BRF1 120 MCB.00910 13.full
User Manual: BRF1- 120
Open the PDF directly: View PDF ![]() .
.
Page Count: 34

1
Mapping the protein interaction network for the TFIIB-related 1
factor Brf1 in the RNA polymerase III pre-initiation complex 2
3
Seok-Kooi Khoo1,2, Chih-Chien Wu2, Yu-Chun Lin2, Jin-Cheng Lee2, and 4
Hung-Ta Chen1,2# 5
6
1. Taiwan International Graduate Program, Graduate Institute of Life 7
Sciences, National Defense Medical Center, Taipei, Taiwan, R.O.C. 8
2. Institute of Molecular Biology, Academia Sinica, Taipei, Taiwan, R.O.C. 9
10
11
# Correspondence to HT Chen, Institute of Molecular Biology, Academia Sinica, 12
128 Sec. 2 Academia Rd., Taipei 115, Taiwan, R.O.C. 13
Phone: + 886 2 27824778, Fax: + 886 2 27826085 14
E-mail: htchen012@gate.sinica.edu.tw 15
16
Running Title: Brf1 protein network in the pre-initiation complex 17
Keywords: Brf1/ RNA Polymerase III/ transcription initiation/ Bdp1/ C34 18
19
20
MCB Accepts, published online ahead of print on 25 November 2013
Mol. Cell. Biol. doi:10.1128/MCB.00910-13
Copyright © 2013, American Society for Microbiology. All Rights Reserved.
on March 4, 2018 by guesthttp://mcb.asm.org/Downloaded from

2
Abstract 21
The TFIIB-related factor Brf1 is essential for RNA polymerase (Pol) III 22
recruitment and open promoter formation in transcription initiation. We site-23
specifically incorporated non-natural amino acid cross-linker to Brf1 to map its 24
protein interaction targets in the pre-initiation complex (PIC). Our cross-linking 25
analysis in the N-terminal domain of Brf1 indicated a pattern of multiple protein 26
interactions reminiscent of TFIIB in the polymerase active site cleft. In addition to 27
the TFIIB-like protein interactions, the Brf1 cyclin repeats subdomain is in contact 28
with the Pol III-specific C34 subunit. With site-directed hydroxyl radical probing, 29
we further revealed the binding between Brf1 cyclin repeats and the highly 30
conserved region connecting C34 winged-helix domains 2 and 3. In contrast to 31
the N-terminal domain of Brf1, the C-terminal domain contains extensive binding 32
sites for TBP and Bdp1 to hold together the TFIIIB complex on the promoter. 33
Overall, the domain architecture of the PIC derived from our cross-linking data 34
explains how individual structural subdomains of Brf1 integrate the protein 35
network from the Pol III active center to the promoter for transcription initiation. 36
37
on March 4, 2018 by guesthttp://mcb.asm.org/Downloaded from

3
Introduction 38
Eukaryotic RNA polymerase (Pol) III transcribes precursor tRNAs, 5S 39
ribosomal RNA, small nuclear RNAs such as U6 and 7SK RNAs, and a number 40
of small nucleolar and microRNAs (1). In yeast (Saccharomyces cerevisiae), the 41
Pol III transcription apparatus consists of the 17-subunit Pol III and three other 42
transcription factors: the single-polypeptide TFIIIA, the three-subunit TFIIIB and 43
the six-subunit TFIIIC (2, 3). TFIIIA and TFIIIC function as the promoter 44
recognition factors, and TFIIIB is recruited to the promoter through TFIIIC. TFIIIB 45
is composed of the TFIIB-related factor Brf1, the TATA-box binding protein TBP, 46
and the SANT domain-containing subunit Bdp1. Previous biochemical studies 47
indicated that Brf1 and TBP cooperatively assemble onto DNA upstream of the 48
transcription start site, and Bdp1 binds to the Brf1-TBP-DNA complex mainly 49
through its SANT domain (4-10). The TFIIIB-DNA assembly is required for 50
subsequent Pol III recruitment and transcript initiation. Both Brf1 and Bdp1 have 51
been found to interact with Pol III and function in promoter opening (4, 11-14). 52
The N-terminal domain of yeast Brf1 (Brf1n; aa. 1-286) contains a zinc 53
ribbon fold (aa. 3-34) and a cyclin-fold repeat subdomain (aa. 83-282) (Figure 54
1A), both of which are homologous to those in the general transcription factor 55
TFIIB of the Pol II system. Based on biochemical and structural analyses, TFIIB 56
ribbon and cyclin-fold repeats are respectively positioned in the RNA exit tunnel 57
and on the wall domain of Pol II (15-20). In addition, the connecting region 58
between the TFIIB ribbon and cyclin repeat domain is structurally resolved to 59
contain B-reader and B-linker motifs interacting with the polymerase active center. 60
on March 4, 2018 by guesthttp://mcb.asm.org/Downloaded from

4
Based on sequence comparison, the connecting region in Brf1n, which we refer 61
to as N-linker, contains low sequence homology with TFIIB. However, this region 62
might also contribute to the binding of the polymerase active center as previous 63
genetic analyses revealed the involvement of ribbon and N-linker in open 64
complex formation (11, 13). 65
The C-terminal half of Brf1 (Brf1c) is Pol III-specific and is not conserved 66
among the TFIIB family, which, in addition to Brf1 and TFIIB, also includes Rrn7 67
(TAF1B in human) in the Pol I system (21-24). Yeast Brf1c (aa. 287-596) contains 68
three homologous sequence blocks, I (aa. 287-304), II (aa. 461-515) and III (aa. 69
570-596) (Figure 1A), that are conserved in S. cerevisiae, Schizosaccharomyces 70
pombe, Candida albicans, Kluyveromyces lactis and Homo sapiens (22, 25). 71
Brf1c exists mostly as a scaffold that holds together the three TFIIIB subunits (12, 72
26). In particular, structural analysis of the Brf1-TBP-DNA complex indicated that 73
homology block II is positioned along the convex and lateral surfaces of TBP, and 74
the block also interacts with Bdp1 (5, 6, 10, 22, 26-28). The homology blocks are 75
separated by two non-conserved connecting regions that we refer to as C-linkers 76
1 and 2 (Figure 1A). 77
Previous genetic and pairwise protein-protein interaction analyses have 78
identified Brf1 interacting partners. In addition to TBP and Bdp1 of the TFIIIB 79
complex, Brf1 interacts with the τ131 (Tfc4) subunit of TFIIIC and two of the Pol 80
III subunits, C34 and C17 (29-33). However, most of the previous studies 81
involved large protein fragments of Brf1, and a detailed and more precise 82
characterization of the Brf1 protein network is not yet available. In this study, we 83
on March 4, 2018 by guesthttp://mcb.asm.org/Downloaded from

5
site-specifically incorporated a non-natural photo-reactive amino acid p-benzoyl-84
L-phenylalanine (BPA) to the yeast Brf1 to map protein-protein interactions within 85
the Pol III pre-initiation complex (PIC). BPA incorporated in the amino acid 86
sequence of Brf1n revealed cross-linking with TBP and the C160 and C128 87
subunits of the Pol III active site cleft as well as two smaller subunits, C34 and 88
C17. The Brf1-C34 interaction was further analyzed by site-specific hydroxyl 89
radical analysis that revealed the connection between the Brf1 cyclin repeat 90
subdomain and a conserved sequence C-terminal to C34 winged-helix domain 2. 91
Our cross-linking results for Brf1c identified additional Bdp1 and TBP interactions 92
in the C-linker 1 region. Mutational analysis indicated that a Bdp1-binding block 93
in C-linker 1 is required for optimal cell growth and in vitro transcription activity. 94
Overall, our work provides a precise mapping of the network of protein-protein 95
interactions for Brf1 and further elucidates the domain architecture of the Pol III 96
PIC. 97
on March 4, 2018 by guesthttp://mcb.asm.org/Downloaded from

6
Materials and Methods 98
Yeast strains and plasmids 99
Yeast strains used for this study were derived from BY4705 with chromosomal 100
disruptions of individual genes by the KanMX4 cassette, yielding Brf1 shuffle 101
strain YSK1 [MAT
α
ade2::his3G his3
Δ
200 leu2
Δ
met15
Δ
lys2
Δ
trp1
Δ
63 ura3
Δ
102
(brf1::KanMX4) Brf1-pRS316 (URA3+)] and C34 shuffle strain YLy3 [MAT
α
103
ade2::his3G his3
Δ
200 leu2
Δ
met15
Δ
lys2
Δ
trp1
Δ
63 ura3
Δ
(Rpc34::KanMX4) 104
Rpc34-pRS316 (URA3+)] (34, 35). Brf1 and Rpc34 (C34) were separately cloned 105
into yeast 2 micron vector pRS425 with LEU2 selection marker (36). Both genes 106
were driven by yeast ADH1 promoter. Brf1 was either V5- or 13-Myc-epitope 107
tagged at the C-terminus via the QuikChange II Site-Directed Mutagenesis Kit 108
(Stratagene), yielding plasmids pSK1 (Adh1-Brf1cV5-pRS425) and pSK2 (Adh1-109
Brf1c13Myc-pRS425), respectively. C34 was C-terminally V5-tagged, yielding 110
pYL5 (Rpc34cV5-pRS425). Each of the constructed plasmids was used to 111
generate individual mutant plasmids containing single “TAG” (amber) nonsense 112
codon substitution at intended amino acid positions. To generate yeast strains for 113
incorporating non-natural amino acids p-benzoyl-L-phenylalanine (BPA) into Brf1 114
and C34, we applied plasmid shuffling to transform individual amber plasmids 115
into yeast YSK1 together with the plasmid pLH157 encoding a suppressor 116
tRNACUA (corresponding to TAG amber codon) and a BPA-tRNA synthetase (16, 117
37). 118
For Brf1 mutagenesis study, the gene encoding Brf1 along with its 119
endogenous promoter was cloned into the vector pRS315 with a single HA 120
on March 4, 2018 by guesthttp://mcb.asm.org/Downloaded from

7
epitope tag at the C-terminus, yielding pSK3 (Brf1-HA, ars cen, LEU2) (38). All 121
Brf1 mutant plasmids were generated based on pSK3, and the plasmids were 122
transformed into the Brf1 shuffle strain to generate mutant strains by the 5-FOA 123
drop-out method. For cells growth assay, both the WT and mutant strains were 124
grown in YPD till OD600 1.0, and the cell cultures were subsequently diluted with 125
the dilution range of 10-2, 10-4 and 10-6. The diluted cells were spotted on the 126
synthetic complete glucose plate lacking leucine, and the growth phenotypes at 127
temperatures 16 °C, 25 °C, 30 °C, and 37 °C were monitored. The incubation 128
time for cell growth at 30 °C and 37 °C was 3 days. For subsequent biochemical 129
studies, yeast whole cell extract (WCE) is prepared. Detailed procedures for 130
preparation of WCE from individual BPA-incorporated or mutant yeast strains 131
have been described previously (14, 39). 132
133
PIC isolation and BPA photo-crosslinking 134
The Pol III pre-initiation complex (PIC) was isolated using the immobilized 135
template assay (IMT) with yeast WCE and DNA template containing either the U6 136
snRNA or SUP4 tRNA promoter immobilized on Streptavidin magnetic beads 137
(DynaI) as previously described (14, 39). For the BPA photo-crosslinking 138
experiment, 800 µg of WCE was incubated with 4 µg of DNA template 139
immobilized on 200 µg of DynaI beads (Invitrogen) at 30oC for 30 min. Each 140
reaction was washed three times with transcription buffer containing 20 mM 141
KHepes (pH7.9), 80 mM KCl, 5 mM MgCl2, 1 mM EDTA, 2%(vol/vol) glycerol, 142
and 0.01% Tween 20. After washing, the reaction was divided into two fractions, 143
on March 4, 2018 by guesthttp://mcb.asm.org/Downloaded from

8
one that would receive UV irradiation (+UV) and the other that would serve as a 144
control (-UV). UV irradiation was conducted with a total energy of 6500 µJ/cm2 in 145
a Spectrolinker XL-1500 UV oven (Spectronics). The samples were then 146
resuspended in NuPAGE sample buffer (Invitrogen) for SDS-PAGE and Western 147
analysis. The Western blot was visualized with the LICOR Odyssey infrared 148
imaging system using fluorescent dye-labeled secondary antibodies. 149
150
In vitro transcription 151
In vitro transcription was conducted with the IMT assay as described above. After 152
washing, the isolated PICs were resuspended in 17 µL of transcription buffer 153
containing 200 ng α-amanitin, 4 units of RNase inhibitor (Promega), and 1 mM 154
DTT. A mixture of NTPs (3 µL) was subsequently added, and the resulting 155
reaction mixture contains 500 µM each of ATP, UTP, CTP, 50 µM GTP and 0.16 156
µM [α-32P] GTP (3000 Ci/mmol). After allowing the reaction to proceed at 30oC 157
for 30 min, transcription was quenched by adding 180 µL of 0.1 M sodium 158
acetate, 10 mM EDTA, 0.5% SDS and 200 µg/mL glycogen. The transcripts were 159
extracted by phenol/chloroform and ethanol precipitated, separated on 6% (wt/vol) 160
denaturing urea polyacrylamide gel and visualized by autoradiogram. 161
Restoration of transcription activity was conducted by adding recombinant Brf1 162
(160 ng) into the Brf1 mutants WCE. 163
164
Immunoprecipitation 165
Brf1 wild-type (WT) and mutant WCEs containing Bdp1 C-terminal Flag-tag and 166
on March 4, 2018 by guesthttp://mcb.asm.org/Downloaded from

9
Brf1 C-terminal HA-tag were used for immunoprecipitation (IP). WCE (1 mg) was 167
mixed with 50 µL of anti-Flag agarose beads (Sigma) in the extract dialysis buffer 168
containing 20 mM KHEPES pH 7.9, 100 mM KCl, 5 mM MgCl2, 1 mM EDTA, 169
and 20% glycerol and incubated overnight at 4oC. Following 3 washes with 500 170
µL of extract dialysis buffer, the bound proteins were eluted by boiling the beads 171
at 95oC for 5 min in 20 µL of 4X NuPAGE buffer (Invitrogen). The eluted proteins 172
were resolved by SDS-PAGE and analyzed by Western blot analysis probing with 173
the following antibodies, anti-Flag (probed for Bdp1), anti-HA (probed for Brf1), 174
anti-TBP, and anti-τ131 (Tfc4; TFIIIC subunit). 175
176
C34 purification and FeBABE conjugation 177
Expression and purification of C34 was as described previously (39). To avoid 178
off-target FeBABE conjugation, three endogenous cysteines were altered to non-179
cysteine residues as follows: Cys124Ala, Cys244Ala and Cys260Ser. All single-180
cysteine C34 variants were derived from the non-cysteine C34. FeBABE 181
conjugation was performed as described previously (14). 182
183
Hydroxyl radical cleavage with C34-FeBABE conjugate 184
Hydroxyl radical probing in the Pol III PIC was conducted based on the 185
previously established protocol using a C82 mutant WCE allowing dissociation of 186
the C82/34/31 subcomplex from the polymerase core (39). In a IMT reaction, 400 187
µg yeast WCE containing C-terminally Flag3-tagged Brf1 and the C82 deletion-188
mutant (50-52) was incubated with 0.72 µg of recombinant C31, 2 µg of 189
on March 4, 2018 by guesthttp://mcb.asm.org/Downloaded from

10
recombinant C82, and 0.94 µg of C34-FeBABE conjugate in a 200 µL reaction 190
containing 2 µg of SUP4 tRNA promoter DNA template. The PICs on beads were 191
washed three times with transcription buffer. After washing, samples were 192
resuspended in 7.5 µL of transcription buffer. The following reagents were added 193
sequentially: 2.5 µL of 50% (vol/vol) glycerol, 1.25 µL of 50 mM sodium ascorbate, 194
and 1.25 µL of H2O2 mix [0.24% (vol/vol) H2O2, 10mM EDTA]. The hydroxyl 195
radical cleavage reaction was conducted at 30oC for 8 min and quenched by 196
adding 4.5 µL NuPAGE LDS sample buffer (Invitrogen) and 1 µL of 1M DTT. The 197
protein cleavage sites in Brf1 were determined based on the method described 198
previously (14). In vitro transcription analysis was also conducted in parallel. The 199
C34-FeBABE conjugates restored transcription activity similar to that of the wild-200
type (data not shown). 201
on March 4, 2018 by guesthttp://mcb.asm.org/Downloaded from

11
Results 202
Brf1 N-terminal domain interacts with Pol III in a similar mode as the TFIIB-203
Pol II complex 204
To map the protein-protein interaction network of Brf1, we applied the 205
nonsense suppression method to incorporate BPA site-specifically to the entire 206
Brf1 (37, 40). We generated individual yeast strains each containing a single TAG 207
amber codon in the Brf1 coding sequence for BPA replacement at the designated 208
amino acid positions. A total of 197 strains were created as listed in Table S1. We 209
isolated yeast WCEs from these Brf1-BPA strains and conducted the immobilized 210
template (IMT) assay coupled with UV-irradiation to allow site-specific photo-211
cross-linking in the isolated PICs. The cross-linking samples were subsequently 212
applied to SDS-PAGE and Western blotting analyses, and protein cross-links 213
were determined based on the appearance of additional low-mobility gel bands 214
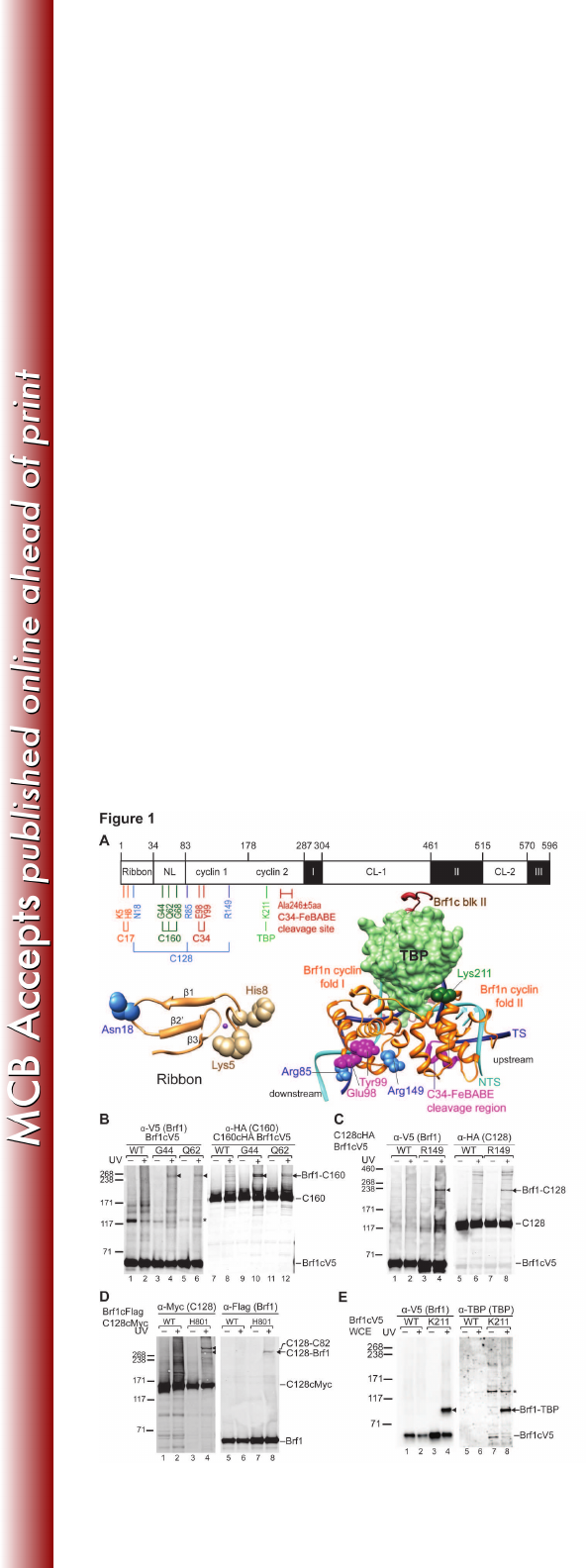
generated by UV-irradiation. As demonstrated in the Western analysis (Figure 215
1B), BPA substitution in residues Gly44 and Gln62 in the N-linker region of Brf1 216
generated protein cross-links of the size of ~240 kDa (Fig. 1B; lanes 4 and 6). By 217
subtracting the apparent molecular weight of Brf1, the polypeptide cross-linked to 218
Brf1 was estimated to have molecular weight in the range of 160 to 180 kDa. We 219
confirmed this crosslinked polypeptide to be the largest subunit C160 of Pol III by 220
repeating the photo-cross-linking experiment using WCEs containing C-terminally 221
HA-tagged C160 and probing with anti-HA antibody (Fig. 1B; lanes 10 and 12). 222
Cross-linking to the second largest subunit C128 of Pol III was also observed for 223
BPA substitution in residues Arg85 and Arg149 of the first cyclin fold and residue 224
on March 4, 2018 by guesthttp://mcb.asm.org/Downloaded from

12
Asn18 of the ribbon fold (Fig. 1C and data not shown). 225
As summarized in Figure 1A, Brf1-C160 and -C128 cross-links are 226
distributed respectively in the N-linker and ribbon/cyclin repeat subdomains. The 227
cross-linking pattern suggests a TFIIB-like binding mode as in the Pol II-TFIIB 228
structural model (20). In the Pol II-TFIIB model, the linker region of TFIIB, 229
including B-reader and B-linker motifs, are positioned in the polymerase active 230
center contacting the lid, rudder, and clamp coiled-coil motifs of Rpb1 231
(homologous to C160). In addition, the first cyclin fold of TFIIB is in close contact 232
with the wall and protrusion domains of Rpb2 (homologous to C128), and the 233
ribbon fold of TFIIB contacts both Rpb1 and Rpb2 in the RNA exit tunnel (16, 20). 234
To further investigate this TFIIB-like binding mode, we conducted another BPA 235
cross-linking analysis in the wall domain of C128. As demonstrated in Figure 1D, 236
a BPA substitution at His801 of the wall domain generated a cross-link with Brf1, 237
supporting the localization of Brf1 on C128. Although further structural and 238
biochemical analyses are required to determine the structural region of Brf1 in 239
contact with the wall domain of C128, our combined photo-cross-linking results 240
with BPA substituted C128 and Brf1 suggest that the Brf1 N-terminal domain 241
likely has a TFIIB-Pol II binding mode in the PIC. 242
In addition to cross-linking with the two largest subunits of Pol III, we also 243
observed Brf1-C17 cross-linking for residues Lys5 and His8 in the zinc-binding 244
knuckle of the ribbon domain (Fig. 1A; data not shown). Since C17 dimerizes 245
with C25 to form the stalk subcomplex that localizes adjacent to the RNA exit 246
tunnel (41), the Brf1-C17 cross-link suggests a potential functional link between 247
on March 4, 2018 by guesthttp://mcb.asm.org/Downloaded from

13
Brf1 and the stalk in transcription initiation. Furthermore, we observed a Brf1-TBP 248
cross-link at Lys211 at the H2’ helix of the second cyclin fold (Fig. 1A & 1E). This 249
cross-link supports the structural model for the binding of cyclin fold repeats with 250
the TBP-DNA complex, where the loop between H2’ and H3’ helices of the 251
second cyclin fold interacts with the C-terminal stirrup and the C-terminus of TBP 252
(42). 253
254
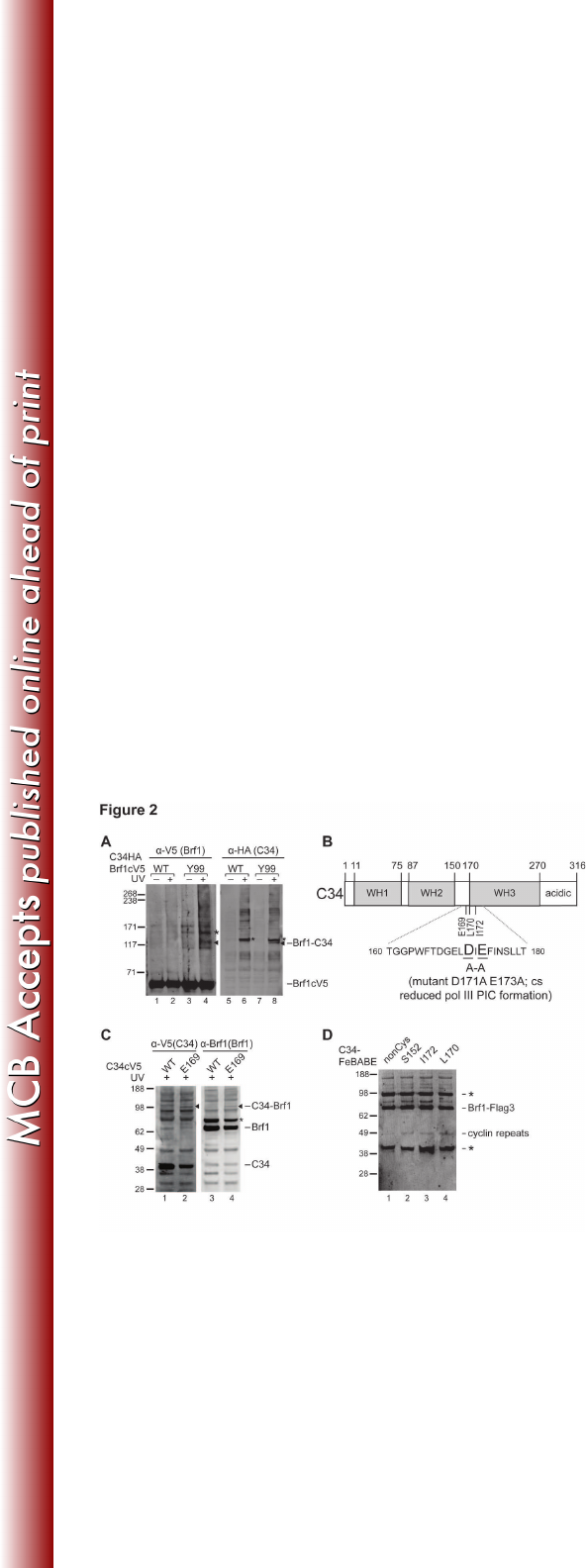
Brf1 cyclin fold repeat subdomain connects with C34 for Pol III recruitment 255
Our BPA cross-linking analysis for Brf1n revealed subdomain-specific 256
interactions with C160, C128, and TBP, suggesting that Brf1n organizes TFIIB-257
like domain architecture in the PIC. Based on previous studies with yeast two-258
hybrid and pull-down analyses, Brf1 also contains a Pol III-system specific 259
interaction with the C34 subunit of the Pol III complex. However, the interaction 260
site for C34 was not precisely mapped as the studies were involved either with 261
the full-length Brf1 protein or with the cyclin fold repeats (aa. 90-262) (22, 43). 262
Consistent with the low-resolution protein mapping data, we observed a weak 263
cross-link between Brf1 and the C34 subunit of Pol III at residue Tyr99 of the H2 264
helix in the first cyclin fold (Figure 2A). 265
Our previous cross-linking analysis on Pol III identified inter-subunit 266
interactions that localize C34 N-terminal winged-helix domains WH1 and WH2 267
above the Pol III active center cleft and the C-terminal region beneath the 268
polymerase clamp domain (Figure 2B). However, it remains unclear how C34 269
provides additional Pol III-Brf1 interaction for Pol III recruitment as indicated in 270
on March 4, 2018 by guesthttp://mcb.asm.org/Downloaded from

14
previous studies (22, 29, 44). To address this, we incorporated BPA in C34 to 271
map Brf1 binding sites. BPA substitution at Glu169, located at the connecting 272
region between WH2 and the predicted WH3, resulted in a weak cross-link with 273
Brf1 (Figure 2C). Surprisingly, Glu169 is located near the amino acid stretch 274
Asp171-Glu173 that is functionally important for Pol III recruitment (44). 275
To further characterize the C34-Brf1 interaction, we applied site-directed 276
hydroxyl radical analysis to probe the structural region of Brf1 near the C34 277
WH2/3 connecting region. We generated C34 single cysteine mutants to 278
conjugate the hydroxyl radical reagent FeBABE at the amino acid positions 279
Leu170 and Ile172. The FeBABE-conjugated C34 variants were applied to the 280
IMT assay for hydroxyl radical protein cleavage analysis in the PIC. In Figure 2D, 281
a Brf1 cleavage fragment was commonly generated by the C34-FeBABE 282
conjugates (Figure 2D; lanes 2, 3, and 4). By comparing with the molecular 283
weight ladder generated from in vitro translated Brf1 peptide fragments, the 284
cleavage site was determined to be in the H4’ helix of Brf1n second cyclin fold. In 285
summary, the combined cross-linking and hydroxyl radical analyses suggest an 286
interaction between the WH2/3 connecting region of C34 and the cyclin fold 287
repeats of Brf1n. As the biochemical probing results were weak, we suspect that 288
C34 might not strongly interact with Brf1 in the PIC. However, as previous studies 289
suggested that BPA is a less efficient cross-linking reagent due to its geometry 290
requirement for hydrogen abstraction by benzophenone (45), the weak C34-Brf1 291
crosslinking could also be attributed to the poor cross-linking efficiency of BPA. 292
293
on March 4, 2018 by guesthttp://mcb.asm.org/Downloaded from

15
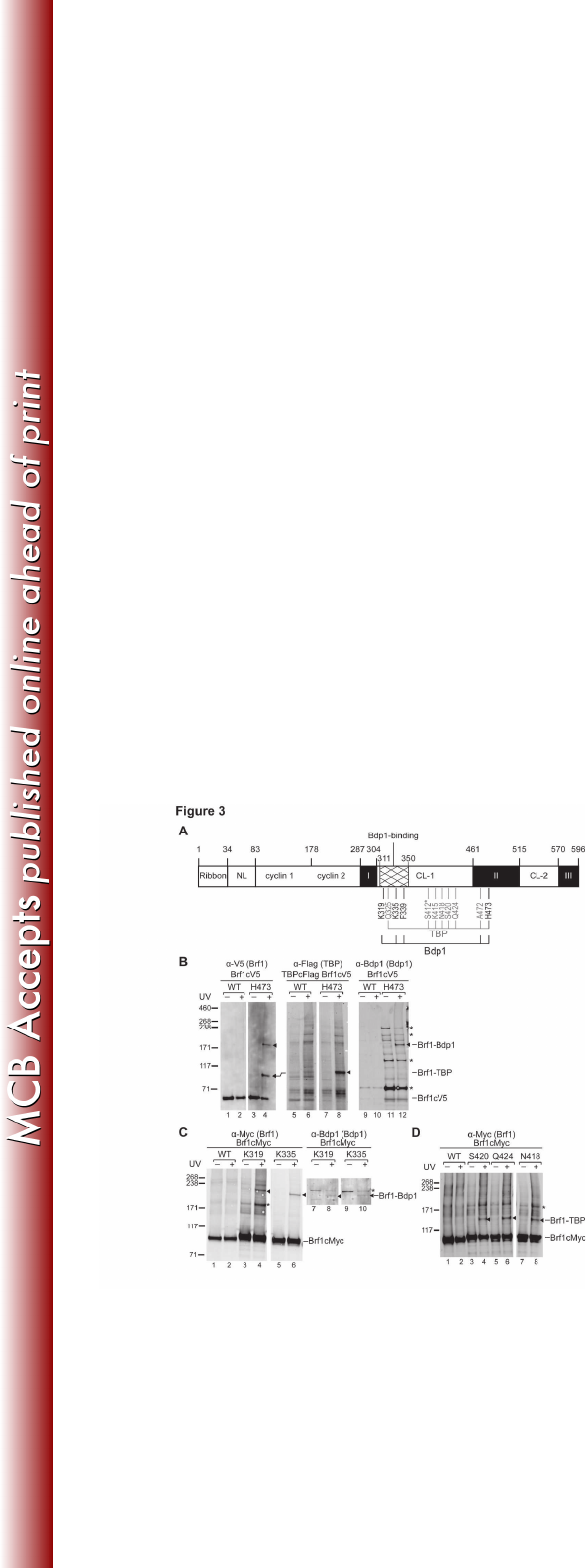
Brf1 C-terminal domain contains extended Bdp1 and TBP binding region 294
The homology block II of Brf1c serves as the dominant binding site for both 295
TBP and Bdp1, and this block adopts a “vine-on-a-tree” conformation to interact 296
with TBP from the convex surface to the lateral surface of the first structural 297
repeat (6, 27, 28). Consistent with the protein interaction model, our BPA cross-298
linking analysis conducted in homology block II revealed cross-links with Bdp1 299
and TBP. As indicated in the summary of Brf1c cross-linking (Figure 3A) and 300
illustrated in Figure 3B, BPA-substitution at residue His473 generates two cross-301
links confirmed to be TBP and Bdp1, indicating simultaneous interactions with 302
both proteins. Similar simultaneous cross-linking was also observed for BPA 303
substitution at the neighboring residue Ala472 (data not shown). In the homology 304
block II-TBP-DNA ternary complex structure, His473 and Ala472 belong to the 305
helix H23 that interacts with the convex surface of the TBP first structural repeat. 306
Our cross-linking results therefore further suggest the localization for Bdp1 on the 307
TBP convex surface. 308
Additional Bdp1 and TBP cross-links were also observed for BPA 309
substitutions in the connecting region between homology blocks I and II, which 310
we refer to as C-linker 1. As shown in Figure 3C and summarized in Figure 3A, 311
BPA incorporated at residues Lys319 and Lys335 generated Bdp1 cross-linking. 312
In contrast to the Brf1-Bdp1 cross-links that are clustered closer to homology 313
block I, Brf1-TBP cross-linking occurs at residues widely distributed in C-linker 1 314
(Figure 3D and summarized in Figure 3A). 315
316
on March 4, 2018 by guesthttp://mcb.asm.org/Downloaded from

16
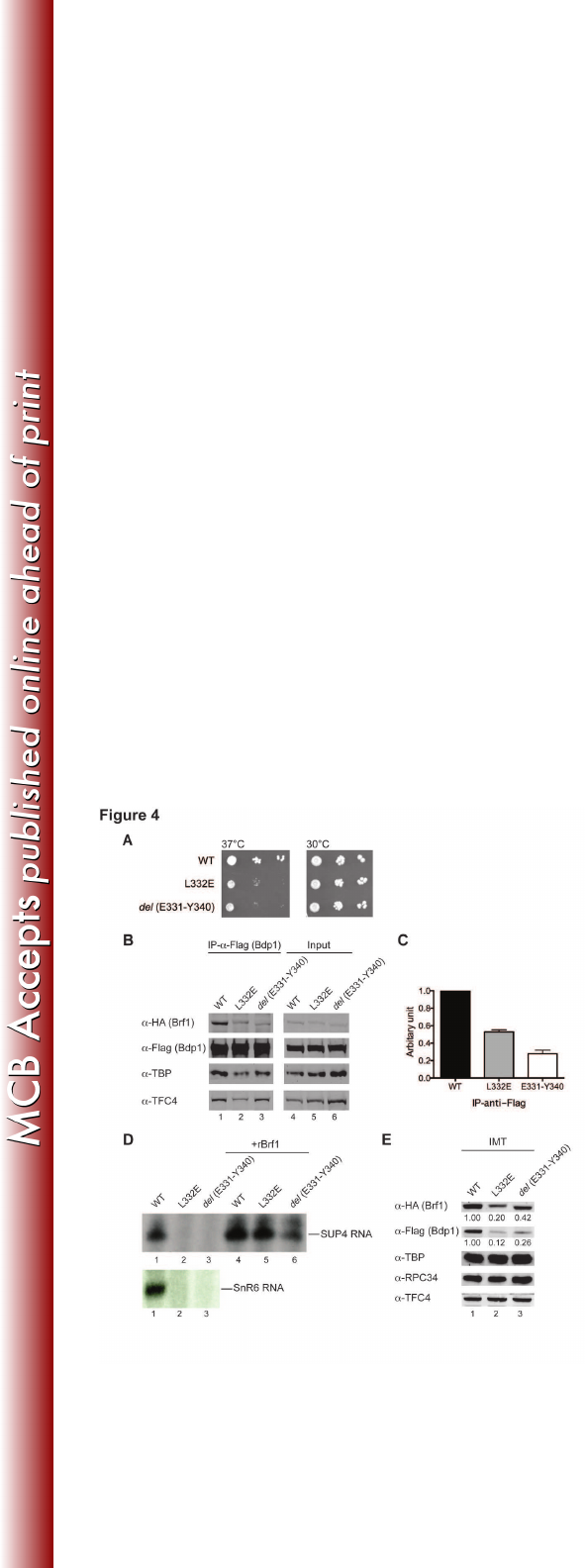
The Bdp1-binding block is important for transcription initiation 317
On the basis of extensive TBP and Bdp1c interactions revealed by BPA 318
cross-linking, we introduced a series of truncations and point mutations in Brf1c. 319
Internal truncations and point mutations were initially introduced in homology 320
block I resulting in cell lethality. In contrast, most of the mutations in C-linker 1 321
resulted in yeast strains without observable temperature-dependent growth 322
defects. However, mutations in the sequence block Gln311-Arg350, which 323
provided multiple cross-linking with Bdp1 (Figure 3A), conferred a temperature-324
sensitive growth phenotype. As demonstrated in Figure 4A, the yeast strains with 325
either Leu332Glu point mutation or del (Glu331-Tyr340) internal truncation 326
showed slow cell growth at the non-permissive temperature 37°C. We isolated 327
WCEs from these two mutant strains and conducted a co-immunoprecipitation 328
assay to analyze Brf1-Bdp1 binding. As shown in Figure 4B and 4C, both Brf1c 329
mutants severely compromised the binding with Bdp1, supporting our cross-link 330
data. We further analyzed this newly identified Bdp1-binding block by in vitro 331
transcription and PIC formation assays on the SUP4 DNA template. Both 332
mutations severely compromised transcription activity (Figure 4D, lanes 2 and 3). 333
The mutations also caused reduced Bdp1 and Brf1 protein levels in the isolated 334
PICs from the IMT assay (Figure 4E, lanes 2 and 3), indicating that both 335
mutations affect stable association of Bdp1 and Brf1 in the PIC. Our results thus 336
suggest that this Bdp1-binding block provides important structural support for 337
stabilizing Brf1 and Bdp1 in the PIC. 338
339
on March 4, 2018 by guesthttp://mcb.asm.org/Downloaded from

17
Discussion 340
In the Pol III transcription machinery, Brf1 together with TBP and Bdp1 341
constitutes transcription factor TFIIIB for Pol III recruitment and open promoter 342
complex formation. Using site-specific biochemical probing analyses in this study, 343
we precisely mapped the network of protein interactions for Brf1 in the PIC. Our 344
cross-linking results suggest that the Brf1 N-terminal domain organizes a TFIIB-345
like domain architecture in the PIC. In contrast, the C-terminal half of Brf1 serves 346
mainly as the interface to hold TBP and Bdp1 for TFIIIB complex. An open 347
promoter model for the Pol III PIC is thus derived based on the x-ray structures of 348
Pol II-TFIIB, TFIIB cyclin folds-TBP-DNA, and Brf1 homology block II-TBP-DNA 349
complexes (Figure 5) (20, 28, 46). In the model, the ribbon and the cyclin fold 350
repeat subdomains are respectively localized in the RNA exit tunnel and on the 351
wall domain of polymerase. TBP contacts a 8-bp-long DNA sequence that starts 352
from 30 bases upstream of the transcription start site, and the Brf1 cyclin folds 353
clamp the second stirrup of TBP and interact with DNA sequences flanking the 354
TBP-binding region. Brf1 N-linker region was not modeled due to the lack of 355
structural information. However, this region likely interacts with the open 356
promoter region as well as structural motifs of the active center based on our 357
Brf1-C160 cross-linking and its functional role, together with the ribbon 358
subdomain, in DNA opening (11, 13, 47, 48). 359
The domain architecture of Pol III derived from our previous study 360
localizes the TFIIE-like C82 and C34 subunits on the polymerase clamp (Figure 361
5). The WH2 domain of C34 is in close contact with the clamp coiled-coil and 362
on March 4, 2018 by guesthttp://mcb.asm.org/Downloaded from

18
further interacts with the upstream edge of the transcription bubble, which is a 363
10~12 base strand-separated promoter region spanning upstream beginning 364
from the transcription start site (39). With the localization of C34 WH2 domain, 365
the functionally important connecting region immediately C-terminal to WH2 is 366
likely positioned adjacent to the Brf1 cyclin fold repeat subdomain. Our site-367
specific cross-linking and hydroxyl radical data support this interaction. Further, 368
this C34 connecting region likely contributes to additional upstream C34-DNA 369
interaction based on the Pol III-DNA topography analysis indicating co-370
localization of C34 and Brf1 in the promoter region spanning ~20 bases upstream 371
of the transcription start site (49, 50). In the Pol II PIC, the TFIIB cyclin folds were 372
found to interact with Tfg1 and Tfg2 subunits of the transcription factor TFIIF (15), 373
which is positioned on the lobe and protrusion domains of polymerase (40). 374
Compared to TFIIE, which also interacts with the polymerase clamp, the 375
localization of TFIIF is on the opposite side of the polymerase cleft. Therefore, 376
the cyclin repeats domain is involved in establishing specific interactions with 377
polypeptides on the polymerase active center cleft for respective transcription 378
systems. 379
Our cross-linking data indicate Brf1c mainly serves as a bipartite interface 380
for TBP and Bdp1. Specifically, our analysis extends Bdp1- and TBP-binding 381
sites to the C-linker 1 region, and we identified a functionally important Bdp1-382
binding sequence block. Although this Bdp1-binding block contains low sequence 383
homology, secondary structure analysis indicates consensus -helical secondary 384
structures in this region. Furthermore, this Bdp1-binding block contains the amino 385
on March 4, 2018 by guesthttp://mcb.asm.org/Downloaded from

19
acid sequence Gly328-Glu329-Gln330-Glu331-Leu332 (GEXEL) that was 386
previously reported to be a conserved short motif in Brf1c (25). The structural 387
region of Bdp1 that interacts with this sequence block remains to be determined. 388
In addition to TBP and Bdp1 interactions, we observed a weak C34 cross-link for 389
BPA substitution at Gln549 adjacent to homology block III (data not shown). This 390
C34 cross-link supports a previous genetic interaction analysis that mapped Brf1-391
C34 interaction to homology blocks II and III (29). 392
The domain architecture of the PIC derived from this study explains 393
respective functional roles in DNA opening for ribbon and N-linker and in 394
organizing TFIIIB-pol III-DNA complex for the cyclin fold repeats subdomain and 395
the C-terminal domain. In the eukaryotic Pol I system, the TFIIB-related factors 396
TAF1B in human and Rrn7 in yeast also contain TFIIB-like ribbon and cyclin 397
repeat subdomains in their N-terminal domains and unique C-terminal domains 398
specific for respective polymerases (23, 24). Genetic analysis for TAF1B 399
indicated that the zinc ribbon and the connecting region (N-terminal linker) mainly 400
function in post-recruitment step(s), reminiscent of Brf1 (23). Although the 401
analysis for domain localization is not available, a conserved binding mechanism 402
may exist for these Pol I factors as suggested by our study for Brf1. 403
on March 4, 2018 by guesthttp://mcb.asm.org/Downloaded from

20
Acknowledgements 404
We thank Dr. George Kassavetis (UC San Diego) for advices on biochemical 405
probing experiments. We thank Yue-Chang Chou for protein purification. We 406
thank AndreAna Peña for English editing. This work was supported by the grant 407
NSC 100-2311-B-001-013-MY3 from National Science Council, R.O.C. and the 408
Career Development Award to H.-T.C. from Academia Sinica. 409
410
on March 4, 2018 by guesthttp://mcb.asm.org/Downloaded from

21
References 411
1. Dieci, G., G. Fiorino, M. Castelnuovo, M. Teichmann, and A. Pagano. 2007. The 412
expanding RNA polymerase III transcriptome. Trends Genet 23:614-622. 413
2. Geiduschek, E. P., and G. A. Kassavetis. 2001. The RNA polymerase III 414
transcription apparatus. J Mol Biol 310:1-26. 415
3. Schramm, L., and N. Hernandez. 2002. Recruitment of RNA polymerase III to its 416
target promoters. Genes Dev 16:2593-2620. 417
4. Ishiguro, A., G. A. Kassavetis, and E. P. Geiduschek. 2002. Essential roles of Bdp1, 418
a subunit of RNA polymerase III initiation factor TFIIIB, in transcription and tRNA 419
processing. Mol Cell Biol 22:3264-3275. 420
5. Kassavetis, G. A., C. Bardeleben, A. Kumar, E. Ramirez, and E. P. Geiduschek. 421
1997. Domains of the Brf component of RNA polymerase III transcription factor 422
IIIB (TFIIIB): functions in assembly of TFIIIB-DNA complexes and recruitment of 423
RNA polymerase to the promoter. Mol Cell Biol 17:5299-5306. 424
6. Kassavetis, G. A., R. Driscoll, and E. P. Geiduschek. 2006. Mapping the principal 425
interaction site of the Brf1 and Bdp1 subunits of Saccharomyces cerevisiae TFIIIB. 426
J Biol Chem 281:14321-14329. 427
7. Kumar, A., A. Grove, G. A. Kassavetis, and E. P. Geiduschek. 1998. Transcription 428
factor IIIB: the architecture of its DNA complex, and its roles in initiation of 429
transcription by RNA polymerase III. Cold Spring Harb Symp Quant Biol 63:121-430
129. 431
8. Kumar, A., G. A. Kassavetis, E. P. Geiduschek, M. Hambalko, and C. J. Brent. 432
1997. Functional dissection of the B" component of RNA polymerase III 433
transcription factor IIIB: a scaffolding protein with multiple roles in assembly and 434
initiation of transcription. Mol Cell Biol 17:1868-1880. 435
9. Librizzi, M. D., M. Brenowitz, and I. M. Willis. 1998. The TATA element and its 436
context affect the cooperative interaction of TATA-binding protein with the TFIIB-437
related factor, TFIIIB70. The Journal of biological chemistry 273:4563-4568. 438
10. Saida, F. 2008. Structural characterization of the interaction between TFIIIB 439
components Bdp1 and Brf1. Biochemistry 47:13197-13206. 440
11. Kassavetis, G. A., A. Kumar, G. A. Letts, and E. P. Geiduschek. 1998. A post-441
recruitment function for the RNA polymerase III transcription-initiation factor IIIB. 442
Proc Natl Acad Sci U S A 95:9196-9201. 443
12. Kassavetis, G. A., A. Kumar, E. Ramirez, and E. P. Geiduschek. 1998. Functional 444
and structural organization of Brf, the TFIIB-related component of the RNA 445
polymerase III transcription initiation complex. Mol Cell Biol 18:5587-5599. 446
13. Hahn, S., and S. Roberts. 2000. The zinc ribbon domains of the general 447
transcription factors TFIIB and Brf: conserved functional surfaces but different 448
roles in transcription initiation. Genes Dev 14:719-730. 449
14. Wu, C. C., Y. C. Lin, and H. T. Chen. 2011. The TFIIF-like Rpc37/53 dimer lies at 450
the center of a protein network to connect TFIIIC, Bdp1, and the RNA polymerase 451
III active center. Molecular and cellular biology 31:2715-2728. 452
15. Chen, H. T., and S. Hahn. 2004. Mapping the location of TFIIB within the RNA 453
on March 4, 2018 by guesthttp://mcb.asm.org/Downloaded from

22
polymerase II transcription preinitiation complex: a model for the structure of 454
the PIC. Cell 119:169-180. 455
16. Chen, H. T., and S. Hahn. 2003. Binding of TFIIB to RNA polymerase II: Mapping 456
the binding site for the TFIIB zinc ribbon domain within the preinitiation complex. 457
Mol Cell 12:437-447. 458
17. Bushnell, D. A., K. D. Westover, R. E. Davis, and R. D. Kornberg. 2004. Structural 459
basis of transcription: an RNA polymerase II-TFIIB cocrystal at 4.5 Angstroms. 460
Science 303:983-988. 461
18. Liu, X., D. A. Bushnell, D. Wang, G. Calero, and R. D. Kornberg. 2010. Structure 462
of an RNA polymerase II-TFIIB complex and the transcription initiation 463
mechanism. Science 327:206-209. 464
19. Kostrewa, D., M. E. Zeller, K. J. Armache, M. Seizl, K. Leike, M. Thomm, and P. 465
Cramer. 2009. RNA polymerase II-TFIIB structure and mechanism of transcription 466
initiation. Nature 462:323-330. 467
20. Sainsbury, S., J. Niesser, and P. Cramer. 2012. Structure and function of the 468
initially transcribing RNA polymerase II-TFIIB complex. Nature. 469
21. Colbert, T., and S. Hahn. 1992. A yeast TFIIB-related factor involved in RNA 470
polymerase III transcription. Genes Dev 6:1940-1949. 471
22. Khoo, B., B. Brophy, and S. P. Jackson. 1994. Conserved functional domains of 472
the RNA polymerase III general transcription factor BRF. Genes Dev 8:2879-2890. 473
23. Naidu, S., J. K. Friedrich, J. Russell, and J. C. Zomerdijk. 2011. TAF1B is a TFIIB-474
like component of the basal transcription machinery for RNA polymerase I. 475
Science 333:1640-1642. 476
24. Knutson, B. A., and S. Hahn. 2011. Yeast Rrn7 and human TAF1B are TFIIB-477
related RNA polymerase I general transcription factors. Science 333:1637-1640. 478
25. Martinez, M. J., and K. U. Sprague. 2003. Cloning of a putative Bombyx mori 479
TFIIB-related factor (BRF). Arch Insect Biochem Physiol 54:55-67. 480
26. Kassavetis, G. A., C. A. Joazeiro, M. Pisano, E. P. Geiduschek, T. Colbert, S. Hahn, 481
and J. A. Blanco. 1992. The role of the TATA-binding protein in the assembly and 482
function of the multisubunit yeast RNA polymerase III transcription factor, TFIIIB. 483
Cell 71:1055-1064. 484
27. Colbert, T., S. Lee, G. Schimmack, and S. Hahn. 1998. Architecture of protein and 485
DNA contacts within the TFIIIB-DNA complex. Mol Cell Biol 18:1682-1691. 486
28. Juo, Z. S., G. A. Kassavetis, J. Wang, E. P. Geiduschek, and P. B. Sigler. 2003. 487
Crystal structure of a transcription factor IIIB core interface ternary complex. 488
Nature 422:534-539. 489
29. Andrau, J. C., A. Sentenac, and M. Werner. 1999. Mutagenesis of yeast TFIIIB70 490
reveals C-terminal residues critical for interaction with TBP and C34. J Mol Biol 491
288:511-520. 492
30. Ferri, M. L., G. Peyroche, M. Siaut, O. Lefebvre, C. Carles, C. Conesa, and A. 493
Sentenac. 2000. A novel subunit of yeast RNA polymerase III interacts with the 494
TFIIB-related domain of TFIIIB70. Mol Cell Biol 20:488-495. 495
31. Moir, R. D., K. V. Puglia, and I. M. Willis. 2000. Interactions between the 496
tetratricopeptide repeat-containing transcription factor TFIIIC131 and its ligand, 497
on March 4, 2018 by guesthttp://mcb.asm.org/Downloaded from

23
TFIIIB70. Evidence for a conformational change in the complex. J Biol Chem 498
275:26591-26598. 499
32. Moir, R. D., K. V. Puglia, and I. M. Willis. 2002. Autoinhibition of TFIIIB70 binding 500
by the tetratricopeptide repeat-containing subunit of TFIIIC. J Biol Chem 277:694-501
701. 502
33. Moir, R. D., I. Sethy-Coraci, K. Puglia, M. D. Librizzi, and I. M. Willis. 1997. A 503
tetratricopeptide repeat mutation in yeast transcription factor IIIC131 (TFIIIC131) 504
facilitates recruitment of TFIIB-related factor TFIIIB70. Mol Cell Biol 17:7119-7125. 505
34. Brachmann, C. B., A. Davies, G. J. Cost, E. Caputo, J. Li, P. Hieter, and J. D. Boeke. 506
1998. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a 507
useful set of strains and plasmids for PCR-mediated gene disruption and other 508
applications. Yeast 14:115-132. 509
35. Wach, A., A. Brachat, R. Pohlmann, and P. Philippsen. 1994. New heterologous 510
modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. 511
Yeast 10:1793-1808. 512
36. Christianson, T. W., R. S. Sikorski, M. Dante, J. H. Shero, and P. Hieter. 1992. 513
Multifunctional yeast high-copy-number shuttle vectors. Gene 110:119-122. 514
37. Chin, J. W., T. A. Cropp, J. C. Anderson, M. Mukherji, Z. Zhang, and P. G. Schultz. 515
2003. An expanded eukaryotic genetic code. Science 301:964-967. 516
38. Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host 517
strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. 518
Genetics 122:19-27. 519
39. Wu, C. C., F. Herzog, S. Jennebach, Y. C. Lin, C. Y. Pai, R. Aebersold, P. Cramer, 520
and H. T. Chen. 2012. RNA polymerase III subunit architecture and implications 521
for open promoter complex formation. Proc Natl Acad Sci U S A 109:19232-19237. 522
40. Chen, H. T., L. Warfield, and S. Hahn. 2007. The positions of TFIIF and TFIIE in the 523
RNA polymerase II transcription preinitiation complex. Nat Struct Mol Biol 524
14:696-703. 525
41. Jasiak, A. J., K. J. Armache, B. Martens, R. P. Jansen, and P. Cramer. 2006. 526
Structural biology of RNA polymerase III: subcomplex C17/25 X-ray structure and 527
11 subunit enzyme model. Mol Cell 23:71-81. 528
42. Nikolov, D. B., H. Chen, E. D. Halay, A. A. Usheva, K. Hisatake, D. K. Lee, R. G. 529
Roeder, and S. K. Burley. 1995. Crystal structure of a TFIIB-TBP-TATA-element 530
ternary complex. Nature 377:119-128. 531
43. Werner, M., N. Chaussivert, I. M. Willis, and A. Sentenac. 1993. Interaction 532
between a complex of RNA polymerase III subunits and the 70-kDa component of 533
transcription factor IIIB. J Biol Chem 268:20721-20724. 534
44. Brun, I., A. Sentenac, and M. Werner. 1997. Dual role of the C34 subunit of RNA 535
polymerase III in transcription initiation. EMBO J 16:5730-5741. 536
45. Tate, J. J., J. Persinger, and B. Bartholomew. 1998. Survey of four different 537
photoreactive moieties for DNA photoaffinity labeling of yeast RNA polymerase 538
III transcription complexes. Nucleic acids research 26:1421-1426. 539
46. Tsai, F. T., and P. B. Sigler. 2000. Structural basis of preinitiation complex 540
assembly on human pol II promoters. Embo J 19:25-36. 541
on March 4, 2018 by guesthttp://mcb.asm.org/Downloaded from

24
47. Kassavetis, G. A., G. A. Letts, and E. P. Geiduschek. 1999. A minimal RNA 542
polymerase III transcription system. EMBO J 18:5042-5051. 543
48. Kassavetis, G. A., G. A. Letts, and E. P. Geiduschek. 2001. The RNA polymerase III 544
transcription initiation factor TFIIIB participates in two steps of promoter opening. 545
EMBO J 20:2823-2834. 546
49. Bartholomew, B., D. Durkovich, G. A. Kassavetis, and E. P. Geiduschek. 1993. 547
Orientation and topography of RNA polymerase III in transcription complexes. 548
Mol Cell Biol 13:942-952. 549
50. Bartholomew, B., G. A. Kassavetis, and E. P. Geiduschek. 1991. Two components 550
of Saccharomyces cerevisiae transcription factor IIIB (TFIIIB) are stereospecifically 551
located upstream of a tRNA gene and interact with the second-largest subunit of 552
TFIIIC. Molecular and cellular biology 11:5181-5189. 553
554
555
556
on March 4, 2018 by guesthttp://mcb.asm.org/Downloaded from

25
Figure Legends 557
Figure 1. Brf1n BPA photo-crosslinking. (A) Schematic of Brf1 domain 558
architecture and summary of Brf1n BPA photo-crosslinking. Residue numbers for 559
the boundaries of individual subdomains are marked. NL, N-linker; CL-1&2, C-560
linker 1&2. BPA-substituted residues are color coded according to respective 561
cross-linked polypeptides indicated below the horizontal connecting lines. Lower 562
panel: models of the ribbon fold (left) and the Brf1c homology block II-TBP-DNA 563
complex (right). The magenta sphere in the ribbon model indicates the zinc ion. 564
TBP is displayed with the molecular surface model in light green. Others are 565
shown as backbone trace with Brf1c homology block II in brown, Brf1n cyclin 566
folds in orange, template DNA (TS) in dark blue, and non-template DNA (NTS) in 567
cyan. BPA-substituted residues with confirmed cross-linking targets are 568
highlighted with spheres. The hydroxyl radical cleavage site (Ala246±5aa) in 569
Brf1n by C34-FeBABE is indicated. (B) Western analysis of Brf1-C160 photo-570
cross-linking. BPA-substituted residues are indicated above the lanes. Brf1-C160 571
cross-linking was identified using anti-V5 antibodies (Brf1) (lanes 1-6) and 572
confirmed with anti-HA antibodies (C160) (lanes 7-12), respectively. Triangles are 573
placed next to the cross-linking gel bands. All cross-linking bands in subsequent 574
figures are marked by triangles. WCE, yeast whole cells extract; UV + or −, with 575
or without UV irradiation; WT, wild-type Brf1 with no BPA replacement; *, non-576
specific background band. (C) Brf1-C128 photo-crosslinking. Brf1-C128 cross-577
linking band was visualized with anti-V5 antibody (Brf1) (lanes 1-4) and 578
confirmed with anti-HA antibody (C128) (lanes 5-8). (D) C128-Brf1 photo-579
on March 4, 2018 by guesthttp://mcb.asm.org/Downloaded from

26
crosslinking. C128-Brf1 cross-linking band was visualized with anti-Myc antibody 580
(C128) (lanes 1-4) and confirmed with anti-Flag antibody (Brf1) (lanes 5-8). The 581
BPA position in C128 additionally cross-links to C82. (E) Brf1-TBP photo-582
crosslinking at BPA substituted residue Lys211 in the second cyclin fold of Brf1. 583
The cross-linked Brf1-TBP was probed with anti-V5 antibody (Brf1) (lane 1-4) and 584
confirmed by anti-TBP antibody (lane 5-8). 585
586
Figure 2. Brf1n cyclin folds interact with C34. (A) Brf1-C34 photo-cross-linking 587
from BPA-substitution at residue Tyr99 of Brf1. The cross-linking was visualized 588
with an antibody against V5 (Brf1) (left panel) and was verified with C34 589
antiserum (right panel). Cross-linking bands are marked with triangles. The 590
bands marked with asterisks (*) are background bands, which appear to be UV-591
specific. (B) Schematic of C34 domain architecture. As highlighted in the 592
sequence of the connecting region between WH2 and 3 domains, Asp171 and 593
Glu173 mutations affect transcription initiation. (C) Western analysis of C34-Brf1 594
cross-linking. BPA-substitution is at the residue Glu169 of C34. Crosslink was 595
visualized by probing with anti-V5 antibody (C34) (left panel) and the identity of 596
the C34-Brf1 cross-linking band was verified by probing with Brf1 antiserum (right 597
panel). Asterisk (*) marks a non-specific background band. (D) Determination of 598
C34-FeBABE hydroxyl radical cleavage site in Brf1. The hydroxyl radical 599
cleavage peptide fragment is revealed in the Western blot analysis with anti-Flag 600
antibody, and the cleavage site is determined to be in the cyclin fold repeat 601
subdomain of Brf1 as indicated. The non-cysteine (nonCys) mutant of C34 does 602
on March 4, 2018 by guesthttp://mcb.asm.org/Downloaded from

27
not contain any cysteine residue for FeBABE conjugation and served as the 603
negative control. Non-specific bands are marked with asterisks. 604
605
Figure 3. BPA photo-cross-linking in Brf1c. (A) Summary of Brf1c BPA photo-606
crosslinking. (B) Western analysis of cross-linking for BPA-substitution at His473 607
of Brf1. The cross-linking results were probed with anti-V5 antibody (Brf1) (left 608
panel), anti-Flag antibody (TBP) (middle panel), and anti-Bdp1 antibody (right 609
panel). The cross-linking bands are marked with triangles. A slight upper mobility 610
shift for the Brf1-TBP cross-link in the middle panel was caused by the use of 611
Flag epitope tagging in TBP. (C) Western analysis of Brf1-Bdp1 cross-linking at 612
Lys319 and Lys335 in the C-linker 1 (CL-1) region. The Western blot was probed 613
with anti-Myc antibody for Myc-epitope tagged Brf1 and anti-Bdp1 antibody to 614
confirm the Bdp1 polypeptide in the cross-linked fusion (lanes 8 and 10). (D) 615
Western analysis of Brf1-TBP cross-linking for BPA-substitution at residues 616
Ser420, Gln424 and Asn418 of Brf1c. 617
618
Figure 4. Mutational analysis of Brf1c homology block I and C-linker 1. (A) Cell 619
growth phenotype was analyzed by the serial dilution spot assay. Both 620
Leu332Glu and del (Glu331-Tyr340) mutants showed slower growth at 37oC. (B) 621
Western blot analysis of co-immunoprecipitation for Brf1 Leu332Glu and del 622
(Glu331-Tyr340) mutants. Co-IP was conducted with anti-Flag agarose to 623
precipitate Flag-tagged Bdp1 and co-immune precipitated polypeptides were 624
probed with respective antibodies indicated on the left. (C) IP-anti-Flag results 625
on March 4, 2018 by guesthttp://mcb.asm.org/Downloaded from

28
are quantified and plotted with WT signals set to 1. Errors bars indicate s.e.m. 626
from four independent experiments. (D) Transcription activity of Brf1 mutants. As 627
indicated, WCEs from wild-type (WT) or mutant yeast strains were used in the in 628
vitro transcription assay. The autoradiograms show the SUP4 pre-tRNA transcript 629
(upper panel) and SnR6 transcript (lower panel). rBrf1, recombinant wild-type 630
Brf1. (E) Immobilized template analysis. Proteins in the isolated Pol III PICs from 631
the IMT assay were probed with antibodies as indicated on the left. The relative 632
protein levels for Brf1 and Bdp1 are listed below each gel band. 633
634
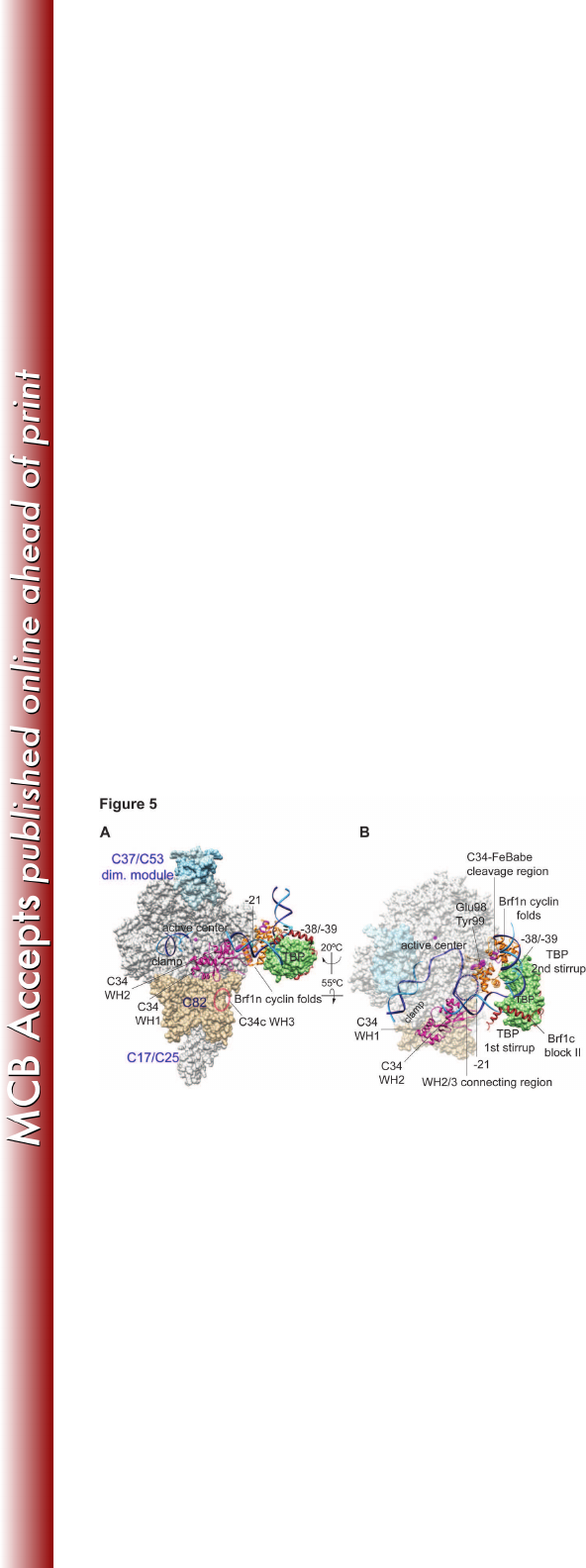
Figure 5. Model of the Pol III open promoter complex. (A) The structural model 635
contains Pol III, Brf1, TBP, and open promoter DNA based on the Pol II-TFIIB-636
TBP open promoter complex (19, 20) and the Brf1c homology block II-TBP-DNA 637
structure (28). Subdomains of Brf1 are displayed with the backbone trace model 638
and are color-coded: Brf1n cyclin repeats in orange and Brf1c homology block II 639
in brown. The molecular surface model of TBP is colored pale green. The Pol III 640
core structure is shown as the white molecular surface, and the magenta sphere 641
in the active center denotes the magnesium ion. Pol III-specific subunits are 642
displayed as follows: C34 WH1 and WH2, magenta backbone trace; C82, tan 643
molecular surface; C37/53 subcomplex, light blue molecular surface. DNA is 644
represented by the phosphate backbone trace with the template strand in blue 645
and non-template strand in cyan. Positions of DNA base-pairs -38/-39 and -21 on 646
the non-template strand (relative to the transcription start site as +1) are also 647
indicated. The localization for the WH3 domain of C34 is indicated as the dashed 648
on March 4, 2018 by guesthttp://mcb.asm.org/Downloaded from

29
oval line in black. The atomic coordinate file for the Pol III PIC model is available 649
upon request. (B) Same as in (A) with rotation as indicated. The molecular 650
surfaces for Pol III core, C37/53 subcomplex, and C82 are semi-transparent. As 651
highlighted with the spheres in the Brf1 cyclin repeats model, Glu98 and Tyr99 652
provide Brf1-C34 BPA-cross-linking and Ala246 (±5aa) is the hydroxyl radical 653
cleavage site by the FeBABE-conjugated C34. The dashed circle represents the 654
potential localization for the connecting region between the WH2 and WH3 655
domains of C34. 656
657
658
on March 4, 2018 by guesthttp://mcb.asm.org/Downloaded from