MER 2.0 Indicator Reference Guide
User Manual:
Open the PDF directly: View PDF ![]() .
.
Page Count: 174 [warning: Documents this large are best viewed by clicking the View PDF Link!]

1
1
January 2018
Version 2.2
Monitoring, Evaluation, and
Reporting (MER 2.0)
Indicator Reference Guide
Updated Release

2
THIS PAGE IS INTENTIONALLY LEFT BLANK.

MER 2.0 INTRODUCTION
3
CONTENTS
Contents ............................................................................................................................................3
Abbreviations ....................................................................................................................................6
Introduction ......................................................................................................................................7
Key Changes: MER 2.0 (V.1) to MER 2.0 (V.2) ......................................................................................8
New Indicators: ......................................................................................................................................... 8
HTS_SELF ............................................................................................................................................... 8
PMTCT_HEI_POS ................................................................................................................................... 8
New Disaggregations: ............................................................................................................................... 8
Age disaggregations .............................................................................................................................. 8
HTS_TST................................................................................................................................................. 9
LAB_PTCQI............................................................................................................................................. 9
Modifications to Existing Indicators .......................................................................................................... 9
OVC_SERV ............................................................................................................................................. 9
Modifications to Existing Disaggregations ................................................................................................ 9
VMMC_CIRC .......................................................................................................................................... 9
PrEP_NEW ............................................................................................................................................. 9
OVC_SERV ............................................................................................................................................. 9
TB_PREV ................................................................................................................................................ 9
TX_TB .................................................................................................................................................. 10
GEND_GBV .......................................................................................................................................... 10
Deleted Indicators ................................................................................................................................... 10
INVS_COMD ........................................................................................................................................ 10
OVC Essential Survey Indicators.......................................................................................................... 10
Deleted Disaggregations ......................................................................................................................... 10
HTS_TST............................................................................................................................................... 10
PMTCT_EID .......................................................................................................................................... 10
HRH_CURR .......................................................................................................................................... 10
Indicator Clarifications ............................................................................................................................ 11
Key Populations................................................................................................................................... 11
PMTCT_STAT ....................................................................................................................................... 11
TB_PREV .............................................................................................................................................. 11
TX_TB .................................................................................................................................................. 11
TX_PVLS: .............................................................................................................................................. 11
PEPFAR Support to Communities and Sites ....................................................................................... 11
DSD: ......................................................................................................................................................... 12
TA-SDI: ..................................................................................................................................................... 12
Support in Centrally Supported Areas .................................................................................................... 12
Disaggregated Monitoring ................................................................................................................ 13
Required Disaggregations ....................................................................................................................... 13
Conditional Disaggregations ................................................................................................................... 13

4
Optional Disaggregations ........................................................................................................................ 13
MER Indicator Narratives ................................................................................................................. 13
Guiding Narrative Questions ................................................................................................................... 14
Implementing Mechanism (IM) Level Narratives ................................................................................... 14
Technical Area Level Narratives .............................................................................................................. 14
National and Subnational Level Results Narratives ................................................................................ 15
Host Country National Program ........................................................................................................ 15
Host Country National and Subnational Results ..................................................................................... 16
Host Country National and Subnational Targets .................................................................................... 16
Host Country indicators by reporting level, targets, and results ............................................................ 16
SIMS in Relation to MER 2.0 ............................................................................................................. 17
DREAMS Specific Guidance ............................................................................................................... 17
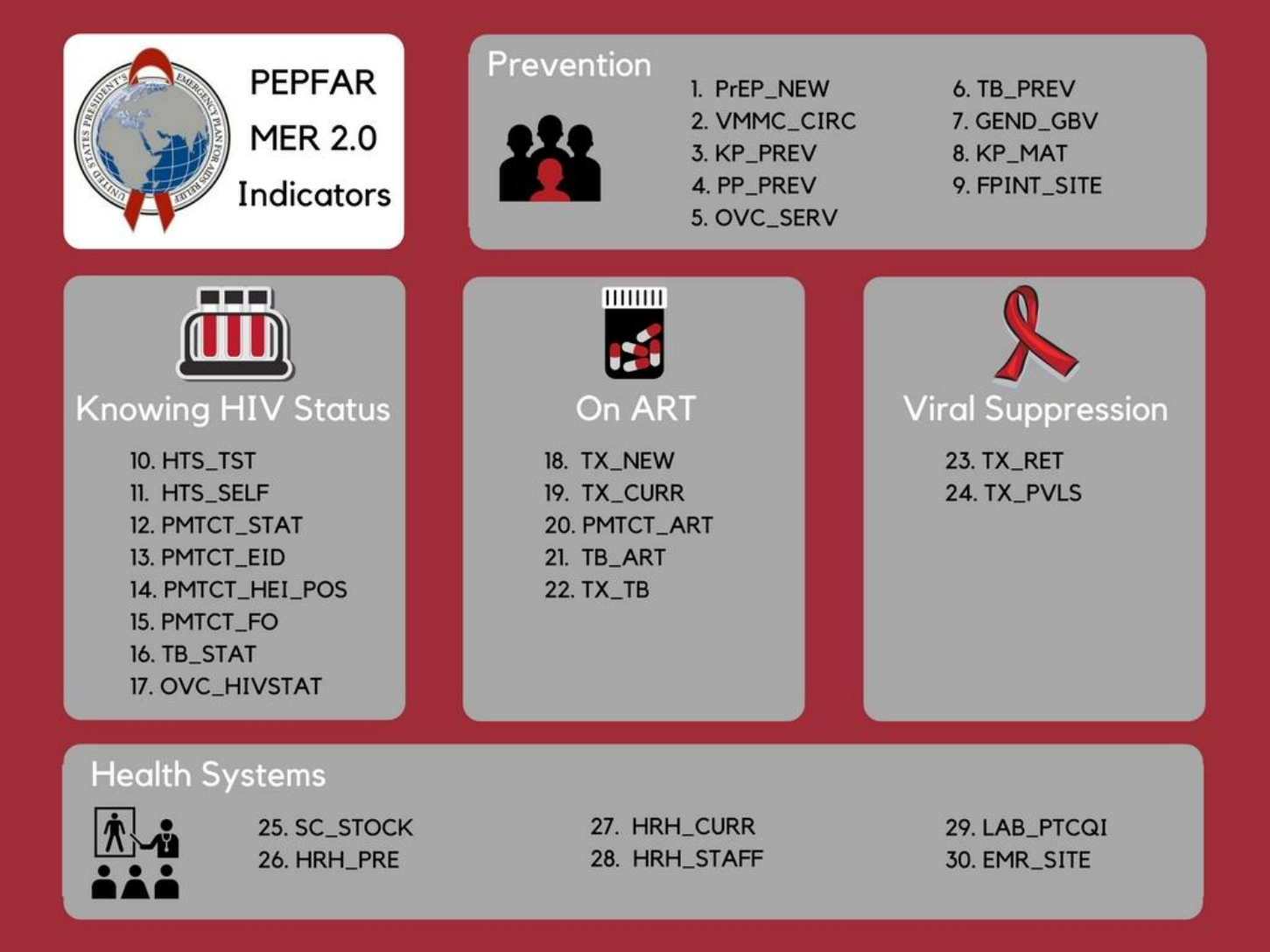
MER 2.0 Infographic ......................................................................................................................... 18
Indicator Reporting Frequency by Program Area ................................................................................... 19
How to read a PEPFAR indicator reference sheet................................................................................... 21
Prevention & Support Indicators ...................................................................................................... 22
PrEP_NEW ............................................................................................................................................... 23
VMMC_CIRC ............................................................................................................................................ 25
KP_PREV .................................................................................................................................................. 27
PP_PREV .................................................................................................................................................. 31
OVC_SERV ............................................................................................................................................... 36
TB_PREV .................................................................................................................................................. 41
KP_MAT ................................................................................................................................................... 44
GEND_GBV .............................................................................................................................................. 46
FPINT_SITE .............................................................................................................................................. 50
Knowing Your HIV Status Indicators .................................................................................................. 55
HTS_TST (including HTS_TST_POS) ......................................................................................................... 56
HTS_SELF ................................................................................................................................................. 65
PMTCT_STAT (including PMTCT_STAT_POS) .......................................................................................... 69
PMTCT_EID .............................................................................................................................................. 72
PMTCT_HEI_POS ..................................................................................................................................... 75
TB_STAT (including TB_STAT_POS) ......................................................................................................... 79
OVC_HIVSTAT .......................................................................................................................................... 81
PMTCT_FO............................................................................................................................................... 85
On ART Indicators ............................................................................................................................ 89
TX_NEW .................................................................................................................................................. 90
TX_CURR ................................................................................................................................................. 93
PMTCT_ART ............................................................................................................................................. 96
TB_ART .................................................................................................................................................... 99
TX_TB .................................................................................................................................................... 101
Viral Suppression Indicators ........................................................................................................... 104
TX_RET .................................................................................................................................................. 105

MER 2.0 INTRODUCTION
5
TX_PVLS ................................................................................................................................................. 110
Health Systems Indicators .............................................................................................................. 114
SC_STOCK .............................................................................................................................................. 115
HRH_PRE ............................................................................................................................................... 118
HRH_STAFF ............................................................................................................................................ 121
HRH_CURR ............................................................................................................................................ 124
EMR_SITE .............................................................................................................................................. 129
LAB_PTCQI ............................................................................................................................................ 132
Host-Country National & Subnational Indicators ............................................................................. 139
DIAGNOSED_NAT/SUBNAT ................................................................................................................... 140
VL_SUPPRESSION_NAT/SUBAT ............................................................................................................. 142
TX_CURR_NAT/SUBNAT ........................................................................................................................ 144
KP_MAT_NAT/SUBNAT ......................................................................................................................... 146
PMTCT_STAT_NAT/SUBNAT ................................................................................................................. 147
PMTCT_ART_NAT/SUBNAT ................................................................................................................... 149
VMMC_CIRC_NAT/SUBNAT .................................................................................................................. 152
VMMC_TOTALCIRC_NAT/SUBNAT ........................................................................................................ 154
Appendices .................................................................................................................................... 156
Appendix 1: Key Population Classification Document .......................................................................... 157
Appendix 2: MER and SIMS Mapping ................................................................................................... 158
Appendix 3: DREAMS and DREAMS-Like SNU Reporting Requirements .............................................. 169
Appendix 4: Frequency & Level of Reporting Table .............................................................................. 171
Appendix 5: Implementation and Planning Attributes (IMPATTS) ....................................................... 172
Appendix 6: HRH_CURR Example Calculation....................................................................................... 173

6
ABBREVIATIONS
CQI continuous quality improvement
DATIM Data for Accountability, Transparency, and Impact
DREAMS Determined, Resilient, Empowered, AIDS-free, Mentored, and Safe
EID early infant diagnosis
EMR electronic medical record
FSW female sex worker
GBV gender-based violence
HEI HIV-exposed infant
HIVST HIV self-testing
HRH human resources for health
HTS HIV testing services
IP implementing partner
KP key populations
MER monitoring, evaluation, and reporting indicators
MOH Ministry of Health
MSM men who have sex with men
OVC orphans and vulnerable children
PEPFAR United States President’s Emergency Plan for AIDS Relief
PITC provider-initiated testing and counseling
PLHIV people living with HIV
PMTCT prevention of mother-to-child transmission
POCT point-of-care testing
PP priority populations
PT proficiency testing
PVLS patient viral load suppression
PWID people who inject drugs
SID sustainability index
SIMS site improvement through monitoring systems
TB tuberculosis
TG transgender people
TX treatment
UNAIDS Joint United Nations Programme on HIV/AIDS
USG United States Government
VL viral load
VMMC voluntary medical male circumcision
WHO World Health Organization
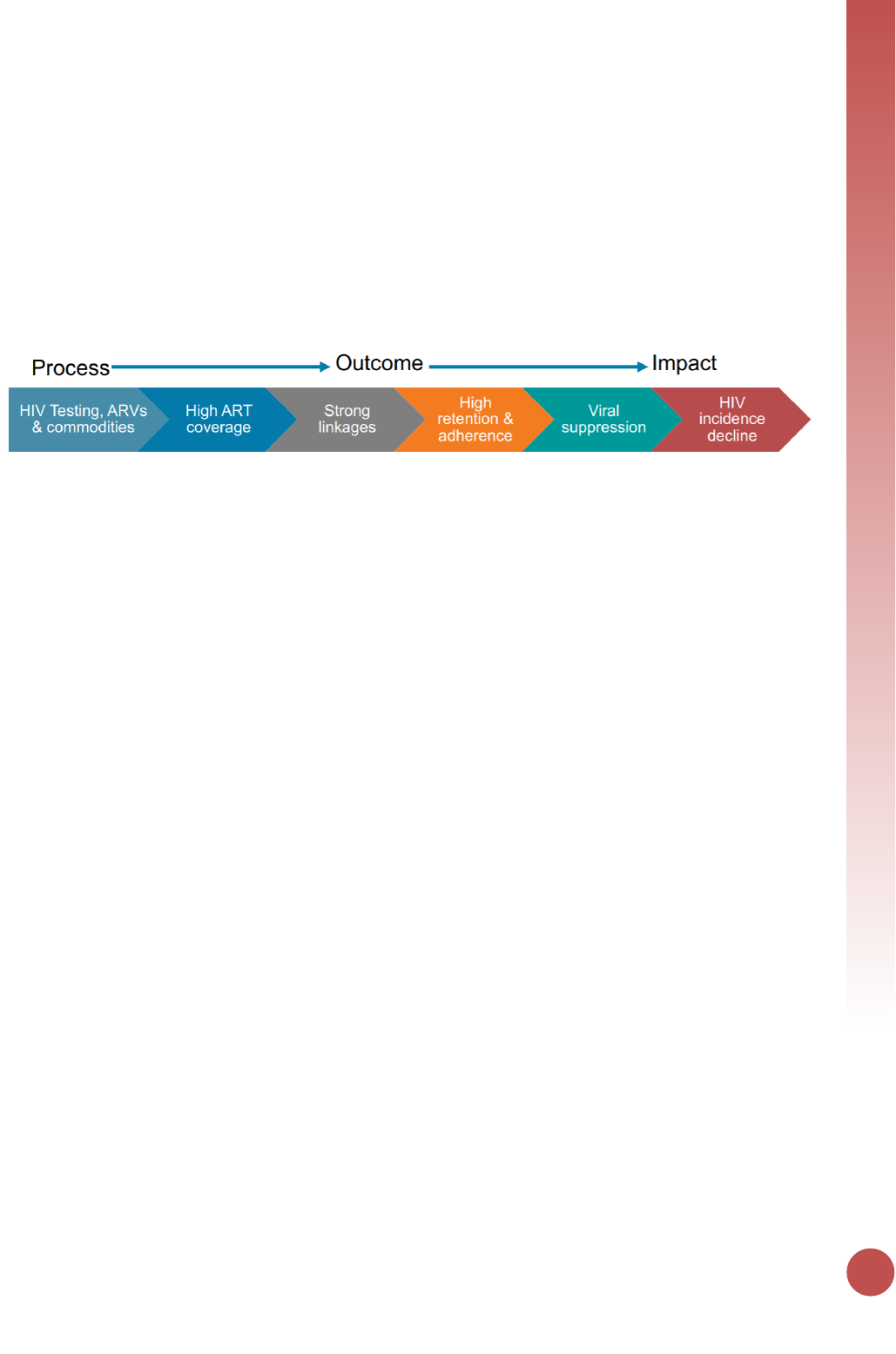
MER 2.0 INTRODUCTION
7
INTRODUCTION
PEPFAR's focus on optimizing impact is a driving force behind global efforts to reach HIV epidemic
control. PEPFAR is partnering with the international community to accelerate towards the UNAIDS 95-
95-95 global goals: 95 percent of people living with HIV know their HIV status, 95 percent of people who
know their HIV status are accessing treatment, and 95 percent of people on treatment have suppressed
viral loads. Progress towards epidemic control will be successfully measured, in part through an effective
strategic information framework that not only monitors program outputs, but also key outcomes and
programmatic impact.
Given the global HIV progress over the past decade, planning, monitoring and resource allocation needs
to occur at the subnational, community, and site levels in order to achieve the greatest impact.
Collection and use of disaggregated data that characterizes the populations served in the lowest
geographic areas where HIV services are being provided is critical in understanding current program
performance and planning for future performance. Consequently, the PEPFAR Monitoring, Evaluation,
and Reporting (MER) indicators continue to evolve in order to reflect the progression of U.S.
government (USG) support and global HIV response guidelines. Measuring the impact of national and
regional above-service delivery area support down to support provided for direct services at the site-
level is paramount to PEPFAR’s monitoring and reporting approach.
The objectives of the MER guidance document are to streamline and prioritize indicators for PEPFAR
programs. As the PEPFAR MER Indicators were being updated the following was taken into
consideration:
• Reduction of indicators to focus program monitoring on what matters most for epidemic
control;
• Standardization of age, sex and key population disaggregations across the prevention and
clinical cascades to monitor which populations are being reached with high quality evidence-
based services, and to identify which populations are not being reached;
• Alignment of indicators with multilaterals and partner governments to avoid duplication of data
collection where possible, and to focus on improved data and programmatic quality;
• Input from community stakeholders, technical experts, implementing partners, and PEPFAR field
staff;
• Alignment with other PEPFAR data streams such as site improvement through monitoring
systems (SIMS), financial monitoring, and the sustainability index (SID).
8
KEY CHANGES: MER 2.0 (V.1) TO MER 2.0 (V.2)
New Indicators:
HTS_SELF: HTS_SELF is a new indicator introduced for reporting beginning in Q1 of FY18. This indicator
assesses the distribution of HIV self-test kits disaggregated by directly assisted versus unassisted self-
testing. While age/sex disaggregates are requested for this indicator, it’s important to remember that
this indicator is assessing the distribution of self-test kits so the disaggregated data should be focused on
the individual the self-test kit was distributed to and not necessarily the end use of the test kit. For more
information and examples, please refer to the indicator reference sheet for HTS_SELF.
PMTCT_HEI_POS: PMTCT_HEI_POS is a new indicator for reporting beginning in Q1 of FY18. This
indicator is being introduced in response to challenges with the former PMTCT_EID_POS indicator
disaggregation in the collection of test results among those tests that were performed within the same
quarter. Previously, a significant proportion of results were reported as “unknown” each quarter since
results reporting was based on the date of DBS collection, but turnaround times from DBS collection to
result return to site are often ≥4 weeks. DBS collected within 4 weeks of the end of the quarter generally
did not have a result reported.
PMTCT_HEI_POS addresses these monitoring challenges by collecting only the positive results that
returned during the reporting period. PMTCT_HEI_POS indicator was introduced to describe both early
testing coverage and linkage of HIV+ infants to ART and to ensure collection of the number of infants
identified as HIV+ in the first year of life that would be accurate and meaningful to program monitoring
and planning. PMTCT_EID will continue to collect the virologic tests performed.
New Disaggregations:
AGE DISAGGREGATIONS: Data from the Population-Based HIV Impact Assessments (PHIA) provided
valuable insight into the progress many PEPFAR countries have made towards achieving the 95-95-95
goals in all ages and sexes. Significant disparities in incidence and viral suppression among adults within
the PEPFAR 25-49-year-old reporting age band lead PEPFAR to reassess the required reporting age
bands and further disaggregate the 25-49-year old age band into the following four age bands: 25-29,
30-34, 35-39, and 40-49. Reporting on the new PEPFAR age bands will commence in FY18 Q2.
New age bands: <1, 1-9, 10-14, 15-19, 20-24, 25-29, 30-34, 35-39, 40-49, and 50+
Previous age bands: <1, 1-9, 10-14, 15-19, 20-24, 25-49, and 50+
Reporting on the new MER 2.0 (v2.2) will be introduced in FY 18. Country teams that are unable to meet
the requirements for reporting the new age bands in FY 18 can continue reporting on the previous
aggregated 25-49 year old age band through FY 18. However, reporting on the new finer
disaggregations to align with targets set is COP 18 is required beginning in FY 19. Country teams should
discuss barriers to reporting on the new disaggregations during COP 18 to determine what systems and
resources can be realigned in FY 18 to ensure seamless reporting on the new age in Q1 of FY 19.
MER 2.0 INTRODUCTION
9
HTS_TST: Two new facility-based testing modalities have been introduced for FY18 reporting:
emergency department and STI clinic. Please refer to the indicator reference sheet for HTS_TST for
additional details on the new facility-based testing modalities.
LAB_PTCQI: A new disaggregate was introduced beginning in FY18 for the number of specimens
received for testing at all PEPFAR-supported laboratories and point-of-care testing (POCT) sites within a
testing category for the following categories: HIV serology/diagnostic testing, HIV IVT/EID, HIV Viral
Load, TB Xpert, TB AFB, TB Culture, and CD4. LAB_PTCQI is an annual indicator so PEPFAR teams will
begin reporting on this change at FY18 Q4.
Modifications to Existing Indicators
OVC_SERV: Requirements for OVC_SERV have changed significantly in FY 2018. The indicator
calculation has been updated and OVC_SERV will return to being a snapshot indicator again for FY 18
reporting. Results should not be summed across reporting periods.
The numerator for OVC_SERV will be auto-calculated using the program participation status
disaggregation for (1) active beneficiaries and (2) graduated beneficiaries. Beneficiaries that transferred
or exited without graduation should no longer be reported in the numerator. However, these data will
still be collected as OVC_SERV disaggregates.
Transferred will be further disaggregated into “transferred to a PEPFAR-supported partner” or
“transferred to a non-PEPFAR-supported partner.”
These changes will be reflected in the data entry screens in DATIM beginning in FY 18 Q2.
Modifications to Existing Disaggregations
VMMC_CIRC: The VMMC follow-up status disaggregate has been updated to capture instances where
post-VMMC follow-up did not take place within 14 days of the procedure or within the reporting period.
PREP_NEW: The KP type disaggregation for this indicator was updated to include ‘Other KP Type’ in
addition to the MSM, TG, and FSW options that were already available.
OVC_SERV: The Age/Sex/Service Area disaggregate [DREAMS Conditional Disaggregate] was updated
to include the age bands for children under 10 (<1, 1-9).
TB_PREV: Corresponding to the sharper focus of the End TB Strategy and the emphasis on TB
prevention, we now report TB_PREV which identifies the proportion of patients that complete or are
maintained on continuous preventive therapy. The disaggregation for “Type of TB preventive therapy”
has been updated for FY18 reporting to include ART start (i.e., newly enrolled on ART vs. previously
enrolled on ART). TB preventive therapy regimen disaggregates include IPT or an alternative TB
preventive therapy regimen by newly or previously enrolled on ART.

10
TX_TB: TX_TB allows us to document the number of patients who are screened for TB and the
proportion of those who are eventually started on TB therapy. This indicator also captures the number
of ART patients who had a specimen sent for bacteriologic diagnosis (and type) of active TB disease. The
denominator disaggregation for ‘Screen Result’ has been updated for FY18 reporting to include ART start
to help understand if patients that screen for TB (i.e., either screen positive or screen negative) are
either newly enrolled or previously enrolled on ART.
GEND_GBV: Age/sex disaggregations were added to the post-exposure prophylaxis (PEP)
disaggregation. This change will help us to better understand which individuals are receiving PEP among
those that have experienced sexual violence. GEND_GBV is an annual indicator so PEPFAR teams will
begin reporting on this change at FY18 Q4.
Deleted Indicators
INVS_COMD: Indicator has been removed due to duplication with quarterly data submitted by
principal supply chain mechanisms.
OVC ESSENTIAL SURVEY INDICATORS: The OVC MER Essential Survey Indicators are currently under
review. Countries that have not yet started data collection should hold on conducting surveys until the
review is complete. Countries that are in the process of data collection, or have already conducted at
least one round, should continue as planned. Questions about the OVC MER essential survey indicators
and related requirements can be directed to SGAC_SI@state.gov.
Deleted Disaggregations
HTS_TST: Home-based testing was removed as a community-based testing modality. Country teams
that targeted for programming for FY18 within the home-based testing modality should assess the
approaches outlined before implementation of these activities begins. Country teams were discouraged
from planning home-based testing activities for COP 17 (FY18 implementation) as previous program
data from this modality yielded sub-optimal results. Door-to-door and family testing activities targeted
under this indicator should be reevaluated and shifted to alternative testing modalities that will lead to
higher yield and greater programmatic progress towards the identification of positives.
PMTCT_EID: Infants’ diagnoses through virologic test results (positive, negative, unknown) are no
longer reported within this indicator beginning in FY18 Q1. PEPFAR is introducing the PMTCT_HEI_POS
indicator which will now be used for reporting on those infants diagnosed HIV positive and their linkage
to treatment. PMTCT_EID will still be collected to monitor the number of EID tests conducted.
HRH_CURR: Changes were made to the above-service delivery area reporting for this indicator. The
‘Cadre Category & Support Type’ disaggregation was updated to remove the ‘Staff Receiving ONLY Non-
Monetary Support (FTE)’ option. Results should be reported at the above-service delivery area by cadre
category and the following support types: ‘Salaried Staff (FTE)’ or ‘Staff Receiving Stipends (FTE).’
Requirements for HRH_CURR reporting at the facility and community-levels remain unchanged. This
change goes into effect with FY17 Q4 reporting.
MER 2.0 INTRODUCTION
11
Indicator Clarifications
KEY POPULATIONS: Language changes for key populations categories were made to align with WHO
guidance. ‘Transgender’ was changed to ‘Transgender People.’ ‘People in prison and other enclosed
setting’ was changed to ‘People in prison and other closed settings.’
In addition, KP guidance has been modified to avoid double-counting and ensure that the KP data
reported can be meaningfully interpreted. Despite persons potentially falling into more than one KP
disaggregate (e.g., FSW who injects drugs, MSM), implementing partners should be instructed to report
an individual in only one KP category with which s/he is most identified. This guidance is applicable to
KP_PREV and the KP disaggregates for PrEP_NEW, HTS_TST, and TX_NEW. To better determine the KPs
of interest for each indicator the key population classification document found in Appendix 1.
PMTCT_STAT: Clarifying language was added to the indicator definition. Data collected for this
indicator should be testing data associated with the first ANC visit (ANC1) of the pregnancy. This
reduces the risk of double counting pregnant women who could be tested multiple times during
pregnancy.
TB_PREV: Language updated to note that this is a snapshot indicator like TX_CURR. Results should not
be summed across reporting periods.
TX_TB: Language updated to note that this is a snapshot indicator like TX_CURR. Results should not be
summed across reporting periods.
TX_PVLS: Clarifying language added to specify that only patients who have been on ART for at least 3
months should be counted under this indicator. This will ensure that all viral load test outcomes
reported will be for patients who have been on ART long enough for it to be efficacious in reducing viral
load. Shift in categorization of follow-up VL test done after an initial VL test result of VL>1,000. Follow-
up viral loads done after an initial VL test result of VL>1,000 should be counted under routine and not
targeted tests since all patients who receive an initial VL test result of VL>1000 should routinely receive
a follow-up VL test after completing some enhanced adherence counseling. Guiding narrative questions
were modified.
PEPFAR SUPPORT TO COMMUNITIES AND SITES
Completing the third year of quarterly site-level monitoring by all PEPFAR implementing agencies and
implementing partners have provided granular data that demonstrate important differences in patient
outcomes and site performance. These results should be used to prioritize resources, staff, and
interventions among sites to determine the appropriate extent of support and monitoring needed based
on site-level outputs and quality outcomes.
There are three categories of PEPFAR support that correspond to attained, scale-up, sustained and
centrally supported areas. In areas where PEPFAR is supporting attained, scale-up, and sustained
services the type of support should be categorized as Direct Service Delivery (DSD) or Technical

12
Assistance-Service Delivery Improvement (TA-SDI). In areas where PEPFAR support is not at the site
level, but is financial support at the national or subnational levels then this support should be
characterized as Central Support (CS). DSD and TA include all sites receiving 1 or more PEPFAR-
supported visits during the year. Importantly, site-level quarterly results and SIMS data should be
analyzed and used to determine the number of program support visits needed each year to optimize the
quality of HIV/AIDS services and impact. PEPFAR teams should work with implementing partners to
ensure that programmatic data (including MER and SIMS results) are being used in this way. The key is
to ensure that PEPFAR-supported sites receive the appropriate number of technical assistance visits
based on their performance.
DSD: Individuals will be counted as receiving direct service delivery support from PEPFAR when BOTH of
the below conditions are met: Provision of key staff or commodities AND support to improve the quality
of services through site visits as often as deemed necessary by the partner and country team.
TA-SDI: Individuals will be counted as supported through TA-SDI when the point of service delivery
receives support from PEPFAR that meets the second criterion ONLY: support to improve the quality of
services through site visits as often as deemed necessary by the partner and country team.
1. PEPFAR is directly interacting with the patient or beneficiary in response to their health
(physical, psychological, etc.) care needs by providing key staff and/or essential commodities for
routine service delivery. Staff who are responsible for the completeness and quality of routine
patient records (paper or electronic) can be counted here; however, staff who exclusively fulfill
MOH and donor reporting requirements cannot be counted. Each indicator reference sheet
includes a list of key staff and/or essential commodities that meet this condition.
AND/OR
2. PEPFAR provides an established presence at and/or routinized support for those services at the
point of service delivery. Each indicator reference sheet includes a list of activities that count
toward support for service delivery improvement.
Support in Centrally Supported Areas: In areas where PEPFAR is providing solely financial support
at the national, regional or district level, site level support will be through annual visits. However, to
support government with quality monitoring results reported through national health information
systems should be jointly monitored with host country government on a quarterly basis. SIMS visits may
be conducted at these sites if quality issues are identified.
While site-specific activities have transitioned to government or other support, PEPFAR continues
provide support for overarching activities, such as quality assurance and quality improvement (QA/QI) to
ensure that patients continue to receive quality services. As such, PEPFAR will continue monitoring
activities in centrally supported sites annually via the following indicators: PMTCT_STAT, PMTCT_ART,
HTS_TST, TX_CURR, TX_NEW, and TX_RET. Due to the financial investments PEPFAR provides at the
above-service delivery area in centrally supported sites and SNUs, it is important that results be
provided to ensure that quality assurance initiatives are having the intended impact. PEPFAR programs

MER 2.0 INTRODUCTION
13
should be focused on moving the national program in their respective country to 90% ART coverage for
PLHIV. Therefore, it is extremely important to have an understanding of the services being provided to
PLHIV in the entire country.
Results for all centrally supported SNUs and sites should be reported for all 23 Standard Process COP
Operating Units (i.e., Botswana, Burundi, Cameroon, Cote d'Ivoire, Democratic Republic of the Congo,
Ethiopia, Haiti, Kenya, Lesotho, Malawi, Mozambique, Namibia, Nigeria, Rwanda, South Africa, South
Sudan, Swaziland, Tanzania, Uganda, Ukraine, Vietnam, Zambia, and Zimbabwe) via the MOH data
alignment process. Standard process countries that did not participate in the MOH data alignment
process in FY 17 will be required to do so in FY 18.
DISAGGREGATED MONITORING
There are 3 categories of MER indicator disaggregations for the MER 2.0, which can be seen in the
indicator reference sheets and the data entry screens.
Required Disaggregations: Required indicates that this indicator disaggregate is required for all
countries that have programming for this area. This means that if the country supports a program area,
defined by budget and targets set during the COP process -- then it is required to report results.
Conditional Disaggregations: Indicator disaggregates that are conditions include those for which
some additional condition must be fulfilled. In MER 2.0 there are no full indicators that are conditional,
but only additional disaggregations that are conditional based on additional funding or programming.
There are two main types of conditional indicator disaggregations:
a. Disaggregations for those programs that have received additional funds for special
programming such as DREAMS
b. Disaggregations that field teams have received permission or a waiver from their OGAC
SI advisor to report on such as reporting on the coarse age disaggregations instead of
the finer age disaggregations. In this case reporting is considered conditional based on
approval from OGAC.
Optional Disaggregations: Optional disaggregates should be completed by those for which the
indicator is useful to determine the success of their program (e.g., KP national and subnational data), for
which the partner has strong methodological sources (e.g., KP catchment area-denominator), or when it
is both relevant and safe to enter the data at the site and/or community level (e.g., KP disaggregations
for PrEP_NEW, HTS_TST, and TX_NEW).
MER INDICATOR NARRATIVES
Three types of narratives are required as part of quarterly submissions: (1) IM level narratives, (2)
technical area level narratives, and (3) national and sub-national level results narratives. Specific
requirements are defined for each type of narrative. In addition, guiding narrative questions have been

14
introduced to provide additional technical detail and continuity within the narrative submitted across
PEPFAR countries.
Guiding Narrative Questions
New for FY18, PEPFAR has included “guiding narrative questions” for each indicator. These questions or
prompts can be found on the subsequent indicator reference sheets and were developed to ensure that
there is continuity in the technical information reported in the narratives that will be most relevant to
subject matter experts in triangulating the narrative data with the quantitative results.
Each indicator has 2-3 questions or prompts that should guide both implementing partners and USG
technical area experts in the development and framing of both the IM and technical area narratives – in
addition to the narrative requirements provided in the paragraphs below.
Implementing Mechanism (IM) Level Narratives
Narratives are required each quarter. These narratives are an opportunity to convey additional context
to accompany the quantitative results. IM level narratives are required for each indicator, and should
describe current quarterly achievements as well as overall achievements against the fiscal year targets,
and provide additional information related to specific data quality concerns or programmatic issues that
may impact the assessment of partner performance. If appropriate, reference specific site-level issues
that were encountered during the reporting period that may prevent achievement of the IM target. If
additional information is useful for the interpretation of the results on an indicator-specific basis, please
add this to the narrative. Please also indicate whether on-the-ground data quality assessments were
conducted during the FY and the impact the assessment had on the results and program.
IM level narratives must also address any result discrepancies that cannot be reconciled after
completing the Data Completeness and Logic Checks. Finally, the IM narratives should specifically
describe the nature of support the partner is providing that qualifies the results to be categorized as
Direct Service Delivery (DSD) or Technical Assistance for Service Delivery Improvement (TA-SDI) in
accordance with PEPFAR guidance.
Technical Area Level Narratives
Technical area level narratives summarize the de-duplicated partner achievements against summary FY
2017 targets. Technical area level narratives are required for each indicator, and should provide an
overall assessment of the performance against FY 2017 targets. These narratives should also provide
additional information related to specific data quality concerns or programmatic issues that may impact
the assessment of overall performance. If additional information is useful for the interpretation of the
results on an indicator-specific basis, please add this to the narrative.
Additionally, the technical area level narratives should specifically describe the nature of support the
partners are providing that qualifies the results to be categorized as Direct Service Delivery (DSD) or
Technical Assistance for Service Delivery Improvement (TA-SDI) in accordance with PEPFAR MER
guidance. Further focus the narratives by describing the following achievements in light of expected

MER 2.0 INTRODUCTION
15
trajectories for the technical area, information related to specific data quality concerns or programmatic
issues that may impact the interpretation of results, data quality assessment (DQA) completion in the
last 12 months, address any result discrepancies that cannot be reconciled (at the interagency level)
after completing the Data Completeness and Logic Checks. Narratives should also address achievements
by prioritization level and DSD and TA-SDI support. For example, is there an overlap between PEPFAR
and the Global Fund in support for ART services?
National and Subnational Level Results Narratives
National level indicator narratives provide an opportunity for teams to discuss the host country
response beyond PEPFAR supported activities. For national indicators, both a justification and a source
narrative are required for each indicator. Also take note that narratives for both National (_NAT) and
Subnational (_SUBNAT) should be recorded in the _NAT narrative section in DATIM.
• Justification Narrative
o How does the national number relate to the PEPFAR number?
o What proportion of results does PEPFAR contribute to the national response
o If the PEPFAR result is larger than the national number please explain
o Note the actual reporting time frame for entered data
• Source narrative
o What is the source of these data?
o When were these data collected/calculated?
HOST COUNTRY NATIONAL PROGRAM
Monitoring the host country HIV response is critical to understanding both the achievements and the
gaps at the subnational level and by population. Host country data are used to inform PEPFAR programs
and guide how PEPFAR resources are allocated at all levels. The key program areas for monitoring host
country targets and results are: prevention of mother to child transmission programs, key populations,
voluntary male medical circumcision and HIV diagnosis and treatment, including viral suppression. Data
are needed from both the national and subnational level. The subnational level is considered the
organizational level in which the country team has prioritized their program (PSNU). Data on the hous
country national program is reported to PEPFAR for all subnational units, regardless of PEPFAR funding
supporting these geographical areas; so that the total of the subnational results or targets should equal
the total number of national results and targets.
At the host country national level, to sufficiently monitor its national response, the host country
government’s national set of indicators should include the minimum set of harmonized global indicators
(Global AIDS Response Reporting) and additional indicators that represent the needs of the country’s
program. The PEPFAR Country team should collaborate with the host country government and other
stakeholders to make sure that PEPFAR reporting requirements are taken into consideration in the host
country’s national set. In constructing its own comprehensive set of requirements for monitoring the
USG response in support of the host country national program, each PEPFAR country team will review all
16
of the PEPFAR essential host country national indicators for applicability to the PEPFAR activities being
conducted in the host country.
The PEPFAR host country national and subnational level indicators represent results obtained within the
entire host country regardless of PEPFAR support. Both Standard Process and STAR Process Countries
should report host country results at Q4 each fiscal year.
Host Country National and Subnational Results
At Q4 of the USG fiscal year, results from the host national systems should be reported up until the most
recent month of collection and include 12 months of data. These may not align with end USG fiscal year
results. These data should be collected continuously at the subnational level as part of service delivery
areas. Data should be in line with GARPR and UNAIDS reported data, where available, although may
differ due to different reporting periods. In the narratives, please indicate what months the data include
(e.g., October 2017-September 2018; or July 2016 to June 2017). Results should be consistently reported
on the same time period to be able to monitor trends over time.
Host Country National and Subnational Targets
Developing targets for the next year at the national and subnational data is an important step in
understanding the national program and determining geographic investments (including host country,
The Global Fund and other donors). When PEPFAR better understands the targets of the national
program setting process, then it is better placed to support the program and to fill necessary impactful
programmatic gaps. Please describe the target setting process that the host country employs in the
narratives and partnering donors). The national targets should cover the next calendar or fiscal year; the
timeframe should be indicated in the narratives. Targets for the host country national and subnational
indicators should be reported into DATIM during COP.
Host Country indicators by reporting level, targets, and results
Indicator
Results
Targets
National
Sub-National
KP_MAT
✓
✓
✓
PMTCT_ART
✓
✓
✓
✓
PMTCT_STAT
✓
✓
✓
✓
TX_CURR
✓
✓
✓
✓
DIAGNOSED
✓
✓
✓
VL_SUPPRESSION
✓
✓
✓
✓
VMMC_CIRC
✓
✓
✓
✓
VMMC_TOTALCIRC
✓
✓
✓
✓
MER 2.0 INTRODUCTION
17
SIMS IN RELATION TO MER 2.0
SIMS evaluates the quality of service delivery or program oversight to identify performance issues that
may impact patient outcomes or the integrity of reporting for MER targets or disaggregates. Low final
scores (reds and yellows) from these CEEs highlight potential issues with service delivery, site
performance or oversight, and/or documentation of patient results. The SIMS 2.0 Linkage Reference
Table in Appendix 2 provides a listing of all SIMS 2.0 CEEs that have been directly linked to a given MER
indicator; linkage data may be used for data triangulation activities to inform and contextualize MER
results.
DREAMS SPECIFIC GUIDANCE
In addition to required MER reporting, it is essential that all DREAMS (Determined, Resilient,
Empowered, AIDS-free, Mentored, and Safe) and DREAMS-like countries ensure that all implementing
Partners in DREAMS SNUs report their results for and use data from all DREAMS-related indicators and
their required disaggregations. DREAMS countries are encouraged to monitor interventions progress
using custom indicators for program components that do not have existing MER indicators (e.g.,
contraceptive method mix, condom promotion and provision). Appendix 3 includes a full list of the
DREAMS-related indicators reported for MER 2.0 and the required disaggregation for each indicator.
Please note there are also specific reporting requirements for DREAMS narratives.
• DREAMS countries: Kenya, Lesotho, Malawi, Mozambique, South Africa, Swaziland, Tanzania,
Uganda, Zambia and Zimbabwe
• DREAMS-like countries: Botswana, Cote d’ Ivoire, Haiti, Namibia, and Rwanda
18
MER 2.0 INFOGRAPHIC
MER 2.0 INTODUCTION
19
Indicator Reporting Frequency by Program Area
#
Program Area Group
Indicator Code
Indicator Name
Reporting
Frequency
1
Knowing Your HIV
Status
HTS_TST
Number of individuals who received HIV Testing Services
(HTS) and received their test results, disaggregated by
HIV result
Quarterly
2
Knowing Your HIV
Status
HTS_SELF
Number of individual HIV self-test kits distributed
Quarterly
3
On ART
PMTCT_ART
Percentage of HIV-positive pregnant women who
received ART to reduce the risk of mother-to-child-
transmission (MTCT) during pregnancy
Quarterly
4
Knowing Your HIV
Status
PMTCT_EID
Percentage of infants born to HIV-positive women who
had a virologic HIV test done within 12 months of birth
Quarterly
5
Knowing Your HIV
Status
PMTCT_HEI_POS
Number of HIV-infected infants identified in the reporting
period, whose diagnostic sample was collected by 12
months of age.
Quarterly
6
Knowing Your HIV
Status
PMTCT_STAT
Percentage of pregnant women with known HIV status at
antenatal care (includes those who already knew their
HIV status prior to ANC), disaggregated by HIV result
Quarterly
7
Prevention
PrEP_NEW
Number of individuals who have received (oral)
antiretroviral pre-exposure prophylaxis (PrEP) to prevent
HIV infection.
Quarterly
8
On ART
TX_CURR
Number of adults and children currently receiving
antiretroviral therapy (ART)
Quarterly
9
On ART
TX_NEW
Number of adults and children newly enrolled on
antiretroviral therapy (ART)
Quarterly
10
Prevention
VMMC_CIRC
Number of males circumcised as part of the voluntary
medical male circumcision for HIV prevention program
Quarterly
11
Prevention
KP_PREV
Number of key populations reached with individual
and/or small group-level HIV prevention interventions
designed for the target population
Semi-Annual
12
Knowing Your HIV
Status
OVC_HIVSTAT
Percentage of orphans and vulnerable children (<18 years
old) with HIV status reported to implementing partner
(including status not reported), disaggregated by status
type
Semi-Annual
13
Prevention
OVC_SERV
Number of beneficiaries served by PEPFAR OVC programs
for children and families affected by HIV
Semi-Annual
14
Prevention
PP_PREV
Number of the priority populations reached with
standardized HIV prevention intervention(s) that are
evidence-based.
Semi-Annual
15
Health Systems
SC_STOCK
Percentage of storage sites where commodities are
stocked according to plan, by level in supply system
Semi-Annual
16
On ART
TB_ART
Percentage of HIV-positive new and relapsed TB cases on
ART during TB treatment
Semi-Annual
17
Prevention
TB_PREV
Proportion of ART patients who completed a standard
course of TB preventive therapy within the reporting
period
Semi-Annual
18
Knowing Your HIV
Status
TB_STAT
Percentage of new and relapse TB cases with
documented HIV status, disaggregated by HIV result
Semi-Annual
19
On ART
TX_TB
The proportion of ART patients who were screened who
are receiving TB treatment
Semi-Annual
20
#
Program Area Group
Indicator Code
Indicator Name
Reporting
Frequency
20
Health Systems
EMR_SITE
Number of PEPFAR-supported facility-based service
delivery points supported by your organization that have
an electronic medical record system
Annual
21
Prevention
FPINT_SITE
Number of HIV service delivery points (SDP) at a site
supported by PEPFAR that are providing integrated
voluntary family planning (FP) services
Annual
22
Prevention
GEND_GBV
Number of people receiving post-gender based violence
(GBV) clinical care based on the minimum package NOTE:
The indicator DOES NOT measure delivery of GBV
prevention activities.
Annual
23
Health Systems
HRH_CURR
Number of health worker full-time equivalents who are
working on any HIV-related activities i.e., prevention,
treatment and other HIV support and are receiving any
type of support from PEPFAR at facility and sites,
community sites, and at the above-service delivery area
level
Annual
24
Health Systems
HRH_PRE
Number of new health workers who graduated from a
pre-service training institution or program as a result of
PEPFAR-supported strengthening efforts, within the
reporting period, by select cadre
Annual
25
Health Systems
HRH_STAFF
Number of health worker full-time equivalents who are
working on any HIV-related activities i.e., prevention,
treatment and other HIV support at PEPFAR-supported
facility sites
Annual
26
Prevention
KP_MAT
Number of people who inject drugs (PWID) on
medication-assisted therapy (MAT) for at least 6 months
within the reporting period
Annual
27
Health Systems
LAB_PTCQI
Number of laboratories and blood centers/banks:
A. Engaged in Continuous Quality Improvement (CQI)
activities
B. Audited and achieved accreditation
C. Performing an HIV-related test and participating in and
passing Proficiency Testing (PT)
Annual
28
Knowing Your HIV
Status
PMTCT_FO
Percentage of final outcomes among HIV exposed infants
registered in a birth cohort
Annual
29
Viral Suppression
TX_PVLS
Percentage of ART patients with a viral load result
documented in the medical record and/or laboratory
information systems (LIS) within the past 12 months with
a suppressed viral load (<1000 copies/ml)
Annual
30
Viral Suppression
TX_RET
Percentage of adults and children known to be on
treatment 12 months after initiation of antiretroviral
therapy (Note: reporting 24 and 36 months is
recommended, but optional)
Annual
MER 2.0 INTODUCTION
21
How to read a PEPFAR indicator reference sheet
All indicators in this guidance are provided in a specific format to allow the reader to easily understand
their specific indicators requirements. Please use this layout as a guide to understand how to read the
reference sheets.
Indicator Code
Description:
Long name of the indicator
Numerator:
Long name of the numerator
Additional information about numerator
definition
Denominator:
Long name of the denominator
Additional information about denominator
definition
Changes in indicator:
Highlights any differences in the indicator from MER 1.0 to 2.0 and between MER 2.0
(versions 2.1 and 2.2)
How to use:
Defines how the data is used to monitor PEPFAR program activities
How to collect:
Defines how the data is collected (highlighting data source, issues with double counting,
and important components of data collection that ensure data quality)
Reporting level:
Defines the level at which the indicator is reported: facility, community, and/or above-
service delivery area
How often to report:
Defines the period at which the indicator is reported: Quarterly, Semi-Annually, or
Annually
How to review for
data quality:
Outlines specific data quality considerations for the indicator
How to calculate
annual total:
Defines how annual totals are calculated for the indicator at the end of the fiscal year.
Data elements
(components of
indicator):
Numerator:
Long name of the
numerator
Disaggregate Groups
Disaggregates
Name of Disaggregate Group(s)
Disaggregations
Denominator:
Long name of the
denominator:
Disaggregate Groups
Disaggregates
Name of Disaggregate Group(s)
Disaggregations
Disaggregate Descriptions & Definitions
Describes and defines the disaggregates relevant to the indicator in greater detail.
PEPFAR-support
definition:
Lists the indicator-specific definition for DSD vs. TA support that differ from the standard
definitions outlined in the introduction section of the guidance.
Guiding narrative
questions:
Lists the indicator-specific questions that implementing partners and USG country teams
should address in the implementing mechanism and technical area summary narratives.

Prevention & Support
Indicators
PREVENTION
23
PrEP_NEW
Description:
Number of individuals who have been newly enrolled on (oral) antiretroviral pre-
exposure prophylaxis (PrEP) to prevent HIV infection in the reporting period
Numerator:
Number of individuals who have
received (oral) antiretroviral pre-
exposure prophylaxis (PrEP) to prevent
HIV infection
The numerator is generated by counting the
number of people newly enrolled in oral PrEP
(including WHO specified regimens
“tenofovir-containing PrEP” which could be
TDF alone, TDF/FTC, or TDF/3TC) during the
reporting period, in accordance with the
demonstration project guidance or the
nationally approved protocol (or
WHO/UNAIDS standards).
Denominator:
N/A
Changes in indicator:
• PrEP_NEW is now reported across PEPFAR programs. It is no longer a DREAMS-
specific indicator (MER 1.0 to MER 2.0).
• A denominator for PrEP_NEW will no longer be collected (MER 1.0 to MER 2.0).
• KP disaggregations were added (MER 1.0 to MER 2.0).
• Age disaggregations updated (MER 2.0 v2.1 to v2.2).
• KP disaggregation updated to include ‘Other KP Type’ (MER 2.0 v2.1 to v2.2).
How to use:
The indicator measures the ongoing growth of PrEP services. This measure is critical to
assess progress in the program’s response to the epidemic in specific geographic areas,
and the uptake and utility of PrEP among persons at substantially increased risk of HIV
infection.
This indicator permits monitoring trends in use, but does not attempt to distinguish
between different modes or regimens of PrEP or to measure the cost, quality or
effectiveness of PrEP provided. These will each vary within and between countries and
are liable to change over time.
PrEP has been shown to reduce incident infections among several populations including
serodiscordant heterosexual couples, MSM, FSW, and transgender people (TG). The
WHO now recommends that oral PrEP containing tenofovir should be offered as an
additional prevention choice for people at substantial risk, defined as HIV incidence >
3/100 person-years.
How to collect:
The numerator can be generated by counting the number of people who are newly
enrolled on PrEP in the reporting period, in accordance with national guidelines (or
WHO/UNAIDS standards). NEW is a state defined by an individual’s beginning in a PrEP
program. It is expected that the characteristics of new clients are recorded at the time
they newly initiate into a program. Patients are “new” on PrEP only if they are naive to
antiretroviral therapy for prevention of HIV infection and have not received oral or
topical prophylaxis previously in any program.
Reporting of the key population disaggregation should be consistent with what is
described under the KP_PREV “How to review for data quality” section on mutual
exclusivity of an individual who falls under multiple KP categories (e.g., FSW who injects
drugs). In such instances, the individual should only be reported in ONE KP
disaggregation category with which this person is most identified. See Appendix 1 to
support the identification of key populations at service delivery.
24
NOTE: In accordance to PrEP guidance, not all PrEP beneficiaries are expected to fall
within the KP disaggregates, therefore the total disaggregations for KP does not have to
sum to the numerator total. Both KP-specific and clinical partners have the option to
complete these KP disaggregation, but only if safe to maintain these files and to report.
Reporting level:
Facility
How often to report:
Quarterly
How to review for
data quality:
Numerator ≥ subtotal of the age/sex disaggregation: The total number people newly
enrolled on PrEP (numerator) should be greater or equal to the subtotal of the age/sex
disaggregate group.
How to calculate
annual total:
Sum results across quarters.
Data elements
(components of
indicator):
Numerator:
Number of
individuals who
have received
(oral)
antiretroviral pre-
exposure
prophylaxis (PrEP)
to prevent HIV
infection.
Disaggregate Groups
Disaggregates
Age/Sex
[Required]
15-19 M, 15-19 F, 20-24 M, 20-
24 F, 25-29 M, 25-29 F, 30-34
M, 30-34 F, 35-39 M, 35-39 F,
40-49 M, 40-49 F, 50+ M, 50+ F
Key Population Type:
[Optional]
MSM: Men who have sex with
men
TG: Transgender people
FSW: Female sex workers
Other KP Type: Other key
population type
Disaggregate Descriptions & Definitions
Age Description: Age is defined as the age at the time of initiation of PrEP. For example,
if a 19-year-old woman begins PrEP and then shortly after turns age 20, she will still be
counted under NEW in the 15-19 F age/sex category.
PEPFAR-support
definition:
Standard definition of DSD and TA used.
Provision of key staff or commodities for PrEP services include: ongoing procurement of
critical commodities such “tenofovir-containing PrEP” which could be TDF alone,
TDF/FTC, or TDF/3TC or funding for salaries of personnel providing any of the prevention
package components (i.e., clinicians, outreach workers, program managers). Staff
responsible for the completeness and quality of routine patient records (paper or
electronic) can be counted here; however, staff who exclusively fulfill MOH and donor
reporting requirements cannot be counted.
Ongoing support for HIV prevention among PrEP services includes: mentoring and
supportive supervision; training; organizational strengthening; QA/QI; program design
like development of training curricula, PrEP guidance development, or standard
operating procedures (SOPs) and follow-up to ensure quality of care; regular assistance
with monitoring and evaluation functions and data quality assessments; or supply chain
management
Guiding narrative
questions:
1. Roughly what proportion of those offered PrEP at the site agrees to start PrEP?
2. Of those initiating PrEP, how many are estimated to continue at one and three
months?
3. What strategy is used to determine PrEP eligibility at the site:
• Screening tool?
• All clients considered at risk and eligible?
• Client request?
PREVENTION
25
VMMC_CIRC
Description:
Number of males circumcised as part of the voluntary medical male circumcision
(VMMC) for HIV prevention program within the reporting period
Numerator:
Number of males circumcised as part of
the voluntary medical male circumcision
(VMMC) for HIV prevention program
The numerator can be generated by counting
the number of males circumcised.
Denominator:
N/A
Changes in indicator:
• Age disaggregations updated (MER 2.0 v2.1 to v2.2).
• Follow-up status disaggregation updated to capture instances where VMMC follow-
up did not take place within 14 days or within the reporting period (MER 2.0 v2.1 to
v2.2).
How to use:
Tracks the number of male circumcisions conducted during the reporting period and
assists in potentially determining coverage of circumcision in the population over time.
The total number of males circumcised indicates a change in the supply of and/or
demand for VMMC services. Additionally, disaggregations are required and are used to
evaluate whether prioritized services have been successful at reaching the intended
population (by age, HIV status, and circumcision technique), targets have been achieved,
and whether modeling inputs should be adjusted. An additional level of disaggregation
below the circumcision technique level is required for follow-up status, since post-
operative clinical assessments are part of good clinical care and low follow-up rates may
indicate a problem in program quality.
How to collect:
The numerator can be generated by counting the number of males circumcised as part
of the VMMC for HIV prevention program. This information can generally be found in
VMMC Register, or client medical records maintained by each program/site/service
provider.
Reporting level:
Facility
How often to report:
Quarterly
How to review for
data quality:
Numerator ≥ subtotal of each of the disaggregation.
How to calculate
annual total:
Sum results across quarters.
Data elements
(components of
indicator):
Numerator:
Number of males
circumcised as
part of the
voluntary medical
male circumcision
(VMMC) for HIV
prevention
program
Disaggregate Groups
Disaggregates
Age
[Required]
0-60 days, 2 months - 9 years,
10-14, 15-19, 20-24, 25-29, 30-
34, 35-39, 40-49, 50+
HIV Status and Outcome
[Required]
• Number of HIV-positive
clients (tested HIV positive at
VMMC site)
• Number of HIV-negative
clients (tested HIV negative
at VMMC site)
• Number of clients with
indeterminate HIV status or
not tested for HIV at site
(regardless of previous
documentation)
Circumcision Technique
[Required]
• Surgical VMMC
• Device-based VMMC
26
Circumcision Technique/Follow-
up Status (Sub-disaggregation
of the VMMC circumcision
technique disaggregation)
[Required]
• Surgical VMMC: Followed-up
within 14 days of surgery;
• Surgical VMMC: Did not
follow-up within 14 days of
surgery or did not follow-up
within the reporting period;
• Device-based VMMC;
Followed-up within 14 days
of device placement. May
include device removal;
• Device-based VMMC: Did
not follow-up within 14 days
of device placement or did
not follow-up within the
reporting period
Disaggregate Descriptions & Definitions
For HIV Status and Outcome: As VMMC_CIRC is a status indicator and not testing
indicator, ALL men tested through the VMMC program should also be counted in the
general HTS indicator “HTS_TST” under the VMMC service delivery modality.
PEPFAR-support
definition:
Standard definition of DSD and TA-SDI used.
Provision of key staff or commodities for VMMC include: medical instruments, supplies,
or medicines needed for the VMMC procedure, or funding for salaries for HCW who
deliver VMMC services.
Ongoing support for VMMC service delivery improvement includes: training of VMMC
service providers; clinical mentoring and supportive supervision of HCW at VMMC sites;
infrastructure/facility renovation; support of VMMC service-related data collection,
reporting, data quality assessments (DQA); CQI/EQA of VMMC services at point of
service delivery; or commodities consumption forecasting and supply chain
management support.
Guiding narrative
questions:
1. Is the age distribution of males 60% or more 15+ years of age?
• Is this age distribution getting older as compared to previous quarters?
2. If OU is using compression collar type device for VMMC
• Are they adhering to WHO Guidelines for tetanus immunization?
• Were there any tetanus AEs reported?
3. What proportion of clients are returning for follow-up? (Should be at least 80%)
4. What barriers are there to further scaling up VMMC services?
PREVENTION
27
KP_PREV
Description:
Number of key populations reached with individual and/or small group-level HIV
prevention interventions designed for the target population
Numerator:
Number of key populations reached
with individual and/or small group-level
HIV prevention interventions designed
for the target population
The numerator can be generated by counting
the number of unique individuals from an
activity who are reached with prevention
interventions designed for the intended key
population.
Denominator:
[Optional,
recommended if
available]
Total estimated number of key
populations in the catchment area
The denominator is the estimated number of
key populations in a defined catchment area.
Programs need to define their geographic
catchment area from which key population
beneficiaries receive HIV prevention services.
Country teams should encourage
methodological harmonization across their
KP partners when estimating KP population
size within a catchment area.
Changes in indicator:
• KP type disaggregations changed, three testing service disaggregations were added,
and HIV testing or referral of an individual to HIV testing services (HTS) is required to
be offered to those who do not know their status or are self-identified as HIV
negative (MER 1.0 to 2.0).
• The denominator is now optional, but recommended for those with good size
estimation metrics (MER 1.0 to 2.0).
How to use:
This indicator provides information on the total number of unique individuals that have
received individual-level and/or small-group level intervention(s). This indicator will help
determine the reach of key populations (if no denominator) and may help understand
the relative saturation (coverage) of PEPFAR-supported KP prevention programs when
reliable population size estimates are included as the denominator.
Small-group intervention is defined as less than or equal to 25 individual attendees in
one setting.
HIV testing services (HTS) or referring an individual to HTS is required to be offered (at
least once during the reporting period and/or in accordance with WHO/national
guidance) unless the individual had previously been tested positive for HIV. If the
individual is self-identified as HIV positive, then HTS provision or referral to HTS will
not be a required element of this indicator.
A partner may count an individual (with unknown HIV serostatus or self-identified as HIV
negative) as having received a prevention activity if they have provided, offered, or
referred to HTS AND at least one additional listed prevention activities below (outside of
HTS) during the reporting period. If an individual is already known to be HIV positive at
the time of the outreach, s/he should receive at least one of the interventions listed in
the table (outside of HTS) to qualify as being counted under this indicator.
The table below lists the prevention interventions that a partner may offer in addition to
HTS (or HTS referral).
28
Prevention Interventions for Key Populations
• Offer or refer to HTS* (Required)
• Targeted information, education, and communication (IEC)
• Outreach/Empowerment
• Condoms
• Lubricant
• Offer or refer to STI screening, prevention, and treatment
• Link or refer to ART
• Offer or refer to prevention, diagnosis, treatment of TB
• Offer or refer to screening and vaccination for viral hepatitis
• Offer or refer to Reproductive Health (Family Planning; PMTCT), if applicable
• Refer to medication-assisted therapy (MAT), if applicable
• Offer or refer to needle syringe program (NSP), if applicable
*Partner should also report the number of individuals tested under the indicator
“HTS_TST” if HTS was conducted (and results were given) as part of the outreach
activity. If it was a documented complete HTS referral to the facility, it can be counted
as HTS_TST_TA. Please refer to the HTS_TST indicator definition sheet for details.
How to collect:
Tracking systems must be able to reduce double-counting of individuals in a reporting
period. The numerator can be generated by counting the number of de-duplicated
individuals who were reached and had completed the appropriate prevention
intervention(s) designed for the intended key population. For example, this means that
when a unique individual receives HTS referral plus condoms and lubricant at more than
one occasion during the reporting period, the person is counted only once for being
reached for this indicator.
Furthermore, de-duplication of all returning beneficiaries within the Q3-Q4 reporting
period (April 1 – September 30) will also need to take place in Q4 reporting if they had
already been counted under KP_PREV in Q1-Q2 of the same fiscal year. For example, if
an individual had received prevention interventions under KP_PREV through PEPFAR-
supported program in January 2017 and was counted as being reached in FY17 Q2
reporting cycle, and this same individual was later reached with prevention services
again by PEPFAR-supported program in June 2017, that individual should NOT be
reported again in the FY17 Q4 reporting period. This de-duplication is critical to
accurately track the ANNUAL number of unique individuals reached by PEPFAR within a
given fiscal year. Trend analysis of past performance of KP_PREV data will be adversely
affected with the change in frequency of KP_PREV reporting from annually to semi-
annually if this de-duplication is ignored (i.e., annual number of KP_PREV reported
within the same fiscal year would be inflated as the same individual would be counted
twice if this de-duplication does not occur at Q4 reporting).
If possible, a unique identifier can be assigned. The use of a unique identifier can help
programs monitor the frequency of contact/outreach of a single individual over time
(i.e., Beneficiary A with unique identifier AW0901 had four documented outreach visits
in FY17 but was only counted once under KP_PREV in FY17).
Reporting level:
Facility & Community
How often to report:
Semi-Annual
How to review for
data quality:
Data should be reviewed regularly for the purposes of program management, to monitor
progress towards achieving targets, and to identify and correct any data quality issues.
Potential data quality issues with KP_PREV are:
• Numerator
PREVENTION
29
o The Numerator is = to the sum of the disaggregation: The number of KP
reached with individual and/or small-group level preventive interventions
should be equal to the sum of KP disaggregates.
o Despite persons potentially falling into more than one KP disaggregate (e.g.
FSW who injects drugs), implementing partners should be instructed to report
an individual in only one KP category with which s/he is most identified.
• Denominator ≥ Numerator: The total estimated number of key populations should
be greater or equal to the number of key populations provided with individual and/or
small group level preventive interventions.
How to calculate
annual total:
Sum across both reporting periods; de-duplicating unique individuals already reached
and reported in Q1-Q2 of the same fiscal year in Q4 reporting.
Data elements
(components of
indicator):
Numerator:
Number of key
populations
reached with
individual and/or
small group-level
HIV prevention
interventions
designed for the
target population
Disaggregate Groups
Disaggregates
KP Type
[Required]
• MSM who are SW;
• MSM who are not SW;
• TG who are SW;
• TG who are not SW;
• Female SW;
• PWID male;
• PWID female;
• People in prisons and other
closed settings
Testing Services
[Required]
• KP known positive;
• KP was newly tested and/or
referred for testing;
• KP declined testing and/or
referral
Denominator:
Total estimated
number of key
populations in the
catchment area.
[Optional,
recommended if
available]
Disaggregate Groups
Disaggregates
KP Type
• MSM who are SW;
• MSM who are not SW;
• TG who are SW;
• TG who are not SW;
• Female SW;
• PWID male;
• PWID female;
• People in prisons and other
closed settings
Disaggregate Descriptions & Definitions
Testing Services Disaggregates Definitions:
• Known Positive: Persons within each key population type for whom HIV testing is
not indicated because they are known to be HIV-positive. HIV-positive test results
should be verified, if possible, for all persons accessing HIV prevention services
during the reporting period. Implementing partners should maintain records
(without personally identifiable information) on whether the HIV-positive client is
linked to treatment. Patients tested positive in previous reporting periods should be
counted as Known Positives.
• Newly Tested and/or Referred for Testing: Persons within each key population type
for whom HIV testing is indicated because they do not know their HIV status or their
last HIV-negative test was more than 3-6 months ago (or more/less frequently as
indicated by National Guidelines) should either be offered an HIV test on site or
given information about where and when they can access an HIV test at another
30
nearby clinic. Every attempt should be made to ensure the client is linked with HIV
testing services that are KP-friendly, and where possible the completed referral
should be documented (i.e., the client accessed HIV testing). Note: Persons who
access testing and whose results are newly tested HIV-positive in the reporting
period should also be counted under “newly tested” even if they return for
additional prevention services during that reporting period.
• Declined Testing and/or Referral: Persons who, after explaining the benefits of HIV
testing and the reason for testing every 3-6 months (or more/less frequently as
indicated by National Guidelines), decline to be tested on-site or referred to a site
where HIV testing is offered. Although every attempt should be made to support key
populations with HIV testing as part of the package of HIV prevention services and
to provide HIV testing on site or KP-friendly sites, programs should also respect the
autonomy of clients to decline this service. Clients who decline testing and/or
referral can still receive other prevention services, as long as the benefits of HIV
testing were explained and testing or a referral for testing was offered.
PEPFAR-support
definition:
Standard definition of DSD and TA-SDI used.
Provision of key staff or commodities for KP receiving HIV prevention services include:
ongoing procurement of critical commodities such as test-kits, condoms, lubricants, or
funding for salaries of personnel providing any of the prevention package components
(i.e., peer navigators, outreach workers, program managers). Staff responsible for the
completeness and quality of routine patient records (paper or electronic) can be
counted here; however, staff who exclusively fulfill MOH and donor reporting
requirements cannot be counted.
Ongoing support for HIV prevention among KP improvement includes: mentoring and
supportive supervision; training; organizational strengthening; QA/QI; program design
like development of training curricula, prevention guidance development, or standard
operating procedures (SOPs) and follow-up to ensure fidelity to the program design;
regular assistance with monitoring and evaluation functions and data quality
assessments; or condom forecasting and supply management.
Guiding narrative
questions:
1. Did the IMs de-duplicate all returning beneficiaries in Q3-Q4 who have already been
counted in Q1-Q2 of this fiscal year? If not, why not?
2. Are there mechanisms in place (i.e. unique identifier) in which IMs can de-duplicate
multiple outreach encounters within a fiscal year? What are these mechanisms? If
mechanisms are not in place, how does the IM report individuals and not encounters
within the fiscal year?
3. Do the testing service disaggregations equal the total number of KP_PREV reported?
If not, why not?
4. What were the barriers in collecting testing service disaggregations for this indicator?
5. If the denominator was reported, what methodology was used to estimating the
number of key populations in a defined catchment area?
PREVENTION
31
PP_PREV
Description:
Number of the priority populations (PP) reached with the standardized, evidence-based
intervention(s) required that are designed to promote the adoption of HIV prevention
behaviors and service uptake
Numerator:
Number of the priority populations
reached with standardized HIV
prevention intervention(s) that are
evidence-based
The numerator is the number of individuals
from each priority population reached with
HIV prevention interventions during the
reporting period. For the purposes of
reporting, the team will sum the numbers
reached in each of the priority populations
and report that total (details of the priority
populations reached should be explained in
the narratives).
Denominator:
[Optional,
recommended if
available]
Total estimated number of priority
populations in the catchment area
The denominator is the estimated number of
individuals in the priority populations.
Programs need to define their geographic
catchment area from which priority
population clients receive HIV prevention
services. Country teams should encourage
methodological harmonization across their
priority partners when estimating population
size within a catchment area.
Changes in indicator:
• The denominator is now optional, but recommended for those with good estimation
metrics (MER 1.0 to 2.0).
• Updated the minimum required standardized HIV prevention interventions and
included the requirement that HIV testing or referral to HIV testing service must be
offered to those who are not known as diagnosed HIV positive (MER 1.0 to 2.0).
• Age/sex disaggregations updated (MER 2.0 v2.1 to v2.2).
• Clarifying language added for Key Populations disaggregation the notes that KP
should be counted in only one KP group to avoid double-counting. More information
is provided below (MER 2.0 v2.1 to v2.2).
How to use:
The indicator represents PEPFAR-supported programming only and helps to determine
PEPFAR’s reach to priority populations (if no denominator). It may also help inform
coverage of PEPFAR-supported programming for priority populations when reliable
population size estimates are included as the denominator.
Priority populations: Priority populations should be defined by each country in the
indicator narrative and must have a documented HIV prevalence or incidence greater
than the general population of the country. Groups that might be counted as priority
populations include:
• Adolescent girls and young women
• Clients of sex workers
• Military and other uniformed services
• Mobile populations (e.g., migrant workers, truck drivers)
• Non-injecting drug users
Size estimation: The IP/country team will estimate the size of each of the priority
populations in the geographic areas where the IP will implement the program. These
areas are chosen based upon epidemiological data with attention to avoiding duplication
32
of activities with those funded by donors (estimating the catchment area should be
explained in the narratives).
Package of interventions: Together with the IP, the country team designs a set of
interventions for each of the priority populations. In a defined catchment area for the
specific priority population, all prevention interventions may not be offered by one IP.
However, all required intervention must be available in the catchment area for the
priority population. Interventions must adhere to written protocols, include goals and
activities, and be designed to promote adoption of key behaviors that support HIV
prevention and service uptake among the priority population(s). The interventions
should comprise multiple encounters with the same individuals or groups.
HIV testing services (HTS) or referring an individual to HTS is required to be offered (at
least once during the reporting period and/or in accordance with WHO/national
guidance) unless the individual had previously been tested positive for HIV. If the
individual is self-identified as HIV positive, then HTS provision or referral to HTS will not
be a required element of this indicator.
The table below lists the interventions that must be offered in addition to HTS (or HTS
referral).
Required Interventions for
Adult Populations
Required Interventions for
Youth Populations
• Promotion of relevant prevention and
clinical services and demand creation to
increase awareness, acceptability, and
uptake of these services.
• Promotion of relevant youth-friendly
prevention and clinical services and
demand creation to increase awareness,
acceptability, and uptake of these
services.
• Information, education, and skills
development to: reduce HIV risk and
vulnerability; correctly identify HIV
prevention methods; adopt and sustain
positive behavior change; and promote
gender equity and supportive norms and
stigma reduction.
• Information, education and skills
development to: reduce HIV risk and
vulnerability; correctly identify HIV
prevention methods; adopt and sustain
positive behavior change; and promote
gender equity and supportive norms and
stigma reduction.
• Referral to or provision of HIV testing;
facilitated linkage to care and
prevention services; and/or support
services to promote use of, retention in,
and adherence to care.
• Referral to or provision of HIV testing;
facilitated linkage to care and
prevention services; and/or support
services to promote use of, retention in,
and adherence to care.
• Condom and lubricant (where feasible)
promotion, skills building, and facilitated
access to condoms and lubricant (where
feasible) through direct provision or
linkages to social marketing and/or
other service outlets.
• Condom and lubricant (where feasible)
promotion, skills training, and facilitated
access to condoms and lubricant (where
feasible) through direct provision or
linkages to social marketing and/or
other youth-friendly, community-based
service outlets.
• Programs targeting adults to raise
awareness of HIV risks for young people,
promote positive parenting and
mentoring practices, and effective adult-
child communication about sexuality
and sexual risk reduction.
PREVENTION
33
How to collect:
Data collection requires reliable tracking systems that are designed to count the number
of one-on-one encounters or participation in group interventions and that reduce
double-counting of individuals in a reporting period. Data should be collected at every
encounter/point of service and aggregated in time for PEPFAR reporting cycles. This
indicator only counts those interventions at the individual and/or group level.
A partner may count an individual (with unknown HIV sero-status or self-identified as
HIV negative) as having received a prevention intervention if they have provided HTS
and/or referral to HTS AND at least one of the other listed prevention interventions
during the reporting period. If an individual is already known to be HIV positive at the
time of service delivery, s/he should receive at least one of the interventions listed in the
table (outside of HTS) to qualify as being counted under this indicator.
Tracking systems must be able to reduce double-counting of individuals in a reporting
period. An individual will be reported when he/she first receives any of the required
interventions in the reporting period; if the same individual receives any subsequent
interventions during the same reporting period they will be reported as a returning
beneficiary and not counted again in the reporting period.
Furthermore, de-duplication of all returning beneficiaries within the Q3-Q4 reporting
period (April 1 – September 30) will also need to take place in Q4 reporting if they had
already been counted under PP_PREV in Q1-Q2 of the same fiscal year. For example, if
an individual had received prevention interventions under PP_PREV through PEPFAR-
supported program in January 2017 and was counted as being reached in FY17 Q2
reporting cycle, and this same individual was later reached with prevention services
again by PEPFAR-supported program in June 2017, that individual should NOT be
reported again in the FY17 Q4 reporting period. This de-duplication is critical to
accurately track the ANNUAL number of unique individuals reached by PEPFAR within a
given fiscal year. Trend analysis of past performance PP_PREV data will be adversely
affected with the change in frequency of PP_PREV reporting from annually to semi-
annually if this de-duplication is ignored (i.e., annual number of PP_PREV reported
within the same fiscal year would be inflated as the same individual would be counted
twice if this de-duplication does not occur at Q4 reporting).
If possible, a unique identifier should be assigned to program participants or names can
be collected to track individual participation in the prevention interventions/sites.
Site (facility and community) level system should maintain accurate registers for each
individual priority population, and sum these individual populations when reporting this
indicator.
Reporting level:
Facility & Community
How often to report:
Semi-Annual
How to review for
data quality:
Data should be reviewed regularly for the purposes of program management, to monitor
progress towards achieving targets, and to identify and correct any data quality issues.
Potential data quality issues for PP_PREV:
Denominator is greater than or equal to the Numerator: The total number of people
from priority populations must be greater than or equal to the total number of
individuals from priority populations who completed a standardized HIV prevention
program.

34
Numerator is greater than or equal to the subtotal of the age/sex disaggregation: The
number of individuals from priority populations who completed a standardized HIV
prevention program should be greater or equal to the sum of the disaggregation by
age/sex.
How to calculate
annual total:
Sum across both reporting periods; de-duplicating unique individuals already reached
and reported in Q1-Q2 of the same fiscal year in Q4 reporting.
Data elements
(components of
indicator):
Numerator:
Number of the
priority
populations
reached with
standardized HIV
prevention
intervention(s)
that are evidence-
based.
Disaggregate Groups
Disaggregates
Age/Sex
[Required]
10-14 M, 10-14 F, 15-19 M, 15-
19 F, 20-24 M, 20-24 F, 25-29
M, 25-29 F, 30-34 M, 30-34 F,
35-39 M, 35-39 F, 40-49 M, 40-
49 F, 50+ M, 50+ F
Testing Services
[Optional]
• Known positive;
• Newly tested and/or
referred for testing;
• Declined testing and/or
referral
Denominator:
Total number of
people in each
priority
populations
[Optional,
recommended if
available]
Disaggregate Groups
Disaggregates
Country teams should
encourage methodological
harmonization across their
priority population partners
when estimating priority
population size within a
catchment area
N/A
Disaggregate Descriptions & Definitions
Testing Services Disaggregates Definitions:
• Known Positive: Persons within each key population type for whom HIV testing is
not indicated because they are known to be HIV-positive. HIV-positive test results
should be verified, if possible, for all persons accessing HIV prevention services
during the reporting period. Implementing partners should maintain records
(without personally identifiable information) on whether the HIV-positive client is
linked to treatment. Patients tested positive in previous reporting periods should be
counted as Known Positives.
• Newly Tested and/or Referred for Testing: Persons within each key population type
for whom HIV testing is indicated because they do not know their HIV status or their
last HIV-negative test was more than 3-6 months ago (or more/less frequently as
indicated by National Guidelines) should either be offered an HIV test on site or
given information about where and when they can access an HIV test at another
nearby clinic. Every attempt should be made to ensure the client is linked with HIV
testing services that are PP-friendly, and where possible the completed referral
should be documented (i.e., the client accessed HIV testing). Note: Persons who
access testing and whose results are newly tested HIV-positive in the reporting
period should also be counted under “newly tested” even if they return for
additional prevention services during that reporting period.
• Declined Testing and/or Referral: Persons who, after explaining the benefits of HIV
testing and the reason for testing every 3-6 months (or more/less frequently as
indicated by National Guidelines), decline to be tested on-site or referred to a site
where HIV testing is offered. Although every attempt should be made to support key
populations with HIV testing as part of the package of HIV prevention services and
to provide HIV testing on site or PP-friendly sites, programs should also respect the
PREVENTION
35
autonomy of clients to decline this service. Clients who decline testing and/or
referral can still receive other prevention services, as long as the benefits of HIV
testing were explained and testing or a referral for testing was offered.
PEPFAR-support
definition:
Standard definition of DSD and TA-SDI used.
Provision of key staff or commodities for priority populations receiving HIV prevention
services includes: ongoing procurement of critical commodities such as condoms,
teaching materials, or community promotion materials; funding for salaries of personnel
who deliver components of the intervention; or paying for transportation of those staff
to the point of Service delivery. Staff responsible for the completeness and quality of
routine patient records (paper or electronic) can be counted here; however, staff who
exclusively fulfill MOH and donor reporting requirements cannot be counted.
For priority populations receiving HIV prevention, ongoing support services service
delivery improvement includes: site supervision; training or assistance with monitoring
and evaluation; QI/QC; and development of materials and protocols.
Guiding narrative
questions:
1. Please indicate how GEND_NORM activities are being tracked and reported by
specifying in the narrative which of the three following options was used:
a. GEND_NORM is tracked as a custom indicator, meets PP_PREV criteria, and is
being included in PP_PREV results. Report the GEND_NORM results in the
narrative.
b. GEND_NORM is a custom indicator but results are not included in PP_PREV
reporting. Report the GEND_NORM results in the narrative.
c. Reporting under PP_PREV alone and not using GEND_NORM as a custom
indicator.
2. Please help us understand what is being tracked or counted under PP_PREV:
a. Describe the types of activities or interventions that are being provided to
beneficiaries.
b. If a specific evidence-based intervention or curriculum is being implemented,
please provide the name.
c. Specify the priority populations counted under PP_PREV and if priority
populations are either receiving the intervention themselves or indirectly
benefiting from intervention, based on other beneficiaries’ receipt or access to
the intervention.
d. If there is “layering” (or combination) of PP_PREV interventions (i.e., various
PP_PREV interventions delivered to benefit one person), please indicate the
priority groups that are receiving layered interventions and if the layered
interventions relate to DREAMS.
3. PP_PREV requires that “HIV testing services (HTS) or referring an individual to HTS (at
least once during the reporting period) unless the individual self-identifies as HIV
positive.
a. Are you tracking the number of HTS referrals generated through PP_PREV
activities? If so, please provide the number.
b. If you are not tracking the HTS referrals please state so and provide an
approximation.
4. If PP_PREV increased or decreased by >25% since the last reporting period, please
explain the reasons (e.g., budget changes, changes to how curriculum-based
interventions are tracked, activities included in PP_PREV that were previously
counted elsewhere, etc.).
36
OVC_SERV
Description:
Number of beneficiaries served by PEPFAR OVC programs for children and families
affected by HIV
Numerator:
Number of beneficiaries served by
PEPFAR OVC programs for children and
families affected by HIV
The numerator is the sum of the following
Program participation disaggregations:
1. Active beneficiaries
2. Graduated beneficiaries
Denominator:
N/A
Changes in indicator:
• Clarifying language added to this indicator reference sheet. Only OVCs that actually
received services in the past three months should be counted in this indicator.
OVCs that have registered for the program, but have not yet received any services
should not be counted in the results (MER 2.0 v2.1 to v2.2).
• The disaggregation for program participation status has been clarified to capture
types of beneficiaries: (1) active beneficiaries and (2) graduated beneficiaries, (MER
2.0 v2.2 Revised Release).
• Beneficiaries that transferred or exited without graduation should no longer be
reported in the numerator (MER 2.0 v2.2 Revised Release). However, these data will
still be collected as disaggregates.
• All indicator changes will be reflected in the data entry screens in DATIM beginning
in FY 18 Q2 (MER 2.0 v2.2 Revised Release).
• The transferred disaggregation was split into two separate disaggregations:
transferred out to a PEPFAR-supported partner and transferred out to non-PEPFAR
supported partner (MER 2.0 v2.2 Revised Release).
• Indicator calculation is updated. Indicator returns to being a snapshot indicator
again for FY 18 reporting. Results should not be summed across reporting periods
(MER 2.0 v 2.2 Revised Release).
How to use:
PEPFAR is mandated to care for children orphaned or made vulnerable by HIV.
Mitigating the impact that HIV is having on children and the families that support them
is integral to a comprehensive HIV response. It is important to note that the definition of
“affected” children includes, but is not limited to, children infected with HIV. PEPFAR
recognizes that individuals, families, and communities are affected by HIV in ways that
may hinder the medical outcomes of HIV-positive persons as well as the emotional and
physical development of children orphaned or made vulnerable by HIV/AIDS. A variety of
services (per Technical Considerations 2017) are supported through PEPFAR to mitigate
these effects in order to improve health and well-being outcomes of adults and children.
The goal of OVC programs is to build stability and resiliency in children and families-
exposed, living with or affected by HIV/AIDS through rigorous case management and
provision and access to health and socio-economic interventions. This indicator, by
disaggregating “active” and “graduated” in the numerator and collecting additional
disaggregates for “transferred out to a PEPFAR-supported partner”, “transferred out to a
non-PEPFAR supported partner”, and “exited without graduation” measures how
successful the OVC program is in building children and their families’ resiliency.
How to collect:
The data sources are the PEPFAR OVC program registers and program data generated by
implementing partners. Implementing partners’ registers need to record names of
children and caregivers who meet the criteria for “active beneficiary” or “graduated” to
generate the numerator total included in this indicator. In addition, implementing
partners should record whether children or caregivers “transferred out to a PEPFAR-
supported partner”, “transferred out to a non-PEPFAR supported partner”, and “exited
without graduation.”
PREVENTION
37
All agencies receiving HKID funding are required to report on this indicator.
This indicator is a direct (output) measure of the number of individuals receiving PEPFAR
OVC program services for children and families affected by HIV/AIDS and tracks progress
on the number of OVC graduating from PEPFAR OVC programs and tracks “exited
without graduation” (such as loss-to-follow up, aging out without transition plan,
moved, or died). Transferred to existing host-country programs, where the host-country
program provides a sustainable response to OVC needs. Transferred to existing PEPFAR-
supported programs to track movement of children and caregivers between PEPFAR-
supported partners. Graduation will vary based on local criteria for achieving stability in
the household.
Reporting level:
Facility & Community
How often to report:
Semi-Annual
How to review for
data quality:
Reviewing PEPFAR OVC implementing partners’ results to ensure that there is no double
counting and changes by Program Completion Status do not show high deviations from
program targets and/or SNU prioritization (scale up, sustained, centrally supported,
sustained commodities.
To ensure completeness, check that OVC_SERV total numerator (autocalculated based
on participation status disaggregates) equals OVC_SERV results by age/sex
disaggregates:
• OVC_SERV total numerator should equal OVC_SERV <1 + 1-9 + 10-14F + 10-14M +
15-17F + 15-17M + 18-24F + 18-24 M + 25+F + 25+M
• OVC_SERV total numerator should equal OVC_SERV<18 + OVC_SERV 18+
• OVC_SERV<18 = OVC_SERV <1 + 1-9 + 10-14F + 10-14M + 15-17F + 15-17M
• OVC_SERV 18+ = OVC_SERV 18-24F + 18-24 M + 25+F + 25+M
How to calculate
annual total:
To calculate data for annual results for OVC_SERV:
Sum Active (children and caregivers received services in the past three months) +
Graduated (OVCs that graduated from the OVC program in the past 12 months).
This indicator should be reported as a snapshot (i.e., report data as of the last day of the
reporting period) in DATIM.
Data elements
(components of
indicator):
Numerator:
Number of
beneficiaries
served by PEPFAR
OVC programs for
children and
families affected
by HIV.
Disaggregate Groups
Disaggregates
Program Participation Status
[Required]
• Active (Received at least one
service in the past 3 months)
• Graduated (At Q2: Report
children and
parents/caregivers that
graduated from the OVC
program in the past 6
months. At Q4: Report
children and
parents/caregivers that
graduated from the OVC
program in the past 12
months.)
Age/Sex (For Active and
Graduated)
[Required]
<1, 1-9, 10-14 M, 10-14 F, 15-17
M, 15-17 F, 18-24 M, 18-24 F,
25+ M, 25+ F
38
Exited or Transferred
[Required]
Disaggregate should be
reported for exited or
transferred, even if no
numerator (active + graduated)
values are reported.
• Transferred out to a PEPFAR-
supported partner (At Q2:
Report children and
parents/caregivers that
transferred out to a PEPFAR-
supported partner in the
past 6 months. At Q4: Report
children and
parents/caregivers that
transferred out to a PEPFAR
supported partner in the
past 12 months.)
• Transferred out to a non-
PEPFAR supported partner
(At Q2: Report children and
parents/caregivers that
transferred out to a non-
PEPFAR-supported partner in
the past 6 months. At Q4:
Report children and
parents/caregivers that
transferred out to a non-
PEPFAR supported partner in
the past 12 months.)
• Exited without graduation
(At Q2: Report children and
parents/caregivers that
exited in the past 6 months.
At Q4: Report children and
parents/aregivers that exited
in the past 12 months.)
Age/Sex/OVC Service Area
[DREAMS Conditional]
• Education Support: <1, 1-9,
10-14 M, 10-14 F, 15-17 M,
15-17 F, 18-24 M, 18-24 F,
25+ M, 25+ F
• Parenting/Caregiver Support:
<1, 1-9, 10-14 M, 10-14 F,
15-17 M, 15-17 F, 18-24 M,
18-24 F, 25+ M, 25+ F
• Social Protection: <1, 1-9, 10-
14 M, 10-14 F, 15-17 M, 15-
17 F, 18-24 M, 18-24 F, 25+
M, 25+ F
• Economic Strengthening: <1,
1-9, 10-14 M, 10-14 F, 15-17
M, 15-17 F, 18-24 M, 18-24
F, 25+ M, 25+ F
• Other Service Areas: <1, 1-9,
10-14 M, 10-14 F, 15-17 M,
15-17 F, 18-24 M, 18-24 F,
25+ M, 25+ F
Disaggregate Descriptions & Definitions
PREVENTION
39
Program Participation Status Definitions:
• “Active beneficiary” is an individual, a child, or parent/caregiver who has received
at least one PEPFAR OVC program service in the last three months. New
beneficiaries registered during the reporting period can be counted as active only if
they have received at least one service in the last three months. Assessment,
enrollment, case plan development, and case plan monitoring are not considered
services. Please refer to the forthcoming OVC Reporting FAQ clarification on what
activities constitute a service for more information.
• “Graduation” is defined as:
1. Graduation is defined as happens when children and parent/caregivers enrolled
in PEPFAR OVC programs are deemed stable and no longer in urgent need of
externally supported services. Criteria for achieving stability in the household
vary and should be defined at the OU-level to be consistent across IPs.
Or
2. Aging out: This only includes children who have reached the age of 18 and who
have a transition plan for successful exiting from the PEPFAR OVC Program. This
does not apply to children > 18 years old enrolled in secondary education.
Exited or Transferred Disaggregate Definitions:
• “Transferred out to a non-PEPFAR-supported partner” happens when children and
families have transitioned to other forms of support programs other than PEPFAR
funded OVC programs. These could include country-led programs or other donor
funded programs.
• “Transferred out to a PEPFAR-supported partner” happens when children and
families have transitioned from the support of one PEPFAR partner to another
PEPFAR-partner.
• “Exited without graduation” This includes children and caregivers who are lost-to-
follow up, re-located, or died and children who aged-out without a graduation plan
from PEPFAR OVC program.
PEPFAR-support
definition:
Standard definition of DSD and TA-SDI used.
Provision of key staff or commodities for OVC beneficiaries receiving care and support
services in the community include: For beneficiaries of OVC services, this can include
funding of salaries (partial or full) for staff of the organization delivering the individual,
small group or community level activity (e.g., psychosocial support, child protection
services, education, etc.) or procurement of critical commodities essential for ongoing
service delivery. Partial salary support may include stipends or incentives for volunteers,
or paying for transportation of those staff to the point of service delivery.
For care and support services, ongoing support for OVC service delivery for
improvement includes: the development of activity-related curricula, education
materials, etc., supportive supervision of volunteers, support for setting quality
standards and/or ethical guidelines, and monitoring visits to assess the quality of the
activity, including a home visit, a visit to a school to verify a child’s attendance and
progress in school or observation of a child’s participation in kids clubs.
Guiding narrative
questions:
1. What is the total achievement of OVC_SERV for <18 years and total numerator?
Please explain partners with highest/lowest performance.
2. Please explain results by participation status disaggregate:
a. What criteria do beneficiaries need to achieve in order to graduate? Is that
standard across partners in your OU?
40
b. How many beneficiaries exited without graduation? Please explain the reasons
for exiting without graduation and try to quantify with percentages if possible. Are
there certain partners with higher rates of exiting without graduation? How are
you managing this with the partner(s)?
c. How many beneficiaries were transitioned? To whom (e.g., other NGOs,
government support, etc.). Where were beneficiaries transferred? Please provide
disaggregates for beneficiaries transferred to specific sources of support.
d. Of those who are reported to be active, what percentage is newly enrolled? Any
re-enrollments of those LTFU? If yes, how many? Are any partners especially
good at finding and re-enrolling those LTFU?
PREVENTION
41
TB_PREV
Description:
The number of ART patients who completed a standard course of TB preventive therapy
within the semiannual reporting period
Numerator:
Number of ART patients who completed
a course of TB preventive therapy
during the reporting period (for
continuous IPT programs, this includes
the patients who have completed the
first 6 months of isoniazid preventive
therapy (IPT))
The numerator can be generated by counting
the number of PLHIV on ART who are
documented as having received at least six
months of IPT or have completed another
standard course of TB preventive therapy.
Denominator:
Number of ART patients who are
expected to complete a course of TB
preventive therapy during the reporting
period (for programs using continuous
IPT, this includes only the patients who
are scheduled to complete the first 6
months of therapy)
The denominator can be generated by
counting the total number of patients who
are scheduled to complete a course of TB
preventive therapy (or at least 6 months of
IPT for those who are on a prolonged or
continuous regimen) in the semiannual
reporting period.
Changes in indicator:
• Type of therapy by ART start disaggregation updated to indicate whether ART
patients started IPT or an alternative TB preventive therapy regimen and whether
they started ART in the same reporting period as TB preventive therapy or if they
were on ART previously. Updated disaggregation titled, “Type of TB Preventative
Therapy (TPT) by ART Start” (MER 2.0 v2.1 to v 2.2).
How to use:
This indicator measures the performance of HIV programs in scaling up TB preventive
therapy, with the goal of preventing progression to active TB disease among PLHIV and
decreasing ongoing TB transmission in this population. As part of a cascade from
TX_CURR to screening (captured in TX_TB), this indicator will inform programs on the
pace of scale-up, and the proportion will allow for monitoring of cohorts through
completion of therapy. New disaggregates on type of therapy will inform programs on
their relative use of different regimens, and the timing of ART will allow the clinical
cascade to follow only those who are newly entering care, which will better demonstrate
program performance, particularly in countries that have already provided TB preventive
therapy for many of their PLHIV in care.
How to collect:
The numerator can be generated by counting the number of PLHIV on ART who are
documented as having received at least six months of IPT or have completed another
standard course of TB preventive therapy. This should include the patients who
completed a shorter alternative course, such as 3 months of isoniazid and rifapentine
(3HP), as well as those who are on prolonged or continuous IPT who have completed
their first 6 months of therapy during the semiannual reporting period. Importantly,
programs should ensure that patients on continuous therapy are counted only once,
and not repeated in future calculations.
The denominator can be generated by counting the total number of patients who are
scheduled to complete a course of TB preventive therapy (or at least 6 months of IPT for
those who are on a prolonged or continuous regimen) in the semiannual reporting
period.
For IPT:
• Patients who are taking a standard 6-month course of IPT would be expected to
complete therapy if they started IPT in the previous reporting period; therefore,
42
all patients who started IPT at any time in the previous 6-month reporting period
(i.e., the 6 months before the start of the current reporting period) should be
included in the denominator. The few patients who start and complete IPT in the
same reporting would also be included.
• Patients who are taking prolonged (9- or 12-month) or continuous IPT would also
be expected to complete the first 6 months of IPT if they started IPT in the
previous reporting period; therefore, all patients who started prolonged or
continuous IPT in the previous 6-month reporting period should be included. The
few patients who start and complete 6 months of IPT in the same reporting would
also be included.
For alternative regimens:
• Patients who are taking a 3-month regimen of isoniazid and rifapentine would be
expected to complete therapy in this reporting period if they started on therapy
at any time in the period starting 3 months prior to the start of the current
reporting period to 3 months prior to the end of the current reporting period; all
such persons should be included in the denominator.
• Patients who are taking a 4-month course of rifampicin would be expected to
complete therapy in this reporting period if they were started on therapy at any
time in the period starting 4 months prior to the start of the current reporting
period to 4 months prior to the end of the current reporting period; all such
persons should be included in the denominator.
These data elements can be collected from the ART register or from separate TB
screening (presumptive TB) or IPT registers.
Reporting level:
Facility
How often to report:
Semi-Annual
How to review for
data quality:
Only one disaggregation type is used for age (coarse disaggregations).
Data Element ≥ subtotal of each of the disaggregations.
How to calculate
annual total:
Snapshot indicator. Use the result reported at Q4.
Data elements
(components of
indicator):
Numerator:
Number of ART
patients who
completed a
course of TB
preventive
therapy during the
reporting period
Disaggregate Groups
Disaggregates
Type of TB Preventative
Therapy (TPT) by ART Start:
[Required]
• IPT by newly enrolled on ART
• IPT by previously enrolled on
ART
• Alternative TPT regimen by
newly enrolled on ART
• Alternative TPT regiment by
previously enrolled on ART
Age/Sex:
[Required]
<15 F, >15 F, <15 M, >15 M
Denominator:
Number of ART
patients who are
expected to
complete a course
of TB preventive
therapy during the
reporting period
Disaggregate Groups
Disaggregates
Type of TB Preventative
Therapy (TPT) by ART Start:
[Required]
• IPT by newly enrolled on ART
• IPT by previously enrolled on
ART
• Alternative TPT regimen by
newly enrolled on ART
• Alternative TPT regiment by
previously enrolled on ART
Age/Sex:
[Required]
<15 F, >15 F, <15 M, >15 M
PREVENTION
43
Disaggregate Descriptions & Definitions
Type of TB Preventative Therapy (TPT) by ART Start Descriptions:
• IPT/Newly enrolled on ART: Among those who completed 6 months of IPT, the
patients who started IPT and ART in the previous reporting period.
• IPT/Previously enrolled on ART: Among those who completed 6 months of IPT,
the patients who started IPT in the previous reporting period, but who started
ART prior to the previous reporting period (i.e., patients who were on ART prior
to the reporting period when they started IPT).
• Alternative TPT regimen/Newly enrolled on ART: Among those who completed
an alternative regimen (e.g., 3-month INH and rifapentine), the patients who
started the regimen and ART in the current or the previous reporting period
• Alternative TPT/Previously enrolled on ART: Among those who completed an
alternative regimen (e.g., 3-month INH and rifapentine), the patients who
started the regimen in the current or the previous reporting period, but started
ART prior to previous reporting period
PEPFAR-support
definition:
Standard definition of DSD and TA-SDI used.
Provision of key staff or commodities for routine HIV-related services include: ongoing
provision of critical re-occurring costs or commodities (such as ARVs, TB preventive
therapy and diagnostic/screening tests) or funding of salaries or provision of Health Care
Workers for HIV clinic services. Staff responsible for maintaining patient records in both
HIV and TB clinics are included in this category however staff responsible for fulfilling
reporting and routine M&E requirements are not included.
Ongoing support for patients receiving routine HIV-related services includes: training of
HIV service providers, clinical mentoring and supportive supervision of staff at HIV sites,
infrastructure/renovation of facilities, support of HIV service data collection, reporting,
data quality, QI/QA of HIV services support, ARV and IPT consumption forecasting and
supply management, support of lab clinical.
Guiding narrative
questions:
1. Roughly what proportion of all PLHIV on treatment have already completed TB
preventive therapy prior to this reporting period?
2. If TB preventive therapy was not provided to all PLHIV in care, what are the main
reasons for limited scale-up?
3. Roughly what proportion of patients who received TB preventive therapy were
treated with the 6-month isoniazid regimen?
44
KP_MAT
Description:
Number of people who inject drugs (PWID) on medication-assisted therapy (MAT) for at
least 6 months within the reporting period
Numerator:
Number of people who inject drugs
(PWID) on medication-assisted therapy
(MAT) for at least 6 months
This indicator provides information on the
total number of individuals who have been
on treatment for at least 6 months within the
reporting period.
Denominator:
N/A
Changes in indicator:
No changes in this indicator.
How to use:
When proper and sufficient dosage is administered, medication-assisted therapy (MAT)
is highly effective in reducing opioid use, reducing injecting behaviors that put opioid-
dependent people at risk for HIV and improving retention for those who are on ART.
Therefore, all people who are dependent on opioids should be offered and have access
to this service. The implementation of MAT programs should facilitate and enhance
access to HIV-specific services for PWID, such as HIV testing services, provision and/or
referral and linkages to ARV treatment programs, PMTCT for female PWID and a range
of other prevention and harm reduction services.
Partners providing MAT referrals only should not use this indicator, unless it also meets
the KP_MAT_TA requirement below. Please see key population indicator “KP_PREV” to
see if services provided meet reporting criteria for that indicator.
How to collect:
This indicator provides information on the total number of individuals who have been on
treatment for at least 6 months since initiation of medication-assisted treatment (e.g.,
methadone, buprenorphine, or buprenorphine/naloxone to treat drug dependency) at
any point in time within the reporting period. Therefore, data for this indicator can be
generated by counting the number of individuals who are currently receiving MAT or
received at least 6 months of MAT in the reporting period in accordance with the
nationally approved treatment protocol (or WHO/UNAIDS standards) at the end of the
reporting period.
Count all individuals who have completed at least 6 months of treatment even if they
drop-out, die, or are otherwise lost to follow-up, as long as they completed the
minimum of 6 months treatment. Do not count individuals who initiate treatment too
late in the reporting period to be able to reach a minimum of 6 months.
Reporting level:
Facility
How often to report:
Annual
How to review for
data quality:
This indicator makes use of program data as part of an on-going cohort, like that used to
monitor ART retention. MAT register and/or patient-level data can be used to
determine the number of people starting MAT in the defined period, as a cohort, and
the number of those who are still in treatment 6 months and who were on MAT for at
least six months during the reporting period.
Data should be reviewed regularly for the purposes of program management, to monitor
progress towards achieving targets, and to identify and correct any data quality issues.
How to calculate
annual total:
Use annual result reported at Q4.
Data elements
(components of
indicator):
Numerator:
Number of people
who inject drugs
(PWID) on
Disaggregate Groups
Disaggregates
Sex
[Required]
Male; Female
PREVENTION
45
medication-
assisted therapy
(MAT) for at least
6 months
Disaggregate Descriptions & Definitions
N/A
PEPFAR-support
definition:
Standard definition of DSD and TA-SDI used:
Provision of key staff or commodities for PWID on MAT includes: procurement of
methadone or any other medication assisted options for the treatment of opioid
dependence, or funding for salaries of personnel delivering the service (i.e., HCW,
program managers). Staff who are responsible for the completeness and quality of
routine patient records (paper or electronic) can be counted here; however, staff who
exclusively fulfill MOH and donor reporting requirements cannot be counted.
Ongoing support for MAT services for PWID service delivery improvement includes:
mentoring and supportive supervision, training, MAT guidance development, site level
QA/QI, regular assistance with monitoring and evaluation functions and data quality
assessments, or MAT consumption forecasting and supply management.
Guiding narrative
questions:
1. Were the individuals who initiated MAT too late in this reporting period (at least 6
months prior) excluded from the results?
46
GEND_GBV
Description:
Number of people receiving post-gender based violence (GBV) clinical care based on the
minimum package
Numerator:
Number of people receiving post-
gender based violence (GBV) clinical
care based on the minimum package
Additional information about numerator
definition
Denominator:
N/A
Changes in indicator:
• Age/sex disaggregations updated (MER 2.0 v2.1 to v2.2).
• Age/sex disaggregations added to the post-exposure prophylaxis (PEP) sub-
disaggregate of the sexual violence disaggregate (MER 2.0 v2.1 to v2.2).
How to use:
This indicator measures delivery of a basic package of post-GBV clinical services
(including PEP and EC). NOTE: This indicator DOES NOT include GBV Prevention
activities or non-clinical community-based GBV response (e.g., shelter programs, case
management).
This indicator will enable PEPFAR to:
• To determine the number of individuals that are suffering from GBV and reporting
to clinical partners
• To assess whether post-GBV clinical services are being used.
• Gain an understanding of the uptake of post-GBV clinical services offered across
PEPFAR countries.
• Provide important information to key stakeholders about PEPFAR programs that
mitigate women and girls’ and other marginalized populations’ vulnerability to
HIV/AIDS.
• Support efforts to assess the impact of post-GBV clinical services by correlating the
reach (i.e., number of people served) of these services over time with outcomes
related to GBV (and HIV/AIDS), as described through other data collection efforts
such as survey data (DHS/PHIA/VACS).
• Identify programmatic gaps by analyzing the number and ages of people receiving
services, as well as the reach of services in particular geographic areas.
How to collect:
Data sources are standard program monitoring tools, such as forms, log books,
spreadsheets and databases that national programs and /or partners develop or already
use.
Data should be collected continuously at the point of service delivery (i.e., ANC, PMTCT,
ART, etc.) and aggregated in time for PEPFAR reporting cycles.
The indicator can be generated by counting the number of persons receiving post-GBV
clinical care, disaggregated by the age group and sex of the client receiving the service,
as well as the type of service (sexual violence or emotional/physical violence) and PEP
provision (see below for disaggregation information).
To adequately capture the provision of these services, logs and monitoring forms will
need to be used wherever the services are offered. These forms will need to track both
the outcome of the initial assessment and the provision of referrals or services. For PEP
specifically, registries should collect both the administration of the PEP as well as its
completion and the patient’s adherence.
Special considerations: As outlined in the Program Guide for Integrating GBV Prevention
and Response in PEPFAR Programs all programs seeking to address GBV must first and
PREVENTION
47
foremost protect the dignity, rights, and well-being of those at risk for, and survivors of,
GBV. There are four fundamental principles for integrating a GBV response into existing
programs and specific actions for putting these principles into practice. These principles
are as follows:
• Do no harm
• Privacy, confidentiality, and informed consent
• Meaningful engagement of people living with HIV (PLHIV) and GBV survivors
• Accountability and M&E
Reporting level:
Facility & Community
How often to report:
Annually
How to review for
data quality:
Numerator ≥ subtotal of each of the disaggregation: The number of people receiving
post-GBV clinical care should be greater or equal to the sum of each individual
disaggregate group.
How to calculate
annual total:
Use annual result reported at Q4.
Data elements
(components of
indicator):
Numerator:
Number of people
receiving post-
GBV clinical care
based on the
minimum package
Disaggregate Groups
Disaggregates
Violence Service Type
[Required]
• Sexual Violence
• Physical and/or Emotional
Violence
Violence Service Type by Age
and Sex
[Required]
• Sexual Violence by: Unknown
Age M, Unknown Age F, <10
M, <10 F, 10-14 M, 10-14 F,
15-19 M, 15-19 F, 20-24 M,
20-24 F, 25-29 M, 25-29 F, 30-
34 M, 30-34 F, 35-39 M, 35-39
F, 40-49 M, 40-49 F, 50+ M,
50+ F
• Physical and/or Emotional
Violence by: Unknown Age M,
Unknown Age F, <10 M, <10 F,
10-14 M, 10-14 F, 15-19 M,
15-19 F, 20-24 M, 20-24 F, 25-
29 M, 25-29 F, 30-34 M, 30-34
F, 35-39 M, 35-39 F, 40-49 M,
40-49 F, 50+ M, 50+ F
Number of People Receiving
Post-Exposure Prophylaxis (PEP)
Services by Age and Sex
(Disaggregate of the Sexual
Violence Service Type)
[Required]
• Received PEP by: Unknown
Age M, Unknown Age F, <10
M, <10 F, 10-14 M, 10-14 F,
15-19 M, 15-19 F, 20-24 M,
20-24 F, 25-29 M, 25-29 F, 30-
34 M, 30-34 F, 35-39 M, 35-39
F, 40-49 M, 40-49 F, 50+ M,
50+ F
Disaggregate Descriptions & Definitions
Violence Service Type Disaggregate Definitions:
Sexual violence (post-rape care): Although guidelines for post-rape care will vary from
country to country, in addition to treatment of serious or life-threatening medical issues
(e.g., lacerations, broken bones) and the necessary forensic interviews and
examinations, the minimum package of post-rape care services should always begin with
an assessment of the client’s specific needs. The following represents the Minimum
Package for post-rape care services that must be in place to count under this indicator:
48
• Provision of Clinical Services: (all of the following must be in place, including
relevant commodities, and ability to count individuals—independent of whether
individuals use the specific service)
• Rapid HIV testing with referral to care and treatment as appropriate
• Post exposure prophylaxis (PEP) for HIV -- if person reached within the first 72
hours
• STI screening/testing and treatment
• Emergency contraception, if person is reached in the first 120 hours. PEPFAR funds
cannot be used to procure EC. EC is legal in all PEPFAR countries except Honduras,
so should be available in all countries except for Honduras
• Counseling (other than counseling for testing, PEP, STI and EC)
Physical and/or emotional violence (other Post-GBV care): GBV can take many forms,
and includes physical and emotional violence. The following services should be available
for persons who have experienced GBV that is not sexual. Services should always begin
with an assessment of the client’s specific needs and include, as appropriate. The
following represents the Minimum Package for other post-GBV care services that must
be in place to count under this indicator:
• Provision of Clinical Services: (all the following must be in place and available to
count persons—independent of whether people use the specific service)
• Rapid HIV testing with referral to care and treatment as appropriate (Please note
that individuals should also be counted under the MER HIV testing and counseling
indicator (i.e., # of individuals who received HIV testing and counseling services and
received their results).
• STI screening/testing and treatment
• Counseling (other than for HIV counseling and testing)
For both Sexual violence and Physical and/or emotional violence: These cannot be
counted for the indicator alone, however where applicable should be offered:
• Longer-term psycho-social support (e.g., peer support groups)
• Legal counsel
• Police
• Child protection services
• Economic empowerment
Number of People Receiving Post-exposure prophylaxis (PEP) Services Description:
PEP service provision should only be counted under this indicator if the individual
receives PEP treatment (i.e., drugs) in accordance with international and/or national
protocols, guidelines, etc., and if the individual completes the full course of treatment. If
an individual is provided with PEP, completes the full course of treatment (and meets
the other criteria detailed within this indicator reference sheet) the individual should be
counted under this GBV care indicator. The individual should not be additionally counted
under other MER treatment indicators (e.g., # of individuals new on ART; # of individuals
ever on ART, etc.) PEP is intended to prevent HIV infection, while other MER treatment
indicators monitor ARV provision to those who are HIV positive.
PEPFAR-support
definition:
Standard definition of DSD and TA-SDI used.
Provision of key staff or commodities for GEND_GBV includes: ongoing procurement of
commodities (e.g., ARVs, rapid HIV test kits, STI testing or treatment commodities) or
funding of salaries (partial or full) for HCW actively delivering the components of GBV
care in accordance with international or national protocols or guidelines [i.e., physicians,
nurses, and other health care workers who can assess GBV and provide treatment and
appropriate referrals.
PREVENTION
49
Ongoing support for GEND_GBV service delivery improvement includes: mentoring and
supportive supervision, training, guidance development, site level QA/QI, regular
assistance with monitoring and evaluation functions and data quality assessments, or
commodity consumption forecasting and supply management.
Guiding narrative
questions:
1. How are GBV cases identified in the community and/or at the facility? If cases are
identified at the community, how are they referred to a facility for post-GBV clinical
care?
2. Of those coming in for services who are screened and disclose sexual violence, what
proportion receive PEP? What proportion of those who disclose sexual violence
refuse PEP?
3. Is site level data on the type of violence disclosed collected? If so, please provide
available data in the narratives on the proportion that disclose physical and/or
emotional violence, and of those choose to receive services.
50
FPINT_SITE
Description:
Number of HIV service delivery points (SDP) at a site supported by PEPFAR that are
providing integrated voluntary family planning (FP) services
Numerator:
Number of service delivery points
supported by PEPFAR that are providing
fully integrated voluntary family
planning services
See definition below for a PEPFAR-supported
service delivery point. Note: a service
delivery point is NOT the same as a site.
There can be numerous service delivery
points within one site.
Denominator:
Number of total service delivery points
at a site supported by PEPFAR
Not collected through the data entry
screened, determined by number of sites
reporting service delivery area.
Changes in indicator:
• This indicator has changed from an absolute count of the number of sites to have
integrated family planning services to the number of service delivery areas within a
site (MER 1.0 to 2.0).
How to use:
This output indicator aims to measure progress towards integrating voluntary FP within
the PEPFAR platform at the service delivery level. It captures information about whether
FP integration is occurring at various HIV service delivery points within PEPFAR
supported sites. Many PEPFAR sites will have numerous service delivery points within
each site. For example, if one hospital receives PEPFAR support for both the HIV
treatment department AND the ANC department, then the FPINT_SITE total for that one
site is 2 service delivery points.
This indicator will enable PEPFAR stakeholders to:
• Gain a basic, but essential, understanding of whether FP services are being
integrated in PEPFAR-supported service delivery points.
• Identify gaps, including service contexts, countries, or regions with low levels of
HIV/FP integration.
Inherent within this indicator is the principle that integrated HIV/FP program activities
must respect a client’s right to make informed decisions about his or her reproductive
life. This means that clients should have access to an appropriate and comprehensive
range of contraceptive options; and/or to safer conception/pregnancy counseling
depending upon their fertility desire and intentions. Judgements and personal opinions
are not appropriate in a clinic setting.
This indicator will be used to monitor coverage of HIV/FP integration at a global level.
Therefore, detailed information on completion of referrals, FP service uptake, types of
contraceptive methods offered on site, and other critical components of integrated
programs will not be captured through this indicator, but should be maintained at the
site or programmatic level.
How to collect:
Definition: Voluntary Family Planning Service Provision
To be considered as a PEPFAR-supported service delivery point that directly provides
fully integrated voluntary FP services, all 3 criteria below must be met. If a service
delivery point provides fewer than 3 of the services noted below, it should not be
counted under this indicator.
The PEPFAR-supported HIV service delivery point must provide for all relevant clients,
including partners in HIV discordant couples (as documented by standard operating
procedures, guidelines, protocols, manuals and/or intake documents, etc.):
PREVENTION
51
1. Assessment of voluntary FP needs through routine screening;
2. Provision of voluntary FP counseling (including safe pregnancy counseling for
those wishing to become pregnant, or those who are pregnant);
3. Provision or referral of a broad range of modern contraceptive methods, in
accordance with the National FP policy guidelines, for clients who voluntarily
wish to delay or prevent pregnancy. It is very much preferred for methods to be
available onsite. If referrals are given, they must include detailed information
(e.g., facility location, hours of operation, etc.) about where methods can be
accessed.
Assess Voluntary Family Planning Needs Through Screening (Number 1 above): In
assessing FP needs, all clients as part of their routine care visit should be asked about
their FP needs and practices. Depending upon the individual client and his or her needs,
these can include: reproductive goals; prior pregnancies; living and family situation; FP
knowledge; previously used FP methods and satisfaction with use; and any FP-related
concerns. These needs should be assessed without expressing any personal biases about
a client’s preference.
Provide Voluntary Family Planning Counseling (including Safe Pregnancy Counseling)
(Number 2 above): Quality voluntary FP counseling should cover a wide range of topics
that are client and context specific, and that include both safe pregnancy counseling for
individuals who wish to become pregnant as well as contraception for individuals who
wish to avoid, space or delay pregnancy. “FP counseling” is not the same as "FP
education". Depending upon the type of FP services that are offered at PEPFAR
supported site; health providers or community mobilizers may provide EDUCATION
and/or COUNSELING on FP.
Education activities may include distribution of printed materials, group health
education and community outreach efforts among other interventions. Education helps
to increase general knowledge on the benefits and importance of FP and increase
support for FP use, as well as to link women and their partners to other FP services,
including contraceptive method provision.
FP counseling is an interpersonal communication between the health provider and client
where topics specific to the clients’ needs are discussed to help them determine if they
want to use FP and if so; to help them choose and use the FP method of their choice. HIV
service providers or all levels can be trained and supported to develop or improve their
skills at FP counseling. A wide array of FP counseling materials exist that can be used in
PEPFAR settings; including national FP flipcharts, counseling cards and informational
handouts
Voluntary FP counseling should follow the standards and best practices outlined in the
“Additional References” section below.
Provision or Referral of a Broad Range of Modern Contraceptive Methods (Number 3
above): Per U.S. Government legislation, and in line with national FP policies, a broad
range of methods should be provided to clients, allowing them to choose the method
most appropriate for them, either directly or through referral. For an SDP to be counted
towards this indicator, at least three modern contraceptive methods should be available
either on site or through referral. Emergency contraception is an important FP method
that should be available in all HIV settings as part of FP and gender based violence (GBV)
services. Information on modern contraceptive methods can be found in the references
52
listed at the end of this sheet. All referrals should include detailed information about
where methods can be accessed (e.g., facility location, operating hours, etc.).
PEPFAR-Supported Service Delivery Point at a site
A PEPFAR-supported service delivery point uses PEPFAR funds to directly provide HIV-
related services. It offers one or more HIV-related services including but not limited to:
HIV testing and counseling; prevention of mother-to-child transmission of HIV (PMTCT);
anti-retroviral treatment (ART); screening and prophylaxis for opportunistic infections
(OI); other health services for people living with HIV (e.g., positive health, dignity and
prevention (PHDP), nutrition support, etc.), and prevention activities for priority
populations (key populations and adolescent girls and young women). It can include
fixed locations and/or mobile operations offering routine and/or regularly scheduled
services. Examples include different HIV services within clinics, hospitals, health facilities
and community-based organizations (government, private or NGO). Individual
community health workers are not considered to be individual service delivery points.
Rather, the organizations with which they are affiliated are considered to be the service
delivery point(s).
PEPFAR service delivery points for FP/HIV integration include the following:
1. Care and Treatment (including Pediatric and Adolescent Care and Treatment
Services) – this includes services where ART is initiated and monitored.
2. Antenatal and/or Maternity services - this includes FP education and healthy
timing and spacing messages in the ANC setting (when a woman in pregnant and
receiving PMTCT services and/or FP counseling and method provision post-
partum.)
3. Priority Population Prevention services – this includes priority population
programming, such as drop in centers and prevention sites focused on adolescent
girls and young women (i.e., DREAMS). FP integration can also take place across
the clinical cascade for priority populations, including care and treatment which
would be recorded under care and treatment service delivery point
4. Key Population Prevention services – this includes programming for Men who
have sex with men, Transgender people, Sex workers, and People who inject
drugs, such as drop in centers. FP integration can also take place across the clinical
cascade for key populations, including care and treatment which would be
recorded under care and treatment service delivery point.
5. HIV Testing services - includes counselling (pre-test information and post-test
counselling); linkage to appropriate HIV services; and coordination with laboratory
services to support quality assurance and the delivery of correct results. FP
services can be made available with HIV testing services, especially for key
populations and adolescent girls and young women as well as for HIV
serodiscordant couples. (even if FP integration is targeting key or priority
populations, if occurring in HTS the integration should be documented under HTS)
Special Considerations:
USG-supported FP and HIV/AIDS programs must adhere to the following principles:
• People living with HIV (PLHIV) and their partners should be provided with
information on, and be able to exercise voluntary choices about their health,
including their reproductive health.
• The USG, including PEPFAR, supports a person‘s right to choose, as a matter of
principle, the number, timing, and spacing of their children, as well as use of FP
methods, regardless of HIV/AIDS status.
• FP use should always be a choice, made freely and voluntarily, independent of the
person‘s HIV status.
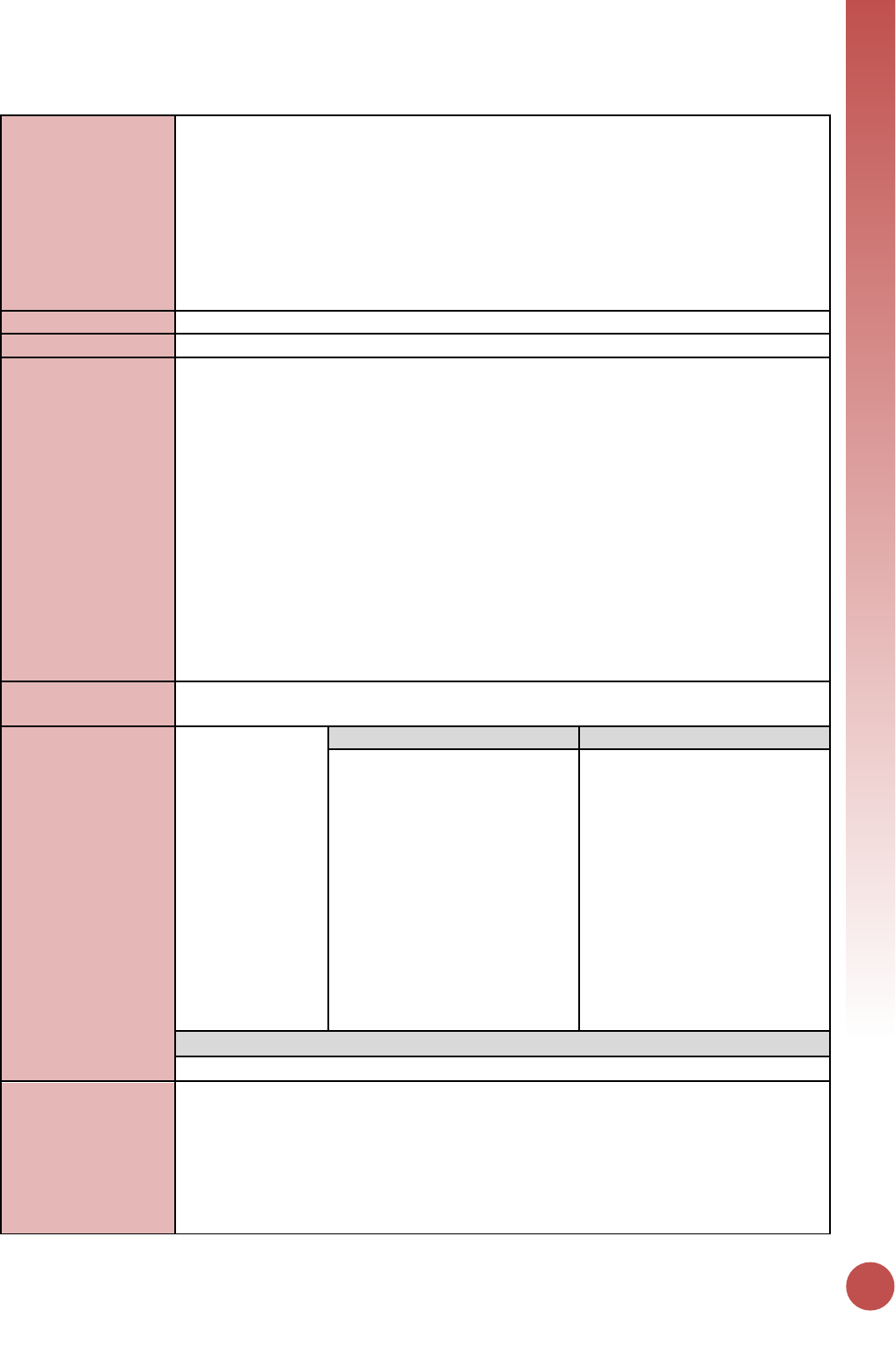
PREVENTION
53
• The decision to use or not to use FP should be free of any discrimination,
judgment, stigma, coercion, duress, or deceit and informed by accurate,
comprehensible information and access to a variety of methods.
• Access to and provision of health services, including antiretroviral treatment, for
PLHIV should never be conditioned on that person's choice to accept or reject any
other service, such as family planning (other than what may be necessary to
ensure the safe use of antiretroviral treatment and other drug interactions).
• PLHIV who wish to have children should have access to safe and non-judgmental
pregnancy counseling services.
Reporting level:
Facility by Service Delivery Area
How often to report:
Annual
How to review for
data quality:
Data should be reviewed regularly for the purposes of program management including
monitoring progress towards achieving targets, and identifying and correcting any data
quality issues. Follow PEPFAR Guidance for data quality review as circulated in Q4
reporting guidance.
Potential data quality issues for FPINT_SITE: Indicator counts individual Service Deliver
Points at Sites: This indicator counts the number of service delivery points (SDP) NOT the
number of sites that integrate FP services. See above for SDP definition.
Denominator is greater than or equal to the Numerator: The total number of PEPFAR-
supported service delivery points (the denominator) must be greater than or equal to
the total number of PEPFAR-supported service delivery points that have integrated
Family Planning (the numerator). (Note: this denominator is not collected through this
indicator, therefore this data quality check would require triangulation with other
indicators and additional data sources)
How to calculate
annual total:
Use annual result reported at Q4.
Data elements
(components of
indicator):
Numerator:
Number of service
delivery points
supported by
PEPFAR that are
providing fully
integrated
voluntary family
planning services
Disaggregate Groups
Disaggregates
Number of Service Delivery
Points by Service Delivery Area
[Required]
• HIV Testing Services service
delivery points
• Care & Treatment (includes
pediatric and adolescent care
and treatment) service
delivery points
• Antenatal Care and/or
Maternity service delivery
points
• Priority Population Prevention
service delivery points
• Key Populations Service
Delivery Points
Disaggregate Descriptions & Definitions
N/A
PEPFAR-support
definition:
The PEPFAR support categories of DSD and TA-SDI do not apply. To report results for this
indicator, it is expected that PEPFAR provides support to the HIV service delivery area
Definition: For this indicator, a “PEPFAR supported site” should include any facility site in
the PEPFAR master facility list in DATIM which also reported any programmatic target or
result during the same reporting period.
54
Definition: For this indicator, a “PEPFAR-Supported Service Delivery Point” at a site is a
service delivery point that uses PEPFAR funds to provide HIV-related services. It offers
one or more HIV-related services including but not limited to: HIV testing and
counseling; prevention of mother-to-child transmission of HIV (PMTCT); anti-retroviral
treatment (ART) and TB/HIV services. Examples include different HIV services within
clinics, hospitals, health facilities and community-based organizations (government,
private or NGO). These can also include fixed locations and/or mobile operations
offering routine and/or regularly scheduled services.
Guiding narrative
questions:
1. Which service delivery points within supported facilities are providing integrated
family planning services to people living with HIV or those at risk of acquiring HIV?
(e.g., HIV prevention, HTS, C&T, PMTCT, KP, etc.)
2. What contraceptive services or methods are provided on site, and which
contraceptive methods are provided through referral? Is there a tracking mechanism
to ensure referrals are completed (e.g. that the client received the service)?
3. How do you ensure the quality of FP services offered at the site?

Knowing Your
HIV Status
Indicators
56
HTS_TST (including HTS_TST_POS)
Description:
Number of individuals who received HIV Testing Services (HTS) and received their test
results
Numerator:
Number of individuals who received HIV
Testing Services (HTS) and received
their test results
The numerator captures the number of
individuals who received HIV Testing Services
(HTS) and received their test results. At a
minimum, this means the person was tested
for HIV and received their HIV test results.
Denominator:
N/A
Changes in indicator:
• Age/sex disaggregates updated (MER 2.0 v2.1 to v2.2).
• HTS community testing modality for home-based testing has been removed (MER
2.0 v2.1 to v2.2).
• Two new HTS facility testing modalities added: STI clinic and emergency department
(MER 2.0 v2.1 to v2.2).
• Clarifying language added for Key Populations disaggregation the notes that KP
should be counted in only one KP group to avoid double-counting. More information
is provided below (MER 2.0 v2.1 to v2.2).
How to use:
This indicator is intended to monitor trends in the uptake of HTS (regardless of the
service delivery modality and population group) within a country.
The disaggregation by test result provides information about the proportion of persons
testing HIV positive and the effectiveness of HTS programs in identifying people living
with HIV (PLHIV) over time.
Further disaggregations are intended to monitor access to and uptake of HTS by
population (age, sex, and test result), HTS setting and service delivery modality. The
findings can support national governments and PEPFAR programs to determine the
coverage and identify gaps in HTS services. These data may also be useful for projecting
programmatic commodities and system needs such as HIV test kits and other staffing
resources, although the numerator reflects the number of individuals tested, not the
number of tests performed.
How to collect:
Existing HTS registers, log books, and reporting forms already in use to capture HTS can
be revised to include the updated disaggregation categories. Examples of data collection
forms include client intake forms, activity report forms, or health registers such as HTS
registers, health information systems and non-governmental organization records. Data
for the numerator should be generated by counting the total number of individuals who
received HTS and their test results.
Note: Although several other MER indicators (see below) may report on the HIV status
of individuals, actual testing of individuals must be reported under HTS_TST. Thus, any
persons who are newly tested as part of the programs linked to the indicators listed
below (i.e., PMTCT, TB, VMMC, Prevention services) must be reported as part of the
HTS_TST indicator.
• PMTCT_STAT
• TB_STAT
• VMMC_CIRC
• PP_PREV
• KP_PREV
• OVC_HIVSTAT
KNOWING HIV STATUS
57
For an individual to be counted under this indicator, that individual’s HIV diagnosis must
be confirmed using a nationally validated testing algorithm. For example, an HIV-positive
rapid HIV test performed at the community- or facility- level must be confirmed with a
second test, which may be performed at the same site or at a different facility. If the
confirmatory test is performed at a different facility, then this may entail follow-up by
implementing partners to confirm the diagnosis before reporting on this indicator.
Note: Serologic testing of pediatric patients should be counted under HTS_TST.
However, HIV virologic testing of HIV-exposed infants should be counted under
PMTCT_EID and PMTCT_HEI_POS.
For children <1, only if serologic tests are used for diagnostic purposes should they be
reported under HTS_TST. Serologic tests for screening infants should be excluded
(including tests to look for HIV exposure at age 9 months or another time point). Since
diagnosis of HIV infection in infants is based on virologic and not serologic tests, the
general expectation is not to see results in the “< 1” disaggregate of the HTS_TST
indicator. However, if the partner/program uses serologic-based testing to confirm
absence of HIV infection in infants <1-year-old who have not breastfed for at least 3
months prior to testing, you may use the HTS_TST <1 indicator to report negative
diagnostic results for such cases.
Note: Retesting for verification of HIV positive status before or at antiretroviral (ART)
initiation should not be counted under HTS_TST, since testing of this individual will
have already been counted at the point of the initial diagnosis. Retesting for verification
is primarily done as a quality assurance activity to avoid misdiagnosis and to ensure
those initiated on ART are indeed HIV positive. Therefore, retesting for verification
should only be performed for persons who have received an HIV diagnosis but have not
yet been initiated on ART.
While verification testing should not be recorded as HTS_TST or HTS_TST_POS, these
data should nevertheless be tracked and rates of discordancy monitored.
Key Populations:
Provision of information (tested, tested positive, tested negative) on key Populations
(FSW, MSM, Transgender people, PWID, and people in prisons and other closed settings)
who were tested and received their results should be reported here. Importantly,
reporting on this disaggregate is optional.
Key population disaggregation* see Appendix 1 to support the identification of key
populations at HTS service delivery. However, reporting of key population
disaggregation should be consistent with what is described under the KP_PREV “How to
review for data quality” section on mutual exclusivity of an individual who falls under
multiple KP categories (e.g., FSW who injects drugs). In such instances, the individual
should only be reported in ONE KP disaggregation category with which s/he is most
identifies in order to avoid double-counting.
Note: Both KP-specific and clinical partners have the option to complete these disaggs,
but only if it is safe to maintain these files and report. Age and sex data on KPs tested
and receiving their results will not be reported—these disaggregates are separate and
distinct from disaggregates for male/female. Please refer to the KP_PREV and PP_PREV
indicator reference sheets for more information on working with KPs.
58
The first priority of data collection and reporting of HTS among key populations must be
to do no harm. These data must be managed confidentially to ensure the identities of
individuals are protected and to prevent further stigma and discrimination of key
populations.
Please also note the misalignment of reporting frequency between HTS_TST [quarterly]
and KP_PREV [semi-annually] and the differences in the process of de-duplication of
individuals (HTS_TST is de-duplicated within the quarter, whereas KP_PREV is de-
duplicated within the fiscal year). For example, if a KP is reached and tested more than
once within the fiscal year, s/he will only be counted once under KP_PREV, but could be
counted multiple times under HTS_TST KP disaggregation during same the fiscal year if
the KP was tested multiple times in different quarters. However, if a KP is tested
multiple times within the same quarter, s/he should be deduplicated (i.e., only be
counted once in the quarter). Please be cognizant of such limitations when interpreting
KP_PREV, HTS_TST, and HTS_TST_POS cascade data by key populations.
Data Systems and Tools
When developing or modifying existing M&E systems and tools to collect and report on
this indicator, the following information should be considered (* designates data
elements that are required for HTS_TST reporting in DATIM):
1. This indicator counts the number of individuals tested not the number of tests
conducted. All efforts should be made to ensure data are collected on individuals
tested vs. number of tests conducted through de-duplication. Within HTS registers,
collecting data on the following variables should be considered to help in these
efforts:
a) Retesting status: new tester, re-tester (i.e., tested in the last 3 months),
retesting to verify an HIV-positive diagnosis before ART initiation
b) HIV testing services - *HIV test results, date of HIV test, receipt of HIV test
results, previously tested during the reporting period
c) Demographic - Client’s Unique ID, name, *sex, and *age at time of HTS services
d) Date HIV-positive individual was linked to treatment
e) Site - *site name and ID, district, region, province, and *service delivery
modality
2. Using unique identifiers for individuals is one way to account for retesting and
avoid double reporting if electronic systems are available to easily link data
through these unique identifiers. Another approach is to record information about
prior testing on the HTS client register.
3. For an individual to be counted under this indicator, their HIV diagnosis must be
confirmed using a nationally validated testing algorithm. For example, an HIV-
positive rapid HIV test performed at the community- or facility- level must be
confirmed with a second test, which may be performed at the same site or at a
different facility. If the confirmatory test is performed at a different facility, then
this may entail follow-up by implementing partners to confirm the diagnosis before
reporting on this indicator.
4. Note: Retesting for verification of HIV positive status before or at antiretroviral
(ART) treatment initiation is only done for persons who have already been
diagnosed HIV-positive as per the national HIV testing guidelines. All clients
diagnosed HIV-positive should be retested for verification before or at ART
initiation with a new specimen and preferably a second operator using the same
national HIV testing strategy. Retesting for verification is primarily done as a quality
assurance activity to avoid misdiagnosis and to ensure those initiated on ART and
treatment services are indeed HIV positive. Thus, HIV testing conducted to verify
KNOWING HIV STATUS
59
status should not be counted under HTS_TST, since their initial HIV diagnosis will
have already been counted at the point of the initial receipt of the HIV diagnosis (as
per the national HIV testing guidelines).
5. Patient level Deduplication: adding “has patient been tested in the last 3 months”
to the HTS facility and community registers can help partners de-duplicate at the
reporting level.
Reporting level:
Facility & Community
How often to report:
Quarterly
How to review for
data quality:
Only one age disaggregation type is used for age/sex/test result received: The number of
individuals newly receiving ART must be disaggregated by age and sex. If possible, the
full age/sex disaggregations should be used. If the full age/sex disaggregations are not
possible, then, and only then, should the aggregated age/sex disaggregations be used.
Do NOT complete both age/sex disaggregations.
Numerator ≥ subtotal of each disaggregate group: The total number of individuals
receiving HTS (numerator) should be equal to the sum of each individual disaggregation
group (age/sex/test result/service delivery modality). If the sum of each individual
disaggregation group (age/sex/test result/service delivery modality) is greater than the
total number of individuals receiving HTS (numerator), then there were more individuals
entered for the disaggregations than for the overall number of individuals receiving HTS.
This should be corrected. If the sum of each individual disaggregation group
(age/sex/test result/service delivery modality) is less than the total number of
individuals receiving HTS, then some data are missing for the disaggregations. This
should also be corrected.
How to calculate
annual total:
Sum results across quarters.
Data elements
(components of
indicator):
Numerator:
Number of
individuals who
received HIV
Testing Services
(HTS) and
received their test
results
Disaggregate Groups
Disaggregates
Age/Sex/Result/HTS Modality
(Community-Level HTS
Reporting)
[Required]
• Index (Positive/Negative):
<1, 1-9, 10-14 M, 10-14 F,
15-19 M, 15_19 F, 20-24 M,
20-24 F, 25-29 M, 25-29 F,
30-34 M, 30-34 F, 35-39 M,
35-39 F, 40-49 M, 40-49 F,
50+ M, 50+ F;
• Mobile (Positive/Negative):
<1, 1-9, 10-14 M, 10-14 F,
15-19 M, 15_19 F, 20-24 M,
20-24 F, 25-29 M, 25-29 F,
30-34 M, 30-34 F, 35-39 M,
35-39 F, 40-49 M, 40-49 F,
50+ M, 50+ F;
• VCT (Positive/Negative): <1,
1-9, 10-14 M, 10-14 F, 15-19
M, 15_19 F, 20-24 M, 20-24
F, 25-29 M, 25-29 F, 30-34
M, 30-34 F, 35-39 M, 35-39
F, 40-49 M, 40-49 F, 50+ M,
50+ F;
• Other Community Testing
Platform: <1, 1-9, 10-14 M,
10-14 F, 15-19 M, 15_19 F,
60
20-24 M, 20-24 F, 25-29 M,
25-29 F, 30-34 M, 30-34 F,
35-39 M, 35-39 F, 40-49 M,
40-49 F, 50+ M, 50+ F
Age/Sex/Result/HTS Modality
(Facility-Level HTS Reporting)
[Required]
• Index (Positive/Negative):
<1, 1-9, 10-14 M, 10-14 F,
15-19 M, 15_19 F, 20-24 M,
20-24 F, 25-29 M, 25-29 F,
30-34 M, 30-34 F, 35-39 M,
35-39 F, 40-49 M, 40-49 F,
50+ M, 50+ F;
• STI (Positive/Negative): <1,
1-9, 10-14 M, 10-14 F, 15-19
M, 15_19 F, 20-24 M, 20-24
F, 25-29 M, 25-29 F, 30-34
M, 30-34 F, 35-39 M, 35-39
F, 40-49 M, 40-49 F, 50+ M,
50+ F;
• Inpatient
(Positive/Negative): <1, 1-9,
10-14 M, 10-14 F, 15-19 M,
15_19 F, 20-24 M, 20-24 F,
25-29 M, 25-29 F, 30-34 M,
30-34 F, 35-39 M, 35-39 F,
40-49 M, 40-49 F, 50+ M,
50+ F;
• Emergency
(Positive/Negative): <1, 1-9,
10-14 M, 10-14 F, 15-19 M,
15_19 F, 20-24 M, 20-24 F,
25-29 M, 25-29 F, 30-34 M,
30-34 F, 35-39 M, 35-39 F,
40-49 M, 40-49 F, 50+ M,
50+ F;
• VCT (Positive/Negative: <1,
1-9, 10-14 M, 10-14 F, 15-19
M, 15_19 F, 20-24 M, 20-24
F, 25-29 M, 25-29 F, 30-34
M, 30-34 F, 35-39 M, 35-39
F, 40-49 M, 40-49 F, 50+ M,
50+ F;
• TB (Positive/Negative): <1, 1-
9, 10-14 M, 10-14 F, 15-19
M, 15_19 F, 20-24 M, 20-24
F, 25-29 M, 25-29 F, 30-34
M, 30-34 F, 35-39 M, 35-39
F, 40-49 M, 40-49 F, 50+ M,
50+ F;
• VMMC (Positive/Negative):
<1, 1-9, 10-14 M, 15-19 M,
20-24 M, 25-29 M, 30-34 M,
35-39 M, 40-49 M, 50+ M;
KNOWING HIV STATUS
61
• PMTCT [ANC Only]
(Positive/Negative): <1, 1-9,
10-14 F, 15-19 F, 20-24 F, 25-
29 F, 30-34 F, 35-39 F, 40-49
F, 50+ F;
• Pediatric (Positive/Negative):
<5
• Malnutrition
(Positive/Negative): <5
• Other PITC
(Positive/Negative): <1, 1-9,
10-14 M, 10-14 F, 15-19 M,
15_19 F, 20-24 M, 20-24 F,
25-29 M, 25-29 F, 30-34 M,
30-34 F, 35-39 M, 35-39 F,
40-49 M, 40-49 F, 50+ M,
50+ F
Key Population by Result
[Optional]
• People who inject drugs
(PWID): Negative, Positive
• Men who have sex with men
(MSM): Negative, Positive
• Transgender people (TG):
Negative, Positive
• Female sex workers (FSW):
Negative, Positive
• People in prison and other
closed settings: Negative,
Positive
Disaggregate Descriptions & Definitions
Disaggregates: Service Delivery Modality
In addition to reporting the total number of individuals tested and receiving their test
results and the total type of test results received (negative, positive), HTS_TST data
should be disaggregated by service delivery modality, and then also by age/sex/test
result within each service delivery modality. Service delivery modalities can reflect a
reason for testing (index partner, STI), as well as, the location/place of testing (e.g.,
inpatient ward, VCT drop-in center). Therefore, please use a hierarchical approach to
determine the appropriate modality, by prioritizing the reason for testing followed by
the location/place of testing.
Service delivery modalities are defined as:
Community-based testing: Applies to any testing done outside of a designated health
facility. Within community-based testing, the following disaggregates are available:
a. Index: Index testing, also referred to as partner testing/partner notification
services, is an approach whereby the exposed contacts (i.e., spouse, sexual
partners, biological children and needle-sharing partners) of an HIV-positive
person, known as the index case, are elicited and offered HIV testing services.
The Index modality is used to define testing of contacts who have been exposed
to HIV through an index case. These contacts include: sexual partners, needle-
sharing partners, and biological children of female index cases. Testing of
persons who have not had exposure through an index case, such as neighbors or
62
family members not born to the index, should not be reported under the Index
modality. Instead, these individuals should be counted under “other community
platforms”. While testing the contacts of an index case may occur in mobile, VCT
or other community testing venue, this testing should be reported under the
index modality, which takes precedence over the other service delivery
modalities. That is, if an individual could be reported under both index testing
and another modality, that individual should only be reported once under index
testing.
b. Mobile: Testing in Mobile ad hoc or temporary testing locations, such as
community centers, schools, workplaces, and includes testing in mobile unit such
as tents and vans. Testing related to VMMC services is not included here. Instead
that should be reported under facility based VMMC modality.
c. VCT: Includes testing conducted in standalone VCT center that exists outside of a
designated health facility (e.g., drop-in-center, wellness clinic where HTS services
are provided, testing sites aimed at key populations, etc.).
d. Other community platforms: Includes all community-based modalities not
captured above (e.g., ad hoc testing campaign that does not satisfy the mobile
testing definition) and community-based OVC testing) should be entered under
this modality.
Facility-based testing: Applies to any testing occurring inside a designated health facility.
Within the facility-based testing, the following disaggregates are available:
a. Index: Index testing, also referred to as partner testing/partner notification
services, is an approach whereby exposed contacts (i.e., spouse, sexual partners,
biological children, and needle-sharing partners) of an HIV-positive person,
known as the index case, are elicited and offered HIV testing services. The Index
modality is used to define testing of contacts who have been exposed to HIV
through an index case. These contacts include: sexual partners, needle-sharing
partners, and biological children of female index cases. Testing of persons who
have not had exposure through an index case (i.e., non-exposed contacts), such
as neighbors or family members not born to an index case, should not be
reported under the Index modality. If these non-exposed contacts come to a
facility for an HIV test, their results should be reported under the “VCT”
modality. Index testing in a facility-based setting (testing the exposed contacts
of an index case) can occur in a variety of service delivery points within a facility
(e.g., TB, VCT, inpatient, etc.). However, all index-based testing should be
reported using the Index modality, which takes precedence over all the other
service delivery modalities That is, if an individual could be reported under both
index testing and another modality, that individual should only be reported once
under index testing
b. Provider Initiated Counseling and Testing (PITC):
i. Malnutrition: Clinics and inpatient wards predominately dedicated to the
treatment of malnourished children. While this service delivery modality
may be part of either inpatient or outpatient services, if an individual could
be reported under both malnutrition and another service delivery modality,
report an individual only once and under malnutrition. However, the
biological children of female index cases should be classified under the
Index testing modality.
ii. Pediatrics: Includes Provider Initiated Counseling and Testing offered to
children under 14 years of age at any service delivery modality within the
health facility (e.g., under 5/EPI clinic (immunization or well child services),
pediatric inpatient wards, etc.). This does not include virologic testing,
KNOWING HIV STATUS
63
which is reported under PMTCT_EID, nor rapid HIV testing used to identify
HIV exposed infants. This modality should also not include children of index
cases who should be classified under the Index modality or malnourished
children who should be classified under Malnutrition.
iii. Inpatient: Includes Provider Initiated Testing & Counseling (PITC) occurring
among those patients admitted in the inpatient and surgery wards.
iv. Emergency: Includes persons tested or seen in a designated emergency
department or ward for the immediate care and treatment of an
unforeseen illness or injury.
v. TB: Includes persons referred for HIV testing because they are a confirmed
or a presumptive TB case. HIV testing may have taken place in a TB clinic, a
co-located VCT or other setting. However, if the reason for the HIV test is
that the client is a TB case or a TB suspect, then it should be classified
under the TB modality. Refer to TB_STAT for guidelines on data collection
for TB.
vi. STI: Includes persons seen in a designated STI clinic as well as patients seen
in the OPD for STI symptoms. This includes suspect and confirmed STI
cases. HIV testing may take place in an STI clinic, an OPD, a co-located VCT
or other setting. However, if the reason for the HIV testing is the individual
is either a suspect or confirmed STI case, then the test should be reported
under the STI modality.
vii. PMTCT (ANC Only): Pregnant women newly tested at antenatal care clinic
(ANC) ANC setting (who would also be reported under PMTCT_STAT)
should be reported under HTS_TST in the facility-based modality of PMTCT
(ANC only). HIV testing for pregnant women as part of the PMTCT program
at antenatal care clinics (ANC) to align with PMTCT_STAT. Refer to
PMTCT_STAT reference sheet for guidelines on data collection. Individuals
counted under PMTCT_STAT who already knew their status should not be
reported under HTS_TST. If a woman is newly tested at a different service
delivery point other than ANC (e.g., labor and delivery, family planning
clinics, etc.), results should be reported under the appropriate facility-
based HTS modality (inpatient, PITC-other, etc.) and not under the PMTCT
(ANC Only) disaggregate and not under PMTCT_STAT. Please note in the
HTS narrative which modality you are using to report new tests at L&D and
any postnatal care (e.g., in-patient, PITC-other).
viii. Other PITC: This includes any other provider-initiated testing and
counseling that is not captured in one of the other testing modalities listed
above. For reporting purposes, this includes testing of patients triaged to
other clinics within the OPD that see patients for routine/chronic care (i.e.,
eye, dental, dermatology, diabetes, etc.). This does not include patients
seen in the OPD for emergency care or an STI. Those patients should be
classified under the emergency and STI modalities, respectively.
c. VMMC: This modality includes HIV testing for males conducted as part of VMMC
programs in both facility and mobile outreach programs. Testing is
recommended through the VMMC program, although not mandatory. Refer to
VMMC_CIRC for guidelines on data collection for VMMC.
d. VCT: Refers to a clinic specifically intended for HIV testing services that is co-
located within a broader health care facility. Use this modality for VCT walk-ins,
client-initiated HIV testing, and clients who have been previously mobilized to
get an HIV test. This should not include testing of patients referred by providers
from other clinical services within the facility (TB, ANC, Inpatient, emergency,
etc.). Even though the actual test may be administered in the VCT clinic, report
64
those individuals under the serviced delivery modality from which they were
referred. This modality should also not include testing of exposed partners and
exposed family members of an index case, who should be reported under the
Index disaggregate.
PEPFAR-support
definition:
Standard definition of DSD and TA-SDI used.
For HTS services, direct service delivery includes: ongoing procurement of critical HTS
related commodities such as rapid HIV test kits or requisite materials (lancets, capillary
tubes), samples and materials for proficiency testing, other HIV diagnostic commodities,
or funding for salaries of HIV testing service providers including counselors, laboratory
technicians, program managers, and/or community health workers. Staff who are
responsible for the completeness and quality of routine patient records (paper or
electronic) can be counted here; however, staff who exclusively fulfill MOH and donor
reporting requirements cannot be counted.
For HTS services, ongoing support for service delivery improvement includes: clinical
mentoring/supportive supervision, HTS training, HTS guidance development,
infrastructure/renovation of facilities (fixed, mobile, and outreach sites), site level
QI/QA, routine support of HTS M&E and reporting, or HIV test kits consumption
forecasting and supply management.
Guiding narrative
questions:
1. Please describe and/or specify any processes or data available for determining rates
of retesting (not including verification testing) of both HIV positives and negatives.
2. Please describe processes/methods and/or quantify any estimation of linkage to
treatment from diagnosis.
3. Please describe and/or quantify (proportions retested prior to ART, concordance or
discordance rates) verification testing occurring prior to ART initiation to minimize
misdiagnosis.
4. Please describe processes/methods for capturing new service delivery modalities (STI
and Emergency) and any challenges with accurately capturing these modalities.
KNOWING HIV STATUS
65
HTS_SELF
Description:
Number of individual HIV self-test kits distributed
Numerator:
Number of individual HIV self-test kits
distributed
This indicator aims to monitor trends in the
distribution of HIV self-test kits within a
country at the lowest distribution point.
Denominator:
N/A
Changes in indicator:
• This is a new indicator for MER 2.0 (version 2.2) and OUs are required to report on it
during FY18 if they support the procurement and/or distribution of HIV self-test kits.
How to use:
This is the first MER indicator to monitor PEPFAR programming of HIV self-testing
approaches and distribution HIV self-test kits.
HIV self-testing refers to a process in which a person collects his or her own specimen
(oral fluid or blood), performs an HIV test, and then interprets the results. This is often
done in a private setting, either alone or with a trusted person. HIV self-testing is a
screening test and requires self-testers with a reactive (preliminary positive) result to
receive further testing from a trained provider using a validated national testing
algorithm. HIV self-testing approaches range from unassisted self-testing (with limited or
no instruction provided) to directly assisted self-testing (where a testing provider
demonstrates how to use the self-test kit). Self-test kits can be distributed in various
ways (i.e., by providers or outreach workers, over-the-counter, etc.). Secondary
distribution of HIV self-test kits may also occur (e.g., to partners of ANC attendees, or
clients of FSWs)
This indicator aims to monitor trends in the distribution of HIV self-test kits within a
country at the lowest distribution point (i.e., between the distributer and the intended
user(s)/recipient). The implementation of HIV self-testing programs should facilitate and
enhance access to and uptake of HIV testing services for populations where HIV test
uptake is low and undiagnosed HIV infection is high (i.e., men, adolescents/young adults,
and key populations).
How to collect:
The suggested data source is a (newly developed) HIVST (HIV self-test) register or
logbook. This will minimize any potential confusion with HTS_TST data collection and
reporting since HIV self-testing is only a screening test and should not be reported under
HTS_TST which only includes diagnostic testing. If a standalone HIVST register or logbook
is not possible, revise existing HTS registers, log books, and reporting forms already in
use to include very clear labels to indicate self-testing to prevent information entered in
an HTS register from being counted and reported under HTS_TST or HTS_TST_POS.
Note that one individual can receive multiple self-test kits (e.g., one for themselves and
one for their partner or partners). Data for the numerator should be generated by
counting the number of individual HIV self-test kits distributed and NOT the number of
individuals receiving an HIV self-test kit. Number of self-test kits distributed should be
captured and reported at the lowest distribution point. The lowest distribution point
refers to the individual/site giving out self-test kits and capturing data for monitoring
purposes. This is to prevent double counting between the various higher supply chain
levels.
For example, the central warehouse distributes 500 self-test kits to an implementing
partner doing outreach for KPs. The implementing partner gives their peer outreach
workers a total of 50 self-test kits to give out during an outreach event. The outreach
66
workers return from their event having given out 30 self-test kits. In this scenario, the
lowest distribution point would be the outreach workers who are capturing the
monitoring data. Therefore, the number of tests kits distributed would be 30. Each of
these lowest distribution counts should be rolled up (aggregated) to create the
numerator for this indicator.
The disaggregation by type of self-testing provides information about the proportion of
test kits distributed through each model (i.e., directly assisted vs. unassisted self-
testing). Further disaggregation by “number of tests distributed to a person by age/sex”
(for both directly assisted and unassisted self-testing) and “test kit distributed for use
by” (for unassisted self-testing) can provide information about what subpopulations are
receiving HIVST kits and who the test kit is intended for use by (i.e., self, sex partner,
other) in the unassisted model. The findings can support national government and
PEPFAR programs to assess how efficient different distribution approaches are at
reaching target populations. These data may also be useful for projecting programmatic
commodities (e.g., self-test kits) and systems needs (e.g., staffing resources). It is
important to note that for the purposes of this indicator, it is assumed that the tests
distributed to individuals and counted in the directly assisted self-testing model are the
used by individuals that received them so the disaggregation for “test kit distributed for
use by” is not requested in the directly assisted model. Please refer to the example
clarification below for additional details.
For example, if an 18-year-old female reports to a testing site and receives a one-on-one
testing demonstration for herself – the test for herself will be reported as directly
assisted and you would provide the age/sex disaggregation data for one test kit
distributed in the 15-19-year-old age band. When she leaves the clinic, she takes two
additional test kits along with her: one for her sex partner and one for her friend to use
at a later time. The two test kits for her sex partner and friend would be counted as
unassisted. For the age/sex breakdown under unassisted, 2 tests would go in the 15-19-
year-old female age band because two tests were distributed to the female in that age
band. The reporting follows the distribution of the test kits and not the age/sex
demographics of the end user of the self-test kit. For the “test kit distributed for use
by” disaggregate, you would indicate a ‘1’ in the ‘sex partner’ disaggregate for the test
she planned to distribute to her sex partner and a ‘1’ in the ‘other’ disaggregate for the
test she planned to distribute to her friend.
It is understood that registers and procedures for HIVST are still relatively new in many
PEPFAR countries and specific distribution methods (e.g., vending machines) may not
always allow for collection of detailed data on self-test kit distribution. As such, the only
required disaggregate for this indicator will be for the type of self-testing (i.e., directly-
assisted vs. unassisted). In addition, age/sex demographic information for test kits
distributed using the directly-assisted self-testing model will also be required as these
individuals should have received an in-person HIV test kit demonstration and
demographic information should be collected at that time
Note: Although not required, implementing partners should attempt to document and
report information about actual use of self-test kits. This includes who used the test kit,
the test result from the self-test and linkage to retesting (if result is reactive),
particularly when directly assisted HIVST occurs. Methods used may include request the
return of the kits or follow up calls to determine outcomes. This information can further
inform whether HIVST services are reaching individuals who may be HIV-positive and if
those individuals are retesting to confirm their diagnosis.
KNOWING HIV STATUS
67
For more information on HIV self-testing, please refer to the “WHO Guidelines on HIV
Self-Testing and Partner Notification” released in December 2016. To review a
repository of country-specific guidance and polices related to HIV self-testing, please
visit the HIV Self-Testing Research and Policy Hub.
Reporting level:
Facility & Community
How often to report:
Quarterly
How to review for
data quality:
Data should be reviewed regularly for the purposes of program management, to monitor
progress towards achieving targets, and to identify and correct any data quality issues.
For example, the number of test kits distributed should not be greater than the number
of test kits a provider allocated during the reporting period. Pay careful attention to the
number of HIVST kits distributed at pharmacies and online.
Implementing partners should review their data to ensure that HTS_SELF is not
reported under HTS_TST (or HTS_TST_POS) results. Further, data should be reviewed
to ensure the numerator does not include the number of HIV self-tests performed or
used, nor does it reflect a definitive diagnosis (which would be reported under HTS_TST).
The “directly-assisted” disaggregate should be reviewed to see if additional information
was collected related to: 1) test result (negative or reactive) and 2) linkage for repeat
testing to confirm a reactive self-test result. While not required for this indicator, this
information should be collected by implementing partners as part of routine program
monitoring.
How to calculate
annual total:
Sum results across quarters.
Data elements
(components of
indicator):
Numerator:
Number of
individual HIV self-
test kits
distributed
Disaggregate Groups
Disaggregates
Type of self-testing [Required]
Directly-assisted; Unassisted
Number of Test Kits Distributed
to a Person by Age/Sex
[Required for Directly Assisted;
Optional for Unassisted]
• Directly-assisted self-testing
by: 10-14 M, 10-14 F, 15-19
M, 15_19 F, 20-24 M, 20-24
F, 25-29 M, 25-29 F, 30-34 M,
30-34 F, 35-39 M, 35-39 F,
40-49 M, 40-49 F, 50+ M, 50+
F;
• Unassisted self-testing by:
10-14 M, 10-14 F, 15-19 M,
15_19 F, 20-24 M, 20-24 F,
25-29 M, 25-29 F, 30-34 M,
30-34 F, 35-39 M, 35-39 F,
40-49 M, 40-49 F, 50+ M, 50+
F
Disaggregate: Number of Test
Kits Distributed to Key
Populations
[Optional for both Directly
Assisted and Unassisted]
• People who inject drugs
(PWID): Directly-assisted,
Unassisted
• Men who have sex with men
(MSM): Directly-assisted,
Unassisted
• Transgender people (TG):
Directly-assisted, Unassisted
• Female sex workers (FSW):
Directly-assisted, Unassisted
68
• People in prison and other
closed settings: Directly-
assisted, Unassisted
Disaggregate: Test kit
distributed for use by
[For Unassisted Only; Reporting
Optional if data are available]
Unassisted self-testing by:
Self, Sex Partner, Other
Disaggregate Descriptions & Definitions
Type of self-testing:
• Directly assisted HIVST refers to trained providers or peers giving individuals an in-
person demonstration before or during HIVST of how to perform the test and
interpret the test result (WHO, 2016).
• Unassisted HIVST refers to when individuals self-test for HIV and only use an HIVST kit
with manufacturer-provided instructions for use. In addition to reporting the total
number of HIV self-test kits distributed to individuals, the HTS_SELF indicator includes
several disaggregates to characterize aspects of distribution (WHO, 2016).
Test kit distributed for use by [For Unassisted Only; Reporting]:
• Self: Individual that HIV self-test kit was distributed to intends to use the test kit on
him- or herself.
• Sex Partner: Individual that HIV self-test kit was distributed to plans to further
distribute the self-test kit for use on his or her sexual partner(s).
• Other: Individual that HIV self-test kit was distributed to plans to further distribute
the test kit to an individual that is not themselves or one of their sex partners (e.g.,
relative, friend)
PEPFAR-support
definition:
Standard definition of DSD and TA-SDI used.
Provision of key staff or commodities for the distribution of HIVST kits includes: ongoing
procurement of HIVST kits or funding for salaries of providers who distribute or directly
assist with HIVST including counselors, laboratory technicians, program managers, and
community health workers. Staff who are responsible for the completeness and quality
of routine patient records (paper or electronic) can be counted here; however, staff who
exclusively fulfill MOH and donor reporting requirements cannot be counted.
For HIVST, ongoing support for service delivery improvement includes: clinical
mentoring/supportive supervision, HIVST training, HIVST guidance development, site
level QI/QA, routine support of HIVST M&E and reporting, or HIVST kit consumption
forecasting and supply management.
Guiding narrative
questions:
1. Please describe process/methods and challenges for tracking distribution of test kits.
2. Please describe process/methods and challenges for tracking use of self-test kits.
3. Please describe process/methods and challenges for tracking linkage of individuals
for repeat testing to confirm a reactive self-test result.
KNOWING HIV STATUS
69
PMTCT_STAT (including PMTCT_STAT_POS)
Description:
Percentage of pregnant women with known HIV status at antenatal care (includes those
who already knew their HIV status prior to ANC)
Numerator:
Number of pregnant women with
known HIV status at first antenatal care
visit (ANC1) (includes those who already
knew their HIV status prior to ANC1)
The numerator is the sum of the following
two data elements:
1) The number of women with a previously
known HIV status (both known HIV
positive and known negative) attending
their first ANC visit (ANC1) for a new
pregnancy over the last reporting period.
2) The number of women attending ANC1
who were tested for HIV and received
results (These women should also be
counted in the general HTS indicator
“HTS_TST”)
Denominator:
Number of new ANC clients in reporting
period
N/A
Changes in indicator:
• Collected at only antenatal care (ANC) sites to better align with upcoming 2016
WHO Consolidated ARV guidelines, reduce burden on data collection, and improve
data quality. No longer collected at L&D. This change is to improve data quality by
aligning with the PMTCT_STAT denominator number of new ANC clients in the
reporting period (MER 1.0 to 2.0).
• Newly tested negative was added as a disaggregate to improve calculated yield
(MER 1.0 to 2.0).
• Language clarified that the collection of this indicator is at the first ANC visit (ANC1)
of the pregnancy reduces the risk of double counting pregnant women who could be
tested multiple times during pregnancy (MER 2.0 v2.1 to v2.2).
• Age disaggregates updated (MER 2.0 v2.1 to v2.2).
How to use:
Track progress toward ensuring that all pregnant women who attend PEPFAR supported
antenatal care (ANC) know their HIV status and are initiated on ART.
How to collect:
The data source is the ANC register. There is a risk of double counting as a pregnant
woman could be tested multiple times during one pregnancy therefore partners should
ensure a data collection and reporting system is in place to minimize double counting
including a longitudinal ANC register (meaning a register that is able to record all
information about one pregnancy in one location, with rows or columns that allow for
recording information on multiple visits during that pregnancy). There is also a risk of
undercounting if those women who already knew their HIV status prior to attending ANC
are not documented, therefore the ANC register should at a minimum should document
both “previously known positive” and “newly tested positive”. Finally, “known negative”
(i.e., women who tested HIV negative prior to current pregnancy) is not reported in
DATIM however it may be appropriate to report “known negative” women as part of the
numerator if: 1) National guidelines do not require retesting women known to be HIV
negative (often women tested in the last 3 months, however exact timing depends on
local guidelines) and 2) ANC registers and reporting systems only capture 1st month or
1st ANC visit.
(As this is a status indicator and not a testing indicator - These women should also be
counted in the general HTS indicator “HTS_TST” PMTCT (ANC Only) service delivery
modality).
70
Women who are newly tested at a different service delivery point (e.g., labor and
delivery (L&D), postnatal clinics, family planning clinics, etc.) should be reported under
the appropriate facility-based HTS modality (inpatient, PITC-other, etc.). If there have
been changes in the MER modality under which L&D and postnatal client testing has
been reported over time, which would affect interpretation of data trends, please note
this in both USG and IM-level narratives under both HTS and PMTCT_STAT.
Reporting level:
Facility
How often to report:
Quarterly
How to review for
data quality:
The % should never be above 100% at a site, and therefore review of the method of data
collection and correction of any errors at sites with greater than 100% coverage is
important to ensuring data quality for this indicator.
Retesting of HIV-negative women during pregnancy, at L&D and through the postpartum
period is an important program strategy, but is not captured in the PMTCT_STAT
indicator. Country teams should collect this data at the country level if it is pertinent to
their country’s epidemic, especially in high HIV burden settings and where there are
concerns of ongoing transmission during the pregnancy and postpartum period.
How to calculate
annual total:
Assuming site level records avoid double counting (as described above) across the
annual reporting cycle, sum numerator and denominator across all reporting periods for
the annual result.
Data elements
(components of
indicator):
Numerator:
Number of
pregnant women
with known HIV
status at first
antenatal care
visit (ANC1)
(includes those
who already knew
their HIV status
prior to ANC)
Disaggregate Groups
Disaggregates
Age
[Required]
Unknown age, <10, 10-14, 15-
19, 20-24, 25-29, 30-34, 35-39,
40-49, 50+
Status and Age
[Required]
• Known Positives: Unknown
age, <10, 10-14, 15-19, 20-24,
25-29, 30-34, 35-39, 40-49,
50+
• Newly Tested Positives:
Unknown age, <10, 10-14, 15-
19, 20-24, 25-29, 30-34, 35-
39, 40-49, 50+
• New Negatives: Unknown
age, <10, 10-14, 15-19, 20-24,
25-29, 30-34, 35-39, 40-49,
50+
Denominator:
Number of new
ANC clients in
reporting period
Disaggregate Groups
Disaggregates
Age
[Required]
Unknown age, <10, 10-14, 15-
19, 20-24, 25-29, 30-34, 35-39,
40-49, 50+
Disaggregate Descriptions & Definitions
Status and Age:
• Known Positive at entry: Number of pregnant women attending ANC for a new
pregnancy who were tested and confirmed HIV-positive at any point prior to the
current pregnancy should be reported as known positive at entry. Pregnant women
with known HIV status attending ANC for a new pregnancy may not need retesting
if they are already on ART, or they may be required to be retesting prior to
initiating ART based on national guidelines. Known positives who are re-tested and
confirmed to be HIV positive prior to initiating ART should still be documented as
known positive at entry.

KNOWING HIV STATUS
71
• Newly tested positive: The number of women attending ANC1 who were tested for
HIV and received a positive result. Women who tested negative prior to this
pregnancy and are tested again at ANC1 for this new pregnancy should be counted
in this indicator. These women should also be counted in the HTS_TST indicator.
• New Negatives: Retesting of HIV-negative women at subsequent ANC visits, L&D,
postnatal clinic or family planning clinic should not be counted in this indicator.
Retesting for verification of positive status prior to initiating ART to reduce
misdiagnosis should not be counted in this indicator.
PEPFAR-support
definition:
Standard definition of DSD and TA-SDI used.
Provision of key staff or commodities for PMTCT include: commodities such as test kits,
ARVs, lab commodities, or funding for salaries of health care workers.
Ongoing support for PMTCT service delivery improvement includes: training of PMTCT
service providers, clinical mentoring and supportive supervision of PTMCT service sites,
infrastructure/renovation of facilities, support for PMTCT service data collection,
reporting, data quality, QI/QA of PMTCT services support, ARV consumption forecasting
and supply management, support of lab clinical monitoring of patients, supporting
patient follow-up/retention, support of mother mentoring programs.
Guiding narrative
questions:
1. Provide context for poor performance in PMTCT_STAT coverage
(Numerator/Denominator = STAT coverage) by geographic area or
partner/implementing mechanism, including any planned activities/remedial actions.
2. For areas where age disaggregates are NOT completely reported, describe challenges
for collecting and/or plan and timeline for collection.
3. PMTCT_STAT is limited to women tested at ANC1 for the current pregnancy. If
additional data is available, provide total # women tested and positive in ANC2 and
beyond, including through labor and delivery and the breastfeeding period (e.g.,
postpartum, MCH settings). This could include women who initially tested negative at
ANC1 or who did not attend ANC. This data may already be reported through MER
HTS modalities, but is not available for review as a specific disaggregate. This will
provide context on quality of care for women and HIV-exposed infants (HEI), and a
better estimate for total HEI.
72
PMTCT_EID
Description:
Percentage of infants born to HIV-positive women who received a first virologic HIV test
(sample collected) by 12 months of age.
This percentage is a proxy measure, since the infants in the numerator could include
infants whose mothers were not included in the PMTCT_STAT denominator.
The numerator is a measure of sample collection for virologic testing. Throughout the
reference guide the term “received a first virologic test” specifically means “had a first
sample collected for virologic testing.” Age refers to age at specimen collection
Numerator:
Number of infants who had a first
virologic HIV test (sample collected) by
12 months of age during the reporting
period
Calculated indicator in DATIM, sum of:
Infants who had a first virologic HIV test
(sample collected) between birth and 2
months of age; Infants who had a first
virologic HIV test (sample collected) between
2 and 12 months of age
Denominator:
PMTCT_STAT_POS (see PMTCT_STAT);
Denominator is no longer collected as
part of indicator, but rather is calculated
as PMTCT_STAT_POS.
Calculated indicator in DATIM, sum of: 1)
Newly Tested Positive, 2) Known Positive at
entry (see PMTCT_STAT, Disaggregate Group
Positivity Status for more details)
Changes in indicator:
• Clarification that reported test is based on infant’s age when the sample was
collected for virologic testing, not based on when sample was sent or result
returned (MER 1.0 to MER 2.0).
• Clarification that 1) PMTCT_STAT_POS is the denominator for this proxy indicator
(MER 1.0 to MER 2.0).
• Infants’ diagnoses through virologic test results (positive, negative, unknown) are no
longer reported within this indicator. Refer to new PMTCT_HEI_POS indicator for
guidance on how to report on infants diagnosed HIV positive as well as confirmation
of their ART initiation (MER 2.0 v2.1 to v2.2).
How to use:
This indicator measures the extent to which HIV-exposed infants receive a first virologic
HIV test to determine their HIV status by 12 months of age. The indicator is
disaggregated by the age of the infant at the time of sample collection, specifically
between birth and 2 months and between 2 and 12 months of age.
Only infants whose samples were collected for the first test for each HIV-exposed infant
should be counted in this indicator, including dried blood spots (DBS) and samples
collected for POC testing (e.g., Alere, Xpert). Even though there is ongoing exposure of
infants to HIV (through breastfeeding), this indicator only measures access to a first test,
and not access to all the recommended HIV tests throughout breastfeeding. HIV status
of infants at the end of the breastfeeding period and the outcomes of the PMTCT
program would be measured in PMTCT_FO.
The positive results of HIV infant virologic testing are collected in a new, separate
indicator in effect for FY18, called PMTCT_HEI_POS. Please see the reference sheet for
PMTCT_HEI_POS for more information, as the definitions for the new indicator are
distinct from PMTCT_EID.
How to collect:
Implementing partners should report on all infants whose samples were collected for a
first virologic test, even if no test result has been recorded in the patient record/register
at the time of reporting.
KNOWING HIV STATUS
73
This indicator should be collected from the clinical source (i.e., HIV-exposed infant
registers or patient records) to ensure unduplicated patient counting. HIV-exposed
infant registers should be used to count exposed infants and samples collected for
virologic testing. (If available, information could come from electronic systems). If the
standard report does not contain all the required information, individual patient files
should be used. Additional supporting information for this indicator can be obtained
from standard laboratory information systems (i.e., DNA PCR or POC/near POC log books
or electronic systems) however, it will be important to ensure that repeat tests of the
same sample or HIV-infected infants receiving a confirmatory virologic HIV test result are
not counted twice.
Only samples collected for a first virologic HIV test should be included in this indicator. A
virologic test is a test used for HIV diagnosis in infants up to 18 months of age. The most
commonly used form of virologic testing or nucleic acid testing (“NAT”) is HIV DNA PCR
on dried blood spots (DBS) but this indicator also includes samples collected for POC
testing. Three other types of testing should not be reported: 1) Serologic testing of
children should not be reported in this indicator. (See HTS_TST for additional details). 2)
Virologic tests conducted with the purpose of confirming the diagnosis of HIV, 3)
Virologic tests used for clinical monitoring of children on ART, such as viral load
quantification Additionally, only the first sample collected should be counted for each
infant, even if they have had more than one virologic test done.
The numerator is divided into first sample collected between birth and 2 months of age
and first sample collected between 2 and 12 months of age. The 0-2 month and 2-12-
month age periods are based on age at collection of sample, not on date of result return
to the facility or caregiver. It is likely that at the time of reporting there will be samples
that have been collected but for which no result is documented in the register or patient
record.
Reporting level:
Facility
How often to report:
Quarterly
How to review for
data quality:
Infant testing coverage (PMTCT_EID / PMTCT_STAT_POS) is a proxy calculation, relying
on PMTCT_STAT_POS as a proxy denominator for the total number of HIV exposed
infants (HEI). Reviewing infants with a first virologic test (N) against PMTCT_STAT_POS
results (D) should be done carefully—see assumptions and limitations below. Review of
outlier percentages for testing coverage by age band is recommended (e.g., review high
and low outliers for 0-≤2-month testing coverage disaggregate).
Assumption: the total number of HIV positive pregnant women, and therefore HEI, does
not significantly vary quarter by quarter. We would not expect all the women reported
under PMTCT_STAT_POS to have given birth to the infants reported under PMTCT_EID.
However, despite that time period mismatch, the assumption is that the total number of
HIV positive women (estimated HEI) does not vary significantly quarter by quarter, so it
is reasonable to compare infants tested to the STAT_POS denominator from the same
reporting time period.
Example Limitations
• PMTCT_STAT_POS could underestimate the number of HEI because it includes
only women who are HIV-positive at ANC1 for the current pregnancy. It does
not include women who attend ANC1 and are HIV+ but are not diagnosed; or
any woman who seroconverts after ANC1, during delivery, or breastfeeding.
74
• PMTCT_STAT_POS could overestimate the number of HEI that should be tested,
because not all pregnancies may come to term.
See the new PMTCT_HEI_POS indicator reference sheet for a description of
considerations and limitations in calculating proxy positivity for HEI (PMTCT_HEI_POS /
PMTCT_EID).
How to calculate
annual total:
Sum results across quarters.
Data elements
(components of
indicator):
Numerator:
Number of infants
who had a first
virologic HIV test
(sample collected)
by 12 months of
age during the
reporting period
Disaggregate Groups
Disaggregates
Infant Test by Age at Sample
Collection
[Required]
• Infants who had a first
virologic test (sample
collected) between birth and
2 months of age (0-≤2mo);
• Infants who had a first
virologic test (sample
collected) between 2 and 12
months of age.
Disaggregate Descriptions & Definitions
Infant Test by Age at Sample Collection: For the numerator to be calculated,
implementing partners are required to report:
• Infants who had a first virologic test (sample collected) between birth and 2
months of age (0-≤2mo): Age at the time the sample is collected should be
reported.
• Infants who had a first virologic test (sample collected) between 2 and 12 months
of age: Age at the time the sample is collected should be reported.
PEPFAR-support
definition:
Standard definition of DSD and TA-SDI used.
Provision of key staff or commodities for PMTCT include: commodities such as test kits,
ARVs including infant prophylaxis, lab commodities, or funding for salaries of health care
workers.
Ongoing support for PMTCT service delivery improvement includes: training of PMTCT
service providers, clinical mentoring and supportive supervision of PTMCT service sites,
infrastructure/renovation of facilities, support for PMTCT service data collection,
reporting, data quality, QI/QA of PMTCT services support, ARV consumption forecasting
and supply management, support of lab clinical monitoring of patients, supporting
patient follow-up/retention, support of mother mentoring programs.
Guiding narrative
questions:
1. Provide context for low EID testing coverage by geographic area or
partner/implementing mechanism, including any planned activities/remedial actions.
For example, PMTCT_EID is lower than previous quarters due to a stock out of DBS
reagent.
2. Provide additional monitoring data related to: turn-around time of virologic test
results back to the facility and results returned to caregiver.
KNOWING HIV STATUS
75
PMTCT_HEI_POS
Description:
Number of HIV-infected infants identified in the reporting period, whose diagnostic
sample was collected by 12 months of age.
This indicator excludes confirmatory testing. It includes 2 required sets of
disaggregations: 1) disaggregation by age for positive infants based on the infant’s age at
specimen collection for virologic testing; 2) Confirmation of ART initiation, also
disaggregated by age at specimen collection.
Numerator:
Number of HIV-infected infants
identified in the reporting period,
whose diagnostic sample was collected
by 12 months of age.
Calculated indicator in DATIM, sum of: HIV-
infected infants whose diagnostic sample was
collected between birth and 2 months of age;
HIV-infected infants whose diagnostic sample
was collected between 2 and 12 months of
age.
Denominator:
N/A
Changes in indicator:
• This is a new indicator for MER 2.0 v2.2.
• Infants’ first virologic test results (positive, negative, and unknown) are no longer
being reported under PMTCT_EID. The total number of positive infants identified
through virologic testing will be collected through the new PMTCT_HEI_POS
indicator, however, the definition for positive infants in the new indicator is
different from the definition for the PMTCT_EID positive infant disaggregate in MER
2.0 (MER 2.0 v2.1 to v2.2).
How to use:
This indicator measures how many HIV-infected infants are identified in a reporting
period, disaggregated by age at sample collection and ART initiation status. Identification
is by virologic HIV testing: DNA PCR testing of dried blood spots (DBS) or point of care
(POC) (e.g., Alere, Xpert) virologic testing. Infants are defined as a child aged between 0
days (newborn) and 12 months of age, and age disaggregation is based on the infant age
at the time of sample collection. The infant age reported should not be based on how
old the infant was when the result was available to the site.
This indicator can include infants identified as HIV-infected on any virologic test by 12
months of age and is not limited to infants identified as HIV-infected on their first
virologic test. Infants may be HIV-uninfected on their first virologic test, but at a later
age be identified as HIV-infected, and they should be counted in this indicator as long as
they were aged 0 - 12 months at the time of subsequent sample collection. Confirmatory
testing (collection of a second sample for repeat virologic testing after the first virologic
test is positive) is excluded.
Positive Infants and Linkage to ART: PMTCT_HEI_POS will be used to track how many
positive infants are identified in a reporting period, and the “ART initiation confirmed”
disaggregate can be compared to PMTCT_HEI_POS positive infants to describe rates of
linkage to ART for HIV-infected infants (PMTCT_HEI_POS_ART / PMTCT_HEI_POS). The
age disaggregate will also help describe linkage rates for very young infants (0-2mo). The
proportion of positive infants confirmed as initiating ART can be used to help identify
sites with potential successes or challenges in documentation, linkage, and/or initiation
of infected infants.
Comparison to TX_NEW age <1: the disaggregate for PMTCT_HEI_POS infants confirmed
as initiating ART (sum of 0-2 and 2-12 months) could be compared to “infants <1-year-
old initiated on ART (TX_NEW <1)." However, equal values for PMTCT_HEI_POS_ART and
76
TX_NEW age <1 may not be expected, as each indicator may not be counting the same
infants. The ART initiation disaggregate within HEI_POS will allow us to report a linked
infant ART initiation outcome for each positive infant reported. For more information,
see section on "How to review for data quality."
Proxy positivity: When quarterly time period results are aggregated, PMTCT_HEI_POS
(numerator) may be able to be compared to PMTCT_EID (numerator) for a proxy
positivity calculation. This comparison will provide a poor proxy for positivity for sites or
areas with a high percent of test results that are unknown. Combining quarters of data
for both PMTCT_HEI_POS and PMTCT_EID for this comparison may reduce the portion
of test results that are unknown, especially for infants whose sample was collected near
the end of a reporting period. It is also important to note that infants reported under
HEI_POS will not be exactly the same as infants reported through PMTCT_EID in the
quarterly time period for several reasons: 1) PMTCT_EID is limited to first virologic tests
whereas HEI_POS reports infants identified on a first or subsequent test 2) PMTCT_EID is
limited to infants with a first virologic test sample collected during the reporting period;
whereas PMTCT_HEI_POS includes infants whose positive diagnosis was established
during the reporting period, but their sample could have been collected in the prior
period.
Birth cohort monitoring: HIV status of infants at the end of the breastfeeding period and
the outcomes of the PMTCT program are measured in the PMTCT Final Outcome
indicator, PMTCT_FO.
This indicator reports HIV-infected infants identified by virologic HIV testing on any
sample collected by 12 months of age: DNA PCR testing of dried blood spots (DBS) or
point of care (POC) (e.g., Alere, Xpert) virologic testing.
Limitations and Considerations:
• This indicator does not collect infants with a negative virologic test result or the
number of infants whose test result is unknown. As such, “percent unknown”
cannot be calculated through the MER indicator, though it is still an important
metric for program monitoring. Notifying caregivers of infant test results remains
important.
• The infants reported as tested under the revised MER 2.2 PMTCT_EID indicator are
not necessarily the same infants whose positive results would be reported under the
new HEI_POS indicator. Dividing HEI_POS by PMTCT_EID will not provide a precise
measure of positivity; however, a proxy positivity could be calculated over a longer
time period. See “How to Review for Data Quality” for more information.
How to collect:
This indicator should be collected from the clinical source (i.e., HIV-exposed infant
registers or patient records) to ensure unduplicated patient counting and patient care.
HIV-exposed infant registers should be used to count HIV-infected infants whose results
were returned in the reporting period and the age at the time of sample collection. (If
available, information could come from electronic systems). If the standard report does
not contain all the required information, individual patient files should be used.
Additional supporting information for this indicator can be obtained from standard
laboratory information systems (i.e., DNA PCR or POC/near POC log books or electronic
systems) however, it will be important to ensure that repeat tests of the same sample or
HIV-infected infants receiving a confirmatory virologic HIV test result are not counted
twice.
KNOWING HIV STATUS
77
Only HIV-infected infants identified as infected by a virologic HIV test on a sample
collected when they were between ages 0 through 12 months should be included in this
indicator. Infants who initially were identified negative from a first virologic test but who
were later identified as HIV-infected after a later virologic test should be included, as
long as the infant was still aged 12 months or less at the time of sample collection.
Currently, the most commonly used form of virologic testing or nucleic acid testing
(“NAT”) is HIV DNA PCR on dried blood spots (DBS) but this indicator also includes HIV-
infected infants identified through POC testing (e.g., Alere, Xpert). Serologic testing or
“rapid” testing cannot diagnose HIV infection in an infant and so infants with a positive
serologic test result and either no virologic test result or a negative virologic test result
should not be included; however, infants with a positive serologic test and a positive
virologic test result should be included.
The numerator is divided into HIV-infected infants who had their diagnostic sample
collected for virologic testing between birth and 2 months of age and those whose
diagnostic sample was collected between 2 and 12 months of age. The 0- ≤2 month and
2-12-month time periods are based on age at sample collection for virologic HIV testing,
not on date of result available to the facility or caregiver. HIV-infected infants should be
reported in the quarterly time period in which they are identified, even if the sample
was collected/sent in the previous quarter; their age should be reported by age at the
time of collection of the sample that produced the positive result, and not the age when
the result was available to the site.
Example scenario to clarify time period and age: an infant has a DBS collected in
quarter 3, aged 11 months. Due to long turnaround times, the positive result returns to
the site in quarter 4 and staff now identify him/her as HIV-infected at 13 months old.
This infant should be counted in quarter 4 as HIV-infected, and his/her age should be
reported as 11 months (2-12mo age band).
ART initiation: An additional disaggregate of the numerator is that the HIV positive
infant is confirmed as having initiated ART. An HIV-infected infant reported as “ART
initiation confirmed” should have documentation of an ART regimen in their record. An
HIV-infected infant whose record includes documentation of “referred to ART” or an ART
clinic number without evidence of receipt of an ART regimen should not be reported as
“ART initiation confirmed.” ART does not include infant ARV prophylaxis regimens for
PMTCT.
Reporting level:
Facility
How often to report:
Quarterly
How to review for
data quality:
Linkage and ART Initiation:
• Compare the PMTCT_HEI_POS ART initiation confirmed (disaggregate) to the
PMTCT_ HEI_POS numerator to calculate linkage to ART. Significantly <100% or
>100% linkage of HIV-infected infants to ART may reflect referrals to different
sites, program weakness, or poor data quality and requires review to confirm.
• TX_NEW comparison: HEI_POS_ART disaggregate is expected to be close in value
to TX_NEW age <1; however, some discrepancies could be expected and
significant discrepancies should be reviewed to confirm. These values may differ
in part because the age disaggregate definitions for these indicators differs.
TX_NEW age is based on age at ART initiation, while PMTCT_HEI_POS is based on
age at virologic sample collection. Scenario: An infant’s virologic sample was
collected when the infant was 11 months old near the end of Q1. The infant’s
positive result was available to the site in Q2 and she started ART in Q2 at 13
78
months of age. Under PMTCT_HEI_POS in Q2, she would be reported as “Positive,
ART initiation confirmed, age 2-12mo;” however, under TX_NEW in Q2 she would
be reported in the 1-9-year age group.
Proxy positivity: it is useful to review proxy positivity (PMTCT_HEI_POS / PMTCT_EID)
across sites or locations to identify potential outliers for further review. Summing
multiple quarters of data is recommended, as quarter-specific comparisons may provide
a less accurate proxy. See “How to use” section for more considerations.
How to calculate
annual total:
Sum results across quarters.
Data elements
(components of
indicator):
Numerator:
Number of HIV-
infected infants
identified in the
reporting period,
whose diagnostic
sample was
collected by 12
months of age.
Disaggregate Groups
Disaggregates
Infant age at virologic sample
collection, for positive infants
[Required]
• Positive, 0 to ≤2 months
• Positive, 2 to 12 months
Positive, confirmed initiated
ART by age at virologic sample
collection
[Required]
• Positive, confirmed initiated
ART, 0-2 months of age.
• Positive, confirmed initiated
ART, 2-12 months
Disaggregate Descriptions & Definitions
Infant age at virologic sample collection, for positive infants Description: For the
numerator to be calculated, implementing partners are required to report:
• HIV-infected infants identified in a quarter, disaggregated by the age at time of
sample collection: 0-2 months of age, or between 2-12 months of age. These
values will auto-sum to the numerator.
Positive, confirmed initiated ART by age at virologic sample collection description:
• Implementing partners are required to note HIV positive infants, disaggregated by
age 0-≤2months and between 2-12 months, who are confirmed as initiating ART by:
a. Positive, confirmed ART initiation, infant was between 0-2 months of age at
age time of virologic sample collection
b. Positive, confirmed ART initiation, infant was between 2-12 months of age at
time of virologic sample collection
PEPFAR-support
definition:
Standard definition of DSD and TA-SDI used.
Provision of key staff or commodities for PMTCT include: commodities such as test kits
(e.g., including but not limited to DBS bundles or collection kit, POC/near POC sample
collection kits and testing devices), ARVs including infant prophylaxis, lab commodities;
or funding for salaries of health care workers.
Ongoing support for PMTCT service delivery improvement includes: training of PMTCT
service providers, clinical mentoring and supportive supervision of PTMCT service sites,
infrastructure/renovation of facilities, support for PMTCT service data collection,
reporting, data quality, QI/QA of PMTCT services support, ARV consumption forecasting
and supply management, support of lab clinical monitoring of patients, supporting
patient follow-up/retention, support of mother mentoring programs.
Guiding narrative
questions:
1. Describe the data source used for reporting on this indicator, and any key
information about data quality that is important for interpretation of quantitative
results.
2. Linkage: (PMTCT_HEI_POS confirmed initiated ART (disaggregation) /
PMTCT_HEI_POS total numerator). Please describe rates of linkage of positive infants
(including young infants, ages 0-2 based on age of virologic sample collection) by
subnational area. Please provide context for areas with low linkage rates, and
describe activities aimed at improving infant ART initiation.
KNOWING HIV STATUS
79
TB_STAT (including TB_STAT_POS)
Description:
Percentage of new and relapse TB cases with documented HIV status
Numerator:
Number of new and relapsed TB cases
with documented HIV status, during the
reporting period
The numerator can be generated by counting
the number of new and relapsed TB cases
with documented HIV test results during the
reporting period.
Denominator:
Total number of new and relapsed TB
cases, during the reporting period
The denominator can be generated by
counting the number of new and relapse TB
cases during the reporting period.
Changes in indicator:
• Now includes an option for “known HIV+ at service entry" (MER 1.0 to MER 2.0).
• Finer age disaggregates no longer required (MER 1.0 to MER 2.0).
• Disaggregates have been added to denominator (MER 1.0 to MER 2.0).
How to use:
This indicator measures the performance of the TB program in ensuring that TB cases
know their HIV status.
How to collect:
The numerator and denominator can be obtained from basic management unit TB
registers as well as additional data collection sources (i.e., HIV testing registers) that may
contain relevant information (i.e., HIV test results, enrollment in HIV care programs).
Programs should modify the register as needed to easily capture this information (<15
M, 15+ M, <15 F, 15+ F)) and (Known HIV-positive at service entry).
The data source is the TB register. There is a risk of double counting as TB patients could
be tested multiple times during their TB treatment, therefore partners should ensure a
data collection and reporting system is in place to minimize double counting. There is
also a risk of undercounting if those patients who already knew their HIV status prior to
attending TB clinic are not documented, therefore the TB register at a minimum should
document “Known HIV-positive at service entry; Newly tested HIV-positive; Tested HIV
negative”.
(As this is a status indicator and not a testing indicator - These patients should also be
counted in the general HTS indicator “HTS_TST” TB service delivery modality).
Reporting level:
Facility
How often to report:
Semi-Annual
How to review for
data quality:
Only one disaggregation type is used for age and gender (coarse age and gender
disaggregations)
• Denominator ≥ Numerator.
• Numerator ≥ subtotal of each of the disaggregations.
• Denominator ≥ subtotal of each of the disaggregations.
How to calculate
annual total:
Sum results across quarters.
Data elements
(components of
indicator):
Numerator:
Number of new
and relapse TB
cases with
documented HIV
test results, during
the reporting
period.
Disaggregate Groups
Disaggregates
Age/Sex/Result
[Required]
• Known Positives: Unknown
age, <15 F, >15 F, <15 M, >15
M
• Newly Tested Positives:
Unknown age, <15 F, >15 F,
<15 M, >15 M
• New Negatives: Unknown
age, <15 F, >15 F, <15 M, >15
M
80
Denominator:
Total number of
new and relapsed
TB cases, during
the reporting
period.
Disaggregate Groups
Disaggregates
Age/Sex
Unknown age, <15 F, >15 F, <15
M, >15 M
Disaggregate Descriptions & Definitions
N/A
PEPFAR-support
definition:
Standard definition of DSD and TA-SDI used.
Provision of key staff or commodities for TB cases receiving HIV-related services include:
funding of test kits, ARVs, ARTs, and lab commodities or funding of salaries or provision
of Health Care Workers for TB/HIV-related services. Staff responsible for maintaining
patient records are included in this category however staff responsible for fulfilling
reporting and routine M&E requirements are not included.
Ongoing support for TB cases receiving HIV-related services includes: training of TB/HIV
service providers, clinical mentoring and supportive supervision of staff at TB/HIV sites,
infrastructure/renovation of facilities, support of TB/HIV service data collection,
reporting, data quality, QI/QA of TB/HIV services support, ARV consumption forecasting
and supply management, support of lab clinical monitoring of patients, supporting
patient follow up/retention, support of other TB/HIV programs.
Guiding narrative
questions:
1. Please describe how the denominator was determined.
2. Describe the sources for the data that you are reporting (i.e., are the data from just
PEPFAR-supported facilities or do the data reflect national-level data, including those
from non-PEPFAR supported facilities)?
KNOWING HIV STATUS
81
OVC_HIVSTAT
Description:
Percentage of orphans and vulnerable children (<18 years old) with HIV status reported
to implementing partner (including report of no status).
Numerator:
Number of orphans and vulnerable
children (<18 years old) with HIV status
reported to implementing partner,
disaggregated by status type.
Data sources for this indicator include HIV
test results that are self-reported by OVC (or
their caregivers), results of HIV Risk
Assessments conducted by implementing
partners, registers, referral forms, client
records, or other confidential case
management and program monitoring tools
that track those in treatment and care.
Denominator:
Number of orphans and vulnerable
children reported under OVC_SERV (<18
years old)
Denominator is not collected again, as part of
this indicator but is collected under the
indicator OVC_SERV.
Changes in indicator:
• This indicator formerly called OVC_ACC (MER 1.0) and OVC_KNOWNSTAT (in the
original MER 2.0 target setting documentation guidance) was changed to
OVC_HIVSTAT to reflect that HIV status is self-reported to the implementing partner
by the OVC or OVC caregiver (MER 1.0 to MER 2.0).
How to use:
This indicator will be tracked through routine program monitoring semi- annually
through the POART process.
Given the elevated risk of HIV infection among children affected by and vulnerable to
HIV, it is imperative for PEPFAR implementing partners to monitor HIV status among
OVC beneficiaries, and to facilitate access and retention in ART treatment for those who
are HIV positive. When the implementing partner knows the HIV status, the program
can contribute to ensuring that the children are linked to appropriate care and
treatment services, all essential elements of quality case management. OVC programs
can also play an important role in family-centered disclosure, for those who are HIV
positive.
• This indicator is NOT intended to be an indicator of HIV tests performed or receipt
of testing results, as these are measured elsewhere and test results are frequently
unavailable to community organizations due to health facility concerns about
patient confidentiality.
• This indicator is NOT intended to imply that all OVC beneficiaries require an HIV
test. OVC with known positive or negative status do not need to be tested. Only
OVC with no HIV status or children reported to be negative and recently
experiencing sexual violence and/or other risk factors in the reporting period should
be assessed for HIV risk. For older children who the IP thinks may be sexually
active, they should be assessed every reporting period.
• Status disclosure to the implementing partner is NOT a prerequisite for enrollment
or continuation in an OVC program. OVC programs serve persons of positive,
negative, and unknown HIV status appropriate to their needs and vulnerability to
HIV. This indicator ensures that IPs are regularly providing outreach to caregivers to
identify children’s HIV status, encourage family disclosure and linkage to care and
treatment as needed.
• This indicator captures if implementing partners are tracking the self-reported HIV
status of the orphans and vulnerable children they serve and enrollment in ART for
those who are positive. Testing results for OVC who are referred for testing should
be reported under HTS_TST based on the service delivery point where they were
tested
82
• This indicator also captures if implementing partners are tracking if the orphans and
vulnerable children they serve who report to be HIV positive are successfully linked
to and retained in treatment and care.
• This indicator is a subset from OVC_SERV. Only OVC who were reported under
OVC_SERV <18 should be included in the denominator for this indicator.
• Since this is not a testing indicator, HIV positivity yield should NOT be calculated
based on this indicator. Yield calculations should only be made by testing partners.
How to collect:
Data sources for this indicator include HIV test results that are self-reported by OVC (or
their caregivers), results of HIV Risk Assessments conducted by implementing partners,
registers, referral forms, client records, or other confidential case management and
program monitoring tools that track those in treatment and care.
Implementation of the HIV risk assessment should be integrated into case management
and on-going case monitoring and should not be conducted separately, if possible. This
will vary by partner and project. The partners should work out a timeline based on their
experience of how long referral completion and status disclosure usually takes and
factor that into their case management processes.
Implementing partners will record the OVC beneficiary’s self-reported HIV status –semi-
annually.
Reporting level:
Facility & Community
How often to report:
Semi-Annual
How to review for
data quality:
The OVC_HIVSTAT total numerator should ideally equal OVC_SERV<18 results. In some
cases, there may be missing data for the following reasons: 1) IP was not able to collect
this information from all caregivers of OVC_SERV<18 within the reporting period, 2) IP
was not able to locate all the caregivers of OVC_SERV<18 (e.g., relocated, migrant work),
3) data entry error and/or 4) Peace Corps is currently not reporting on this indicator so
OVC served <18 under PC would be missing.
Review any site with the following reporting issues: 1) numerator greater than 100% of
OVC_SERV <age 18, 2) very low coverage of OVC_HIVSTAT, 3) sum of “Currently on ART”
and “Not currently on ART” do no equal the “Reported HIV positive to IP” results and 5)
sum of “Test not indicated” and “Other reasons” do not equal “Reported No Status to
IP”.
How to calculate
annual total:
Use result reported at Q4.
Data elements
(components of
indicator):
Numerator:
Number of
orphans and
vulnerable
children (<18
years old) with
HIV status
reported to
implementing
partner,
disaggregated by
status type.
Disaggregate Groups
Disaggregates
Status Type
[Required]
• Reported HIV positive to
implementing partner
o Currently receiving ART
o Not currently receiving
ART
• Reported HIV negative to
implementing partner
• No HIV status reported to the
implementing partner
o Test not indicated based
on HIV risk assessment
o Other reasons
Disaggregate Descriptions & Definitions
Status Type Disaggregate Definitions:
• “Reported HIV positive to IP” includes beneficiaries <age 18 who report to the IP
that they are HIV positive based on an HIV test conducted during or prior to the
KNOWING HIV STATUS
83
reporting period (regardless of where the test occurred). All entries for “reported
HIV positive to IP” should be further disaggregated as “currently receiving ART” or
“not currently receiving ART.” This also includes beneficiaries <age 18 who report
that they are HIV positive based on an HIV test conducted during previous project
reporting periods. OVC entered as “Reported HIV positive to IP” in the previous
reporting period, should continue to be reported as positive during the current
reporting period and their enrollment in ART noted.
• “Reported HIV negative to IP” includes beneficiaries <age 18 who report that they
are HIV negative to the IP based on an HIV test conducted during the reporting
period (regardless of where the test occurred). For a child who reports multiple tests
within the current period, use most recent test. For beneficiaries entered as
“Reported HIV negative to IP” in a previous reporting period—if the IP believes the
child’s risk has not changed in the last six months, they should continue to report the
child as negative during the current reporting period. However, if the IP believes that
the child has recently been exposed to risk of HIV infection (e.g., sexual violence) or if
an adolescent has become sexually active, then the IP should conduct the HIV risk
assessment. Potential outcomes reported after the HIV risk assessment include 1)
the child is tested and reported as HIV positive and either currently receiving ART or
not receiving ART, or 2) the child is tested and reported as HIV negative, or 3) the
child is reported as “No Status” and under one of its disaggregates (“Test not
indicated” or “Other reasons”).
• “No HIV status reported to the IP” includes beneficiaries who fall into one of the
below described categories:
o “Test not indicated” – includes beneficiaries (OVC_SERV<age 18) who based on
a risk assessment made by the implementing partner do not require a test
during the reporting period. (Consensus Conference Technical Report on the
Role of OVC Programs Supported by PEPFAR in Extending Access to HTS includes
further information on determining whether a test is indicated)
o “Other reasons” – includes all beneficiaries (OVC_SERV <age 18) not entered in
above categories. Potential scenarios included in other reasons include:
i. Caregiver refuses to disclose whether the child has been tested and his/her
current HIV status in the reporting period
ii. Caregiver refuses to let the IP conduct a risk assessment on the child in the
reporting period.
iii. Caregiver recommended by IP to have child tested base on risk assessment,
but refuses to test the child in the reporting period OR does take child to
test but doesn't report results to IP in the reporting period.
iv. The IP is still in the process of convincing the caregiver to get the child
assessed, tested and/or disclosure of status. Since this is a new indicator
and takes time, IPs may not be positioned to report within the reporting
period and would be captured under – Undisclosed to IP - Other Reasons.
The IP should monitor these children and provide services to encourage
referral completion and disclosure in the next reporting period.
• Children entered as “No HIV status reported to the IP” with the disaggregate “Other
reasons” in the previous reporting period should receive follow-up services to
encourage referral completion/disclosure of status to the IP. Children reported as
“No HIV Status to the IP” with the disaggregate “Test not indicated” with no changes
in their risk situation for past six months, don’t need to be reassessed. If the IP
believes the child’s risk situation has changed in the last six months, then the child
should be reassessed by the implementing partner to determine whether testing is
84
indicated and the results entered as outline above, and the child should receive
appropriate follow-up.
PEPFAR-support
definition:
Standard definition of DSD and TA-SDI used.
Provision of key staff or commodities for OVC beneficiaries receiving care and support
services in the community include: For beneficiaries of OVC services, this can include
funding of salaries (partial or full) for staff of the organization delivering the individual,
small group or community level activity (e.g., psychosocial support, child protection
services, education, etc.) or procurement of critical commodities essential for ongoing
service delivery. Partial salary support may include stipends or incentives for volunteers,
or paying for transportation of those staff to the point of service delivery.
For care and support services, ongoing support for OVC service delivery for
improvement includes: the development of activity-related curricula, education
materials, etc., supportive supervision of volunteers, support for setting quality
standards and/or ethical guidelines, and monitoring visits to assess the quality of the
activity, including a home visit, a visit to a school to verify a child’s attendance and
progress in school or observation of a child’s participation in kids clubs.
Guiding narrative
questions:
1. For OVC_HIVSTAT, if less than 100% of caregivers have reported their child's status,
please explain the percentage that have not reported to the IP their child's status
and the plan to get closer to 100% coverage. Are there certain partners that are
struggling and how the Mission is responding?
2. For children reported as not currently on ART, what are efforts are being undertaken
in response? Are there certain partners with low ART coverage, why?
3. Please explain the breakdown of those reported under No Status. What percentage
were: 1) risk assessed and reported as test not indicated and 2) test indicated, 3)
caregivers unwilling to disclose status; 4) incomplete referrals for testing; 5) Other
reasons (please specify).
KNOWING HIV STATUS
85
PMTCT_FO
Description:
Percentage of final outcomes among HIV exposed infants registered in a birth cohort
Numerator:
Number of HIV-exposed infants with a
documented outcome by 18 months of
age disaggregated by outcome type.
(Note: Collection of 18 month visit
outcomes is recommended at 24
months of age, see additional
explanation to the right.)
Calculated indicator in DATIM, sum of: HIV-
infected, HIV-uninfected, HIV-final status
unknown, died without status known.
It is recommended to wait to collect the 18
month visit outcomes until the patient is 24
months old for the following reasons: 1) this
allows for children who present several
months late to their 18 month visit to be
included in the numerator and 2) cohort
reporting is easiest when monthly reporting
by facilities is used and where the birth
month and the reporting month are the same
calendar month (i.e., for infants born in
January 2012, their 24 month reporting
month would be January 2014, rather than
using the 18 month reporting month of July
2013).
Denominator:
Number of HIV-exposed infants who
were born 24 months prior to the
reporting period and registered in the
birth cohort.
Only those HIV-exposed infants registered in
the birth cohort at any time between 0 and
18 months of age (including transfers-ins)
who were born 24 months prior to the
reporting period are included in the
denominator.
Changes in indicator:
N/A
How to use:
In settings where national guidelines support breastfeeding of HIV-exposed infants,
antibody testing of all HIV-exposed children at 18 months of age and/or 6 weeks after
cessation of breastfeeding is recommended to determine final HIV status (‘final
outcome’/FO) of HIV-exposed children. To accomplish this goal, it is recommended to
identify infants at birth or at the first infant follow-up visit and track them through the
end of the breastfeeding period. This indicator measures progress toward ensuring that
all infants born to HIV-positive women have an outcome documented. In settings where
a mother-infant register is utilized and/or it is common practice for HIV-infected women
to breastfeed less than or more than 18 months please describe in the narrative the final
outcome time point.
How to collect:
To report on this indicator PEPFAR supported sites would ideally use registers or facility
held cards for HIV exposed infants that collect longitudinal information on follow-up and
are organized by birth month of infants. This methodology is referred to as birth cohort
reporting.
Two examples of birth cohort reporting:
1. In Kenya, this indicator was first piloted by PEPFAR and the Ministry of Health in
Western Kenya and is currently integrated into the national HIV summary
reporting tool. Data from the facility HIV exposed infant longitudinal follow-up
register, which organizes infants by birth-month cohorts, are aggregated into a
report summarizing outcomes for infants reaching 24 months of age during
each month.
86
2. In Malawi, clinic staff complete monthly follow up reporting forms as part of
the national quarterly supervision visits using data collected directly from HIV-
exposed infant cards which are kept in a binder that is organized by birth month
(no HIV exposed register is used).
As an example, for those infants born in FY 2015, the outcomes would be reported in FY
2017.
FY2017 (Report results for the entire 12-month
reporting period for these indicators at the Q4
reporting cycle)
Reporting Month
(FY 2017)
O
c
t
N
o
v
D
e
c
J
a
n
F
e
b
M
a
r
A
p
r
M
a
y
J
u
n
J
u
l
A
u
g
S
e
p
↓
↓
↓
↓
↓
↓
↓
↓
↓
↓
↓
↓
Birth Month
(FY 2015)
O
c
t
N
o
v
D
e
c
J
a
n
F
e
b
M
a
r
A
p
r
M
a
y
J
u
n
J
u
l
A
u
g
S
e
p
Both approaches allow a paper-based health facility records to quickly identify the
number of HIV-exposed infants registered in the birth cohort at any time between 0 and
18 months of age (denominator).
Reporting level:
Facility
How often to report:
Annual
How to review for
data quality:
By design this indicator should equal 100% if all outcomes are known regardless of
outcome type. This allows for facilities to check that all HIV-exposed infants have an
outcome assigned to them during the reporting process. Data utilization requires
reviewing the disaggregated data to understand the specific outcomes of interest. In
settings where HIV-exposed infant registers do not allow for documentation of all
disaggregated outcomes, country teams should report only on available disaggregates
even if the aggregate indicator is less than 100%, however this should be specified in the
narrative.
The denominator should include those “Transferred In” and those “Transferred Out” as
long as for “Transferred In” there is documentation that HIV-exposed infants were
registered at their original site in the birth cohort at any time between 0 and 18 months
of age and were born 24 months prior to the reporting period. “Transferred Out” should
be reported under HIV status unknown. The inclusion of Transfers-In/Out provides a
quality check to ensure that all exposed infants have an outcome assigned to them
during the reporting process such that the sum of the numerator disaggregation equals
the denominator. However, this may lead to outcomes for >100% of HIV positive
pregnant women (PMTCT_STAT_POS) identified at a site so this comparison should not
be used as a logic check.
How to calculate
annual total:
Use annual result reported at Q4.
Data elements
(components of
indicator):
Numerator:
Number of HIV-
exposed infants
with a
Disaggregate Groups
Disaggregates
Outcome Type
[Required]
• HIV-infected;
• HIV-uninfected;
• HIV-final status unknown;
KNOWING HIV STATUS
87
documented
outcome by 18
months of age
disaggregated by
outcome type.
• Died without status known
Denominator:
Number of HIV-
exposed infants
who were born 24
months prior to
the reporting
period and
registered in the
birth cohort.
Disaggregate Groups
Disaggregates
N/A
N/A
Disaggregate Descriptions & Definitions
Outcome Type:
For the numerator to be calculated, implementing partners are required to report:
• HIV-infected: Number of HIV-exposed infants identified as HIV-infected at any point
during follow-up. HIV-infected includes infants and children with diagnostic
virologic or serologic confirmation of HIV-infection (DNA PCR before 18 months;
rapid test at 18 months) and those with a presumptive HIV diagnosis where DNA
PCR is not available. Site should also maintain data on HIV infected infants and
whether they are linked or not linked to ART services, or whether they have no
information on patient linkage to ART programs.
• HIV-uninfected: Number of HIV-exposed infants with a negative 18-month antibody
test documented. Based on national guidelines, countries should determine if “HIV-
uninfected” includes infants with a documented negative antibody test that was
done at least 6 weeks after cessation of breastfeeding but before 18 months of age.
• HIV final status unknown: Sum of the following disaggregates (not reported in
DATIM but should be documented at site level)
o In care but no test done: Number of HIV-exposed infants who attended 18-
month visit but no antibody test result is documented (unknown FO)
o Lost to follow-up: Number of HIV-exposed infants who did not attend the
18-month visit (unknown FO)
o Transferred out (unknown FO): Number of HIV-exposed infants who
transferred out between 0 and 18 months without confirmation of HIV-
infection (unknown FO)
• Died without status known: Number of HIV-exposed infants who are documented
to have died without confirmation of HIV-infection between 0 and 18 months. Note:
HIV-exposed infants who are HIV infected and later confirmed to have died or
transferred out during follow-up are still counted under HIV infected and not died or
transferred out.
Every infant in a given cohort should be assigned one outcome only.
PEPFAR-support
definition:
Standard definition of DSD and TA-SDI used.
Provision of key staff or commodities for PMTCT include: commodities such as test kits,
ARVs, lab commodities, or funding for salaries of health care workers.
Ongoing support for PMTCT service delivery improvement includes: training of PMTCT
service providers, clinical mentoring and supportive supervision of PTMCT service sites,
88
infrastructure/renovation of facilities, support for PMTCT service data collection,
reporting, data quality, QI/QA of PMTCT services support, ARV consumption forecasting
and supply management, support of lab clinical monitoring of patients, supporting
patient follow-up/retention, support of mother mentoring programs.
Guiding narrative
questions:
1. Provide context for PMTCT_FO results (e.g., PMTCT_FO not equal to 100%, low or
high rate of HIV-uninfected infants) and describe how this data being use for program
management?
2. Provide context on:
• The status of birth cohort monitoring in your operating unit, geographic area or
partner/implementing mechanism, including any planned activities.
• The data source used for reporting, and any key information about data quality
that is important for interpretation of results (see MER reference sheet for
examples).
• The number and proportion of PEPFAR-supported PMTCT sites implementing
cohort monitoring and able to (1) report on PMTCT_FO and (2) longitudinally track
mothers to assess retention/viral suppression

On ART
Indicators
90
TX_NEW
Description:
Number of adults and children newly enrolled on antiretroviral therapy (ART)
Numerator:
Number of adults and children newly
enrolled on antiretroviral therapy (ART)
The indicator measures the ongoing scale-up
and uptake of ART programs.
Denominator:
N/A
Changes in indicator:
• TB disaggregate added to the indicator (MER 1.0 to MER 2.0).
• Key population disaggregate added to the indicator (MER 1.0 to MER 2.0).
• Age/sex disaggregates updated (MER 2.0 v2.1 to v2.2).
• Clarifying language added for Key Populations disaggregation the notes that KP
should be counted in only one KP group to avoid double-counting. More information
is provided below (MER 2.0 v2.1 to v2.2).
How to use:
The indicator measures the ongoing scale-up and uptake of ART programs. This measure
is critical to monitor along with number of patients currently on ART in relation to the
number of PLHIV that are estimated to be eligible for treatment to assess progress in the
program’s response to the epidemic in specific geographic areas and populations as well
as at the national level. This is particularly critical in the context of current revisions to
country-specific ART eligibility.
Reporting the number of new patients enrolled on ART at both the national and overall
PEPFAR program levels is critical to monitoring the HIV services cascade, specifically the
successful linkage between HIV diagnosis and initiating ART. Disaggregation of new on
ART by age/sex at ART initiation, pregnancy status at ART initiation, and breastfeeding
status at ART initiation is important to understand the percentage of new ART initiations
coming from priority populations.
How to collect:
Facility ART registers/databases, program monitoring tools, or drug supply management
systems.
• The numerator can be generated by counting the number of adults and children
who are newly enrolled in ART in the reporting period, in accordance with the
nationally approved treatment protocol (or WHO/UNAIDS standards).
• Patients who known to transfer in from another facility, or who temporarily stopped
therapy and have started again should not be counted as new patients.
• NEW is a state defined by an individual initiating ART during the reporting period.
It is expected that the characteristics of new clients are recorded at the time they
newly initiate life-long ART. For example, patients who receive post-exposure
prophylaxis (PEP), short term ART only for prevention (PREP), or ART starter pack
alone should not be used to count individuals reached with this indicator.
TB/ HIV disaggregation: At initiation of ART, number of patients with a confirmed
diagnosis of TB (new and relapsed) and/or on TB treatment collected from TB/HIV
registers;
Pregnant/BF disaggregation: Women who initiate ART while breastfeeding should be
counted under this indicator but not in PMTCT_ART. Women who initiate during
pregnancy and are reported under PMTCT_ART should also be reported here.
Key population disaggregation* see Appendix 1 to support the identification of key
populations at ART initiation. However, reporting of key population disaggregation
should be consistent with what is described under the KP_PREV “How to review for data
quality” section on mutual exclusivity of an individual who falls under multiple KP
categories (e.g., FSW who injects drugs). In such instances, the individual should only be
ON ART
91
reported in ONE KP disaggregation category with which s/he is most identified in order
to avoid double-counting.
NOTE: both KP-specific and clinical partners have the option to complete these disaggs,
but only if safe to maintain these files and to report.
Reporting level:
Facility
How often to report:
Quarterly
How to review for
data quality:
• Confirm that TX_CURR ≥ TX_NEW
• Only one age disaggregation type is used for age/sex: The number of individuals
newly receiving ART must be disaggregated by age and sex. If possible, the full
age/sex disaggregations should be used. If the full age/sex disaggregations are not
possible, then, and only then, should the aggregated age/sex disaggregations be
used, do NOT complete both age/sex disaggregations.
• Numerator ≥ subtotal of each disaggregation: The total number of adults and
children newly enrolled on ART should be greater or equal to the sum of all of the
age/sex disaggregations and pregnancy/ breastfeeding status.
How to calculate
annual total:
Sum across all reporting periods
Data elements
(components of
indicator):
Numerator:
Number of adults
and children
newly enrolled on
antiretroviral
therapy (ART)
Disaggregate Groups
Disaggregates
Age/Sex
[Required]
<1, 1-9, 10-14 M, 10-14 F, 15-19
M, 15-19 F, 20-24 M, 20-24 F,
25-29 M, 25-29 F, 30-34 M, 30-
34 F, 35-39 M, 35-39 F, 40-49
M, 40-49 F, 50+ M, 50+ F
TB/HIV Status
[Required}
Number new on treatment with
confirmed diagnosis of TB (new
and relapsed) and/or TB treated
Pregnancy and breastfeeding
status at ART initiation
[Required]
• Pregnant at initiation of ART;
• Breastfeeding at initiation of
ART
Key Population Type
[Optional]
• People who inject drugs
(PWID)
• Men who have sex with men
(MSM)
• Transgender people (TG)
• Female sex workers (FSW)
• People in prison and other
closed settings
Disaggregate Descriptions & Definitions
Age/Sex: Age is defined as the age of the patient at the date of initiation on ART, not the
age at the date of reporting.
PEPFAR-support
definition:
Standard definition of DSD and TA-SDI used.
Provision of key staff or commodities for PLHIV receiving ART include: the provision of
key staff and/or commodities can include ongoing procurement of critical commodities,
such as ARVs, or funding for salaries of HCW who deliver HIV treatment services. Staff
who are responsible for the completeness and quality of routine patient records (paper
or electronic) can be counted here; however, staff who exclusively fulfill MOH and donor
reporting requirements cannot be counted.

92
Ongoing support for PLHIV receiving ART service delivery improvement includes: clinical
mentoring and supportive supervision of staff at HIV sites providing ART, support for
quality improvement activities, patient tracking system support, routine support of ART
M&E and reporting, commodities consumption forecasting and supply management.
Guiding narrative
questions:
1. If TX_NEW does NOT equal HTS_TST_POS, explain why.
2. If TX_NEW result is markedly different from targets, explain why.
ON ART
93
TX_CURR
Description:
Number of adults and children currently receiving antiretroviral therapy (ART)
Numerator:
Number of adults and children currently
receiving antiretroviral therapy (ART)
The current on ART count should equal the
number of adults and children with HIV
infection who ever started ART MINUS those
patients who are not currently on treatment
at the end of the reporting period.
Denominator:
N/A
Changes in indicator:
• Age/sex disaggregates updated (MER 2.0 v2.1 to v2.2).
How to use:
This indicator measures the ongoing scale-up and uptake of ART and retention in ART
programs as a critical step in the HIV service cascade and assesses progress towards
coverage of ART for all eligible HIV-positive individuals when reviewed against the
number of PLHIV that are estimated to be eligible for treatment. It allows us to track the
response to the epidemic in specific geographic areas and among specific populations as
well as at the national level.
How to collect:
This indicator should be collected from facility ART registers/databases, program
monitoring tools, and drug supply management systems. Count the number of adults
and children who are currently receiving ART in accordance with the nationally approved
treatment protocol (or WHO/UNAIDS standards) at the end of the reporting period.
The current on ART count should equal the number of adults and children with HIV
infection who ever started ART minus those patients who are not currently on treatment
at the end of the reporting period.
• Patients on ART who initiated or transferred-in during the reporting period
should be counted.
• Patients who have received enough ARVs to last to the end of the reporting
period should be counted including those patients that pick up several months of
antiretroviral drugs at one visit
• HIV-positive pregnant women who are eligible for and are receiving antiretroviral
drugs for their own treatment are included. HIV-positive pregnant women
initiating lifelong ART through PMTCT (Option B+) will count as “current” on ART
under this indicator. These include HIV-infected pregnant women who:
o Have newly initiated ART during the current pregnancy
o Are already on ART at the beginning of the current pregnancy
Patients excluded from the Current on ART count are patients who died, stopped
treatment, transferred out, or are lost to follow-up (LTFU). LTFU is defined as a patient
who has not received ARVs in the last 90 days (three months) following their last missed
appointment or missed drug pick-up. (Note: As models of service delivery change to
reflect longer visit intervals for stable patients, it is important to emphasize the
definition of LTFU applies to both missed visits or missed drug pick-up, but does not
apply who have not received ARVs in the last 90 days (three months) following their last
attended appointment or attended drug pick-up. As that interval between scheduled
visits for stable patients maybe longer than 3 months.)
This indicator should be reported from both PEPFAR-supported sites in the private or
public sector. Patients currently receiving treatment from mobile clinics can be reported
in two ways. Firstly, if the mobile clinic is associated (receives commodities, reports to, is
staff by) a nearby health facility, then these individuals should be reported by that
94
facility. Secondly, if a mobile clinic is stationary for more than 2 reporting periods, it
should be added to the PEPFAR facility list with geocodes and data should be reported
for this mobile clinic directly.
For age /sex disaggregates:
CURRENT is a state defined by treatment status when last seen, so it is expected that
characteristics of these clients would be updated each time they are seen by a program.
Age represents an individual’s age at the end of the reporting period or when last seen
at the facility. For example, a 14-year-old child will be counted as currently receiving
treatment in the <15 age category at the end of reporting period “A”. During reporting
period “B” the child turns age 15 and so at the end of this reporting period the child will
be counted under the 15+ age category.
DO NOT include:
Patients who receive ARVs for post-exposure prophylaxis (PEP) or short-term ART only
for prevention (PREP) should not be reported in this indicator.
Reporting level:
Facility
How often to report:
Quarterly
How to review for
data quality:
• Confirm that TX_CURR ≥ TX_NEW
• Only one age disaggregation type is used for age/sex: The number of individuals
newly receiving ART must be disaggregated by age and sex. If possible, the full
age/sex disaggregations should be used. If the full age/sex disaggregations are not
possible, then, and only then, should the aggregated age/sex disaggregations be
used, do NOT complete both age/sex disaggregations.
• Numerator ≥ subtotal of age/sex disaggregation: The total number of adults and
children newly enrolled on ART should be greater or equal to the sum of the
age/sex disaggregations
• Net new of TX_CURR between reporting periods should be less than TX_NEW in
that time period
How to calculate
annual total:
Snapshot indicator. Use the result reported at Q4.
Data elements
(components of
indicator):
Numerator:
Number of adults
and children
currently receiving
antiretroviral
therapy (ART)
Disaggregate Groups
Disaggregates
Age/Sex
[Required]
<1, 1-9, 10-14 M, 10-14 F, 15-19
M, 15-19 F, 20-24 M, 20-24 F,
25-29 M, 25-29 F, 30-34 M, 30-
34 F, 40-49 M, 40-49 F, 50+ M,
50+ F
Disaggregate Descriptions & Definitions
Age/Sex: Age is defined as the age of the patient at the date of reporting, not the age at
the date of initiation on ART.
PEPFAR-support
definition:
Standard definition of DSD and TA-SDI used.
Provision of key staff or commodities for PLHIV receiving ART include: the provision of
key staff and/or commodities can include ongoing procurement of critical commodities,
such as ARVs, or funding for salaries of HCW who deliver HIV treatment services. Staff
who are responsible for the completeness and quality of routine patient records (paper
or electronic) can be counted here; however, staff who exclusively fulfill MOH and donor
reporting requirements cannot be counted.
Ongoing support for PLHIV receiving ART service delivery improvement includes: clinical
mentoring and supportive supervision of staff at HIV sites providing ART, support for

ON ART
95
quality improvement activities, patient tracking system support, routine support of ART
M&E and reporting, commodities consumption forecasting and supply management
Guiding narrative
questions:
1. If the change in TX_CURR from the previous reporting period (TX_NET_NEW) is
substantially different from TX_NEW, explain why (i.e., if you can, estimate or
comment on the numbers of patients who died, transferred or were lost to follow-
up).
2. Please describe the reasoning for any net losses in treatment from the previous
quarter.
96
PMTCT_ART
Description:
Percentage of HIV-positive pregnant women who received ART to reduce the risk of
mother-to-child-transmission (MTCT) during pregnancy
Numerator:
Number of HIV-positive pregnant
women who received ART to reduce the
risk of mother-to-child-transmission
during pregnancy
Auto-Calculated indicator in DATIM, sum of:
1) New on life-long ART, 2) Already on life-
long ART at the beginning of the current
pregnancy
Denominator:
PMTCT_STAT_POS (see PMTCT_STAT):
Denominator is no longer collected as
part of indicator, but rather is calculated
as PMTCT_STAT_POS.
Collected as part of PMTCT_STAT. Calculated
indicator in DATIM, sum of: 1) New Positives,
2) Known Positive at entry (see
PMTCT_STAT, Disaggregate Group Positivity
Status for more details)
Changes in indicator:
• Collect only ART disaggregates and collected only at antenatal care (ANC) sites to
better align with 2016 Consolidated WHO ARV guidelines, reduce burden on data
collection, and improve data quality (MER 1.0 to MER 2.0).
• Denominator is no longer collected as part of indicator, but rather is calculated as
PMTCT_STAT_POS (MER 1.0 to MER 2.0).
How to use:
Track progress toward ensuring that all pregnant women who attend PEPFAR supported
antenatal care (ANC) know their HIV status and are initiated on ART.
How to collect:
Data source is the ANC or PMTCT register depending on country context (in many high
HIV prevalence settings information on the number of women receiving ART regimens is
integrated into the ANC register). There is a risk of double counting as a pregnant
woman receiving ART at ANC should have multiple visits for each pregnancy therefore
partners should ensure a data collection and reporting system is in place to minimize
double counting of the same pregnant women across visits including a paper based
longitudinal ANC or PMTCT register (meaning a register that is able to record all
information about 1 pregnancy in one location, with rows or columns that allow for
recording information on multiple visits during that pregnancy) or an electronic medical
record/patient tracking system. There is also a risk of undercounting if those women
who already on ART prior to attending ANC are not documented, therefore the ANC
register should document both “New on ART” and “Already on ART at the beginning of
the current pregnancy”. Women who initiate ART while breastfeeding should not be
counted under this indicator, and should instead be reported as part of the TX_NEW
indicator (see TX_NEW; disaggregate group pregnancy/breastfeeding status).
Note: Those women reported in PMTCT_ART including newly enrolled on ART and
already on ART at the beginning of pregnancy should also be reported in the TX_NEW
and TX_CURR indicators, respectively. Women who are already on ART should not be
counted in TX_NEW.
Reporting level:
Facility
How often to report:
Quarterly
How to review for
data quality:
Review any site with over 100% coverage or very low coverage to ensure they reflect
expected results. In general, services should be reported at the site where they are
delivered (however PMTCT_ART- “already on treatment” and PMTCT_STAT_POS “known
positive at entry” are exceptions, see details under description of disaggregate below).
Therefore, coverage at site level must be understood within the context of the service
delivery model at that site. For example, in local areas where ART is integrated into ANC
and low volume PMTCT sites are only testing for HIV and then referring women to other
facilities for ART, the expectation is that for one individual PMTCT_STAT_POS (newly
tested) will be documented at one facility and PMTCT_ART (new on ART) would be
ON ART
97
documented at another facility leading to the appearance of greater than >100%
coverage at one site and 0% coverage at another.
Compare the number of HIV-positive pregnant women newly initiating ART (PMTCT_ART
disaggregate) and the number individuals newly initiated on ART (TX_NEW disaggregate)
who are pregnant (disaggregation of the new on treatment indicator). It is expected that
women are new ART initiations are reported in both indicators; however the data source
is often different (ANC/PMTCT register for PMTCT_ART and ART register for TX_NEW)
and to discrepancies can provide better understanding of data quality.
How to calculate
annual total:
Assuming site level records avoid double counting (as described above) across the
annual reporting cycle, sum numerator and denominator across all reporting periods for
the annual result
Data elements
(components of
indicator):
Numerator:
Number of HIV-
positive pregnant
women who
received ART to
reduce the risk of
mother-to-child-
transmission
during pregnancy
Disaggregate Groups
Disaggregates
Maternal Regimen Type
[Required]
• New on ART
• Already on ART at the
beginning of current
pregnancy
Denominator:
PMTCT_STAT_POS
Disaggregate Groups
Disaggregates
See PMTCT_STAT.
See PMTCT_STAT.
Disaggregate Descriptions & Definitions
Maternal Regimen Type:
For the numerator to be calculated, implementing partners are required to report:
• The number of HIV-positive pregnant women newly initiated on ART. (These should
also be counted in “TX_NEW” see TX_NEW, Disaggregate group
breastfeeding/pregnancy status): Should only be counted in a regimen category if she
actually received the regimen. Referral alone for ART should not be counted.
Additionally, a woman who temporarily stopped ART and has started again during the
same pregnancy should not be counted as new on treatment.
• The number of HIV-positive pregnant women already on ART at beginning of
pregnancy: Maybe counted even if ART is continuing to be received at another
facility. For example, a woman, who is already on treatment, becomes pregnant and
enrolls in ANC/PMTCT because she is HIV-positive but is continuing to receive her
ART at a nearby treatment clinic should be counted within this disaggregate.
However, if a woman was initiated on ART at another facility during this pregnancy
and then transfers-in to the ANC site, she should not be counted. (since she was
already counted at the first ANC site for this pregnancy)
PEPFAR-support
definition:
Standard definition of DSD and TA-SDI used.
Provision of key staff or commodities for PMTCT include: commodities such as test kits,
ARVs, lab commodities, or funding for salaries of health care workers.
Ongoing support for PMTCT service delivery improvement includes: training of PMTCT
service providers, clinical mentoring and supportive supervision of PTMCT service sites,
infrastructure/renovation of facilities, support for PMTCT service data collection,
reporting, data quality, QI/QA of PMTCT services support, ARV consumption forecasting
and supply management, support of lab clinical monitoring of patients, supporting
patient follow-up/retention, support of mother mentoring programs.

98
Guiding narrative
questions:
1. Provide context for low PMTCT_ART coverage (PMTCT_ART / PMTCT_STAT_POS =
ART coverage) by geographic area or partner/implementing mechanism, including
any planned activities/remedial actions.
2. Describe activities related to ensuring retention through the breastfeeding period. If
additional data available in country, describe retention rates or rates of LTFU among
pregnant women continuing or starting ART as of ANC1.
3. Explain any differences in PMTCT_ART coverage among newly identified HIV positive
women initiating ART compared to known positives already on ART.
ON ART
99
TB_ART
Description:
The number of HIV-positive new and relapsed TB cases on ART during TB treatment
Numerator:
Number of TB cases with documented
HIV-positive status who start or
continue ART during the reporting
period
The numerator is generated by counting the
total number of TB patients (new and relapse
TB cases) with documented HIV-positive
status during TB treatment who are newly
initiated or already on ART.
Denominator:
TB_STAT_POS (see TB_STAT): Number
of registered TB cases with documented
HIV-positive status during the reporting
period.
Denominator is not collected as part of this
indicator, but is TB_STAT_POS.
Changes in indicator:
▪ HIV treatment disaggregate revised to be already on ART/new on ART (MER 1.0 to
MER 2.0).
▪ TB_ART denominator entry removed from DATIM. TB_ART denominator is
TB_STAT_POS. (MER 2.0 v2.1 to v2.2).
How to use:
This indicator will measure the extent to which programs effectively link HIV-infected TB
patients to appropriate HIV treatment. The HIV status of TB patients is often determined
at the TB clinics (and will be captured with TB_STAT), but ART for TB cases is frequently
provided by the HIV program. Therefore, provision of ART for this population often
implies successful linkage between the TB and HIV program, which should be followed
from TB_STAT_POS to TB_ART.
How to collect:
The numerator is generated by counting the total number of TB patients (new and
relapse TB cases) with documented HIV-positive status during TB treatment who are
newly initiated or already on ART.
Reporting level:
Facility
How often to report:
Semi-Annual
How to review for
data quality:
Only one disaggregation type is used for age/sex. Numerator ≥ subtotal of each of the
disaggregation.
How to calculate
annual total:
Sum across both reporting periods.
Data elements
(components of
indicator):
Numerator:
Number of TB
cases with
documented HIV-
positive status
who start or
continue ART
during the
reporting period
Disaggregate Groups
Disaggregates
ART Status
[Required]
• New on ART
• Already on ART
Age/Sex
[Required]
<1, 1-9, 10-14 M, 10-14 F, 15-19
M, 15-19 F, 20-24 M, 20-24 F,
25-29 M, 25-29 F, 30-34 M, 30-
34 F, 35-39 M, 35-39 F, 40-49
M, 40-49 F, 50+ M, 50+ F
Disaggregate Descriptions & Definitions
Age Description: Age is defined as the age at the date of initiation on ART or current
age, not the age at the date of reporting.
ART Status Definition: This disaggregation should distinguish those who started ART
during the reporting period (this should also be reported under TX_NEW) from those
who were already on it at the beginning of the reporting period.
PEPFAR-support
definition:
Provision of key staff or commodities for TB cases receiving HIV-related services include:
ongoing provision of critical re-occurring costs or commodities (such as ARVs) or funding
of salaries or provision of Health Care Workers for TB/HIV clinic services. Where TB and
100
HIV services are not integrated, this can include support for system/personnel critical to
patient referral, transfer or tracking that ensures patient linkage between the TB and
HIV programs/facilities that is required to accomplish the delivery of the service. Staff
responsible for maintaining patient records are included in this category however staff
responsible for fulfilling reporting and routine M&E requirements are not included.
Ongoing support for TB cases receiving HIV-related services includes: Clinical mentoring
and supportive supervision of staff at ART sites, Quality Improvement services support,
patient tracking/referral system support, routine support of ART M&E and reporting,
commodities consumption forecasting and supply management.
Guiding narrative
questions:
1. Describe the sources for the data that you are reporting (i.e., are the data from just
PEPFAR-supported facilities or do the data reflect national-level data, including those
from non-PEPFAR supported facilities)? As above, please describe the sources of the
data you are reporting.
ON ART
101
TX_TB
Description:
The proportion of ART patients screened for TB in the semiannual reporting period who
are receiving TB treatment.
Numerator:
The number of ART patients who were
started on TB treatment during the
semiannual reporting period.
The numerator can be generated by counting
the number of screened ART patients who
were diagnosed with TB and started on anti-
TB therapy during the reporting period.
Denominator:
The number of ART patients who were
screened for TB at least once during the
semiannual reporting period.
The denominator can be generated by
counting the number of ART patients who
were screened for TB symptoms at least once
during the reporting period.
Changes in indicator:
• Denominator disaggregate for TB screen results has been updated to include Start
of ART by TB screen result (MER 2.0 v2.1 to v2.2).
How to use:
This indicator documents the TB screening of ART patients as well as the proportion who
were diagnosed and started on TB therapy. The disaggregates demonstrate the cascade
from screening to testing and can be used to identify gaps and challenges in TB
diagnostic activities.
How to collect:
The denominator can be generated by counting the number of ART patients who were
screened for TB symptoms at least once during the reporting period. This includes newly
enrolling ART patients as well as those previously started on ART.
The numerator can be generated by counting the number of screened ART patients who
were diagnosed with TB and started on anti-TB therapy during the reporting period.
These data should be captured in ART registers as well as additional data collection
sources (e.g., facility-based TB screening registers or forms, TB specimen registers, TB
microscopy result registers, GeneXpert data collection systems) that may contain
relevant information (e.g., TB screening results, TB specimen testing results). Programs
should modify the register as needed to easily capture this information.
Screening for TB and/or initiation of anti-TB therapy might not happen at the same time
that ART is started. For PLHIV new to HIV care, those who are diagnosed with TB are
usually started on anti-TB therapy before they initiate ART (e.g., 2-8 weeks as per
current recommendations). Regardless of when they occur relative to ART initiation, TB
screening and initiation of TB therapy should be included for all patients who were
currently on ART or who started ART at any time during the reporting period.
Reporting level:
Facility
How often to report:
Semi-Annual
How to review for
data quality:
Only one disaggregation type is used for age (coarse disaggregates).
Numerator ≥ subtotal of each of the disaggregations.
How to calculate
annual total:
Snapshot indicator. Use the result reported at Q4.
Data elements
(components of
indicator):
Numerator:
Number of ART
patients who were
started on TB
treatment during
the semiannual
reporting period.
Disaggregate Groups
Disaggregates
ART Status (Current/New on
ART)
[Required]
• The number of patients
starting TB treatment who
newly started ART during the
reporting period
• The number of patients
starting TB treatment who
were already on ART prior to
102
the start of the reporting
period
Age/Sex
[Required]
<15 F, 15+ F, <15 M, 15+ M
Denominator:
The number of
ART patients who
were screened for
TB at least once
during the
semiannual
reporting period.
Disaggregate Groups
Disaggregates
Start of ART by Screen Result
[Required]
• New on ART/Screen Positive;
• New on ART/Screen
Negative;
• Previously on ART/Screen
Positive;
• Previously on ART/Screen
Negative
Specimen Sent
[Required]
Number of ART patients who
had a specimen sent for
bacteriologic diagnosis of active
TB disease.
Diagnostic Test (Disaggregation
of Specimen Sent)
[Required]
• GeneXpert MTB/RIF assay
(with or without other
testing)
• Smear microscopy only
• Additional test other than
GeneXpert
Age/Sex
[Required]
<15 F, 15+ F, <15 M, 15+ M
Disaggregate Descriptions & Definitions
Start of ART by Screen Result:
• New on ART/Screen Positive: The number of patients who started ART in the
reporting period and who screened with least one positive symptom during the
reporting period.
• New on ART/Screen Negative: The number of ART patients who started ART in the
reporting period and who had all negative symptom screens during the reporting
period.
• Previously on ART/Screen Positive: The number of patients who were on ART prior
to the reporting period and who had at least one positive symptom screen during
the reporting period.
• Previously on ART/Screen Negative: The number of ART patients who were on ART
prior to the reporting period and who had all negative symptom screens during the
reporting period.
PEPFAR-support
definition:
For DSD for HIV-related services, the provision of key staff and/or commodities can
include ongoing provision of critical re-occurring costs or commodities (such as
laboratory supplies, GeneXpert cartridges etc.) and/or delivery of TB symptom screening
and bacteriological testing to the counted individuals, such as through funding of salaries
or provision of Health Care Workers for TB services. Staff responsible for maintaining
patient records are included in this category however staff responsible for fulfilling
reporting and routine M&E requirements are not included.
For DSD and TA for TB/HIV-related services, TB and HIV clinical care facilities and
community-based services will be counted as supported by TA/QI when PEPFAR
provides established presence and/or routinized, frequent (at least quarterly) support
for the services by PEPFAR at the point of service delivery, clinical mentoring and
supportive supervision of staff providing TB/HIV services, Quality Improvement services,

ON ART
103
routine support of M&E, TB screening and bacteriologic testing, commodities
consumption forecasting and supply management, or specimen transport and result
return.
Guiding narrative
questions:
1. If the denominator does not roughly equal TX_CURR, please describe the main
reasons.
2. If there are issues with reporting the disaggregations, please describe.
3. Are the patients in the numerator all receiving care from PEPFAR-supported sites?
Are they receiving TB and HIV care from the same site?

Viral Suppression
Indicators
VIRAL SUPPRESSION
105
TX_RET
Description:
Percentage of adults and children known to be on treatment 12 months after initiation
of antiretroviral therapy (Note: reporting 24 and 36 months is recommended, but
optional)
Numerator:
Number of adults and children who are
still on treatment at 12 months after
initiating ART
The numerator is defined as the number of
adults and children who are still on
treatment twelve months after initiating ART.
Denominator:
Total number of adults and children
who initiated ART in the 12 months
prior to the beginning of the reporting
period, including those who have died
and those who have stopped ART. Does
not include transfer outs.
The denominator is defined as the number of
all adults and children who were initiated on
treatment in the 12-month period before the
reporting period. The denominator includes
those “New” on ART as well as those who
“Transferred In” if they have a cohort-start
date within the reporting period of interest.
However, transfers-out should be taken out
of both the denominator as well as the
numerator. It is assumed that if a patient
transfers out from an ART facility, that
patient will be a “transfer in” at a new ART
facility.
Changes in indicator:
• 24 and 36 months were added as optional time periods to monitor changes to
retention of these patients as models of service delivery change for stable patients
on ART (the definition of stable varies across contexts, but often excludes patients
on ART for less than 12 months) (MER 1.0 to MER 2.0).
• As models of service delivery change to reflect longer visit intervals for stable
patients, it is important to emphasize the definition of LTFU applies to both missed
visits or missed drug pick-up, but does not apply who have not received ARVs in the
last 90 days (three months) following their last attended appointment or attended
drug pick-up. As that interval between scheduled visits for stable patients maybe
longer than 3 months.
• Age disaggregations updated (MER 2.0 v2.1 to v2.2).
How to use:
This indicator measures the proportion of individuals who have been retained on
antiretroviral therapy (ART). ART is viewed by the scientific community and PEPFAR not
only as essential for decreasing morbidity and mortality, but also as a highly effective
approach to prevent HIV transmission. High retention is one important measure of
program success, specifically in reducing morbidity and mortality, and is a proxy for
overall quality of the ART program. Monitoring the program level retention is a critical
quality of service indicator at the site, national and PEPFAR program levels as it can
highlight barriers to health seeking behaviors and/or gaps in access to and provision of
health services.
How to collect:
Information should come from electronic systems (EMR) if possible. Where electronic
systems do not exist ART registers/databases and cohort/group analysis forms can be
used to count patients that have been retained after 12, 24 or 36 months on ART. This
indicator should NOT be estimated. This indicator should be calculated directly from
information gathered in standard cohort ART registers or electronic patient level
databases.
106
While reporting on 24 and 36 month retention is optional for PEPFAR reporting, it is
strongly recommended so that programs can have a better understanding of longer
term outcomes for patients on ART.
Sites are required to disaggregate retention by pregnancy and breastfeeding and specific
age/sex disaggregates (see data element below). In order to collect this information ART
registers, cohort/group analysis forms, and EMRs must document age, sex, pregnancy
status, and breastfeeding status on the date of ART initiation.
Of note, for reporting purposes a three-month grace period should be observed
following drug pick-up, before concluding a patient is actually LTFU. However, while
practical, if follow-up of patients is delayed until LTFU is official, the majority of clients
who do not present by three months of last missed appointment/drug pick-up are very
unlikely to return thereafter. Therefore, for patient management, the facility should
make every effort to contact a patient as soon as s/he misses an appointment and/ or
drug pick-up (by phone, via community health worker) rather than waiting for the
prescribed 90 days. This is particularly important when patients are routinely seen every
three to six months (a patient may not have been seen for up to nine months if the
facility adheres to the waiting period before attempting contact). LTFU is an ambiguous
outcome that may often include patients who have self-transferred (silent transfer,
without proper documentation or referral from their original primary care facility) or
have died for which there is no documentation. Every effort should be made to
document the more concreate outcomes for those not on ART (i.e., died, stopped ART,
transfer out) to make the information more useful.
The numerator is defined as the number of adults and children who are still on
treatment twelve months after initiating ART.
For example, if the PEPFAR reporting period is 1 October 2016 to 30 September 2017,
countries will calculate this numerator by using all patients who started ART any time
during the 12-month period from 1 October 2015 to 30 September 2016. The 12-month
outcomes are defined as 1) on ART and 2) not on ART because patient died, stopped ART
or was lost to follow-up (LTFU), (including silent transfers).
On ART is defined as those patients who had received enough ARVs to last to the end of
the reporting period. See example below for more details.
• LTFU is defined as a patient who has not received ARVs in the last 90 days
(three months) following their last missed appointment or missed drug pick-up.
• Died: Patients that are documented death during the previous 12 months
period.
• Stopped ART: Patient intentionally stops ART, usually, but not always in
discussion with the clinical team.
• Known Transfers: Patients who have transferred in with a known treatment
initiation date that falls within the reporting period should be counted.
Conversely, patients who transferred out of the facility should not be counted
in the numerator (or denominator, see below)
Note: this indicator does not collect adherence information, but only retention,
therefore the numerator does not require patients to have been on ART continuously for
the 12-month period. Patients may be included in the numerator (and denominator) if
they have missed an appointment or drug pick-up or temporarily stopped treatment
VIRAL SUPPRESSION
107
during the 12 months since initiating treatment, as long as they are recorded as still
being on treatment at month 12.
For example. A patient who started ART in September 2016 would be considered “on
ART at 12 months” (in September 2017) if:
• The patient visited the facility and received ARVs in September 2017; OR
• The patient had enough ARVs to last through the end of September 2017
(month 12) based on the last drug pick-up (e.g., patient received 60 days of drug
on August 15th, or patient received 90 days of drug on July 1st, etc.).
However, the patient would NOT be considered “on ART at 12 months” if:
• The patient did NOT have enough ARVs to last through the end of September
2017 (e.g., patient received 30 days of drug on August 1st); OR
• The patient had died, transferred out, stopped ART, or was lost to follow-up at
the end of September 2017.
The denominator is defined as the number of all adults and children who were initiated
on treatment in the 12-month period before the reporting period. The denominator
includes those “New” on ART as well as those who “Transferred In” if they have a
cohort-start date within the reporting period of interest. However, transfers-out should
be taken out of both the denominator as well as the numerator. It is assumed that if a
patient transfers out from an ART facility, that patient will be a “transfer in” at a new
ART facility.
For example, for the reporting period October 1, 2016 to September 30, 2017, this will
include all patients who started ART during the 12-month period from October 1, 2015
to September 30, 2016. This includes all patients, both those on ART as well as those
who have died, stopped ART or were lost to follow-up (LTFU).
Only sites that have been operational for at least 24 months prior to the end of the
reporting period should report. PEPFAR country teams may use the USG FY reporting
period as the timeframe for the 12-month cohort. Teams may also wish to ‘lag’ by 1-3
months the cohort-months comprising the annual cohort, in order to allow sufficient
time for reporting from data sources (i.e., implementing partners and/or national
systems).
Reporting level:
Facility
How often to report:
Annually
How to review for
data quality:
• TX_RET Denominator ≥ TX_RET Numerator
• Denominator ≥ subtotal of each disaggregation: The total number of adults and
children who initiated ART in the past 12 months should be greater or equal to the
sum of the disaggregations by (1) Pregnancy/breastfeeding status and (2) age/sex
• Numerator ≥ subtotal of each disaggregation: The total number of adults and
children still on treatment at 12 months should be greater or equal to the sum of
the disaggregations by (1) Pregnancy/ breastfeeding status and (2) age/sex
• Number of PEPFAR supported sites that report TX_RET vs number of sites that
report TX_CURR by region to determine completeness of reporting
How to calculate
annual total:
Use result reported at Q4/APR.
Numerator should be divided by denominator to determine % retained; % retained for
pregnant and breastfeed women; as well as children <15 % retained should be
calculated separately and used to assess these programs.
Numerator:
Disaggregate Groups
Disaggregates
Longer term retention
• 24-month retention
108
Data elements
(components of
indicator):
Number of adults
and children in
the cohort, who
are still on
treatment at 12
months after
initiating ART.
[Optional]
• 36-month retention
Pregnant/Breastfeeding
[Required]
• Pregnant
• Breastfeeding
Age/Sex
[Required]
<1, 1-9, 10-14 M, 10-14 F, 15-19
M, 15-19 F, 20-24 M, 20-24 F,
25-29 M, 25-29 F, 30-34 M, 30-
34 F, 35-39 M, 35-39 F, 40-49
M, 40-49 F, 50+ M, 50+ F
Denominator:
Total number of
adults and
children who
initiated ART in
the in the 12
months prior to
the beginning of
the reporting
period, including
those who have
died, those who
have stopped ART,
and those lost to
follow-up during
the subsequent 12
months.
Disaggregate Groups
Disaggregates
Longer term retention
[Optional]
• 24-month retention
• 36-month retention
Pregnant/Breastfeeding
[Required]
• Pregnant
• Breastfeeding
Age/Sex
[Required]
<1, 1-9, 10-14 M, 10-14 F, 15-19
M, 15-19 F, 20-24 M, 20-24 F,
25-29 M, 25-29 F, 30-34 M, 30-
34 F, 35-39 M, 35-39 F, 40-49
M, 40-49 F, 50+ M, 50+ F
Disaggregate Descriptions & Definitions
Longer term retention: Although optional, it is recommended for sites to include their
longer-term ART retention numbers (including retention at 24 and 36 months).
Pregnant/Breastfeeding: Pregnancy and Breastfeeding status is defined as the status at
the date of initiation on ART, not the status at the date of reporting.
Age/sex: Age is defined as the age at the date of initiation on ART, not the age at the
date of reporting.
PEPFAR-support
definition:
Standard definition of DSD and TA-SDI used.
Provision of key staff or commodities for PLHIV receiving ART include: the provision of
key staff and/or commodities can include ongoing procurement of critical commodities,
such as ARVs, or funding for salaries of HCW who deliver HIV treatment services. Staff
who are responsible for the completeness and quality of routine patient records (paper
or electronic) can be counted here; however, staff who exclusively fulfill MOH and donor
reporting requirements cannot be counted.
Ongoing support for PLHIV receiving ART service delivery improvement includes: clinical
mentoring and supportive supervision of staff at HIV sites providing ART, support for
quality improvement activities, patient tracking system support, routine support of ART
M&E and reporting, commodities consumption forecasting and supply management
Guiding narrative
questions:
1. If TX_RET is below 85%, describe the main reasons for non-retention or difficulties in
capturing retention.
2. If there are geographic, age or sex differences in TX_RET, describe the most likely
reasons

VIRAL SUPPRESSION
109
3. Describe the definition of LTFU utilized by the team to determine if a patient has
been retained on treatment. What systems, registers, or tools were used to calculate
this indicator?
110
TX_PVLS
Description:
Percentage of ART patients with a viral load result documented in the medical record
and/or laboratory information systems (LIS) within the past 12 months with a
suppressed viral load (<1000 copies/ml)
Numerator:
Number of adult and pediatric patients
on ART with suppressed viral load
results (<1,000 copies/ml) documented
in the medical records and /or
supporting laboratory results within the
past 12 months
If there is more than one VL test during the
last 12 months, report the most recent test.
Only patients who have been on ART for at
least 3 months should be counted.
Denominator:
Number of adult and pediatric ART
patients with a viral load result
documented in the patient medical
record and/or laboratory records in the
past 12 months.
Only patients who have been on ART for at
least 3 months should be counted.
Changes in indicator:
• The indicator now requires the suppressed viral load result to be documented in the
clinic patient record and only use the laboratory system for results if it can be linked
back to the individual patient file (MER 1.0 to MER 2.0).
• Age disaggregations updated (MER 2.0 v2.1 to v2.2).
• The indicator now requires that patients be on ART for at least 3 months to be
reported on under TX_PVLS (MER 2.0 v2.2 Revised Release).
• Shift in categorization of follow-up VL test done after an initial VL test result of
VL>1,000. Follow-up viral loads done after an initial VL test result of VL>1,000 should
be counted under routine and not targeted since all patients who receive an initial
VL test result of VL>1,000 should be routinely receive a follow-up VL test after some
enhanced adherence counseling (MER 2.0 v2.2 Revised Release).
How to use:
This indicator monitors the proportion of documented viral load tests from adult and
pediatric ART patients who have been on ART for at least 3 months with a suppressed
result (<1,000 copies/ml), allowing ART programs to monitor individual and overall
programmatic response to ART as measured by virologic suppression. Comparison of the
denominator for this indicator with the result for TX_CURR can be used to estimate viral
load testing coverage supported by PEPFAR.
How to collect:
This indicator should be collected from the clinical source to assure unduplicated patient
counting and receipt of results to inform patient care. Information should come from
electronic systems (EMR) if possible. Where electronic systems do not exist patient
registers can be used to count patients and VL collected/sent VL test (denominator) or
VL results (numerator). If the standard registers or reports do not contain all the
required information, individual patient files should be reviewed. To determine if a lab
test was collected/sent additional supporting information for this indicator can be
obtained from standard laboratory information systems (including electronic systems or
paper-based registries or logbooks), but the viral load test submission and result must be
able to be linked to specific patient.
VL results should be reported for patients who have been on ART for at least 3 months.
It is important to ensure that the data sources used to collect and aggregate data are
updated to be able to report VL results data for patients who have been on ART for at
least three months.
VIRAL SUPPRESSION
111
NOTE: IF the patient file does not include this information (collected/sent VL test or VL
results) but the information was reported from the laboratory information system; then
it is strongly recommended that IP ensure that this information is transcribed to the
patient file for improved quality care and treatment services.
This indicator should be reported for all PEPFAR supported treatment sites (reported
TX_CURR and TX_NEW) with VL monitoring to promote site level use and reporting of
patient viral suppression information. If a PEPFAR supported treatment site has not
conducted any viral load testing, a 0 should be entered for both the denominator, as
well as the numerator. Where more than one result is available for the reporting period,
the most recent result should be reported. If viral load sample has been sent for testing,
but no result has been recorded, this should not be included in the numerator or
denominator of this indicator. Programs should describe the method(s) of data
collection in their APR narratives, along with describing methodology for de-duplication
of results.
Reporting level:
Facility
How often to report:
Annually
How to review for
data quality:
• Denominator ≥ Numerator: The number of viral load tests performed from adults and
children on ART must be greater than or equal to the number of viral load tests from
adult and pediatric ART patients with a viral load <1,000 copies/ml.
• Numerator ≥ subtotal of each disaggregation: The total number of viral load tests
from adult and pediatric ART patients with a viral load <1,000 copies/ml should be
greater than or equal to the sum of all of the disaggregation by age/sex,
pregnancy/breastfeeding status, and test indication.
How to calculate
annual total:
Use result reported at Q4/APR.
Data elements
(components of
indicator):
Numerator:
Number of adult
and pediatric
patients on ART
with suppressed
viral load results
(<1,000
copies/ml)
documented in
the medical
records and /or
supporting
laboratory results
within the past 12
months
Disaggregate Groups
Disaggregates
Indication
[Required]
• Routine;
• Targeted;
• Not Documented
Pregnant/Breastfeeding
Indication
[Required]
• Pregnant Routine;
• Pregnant Targeted;
• Pregnant Not Documented;
• Breastfeeding Routine;
• Breastfeeding Targeted;
• Breastfeeding Not
Documented
Age/Sex/Indication
[Required]
• Routine: <1, 1-9, 10-14 M,
10-14 F, 15-19 M, 15_19 F,
20-24 M, 20-24 F, 25-29 M,
25-29 F, 30-34 M, 30-34 F,
35-39 M, 35-39 F, 40-49 M,
40-49 F, 50+ M, 50+ F;
• Targeted: <1, 1-9, 10-14 M,
10-14 F, 15-19 M, 15_19 F,
20-24 M, 20-24 F, 25-29 M,
25-29 F, 30-34 M, 30-34 F,
35-39 M, 35-39 F, 40-49 M,
40-49 F, 50+ M, 50+ F;
• Not Documented: <1, 1-9,
10-14 M, 10-14 F, 15-19 M,
112
15_19 F, 20-24 M, 20-24 F,
25-29 M, 25-29 F, 30-34 M,
30-34 F, 35-39 M, 35-39 F,
40-49 M, 40-49 F, 50+ M,
50+ F
Denominator:
Number of adult
and pediatric ART
patients with a
viral load result
documented in
the patient
medical record
and /or laboratory
records in the past
12 months.
Disaggregate Groups
Disaggregates
Indication
[Required]
• Routine;
• Targeted;
• Not Documented
Pregnant/Breastfeeding
Indication
[Required]
• Pregnant Routine;
• Pregnant Targeted;
• Pregnant Not Documented;
• Breastfeeding Routine;
• Breastfeeding Targeted;
• Breastfeeding Not
Documented
Age/Sex/Indication
[Required]
• Routine: <1, 1-9, 10-14 M,
10-14 F, 15-19 M, 15_19 F,
20-24 M, 20-24 F, 25-29 M,
25-29 F, 30-34 M, 30-34 F,
35-39 M, 35-39 F, 40-49 M,
40-49 F, 50+ M, 50+ F;
• Targeted: <1, 1-9, 10-14 M,
10-14 F, 15-19 M, 15_19 F,
20-24 M, 20-24 F, 25-29 M,
25-29 F, 30-34 M, 30-34 F,
35-39 M, 35-39 F, 40-49 M,
40-49 F, 50+ M, 50+ F;
• Not Documented: <1, 1-9,
10-14 M, 10-14 F, 15-19 M,
15_19 F, 20-24 M, 20-24 F,
25-29 M, 25-29 F, 30-34 M,
30-34 F, 35-39 M, 35-39 F,
40-49 M, 40-49 F, 50+ M,
50+ F
Disaggregate Descriptions & Definitions
Indication Disaggregate Definitions:
• Routine: Refers to viral load tests obtained at standard intervals following ART
initiation to monitor virologic response to ART (Timing is dependent on the National
guidelines, but should be recommended to occur at least annually). This includes
follow-up viral loads done after an initial VL test result of VL>1000 since follow-up
tests should routinely done on patients with an initial VL>1000.
• Targeted: refers to viral load tests obtained based on a specific clinical indication,
e.g., concern about disease progression or failure to respond to ART.
• Not documented: not indicated in the patient file, registry, or log book whether this
test was targeted or routine.
PEPFAR-support
definition:
Standard definition of DSD and TA-SDI used.
Provision of key staff or commodities for PLHIV receiving ART include: the provision of
key staff and/or commodities can include ongoing procurement of critical commodities,
such as ARVs, or funding for salaries of HCW who deliver HIV treatment services. Staff
who are responsible for the completeness and quality of routine patient records (paper
VIRAL SUPPRESSION
113
or electronic) can be counted here; however, staff who exclusively fulfill MOH and donor
reporting requirements cannot be counted.
Ongoing support for PLHIV receiving ART service delivery improvement includes: clinical
mentoring and supportive supervision of staff at HIV sites providing ART, support for
quality improvement activities, patient tracking system support, routine support of ART
M&E and reporting, commodities consumption forecasting and supply management
Guiding narrative
questions:
1. Please describe the overall proportion of patients who received a VL (i.e., describe
the overall coverage of VL testing in the country, with any differences by region or
age).
2. If there were lower-than-expected numbers of targeted or routine VL testing, explain.
3. Describe any association of ART regimen type with TX_PVLS.
4. Describe the data sources used to report on this indicator (e.g. EMR, LIS, DHIS2 etc.)
and efforts made to ensure individual, not tests are being reported.
5. Clarify how the program is able to ensure that only patients who have been on ART
for at least 3 months are being reported.
6. Briefly describe the VL testing algorithm used in country.

Health Systems
Indicators
HEALTH SYSTEMS
115
SC_STOCK
Description:
Percentage of stock status observations from storage sites where commodities are
stocked according to plan, by level in supply system
Numerator:
Number of stock status observations
per tracer commodity that are between
the designed minimum and maximum
quantities/months of stock from storage
sites at a given level (Central, Regional,
etc.) of the system.
Checking this data frequently can help to
avoid stock-outs through active supply chain
management.
Denominator:
Total number of stock status
observations per tracer commodity
from storage sites at a given level
(Central, Regional, etc.) of the system.
Total observations available are the
denominator.
Changes in indicator:
• Semi-Annual reporting is required for this indicator (MER 1.0 to MER 2.0).
How to use:
This indicator checks to see if the supply chain system is functioning as it was designed
and if storage sites at all levels are able to maintain the designed quantity of
stock/months of stock to treat patients and distribute to lower level facilities which treat
patients. Checking this frequently can help to avoid stock-outs through active supply
chain management.
A view of each level of the system (Central and Intermediate sites), using this metric can
also help to locate bottlenecks within the system, which could prevent patients from
receiving needed commodities; cause needless stock-outs, or unnecessary expiries.
How to collect:
The country’s supply chain standard operating procedures should outline the min and
max levels for each level of the system. These levels were defined by the needed
throughput (the amount of pharmaceuticals intended to flow through the system in a
given period), the space available and the frequency of distribution.
Observations of storage site and level-specific quantity of stock should be available
through one or several of the following: The Procurement Planning and Monitoring
Report for HIV and FP commodities (for condoms), a warehouse monitoring system,
regular program monitoring reports, an existing logistics management information
system, stock status reports/stock keeping records/regular physical counts, order forms
from the central/regional/district/other levels, or regular supervision visits.
For the required central level and at least one intermediate level, there may be
numerous observations (through physical counts performed or spot checks) of stock
status for the products of interest annually, or there may be monthly counts, either way,
the stock status will be monitored closely and updated with each transaction. These
observations should be analyzed in this fashion:
• Document observations for each product of interest.
• Sort observations for each product into “quantities between maximum and
minimum quantities/months of stock” and quantities above or below maximum
and minimum.
• Number of observations where quantities are between maximum and minimum
are the numerator.
• Total observations available are the denominator.
116
Example 1: if the Central Medical Store (CMS) has monthly stock observations for RTKs,
and nine of which are within max and min levels but the remaining three represent a
stock-out then for the CMS the resulting measurement would be 9/12 or 75%
Example 2: If there are ten regions in a country and the regional medical stores report
to the CMS quarterly, then ideally there should be 40 observations. Of these
observations 25 are stocked according to plan for ARVs. In this scenario, the resulting
measurement for ARVs at the regional level is 25/40 or 62.5%.
Reporting level:
Facility (Medical Stores including Central Medical Stores, Regional Medical Stores, and
District sites which supply commodities to lower health facility)
How often to report:
Semi-Annual
How to review for
data quality:
Cross-reference data with shipments arriving, as shipments arrive the quantity of stock
or the months of stock should increase. Ensure the data comes from the warehouse
management system. Consult with supply chain stakeholders to ensure that data is
consistent.
How to calculate
annual total:
N/A
Data elements
(components of
indicator):
Numerator:
Sum the
observations of
stock status for
tracer
commodities that
are between
maximum and
minimum
quantities/months
of stock from
storage sites
within a given
level of the
system during the
reporting period
Disaggregate Groups
Disaggregates
System Level
[Required]
System Level: Central Medical
Stores (CMS), Regional Medical
Stores, District sites which
supply commodities to lower
Health Facility
Commodity
[Required]
• Condoms
• ARV drugs
• Rapid test kits
• OI drugs
• Other
Denominator:
Total number of
observations of
stock status for
tracer
commodities at
the same level of
the system during
the same
reporting period.
Disaggregate Groups
Disaggregates
System Level
[Required]
System Level: Central Medical
Stores (CMS), Regional Medical
Stores, District sites which
supply commodities to lower
Health Facility
Commodity
[Required]
• Condoms
• ARV drugs
• Rapid test kits
• OI drugs
• Other
Disaggregate Descriptions & Definitions
PEPFAR Warehouses in DATIM: Warehouses in the PEPFAR master facility list should be
entered at each system level (this does not have to be re-entered on the entry screen;
however, please ensure that the site has been allocated to one of the system levels)
PEPFAR-support
definition:
Nonstandard definition of DSD and TA-SDI:
PEPFAR Support: PEPFAR direct support to sites within the fiscal year is to ensure
continuous access to commodities for HIV/AIDS patient diagnosis, care, and treatment.
HEALTH SYSTEMS
117
Reasons why access to commodities may be interrupted include poor infrastructure,
inconsistent transportation or distribution practices, lack of equipment, poor ordering
procedures, personnel and technical skills issues, or stock-outs due to any one of the
above from the distribution site. PEPFAR support for supply chain sites should provide
consistent access to commodities needed for care and treatment.
Direct Service Delivery (DSD)
Supply chain sites can be counted as directly supported by PEPFAR when the following
conditions apply:
1) PEPFAR pays for recurrent maintenance, operations, personnel such as those
who are seconded or regular provision of HIV and AIDS commodities.
AND
2) 2) There is at least annual technical support to monitor the support to the
system.
Both conditions must be met in order to count the site as directly supported (DSD) by
PEPFAR.
Technical Assistance-only Support (TA-only)
Supply chain sites can be counted as directly supported through technical assistance-
only when the site receives recurrent (at least quarterly) technical support.
Guiding narrative
questions:
1. Please provide background information to explain observations which were not
stocked according to plan.
a. Indicate if these instances were due to: understock, overstock, or stock-out and
if these challenges lead to rationing of the product from that site or any known
waste or expiries.
b. Provide some root cause for the instances when a site was not stocked
according to plan.
i. Was the problem in-country transportation?
ii. Were sites overstocked in preparation for a testing campaign, Test and
Start or Multi-Month Scripting?
iii. Was there a late international procurement? If so, how late (in days if
possible) and which procurement services agent was responsible for the
late procurement? Likewise, were there ordering or reporting challenges?
118
HRH_PRE
Description:
Number of new health workers who graduated from a pre-service training institution or
program as a result of PEPFAR-supported strengthening efforts, within the reporting
period, by select cadre
Numerator:
Number of new health workers who
graduated from a pre-service training
institution or program as a result of
PEPFAR-supported strengthening
efforts, within the reporting period, by
select cadre
The numerator is the sum of new health
workers from the host country who
graduated from a pre-service training
institution within the reporting period with
full or partial PEPFAR support. Individuals
may be in pre-service training over a number
of years, but can be counted as graduated
when they have completed their program.
Graduates do not need to attend a formal
ceremony – completing the program and
receiving documentation
Denominator:
N/A
Changes in indicator:
No change.
How to use:
It is widely acknowledged that the lack of trained health workers is a major barrier to
scaling up health services. The lack of a sufficient workforce in countries presents a
serious challenge to every area of health. The data will tell us the number of new health
workers who are available to enter the health workforce each year as a result of PEPFAR
support.
How to collect:
Training under this indicator is defined as “pre-service” training – the training of “new”
health workers (see definition below). Training generally occurs prior to the individual
entering the health workforce in his or her new position (with the exception of certain
training that may occur on-the job but that prepares health workers to function as a new
cadre or with an expanded scope of practice in the health system). A health worker who
advances to a higher cadre (e.g., a clinical assistant who completes training to become a
clinical officer) shall be counted as a “new” health worker for the purposes of this
indicator. The HRH goal is to expand the number of workers in the workforce and
increase access to care through the advancement of current workers to higher level
cadres through additional training and education.
Pre-service training institutions are university-based or affiliated schools of medicine,
nursing, public health, social work, laboratory science, pharmacy, and other health-
related fields. Non-professional or paraprofessional training would be any accredited
and nationally recognized pre-service program that is a requirement for this cadre’s
entry into the workforce.
“In-service” and “continuing education” training should not be included in the count for
this indicator, but continue
to be encouraged. These types of training may be captured by other indicators within
program areas (e.g., supply chain).
In order to count the duration of training must meet or exceed a minimum of 6 months.
For example, community health workers who receive a 3-month training course cannot
be counted here. The training duration may be a combination of classroom and practical
field time to arrive at six months.
HEALTH SYSTEMS
119
A pre-service training program must be nationally accredited, or at the minimum meet
national and international standards. The program must also have specific learning
objectives, a course curriculum, expected knowledge, skills, and competencies to be
gained by participants, as well as documented minimum requirements for course
completion. The duration and intensity of training will vary by cadre; however, all
training programs should have at a minimum the criteria listed above.
Individuals may be in training over many reporting periods; however, only participants
who have successfully completed their training should be counted.
Successful completion of training may be documented by diploma, certificate or other
evidence of completion of the program and subsequent eligibility to enter service.
Individuals not meeting these documented requirements should not be counted in this
indicator.
“Health workers” refers to individuals involved in safeguarding and contributing to the
prevention, promotion and protection of the health of the population (both professional
and auxiliary-professionals). The categories below describe the different types of health
workers to be considered under this indicator. This is not an exhaustive list of all health
workers and position titles may vary from country to country. For the purposes of this
indicator, health workers may include the following but is not limited to:
• Clinical professionals, including doctors, nurses, midwives, laboratory scientists,
pharmacists, medical technologists, and psychologists. They usually have a
tertiary education and most countries have a formal method of certifying their
qualifications.
• Clinical officers, medical and nursing assistants, lab and pharmacy technicians,
auxiliary nurses, auxiliary midwives, T&C counselors. They should have
completed a diploma or certificate program according to a standardized or
accredited curriculum and support or substitute for university-trained
professionals.
• Workers in a health ministry, hospital and facility administrators, human
resource managers, monitoring and evaluation advisors, epidemiologists and
other professional staff critical to health service delivery and program support.
• Social service workers including social workers, child and youth development
workers, social welfare assistants.
PEPFAR support includes funding in the areas of curriculum development, teacher
training and support, tuition/scholarships, infrastructure, materials/equipment, and
practica/internships. For example, full or partial support of student tuition or
scholarships, teacher salaries, and expansion/refurbishment of pre-service training
facilities could all count under this indicator depending on the investment.
Data sources: MOH Human Resource Information Systems (HRIS), pre-service training
institutions, Ministry of Education, Public Service, and/or private sector HRIS, Ministry of
Social Welfare HRIS, professional boards and councils, alumni or graduate networks.
Reporting level:
Above-service Delivery Area
How often to report:
Annually
How to review for
data quality:
N/A
120
How to calculate
annual total:
N/A
Data elements
(components of
indicator):
Numerator:
Number of new
health workers
who graduated
from a pre-service
training institution
or program as a
result of PEPFAR-
supported
strengthening
efforts, within the
reporting period,
by select cadre
Disaggregate Groups
Disaggregates
By Cadre:
[Required]
• Doctors
• Nurses
• Midwives
• Social Service Workers
• Laboratory Professionals
• Other
Disaggregate Descriptions & Definitions
N/A
PEPFAR-support
definition:
As an service delivery area indicator, the PEPFAR support categories of DSD and TA-SDI
do not apply. To report results for this indicator, it is expected that PEPFAR provides
support for this activity as defined below.
New health worker graduates of pre-service training institution or program will be
counted as PEPFAR supported when PEPFAR is supporting the training of new health
worker graduates, including:
• Tuition and fees - At least 50% of the students' tuition and fees were or will be
provided by PEPFAR for at least six months of their education
• Curriculum development - The students received or will receive training where
PEPFAR curriculum development was essential to qualify them for their trained role
• Infrastructure - The students received or will receive six months or more of education
at an institution that could not have supported their education without PEPFAR-
supported infrastructure improvements (classrooms, dormitories, utilities)
• Faculty support - The students received or will receive six months of more of
education at an institution that could not have supported their education without one
or more faculty members present and qualified due to PEPFAR support
• Practica / internship support - The students would not have received or will not
receive adequate practica or internship training without PEPFAR support (including
transportation to or sufficient resources at the practicum facility)
• Materials / equipment - The students would not have received or will not receive
education without materials or equipment (including books and supplies) provided by
PEPFAR
• PEPFAR educational programs (for non-university-based training institutions) - The
students received or will receive their education in a PEPFAR-funded, non-university-
based education program for one or more courses without which they would not
graduate or be qualified for the intended role
• Please refer to the HRH flowchart and worksheet for further information
(https://www.pepfarii.net/twg/hrh/SitePages/Home.aspx)
Guiding narrative
questions:
None.
HEALTH SYSTEMS
121
HRH_STAFF
Description:
Number of health worker full-time equivalents who are working on any HIV-related
activities (i.e., prevention, treatment and other HIV support) at PEPFAR-supported
facility sites
Numerator:
Number of health worker full-time
equivalents who are working on any
HIV-related activities (i.e., prevention,
treatment and other HIV support) at
PEPFAR-supported facility sites
This indicator is the number of full-time
equivalent positions (FTE) working on HIV
(“HIV FTE”) at PEPFAR facility sites.
Denominator:
N/A
Changes in indicator:
No changes in this indicator.
How to use:
This indicator is the number of full-time equivalent positions (FTE) working on HIV (“HIV
FTE”) at PEPFAR facility sites. Calculate part-time positions working exclusively on HIV,
or full-time positions working on several areas including HIV and other illnesses, as
fractions, based on hours worked relative to full-time equivalency hours. Full time
equivalency hours should be the standard listed in the cadre’s scheme of service and/or
Ministry of Health guidelines.
This is NOT a cumulative total, but a one-time count undertaken during the final quarter.
Only filled staff positions at respective facility should be counted.
For this indicator, a “PEPFAR supported site” should include any facility site in the
PEPFAR geographic organizational hierarchy list in DATIM, which also reported any site-
level programmatic target or result during the same reporting period. Omit community
sites. Omit facilities which were previously supported by PEPFAR, but were not assigned
any targets nor reported any results for any program area during the same reporting
period. Include all health care workers irrespective of whether any or all are receiving
PEPFAR support (this is captured in HRH_CURR.)
HIV/AIDS has placed significant demands on the already constrained health workforce in
many low-income countries. The rapid scale-up of ART is placing additional demands on
the health workforce.
In the majority of PEPFAR countries, there are overall shortages of HRH, particularly in
rural and remote areas, leading to insufficient numbers of health workers according to
internationally recommended levels (2.3 doctors, nurses, midwives/1,000 population).
Many countries experience HRH shortages and/or imbalances by population densities
(e.g., HRH shortages in rural areas) that are not related to population health needs,
including HIV epidemiology. Addressing density, distribution, and overall utilization of
HRH is important in increasing access to HIV services.
This indicator allows PEPFAR to analyze the availability of staff to provide HIV services at
PEPFAR supported facilities. Data should be reviewed against site target achievement
and investment. The first year of data collection will serve as an Integral benchmark for
continued analysis.
Teams can also look at this indicator in conjunction with HRH_CURR that captures
number of PEPFAR supported workers at PEPFAR-supported sites. This will allow PEPFAR
to conduct analysis to determine if the number of PEPFAR-supported staff is appropriate
vis-à-vis the number of other staff at the facility providing HIV services.
122
There is no universal benchmark against which to measure these data and no ideal
PEPFAR to non-PEPFAR ratio. However, over time we would hope to see a decrease in
the number of PEPFAR-supported staff. As this happens countries should carefully
monitor any changes total number of staff working in HIV service delivery at sites and
quality of services.
How to collect:
PEPFAR team or Implementing Partners (IP) should collect and report on this data during
the last quarter of the year. Designate one IP per site to collect HRH_STAFF. If more
than one IP is working at the same PEPFAR supported facility, teams should determine
which IP will collect data for HRH_STAFF. Country teams need to collect data from all
PEPFAR-supported sites irrespective of PEPFAR’s financial support of health workers at a
site (as captured by HRH_CURR.)
Number of health workers reported should be expressed as full-time equivalency (FTE)
positions, including part-time health workers or health workers who work part-time on
HIV, expressed as fractions of FTE corresponding to estimated hours worked on HIV per
week out of total hours per week prescribed as full-time for that cadre in the national
scheme of service, or other Ministry of Health guidelines.
Report HRH who are actively working on services or programs related to HIV at the time
of data collection, not including staff who have resigned, absconded, are dismissed, are
pending hiring, or are on extended leave (e.g., for graduate studies). Unfilled positions
or vacancies should not be included.
If possible, avoid collecting data across a period which spans across a major budgetary
change or a health worker graduation and placement period.
Reporting level:
Facility
How often to report:
Annual
How to review for
data quality:
Numerator auto-calculates based on the sum of the cadre group type disaggregation.
How to calculate
annual total:
Use results reported at Q4.
Data elements
(components of
indicator):
Numerator:
Number of health
worker full-time
equivalents who
are working on
any HIV-related
activities (i.e.,
prevention,
treatment and
other HIV support)
at PEPFAR-
supported facility
sites
Disaggregate Groups
Disaggregates
By Cadre Group Type:
[Required]
• Clinical
• Clinical Support
• Management
• Social Service
• Lay
• Other
Disaggregate Descriptions & Definitions
Cadre Group Type Definitions:
Note: In the indicator narrative, please specify which cadres you included in each cadre
group.
• Clinical workers are those who provide a direct clinical service to clients:
(Clinical professionals, including doctors, nurses, midwives, clinical officers, medical
and nursing assistants, auxiliary nurses, auxiliary midwives, testing and counseling
providers. They should have completed a diploma or certificate program according to
HEALTH SYSTEMS
123
a standardized or accredited curriculum and support or substitute for university-
trained professionals.)
• Clinical Support workers are those who support clinical services at the site but do not
directly provide services to clients: (Pharmacists, medical technologists,
laboratorians, lab and pharmacy technicians)
• Management workers are those who provide support to the site for administrative
needs but not directly provide services to clients: (Facility administrators, human
resource managers, monitoring and evaluation advisors, epidemiologists and other
professional staff critical to health service delivery and program support.)
• Social Service workers are those who have advanced training in social services and
provide services directly to clients: Social service workers including social workers,
child and youth development workers, social welfare assistants.
• Lay workers are those who have non-clinical training and provide services directly to
clients: (Health workers who provide important services for the continuum of care
within facilities and/or communities. These include (but are not limited to) adherence
support, mother mentors, cough monitors, expert clients, lay counselors, peer
educators, community health workers and other community-based cadres)
• Other – workers who do not fit into any of the categories above.
PEPFAR-support
definition:
A “PEPFAR supported site” for the purpose of this indicator includes any facility site in
the PEPFAR master facility list in DATIM which also reported any programmatic target or
result during the same reporting period.
Report all HRH at those sites who are working in HIV-related activities, regardless of
whether they are supported by PEPFAR or not.
Guiding narrative
questions:
1. Please provide description of how FTE was calculated.
2. For all categories of workers, including other, please provide description of specific
cadres in the narrative when reporting.
124
HRH_CURR
Description:
Number of health worker full-time equivalents who are working on any HIV-related
activities i.e., prevention, treatment and other HIV support and are receiving any type of
support from PEPFAR
Numerator:
Number of health worker full-time
equivalents who are working on any
HIV-related activities i.e., prevention,
treatment and other HIV support and
are receiving any type of support from
PEPFAR
This indicator is reported at the facility,
community, and above-service delivery
areas.
Denominator:
N/A
Changes in indicator:
• HRH_CURR was previously reported at the facility site and community site levels by
type of cadre and type of support. Above service delivery area workers are now
included in this indicator (MER 1.0 to MER 2.0).
• Added new types of staffing support (Salaried staff, Staff receiving Stipends, Staff
receiving non-monetary support) (MER 1.0 to MER 2.0).
How to use:
Many countries experience HRH shortages and/or imbalances by population density
(e.g., HRH shortages in rural areas) that are not related to population health needs,
including HIV epidemiology; addressing density and distribution of HRH is important in
increasing access to HIV services.
In many PEPFAR countries, there are overall shortages of HRH, particularly in rural and
remote areas, leading to insufficient numbers of health workers according to
internationally recommended levels (2.3 doctors, nurses, midwives/1,000 population).
There are also countries where there is large overproduction of health workers, with
medical unemployment in urban areas, and at the same time with shortages in rural
areas.
Furthermore, different types of health workers receive different types and amounts of
support that may vary by geographic location, cadre, workload, and other factors.
Understanding the ways in which different cadres are supported is important for
mobilizing differential models of service delivery under different circumstances.
This indicator measures the person-time that PEPFAR-supported health workers
contribute to providing HIV services at facility and community sites. It allows us to track
our level of support and continuously calibrate it based on impact. It also allows us, over
time, to measure the transition from PEPFAR support to host country support.
How to collect:
Data on total numbers of positions or FTEs supported should be tracked by
implementing partner’s record-keeping systems, for example, personnel databases,
human resources records, and financial records that show salary or stipend payments,
including information on non-monetary support to volunteers. Leverage the same
records and systems partners already use to report dollar amounts for EA reporting, to
identify PEPFAR support of HRH. Hours worked on HIV may be estimated using staff
work-week scheduling calendars and HIV clinic/lab opening hours, and speaking with
facility in-charges. For community sites, hours worked on HIV can be estimated using
average beneficiary consultation times, and average number of consultations.
For non-monetary supported personnel, partners should cross-reference expense
reports and registers against the cadre types who received the corresponding non-
monetary benefits. For example, receipts showing transportation allowances were
HEALTH SYSTEMS
125
provided to attend meetings could be cross-referenced with the attendance listed in the
minutes for community lay workers.
Facility and community workers are reported by IM, Site ID, facility and community site
affiliation, and cadre type. All PEPFAR-supported workers at the facility and community
should be reported.
We recommend that PEPFAR implementing partners following these steps:
1) Identify all facility and community sites where you work.
2) Identify and count the number of health workers (individuals) you support at
each site.
3) Group these health workers into their most appropriate, mutually exclusive
cadre (doctor, nurse, lay counselor, lab technician).
4) List all types of monetary and non-monetary support that were provided to
health workers at any of those sites in the current fiscal year (as incentive or
compensation for time spent on HIV services at those sites).
5) Assign those types of support to the health workers identified on your site lists.
Create a matrix of supported health workers by cadre and support type:
6) Further split the health workers into sub-groups based on the most appropriate
mutually exclusive type of PEPFAR support. (*Assign FTE to the “highest”
category - Non-monetary support should be reported if you provide only non-
monetary support, with no salary or stipend
7) Calculate the FTE: Hours per week that this mechanism supports for HIV-related
services at this site / Hours in a full-time work week
Repeat this separately for the three types of support:
8) Take the average FTE for each cadre
9) Add up the total FTE within each broader cadre category (clinical, clinical
support, management, lay, social service, other)
10) Enter this amount in DATIM in the corresponding box for cadre category –
support type.
Above-service delivery area support may include Ministry of Health or other government
staff who work at the district or provincial level, or at the national level, including
Ministry of Health office, National Reference Laboratories, or at national research
centers not otherwise providing HIV services directly to beneficiaries.
Reporting level:
Facility, Community, and Above-Service Delivery Area.
How often to report:
Annual
How to review for
data quality:
Appendix 6 outlines an example HRH_CURR calculation that helps to articulate the
reporting structure of this indicator.
How to calculate
annual total:
Fill out disaggregated data entry form first, annual total will auto-calculate from
disaggregates. Data should capture health workers for whom PEPFAR provided support
in the same reporting period (fiscal year), and who have not been transitioned by the
end of the fiscal year. Unfilled positions or vacancies should not be included.
Data elements
(components of
indicator):
Numerator:
Number of health
worker full-time
equivalents who
are working on
any HIV-related
activities i.e.,
prevention,
treatment and
Disaggregate Groups
Disaggregates
By Cadre Category (Facility &
Community-Level) by type of
support provided by PEPFAR to
the staff
[Required]
• Clinical: Salaried Staff (FTE);
Staff Receiving Stipends
(FTE); Staff Receiving ONLY
Non-Monetary Support
(FTE);
• Clinical Support: Salaried
Staff (FTE); Staff Receiving
Stipends (FTE); Staff
126
other HIV support
and are receiving
any type of
support from
PEPFAR at facility
sites, community
sites, and at the
above-service
delivery area
level.
Receiving ONLY Non-
Monetary Support (FTE);
• Management: Salaried Staff
(FTE); Staff Receiving
Stipends (FTE); Staff
Receiving ONLY Non-
Monetary Support (FTE);
• Social Service: Salaried Staff
(FTE); Staff Receiving
Stipends (FTE); Staff
Receiving ONLY Non-
Monetary Support (FTE);
• Lay: Salaried Staff (FTE); Staff
Receiving Stipends (FTE);
Staff Receiving ONLY Non-
Monetary Support (FTE);
• Other: Salaried Staff (FTE);
Staff Receiving Stipends
(FTE); Staff Receiving ONLY
Non-Monetary Support (FTE)
By Cadre Category (Above-
Service Delivery Area) by type
of support provided by PEPFAR
to the staff
[Required]
• Management (Central Level):
Salaried Staff (FTE); Staff
Receiving Stipends (FTE);
• Management (Subnational
Unit Level): Salaried Staff
(FTE); Staff Receiving
Stipends (FTE);
• Epidemiologist/Surveillance:
Management (Central Level):
Salaried Staff (FTE); Staff
Receiving Stipends (FTE);
• Faculty/Tutors: Management
(Central Level): Salaried Staff
(FTE); Staff Receiving
Stipends (FTE);
• Other: Management (Central
Level): Salaried Staff (FTE);
Staff Receiving Stipends
(FTE)
Disaggregate Descriptions & Definitions
Cadre Category (Facility & Community Level) Descriptions:
• Clinical workers are those who provide a direct clinical service to clients: Clinical
professionals, including doctors, nurses, midwives, clinical officers, medical and
nursing assistants, auxiliary nurses, auxiliary midwives, testing and counseling
providers. They should have completed a diploma or certificate program
according to a standardized or accredited curriculum and support or substitute
for university-trained professionals.
• Clinical Support workers are those who support clinical services at the site but do
not directly provide services to clients: Pharmacists, medical technologists,
laboratorians, lab and pharmacy technicians
• Management workers are those who provide support to the site for
administrative needs but not directly provide services to clients: Facility
HEALTH SYSTEMS
127
administrators, human resource managers, monitoring and evaluation advisors,
epidemiologists and other professional staff critical to health service delivery and
program support.
• Social Service workers are those who have advanced training in social services
and provide services directly to clients: Social service workers including social
workers, child and youth development workers, social welfare assistants.
• Lay workers are those who have non-clinical training and provide services
directly to clients: Health workers who provide important services for the
continuum of care within facilities and/or communities. These include but are
not limited to adherence support, mother mentors, cough monitors, expert
clients, lay counselors, peer educators, community health workers and other
community-based cadres.
• Other: workers who do not fit into any of the categories above.
Cadre Category (Above Service Delivery Area) Descriptions:
• Management central level are those staff supporting management functions at
national level. Examples may be development and implementation of policies,
guidelines, quality standards, health or HIV budgeting and financing. The work of
these staff has a national scope and affect all (or multiple) districts or regions.
• Management sub-national unit are those staff supporting management functions
for one geographic area at the sub-national level. Examples may include district-
level health planning and coordination, district-level quality improvement,
training or mentoring (e.g., district health office, provincial coordinating
authority)
• Faculty (Tutors and Trainers) are those staff working at pre-service institutions
and training centers/departments.
• Epi/Surveillance staff are those collecting and/or analyzing HIV epidemiologic
data at the above-service delivery area level. This may include making national
or district-level estimates of PLHIV or key populations, incidence modeling, ANC
or sentinel surveillance, integrated behavioral and biological surveys, drug
resistance estimates.
• Other types of staff not covered by the above categories.
Type of Support Provided by PEPFAR to the Staff: For each cadre category supported by
PEPFAR at the site level, further disaggregate the HIV FTE by the type of support
provided by PEPFAR. The total HIV FTE should equal the sum of the HIV FTE by three
types of support. Do not disaggregate the above-service delivery area cadre category
FTE by type of support.
• Salary – Total number of HIV FTE positions for which PEPFAR is providing any
level of financial support toward their regular salary. Include all HIV FTE (all
person-time spent on HIV) if any amount of salary support is provided, even if
they also receive support from sources other than PEPFAR. This represents the
total FTE that are “touched” by PEPFAR salary support. PEPFAR salary support is
any ongoing monetary contribution bench marked toward a total salary which is
benchmarked toward, a government salary scale or international salary
standard). A salary is characterized by being disbursed at regularly scheduled
intervals in expected denominations.
• Stipend – Total number of HIV FTE positions for which PEPFAR does not provide
salary support but does provide monetary payments in connection with the
provision of HIV services. Stipend payments are not necessarily disbursed in
regularly scheduled intervals, and are not necessarily commensurate with, nor
benchmarked toward, a government salary scale or international salary standard.
These include one-time reimbursements for expenses connected to travel or
training (per diems); and supplementary payments, for example, for overtime
128
worked due to HIV case burden. Payment could be made at regular intervals
depending on agreement.
• Non-monetary only – Total number of HIV FTE positions for which PEPFAR
provides only non-monetary support. Report if PEPFAR provides only non-
monetary forms of support that do not involve currency, in connection with or in
support of the provision of HIV services. These include mobile phone credits,
meals, general modes of transportation like bicycle or motorbike, job aids or
equipment that can be used outside of HIV or in other jobs (such as in private
practice), or other in-kind support. Include volunteers who work on HIV and
receive only non-monetary support from PEPFAR.
PEPFAR-support
definition:
No additional requirements needed outside of the standard definition.
Guiding narrative
questions:
1. Please provide description of how FTE was calculated.
2. For all categories of workers, including other, please provide description of specific
cadres in the narrative.
3. Please include description of what type types of non-monetary support are captured.
4. Please confirm that workers listed as under non-monetary receiving only non-
monetary support (not in addition to salary or stipend)?
HEALTH SYSTEMS
129
EMR_SITE
Description:
Number of PEPFAR-supported facilities that have an electronic medical record system
within the following service delivery areas: HIV Testing Services, Care & Treatment,
Antenatal or Maternity Services, Early Infant Diagnosis or Under Five Clinic, or TB/HIV
Services
Numerator:
Number of PEPFAR-supported facilities
that have an electronic medical record
system within the following service
delivery areas: HIV Testing Services,
Care & Treatment, Antenatal or
Maternity Services, Early Infant
Diagnosis or Under Five Clinic, or TB/HIV
Services
Answer recorded separately for each service
delivery area.
Denominator:
Denominator is not collected as part of this indicator. However, it should be the total
number of PEPFAR supported active service delivery areas (those sites that reported
either targets or results for indicators related to that service delivery area at each site).
Changes in indicator:
None
How to use:
This indicator can be used as a cross-sectional indicator at Q4. It can be used to better
understand PEPFAR’s investments in Strategic information and to support a broader
understanding of data quality challenges for other indicators. Timely access to up-to-
date patient information plays a vital role in the provision of effective clinical care by
health professionals. Diagnosis and treatment can be improved if health professionals
have easy access to accurate and comprehensive medical records of patients.
How to collect:
The implementing partner should indicate whether the PEPFAR-Supported service
delivery areas have implemented and are actively using an electronic medical record
system to assist clinical service provision or patient/program monitoring and reporting.
Specifically, for PEPFAR reporting a minimum of 6 months of retrospective data should
be included in the EMR. (For example, an ART EMR set up in September 2018 to contain
at least 6 months of retrospective data (current patients that have been enrolled on
ART) could be counted in the reporting at FY18 APR.
For example, if services are integrated, for example EID as part of the Treatment
services, then as long as EID is captured in the treatment services EMR or a separate
EMR for EID is available within these services, then this would be counted as an EID EMR
as well.
Definition of an Electronic Medical Record (EMR):
An EMR is a longitudinal electronic record of an individual patient's health information
that can assist health professionals with decision-making and treatment. Data found in a
record may include patient demographics, past medical history, vital signs, examination
and progress notes, medications, allergies, immunizations, laboratory test results, other
test results. It can also support the collection of data for other uses such as quality
management, public health disease surveillance and reporting. < WHO: Global
Observatory for eHealth > EMR can include real-time point-of-care data entry as well as
retrospective data entry. An electronic medical record (EMR) is a digital version of a
paper chart that contains key information in a patient’s medical history from one service
delivery point or site.
Individual service delivery area/point EMR versus Integrated Health EMR:
130
EMRs are typically for all health areas, but PEPFAR is interested in better understanding
whether EMRs are available for the service delivery areas where PEPFAR focusses its
work (presented in the disaggregation below). If a service delivery area is incorporated
in a larger integrated health EMR, then it should be included this indicator. If two or
more service areas are in an integrated EMR, both areas should be included in this
indicator. A site service delivery area should be included in this indicator if the EMR is on
site (Server and Computer entry screen or there is a central server at a hub facility, that
includes all data from all the “spokes” for that facility’s catchment area. As long as the
data for patient management and reporting comes from the EMR system as one source.
Registries:
Some sites maintain types of e-Registers (which might provide basic functionality like
reporting, default tracing, etc.). However, if these e-Registers do not capture
longitudinal clinical information, they should not be included in this indicator.
Reporting level:
Facility-level by service delivery area
How often to report:
Annually
How to review for
data quality:
If a site does not report ART (PEPFAR-supported ART site), then it should not be included
as having an ART EMR. Number of service delivery area with an EMR should not exceed
the number of service delivery areas reporting results/targets.
How to calculate
annual total:
Use annual result reported at Q4.
Data elements
(components of
indicator):
Numerator:
Number of
PEPFAR-supported
facilities that have
an electronic
medical record
system
Disaggregate Groups
Disaggregates
Service Delivery Area [Required]
• HIV Testing Services;
• Care & Treatment (includes
Pediatric and Adolescent
Care and Treatment Services;
• Antenatal and/or Maternity
Services;
• Early Infant Diagnosis and/or
Under Five Clinic (not
Pediatric ART Services);
• TB/HIV Services
Disaggregate Descriptions & Definitions
Service Delivery Area:
• HIV Testing services: includes counselling (pre-test information and post-test
counselling); linkage to appropriate HIV services; and coordination with laboratory
services to support quality assurance and the delivery of correct results.
• Treatment services: includes services where ART is initiated and monitored.
• Antenatal/maternity services: HIV Testing and treatment in an ANC and/or maternity
setting
• EID services: HIV testing and care for infants of HIV positive women, often linked to
<5 children services and/or maternity services, but can also be part of an ART clinic,
but with its own EMR EID
• TB/HIV services: includes routine screening, diagnosis, treatment, and prevention of
TB among PLWHA or routine HIV testing and counseling and appropriate referral in
persons with TB
PEPFAR-support
definition:
The PEPFAR support categories of DSD and TA-SDI do not apply to this indicator. To
report results for this indicator, it is expected that PEPFAR provides support to the HIV
service delivery area. PEPFAR did not have to support the development of the EMR in
order for it to be counted. EMRs supported by other donors or Ministries of Health
should be included in this indicator. It is highly recommended that service delivery
HEALTH SYSTEMS
131
areas that have functional EMRs use these both for patient management as well as
reporting.
Definitions:
What is a PEPFAR supported site for the purpose of this indicator?
A “PEPFAR supported site” for the purpose of this indicator should include any facility in
the PEPFAR master facility list in DATIM which also reported any programmatic target or
result during the same reporting period.
What is a PEPFAR-Supported Service Delivery area at a site for the purpose of this
indicator?
A PEPFAR-supported facility-based service delivery area uses PEPFAR funds to provide
HIV-related services at service delivery points within the facility. It offers one or more
HIV-related services including but not limited to: HIV testing and counseling; prevention
of mother-to-child transmission of HIV (PMTCT); anti-retroviral treatment (ART) and
TB/HIV services. Examples include different HIV services within clinics, hospitals, health
facilities and community-based organizations (government, private or NGO). These can
also include fixed locations and/or mobile operations offering routine and/or regularly
scheduled services.
Guiding narrative
questions:
1. In the narrative, implementing partners should describe the primary EMR(s) in use for
each the service delivery areas within the sites they support. Indicate the platforms
that these EMRS were created on and who the primary partner, developer, or donor
is that is responsible for maintaining these EMRs at the sites.
132
LAB_PTCQI
Description:
Number of PEPFAR-supported laboratory-based testing and/or Point-of-Care Testing
(POCT) sites engaged in continuous quality Improvement (CQI) and proficiency testing
(PT) activities.
Numerator:
• Number of PEPFAR-supported
laboratory-based testing and/or
Point-of-Care Testing sites engaged
in CQI activities.
• Number of PEPFAR-supported
laboratory-based testing and/or
Point-of-Care Testing sites engaged
in PT activities.
• Number of specimens received for
testing at all PEPFAR-supported
laboratory-based testing and/or
Point-of-Care Testing sites within a
testing category.
The numerator is generated by counting the
number of PEPFAR-supported laboratory-
based testing and point-of-care testing sites
for each testing category by their level of
engagement in CQI and PT activities; and the
number of specimens received for testing at
laboratory-based testing and point-of-care
testing sites within each testing category.
Denominator:
N/A
Changes in indicator:
• LAB_PTCQI will now capture the volume of specimens received for testing at
laboratory-based testing sites in each testing category (MER 2.0 v2.1 to v2.2).
How to use:
The intent of this indicator is to monitor the level of engagement in CQI and PT activities
at PEPFAR-supported laboratory-based testing and/or POCT sites by testing category as
well as the number of specimens received for testing at those sites. CQI and PT
programs are critical to ensure efficient and quality assured laboratory testing. By
monitoring the level of engagement in CQI and PT, this indicator will encourage sites to
participate in CQI and PT for the first time and/or enhance their level of engagement in
CQI and PT.
How to collect:
Which facilities are counted?
Collect data for the LAB_PTCQI, both laboratory and POCT, indicator at facilities with
PEPFAR-supported laboratories. See definitions for ‘laboratory’ and ‘POCT site’ below.
How many laboratory-based testing sites are in the facility?
A facility may have one laboratory-based testing site (e.g., HIV Viral Load laboratory-
based testing site), multiple laboratory-based testing sites with different testing
categories (e.g., HIV Serology/Diagnostic and HIV Viral Load laboratory-based testing
sites), and/or multiple laboratory-based testing sites with the same testing category
(e.g., Two HIV Viral Load laboratory-based testing sites - each under a distinct
entity/department within the facility).
How many POCT sites are in the facility?
A facility may have one POCT site (e.g., HIV Rapid Test POCT site), multiple POCT sites
with different testing categories (e.g., HIV Rapid Test POCT site and CD4 POCT site),
and/or multiple POCT sites with the same testing category (e.g., Two HIV
Serology/Diagnostic test POCT sites – one associated with the PMTCT program and the
other associated with the TB program).
Where can data for this indicator be found?
Data on engagement in CQI and PT can be obtained from program records of PEPFAR-
funded partners. Additionally, laboratory-based testing and POCT site-level
documentation can be used to assess CQI engagement and PT results. Data on the
HEALTH SYSTEMS
133
number of specimens received for testing can be obtained from specimen registers/log
books and/or laboratory information systems (LIS).
How are data interpreted and reported (Laboratory-Based Testing)?
Identify the level of engagement in CQI activities for each laboratory-based testing site
by choosing one of the following:
• Performs this test, but does not participate in CQI (see definition of ‘CQI
participation’ below).
• Performs this test and participates in CQI, but has not been externally audited (see
definition of ‘external audit’ below).
• Performs this test, participates in CQI, and has been externally audited, but does not
meet full accreditation standards (see definition of ‘accreditation’ below).
• Performs this test, participates in CQI, has been externally audited, and is fully
accredited.
• Identify the level of engagement in PT activities for each laboratory-based testing site
by choosing one of the following:
• Performs this test, but does not participate in PT (see definition of ‘PT participation’
below).
• Performs this test, participates in PT, but did not pass the last round (see definition of
‘passing PT’ below).
• Performs this test, participates in PT, and passed the last round.
Sum the number of specimens received for testing at all laboratory-based testing sites
within a testing category. See definition for ‘specimens received for testing’.
How are data interpreted and reported (Point-of-Care Testing)?
Identify the level of engagement in CQI activities for each POCT site by choosing one of
the following:
• Performs this test, but does not participate in CQI.
• Performs this test and participates in CQI, but has not been externally audited.
• Performs this test, participates in CQI, has been externally audited, and achieved a
score of 0-1 (≤ 59%)
• Performs this test, participates in CQI, has been externally audited, and achieved a
score of 2-3 (60%-89%)
• Performs this test, participates in CQI, has been externally audited, and achieved a
score of 4-certified (≥ 90%)
• Identify the level of engagement in PT activities for each POCT site by choosing one of
the following:
• Performs this test, but does not participate in PT (see definition of ‘PT participation’
below).
• Performs this test, participates in PT, but did not pass the last round (see definition of
‘passing PT’ below).
• Performs this test, participates in PT, and passed the last round.
Sum the number of specimens received for testing at all POCT sites within a testing
category. See definition for ‘specimens received for testing’.
DEFINITIONS (LABORATORY-BASED TESTING SITES):
Laboratory:
A. Having dedicated physical laboratory infrastructure
B. Having dedicated trained laboratory professionals performing testing.
134
C. Conducting laboratory testing in one or more of the following areas:
a. Diagnosis of HIV infection with rapid test kits, EIA, WB or other molecular
methods
b. Infant Virologic Testing / Early Infant Diagnosis (IVT/EID)
c. HIV viral load
d. TB diagnostics: Xpert, AFB, or culture
e. CD4 testing
f. Others, including:
g. Blood bank screening and/or cross-matching
b. Hematology
c. Clinical chemistry
d. Serology
e. Microbiology
f. Malaria infection diagnostics
g. STI diagnostics
h. OI (Opportunistic Infection) diagnostics, including Cryptococcal antigen
Note: If a point-of-care assay (such as a rapid diagnostic test or Pima CD4) is performed
at a laboratory-based testing site, as defined above, data should be reported in the
laboratory portion of the indicator LAB_PTCQI indicator.
Laboratory-based testing site:
A point within a facility (with a PEPFAR-supported laboratory) that performs one of the
tests defined in the testing categories within a laboratory.
Blood centers/banks:
Perform any service involved in blood donor recruitment, blood and plasma collection,
testing, processing, storage, and distribution of blood and blood products. Stand-alone
blood center/banks conducting testing such as screening and/or cross-matching are
considered laboratories for this indicator.
CQI Participation:
CQI activities implement, improve, or maintain a Quality Management System (QMS). A
functioning QMS is essential to provide accurate and reliable results with safety,
efficiency, monitoring, and accountability throughout the testing process.
A laboratory-based testing site is counted as participating in CQI if they are engaged in
activities within the testing category that are supported by a locally, nationally,
regionally or internationally recognized CQI or accreditation preparedness program.
Examples of recognized programs:
A. Strengthening Laboratory Management Towards Accreditation (SLMTA)
B. Other established programs that utilize an auditing process such as WHO AFRO
Stepwise Laboratory Quality Improvement Process Towards accreditation (SLIPTA)
stepwise processes or CDC/PAHO Caribbean Laboratory Quality Management System
Stepwise Improvement Process towards Accreditation (CDC/PAHO LQMS-SIP).
C. Locally-recognized basic laboratory quality management system programs
D. Participation in laboratory accreditation programs based on recognized laboratory
standards such as African Society for Blood Transfusion (AfSBT), College of American
Pathologists (CAP), or International Organization for Standardization (ISO).
External Audit:
Refers to a documented assessment conducted by a qualified external auditor. External
audits can either be those for accreditation or those to assess readiness for accreditation
such as WHO AFRO Stepwise Laboratory Quality Improvement Process Towards
Accreditation (SLIPTA) and CDC/PAHO Caribbean Laboratory Quality Management
System Stepwise Improvement Process towards Accreditation (CDC/PAHO LQMS-SIP).
HEALTH SYSTEMS
135
Internal assessments and audits, including those conducted as part of a training program
curriculum; do not count towards this indicator.
Accreditation:
Refers to accreditation by a national, regional or internationally recognized accreditation
body, such as College of American Pathologists (CAP), International Organization for
Standardization (ISO) accreditation programs, regional accreditation bodies such as the
South African National Accreditation System (SANAS), African Society for Blood
Transfusion (AfSBT), or other approved accreditation organizations. A laboratory-based
testing site is assessed by a standardized set of criteria defined by an acceptable
national, regional, or international organization. Accreditation certificates are a formal
recognition that a laboratory is competent to perform clinical testing. Laboratory-based
testing site accreditation status must be current.
PT Participation:
Defined as enrollment/participation in a local, national, regional, and/or international
external quality assurance or proficiency testing program.
Passing PT:
A laboratory-based testing site is counted as passing PT if the last scored PT panel is
acceptable, successful, or satisfactory as scored by the PT provider. Be aware that
scoring systems between PT providers and with test categories may differ.
Specimen received for testing:
A specimen is received for testing if its arrival at the laboratory-based testing site was
recorded in a register/log book and/or LIS within the reporting timeframe. A specimen
received for testing may or may not have been tested/analyzed.
DEFINITIONS (POINT-OF-CARE TESTING SITES):
POCT site:
A. The site performs testing near or at the place of interaction with the patient/client.
B. The site performs testing in an environment which does not have a formal laboratory
infrastructure.
C. Testing at the POCT site is performed by healthcare workers who may not be
laboratorians.
D. Conducting POCT in one or more of the following areas:
a. HIV rapid test
b. Infant Virologic Testing / Early Infant Diagnosis (IVT/EID)
c. HIV viral load
d. TB diagnostics: Xpert or AFB
e. CD4 testing
Notes: A laboratory-based testing site and POCT site may both be present at a facility. If
a point-of-care assay (such as an HIV rapid test or Pima CD4) is performed at a
laboratory-based testing site, CQI and PT data should be reported in the laboratory
portion of the indicator (LAB_PTCQI (Laboratory)).
CQI Participation:
A POCT site is counted as participating in CQI if they are engaged in activities within the
defined test category that are supported by a locally, nationally, regionally or
internationally recognized CQI or certification preparedness program.
Examples of POCT CQI programs:
A. Rapid Testing Continuous Quality Improvement (RT-CQI)
B. Other established programs that utilize WHO/CDC Stepwise Process for Improving
the Quality of HIV rapid testing (SPI-RT) or the WHO/CDC Stepwise process for
136
Improving the Quality of HIV-Related Point-of-Care-Testing (SPI-POCT) Checklists to
audit the POCT sites.
C. Locally-recognized basic quality management system programs
External Audit or Certification:
Refers to a documented assessment conducted by a qualified external auditor. These
audits include those for national POCT site certification or for a stepwise quality
improvement approaches such as the WHO/CDC Stepwise Process for Improving the
Quality of HIV rapid testing (SPI-RT) or the WHO/CDC Stepwise process for Improving the
Quality of HIV-Related Point-of-Care-Testing (SPI-POCT) Checklists. Internal assessments
and audits, including those conducted as part of a training program curriculum; do not
count towards this indicator.
PT Participation:
Defined as enrollment/participation in a local, national, regional, and/or international
external quality assurance or proficiency testing program.
Passing PT:
A POCT site is counted as passing PT if the last scored PT panel is acceptable, successful,
or satisfactory as scored by the PT provider. If multiple testers participate in the same
round of PT for the same test category for a single POCT site, >80% of testers must
receive a passing PT score for the POCT site to be reported as passing PT. Scoring
systems between PT providers and with test categories may differ.
Specimen received for testing:
A specimen is received for testing if its arrival at the POCT site was recorded in a
register/log book and/or LIS within the reporting timeframe. A specimen received for
testing may or may not have been tested/analyzed.
Reporting level:
Facility
How often to report:
Annual
How to review for
data quality:
The total numerator is automatically summed across the CQI and PT data elements for
each laboratory-based testing category. This sum should equal the total number of
laboratory-based testing and/or POCT sites for in each testing category at the facility,
and should be the same between the CQI and PT sections.
How to calculate
annual total:
N/A
Data elements
(components of
indicator):
Numerator:
Number of
PEPFAR-supported
laboratories
and/or POCT
engaged in CQI
and PT activities
for each test
category:
HIV Serology/
Diagnostic Testing
HIV IVT/EID
HIV Viral Load
TB Xpert
TB AFB
TB Culture
CD4
Disaggregate Groups
Disaggregates
CQI at laboratory-based testing
sites by test category: HIV
serology/diagnostic testing, HIV
IVT/EID, HIV Viral Load, TB
Xpert, TB AFB, TB Culture, CD4)
[Required]
1. How many sites perform this
test but do not participate in
CQI?
2. How many sites perform this
test and participate in CQI,
but have not been externally
audited or accredited?
3. How many sites perform this
test, participate in CQI, have
been externally audited, but
do not meet full
accreditation standards?
4. How many sites perform this
test, participate in CQI, have
been externally audited &
are fully Accredited?
CQI at point-of-care-based
testing sites by test category:
HIV serology/diagnostic testing,
1. How many POCT sites
perform this test but do not
participate in CQI?
HEALTH SYSTEMS
137
HIV IVT/EID, HIV Viral Load, TB
Xpert, TB AFB, TB Culture, CD4)
[Required]
2. How many POCT sites
perform this test and
participate in CQI, but have
not been externally audited
or certified?
3. How many POCT sites
perform this test, participate
in CQI, and have been
externally audited &
achieved a score of 0-1 (≤
59%)?
4. How many POCT sites
perform this test, participate
in CQI, have been externally
audited & achieved a score
of 2-3 (60%-89%)?
5. How many POCT sites
perform this test, participate
in CQI, have been externally
audited & achieved a score
of 4-certified (≥ 90%)?
PT at laboratory-based testing
sites by test category: HIV
serology/diagnostic testing, HIV
IVT/EID, HIV Viral Load, TB
Xpert, TB AFB, TB Culture, CD4)
[Required]
1. How many sites performed
this test but do not
participate in PT?
2. How many sites perform this
test and participate in PT,
but did not pass last round?
3. How many sites perform this
test, participate in PT and
passed last round?
PT at point-of-care-based
testing sites by test category:
HIV serology/diagnostic testing,
HIV IVT/EID, HIV Viral Load, TB
Xpert, TB AFB, TB Culture, CD4)
[Required]
1. How many POCT sites
performed this test but do
not participate in PT?
2. How many POCT sites
perform this test and
participate in PT, but did not
pass last round?
3. How many POCT sites
perform this test, participate
in PT and passed last round?
Testing Volume (By laboratory
vs. point-of-care testing and
test category: HIV
serology/diagnostic testing, HIV
IVT/EID, HIV Viral Load, TB
Xpert, TB AFB, TB Culture, CD4)
[Required]
Number of specimens received
for testing at all PEPFAR-
supported laboratory-based
testing sites within a testing
category
Disaggregate Descriptions & Definitions
• For both CQI and PT disaggregate groups, testing category disaggregations are only
applicable if specific test category is performed by the laboratory.

138
• The most recent PT panel with a score must be satisfactory/acceptable/successful to
be counted as a passing score.
PEPFAR-support
definition:
Standard definition of DSD and TA-SDI used.
Guiding narrative
questions:
1. In the narrative, please define which clinical laboratory tests were included in the
“other” category.
2. In the narrative, please define how the specimen volume was counted (i.e., specimen
log, LIS, etc.).

Host-Country
National & Subnational
Indicators
140
DIAGNOSED_NAT/SUBNAT
Description:
The percentage of adults and children living with HIV who know their status (have been
diagnosed)
Numerator:
Among people living with HIV, the
number who know their HIV status
Disaggregation: Disaggregated data is
required. If data is available use the Age/ex
disaggregates, if not available use the Sex
disaggregate. Do not enter both.
• Sex: Male, Female
• Coarse Age/Sex Disaggregation:
Female<15, Male <15, Female 15+,
Male 15+
Denominator:
Estimated number of adults and
children living with HIV (PLHIV Estimate)
Denominator is not collected as part of
indicator, but rather is submitted in DATIM
during COP planning [PLHIV estimates
submitted in the PEPFAR Implementation
and Planning Attributes].
How to collect:
Diagnosed is the first 90 of the global targets. To ensure people living with HIV receive
the care and treatment required to live healthy, productive lives, and to reduce the
chance of transmitting HIV, it is critical that they know their status. In many countries,
targeting testing and counselling at locations and populations with the highest HIV
burden will be the most efficient way to reach people living with HIV and ensure they
are aware of their status. This indicator captures the efficacy and coverage of HIV testing
interventions.
This indicator is harmonized with GARPR indicator 1.5
(https://aidsreportingtool.unaids.org/static/docs/GARPR_Guidelines_2016_EN.pdf).
Numerator:
There are multiple methods to estimate the number of people living with HIV who know
their status.
• Case-based surveillance: In countries with well-functioning HIV reporting
systems, the number of people diagnosed can be estimated from national case-
based data. The number of deaths among PLHIV must be subtracted from the
cumulative number diagnosed to calculate the number of people living with HIV
who know their status.
• Survey-based reporting:
o Certain population-based surveys include questions about known HV
status. Although this information may be subject to under-reporting bias,
when combined with survey-related HIV testing it can provide an estimate
of known status among survey respondents.
o Many population-based surveys include questions on HIV testing history.
These questions can provide a range for the proportion of PLHIV with
known status. The percentage of people living with HIV in the survey who
have been tested in the past 12 months and received the results provides
the upper range of known status (there will be a small proportion equal to
the annual incidence rate – less than 2% in most cases – of people who
might have converted in the 12 months after being tested). The
percentage of people living with HIV in the survey who have ever been
tested and received the results provides the lower range of known status.
o When using survey-based methods, note that:
HOST-COUNTRY INDICATORS
141
▪ Household surveys are often restricted to respondents of
reproductive age (15– 49), and so may not be representative of
people living with HIV <15 years and >49 years.
▪ Because household surveys are typically only done every five years,
data from non-recent surveys may not reflect current levels of
testing coverage.
Reporting level:
National-Level
How often to report:
Annually
Subnational
reporting:
This data should be entered for all SNUs, regardless of PEPFAR funding supporting these
geographical areas; so that the total of the sub-National number should equal the total
number of National number.
Entered by:
This data should be entered in DATIM by the USG country team.
Targets:
Not required.
Guiding narrative
questions:
1. Narratives should include information on how the number of individuals diagnosed
was calculated or estimated.
2. Narratives should also discuss how national PLHIV estimates were derived.
142
VL_SUPPRESSION_NAT/SUBAT
Description:
Percentage of people living with HIV on ART with a suppressed viral load
Numerator:
Number of people living with HIV and
on ART [in the reporting period] who
have a suppressed viral load (<1000
copies/mL)
Disaggregation: Disaggregated data is
required. If data is available use the Age/Sex
disaggregate, if not available use the Sex
disaggregate. Do not enter both.
• Sex: Male, Female
• Coarse Age/Sex Disaggregation:
Female<15, Male <15, Female 15+, Male
15+
Denominator:
TX_CURR_NAT
Denominator is not collected as part of
indicator, but rather is calculated as
TX_CURR_NAT Numerator.
How to collect:
Viral suppression is the third and last 90 of the global target, and the ultimate goal of the
HIV treatment cascade. Patients on ART who achieve and maintain viral suppression
minimize their risk of disease progression and HIV transmission. Viral suppression is a
critical quality of service quality; unsuppressed viral load can be indicative of suboptimal
treatment adherence, and can lead to the development and spread of drug resistance.
This indicator is harmonized with GARPR indicator 4.6
(https://aidsreportingtool.unaids.org/static/docs/GARPR_Guidelines_2016_EN.pdf)
Numerator: The numerator can be generated by counting the number of adults and
children receiving antiretroviral therapy at the end of the reporting period. Count the
patient if, during the reporting months, viral load has been recorded and is <1000
copies/mL. For countries with other thresholds (e.g., undetectable <50 copies/ml or
<400 copies/ml), preliminary evidence from several studies suggests the proportion of
those with 50 copies/ml or above and less than 1000 copies/ml is small, so no
adjustment is required. The testing threshold value should be reported in the narrative
for countries with thresholds other than <1000 copies/ml.
Viral-load testing should be routine rather than episodic; for example, when treatment
failure is suspected. If multiple viral-load tests are done annually for a person, only the
last routine test result should be reported. Results from episodic viral loads should not
be reported. If viral-load testing coverage is less than 75% of those receiving
antiretroviral therapy in the reporting year, results should be interpreted with caution.
Tools for measuring viral load may vary across countries. Routine viral-load suppression
tests from clinical and program data should be reported where available. In countries
where such data are not available, results from HIV population-based surveys or drug-
resistance surveys based on a random sample of people on antiretroviral therapy may
be reported. Countries should report the source of the numerator and denominator
data, and data from both sources should be reported if available, although clinical and
program data are preferred. If results from a survey are used, that should be included
when reporting.
Where clinical and program data are available from routine monitoring systems, results
will be recorded in patient files or in a laboratory system. Data should be de-duplicated
where patients receive multiple viral-load tests in a year.
HOST-COUNTRY INDICATORS
143
If an HIV population-based or drug-resistance survey is used in place of routine program
monitoring data, measurement of viral load should be done for the entire population of
HIV- positive individuals where ARV is detected in specimens. Self-reported treatment
status has been shown to be of limited quality. Therefore, viral-load estimates among
those who report receiving antiretroviral therapy should not be used.
Reporting level:
National-Level
How often to report:
Annually
Subnational
reporting:
This data should be entered for all SNUs, regardless of PEPFAR funding supporting these
geographical areas; so that the total of the sub-National number should equal the total
number of National number.
Entered by:
This data should be entered in DATIM by the USG country team.
Targets:
Host country teams often set targets by OU level. Targets should be aligned with the 90-
90-90 UNAIDS HIV response initiative. If the host country does not develop targets for
this indicator, then for planning purposes, data should be entered that includes MOH
results from the previous reporting period in addition to, at a minimum, the PEPFAR
planned targets.
Guiding narrative
questions:
1. Narratives should include information on how the number of HIV+ individuals
diagnosed was calculated or estimated.
144
TX_CURR_NAT/SUBNAT
Description:
Percentage of adults and children receiving antiretroviral therapy
Numerator:
Number of adults and children on ART
at the end of the reporting period
Disaggregation: Disaggregated data is
required. If data is available use the Age/ex
disaggregates, if not available use the Sex
disaggregate. Do not enter both.
• Sex: Male, Female
• Coarse Age/Sex Disaggregation:
Female<15, Male <15, Female 15+,
Male 15+
Denominator:
Estimated number of adults and
children living with HIV (PLHIV Estimate)
Denominator is not collected as part of
indicator, but rather is submitted in DATIM
during COP planning [PLHIV estimates
submitted in the PEPFAR Implementation
and Planning Attributes].
How to collect:
ART coverage is the second 90 of the global target, and an important step in ending the
AIDS epidemic. Antiretroviral therapy has been shown to reduce HIV-related morbidity
and mortality among those living with HIV, and onward HIV transmission. Studies have
also shown that early initiation, regardless of an individual’s CD4 cell count, can enhance
treatment benefits and save lives, and WHO currently recommends treatment for all.
The percentage of adults and children receiving antiretroviral therapy among all adults
and children living with HIV provides a benchmark for monitoring global targets over
time, and comparing progress across countries. It is one of the 10 global indicators in
WHO’s 2015 Consolidated strategic information guidelines for HIV in the health sector.
This indicator is harmonized with GARPR indicator 4.1
(https://aidsreportingtool.unaids.org/static/docs/GARPR_Guidelines_2016_EN.pdf).
This indicator measures the progress towards providing antiretroviral therapy to all
people living with HIV. The data source for this indicator is ART program monitoring
tools, such as ART patient registers, pharmacy dispensing records, and summary
reporting forms.
The number of adults and children receiving treatment can be obtained through data
from facility- based antiretroviral therapy registers or drug supply management systems.
Data should be collected continuously and aggregated on a monthly or quarterly basis to
obtain subnational and national totals. The most recent full year of data should be used
for annual reporting. Data should be collected from health facility recording and
reporting forms, program data, health information system.
This indicator can be generated by counting the number of adults and children receiving
antiretroviral therapy at the end of the reporting period. This value should equal the
number of adults and children who have ever started antiretroviral therapy minus those
not currently on treatment prior to the end of the reporting period. This will exclude
those who died, stopped treatment or were lost to follow-up during the year.
Some people pick up several months of antiretroviral medicines (ARVs) at one visit,
which could cover the last months of the reporting period. Efforts should be made to
include these people in the numerator as receiving antiretrovirals even if they do not
attend the clinic in the last month of the reporting period.
HOST-COUNTRY INDICATORS
145
When disaggregating the numerator by age, people receiving antiretroviral therapy
should be reported in the relevant age category based on their age at the end of the
reporting year. HIV- positive pregnant women who are on antiretroviral therapy should
be included in the numerator.
People receiving antiretroviral therapy in the private and public sectors should be
included where data are available.
Reporting level:
National and Subnational-Levels
How often to report:
Annually
Subnational
reporting:
To adequately plan the ART program, these numbers are needed from both the National
and subnational level. The subnational level is considered that in which the country
team has prioritized their program (PSNU).
This data should be entered for all SNUs, regardless of PEPFAR funding supporting these
geographical areas; so that the total of the sub-National number should equal the total
number of National number.
Entered by:
This data should be entered in DATIM by the USG country team.
Targets:
Host country teams often set targets by OU, and SNU level to plan their programs
(please describe the target setting process that the host country employs in the
narratives). Targets should align with the 90-90-90 UNAIDS HIV response initiative. If the
host country does not develop targets for this indicator, then for planning purposes,
data should be entered that includes MOH results from the previous reporting period in
addition to, at a minimum, the PEPFAR planned targets.
Guiding narrative
questions:
1. Narratives should include information on how national and subnational totals have
been derived for both results and targets.
2. Narratives should describe data systems used to aggregate treatment results at the
national and subnational levels and any work that country teams have conducted to
endure reporting results are accurate.
146
KP_MAT_NAT/SUBNAT
Description:
Percentage of people who inject drugs (PWID) on medication assisted therapy
Numerator:
Number of people who inject drugs
(PWID) on medication assisted therapy
The numerator is generated by counting the
total number of individuals who have been
on treatment for at least 6 months since
initiation of medication-assisted treatment
(e.g., using methadone or buprenorphine to
treat drug dependency) at any point in time
within the reporting period. The numerator
should equal the number of adults who
initiated and remain on medication-assisted
treatment for at least 6 months prior to the
end of the reporting period
Denominator:
Estimated number PWID
Denominator is not collected as part of
indicator, but rather is submitted in DATIM
during COP planning [PWID KP estimates
submitted in the PEPFAR Implementation
and Planning Attributes].
How to collect:
Medication assisted therapy programs should be an access point for PWID and the
program should refer and link to ARV treatment programs, PMTCT for female PWID and
a range of other prevention services.
It is important to know how many people are reached in order to monitor how well
programs are reaching PWIDs with medication-assisted treatment. This information can
be used to plan and make decisions on how well the PWID audience is being reached
with medication-assisted treatment. If a small percentage of the intended audience is
being reached, then it would be recommended that activities are adjusted to improve
reach. If a large percentage of the intended audience is being reached, then
headquarters staff would want to take these lessons learned and disseminate them to
other countries. The country can use the information to improve upon the quality of the
program as well as scale-up successful models.
Data should be collected continuously at the organization level as part of service delivery
and aggregated in time for national reporting cycles.
Reporting level:
National and Subnational-Levels
How often to report:
Annual
Subnational
reporting:
To adequately plan the key populations medication-assisted therapy (MAT) program,
these numbers are needed from both the national and subnational level. The
subnational level is considered that in which the country team has prioritized their
program (PSNU; district, province etc.). This data should be entered for all SNUs,
regardless of PEPFAR funding supporting these geographical area; so that the total of
the sub-national number should equal the total number of national number.
Entered by:
This data should be entered in DATIM by the USG country team.
Targets:
Not required.
Guiding narrative
questions:
1. Narratives should include information on how national and subnational totals have
been derived for results.
2. Narratives should discuss the national policy environment and future plans for MAT
at the national level.
HOST-COUNTRY INDICATORS
147
PMTCT_STAT_NAT/SUBNAT
Description:
Percentage of pregnant women with known HIV status
Numerator:
Number of pregnant women attending
antenatal clinics (ANC) and/or had a
facility-based delivery and were tested
for HIV during pregnancy, or already
knew they were HIV positive
Disaggregation: Disaggregated data is
required. This indicator should be
disaggregated by:
HIV status/test results:
• Known HIV infection at antenatal clinic
entry (Known Positive)
• Tested HIV positive at ANC during current
pregnancy (Newly tested positive)
• Tested HIV negative at ANC during current
pregnancy (Newly tested negative)
Denominator:
Number of pregnant women who
attended ANC or had a facility-based
delivery in the past 12 months
N/A
How to collect:
The risk of mother-to-child transmission (MTCT) can be significantly reduced by
providing ARVs to the mother during pregnancy, delivery and (if applicable)
breastfeeding. This indicator provides information on coverage of the first step in the
prevention of mother-to-child transmission (PMTCT) cascade. High coverage enables
early initiation of care and treatment for HIV-positive mothers. The total number of
identified HIV-positive women provides the facility-specific number of pregnant women
with HIV to start a facility-based PMTCT cascade. This indicator is harmonized with
GARPR indicators 3.4
(https://aidsreportingtool.unaids.org/static/docs/GARPR_Guidelines_2016_EN.pdf).
For the numerator and denominator: The data source is ANC, PMTCT and L&D program
monitoring tools, such as patient registers and summary reporting forms.
Numerator: Count all women who were enrolled in ANC during the 12-month reporting
period whose HIV status is known positive, or who received an HIV test result (positive
or negative) during ANC. Reconcile with all women in the L&D register who whose date
of delivery was in the 12 months reporting period and whose HIV status at L&D was
known positive, or who received an HIV test result (positive or negative) at ANC or L&D
to avoid double counting.
The numerator is a composite of the following two data components:
1) The number of women with known (positive) HIV infection attending ANC for a
new pregnancy over the last reporting period
2) The number of women attending ANC, L&D who were tested for HIV and
received results
The numerator can be summed from categories a-d below:
a) Number of pregnant women with unknown HIV status attending ANC who
received an HIV test and result during the current pregnancy
b) Pregnant women with known HIV infection attending ANC for a new pregnancy
c) Number of pregnant women with unknown HIV status attending L&D who
received an HIV test and result during their current pregnancy
d) Women with unknown HIV status attending postpartum services within 72
hours of delivery who were tested for the first time in the current pregnancy
and received results.
148
A “status” is defined as a confirmed test result from a test during this pregnancy (either
positive or negative) or already known HIV infection at antenatal clinic entry. An
indeterminate test result should not be counted or reported as a part of this indicator.
For the denominator: Count all women who were enrolled in ANC during the 12-month
reporting period OR delivered at the facility (recorded in the L&D register), reconciling
the latter with the former using the ANC No. to avoid double counting.
As per global guidance (see GARPR indicator 3.4, link above), it is expected that the
national program can reconcile information collected from ANC with L&D records.
However, in MER 2.0 the PEPFAR indicator for PMTCT_ART has been simplified to collect
information only at antenatal care (ANC) sites to better align with 2016 WHO
Consolidated ARV guidelines, reduce burden on data collection, and improve data
quality. Therefore, in reporting this indicator PEPFAR operating units should 1) utilize the
national system whether it is able avoid double counting or not and are not expected to
collect or report this information through a separate system 2) if it this is not possible to
report individuals from both ANC and L&D, please include an explanation in the
narrative whether the data is from ANC, L&D and/or both.
Pregnant women’s HIV status should be counted only once per pregnancy. This may be
difficult if national guidelines recommend testing a pregnant woman more than once
during a pregnancy or if a woman seroconverts during her pregnancy and has multiple
tests.
Reporting level:
National and Subnational-Levels
How often to report:
Annual
Subnational
reporting:
To adequately plan the PMTCT program, these numbers are needed from both the
National and subnational level. The subnational level is considered that in which the
country team has prioritized their program (PSNU; District, province etc.). This data
should be entered for all SNUs, regardless of PEPFAR funding supporting these
geographical area; so that the total of the subnational number should equal the total
number of National number.
Entered by:
This data should be entered in DATIM by the USG country team.
Targets:
Host country teams often set targets by OU, and SNU level to plan their programs
(please describe the target setting process that the host country employs in the
narratives). Targets should be aligned with the START free, STAY free, AIDS-free super-
FAST TRACK initiative.
If the host country does not develop targets for this indicator, then for planning
purposes, data should be entered that includes MOH results from the previous reporting
period in addition to, at a minimum, the PEPFAR planned targets.
Guiding narrative
questions:
1. Narratives should include information on how national and subnational totals have
been derived for both results and targets.
2. Provide context for poor performance in PMTCT_STAT coverage
(Numerator/Denominator = STAT coverage) by geographic area. Include any planned
activities/remedial actions.
HOST-COUNTRY INDICATORS
149
PMTCT_ART_NAT/SUBNAT
Description:
Number and percentage of HIV-positive pregnant women who received antiretroviral
medicine (ARV) during pregnancy to reduce the risk of mother-to-child transmission
Numerator:
Number of HIV-positive pregnant
women who delivered and received ARV
to reduce the risk of mother-to- child
transmission during pregnancy and
delivery.
Disaggregation: Disaggregated data is
required. The numerator should be
disaggregated by the three categories below
for HIV- positive pregnant women for the
prevention of mother-to-child transmission:
1. Newly initiated on antiretroviral therapy
during the current pregnancy (New on
ART, includes Maternal triple ARV
prophylaxis)
2. Already on antiretroviral therapy before
the current pregnancy (Already on ART)
3. Other: All other options including
• Maternal AZT (prophylaxis
component during pregnancy and
delivery of WHO Option A or WHO
2006 guidelines)
• Single dose nevirapine (with or
without tail) only
• Any other regimen not listed above
Denominator:
Estimated number of HIV-positive
pregnant women
The number of HIV positive pregnant women
who delivered within the past 12 months is
also referred to as the number of pregnant
women living with HIV needing
antiretrovirals for preventing mother-to-child
transmission
How to collect:
The risk of mother-to-child transmission can be significantly reduced by providing ARVs
for the mother during pregnancy and delivery, with antiretroviral prophylaxis for the
infant, and antiretroviral medicines to the mother or child if breastfeeding, and the use
of safe delivery practices and safer infant feeding. The data will be used to track
progress towards global and national goals of eliminating mother-to-child transmission;
to inform policy and strategic planning; for advocacy; and for leveraging resources for
accelerated scale-up. It will help measure trends in coverage of antiretroviral prophylaxis
and treatment, and when disaggregated by regimen type, will also assess progress in
implementing more effective antiretroviral therapy regimens. As the indicator usually
measures ARVs dispensed and not those consumed, it is not possible to determine
adherence to the regimen in most cases.
This indicator is harmonized with GARPR indicator 3.1
(https://aidsreportingtool.unaids.org/static/docs/GARPR_Guidelines_2016_EN.pdf).
For the numerator: the source of this information is national program records
aggregated from program monitoring tools, such as patient registers and summary
reporting forms. The numerator can be generated by counting the number of HIV-
positive pregnant women who received antiretrovirals to reduce MTCT in the reporting
period, by regimen.
Disaggregation of regimen definitions:
150
Categories
Further Clarification
Common Examples
The first two
options include
women
receiving
lifelong
antiretroviral
therapy
(including
Option B+)
1) newly
initiated on
treatment
during the
current
pregnancy
(new on ART)
2) already on
treatment
before the
pregnancy
(Already on
ART)
A three-drug regimen intended to
provide antiretroviral therapy for life
1) Number of HIV-positive pregnant
women identified in the reporting
period newly initiated on
antiretroviral therapy for life
2) Number of HIV-positive pregnant
women identified in the reporting
period who were already on
antiretroviral therapy at their first
antenatal clinic visit.
If a woman is initiating antiretroviral
therapy for life during labor, she would
be counted in category 1.
-If the number of women on
antiretroviral therapy is not available
by the timing of when they started
antiretroviral therapy the number can
be included in the cell titled total
number of pregnant women on
lifelong antiretroviral therapy.
-If a woman is initiating a 3-drug
regimen provided for MTCT
prophylaxis started during pregnancy
or as late as during labor or delivery
with the intention of stopping at the
end of the breastfeeding period (or
stopping at delivery if not
breastfeeding) (previously known as
Option B), she would be counted in
category 1.
Standard national treatment
regimen, for example:
• TDF+3TC+EFV
• AZT+3TC+NVP
Other
All other suboptimal regimens are
counted here including:
1) Maternal AZT (prophylaxis
component of WHO Option A
during pregnancy and delivery)
2) Single-dose nevirapine (sd- NVP) to
the mother during pregnancy or
delivery
3) Any other regimen that is not ART
and/or one of the two options
listed above
• AZT at any point before labor
+ intrapartum NVP
• AZT at any point before labor
+ intrapartum NVP +7-day
post-partum tail of AZT/3TC
• sd-NVP for mother only at
onset of labor
• sd-NVP + 7-day AZT/3TC tail
ONLY
• sd-NVP for mother at onset
of labor and sd-NVP for baby
ONLY
For the denominator: Two methods can be used to estimate the denominator: an
estimation model, such as Spectrum, using the output, number of pregnant women
needing PMTCT; or, if Spectrum estimates are not available, by multiplying the number
of women giving birth in the past 12 months (which can be obtained from estimates of
the central statistics office, United Nations Population Division or pregnancy registration
systems with complete data) by the most recent national estimate of HIV prevalence in
pregnant women (which can be derived from HIV sentinel surveillance in ANC and
appropriate adjustments related to coverage of ANC surveys).
Reporting level:
National and Subnational-Levels
HOST-COUNTRY INDICATORS
151
How often to report:
Annual
Subnational
reporting:
To adequately plan the PMTCT program, these numbers are needed from both the
National and subnational level. The subnational level is considered that in which the
country team has prioritized their program (PSNU; District, province etc.). This data
should be entered for all SNUs, regardless of PEPFAR funding supporting these
geographical area; so that the total of the subnational number should equal the total
number of National number.
Entered by:
This data should be entered in DATIM by the USG country team.
Targets:
Host country teams often set targets by OU, and SNU level to plan their programs
(please describe the target setting process that the host country employs in the
narratives). Targets should be aligned with the START free, STAY free, AIDS-free super-
FAST TRACK initiative.
If the host country does not develop targets for this indicator, then for planning
purposes, data should be entered that includes MOH results from the previous reporting
period in addition to, at a minimum, the PEPFAR planned targets.
Guiding narrative
questions:
1. Narratives should include information on how national and subnational totals have
been derived for both results and targets.
2. Provide context for low PMTCT_ART coverage (PMTCT_ART_NAT /
PMTCT_STAT_POS_NAT = ART coverage) by geographic area or
partner/implementing mechanism, including any planned activities/remedial actions.
152
VMMC_CIRC_NAT/SUBNAT
Description:
Number of males circumcised during the reporting period according to national
standards
Numerator:
Number of males circumcised during
the reporting period according to
national standards
Disaggregation: Disaggregated data is
required. Enter data disaggregated by age.
• Age (<15, 15-29, 30+)
Denominator:
N/A
How to collect:
There is compelling evidence that male circumcision provided by well-trained health
professionals in properly equipped settings is safe and can reduce the risk of
heterosexually acquired HIV infection in men by approximately 60%. WHO/UNAIDS
recommendations emphasize that male circumcision should be considered an
efficacious intervention for HIV prevention in countries and regions in which
heterosexual activity plays a significant role in HIV transmission.
This indicator is harmonized with GARPR indicator 1.23
(https://aidsreportingtool.unaids.org/static/docs/GARPR_Guidelines_2016_EN.pdf).
Males should be provided with circumcision as part of the VMMC for HIV prevention
program and in accordance with the WHO/UNAIDS/Jhpiego Manual for Male
Circumcision Under Local Anesthesia, or other WHO normative guidance (in the case of
device-based VMMC), and per national standards by funded programs/sites in the
reporting period meet the definition for the numerator. Males who are provided with
circumcision using a medical device by funded programs/sites in the reporting period
also meet the definition for the numerator as long as the device used is recognized or
pre- qualified by WHO.
This indicator measures the progress in scaling up male circumcision services and should
be calculated by counting male clients documented as having received VMMC within the
reporting period from VMMC Registries or clients’ medical records maintained by
programs at Priority SNU level.
Data should be collected from health facility recording and reporting forms, program
data, health information system, or data maintained at Priority SNU level.
Reporting level:
National and Subnational-Levels
How often to report:
Annual
Subnational
reporting:
To adequately plan the VMMC program, these numbers are needed from both the
National and subnational level. The subnational level is considered that in which the
country team has prioritized their program (PSNU; District, province etc.).
This data should be entered for all SNUs, regardless of PEPFAR funding supporting these
geographical areas; so that the total of the sub-National number should equal the total
number of National number.
Entered by:
This data should be entered in DATIM by the USG country team.
Targets:
Host country teams often set targets by OU, and SNU level to plan their programs
(please describe the target setting process that the host country employs in the
narratives).
If the host country does not develop targets for this indicator, then for planning
purposes, data should be entered that includes MOH results from the previous reporting
period in addition to, at a minimum, the PEPFAR planned targets.

HOST-COUNTRY INDICATORS
153
Guiding narrative
questions:
1. Narratives should include information on how national and subnational totals have
been derived for both results and targets.
2. What barriers are there to further scaling up VMMC services in the country?
154
VMMC_TOTALCIRC_NAT/SUBNAT
Description:
Total number of men ever circumcised
Numerator:
Total number of men ever circumcised
Disaggregation: Disaggregated data is
optional. If data is available enter by age.
• Age (<15, 15-29, 30+)
Denominator:
Total population of men in the
corresponding age category
Denominator is not collected as part of
indicator, but rather is submitted in DATIM
during COP planning [Population estimates
submitted in the PEPFAR Implementation
and Planning Attributes].
How to collect:
There is compelling evidence that male circumcision provided by well-trained health
professionals in properly equipped settings is safe and can reduce the risk of
heterosexually acquired HIV infection in men by approximately 60%. WHO/UNAIDS
recommendations emphasize that male circumcision should be considered an
efficacious intervention for HIV prevention in countries and regions in which
heterosexual activity plays a significant role in HIV transmission.
This indicator is harmonized with GARPR indicator 1.22
(https://aidsreportingtool.unaids.org/static/docs/GARPR_Guidelines_2016_EN.pdf).
The denominator for this indicator is the number of male populations estimates,
disaggregated by age (<15, 15-29, 30+). This information is collected under the
population estimates indicator in the IMPATTS (Implementation and Planning
Attributes).
A guide to indicators for male circumcision programs in the formal health care system.
Geneva, World Health Organization/UNAIDS, 2009.
http://whqlibdoc.who.int/publications/2009/9789241598262_eng.pdf
Estimates derived from population-based surveys (Demographic and Health Survey, AIDS
Indicator Survey, Multiple Indicator Cluster Surveys or other representative surveys); this
indicator will help to determine male circumcision prevalence. The total number of men
circumcised should include all men circumcised regardless if circumcised at birth, as part
of the VMMC program or at any other time during their lifetime.
Reporting level:
National and Subnational-Levels
How often to report:
Annual
Subnational
reporting:
To adequately plan the VMMC program, these numbers are needed from both the
National and subnational level. The subnational level is considered that in which the
country team has prioritized their program (PSNU).
This data should be entered for all subnational units, regardless of PEPFAR funding
supporting these geographical areas, if there are no achievements, enter 0; so that the
total of the subnational number should equal the total number of National number.
Entered by:
This data should be entered in DATIM by the USG country team.
Targets:
Host country teams often set targets by OU, and SNU level to plan their programs
(please describe the target setting process that the host country employs in the
narratives).
If the host country does not develop targets for this indicator, then for planning
purposes, data should be entered that includes MOH results from the previous reporting

HOST-COUNTRY INDICATORS
155
with the PEPFAR planned targets (at the least) should constitute the host country
targets.
Guiding narrative
questions:
1. Narratives should include information on how national and subnational totals have
been derived for both results and targets.

Appendices

APPENDICES
157
Appendix 1: Key Population Classification Document
Key Population Classification (core)
6/14/2016
This assessment was developed to be used in both community and facility health care settings for the purpose of
helping providers identify the types of services needed by the client. The complete form should be offered to all
clients, regardless of providers’ assumptions about whether the client is a key population member or not. Note- all
script in normal text should be read out loud to the client, italicized text is instruction to the provider.
Health Care Provider script to Client: “I will be asking you about some sexual and drug using risk behaviors. Your
responses will help me/us provide you with better care. Your answers to these questions will be kept in your
confidential clinic record. Answering these questions is voluntary and you can refuse to answer any question and
still receive the service you’ve come here for today.”
1. Do you consider yourself: male, female, transgender or
other?
□ MALE
□ FEMALE
□ TRANSGENDER (male
to) FEMALE
□ TRANSGENDER (female to) MALE
□ ______________________OTHER
□ REFUSE TO ANSWER
If TRANSGENDER (male to) FEMALE:
client was born a boy, but identifies as a
woman
If TRANSGENDER (female to) MALE:
client was born a girl, but identifies as a
man
2. What was your sex at birth: male or female?
□ MALE
□ FEMALE
□ ______________________OTHER
□ REFUSE TO ANSWER
3. Do you have sex with: men, women or both?
□ MEN ONLY
□ WOMEN ONLY
□ BOTH MEN AND WOMEN
□ REFUSE TO ANSWER
4. Is selling sex your main source of income?
□ YES
□ NO
□ REFUSE TO ANSWER
5. In the last 6 months, have you injected illicit or
illegal drugs?
□ YES
□ NO
□ REFUSE TO ANSWER
Key Populations Team, HIV Prevention Branch, CDC-Atlanta (Version 3.1)
Key Population Classification
If client answers Male to Q1 and answers Men Only or Men and Women to Q3, then classify as MSM
□
If client answers Transgender MTF or FTM to Q1, or if client identifies as a gender different from their birth
sex, then classify as TG
□
If client answers Yes to Q4, then categorize as SW
□
If client answers Yes to Q5, then classify as PWID
□
If client is currently incarcerated, then classify as Person in Prison
□
Final Classification: (mark *ALL* that apply) □MSM □TG □SW □PWID □Person in Prison □NONE
*Some clients may belong to more than one category due to overlapping vulnerabilities and behavior

158
Appendix 2: MER and SIMS Mapping
MER Indicators and Corresponding SIMS Core Essential Elements (CEEs)
# Linkages
PrEP_NEW
2
C_04.04 [262] Monitoring Outreach for Key Populations [KP]
C_04.07 [264] Service Referral System [KP]
VMMC_CIRC
6
F_01.13 [013] Data Reporting Consistency – VMMC_CIRC [ALL FACILITIES]
F_05.01 [069] VMMC Registers-Paper [VMMC]
F_05.02 [070] VMMC Register-Electronic [VMMC]
F_05.03 [071] Adverse Event (AE) Prevention and Management [VMMC]
F_05.04 [072] Voluntarism and Informed Consent [VMMC]
F_05.05 [073] VMMC Clinical Follow-Up [VMMC]
KP_PREV
17
A_04.01 [430] National Guidelines for Key Populations (National level) [GUIDE]
C_01.12 [212] Facilitation of Small Group Sessions for HIV Prevention [AP]
C_01.13 [213] Small Group Sessions for HIV Prevention [AP]
C_04.01 [226] Condom Availability [KP]
C_04.02 [249] Lubricant Availability [KP]
C_04.03 [261] STI Screening and Management Among Key Populations [KP]
C_04.04 [262] Monitoring Outreach for Key Populations [KP]
C_04.05 [263] Peer Outreach Management [KP]
C_04.06 [250] Family Planning/HIV Integration Service Delivery in Community Settings [KP]
C_04.07 [264] Service Referral System [KP]
C_04.08 [265] Data Reporting Consistency – KP_PREV [KP]
F_03.01 [049] Lubricant Availability at Point of Service [KP]
F_03.02 [050] STI Screening and Management for Key Populations [KP]
F_03.03 [051] Service Referral System [KP]
F_03.19 [105] Systems for Family Planning (FP)/HIV Integration [C&T KP]
F_03.20 [106] Family Planning (FP)/HIV Integration Service Delivery [C&T KP]
F_03.21 [032] Partner HIV Testing [C&T KP]
PP_PREV
6
C_01.12 [212] Facilitation of Small Group Sessions for HIV Prevention [AP]
C_01.13 [213] Small Group Sessions for HIV Prevention [AP]
C_01.26 [226] Condom Availability (at the Service Delivery Point) [AP-HTC]
C_05.02 [255] Preventing HIV in Girls [OPP]
C_05.03 [254] Girls Secondary Education Transition [OPP]
C_05.06 [226] Condom Availability [OPP]
TB_PREV
3

APPENDICES
159
F_02.17 [037] Isoniazid Preventive Therapy (IPT) [C&T GEN POP]
F_03.17 [037] Isoniazid Preventive Therapy (IPT) [C&T KP]
F_04.14 [037] Isoniazid Preventive Therapy (IPT) [PMTCT-ANC]
KP_MAT
9
A_04.01 [430] National Guidelines for Key Populations (National level) [GUIDE]
C_04.07 [264] Service Referral System [KP]
F_09.01 [084] Intake Treatment Plan Development [MAT]
F_09.02 [085] TB screening and Management in MAT Facilities [MAT]
F_09.03 [086] Psychosocial Support for MAT Clients [MAT]
F_09.04 [087] Induction-[MAT]
F_09.05 [088] Stabilization [MAT]
F_09.06 [089] Dose Reduction and Termination [MAT]
F_09.08 [091] Supply Chain Reliability (methadone and buprenorphine) [MAT]
GEND_GBV
4
C_01.17 [217] Standard Guidance for Gender-Based Violence Response in Community Setting [AP]
C_01.18 [218] Gender-Based Violence Referrals in Community Setting [AP]
F_06.01 [074] Capacity to Provide Post-Violence Care Services [GBV]
F_06.02 [075] Availability of Post-Violence Care Services [GBV]
OVC_SERV
13
A_05.01 [440] Management and Planning – strategic planning (Social Services) (National level) [SOC OVC]
A_05.05 [444] Management and Planning – operational planning (Social Services) (Sub-national level) [SOC OVC]
C_03.01 [252] Case Management Services [OVC]
C_03.02 [255] Preventing HIV in Girls [OVC]
C_03.03 [257] Linkages to HIV Testing [OVC]
C_03.04 [258] Child Protection Services [OVC]
C_03.05 [253] Education Services [OVC]
C_03.06 [254] Girls Secondary Education Transition [OVC]
C_03.07 [256] Economic Strengthening and Social Protection Services [OVC]
C_03.08 [259] Early Childhood Development Services [OVC]
C_03.09 [246] Community Pediatric Nutrition Screening & Referral to Clinical Services [OVC]
C_03.10 [250] Family Planning/HIV Integration Service Delivery in Community Settings [OVC]
C_05.02 [255] Preventing HIV in Girls [OPP]
FPINT_SITE
6
F_02.20 [040] Systems for Family Planning (FP)/HIV Integration [C&T GEN POP]
F_02.21 [041] Family Planning (FP)/HIV Integration Service Delivery [C&T GEN POP]
F_03.19 [105] Systems for Family Planning (FP)/HIV Integration [C&T KP]
F_03.20 [106] Family Planning (FP)/HIV Integration Service Delivery [C&T KP]
F_04.17 [040] Systems for Family Planning (FP)/HIV Integration [PMTCT]
F_04.18 [041] Family Planning (FP)/HIV Integration Service Delivery [PMTCT]

160
HTS_TST
39
A_01.04 [404] Quality Assurance of HIV Testing Services (National level) [LAB]
A_01.09 [409] Quality Assurance of HIV Testing Services (Sub-national level) [LAB]
A_10.07 [496] Data Use for RTK Distribution Decision making (National level) [SC RTK NATL]
A_10.08 [497] Supervision/Monitoring for RTK Supply Chain (National level) [SC RTK NATL]
A_10.09 [498] Data Use for RTK Distribution Decision making (Sub-national level) [SC RTK SNU]
A_10.10 [499] Supervision/Monitoring for RTK Supply Chain (Sub-national level) [SC RTK SNU]
C_01.13 [213] Small Group Sessions for HIV Prevention [AP]
C_01.20 [220] HIV Proficiency Testing at the Organization Assessment Point [AP-HTC]
C_01.21 [221] Supply Chain Reliability (Rapid Test Kits) at the Organization Assessment Point [AP-HTC]
C_01.23 [223] HIV Testing Quality Assurance at the Organization Assessment Point [AP-HTC]
C_01.25 [225] Confidentiality of HIV Testing Services at the Organization Assessment Point [AP-HTC]
C_01.33 [233] Compliance with National Testing Algorithm and Strategy [AP HTC]
C_01.34 [234] HIV Testing Quality Assurance at the Service Delivery Point [AP HTC]
C_01.36 [236] Confidentiality of HIV Testing Services at the Service Delivery Point [AP HTC]
C_02.02 [243] Partner HIV Testing [PLHIV]
C_03.03 [257] Linkages to HIV Testing [OVC]
F_01.11 [011] Data Reporting Consistency – HTC_TST [ALL FACILITIES]
F_01.14 [014] Supply Chain Management [ALL FACILITIES-COMM]
F_01.20 [020] Supply Chain Reliability-Rapid Test Kits [ALL FACILITIES-COMM]
F_02.12 [032] Partner HIV Testing [C&T GEN POP]
F_02.13 [033] Routine HIV testing of Children of Adult Patients [C&T GEN POP]
F_02.22 [042] Routine HIV Testing for Children [C&T PEDS]
F_03.21 [032] Partner HIV Testing [C&T KP]
F_03.22 [033] Routine HIV testing of Children of Adult Patients [C&T KP]
F_04.01 [052] ANC Register-Paper [PMTCT-ANC]
F_04.02 [053] ANC Register-Electronic [PMTCT-ANC]
F_04.11 [032] Partner HIV Testing [PMTCT-ANC]
F_04.21 [058] PITC for Maternity Patients [PMTCT-L&D]
F_04.32 [033] Routine HIV testing of Children of Adult Patients [PMTCT-ANC]
F_07.01 [076] Compliance with National Testing Algorithm and Strategy [HTC]
F_07.02 [077] Quality Assurance of HIV Testing Services [HTC]
F_07.04 [079] Facility Level HIV Proficiency Testing [HTC]
F_08.01 [080] Routine PITC for Adult TB Patients [TB]
F_08.03 [082] Routine PITC for Pediatric TB Patients [TB]
F_09.07 [090] HIV Testing [MAT]
F_10.03 [094] Test SOPs [LAB]
F_10.04 [095] Quality Testing Monitoring [LAB]
F_10.05 [096] Results and Information Management [LAB]
F_10.06 [097] Testing Interruptions [LAB]

APPENDICES
161
PMTCT_STAT
7
F_01.12 [012] Data Reporting Consistency – PMTCT_STAT [ALL FACILITIES]
F_04.01 [052] ANC Register-Paper [PMTCT-ANC]
F_04.02 [053] ANC Register-Electronic [PMTCT-ANC]
F_04.06 [021] Patient/Beneficiary Records for ART/pre-ART /PMTCT B+ Facilities
F_04.07 [024] ART Register-Paper [PMTCT-ANC]
F_04.08 [025] ART Register-Electronic [PMTCT-ANC]
F_04.21 [058] PITC for Maternity Patients [PMTCT-L&D]
PMTCT_EID
14
A_01.03 [403] Specimen Referrals (National level) [LAB]
A_01.08 [408] Specimen Referrals (Sub-national level) [LAB]
F_04.01 [052] ANC Register-Paper [PMTCT-ANC]
F_04.02 [053] ANC Register-Electronic [PMTCT-ANC]
F_04.19 [057] Patient Tracking -HIV+ Breastfeeding Women [PMTCT]
F_04.25 [062] Early Infant Diagnosis [HEI]
F_04.27 [064] Tracking HIV-Exposed Infants [HEI]
F_04.29 [066] HIV Exposed Infant/Early Infant Diagnosis Registers-Paper [HEI]
F_04.30 [067] HIV Exposed Infant/Early Infant Diagnosis Register-Electronic [HEI]
F_04.31 [068] Supply Chain Reliability (Early Infant Diagnosis) [HEI]
F_10.03 [094] Test SOPs [LAB]
F_10.04 [095] Quality Testing Monitoring [LAB]
F_10.05 [096] Results and Information Management [LAB]
F_10.06 [097] Testing Interruptions [LAB]
TB_STAT
2
F_08.01 [080] Routine PITC for Adult TB Patients [TB]
F_08.03 [082] Routine PITC for Pediatric TB Patients [TB]
OVC_HIVSTAT
2
C_03.01 [252] Case Management Services [OVC]
C_03.03 [257] Linkages to HIV Testing [OVC]
PMTCT_FO
15
A_01.03 [403] Specimen Referrals (National level) [LAB]
A_01.08 [408] Specimen Referrals (Sub-national level) [LAB]
F_04.01 [052] ANC Register-Paper [PMTCT-ANC]
F_04.02 [053] ANC Register-Electronic [PMTCT-ANC]
F_04.06 [021] Patient/Beneficiary Records for ART/pre-ART /PMTCT B+ Facilities
F_04.19 [057] Patient Tracking -HIV+ Breastfeeding Women [PMTCT]
F_04.25 [062] Early Infant Diagnosis [HEI]
F_04.27 [064] Tracking HIV-Exposed Infants [HEI]
F_04.29 [066] HIV Exposed Infant/Early Infant Diagnosis Registers-Paper [HEI]

162
F_04.30 [067] HIV Exposed Infant/Early Infant Diagnosis Register-Electronic [HEI]
F_04.31 [068] Supply Chain Reliability (Early Infant Diagnosis) [HEI]
F_10.03 [094] Test SOPs [LAB]
F_10.04 [095] Quality Testing Monitoring [LAB]
F_10.05 [096] Results and Information Management [LAB]
F_10.06 [097] Testing Interruptions [LAB]
TX_NEW
37
A_10.01 [490] Supply Chain: ARVs (National level) [SC ARV NATL]
A_10.02 [491] Data Use for ARV Distribution Decision making (National level) [SC ARV NATL]
A_10.03 [492] Supervision/Monitoring for ARV Supply Chain (National Level) [SC-ARV NATL]
A_10.04 [493] Data Use for ARV Distribution Decision making (Sub-national level) [SC ARV SNU]
A_10.05 [494] Supervision/Monitoring for ARV Supply Chain (Sub-national level) [SC ARV SNU]
C_01.19 [219] HTC Referrals to HIV Care and Treatment at the Organization Assessment Point [AP-HTC]
C_01.32 [232] POCT Referral and Linkages [AP-POCT]
C_02.06 [247] Community-Based Linkage and Retention Support Services [PLHIV]
F_01.10 [010] Data Reporting Consistency – TX_NEW-C&T [ALL FACILITIES]
F_01.14 [014] Supply Chain Management [ALL FACILITIES-COMM]
F_01.15 [015] Medication Dispensing [ALL FACILITIES-COMM]
F_01.16 [016] Supply Chain Reliability-Adult ARVs [ALL FACILITIES-COMM]
F_01.18 [018] Supply Chain Reliability -Pediatric ARVs [ALL FACILITIES-COMM]
F_02.01 [021] Patient/Beneficiary Records [C&T GEN POP]
F_02.03 [023] Patient Tracking-Pre-ART Patients [C&T GEN POP]
F_02.04 [024] ART Register-Paper [C&T GEN POP]
F_02.05 [025] ART Register-Electronic [C&T GEN POP]
F_02.06 [026] Pre-ART Register-Paper [C&T GEN POP]
F_02.07 [027] Pre-ART Register-Electronic [C&T GEN POP]
F_02.08 [028] ART Eligibility [C&T GEN POP]
F_03.04 [021] Patient/Beneficiary Records [C&T KP]
F_03.06 [023] Patient Tracking-Pre-ART Patients [C&T KP]
F_03.07 [024] ART Register-Paper [C&T KP]
F_03.08 [025] ART Register-Electronic [C&T KP]
F_03.09 [026] Pre-ART Register-Paper [C&T KP]
F_03.10 [027] Pre-ART Register-Electronic [C&T KP]
F_03.11 [028] ART Eligibility [C&T KP]
F_04.06 [021] Patient/Beneficiary Records for ART/pre-ART /PMTCT B+ Facilities
F_04.07 [024] ART Register-Paper [PMTCT-ANC]
F_04.08 [025] ART Register-Electronic [PMTCT-ANC]
F_04.27 [064] Tracking HIV-Exposed Infants [HEI]
F_04.28 [065] Enrollment of HIV-Infected Infants Identified through Early Infant Diagnosis (EID) Services into ART Services [HEI]
F_04.29 [066] HIV Exposed Infant/Early Infant Diagnosis Registers-Paper [HEI]

APPENDICES
163
F_04.30 [067] HIV Exposed Infant/Early Infant Diagnosis Register-Electronic [HEI]
F_07.03 [078] HTC Referrals to HIV Care and Treatment [HTC]
F_08.02 [081] ART Provision for HIV-Positive Adult TB Patients [TB]
F_08.04 [083] ART Provision for HIV-Positive Pediatric TB Patients [TB]
TX_CURR
30
A_10.01 [490] Supply Chain: ARVs (National level) [SC ARV NATL]
A_10.02 [491] Data Use for ARV Distribution Decision making (National level) [SC ARV NATL]
A_10.03 [492] Supervision/Monitoring for ARV Supply Chain (National Level) [SC-ARV NATL]
A_10.04 [493] Data Use for ARV Distribution Decision making (Sub-national level) [SC ARV SNU]
A_10.05 [494] Supervision/Monitoring for ARV Supply Chain (Sub-national level) [SC ARV SNU]
C_02.01 [242] Adherence Support [PLHIV]
C_02.06 [247] Community-Based Linkage and Retention Support Services [PLHIV]
F_01.14 [014] Supply Chain Management [ALL FACILITIES-COMM]
F_01.15 [015] Medication Dispensing [ALL FACILITIES-COMM]
F_01.16 [016] Supply Chain Reliability-Adult ARVs [ALL FACILITIES-COMM]
F_01.18 [018] Supply Chain Reliability -Pediatric ARVs [ALL FACILITIES-COMM]
F_02.01 [021] Patient/Beneficiary Records [C&T GEN POP]
F_02.02 [022] Patient Tracking-ART Patients [C&T GEN POP]
F_02.04 [024] ART Register-Paper [C&T GEN POP]
F_02.05 [025] ART Register-Electronic [C&T GEN POP]
F_02.10 [030] Adherence Support-[C&T GEN POP]
F_03.04 [021] Patient/Beneficiary Records [C&T KP]
F_03.05 [022] Patient Tracking-ART Patients [C&T KP]
F_03.07 [024] ART Register-Paper [C&T KP]
F_03.08 [025] ART Register-Electronic [C&T KP]
F_03.13 [030] Adherence Support [C&T KP]
F_04.03 [054] ART in PMTCT Facilities [PMTCT-ANC]
F_04.05 [056] Patient Tracking-HIV+ Pregnant Women [PMTCT-ANC]
F_04.06 [021] Patient/Beneficiary Records for ART/pre-ART /PMTCT B+ Facilities
F_04.07 [024] ART Register-Paper [PMTCT-ANC]
F_04.08 [025] ART Register-Electronic [PMTCT-ANC]
F_04.09 [030] Adherence Support-[PMTCT-ANC]
F_04.19 [057] Patient Tracking -HIV+ Breastfeeding Women [PMTCT]
F_04.29 [066] HIV Exposed Infant/Early Infant Diagnosis Registers-Paper [HEI]
F_04.30 [067] HIV Exposed Infant/Early Infant Diagnosis Register-Electronic [HEI]
PMTCT_ART
15
C_02.01 [242] Adherence Support [PLHIV]
F_01.14 [014] Supply Chain Management [ALL FACILITIES-COMM]
F_01.15 [015] Medication Dispensing [ALL FACILITIES-COMM]

164
F_01.16 [016] Supply Chain Reliability-Adult ARVs [ALL FACILITIES-COMM]
F_02.04 [024] ART Register-Paper [C&T GEN POP]
F_02.05 [025] ART Register-Electronic [C&T GEN POP]
F_04.01 [052] ANC Register-Paper [PMTCT-ANC]
F_04.02 [053] ANC Register-Electronic [PMTCT-ANC]
F_04.03 [054] ART in PMTCT Facilities [PMTCT-ANC]
F_04.05 [056] Patient Tracking-HIV+ Pregnant Women [PMTCT-ANC]
F_04.06 [021] Patient/Beneficiary Records for ART/pre-ART /PMTCT B+ Facilities
F_04.07 [024] ART Register-Paper [PMTCT-ANC]
F_04.08 [025] ART Register-Electronic [PMTCT-ANC]
F_04.09 [030] Adherence Support-[PMTCT-ANC]
F_04.22 [059] ARVs at Labor and Delivery [PMTCT-L&D]
TX_TB
10
A_01.03 [403] Specimen Referrals (National level) [LAB]
A_01.08 [408] Specimen Referrals (Sub-national level) [LAB]
F_02.16 [036] TB Screening [C&T GEN POP]
F_02.18 [038] TB Diagnostic Evaluation Cascade [C&T GEN POP]
F_02.24 [044] Pediatric TB Screening [C&T PEDS]
F_03.16 [036] TB Screening [C&T KP]
F_03.18 [038] TB Diagnostic Evaluation Cascade [C&T KP]
F_04.13 [036] TB Screening [PMTCT-ANC]
F_04.15 [038] TB Diagnostic Evaluation Cascade [PMTCT-ANC]
F_09.02 [085] TB screening and Management in MAT Facilities [MAT]
TB_ART
13
C_01.19 [219] HTC Referrals to HIV Care and Treatment at the Organization Assessment Point [AP-HTC]
C_01.32 [232] POCT Referral and Linkages [AP-POCT]
C_02.01 [242] Adherence Support [PLHIV]
C_02.06 [247] Community-Based Linkage and Retention Support Services [PLHIV]
F_01.14 [014] Supply Chain Management [ALL FACILITIES-COMM]
F_01.15 [015] Medication Dispensing [ALL FACILITIES-COMM]
F_01.16 [016] Supply Chain Reliability-Adult ARVs [ALL FACILITIES-COMM]
F_01.18 [018] Supply Chain Reliability -Pediatric ARVs [ALL FACILITIES-COMM]
F_02.04 [024] ART Register-Paper [C&T GEN POP]
F_02.05 [025] ART Register-Electronic [C&T GEN POP]
F_02.10 [030] Adherence Support-[C&T GEN POP]
F_08.02 [081] ART Provision for HIV-Positive Adult TB Patients [TB]
F_08.04 [083] ART Provision for HIV-Positive Pediatric TB Patients [TB]
TX_RET
28
A_10.01 [490] Supply Chain: ARVs (National level) [SC ARV NATL]
A_10.02 [491] Data Use for ARV Distribution Decision making (National level) [SC ARV NATL]

APPENDICES
165
A_10.03 [492] Supervision/Monitoring for ARV Supply Chain (National Level) [SC-ARV NATL]
A_10.04 [493] Data Use for ARV Distribution Decision making (Sub-national level) [SC ARV SNU]
A_10.05 [494] Supervision/Monitoring for ARV Supply Chain (Sub-national level) [SC ARV SNU]
C_02.01 [242] Adherence Support [PLHIV]
C_02.06 [247] Community-Based Linkage and Retention Support Services [PLHIV]
F_01.14 [014] Supply Chain Management [ALL FACILITIES-COMM]
F_01.15 [015] Medication Dispensing [ALL FACILITIES-COMM]
F_01.16 [016] Supply Chain Reliability-Adult ARVs [ALL FACILITIES-COMM]
F_01.18 [018] Supply Chain Reliability -Pediatric ARVs [ALL FACILITIES-COMM]
F_02.01 [021] Patient/Beneficiary Records [C&T GEN POP]
F_02.02 [022] Patient Tracking-ART Patients [C&T GEN POP]
F_02.04 [024] ART Register-Paper [C&T GEN POP]
F_02.05 [025] ART Register-Electronic [C&T GEN POP]
F_02.10 [030] Adherence Support-[C&T GEN POP]
F_02.19 [039] Facility Linkage to Community Care & Support Services for Adult/Child PLHIV [C&T GEN POP]
F_03.04 [021] Patient/Beneficiary Records [C&T KP]
F_03.05 [022] Patient Tracking-ART Patients [C&T KP]
F_03.07 [024] ART Register-Paper [C&T KP]
F_03.08 [025] ART Register-Electronic [C&T KP]
F_03.13 [030] Adherence Support [C&T KP]
F_04.05 [056] Patient Tracking-HIV+ Pregnant Women [PMTCT-ANC]
F_04.06 [021] Patient/Beneficiary Records for ART/pre-ART /PMTCT B+ Facilities
F_04.07 [024] ART Register-Paper [PMTCT-ANC]
F_04.08 [025] ART Register-Electronic [PMTCT-ANC]
F_04.09 [030] Adherence Support-[PMTCT-ANC]
F_04.19 [057] Patient Tracking -HIV+ Breastfeeding Women [PMTCT]
TX_PVLS
20
A_01.03 [403] Specimen Referrals (National level) [LAB]
A_01.08 [408] Specimen Referrals (Sub-national level) [LAB]
C_02.01 [242] Adherence Support [PLHIV]
C_02.06 [247] Community-Based Linkage and Retention Support Services [PLHIV]
F_02.01 [021] Patient/Beneficiary Records [C&T GEN POP]
F_02.04 [024] ART Register-Paper [C&T GEN POP]
F_02.05 [025] ART Register-Electronic [C&T GEN POP]
F_02.10 [030] Adherence Support-[C&T GEN POP]
F_02.11 [031] ART Monitoring [C&T GEN POP]
F_02.26 [046] Pediatric ART Monitoring [C&T PEDS]
F_03.04 [021] Patient/Beneficiary Records [C&T KP]
F_03.07 [024] ART Register-Paper [C&T KP]

166
F_03.08 [025] ART Register-Electronic [C&T KP]
F_03.13 [030] Adherence Support [C&T KP]
F_03.14 [031] ART Monitoring [C&T KP]
F_04.06 [021] Patient/Beneficiary Records for ART/pre-ART /PMTCT B+ Facilities
F_04.07 [024] ART Register-Paper [PMTCT-ANC]
F_04.08 [025] ART Register-Electronic [PMTCT-ANC]
F_04.09 [030] Adherence Support-[PMTCT-ANC]
F_04.20 [031] ART Monitoring [PMTCT]
LAB_PTCQI
11
A_01.01 [401] Proficiency Testing (PT)/External Quality Assurance (EQA) (National level) [LAB]
A_01.02 [402] Laboratory/Point-of-Care Technology (POCT) Quality Improvement (QI) Program (National level) [LAB]
A_01.03 [403] Specimen Referrals (National level) [LAB]
A_01.04 [404] Quality Assurance of HIV Testing Services (National level) [LAB]
A_01.05 [405] National Blood Transfusion Service Accreditation (National level) [LAB]
A_01.06 [406] Proficiency Testing (PT)/External Quality Assurance (EQA) (Sub-national level) [LAB]
A_01.07 [407] Laboratory/Point-of-Care Technology (POCT) Quality Improvement (QI) Program (Sub-national level) [LAB]
A_01.08 [408] Specimen Referrals (Sub-national level) [LAB]
A_01.09 [409] Quality Assurance of HIV Testing Services (Sub-national level) [LAB]
F_10.04 [095] Quality Testing Monitoring [LAB]
F_11.04 [103] Quality Assurance [POCT]
SC_STOCK
38
A_10.01 [490] Supply Chain: ARVs (National level) [SC ARV NATL]
A_10.02 [491] Data Use for ARV Distribution Decision making (National level) [SC ARV NATL]
A_10.03 [492] Supervision/Monitoring for ARV Supply Chain (National Level) [SC-ARV NATL]
A_10.04 [493] Data Use for ARV Distribution Decision making (Sub-national level) [SC ARV SNU]
A_10.05 [494] Supervision/Monitoring for ARV Supply Chain (Sub-national level) [SC ARV SNU]
A_10.06 [495] Supply Chain: Rapid Test Kits/Diagnostics (National level) [SC RTK NATL]
A_10.07 [496] Data Use for RTK Distribution Decision making (National level) [SC RTK NATL]
A_10.08 [497] Supervision/Monitoring for RTK Supply Chain (National level) [SC RTK NATL]
A_10.09 [498] Data Use for RTK Distribution Decision making (Sub-national level) [SC RTK SNU]
A_10.10 [499] Supervision/Monitoring for RTK Supply Chain (Sub-national level) [SC RTK SNU]
A_10.11 [500] Supply Chain: Food and Nutrition (National level) [SC FN NATL]
A_10.12 [501] Data Use for Food and Nutrition Commodity Distribution Decision making (National level) [SC FN NATL]
A_10.13 [502] Supervision/Monitoring for Food and Nutrition Supply Chain (National level) [SC FN NATL]
A_10.14 [503] Data Use for Food and Nutrition Commodity Distribution Decision Making (Sub-national level) [SC FN SNU]
A_10.15 [504] Supervision/Monitoring for Food and Nutrition Supply Chain (Sub-national level) [SC FN SNU]
A_10.16 [510] Medicines Regulatory System - Registration (National level) [MED REG]
A_10.17 [511] Medicines Regulatory System – Quality Assurance / Quality Control (National level) [MED REG]
A_10.18 [512] Medicines Regulatory System – Pharmacovigilance (National level) [MED REG]
C_01.21 [221] Supply Chain Reliability (Rapid Test Kits) at the Organization Assessment Point [AP-HTC]

APPENDICES
167
C_01.26 [226] Condom Availability (at the Service Delivery Point) [AP-HTC]
C_01.28 [228] POCT Supplies, Reagents and Equipment [AP-POCT]
C_02.08 [226] Condom Availability [PLHIV]
C_02.09 [249] Lubricant Availability [PLHIV]
C_04.01 [226] Condom Availability [KP]
C_04.02 [249] Lubricant Availability [KP]
C_05.06 [226] Condom Availability [OPP]
F_01.03 [003] Risk Reduction Counseling and Condom Availability [ALL FACILITIES]
F_01.14 [014] Supply Chain Management [ALL FACILITIES-COMM]
F_01.16 [016] Supply Chain Reliability-Adult ARVs [ALL FACILITIES-COMM]
F_01.17 [017] Supply Chain Reliability-Cotrimoxazole [ALL FACILITIES-COMM]
F_01.18 [018] Supply Chain Reliability -Pediatric ARVs [ALL FACILITIES-COMM]
F_01.19 [019] Supply Chain-Pediatric Cotrimoxazole (ALL FACILITIES-COMM)
F_01.20 [020] Supply Chain Reliability-Rapid Test Kits [ALL FACILITIES-COMM]
F_03.01 [049] Lubricant Availability at Point of Service [KP]
F_04.31 [068] Supply Chain Reliability (Early Infant Diagnosis) [HEI]
F_09.08 [091] Supply Chain Reliability (methadone and buprenorphine) [MAT]
F_10.06 [097] Testing Interruptions [LAB]
F_11.05 [104] Supplies, Reagents and Equipment [POCT]
HRH_PRE
1
A_03.04 [423] HRH Regulation (National level) [HRH]
HRH_CURR
2
A_03.04 [423] HRH Regulation (National level) [HRH]
F_01.05 [005] Support and Assessment of Staff Performance [ALL FACILITIES]
HRH_STAFF
1
A_03.04 [423] HRH Regulation (National level) [HRH]
EMR_SITE
11
C_01.05 [205] Beneficiary/Client Records [AP]
C_01.08 [208] Data Quality Assurance [AP]
F_01.09 [009] Data Quality Assurance [ALL FACILITIES]
F_02.05 [025] ART Register-Electronic [C&T GEN POP]
F_02.07 [027] Pre-ART Register-Electronic [C&T GEN POP]
F_03.08 [025] ART Register-Electronic [C&T KP]
F_03.10 [027] Pre-ART Register-Electronic [C&T KP]
F_04.02 [053] ANC Register-Electronic [PMTCT-ANC]
F_04.08 [025] ART Register-Electronic [PMTCT-ANC]
F_04.24 [061] L&D Register-Electronic [PMTCT-L&D]
F_04.30 [067] HIV Exposed Infant/Early Infant Diagnosis Register-Electronic [HEI]

168

APPENDICES
169
Appendix 3: DREAMS and DREAMS-Like SNU Reporting Requirements
Indicator
Required Disaggregations for DREAMS
Who Should Report?
PMTCT_STAT
POSITIVITY STATUS/AGE:
Females: Known at Entry Positive: 10-14, 15-19, 20-24, 25-
29, 30-34, 35-39; Newly Tested Positive: 10-14, 15-19, 20-
24, 25-29, 30-34, 35-39; Known Negatives: 10-14, 15-19,
20-24, 25-29, 30-34, 35-39
All IPs delivering
PMTCT Services
PrEP_NEW
AGE/SEX:
Females: 15-19, 20-24, 25-29, 30-34, 35-39
All IPs delivering PrEP
HTS_TST
SERVICE DELIVERY MODALITY/AGE/SEX/RESULT:
Service Delivery Modalities: Index testing, Mobile testing,
VCT testing, Other community testing platforms, Inpatient,
PMTCT (ANC only), TB, VMMC, other PITC, VCT, Index
testing, STI, Emergency
*For each service delivery modality listed above,
disaggregate by Age/Sex/Result below:
Females:
Positive: 10-14, 15-19, 20-24, 25-29, 30-34, 35-39
Negative: 10-14, 15-19, 20-24, 25-29, 30-34, 35-39
Males:
Positive: 10-14, 15-19, 20-24, 25-29, 30-34, 35-39
Negative: 10-14, 15-19, 20-24, 25-29, 30-34, 35-39
All IPs delivering HTS
KP_PREV
KEY POPULATION TYPE:
Key population type: Female Sex Worker (FSW)
All IPs delivering KP
prevention services
PP_PREV
AGE/SEX:
Females: 10-14, 15-19, 20-24, 25-29, 30-34, 35-39
Males: 10-14, 15-19, 20-24, 25-29, 30-34, 35-39
All IPs delivering
prevention services
GEND_GBV
VIOLENCE SERVICE TYPE/AGE/SEX:
Sexual Violence:
Females: 10-14, 15-19, 20-24, 25-29, 30-34, 35-39
Physical and/or emotional violence:
Females: 10-14, 15-19, 20-24, 25-29, 30-34, 35-39
All IPs delivering post
violence care services
VMMC_CIRC
AGE:
Males: 15-19, 20-24, 25-29, 30-34, 35-39
All IPs delivering
male circumcision
services
OVC_SERV
AGE/SEX/SERVICE AREA:
Education Support:
Females: 10-14, 15-17, 18-24, 25+
Males: 10-14, 15-17, 18-24, 25+
Parenting/Caregiver program:
Females: 10-14, 15-17, 18-24, 25+
Males: 10-14, 15-17, 18-24, 25+
Social Protection (including cash transfer):
Females: 10-14, 15-17, 18-24, 25+
Males: 10-14, 15-17, 18-24, 25+
Economic Strengthening:
Only DREAMS-
funded partners
providing OVC
services in DREAMS
SNUs should report
170
Indicator
Required Disaggregations for DREAMS
Who Should Report?
Females: 10-14, 15-17, 18-24, 25+
Males: 10-14, 15-17, 18-24, 25+
Other service areas:
Females: 10-14, 15-17, 18-24, 25+
Males: 10-14, 15-17, 18-24, 25+
TX_NEW
AGE/SEX:
Females: 15-19, 20-24, 25-29, 30-34, 35-39
Males: 15-19, 20-24, 25-29, 30-34, 35-39
All IPs providing
treatment services
TX_CURR
AGE/SEX:
Females: 15-19, 20-24, 25-29, 30-34, 35-39
Males: 15-19, 20-24, 25-29, 30-34, 35-39
All IPs providing
treatment services
TX_RET
AGE/SEX:
Females: 15-19, 20-24, 25-29, 30-34, 35-39
Males: 15-19, 20-24, 25-29, 30-34, 35-39
All IPs providing
treatment services
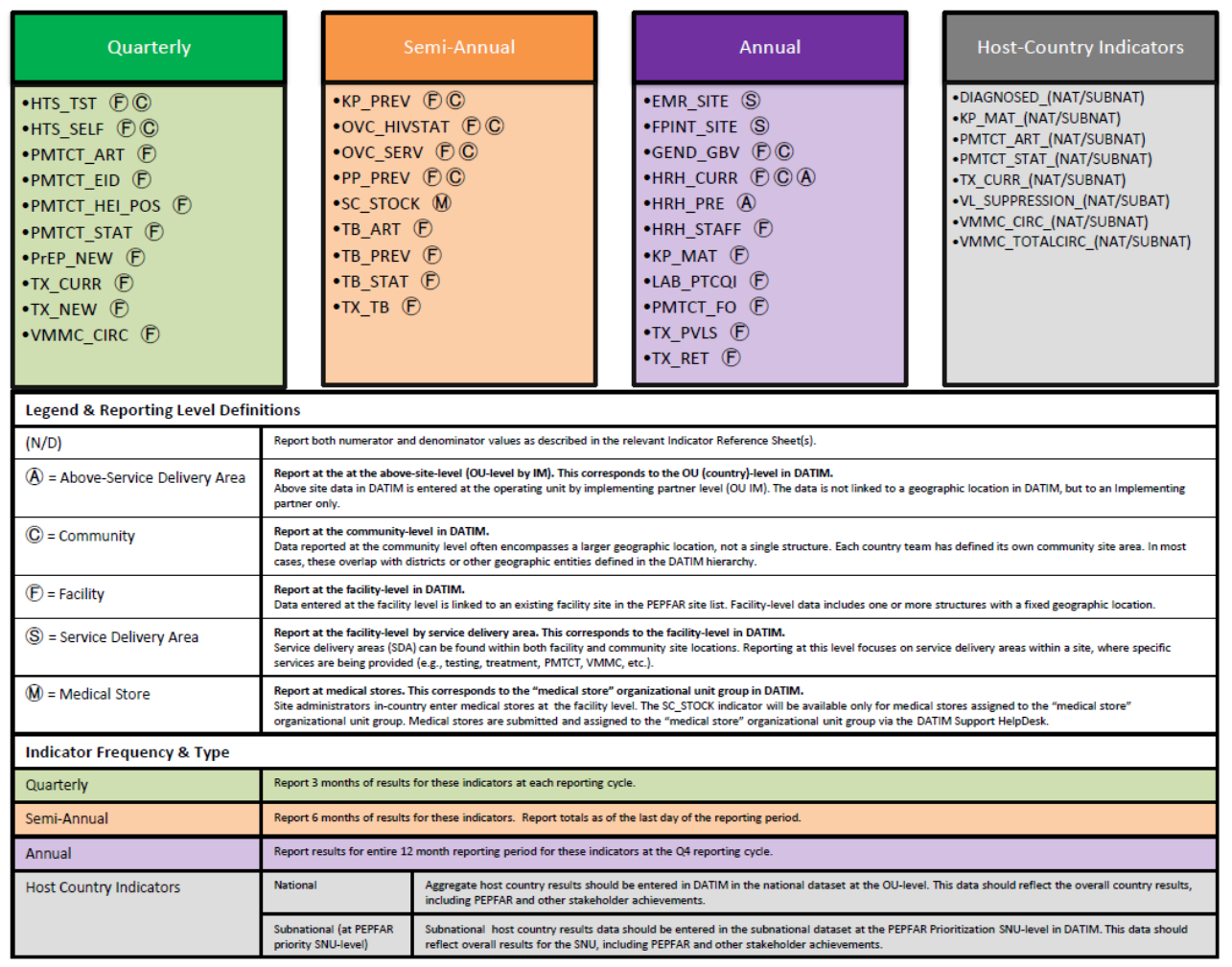
Appendix 4: Frequency & Level of Reporting Table
172
Appendix 5: Implementation and Planning Attributes (IMPATTS)
Indicators to be used to analyze program coverage levels:
Indicators
Numerator and Denominator
(Disaggregations)
Description
POPULATION
ESTIMATE_NAT /
SUBNAT
The total midyear population estimate
Disaggregation:
• Sex
• Adults/Children
These figures provide the denominators for the
calculation of multiple epidemiological
parameters
HIV PREVALENCE
ESTIMATE_NAT /
SUBNAT
The prevalence of HIV in the adult population
Disaggregation:
• Sex
• Adults/Children
Knowing the percentage of adults in a country
who are living with HIV is fundamental for
understanding the burden of HIV at the national
and sub-national levels, for planning programs
to serve people living with HIV, and for
monitoring the impact of HIV programs.
Disaggregating prevalence estimates by sex, and
geographical distribution is crucial for tailoring a
country’s response to needs. Disaggregation is
also necessary for monitoring program coverage
and impact.
KP ESTIMATE_NAT /
SUBNAT
Number of people engaging in defined
behaviors (men who have sex with men, sex
workers, people who inject drugs), or belonging
to defined groups (transgender people,
inmates/detainees), associated with increased
risk of HIV infection
Disaggregation: By defined key population:
• Sex workers
• Men who have sex with men
• People who inject drugs
• Transgender people
• Persons in prisons or other closed
settings
Program planning for key populations can be
more efficient if there are accurate estimates of
the size of these populations. The figures enable
national AIDS programs, ministries of health,
donors and non-profit and multilateral
organizations to efficiently allocate resources to
adequately meet the prevention needs of key
populations. Size estimates are also important
for modelling the HIV epidemic.
PLHIV ESTIMATE_NAT
/ SUBNAT
The number of adults and children living with
HIV
Disaggregating people living with HIV estimates
by sex, age, and geographical distribution is
crucial for tailoring a country’s response to
needs. Disaggregation is also necessary for
monitoring program coverage and impact.
Disaggregation:
• Sex
• Adults/children
Knowing the number of adults and children in a
country who are living with HIV is fundamental
for understanding the burden of HIV at the
national and sub-national levels, for planning
programs to serve people living with HIV, and
for monitoring the impact of HIV programs. The
estimated number of people living with HIV
provides the potential size of the group entering
the care and treatment cascade, and it also
serves as the denominator for the first two of
the 95–95–95 treatment targets.
APPENDICES
173
Appendix 6: HRH_CURR Example Calculation
Category Cadre / specialization / role
Number of
persons
Average percent of
full-time work
week spent
providing HIV
treatment
prevention and
support
HIV FTE
Persons
receiving
stipend, not
salary
Average percent of
full-time work
week spent
providing HIV
treatment
prevention and
support
HIV FTE
Persons
receiving
only non-
monetary
support
Average percent of
full-time work
week spent
providing HIV
treatment
prevention and
support
HIV FTE
Total persons
receiving any
PEPFAR
support
Total HIV FTE
Clinical MCH Nurse 2 25% 0.500 2 0.500
Pediatric nurse 3 10% 0.300 3 0.300
General nurse 0 0.000
Infectious disease nurse 1 100% 1.000 1 1.000
Midwife 0 0.000
Doctor (part-time) 1 10% 0.100 210% 0.200 3 0.300
Medical officer 1 25% 0.250 1 0.250
(sum of all clinical) 1.350 1.000 0.000
Lay Community health worker 2 50% 1.000 833% 2.664 10 3.664
Adherence counselor 4 100% 4.000 4 4.000
Outreach worker, part-time 5 20% 1.000 5 1.000
MSM peer navigator 3 100% 3.000 3 3.000
(sum of all lay) 0.000 2.000 9.664
= to enter in DATIM Grand Total 32 14.014
Receiving any PEPFAR salary support
Received stipend; non-salary,
monetary
Receiving ONLY non-monetary
