MP Trt Guide 2015
MP_trt_guide_2015
User Manual:
Open the PDF directly: View PDF ![]() .
.
Page Count: 37

Treatment of Symptoms of the Menopause: An
Endocrine Society Clinical Practice Guideline
Cynthia A. Stuenkel, Susan R. Davis, Anne Gompel, Mary Ann Lumsden,
M. Hassan Murad, JoAnn V. Pinkerton, and Richard J. Santen
University of California, San Diego, Endocrine/Metabolism (C.A.S.), La Jolla, California 92093; Monash
University, School of Public Health and Preventive Medicine (S.R.D.), Melbourne 03004, Australia;
Universite´ Paris Descartes, Hoˆ pitaux Universitaires Port Royal-Cochin Unit de Gyne´ cologie Endocrnienne
(A.G.), Paris 75014, France; University of Glasgow School of Medicine (M.A.L.), Glasgow G31 2ER,
Scotland; Mayo Clinic, Division of Preventive Medicine (M.H.M.), Rochester, Minnesota 55905; University
of Virginia, Obstetrics and Gynecology (J.V.P.), Charlottesville, Virginia 22908; and University of Virginia
Health System (R.J.S.), Charlottesville, Virginia 22903
Objective: The objective of this document is to generate a practice guideline for the management
and treatment of symptoms of the menopause.
Participants: The Treatment of Symptoms of the Menopause Task Force included six experts, a
methodologist, and a medical writer, all appointed by The Endocrine Society.
Evidence: The Task Force developed this evidenced-based guideline using the Grading of Recom-
mendations, Assessment, Development, and Evaluation (GRADE) system to describe the strength
of recommendations and the quality of evidence. The Task Force commissioned three systematic
reviews of published data and considered several other existing meta-analyses and trials.
Consensus Process: Multiple e-mail communications, conference calls, and one face-to-face meet-
ing determined consensus. Committees of The Endocrine Society, representatives from endorsing
societies, and members of The Endocrine Society reviewed and commented on the drafts of the
guidelines. The Australasian Menopause Society, the British Menopause Society, European Meno-
pause and Andropause Society, the European Society of Endocrinology, and the International
Menopause Society (co-sponsors of the guideline) reviewed and commented on the draft.
Conclusions: Menopausal hormone therapy (MHT) is the most effective treatment for vasomotor
symptoms and other symptoms of the climacteric. Benefits may exceed risks for the majority of symp-
tomatic postmenopausal women who are under age 60 or under 10 years since the onset of meno-
pause. Health care professionals should individualize therapy based on clinical factors and patient
preference. They should screen women before initiating MHT for cardiovascular and breast cancer risk
and recommend the most appropriate therapy depending on risk/benefit considerations. Current
evidence does not justify the use of MHT to prevent coronary heart disease, breast cancer, or dementia.
Other options are available for those with vasomotor symptoms who prefer not to use MHT or who have
contraindications because these patients should not use MHT. Low-dose vaginal estrogen and ospemifene
provide effective therapy for the genitourinary syndrome of menopause, and vaginal moisturizers and
lubricants are available for those not choosing hormonal therapy. All postmenopausal women should
embrace appropriate lifestyle measures. (J Clin Endocrinol Metab 100: 3975–4011, 2015)
ISSN Print 0021-972X ISSN Online 1945-7197
Printed in USA
Copyright © 2015 by the Endocrine Society
Received May 7, 2015. Accepted August 28, 2015.
First Published Online October 7, 2015
Abbreviations: BZA, bazedoxifene; CEE, conjugated equine estrogens; CHD, coronary heart
disease; CI, confidence interval; CVD, cardiovascular disease; DVT, deep vein thrombosis; EPT,
estrogen plus progestogen therapy; ET, estrogen therapy; GSM, genitourinary syndrome of
menopause; HR, hazard ratio; MetS, metabolic syndrome; MHT, menopausal hormone ther-
apy; MI, myocardial infarction; MPA, medroxyprogesterone acetate; OTC, over the counter; PE,
pulmonary embolism; POI, primary ovarian insufficiency; QOL, quality of life; RCT, randomized
controlled trial; SERM, selective estrogen receptor modulator; SSRI, selective serotonin re-
uptake inhibitor; SNRI, serotonin-norepinephrine reuptake inhibitor; VMS, vasomotor symp-
toms; VTE, venous thromboembolism; VVA, vulvovaginal atrophy.
SPECIAL FEATURE
Clinical Practice Guideline
doi: 10.1210/jc.2015-2236 J Clin Endocrinol Metab, November 2015, 100(11):3975–4011 press.endocrine.org/journal/jcem 3975
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018

Summary of Recommendations
1.0 Diagnosis and symptoms of menopause
1.1 We suggest diagnosing menopause based on the
clinical criteria of the menstrual cycle. (2ⱍQQEE)
1.2 If establishing a diagnosis of menopause is neces-
sary for patient management in women having undergone
a hysterectomy without bilateral oophorectomy or pre-
senting with a menstrual history that is inadequate to as-
certain menopausal status, we suggest making a presump-
tive diagnosis of menopause based on the presence of
vasomotor symptoms (VMS) and, when indicated, labo-
ratory testing that includes replicate measures of FSH and
serum estradiol. (2ⱍQQEE)
2.0 Health considerations for all menopausal
women
2.1 When women present during the menopausal tran-
sition, we suggest using this opportunity to address bone
health, smoking cessation, alcohol use, cardiovascular
risk assessment and management, and cancer screening
and prevention. (Ungraded best practice statement)
3.0 Hormone therapy for menopausal symptom
relief
3.1 Estrogen and progestogen therapy
3.1a For menopausal women ⬍60 years of age or ⬍10
years past menopause with bothersome VMS (with or
without additional climacteric symptoms) who do not
have contraindications or excess cardiovascular or breast
cancer risks and are willing to take menopausal hormone
therapy (MHT), we suggest initiating estrogen therapy
(ET) for those without a uterus and estrogen plus prog-
estogen therapy (EPT) for those with a uterus. (2ⱍQQEE)
Cardiovascular risk
3.1b For women ⬍age 60 or ⬍10 years past meno-
pause onset considering MHT for menopausal symptom
relief, we suggest evaluating the baseline risk of cardio-
vascular disease (CVD) and taking this risk into consid-
eration when advising for or against MHT and when se-
lecting type, dose, and route of administration. (2ⱍQQEE)
3.1c For women at high risk of CVD, we suggest initi-
ating nonhormonal therapies to alleviate bothersome
VMS (with or without climacteric symptoms) over MHT.
(2ⱍQQEE)
3.1d For women with moderate risk of CVD, we sug-
gest transdermal estradiol as first-line treatment, alone for
women without a uterus or combined with micronized
progesterone (or another progestogen that does not ad-
versely modify metabolic parameters) for women with a
uterus, because these preparations have less untoward ef-
fect on blood pressure, triglycerides, and carbohydrate
metabolism. (2ⱍQQEE)
Venous thromboembolic events
3.1e For women at increased risk of venous thrombo-
embolism (VTE) who request MHT, we recommend a
nonoral route of ET at the lowest effective dose, if not
contraindicated (1ⱍQQEE); for women with a uterus, we
recommend a progestogen (for example, progesterone and
dydrogestone) that is neutral on coagulation parameters.
(1ⱍQQQE)
Breast cancer
3.1f For women considering MHT for menopausal
symptom relief, we suggest evaluating the baseline risk of
breast cancer and taking this risk into consideration when
advising for or against MHT and when selecting type,
dose, and route of administration. (2ⱍQQEE)
3.1g For women at high or intermediate risk of breast
cancer considering MHT for menopausal symptom relief,
we suggest nonhormonal therapies over MHT to alleviate
bothersome VMS. (2ⱍQQEE)
Tailoring MHT
3.1h We suggest a shared decision-making approach to
decide about the choice of formulation, starting dose, the
route of administration of MHT, and how to tailor MHT
to each woman’s individual situation, risks, and treatment
goals. (Ungraded best practice statement)
Custom-compounded hormones
3.1i We recommend using MHT preparations ap-
proved by the US Food and Drug Administration (FDA)
and comparable regulating bodies outside the United
States and recommend against the use of custom-com-
pounded hormones. (Ungraded best practice statement)
3.2 Conjugated equine estrogens with bazedoxifene
3.2 For symptomatic postmenopausal women with a
uterus and without contraindications, we suggest the com-
bination of conjugated equine estrogens (CEE)/bazedox-
ifene (BZA) (where available) as an option for relief of
VMS and prevention of bone loss. (2ⱍQQQE)
3.3 Tibolone
3.3a For women with bothersome VMS and climacteric
symptoms and without contraindications, we suggest
tibolone (in countries where available) as an alternative to
MHT. (2ⱍQQEE)
3.3b We recommend against adding tibolone to other
forms of MHT. (1ⱍQQEE)
3.3c We recommend against using tibolone in women
with a history of breast cancer. (1ⱍQQEE)
3976 Stuenkel et al Guideline on Menopause J Clin Endocrinol Metab, November 2015, 100(11):3975–4011
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018

3.4 Clinical management of patients taking hormone
therapies
Monitoring during therapy
3.4a For women with persistent unscheduled bleeding
while taking MHT, we recommend evaluation to rule out
pelvic pathology, most importantly, endometrial hyper-
plasia and cancer. (1ⱍQQQE)
3.4b We recommend informing women about the
possible increased risk of breast cancer during and after
discontinuing EPT and emphasizing the importance of
adhering to age-appropriate breast cancer screening.
(1ⱍQQQE)
3.4c We suggest that the decision to continue MHT be
revisited at least annually, targeting the shortest total du-
ration of MHT consistent with the treatment goals and
evolving risk assessment of the individual woman. (Un-
graded best practice statement)
3.4d For young women with primary ovarian insuffi-
ciency (POI), premature or early menopause, without con-
traindications, we suggest taking MHT until the time of
anticipated natural menopause, when the advisability of
continuing MHT can be reassessed. (2ⱍQQEE)
Stopping considerations
3.4e For women preparing to discontinue MHT, we
suggest a shared decision-making approach to elicit indi-
vidual preference about adopting a gradual taper vs
abrupt discontinuation. (2ⱍQQEE)
4.0 Nonhormonal therapies for VMS
4.0 For postmenopausal women with mild or less both-
ersome hot flashes, we suggest a series of steps that do not
involve medication, such as turning down the thermostat,
dressing in layers, avoiding alcohol and spicy foods, and
reducing obesity and stress. (2ⱍQQEE)
4.1 Nonhormonal prescription therapies for VMS
4.1a For women seeking pharmacological management
for moderate to severe VMS for whom MHT is contrain-
dicated, or who choose not to take MHT, we recommend
selective serotonin reuptake inhibitors (SSRIs)/serotonin-
norepinephrine reuptake inhibitors (SNRIs) or gabapen-
tin or pregabalin (if there are no contraindications).
(1ⱍQQQE)
4.1b For those women seeking relief of moderate to
severe VMS who are not responding to or tolerating the
nonhormonal prescription therapies, SSRIs/SNRIs or ga-
bapentin or pregabalin, we suggest a trial of clonidine (if
there are no contraindications). (2ⱍQQEE)
4.2 Over-the-counter and alternative nonhormonal
therapies for VMS
4.2 For women seeking relief of VMS with over-the-
counter (OTC) or complementary medicine therapies,
we suggest counseling regarding the lack of consistent
evidence for benefit for botanicals, black cohosh,
omega-3-fatty acids, red clover, vitamin E, and mind/
body alternatives including anxiety control, acupunc-
ture, paced breathing, and hypnosis. (2ⱍQQEE)
5.0 Treatment of genitourinary syndrome of
menopause
5.1 Vaginal moisturizers and lubricants
5.1a For postmenopausal women with symptoms of
vulvovaginal atrophy (VVA), we suggest a trial of vaginal
moisturizers to be used at least twice weekly. (2ⱍQQEE)
5.1b For women who do not produce sufficient vaginal
secretions for comfortable sexual activity, we suggest vag-
inal lubricants. (2ⱍQQEE)
5.2 Vaginal estrogen therapies
5.2a For women without a history of hormone- (estro-
gen) dependent cancers who are seeking relief from symp-
toms of genitourinary syndrome of menopause (GSM) (in-
cluding VVA) that persist despite using vaginal lubricants
and moisturizers, we recommend low-dose vaginal ET.
(1ⱍQQQE)
Practice statement
5.2b In women with a history of breast or endometrial
cancer, who present with symptomatic GSM (including
VVA), that does not respond to nonhormonal therapies,
we suggest a shared decision-making approach that in-
cludes the treating oncologist to discuss using low-dose
vaginal ET. (Ungraded best practice statement)
5.2c For women taking raloxifene, without a history of
hormone- (estrogen) dependent cancers, who develop
symptoms of GSM (including VVA) that do not respond
to nonhormonal therapies, we suggest adding low-dose
vaginal ET. (2ⱍQQEE)
5.2d For women using low-dose vaginal ET, we suggest
against adding a progestogen (ie, no need for adding pro-
gestogen to prevent endometrial hyperplasia). (2ⱍQEEE)
5.2e For women using vaginal ET who report post-
menopausal bleeding or spotting, we recommend prompt
evaluation for endometrial pathology. (1ⱍQQEE)
5.3 Ospemifene
5.3a For treatment of moderate to severe dyspareunia
associated with vaginal atrophy in postmenopausal
women without contraindications, we suggest a trial of
ospemifene. (2ⱍQQQE)
doi: 10.1210/jc.2015-2236 press.endocrine.org/journal/jcem 3977
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018

5.3b For women with a history of breast cancer pre-
senting with dyspareunia, we recommend against os-
pemifene. (1ⱍQEEE)
Method of Development of Evidence-
based Clinical Practice Guidelines
The Clinical Guidelines Subcommittee (CGS) of The En-
docrine Society deemed management of menopause a pri-
ority area in need of a practice guideline and appointed a
Task Force to formulate evidence-based recommenda-
tions. The Task Force followed the approach recom-
mended by the Grading of Recommendations, Assess-
ment, Development, and Evaluation (GRADE) group, an
international group with expertise in development and
implementation of evidence-based guidelines (1). A de-
tailed description of the grading scheme has been pub-
lished elsewhere (2). The Task Force used the best avail-
able research evidence to develop the recommendations.
The Task Force commissioned three systematic reviews of
the literature to inform its key recommendations. The
Task Force used consistent language and graphical de-
scriptions of both the strength of a recommendation and
the quality of evidence using the recommendations of the
GRADE system. In terms of the strength of the recom-
mendation, strong recommendations use the phrase “we
recommend” or “we recommend against” and the number
1, and weak recommendations use the phrase “we sug-
gest” or “we suggest against” and the number 2. Cross-
filled circles indicate the quality of the evidence, such that
QEEE denotes very low quality evidence; QQEE, low
quality; QQQE, moderate quality; and QQQQ, high qual-
ity. The Task Force has confidence that persons who re-
ceive care according to the strong recommendations will
derive, on average, more good than harm. Weak recom-
mendations require more careful consideration of the per-
son’s circumstances, values, and preferences to determine
the best course of action. Linked to each recommendation
is a description of the evidence and the values the panelists
considered when making the recommendation. In some
instances, there are remarks, a section in which panelists
offer technical suggestions for testing conditions, dosing,
and monitoring. These technical comments reflect the best
available evidence applied to a typical person being
treated. Often this evidence comes from the unsystematic
observations of the panelists and their values and prefer-
ences; therefore, these remarks should be considered sug-
gestions. In this guideline, the Task Force made several
statements to emphasize the importance of shared decision
making, general preventive care measures, and basic prin-
ciples of women’s health. These were labeled as ungraded
best practice statements. Direct evidence for these statements
was either unavailable or not systematically appraised and
was considered out of the scope of this guideline. The inten-
tion of these statements is to draw attention and remind pro-
viders of these principles, and these statements should not be
considered as graded recommendations (3).
The 2013 Appraisal of Guidelines for Research and
Evaluation II (AGREEII) criteria (23 items) were satisfied,
with three exceptions. Item 5 stipulates that the views and
preferences of the target population (patients, public, etc)
have been sought. The Task Force did not conduct specific
polling/outreach to the public in anticipation of this guide-
line. Item 14 states that a procedure for updating the
guideline is provided. This process has not been formal-
ized. Item 20 suggests that the potential resource implica-
tions of applying the recommendations have been consid-
ered. The Task Force did not include cost analysis of risk
assessment tools or prescription drug therapies.
The Endocrine Society maintains a rigorous conflict-
of-interest review process for the development of clinical
practice guidelines. All Task Force members must declare
any potential conflicts of interest, which are reviewed
before the members are approved to serve on the Task
Force and periodically during the development of the
guideline. The conflict-of-interest forms are vetted by the
CGS before the members are approved by the Society’s
Council to participate on the guideline Task Force. Par-
ticipants in the guideline development must include a ma-
jority of individuals without conflict of interest in the mat-
ter under study. Participants with conflicts of interest may
participate in the development of the guideline, but they
must have disclosed all conflicts. The CGS and the Task
Force have reviewed all disclosures for this guideline and
resolved or managed all identified conflicts of interest.
Conflicts of interest are defined by remuneration in any
amount from the commercial interest(s) in the form of
grants; research support; consulting fees; salary; owner-
ship interest (eg, stocks, stock options, or ownership in-
terest excluding diversified mutual funds); honoraria or
other payments for participation in speakers’ bureaus, ad-
visory boards, or boards of directors; or other financial
benefits. Completed forms are available through the En-
docrine Society office.
Funding for this guideline was derived solely from the
Endocrine Society, and thus the Task Force received no
funding or remuneration from commercial or other
entities.
Commissioned systematic reviews
The Task Force formulated three questions for system-
atic reviews to provide evidence supporting this guideline.
The first compared the effect of oral vs transdermal es-
3978 Stuenkel et al Guideline on Menopause J Clin Endocrinol Metab, November 2015, 100(11):3975–4011
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018

trogens on the risk of venous and arterial thrombotic
events. Low-quality evidence derived from 15 observa-
tional studies suggested that, compared with transdermal
MHT, oral MHT was associated with increased risk of
VTE, deep vein thrombosis (DVT), and possibly stroke,
but not myocardial infarction (MI) (4). The second ques-
tion evaluated the effect of MHT on mortality. Data from
43 randomized controlled trials (RCTs) demonstrated no
association between all-cause mortality, regardless of hor-
mone type, the presence of pre-existing heart disease, or
length of follow-up (5). Meta-analysis of 2 RCTs in which
MHT was started at a mean age less than 60 and 3 RCTs
in which MHT was started less than10 years after meno-
pause suggested possible reduction of mortality with
MHT. The third question compared the effect of MHT
with natural progesterone vs synthetic progestins on
breast cancer risk. Low-quality evidence from two obser-
vational studies suggested that natural progesterone may
be associated with a reduced risk for breast cancer com-
pared with synthetic progestins, but data were insufficient
to draw a firm conclusion.
Introduction and background
VMS, hot flashes, and night sweats, are the hallmarks
of menopause, although not all women experience these
symptoms. Other climacteric symptoms include sleep dis-
turbance (6, 7), arthralgia (7–9), and vaginal dryness and
dyspareunia (7, 10, 11). It is less clear whether anxiety,
irritability, depression, palpitations, skin dryness, loss of
libido, and fatigue can be attributed to menopause (7, 9,
12). Symptoms frequently start in the years before the final
menstrual period and can last, with unpredictable dura-
tion, from a few years to more than 13 years (13–16).
ET has long been recognized as the most effective treat-
ment for the relief of bothersome vasomotor and vaginal
symptoms associated with menopause. However, pre-
scriptions for MHT declined considerably after the 2002
publication of the Women’s Health Initiative (WHI) RCT.
This study determined that for postmenopausal women
(average age, 63 y), oral CEE alone after hysterectomy
(17), or coupled with daily medroxyprogesterone acetate
(MPA) in women with a uterus (18), was associated with
risks disproportionate to preventive benefits (17, 18).
During ensuing years, a consensus arose that most healthy
symptomatic women, without contraindications and
closer to the time of menopause (⬍10 y after menopause
onset or age ⬍60 y), were appropriate candidates for
MHT for symptom relief (19, 20). Post hoc WHI analyses
and observational data suggest that benefits exceed risks
in most of these women. At this juncture, women in the
United States and some other countries have a broader
range of therapeutic choices than ever before, including:
MHT dose, type, and route of administration; new selec-
tive estrogen receptor modulators (SERMs) as solo or
combination therapies; and expanded choices of nonhor-
monal prescription medications. In this guideline, we em-
phasize safety in identifying which late perimenopausal
and recently postmenopausal women are candidates for
various therapeutic agents. Considerations include the
risks and benefits of each available therapy, the expected
duration of treatment, the intensity of monitoring during
therapy, and most importantly, individualizing the
course of therapy to reflect the specific characteristics of
the patient who is making decisions regarding symptom
management.
This guideline covers the full spectrum of therapies for
relief of the most common and bothersome menopausal
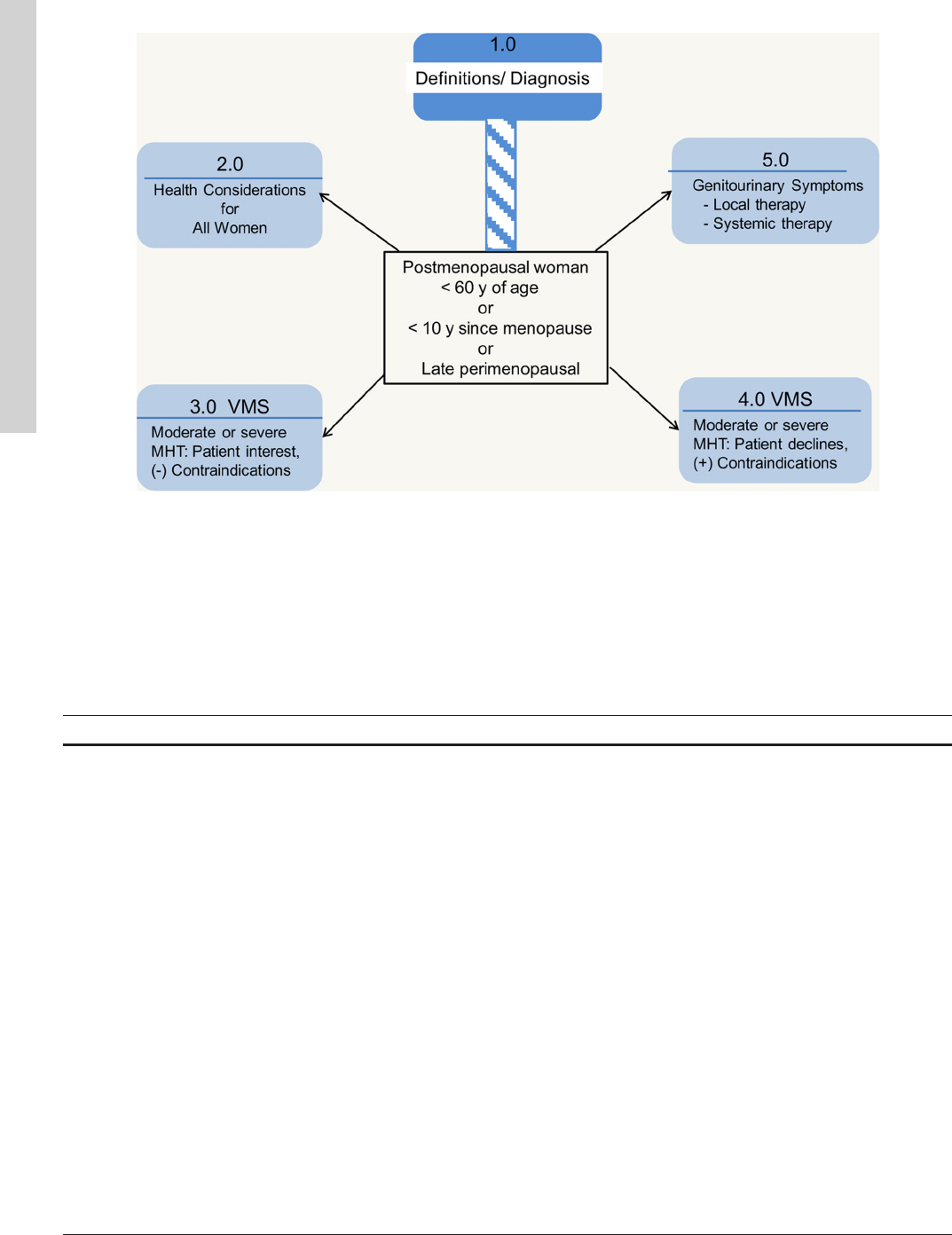
symptoms (Figure 1). (The detailed management of early
menopause transition, primary ovarian insufficiency, and
prevention of osteoporosis and fracture are considered
beyond the current scope.) Choice of therapy is ideally
based on available evidence regarding safety and efficacy
and is generally a shared decision including both patient
and provider. The treatment selected should be tailored to
the individual patient and will vary according to each
woman’s symptom severity, age, medical profile, personal
preference, and estimated benefit/risk ratio. The impact of
severe menopausal symptoms on quality of life (QOL) can
be substantial, and there are instances in which a woman
with a history of coronary heart disease (CHD) or breast
cancer, for example, will choose to accept a degree of risk
that might otherwise be considered to outweigh the ben-
efits of MHT. An accepted philosophy is that a fully in-
formed patient should be empowered to make a decision
that best balances individual QOL benefits against poten-
tial health risks (21).
1.0 Diagnosis and symptoms of menopause
1.1 We suggest diagnosing menopause based on the
clinical criteria of the menstrual cycle. (2ⱍQQEE)
1.2 If establishing a diagnosis of menopause is neces-
sary for patient management in women having undergone
a hysterectomy without bilateral oophorectomy or pre-
senting with a menstrual history that is inadequate to as-
certain menopausal status, we suggest making a presump-
tive diagnosis of menopause based on the presence of VMS
and, when indicated, laboratory testing that includes rep-
licate measures of FSH and serum estradiol. (2ⱍQQEE)
Technical remark
Table 1 summarizes other etiologies of secondary
amenorrhea to be considered in the differential diagnosis.
doi: 10.1210/jc.2015-2236 press.endocrine.org/journal/jcem 3979
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018
Diagnosis
Table 1 lists definitions of the clinical spectrum of
menopause. In a woman with an intact uterus, menopause
is a clinical diagnosis based upon cessation of menses for
at least 12 months. Sex steroids, gonadotropins, inhibin B,
or anti-Mullerian hormone measurements do not further
inform the diagnosis, do not indicate precisely when the
final menstrual period will occur, and will not influence
management unless a woman is seeking fertility. In women
having undergone a hysterectomy but not bilateral oo-
phorectomy, elevated FSH levels and estradiol concentra-
tions ⬍20 pg/mL on several occasions support but do not
Table 1. Definitions of Spectrum of Menopause
Menopause
Clinical status after the final menstrual period, diagnosed retrospectively after cessation of menses for 12 mo in a previously
cycling woman and reflecting complete or nearly complete permanent cessation of ovarian function and fertility.
Spontaneous menopause
Cessation of menses that occurs at an average age of 51 y in the absence of surgery or medication (316–318).
Menopausal transition (or perimenopause)
An interval preceding the menopause characterized by variations in menstrual cycle length and bleeding pattern, mood
shifts, vasomotor, and vaginal symptoms and with rising FSH levels and falling anti-Mullerian hormone and inhibin B
levels, which starts during the late reproductive stage and progresses during the menopause transition (15, 319).
Climacteric
The phase in the aging of women marking the transition from the reproductive phase to the nonreproductive state. This
phase incorporates the perimenopause by extending for a longer variable period before and after the perimenopause.
Climacteric syndrome
When the climacteric is associated with symptomatology.
Menopause after hysterectomy without oophorectomy
Spontaneous cessation of ovarian function without the clinical signal of cessation of menses.
Induced menopause
Cessation of ovarian function induced by chemotherapy, radiotherapy, or bilateral oophorectomy.
Early menopause
Cessation of ovarian function occurring between ages 40 and 45 in the absence of other etiologies for secondary
amenorrhea (pregnancy, hyperprolactinemia, and thyroid disorders).
POI
Loss of ovarian function before the age of 40 y with waxing and waning course and potential resumption of menses,
conception, and pregnancy (320). The prevalence of POI is approximately 1% (321) and is differentiated into idiopathic,
autoimmune (associated with polyglandular autoimmune syndromes), metabolic disorders, and genetic abnormalities
(including fragile X premutation).
Figure 1. Approach to menopause guideline. Numbers correspond to section of text addressing selected clinical issue.
3980 Stuenkel et al Guideline on Menopause J Clin Endocrinol Metab, November 2015, 100(11):3975–4011
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018

confirm the diagnosis. A distinction between the late peri-
menopause transition, marked by episodes of ⬎60 days of
amenorrhea and increasing severity of VMS (15), and
early postmenopause cannot be made on the sole basis of
hormone measurements. With radiotherapy- or chemo-
therapy-induced menopause, it is important to recognize
that ovarian function may resume after 12 months of
amenorrhea (22), depending on the age of the woman and
the dose and duration of treatment (22). For POI, persis-
tent FSH elevation in women ⬍age 40 provides a tentative
diagnosis (Table 1).
Signs and symptoms
Vasomotor symptoms
Prevalence. Hot flashes (also called hot flushes) occur in
approximately 75% of postmenopausal women in the
United States (23). In the Study of Women Across the
Nation (SWAN), after controlling for age, education,
health, and economic strain, researchers found that US
Caucasian women report more psychosomatic symptoms,
African American and Hispanic women report more
VMS, and Asian women report more somatic complaints
(16, 24). Notably, across countries and ethnic back-
grounds, the percentage of women reporting hot flashes
varies (25–27). In a cross-sectional study of premeno-
pausal women (mean age, 48 y), one-third reported “ever”
experiencing hot flashes (28). A comparison between
VMS experienced during the premenopause vs the post-
menopause may be informative when counseling a post-
menopausal woman regarding symptom relief, although
to our knowledge, the presence and frequency of premeno-
pausal hot flashes have not been studied as being predic-
tive of response to therapy in the postmenopause. Persis-
tence of hot flashes may also vary depending upon when
in the menopausal transition VMS were first noted. In
SWAN, earlier onset of VMS was associated with longer
postmenopausal duration (16).
Clinical manifestations. Hot flashes typically begin as the
sudden sensation of heat centered on the upper chest and
face. When moderate or severe, the hot flash rapidly be-
comes generalized, lasts from 2 to 4 minutes, and can be
associated with profuse perspiration, palpitations, or anx-
iety. Triggers include spicy food or alcohol. At night, va-
somotor instability manifests as hot flashes or night
sweats, which may represent different physiological mech-
anisms. The differential diagnosis includes several entities
distinguishable by clinical features (Table 2). New-onset
VMS in older (age, ⱖ65 y) postmenopausal women may
be associated with, but not necessarily causally related to,
increased risk of major CHD and all-cause mortality (29).
Association with sleep. In polysomnography studies, noc-
turnal hot flashes are more common during the first 4
hours of sleep, whereas subsequent rapid eye movement
sleep suppresses hot flashes, arousals, and awakenings
(30). A recent study that induced estrogen deficiency in
healthy premenopausal women with a GnRH agonist di-
rectly demonstrated that hot flashes are associated with
three factors: 1) an increase in episodes of waking after
sleep-onset; 2) a decrease in perceived sleep efficiency; and
3) a statistically significant correlation between nocturnal
VMS and sleep disruption (31). Although these data are
informative, it has not been substantiated whether they
apply in naturally postmenopausal women with continu-
ously high gonadotropins. An important contributing fac-
tor is aging, which likely is also involved in sleep distur-
bances in menopausal women.
Mechanisms. VMS appear to involve the central nervous
system (32) because: 1) hot flashes occur simultaneously
with, but are not caused by, LH pulses (33, 34); and 2)
research has shown an association with the neuroregula-
tors kisspeptin, neurokinin B, and dynorphin (35). Alter-
ations of thermoregulatory systems are mechanistically
involved because women with hot flashes exhibit a nar-
rowing of the thermoregulatory-neutral zone (32).
Whereas premenopausal women initiate mechanisms to
dissipate heat when the core body temperature increases
Table 2. Conditions That May Cause or Mimic
Vasomotor Events and That Can Be Distinguished From
Menopausal Symptoms by History, Examination, and
Investigations, as Indicated
Hormone excess
Thyroid hormone excess
Carcinoid syndrome (flushing without sweating)
Pheochromocytoma (hypertension, flushing, and profuse
sweating)
Dietary factors
Alcohol
Spicy food
Food additives (eg, monosodium glutamate, sulfites)
Pharmaceuticals
Chronic opioid use
Opiate withdrawal
SSRIs (may cause sweats)
Nicotinic acid (intense warmth, itching lasting up to 30 min)
Calcium channel blockers
Medications that block estrogen action or biosynthesis
Chronic infection (increased body temperature)
Other medical conditions
Postgastric surgery dumping syndrome
Mastocytosis and mast cell disorders (usually with
gastrointestinal symptoms)
Some cancers: medullary carcinoma of the thyroid,
pancreatic islet-cell tumors, renal cell carcinoma,
lymphoma
Anxiety disorders
doi: 10.1210/jc.2015-2236 press.endocrine.org/journal/jcem 3981
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018

by 0.4°C, this happens with much lower increases in tem-
perature in menopausal women (36). Core body temper-
ature is usually still within the normal range at the onset
of the flash, but inappropriate peripheral vasodilatation
with increased digital and cutaneous blood flow and per-
spiration results in rapid heat loss and a fall in core body
temperature (32). Shivering may occur to restore the core
temperature (36).
Genitourinary syndrome of menopause
This new term “genitourinary syndrome of meno-
pause” (GSM) combines the conditions of VVA and uri-
nary tract dysfunction (Table 3) (37). VVA most often
presents in the late postmenopausal stage, when VMS may
have abated (15). When VVA is severe, women may have
discomfort wearing tight-fitting clothing or while sitting
or exercising. Sexual activity is not required for patients to
experience vaginal or genital discomfort. Urinary symp-
toms—dysuria, urinary frequency, and recurrent urinary
tract infections—increase in severity with time since
menopause.
Other signs and symptoms
The menopausal decline of estradiol increases bone re-
sorption and contributes to fractures (38).
Possible related signs and symptoms
Research has suggested (but not proven) a direct rela-
tionship between menopause and mood changes, mild de-
pressive symptoms, anxiety, irritability, arthralgias, loss
of libido, palpitations, skin dryness, fatigue, and reduction
in QOL (38, 39). As opposed to the conclusions in the
2005 National Institutes of Health State of the Science
consensus regarding the uncertain relationship between
mood and menopause, more recent longitudinal studies
now support an association of the menopause transition
with depressed mood, major depressive episodes, and
anxiety.
2.0 Health considerations for all menopausal
women
2.1 When women present during the menopausal tran-
sition, we suggest using this opportunity to address bone
health, smoking cessation, alcohol use, cardiovascular
risk assessment and management, and cancer screening
and prevention. (Ungraded best practice statement)
Evidence
The menopause transition, a portal to the second half
of life, is a critical window to reassess lifestyle, recognize
ongoing and potential health concerns, and encourage a
proactive approach to future well-being, regardless of
menopausal symptoms. To decrease morbidity and mor-
tality from CVD and cancer and maintain QOL, optimiz-
ing diet and exercise to maintain healthy weight are
important measures, as are counseling regarding alco-
hol use and smoking cessation and identifying and treat-
ing hypertension, glucose intolerance, and dyslipi-
demias (40, 41).
Adequate intake of calcium and vitamin D, along with
limiting alcohol consumption will minimize bone loss and
reduce the risk of falls and fractures (42). For postmeno-
pausal women ⬍65 years of age and at high risk of os-
teoporosis, dual-energy x-ray absorptiometry assessment
of bone mineral density contributes to risk assessment. ET
for the relief of menopausal symptoms prevents bone loss
and reduces fracture risk (43). Women without VMS and
at significant risk of osteoporosis can discuss the merits of
ET for bone preservation. Recent guidelines address bone-
specific therapies (43).
3.0 Hormone therapy for menopausal symptom
relief
3.1 Estrogen and progestogen therapy
3.1a For menopausal women ⬍60 years of age or ⬍10
years past menopause with bothersome VMS (with or
without additional climacteric symptoms) who do not
have contraindications or excess cardiovascular or breast
cancer risks and are willing to take MHT, we suggest ini-
tiating ET for those without a uterus and EPT for those
with a uterus. (2ⱍQQEE)
Table 3. Genitourinary Syndrome of Menopause
Symptoms
Vulvar pain, burning, or itching
Vaginal dryness
Vaginal discharge
Dyspareunia
Spotting or bleeding after intercourse
Dysuria, urinary frequency, urgency
Recurrent urinary tract infections
Signs, external genitalia
Decreased labial size
Loss of vulvar fat pads
Vulvar fissures
Receded or phimotic clitoris
Prominent urethra with mucosal eversion or prolapse
Signs, vagina
Introital narrowing
Loss of elasticity with constriction
Thin vaginal epithelial lining
Loss of mature squamous epithelium
Pale or erythematous appearance
Petechiae, ulcerations, or tears
Alkaline pH (⬎5.5)
Infection (yellow or greenish discharge)
Derived from D. J. Portman et al: Genitourinary syndrome of
menopause: new terminology for vulvovaginal atrophy from the
International Society for the Study of Women’s Sexual Health and the
North American Menopause Society. Menopause. 2014;21:1063–1068
(37), with permission.
3982 Stuenkel et al Guideline on Menopause J Clin Endocrinol Metab, November 2015, 100(11):3975–4011
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018
Evidence
In postmenopausal women, ET improves menopause-
associated (climacteric) symptoms (eg, VMS, genitouri-
nary symptoms, sleep disturbance, menopause-associated
anxiety and depressive symptoms, and arthralgias). ET
also reduces menopause-related bone loss, lowers the risk
of fragility fractures in older women, and reduces the in-
cidence of self-reported diabetes. In addition, combined
EPT reduced the risk of colorectal cancer and, in cumu-
lative follow-up of the WHI, endometrial cancer (38, 44).
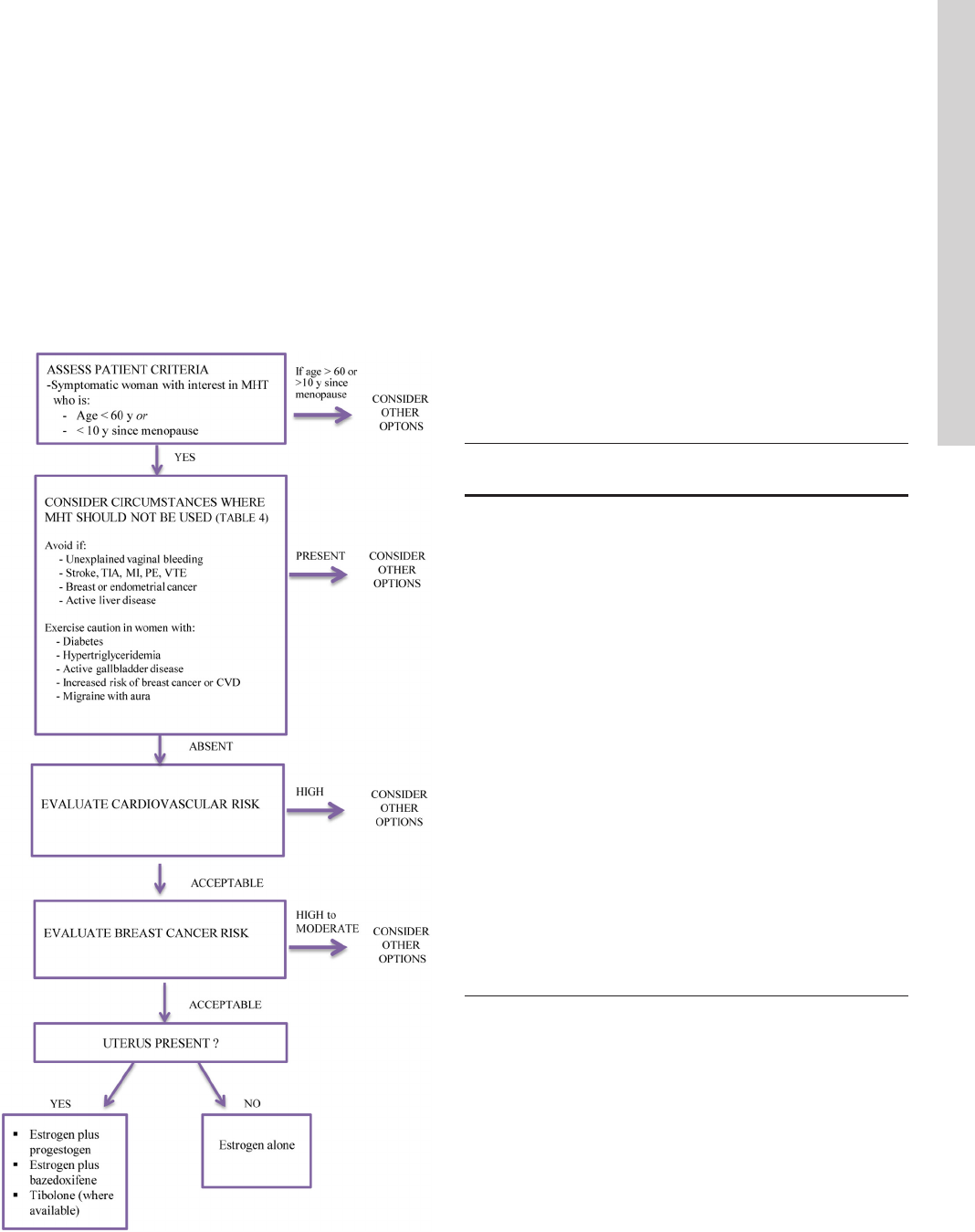
MHT is not appropriate for all symptomatic meno-
pausal women (Figure 2). There are no commonly recog-
nized lists of absolute or relative contraindications to
MHT as published in professional society guidelines. And
whereas US product labeling (regulated by the FDA) does
include contraindications to MHT (Table 4), caution is
also advised for women with certain additional medical
conditions (Table 4). Risk/benefit assessment is the most
important consideration, and QOL may be an important
issue in a decision to recommend MHT. Women with con-
ditions precluding MHT (Table 4) who are unwilling to
take MHT, or at substantial risk for breast cancer or CVD,
can consider nonhormonal options for symptom relief
(Section 4.0).
Risks and benefit overview
Healthcare providers and patients should choose MHT
based on individual risks and benefits utilizing a shared
Figure 2. Approach to the patient with VMS contemplating MHT.
TIA, transient ischemic attack.
Table 4. Specific Cautions to Use of Systemic MHT or
SERMs
a,b
for Treatment of Menopausal Symptoms
In general, ET should not be used in women with any
of the following conditions:
Undiagnosed abnormal genital bleeding
Known, suspected, or history of cancer of the breast
Known or suspected estrogen-dependent neoplasia
including endometrial cancer
Active DVT, pulmonary embolism, or history of these
conditions
Active arterial thromboembolic disease (for example,
stroke, MI) or a history of these conditions
Known anaphylactic reaction or angioedema in
response to any ingredient in the medication
c
Known liver impairment or disease
Known protein C, protein S, or antithrombin deficiency,
or other known thrombophilic disorders
c
Known or suspected pregnancy
Caution should also be exercised in women with:
Gallbladder disease (oral ET)
Hypertriglyceridemia (⬎400 mg/d) (oral ET)
Diabetes
Hypoparathyroidism (risk of hypocalcemia)
Benign meningioma
Intermediate or high risk of breast cancer
High risk of heart disease
Migraine with aura (oral ET)
Other conditions
d
a
Also apply to conjugated estrogens/BZA, ospemifene, and tibolone
therapies.
b
Advice not to use estrogens in the specific conditions listed is based
on FDA recommendations and package labeling in the United States.
The advice to exercise caution is based on a review of the literature
(including package labeling) and not dictums generally included in
various Menopause Society guidelines. Because these guidelines are
meant to be used internationally, it should be noted that these
considerations may vary from country to country.
c
Specific to CEE ⫾combination with BZA.
d
Estrogen therapy may cause an exacerbation of asthma, diabetes
mellitus, epilepsy, migraine, porphyria, systemic lupus erythematosus,
and hepatic hemangiomas and should be used with caution in women
with these conditions.
doi: 10.1210/jc.2015-2236 press.endocrine.org/journal/jcem 3983
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018
decision-making approach. Current recommendations
suggest that the initiation of MHT should generally be
limited to women ⬍60 years of age or ⬍10 years after
menopause onset. Accordingly, data are needed to esti-
mate risks and benefits in this specific population. No
adequately powered RCTs with clinical outcomes have
been specifically conducted with younger, symptomatic
women, however, and data for women ⬍50 years old are
limited. The best available evidence comes from subgroup
analyses of WHI data, which provide information specif-
ically in women 50 to 59 years of age or ⬍10 years since
menopause onset. Because of the number of women par-
ticipants ages 50 to 59 (5520 in the combined therapy arm
and 3313 in the estrogen-alone arm),
and the low event rate for MI and
stroke in this age group, such data
provide trends but few statistically
significant differences. Findings from
observational studies, case reports,
and clinical expertise, both from the
United States and other countries,
provide additional sources of evi-
dence regarding younger postmeno-
pausal women.
Estimations of risks and benefits
previously published in The Endo-
crine Society’s 2010 Scientific State-
ment utilized both observational
and RCT data. However, updated
outcomes from the WHI are now
available. Accordingly, the up-
dated reanalysis of the WHI (44) is
considered by many to provide the
best available data on risks and ben-
efits in women ages 50 to 59, but not
in those younger than age 50. The
2010 Statement expresses attribut-
able (excess) benefits and risks as the
number of affected women/1000 us-
ers/5 years of therapy, assuming that
most women initiating MHT will
consider therapy for 5 years. Main-
taining this format, the risks and
benefits (as reported in the WHI and
reflecting the specific oral therapies
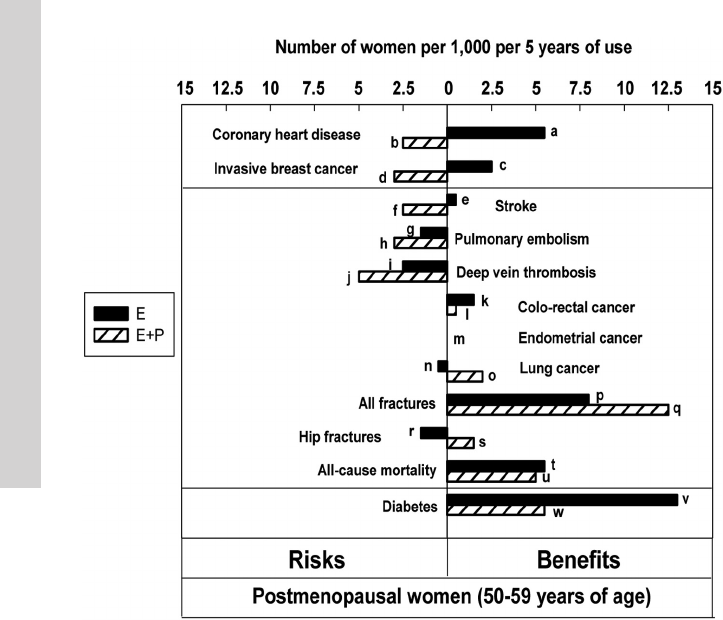
studied) are presented in Figure 3
and are not repeated in the text of
this guideline. These data, represent-
ing the effects of CEE with or with-
out MPA, cannot be extrapolated to
other MHT regimens. However, in
the absence of RCTs with other spe-
cific agents, they provide the most
conservative estimates. Notably, the baseline risk of most
adverse events is lower in younger vs older women and
results in lower attributable risk although relative risks
may be similar among various age groups. The converse is
also true for benefits, such as fracture reduction.
Benefits of MHT
Vasomotor symptoms
ET is the most effective treatment for VMS and im-
proving QOL in symptomatic women (38). In a dose-de-
pendent manner, MHT reduces hot flash frequency by
approximately 75% and severity by 87%, compared with
50% with placebo (38, 45, 46).
Figure 3. Updated summary of the effects of orally administered CEE alone or combined with
MPA in women ages 50–59 years during intervention phase of WHI. One set of analyses
examined the risks and benefits of these agents in women ages 50–59 years. This figure plots
these data, which are expressed here as excess risks and benefits per 1000 women using MHT
for 5 years. Because women deciding to use MHT are more likely to continue this for a period of
years rather than 1 year, this figure is constructed according to that assumption. WHI studies
were not powered for age-related subset analyses, and none of the data presented in the figure
are statistically significant. Nonetheless, this figure represents the best estimates that are
available at the present time and are likely more reliable than similar estimates based on
observational studies as reported previously in The Endocrine Society Scientific Statement (38).
The HR (95% CI) values for the bars in the figure are listed here with reference to the
alphabetical designations shown next to the bars: a, HR, 0.60 (0.35–1.04); b, HR, 1.34 (0.82–
2.19); c, HR, 0.82 (0.50–1.34); d, HR, 1.21 (0.81–1.80); e, HR, 0.99 (0.53–1.85); f, HR, 1.51
(0.81–2.82); g, HR, 1.53 (0.63–3.75); h, HR, 2.05 (0.89–4.71); i, HR, 1.66 (0.76–3.67); j, HR,
3.01 (1.36– 6.66); k, HR, 0.71 (0.30–1.67); l, HR, 0.79 (0.29 –2.18); m, HR, 1.00 (ns-ns); n, HR,
1.12 (0.45–2.75); o, HR, 0.62 (0.30–1.29); p, HR, 0.90 (0.72–1.11); q, HR, 0.82 (0.68 –1.00); r,
HR, 5.01 (0.59– 42.9); s, HR, 0.17 (0.02–1.45); t, HR, 0.70 (0.46–1.09); u, HR, 0.67 (0.43–1.04);
v, HR, 0.83 (0.67–1.04); and w, HR, 0.85 (0.66–1.09). [RJ Santen, et al: Competency in
menopause management: whither goest the internist? J Womens Health (Larchmt). 2014;23(4):
281–285, courtesy of Mary Ann Liebert, Inc].
3984 Stuenkel et al Guideline on Menopause J Clin Endocrinol Metab, November 2015, 100(11):3975–4011
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018

Genitourinary syndrome of menopause
Systemic estrogen administration effectively treats
VVA and improves symptoms of overactive bladder and
recurrent urinary tract infections (47, 48). With lower
doses of systemic MHT, vaginal symptoms may persist
and local therapy may be needed (Section 5).
Sleep disruption
Large placebo-controlled trials reported significantly
fewer sleep disturbances with MHT use (44), but addi-
tional data are required for definitive conclusions.
Anxiety and depressive symptoms
Anxiety symptoms increase during the menopause
transition and are associated with an increased likelihood
of a major depressive disorder (49). ET may improve mild-
to-moderate depressive symptoms during or shortly after
the menopause transition, whereas antidepressant ther-
apy remains appropriate treatment for major depres-
sion (50, 51).
Arthralgia
Joint pain or stiffness and general aches or pains were
improved in women receiving EPT (38, 44, 52). Joint pain
increased slightly after discontinuation of treatment (44).
Potential preventive benefits of menopausal hor-
mone therapy
Although studies have suggested certain preventive
benefits, the U.S. Preventive Services Task Force (53) and
many expert groups (40, 54–56) recommend against
MHT for primary or secondary disease prevention,
whereas other experts disagree (57).
Bone loss and fracture. RCTs, observational studies, and
meta-analyses consistently report reduction in bone loss
with ET (38). The updated WHI analysis reports a signif-
icant reduction in vertebral fractures and a borderline sig-
nificant reduction for all fractures with EPT in women
ages 50 to 59 years (Figure 3); this effect was greater than
with ET (44). Benefit may also be dose-related (38).
Type 2 diabetes. RCTs (58–60) and large observational
studies (61, 62) reported that MHT reduced the preva-
lence of self-reported diabetes by 14 to 19% (44), an effect
that did not persist after therapy was discontinued (44).
Colorectal cancer. In clinical trials, EPT was associated
with a nonsignificant lower incidence of colorectal cancer
in women ages 50 to 59 (44). Cancers that did occur in
women receiving EPT, however, were diagnosed at a more
advanced stage when all age groups were considered (64).
The reduction in cancer during active therapy did not per-
sist after discontinuation (44).
Endometrial cancer. During 13 years of cumulative fol-
low-up of the WHI, combined CEE and MPA was asso-
ciated with a 35% reduction in endometrial cancer in
women ages 50 to 59 years (hazard ratio [HR], 0.65; 95%
confidence interval [CI], 0.37–1.12) (44). This finding
may be unique to the specific type, dose, and regimen
utilized.
Risks of MHT
Endometrial cancer
Unopposed ET increases the risk of endometrial hyper-
plasia and cancer (38, 65, 66), whereas concurrent prog-
estogen therapy (Table 5) for at least 12 days per month
reduces this risk (18, 44, 67) and is recommended for all
women with a uterus. Continuous combined CEE and
MPA was associated with a reduced risk of endometrial
cancer over 13 years of cumulative follow-up (44). After
6 to 10 years, sequential regimens may be associated with
a 2-fold increased risk of endometrial cancer, particularly
in thin women (38). Micronized progesterone and dydro-
gesterone, in combination with estrogen, have been asso-
ciated with an approximate 2-fold increase in endometrial
cancer when continued beyond 5 years in a large obser-
vational study (68). In contrast, one RCT comparing mi-
cronized progesterone with MPA (3 y) (69), a second RCT
comparing micronized progesterone with chlormadinone
acetate (18 mo) (70), and a third trial of single-tablet for-
mulation of cyclical estradiol-dydrogesterone (2 y) (71)
each demonstrated endometrial safety. The difference in
outcome may reflect enhanced patient compliance with
progestogen therapies when formulated in combination.
Limited information is available about the safety of long
cycle intermittent use of progestogens, but concern has
been raised about increased risk of endometrial cancer
(72, 73).
The levonorgestrel intrauterine device (not approved
for a postmenopausal indication in the United States, but
widely used in other countries and, increasingly, off-label
in United States) appears effective at minimizing hyper-
plasia and endometrial cancer risk, especially in obese
women (74–76).
Breast cancer
Estrogen therapy. Most, but not all, observational studies
report an increased breast cancer risk with oral or trans-
dermal estradiol when initiated in recently menopausal
women (77–79). This increase occurs as a function of du-
ration of ET (38, 80–82) with a linear trend in the largest
study (83). Insufficient numbers of patients may confound
doi: 10.1210/jc.2015-2236 press.endocrine.org/journal/jcem 3985
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018

Table 5. Commonly Prescribed Hormone Therapies
Preparation Doses Comments
Systemic estrogen therapies
a
Oral estrogen tablets
Micronized E2 0.5, 1.0, 2.0 mg/d
Estradiol valerate
b
1.5 mg/d
CEE 0.3, 0.45, 0.625 mg/d Higher doses available
Preparation used in WHI
Transdermal estrogens
Estradiol patch 0.025 to 0.1 mg once or twice weekly
depending on preparation
Corresponds to 0.5 to 2.0 mg estradiol tablets
Diffusion can be different from one patch to another
0.014 mg/wk Preserved bone in women ⬎60 y old
Estradiol percutaneous gel 0.25–1.5 mg qd Corresponds to 0.5 to 2.0 mg estradiol tablets
Can be transferred to persons and pets by skin
contact
Estradiol transdermal spray 1.5 mg qd Estradiol via spray
Can be transferred to persons and pets by skin
contact
Vaginal ring
Estradiol acetate 0.05–0.10 mg/d Systemic levels of estradiol provide relief of VMS;
90-d duration/ring
Progestogen therapies
Oral progestin tablets
Medroxyprogesterone acetate 2.5, 5, 10 mg/d Utilized in WHI
Norethindrone 0.35 mg/d
Neta 5.0 mg/d
Megestrol acetate 20, 40 mg/d
Dydrogesterone
b
10 mg/d
Chlormadinone acetate
b
5, 10 mg/d
Medrogestone
b
5 mg/d
Nomegestrol acetate
b
3.75, 5 mg/d
Promegestone
b
0.125, 0.25, 0.5 mg/d
Oral progesterone capsule
Micronized progesterone 100, 200 mg/d In peanut oil; avoid if peanut allergy. May cause
drowsiness and should be taken at bedtime
Intrauterine system progestin
c
LNorg 20
g released/d IUD for 5-y use
6
g/d IUD for 3-y use
Vaginal gel progesterone
c
4%, 8% 45- or 90-mg applicator
Combination hormone therapies
Oral
CEE ⫹MPA 0.3–0.625 mg/1.5–5 mg/d Cyclic or continuous
E2 ⫹Neta 0.5–1 mg/0.1–0.5 mg/d Continuous
E2 ⫹drospirenone 0.5–1 mg/0.25–1 mg/d Continuous
E2 ⫹norgestimate 1 mg/0.09 mg/d Cycle3dEalone,3dE⫹progesterone
E2 ⫹dydrogesterone
b
1–2 mg/5–10 mg/d Cyclic and continuous
E2 ⫹cyproterone acetate
b
2 mg/1 mg/d Continuous
E2 ⫹MPA
b
1–2 mg/2–10 mg/d Continuous
CEE ⫹BZA
d
0.45 mg/20 mg/d Continuous
Transdermal
E2 ⫹Neta 50
g/0.14–0.25 mg/patch Twice weekly
E2 ⫹LNorg 45
g/0.015 mg/patch Once weekly
Abbreviations: IUD, intrauterine device; E, estrogen; E2, 17-

estradiol; LNorg, levonorgestrel; Neta, norethindrone acetate or norethisterone
acetate; qd, once daily.
a
Not all preparations and doses are available in all countries.
b
Only available outside the United States.
c
Not approved in the United States for endometrial protection when administered with postmenopausal estrogen.
d
Approved indications in the United States include treatment of moderate to severe VMS associated with menopause and prevention of
postmenopausal osteoporosis. In the European Union, the indications state: treatment of estrogen deficiency symptoms in postmenopausal
women with a uterus (with at least 12 mo since the last menses) for whom treatment with progestin-containing therapy is not appropriate. The
experience treating women older than 65 years is limited.
3986 Stuenkel et al Guideline on Menopause J Clin Endocrinol Metab, November 2015, 100(11):3975–4011
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018

the interpretation of these data on ET alone (ie, type II
statistical error). It is possible that in observational studies
mammographic surveillance differed between users and
nonusers of MHT. The finding of increased risk in recently
menopausal women is controversial, however. In women
in the WHI ages 50 to 59 or ⬍10 years after menopause
onset, CEE did not increase risk (44, 84). The statistically
significant 21% reduction of invasive breast cancer in the
13-year cumulative follow-up of all women in the estro-
gen-alone arm of the WHI was of similar magnitude in
each age group (44), but some analyses have suggested less
reduction or an increase in risk among women starting ET
close to menopause (77, 85).
The presence or absence of obesity confounds the in-
terpretation of existing data. The aromatase enzyme,
which increases with obesity, results in enhanced endog-
enous estrogen production, which may minimize the ad-
ditional effects of exogenous ET. The insulin resistance
associated with obesity also confounds the relationship
between obesity and breast cancer risk (86). Therefore,
increased breast cancer risk with ET in non-US studies
might reflect differing levels of obesity between US and
European populations. CEE and estradiol may also have
differential effects as suggested by in vitro (87) and pri-
mate (88) studies. In summary, the risk of breast cancer
from estrogen alone, taken for 5 years, appears to be small.
Combined EPT. Studies examining the effects of com-
bined therapy report a consistent increase in breast cancer
risk (38, 89, 90). It should be noted that the original WHI
study did not report any increase overall in women who
had not previously used MHT (hormone naive), but data
on this issue are not available for women ages 50 to 59
or ⬍10 years postmenopausal (18, 91), and there are no
reported follow-up data for the hormone-naive women. In
women ages 50 to 59 in the WHI, the excess risk of inva-
sive breast cancer during the intervention phase persisted
7 years after the cessation of EPT, with 4.5 excess cases/
1000 over 5 years (HR, 1.34; 95% CI, 1.03–1.75) (44).
Studies have reported similar findings with most other
estrogen/progestogen combinations (38, 89, 92). How-
ever, observational data suggest that progesterone or dy-
drogesterone (5, 89) may be associated with a lower risk,
but further studies are required to confirm this. Observa-
tional studies also report a greater risk when EPT is started
close to menopause (79, 85, 93) and with continuous
rather than with cyclic regimens (78, 82, 94).
Lung cancer
In the 50- to 59-year age group in the WHI study, the
incidence of lung cancer was not significantly increased or
decreased in either treatment arm (44).
Ovarian cancer
In the 50- to 59-year age group of the WHI, the HR of
ovarian cancer with EPT was 0.30 (two vs six cases; 95%
CI, 0.06–1.47), with 1.5 fewer cases/1000 per 5 years of
treatment (44). No data have been reported for ET. A
controversial meta-analysis of 52 observational studies
(95–97) showed an increase of 0.52 cases/1000 in women
starting MHT (no difference in risk between ET and EPT)
at age 50 and continuing therapy for 5 years. Risk per-
sisted 5 years after stopping MHT, with 0.37 cases/1000
in the same women when ages 55 to 59 (95). Of note, the
overall risk of ovarian cancer with EPT in the WHI (HR,
1.41), although not statistically significant, was compa-
rable to findings in the meta-analysis, as was the rate in the
cumulative follow-up (HR, 1.24). Based on current data,
adequately powered RCTs are needed to fully ascertain
ovarian cancer risk in symptomatic, recently postmeno-
pausal women.
Coronary heart disease
Estrogen therapy. The age at initiation of ET influences
risk. In the WHI, there was a trend toward a reduction in
CHD and total MI in women aged 50 to 59 years at trial
enrollment (44). Composite outcomes, including revascu-
larization (98) and coronary artery calcium scores (99),
were lower with ET than with placebo.
Observational studies of ET suggest the potential for
CHD benefit in some women, although a number of biases
might have contributed to those conclusions (100). In
summary, ET does not increase CHD risk in women start-
ing therapy at ages ⬍60 years and may possibly reduce
this risk.
Although observational studies suggest that a dermal
route of ET may carry a lower risk of MI (101, 102), a
meta-analysis reported no significant difference in CHD
outcomes between oral and transdermal MHT (4). No
associations with estrogen dose were reported (101, 102).
Combined EPT. Age at initiation of EPT does not appear
to influence the relative risk of CHD, based on the most
recent WHI data (44) and a meta-analysis (4). In women
in the WHI aged 50 to 59, there was a trend toward excess
risk of CHD, but no increased risk was apparent in
women ⬍10 years since menopause onset (44). These
findings and those of several recent studies have been con-
troversial. A randomized osteoporosis trial that did not
have CHD as a predefined primary endpoint reported that
10 years of MHT treatment in women ⬍50 years old at
study onset was associated with the reduction of a com-
posite safety endpoint (death, hospital admission for MI,
or heart failure) (103). This study has been criticized for its
composite index and nonblinded nature. A primary pre-
doi: 10.1210/jc.2015-2236 press.endocrine.org/journal/jcem 3987
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018

vention RCT of recently (⬍3 y) postmenopausal women
ages 42 to 58 failed to detect a difference in progression of
atherosclerosis (as assessed by carotid intima-medial
thickness and coronary artery calcium determinations) af-
ter 4 years of therapy (104) but may have been underpow-
ered to detect significant differences (ie, type II error). In
summary, EPT does not appear to be associated with an
increased risk of CHD among women close to the onset of
menopause, and if any risk elevation is present in women
younger than 60 years, its magnitude is small. A definitive
conclusion regarding CHD risk requires an appropriately
powered RCT.
Stroke
Researchers reported a nonsignificant trend toward an
increase in stroke risk with EPT in women ages 50 to 59
in the WHI (44) but did not report an adverse effect with
ET. When examined by years since menopause, ET in-
creased stroke risk in women ⬍10 years since menopause
(6.5 women/1000 over 5 y) (44). The differences between
these two groups might reflect the difficulty in establishing
time of menopause in women with a hysterectomy.
No RCTs have evaluated stroke risk according to es-
trogen type, dose, or route of administration. Some ob-
servational studies suggest that transdermal estradiol in
doses ⱕ50
g may confer a lower risk compared with
higher dose transdermal or oral therapies (4, 105). Other
studies are conflicting regarding effects of estrogen type
(102, 106) and dose (101, 105, 107). In summary, MHT
may confer a small risk of stroke.
Venous thromboembolic events
Estrogen therapy. RCTs demonstrate that oral ET in-
creases VTE risk in women ages 50 to 59 (44). These data
are supported by observational studies (106, 108, 109).
Risk declined after discontinuing therapy (44). Observa-
tional studies (108–112) and meta-analyses (4, 113) sug-
gest that transdermal ET does not increase VTE risk, even
in women with thrombophilia or obesity (114–117). In an
observational study, oral CEE was associated with a
2-fold increase in VTE compared with oral estradiol (106).
Combined EPT. The WHI trial found an association be-
tween EPT and both DVT and pulmonary embolism (PE)
in women ages 50 to 59 (44). Risks resolved when therapy
was discontinued. Observational studies suggest that for-
mulations containing MPA and normethytestosterone de-
rivatives appear to be associated with greater risk than
other progestogens (108, 109, 111). A recent meta-anal-
ysis comparing ET and EPT did not report any statistically
significant differences in risk (4).
Gallbladder disease
No data are available specifically for women ages 50 to
59; conclusions regarding gallbladder disease rely on over-
all findings of the WHI. ET resulted in 29 excess cases/
1000 women over 5 years (44). This risk did not persist
after discontinuation (44, 118). With EPT, the excess risk
was 23 women/1000 (44), similar to another trial (119).
Risk persisted at least 5 years after cessation of EPT (44,
120). Observational studies report increased risk with
oral, but not transdermal, estradiol (121, 122) and in-
creased dose and duration (120, 123).
Incontinence
Stress urinary incontinence, urge urinary incontinence,
and mixed urinary incontinence increase in women taking
oral ET and EPT (124, 125). An increased risk may persist
after discontinuation (44).
Uncertain benefits of hormone therapy
Mortality
A meta-analysis of RCTs demonstrated no significant
effect on all-cause mortality with MHT use, but these data
included women ⬍and ⬎60 years of age (5). A recent
Cochrane collaboration review reported a 30% relative
risk reduction (HR, 0.70; 95% CI, 0.52–0.95) of all-cause
mortality in women starting MHT ⬍10 years since meno-
pause (or ⬍age 60) (127). Comparison of the ET and EPT
groups in the WHI suggested a stronger trend by age group
among those on ET, with a statistically significant trend by
age in the ET trial but not in the EPT trial (44). Observa-
tional studies (128–130) reported a reduction in mortality
with MHT, as did one small RCT with composite end-
points (103). This is consistent with meta-analyses that
reported a 30–40% mortality reduction (131, 132). In
summary, further data are required for definitive conclu-
sions about mortality in younger women.
Dementia
Observational studies suggest a possible benefit of
MHT if started in younger women closer to menopause
(133), as opposed to the detrimental effects reported in
clinical trials when MHT is initiated in women ⬎65 years
old (134). Some studies of postmenopausal women treated
with estradiol reported an improvement in verbal memory
and executive function (135–138), whereas other studies
did not associate CEE therapy with cognitive improve-
ment (139, 140). Definitive conclusions about MHT in
women ⬍age 60, therefore, are lacking.
Individual baseline risk assessment and therapeutic
decisions
Evaluating risk facilitates individual counseling and de-
cisions regarding MHT for symptom relief (Figure 2).
3988 Stuenkel et al Guideline on Menopause J Clin Endocrinol Metab, November 2015, 100(11):3975–4011
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018

However, no clinical trial evidence is available to support
the practice of incorporating risk assessment instru-
ments for quantifying cardiovascular (CHD, stroke,
and VTE) and breast cancer risks among women con-
sidering MHT. Nevertheless, we feel that risk assess-
ment instruments are useful to facilitate decision-mak-
ing regarding MHT.
Cardiovascular risk
3.1b For women ⬍age 60 or ⬍10 years past meno-
pause onset considering MHT for menopausal symptom
relief, we suggest evaluating the baseline risk of CVD and
taking this risk into consideration when advising for or
against MHT and when selecting type, dose, and route of
administration. (2ⱍQQEE)
3.1c For women at high risk of CVD, we suggest initi-
ating nonhormonal therapies to alleviate bothersome
VMS (with or without climacteric symptoms) over MHT.
(2ⱍQQEE)
Technical remarks
High risk includes known MI, cerebrovascular disease,
and peripheral arterial disease, abdominal aortic aneu-
rysm, diabetes mellitus, chronic kidney disease, and 10-
year CVD risk ⬎10% (40).
3.1d For women with moderate risk of CVD, we sug-
gest transdermal estradiol as first-line treatment, alone for
women without a uterus or combined with micronized
progesterone (or another progestogen that does not ad-
versely modify metabolic parameters) for women with a
uterus because these preparations have less untoward ef-
fect on blood pressure, triglycerides, and carbohydrate
metabolism. (2ⱍQQEE)
Evidence
Cardiovascular risk
Results showing fewer excess CHD and stroke events
when MHT was initiated in younger rather than older
study participants in the WHI (141) provide the founda-
tion for the widely accepted consensus that MHT should
be initiated primarily in younger women (age ⬍60 y) close
in time (⬍10 y) to menopause onset, when women likely
have less baseline atherosclerosis (19, 20). The population
prevalence of obesity, hypertension, dyslipidemia, and di-
abetes continues to increase. Accordingly, baseline CVD
risk evaluation is important in women considering MHT.
As reviewed in recent statements, CHD and stroke are
associated with a wide range of risk factors, many unique
to women (40, 41). Notably, a prior history of CHD con-
veys the highest risk of subsequent MI and stroke (142).
We feel that methods to integrate these factors to catego-
rize individual risk as minimal, moderate, and high are
useful and can be accomplished qualitatively by clinical
judgment or quantitatively by risk assessment tools.
Country- and population-specific CVD risk calculators
are available to quantify individual risk per local guide-
lines (143). However, specific cutoffs for the safe use of
MHT have not been formally validated, and practice dif-
fers from country to country.
The Menopause Decision-Support Algorithm (63)
starts with calculating the American College of Cardiol-
ogy (ACC)/American Heart Association (AHA) 10-year
CVD risk (144), then stratifies by years since menopause
to suggest appropriateness of MHT (Table 6) (63). For a
woman at intermediate risk, family history, coronary ar-
tery calcium score, C-reactive protein, and ankle-brachial
index can further stratify risk (144); inflammatory mark-
ers and lipid ratios predict treatment-related CHD events
(145).
Metabolic syndrome. The metabolic syndrome (MetS) is
associated with higher risk of cardiovascular events and
breast and colon cancers (146). In a nested case-control
study in the WHI, women with MetS at baseline were
twice as likely to have CHD events while taking oral MHT
as with placebo (147). In contrast, women without MetS
had no increase in CHD risk on MHT. Transdermal es-
tradiol with micronized progesterone might have less del-
eterious metabolic effects than oral therapies, but there are
no RCTs that have evaluated the safety of these prepara-
tions in women with MetS.
Diabetes. Diabetes is considered by the AHA to be a CHD
risk equivalent (40), which would suggest that women
with diabetes should not take MHT. However, clinical
trial evidence of CVD outcomes associated with MHT in
women with diabetes is mostly lacking. Some diabetic
women were included in RCTs (Heart and Estrogen/Pro-
gestin Replacement Study [19%]; WHI [4.4–7.7%]), but
these trials were not powered to assess differences in CVD
Table 6. Evaluating CVD Risk in Women
Contemplating MHT
10-y CVD Risk
Years Since Menopause Onset
<5y 6to10y
Low (⬍5%) MHT ok MHT ok
Moderate (5–10%) MHT ok (choose
transdermal)
MHT ok (choose
transdermal)
High (⬎10%)
a
Avoid MHT Avoid MHT
CVD risk calculated by ACC/AHA Cardiovascular Risk Calculator (144).
Methods to calculate risk and risk stratification vary among countries.
Derived from J. E. Manson: Current recommendations: what is the
clinician to do? Fertil Steril. 2014;101:916–921 (63), with permission.
© Elsevier Inc.
a
High risk includes known MI, stroke, peripheral artery disease, etc.
doi: 10.1210/jc.2015-2236 press.endocrine.org/journal/jcem 3989
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018

outcomes. A few short-term RCTs have evaluated glucose
control in diabetic women taking a variety of MHT prep-
arations and showed either no effect or improved control
(148). The evidence at this time is inadequate to make firm
recommendations. An individualized approach to treating
menopausal symptoms could be considered, with a low
threshold to recommend nonhormonal therapies, partic-
ularly in women with concurrent CVD. However, some
diabetic women, after careful evaluation of cardiovascular
risk, may be candidates for MHT, preferably transdermal
estrogen and micronized progesterone or another less met-
abolically active progestogen.
Venous thromboembolic events
3.1e For women at increased risk of VTE who request
MHT, we recommend a nonoral route of ET at the lowest
effective dose, if not contraindicated (1ⱍQQEE); for
women with a uterus, we recommend a progestogen (for
example, progesterone and dydrogestone) that is neutral
on coagulation parameters. (1ⱍQQQE)
Evidence
Obesity, age, and thrombophilia are associated with
increased risk of VTE. An approximately 2-fold increased
risk of VTE (both DVT and PE) with oral MHT is similar
among women at low, intermediate, or high risk (149,
150). Accordingly, the attributable risk of MHT will be
higher in those at high or intermediate baseline risk.
A prior history of VTE confers the highest risk. If the
patient has a known inherited coagulation defect, such as
Factor V Leiden, oral ET or EPT should be avoided be-
cause research has shown a high risk of VTE recurrence
(114). A history of VTE due to pregnancy, oral contra-
ceptives, unknown etiology, or blood clotting disorders
poses a contraindication to any ET, whereas VTE due to
past immobility, surgery, or bone fracture would be a con-
traindication to oral but not necessarily transdermal MHT
(151). In some countries, a history of any VTE is a con-
traindication to oral but not low-dose transdermal ET.
Breast cancer
3.1f For women considering MHT for menopausal
symptom relief, we suggest evaluating the baseline risk of
breast cancer and taking this risk into consideration when
advising for or against MHT and when selecting type,
dose, and route of administration. (2ⱍQQEE)
3.1g For women at high or intermediate risk of breast
cancer considering MHT for menopausal symptom relief,
we suggest nonhormonal therapies over MHT to alleviate
bothersome VMS. (2ⱍQQEE)
Technical remarks
High or intermediate risk includes calculated level of
risk that would qualify for risk-reducing medications.
Evidence
There are no established clear criteria for recommend-
ing (or avoiding) MHT based on a woman’s risk of breast
cancer. Nonsignificant trends from the WHI suggest that
the relative risk of breast cancer in association with MHT
remains stable or increases in the 5-year Gail model breast
risk categories of ⬍1.25 vs ⱖ1.75. On this basis, the
excess or attributable risk should increase in women at
higher categories of risk (90, 152). As another consider-
ation, it seems prudent not to recommend MHT for
women whose risk meets the criteria for breast cancer
prevention with SERMs or aromatase inhibitors. The U.S.
Preventive Services 2013 Task Force recommends that
women with a 5-year risk of ⱖ3% should be considered
for preventive therapy with tamoxifen or raloxifene (126),
whereas the American Society of Clinical Oncology guide-
lines suggest discussing such therapy in women with a risk
of ⱖ1.67% (153), consistent with enrollment criteria of
breast cancer prevention trials. Prevention recommenda-
tions differ outside the United States. Another consider-
ation is to take into account the data suggesting that breast
cancer risk is associated with combined estrogen/proges-
togen use, but less so, if at all, with CEE alone.
We suggest one potential algorithm for MHT counsel-
ing, extrapolated from breast cancer prevention trial en-
rollment criteria (Table 7); however, it is not validated in
clinical trials or widely utilized. This algorithm requires
the assessment of breast cancer risk, which can be accom-
plished by qualitative methods or preferably with readily
available quantitative risk assessment tools. The National
Cancer Institute Breast Cancer Risk Assessment Tool pro-
Table 7. Breast Cancer Risk Cutoffs for Counseling
Before Recommending MHT
a
Risk
Category
a
5-y NCI or IBIS Breast Cancer
Risk Assessment, %
Suggested
Approach
Low ⬍1.67 MHT ok
Intermediate 1.67–5 Caution
b
High ⬎5 Avoid
Abbreviations: IBIS, International Breast Intervention Study; NCI,
National Cancer Institute.
a
Categories here are newly defined for these guidelines and based on
recommendations published for use of antiestrogens for breast cancer
prevention (126, 153, 322, 323). The assumption is that candidates for
breast cancer prevention with antiestrogens should not be candidates
for initiating MHT. Method to calculate risk varies among countries.
b
Caution indicates need for detailed counseling regarding anticipated
benefits and risks of MHT with strong consideration of nonhormonal
therapies for symptom relief, and possible consideration of
chemopreventive strategies for women who meet suggested criteria.
3990 Stuenkel et al Guideline on Menopause J Clin Endocrinol Metab, November 2015, 100(11):3975–4011
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018

vides a standardized online risk calculator for 5-year risk
of invasive breast cancer (154). The International Breast
Intervention Study calculator predicts 10-year and life-
time risk (155, 156). For women with strong family his-
tories of breast cancer, several other methods are available
(155). Although these provide useful predictive informa-
tion, all are limited by only moderate discriminatory ac-
curacy (155). Mammographic breast density, when added
to these methods, may emerge as an important objective
risk for women contemplating MHT (157–159).
Although a history of breast cancer is considered by
most to be a contraindication to MHT, the severity of
menopausal symptoms, the compromise in QOL experi-
enced by breast cancer survivors, and limitations of non-
hormonal therapies for relief of VMS present a persistent
clinical challenge. As recently summarized, it is not pos-
sible from currently available studies to draw firm con-
clusions regarding the risks of MHT in this population
(38), but adding estrogen seems counterintuitive when
current breast cancer therapies interrupt or decrease es-
trogen levels. Future studies taking into account estrogen
receptor status, time since diagnosis and therapy, mastec-
tomy status, and risks for breast cancer recurrence might
better inform decision-making.
Tailoring menopausal hormone therapy
3.1h We suggest a shared decision-making approach to
decide about the choice of formulation, starting dose, the
route of administration of MHT, and how to tailor MHT
to each woman’s individual situation, risks, and treatment
goals. (Ungraded best practice statement)
Clinicians prescribe estrogen alone for women without
a uterus. Starting dosages are generally lower than those in
the WHI (Table 5), and the overarching principle is to use
the lowest effective dose with upward titration based on
clinical response. Clinicians usually do not measure estra-
diol levels to monitor therapy except when symptoms do
not improve with escalating doses, particularly after
changing the mode of administration from oral to trans-
dermal. For younger women with surgical menopause or
those with POI who are accustomed to higher baseline
endogenous estradiol levels, clinicians often prescribe
higher starting doses of ET (eg, transdermal estradiol, 100
g), and then slowly lower the dose as tolerated. When
women with premature menopause approach the age of
natural menopause, the reassessment and tapering of
MHT dose seems reasonable.
Estrogen preparations
Oral estrogens. Estradiol tablets or conjugated estrogens
(synthetic or equine) are convenient, are studied most ex-
tensively, and alleviate climacteric symptoms in a dose-
dependent fashion. CEE, derived from pregnant mares’
urine and used for decades, contain more than 200 com-
pounds with varying estrogenic potency (160). Oral mi-
cronized estradiol and other oral estrogen preparations
may result in up to 5-fold higher levels of circulating es-
trone and 10- to 20-fold higher estrone sulfate than trans-
dermally administered estradiol at comparable or even
higher doses. The biological effects of these estrone and
estrone-sulfate increments are unknown (161–163).
Cutaneous and transdermal estradiol. Cutaneous and
transdermal estradiol, administered via percutaneous gels,
sprays, emulsions, or transdermal patches, have a similar
efficacy as oral ET in reducing climacteric symptoms and
are easily tailored to the individual (164, 165). The pri-
mary advantage of transdermal ET is to alleviate the first-
pass hepatic metabolic effect (166) of oral estrogens re-
sulting in a procoagulant effect and increases in SHBG,
thyroid-binding globulin, cortisol-binding globulin (167,
168), triglycerides, and markers of inflammation such as
C-reactive protein (167, 169).
Transdermal therapies, at low doses, are preferable for
women with a VTE risk, as evidenced by a recent meta-
analysis commissioned for these guidelines (4), and they
may also be preferable in patients with hypertension, hy-
pertriglyceridemia, obesity, MetS, diabetes, or a history of
gallbladder disease. Clinicians should keep in mind that
there are no existing head-to-head RCTs with clinical out-
comes that compare transdermal with oral therapies.
Vaginal delivery of systemic estrogens. Estradiol acetate
vaginal rings, delivering 50 or 100
g of estradiol daily
(Table 5), provide consistent systemic estradiol levels for
3 months per ring insertion. They are indicated for treat-
ment of moderate to severe VMS and VVA due to meno-
pause (170, 171). High-dose vaginal creams containing
estradiol or CEE (ie, 1–2 g) also result in systemic estrogen
levels. Concomitant progestogen is needed with these
preparations to abrogate endometrial stimulation. We dis-
cuss low-dose vaginal ETs for the specific treatment of
GSM in Section 5.0.
Progestogen administration
In women with a uterus, a progestogen must be added
to prevent endometrial hyperplasia and cancer. The var-
ious formulations (Table 5) are administered in two reg-
imens. The combined sequential regimen includes estro-
gen for 20 to 25 days and a progestogen for 12 to 15 days
each month. This approach is preferred for recently meno-
pausal woman who are prone to breakthrough bleeding
during the first year or two of therapy. The combined
continuous regimen utilizes both an estrogen and pro-
doi: 10.1210/jc.2015-2236 press.endocrine.org/journal/jcem 3991
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018

gestogen daily on a continuous basis. Clinicians can
administer progestogen orally, transdermally by patch,
vaginally, or by intrauterine administration (172). The
levonorgestrel intrauterine device minimizes systemic
progestogen absorption, but increased blood levels do oc-
cur, and one observational study reported higher breast
cancer incidence (173).
Progestogen alone. For those who do not tolerate ET, pro-
gestogens can relieve VMS. In RCTs, oral synthetic pro-
gestogens (Table 5) (174, 175) and micronized progester-
one (176) were effective. Clinical outcome trials are
lacking in women with breast cancer; thus, progestogen
therapy is best avoided, except under limited circum-
stances in these patients, because the effect on recurrence
is unclear (80).
Custom-compounded hormones
3.1i We recommend using MHT preparations ap-
proved by the FDA and comparable regulating bodies out-
side the United States and recommend against the use of
custom-compounded hormones. (Ungraded best practice
statement)
Evidence
A number of FDA-approved hormonal therapies are
“biochemically identical” to endogenous estradiol and
progesterone and are preferred to custom-compounded
options. Custom-compounded hormone therapies have
become increasingly popular but are not recommended
because the manufacturing process lacks FDA oversight
(177). Clinical trials documenting the efficacy and safety
of compounded progesterone for endometrial protection
are lacking. Proponents of custom-compounded hormone
therapies often advise measuring salivary hormone levels
to monitor therapy. However, scientific evidence is lack-
ing to justify salivary measurements due to inter- and in-
tra-assay variability, variable salivary flow rates depen-
dent upon hydration, food intake, and other factors, and
the inability to predict the pharmacokinetics of a custom-
compounded hormone dose in a manner that would allow
for valid salivary sampling.
3.2 Conjugated equine estrogens with bazedoxifene
3.2 For symptomatic postmenopausal women with a
uterus and without contraindications, we suggest the com-
bination of CEE/BZA (where available) as an option for
relief of VMS and prevention of bone loss. (2ⱍQQQE)
Evidence
The combination of CEE with the SERM/BZA (avail-
able in the United States and licensed in the European
Union) relieves VMS and vaginal atrophy and reduces
bone resorption in women with a uterus; it provides an
alternative to progestogen therapy for women averse to
vaginal bleeding, breast tenderness, or altered mood. A
series of RCTs up to 2 years in duration evaluated effects
of CEE/BZA (0.45 mg/20 mg, the approved dose) com-
pared with MHT (CEE 0.45 mg/MPA 1.5 mg) (178–180).
Benefits
Vasomotor symptoms. The number and severity of mod-
erate-to-severe VMS were significantly decreased at 12
weeks; hot flash frequency was reduced by 74% compared
with 51% for placebo, and hot flash severity was reduced
up to 54%. Hot flash reduction was sustained at 12
months (P⬍.05) (181).
Bone loss. Bone loss at the lumbar spine and hip was pre-
vented in postmenopausal women at risk for osteoporosis
(182), as reflected by reduction of serum bone turnover
markers and enhancement of bone mineral density vs pla-
cebo (180, 181). At 12 months, CEE/BZA was less effec-
tive at the lumbar spine than CEE/MPA (180). Fracture
data are lacking.
Vaginal effects. Treating postmenopausal women ages 40
to 65 with VVA at baseline (183) improved vaginal mat-
uration at 12 weeks (181). Women reported a lower in-
cidence of dyspareunia.
Quality of life. Secondary endpoints included improve-
ments in sleep, health-related QOL, and improved treat-
ment satisfaction (184, 185). In RCTs, both CEE/BZA and
CEE/MPA improved sleep disturbance and time to fall
asleep (185).
Safety considerations
Breast. The incidence of breast pain and tenderness was
similar for CEE/BZA and placebo (185–187) and was less
than with CEE/MPA. After 1 year of therapy with CEE/
BZA, mammographic breast density was not appreciably
different than with placebo, whereas it increased with
CEE/MPA (184). In trials up to 2 years, the rates of breast
cancer (reported as adverse events, not clinical outcomes)
were not sufficient to assess risk or benefit (186, 187).
Endometrium. Cumulative amenorrhea rates for CEE/
BZA were comparable with placebo and greater than for
CEE/MPA (188). At 2 years, the incidence of neither en-
dometrial hyperplasia nor endometrial cancer was in-
creased (180, 189).
Potential risks
Adverse events. Although an osteoporosis trial found a
2-fold risk of VTE with BZA 20-mg therapy alone (190),
3992 Stuenkel et al Guideline on Menopause J Clin Endocrinol Metab, November 2015, 100(11):3975–4011
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018

there was no additive effect on VTE when BZA was com-
bined with CEE, although adequately powered studies are
necessary (181). In trials of up to 2 years in women ages 40
to 65, rates of cardiovascular events, cancers (breast, en-
dometrial, ovarian), and mortality were similar to placebo
(191), but studies were underpowered to draw firm con-
clusions regarding these endpoints.
3.3 Tibolone
3.3a For women with bothersome VMS and climacteric
symptoms and without contraindications, we suggest ti-
bolone (in countries where available) as an alternative to
MHT. (2ⱍQQEE)
3.3b We recommend against adding tibolone to other
forms of MHT. (1ⱍQQEE)
3.3c We recommend against using tibolone in women
with a history of breast cancer. (1ⱍQQEE)
Evidence
Tibolone belongs to the group of normethyltestoster-
one progestogen derivatives and has metabolites that ex-
hibit estrogenic, progestogenic, and androgenic effects
(192). This agent (193) is available in many countries out-
side of the United States at doses of 1.25–2.5 mg/d.
Benefits
Menopausal symptoms. Tibolone alleviates VMS with
equivalent or lesser potency than conventional MHT. Ti-
bolone also improves sleep, mood, and urogenital atrophy
and may improve libido (194–197).
Bone loss and fracture. Tibolone prevents postmeno-
pausal bone loss and osteoporotic fractures in women
with osteoporosis (198, 199), but is not approved for this
purpose because of the increased risk of stroke in older
women with osteoporosis initiating therapy at ages ⱖ60
years (199).
Possible risks
Endometrium. There is no endometrial thickening (197)
or increase in myoma with tibolone (200). A Cochrane
analysis concluded that there was no clear evidence of
endometrial cancer with tibolone therapy (seven RCTs,
n⫽8152; odds ratio, 1.98; 95% CI, 0.73–5.32) (194).
Thrombosis and CVD. In an observational study (110),
tibolone did not increase the risk of thrombosis. In an RCT
of older women with osteoporosis, tibolone increased
stroke (199).
Breast and colon cancers. The incidence of breast tender-
ness is low (around 3%), (201, 202), and neither mam-
mographic density nor invasive breast cancer was in-
creased; however, the risk of colon cancer was decreased
(199, 201). An RCT of women with a history of breast
cancer, after a median follow-up of 3.1 years, reported a
higher rate of breast cancer recurrence with tibolone (HR,
1.40; 95% CI, 1.14–1.70) (203). The study reported the
greatest increase for women taking an aromatase inhibitor
(HR, 2.42; 95% CI, 1.01–5.79).
3.4 Clinical management of patients taking hormone
therapies
Monitoring during therapy
3.4a For women with persistent unscheduled bleeding
while taking MHT, we recommend evaluation to rule out
pelvic pathology, most importantly, endometrial hyper-
plasia and cancer. (1ⱍQQQE)
3.4b We recommend informing women about the pos-
sible increased risk of breast cancer during and after dis-
continuing EPT and emphasizing the importance of ad-
hering to age-appropriate breast cancer screening.
(1ⱍQQQE)
Technical remarks
Regular clinical follow-up, initially, within 1 to 3
months after starting MHT, and then every 6 to 12
months, depending upon the individual (and health care
system), allows for monitoring efficacy and side effects
(abdominal/pelvic pain, mastalgia, metrorrhagia, weight
gain, mood changes, blood pressure), and if necessary,
making treatment adjustments (Table 8).
Duration of therapy
3.4c We suggest that the decision to continue MHT be
revisited at least annually, targeting the shortest total du-
ration of MHT consistent with the treatment goals and
evolving risk assessment of the individual woman. (Un-
graded best practice statement)
Technical remarks
Most published recommendations suggest using MHT
for the shortest duration possible, but strong evidence is
lacking to support this recommendation. Current pro-
posed limits on duration of therapy are informed by large
intervention trials (5 to 7 y) with extended follow-up for
13 years (44). Regarding duration of use, these data sug-
gest that risk rates for breast cancer and CVD increase
with age and time since menopause, although the risks
with ET appear to be less than with EPT. Ovarian cancer
risk may also increase relative to duration of MHT (95).
We conclude, and guidelines from other societies concur,
that clinicians and patients should reassess MHT contin-
uation yearly and discuss the risks (and individual bene-
fits) beyond 5 years (55, 56). Patients likely to consider
doi: 10.1210/jc.2015-2236 press.endocrine.org/journal/jcem 3993
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018

continuing therapy include those who fail an attempt to
stop EPT, who are at high risk for fracture, or for whom
alternative therapies are not appropriate.
3.4d For young women with POI, premature, or early
menopause, without contraindications, we suggest taking
MHT until the time of anticipated natural menopause,
when the advisability of continuing MHT can be reas-
sessed. (2ⱍQQEE)
Stopping considerations
3.4e For women preparing to discontinue MHT, we
suggest a shared decision-making approach to elicit indi-
vidual preference about adopting a gradual taper vs
abrupt discontinuation. (2ⱍQQEE)
Evidence
A number of studies have compared methods (ie, taper
protocols vs abrupt cessation) to facilitate the discontinua-
tion of MHT (204–207) and have detected no differences.
Therefore, the approach to discontinuation is an individual
choice. Anecdotally, some women find that a very low dose
of ET maintains adequate symptom relief and well-being and
prefer that to complete discontinuation.
Menopausal symptoms and joint pain can recur when
MHT is discontinued (44). Depending on the severity of
the symptoms, women may elect to restart MHT, perhaps
at a lower dose, or seek relief with nonhormonal therapies.
Accelerated bone loss was reported after the discontinu-
ation of MHT, whereas in contrast, bone density is stable
for some years after discontinuing bisphosphonate ther-
apy. Bisphosphonates, however, remain in bone indefi-
nitely, and most expert groups do not recommend initi-
ating bisphosphonate therapy for osteoporosis prevention
in women aged 50 to 59. Adverse effects such as osteone-
crosis of the jaw and atypical femur fractures, while rare,
increase with the duration of therapy. Furthermore, as
opposed to reports from observational studies (208), in
the long-term follow-up of the WHI, hip fracture rates did
not increase during 5 to 7 years of observation after MHT
was discontinued (44). Breast cancer risk after 5 years of
EPT in the WHI persisted 7 years after discontinuation. A
large meta-analysis of observational studies found a per-
sistent risk of ovarian cancer up to a decade after discon-
tinuing MHT (95). Urinary incontinence persisted after
oral MHT was discontinued; however, the percentage of
affected women was approximately one-third less than
during active treatment (44). MHT discontinuation may
result in symptoms of VVA (Section 5.0), and when oral
therapy is discontinued, glucose, cholesterol, triglycerides,
calcium, and TSH (209) levels may change.
4.0 Nonhormonal therapies for VMS
4.0 For postmenopausal women with mild or less both-
ersome hot flashes, we suggest a series of steps that do not
involve medication, such as turning down the thermostat,
dressing in layers, avoiding alcohol and spicy foods, and
reducing obesity and stress. (2ⱍQQEE)
Table 8. Clinical Caveats During Treatment With MHT
Symptom/Condition When MHT
Started Approach to Resolution
Persistent, intolerable VMS Switch mode of administration or adjust dose of estrogen and/or progestogen.
Hot flashes that persist after treatment
adjustment
Consider another etiology of flashes (Table 2).
Ensure absorption: if transdermal, consider serum estradiol determination.
Bleeding: approach depends on time since
menopause, MHT regimen, duration of
therapy, duration and character of
bleeding
Sequential regimen may be more appropriate for recently menopausal (⬍2 y),
because unscheduled bleeding with continuous combined MHT can be
problematic.
Persistent irregular bleeding (⬎6 mo) should be evaluated for endometrial
pathology; if obese, diabetic, or having family history for endometrial cancer,
evaluate sooner.
Atrophic endometrium in women more remote from menopause may respond
to increased estrogen dose if otherwise appropriate.
Breast tenderness Usually responds to a reduction in estrogen dose or change in progestogen
preparation.
CEE/BZA may improve symptoms.
Changing to tibolone may be helpful in women who develop mastalgia on
conventional MHT.
Baseline TG level ⬎200 mg/dL Review family history and seek contributing factors.
Transdermal ET is preferred.
If oral estrogen is selected, monitor serum TG levels 2 wk after starting
therapy.
Hypothyroid on thyroid replacement Monitor TSH 6 to 12 wk after starting oral MHT; T
4
dose may need to be
increased (209).
Abbreviation: TG, triglycerides.
3994 Stuenkel et al Guideline on Menopause J Clin Endocrinol Metab, November 2015, 100(11):3975–4011
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018

Evidence
As hot flashes result from alterations of the thermoreg-
ulatory neutral zone, shedding layers of clothing, using
fans, keeping the bedroom cool (30), avoiding alcohol and
spicy foods, and reducing stress may be effective. Being
overweight or obese is a risk factor for VMS (26, 210,
211), and weight loss may reduce hot flash frequency
(212, 214).
4.1 Nonhormonal prescription therapies for VMS
4.1a For women seeking pharmacological management
for moderate to severe VMS for whom MHT is contrain-
dicated, or who choose not to take MHT, we recommend
SSRIs/SNRIs or gabapentin or pregabalin (if there are no
contraindications). (1ⱍQQQE)
Evidence
The interpretation of hot flash efficacy studies requires
an appreciation of an important confounding factor.
There is a strong, consistently reported placebo effect,
which averages 30% (range, 4–57%; Figure 4) and occurs
more often in women with high anxiety and stress scores
(215–220). Clinical trials of paroxetine, venlafaxine, des-
venlafaxine, citalopram, and escitalopram demonstrate
statistically significant efficacy with a reduction of fre-
quency of hot flashes ranging from 25 to 69% (Figure 4).
The composite score of hot flash frequency and severity is
reduced by 27–61%. Other agents such as sertraline and
fluoxetine are associated with non-statistically significant
trends toward the reduction of hot flashes and inconsistent
results (221–223).
Meta-analyses and a Cochrane review concluded that
SSRIs and SNRIs exert mild-to-moderate effects to reduce
hot flashes in women with a history of breast cancer (217,
224–227). Each of these agents appears to have similar
efficacy in breast cancer survivors as in healthy meno-
pausal women, although studies are small (213, 217, 228–
234). Caution is advised in the use of paroxetine in pa-
tients taking tamoxifen because paroxetine markedly
interferes with the metabolism of tamoxifen to its metab-
olite, endoxifen (221, 222, 224, 235–237).
The only FDA-approved agent in this class is low-dose
paroxetine mesylate, but others have been used off-label in
the United States. No direct trials are available to deter-
mine the relative efficacy of one over another. We describe
suggested daily doses, efficacy, side effects, and contrain-
dications in Figure 4. In general, the evidence suggests that
these agents are effective and well tolerated.
Gabapentin
Four RCTs confirmed moderate efficacy in relieving
hot flashes (238–241). On the basis of clinical experience,
women whose hot flashes occur primarily at night respond
well to a single bedtime dose. Individual dose require-
ments vary widely, as determined by empiric dose escala-
tion, and range from 300 to 1200 mg. Gabapentin effects
as a sedative and a reducer of vasomotor instability work
well together when used at bedtime because sedating side
effects dissipate by morning. However, when used during
the day, gabapentin may result in a level of lethargy that
is not tolerable.
Pregabalin
In one 6-week RCT, pregabalin (75–150 mg twice
daily) decreased mean hot flash scores by 65 and 71%,
compared with 50% by placebo (242), and was reason-
ably well tolerated.
Choice of SSRI/SNRI vs gabapentin/pregabalin
A randomized, crossover, multicenter trial that com-
pared recommended doses of venlafaxine vs gabapentin,
300 mg three times a day (243), reported that both agents
reduced hot flash scores by 66%, but two-thirds of pa-
tients preferred venlafaxine over gabapentin. The quality
of this comparative evidence is low due to imprecision.
Relative efficacy of nonhormonal prescription ther-
apies vs estrogens
A limited number of head-to-head RCTs have com-
pared varying estrogen doses, preparations, and routes of
administration with nonhormonal agents (213, 240, 244).
None of the RCTs established statistically significant su-
periority of one treatment regimen over another. How-
ever, when these and other published data are taken into
account (213, 217, 236, 245), the limited evidence avail-
able suggests that standard-dose MHT is more effective
than nonhormonal agents.
4.1b For those women seeking relief of moderate to
severe VMS who are not responding to or tolerating the
nonhormonal prescription therapies SSRIs/SNRIs or ga-
bapentin or pregabalin, we suggest a trial of clonidine (if
there are no contraindications). (2ⱍQQEE)
Evidence
Clonidine
Several RCTs demonstrated that this
␣
-2-adrenergic
receptor agonist reduced hot flashes, but less effectively
than the SSRI/SNRIs, gabapentin, and pregabalin, and
with more side effects (Figure 4) (217, 236). Clonidine
transdermal patches are preferred over tablets because of
more stable blood levels.
4.2 OTC and alternative nonhormonal therapies for
VMS
4.2 For women seeking relief of VMS with OTC or com-
plementary medicine therapies, we suggest counseling re-
doi: 10.1210/jc.2015-2236 press.endocrine.org/journal/jcem 3995
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018
garding the lack of consistent evidence for benefit for botan-
icals, black cohosh, omega-3 fatty acids, red clover, vitamin
E, and mind/body alternatives including anxiety control,
acupuncture, paced breathing, and hypnosis. (2ⱍQQEE)
Evidence
Clinical trials with these agents have reported incon-
sistent efficacy over placebo, but individual patients
may experience benefit (Table 9). The MSFLASH trial
showed that omega-3 fatty acids do not improve VMS
(246). In a randomized trial of 187 symptomatic meno-
pausal women, clinical hypnosis was associated with a
74.2% reduction in hot flashes compared with a 17.1%
reduction in women randomized to structured attention
control (P⬍.001) (247). The phytoestrogens are non-
steroidal compounds that have both estrogenic and anti-
Figure 4. Hot flash frequency and composite score with nonhormonal prescription therapies for relief of VMS. Upper panel, Effect on frequency
of VMS; lower panel, effect on composite score (severity times frequency; best representation of effect); open bars, placebo; colored bars,
therapies; length of bars, ranges in studies; horizontal bar, means. All of these agents are generally well tolerated (226). Hypersensitivity or prior
adverse drug reactions to each of these agents represent contraindications. For the SSRI/SNRIs, prior neuroleptic syndrome, serotonin syndrome,
and concurrent use of monoamine oxidase inhibitors are also contraindications. SSRI/SNRIs should be used with caution in patients with bipolar
disease, uncontrolled seizures, hepatic or renal insufficiency, uncontrolled hyponatremia, concurrent use of other SSRI/SNRIs, or poorly controlled
hypertension. These agents uncommonly induce suicidal thoughts within the first few months of treatment. Preliminary evidence suggests a
possible increase in risk of bone fracture. Gabapentin and pregabalin may increase suicidal thoughts and behaviors, cause drowsiness or dizziness,
and impair balance and coordination. Pregabalin may impair memory and concentration. Clonidine is contraindicated in patients with low blood
pressure and may cause lightheadedness, hypotension, headache, and constipation; sudden cessation of treatment can be associated with
significant increments in blood pressure (63).
3996 Stuenkel et al Guideline on Menopause J Clin Endocrinol Metab, November 2015, 100(11):3975–4011
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018

estrogenic properties. Caution is advised because some of
these agents, when consumed as supplements, can exert
estrogenic effects, a concern in breast cancer survivors al-
though dietary soy appears to have no adverse effects on
breast cancer prognosis (248).
5.0 Treatment of genitourinary syndrome of
menopause
5.1 Vaginal moisturizers and lubricants
5.1a For postmenopausal women with symptoms of
VVA, we suggest a trial of vaginal moisturizers to be used
at least twice weekly. (2ⱍQQEE)
Evidence
Vaginal moisturizers (eg, polycarbophil-based mois-
turizer, hyaluronic acid-based preparations, and a pectin-
based preparation), when used regularly (at least twice
weekly), may provide an effective nonhormonal approach
to alleviating symptoms of vaginal atrophy. However,
studies have been small, mostly open-labeled, and limited
to 12 weeks (249–257). Although helpful, these ap-
proaches are not likely as effective as vaginal ET. Vaginal
moisturizers have not been shown to reduce urinary tract
symptoms or asymptomatic bacteriuria. Use of a vaginal
moisturizer may not eliminate the need for a vaginal lu-
bricant during intercourse.
5.1b For women who do not produce sufficient vaginal
secretions for comfortable sexual activity, we suggest vag-
inal lubricants. (2ⱍQQEE)
Evidence
Vaginal lubricants are used to enhance the sexual ex-
perience in women with symptoms of VVA by alleviating
vaginal dryness and preventing dyspareunia (258). Lubri-
cants do not treat the underlying problem and only briefly
alleviate symptoms. Several OTC options are available. Be-
cause data do not demonstrate the superiority of one to an-
other, women can experiment with these products. Olive oil
is also effective (259). Petroleum jelly has been associated
with an increased rate of bacterial vaginosis (260).
5.2 Vaginal estrogen therapies
5.2a For women without a history of hormone- (estro-
gen) dependent cancers who are seeking relief from symp-
toms of GSM (including VVA) that persist despite using
Table 9. Alternative Therapies for Treatment of VMS
Agents Comments Refs.
Agents with inconsistent reports of benefit
Genistein Purified isoflavone 324–336
⫾Estrogenically active
Breast safety not established
Daidzein Purified isoflavone 324–336
⫾Estrogenically active
Breast safety not established
S-equol Metabolite of daidzein 337
Nonpurified isoflavones Breast safety not established 338
Flaxseed 225, 236, 328, 339–341
Red clover Breast safety not established 225, 236, 328, 339–341
High-dose extracted or synthesized
phytoestrogen
225, 236, 328, 339–341
Dietary soy Agreement about breast safety 248
Vitamin E 10% benefit in some studies 217, 342, 343
Reports with predominantly no benefit
Black cohosh Some short-term trials report benefit, most report no
benefit
225, 344–352
Breast safety not established
Reports of liver toxicity
Omega-3 fatty acids No benefit in MSFLASH trial 246
Acupuncture Not effective when compared to “sham acupuncture”
controls
353–356
Exercise Exercise with sweating may increase hot flashes 357
Other complementary approaches Ginseng, dong quai, wild yam, progesterone creams,
traditional Chinese herbs, reflexology, magnetic devices
225, 332
Agents requiring further study
Stellate ganglion block Need further RCTs to establish lack of complications 358
Guided relaxation Stress management, deep breathing, paced respiration,
guided imagery, mindfulness training
217, 225, 247, 359–365
Hypnosis Recent studies suggest efficacy 247
Cognitive behavior modification Recent studies suggest efficacy with trained practitioners 366, 367
doi: 10.1210/jc.2015-2236 press.endocrine.org/journal/jcem 3997
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018

vaginal lubricants and moisturizers, we recommend low-
dose vaginal ET. (1ⱍQQQE)
Evidence
A 2006 Cochrane meta-analysis of vaginal estrogens
(261) compared 19 efficacy trials and found that all prod-
ucts effectively alleviated symptoms, but study differences
limited comparisons among agents. As a guiding principle,
we recommend using the lowest effective dose.
RCTs of low-dose vaginal estrogen products (262–
267) report rapid improvement of vaginal symptoms (vag-
inal dryness or dyspareunia) and urinary symptoms (dys-
uria and urge incontinence) within 2 to 3 weeks. Objective
improvements continue at 12 weeks and are maintained to
1 year. Limited evidence suggests that vaginal ET may
prevent recurrent urinary tract infections (268, 269) and
overactive bladder (270, 271). No clear proof exists that
vaginal ET prevents or improves pelvic prolapse (272),
but it may be advantageous preoperatively (273). Ad-
verse effects include potential transfer to partner via
penile or oral absorption and, with vaginal creams, res-
idue on undergarments.
Vaginal estrogens
Vaginal estrogen preparations have been categorized
as: 1) low, 2) intermediate, and 3) systemic doses (274)
(Table 10). By using the lowest effective doses, systemic
absorption is minimized. During the initiation of therapy,
vaginal atrophy may enhance systemic absorption, al-
though not all studies demonstrate this effect (267, 275).
When vaginal epithelium is restored (after several weeks of
ET), systemic absorption may decrease (276, 277).
Low-dose therapies
Low-dose vaginal ring. Low-dose vaginal rings result in
estradiol levels that remain within the normal postmeno-
pausal range; however, bone resorption and lipid levels
decrease, suggesting possible systemic effects (278, 279).
Insertion and removal at 3-month intervals may be diffi-
cult, the ring can be sensed during intercourse, and it can
be expelled, particularly in women who have undergone a
hysterectomy (265).
Vaginal estradiol tablets. The 10-
g tablet provides stan-
dard twice weekly dosing, relieves vaginal symptoms by 8
weeks, and is effective for at least 52 weeks (263, 275, 280,
281). Therapy is initiated with daily administration for 2
weeks, and then twice weekly thereafter. Vaginal place-
ment of the tablet may provide less introital benefit than
creams.
Promestriene (estradiol diether). This is a low-dose estro-
gen used outside the United States. Evidence is limited to
studies of poor quality and very few RCTs (282).
Intermediate-dose vaginal estrogen
The 25-
g estradiol tablets increase plasma estradiol
from 3.1 ⫾0.83 to 19.8 ⫾6.1 pg/mL by 7 days (283). An
RCT of CEE vaginal cream ⱖ0.3 mg applied daily or twice
weekly reported an improvement in VVA by 12 weeks that
was sustained for 52 weeks without reports of endometrial
effects (266). Intermediate-dose estradiol and CEE creams
provide flexibility of dosing, allow treatment from the in-
troitus to the vaginal apex, and provide the emollient effect
of vehicle. Some systemic absorption exists (284, 285).
Table 10. Classification of Government-Approved Vaginal Estrogens
Type Dose Serum Estradiol Level
Low dose ⬍20 pg/mL
Silastic estradiol vaginal ring 7.5
g
Estradiol vaginal tablet 10
g
Promestriene (estradiol diether) ovule
a
10 mg
Estriol ovule
a
0.5 mg
Estriol ⫹progesterone ⫹Lactobacillus Doderleini ovule
a
0.2 mg ⫹2mg⫹341 mg
Promestriene cream
a
3mg
Estriol cream
a
0.015–0.03 mg
Intermediate dose ⬎20 pg/mL
CEE vaginal cream ⬎0.3-mg dose 5–50 pg/mL
Estradiol vaginal tablet 25
g
b
Some ⬎20 pg/mL
High dose (systemic) 35–200 pg/mL
Estradiol vaginal ring 50 and 100
g
Vaginal estradiol ⬎0.5 mg
Vaginal CEE ⬎0.5 mg
c
a
Not approved or recommended in United States.
b
No longer available in United States.
c
Predominantly estrone sulfate; LH suppression reflects systemic absorption.
3998 Stuenkel et al Guideline on Menopause J Clin Endocrinol Metab, November 2015, 100(11):3975–4011
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018

Systemic-dose vaginal estrogen
CEE 0.625- to 2.5-mg vaginal cream, administered
daily, results in systemic effects as evidenced by LH and
FSH suppression (285). No RCT data are available re-
garding the FDA-approved dosing of estradiol 2- to 4-g
vaginal cream, administered daily for 1 to 2 weeks, fol-
lowed by a maintenance dosage of 1 g, one to three times
a week.
Other hormonal agents
Estriol vaginal preparations (gels and suppositories)
are manufactured and government regulated in a number
of countries outside the United States. Estriol is considered
a low-affinity estrogen and, despite increased plasma con-
centration after repeated vaginal administration, is not
considered to have substantial systemic effects (286, 287).
Adverse events
Because serum estradiol levels during therapy usually
fall within the normal postmenopausal range, the risk pro-
file with low-dose vaginal ET is expected to be lower than
with systemic ET (288). However, long-term endometrial
safety data are lacking, and 1 year is the maximum dura-
tion of RCTs of vaginal ET (261). Side effects include
vulvovaginal candidiasis (289, 290) and, with higher dos-
ing and systemic absorption, vaginal bleeding and breast
pain (289). Increased CVD or VTE risk has not been re-
ported (261). This may reflect an actual neutral effect due
to the absence of a first-pass hepatic effect by vaginal es-
trogens, or that studies of women at high CVD or VTE risk
are lacking (281). Available evidence does not support the
boxed warning on low-dose vaginal estrogen regarding an
increased risk of CHD, stroke, VTE, dementia, and breast
cancer, and efforts to modify the labeling of these products
are in progress (288).
Practice statement
5.2b In women with a history of breast or endometrial
cancer, who present with symptomatic GSM (including
VVA), that does not respond to nonhormonal therapies,
we suggest a shared decision-making approach that in-
cludes the treating oncologist to discuss using low-dose
vaginal ET. (Ungraded best practice statement)
Evidence
Breast cancer
Whether small increases in circulating estrogens from
low-dose vaginal estrogen can stimulate the growth of
residual breast cancer cells (280, 291–293) remains an
unanswered question. However, for women taking aro-
matase inhibitors, the effectiveness of which depends upon
blocking up to 95% of estrogen synthesis and reducing
circulating estradiol levels to ⬍1 pg/mL (250), caution is
raised because minimal amounts of estrogen can be ab-
sorbed with low-dose vaginal ET. In a cohort case-control
study of 13 479 breast cancer survivors taking adjuvant
tamoxifen or aromatase inhibitor therapy for at least 1
year, after 3.5 years of concurrent administration of the
low-dose estrogen ring or 10-
g vaginal tablet, breast can-
cer recurrence did not increase (relative risk, 0.78; 95%
CI, 0.48–1.25) (294). These data are insufficient, how-
ever, to conclude safety and to recommend this approach.
Endometrial cancer
The effect of low-dose vaginal ET on endometrial can-
cer recurrence is unknown. The only RCT attempting to
evaluate the effect of systemic ET on recurrence rate and
survival in women after surgery for stage I or II endome-
trial cancer was closed prematurely without complete en-
rollment (295). In the absence of RCT findings to guide
practice recommendations, the decision to use ET remains
controversial and involves assessing the severity of post-
menopausal symptoms and tumor characteristics (296,
297).
5.2c For women taking raloxifene, without a history of
hormone- (estrogen) dependent cancers, who develop
symptoms of GSM (including VVA) that do not respond
to nonhormonal therapies, we suggest adding low-dose
vaginal ET. (2ⱍQQEE)
Evidence
Raloxifene has neutral vaginal effects (298–300). In
two clinical trials, vaginal, but not oral (301) ET, was
safely used to treat vaginal symptoms in women taking
raloxifene without untoward endometrial effects (302,
303).
5.2d For women using low-dose vaginal ET, we suggest
against adding a progestogen (ie, no need for adding pro-
gestogen to prevent endometrial hyperplasia). (2ⱍQEEE)
5.2e For women using vaginal ET who report post-
menopausal bleeding or spotting, we recommend prompt
evaluation for endometrial pathology. (1ⱍQQEE)
Evidence
Bleeding or spotting in a woman using only vaginal
estrogens is uncommon in the absence of endometrial pa-
thology. The 2006 Cochrane review of 19 studies found
no significant difference among vaginal creams, tablets, or
rings in terms of endometrial thickness or hyperplasia or
in the proportion of women with adverse events (261).
Recent 1-year-long studies of vaginal CEE cream and low-
dose vaginal estradiol tablets revealed no cases of endo-
metrial hyperplasia or cancer as determined by endome-
trial biopsy (263, 266, 304). Vaginal administration of
doi: 10.1210/jc.2015-2236 press.endocrine.org/journal/jcem 3999
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018

estradiol tablets, when placed in the upper third of the
vagina, may result in a uterine first-pass effect resulting in
a higher degree of uterine stimulation (305–309). It is un-
known whether endometrial proliferation, hyperplasia, or
cancer can occur after long-duration treatment (⬎1y)or
in women with risk factors (late menopause, higher body
mass index, higher dosing). For women at higher risk of
endometrial cancer, surveillance using transvaginal ultra-
sound, followed by endometrial biopsy if endometrial
thickening is present, may be prudent. Intermittent (pos-
sibly annual) progestogen withdrawal may be considered
to assess endometrial status (261, 280).
5.3 Ospemifene
5.3a For treatment of moderate to severe dyspareunia
associated with vaginal atrophy in postmenopausal
women without contraindications, we suggest a trial of
ospemifene. (2ⱍQQQE)
5.3b For women with a history of breast cancer pre-
senting with dyspareunia, we recommend against os-
pemifene. (1ⱍQEEE)
Evidence
Benefits
Not all women are comfortable using vaginal ET, and
women may prefer an oral medication specifically indi-
cated for dyspareunia.
Vaginal symptoms and sexual function. Two 12-week
RCTs of ospemifene reported improvements in pH and
vaginal maturation index, severity of dyspareunia (310,
311), and standardized measures of sexual function (in-
cluding desire, arousal, orgasm, and satisfaction) (312).
Two year-long studies (313, 314) demonstrated sustained
vaginal benefits.
Risks
Vasomotor symptoms. The most common adverse effect
was VMS (7.2% of women taking ospemifene compared
with 2% taking placebo) (314).
Cardiovascular. Ospemifene involves risk of VTE (315)
and is contraindicated in women at risk for venous or
arterial thrombosis or stroke. In safety studies, incidence
rates for thromboembolic stroke, hemorrhagic stroke, and
DVT were 0.72, 1.45, and 1.45/1000, respectively, in
women receiving ospemifene 60 mg vs 1.04, 0, and 1.04/
1000, respectively, in women assigned to placebo (310).
Endometrium. No cases of endometrial carcinoma have
been reported. Studies reported endometrial thickening
of ⱖ5 mm at a rate of 60.1/1000 women per year of
therapy with ospemifene vs 21.2/1000 women per year of
therapy with placebo. The incidence of proliferative en-
dometrium (weakly plus active plus disordered) was 86.1/
1000 women with ospemifene vs 13.3/1000 with placebo
(315). The incidence of uterine polyps was 5.9 cases/1000
women with ospemifene vs 1.8/1000 women with placebo
(315).
Breast. Data on breast density or breast cancer risk are lack-
ing. Estrogen-dependent neoplasia is a contraindication.
Future research
There are numerous gaps in our knowledge regarding
menopause symptoms. Some of these include a lack of the
most basic understanding of what causes hot flashes, ques-
tions regarding the potential link between VMS and CVD
in older vs younger postmenopausal women, and a poor
understanding of the relationships between menopause
and sleep and hormonal transitions and mood, which have
significant social and economic implications. Given the
uncertainties regarding the precise neuroendocrine events
that cause VMS, developing specific targeted therapies is
challenging. Establishing appropriate animal models and
expanding recent research involving the neuroregulators
kisspeptin, neurokinin B, and dynorphin may help de-
velop new effective treatments (35).
Management of the transition to menopause remains
uncharted territory. The SWAN and the Melbourne
Women’s Midlife Health Project provide extensive epide-
miological, physiological, and descriptive data character-
izing reproductive changes that occur during the transi-
tion to menopause. However, clinical management
decisions are often based on the extrapolation of obser-
vational data collected from studies conducted in younger,
reproductive age women. RCTs of frequently prescribed
therapies, such as oral contraceptives, MHT, and mea-
sures to control mood, with clinical outcomes relevant to
women of relatively advanced age are sorely needed to
confidently advise patients regarding the safest and most
effective therapies to use during this transition.
Managing the loss of ovarian function in premeno-
pausal women due to surgery, the range of disorders man-
ifesting as POI, or the sequelae of treatment for breast
cancer and other malignancies remains challenging. This
is due to a dearth of quality data assessing the long-term
risks and benefits of MHT or other options for symptom
relief and prevention of chronic diseases in these groups.
Fertility issues can be managed with modern assisted re-
productive technology, but we fall short on adequately
managing estrogen deficiency. Pressing questions remain
4000 Stuenkel et al Guideline on Menopause J Clin Endocrinol Metab, November 2015, 100(11):3975–4011
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018

regarding optimal treatment preparation, dosing and reg-
imens, and the merits of long-term MHT, even in women
without menopausal symptoms. International registries
and clinical trials are overdue to address the long-reaching
implications of these important issues.
The most persistent question for naturally postmeno-
pausal women is how to balance menopausal symptom
relief with the prevention of chronic diseases of aging such
as CHD, osteoporotic fractures, and dementia. ET has
long been hypothesized to meet this goal, although con-
clusive evidence remains elusive, and questions persist re-
garding the interaction between EPT and these outcomes,
as well as breast cancer. Observational data suggesting
differences in VTE risk and other CVD outcomes continue
to accumulate, suggesting a significant need for ade-
quately powered clinical trials comparing the safety and
efficacy of oral with transdermal therapies in younger,
recently postmenopausal women.
Finally, new SERM therapies (alone and partnered with
estrogens) are promising, but larger, longer trials are
needed to fully characterize the benefit/risk profiles of
these new treatments and inform the clinician as to which
patients stand to benefit the most from their use.
Financial Disclosures of the Task Force*
Financial Disclosure of Task Force:* Cynthia A. Stuenkel,
MD. (chair)—Financial or business/organizational inter-
ests: North American Menopause Society (Chair, Exam
Committee), National Women’s Law Center-Well Wom-
en’s Project; Significant financial interest or leadership
position: none declared. Susan R. Davis, MBBS, PhD—
Financial or Business/Organizational Interests: Interna-
tional Menopause Society, North American Menopause
Society, Menopause, Maturitas, Climacteric, Trimel Phar-
maceuticals Canada, Lawley Pharmaceuticals Australia,
Abbott Pharmaceuticals; Significant Financial Interest or
Leadership Position: International Menopause Society,
National Health and Medical Research Council, Austra-
lia, Bupa Health Foundation. Anne Gompel, MD, PhD—
Financial or Business/Organizational Interests: European
Society for Contraception, European Society of Endocri-
nology, Groupe d’Etude sur la Ménopause et le Vieillisse-
ment Hormonal, Société Française de Sénologie et Pa-
thologie Mammaire; Significant financial interest or
leadership position: none declared. Mary Ann Lumsden,
MD, PhD—Financial or Business/Organizational Inter-
ests: —Financial or Business/Organizational Interests: In-
ternational Menopause Society, British Menopause Soci-
ety; Significant Financial Interest or Leadership Position:
National Institute of Health and Clinical Excellence. M.
Hassan Murad, M.D., M.P.H.** —Financial or business/
organizational interests: Mayo Clinic, Division of Preven-
tive Medicine; Significant financial interest or leadership
position: none declared. JoAnn V. Pinkerton, MD—Fi-
nancial or business/organizational interests: North Amer-
ican Menopause Society, Menopause Journal, OBG Man-
agement, Climacteric Journal, Journal of Women’s
Health, University of Virginia Board of Visitors (Noven
Pharmaceuticals, Pfizer, Inc., Shionogi, Therapeutics
MD), University of Virginia Clinical Trials (Therapeutics
MD); Significant Financial Interest or Leadership Posi-
tion: North American Menopause Society, Academy of
Women’s Health, South Atlantic Association of ObGyn.
Richard J. Santen, MD—Financial or business/organiza-
tional interests: American Society of Clinical Oncology,
Up-to-Date (Author/Honorarium); Significant Financial
Interest or Leadership Position: Pfizer (Advisory Board,
Research Grant).
* Financial, business, and organizational disclosures of
the Task Force cover the year prior to publication. Dis-
closures prior to this time period are archived.
** Evidence-based reviews for this guideline were pre-
pared under contract with The Endocrine Society.
Acknowledgments
Special thanks are extended to Drs. David F. Archer, Gloria A.
Bachmann, Henry Burger, Roger A. Lobo, Charles L. Loprinzi,
JoAnn E. Manson, Kathryn A. Martin, Nanette F. Santoro,
Hugh S. Taylor, and Nelson B. Watts for careful review and
thoughtful suggestions.
Address all correspondence and requests for reprints to: The
Endocrine Society, 2055 L Street NW, Suite 600, Washington,
DC 20036. E-mail: govt-prof@endocrine.org; Phone: 202–971-
3636. Send commercial reprint requests for orders over 100 to:
https://www.endocrine.org/corporate-
relations/commercial-reprints. Send commercial reprint requests
for orders under 100 to: Society Services, E-mail: society
services@endocrine.org; Phone: 202–971-3636; Fax:
202–736-9705.
Cosponsoring Associations: The Australasian Menopause
Society, the British Menopause Society, European Menopause
and Andropause Society, the European Society of Endocrinol-
ogy, and the International Menopause Society.
References
1. Atkins D, Best D, Briss PA, et al. Grading quality of evidence and
strength of recommendations. BMJ. 2004;328:1490.
2. Swiglo BA, Murad MH, Schu¨ nemann HJ, et al. A case for clarity,
consistency, and helpfulness: state-of-the-art clinical practice
guidelines in endocrinology using the grading of recommendations,
assessment, development, and evaluation system. J Clin Endocrinol
Metab. 2008;93:666– 673.
doi: 10.1210/jc.2015-2236 press.endocrine.org/journal/jcem 4001
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018

3. Guyatt GH, Schu¨ nemann HJ, Djulbegovic B, Akl EA. Guideline
panels should not GRADE good practice statements. J Clin Epi-
demiol. 2015;68:597–600.
4. Mohammed K, Benkhadra K, et al. Oral vs. transdermal estrogen
and the risk of venous and arterial thrombotic events: a systematic
review and meta-analysis. J Clin Endocrinol Metab. (To be sub-
mitted 2015).
5. Benkhadra KM, Nofal AA, Carranza Leon BG, Alahdab F, Abu
Dabrh AM. Menopausal hormonal therapy and mortality: a sys-
tematic review and meta-analysis. J Clin Endocrinol Metab (to be
submitted 2015).
6. Tom SE, Kuh D, Guralnik JM, Mishra GD. Self-reported sleep
difficulty during the menopausal transition: results from a prospec-
tive cohort study. Menopause. 2010;17:1128–1135.
7. Mishra GD, Kuh D. Health symptoms during midlife in relation to
menopausal transition: British prospective cohort study. BMJ.
2012;344:e402.
8. Syed Alwi SA, Lee PY, Awi I, Mallik PS, Md Haizal MN. The
menopausal experience among indigenous women of Sarawak,
Malaysia. Climacteric. 2009;12:548–556.
9. Liu M, Wang Y, Li X, et al. A health survey of Beijing middle-aged
registered nurses during menopause. Maturitas. 2013;74:84– 88.
10. Nappi RE, Davis SR. The use of hormone therapy for the main-
tenance of urogynecological and sexual health post WHI.
Climacteric. 2012;15:267–274.
11. Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A
prospective population-based study of menopausal symptoms.
Obstet Gynecol. 2000;96:351–358.
12. Freeman EW, Sammel MD, Lin H. Temporal associations of hot
flashes and depression in the transition to menopause. Menopause.
2009;16:728–734.
13. Herber-Gast GC, Mishra GD, van der Schouw YT, Brown WJ,
Dobson AJ. Risk factors for night sweats and hot flushes in midlife:
results from a prospective cohort study. Menopause. 2013;20(9):
953–959.
14. Freeman EW. Hot flushes and the menopause: how long should
they be expected to last? Maturitas. 2014;78:153–154.
15. Harlow SD, Gass M, Hall JE, et al. Executive summary of the Stages
of Reproductive Aging Workshop ⫹10: addressing the unfinished
agenda of staging reproductive aging. J Clin Endocrinol Metab.
2012;97:1159–1168.
16. Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal
vasomotor symptoms over the menopause transition. JAMA Intern
Med. 2015;175:531–539.
17. Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated
equine estrogen in postmenopausal women with hysterectomy: the
Women’s Health Initiative randomized controlled trial. JAMA.
2004;291:1701–1712.
18. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits
of estrogen plus progestin in healthy postmenopausal women: prin-
cipal results From the Women’s Health Initiative randomized con-
trolled trial. JAMA. 2002;288:321–333.
19. Stuenkel CA, Gass ML, Manson JE, et al. A decade after the
women’s health initiative–the experts do agree. J Clin Endocrinol
Metab. 2012;97:2617–2618.
20. de Villiers TJ, Gass ML, Haines CJ, et al. Global consensus state-
ment on menopausal hormone therapy. Maturitas. 2013;74:391–
392.
21. Santen R, Pritchard K, Burger H. The consensus conference on
treatment of estrogen deficiency symptoms in women surviving
breast cancer. Obstet Gynecol Surv. 1998;53:S1–S83.
22. Goodwin PJ, Ennis M, Pritchard KI, Trudeau M, Hood N. Risk of
menopause during the first year after breast cancer diagnosis. J Clin
Oncol. 1999;17:2365–2370.
23. Woods NF, Mitchell ES. Symptoms during the perimenopause:
prevalence, severity, trajectory, and significance in women’s lives.
Am J Med. 2005;118(suppl 12B):14–24.
24. Avis NE, Stellato R, Crawford S, et al. Is there a menopausal syn-
drome? Menopausal status and symptoms across racial/ethnic
groups. Soc Sci Med. 2001;52:345–356.
25. Freeman EW, Sherif K. Prevalence of hot flushes and night sweats
around the world: a systematic review. Climacteric. 2007;10:197–
214.
26. Gartoulla P, Islam MR, Bell RJ, Davis SR. Prevalence of meno-
pausal symptoms in Australian women at midlife: a systematic
review. Climacteric. 2014;17:529–539.
27. Islam MR, Gartoulla P, Bell RJ, Fradkin P, Davis SR. Prevalence of
menopausal symptoms in Asian midlife women: a systematic re-
view. Climacteric. 2015;18:157–176.
28. Reed SD, Lampe JW, Qu C, et al. Premenopausal vasomotor symp-
toms in an ethnically diverse population. Menopause. 2014;21:
153–158.
29. Szmuilowicz ED, Manson JE, Rossouw JE, et al. Vasomotor
symptoms and cardiovascular events in postmenopausal
women. Menopause. 2011;18:603–610.
30. Freedman RR, Roehrs TA. Effects of REM sleep and ambient tem-
perature on hot flash-induced sleep disturbance. Menopause.
2006;13:576–583.
31. Joffe H, Crawford S, Economou N, et al. A gonadotropin-releasing
hormone agonist model demonstrates that nocturnal hot flashes
interrupt objective sleep. Sleep. 2013;36:1977–1985.
32. Freedman RR. Hot flashes: behavioral treatments, mechanisms,
and relation to sleep. Am J Med. 2005;118(suppl 12B):124–130.
33. Casper RF, Yen SS, Wilkes MM. Menopausal flushes: a neuroen-
docrine link with pulsatile luteinizing hormone secretion. Science.
1979;205:823–825.
34. Tataryn IV, Meldrum DR, Lu KH, Frumar AM, Judd HL. LH, FSH
and skin temperature during the menopausal hot flash. J Clin En-
docrinol Metab. 1979;49:152–154.
35. Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA,
Krajewski-Hall SJ. Modulation of body temperature and LH se-
cretion by hypothalamic KNDy (kisspeptin, neurokinin B and
dynorphin) neurons: a novel hypothesis on the mechanism of hot
flushes. Front Neuroendocrinol. 2013;34:211–227.
36. Casper RF, Yen SS. Neuroendocrinology of menopausal flushes: an
hypothesis of flush mechanism. Clin Endocrinol (Oxf). 1985;22:
293–312.
37. Portman DJ, Gass ML, Vulvovaginal Atrophy Terminology Con-
sensus Conference Panel. Genitourinary syndrome of menopause:
new terminology for vulvovaginal atrophy from the International
Society for the Study of Women’s Sexual Health and the North
American Menopause Society. Menopause. 2014;21:1063–1068.
38. Santen RJ, Allred DC, Ardoin SP, et al. Postmenopausal hormone
therapy: an Endocrine Society scientific statement. J Clin Endocri-
nol Metab. 2010;95:s1–s66.
39. The Study of Women’s Health Across the Nation. SWAN Research
Findings Publication List. Pages 1–37. Updated September 7, 2015.
http://www.swanstudy.org/publications/swan-research-findings/. Ac-
cessed September 15, 2015.
40. Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines
for the prevention of cardiovascular disease in women–2011 update:
a guideline from the American Heart Association. Circulation. 2011;
123:1243–1262.
41. Bushnell C, McCullough LD, Awad IA, et al. Guidelines for the
prevention of stroke in women: a statement for healthcare profes-
sionals from the American Heart Association/American Stroke As-
sociation. Stroke. 2014;45:1545–1588.
42. National Osteoporosis Foundation. 2014 Clinician’s Guide to Pre-
vention and Treatment of Osteoporosis.http://nof.org/files/nof/
public/content/file/2791/upload/919.pdf. Accessed April 12, 2014.
43. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to pre-
vention and treatment of osteoporosis. Osteoporos Int. 2014;25:
2359–2381.
44. Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal
hormone therapy and health outcomes during the intervention and
4002 Stuenkel et al Guideline on Menopause J Clin Endocrinol Metab, November 2015, 100(11):3975–4011
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018

extended poststopping phases of the Women’s Health Initiative
randomized trials. JAMA. 2013;310:1353–1368.
45. Maclennan AH, Broadbent JL, Lester S, Moore V. Oral oestrogen
and combined oestrogen/progestogen therapy versus placebo for
hot flushes. Cochrane Database Syst Rev. 2004;4:CD002978.
46. Utian WH, Shoupe D, Bachmann G, Pinkerton JV, Pickar JH.
Relief of vasomotor symptoms and vaginal atrophy with lower
doses of conjugated equine estrogens and medroxyprogesterone
acetate. Fertil Steril. 2001;75:1065–1079.
47. Cardozo L, Bachmann G, McClish D, Fonda D, Birgerson L. Meta-
analysis of estrogen therapy in the management of urogenital at-
rophy in postmenopausal women: second report of the Hormones
and Urogenital Therapy Committee. Obstet Gynecol. 1998;92:
722–727.
48. Perrotta C, Aznar M, Mejia R, Albert X, Ng CW. Oestrogens for
preventing recurrent urinary tract infection in postmenopausal
women. Cochrane Database Syst Rev. 2008;2:CD005131.
49. Kravitz HM, Schott LL, Joffe H, Cyranowski JM, Bromberger JT.
Do anxiety symptoms predict major depressive disorder in midlife
women? The Study of Women’s Health Across the Nation (SWAN)
Mental Health Study (MHS). Psychol Med. 2014;44:2593–2602.
50. Soares CN. Mood disorders in midlife women: understanding the
critical window and its clinical implications. Menopause. 2014;
21:198–206.
51. Worsley R, Davis SR, Gavrilidis E, et al. Hormonal therapies for
new onset and relapsed depression during perimenopause.
Maturitas. 2012;73:127–133.
52. Barnabei VM, Cochrane BB, Aragaki AK, et al. Menopausal symp-
toms and treatment-related effects of estrogen and progestin in the
Women’s Health Initiative. Obstet Gynecol. 2005;105:1063–
1073.
53. Moyer VA, U.S. Preventive Services Task Force. Menopausal hor-
mone therapy for the primary prevention of chronic conditions:
U.S. Preventive Services Task Force recommendation statement.
Ann Intern Med. 2013;158:47–54.
54. Marjoribanks J, Farquhar C, Roberts H, Lethaby A. Long term
hormone therapy for perimenopausal and postmenopausal
women. Cochrane Database Syst Rev. 2012;7:CD004143.
55. North American Menopause Society. The 2012 hormone therapy
position statement of: The North American Menopause Society.
Menopause. 2012;19:257–271.
56. ACOG Practice Bulletin No. 141: management of menopausal
symptoms. Obstet Gynecol. 2014;123:202–216.
57. Lobo RA, Davis SR, De Villiers TJ, et al. Prevention of diseases
after menopause. Climacteric. 2014;17:540–556.
58. Kanaya AM, Herrington D, Vittinghoff E, et al. Glycemic effects of
postmenopausal hormone therapy: the Heart and Estrogen/Pro-
gestin Replacement Study. A randomized, double-blind, placebo-
controlled trial. Ann Intern Med. 2003;138:1–9.
59. Margolis KL, Bonds DE, Rodabough RJ, et al. Effect of oestrogen
plus progestin on the incidence of diabetes in postmenopausal
women: results from the Women’s Health Initiative Hormone
Trial. Diabetologia. 2004;47:1175–1187.
60. Bonds DE, Lasser N, Qi L, et al. The effect of conjugated equine
oestrogen on diabetes incidence: the Women’s Health Initiative
randomised trial. Diabetologia. 2006;49:459– 468.
61. Manson JE, Rimm EB, Colditz GA, et al. A prospective study of
postmenopausal estrogen therapy and subsequent incidence of
non-insulin-dependent diabetes mellitus. Ann Epidemiol. 1992;2:
665–673.
62. de Lauzon-Guillain B, Fournier A, Fabre A, et al. Menopausal
hormone therapy and new-onset diabetes in the French Etude Epi-
demiologique de Femmes de la Mutuelle Ge´ne´rale de l’Education
Nationale (E3N) cohort. Diabetologia. 2009;52:2092–2100.
63. Manson JE. Current recommendations: what is the clinician to do?
Fertil Steril. 2014;101:916–921.
64. Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, et al. Estro-
gen plus progestin and colorectal cancer in postmenopausal
women. N Engl J Med. 2004;350:991–1004.
65. Weiss NS, Szekely DR, Austin DF. Increasing incidence of endo-
metrial cancer in the United States. N Engl J Med. 1976;294:1259–
1262.
66. Mack TM, Pike MC, Henderson BE, et al. Estrogens and endo-
metrial cancer in a retirement community. N Engl J Med. 1976;
294:1262–1267.
67. Effects of estrogen or estrogen/progestin regimens on heart disease
risk factors in postmenopausal women. The Postmenopausal Es-
trogen/Progestin Interventions (PEPI) Trial. The Writing Group for
the PEPI Trial. JAMA. 1995;273:199–208.
68. Fournier A, Dossus L, Mesrine S, et al. Risks of endometrial cancer
associated with different hormone replacement therapies in the
E3N cohort, 1992–2008. Am J Epidemiol. 2014;180:508–517.
69. Effects of hormone replacement therapy on endometrial histology
in postmenopausal women. The Postmenopausal Estrogen/Proges-
tin Interventions (PEPI) Trial. The Writing Group for the PEPI
Trial. JAMA. 1996;275:370–375.
70. Jondet M, Maroni M, Yaneva H, Brin S, Peltier-Pujol F, Pe´lissier
C. Comparative endometrial histology in postmenopausal women
with sequential hormone replacement therapy of estradiol and, either
chlormadinone acetate or micronized progesterone. Maturitas. 2002;
41:115–121.
71. Ferenczy A, Gelfand MM, van de Weijer PH, Rioux JE. Endome-
trial safety and bleeding patterns during a 2-year study of 1 or 2 mg
17

-estradiol combined with sequential 5–20 mg dydrogesterone.
Climacteric. 2002;5:26–35.
72. Pukkala E, Tulenheimo-Silfvast A, Leminen A. Incidence of cancer
among women using long versus monthly cycle hormonal replace-
ment therapy, Finland 1994–1997. Cancer Causes Control. 2001;
12:111–115.
73. Jaakkola S, Lyytinen HK, Dyba T, Ylikorkala O, Pukkala E. En-
dometrial cancer associated with various forms of postmenopausal
hormone therapy: a case control study. Int J Cancer. 2011;128:
1644–1651.
74. Wildemeersch D, Pylyser K, De Wever N, Pauwels P, Tjalma W.
Endometrial safety after 5 years of continuous combined transder-
mal estrogen and intrauterine levonorgestrel delivery for post-
menopausal hormone substitution. Maturitas. 2007;57:205–209.
75. Orbo A, Vereide A, Arnes M, Pettersen I, Straume B. Levonorg-
estrel-impregnated intrauterine device as treatment for endome-
trial hyperplasia: a national multicentre randomised trial. BJOG.
2014;121:477–486.
76. Morelli M, Di Cello A, Venturella R, Mocciaro R, D’Alessandro P,
Zullo F. Efficacy of the levonorgestrel intrauterine system (LNG-
IUS) in the prevention of the atypical endometrial hyperplasia and
endometrial cancer: retrospective data from selected obese meno-
pausal symptomatic women. Gynecol Endocrinol. 2013;29:156–
159.
77. Beral V, Reeves G, Bull D, Green J, Million Women Study Col-
laborators. Breast cancer risk in relation to the interval between
menopause and starting hormone therapy. J Natl Cancer Inst.
2011;103:296–305.
78. Bakken K, Fournier A, Lund E, et al. Menopausal hormone therapy
and breast cancer risk: impact of different treatments. The Euro-
pean Prospective Investigation into Cancer and Nutrition. Int J
Cancer. 2011;128:144–156.
79. Fournier A, Mesrine S, Boutron-Ruault MC, Clavel-Chapelon F.
Estrogen-progestagen menopausal hormone therapy and breast
cancer: does delay from menopause onset to treatment initiation
influence risks? J Clin Oncol. 2009;27:5138–5143.
80. Beral V, Million Women Study Collaborators. Breast cancer and
hormone-replacement therapy in the Million Women Study.
Lancet. 2003;362:419– 427.
81. Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen
therapy and the risk of invasive breast cancer. Arch Intern Med.
2006;166:1027–1032.
doi: 10.1210/jc.2015-2236 press.endocrine.org/journal/jcem 4003
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018

82. Saxena T, Lee E, Henderson KD, et al. Menopausal hormone ther-
apy and subsequent risk of specific invasive breast cancer subtypes
in the California Teachers Study. Cancer Epidemiol Biomarkers
Prev. 2010;19:2366–2378.
83. Breast cancer and hormone replacement therapy: collaborative re-
analysis of data from 51 epidemiological studies of 52,705 women
with breast cancer and 108,411 women without breast cancer.
Collaborative Group on Hormonal Factors in Breast Cancer.
Lancet. 1997;350:1047–1059.
84. Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated
equine oestrogen and breast cancer incidence and mortality in post-
menopausal women with hysterectomy: extended follow-up of the
Women’s Health Initiative randomised placebo-controlled trial.
Lancet Oncol. 2012;13:476– 486.
85. Prentice RL, Manson JE, Langer RD, et al. Benefits and risks of
postmenopausal hormone therapy when it is initiated soon after
menopause. Am J Epidemiol. 2009;170:12–23.
86. Goodwin PJ, Stambolic V. Obesity and insulin resistance in breast
cancer–chemoprevention strategies with a focus on metformin.
Breast. 2011;20(suppl 3):S31–S35.
87. Song Y, Santen RJ, Wang JP, Yue W. Effects of the conjugated equine
estrogen/bazedoxifene tissue-selective estrogen complex (TSEC) on
mammary gland and breast cancer in mice. Endocrinology. 2012;
153:5706–5715.
88. Wood CE, Clarkson TB, Chen H, et al. Comparative effects of oral
conjugated equine estrogens and micronized 17

-estradiol on
breast proliferation: a retrospective analysis. Menopause. 2008;
15:890– 898.
89. Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast
cancer associated with different hormone replacement therapies:
results from the E3N cohort study. Breast Cancer Res Treat. 2008;
107:103–111.
90. Chlebowski RT, Anderson GL, Gass M, et al. Estrogen plus pro-
gestin and breast cancer incidence and mortality in postmeno-
pausal women. JAMA. 2010;304:1684–1692.
91. Anderson GL, Chlebowski RT, Rossouw JE, et al. Prior hormone
therapy and breast cancer risk in the Women’s Health Initiative
randomized trial of estrogen plus progestin. Maturitas. 2006;55:
103–115.
92. Lyytinen H, Dyba T, Pukkala E, Ylikorkala O. Do the dose or route
of administration of norethisterone acetate as a part of hormone
therapy play a role in risk of breast cancer: national-wide case-
control study from Finland. Int J Cancer. 2010;127:185–189.
93. Chlebowski RT, Manson JE, Anderson GL, et al. Estrogen plus
progestin and breast cancer incidence and mortality in the
Women’s Health Initiative Observational Study. J Natl Cancer
Inst. 2013;105:526–535.
94. Cordina-Duverger E, Truong T, Anger A, et al. Risk of breast
cancer by type of menopausal hormone therapy: a case-control
study among post-menopausal women in France. PLoS One. 2013;
8:e78016.
95. Collaborative Group On Epidemiological Studies Of Ovarian Can-
cer, Beral V, Gaitskell K, et al. Menopausal hormone use and ovar-
ian cancer risk: individual participant meta-analysis of 52 epide-
miological studies. Lancet. 2015;385:1835–1842.
96. Gompel A, Burger H. A commentary on a recent update of the
ovarian cancer risk attributable to menopausal hormone therapy.
Climacteric. 2015;18:376–378.
97. Davis SR, Baber R. Reproductive endocrinology: menopausal hor-
mone therapy-ovarian cancer risk revisited. Nat Rev Endocrinol.
2015;11:322–323.
98. Hsia J, Langer RD, Manson JE, et al. Conjugated equine estrogens
and coronary heart disease: the Women’s Health Initiative. Arch
Intern Med. 2006;166:357–365.
99. Manson JE, Allison MA, Rossouw JE, et al. Estrogen therapy and
coronary-artery calcification. N Engl J Med. 2007;356:2591–
2602.
100. Barrett-Connor E, Grady D. Hormone replacement therapy, heart
disease, and other considerations. Annu Rev Public Health. 1998;
19:55–72.
101. Løkkegaard E, Andreasen AH, Jacobsen RK, Nielsen LH, Agger C,
Lidegaard Ø. Hormone therapy and risk of myocardial infarction:
a national register study. Eur Heart J. 2008;29:2660–2668.
102. Shufelt CL, Merz CN, Prentice RL, et al. Hormone therapy dose,
formulation, route of delivery, and risk of cardiovascular events in
women: findings from the Women’s Health Initiative Observa-
tional Study. Menopause. 2014;21:260–266.
103. Schierbeck LL, Rejnmark L, Tofteng CL, et al. Effect of hormone
replacement therapy on cardiovascular events in recently post-
menopausal women: randomised trial. BMJ. 2012;345:e6409.
104. Harman SM, Black DM, Naftolin F, et al. Arterial imaging out-
comes and cardiovascular risk factors in recently menopausal
women: a randomized trial. Ann Intern Med. 2014;161:249–260.
105. Renoux C, Dell’aniello S, Garbe E, Suissa S. Transdermal and oral
hormone replacement therapy and the risk of stroke: a nested case-
control study. BMJ. 2010;340:c2519.
106. Smith NL, Blondon M, Wiggins KL, et al. Lower risk of cardio-
vascular events in postmenopausal women taking oral estradiol
compared with oral conjugated equine estrogens. JAMA Intern
Med. 2014;174:25–31.
107. Grodstein F, Manson JE, Stampfer MJ, Rexrode K. Postmeno-
pausal hormone therapy and stroke: role of time since menopause
and age at initiation of hormone therapy. Arch Intern Med. 2008;
168:861–866.
108. Canonico M, Oger E, Plu-Bureau G, et al. Hormone therapy and
venous thromboembolism among postmenopausal women: im-
pact of the route of estrogen administration and progestogens: the
ESTHER study. Circulation. 2007;115:840– 845.
109. Sweetland S, Beral V, Balkwill A, et al. Venous thromboembolism
risk in relation to use of different types of postmenopausal hor-
mone therapy in a large prospective study. J Thromb Haemost.
2012;10:2277–2286.
110. Renoux C, Dell’Aniello S, Suissa S. Hormone replacement therapy
and the risk of venous thromboembolism: a population-based
study. J Thromb Haemost. 2010;8:979–986.
111. Canonico M, Fournier A, Carcaillon L, et al. Postmenopausal hor-
mone therapy and risk of idiopathic venous thromboembolism:
results from the E3N cohort study. Arterioscler Thromb Vasc Biol.
2010;30:340–345.
112. Roach RE, Lijfering WM, Helmerhorst FM, Cannegieter SC,
Rosendaal FR, van Hylckama Vlieg A. The risk of venous throm-
bosis in women over 50 years old using oral contraception or post-
menopausal hormone therapy. J Thromb Haemost. 2013;11:124–
131.
113. Canonico M, Plu-Bureau G, Lowe GD, Scarabin PY. Hormone
replacement therapy and risk of venous thromboembolism in post-
menopausal women: systematic review and meta-analysis. BMJ.
2008;336:1227–1231.
114. Høibraaten E, Qvigstad E, Arnesen H, Larsen S, Wickstrøm E,
Sandset PM. Increased risk of recurrent venous thromboembolism
during hormone replacement therapy–results of the randomized,
double-blind, placebo-controlled estrogen in venous thromboem-
bolism trial (EVTET). Thromb Haemost. 2000;84:961–967.
115. Olie´ V, Plu-Bureau G, Conard J, Horellou MH, Canonico M,
Scarabin PY. Hormone therapy and recurrence of venous throm-
boembolism among postmenopausal women. Menopause. 2011;
18:488– 493.
116. Straczek C, Oger E, Yon de Jonage-Canonico MB, et al. Prothrom-
botic mutations, hormone therapy, and venous thromboembolism
among postmenopausal women: impact of the route of estrogen
administration. Circulation. 2005;112:3495–3500.
117. Canonico M, Oger E, Conard J, et al. Obesity and risk of venous
thromboembolism among postmenopausal women: differential
impact of hormone therapy by route of estrogen administration.
The ESTHER Study. J Thromb Haemost. 2006;4:1259–1265.
4004 Stuenkel et al Guideline on Menopause J Clin Endocrinol Metab, November 2015, 100(11):3975–4011
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018

118. Cirillo DJ, Wallace RB, Rodabough RJ, et al. Effect of estrogen
therapy on gallbladder disease. JAMA. 2005;293:330–339.
119. Simon JA, Hunninghake DB, Agarwal SK, et al. Effect of estrogen
plus progestin on risk for biliary tract surgery in postmenopausal
women with coronary artery disease. The Heart and Estrogen/
progestin Replacement Study. Ann Intern Med. 2001;135:493–
501.
120. Grodstein F, Colditz GA, Stampfer MJ. Postmenopausal hormone
use and cholecystectomy in a large prospective study. Obstet Gy-
necol. 1994;83:5–11.
121. Liu B, Beral V, Balkwill A, et al. Gallbladder disease and use of
transdermal versus oral hormone replacement therapy in post-
menopausal women: prospective cohort study. BMJ. 2008;337:
a386.
122. Racine A, Bijon A, Fournier A, et al. Menopausal hormone therapy
and risk of cholecystectomy: a prospective study based on the
French E3N cohort. CMAJ. 2013;185:555–561.
123. Hart AR, Luben R, Welch A, Bingham S, Khaw KT. Hormone
replacement therapy and symptomatic gallstones - a prospective
population study in the EPIC-Norfolk cohort. Digestion. 2008;
77:4–9.
124. Hendrix SL, Cochrane BB, Nygaard IE, et al. Effects of estrogen
with and without progestin on urinary incontinence. JAMA. 2005;
293:935–948.
125. Steinauer JE, Waetjen LE, Vittinghoff E, et al. Postmenopausal
hormone therapy: does it cause incontinence? Obstet Gynecol.
2005;106:940–945.
126. Moyer VA, U.S, Preventive Services Task Force. Medications to de-
crease the risk for breast cancer in women: recommendations from the
U.S. Preventive Task Force Recommendation Statement. Ann Intern
Med. 2013;159(10):698–708. http://www.ncbi.nlm.nih.gov/pubmed/
24061412 “Annals of internal medicine”
127. Boardman HM, Hartley L, Eisinga A, et al. Hormone therapy for
preventing cardiovascular disease in post-menopausal women. Co-
chrane Database Syst Rev. 2015;3:CD002229.
128. Grodstein F, Stampfer MJ, Colditz GA, et al. Postmenopausal hor-
mone therapy and mortality. N Engl J Med. 1997;336:1769 –1775.
129. Berglind IA, Andersen M, Citarella A, Linder M, Sundstro¨m A,
Kieler H. Hormone therapy and risk of cardiovascular outcomes
and mortality in women treated with statins. Menopause. 2015;
22:369–376.
130. Mikkola TS, Tuomikoski P, Lyytinen H, et al. Estradiol-based
postmenopausal hormone therapy and risk of cardiovascular and
all-cause mortality. Menopause. 2015;22(9):976–983.
131. Salpeter SR, Cheng J, Thabane L, Buckley NS, Salpeter EE. Bayes-
ian meta-analysis of hormone therapy and mortality in younger
postmenopausal women. Am J Med. 2009;122:1016–1022.e1.
132. Salpeter SR, Walsh JM, Greyber E, Ormiston TM, Salpeter EE.
Mortality associated with hormone replacement therapy in
younger and older women: a meta-analysis. J Gen Intern Med.
2004;19:791–804.
133. Maki PM. Critical window hypothesis of hormone therapy and
cognition: a scientific update on clinical studies. Menopause. 2013;
20:695–709.
134. Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin
and the incidence of dementia and mild cognitive impairment in
postmenopausal women: the Women’s Health Initiative Memory
Study: a randomized controlled trial. JAMA. 2003;289:2651–
2662.
135. Carlson MC, Zandi PP, Plassman BL, et al. Hormone replacement
therapy and reduced cognitive decline in older women: the Cache
County Study. Neurology. 2001;57:2210–2216.
136. Jacobs DM, Tang MX, Stern Y, et al. Cognitive function in non-
demented older women who took estrogen after menopause.
Neurology. 1998;50:368–373.
137. Sherwin BB, Tulandi T. “Add-back” estrogen reverses cognitive
deficits induced by a gonadotropin-releasing hormone agonist in
women with leiomyomata uteri. J Clin Endocrinol Metab. 1996;
81:2545–2549.
138. Phillips SM, Sherwin BB. Effects of estrogen on memory function
in surgically menopausal women. Psychoneuroendocrinology.
1992;17:485–495.
139. Kang JH, Weuve J, Grodstein F. Postmenopausal hormone therapy
and risk of cognitive decline in community-dwelling aging women.
Neurology. 2004;63:101–107.
140. Espeland MA, Rapp SR, Shumaker SA, et al. Conjugated equine
estrogens and global cognitive function in postmenopausal
women: Women’s Health Initiative Memory Study. JAMA. 2004;
291:2959–2968.
141. Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hor-
mone therapy and risk of cardiovascular disease by age and years
since menopause. JAMA. 2007;297:1465–1477.
142. Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke
statistics–2008 update: a report from the American Heart Associ-
ation Statistics Committee and Stroke Statistics Subcommittee.
Circulation. 2008;117:e25–e146.
143. Kariuki JK, Stuart-Shor EM, Leveille SG, Hayman LL. Evaluation
of the performance of existing non-laboratory based cardiovascu-
lar risk assessment algorithms. BMC Cardiovasc Disord. 2013;13:
123.
144. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA
guideline on the assessment of cardiovascular risk: a report of the
American College of Cardiology/American Heart Association
Task Force on Practice Guidelines. Circulation. 2014;129:S49–
S73.
145. Rossouw JE, Cushman M, Greenland P, et al. Inflammatory, lipid,
thrombotic, and genetic markers of coronary heart disease risk in
the Women’s Health Initiative trials of hormone therapy. Arch
Intern Med. 2008;168:2245–2253.
146. Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic
syndrome and risk of cancer: a systematic review and meta-anal-
ysis. Diabetes Care. 2012;35:2402–2411.
147. Wild RA, Wu C, Curb JD, et al. Coronary heart disease events in
the Women’s Health Initiative hormone trials: effect modification
by metabolic syndrome: a nested case-control study within the
Women’s Health Initiative randomized clinical trials. Menopause.
2013;20:254–260.
148. Szmuilowicz ED, Stuenkel CA, Seely EW. Influence of menopause
on diabetes and diabetes risk. Nat Rev Endocrinol. 2009;5:553–
558.
149. Curb JD, Prentice RL, Bray PF, et al. Venous thrombosis and con-
jugated equine estrogen in women without a uterus. Arch Intern
Med. 2006;166:772–780.
150. Cushman M, Kuller LH, Prentice R, et al. Estrogen plus progestin
and risk of venous thrombosis. JAMA. 2004;292:1573–1580.
151. Manson JE, Bassuk SS. The menopause transition and postmeno-
pausal hormone therapy. In: Longo DL, Fauci AS, Kasper DL, et al.
Harrison’s Principles of Internal Medicine. New York, NY:
McGraw Hill; 2012;3040–3045.
152. Stefanick ML, Anderson GL, Margolis KL, et al. Effects of conju-
gated equine estrogens on breast cancer and mammography
screening in postmenopausal women with hysterectomy. JAMA.
2006;295:1647–1657.
153. Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic
interventions for breast cancer risk reduction: American Society of
Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;
31:2942–2962.
154. National Cancer Institute. Breast Cancer Risk Assessment Tool.
http://www.cancer.gov/bcrisktool/. Accessed April 12, 2015.
155. Amir E, Freedman OC, Seruga B, Evans DG. Assessing women at
high risk of breast cancer: a review of risk assessment models. J Natl
Cancer Inst. 2010;102:680– 691.
156. Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model
incorporating familial and personal risk factors. Stat Med. 2004;
23:1111–1130.
doi: 10.1210/jc.2015-2236 press.endocrine.org/journal/jcem 4005
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018

157. Kerlikowske K, Cook AJ, Buist DS, et al. Breast cancer risk by
breast density, menopause, and postmenopausal hormone therapy
use. J Clin Oncol. 2010;28:3830–3837.
158. Hou N, Hong S, Wang W, Olopade OI, Dignam JJ, Huo D. Hor-
mone replacement therapy and breast cancer: heterogeneous risks
by race, weight, and breast density. J Natl Cancer Inst. 2013;105:
1365–1372.
159. Chen J, Pee D, Ayyagari R, et al. Projecting absolute invasive breast
cancer risk in white women with a model that includes mammo-
graphic density. J Natl Cancer Inst. 2006;98:1215–1226.
160. Bhavnani BR. Pharmacokinetics and pharmacodynamics of con-
jugated equine estrogens: chemistry and metabolism. Proc Soc Exp
Biol Med. 1998;217:6–16.
161. Torres-Santiago L, Mericq V, Taboada M, et al. Metabolic effects
of oral versus transdermal 17

-estradiol (E
2
): a randomized clin-
ical trial in girls with Turner syndrome. J Clin Endocrinol Metab.
2013;98:2716–2724.
162. Slater CC, Hodis HN, Mack WJ, Shoupe D, Paulson RJ, Stanczyk
FZ. Markedly elevated levels of estrone sulfate after long-term oral,
but not transdermal, administration of estradiol in postmeno-
pausal women. Menopause. 2001;8:200–203.
163. Nachtigall LE, Raju U, Banerjee S, Wan L, Levitz M. Serum es-
tradiol-binding profiles in postmenopausal women undergoing
three common estrogen replacement therapies: associations
with sex hormone-binding globulin, estradiol, and estrone lev-
els. Menopause. 2000;7:243–250.
164. Nelson HD. Commonly used types of postmenopausal estrogen for
treatment of hot flashes: scientific review. JAMA. 2004;291:1610 –
1620.
165. Crandall C. Low-dose estrogen therapy for menopausal women: a
review of efficacy and safety. J Womens Health (Larchmt). 2003;
12:723–747.
166. Mauvais-Jarvis P, Bercovici JP. Hormone therapy by percutane-
ous route. Physiological bases. Clinical applications [in French].
Therapeutique. 1972;48:403–406.
167. Vehkavaara S, Silveira A, Hakala-Ala-Pietila¨ T,etal.Effects of oral
and transdermal estrogen replacement therapy on markers of co-
agulation, fibrinolysis, inflammation and serum lipids and lipo-
proteins in postmenopausal women. Thromb Haemost. 2001;85:
619– 625.
168. Scarabin PY, Alhenc-Gelas M, Plu-Bureau G, Taisne P, Agher R,
Aiach M. Effects of oral and transdermal estrogen/progesterone
regimens on blood coagulation and fibrinolysis in postmenopausal
women. A randomized controlled trial. Arterioscler Thromb Vasc
Biol. 1997;17:3071–3078.
169. Loeper J, Loeper MJ, Ohlghiesser C, de Lignie`res B, Mauvais-
Jarvis P. The influence of estrogen therapy on triglycerides. Im-
portance of the choice of substance and the route of administration
(author’s translation) [in French]. Nouv Presse Med. 1977;6:
2747–2750.
170. Speroff L. Efficacy and tolerability of a novel estradiol vaginal ring
for relief of menopausal symptoms. Obstet Gynecol. 2003;102:
823–834.
171. Al-Azzawi F, Buckler HM, United Kingdom Vaginal Ring Inves-
tigator Group. Comparison of a novel vaginal ring delivering es-
tradiol acetate versus oral estradiol for relief of vasomotor meno-
pausal symptoms. Climacteric. 2003;6:118–127.
172. Jaakkola S, Lyytinen H, Pukkala E, Ylikorkala O. Endometrial
cancer in postmenopausal women using estradiol-progestin ther-
apy. Obstet Gynecol. 2009;114:1197–1204.
173. Soini T, Hurskainen R, Gre´nman S, Ma¨enpa¨a¨ J, Paavonen J, Puk-
kala E. Cancer risk in women using the levonorgestrel-releasing
intrauterine system in Finland. Obstet Gynecol. 2014;124:292–
299.
174. Schiff I, Tulchinsky D, Cramer D, Ryan KJ. Oral medroxyproges-
terone in the treatment of postmenopausal symptoms. JAMA.
1980;244:1443–1445.
175. Prior JC, Nielsen JD, Hitchcock CL, Williams LA, Vigna YM,
Dean CB. Medroxyprogesterone and conjugated oestrogen are
equivalent for hot flushes: a 1-year randomized double-blind trial
following premenopausal ovariectomy. Clin Sci. 2007;112:517–
525.
176. Hitchcock CL, Prior JC. Oral micronized progesterone for vaso-
motor symptoms–a placebo-controlled randomized trial in healthy
postmenopausal women. Menopause. 2012;19:886– 893.
177. The Endocrine Society. The Endocrine Society re-issues position
statement on bioidentical hormones. Press release. https://www.
endocrine.org/news-room/press-release-archives/2009/society
reissuespositionstatementonbioidenticalhormones. Published Febru-
ary 5, 2009. Accessed February 13, 2015.
178. Sharifi M, Lewiecki EM. Conjugated estrogens combined with ba-
zedoxifene: the first approved tissue selective estrogen complex
therapy. Expert Rev Clin Pharmacol. 2014;7:281–291.
179. Mirkin S, Komm BS, Pan K, Chines AA. Effects of bazedoxifene/
conjugated estrogens on endometrial safety and bone in postmeno-
pausal women. Climacteric. 2013;16:338–346.
180. Pinkerton JV, Harvey JA, Lindsay R, et al. Effects of bazedoxifene/
conjugated estrogens on the endometrium and bone: a randomized
trial. J Clin Endocrinol Metab. 2014;99:E189–E198.
181. Lobo RA, Pinkerton JV, Gass ML, et al. Evaluation of bazedox-
ifene/conjugated estrogens for the treatment of menopausal symp-
toms and effects on metabolic parameters and overall safety pro-
file. Fertil Steril. 2009;92:1025–1038.
182. Lindsay R, Gallagher JC, Kagan R, Pickar JH, Constantine G.
Efficacy of tissue-selective estrogen complex of bazedoxifene/con-
jugated estrogens for osteoporosis prevention in at-risk postmeno-
pausal women. Fertil Steril. 2009;92:1045–1052.
183. Kagan R, Williams RS, Pan K, Mirkin S, Pickar JH. A randomized,
placebo- and active-controlled trial of bazedoxifene/conjugated es-
trogens for treatment of moderate to severe vulvar/vaginal atrophy
in postmenopausal women. Menopause. 2010;17:281–289.
184. Utian W, Yu H, Bobula J, Mirkin S, Olivier S, Pickar JH. Baze-
doxifene/conjugated estrogens and quality of life in postmeno-
pausal women. Maturitas. 2009;63:329–335.
185. Abraham L, Pinkerton JV, Messig M, Ryan KA, Komm BS, Mirkin
S. Menopause-specific quality of life across varying menopausal
populations with conjugated estrogens/bazedoxifene. Maturitas.
2014;78:212–218.
186. Pinkerton JV, Harvey JA, Pan K, et al. Breast effects of bazedox-
ifene-conjugated estrogens: a randomized controlled trial. Obstet
Gynecol. 2013;121:959–968.
187. Harvey JA, Pinkerton JV, Baracat EC, Shi H, Chines AA, Mirkin
S. Breast density changes in a randomized controlled trial evalu-
ating bazedoxifene/conjugated estrogens. Menopause. 2013;20:
138–145.
188. Mirkin S, Archer DF, Taylor HS, Pickar JH, Komm BS. Differential
effects of menopausal therapies on the endometrium. Menopause.
2014;21:899–908.
189. Pickar JH, Yeh IT, Bachmann G, Speroff L. Endometrial effects of
a tissue selective estrogen complex containing bazedoxifene/con-
jugated estrogens as a menopausal therapy. Fertil Steril. 2009;92:
1018–1024.
190. de Villiers TJ, Chines AA, Palacios S, et al. Safety and tolerability
of bazedoxifene in postmenopausal women with osteoporosis: re-
sults of a 5-year, randomized, placebo-controlled phase 3 trial.
Osteoporos Int. 2011;22:567–576.
191. Komm BS, Thompson JR, Mirkin S. Cardiovascular safety of con-
jugated estrogens plus bazedoxifene: meta-analysis of the SMART
trials. Climacteric. 2015;18:503–511.
192. Kloosterboer HJ. Intracrinology: the secret of the tissue-specificity
of tibolone. J Br Menopause Soc. 2000;6:23–27.
193. Tiefert MA, Roy H, Moudrianakis EN. Binding of adenine nucle-
otides and pyrophosphate by the purified coupling factor of pho-
tophosphorylation. Biochemistry. 1977;16:2396–2404.
194. Formoso G, Perrone E, Maltoni S, et al. Short and long term effects
4006 Stuenkel et al Guideline on Menopause J Clin Endocrinol Metab, November 2015, 100(11):3975–4011
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018

of tibolone in postmenopausal women. Cochrane Database Syst
Rev. 2012;2:CD008536.
195. Davis SR. The effects of tibolone on mood and libido. Menopause.
2002;9:153–155.
196. Botsis D, Kassanos D, Kalogirou D, Antoniou G, Vitoratos N,
Karakitsos P. Vaginal ultrasound of the endometrium in post-
menopausal women with symptoms of urogenital atrophy on low-
dose estrogen or tibolone treatment: a comparison. Maturitas.
1997;26:57–62.
197. Swanson SG, Drosman S, Helmond FA, Stathopoulos VM. Tibo-
lone for the treatment of moderate to severe vasomotor symptoms
and genital atrophy in postmenopausal women: a multicenter, ran-
domized, double-blind, placebo-controlled study. Menopause.
2006;13:917–925.
198. Delmas PD, Davis SR, Hensen J, Adami S, van Os S, Nijland EA.
Effects of tibolone and raloxifene on bone mineral density in os-
teopenic postmenopausal women. Osteoporos Int. 2008;19:
1153–1160.
199. Cummings SR, Ettinger B, Delmas PD, et al. The effects of tibolone
in older postmenopausal women. N Engl J Med. 2008;359:697–
708.
200. Fedele L, Bianchi S, Raffaelli R, Zanconato G. A randomized study
of the effects of tibolone and transdermal estrogen replacement
therapy in postmenopausal women with uterine myomas. Eur J
Obstet Gynecol Reprod Biol. 2000;88:91–94.
201. Hammar M, Christau S, Nathorst-Bo¨o¨ s J, Rud T, Garre K. A
double-blind, randomised trial comparing the effects of tibolone
and continuous combined hormone replacement therapy in post-
menopausal women with menopausal symptoms. Br J Obstet
Gynaecol. 1998;105:904–911.
202. Hammar ML, van de Weijer P, Franke HR, et al. Tibolone and
low-dose continuous combined hormone treatment: vaginal bleed-
ing pattern, efficacy and tolerability. BJOG. 2007;114:1522–
1529.
203. Kenemans P, Bundred NJ, Foidart JM, et al. Safety and efficacy of
tibolone in breast-cancer patients with vasomotor symptoms: a
double-blind, randomised, non-inferiority trial. Lancet Oncol.
2009;10:135–146.
204. Haskell SG, Bean-Mayberry B, Gordon K. Discontinuing post-
menopausal hormone therapy: an observational study of tapering
versus quitting cold turkey: is there a difference in recurrence of
menopausal symptoms? Menopause. 2009;16:494– 499.
205. Suffoletto JA, Hess R. Tapering versus cold turkey: symptoms ver-
sus successful discontinuation of menopausal hormone therapy.
Menopause. 2009;16:436– 437.
206. Aslan E, Bagis T, Kilicdag EB, Tarim E, Erkanli S, Kuscu E. How
best is to discontinue postmenopausal hormone therapy: immedi-
ate or tapered? Maturitas. 2007;56:78– 83.
207. Haimov-Kochman R, Barak-Glantz E, Arbel R, et al. Gradual dis-
continuation of hormone therapy does not prevent the reappear-
ance of climacteric symptoms: a randomized prospective study.
Menopause. 2006;13:370–376.
208. Karim R, Dell RM, Greene DF, Mack WJ, Gallagher JC, Hodis
HN. Hip fracture in postmenopausal women after cessation of
hormone therapy: results from a prospective study in a large health
management organization. Menopause. 2011;18:1172–1177.
209. Arafah BM. Increased need for thyroxine in women with hypo-
thyroidism during estrogen therapy. N Engl J Med. 2001;344:
1743–1749.
210. Thurston RC, Sowers MR, Chang Y, et al. Adiposity and reporting
of vasomotor symptoms among midlife women: the study of wom-
en’s health across the nation. Am J Epidemiol. 2008;167:78– 85.
211. Thurston RC, Sowers MR, Sternfeld B, et al. Gains in body fat and
vasomotor symptom reporting over the menopausal transition: the
study of women’s health across the nation. Am J Epidemiol. 2009;
170:766–774.
212. Huang AJ, Subak LL, Wing R, et al. An intensive behavioral weight
loss intervention and hot flushes in women. Arch Intern Med.
2010;170:1161–1167.
213. Joffe H, Guthrie KA, LaCroix AZ, et al. Low-dose estradiol and the
serotonin-norepinephrine reuptake inhibitor venlafaxine for va-
somotor symptoms: a randomized clinical trial. JAMA Intern Med.
2014;174:1058–1066.
214. Kroenke CH, Caan BJ, Stefanick ML, et al. Effects of a dietary
intervention and weight change on vasomotor symptoms in the
Women’s Health Initiative. Menopause. 2012;19:980–988.
215. van Die MD, Teede HJ, Bone KM, Reece JE, Burger HG. Predictors
of placebo response in a randomized, controlled trial of phyto-
therapy in menopause. Menopause. 2009;16:792–796.
216. Villaseca P. Non-estrogen conventional and phytochemical treat-
ments for vasomotor symptoms: what needs to be known for prac-
tice. Climacteric. 2012;15:115–124.
217. Rada G, Capurro D, Pantoja T, et al. Non-hormonal interventions
for hot flushes in women with a history of breast cancer. Cochrane
Database Syst Rev. 2010;9:CD004923.
218. Albertazzi P. Non-estrogenic approaches for the treatment of cli-
macteric symptoms. Climacteric. 2007;10(suppl 2):115–120.
219. Loprinzi CL, Barton DL, Sloan JA, et al. Mayo Clinic and North
Central Cancer Treatment Group hot flash studies: a 20-year ex-
perience. Menopause. 2008;15:655–660.
220. Guttuso T Jr. Effective and clinically meaningful non-hormonal
hot flash therapies. Maturitas. 2012;72:6–12.
221. Grady D, Cohen B, Tice J, Kristof M, Olyaie A, Sawaya GF. In-
effectiveness of sertraline for treatment of menopausal hot flushes:
a randomized controlled trial. Obstet Gynecol. 2007;109:823–
830.
222. Kerwin JP, Gordon PR, Senf JH. The variable response of
women with menopausal hot flashes when treated with sertra-
line. Menopause. 2007;14:841–845.
223. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of
fluoxetine for treatment of hot flashes. J Clin Oncol. 2002;20:
1578–1583.
224. Loprinzi CL, Sloan J, Stearns V, et al. Newer antidepressants and
gabapentin for hot flashes: an individual patient pooled analysis.
J Clin Oncol. 2009;27:2831–2837.
225. Nedrow A, Miller J, Walker M, Nygren P, Huffman LH, Nelson
HD. Complementary and alternative therapies for the manage-
ment of menopause-related symptoms: a systematic evidence re-
view. Arch Intern Med. 2006;166:1453–1465.
226. Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: a sys-
tematic review and meta-analysis of randomized trials. J Gen
Intern Med. 2014;29:204–213.
227. Sun Z, Hao Y, Zhang M. Efficacy and safety of desvenlafaxine
treatment for hot flashes associated with menopause: a meta-anal-
ysis of randomized controlled trials. Gynecol Obstet Invest. 2013;
75:255–262.
228. Bardia A, Novotny P, Sloan J, Barton D, Loprinzi C. Efficacy of
nonestrogenic hot flash therapies among women stratified by breast
cancer history and tamoxifen use: a pooled analysis. Menopause.
2009;16:477–483.
229. Stearns V, Beebe KL, Iyengar M, Dube E. Paroxetine controlled
release in the treatment of menopausal hot flashes: a randomized
controlled trial. JAMA. 2003;289:2827–2834.
230. Stearns V, Slack R, Greep N, et al. Paroxetine is an effective treat-
ment for hot flashes: results from a prospective randomized clinical
trial. J Clin Oncol. 2005;23:6919– 6930.
231. Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in manage-
ment of hot flashes in survivors of breast cancer: a randomised
controlled trial. Lancet. 2000;356:2059–2063.
232. Evans ML, Pritts E, Vittinghoff E, McClish K, Morgan KS, Jaffe
RB. Management of postmenopausal hot flushes with venlafaxine
hydrochloride: a randomized, controlled trial. Obstet Gynecol.
2005;105:161–166.
233. Carpenter JS, Storniolo AM, Johns S, et al. Randomized, double-
doi: 10.1210/jc.2015-2236 press.endocrine.org/journal/jcem 4007
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018

blind, placebo-controlled crossover trials of venlafaxine for hot
flashes after breast cancer. Oncologist. 2007;12:124–135.
234. Simon JA, Portman DJ, Kaunitz AM, et al. Low-dose paroxetine
7.5 mg for menopausal vasomotor symptoms: two randomized
controlled trials. Menopause. 2013;20:1027–1035.
235. Kalay AE, Demir B, Haberal A, Kalay M, Kandemir O. Efficacy of
citalopram on climacteric symptoms. Menopause. 2007;14:223–
229.
236. Nelson HD, Vesco KK, Haney E, et al. Nonhormonal therapies for
menopausal hot flashes: systematic review and meta-analysis.
JAMA. 2006;295:2057–2071.
237. Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram
for hot flashes in healthy menopausal women: a randomized con-
trolled trial. JAMA. 2011;305:267–274.
238. Guttuso T Jr, Kurlan R, McDermott MP, Kieburtz K. Gabapentin’s
effects on hot flashes in postmenopausal women: a randomized
controlled trial. Obstet Gynecol. 2003;101:337–345.
239. Butt DA, Lock M, Lewis JE, Ross S, Moineddin R. Gabapentin for
the treatment of menopausal hot flashes: a randomized controlled
trial. Menopause. 2008;15:310–318.
240. Reddy SY, Warner H, Guttuso T Jr, et al. Gabapentin, estrogen,
and placebo for treating hot flushes: a randomized controlled trial.
Obstet Gynecol. 2006;108:41–48.
241. Pandya KJ, Morrow GR, Roscoe JA, et al. Gabapentin for hot
flashes in 420 women with breast cancer: a randomised double-
blind placebo-controlled trial. Lancet. 2005;366:818– 824.
242. Loprinzi CL, Qin R, Balcueva EP, et al. Phase III, randomized,
double-blind, placebo-controlled evaluation of pregabalin for al-
leviating hot flashes, N07C1. J Clin Oncol. 2010;28:641–647.
243. Bordeleau L, Pritchard KI, Loprinzi CL, et al. Multicenter, ran-
domized, cross-over clinical trial of venlafaxine versus gabapentin
for the management of hot flashes in breast cancer survivors. J Clin
Oncol. 2010;28:5147–5152.
244. Aguirre W, Chedraui P, Mendoza J, Ruilova I. Gabapentin vs.
low-dose transdermal estradiol for treating post-menopausal
women with moderate to very severe hot flushes. Gynecol Endo-
crinol. 2010;26:333–337.
245. Notelovitz M, Mattox JH. Suppression of vasomotor and vulvovag-
inal symptoms with continuous oral 17

-estradiol. Menopause.
2000;7:310–317.
246. Cohen LS, Joffe H, Guthrie KA, et al. Efficacy of omega-3 for
vasomotor symptoms treatment: a randomized controlled trial.
Menopause. 2014;21:347–354.
247. Elkins GR, Fisher WI, Johnson AK, Carpenter JS, Keith TZ. Clin-
ical hypnosis in the treatment of postmenopausal hot flashes: a
randomized controlled trial. Menopause. 2013;20:291–298.
248. Caan BJ, Natarajan L, Parker B, et al. Soy food consumption and
breast cancer prognosis. Cancer Epidemiol Biomarkers Prev.
2011;20:854– 858.
249. Loprinzi CL, Abu-Ghazaleh S, Sloan JA, et al. Phase III randomized
double-blind study to evaluate the efficacy of a polycarbophil-
based vaginal moisturizer in women with breast cancer. J Clin
Oncol. 1997;15:969–973.
250. Biglia N, Peano E, Sgandurra P, et al. Low-dose vaginal estrogens
or vaginal moisturizer in breast cancer survivors with urogenital
atrophy: a preliminary study. Gynecol Endocrinol. 2010;26:404–
412.
251. van der Laak JA, de Bie LM, de Leeuw H, de Wilde PC, Hanselaar
AG. The effect of Replens on vaginal cytology in the treatment of
postmenopausal atrophy: cytomorphology versus computerised
cytometry. J Clin Pathol. 2002;55:446– 451.
252. Nachtigall LE. Comparative study: Replens versus local estrogen in
menopausal women. Fertil Steril. 1994;61:178–180.
253. Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the
symptomatic treatment of vaginal atrophy in postmenopausal
women. Maturitas. 1996;23:259–263.
254. Fiorilli A, Molteni B, Milani M. Successful treatment of bacterial
vaginosis with a policarbophil-carbopol acidic vaginal gel: results
from a randomised double-blind, placebo-controlled trial. Eur J
Obstet Gynecol Reprod Biol. 2005;120:202–205.
255. Le Donne M, Caruso C, Mancuso A, et al. The effect of vaginally
administered genistein in comparison with hyaluronic acid on atro-
phic epithelium in postmenopause. Arch Gynecol Obstet. 2011;
283:1319–1323.
256. Ekin M, Yas¸ar L, Savan K, et al. The comparison of hyaluronic acid
vaginal tablets with estradiol vaginal tablets in the treatment of
atrophic vaginitis: a randomized controlled trial. Arch Gynecol
Obstet. 2011;283:539–543.
257. Caswell M, Kane M. Comparison of the moisturization efficacy of
two vaginal moisturizers: pectin versus polycarbophil technolo-
gies. J Cosmet Sci. 2002;53:81–87.
258. Jozkowski KN, Herbenick D, Schick V, Reece M, Sanders SA,
Fortenberry JD. Women’s perceptions about lubricant use and vag-
inal wetness during sexual activities. J Sex Med. 2013;10:484–
492.
259. Juraskova I, Jarvis S, Mok K, et al. The acceptability, feasibility,
and efficacy (phase I/II study) of the OVERcome (Olive Oil, Vag-
inal Exercise, and MoisturizeR) intervention to improve dyspareu-
nia and alleviate sexual problems in women with breast cancer.
J Sex Med. 2013;10:2549–2558.
260. Brown JM, Hess KL, Brown S, Murphy C, Waldman AL, Hezareh
M. Intravaginal practices and risk of bacterial vaginosis and can-
didiasis infection among a cohort of women in the United States.
Obstet Gynecol. 2013;121:773–780.
261. Suckling J, Lethaby A, Kennedy R. Local oestrogen for vaginal
atrophy in postmenopausal women. Cochrane Database Syst Rev.
2006;4:CD001500.
262. Bachmann G, Lobo RA, Gut R, Nachtigall L, Notelovitz M. Effi-
cacy of low-dose estradiol vaginal tablets in the treatment of atro-
phic vaginitis: a randomized controlled trial. Obstet Gynecol.
2008;111:67–76.
263. Simon J, Nachtigall L, Gut R, Lang E, Archer DF, Utian W. Ef-
fective treatment of vaginal atrophy with an ultra-low-dose estra-
diol vaginal tablet. Obstet Gynecol. 2008;112:1053–1060.
264. Lose G, Englev E. Oestradiol-releasing vaginal ring versus oestriol
vaginal pessaries in the treatment of bothersome lower urinary
tract symptoms. BJOG. 2000;107:1029–1034.
265. Crandall C. Vaginal estrogen preparations: a review of safety and
efficacy for vaginal atrophy. J Womens Health (Larchmt). 2002;
11:857–877.
266. Bachmann G, Bouchard C, Hoppe D, et al. Efficacy and safety of
low-dose regimens of conjugated estrogens cream administered
vaginally. Menopause. 2009;16:719–727.
267. Santen RJ, Pinkerton JV, Conaway M, et al. Treatment of uro-
genital atrophy with low-dose estradiol: preliminary results.
Menopause. 2002;9:179–187.
268. Raz R, Stamm WE. A controlled trial of intravaginal estriol in
postmenopausal women with recurrent urinary tract infections.
N Engl J Med. 1993;329:753–756.
269. Eriksen B. A randomized, open, parallel-group study on the pre-
ventive effect of an estradiol-releasing vaginal ring (Estring) on
recurrent urinary tract infections in postmenopausal women. Am J
Obstet Gynecol. 1999;180:1072–1079.
270. Nelken RS, Ozel BZ, Leegant AR, Felix JC, Mishell DR Jr. Ran-
domized trial of estradiol vaginal ring versus oral oxybutynin for
the treatment of overactive bladder. Menopause. 2011;18:962–
966.
271. Cody JD, Jacobs ML, Richardson K, Moehrer B, Hextall A. Oes-
trogen therapy for urinary incontinence in post-menopausal
women. Cochrane Database Syst Rev. 2012;10:CD001405.
272. de Tayrac R, Sentilhes L. Complications of pelvic organ prolapse
surgery and methods of prevention. Int Urogynecol J. 2013;24:
1859–1872.
273. Rahn DD, Good MM, Roshanravan SM, et al. Effects of preop-
erative local estrogen in postmenopausal women with prolapse: a
randomized trial. J Clin Endocrinol Metab. 2014;99:3728–3736.
4008 Stuenkel et al Guideline on Menopause J Clin Endocrinol Metab, November 2015, 100(11):3975–4011
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018

274. Santen RJ. Vaginal administration of estradiol: effects of dose,
preparation and timing on plasma estradiol levels. Climacteric.
2015;18:121–134.
275. Eugster-Hausmann M, Waitzinger J, Lehnick D. Minimized estra-
diol absorption with ultra-low-dose 10 microg 17

-estradiol vag-
inal tablets. Climacteric. 2010;13:219–227.
276. Dorr MB, Nelson AL, Mayer PR, et al. Plasma estrogen concen-
trations after oral and vaginal estrogen administration in women
with atrophic vaginitis. Fertil Steril. 2010;94:2365–2368.
277. Pschera H, Hjerpe A, Carlstro¨mK.Influence of the maturity of the
vaginal epithelium upon the absorption of vaginally administered
estradiol-17

and progesterone in postmenopausal women.
Gynecol Obstet Invest. 1989;27:204–207.
278. Naessen T, Rodriguez-Macias K, Lithell H. Serum lipid profile
improved by ultra-low doses of 17

-estradiol in elderly women.
J Clin Endocrinol Metab. 2001;86:2757–2762.
279. Ballagh SA. Vaginal hormone therapy for urogenital and meno-
pausal symptoms. Semin Reprod Med. 2005;23:126–140.
280. Management of symptomatic vulvovaginal atrophy: 2013 po-
sition statement of The North American Menopause Society.
Menopause. 2013;20:888–902; quiz 903–904.
281. Archer DF. Efficacy and tolerability of local estrogen therapy for
urogenital atrophy. Menopause. 2010;17:194–203.
282. Del Pup L, Di Francia R, Cavaliere C, et al. Promestriene, a specific
topic estrogen. Review of 40 years of vaginal atrophy treatment: is
it safe even in cancer patients? Anticancer Drugs. 2013;24:989–
998.
283. Labrie F, Cusan L, Gomez JL, et al. Effect of one-week treatment
with vaginal estrogen preparations on serum estrogen levels in
postmenopausal women. Menopause. 2009;16:30–36.
284. Rigg LA, Hermann H, Yen SS. Absorption of estrogens from vag-
inal creams. N Engl J Med. 1978;298:195–197.
285. Mandel FP, Geola FL, Meldrum DR, et al. Biological effects of
various doses of vaginally administered conjugated equine estro-
gens in postmenopausal women. J Clin Endocrinol Metab. 1983;
57:133–139.
286. van Haaften M, Donker GH, Haspels AA, Thijssen JH. Oestrogen
concentrations in plasma, endometrium, myometrium and vagina
of postmenopausal women, and effects of vaginal oestriol (E3) and
oestradiol (E2) applications. J Steroid Biochem. 1989;33:647–
653.
287. Kicovic PM, Cortes-Prieto J, Milojeviæ S, Haspels AA, Aljinovic A.
The treatment of postmenopausal vaginal atrophy with Ovestin
vaginal cream or suppositories: clinical, endocrinological and
safety aspects. Maturitas. 1980;2:275–282.
288. Manson JE, Goldstein SR, Kagan R, et al. Why the product labeling
for low-dose vaginal estrogen should be changed. Menopause.
2014;21:911–916.
289. Fischer G, Bradford J. Vulvovaginal candidiasis in postmeno-
pausal women: the role of hormone replacement therapy. J Low
Genit Tract Dis. 2011;15:263–267.
290. Dennerstein GJ, Ellis DH. Oestrogen, glycogen and vaginal can-
didiasis. Aust N Z J Obstet Gynaecol. 2001;41:326–328.
291. Obiorah I, Jordan VC. Scientific rationale for postmenopause de-
lay in the use of conjugated equine estrogens among postmeno-
pausal women that causes reduction in breast cancer incidence and
mortality. Menopause. 2013;20:372–382.
292. Mastaglia SR, Bagur A, Royer M, Yankelevich D, Sayegh F, Oliveri
B. Effect of endogenous estradiol levels on bone resorption and
bone mineral density in healthy postmenopausal women: a pro-
spective study. Climacteric. 2009;12:49–58.
293. Bagur A, Oliveri B, Mautalen C, et al. Low levels of endogenous
estradiol protect bone mineral density in young postmenopausal
women. Climacteric. 2004;7:181–188.
294. Le Ray I, Dell’Aniello S, Bonnetain F, Azoulay L, Suissa S. Local
estrogen therapy and risk of breast cancer recurrence among hor-
mone-treated patients: a nested case-control study. Breast Cancer
Res Treat. 2012;135:603–609.
295. Barakat RR, Bundy BN, Spirtos NM, et al. Randomized double-
blind trial of estrogen replacement therapy versus placebo in stage
I or II endometrial cancer: a Gynecologic Oncology Group Study.
J Clin Oncol. 2006;24:587–592.
296. Guidozzi F. Estrogen therapy in gynecological cancer survivors.
Climacteric. 2013;16:611–617.
297. North American Menopause Society. Menopause Practice: A Cli-
nician’s Guide. 5th ed. Cleveland, OH: North American Meno-
pause Society; 2014:152.
298. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamox-
ifen vs raloxifene on the risk of developing invasive breast cancer
and other disease outcomes: the NSABP Study of Tamoxifen and
Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–2741.
299. Land SR, Wickerham DL, Costantino JP, et al. Patient-reported
symptoms and quality of life during treatment with tamoxifen or
raloxifene for breast cancer prevention: the NSABP Study of
Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:
2742–2751.
300. Davies GC, Huster WJ, Lu Y, Plouffe L Jr, Lakshmanan M. Ad-
verse events reported by postmenopausal women in controlled tri-
als with raloxifene. Obstet Gynecol. 1999;93:558–565.
301. Stovall DW, Utian WH, Gass ML, et al. The effects of combined
raloxifene and oral estrogen on vasomotor symptoms and endo-
metrial safety. Menopause. 2007;14:510–517.
302. Pinkerton JV, Shifren JL, La Valleur J, Rosen A, Roesinger M,
Siddhanti S. Influence of raloxifene on the efficacy of an estradiol-
releasing ring for treating vaginal atrophy in postmenopausal
women. Menopause. 2003;10:45–52.
303. Parsons A, Merritt D, Rosen A, et al. Effect of raloxifene on the
response to conjugated estrogen vaginal cream or nonhormonal
moisturizers in postmenopausal vaginal atrophy. Obstet Gynecol.
2003;101:346–352.
304. Ulrich LS, Naessen T, Elia D, et al. Endometrial safety of ultra-
low-dose Vagifem 10 microg in postmenopausal women with vag-
inal atrophy. Climacteric. 2010;13:228–237.
305. Cicinelli E, Di Naro E, De Ziegler D, et al. Placement of the vaginal
17

-estradiol tablets in the inner or outer one third of the vagina
affects the preferential delivery of 17

-estradiol toward the uterus
or periurethral areas, thereby modifying efficacy and endometrial
safety. Am J Obstet Gynecol. 2003;189:55–58.
306. Tourgeman DE, Boostanfar R, Chang L, Lu J, Stanczyk FZ,
Paulson RJ. Is there evidence for preferential delivery of ovarian
estradiol to the endometrium? Fertil Steril. 2001;75:1156–1158.
307. Fanchin R, De Ziegler D, Bergeron C, Righini C, Torrisi C, Fry-
dman R. Transvaginal administration of progesterone. Obstet Gy-
necol. 1997;90:396– 401.
308. Ross D, Cooper AJ, Pryse-Davies J, Bergeron C, Collins WP,
Whitehead MI. Randomized, double-blind, dose-ranging study of
the endometrial effects of a vaginal progesterone gel in estrogen-
treated postmenopausal women. Am J Obstet Gynecol. 1997;177:
937–941.
309. De Ziegler D, Bulletti C, De Monstier B, Ja¨a¨skela¨inen AS. The first
uterine pass effect. Ann NY Acad Sci. 1997;828:291–299.
310. Portman DJ, Bachmann GA, Simon JA, Ospemifene Study Group.
Ospemifene, a novel selective estrogen receptor modulator for
treating dyspareunia associated with postmenopausal vulvar and
vaginal atrophy. Menopause. 2013;20:623–630.
311. Bachmann GA, Komi JO, Ospemifene Study Group. Ospemifene
effectively treats vulvovaginal atrophy in postmenopausal women:
results from a pivotal phase 3 study. Menopause. 2010;17:480–
486.
312. Constantine G, Graham S, Portman DJ, Rosen RC, Kingsberg SA.
Female sexual function improved with ospemifene in postmeno-
pausal women with vulvar and vaginal atrophy: results of a ran-
domized, placebo-controlled trial. Climacteric. 2015;18:226–
232.
313. Simon JA, Lin VH, Radovich C, Bachmann GA, Ospemifene Study
Group. One-year long-term safety extension study of ospemifene
doi: 10.1210/jc.2015-2236 press.endocrine.org/journal/jcem 4009
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018

for the treatment of vulvar and vaginal atrophy in postmenopausal
women with a uterus. Menopause. 2013;20:418– 427.
314. Simon J, Portman D, Mabey RG Jr, Ospemifene Study Group.
Long-term safety of ospemifene (52-week extension) in the treat-
ment of vulvar and vaginal atrophy in hysterectomized postmeno-
pausal women. Maturitas. 2014;77:274–281.
315. Shionogi Inc. Highlights of prescribing information for Osphena.
http://www.shionogi.com/pdf/PI/Osphena-PI.pdf. Accessed Sep-
tember 15, 2015.
316. Singh M. Early age of natural menopause in India, a biological
marker for early preventive health programs. Climacteric. 2012;
15:581–586.
317. Ang SB, How CH. Menopause: an important milestone in women’s
health. Singapore Med J. 2013;54:60– 63.
318. Gold EB, Crawford SL, Avis NE, et al. Factors related to age at
natural menopause: longitudinal analyses from SWAN. Am J Epi-
demiol. 2013;178:70– 83.
319. Hale GE, Robertson DM, Burger HG. The perimenopausal wom-
an: endocrinology and management. J Steroid Biochem Mol Biol.
2014;142:121–131.
320. Nelson LM. Clinical practice. Primary ovarian insufficiency.
N Engl J Med. 2009;360:606– 614.
321. Kalantaridou SN, Davis SR, Nelson LM. Premature ovarian fail-
ure. Endocrinol Metab Clin North Am. 1998;27:989–1006.
322. Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for
breast cancer chemoprevention with raloxifene or tamoxifen for
women age 50 years or older. J Clin Oncol. 2011;29:2327–2333.
323. Levine M, Moutquin JM, Walton R, et al. Chemoprevention of
breast cancer. A joint guideline from the Canadian Task Force on
Preventive Health Care and the Canadian Breast Cancer Initiative’s
Steering Committee on Clinical Practice Guidelines for the Care
and Treatment of Breast Cancer. CMAJ. 2001;164:1681–1690.
324. Taku K, Melby MK, Kronenberg F, Kurzer MS, Messina M. Ex-
tracted or synthesized soybean isoflavones reduce menopausal hot
flash frequency and severity: systematic review and meta-analysis
of randomized controlled trials. Menopause. 2012;19:776–790.
325. Bolan˜ os R, Del Castillo A, Francia J. Soy isoflavones versus pla-
cebo in the treatment of climacteric vasomotor symptoms: system-
atic review and meta-analysis. Menopause. 2010;17:660– 666.
326. Jacobs A, Wegewitz U, Sommerfeld C, Grossklaus R, Lampen A.
Efficacy of isoflavones in relieving vasomotor menopausal symp-
toms - a systematic review. Mol Nutr Food Res. 2009;53:1084–
1097.
327. Eden JA. Phytoestrogens for menopausal symptoms: a review.
Maturitas. 2012;72:157–159.
328. Lethaby AE, Marjoribanks J, Kronenberg F, Roberts H, Eden J,
Brown J. Phytoestrogens for vasomotor menopausal symptoms.
Cochrane Database Syst Rev. 2007;12:CD001395.
329. Van Patten CL, Olivotto IA, Chambers GK, et al. Effect of soy
phytoestrogens on hot flashes in postmenopausal women with
breast cancer: a randomized, controlled clinical trial. J Clin Oncol.
2002;20:1449–1455.
330. Upmalis DH, Lobo R, Bradley L, Warren M, Cone FL, Lamia CA.
Vasomotor symptom relief by soy isoflavone extract tablets in
postmenopausal women: a multicenter, double-blind, random-
ized, placebo-controlled study. Menopause. 2000;7:236–242.
331. Quella SK, Loprinzi CL, Barton DL, et al. Evaluation of soy phy-
toestrogens for the treatment of hot flashes in breast cancer sur-
vivors: a North Central Cancer Treatment Group Trial. J Clin
Oncol. 2000;18:1068–1074.
332. Kronenberg F, Fugh-Berman A. Complementary and alternative
medicine for menopausal symptoms: a review of randomized, con-
trolled trials. Ann Intern Med. 2002;137:805–813.
333. Nikander E, Kilkkinen A, Metsa¨ -Heikkila¨ M,etal.A randomized
placebo-controlled crossover trial with phytoestrogens in treat-
ment of menopause in breast cancer patients. Obstet Gynecol.
2003;101:1213–1220.
334. MacGregor CA, Canney PA, Patterson G, McDonald R, Paul J. A
randomised double-blind controlled trial of oral soy supplements
versus placebo for treatment of menopausal symptoms in patients
with early breast cancer. Eur J Cancer. 2005;41:708–714.
335. Levis S, Strickman-Stein N, Ganjei-Azar P, Xu P, Doerge DR,
Krischer J. Soy isoflavones in the prevention of menopausal bone
loss and menopausal symptoms: a randomized, double-blind trial.
Arch Intern Med. 2011;171:1363–1369.
336. Murkies AL, Wilcox G, Davis SR. Clinical review 92: Phytoestro-
gens. J Clin Endocrinol Metab. 1998;83:297–303.
337. Jenks BH, Iwashita S, Nakagawa Y, et al. A pilot study on the
effects of S-equol compared to soy isoflavones on menopausal hot
flash frequency. J Womens Health (Larchmt). 2012;21:674– 682.
338. North American Menopause Society. The role of soy isoflavones in
menopausal health: report of The North American Menopause
Society/Wulf H. Utian Translational Science Symposium in Chi-
cago, IL (October 2010). Menopause. 2011;18:732–753.
339. Dodin S, Lemay A, Jacques H, Le´gare´ F, Forest JC, Maˆsse B. The
effects of flaxseed dietary supplement on lipid profile, bone mineral
density, and symptoms in menopausal women: a randomized, dou-
ble-blind, wheat germ placebo-controlled clinical trial. J Clin En-
docrinol Metab. 2005;90:1390–1397.
340. Pruthi S, Qin R, Terstreip SA, et al. A phase III, randomized, pla-
cebo-controlled, double-blind trial of flaxseed for the treatment of
hot flashes: North Central Cancer Treatment Group N08C7.
Menopause. 2012;19:48–53.
341. Tice JA, Ettinger B, Ensrud K, Wallace R, Blackwell T, Cummings
SR. Phytoestrogen supplements for the treatment of hot flashes: the
Isoflavone Clover Extract (ICE) Study: a randomized controlled
trial. JAMA. 2003;290:207–214.
342. Barton DL, Loprinzi CL, Quella SK, et al. Prospective evaluation
of vitamin E for hot flashes in breast cancer survivors. J Clin Oncol.
1998;16:495–500.
343. Ziaei S, Kazemnejad A, Zareai M. The effect of vitamin E on hot
flashes in menopausal women. Gynecol Obstet Invest. 2007;64:
204–207.
344. Newton KM, Buist DS, Keenan NL, Anderson LA, LaCroix AZ.
Use of alternative therapies for menopause symptoms: results of a
population-based survey. Obstet Gynecol. 2002;100:18–25.
345. Keenan NL, Mark S, Fugh-Berman A, Browne D, Kaczmarczyk J,
Hunter C. Severity of menopausal symptoms and use of both con-
ventional and complementary/alternative therapies. Menopause.
2003;10:507–515.
346. Leach MJ, Moore V. Black cohosh (Cimicifuga spp.) for menopausal
symptoms. Cochrane Database Syst Rev. 2012;9:CD007244.
347. Laakmann E, Grajecki D, Doege K, zu Eulenburg C, Buhling KJ.
Efficacy of Cimicifuga racemosa,Hypericum perforatum and Ag-
nus castus in the treatment of climacteric complaints: a systematic
review. Gynecol Endocrinol. 2012;28:703–709.
348. Frei-Kleiner S, Schaffner W, Rahlfs VW, Bodmer Ch, Birkha¨user M.
Cimicifuga racemosa dried ethanolic extract in menopausal disorders:
a double-blind placebo-controlled clinical trial. Maturitas. 2005;51:
397–404.
349. Osmers R, Friede M, Liske E, Schnitker J, Freudenstein J, Henne-
icke-von Zepelin HH. Efficacy and safety of isopropanolic black
cohosh extract for climacteric symptoms. Obstet Gynecol. 2005;
105:1074–1083.
350. Verhoeven MO, van der Mooren MJ, van de Weijer PH, et al. Effect
of a combination of isoflavones and Actaea racemosa Linnaeus on
climacteric symptoms in healthy symptomatic perimenopausal
women: a 12-week randomized, placebo-controlled, double-blind
study. Menopause. 2005;12:412–420.
351. Pockaj BA, Gallagher JG, Loprinzi CL, et al. Phase III double-
blind, randomized, placebo-controlled crossover trial of black co-
hosh in the management of hot flashes: NCCTG Trial N01CC1.
J Clin Oncol. 2006;24:2836–2841.
352. Newton KM, Reed SD, LaCroix AZ, Grothaus LC, Ehrlich K,
Guiltinan J. Treatment of vasomotor symptoms of menopause
4010 Stuenkel et al Guideline on Menopause J Clin Endocrinol Metab, November 2015, 100(11):3975–4011
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018

with black cohosh, multibotanicals, soy, hormone therapy, or pla-
cebo: a randomized trial. Ann Intern Med. 2006;145:869– 879.
353. Kim DI, Jeong JC, Kim KH, et al. Acupuncture for hot flushes in
perimenopausal and postmenopausal women: a randomised,
sham-controlled trial. Acupunct Med. 2011;29:249–256.
354. Avis NE, Legault C, Coeytaux RR, et al. A randomized, controlled
pilot study of acupuncture treatment for menopausal hot flashes.
Menopause. 2008;15:1070–1078.
355. Cho SH, Whang WW. Acupuncture for vasomotor menopausal
symptoms: a systematic review. Menopause. 2009;16:1065–1073.
356. Lee MS, Shin BC, Ernst E. Acupuncture for treating menopausal
hot flushes: a systematic review. Climacteric. 2009;12:16–25.
357. Daley A, Stokes-Lampard H, Macarthur C. Exercise for vasomotor
menopausal symptoms. Cochrane Database Syst Rev. 2011;5:
CD006108.
358. van Gastel P, Kallewaard JW, van der Zanden M, de Boer H.
Stellate-ganglion block as a treatment for severe postmenopausal
flushing. Climacteric. 2013;16:41–47.
359. Carpenter JS, Burns DS, Wu J, et al. Paced respiration for vaso-
motor and other menopausal symptoms: a randomized, controlled
trial. J Gen Intern Med. 2013;28:193–200.
360. Sood R, Sood A, Wolf SL, et al. Paced breathing compared with
usual breathing for hot flashes. Menopause. 2013;20:179–184.
361. Carmody JF, Crawford S, Salmoirago-Blotcher E, Leung K,
Churchill L, Olendzki N. Mindfulness training for coping with hot
flashes: results of a randomized trial. Menopause. 2011;18:611–
620.
362. Ayers B, Smith M, Hellier J, Mann E, Hunter MS. Effectiveness of
group and self-help cognitive behavior therapy in reducing prob-
lematic menopausal hot flushes and night sweats (MENOS 2): a
randomized controlled trial. Menopause. 2012;19:749–759.
363. Maki PM. New data on mindfulness-based stress reduction for hot
flashes: how do alternative therapies compare with selective sero-
tonin reuptake inhibitors? Menopause. 2011;18:596–598.
364. Duijts SF, van Beurden M, Oldenburg HS, et al. Efficacy of cog-
nitive behavioral therapy and physical exercise in alleviating treat-
ment-induced menopausal symptoms in patients with breast can-
cer: results of a randomized, controlled, multicenter trial. J Clin
Oncol. 2012;30:4124– 4133.
365. Freedman RR, Woodward S. Behavioral treatment of menopausal
hot flushes: evaluation by ambulatory monitoring. Am J Obstet
Gynecol. 1992;167:436– 439.
366. Norton S, Chilcot J, Hunter MS. Cognitive-behavior therapy for
menopausal symptoms (hot flushes and night sweats): moderators
and mediators of treatment effects. Menopause. 2014;21:574–
578.
367. Chilcot J, Norton S, Hunter MS. Cognitive behaviour therapy for
menopausal symptoms following breast cancer treatment: who
benefits and how does it work? Maturitas. 2014;78:56– 61.
368. Santen RJ, Stuenkel CA, Burger HG, Manson JE. Competency in
menopause management: whither goest the internist? J Womens
Health (Larchmt). 2014;23(4):281–285.
doi: 10.1210/jc.2015-2236 press.endocrine.org/journal/jcem 4011
Downloaded from https://academic.oup.com/jcem/article-abstract/100/11/3975/2836060
by guest
on 04 April 2018