369).
a. This code SHALL contain exactly one [1..1] @code="11369-6"
Immunizations (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:15370).
4. SHALL contain exactly one [1..1] title (CONF:9017)
5. SHALL contain exactly one [1..1] text (CONF:9018)
6. SHALL contain at least one [1..*] entry (CONF:9019) such that it
a. SHALL contain exactly one [1..1] Immunization Activity
(2.16.840.1.113883.10.20.22.4.52) (CONF:9020)

Page 246 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 109: Immunization section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.2"/>
<!-- ******** Immunizations section template ******** -->
<code code="11369-6"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="History of immunizations"/>
<title>Immunizations</title>
<text>
<table border="1" width="100%">
<thead>
<tr>
<th>Vaccine</th>
<th>Date</th>
<th>Status</th>
</tr>
</thead>
<tbody>
<tr>
<td>
<content ID="immun1"/>Influenza virus vaccine, IM</td>
<td>Nov 1999</td>
<td>Completed</td>
</tr>
<tr>
<td>
<content ID="immun2"/>Influenza virus vaccine, IM</td>
<td>Dec 1998</td>
<td>Completed</td>
</tr>
<tr>
<td>
<content ID="immun3"/>
Pneumococcal polysaccharide vaccine, IM</td>
<td>Dec 1998</td>
<td>Completed</td>
</tr>
<tr>
<td>
<content ID="immun4"/>Tetanus and diphtheria toxoids, IM</td>
<td>1997</td>
<td>Refused</td>
</tr>
</tbody>
</table>
</text>
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 247
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
<entry typeCode="DRIV">
<substanceAdministration classCode="SBADM" moodCode="EVN"
negationInd="false">
<templateId root="2.16.840.1.113883.10.20.22.4.52"/>
<!-- **** Immunization activity template **** -->
...
</substanceAdministration>
</entry>
...
</section>
5.28 Instructions Section 69730-0
[section: templateId 2.16.840.1.113883.10.20.22.2.45 (open)]
Table 74: Interventions Section Contexts
Used By:
Contains Entries:
History and Physical (optional)
Progress Note (optional)
Instructions
The Instructions section records instructions given to a patient. List patient
decision aids here.
1. SHALL contain exactly one [1..1] templateId (CONF:10112) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.45" (CONF:10402).
2. SHALL contain exactly one [1..1] code (CONF:15375).
a. This code SHALL contain exactly one [1..1] @code="69730-0"
Instructions (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:15376).
3. SHALL contain exactly one [1..1] title (CONF:10114).
4. SHALL contain exactly one [1..1] text (CONF:10115).
5. SHOULD contain zero or more [0..*] entry (CONF:10116).
a. SHALL contain exactly one [1..1] Instructions
(2.16.840.1.113883.10.20.22.4.20) (CONF:10117).
Page 248 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 110: Instructions section example
<section>
<templateId root="2.16.840.1.113883.10.20.21.2.45"/>
<code code="69730-0" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="INSTRUCTIONS"/>
<title>INSTRUCTIONS</title>
<text>
Patient may have low grade fever, mild joint pain and injection area
tenderness
</text>
<entry typeCode="DRIV">
<act classCode="ACT" moodCode="INT">
<templateId root="2.16.840.1.113883.10.20.22.4.20"/>
<!-- *** Instructions template *** -->
...
</supply>
</act>
</section>
5.29 Interventions Section 62387-6
[section: templateId 2.16.840.1.113883.10.20.21.2.3(open)]
Table 75: Interventions Section Contexts
Used By:
Contains Entries:
Progress Note (optional)
The Interventions section contains information about the specific interventions
provided during the healthcare visit. Depending on the type of intervention(s)
provided (procedural, education, application of assistive equipment, etc.), the
details will vary but may include specification of frequency, intensity, and
duration.
1. SHALL contain exactly one [1..1] templateId (CONF:8680) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.21.2.3" (CONF:10461).
2. SHALL contain exactly one [1..1] code (CONF:15377).
a. This code SHALL contain exactly one [1..1] @code="62387-6"
Interventions Provided (CodeSystem: LOINC
2.16.840.1.113883.6.1) (CONF:15378).
3. SHALL contain exactly one [1..1] title (CONF:8682).
4. SHALL contain exactly one [1..1] text (CONF:8683).
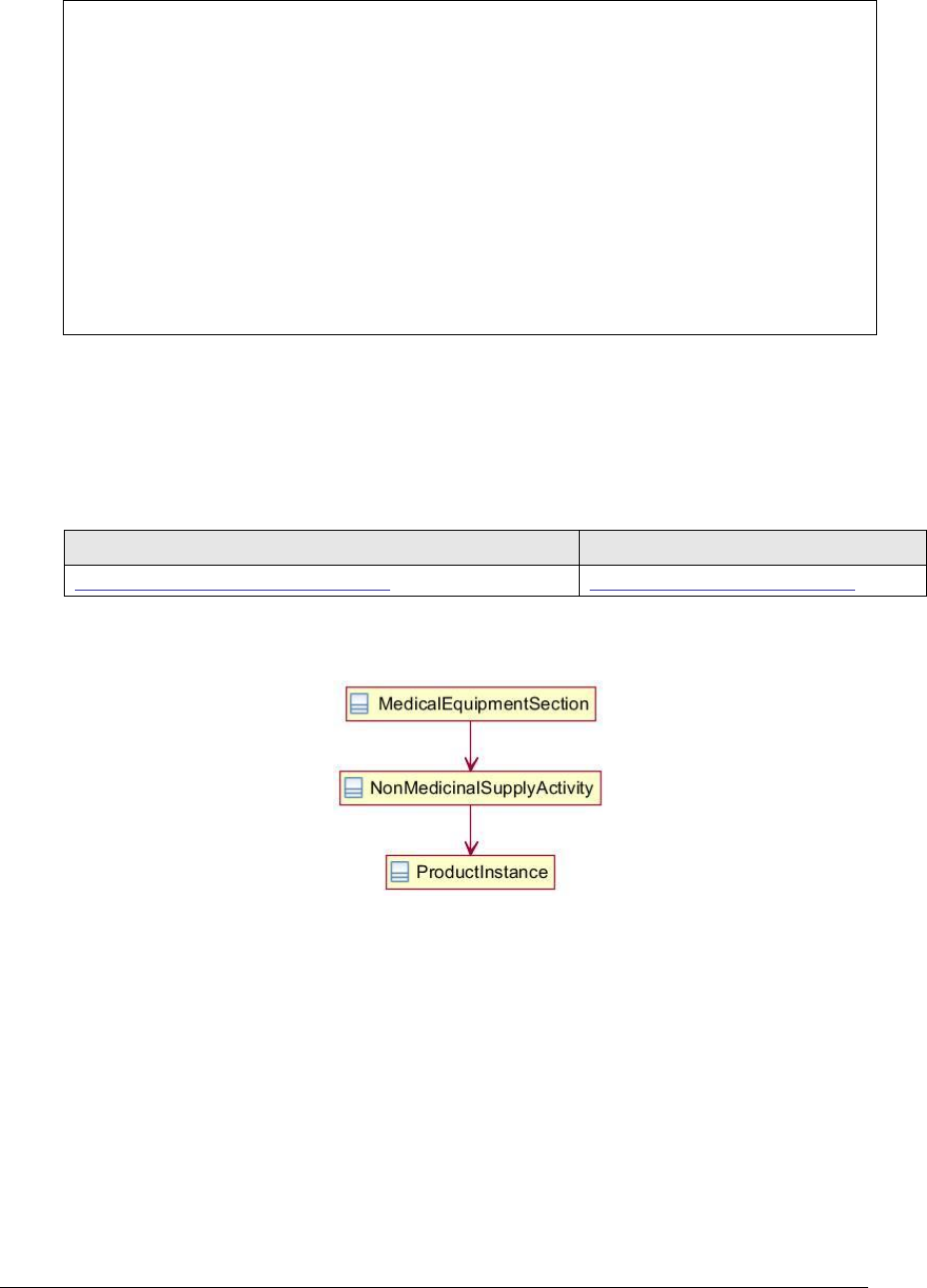
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 249
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 111: Interventions section example
<section>
<templateId root="2.16.840.1.113883.10.20.21.2.3"/>
<code code="62387-6" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="INTERVENTIONS PROVIDED"/>
<title>INTERVENTIONS PROVIDED</title>
<text>
<list listType="ordered">
<item>Therapeutic exercise intervention: knee
extension, 3 sets, 10 repetitions, 10-lb weight. </item>
<item>Therapeutic exercise intervention: arm curl, 3 sets, 10
repetitions, 15-lb weight </item>
</list>
</text>
</section>
5.30 Medical Equipment Section 46264-8
[section: templateId 2.16.840.1.113883.10.20.22.2.23(open)]
Table 76: Medical Equipment Section Contexts
Used By:
Contains Entries:
Continuity of Care Document (CCD) (optional)
Non-Medicinal Supply Activity
Figure 112: Medical equipment section UML diagram
The Medical Equipment section defines a patient’s implanted and external
medical devices and equipment that their health status depends on, as well as
any pertinent equipment or device history. This section is also used to itemize
any pertinent current or historical durable medical equipment (DME) used to
help maintain the patient’s health status. All pertinent equipment relevant to
the diagnosis, care, and treatment of a patient should be included.
1. SHALL contain exactly one [1..1] templateId (CONF:7944) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.23" (CONF:10404).
2. SHALL contain exactly one [1..1] code (CONF:15381).
Page 250 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
a. This code SHALL contain exactly one [1..1] @code="46264-8" Medical
Equipment (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:15382).
3. SHALL contain exactly one [1..1] title (CONF:7946).
4. SHALL contain exactly one [1..1] text (CONF:7947).
5. SHOULD contain zero or more [0..*] entry (CONF:7948) such that it
a. SHALL contain exactly one [1..1] Non-Medicinal Supply Activity
(2.16.840.1.113883.10.20.22.4.50) (CONF:8755).
Figure 113: Medical equipment section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.23"/>
<!-- *** Medical equipment section template *** -->
<code code="46264-8" codeSystem="2.16.840.1.113883.6.1"/>
<title>Medical Equipment</title>
<text>
...
</text>
<entry typeCode="DRIV">
<supply classCode="SPLY" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.50"/>
<!-- *** Non-medicinal supply activity template *** -->
...
</supply>
</entry>
</section>
5.31 Medical (General) History Section 11329-0
[section: templateId 2.16.840.1.113883.10.20.22.2.39(open)]
Table 77: Medical (General) History Section Contexts
Used By:
Contains Entries:
Procedure Note (optional)
The Medical History section describes all aspects of the medical history of the
patient even if not pertinent to the current procedure, and may include chief
complaint, past medical history, social history, family history, surgical or
procedure history, medication history, and other history information. The
history may be limited to information pertinent to the current procedure or may
be more comprehensive. The history may be reported as a collection of random
clinical statements or it may be reported categorically. Categorical report
formats may be divided into multiple subsections including Past Medical
History, Social History.
1. SHALL contain exactly one [1..1] templateId (CONF:8160) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.39" (CONF:10403).
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 251
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
2. SHALL contain exactly one [1..1] code (CONF:15379).
a. This code SHALL contain exactly one [1..1] @code="11329-0" Medical
(General) History (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:15380).
3. SHALL contain exactly one [1..1] title (CONF:8162).
4. SHALL contain exactly one [1..1] text (CONF:8163).
Figure 114: Medical (general) history section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.39"/>
<code code="11329-0" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="MEDICAL (GENERAL) HISTORY"/>
<title>MEDICAL (GENERAL) HISTORY</title>
<text>
<list listType="ordered">
<item>Patient has had recent issue with acne that does not seem to
be related to any particular cause.</item>
<item>Previous concerns of oral cancer was actually irritated gums
as a result of mild food allergy.</item>
<item>Patient had recent weight gain due to sedentary lifestyle and
new job.</item>
</list>
</text>
</section>
5.32 Medications Administered Section 29549-3
[section: templateId 2.16.840.1.113883.10.20.22.2.38(open)]
Table 78: Medications Administered Section Contexts
Used By:
Contains Entries:
Procedure Note (optional)
Medication Activity
The Medications Administered section defines medications and fluids
administered during the procedure, encounter, or other activity excluding
anesthetic medications. This guide recommends anesthesia medications be
documented as described in the section on Anesthesia.
1. SHALL contain exactly one [1..1] templateId (CONF:8152) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.38" (CONF:10405).
2. SHALL contain exactly one [1..1] code (CONF:15383).
a. This code SHALL contain exactly one [1..1] @code="29549-3"
Medications Administered (CodeSystem: LOINC
2.16.840.1.113883.6.1) (CONF:15384).
3. SHALL contain exactly one [1..1] title (CONF:8154).
4. SHALL contain exactly one [1..1] text (CONF:8155).
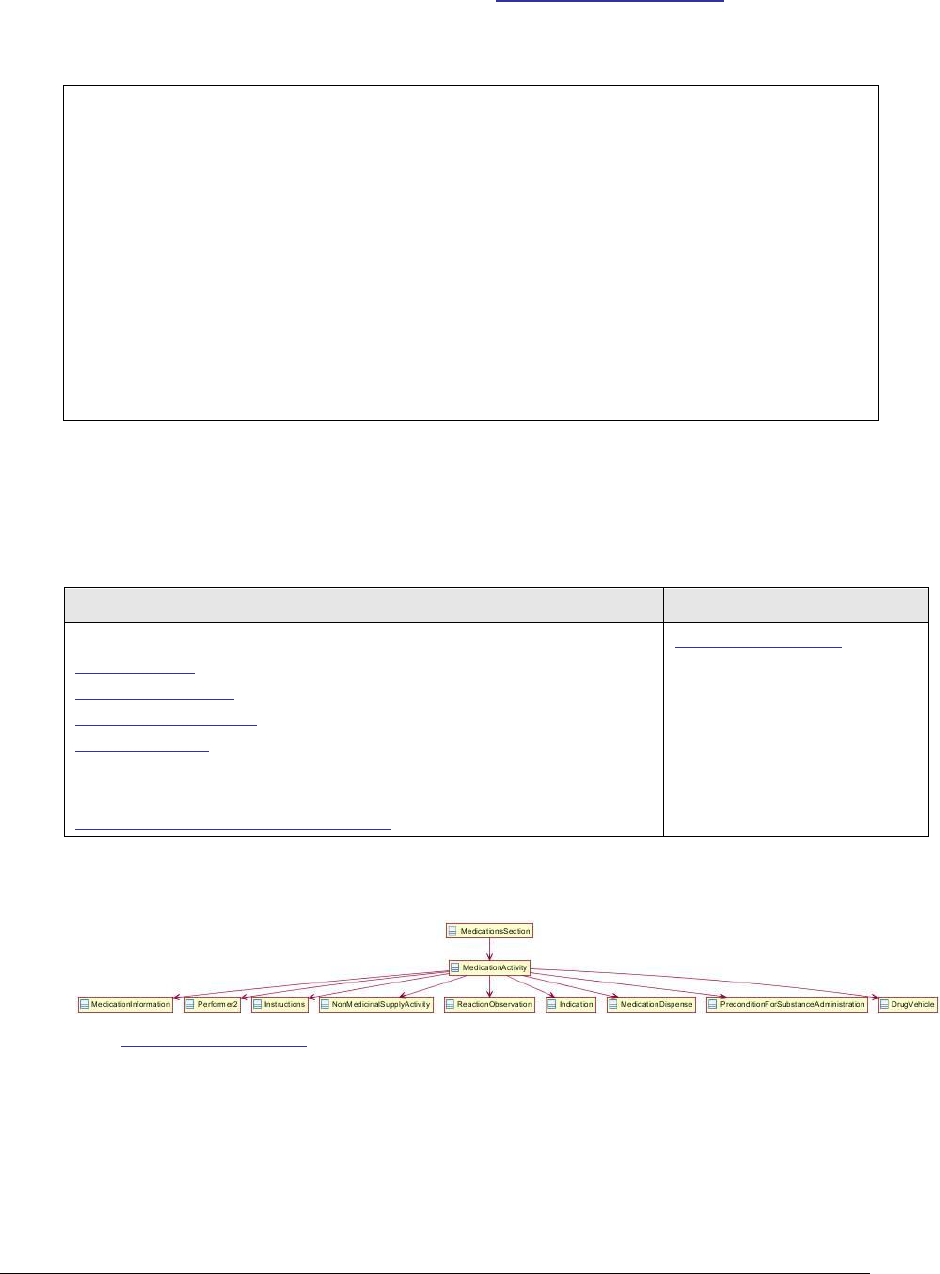
Page 252 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
5. MAY contain zero or more [0..*] entry (CONF:8156).
a. SHALL contain exactly one [1..1] Medication Activity
(2.16.840.1.113883.10.20.22.4.16) (CONF:8157).
Figure 115: Medications administered section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.38" />
<code code="29549-3"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="MEDICATIONS ADMINISTERED" />
<title>Medications Administered</title>
<text>Secretin 100 IU administered IV</text>
<entry>
<substanceAdministration classCode="SBADM" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.16"/>
<!-- Medication Activity template -->
...
</entry>
</section>
5.33 Medications Section 10160-0
Table 79: Medications Section Contexts
Used By:
Contains Entries:
Coded entries optional:
Progress Note (optional)
Consultation Note (optional)
History and Physical (required)
Procedure Note (optional)
Coded entries required:
Continuity of Care Document (CCD) (required)
Medication Activity
Figure 116: Medications section UML diagram
*The Large UML Diagrams appendix provides a larger version of this diagram
The Medications section defines a patient's current medications and pertinent
medication history. At a minimum, the currently active medications are to be
listed, with an entire medication history as an option. The section may also
include a patient's prescription and dispense history.

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 253
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
This section requires that there be either an entry indicating the subject is not
known to be on any medications, or that there be entries summarizing the
subject's medications.
Medications Section With Coded Entries Optional
[section: templateId 2.16.840.1.113883.10.20.22.2.1(open)]
The following constraints apply to a Medications section in which entries are not
required.
1. SHALL contain exactly one [1..1] templateId (CONF:7791) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.1" (CONF:10432).
2. SHALL contain exactly one [1..1] @code="10160-0" History of medication use
(CodeSystem: LOINC 2.16.840.1.113883.6.1) (CONF:7792).
3. SHALL contain exactly one [1..1] title="Medications" (CONF:7793).
4. SHALL contain exactly one [1..1] text (CONF:7794).
5. SHOULD contain zero or more [0..*] entry (CONF:7795) such that it
a. SHALL contain exactly one [1..1] Medication Activity
(2.16.840.1.113883.10.20.22.4.16) (CONF:7796).
b. If medication use is unknown, the appropriate nullFlavor MAY be
present (see unknown information in Section 1) (CONF:10076).
Medications Section With Coded Entries Required
[section: templateId 2.16.840.1.113883.10.20.22.2.1.1(open)]
The following constraints apply to a Medications section in which entries are
required.
1. Conforms to Medications Section (entries optional) template
(2.16.840.1.113883.10.20.22.2.1).
2. SHALL contain exactly one [1..1] templateId (CONF:7568) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.1.1" (CONF:10433).
3. SHALL contain exactly one [1..1] @code="10160-0" History of medication use
(CodeSystem: LOINC 2.16.840.1.113883.6.1) (CONF:7569).
4. SHALL contain exactly one [1..1] title="Medications" (CONF:7570).
5. SHALL contain exactly one [1..1] text (CONF:7571).
6. SHALL contain at least one [1..*] entry (CONF:7572) such that it
a. SHALL contain exactly one [1..1] Medication Activity
(2.16.840.1.113883.10.20.22.4.16) (CONF:7573).
b. If medication use is unknown, the appropriate nullFlavor MAY be
present (see unknown information in Section 1) (CONF:10077).
Page 254 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 117: Medications section entries example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.1"/>
<code code="10160-0"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="HISTORY OF MEDICATION USE"/>
<title>MEDICATIONS</title>
<text>
...
</text>
<entry typeCode="DRIV">
<substanceAdministration classCode="SBADM" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.16"/>
<!-- Medication Activity template -->
...
</substanceAdministration>
</entry>
</section>
5.34 Objective Section 61149-1
[section: templateId 2.16.840.1.113883.10.20.21.2.1(open)]
Table 80: Objective Section Contexts
Used By:
Contains Entries:
Progress Note (optional)
The Objective section contains data about the patient gathered through tests,
measures, or observations that produce a quantified or categorized result. It
includes important and relevant positive and negative test results, physical
findings, review of systems, and other measurements and observations.
1. SHALL contain exactly one [1..1] templateId (CONF:7869) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.21.2.1" (CONF:10462).
2. SHALL contain exactly one [1..1] code (CONF:15389).
a. This code SHALL contain exactly one [1..1] @code="61149-1"
Objective (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:15390).
3. SHALL contain exactly one [1..1] title (CONF:7871).
4. SHALL contain exactly one [1..1] text (CONF:7872).
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 255
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 118: Objective section example
<section>
<templateId root="2.16.840.1.113883.10.20.21.2.1"/>
<code code="61149-1 " codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="OBJECTIVE DATA "/>
<title>OBJECTIVE DATA</title>
<text>
<list listType="ordered">
<item>Chest: clear to ausc. No rales, normal breath sounds</item>
<item>Heart: RR, PMI in normal location and no heave or evidence
of
cardiomegaly,normal heart sounds, no murm or gallop</item>
</list>
</text>
</section>
5.35 Operative Note Fluid Section 10216-0
[section: templateId 2.16.840.1.113883.10.20.7.12(open)]
Table 81: Operative Note Fluids Section Contexts
Used By:
Contains Entries:
Operative Note (optional)
The Operative Note Fluids section may be used to record fluids administered
during the surgical procedure.
1. SHALL contain exactly one [1..1] templateId (CONF:8030) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.7.12" (CONF:10463).
2. SHALL contain exactly one [1..1] code (CONF:15391).
a. This code SHALL contain exactly one [1..1] @code="10216-0"
Operative Note Fluids (CodeSystem: LOINC
2.16.840.1.113883.6.1) (CONF:15392).
3. SHALL contain exactly one [1..1] title (CONF:8032).
4. SHALL contain exactly one [1..1] text (CONF:8033).
5. If the Operative Note Fluids section is present, there SHALL be a statement
providing details of the fluids administered or SHALL explicitly state there
were no fluids administered (CONF:8052).

Page 256 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 119: Operative Note fluid section example
<section>
<templateId root="2.16.840.1.113883.10.20.7.12"/>
<code code="10216-0"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="SURGICAL OPERATION NOTE FLUIDS"/>
<title>Operative Note Fluids</title>
<text>250 ML Ringers Lactate</text>
</section>
5.36 Operative Note Surgical Procedure Section 10223-6
[section: templateId 2.16.840.1.113883.10.20.7.14(open)]
Table 82: Operative Note Surgical Procedure Section Contexts
Used By:
Contains Entries:
Operative Note (optional)
The Operative Note Surgical Procedure section can be used to restate the
procedures performed if appropriate for an enterprise workflow. The
procedure(s) performed associated with the Operative Note are formally modeled
in the header using serviceEvent.
1. SHALL contain exactly one [1..1] templateId (CONF:8034) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.7.14" (CONF:10464).
2. SHALL contain exactly one [1..1] code (CONF:15393).
a. This code SHALL contain exactly one [1..1] @code="10223-6"
Operative Note Surgical Procedure (CodeSystem: LOINC
2.16.840.1.113883.6.1) (CONF:15394).
3. SHALL contain exactly one [1..1] title (CONF:8036).
4. SHALL contain exactly one [1..1] text (CONF:8037).
5. If the surgical procedure section is present there SHALL be text indicating the
procedure performed (CONF:8054).
Figure 120: Operative Note surgical procedure section example
<section>
<templateId root="2.16.840.1.113883.10.20.7.14"/>
<code code="10223-6"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="SURGICAL OPERATION NOTE SURGICAL PROCEDURE"/>
<title>Surgical Procedure</title>
<text>Laparoscopic Appendectomy</text>
</section>
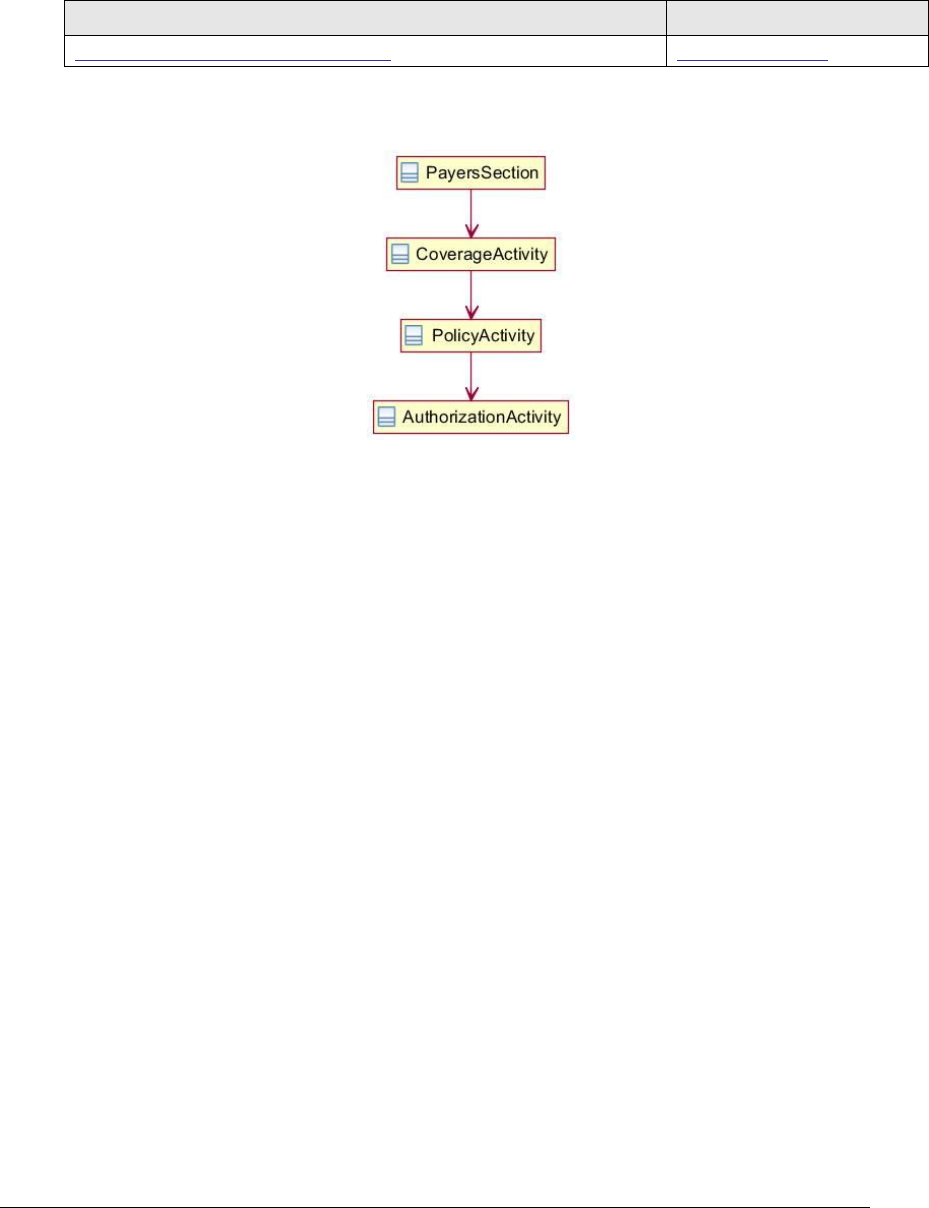
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 257
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
5.37 Payers Section 48768-6
[section: templateId 2.16.840.1.113883.10.20.22.2.18(open)]
Table 83: Payers Section Contexts
Used By:
Contains Entries:
Continuity of Care Document (CCD) (optional)
Coverage Activity
Figure 121: Payers section UML diagram
The Payers section contains data on the patient’s payers, whether a ‘third party’
insurance, self-pay, other payer or guarantor, or some combination of payers,
and is used to define which entity is the responsible fiduciary for the financial
aspects of a patient’s care.
Each unique instance of a payer and all the pertinent data needed to contact,
bill to, and collect from that payer should be included. Authorization information
that can be used to define pertinent referral, authorization tracking number,
procedure, therapy, intervention, device, or similar authorizations for the patient
or provider, or both should be included. At a minimum, the patient’s pertinent
current payment sources should be listed.
The sources of payment are represented as a Coverage Activity, which identifies
all of the insurance policies or government or other programs that cover some or
all of the patient's healthcare expenses. The policies or programs are sequenced
by preference. The Coverage Activity has a sequence number that represents the
preference order. Each policy or program identifies the covered party with
respect to the payer, so that the identifiers can be recorded.
1. SHALL contain exactly one [1..1] templateId (CONF:7924) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.18" (CONF:10434).
2. SHALL contain exactly one [1..1] code (CONF:15395).
a. This code SHALL contain exactly one [1..1] @code="48768-6" Payers
(CodeSystem: LOINC 2.16.840.1.113883.6.1) (CONF:15396).
3. SHALL contain exactly one [1..1] title (CONF:7926).
4. SHALL contain exactly one [1..1] text (CONF:7927).

Page 258 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
5. SHOULD contain zero or more [0..*] entry (CONF:7959) such that it
a. SHALL contain exactly one [1..1] Coverage Activity
(2.16.840.1.113883.10.20.22.4.60) (CONF:8905).
Figure 122: Payers section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.18"/>
<!-- ******** Payers section template ******** -->
<code code="48768-6" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC" displayName="Payments"/>
<title>Insurance Providers</title>
<text>
<table border="1" width="100%">
<thead>
<tr>
<th>Payer name</th>
<th>Policy type / Coverage type</th>
<th>Policy ID</th>
<th>Covered party ID</th>
<th>Policy Holder</th>
</tr>
</thead>
<tbody>
<tr>
<td>Good Health Insurance</td>
<td>Extended healthcare / Family</td>
<td>Contract Number</td>
<td>1138345</td>
<td>Patient's Mother</td>
</tr>
</tbody>
</table>
</text>
<entry typeCode="DRIV">
<act classCode="ACT" moodCode="DEF">
<templateId root="2.16.840.1.113883.10.20.22.4.60"/>
<!-- **** Coverage entry template **** -->
...
</act>
</entry>
</section>
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 259
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
5.38 Physical Exam Section 29545-1
[section: templateId 2.16.840.1.113883.10.20.2.10 (open)]
Table 84: Physical Exam Section Contexts
Used By:
Contains Entries:
Progress Note (optional)
Consultation Note (optional)
History and Physical (required)
Procedure Note (optional)
Highest Pressure Ulcer Stage
Number of Pressure Ulcers Observation
Pressure Ulcer Observation
The Physical Exam section includes direct observations made by the clinician.
The examination may include the use of simple instruments and may also
describe simple maneuvers performed directly on the patient’s body. This
section includes only observations made by the examining clinician using
inspection, palpation, auscultation, and percussion; it does not include
laboratory or imaging findings. The exam may be limited to pertinent body
systems based on the patient’s chief complaint or it may include a
comprehensive examination. The examination may be reported as a collection of
random clinical statements or it may be reported categorically.
The Physical Exam section may contain multiple nested subsections: Vital
Signs, General Status, and those listed in the Additional Physical Examination
Subsections appendix.
1. SHALL contain exactly one [1..1] templateId (CONF:7806) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.2.10" (CONF:10465).
2. SHALL contain exactly one [1..1] code (CONF:15397).
a. This code SHALL contain exactly one [1..1] @code="29545-1" Physical
Findings (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:15398).
3. SHALL contain exactly one [1..1] title (CONF:7808).
4. SHALL contain exactly one [1..1] text (CONF:7809).
5. MAY contain zero or more [0..*] entry (CONF:17094) such that it
a. SHALL contain exactly one [1..1] Pressure Ulcer Observation
(templateId:2.16.840.1.113883.10.20.22.4.70) (CONF:17095).
6. MAY contain zero or more [0..*] entry (CONF:17096) such that it
a. SHALL contain exactly one [1..1] Number of Pressure Ulcers
Observation (templateId:2.16.840.1.113883.10.20.22.4.76)
(CONF:17097).
7. MAY contain zero or more [0..*] entry (CONF:17098) such that it
a. SHALL contain exactly one [1..1] Highest Pressure Ulcer Stage
(templateId:2.16.840.1.113883.10.20.22.4.77) (CONF:17099).

Page 260 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 123: Physical exam section example
<section>
<templateId root="2.16.840.1.113883.10.20.2.10"/>
<code codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"
code="29545-1" displayName="PHYSICAL FINDINGS"/>
<title>PHYSICAL EXAMINATION</title>
<text>
<paragraph>All normal to examination.</paragraph>
</text>
</section>
5.39 Plan of Care Section 18776-5
[section: templateId 2.16.840.1.113883.10.20.22.2.10(open)]
Table 85: Plan of Care Section Contexts
Used By:
Contains Entries:
Progress Note (optional)
Consultation Note (optional)
Discharge Summary (required)
History and Physical (optional)
Procedure Note (optional)
Operative Note (optional)
Continuity of Care Document (CCD) (optional)
Instructions
Plan of Care Activity Act
Plan of Care Activity Encounter
Plan of Care Activity Observation
Plan of Care Activity Procedure
Plan of Care Activity Substance Administration
Plan of Care Activity Supply
Figure 124: Plan of care section UML diagram
*The Large UML Diagrams appendix provides a larger version of this diagram
The Plan of Care section contains data that defines pending orders,
interventions, encounters, services, and procedures for the patient. It is limited
to prospective, unfulfilled, or incomplete orders and requests only, which are
indicated by the @moodCode of the entries within this section. All active,
incomplete, or pending orders, appointments, referrals, procedures, services, or
any other pending event of clinical significance to the current care of the patient
should be listed unless constrained due to privacy issues. The plan may also
contain information about ongoing care of the patient and information regarding
goals and clinical reminders. Clinical reminders are placed here to provide
prompts for disease prevention and management, patient safety, and health-care
quality improvements, including widely accepted performance measures. The
plan may also indicate that patient education will be provided.
1. SHALL contain exactly one [1..1] templateId (CONF:7723) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.10" (CONF:10435).
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 261
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
2. SHALL contain exactly one [1..1] code (CONF:14749).
a. This code SHALL contain exactly one [1..1] @code="18776-5" Plan of
Care (CodeSystem: LOINC 2.16.840.1.113883.6.1) (CONF:14750).
3. SHALL contain exactly one [1..1] title (CONF:16986).
4. SHALL contain exactly one [1..1] text (CONF:7725).
5. MAY contain zero or more [0..*] entry (CONF:7726) such that it
a. SHALL contain exactly one [1..1] Plan of Care Activity Act
(2.16.840.1.113883.10.20.22.4.39) (CONF:8804).
6. MAY contain zero or more [0..*] entry (CONF:8805) such that it
a. SHALL contain exactly one [1..1] Plan of Care Activity
Encounter (2.16.840.1.113883.10.20.22.4.40) (CONF:8806).
7. MAY contain zero or more [0..*] entry (CONF:8807) such that it
a. SHALL contain exactly one [1..1] Plan of Care Activity
Observation (2.16.840.1.113883.10.20.22.4.44) (CONF:8808).
8. MAY contain zero or more [0..*] entry (CONF:8809) such that it
a. SHALL contain exactly one [1..1] Plan of Care Activity
Procedure (2.16.840.1.113883.10.20.22.4.41) (CONF:8810).
9. MAY contain zero or more [0..*] entry (CONF:8811) such that it
a. SHALL contain exactly one [1..1] Plan of Care Activity
Substance Administration
(2.16.840.1.113883.10.20.22.4.42) (CONF:8812).
10. MAY contain zero or more [0..*] entry (CONF:8813) such that it
a. SHALL contain exactly one [1..1] Plan of Care Activity Supply
(templateId:2.16.840.1.113883.10.20.22.4.43) (CONF:14756).
11. MAY contain zero or more [0..*] entry (CONF:14695) such that it
a. SHALL contain exactly one [1..1] Instructions
(templateId:2.16.840.1.113883.10.20.22.4.20) (CONF:16751).
Figure 125: Plan of care section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.10" />
<!-- **** Plan of Care section template **** -->
<code code="18776-5" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC" displayName="Treatment plan"/>
<title>Plan of Care</title>
<text>
...
</text>
<entry typeCode="DRIV">
<observation classCode="OBS" moodCode="RQO">
<templateId root="2.16.840.1.113883.10.20.22.4.44"/>
<!-- **** Plan of Care Activity Observation template **** --
>
...
</observation>
</entry>

Page 262 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
<entry>
<act moodCode="RQO" classCode="ACT">
<templateId root="2.16.840.1.113883.10.20.22.4.39"/>
<!-- **** Plan of Care Activity Act template **** -->
...
</act>
</entry>
<entry>
<encounter moodCode="INT" classCode="ENC">
<templateId root="2.16.840.1.113883.10.20.22.4.40"/>
<!-- **** Plan of Care Activity Encounter template **** -->
...
</encounter>
</entry>
<entry>
<procedure moodCode="RQO" classCode="PROC">
<templateId root="2.16.840.1.113883.10.20.22.4.41"/>
<!-- **** Plan of Care Activity Procedure Template **** -->
...
</procedure>
</entry>
<entry>
<substanceAdministration moodCode="RQO" classCode="SBADM">
<templateId root="2.16.840.1.113883.10.20.22.4.42"/>
<!-- **** Plan of Care Activity Substance Administration **** --
>
...
</substanceAdministration>
</entry>
<entry>
<supply moodCode="INT" classCode="SPLY">
<templateId root="2.16.840.1.113883.10.20.22.4.43"/>
<!-- ** Plan of Care Activity Supply ** -->
...
</supply>
</entry>
</section>
5.40 Planned Procedure Section 59772-4
[section: templateId 2.16.840.1.113883.10.20.22.2.30(open)]
Table 86: Planned Procedure Section Contexts
Used By:
Contains Entries:
Procedure Note (optional)
Operative Note (optional)
Plan of Care Activity Procedure
The Planned Procedure section records the procedure(s) that a clinician thought
would need to be done based on the preoperative assessment. It may be
important to record the procedure(s) that were originally planned for, consented
to, and perhaps pre-approved by the payor, particularly if different from the
actual procedure(s) and procedure details, to provide evidence to various
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 263
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
stakeholders that the providers are aware of the discrepancy and the
justification can be found in the procedure details.
1. SHALL contain exactly one [1..1] templateId (CONF:8082) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.30" (CONF:10436).
2. SHALL contain exactly one [1..1] code (CONF:15399).
a. This code SHALL contain exactly one [1..1] @code="59772-4" Planned
Procedure (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:15400).
3. SHALL contain exactly one [1..1] title (CONF:8084).
4. SHALL contain exactly one [1..1] text (CONF:8085).
5. MAY contain zero or more [0..*] entry (CONF:8744) such that it
a. SHALL contain exactly one [1..1] Plan of Care Activity
Procedure (2.16.840.1.113883.10.20.22.4.41) (CONF:8766).
Figure 126: Planned procedure section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.30"/>
<!-- ******** Planned Procedure Section template ******** -->
<code code="59772-4" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC" displayName="Planned Procedure"/>
<title>Planned Procedure</title>
<text>
...
</text>
<entry>
<procedure moodCode="RQO" classCode="PROC">
<templateId root="2.16.840.1.113883.10.20.22.4.41"/>
<!-- ** Plan of Care Activity Procedure Template ** -->
...
</procedure>
</entry>
</section>
5.41 Postoperative Diagnosis Section 10218-6
[section: templateId 2.16.840.1.113883.10.20.22.2.35(open)]
Table 87: Postoperative Diagnosis Section Contexts
Used By:
Contains Entries:
Operative Note (required)
The Postoperative Diagnosis section records the diagnosis or diagnoses
discovered or confirmed during the surgery. Often it is the same as the
preoperative diagnosis.
1. SHALL contain exactly one [1..1] templateId (CONF:8101) such that it

Page 264 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.35" (CONF:10437).
2. SHALL contain exactly one [1..1] code (CONF:15401).
a. This code SHALL contain exactly one [1..1] @code="10218-6"
Postoperative Diagnosis (CodeSystem: LOINC
2.16.840.1.113883.6.1) (CONF:15402).
3. SHALL contain exactly one [1..1] title (CONF:8103).
4. SHALL contain exactly one [1..1] text (CONF:8104).
Figure 127: Postoperative diagnosis section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.35"/>
<code code="10218-6"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="POSTOPERATIVE DIAGNOSIS"/>
<title>Postoperative Diagnosis</title>
<text>Appendicitis with periappendiceal abscess</text>
</section>
5.42 Postprocedure Diagnosis Section 59769-0
[section: templateId 2.16.840.1.113883.10.20.22.2.36(open)]
Table 88: Postprocedure Diagnosis Section Contexts
Used By:
Contains Entries:
Procedure Note (required)
Postprocedure Diagnosis
The Postprocedure Diagnosis section records the diagnosis or diagnoses
discovered or confirmed during the procedure. Often it is the same as the pre-
procedure diagnosis or indication.
1. SHALL contain exactly one [1..1] templateId (CONF:8167) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.36" (CONF:10438).
2. SHALL contain exactly one [1..1] code (CONF:15403).
a. This code SHALL contain exactly one [1..1] @code="59769-0"
Postprocedure Diagnosis (CodeSystem: LOINC
2.16.840.1.113883.6.1) (CONF:15404).
3. SHALL contain exactly one [1..1] title (CONF:8170).
4. SHALL contain exactly one [1..1] text (CONF:8171).
5. SHOULD contain zero or one [0..1] entry (CONF:8762) such that it
a. SHALL contain exactly one [1..1] Postprocedure Diagnosis
(2.16.840.1.113883.10.20.22.4.51) (CONF:8764).
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 265
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 128: Postprocedure diagnosis section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.36"/>
<code code="59769-0" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC" displayName="POSTPROCEDURE DIAGNOSIS"/>
<title>Postprocedure Diagnosis</title>
<text>
...
</text>
<entry>
<act moodCode="EVN" classCode="ACT">
<templateId root="2.16.840.1.113883.10.20.22.4.51"/>
<!-- ** Postprocedure Diagnosis Entry ** -->
...
</act>
</entry>
</section>
5.43 Preoperative Diagnosis Section 10219-4
[section: templateId 2.16.840.1.113883.10.20.22.2.34(open)]
Table 89: Preoperative Diagnosis Section Contexts
Used By:
Contains Entries:
Operative Note (required)
Preoperative Diagnosis
The Preoperative Diagnosis section records the surgical diagnosis or diagnoses
assigned to the patient before the surgical procedure and is the reason for the
surgery. The preoperative diagnosis is, in the opinion of the surgeon, the
diagnosis that will be confirmed during surgery.
1. SHALL contain exactly one [1..1] templateId (CONF:8097) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.34" (CONF:10439).
2. SHALL contain exactly one [1..1] code (CONF:15405).
a. This code SHALL contain exactly one [1..1] @code="10219-4"
Preoperative Diagnosis (CodeSystem: LOINC
2.16.840.1.113883.6.1) (CONF:15406).
3. SHALL contain exactly one [1..1] title (CONF:8099).
4. SHALL contain exactly one [1..1] text (CONF:8100).
5. SHOULD contain zero or one [0..1] entry (CONF:10096) such that it
a. SHALL contain exactly one [1..1] Preoperative Diagnosis
(2.16.840.1.113883.10.20.22.4.65) (CONF:10097).
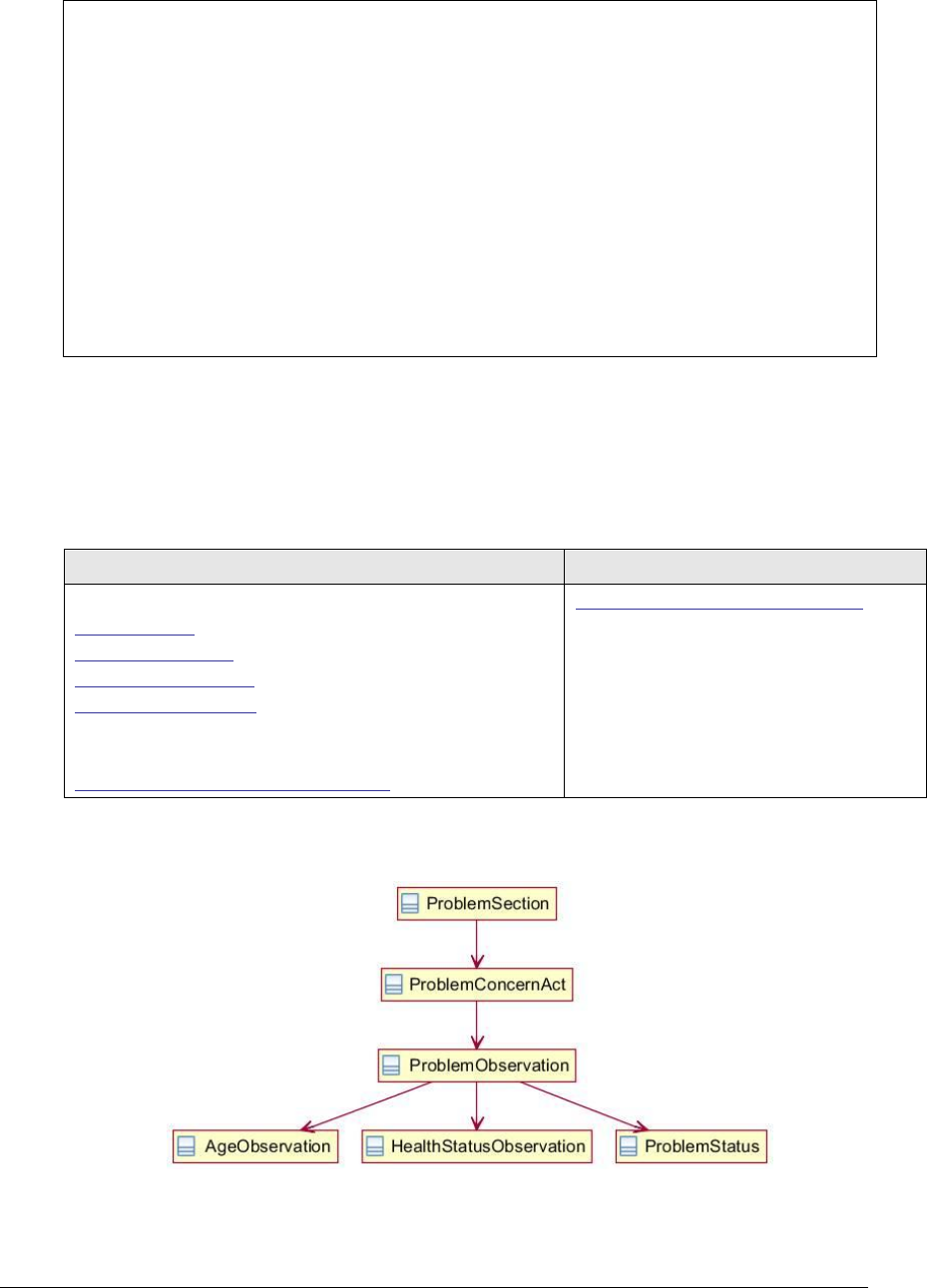
Page 266 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 129: Preoperative diagnosis section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.34"/>
<code code="10219-4"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="SURGICAL OPERATION NOTE PREOPERATIVE DX"/>
<title>Preoperative Diagnosis</title>
<text>Appendicitis</text>
<entry>
<act moodCode="EVN" classCode="ACT">
<templateId root="2.16.840.1.113883.10.20.22.4.65"/>
<!-- ** Preoperative Diagnosis Entry ** -->
...
</act>
</entry>
</section>
5.44 Problem Section 11450-4
Table 90: Problem Section Contexts
Used By:
Contains Entries:
Entries optional:
Progress Note (optional)
Consultation Note (optional)
Discharge Summary (optional)
History and Physical (optional)
Entries required:
Continuity of Care Document (CCD) (required)
Problem Concern Act (Condition)
Figure 130: Problem section UML diagram

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 267
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
This section lists and describes all relevant clinical problems at the time the
document is generated. At a minimum, all pertinent current and historical
problems should be listed.
Problem Section with Coded Entries Optional
[section: templateId 2.16.840.1.113883.10.20.22.2.5(open)]
The following constraints apply to a Problem section in which entries are not
required.
1. SHALL contain exactly one [1..1] templateId (CONF:7877) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.5" (CONF:10440).
2. SHALL contain exactly one [1..1] code (CONF:15407).
a. This code SHALL contain exactly one [1..1] @code="11450-4" Problem
List (CodeSystem: LOINC 2.16.840.1.113883.6.1) (CONF:15408).
3. SHALL contain exactly one [1..1] title (CONF:7879).
4. SHALL contain exactly one [1..1] text (CONF:7880).
5. SHOULD contain zero or more [0..*] entry (CONF:7881).
a. SHALL contain exactly one [1..1] Problem Concern Act
(Condition) (2.16.840.1.113883.10.20.22.4.3) (CONF:7882).
Problem Section with Coded Entries Required
[section: templateId 2.16.840.1.113883.10.20.22.2.5.1(open)]
The following constraints apply to a Problem section in which entries are
required.
1. Conforms to Problem Section (entries optional) template
(2.16.840.1.113883.10.20.22.2.5).
2. SHALL contain exactly one [1..1] templateId (CONF:9179) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.5.1" (CONF:10441).
3. SHALL contain exactly one [1..1] code (CONF:15409).
a. This code SHALL contain exactly one [1..1] @code="11450-4" Problem
List (CodeSystem: LOINC 2.16.840.1.113883.6.1) (CONF:15410).
4. SHALL contain exactly one [1..1] title (CONF:9181).
5. SHALL contain exactly one [1..1] text (CONF:9182).
6. SHALL contain at least one [1..*] entry (CONF:9183).
a. SHALL contain exactly one [1..1] Problem Concern Act
(Condition) (2.16.840.1.113883.10.20.22.4.3) (CONF:9184).

Page 268 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 131: Problem section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.5"/>
<code code="11450-4" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="PROBLEM LIST"/>
<title>PROBLEMS</title>
<text>
<list listType="ordered">
<item>Pneumonia: Resolved in March 1998 </item>
<item>...</item>
</list>
</text>
<entry typeCode="DRIV">
<act classCode="ACT" moodCode="EVN">
<!-- Problem Concern Act (Condition) template -->
...
</act>
</entry>
</section>
Figure 132: Pressure ulcer on a problem list example
<component>
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.5"/>
<code code="11450-4" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC" displayName="PROBLEM LIST"/>
<title>Problems</title>
<text>
<list listType="ordered">
<item>2 Stage 3 Pressure Ulcers</item>
<item>...</item>
</list>
</text>
<entry typeCode="DRIV">
<act classCode="ACT" moodCode="EVN">
<!-- Problem act template -->
<templateId root="2.16.840.1.113883.10.20.22.4.3"/>
<id root="ec8a6ff8-ed4b-4f7e-82c3-e98e58b45de7"/>
<code code="CONC" displayName="Concern"
codeSystem="2.16.840.1.113883.5.6"
codeSystemName="HL7ActClass"/>
<statusCode code="completed"/>
<effectiveTime>
<!-- date of onset -->
<low value="20120101"/>
</effectiveTime>
</act>
</entry>
</section>
</component>

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 269
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
5.45 Procedure Description Section 29554-3
[section: templateId 2.16.840.1.113883.10.20.22.2.27(open)]
Table 91: Procedure Description Section Contexts
Used By:
Contains Entries:
Procedure Note (required)
Operative Note (required)
The Procedure Description section records the particulars of the procedure and
may include procedure site preparation, surgical site preparation, pertinent
details related to sedation/anesthesia, pertinent details related to measurements
and markings, procedure times, medications administered, estimated blood loss,
specimens removed, implants, instrumentation, sponge counts, tissue
manipulation, wound closure, sutures used, vital signs and other monitoring
data. Local practice often identifies the level and type of detail required based on
the procedure or specialty.
1. SHALL contain exactly one [1..1] templateId (CONF:8062) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.27" (CONF:10442).
2. SHALL contain exactly one [1..1] code (CONF:15411).
a. This code SHALL contain exactly one [1..1] @code="29554-3"
Procedure Description (CodeSystem: LOINC
2.16.840.1.113883.6.1) (CONF:15412).
3. SHALL contain exactly one [1..1] title (CONF:8064).
4. SHALL contain exactly one [1..1] text (CONF:8065).
Figure 133: Procedure description section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.27" />
<code code="29554-3"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="PROCEDURE DESCRIPTION" />
<title>Procedure Description</title>
<text>The patient was taken to the endoscopy suite where ...
</text>
</section>
Page 270 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
5.46 Procedure Disposition Section 59775-7
[section: templateId 2.16.840.1.113883.10.20.18.2.12(open)]
Table 92: Procedure Disposition Section Contexts
Used By:
Contains Entries:
Procedure Note (optional)
Operative Note (optional)
The Procedure Disposition section records the status and condition of the
patient at the completion of the procedure or surgery. It often also states where
the patent was transferred to for the next level of care.
1. SHALL contain exactly one [1..1] templateId (CONF:8070) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.18.2.12" (CONF:10466).
2. SHALL contain exactly one [1..1] code (CONF:15413).
a. This code SHALL contain exactly one [1..1] @code="59775-7"
Procedure Disposition (CodeSystem: LOINC
2.16.840.1.113883.6.1) (CONF:15414).
3. SHALL contain exactly one [1..1] title (CONF:8072).
4. SHALL contain exactly one [1..1] text (CONF:8073).
Figure 134: Procedure disposition section example
<section>
<templateId root="2.16.840.1.113883.10.20.18.2.12"/>
<code code="59775-7" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="PROCEDURE DISPOSITION"/>
<title>PROCEDURE DISPOSITION</title>
<text>The patient was taken to the Endoscopy Recovery Unit in stable
condition.</text>
</section>
5.47 Procedure Estimated Blood Loss Section 59770-8
[section: templateId 2.16.840.1.113883.10.20.18.2.9(open)]
Table 93: Procedure Estimated Blood Loss Section Contexts
Used By:
Contains Entries:
Procedure Note (optional)
Operative Note (required)
The Estimated Blood Loss section may be a subsection of another section such
as the Procedure Description section. The Estimated Blood Loss section records
the approximate amount of blood that the patient lost during the procedure or
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 271
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
surgery. It may be an accurate quantitative amount, e.g., 250 milliliters, or it
may be descriptive, e.g., “minimal” or “none”.
1. SHALL contain exactly one [1..1] templateId (CONF:8074) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.18.2.9" (CONF:10467).
2. SHALL contain exactly one [1..1] code (CONF:15415).
a. This code SHALL contain exactly one [1..1] @code="59770-8"
Procedure Estimated Blood Loss (CodeSystem: LOINC
2.16.840.1.113883.6.1) (CONF:15416).
3. SHALL contain exactly one [1..1] title (CONF:8076).
4. SHALL contain exactly one [1..1] text (CONF:8077).
5. The Estimated Blood Loss section SHALL include a statement providing an
estimate of the amount of blood lost during the procedure, even if the
estimate is text, such as "minimal" or "none" (CONF:8741).
Figure 135: Procedure estimated blood loss section example
<section>
<templateId root="2.16.840.1.113883.10.20.18.2.9"/>
<code code="59770-8" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC" displayName="PROCEDURE ESTIMATED BLOOD
LOSS"/>
<title>Procedure Estimated Blood Loss</title>
<text>Minimal</text>
</section>
5.48 Procedure Findings Section 59776-5
[section: templateId 2.16.840.1.113883.10.20.22.2.28(open)]
Table 94: Procedure Findings Section Contexts
Used By:
Contains Entries:
Procedure Note (optional)
Operative Note (required)
Problem Observation
The Procedure Findings section records clinically significant observations
confirmed or discovered during the procedure or surgery.
1. SHALL contain exactly one [1..1] templateId (CONF:8078) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.28" (CONF:10443).
2. SHALL contain exactly one [1..1] code (CONF:15417).
a. This code SHALL contain exactly one [1..1] @code="59776-5"
Procedure Findings (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:15418).
3. SHALL contain exactly one [1..1] title (CONF:8080).
4. SHALL contain exactly one [1..1] text (CONF:8081).
Page 272 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
5. MAY contain zero or more [0..*] entry (CONF:8090) such that it
a. SHALL contain exactly one [1..1] Problem Observation
(2.16.840.1.113883.10.20.22.4.4) (CONF:8091).
Figure 136: Procedure findings section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.28" />
<code code="59776-5"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="PROCEDURE FINDINGS" />
<title>Procedure Findings</title>
<text>A 6 mm sessile polyp was found in the ascending colon and
removed by
snare, no cautery. Bleeding was controlled. Moderate
diverticulosis
and hemorrhoids were incidentally noted.</text>
<entry>
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.4"/>
<!-- Problem Observation -->
...
</observation>
</entry>
</section>
5.49 Procedure Implants Section 59771-6
[section: templateId 2.16.840.1.113883.10.20.22.2.40(open)]
Table 95: Procedure Implants Section Contexts
Used By:
Contains Entries:
Procedure Note (optional)
Operative Note (optional)
The Procedure Implants section records any materials placed during the
procedure including stents, tubes, and drains.
1. SHALL contain exactly one [1..1] templateId (CONF:8178) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.40" (CONF:10444).
2. SHALL contain exactly one [1..1] code (CONF:15373).
a. This code SHALL contain exactly one [1..1] @code="59771-6"
Procedure Implants (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:15374).
3. SHALL contain exactly one [1..1] title (CONF:8180).
4. SHALL contain exactly one [1..1] text (CONF:8181).
5. The Implants section SHALL include a statement providing details of the
implants placed, or assert no implants were placed (CONF:8769).
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 273
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 137: Procedure implants section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.40"/>
<code code="59771-6" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC" displayName="PROCEDURE IMPLANTS"/>
<title>Procedure Implants</title>
<text>No implants were placed.</text>
</section>
5.50 Procedure Indications Section 59768-2
[section: templateId 2.16.840.1.113883.10.20.22.2.29(open)]
Table 96: Procedure Indications Section Contexts
Used By:
Contains Entries:
Procedure Note (required)
Operative Note (optional)
Indication
The Procedure Indications section records details about the reason for the
procedure or surgery. This section may include the pre-procedure diagnosis or
diagnoses as well as one or more symptoms that contribute to the reason the
procedure is being performed.
1. SHALL contain exactly one [1..1] templateId (CONF:8058) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.29" (CONF:10445).
2. SHALL contain exactly one [1..1] code (CONF:15419).
a. This code SHALL contain exactly one [1..1] @code="59768-2"
Procedure Indications (CodeSystem: LOINC
2.16.840.1.113883.6.1) (CONF:15420).
3. SHALL contain exactly one [1..1] title (CONF:8060).
4. SHALL contain exactly one [1..1] text (CONF:8061).
5. MAY contain zero or more [0..*] entry (CONF:8743) such that it
a. SHALL contain exactly one [1..1] Indication
(2.16.840.1.113883.10.20.22.4.19) (CONF:8765).
Page 274 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 138: Procedure indications section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.29"/>
<code code="59768-2" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC" displayName="PROCEDURE INDICATIONS"/>
<title>Procedure Indications</title>
<text>The procedure is performed for screening in a low risk
individual.
</text>
<entry>
<observation classCode="OBS" moodCode="EVN">
<!-- Indication Entry -->
<templateId root="2.16.840.1.113883.10.20.22.4.19"/>
...
</observation>
</entry>
</section>
5.51 Procedure Specimens Taken Section 59773-2
[section: templateId 2.16.840.1.113883.10.20.22.2.31(open)]
Table 97: Procedure Specimens Taken Section Contexts
Used By:
Contains Entries:
Procedure Note (optional)
Operative Note (required)
The Procedure Specimens Taken section records the tissues, objects, or samples
taken from the patient during the procedure including biopsies, aspiration fluid,
or other samples sent for pathological analysis. The narrative may include a
description of the specimens.
1. SHALL contain exactly one [1..1] templateId (CONF:8086) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.31" (CONF:10446).
2. SHALL contain exactly one [1..1] code (CONF:15421).
a. This code SHALL contain exactly one [1..1] @code="59773-2"
Procedure Specimens Taken (CodeSystem: LOINC
2.16.840.1.113883.6.1) (CONF:15422).
3. SHALL contain exactly one [1..1] title (CONF:8088).
4. SHALL contain exactly one [1..1] text (CONF:8089).
5. 5. The Procedure Specimens Taken section SHALL list all specimens removed
or SHALL explicitly state that no specimens were taken (CONF:8742).
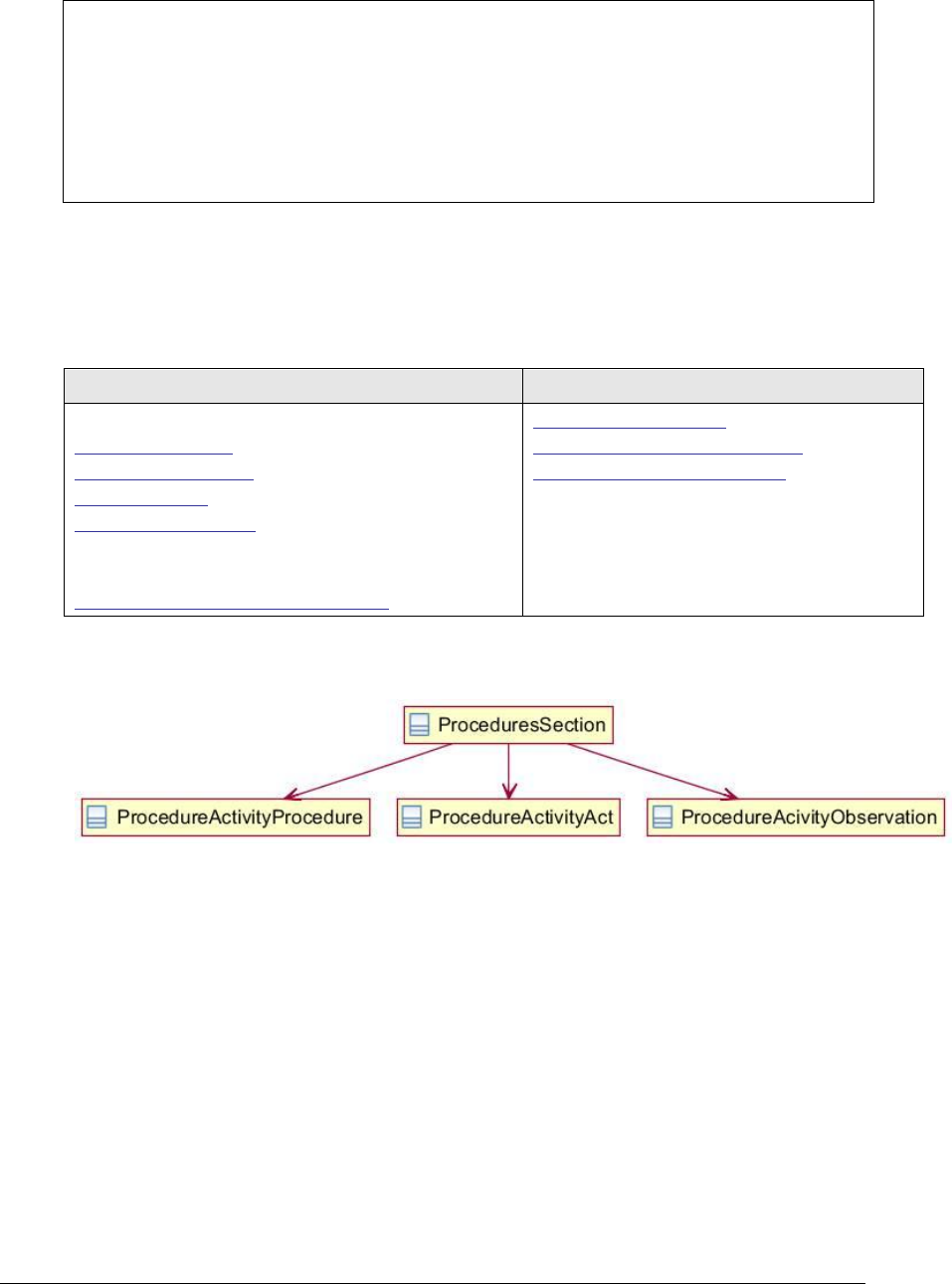
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 275
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 139: Procedure specimens taken section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.31"/>
<code code="59773-2"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="PROCEDURE SPECIMENS TAKEN"/>
<title>Procedure Specimens Taken</title>
<text>Ascending colon polyp</text>
</section>
5.52 Procedures Section 47519-4
Table 98: Procedures Section Contexts
Used By:
Contains Entries:
Entries optional:
Consultation Note (optional)
Discharge Summary (optional)
Procedure Note (optional)
History and Physical (optional)
Entries required:
Continuity of Care Document (CCD) (optional)
Procedure Activity Act
Procedure Activity Observation
Procedure Activity Procedure
Figure 140: Procedures section UML diagram
This section defines all interventional, surgical, diagnostic, or therapeutic
procedures or treatments pertinent to the patient historically at the time the
document is generated. The section is intended to include notable procedures,
but can contain all procedures for the period of time being summarized. The
common notion of "procedure" is broader than that specified by the HL7 Version
3 Reference Information Model (RIM). Therefore this section contains procedure
templates represented with three RIM classes: Act. Observation, and Procedure.
Procedure act is for procedures the alter that physical condition of a patient
(Splenectomy). Observation act is for procedures that result in new information
about a patient but do not cause physical alteration (EEG). Act is for all other
types of procedures (dressing change).

Page 276 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
The length of an encounter is documented in the
documentationOf/encompassingEncounter/effectiveTime and length of
service in documentationOf/ServiceEvent/effectiveTime.
Procedures Section with Coded Entries Optional
[section: templateId 2.16.840.1.113883.10.20.22.2.7(open)]
The following constraints apply to a Procedures section in which entries are not
required.
1. SHALL contain exactly one [1..1] templateId (CONF:6270) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.7" (CONF:6271).
2. SHALL contain exactly one [1..1] code (CONF:15423).
a. This code SHALL contain exactly one [1..1] @code="47519-4" History
of Procedures (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:15424).
3. SHALL contain exactly one [1..1] title (CONF:17184).
4. SHALL contain exactly one [1..1] text (CONF:6273).
5. MAY contain zero or more [0..*] entry (CONF:6274) such that it
a. SHALL contain exactly one [1..1] Procedure Activity Procedure
(templateId:2.16.840.1.113883.10.20.22.4.14) (CONF:15509).
6. MAY contain zero or one [0..1] entry (CONF:6278) such that it
a. SHALL contain exactly one [1..1] Procedure Activity Observation
(templateId:2.16.840.1.113883.10.20.22.4.13) (CONF:15510).
7. MAY contain zero or one [0..1] entry (CONF:8533) such that it
a. SHALL contain exactly one [1..1] Procedure Activity Act
(templateId:2.16.840.1.113883.10.20.22.4.12) (CONF:15511).
Procedures Section with Coded Entries Required
[section: templateId 2.16.840.1.113883.10.20.22.2.7.1(open)]
The following constraints apply to a Procedures section in which entries are
required.
1. Conforms to Procedures Section (entries optional) template
(2.16.840.1.113883.10.20.22.2.7)
2. SHALL contain exactly one [1..1] templateId (CONF:7891) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.7.1" (CONF:10447).
3. SHALL contain exactly one [1..1] code (CONF:15425).
a. This code SHALL contain exactly one [1..1] @code="47519-4" History
of Procedures (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:15426).
4. SHALL contain exactly one [1..1] title (CONF:7893).
5. SHALL contain exactly one [1..1] text (CONF:7894).
6. MAY contain zero or more [0..*] entry (CONF:7895) such that it
a. SHALL contain exactly one [1..1] Procedure Activity Procedure
(2.16.840.1.113883.10.20.22.4.14) (CONF:7896).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 277
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
7. MAY contain zero or more [0..*] entry (CONF:8017) such that it
a. SHALL contain exactly one [1..1] Procedure Activity Observation
(2.16.840.1.113883.10.20.22.4.13) (CONF:8018).
8. MAY contain zero or more [0..*] entry (CONF:8019) such that it
a. SHALL contain exactly one [1..1] Procedure Activity Act
(2.16.840.1.113883.10.20.22.4.12) (CONF:8020).
9. There SHALL be at least one procedure, observation or act entry conformant
to Procedure Activity Procedure template, Procedure Activity Observation
template or Procedure Activity Act template in the Procedure Section
(CONF:8021).
Figure 141: Procedures section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.7"/>
<!-- Procedures section template -->
<code code="47519-4"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="PROCEDURES" />
<title>Procedures</title>
<text>
...
</text>
<entry typeCode="DRIV">
<procedure classCode="PROC" moodCode="EVN">
<!-- Procedure Activity Procedure template -->
<templateId root="2.16.840.1.113883.10.20.22.4.14"/>
...
</procedure>
</entry>
</entry>
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.13"/>
<!-- Procedure Activity Observation template -->
...
</observation>
</entry>
<entry>
<act classCode="ACT" moodCode="INT">
<templateId root="2.16.840.1.113883.10.20.22.4.12"/>
<!-- Procedure Activity Act template -->
...
</act>
</entry>
</section>

Page 278 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
5.53 Reason for Referral Section 42349-1
[section: templateId 1.3.6.1.4.1.19376.1.5.3.1.3.1(open)]
Table 99: Reason for Referral Section Contexts
Used By:
Contains Entries:
Consultation Note (optional)
A Reason for Referral section records the reason the patient is being referred for
a consultation by a provider. An optional Chief Complaint section may capture
the patient’s description of the reason for the consultation.
1. SHALL contain exactly one [1..1] templateId (CONF:7844) such that it
a. SHALL contain exactly one [1..1]
@root="1.3.6.1.4.1.19376.1.5.3.1.3.1" (CONF:10468).
2. SHALL contain exactly one [1..1] code (CONF:15427).
a. This code SHALL contain exactly one [1..1] @code="42349-1" Reason
for Referral (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:15428).
3. SHALL contain exactly one [1..1] title (CONF:7846).
4. SHALL contain exactly one [1..1] text (CONF:7847).
Figure 142: Reason for referral section example
<section>
<templateId root="1.3.6.1.4.1.19376.1.5.3.1.3.1"/>
<!-- ** Reason for Referral Section Template ** -->
<code codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC" code="42349-1"
displayName="REASON FOR REFERRAL"/>
<title>REASON FOR REFERRAL</title>
<text>
<paragraph>Lumbar spinal stenosis with radiculopathy.</paragraph>
</text>
</section>

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 279
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
5.54 Reason for Visit Section 29299-5
[section: templateId 2.16.840.1.113883.10.20.22.2.12(open)]
Table 100: Reason for Visit Section Contexts
Used By:
Contains Entries:
Consultation Note (optional)
Discharge Summary (optional)
History and Physical (required)
Procedure Note (optional)
This section records the patient’s reason for the patient's visit (as documented
by the provider). Local policy determines whether Reason for Visit and Chief
Complaint are in separate or combined sections.
1. SHALL contain exactly one [1..1] templateId (CONF:7836) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.12" (CONF:10448).
2. SHALL contain exactly one [1..1] code (CONF:15429).
a. This code SHALL contain exactly one [1..1] @code="29299-5" Reason
for Visit (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:15430).
3. SHALL contain exactly one [1..1] title (CONF:7838).
4. SHALL contain exactly one [1..1] text (CONF:7839).
Figure 143: Reason for visit section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.12"/>
<code code="29299-5"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="REASON FOR VISIT"/>
<title>REASON FOR VISIT</title>
<text>
<paragraph>Dark stools.</paragraph>
</text>
</section>
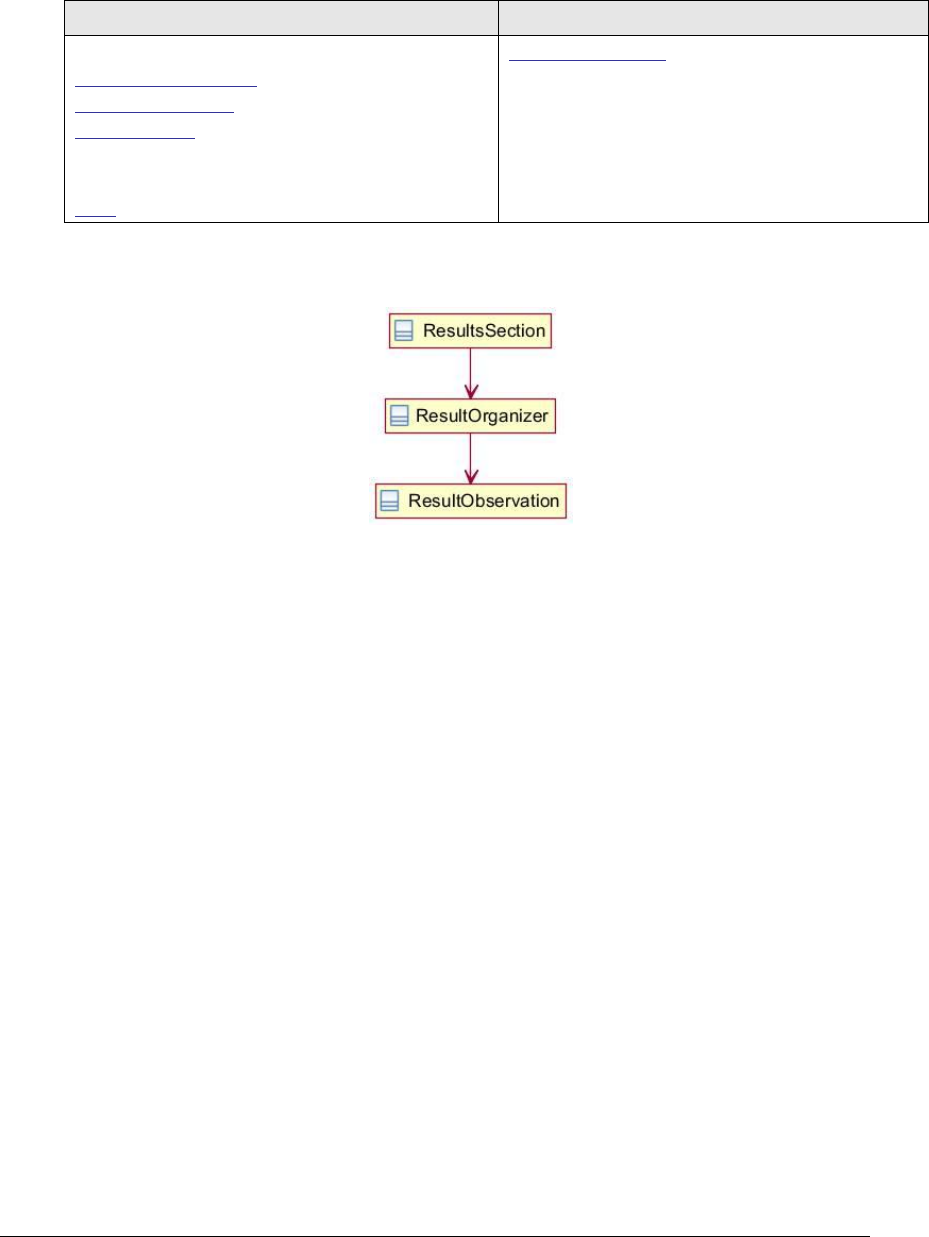
Page 280 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
5.55 Results Section 30954-2
Table 101: Results Section Contexts
Used by:
Contains entries:
Coded entries optional:
History and Physical (required)
Consultation Note (optional)
Progress Note (optional)
Coded entries required:
CCD (required)
Results Organizer
Figure 144: Results section UML diagram
The Results section contains the results of observations generated by
laboratories, imaging procedures, and other procedures. The scope includes
observations such as hematology, chemistry, serology, virology, toxicology,
microbiology, plain x-ray, ultrasound, CT, MRI, angiography, echocardiography,
nuclear medicine, pathology, and procedure observations. The section often
includes notable results such as abnormal values or relevant trends, and could
contain all results for the period of time being documented.
Laboratory results are typically generated by laboratories providing analytic
services in areas such as chemistry, hematology, serology, histology, cytology,
anatomic pathology, microbiology, and/or virology. These observations are based
on analysis of specimens obtained from the patient and submitted to the
laboratory.
Imaging results are typically generated by a clinician reviewing the output of an
imaging procedure, such as where a cardiologist reports the left ventricular
ejection fraction based on the review of a cardiac echocardiogram.
Procedure results are typically generated by a clinician to provide more granular
information about component observations made during a procedure, such as
where a gastroenterologist reports the size of a polyp observed during a
colonoscopy.
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 281
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Results Section with Coded Entries Optional
[section: templateId 2.16.840.1.113883.10.20.22.2.3(open)]
The following constraints apply to a Results section in which entries are not
required.
1. SHALL contain exactly one [1..1] templateId (CONF:7116) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.3" (CONF:9136).
2. SHALL contain exactly one [1..1] code (CONF:15431).
a. This code SHALL contain exactly one [1..1] @code="30954-2" Relevant
diagnostic tests and/or laboratory data (CodeSystem: LOINC
2.16.840.1.113883.6.1) (CONF:15432).
3. SHALL contain exactly one [1..1] title (CONF:8891).
4. SHALL contain exactly one [1..1] text (CONF:7118).
5. SHOULD contain zero or more [0..*] entry (CONF:7119) such that it
a. SHALL contain exactly one [1..1] Result Organizer
(2.16.840.1.113883.10.20.22.4.1) (CONF:7120).
Results Section with Coded Entries Required
[section: templateId 2.16.840.1.113883.10.20.22.2.3.1(open)]
The following constraints apply to a Results section in which entries are
required.
1. Conforms to Results Section (entries optional) template
(2.16.840.1.113883.10.20.22.2.3)
2. SHALL contain exactly one [1..1] templateId (CONF:7108) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.3.1" (CONF:9137).
3. SHALL contain exactly one [1..1] code (CONF:15433).
a. This code SHALL contain exactly one [1..1] @code="30954-2" Relevant
diagnostic tests and/or laboratory data (CodeSystem: LOINC
2.16.840.1.113883.6.1) (CONF:15434).
4. SHALL contain exactly one [1..1] title (CONF:8892).
5. SHALL contain exactly one [1..1] text (CONF:7111).
6. SHALL contain at least one [1..*] entry (CONF:7112) such that it
a. SHALL contain exactly one [1..1] Result Organizer
(2.16.840.1.113883.10.20.22.4.1) (CONF:7113).
Page 282 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 145: Results section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.3.1"/>
<code code="30954-2"
codeSystem="2.16.840.1.113883.6.1"/>
codeSystemName="LOINC"
displayName="RESULTS" />
<title>Results</title>
<text>
...
</text>
<entry typeCode="DRIV">
<organizer classCode="BATTERY" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.1"/>
...
</organizer>
</entry>
</section>
5.56 Review of Systems Section 10187-3
[section: templateId 1.3.6.1.4.1.19376.1.5.3.1.3.18(open)]
Table 102: Review of Systems Section Contexts
Used By:
Contains Entries:
Progress Note (optional)
Consultation Note (optional)
Discharge Summary (optional)
History and Physical (required)
Procedure Note (optional)
The Review of Systems section contains a relevant collection of symptoms and
functions systematically gathered by a clinician. It includes symptoms the
patient is currently experiencing, some of which were not elicited during the
history of present illness, as well as a potentially large number of pertinent
negatives, for example, symptoms that the patient denied experiencing.
1. SHALL contain exactly one [1..1] templateId (CONF:7812) such that it
a. SHALL contain exactly one [1..1]
@root="1.3.6.1.4.1.19376.1.5.3.1.3.18" (CONF:10469).
2. SHALL contain exactly one [1..1] code (CONF:15435).
a. This code SHALL contain exactly one [1..1] @code="10187-3" Review
of Systems (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:15436).
3. SHALL contain exactly one [1..1] title (CONF:7814).
4. SHALL contain exactly one [1..1] text (CONF:7815).
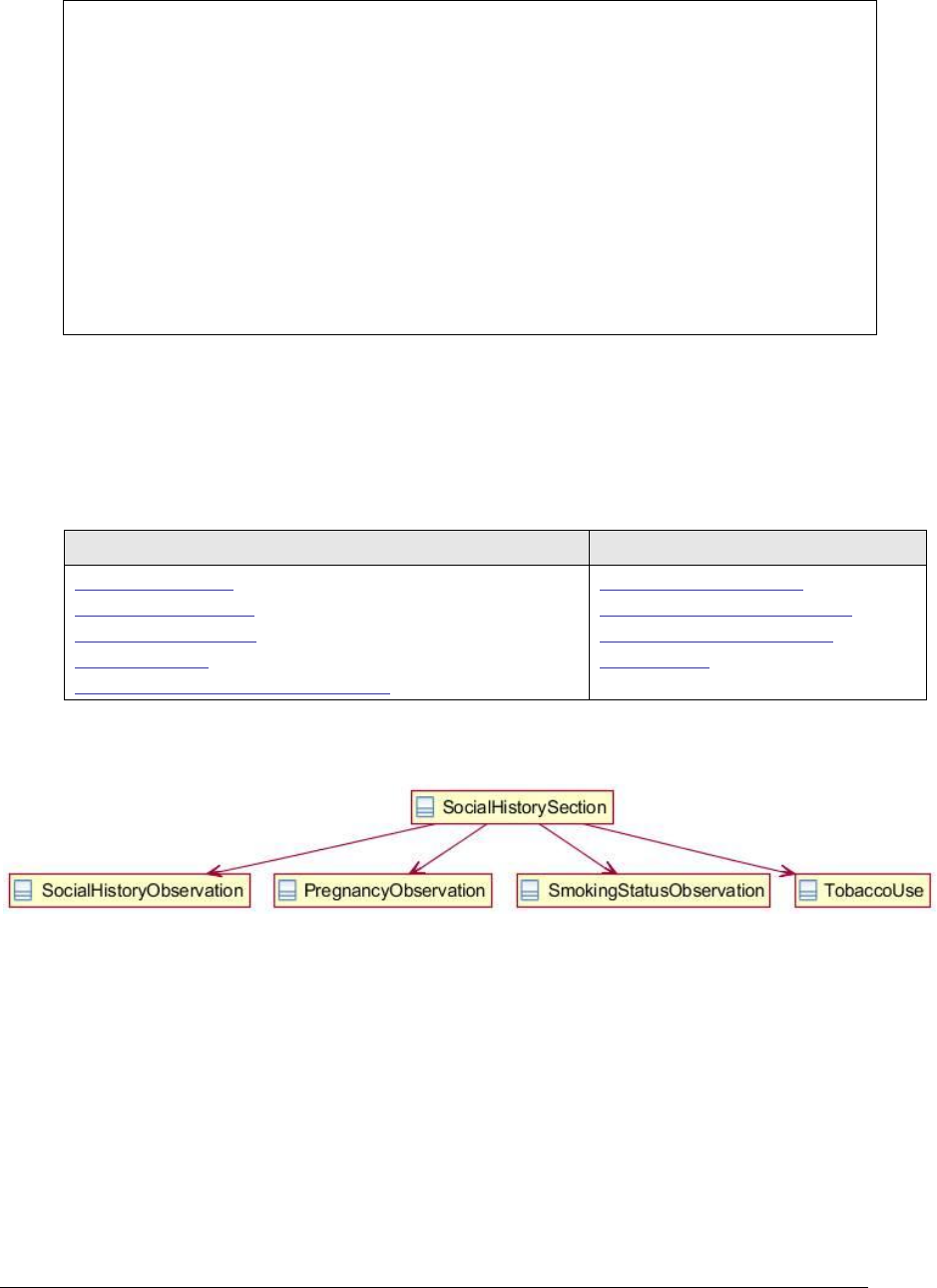
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 283
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 146: Review of systems section example
<section>
<templateId root="1.3.6.1.4.1.19376.1.5.3.1.3.18"/>
<code code="10187-3" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="REVIEW OF SYSTEMS"/>
<title>REVIEW OF SYSTEMS</title>
<text>
<paragraph>
Patient denies recent history of fever or malaise. Positive
For weakness and shortness of breath. One episode of melena. No
recent
headaches. Positive for osteoarthritis in hips, knees and hands.
</paragraph>
</text>
</section>
5.57 Social History Section 29762-2
[section: templateId 2.16.840.1.113883.10.20.22.2.17(open)]
Table 103: Social History Section Contexts
Used By:
Contains Entries:
Consultation Note (optional)
Discharge Summary (optional)
History and Physical (required)
Procedure Note (optional)
Continuity of Care Document (CCD) (optional)
Pregnancy Observation
Smoking Status Observation
Social History Observation
Tobacco Use
Figure 147: Social history section UML diagram
This section contains data defining the patient’s occupational, personal (e.g.
lifestyle), social, and environmental history and health risk factors, as well as
administrative data such as marital status, race, ethnicity and religious
affiliation. Social history can have significant influence on a patient’s physical,
psychological and emotional health and wellbeing so should be considered in the
development of a complete record.
1. SHALL contain exactly one [1..1] templateId (CONF:7936) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.17" (CONF:10449).

Page 284 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
2. SHALL contain exactly one [1..1] code (CONF:14819).
a. This code SHALL contain exactly one [1..1] @code="29762-2" Social
History (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:14820).
3. SHALL contain exactly one [1..1] title (CONF:7938).
4. SHALL contain exactly one [1..1] text (CONF:7939).
5. MAY contain zero or more [0..*] entry (CONF:7953) such that it
a. SHALL contain at least one [1..*] Social History Observation
(2.16.840.1.113883.10.20.22.4.38) (CONF:7954).
6. MAY contain zero or more [0..*] entry (CONF:9132) such that it
a. SHALL contain exactly one [1..1] Pregnancy Observation
(2.16.840.1.113883.10.20.15.3.8) (CONF:9133).
7. SHOULD contain zero or more [0..*] entry (CONF:14823) such that it
a. SHALL contain exactly one [1..1] Smoking Status Observation
(templateId:2.16.840.1.113883.10.22.4.78) (CONF:14824).
8. MAY contain zero or more [0..*] entry (CONF:16816) such that it
a. SHALL contain exactly one [1..1] Tobacco Use
(templateId:2.16.840.1.113883.10.20.22.4.85) (CONF:16817).
Figure 148: Social history section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.17"/>
<!-- ** Social history section template ** -->
<code code="29762-2" codeSystem="2.16.840.1.113883.6.1"
displayName="Social History"/>
<title>Social History</title>
<text>
...
</text>
<entry typeCode="DRIV">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.38"/>
<!-- ** Social history observation template ** -->
...
</observation>
</entry>
<entry typeCode="DRIV">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.38"/>
<!-- ** Social history observation template ** -->
...
</observation>
</entry>
</section>

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 285
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
5.58 Subjective Section 61150-9
[section: templateId 2.16.840.1.113883.10.20.21.2.2(open)]
Table 104: Subjective Section Contexts
Used By:
Contains Entries:
Progress Note (optional)
The Subjective section describes in a narrative format the patient’s current
condition and/or interval changes as reported by the patient or by the patient’s
guardian or another informant.
1. SHALL contain exactly one [1..1] templateId (CONF:7873) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.21.2.2" (CONF:10470).
2. SHALL contain exactly one [1..1] code (CONF:15437).
a. This code SHALL contain exactly one [1..1] @code="61150-9"
Subjective (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:15438).
3. SHALL contain exactly one [1..1] title (CONF:7875).
4. SHALL contain exactly one [1..1] text (CONF:7876).
Figure 149: Subjective section example
<section>
<templateId root="2.16.840.1.113883.10.20.21.2.2"/>
<code code="61150-9" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="SUBJECTIVE"/>
<title>SUBJECTIVE DATA</title>
<text>
<paragraph>
I have used the peripheral nerve stimulator in my back for five
days.
While using it I found that I was able to do physical activity
without pain. However, afterwards for one day, I would feel pain
but
then it would go away. I also noticed that I didn’t have to take
the
Vicodin as much. I took 2 less Vicodin per day and 2 less
tramadol
everyday. I have not lain in my bed in a year and a half. I sleep
in
a recliner.
</paragraph>
</text>
</section>

Page 286 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
5.59 Surgical Drains Section 11537-8
[section: templateId 2.16.840.1.113883.10.20.7.13(open)]
Table 105: Surgical Drains Section Contexts
Used By:
Contains Entries:
Operative Note (optional)
The Surgical Drains section may be used to record drains placed during the
surgical procedure. Optionally, surgical drain placement may be represented
with a text element in the Procedure Description Section.
1. SHALL contain exactly one [1..1] templateId (CONF:8038) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.7.13" (CONF:10473).
2. SHALL contain exactly one [1..1] code (CONF:15441).
a. This code SHALL contain exactly one [1..1] @code="11537-8" Surgical
Drains (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:15442).
3. SHALL contain exactly one [1..1] title (CONF:8040).
4. SHALL contain exactly one [1..1] text (CONF:8041).
5. If the Surgical Drains section is present, there SHALL be a statement
providing details of the drains placed or SHALL explicitly state there were no
drains placed (CONF:8056).
Figure 150: Surgical drains section example
<section>
<templateId root="2.16.840.1.113883.10.20.7.13"/>
<code code="11537-8"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="SURGICAL DRAINS"/>
<title>Surgical Drains</title>
<text>Penrose drain placed</text>
</section>
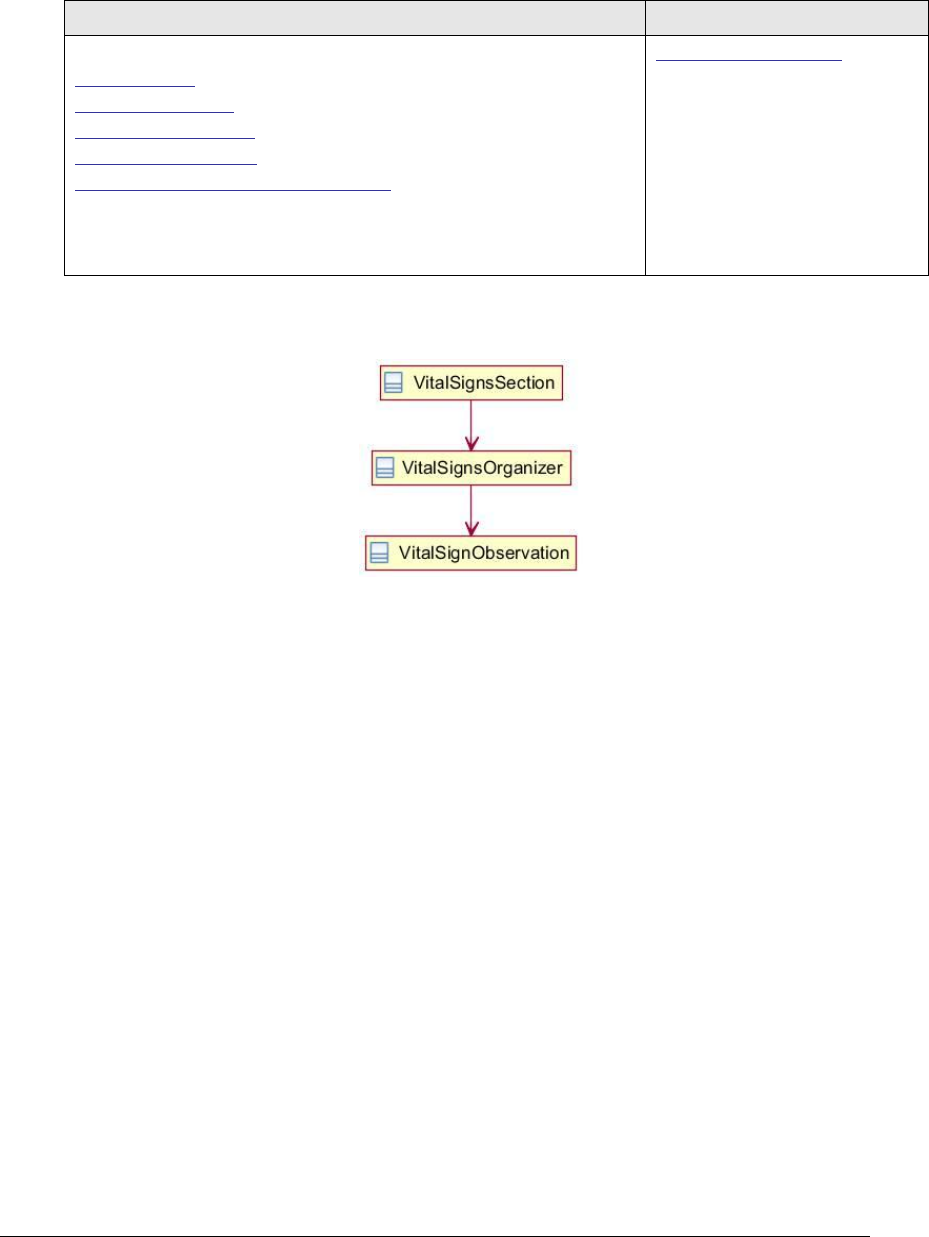
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 287
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
5.60 Vital Signs Section 8716-3
Table 106: Vital Signs Section Contexts
Used By:
Contains Entries:
Entries optional:
Progress Note (optional)
Consultation Note (optional)
Discharge Summary (optional)
History and Physical (required)
Continuity of Care Document (CCD) (optional)
Entries required:
---
Vital Signs Organizer
Figure 151: Vital signs section UML diagram
The Vital Signs section contains relevant vital signs for the context and use case
of the document type, such as blood pressure, heart rate, respiratory rate,
height, weight, body mass index, head circumference, and pulse oximetry. The
section should include notable vital signs such as the most recent, maximum
and/or minimum, baseline, or relevant trends.
Vital signs are represented in the same way as other results, but are aggregated
into their own section to follow clinical conventions.
Vital Signs Section with Coded Entries Optional
[section: templateId 2.16.840.1.113883.10.20.22.2.4(open)]
The following constraints apply to a Vital Signs section in which entries are not
required.
1. SHALL contain exactly one [1..1] templateId (CONF:7268) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.4" (CONF:10451).
2. SHALL contain exactly one [1..1] code (CONF:15242).
a. This code SHALL contain exactly one [1..1] @code="8716-3" Vital
Signs (CodeSystem: LOINC 2.16.840.1.113883.6.1) (CONF:15243).
3. SHALL contain exactly one [1..1] title (CONF:9966).
4. SHALL contain exactly one [1..1] text (CONF:7270).
Page 288 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
5. SHOULD contain zero or more [0..*] entry (CONF:7271) such that it
a. SHALL contain exactly one [1..1] Vital Signs Organizer
(2.16.840.1.113883.10.20.22.4.26) (CONF:7272).
Vital Signs Section with Coded Entries Required
[section: templateId 2.16.840.1.113883.10.20.22.2.4.1(open)]
The following constraints apply to a Vital Signs section in which entries are
required.
1. Conforms to Vital Signs Section (entries optional) template
(2.16.840.1.113883.10.20.22.2.4)
2. SHALL contain exactly one [1..1] templateId (CONF:7273) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.2.4.1" (CONF:10452).
3. SHALL contain exactly one [1..1] code (CONF:15962).
a. This code SHALL contain exactly one [1..1] @code="8716-3" Vital
Signs (CodeSystem: LOINC 2.16.840.1.113883.6.1) (CONF:15963).
4. SHALL contain exactly one [1..1] title (CONF:9967).
5. SHALL contain exactly one [1..1] text (CONF:7275).
6. SHALL contain at least one [1..*] entry (CONF:7276) such that it
a. SHALL contain exactly one [1..1] Vital Signs Organizer
(2.16.840.1.113883.10.20.22.4.26) (CONF:7277).
Figure 152: Vital signs section example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.4.1"/>
<code code="8716-3"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="VITAL SIGNS" />
<title>Vital Signs</title>
<text>
...
</text>
<entry typeCode="DRIV">
<organizer classCode="CLUSTER" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.26"/>
<!-- Vital Signs Organizer template -->
...
</organizer>
</entry>
</section>
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 289
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
6 ENTRY- L EVEL TEMPLATES
This part of the guide describes the clinical statement entry templates used
within the sections of the consolidated documents. Entry templates contain
constraints that are required for conformance. Note that the clinical statement
templates are presented in alphabetical order; templates are not grouped by
possible containing templates.
Entry-level templates are always allowed in sections.
Each entry-level template description contains the following information:
Key template metadata (e.g., templateId, etc.)
Description and explanatory narrative.
Required CDA acts, participants and vocabularies.
Optional CDA acts, participants and vocabularies.
Several entry-level templates require an effectiveTime:
The effectiveTime of an observation is the time interval over which the
observation is known to be true. The low and high values should be as
precise as possible, but no more precise than known. While CDA has
multiple mechanisms to record this time interval (e.g., by low and high
values, low and width, high and width, or center point and width), we
constrain most to use only the low/high form. The low value is the
earliest point for which the condition is known to have existed. The high
value, when present, indicates the time at which the observation was no
longer known to be true. The full description of effectiveTime and time
intervals is contained in the CDA R2 normative edition32.
Entry-level templates may also describe an id element, which is an identifier for
that entry. This id may be referenced within the document, or by the system
receiving the document. The id assigned must be globally unique.
6.1 Admission Medication
[act: templateId 2.16.840.1.113883.10.20.22.4.36 (open)]
Table 107: Admission Medication Contexts
Used By:
Contains Entries:
Hospital Admission Medications Section (entries optional) (optional)
Medication Activity
The Admission Medications entry codes medications that the patient took prior
to admission.
32 HL7 Clinical Document Architecture (CDA Release 2).
http://www.hl7.org/implement/standards/cda.cfm
Page 290 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 108: Admission Medication Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
act[templateId/@root = '2.16.840.1.113883.10.20.22.4.36']
@classCode
1..1
SHALL
7698
2.16.840.1.113883.5.6
(HL7ActClass) = ACT
@moodCode
1..1
SHALL
7699
2.16.840.1.113883.5.1001
(ActMood) = EVN
templateId
1..1
SHALL
16758
@root
1..1
SHALL
16759
2.16.840.1.113883.10.20.2
2.4.36
code
1..1
SHALL
15518
@code
0..1
MAY
15519
2.16.840.1.113883.6.1
(LOINC) = 42346-7
entryRelationship
1..*
SHALL
7701
@typeCode
1..1
SHALL
7702
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) =
SUBJ
substanceAdministration
1..1
SHALL
15520
1. SHALL contain exactly one [1..1] @classCode="ACT" (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:7698).
2. SHALL contain exactly one [1..1] @moodCode="EVN" (CodeSystem: ActMood
2.16.840.1.113883.5.1001) (CONF:7699).
3. SHALL contain exactly one [1..1] templateId (CONF:16758) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.36" (CONF:16759).
4. SHALL contain exactly one [1..1] code (CONF:15518).
a. This code MAY contain zero or one [0..1] @code="42346-7"
Medications on Admission (CodeSystem: LOINC
2.16.840.1.113883.6.1) (CONF:15519).
5. SHALL contain at least one [1..*] entryRelationship (CONF:7701) such that
it
a. SHALL contain exactly one [1..1] @typeCode="SUBJ" (CodeSystem:
HL7ActRelationshipType 2.16.840.1.113883.5.1002)
(CONF:7702).
b. SHALL contain exactly one [1..1] Medication Activity
(templateId:2.16.840.1.113883.10.20.22.4.16) (CONF:15520).
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 291
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 153: Admission medication entry example
<entry>
<act classCode="ACT" moodCode="EVN">
<!-- Admission Medication Entry -->
<templateId root="2.16.840.1.113883.10.20.22.4.36"/>
<id root="5a784260-6856-4f38-9638-80c751aff2fb"/>
<code code="42346-7"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="Admission medication"/>
<statusCode code="active"/>
<effectiveTime>
<low value="20903003"/>
</effectiveTime>
<entryRelationship typeCode="SUBJ">
<substanceAdministration moodCode="" classCode="SBADM">
<templateId root="2.16.840.1.113883.10.20.22.4.16"/>
<!-- Medication Activity -->
...
</substanceAdministration>
</entryRelationship>
</act>
</entry>
6.2 Advance Directive Observation
[observation: templateId 2.16.840.1.113883.10.20.22.4.48(open)]
Table 109: Advance Directive Observation Contexts
Used By:
Contains Entries:
Advance Directives Section (entries optional)
Advance Directives Section (entries required)
Advance Directives Observatations assert findings (e.g., “resuscitation status is
Full Code”) rather than orders, and should not be considered legal documents. A
legal document can be referenced using the reference/externalReference
construct.
Page 292 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 110: Advance Directive Observation Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
observation[templateId/@root = '2.16.840.1.113883.10.20.22.4.48']
@classCode
1..1
SHALL
8648
2.16.840.1.113883.5.6
(HL7ActClass) = OBS
@moodCode
1..1
SHALL
8649
2.16.840.1.113883.5.1001
(ActMood) = EVN
templateId
1..1
SHALL
SET<II>
8655
@root
1..1
SHALL
10485
2.16.840.1.113883.10.20.2
2.4.48
id
1..*
SHALL
II
8654
Advance
Directive
Type
code
1..1
SHALL
CD
8651
2.16.840.1.113883.1.11.20.
2
(AdvanceDirectiveTypeCode)
statusCode
1..1
SHALL
CS
8652
2.16.840.1.113883.5.14
(ActStatus) = completed
effective
Date
effectiveTime
1..1
SHALL
TS or
IVL<TS>
8656
low
1..1
SHALL
TS
8657
high
1..1
SHALL
TS
8659
participant
1..*
SHOULD
8662
@typeCode
1..1
SHALL
8663
2.16.840.1.113883.5.90
(HL7ParticipationType) =
VRF
templateId
1..1
SHALL
SET<II>
8664
@root
1..1
SHALL
10486
2.16.840.1.113883.10.20.1.
58
time
0..1
SHOULD
IVL<TS>
8665
participant
Role
1..1
SHALL
8825
custodian
of the
Document
participant
1..1
SHOULD
8667
@typeCode
1..1
SHALL
8668
2.16.840.1.113883.5.90
(HL7ParticipationType) =
CST
participant
Role
1..1
SHALL
8669
@classCode
1..1
SHALL
8670
2.16.840.1.113883.5.110
(RoleClass) = AGNT
addr
0..1
SHOULD
SET<AD>
8671
telecom
0..1
SHOULD
SET<TEL
>
8672
playingEntity
1..1
SHALL
8824
name
1..1
SHALL
PN
8673
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 293
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
reference
1..*
SHOULD
8692
@typeCode
1..1
SHALL
8694
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) =
REFR
external
Document
1..1
SHALL
8693
id
1..*
SHALL
II
8695
text
0..1
MAY
ED
8696
@mediaType
0..1
MAY
8703
reference
0..1
MAY
8697
1. SHALL contain exactly one [1..1] @classCode="OBS" Observation
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6) (CONF:8648).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001) (CONF:8649).
3. SHALL contain exactly one [1..1] templateId (CONF:8655) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.48" (CONF:10485).
4. SHALL contain at least one [1..*] id (CONF:8654).
5. SHALL contain exactly one [1..1] code, where the @code SHOULD be selected
from ValueSet AdvanceDirectiveTypeCode
2.16.840.1.113883.1.11.20.2 STATIC 2006-10-17 (CONF:8651).
6. SHALL contain exactly one [1..1] statusCode="completed" Completed
(CodeSystem: ActStatus 2.16.840.1.113883.5.14) (CONF:8652).
7. SHALL contain exactly one [1..1] effectiveTime (CONF:8656).
a. This effectiveTime SHALL contain exactly one [1..1] low (CONF:8657).
i. If the starting time is unknown, the <low> element SHALL have
the nullFlavor attribute set to UNK (CONF:8658).
b. This effectiveTime SHALL contain exactly one [1..1] high (CONF:8659).
i. If the ending time is unknown, the <high> element SHALL have
the nullFlavor attribute set to UNK (CONF:8660).
ii. If the Advance Directive does not have a specified ending time,
the <high> element SHALL have the nullFlavor attribute set to
NA (CONF:8661).
8. SHOULD contain at least one [1..*] participant (CONF:8662) such that it
a. SHALL contain exactly one [1..1] @typeCode="VRF" Verifier
(CodeSystem: HL7ParticipationType 2.16.840.1.113883.5.90)
(CONF:8663).
b. SHALL contain exactly one [1..1] templateId (CONF:8664) such that
it
i. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.1.58" (CONF:10486).

Page 294 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
c. SHOULD contain zero or one [0..1] time (CONF:8665).
i. The data type of Observation/participant/time in a
verification SHALL be TS (time stamp) (CONF:8666).
d. SHALL contain exactly one [1..1] participantRole (CONF:8825).
9. SHOULD contain exactly one [1..1] participant (CONF:8667) such that it
a. SHALL contain exactly one [1..1] @typeCode="CST" Custodian
(CodeSystem: HL7ParticipationType 2.16.840.1.113883.5.90)
(CONF:8668).
b. SHALL contain exactly one [1..1] participantRole (CONF:8669).
i. This participantRole SHALL contain exactly one [1..1]
@classCode="AGNT" Agent (CodeSystem: RoleClass
2.16.840.1.113883.5.110) (CONF:8670).
ii. This participantRole SHOULD contain zero or one [0..1] addr
(CONF:8671).
iii. This participantRole SHOULD contain zero or one [0..1]
telecom (CONF:8672).
iv. This participantRole SHALL contain exactly one [1..1]
playingEntity (CONF:8824).
1. This playingEntity SHALL contain exactly one [1..1]
name (CONF:8673).
a. The name of the agent who can provide a copy
of the Advance Directive SHALL be recorded in
the <name> element inside the <playingEntity>
element (CONF:8674).
10. SHOULD contain at least one [1..*] reference (CONF:8692) such that it
a. SHALL contain exactly one [1..1] @typeCode="REFR" Refers to
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002) (CONF:8694).
b. SHALL contain exactly one [1..1] externalDocument (CONF:8693).
i. This externalDocument SHALL contain at least one [1..*] id
(CONF:8695).
ii. This externalDocument MAY contain zero or one [0..1] text
(CONF:8696).
1. The text, if present, MAY contain zero or one [0..1]
@mediaType (CONF:8703).
2. The text, if present, MAY contain zero or one [0..1]
reference (CONF:8697).
a. The URL of a referenced advance directive
document MAY be present, and SHALL be
represented in
Observation/reference/ExternalDocument/text
/reference (CONF:8698).
b. If a URL is referenced, then it SHOULD have a
corresponding linkHTML element in narrative
block (CONF:8699).
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 295
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 111: Advance Directive Type Code Value Set
Value Set: AdvanceDirectiveTypeCode 2.16.840.1.113883.1.11.20.2 STATIC 2006-10-17
Code System(s):
SNOMED CT 2.16.840.1.113883.6.96
Code
Code System
Print Name
52765003
SNOMED CT
Intubation
61420007
SNOMED CT
Tube Feedings
71388002
SNOMED CT
Other Directive
78823007
SNOMED CT
Life Support
89666000
SNOMED CT
CPR
225204009
SNOMED CT
IV Fluid and Support
281789004
SNOMED CT
Antibiotics
304251008
SNOMED CT
Resuscitation
Figure 154: Advance directive observation example
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.48"/>
<id root="9b54c3c9-1673-49c7-aef9-b037ed72ed27"/>
<code code="304251008"
codeSystem="2.16.840.1.113883.6.96"
displayName="Resuscitation"/>
<statusCode code="completed"/>
<effectiveTime>
<low value="20110213"/>
<high nullFlavor="NA"/>
</effectiveTime>
<value xsi:type="CD" code="304253006"
codeSystem="2.16.840.1.113883.6.96"
displayName="Do not resuscitate">
<originalText>
<reference value="#AD1"/>
</originalText>
</value>
<participant typeCode="VRF">
<templateId root="2.16.840.1.113883.10.20.1.58"/>
<time value="201102013"/>
<participantRole>
<id root="20cf14fb-b65c-4c8c-a54d-b0cca834c18c"/>
<playingEntity>
<name>
<prefix>Dr.</prefix>
<family>Dolin</family>
<given>Robert</given>
</name>
</playingEntity>
</participantRole>
</participant>
Page 296 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
<participant typeCode="CST">
<participantRole classCode="AGNT">
<addr>
<streetAddressLine>21 North Ave.</streetAddressLine>
<city>Burlington</city>
<state>MA</state>
<postalCode>02368</postalCode>
<country>US</country>
</addr>
<telecom value="tel:(555)555-1003"/>
<playingEntity>
<name>
<prefix>Dr.</prefix>
<family>Dolin</family>
<given>Robert</given>
</name>
</playingEntity>
</participantRole>
</participant>
<reference typeCode="REFR">
<seperatableInd value="false"/>
<externalDocument>
<id root="b50b7910-7ffb-4f4c-bbe4-177ed68cbbf3"/>
<text mediaType="application/pdf">
<reference
value="AdvanceDirective.b50b7910-7ffb-4f4c-bbe4-
177ed68cbbf3.pdf"/>
</text>
</externalDocument>
</reference>
</observation>
6.3 Age Observation
[observation: templateId 2.16.840.1.113883.10.20.22.4.31(open)]
Table 112: Age Observation Contexts
Used By:
Contains Entries:
Family History Observation
Problem Observation
This Age Observation represents the subject's age at onset of an event or
observation. The age of a relative in a Family History Observation at the time of
that observation could also be inferred by comparing
RelatedSubject/subject/birthTime with Observation/effectiveTime.
However, a common scenario is that a patient will know the age of a relative
when the relative had a certain condition or when the relative died, but will not
know the actual year (e.g., "grandpa died of a heart attack at the age of 50").
Often times, neither precise dates nor ages are known (e.g. "cousin died of
congenital heart disease as an infant").
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 297
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 113: Age Observation Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
observation[templateId/@root = '2.16.840.1.113883.10.20.22.4.31']
@classCode
1..1
SHALL
7613
2.16.840.1.113883.5.6 (HL7ActClass)
= OBS
@moodCode
1..1
SHALL
7614
2.16.840.1.113883.5.1001 (ActMood)
= EVN
templateId
1..1
SHALL
SET<II>
7899
@root
1..1
SHALL
10487
2.16.840.1.113883.10.20.22.4.31
code
1..1
SHALL
7615
@code
1..1
SHALL
16776
2.16.840.1.113883.6.96 (SNOMED-
CT) = 445518008
statusCode
1..1
SHALL
15965
@code
1..1
SHALL
15966
2.16.840.1.113883.5.14 (ActStatus) =
completed
value
1..1
SHALL
PQ
7617
@unit
1..1
SHALL
7618
2.16.840.1.113883.11.20.9.21
(AgePQ_UCUM) = 1
1. SHALL contain exactly one [1..1] @classCode="OBS" Observation
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6) (CONF:7613).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001) (CONF:7614).
3. SHALL contain exactly one [1..1] templateId (CONF:7899) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.31" (CONF:10487).
4. SHALL contain exactly one [1..1] code (CONF:7615).
a. This code SHALL contain exactly one [1..1] @code="445518008" Age
At Onset (CodeSystem: SNOMED-CT 2.16.840.1.113883.6.96)
(CONF:16776).
5. SHALL contain exactly one [1..1] statusCode (CONF:15965).
a. This statusCode SHALL contain exactly one [1..1] @code="completed"
Completed (CodeSystem: ActStatus 2.16.840.1.113883.5.14)
(CONF:15966).
6. SHALL contain exactly one [1..1] value with @xsi:type="PQ" (CONF:7617).
a. This value SHALL contain exactly one [1..1] @unit="1", which SHALL
be selected from ValueSet AgePQ_UCUM
2.16.840.1.113883.11.20.9.21 DYNAMIC (CONF:7618).
Page 298 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 114: AgePQ_UCUM Value Set
Value Set: AgePQ_UCUM 2.16.840.1.113883.11.20.9.21 DYNAMIC
Code System(s):
Unified Code for Units of Measure (UCUM) 2.16.840.1.113883.6.8
Description:
A valueSet of UCUM codes for representing age value units
Code
Code System
Print Name
min
UCUM
Minute
h
UCUM
Hour
d
UCUM
Day
wk
UCUM
Week
mo
UCUM
Month
a
UCUM
Year
Figure 155: Age observation example
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.31"/>
<!-- Age observation template -->
<code code="397659008"
codeSystem="2.16.840.1.113883.6.96"
displayName="Age"
codeSystemName="SNOMED CT"/>
<statusCode code="completed"/>
<value xsi:type="PQ" value="57" unit="a"/>
</observation>
6.4 Allergy - Intolerance Observation
[observation: templateId 2.16.840.1.113883.10.20.22.4.7(open)]
Table 115: Allergy - Intolerance Observation Contexts
Used By:
Contains Entries:
Allergy Problem Act (required)
Allergy Status Observation
Reaction Observation
Severity Observation
This clinical statement represents that an allergy or adverse reaction exists or
does not exist. The agent that is the cause of the allergy or adverse reaction is
represented as a manufactured material participant playing entity in the allergy
- intolerance observation. While the agent is often implicit in the alert
observation (e.g. "allergy to penicillin"), it should also be asserted explicitly as an
entity. The manufactured material participant is used to represent natural and
non-natural occurring substances.
NOTE: The agent responsible for an allergy or adverse reaction is not always a
manufactured material (for example, food allergies), nor is it necessarily
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 299
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
consumed. The following constraints reflect limitations in the base CDA R2
specification, and should be used to represent any type of responsible agent.
Table 116: Allergy - Intolerance Observation Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
Allergy –
Intolerance
Observation
observation[templateId/@root = '2.16.840.1.113883.10.20.22.4.7']
@classCode
1..1
SHALL
7379
2.16.840.1.113883.5.6
(HL7ActClass) = OBS
@moodCode
1..1
SHALL
7380
2.16.840.1.113883.5.1001
(ActMood) = EVN
templateId
1..1
SHALL
SET<I
I>
7381
@root
1..1
SHALL
10488
2.16.840.1.113883.10.20.22.4.
7
id
1..*
SHALL
7382
code
1..1
SHALL
15947
@code
1..1
SHALL
15948
2.16.840.1.113883.5.4
(ActCode) = ASSERTION
statusCode
1..1
SHALL
7386
2.16.840.1.113883.5.14
(ActStatus) = completed
adverseEve
nt
Date
effectiveTime
1..1
SHALL
TS or
IVL<T
S>
7387
value
1..1
SHALL
CD
7390
adverseEve
nt
Type
@code
1..1
SHALL
9139
2.16.840.1.113883.3.88.12.32
21.6.2 (Allergy/Adverse Event
Type)
originalText
0..1
SHOULD
7422
reference
0..1
MAY
15949
@value
0..1
SHOULD
15950
product
participant
0..1
SHOULD
7402
@typeCode
1..1
SHALL
7403
2.16.840.1.113883.5.90
(HL7ParticipationType) = CSM
productDet
ail
participantRole
1..1
SHALL
7404
Page 300 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
@classCode
1..1
SHALL
7405
2.16.840.1.113883.5.110
(RoleClass) = MANU
playingEntity
1..1
SHALL
7406
@classCode
1..1
SHALL
7407
2.16.840.1.113883.5.41
(EntityClass) = MMAT
product
Coded
code
1..1
SHALL
7419
productFree
Text
originalText
0..1
SHOULD
7424
reference
0..1
SHOULD
7425
@value
0..1
SHOULD
15952
translation
0..*
MAY
SET<P
QR>
7431
entryRelations
hip
0..1
MAY
7440
@typeCode
1..1
SHALL
7906
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) =
SUBJ
@inversionInd
1..1
SHALL
7446
true
observation
1..1
SHALL
15954
reaction
entryRelations
hip
0..*
SHOULD
7447
@typeCode
1..1
SHALL
7907
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) =
MFST
@inversionInd
1..1
SHALL
7449
true
observation
1..1
SHALL
15955
severity
entryRelations
hip
0..1
SHOULD
9961
@typeCode
1..1
SHALL
9962
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) =
SUBJ
@inversionInd
1..1
SHALL
9964
true
observation
1..1
SHALL
15956
1. Conforms to Substance or Device Allergy - Intolerance
Observation template (2.16.840.1.113883.10.20.24.3.90).
2. SHALL contain exactly one [1..1] @classCode="OBS" Observation
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6) (CONF:7379).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 301
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
3. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001) (CONF:7380).
4. SHALL contain exactly one [1..1] templateId (CONF:7381) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.7" (CONF:10488).
5. SHALL contain at least one [1..*] id (CONF:7382).
6. SHALL contain exactly one [1..1] code (CONF:15947).
a. This code SHALL contain exactly one [1..1] @code="ASSERTION"
Assertion (CodeSystem: ActCode 2.16.840.1.113883.5.4)
(CONF:15948).
7. SHALL contain exactly one [1..1] statusCode="completed" Completed
(CodeSystem: ActStatus 2.16.840.1.113883.5.14 STATIC) (CONF:7386).
8. SHALL contain exactly one [1..1] effectiveTime (CONF:7387).
a. If it is unknown when the allergy began, this effectiveTime SHALL
contain low/@nullFLavor="UNK" (CONF:9103).
b. If the allergy is no longer a concern, this effectiveTime MAY contain
zero or one [0..1] high (CONF:10082).
9. SHALL contain exactly one [1..1] value with @xsi:type="CD" (CONF:7390).
a. This value SHALL contain exactly one [1..1] @code, which SHALL be
selected from ValueSet Allergy/Adverse Event Type
2.16.840.1.113883.3.88.12.3221.6.2 DYNAMIC (CONF:9139).
b. This value SHOULD contain zero or one [0..1] originalText
(CONF:7422).
i. The originalText, if present, MAY contain zero or one [0..1]
reference (CONF:15949).
1. The reference, if present, SHOULD contain zero or one
[0..1] @value (CONF:15950).
a. This reference/@value SHALL begin with a '#'
and SHALL point to its corresponding narrative
(using the approach defined in CDA Release 2,
section 4.3.5.1) (CONF:15951).
10. SHOULD contain zero or one [0..1] participant (CONF:7402) such that it
a. SHALL contain exactly one [1..1] @typeCode="CSM" Consumable
(CodeSystem: HL7ParticipationType 2.16.840.1.113883.5.90
STATIC) (CONF:7403).
b. SHALL contain exactly one [1..1] participantRole (CONF:7404).
i. This participantRole SHALL contain exactly one [1..1]
@classCode="MANU" Manufactured Product (CodeSystem:
RoleClass 2.16.840.1.113883.5.110 STATIC) (CONF:7405).
ii. This participantRole SHALL contain exactly one [1..1]
playingEntity (CONF:7406).
1. This playingEntity SHALL contain exactly one [1..1]
@classCode="MMAT" Manufactured Material
(CodeSystem: EntityClass
2.16.840.1.113883.5.41 STATIC) (CONF:7407).

Page 302 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
2. This playingEntity SHALL contain exactly one [1..1]
code (CONF:7419).
a. This code SHOULD contain zero or one [0..1]
originalText (CONF:7424).
i. The originalText, if present, SHOULD
contain zero or one [0..1] reference
(CONF:7425).
1. The reference, if present, SHOULD
contain zero or one [0..1] @value
(CONF:15952).
1. This reference/@value
SHALL begin with a '#' and SHALL
point to its corresponding
narrative (using the approach
defined in CDA Release 2,
section 4.3.5.1) (CONF:15953).
b. This code MAY contain zero or more [0..*]
translation (CONF:7431).
c. In an allergy to a specific medication the code
SHALL be selected from the ValueSet
2.16.840.1.113883.3.88.12.80.16 Medication
Brand Name DYNAMIC or the ValueSet
2.16.840.1.113883.3.88.12.80.17 Medication
Clinical Drug DYNAMIC (CONF:7421).
d. In an allergy to a class of medications the code
SHALL be selected from the ValueSet
2.16.840.1.113883.3.88.12.80.18 Medication
Drug Class DYNAMIC (CONF:10083).
e. In an allergy to a food or other substance the
code SHALL be selected from the ValueSet
2.16.840.1.113883.3.88.12.80.20 Ingredient
Name DYNAMIC (CONF:10084).
11. MAY contain zero or one [0..1] entryRelationship (CONF:7440) such that it
a. SHALL contain exactly one [1..1] @typeCode="SUBJ" Has subject
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002 STATIC) (CONF:7906).
b. SHALL contain exactly one [1..1] @inversionInd="true" True
(CONF:7446).
c. SHALL contain exactly one [1..1] Allergy Status Observation
(templateId:2.16.840.1.113883.10.20.22.4.28) (CONF:15954).
12. SHOULD contain zero or more [0..*] entryRelationship (CONF:7447) such
that it
a. SHALL contain exactly one [1..1] @typeCode="MFST" Is Manifestation
of (CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002 STATIC) (CONF:7907).
b. SHALL contain exactly one [1..1] @inversionInd="true" True
(CONF:7449).
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 303
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
c. SHALL contain exactly one [1..1] Reaction Observation
(templateId:2.16.840.1.113883.10.20.22.4.9) (CONF:15955).
13. SHOULD contain zero or one [0..1] entryRelationship (CONF:9961) such
that it
a. SHALL contain exactly one [1..1] @typeCode="SUBJ" Has Subject
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002 STATIC) (CONF:9962).
b. SHALL contain exactly one [1..1] @inversionInd="true" True
(CONF:9964).
c. SHALL contain exactly one [1..1] Severity Observation
(templateId:2.16.840.1.113883.10.20.22.4.8) (CONF:15956).
Table 117: Allergy/Adverse Event Type Value Set
Value Set: Allergy/Adverse Event Type 2.16.840.1.113883.3.88.12.3221.6.2 DYNAMIC
Code System(s):
SNOMED CT 2.16.840.1.113883.6.96
Description:
This describes the type of product and intolerance suffered by the patient
http://phinvads.cdc.gov/vads/ViewValueSet.action?id=7AFDBFB5-A277-
DE11-9B52-0015173D1785
Code
Code System
Print Name
420134006
SNOMED CT
Propensity to adverse reactions (disorder)
418038007
SNOMED CT
Propensity to adverse reactions to substance
(disorder)
419511003
SNOMED CT
Propensity to adverse reactions to drug (disorder)
418471000
SNOMED CT
Propensity to adverse reactions to food (disorder)
419199007
SNOMED CT
Allergy to substance (disorder)
416098002
SNOMED CT
Drug allergy (disorder)
414285001
SNOMED CT
Food allergy (disorder)
59037007
SNOMED CT
Drug intolerance (disorder)
235719002
SNOMED CT
Food intolerance (disorder)
Table 118: Medication Brand Name Value Set (excerpt)
Value Set: Medication Brand Name 2.16.840.1.113883.3.88.12.80.16 DYNAMIC
Code System(s):
RxNorm 2.16.840.1.113883.6.88
Description:
Brand names
http://phinvads.cdc.gov/vads/ViewValueSet.action?id=229BEF3E-971C-
DF11-B334-0015173D1785
Code
Code System
Print Name
205734
RxNorm
Amoxicillin 25 MG/ML Oral Suspension [Amoxil]
856537
RxNorm
24 HR Propranolol Hydrochloride 60 MG Extended
Release Capsule [Inderal]
104700
RxNorm
Diazepam 5 MG Oral Tablet [Valium]
…
Page 304 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 119: Medication Clinical Drug Value Set (excerpt)
Value Set: Medication Clinical Drug 2.16.840.1.113883.3.88.12.80.17 DYNAMIC
Code System(s):
RxNorm 2.16.840.1.113883.6.88
Description:
Clinical drug names
http://phinvads.cdc.gov/vads/ViewValueSet.action?id=239BEF3E-971C-
DF11-B334-0015173D1785
Code
Code System
Print Name
313850
RxNorm
Amoxicillin 40 MG/ML Oral Suspension
856448
RxNorm
Propranolol Hydrochloride 10 MG Oral Tablet
197589
RxNorm
Diazepam 10 MG Oral Tablet
…
Table 120: Medication Drug Class Value Set (excerpt)
Value Set: Medication Drug Class 2.16.840.1.113883.3.88.12.80.18 DYNAMIC
Code System(s):
NDF-RT 2.16.840.1.113883.3.26.1.5
Description:
This identifies the pharmacological drug class, such as Cephalosporins.
Shall contain a value descending from the NDF-RT concept types of
“Mechanism of Action - N0000000223”, “Physiologic Effect -
N0000009802” or “Chemical Structure - N0000000002”`. NUI will be used
as the concept code.
http://phinvads.cdc.gov/vads/ViewValueSet.action?id=77FDBFB5-A277-
DE11-9B52-0015173D1785
Code
Code System
Print Name
N0000011161
NDF-RT
Cephalosporins
N0000005909
NDF-RT
2-Propanol
N0000006629
NDF-RT
Filgrastim
…

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 305
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 121: Ingredient Name Value Set (excerpt)
Value Set: Ingredient Name 2.16.840.1.113883.3.88.12.80.20 DYNAMIC
Code System(s):
Unique Ingredient Identifier (UNII) 2.16.840.1.113883.4.9
Description:
Unique ingredient identifiers (UNIIs) for substances in drugs, biologics,
foods, and devices.
http://www.fda.gov/ForIndustry/DataStandards/StructuredProductLabe
ling/ucm162523.htm
Code
Code System
Print Name
OLT4M28U3Z
UNII
((3-TRIFLUOROMETHYL)PHENYL)METHYL-
PHOSPHONIC ACID
L0VRY82PKO
UNII
CYCLOHEXENE, 4-[(1Z)-1,5-DIMETHYL-1,4-
HEXADIEN-1-YL]-1-METHYL-
62H4W26906
UNII
BISNAFIDE
QE1QX6B99R
UNII
PEANUT
…
Figure 156: Allergy - intolerance observation example
<observation classCode="OBS" moodCode="EVN">
<!-- allergy - intolerance observation template -->
<templateId root="2.16.840.1.113883.10.20.22.4.7"/>
<id root="4adc1020-7b14-11db-9fe1-0800200c9a66"/>
<code code="ASSERTION" codeSystem="2.16.840.1.113883.5.4"/>
<statusCode code="completed"/>
<effectiveTime>
<low value="20110215"/>
</effectiveTime>
<value xsi:type="CD" code="282100009"
displayName="Adverse reaction to substance"
codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT">
<originalText>
<reference value=""/>
</originalText>
</value>
Page 306 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
<participant typeCode="CSM">
<participantRole classCode="MANU">
<playingEntity classCode="MMAT">
<code code="QE1QX6B99R" displayName="PEANUT"
codeSystem="2.16.840.1.113883.4.9" codeSystemName=" UNII">
<originalText>
<reference value=""/>
</originalText>
</code>
<name>Penicillin</name>
</playingEntity>
</participantRole>
</participant>
</observation>
6.5 Allergy Problem Act
[act: templateId 2.16.840.1.113883.10.20.22.4.30(open)]
Table 122: Allergy Problem Act Contexts
Used By:
Contains Entries:
Allergies Section (entries required)
Allergies Section (entries optional)
Allergy - Intolerance Observation
This clinical statement act represents a concern relating to a patient's allergies
or adverse events. A concern is a term used when referring to patient's problems
that are related to one another. Observations of problems or other clinical
statements captured at a point in time are wrapped in a Allergy Problem Act, or
"Concern" act, which represents the ongoing process tracked over time. This
outer Allergy Problem Act (representing the "Concern") can contain nested
problem observations or other nested clinical statements relevant to the allergy
concern.
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 307
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 123: Allergy Problem Act Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
act[templateId/@root = '2.16.840.1.113883.10.20.22.4.30']
@classCode
1..1
SHALL
7469
2.16.840.1.113883.5.6 (HL7ActClass) =
ACT
@moodCode
1..1
SHALL
7470
2.16.840.1.113883.5.1001 (ActMood) =
EVN
templateId
1..1
SHALL
SET<II>
7471
@root
1..1
SHALL
10489
2.16.840.1.113883.10.20.22.4.30
id
1..*
SHALL
II
7472
code
1..1
SHALL
CD
7477
2.16.840.1.113883.6.1 (LOINC) = 48765-
2
statusCode
1..1
SHALL
CS
7485
2.16.840.1.113883.11.20.9.19
(ProblemAct statusCode)
effectiveTime
1..1
SHALL
TS or
IVL<TS>
7498
Entry
Relationship
1..*
SHALL
7509
@typeCode
1..1
SHALL
7915
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) = SUBJ
1. SHALL contain exactly one [1..1] @classCode="ACT" (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:7469).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001) (CONF:7470).
3. SHALL contain exactly one [1..1] templateId (CONF:7471) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.30" (CONF:10489).
4. SHALL contain at least one [1..*] id (CONF:7472).
5. SHALL contain exactly one [1..1] code="48765-2" Allergies, adverse
reactions, alerts (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:7477).
6. SHALL contain exactly one [1..1] statusCode, which SHALL be selected from
ValueSet ProblemAct statusCode 2.16.840.1.113883.11.20.9.19
STATIC 2011-09-09 (CONF:7485).
7. SHALL contain exactly one [1..1] effectiveTime (CONF:7498).
a. If statusCode="active" Active, then effectiveTime SHALL contain [1..1]
low (CONF:7504).
b. If statusCode="completed" Completed, then effectiveTime SHALL
contain [1..1] high (CONF:10085).
8. SHALL contain at least one [1..*] entryRelationship (CONF:7509) such that
it
Page 308 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
a. SHALL contain exactly one [1..1] @typeCode="SUBJ" Has subject
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002) (CONF:7915).
b. SHALL contain exactly one [1..1] Allergy - Intolerance
Observation (templateId:2.16.840.1.113883.10.20.22.4.7)
(CONF:14925).
Table 124: ProblemAct statusCode Value Set
Value Set: ProblemAct statusCode 2.16.840.1.113883.11.20.9.19 STATIC 2011-09-09
Code System(s):
ActStatus 2.16.840.1.113883.5.14
Description:
This value set indicates the status of the problem concern act
Code
Code System
Print Name
active
ActStatus
active
suspended
ActStatus
suspended
aborted
ActStatus
aborted
completed
ActStatus
completed
Figure 157: Allergy problem act example
<entry typeCode="DRIV">
<act classCode="ACT" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.30"/>
<id root="36e3e930-7b14-11db-9fe1-0800200c9a66"/>
<code code="48765-2"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="Allergies, adverse reactions, alerts"/>
<statusCode code="active"/>
<effectiveTime>
<low value="20090902"/>
<high value="20100103"/>
</effectiveTime>
<entryRelationship typeCode="SUBJ">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.7"/>
<!-- Allergy - intolerance observation template -->
...
</observation>
</entryRelationship>
</act>
</entry>
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 309
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
6.6 Allergy Status Observation
[observation: templateId 2.16.840.1.113883.10.20.22.4.28(open)]
Table 125: Allergy Status Observation Contexts
Used By:
Contains Entries:
Allergy - Intolerance Observation
This template represents the status of the allergy indicating whether it is active,
no longer active, or is an historic allergy. There can be only one allergy status
observation per alert observation.
Table 126: Allergy Status Observation Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
observation[templateId/@root = '2.16.840.1.113883.10.20.22.4.28']
@classCode
1..1
SHALL
7318
2.16.840.1.113883.5.6 (HL7ActClass) =
OBS
@moodCode
1..1
SHALL
7319
2.16.840.1.113883.5.1001 (ActMood) =
EVN
templateId
1..1
SHALL
SET<II>
7317
@root
1..1
SHALL
10490
2.16.840.1.113883.10.20.22.4.28
code
1..1
SHALL
7320
2.16.840.1.113883.6.1 (LOINC) =
33999-4
statusCode
1..1
SHALL
7321
2.16.840.1.113883.5.14 (ActStatus) =
completed
value
1..1
SHALL
CE
7322
2.16.840.1.113883.3.88.12.80.68
(HITSPProblemStatus)
1. SHALL contain exactly one [1..1] @classCode="OBS" Observation
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6 STATIC) (CONF:7318).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001 STATIC) (CONF:7319).
3. SHALL contain exactly one [1..1] templateId (CONF:7317) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.28" (CONF:10490).
4. SHALL contain exactly one [1..1] code="33999-4" Status (CodeSystem:
LOINC 2.16.840.1.113883.6.1 STATIC) (CONF:7320).
5. SHALL contain exactly one [1..1] statusCode="completed" Completed
(CodeSystem: ActStatus 2.16.840.1.113883.5.14 STATIC) (CONF:7321).
6. SHALL contain exactly one [1..1] value with @xsi:type="CE", where the @code
SHALL be selected from ValueSet HITSPProblemStatus
2.16.840.1.113883.3.88.12.80.68 DYNAMIC (CONF:7322).

Page 310 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 127: HITSP Problem Status Value Set
Value Set: HITSPProblemStatus 2.16.840.1.113883.3.88.12.80.68 DYNAMIC
Code System: SNOMED CT 2.16.840.1.113883.6.96
Code
Code System
Display Name
55561003
SNOMED CT
Active
73425007
SNOMED CT
Inactive*
413322009
SNOMED CT
Resolved**
* An inactive problems refers to one that is quiescent, and may appear again in future.
** A resolved problem refers to one that used to affect a patient, but does not any more.
Figure 158: Allergy status observation example
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.28"/>
<!-- Allergy status observation template -->
<code code="33999-4"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="Status"/>
<statusCode code="completed"/>
<value xsi:type="CE" code="55561003"
codeSystem="2.16.840.1.113883.6.96"
displayName="Active"/>
</observation>
6.7 Assessment Scale Observation
[observation: templateId 2.16.840.1.113883.10.20.22.4.69 (open)]
Table 128: Assessment Scale Observation Contexts
Used By:
Contains Entries:
Functional Status Problem Observation (optional)
Functional Status Result Observation (optional)
Cognitive Status Problem Observation (optional)
Cognitive Status Result Observation (optional)
Functional Status Section (optional)
Assessment Scale Supporting Observation
An assessment scale is a collection of observations that together yield a
summary evaluation of a particular condition. Examples include the Braden
Scale (assesses pressure ulcer risk), APACHE Score (estimates mortality in
critically ill patients), Mini-Mental Status Exam (assesses cognitive function),
APGAR Score (assesses the health of a newborn), and Glasgow Coma Scale
(assesses coma and impaired consciousness.)
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 311
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 129: Assessment Scale Observation Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
observation[templateId/@root = '2.16.840.1.113883.10.20.22.4.69']
@classCode
1..1
SHALL
14434
2.16.840.1.113883.5.6
(HL7ActClass) = OBS
@moodCode
1..1
SHALL
14435
2.16.840.1.113883.5.1001
(ActMood) = EVN
templateId
1..1
SHALL
14436
@root
1..1
SHALL
14437
2.16.840.1.113883.10.20.22.4.69
id
1..*
SHALL
14438
code
1..1
SHALL
14439
derivationExpr
0..1
MAY
14637
statusCode
1..1
SHALL
14444
2.16.840.1.113883.5.14
(ActStatus) = completed
effectiveTime
1..1
SHALL
14445
value
1..1
SHALL
14450
interpretationCode
0..*
MAY
14459
translation
0..*
MAY
14888
author
0..*
MAY
14460
entryRelationship
0..*
SHOULD
14451
@typeCode
1..1
SHALL
16741
COMP
observation
1..1
SHALL
16742
referenceRange
0..*
MAY
16799
observationRange
1..1
SHALL
16800
text
0..1
SHOULD
16801
reference
0..1
SHOULD
16802
@value
0..1
MAY
16803
1. SHALL contain exactly one [1..1] @classCode="OBS" (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:14434).
2. SHALL contain exactly one [1..1] @moodCode="EVN" (CodeSystem: ActMood
2.16.840.1.113883.5.1001) (CONF:14435).
3. SHALL contain exactly one [1..1] templateId (CONF:14436) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.69" (CONF:14437).
4. SHALL contain at least one [1..*] id (CONF:14438).
5. SHALL contain exactly one [1..1] code (CONF:14439).
a. SHOULD be from LOINC (CodeSystem: 2.16.840.1.113883.6.1) or
SNOMED CT (CodeSystem: 2.16.840.1.113883.6.96) identifying the
assessment scale (CONF:14440).

Page 312 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Such derivation expression can contain a text calculation of how the
components total up to the summed score
6. MAY contain zero or one [0..1] derivationExpr (CONF:14637).
7. SHALL contain exactly one [1..1] statusCode="completed" (CodeSystem:
ActStatus 2.16.840.1.113883.5.14) (CONF:14444).
Represents clinically effective time of the measurement, which may be when the
measurement was performed (e.g., a BP measurement), or may be when sample
was taken (and measured some time afterwards)
8. SHALL contain exactly one [1..1] effectiveTime (CONF:14445).
9. SHALL contain exactly one [1..1] value (CONF:14450).
10. MAY contain zero or more [0..*] interpretationCode (CONF:14459).
a. The interpretationCode, if present, MAY contain zero or more [0..*]
translation (CONF:14888).
11. MAY contain zero or more [0..*] author (CONF:14460).
12. SHOULD contain zero or more [0..*] entryRelationship (CONF:14451) such
that it
a. SHALL contain exactly one [1..1] @typeCode="COMP" has component
(CONF:16741).
b. SHALL contain exactly one [1..1] Assessment Scale Supporting
Observation (templateId:2.16.840.1.113883.10.20.22.4.86)
(CONF:16742).
The referenceRange/observationRange/text, if present, MAY contain a
description of the scale (e.g. for a Pain Scale 1 to 10: 1 to 3 = little pain, 4 to 7=
moderate pain, 8 to 10 = severe pain)
13. MAY contain zero or more [0..*] referenceRange (CONF:16799).
a. The referenceRange, if present, SHALL contain exactly one [1..1]
observationRange (CONF:16800).
i. This observationRange SHOULD contain zero or one [0..1] text
(CONF:16801).
1. The text, if present, SHOULD contain zero or one [0..1]
reference (CONF:16802).
a. The reference, if present, MAY contain zero or
one [0..1] @value (CONF:16803).
i. This reference/@value SHALL begin with
a '#' and SHALL point to its
corresponding narrative (using the
approach defined in CDA Release 2,
section 4.3.5.1) (CONF:16804).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 313
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 159: Assessment scale observation example
<section>
...
<entry typeCode="DRIV">
<observation classCode="OBS" moodCode="EVN">
<!—Assessment Scale Observation -->
<templateId root="2.16.840.1.113883.10.20.22.4.69"/>
<code code="248241002" displayName="Brief Interview for Mental
Status"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"/>
<derivationExpr>Text description of the
calculation</derivationExpr>
<statusCode code="completed"/>
<effectiveTime value="20120214"/>
<!-- Summed score of the component values -->
<value xsi:type="INT" value="7"/>
<entryRelationship typeCode="COMP">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.86"/>
<id root="f4dce790-8328-11db-9fe1-0800200c9a33"/>
<code code="52731-7" displayName="Repetition of Three Words"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"/>
<statusCode code="completed"/>
<value xsi:type="CD" code="LA6395-3" displayName="Three"
codeSystem="2.16.840.1.113883.6.1"/>
</observation
</entryRelationship>
<entryRelationship typeCode="COMP">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.86"/>
<id root="f4dce790-8328-11db-9fe1-0800200c9a22"/>
<code code="52732-5"
displayName="Temporal orientation - current year"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"/>
<statusCode code="completed"/>
<value xsi:type="CD" code="LA10966-2"
displayName="Missed by 2-5 years"
codeSystem="2.16.840.1.113883.6.1"/>
</observation>
</entryRelationship>
Page 314 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
<entryRelationship typeCode="COMP">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.86"/>
<id root="f4dce790-8328-11db-9fe1-0800200c9a44"/>
<code code="248240001" displayName="motor response"
codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED"/>
<statusCode code="completed"/>
<value xsi:type="INT" value="3"/>
</observation>
</entryRelationship>
</observation>
</entry>
...
6.8 Assessment Scale Supporting Observation
[observation: templateId 2.16.840.1.113883.10.20.22.4.86 (open)]
Table 130: Assessment Scale Supporting Observation Contexts
Used By:
Contains Entries:
Assessment Scale Observation (required)
An Assessment Scale Supporting observation represents the components of a
scale used in an Assessment Scale Observation. The individual parts that make
up the component may be a group of cognitive or functional status observations.
Table 131: Assessment Scale Supporting Observation Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
observation[templateId/@root = '2.16.840.1.113883.10.20.22.4.86']
@classCode
1..1
SHALL
16715
2.16.840.1.113883.5.6 (HL7ActClass) =
OBS
@moodCode
1..1
SHALL
16716
2.16.840.1.113883.5.1001 (ActMood) =
EVN
templateId
1..1
SHALL
SET<II>
16722
@root
1..1
SHALL
16723
2.16.840.1.113883.10.20.22.4.86
id
1..*
SHALL
16724
code
1..1
SHALL
CE
16717
@code
1..1
SHALL
16738
statusCode
1..1
SHALL
16720
2.16.840.1.113883.5.14 (ActStatus) =
completed
value
1..*
SHALL
16754
1. SHALL contain exactly one [1..1] @classCode="OBS" Observation
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6) (CONF:16715).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 315
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001) (CONF:16716).
3. SHALL contain exactly one [1..1] templateId (CONF:16722) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.86" (CONF:16723).
4. SHALL contain at least one [1..*] id (CONF:16724).
5. SHALL contain exactly one [1..1] code with @xsi:type="CE" (CONF:16717).
a. This code SHALL contain exactly one [1..1] @code (CONF:16738).
i. Such that observation/code SHALL be from LOINC
(CodeSystem: 2.16.840.1.113883.6.1) or SNOMED CT
(CodeSystem: 2.16.840.1.113883.6.96) and represents
components of the scale (CONF:14458) (CONF:16739).
6. SHALL contain exactly one [1..1] statusCode="completed" (CodeSystem:
ActStatus 2.16.840.1.113883.5.14) (CONF:16720).
7. SHALL contain at least one [1..*] value (CONF:16754).
a. If xsi:type="CD" , MAY have a translation code to further specify the
source if the instrument has an applicable code system and valueSet
for the integer (CONF:14639) (CONF:16755).
Figure 160: Assessment scale supporting observation example
<entryRelationship typeCode="COMP">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.86"/>
<id root="f4dce790-8328-11db-9fe1-0800200c9a44"/>
<code code="248240001" displayName="motor response"
codeSystem="2.16.840.1.113883.6.96" codeSystemName="SNOMED"/>
<statusCode code="completed"/>
<value xsi:type="INT" value="3"/>
</observation>
</entryRelationship>
6.9 Authorization Activity
[act: templateId 2.16.840.1.113883.10.20.1.19(open)]
Table 132: Authorization Activity Contexts
Used By:
Contains Entries:
Policy Activity (optional)
An Authorization Activity represents authorizations or pre-authorizations
currently active for the patient for the particular payer.
Authorizations are represented using an act subordinate to the policy or
program that provided it. The authorization refers to the policy or program.
Authorized treatments can be grouped into an organizer class, where common
properties, such as the reason for the authorization, can be expressed.
Subordinate acts represent what was authorized.
Page 316 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 133: Authorization Activity Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
act[templateId/@root = '2.16.840.1.113883.10.20.1.19']
@classCode
1..1
SHALL
8944
2.16.840.1.113883.5.6 (HL7ActClass) =
ACT
@moodCode
1..1
SHALL
8945
2.16.840.1.113883.5.6 (HL7ActClass) =
EVN
templateId
1..1
SHALL
SET<II>
8946
@root
1..1
SHALL
10529
2.16.840.1.113883.10.20.1.19
id
1..1
SHALL
II
8947
entry
Relationship
1..*
SHALL
8948
@typeCode
1..1
SHALL
8949
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) = SUBJ
1. SHALL contain exactly one [1..1] @classCode="ACT" Act (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:8944).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:8945).
3. SHALL contain exactly one [1..1] templateId (CONF:8946) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.1.19" (CONF:10529).
4. SHALL contain exactly one [1..1] id (CONF:8947).
5. SHALL contain at least one [1..*] entryRelationship (CONF:8948) such that
it
a. SHALL contain exactly one [1..1] @typeCode="SUBJ" Has Subject
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002) (CONF:8949).
b. The target of an authorization activity with
act/entryRelationship/@typeCode="SUBJ" SHALL be a clinical
statement with moodCode="PRMS" Promise (CONF:8951).
c. The target of an authorization activity MAY contain one or more
performer, to indicate the providers that have been authorized to
provide treatment (CONF:8952).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 317
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 161: Authorization activity example
<act classCode="ACT" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.1.19"/>
<!-- **** Authorization activity template **** -->
<id root="f4dce790-8328-11db-9fe1-0800200c9a66"/>
<code nullFlavor="NA"/>
<entryRelationship typeCode="SUBJ">
<procedure classCode="PROC" moodCode="PRMS">
<code code="73761001"
codeSystem="2.16.840.1.113883.6.96"
displayName="Colonoscopy"/>
</procedure>
</entryRelationship>
</act>
6.10 Boundary Observation
[observation: templateId 2.16.840.1.113883.10.20.6.2.11(open)]
Table 134: Boundary Observation Contexts
Used By:
Contains Entries:
Referenced Frames Observation
A Boundary Observation contains a list of integer values for the referenced
frames of a DICOM multiframe image SOP instance. It identifies the frame
numbers within the referenced SOP instance to which the reference applies. The
CDA Boundary Observation numbers frames using the same convention as
DICOM, with the first frame in the referenced object being Frame 1. A Boundary
Observation must be used if a referenced DICOM SOP instance is a multiframe
image and the reference does not apply to all frames.
Table 135: Boundary Observation Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
observation[templateId/@root = '2.16.840.1.113883.10.20.6.2.11']
@classCode
1..1
SHALL
9282
2.16.840.1.113883.5.6 (HL7ActClass) =
OBS
@moodCode
1..1
SHALL
9283
2.16.840.1.113883.5.6 (HL7ActClass) =
EVN
code
1..1
SHALL
CD
9284
1.2.840.10008.2.16.4 (DCM) = 113036
value
1..*
SHALL
9285
1. SHALL contain exactly one [1..1] @classCode="OBS" Observation
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6) (CONF:9282).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:9283).
Page 318 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
3. SHALL contain exactly one [1..1] code="113036" Frames for Display
(CodeSystem: DCM 1.2.840.10008.2.16.4) (CONF:9284).
Each number represents a frame for display.
4. SHALL contain at least one [1..*] value with @xsi:type="INT" (CONF:9285).
Figure 162: Boundary observation example
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.6.2.11"/>
<code code="113036"
codeSystem="1.2.840.10008.2.16.4"
displayName="Frames for Display"/>
<value xsi:type="INT" value="1"/>
</observation>
6.11 Caregiver Characteristics
[observation: templateId 2.16.840.1.113883.10.20.22.4.72 (open)]
Table 136: Caregiver Characteristics Contexts
Used By:
Contains Entries:
Functional Status Result Observation (optional)
Cognitive Status Result Observation (optional)
Functional Status Problem Observation (optional)
Cognitive Status Problem Observation (required)
Functional Status Section (optional)
This clinical statement represents a caregiver’s willingness to provide care and
the abilities of that caregiver to provide assistance to a patient in relation to a
specific need.

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 319
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 137: Caregiver Characteristics Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
observation[templateId/@root = '2.16.840.1.113883.10.20.22.4.72']
@classCode
1..1
SHALL
14219
2.16.840.1.113883.5.6 (HL7ActClass)
= OBS
@moodCode
1..1
SHALL
14220
2.16.840.1.113883.5.1001 (ActMood)
= EVN
templateId
1..1
SHALL
14221
@root
1..1
SHALL
14222
2.16.840.1.113883.10.20.22.4.72
id
1..*
SHALL
14223
code
1..1
SHALL
14230
statusCode
1..1
SHALL
14233
2.16.840.1.113883.5.14 (ActStatus) =
Completed
value
1..1
SHALL
14599
participant
0..*
SHALL
14227
time
0..1
MAY
14830
low
1..1
SHALL
14831
high
0..1
MAY
14832
participantRole
1..1
SHALL
14228
@classCode
1..1
SHALL
14229
IND
1. SHALL contain exactly one [1..1] @classCode="OBS" (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:14219).
2. SHALL contain exactly one [1..1] @moodCode="EVN" (CodeSystem: ActMood
2.16.840.1.113883.5.1001) (CONF:14220).
3. SHALL contain exactly one [1..1] templateId (CONF:14221) such that it
a. This templateId SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.72" (CONF:14222).
4. SHALL contain at least one [1..*] id (CONF:14223).
5. SHALL contain exactly one [1..1] code (CONF:14230).
6. SHALL contain exactly one [1..1] statusCode="Completed" (CodeSystem:
ActStatus 2.16.840.1.113883.5.14) (CONF:14233).
7. SHALL contain exactly one [1..1] value (CONF:14599).
a. Where the @code SHALL be selected from LOINC (codeSystem:
2.16.840.1.113883.6.1) or SNOMED CT (CodeSystem:
2.16.840.1.113883.6.96 (CONF:14600).
8. SHALL contain at least one [1..*] participant (CONF:14227).
a. Such participants MAY contain zero or one [0..1] time (CONF:14830).
i. The time, if present, SHALL contain exactly one [1..1] low
(CONF:14831).
ii. The time, if present, MAY contain zero or one [0..1] high
(CONF:14832).

Page 320 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
b. Such participants SHALL contain exactly one [1..1] participantRole
(CONF:14228).
i. This participantRole SHALL contain exactly one [1..1]
@classCode="IND" (CONF:14229).
Figure 163: Caregiver characteristics example with assertion
<section>
...
<entry typeCode="DRIV">
<observation classCode="OBS" moodCode="EVN">
<!-- Functional Status Result Observation -->
<templateId root="2.16.840.1.113883.10.20.22.4.74"/>
...
<entryRelationship typeCode="REFR">
<observation classCode="OBS" moodCode="EVN">
<!-- Caregiver Characteristics -->
<templateId root="2.16.840.1.113883.10.20.22.4.72"/>
<code code="ASSERTION" codeSystem="2.16.840.1.113883.5.4"/>
<statusCode code="completed"/>
<value xsi:type="CD" code="422615001"
codeSystem="2.16.840.1.113883.6.96"
displayName="caregiver difficulty providing physical
care"/>
<participant typeCode="IND">
<participantRole classCode="CAREGIVER">
<code code="MTH" codeSystem="2.16.840.1.113883.5.111"
displayName="Mother"/>
</participantRole>
</participant>
</observation>
</entryRelationship>
</observation>
</entry>
...
</section>
Figure 164: Caregiver characteristics example without assertion
<entryRelationship typeCode="SUBJ">
<observation classCode="OBS" moodCode="EVN">
<!-- Functional Status Problem observation template -->
<templateId root="2.16.840.1.113883.10.20.22.4.67"/>
<id root="08edb7c0-2111-43f2-a784-9a5fdfaa67f0"/>
<code code="404684003"
codeSystem="2.16.840.1.113883.6.96"
displayName="Finding of Functional Performance and activity"/>
<statusCode code="completed"/>
<effectiveTime>
<low value="200702"/>
</effectiveTime>
<value xsi:type="CD" code=" 424445006"
codeSystem="2.16.840.1.113883.6.96"
displayName="difficulty with dressing upper body"/>
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 321
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
<entryRelationship typeCode="REFR">
<observation classCode="OBS" moodCode="EVN">
<!-- Caregiver Characteristics -->
<templateId root="2.16.840.1.113883.10.20.22.4.9999999"/>
<code code=" 5267-7" codeSystem="2.16.840.1.113883.6.1"
displayName=" ADL or IADL assistance from any caregiver”
<statusCode code="completed"/>
<value xsi:type="CD" code=" 422615001"
codeSystem="2.16.840.1.113883.6.96"
displayName="caregiver difficulty providing physical care"/>
<participant typeCode="IND">
<participantRole classCode="CAREGIVER">
<code code="MTH" codeSystem="2.16.840.1.113883.5.111"
displayName="Mother"/>
</participantRole>
</participant>
</observation>
</entryRelationship>
</observation>
</entryRelationship>
6.12 Code Observations
[observation: templateId 2.16.840.1.113883.10.20.6.2.13(open)]
Table 138: Code Observations Contexts
Used By:
Contains Entries:
Quantity Measurement Observation
SOP Instance Observation
DICOM Template 2000 specifies that Imaging Report Elements of Value Type
Code are contained in sections. The Imaging Report Elements are inferred from
Basic Diagnostic Imaging Report Observations that consist of image references
and measurements (linear, area, volume, and numeric). Coded DICOM Imaging
Report Elements in this context are mapped to CDA-coded observations that are
section components and are related to the SOP Instance Observations
(templateId 2.16.840.1.113883.10.20.6.2.8) or Quantity Measurement
Observations (templateId 2.16.840.1.113883.10.20.6.2.14) by the SPRT
(Support) act relationship.
Page 322 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 139: Code Observations Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
observation[templateId/@root = '2.16.840.1.113883.10.20.6.2.13']
@classCode
1..1
SHALL
9304
2.16.840.1.113883.5.6
(HL7ActClass) = OBS
@moodCode
1..1
SHALL
9305
2.16.840.1.113883.5.1001
(ActMood) = EVN
templateId
1..1
SHALL
15523
@root
1..1
SHALL
15524
2.16.840.1.113883.10.20.6.2.13
code
1..1
SHALL
CD
9307
effectiveTime
0..1
SHOULD
TS or
IVL<TS>
9309
value
1..1
SHALL
9308
entryRelationship
0..*
MAY
9311
@typeCode
1..1
SHALL
9312
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) = SPRT
entryRelationship
0..*
MAY
9314
@typeCode
1..1
SHALL
9315
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) = SPRT
1. SHALL contain exactly one [1..1] @classCode="OBS" Observation
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6) (CONF:9304).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001) (CONF:9305).
3. SHALL contain exactly one [1..1] templateId (CONF:15523).
a. This templateId SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.6.2.13" (CONF:15524).
4. SHALL contain exactly one [1..1] code with @xsi:type="CE" (CONF:9307).
5. SHOULD contain zero or one [0..1] effectiveTime (CONF:9309).
6. SHALL contain exactly one [1..1] value (CONF:9308).
7. Code Observations SHALL be rendered into section/text in separate
paragraphs (CONF:9310).
8. MAY contain zero or more [0..*] entryRelationship (CONF:9311) such that
it
a. SHALL contain exactly one [1..1] @typeCode="SPRT" Has Support
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002) (CONF:9312).
b. SHALL contain exactly one [1..1] SOP Instance Observation
(2.16.840.1.113883.10.20.6.2.8) (CONF:9313).
9. MAY contain zero or more [0..*] entryRelationship (CONF:9314) such that
it
a. SHALL contain exactly one [1..1] @typeCode="SPRT" Has Support
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002) (CONF:9315).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 323
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
b. SHALL contain exactly one [1..1] Quantity Measurement
Observation (2.16.840.1.113883.10.20.6.2.14) (CONF:9316).
Figure 165: Code observation example
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.6.2.13"/>
<code code="18782-3" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="Study observation"/>
<statusCode code="completed"/>
<value xsi:type="CD" code="309530007"
codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT"
displayName="Hilar mass"/>
<!-- entryRelationship elements referring to SOP Instance Observations
or Quantity Measurement Observations may appear here -->
</observation>
6.13 Cognitive Status Problem Observation
[observation: templateId 2.16.840.1.113883.10.20.22.4.73 (open)]
Table 140: Cognitive Status Problem Observation Contexts
Used By:
Contains Entries:
Functional Status Section (optional)
Assessment Scale Observation
Caregiver Characteristics
Non-Medicinal Supply Activity
A cognitive status problem observation is a clinical statement that describes a
patient's cognitive condition, findings, or symptoms. Examples of cognitive
problem observations are inability to recall, amnesia, dementia, and aggressive
behavior.
A cognitive problem observation is a finding or medical condition. This is
different from a cognitive result observation, which is a response to a question
that provides insight into the patient's cognitive status, judgement,
comprehension ability, or response speed.
Page 324 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 141: Cognitive Status Problem Observation Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
observation[templateId/@root = '2.16.840.1.113883.10.20.22.4.73']
@classCode
1..1
SHALL
14319
2.16.840.1.113883.5.6
(HL7ActClass) = OBS
@mood
Code
1..1
SHALL
14320
2.16.840.1.113883.5.1001
(ActMood) = EVN
@negation
Ind
0..1
MAY
14344
templateId
1..1
SHALL
14346
@root
1..1
SHALL
14347
2.16.840.1.113883.10.20.22.4.73
id
1..1
SHALL
14321
code
1..1
SHALL
14804
@code
0..1
SHOULD
14805
2.16.840.1.113883.6.96 (SNOMED-
CT) = 373930000
text
0..1
SHOULD
14341
reference/
@value
0..1
SHOULD
14342
statusCode
1..1
SHALL
14323
2.16.840.1.113883.5.14 (ActStatus)
= completed
effective
Time
0..1
SHOULD
TS or
IVL<TS>
14324
value
1..1
SHALL
CD
14349
2.16.840.1.113883.3.88.12.3221.7.
4 (Problem)
Method
Code
0..*
MAY
14693
entry
Relationship
0..*
MAY
14331
@typeCode
1..1
SHALL
14588
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) = REFR
supply
1..1
SHALL
14351
entry
Relationship
0..*
SHALL
14335
@typeCode
1..1
SHALL
14589
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) = REFR
observation
1..1
SHALL
14352
entry
Relationship
0..*
SHALL
14467
@typeCode
1..1
SHALL
14590
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) = COMP
observation
1..1
SHALL
14468
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 325
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
1. Conforms to Problem Observation template
(2.16.840.1.113883.10.20.22.4.4).
2. SHALL contain exactly one [1..1] @classCode="OBS" Observation
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6) (CONF:14319).
3. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001) (CONF:14320).
Use negationInd="true" to indicate that the problem was not observed.
4. MAY contain zero or one [0..1] @negationInd (CONF:14344).
5. SHALL contain exactly one [1..1] templateId (CONF:14346) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.73" (CONF:14347).
6. SHALL contain exactly one [1..1] id (CONF:14321).
7. SHALL contain exactly one [1..1] code (CONF:14804).
a. This code SHOULD contain zero or one [0..1] @code="373930000"
Cognitive function finding (CodeSystem: SNOMED-CT
2.16.840.1.113883.6.96) (CONF:14805).
8. SHOULD contain zero or one [0..1] text (CONF:14341).
a. The text, if present, SHOULD contain zero or one [0..1]
reference/@value (CONF:14342).
i. This reference/@value SHALL begin with a '#' and SHALL point
to its corresponding narrative (using the approach defined in
CDA Release 2, section 4.3.5.1) (CONF:14343).
9. SHALL contain exactly one [1..1] statusCode="completed" Completed
(CodeSystem: ActStatus 2.16.840.1.113883.5.14) (CONF:14323).
10. SHOULD contain zero or one [0..1] effectiveTime (CONF:14324).
a. The onset date SHALL be recorded in the low element of the
effectiveTime element when known (CONF:14325).
b. The resolution date SHALL be recorded in the high element of the
effectiveTime element when known (CONF:14326).
c. If the problem is known to be resolved, but the date of resolution is
not known, then the high element SHALL be present, and the
nullFlavor attribute SHALL be set to 'UNK'. Therefore, the existence of
a high element within a problem does indicate that the problem has
been resolved (CONF:14327).
11. SHALL contain exactly one [1..1] value with @xsi:type="CD", where the @code
SHOULD be selected from ValueSet Problem
2.16.840.1.113883.3.88.12.3221.7.4 DYNAMIC (CONF:14349).
12. MAY contain zero or more [0..*] methodCode (CONF:14693).
13. MAY contain zero or more [0..*] entryRelationship (CONF:14331) such that
it
a. SHALL contain exactly one [1..1] @typeCode="REFR" refers to
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002) (CONF:14588).
b. SHALL contain exactly one [1..1] Non-Medicinal Supply Activity
(templateId:2.16.840.1.113883.10.20.22.4.50) (CONF:14351).

Page 326 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
14. SHALL contain zero or more [0..*] entryRelationship (CONF:14335) such
that it
a. SHALL contain exactly one [1..1] @typeCode="REFR" refers to
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002) (CONF:14589).
b. SHALL contain exactly one [1..1] Caregiver Characteristics
(templateId:2.16.840.1.113883.10.20.22.4.72) (CONF:14352).
15. SHALL contain zero or more [0..*] entryRelationship (CONF:14467) such
that it
a. SHALL contain exactly one [1..1] @typeCode="COMP" has component
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002) (CONF:14590).
b. SHALL contain exactly one [1..1] Assessment Scale Observation
(templateId:2.16.840.1.113883.10.20.22.4.69) (CONF:14468).
Table 142: Problem type value set
Value Set: Problem Type 2.16.840.1.113883.3.88.12.3221.7.2 STATIC 2012-06-01
Code System(s):
SNOMED CT 2.16.840.1.113883.6.96
Description:
This value set indicates the level of medical judgment used to determine
the existence of a problem.
Code
Code System
Print Name
404684003
SNOMED CT
Finding
409586006
SNOMED CT
Complaint
282291009
SNOMED CT
Diagnosis
64572001
SNOMED CT
Condition
248536006
SNOMED CT
Finding of functional performance and activity
418799008
SNOMED CT
Symptom
55607006
SNOMED CT
Problem
373930000
SNOMED CT
Cognitive function finding
Table 143: Problem Value Set (excerpt)
Value Set: Problem 2.16.840.1.113883.3.88.12.3221.7.4 DYNAMIC
Code System(s):
SNOMED CT 2.16.840.1.113883.6.96
Description:
Problems and diagnoses. Limited to terms descending from the Clinical
Findings (404684003) or Situation with Explicit Context (243796009)
hierarchies.
http://phinvads.cdc.gov/vads/ViewValueSet.action?id=70FDBFB
5-A277-DE11-9B52-0015173D1785
Code
Code System
Print Name
46635009
SNOMED CT
Diabetes mellitus type 1
234422006
SNOMED CT
Acute porphyria
31712002
SNOMED CT
Primary biliary cirrhosis
302002000
SNOMED CT
Difficulty moving
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 327
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Value Set: Problem 2.16.840.1.113883.3.88.12.3221.7.4 DYNAMIC
Code System(s):
SNOMED CT 2.16.840.1.113883.6.96
Description:
Problems and diagnoses. Limited to terms descending from the Clinical
Findings (404684003) or Situation with Explicit Context (243796009)
hierarchies.
http://phinvads.cdc.gov/vads/ViewValueSet.action?id=70FDBFB
5-A277-DE11-9B52-0015173D1785
Code
Code System
Print Name
15188001
SNOMED CT
Hearing loss
48167000
SNOMED CT
Amnesia
…
Figure 166:Cognitive status problem observation example
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.68"/>
<!-- Cognitive Status Problem observation template -->
<id root="08edb7c0-2111-43f2-a784-9a5fdfaa67f0"/>
<code code="373930000"
codeSystem="2.16.840.1.113883.6.96"
displayName="Cognitive Function Finding"/>
<text>
...
</text>
<statusCode code="completed"/>
<effectiveTime>
<low value="200704"/>
</effectiveTime>
<value xsi:type="CD" code=" 371632003"
codeSystem="2.16.840.1.113883.6.96" displayName=" Comatose"/>
<entryRelationship typeCode="SUBJ">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.72"/>
<!—Caregiver Characteristics -->
...
</observation>
</entryRelationship>
<entryRelationship typeCode="SUBJ">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.69"/>
<!—Assessment Scale Observation -->
...
</observation>
</entryRelationship>
</observation>
Page 328 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
6.14 Cognitive Status Result Observation
[observation: templateId 2.16.840.1.113883.10.20.22.4.74 (open)]
Table 144: Cognitive Status Result Observation Contexts
Used By:
Contains Entries:
Cognitive Status Result Organizer (required)
Functional Status Section (optional)
Assessment Scale Observation
Caregiver Characteristics
Non-Medicinal Supply Activity
This clinical statement contains details of an evaluation or assessment of a
patient’s cognitive status. The evaluation may include assessment of a patient's
mood, memory, and ability to make decisions. The statement, if present, will
include supporting caregivers, non-medical devices, and the time period for
which the evaluation and assessment were performed.
This is different from a cognitive status problem observation, which is a clinical
statement that describes a patient's cognitive condition, findings, or symptoms.
Examples of cognitive problem observations are inability to recall, amnesia,
dementia, and aggressive behavior.
Table 145: Cognitive Status Result Observation Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
observation[templateId/@root = '2.16.840.1.113883.10.20.22.4.74']
@classCode
1..1
SHALL
14249
2.16.840.1.113883.5.6 (HL7ActClass) =
OBS
@moodCode
1..1
SHALL
14250
2.16.840.1.113883.5.1001 (ActMood) =
EVN
templateId
1..1
SHALL
SET<II>
14255
@root
1..1
SHALL
14256
2.16.840.1.113883.10.20.22.4.74
id
1..*
SHALL
14257
code
1..1
SHALL
14591
@code
0..1
SHOULD
14592
2.16.840.1.113883.6.96 (SNOMED-CT)
= 373930000
text
0..1
SHOULD
14258
reference/
@value
0..1
SHOULD
14259
statusCode
1..1
SHALL
14254
2.16.840.1.113883.5.14 (ActStatus) =
completed
effectiveTime
1..1
SHALL
TS or
IVL<TS>
14261
value
1..1
SHALL
14263
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 329
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
interpretationCode
0..*
SHOULD
14264
methodCode
0..1
MAY
SET<CE>
14265
targetSiteCode
0..1
MAY
SET<CD>
14270
author
0..1
MAY
14266
entryRelationship
0..*
MAY
14272
@typeCode
1..1
SHALL
14593
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) = REFR
supply
1..1
SHALL
14273
entryRelationship
0..*
MAY
14276
@typeCode
1..1
SHALL
14594
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) = REFR
observation
1..1
SHALL
14277
entryRelationship
0..*
MAY
14469
@typeCode
1..1
SHALL
14595
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) = COMP
observation
1..1
SHALL
14470
referenceRange
0..*
SHOULD
14267
observationRange
1..1
SHALL
14268
code
0..0
SHALL
NOT
14269
1. Conforms to Result Observation template
(2.16.840.1.113883.10.20.22.4.2).
2. SHALL contain exactly one [1..1] @classCode="OBS" Observation
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6) (CONF:14249).
3. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001) (CONF:14250).
4. SHALL contain exactly one [1..1] templateId (CONF:14255) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.74" (CONF:14256).
5. SHALL contain at least one [1..*] id (CONF:14257).
6. SHALL contain exactly one [1..1] code (CONF:14591).
a. This code SHOULD contain zero or one [0..1] @code="373930000"
Cognitive function finding (CodeSystem: SNOMED-CT
2.16.840.1.113883.6.96) (CONF:14592).
7. SHOULD contain zero or one [0..1] text (CONF:14258).
a. The text, if present, SHOULD contain zero or one [0..1]
reference/@value (CONF:14259).
i. This reference/@value SHALL begin with a '#' and SHALL point
to its corresponding narrative (using the approach defined in
CDA Release 2, section 4.3.5.1) (CONF:14260).

Page 330 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
8. SHALL contain exactly one [1..1] statusCode="completed" Completed
(CodeSystem: ActStatus 2.16.840.1.113883.5.14 STATIC) (CONF:14254).
Represents clinically effective time of the measurement, which may be the time
the measurement was performed (e.g., a BP measurement), or may be the time
the sample was taken (and measured some time afterwards).
9. SHALL contain exactly one [1..1] effectiveTime (CONF:14261).
10. SHALL contain exactly one [1..1] value (CONF:14263).
a. If xsi:type=“CD”, SHOULD contain a code from SNOMED CT
(CodeSystem: 2.16.840.1.113883.6.96) (CONF:14271).
11. SHOULD contain zero or more [0..*] interpretationCode (CONF:14264).
12. MAY contain zero or one [0..1] methodCode (CONF:14265).
13. MAY contain zero or one [0..1] targetSiteCode (CONF:14270).
14. MAY contain zero or one [0..1] author (CONF:14266).
15. MAY contain zero or more [0..*] entryRelationship (CONF:14272) such that
it
a. SHALL contain exactly one [1..1] @typeCode="REFR" refers to
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002) (CONF:14593).
b. SHALL contain exactly one [1..1] Non-Medicinal Supply Activity
(templateId:2.16.840.1.113883.10.20.22.4.50) (CONF:14273).
16. MAY contain zero or more [0..*] entryRelationship (CONF:14276) such that
it
a. SHALL contain exactly one [1..1] @typeCode="REFR" refers to
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002) (CONF:14594).
b. SHALL contain exactly one [1..1] Caregiver Characteristics
(templateId:2.16.840.1.113883.10.20.22.4.72) (CONF:14277).
17. MAY contain zero or more [0..*] entryRelationship (CONF:14469) such that
it
a. SHALL contain exactly one [1..1] @typeCode="COMP" has component
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002) (CONF:14595).
b. SHALL contain exactly one [1..1] Assessment Scale Observation
(templateId:2.16.840.1.113883.10.20.22.4.69) (CONF:14470).
18. SHOULD contain zero or more [0..*] referenceRange (CONF:14267).
a. The referenceRange, if present, SHALL contain exactly one [1..1]
observationRange (CONF:14268).
i. This observationRange SHALL NOT contain [0..0] code
(CONF:14269).
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 331
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 167: Cognitive status result observation example
<observation classCode="OBS" moodCode="EVN">
<!—Cognitive Status Result Oservation template -->
<templateId root="2.16.840.1.113883.10.20.22.4.74"/>
<id root=" c6b5a04b-2bf4-49d1-8336-636a3813df0a"/>
<code code="5249-2"
displayName="Observational Assessment of Cognitive Status
at 2D Assessment"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"/>
<statusCode code="completed"/>
<effectiveTime value="200903111230"/>
<value xsi:type="CD"/>
<code code="61372001" displayName="Aggressive behavior"
codeSystem="2.16.840.1.113883.5.83"
codeSystemName="SNOMED CT"/>
<entryRelationship typeCode="SUBJ">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.69"/>
<!—- Assessment Scale Observation -->
...
</observation>
</entryRelationship>
<entryRelationship typeCode="SUBJ">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.72"/>
<!—Caregiver Support and Ablility -->
...
</observation>
</entryRelationship>
</observation>
6.15 Cognitive Status Result Organizer
[organizer: templateId 2.16.840.1.113883.10.20.22.4.75 (open)]
Table 146: Cognitive Status Result Organizer Contexts
Used By:
Contains Entries:
Functional Status Section (optional)
Cognitive Status Result Observation
This clinical statement identifies a set of cognitive status result observations. It
contains information applicable to all of the contained cognitive status result
observations. A result organizer may be used to group questions in a Patient
Health Questionaire (PHQ).
An appropriate nullFlavor can be used when the organizer/code or
organizer/id is unknown.

Page 332 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 147: Cognitive Status Result Organizer Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
organizer[templateId/@root = '2.16.840.1.113883.10.20.22.4.75']
@classCode
1..1
SHALL
14369
2.16.840.1.113883.5.6 (HL7ActClass) =
CLUSTER
@moodCode
1..1
SHALL
14371
2.16.840.1.113883.5.1001 (ActMood) = EVN
templateId
1..1
SHALL
SET<II>
14375
@root
1..1
SHALL
14376
2.16.840.1.113883.10.20.22.4.75
id
1..*
SHALL
14377
code
1..1
SHALL
14378
@code
0..1
SHOULD
14697
statusCode
1..1
SHALL
14372
2.16.840.1.113883.5.14 (ActStatus) =
completed
component
1..*
SHALL
14373
observation
1..1
SHALL
14381
1. Conforms to Result Organizer template
(2.16.840.1.113883.10.20.22.4.1).
2. SHALL contain exactly one [1..1] @classCode, which SHALL be selected from
CodeSystem HL7ActClass (2.16.840.1.113883.5.6)="CLUSTER"
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6) (CONF:14369).
3. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001) (CONF:14371).
4. SHALL contain exactly one [1..1] templateId (CONF:14375) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.75" (CONF:14376).
5. SHALL contain at least one [1..*] id (CONF:14377).
6. SHALL contain exactly one [1..1] code (CONF:14378).
a. This code SHOULD contain zero or one [0..1] @code (CONF:14697).
i. Should be selected from ICF (codeSystem
2.16.840.1.113883.6.254) or SNOMED CT (codeSystem
2.16.840.1.113883.6.96) (CONF:14698).
7. SHALL contain exactly one [1..1] statusCode="completed" Completed
(CodeSystem: ActStatus 2.16.840.1.113883.5.14) (CONF:14372).
8. SHALL contain at least one [1..*] component (CONF:14373) such that it
a. SHALL contain exactly one [1..1] Cognitive Status Result
Observation (templateId:2.16.840.1.113883.10.20.22.4.74)
(CONF:14381).
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 333
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 168 Cognitive status result organizer example
<organizer classCode="CLUSTER" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.75"/>
<!-- Cognitive Status Result Organizer template -->
<id root="9295dba4-df05-46bb-b94e-f2c4e4b156f8"/>
<code code="d3" displayName="Communication"
codeSystem="2.16.840.1.113883.6.254" codeSystemName="ICF"/>
<statusCode code="completed"/>
<component>
<observation classCode="OBS" moodCode="EVN">
<!-- Cognitive Status Result observation
(Understanding Verbal Content) -->
<templateId root="2.16.840.1.113883.10.20.22.4.74"/>
...
</observation>
</component>
<component>
<observation classCode="OBS" moodCode="EVN">
<!-- Cognitive Status Result observation(Expression of Ideas) -->
<templateId root="2.16.840.1.113883.10.20.22.4.74"/>
...
</observation>
</component>
</organizer>
6.16 Comment Activity
[act: templateId 2.16.840.1.113883.10.20.22.4.64(open)]
Table 148: Comment Activity Contexts
Used By:
Contains Entries:
Any document
Comments are free text data that cannot otherwise be recorded using data
elements already defined by this specification. They are not to be used to record
information that can be recorded elsewhere. For example, a free text description
of the severity of an allergic reaction would not be recorded in a comment.
Page 334 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 149: Comment Activity Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
act[templateId/@root = '2.16.840.1.113883.10.20.22.4.64']
@classCode
1..1
SHALL
9425
2.16.840.1.113883.5.6
(HL7ActClass) = ACT
@moodCode
1..1
SHALL
9426
2.16.840.1.113883.5.1001
(ActMood) = EVN
templateId
1..1
SHALL
SET<II>
9427
@root
1..1
SHALL
10491
2.16.840.1.113883.10.20.22.4
.64
code
1..1
SHALL
CD
9428
2.16.840.1.113883.6.1
(LOINC) = 48767-8
text
1..1
SHALL
ED
9430
free
Text
Comment
reference/@value
1..1
SHALL
9431
author
author
0..1
MAY
9433
time
1..1
SHALL
IVL<TS>
9434
assignedAuthor
1..1
SHALL
9435
id
1..1
SHALL
II
9436
addr
1..1
SHALL
SET<AD>
9437
1. Data elements defined elsewhere in the specification SHALL NOT be recorded
using the Comment Activity (CONF:9429).
2. SHALL contain exactly one [1..1] @classCode="ACT" Act (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:9425).
3. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001) (CONF:9426).
4. SHALL contain exactly one [1..1] templateId (CONF:9427) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.64" (CONF:10491).
5. SHALL contain exactly one [1..1] code="48767-8" Annotation Comment
(CodeSystem: LOINC 2.16.840.1.113883.6.1) (CONF:9428).
a. This text SHALL contain exactly one [1..1] reference (CONF:15967).
i. This reference SHALL contain exactly one [1..1] @value
(CONF:15968).
1. This reference/@value SHALL begin with a '#' and SHALL
point to its corresponding narrative (using the
approach defined in CDA Release 2, section 4.3.5.1)
(CONF:15969).
6. MAY contain zero or one [0..1] author (CONF:9433).
a. The author, if present, SHALL contain exactly one [1..1] time
(CONF:9434).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 335
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
b. The author, if present, SHALL contain exactly one [1..1]
assignedAuthor (CONF:9435).
i. This assignedAuthor SHALL contain exactly one [1..1] id
(CONF:9436).
ii. This assignedAuthor SHALL contain exactly one [1..1] addr
(CONF:9437).
1. The content of addr SHALL be a conformant US Realm
Address (AD.US.FIELDED)
(2.16.840.1.113883.10.20.22.5.2) (CONF:10480).
iii. SHALL include assignedPerson/name or
representedOrganization/name (CONF:9438).
iv. An assignedPerson/name SHALL be a conformant US Realm
Person Name (PN.US.FIELDED)
(2.16.840.1.113883.10.20.22.5.1.1) (CONF:9439).
Figure 169: Comment act example
<act classCode="ACT" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.64"/>
<!-- Comment template -->
<code code="48767-8" displayName="comment"
codeSystemName="LOINC"
codeSystem="2.16.840.1.113883.6.1"/>
<text>The patient stated that he was looking forward to an upcoming
vacation to New York with his family. He was concerned that he may
not have enough medication for the trip. An additional prescription
was provided to cover that period of time.
<reference value="#PntrtoSectionText"/>
</text>
<author>
<time value="20050329224411+0500"/>
<assignedAuthor>
<id extension="KP00017" root="2.16.840.1.113883.19.5"/>
<addr>
<streetAddressLine>21 North Ave.</streetAddressLine>
<city>Burlington</city>
<state>MA</state>
<postalCode>02368</postalCode>
<country>US</country>
</addr>
<telecom use="WP" value="tel:(555)555-1003"/>
<assignedPerson>
<name>
<given>Henry</given>
<family>Seven</family>
</name>
</assignedPerson>
</assignedAuthor>
</author>
</act>

Page 336 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
6.17 Coverage Activity
[act: templateId 2.16.840.1.113883.10.20.22.4.60 (open)]
Table 150: Coverage Activity Contexts
Used By:
Contains Entries:
Payers Section (optional)
Policy Activity
A Coverage Activity groups the policy and authorization acts within a Payers
Section to order the payment sources. A Coverage Activity contains one or more
policy activities, each of which contains zero or more authorization activities.
The Coverage Activity id is the Id from the patient's insurance card. The
sequenceNumber/@value shows the policy order of preference.
Table 151: Coverage Activity Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
act[templateId/@root = '2.16.840.1.113883.10.20.22.4.60']
@classCode
1..1
SHALL
8872
2.16.840.1.113883.5.6
(HL7ActClass) = ACT
@moodCode
1..1
SHALL
8873
2.16.840.1.113883.5.1001
(ActMood) = EVN
templateId
1..1
SHALL
SET<II>
8897
@root
1..1
SHALL
10492
2.16.840.1.113883.10.20.22.4.60
id
1..*
SHALL
8874
code
1..1
SHALL
CE
8876
2.16.840.1.113883.6.1 (LOINC) =
48768-6
statusCode
1..1
SHALL
8875
2.16.840.1.113883.5.14 (ActStatus)
= completed
entryRelationship
1..*
SHALL
8878
@typeCode
1..1
SHALL
8879
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) = COMP
act
1..1
SHALL
15528
1. SHALL contain exactly one [1..1] @classCode="ACT" Act (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:8872).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001) (CONF:8873).
3. SHALL contain exactly one [1..1] templateId (CONF:8897) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.60" (CONF:10492).
4. SHALL contain at least one [1..*] id (CONF:8874).
5. SHALL contain exactly one [1..1] code="48768-6" Payment Sources with
@xsi:type="CE" (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:8876).
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 337
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
6. SHALL contain exactly one [1..1] statusCode="completed" Completed
(CodeSystem: ActStatus 2.16.840.1.113883.5.14) (CONF:8875).
7. SHALL contain at least one [1..*] entryRelationship (CONF:8878) such that
it
a. SHALL contain exactly one [1..1] @typeCode="COMP" has component
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002) (CONF:8879).
b. SHALL contain exactly one [1..1] Policy Activity
(templateId:2.16.840.1.113883.10.20.22.4.61) (CONF:15528).
Figure 170: Coverage activity example
<act classCode="ACT" moodCode="DEF">
<templateId root="2.16.840.1.113883.10.20.22.4.60"/>
<!-- **** Coverage activity template **** -->
<id root="1fe2cdd0-7aad-11db-9fe1-0800200c9a66"/>
<code code="48768-6" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC" displayName="Payment sources"/>
<statusCode code="completed"/>
<entryRelationship typeCode="COMP">
<act classCode="ACT" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.61"/>
<!-- **** Policy Activity template **** -->
...
</act>
</entryRelationship>
</act>
6.18 Deceased Observation
[observation: templateId 2.16.840.1.113883.10.20.22.4.79 (open)]
Table 152: Deceased Observation Contexts
Used By:
Contains Entries:
Problem Observation
This clinical statement represents the observation that a patient has expired. It
also represents the cause of death, indicated by an entryRelationship type
of “CAUS”.
Page 338 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 153: Deceased Observation Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
observation[templateId/@root = '2.16.840.1.113883.10.20.22.4.79']
@classCode
1..1
SHALL
14851
2.16.840.1.113883.5.6
(HL7ActClass) = OBS
@moodCode
1..1
SHALL
14852
2.16.840.1.113883.5.1001
(ActMood) = EVN
templateId
1..1
SHALL
14871
@root
1..1
SHALL
14872
2.16.840.1.113883.10.20.22.4
.79
id
1..*
SHALL
14873
code
1..1
SHALL
14853
2.16.840.1.113883.5.4
(ActCode) = ASSERTION
statusCode
1..1
SHALL
14854
2.16.840.1.113883.5.14
(ActStatus) = completed
effectiveTime
1..1
SHALL
14855
low
1..1
SHALL
14874
value
1..1
SHALL
CD
14857
@code
1..1
SHALL
15142
2.16.840.1.113883.6.96
(SNOMED-CT) = 419099009
entryRelationship
0..1
SHOULD
14868
@typeCode
1..1
SHALL
14875
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) =
CAUS
observation
1..1
SHALL
14870
1. SHALL contain exactly one [1..1] @classCode="OBS" Observation
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6 STATIC)
(CONF:14851).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001 STATIC) (CONF:14852).
3. SHALL contain exactly one [1..1] templateId (CONF:14871) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.79" (CONF:14872).
4. SHALL contain at least one [1..*] id (CONF:14873).
5. SHALL contain exactly one [1..1] code="ASSERTION" Assertion (CodeSystem:
ActCode 2.16.840.1.113883.5.4 STATIC) (CONF:14853).
6. SHALL contain exactly one [1..1] statusCode="completed" Completed
(CodeSystem: ActStatus 2.16.840.1.113883.5.14 STATIC) (CONF:14854).
7. SHALL contain exactly one [1..1] effectiveTime (CONF:14855).
a. This effectiveTime SHALL contain exactly one [1..1] low (CONF:14874).
8. SHALL contain exactly one [1..1] value with @xsi:type="CD" (CONF:14857).
a. This value SHALL contain exactly one [1..1] @code="419099009" Dead
(CodeSystem: SNOMED-CT 2.16.840.1.113883.6.96) (CONF:15142).
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 339
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
9. SHOULD contain zero or one [0..1] entryRelationship (CONF:14868) such
that it
a. SHALL contain exactly one [1..1] @typeCode="CAUS" Is etiology for
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002) (CONF:14875).
b. SHALL contain exactly one [1..1] Problem Observation
(templateId:2.16.840.1.113883.10.20.22.4.4) (CONF:14870).
Figure 171: Deceased observation example
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.79"/>
<!-- Deceased observation template -->
<id root="6898fae0-5c8a-11db-b0de-0800200c9a77"/>
<code code="ASSERTION" codeSystem="2.16.840.1.113883.5.4"/>
<statusCode code="completed"/>
<effectiveTime>
<low value="20100303"/>
</effectiveTime>
<value xsi:type="CD" code="419099009"
codeSystem="2.16.840.1.113883.6.96"
displayName="Dead"/>
</observation>
6.19 Discharge Medication
[act: templateId 2.16.840.1.113883.10.20.22.4.35(open)]
Table 154: Discharge Medication Contexts
Used By:
Contains Entries:
Hospital Discharge Medications Section (entries required)
Hospital Discharge Medications Section (entries optional)
Medication Activity
The Discharge Medications entry codes medications that the patient is intended
to take (or stop) after discharge.
Page 340 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 155: Discharge Medication Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
act[templateId/@root = '2.16.840.1.113883.10.20.22.4.35']
@classCode
1..1
SHALL
7689
2.16.840.1.113883.5.6
(HL7ActClass) = ACT
@moodCode
1..1
SHALL
7690
2.16.840.1.113883.5.1001
(ActMood) = EVN
templateId
1..1
SHALL
16760
@root
1..1
SHALL
16761
2.16.840.1.113883.10.20.22.4.35
code
1..1
SHALL
CD
7691
2.16.840.1.113883.6.1 (LOINC) =
10183-2
entryRelationship
1..1
SHALL
7692
@typeCode
1..1
SHALL
7693
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) =
SUBJ
substanceAdministration
1..1
SHALL
15525
1. SHALL contain exactly one [1..1] @classCode="ACT" (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:7689).
2. SHALL contain exactly one [1..1] @moodCode="EVN" (CodeSystem: ActMood
2.16.840.1.113883.5.1001) (CONF:7690).
3. SHALL contain exactly one [1..1] templateId (CONF:16760) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.35" (CONF:16761).
4. SHALL contain exactly one [1..1] code="10183-2" Discharge medication
(CodeSystem: LOINC 2.16.840.1.113883.6.1) (CONF:7691).
5. SHALL contain at least one [1..*] entryRelationship (CONF:7692) such that
it
a. SHALL contain exactly one [1..1] @typeCode="SUBJ" Has Subject
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002) (CONF:7693).
b. SHALL contain exactly one [1..1] Medication Activity
(templateId:2.16.840.1.113883.10.20.22.4.16) (CONF:15525).
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 341
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 172: Discharge medication entry example
<entry>
<act classCode="ACT" moodCode="EVN">
<!-- Discharge Medication Entry -->
<templateId root="2.16.840.1.113883.10.20.22.4.35"/>
<id root="5a784260-6856-4f38-9638-80c751aff2fb"/>
<code code="10183-2"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="Discharge medication"/>
<statusCode code="active"/>
<effectiveTime>
<low value="20030303"/>
</effectiveTime>
<entryRelationship typeCode="SUBJ">
<substanceAdministration moodCode="" classCode="SBADM">
<templateId root="2.16.840.1.113883.10.20.22.4.16"/>
<!-- Medication Activity -->
...
</substanceAdministration>
</entryRelationship>
</act>
</entry>
6.20 Drug Vehicle
[participantRole: templateId 2.16.840.1.113883.10.20.22.4.24(open)]
Table 156: Drug Vehicle Contexts
Used By:
Contains Entries:
Medication Activity
Immunization Activity
This template represents the vehicle (e.g., saline, dextrose) for administering a
medication.
Page 342 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 157: Drug Vehicle Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
participantRole[templateId/@root = '2.16.840.1.113883.10.20.22.4.24']
@classCode
1..1
SHALL
7490
2.16.840.1.113883.5.110 (RoleClass) =
MANU
templateId
1..1
SHALL
SET<II>
7495
@root
1..1
SHALL
10493
2.16.840.1.113883.10.20.22.4.24
code
1..1
SHALL
7491
2.16.840.1.113883.6.96 (SNOMED-CT)
= 412307009
playingEntity
1..1
SHALL
7492
code
1..1
SHALL
7493
name
0..1
MAY
7494
1. SHALL contain exactly one [1..1] @classCode="MANU" (CodeSystem:
RoleClass 2.16.840.1.113883.5.110) (CONF:7490).
2. SHALL contain exactly one [1..1] templateId (CONF:7495) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.24" (CONF:10493).
3. SHALL contain exactly one [1..1] code="412307009" Drug Vehicle
(CodeSystem: SNOMED-CT 2.16.840.1.113883.6.96) (CONF:7491).
4. SHALL contain exactly one [1..1] playingEntity (CONF:7492).
This playingEntity/code is used to supply a coded term for the drug vehicle.
a. This playingEntity SHALL contain exactly one [1..1] code
(CONF:7493).
b. This playingEntity MAY contain zero or one [0..1] name (CONF:7494).
i. This playingEntity/name MAY be used for the vehicle name in
text, such as Normal Saline (CONF:10087).
Figure 173: Drug vehicle entry example
<participantRole classCode="MANU">
<templateId root="2.16.840.1.113883.10.20.22.4.24"/>
<code code="412307009"
codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT"
displayName="drug vehicle" />
<playingEntity classCode="MMAT">
<code code="125464" displayName="Normal Saline"
codeSystem="2.16.840.1.113883.6.88"
codeSystemName="RxNorm"/>
<name>Normal Saline</name>
</playingEntity>
</participantRole>
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 343
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
6.21 Encounter Activities
[encounter: templateId 2.16.840.1.113883.10.20.22.4.49(open)]
Table 158: Encounter Activities Contexts
Used By:
Contains Entries:
Encounters Section (entries optional) (optional)
Encounters Section (entries required) (required)
Encounter Diagnosis
Indication
Service Delivery Location
This clinical statement describes the interactions between the patient and
clinicians. Interactions include in-person encounters, telephone conversations,
and email exchanges.
Table 159: Encounter Activities Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF
#
Fixed Value
Green
Encounter
Activities
encounter[templateId/@root = '2.16.840.1.113883.10.20.22.4.49']
@classCode
1..1
SHALL
8710
2.16.840.1.113883.5.6
(HL7ActClass) = ENC
@moodCode
1..1
SHALL
8711
2.16.840.1.113883.5.1001
(ActMood) = EVN
templateId
1..1
SHALL
SET<
II>
8712
encounter
ID
id
1..*
SHALL
8713
encounter
Type
code
0..1
SHOULD
8714
2.16.840.1.113883.3.88.1
2.80.32
(EncounterTypeCode)
originalText
0..1
SHOULD
8719
reference
0..1
SHOULD
15970
@value
0..1
SHOULD
15971
encounter
FreeText
Type
reference/@value
0..1
SHOULD
8720
encounter
DateTime
effectiveTime
1..1
SHALL
TS or
IVL<
TS>
8715
performer
0..*
MAY
8725
encounter
Provider
assignedEntity
1..1
SHALL
8726
code
0..1
MAY
8727
facility
Location
participant
0..*
MAY
8738
@typeCode
1..1
SHALL
8740
2.16.840.1.113883.5.1002
(HL7ActRelationshipType)
Page 344 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Name
XPath
Card.
Verb
Data
Type
CONF
#
Fixed Value
= LOC
participantRole
1..1
SHALL
14903
reasonFor
Visit
entryRelationship
0..*
MAY
8722
@typeCode
1..1
SHALL
8723
2.16.840.1.113883.5.1002
(HL7ActRelationshipType)
= RSON
observation
1..1
SHALL
14899
entryRelationship
0..*
MAY
15492
act
1..1
SHALL
15973
1. SHALL contain exactly one [1..1] @classCode="ENC" (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:8710).
2. SHALL contain exactly one [1..1] @moodCode="EVN" (CodeSystem: ActMood
2.16.840.1.113883.5.1001) (CONF:8711).
3. SHALL contain exactly one [1..1] templateId (CONF:8712) such that it
4. SHALL contain at least one [1..*] id (CONF:8713).
5. SHOULD contain zero or one [0..1] code, which SHOULD be selected from
ValueSet EncounterTypeCode 2.16.840.1.113883.3.88.12.80.32
DYNAMIC (CONF:8714).
a. The code, if present, SHOULD contain zero or one [0..1] originalText
(CONF:8719).
i. The originalText, if present, SHOULD contain zero or one [0..1]
reference (CONF:15970).
1. The reference, if present, SHOULD contain zero or one
[0..1] @value (CONF:15971).
a. This reference/@value SHALL begin with a '#'
and SHALL point to its corresponding narrative
(using the approach defined in CDA Release 2,
section 4.3.5.1) (CONF:15972).
ii. The originalText, if present, SHOULD contain zero or one [0..1]
reference/@value (CONF:8720).
6. SHALL contain exactly one [1..1] effectiveTime (CONF:8715).
7. MAY contain zero or more [0..*] performer (CONF:8725).
a. The performer, if present, SHALL contain exactly one [1..1]
assignedEntity (CONF:8726).
i. This assignedEntity MAY contain zero or one [0..1] code
(CONF:8727).
8. MAY contain zero or more [0..*] participant (CONF:8738) such that it
a. SHALL contain exactly one [1..1] @typeCode="LOC" Location
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002 STATIC) (CONF:8740).
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 345
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
b. SHALL contain exactly one [1..1] Service Delivery Location
(templateId:2.16.840.1.113883.10.20.22.4.32) (CONF:14903).
9. MAY contain zero or more [0..*] entryRelationship (CONF:8722) such that
it
a. SHALL contain exactly one [1..1] @typeCode="RSON" Has Reason
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002) (CONF:8723).
b. SHALL contain exactly one [1..1] Indication
(templateId:2.16.840.1.113883.10.20.22.4.19) (CONF:14899).
10. MAY contain zero or more [0..*] entryRelationship (CONF:15492) such that
it
a. SHALL contain exactly one [1..1] Encounter Diagnosis
(templateId:2.16.840.1.113883.10.20.22.4.80 )
(CONF:15973).
11. MAY contain zero or one [0..1] sdtc:dischargeDispositionCode, which SHALL
be selected from ValueSet 2.16.840.1.113883.3.88.12.80.33 NUBC UB-04
FL17-Patient Status DYNAMIC or, if access to NUBC is unavailable, from
CodeSystem 2.16.840.1.113883.12.112 HL7 Discharge Disposition. The
prefix sdtc: SHALL be bound to the namespace “urn:hl7-org:sdtc”. The use of
the namespace provides a necessary extension to CDA R2 for the use of the
dischargeDispositionCode element (CONF:9929).
Table 160: Encounter Type Value Set
Value Set: EncounterTypeCode 2.16.840.1.113883.3.88.12.80.32 DYNAMIC
Code System: CPT-4 2.16.840.1.113883.6.12
This value set includes only the codes of the Current Procedure and Terminology designated for
Evaluation and Management (99200 – 99607) (subscription to AMA Required
http://www.amacodingonline.com/)
Code
Code System
Print Name
99201
CPT-4
Office or other outpatient visit (problem
focused)
99202
CPT-4
Office or other outpatient visit (expanded
problem (expanded)
99203
CPT-4
Office or other outpatient visit (detailed)
99204
CPT-4
Office or other outpatient visit
(comprehensive, (comprehensive -
moderate)
99205
CPT-4
Office or other outpatient visit
(comprehensive, comprehensive-high)
…
CPT-4
…

Page 346 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 174: Encounter activities example
<entry typeCode="DRIV">
<encounter classCode="ENC" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.49"/>
<!-- Encounter Activities -->
<id root="2a620155-9d11-439e-92b3-5d9815ff4de8"/>
<code code="99241"
displayName="Office consultation - 15 minutes"
codeSystemName="CPT-4"
codeSystem="2.16.840.1.113883.6.12"
codeSystemVersion="4">
<originalText>Checkup Examination<reference
value="#Encounter1"/>
</originalText>
<translation code="AMB"
codeSystem="2.16.840.1.113883.5.4"
displayName="Ambulatory"
codeSystemName="HL7ActEncounterCode"/>
</code>
<effectiveTime value="20000407"/>
<performer>
<assignedEntity>
<code code="59058001"
codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT"
displayName="General Physician"/>
</assignedEntity>
</performer>
<participant typeCode="LOC">
<participantRole classCode="SDLOC">
<templateId root="2.16.840.1.113883.10.20.22.4.32"/>
...
</participantRole>
</participant>
<entryRelationship typeCode="RSON">
<observation classCode="OBS" moodCode="EVN">
<!-- Indication -->
<templateId root="2.16.840.1.113883.10.20.22.4.19"/>
...
</observation>
</entryRelationship>
</encounter>
</entry>
6.22 Encounter Diagnosis
[act: templateId 2.16.840.1.113883.10.20.22.4.80 (open)]
Table 161: Encounter Diagnosis Contexts
Used By:
Contains Entries:
Encounter Activities (optional)
Problem Observation
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 347
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
This template wraps relevant problems or diagnoses at the close of a visit or that
need to be followed after the visit. If the encounter is associated with a Hospital
Discharge, the Hospital Discharge Diagnosis must be used. This entry requires
at least one Problem Observation entry.
Table 162: Encounter Diagnosis Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
act[templateId/@root = '2.16.840.1.113883.10.20.22.4.80 ']
@classCode
1..1
SHALL
14889
2.16.840.1.113883.5.6 (HL7ActClass) =
ACT
@moodCode
1..1
SHALL
14890
2.16.840.1.113883.5.1001 (ActMood) =
EVN
templateId
1..1
SHALL
14895
@root
1..1
SHALL
14896
2.16.840.1.113883.10.20.22.4.80
code
1..1
SHALL
CE
14891
@code
1..1
SHALL
14897
2.16.840.1.113883.6.1 (LOINC) = 29308-4
entry
Relationship
1..*
SHALL
14892
@typeCode
1..1
SHALL
14893
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) = SUBJ
observation
1..1
SHALL
14898
1. SHALL contain exactly one [1..1] @classCode="ACT" (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:14889).
2. SHALL contain exactly one [1..1] @moodCode="EVN" (CodeSystem: ActMood
2.16.840.1.113883.5.1001) (CONF:14890).
3. SHALL contain exactly one [1..1] templateId (CONF:14895) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.80" (CONF:14896).
4. SHALL contain exactly one [1..1] code with @xsi:type="CE" (CONF:14891).
a. This code SHALL contain exactly one [1..1] @code="29308-4"
Diagnosis (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:14897).
5. SHALL contain at least one [1..*] entryRelationship (CONF:14892) such
that it
a. SHALL contain exactly one [1..1] @typeCode="SUBJ" (CodeSystem:
HL7ActRelationshipType 2.16.840.1.113883.5.1002)
(CONF:14893).
b. SHALL contain exactly one [1..1] Problem Observation
(templateId:2.16.840.1.113883.10.20.22.4.4) (CONF:14898).
Page 348 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 175: Encounter diagnosis act example
<act classCode="ACT" moodCode="EVN">
<!—Encounter diagnosis act -->
<templateId root="2.16.840.1.113883.10.20.22.4.80"/>
<id root="5a784260-6856-4f38-9638-80c751aff2fb"/>
<code code="29038-4"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="ENCOUNTER DIAGNOSIS"/>
<statusCode code="active"/>
<effectiveTime>
<low value="20903003"/>
</effectiveTime>
<entryRelationship typeCode="SUBJ" inversionInd="false">
<observation classCode="OBS" moodCode="EVN" negationInd="false">
<templateId root="2.16.840.1.113883.10.20.22.4.4"/>
<!-- Problem Observation -->
...
</observation>
</entryRelationship>
</act>
6.23 Estimated Date of Delivery
[observation: templateId 2.16.840.1.113883.10.20.15.3.1(closed)]
Table 163: Estimated Date of Delivery Contexts
Used By:
Contains Entries:
Pregnancy Observation
This clinical statement represents the anticipated date when a woman will give
birth.
Table 164: Estimated Date of Delivery Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
observation[templateId/@root = '2.16.840.1.113883.10.20.15.3.1']
@classCode
1..1
SHALL
444
2.16.840.1.113883.5.6 (HL7ActClass) = OBS
@moodCode
1..1
SHALL
445
2.16.840.1.113883.5.1001 (ActMood) = EVN
templateId
1..1
SHALL
16762
@root
1..1
SHALL
16763
2.16.840.1.113883.10.20.15.3.1
code
1..1
SHALL
CE
446
2.16.840.1.113883.6.1 (LOINC) = 11778-8
statusCode
1..1
SHALL
448
2.16.840.1.113883.5.14 (ActStatus) =
completed
value
1..1
SHALL
TS
450
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 349
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
1. SHALL contain exactly one [1..1] @classCode="OBS" Observation
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6) (CONF:444).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001 STATIC) (CONF:445).
3. SHALL contain exactly one [1..1] templateId (CONF:16762) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.15.3.1" (CONF:16763).
4. SHALL contain exactly one [1..1] code="11778-8" Estimated date of delivery
(CodeSystem: LOINC 2.16.840.1.113883.6.1) (CONF:446).
5. SHALL contain exactly one [1..1] statusCode="completed" Completed
(CodeSystem: ActStatus 2.16.840.1.113883.5.14) (CONF:448).
6. SHALL contain exactly one [1..1] value with @xsi:type="TS" (CONF:450).
Figure 176: Estimated date of delivery example
<observation classCode="OBS" moodCode="EVN">
<!-- Estimated Date of Delivery observation template -->
<templateId root="2.16.840.1.113883.10.20.15.3.1"/>
<id extension="123456789" root="2.16.840.1.113883.19"/>
<code code="11778-8" codeSystem="2.16.840.1.113883.6.1"
displayName="Estimated date of delivery"/>
<statusCode code="completed"/>
<value xsi:type="TS">20110919</value>
</observation>
6.24 Family History Death Observation
[observation: templateId 2.16.840.1.113883.10.20.22.4.47(open)]
Table 165: Family History Death Observation Contexts
Used By:
Contains Entries:
Family History Observation
This clinical statement records whether the family member is deceased.

Page 350 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 166: Family History Death Observation Constraints Overview
Name
XPath
Card
.
Verb
Data
Type
CONF#
Fixed Value
observation[templateId/@root = '2.16.840.1.113883.10.20.22.4.47']
@classCode
1..1
SHALL
8621
2.16.840.1.113883.5.6
(HL7ActClass) = OBS
@moodCode
1..1
SHALL
8622
2.16.840.1.113883.5.1001
(ActMood) = EVN
templateId
1..1
SHALL
SET<I
I>
8623
@root
1..1
SHALL
10495
2.16.840.1.113883.10.20.22.4.4
7
code
1..1
SHALL
16889
2.16.840.1.113883.5.4 (ActCode)
= ASSERTION
statusCode
1..1
SHALL
8625
2.16.840.1.113883.5.14
(ActStatus) = completed
value
1..1
SHALL
CD
8626
2.16.840.1.113883.6.96
(SNOMED-CT) = 419099009
1. SHALL contain exactly one [1..1] @classCode="OBS" Observation
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6) (CONF:8621).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001) (CONF:8622).
3. SHALL contain exactly one [1..1] templateId (CONF:8623) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.47" (CONF:10495).
4. SHALL contain exactly one [1..1] code="ASSERTION" Assertion (CodeSystem:
ActCode 2.16.840.1.113883.5.4) (CONF:16889).
5. SHALL contain exactly one [1..1] statusCode="completed" (CodeSystem:
ActStatus 2.16.840.1.113883.5.14) (CONF:8625).
6. SHALL contain exactly one [1..1] value="419099009" Dead with
@xsi:type="CD" (CodeSystem: SNOMED-CT 2.16.840.1.113883.6.96)
(CONF:8626).
Figure 177: Family history death observation example
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.47"/>
<!-- Family history death observation template -->
<id root="6898fae0-5c8a-11db-b0de-0800200c9a66"/>
<code code="ASSERTION" codeSystem="2.16.840.1.113883.5.4"/>
<statusCode code="completed"/>
<value xsi:type="CD" code="419099009"
codeSystem="2.16.840.1.113883.6.96"
displayName="Dead"/>
</observation>
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 351
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
6.25 Family History Observation
[observation: templateId 2.16.840.1.113883.10.20.22.4.46(open)]
Table 167: Family History Observation Contexts
Used By:
Contains Entries:
Family History Organizer (optional)
Age Observation
Family History Death Observation
Family History Observations related to a particular family member are contained
within a Family History Organizer. The effectiveTime in the Family History
Observation is the biologically or clinically relevant time of the observation. The
biologically or clinically relevant time is the time at which the observation holds
(is effective) for the family member (the subject of the observation).
Table 168: Family History Observation Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
Green Family
History
Observation
observation[templateId/@root = '2.16.840.1.113883.10.20.22.4.46']
@classCode
1..1
SHALL
8586
2.16.840.1.113883.5.6
(HL7ActClass) = OBS
@moodCode
1..1
SHALL
8587
2.16.840.1.113883.5.100
1 (ActMood) = EVN
templateId
1..1
SHALL
SET<
II>
8599
@root
1..1
SHALL
10496
2.16.840.1.113883.10.20.
22.4.46
id
1..*
SHALL
8592
code
1..1
SHALL
8589
2.16.840.1.113883.3.88.1
2.3221.7.2 (Problem
Type)
statusCode
1..1
SHALL
8590
2.16.840.1.113883.5.14
(ActStatus) = completed
effectiveTime
0..1
SHOULD
TS or
IVL<
TS>
8593
value
1..1
SHALL
CD
8591
2.16.840.1.113883.3.88.1
2.3221.7.4 (Problem)
entryRelationship
0..1
MAY
8675
@typeCode
1..1
SHALL
8676
2.16.840.1.113883.5.90
(HL7ParticipationType) =
SUBJ
@inversionInd
1..1
SHALL
8677
true
observation
1..1
SHALL
15526

Page 352 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
entryRelationship
0..1
MAY
8678
@typeCode
1..1
SHALL
8679
2.16.840.1.113883.5.90
(HL7ParticipationType) =
CAUS
observation
1..1
SHALL
15527
1. SHALL contain exactly one [1..1] @classCode="OBS" Observation
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6) (CONF:8586).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001) (CONF:8587).
3. SHALL contain exactly one [1..1] templateId (CONF:8599) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.46" (CONF:10496).
4. SHALL contain at least one [1..*] id (CONF:8592).
5. SHALL contain exactly one [1..1] code, which SHOULD be selected from
ValueSet Problem Type 2.16.840.1.113883.3.88.12.3221.7.2 STATIC
2012-06-01 (CONF:8589).
6. SHALL contain exactly one [1..1] statusCode="completed" Completed
(CodeSystem: ActStatus 2.16.840.1.113883.5.14) (CONF:8590).
7. SHOULD contain zero or one [0..1] effectiveTime (CONF:8593).
8. SHALL contain exactly one [1..1] value with @xsi:type="CD", where the @code
SHALL be selected from ValueSet Problem
2.16.840.1.113883.3.88.12.3221.7.4 DYNAMIC (CONF:8591).
9. MAY contain zero or one [0..1] entryRelationship (CONF:8675) such that it
a. SHALL contain exactly one [1..1] @typeCode="SUBJ" Subject
(CodeSystem: HL7ParticipationType 2.16.840.1.113883.5.90)
(CONF:8676).
b. SHALL contain exactly one [1..1] @inversionInd="true" True
(CONF:8677).
c. SHALL contain exactly one [1..1] Age Observation
(templateId:2.16.840.1.113883.10.20.22.4.31) (CONF:15526).
10. MAY contain zero or one [0..1] entryRelationship (CONF:8678) such that it
a. SHALL contain exactly one [1..1] @typeCode="CAUS" Causal or
Contributory (CodeSystem: HL7ParticipationType
2.16.840.1.113883.5.90) (CONF:8679).
b. SHALL contain exactly one [1..1] Family History Death
Observation (templateId:2.16.840.1.113883.10.20.22.4.47)
(CONF:15527).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 353
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 178: Family history observation scenario
SCENARIO
A patient's father was diagnosed with diabetes at the age of 40. He died
of Myocardial Infarction at the age of 57. If the patient's father was
born in 1910, the family history organizer for the father would contain
the following items:
The Date of Birth
RelatedSubject/subject/birthTime => 1910
The Date of Death
RelatedSubject/subject/sdtc:deceasedInd => true
RelatedSubject/subject/sdtc:deceasedTime => 1967
The Diabetes Diagnosis
component/observation/effectiveTime => 1950
component/observation/value => contains the code and displayName for
diabetes
component/observation/entryRelationship/observation/value/@value => 40
with the unit set to "a" to indicate years
The Myocardial Infarction Diagnosis and Cause of Death
component/observation/effectiveTime => 1967
component/observation/value => contains the code and displayName for MI
component/observation/entryRelationship/observation/value/@value => 57
with the unit set to "a" to indicate years
component/observation/entryRelationship/@typeCode => "CAUS". This
second entryRelationship shows that the MI was the cause of death.
The next example uses the above scenario .

Page 354 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 179: Family history observation example
<section>
<templateId root="2.16.840.1.113883.10.20.22.2.15"/>
<!-- ******** Family history section template ******** -->
<code code="10157-6" codeSystem="2.16.840.1.113883.6.1"/>
<title>FAMILY HISTORY</title>
<text>
<paragraph>Father (deceased)</paragraph>
<table border="1" width="100%">
<thead>
<tr>
<th>Diagnosis</th>
<th>Age At Onset</th>
</tr>
</thead>
<tbody>
<tr>
<td>Myocardial Infarction (cause of death)</td>
<td>57</td>
</tr>
<tr>
<td>Diabetes</td>
<td>40</td>
</tr>
</tbody>
</table>
</text>
<entry typeCode="DRIV">
<organizer moodCode="EVN" classCode="CLUSTER">
<templateId root="2.16.840.1.113883.10.20.22.4.45"/>
<!-- ******** Family history organizer template ******** -->
<statusCode code="completed"/>
<subject>
<relatedSubject classCode="PRS">
<code code="FTH" displayName="Father"
codeSystemName="HL7 FamilyMember"
codeSystem="2.16.840.1.113883.5.111">
<translation code="9947008"
displayName="Biological father"
codeSystemName="SNOMED"
codeSystem="2.16.840.1.113883.6.96"/>
</code>
<subject>
<administrativeGenderCode code="M"
codeSystem="2.16.840.1.113883.5.1"
displayName="Male"/>
<birthTime value="1910"/>
<sdtc:deceasedInd value="true"/>
<sdtc:deceasedTime value="1967"/>
</subject>
</relatedSubject>
</subject>

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 355
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
<component>
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.46"/>
<!-- Family History Observation template -->
<id root="d42ebf70-5c89-11db-b0de-0800200c9a66"/>
<code code="55561003" displayName="Active"
codeSystemName="SNOMED CT"
codeSystem="2.16.840.1.113883.6.96"/>
<statusCode code="completed"/>
<effectiveTime value="1967"/>
<value xsi:type="CD" code="22298006"
codeSystem="2.16.840.1.113883.6.96"
displayName="Myocardial infarction"/>
<entryRelationship typeCode="CAUS">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.47"/>
<!-- ******** Family history death observation template ******** --
>
<id root="6898fae0-5c8a-11db-b0de-0800200c9a66"/>
<code code="ASSERTION"
codeSystem="2.16.840.1.113883.5.4"/>
<statusCode code="completed"/>
<value xsi:type="CD" code="419099009"
codeSystem="2.16.840.1.113883.6.96"
displayName="Dead"/>
</observation>
</entryRelationship>
<entryRelationship typeCode="SUBJ" inversionInd="true">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.31"/>
<!-- ******** Age observation template ******** -->
<code code="397659008"
codeSystem="2.16.840.1.113883.6.96"
displayName="Age"/>
<statusCode code="completed"/>
<value xsi:type="PQ" value="57" unit="a"/>
</observation>
</entryRelationship>
</observation>
</component>
<component>
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.46"/>
<!-- ******** Family history observation template ******** -->
<id root="5bfe3ec0-5c8b-11db-b0de-0800200c9a66"/>
<code code="7087005" displayName="Intermittent"
codeSystemName="SNOMED CT"
codeSystem="2.16.840.1.113883.6.96"/>
<statusCode code="completed"/>
<effectiveTime value="1950"/>
<value xsi:type="CD" code="46635009"
codeSystem="2.16.840.1.113883.6.96"
displayName="Diabetes mellitus type 1"/>
Page 356 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
<entryRelationship typeCode="SUBJ" inversionInd="true">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.31"/>
<!-- ******** Age observation template ******** -->
<code code="397659008"
codeSystem="2.16.840.1.113883.6.96"
displayName="Age"/>
<statusCode code="completed"/>
<value xsi:type="PQ" value="40" unit="a"/>
</observation>
</entryRelationship>
</observation>
</component>
</organizer>
</entry>
</section>
6.26 Family History Organizer
[organizer: templateId 2.16.840.1.113883.10.20.22.4.45(open)]
Table 169: Family History Organizer Contexts
Used By:
Contains Entries:
Family History Section
Family History Observation
The Family History Organizer associates a set of observations with a family
member. For example, the Family History Organizer can group a set of
observations about the patient’s father.
Table 170: Family History Organizer Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
Green Family
History
Organizer
organizer[templateId/@root = '2.16.840.1.113883.10.20.22.4.45']
@classCode
1..1
SHALL
8600
2.16.840.1.113883.5.6
(HL7ActClass) = CLUSTER
@moodCode
1..1
SHALL
8601
2.16.840.1.113883.5.1001
(ActMood) = EVN
templateId
1..1
SHALL
SET<
II>
8604
@root
1..1
SHALL
10497
2.16.840.1.113883.10.20.2
2.4.45
statusCode
1..1
SHALL
8602
2.16.840.1.113883.5.14
(ActStatus) = completed
familyMember
Demographics
subject
1..1
SHALL
8609
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 357
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
relatedSubject
1..1
SHALL
15244
@classCode
1..1
SHALL
15245
2.16.840.1.113883.5.41
(EntityClass) = PRS
code
1..1
SHALL
15246
@code
0..1
SHALL
15247
2.16.840.1.113883.1.11.19
579
(FamilyHistoryRelatedSubje
ctCode)
subject
0..1
SHOULD
15248
administrative
GenderCode
1..1
SHALL
15974
@code
1..1
SHALL
15975
2.16.840.1.113883.1.11.1
(Administrative Gender
(HL7 V3))
birthTime
0..1
SHOULD
15976
relatedSubject
/@classCode
1..1
SHALL
8610
2.16.840.1.113883.5.41
(EntityClass) = PRS
familyMember
Relationship
ToPatient
code
1..1
SHALL
CE
8611
familyMember
Person
Information
subject
0..1
SHOULD
8613
familyMember
Administrative
Gender
administrative
GenderCode
1..1
SHALL
CE
8614
2.16.840.1.113883.1.11.1
(Administrative Gender
(HL7 V3))
familyMember
DateOfBirth
birthTime
0..1
SHOULD
TS
8615
familyMember
MedicalHistory
component
1..*
SHALL
8607
observation
1..1
SHOULD
16888
1. SHALL contain exactly one [1..1] @classCode="CLUSTER" Cluster
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6) (CONF:8600).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001) (CONF:8601).
3. SHALL contain exactly one [1..1] templateId (CONF:8604) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.45" (CONF:10497).
4. SHALL contain exactly one [1..1] statusCode="completed" Completed
(CodeSystem: ActStatus 2.16.840.1.113883.5.14) (CONF:8602).
5. SHALL contain exactly one [1..1] subject (CONF:8609).

Page 358 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
a. This subject SHALL contain exactly one [1..1] relatedSubject
(CONF:15244).
i. This relatedSubject SHALL contain exactly one [1..1]
@classCode="PRS" Person (CodeSystem: EntityClass
2.16.840.1.113883.5.41) (CONF:15245).
ii. This relatedSubject SHALL contain exactly one [1..1] code
(CONF:15246).
1. This code SHALL contain zero or one [0..1] @code,
which SHOULD be selected from ValueSet
FamilyHistoryRelatedSubjectCode
2.16.840.1.113883.1.11.19579 DYNAMIC
(CONF:15247).
iii. This relatedSubject SHOULD contain zero or one [0..1] subject
(CONF:15248).
1. The subject, if present, SHALL contain exactly one [1..1]
administrativeGenderCode (CONF:15974).
a. This administrativeGenderCode SHALL contain
exactly one [1..1] @code, which SHALL be
selected from ValueSet Administrative
Gender (HL7 V3)
2.16.840.1.113883.1.11.1 (CONF:15975).
2. The subject, if present, SHOULD contain zero or one
[0..1] birthTime (CONF:15976).
3. The subject SHOULD contain zero or more [0..*] sdtc:id.
The prefix sdtc: SHALL be bound to the namespace
“urn:hl7-org:sdtc”. The use of the namespace provides
a necessary extension to CDA R2 for the use of the id
element (CONF:15249).
4. The subject MAY contain zero or one sdtc:deceasedInd.
The prefix sdtc: SHALL be bound to the namespace
“urn:hl7-org:sdtc”. The use of the namespace provides
a necessary extension to CDA R2 for the use of the
deceasedInd element (CONF:15981).
5. The subject MAY contain zero or one
sdtc:deceasedTime. The prefix sdtc: SHALL be bound to
the namespace “urn:hl7-org:sdtc”. The use of the
namespace provides a necessary extension to CDA R2
for the use of the deceasedTime element
(CONF:15982).
6. The age of a relative at the time of a family history
observation SHOULD be inferred by comparing
RelatedSubject/subject/birthTime with
Observation/effectiveTime (CONF:15983).
6. SHALL contain at least one [1..*] component (CONF:8607).
a. Such components SHALL contain exactly one [1..1] Family History
Observation (templateId:2.16.840.1.113883.10.20.22.4.46)
(CONF:16888).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 359
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 171: Family History Related Subject Value Set (excerpt)
Value Set: FamilyHistoryRelatedSubjectCode 2.16.840.1.113883.1.11.19579 DYNAMIC
Code System: RoleCode 2.16.840.1.113883.5.111 (any subtype of RoleCode: FAMMEMB)
See HL7 Vocabulary Domains included in the CDA R2 Normative Web Edition
http://www.hl7.org/documentcenter/private/standards/cda/r2/cda_r2_normativewebeditio
n2010.zip
Code
Code System
Print Name
CHILD
RoleCode
Child
CHLDADOPT
RoleCode
Adopted Child
DAUADOPT
RoleCode
Adopted Daughter
SONADOPT
RoleCode
Adopted Son
CHLDINLAW
RoleCode
Child in-law
…
Figure 180: Family history organizer example
<entry typeCode="DRIV">
<organizer moodCode="EVN" classCode="CLUSTER">
<templateId root="2.16.840.1.113883.10.20.22.4.45"/>
<!-- Family history organizer template -->
<statusCode code="completed"/>
<subject>
<relatedSubject classCode="PRS">
<code code="FTH" displayName="Father"
codeSystemName="HL7RoleCode"
codeSystem="2.16.840.1.113883.5.111">
<translation code="9947008"
displayName="Biological father"
codeSystemName="SNOMED CT"
codeSystem="2.16.840.1.113883.6.96"/>
</code>
<subject>
<administrativeGenderCode
code="M" codeSystem="2.16.840.1.113883.5.1"
codeSystemName="HL7AdministrativeGender"
displayName="Male"/>
<birthTime value="1912"/>
</subject>
</relatedSubject>
</subject>
<component>
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.46"/>
<!-- Family history observation template -->
...
</observation>
</component>
</organizer>
</entry>
Page 360 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
6.27 Functional Status Problem Observation
[observation: templateId 2.16.840.1.113883.10.20.22.4.68 (open)]
Table 172: Functional Status Problem Observation Contexts
Used By:
Contains Entries:
Functional Status Section (optional)
Assessment Scale Observation
Caregiver Characteristics
Non-Medicinal Supply Activity
A functional status problem observation is a clinical statement that represents a
patient’s functional perfomance and ability.
Table 173: Functional Status Problem Observation Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
observation[templateId/@root = '2.16.840.1.113883.10.20.22.4.68']
@classCode
1..1
SHALL
14282
2.16.840.1.113883.5.6
(HL7ActClass) = OBS
@moodCode
1..1
SHALL
14283
2.16.840.1.113883.5.1001
(ActMood) = EVN
@negationInd
0..1
MAY
14307
templateId
1..1
SHALL
14312
@root
1..1
SHALL
14313
2.16.840.1.113883.10.20.22.4.68
id
1..*
SHALL
14284
code
1..1
SHALL
14314
@code
0..1
SHOULD
14315
2.16.840.1.113883.6.96
(SNOMED-CT) = 248536006
text
0..1
SHOULD
14304
reference
1..1
SHOULD
15552
@value
0..1
SHOULD
15553
statusCode
1..1
SHALL
14286
2.16.840.1.113883.5.14
(ActStatus) = completed
effectiveTime
0..1
SHOULD
TS or
IVL<T
S>
14287
value
1..1
SHALL
CD
14291
2.16.840.1.113883.3.88.12.3221.7
.4 (Problem)
@nullFlavor
0..1
MAY
14292
methodCode
0..1
MAY
14316
entryRelationship
0..*
MAY
14294
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 361
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
@typeCode
1..1
SHALL
14584
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) = REFR
supply
1..1
SHALL
14317
entryRelationship
0..*
MAY
14298
@typeCode
1..1
SHALL
14586
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) = REFR
observation
1..1
SHALL
14318
entryRelationship
0..*
MAY
14463
@typeCode
1..1
SHALL
14587
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) = COMP
observation
1..1
SHALL
14464
1. Conforms to Problem Observation template
(2.16.840.1.113883.10.20.22.4.4).
2. SHALL contain exactly one [1..1] @classCode="OBS" (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:14282).
3. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001 STATIC) (CONF:14283).
Use negationInd="true" to indicate that the problem was not observed.
4. MAY contain zero or one [0..1] @negationInd (CONF:14307).
5. SHALL contain exactly one [1..1] templateId (CONF:14312) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.68" (CONF:14313).
6. SHALL contain at least one [1..*] id (CONF:14284).
7. SHALL contain exactly one [1..1] code (CONF:14314).
a. This code SHOULD contain zero or one [0..1] @code="248536006"
finding of functional performance and activity (CodeSystem: SNOMED-
CT 2.16.840.1.113883.6.96) (CONF:14315).
8. SHOULD contain zero or one [0..1] text (CONF:14304).
a. The text, if present, SHOULD contain exactly one [1..1] reference
(CONF:15552).
i. This reference SHOULD contain zero or one [0..1] @value
(CONF:15553).
1. This reference/@value SHALL begin with a '#' and SHALL
point to its corresponding narrative (using the
approach defined in CDA Release 2, section 4.3.5.1)
(CONF:15554).
9. SHALL contain exactly one [1..1] statusCode="completed" Completed
(CodeSystem: ActStatus 2.16.840.1.113883.5.14 STATIC) (CONF:14286).
10. SHOULD contain zero or one [0..1] effectiveTime (CONF:14287).
a. The onset date SHALL be recorded in the low element of the
effectiveTime element when known (CONF:14288).
Page 362 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
b. The resolution date SHALL be recorded in the high element of the
effectiveTime element when known (CONF:14289).
c. If the problem is known to be resolved, but the date of resolution is
not known, then the high element SHALL be present, and the
nullFlavor attribute SHALL be set to 'UNK'. Therefore, the existence of
an high element within a problem does indicate that the problem has
been resolved (CONF:14290).
11. SHALL contain exactly one [1..1] value with @xsi:type="CD", where the @code
SHOULD be selected from ValueSet Problem
2.16.840.1.113883.3.88.12.3221.7.4 DYNAMIC (CONF:14291).
a. This value MAY contain zero or one [0..1] @nullFlavor
(CONF:14292).
i. If the diagnosis is unknown or the SNOMED code is
unknown, @nullFlavor SHOULD be “UNK”. If the code is
something other than SNOMED, @nullFlavor SHOULD be
“OTH” and the other code SHOULD be placed in the translation
element (CONF:14293).
12. MAY contain zero or one [0..1] methodCode (CONF:14316).
13. MAY contain zero or more [0..*] entryRelationship (CONF:14294) such that
it
a. SHALL contain exactly one [1..1] @typeCode="REFR" refers to
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002) (CONF:14584).
b. SHALL contain exactly one [1..1] Non-Medicinal Supply Activity
(templateId:2.16.840.1.113883.10.20.22.4.50) (CONF:14317).
14. MAY contain zero or more [0..*] entryRelationship (CONF:14298) such that
it
a. SHALL contain exactly one [1..1] @typeCode="REFR" refers to
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002) (CONF:14586).
b. SHALL contain exactly one [1..1] Caregiver Characteristics
(templateId:2.16.840.1.113883.10.20.22.4.72) (CONF:14318).
15. MAY contain zero or more [0..*] entryRelationship (CONF:14463) such that
it
a. SHALL contain exactly one [1..1] @typeCode="COMP" has component
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002) (CONF:14587).
b. SHALL contain exactly one [1..1] Assessment Scale Observation
(templateId:2.16.840.1.113883.10.20.22.4.69) (CONF:14464).
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 363
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 181: Functional status problem observation example
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.68"/>
<!-- Functional Status Problem observation template -->
<id root="08edb7c0-2111-43f2-a784-9a5fdfaa67f0"/>
<code code="404684003"
codeSystem="2.16.840.1.113883.6.96"
displayName="Finding of Functional Performance and activity"/>
<text>
...
</text>
<statusCode code="completed"/>
<effectiveTime>
<low value="200702"/>
</effectiveTime>
<value xsi:type="CD" code=" 162891007"
codeSystem="2.16.840.1.113883.6.96"
displayName="dyspnea"/>
<entryRelationship typeCode="SUBJ">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.72"/>
<!—Caregiver Characteristics -->
...
</observation>
</entryRelationship>
<entryRelationship typeCode="SUBJ">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.69"/>
<!— Assessment Scale Observation -->
...
</observation>
</entryRelationship>
</observation>
6.28 Functional Status Result Observation
[observation: templateId 2.16.840.1.113883.10.20.22.4.67 (open)]
Table 174: Functional Status Result Observation Contexts
Used By:
Contains Entries:
Functional Status Result Organizer (required)
Functional Status Section (optional)
Assessment Scale Observation
Caregiver Characteristics
Non-Medicinal Supply Activity
This clinical statement represents details of an evaluation or assessment of a
patient's functional status. The evaluation may include assessment of a patient's
language, vision, hearing, activities of daily living, behavior, general function,
mobility, and self-care status. The statement, if present, will include supporting
caregivers, non-medical devices, and the time period for which the evaluation
and assessment were performed.
Page 364 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 175: Functional Status Result Observation Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
observation[templateId/@root = '2.16.840.1.113883.10.20.22.4.67']
@classCode
1..1
SHALL
13905
2.16.840.1.113883.5.6
(HL7ActClass) = OBS
@moodCode
1..1
SHALL
13906
2.16.840.1.113883.5.1001
(ActMood) = EVN
templateId
1..1
SHALL
13889
@root
1..1
SHALL
13890
2.16.840.1.113883.10.20.22.
4.67
id
1..*
SHALL
13907
code
1..1
SHALL
CE
13908
2.16.840.1.113883.6.1
(LOINC)
text
0..1
SHOULD
13926
reference
0..1
SHOULD
13927
statusCode
1..1
SHALL
13929
Completed
effectiveTime
1..1
SHALL
13930
value
1..1
SHALL
13932
interpretationCode
0..*
SHOULD
13933
methodCode
0..1
MAY
13934
targetSiteCode
0..1
MAY
13935
author
0..1
MAY
13936
entryRelationship
0..1
MAY
13892
@typeCode
1..1
SHALL
14596
REFR
supply
1..1
SHALL
14218
entryRelationship
0..1
MAY
13895
@typeCode
1..1
SHALL
14597
REFR
observation
1..1
SHALL
13897
entryRelationship
0..1
MAY
14465
@typeCode
1..1
SHALL
14598
COMP
observation
1..1
SHALL
14466
referenceRange
0..*
SHOULD
13937
observationRange
1..1
SHALL
13938
1. Conforms to Result Observation template
(2.16.840.1.113883.10.20.22.4.2).
2. SHALL contain exactly one [1..1] @classCode="OBS" (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:13905).
3. SHALL contain exactly one [1..1] @moodCode="EVN" (CodeSystem: ActMood
2.16.840.1.113883.5.1001) (CONF:13906).
4. SHALL contain exactly one [1..1] templateId (CONF:13889) such that it
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 365
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.67" (CONF:13890).
5. SHALL contain at least one [1..*] id (CONF:13907).
6. SHALL contain exactly one [1..1] code with @xsi:type="CE", where the @code
SHOULD be selected from CodeSystem LOINC (2.16.840.1.113883.6.1)
(CONF:13908).
7. SHOULD contain zero or one [0..1] text (CONF:13926).
a. The text, if present, SHOULD contain zero or one [0..1] reference
(CONF:13927).
i. This reference/@value SHALL begin with a '#' and SHALL point
to its corresponding narrative (using the approach defined in
CDA Release 2, section 4.3.5.1) (CONF:13928).
8. SHALL contain exactly one [1..1] statusCode="Completed" (CONF:13929).
Represents clinically effective time of the measurement, which may be when the
measurement was performed (e.g., a BP measurement), or may be when sample
was taken (and measured some time afterwards)
9. SHALL contain exactly one [1..1] effectiveTime (CONF:13930).
10. SHALL contain exactly one [1..1] value (CONF:13932).
a. If xsi:type=“CD”, SHOULD contain a code from SNOMED CT
(CodeSystem: 2.16.840.1.113883.6.96) (CONF:14234).
11. SHOULD contain zero or more [0..*] interpretationCode (CONF:13933).
12. MAY contain zero or one [0..1] methodCode (CONF:13934).
13. MAY contain zero or one [0..1] targetSiteCode (CONF:13935).
14. MAY contain zero or one [0..1] author (CONF:13936).
15. MAY contain zero or one [0..1] entryRelationship (CONF:13892) such that
it
a. SHALL contain exactly one [1..1] @typeCode="REFR" refers to
(CONF:14596).
b. SHALL contain exactly one [1..1] Non-Medicinal Supply Activity
(templateId:2.16.840.1.113883.10.20.22.4.50) (CONF:14218).
16. MAY contain zero or one [0..1] entryRelationship (CONF:13895) such that
it
a. SHALL contain exactly one [1..1] @typeCode="REFR" refers to
(CONF:14597).
b. SHALL contain exactly one [1..1] Caregiver Characteristics
(templateId:2.16.840.1.113883.10.20.22.4.72) (CONF:13897).
17. MAY contain zero or one [0..1] entryRelationship (CONF:14465) such that
it
a. SHALL contain exactly one [1..1] @typeCode="COMP" has component
(CONF:14598).
b. SHALL contain exactly one [1..1] Assessment Scale Observation
(templateId:2.16.840.1.113883.10.20.22.4.69) (CONF:14466).
18. SHOULD contain zero or more [0..*] referenceRange (CONF:13937).
a. The referenceRange, if present, SHALL contain exactly one [1..1]
observationRange (CONF:13938).
Page 366 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
i. This observationRange SHALL NOT contain [0..0] code
(CONF:13939).
Figure 182: Functional status result observation example
<observation classCode="OBS" moodCode="EVN">
<!—Cognitive Status Result Oservation template -->
<templateId root="2.16.840.1.113883.10.20.22.4.74"/>
<id root=" c6b5a04b-2bf4-49d1-8336-636a3813df0a"/>
<code code="54744-8"
displayName="Dressing upper body in last 7D(MDSv3)"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"/>
<statusCode code="completed"/>
<effectiveTime value="200903111230"/>
<value xsi:type="CD"/>
<code code="371153006" displayName=" Independently able"
codeSystem="2.16.840.1.113883.5.83"
codeSystemName="SNOMED CT"/>
<entryRelationship typeCode="SUBJ">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.69"/>
<!—- Assessment Scale Observation -->
...
</observation>
</entryRelationship>
<entryRelationship typeCode="SUBJ">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.72"/>
<!—Caregiver Support and Ablility -->
...
</observation>
</entryRelationship>
</observation>
6.29 Functional Status Result Organizer
[organizer: templateId 2.16.840.1.113883.10.20.22.4.66 (open)]
Table 176: Functional Status Result Organizer Contexts
Used By:
Contains Entries:
Functional Status Section (optional)
Functional Status Result Observation
This clinical statement identifies a set of functional status result observations. It
contains information applicable to all of the contained functional status result
observations. A functional status organizer may group self-care observations
related to a patient's ability to feed, bathe, and dress.
An appropriate nullFlavor can be used when the organizer/code or
organizer/id is unknown.
HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 367
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 177: Functional Status Result Organizer Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
organizer[templateId/@root = '2.16.840.1.113883.10.20.22.4.66']
@classCode
1..1
SHALL
14355
2.16.840.1.113883.5.6 (HL7ActClass)
= CLUSTER
@mood
Code
1..1
SHALL
14357
2.16.840.1.113883.5.1001 (ActMood)
= EVN
templateId
1..1
SHALL
SET<II>
14361
@root
1..1
SHALL
14362
2.16.840.1.113883.10.20.22.4.66
id
1..*
SHALL
14363
code
1..1
SHALL
14364
@code
0..1
SHOULD
14747
statusCode
1..1
SHALL
14358
2.16.840.1.113883.5.14 (ActStatus) =
completed
component
1..*
SHALL
14359
observation
1..1
SHALL
14368
1. Conforms to Result Organizer template
(2.16.840.1.113883.10.20.22.4.1).
2. SHALL contain exactly one [1..1] @classCode, which SHALL be selected from
CodeSystem HL7ActClass (2.16.840.1.113883.5.6)="CLUSTER"
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6) (CONF:14355).
3. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001) (CONF:14357).
4. SHALL contain exactly one [1..1] templateId (CONF:14361) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.66" (CONF:14362).
5. SHALL contain at least one [1..*] id (CONF:14363).
6. SHALL contain exactly one [1..1] code (CONF:14364).
a. This code SHOULD contain zero or one [0..1] @code (CONF:14747).
i. SHOULD be selected from ICF (codeSystem
2.16.840.1.113883.6.254) or SNOMED CT (codeSystem
2.16.840.1.113883.6.96) (CONF:14748).
7. SHALL contain exactly one [1..1] statusCode="completed" Completed
(CodeSystem: ActStatus 2.16.840.1.113883.5.14) (CONF:14358).
8. SHALL contain at least one [1..*] component (CONF:14359) such that it
a. SHALL contain exactly one [1..1] Functional Status Result
Observation (templateId:2.16.840.1.113883.10.20.22.4.67)
(CONF:14368).

Page 368 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 183: Functional status result organizer example
<organizer classCode="CLUSTER" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.75"/>
<!-- Cognitive Status Result Organizer template -->
<id root="9295dba4-df05-46bb-b94e-f2c4e4b156f8"/>
<code code="d5" displayName="Self-Care"
codeSystem="2.16.840.1.113883.6.254"
codeSystemName="ICF"/>
<statusCode code="completed"/>
<component>
<observation classCode="OBS" moodCode="EVN">
<!-- Functional Status Result observation(such as toileting) -->
<templateId root="2.16.840.1.113883.10.20.22.4.74"/>
...
</observation>
</component>
<component>
<observation classCode="OBS" moodCode="EVN">
<!-- Functional Status Result observation(such as eating) -->
<templateId root="2.16.840.1.113883.10.20.22.4.74"/>
...
</observation>
</component>
</organizer>
6.30 Health Status Observation
[Observation: templateId 2.16.840.1.113883.10.20.22.4.5(open)]
Table 178: Health Status Observation Contexts
Used By:
Contains Entries:
Problem Observation
The Health Status Observation records information about the current health
status of the patient.
Table 179: Health Status Observation Constraints Overview

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 369
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
observation[templateId/@root = '2.16.840.1.113883.10.20.22.4.5']
@classCode
1..1
SHALL
9057
2.16.840.1.113883.5.6 (HL7ActClass) =
OBS
@moodCode
1..1
SHALL
9072
2.16.840.1.113883.5.1001 (ActMood) =
EVN
templateId
1..1
SHALL
16756
@root
1..1
SHALL
16757
2.16.840.1.113883.10.20.22.4.5
code
1..1
SHALL
CE
9073
2.16.840.1.113883.6.1 (LOINC) =
11323-3
text
0..1
SHOULD
9270
reference
0..1
SHOULD
15529
@value
0..1
SHOULD
15530
statusCode
1..1
SHALL
9074
2.16.840.1.113883.5.14 (ActStatus) =
completed
value
1..1
SHALL
CD
9075
2.16.840.1.113883.1.11.20.12
(HealthStatus)
1. SHALL contain exactly one [1..1] @classCode="OBS" Observation
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6) (CONF:9057).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001) (CONF:9072).
3. SHALL contain exactly one [1..1] templateId (CONF:16756) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.5" (CONF:16757).
4. SHALL contain exactly one [1..1] code="11323-3" Health status with
@xsi:type="CE" (CodeSystem: LOINC 2.16.840.1.113883.6.1 STATIC)
(CONF:9073).
5. SHOULD contain zero or one [0..1] text (CONF:9270).
a. The text, if present, SHOULD contain zero or one [0..1] reference
(CONF:15529).
i. The reference, if present, SHOULD contain zero or one [0..1]
@value (CONF:15530).
1. SHALL begin with a '#' and SHALL point to its
corresponding narrative (using the approach defined
in CDA Release 2, section 4.3.5.1) (CONF:15531).
6. SHALL contain exactly one [1..1] statusCode="completed" Completed
(CodeSystem: ActStatus 2.16.840.1.113883.5.14 STATIC) (CONF:9074).
7. SHALL contain exactly one [1..1] value with @xsi:type="CD", where the @code
SHALL be selected from ValueSet HealthStatus
2.16.840.1.113883.1.11.20.12 DYNAMIC (CONF:9075).
Table 180: HealthStatus Value Set

Page 370 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Value Set: HealthStatus 2.16.840.1.113883.1.11.20.12 DYNAMIC
Code System(s):
SNOMED CT 2.16.840.1.113883.6.96
Description:
Represents the general health status of the patient.
Code
Code System
Print Name
81323004
SNOMED CT
Alive and well
313386006
SNOMED CT
In remission
162467007
SNOMED CT
Symptom free
161901003
SNOMED CT
Chronically ill
271593001
SNOMED CT
Severely ill
21134002
SNOMED CT
Disabled
161045001
SNOMED CT
Severely disabled
Figure 184: Health status observation example
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.5"/>
<!-- Health status observation template -->
<code code="11323-3"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="Health status"/>
<statusCode code="completed"/>
<value xsi:type="CE" code="313386006"
codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT"
displayName="In Remission"/>
</observation>

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 371
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
6.31 Highest Pressure Ulcer Stage
[observation: templateId 2.16.840.1.113883.10.20.22.4.77 (open)]
Table 181: Highest Pressure Ulcer Stage Contexts
Used By:
Contains Entries:
Functional Status Section (optional)
Physical Exam Section (optional)
This observation contains a description of the wound tissue of the most severe
or highest staged pressure ulcer observed on a patient.
Table 182: Highest Pressure Ulcer Stage Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
observation[templateId/@root = '2.16.840.1.113883.10.20.22.4.77']
@classCode
1..1
SHALL
14726
2.16.840.1.113883.5.6 (HL7ActClass) =
OBS
@moodCode
1..1
SHALL
14727
2.16.840.1.113883.5.1001 (ActMood) =
EVN
templateId
1..1
SHALL
14728
@root
1..1
SHALL
14729
2.16.840.1.113883.10.20.22.4.77
id
1..*
SHALL
14730
code
1..1
SHALL
14731
@code
1..1
SHALL
14732
2.16.840.1.113883.6.96 (SNOMED-CT) =
420905001
value
1..1
SHALL
14733
1. SHALL contain exactly one [1..1] @classCode="OBS" (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:14726).
2. SHALL contain exactly one [1..1] @moodCode="EVN" (CodeSystem: ActMood
2.16.840.1.113883.5.1001) (CONF:14727).
3. SHALL contain exactly one [1..1] templateId (CONF:14728) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.77" (CONF:14729).
4. SHALL contain at least one [1..*] id (CONF:14730).
5. SHALL contain exactly one [1..1] code (CONF:14731).
a. This code SHALL contain exactly one [1..1] @code="420905001"
Highest Pressure Ulcer Stage (CodeSystem: SNOMED-CT
2.16.840.1.113883.6.96) (CONF:14732).
6. SHALL contain exactly one [1..1] value (CONF:14733).

Page 372 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
<entry typeCode="DRIV">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.77"/>
<id root="08edb7c0-2111-43f2-a784-9a5fdfaa67f0"/>
<code code="420905001" codeSystem="2.16.840.1.113883.6.96"
displayName=" Highest Pressure Ulcer Stage"/>
<statusCode code="completed"/>
<value xsi:type="CD" code="421306004"
codeSystem="2.16.840.1.113883.6.96"
displayName="necrotic eschar"/>
</observation>
</entry>
6.32 Hospital Admission Diagnosis
[act: templateId 2.16.840.1.113883.10.20.22.4.34(open)
Table 183: Hospital Admission Diagnosis Contexts
Used By:
Contains Entries:
Hospital Admission Diagnosis Section
Problem Observation
The Hospital Admission Diagnosis entry describes the relevant problems or
diagnoses at the time of admission.
Table 184: Hospital Admission Diagnosis Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
act[templateId/@root = '2.16.840.1.113883.10.20.22.4.34']
@classCode
1..1
SHALL
7671
2.16.840.1.113883.5.6 (HL7ActClass)
= ACT
@moodCode
1..1
SHALL
7672
2.16.840.1.113883.5.1001 (ActMood)
= EVN
templateId
1..1
SHALL
16747
@root
1..1
SHALL
16748
2.16.840.1.113883.10.20.22.4.34
code
1..1
SHALL
CE
7673
2.16.840.1.113883.6.1 (LOINC) =
46241-6
entryRelationship
1..*
SHALL
7674
@typeCode
1..1
SHALL
7675
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) = SUBJ
observation
1..1
SHALL
15535
1. SHALL contain exactly one [1..1] @classCode="ACT" (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:7671).
2. SHALL contain exactly one [1..1] @moodCode="EVN" (CodeSystem: ActMood
2.16.840.1.113883.5.1001) (CONF:7672).
3. SHALL contain exactly one [1..1] templateId (CONF:16747) such that it

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 373
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.34" (CONF:16748).
4. SHALL contain exactly one [1..1] code="46241-6" Admission diagnosis
(CodeSystem: LOINC 2.16.840.1.113883.6.1) (CONF:7673).
5. SHALL contain at least one [1..*] entryRelationship (CONF:7674) such that
it
a. SHALL contain exactly one [1..1] @typeCode="SUBJ" Has Subject
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002) (CONF:7675).
b. SHALL contain exactly one [1..1] Problem Observation
(templateId:2.16.840.1.113883.10.20.22.4.4) (CONF:15535).
Figure 185: Hospital admission diagnosis example
<act classCode="ACT" moodCode="EVN">
<!-- Admission Diagnosis template -->
<templateId root="2.16.840.1.113883.10.20.22.4.34"/>
<id root="5a784260-6856-4f38-9638-80c751aff2fb"/>
<code code="46241-6"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="Hospital Admission Diagnosis"/>
<statusCode code="active"/>
<effectiveTime>
<low value="20090303"/>
</effectiveTime>
<entryRelationship typeCode="SUBJ" inversionInd="false">
<observation classCode="OBS" moodCode="EVN" negationInd="false">
<templateId root="2.16.840.1.113883.10.20.22.4.4"/>
<!-- Problem Observation -->
...
</observation>
</entryRelationship>
</act>
6.33 Hospital Discharge Diagnosis
[act: templateId 2.16.840.1.113883.10.20.22.4.33(open)
Table 185: Hospital Discharge Diagnosis Contexts
Used By:
Contains Entries:
Hospital Discharge Diagnosis Section
Problem Observation
The Hospital Discharge Diagnosis act wraps relevant problems or diagnoses at
the time of discharge that occurred during the hospitalization or that need to be
followed after hospitalization. This entry requires at least one Problem
Observation entry.

Page 374 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 186: Hospital Discharge Diagnosis Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
act[templateId/@root = '2.16.840.1.113883.10.20.22.4.33']
@classCode
1..1
SHALL
7663
2.16.840.1.113883.5.6 (HL7ActClass)
= ACT
@moodCode
1..1
SHALL
7664
2.16.840.1.113883.5.1001 (ActMood)
= EVN
templateId
1..1
SHALL
16764
@root
1..1
SHALL
16765
2.16.840.1.113883.10.20.22.4.33
code
1..1
SHALL
CE
7665
2.16.840.1.113883.6.1 (LOINC) =
11535-2
entryRelationship
1..*
SHALL
7666
@typeCode
1..1
SHALL
7667
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) = SUBJ
observation
1..1
SHALL
15536
1. SHALL contain exactly one [1..1] @classCode="ACT" (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:7663).
2. SHALL contain exactly one [1..1] @moodCode="EVN" (CodeSystem: ActMood
2.16.840.1.113883.5.1001) (CONF:7664).
3. SHALL contain exactly one [1..1] templateId (CONF:16764) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.33" (CONF:16765).
4. SHALL contain exactly one [1..1] code="11535-2" Hospital discharge
diagnosis (CodeSystem: LOINC 2.16.840.1.113883.6.1) (CONF:7665).
5. SHALL contain at least one [1..*] entryRelationship (CONF:7666) such that
it
a. SHALL contain exactly one [1..1] @typeCode="SUBJ" Has Subject
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002) (CONF:7667).
b. SHALL contain exactly one [1..1] Problem Observation
(templateId:2.16.840.1.113883.10.20.22.4.4) (CONF:15536).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 375
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 186: Hospital discharge diagnosis act example
<act classCode="ACT" moodCode="EVN">
<!—Hospital discharge diagnosis act -->
<templateId root="2.16.840.1.113883.10.20.22.4.33"/>
<id root="5a784260-6856-4f38-9638-80c751aff2fb"/>
<code code="11535-2"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="HOSPITAL DISCHARGE DIAGNOSIS"/>
<statusCode code="active"/>
<effectiveTime>
<low value="20903003"/>
</effectiveTime>
<entryRelationship typeCode="SUBJ" inversionInd="false">
<observation classCode="OBS" moodCode="EVN" negationInd="false">
<templateId root="2.16.840.1.113883.10.20.22.4.4"/>
<!-- Problem Observation -->
...
</observation>
</entryRelationship>
</act>
6.34 Immunization Activity
[substanceAdministration: templateId
2.16.840.1.113883.10.20.22.4.52 (open)]
Table 187: Immunization Activity Contexts
Used By:
Contains Entries:
Immunizations Section (entries optional) (optional)
Immunizations Section (entries required) (required)
Drug Vehicle
Immunization Medication Information
Immunization Refusal Reason
Indication
Instructions
Medication Dispense
Medication Supply Order
Precondition for Substance
Administration
Reaction Observation
An Immunization Activity describes immunization substance administrations
that have actually occurred or are intended to occur. Immunization Activities in
"INT" mood are reflections of immunizations a clinician intends a patient to
receive. Immunization Activities in "EVN" mood reflect immunizations actually
received.
An Immunization Activity is very similar to a Medication Activity with some key
differentiators. The drug code system is constrained to CVX codes.
Administration timing is less complex. Patient refusal reasons should be
captured. All vaccines administered should be fully documented in the patient's

Page 376 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
permanent medical record. Healthcare providers who administer vaccines
covered by the National Childhood Vaccine Injury Act are required to ensure
that the permanent medical record of the recipient indicates:
1) Date of administration
2) Vaccine manufacturer
3) Vaccine lot number
4) Name and title of the person who administered the vaccine and the
address of the clinic or facility where the permanent record will reside
5) Vaccine information statement (VIS)
a. date printed on the VIS
b. date VIS given to patient or parent/guardian.
This information should be included in an Immunization Activity when available.
33
Table 188: Immunization Activity Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
Green
Immunization
Activity
substanceAdministration[templateId/@root = '2.16.840.1.113883.10.20.22.4.52']
@classCode
1..1
SHALL
8826
2.16.840.1.113883.5.6
(HL7ActClass) = SBADM
@moodCode
1..1
SHALL
8827
2.16.840.1.113883.11.20.9.
18 (MoodCodeEvnInt)
refusal
@negationInd
1..1
SHALL
8985
templateId
1..1
SHALL
SET<
II>
8828
@root
1..1
SHALL
10498
2.16.840.1.113883.10.20.2
2.4.52
id
1..*
SHALL
8829
code
0..1
MAY
CE
8830
text
0..1
SHOULD
8831
reference
0..1
SHOULD
15543
@value
0..1
SHOULD
15544
statusCode
1..1
SHALL
8833
administered
Date
effectiveTime
1..1
SHALL
TS or
IVL<
TS>
8834
medication
SeriesNumber
repeatNumber
0..1
MAY
IVL<I
NT>
8838
routeCode
0..1
MAY
8839
2.16.840.1.113883.3.88.12.
3221.8.7 (Medication Route
FDA Value Set)
approachSiteCode
0..1
MAY
SET<
8840
2.16.840.1.113883.3.88.12.
3221.8.9 (Body Site Value
33 Vaccine Administration Guidelines.
http://www.cdc.gov/vaccines/pubs/pinkbook/downloads/appendices/D/vacc_admin.
pdf

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 377
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
CD>
Set)
doseQuantity
0..1
SHOULD
IVL<
PQ>
8841
@unit
0..1
SHOULD
8842
2.16.840.1.113883.1.11.12
839 (UCUM Units of
Measure (case sensitive)) =
1
administrationUni
tCode
0..1
MAY
8846
2.16.840.1.113883.3.88.12.
3221.8.11 (Medication
Product Form)
medication
Information
consumable
1..1
SHALL
8847
manufacturedPro
duct
1..1
SHALL
15546
performer
performer
0..1
SHOULD
8849
participant
0..*
MAY
8850
@typeCode
1..1
SHALL
8851
2.16.840.1.113883.5.90
(HL7ParticipationType) =
CSM
participantRole
1..1
SHALL
15547
entryRelationship
0..*
MAY
8853
@typeCode
1..1
SHALL
8854
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) =
RSON
observation
1..1
SHALL
15537
entryRelationship
0..1
MAY
8856
@typeCode
1..1
SHALL
8857
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) =
SUBJ
@inversionInd
1..1
SHALL
8858
true
act
1..1
SHALL
15538
entryRelationship
0..1
MAY
8860
@typeCode
1..1
SHALL
8861
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) =
REFR
supply
1..1
SHALL
15539
entryRelationship
0..1
MAY
8863
@typeCode
1..1
SHALL
8864
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) =
REFR
supply
1..1
SHALL
15540
reaction
entryRelationship
0..1
MAY
8866
@typeCode
1..1
SHALL
8867
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) =
CAUS

Page 378 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
observation
1..1
SHALL
15541
refusal
Reason
entryRelationship
0..1
MAY
8988
@typeCode
1..1
SHALL
8989
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) =
RSON
observation
1..1
SHALL
15542
precondition
0..*
MAY
8869
@typeCode
1..1
SHALL
8870
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) =
PRCN
criterion
1..1
SHALL
15548
1. SHALL contain exactly one [1..1] @classCode="SBADM" (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6 STATIC) (CONF:8826).
2. SHALL contain exactly one [1..1] @moodCode, which SHALL be selected from
ValueSet MoodCodeEvnInt 2.16.840.1.113883.11.20.9.18 STATIC
(CONF:8827).
Use negationInd="true" to indicate that the immunization was not given.
3. SHALL contain exactly one [1..1] @negationInd (CONF:8985).
4. SHALL contain exactly one [1..1] templateId (CONF:8828) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.52" (CONF:10498).
5. SHALL contain at least one [1..*] id (CONF:8829).
6. MAY contain zero or one [0..1] code with @xsi:type="CE" (CONF:8830).
7. SHOULD contain zero or one [0..1] text (CONF:8831).
a. The text, if present, SHOULD contain zero or one [0..1] reference
(CONF:15543).
i. The reference, if present, SHOULD contain zero or one [0..1]
@value (CONF:15544).
1. This reference/@value SHALL begin with a '#' and SHALL
point to its corresponding narrative (using the
approach defined in CDA Release 2, section 4.3.5.1
(CONF:15545).
8. SHALL contain exactly one [1..1] statusCode (CONF:8833).
9. SHALL contain exactly one [1..1] effectiveTime (CONF:8834).
In "INT" (intent) mood, the repeatNumber defines the number of allowed
administrations. For example, a repeatNumber of "3" means that the substance
can be administered up to 3 times. In "EVN" (event) mood, the repeatNumber is
the number of occurrences. For example, a repeatNumber of "3" in a dispense
act means that the current dispensation is the 3rd. A repeatNumber of "3" in a
substance administration event means that the current administration is the
3rd in a series.

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 379
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
10. MAY contain zero or one [0..1] repeatNumber (CONF:8838).
11. MAY contain zero or one [0..1] routeCode, which SHALL be selected from
ValueSet Medication Route FDA Value Set
2.16.840.1.113883.3.88.12.3221.8.7 DYNAMIC (CONF:8839).
12. MAY contain zero or one [0..1] approachSiteCode, where the @code SHALL be
selected from ValueSet Body Site Value Set
2.16.840.1.113883.3.88.12.3221.8.9 DYNAMIC (CONF:8840).
13. SHOULD contain zero or one [0..1] doseQuantity (CONF:8841).
a. The doseQuantity, if present, SHOULD contain zero or one [0..1]
@unit="1", which SHALL be selected from ValueSet UCUM Units of
Measure (case sensitive) 2.16.840.1.113883.1.11.12839
DYNAMIC (CONF:8842).
14. MAY contain zero or one [0..1] administrationUnitCode, which SHALL be
selected from ValueSet Medication Product Form
2.16.840.1.113883.3.88.12.3221.8.11 DYNAMIC (CONF:8846).
15. SHALL contain exactly one [1..1] consumable (CONF:8847).
a. This consumable SHALL contain exactly one [1..1] Immunization
Medication Information
(templateId:2.16.840.1.113883.10.20.22.4.54) (CONF:15546).
16. SHOULD contain zero or one [0..1] performer (CONF:8849).
17. MAY contain zero or more [0..*] participant (CONF:8850).
a. The participant, if present, SHALL contain exactly one [1..1]
@typeCode="CSM" (CodeSystem: HL7ParticipationType
2.16.840.1.113883.5.90 STATIC) (CONF:8851).
b. The participant, if present, SHALL contain exactly one [1..1] Drug
Vehicle (templateId:2.16.840.1.113883.10.20.22.4.24)
(CONF:15547).
18. MAY contain zero or more [0..*] entryRelationship (CONF:8853) such that
it
a. SHALL contain exactly one [1..1] @typeCode="RSON" (CodeSystem:
HL7ActRelationshipType 2.16.840.1.113883.5.1002 STATIC)
(CONF:8854).
b. SHALL contain exactly one [1..1] Indication
(templateId:2.16.840.1.113883.10.20.22.4.19) (CONF:15537).
19. MAY contain zero or one [0..1] entryRelationship (CONF:8856) such that it
a. SHALL contain exactly one [1..1] @typeCode="SUBJ" (CodeSystem:
HL7ActRelationshipType 2.16.840.1.113883.5.1002 STATIC)
(CONF:8857).
b. SHALL contain exactly one [1..1] @inversionInd="true" True
(CONF:8858).
c. SHALL contain exactly one [1..1] Instructions
(templateId:2.16.840.1.113883.10.20.22.4.20) (CONF:15538).
20. MAY contain zero or one [0..1] entryRelationship (CONF:8860) such that it
a. SHALL contain exactly one [1..1] @typeCode="REFR" (CodeSystem:
HL7ActRelationshipType 2.16.840.1.113883.5.1002 STATIC)
(CONF:8861).

Page 380 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
b. SHALL contain exactly one [1..1] Medication Supply Order
(templateId:2.16.840.1.113883.10.20.22.4.17) (CONF:15539).
21. MAY contain zero or one [0..1] entryRelationship (CONF:8863) such that it
a. SHALL contain exactly one [1..1] @typeCode="REFR" (CodeSystem:
HL7ActRelationshipType 2.16.840.1.113883.5.1002 STATIC)
(CONF:8864).
b. SHALL contain exactly one [1..1] Medication Dispense
(templateId:2.16.840.1.113883.10.20.22.4.18) (CONF:15540).
22. MAY contain zero or one [0..1] entryRelationship (CONF:8866) such that it
a. SHALL contain exactly one [1..1] @typeCode="CAUS" (CodeSystem:
HL7ActRelationshipType 2.16.840.1.113883.5.1002 STATIC)
(CONF:8867).
b. SHALL contain exactly one [1..1] Reaction Observation
(templateId:2.16.840.1.113883.10.20.22.4.9) (CONF:15541).
23. MAY contain zero or one [0..1] entryRelationship (CONF:8988) such that it
a. SHALL contain exactly one [1..1] @typeCode="RSON" (CodeSystem:
HL7ActRelationshipType 2.16.840.1.113883.5.1002 STATIC)
(CONF:8989).
b. SHALL contain exactly one [1..1] Immunization Refusal Reason
(templateId:2.16.840.1.113883.10.20.22.4.53) (CONF:15542).
24. MAY contain zero or more [0..*] precondition (CONF:8869) such that it
a. SHALL contain exactly one [1..1] @typeCode="PRCN" (CodeSystem:
HL7ActRelationshipType 2.16.840.1.113883.5.1002 STATIC)
(CONF:8870).
b. SHALL contain exactly one [1..1] Precondition for Substance
Administration
(templateId:2.16.840.1.113883.10.20.22.4.25) (CONF:15548).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 381
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 187: Immunization activity example
<substanceAdministration classCode="SBADM" moodCode="EVN"
negationInd="false">
<templateId root="2.16.840.1.113883.10.20.22.4.52"/>
<!-- **** Immunization activity template **** -->
<id root="e6f1ba43-c0ed-4b9b-9f12-f435d8ad8f92"/>
<text>
<reference value="#immun3"/>
</text>
<statusCode code="completed"/>
<effectiveTime xsi:type="IVL_TS" value="19981215"/>
<routeCode code="IM" codeSystem="2.16.840.1.113883.5.112"
codeSystemName="RouteOfAdministration"
displayName="Intramuscular injection"/>
<doseQuantity nullFlavor="UNK"/>
<consumable>
<manufacturedProduct>
<templateId root="2.16.840.1.113883.10.20.22.4.54"/>
<!-- **** Immunization Medication Information **** -->
<manufacturedMaterial>
...
</manufacturedMaterial>
</manufacturedProduct>
</consumable>
<entryRelationship typeCode="SUBJ">
<act classCode="ACT" moodCode="INT">
<templateId root="2.16.840.1.113883.10.20.22.4.20"/>
<!-- ** Instructions Template ** -->
...
</act>
</entryRelationship>
<entryRelationship typeCode="RSON">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.53"/>
<!-- Immunization Refusal -->
...
</observation>
</entryRelationship>
</substanceAdministration>
6.35 Immunization Medication Information
[manufacturedProduct: templateId
2.16.840.1.113883.10.20.22.4.54(open)]
Table 189: Immunization Medication Information Contexts
Used By:
Contains Entries:
Immunization Activity
Medication Dispense
Medication Supply Order

Page 382 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
The Immunization Medication Information represents product information about
the immunization substance. The vaccine manufacturer and vaccine lot number
are typically recorded in the medical record and should be included if known.34
Table 190: Immunization Medication Information Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
Green
Immunization
Medication
Information
manufacturedProduct[templateId/@root = '2.16.840.1.113883.10.20.22.4.54']
@classCode
1..1
SHALL
9002
2.16.840.1.113883.5.11
0 (RoleClass) = MANU
templateId
1..1
SHALL
SET<
II>
9004
@root
1..1
SHALL
10499
2.16.840.1.113883.10.2
0.22.4.54
id
0..*
MAY
9005
manufactured
Material
1..1
SHALL
9006
codedProduct
Name
code
1..1
SHALL
9007
2.16.840.1.113883.3.88
.12.80.22 (Vaccine
Administered Value Set)
freeText
ProductName
originalText
0..1
SHOULD
9008
reference
0..1
SHOULD
15555
@value
0..1
SHOULD
15556
translation
0..*
MAY
SET<
PQR
>
9011
lotNumber
lotNumberText
0..1
SHOULD
9014
drug
Manufacturer
manufacturer
Organization
0..1
SHOULD
9012
1. SHALL contain exactly one [1..1] @classCode="MANU" (CodeSystem:
RoleClass 2.16.840.1.113883.5.110 STATIC) (CONF:9002).
2. SHALL contain exactly one [1..1] templateId (CONF:9004) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.54" (CONF:10499).
3. MAY contain zero or more [0..*] id (CONF:9005).
4. SHALL contain exactly one [1..1] manufacturedMaterial (CONF:9006).
a. This manufacturedMaterial SHALL contain exactly one [1..1] code,
which SHALL be selected from ValueSet Vaccine Administered
34 Vaccine Administration Guidelines.
http://www.cdc.gov/vaccines/pubs/pinkbook/downloads/appendices/D/vacc_admin.
pdf

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 383
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Value Set 2.16.840.1.113883.3.88.12.80.22 DYNAMIC
(CONF:9007).
i. This code SHOULD contain zero or one [0..1] originalText
(CONF:9008).
1. The originalText, if present, SHOULD contain zero or one
[0..1] reference (CONF:15555).
a. The reference, if present, SHOULD contain zero
or one [0..1] @value (CONF:15556).
i. This reference/@value SHALL begin with
a '#' and SHALL point to its
corresponding narrative (using the
approach defined in CDA Release 2,
section 4.3.5.1) (CONF:15557).
Translations can be used to represent generic product name, packaged product
code, etc.
ii. This code MAY contain zero or more [0..*] translation
(CONF:9011).
b. This manufacturedMaterial SHOULD contain zero or one [0..1]
lotNumberText (CONF:9014).
5. SHOULD contain zero or one [0..1] manufacturerOrganization (CONF:9012).
Table 191: Vaccine Administered (Hepatitis B) Value Set (excerpt)
Value Set: Vaccine Administered Value Set 2.16.840.1. 113883.3.88.12.80.22 DYNAMIC
Code System(s):
Vaccines administered (CVX) 2.16.840.1.113883.12.292
http://phinvads.cdc.gov/vads/ViewCodeSystem.action?id=2.16.840.1.113883.12.292
Code
Code System
Print Name
82
CVX
adenovirus vaccine, NOS
54
CVX
adenovirus vaccine, type 4, live, oral
55
CVX
adenovirus vaccine, type 7, live, oral
24
CVX
anthrax vaccine
…

Page 384 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 188: Immunization medication information example
<manufacturedProduct>
<templateId root="2.16.840.1.113883.10.20.22.4.54"/>
<!-- **** Immunization Medication Information **** -->
<manufacturedMaterial>
<code code="103"
codeSystem="2.16.840.1.113883.6.59"
codeSystemName="CVX"
displayName="Tetanus and diphtheria toxoids –
preservative free" codeSystemName="CVX">
<originalText>Tetanus and diphtheria toxoids - preservative
free</originalText>
<translation code="09"
displayName="Tetanus and diphtheria toxoids – preservative
free"
codeSystemName="CVX"
codeSystem="2.16.840.1.113883.6.59"/>
</code>
</manufacturedMaterial>
</manufacturedProduct>
6.36 Immunization Refusal Reason
[observation: templateId 2.16.840.1.113883.10.20.22.4.53(open)]
Table 192: Immunization Refusal Reason Contexts
Used By:
Contains Entries:
Immunization Activity
The Immunization Refusal Reason Observation documents the rationale for the
patient declining an immunization.
Table 193: Immunization Refusal Reason Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
observation[templateId/@root = '2.16.840.1.113883.10.20.22.4.53']
@classCode
1..1
SHALL
8991
2.16.840.1.113883.5.6 (HL7ActClass) =
OBS
@moodCode
1..1
SHALL
8992
2.16.840.1.113883.5.1001 (ActMood) =
EVN
templateId
1..1
SHALL
SET<II>
8993
@root
1..1
SHALL
10500
2.16.840.1.113883.10.20.22.4.53
id
1..*
SHALL
II
8994
code
1..1
SHALL
CD
8995
2.16.840.1.113883.1.11.19717 (No
Immunization Reason Value Set)
statusCode
1..1
SHALL
CS
8996
2.16.840.1.113883.5.14 (ActStatus) =
completed

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 385
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
1. SHALL contain exactly one [1..1] @classCode="OBS" Observation
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6) (CONF:8991).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001) (CONF:8992).
3. SHALL contain exactly one [1..1] templateId (CONF:8993) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.53" (CONF:10500).
4. SHALL contain at least one [1..*] id (CONF:8994).
5. SHALL contain exactly one [1..1] code, where the @code SHALL be selected
from ValueSet No Immunization Reason Value Set
2.16.840.1.113883.1.11.19717 DYNAMIC (CONF:8995).
6. SHALL contain exactly one [1..1] statusCode="completed" Completed
(CodeSystem: ActStatus 2.16.840.1.113883.5.14) (CONF:8996).
Table 194: No Immunization Reason Value Set
Value Set: No Immunization Reason Value Set 2.16.840.1.113883.1.11.19717 DYNAMIC
Code System(s):
ActReason 2.16.840.1.113883.5.8
Code
Code System
Print Name
IMMUNE
ActReason
Immunity
MEDPREC
ActReason
Medical precaution
OSTOCK
ActReason
Out of stock
PATOBJ
ActReason
Patient objection
PHILISOP
ActReason
Philosophical objection
RELIG
ActReason
Religious objection
VACEFF
ActReason
Vaccine efficacy concerns
VACSAF
ActReason
Vaccine safety concerns
Figure 189: Immunization refusal reason
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.53"/>
<!-- Immunization Refusal -->
<id root="350a25a0-5e69-11e1-b86c-0800200c9a66"/>
<code displayName="Patient Objection" code="PATOBJ"
codeSystemName="HL7 ActNoImmunizationReason"
codeSystem="2.16.840.1.113883.11.19725"/>
<statusCode code="completed"/>
</observation>

Page 386 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
6.37 Indication
[observation: templateId 2.16.840.1.113883.10.20.22.4.19 (open)]
Table 195: Indication Contexts
Used By:
Contains Entries:
Encounter Activities (optional)
Procedure Indications Section (optional)
Immunization Activity (optional)
Procedure Activity Act (optional)
Procedure Activity Observation (optional)
Procedure Activity Procedure (optional)
Medication Activity (optional)
The Indication Observation documents the rationale for an activity. It can do
this with the id element to reference a problem recorded elsewhere in the
document or with a code and value to record the problem type and problem
within the Indication. For example, the indication for a prescription of a
painkiller might be a headache that is documented in the Problems Section.
Table 196: Indication Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
observation[templateId/@root = '2.16.840.1.113883.10.20.22.4.19']
@classCode
1..1
SHALL
7480
2.16.840.1.113883.5.6 (HL7ActClass)
= OBS
@moodCode
1..1
SHALL
7481
2.16.840.1.113883.5.1001 (ActMood)
= EVN
templateId
1..1
SHALL
SET<II>
7482
@root
1..1
SHALL
10502
2.16.840.1.113883.10.20.22.4.19
id
1..1
SHALL
7483
code
1..1
SHALL
16886
2.16.840.1.113883.3.88.12.3221.7.2
(Problem Type)
statusCode
1..1
SHALL
7487
2.16.840.1.113883.5.14 (ActStatus) =
completed
effectiveTime
0..1
SHOULD
TS or
IVL<TS>
7488
value
0..1
SHOULD
CD
7489
@nullFlavor
0..1
MAY
15990
@code
0..1
SHOULD
15985
2.16.840.1.113883.3.88.12.3221.7.4
(Problem)
1. SHALL contain exactly one [1..1] @classCode="OBS" (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:7480).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 387
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
2. SHALL contain exactly one [1..1] @moodCode="EVN" (CodeSystem: ActMood
2.16.840.1.113883.5.1001) (CONF:7481).
3. SHALL contain exactly one [1..1] templateId (CONF:7482) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.19" (CONF:10502).
4. SHALL contain exactly one [1..1] id (CONF:7483).
a. Set the observation/id equal to an ID on the problem list to signify
that problem as an indication (CONF:16885).
5. SHALL contain exactly one [1..1] code, which SHOULD be selected from
ValueSet Problem Type 2.16.840.1.113883.3.88.12.3221.7.2 STATIC
2012-06-01 (CONF:16886).
6. SHALL contain exactly one [1..1] statusCode="completed" Completed
(CodeSystem: ActStatus 2.16.840.1.113883.5.14) (CONF:7487).
7. SHOULD contain zero or one [0..1] effectiveTime (CONF:7488).
8. SHOULD contain zero or one [0..1] value with @xsi:type="CD" (CONF:7489).
a. The value, if present, MAY contain zero or one [0..1] @nullFlavor
(CONF:15990).
i. If the diagnosis is unknown or the SNOMED code is
unknown, @nullFlavor SHOULD be “UNK”. If the code is
something other than SNOMED, @nullFlavor SHOULD be
“OTH” and the other code SHOULD be placed in the translation
element (CONF:15991).
b. The value, if present, SHOULD contain zero or one [0..1] @code
(ValueSet: Problem 2.16.840.1.113883.3.88.12.3221.7.4
DYNAMIC) (CONF:15985).
Figure 190: Indication entry example
<observation classCode="OBS" moodCode="EVN">
<!-- Indication -->
<templateId root="2.16.840.1.113883.10.20.22.4.19"/>
<id extension="123456789" root="2.16.840.1.113883.19"/>
<code code="409586006"
codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT"
displayName="Complaint"/>
<statusCode code="completed"/>
<value xsi:type="CD"
code="195967001"
codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT"
displayName="Asthma"/>
</observation>

Page 388 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
6.38 Instructions
[act: templateId 2.16.840.1.113883.10.20.22.4.20(open)]
Table 197: Instructions Contexts
Used By:
Contains Entries:
Medication Supply Order
Medication Activity
Procedure Activity Procedure
Procedure Activity Observation
Procedure Activity Act
Immunization Activity
Instructions Section
Plan of Care Section
The Instructions template can be used in several ways, such as to record patient
instructions within a Medication Activity or to record fill instructions within a
supply order. The act/code defines the type of instruction. Though not defined
in this template, a Vaccine Information Statement (VIS) document could be
referenced through act/reference/externalDocument, and patient awareness of
the instructions can be represented with the generic participant and the
participant/awarenessCode.
Table 198: Instructions Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
act[templateId/@root = '2.16.840.1.113883.10.20.22.4.20']
@classCode
1..1
SHALL
7391
2.16.840.1.113883.5.6 (HL7ActClass)
= ACT
@moodCode
1..1
SHALL
7392
2.16.840.1.113883.5.1001 (ActMood)
= INT
templateId
1..1
SHALL
SET<II>
7393
@root
1..1
SHALL
10503
2.16.840.1.113883.10.20.22.4.20
code
1..1
SHALL
CE
7394
2.16.840.1.113883.11.20.9.34
(Patient Education)
text
0..1
SHOULD
7395
reference
1..1
SHOULD
15577
@value
1..1
SHOULD
15578
statusCode
1..1
SHALL
7396
2.16.840.1.113883.5.14 (ActStatus) =
completed
1. SHALL contain exactly one [1..1] @classCode="ACT" (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:7391).
2. SHALL contain exactly one [1..1] @moodCode="INT" (CodeSystem: ActMood
2.16.840.1.113883.5.1001) (CONF:7392).
3. SHALL contain exactly one [1..1] templateId (CONF:7393) such that it

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 389
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.20" (CONF:10503).
4. SHALL contain exactly one [1..1] code with @xsi:type="CE", where the @code
SHOULD be selected from ValueSet Patient Education
2.16.840.1.113883.11.20.9.34 DYNAMIC (CONF:7394).
5. SHOULD contain zero or one [0..1] text (CONF:7395).
a. The text, if present, SHOULD contain exactly one [1..1] reference
(CONF:15577).
i. This reference SHOULD contain exactly one [1..1] @value
(CONF:15578).
1. This reference/@value SHALL begin with a '#' and SHALL
point to its corresponding narrative (using the
approach defined in CDA Release 2, section 4.3.5.1)
(CONF:15579).
6. SHALL contain exactly one [1..1] statusCode="completed" Completed
(CodeSystem: ActStatus 2.16.840.1.113883.5.14) (CONF:7396).
Figure 191: Instructions entry example
<act classCode="ACT" moodCode="INT">
<templateId root="2.16.840.1.113883.10.20.22.4.20"/>
<code code="171044003"
codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT"
displayName="Immunization Education"/>
<text>
<reference value="#sect1"/>
Patient may have low grade fever, mild joint pain and injection
area tenderness .
</text>
<statusCode code="completed"/>
</act>
Table 199: Patient Education Value Set
Value Set: Patient Education 2.16.840.1.113883.11.20.9.34 DYNAMIC
Code System(s):
SNOMED CT 2.16.840.1.113883.6.96
Description:
Limited to terms descending from the Education (409073007) hierarchy.
Code system browser: https://uts.nlm.nih.gov/snomedctBrowser.html
Code
Code System
Print Name
311401005
SNOMED CT
Patient Education
171044003
SNOMED CT
Immunization Education
243072006
SNOMED CT
Cancer Education
…

Page 390 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
6.39 Medication Activity
[substanceAdministration: templateId
2.16.840.1.113883.10.20.22.4.16(open)]
Table 200: Medication Activity Contexts
Used By:
Contains Entries:
Reaction Observation
Medications Section (entries required)
Discharge Medication
Admission Medication
Medications Section (entries optional)
Procedure Activity Procedure
Anesthesia Section
Medications Administered Section
Procedure Activity Observation
Procedure Activity Act
Drug Vehicle
Indication
Instructions
Medication Dispense
Medication Information
Medication Supply Order
Precondition for Substance Administration
Reaction Observation
A medication activity describes substance administrations that have actually
occurred (e.g. pills ingested or injections given) or are intended to occur (e.g.
"take 2 tablets twice a day for the next 10 days"). Medication activities in "INT"
mood are reflections of what a clinician intends a patient to be taking.
Medication activities in "EVN" mood reflect actual use.
Medication timing is complex. This template requires that there be a
substanceAdministration/effectiveTime valued with a time interval, representing
the start and stop dates. Additional effectiveTime elements are optional, and can
be used to represent frequency and other aspects of more detailed dosing
regimens.
Table 201: Medication Activity Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF
#
Fixed Value
Green
Medication
Activity
substanceAdministration[templateId/@root = '2.16.840.1.113883.10.20.22.4.16']
@classCode
1..1
SHALL
7496
2.16.840.1.113883.5.6
(HL7ActClass) = SBADM
@moodCode
1..1
SHALL
7497
2.16.840.1.113883.11.20.
9.18 (MoodCodeEvnInt)
templateId
1..1
SHALL
SET<
II>
7499
@root
1..1
SHALL
10504
2.16.840.1.113883.10.20.
22.4.16
id
1..*
SHALL
7500
delivery
Method
code
0..1
MAY
7506
freeTextSig
text
0..1
SHOULD
7501

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 391
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Name
XPath
Card.
Verb
Data
Type
CONF
#
Fixed Value
reference
0..1
SHOULD
15977
@value
0..1
SHOULD
15978
statusCode
1..1
SHALL
7507
effectiveTime
1..1
SHALL
TS or
IVL<
TS>
7508
indicate
Medication
Started
low
1..1
SHALL
TS
7511
indicate
Medication
Stopped
high
1..1
SHALL
TS
7512
administra-
tionTiming
effectiveTime
0..1
SHOULD
TS or
IVL<
TS>
7513
@operator
1..1
SHALL
9106
A
repeatNumber
0..1
MAY
IVL<I
NT>
7555
route
routeCode
0..1
MAY
7514
2.16.840.1.113883.3.88.1
2.3221.8.7 (Medication
Route FDA Value Set)
site
approachSiteCode
0..1
MAY
SET<
CD>
7515
2.16.840.1.113883.3.88.1
2.3221.8.9 (Body Site
Value Set)
dose
doseQuantity
0..1
SHOULD
IVL<
PQ>
7516
@unit
0..1
SHOULD
7526
2.16.840.1.113883.1.11.1
2839 (UCUM Units of
Measure (case sensitive)) =
1
rateQuantity
0..1
MAY
IVL<
PQ>
7517
@unit
1..1
SHALL
7525
2.16.840.1.113883.1.11.1
2839 (UCUM Units of
Measure (case sensitive)) =
1
dose
Restriction
maxDoseQuantity
0..1
MAY
RTO<
PQ,
PQ>
7518
product
Form
administrationUni
tCode
0..1
MAY
7519
2.16.840.1.113883.3.88.1
2.3221.8.11 (Medication
Product Form)
medication
Information
consumable
1..1
SHALL
7520
manufacturedPro
duct
1..1
SHALL
16085
performer
0..1
MAY
7522

Page 392 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Name
XPath
Card.
Verb
Data
Type
CONF
#
Fixed Value
vehicle
participant
0..*
MAY
7523
@typeCode
1..1
SHALL
7524
2.16.840.1.113883.5.90
(HL7ParticipationType) =
CSM
participantRole
1..1
SHALL
16086
indication
entryRelationship
0..*
MAY
7536
@typeCode
1..1
SHALL
7537
2.16.840.1.113883.5.1002
(HL7ActRelationshipType)
= RSON
observation
1..1
SHALL
16087
patient
Instructions
entryRelationship
0..1
MAY
7539
@typeCode
1..1
SHALL
7540
2.16.840.1.113883.5.1002
(HL7ActRelationshipType)
= SUBJ
@inversionInd
1..1
SHALL
7542
true
act
1..1
SHALL
16088
order
Information
entryRelationship
0..1
MAY
7543
@typeCode
1..1
SHALL
7547
2.16.840.1.113883.5.1002
(HL7ActRelationshipType)
= REFR
supply
1..1
SHALL
16089
fulfillment
Instructions
entryRelationship
0..*
MAY
7549
@typeCode
1..1
SHALL
7553
2.16.840.1.113883.5.1002
(HL7ActRelationshipType)
= REFR
supply
1..1
SHALL
16090
reaction
entryRelationship
0..1
MAY
7552
@typeCode
1..1
SHALL
7544
2.16.840.1.113883.5.1002
(HL7ActRelationshipType)
= CAUS
observation
1..1
SHALL
16091
precondition
0..*
MAY
7546
@typeCode
1..1
SHALL
7550
2.16.840.1.113883.5.1002
(HL7ActRelationshipType)
= PRCN
criterion
1..1
SHALL
16092
1. SHALL contain exactly one [1..1] @classCode="SBADM" (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:7496).
2. SHALL contain exactly one [1..1] @moodCode, which SHALL be selected from
ValueSet MoodCodeEvnInt 2.16.840.1.113883.11.20.9.18 STATIC 2011-
04-03 (CONF:7497).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 393
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
3. SHALL contain exactly one [1..1] templateId (CONF:7499) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.16" (CONF:10504).
4. SHALL contain at least one [1..*] id (CONF:7500).
5. MAY contain zero or one [0..1] code (CONF:7506).
6. SHOULD contain zero or one [0..1] text (CONF:7501).
a. The text, if present, SHOULD contain zero or one [0..1] reference
(CONF:15977).
i. The reference, if present, SHOULD contain zero or one [0..1]
@value (CONF:15978).
1. This reference/@value SHALL begin with a '#' and SHALL
point to its corresponding narrative (using the
approach defined in CDA Release 2, section 4.3.5.1)
(CONF:15979).
7. SHALL contain exactly one [1..1] statusCode (CONF:7507).
8. SHALL contain exactly one [1..1] effectiveTime (CONF:7508) such that it
a. SHALL contain exactly one [1..1] low (CONF:7511).
b. SHALL contain exactly one [1..1] high (CONF:7512).
9. SHOULD contain zero or one [0..1] effectiveTime (CONF:7513) such that it
a. SHALL contain exactly one [1..1] @operator="A" (CONF:9106).
b. SHALL contain exactly one [1..1] @xsi:type=”PIVL_TS” or “EIVL_TS”
(CONF:9105).
10. MAY contain zero or one [0..1] repeatNumber (CONF:7555).
a. In "INT" (intent) mood, the repeatNumber defines the number of
allowed administrations. For example, a repeatNumber of "3" means
that the substance can be administered up to 3 times. In "EVN"
(event) mood, the repeatNumber is the number of occurrences. For
example, a repeatNumber of "3" in a substance administration event
means that the current administration is the 3rd in a series
(CONF:16877).
11. MAY contain zero or one [0..1] routeCode, which SHALL be selected from
ValueSet Medication Route FDA Value Set
2.16.840.1.113883.3.88.12.3221.8.7 DYNAMIC (CONF:7514).
12. MAY contain zero or one [0..1] approachSiteCode, where the @code SHALL be
selected from ValueSet Body Site Value Set
2.16.840.1.113883.3.88.12.3221.8.9 DYNAMIC (CONF:7515).
13. SHOULD contain zero or one [0..1] doseQuantity (CONF:7516).
a. The doseQuantity, if present, SHOULD contain zero or one [0..1]
@unit="1", which SHALL be selected from ValueSet UCUM Units of
Measure (case sensitive) 2.16.840.1.113883.1.11.12839
DYNAMIC (CONF:7526).
b. Pre-coordinated consumable: If the consumable code is a
precoordinated unit dose (e.g. "metoprolol 25mg tablet") then
doseQuantity is a unitless number that indicates the number of
products given per administration (e.g. "2", meaning 2 x "metoprolol
25mg tablet") (CONF:16878).

Page 394 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
c. Not pre-coordinated consumable: If the consumable code is not pre-
coordinated (e.g. is simply "metoprolol"), then doseQuantity must
represent a physical quantity with @unit, e.g. "25" and "mg",
specifying the amount of product given per administration
(CONF:16879).
14. MAY contain zero or one [0..1] rateQuantity (CONF:7517).
a. The rateQuantity, if present, SHALL contain exactly one [1..1]
@unit="1", which SHALL be selected from ValueSet UCUM Units of
Measure (case sensitive) 2.16.840.1.113883.1.11.12839
DYNAMIC (CONF:7525).
15. MAY contain zero or one [0..1] maxDoseQuantity (CONF:7518).
16. MAY contain zero or one [0..1] administrationUnitCode, which SHALL be
selected from ValueSet Medication Product Form
2.16.840.1.113883.3.88.12.3221.8.11 DYNAMIC (CONF:7519).
17. SHALL contain exactly one [1..1] consumable (CONF:7520).
a. This consumable SHALL contain exactly one [1..1] Medication
Information (templateId:2.16.840.1.113883.10.20.22.4.23)
(CONF:16085).
18. MAY contain zero or one [0..1] performer (CONF:7522).
19. MAY contain zero or more [0..*] participant (CONF:7523) such that it
a. SHALL contain exactly one [1..1] @typeCode="CSM" (CodeSystem:
HL7ParticipationType 2.16.840.1.113883.5.90) (CONF:7524).
b. SHALL contain exactly one [1..1] Drug Vehicle
(templateId:2.16.840.1.113883.10.20.22.4.24) (CONF:16086).
20. MAY contain zero or more [0..*] entryRelationship (CONF:7536) such that
it
a. SHALL contain exactly one [1..1] @typeCode="RSON" (CodeSystem:
HL7ActRelationshipType 2.16.840.1.113883.5.1002)
(CONF:7537).
b. SHALL contain exactly one [1..1] Indication
(templateId:2.16.840.1.113883.10.20.22.4.19) (CONF:16087).
21. MAY contain zero or one [0..1] entryRelationship (CONF:7539) such that it
a. SHALL contain exactly one [1..1] @typeCode="SUBJ" (CodeSystem:
HL7ActRelationshipType 2.16.840.1.113883.5.1002)
(CONF:7540).
b. SHALL contain exactly one [1..1] @inversionInd="true" True
(CONF:7542).
c. SHALL contain exactly one [1..1] Instructions
(templateId:2.16.840.1.113883.10.20.22.4.20) (CONF:16088).
22. MAY contain zero or one [0..1] entryRelationship (CONF:7543) such that it
a. SHALL contain exactly one [1..1] @typeCode="REFR" (CodeSystem:
HL7ActRelationshipType 2.16.840.1.113883.5.1002)
(CONF:7547).
b. SHALL contain exactly one [1..1] Medication Supply Order
(templateId:2.16.840.1.113883.10.20.22.4.17) (CONF:16089).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 395
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
23. MAY contain zero or more [0..*] entryRelationship (CONF:7549) such that
it
a. SHALL contain exactly one [1..1] @typeCode="REFR" (CodeSystem:
HL7ActRelationshipType 2.16.840.1.113883.5.1002)
(CONF:7553).
b. SHALL contain exactly one [1..1] Medication Dispense
(templateId:2.16.840.1.113883.10.20.22.4.18) (CONF:16090).
24. MAY contain zero or one [0..1] entryRelationship (CONF:7552) such that it
a. SHALL contain exactly one [1..1] @typeCode="CAUS" (CodeSystem:
HL7ActRelationshipType 2.16.840.1.113883.5.1002)
(CONF:7544).
b. SHALL contain exactly one [1..1] Reaction Observation
(templateId:2.16.840.1.113883.10.20.22.4.9) (CONF:16091).
25. MAY contain zero or more [0..*] precondition (CONF:7546) such that it
a. SHALL contain exactly one [1..1] @typeCode="PRCN" (CodeSystem:
HL7ActRelationshipType 2.16.840.1.113883.5.1002)
(CONF:7550).
b. SHALL contain exactly one [1..1] Precondition for Substance
Administration
(templateId:2.16.840.1.113883.10.20.22.4.25) (CONF:16092).
26. Medication Activity SHOULD include doseQuantity OR rateQuantity
(CONF:7529).
Table 202: MoodCodeEvnInt Value Set
Value Set: MoodCodeEvnInt 2.16.840.1.113883.11.20.9.18 STATIC 2011-04-03
Code System(s):
ActMood 2.16.840.1.113883.5.1001
Description:
Subset of HL7 ActMood codes, constrained to represent event (EVN) and
intent (INT) moods
Code
Code System
Print Name
EVN
ActMood
Event
INT
ActMood
Intent

Page 396 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 203: Medication Route FDA Value Set (excerpt)
Value Set: Medication Route FDA Value Set 2.16.840.1.113883.3.88.12.3221.8.7 DYNAMIC
Code System(s):
National Cancer Institute (NCI) Thesaurus 2.16.840.1.113883.3.26.1.1
Description:
This indicates the method for the medication received by the individual (e.g.,
by mouth, intravenously, topically, etc.). NCI concept code for route of
administration: C38114
http://www.fda.gov/ForIndustry/DataStandards/StructuredProductLabeling
/ucm162034.htm
Code
Code System
Print Name
C38229
NCI Thesaurus
INTRACAUDAL
C38276
NCI Thesaurus
INTRAVENOUS
C38288
NCI Thesaurus
ORAL
C38295
NCI Thesaurus
RECTAL
…
Table 204: Body Site Value Set (excerpt)
Value Set: Body Site Value Set 2.16.840.1.113883.3.88.12.3221.8.9 DYNAMIC
Code System(s):
SNOMED CT 2.16.840.1.113883.6.96
Description:
Contains values descending from the SNOMED CT® Anatomical Structure
(91723000) hierarchy or Acquired body structure (body structure)
(280115004) or Anatomical site notations for tumor staging (body structure)
(258331007) or Body structure, altered from its original anatomical structure
(morphologic abnormality) (118956008) or Physical anatomical entity (body
structure) (91722005) This indicates the anatomical site.
http://www.nlm.nih.gov/research/umls/Snomed/snomed_main.html
Code
Code System
Print Name
361316009
SNOMED CT
entire embryonic artery
38033009
SNOMED CT
amputation stump
9550003
SNOMED CT
bronchogenic cyst
302509004
SNOMED CT
heart
…

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 397
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 205: Medication Product Form Value Set (excerpt)
Value Set: Medication Product Form 2.16.840.1.113883.3.88.12.3221.8.11 DYNAMIC
Code System(s):
National Cancer Institute (NCI) Thesaurus 2.16.840.1.113883.3.26.1.1
Description:
This is the physical form of the product as presented to the individual.
For example: tablet, capsule, liquid or ointment.
http://www.fda.gov/ForIndustry/DataStandards/StructuredProductLabeling
/ucm162038.htm
Code
Code System
Print Name
C42887
NCI Thesaurus
AEROSOL
C42909
NCI Thesaurus
GRANULE, EFFERVESCENT
C42998
NCI Thesaurus
TABLET
…
Table 206: Unit of Measure Value Set (excerpt)
Value Set: UCUM Units of Measure (case sensitive) 2.16.840.1.113883.1.11.12839 DYNAMIC
Code System(s):
Unified Code for Units of Measure (UCUM) 2.16.840.1.113883.6.8
Description:
UCUM codes include all units of measures being contemporarily used in
international science, engineering, and business. The purpose is to facilitate
unambiguous electronic communication of quantities together with their
units. The focus is on electronic communication, as opposed to
communication between humans.
http://www.regenstrief.org/medinformatics/ucum
Code
Code System
Print Name
mmol/kg
UCUM
MilliMolesPerKiloGram
fL
UCUM
FemtoLiter
ug/mL
UCUM
MicroGramsPerMilliLiter
…
Figure 192: Medication activity example
<substanceAdministration classCode="SBADM" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.16"/>
<id root="cdbd33f0-6cde-11db-9fe1-0800200c9a66"/>
<text>
<reference value="#med1/>
Proventil 0.09 MG/ACTUAT inhalant solution, 2 puffs QID PRN wheezing
</text>
<statusCode code="completed"/>
<effectiveTime xsi:type="IVL_TS">
<low value="20110301"/>
<high value="20120301"/>
</effectiveTime>
<effectiveTime xsi:type="PIVL_TS" institutionSpecified="true"
operator="A">
<period value="6" unit="h"/>
</effectiveTime>

Page 398 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
<routeCode code="C38216" codeSystem="2.16.840.1.113883.3.26.1.1"
codeSystemName="NCI Thesaurus"
displayName="RESPIRATORY (INHALATION)"/>
<doseQuantity value="1"/>
<rateQuantity value="90" unit="ml/min"/>
<maxDoseQuantity nullFlavor="UNK">
<numerator nullFlavor="UNK"/>
<denominator nullFlavor="UNK"/>
</maxDoseQuantity>
<administrationUnitCode code="C42944"
displayName="INHALANT"
codeSystem="2.16.840.1.113883.3.26.1.1"
codeSystemName="NCI Thesaurus"/>
<consumable>
<manufacturedProduct>
<templateId root="2.16.840.1.113883.10.20.22.4.23"/>
<!-- Medication Information template -->
...
</manufacturedMaterial>
<manufacturerOrganization/>
</manufacturedProduct>
</consumable>
<performer>
...
</performer>
<participant typeCode="CSM">
<participantRole classCode="MANU">
<templateId root="2.16.840.1.113883.10.20.22.4.24"/>
<!-- Drug Vehicle template -->
...
</participantRole>
</participant>
<entryRelationship typeCode="RSON">
<observation classCode="COND" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.19"/>
<!-- Indication template -->
...
</observation>
</entryRelationship>
<entryRelationship typeCode="REFR">
<supply classCode="SPLY" moodCode="INT">
<templateId root="2.16.840.1.113883.10.20.22.4.17"/>
<!-- Medication Supply Order template -->
...
<entryRelationship typeCode="SUBJ" inversionInd="true">
<act classCode="ACT" moodCode="INT">
<templateId root="2.16.840.1.113883.10.20.22.4.20"/>
<!-- Instructions template -->
...
</act>
</entryRelationship>
</supply>
</entryRelationship>

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 399
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
<entryRelationship typeCode="REFR">
<supply classCode="SPLY" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.18"/>
<!-- Medication Dispense template -->
...
</supply>
</entryRelationship>
<precondition typeCode="PRCN">
<templateId root="2.16.840.1.113883.10.20.22.4.25"/>
<!-- Precondition for Substance Administration template -->
...
</precondition>
</substanceAdministration>
6.40 Medication Dispense
[supply: templateId 2.16.840.1.113883.10.20.22.4.18(open)]
Table 207: Medication Dispense Contexts
Used By:
Contains Entries:
Medication Activity
Immunization Activity
Immunization Medication Information
Medication Information
Medication Supply Order
This template records the act of supplying medications (i.e., dispensing).

Page 400 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 208: Medication Dispense Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF
#
Fixed Value
Green
Medication
Dispense
supply[templateId/@root = '2.16.840.1.113883.10.20.22.4.18']
@classCode
1..1
SHALL
7451
2.16.840.1.113883.5.6
(HL7ActClass) = SPLY
@moodCode
1..1
SHALL
7452
2.16.840.1.113883.5.100
1 (ActMood) = EVN
templateId
1..1
SHALL
SET<II>
7453
@root
1..1
SHALL
10505
2.16.840.1.113883.10.20
.22.4.18
prescriptio
nNumber
id
1..*
SHALL
7454
statusCode
1..1
SHALL
7455
2.16.840.1.113883.3.88.
12.80.64 (Medication Fill
Status)
dispense
Date
effectiveTime
0..1
SHOULD
TS or
IVL<TS>
7456
fillNumber
repeatNumber
0..1
SHOULD
IVL<INT
>
7457
quantity
Dispensed
quantity
0..1
SHOULD
7458
product
0..1
MAY
7459
manufactured
Product
1..1
SHALL
15607
product
0..1
MAY
9331
manufactured
Product
1..1
SHALL
15608
performer
0..1
MAY
7461
provider
assignedEntity
1..1
SHALL
7467
addr
0..1
SHOULD
SET<AD
>
7468
order
Information
entryRelationship
0..1
MAY
7473
@typeCode
1..1
SHALL
7474
2.16.840.1.113883.5.100
2
(HL7ActRelationshipType)
= REFR
supply
1..1
SHALL
15606
1. SHALL contain exactly one [1..1] @classCode="SPLY" (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:7451).
2. SHALL contain exactly one [1..1] @moodCode="EVN" (CodeSystem: ActMood
2.16.840.1.113883.5.1001) (CONF:7452).
3. SHALL contain exactly one [1..1] templateId (CONF:7453) such that it

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 401
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.18" (CONF:10505).
4. SHALL contain at least one [1..*] id (CONF:7454).
5. SHALL contain exactly one [1..1] statusCode, which SHALL be selected from
ValueSet Medication Fill Status 2.16.840.1.113883.3.88.12.80.64
DYNAMIC (CONF:7455).
6. SHOULD contain zero or one [0..1] effectiveTime (CONF:7456).
7. SHOULD contain zero or one [0..1] repeatNumber (CONF:7457).
a. In "EVN" (event) mood, the repeatNumber is the number of
occurrences. For example, a repeatNumber of "3" in a dispense act
means that the current dispensation is the 3rd (CONF:16876).
8. SHOULD contain zero or one [0..1] quantity (CONF:7458).
9. MAY contain zero or one [0..1] product (CONF:7459) such that it
a. SHALL contain exactly one [1..1] Medication Information
(templateId:2.16.840.1.113883.10.20.22.4.23) (CONF:15607).
10. MAY contain zero or one [0..1] product (CONF:9331) such that it
a. SHALL contain exactly one [1..1] Immunization Medication
Information (templateId:2.16.840.1.113883.10.20.22.4.54)
(CONF:15608).
11. MAY contain zero or one [0..1] performer (CONF:7461).
a. The performer, if present, SHALL contain exactly one [1..1]
assignedEntity (CONF:7467).
i. This assignedEntity SHOULD contain zero or one [0..1] addr
(CONF:7468).
1. The content of addr SHALL be a conformant US Realm
Address (AD.US.FIELDED)
(2.16.840.1.113883.10.20.22.5.2) (CONF:10565).
12. MAY contain zero or one [0..1] entryRelationship (CONF:7473) such that it
a. SHALL contain exactly one [1..1] @typeCode="REFR" (CodeSystem:
HL7ActRelationshipType 2.16.840.1.113883.5.1002)
(CONF:7474).
b. SHALL contain exactly one [1..1] Medication Supply Order
(templateId:2.16.840.1.113883.10.20.22.4.17) (CONF:15606).
13. A supply act SHALL contain one product/Medication Information or one
product/Immunization Medication Information template (CONF:9333).
Table 209: Medication Fill Status Value Set
Value Set: Medication Fill Status 2.16.840.1.113883.3.88.12.80.64 DYNAMIC
Code System: ActStatus 2.16.840.1.113883.5.14
Code
Code System
Print Name
aborted
ActStatus
Aborted
completed
ActStatus
Completed

Page 402 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 193: Medication dispense example
<supply classCode="SPLY" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.18"/>
<id root="1.2.3.4.56789.1" extension="cb734647-fc99-424c-a864-
7e3cda82e704"/>
<statusCode code="completed"/>
<effectiveTime value="20020101"/>
<repeatNumber value="1"/>
<quantity value="75"/>
<performer>
<time nullFlavor="UNK"/>
<assignedEntity>
<id/>
<addr>
<streetAddressLine>17 Daws Rd.</streetAddressLine>
<city>Blue Bell</city>
<state>MA</state>
<postalCode>02368</postalCode>
<country>US</country>
</addr>
<telecom nullFlavor="UNK"/>
<assignedPerson>
<name>
<prefix>Dr.</prefix>
<given>Robert</given>
<family>Michaels</family>
</name>
</assignedPerson>
<representedOrganization>
<id root="2.16.840.1.113883.19.5"/>
<name>Good Health Clinic</name>
<telecom nullFlavor="UNK"/>
<addr nullFlavor="UNK"/>
</representedOrganization>
</assignedEntity>
</performer>
</supply>
6.41 Medication Information
[manufacturedProduct: templateId
2.16.840.1.113883.10.20.22.4.23(open)]
Table 210: Medication Information Contexts
Used By:
Contains Entries:
Medication Supply Order
Medication Dispense
Medication Activity
The medication can be recorded as a precoordinated product strength, product
form, or product concentration (e.g., "metoprolol 25mg tablet", "amoxicillin
400mg/5mL suspension"); or not pre-coordinated (e.g., "metoprolol product").

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 403
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 211: Medication Information Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF
#
Fixed Value
Green
Medication
Information
manufacturedProduct[templateId/@root = '2.16.840.1.113883.10.20.22.4.23']
@classCode
1..1
SHALL
7408
2.16.840.1.113883.5.110
(RoleClass) = MANU
templateId
1..1
SHALL
SET<
II>
7409
@root
1..1
SHALL
10506
2.16.840.1.113883.10.20.2
2.4.23
id
0..*
MAY
7410
manufactured
Material
1..1
SHALL
7411
codedProduct
Name
code
1..1
SHALL
7412
2.16.840.1.113883.3.88.12.
80.17 (Medication Clinical
Drug)
freeText
ProductName
originalText
0..1
SHOULD
7413
reference
0..1
SHOULD
15986
@value
0..1
SHOULD
15987
codedBrand
Name
translation
0..*
MAY
SET<
PQR>
7414
drug
Manufacturer
Manufacturer
Organization
0..1
MAY
7416
1. SHALL contain exactly one [1..1] @classCode="MANU" (CodeSystem:
RoleClass 2.16.840.1.113883.5.110) (CONF:7408).
2. SHALL contain exactly one [1..1] templateId (CONF:7409) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.23" (CONF:10506).
3. MAY contain zero or more [0..*] id (CONF:7410).
4. SHALL contain exactly one [1..1] manufacturedMaterial (CONF:7411).
a. This manufacturedMaterial SHALL contain exactly one [1..1] code,
which SHALL be selected from ValueSet Medication Clinical Drug
2.16.840.1.113883.3.88.12.80.17 DYNAMIC (CONF:7412).
i. This code SHOULD contain zero or one [0..1] originalText
(CONF:7413).
1. The originalText, if present, SHOULD contain zero or one
[0..1] reference (CONF:15986).
a. The reference, if present, SHOULD contain zero
or one [0..1] @value (CONF:15987).
i. This reference/@value SHALL begin with
a '#' and SHALL point to its
corresponding narrative (using the

Page 404 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
approach defined in CDA Release 2,
section 4.3.5.1) (CONF:15988).
ii. This code MAY contain zero or more [0..*] translation
(CONF:7414).
1. Translations can be used to represent generic product
name, packaged product code, etc (CONF:16875).
5. MAY contain zero or one [0..1] manufacturerOrganization (CONF:7416).
Figure 194: Medication information example
<manufacturedProduct classCode="MANU">
<templateId root="2.16.840.1.113883.10.20.22.4.23"/>
<id/>
<manufacturedMaterial>
<code code="329498"
codeSystem="2.16.840.1.113883.6.88"
codeSystemName="RxNorm"
displayName="Albuterol 0.09 MG/ACTUAT inhalant solution">
<originalText><reference value="#manmat1"/></originalText>
<translation code="573621"
codeSystem="2.16.840.1.113883.6.88" codeSystemName="RxNorm"
displayName="Proventil 0.09 MG/ACTUAT inhalant solution"
sdtc:valueSet="{$QDMElementValueSetOID}/>
<!-- Would be actual valueSetOID -->
</code>
</manufacturedMaterial>
<manufacturerOrganization>...</manufacturerOrganization>
</manufacturedProduct>
6.42 Medication Supply Order
[supply: templateId 2.16.840.1.113883.10.20.22.4.17(open)]
Table 212: Medication Supply Order Contexts
Used By:
Contains Entries:
Medication Dispense
Medication Activity
Immunization Activity
Immunization Medication Information
Instructions
Medication Information
This template records the intent to supply a patient with medications.

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 405
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 213: Medication Supply Order Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
Green
Medication
Supply
Order
supply[templateId/@root = '2.16.840.1.113883.10.20.22.4.17']
@classCode
1..1
SHALL
7427
2.16.840.1.113883.5.6
(HL7ActClass) = SPLY
@moodCode
1..1
SHALL
7428
2.16.840.1.113883.5.100
1 (ActMood) = INT
templateId
1..1
SHALL
SET<II>
7429
@root
1..1
SHALL
10507
2.16.840.1.113883.10.20.
22.4.17
order
Number
id
1..*
SHALL
7430
statusCode
1..1
SHALL
7432
effectiveTime
0..1
SHOULD
IVL_TS
15143
high
1..1
SHALL
15144
fills
repeatNumber
0..1
SHOULD
IVL<INT>
7434
quantity
Ordered
quantity
0..1
SHOULD
7436
product
0..1
MAY
7439
manufactured
Product
1..1
SHALL
16093
product
0..1
MAY
9334
manufactured
Product
1..1
SHALL
16094
ordering
Provider
author
0..1
MAY
7438
patient
Instructions
entry
Relationship
0..1
MAY
7442
@typeCode
1..1
SHALL
7444
2.16.840.1.113883.5.100
2
(HL7ActRelationshipType)
= SUBJ
@inversionInd
1..1
SHALL
7445
true
act
1..1
SHALL
16095
1. SHALL contain exactly one [1..1] @classCode="SPLY" (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:7427).
2. SHALL contain exactly one [1..1] @moodCode="INT" (CodeSystem: ActMood
2.16.840.1.113883.5.1001) (CONF:7428).
3. SHALL contain exactly one [1..1] templateId (CONF:7429) such that it

Page 406 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.17" (CONF:10507).
4. SHALL contain at least one [1..*] id (CONF:7430).
5. SHALL contain exactly one [1..1] statusCode (CONF:7432).
6. SHOULD contain zero or one [0..1] effectiveTime (CONF:15143) such that it
a. SHALL contain exactly one [1..1] high (CONF:15144).
7. SHOULD contain zero or one [0..1] repeatNumber (CONF:7434).
a. In "INT" (intent) mood, the repeatNumber defines the number of
allowed fills. For example, a repeatNumber of "3" means that the
substance can be supplied up to 3 times (or, can be dispensed, with
2 refills) (CONF:16869).
8. SHOULD contain zero or one [0..1] quantity (CONF:7436).
9. MAY contain zero or one [0..1] product (CONF:7439) such that it
a. SHALL contain exactly one [1..1] Medication Information
(templateId:2.16.840.1.113883.10.20.22.4.23) (CONF:16093).
10. MAY contain zero or one [0..1] product (CONF:9334) such that it
a. SHALL contain exactly one [1..1] Immunization Medication
Information (templateId:2.16.840.1.113883.10.20.22.4.54)
(CONF:16094).
i. A supply act SHALL contain one product/Medication
Information or one product/Immunization Medication
Information template (CONF:16870).
11. MAY contain zero or one [0..1] author (CONF:7438).
12. MAY contain zero or one [0..1] entryRelationship (CONF:7442).
a. The entryRelationship, if present, SHALL contain exactly one [1..1]
@typeCode="SUBJ" (CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002) (CONF:7444).
b. The entryRelationship, if present, SHALL contain exactly one [1..1]
@inversionInd="true" True (CONF:7445).
c. The entryRelationship, if present, SHALL contain exactly one [1..1]
Instructions
(templateId:2.16.840.1.113883.10.20.22.4.20) (CONF:16095).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 407
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 195: Medication supply order example
<supply classCode="SPLY" moodCode="INT">
<templateId root="2.16.840.1.113883.10.20.22.4.17"/>
<id root="1.2.3.4.5.6.7" extension="1234567"/>
<statusCode code="completed"/>
<effectiveTime xsi:type="IVL_TS">
<width value="10" unit="d"/>
<high value="20121012" />
</effectiveTime>
<repeatNumber value="1"/>
<quantity value="75"/>
<product>
<manufacturedProduct>
<templateId root="2.16.840.1.113883.10.20.22.4.23"/>
<!-- Medication Information template -->
...
</manufacturedProduct>
</product>
<author>
...
</author>
<entryRelationship typeCode="SUBJ" inversionInd="true">
<act classCode="ACT" moodCode="INT">
<templateId root="2.16.840.1.113883.10.20.22.4.20"/>
<!-- Instructions template -->
...
</act>
</entryRelationship>
</supply>
6.43 Medication Use – None Known (deprecated)
[observation: templateId 2.16.840.1.113883.10.20.22.4.29(open)]
The recommended approach to stating no known medications is to use the
appropriate nullFlavor instead of this template. See "Unknown Information" in
Section 1.
This template indicates that the subject is not known to be on any medications.
1. SHALL contain exactly one [1..1] @classCode="OBS" (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:7557).
2. SHALL contain exactly one [1..1] @moodCode="EVN" (CodeSystem: ActMood
2.16.840.1.113883.5.1001) (CONF:7558).
3. SHALL contain exactly one [1..1] templateId (CONF:7559) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.29" (CONF:10508).
4. SHALL contain at least one [1..*] id (CONF:7560).
5. SHALL contain exactly one [1..1] code="ASSERTION" (CodeSystem: ActCode
2.16.840.1.113883.5.4) (CONF:7561).
6. MAY contain zero or one [0..1] text (CONF:7565).

Page 408 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
a. The text, if present, SHOULD contain zero or one [0..1] reference
(CONF:15580).
i. The reference, if present, SHOULD contain zero or one [0..1]
@value (CONF:15581).
1. This reference/@value SHALL begin with a '#' and SHALL
point to its corresponding narrative (using the
approach defined in CDA Release 2, section 4.3.5.1)
(CONF:15582).
7. SHALL contain exactly one [1..1] statusCode="completed" Completed
(CodeSystem: ActStatus 2.16.840.1.113883.5.14) (CONF:7562).
8. SHOULD contain zero or one [0..1] effectiveTime (CONF:7563).
9. SHALL contain exactly one [1..1] value with @xsi:type="ANY"="182904002"
Drug treatment unknown (CodeSystem: SNOMEDCT
2.16.840.1.113883.6.96) (CONF:7564).
Figure 196: Medication use – none known example
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.29"/>
<id root="db734647-fc99-424c-a864-7e3cda82e703" extension="45665"/>
<code code="ASSERTION" codeSystem="2.16.840.1.113883.5.4"/>
<text><reference value="#med_text1"/></text>
<statusCode code="completed"
codeSystem="2.16.840.1.113883.5.4"/>
<effectiveTime value="20110203"/>
<value code="182904002"
displayName="Drug treatment unknown"
codeSystem="2.16.840.1.113883.6.96"/>
</observation>
6.44 Non-Medicinal Supply Activity
[supply: templateId 2.16.840.1.113883.10.20.22.4.50(open)]
Table 214: Non-Medicinal Supply Activity Contexts
Used By:
Contains Entries:
Medical Equipment Section
Functional Status Result Observation (optional)
Cognitive Status Problem Observation
Cognitive Status Result Observation
Functional Status Problem Observation
Functional Status Result Observation
Product Instance
This template records non-medicinal supplies provided, such as medical
equipment.

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 409
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 215: Non-Medicinal Supply Activity Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
supply[templateId/@root = '2.16.840.1.113883.10.20.22.4.50']
@classCode
1..1
SHALL
8745
2.16.840.1.113883.5.6
(HL7ActClass) = SPLY
@moodCode
1..1
SHALL
8746
2.16.840.1.113883.11.20.9.18
(MoodCodeEvnInt)
templateId
1..1
SHALL
SET<
II>
8747
@root
1..1
SHALL
10509
2.16.840.1.113883.10.20.22.4.50
id
1..*
SHALL
8748
statusCode
1..1
SHALL
8749
effectiveTime
0..1
SHOULD
IVL_T
S
15498
quantity
0..1
SHOULD
8751
participant
0..1
MAY
8752
@typeCode
1..1
SHALL
8754
2.16.840.1.113883.5.90
(HL7ParticipationType) = PRD
participantRole
1..1
SHALL
15900
1. SHALL contain exactly one [1..1] @classCode="SPLY" (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:8745).
2. SHALL contain exactly one [1..1] @moodCode, which SHALL be selected from
ValueSet MoodCodeEvnInt 2.16.840.1.113883.11.20.9.18 STATIC 2011-
04-03 (CONF:8746).
3. SHALL contain exactly one [1..1] templateId (CONF:8747) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.50" (CONF:10509).
4. SHALL contain at least one [1..*] id (CONF:8748).
5. SHALL contain exactly one [1..1] statusCode (CONF:8749).
6. SHOULD contain zero or one [0..1] effectiveTime (CONF:15498).
a. The effectiveTime, if present, SHOULD contain zero or one [0..1] high
(CONF:16867).
7. SHOULD contain zero or one [0..1] quantity (CONF:8751).
8. MAY contain zero or one [0..1] participant (CONF:8752) such that it
a. SHALL contain exactly one [1..1] @typeCode="PRD" Product
(CodeSystem: HL7ParticipationType 2.16.840.1.113883.5.90)
(CONF:8754).
b. SHALL contain exactly one [1..1] Product Instance
(templateId:2.16.840.1.113883.10.20.22.4.37) (CONF:15900).

Page 410 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 197: Non-medicinal supply activity example
<supply classCode="SPLY" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.50"/>
<!-- Non-Medicinal Supply Activity template -->
<id root="2413773c-2372-4299-bbe6-5b0f60664446"/>
<statusCode code="completed"/>
<effectiveTime xsi:type="IVL_TS">
<center value="199911"/>
</effectiveTime>
<quantity value="2"/>
<participant typeCode="PRD">
<participantRole classCode="MANU">
<templateId root="2.16.840.1.113883.10.20.22.4.37"/>
<!-- *** Product Instance template *** -->
...
</participantRole>
</participant>
</supply>
6.45 Number of Pressure Ulcers Observation
[observation: templateId 2.16.840.1.113883.10.20.22.4.76 (open)]
Table 216: Number of Pressure Ulcers Observation Contexts
Used By:
Contains Entries:
Functional Status Section (optional)
This clinical statement enumerates the number of pressure ulcers observed in a
particular stage.
Table 217: Number of Pressure Ulcers Observation Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
observation[templateId/@root = '2.16.840.1.113883.10.20.22.4.76']
@classCode
1..1
SHALL
14705
2.16.840.1.113883.5.6 (HL7ActClass) =
OBS
@moodCode
1..1
SHALL
14706
2.16.840.1.113883.5.1001 (ActMood) =
EVN
templateId
1..1
SHALL
14707
@root
1..1
SHALL
14708
2.16.840.1.113883.10.20.22.4.76
id
1..*
SHALL
14709
code
1..1
SHALL
14767
@code
1..1
SHALL
14768
2264892003
statusCode
1..1
SHALL
14714
2.16.840.1.113883.5.14 (ActStatus) =
completed
effectiveTime
1..1
SHALL
14715
value
1..1
SHALL
INT
14771

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 411
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
author
0..1
MAY
14717
entry
Relationship
1..1
SHALL
14718
@typeCode
1..1
SHALL
14719
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) = SUBJ
observation
1..1
SHALL
14720
@classCode
1..1
SHALL
14721
2.16.840.1.113883.5.6 (HL7ActClass) =
OBS
@moodCode
1..1
SHALL
14722
2.16.840.1.113883.5.1001 (ActMood) =
EVN
value
1..1
SHALL
CD
14725
2.16.840.1.113883.11.20.9.35 (Pressure
Ulcer Stage)
1. SHALL contain exactly one [1..1] @classCode="OBS" (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:14705).
2. SHALL contain exactly one [1..1] @moodCode="EVN" (CodeSystem: ActMood
2.16.840.1.113883.5.1001) (CONF:14706).
3. SHALL contain exactly one [1..1] templateId (CONF:14707) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.76" (CONF:14708).
4. SHALL contain at least one [1..*] id (CONF:14709).
5. SHALL contain exactly one [1..1] code (CONF:14767).
a. This code SHALL contain exactly one [1..1] @code="2264892003"
number of pressure ulcers (CONF:14768).
6. SHALL contain exactly one [1..1] statusCode="completed" (CodeSystem:
ActStatus 2.16.840.1.113883.5.14) (CONF:14714).
7. SHALL contain exactly one [1..1] effectiveTime (CONF:14715).
8. SHALL contain exactly one [1..1] value with @xsi:type="INT" (CONF:14771).
9. MAY contain zero or one [0..1] author (CONF:14717).
10. SHALL contain exactly one [1..1] entryRelationship (CONF:14718).
a. This entryRelationship SHALL contain exactly one [1..1]
@typeCode="SUBJ" Has subject (CodeSystem:
HL7ActRelationshipType 2.16.840.1.113883.5.1002)
(CONF:14719).
b. This entryRelationship SHALL contain exactly one [1..1] observation
(CONF:14720).
i. This observation SHALL contain exactly one [1..1]
@classCode="OBS" (CodeSystem: HL7ActClass
2.16.840.1.113883.5.6) (CONF:14721).
ii. This observation SHALL contain exactly one [1..1]
@moodCode="EVN" (CodeSystem: ActMood
2.16.840.1.113883.5.1001) (CONF:14722).
iii. This observation SHALL contain exactly one [1..1] value with
@xsi:type="CD", where the @code SHOULD be selected from

Page 412 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
ValueSet Pressure Ulcer Stage
2.16.840.1.113883.11.20.9.35 (CONF:14725).
Figure 198: Number of pressure ulcers example
<entry typeCode="DRIV">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.70"/>
<id root="08edb7c0-2111-43f2-a784-9a5fdfaa67f0"/>
<code code="2264892003"
codeSystem="2.16.840.1.113883.6.96"
displayName=" number of pressure ulcers"/>
<statusCode code="completed"/>
<value xsi:type="INT" value="3"/>
<entryRelationship typeCode="SUBJ">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.76"/>
<value xsi:type="CD" code="421927004"
codeSystem="2.16.840.1.113883.6.96"
displayName="Pressure ulcer stage 3"/>
</observation>
</entryRelationship>
</observation>
</entry>
6.46 Plan of Care Activity Act
[act: templateId 2.16.840.1.113883.10.20.22.4.39(open)]
Table 218: Plan of Care Activity Act Contexts
Used By:
Contains Entries:
Assessment and Plan Section
Plan of Care Section
This is the generic template for the Plan of Care Activity.
Table 219: Plan of Care Activity Act Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
act[templateId/@root = '2.16.840.1.113883.10.20.22.4.39']
@classCode
1..1
SHALL
8538
2.16.840.1.113883.5.6 (HL7ActClass) =
ACT
@moodCode
1..1
SHALL
8539
2.16.840.1.113883.11.20.9.23 (Plan of
Care moodCode
(Act/Encounter/Procedure))
templateId
1..1
SHALL
SET<II>
8544
@root
1..1
SHALL
10510
2.16.840.1.113883.10.20.22.4.39
id
1..*
SHALL
II
8546

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 413
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
1. SHALL contain exactly one [1..1] @classCode="ACT" (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:8538).
2. SHALL contain exactly one [1..1] @moodCode, which SHALL be selected from
ValueSet Plan of Care moodCode (Act/Encounter/Procedure)
2.16.840.1.113883.11.20.9.23 STATIC 2011-09-30 (CONF:8539).
3. SHALL contain exactly one [1..1] templateId (CONF:8544) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.39" (CONF:10510).
4. SHALL contain at least one [1..*] id (CONF:8546).
Table 220: Plan of Care moodCode (Act/Encounter/Procedure)
Value Set: Plan of Care moodCode (Act/Encounter/Procedure) 2.16.840.1.113883.11.20.9.23
STATIC 2011-09-30
Code System(s):
HL7 ActMood 2.16.840.1.113883.5.1001
Code
Code System
Print Name
INT
HL7 ActMood
Intent
ARQ
HL7 ActMood
Appointment Request
PRMS
HL7 ActMood
Promise
PRP
HL7 ActMood
Proposal
RQO
HL7 ActMood
Request
Figure 199: Plan of care activity act example
<act moodCode="RQO" classCode="ACT">
<templateId root="2.16.840.1.113883.10.20.22.4.39"/>
<!-- Plan of Care Activity Act -->
<id root="9a6d1bac-17d3-4195-89a4-1121bc809a5c"/>
<code code="310634005" codeSystem="2.16.840.1.113883.6.96"
displayName="Colonoscopy"/>
<statusCode code="new"/>
<effectiveTime>
<center value="20000421"/>
</effectiveTime>
</act>
6.47 Plan of Care Activity Encounter
[encounter: templateId 2.16.840.1.113883.10.20.22.4.40(open)]
Table 221: Plan of Care Activity Encounter Contexts
Used By:
Contains Entries:
Plan of Care Section
This is the template for the Plan of Care Activity Encounter.

Page 414 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 222: Plan of Care Activity Encounter Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
encounter[templateId/@root = '2.16.840.1.113883.10.20.22.4.40']
@classCode
1..1
SHALL
8564
2.16.840.1.113883.5.6 (HL7ActClass) =
ENC
@moodCode
1..1
SHALL
8565
2.16.840.1.113883.11.20.9.23 (Plan of
Care moodCode
(Act/Encounter/Procedure))
templateId
1..1
SHALL
SET<II>
8566
@root
1..1
SHALL
10511
2.16.840.1.113883.10.20.22.4.40
id
1..*
SHALL
II
8567
1. SHALL contain exactly one [1..1] @classCode="ENC" (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:8564).
2. SHALL contain exactly one [1..1] @moodCode, which SHALL be selected from
ValueSet Plan of Care moodCode (Act/Encounter/Procedure)
2.16.840.1.113883.11.20.9.23 STATIC 2011-09-30 (CONF:8565).
3. SHALL contain exactly one [1..1] templateId (CONF:8566) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.40" (CONF:10511).
4. SHALL contain at least one [1..*] id (CONF:8567).
Figure 200: Plan of care activity encounter example
<encounter moodCode="INT" classCode="ENC">
<templateId root="2.16.840.1.113883.10.20.22.4.40"/>
<!-- **** Plan of Care Activity Encounter template **** -->
<id root="9a6d1bac-17d3-4195-89a4-1121bc809b4d"/>
</encounter>
6.48 Plan of Care Activity Observation
[observation: templateId 2.16.840.1.113883.10.20.22.4.44(open)]
Table 223: Plan of Care Activity Observation Contexts
Used By:
Contains Entries:
Plan of Care Section
This is the template for the Plan of Care Activity Observation.

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 415
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 224: Plan of Care Activity Observation Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
observation[templateId/@root = '2.16.840.1.113883.10.20.22.4.44']
@classCode
1..1
SHALL
8581
2.16.840.1.113883.5.6 (HL7ActClass) =
OBS
@moodCode
1..1
SHALL
8582
2.16.840.1.113883.11.20.9.25 (Plan of
Care moodCode (Observation))
templateId
1..1
SHALL
SET<II>
8583
@root
1..1
SHALL
10512
2.16.840.1.113883.10.20.22.4.44
id
1..*
SHALL
II
8584
1. SHALL contain exactly one [1..1] @classCode="OBS" (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:8581).
2. SHALL contain exactly one [1..1] @moodCode, which SHALL be selected from
ValueSet Plan of Care moodCode (Observation)
2.16.840.1.113883.11.20.9.25 STATIC 2011-09-30 (CONF:8582).
3. SHALL contain exactly one [1..1] templateId (CONF:8583) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.44" (CONF:10512).
4. SHALL contain at least one [1..*] id (CONF:8584).
Table 225: Plan of Care moodCode (Observation) Value Set
Value Set: Plan of Care moodCode (Observation) 2.16.840.1.113883.11.20.9.25 STATIC 2011-
09-30
Code System(s):
HL7 ActMood 2.16.840.1.113883.5.1001
Code
Code System
Print Name
INT
ActMood
Intent
GOL
ActMood
Goal
PRMS
ActMood
Promise
PRP
ActMood
Proposal
RQO
ActMood
Request

Page 416 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 201: Plan of care activity observation example
<observation classCode="OBS" moodCode="RQO">
<templateId root="2.16.840.1.113883.10.20.22.4.44"/>
<!-- Plan of Care Activity Observation template -->
<id root="9a6d1bac-17d3-4195-89a4-1121bc809b4a"/>
<code code="23426006" codeSystem="2.16.840.1.113883.6.96"
displayName="Pulmonary function test"/>
<statusCode code="new"/>
<effectiveTime>
<center value="20000421"/>
</effectiveTime>
</observation>
6.49 Plan of Care Activity Procedure
[procedure: templateId 2.16.840.1.113883.10.20.22.4.41(open)]
Table 226: Plan of Care Activity Procedure Contexts
Used By:
Contains Entries:
Planned Procedure Section
Plan of Care Section
This is the template for the Plan of Care Activity Procedure.
Table 227: Plan of Care Activity Procedure Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
procedure[templateId/@root = '2.16.840.1.113883.10.20.22.4.41']
@classCode
1..1
SHALL
8568
2.16.840.1.113883.5.6 (HL7ActClass) =
PROC
@moodCode
1..1
SHALL
8569
2.16.840.1.113883.11.20.9.23 (Plan of
Care moodCode
(Act/Encounter/Procedure))
templateId
1..1
SHALL
SET<II>
8570
@root
1..1
SHALL
10513
2.16.840.1.113883.10.20.22.4.41
id
1..*
SHALL
II
8571
1. SHALL contain exactly one [1..1] @classCode="PROC" (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:8568).
2. SHALL contain exactly one [1..1] @moodCode, which SHALL be selected from
ValueSet Plan of Care moodCode (Act/Encounter/Procedure)
2.16.840.1.113883.11.20.9.23 STATIC 2011-09-30 (CONF:8569).
3. SHALL contain exactly one [1..1] templateId (CONF:8570) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.41" (CONF:10513).
4. SHALL contain at least one [1..*] id (CONF:8571).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 417
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 202: Plan of care activity procedure example
<procedure moodCode="RQO" classCode="PROC">
<templateId root="2.16.840.1.113883.10.20.22.4.41"/>
<!-- ** Plan of Care Activity Procedure template ** -->
<id root="9a6d1bac-17d3-4195-89c4-1121bc809b5a"/>
<code code="23426006" codeSystem="2.16.840.1.113883.6.96"
displayName="Pulmonary function test"/>
<statusCode code="new"/>
<effectiveTime>
<center value="20000421"/>
</effectiveTime>
</procedure>
6.50 Plan of Care Activity Substance Administration
[substanceAdministration: templateId
2.16.840.1.113883.10.20.22.4.42(open)]
Table 228: Plan of Care Activity Substance Administration Contexts
Used By:
Contains Entries:
Plan of Care Section
This is the template for the Plan of Care Activity Substance Administration
Table 229: Plan of Care Activity Substance Administration Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
substanceAdministration[templateId/@root = '2.16.840.1.113883.10.20.22.4.42']
@classCode
1..1
SHALL
8572
2.16.840.1.113883.5.6
(HL7ActClass) = SBADM
@moodCode
1..1
SHALL
8573
2.16.840.1.113883.11.20.9.24
(Plan of Care moodCode
(SubstanceAdministration/Supply))
templateId
1..1
SHALL
SET<II>
8574
@root
1..1
SHALL
10514
2.16.840.1.113883.10.20.22.4.42
id
1..*
SHALL
II
8575
1. SHALL contain exactly one [1..1] @classCode="SBADM" (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:8572).
2. SHALL contain exactly one [1..1] @moodCode, which SHALL be selected from
ValueSet Plan of Care moodCode (SubstanceAdministration/Supply)
2.16.840.1.113883.11.20.9.24 STATIC 2011-09-30 (CONF:8573).
3. SHALL contain exactly one [1..1] templateId (CONF:8574) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.42" (CONF:10514).
4. SHALL contain at least one [1..*] id (CONF:8575).

Page 418 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 230: Plan of Care moodCode (SubstanceAdministration/Supply) Value Set
Value Set: Plan of Care moodCode (SubstanceAdministration/Supply)
2.16.840.1.113883.11.20.9.24 STATIC 2011-09-30
Code System(s):
HL7 ActMood 2.16.840.1.113883.5.1001
Code
Code System
Print Name
INT
ActMood
Intent
PRMS
ActMood
Promise
PRP
ActMood
Proposal
RQO
ActMood
Request
Figure 203: Plan of care activity substance administration example
<substanceAdministration moodCode="RQO" classCode="SBADM">
<templateId root="2.16.840.1.113883.10.20.22.4.42"/>
<!-- ** Plan of Care Activity Substance Administration template **-->
<id root="9a6d1bac-17d3-4195-89c4-1121bc809b5b"/>
<consumable>
<manufacturedProduct>
<manufacturedLabeledDrug .../>
</manufacturedProduct>
</consumable>
</substanceAdministration>
6.51 Plan of Care Activity Supply
[supply: templateId 2.16.840.1.113883.10.20.22.4.43(open)]
Table 231: Plan of Care Activity Supply Contexts
Used By:
Contains Entries:
Plan of Care Section
This is the template for the Plan of Care Activity Supply.

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 419
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 232: Plan of Care Activity Supply Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
supply[templateId/@root = '2.16.840.1.113883.10.20.22.4.43']
@classCode
1..1
SHALL
8577
2.16.840.1.113883.5.6 (HL7ActClass) =
SPLY
@moodCode
1..1
SHALL
8578
2.16.840.1.113883.11.20.9.24 (Plan of
Care moodCode
(SubstanceAdministration/Supply))
templateId
1..1
SHALL
SET<II>
8579
@root
1..1
SHALL
10515
2.16.840.1.113883.10.20.22.4.43
id
1..*
SHALL
II
8580
1. SHALL contain exactly one [1..1] @classCode="SPLY" (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:8577).
2. SHALL contain exactly one [1..1] @moodCode, which SHALL be selected from
ValueSet Plan of Care moodCode (SubstanceAdministration/Supply)
2.16.840.1.113883.11.20.9.24 STATIC 2011-09-30 (CONF:8578).
3. SHALL contain exactly one [1..1] templateId (CONF:8579) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.43" (CONF:10515).
4. SHALL contain at least one [1..*] id (CONF:8580).
Figure 204: Plan of care activity supply example
<supply moodCode="INT" classCode="SPLY">
<templateId root="2.16.840.1.113883.10.20.22.4.43"/>
<!-- ** Plan of Care Activity Supply ** -->
<id root="9a6d1bac-17d3-4195-89c4-1121bc809b5d"/>
<code .../>
</supply>
6.52 Policy Activity
[act: templateId 2.16.840.1.113883.10.20.22.4.61 (closed)]
Table 233: Policy Activity Contexts
Used By:
Contains Entries:
Coverage Activity (required)
A policy activity represents the policy or program providing the coverage. The
person for whom payment is being provided (i.e., the patient) is the covered
party. The subscriber of the policy or program is represented as a participant
that is the holder the coverage. The payer is represented as the performer of the
policy activity.

Page 420 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 234: Policy Activity Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
Green Policy
Activity
act[templateId/@root = '2.16.840.1.113883.10.20.22.4.61']
@classCode
1..1
SHALL
8898
2.16.840.1.113883.5.6
(HL7ActClass) = ACT
@moodCode
1..1
SHALL
8899
2.16.840.1.113883.5.1
001 (ActMood) = EVN
templateId
1..1
SHALL
SET<
II>
8900
@root
1..1
SHALL
10516
2.16.840.1.113883.10.
20.22.4.61
resultId
id
1..*
SHALL
8901
code
0..1
SHOULD
CE
8903
@code
0..1
SHOULD
15993
2.16.840.1.113883.3.8
8.12.3221.5.2 (Health
Insurance Type Value
Set)
health
InsuranceType
code
0..1
SHOULD
8904
2.16.840.1.113883.3.8
8.12.3221.5.2 (Health
Insurance Type Value
Set)
statusCode
1..1
SHALL
8902
2.16.840.1.113883.5.1
4 (ActStatus) =
completed
performer
1..1
SHALL
8906
@typeCode
1..1
SHALL
8907
2.16.840.1.113883.5.9
0
(HL7ParticipationType)
= PRF
templateId
1..1
SHALL
16808
@root
1..1
SHALL
16809
2.16.840.1.113883.10.
20.22.4.87
payer
assignedEntity
1..1
SHALL
8908
healthPlan
Insurance
Information
SourceId
id
1..*
SHALL
8909
code
0..1
SHOULD
8914
@code
0..1
SHALL
15992
2.16.840.1.113883.1.1
1.10416
(HL7FinanciallyRespo
nsiblePartyType)
healthPlan
Insurance
Information
SourceAddress
addr
0..1
MAY
SET<
AD>
8910

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 421
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
healthPlan
Insurance
Information
SourcePhone
EmailURL
telecom
0..1
MAY
SET<
TEL>
8911
representedOr
ganization
0..1
SHOULD
8912
healthPlan
Insurance
Information
SourceName
name
0..1
SHOULD
PN
8913
guarantorInfor
mation
performer
0..1
SHOULD
8961
2.16.840.1.113883.5.9
0
(HL7ParticipationType)
= PRF
templateId
1..1
SHALL
16810
@root
1..1
SHALL
16811
2.16.840.1.113883.10.
20.22.4.88
effectiveDateOf
Financial
Responsibility
time
0..1
SHOULD
IVL<
TS>
8963
assignedEntity
1..1
SHALL
8962
code
1..1
SHALL
8968
@code
1..1
SHALL
16096
2.16.840.1.113883.5.1
11 (RoleCode) = GUAR
financial
Responsibility
PartyAddress
addr
0..1
SHOULD
SET<
AD>
8964
financial
Responsibility
PartyPhone
EmailURL
telecom
0..1
SHOULD
SET<
TEL>
8965
participant
1..1
SHALL
8916
member
Information
@typeCode
1..1
SHALL
8917
2.16.840.1.113883.5.9
0
(HL7ParticipationType)
= COV
templateId
1..1
SHALL
16812
@root
1..1
SHALL
16814
2.16.840.1.113883.10.
20.22.4.89
healthPlan
CoverageDates
time
0..1
SHOULD
IVL<
TS>
8918
low
0..1
SHOULD
TS
8919
high
0..1
SHOULD
TS
8920
patient
participantRole
1..1
SHALL
8921

Page 422 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
memberId
id
1..*
SHALL
8922
code
1..1
SHALL
8923
@code
0..1
SHOULD
16078
2.16.840.1.113883.1.1
1.18877 (Coverage
Role Type Value Set)
patient
RelationshipTo
Subscriber
code
1..1
SHALL
CE
8924
2.16.840.1.113883.1.1
1.18877 (Coverage
Role Type Value Set)
patientAddress
addr
0..1
SHOULD
SET<
AD>
8956
playingEntity
0..1
SHOULD
8932
patientName
name
1..1
SHALL
8930
participant
0..1
SHOULD
8934
@typeCode
1..1
SHALL
8935
2.16.840.1.113883.5.9
0
(HL7ParticipationType)
= HLD
templateId
1..1
SHALL
16813
@root
1..1
SHALL
16815
2.16.840.1.113883.10.
20.22.4.90
time
0..1
MAY
IVL<
TS>
8938
subscriber
Information
participantRole
1..1
SHALL
8936
subscriberId
id
1..*
SHALL
8937
subscriber
Address
addr
0..1
SHOULD
SET<
AD>
8925
healthPlan
entryRelations
hip
1..*
SHALL
8939
@typeCode
1..1
SHALL
8940
2.16.840.1.113883.5.1
002
(HL7ActRelationshipTy
pe) = REFR
1. SHALL contain exactly one [1..1] @classCode="ACT" Act (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:8898).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001) (CONF:8899).
3. SHALL contain exactly one [1..1] templateId (CONF:8900) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.61" (CONF:10516).
This id is a unique identifier for the policy or program providing the coverage
4. SHALL contain at least one [1..*] id (CONF:8901).
5. SHOULD contain zero or one [0..1] code with @xsi:type="CE" (CONF:8903).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 423
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
a. The code, if present, SHOULD contain zero or one [0..1] @code
(ValueSet: Health Insurance Type Value Set
2.16.840.1.113883.3.88.12.3221.5.2 DYNAMIC) (CONF:15993).
b. The code, if present, SHOULD contain zero or one [0..1] code, which
SHOULD be selected from ValueSet Health Insurance Type Value
Set 2.16.840.1.113883.3.88.12.3221.5.2 DYNAMIC (CONF:8904).
6. SHALL contain exactly one [1..1] statusCode="completed" Completed
(CodeSystem: ActStatus 2.16.840.1.113883.5.14) (CONF:8902).
This performer represents the Payer.
7. SHALL contain exactly one [1..1] performer (CONF:8906) such that it
a. SHALL contain exactly one [1..1] @typeCode="PRF" Performer
(CodeSystem: HL7ParticipationType 2.16.840.1.113883.5.90)
(CONF:8907).
b. SHALL contain exactly one [1..1] templateId (CONF:16808) such that
it
i. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.87" Payer
Performer (CONF:16809).
c. SHALL contain exactly one [1..1] assignedEntity (CONF:8908).
i. This assignedEntity SHALL contain at least one [1..*] id
(CONF:8909).
ii. This assignedEntity SHOULD contain zero or one [0..1] code
(CONF:8914).
1. The code, if present, SHALL contain zero or one [0..1]
@code (ValueSet:
HL7FinanciallyResponsiblePartyType
2.16.840.1.113883.1.11.10416 DYNAMIC)
(CONF:15992).
iii. This assignedEntity MAY contain zero or one [0..1] addr
(CONF:8910).
1. The content of addr SHALL be a conformant US Realm
Address (AD.US.FIELDED)
(2.16.840.1.113883.10.20.22.5.2) (CONF:10481).
iv. This assignedEntity MAY contain zero or one [0..1] telecom
(CONF:8911).
v. This assignedEntity SHOULD contain zero or one [0..1]
representedOrganization (CONF:8912).
1. The representedOrganization, if present, SHOULD
contain zero or one [0..1] name (CONF:8913).
This performer represents the Guarantor.
8. SHOULD contain zero or one [0..1] performer="PRF" Performer (CodeSystem:
HL7ParticipationType 2.16.840.1.113883.5.90) (CONF:8961) such that
it
a. SHALL contain exactly one [1..1] templateId (CONF:16810) such that
it

Page 424 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
i. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.88" Guarantor
Performer (CONF:16811).
b. SHOULD contain zero or one [0..1] time (CONF:8963).
c. SHALL contain exactly one [1..1] assignedEntity (CONF:8962).
i. This assignedEntity SHALL contain exactly one [1..1] code
(CONF:8968).
1. This code SHALL contain exactly one [1..1]
@code="GUAR" Guarantor (CodeSystem: RoleCode
2.16.840.1.113883.5.111) (CONF:16096).
ii. This assignedEntity SHOULD contain zero or one [0..1] addr
(CONF:8964).
1. The content of addr SHALL be a conformant US Realm
Address (AD.US.FIELDED)
(2.16.840.1.113883.10.20.22.5.2) (CONF:10482).
iii. This assignedEntity SHOULD contain zero or one [0..1] telecom
(CONF:8965).
iv. SHOULD include assignedEntity/assignedPerson/name
AND/OR assignedEntity/representedOrganization/name
(CONF:8967).
9. SHALL contain exactly one [1..1] participant (CONF:8916) such that it
a. SHALL contain exactly one [1..1] @typeCode="COV" Coverage target
(CodeSystem: HL7ParticipationType 2.16.840.1.113883.5.90)
(CONF:8917).
b. SHALL contain exactly one [1..1] templateId (CONF:16812) such that
it
i. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.89" Covered Party
Participant (CONF:16814).
c. SHOULD contain zero or one [0..1] time (CONF:8918).
i. The time, if present, SHOULD contain zero or one [0..1] low
(CONF:8919).
ii. The time, if present, SHOULD contain zero or one [0..1] high
(CONF:8920).
d. SHALL contain exactly one [1..1] participantRole (CONF:8921).
i. This participantRole SHALL contain at least one [1..*] id
(CONF:8922).
1. This id is a unique identifier for the covered party
member. Implementers SHOULD use the same GUID for
each instance of a member identifier from the same
health plan (CONF:8984).
ii. This participantRole SHALL contain exactly one [1..1] code
(CONF:8923).
1. This code SHOULD contain zero or one [0..1] @code
(ValueSet: Coverage Role Type Value Set

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 425
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
2.16.840.1.113883.1.11.18877 DYNAMIC)
(CONF:16078).
2. This code SHALL contain exactly one [1..1] code with
@xsi:type="CE", where the @code SHOULD be selected
from ValueSet Coverage Role Type Value Set
2.16.840.1.113883.1.11.18877 DYNAMIC
(CONF:8924).
iii. This participantRole SHOULD contain zero or one [0..1] addr
(CONF:8956).
1. The content of addr SHALL be a conformant US Realm
Address (AD.US.FIELDED)
(2.16.840.1.113883.10.20.22.5.2) (CONF:10484).
iv. This participantRole SHOULD contain zero or one [0..1]
playingEntity (CONF:8932).
1. The playingEntity, if present, SHALL contain exactly
one [1..1] name (CONF:8930).
a. If the member name as recorded by the health
plan differs from the patient name as recorded
in the registration/medication summary (e.g.,
due to marriage or for other reasons), then the
member name SHALL be recorded in the name
element (CONF:8931).
2. If the member date of birth as recorded by the health
plan differs from the patient date of birth as recorded
in the registration/medication summary, then the
member date of birth SHALL be recorded in
sdwg:birthTime. The prefix sdtc: SHALL be bound to
the namespace “urn:hl7-org:sdtc”. The use of the
namespace provides a necessary extension to CDA R2
for the use of the birthTime element (CONF:8933).
When the Subscriber is the patient, the participant element describing the
subscriber SHALL NOT be present. This information will be recorded instead in
the data elements used to record member information.
10. SHOULD contain zero or one [0..1] participant (CONF:8934) such that it
a. SHALL contain exactly one [1..1] @typeCode="HLD" Holder
(CodeSystem: HL7ParticipationType 2.16.840.1.113883.5.90)
(CONF:8935).
b. SHALL contain exactly one [1..1] templateId (CONF:16813) such that
it
i. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.90" Policy Holder
Participant (CONF:16815).
c. MAY contain zero or one [0..1] time (CONF:8938).
d. SHALL contain exactly one [1..1] participantRole (CONF:8936).
i. This participantRole SHALL contain at least one [1..*] id
(CONF:8937).

Page 426 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
1. This id is a unique identifier for the subscriber of the
coverage (CONF:10120).
ii. This participantRole SHOULD contain zero or one [0..1] addr
(CONF:8925).
1. The content of addr SHALL be a conformant US Realm
Address (AD.US.FIELDED)
(2.16.840.1.113883.10.20.22.5.2) (CONF:10483).
11. SHALL contain at least one [1..*] entryRelationship (CONF:8939) such that
it
a. SHALL contain exactly one [1..1] @typeCode="REFR" Refers to
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002) (CONF:8940).
b. The target of a policy activity with
act/entryRelationship/@typeCode="REFR" SHALL be an authorization
activity (templateId 2.16.840.1.113883.10.20.1.19) OR an act, with
act[@classCode="ACT"] and act[@moodCode="DEF"], representing a
description of the coverage plan (CONF:8942).
c. A description of the coverage plan SHALL contain one or more act/id,
to represent the plan identifier, and an act/text with the name of the
plan (CONF:8943).
Table 235: Health Insurance Type Value Set (excerpt)
Value Set: Health Insurance Type Value Set 2.16.840.1.113883.3.88.12.3221.5.2 DYNAMIC
Code System(s):
ASC X12 2.16.840.1.113883.6.255.1336
The full value set is available in HITSP C80 (see HITSP.org).
Code
Code System
Print Name
12
ASC X12
Medicare Secondary Working Aged Beneficiary or
Spouse with Employer Group Health Plan
13
ASC X12
Medicare Secondary End-Stage Renal Disease
Beneficiary in the 12 month coordination period with
an employer’s group health plan
14
ASC X12
Medicare Secondary, No-fault Insurance including Auto
is Primary
…

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 427
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 236: Coverage Type Value Set
Value Set: Coverage Role Type Value Set 2.16.840.1.113883.1.11.18877 DYNAMIC
Code System(s):
RoleCode 2.16.840.1.113883.5.111
Code
Code System
Print Name
FAMDEP
RoleCode
Family dependent
FSTUD
RoleCode
Full-time student
HANDIC
RoleCode
Handicapped dependent
INJ
RoleCode
Injured plaintiff
PSTUD
RoleCode
Part-time student
SELF
RoleCode
Self
SPON
RoleCode
Sponsored dependent
STUD
RoleCode
Student
Table 237: Financially Responsible Party Value Set (excerpt)
Value Set: FinanciallyResponsiblePartyType 2.16.840.1.113883.1.11.10416 DYNAMIC
Code System(s):
RoleCode 2.16.840.1.113883.5.110
http://www.hl7.org/memonly/downloads/v3edition.cfm#V32008
Code
Code System
Print Name
EMP
RoleCode
employee
GUAR
RoleCode
guarantor
INVSBJ
RoleCode
Investigation Subject
COVPTY
RoleCode
Covered party
…
Figure 205: Policy activity example
<act classCode="ACT" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.61"/>
<!-- ******** Policy Activity template ******** -->
<id root="3e676a50-7aac-11db-9fe1-0800200c9a66"/>
<code code="SELF" codeSystemName="HL7RoleClass"
codeSystem="2.16.840.1.113883.5.110">
</code>
<statusCode code="completed"/>

Page 428 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
<!-- Insurance Company Information -->
<performer typeCode="PRF">
<time/>
<assignedEntity>
<id root="2.16.840.1.113883.19"/>
<code code="PAYOR" codeSystem="2.16.840.1.113883.5.111"
codeSystemName="RoleCode"/>
<addr use="WP">
<!-- HP is "primary home" from codeSystem
2.16.840.1.113883.5.1119 -->
<streetAddressLine>123 Insurance Road</streetAddressLine>
<city>Blue Bell</city>
<state>MA</state>
<postalCode>02368</postalCode>
<country>US</country>
<!--US is "United States" from ISO 3166-1 Country Codes:
1.0.3166.1-->
</addr>
<telecom value="tel:(781)555-1515" use="WP"/>
<representedOrganization>
<name>Good Health Insurance</name>
<telecom/>
<addr/>
</representedOrganization>
</assignedEntity>
</performer>
<!-- Guarantor Information The person responsible for the final bill. -
->
<performer typeCode="PRF">
<time/>
<assignedEntity>
<id root="329fcdf0-7ab3-11db-9fe1-0800200c9a66"/>
<code code="GUAR" codeSystem="2.16.840.1.113883.5.110"
codeSystemName="HL7RoleClass"/>
<addr use="HP">
<streetAddressLine>17 Daws Rd.</streetAddressLine>
<city>Blue Bell</city>
<state>MA</state>
<postalCode>02368</postalCode>
<country>US</country>
</addr>
<telecom value="tel:(781)555-1212" use="HP"/>
<assignedPerson>
<name>
<prefix>Mr.</prefix>
<given>Adam</given>
<given>Frankie</given>
<family>Everyman</family>
</name>
</assignedPerson>
</assignedEntity>
</performer>
<participant typeCode="COV">
<time>
<low nullFlavor="UNK"/>
<high nullFlavor="UNK"/>
</time>

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 429
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
<participantRole classCode="PAT">
<id root="14d4a520-7aae-11db-9fe1-0800200c9a66"
extension="1138345"/>
<!-- Health plan ID for patient. -->
<code code="SELF" codeSystem="2.16.840.1.113883.5.111"
displayName="Self"/>
<addr use="HP">
<streetAddressLine>17 Daws Rd.</streetAddressLine>
<city>Blue Bell</city>
<state>MA</state>
<postalCode>02368</postalCode>
<country>US</country>
</addr>
<playingEntity>
<name>
<!-- Name is needed if different than health plan name. -->
<prefix>Mr.</prefix>
<given>Frank</given>
<given>A.</given>
<family>Everyman</family>
</name>
</playingEntity>
</participantRole>
</participant>
<participant typeCode="HLD">
<participantRole>
<id extension="1138345" root="2.16.840.1.113883.19"/>
<addr use="HP">
<streetAddressLine>17 Daws Rd.</streetAddressLine>
<city>Blue Bell</city>
<state>MA</state>
<postalCode>02368</postalCode>
<country>US</country>
</addr>
</participantRole>
</participant>
<entryRelationship typeCode="REFR">
<act classCode="ACT" moodCode="DEF">
<templateId root="2.16.840.1.113883.10.20.1.19"/>
<!-- **** Authorization activity template **** -->
...
</act>
</entryRelationship>
...
</entryRelationship>
</act>

Page 430 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
6.53 Postprocedure Diagnosis
[act: templateId 2.16.840.1.113883.10.20.22.4.51 (open)]
Table 238: Postprocedure Diagnosis Contexts
Used By:
Contains Entries:
Postprocedure Diagnosis Section (optional)
Problem Observation
The Postprocedure Diagnosis entry encodes the diagnosis or diagnoses
discovered or confirmed during the procedure. Often it is the same as the pre-
procedure diagnosis or indication.
Table 239: Postprocedure Diagnosis Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
act[templateId/@root = '2.16.840.1.113883.10.20.22.4.51']
@classCode
1..1
SHALL
8756
ACT
@moodCode
1..1
SHALL
8757
EVN
templateId
1..1
SHALL
16766
@root
1..1
SHALL
16767
2.16.840.1.113883.10.20.22.4.51
code
1..1
SHALL
CE
8758
2.16.840.1.113883.6.1 (LOINC) =
59769-0
entryRelationship
1..*
SHALL
8759
@typeCode
1..1
SHALL
8760
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) = SUBJ
observation
1..1
SHALL
15583
1. SHALL contain exactly one [1..1] @classCode="ACT" (CONF:8756).
2. SHALL contain exactly one [1..1] @moodCode="EVN" (CONF:8757).
3. SHALL contain exactly one [1..1] templateId (CONF:16766) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.51" (CONF:16767).
4. SHALL contain exactly one [1..1] code="59769-0" Postprocedure Diagnosis
with @xsi:type="CE" (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:8758).
5. SHALL contain at least one [1..*] entryRelationship (CONF:8759).
a. Such entryRelationships SHALL contain exactly one [1..1]
@typeCode="SUBJ" Has subject (CodeSystem:
HL7ActRelationshipType 2.16.840.1.113883.5.1002)
(CONF:8760).
b. Such entryRelationships SHALL contain exactly one [1..1] Problem
Observation (templateId:2.16.840.1.113883.10.20.22.4.4)
(CONF:15583).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 431
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 206: Postprocedure diagnosis example
<act moodCode="EVN" classCode="ACT">
<templateId root="2.16.840.1.113883.10.20.22.4.51"/>
<!-- ** Postprocedure Diagnosis Entry ** -->
<code code="59769-0" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="Postprocedure Diagnosis"/>
<entryRelationship typeCode="SUBJ">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.4"/>
<!-- Problem Observation template -->
...
</observation>
</entryRelationship>
</act>
6.54 Precondition for Substance Administration
[precondition: templateId 2.16.840.1.113883.10.20.22.4.25(open)]
Table 240: Precondition for Substance Administration Contexts
Used By:
Contains Entries:
Medication Activity
Immunization Activity
A criterion for administration can be used to record that the medication is to be
administered only when the associated criteria are met.
Table 241: Precondition for Substance Administration Constraints Overview
Name
XPath
Card.
Verb
Data Type
CONF#
Fixed Value
criterion[templateId/@root = '2.16.840.1.113883.10.20.22.4.25']
templateId
1..1
SHALL
SET<II>
7372
@root
1..1
SHALL
10517
2.16.840.1.113883.10.20.22.4.25
code
0..1
SHOULD
16854
text
0..1
MAY
7373
value
0..1
SHOULD
CD
7369
1. SHALL contain exactly one [1..1] templateId (CONF:7372) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.25" (CONF:10517).
2. SHOULD contain zero or one [0..1] code (CONF:16854).
3. MAY contain zero or one [0..1] text (CONF:7373).
4. SHOULD contain zero or one [0..1] value with @xsi:type="CD" (CONF:7369).

Page 432 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 207: Precondition for substance administration example
<precondition typeCode="PRCN">
<templateId root="2.16.840.1.113883.10.20.22.4.25"/>
<criterion>
<code code="ASSERTION"
codeSystem="2.16.840.1.113883.5.4"
codeSystemName="HL7ActCode"/>
<text>...</text>
<value xsi:type="CD" code="56018004"
codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT"
displayName="Wheezing"/>
</criterion>
</precondition>
6.55 Pregnancy Observation
[observation: templateId 2.16.840.1.113883.10.20.15.3.8 (open)]
Table 242: Pregnancy Observation Contexts
Used By:
Contains Entries:
Social History Section (optional)
Estimated Date of Delivery
This clinical statement represents current and/or prior pregnancy dates
enabling investigators to determine if the subject of the case report was
pregnant during the course of a condition.
Table 243: Pregnancy Observation Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF
#
Fixed Value
Green
Pregnancy
Observation
observation[templateId/@root = '2.16.840.1.113883.10.20.15.3.8']
@classCode
1..1
SHALL
451
2.16.840.1.113883.5.6
(HL7ActClass) = OBS
@moodCode
1..1
SHALL
452
2.16.840.1.113883.5.1001
(ActMood) = EVN
templateId
1..1
SHALL
16768
@root
1..1
SHALL
16868
2.16.840.1.113883.10.20.15.3.
8
code
1..1
SHALL
CE
454
2.16.840.1.113883.5.4
(ActCode) = ASSERTION
statusCode
1..1
SHALL
455
2.16.840.1.113883.5.14
(ActStatus) = completed
effectiveTime
0..1
SHOULD
TS or
IVL<
TS>
2018

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 433
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Name
XPath
Card.
Verb
Data
Type
CONF
#
Fixed Value
pregnancy
value
1..1
SHALL
CD
457
2.16.840.1.113883.6.96
(SNOMED-CT) = 77386006
entryRelationship
0..1
MAY
458
@typeCode
1..1
SHALL
459
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) =
REFR
observation
1..1
SHALL
15584
1. SHALL contain exactly one [1..1] @classCode="OBS" Observation
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6 STATIC) (CONF:451).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001 STATIC) (CONF:452).
3. SHALL contain exactly one [1..1] templateId (CONF:16768) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.15.3.8" (CONF:16868).
4. SHALL contain exactly one [1..1] code="ASSERTION" Assertion with
@xsi:type="CE" (CodeSystem: ActCode 2.16.840.1.113883.5.4 STATIC)
(CONF:454).
5. SHALL contain exactly one [1..1] statusCode="completed" Completed
(CodeSystem: ActStatus 2.16.840.1.113883.5.14 STATIC) (CONF:455).
6. SHOULD contain zero or one [0..1] effectiveTime (CONF:2018).
7. SHALL contain exactly one [1..1] value="77386006" Pregnant with
@xsi:type="CD" (CodeSystem: SNOMED-CT 2.16.840.1.113883.6.96 STATIC)
(CONF:457).
8. MAY contain zero or one [0..1] entryRelationship (CONF:458) such that it
a. SHALL contain exactly one [1..1] @typeCode="REFR" Refers to
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002 STATIC) (CONF:459).
b. SHALL contain exactly one [1..1] Estimated Date of Delivery
(templateId:2.16.840.1.113883.10.20.15.3.1) (CONF:15584).

Page 434 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 208: Pregnancy observation example
<observation classCode="OBS" moodCode="EVN">
<!-- Pregnancy observation template -->
<templateId root="2.16.840.1.113883.10.20.15.3.8"/>
<id extension="123456789" root="2.16.840.1.113883.19"/>
<code code="ASSERTION" codeSystem="2.16.840.1.113883.5.4"/>
<statusCode code="completed"/>
<effectiveTime>
<low value="20110410"/>
</effectiveTime>
<value xsi:type="CD" code="77386006"
displayName="pregnant"
codeSystem="2.16.840.1.113883.6.96"/>
<entryRelationship typeCode="REFR">
<!-- Estimated Date of Delivery template -->
<templateId root="2.16.840.1.113883.10.20.15.3.1"/>
...
</entryRelationship>
</observation>
6.56 Preoperative Diagnosis
[act: templateId 2.16.840.1.113883.10.20.22.4.65 (open)]
Table 244: Preoperative Diagnosis Contexts
Used By:
Contains Entries:
Preoperative Diagnosis Section (optional)
Problem Observation
The Preoperative Diagnosis entry encodes the surgical diagnosis or diagnoses
assigned to the patient before the surgical procedure and is the reason for the
surgery. The preoperative diagnosis is, in the opinion of the surgeon, the
diagnosis that will be confirmed during surgery.

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 435
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 245: Preoperative Diagnosis Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
act[templateId/@root = '2.16.840.1.113883.10.20.22.4.65']
@classCode
1..1
SHALL
10090
ACT
@moodCode
1..1
SHALL
10091
EVN
templateId
1..1
SHALL
16770
@root
1..1
SHALL
16771
2.16.840.1.113883.10.20.22.4.65
code
1..1
SHALL
CE
10092
2.16.840.1.113883.6.1 (LOINC) =
10219-4
entryRelationship
1..*
SHALL
10093
@typeCode
1..1
SHALL
10094
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) = SUBJ
observation
1..1
SHALL
15605
1. SHALL contain exactly one [1..1] @classCode="ACT" (CONF:10090).
2. SHALL contain exactly one [1..1] @moodCode="EVN" (CONF:10091).
3. SHALL contain exactly one [1..1] templateId (CONF:16770) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.65" (CONF:16771).
4. SHALL contain exactly one [1..1] code="10219-4" Preoperative Diagnosis
with @xsi:type="CE" (CodeSystem: LOINC 2.16.840.1.113883.6.1)
(CONF:10092).
5. SHALL contain at least one [1..*] entryRelationship (CONF:10093).
a. Such entryRelationships SHALL contain exactly one [1..1]
@typeCode="SUBJ" Has subject (CodeSystem:
HL7ActRelationshipType 2.16.840.1.113883.5.1002)
(CONF:10094).
b. Such entryRelationships SHALL contain exactly one [1..1] Problem
Observation (templateId:2.16.840.1.113883.10.20.22.4.4)
(CONF:15605).

Page 436 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 209: Preoperative diagnosis example
<act moodCode="EVN" classCode="ACT">
<templateId root="2.16.840.1.113883.10.20.22.4.65"/>
<!-- ** Preoperative Diagnosis Entry ** -->
<code code="10219-4" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="Preoperative Diagnosis"/>
<entryRelationship typeCode="SUBJ">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.4"/>
<!-- Problem Observation template -->
...
</observation>
</entryRelationship>
</act>
6.57 Pressure Ulcer Observation
[observation: templateId 2.16.840.1.113883.10.20.22.4.70 (open)]
Table 246: Pressure Ulcer Observation Contexts
Used By:
Contains Entries:
Functional Status Section (optional)
The pressure ulcer observation contains details about the pressure ulcer such
as the stage of the ulcer, location, and dimensions. If the pressure ulcer is a
diagnosis, you may find this on the problem list. An example of how this would
appear is in the Problem Section.
Table 247: Pressure Ulcer Observation Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
observation[templateId/@root = '2.16.840.1.113883.10.20.22.4.70']
@classCode
1..1
SHALL
14383
2.16.840.1.113883.5.6 (HL7ActClass)
= OBS
@moodCode
1..1
SHALL
14384
2.16.840.1.113883.5.1001 (ActMood)
= EVN
@negationInd
0..1
MAY
14385
templateId
1..1
SHALL
14387
@root
1..1
SHALL
14388
2.16.840.1.113883.10.20.22.4.70
id
1..*
SHALL
14389
code
1..1
SHALL
14759
@code
1..1
SHOULD
14760
2.16.840.1.113883.5.4 (ActCode) =
ASSERTION
text
0..1
SHOULD
14391
reference
0..1
SHOULD
14392

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 437
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
@value
1..1
SHALL
15585
statusCode
1..1
SHALL
14394
2.16.840.1.113883.5.14 (ActStatus) =
completed
effectiveTime
1..1
SHALL
14395
value
1..1
SHALL
CD
14396
2.16.840.1.113883.11.20.9.35
(Pressure Ulcer Stage)
@nullFlavor
0..1
MAY
14397
targetSiteCode
1..*
SHOULD
14797
@code
1..1
SHALL
14798
2.16.840.1.113883.11.20.9.36
(Pressure Point )
qualifier
1..1
MAY
14799
name
1..1
SHALL
14800
@code
1..1
MAY
14801
2.16.840.1.113883.6.96 (SNOMED-
CT) = 272741003
value
1..1
SHALL
14802
@code
1..1
SHOULD
14803
2.16.840.1.113883.11.20.9.37
(TargetSite Qualifiers )
entry
Relationship
0..1
SHOULD
14410
@typeCode
1..1
SHALL
14411
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) = COMP
observation
1..1
SHALL
14619
@classCode
1..1
SHALL
14685
2.16.840.1.113883.5.6 (HL7ActClass)
= OBS
@moodCode
1..1
SHALL
14686
2.16.840.1.113883.5.1001 (ActMood)
= EVN
code
1..1
SHALL
14620
@code
1..1
SHALL
14621
2.16.840.1.113883.6.96 (SNOMED-
CT) = 401238003
value
1..1
SHALL
PQ
14622
entry
Relationship
0..1
SHOULD
14601
@typeCode
1..1
SHALL
14602
COMP
observation
1..1
SHALL
14623
@classCode
1..1
SHALL
14687
2.16.840.1.113883.5.6 (HL7ActClass)
= OBS
@moodCode
1..1
SHALL
14688
2.16.840.1.113883.5.1001 (ActMood)
= EVN
code
1..1
SHALL
14624
@code
1..1
SHALL
14625
2.16.840.1.113883.6.96 (SNOMED-
CT) = 401239006
value
1..1
SHALL
PQ
14626
entry
Relationship
0..1
SHOULD
14605

Page 438 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
@typeCode
1..1
SHALL
14606
COMP
observation
1..1
SHALL
14627
@classCode
1..1
SHALL
14689
2.16.840.1.113883.5.6 (HL7ActClass)
= OBS
@moodCode
1..1
SHALL
14690
2.16.840.1.113883.5.1001 (ActMood)
= EVN
code
1..1
SHALL
14628
@code
1..1
SHALL
14629
2.16.840.1.113883.6.96 (SNOMED-
CT) = 425094009
value
1..1
SHALL
PQ
14630
1. SHALL contain exactly one [1..1] @classCode="OBS" (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:14383).
2. SHALL contain exactly one [1..1] @moodCode="EVN" (CodeSystem: ActMood
2.16.840.1.113883.5.1001) (CONF:14384).
3. MAY contain zero or one [0..1] @negationInd (CONF:14385).
a. NegationInd="true" SHALL be used to represent that the problem was
not observed (CONF:14386).
4. SHALL contain exactly one [1..1] templateId (CONF:14387) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.70" (CONF:14388).
5. SHALL contain at least one [1..*] id (CONF:14389).
6. SHALL contain exactly one [1..1] code (CONF:14759).
a. This code SHOULD contain exactly one [1..1] @code="ASSERTION"
Assertion (CodeSystem: ActCode 2.16.840.1.113883.5.4)
(CONF:14760).
7. SHOULD contain zero or one [0..1] text (CONF:14391).
a. The text, if present, SHOULD contain zero or one [0..1] reference
(CONF:14392).
i. The reference, if present, SHALL contain exactly one [1..1]
@value (CONF:15585).
1. This reference/@value SHALL begin with a '#' and
SHALL point to its corresponding narrative (using the
approach defined in CDA Release 2, section 4.3.5.1)
(CONF:15586).
8. SHALL contain exactly one [1..1] statusCode="completed" (CodeSystem:
ActStatus 2.16.840.1.113883.5.14) (CONF:14394).
9. SHALL contain exactly one [1..1] effectiveTime (CONF:14395).
10. SHALL contain exactly one [1..1] value with @xsi:type="CD", where the @code
SHOULD be selected from ValueSet Pressure Ulcer Stage
2.16.840.1.113883.11.20.9.35 (CONF:14396).
a. This value MAY contain zero or one [0..1] @nullFlavor
(CONF:14397).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 439
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
i. If the stage unknown or the SNOMED code is unknown,
@nullFlavor SHOULD be “UNK”. If the code is something other
than SNOMED, @nullFlavor SHOULD be “OTH” and the other
code SHOULD be placed in the translation element
(CONF:14398).
11. SHOULD contain at least one [1..*] targetSiteCode (CONF:14797).
a. Such targetSiteCodes SHALL contain exactly one [1..1] @code, which
SHOULD be selected from ValueSet Pressure Point
2.16.840.1.113883.11.20.9.36 (CONF:14798).
b. Such targetSiteCodes MAY contain exactly one [1..1] qualifier
(CONF:14799).
i. This qualifier SHALL contain exactly one [1..1] name
(CONF:14800).
1. This name MAY contain exactly one [1..1]
@code="272741003" laterality (CodeSystem: SNOMED-
CT 2.16.840.1.113883.6.96) (CONF:14801).
ii. This qualifier SHALL contain exactly one [1..1] value
(CONF:14802).
1. This value SHOULD contain exactly one [1..1] @code,
which SHOULD be selected from ValueSet TargetSite
Qualifiers 2.16.840.1.113883.11.20.9.37
(CONF:14803).
12. SHOULD contain zero or one [0..1] entryRelationship (CONF:14410) such
that it
a. SHALL contain exactly one [1..1] @typeCode="COMP" (CodeSystem:
HL7ActRelationshipType 2.16.840.1.113883.5.1002)
(CONF:14411).
b. SHALL contain exactly one [1..1] observation (CONF:14619).
i. This observation SHALL contain exactly one [1..1]
@classCode="OBS" (CodeSystem: HL7ActClass
2.16.840.1.113883.5.6) (CONF:14685).
ii. This observation SHALL contain exactly one [1..1]
@moodCode="EVN" (CodeSystem: ActMood
2.16.840.1.113883.5.1001) (CONF:14686).
iii. This observation SHALL contain exactly one [1..1] code
(CONF:14620).
1. This code SHALL contain exactly one [1..1]
@code="401238003" Length of Wound (CodeSystem:
SNOMED-CT 2.16.840.1.113883.6.96)
(CONF:14621).
iv. This observation SHALL contain exactly one [1..1] value with
@xsi:type="PQ" (CONF:14622).
13. SHOULD contain zero or one [0..1] entryRelationship (CONF:14601) such
that it
a. SHALL contain exactly one [1..1] @typeCode="COMP" (CONF:14602).
b. SHALL contain exactly one [1..1] observation (CONF:14623).

Page 440 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
i. This observation SHALL contain exactly one [1..1]
@classCode="OBS" (CodeSystem: HL7ActClass
2.16.840.1.113883.5.6) (CONF:14687).
ii. This observation SHALL contain exactly one [1..1]
@moodCode="EVN" (CodeSystem: ActMood
2.16.840.1.113883.5.1001) (CONF:14688).
iii. This observation SHALL contain exactly one [1..1] code
(CONF:14624).
1. This code SHALL contain exactly one [1..1]
@code="401239006" Width of Wound (CodeSystem:
SNOMED-CT 2.16.840.1.113883.6.96)
(CONF:14625).
iv. This observation SHALL contain exactly one [1..1] value with
@xsi:type="PQ" (CONF:14626).
14. SHOULD contain zero or one [0..1] entryRelationship (CONF:14605) such
that it
a. SHALL contain exactly one [1..1] @typeCode="COMP" (CONF:14606).
b. SHALL contain exactly one [1..1] observation (CONF:14627).
i. This observation SHALL contain exactly one [1..1]
@classCode="OBS" (CodeSystem: HL7ActClass
2.16.840.1.113883.5.6) (CONF:14689).
ii. This observation SHALL contain exactly one [1..1]
@moodCode="EVN" (CodeSystem: ActMood
2.16.840.1.113883.5.1001) (CONF:14690).
iii. This observation SHALL contain exactly one [1..1] code
(CONF:14628).
1. This code SHALL contain exactly one [1..1]
@code="425094009" Depth of Wound (CodeSystem:
SNOMED-CT 2.16.840.1.113883.6.96)
(CONF:14629).
iv. This observation SHALL contain exactly one [1..1] value with
@xsi:type="PQ" (CONF:14630).
Table 248 Pressure Ulcer Stage Value Set
Value Set: Pressure Ulcer Stage 2.16.840.1.113883.11.20.9.35 DYNAMIC
Code System: SNOMED CT 2.16.840.1.113883.6.96
Descriptions: This value set enumerates the type of a pressure ulcer.
Code
Code System
Print Name
421076008
SNOMED CT
Pressure Ulcer Stage 1
420324007
SNOMED CT
Pressure Ulcer Stage 2
421927004
SNOMED CT
Pressure Ulcer Stage 3
420597008
SNOMED CT
Pressure Ulcer Stage 4
421594008
SNOMED CT
Nonstageable pressure ulcer

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 441
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
The Pressure Point Value Set contains a list of body structures from the
Pressure Ulcer Prevention Domain Analysis Model (DAM), Informative Ballot
published May 2011 35by HL7 combined with a list of structures suggested in
the DAM currently available on HL7.36 HL7 is consulting with the National Skin
Assessment team to reconcile the DAM. The vocabulary is in review by the
International Health Terminology Standards Development Organisation
(IHTSDO) Nursing Sig for international standardization.
Table 249: Pressure Point Value Set
Value Set: Pressure Point 2.16.840.1.113883.11.20.9.36 DYNAMIC
Code System: SNOMED CT 2.16.840.1.113883.6.96
Description: This value set represents points on the body that are susceptible to pressure ulcer
development
Code (CID)
Code System
Print Name
79951008
SNOMED CT
skin of occipital region (body structure)
23747009
SNOMED CT
skin structure of chin (body structure)
76552005
SNOMED CT
skin structure of shoulder (body structure)
45980000
SNOMED CT
skin structure of scapular region of back (body structure)
74757004
SNOMED CT
skin structure of elbow (body structure)
51027004
SNOMED CT
skin structure of sacral region (body structure)
304037003
SNOMED CT
thoracic region back structure (body structure)
286591006
SNOMED CT
skin of lumbar region (body structure)
49812005
SNOMED CT
skin structure of hip (body structure)
29850006
SNOMED CT
iliac crest structure (body structure)*
22180002
SNOMED CT
skin structure of buttock (body structure)
63464009
SNOMED CT
skin structure of knee (body structure)
84607009
SNOMED CT
skin structure of heel (body structure)
67269001
SNOMED CT
skin structure of ankle (body structure)
50938007
SNOMED CT
skin structure of sacrococcygeal region (body structure)
181512003
SNOMED CT
skin of dorsal region (body structure)
1902009
SNOMED CT
skin structure of ear (body structure)
36141000
SNOMED CT
skin structure of cheek (body structure)
113179006
SNOMED CT
skin structure of nose (body structure)
6141000
SNOMED CT
skin structure of cheek (body structure)
113179006
SNOMED CT
skin structure of nose (body structure)
1797002
SNOMED CT
structure of anterior naris (body structure)
…
*mapped to parent and not to “posterior” iliac crest structure
35
http://wiki.hl7.org/images/b/be/PressureUlcerPreventionDomainAnalysisModel_May2011.pdf
Accessed May 2, 2012
36 Domain Analysis Model, HL7 http://pressureulcerpreventionmodel.com/DAM20110325/
Accessed May 2, 2012.

Page 442 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 250: Target Site Qualifiers Value Set
Value Set: TargetSite Qualifiers 2.16.840.1.113883.11.20.9.37 DYNAMIC
Code System: SNOMED CT 2.16.840.1.113883.6.96
Code
Code System
Print Name
255549009
SNOMED CT
anterior
7771000
SNOMED CT
left
255561001
SNOMED CT
medial
255551008
SNOMED CT
posterior
24028007
SNOMED CT
right

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 443
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 210: Pressure ulcer observation example
<entry typeCode="DRIV">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.70"/>
<!-- Pressure Ulcer Observation in Plan of Care template -->
<id root="e2292075-9183-4a25-b8c3-df8521130443"/>
<code code="ASSERTION" codeSystem="2.16.840.1.113883.5.4"/>
<statusCode code="completed"/>
<effectiveTime value="20120328"/>
<value xsi:type="CD" code="421927004"
codeSystem="2.16.840.1.113883.6.96"
displayName="Pressure ulcer stage 3"/>
<targetSiteCode code="76552005" codeSystem="2.16.840.1.113883.6.96"
displayName="skin structure of shoulder"/>
<qualifier>
<name code="272741003" displayName="Laterality"/>
<value code="7771000" displayName="Left"/>
</qualifier>
<entryRelationship typeCode="COMP">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.70"/>
<id root="737b4094-ebb1-41ed-8fcd-f3c53f649e3b"/>
<code code="401239006" codeSystem="2.16.840.1.113883.6.96"
displayName="Width of Wound"/>
<statusCode code="completed"/>
<value xsi:type="PQ" value="1" unit="[in_i]"/>
</observation>
</entryRelationship>
<entryRelationship typeCode="COMP">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.70"/>
<id root="737b4094-ebb1-41ed-8fcd-f3c53f649e3b"/>
<code code=" 401238003 " codeSystem="2.16.840.1.113883.6.96"
displayName="Length of Wound"/>
<statusCode code="completed"/>
<value xsi:type="PQ" value="2" unit="[in_i]"/>
</observation>
</entryRelationship>
<entryRelationship typeCode="COMP">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.70"/>
<id root="737b4094-ebb1-41ed-8fcd-f3c53f649e3b"/>
<code code="425094009" codeSystem="2.16.840.1.113883.6.96"
displayName="Depth of Wound"/>
<statusCode code="completed"/>
<value xsi:type="PQ" value="0.5" unit="[in_i]"/>
</observation>
</entryRelationship>

Page 444 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
6.58 Problem Concern Act (Condition)
[act: templateId 2.16.840.1.113883.10.20.22.4.3(open)]
Table 251: Problem Concern Act (Condition) Contexts
Used By:
Contains Entries:
Problem Section (entries optional)
Problem Section (entries required)
Problem Observation
Observations of problems or other clinical statements captured at a point in
time are wrapped in a "Concern" act, which represents the ongoing process
tracked over time. This allows for binding related observations of problems. For
example, the observation of "Acute MI" in 2004 can be related to the observation
of "History of MI" in 2006 because they are the same concern. The conformance
statements in this section define an outer "problem act" (representing the
"Concern") that can contain a nested "problem observation" or other nested
clinical statements.
Table 252: Problem Concern Act (Condition) Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
act[templateId/@root = '2.16.840.1.113883.10.20.22.4.3']
@classCode
1..1
SHALL
9024
2.16.840.1.113883.5.6
(HL7ActClass) = ACT
@moodCode
1..1
SHALL
9025
2.16.840.1.113883.5.1001
(ActMood) = EVN
id
1..*
SHALL
II
9026
code
1..1
SHALL
CD
9027
@code
1..1
SHALL
9440
2.16.840.1.113883.5.6
(HL7ActClass) = CONC
statusCode
1..1
SHALL
CS
9029
2.16.840.1.113883.11.20.9.19
(ProblemAct statusCode)
effectiveTime
1..1
SHALL
TS or
IVL<TS>
9030
low
1..1
SHALL
TS
9032
high
0..1
SHOULD
TS
9033
entryRelationship
1..*
SHALL
9034
@typeCode
1..1
SHALL
9035
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) =
SUBJ
observation
1..1
SHALL
15980
1. SHALL contain exactly one [1..1] @classCode="ACT" Act (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:9024).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001) (CONF:9025).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 445
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
3. SHALL contain exactly one [1..1] templateId (CONF:16772) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.3" (CONF:16773).
4. SHALL contain at least one [1..*] id (CONF:9026).
5. SHALL contain exactly one [1..1] code (CONF:9027).
a. This code SHALL contain exactly one [1..1] @code="CONC" Concern
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6) (CONF:9440).
6. SHALL contain exactly one [1..1] statusCode, where the @code SHALL be
selected from ValueSet ProblemAct statusCode
2.16.840.1.113883.11.20.9.19 STATIC 2011-09-09 (CONF:9029).
The effectiveTime element records the starting and ending times during which the
concern was active on the Problem List.
7. SHALL contain exactly one [1..1] effectiveTime (CONF:9030).
a. This effectiveTime SHALL contain exactly one [1..1] low (CONF:9032).
b. This effectiveTime SHOULD contain zero or one [0..1] high
(CONF:9033).
8. SHALL contain at least one [1..*] entryRelationship (CONF:9034) such that
it
a. SHALL contain exactly one [1..1] @typeCode="SUBJ" Has subject
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002) (CONF:9035).
b. SHALL contain exactly one [1..1] Problem Observation
(2.16.840.1.113883.10.20.22.4.4) (CONF:15980).
Figure 211: Problem concern act (condition) example
<entry typeCode="DRIV">
<act classCode="ACT" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.3"/>
<id root="36e3e930-7b14-11db-9fe1-0800200c9a66"/>
<code code="CONC" codeSystem="2.16.840.1.113883.5.6"/>
<statusCode code="active"/>
<effectiveTime>
<low value="20090902"/>
</effectiveTime>
<entryRelationship typeCode="SUBJ">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.4"/>
<!-- Problem Observation template-->
...
</observation>
</act>
</entry>

Page 446 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
6.59 Problem Observation
[Observation: templateId 2.16.840.1.113883.10.20.22.4.4(open)]
Table 253: Problem Observation Contexts
Used By:
Contains Entries:
Hospital Discharge Diagnosis
Hospital Admission Diagnosis
Procedure Findings Section
Postprocedure Diagnosis
History of Past Illness Section
Complications Section
Problem Concern Act (Condition)
Functional Status Section
Preoperative Diagnosis
Age Observation
Health Status Observation
Problem Status
A problem is a clinical statement that a clinician has noted. In health care it is a
condition that requires monitoring or diagnostic, therapeutic, or educational
action. It also refers to any unmet or partially met basic human need.
A Problem Observation is required to be wrapped in an act wrapper in locations
such as the Problem Section, Allergies Section, and Hospital Discharge
Diagnosis Section, where the type of problem needs to be identified or the
condition tracked.
A Problem Observation can be a valid "standalone" template instance in cases
where a simple problem observation is to be sent.
The negationInd attribute, if true, specifies that the problem indicated was
observed to not have occurred (which is subtly but importantly different from
having not been observed). NegationInd='true' is an acceptable way to make a
clinical assertion that something did not occur, for example, "no diabetes".
Table 254: Problem Observation Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
Green
Problem
Observation
observation[templateId/@root = '2.16.840.1.113883.10.20.22.4.4']
@classCode
1..1
SHALL
9041
2.16.840.1.113883.5.6
(HL7ActClass) = OBS
@moodCode
1..1
SHALL
9042
2.16.840.1.113883.5.1001
(ActMood) = EVN
@negationInd
0..1
MAY
10139
templateId
1..1
SHALL
14926
@root
1..1
SHALL
14927
2.16.840.1.113883.10.20.
22.4.4
id
1..*
SHALL
9043
problem
code
1..1
SHALL
9045
2.16.840.1.113883.3.88.1

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 447
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
Type
2.3221.7.2 (Problem Type)
problem
Name
text
0..1
SHOULD
9185
reference
0..1
SHOULD
15587
@value
1..1
SHALL
15588
statusCode
1..1
SHALL
9049
2.16.840.1.113883.5.14
(ActStatus) = completed
problem
Date
effectiveTime
0..1
SHOULD
TS or
IVL<T
S>
9050
low
1..1
SHALL
15603
high
0..1
SHOULD
15604
problem
Code
value
1..1
SHALL
CD
9058
2.16.840.1.113883.3.88.1
2.3221.7.4 (Problem)
@nullFlavor
0..1
MAY
10141
translation
0..*
MAY
16749
@code
0..1
MAY
16750
2.16.840.1.113883.6.3
(ICD10)
ageAtOnset
entryRelationship
0..1
MAY
9059
@typeCode
1..1
SHALL
9060
2.16.840.1.113883.5.1002
(HL7ActRelationshipType)
= SUBJ
@inversionInd
1..1
SHALL
9069
true
observation
1..1
SHALL
15590
problem
Status
entryRelationship
0..1
MAY
9063
@typeCode
1..1
SHALL
9068
2.16.840.1.113883.5.1002
(HL7ActRelationshipType)
= REFR
observation
1..1
SHALL
15591
entryRelationship
0..1
MAY
9067
@typeCode
1..1
SHALL
9064
2.16.840.1.113883.5.1002
(HL7ActRelationshipType)
= REFR
observation
1..1
SHALL
15592
1. SHALL contain exactly one [1..1] @classCode="OBS" Observation
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6) (CONF:9041).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001 STATIC) (CONF:9042).
3. MAY contain zero or one [0..1] @negationInd (CONF:10139).
a. Use negationInd="true" to indicate that the problem was not observed
(CONF:16880).
4. SHALL contain exactly one [1..1] templateId (CONF:14926) such that it

Page 448 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.4" (CONF:14927).
5. SHALL contain at least one [1..*] id (CONF:9043).
6. SHALL contain exactly one [1..1] code, which SHOULD be selected from
ValueSet Problem Type 2.16.840.1.113883.3.88.12.3221.7.2 STATIC
2012-06-01 (CONF:9045).
7. SHOULD contain zero or one [0..1] text (CONF:9185).
a. The text, if present, SHOULD contain zero or one [0..1] reference
(CONF:15587).
i. The reference, if present, SHALL contain exactly one [1..1]
@value (CONF:15588).
1. This reference/@value SHALL begin with a '#' and SHALL
point to its corresponding narrative (using the
approach defined in CDA Release 2, section 4.3.5.1)
(CONF:15589).
8. SHALL contain exactly one [1..1] statusCode="completed" Completed
(CodeSystem: ActStatus 2.16.840.1.113883.5.14 STATIC) (CONF:9049).
9. SHOULD contain zero or one [0..1] effectiveTime (CONF:9050).
a. The effectiveTime, if present, SHALL contain exactly one [1..1] low
(CONF:15603).
i. This field represents the onset date (CONF:16882).
b. The effectiveTime, if present, SHOULD contain zero or one [0..1] high
(CONF:15604).
i. This field represents the resolution date (CONF:16883).
c. If the problem is known to be resolved, but the date of resolution is
not known, then the high element SHALL be present, and the
nullFlavor attribute SHALL be set to 'UNK'. Therefore, the existence of
an high element within a problem does indicate that the problem has
been resolved (CONF:16881).
10. SHALL contain exactly one [1..1] value with @xsi:type="CD", where the @code
SHOULD be selected from ValueSet Problem
2.16.840.1.113883.3.88.12.3221.7.4 DYNAMIC (CONF:9058).
a. This value MAY contain zero or one [0..1] @nullFlavor
(CONF:10141).
i. If the diagnosis is unknown or the SNOMED code is
unknown, @nullFlavor SHOULD be “UNK”. If the code is
something other than SNOMED, @nullFlavor SHOULD be
“OTH” and the other code SHOULD be placed in the translation
element (CONF:10142).
b. This value MAY contain zero or more [0..*] translation
(CONF:16749).
i. The translation, if present, MAY contain zero or one [0..1]
@code (CodeSystem: ICD10 2.16.840.1.113883.6.3)
(CONF:16750).
11. MAY contain zero or one [0..1] entryRelationship (CONF:9059) such that it

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 449
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
a. SHALL contain exactly one [1..1] @typeCode="SUBJ" Has subject
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002 STATIC) (CONF:9060).
b. SHALL contain exactly one [1..1] @inversionInd="true" True
(CONF:9069).
c. SHALL contain exactly one [1..1] Age Observation
(templateId:2.16.840.1.113883.10.20.22.4.31) (CONF:15590).
12. MAY contain zero or one [0..1] entryRelationship (CONF:9063) such that it
a. SHALL contain exactly one [1..1] @typeCode="REFR" Refers to
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002 STATIC) (CONF:9068).
b. SHALL contain exactly one [1..1] Problem Status
(templateId:2.16.840.1.113883.10.20.22.4.6) (CONF:15591).
13. MAY contain zero or one [0..1] entryRelationship (CONF:9067) such that it
a. SHALL contain exactly one [1..1] @typeCode="REFR" Refers to
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002 STATIC) (CONF:9064).
b. SHALL contain exactly one [1..1] Health Status Observation
(templateId:2.16.840.1.113883.10.20.22.4.5) (CONF:15592).
Figure 212: Problem observation example
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.4"/>
<!-- Problem Observation template -->
<id root="d11275e7-67ae-11db-bd13-0800200c9a66"/>
<code code="409586006"
codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT"
displayName="Complaint"/>
<text>
...
</text>
<statusCode code="completed"/>
<effectiveTime>
<low value="1950"/>
</effectiveTime>
<value xsi:type="CD" code="195967001"
codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT"
displayName="Asthma"/>
<entryRelationship typeCode="SUBJ" inversionInd="true">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.31"/>
<!-- Age observation template -->
...
</observation>
</entryRelationship>

Page 450 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
<entryRelationship typeCode="REFR" inversionInd="true">
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.5"/>
<!-- Health status observation template -->
...
</observation>
</entryRelationship>
</observation>
Figure 213: Problem observation with specific problem not observed
<observation classCode="OBS" moodCode="EVN" nullFlavor="NI">
<code code="ASSERTION"
codeSystem="2.16.840.1.113883.5.4"
codeSystemName="HL7ActCode" />
<text> No known problems</text>
<statusCode code="completed" />
<value xsi:type="CD" code="195967001"
codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT"
displayName="Asthma" />
</observation>
Figure 214: Problem observation for no known problems
<observation classCode="OBS" moodCode="EVN" negationInd="true">
<!-- Problem Observation template -->
<templateId root="2.16.840.1.113883.10.20.22.4.4" />
<code code="ASSERTION"
codeSystem="2.16.840.1.113883.5.4"
codeSystemName="HL7ActCode" />
<statusCode code="completed" />
<value xsi:type="CD" code="55607006"
codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT"
displayName="Problem" />
</observation>
Figure 215: NullFlavor example
<value nullFlavor="OTH">
<translation code="1234"
displayName="Example"
codeSystem="2.16.840.1.113883.19.5"
codeSystemName="Non-SNOMED"/>
</value>

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 451
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
6.60 Problem Status
[observation: templateId 2.16.840.1.113883.10.20.22.4.6(open)]
Table 255: Problem Status Contexts
Used By:
Contains Entries:
Problem Observation
The Problem Status records whether the indicated problem is active, inactive, or
resolved.
Table 256: Problem Status Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
observation[templateId/@root = '2.16.840.1.113883.10.20.22.4.6']
@classCode
1..1
SHALL
7357
2.16.840.1.113883.5.6 (HL7ActClass)
= OBS
@moodCode
1..1
SHALL
7358
2.16.840.1.113883.5.1001 (ActMood)
= EVN
templateId
1..1
SHALL
SET<II>
7359
@root
1..1
SHALL
10518
2.16.840.1.113883.10.20.22.4.6
code
1..1
SHALL
CE
7361
2.16.840.1.113883.6.1 (LOINC) =
33999-4
text
0..1
SHOULD
7362
reference
0..1
SHOULD
15593
@value
1..1
SHALL
15594
statusCode
1..1
SHALL
7364
2.16.840.1.113883.5.14 (ActStatus) =
completed
value
1..1
SHALL
CD
7365
2.16.840.1.113883.3.88.12.80.68
(HITSPProblemStatus)
1. SHALL contain exactly one [1..1] @classCode="OBS" Observation
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6 STATIC) (CONF:7357).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001 STATIC) (CONF:7358).
3. SHALL contain exactly one [1..1] templateId (CONF:7359) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.6" (CONF:10518).
4. SHALL contain exactly one [1..1] code="33999-4" Status with @xsi:type="CE"
(CodeSystem: LOINC 2.16.840.1.113883.6.1 STATIC) (CONF:7361).
5. SHOULD contain zero or one [0..1] text (CONF:7362).
a. The text, if present, SHOULD contain zero or one [0..1] reference
(CONF:15593).

Page 452 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
i. The reference, if present, SHALL contain exactly one [1..1]
@value (CONF:15594).
1. This reference/@value SHALL begin with a '#' and SHALL
point to its corresponding narrative (using the
approach defined in CDA Release 2, section 4.3.5.1)
(CONF:15595).
6. SHALL contain exactly one [1..1] statusCode="completed" Completed
(CodeSystem: ActStatus 2.16.840.1.113883.5.14 STATIC) (CONF:7364).
7. SHALL contain exactly one [1..1] value with @xsi:type="CD", where the @code
SHALL be selected from ValueSet HITSPProblemStatus
2.16.840.1.113883.3.88.12.80.68 DYNAMIC (CONF:7365).
Figure 216: Problem status example
<observation classCode="OBS" moodCode="EVN">
<!-- Status observation template -->
<templateId root="2.16.840.1.113883.10.20.22.4.6"/>
<code code="33999-4"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="Status"/>
<statusCode code="completed"/>
<value xsi:type="CD"
code="55561003"
codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT"
displayName="Active"/>
</observation>
6.61 Procedure Activity Act
[act: templateId 2.16.840.1.113883.10.20.22.4.12(open)]
Table 257: Procedure Activity Act Contexts
Used By:
Contains Entries:
Procedures Section (entries required)
Procedures Section (entries optional)
Indication
Instructions
Medication Activity
Service Delivery Location
The common notion of "procedure" is broader than that specified by the HL7
Version 3 Reference Information Model (RIM). Therefore procedure templates can
be represented with various RIM classes: act (e.g., dressing change), observation
(e.g., EEG), procedure (e.g. splenectomy).
This clinical statement represents any procedure that cannot be classified as an
observation or a procedure according to the HL7 RIM. Examples of these

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 453
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
procedures are a dressing change, teaching or feeding a patient or providing
comfort measures.

Page 454 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 258: Procedure Activity Act Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
Green
Procedure
Activity
Act
act[templateId/@root = '2.16.840.1.113883.10.20.22.4.12']
@classCode
1..1
SHALL
8289
2.16.840.1.113883.5.6
(HL7ActClass) = ACT
@moodCode
1..1
SHALL
8290
2.16.840.1.113883.11.20.
9.18 (MoodCodeEvnInt)
templateId
1..1
SHALL
SET<II>
8291
@root
1..1
SHALL
10519
2.16.840.1.113883.10.20.
22.4.12
procedure
Id
id
1..*
SHALL
8292
procedure
Type
code
1..1
SHALL
CE
8293
procedure
FreeText
Type
originalText
0..1
SHOULD
8295
reference
0..1
MAY
15596
@value
0..1
MAY
15597
statusCode
1..1
SHALL
8298
2.16.840.1.113883.11.20.
9.22 (ProcedureAct
statusCode)
procedure
DateTime
effectiveTime
0..1
SHOULD
TS or
IVL<TS>
8299
priorityCode
0..1
MAY
8300
2.16.840.1.113883.1.11.1
6866 (ActPriority)
performer
0..*
SHOULD
8301
procedure
Performer
assignedEntity
1..1
SHALL
8302
id
1..*
SHALL
8303
addr
1..1
SHALL
SET<AD
>
8304
telecom
1..1
SHALL
SET<TE
L>
8305
represented
Organization
0..1
SHOULD
8306
id
0..*
SHOULD
8307
name
0..*
MAY
PN
8308
telecom
1..1
SHALL
SET<TE
L>
8310
addr
1..1
SHALL
SET<AD
>
8309
participant
0..*
MAY
8311

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 455
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
@typeCode
1..1
SHALL
8312
2.16.840.1.113883.5.1002
(HL7ActRelationshipType)
= LOC
participantRole
1..1
SHALL
15599
entry
Relationship
0..*
MAY
8314
@typeCode
1..1
SHALL
8315
2.16.840.1.113883.5.1002
(HL7ActRelationshipType)
= COMP
@inversionInd
1..1
SHALL
8316
true
encounter
1..1
SHALL
8317
@classCode
1..1
SHALL
8318
2.16.840.1.113883.5.6
(HL7ActClass) = ENC
@moodCode
1..1
SHALL
8319
2.16.840.1.113883.5.1001
(ActMood) = EVN
id
1..1
SHALL
8320
entry
Relationship
0..1
MAY
8322
@typeCode
1..1
SHALL
8323
2.16.840.1.113883.5.1002
(HL7ActRelationshipType)
= SUBJ
@inversionInd
1..1
SHALL
8324
true
act
1..1
SHALL
15600
entry
Relationship
0..*
MAY
8326
@typeCode
1..1
SHALL
8327
2.16.840.1.113883.5.1002
(HL7ActRelationshipType)
= RSON
observation
1..1
SHALL
15601
entry
Relationship
0..1
MAY
8329
@typeCode
1..1
SHALL
8330
2.16.840.1.113883.5.1002
(HL7ActRelationshipType)
= COMP
substance
Administration
1..1
SHALL
15602
1. SHALL contain exactly one [1..1] @classCode="ACT" Act (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6 STATIC) (CONF:8289).
2. SHALL contain exactly one [1..1] @moodCode, which SHALL be selected from
ValueSet MoodCodeEvnInt 2.16.840.1.113883.11.20.9.18 STATIC 2011-
04-03 (CONF:8290).
3. SHALL contain exactly one [1..1] templateId (CONF:8291) such that it

Page 456 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.12" (CONF:10519).
4. SHALL contain at least one [1..*] id (CONF:8292).
5. SHALL contain exactly one [1..1] code with @xsi:type="CE" (CONF:8293).
a. This code SHOULD contain zero or one [0..1] originalText
(CONF:8295).
i. The originalText, if present, MAY contain zero or one [0..1]
reference (CONF:15596).
1. The reference, if present, MAY contain zero or one [0..1]
@value (CONF:15597).
a. This reference/@value SHALL begin with a '#'
and SHALL point to its corresponding narrative
(using the approach defined in CDA Release 2,
section 4.3.5.1) (CONF:15598).
b. This code in a procedure activity observation SHOULD be selected from
LOINC (CodeSystem: 2.16.840.1.113883.6.1) or SNOMED CT
(CodeSystem: 2.16.840.1.113883.6.96) (CONF:8294).
6. SHALL contain exactly one [1..1] statusCode, which SHALL be selected from
ValueSet ProcedureAct statusCode 2.16.840.1.113883.11.20.9.22
DYNAMIC (CONF:8298).
7. SHOULD contain zero or one [0..1] effectiveTime (CONF:8299).
8. MAY contain zero or one [0..1] priorityCode, which SHALL be selected from
ValueSet ActPriority 2.16.840.1.113883.1.11.16866 DYNAMIC
(CONF:8300).
9. SHOULD contain zero or more [0..*] performer (CONF:8301).
a. The performer, if present, SHALL contain exactly one [1..1]
assignedEntity (CONF:8302).
i. This assignedEntity SHALL contain at least one [1..*] id
(CONF:8303).
ii. This assignedEntity SHALL contain exactly one [1..1] addr
(CONF:8304).
iii. This assignedEntity SHALL contain exactly one [1..1] telecom
(CONF:8305).
iv. This assignedEntity SHOULD contain zero or one [0..1]
representedOrganization (CONF:8306).
1. The representedOrganization, if present, SHOULD
contain zero or more [0..*] id (CONF:8307).
2. The representedOrganization, if present, MAY contain
zero or more [0..*] name (CONF:8308).
3. The representedOrganization, if present, SHALL contain
exactly one [1..1] telecom (CONF:8310).
4. The representedOrganization, if present, SHALL contain
exactly one [1..1] addr (CONF:8309).
10. MAY contain zero or more [0..*] participant (CONF:8311).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 457
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
a. The participant, if present, SHALL contain exactly one [1..1]
@typeCode="LOC" Location (CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002 STATIC) (CONF:8312).
b. The participant, if present, SHALL contain exactly one [1..1] Service
Delivery Location
(templateId:2.16.840.1.113883.10.20.22.4.32) (CONF:15599).
11. MAY contain zero or more [0..*] entryRelationship (CONF:8314).
a. The entryRelationship, if present, SHALL contain exactly one [1..1]
@typeCode="COMP" Has Component (CodeSystem:
HL7ActRelationshipType 2.16.840.1.113883.5.1002 STATIC)
(CONF:8315).
b. The entryRelationship, if present, SHALL contain exactly one [1..1]
@inversionInd="true" true (CONF:8316).
c. The entryRelationship, if present, SHALL contain exactly one [1..1]
encounter (CONF:8317).
i. This encounter SHALL contain exactly one [1..1]
@classCode="ENC" Encounter (CodeSystem: HL7ActClass
2.16.840.1.113883.5.6 STATIC) (CONF:8318).
ii. This encounter SHALL contain exactly one [1..1]
@moodCode="EVN" Event (CodeSystem: ActMood
2.16.840.1.113883.5.1001 STATIC) (CONF:8319).
iii. This encounter SHALL contain exactly one [1..1] id
(CONF:8320).
1. Set the encounter ID to the ID of an encounter in
another section to signify they are the same encounter
(CONF:16849).
12. MAY contain zero or one [0..1] entryRelationship (CONF:8322).
a. The entryRelationship, if present, SHALL contain exactly one [1..1]
@typeCode="SUBJ" Has Subject (CodeSystem:
HL7ActRelationshipType 2.16.840.1.113883.5.1002 STATIC)
(CONF:8323).
b. The entryRelationship, if present, SHALL contain exactly one [1..1]
@inversionInd="true" true (CONF:8324).
c. The entryRelationship, if present, SHALL contain exactly one [1..1]
Instructions
(templateId:2.16.840.1.113883.10.20.22.4.20) (CONF:15600).
13. MAY contain zero or more [0..*] entryRelationship (CONF:8326).
a. The entryRelationship, if present, SHALL contain exactly one [1..1]
@typeCode="RSON" Has Reason (CodeSystem:
HL7ActRelationshipType 2.16.840.1.113883.5.1002 STATIC)
(CONF:8327).
b. The entryRelationship, if present, SHALL contain exactly one [1..1]
Indication (templateId:2.16.840.1.113883.10.20.22.4.19)
(CONF:15601).
14. MAY contain zero or one [0..1] entryRelationship (CONF:8329).
a. The entryRelationship, if present, SHALL contain exactly one [1..1]
@typeCode="COMP" Has Component (CodeSystem:

Page 458 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
HL7ActRelationshipType 2.16.840.1.113883.5.1002 STATIC)
(CONF:8330).
b. The entryRelationship, if present, SHALL contain exactly one [1..1]
Medication Activity
(templateId:2.16.840.1.113883.10.20.22.4.16) (CONF:15602).
Table 259: Procedure Act Status Code Value Set
Value Set: ProcedureAct statusCode 2.16.840.1.113883.11.20.9.22 DYNAMIC
Code System(s):
ActStatus 2.16.840.1.113883.5.14
Description:
A ValueSet of HL7 actStatus codes for use with a procedure activity
Code
Code System
Print Name
completed
ActStatus
Completed
active
ActStatus
Active
aborted
ActStatus
Aborted
cancelled
ActStatus
Cancelled
Table 260: Act Priority Value Set
Value Set: ActPriority 2.16.840.1.113883.1.11.16866 DYNAMIC
Code System(s):
ActPriority 2.16.840.1.113883.5.7
Description:
A code or set of codes (e.g., for routine, emergency,) specifying the urgency under
which the Act happened, can happen, is happening, is intended to happen, or is
requested/demanded to happen.
Code
Code System
Print Name
A
ActPriority
ASAP
CR
ActPriority
Callback results
CS
ActPriority
Callback for scheduling
CSP
ActPriority
Callback placer for scheduling
CSR
ActPriority
Contact recipient for scheduling
EL
ActPriority
Elective
EM
ActPriority
Emergency
P
ActPriority
Preoperative
PRN
ActPriority
As needed
R
ActPriority
Routine
RR
ActPriority
Rush reporting
S
ActPriority
Stat
T
ActPriority
Timing critical
UD
ActPriority
Use as directed
UR
ActPriority
Urgent

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 459
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 217: Procedure activity act example
<act classCode="ACT" moodCode="INT">
<templateId root="2.16.840.1.113883.10.20.22.4.12"/>
<id root="1.2.3.4.5.6.7.8" extension="1234567"/>
<code code="80146002"
codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT"
displayName="Appendectomy">
<originalText>
<reference value="#proc1"/>
</originalText>
</code>
<statusCode code="completed"/>
<effectiveTime value="20110203"/>
<priorityCode code="CR"
codeSystem="2.16.840.1.113883.5.7"
codeSystemName="HL7ActPriority"
displayName="Callback results"/>
<performer>
<assignedEntity>
<id root="1.2.3.4" extension="1234"/>
<addr>
<streetAddressLine>17 Daws Rd.</streetAddressLine>
<city>Blue Bell</city>
<state>MA</state>
<postalCode>02368</postalCode>
<country>US</country>
</addr>
<telecom use="WP" value="(555)555-555-1234"/>
<representedOrganization>
<id root="2.16.840.1.113883.19.5"/>
<name>Good Health Clinic</name>
<telecom nullFlavor="UNK"/>
<addr nullFlavor="UNK"/>
</representedOrganization>
</assignedEntity>
</performer>
<participant typeCode="LOC">
<participantRole classCode="SDLOC">
<templateId root="2.16.840.1.113883.10.20.22.4.32"/>
<!-- Service Delivery Location template -->
...
</participantRole>
</participant>
<entryRelationship typeCode="COMP">
<substanceAdministration classCode="SBADM" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.16"/>
<!-- Medication Activity template -->
...
</substanceAdministration>
</entryRelationship>
</act>

Page 460 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
6.62 Procedure Activity Observation
[observation: templateId 2.16.840.1.113883.10.20.22.4.13(open)]
Table 261: Procedure Activity Observation Contexts
Used By:
Contains Entries:
Procedures Section (entries optional)
Procedures Section (entries required)
Indication
Instructions
Medication Activity
Service Delivery Location
The common notion of "procedure" is broader than that specified by the HL7
Version 3 Reference Information Model (RIM). Therefore procedure templates can
be represented with various RIM classes: act (e.g., dressing change), observation
(e.g., EEG), procedure (e.g. splenectomy).
This clinical statement represents procedures that result in new information
about the patient that cannot be classified as a procedure according to the HL7
RIM. Examples of these procedures are diagnostic imaging procedures, EEGs
and EKGs.
Table 262: Procedure Activity Observation Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
Green
Procedure
Activity
Observation
observation[templateId/@root = '2.16.840.1.113883.10.20.22.4.13']
@classCode
1..1
SHALL
8282
2.16.840.1.113883.5.6
(HL7ActClass) = OBS
@moodCode
1..1
SHALL
8237
2.16.840.1.113883.11.20.9.
18 (MoodCodeEvnInt)
templateId
1..1
SHALL
SET<II
>
8238
@root
1..1
SHALL
10520
2.16.840.1.113883.10.20.22
.4.13
procedureId
id
1..*
SHALL
8239
procedure
Type
code
1..1
SHALL
CE
8240
procedure
FreeText
Type
originalText
0..1
SHOULD
8242
reference
0..1
SHOULD
15901
@value
0..1
SHOULD
15902
statusCode
1..1
SHALL
8245
2.16.840.1.113883.11.20.9.
22 (ProcedureAct
statusCode)
procedure
effectiveTime
0..1
SHOULD
TS or
IVL<T
8246

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 461
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
DateTime
S>
priorityCode
0..1
MAY
8247
2.16.840.1.113883.1.11.168
66 (ActPriority)
value
1..1
SHALL
16846
methodCode
0..1
MAY
SET<
CE>
8248
procedure
BodyType
targetSiteCode
0..*
SHOULD
SET<
CD>
8250
@code
1..1
SHALL
16071
2.16.840.1.113883.3.88.12.
3221.8.9 (Body Site Value
Set)
performer
0..*
SHOULD
8251
procedure
Provider
assignedEntity
1..1
SHALL
8252
id
1..*
SHALL
8253
addr
1..1
SHALL
SET<A
D>
8254
telecom
1..1
SHALL
SET<T
EL>
8255
representedOrg
anization
0..1
SHOULD
8256
id
0..*
SHOULD
8257
name
0..*
MAY
PN
8258
telecom
1..1
SHALL
SET<T
EL>
8260
addr
1..1
SHALL
SET<A
D>
8259
participant
0..*
MAY
8261
@typeCode
1..1
SHALL
8262
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) =
LOC
participantRole
1..1
SHALL
15904
entryRelations
hip
0..*
MAY
8264
@typeCode
1..1
SHALL
8265
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) =
COMP
@inversionInd
1..1
SHALL
8266
true
encounter
1..1
SHALL
8267
@classCode
1..1
SHALL
8268
2.16.840.1.113883.5.6
(HL7ActClass) = ENC
@moodCode
1..1
SHALL
8269
2.16.840.1.113883.5.1001
(ActMood) = EVN

Page 462 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
id
1..1
SHALL
8270
entryRelations
hip
0..1
MAY
8272
@typeCode
1..1
SHALL
8273
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) =
SUBJ
@inversionInd
1..1
SHALL
8274
true
act
1..1
SHALL
15905
entryRelations
hip
0..*
MAY
8276
@typeCode
1..1
SHALL
8277
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) =
RSON
observation
1..1
SHALL
15906
entryRelations
hip
0..1
MAY
8279
@typeCode
1..1
SHALL
8280
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) =
COMP
substanceAdmi
nistration
1..1
SHALL
15907
1. SHALL contain exactly one [1..1] @classCode="OBS" Observation
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6 STATIC) (CONF:8282).
2. SHALL contain exactly one [1..1] @moodCode, which SHALL be selected from
ValueSet MoodCodeEvnInt 2.16.840.1.113883.11.20.9.18 STATIC 2011-
04-03 (CONF:8237).
3. SHALL contain exactly one [1..1] templateId (CONF:8238) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.13" (CONF:10520).
4. SHALL contain at least one [1..*] id (CONF:8239).
5. SHALL contain exactly one [1..1] code with @xsi:type="CE" (CONF:8240).
a. This code SHOULD contain zero or one [0..1] originalText
(CONF:8242).
i. The originalText, if present, SHOULD contain zero or one [0..1]
reference (CONF:15901).
1. The reference, if present, SHOULD contain zero or one
[0..1] @value (CONF:15902).
a. This reference/@value SHALL begin with a '#'
and SHALL point to its corresponding narrative
(using the approach defined in CDA Release 2,
section 4.3.5.1) (CONF:15903).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 463
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
b. This code in a procedure activity SHOULD be selected from LOINC
(CodeSystem: 2.16.840.1.113883.6.1) or SNOMED CT (CodeSystem:
2.16.840.1.113883.6.96), and MAY be selected from CPT-4
(CodeSystem: 2.16.840.1.113883.6.12), ICD9 Procedures
(CodeSystem: 2.16.840.1.113883.6.4) (CONF:8241).
6. SHALL contain exactly one [1..1] statusCode, which SHALL be selected from
ValueSet ProcedureAct statusCode 2.16.840.1.113883.11.20.9.22
DYNAMIC (CONF:8245).
7. SHOULD contain zero or one [0..1] effectiveTime (CONF:8246).
8. MAY contain zero or one [0..1] priorityCode, which SHALL be selected from
ValueSet ActPriority 2.16.840.1.113883.1.11.16866 DYNAMIC
(CONF:8247).
9. SHALL contain exactly one [1..1] value (CONF:16846).
10. MAY contain zero or one [0..1] methodCode (CONF:8248).
a. MethodCode SHALL NOT conflict with the method inherent in
Observation / code (CONF:8249).
11. SHOULD contain zero or more [0..*] targetSiteCode (CONF:8250).
a. The targetSiteCode, if present, SHALL contain exactly one [1..1] @code,
which SHALL be selected from ValueSet Body Site Value Set
2.16.840.1.113883.3.88.12.3221.8.9 DYNAMIC (CONF:16071).
12. SHOULD contain zero or more [0..*] performer (CONF:8251).
a. The performer, if present, SHALL contain exactly one [1..1]
assignedEntity (CONF:8252).
i. This assignedEntity SHALL contain at least one [1..*] id
(CONF:8253).
ii. This assignedEntity SHALL contain exactly one [1..1] addr
(CONF:8254).
iii. This assignedEntity SHALL contain exactly one [1..1] telecom
(CONF:8255).
iv. This assignedEntity SHOULD contain zero or one [0..1]
representedOrganization (CONF:8256).
1. The representedOrganization, if present, SHOULD
contain zero or more [0..*] id (CONF:8257).
2. The representedOrganization, if present, MAY contain
zero or more [0..*] name (CONF:8258).
3. The representedOrganization, if present, SHALL contain
exactly one [1..1] telecom (CONF:8260).
4. The representedOrganization, if present, SHALL contain
exactly one [1..1] addr (CONF:8259).
13. MAY contain zero or more [0..*] participant (CONF:8261).
a. The participant, if present, SHALL contain exactly one [1..1]
@typeCode="LOC" Location (CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002 STATIC) (CONF:8262).
b. The participant, if present, SHALL contain exactly one [1..1] Service
Delivery Location
(templateId:2.16.840.1.113883.10.20.22.4.32) (CONF:15904).

Page 464 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
14. MAY contain zero or more [0..*] entryRelationship (CONF:8264).
a. The entryRelationship, if present, SHALL contain exactly one [1..1]
@typeCode="COMP" Component (CodeSystem:
HL7ActRelationshipType 2.16.840.1.113883.5.1002 STATIC)
(CONF:8265).
b. The entryRelationship, if present, SHALL contain exactly one [1..1]
@inversionInd="true" true (CONF:8266).
c. The entryRelationship, if present, SHALL contain exactly one [1..1]
encounter (CONF:8267).
i. This encounter SHALL contain exactly one [1..1]
@classCode="ENC" Encounter (CodeSystem: HL7ActClass
2.16.840.1.113883.5.6 STATIC) (CONF:8268).
ii. This encounter SHALL contain exactly one [1..1]
@moodCode="EVN" Event (CodeSystem: ActMood
2.16.840.1.113883.5.1001 STATIC) (CONF:8269).
iii. This encounter SHALL contain exactly one [1..1] id
(CONF:8270).
1. Set encounter/id to the id of an encounter in another
section to signify they are the same encounter
(CONF:16847).
15. MAY contain zero or one [0..1] entryRelationship (CONF:8272) such that it
a. SHALL contain exactly one [1..1] @typeCode="SUBJ" Has Subject
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002 STATIC) (CONF:8273).
b. SHALL contain exactly one [1..1] @inversionInd="true" true
(CONF:8274).
c. SHALL contain exactly one [1..1] Instructions
(templateId:2.16.840.1.113883.10.20.22.4.20) (CONF:15905).
16. MAY contain zero or more [0..*] entryRelationship (CONF:8276) such that
it
a. SHALL contain exactly one [1..1] @typeCode="RSON" Has Reason
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002 STATIC) (CONF:8277).
b. SHALL contain exactly one [1..1] Indication
(templateId:2.16.840.1.113883.10.20.22.4.19) (CONF:15906).
17. MAY contain zero or one [0..1] entryRelationship (CONF:8279) such that it
a. SHALL contain exactly one [1..1] @typeCode="COMP" Has Component
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002 STATIC) (CONF:8280).
b. SHALL contain exactly one [1..1] Medication Activity
(templateId:2.16.840.1.113883.10.20.22.4.16) (CONF:15907).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 465
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 218: Procedure activity observation example
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.13"/>
<!-- Procedure Activity Observation -->
<id extension="123456789" root="2.16.840.1.113883.19"/>
<code code="80146002"
codeSystem="2.16.840.1.113883.6.96"
displayName="Appendectomy"
codeSystemName="SNOMED CT">
<originalText>
<reference value="#proc1"/>
</originalText>
</code>
<statusCode code="aborted"
codeSystem="2.16.840.1.113883.5.14"
codeSystemName="HL7ActStatus"/>
<effectiveTime value="20110203"/>
<priorityCode code="CR"
codeSystem="2.16.840.1.113883.5.7"
codeSystemName="HL7ActPriority"
displayName="Callback results"/>
<value xsi:type="CD"/>
<methodCode nullFlavor="UNK"/>
<targetSiteCode code="416949008"
codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT"
displayName="Abdomen and pelvis" />
<performer>
<assignedEntity>
<id root="1.2.3.4" extension="1234"/>
<addr>
<streetAddressLine>17 Daws Rd.</streetAddressLine>
<city>Blue Bell</city>
<state>MA</state>
<postalCode>02368</postalCode>
<country>US</country>
</addr>
<telecom use="WP" value="(555)555-555-1234"/>
<representedOrganization>
<id root="2.16.840.1.113883.19.5"/>
<name>Good Health Clinic</name>
<telecom nullFlavor="UNK"/>
<addr nullFlavor="UNK"/>
</representedOrganization>
</assignedEntity>
</performer>
<entryRelationship typeCode="COMP">
<substanceAdministration classCode="SBADM" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.16"/>
<!-- Medication Activity template -->
...
</substanceAdministration>
</entryRelationship>
</observation>

Page 466 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
6.63 Procedure Activity Procedure
[procedure: templateId 2.16.840.1.113883.10.20.22.4.14(open)]
Table 263: Procedure Activity Procedure Contexts
Used By:
Contains Entries:
Procedures Section (entries optional)
Reaction Observation
Procedures Section (entries required)
Anesthesia Section
Indication
Instructions
Medication Activity
Product Instance
Service Delivery Location
The common notion of "procedure" is broader than that specified by the HL7
Version 3 Reference Information Model (RIM). Therefore procedure templates can
be represented with various RIM classes: act (e.g., dressing change), observation
(e.g., EEG), procedure (e.g. splenectomy).
This clinical statement represents procedures whose immediate and primary
outcome (post-condition) is the alteration of the physical condition of the
patient. Examples of these procedures are an appendectomy, hip replacement
and a creation of a gastrostomy.
Table 264: Procedure Activity Procedure Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF
#
Fixed Value
Green
Procedure
Activity
Procedure
procedure[templateId/@root = '2.16.840.1.113883.10.20.22.4.14']
@classCode
1..1
SHALL
7652
2.16.840.1.113883.5.6
(HL7ActClass) = PROC
@moodCode
1..1
SHALL
7653
2.16.840.1.113883.11.20.
9.18 (MoodCodeEvnInt)
templateId
1..1
SHALL
SET<
II>
7654
@root
1..1
SHALL
10521
2.16.840.1.113883.10.20.
22.4.14
procedure
Id
id
1..*
SHALL
7655
procedure
Type
code
1..1
SHALL
CE
7656
originalText
0..1
SHOULD
7658
reference
0..1
SHOULD
15908
@value
0..1
SHOULD
15909
procedure
FreeText
Type
reference/@value
0..1
SHOULD
7659
statusCode
1..1
SHALL
7661
2.16.840.1.113883.11.20.

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 467
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Name
XPath
Card.
Verb
Data
Type
CONF
#
Fixed Value
9.22 (ProcedureAct
statusCode)
procedure
DateTime
effectiveTime
0..1
SHOULD
TS or
IVL<
TS>
7662
priorityCode
0..1
MAY
7668
2.16.840.1.113883.1.11.1
6866 (ActPriority)
methodCode
0..1
MAY
SET<
CE>
7670
bodySite
targetSiteCode
0..*
SHOULD
7683
@code
1..1
SHALL
16082
2.16.840.1.113883.3.88.1
2.3221.8.9 (Body Site
Value Set)
specimen
0..*
MAY
7697
specimenRole
1..1
SHALL
7704
id
0..*
SHOULD
7716
performer
0..*
SHOULD
7718
procedure
Provider
assignedEntity
1..1
SHALL
7720
id
1..*
SHALL
7722
addr
1..1
SHALL
SET<
AD>
7731
telecom
1..1
SHALL
SET<
TEL>
7732
represented
Organization
0..1
SHOULD
7733
id
0..*
SHOULD
7734
name
0..*
MAY
PN
7735
telecom
1..1
SHALL
SET<
TEL>
7737
addr
1..1
SHALL
SET<
AD>
7736
participant
0..*
MAY
7751
@typeCode
1..1
SHALL
7752
2.16.840.1.113883.5.1002
(HL7ActRelationshipType)
= DEV
participantRole
1..1
SHALL
15911
participant
0..*
MAY
7765
@typeCode
1..1
SHALL
7766
2.16.840.1.113883.5.90
(HL7ParticipationType) =
LOC
participantRole
1..1
SHALL
15912
entryRelationship
0..*
MAY
7768
@typeCode
1..1
SHALL
7769
2.16.840.1.113883.5.1002
(HL7ActRelationshipType)

Page 468 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Name
XPath
Card.
Verb
Data
Type
CONF
#
Fixed Value
= COMP
@inversionInd
1..1
SHALL
8009
true
encounter
1..1
SHALL
7770
@classCode
1..1
SHALL
7771
2.16.840.1.113883.5.6
(HL7ActClass) = ENC
@moodCode
1..1
SHALL
7772
2.16.840.1.113883.5.1001
(ActMood) = EVN
id
1..1
SHALL
7773
entryRelationship
0..1
MAY
7775
@typeCode
1..1
SHALL
7776
2.16.840.1.113883.5.1002
(HL7ActRelationshipType)
= SUBJ
@inversionInd
1..1
SHALL
7777
true
act
1..1
SHALL
15913
entryRelationship
0..*
MAY
7779
@typeCode
1..1
SHALL
7780
2.16.840.1.113883.5.1002
(HL7ActRelationshipType)
= RSON
observation
1..1
SHALL
15914
entryRelationship
0..1
MAY
7886
@typeCode
1..1
SHALL
7887
2.16.840.1.113883.5.1002
(HL7ActRelationshipType)
= COMP
substance
Administration
1..1
SHALL
15915
1. SHALL contain exactly one [1..1] @classCode="PROC" Procedure
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6 STATIC) (CONF:7652).
2. SHALL contain exactly one [1..1] @moodCode, which SHALL be selected from
ValueSet MoodCodeEvnInt 2.16.840.1.113883.11.20.9.18 STATIC 2011-
04-03 (CONF:7653).
3. SHALL contain exactly one [1..1] templateId (CONF:7654) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.14" (CONF:10521).
4. SHALL contain at least one [1..*] id (CONF:7655).
5. SHALL contain exactly one [1..1] code with @xsi:type="CE" (CONF:7656).
a. This code SHOULD contain zero or one [0..1] originalText
(CONF:7658).
i. The originalText, if present, SHOULD contain zero or one [0..1]
reference (CONF:15908).
1. The reference, if present, SHOULD contain zero or one
[0..1] @value (CONF:15909).
a. This reference/@value SHALL begin with a '#'
and SHALL point to its corresponding narrative

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 469
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
(using the approach defined in CDA Release 2,
section 4.3.5.1) (CONF:15910).
ii. The originalText, if present, SHOULD contain zero or one [0..1]
reference/@value (CONF:7659).
b. This code in a procedure activity SHOULD be selected from LOINC
(codeSystem 2.16.840.1.113883.6.1) or SNOMED CT (CodeSystem:
2.16.840.1.113883.6.96), and MAY be selected from CPT-4
(CodeSystem: 2.16.840.1.113883.6.12), ICD9 Procedures
(CodeSystem: 2.16.840.1.113883.6.104), ICD10 Procedure Coding
System (CodeSystem: 2.16.840.1.113883.6.4) (CONF:7657).
6. SHALL contain exactly one [1..1] statusCode, which SHALL be selected from
ValueSet ProcedureAct statusCode 2.16.840.1.113883.11.20.9.22
DYNAMIC (CONF:7661).
7. SHOULD contain zero or one [0..1] effectiveTime (CONF:7662).
8. MAY contain zero or one [0..1] priorityCode, which SHALL be selected from
ValueSet ActPriority 2.16.840.1.113883.1.11.16866 DYNAMIC
(CONF:7668).
9. MAY contain zero or one [0..1] methodCode (CONF:7670).
a. MethodCode SHALL NOT conflict with the method inherent in
Procedure / code (CONF:7890).
10. SHOULD contain zero or more [0..*] targetSiteCode (CONF:7683).
a. The targetSiteCode, if present, SHALL contain exactly one [1..1] @code,
which SHALL be selected from ValueSet Body Site Value Set
2.16.840.1.113883.3.88.12.3221.8.9 DYNAMIC (CONF:16082).
11. MAY contain zero or more [0..*] specimen (CONF:7697).
a. The specimen, if present, SHALL contain exactly one [1..1]
specimenRole (CONF:7704).
i. This specimenRole SHOULD contain zero or more [0..*] id
(CONF:7716).
1. If you want to indicate that the Procedure and the
Results are referring to the same specimen, the
Procedure/specimen/specimenRole/id SHOULD be set
to equal an Organizer/specimen/ specimenRole/id
(CONF:7717).
b. This specimen is for representing specimens obtained from a
procedure (CONF:16842).
12. SHOULD contain zero or more [0..*] performer (CONF:7718) such that it
a. SHALL contain exactly one [1..1] assignedEntity (CONF:7720).
i. This assignedEntity SHALL contain at least one [1..*] id
(CONF:7722).
ii. This assignedEntity SHALL contain exactly one [1..1] addr
(CONF:7731).
iii. This assignedEntity SHALL contain exactly one [1..1] telecom
(CONF:7732).
iv. This assignedEntity SHOULD contain zero or one [0..1]
representedOrganization (CONF:7733).

Page 470 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
1. The representedOrganization, if present, SHOULD
contain zero or more [0..*] id (CONF:7734).
2. The representedOrganization, if present, MAY contain
zero or more [0..*] name (CONF:7735).
3. The representedOrganization, if present, SHALL contain
exactly one [1..1] telecom (CONF:7737).
4. The representedOrganization, if present, SHALL contain
exactly one [1..1] addr (CONF:7736).
13. MAY contain zero or more [0..*] participant (CONF:7751) such that it
a. SHALL contain exactly one [1..1] @typeCode="DEV" Device
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002 STATIC) (CONF:7752).
b. SHALL contain exactly one [1..1] Product Instance
(templateId:2.16.840.1.113883.10.20.22.4.37) (CONF:15911).
14. MAY contain zero or more [0..*] participant (CONF:7765) such that it
a. SHALL contain exactly one [1..1] @typeCode="LOC" Location
(CodeSystem: HL7ParticipationType 2.16.840.1.113883.5.90
STATIC) (CONF:7766).
b. SHALL contain exactly one [1..1] Service Delivery Location
(templateId:2.16.840.1.113883.10.20.22.4.32) (CONF:15912).
15. MAY contain zero or more [0..*] entryRelationship (CONF:7768) such that
it
a. SHALL contain exactly one [1..1] @typeCode="COMP" Has Component
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002 STATIC) (CONF:7769).
b. SHALL contain exactly one [1..1] @inversionInd="true" true
(CONF:8009).
c. SHALL contain exactly one [1..1] encounter (CONF:7770).
i. This encounter SHALL contain exactly one [1..1]
@classCode="ENC" Encounter (CodeSystem: HL7ActClass
2.16.840.1.113883.5.6 STATIC) (CONF:7771).
ii. This encounter SHALL contain exactly one [1..1]
@moodCode="EVN" Event (CodeSystem: ActMood
2.16.840.1.113883.5.1001 STATIC) (CONF:7772).
iii. This encounter SHALL contain exactly one [1..1] id
(CONF:7773).
1. Set the encounter ID to the ID of an encounter in
another section to signify they are the same encounter
(CONF:16843).
16. MAY contain zero or one [0..1] entryRelationship (CONF:7775) such that it
a. SHALL contain exactly one [1..1] @typeCode="SUBJ" Has Subject
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002 STATIC) (CONF:7776).
b. SHALL contain exactly one [1..1] @inversionInd="true" true
(CONF:7777).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 471
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
c. SHALL contain exactly one [1..1] Instructions
(templateId:2.16.840.1.113883.10.20.22.4.20) (CONF:15913).
17. MAY contain zero or more [0..*] entryRelationship (CONF:7779) such that
it
a. SHALL contain exactly one [1..1] @typeCode="RSON" Has Reason
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002 STATIC) (CONF:7780).
b. SHALL contain exactly one [1..1] Indication
(templateId:2.16.840.1.113883.10.20.22.4.19) (CONF:15914).
18. MAY contain zero or one [0..1] entryRelationship (CONF:7886) such that it
a. SHALL contain exactly one [1..1] @typeCode="COMP" Has Component
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002 STATIC) (CONF:7887).
b. SHALL contain exactly one [1..1] Medication Activity
(templateId:2.16.840.1.113883.10.20.22.4.16) (CONF:15915).
Figure 219: Procedure activity procedure example
<procedure classCode="PROC" moodCode="EVN">
<!-- Procedure activity procedure template -->
<templateId root="2.16.840.1.113883.10.20.22.4.14"/>
<id root="e401f340-7be2-11db-9fe1-0800200c9a66"/>
<code code="397394009"
codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT"
displayName="Bronchoalveolar lavage">
<originalText>Bronchoalveolar<reference
value="procedure1"/></originalText>
</code>
<text>
<reference value="procedure1"/>
</text>
<statusCode code="completed"/>
<effectiveTime value="1998"/>
<methodCode code="168731009"
codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT"
displayName="Standard chest X-ray"/>
<targetSiteCode code="82094008"
codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT"
displayName="Lower respiratory tract structure"/>
<specimen>
<specimenRole>
<id extension="234234"/>
</specimenRole>
</specimen>

Page 472 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
<participant typeCode="DEV">
<participantRole classCode="MANU">
<!-- Product instance template -->
<templateId root="2.16.840.1.113883.10.20.22.4.37"/>
...
</participantRole>
</participant>
<entryRelationship typeCode="COMP" inversionInd="true">
<substanceAdministration classCode="SBADM" moodCode="INT">
<!-- Medication activity template -->
<templateId root=" 2.16.840.1.113883.10.20.22.4.16"/>
...
</substanceAdministration>
</entryRelationship>
</procedure>
6.64 Procedure Context
[act: templateId 2.16.840.1.113883.10.20.6.2.5(open)]
Table 265: Procedure Context Contexts
Used By:
Contains Entries:
Diagnostic Imaging Report (optional)
The ServiceEvent Procedure Context of the document header may be overridden
in the CDA structured body if there is a need to refer to multiple imaging
procedures or acts. The selection of the Procedure or Act entry from the clinical
statement choice box depends on the nature of the imaging service that has
been performed. The Procedure entry shall be used for image-guided
interventions and minimal invasive imaging services, whereas the Act entry shall
be used for diagnostic imaging services.
Table 266: Procedure Context Constraints Overview
Name
XPath
Card.
Verb
Data Type
CONF#
Fixed Value
act[templateId/@root = '2.16.840.1.113883.10.20.6.2.5']
templateId
1..1
SHALL
SET<II>
9200
@root
1..1
SHALL
10530
2.16.840.1.113883.10.20.6.2.5
code
1..1
SHALL
CD
9201
effectiveTime
0..1
SHOULD
TS
9203
@value
1..1
SHALL
17173
1. Procedure Context SHALL be represented with the procedure or act elements
depending on the nature of the procedure (CONF:9199).
2. SHALL contain exactly one [1..1] templateId (CONF:9200) such that it

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 473
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.6.2.5" (CONF:10530).
3. SHALL contain exactly one [1..1] code (CONF:9201).
4. SHOULD contain zero or one [0..1] effectiveTime (CONF:9203).
a. The effectiveTime, if present, SHALL contain exactly one [1..1] @value
(CONF:17173).
Figure 220: Procedure context template example
<act moodCode="EVN" classCode="ACT">
<templateId root="2.16.840.1.113883.10.20.6.2.5"/>
<!-- Procedure Context template -->
<code code="70548"
displayName="Magnetic resonance angiography, head; with contrast
material(s)"
codeSystem="2.16.840.1.113883.6.12" codeSystemName="CPT4"/>
<!-- Note: This code is slightly different from the code used in the
header documentationOf and overrides it, which is what this entry
is for. -->
<effectiveTime value="20060823222400"/>
</act>
6.65 Product Instance
[participantRole: templateId 2.16.840.1.113883.10.20.22.4.37(open)]
Table 267: Product Instance Contexts
Used By:
Contains Entries:
Procedure Activity Procedure
Non-Medicinal Supply Activity
This clinical statement represents a particular device that was placed in or used
as part of a procedure or other act. This provides a record of the identifier and
other details about the given product that was used. For example, it is important
to have a record that indicates not just that a hip prostheses was placed in a
patient but that it was a particular hip prostheses number with a unique
identifier.
The FDA Amendments Act specifies the creation of a Unique Device
Identification (UDI) System that requires the label of devices to bear a unique
identifier that will standardize device identification and identify the device
through distribution and use.
The UDI should be sent in the participantRole/id.

Page 474 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 268: Product Instance Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
participantRole[templateId/@root = '2.16.840.1.113883.10.20.22.4.37']
@classCode
1..1
SHALL
7900
2.16.840.1.113883.5.110 (RoleClass)
= MANU
templateId
1..1
SHALL
SET<II>
7901
@root
1..1
SHALL
10522
2.16.840.1.113883.10.20.22.4.37
id
1..*
SHALL
II
7902
playing
Device
1..1
SHALL
7903
code
0..1
SHOULD
CE
7904
scoping
Entity
1..1
SHALL
7905
id
1..*
SHALL
II
7908
1. SHALL contain exactly one [1..1] @classCode="MANU" Manufactured Product
(CodeSystem: RoleClass 2.16.840.1.113883.5.110) (CONF:7900).
2. SHALL contain exactly one [1..1] templateId (CONF:7901) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.37" (CONF:10522).
3. SHALL contain at least one [1..*] id (CONF:7902).
4. SHALL contain exactly one [1..1] playingDevice (CONF:7903).
a. This playingDevice SHOULD contain zero or one [0..1] code
(CONF:7904).
5. SHALL contain exactly one [1..1] scopingEntity (CONF:7905).
a. This scopingEntity SHALL contain at least one [1..*] id (CONF:7908).
Figure 221: Product instance example
<participantRole classCode="MANU">
<templateId root="2.16.840.1.113883.10.20.22.4.37"/>
<!-- Product instance template -->
<id root="eb936010-7b17-11db-9fe1-0800200c9a68"/>
<playingDevice>
<code code="72506001"
codeSystem="2.16.840.1.113883.6.96"
displayName="Automatic implantable
cardioverter/defibrillator"/>
</playingDevice>
<scopingEntity>
<id root="eb936010-7b17-11db-9fe1-0800200c9b65"/>
</scopingEntity>
</participantRole>

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 475
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
6.66 Purpose of Reference Observation
[observation: templateId 2.16.840.1.113883.10.20.6.2.9(open)]
Table 269: Purpose of Reference Observation Contexts
Used By:
Contains Entries:
SOP Instance Observation
A Purpose of Reference Observation describes the purpose of the DICOM
composite object reference. Appropriate codes, such as externally defined
DICOM codes, may be used to specify the semantics of the purpose of reference.
When this observation is absent, it implies that the reason for the reference is
unknown.
Table 270: Purpose of Reference Observation Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
observation[templateId/@root = '2.16.840.1.113883.10.20.6.2.9']
@classCode
1..1
SHALL
9264
2.16.840.1.113883.5.6 (HL7ActClass)
= OBS
@moodCode
1..1
SHALL
9265
2.16.840.1.113883.5.1001 (ActMood)
= EVN
templateId
1..1
SHALL
SET<II>
9266
@root
1..1
SHALL
10531
2.16.840.1.113883.10.20.6.2.9
code
1..1
SHALL
CD
9267
code
0..1
SHOULD
9268
2.16.840.1.113883.5.4 (ActCode) =
ASSERTION
value
0..1
SHOULD
CD
9273
2.16.840.1.113883.11.20.9.28
(DICOMPurposeOfReference)
1. SHALL contain exactly one [1..1] @classCode="OBS" Observation
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6) (CONF:9264).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001) (CONF:9265).
3. SHALL contain exactly one [1..1] templateId (CONF:9266) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.6.2.9" (CONF:10531).
4. SHALL contain exactly one [1..1] code (CONF:9267).
a. This code SHOULD contain zero or one [0..1] code="ASSERTION"
(CodeSystem: ActCode 2.16.840.1.113883.5.4) (CONF:9268).
b. For backwards compatibility with the DICOM CMET, the code MAY be
drawn from ValueSet 2.16.840.1.113883.11.20.9.28
DICOMPurposeOfReference DYNAMIC (CONF:9269).
5. SHOULD contain zero or one [0..1] value with @xsi:type="CD", where the
@code SHOULD be selected from ValueSet DICOMPurposeOfReference
2.16.840.1.113883.11.20.9.28 DYNAMIC (CONF:9273).

Page 476 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
a. The value element is a SHOULD to allow backwards compatibility with
the DICOM CMET. Note that the use of ASSERTION for the code
differs from the DICOM CMET. This is intentional. The DICOM CMET
was created before the Term Info guidelines describing the use of the
assertion pattern were released. It was determined that this IG
should follow the latest Term Info guidelines. Implementers using
both this IG and the DICOM CMET will need to be aware of this
difference and apply appropriate transformations (CONF:9274).
Table 271: DICOM Purpose of Reference Value Set
Value Set: DICOMPurposeOfReference 2.16.840.1.113883.11.20.9.28 DYNAMIC
Code System(s):
DCM 1.2.840.10008.2.16.4
Code
Code System
Print Name
121079
DCM
Baseline
121080
DCM
Best illustration of finding
121112
DCM
Source of Measurement
Figure 222: Purpose of reference example
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.6.2.9"/>
<code code="ASSERTION" codeSystem="2.16.840.1.113883.5.4"/>
<value xsi:type="CD" code="121112"
codeSystem="1.2.840.10008.2.16.4"
codeSystemName="DCM"
displayName="Source of Measurement"/>
</observation>
6.67 Quantity Measurement Observation
[observation: templateId 2.16.840.1.113883.10.20.6.2.14(open)]
Table 272: Quantity Measurement Observation Contexts
Used By:
Contains Entries:
Text Observation
Code Observations
SOP Instance Observation
A Quantity Measurement Observation records quantity measurements based on
image data such as linear, area, volume, and numeric measurements. The codes
in DIRQuantityMeasurementTypeCodes (ValueSet:
2.16.840.1.113883.11.20.9.29) are from the qualifier hierarchy of SNOMED CT
and are not valid for observation/code according to the Term Info guidelines.
These codes can be used for backwards compatibility, but going forward, codes
from the observable entity hierarchy will be requested and used.

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 477
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 273: Quantity Measurement Observation Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
observation[templateId/@root = '2.16.840.1.113883.10.20.6.2.14']
@classCode
1..1
SHALL
9317
2.16.840.1.113883.5.6
(HL7ActClass) = OBS
@moodCode
1..1
SHALL
9318
2.16.840.1.113883.5.1001
(ActMood) = EVN
templateId
1..1
SHALL
SET<II>
9319
@root
1..1
SHALL
10532
2.16.840.1.113883.10.20.6.2.14
code
1..1
SHALL
CD
9320
code
0..1
SHOULD
9322
2.16.840.1.113883.11.20.9.29
(DIRQuantityMeasurementTypeCod
es)
code
0..1
SHOULD
9323
2.16.840.1.113883.11.20.9.30
(DICOMQuantityMeasurementType
Codes)
value
1..1
SHALL
9324
@xsi:type
1..1
SHALL
9325
PQ
effectiveTime
0..1
SHOULD
TS or
IVL<TS>
9326
entry
Relationship
0..*
MAY
9327
@typeCode
1..1
SHALL
9328
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) = SPRT
1. SHALL contain exactly one [1..1] @classCode="OBS" Observation
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6) (CONF:9317).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001) (CONF:9318).
3. SHALL contain exactly one [1..1] templateId (CONF:9319) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.6.2.14" (CONF:10532).
4. SHALL contain exactly one [1..1] code (CONF:9320).
a. This code SHOULD contain zero or one [0..1] code, which SHALL be
selected from ValueSet DIRQuantityMeasurementTypeCodes
2.16.840.1.113883.11.20.9.29 DYNAMIC (CONF:9322).
b. This code SHOULD contain zero or one [0..1] code, which SHALL be
selected from ValueSet DICOMQuantityMeasurementTypeCodes
2.16.840.1.113883.11.20.9.30 DYNAMIC (CONF:9323).
c. The value set of the observation/code includes numeric measurement
types for linear dimensions, areas, volumes, and other numeric
measurements. This value set is extensible and comprises the union
of SNOMED codes for observable entities as reproduced in
DIRQuantityMeasurementTypeCodes (ValueSet:
2.16.840.1.113883.11.20.9.29) and DICOM Codes in

Page 478 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
DICOMQuantityMeasurementTypeCodes (ValueSet:
2.16.840.1.113883.11.20.9.30) (CONF:9330).
5. SHALL contain exactly one [1..1] value (CONF:9324).
a. This value SHALL contain exactly one [1..1] @xsi:type, where the
@code="PQ" (CONF:9325).
6. SHOULD contain zero or one [0..1] effectiveTime (CONF:9326).
7. MAY contain zero or more [0..*] entryRelationship (CONF:9327) such that
it
a. SHALL contain exactly one [1..1] @typeCode="SPRT" Has Support
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002) (CONF:9328).
b. SHALL contain exactly one [1..1] SOP Instance Observation
(2.16.840.1.113883.10.20.6.2.8) (CONF:9329).
Table 274: DIR Quantity Measurement Type Value Set
Value Set: DIRQuantityMeasurementTypeCodes 2.16.840.1.113883.11.20.9.29 DYNAMIC
Code System(s):
SNOMED CT 2.16.840.1.113883.6.96
Code
Code System
Print Name
439932008
SNOMED CT
Length of structure
440357003
SNOMED CT
Width of structure
439934009
SNOMED CT
Depth of structure
439984002
SNOMED CT
Diameter of structure
439933003
SNOMED CT
Long axis length of structure
439428006
SNOMED CT
Short axis length of structure
439982003
SNOMED CT
Major axis length of structure
439983008
SNOMED CT
Minor axis length of structure
440356007
SNOMED CT
Perpendicular axis length of structure
439429003
SNOMED CT
Radius of structure
440433004
SNOMED CT
Perimeter of non-circular structure
439747008
SNOMED CT
Circumference of circular structure
439748003
SNOMED CT
Diameter of circular structure
439746004
SNOMED CT
Area of structure
439985001
SNOMED CT
Area of body region
439749006
SNOMED CT
Volume of structure

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 479
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 275: DICOM Quantity Measurement Type Value Set
Value Set: DICOMQuantityMeasurementTypeCodes 2.16.840.1.113883.11.20.9.30 DYNAMIC
Code System(s):
DCM 1.2.840.10008.2.16.4
Code
Code
System
Print Name
Measurement
Type
121211
DCM
Path length
Linear
121206
DCM
Distance
Linear
121207
DCM
Height
Linear
121216
DCM
Volume estimated from single 2D region
Volume
121218
DCM
Volume estimated from two non-coplanar
2D regions
Volume
121217
DCM
Volume estimated from three or more non-
coplanar 2D regions
Volume
121222
DCM
Volume of sphere
Volume
121221
DCM
Volume of ellipsoid
Volume
121220
DCM
Volume of circumscribed sphere
Volume
121219
DCM
Volume of bounding three dimensional
region
Volume
Figure 223: Quantity measurement observation example
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.6.2.14"/>
<code code="439984002" codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNM3"
displayName="Diameter of structure">
<originalText>
<reference value="#Diam2"/>
</originalText>
</code>
<statusCode code="completed"/>
<effectiveTime value="20060823223912"/>
<value xsi:type="PQ" value="45" unit="mm">
codeSystemVersion="1.5"/>
</value>
<!-- entryRelationships to SOP Instance Observations may go here -->
</observation>

Page 480 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
6.68 Reaction Observation
[observation: templateId 2.16.840.1.113883.10.20.22.4.9(open)]
Table 276: Reaction Observation Contexts
Used By:
Contains Entries:
Allergy - Intolerance Observation
Medication Activity
Immunization Activity
Medication Activity
Procedure Activity Procedure
Severity Observation
This clinical statement represents an undesired symptom, finding, etc., due to
an administered or exposed substance. A reaction can be defined with respect to
its severity, and can have been treated by one or more interventions.
Table 277: Reaction Observation Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF
#
Fixed Value
Green
Reaction
Observation
observation[templateId/@root = '2.16.840.1.113883.10.20.22.4.9']
@classCode
1..1
SHALL
7325
2.16.840.1.113883.5.6
(HL7ActClass) = OBS
@moodCode
1..1
SHALL
7326
2.16.840.1.113883.5.10
01 (ActMood) = EVN
templateId
1..1
SHALL
SET<II>
7323
@root
1..1
SHALL
10523
2.16.840.1.113883.10.2
0.22.4.9
id
1..1
SHALL
7329
code
1..1
SHALL
16851
reaction
FreeText
text
0..1
SHOULD
7330
reference
0..1
SHOULD
15917
@value
0..1
SHOULD
15918
statusCode
1..1
SHALL
7328
2.16.840.1.113883.5.14
(ActStatus) = completed
effectiveTime
0..1
SHOULD
TS or
IVL<TS>
7332
low
0..1
SHOULD
TS
7333
high
0..1
SHOULD
TS
7334
reaction
Coded
value
1..1
SHALL
CD
7335
2.16.840.1.113883.3.88.
12.3221.7.4 (Problem)
entryRelationship
0..*
MAY
7337
@typeCode
1..1
SHALL
7338
2.16.840.1.113883.5.10
02
(HL7ActRelationshipTyp
e) = RSON
@inversionInd
1..1
SHALL
7343
true

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 481
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Name
XPath
Card.
Verb
Data
Type
CONF
#
Fixed Value
procedure
1..1
SHALL
15920
entryRelationship
0..*
MAY
7340
@typeCode
1..1
SHALL
7341
2.16.840.1.113883.5.10
02
(HL7ActRelationshipTyp
e) = RSON
@inversionInd
1..1
SHALL
7344
true
substance
Administration
1..1
SHALL
15921
severity
entryRelationship
0..1
SHOULD
7580
@typeCode
1..1
SHALL
7581
2.16.840.1.113883.5.10
02
(HL7ActRelationshipTyp
e) = SUBJ
@inversionInd
1..1
SHALL
10375
true
observation
1..1
SHALL
15922
1. SHALL contain exactly one [1..1] @classCode="OBS" Observation
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6 STATIC) (CONF:7325).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001 STATIC) (CONF:7326).
3. SHALL contain exactly one [1..1] templateId (CONF:7323) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.9" (CONF:10523).
4. SHALL contain exactly one [1..1] id (CONF:7329).
5. SHALL contain exactly one [1..1] code (CONF:16851).
a. The value set for this code element has not been specified.
Implementers are allowed to use any code system, such as SNOMED
CT, a locally determined code, or a nullFlavor (CONF:16852).
6. SHOULD contain zero or one [0..1] text (CONF:7330).
a. The text, if present, SHOULD contain zero or one [0..1] reference
(CONF:15917).
i. The reference, if present, SHOULD contain zero or one [0..1]
@value (CONF:15918).
1. This reference/@value SHALL begin with a '#' and SHALL
point to its corresponding narrative (using the
approach defined in CDA Release 2, section 4.3.5.1)
(CONF:15919).
7. SHALL contain exactly one [1..1] statusCode="completed" Completed
(CodeSystem: ActStatus 2.16.840.1.113883.5.14 STATIC) (CONF:7328).
8. SHOULD contain zero or one [0..1] effectiveTime (CONF:7332).
a. The effectiveTime, if present, SHOULD contain zero or one [0..1] low
(CONF:7333).

Page 482 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
b. The effectiveTime, if present, SHOULD contain zero or one [0..1] high
(CONF:7334).
9. SHALL contain exactly one [1..1] value with @xsi:type="CD", where the @code
SHALL be selected from ValueSet Problem
2.16.840.1.113883.3.88.12.3221.7.4 DYNAMIC (CONF:7335).
10. MAY contain zero or more [0..*] entryRelationship (CONF:7337) such that
it
a. SHALL contain exactly one [1..1] @typeCode="RSON" Has reason
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002 STATIC) (CONF:7338).
b. SHALL contain exactly one [1..1] @inversionInd="true" True
(CONF:7343).
c. SHALL contain exactly one [1..1] Procedure Activity Procedure
(templateId:2.16.840.1.113883.10.20.22.4.14) (CONF:15920).
i. This procedure activity is intended to contain information
about procedures that were performed in response to an
allergy reaction (CONF:16853).
11. MAY contain zero or more [0..*] entryRelationship (CONF:7340) such that
it
a. SHALL contain exactly one [1..1] @typeCode="RSON" Has reason
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002 STATIC) (CONF:7341).
b. SHALL contain exactly one [1..1] @inversionInd="true" True
(CONF:7344).
c. SHALL contain exactly one [1..1] Medication Activity
(templateId:2.16.840.1.113883.10.20.22.4.16) (CONF:15921).
i. This medication activity is intended to contain information
about medications that were administered in response to an
allergy reaction (CONF:16840).
12. SHOULD contain zero or one [0..1] entryRelationship (CONF:7580) such
that it
a. SHALL contain exactly one [1..1] @typeCode="SUBJ" Has subject
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002) (CONF:7581).
b. SHALL contain exactly one [1..1] @inversionInd="true" TRUE
(CONF:10375).
c. SHALL contain exactly one [1..1] Severity Observation
(templateId:2.16.840.1.113883.10.20.22.4.8) (CONF:15922).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 483
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 224: Reaction observation example
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.9"/>
<!-- Reaction observation template -->
<id root="350a25a1-5e69-11e1-b86c-0800200c9a66"/>
<code code="ASSERTION"
codeSystem="2.16.840.1.113883.5.4"/>
<statusCode code="completed"/>
<value xsi:type="CD"
code="56018004"
codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT"
displayName="Wheezing"/>
</observation>
6.69 Referenced Frames Observation
[observation: templateId 2.16.840.1.113883.10.20.6.2.10(open)]
A Referenced Frames Observation is used if the referenced DICOM SOP instance
is a multiframe image and the reference does not apply to all frames. The list of
integer values for the referenced frames of a DICOM multiframe image SOP
instance is contained in a Boundary Observation nested inside this class.
Table 278: Referenced Frames Observation Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
observation[templateId/@root = '2.16.840.1.113883.10.20.6.2.10']
@classCode
1..1
SHALL
9276
2.16.840.1.113883.5.6
(HL7ActClass) = ROIBND
@moodCode
1..1
SHALL
9277
2.16.840.1.113883.5.1001
(ActMood) = EVN
code
1..1
SHALL
CE
9278
1.2.840.10008.2.16.4 (DCM) =
121190
entryRelationship
1..1
SHALL
9279
@typeCode
1..1
SHALL
9280
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) = COMP
observation
1..1
SHALL
15923
1. SHALL contain exactly one [1..1] @classCode="ROIBND" Bounded Region of
Interest (CodeSystem: 2.16.840.1.113883.5.6 HL7ActClass)
(CONF:9276).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
2.16.840.1.113883.5.1001 ActMood) (CONF:9277).
3. SHALL contain exactly one [1..1] code="121190" Referenced Frames
(CodeSystem: 1.2.840.10008.2.16.4 DCM) (CONF:9278).
4. SHALL contain exactly one [1..1] entryRelationship (CONF:9279).

Page 484 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
a. This entryRelationship SHALL contain exactly one [1..1]
@typeCode="COMP" Component (CodeSystem:
2.16.840.1.113883.5.1002 HL7ActRelationshipType)
(CONF:9280).
b. This entryRelationship SHALL contain exactly one [1..1] Boundary
Observation (templateId:2.16.840.1.113883.10.20.6.2.11)
(CONF:9281).
Figure 225: Referenced frames observation example
<observation classCode="ROIBND" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.6.2.10"/>
<code code="121190" codeSystem="1.2.840.10008.2.16.4"
displayName="Referenced Frames"/>
<entryRelationship typeCode="COMP">
<!-- Boundary Observation -->
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.6.2.11"/>
<code code="113036" codeSystem="1.2.840.10008.2.16.4"
displayName="Frames for Display"/>
<value xsi:type="INT" value="1"/>
</observation>
</entryRelationship>
</observation>
6.70 Result Observation
[observation: templateId 2.16.840.1.113883.10.20.22.4.2(open)]
Table 279: Result Observation Contexts
Used By:
Contains Entries:
Result Organizer
Functional Status Section
This clinical statement represents details of a lab, radiology, or other study
performed on a patient.
The result observation includes a statusCode to allow recording the status of an
observation. If a Results Observation is not completed, the Result Organizer
must include corresponding statusCode. “Pending” results (e.g., a test has been
run but results have not been reported yet) should be represented as “active”
ActStatus.

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 485
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 280: Result Observation Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
Green
Result
Observation
observation[templateId/@root = '2.16.840.1.113883.10.20.22.4.2']
@classCode
1..1
SHALL
7130
2.16.840.1.113883.5.
6 (HL7ActClass) =
OBS
@moodCode
1..1
SHALL
7131
2.16.840.1.113883.5.
1001 (ActMood) =
EVN
templateId
1..1
SHALL
SET<
II>
7136
@root
1..1
SHALL
9138
2.16.840.1.113883.1
0.20.22.4.2
resultID
id
1..*
SHALL
7137
resultType
code
1..1
SHALL
CE
7133
text
0..1
SHOULD
7138
reference
0..1
SHOULD
15924
@value
0..1
SHOULD
15925
resultStatus
statusCode
1..1
SHALL
7134
@code
1..1
SHALL
14849
2.16.840.1.113883.1
1.20.9.39 (Result
Status)
resultDate
Time
effectiveTime
1..1
SHALL
TS or
IVL<
TS>
7140
resultValue
value
1..1
SHALL
7143
resultInterp
retation
interpretationCode
0..*
SHOULD
7147
methodCode
0..1
MAY
SET<
CE>
7148
targetSiteCode
0..1
MAY
SET<
CD>
7153
author
0..1
MAY
7149
resultRefere
nceRange
referenceRange
0..*
SHOULD
7150
observationRange
1..1
SHALL
7151
code
0..0
SHALL
NOT
7152
1. SHALL contain exactly one [1..1] @classCode="OBS" Observation
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6) (CONF:7130).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001) (CONF:7131).

Page 486 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
3. SHALL contain exactly one [1..1] templateId (CONF:7136) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.2" (CONF:9138).
4. SHALL contain at least one [1..*] id (CONF:7137).
5. SHALL contain exactly one [1..1] code with @xsi:type="CE" (CONF:7133).
a. SHOULD be from LOINC (CodeSystem: 2.16.840.1.113883.6.1) or
SNOMED CT (CodeSystem: 2.16.840.1.113883.6.96) (CONF:7166).
b. Laboratory results SHOULD be from LOINC (CodeSystem:
2.16.840.1.113883.6.1) or other constrained terminology named by
the US Department of Health and Human Services Office of National
Coordinator or other federal agency. Local and/or regional codes for
laboratory results are allowed. The Local and/or regional codes
SHOULD be sent in the translation element. See the Local code
example figure (CONF:9109).
6. SHOULD contain zero or one [0..1] text (CONF:7138).
a. The text, if present, SHOULD contain zero or one [0..1] reference
(CONF:15924).
i. The reference, if present, SHOULD contain zero or one [0..1]
@value (CONF:15925).
1. This reference/@value SHALL begin with a '#' and SHALL
point to its corresponding narrative (using the
approach defined in CDA Release 2, section 4.3.5.1)
(CONF:15926).
7. SHALL contain exactly one [1..1] statusCode (CONF:7134).
a. This statusCode SHALL contain exactly one [1..1] @code, which SHALL
be selected from ValueSet Result Status
2.16.840.1.113883.11.20.9.39 STATIC (CONF:14849).
8. SHALL contain exactly one [1..1] effectiveTime (CONF:7140).
a. Represents clinically effective time of the measurement, which may
be when the measurement was performed (e.g., a BP measurement),
or may be when sample was taken (and measured some time
afterwards) (CONF:16838).
9. SHALL contain exactly one [1..1] value (CONF:7143).
10. SHOULD contain zero or more [0..*] interpretationCode (CONF:7147).
11. MAY contain zero or one [0..1] methodCode (CONF:7148).
12. MAY contain zero or one [0..1] targetSiteCode (CONF:7153).
13. MAY contain zero or one [0..1] author (CONF:7149).
14. SHOULD contain zero or more [0..*] referenceRange (CONF:7150).
a. The referenceRange, if present, SHALL contain exactly one [1..1]
observationRange (CONF:7151).
i. This observationRange SHALL NOT contain [0..0] code
(CONF:7152).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 487
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 281: Result Status Value Set
Value Set: Result Status 2.16.840.1.113883.11.20.9.39 STATIC 2012-07-01
Code System(s):
ActStatus 2.16.840.1.113883.5.14
Description:
This value set indicates the status of the results observation or
organizer
Code
Code System
Print Name
aborted
ActStatus
aborted
active
ActStatus
active
cancelled
ActStatus
cancelled
completed
ActStatus
completed
held
ActStatus
held
suspended
ActStatus
suspended
Figure 226: Result observation example
<observation classCode="OBS" moodCode="EVN">
<!-- Result observation template -->
<templateId root="2.16.840.1.113883.10.20.22.4.2"/>
<id root="107c2dc0-67a5-11db-bd13-0800200c9a66"/>
<code code="30313-1"
displayName="HGB"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"/>
<statusCode code="completed"/>
<effectiveTime value="200003231430"/>
<value xsi:type="PQ" value="13.2" unit="g/dl"/>
<interpretationCode code="N" codeSystem="2.16.840.1.113883.5.83"/>
<methodCode/>
<targetSiteCode/>
<author>
<time/>
<assignedAuthor>
<id/>
</assignedAuthor>
</author>
<referenceRange>
<observationRange>
<text>M 13-18 g/dl; F 12-16 g/dl</text>
</observationRange>
</referenceRange>
</observation>

Page 488 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 227: No evaluation procedures (e.g., labs/x-rays) performed example
<entry>
<act classCode="ACT" moodCode="EVN" negationInd="true">
<code code="386053000" codeSystem="2.16.840.1.113883.6.96"
displayName="evaluation procedure"/>
<text>No Evaluation Procedures Performed</text>
<statusCode code="completed"/>
</act>
</entry>
Figure 228: Local code example
<code code="30313-1" displayName="HGB" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC">
<translation code="123-4"
displayName="Example"
codeSystem="2.16.840.1.113883.19.5"
codeSystemName="Regional Example Code System"/>
</code>
6.71 Result Organizer
[organizer: templateId 2.16.840.1.113883.10.20.22.4.1(open)]
Table 282: Result Organizer Contexts
Used By:
Contains Entries:
Results Section (entries required)
Results Section (entries optional)
Result Observation
This clinical statement identifies set of result observations. It contains
information applicable to all of the contained result observations. Result type
codes categorize a result into one of several commonly accepted values (e.g.,
“Hematology”, “Chemistry”, “Nuclear Medicine”). These values are often implicit
in the Organizer/code (e.g., an Organizer/code of “complete blood count”
implies a ResultTypeCode of “Hematology”). This template requires
Organizer/code to include a ResultTypeCode either directly or as a
translation of a code from some other code system.
An appropriate nullFlavor can be used when the organizer/code or
organizer/id is unknown.
If any Results Observation within the organizer has a statusCode of ‘active’, the
Result Organizer must also have as statusCode of ‘active.

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 489
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 283: Result Organizer Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
organizer[templateId/@root = '2.16.840.1.113883.10.20.22.4.1']
@classCode
1..1
SHALL
7121
2.16.840.1.113883.5.6 (HL7ActClass)
@moodCode
1..1
SHALL
7122
2.16.840.1.113883.5.1001 (ActMood) =
EVN
templateId
1..1
SHALL
SET<II>
7126
@root
1..1
SHALL
9134
2.16.840.1.113883.10.20.22.4.1
id
1..*
SHALL
7127
code
1..1
SHALL
CE
7128
statusCode
1..1
SHALL
7123
@code
1..1
SHALL
14848
2.16.840.1.113883.11.20.9.39 (Result
Status)
component
1..*
SHALL
7124
observation
1..1
SHALL
14850
1. SHALL contain exactly one [1..1] @classCode (CONF:7121).
a. SHOULD contain zero or one [0..1] @classCode="CLUSTER" Cluster
(CodeSystem: 2.16.840.1.113883.5.6 HL7ActClass) OR SHOULD
contain zero or one [0..1] @classCode="BATTERY" Battery
(CodeSystem: 2.16.840.1.113883.5.6 HL7ActClass) (CONF:7165).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001) (CONF:7122).
3. SHALL contain exactly one [1..1] templateId (CONF:7126) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.1" (CONF:9134).
4. SHALL contain at least one [1..*] id (CONF:7127).
5. SHALL contain exactly one [1..1] code with @xsi:type="CE" (CONF:7128).
a. SHOULD be selected from LOINC (codeSystem 2.16.840.1.113883.6.1)
or SNOMED CT (codeSystem 2.16.840.1.113883.6.96), and MAY be
selected from CPT-4 (codeSystem 2.16.840.1.113883.6.12)
(CONF:7164).
b. Laboratory results SHOULD be from LOINC (CodeSystem:
2.16.840.1.113883.6.1) or other constrained terminology named by
the US Department of Health and Human Services Office of National
Coordinator or other federal agency. Local and/or regional codes for
laboratory results SHOULD also be allowed (CONF:9108).
6. SHALL contain exactly one [1..1] statusCode (CONF:7123).
a. This statusCode SHALL contain exactly one [1..1] @code, which SHALL
be selected from ValueSet Result Status
2.16.840.1.113883.11.20.9.39 STATIC 2012-04-27 (CONF:14848).
7. SHALL contain at least one [1..*] component (CONF:7124) such that it

Page 490 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
a. SHALL contain exactly one [1..1] Result Observation
(templateId:2.16.840.1.113883.10.20.22.4.2) (CONF:14850).
Figure 229: Result organizer example
<organizer classCode="BATTERY" moodCode="EVN">
<!-- Result organizer template -->
<templateId root="2.16.840.1.113883.10.20.22.4.1"/>
<id root="7d5a02b0-67a4-11db-bd13-0800200c9a66"/>
<code code="57021-8" displayName="CBC W Auto Differential panel"
codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"/>
<statusCode code="completed"/>
<component>
<observation classCode="OBS" moodCode="EVN">
<!-- Result observation template -->
<templateId root="2.16.840.1.113883.10.20.22.4.2"/>
...
</observation>
</component>
<component>
<observation classCode="OBS" moodCode="EVN">
<!-- Result observation template -->
<templateId root="2.16.840.1.113883.10.20.22.4.2"/>
...
</observation>
</component>
...
</organizer>
6.72 Series Act
[act: templateId 2.16.840.1.113883.10.20.22.4.63 (open)]
Table 284: Series Act Contexts
Used By:
Contains Entries:
Study Act (required)
SOP Instance Observation
A Series Act contains the DICOM series information for referenced DICOM
composite objects. The series information defines the attributes that are used to
group composite instances into distinct logical sets. Each series is associated
with exactly one study. Series Act clinical statements are only instantiated in the
DICOM Object Catalog section inside a Study Act, and thus do not require a
separate templateId; in other sections, the SOP Instance Observation is included
directly.

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 491
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 285: Series Act Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
act[templateId/@root = '2.16.840.1.113883.10.20.22.4.63']
@classCode
1..1
SHALL
9222
2.16.840.1.113883.5.6
(HL7ActClass) = ACT
@moodCode
1..1
SHALL
9223
2.16.840.1.113883.5.1001
(ActMood) = EVN
templateId
1..1
SHALL
10918
@root
1..1
SHALL
10919
2.16.840.1.113883.10.20.22.4.63
id
1..*
SHALL
9224
@root
1..1
SHALL
9225
@extension
0..0
SHALL
NOT
9226
code
1..1
SHALL
CE
9228
1.2.840.10008.2.16.4 (DCM) =
113015
qualifier
1..1
SHALL
SET<CS>
9229
name
1..1
SHALL
PN
9230
1.2.840.10008.2.16.4 (DCM) =
121139
value
1..1
SHALL
ANY
9231
text
0..1
MAY
9233
effectiveTime
0..1
SHOULD
TS or
IVL<TS>
9235
entryRelationship
1..*
SHALL
9237
@typeCode
1..1
SHALL
9238
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) =
COMP
observation
1..1
SHALL
15927
1. SHALL contain exactly one [1..1] @classCode="ACT" Act (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:9222).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001) (CONF:9223).
3. SHALL contain exactly one [1..1] templateId (CONF:10918) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.63" (CONF:10919).
4. SHALL contain at least one [1..*] id (CONF:9224).
The @root contains the OID of the study instance UID since DICOM study ids
consist only of an OID
a. Such ids SHALL contain exactly one [1..1] @root (CONF:9225).
b. Such ids SHALL NOT contain [0..0] @extension (CONF:9226).
5. SHALL contain exactly one [1..1] code="113015" with @xsi:type="CE"
(CodeSystem: DCM 1.2.840.10008.2.16.4) (CONF:9228).
a. This code SHALL contain exactly one [1..1] qualifier (CONF:9229).

Page 492 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
i. This qualifier SHALL contain exactly one [1..1] name="121139"
Modality (CodeSystem: DCM 1.2.840.10008.2.16.4)
(CONF:9230).
The value element code contains a modality code and codeSystem is
1.2.840.10008.2.16.4
ii. This qualifier SHALL contain exactly one [1..1] value with
@xsi:type="ANY" (CONF:9231).
If present, the text element contains the description of the series
6. MAY contain zero or one [0..1] text (CONF:9233).
If present, the effectiveTime contains the time the series was started
7. SHOULD contain zero or one [0..1] effectiveTime (CONF:9235).
8. SHALL contain at least one [1..*] entryRelationship (CONF:9237) such that
it
a. SHALL contain exactly one [1..1] @typeCode="COMP" Component
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002) (CONF:9238).
b. SHALL contain exactly one [1..1] SOP Instance Observation
(templateId:2.16.840.1.113883.10.20.6.2.8) (CONF:15927).
Figure 230: Series act example
<act classCode="ACT" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.63"/>
<id root="1.2.840.113619.2.62.994044785528.20060823223142485051"/>
<code code="113015" codeSystem="1.2.840.10008.2.16.4"
codeSystemName="DCM" displayName="Series">
<qualifier>
<name code="121139" codeSystem="1.2.840.10008.2.16.4"
codeSystemName="DCM"
displayName="Modality"> </name>
<value code="CR" codeSystem="1.2.840.10008.2.16.4"
codeSystemName="DCM"
displayName="Computed Radiography"> </value>
</qualifier>
</code>
<!-- **** SOP Instance UID *** -->
<entryRelationship typeCode="COMP">
<observation classCode="DGIMG" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.6.2.8"/>
...
</observation>
</entryRelationship>
</act>

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 493
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
6.73 Service Delivery Location
[participantRole: templateId 2.16.840.1.113883.10.20.22.4.32(open)]
Table 286: Service Delivery Location Contexts
Used By:
Contains Entries:
Procedure Activity Procedure
Procedure Activity Observation
Procedure Activity Act
Encounter Activities
This clinical statement represents the location of a service event where an act,
observation or procedure took place.
Table 287: Service Delivery Location Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
participantRole[templateId/@root = '2.16.840.1.113883.10.20.22.4.32']
@classCode
1..1
SHALL
7758
2.16.840.1.113883.5.111
(RoleCode) = SDLOC
templateId
1..1
SHALL
SET<II
>
7635
@root
1..1
SHALL
10524
2.16.840.1.113883.10.20.22.4.
32
code
1..1
SHALL
16850
2.16.840.1.113883.1.11.20275
(HealthcareServiceLocation)
addr
0..*
SHOULD
SET<A
D>
7760
telecom
0..*
SHOULD
SET<T
EL>
7761
playingEntity
0..1
MAY
7762
@classCode
1..1
SHALL
7763
2.16.840.1.113883.5.41
(EntityClass) = PLC
name
0..1
MAY
16037
1. SHALL contain exactly one [1..1] @classCode="SDLOC" (CodeSystem:
RoleCode 2.16.840.1.113883.5.111 STATIC) (CONF:7758).
2. SHALL contain exactly one [1..1] templateId (CONF:7635) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.32" (CONF:10524).
3. SHALL contain exactly one [1..1] code, which SHALL be selected from ValueSet
HealthcareServiceLocation 2.16.840.1.113883.1.11.20275
(CONF:16850).
4. SHOULD contain zero or more [0..*] addr (CONF:7760).
5. SHOULD contain zero or more [0..*] telecom (CONF:7761).

Page 494 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
6. MAY contain zero or one [0..1] playingEntity (CONF:7762).
a. The playingEntity, if present, SHALL contain exactly one [1..1]
@classCode="PLC" (CodeSystem: EntityClass
2.16.840.1.113883.5.41 STATIC) (CONF:7763).
b. The playingEntity, if present, MAY contain zero or one [0..1] name
(CONF:16037).
Table 288: HealthcareServiceLocation Value Set (excerpt)
Value Set: HealthcareServiceLocation 2.16.840.1.113883.1.11.20275 DYNAMIC
Code System(s):
HealthcareServiceLocation 2.16.840.1.113883.6.259
Description:
A comprehensive classification of locations and settings where healthcare services
are provided. This value set is based on the National Healthcare Safety Network
(NHSN) location code system that has been developed over a number of years
through CDC's interaction with a variety of healthcare facilities and is intended to
serve a variety of reporting needs where coding of healthcare service locations is
required.
Full value set may be found at:
http://phinvads.cdc.gov/vads/SearchAllVocab_search.action?searchOption
s.searchText=Healthcare+Service+Location+%28NHSN%29
Code
Code System
Print Name
1024-9
HealthcareServiceLocation
Critical Care Unit
1117-1
HealthcareServiceLocation
Family Medicine Clinic
1128-8
HealthcareServiceLocation
Pediatric Clinic
1160-1
HealthcareServiceLocation
Urgent Care Center
…
Figure 231: Service delivery location example
<participantRole classCode="SDLOC">
<templateId root="2.16.840.1.113883.10.20.22.4.32"/>
<code code="GACH"
codeSystem="2.16.840.1.113883.5.111"
codeSystemName="HL7RoleCode"
displayName="General Acute Care Hospital"/>
<addr>
<streetAddressLine>17 Daws Rd.</streetAddressLine>
<city>Blue Bell</city>
<state>MA</state>
<postalCode>02368</postalCode>
<country>US</country>
</addr>
<telecom nullFlavor="UNK"/>
<playingEntity classCode="PLC">
<name>Good Health Clinic</name>
</playingEntity>
</participantRole>

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 495
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
6.74 Severity Observation
[observation: templateId 2.16.840.1.113883.10.20.22.4.8(open)]
Table 289: Severity Observation Contexts
Used By:
Contains Entries:
Reaction Observation
Allergy - Intolerance Observation
This clinical statement represents the gravity of the problem, such as allergy or
reaction, in terms of its actual or potential impact on the patient. The Severity
Observation can be associated with an Allergy Observation, Reaction
Observation or both. When the Severity Observation is associated directly with
an Allergy it characterizes the Allergy. When the Severity Observation is
associated with a Reaction Observation it characterizes a Reaction. A person
may manifest many symptoms in a reaction to a single substance, and each
reaction to the substance can be represented. However, each reaction
observation can have only one severity observation associated with it. For
example, someone may have a rash reaction observation as well as an itching
reaction observation, but each can have only one level of severity.
Table 290: Severity Observation Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
Green
Severity
Observation
observation[templateId/@root = '2.16.840.1.113883.10.20.22.4.8']
@classCode
1..1
SHALL
7345
2.16.840.1.113883.5.6
(HL7ActClass) = OBS
@moodCode
1..1
SHALL
7346
2.16.840.1.113883.5.1001
(ActMood) = EVN
templateId
1..1
SHALL
SET<
II>
7347
@root
1..1
SHALL
10525
2.16.840.1.113883.10.20.22.
4.8
code
1..1
SHALL
CE
7349
2.16.840.1.113883.5.4
(ActCode) = SEV
severityFree
Text
text
0..1
SHOULD
7350
reference
0..1
SHOULD
15928
@value
0..1
SHOULD
15929
statusCode
1..1
SHALL
7352
2.16.840.1.113883.5.14
(ActStatus) = completed

Page 496 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
severity
Coded
value
1..1
SHALL
CD
7356
2.16.840.1.113883.3.88.12.3
221.6.8 (Problem Severity)
interpretation
Code
0..*
SHOULD
9117
@code
0..1
SHOULD
16038
2.16.840.1.113883.1.11.78
(Observation Interpretation
(HL7))
1. SHALL contain exactly one [1..1] @classCode="OBS" Observation
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6 STATIC) (CONF:7345).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001 STATIC) (CONF:7346).
3. SHALL contain exactly one [1..1] templateId (CONF:7347) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.8" (CONF:10525).
4. SHALL contain exactly one [1..1] code="SEV" Severity Observation with
@xsi:type="CE" (CodeSystem: ActCode 2.16.840.1.113883.5.4 STATIC)
(CONF:7349).
5. SHOULD contain zero or one [0..1] text (CONF:7350).
a. The text, if present, SHOULD contain zero or one [0..1] reference
(CONF:15928).
i. The reference, if present, SHOULD contain zero or one [0..1]
@value (CONF:15929).
1. This reference/@value SHALL begin with a '#' and SHALL
point to its corresponding narrative (using the
approach defined in CDA Release 2, section 4.3.5.1)
(CONF:15930).
6. SHALL contain exactly one [1..1] statusCode="completed" Completed
(CodeSystem: ActStatus 2.16.840.1.113883.5.14 STATIC) (CONF:7352).
7. SHALL contain exactly one [1..1] value with @xsi:type="CD", where the @code
SHALL be selected from ValueSet Problem Severity
2.16.840.1.113883.3.88.12.3221.6.8 DYNAMIC (CONF:7356).
8. SHOULD contain zero or more [0..*] interpretationCode (CONF:9117).
a. The interpretationCode, if present, SHOULD contain zero or one [0..1]
@code, which SHOULD be selected from ValueSet Observation
Interpretation (HL7) 2.16.840.1.113883.1.11.78 DYNAMIC
(CONF:16038).
Table 291: Problem Severity Value Set

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 497
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Value Set: Problem Severity 2.16.840.1.113883.3.88.12.3221.6.8 DYNAMIC
Code System(s):
SNOMED CT 2.16.840.1.113883.6.96
Description:
This is a description of the level of the severity of the problem.
Code
Code System
Print Name
255604002
SNOMED CT
Mild (qualifier value)
371923003
SNOMED CT
Mild to moderate (qualifier value)
6736007
SNOMED CT
Moderate (severity modifier) (qualifier value)
371924009
SNOMED CT
Moderate to severe (qualifier value)
24484000
SNOMED CT
Severe (severity modifier) (qualifier value)
399166001
SNOMED CT
Fatal (qualifier value)
Figure 232: Severity observation example
<observation classCode="OBS" moodCode="EVN">
<!-- Severity observation template -->
<templateId root=" 2.16.840.1.113883.10.20.22.4.8"/>
<code code="SEV"
displayName="Severity Observation"
codeSystem="2.16.840.1.113883.5.4"
codeSystemName="HL7ActCode"/>
<text>
<reference value="#severity"/>
</text>
<statusCode code="completed"/>
<value xsi:type="CD" code="371924009" displayName="Moderate to severe"
codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT"/>
</observation>
6.75 Smoking Status Observation
[observation: templateId 2.16.840.1.113883.10.22.4.78 (open)]
Table 292: Smoking Status Observation Contexts
Used By:
Contains Entries:
Social History Section (optional)
This clinical statement represents a patient’s current smoking status. The
vocabulary selected for this clinical statement is the best approximation of the
statuses in Meaningful Use (MU) Stage 1.
If the patient is a smoker (77176002), the effectiveTime/low element must be
present. If the patient is an ex-smoker (8517006), both the effectiveTime/low
and effectiveTime/high element must be present.
The smoking status value set includes a special code to communicate if the
smoking status is unknown which is different from how Consolidated CDA
generally communicates unknown information.

Page 498 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 293: Smoking Status Observation Constraints Overview
Name
XPath
Card.
Verb
Data Type
CONF#
Fixed Value
observation[templateId/@root = '2.16.840.1.113883.10.22.4.78']
@classCode
1..1
SHALL
14806
2.16.840.1.113883.5.6 (HL7ActClass)
= OBS
@moodCode
1..1
SHALL
14807
2.16.840.1.113883.5.1001 (ActMood)
= EVN
templateId
1..1
SHALL
14815
@root
1..1
SHALL
14816
2.16.840.1.113883.10.22.4.78
code
1..1
SHALL
14808
2.16.840.1.113883.5.4 (ActCode) =
ASSERTION
statusCode
1..1
SHALL
14809
2.16.840.1.113883.5.14 (ActStatus) =
completed
effectiveTime
1..1
SHALL
TS or
IVL<TS>
14814
low
1..1
SHALL
14818
value
1..1
SHALL
CD
14810
@code
1..1
SHALL
14817
2.16.840.1.113883.10.22.4.78
(Smoking Status)
1. Conforms to Tobacco Use template
(2.16.840.1.113883.10.20.22.4.85).
2. SHALL contain exactly one [1..1] @classCode="OBS" Observation
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6) (CONF:14806).
3. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001) (CONF:14807).
4. SHALL contain exactly one [1..1] templateId (CONF:14815) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.22.4.78" (CONF:14816).
5. SHALL contain exactly one [1..1] code="ASSERTION" Assertion (CodeSystem:
ActCode 2.16.840.1.113883.5.4) (CONF:14808).
6. SHALL contain exactly one [1..1] statusCode="completed" Completed
(CodeSystem: ActStatus 2.16.840.1.113883.5.14) (CONF:14809).
7. SHALL contain exactly one [1..1] effectiveTime (CONF:14814).
a. This effectiveTime SHALL contain exactly one [1..1] low (CONF:14818).
8. SHALL contain exactly one [1..1] value with @xsi:type="CD" (CONF:14810).
a. This value SHALL contain exactly one [1..1] @code, which SHALL be
selected from ValueSet Smoking Status
2.16.840.1.113883.10.22.4.78 DYNAMIC (CONF:14817).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 499
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 294: Smoking Status Value Set
Value Set: Smoking Status 2.16.840.1.113883.11.20.9.38 STATIC 2012-07-01
Code System(s):
SNOMED CT 2.16.840.1.113883.6.96
Description:
This value set indicates the current smoking status of a patient
Code
Code System
Print Name
449868002
SNOMED CT
Current every day smoker
428041000124106
SNOMED CT
Current some day smoker
8517006
SNOMED CT
Former smoker
266919005
SNOMED CT
Never smoker (Never Smoked)
77176002
SNOMED CT
Smoker, current status unknown
266927001
SNOMED CT
Unknown if ever smoked
Figure 233: Smoking status observation example
<observation classCode="OBS" moodCode="EVN">
<!-- Smoking status observation template -->
<templateId root="2.16.840.1.113883.10.22.4.78"/>
<id extension="123456789" root="2.16.840.1.113883.19"/>
<code code="ASSERTION" codeSystem="2.16.840.1.113883.5.4"/>
<statusCode code="completed"/>
<effectiveTime>
<low value="20110410"/>
</effectiveTime>
<value xsi:type="CD" code="266919005"
displayName="Never Smoked"
codeSystem="2.16.840.1.113883.6.96"/>
</observation>
Figure 234: Unknown if ever smoked
<observation classCode="OBS" moodCode="EVN">
<!-- Smoking status observation template -->
<templateId root="2.16.840.1.113883.10.22.4.78"/>
<id extension="123456789" root="2.16.840.1.113883.19"/>
<code code="ASSERTION" codeSystem="2.16.840.1.113883.5.4"/>
<statusCode code="completed"/>
<effectiveTime>
<low value="20110410"/>
</effectiveTime>
<value xsi:type="CD" code="266927001"
displayName="Unknown if ever smoked"
codeSystem="2.16.840.1.113883.6.96"/>
</observation>

Page 500 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
6.76 Social History Observation
[observation: templateId 2.16.840.1.113883.10.20.22.4.38(open)]
Table 295: Social History Observation Contexts
Used By:
Contains Entries:
Social History Section
This Social History Observation defines the patient’s occupational, personal
(e.g., lifestyle), social, and environmental history and health risk factors, as well
as administrative data such as marital status, race, ethnicity, and religious
affiliation.
Table 296: Social History Observation Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
observation[templateId/@root = '2.16.840.1.113883.10.20.22.4.38']
@classCode
1..1
SHALL
8548
2.16.840.1.113883.5.6
(HL7ActClass) = OBS
@moodCode
1..1
SHALL
8549
2.16.840.1.113883.5.1001
(ActMood) = EVN
templateId
1..1
SHALL
SET<II
>
8550
@root
1..1
SHALL
10526
2.16.840.1.113883.10.20.22.
4.38
id
1..*
SHALL
II
8551
code
0..1
SHOULD
CD
8558
social
History
Type
code
0..1
SHOULD
8896
2.16.840.1.113883.3.88.12.8
0.60 (Social History Type Set
Definition)
social
History
FreeText
original
Text
0..1
SHOULD
ED
8893
reference
/@value
0..1
SHOULD
8894
statusCode
1..1
SHALL
CS
8553
2.16.840.1.113883.5.14
(ActStatus) = completed
social
History
Observed
Value
value
0..1
SHOULD
ANY
8559
1. SHALL contain exactly one [1..1] @classCode="OBS" Observation
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6) (CONF:8548).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001) (CONF:8549).
3. SHALL contain exactly one [1..1] templateId (CONF:8550) such that it

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 501
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.38" (CONF:10526).
4. SHALL contain at least one [1..*] id (CONF:8551).
5. SHOULD contain zero or one [0..1] code (CONF:8558).
a. The code, if present, SHOULD contain zero or one [0..1] code, where
the @code SHOULD be selected from ValueSet Social History Type
Set Definition 2.16.840.1.113883.3.88.12.80.60 STATIC
(2008-12-18 CONF:8896).
b. The code, if present, SHOULD contain zero or one [0..1] originalText
(CONF:8893).
i. The originalText, if present, SHOULD contain zero or one [0..1]
reference/@value (CONF:8894).
1. This reference/@value SHALL begin with a '#' and SHALL
point to its corresponding narrative (using the
approach defined in CDA Release 2, section 4.3.5.1)
(CONF:8895).
6. SHALL contain exactly one [1..1] statusCode="completed" (CodeSystem:
ActStatus 2.16.840.1.113883.5.14) (CONF:8553).
7. SHOULD contain zero or one [0..1] value with @xsi:type="ANY" (CONF:8559).
a. Observation/value can be any data type. Where Observation/value is
a physical quantity, the unit of measure SHALL be expressed using a
valid Unified Code for Units of Measure (UCUM) expression
(CONF:8555).
Table 297: Social History Type Set Definition Value Set
Value Set: Social History Type Set Definition 2.16.840.1.113883.3.88.12.80.60
STATIC 2008-12-18
Code System(s):
SNOMED CT 2.16.840.1.113883.6.96
Code
Code System
Print Name
229819007
SNOMED CT
Tobacco use and exposure
256235009
SNOMED CT
Exercise
160573003
SNOMED CT
Alcohol intake
364393001
SNOMED CT
Nutritional observable
364703007
SNOMED CT
Employment detail
425400000
SNOMED CT
Toxic exposure status
363908000
SNOMED CT
Details of drug misuse behavior
228272008
SNOMED CT
Health-related behavior
105421008
SNOMED CT
Educational Achievement

Page 502 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 235: Social history observation template example
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.38"/>
<!-- ** Social history observation template ** -->
<id root="45efb604-7049-4a2e-ad33-d38556c9636c"/>
<code code="230056004" codeSystem="2.16.840.1.113883.6.96"
displayName="Cigarette smoking">
<originalText>
<reference value="#soc2"/>
</originalText>
</code>
<statusCode code="completed"/>
<effectiveTime>
<low value="1973"/>
</effectiveTime>
<value xsi:type="ST">None</value>
</observation>
6.77 SOP Instance Observation
[observation: templateId 2.16.840.1.113883.10.20.6.2.8(open)]
Table 298: SOP Instance Observation Contexts
Used By:
Contains Entries:
Series Act
Text Observation
Code Observations
Quantity Measurement Observation
Purpose of Reference Observation
Referenced Frames Observation
SOP Instance Observation
A SOP Instance Observation contains the DICOM Service Object Pair (SOP)
Instance information for referenced DICOM composite objects. The SOP Instance
act class is used to reference both image and non-image DICOM instances. The
text attribute contains the DICOM WADO reference.
Table 299: SOP Instance Observation Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
observation[templateId/@root = '2.16.840.1.113883.10.20.6.2.8']
@classCode
1..1
SHALL
9240
2.16.840.1.113883.5.6
(HL7ActClass) = DGIMG
@moodCode
1..1
SHALL
9241
2.16.840.1.113883.5.1001
(ActMood) = EVN
id
1..*
SHALL
II
9242
code
1..1
SHALL
CD
9244
text
0..1
SHOULD
ED
9246
@mediaType
1..1
SHALL
9247
application/dicom
reference
1..1
SHALL
9248

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 503
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
effectiveTime
0..1
SHOULD
TS or
IVL<TS>
9250
@value
1..1
SHALL
9251
low
0..0
SHALL
NOT
TS
9252
high
0..0
SHALL
NOT
TS
9253
entryRelationship
0..*
MAY
9254
@typeCode
1..1
SHALL
9255
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) =
SUBJ
entryRelationship
0..*
MAY
9257
@typeCode
1..1
SHALL
9258
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) =
RSON
observation
1..1
SHALL
15935
entryRelationship
0..*
MAY
9260
@typeCode
1..1
SHALL
9261
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) =
COMP
observation
1..1
SHALL
15936
1. SHALL contain exactly one [1..1] @classCode="DGIMG" Diagnostic Image
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6) (CONF:9240).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001) (CONF:9241).
3. SHALL contain at least one [1..*] id (CONF:9242).
a. The @root contains an OID representing the DICOM SOP Instance
UID (CONF:9243).
4. SHALL contain exactly one [1..1] code (CONF:9244).
a. SHALL contain codeSystem 1.2.840.10008.2.6.1 DCMUID and code is
an OID for a valid SOP class name UID (CONF:9245).
5. SHOULD contain zero or one [0..1] text (CONF:9246).
a. The text, if present, SHALL contain exactly one [1..1]
@mediaType="application/dicom" (CONF:9247).
b. The text, if present, SHALL contain exactly one [1..1] reference
(CONF:9248).
i. SHALL contain a @value that contains a WADO reference as a
URI (CONF:9249).
6. SHOULD contain zero or one [0..1] effectiveTime (CONF:9250).
a. The effectiveTime, if present, SHALL contain exactly one [1..1] @value
(CONF:9251).
b. The effectiveTime, if present, SHALL NOT contain [0..0] low
(CONF:9252).

Page 504 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
c. The effectiveTime, if present, SHALL NOT contain [0..0] high
(CONF:9253).
7. MAY contain zero or more [0..*] entryRelationship (CONF:9254) such that
it
a. SHALL contain exactly one [1..1] @typeCode="SUBJ" Has Subject
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002) (CONF:9255).
b. SHALL contain exactly one [1..1] SOP Instance Observation
(2.16.840.1.113883.10.20.6.2.8) (CONF:9256).
8. MAY contain zero or more [0..*] entryRelationship (CONF:9257) such that
it
a. SHALL contain exactly one [1..1] @typeCode="RSON" Has Reason
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002) (CONF:9258).
b. SHALL contain exactly one [1..1] Purpose of Reference
Observation (2.16.840.1.113883.10.20.6.2.9) (CONF:15935).
9. MAY contain zero or more [0..*] entryRelationship (CONF:9260) such that
it
a. This entryRelationship SHALL be present if the referenced DICOM
object is a multiframe object and the reference does not apply to all
frames (CONF:9263).
b. SHALL contain exactly one [1..1] @typeCode="COMP" Has Component
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002) (CONF:9261).
c. SHALL contain exactly one [1..1] Referenced Frames Observation
(2.16.840.1.113883.10.20.6.2.10) (CONF:15936).
Figure 236: SOP instance observation example
<observation classCode="DGIMG" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.6.2.8"/>
<id
root="1.2.840.113619.2.62.994044785528.20060823.200608232232322.3"/>
<code code="1.2.840.10008.5.1.4.1.1.1"
codeSystem="1.2.840.10008.2.6.1" codeSystemName="DCMUID"
displayName="Computed Radiography Image Storage">
</code>
<text mediaType="application/dicom">
<reference
value="http://www.example.org/wado?requestType=WADO&studyUID=1.2.840.
113619.2.62.994044785528.114289542805&seriesUID=1.2.840.113619.2.62.9
94044785528.20060823223142485051&objectUID=1.2.840.113619.2.62.994044
785528.20060823.200608232232322.3&contentType=application/dicom"/>
<!--reference to image 1 (PA) -->
</text>
<effectiveTime value="20060823223232"/>
</observation>

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 505
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
6.78 Study Act
[act: templateId 2.16.840.1.113883.10.20.6.2.6 (open)]
Table 300: Study Act Contexts
Used By:
Contains Entries:
DICOM Object Catalog Section - DCM 121181 (required)
Series Act
A Study Act contains the DICOM study information that defines the
characteristics of a referenced medical study performed on a patient. A study is
a collection of one or more series of medical images, presentation states, SR
documents, overlays, and/or curves that are logically related for the purpose of
diagnosing a patient. Each study is associated with exactly one patient. A study
may include composite instances that are created by a single modality, multiple
modalities, or by multiple devices of the same modality. The study information is
modality-independent. Study Act clinical statements are only instantiated in the
DICOM Object Catalog section; in other sections, the SOP Instance Observation
is included directly.

Page 506 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 301: Study Act Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
act[templateId/@root = '2.16.840.1.113883.10.20.6.2.6']
@classCode
1..1
SHALL
9207
2.16.840.1.113883.5.6
(HL7ActClass) = ACT
@moodCode
1..1
SHALL
9208
2.16.840.1.113883.5.1001
(ActMood) = EVN
templateId
1..1
SHALL
SET<II>
9209
@root
1..1
SHALL
10533
2.16.840.1.113883.10.20.6.2.6
id
1..*
SHALL
9210
@root
1..1
SHALL
9213
@extension
0..0
SHALL
NOT
9211
code
1..1
SHALL
CE
9214
1.2.840.10008.2.16.4 (DCM) =
113014
text
0..1
MAY
9215
reference
0..1
SHOULD
15995
@value
0..1
SHOULD
15996
effectiveTime
0..1
SHOULD
TS or
IVL<TS>
9216
entryRelationship
1..*
SHALL
9219
@typeCode
1..1
SHALL
9220
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) =
COMP
act
1..1
SHALL
15937
1. SHALL contain exactly one [1..1] @classCode="ACT" (CodeSystem:
HL7ActClass 2.16.840.1.113883.5.6) (CONF:9207).
2. SHALL contain exactly one [1..1] @moodCode="EVN" (CodeSystem: ActMood
2.16.840.1.113883.5.1001) (CONF:9208).
3. SHALL contain exactly one [1..1] templateId (CONF:9209) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.6.2.6" (CONF:10533).
4. SHALL contain at least one [1..*] id (CONF:9210).
The @root contains the OID of the study instance UID since DICOM study ids
consist only of an OID
a. Such ids SHALL contain exactly one [1..1] @root (CONF:9213).
b. Such ids SHALL NOT contain [0..0] @extension (CONF:9211).
5. SHALL contain exactly one [1..1] code="113014" with @xsi:type="CE"
(CodeSystem: DCM 1.2.840.10008.2.16.4) (CONF:9214).
If present, the text element contains the description of the study.
6. MAY contain zero or one [0..1] text (CONF:9215).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 507
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
a. The text, if present, SHOULD contain zero or one [0..1] reference
(CONF:15995).
i. The reference, if present, SHOULD contain zero or one [0..1]
@value (CONF:15996).
1. This reference/@value SHALL begin with a '#' and SHALL
point to its corresponding narrative (using the
approach defined in CDA Release 2, section 4.3.5.1)
(CONF:15997).
If present, the effectiveTime contains the time the study was started
7. SHOULD contain zero or one [0..1] effectiveTime (CONF:9216).
8. SHALL contain at least one [1..*] entryRelationship (CONF:9219) such that
it
a. SHALL contain exactly one [1..1] @typeCode="COMP" Component
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002) (CONF:9220).
b. SHALL contain exactly one [1..1] Series Act
(templateId:2.16.840.1.113883.10.20.22.4.63) (CONF:15937).
Figure 237: Study act example
<act classCode="ACT" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.6.2.6"/>
<id root="1.2.840.113619.2.62.994044785528.114289542805"/>
<code code="113014" codeSystem="1.2.840.10008.2.16.4"
codeSystemName="DCM" displayName="Study"/>
<!-- **** Series ****-->
<entryRelationship typeCode="COMP">
<act classCode="ACT" moodCode="EVN">
...
</act>
</entryRelationship>
</act>
6.79 Text Observation
[observation: templateId 2.16.840.1.113883.10.20.6.2.12 (open)]
Table 302: Text Observation Contexts
Used By:
Contains Entries:
Quantity Measurement Observation
SOP Instance Observation
DICOM Template 2000 specifies that Imaging Report Elements of Value Type
Text are contained in sections. The Imaging Report Elements are inferred from
Basic Diagnostic Imaging Report Observations that consist of image references
and measurements (linear, area, volume, and numeric). Text DICOM Imaging
Report Elements in this context are mapped to CDA text observations that are

Page 508 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
section components and are related to the SOP Instance Observations
(templateId 2.16.840.1.113883.10.20.6.2.8) or Quantity Measurement
Observations (templateId 2.16.840.1.113883.10.20.6.2.14) by the SPRT
(Support) act relationship.
A Text Observation is required if the findings in the section text are represented
as inferred from SOP Instance Observations.
Table 303: Text Observation Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
observation[templateId/@root = '2.16.840.1.113883.10.20.6.2.12']
@classCode
1..1
SHALL
9288
2.16.840.1.113883.5.4 (ActCode)
= OBS
@moodCode
1..1
SHALL
9289
2.16.840.1.113883.5.1001
(ActMood) = EVN
templateId
1..1
SHALL
SET<II>
9290
@root
1..1
SHALL
10534
2.16.840.1.113883.10.20.6.2.12
code
1..1
SHALL
CE
9291
text
0..1
MAY
9295
reference
0..1
SHOULD
15938
@value
0..1
SHOULD
15939
effectiveTime
0..1
SHOULD
TS or
IVL<TS>
9294
value
1..1
SHALL
ED
9292
entryRelationship
0..*
MAY
9298
@typeCode
1..1
SHALL
9299
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) =
SPRT
observation
1..1
SHALL
15941
entryRelationship
0..*
MAY
9301
@typeCode
1..1
SHALL
9302
2.16.840.1.113883.5.1002
(HL7ActRelationshipType) =
SPRT
observation
1..1
SHALL
15942
1. SHALL contain exactly one [1..1] @classCode="OBS" Observation
(CodeSystem: ActCode 2.16.840.1.113883.5.4) (CONF:9288).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001) (CONF:9289).
3. SHALL contain exactly one [1..1] templateId (CONF:9290) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.6.2.12" (CONF:10534).
4. SHALL contain exactly one [1..1] code with @xsi:type="CE" (CONF:9291).
5. MAY contain zero or one [0..1] text (CONF:9295).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 509
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
a. The text, if present, SHOULD contain zero or one [0..1] reference
(CONF:15938).
i. The reference, if present, SHOULD contain zero or one [0..1]
@value (CONF:15939).
1. This reference/@value SHALL begin with a '#' and SHALL
point to its corresponding narrative (using the
approach defined in CDA Release 2, section 4.3.5.1)
(CONF:15940).
6. SHOULD contain zero or one [0..1] effectiveTime (CONF:9294).
7. SHALL contain exactly one [1..1] value with @xsi:type="ED" (CONF:9292).
8. MAY contain zero or more [0..*] entryRelationship (CONF:9298) such that
it
a. SHALL contain exactly one [1..1] @typeCode="SPRT" Has Support
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002) (CONF:9299).
b. SHALL contain exactly one [1..1] SOP Instance Observation
(templateId:2.16.840.1.113883.10.20.6.2.8) (CONF:15941).
9. MAY contain zero or more [0..*] entryRelationship (CONF:9301) such that
it
a. SHALL contain exactly one [1..1] @typeCode="SPRT" Has Support
(CodeSystem: HL7ActRelationshipType
2.16.840.1.113883.5.1002) (CONF:9302).
b. SHALL contain exactly one [1..1] Quantity Measurement
Observation (templateId:2.16.840.1.113883.10.20.6.2.14)
(CONF:15942).

Page 510 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 238: Text observation example
<text>
<paragraph>
<caption>Finding</caption>
<content ID="Fndng2">The cardiomediastinum is within normal limits.
The trachea is midline. The previously described opacity at the medial
right lung base has cleared. There are no new infiltrates. There is a new
round density at the left hilus, superiorly (diameter about 45mm). A CT
scan is recommended for further evaluation. The pleural spaces are clear.
The visualized musculoskeletal structures and the upper abdomen are
stable and unremarkable.</content>
</paragraph>
...
</text>
<entry>
<observation classCode="OBS" moodCode="EVN">
<!-- Text Observation -->
<templateId root="2.16.840.1.113883.10.20.6.2.12"/>
<code code="121071" codeSystem="1.2.840.10008.2.16.4"
codeSystemName="DCM" displayName="Finding"/>
<value xsi:type="ED"><reference value="#Fndng2"/></value>
...
<!-- entryRelationships to SOP Instance Observations and Quantity
Measurement Observations may go here -->
</observation>
</entry>
6.80 Tobacco Use
[observation: templateId 2.16.840.1.113883.10.20.22.4.85 (open)]
Table 304: Tobacco Use Observation Contexts
Used By:
Contains Entries:
Social History Section (optional)
This clinical statement represents a patient’s tobacco use. All types of tobacco
use are represented using the codes from the tobacco use and exposure - finding
hierarchy in SNOMED CT.

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 511
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 305: Tobacco Use Constraints Overview
Name
XPath
Card.
Verb
Data Type
CONF#
Fixed Value
observation[templateId/@root = '2.16.840.1.113883.10.20.22.4.85']
@classCode
1..1
SHALL
16558
2.16.840.1.113883.5.6 (HL7ActClass)
= OBS
@moodCode
1..1
SHALL
16559
2.16.840.1.113883.5.1001 (ActMood)
= EVN
templateId
1..1
SHALL
16566
@root
1..1
SHALL
16567
2.16.840.1.113883.10.22.4.78
code
1..1
SHALL
16560
2.16.840.1.113883.5.4 (ActCode) =
ASSERTION
statusCode
1..1
SHALL
16561
2.16.840.1.113883.5.14 (ActStatus) =
completed
effectiveTime
1..1
SHALL
TS or
IVL<TS>
16564
low
1..1
SHALL
16565
value
1..1
SHALL
CD
16562
@code
1..1
SHALL
16563
2.16.840.1.113883.11.20.9.41
(Tobacco Use)
1. SHALL contain exactly one [1..1] @classCode="OBS" Observation
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6) (CONF:16558).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001) (CONF:16559).
3. SHALL contain exactly one [1..1] templateId (CONF:16566) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.22.4.78" (CONF:16567).
4. SHALL contain exactly one [1..1] code="ASSERTION" Assertion (CodeSystem:
ActCode 2.16.840.1.113883.5.4) (CONF:16560).
5. SHALL contain exactly one [1..1] statusCode="completed" Completed
(CodeSystem: ActStatus 2.16.840.1.113883.5.14 STATIC) (CONF:16561).
6. SHALL contain exactly one [1..1] effectiveTime (CONF:16564).
a. This effectiveTime SHALL contain exactly one [1..1] low (CONF:16565).
7. SHALL contain exactly one [1..1] value with @xsi:type="CD" (CONF:16562).
a. This value SHALL contain exactly one [1..1] @code, which SHALL be
selected from ValueSet Tobacco Use
2.16.840.1.113883.11.20.9.41 DYNAMIC (CONF:16563).

Page 512 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 306: Tobacco Use Value Set
Value Set: Tobacco Use 2.16.840.1.113883.11.20.9.41 DYNAMIC
Code System(s):
SNOMED CT 2.16.840.1.113883.6.96
Description:
Contains all values descending from the SNOMED CT® 365980008 tobacco
use and exposure - finding hierarchy
http://www.nlm.nih.gov/research/umls/Snomed/snomed_main.html
Code
Code System
Print Name
81703003
SNOMED-CT
Chews tobacco
228494002
SNOMED-CT
Snuff user
59978006
SNOMED-CT
Cigar smoker
43381005
SNOMED-CT
Passive smoker
449868002
SNOMED CT
Current every day smoker
428041000124
106
SNOMED CT
Current some day smoker
8517006
SNOMED CT
Former smoker
266919005
SNOMED CT
Never smoker
77176002
SNOMED CT
Smoker, current status unknown
266927001
SNOMED CT
Unknown if ever smoked
…
Figure 239: Tobacco use entry example
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.22.4.9999"/>
<id extension="123456789" root="2.16.840.1.113883.19"/>
<code code="ASSERTION"
displayName="Assertion"
codeSystem="2.16.840.1.113883.5.4"
codeSystemName="ActCode"/>
<statusCode code="completed"/>
<effectiveTime>
<low value="20110410"/>
</effectiveTime>
<value xsi:type="CD" code="266919005"
displayName="Never Smoked"
codeSystem="2.16.840.1.113883.6.96"
</observation>
6.81 Vital Sign Observation
[observation: templateId 2.16.840.1.113883.10.20.22.4.27(open)]
Table 307: Vital Sign Observation Contexts
Used By:
Contains Entries:
Vital Signs Organizer

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 513
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Vital signs are represented as are other results, with additional vocabulary
constraints.
Table 308: Vital Sign Observation Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
observation[templateId/@root = '2.16.840.1.113883.10.20.22.4.27']
@classCode
1..1
SHALL
7297
2.16.840.1.113883.5.6
(HL7ActClass) = OBS
@moodCode
1..1
SHALL
7298
2.16.840.1.113883.5.1001
(ActMood) = EVN
templateId
1..1
SHALL
SET<II
>
7299
@root
1..1
SHALL
10527
2.16.840.1.113883.10.20.22
.4.27
id
1..*
SHALL
7300
code
1..1
SHALL
CD
7301
2.16.840.1.113883.3.88.12.
80.62 (HITSP Vital Sign
Result Type)
text
0..1
SHOULD
7302
reference
0..1
SHOULD
15943
@value
0..1
SHOULD
15944
statusCode
1..1
SHALL
7303
2.16.840.1.113883.5.14
(ActStatus) = completed
effectiveTime
1..1
SHALL
TS or
IVL<TS
>
7304
value
1..1
SHALL
PQ
7305
interpretationCode
0..1
MAY
7307
methodCode
0..1
MAY
SET<C
E>
7308
targetSiteCode
0..1
MAY
SET<C
D>
7309
author
0..1
MAY
7310
1. SHALL contain exactly one [1..1] @classCode="OBS" Observation
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6) (CONF:7297).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001) (CONF:7298).
3. SHALL contain exactly one [1..1] templateId (CONF:7299) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.27" (CONF:10527).
4. SHALL contain at least one [1..*] id (CONF:7300).
5. SHALL contain exactly one [1..1] code, where the @code SHOULD be selected
from ValueSet HITSP Vital Sign Result Type
2.16.840.1.113883.3.88.12.80.62 DYNAMIC (CONF:7301).

Page 514 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
6. SHOULD contain zero or one [0..1] text (CONF:7302).
a. The text, if present, SHOULD contain zero or one [0..1] reference
(CONF:15943).
i. The reference, if present, SHOULD contain zero or one [0..1]
@value (CONF:15944).
1. This reference/@value SHALL begin with a '#' and SHALL
point to its corresponding narrative (using the
approach defined in CDA Release 2, section 4.3.5.1)
(CONF:15945).
7. SHALL contain exactly one [1..1] statusCode="completed" Completed
(CodeSystem: ActStatus 2.16.840.1.113883.5.14) (CONF:7303).
8. SHALL contain exactly one [1..1] effectiveTime (CONF:7304).
9. SHALL contain exactly one [1..1] value with @xsi:type="PQ" (CONF:7305).
10. MAY contain zero or one [0..1] interpretationCode (CONF:7307).
11. MAY contain zero or one [0..1] methodCode (CONF:7308).
12. MAY contain zero or one [0..1] targetSiteCode (CONF:7309).
13. MAY contain zero or one [0..1] author (CONF:7310).
Table 309: Vital Sign Result Type Value Set
Value Set: HITSP Vital Sign Result Type 2.16.840.1.113883.3.88.12.80.62 DYNAMIC
Code System(s):
LOINC 2.16.840.1.113883.6.1
Description:
This identifies the vital sign result type
Code
Code System
Print Name
9279-1
LOINC
Respiratory Rate
8867-4
LOINC
Heart Rate
2710-2
LOINC
O2 % BldC Oximetry
8480-6
LOINC
BP Systolic
8462-4
LOINC
BP Diastolic
8310-5
LOINC
Body Temperature
8302-2
LOINC
Height
8306-3
LOINC
Height (Lying)
8287-5
LOINC
Head Circumference
3141-9
LOINC
Weight Measured
39156-5
LOINC
BMI (Body Mass Index)
3140-1
LOINC
BSA (Body Surface Area)

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 515
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 240: Vital sign observation example
<component>
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.27"/>
<!-- Vital Sign Observation template -->
<id root="c6f88321-67ad-11db-bd13-0800200c9a66"/>
<code code="8302-2"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"
displayName="Height"/>
<text><reference value="#height1"/></text>
<statusCode code="completed"/>
<effectiveTime value="19991114"/>
<value xsi:type="PQ" value="177" unit="cm"/>
<interpretationCode code="N" codeSystem="2.16.840.1.113883.5.83"/>
</observation>
</component>
6.82 Vital Signs Organizer
[organizer: templateId 2.16.840.1.113883.10.20.22.4.26(open)]
Table 310: Vital Signs Organizer Contexts
Used By:
Contains Entries:
Vital Signs Section (entries optional)
Vital Signs Section (entries required)
Vital Sign Observation
The Vital Signs Organizer groups vital signs, which is similar to the Result
Organizer, but with further constraints.
An appropriate nullFlavor can be used when the organizer/code or
organizer/id is unknown.

Page 516 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 311: Vital Signs Organizer Constraints Overview
Name
XPath
Card.
Verb
Data
Type
CONF#
Fixed Value
organizer[templateId/@root = '2.16.840.1.113883.10.20.22.4.26']
@classCode
1..1
SHALL
7279
2.16.840.1.113883.5.6 (HL7ActClass)
= CLUSTER
@moodCode
1..1
SHALL
7280
2.16.840.1.113883.5.1001 (ActMood)
= EVN
templateId
1..1
SHALL
SET<II>
7281
@root
1..1
SHALL
10528
2.16.840.1.113883.10.20.22.4.26
id
1..*
SHALL
II
7282
code
1..1
SHALL
CD
7283
2.16.840.1.113883.6.96 (SNOMEDCT)
= 46680005
statusCode
1..1
SHALL
CS
7284
2.16.840.1.113883.5.14 (ActStatus) =
completed
effectiveTime
1..1
SHALL
TS or
IVL<TS>
7288
component
1..*
SHALL
7285
observation
1..1
SHALL
15946
1. SHALL contain exactly one [1..1] @classCode="CLUSTER" CLUSTER
(CodeSystem: HL7ActClass 2.16.840.1.113883.5.6) (CONF:7279).
2. SHALL contain exactly one [1..1] @moodCode="EVN" Event (CodeSystem:
ActMood 2.16.840.1.113883.5.1001) (CONF:7280).
3. SHALL contain exactly one [1..1] templateId (CONF:7281) such that it
a. SHALL contain exactly one [1..1]
@root="2.16.840.1.113883.10.20.22.4.26" (CONF:10528).
4. SHALL contain at least one [1..*] id (CONF:7282).
5. SHALL contain exactly one [1..1] code="46680005" Vital Signs (CodeSystem:
SNOMED CT 2.16.840.1.113883.6.96) (CONF:7283).
6. SHALL contain exactly one [1..1] statusCode="completed" Completed
(CodeSystem: ActStatus 2.16.840.1.113883.5.14) (CONF:7284).
The effectivetime represents clinically effective time of the measurement,
which is most likely when the measurement was performed (e.g., a BP
measurement).
7. SHALL contain exactly one [1..1] effectiveTime (CONF:7288).
8. SHALL contain at least one [1..*] component (CONF:7285) such that it
a. SHALL contain exactly one [1..1] Vital Sign Observation
(2.16.840.1.113883.10.20.22.4.27) (CONF:15946).

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 517
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Figure 241: Vital signs organizer example
<organizer classCode="CLUSTER" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.26"/>
<!-- Vital signs organizer template -->
<id root="c6f88320-67ad-11db-bd13-0800200c9a66"/>
<code code="46680005" codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT" displayName="Vital signs"/>
<statusCode code="completed"/>
<effectiveTime value="19991114"/>
<component>
<observation classCode="OBS" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.20.22.4.27"/>
...
</observation>
</component>
</observation>

Page 518 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
7 REFERENCES
American Recovery And Reinvestment Act of 2009,
http://www.gpo.gov/fdsys/pkg/PLAW-111publ5/content-detail.html
CDC, Epidemiology and Prevention of Vaccine-Preventable Diseases (The
Pink Book), Appendix D: Vaccine Administration Guidelines.
http://www.cdc.gov/vaccines/pubs/pinkbook/downloads/appendices/
D/vacc_admin.pdf
Cross Transaction Specifications and Content Specifications. IHE ITI
Technical Framework, Volume 3 (ITI TF-3) (see 5.2 Scanned Documents
Content Model).
http://www.ihe.net/Technical_Framework/upload/IHE_ITI_TF_6-
0_Vol3_FT_2009-08-10.pdf
Extensible Markup Language (XML) 1.0 (Fifth Edition),
http://www.w3c.org/TR/2008/REC-xml-20081126/
Final Rules for Stage 1 Meaningful Use,
http://edocket.access.gpo.gov/2010/pdf/2010-17207.pdf
Health Information Technology: Initial Set of Standards, Implementation
Specifications, and Certification Criteria for Electronic Health Record
Technology; 45 CFR Part 170, Final Rule,
http://edocket.access.gpo.gov/2010/pdf/2010-17210.pdf
HITSP Summary Documents Using HL7 Continuity of Care Document
(CCD) Component; (HITSP/C32); Versions 2.1, 2.2, 2.3, 2.5; December
13, 2007 - July 8, 2009
HL7 Clinical Document Architecture (CDA Release 2).
http://www.hl7.org/implement/standards/cda.cfm
HL7 Implementation Guide for CDA Release 2: Consultation Notes, (U.S.
Realm), Draft Standard for Trial Use, Release 1, Levels 1, 2, and 3, DSTU
Updated: January 2010
HL7 Implementation Guide for CDA Release 2: History and Physical (H&P)
Notes (U.S. Realm) Draft Standard for Trial Use, Release 1, Levels 1, 2,
and 3 A CDA Implementation guide for History and Physical Notes, DSTU
Updated: January 2010
HL7 Implementation Guide for CDA Release 2: Procedure Note (Universal
Realm), Draft Standard for Trial Use, Release 1, Levels 1, 2, and 3, July
2010
HL7 Implementation Guide for CDA Release 2: Unstructured Documents,
Release 1, Level 1 (Universal Realm), Draft Standard for Trial Use,
September 2010
HL7 Implementation Guide: CDA Release 2 – Continuity of Care Document
(CCD) A CDA implementation of ASTM E2369-05 Standard Specification
for Continuity of Care Record© (CCR), April 01, 2007

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 519
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
HL7 Version 3 Interoperability Standards, Normative Edition 2010.
http://www.hl7.org/memonly/downloads/v3edition.cfm - V32010
HL7 Version 3 Publishing Facilitator's Guide.
http://www.hl7.org/v3ballot/html/help/pfg/pfg.htm
Implementation Guide for CDA Release 2.0 Operative Note, (U.S. Realm),
Draft Standard for Trial Use, Release 1, Levels 1, 2 and 3, Published,
March 2009
Implementation Guide for CDA Release 2.0, Care Record Summary Release
2
Discharge Summary, (U.S. Realm) Draft Standard for Trial Use, Levels 1,
2 and 3, December 2009
Implementation Guide for CDA Release 2.0, Progress Note (U.S. Realm),
Draft Standard for Trial Use, Levels 1, 2, and 3, January 2011
Implementation Guide for CDA Release 2: Imaging Integration, Levels 1, 2,
and 3, Basic Imaging Reports in CDA and DICOM Diagnostic Imaging
Reports (DIR) – Universal Realm, Based on HL7 CDA Release 2.0, Release
1.0, Informative Document, First Release, March 2009
Joint Commission Requirements for Discharge Summary (JCAHO
IM.6.10 EP7). See
http://www.jointcommission.org/NR/rdonlyres/C9298DD0-6726-4105-
A007-FE2C65F77075/0/CMS_New_Revised_HAP_FINAL_withScoring.pdf
(page 26).
Mosby's Medical Dictionary, 8th edition. © 2009, Elsevier.
Pressure Ulcer Prevention Domain Analysis Model May 2011.
http://wiki.hl7.org/images/b/be/PressureUlcerPreventionDomainAnalys
isModel_May2011.pdf
Pressure Ulcer Prevention Domain Analysis Model, current, accessed
May 2, 2012.
http://pressureulcerpreventionmodel.com/DAM20110325/
Taber's Cyclopedic Medical Dictionary, 21st Edition, F.A. Davis
Company. http://www.tabers.com
Term Info. http://www.hl7.org/special/committees/terminfo/index.cfm
Trifolia Workbench (Consolidation Project Edition). Open source template
database. http://www.lantanagroup.com/newsroom/press-
releases/trifolia-workbench/
You must be logged in as a member of HL7.org to access this resource:
http://www.hl7.org/login/singlesignon.cfm?next=/documentcenter/priv
ate/standards/cda/Trifolia_HL7_Consolidation_20110712-dist.zip
XML Path Language (XPath), Version 1.0. http://www.w3.org/TR/xpath/

Page 520 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
APPENDIX A — ACRONYMS AND ABBREVIATIONS
ADL Activities of Daily Living
AMA American Medical Association
CCD Continuity of Care Document
CDA Clinical Document Architecture
CDC Centers for Disease Control and Prevention
DAM Domain Analysis Model
DICOM Digital Imaging and Communications in Medicine
DIR Diagnostic Imaging Report
EHR electronic health record
DSTU Draft Standard for Trial Use
H&P History and Physical
HIMSS Healthcare Information and Management Systems Society
HIT healthcare information technology
HITECH Health Information Technology for Economic and Clinical Health
HITSP Health Information Technology Standards Panel
HL7 Health Level Seven
HSS U.S. Department of Health and Human Services
HTML Hypertext Markup Language
IG implementation guide
IHE Integrating the Healthcare Enterprise
IHTSDO International Health Terminology Standard Development
Organisation
LOINC Logical Observation Identifiers Names and Codes
MDHT Model-Driven Health Tools
MIME Multipurpose Internet Mail Extensions
NPP non-physician providers
NUCC Healthcare Provider Taxonomy Code
ONC Office of National Coordinator
PCP primary care provider
PDF portable document format
PHCR Public Health case reports
PHQ Patient Health Questionnaire
PHR personal health record

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 521
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
PPRF primary performers
RIM Reference Information Model
RTF rich text format
S&I Standards and Interoperability
SCOORD Spatial Coordinates
SDWG Structured Documents Working Group
SDO Standards Development Organization
SNOMED CT Systemized Nomenclature for Medicine – Clinical Terms
SOP Service Object Pair
SR Structured Report
Tdb Template Database
TIFF tagged-image file format
UCUM Unified Code for Units of Measure
UD Unstructured Document
UDI Unique Device Identification
UML Unified Modeling Language
URL Uniform Resource Locator
VIS Vaccine Information Statement
WADO Web Access to Persistent DICOM Objects
XPath XML Path Language

Page 522 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
APPENDIX B — CHANGES FROM PREVIOUS GUIDES
The first subsection below lists the changes in the May 2012 ballot. The
remainder of the sections in this appendix explain all changes in the
consolidation of the HL7 Health Story guides, HITSP C32, related components of
IHE PCC, and CCD.
New and Updated Templates
Table 312: Templates Added and Updated in May 2012 Ballot
Template
Type
TemplateID
The following templates were updated for this version.
US Realm Header
documentOf/serviceEvent
Header
Not applicable
DIR Header documentOf
Header
Not applicable
Assessment Section
Section
2.16.840.1.113883.10.20.22.2.8
Functional Status Section
Section
2.16.840.1.113883.10.20.22.2.14
Plan of Care Section
Section
2.16.840.1.113883.10.20.22.2.10
Social History Section
Section
2.16.840.1.113883.10.20.22.2.17
Instructions Section
Section
2.16.840.1.113883.10.20.22.2.45
Encounter Activities
Entry
2.16.840.1.113883.10.20.22.4.49
Result Observation
Entry
2.16.840.1.113883.10.20.22.4.2
Result Organizer
Entry
2.16.840.1.113883.10.20.22.4.1
The following templates were added for this version.
Assessment Scale Observation
Entry
2.16.840.1.113883.10.20.22.4.69
Deceased Observation
Entry
2.16.840.1.113883.10.20.22.4.79
Caregiver Characteristics
Entry
2.16.840.1.113883.10.20.22.4.72
Cognitive Status Problem
Observation
Entry
2.16.840.1.113883.10.20.22.4.73
Cognitive Status Result
Observation
Entry
2.16.840.1.113883.10.20.22.4.74
Cognitive Status Result Organizer
Entry
2.16.840.1.113883.10.20.22.4.75
Encounter Diagnosis
Entry
2.16.840.1.113883.10.20.22.4.80
Functional Status Problem
Observation
Entry
2.16.840.1.113883.10.20.22.4.68
Functional Status Result
Observation
Entry
2.16.840.1.113883.10.20.22.4.67
Functional Status Result
Organizer
Entry
2.16.840.1.113883.10.20.22.4.66
Health Status Observation
Entry
2.16.840.1.113883.10.20.22.4.5
Highest Pressure Ulcer Stage
Entry
2.16.840.1.113883.10.20.22.4.77
Number of Pressure Ulcers
Observation
Entry
2.16.840.1.113883.10.20.22.4.76

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 523
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Template
Type
TemplateID
Pressure Ulcer Observation
Entry
2.16.840.1.113883.10.20.22.4.70
Smoking Status Observation
Entry
2.16.840.1.113883.10.22.4.78
Cardinality Changes
The next three tables show updates for H&P, Consultation Note, and Discharge
Summary cardinality.
Table 313: H&P Cardinality Updates
Sections Names
HITSP
(C84)
HL7
(H&P)
Current Cardinality
Problems
R
O
O
Resolved Problems
R
-
Vital Signs
-
R
R
Past Medical History
-
R
R
Table 314: Consultation Note Cardinality Updates
Sections Names
HITSP
(C84)
HL7
(H&P)
Current Cardinality
Active Problems
R
O
O – Problems
Resolved Problems
R2
-
Allergies
R
O
O
Current Meds
R
O
O
Past Medical History
-
O
O
Chief Complaint
-
O
O
Functional Status
R2
-
-
Advance Directives
R
-
-
Pertinent Insurance
Information (Payers)
R2
-
-

Page 524 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 315: Discharge Summary Cardinality Updates
Sections Names
HITSP
(C48)
HL7
(H&P)
Current Cardinality
Problems
R
O
O
Hospital Admission
Diagnosis Section
R
-
O
Section Code Changes
The following table documents changes to section codes used in the current
Operative Note templates to conform to those in use for general procedures.
Table 316: Surgical Operative Codes Mapping to Generic Procedure Codes
Sections Names
Section Codes
Sections Names
Section Codes
Previous Operative Section Codes
Now Using
Surgical Operation Note
Anesthesia
10213-7
Anesthesia
59774-0
Surgical Operation Note
Description
8724-7
Procedure Description
29554-3
Surgical Operation Note
Disposition
55102-8
Procedure Disposition
59775-7
Surgical Operation Note
Estimated Blood Loss
55103-6
Procedure Estimated Blood
Loss
59770-8
Surgical Operation Note
Findings
10215-2
Procedure Findings
59776-5
Surgical Operation Note
Indications
10217-8
Procedure Indications
59768-2
Surgical Operation Note
Planned Procedure
55104-4
Planned Procedure
59772-4
Surgical Operation Note
Specimens Taken
10221-0
Procedure Specimens taken
59773-2

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 525
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Conformance Verbs
The next table represents a matrix of the conformance verbs used across the standards reviewed for the consolidation
guide. Cells with a dash (-) did not have an equivalent conformance convention.
Table 317: Consolidated Conformance Verb Matrix
RFC 2119
HL7
IHE
HITSP
Workgroup Consensus
SHALL
Absolute requirement
of the specification
SHALL
Required/Mandatory
R (Required)
Element must be present
but can be NULL
R (Required)
Data elements must
always be sent. A NULL
can be sent.
SHALL
Element must be present but can be
NULL
Where necessary to explicitly
preclude nullFlavor (e.g. where you
want to preclude nullFlavor on
observation/value), can include
something like "SHALL NOT include
nullFlavor".
Where SHALL is applied to an
attribute, it must be present and
cannot be a NULL
SHALL NOT
Absolute prohibition
of the specification
SHALL NOT
Not
Required/Mandatory
-
-
SHALL NOT
Absolute prohibition against
inclusion

Page 526 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
RFC 2119
HL7
IHE
HITSP
Workgroup Consensus
SHOULD
Recommended
There may exist valid
reasons in particular
circumstances to
ignore a particular
item, but the full
implications must be
understood and
carefully weighed
before choosing a
different course.
SHOULD
Best Practice or
Recommendation
R2 (Required if known)
The sending application
must be able to
demonstrate that it can
send all required if known
elements, unless it does
not in fact gather that
data. If the information
cannot be transmitted, the
data element shall contain
a value indicating the
reason for omission of the
data.
R2 (Required if known)
If the sending
application has data for
the data element, it is
REQUIRED to populate
the data element. If the
value is not known, the
data element need not be
sent
SHOULD
Best Practice or Recommendation
There may exist valid reasons in
particular circumstances to ignore a
particular item, but the full
implications must be understood and
carefully weighed before choosing a
different course
SHOULD NOT
Not Recommended
SHOULD NOT
Not Recommended
-
-
SHOULD NOT
Not Recommended
MAY
Optional
MAY
Accepted/Permitted
O (Optional)
O (Optional)
MAY
Optional
-
-
C (Conditional)
A conditional data element
is one that is required,
required if known or
optional depending upon
other conditions.
C (Conditional)
Required to be sent when
the conditions specified
in the HITSP additional
specifications column are
true
-

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 527
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Template ID Changes
The following table tracks changes in template IDs, for the most part representing a consolidation of separate templates
into a single template. In some cases, two new template IDs are assigned to distinguish sections where computable data
entries are required and those where entries are optional and only the human-readable narrative is required. To meet
future requirements, an Entry Required template may be added to a section that doesn’t currently contain one.
Table 318: Section Template Change Tracking
Section
LOINC
Code(s)
Consolidated Entry Optional
templateId
Consolidated Entry Required
templateId
Previous templateIds
Was
Medications Category
Medications
Section
10160-0
2.16.840.1.113883.10.20.22.2
.1
2.16.840.1.113883.10.20.22.2.
1.1
2.16.840.1.113883.10.20.1.8 (CCD)
HL7
2.16.840.1.113883.3.88.11.83.112
HITSP
1.3.6.1.4.1.19376.1.5.3.1.3.19
IHE
Hospital
Discharge
Medications
Section
10183-2
2.16.840.1.113883.10.20.22.2
.11
2.16.840.1.113883.10.20.22.2.
11.1
2.16.840.1.113883.10.20.16.2.2
(DS)
HL7
2.16.840.1.113883.3.88.11.83.114
HITSP
1.3.6.1.4.1.19376.1.5.3.1.3.22
IHE

Page 528 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Section
LOINC
Code(s)
Consolidated Entry Optional
templateId
Consolidated Entry Required
templateId
Previous templateIds
Was
Medications
Administere
d Section37
29549-3
18610-6
2.16.840.1.113883.10.20.22.2
.38
Future assignment
2.16.840.1.113883.10.20.18.2.8
(Proc Note)
HL7
2.16.840.1.113883.3.88.11.83.115
HITSP
1.3.6.1.4.1.19376.1.5.3.1.3.21
IHE
Immunizatio
ns Section
11369-6
2.16.840.1.113883.10.20.22.2
.2
Future assignment
2.16.840.1.113883.10.20.1.6 (CCD)
HL7
2.16.840.1.113883.3.88.11.83.117
HITSP
1.3.6.1.4.1.19376.1.5.3.1.3.23
IHE
Conditions/Concern Category
Allergies
Section
(2.2.1.2)
48765-2
2.16.840.1.113883.10.20.22.2
.6
2.16.840.1.113883.10.20.22.2.
6.1
2.16.840.1.113883.10.20.1.2 (CCD)
HL7
2.16.840.1.113883.3.88.11.83.102
HITSP
1.3.6.1.4.1.19376.1.5.3.1.3.13
IHE
Problem
Section
11450-4
2.16.840.1.113883.10.20.22.2
.5
2.16.840.1.113883.10.20.22.2.
5.1
2.16.840.1.113883.10.20.1.11
HL7
2.16.840.1.113883.3.88.11.83.103
HITSP
1.3.6.1.4.1.19376.1.5.3.1.3.6
IHE
History of
Past Illness
Section
(2.2.1.4)
11348-0
2.16.840.1.113883.10.20.22.2
.20
2.16.840.1.113883.10.20.2.9 (H&P)
HL7
2.16.840.1.113883.3.88.11.83.104
HITSP
1.3.6.1.4.1.19376.1.5.3.1.3.8
IHE
Hospital
Discharge
Diagnosis
Section
11535-2
2.16.840.1.113883.10.20.22.2
.24
2.16.840.1.113883.10.20.16.2.1
(DS)
HL7
2.16.840.1.113883.3.88.11.83.111
HITSP
1.3.6.1.4.1.19376.1.5.3.1.3.7
IHE
Preoperative
Diagnosis
Section
10219-4
2.16.840.1.113883.10.20.22.2
.34
2.16.840.1.113883.10.20.7.1
(OpNote)
HL7
2.16.840.1.113883.3.88.11.83.129
HITSP
Post-
operative
Diagnosis
Section
10218-6
2.16.840.1.113883.10.20.22.2
.35
2.16.840.1.113883.10.20.7.2
(OpNote)
HL7
2.16.840.1.113883.3.88.11.83.130
HITSP
37 Requires further discussion and resolution.

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 529
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Section
LOINC
Code(s)
Consolidated Entry Optional
templateId
Consolidated Entry Required
templateId
Previous templateIds
Was
Chief
Complaint
Section /
Reason for
Visit
10154-3
29299-5
46239-0
Chief complaint
(1.3.6.1.4.1.19376.1.5.3.1.1.1
3.2.1)
Reason for Visit
(2.16.840.1.113883.10.20.22.
2.12)
Chief Complaint + Reason for
Visit
(2.16.840.1.113883.10.20.22.
2.13)
N/A (narrative-only)
2.16.840.1.113883.10.20.2.8 (H&P)
2.16.840.1.113883.10.20.18.2.16
(Proc Note)
HL7
2.16.840.1.113883.3.88.11.83.105
HITSP
1.3.6.1.4.1.19376.1.5.3.1.1.13.2.1
IHE
Reason for
Referral
Section
42349-1
1.3.6.1.4.1.19376.1.5.3.1.3.1
N/A (narrative-only)
2.16.840.1.113883.10.20.4.8
(Consult Note)
HL7
2.16.840.1.113883.3.88.11.83.106
HITSP
1.3.6.1.4.1.19376.1.5.3.1.3.1
(narrative-only)
1.3.6.1.4.1.19376.1.5.3.1.3.2
(coded)
IHE
History of
Present
Illness
Section
10164-2
N/A (use IHE
1.3.6.1.4.1.19376.1.5.3.1.3.4)
N/A (narrative-only)
1.3.6.1.4.1.19376.1.5.3.1.3.4
HL7
2.16.840.1.113883.3.88.11.83.107
HITSP
1.3.6.1.4.1.19376.1.5.3.1.3.4
IHE
Medical
(General)
History
Section
11329-0
2.16.840.1.113883.10.20.22.2
.39
2.16.840.1.113883.10.20.18.2.5
(Proc Note)
HL7
Procedure and Surgery Category
Procedures
Section (List
of Surgeries)
(History of
Procedures)
47519-4
2.16.840.1.113883.10.20.22.2
.7
N/A (narrative-only)
2.16.840.1.113883.10.20.1.12
(CCD)
HL7:2.16.840.1.113883.10.20.18.2
.18 (Proc Note)
HL7
2.16.840.1.113883.3.88.11.83.108
HITSP
1.3.6.1.4.1.19376.1.5.3.1.3.12
IHE
Operative
Note Fluids
Section
10216-0
2.16.840.1.113883.10.20.7.12
2.16.840.1.113883.10.20.7.12
(OpNote)
HL7

Page 530 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Section
LOINC
Code(s)
Consolidated Entry Optional
templateId
Consolidated Entry Required
templateId
Previous templateIds
Was
Operative
Note
Surgical
Procedure
Section
10223-6
2.16.840.1.113883.10.20.7.14
2.16.840.1.113883.10.20.7.14
(OpNote)
HL7
Surgical
Drains
Section
11537-8
2.16.840.1.113883.10.20.7.13
2.16.840.1.113883.10.20.7.13
(OpNote)
HL7
Procedure
Indications
Section
59768-2
2.16.840.1.113883.10.20.22.2
.29
2.16.840.1.113883.10.20.18.2.1
(Proc Note)
HL7
Procedure
Description
Section
29554-3
2.16.840.1.113883.10.20.22.2
.27
2.16.840.1.113883.10.20.18.2.2
(Proc Note)
HL7
Post-
procedure
Diagnosis
Section
59769-0
2.16.840.1.113883.10.20.22.2
.36
2.16.840.1.113883.10.20.18.2.3
(Proc Note)
HL7
Complica-
tions
Section
55109-3
2.16.840.1.113883.10.20.22.2
.37
2.16.840.1.113883.10.20.18.2.4
(Proc Note)
2.16.840.1.113883.10.20.7.10
(OpNote)
HL7
Anesthesia
Section
59774-0
2.16.840.1.113883.10.20.22.2
.25
2.16.840.1.113883.10.20.18.2.7
(Proc Note)
2.16.840.1.113883.10.20.7.5
(OpNote)
HL7
Procedure
Disposition
Section
59775-7
2.16.840.1.113883.10.20.18.2
.12
2.16.840.1.113883.10.20.18.2.12
(Proc Note)
HL7
Procedure
Estimated
Blood Loss
Section
59770-8
2.16.840.1.113883.10.20.18.2
.9
2.16.840.1.113883.10.20.18.2.9
(Proc Note)
HL7
Procedure
Findings
59776-5
2.16.840.1.113883.10.20.22.2
.28
2.16.840.1.113883.10.20.18.2.15
(Proc Note)
HL7

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 531
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Section
LOINC
Code(s)
Consolidated Entry Optional
templateId
Consolidated Entry Required
templateId
Previous templateIds
Was
Section
Procedure
Implants
Section
59771-6
2.16.840.1.113883.10.20.22.2
.40
2.16.840.1.113883.10.20.18.2.11
(Proc Note)
HL7
Planned
Procedure
Section
59772-4
2.16.840.1.113883.10.20.22.2
.30
2.16.840.1.113883.10.20.18.2.6
(Proc Note)
HL7
Procedure
Specimens
Taken
Section
59773-2
2.16.840.1.113883.10.20.22.2
.31
2.16.840.1.113883.10.20.18.2.10
(Proc Note)
HL7
Care Planning/Assessment Category
Assessment
s Section
51848-0
2.16.840.1.113883.10.20.22.2
.8
-
2.16.840.1.113883.10.20.2.7 (H&P)
2.16.840.1.113883.10.20.18.2.13
(Proc Note)
HL7
1.3.6.1.4.1.19376.1.5.3.1.1.13.2.4
HITSP
1.3.6.1.4.1.19376.1.5.3.1.1.13.2.4
IHE
Assessment
and Plan
Section
51847-2
2.16.840.1.113883.10.20.22.2
.9
-
2.16.840.1.113883.10.20.2.7 (H&P)
2.16.840.1.113883.10.20.18.2.14
(Proc Note)
HL7
1.3.6.1.4.1.19376.1.5.3.1.1.13.2.5
IHE
Plan of Care
Section
(may be
used for
Discharge
Instructions)
18776-5
2.16.840.1.113883.10.20.22.2
.10
-
2.16.840.1.113883.10.20.2.7 (H&P)
2.16.840.1.113883.10.20.1.10
(CCD)
HL7
2.16.840.1.113883.3.88.11.83.124
HITSP
1.3.6.1.4.1.19376.1.5.3.1.3.31
IHE
Functional
Status
Section
47420-5
2.16.840.1.113883.10.20.22.2
.14
-
2.16.840.1.113883.10.20.1.5
(CCD)
HL7
2.16.840.1.113883.3.88.11.83.109
HITSP
1.3.6.1.4.1.19376.1.5.3.1.3.17
IHE
Results Category
Results
30954-2
2.16.840.1.113883.10.20.22.2
2.16.840.1.113883.10.20.22.2.
2.16.840.1.113883.10.20.1.14
HL7

Page 532 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Section
LOINC
Code(s)
Consolidated Entry Optional
templateId
Consolidated Entry Required
templateId
Previous templateIds
Was
Section
(Diagnostic
Results in
HITSP)
.3
3.1
(CCD)
2.16.840.1.113883.3.88.11.83.122
HITSP
1.3.6.1.4.1.19376.1.5.3.1.3.28
IHE
Vital Signs
Section
8716-3
2.16.840.1.113883.10.20.22.2
.4
2.16.840.1.113883.10.20.22.2.
4.1
2.16.840.1.113883.10.20.1.16
(CCD)
2.16.840.1.113883.10.20.2.4 (H&P)
HL7
2.16.840.1.113883.3.88.11.83.119
HITSP
1.3.6.1.4.1.19376.1.5.3.1.3.25
IHE
DICOM
Object
Catalog
Section
121181
N/A
2.16.840.1.113883.10.20.6.1.1
2.16.840.1.113883.10.20.6.1.1
HL7
Findings
(DIR)
(Radiology
Comparison
Study -
Observation)
Section
18782-3
2.16.840.1.113883.10.20.6.1.
2
2.16.840.1.113883.10.20.6.1.2
HL7
Other Templates
Payers
Section
48768-6
2.16.840.1.113883.10.20.22.2
.18
-
2.16.840.1.113883.10.20.1.9 (CCD)
HL7
2.16.840.1.113883.3.88.11.83.101.
1
HITSP
1.3.6.1.4.1.19376.1.5.3.1.1.5.3.7
IHE
Advance
Directives
Section
42348-3
2.16.840.1.113883.10.20.22.2
.21
-
2.16.840.1.113883.10.20.1.1 (CCD)
HL7
2.16.840.1.113883.3.88.11.83.116
HITSP
1.3.6.1.4.1.19376.1.5.3.1.3.34
(narrative-only)
1.3.6.1.4.1.19376.1.5.3.1.3.35
(coded)
IHE
Physical
Exam
29545-1
2.16.840.1.113883.10.20.2.10
-
2.16.840.1.113883.10.20.2.10
(H&P)
HL7

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 533
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Section
LOINC
Code(s)
Consolidated Entry Optional
templateId
Consolidated Entry Required
templateId
Previous templateIds
Was
Section
2.16.840.1.113883.3.88.11.83.118
HITSP
1.3.6.1.4.1.19376.1.5.3.1.3.24
(narrative-only)
1.3.6.1.4.1.19376.1.5.3.1.1.9.15
(coded)
IHE
Review of
Systems
Section
10187-3
1.3.6.1.4.1.19376.1.5.3.1.3.18
N/A (narrative-only)
2.16.840.1.113883.10.20.4.10
(Consult)
HL7
2.16.840.1.113883.3.88.11.83.120
HITSP
1.3.6.1.4.1.19376.1.5.3.1.3.18
IHE
Hospital
Course
Section
(may be
used as part
of Discharge
Summary)
8648-8
1.3.6.1.4.1.19376.1.5.3.1.3.5
N/A (narrative-only)
1.3.6.1.4.1.19376.1.5.3.1.3.5
HL7
2.16.840.1.113883.3.88.11.83.121
HITSP
1.3.6.1.4.1.19376.1.5.3.1.3.5
IHE
Family
History
Section
10157-6
2.16.840.1.113883.10.20.22.2
.15
-
2.16.840.1.113883.10.20.1.4 (CCD)
2.16.840.1.113883.10.20.18.2.17
(Proc Note)
HL7
2.16.840.1.113883.3.88.11.83.125
HITSP
1.3.6.1.4.1.19376.1.5.3.1.3.14
(narrative-only)
1.3.6.1.4.1.19376.1.5.3.1.3.15
(coded)
IHE
Social
History
Section(incl.
smoking)
29762-2
2.16.840.1.113883.10.20.22.2
.17
N/A (no stds require entry)
2.16.840.1.113883.10.20.1.15
(CCD)
HL7
2.16.840.1.113883.3.88.11.83.126
HITSP
1.3.6.1.4.1.19376.1.5.3.1.3.16
IHE
Encounters
Section
46240-8
2.16.840.1.113883.10.20.22.2
.22
-
2.16.840.1.113883.10.20.1.3 (CCD)
HL7
2.16.840.1.113883.3.88.11.83.127
HITSP
1.3.6.1.4.1.19376.1.5.3.1.1.5.3.3
IHE
Medical
46264-8
2.16.840.1.113883.10.20.22.2
-
2.16.840.1.113883.10.20.1.7 (CCD)
HL7

Page 534 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Section
LOINC
Code(s)
Consolidated Entry Optional
templateId
Consolidated Entry Required
templateId
Previous templateIds
Was
Equipment
Section
.23
2.16.840.1.113883.3.88.11.83.128
HITSP
1.3.6.1.4.1.19376.1.5.3.1.1.5.3.5
IHE
Hospital
Discharge
Physical
Section
10184-0
N/A
(1.3.6.1.4.1.19376.1.5.3.1.3.2
6)
N/A (narrative-only)
N/A – Used IHE
HL7
1.3.6.1.4.1.19376.1.5.3.1.3.26
IHE
General
Status
Section
10210-3
N/A
(2.16.840.1.113883.10.20.2.5)
N/A (narrative-only)
2.16.840.1.113883.10.20.2.5 (H&P)
HL7
Objective
Section
61149-1
N/A
(2.16.840.1.113883.10.20.21.
2.1)
N/A (narrative-only)
2.16.840.1.113883.10.20.22.2.1
(Prog Note)
HL7
Subjective
Section
61150-9
N/A
(2.16.840.1.113883.10.20.21.
2.2)
N/A (narrative-only)
2.16.840.1.113883.10.20.22.2.2
(Prog Note)
HL7
Discharge
Diet
42344-2
N/A
(1.3.6.1.4.1.19376.1.5.3.1.3.3
3)
N/A (narrative-only)
N/A – Used IHE
HL7
1.3.6.1.4.1.19376.1.5.3.1.3.33
IHE
Hospital
Discharge
Studies
Summary
Section
11493-4
2.16.840.1.113883.10.20.22.2
.16
N/A (no stds require entry)
2.16.840.1.113883.10.20.16.2.3
(DS)
HL7

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 535
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Consolidated Entries
The following table tracks changes made to consolidate templates originating in HL7, IHE, and HITSP.
Table 319: Entry Change Tracking Table
Entry
New templateId
Previous Title
Previous templateId
Previous Template
Organization
Result Organizer
2.16.840.1.113883.10.20.22.4.1
Result Organizer
2.16.840.1.113883.10.20.1.32
CCD
Result Observation
2.16.840.1.113883.10.20.22.4.2
Result Observation
Result Entry Content
Module
2.16.840.1.113883.10.20.1.31
2.16.840.1.113883.3.88.11.83.15.1
CCD
HITSP C83
Problem Concern Act
(Condition)
2.16.840.1.113883.10.20.22.4.3
Problem Act
Concern Entry
Problem Concern
Entry
Condition Entry
Module
2.16.840.1.113883.10.20.1.27
1.3.6.1.4.1.19376.1.5.3.1.4.5.1
1.3.6.1.4.1.19376.1.5.3.1.4.5.2
2.16.840.1.113883.3.88.11.83.7
CCD
IHE PCC R6-0 V2
IHE PCC R6-0 V2
HITSP C83
Problem Observation
2.16.840.1.113883.10.20.22.4.4
Problem Observation
Problem Entry
2.16.840.1.113883.10.20.1.28
1.3.6.1.4.1.19376.1.5.3.1.4.5
CCD
IHE PCC R6-0 V2
Health Status
Observation
2.16.840.1.113883.10.20.22.4.5
Problem Healthstatus
observation
Health Status
2.16.840.1.113883.10.20.1.51
1.3.6.1.4.1.19376.1.5.3.1.4.1.2
CCD
IHE PCC R6-0 V2
Problem Status
Observation
2.16.840.1.113883.10.20.22.4.6
Problem status
observation
Problem Status
Observation
2.16.840.1.113883.10.20.1.50
1.3.6.1.4.1.19376.1.5.3.1.4.1.1
CCD
IHE PCC R6-0 V2
Allergy - Intolerance
Observation
2.16.840.1.113883.10.20.22.4.7
Allergy/Alert
Observation
Alert observation
Allergy and
Intolerance Concern
Allergy/Drug
Sensitivity Module
2.16.840.1.113883.10.20.22.4.7
2.16.840.1.113883.10.20.1.18
1.3.6.1.4.1.19376.1.5.3.1.4.5.3
2.16.840.1.113883.3.88.11.83.6
Consolidated IG
CCD
IHE PCC R6-0 V2
HITSP C83
Severity Observation
2.16.840.1.113883.10.20.22.4.8
Severity observation
Severity
2.16.840.1.113883.10.20.1.55
1.3.6.1.4.1.19376.1.5.3.1.4.1
CCD
IHE PCC R6-0 V2

Page 536 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Entry
New templateId
Previous Title
Previous templateId
Previous Template
Organization
Reaction Observation
2.16.840.1.113883.10.20.22.4.9
Reaction Observation
2.16.840.1.113883.10.20.1.54
CCD
Procedure Activity
2.16.840.1.113883.10.20.22.4.12
Procedure activity
2.16.840.1.113883.10.20.1.29
CCD
Procedure Activity
Observation
2.16.840.1.113883.10.20.22.4.13
Procedure activity
2.16.840.1.113883.10.20.1.29
CCD
Procedure Activity
Procedure
2.16.840.1.113883.10.20.22.4.14
Procedure activity
Procedure Entry
Procedure
2.16.840.1.113883.10.20.1.29
1.3.6.1.4.1.19376.1.5.3.1.4.19
2.16.840.1.113883.3.88.11.83.17
CCD
IHE PCC R6-0 V2
HITSP C83
Immunization
SubstanceAdministra
tion
2.16.840.1.113883.10.20.22.4.52
Medication Activity
(for immunization)
Immunization
Immunization
2.16.840.1.113883.10.20.1.24
1.3.6.1.4.1.19376.1.5.3.1.4.12
2.16.840.1.113883.3.88.11.83.13
CCD
IHE PCC R6-0 V2
HITSP C83
Medication Activity
2.16.840.1.113883.10.20.22.4.16
Medication Activity
Medication
Medication
2.16.840.1.113883.10.20.1.24
1.3.6.1.4.1.19376.1.5.3.1.4.7
2.16.840.1.113883.3.88.11.83.8
CCD
IHE PCC R6-0 V2
HITSP C83
Medication Supply
Order
2.16.840.1.113883.10.20.22.4.17
Supply Activity
Supply entry
Order Information
Constraint
2.16.840.1.113883.10.20.1.34
1.3.6.1.4.1.19376.1.5.3.1.4.7.3
2.16.840.1.113883.3.88.11.83.8.3
CCD
IHE PCC R6-0 V2
HITSP C83
Medication Dispense
2.16.840.1.113883.10.20.22.4.18
Supply Activity
Supply entry
2.16.840.1.113883.10.20.1.34
1.3.6.1.4.1.19376.1.5.3.1.4.7.3
CCD
IHE PCC R6-0 V2
Indication
2.16.840.1.113883.10.20.22.4.19
Indications
2.16.840.1.113883.3.88.11.83.138
HITSP C83
Instructions
2.16.840.1.113883.10.20.22.4.20
Patient instruction
Patient Medication
Instructions
2.16.840.1.113883.10.20.1.49
1.3.6.1.4.1.19376.1.5.3.1.4.3
CCD
IHE PCC R6-0 V2
Sequence Number
2.16.840.1.113883.10.20.22.4.22
Medication
Information
(manufacturedMateri
al)
2.16.840.1.113883.10.20.22.4.23
Product
Product Entry
Medication
Information
Constraints
2.16.840.1.113883.10.20.1.53
1.3.6.1.4.1.19376.1.5.3.1.4.7.2
2.16.840.1.113883.3.88.11.83.8.2
CCD
IHE PCC R6-0 V2
HITSP C83

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 537
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Entry
New templateId
Previous Title
Previous templateId
Previous Template
Organization
Drug Vehicle
(participant)
2.16.840.1.113883.10.20.22.4.24
Precondition
(criterion)
2.16.840.1.113883.10.20.22.4.25
Medication Use –
None known
(deprecated)
2.16.840.1.113883.10.20.22.4.29
Vital Signs Organizer
2.16.840.1.113883.10.20.22.4.26
Vital signs organizer
Vital Signs Organizer
2.16.840.1.113883.10.20.1.35
1.3.6.1.4.1.19376.1.5.3.1.4.13.1
CCD
IHE PCC R6-0 V2
Vital Signs
Observation
2.16.840.1.113883.10.20.22.4.27
Vital Signs
Observation
1.3.6.1.4.1.19376.1.5.3.1.4.13.2
IHE PCC R6-0 V2
Allergy Status
Observation
2.16.840.1.113883.10.20.22.4.28
Alert Status
2.16.840.1.113883.10.20.1.39
CCD
Allergy Problem Act
2.16.840.1.113883.10.20.22.4.30
Age Observation
2.16.840.1.113883.10.20.22.4.31
Age Observation
2.16.840.1.113883.10.20.22.4.38
CCD
Encounter Location
2.16.840.1.113883.10.20.22.4.32
Encounter Location
2.16.840.1.113883.10.20.1.45
CCD
Hospital Discharge
Diagnosis
2.16.840.1.113883.10.20.22.4.33
Discharge Diagnosis
Hospital Admission
Diagnosis
2.16.840.1.113883.10.20.22.4.34
Admission Diagnosis
Discharge medication
2.16.840.1.113883.10.20.22.4.35
Admission medication
2.16.840.1.113883.10.20.22.4.36
Product Instance
2.16.840.1.113883.10.20.22.4.37
Social History
Observation
2.16.840.1.113883.10.20.22.4.38
Social History
Observation
Social History
Social History
Observation
2.16.840.1.113883.10.20.1.33
2.16.840.1.113883.3.88.11.83.19
1.3.6.1.4.1.19376.1.5.3.1.4.13.4
CCD
HITSP C83
IHE PCC R6-0 V2
Family History
Organizer
2.16.840.1.113883.10.20.22.4.45
Family History
Organizer
Family Member
Information
2.16.840.1.113883.10.20.1.23
2.16.840.1.113883.3.88.11.83.18
CCD
HITSP C83

Page 538 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Entry
New templateId
Previous Title
Previous templateId
Previous Template
Organization
Family History
Observation
2.16.840.1.113883.10.20.22.4.46
Family History
Observation
2.16.840.1.113883.10.20.1.22
CCD
Family History Death
Observation
2.16.840.1.113883.10.20.22.4.47
Family History Cause
of Death Observation
2.16.840.1.113883.10.20.1.42
CCD
Advance Directive
Observation
2.16.840.1.113883.10.20.22.4.48
Advance Directive
Observation
2.16.840.1.113883.10.20.1.17
CCD
Comment Template
2.16.840.1.113883.10.20.22.4.64
Comments
Comment Module
IHE Comment
Module
2.16.840.1.113883.10.20.1.40
2.16.840.1.113883.3.88.11.83.11
1.3.6.1.4.1.19376.1.5.3.1.4.2
CCD
HITSP C83
IHE PCC R6-0 V2

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 539
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Changes Within Sections
The next five tables show changes in the Results, Problems, Vital Signs, Procedures, and Medications sections.
Table 320: Result Section Changes
Title
Previous Templates
New Templates
Changes from HITSP C83
Changes from CCD
Results
Section
2.16.840.1.113883.10.20.1.14
(HL7)
2.16.840.1.113883.3.88.11.83.122
(HITSP)
1.3.6.1.4.1.19376.1.5.3.1.3.28 (IHE)
2.16.840.1.113883.10.20.22.2.3
(optional entries)
2.16.840.1.113883.10.20.22.2.3.
1 (requires entries)
1. IHE Coded Results template
(1.3.6.1.4.1.19376.1.5.3.1.3.28)
is not required
2. The C83 Procedure Module
(2.16.840.1.113883.3.88.11.83.
17 ) is not required
3. Result Organizer
(section/entry/organizer) is
required for all coded results
1. Result Organizer
(section/entry/organize
r) is required for all
coded results
Result
Organizer
2.16.840.1.113883.10.20.1.32
(CCD)
2.16.840.1.113883.10.20.22.4.1
1. Requires new Result
Observation
(2.16.840.1.113883.10.20.22.4.
2)
1. Requires new Result
Observation
(2.16.840.1.113883.10.
20.22.4.2)
Result
Observa-
tion
2.16.840.1.113883.10.20.1.31
(CCD)
2.16.840.1.113883.3.88.11.83.15.1
(HITSP)
1.3.6.1.4.1.19376.1.5.3.1.4.13 (IHE)
2.16.840.1.113883.10.20.22.4.2
1. Narrative-entry link is not
required.
2. observation “INT” and “PRP”
@moodCodes no longer included
1. Requires
observation/statusCode
2. Requires
observation/effectiveTi
me

Page 540 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Table 321: Problems Section Changes
Title
(templateId)
Previous templateId
Changes from IHE
Changes from HITSP C83
Changes from CCD
Problems Section
(Entries Optional)
(2.16.840.1.11388
3.10.20.22.2.5)
2.16.840.1.113883.10.20.1.11
(CCD)
1.3.6.1.4.1.19376.1.5.3.1.3.6 (IHE)
2.16.840.1.113883.3.88.11.83.103
(C83)
1. IHE templateId(s) no longer
required
2. title updated to SHALL
[1..1] be present
3. entry should be present
and contain Problem
Concern Act
1. C83 templateId(s) no
longer required
2. title updated to SHALL
[1..1] be present
3. entry should be present
and contain Problem
Concern Act
1. CCD templateId no
longer required
2. title updated to not
require inclusion of
"problems"
3. text updated to SHALL
[1..1] be present
4. entry should be
present and contain
Problem Concern Act
Problems Section
(Entries Required)
(2.16.840.1.11388
3.10.20.22.2.5.1)
2.16.840.1.113883.10.20.1.11
(CCD)
1.3.6.1.4.1.19376.1.5.3.1.3.6 (IHE)
2.16.840.1.113883.3.88.11.83.103
(C83)
1. IHE templateId(s) no longer
required
2. title updated to SHALL
[1..1] be present
3. entry updated to SHALL
[1..*] contain Problem
Concern Act
1. C83 templateId(s) no
longer required
2. title updated to SHALL
[1..1] be present
3. entry updated to SHALL
[1..*] contain Problem
Concern Act
1. CCD templateId no
longer required
2. title updated to not
require inclusion of
"problems"
3. entry updated to SHALL
[1..*] contain Problem
Concern Act
Problem Concern
Act
(2.16.840.1.11388
3.10.20.22.4.3)
2.16.840.1.113883.10.20.1.27
(CCD)
1.3.6.1.4.1.19376.1.5.3.1.4.5.1
(IHE)
1.3.6.1.4.1.19376.1.5.3.1.4.5.2
(IHE)
2.16.840.1.113883.3.88.11.83.7
(C83)
1. IHE templateId(s) no longer
required
2. id updated to SHALL [1..*]
3. code updated to SHALL
[1..1] 'CONC' from
2.16.840.1.113883.5.6
4. statusCode updated to
SHALL [1..1] be from
2.16.840.1.113883.11.20.9.
19
5. effectiveTime/high updated
to remove dependency on
status
1. C83 templateId(s) no
longer required
2. id updated to SHALL [1..*]
3. code updated to SHALL
[1..1] 'CONC' from
2.16.840.1.113883.5.6
4. statusCode updated to
SHALL [1..1] be from
2.16.840.1.113883.11.20.
9.19
5. effectiveTime updated to
SHALL [1..1]
1. CCD templateId no
longer required
2. id updated to SHALL
[1..*]
3. code updated to
SHALL [1..1] 'CONC'
from
2.16.840.1.113883.5.6
4. statusCode updated to
SHALL [1..1] be from
2.16.840.1.113883.11.
20.9.19
5. effectiveTime updated
to SHALL [1..1]

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 541
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Title
(templateId)
Previous templateId
Changes from IHE
Changes from HITSP C83
Changes from CCD
Problem
Observation
(2.16.840.1.11388
3.10.20.22.4.4)
2.16.840.1.113883.10.20.1.28
(CCD)
1.3.6.1.4.1.19376.1.5.3.1.4.5 (IHE)
1. IHE templateId(s) no longer
required
2. id updated to SHALL [1..*]
3. code updated to SHALL
[1..1] be from
2.16.840.1.113883.3.88.12.
3221.7.2
4. Text and
text/reference/@value
updated to SHOULD [0..1]
be present
5. effectiveTime must be
updated for semantic
differences
6. value updated to SHALL
[1..1] be CD from
2.16.840.1.113883.3.88.12.
3221.7.4
7. entryRelationships must be
updated for differences
New requirement, no
mappings required
1. CCD templateId no
longer required
2. id updated to SHALL
[1..*]
3. code updated to
SHALL [1..1] be from
2.16.840.1.113883.3.8
8.12.3221.7.2
4. Text and
text/reference/@value
SHOULD be present
5. effectiveTime and child
elements updated to
SHOULD [0..1] be
present
6. value updated to
SHALL [1..1] be CD
from
2.16.840.1.113883.3.8
8.12.3221.7.4
7. entryRelationships
must be updated for
differences
8. Sources of information
differences are allowed
under open template
rules.
Problem Status
Entry
(2.16.840.1.11388
3.10.20.22.4.6)
2.16.840.1.113883.10.20.1.50
(CCD)
1.3.6.1.4.1.19376.1.5.3.1.4.1.1
(IHE)
1. IHE templateId(s) no longer
required
2. text updated to SHOULD
[0..1] (not SHALL)
3. value updated to SHALL be
[1..1] CD from
2.16.840.1.113883.3.88.12.
80.68
New requirement, no
mappings required
1. CCD templateId no
longer required
2. text updated to
SHOULD [0..1] be
present
3. value updated to
SHALL be [1..1] CD
from
2.16.840.1.113883.3.8
8.12.80.68

Page 542 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Title
(templateId)
Previous templateId
Changes from IHE
Changes from HITSP C83
Changes from CCD
Health Status
Observation
(2.16.840.1.11388
3.10.20.22.4.5)
2.16.840.1.113883.10.20.1.51
(CCD)
1.3.6.1.4.1.19376.1.5.3.1.4.1.2
(IHE)
1. IHE templateId(s) no longer
required
2. text and
text/reference/@value
updated to SHOULD [0..1] be
present
3. value updated to SHALL be
[1..1] CD from
2.16.840.1.113883.3.88.12.8
0.68
4. entryRelationships must be
updated for differences
New requirement, no
mappings required
1. CCD templateId no
longer required
2. id updated to SHALL
[1..*]
3. text and text/@value
updated to SHOULD
[0..1] be present
4. effectiveTime and child
elements updated to
SHOULD [0..1] be
present
5. value updated to SHALL
be [1..1] CD from
2.16.840.1.113883.3.88
.12.80.68
6. entryRelationships
must be updated for
differences
7. Sources of information
differences are allowed
under open template
rules.

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 543
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 322: Vital Signs Section Changes
Title
(templateId)
Previous templateId
Changes from IHE
Changes from HITSP C83
Changes from CCD
Vital Signs Section
(Entries Optional)
(2.16.840.1.11388
3.10.20.22.4)
2.16.840.1.113883.10.20.1.16
(CCD)
1.3.6.1.4.1.19376.1.5.3.1.1.5.3.25
(IHE)
2.16.840.1.113883.3.88.11.83.119
(C83)
1. IHE templateId(s) no longer
required
2. title updated to not require
inclusion of "vital signs"
1. HITSP templateID(s) no
longer required
2. code/@code updated to
SHALL be [1..1] 8716-3
from
2.16.840.1.113883.6.1
3. title updated to SHALL
[1..1] occur
1. CCD templateId no
longer required
2. title updated to not
require inclusion of
"vital signs"
Vital Signs Section
(Entries Required)
(2.16.840.1.11388
3.10.20.22.4.1)
2.16.840.1.113883.10.20.1.16
(CCD)
1.3.6.1.4.1.19376.1.5.3.1.1.5.3.25
(IHE)
2.16.840.1.113883.3.88.11.83.119
(C83)
1. IHE templateId(s) no longer
required
2. title updated to not require
inclusion of "vital signs"
3. Section updated to SHALL
contain [1..*] Vital Signs
Organizer
1. HITSP templateID(s) no
longer required
2. code/@code updated to
SHALL be [1..1] 8716-3
from
2.16.840.1.113883.6.1
3. title updated to SHALL
[1..1] occur
4. Section updated to SHALL
contain [1..*] Vital Signs
Organizer
1. CCD templateId no
longer required
2. title updated to not
require inclusion of
"vital signs"
3. Section updated to
SHALL contain [1..*]
Vital Signs Organizer
Vital Signs
Organizer
(2.16.840.1.11388
3.10.20.22.4.26)
1.3.6.1.4.1.19376.1.5.3.1.4.13.1
(IHE)
2.16.840.1.113883.10.20.1.35
(CCD)
1. IHE templateId(s) no longer
required
2. Requires [1..*] organizer/id
3. effectiveTime updated to
SHALL [1..1]
1. HITSP templateID(s) no
longer required
2. Requires [1..*]
organizer/id
3. effectiveTime updated to
SHALL [1..1]
1. CCD templateId no
longer required
2. @classCode updated to
CLUSTER
3. code updated to
46680005 from
2.16.840.1.113883.6.9
6
4. statusCode updated to
"completed"
5. effectiveTime updated
to SHALL [1..1]

Page 544 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Title
(templateId)
Previous templateId
Changes from IHE
Changes from HITSP C83
Changes from CCD
Vital Signs
Observation
(2.16.840.1.11388
3.10.20.22.4.27)
1.3.6.1.4.1.19376.1.5.3.1.4.13.2
(IHE)
1. IHE templateId(s) no longer
required
2. id updated to SHALL [1..*]
3. code replaced with value
from value set
2.16.840.1.113883.3.88.12.
80.62
4. text updated to SHOULD
[0..1]
5. text/reference/@value
updated to SHOULD [0..1]
6. statusCode updated to
SHALL [1..1] 'completed'
7. effectiveTime updated to
SHALL [1..1]
New requirement, no
mappings required
New requirement, no
mappings required

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 545
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Table 323: Procedures Section Changes
Title
(templateId)
Previous templateId
Changes from IHE
Changes from HITSP C83
Changes from CCD
Procedures Section
(Entries Optional)
(2.16.840.1.11388
3.10.20.22.2.7)
2.16.840.1.113883.10.20.1.12
(CCD)
No equivalent template
No equivalent template
1. CCD templateId no longer
required
2. Remove title requirements
CONF-425 and CONF 426
3. Add SHALL requirement for text
Procedures Section
(Entries Required)
(2.16.840.1.11388
3.10.20.22.2.7.1)
2.16.840.1.113883.10.20.1.12
(CCD)
1.3.6.1.4.1.19376.1.5.3.1.3.11
(IHE)
1.3.6.1.4.1.19376.1.5.3.1.3.12
(IHE)
2.16.840.1.113883.3.88.11.83.
145 (C83)
1. IHE templateId(s) no
longer required
2. Explicit that multiple
entries are allowed.
3. Remove constraint for
References Entry
4. Add text SHALL be [1..1]
present
1. C83 templateId(s) no
longer required
2. Add SHALL be [1..1]
present and
@code='45719-4' LOINC.
3. Add text SHALL be [1..1]
present
1. CCD templateId no longer
required
2. Remove title requirements
CONF-425 and CONF 426
3. Add text SHALL be [1..1]
present
4. Add requirement for at least one
Procedure Activity Act,
Procedure Activity Observation,
or Procedure Activity Procedure.
Procedure Activity
Procedure Entry
(2.16.840.1.11388
3.10.20.22.4.14)
2.16.840.1.113883.10.20.1.29
(CCD)
2.16.840.1.113883.11.83.17
(C83)
1.3.6.1.4.1.19376.1.5.3.1.4.19
(C83)
No template supplied in
guide.
1. C83 templateId(s) no
longer required.
2. Constrain @classCode to
PROC
3. Replace @moodCode with
value from
2.16.840.1.113883.11.20.
9.18
4. Relax code/originalText
to SHOULD contain
reference/@value
5. Add statusCode SHALL
be [1..1] present from
2.16.840.1.113883.11.20.
9.22
6. Add priorityCode MAY be
[0..1] present from
2.16.840.1.113883.1.11.1
6866
1. CCD templateId no longer
required
2. Constrain @classCode to PROC
3. Replace @moodCode with value
from
2.16.840.1.113883.11.20.9.18
4. Add code SHOULD contain
originalText
5. Add code/originalText SHOULD
contain reference/@value
6. Replace statusCode with value
from
2.16.840.1.113883.11.20.9.22
7. Add priorityCode MAY [0..1] be
present from
2.16.840.1.113883.1.11.16866
8. Constrain methodCode to MAY
[0..1]
9. Replace targetSiteCode with

Page 546 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Title
(templateId)
Previous templateId
Changes from IHE
Changes from HITSP C83
Changes from CCD
7. Add methodCode MAY be
[0..1] present
8. Add specimen and
children MAY be [0..*]
present.
9. Add required attributes
for
performer/assignedEntity
10. Add MAY [0..*]
participant/@typeCode='D
EV' and allow
corresponding Product
Instance
11. Add MAY [0..*]
participant/@typeCode='L
OC' and allow
corresponding Service
Delivery Location
12. Add MAY [0..1]
entryRelationship with
@typeCode='COMP'
@inversionInd='TRUE' to
encounter with required id.
13. Add MAY [0..1]
entryRelationship with
@typeCode='SUBJ'
@inversionInd='TRUE' to
Instructions with required
templateId.
14. Add MAY [0..1]
entryRelationship with
@typeCode='RSON' to
Indication with required
templateId.
15. Add MAY [0..1]
entryRelationship with
@typeCode='COMP' to
Medication Activity with
SHOULD [0..1] from
2.16.840.1.113883.3.88.12.322
1.8.9
10. Add required attributes for
performer/assignedEntity
11. Add MAY [0..*]
participant/@typeCode='DEV'
and allow corresponding
Product Instance
12. Add MAY [0..1]
entryRelationship with
@typeCode='COMP'
@inversionInd='TRUE' to
encounter with required
templateId.
13. Add required attributes for
entryRelationship/encounter
14. Replace templateId for
Encounter Location with that of
Service Delivery Location
15. Constrain entryRelationship to
instructions to [0..1]
16. Replace Instructions
templateId with
2.16.840.1.113883.10.20.22.4.
20
17. Add templateId
2.16.840.1.113883.10.20.22.4.
19 entryRelationship/Indication
(if required) and remove existing
templateId(s).
18. Replace
2.16.840.1.113883.10.20.1.24.
20 templateId with
2.16.840.1.113883.10.20.22.4.
16 Medication Activity and
constrain to MAY be [0..1]

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 547
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Title
(templateId)
Previous templateId
Changes from IHE
Changes from HITSP C83
Changes from CCD
required templateId.
present.
ProcedureActivity
Observation Entry
(2.16.840.1.11388
3.10.20.22.4.13)
2.16.840.1.113883.10.20.1.29
(CCD)
2.16.840.1.113883.11.83.17
(C83)
1.3.6.1.4.1.19376.1.5.3.1.4.19
(C83)
No template supplied in
guide.
Same as
2.16.840.1.113883.11.83.17
changes EXCEPT:
1. Constrain @classCode to
OBS
2. specimen and children
MAY be [0..*] present
constraint is not
applicable
3. participant/@typeCode='
DEV' and corresponding
Product Instance template
are not applicable.
Same as
2.16.840.1.113883.10.20.1.29
changes EXCEPT:
1. Constrain @classCode to OBS
2. specimen and children MAY be
[0..*] present constraint is not
applicable
3. participant/@typeCode='DEV'
and corresponding Product
Instance template are not
applicable.
ProcedureActivity
Act Entry
(2.16.840.1.11388
3.10.20.22.4.12)
2.16.840.1.113883.10.20.1.29
(CCD)
2.16.840.1.113883.11.83.17
(C83)
1.3.6.1.4.1.19376.1.5.3.1.4.19
(C83)
No template supplied in
guide.
Same as
2.16.840.1.113883.11.83.17
changes EXCEPT:
1. Constrain @classCode to
ACT
2. specimen and children
MAY be [0..*] present
constraint is not
applicable
3. participant/@typeCode='
DEV' and corresponding
Product Instance template
are not applicable.
4. methodCode constraint is
not applicable
5. targetSiteCode constraint
is not applicable
Same as
2.16.840.1.113883.10.20.1.29
changes EXCEPT:
1. Constrain @classCode to ACT
2. specimen and children MAY be
[0..*] present constraint is not
applicable
3. participant/@typeCode='DEV'
and corresponding Product
Instance template are not
applicable.
4. methodCode constraint is not
applicable
5. targetSiteCode constraint is not
applicable

Page 548 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Title
(templateId)
Previous templateId
Changes from IHE
Changes from HITSP C83
Changes from CCD
Service Delivery
Location Entry
(2.16.840.1.11388
3.10.20.22.4.32)
2.16.840.1.113883.10.20.1.45
(CCD)
No equivalent template.
No equivalent template.
1. CCD templateId no longer
required.
2. Constrain code to SHALL be
[1..1] from
2.16.840.1.113883.1.11.20275
3. Update addr to SHOULD be
[0..1] present
4. Update telecom to SHOULD be
[0..1] present
5. Add playingEntity/name MAY
be [0..1] present.
Product Instance
Entry
(2.16.840.1.11388
3.10.20.22.4.37)
2.16.840.1.113883.10.20.1.53
(CCD)
1.3.6.1.4.1.19376.1.5.3.1.4.7.
2 (IHE)
1. IHE templateId(s) no
longer required.
2. Replace entire IHE
template with
Consolidated template
and templateId.
(differences in classes
in templates – IHE
product entry cannot be
attached to procedures
in CDA)
1. C83 templateId(s) no
longer required.
2. Replace entire C83
template with
Consolidated template
and templateId.
(differences in classes in
templates – C83 product
form cannot be attached
to procedures in CDA)
1. CCD templateId no longer
required.
2. Replace entire CCD template
with Consolidated template
and templateId. (differences in
classes in templates – CCD
product instance cannot be
attached to procedures in
CDA)
Table 324: Medications Section Changes
Title
(templateId)
Previous templateId
Changes from IHE
Changes from HITSP C83
Changes from CCD
Medications
Section (Entries
Optional)
(2.16.840.1.113
883.10.20.22.2.
1)
2.16.840.1.113883.10.20.1.8
(CCD)
No equivalent template
No equivalent template
1. CCD templateId no longer
required
2. title updated to not require
inclusion of "medication"
3. text updated to SHALL [1..1] be
present

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 549
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Title
(templateId)
Previous templateId
Changes from IHE
Changes from HITSP C83
Changes from CCD
4. entry should be present and
contain Medication Activity
5. entry should be present and
contain Medication Use – None
Known. This is in place of the
simple statement that absence
of known medications SHALL
be explicitly asserted
Medications
Section (Entries
Required)
(2.16.840.1.113
883.10.20.22.2.
1.1)
1.3.6.1.4.1.19376.1.5.3.1.3.19
(IHE)
2.16.840.1.113883.3.88.11.83.
112 (C83)
1. IHE templateId(s) no
longer required
2. title updated to SHALL
[1..1] be present
3. text updated to SHALL
[1..1] be present
4. entry SHALL be present
and contain Medication
Activity or and contain
Medication Use – None
Known
1. C83 templateId(s) no
longer required
2. title updated to SHALL
[1..1] be present
3. text updated to SHALL
[1..1] be present
4. entry SHALL be present
and contain Medication
Activity or and contain
Medication Use – None
Known
No equivalent template
Medication
Activity
(2.16.840.1.113
883.10.20.22.4.
16)
2.16.840.1.113883.10.20.1.24
(CCD)
1.3.6.1.4.1.19376.1.5.3.1.4.7
(IHE)
1.3.6.1.4.1.19376.1.5.3.1.4.7.1
(IHE)
1.3.6.1.4.1.19376.1.5.3.1.4.8
(IHE)
1.3.6.1.4.1.19376.1.5.3.1.4.9
(IHE)
1.3.6.1.4.1.19376.1.5.3.1.4.10
(IHE)
1.3.6.1.4.1.19376.1.5.3.1.4.11
(IHE)
2.16.840.1.113883.3.88.11.83.
8 (C83)
1. IHE templateId(s) no
longer required
2. @moodCode changed to
require value from
2.16.840.1.113883.11.2
0.9.18 (effect is same).
3. id SHALL [1..*] be
present
4. relax code to MAY BE
[0..1] present, and use
template Medication Use
– None Known to assert
no meds.
5. Relax
text/reference/@value to
SHOULD [0..1] be
present.
6. Relax statusCode to
1. C83 templateId(s) no
longer required
2. @moodCode changed to
require value from
2.16.840.1.113883.11.20.
9.18 (effect is same).
3. id SHALL [1..*] be present
4. relax entry SHALL to
SHOULD be present and
contain Medication Activity
5. Relax
text/reference/@value to
SHOULD [0..1] be present.
6. Add statusCode SHALL
be [1..1] present
7. effectiveTime updated to
SHALL be [1..1] present
1. CCD templateId no longer
required
2. @classsCode updated to
SHALL [1..1] be 'SBADM'
3. @moodCode changed to
require value from
2.16.840.1.113883.11.20.9.18
(effect is same).
4. code may be present
5. add text SHOULD [0..1] be
present.
6. Replace statusCode SHOULD
with SHALL be [1..1] present.
7. effectiveTime updated to
SHALL be [1..1] present and
SHOULD [0..*]. See guides for
usage.

Page 550 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Title
(templateId)
Previous templateId
Changes from IHE
Changes from HITSP C83
Changes from CCD
SHALL be [1..1] present
7. effectiveTime updated to
SHALL be [1..1] present
and SHOULD [0..*]. See
guides for usage.
8. Add repeatNumber MAY
[0..1] be present
9. Constrain routeCode to
values from
2.16.840.1.113883.3.88.
12.3221.8.7
10. Constrain
approachSiteCode to
values from
2.16.840.1.113883.3.88.
12.3221.8.9
11. Simplify doseQuantity to
single element. See
guides for usage.
12. Update rateQuantity to
MAY be [0..1] present.
See guides for usage.
13. Update rateQuantity to
contain @unit from
2.16.840.1.113883.1.11.
12839
14. add constraint that
exactly one doseQuantity
or rateQuantity SHOULD
be present
15. add maxDoseQuantity
MAY be [0..1] present
16. add
administrationUnitCode
MAY be [0..1] present
17. update consumable to
include Medication
and SHOULD [0..*]. See
guides for usage.
8. Add repeatNumber MAY
[0..1] be present
9. Update routeCode
constraint to MAY be [0..1]
10. update doseQuantity to
SHOULD be [0..1] present
11. add rateQuantity MAY be
[0..1] present and contain
@unit from
2.16.840.1.113883.1.11.1
2839
12. add constraint that
exactly one doseQuantity
or rateQuantity SHOULD
be present
13. update maxDoseQuantity
to MAY be [0..1] present
14. update consumable to
include Medication
Information template. C83
templateId is no longer
required.
15. Add performer MAY be
[0..1] be present.
16. Update participant to
Drug Vehicle template,
replacing inline
constraints.
17. Replace
entryRelationship/[RSON]
distal Observation with
Indication template. C83
templateId no longer
required.
18. Replace
8. Add repeatNumber MAY [0..1]
be present
9. Relax routeCode to MAY be
[0..1] present and update to
values from
2.16.840.1.113883.3.88.12.32
21.8.7
10. approachSiteCode MAY be
[0..1] present with values from
2.16.840.1.113883.3.88.12.32
21.8.9
11. add doseQuantity SHOULD
[0..1] contain @unit and be
from
2.16.840.1.113883.1.11.12839
12. update rateQuantity to MAY
be [0..1] present and contain
@unit from
2.16.840.1.113883.1.11.12839
13. add administrationUnitCode
MAY be [0..1] present
14. add consumable SHALL [1..1]
to include Medication
Information
15. update performer MAY be
[0..1] be present.
16. Add participant MAY be [0..*]
present to Drug Vehicle
17. Replace
entryRelationship/[RSON]
distal end with Indication
template. CCD templateId no
longer required.
18. Replace
entryRelationship/[SUBJ]
distal end with Instructions
template. CCD templateId(s) no

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 551
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Title
(templateId)
Previous templateId
Changes from IHE
Changes from HITSP C83
Changes from CCD
Information template.
IHE templateId is no
longer required.
18. Add performer MAY be
[0..1] be present.
19. Add participant MAY be
[0..*] present to Drug
Vehicle
20. Replace
entryRelationship/[RSO
N] distal Act with
Indication template. IHE
templateId no longer
required.
21. Replace
entryRelationship/[SUBJ
] distal end with
Instructions template.
IHE templateId no longer
required.
22. Replace
entryRelationship/[REFR
] distal end with
Medication Supply Order
template. IHE
templateId(s) no longer
required.
23. Add
entryRelationship/[REFR
] to Medication Dispense
template MAY be [0..1]
present.
24. Add entryRelationship/
[CAUS] to Reaction
Observation template
MAY be [0..1] present.
25. Update precondition to
use Precondition for
entryRelationship/[SUBJ]
distal end with
Instructions template. C83
templateId(s) no longer
required.
19. Replace
entryRelationship/[REFR]
distal end with Medication
Supply Order template.
C83 templateId(s) no
longer required.
20. Replace
entryRelationship/[REFR]
with Medication Dispense
template. C83
templateId(s) no longer
required.
21. Replace
entryRelationship/[CAUS]
with Reaction Observation
template. C83
templateId(s) no longer
required.
22. Add precondition to
Precondition for Substance
Administration template.
longer required.
19. Replace
entryRelationship/[REFR]
distal end with Medication
Supply Order template. CCD
templateId(s) no longer
required.
20. Add entryRelationship/[REFR]
to Medication Dispense
template MAY be [0..1] present.
21. Add entryRelationship/[CAUS]
to Reaction Observation
template MAY be [0..1] present.
22. Add precondition to
Precondition for Substance
Administration template.

Page 552 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Title
(templateId)
Previous templateId
Changes from IHE
Changes from HITSP C83
Changes from CCD
Substance
Administration template.
Remove existing
precondition elements.
26. Remove
entryRelationship/
[COMP] constraint.
Medication Use –
None Known
(2.16.840.1.113
883.10.20.22.4.
29)
No equivalent template.
No equivalent template.
No equivalent template.
Medication
Information
(2.16.840.1.113
883.10.20.22.4.
23)
2.16.840.1.113883.10.20.1.53
(CCD)
1.3.6.1.4.1.19376.1.5.3.1.4.7.2
(IHE)
2.16.840.1.113883.3.88.11.83.
8.2 (C83)
1. IHE templateId(s) no
longer required
2. consumable/manufactur
edProduct/manufacture
dMaterial/code SHALL
be selected from
2.16.1.113883.3.88.12.8
0.17. Remove any
conflicting code
constraints.
1. C83 templateId(s) no
longer required.
2. consumable/manufacture
dProduct/manufacturedM
aterial/code SHALL be
selected from
2.16.1.113883.3.88.12.80
.17. Remove any
conflicting code
constraints.
1. CCD templateId no longer
required
2. consumable/manufacturedPro
duct/manufacturedMaterial/c
ode SHALL be selected from
2.16.1.113883.3.88.12.80.17.
Remove any conflicting code
constraints.
Drug Vehicle
(2.16.840.1.113
883.10.20.22.4.
24)
No equivalent template.
1. relax name element to
MAY be [0..1] present (in
playingEntity)
2. update code element to
SHALL be [1..1] present
(in playingEntity).
No equivalent template.
Indication
(2.16.840.1.113
883.10.20.22.4.
19)
2.16.840.1.113883.10.20.1.27
(CCD)
1.3.6.1.4.1.19376.1.5.3.1.4.4.1
(IHE)
2.16.840.1.113883.10.20.1.28
(C83)
1. IHE templateId(s) no
longer required.
2. Replace IHE template
with Indication template.
1. C83 templateId(s) no
longer required
2. Remove observation/text
constraint
1. CCD templateId(s) no longer
required.
2. Replace CCD template with
Indication.

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 553
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Title
(templateId)
Previous templateId
Changes from IHE
Changes from HITSP C83
Changes from CCD
Instructions
(2.16.840.1.113
883.10.20.22.4.
20)
2.16.840.1.113883.10.20.1.49
(CCD)
1.3.6.1.4.1.19376.1.5.3.1.4.3
(IHE)
1. IHE templateId(s) no
longer required.
2. Remove code SHOULD
be 'PINSTRUCT'
codeSystem
1.3.6.1.4.1.19376.1.5.3.
2
1. C83 templateId(s) no
longer required
2. Remove code SHOULD be
'PINSTRUCT' codeSystem
1.3.6.1.4.1.19376.1.5.3.2
1. CCD templateId(s) no longer
required.
2. Add text SHOULD be [0..1]
present and add dependent
constraints
3. Add statusCode SHALL be
[1..1] present with value
'completed'.
Precondition for
Substance
Administration
(2.16.840.1.113
883.10.20.22.4.
25)
No equivalent template.
No equivalent template.
No equivalent template.
Reaction
Observation
(2.16.840.1.113
883.10.20.22.4.
9)
2.16.840.1.113883.10.20.1.54
(CCD)
1.3.6.1.4.1.19376.1.5.3.1.4.5
(IHE)
1. IHE templateId(s) no
longer required.
2. Add id SHALL be [1..1]
present
3. Add code SHALL be [1..1]
present
4. Add text SHOULD be
[0..1] present, and child
constraints
5. Add effectiveTime
SHOULD be [0..1]
present and child
constraints
6. Add value SHALL be
[1..1] present with type
of 'CD' and value from
2.16.3.88.12.3221.7.4
7. Replace
entryRelationship/[SUBJ
] templateId with
Severity Observation
2.16.840.1.113883.10.2
No existing template.
1. CCD templateId(s) no longer
required.
2. Add id SHALL be [1..1] present
3. Add code SHALL be [1..1]
present
4. Add text SHOULD be [0..1]
present, and child constraints
5. Add effectiveTime SHOULD be
[0..1] present and child
constraints
6. Add value SHALL be [1..1]
present with type of 'CD' and
value from
2.16.3.88.12.3221.7.4
7. Add entryRelationship/[RSON]
to template Procedure Activity
Procedure MAY be [0..1]
present
8. Add entryRelationship/[RSON]
to template Medication Activity
MAY be [0..1] present.

Page 554 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Title
(templateId)
Previous templateId
Changes from IHE
Changes from HITSP C83
Changes from CCD
0.22.4.8
8. Add
entryRelationship/[RSO
N] to template Procedure
Activity Procedure MAY
be [0..1] present
9. Add
entryRelationship/[RSO
N] to template
Medication Activity MAY
be [0..1] present.
10. Remove
entryRelationship/[REFR
] constraint
11. Remove
entryRelationship/[SUBJ
] to comments template
constraint.
Severity
Observation
(2.16.840.1.113
883.10.20.22.4.
8)
2.16.840.1.113883.10.20.1.55
(CCD)
1.3.6.1.4.1.19376.1.5.3.1.4.1
(IHE)
1. IHE templateId(s) no
longer required.
2. Relax text to SHOULD be
[0..1] present
3. Update value to be from
2.16.840.1.113883.3.88.
12.3221.6.8
No existing template.
1. CCD templateId(s) no longer
required.
2. Add text SHOULD be [0..1]
present and child constraints.
3. Update value to be from
2.16.840.1.113883.3.88.12.32
21.6.8
Medication
Supply Order
Entry
(2.16.840.1.113
883.10.20.22.4.
17)
2.16.840.1.113883.10.20.1.34
(CCD)
1.3.6.1.4.1.19376.1.5.3.1.4.7.3
(IHE)
2.16.840.1.113883.3.88.11.83.
8.3 (C83)
1. IHE templateId(s) no
longer required.
2. Constrain @moodCode to
INT
3. Update id to SHALL [1..*]
4. Add statusCode SHALL
be [1..1] present
5. Add effectiveTime/high
SHOULD be [0..1]
present
6. Add constraint that at
1. C83 templateId(s) no
longer required.
2. Constrain @moodCode to
INT
3. Update id to SHALL [1..*];
remove additional
constraints
4. Add statusCode SHALL be
[1..1] present
5. Add effectiveTime/high
SHOULD be [0..1] present
1. CCD templateId(s) no longer
required.
2. Constrain @moodCode to INT
3. Update statusCode to SHALL
be [1..1] present
4. Add effectiveTime/high
SHOULD be [0..1] present
5. Update repeatNumber to
SHOULD be [0..1] present
6. Update quantity to SHOULD be
[0..1] present

HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1 Page 555
July 2012 © 2012 Health Level Seven, Inc. All rights reserved.
Title
(templateId)
Previous templateId
Changes from IHE
Changes from HITSP C83
Changes from CCD
least 1 of
product/Medication
Information or
product/Immunization
Medication Information
SHALL be [1..1] present
7. Add
entryRelationship/[SUBJ
] to Instructions MAY be
[0..1] present along with
child constraints
8. Remove all other
differing constraints
6. Update repeatNumber to
SHOULD be [0..1] present
7. Update quantity to
SHOULD be [0..1] present
8. Add constraint that at
least 1 of
product/Medication
Information or
product/Immunization
Medication Information
SHALL be [1..1] present
9. Add
entryRelationship/[SUBJ]
to Instructions MAY be
[0..1] present along with
child constraints
10. Remove all other differing
constraints
7. Add constraint that at least 1
of product/Medication
Information or
product/Immunization
Medication Information SHALL
be [1..1] present
8. Add entryRelationship/[SUBJ]
to Instructions MAY be [0..1]
present along with child
constraints
9. Remove all other differing
constraints
Medication
Dispense Entry
(2.16.840.1.113
883.10.20.22.4.
18)
2.16.840.1.113883.10.20.1.34
(CCD)
1.3.6.1.4.1.19376.1.5.3.1.4.7.3
(IHE)
2.16.840.1.113883.3.88.11.83.
8.3 (C83)
1. IHE templateId(s) no
longer required.
2. Constrain @moodCode to
EVN
3. Update id to SHALL [1..*]
4. Add statusCode SHALL
be [1..1] present and
from
2.16.840.1.113883.3.88.
12.80.64
5. Add effectiveTime
SHOULD be [0..1]
present
6. Add constraint that at
least 1 of
product/Medication
Information or
product/Immunization
Medication Information
1. C83 templateId(s) no
longer required.
2. Constrain @moodCode to
EVN
3. Update id to SHALL [1..*];
remove additional
constraints
4. Add statusCode SHALL be
[1..1] present and from
2.16.840.1.113883.3.88.1
2.80.64
5. Add effectiveTime
SHOULD be [0..1] present
6. update repeatNumber
SHOULD be [0..1] present
7. Update quantity to
SHOULD be [0..1] present
8. Add constraint that at
least 1 of
1. CCD templateId(s) no longer
required.
2. Constrain @moodCode to EVN
3. Update statusCode to SHALL
be [1..1] present
4. Update repeatNumber to
SHOULD be [0..1] present
5. Update quantity to SHOULD be
[0..1] present
6. Add constraint that at least 1
of product/Medication
Information or
product/Immunization
Medication Information SHALL
be [1..1] present
7. Add entryRelationship/[REFR]
to Medication Supply Order
MAY be [0..1] present along
with child constraints

Page 556 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Title
(templateId)
Previous templateId
Changes from IHE
Changes from HITSP C83
Changes from CCD
SHALL be [1..1] present
7. Add
entryRelationship/[REFR
] to Medication Supply
Order MAY be [0..1]
present along with child
constraints
8. Add
assignedEntity/Consolid
ated US Realm Header
Address template
SHOULD be [0..1]
present
9. Remove all other
differing constraints
product/Medication
Information or
product/Immunization
Medication Information
SHALL be [1..1] present
9. Add performer MAY be
[0..1] present
10. Add
entryRelationship/[REFR]
to Medication Supply
Order MAY be [0..1]
present along with child
constraints
11. Add
assignedEntity/Consolida
ted US Realm Header
Address template
SHOULD be [0..1] present
12. Remove all other differing
constraints
8. Add
assignedEntity/Consolidated
US Realm Header Address
template SHOULD be [0..1]
present
9. Remove all other differing
constraints

Page 557 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
APPENDIX C — TEMPLATE IDS IN THIS GUIDE
The following table lists all templateIds in this guide. The sections for document types
include tables showing the template containment within each document type:
CCD
Consultation Note
Diagnostic Imaging Report
Discharge Summary
History and Physical
Operative Note
Procedure Note
Progress Note
Table 325: Template Ids Alphabetically by Template Type
Template Title
Template
Type
templateId
US Realm Address (AD.US.FIELDED)
header
2.16.840.1.113883.10.20.22.5.2
US Realm Date and Time
(DT.US.FIELDED)
2.16.840.1.113883.10.20.22.5.3
US Realm Date and Time
(DTM.US.FIELDED)
2.16.840.1.113883.10.20.22.5.4
US Realm Patient Name
(PTN.US.FIELDED)
2.16.840.1.113883.10.20.22.5.1
US Realm Person Name
(PN.US.FIELDED)
2.16.840.1.113883.10.20.22.5.1.1
Consultation Note
document
2.16.840.1.113883.10.20.22.1.4
Continuity of Care Document (CCD)
document
2.16.840.1.113883.10.20.22.1.2
Diagnostic Imaging Report
document
2.16.840.1.113883.10.20.22.1.5
Discharge Summary
document
2.16.840.1.113883.10.20.22.1.8
History and Physical
document
2.16.840.1.113883.10.20.22.1.3
Operative Note
document
2.16.840.1.113883.10.20.22.1.7
Procedure Note
document
2.16.840.1.113883.10.20.22.1.6
Progress Note
document
2.16.840.1.113883.10.20.22.1.9
Unstructured Document
document
2.16.840.1.113883.10.20.22.1.10
US Realm Header
document
2.16.840.1.113883.10.20.22.1.1
Advance Directives Section (entries
optional)
section
2.16.840.1.113883.10.20.22.2.21
Advance Directives Section (entries
required)
section
2.16.840.1.113883.10.20.22.2.21.1
Allergies Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.6
Allergies Section (entries required)
section
2.16.840.1.113883.10.20.22.2.6.1

Page 558 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Template Title
Template
Type
templateId
Anesthesia Section
section
2.16.840.1.113883.10.20.22.2.25
Assessment and Plan Section
section
2.16.840.1.113883.10.20.22.2.9
Assessment Section
section
2.16.840.1.113883.10.20.22.2.8
Chief Complaint and Reason for Visit
Section
section
2.16.840.1.113883.10.20.22.2.13
Chief Complaint Section
section
1.3.6.1.4.1.19376.1.5.3.1.1.13.2.1
Complications Section
section
2.16.840.1.113883.10.20.22.2.37
DICOM Object Catalog Section - DCM
121181
section
2.16.840.1.113883.10.20.6.1.1
Discharge Diet Section
section
1.3.6.1.4.1.19376.1.5.3.1.3.33
Encounters Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.22
Encounters Section (entries required)
section
2.16.840.1.113883.10.20.22.2.22.1
Family History Section
section
2.16.840.1.113883.10.20.22.2.15
Fetus Subject Context
section
2.16.840.1.113883.10.20.6.2.3
Findings Section (DIR)
section
2.16.840.1.113883.10.20.6.1.2
Functional Status Section
section
2.16.840.1.113883.10.20.22.2.14
General Status Section
section
2.16.840.1.113883.10.20.2.5
History of Past Illness Section
section
2.16.840.1.113883.10.20.22.2.20
History of Present Illness Section
section
1.3.6.1.4.1.19376.1.5.3.1.3.4
Hospital Admission Diagnosis Section
section
2.16.840.1.113883.10.20.22.2.43
Hospital Admission Medications Section
(entries optional)
section
2.16.840.1.113883.10.20.22.2.44
Hospital Consultations Section
section
2.16.840.1.113883.10.20.22.2.42
Hospital Course Section
section
1.3.6.1.4.1.19376.1.5.3.1.3.5
Hospital Discharge Diagnosis Section
section
2.16.840.1.113883.10.20.22.2.24
Hospital Discharge Instructions Section
section
2.16.840.1.113883.10.20.22.2.41
Hospital Discharge Medications Section
(entries optional)
section
2.16.840.1.113883.10.20.22.2.11
Hospital Discharge Medications Section
(entries required)
section
2.16.840.1.113883.10.20.22.2.11.1
Hospital Discharge Physical Section
section
1.3.6.1.4.1.19376.1.5.3.1.3.26
Hospital Discharge Studies Summary
Section
section
2.16.840.1.113883.10.20.22.2.16
Immunizations Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.2
Immunizations Section (entries required)
section
2.16.840.1.113883.10.20.22.2.2.1
Implants Section
section
2.16.840.1.113883.10.20.22.2.33
Instructions Section
section
2.16.840.1.113883.10.20.22.2.45
Interventions Section
section
2.16.840.1.113883.10.20.21.2.3
Medical (General) History Section
section
2.16.840.1.113883.10.20.22.2.39
Medical Equipment Section
section
2.16.840.1.113883.10.20.22.2.23

Page 559 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Template Title
Template
Type
templateId
Medications Administered Section
section
2.16.840.1.113883.10.20.22.2.38
Medications Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.1
Medications Section (entries required)
section
2.16.840.1.113883.10.20.22.2.1.1
Objective Section
section
2.16.840.1.113883.10.20.21.2.1
Observer Context
section
2.16.840.1.113883.10.20.6.2.4
Operative Note Fluids Section
section
2.16.840.1.113883.10.20.7.12
Operative Note Surgical Procedure
Section
section
2.16.840.1.113883.10.20.7.14
Payers Section
section
2.16.840.1.113883.10.20.22.2.18
Physical Exam Section
section
2.16.840.1.113883.10.20.2.10
Plan of Care Section
section
2.16.840.1.113883.10.20.22.2.10
Planned Procedure Section
section
2.16.840.1.113883.10.20.22.2.30
Postoperative Diagnosis Section
section
2.16.840.1.113883.10.20.22.2.35
Postprocedure Diagnosis Section
section
2.16.840.1.113883.10.20.22.2.36
Preoperative Diagnosis Section
section
2.16.840.1.113883.10.20.22.2.34
Problem Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.5
Problem Section (entries required)
section
2.16.840.1.113883.10.20.22.2.5.1
Procedure Description Section
section
2.16.840.1.113883.10.20.22.2.27
Procedure Disposition Section
section
2.16.840.1.113883.10.20.18.2.12
Procedure Estimated Blood Loss Section
section
2.16.840.1.113883.10.20.18.2.9
Procedure Findings Section
section
2.16.840.1.113883.10.20.22.2.28
Procedure Implants Section
section
2.16.840.1.113883.10.20.22.2.40
Procedure Indications Section
section
2.16.840.1.113883.10.20.22.2.29
Procedure Specimens Taken Section
section
2.16.840.1.113883.10.20.22.2.31
Procedures Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.7
Procedures Section (entries required)
section
2.16.840.1.113883.10.20.22.2.7.1
Reason for Referral Section
section
1.3.6.1.4.1.19376.1.5.3.1.3.1
Reason for Visit Section
section
2.16.840.1.113883.10.20.22.2.12
Results Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.3
Results Section (entries required)
section
2.16.840.1.113883.10.20.22.2.3.1
Review of Systems Section
section
1.3.6.1.4.1.19376.1.5.3.1.3.18
Social History Section
section
2.16.840.1.113883.10.20.22.2.17
Subjective Section
section
2.16.840.1.113883.10.20.21.2.2
Surgery Description Section
section
2.16.840.1.113883.10.20.22.2.26
Surgical Drains Section
section
2.16.840.1.113883.10.20.7.13
Vital Signs Section (entries optional)
section
2.16.840.1.113883.10.20.22.2.4
Vital Signs Section (entries required)
section
2.16.840.1.113883.10.20.22.2.4.1
Admission Medication
entry
2.16.840.1.113883.10.20.22.4.36
Advance Directive Observation
entry
2.16.840.1.113883.10.20.22.4.48

Page 560 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Template Title
Template
Type
templateId
Age Observation
entry
2.16.840.1.113883.10.20.22.4.31
Allergy - Intolerance Observation
entry
2.16.840.1.113883.10.20.22.4.7
Allergy Problem Act
entry
2.16.840.1.113883.10.20.22.4.30
Allergy Status Observation
entry
2.16.840.1.113883.10.20.22.4.28
Assessment Scale Observation
entry
2.16.840.1.113883.10.20.22.4.69
Authorization Activity
entry
2.16.840.1.113883.10.20.1.19
Boundary Observation
entry
2.16.840.1.113883.10.20.6.2.11
Caregiver Characteristics
entry
2.16.840.1.113883.10.20.22.4.72
Code Observations
entry
2.16.840.1.113883.10.20.6.2.13
Cognitive Status Problem Observation
entry
2.16.840.1.113883.10.20.22.4.73
Cognitive Status Result Observation
entry
2.16.840.1.113883.10.20.22.4.74
Cognitive Status Result Organizer
entry
2.16.840.1.113883.10.20.22.4.75
Comment Activity
entry
2.16.840.1.113883.10.20.22.4.64
Coverage Activity
entry
2.16.840.1.113883.10.20.22.4.60
Deceased Observation
entry
2.16.840.1.113883.10.20.22.4.79
Discharge Medication
entry
2.16.840.1.113883.10.20.22.4.35
Drug Vehicle
entry
2.16.840.1.113883.10.20.22.4.24
Encounter Activities
entry
2.16.840.1.113883.10.20.22.4.49
Encounter Diagnosis
entry
2.16.840.1.113883.10.20.22.4.80
Estimated Date of Delivery
entry
2.16.840.1.113883.10.20.15.3.1
Family History Death Observation
entry
2.16.840.1.113883.10.20.22.4.47
Family History Observation
entry
2.16.840.1.113883.10.20.22.4.46
Family History Organizer
entry
2.16.840.1.113883.10.20.22.4.45
Functional Status Problem Observation
entry
2.16.840.1.113883.10.20.22.4.68
Functional Status Result Observation
entry
2.16.840.1.113883.10.20.22.4.67
Functional Status Result Organizer
entry
2.16.840.1.113883.10.20.22.4.66
5.19
Observation
entry
2.16.840.1.113883.10.20.22.4.5
Highest Pressure Ulcer Stage
entry
2.16.840.1.113883.10.20.22.4.77
Hospital Admission Diagnosis
entry
2.16.840.1.113883.10.20.22.4.34
Hospital Discharge Diagnosis
entry
2.16.840.1.113883.10.20.22.4.33
Immunization Activity
entry
2.16.840.1.113883.10.20.22.4.52
Immunization Medication Information
entry
2.16.840.1.113883.10.20.22.4.54
Immunization Refusal Reason
entry
2.16.840.1.113883.10.20.22.4.53
Indication
entry
2.16.840.1.113883.10.20.22.4.19
Instructions
entry
2.16.840.1.113883.10.20.22.4.20
Medication Activity
entry
2.16.840.1.113883.10.20.22.4.16
Medication Dispense
entry
2.16.840.1.113883.10.20.22.4.18
Medication Information
entry
2.16.840.1.113883.10.20.22.4.23

Page 561 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Template Title
Template
Type
templateId
Medication Supply Order
entry
2.16.840.1.113883.10.20.22.4.17
Medication Use - None Known
(deprecated)
entry
2.16.840.1.113883.10.20.22.4.29
Non-Medicinal Supply Activity
entry
2.16.840.1.113883.10.20.22.4.50
Number of Pressure Ulcers Observation
entry
2.16.840.1.113883.10.20.22.4.76
Plan of Care Activity Act
entry
2.16.840.1.113883.10.20.22.4.39
Plan of Care Activity Encounter
entry
2.16.840.1.113883.10.20.22.4.40
Plan of Care Activity Observation
entry
2.16.840.1.113883.10.20.22.4.44
Plan of Care Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.41
Plan of Care Activity Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.42
Plan of Care Activity Supply
entry
2.16.840.1.113883.10.20.22.4.43
Policy Activity
entry
2.16.840.1.113883.10.20.22.4.61
Postprocedure Diagnosis
entry
2.16.840.1.113883.10.20.22.4.51
Precondition for Substance
Administration
entry
2.16.840.1.113883.10.20.22.4.25
Pregnancy Observation
entry
2.16.840.1.113883.10.20.15.3.8
Preoperative Diagnosis
entry
2.16.840.1.113883.10.20.22.4.65
Pressure Ulcer Observation
entry
2.16.840.1.113883.10.20.22.4.70
Problem Concern Act (Condition)
entry
2.16.840.1.113883.10.20.22.4.3
Problem Observation
entry
2.16.840.1.113883.10.20.22.4.4
Problem Status
entry
2.16.840.1.113883.10.20.22.4.6
Procedure Activity Act
entry
2.16.840.1.113883.10.20.22.4.12
Procedure Activity Observation
entry
2.16.840.1.113883.10.20.22.4.13
Procedure Activity Procedure
entry
2.16.840.1.113883.10.20.22.4.14
Procedure Context
entry
2.16.840.1.113883.10.20.6.2.5
Product Instance
entry
2.16.840.1.113883.10.20.22.4.37
Purpose of Reference Observation
entry
2.16.840.1.113883.10.20.6.2.9
Quantity Measurement Observation
entry
2.16.840.1.113883.10.20.6.2.14
Reaction Observation
entry
2.16.840.1.113883.10.20.22.4.9
Referenced Frames Observation
entry
2.16.840.1.113883.10.20.6.2.10
Result Observation
entry
2.16.840.1.113883.10.20.22.4.2
Result Organizer
entry
2.16.840.1.113883.10.20.22.4.1
Series Act
entry
2.16.840.1.113883.10.20.22.4.63
Service Delivery Location
entry
2.16.840.1.113883.10.20.22.4.32
Severity Observation
entry
2.16.840.1.113883.10.20.22.4.8
Smoking Status Observation
entry
2.16.840.1.113883.10.22.4.78
Social History Observation
entry
2.16.840.1.113883.10.20.22.4.38
SOP Instance Observation
entry
2.16.840.1.113883.10.20.6.2.8

Page 562 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Template Title
Template
Type
templateId
Study Act
entry
2.16.840.1.113883.10.20.6.2.6
Text Observation
entry
2.16.840.1.113883.10.20.6.2.12
Tobacco Use
entry
2.16.840.1.113883.10.20.22.4.85
Vital Sign Observation
entry
2.16.840.1.113883.10.20.22.4.27
Vital Signs Organizer
entry
2.16.840.1.113883.10.20.22.4.26
Physician of Record Participant
unspecified
2.16.840.1.113883.10.20.6.2.2
Physician Reading Study Performer
unspecified
2.16.840.1.113883.10.20.6.2.1

Page 563 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
APPENDIX D — CODE SYSTEMS IN THIS GUIDE
The following table lists all the code systems in this guide. The next two appendices list
all value sets (vocabularies) and single-value bindings.
Table 326: Code Systems in This Guide
Code System OID
Code System Name
1.0.3166.1
ISO 3166-1 Country Codes
1.2.840.10008.2.16.4
DCM
2.16.840.1.113883.1.11.11526
Internet Society Language
2.16.840.1.113883.12.292
Vaccines administered (CVX)
2.16.840.1.113883.5.1
Administrative Gender
2.16.840.1.113883.5.1001
ActMood
2.16.840.1.113883.5.1076
Religious Affiliation
2.16.840.1.113883.5.110
RoleClass
2.16.840.1.113883.5.111
RoleCode
2.16.840.1.113883.5.1119
AddressUse
2.16.840.1.113883.5.14
ActStatus
2.16.840.1.113883.5.2
MaritalStatus
2.16.840.1.113883.6.1
LOINC
2.16.840.1.113883.6.101
NUCC Health Care Provider Taxonomy
2.16.840.1.113883.6.104
ICD9 CM Procedures
2.16.840.1.113883.6.12
CPT-4
2.16.840.1.113883.5.25
Confidentiality Code
2.16.840.1.113883.3.26.1.1
National Cancer Institute (NCI) Thesaurus
2.16.840.1.113883.6.231
US Postal Codes
2.16.840.1.113883.6.238
Race and Ethnicity - CDC
2.16.840.1.113883.6.259
HealthcareServiceLocation
2.16.840.1.113883.5.4
ActCode
2.16.840.1.113883.5.43
EntityNamePartQualifier
2.16.840.1.113883.5.45
EntityNameUse
2.16.840.1.113883.6.255.1336
ASC X12
2.16.840.1.113883.5.6
HL7ActClass
2.16.840.1.113883.5.60
LanguageAbilityMode
2.16.840.1.113883.5.61
LanguageAbilityProficiency
2.16.840.1.113883.3.26.1.5
NDF-RT
2.16.840.1.113883.5.7
ActPriority
2.16.840.1.113883.4.9
Unique Ingredient Identifier (UNII)
2.16.840.1.113883.5.8
ActReason
2.16.840.1.113883.5.83
ObservationInterpretation
2.16.840.1.113883.5.88
ParticipationFunction

Page 564 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Code System OID
Code System Name
2.16.840.1.113883.5.89
Participationsignature
2.16.840.1.113883.6.8
Unified Code for Units of Measure (UCUM)
2.16.840.1.113883.6.88
RXNorm
2.16.840.1.113883.6.92
FIPS 5-2 (State)
2.16.840.1.113883.6.96
SNOMED CT
2.16.840.1.113883.4.6
National Provider ID (NPI)

Page 565 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
APPENDIX E — VALUE SETS IN THIS GUIDE
The following table lists all the value sets (vocabularies) in this guide.
2.16.840.1.113883.11.20.9.41
Table 327: Value Sets in This Guide
ValueSet OID
ValueSet Name
Binding
2.16.840.1.113883.1.11.16866
ActPriority
DYNAMIC
2.16.840.1.113883.1.11.1
Administrative Gender (HL7 V3)
DYNAMIC
2.16.840.1.113883.1.11.20.2
AdvanceDirectiveTypeCode
STATIC
2.16.840.1.113883.11.20.9.21
AgePQ_UCUM
DYNAMIC
2.16.840.1.113883.3.88.12.3221.6.2
Allergy/Adverse Event Type
DYNAMIC
2.16.840.1.113883.3.88.12.3221.8.9
Body Site Value Set
DYNAMIC
2.16.840.1.113883.11.20.9.31
ConsultDocumentType
DYNAMIC
2.16.840.1.113883.3.88.12.80.63
CountryValueSet
DYNAMIC
2.16.840.1.113883.1.11.18877
Coverage Role Type Value Set
DYNAMIC
2.16.840.1.113883.11.20.9.28
DICOMPurposeOfReference
DYNAMIC
2.16.840.1.113883.11.20.9.30
DICOMQuantityMeasurementType
Codes
DYNAMIC
2.16.840.1.113883.11.20.9.32
DIRDocumentTypeCodes
DYNAMIC
2.16.840.1.113883.11.20.9.29
DIRQuantityMeasurementTypeCodes
DYNAMIC
2.16.840.1.113883.11.20.4.1
DischargeSummary
DocumentTypeCode
DYNAMIC
2.16.840.1.113883.3.88.12.80.32
EncounterTypeCode
DYNAMIC
2.16.840.1.113883.1.11.15913
EntityNameUse
STATIC
2.16.840.1.113883.11.20.9.26
EntityPersonNamePartQualifier
STATIC
2.16.840.1.113883.1.11.19579
FamilyHistoryRelatedSubjectCode
DYNAMIC
2.16.840.1.113883.1.11.10416
FinanciallyResponsiblePartyType
DYNAMIC
2.16.840.1.113883.3.88.12.3221.5.2
Health Insurance Type Value Set
DYNAMIC
2.16.840.1.114222.4.11.1066
Healthcare Provider Taxonomy (NUCC
– HIPAA)
DYNAMIC
2.16.840.1.113883.1.11.20275
HealthcareServiceLocation
DYNAMIC
2.16.840.1.113883.1.11.20.12
HealthStatus
DYNAMIC
2.16.840.1.113883.1.11.15836
HITSP Ethnicity Value Set
DYNAMIC
2.16.840.1.113883.3.88.12.80.62
HITSP Vital Sign Result Type
DYNAMIC

Page 566 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
ValueSet OID
ValueSet Name
Binding
2.16.840.1.113883.3.88.12.80.68
HITSPProblemStatus
DYNAMIC
2.16.840.1.113883.1.11.16926
HL7 BasicConfidentialityKind
STATIC
2.16.840.1.113883.1.11.12249
HL7 LanguageAbilityMode
DYNAMIC
2.16.840.1.113883.1.11.12212
HL7 Marital Status
DYNAMIC
2.16.840.1.113883.1.11.19185
HL7 Religious Affiliation
DYNAMIC
2.16.840.1.113883.1.11.20.22
HPDocumentType
DYNAMIC
2.16.840.1.113883.11.20.9.33
INDRoleclassCodes
STATIC
2.16.840.1.113883.3.88.12.80.20
Ingredient Name
DYNAMIC
2.16.840.1.113883.1.11.11526
Language
DYNAMIC
2.16.840.1.113883.1.11.12199
LanguageAbilityProficiency
DYNAMIC
2.16.840.1.113883.3.88.12.80.16
Medication Brand Name
DYNAMIC
2.16.840.1.113883.3.88.12.80.17
Medication Clinical Drug
DYNAMIC
2.16.840.1.113883.3.88.12.80.18
Medication Drug Class
DYNAMIC
2.16.840.1.113883.3.88.12.80.64
Medication Fill Status
DYNAMIC
2.16.840.1.113883.3.88.12.3221.8.11
Medication Product Form
DYNAMIC
2.16.840.1.113883.3.88.12.3221.8.7
Medication Route FDA Value Set
DYNAMIC
2.16.840.1.113883.11.20.9.18
MoodCodeEvnInt
STATIC
2.16.840.1.113883.1.11.19717
No Immunization Reason Value Set
DYNAMIC
2.16.840.1.113883.3.88.12.80.33
NUBC UB-04 FL17-Patient Status
DYNAMIC
2.16.840.1.113883.11.20.9.34
Patient Education
DYNAMIC
2.16.840.1.113883.1.11.19563
Personal Relationship Role Type
DYNAMIC
2.16.840.1.113883.11.20.9.23
Plan of Care moodCode
(Act/Encounter/Procedure)
STATIC
2.16.840.1.113883.11.20.9.25
Plan of Care moodCode (Observation)
STATIC
2.16.840.1.113883.11.20.9.24
Plan of Care moodCode
(SubstanceAdministration/Supply)
STATIC
2.16.840.1.113883.1.11.10637
PostalAddressUse
STATIC
2.16.840.1.113883.3.88.12.80.2
PostalCodeValueSet
DYNAMIC
2.16.840.1.113883.11.20.9.36
Pressure Point
DYNAMIC
2.16.840.1.113883.11.20.9.35
Pressure Ulcer Stage
DYNAMIC
2.16.840.1.113883.3.88.12.3221.7.4
Problem
STATIC
2.16.840.1.113883.3.88.12.3221.6.8
Problem Severity
DYNAMIC
2.16.840.1.113883.3.88.12.3221.7.2
Problem Type
STATIC

Page 567 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
ValueSet OID
ValueSet Name
Binding
2.16.840.1.113883.11.20.9.19
ProblemAct statusCode
STATIC
2.16.840.1.113883.3.88.12.80.28
Procedure
DYNAMIC
2.16.840.1.113883.11.20.9.22
ProcedureAct statusCode
DYNAMIC
2.16.840.1.113883.11.20.6.1
ProcedureNoteDocument
TypeCodes
DYNAMIC
2.16.840.1.113883.11.20.8.1
ProgressNoteDocumentTypeCode
DYNAMIC
2.16.840.1.113883.3.88.12.3221.4
Provider Type
DYNAMIC
2.16.840.1.113883.1.11.14914
Race
DYNAMIC
2.16.840.1.113883.11.20.9.39
Result Status
STATIC
2.16.840.1.113883.11.20.9.38
Smoking Status
STATIC
2.16.840.1.113883.3.88.12.80.60
Social History Type Set Definition
STATIC
2.16.840.1.113883.3.88.12.80.1
StateValueSet
DYNAMIC
2.16.840.1.113883.11.20.7.1
SupportedFileFormats
STATIC
2.16.840.1.113883.11.20.1.1
SurgicalOperationNote
DocumentTypeCode
DYNAMIC
2.16.840.1.113883.11.20.9.37
TargetSite Qualifiers
DYNAMIC
2.16.840.1.113883.11.20.9.20
Telecom Use (US Realm Header)
DYNAMIC
2.16.840.1.113883.11.20.9.41
Tobacco Use
DYNAMIC
2.16.840.1.113883.1.11.12839
UCUM Units of Measure (case
sensitive)
DYNAMIC
2.16.840.1. 113883.3.88.12.80.22
Vaccine Administered Value Set
DYNAMIC

Page 568 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
APPENDIX F — SINGLE-VALUE BINDINGS IN THIS GUIDE
Table 328: Single-Value Bindings in This Guide
Code
Display Name
Code System OID
121181
Dicom Object Catalog
1.2.840.10008.2.16.4
182904002
Drug treatment unknown
2.16.840.1.113883.6.96
397659008
Age at Onset
2.16.840.1.113883.6.96
412307009
Drug vehicle
2.16.840.1.113883.6.96
419099009
Dead
2.16.840.1.113883.6.96
46680005
Vital Signs
2.16.840.1.113883.6.96
77386006
Pregnant
2.16.840.1.113883.6.96
ASSERTION
Assertion
2.16.840.1.113883.5.4
SEV
Severity Observation
2.16.840.1.113883.5.4
S
2.16.840.1.113883.5.89

Page 569 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
APPENDIX G — EXTENSIONS TO CDA R2
Where there is a need to communicate information for which there is no suitable
representation in CDA R2, extensions to CDA R2 have been developed. These
extensions are described above in the context of the section where they are used. This
section serves to summarize the extensions and provide implementation guidance.
Extensions created for this guide include:
sdtc:raceCode - The raceCode extension allows for multiple races to be reported
for a patient.
sdtc:id - The id extension in the family history organizer on the related subject
allows for unique identification of the family member(s).
sdtc:deceasedInd - The deceasedIndextension (= “true” or “false”) in the family
history organizer on the related subject is used inside to indicate if a family
member is deceased.
sdtc:deceasedTime - The deceasedTime extension in the family history organizer
on the related subject allows for reporting the date and time a family member
died.
sdtc:birthTime - The <sdtc:birthTime> element allows for the birth date of
any person to be recorded. The purpose of this extension is to allow the
recording of the subscriber or member of a health plan in cases where the health
plan eligibility system has different information on file than the provider does for
the patient.
sdtc:dischargeDispositionCode - The sdtc:dischargeDispositionCode element
allows the provider to record a discharge disposition in an encounter activity.
To resolve issues that need to be addressed by extension, the developers of this guide
chose to approach extensions as follows:
An extension is a collection of element or attribute declarations and rules for
their application to the CDA Release 2.0.
All extensions are optional. An extension may be used, but need not be under
this guide.
A single namespace for all extension elements or attributes that may be used by
this guide will be defined.
The namespace for extensions created by the HL7 Structured Documents
Working Group (formerly Structured Documents Technical Committee) shall be
urn:hl7-org:sdtc.
This namespace shall be used as the namespace for any extension elements or
attributes that are defined by this implementation guide.
Each extension element shall use the same HL7 vocabularies and data types
used by CDA Release 2.0.

Page 570 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Each extension element shall use the same conventions for order and naming as
is used by the current HL7 tooling.
An extension element shall appear in the XML where the expected RIM element
of the same name would have appeared had that element not been otherwise
constrained from appearing in the CDA XML schema.

Page 571 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
APPENDIX H — XDS-SD AND US REALM CLINICAL
DOCUMENT HEADER COMPARISON
The following table can help the implementer familiar with XDS-SD decide whether to
assert conformance to Unstructured Documents and the US Realm Clinical Document
Header constraints specified in this guide. [See References for a link to XDS-SD (Cross-
Transaction Specifications and Content Specifications, Scanned Documents Module).]
Areas where this Unstructured Document specification and the Clinical Document
Header constraints are more restrictive than XDS-SD have been highlighted in yellow.
Table 329: Comparison of XDS-SD and Clinical Document Header
CDA
XDS-SD
Clinical Document Header
ClinicalDocument
SHALL
SHALL
ClinicalDocument/ realmcode
SHALL
SHALL
ClinicalDocument/ typeId
SHALL
SHALL
ClinicalDocument/ templateId
SHALL
SHALL
ClinicalDocument/ id
SHALL
SHALL
ClinicalDocument/ code
SHALL
SHALL
ClinicalDocument/ title
SHOULD
SHALL
ClinicalDocument/ effectiveTime
SHALL
SHALL
ClinicalDocument/
confidentialityCode
SHALL
SHALL
ClinicalDocument/ languageCode
SHALL
SHALL
ClinicalDocument/
documentationOf/ serviceEvent/
effectiveTime
SHALL
Not required
ClinicalDocument/ recordTarget
SHALL
SHALL
ClinicalDocument/ recordTarget/
patientRole
SHALL
SHALL
ClinicalDocument/ recordTarget/
patientRole/ addr
SHALL
SHALL
ClinicalDocument/ recordTarget/
patientRole/ telecom
Not required
SHALL
ClinicalDocument/ recordTarget/
patientRole/ patient/ name
SHALL
SHALL
ClinicalDocument/ recordTarget/
patientRole/ patient/
administrativeGenderCode
SHALL
SHALL
ClinicalDocument/ recordTarget/
patientRole/ patient/ birthTime
SHALL
SHALL
ClinicalDocument/ author/ time
Not required
SHALL
ClinicalDocument/ author/
assignedAuthor
SHALL
SHALL

Page 572 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
CDA
XDS-SD
Clinical Document Header
ClinicalDocument/ author/
assignedAuthor/ id
assignedPerson:
SHOULD
assignedAuthoringDevice:
SHALL
SHALL
ClinicalDocument/ author/
assignedAuthor/ addr
Not required
SHALL
ClinicalDocument/ author/
assignedAuthor/ telecom
Not required
SHALL
ClinicalDocument/ custodian
SHALL
SHALL
ClinicalDocument/ custodian/
assignedCustodian/
representedCustodianOrganization/
name
SHALL
SHALL
ClinicalDocument/ custodian/
assignedCustodian/
representedCustodianOrganization/
addr
SHALL
SHALL
ClinicalDocument/ custodian/
assignedCustodian/
representedCustodianOrganization/
telecom
Not required
SHALL
ClinicalDocument/ author
(scanner)
SHALL
ClinicalDocument/ author/
assignedAuthor/ authoringDevice
(scanner)
SHALL
ClinicalDocument/ dataEnterer
SHALL
ClinicalDocument/
legalAuthenticator
SHOULD
ClinicalDocument/ component/
nonXMLBody
SHALL

Page 573 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
APPENDIX I — MIME MULTIPART/RELATED MESSAGES
The following text is taken from the Claims Attachments Implementation Guide
(AIS00000) in Section 2.4
http://www.hl7.org/documentcenter/public/wg/ca/CDAR2AIS0000R030_Implementat
ionGuideDraft.pdf. For up-to-date guidance, refer to the latest edition of that
specification.
MIME Multipart/Related Messages
An attachment is comprised of the CDA document, including any supporting files
necessary to render the attested content of the document. Two Internet request for
comments (RFCs) are needed to properly construct the mime multipart message. When
supporting files are needed, the collection of information shall be organized using a
MIME multipart/related package constructed according to RFC 2557. Within the MIME
package, supporting files must be encoded using Base-64. RFC-4648 should be used
when encoding the contents of the MIME package using Base-64. Finally, RFC-2392
may be used to reference other content that appears in the same X12 transaction to use
the same content to answer multiple questions for a single claim. Internet RFCs can be
downloaded from the RFC editor page at http://www.rfc-editor.org.
RFC-2557 MIME Encapsulation of Aggregate Documents, Such as HTML (MHTML)
This RFC describes how to construct a MIME multipart/related package, and how URLs
are resolved within content items of that package. RFC-2557 can be obtained at:
http://www.rfc-editor.org/rfc/rfc2557.txt
A MIME multipart/related package is made up of individual content items. Each
content item has a MIME header identifying the item. Each content item is delimited
from other content items using a string of application specified text. In addition, there
must be an ending boundary. The actual content is recorded between these delimiter
strings using a BASE-64 encoding of the content item. There is also a MIME header for
the entire package.
The first content item of a multipart/related message supporting attachments is the
CDA document, containing the header and structured or non-structured body.
Subsequent content items included in this package will contain additional content that
appears within the body of the document. The CDA document will reference these
additional content items by their URLs.
Referencing Supporting Files in Multipart/Related Messages
Because the CDA document and its supporting files may have already existed in a
clinical information system, references may already exist within the CDA document to
URLs that are not accessible outside of the clinical information system that created the
document. When the CDA document is sent via attachments, these URLs may no
longer be accessible by the receiving information system. Therefore, each content item
that is referenced by a URL within the CDA document must be included as a content
item in the MIME package. Each content item may specify the URL by which it is

Page 574 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
known using the Content-Location header. The receiver of this MIME package shall
translate URL references according the RFC-2557. This will ensure resolution of the
original URL to the correct content item within the MIME package. Thus, URL
references contained within an original document need not be rewritten when the CDA
package is transmitted. Instead, these URLs are simply supplied as the value of the
Content-Location header in the MIME package.
This capability allows for the same content item to be referred to more than once in a
MIME multipart/related package without requiring the content item to be supplied
twice. However, it does not allow a separate MIME multipart/related package to
contain references to information sent in a previously recorded package.
Referencing Documents from Other Multiparts within the Same X12 Transactions
RFC-2392 is used when referencing content across MIME package boundaries, but still
contained within the same X12 transaction (ST to SE). This can occur when the same
document answers multiple questions for a single claim. Each component of a MIME
package may be assigned a content identifier using the Content-ID header for the
content item. For example, this header would appear as:
Content-ID: <07EE4DAC-76C4-4a98-967E-F6EF9667DED1>
This content identifier is a unique identifier for the content item, which means it must
never be used to refer to any other content item. RFC-2392 defines the cid: URL
scheme (http: and ftp: are two other URL schemes). This URL scheme allows for
references by the Content-ID header to be resolved. The URL for the content item
identified above would be:
cid:07EE4DAC-76C4-4a98-967E-F6EF9667DED1
Receivers of the MIME multipart message must be able to resolve a cid: URL to the
content item that it identifies. Senders must ensure that they only refer to items that
have already been transmitted to the receiver by their cid: URL. Thus, this
implementation guide prohibits forward URL references using the cid: URL scheme.
Content items shall not be referenced across X12 transactions using the cid: URL
scheme. For example, if the payer previously requested information using a 277, and
the provider returned that information in a MIME multipart/related package in a 275,
and then the payer requested additional information in another 277, the provider may
not refer to the content item previously returned in the prior 275 transaction.

Page 575 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
APPENDIX J — ADDITIONAL PHYSICAL EXAMINATION
SUBSECTIONS
Below is the list of additional optional subsections that may be used under the Physical
Examination section. Most of the codes for these subsections are included in the HL7
document titled “CDAR2AIS0004R030, Additional Information Specification 0004:
Clinical Reports Attachment,” which also lists General Status (10210-3) and Vital Signs
(8716-3)—defined in this guide.
10190-7
MENTAL STATUS
11451-2
PSYCHIATRIC FINDINGS
10199-8
HEAD, PHYSICAL FINDINGS
10197-2
EYE, PHYSICAL FINDINGS
10195-6
EAR, PHYSICAL FINDINGS
10203-8
NOSE, PHYSICAL FINDINGS
11393-6
EARS & NOSE & MOUTH & THROAT, PHYSICAL FINDINGS
10201-2
MOUTH & THROAT & TEETH, PHYSICAL FINDINGS
51850-6
HEAD & EARS & EYES & NOSE & THROAT, PHYSICAL FINDINGS
11411-6
NECK, PHYSICAL FINDINGS
10207-9
THORAX & LUNGS, PHYSICAL FINDINGS
11391-0
CHEST, PHYSICAL FINDINGS
11392-8
CHEST WALL, PHYSICAL FINDINGS
10200-4
HEART, PHYSICAL FINDINGS
10193-1
BREASTS, PHYSICAL FINDINGS
10192-3
BACK, PHYSICAL FINDINGS
10191-5
ABDOMEN, PHYSICAL FINDINGS
10204-6
PELVIS, PHYSICAL FINDINGS
11403-3
GROIN, PHYSICAL FINDINGS
10198-0
GENITOURINARY TRACT, PHYSICAL FINDINGS
11400-9
GENITALIA, PHYSICAL FINDINGS
11401-7
GENITALIA FEMALE, PHYSICAL FINDINGS
11402-5
GENITALIA MALE, PHYSICAL FINDINGS
11388-6
BUTTOCKS, PHYSICAL FINDINGS
10205-3
RECTUM, PHYSICAL FINDINGS
10196-4
EXTREMITIES, PHYSICAL FINDINGS
11413-2
SHOULDER, PHYSICAL FINDINGS
11387-8
AXILLA, PHYSICAL FINDINGS
11386-0
UPPER ARM, PHYSICAL FINDINGS
11394-4
ELBOW, PHYSICAL FINDINGS
11398-5
FOREARM, PHYSICAL FINDINGS
11415-7
WRIST, PHYSICAL FINDINGS
11404-1
HAND, PHYSICAL FINDINGS
11406-6
HIP, PHYSICAL FINDINGS

Page 576 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
11414-0
THIGH, PHYSICAL FINDINGS
11407-4
KNEE, PHYSICAL FINDINGS
11389-4
CALF, PHYSICAL FINDINGS
11385-2
ANKLE, PHYSICAL FINDINGS
11397-7
FOOT, PHYSICAL FINDINGS
10209-5
BALANCE+COORDINATION, PHYSICAL FINDINGS
10212-9
STRENGTH PHYSICAL FINDINGS
10211-1
SENSATION, PHYSICAL FINDINGS
10206-1
SKIN, PHYSICAL FINDINGS
10194-9
DEEP TENDON REFLEXES, PHYSICAL FINDINGS
10208-7
VESSELS, PHYSICAL FINDINGS
11384-5
PHYSICAL EXAMINATION BY ORGAN SYSTEMS
11447-0
HEMATOLOGIC+LYMPHATIC+IMMUNOLOGIC PHYSICAL FINDINGS
11390-2
CARDIOVASCULAR SYSTEM, PHYSICAL FINDINGS
11399-3
GASTROINTESTINAL SYSTEM, PHYSICAL FINDINGS
10202-0
NEUROLOGIC SYSTEM, PHYSICAL FINDINGS
11410-8
MUSCULOSKELETAL SYSTEM, PHYSICAL FINDINGS

Page 577 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
APPENDIX K — ADDITIONAL EXAMPLES
This appendix contains various examples of use from this guide.
Names Examples
Figure 242: Correct use of name example 1
<name><given>John</given><given>Q.</given><family>Doe</family></name>
The name element in CDA contains mixed content. In XML, this means that name can
contain a mix of character data and element markup in any order. The consequence of
this is that all whitespace is significant, thus tab characters, carriage returns, space
characters, etc. all become “part” of the person’s name.
Figure 243: Incorrect use of name example 1 - whitespace
<name>
<given>John</given>
<given>Q.</given>
<family>Doe</family>
</name>
Figure 244: Incorrect use of Patient name example 2 - no tags
<name>John Q. Doe</name>
Addresses Examples
Figure 245: Correct use telecom address example
<telecom use="WP" value="tel:555-555-1212"/>
Figure 246: Correct use postal address example
<addr use="H"><streetAddressLine>17 Daws Rd.</streetAddressLine><city>Blue
Bell</city><state>MA</state><postalCode>02368</postalCode><country>US</country>
</addr>

Page 578 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Time Examples
Figure 247: Correct use of IVL_TS example
<effectiveTime>
<low value='20110907'/>
<high value='20110909'/>
</effectiveTime>
Figure 248: Correct use of TS with precision to minute example
<effectiveTime value='201109071023'/>
Figure 249: Correct use of TS with time zone offset example
<effectiveTime value='201109071023-0500'/>
Figure 250: Incorrect use of IVL_TS example
<effectiveTime value='20110907'/>
Figure 251: Incorrect use of TS - insufficient precision example
<effectiveTime value='20110907'/> (must be precise to the minute)
Figure 252: Incorrect use of TS when time zone offset required example
<effectiveTime value='20110907'/>
Use of effectiveTime with time zone where not relevant (precise only to the day)
Figure 253: Incorrect use of time zone offset - not enough precision example
<effectiveTime value="20110907-0500'/>
CD Examples
Figure 254: Correct use of CD with no code example
<code nullFlavor='NI'>
<originalText><reference value='#problem-1'/></originalText>
</code>

Page 579 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
Figure 255: Incorrect use of CD with no code - missing nullFlavor attribute example
<code>
<originalText><reference value='#problem-1'/></originalText>
</code>

Page 580 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012
APPENDIX L — LARGE UML DIAGRAMS
This appendix provides larger versions of three hard-to-read UML diagrams.
Figure 256: Immunizations section UML diagram (larger copy)
Figure 257: Functional Status section UML diagram (larger copy)
Figure 258: Medications section UML diagram (larger copy)
Figure 259: Plan of care section UML diagram (larger copy)

Page 581 HL7 Implementation Guide for CDA R2: IHE Health Story Consolidation, DSTU R1.1
© 2012 Health Level Seven, Inc. All rights reserved. July 2012