Telecommunication.Imp.Guide.VD.0
User Manual:
Open the PDF directly: View PDF ![]() .
.
Page Count: 1004 [warning: Documents this large are best viewed by clicking the View PDF Link!]

TELECOMMUNICATION STANDARD
IMPLEMENTATION GUIDE
VERSION D.Ø
This document provides guidelines for implementing the NCPDP Telecommunication Standard Format to ensure a
consistent implementation of the standard.
Approval Date for ANS: August 7, 2ØØ7
August 2ØØ7
National Council for Prescription Drug Programs
924Ø East Raintree Drive
Scottsdale, AZ 8526Ø
Phone: (48Ø) 477-1ØØØ
Fax: (48Ø) 767-1Ø42
E-mail: ncpdp@ncpdp.org
http: www.ncpdp.org
Version D.Ø August 2ØØ7
**OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 2 -
Telecommunication Standard Implementation Guide
Version D.Ø
NCPDP recognizes the confidentiality of certain information exchanged electronically through the use of its standards.
Users should be familiar with the federal, state, and local laws, regulations and codes requiring confidentiality of this
information and should utilize the standards accordingly.
NOTICE: In addition, this NCPDP Standard contains certain data fields and elements that may be completed by users
with the proprietary information of third parties. The use and distribution of third parties' proprietary information without
such third parties' consent, or the execution of a license or other agreement with such third party, could subject the user to
numerous legal claims. All users are encouraged to contact such third parties to determine whether such
information is proprietary and if necessary, to consult with legal counsel to make arrangements for the use and
distribution of such proprietary information.
Published by:
National Council for Prescription Drug Programs
Publication History:
Version 1.Ø September 1, 1988
Version 2.Ø December 1, 1989
Version 3.1 February 5, 1991
Version 3.2 February 11, 1992
Version 5.Ø June 1999
Version 5.1, September 1999
Version 5.2, May 2ØØØ
Version 5.3, June 2ØØØ
Version 5.4, September 2ØØØ
Version 5.5, November 2ØØØ
Version 5.6, August 2ØØ1
Version 6.Ø January, 2ØØ2
Version 7.Ø January, 2ØØ2
Version 7.1, June, 2ØØ2
Version 8.Ø February, 2ØØ3
Version 8.1 August, 2ØØ3
Version 8.2 October, 2ØØ3
Version 8.3 October, 2ØØ3
Version 9.Ø May, 2ØØ4
Version A.Ø August, 2ØØ4
Version A.1 October, 2ØØ4
Version B.Ø May, 2ØØ5
Version C.Ø July, 2ØØ5
Version C.1 October, 2ØØ5
Version C.2 June 2ØØ6
Version C.3 September 2ØØ6
Version C.4 January 2ØØ7
Version D.ØJuly, 2ØØ7, August 2ØØ7
Copyright © 2ØØ7
All rights reserved.
No part of this manual may be reproduced in any form
or by any means without permission in writing from:
National Council for Prescription Drug Programs
924Ø E. Raintree Drive
Scottsdale, AZ 8526Ø
(48Ø) 477-1ØØØ
ncpdp@ncpdp.org

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 3 -
TABLE OF CONTENTS
1. INTRODUCTION ................................................................................................................................................................................................................31
1.1 DOCUMENT SCOPE ...............................................................................................................................................................................31
2. BACKGROUND..................................................................................................................................................................................................................33
3. BUSINESS ENVIRONMENT............................................................................................................................................................................................34
3.1 OBJECTIVES .........................................................................................................................................................................................34
3.2 PARTICIPANTS ......................................................................................................................................................................................34
3.2.1 Between Providers and Adjudicators.........................................................................................................................................34
3.2.2 Between Adjudicators (Payer-to-Payer).....................................................................................................................................35
4. BUSINESS FUNCTIONS..................................................................................................................................................................................................37
4.1 INTRODUCTION .....................................................................................................................................................................................37
4.2 MEDICAID SUBROGATION ......................................................................................................................................................................37
5. TERMINOLOGY USED THROUGHOUT.......................................................................................................................................................................38
5.1 TABLE DESIGNATION – LEGEND ............................................................................................................................................................38
5.2 TABLE DESIGNATION.............................................................................................................................................................................39
5.3 TRANSMISSION DISCUSSION..................................................................................................................................................................39
6. ELIGIBILITY VERIFICATION INFORMATION.............................................................................................................................................................41
6.1 ELIGIBILITY VERIFICATION.....................................................................................................................................................................41
6.1.1 Medicare Part D Eligibility ...........................................................................................................................................................41
6.1.1.1 Business Rules for Medicare Part D Eligibility Transactions between the Pharmacy and the Facilitator ................................. 41
6.2 ELIGIBILITY VERIFICATION REQUEST DIAGRAMS ....................................................................................................................................42
6.2.1 Diagram For Transmission Of Eligibility Verification Transaction ..........................................................................................42
6.3 ELIGIBILITY VERIFICATION REQUEST SEGMENTS....................................................................................................................................42
6.3.1 Transaction Header Segment (Eligibility Verification)..............................................................................................................42
6.3.2 Insurance Segment (Eligibility Verification)...............................................................................................................................43
6.3.3 Patient Segment (Eligibility Verification)....................................................................................................................................44
6.3.4 Pharmacy Provider Segment (Eligibility Verification)...............................................................................................................45
6.3.5 Prescriber Segment (Eligibility Verification)..............................................................................................................................45
6.3.6 Additional Documentation Segment (Eligibility Verification)...................................................................................................46
6.4 ELIGIBILITY VERIFICATION RESPONSE DIAGRAMS AND SEGMENTS .........................................................................................................47
6.4.1 Transmission Accepted/Transaction Approved........................................................................................................................47
6.4.1.1 Diagram For Transmission Of Eligibility Verification Response (Transmission Accepted/Transaction Approved) .................47
6.4.1.2 Eligibility Verification Response Segments (Transmission Accepted/Transaction Approved).................................................... 48
6.4.1.2.1 Response Header Segment (Eligibility Verification) (Transmission Accepted/Transaction Approved)................................48
6.4.1.2.2 Response Message Segment (Eligibility Verification) (Transmission Accepted/Transaction Approved) ............................48
6.4.1.2.3 Response Insurance Segment (Eligibility Verification) (Transmission Accepted/Transaction Approved) ........................... 48
6.4.1.2.4 Response Insurance Additional Information Segment (Eligibility Verification) (Transmission Accepted/Transaction
Approved) 49
6.4.1.2.5 Response Patient Segment (Eligibility Verification) (Transmission Accepted/Transaction Approved) ................................ 50
6.4.1.2.6 Response Status Segment (Eligibility Verification) (Transmission Accepted/Transaction Approved) .................................50
6.4.1.2.7 Response Coordination of Benefits/Other Payers Segment (Eligibility Verification) (Transmission Accepted/Transaction
Approved) 51
6.4.2 Transmission Accepted/Transaction Rejected..........................................................................................................................52
6.4.2.1 Diagram For Transmission Of Eligibility Verification Response (Transmission Accepted/Transaction Rejected)...................53
6.4.2.2 Eligibility Verification Response Segments (Transmission Accepted/Transaction Rejected) ..................................................... 53
6.4.2.2.1 Response Header Segment (Eligibility Verification) (Transmission Accepted/Transaction Rejected).................................53
6.4.2.2.2 Response Message Segment (Eligibility Verification) (Transmission Accepted/Transaction Rejected)..............................53
6.4.2.2.3 Response Insurance Additional Information Segment (Eligibility Verification) (Transmission Accepted/Transaction
Rejected) 54
6.4.2.2.4 Response Patient Segment (Eligibility Verification) (Transmission Accepted/Transaction Rejected).................................. 54
6.4.2.2.5 Response Status Segment (Eligibility Verification) (Transmission Accepted/Transaction Rejected)................................... 55
6.4.2.2.6 Response Coordination of Benefits/Other Payers Segment (Eligibility Verification) (Transmission Accepted/Transaction
Rejected) 56
6.4.3 Transmission Rejected/Transaction Rejected...........................................................................................................................57
6.4.3.1 Diagram For Transmission Of Eligibility Verification Response (Transmission Rejected/Transaction Rejected) ...................57
6.4.3.2 Eligibility Verification Response Segments (Transmission Rejected/Transaction Rejected) ...................................................... 58
6.4.3.2.1 Response Header Segment (Eligibility Verification) (Transmission Rejected/Transaction Rejected).................................. 58
6.4.3.2.2 Response Message Segment (Eligibility Verification) (Transmission Rejected/Transaction Rejected)............................... 58
6.4.3.2.3 Response Status Segment (Eligibility Verification) (Transmission Rejected/Transaction Rejected)....................................58

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 4 -
7. CLAIM BILLING OR ENCOUNTER INFORMATION..................................................................................................................................................60
7.1 CLAIM BILLING .....................................................................................................................................................................................60
7.2 ENCOUNTER .........................................................................................................................................................................................60
7.2.1 Encounter Diagrams ....................................................................................................................................................................61
7.2.1.1 Diagram For Transmission Of One, Two, Three, or Four Encounter Transactions....................................................................... 61
7.2.1.2 Diagram For Transmission Of One, Two, Three, or Four Encounter Response Transactions ...................................................61
7.3 CLAIM BILLING OR ENCOUNTER REQUEST DIAGRAMS ...........................................................................................................................61
7.3.1 Diagram For Transmission Of One Claim Billing or Encounter Transaction..........................................................................61
7.3.2 Diagram For Transmission of Two Claim Billing or Encounter Transactions........................................................................62
7.3.3 Diagram For Transmission of Three Claim Billing or Encounter Transactions .....................................................................63
7.3.4 Diagram For Transmission of Four Claim Billing or Encounter Transactions .......................................................................64
7.4 CLAIM BILLING OR ENCOUNTER REQUEST SEGMENTS ...........................................................................................................................66
7.4.1 Transaction Header Segment (Claim Billing or Encounter) .....................................................................................................66
7.4.2 Insurance Segment (Claim Billing or Encounter)......................................................................................................................66
7.4.2.1 Insurance Segment (Medicaid Subrogation Claim Billing or Encounter) ........................................................................................67
7.4.3 Patient Segment (Claim Billing or Encounter)...........................................................................................................................67
7.4.3.1 Patient Segment (Medicaid Subrogation Claim Billing or Encounter) .............................................................................................68
7.4.4 Claim Segment (Claim Billing or Encounter) .............................................................................................................................69
7.4.4.1 Claim Segment (Medicaid Subrogation Claim Billing or Encounter)................................................................................................ 71
7.4.5 Pricing Segment (Claim Billing or Encounter)...........................................................................................................................72
7.4.5.1 Pricing Segment (Medicaid Subrogation Claim Billing or Encounter).............................................................................................. 73
7.4.6 Pharmacy Provider Segment (Claim Billing or Encounter) ......................................................................................................73
7.4.7 Prescriber Segment (Claim Billing or Encounter).....................................................................................................................73
7.4.8 Coordination of Benefits/Other Payments Segment (Claim Billing or Encounter).................................................................74
7.4.9 Workers’ Compensation Segment (Claim Billing or Encounter)..............................................................................................76
7.4.10 DUR/PPS Segment (Claim Billing or Encounter) ..................................................................................................................77
7.4.11 Coupon Segment (Claim Billing or Encounter).....................................................................................................................78
7.4.12 Compound Segment (Claim Billing or Encounter) ...............................................................................................................78
7.4.13 Clinical Segment (Claim Billing or Encounter)......................................................................................................................79
7.4.14 Additional Documentation Segment (Claim Billing or Encounter)......................................................................................80
7.4.15 Facility Segment (Claim Billing or Encounter)......................................................................................................................81
7.4.16 Narrative Segment (Claim Billing or Encounter)...................................................................................................................82
7.5 CLAIM BILLING OR ENCOUNTER RESPONSE DIAGRAMS AND SEGMENTS ................................................................................................82
7.5.1 Transmission Accepted/Transaction Paid .................................................................................................................................82
7.5.1.1 Diagram For Transmission of One Claim Billing or Encounter Response (Transmission Accepted/Transaction Paid).........82
7.5.1.2 Diagram For Transmission of Two Claim Billing Or Encounter Responses (Transmission Accepted/Transaction Paid) ......82
7.5.1.3 Diagram For Transmission of Three Claim Billing Or Encounter Responses (Transmission Accepted/Transaction Paid) ... 83
7.5.1.4 Diagram For Transmission of Four Claim Billing Or Encounter Responses (Transmission Accepted/Transaction Paid) .....84
7.5.1.5 Claim Billing Or Encounter Response Segments (Transmission Accepted/Transaction Paid)................................................... 85
7.5.1.5.1 Response Header Segment (Claim Billing or Encounter) (Transmission Accepted/Transaction Paid) ............................... 85
7.5.1.5.2 Response Message Segment (Claim Billing or Encounter) (Transmission Accepted/Transaction Paid) ............................85
7.5.1.5.3 Response Insurance Segment (Claim Billing or Encounter) (Transmission Accepted/Transaction Paid)........................... 86
7.5.1.5.3.1 Response Insurance Segment (Medicaid Subrogation Claim Billing or Encounter) (Transmission
Accepted/Transaction Paid) ..............................................................................................................................................................................87
7.5.1.5.4 Response Patient Segment (Claim Billing or Encounter) (Transmission Accepted/Transaction Paid)................................ 87
7.5.1.5.5 Response Status Segment (Claim Billing or Encounter) (Transmission Accepted/Transaction Paid).................................87
7.5.1.5.6 Response Claim Segment (Claim Billing or Encounter) (Transmission Accepted/Transaction Paid) .................................. 88
7.5.1.5.6.1 Response Claim Segment (Medicaid Subrogation Claim Billing or Encounter) (Transmission Accepted/Transaction
Paid) 89
7.5.1.5.7 Response Pricing Segment (Claim Billing or Encounter) (Transmission Accepted/Transaction Paid) ................................ 89
7.5.1.5.8 Response DUR/PPS Segment (Claim Billing or Encounter) (Transmission Accepted/Transaction Paid) ..........................92
7.5.1.5.9 Response Coordination of Benefits/Other Payers Segment (Claim Billing or Encounter) (Transmission
Accepted/Transaction Paid) ...................................................................................................................................................................................93
7.5.2 Transmission Accepted/Transaction Captured.........................................................................................................................94
7.5.2.1 Diagram For Transmission of One Claim Billing Or Encounter Response (Transmission Accepted/Transaction Captured) 94
7.5.2.2 Diagram For Transmission of Two Claim Billing or Encounter Responses (Transmission Accepted/Transaction Captured)
94
7.5.2.3 Diagram For Transmission of Three Claim Billing or Encounter Responses (Transmission Accepted/Transaction Captured)
95
7.5.2.4 Diagram For Transmission of Four Claim Billing or Encounter Responses (Transmission Accepted/Transaction Captured)
96
7.5.2.5 Claim Billing or Encounter Response Segments (Transmission Accepted/Transaction Captured)...........................................97
7.5.2.5.1 Response Header Segment (Claim Billing or Encounter) (Transmission Accepted/Transaction Captured)....................... 97
7.5.2.5.2 Response Message Segment (Claim Billing or Encounter) (Transmission Accepted/Transaction Captured).................... 97
7.5.2.5.3 Response Insurance Segment (Claim Billing or Encounter) (Transmission Accepted/Transaction Captured) .................. 97
7.5.2.5.3.1 Response Insurance Segment (Medicaid Subrogation Claim Billing or Encounter) (Transmission
Accepted/Transaction Captured)......................................................................................................................................................................98

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 5 -
7.5.2.5.4 Response Patient Segment (Claim Billing or Encounter) (Transmission Accepted/Transaction Captured) ....................... 98
7.5.2.5.5 Response Status Segment (Claim Billing or Encounter) (Transmission Accepted/Transaction Captured) ........................99
7.5.2.5.6 Response Claim Segment (Claim Billing or Encounter) (Transmission Accepted/Transaction Captured)........................100
7.5.2.5.7 Response Pricing Segment (Claim Billing or Encounter) (Transmission Accepted/Transaction Captured)...................... 101
7.5.2.5.8 Response DUR/PPS Segment (Claim Billing or Encounter) (Transmission Accepted/Transaction Captured) ................104
7.5.3 Transmission Accepted/Transaction Rejected........................................................................................................................104
7.5.3.1 Diagram For Transmission Of One Claim Billing or Encounter Response (Transmission Accepted/Transaction Rejected)
104
7.5.3.2 Diagram For Transmission Of Two Claim Billing or Encounter Responses (Transmission Accepted/Transaction Rejected)
105
7.5.3.3 Diagram For Transmission Of Three Claim Billing or Encounter Responses (Transmission Accepted/Transaction Rejected)
105
7.5.3.4 Diagram For Transmission Of Four Claim Billing or Encounter Responses (Transmission Accepted/Transaction Rejected)
106
7.5.3.5 Claim Billing or Encounter Response Segments (Transmission Accepted/Transaction Rejected)..........................................107
7.5.3.5.1 Response Header Segment (Claim Billing or Encounter) (Transmission Accepted/Transaction Rejected) ..................... 107
7.5.3.5.2 Response Message Segment (Claim Billing or Encounter) (Transmission Accepted/Transaction Rejected) ..................108
7.5.3.5.3 Response Insurance Segment (Claim Billing or Encounter) (Transmission Accepted/Transaction Rejected)................. 108
7.5.3.5.3.1 Response Insurance Segment (Medicaid Subrogation Claim Billing or Encounter) (Transmission
Accepted/Transaction Rejected) ....................................................................................................................................................................109
7.5.3.5.4 Response Patient Segment (Claim Billing or Encounter) (Transmission Accepted/Transaction Rejected) ......................109
7.5.3.5.5 Response Status Segment (Claim Billing or Encounter) (Transmission Accepted/Transaction Rejected) ....................... 109
7.5.3.5.6 Response Claim Segment (Claim Billing or Encounter) (Transmission Accepted/Transaction Rejected) ........................ 111
7.5.3.5.6.1 Response Claim Segment (Medicaid Subrogation Claim Billing or Encounter) (Transmission Accepted/Transaction
Rejected) 111
7.5.3.5.7 Response DUR/PPS Segment (Claim Billing or Encounter) (Transmission Accepted/Transaction Rejected).................112
7.5.3.5.8 Response Prior Authorization Segment (Claim Billing or Encounter) (Transmission Accepted/Transaction Rejected) . 112
7.5.3.5.9 Response Coordination of Benefits/Other Payers Segment (Claim Billing or Encounter) (Transmission
Accepted/Transaction Rejected) .........................................................................................................................................................................113
7.5.4 Transmission Rejected/Transaction Rejected.........................................................................................................................114
7.5.4.1 Diagram For Transmission Of One Claim Billing or Encounter Response (Transmission Rejected/Transaction Rejected)114
7.5.4.2 Diagram For Transmission Of Two Claim Billing or Encounter Responses (Transmission Rejected/Transaction Rejected)
114
7.5.4.3 Diagram For Transmission Of Three Claim Billing or Encounter Responses (Transmission Rejected/Transaction Rejected)
114
7.5.4.4 Diagram For Transmission Of Four Claim Billing or Encounter Responses (Transmission Rejected/Transaction Rejected)
115
7.5.4.5 Claim Billing or Encounter Response Segments (Transmission Rejected/Transaction Rejected)...........................................115
7.5.4.5.1 Response Header Segment (Claim Billing or Encounter) (Transmission Rejected/Transaction Rejected) ......................115
7.5.4.5.2 Response Message Segment (Claim Billing or Encounter) (Transmission Rejected/Transaction Rejected) ................... 116
7.5.4.5.3 Response Status Segment (Claim Billing or Encounter) (Transmission Rejected/Transaction Rejected) ........................116
8. PREDETERMINATION OF BENEFITS INFORMATION.......................................................................................................................................... 118
8.1 PREDETERMINATION OF BENEFITS REQUEST DIAGRAMS......................................................................................................................118
8.1.1 Diagram For Transmission Of One Predetermination of Benefits Transaction....................................................................118
8.1.2 Diagram For Transmission Of Two Predetermination of Benefits Transactions .................................................................119
8.1.3 Diagram For Transmission Of Three Or Four Predetermination of Benefits Transactions.................................................119
8.2 PREDETERMINATION OF BENEFITS REQUEST SEGMENTS .....................................................................................................................120
8.2.1 Pricing Segment (Predetermination Of Benefits) ....................................................................................................................120
8.3 PREDETERMINATION OF BENEFITS RESPONSE DIAGRAMS AND SEGMENTS ..........................................................................................121
8.3.1 Transmission Accepted/Transaction Benefit...........................................................................................................................121
8.3.1.1 Diagram For Transmission of One Predetermination Of Benefit Response (Transmission Accepted/Transaction Benefit)121
8.3.1.2 Diagram For Transmission of Two Predetermination Of Benefit Responses (Transmission Accepted/Transaction Benefit)
121
8.3.1.3 Diagram For Transmission of Three Or Four Predetermination Of Benefit Responses (Transmission Accepted/Transaction
Benefit) 122
8.3.1.4 Predetermination Of Benefits Response Segments (Transmission Accepted/Transaction Benefit)........................................122
8.3.1.4.1 Response Pricing Segment (Predetermination Of Benefits) (Transmission Accepted/Transaction Benefit) .................... 122
8.3.2 Transmission Accepted/Transaction Rejected........................................................................................................................124
8.3.2.1 Diagram For Transmission Of One Predetermination Of Benefits Response (Transmission Accepted/Transaction Rejected)
124
8.3.2.2 Diagram For Transmission Of Two Predetermination Of Benefits Responses (Transmission Accepted/Transaction
Rejected) 124
8.3.2.3 Diagram For Transmission Of Three Or Four Predetermination Of Benefit Responses (Transmission Accepted/Transaction
Rejected) 125
8.3.2.4 Predetermination Of Benefits Response Segments (Transmission Accepted/Transaction Rejected) .................................... 125
8.3.3 Transmission Rejected/Transaction Rejected.........................................................................................................................125

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 6 -
8.3.3.1 Diagram For Transmission Of One Predetermination Of Benefits Response (Transmission Rejected/Transaction Rejected)
125
8.3.3.2 Diagram For Transmission Of Two Predetermination Of Benefits Responses (Transmission Rejected/Transaction
Rejected) 125
8.3.3.3 Diagram For Transmission Of Three Or Four Predetermination Of Benefits Responses (Transmission
Rejected/Transaction Rejected) ...............................................................................................................................................................................126
8.3.3.4 Predetermination Of Benefits Response Segments (Transmission Rejected/Transaction Rejected) .....................................126
9. SERVICE BILLING (PROFESSIONAL PHARMACY SERVICE) INFORMATION .............................................................................................. 127
9.1 SERVICE BILLING................................................................................................................................................................................127
9.2 SERVICE BILLING REQUEST DIAGRAMS ...............................................................................................................................................127
9.2.1 Diagram For Transmission Of One Service Billing Transaction............................................................................................127
9.2.2 Diagram For Transmission Of Two Service Billing Transactions..........................................................................................128
9.2.3 Diagram For Transmission Of Three Service Billing Transactions .......................................................................................129
9.2.4 Diagram For Transmission Of Four Service Billing Transactions.........................................................................................130
9.3 SERVICE BILLING REQUEST SEGMENTS...............................................................................................................................................132
9.3.1 Transaction Header Segment (Service Billing)........................................................................................................................132
9.3.2 Insurance Segment (Service Billing) ........................................................................................................................................132
9.3.3 Patient Segment (Service Billing) .............................................................................................................................................133
9.3.4 Claim Segment (Service Billing) ...............................................................................................................................................134
9.3.5 Pricing Segment (Service Billing).............................................................................................................................................136
9.3.6 Pharmacy Provider Segment (Service Billing) ........................................................................................................................137
9.3.7 Prescriber Segment (Service Billing) .......................................................................................................................................138
9.3.8 Coordination of Benefits /Other Payments Segment (Service Billing)..................................................................................139
9.3.9 Workers’ Compensation Segment (Service Billing)................................................................................................................140
9.3.10 DUR/PPS Segment (Service Billing).....................................................................................................................................141
9.3.11 Clinical Segment (Service Billing)........................................................................................................................................142
9.3.12 Additional Documentation Segment (Service Billing)........................................................................................................143
9.3.13 Facility Segment (Service Billing) ........................................................................................................................................144
9.3.14 Narrative Segment (Service Billing) .....................................................................................................................................145
9.4 SERVICE BILLING RESPONSE DIAGRAMS AND SEGMENTS ....................................................................................................................145
9.4.1 Transmission Accepted/Transaction Paid ...............................................................................................................................145
9.4.1.1 Diagram For Transmission Of One Service Billing Response (Transmission Accepted/Transaction Paid) ...........................145
9.4.1.2 Diagram For Transmission Of Two Service Billing Responses (Transmission Accepted/Transaction Paid) .........................145
9.4.1.3 Diagram For Transmission Of Three Service Billing Responses (Transmission Accepted/Transaction Paid) ......................146
9.4.1.4 Diagram For Transmission Of Four Service Billing Responses (Transmission Accepted/Transaction Paid) ........................ 147
9.4.1.5 Service Billing Response Segments (Transmission Accepted/Transaction Paid).......................................................................148
9.4.1.5.1 Response Header Segment (Service Billing) (Transmission Accepted/Transaction Paid) .................................................. 148
9.4.1.5.2 Response Message Segment (Service Billing) (Transmission Accepted/Transaction Paid) ...............................................148
9.4.1.5.3 Response Insurance Segment (Service Billing) (Transmission Accepted/Transaction Paid)..............................................149
9.4.1.5.4 Response Patient Segment (Service Billing) (Transmission Accepted/Transaction Paid)................................................... 149
9.4.1.5.5 Response Status Segment (Service Billing) (Transmission Accepted/Transaction Paid) ....................................................150
9.4.1.5.6 Response Claim Segment (Service Billing) (Transmission Accepted/Transaction Paid) .....................................................151
9.4.1.5.7 Response Pricing Segment (Service Billing) (Transmission Accepted/Transaction Paid) ................................................... 152
9.4.1.5.8 Response DUR/PPS Segment (Service Billing) (Transmission Accepted/Transaction Paid)..............................................154
9.4.1.5.9 Response Coordination of Benefits/Other Payers Segment (Service Billing) (Transmission Accepted/Transaction Paid)
155
9.4.2 Transmission Accepted/Transaction Captured.......................................................................................................................156
9.4.2.1 Diagram For Transmission Of One Service Billing Response (Transmission Accepted/Transaction Captured)................... 156
9.4.2.2 Diagram For Transmission Of Two Service Billing Responses (Transmission Accepted/Transaction Captured)................. 156
9.4.2.3 Diagram For Transmission Of Three Service Billing Responses (Transmission Accepted/Transaction Captured)..............157
9.4.2.4 Diagram For Transmission Of Four Service Billing Responses (Transmission Accepted/Transaction Captured)................157
9.4.2.5 Service Billing Response Segments (Transmission Accepted/Transaction Captured) ..............................................................158
9.4.2.5.1 Response Header Segment (Service Billing) (Transmission Accepted/Transaction Captured)..........................................158
9.4.2.5.2 Response Message Segment (Service Billing) (Transmission Accepted/Transaction Captured)....................................... 158
9.4.2.5.3 Response Insurance Segment (Service Billing) (Transmission Accepted/Transaction Captured) ..................................... 159
9.4.2.5.4 Response Patient Segment (Service Billing) (Transmission Accepted/Transaction Captured) ..........................................160
9.4.2.5.5 Response Status Segment (Service Billing) (Transmission Accepted/Transaction Captured)............................................160
9.4.2.5.6 Response Claim Segment (Service Billing) (Transmission Accepted/Transaction Captured).............................................161
9.4.2.5.7 Response Pricing Segment (Service Billing) (Transmission Accepted/Transaction Captured)...........................................161
9.4.3 Transmission Accepted/Transaction Rejected........................................................................................................................164
9.4.3.1 Diagram for Transmission Of One Service Billing Response (Transmission Accepted/Transaction Rejected)..................... 164
9.4.3.2 Diagram for Transmission Of Two Service Billing Responses (Transmission Accepted/Transaction Rejected)...................164
9.4.3.3 Diagram for Transmission Of Three Service Billing Responses (Transmission Accepted/Transaction Rejected)................165
9.4.3.4 Diagram for Transmission Of Four Service Billing Responses (Transmission Accepted/Transaction Rejected).................. 166
9.4.3.5 Service Billing Response Segments (Transmission Accepted/Transaction Rejected)...............................................................167
9.4.3.5.1 Response Header Segment (Service Billing) (Transmission Accepted/Transaction Rejected) ..........................................167

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 7 -
9.4.3.5.2 Response Message Segment (Service Billing) (Transmission Accepted/Transaction Rejected) ....................................... 167
9.4.3.5.3 Response Insurance Segment (Service Billing) (Transmission Accepted/Transaction Rejected)...................................... 167
9.4.3.5.4 Response Patient Segment (Service Billing) (Transmission Accepted/Transaction Rejected) ...........................................168
9.4.3.5.5 Response Status Segment (Service Billing) (Transmission Accepted/Transaction Rejected) ............................................168
9.4.3.5.6 Response Claim Segment (Service Billing) (Transmission Accepted/Transaction Rejected) .............................................170
9.4.3.5.7 Response Prior Authorization Segment (Service Billing) (Transmission Accepted/Transaction Rejected) ......................170
9.4.3.5.8 Response Coordination of Benefits/Other Payers Segment (Service Billing) (Transmission Accepted/Transaction
Rejected) 171
9.4.4 Transmission Rejected/Transaction Rejected.........................................................................................................................172
9.4.4.1 Diagram For Transmission Of One Service Billing Response (Transmission Rejected/Transaction Rejected) .................... 172
9.4.4.2 Diagram For Transmission Of Two Service Billing Responses (Transmission Rejected/Transaction Rejected) .................. 172
9.4.4.3 Diagram For Transmission Of Three Service Billing Responses (Transmission Rejected/Transaction Rejected) ............... 172
9.4.4.4 Diagram For Transmission Of Four Service Billing Responses (Transmission Rejected/Transaction Rejected) .................172
9.4.4.5 Service Billing Response Segments (Transmission Rejected/Transaction Rejected)................................................................ 173
9.4.4.5.1 Response Header Segment (Service Billing) (Transmission Rejected/Transaction Rejected) ...........................................173
9.4.4.5.2 Response Message Segment (Service Billing) (Transmission Rejected/Transaction Rejected) ........................................173
9.4.4.5.3 Response Status Segment (Service Billing) (Transmission Rejected/Transaction Rejected) .............................................174
10. REVERSAL INFORMATION.....................................................................................................................................................................................176
10.1 CLAIM OR SERVICE REVERSAL ...........................................................................................................................................................176
10.2 CLAIM REVERSAL REQUEST DIAGRAMS...............................................................................................................................................177
10.2.1 Diagram For Transmission Of One Claim Reversal Transaction ......................................................................................177
10.2.2 Diagram For Transmission Of Two Claim Reversal Transactions ....................................................................................177
10.2.3 Diagram For Transmission Of Three Claim Reversal Transactions..................................................................................178
10.2.4 Diagram For Transmission Of Four Claim Reversal Transactions ...................................................................................178
10.3 CLAIM REVERSAL REQUEST SEGMENTS ..............................................................................................................................................179
10.3.1 Transaction Header Segment (Claim Reversal) ..................................................................................................................179
10.3.2 Insurance Segment (Claim Reversal)...................................................................................................................................180
10.3.2.1 Insurance Segment (Medicaid Subrogation Claim Reversal) .........................................................................................................180
10.3.3 Claim Segment (Claim Reversal)..........................................................................................................................................181
10.3.4 DUR/PPS Segment (Claim Reversal)....................................................................................................................................182
10.3.5 Pricing Segment (Claim Reversal)........................................................................................................................................183
10.3.5.1 Example 1: Reporting a DUR event on a Claim Reversal without any incentive submitted ......................................................183
10.3.5.2 Example 2: No Incentive Amount Submitted (438-E3) for a Claim Reversal. Incentive Paid.................................................... 184
10.3.5.3 Example 3: Incentive Amount Submitted (438-E3) for a Claim Reversal .....................................................................................184
10.3.5.4 Example 4: Incentive Amount Submitted (438-E3) for a Claim Reversal .....................................................................................185
10.3.6 Coordination of Benefits/Other Payments Segment (Claim Reversal) .............................................................................185
10.3.6.1 Coordination of Benefits/Other Payments Segment Usage in Claim Reversal............................................................................ 186
10.3.6.1.1 Excerpt Example 1........................................................................................................................................................................... 186
10.3.6.1.2 Excerpt Example 2........................................................................................................................................................................... 186
10.4 CLAIM REVERSAL RESPONSE DIAGRAMS AND SEGMENTS....................................................................................................................186
10.4.1 Transmission Accepted/Transaction Approved .................................................................................................................186
10.4.1.1 Diagram For Transmission Of One Claim Reversal Response (Transmission Accepted/Transaction Approved)................186
10.4.1.2 Diagram For Transmission Of Two Claim Reversal Responses (Transmission Accepted/Transaction Approved)..............187
10.4.1.3 Diagram For Transmission Of Three Claim Reversal Responses (Transmission Accepted/Transaction Approved) ...........187
10.4.1.4 Diagram For Transmission Of Four Claim Reversal Responses (Transmission Accepted/Transaction Approved) ............. 188
10.4.1.5 Claim Reversal Response Segments (Transmission Accepted/Transaction Approved) ........................................................... 189
10.4.1.5.1 Response Header Segment (Claim Reversal) (Transmission Accepted/Transaction Approved) .....................................189
10.4.1.5.2 Response Message Segment (Claim Reversal) (Transmission Accepted/Transaction Approved).................................. 189
10.4.1.5.3 Response Status Segment (Claim Reversal) (Transmission Accepted/Transaction Approved).......................................189
10.4.1.5.4 Response Claim Segment (Claim Reversal) (Transmission Accepted/Transaction Approved)........................................190
10.4.1.5.4.1 Response Claim Segment (Medicaid Subrogation Claim Reversal) (Transmission Accepted/Transaction
Approved) 191
10.4.1.5.5 Response Pricing Segment (Claim Reversal) (Transmission Accepted/Transaction Approved)......................................191
10.4.2 Transmission Accepted/Transaction Captured ..................................................................................................................193
10.4.2.1 Diagram For Transmission Of One Claim Reversal Response (Transmission Accepted/Transaction Captured).................193
10.4.2.2 Diagram For Transmission Of Two Claim Reversal Responses (Transmission Accepted/Transaction Captured)...............193
10.4.2.3 Diagram For Transmission Of Three Claim Reversal Responses (Transmission Accepted/Transaction Captured)............ 193
10.4.2.4 Diagram For Transmission Of Four Claim Reversal Responses (Transmission Accepted/Transaction Captured)..............194
10.4.2.5 Claim Reversal Response Segments (Transmission Accepted/Transaction Captured) ............................................................194
10.4.2.5.1 Response Header Segment (Claim Reversal) (Transmission Accepted/Transaction Captured)......................................195
10.4.2.5.2 Response Message Segment (Claim Reversal) (Transmission Accepted/Transaction Captured)................................... 195
10.4.2.5.3 Response Status Segment (Claim Reversal) (Transmission Accepted/Transaction Captured)........................................195
10.4.2.5.4 Response Claim Segment (Claim Reversal) (Transmission Accepted/Transaction Captured).........................................196
10.4.3 Transmission Accepted/Transaction Rejected ...................................................................................................................197
10.4.3.1 Diagram For Transmission Of One Claim Reversal Response (Transmission Accepted/Transaction Rejected) .................197
10.4.3.2 Diagram For Transmission Of Two Claim Reversal Responses (Transmission Accepted/Transaction Rejected) ...............197

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 8 -
10.4.3.3 Diagram For Transmission Of Three Claim Reversal Responses (Transmission Accepted/Transaction Rejected) ............ 198
10.4.3.4 Diagram For Transmission Of Four Claim Reversal Responses (Transmission Accepted/Transaction Rejected)............... 198
10.4.3.5 Claim Reversal Response Segments (Transmission Accepted/Transaction Rejected)............................................................. 199
10.4.3.5.1 Response Header Segment (Claim Reversal) (Transmission Accepted/Transaction Rejected) ...................................... 199
10.4.3.5.2 Response Message Segment (Claim Reversal) (Transmission Accepted/Transaction Rejected) ................................... 199
10.4.3.5.3 Response Status Segment (Claim Reversal) (Transmission Accepted/Transaction Rejected) ........................................199
10.4.3.5.4 Response Claim Segment (Claim Reversal) (Transmission Accepted/Transaction Rejected) ......................................... 200
10.4.4 Transmission Rejected/Transaction Rejected ....................................................................................................................201
10.4.4.1 Diagram For Transmission Of One Claim Reversal Response (Transmission Rejected/Transaction Rejected) ..................201
10.4.4.2 Diagram For Transmission Of Two Claim Reversal Responses (Transmission Rejected/Transaction Rejected) ................201
10.4.4.3 Diagram For Transmission Of Three Claim Reversal Responses (Transmission Rejected/Transaction Rejected) ............. 202
10.4.4.4 Diagram For Transmission Of Four Claim Reversal Responses (Transmission Rejected/Transaction Rejected) ...............202
10.4.4.5 Claim Reversal Response Segments (Transmission Rejected/Transaction Rejected)..............................................................203
10.4.4.5.1 Response Header Segment (Claim Reversal) (Transmission Rejected/Transaction Rejected) .......................................203
10.4.4.5.2 Response Message Segment (Claim Reversal) (Transmission Rejected/Transaction Rejected) .................................... 203
10.4.4.5.3 Response Status Segment (Claim Reversal) (Transmission Rejected/Transaction Rejected) .........................................203
10.5 SERVICE REVERSAL REQUEST DIAGRAMS ...........................................................................................................................................205
10.5.1 Diagram For Transmission Of One Service Reversal Transaction ...................................................................................205
10.5.2 Diagram For Transmission Of Two Service Reversal Transactions .................................................................................205
10.5.3 Diagram For Transmission Of Three Service Reversal Transactions...............................................................................205
10.5.4 Diagram For Transmission Of Four Service Reversal Transactions ................................................................................206
10.6 SERVICE REVERSAL REQUEST SEGMENTS...........................................................................................................................................206
10.6.1 Transaction Header Segment (Service Reversal) ...............................................................................................................206
10.6.2 Insurance Segment (Service Reversal)................................................................................................................................207
10.6.3 Claim Segment (Service Reversal).......................................................................................................................................207
10.6.4 Coordination of Benefits/Other Payments Segment (Service Reversal) ..........................................................................209
10.6.4.1 Coordination of Benefits/Other Payments Segment Usage in Service Reversal ........................................................................210
10.6.4.1.1 Excerpt Example 1........................................................................................................................................................................... 210
10.6.4.1.2 Excerpt Example 2........................................................................................................................................................................... 210
10.7 SERVICE REVERSAL RESPONSE DIAGRAMS AND SEGMENTS................................................................................................................210
10.7.1 Transmission Accepted/Transaction Approved .................................................................................................................210
10.7.1.1 Diagram For Transmission Of One Service Reversal Response (Transmission Accepted/Transaction Approved).............211
10.7.1.2 Diagram For Transmission Of Two Service Reversal Responses (Transmission Accepted/Transaction Approved)...........211
10.7.1.3 Diagram For Transmission Of Three Service Reversal Responses (Transmission Accepted/Transaction Approved)........ 211
10.7.1.4 Diagram For Transmission Of Four Service Reversal Responses (Transmission Accepted/Transaction Approved) .......... 212
10.7.1.5 Service Reversal Response Segments (Transmission Accepted/Transaction Approved) ........................................................ 212
10.7.1.5.1 Response Header Segment (Service Reversal) (Transmission Accepted/Transaction Approved)..................................212
10.7.1.5.2 Response Message Segment (Service Reversal) (Transmission Accepted/Transaction Approved)...............................212
10.7.1.5.3 Response Status Segment (Service Reversal) (Transmission Accepted/Transaction Approved)....................................213
10.7.1.5.4 Response Claim Segment (Service Reversal) (Transmission Accepted/Transaction Approved).....................................214
10.7.2 Transmission Accepted/Transaction Captured ..................................................................................................................214
10.7.2.1 Diagram For Transmission Of One Service Reversal Response (Transmission Accepted/Transaction Captured) .............215
10.7.2.2 Diagram For Transmission Of Two Service Reversal Responses (Transmission Accepted/Transaction Captured) ...........215
10.7.2.3 Diagram For Transmission Of Three Service Reversal Responses (Transmission Accepted/Transaction Captured).........215
10.7.2.4 Diagram For Transmission Of Four Service Reversal Responses (Transmission Accepted/Transaction Captured)...........216
10.7.2.5 Service Reversal Response Segments (Transmission Accepted/Transaction Captured).........................................................216
10.7.2.5.1 Response Header Segment (Service Reversal) (Transmission Accepted/Transaction Captured)...................................216
10.7.2.5.2 Response Message Segment (Service Reversal) (Transmission Accepted/Transaction Captured) ...............................217
10.7.2.5.3 Response Status Segment (Service Reversal) (Transmission Accepted/Transaction Captured) .................................... 217
10.7.2.5.4 Response Claim Segment (Service Reversal) (Transmission Accepted/Transaction Captured) .....................................218
10.7.3 Transmission Accepted/Transaction Rejected ...................................................................................................................219
10.7.3.1 Diagram For Transmission Of One Service Reversal Response (Transmission Accepted/Transaction Rejected) .............. 219
10.7.3.2 Diagram For Transmission Of Two Service Reversal Responses (Transmission Accepted/Transaction Rejected) ............ 219
10.7.3.3 Diagram For Transmission Of Three Service Reversal Responses (Transmission Accepted/Transaction Rejected) .........219
10.7.3.4 Diagram For Transmission Of Four Service Reversal Responses (Transmission Accepted/Transaction Rejected) ...........220
10.7.3.5 Service Reversal Response Segments (Transmission Accepted/Transaction Rejected).......................................................... 220
10.7.3.5.1 Response Header Segment (Service Reversal) (Transmission Accepted/Transaction Rejected) ................................... 220
10.7.3.5.2 Response Message Segment (Service Reversal) (Transmission Accepted/Transaction Rejected) ................................ 221
10.7.3.5.3 Response Status Segment (Service Reversal) (Transmission Accepted/Transaction Rejected) .....................................221
10.7.3.5.4 Response Claim Segment (Service Reversal) (Transmission Accepted/Transaction Rejected) ......................................222
10.7.4 Transmission Rejected/Transaction Rejected ....................................................................................................................223
10.7.4.1 Diagram For Transmission Of One Service Reversal Response (Transmission Rejected/Transaction Rejected) ...............223
10.7.4.2 Diagram For Transmission Of Two Service Reversal Responses (Transmission Rejected/Transaction Rejected) .............223
10.7.4.3 Diagram For Transmission Of Three Service Reversal Responses (Transmission Rejected/Transaction Rejected) .......... 223
10.7.4.4 Diagram For Transmission Of Four Service Reversal Responses (Transmission Rejected/Transaction Rejected) ............ 224
10.7.4.5 Service Reversal Response Segments (Transmission Rejected/Transaction Rejected) ..........................................................224
10.7.4.5.1 Response Header Segment (Service Reversal) (Transmission Rejected/Transaction Rejected) ....................................224

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 9 -
10.7.4.5.2 Response Message Segment (Service Reversal) (Transmission Rejected/Transaction Rejected) .................................224
10.7.4.5.3 Response Status Segment (Service Reversal) (Transmission Rejected/Transaction Rejected)......................................225
11. REBILL INFORMATION............................................................................................................................................................................................227
11.1 CLAIM OR SERVICE REBILL.................................................................................................................................................................227
11.2 CLAIM REBILL REQUEST DIAGRAMS ....................................................................................................................................................227
11.2.1 Diagram For Transmission Of One Claim Rebill Transaction............................................................................................227
11.2.2 Diagram For Transmission Of Two Claim Rebill Transactions .........................................................................................228
11.2.3 Diagram For Transmission Of Three Claim Rebill Transactions.......................................................................................229
11.2.4 Diagram For Transmission Of Four Claim Rebill Transactions.........................................................................................230
11.3 CLAIM REBILL REQUEST SEGMENTS ...................................................................................................................................................232
11.3.1 Transaction Header Segment (Claim Rebill) .......................................................................................................................232
11.3.2 Insurance Segment (Claim Rebill)........................................................................................................................................233
11.3.2.1 Insurance Segment (Medicaid Subrogation Claim Rebill) ............................................................................................................... 233
11.3.3 Patient Segment (Claim Rebill).............................................................................................................................................234
11.3.3.1 Patient Segment (Medicaid Subrogation Claim Rebill) .................................................................................................................... 235
11.3.4 Claim Segment (Claim Rebill) ...............................................................................................................................................235
11.3.4.1 Claim Segment (Medicaid Subrogation Claim Rebill).......................................................................................................................238
11.3.5 Pricing Segment (Claim Rebill).............................................................................................................................................238
11.3.5.1 Pricing Segment (Medicaid Subrogation Claim Rebill)..................................................................................................................... 239
11.3.6 Pharmacy Provider Segment (Claim Rebill) ........................................................................................................................239
11.3.7 Prescriber Segment (Claim Rebill) .......................................................................................................................................240
11.3.8 Coordination of Benefits/Other Payments Segment (Claim Rebill)...................................................................................241
11.3.9 Workers’ Compensation Segment (Claim Rebill)................................................................................................................243
11.3.10 DUR/PPS Segment (Claim Rebill).........................................................................................................................................243
11.3.11 Coupon Segment (Claim Rebill) ...........................................................................................................................................244
11.3.12 Compound Segment (Claim Rebill)......................................................................................................................................245
11.3.13 Clinical Segment (Claim Rebill) ............................................................................................................................................245
11.3.14 Additional Documentation Segment (Claim Rebill) ............................................................................................................246
11.3.15 Facility Segment (Claim Rebill).............................................................................................................................................247
11.3.16 Narrative Segment (Claim Rebill) .........................................................................................................................................248
11.4 CLAIM REBILL RESPONSE DIAGRAMS AND SEGMENTS.........................................................................................................................248
11.4.1 Transmission Accepted/Transaction Paid...........................................................................................................................248
11.4.1.1 Diagram For Transmission of One Claim Rebill Response (Transmission Accepted/Transaction Paid)................................248
11.4.1.2 Diagram For Transmission of Two Claim Rebill Responses (Transmission Accepted/Transaction Paid)..............................249
11.4.1.3 Diagram For Transmission of Three Claim Rebill Responses (Transmission Accepted/Transaction Paid)........................... 249
11.4.1.4 Diagram For Transmission of Four Claim Rebill Responses (Transmission Accepted/Transaction Paid) ............................. 250
11.4.2 Claim Rebill Response Segments (Transmission Accepted/Transaction Paid) ..............................................................251
11.4.2.1.1 Response Header Segment (Claim Rebill) (Transmission Accepted/Transaction Paid) ....................................................251
11.4.2.1.2 Response Message Segment (Claim Rebill) (Transmission Accepted/Transaction Paid)................................................. 252
11.4.2.1.3 Response Insurance Segment (Claim Rebill) (Transmission Accepted/Transaction Paid)................................................252
11.4.2.1.3.1 Response Insurance Segment (Medicaid Subrogation Claim Rebill) (Transmission Accepted/Transaction Paid)253
11.4.2.1.4 Response Patient Segment (Claim Rebill) (Transmission Accepted/Transaction Paid).....................................................253
11.4.2.1.5 Response Status Segment (Claim Rebill) (Transmission Accepted/Transaction Paid)......................................................253
11.4.2.1.6 Response Claim Segment (Claim Rebill) (Transmission Accepted/Transaction Paid).......................................................255
11.4.2.1.6.1 Response Claim Segment (Medicaid Subrogation Claim Rebill) (Transmission Accepted/Transaction Paid)....... 255
11.4.2.1.7 Response Pricing Segment (Claim Rebill) (Transmission Accepted/Transaction Paid)..................................................... 255
11.4.2.1.8 Response DUR/PPS Segment (Claim Rebill) (Transmission Accepted/Transaction Paid) ...............................................259
11.4.2.1.9 Response Coordination of Benefits/Other Payers Segment (Claim Rebill) (Transmission Accepted/Transaction Paid)
259
11.4.3 Transmission Accepted/Transaction Captured ..................................................................................................................260
11.4.3.1 Diagram For Transmission of One Claim Rebill Response (Transmission Accepted/Transaction Captured) .......................260
11.4.3.2 Diagram For Transmission of Two Claim Rebill Responses (Transmission Accepted/Transaction Captured) .....................261
11.4.3.3 Diagram For Transmission of Three Claim Rebill Responses (Transmission Accepted/Transaction Captured) .................. 261
11.4.3.4 Diagram For Transmission of Four Claim Rebill Responses (Transmission Accepted/Transaction Captured).....................262
11.4.3.5 Claim Rebill Response Segments (Transmission Accepted/Transaction Captured)..................................................................263
11.4.3.5.1 Response Header Segment (Claim Rebill) (Transmission Accepted/Transaction Captured)............................................ 263
11.4.3.5.2 Response Message Segment (Claim Rebill) (Transmission Accepted/Transaction Captured) ........................................ 263
11.4.3.5.3 Response Insurance Segment (Claim Rebill) (Transmission Accepted/Transaction Captured) .......................................264
11.4.3.5.3.1 Response Insurance Segment (Medicaid Subrogation Claim Rebill) (Transmission Accepted/Transaction
Captured) 264
11.4.3.5.4 Response Patient Segment (Claim Rebill) (Transmission Accepted/Transaction Captured) ............................................264
11.4.3.5.5 Response Status Segment (Claim Rebill) (Transmission Accepted/Transaction Captured) .............................................265
11.4.3.5.6 Response Claim Segment (Claim Rebill) (Transmission Accepted/Transaction Captured) ..............................................266
11.4.3.5.7 Response Pricing Segment (Claim Rebill) (Transmission Accepted/Transaction Captured) ............................................267
11.4.3.5.8 Response DUR/PPS Segment (Claim Rebill) (Transmission Accepted/Transaction Captured).......................................270
11.4.4 Transmission Accepted/Transaction Rejected ...................................................................................................................270

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 10 -
11.4.4.1 Diagram For Transmission Of One Claim Rebill Response (Transmission Accepted/Transaction Rejected) .......................270
11.4.4.2 Diagram For Transmission Of Two Claim Rebill Responses (Transmission Accepted/Transaction Rejected) .....................271
11.4.4.3 Diagram For Transmission Of Three Claim Rebill Responses (Transmission Accepted/Transaction Rejected) .................. 271
11.4.4.4 Diagram For Transmission Of Four Claim Rebill Responses (Transmission Accepted/Transaction Rejected) .................... 272
11.4.4.5 Claim Rebill Response Segments (Transmission Accepted/Transaction Rejected)...................................................................273
11.4.4.5.1 Response Header Segment (Claim Rebill) (Transmission Accepted/Transaction Rejected) ............................................273
11.4.4.5.2 Response Message Segment (Claim Rebill) (Transmission Accepted/Transaction Rejected) .........................................273
11.4.4.5.3 Response Insurance Segment (Claim Rebill) (Transmission Accepted/Transaction Rejected)........................................274
11.4.4.5.3.1 Response Insurance Segment (Medicaid Subrogation Claim Rebill) (Transmission Accepted/Transaction
Rejected) 275
11.4.4.5.4 Response Patient Segment (Claim Rebill) (Transmission Accepted/Transaction Rejected).............................................275
11.4.4.5.5 Response Status Segment (Claim Rebill) (Transmission Accepted/Transaction Rejected)..............................................275
11.4.4.5.6 Response Claim Segment (Claim Rebill) (Transmission Accepted/Transaction Rejected) ...............................................276
11.4.4.5.7 Response DUR/PPS Segment (Claim Rebill) (Transmission Accepted/Transaction Rejected) .......................................277
11.4.4.5.8 Response Prior Authorization Segment (Claim Rebill) (Transmission Accepted/Transaction Rejected) ........................ 278
11.4.4.5.9 Response Coordination of Benefits/Other Payers Segment (Claim Rebill) (Transmission Accepted/Transaction
Rejected) 278
11.4.5 Transmission Rejected/Transaction Rejected ....................................................................................................................279
11.4.5.1 Diagram For Transmission Of One Claim Rebill Response (Transmission Rejected/Transaction Rejected)........................279
11.4.5.2 Diagram For Transmission Of Two Claim Rebill Responses (Transmission Rejected/Transaction Rejected)......................280
11.4.5.3 Diagram For Transmission Of Three Claim Rebill Responses (Transmission Rejected/Transaction Rejected) ...................280
11.4.5.4 Diagram For Transmission Of Four Claim Rebill Responses (Transmission Rejected/Transaction Rejected) .....................280
11.4.5.5 Claim Rebill Response Segments (Transmission Rejected/Transaction Rejected) ...................................................................281
11.4.5.5.1 Response Header Segment (Claim Rebill) (Transmission Rejected/Transaction Rejected) ............................................. 281
11.4.5.5.2 Response Message Segment (Claim Rebill) (Transmission Rejected/Transaction Rejected)..........................................281
11.4.5.5.3 Response Status Segment (Claim Rebill) (Transmission Rejected/Transaction Rejected)............................................... 281
11.5 SERVICE REBILL REQUEST DIAGRAMS ................................................................................................................................................283
11.5.1.1 Diagram For Transmission Of One Service Rebill Transaction ...................................................................................................... 283
11.5.1.2 Diagram For Transmission Of Two Service Rebill Transactions ....................................................................................................283
11.5.1.3 Diagram For Transmission Of Three Service Rebill Transactions .................................................................................................284
11.5.1.4 Diagram For Transmission Of Four Service Rebill Transactions ................................................................................................... 285
11.6 SERVICE REBILL REQUEST SEGMENTS................................................................................................................................................287
11.6.1 Transaction Header Segment (Service Rebill) ....................................................................................................................287
11.6.2 Insurance Segment (Service Rebill).....................................................................................................................................287
11.6.3 Patient Segment (Service Rebill)..........................................................................................................................................288
11.6.4 Claim Segment (Service Rebill) ............................................................................................................................................289
11.6.5 Pricing Segment (Service Rebill)..........................................................................................................................................292
11.6.6 Pharmacy Provider Segment (Service Rebill) .....................................................................................................................293
11.6.7 Prescriber Segment (Service Rebill)....................................................................................................................................293
11.6.8 Coordination of Benefits/Other Payments Segment (Service Rebill)................................................................................294
11.6.9 Workers’ Compensation Segment (Service Rebill).............................................................................................................296
11.6.10 DUR/PPS Segment (Service Rebill)......................................................................................................................................297
11.6.11 Clinical Segment (Service Rebill) .........................................................................................................................................297
11.6.12 Additional Documentation Segment (Service Rebill) .........................................................................................................298
11.6.13 Facility Segment (Service Rebill)..........................................................................................................................................299
11.6.14 Narrative Segment (Service Rebill) ......................................................................................................................................300
11.7 SERVICE REBILL RESPONSE DIAGRAMS AND SEGMENTS .....................................................................................................................300
11.7.1 Transmission Accepted/Transaction Paid...........................................................................................................................300
11.7.1.1 Diagram For Transmission Of One Service Rebill Response (Transmission Accepted/Transaction Paid)............................ 300
11.7.1.2 Diagram For Transmission Of Two Service Rebill Responses (Transmission Accepted/Transaction Paid).......................... 301
11.7.1.3 Diagram For Transmission Of Three Service Rebill Responses (Transmission Accepted/Transaction Paid).......................301
11.7.1.4 Diagram For Transmission Of Four Service Rebill Responses (Transmission Accepted/Transaction Paid) .........................302
11.7.1.5 Service Rebill Response Segments (Transmission Accepted/Transaction Paid) .......................................................................303
11.7.1.5.1 Response Header Segment (Service Rebill) (Transmission Accepted/Transaction Paid)................................................. 303
11.7.1.5.2 Response Message Segment (Service Rebill) (Transmission Accepted/Transaction Paid).............................................. 304
11.7.1.5.3 Response Insurance Segment (Service Rebill) (Transmission Accepted/Transaction Paid) ............................................ 304
11.7.1.5.4 Response Patient Segment (Service Rebill) (Transmission Accepted/Transaction Paid)..................................................305
11.7.1.5.5 Response Status Segment (Service Rebill) (Transmission Accepted/Transaction Paid)...................................................305
11.7.1.5.6 Response Claim Segment (Service Rebill) (Transmission Accepted/Transaction Paid)....................................................306
11.7.1.5.7 Response Pricing Segment (Service Rebill) (Transmission Accepted/Transaction Paid).................................................. 307
11.7.1.5.8 Response DUR/PPS Segment (Service Rebill) (Transmission Accepted/Transaction Paid) ............................................309
11.7.1.5.9 Response Coordination of Benefits/Other Payers Segment (Service Rebill) (Transmission Accepted/Transaction Paid)
310
11.7.2 Transmission Accepted/Transaction Captured ..................................................................................................................310
11.7.2.1 Diagram For Transmission Of One Service Rebill Response (Transmission Accepted/Transaction Captured) ...................310
11.7.2.2 Diagram For Transmission Of Two Service Rebill Responses (Transmission Accepted/Transaction Captured) ................. 311
11.7.2.3 Diagram For Transmission Of Three Service Rebill Responses (Transmission Accepted/Transaction Captured) .............. 311

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 11 -
11.7.2.4 Diagram For Transmission Of Four Service Rebill Responses (Transmission Accepted/Transaction Captured)................. 312
11.7.2.5 Service Rebill Response Segments (Transmission Accepted/Transaction Captured)............................................................... 313
11.7.2.5.1 Response Header Segment (Service Rebill) (Transmission Accepted/Transaction Captured) ........................................313
11.7.2.5.2 Response Message Segment (Service Rebill) (Transmission Accepted/Transaction Captured) ..................................... 313
11.7.2.5.3 Response Insurance Segment (Service Rebill) (Transmission Accepted/Transaction Captured).................................... 314
11.7.2.5.4 Response Patient Segment (Service Rebill) (Transmission Accepted/Transaction Captured) .........................................314
11.7.2.5.5 Response Status Segment (Service Rebill) (Transmission Accepted/Transaction Captured) ..........................................315
11.7.2.5.6 Response Claim Segment (Service Rebill) (Transmission Accepted/Transaction Captured) ...........................................316
11.7.2.5.7 Response Pricing Segment (Service Rebill) (Transmission Accepted/Transaction Captured) ......................................... 316
11.7.3 Transmission Accepted/Transaction Rejected ...................................................................................................................319
11.7.3.1 Diagram for Transmission Of One Service Rebill Response (Transmission Accepted/Transaction Rejected) ..................... 319
11.7.3.2 Diagram for Transmission Of Two Service Rebill Responses (Transmission Accepted/Transaction Rejected) ................... 319
11.7.3.3 Diagram for Transmission Of Three Service Rebill Responses (Transmission Accepted/Transaction Rejected) ................320
11.7.3.4 Diagram for Transmission Of Four Service Rebill Responses (Transmission Accepted/Transaction Rejected)................... 321
11.7.3.5 Service Rebill Response Segments (Transmission Accepted/Transaction Rejected) ............................................................... 321
11.7.3.5.1 Response Header Segment (Service Rebill) (Transmission Accepted/Transaction Rejected) .........................................322
11.7.3.5.2 Response Message Segment (Service Rebill) (Transmission Accepted/Transaction Rejected)...................................... 322
11.7.3.5.3 Response Insurance Segment (Service Rebill) (Transmission Accepted/Transaction Rejected).....................................322
11.7.3.5.4 Response Patient Segment (Service Rebill) (Transmission Accepted/Transaction Rejected)..........................................323
11.7.3.5.5 Response Status Segment (Service Rebill) (Transmission Accepted/Transaction Rejected)...........................................323
11.7.3.5.6 Response Claim Segment (Service Rebill) (Transmission Accepted/Transaction Rejected)............................................324
11.7.3.5.7 Response Prior Authorization Segment (Service Rebill) (Transmission Accepted/Transaction Rejected) .....................325
11.7.3.5.8 Response Coordination of Benefits/Other Payers Segment (Service Rebill) (Transmission Accepted/Transaction
Rejected) 325
11.7.4 Transmission Rejected/Transaction Rejected ....................................................................................................................326
11.7.4.1 Diagram For Transmission Of One Service Rebill Response (Transmission Rejected/Transaction Rejected).....................327
11.7.4.2 Diagram For Transmission Of Two Service Rebill Responses (Transmission Rejected/Transaction Rejected)...................327
11.7.4.3 Diagram For Transmission Of Three Service Rebill Responses (Transmission Rejected/Transaction Rejected)................ 327
11.7.4.4 Diagram For Transmission Of Four Service Rebill Responses (Transmission Rejected/Transaction Rejected) .................. 327
11.7.4.5 Service Rebill Response Segments (Transmission Rejected/Transaction Rejected) ................................................................328
11.7.4.5.1 Response Header Segment (Service Rebill) (Transmission Rejected/Transaction Rejected)..........................................328
11.7.4.5.2 Response Message Segment (Service Rebill) (Transmission Rejected/Transaction Rejected).......................................328
11.7.4.5.3 Response Status Segment (Service Rebill) (Transmission Rejected/Transaction Rejected)............................................ 329
12. PRIOR AUTHORIZATION INFORMATION............................................................................................................................................................ 331
13. PRIOR AUTHORIZATION REQUEST AND BILLING INFORMATION............................................................................................................332
13.1 PRIOR AUTHORIZATION REQUEST AND BILLING REQUEST DIAGRAMS...................................................................................................332
13.1.1 Diagram For Transmission Of One Prior Authorization Request And Billing Transaction.............................................332
13.2 PRIOR AUTHORIZATION REQUEST AND BILLING REQUEST SEGMENTS ..................................................................................................333
13.2.1 Transaction Header Segment (Prior Authorization Request And Billing) ........................................................................333
13.2.2 Insurance Segment (Prior Authorization Request And Billing).........................................................................................333
13.2.3 Patient Segment (Prior Authorization Request And Billing)..............................................................................................334
13.2.4 Claim Segment (Prior Authorization Request And Billing)................................................................................................335
13.2.5 Pricing Segment (Prior Authorization Request And Billing)..............................................................................................339
13.2.6 Prior Authorization Segment (Prior Authorization Request And Billing) .........................................................................341
13.2.7 Pharmacy Provider Segment (Prior Authorization Request And Billing).........................................................................342
13.2.8 Prescriber Segment (Prior Authorization Request And Billing)........................................................................................342
13.2.9 Coordination of Benefits/Other Payments Segment (Prior Authorization Request And Billing) ...................................343
13.2.10 Workers’ Compensation Segment (Prior Authorization Request And Billing).................................................................344
13.2.11 DUR/PPS Segment (Prior Authorization Request And Billing)..........................................................................................345
13.2.12 Compound Segment (Prior Authorization Request And Billing).......................................................................................346
13.2.13 Clinical Segment (Prior Authorization Request And Billing).............................................................................................347
13.2.14 Additional Documentation Segment (Prior Authorization Request And Billing) .............................................................348
13.2.15 Facility Segment (Prior Authorization Request And Billing) .............................................................................................349
13.2.16 Narrative Segment (Prior Authorization Request And Billing) ..........................................................................................350
13.3 PRIOR AUTHORIZATION REQUEST AND BILLING RESPONSE DIAGRAMS AND SEGMENTS .......................................................................350
13.3.1 Transmission Accepted/Transaction Paid...........................................................................................................................350
13.3.1.1 Diagram For Transmission Of One Prior Authorization Request And Billing Response (Transmission Accepted/Transaction
Paid) 350
13.3.1.2 Prior Authorization Request And Billing Response Segments (Transmission Accepted/Transaction Paid) .......................... 350
13.3.1.2.1 Response Header Segment (Prior Authorization Request And Billing) (Transmission Accepted/Transaction Paid) ....350
13.3.1.2.2 Response Message Segment (Prior Authorization Request And Billing) (Transmission Accepted/Transaction Paid).351
13.3.1.2.3 Response Insurance Segment (Prior Authorization Request And Billing) (Transmission Accepted/Transaction Paid) 351
13.3.1.2.4 Response Patient Segment (Prior Authorization Request And Billing) (Transmission Accepted/Transaction Paid).....352
13.3.1.2.5 Response Status Segment (Prior Authorization Request And Billing) (Transmission Accepted/Transaction Paid)......352
13.3.1.2.6 Response Claim Segment (Prior Authorization Request And Billing) (Transmission Accepted/Transaction Paid).......353

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 12 -
13.3.1.2.7 Response Pricing Segment (Prior Authorization Request And Billing) (Transmission Accepted/Transaction Paid)..... 354
13.3.1.2.8 Response Prior Authorization Segment (Prior Authorization Request And Billing) (Transmission Accepted/Transaction
Paid) 358
13.3.1.2.9 Response DUR/PPS Segment (Prior Authorization Request And Billing) (Transmission Accepted/Transaction Paid)359
13.3.1.2.10 Response Coordination of Benefits/Other Payers Segment (Prior Authorization Request And Billing) (Transmission
Accepted/Transaction Paid) .................................................................................................................................................................................360
13.3.2 Transmission Accepted/Transaction Captured ..................................................................................................................361
13.3.2.1 Diagram For Transmission Of One Prior Authorization Request And Billing Response (Transmission Accepted/Transaction
Captured) 361
13.3.2.2 Prior Authorization Request And Billing Response Segments (Transmission Accepted/Transaction Captured).................. 361
13.3.2.2.1 Response Header Segment (Prior Authorization Request And Billing) (Transmission Accepted/Transaction Captured)
362
13.3.2.2.2 Response Message Segment (Prior Authorization Request And Billing) (Transmission Accepted/Transaction
Captured) 362
13.3.2.2.3 Response Insurance Segment (Prior Authorization Request And Billing) (Transmission Accepted/Transaction
Captured) 362
13.3.2.2.4 Response Patient Segment (Prior Authorization Request And Billing) (Transmission Accepted/Transaction Captured)
363
13.3.2.2.5 Response Status Segment (Prior Authorization Request And Billing) (Transmission Accepted/Transaction Captured)
363
13.3.2.2.6 Response Claim Segment (Prior Authorization Request And Billing) (Transmission Accepted/Transaction Captured)
364
13.3.2.2.7 Response DUR/PPS Segment (Prior Authorization Request And Billing) (Transmission Accepted/Transaction
Captured) 365
13.3.3 Transmission Accepted/Transaction Deferred ...................................................................................................................367
13.3.3.1 Diagram For Transmission Of One Prior Authorization Request And Billing Response (Transmission Accepted/Transaction
Deferred) 367
13.3.3.2 Prior Authorization Request And Billing Response Segments (Transmission Accepted/Transaction Deferred)...................367
13.3.3.2.1 Response Header Segment (Prior Authorization Request And Billing) (Transmission Accepted/Transaction Deferred)
367
13.3.3.2.2 Response Message Segment (Prior Authorization Request And Billing) (Transmission Accepted/Transaction Deferred)
368
13.3.3.2.3 Response Insurance Segment (Prior Authorization Request And Billing) (Transmission Accepted/Transaction
Deferred) 368
13.3.3.2.4 Response Patient Segment (Prior Authorization Request And Billing) (Transmission Accepted/Transaction Deferred)
369
13.3.3.2.5 Response Status Segment (Prior Authorization Request And Billing) (Transmission Accepted/Transaction Deferred)
369
13.3.3.2.6 Response Claim Segment (Prior Authorization Request And Billing) (Transmission Accepted/Transaction Deferred)370
13.3.3.2.7 Response Prior Authorization Segment (Prior Authorization Request And Billing) (Transmission Accepted/Transaction
Deferred) 371
13.3.3.2.8 Response DUR/PPS Segment (Prior Authorization Request And Billing) (Transmission Accepted/Transaction
Deferred) 371
13.3.4 Transmission Accepted/Transaction Rejected Response.................................................................................................372
13.3.4.1 Diagram For Transmission Of One Prior Authorization Request And Billing Response (Transmission Accepted/Transaction
Rejected) 373
13.3.4.2 Prior Authorization Request And Billing Response Segments (Transmission Accepted/Transaction Rejected)...................373
13.3.4.2.1 Response Header Segment (Prior Authorization Request And Billing) (Transmission Accepted/Transaction Rejected)
373
13.3.4.2.2 Response Message Segment (Prior Authorization Request And Billing) (Transmission Accepted/Transaction Rejected)
373
13.3.4.2.3 Response Insurance Segment (Prior Authorization Request And Billing) (Transmission Accepted/Transaction
Rejected) 374
13.3.4.2.4 Response Patient Segment (Prior Authorization Request And Billing) (Transmission Accepted/Transaction Rejected)
375
13.3.4.2.5 Response Status Segment (Prior Authorization Request And Billing) (Transmission Accepted/Transaction Rejected)
375
13.3.4.2.6 Response Claim Segment (Prior Authorization Request And Billing) (Transmission Accepted/Transaction Rejected)376
13.3.4.2.7 Response DUR/PPS Segment (Prior Authorization Request And Billing) (Transmission Accepted/Transaction
Rejected) 377
13.3.4.2.8 Response Coordination of Benefits/Other Payers Segment (Prior Authorization Request And Billing) (Transmission
Accepted/Transaction Rejected) .........................................................................................................................................................................379
13.3.5 Transmission Rejected/Transaction Rejected ....................................................................................................................380
13.3.5.1 Diagram For Transmission Of One Prior Authorization Request And Billing Response (Transmission Rejected/Transaction
Rejected) 380
13.3.5.2 Prior Authorization Request And Billing Response Segments (Transmission Rejected/Transaction Rejected) ...................380
13.3.5.2.1 Response Header Segment (Prior Authorization Request And Billing) (Transmission Rejected/Transaction Rejected)
380

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 13 -
13.3.5.2.2 Response Message Segment (Prior Authorization Request And Billing) (Transmission Rejected/Transaction Rejected)
380
13.3.5.2.3 Response Status Segment (Prior Authorization Request And Billing) (Transmission Rejected/Transaction Rejected)381
14. PRIOR AUTHORIZATION REVERSAL INFORMATION.....................................................................................................................................383
14.1 PRIOR AUTHORIZATION REVERSAL REQUEST DIAGRAMS......................................................................................................................383
14.1.1 Diagram For Transmission Of One Prior Authorization Reversal Transaction................................................................383
14.2 PRIOR AUTHORIZATION REVERSAL REQUEST SEGMENTS.....................................................................................................................383
14.2.1 Transaction Header Segment (Prior Authorization Reversal) ...........................................................................................383
14.2.2 Insurance Segment (Prior Authorization Reversal)............................................................................................................384
14.2.3 Prior Authorization Segment (Prior Authorization Reversal) ............................................................................................384
14.2.4 Prior Authorization Reversal Response Diagrams And Segments...................................................................................385
14.2.5 Transmission Accepted/Transaction Approved .................................................................................................................385
14.2.5.1 Diagram For Transmission Of One Prior Authorization Reversal Response (Transmission Accepted/Transaction Approved)
385
14.2.5.2 Prior Authorization Reversal Response Segments (Transmission Accepted/Transaction Approved).....................................385
14.2.5.2.1 Response Header Segment (Prior Authorization Reversal) (Transmission Accepted/Transaction Approved) ..............385
14.2.5.2.2 Response Message Segment (Prior Authorization Reversal) (Transmission Accepted/Transaction Approved) ...........386
14.2.5.2.3 Response Status Segment (Prior Authorization Reversal) (Transmission Accepted/Transaction Approved)................386
14.2.6 Transmission Accepted/Transaction Captured ..................................................................................................................387
14.2.6.1 Diagram For Transmission Of One Prior Authorization Reversal Response (Transmission Accepted/Transaction Captured)
387
14.2.6.2 Prior Authorization Reversal Response Segments (Transmission Accepted/Transaction Captured) .....................................388
14.2.6.2.1 Response Header Segment (Prior Authorization Reversal) (Transmission Accepted/Transaction Captured)............... 388
14.2.6.2.2 Response Message Segment (Prior Authorization Reversal) (Transmission Accepted/Transaction Captured)............ 388
14.2.6.2.3 Response Status Segment (Prior Authorization Reversal) (Transmission Accepted/Transaction Captured).................388
14.2.7 Transmission Accepted/Transaction Rejected ...................................................................................................................389
14.2.7.1 Diagram For Transmission Of One Prior Authorization Reversal Response (Transmission Accepted/Transaction Rejected)
390
14.2.7.2 Prior Authorization Reversal Response Segments (Transmission Accepted/Transaction Rejected)...................................... 390
14.2.7.2.1 Response Header Segment (Prior Authorization Reversal) (Transmission Accepted/Transaction Rejected)................390
14.2.7.2.2 Response Message Segment (Prior Authorization Reversal) (Transmission Accepted/Transaction Rejected) ............ 390
14.2.7.2.3 Response Status Segment (Prior Authorization Reversal) (Transmission Accepted/Transaction Rejected) .................391
14.2.8 Transmission Rejected/Transaction Rejected ....................................................................................................................392
14.2.8.1 Diagram For Transmission Of One Prior Authorization Reversal Response (Transmission Rejected/Transaction Rejected)
392
14.2.8.2 Prior Authorization Reversal Response Segments (Transmission Rejected/Transaction Rejected).......................................392
14.2.8.2.1 Response Header Segment (Prior Authorization Reversal) (Transmission Rejected/Transaction Rejected) ................392
14.2.8.2.2 Response Message Segment (Prior Authorization Reversal) (Transmission Rejected/Transaction Rejected) ............. 392
14.2.8.2.3 Response Status Segment (Prior Authorization Reversal) (Transmission Rejected/Transaction Rejected) ..................393
15. PRIOR AUTHORIZATION INQUIRY INFORMATION.......................................................................................................................................... 395
15.1 PRIOR AUTHORIZATION INQUIRY REQUEST DIAGRAMS .........................................................................................................................395
15.1.1 Diagram For Transmission Of One Prior Authorization Inquiry Transaction...................................................................395
15.2 PRIOR AUTHORIZATION INQUIRY REQUEST SEGMENTS.........................................................................................................................396
15.2.1 Transaction Header Segment (Prior Authorization Inquiry) ..............................................................................................396
15.2.2 Insurance Segment (Prior Authorization Inquiry)...............................................................................................................396
15.2.3 Prior Authorization Segment (Prior Authorization Inquiry) ...............................................................................................397
15.3 PRIOR AUTHORIZATION INQUIRY RESPONSE DIAGRAMS AND SEGMENTS ..............................................................................................397
15.3.1 Transmission Accepted/Transaction Paid...........................................................................................................................397
15.3.1.1 Diagram For Transmission Of One Prior Authorization Inquiry Response (Transmission Accepted/Transaction Paid) ...... 397
15.3.1.2 Prior Authorization Inquiry Response Segments (Transmission Accepted/Transaction Paid)..................................................398
15.3.1.2.1 Response Header Segment (Prior Authorization Inquiry) (Transmission Accepted/Transaction Paid) ...........................398
15.3.1.2.2 Response Message Segment (Prior Authorization Inquiry) (Transmission Accepted/Transaction Paid) ........................398
15.3.1.2.3 Response Insurance Segment (Prior Authorization Inquiry) (Transmission Accepted/Transaction Paid).......................399
15.3.1.2.4 Response Patient Segment (Prior Authorization Inquiry) (Transmission Accepted/Transaction Paid)............................ 399
15.3.1.2.5 Response Status Segment (Prior Authorization Inquiry) (Transmission Accepted/Transaction Paid).............................400
15.3.1.2.6 Response Claim Segment (Prior Authorization Inquiry) (Transmission Accepted/Transaction Paid) .............................. 401
15.3.1.2.7 Response Pricing Segment (Prior Authorization Inquiry) (Transmission Accepted/Transaction Paid) ............................402
15.3.1.2.8 Response Prior Authorization Segment (Prior Authorization Inquiry) (Transmission Accepted/Transaction Paid) ....... 406
15.3.1.2.9 Response DUR/PPS Segment (Prior Authorization Inquiry) (Transmission Accepted/Transaction Paid).......................406
15.3.1.2.10 Response Coordination of Benefits/Other Payers Segment (Prior Authorization Inquiry) (Transmission
Accepted/Transaction Paid) .................................................................................................................................................................................407
15.3.2 Transmission Accepted/Transaction Captured ..................................................................................................................408
15.3.2.1 Diagram For Transmission Of One Prior Authorization Inquiry Response (Transmission Accepted/Transaction Captured)
408
15.3.2.2 Prior Authorization Inquiry Response Segments (Transmission Accepted/Transaction Captured) .........................................408

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 14 -
15.3.2.2.1 Response Header Segment (Prior Authorization Inquiry) (Transmission Accepted/Transaction Captured)................... 409
15.3.2.2.2 Response Message Segment (Prior Authorization Inquiry) (Transmission Accepted/Transaction Captured)................409
15.3.2.2.3 Response Status Segment (Prior Authorization Inquiry) (Transmission Accepted/Transaction Captured).....................409
15.3.3 Transmission Accepted/Transaction Approved .................................................................................................................410
15.3.3.1 Diagram For Transmission Of One Prior Authorization Inquiry Response (Transmission Accepted/Transaction Approved)
411
15.3.3.2 Prior Authorization Inquiry Response Segments (Transmission Accepted/Transaction Approved) ........................................411
15.3.3.2.1 Response Header Segment (Prior Authorization Inquiry) (Transmission Accepted/Transaction Approved) ..................411
15.3.3.2.2 Response Message Segment (Prior Authorization Inquiry) (Transmission Accepted/Transaction Approved)............... 411
15.3.3.2.3 Response Status Segment (Prior Authorization Inquiry) (Transmission Accepted/Transaction Approved)....................412
15.3.3.2.4 Response Claim Segment (Prior Authorization Inquiry) (Transmission Accepted/Transaction Approved).....................413
15.3.3.2.5 Response Prior Authorization Segment (Prior Authorization Inquiry) (Transmission Accepted/Transaction Approved)
414
15.3.4 Transmission Accepted/Transaction Deferred ...................................................................................................................414
15.3.4.1 Diagram For Transmission Of One Prior Authorization Inquiry Response (Transmission Accepted/Transaction Deferred)
414
15.3.4.2 Prior Authorization Inquiry Response Segments (Transmission Accepted/Transaction Deferred)..........................................415
15.3.4.2.1 Response Header Segment (Prior Authorization Inquiry) (Transmission Accepted/Transaction Deferred)....................415
15.3.4.2.2 Response Message Segment (Prior Authorization Inquiry) (Transmission Accepted/Transaction Deferred) ................415
15.3.4.2.3 Response Status Segment (Prior Authorization Inquiry) (Transmission Accepted/Transaction Deferred) ..................... 415
15.3.4.2.4 Response Claim Segment (Prior Authorization Inquiry) (Transmission Accepted/Transaction Deferred).......................417
15.3.4.2.5 Response Prior Authorization Segment (Prior Authorization Inquiry) (Transmission Accepted/Transaction Deferred) 417
15.3.5 Transmission Accepted/Transaction Rejected ...................................................................................................................418
15.3.5.1 Diagram For Transmission Of One Prior Authorization Inquiry Response (Transmission Accepted/Transaction Rejected)
418
15.3.5.2 Prior Authorization Inquiry Response Segments (Transmission Accepted/Transaction Rejected)..........................................418
15.3.5.2.1 Response Header Segment (Prior Authorization Inquiry) (Transmission Accepted/Transaction Rejected) ................... 418
15.3.5.2.2 Response Message Segment (Prior Authorization Inquiry) (Transmission Accepted/Transaction Rejected) ................419
15.3.5.2.3 Response Status Segment (Prior Authorization Inquiry) (Transmission Accepted/Transaction Rejected) ..................... 419
15.3.5.2.4 Response Claim Segment (Prior Authorization Inquiry) (Transmission Accepted/Transaction Rejected) ...................... 420
15.3.5.2.5 Response Coordination of Benefits/Other Payers Segment (Prior Authorization Inquiry) (Transmission
Accepted/Transaction Rejected) .........................................................................................................................................................................421
15.3.6 Transmission Rejected/Transaction Rejected ....................................................................................................................422
15.3.6.1 Diagram For Transmission Of One Prior Authorization Inquiry Response (Transmission Rejected/Transaction Rejected)422
15.3.6.2 Prior Authorization Inquiry Response Segments (Transmission Rejected/Transaction Rejected)........................................... 422
15.3.6.2.1 Response Header Segment (Prior Authorization Inquiry) (Transmission Rejected/Transaction Rejected) .................... 423
15.3.6.2.2 Response Message Segment (Prior Authorization Inquiry) (Transmission Rejected/Transaction Rejected) ................. 423
15.3.6.2.3 Response Status Segment (Prior Authorization Inquiry) (Transmission Rejected/Transaction Rejected) ......................423
16. PRIOR AUTHORIZATION REQUEST ONLY INFORMATION........................................................................................................................... 425
16.1 PRIOR AUTHORIZATION REQUEST ONLY REQUEST DIAGRAMS..............................................................................................................425
16.1.1 Diagram For Transmission Of One Prior Authorization Request Only (Claim) Transaction ..........................................425
16.1.2 Diagram For Transmission Of One Prior Authorization Request Only (Service) Transaction .......................................425
16.2 PRIOR AUTHORIZATION REQUEST ONLY REQUEST SEGMENTS .............................................................................................................426
16.2.1 Transaction Header Segment (Prior Authorization Request Only) ...................................................................................426
16.2.2 Insurance Segment (Prior Authorization Request Only)....................................................................................................426
16.2.3 Patient Segment (Prior Authorization Request Only).........................................................................................................428
16.2.4 Claim Segment (Prior Authorization Request Only)...........................................................................................................430
16.2.5 Prior Authorization Segment (Prior Authorization Request Only) ....................................................................................432
16.2.6 Prescriber Segment (Prior Authorization Request Only)...................................................................................................433
16.2.7 Workers’ Compensation Segment (Prior Authorization Request Only)............................................................................434
16.2.8 DUR/PPS Segment (Prior Authorization Request Only).....................................................................................................434
16.2.9 Compound Segment (Prior Authorization Request Only)..................................................................................................435
16.2.10 Clinical Segment (Prior Authorization Request Only)........................................................................................................435
16.3 PRIOR AUTHORIZATION REQUEST ONLY RESPONSE DIAGRAMS AND SEGMENTS ..................................................................................437
16.3.1 Transmission Accepted/Transaction Approved .................................................................................................................437
16.3.1.1 Diagram For Transmission Of One Prior Authorization Request Only Response (Transmission Accepted/Transaction
Approved) 437
16.3.1.2 Prior Authorization Request Only Response Segments (Transmission Accepted/Transaction Approved) ............................437
16.3.1.2.1 Response Header Segment (Prior Authorization Request Only) (Transmission Accepted/Transaction Approved)......437
16.3.1.2.2 Response Message Segment (Prior Authorization Request Only) (Transmission Accepted/Transaction Approved)...437
16.3.1.2.3 Response Status Segment (Prior Authorization Request Only) (Transmission Accepted/Transaction Approved) .......438
16.3.1.2.4 Response Claim Segment (Prior Authorization Request Only) (Transmission Accepted/Transaction Approved).........439
16.3.1.2.5 Response Prior Authorization Segment (Prior Authorization Request Only) (Transmission Accepted/Transaction
Approved) 440
16.3.1.2.6 Response Coordination of Benefits/Other Payers Segment (Prior Authorization Request Only) (Transmission
Accepted/Transaction Approved) ........................................................................................................................................................................ 440

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 15 -
16.3.2 Transmission Accepted/Transaction Captured ..................................................................................................................441
16.3.2.1 Diagram For Transmission Of One Prior Authorization Request Only Response (Transmission Accepted/Transaction
Captured) 441
16.3.2.2 Prior Authorization Request Only Response Segments (Transmission Accepted/Transaction Captured)............................. 442
16.3.2.2.1 Response Header Segment (Prior Authorization Request Only) (Transmission Accepted/Transaction Captured) ...... 442
16.3.2.2.2 Response Message Segment (Prior Authorization Request Only) (Transmission Accepted/Transaction Captured) ...442
16.3.2.2.3 Response Status Segment (Prior Authorization Request Only) (Transmission Accepted/Transaction Captured) ........443
16.3.2.2.4 Response Claim Segment (Prior Authorization Request Only) (Transmission Accepted/Transaction Captured) ......... 444
16.3.3 Transmission Accepted/Transaction Deferred ...................................................................................................................444
16.3.3.1 Diagram For Transmission Of One Prior Authorization Request Only Response (Transmission Accepted/Transaction
Deferred) 444
16.3.3.2 Prior Authorization Request Only Response Segments (Transmission Accepted/Transaction Deferred).............................. 445
16.3.3.2.1 Response Header Segment (Prior Authorization Request Only) (Transmission Accepted/Transaction Deferred) .......445
16.3.3.2.2 Response Message Segment (Prior Authorization Request Only) (Transmission Accepted/Transaction Deferred) .... 445
16.3.3.2.3 Response Status Segment (Prior Authorization Request Only) (Transmission Accepted/Transaction Deferred) .........446
16.3.3.2.4 Response Claim Segment (Prior Authorization Request Only) (Transmission Accepted/Transaction Deferred) ..........447
16.3.3.2.5 Response Prior Authorization Segment (Prior Authorization Request Only) (Transmission Accepted/Transaction
Deferred) 447
16.3.4 Transmission Accepted/Transaction Rejected ...................................................................................................................448
16.3.4.1 Diagram For Transmission Of One Prior Authorization Request Only Response (Transmission Accepted/Transaction
Rejected) 448
16.3.4.2 Prior Authorization Request Only Response Segments (Transmission Accepted/Transaction Rejected) .............................448
16.3.4.2.1 Response Header Segment (Prior Authorization Request Only) (Transmission Accepted/Transaction Rejected) .......448
16.3.4.2.2 Response Message Segment (Prior Authorization Request Only) (Transmission Accepted/Transaction Rejected)....449
16.3.4.2.3 Response Status Segment (Prior Authorization Request Only) (Transmission Accepted/Transaction Rejected).........449
16.3.4.2.4 Response Claim Segment (Prior Authorization Request Only) (Transmission Accepted/Transaction Rejected).......... 450
16.3.4.2.5 Response Coordination of Benefits/Other Payers Segment (Prior Authorization Request Only) (Transmission
Accepted/Transaction Approved) ........................................................................................................................................................................ 451
16.3.5 Transmission Rejected/Transaction Rejected Response ..................................................................................................452
16.3.5.1 Diagram For Transmission Of One Prior Authorization Request Only Response (Transmission Rejected/Transaction
Rejected) 452
16.3.5.2 Prior Authorization Request Only Response Segments (Transmission Rejected/Transaction Rejected) .............................. 452
16.3.5.2.1 Response Header Segment (Prior Authorization Request Only) (Transmission Rejected/Transaction Rejected)........452
16.3.5.2.2 Response Message Segment (Prior Authorization Request Only) (Transmission Rejected/Transaction Rejected)..... 453
16.3.5.2.3 Response Status Segment (Prior Authorization Request Only) (Transmission Rejected/Transaction Rejected)..........453
17. PRIOR AUTHORIZATION TRANSACTION DISCUSSION.................................................................................................................................455
17.1 TRANSACTION USAGE.........................................................................................................................................................................455
17.1.1 Prior Authorization Request And Billing .............................................................................................................................455
17.1.2 Prior Authorization Request Only ........................................................................................................................................455
17.1.3 Prior Authorization Inquiry....................................................................................................................................................456
17.1.4 Prior Authorization Reversal.................................................................................................................................................457
17.2 FIELD CLARIFICATION .........................................................................................................................................................................457
17.2.1 Prior Authorization Fields .....................................................................................................................................................457
17.2.2 Prior Authorization Number-Assigned (498-PY) in Response Prior Authorization Segment) and Authorization Number
(5Ø3-F3) in Response Status Segment..................................................................................................................................................457
17.2.3 Authorization Number (5Ø3-F3) In Prior Authorization Segment......................................................................................458
17.2.4 Prior Authorization Number Submitted (462-EV) In Claim Segment.................................................................................458
17.3 SCENARIO EXAMPLES .........................................................................................................................................................................458
17.3.1 Prior Authorization Request And Billing Responses .........................................................................................................458
17.3.1.1 Scenarios For Prior Authorization Request And Billing....................................................................................................................459
17.3.2 Prior Authorization Request Only Responses ....................................................................................................................459
17.3.2.1 Scenarios For Prior Authorization Request Only............................................................................................................................... 460
18. CONTROLLED SUBSTANCE REPORTING INFORMATION............................................................................................................................461
18.1 CONTROLLED SUBSTANCE REPORTING ...............................................................................................................................................461
18.2 CONTROLLED SUBSTANCE REPORTING REQUEST DIAGRAMS...............................................................................................................461
18.2.1 Diagram For Transmission Of One Controlled Substance Reporting Transaction .........................................................461
18.2.2 Diagram For Transmission Of Two Controlled Substance Reporting Transactions.......................................................462
18.2.3 Diagram For Transmission Of Three Or Four Controlled Substance Reporting Transactions ......................................462
18.3 CONTROLLED SUBSTANCE REPORTING RESPONSE DIAGRAMS.............................................................................................................462
18.3.1 Transmission Accepted/Transaction Captured, Approved, Rejected...............................................................................462
18.3.1.1 Diagram For Transmission Of One Controlled Substance Reporting Responses (Transmission Accepted/Transaction
Captured, Approved, Rejected) ................................................................................................................................................................................ 462
18.3.1.2 Diagram For Transmission Of Two Controlled Substance Reporting Responses (Transmission Accepted/Transaction
Captured, Approved, Rejected) ................................................................................................................................................................................ 463

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 16 -
18.3.1.3 Diagram For Transmission Of Three or Four Controlled Substance Reporting Responses (Transmission
Accepted/Transaction Captured, Approved, Rejected) ........................................................................................................................................463
18.3.2 Transmission Rejected/Transaction Rejected ....................................................................................................................463
18.3.2.1 Diagram For Transmission Of One Controlled Substance Reporting Response (Transmission Rejected/Transaction
Rejected) 463
18.3.2.2 Diagram For Transmission Of Two Controlled Substance Reporting Responses (Transmission Rejected/Transaction
Rejected) 463
18.3.2.3 Diagram For Transmission Of Three or Four Controlled Substance Reporting Responses (Transmission
Rejected/Transaction Rejected) ...............................................................................................................................................................................464
19. CONTROLLED SUBSTANCE REPORTING REVERSAL INFORMATION.....................................................................................................465
19.1 CONTROLLED SUBSTANCE REPORTING REVERSAL ..............................................................................................................................465
19.2 CONTROLLED SUBSTANCE REPORTING REVERSAL REQUEST DIAGRAMS..............................................................................................465
19.2.1 Diagram For Transmission Of One Controlled Substance Reporting Reversal Transaction .........................................465
19.2.2 Diagram For Transmission Of Two Controlled Substance Reporting Reversal Transactions.......................................466
19.2.3 Diagram for Transmission Of Three or Four Controlled Substance Reporting Reversal Transactions........................466
19.3 CONTROLLED SUBSTANCE REPORTING REVERSAL RESPONSE DIAGRAMS............................................................................................466
19.3.1 Transmission Accepted/Transaction Approved, Captured, Rejected...............................................................................466
19.3.1.1 Diagram For Transmission Of One Controlled Substance Reporting Reversal Response (Transmission
Accepted/Transaction Approved, Captured, Rejected) ........................................................................................................................................466
19.3.1.2 Diagram For Transmission Of Two Controlled Substance Reporting Reversal Responses (Transmission
Accepted/Transaction Approved, Captured, Rejected) ........................................................................................................................................467
19.3.1.3 Diagram for Transmission Of Three or Four Controlled Substance Reporting Reversal Responses (Transmission
Accepted/Transaction Approved, Captured, Rejected) ........................................................................................................................................467
19.3.2 Transmission Rejected/Transaction Rejected ....................................................................................................................467
19.3.2.1 Diagram For Transmission Of One Controlled Substance Reporting Reversal Response (Transmission
Rejected/Transaction Rejected) ...............................................................................................................................................................................467
19.3.2.2 Diagram For Transmission Of Two Controlled Substance Reporting Reversal Responses (Transmission
Rejected/Transaction Rejected) ...............................................................................................................................................................................467
19.3.2.3 Diagram For Transmission Of Three Or Four Controlled Substance Reporting Reversal Responses (Transmission
Rejected/Transaction Rejected) ...............................................................................................................................................................................468
20. CONTROLLED SUBSTANCE REPORTING REBILL INFORMATION............................................................................................................469
20.1 CONTROLLED SUBSTANCE REPORTING REBILL ...................................................................................................................................469
20.2 CONTROLLED SUBSTANCE REPORTING REBILL REQUEST DIAGRAMS ...................................................................................................469
20.2.1 Diagram For Transmission Of One Controlled Substance Reporting Rebill Transaction ..............................................469
20.2.2 Diagram For Transmission Of Two Controlled Substance Reporting Rebill Transactions ............................................469
20.2.3 Diagram For Transmission Of Three Or Four Controlled Substance Reporting Rebill Transactions ...........................470
20.3 CONTROLLED SUBSTANCE REPORTING REBILL RESPONSE DIAGRAMS.................................................................................................470
20.3.1 Transmission Accepted/Transaction Captured, Approved, Rejected...............................................................................470
20.3.1.1 Diagram For Transmission Of One Controlled Substance Reporting Rebill Response (Transmission Accepted/Transaction
Captured, Approved, Rejected) ................................................................................................................................................................................ 470
20.3.1.2 Diagram For Transmission Of Two Controlled Substance Reporting Rebill Responses (Transmission Accepted/Transaction
Captured, Approved, Rejected) ................................................................................................................................................................................ 470
20.3.1.3 Diagram For Transmission Of Three Or Four Controlled Substance Reporting Rebill Responses (Transmission
Accepted/Transaction Captured, Approved, Rejected) ........................................................................................................................................471
20.3.2 Transmission Rejected/Transaction Rejected ....................................................................................................................471
20.3.2.1 Diagram For Transmission Of One Controlled Substance Reporting Rebill Response (Transmission Rejected/Transaction
Rejected) 471
20.3.2.2 Diagram For Transmission Of Two Controlled Substance Reporting Rebill Responses (Transmission Rejected/Transaction
Rejected) 471
20.3.2.3 Diagram For Transmission Of Three or Four Controlled Substance Reporting Rebill Responses (Transmission
Rejected/Transaction Rejected) ...............................................................................................................................................................................471
21. INFORMATION REPORTING INFORMATION ..................................................................................................................................................... 472
21.1 INFORMATION REPORTING...................................................................................................................................................................472
21.2 INFORMATION REPORTING REQUEST DIAGRAMS ..................................................................................................................................472
21.2.1 Diagram For Transmission Of One Information Reporting Transaction...........................................................................472
21.2.2 Diagram For Transmission Of Two Information Reporting Transactions ........................................................................473
21.2.3 Diagram For Transmission Of Three Information Reporting Transactions......................................................................474
21.2.4 Diagram For Transmission Of Four Information Reporting Transactions........................................................................475
21.3 INFORMATION REPORTING REQUEST SEGMENTS..................................................................................................................................476
21.3.1 Transaction Header Segment (Information Reporting) ......................................................................................................476
21.3.2 Insurance Segment (Information Reporting).......................................................................................................................476
21.3.3 Patient Segment (Information Reporting)............................................................................................................................477
21.3.4 Claim Segment (Information Reporting)..............................................................................................................................479
21.3.5 Pharmacy Provider Segment (Information Reporting).......................................................................................................482

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 17 -
21.3.6 Prescriber Segment (Information Reporting)......................................................................................................................482
21.3.7 Workers’ Compensation Segment (Information Reporting)...............................................................................................483
21.3.8 DUR/PPS Segment (Information Reporting)........................................................................................................................484
21.3.9 Pricing Segment (Information Reporting)............................................................................................................................485
21.3.10 Clinical Segment (Information Reporting)...........................................................................................................................486
21.4 INFORMATION REPORTING RESPONSE DIAGRAMS AND SEGMENTS.......................................................................................................487
21.4.1 Transmission Accepted/Transaction Captured ..................................................................................................................487
21.4.1.1 Diagram For Transmission Of One Information Reporting Response (Transmission Accepted/Transaction Captured) ..... 487
21.4.1.2 Diagram For Transmission Of Two Information Reporting Responses (Transmission Accepted/Transaction Captured) ...487
21.4.1.3 Diagram For Transmission Of Three Information Reporting Responses (Transmission Accepted/Transaction Captured) 488
21.4.1.4 Diagram For Transmission Of Four Information Reporting Responses (Transmission Accepted/Transaction Captured)... 488
21.4.1.5 Information Reporting Response Segments (Transmission Accepted/Transaction Captured).................................................489
21.4.1.5.1 Response Header Segment (Information Reporting) (Transmission Accepted/Transaction Captured) ..........................489
21.4.1.5.2 Response Message Segment (Information Reporting) (Transmission Accepted/Transaction Captured) .......................489
21.4.1.5.3 Response Insurance Segment (Information Reporting) (Transmission Accepted/Transaction Captured)...................... 490
21.4.1.5.4 Response Patient Segment (Information Reporting) (Transmission Accepted/Transaction Captured) ...........................490
21.4.1.5.5 Response Status Segment (Information Reporting) (Transmission Accepted/Transaction Captured) ............................ 491
21.4.1.5.6 Response Claim Segment (Information Reporting) (Transmission Accepted/Transaction Captured) .............................492
21.4.1.5.7 Response DUR/PPS Segment (Information Reporting) (Transmission Accepted/Transaction Captured)...................... 492
21.4.2 Transmission Accepted/Transaction Approved .................................................................................................................494
21.4.2.1 Diagram For Transmission Of One Information Reporting Response (Transmission Accepted/Transaction Approved).....494
21.4.2.2 Diagram For Transmission Of Two Information Reporting Responses (Transmission Accepted/Transaction Approved)...494
21.4.2.3 Diagram For Transmission Of Three Information Reporting Responses (Transmission Accepted/Transaction Approved) 495
21.4.2.4 Diagram For Transmission Of Four Information Reporting Responses (Transmission Accepted/Transaction Approved)..495
21.4.2.5 Information Reporting Response Segments (Transmission Accepted/Transaction Approved)................................................496
21.4.2.5.1 Response Header Segment (Information Reporting) (Transmission Accepted/Transaction Approved)..........................496
21.4.2.5.2 Response Message Segment (Information Reporting) (Transmission Accepted/Transaction Approved) ......................496
21.4.2.5.3 Response Insurance Segment (Information Reporting) (Transmission Accepted/Transaction Approved) .....................497
21.4.2.5.4 Response Patient Segment (Information Reporting) (Transmission Accepted/Transaction Approved) ..........................497
21.4.2.5.5 Response Status Segment (Information Reporting) (Transmission Accepted/Transaction Approved) ...........................498
21.4.2.5.6 Response Claim Segment (Information Reporting) (Transmission Accepted/Transaction Approved).............................499
21.4.2.5.7 Response DUR/PPS Segment (Information Reporting) (Transmission Accepted/Transaction Approved).....................499
21.4.3 Transmission Accepted/Transaction Rejected ...................................................................................................................501
21.4.3.1 Diagram For Transmission Of One Information Reporting Response (Transmission Accepted/Transaction Rejected)...... 501
21.4.3.2 Diagram For Transmission Of Two Information Reporting Responses (Transmission Accepted/Transaction Rejected).... 501
21.4.3.3 Diagram For Transmission Of Three Information Reporting Responses (Transmission Accepted/Transaction Rejected).501
21.4.3.4 Diagram For Transmission Of Four Information Reporting Responses (Transmission Accepted/Transaction Rejected) ...502
21.4.3.5 Information Reporting Response Segments (Transmission Accepted/Transaction Rejected) .................................................502
21.4.3.5.1 Response Header Segment (Information Reporting) (Transmission Accepted/Transaction Rejected)...........................502
21.4.3.5.2 Response Message Segment (Information Reporting) (Transmission Accepted/Transaction Rejected)........................503
21.4.3.5.3 Response Insurance Segment (Information Reporting) (Transmission Accepted/Transaction Rejected) ......................503
21.4.3.5.4 Response Patient Segment (Information Reporting) (Transmission Accepted/Transaction Rejected)............................504
21.4.3.5.5 Response Status Segment (Information Reporting) (Transmission Accepted/Transaction Rejected).............................504
21.4.3.5.6 Response Claim Segment (Information Reporting) (Transmission Accepted/Transaction Rejected).............................. 505
21.4.4 Transmission Rejected/Transaction Rejected ....................................................................................................................506
21.4.4.1 Diagram For Transmission Of One Information Reporting Response (Transmission Rejected/Transaction Rejected).......506
21.4.4.2 Diagram For Transmission Of Two Information Reporting Responses (Transmission Rejected/Transaction Rejected).....506
21.4.4.3 Diagram For Transmission Of Three Information Reporting Responses (Transmission Rejected/Transaction Rejected)..506
21.4.4.4 Diagram For Transmission Of Four Information Reporting Responses (Transmission Rejected/Transaction Rejected) .... 507
21.4.4.5 Information Reporting Response Segments (Transmission Rejected/Transaction Rejected) .................................................. 507
21.4.4.5.1 In Response Header Segment (Information Reporting) (Transmission Rejected/Transaction Rejected) .......................507
21.4.4.5.2 Response Message Segment (Information Reporting) (Transmission Rejected/Transaction Rejected)......................... 508
21.4.4.5.3 Response Status Segment (Information Reporting) (Transmission Rejected/Transaction Rejected)..............................508
22. INFORMATION REPORTING REVERSAL INFORMATION .............................................................................................................................. 510
22.1 INFORMATION REPORTING REVERSAL..................................................................................................................................................510
22.2 INFORMATION REPORTING REVERSAL (CLAIM) REQUEST DIAGRAMS ....................................................................................................510
22.2.1 Diagram For Transmission Of One Information Reporting Reversal (Claim) Transaction .............................................510
22.2.2 Diagram For Transmission Of Two Information Reporting Reversal (Claim) Transactions ...........................................511
22.2.3 Diagram For Transmission Of Three Information Reporting Reversal (Claim) Transactions.........................................511
22.2.4 Diagram For Transmission Of Four Information Reporting Reversal (Claim) Transactions ..........................................511
22.3 INFORMATION REPORTING REVERSAL (CLAIM) REQUEST SEGMENTS....................................................................................................512
22.3.1 Transaction Header Segment (Information Reporting Reversal (Claim)) .........................................................................512
22.3.2 Insurance Segment (Information Reporting Reversal (Claim))..........................................................................................512
22.3.3 Claim Segment (Information Reporting Reversal (Claim)).................................................................................................513
22.4 INFORMATION REPORTING REVERSAL (SERVICE) REQUEST DIAGRAMS.................................................................................................515
22.4.1 Diagram For Transmission Of One Information Reporting Reversal (Service) Transaction ..........................................515

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 18 -
22.4.2 Diagram For Transmission Of Two Information Reporting Reversal (Service) Transactions ........................................515
22.4.3 Diagram For Transmission Of Three Information Reporting Reversal (Service) Transactions......................................515
22.4.4 Diagram For Transmission Of Four Information Reporting Reversal (Service) Transactions .......................................516
22.5 INFORMATION REPORTING REVERSAL (SERVICE) SEGMENTS ...............................................................................................................516
22.5.1 Transaction Header Segment (Information Reporting Reversal (Service)) ......................................................................516
22.5.2 Insurance Segment (Information Reporting Reversal (Service)).......................................................................................516
22.5.3 Claim Segment (Information Reporting Reversal (Service))..............................................................................................517
22.6 INFORMATION REPORTING REVERSAL (CLAIM/SERVICE) RESPONSE DIAGRAMS....................................................................................519
22.6.1 Transmission Accepted/Transaction Approved .................................................................................................................519
22.6.1.1 Diagram For Transmission Of One Information Reporting Reversal Response (Claim/Service) (Transmission
Accepted/Transaction Approved) .............................................................................................................................................................................519
22.6.1.2 Diagram For Transmission Of Two Information Reporting Reversal Responses (Claim/Service) (Transmission
Accepted/Transaction Approved) .............................................................................................................................................................................519
22.6.1.3 Diagram For Transmission Of Three Information Reporting Reversal Responses (Claim/Service) (Transmission
Accepted/Transaction Approved) .............................................................................................................................................................................519
22.6.1.4 Diagram For Transmission Of four Information Reporting Reversal Responses (Claim/Service) (Transmission
Accepted/Transaction Approved) .............................................................................................................................................................................520
22.6.1.5 Information Reporting Reversal Response Segments (Claim/Service) (Transmission Accepted/Transaction Approved) ..520
22.6.1.5.1 Response Header Segment (Information Reporting Reversal (Claim/Service)) (Transmission Accepted/Transaction
Approved) 520
22.6.1.5.2 Response Message Segment (Information Reporting Reversal (Claim/Service)) (Transmission Accepted/Transaction
Approved) 521
22.6.1.5.3 Response Status Segment (Information Reporting Reversal (Claim/Service)) (Transmission Accepted/Transaction
Approved) 521
22.6.1.5.4 Response Claim Segment (Information Reporting Reversal (Claim/Service)) (Transmission Accepted/Transaction
Approved) 522
22.6.2 Transmission Accepted/Transaction Captured ..................................................................................................................523
22.6.2.1 Diagram For Transmission Of One Information Reporting Reversal Response (Claim/Service) (Transmission
Accepted/Transaction Captured).............................................................................................................................................................................. 523
22.6.2.2 Diagram For Transmission Of Two Information Reporting Reversal Responses (Claim/Service) (Transmission
Accepted/Transaction Captured).............................................................................................................................................................................. 523
22.6.2.3 Diagram For Transmission Of Three Information Reporting Reversal Responses (Claim/Service) (Transmission
Accepted/Transaction Captured).............................................................................................................................................................................. 524
22.6.2.4 Diagram For Transmission Of Four Information Reporting Reversal Responses (Claim/Service) (Transmission
Accepted/Transaction Captured).............................................................................................................................................................................. 524
22.6.2.5 Information Reporting Reversal Response Segments (Claim/Service) (Transmission Accepted/Transaction Captured)... 525
22.6.2.5.1 Response Header Segment (Information Reporting Reversal (Claim/Service)) (Transmission Accepted/Transaction
Captured) 525
22.6.2.5.2 Response Message Segment (Information Reporting Reversal (Claim/Service)) (Transmission Accepted/Transaction
Captured) 525
22.6.2.5.3 Response Status Segment (Information Reporting Reversal (Claim/Service)) (Transmission Accepted/Transaction
Captured) 525
22.6.2.5.4 Response Claim Segment (Information Reporting Reversal (Claim/Service)) (Transmission Accepted/Transaction
Captured) 526
22.6.3 Transmission Accepted/Transaction Rejected ...................................................................................................................527
22.6.3.1 Diagram For Transmission Of One Information Reporting Reversal Response (Claim/Service) (Transmission
Accepted/Transaction Rejected)...............................................................................................................................................................................527
22.6.3.2 Diagram For Transmission Of Two Information Reporting Reversal Responses (Claim/Service) (Transmission
Accepted/Transaction Rejected)...............................................................................................................................................................................527
22.6.3.3 Diagram For Transmission Of Three Information Reporting Reversal Responses (Claim/Service) (Transmission
Accepted/Transaction Rejected)...............................................................................................................................................................................528
22.6.3.4 Diagram For Transmission Of Four Information Reporting Reversal Responses (Claim/Service) (Transmission
Accepted/Transaction Rejected)...............................................................................................................................................................................528
22.6.3.5 Information Reporting Reversal Response Segments (Claim/Service) (Transmission Accepted/Transaction Rejected)....529
22.6.3.5.1 Response Header Segment (Information Reporting Reversal (Claim/Service)) (Transmission Accepted/Transaction
Rejected) 529
22.6.3.5.2 Response Message Segment (Information Reporting Reversal (Claim/Service)) (Transmission Accepted/Transaction
Rejected) 529
22.6.3.5.3 Response Status Segment (Information Reporting Reversal (Claim/Service)) (Transmission Accepted/Transaction
Rejected) 529
22.6.3.5.4 Response Claim Segment (Information Reporting Reversal (Claim/Service)) (Transmission Accepted/Transaction
Rejected) 531
22.6.4 Transmission Rejected/Transaction Rejected ....................................................................................................................531
22.6.4.1 Diagram For Transmission Of One Information Reporting Reversal Response (Claim/Service) (Transmission
Rejected/Transaction Rejected) ...............................................................................................................................................................................531
22.6.4.2 Diagram For Transmission Of Two Information Reporting Reversal Responses (Claim/Service) (Transmission
Rejected/Transaction Rejected) ...............................................................................................................................................................................531

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 19 -
22.6.4.3 Diagram For Transmission Of Three Information Reporting Reversal Responses (Claim/Service) (Transmission
Rejected/Transaction Rejected) ...............................................................................................................................................................................532
22.6.4.4 Diagram For Transmission Of Four Information Reporting Reversal Responses (Claim/Service) (Transmission
Rejected/Transaction Rejected) ...............................................................................................................................................................................532
22.6.4.5 Information Reporting Reversal Response Segments (Claim/Service) (Transmission Rejected/Transaction Rejected) ....532
22.6.4.5.1 Response Header Segment (Information Reporting Reversal (Claim/Service)) (Transmission Rejected/Transaction
Rejected) 532
22.6.4.5.2 Response Message Segment (Information Reporting Reversal (Claim/Service)) (Transmission Rejected/Transaction
Rejected) 533
22.6.4.5.3 Response Status Segment (Information Reporting Reversal (Claim/Service)) (Transmission Rejected/Transaction
Rejected) 533
23. INFORMATION REPORTING REBILL INFORMATION...................................................................................................................................... 535
23.1 INFORMATION REPORTING REBILL.......................................................................................................................................................535
23.2 INFORMATION REPORTING REBILL (CLAIM/SERVICE) REQUEST DIAGRAMS ...........................................................................................535
23.2.1 Diagram For Transmission Of One Information Reporting Rebill Transaction................................................................535
23.2.2 Diagram For Transmission Of Two Information Reporting Rebill Transactions..............................................................536
23.2.3 Diagram For Transmission Of Three Information Reporting Rebill Transactions...........................................................536
23.2.4 Diagram For Transmission Of Four Information Reporting Transactions........................................................................537
23.3 INFORMATION REPORTING REBILL REQUEST SEGMENTS......................................................................................................................539
23.3.1 Transaction Header Segment (Information Reporting Rebill)............................................................................................539
23.3.2 Insurance Segment (Information Reporting Rebill)............................................................................................................539
23.3.3 Patient Segment (Information Reporting Rebill).................................................................................................................540
23.3.4 Claim Segment (Information Reporting Rebill) ...................................................................................................................541
23.3.5 Pharmacy Provider Segment (Information Reporting Rebill) ............................................................................................545
23.3.6 Prescriber Segment (Information Reporting Rebill) ...........................................................................................................545
23.3.7 Workers’ Compensation Segment (Information Reporting Rebill)....................................................................................546
23.3.8 DUR/PPS Segment (Information Reporting Rebill).............................................................................................................547
23.3.9 Pricing Segment (Information Reporting Rebill).................................................................................................................548
23.3.10 Clinical Segment (Information Reporting Rebill) ................................................................................................................548
23.4 INFORMATION REPORTING REBILL RESPONSE DIAGRAMS AND SEGMENTS ...........................................................................................549
23.4.1 Transmission Accepted/Transaction Captured ..................................................................................................................549
23.4.1.1 Diagram For Transmission Of One Information Reporting Rebill Response (Transmission Accepted/Transaction Captured)
550
23.4.1.2 Diagram For Transmission Of Two Information Reporting Rebill Responses (Transmission Accepted/Transaction
Captured) 550
23.4.1.3 Diagram For Transmission Of Three Information Reporting Rebill Responses (Transmission Accepted/Transaction
Captured) 550
23.4.1.4 Diagram For Transmission Of Four Information Reporting Rebill Responses (Transmission Accepted/Transaction
Captured) 551
23.4.1.5 Information Reporting Response Rebill Response Segments (Transmission Accepted/Transaction Captured) ..................552
23.4.1.5.1 Response Header Segment (Information Reporting Rebill) (Transmission Accepted/Transaction Captured) ............... 552
23.4.1.5.2 Response Message Segment (Information Reporting Rebill) (Transmission Accepted/Transaction Captured)............552
23.4.1.5.3 Response Insurance Segment (Information Reporting Rebill) (Transmission Accepted/Transaction Captured)...........553
23.4.1.5.4 Response Patient Segment (Information Reporting Rebill) (Transmission Accepted/Transaction Captured)................553
23.4.1.5.5 Response Status Segment (Information Reporting Rebill) (Transmission Accepted/Transaction Captured)................. 554
23.4.1.5.6 Response Claim Segment (Information Reporting Rebill) (Transmission Accepted/Transaction Captured)..................555
23.4.1.5.7 Response DUR/PPS Segment (Information Reporting Rebill) (Transmission Accepted/Transaction Captured) .......... 555
23.4.2 Transmission Accepted/Transaction Approved .................................................................................................................556
23.4.2.1 Diagram For Transmission Of One Information Reporting Rebill Response (Transmission Accepted/Transaction Approved)
556
23.4.2.2 Diagram For Transmission Of Two Information Reporting Rebill Responses (Transmission Accepted/Transaction
Approved) 557
23.4.2.3 Diagram For Transmission Of Three Information Reporting Rebill Responses (Transmission Accepted/Transaction
Approved) 557
23.4.2.4 Diagram For Transmission Of Four Information Reporting Rebill Responses (Transmission Accepted/Transaction
Approved) 558
23.4.2.5 Information Reporting Rebill Response Segments (Transmission Accepted/Transaction Approved)..................................... 559
23.4.2.5.1 Response Header Segment (Information Reporting Rebill) (Transmission Accepted/Transaction Approved) ..............559
23.4.2.5.2 Response Message Segment (Information Reporting Rebill) (Transmission Accepted/Transaction Approved) ........... 559
23.4.2.5.3 Response Insurance Segment (Information Reporting Rebill) (Transmission Accepted/Transaction Approved)..........560
23.4.2.5.4 Response Patient Segment (Information Reporting Rebill) (Transmission Accepted/Transaction Approved) ...............560
23.4.2.5.5 Response Status Segment (Information Reporting Rebill) (Transmission Accepted/Transaction Approved) ................560
23.4.2.5.6 Response Claim Segment (Information Reporting Rebill) (Transmission Accepted/Transaction Approved) .................562
23.4.2.5.7 Response DUR/PPS Segment (Information Reporting Rebill) (Transmission Accepted/Transaction Approved).......... 562
23.4.3 Transmission Accepted/Transaction Rejected ...................................................................................................................563

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 20 -
23.4.3.1 Diagram For Transmission Of One Information Reporting Rebill Response (Transmission Accepted/Transaction Rejected)
563
23.4.3.2 Diagram For Transmission Of Two Information Reporting Rebill Responses (Transmission Accepted/Transaction Rejected)
564
23.4.3.3 Diagram For Transmission Of Three Information Reporting Rebill Responses (Transmission Accepted/Transaction
Rejected) 564
23.4.3.4 Diagram For Transmission Of Four Information Reporting Rebill Responses (Transmission Accepted/Transaction
Rejected) 565
23.4.3.5 Information Reporting Rebill Response Segments (Transmission Accepted/Transaction Rejected) ......................................565
23.4.3.5.1 Response Header Segment (Information Reporting Rebill) (Transmission Accepted/Transaction Rejected)................565
23.4.3.5.2 Response Message Segment (Information Reporting Rebill) (Transmission Accepted/Transaction Rejected)............. 566
23.4.3.5.3 Response Insurance Segment (Information Reporting Rebill) (Transmission Accepted/Transaction Rejected) ........... 566
23.4.3.5.4 Response Patient Segment (Information Reporting Rebill) (Transmission Accepted/Transaction Rejected) ................ 567
23.4.3.5.5 Response Status Segment (Information Reporting Rebill) (Transmission Accepted/Transaction Rejected)..................567
23.4.3.5.6 Response Claim Segment (Information Reporting Rebill) (Transmission Accepted/Transaction Rejected)...................568
23.4.4 Transmission Rejected/Transaction Rejected ....................................................................................................................569
23.4.4.1 Diagram For Transmission Of One Information Reporting Rebill Response (Transmission Rejected/Transaction Rejected)
569
23.4.4.2 Diagram For Transmission Of Two Information Reporting Rebill Responses (Transmission Rejected/Transaction Rejected)
569
23.4.4.3 Diagram For Transmission Of Three Information Reporting Rebill Responses (Transmission Rejected/Transaction
Rejected) 569
23.4.4.4 Diagram For Transmission Of Four Information Reporting Rebill Responses (Transmission Rejected/Transaction Rejected)
570
23.4.4.5 Information Reporting Rebill Responses (Transmission Rejected/Transaction Rejected) ........................................................ 570
23.4.4.5.1 In Response Header Segment (Information Reporting Rebill) (Transmission Rejected/Transaction Rejected) ............570
23.4.4.5.2 Response Message Segment (Information Reporting Rebill) (Transmission Rejected/Transaction Rejected) .............570
23.4.4.5.3 Response Status Segment (Information Reporting Rebill) (Transmission Rejected/Transaction Rejected) .................. 571
24. TRANSMISSION STRUCTURE............................................................................................................................................................................... 573
24.1 REQUEST SEGMENT MATRICES BY FIELD WITHIN SEGMENT - LEGEND .................................................................................................573
24.2 REQUEST SEGMENT MATRICES BY FIELD WITHIN SEGMENT.................................................................................................................574
24.2.1 Eligibility/Claim Billing/Claim Rebill/Encounter/Service Billing/Service Rebill/Claim Reversal/Service Reversal Matrix
574
24.2.2 Prior Authorization Request And Billing (Claim/Service)/Prior Authorization Reversal/Prior Authorization
Inquiry/Prior Authorization Request Only (Claim/Service) Matrix.......................................................................................................582
24.2.3 Information Reporting (Claim/Service)/Information Reporting Rebill (Claim/Service)/Information Reporting Reversal
(Claim/Service) Matrix..............................................................................................................................................................................590
24.2.4 Controlled Substance Reporting/Controlled Substance Reporting Rebill/Controlled Substance Reporting Reversal
Matrix 598
24.3 REQUEST SEGMENT MATRICES BY SEGMENT - LEGEND.......................................................................................................................606
24.4 REQUEST SEGMENT MATRICES BY SEGMENT ......................................................................................................................................607
24.4.1 Eligibility/Billing/Encounter/Rebill/Reversal Matrix............................................................................................................607
24.4.2 Prior Authorization Request And Billing/Prior Authorization Reversal/Prior Authorization Inquiry/Prior Authorization
Request Only Matrix ................................................................................................................................................................................607
24.4.3 Information Reporting/Information Reporting Reversal/Information Reporting Rebill/Controlled Substance
Reporting/Controlled Substance Reversal/Controlled Substance Rebill ...........................................................................................608
24.5 RESPONSE SEGMENT MATRICES BY FIELD WITHIN SEGMENT - LEGEND ...............................................................................................609
24.6 RESPONSE SEGMENT MATRICES BY FIELD WITHIN SEGMENT...............................................................................................................610
24.6.1 Eligibility Matrix......................................................................................................................................................................610
24.6.2 Claim Billing/Claim Rebill/Encounter/Service Billing/Service Rebill Matrix.....................................................................615
24.6.3 Predetermination Of Benefits (Claim) Matrix.......................................................................................................................620
24.6.4 Claim Reversal/Service Reversal Matrix..............................................................................................................................625
24.6.5 Prior Authorization Request And Billing (Claim/Service) Matrix.......................................................................................630
24.6.6 Prior Authorization Reversal Matrix.....................................................................................................................................636
24.6.7 Prior Authorization Inquiry (Claim/Service) Matrix.............................................................................................................641
24.6.8 Prior Authorization Request Only (Claim) Matrix................................................................................................................652
24.6.9 Prior Authorization Request Only (Service) Matrix.............................................................................................................657
24.6.10 Information Reporting/Information Reporting Rebill (Claim/Service) Matrix ...................................................................663
24.6.11 Information Reporting Reversal (Claim/Service) Matrix.....................................................................................................668
24.6.12 Controlled Substance Reporting/Controlled Substance Reporting Rebill Matrix............................................................673
24.6.13 Controlled Substance Reporting Reversal Matrix ..............................................................................................................679
24.7 RESPONSE SEGMENT MATRICES BY SEGMENT – LEGEND....................................................................................................................684
24.8 RESPONSE SEGMENT MATRICES BY SEGMENT....................................................................................................................................685
24.8.1 Transmission Accepted; Transaction Paid Or Duplicate Of Paid .....................................................................................685
24.8.2 Transmission Accepted; Transaction Benefit Matrix .........................................................................................................685
24.8.3 Transmission Accepted; Transaction Captured Or Duplicate Of Capture Matrix............................................................686

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 21 -
24.8.4 Transmission Accepted; Transaction Approved Or Duplicate Of Approved Matrix........................................................688
24.8.5 Transmission Accepted; Transaction Deferred Matrix.......................................................................................................690
24.8.6 Transmission Accepted; Transaction Rejected Matrix ......................................................................................................691
24.8.7 Transmission Rejected; Transaction Rejected Matrix........................................................................................................692
25. RESPONSE OVERVIEW...........................................................................................................................................................................................695
25.1 RESPONSE STATUS BY TRANSACTION TYPE ........................................................................................................................................695
26. RESPONSE PROCESSING GUIDELINES............................................................................................................................................................ 698
26.1 TRANSACTION RESPONSE STATUS (112-AN).......................................................................................................................................698
26.1.1 Approved ................................................................................................................................................................................698
26.1.2 Reject ......................................................................................................................................................................................698
26.1.3 Deferred ..................................................................................................................................................................................698
26.1.4 Benefit.....................................................................................................................................................................................698
26.1.5 Captured .................................................................................................................................................................................698
26.1.5.1 Business Function Of Capture..............................................................................................................................................................698
26.1.5.1.1 Valid Uses ......................................................................................................................................................................................... 698
26.1.5.1.2 Capture Consistency.......................................................................................................................................................................699
26.1.5.2 Reversals And Capture ..........................................................................................................................................................................699
26.1.5.3 Business Functions Not Supported For Capture...............................................................................................................................700
26.1.6 Paid .........................................................................................................................................................................................700
26.2 PRICING GUIDELINES (CLAIM/SERVICE) ...............................................................................................................................................700
26.2.1 Definitions...............................................................................................................................................................................700
26.2.2 Other Pricing Information......................................................................................................................................................700
26.2.3 CLAIM......................................................................................................................................................................................700
26.2.3.1 Corresponding Pricing Fields (Claim)..................................................................................................................................................700
26.2.4 Patient Financial Responsibility (Claim)..............................................................................................................................701
26.2.5 Service ....................................................................................................................................................................................701
26.2.5.1 Corresponding Pricing Fields (Service)...............................................................................................................................................701
26.2.5.2 Patient Financial Responsibility (Service)...........................................................................................................................................702
26.3 DUPLICATE TRANSACTIONS.................................................................................................................................................................702
26.3.1 Duplicate Transmission For A Primary Payer.....................................................................................................................702
26.3.2 Duplicate Transmission For A Downstream Payer.............................................................................................................702
26.3.2.1 Excerpt Example 1..................................................................................................................................................................................703
26.3.2.2 Excerpt Example 2..................................................................................................................................................................................703
26.3.3 Duplicate Transmission For A Reversal For A Primary Payer...........................................................................................704
26.3.4 Duplicate Transmission For A Reversal For A Downstream Payer ..................................................................................704
26.3.4.1 Excerpt Example 1..................................................................................................................................................................................704
26.3.4.2 Excerpt Example 2..................................................................................................................................................................................705
26.3.5 Duplicate Information For Other Transactions ...................................................................................................................705
26.4 DUPLICATE PROCESSING FOR ALL REBILL TRANSACTIONS..................................................................................................................705
27. STRUCTURE QUICK REFERENCE.......................................................................................................................................................................707
27.1 REQUEST SEGMENTS..........................................................................................................................................................................707
27.1.1 Transmission Level................................................................................................................................................................707
27.1.2 Transaction Level...................................................................................................................................................................708
27.2 RESPONSE SEGMENTS........................................................................................................................................................................713
27.2.1 Transmission Level................................................................................................................................................................713
27.2.2 Transaction Level...................................................................................................................................................................714
28. SPECIFIC SEGMENT DISCUSSION......................................................................................................................................................................718
28.1 REQUEST SEGMENTS..........................................................................................................................................................................718
28.1.1 Transaction Header Segment ...............................................................................................................................................718
28.1.1.1 Transaction Count...................................................................................................................................................................................718
28.1.2 Patient Segment.....................................................................................................................................................................718
28.1.3 Insurance Segment................................................................................................................................................................718
28.1.3.1 Medicare Part D Information Reporting Usage..................................................................................................................................718
28.1.4 Pharmacy Provider Segment................................................................................................................................................718
28.1.5 Prescriber Segment...............................................................................................................................................................718
28.1.6 Coordination of Benefits/Other Payments Segment ..........................................................................................................718
28.1.6.1 To Denote a Total Amount of Patient Financial Responsibility as Reported from a Previous Payer.......................................719
28.1.6.2 To Denote Individual Amounts of Patient Financial Responsibility as Reported from a Previous Payer................................719
28.1.6.3 When the Previous Payer has Rejected the Service or Claim........................................................................................................720
28.1.6.4 Medicare Part D.......................................................................................................................................................................................720
28.1.6.5 Payer-to-Payer Usage Of Internal Control Number (993-A7).......................................................................................................... 720
28.1.7 Workers’ Compensation Segment........................................................................................................................................721

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 22 -
28.1.8 DUR/PPS Segment.................................................................................................................................................................722
28.1.8.1 Terminology.............................................................................................................................................................................................. 722
28.1.8.2 Specific Discussion – DUR.................................................................................................................................................................... 722
28.1.8.2.1 The Problem Of Noise.....................................................................................................................................................................722
The following chart illustrates alerts that contribute to DUR Noise ..................................................................................................... 723
28.1.8.2.2 DUR Inputs........................................................................................................................................................................................ 724
28.1.8.2.3 ORDUR Screening .......................................................................................................................................................................... 724
28.1.8.2.4 Dosing/Limits ....................................................................................................................................................................................724
28.1.8.2.5 Drug Interactions.............................................................................................................................................................................. 724
28.1.8.2.6 Drug Conflicts ...................................................................................................................................................................................724
28.1.8.2.7 Duplicate Therapy............................................................................................................................................................................724
28.1.8.2.8 Precautionary Screenings ..............................................................................................................................................................724
28.1.8.3 Specific Discussion-Professional Pharmacy Services ..................................................................................................................... 725
28.1.8.3.1 PPS Processing ...............................................................................................................................................................................725
28.1.8.4 Special Considerations ..........................................................................................................................................................................726
28.1.9 Claim Segment.......................................................................................................................................................................727
28.1.9.1 Partial Fill..................................................................................................................................................................................................728
28.1.9.1.1 PARTIAL FILL ASSUMPTIONS & RECOMMENDATIONS .....................................................................................................729
28.1.9.2 Other Coverage Code (3Ø8-C8) ..........................................................................................................................................................729
28.1.9.3 Split Billing In Long Term Care .............................................................................................................................................................730
28.1.10 Pricing Segment.....................................................................................................................................................................730
28.1.10.1 Prescription Claim Request Formula..............................................................................................................................................730
28.1.10.2 Service Claim Request Formula...................................................................................................................................................... 730
28.1.10.3 Other Information ............................................................................................................................................................................... 731
28.1.11 Coupon Segment ...................................................................................................................................................................731
28.1.12 Compound Segment..............................................................................................................................................................731
28.1.12.1 Claim and Pricing Segment Fields..................................................................................................................................................732
28.1.12.2 Definitions............................................................................................................................................................................................ 732
28.1.12.3 Use Of Compound Fields .................................................................................................................................................................733
28.1.12.4 Compound Ingredient Calculates To Be Less Than $Ø.ØØ5 ....................................................................................................733
28.1.12.5 Support Of A Single Ingredient Compound ...................................................................................................................................733
28.1.12.6 Multi-Ingredient Compound And Rejects .......................................................................................................................................733
28.1.12.7 Multi-Ingredient Compounds and DUR Rejects............................................................................................................................734
28.1.12.7.1 Scenario One..................................................................................................................................................................................734
28.1.12.7.2 Scenario Two..................................................................................................................................................................................735
28.1.12.7.3 Scenario Three...............................................................................................................................................................................735
28.1.12.7.4 Scenario Four .................................................................................................................................................................................735
28.1.12.7.5 Scenario Five..................................................................................................................................................................................735
28.1.12.7.6 Recommendations......................................................................................................................................................................... 735
28.1.12.8 Shared Reject Codes ........................................................................................................................................................................ 736
28.1.13 Prior Authorization Segment ................................................................................................................................................737
28.1.14 Clinical Segment....................................................................................................................................................................737
28.1.15 Additional Documentation Segment ....................................................................................................................................737
28.1.16 Facility Segment.....................................................................................................................................................................738
28.1.17 Narrative Segment .................................................................................................................................................................738
28.2 RESPONSE SEGMENTS........................................................................................................................................................................738
28.2.1 Response Header Segment...................................................................................................................................................738
28.2.2 Response Patient Segment...................................................................................................................................................738
28.2.3 Response Insurance Segment..............................................................................................................................................739
28.2.4 Response Insurance Additional Information Segment ......................................................................................................739
28.2.5 Response Status Segment....................................................................................................................................................739
28.2.5.1 Reject Field Occurrence Indicator (546-4F) .......................................................................................................................................739
28.2.5.2 Shared Reject Codes .............................................................................................................................................................................739
28.2.5.3 Additional Message Information Fields ...............................................................................................................................................739
28.2.5.3.1 Free Text Messages........................................................................................................................................................................740
28.2.5.3.2 Structured Messages ......................................................................................................................................................................740
28.2.5.3.3 Example 1: One Free Text Message is Sent, Less Than 4Ø Bytes .......................................................................................740
28.2.5.3.4 Example 2: One Free Text Message is Sent, Longer Than 4Ø Bytes; No Continuation Needed.....................................740
28.2.5.3.5 Example 3: Three Free Text Messages; Continuity Character Needed.................................................................................741
28.2.5.3.6 Example 4: One Free Text Message, Less Than 4Ø Bytes.....................................................................................................741
28.2.5.3.7 Example 5: Two Free Text Messages; Continuity Character Needed ................................................................................... 742
28.2.5.4 Transaction Reference Number (88Ø-K5)..........................................................................................................................................742
28.2.6 Response Pricing Segment...................................................................................................................................................742
28.2.6.1 Prescription Response Formula ........................................................................................................................................................... 742
28.2.6.2 Service Response Formula ...................................................................................................................................................................742
28.2.6.3 Patient Pay Amount (5Ø5-F5) Formula...............................................................................................................................................743
28.2.6.3.1 Example #1 ....................................................................................................................................................................................... 743

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 23 -
28.2.6.3.2 Example #2 ....................................................................................................................................................................................... 743
28.2.6.3.3 Example #3 ....................................................................................................................................................................................... 744
28.2.6.3.4 Example #4 ....................................................................................................................................................................................... 744
28.2.6.4 Medicare Part D.......................................................................................................................................................................................746
28.2.6.4.1 Excerpt Examples............................................................................................................................................................................746
28.2.6.4.1.1 Example 1 Brand Selection....................................................................................................................................................746
28.2.6.4.1.2 Example 2 Deductible Not Met.............................................................................................................................................. 747
28.2.6.4.1.3 Example 3 Coverage Gap......................................................................................................................................................748
28.2.6.4.1.4 Example 4 Non-preferred Formulary Selection..................................................................................................................749
28.2.6.5 Healthcare Reimbursement Account (HRA), Health Savings Accounts (HSAs), and Healthcare Flexible Spending Account
(FSA) 750
28.2.6.5.1 Healthcare Reimbursement Account (HRA) – based plan designs........................................................................................750
28.2.6.5.2 Health Savings Accounts (HSAs) and Qualifying Health Plans ..............................................................................................750
28.2.6.5.3 Healthcare Flexible Spending Account (FSA) ............................................................................................................................750
28.2.6.5.4 Primary Pays The Claim Using Plan-Funded Health Reimbursement Account ................................................................... 750
28.2.6.5.4.1 SCENARIO 1A: PHARMACY BILLS SECONDARY INSURANCE – HRA used in PRIMARY PAYMENT ............751
28.2.6.5.4.2 SCENARIO 1B: SECONDARY INSURANCE PAYS THE CLAIM................................................................................. 752
28.2.6.5.4.3 SCENARIO 2A: PHARMACY BILLS SECONDARY INSURANCE – HRA used in PRIMARY PAYMENT ............752
28.2.6.5.4.4 SCENARIO 2A-1: BILLING FOR “LUMP SUM” PATIENT RESPONSIBILITY AMOUNT AS REPORTED BY LAST
PAYER 752
28.2.6.5.4.5 SCENARIO 2A-2: SECONDARY INSURANCE PAYS THE CLAIM RESULTING IN REDUCED PATIENT
RESPONSIBILITY.............................................................................................................................................................................................753
28.2.6.5.4.6 SCENARIO 2B-1: BILLING FOR “PARTS” OF PATIENT RESPONSIBILITY AMOUNT AS REPORTED BY LAST
PAYER. 753
28.2.6.5.4.7 SCENARIO 2B-2: SECONDARY INSURANCE PAYS THE DETAILED PATIENT RESPONSIBILITY CLAIM
RESULTING IN REDUCED PATIENT RESPONSIBILITY ........................................................................................................................754
28.2.7 Response Claim Segment.....................................................................................................................................................754
28.2.8 Response DUR/PPS Segment...............................................................................................................................................754
28.2.8.1 DUR/PPS And Multi-Ingredient Compounds...................................................................................................................................... 754
28.2.8.2 DUR/PPS Claims Data And Responses In Batch Transactions.....................................................................................................755
28.2.9 Response Prior Authorization Segment ..............................................................................................................................755
28.2.10 Response Coordination of Benefits/Other Payers Segment .............................................................................................755
29. VERSION IDENTIFICATION SYSTEM................................................................................................................................................................... 757
30. FRAMEWORK.............................................................................................................................................................................................................758
30.1 TECHNICAL FRAMEWORK ....................................................................................................................................................................758
30.2 SCOPE ...............................................................................................................................................................................................758
30.3 TECHNICAL DEFINITIONS .....................................................................................................................................................................758
30.4 CONNECTIVITY BETWEEN PARTICIPANTS .............................................................................................................................................758
30.5 SOFTWARE/SYSTEM DEVELOPMENT....................................................................................................................................................760
30.6 RESPONSIBILITIES OF THE PARTICIPANTS ...........................................................................................................................................760
30.6.1 Responsibilities Of The Originator.......................................................................................................................................760
30.6.2 Responsibilities Of The Switch.............................................................................................................................................760
30.6.3 Responsibilities Of The Receiver .........................................................................................................................................760
30.6.4 Responsibilities Of The Facilitator.......................................................................................................................................760
30.7 PROCESSOR IMPLEMENTATION............................................................................................................................................................761
30.7.1 Transmitting A Response......................................................................................................................................................761
30.7.2 Other considerations.............................................................................................................................................................761
30.8 SWITCH IMPLEMENTATION...................................................................................................................................................................761
31. GENERAL STRUCTURAL OVERVIEW.................................................................................................................................................................762
31.1 OVERVIEW..........................................................................................................................................................................................762
31.1.1 Transmission..........................................................................................................................................................................762
31.1.2 Transaction.............................................................................................................................................................................762
31.1.2.1 Segments.................................................................................................................................................................................................. 762
31.2 TRANSMISSION LEVEL FOR A REQUEST...............................................................................................................................................762
31.2.1 Rules For 2, 3 Or 4 Transaction Formats.............................................................................................................................763
31.3 TRANSACTION LEVEL FOR A REQUEST................................................................................................................................................763
31.4 TRANSMISSION LEVEL FOR A RESPONSE.............................................................................................................................................763
31.5 TRANSACTION LEVEL FOR A RESPONSE..............................................................................................................................................763
32. NOTABLE CHANGES FROM PREVIOUS TELECOMMUNICATION VERSIONS........................................................................................ 765
33. STANDARD CONVENTIONS...................................................................................................................................................................................766
33.1 VARIABLE USAGE GUIDELINES ............................................................................................................................................................766

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 24 -
33.2 GENERAL SYNTAX OUTLINE ................................................................................................................................................................766
33.2.1 Header Segment.....................................................................................................................................................................766
33.2.2 Other Segments .....................................................................................................................................................................766
33.2.3 A Transmission......................................................................................................................................................................766
33.2.4 A Transaction.........................................................................................................................................................................766
33.2.5 Order of Segments.................................................................................................................................................................767
33.3 EXPLANATION OF SEGMENT AND FIELD DESIGNATION ..........................................................................................................................768
33.4 SEPARATOR CHARACTERS..................................................................................................................................................................768
33.4.1 Separator Character Rules....................................................................................................................................................770
33.5 FIELD DEFINITIONS AND VALUES.........................................................................................................................................................770
33.6 CHARACTER SETS DESIGNATION.........................................................................................................................................................771
33.7 CHARACTER SET DESIGNATION TRUNCATION ......................................................................................................................................771
33.7.1 Overview.................................................................................................................................................................................771
33.7.2 Numeric...................................................................................................................................................................................771
33.7.2.1 Numeric Truncation.................................................................................................................................................................................771
33.7.3 Dollar.......................................................................................................................................................................................771
33.7.3.1 Dollar Truncation .....................................................................................................................................................................................772
33.7.4 Alphanumeric.........................................................................................................................................................................772
33.7.4.1 Alphanumeric Truncation.......................................................................................................................................................................772
33.8 DEFAULT VALUES ...............................................................................................................................................................................772
33.9 INTERNAL REPRESENTATION OF OVERPUNCH SIGNS ...........................................................................................................................772
33.10 DATE FORMAT................................................................................................................................................................................773
33.10.1 Default Date Format...............................................................................................................................................................773
33.11 IMPLIED DECIMAL POINTS...............................................................................................................................................................773
33.12 EXPLICIT HYPHENS.........................................................................................................................................................................773
33.13 QUALIFIERS ...................................................................................................................................................................................773
33.14 REPETITION AND MULTIPLE OCCURRENCES....................................................................................................................................773
33.14.1 Multiple Occurrences Of Segments .....................................................................................................................................773
33.14.2 Repeating Data Elements......................................................................................................................................................774
33.14.2.1 Count Fields ........................................................................................................................................................................................ 774
33.14.2.2 Counter Fields.....................................................................................................................................................................................775
33.14.2.3 Usage ...................................................................................................................................................................................................776
33.14.2.4 Request Segments ............................................................................................................................................................................777
33.14.2.4.1 Coordination of Benefits/Other Payments Segment................................................................................................................ 777
33.14.2.4.1.1 In Payment Scenarios...........................................................................................................................................................777
33.14.2.4.1.1.1 1. Other Payer Amount Paid Repetitions Only......................................................................................................... 777
33.14.2.4.1.1.2 2. Other Payer-Patient Responsibility Amount Repetitions Only ..........................................................................777
33.14.2.4.1.1.3 3. Other Payer Amount Paid, Other Payer-Patient Responsibility Amount, and Benefit Stage Repetitions
Present (Government Programs) ..............................................................................................................................................................777
33.14.2.4.1.2 General Information...............................................................................................................................................................778
33.14.2.4.1.3 In Reject Scenarios ...............................................................................................................................................................778
33.14.2.4.1.3.1 Other Payer Reject Fields............................................................................................................................................ 778
33.14.2.4.2 Claim Segment............................................................................................................................................................................... 779
33.14.2.4.2.1 Procedure Modifier Code Count .........................................................................................................................................779
33.14.2.4.2.2 Submission Clarification Code Count ................................................................................................................................ 779
33.14.2.4.3 DUR/PPS Segment ....................................................................................................................................................................... 779
33.14.2.4.3.1 DUR/PPS Code Counter ......................................................................................................................................................779
33.14.2.4.4 Compound Segment .....................................................................................................................................................................779
33.14.2.4.4.1 Compound Ingredient Component Count..........................................................................................................................779
33.14.2.4.5 Pricing Segment............................................................................................................................................................................. 780
33.14.2.4.5.1 Other Amount Claimed Submitted Count..........................................................................................................................780
33.14.2.4.6 Clinical Segment ............................................................................................................................................................................780
33.14.2.4.6.1 Diagnosis Code Count..........................................................................................................................................................780
33.14.2.4.6.2 Clinical Information Counter ................................................................................................................................................780
33.14.2.4.7 Additional Documentation Segment ...........................................................................................................................................780
33.14.2.4.7.1 Question Number/Letter Count ...........................................................................................................................................780
33.14.2.5 Response Segments .........................................................................................................................................................................780
33.14.2.5.1 Response Status Segment ..........................................................................................................................................................780
33.14.2.5.1.1 Approved Message Code Count.........................................................................................................................................780
33.14.2.5.1.2 Reject Count...........................................................................................................................................................................781
33.14.2.5.1.3 Additional Message Information Count.............................................................................................................................. 781
33.14.2.5.2 Response Claim Segment ...........................................................................................................................................................781
33.14.2.5.2.1 Preferred Product Count ......................................................................................................................................................781
33.14.2.5.3 Response Pricing Segment .........................................................................................................................................................781
33.14.2.5.3.1 Other Amount Paid Repetitions Only .................................................................................................................................781
33.14.2.5.3.2 Benefit Stage Repetitions Only ...........................................................................................................................................781
33.14.2.5.4 Response DUR/PPS Segment....................................................................................................................................................782

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 25 -
33.14.2.5.4.1 DUR/PPS Response Code Counter................................................................................................................................... 782
33.14.2.5.5 Response Coordination of Benefits/Other Payers Segment..................................................................................................782
33.14.2.5.5.1 Other Payer ID Count ...........................................................................................................................................................782
33.14.3 Reject Field Occurrence Indicator........................................................................................................................................782
33.14.3.1 Reject Field Occurrence Indicator Use for Multi Ingredient Compound Transaction.............................................................783
34. TRANSMISSION EXAMPLES..................................................................................................................................................................................785
34.1 EXAMPLE CONVENTIONS.....................................................................................................................................................................785
34.1.1 Raw Data Streams..................................................................................................................................................................785
34.1.2 Category (CAT) Column ........................................................................................................................................................785
34.1.3 “Mandatory” Categorization Examples................................................................................................................................786
34.1.4 “Required” Categorization Examples ..................................................................................................................................786
34.1.5 “Qualified Requirement” Categorization Examples ...........................................................................................................787
34.1.6 “Optional” Categorization Examples ...................................................................................................................................788
34.1.7 “Informational” Categorization Examples...........................................................................................................................789
34.2 ELIGIBILITY VERIFICATION - TRANSACTION CODE E1............................................................................................................................789
34.2.1 Eligibility Verification Accepted Response .........................................................................................................................789
34.3 ELIGIBILITY VERIFICATION - TRANSMISSION REJECTED.........................................................................................................................790
34.3.1 Eligibility Verification Transmission Rejected Response..................................................................................................790
34.4 ELIGIBILITY VERIFICATION TRANSACTION REJECTED............................................................................................................................790
34.4.1 Eligibility Verification Transaction Rejected Response.....................................................................................................791
34.5 BILLING - TRANSACTION CODE B1 ......................................................................................................................................................791
34.5.1 Billing With Intermediary Processing Override Codes - Transaction B1 .........................................................................793
34.5.2 Billing Accepted Response- Paid (Duplicate of Paid) ........................................................................................................794
34.5.3 Billing Accepted Response-Captured..................................................................................................................................795
34.5.4 Billing Accepted Response With Approved Message Codes............................................................................................796
34.5.5 Billing Transmission Rejected Response............................................................................................................................797
34.5.6 Billing Transaction Rejected Response...............................................................................................................................797
34.6 BILLING – TRANSACTION CODE B1 – COORDINATION OF BENEFITS SCENARIOS PHARMACY BILLS TO INSURANCE DESIGNATED BY
PATIENT 798
34.6.1 Billing Accepted Response – Payer Rejects Indicating Other Coverage Exists..............................................................798
34.6.2 Billing – Transaction Code B1 – Pharmacy Bills To Other Insurance ..............................................................................799
34.6.2.1 Billing Accepted Response – Paid - Primary Insurance Pays The Claim .....................................................................................800
34.6.3 Billing – Transaction Code B1 – Coordination of Benefits – Scenario 1: Pharmacy Bills Secondary Insurance........801
34.6.3.1 Scenario 1 Response: Secondary Insurance Pays The Claim Submitted With Amount Paid By Other Payer......................802
34.6.4 Billing – Transaction Code B1 – Coordination of Benefits – Scenario 2: Pharmacy Bills Secondary Insurance........803
34.6.4.1 Scenario 2 Response: Secondary Insurance Pays The Claim Submitted With Net Other Payer Patient Responsibility
Amount 804
34.6.5 Scenario 3: Pharmacy Bills Secondary Insurance.............................................................................................................805
34.6.5.1 Scenario 3 Response: Secondary Insurance Pays The Claim Submitted With The “Pieces” Of Other Payer-Patient
Responsibility Amount ................................................................................................................................................................................................806
34.7 BILLING W/SUBMITTED DUR OVERRIDE - TRANSACTION CODE B1 .......................................................................................................807
34.7.1 Billing w/Submitted DUR Override Accepted Response- Paid..........................................................................................808
34.7.2 Billing w/Submitted DUR Override Rejected Response.....................................................................................................809
34.8 BILLING W/DUR CONFLICTS - TRANSACTION CODE B1........................................................................................................................810
34.8.1 Billing w/Information DUR Accepted Response- Paid........................................................................................................811
34.8.2 Billing w/DUR Conflicts Rejected Response.......................................................................................................................813
34.9 SERVICE BILLING - TRANSACTION CODE S1 (Ø1/Ø2)...........................................................................................................................814
34.9.1 Service Billing Accepted Response- Paid (Duplicate of Paid)...........................................................................................816
34.9.2 Service Billing Transmission Rejected Response..............................................................................................................817
34.9.3 Service Billing Transmission – One Rejected, One Paid Response .................................................................................817
34.10 COMPOUNDED RX BILLING - TRANSACTION CODE B1 (Ø1)..............................................................................................................818
34.10.1 Compounded Rx Billing Accepted Response- Paid (Duplicate of Paid)...........................................................................820
34.10.2 Compounded Rx Billing Rejected Response ......................................................................................................................821
34.10.3 Billing Resubmission w/DUR Resolution ............................................................................................................................821
34.10.4 Billing Resubmission w/DUR Accepted Response- Paid (Duplicate of Paid) ..................................................................823
34.11 BILLING, PARTIAL FILL-INITIAL - TRANSACTION CODE B1 ................................................................................................................823
34.11.1 Billing, Initial Partial Fill Accepted Response- Paid (Duplicate of Paid)...........................................................................825
34.12 BILLING, PARTIAL FILL-COMPLETION - TRANSACTION CODE B1.......................................................................................................826
34.12.1 Billing, Completion Partial Fill Accepted Response- Paid.................................................................................................828
34.13 REVERSAL – PARTIAL FILL TRANSACTIONS .....................................................................................................................................829
34.14 WORKERS’ COMPENSATION BILLING - TRANSACTION CODE B1 .......................................................................................................829
34.14.1 Workers’ Compensation Billing Accepted Response- Paid (Duplicate of Paid) ..............................................................830
34.15 BILLING W/COUPON (FREE PRODUCT) - TRANSACTION CODE B1-BILLING TO COUPON PROCESSOR.................................................831
34.15.1 Billing w/Coupon (Free Product) Accepted Response- Paid (Duplicate of Paid).............................................................832
34.16 BILLING TO A COUPON PROCESSOR TO REDUCE A PATIENT RESPONSIBILITY AMOUNT .....................................................................832

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 26 -
34.16.1 Bill “Patient Responsibility Amount” to Coupon Processor Using the Patient Pay Amount (5Ø5-F5) as Returned by
Prior Payer................................................................................................................................................................................................833
34.16.2 Billing w/Coupon Accepted Response—Paid .....................................................................................................................834
34.17 REVERSAL - TRANSACTION CODE B2..............................................................................................................................................835
34.17.1 Reversal with Situational Fields Submitted - Transaction Code B2..................................................................................836
34.17.2 Reversal Accepted Response-Captured, Approved...........................................................................................................836
34.17.3 Reversal Accepted Response-Duplicate of Approved .......................................................................................................837
34.17.4 Reversal Rejected Response................................................................................................................................................837
34.18 CLAIM REBILL - TRANSACTION CODE B3.........................................................................................................................................838
34.18.1 Rebill Accepted Response-Captured...................................................................................................................................839
34.18.2 Rebill Accepted Response-Paid ...........................................................................................................................................840
34.18.3 Rebill Rejected Response .....................................................................................................................................................841
34.19 PRIOR AUTHORIZATION REQUEST AND BILLING (CLAIM) - TRANSACTION CODE P1...........................................................................841
34.19.1 Prior Authorization Request And Billing Accepted Response-Captured .........................................................................843
34.19.2 Prior Authorization Request And Billing Accepted Response-Paid .................................................................................843
34.19.3 Prior Authorization Request And Billing Rejected Response ...........................................................................................844
34.19.4 Prior Authorization Request And Billing Duplicate of Paid Response.............................................................................845
34.20 PRIOR AUTHORIZATION REVERSAL - TRANSACTION CODE P2 ..........................................................................................................846
34.20.1 Prior Authorization Reversal Accepted Response-Captured, Approved .........................................................................847
34.21 PRIOR AUTHORIZATION INQUIRY - TRANSACTION CODE P3 ..............................................................................................................847
34.21.1 Prior Authorization Inquiry Accepted Response-Paid .......................................................................................................848
34.22 PRIOR AUTHORIZATION REQUEST ONLY (CLAIM) - TRANSACTION CODE P4......................................................................................849
34.22.1 Prior Authorization Request Only Accepted Response-Approved...................................................................................849
34.22.2 Prior Authorization Request Only Rejected Response ......................................................................................................850
34.23 INFORMATION REPORTING (SERVICE – DUR/PPS) - TRANSACTION CODE N1...................................................................................851
34.23.1 Information Reporting Accepted Response-Captured, Approved ....................................................................................853
34.24 INFORMATION REPORTING REVERSAL - TRANSACTION CODE N2......................................................................................................853
34.24.1 Information Reporting Reversal Accepted Response—Captured or Approved (or Duplicate) ......................................854
34.24.2 Information Reporting Reversal Rejected Response .........................................................................................................854
34.25 INFORMATION REPORTING REBILL (SERVICE – DUR/PPS) - TRANSACTION CODE N3.......................................................................855
34.25.1 Information Reporting Rebill Accepted Response-Captured ............................................................................................855
34.25.2 Information Reporting Rebill Accepted Response-Captured ............................................................................................856
34.25.3 Information Reporting Rebill Rejected Response ..............................................................................................................856
34.26 CONTROLLED SUBSTANCE REPORTING - TRANSACTION CODE C1 ...................................................................................................857
34.26.1 Controlled Substance Reporting Accepted Response-Captured, Approved...................................................................858
34.27 CONTROLLED SUBSTANCE REPORTING REVERSAL - TRANSACTION CODE C2 ..................................................................................858
34.27.1 Controlled Substance Reporting Reversal Accepted Response-Captured, Approved...................................................859
34.28 CONTROLLED SUBSTANCE REPORTING REBILL - TRANSACTION CODE C3.......................................................................................859
34.28.1 Controlled Substance Reporting Rebill Accepted Response-Captured, Approved ........................................................860
34.28.2 Controlled Substance Reporting Rebill Rejected Response.............................................................................................860
34.29 BILLING WITH DUR SEGMENT USING CO-AGENT FIELDS - TRANSACTION CODE B1 (Ø1/Ø2) ...........................................................861
34.29.1 Billing With DUR Segment Using Co-Agent Fields —Paid (Duplicate of Paid) ................................................................863
34.29.2 Billing With DUR Segment Using Co-Agent Fields —Paid, But With A DIFFERENT DUR Message Reported .............863
34.30 BILLING PAID RESPONSE USING DUR ADDITIONAL TEXT – TRANSACTION CODE B1 (Ø1/Ø2)...........................................................864
34.31 BILLING - TRANSACTION CODE B1 WITH ADDITIONAL DOCUMENTATION SEGMENT ...........................................................................865
34.31.1 Billing Accepted Response- Paid.........................................................................................................................................868
34.32 BILLING - TRANSACTION CODE B1 WITH FACILITY INFORMATION ....................................................................................................869
34.33 BILLING - TRANSACTION CODE B1 WITH ADDITIONAL DOCUMENTATION AND FACILITY INFORMATION................................................871
34.34 BILLING - TRANSACTION CODE B1 WITH NARRATIVE INFORMATION .................................................................................................873
34.35 BILLING - TRANSACTION CODE B1 WITH FACILITY INFORMATION AND NARRATIVE INFORMATION ......................................................875
34.36 BILLING - TRANSACTION CODE B1 WITH ADDITIONAL DOCUMENTATION AND NARRATIVE INFORMATION............................................877
34.37 PRIMARY CLAIM FROM PHARMACY TO PDP....................................................................................................................................879
34.37.1 Response From PDP To Pharmacy On Primary Claim.......................................................................................................880
34.38 MEDICARE PART D - 1- CLAIM SUBMITTED TO SECONDARY PAYER FROM PHARMACY......................................................................882
34.38.1 Medicare Part D - 2 – Response From Secondary Payer To Pharmacy For Secondary Claim.......................................883
34.39 MEDICARE PART D - 3 – INFORMATION REPORTING (N1) FROM FACILITATOR TO PDP FOR SECONDARY CLAIM................................884
34.39.1 Medicare Part D - 4 – Response From PDP To Facilitator For Information Reporting (N1) ............................................885
34.40 MEDICARE PART D - 5 – CLAIM SUBMITTED FROM PHARMACY TO TERTIARY PAYER WITHOUT UNIQUE BIN/PCN COMBINATION.......886
34.40.1 Medicare Part D - 6 – Response From Tertiary Payer To Pharmacy For Tertiary Claim .................................................887
34.41 MEDICARE PART D – 7 – INFORMATION REPORTING TRANSACTION SUBMITTED FROM TERTIARY PAYER TO FACILITATOR .................889
34.41.1 Medicare Part D - 8 – Information Reporting Transaction Submitted From Facilitator To PDP With Tertiary TrOOP
Update 890
34.41.2 Medicare Part D - 9 – Response For Information Reporting Transaction From PDP To Facilitator...............................891
34.41.3 Medicare Part D - 10 – Response For Information Reporting Transaction From Facilitator To Tertiary Payer ............892
34.42 MEDICARE PART D - 11 – B2 TRANSACTION REVERSAL FROM PHARMACY TO TERTIARY PAYER WITHOUT UNIQUE BIN/PCN
COMBINATION ..................................................................................................................................................................................................893
34.42.1 Medicare Part D - 12 – Response From Tertiary Payer To Pharmacy For Tertiary Reversal..........................................893
34.43 MEDICARE PART D -13 – INFORMATION REPORTING REVERSAL SUBMITTED FROM TERTIARY PAYER TO FACILITATOR......................894

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 27 -
34.43.1 Medicare Part D - 14 – Information Reporting Reversal Submitted From Facilitator To PDP For Reversal Of Tertiary
Claim 895
34.43.2 Medicare Part D - 15 – Response For Information Reporting Reversal From PDP To Facilitator For Tertiary Claim ..896
34.43.3 Medicare Part D - 16 – Response For Information Reporting Reversal From Facilitator To Tertiary Payer Of Tertiary
Claim 897
34.44 MEDICARE PART D - 17 – REVERSAL SUBMITTED FROM PHARMACY TO SECONDARY PAYER............................................................898
34.44.1 Medicare Part D - 18 – Response From Secondary Payer To Pharmacy For Reversal Of Secondary Claim................899
34.44.2 Medicare Part D - 19 – Information Reporting Reversal Submitted From Facilitator To PDP For Reversal Of Secondary
Claim 899
34.44.3 Medicare Part D – 2Ø – Response For Information Reporting Reversal From PDP To Facilitator For Secondary Claim
900
34.45 COMPOUNDED RX BILLING - TRANSACTION CODE B1 (Ø1) – COORDINATION OF BENEFITS SCENARIO..............................................902
34.45.1 Compounded Rx Billing Accepted Response- Paid ...........................................................................................................903
34.45.2 Billing – Transaction Code B1 – Compound – Coordination of Benefits –Pharmacy Bills Secondary Insurance .......904
34.45.2.1 Secondary Insurance Pays The Claim Submitted With Amount Paid By Other Payer.......................................................... 905
34.46 PREDETERMINATION OF BENEFITS - TRANSACTION CODE D1...........................................................................................................906
34.46.1 Predetermination Accepted Response - Benefit.................................................................................................................907
34.46.2 Predetermination of Benefits Transmission Rejected Response .....................................................................................908
34.46.3 Predetermination of Benefits Transaction Rejected Response ........................................................................................909
34.47 ELIGIBILITY MEDICARE PART D TO FACILITATOR – REQUEST ...........................................................................................................909
34.47.1 Scenario 1 – Could not find this member ............................................................................................................................909
34.48 ELIGIBILITY MEDICARE PART D TO FACILITATOR – REJECT RESPONSE ............................................................................................910
34.48.1 Scenario 1 – Could Not Find This Member..........................................................................................................................910
34.49 ELIGIBILITY MEDICARE PART D TO FACILITATOR – REQUEST ...........................................................................................................910
34.49.1 Scenario 2 – Found Member But No Coverage...................................................................................................................910
34.50 ELIGIBILITY MEDICARE PART D TO FACILITATOR – REJECT RESPONSE ............................................................................................911
34.50.1 Scenario 2 – Found Member But No Coverage...................................................................................................................911
34.51 ELIGIBILITY MEDICARE PART D TO FACILITATOR – REQUEST ...........................................................................................................912
34.51.1 Scenario 3 - Member Has Current Medicare Part D Coverage and No Other Coverage..................................................912
34.52 ELIGIBILITY MEDICARE PART D TO FACILITATOR – APPROVED RESPONSE........................................................................................912
34.52.1 Scenario 3 - Member has Current Medicare Part D Coverage and No Other Coverage..................................................912
34.53 ELIGIBILITY MEDICARE PART D TO FACILITATOR – REQUEST ...........................................................................................................913
34.53.1 Scenario 4 – Member Has Current Medicare Part D Coverage (Primary) and Current Other Coverage........................913
34.54 ELIGIBILITY MEDICARE PART D TO FACILITATOR – APPROVED RESPONSE........................................................................................914
34.54.1 Scenario 4 – Member Has Current Medicare Part D Coverage (Primary) and Current Other Coverage........................914
34.55 ELIGIBILITY MEDICARE PART D TO FACILITATOR – REQUEST ...........................................................................................................916
34.55.1 Scenario 5 – Future Effective with Medicare Part D ...........................................................................................................916
34.56 ELIGIBILITY MEDICARE PART D TO FACILITATOR – REJECTED RESPONSE ........................................................................................916
34.56.1 Scenario 5 – Future Effective with Medicare Part D ...........................................................................................................916
34.57 ELIGIBILITY MEDICARE PART D TO FACILITATOR – REQUEST ...........................................................................................................917
34.57.1 Scenario 6 – Adjusted Request to Scenario 5.....................................................................................................................917
34.58 ELIGIBILITY MEDICARE PART D TO FACILITATOR – APPROVED RESPONSE........................................................................................917
34.58.1 Scenario 6 – Adjusted Request to Scenario 5.....................................................................................................................917
34.59 BILLING - TRANSACTION CODE B1 - COB SCENARIO - PHARMACY BILLS REPORTING AMOUNT PAID BY PREVIOUS PAYER ONLY ......919
34.59.1 Pharmacy Bills Secondary Insurance..................................................................................................................................919
34.59.1.1 Secondary Response - Paid.............................................................................................................................................................920
34.60 BILLING – TRANSACTION CODE B1 – COORDINATION OF BENEFITS..................................................................................................921
34.60.1 Pharmacy Bills Secondary Insurance..................................................................................................................................922
34.60.1.1 Secondary Response - Paid.............................................................................................................................................................923
34.61 BILLING – TRANSACTION CODE B1 – COORDINATION OF BENEFITS – REIMBURSEMENT BASED ON THE OTHER PAYER PATIENT
RESPONSIBILITY AMOUNT (352-NQ) AND PATIENT REQUEST OF BRAND ............................................................................................................924
34.61.1 Pharmacy Bills Secondary Insurance..................................................................................................................................924
34.61.1.1 Secondary Response - Paid.............................................................................................................................................................925
34.62 BILLING – TRANSACTION CODE B1 – COORDINATION OF BENEFITS SCENARIO PHARMACY BILLS TO SECONDARY WHICH MEETS
DESIGNATION AS GOVERNMENT PAYER , PATIENT REQUESTS BRAND................................................................................................................926
34.62.1 Billing – Transaction Code B1 – Coordination of Benefits Scenario, Pharmacy Bills To Secondary Which Meets
Designation As Government Payer........................................................................................................................................................926
34.62.1.1 Response From Secondary Payer– Paid.......................................................................................................................................927
34.63 BILLING - TRANSACTION CODE B1 - REIMBURSEMENT BASED ON PATIENT PAY AMOUNT (5Ø5-F5)..................................................928
34.63.1 Billing - Accepted Response- Paid (Duplicate of Paid) ......................................................................................................930
34.64 SERVICE BILLING – TRANSACTION CODE S1 WITH CPT CODES.......................................................................................................930
34.64.1 Scenario using CPT Codes ...................................................................................................................................................930
34.64.1.1 Paid Response ...................................................................................................................................................................................932
34.64.2 Scenario Using CPT Codes With DUR/PPS Segment.........................................................................................................933
34.64.2.1 Paid Response ...................................................................................................................................................................................934
35. FREQUENTLY ASKED QUESTIONS..................................................................................................................................................................... 936

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 28 -
35.1 NOTABLE CHANGES FROM VERSION 5.1 TO VERSION D.Ø ...................................................................................................................936
35.2 UNUSUAL PACKAGE SIZE....................................................................................................................................................................936
35.3 COMPOUNDED PRESCRIPTIONS ...........................................................................................................................................................936
35.4 COMPOUND INGREDIENTS IN SEPARATE TRANSACTIONS ......................................................................................................................936
35.5 NON-COVERED INGREDIENTS IN A COMPOUND ....................................................................................................................................936
35.6 ELIGIBILITY CHECK .............................................................................................................................................................................936
35.7 BILLING FOR PARTIAL FILLS ...............................................................................................................................................................936
35.8 PRESCRIPTION AND SERVICE PRICING FORMULAE...............................................................................................................................936
35.9 CALCULATE NET AMOUNT DUE ...........................................................................................................................................................937
35.10 DUPLICATE TRANSACTIONS ............................................................................................................................................................937
35.11 PRESCRIPTION AND SERVICE BILLINGS IN ONE TRANSACTION.........................................................................................................938
35.12 REVERSING PRIOR AUTHORIZATION REQUEST AND BILLING TRANSACTIONS....................................................................................938
35.13 PRIOR AUTHORIZATION NUMBER-ASSIGNED (462-EV) ....................................................................................................................938
35.14 TRUNCATION IN THE HEADER SEGMENTS........................................................................................................................................938
35.15 SITUATIONAL/OPTIONAL FIELD POSITIONING ...................................................................................................................................938
35.16 SYNTAX ERRORS............................................................................................................................................................................938
35.17 USE OF COUNTERS ........................................................................................................................................................................938
35.18 PARTIAL FILL AND CHANGE OF COVERAGE.....................................................................................................................................938
35.19 ZERO DOLLAR AMOUNTS................................................................................................................................................................939
35.20 IDENTIFIER OF AN INGREDIENT .......................................................................................................................................................939
35.21 BILLING FOR PARTIAL FILL COMPOUND ..........................................................................................................................................939
35.22 RESPONSE PRICING SEGMENT IN CAPTURED RESPONSE.................................................................................................................939
35.23 PRIOR AUTHORIZATION INQUIRY AND CAPTURED RESPONSE...........................................................................................................939
35.24 RESPONSE HEADER SEGMENT FIELDS............................................................................................................................................939
35.25 ACCEPTED AND REJECTED INFORMATION IN ONE RESPONSE ..........................................................................................................940
35.26 DUR IN A COMPOUND....................................................................................................................................................................940
35.27 AN ORDER TO COMPOUND INGREDIENTS ........................................................................................................................................940
35.28 FORMAT OF PERCENTAGE SALES TAX FIELDS ................................................................................................................................940
35.29 ELIGIBILITY TRANSACTION AND THE GROUP SEPARATOR................................................................................................................940
35.30 REJECTING FOR INVALID HEADER FIELDS .......................................................................................................................................941
35.31 PRIOR AUTHORIZATION REQUEST AND BILLING – PRIOR AUTHORIZATION NOT REQUIRED................................................................941
35.32 PAYMENT AMOUNT BASED ON DISPENSED OR INTENDED................................................................................................................941
35.33 COORDINATION OF BENEFITS AND PARTIAL FILLS...........................................................................................................................941
35.34 NATIONAL DRUG CODES (NDCS) AND PROCEDURE CODE MODIFIERS.............................................................................................941
35.35 INVALID PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER (455-EM) ..................................................................................941
35.36 PREDETERMINATION OF BENEFITS DIFFERENCE TO CLAIM ..............................................................................................................942
35.37 COUPONS NOT SUBMITTED AFTER BILLING PRIMARY INSURANCE....................................................................................................942
35.38 FREE PRODUCT DEFINITION............................................................................................................................................................942
35.39 COUPONS AND REPLACEMENT OF INVENTORY................................................................................................................................942
35.40 MANUFACTURER CARDS AND COUPONS .........................................................................................................................................942
35.41 COUPONS AND PATIENT IDENTIFICATION.........................................................................................................................................942
35.42 PROCESS COUPONS WITHOUT COUPON SEGMENT..........................................................................................................................942
36. UPDATES AND CORRECTIONS TO STANDARDS ...........................................................................................................................................943
37. APPENDIX A. HISTORY OF DOCUMENT CHANGES.......................................................................................................................................944
37.1 VERSION 5.1.......................................................................................................................................................................................944
37.2 VERSION 5.2.......................................................................................................................................................................................944
37.3 VERSION 5.3.......................................................................................................................................................................................944
37.4 VERSION 5.4.......................................................................................................................................................................................944
37.5 VERSION 5.5.......................................................................................................................................................................................944
37.6 VERSION 5.6.......................................................................................................................................................................................944
37.7 VERSION 6.Ø......................................................................................................................................................................................945
37.8 VERSION 7.Ø......................................................................................................................................................................................946
37.9 VERSION 7.1.......................................................................................................................................................................................947
37.10 VERSION 8.Ø .................................................................................................................................................................................948
37.11 VERSION 8.1 ..................................................................................................................................................................................949
37.12 VERSION 8.2 ..................................................................................................................................................................................949
37.13 VERSION 8.3 ..................................................................................................................................................................................950
37.14 VERSION 9.Ø .................................................................................................................................................................................950
37.15 VERSION A.Ø.................................................................................................................................................................................950
37.16 VERSION A.1..................................................................................................................................................................................951
37.17 VERSION B.Ø.................................................................................................................................................................................952
37.18 VERSION C.Ø.................................................................................................................................................................................952
37.19 VERSION C.1..................................................................................................................................................................................954
37.20 VERSION C.2..................................................................................................................................................................................955
37.21 VERSION C.3..................................................................................................................................................................................956

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 29 -
37.22 VERSION C.4..................................................................................................................................................................................957
37.23 VERSION D.Ø.................................................................................................................................................................................960
37.23.1 August 2ØØ6 DERF Approvals.............................................................................................................................................960
37.23.2 November 2ØØ6 Approvals ..................................................................................................................................................962
37.23.3 Request Segment Matrices Modifications...........................................................................................................................969
37.23.3.1 Request Segment Matrices By Segment.......................................................................................................................................969
37.23.3.1.1 Eligibility/Billing/Encounter/Rebill/Reversal Matrix...................................................................................................................969
37.23.3.1.2 Prior Authorization Request And Billing/Prior Authorization Reversal/Prior Authorization Inquiry/Prior Authorization
Request Only Matrix .............................................................................................................................................................................................. 969
37.23.3.1.3 Information Reporting/Information Reporting Reversal/Information Reporting Rebill/Controlled Substance
Reporting/Controlled Substance Reversal/Controlled Substance Rebill......................................................................................................970
37.23.4 Response Segment Matrices Modifications........................................................................................................................971
37.23.4.1 Response Segment Matrices By Segment....................................................................................................................................971
37.23.4.1.1 Transmission Accepted; Transaction Paid Or Duplicate Of Paid, or Benefit Matrix ..........................................................971
37.23.4.1.2 Transmission Accepted; Transaction Captured Or Duplicate Of Capture Matrix............................................................... 972
37.23.4.1.3 Transmission Accepted; Transaction Approved Or Duplicate Of Approved Matrix ........................................................... 973
37.23.4.1.4 Transmission Accepted; Transaction Deferred Matrix ............................................................................................................ 974
37.23.4.1.5 Transmission Accepted; Transaction Rejected Matrix............................................................................................................ 975
37.23.4.1.6 Transmission Rejected; Transaction Rejected Matrix............................................................................................................. 977
37.23.5 August 2ØØ7 Approvals........................................................................................................................................................978
38. APPENDIX B. REVISION INFORMATION.............................................................................................................................................................979
39. APPENDIX C. DATA DICTIONARY FIELD DELETIONS................................................................................................................................... 980
40. APPENDIX D. WHAT IS THE 11-DIGIT FORMAT FOR AN NDC, UPC, OR HRI? ....................................................................................... 981
40.1 NATIONAL DRUG CODES (NDC)..........................................................................................................................................................981
40.2 UNIVERSAL PRODUCT CODES (UPC)...................................................................................................................................................981
40.3 NATIONAL HEALTH RELATED ITEM CODES (NHRIC OR HRI) ................................................................................................................982
40.4 NON STANDARD PRODUCT CODES ......................................................................................................................................................982
41. APPENDIX E. USE OF INFORMATION REPORTING (N1, N2, N3) FUNCTIONALITY FOR MEDICARE PART D PROCESSING...983
41.1 BACKGROUND ....................................................................................................................................................................................983
41.2 INFORMATION REPORTING...................................................................................................................................................................983
42. APPENDIX F. ORDUR (ONLINE REAL-TIME DRUG UTILIZATION REVIEW).............................................................................................985
42.1 INTRODUCTION ...................................................................................................................................................................................985
42.2 CHAPTER 1. ORDUR PROCESSING DESIGN AND IMPLEMENTATION .....................................................................................................985
42.2.1 Information Categories..........................................................................................................................................................985
42.2.1.1 Member Information................................................................................................................................................................................985
42.2.1.2 Prescription Information ......................................................................................................................................................................... 986
42.2.1.3 Prescriber Identification.......................................................................................................................................................................... 986
42.2.1.4 Pharmacy Identification..........................................................................................................................................................................986
42.2.2 DUR System Support Files....................................................................................................................................................986
42.2.3 Design Discussion Summary................................................................................................................................................989
42.3 CHAPTER 2. ORDUR MESSAGE FORMATS .........................................................................................................................................989
42.3.1 Standard DUR Message.........................................................................................................................................................989
42.3.2 DUR Action Code Messages.................................................................................................................................................992
42.3.3 DUR Information Entry ..........................................................................................................................................................992
43. APPENDIX G. TWO-WAY COMMUNICATION TO INCREASE THE VALUE OF ON-LINE MESSAGING .............................................. 994
43.1 BACKGROUND ....................................................................................................................................................................................994
43.2 SPECIFIC DATA FIELD USE RECOMMENDATIONS ..................................................................................................................................994
43.2.1 Benefit- or Plan-Generated Rejections ................................................................................................................................995
43.2.1.1 Reject Code “76 “ (Plan Limitations Exceeded).................................................................................................................................995
43.2.1.2 Reject Code “79 “ (Refill Too Soon).....................................................................................................................................................995
43.2.1.3 Reject Code “52 “ (Non-Matched Cardholder ID)..............................................................................................................................995
43.2.1.4 Reject Code “69 “ (Filled After Coverage Terminated).....................................................................................................................996
43.2.1.5 Reject Code “68 “ (Filled After Coverage Expired)............................................................................................................................996
43.2.1.6 Reject Code “7Ø “ (Product/Service Not Covered) ........................................................................................................................... 996
43.2.1.7 Reject Code “Ø6 “ (M/I Group ID) ........................................................................................................................................................996
43.2.1.8 Reject Code “19 “ (M/I Days Supply) ...................................................................................................................................................996
43.2.1.9 Reject Code “88 “ (DUR Reject Error) .................................................................................................................................................996
43.2.1.10 Reject Code “65 “ (Patient Is Not Covered)................................................................................................................................... 997
43.2.1.11 Reject Code “Ø7 “ (M/I Cardholder ID)...........................................................................................................................................997
43.2.1.12 Reject Code “54 “ (Non-Matched Product/Service ID Number)................................................................................................. 997

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 30 -
43.2.1.13 Reject Code “75 “ (Prior Authorization Required).........................................................................................................................997
43.2.1.14 Reject Code “Ø9 “ (M/I Date Of Birth) ............................................................................................................................................998
43.2.1.15 Reject Code “51 “ (Non-Matched Group ID)..................................................................................................................................998
43.2.1.16 Reject Code “92 “ (System Unavailable/Host Unavailable)........................................................................................................998
43.2.2 Other Notable Reject Codes..................................................................................................................................................998
43.2.2.1 Reject Code “83 “ (Duplicate Paid/Captured Claim) .........................................................................................................................998
43.2.2.2 Reject Code “53 “ (Non-Matched Person Code)................................................................................................................................998
43.2.2.3 Reject Code “4Ø “ (Pharmacy Not Contracted with Plan)................................................................................................................ 999
43.3 DUR-GENERATED REJECTIONS ..........................................................................................................................................................999
43.4 PARTICIPATING ORGANIZATIONS .......................................................................................................................................................1000
43.5 LONG TERM CARE TRANSITION, EMERGENCY FILL AND CHANGE IN LEVEL OF CARE MESSAGING FOR REJECTED AND PAID CLAIMS.....1000
43.5.1 Background..........................................................................................................................................................................1000
43.5.2 Rejected Claim Option.........................................................................................................................................................1001
43.5.2.1 When Prior Authorization Number (498-PY) Required................................................................................................................... 1001
43.5.2.2 Transition and Safety-Related Rejects..............................................................................................................................................1002
43.5.3 Claims Paid Due To CMS Initial Eligibility Transition Period...........................................................................................1002
43.5.3.1 Approved Message Code “ØØ4” (Filled During Transition Benefit).............................................................................................1002
43.5.4 Claims Paid Due To CMS Emergency Fill Requirement...................................................................................................1002
43.5.4.1 Approved Message Code “ØØ8” (Emergency Fill Situation).........................................................................................................1002
43.5.5 Claims Paid Due To CMS Change In Level Of Care Requirement...................................................................................1003
43.5.5.1 Approved Message Code “Ø12” (Level of Care Change)..............................................................................................................1003
44. APPENDIX H. ROUTE OF ADMINISTRATION TRANSITION.........................................................................................................................1004

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 31 -
1. INTRODUCTION
The Standardization Committee within the National Council for Prescription Drug Programs (NCPDP) is responsible for maintaining standard
formats for the electronic submission of third party drug claims and other related transactions. NCPDP revises the standard format as industry
requirements change and as new technology becomes available.
The Standardization Committee within NCPDP, in conjunction with Work Group members, develops the telecommunication standard to provide
a consistent format for electronic pharmacy transaction processing. The NCPDP Telecommunication Work Group receives input from all aspects of
the prescription drug program administration industry, and the standard is designed to be easy to implement and yet flexible enough to respond as
the needs and technology change. The Telecommunication Work Group continually reviews the format design and recommends revisions when
appropriate.
The Standardization Committee also pursues standardization of other requirements in the pharmacy industry. NCPDP recommends the use of a
standardized format for electronic communication of pharmacy service-related billing, prior authorization processing, and information reporting
between pharmacies and other responsible parties. This standard addresses the data format and content, the transmission protocol, and other
appropriate telecommunication requirements. NCPDP does not endorse any specific electronic device or network that is used to support these
communication vehicles.
If you have any questions regarding the availability or content of the NCPDP Telecommunication Standard Implementation Guide, see
www.ncpdp.org, or contact the Council office at (48Ø) 477-1ØØØ or via e-mail at ncpdp@ncpdp.org.
1.1 DOCUMENT SCOPE
This document contains the standard formats and implementation guide. Users of this document should consult the NCPDP documents listed
below for further information and clarification.
BILLING UNIT STANDARD IMPLEMENTATION GUIDE
Standard billing units used for claim submission.
. DATA DICTIONARY
Full reference to all fields and values (contained within or reference to the External Code List) used in the NCPDP
standard with examples.
EXTERNAL CODE LIST
Full reference to values used in the NCPDP standard.
STANDARDS MATRIX
This document contains charts that list the Standards and Implementation Guides versions approved or under
consideration by NCPDP, with reference to the Data Dictionary and External Code List documents appropriate for use.
EDITORIAL DOCUMENT
This document contains clarifications, corrections, examples, and questions/answers that were obtained after the
publication of the NCPDP Telecommunication Standard Implementation Guide. It must be used as a reference between
official publications of the implementation guide. This document may be updated as often as quarterly and new versions
should be downloaded. It is available from the public and members only sections of the NCPDP website.
BATCH IMPLEMENTATION GUIDE
This document supports the business need to support the same functionality as the NCPDP Telecommunication Standard
Implementation Guide, except in a batch environment.
MEDICAID SUBROGATION IMPLEMENTATION GUIDE
The NCPDP Medicaid Subrogation Implementation Guide provides guidelines for the process whereby a Medicaid agency
can communicate to a processor for reimbursement. The state has reimbursed the pharmacy provider for covered
services and now is pursuing reimbursement from other payers for these services.
NCPDP produces a comprehensive Data Dictionary for all approved standards. The NCPDP Data Dictionary document specifies valid field
values and definitions for all elements in this standard as well as other NCPDP approved standards. The NCPDP Data Dictionary has been
modified to remove some data elements contained in the previous releases of the standard that were considered impractical and unnecessary
for the new standard. Data elements that were not brought forward are noted in the appendix “Data Dictionary Field Deletions” section of this
document.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 32 -
These documents are available with NCPDP membership; contact the NCPDP office at 48Ø-477-1ØØØ, or via Internet e-mail at
ncpdp@ncpdp.org. The documents are available in the “Members” section of the website at www.ncpdp.org.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 33 -
2. BACKGROUND
This document describes NCPDP Telecommunication Standard Implementation Guide (Version D and above) standards for the transmission
of transactions via telecommunication facilities among health care entities. It is intended for use by organizational decision makers who need
to understand the essential features of electronic transmission of transactions and as a guide for software developers and others who must
implement the standard.
To understand the development and intent of this format and implementation, it is necessary to first review its background and objective, the
framework within which it has been developed, and its intended use in the third party environment.
This version of the standard has been developed for the use of direct electronic submission and adjudication of transactions in an on-line, real-
time environment. It is the next logical step in an evolutionary process marked by the following key events:
Submission of paper claims using claim forms unique to each carrier or administrator.
• Development of a Universal Claim Form by NCPDP (198Ø).
• Submission of claims via magnetic tape and diskette using a format unique to each carrier or administrator (1984).
• Direct electronic submission and adjudication of claims in an on-line, real-time environment using processor-specific formats (1988).
• Development of a telecommunication standard format (version 1.Ø) by NCPDP (1989).
• Development of an enhanced telecommunication standard format (version 3.2) by NCPDP (1992).
• Development of on-line, real-time compound claim submission within the telecommunication standard format (version 3.3) by
NCPDP (1996).
• Development of prior authorization transaction sets within the telecommunication standard format (version 3.4) by NCPDP (1996).
• Development of enhanced, variable telecommunication standard format (version 5.Ø) by NCPDP (June 1999).
• The naming of the Telecommunication Standard Version 5.1 in the Health Information Portability and Accountability Act (HIPAA)
(2ØØØ).
Usage of a common transaction format brings advantages to participants in the pharmacy industry. There are significant advantages to both
the Originator of the claim and the Processor of the transaction by adopting this version of the standard, such as:
• Common syntax and dictionary
• Adaptability
• Reduced system development expense
• Reduced equipment requirements
• Reduced errors
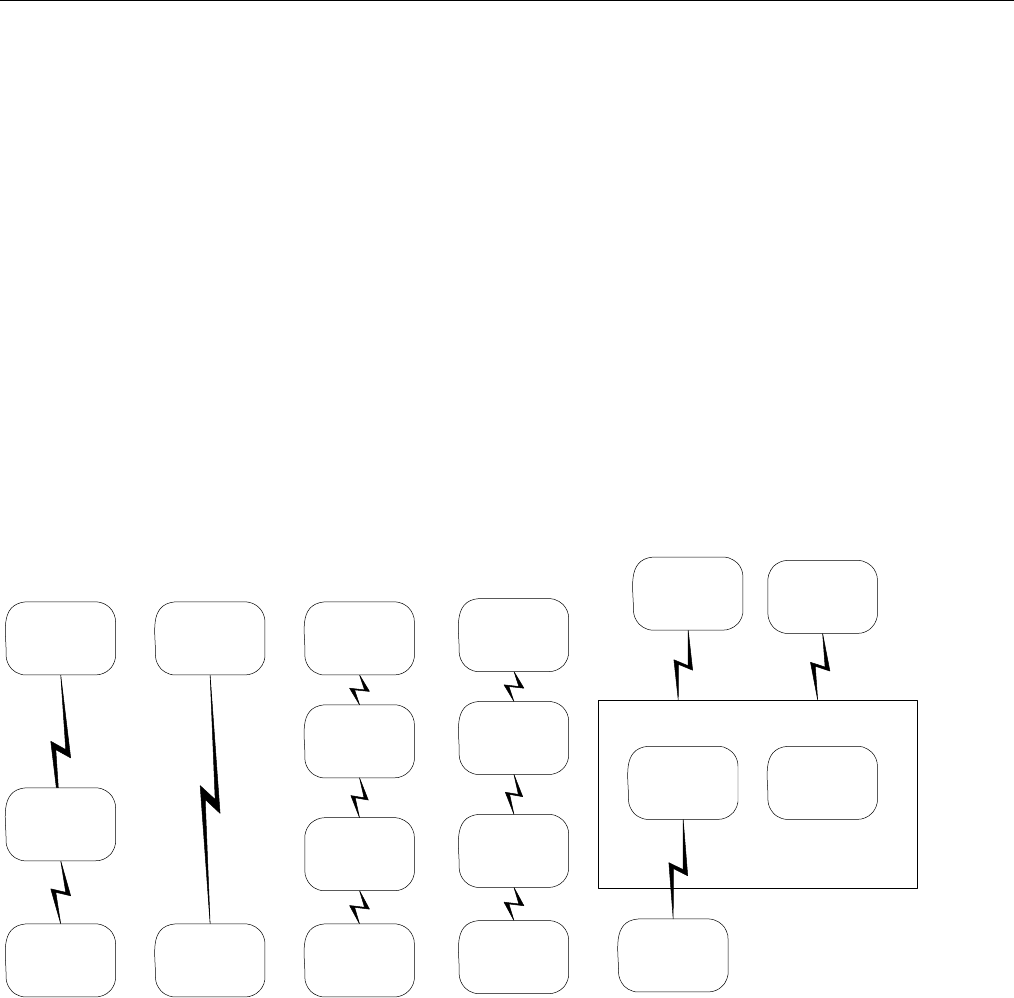
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 34 -
3. BUSINESS ENVIRONMENT
3.1 OBJECTIVES
The NCPDP Telecommunication Standard Implementation Guide (Version D and above) is intended to meet two needs within the pharmacy
drug claim industry: to provide practical guidelines for software developers throughout the industry as they implement the Version D and above
Standard, and to ensure a consistent implementation of the Version D and above Standard.
This version of the standard facilitates a specific type of business communication among diverse parties within the third party environment. To
do this successfully, it must accomplish the following goals:
• Support the needs of a wide base of potential users.
• Maximize use of existing relevant standards wherever possible.
• Be flexible enough to change as needs and technology change.
• Be unambiguous.
• Be easy to implement by carriers and vendors.
3.2 PARTICIPANTS
The NCPDP Telecommunication Standard Implementation Guide (Version D and above) supports prescription claim transactions between the
following industry participants:
• Between Providers and Adjudicators, and
• Between Adjudicators (aka Payer-to-Payer)
3.2.1 BETWEEN PROVIDERS AND ADJUDICATORS
The communication between Providers and Adjudicators is two-way and the record layout for the transmitted claim and the response to the
claim are defined by the Version D and above standard. The diagram below illustrates the typical business environments in which the NCPDP
Telecommunication Standard Implementation Guide (Version D and above) is employed between providers and adjudicators.
Adjudicator
(Processor)
Switch
Provider
Adjudicator
(Processor)
Provider
Intermediary
Adjudicator
(Processor)
Switch
Provider
Switch
Adjudicator
(Processor)
Intermediary
Provider
Facilitator
Secondary
Adjudicator
(Processor)
Switch
Provider
Primary
Adjudicator
(Processor)
Figure 1. Provider/Adjudicator Participants
A “PROVIDER” may be a retail pharmacy, mail order pharmacy, doctor’s office, clinic, hospital, long-term care facility, or any other entity, which
dispenses prescription drugs and submits those prescriptions to a payer for reimbursement.
The “ADJUDICATOR” (hereinafter referred to as the “PROCESSOR”) is often a third-party administrator of prescription drug programs on behalf of
insurers. The Adjudicator also may be an insurer, a governmental program or any other entity, which receives prescription drug claims, makes
a decision regarding the level of reimbursement to the provider, and transmits a response to the provider.
Providers may choose to transmit certain prescription drug claims to an “INTERMEDIARY”. Intermediaries receive claims from switches or
providers, perform editing/messaging and then either pass the claims to the appropriate switch or adjudicator or return (reject) claims to the
providers. The reply from the adjudicator also may pass to an intermediary for editing and messaging on its return to the provider.
Providers may choose to transmit claims to an intermediary for a number of reasons, including the following:
• Consolidated provider reporting
• Inventory tracking
• Consolidated claim editing and messaging
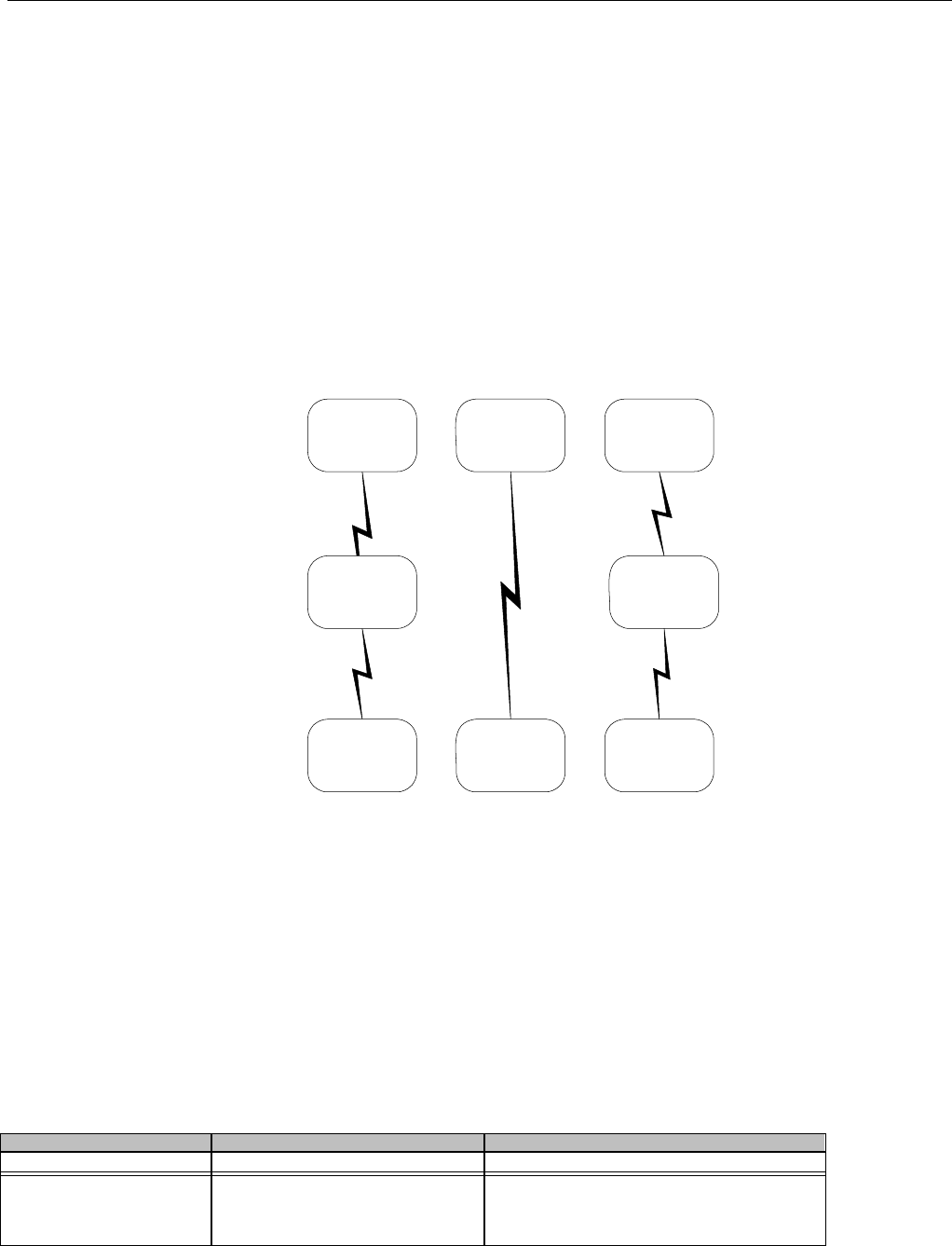
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 35 -
The “SWITCH” also receives transactions from providers and intermediaries as claims pass from providers to adjudicators. Switching
companies accept claims, optionally perform format conversions, may perform pre-editing, and then pass the claims to the appropriate
processor. The reply from the processor also may pass through the switch on its return to the provider.
Providers utilize the services of a switch for a number of reasons, including the following:
• A processor may not support “Dial-Up” communications
• All claims can be transmitted to one central point, the “Switch”
• Increased reliability of communications
3.2.2 BETWEEN ADJUDICATORS (PAYER-TO-PAYER)
The communication between Adjudicators is two-way. The record layout for the transmitted claim and the response to the claim (if supported)
are defined by the Version D and above standard. Uses, for example:
• Medicare Crossover - Coordination of benefits of claims between Medicare and other payers. This is referred to as “payer-to-
payer”.
• Information Reporting transactions for Medicare Part D from payer to facilitator to payer. This is referred to as “Medicare
Part D payer-to-payer facilitation”.
The diagram below illustrates the typical business environments in which the NCPDP Telecommunication Standard Implementation Guide
(Version D and above) is employed between adjudicators (payer-to-payer).
Adjudicator
(Processor)
Switch
Adjudicator
(Processor)
Adjudicator
(Processor)
Adjudicator
(Processor)
Facilitator
Adjudicator
(Processor)
Adjudicator
(Processor)
Figure 2. Between Adjudicator Participants
The sections that follow address a variety of issues including the following:
• Implementation practices which are generally accepted throughout the industry although they may not be defined as part of the
standard.
• Updates and corrections to the document that defines the Version D and above standard.
• Sample transactions using the Version D and above standard.
• Answers to frequently asked questions regarding the standard.
There is a unique communication occurring for Medicare Part D claims involving true-out-of-pocket (TrOOP) facilitation that introduces the
“FACILITATOR” entity. There is a need for the primary processor Part D plan, hereafter referred to as the Prescription Drug Plan (PDP) to know
the patient’s pay amount from all other payers.
The Provider transmits all non-primary claims to the Switch, which routes them to both the Secondary, Tertiary, etc. Adjudicator and to the
Facilitator. The Facilitator creates and sends reporting transactions containing Secondary, Tertiary, etc. patient’s pay amount information to the
Primary Adjudicator (Processor).
Third-party activities, as they pertain to the prescription drug industry, can have the following key participants:
Participant Description Functions Performed
Patient Recipient of Service • Request for service
Insurer Provider of Insurance Plan Definition
Covered Group
Covered Services
Benefit Level
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 36 -
Participant Description Functions Performed
• Pricing of Contract (Premiums)
• Assume Risk of Actual Plan Experience
Administrator Delivers Administrative Services • Authorization of Individual Patient
• Adjudicator of Claim or Service
Processor Authorization/Adjudication of Services • Authorization of Individual Service
• Adjudication (Claim or Service Processing)
• Predetermination of Benefits information
(Claim only)
Reporting Entity Contractual Service • Record Keeping
• Authorization of Individual Service
• Auditing
Provider/Originator Provider of Service Claims/Service Submission
• Information Reporting Submission
• Controlled Substance Reporting
Submission
• Prior Authorization Submission
• Predetermination of Benefits information
(Claim only)
Switch Communication/Translation Service
• Network or communication services
• Format/syntax translation
Intermediary Contractual Service • Reconciliation Services
• Formulary Services
• Pre-Claim editing
Facilitator Contractual Service
• Eligibility Inquiry
• Reporting to the Prescription Drug Plan
(PDP) for True Out-Of-Pocket (TrOOP)
calculation
The following information may be true of business framework relationships:
• A given entity might serve multiple roles (for example, Insurer and Processor).
• Certain roles might be split among multiple organizations, for example, the Administrator and Processor could be different.
• This version of the telecommunication standard addresses the submission of a claim or service by an Originator to an
Administrator/Processor, and identifies the response of the Administrator/Processor to the Originator. For the purpose of this
document, the term "Processor" will be used to identify the entity performing the authorization/adjudication function.
• This version of the standard also addresses the submission of an information or controlled substance reporting action by an
Originator to a Reporting Entity. This action may be separate from the actual dispensing of a product or service.
• This version of the standard also addresses the trading partner requirement of prior authorizations performed by an Originator to an
Administrator/Processor prior to a claim or service being performed.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 37 -
4. BUSINESS FUNCTIONS
4.1 INTRODUCTION
This version of the standard addresses the types of communication between Originators and Processors, Administrators, or Reporting Entities
(receivers). It is not expected that all transaction types will be used by all Processors, Providers, or Switches. Trading partner and business
needs will determine transaction type usage.
This section describes the different functional transactions defined in this version of the standard.
It is expected that endorsement of this version of the standard will ensure that whenever a Processor needs to process transactions defined by
this standard, they will do so only in the formats defined herein.
Please refer to the section “Transmission Structure” for a list of the mandatory, situational, and optional segments that may be used in the different
transaction types. Also, each transaction has an information section (for example “Claim or Encounter Information” that describes the transactions,
segments, and fields usage.
The formats for telecommunicated information include the following situations:
1. Eligibility verification.
2. Claim or Service(s) billings.
3. Eligibility verification as part of full claim or service (s) adjudication as dictated by plan parameters.
4. Claim or service reversals for previously captured or adjudicated claims or services.
5. Claim or service(s) rebilling with an implied reversal - A combination of items 2 or 3, and 4 above. Previously captured or
adjudicated claims or services are reversed and then the new claims or services are processed.
6. Information reporting - An example of this type of transmission may occur when a drug conflict has been identified and a pharmacist
has executed some form of intervention with a specific outcome or to capture other DUR information.
a. This type of transaction can be used for a variety of Drug Use Review (DUR) activities.
b. Examples of these transactions include capture of dispensed non-covered prescriptions, override of DUR information-only
alerts, or Medicare Part D notifications to PDP regarding supplemental claims.
7. Information reporting reversals for previously captured information.
8. Information reporting rebilling with an implied reversal - A combination of items 6 and 7 above. Previously captured information is
reversed and then the new information is processed.
9. Prior authorization request and billing - Transaction to request simultaneous adjudication/capture of the transaction by the processor
upon approval of the prior authorization.
10. Prior authorization reversal for previously captured authorizations.
11. Prior authorization inquiry - Transaction to request the status of a previously submitted prior authorization request that was pended
by the processor.
12. Prior authorization request only - Transaction to request a prior authorization only and exclude the processing of a claim or service.
13. Controlled substance reporting - Transactions which allow Processors to collect information about prescribing, dispensing, and
consumption of dangerous or abusable drug.
14. Controlled substance reporting reversals for previously captured reporting.
15. Controlled substance reporting rebilling with an implied reversal - A combination of items 13 and 14 above. Previously captured
controlled substance reporting transactions are reversed and then the new controlled substance reporting transactions are
processed.
16. Medicaid Subrogation – See below in section “Medicaid Subrogation”. More information on this business function is found in the
NCPDP “Medicaid Subrogation Implementation Guide”.
17. Predetermination of benefits – Transaction for a provider to assist the patient in determining if a given prescription would be covered
under their program and to provide guidance on patient responsibility costs to make an informed decision about whether the patient
would proceed.
The mandatory and situational fields and segment designations as noted in this document must be followed. Though some fields are
designated as situational in this document, the receiver/processor/payer/adjudicator may choose to “require” the field, provided the situation(s)
stated for that field in this document (and the requirements of the Implementation Guide) are met. When a situational field is used, the situation
must be noted in the plan sheet or provider manual.
4.2 MEDICAID SUBROGATION
Medicaid Subrogation is a process whereby Medicaid is the payer of last resort. The state has reimbursed the pharmacy provider for covered
claims and now is pursuing reimbursement from other payers for these claims. Some states may choose to “Pay” all claims in full, through a
federal waiver, at the point of receipt and “Chase” reimbursements from responsible third parties after the fact. After the Claim Billing, Claim
Rebill, or Encounter transactions, where situations are defined for Medicaid Subrogation, separate Segment tables will be shown. (Note the
membership determined that Medicaid Subrogation was not applicable for Service Billings.)
Since the Medicaid Subrogation transactions use the Telecommunication Standard transactions, where situations have been defined for fields
specifically for subrogation purposes, they are included in this guide as separate charts. More information on this business function is found in
the NCPDP “Medicaid Subrogation Implementation Guide”.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 38 -
5. TERMINOLOGY USED THROUGHOUT
Standard –
In this document “standard” as in “Standard Rejected Response” is meant as a generic transaction response that does not need to
be differentiated as emanating from a Receiver.
Grouped -
Defined in the Situational column of the charts, as in “Grouped with Other Payer ID Qualifier, Other Payer ID, Other Payer Date, and
either Other Payer Amount Paid Count and its grouping, or Other Payer Reject Count and it’s grouping.” Grouped refers to a natural
occurrence of fields that together form valuable information. The Group does not mean that all fields must be present. Refer to each
field for instructions.
Required if –
Used in the Situation column and Notes sections, “Required if” designates the field is to be used if the requirement is satisfied. For
example, “Required if Patient ID (332-CY) is used” means that if the Patient ID (332-CY) field contains a value, the Patient ID
Qualifier (331-CX) must also contain a valid value.
Not required if –
Used in the Situation column and Notes sections, “Not required if” designates the field is not to be used if the requirement is
satisfied. For example, “Not required if Prescription/Service Reference Number Qualifier (455-EM) = “1” (Rx Billing)” means that the
Procedure Modifier Code Count is not to be used if Prescription/Service Reference Number Qualifier = “1”. However in other
situations, the Procedure Modifier Code Count may be used.
Not used –
Used in the Situation column and Notes sections, “Not used if/for” designates the field is not available for usage in this transaction.
For example, “Not used for a Transaction Code = “E1” (Eligibility Verification)” for field Approved Message Code Count means the
Approved Message Code Count field is not to be used in an Eligibility Verification transaction.
Optional –
Used when no situations are defined but the field is to be used. The use of the field is left to trading partner agreement.
Sender and Receiver –
For this situational usage charts, the following definitions are used for these entities, depending on the role they are taking at a given
moment in time.
Sender – initiates the request
Receiver – receives the request
Sender – initiates the response
Receiver – receives the response
For example, when a pharmacy submits an Eligibility Verification request to the health plan, the pharmacy is the sender; the health
plan is the receiver.
When the health plan returns a response to the pharmacy, the health plan is the sender; the pharmacy is the receiver.
Commercial Health Plan –
In this document Commercial Health Plan is meant as a non-Medicaid agency that is processing pharmacy transactions.
Health Plan –
When used by itself, “Health Plan” means either Commercial or Medicaid Health Plan, as when used in “Standard
Rejected Response from a Health Plan to a Pharmacy”. The response in this scenario is not different enough when
emanating from a Medicaid or a Commercial Health Plan to warrant a separate scenario.
Claim – Throughout the situational fields if the terminology “Claim” or “Claim Billing” is used, this means if the Transaction Code (1Ø3-A3) =
“B1” or “B3” and the Prescription/Service Reference Number Qualifier (455-EM) = “1” (Rx Billing). For example, “Claim Billing/Claim
Rebill/Encounter:” Or the Transaction Code (1Ø3-A3) is specific to a transaction, but the Prescription/Service Reference Number Qualifier
(455-EM) = “1” (Rx Billing) – for example “Prior Authorization Inquiry (Claim):”
Service – Throughout the situational fields, if the terminology “Service” or “Service Billing” is used, this means if the Transaction Code (1Ø3-
A3) = “S1” or “S3” and the Prescription/Service Reference Number Qualifier (455-EM) = “2” (Service Billing). For example “Service
Billing/Service Rebill”. Or the Transaction Code (1Ø3-A3) is specific to a transaction, but the Prescription/Service Reference Number Qualifier
(455-EM) = “1” (Rx Billing) – for example “Prior Authorization Inquiry (Service):”
In cases where the Claim or Service situations are the same, the terminology does not specify a function. For example, “Prior Authorization
Inquiry:” or “Prior Authorization Reversal:” is synonymous with “Prior Authorization Inquiry (Claim/Service)” or “Prior Authorization Reversal
(Claim/Service)”. This means that for a claim or service, the situation applies.
5.1 TABLE DESIGNATION – LEGEND
Designation Value Explanation
MANDATORY M
The Segment is mandatory for the Transaction
or
The Field is mandatory for the Segment for the Transaction.
Mandatory elements have structural requirements.
SITUATIONAL The Segment has been further designated for usage for the Transaction
or
The Field has been further designated for usage for the Transaction.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 39 -
Designation Value Explanation
Required R
The Field has been designated with the situation of "Required" for the Segment for
the Transaction.
Required for Medicaid Subrogation only RM The Field has been designated with the situation of "Required" for the Segment for
the Transaction for Medicaid Subrogation usage only.
Qualified Requirement Q The situations designated have qualifications for usage ("Required if x", "Not
required if y").
Qualified Requirement for Medicaid
Subrogation only QM The situations designated have qualifications for usage ("Required if x", "Not
required if y") for Medicaid Subrogation.
INFORMATIONAL ONLY I The Field is for informational purposes only for the Transaction.
OPTIONAL O The Field has been designated as optional usage (situations were not intentionally
defined).
NOT USED N The Segment is not used for the Transaction
or
The Field is not used for the Segment for the Transaction.
Not used are shaded for clarity.
Repeating ***R*** The three asterisks, “R”, and three asterisks designates a field is repeating.
Example “Q***R***” means a situationally qualified field that repeats. Example
“N***R***” means a not used field that repeats when used.
5.2 TABLE DESIGNATION
Throughout the document, font color is used to designate a field that is not used in the specific transaction.
Reject Count (51Ø-FA) and Reject Code (511-FB) are not used in this specific example and are therefore shown in gray. The gray designation
is only used when a field is not used in the specific transaction. Note the example below.
Field Field Name Mandatory or
Situational Situation
5Ø3-F3 AUTHORIZATION NUMBER Q Claim Billing/Encounter:
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT N Claim Billing/Encounter:
Not used.
511-FB REJECT CODE N**R*** Claim Billing/Encounter:
Not used.
Note also that Reject Code (511-FB) is a repeating field (***R***) but in this transaction, the field is not used (N***R***).
When a field is not used for a particular situation, such as below, the gray designation is not used. For example below, even though the Prior
Authorization Inquiry (Service) situation is “Not used”, the field is not gray because other situations apply to this field (for the Claim). Note in
this example, the Claim and Service have different situations, so the “Mandatory or Situational” column shows both designations (Q and N).
Field Field Name Mandatory or
Situational Situation
5Ø6-F6 INGREDIENT COST PAID Q
N
Prior Authorization Inquiry (Claim):
Required if this value is used to arrive at the final
reimbursement.
Service:
Not used.
5Ø7-F7 DISPENSING FEE PAID Q
N
Prior Authorization Inquiry (Claim):
Required if this value is used to arrive at the final
reimbursement.
Service:
Not used.
5.3 TRANSMISSION DISCUSSION
In each Transmission defined, there is general overview information, followed by the Segment Usage in table form.
Next follows the actual Segments, denoted as Mandatory or Situational, and each field within the Segment. Each field is designated as to its
use.
Each section lists each segment, with all fields included in this segment. In some cases, fields within that segment are not used for the
particular transaction or scenario. All fields are shown to illustrate that each field was reviewed with recommendations, whereas the absence
of a field might lead a reader to wonder if the field was left off intentionally.
Note if the sender chooses to send in more fields than are required or situational by the receiver, but which the sender needs for their
business, the receiver is to ignore these fields or segments.
After each Segment is a “Notes” section, that further explains any rules, situations, or notes on this Segment.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 40 -
The Transaction Header Segment and the Response Header Segment are mandatory, fixed length segments. In the segment usage charts for
the Transaction Header Segment and the Response Header Segment, the column denoted “Situation” is not applicable.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 41 -
6. ELIGIBILITY VERIFICATION INFORMATION
6.1 ELIGIBILITY VERIFICATION
This transaction is used by the Originator to request that the Administrator, Processor, or Reporting Entity verify the eligibility of a specific
patient according to appropriate plan parameters. This transaction is used to request verification of a patient’s or cardholder’s status for a
given benefit program. Only one transaction per transmission is permitted.
An eligibility verification request occurs once per transmission. The Transaction Code is “E1”.
The Processor responds with either of the following:
Approved Response - The patient is eligible for service.
Rejected Response - The patient is not eligible for service, or the transaction is in error.
If a duplicate transmission occurs, the returned response must be the same as the original transmission response. See section “Response
Processing Guidelines”, “Duplicate Transactions”.
6.1.1 MEDICARE PART D ELIGIBILITY
For Medicare Part D the Eligibility transaction (E1) is used to determine patient eligibility. If a patient enrolled in Medicare Part D does not
present a Medicare Part D ID card to the pharmacy provider or the pharmacy provider wants to verify coverage, this transaction can be used to
determine which plan(s) to bill and if known, in what order. The Facilitator provides this information on the E1 response to the pharmacy
provider.
This Eligibility enrollment response will be different than a normal Eligibility response from a Processor. In the normal Eligibility Response, the
Processor supplies Eligibility information specific to coverage provided under that Plan. In the Medicare Part D Eligibility Response, the
Facilitator supplies Eligibility Enrollment information for Medicare Part D coverage and Other Health Insurance coverage via the Eligibility
request by the Pharmacy Provider. CMS provides to the Facilitator eligibility enrollment data, which includes plans in which the Patient is
enrolled.
6.1.1.1 BUSINESS RULES FOR MEDICARE PART D ELIGIBILITY TRANSACTIONS BETWEEN THE
PHARMACY AND THE FACILITATOR
• The search will always be based on Date of Service
• Future Coverage from the Date of Service will only be provided for Part D and will only be provided in the Next Medicare Part D
Effective Date (14Ø-US) and Next Medicare Part D Termination Date (141-UT) in the Response Insurance Additional Information
Segment
o Future means—Future Eligibility coverage from date of service
o The future date closest to the date of service requested will be returned when more than one future coverage exist.
• Coverage other than Part D will be sent if the following criteria are met:
o There is Medicare Part D coverage as of date of service and
o The other coverage is effective as of date of service
• The most current information as of the date of request for that date of service will be returned
• The response will be based on Date of Service for Part D coverage
o If patient is not found, then
Rejected response
Response Patient Segment Not Returned
o If patient is FOUND (Patient that has had Medicare Part D coverage at some point within the search parameters
timeframe determined by the Facilitator), then
If patient has current Part D Coverage based on Date of Service
• Approved response
• Response Patient Segment will be returned with data from the Facilitator system –not the submitted
data
If patient has future Part D coverage, but no current coverage based on Date of Service
• Rejected response
• Response Patient Segment will be returned with data from the Facilitator system-not the submitted
data,
If patient had Part D, but does not have current or future coverage based on Date of Service
• Rejected response
• Response Patient Segment will be returned with data from the Facilitator system-not the submitted
data
• If more than one payer exists in the Coordination Of Benefits/Other Payments Segment, the values within the Other Payer Coverage
Type (338-5C) and Medicare Part D Coverage Code (139-UR) reflect the payer order determined by CMS.
• If the date of service requested exceeds the available search data for the Facilitator, a rejected response will be returned with Reject
Code of “VD “ (Eligibility Search Time Frame Exceeded)
The Facilitator uses the following fields from the Eligibility Transaction to match to the Eligibility Enrollment database provided by CMS.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 42 -
• Cardholder ID (3Ø2-C2) populated as any of:
o The Health Insurance Claim Number (HICN), Part A, B, or C
OR
o Last 4 digits of Patient Social Security Number (SSN)
OR
o Entire Patient Social Security Number (SSN)
OR
o Railroad Retirement Board Number
• Patient ZIP/Postal Zone (325-CP)
• Patient Last Name (311-CB)
• Patient First Name (31Ø-CA)
• Patient Gender Code (3Ø5-C5)
• Date Of Birth (3Ø4-C4)
The Facilitator uses the Date of Service (4Ø1-D1) sent by the pharmacy to determine eligibility timeframe of the request and returns the most
current information as of the date of request for that date of service. The Date of Service (4Ø1-D1) can be up to 9Ø days prior to or later than
the current date (based on Facilitator rules). The Facilitator will use the submitted Date of Service to find the Part D coverage that has an
Effective Date on or before the Date of Service and has a Termination Date after the Date of Service. It is recommended that when a
pharmacy has multiple eligibility periods to check, the Eligibility inquiry should be from the oldest date of service forward. For example, if the
current date is Ø2/Ø1/2ØØ7, and the pharmacy needs to verify eligibility for past claims of 11/22/2ØØ6, 12/15/2ØØ6, and Ø2/Ø1/2ØØ7, the
first eligibility verification is submitted with a Date of Service of 11/22/2ØØ6.
If known, the Facilitator will return primary processor information and secondary processor information in the Other Payer fields of the
Response Coordination of Benefits/Other Payers Segment. The order of the Other Payer fields in the Response Coordination of
Benefits/Other Payers Segment are positional for a Medicare Part D Eligibility response—primary, secondary, etc. must appear in
that order. Additional information is returned in the Response Insurance Additional Information Segment fields.
6.2 ELIGIBILITY VERIFICATION REQUEST DIAGRAMS
6.2.1 DIAGRAM FOR TRANSMISSION OF ELIGIBILITY VERIFICATION TRANSACTION
For Eligibility, the scenarios defined include
Eligibility Request from a Sender to a Receiver
Eligibility Accepted Response from a Sender to a Receiver Approved/Rejected
Eligibility Transmission Reject Response from a Sender to a Receiver
The transmission of the Eligibility request does not have a Group Separator. The members discussed putting the Group Separator in the
Eligibility request, but determined it was extraneous since the only “transaction level” segments were the Patient Segment, Pharmacy Provider
Segment, Prescriber Segment, and Additional Documentation Segment and as situational, may not be sent. The Group Separator is therefore
not supported in the Eligibility Verification request.
Mandatory
Transaction Header Segment
Segment Separator
Insurance Segment
Situational
Segment Separator
Patient Segment
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Additional Documentation Segment
6.3 ELIGIBILITY VERIFICATION REQUEST SEGMENTS
6.3.1 TRANSACTION HEADER SEGMENT (ELIGIBILITY VERIFICATION)
TRANSACTION HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø1-A1 BIN NUMBER M
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø4-A4 PROCESSOR CONTROL NUMBER M
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 43 -
TRANSACTION HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø9-A9 TRANSACTION COUNT M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M
Notes on Transaction Header Segment on an Eligibility Verification Request:
The Transaction Header Segment is a mandatory, fixed length segment for an Eligibility Verification request. The “Situation” column is not
applicable.
6.3.2 INSURANCE SEGMENT (ELIGIBILITY VERIFICATION)
INSURANCE SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø2-C2 CARDHOLDER ID M
312-CC CARDHOLDER FIRST NAME Q Eligibility Verification:
Required if needed for receiver inquiry validation and/or
determination.
Required if the Patient is the Cardholder, and Date of Birth
(3Ø4-C4) is not available. (Note: Cardholder ID (3Ø2-C2) is
mandatory.)
Required if necessary for state/federal/regulatory agency or
Workers’ Compensation programs.
Required if multiple people have the same Cardholder ID.
313-CD CARDHOLDER LAST NAME Q Eligibility Verification:
Required if needed for inquiry validation and/or
determination.
Required if the Patient is the Cardholder, and Date of Birth
(3Ø4-C4) is not available. (Note: Cardholder ID is
mandatory.)
Required if necessary for state/federal/regulatory agency or
Workers’ Compensation programs.
Required if multiple people have the same Cardholder ID.
314-CE HOME PLAN Q Eligibility Verification:
Required if needed for receiver inquiry validation and/or
determination for Blue Cross or Blue Shield, if a Patient has
coverage under more than one plan, to distinguish each
plan.
524-FO PLAN ID N Eligibility Verification:
Not used.
3Ø9-C9 ELIGIBILITY CLARIFICATION CODE Q Eligibility Verification:
Required if needed for receiver inquiry validation and/or
determination, when eligibility is not maintained at the
dependent level. Required in special situations as defined by
the code to clarify the eligibility of an individual, which may
extend coverage.
3Ø1-C1 GROUP ID Q Eligibility Verification:
Required if needed for receiver inquiry validation and/or
determination.
Required if necessary for state/federal/regulatory agency
programs.
3Ø3-C3 PERSON CODE Q Eligibility Verification:
Required if needed to uniquely identify the family members
within the Cardholder ID.
3Ø6-C6 PATIENT RELATIONSHIP CODE Q Eligibility Verification:
Required if needed to uniquely identify the relationship of the
Patient to the Cardholder ID.
99Ø-MG OTHER PAYER BIN NUMBER N Eligibility Verification:
Not used.
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER N Eligibility Verification:
Not used.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 44 -
INSURANCE SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
356-NU OTHER PAYER CARDHOLDER ID N Eligibility Verification:
Not used.
992-MJ OTHER PAYER GROUP ID N Eligibility Verification:
Not used.
359-2A MEDIGAP ID N Eligibility Verification:
Not used.
36Ø-2B MEDICAID INDICATOR N Eligibility Verification:
Not used.
361-2D PROVIDER ACCEPT ASSIGNMENT INDICATOR N Eligibility Verification:
Not used.
997-G2 CMS PART D DEFINED QUALIFIED FACILITY N Eligibility Verification:
Not used.
115-N5
MEDICAID ID NUMBER N Eligibility Verification
Not used.
116-N6
MEDICAID AGENCY NUMBER N Eligibility Verification:
Not used.
Notes on Insurance Segment on an Eligibility Verification Request:
The Insurance Segment is mandatory for an Eligibility Verification request. Fields defined as Mandatory are required to be submitted when the
segment is sent.
6.3.3 PATIENT SEGMENT (ELIGIBILITY VERIFICATION)
PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
331-CX PATIENT ID QUALIFIER N Eligibility Verification:
Not used.
332-CY PATIENT ID N Eligibility Verification:
Not used.
3Ø4-C4 DATE OF BIRTH Q Eligibility Verification:
Required if needed for receiver inquiry validation and/or
determination.
Required if necessary for state/federal/regulatory agency
programs.
3Ø5-C5 PATIENT GENDER CODE Q Eligibility Verification:
Required if needed for receiver inquiry validation and/or
determination.
Required if additional verification of the submitted eligibility
information is needed.
31Ø-CA PATIENT FIRST NAME Q Eligibility Verification:
Required if the Patient is not the Cardholder and Date of
Birth (3Ø4-C4) is not available.
Required if necessary for state/federal/regulatory agency
programs.
Required if additional verification of the submitted eligibility
information is needed.
311-CB PATIENT LAST NAME Q Eligibility Verification:
Required if the Patient is not the Cardholder and Date of
Birth (3Ø4-C4) is not available.
Required if necessary for state/federal/regulatory agency
programs.
Required if additional verification of the submitted eligibility
information is needed.
322-CM PATIENT STREET ADDRESS Q Eligibility Verification:
Required if necessary for state/federal/regulatory agency
programs.
323-CN PATIENT CITY ADDRESS Q Eligibility Verification:
Required if necessary for state/federal/regulatory agency
programs.
324-CO PATIENT STATE / PROVINCE ADDRESS Q Eligibility Verification:
Required if necessary for state/federal/regulatory agency
programs.
325-CP PATIENT ZIP/POSTAL ZONE Q Eligibility Verification:

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 45 -
PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if necessary for state/federal/regulatory agency
programs.
326-CQ PATIENT PHONE NUMBER N Eligibility Verification:
Not used.
3Ø7-C7 PLACE OF SERVICE Q Eligibility Verification:
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
333-CZ EMPLOYER ID N Eligibility Verification:
Not used.
334-1C SMOKER / NON-SMOKER CODE N Eligibility Verification:
Not used.
335-2C PREGNANCY INDICATOR Q Eligibility Verification:
Required if necessary for state/federal/regulatory agency
programs.
35Ø-HN PATIENT E-MAIL ADDRESS N Eligibility Verification:
Not used.
384-4X PATIENT RESIDENCE Q Eligibility Verification:
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
Notes on Patient Segment on an Eligibility Verification Request:
The Patient Segment is situational for an Eligibility Verification request. It is used when a receiver needs some of the patient demographic
information to perform eligibility determination. The Patient Segment must be submitted when needed to differentiate between the patient and
the cardholder. If the cardholder and the patient are the same, then the Patient Segment is not submitted unless additional information about
the patient is needed to clarify the eligibility inquiry. Fields defined as Mandatory are required to be submitted when the segment is sent.
6.3.4 PHARMACY PROVIDER SEGMENT (ELIGIBILITY VERIFICATION)
PHARMACY PROVIDER SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
465-EY PROVIDER ID QUALIFIER Q Eligibility Verification:
Required if Provider ID (444-E9) is used.
444-E9 PROVIDER ID Q Eligibility Verification:
Required if pharmacy provider needed for receiver inquiry
validation and/or determination.
Notes on Pharmacy Provider Segment on an Eligibility Verification Request:
The Pharmacy Provider Segment is situational for an Eligibility Verification request. It is used when a receiver needs pharmacy provider
information to perform eligibility determination. Fields defined as Mandatory are required to be submitted when the segment is sent.
6.3.5 PRESCRIBER SEGMENT (ELIGIBILITY VERIFICATION)
PRESCRIBER SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
466-EZ PRESCRIBER ID QUALIFIER Q Eligibility Verification:
Required if Prescriber ID (411-DB) is used.
411-DB PRESCRIBER ID Q Eligibility Verification:
Required if this field could result in different coverage or
patient financial responsibility.
Required if necessary for state/federal/regulatory agency
programs.
427-DR PRESCRIBER LAST NAME Q Eligibility Verification:
Required when the Prescriber ID (411-DB) is not known.
Required if needed for Prescriber ID (411-DB)
validation/clarification.
498-PM PRESCRIBER PHONE NUMBER N Eligibility Verification:
Not used.
468-2E PRIMARY CARE PROVIDER ID QUALIFIER Q Eligibility Verification:
Required if Primary Care Provider ID (421-DL) is used.
421-DL PRIMARY CARE PROVIDER ID Q Eligibility Verification:
Required if needed for receiver eligibility determination, if
known and available.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 46 -
PRESCRIBER SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if this field could result in different coverage or
patient financial responsibility.
Required if necessary for state/federal/regulatory agency
programs.
47Ø-4E PRIMARY CARE PROVIDER LAST NAME Q Eligibility Verification:
Required if this field is used as an alternative for Primary
Care Provider ID (421-DL) when ID is not known.
Required if needed for Primary Care Provider ID (421-DL)
validation/clarification.
364-2J PRESCRIBER FIRST NAME Q Eligibility Verification:
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
365-2K PRESCRIBER STREET ADDRESS N Eligibility Verification:
Not used.
366-2M PRESCRIBER CITY ADDRESS N Eligibility Verification:
Not used.
367-2N PRESCRIBER STATE/PROVINCE ADDRESS N Eligibility Verification:
Not used.
368-2P PRESCRIBER ZIP/POSTAL ZONE N Eligibility Verification:
Not used.
Notes on Prescriber Segment on an Eligibility Verification:
The Prescriber Segment is situational for an Eligibility Verification request. It is used when prescriber information is needed to perform
eligibility determination. The Segment is mandatory if required under provider payer contract or mandatory on eligibility verification where this
information is necessary for eligibility determination.
When checking eligibility for a recipient under various restricted programs, the ordering provider (Prescriber ID (411-DB)) and referring
provider (Primary Care Provider ID (421-DL)) may be validated by the recipient eligibility check to verify that the recipient is eligible for
services.
Fields defined as Mandatory are required to be submitted when the segment is sent.
6.3.6 ADDITIONAL DOCUMENTATION SEGMENT (ELIGIBILITY VERIFICATION)
ADDITIONAL DOCUMENTATION SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
369-2Q ADDITIONAL DOCUMENTATION TYPE ID M
374-2V REQUEST PERIOD BEGIN DATE Q Eligibility Verification:
Required if necessary for state/federal/regulatory agency
programs.
375-2W REQUEST PERIOD RECERT/REVISED DATE Q Eligibility Verification:
Required if necessary for state/federal/regulatory agency
programs.
Required if the Request Status (373-2U) = “2” (Revision) or
“3” (Recertification).
373-2U REQUEST STATUS Q Eligibility Verification:
Required if necessary for state/federal/regulatory agency
programs.
371-2S LENGTH OF NEED QUALIFIER Q Eligibility Verification:
Required if Length of Need (37Ø-2R) is used.
37Ø-2R LENGTH OF NEED Q Eligibility Verification:
Required if necessary for state/federal/regulatory agency
programs.
372-2T PRESCRIBER/SUPPLIER DATE SIGNED Q Eligibility Verification:
Required if necessary for state/federal/regulatory agency
programs.
376-2X SUPPORTING DOCUMENTATION Q Eligibility Verification:
Required if necessary for state/federal/regulatory agency
programs (using Section C of Medicare’s CMN forms).
377-2Z QUESTION NUMBER/LETTER COUNT Q Eligibility Verification:
Maximum count of 5Ø.
Required if needed to provide response to narratives.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 47 -
ADDITIONAL DOCUMENTATION SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
378-4B QUESTION NUMBER/LETTER Q***R*** Eligibility Verification:
Required if necessary for state/federal/regulatory agency
programs to respond to questions included on a Medicare
form.
Required if Question Number/Letter
Count (377-2Z) is greater than Ø.
379-4D QUESTION PERCENT RESPONSE Q***R*** Eligibility Verification:
Required if necessary for state/federal/regulatory agency
programs to respond to questions included on a Medicare
form that requires a percent as the response. (At least one
response is required per question.)
38Ø-4G QUESTION DATE RESPONSE Q***R*** Eligibility Verification:
Required if necessary for state/federal/regulatory agency
programs to respond to questions included on a Medicare
form that requires a date as the response. (At least one
response is required per question.)
381-4H QUESTION DOLLAR AMOUNT RESPONSE Q***R*** Eligibility Verification:
Required if necessary for state/federal/regulatory agency
programs to respond to questions included on a Medicare
form that requires a dollar amount as the response. (At least
one response is required per question.)
382-4J QUESTION NUMERIC RESPONSE Q***R*** Eligibility Verification:
Required if necessary for
State/federal/regulatory agency programs to respond to
questions included on a Medicare form that requires a
numeric as the response. (At least one response is required
per question.)
383-4K QUESTION ALPHANUMERIC RESPONSE Q***R*** Eligibility Verification:
Required if necessary for state/federal/regulatory agency
programs to respond to questions included on a Medicare
form that requires an alphanumeric as the response. (At
least one response is required per question.)
Notes on Additional Documentation Segment on a Eligibility Verification Request:
The Additional Documentation Segment is situational for Eligibility Verification request. It is used to provide additional information on Medicare
forms. Fields defined as Mandatory are required to be submitted when the segment is sent.
6.4 ELIGIBILITY VERIFICATION RESPONSE DIAGRAMS AND SEGMENTS
6.4.1 TRANSMISSION ACCEPTED/TRANSACTION APPROVED
6.4.1.1 DIAGRAM FOR TRANSMISSION OF ELIGIBILITY VERIFICATION RESPONSE (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
Eligibility Verification transmission response
Header Response Status (5Ø1-F1) of "A" (Accepted) and
Transaction Response Status (112-AN) of "A" (Approved)
A value of “A” (Accepted) in the Header Response Status (5Ø1-F1) indicates that the transmission was accepted. A value of “A” (Approved)
in the Transaction Response Status (112-AN) indicates the transaction was approved.
A value of “A” in the Transaction Response Status (112-AN) indicates the Patient is eligible.
The transmission of the Eligibility response has a Group Separator, so that all response transmissions are parsed the same way (with the
Response Status Segment coming after the Group Separator).
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate Eligibility transaction.
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Insurance Additional Information Segment
Segment Separator
Response Patient Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 48 -
Mandatory
Group Separator
Segment Separator
Response Status Segment
Situational
Segment Separator
Response Coordination of Benefits/Other Payers Segment
6.4.1.2 ELIGIBILITY VERIFICATION RESPONSE SEGMENTS (TRANSMISSION ACCEPTED/TRANSACTION
APPROVED)
6.4.1.2.1 RESPONSE HEADER SEGMENT (ELIGIBILITY VERIFICATION) (TRANSMISSION ACCEPTED/TRANSACTION
APPROVED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on an Eligibility Verification Response:
The Response Header Segment is a mandatory, fixed length segment for Eligibility Verification when the Header Response Status (5Ø1-F1) of
"A" (Accepted) and Transaction Response Status (112-AN) of “A” (Approved). The “Situation” column is not applicable.
6.4.1.2.2 RESPONSE MESSAGE SEGMENT (ELIGIBILITY VERIFICATION) (TRANSMISSION ACCEPTED/TRANSACTION
APPROVED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Eligibility Verification:
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on an Eligibility Verification Response:
The Response Message Segment is situational for Eligibility Verification when the Header Response Status (5Ø1-F1) of "A" (Accepted) and
Transaction Response Status (112-AN) of “A” (Approved). It is used when additional text information needs to be sent. Fields defined as
Mandatory are required to be submitted when the segment is sent.
6.4.1.2.3 RESPONSE INSURANCE SEGMENT (ELIGIBILITY VERIFICATION) (TRANSMISSION ACCEPTED/TRANSACTION
APPROVED)
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 49 -
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø1-C1 GROUP ID Q Eligibility Verification:
Required if needed to identify the cardholder or employer
group, to identify appropriate group number for billing.
524-FO PLAN ID Q Eligibility Verification:
Required if needed to identify a set of parameters, benefit,
or coverage criteria.
545-2F NETWORK REIMBURSEMENT ID Q Eligibility Verification:
Required if needed to identify the network for the covered
member.
568-J7 PAYER ID QUALIFIER N Eligibility Verification:
Not used.
569-J8 PAYER ID N Eligibility Verification:
Not used.
115-N5
MEDICAID ID NUMBER N Eligibility Verification:
Not used.
116-N6
MEDICAID AGENCY NUMBER N Eligibility Verification:
Not used.
3Ø2-C2
CARDHOLDER ID Q Eligibility Verification:
Required if the identification to be used in future
transactions is different than what was submitted on the
request.
Notes on Response Insurance Segment on an Eligibility Verification Response:
The Response Insurance Segment is situational for Eligibility Verification transmission response Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “A” (Approved). It is used when coverage parameters or identifiers need to be sent
from the sender to the receiver. Fields defined as Mandatory are required to be submitted when the segment is sent.
6.4.1.2.4 RESPONSE INSURANCE ADDITIONAL INFORMATION SEGMENT (ELIGIBILITY VERIFICATION)
(TRANSMISSION ACCEPTED/TRANSACTION APPROVED)
RESPONSE INSURANCE ADDITIONAL INFORMATION SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
139-UR MEDICARE PART D COVERAGE CODE Q Eligibility Verification:
Required when needed to supply the provider with
additional Medicare Part D Eligibility information. Used only
in Eligibility Transaction. The value of the code is the
pointer for the Other Payer Coverage Type (338-5C) in one
of the response loops which designates the Medicare Part
D coverage.
138-UQ CMS LOW INCOME COST SHARING (LICS) LEVEL Q Eligibility Verification:
Required when needed to supply the provider with
additional Medicare Part D Eligibility information. Used only
in Eligibility Transaction.
24Ø-U1 CONTRACT NUMBER Q Eligibility Verification:
Required if needed to identify the contract of the covered
member. Used only in Eligibility Transaction.
926-FF FORMULARY ID Q Eligibility Verification:
Required if known to identify the formulary of the covered
member. Used only in Eligibility Transaction.
757-U6 BENEFIT ID Q Eligibility Verification:
Required when known for Part D to identify the PBP (Plan
Benefit Package) Number. Used only in Eligibility
Transaction.
14Ø-US NEXT MEDICARE PART D EFFECTIVE DATE Q Eligibility Verification:
Required when future Medicare Part D coverage is known
which is after the Date of Service submitted. Used only in
Eligibility Transaction.
141-UT NEXT MEDICARE PART D TERMINATION DATE Q Eligibility Verification:
Required when future Medicare Part D coverage is known
which is after the Date of Service submitted. Used only in
Eligibility Transaction.
Notes on Response Insurance Additional Information Segment on an Eligibility Verification Response:
The Response Insurance Additional Information Segment is mandatory for Eligibility Verification transmission response Header Response
Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “A” (Approved) for Medicare Part D. This segment is used
solely for Medicare Part D Eligibility transactions between the pharmacy and the Facilitator to provide Medicare specific benefit information.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 50 -
The Response Insurance Additional Information Segment is not used for other than Medicare Part D Eligibility transactions between the
pharmacy and the Facilitator.
Fields defined as Mandatory are required to be submitted when the segment is sent.
6.4.1.2.5 RESPONSE PATIENT SEGMENT (ELIGIBILITY VERIFICATION) (TRANSMISSION ACCEPTED/TRANSACTION
APPROVED)
RESPONSE PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
31Ø-CA PATIENT FIRST NAME Q Eligibility Verification:
Required if known.
311-CB PATIENT LAST NAME Q Eligibility Verification:
Required if known.
3Ø4-C4 DATE OF BIRTH Q Eligibility Verification:
Required if known.
Notes on Response Patient Segment on an Eligibility Verification Response:
The Response Patient Segment is situational for Eligibility Verification transmission response Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “A” (Approved) when patient demographic information needs to be sent from the
sender to the receiver.
Medicare Part D Eligibility Transactions from Sender to Facilitator:
This segment is used for Medicare Part D Eligibility transactions to provide patient name and date of birth in order to provide
additional patient information. This information could assist in the verification that the eligibility information returned is indeed the
patient for which the eligibility request was intended. It is used only when the patient has had Medicare Part D eligibility at some
point within the Facilitator’s files and within the search parameters established. The data returned is based on information within the
Facilitator’s files and not on information sent on the Eligibility Request.
The response will be based on Date of Service for Part D coverage
If patient is not found, then
Rejected response
Response Patient Segment Not Returned
If patient is FOUND (Patient that has had Medicare Part D coverage at some point within the search parameters
timeframe determined by the Facilitator), then
If patient has current Part D Coverage based on Date of Service
Approved response
Response Patient Segment will be returned with data from the Facilitator system –not the
submitted data
If patient has future Part D coverage, but no current coverage based on Date of Service
Rejected response
Response Patient Segment will be returned with data from the Facilitator system-not the submitted
data,
If patient had Part D, but does not have current or future coverage based on Date of Service
Rejected response
Response Patient Segment will be returned with data from the Facilitator system-not the submitted
data
Fields defined as Mandatory are required to be submitted when the segment is sent.
6.4.1.2.6 RESPONSE STATUS SEGMENT (ELIGIBILITY VERIFICATION) (TRANSMISSION ACCEPTED/TRANSACTION
APPROVED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Eligibility Verification:
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT N Eligibility Verification:
Not used.
511-FB REJECT CODE N***R*** Eligibility Verification:
Not used.
546-4F REJECT FIELD OCCURRENCE INDICATOR N***R*** Eligibility Verification:
Not used.
547-5F APPROVED MESSAGE CODE COUNT N Eligibility Verification:
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 51 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Eligibility Verification:
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Eligibility Verification:
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Eligibility Verification:
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Eligibility Verification:
Required if additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Eligibility Verification:
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Eligibility Verification:
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER Q Eligibility Verification:
Required if needed to provide a support telephone number
to the receiver.
For Medicare Part D Eligibility Transactions returned by a
CMS certified Eligibility Facilitator, the Help Desk Phone
Number (55Ø-8F) will always reflect the CMS phone
number.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Eligibility Verification:
Not used.
993-A7 INTERNAL CONTROL NUMBER N Eligibility Verification:
Not used.
987-MA URL N Eligibility Verification:
Not used.
Notes on Response Status Segment on an Eligibility Verification Response:
The Response Status Segment is mandatory for an Eligibility Verification response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “A” (Approved). The Response Status Segment is sent from the sender to the
receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.
6.4.1.2.7 RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS SEGMENT (ELIGIBILITY VERIFICATION)
(TRANSMISSION ACCEPTED/TRANSACTION APPROVED)
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS
SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 52 -
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS
SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
355-NT OTHER PAYER ID COUNT M Eligibility Verification:
Maximum count of 3.
338-5C OTHER PAYER COVERAGE TYPE M***R***
339-6C OTHER PAYER ID QUALIFIER Q***R*** Eligibility Verification:
Required if Other Payer ID (34Ø-7C) is used.
34Ø-7C OTHER PAYER ID Q***R*** Eligibility Verification:
Required if known. For Medicare Part D Eligibility
Transaction this field must contain the BIN (with
appropriate Other Payer ID Qualifier (339-6C)).
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER Q***R*** Eligibility Verification:
Required if other insurance information is available for
coordination of benefits.
356-NU OTHER PAYER CARDHOLDER ID Q***R*** Eligibility Verification:
Required if other insurance information is available for
coordination of benefits.
992-MJ OTHER PAYER GROUP ID Q***R*** Eligibility Verification:
Required if other insurance information is available for
coordination of benefits.
142-UV OTHER PAYER PERSON CODE Q***R*** Eligibility Verification:
Required if needed to uniquely identify the family members
within the Cardholder ID, as assigned by the other payer.
127-UB OTHER PAYER HELP DESK PHONE NUMBER Q***R*** Eligibility Verification:
Required if needed to provide a support telephone number
of the other payer to the receiver.
143-UW OTHER PAYER PATIENT RELATIONSHIP CODE Q***R*** Eligibility Verification:
Required if needed to uniquely identify the relationship of
the patient to the cardholder ID, as assigned by the other
payer.
144-UX OTHER PAYER BENEFIT EFFECTIVE DATE Q***R*** Eligibility Verification:
Required when other coverage is known which is before,
on, or after the Date of Service submitted.
145-UY OTHER PAYER BENEFIT TERMINATION DATE Q***R*** Eligibility Verification:
Required when other coverage is known which is before,
on, or after the Date of Service submitted.
Notes on Response Coordination of Benefits/Other Payers Segment on an Eligibility Verification Response:
The Response Coordination of Benefits/Other Payers Segment is situational for an Eligibility Verification response when the Header Response
Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “A” (Approved) when other insurance information is available
for coordination of benefits.
If additional payer(s) for this patient is not known, the Other Payer information is not sent.
If additional payer(s) for this patient is known, the following may be sent:
• Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C),
• Other Payer Group ID (992-MJ),
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU)
• And other Other Payer fields.
In addition, if any of the following three fields are sent:
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU),
• Other Payer Group ID (992-MJ),
then the Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C) must be sent.
Fields defined as Mandatory are required to be submitted when the segment is sent.
For Medicare Part D Eligibility Transactions returned by a CMS certified Eligibility Facilitator,
For Medicare Part D - If known, the Facilitator will return primary processor information and secondary processor information in the
Other Payer fields of the Response Coordination of Benefits/Other Payers Segment. The order of the Other Payer fields in the
Response Coordination of Benefits/Other Payers Segment are positional for a Medicare Part D Eligibility response—primary,
secondary, etc. must appear in that order. Additional information is returned in the Response Insurance Additional Information
Segment fields.
The Help Desk Phone Number (55Ø-8F) in the Response Status Segment will always reflect the CMS phone number.
6.4.2 TRANSMISSION ACCEPTED/TRANSACTION REJECTED
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 53 -
6.4.2.1 DIAGRAM FOR TRANSMISSION OF ELIGIBILITY VERIFICATION RESPONSE (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
Eligibility Verification transmission response
Header Response Status (5Ø1-F1) of "A" (Accepted) and
Transaction Response Status (112-AN) of "R" (Rejected)
A value of “A” in the Header Response Status (5Ø1-F1) indicates that the transmission was accepted. A value of “R” in the Transaction
Response Status (112-AN) indicates the transaction was rejected.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate Eligibility transaction.
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Additional Information Segment
Segment Separator
Response Patient Segment
Mandatory
Group Separator
Segment Separator
Response Status Segment
Situational
Segment Separator
Response Coordination of Benefits/Other Payers Segment
6.4.2.2 ELIGIBILITY VERIFICATION RESPONSE SEGMENTS (TRANSMISSION ACCEPTED/TRANSACTION
REJECTED)
6.4.2.2.1 RESPONSE HEADER SEGMENT (ELIGIBILITY VERIFICATION) (TRANSMISSION ACCEPTED/TRANSACTION
REJECTED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on an Eligibility Verification Response:
The Response Header Segment is a mandatory, fixed length segment for Eligibility Verification when the Header Response Status (5Ø1-F1) of
"A" (Accepted) and Transaction Response Status (112-AN) of “R" (Rejected). The “Situation” column is not applicable.
6.4.2.2.2 RESPONSE MESSAGE SEGMENT (ELIGIBILITY VERIFICATION) (TRANSMISSION ACCEPTED/TRANSACTION
REJECTED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Eligibility Verification:
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single transaction
per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain transmission-
level text and Additional Message Information
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 54 -
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
(526-FQ) will contain transaction-level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on an Eligibility Verification Response:
The Response Message Segment is situational for Eligibility Verification when the Header Response Status (5Ø1-F1) of "A" (Accepted) and
Transaction Response Status (112-AN) of “R" (Rejected). It is used when additional text information needs to be sent. Fields defined as
Mandatory are required to be submitted when the segment is sent.
6.4.2.2.3 RESPONSE INSURANCE ADDITIONAL INFORMATION SEGMENT (ELIGIBILITY VERIFICATION)
(TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
RESPONSE INSURANCE ADDITIONAL INFORMATION SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
139-UR MEDICARE PART D COVERAGE CODE N Eligibility Verification:
Not used.
138-UQ CMS LOW INCOME COST SHARING (LICS) LEVEL N Eligibility Verification:
Not used.
24Ø-U1 CONTRACT NUMBER N Eligibility Verification:
Not used.
926-FF FORMULARY ID N Eligibility Verification:
Not used.
757-U6 BENEFIT ID N Eligibility Verification:
Not used.
14Ø-US NEXT MEDICARE PART D EFFECTIVE DATE Q Eligibility Verification:
Required when future Part D coverage is known which is
after the Date of Service submitted.
141-UT NEXT MEDICARE PART D TERMINATION DATE Q Eligibility Verification:
Required when future Part D coverage is known which is
after the Date of Service submitted.
Notes on Response Insurance Additional Information Segment on an Eligibility Verification Response:
The Response Insurance Additional Information Segment is situational for Eligibility Verification transmission response Header Response
Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “R” (Rejected) for Medicare Part D between the pharmacy
and the Facilitator to relay dates.
Medicare Part D Eligibility Transactions from Sender to Facilitator:
This segment is used solely for Medicare Part D Eligibility transactions to provide Medicare specific date information between
sender and Facilitator.
The Response Insurance Additional Information Segment is not used for other than Medicare Part D Eligibility.
Fields defined as Mandatory are required to be submitted when the segment is sent.
6.4.2.2.4 RESPONSE PATIENT SEGMENT (ELIGIBILITY VERIFICATION) (TRANSMISSION ACCEPTED/TRANSACTION
REJECTED)
RESPONSE PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
31Ø-CA PATIENT FIRST NAME Q Eligibility Verification:
Required if known.
311-CB PATIENT LAST NAME Q Eligibility Verification:
Required if known.
3Ø4-C4 DATE OF BIRTH Q Eligibility Verification:
Required if known.
Notes on Response Patient Segment on an Eligibility Verification Response:
The Response Patient Segment is situational for Eligibility Verification transmission response Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “R” (Rejected) when patient demographic information needs to be sent from the
sender to the receiver.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 55 -
Medicare Part D Eligibility Transactions from Sender to Facilitator:
This segment is used for Medicare Part D Eligibility transactions to provide patient name and date of birth in order to provide
additional patient information. This information could assist in the verification that the eligibility information returned is indeed the
patient for which the eligibility request was intended. It is used only when the patient has had Medicare Part D eligibility at some
point within the Facilitator’s files and within the search parameters established. The data returned is based on information within the
Facilitator’s files and not on information sent on the Eligibility Request.
The response will be based on Date of Service for Part D coverage
If patient is not found, then
Rejected response
Response Patient Segment Not Returned
If patient is FOUND (Patient that has had Medicare Part D coverage at some point within the search parameters
timeframe determined by the Facilitator), then
If patient has current Part D Coverage based on Date of Service
Approved response
Response Patient Segment will be returned with data from the Facilitator system –not the submitted
data
If patient has future Part D coverage, but no current coverage based on Date of Service
Rejected response
Response Patient Segment will be returned with data from the Facilitator system-not the
submitted data,
If patient had Part D, but does not have current or future coverage based on Date of Service
Rejected response
Response Patient Segment will be returned with data from the Facilitator system-not the
submitted data
Fields defined as Mandatory are required to be submitted when the segment is sent.
6.4.2.2.5 RESPONSE STATUS SEGMENT (ELIGIBILITY VERIFICATION) (TRANSMISSION ACCEPTED/TRANSACTION
REJECTED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Eligibility Verification:
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT R Eligibility Verification:
Maximum count of 5.
Required.
511-FB REJECT CODE R***R*** Eligibility Verification:
Required.
546-4F REJECT FIELD OCCURRENCE INDICATOR Q***R*** Eligibility Verification:
Required if a repeating field is in error, to identify repeating
field occurrence.
This field must be sent when relaying error information about
a repeating field or set. Note, if the Reject Code is not
denoting a repeating field or set, the Reject Field Occurrence
Indicator must not be sent.
547-5F APPROVED MESSAGE CODE COUNT N Eligibility Verification:
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Eligibility Verification:
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Eligibility Verification:
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Eligibility Verification:
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Eligibility Verification:
Required if additional text is needed for clarification or
detail.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 56 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Eligibility Verification:
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Eligibility Verification:
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER Q Eligibility Verification:
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Eligibility Verification:
Not used.
993-A7 INTERNAL CONTROL NUMBER N Eligibility Verification:
Not used.
987-MA URL I Eligibility Verification:
Provided for informational purposes only to relay health
care communications via the Internet.
Notes on Response Status Segment on an Eligibility Verification Response:
The Response Status Segment is mandatory for an Eligibility Verification response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “R" (Rejected). The Response Status Segment is sent from the sender to the
receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.
6.4.2.2.6 RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS SEGMENT (ELIGIBILITY VERIFICATION)
(TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS
SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
355-NT OTHER PAYER ID COUNT M Eligibility Verification:
Maximum count of 3.
338-5C OTHER PAYER COVERAGE TYPE M***R***
339-6C OTHER PAYER ID QUALIFIER Q***R*** Eligibility Verification:
Required if Other Payer ID (34Ø-7C) is used.
34Ø-7C OTHER PAYER ID Q***R*** Eligibility Verification:
Required if other insurance information is available for
coordination of benefits.
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER Q***R*** Eligibility Verification:
Required if other insurance information is available for
coordination of benefits.
356-NU OTHER PAYER CARDHOLDER ID Q***R*** Eligibility Verification:
Required if other insurance information is available for
coordination of benefits.
992-MJ OTHER PAYER GROUP ID Q***R*** Eligibility Verification:
Required if other insurance information is available for
coordination of benefits.
142-UV OTHER PAYER PERSON CODE Q***R*** Eligibility Verification:
Required if needed to uniquely identify the family members
within the Cardholder ID, as assigned by the other payer.
127-UB OTHER PAYER HELP DESK PHONE NUMBER Q***R*** Eligibility Verification:
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 57 -
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS
SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if needed to provide a support telephone number
of the other payer to the receiver.
143-UW OTHER PAYER PATIENT RELATIONSHIP CODE Q***R*** Eligibility Verification:
Required if needed to uniquely identify the relationship of
the patient to the cardholder ID, as assigned by the other
payer.
144-UX OTHER PAYER BENEFIT EFFECTIVE DATE Q***R*** Eligibility Verification:
Required when other coverage is known which is before,
on, or after the Date of Service submitted.
145-UY OTHER PAYER BENEFIT TERMINATION DATE Q***R*** Eligibility Verification:
Required when other coverage is known which is before,
on, or after the Date of Service submitted.
Notes on Response Coordination of Benefits/Other Payers Segment on an Eligibility Verification Response:
The Response Coordination of Benefits/Other Payers Segment is situational for an Eligibility Verification response when the Header Response
Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “R” (Rejected) when other insurance information is available
for coordination of benefits.
If additional payer(s) for this patient is not known, the Other Payer information is not sent.
If additional payer(s) for this patient is known, the following may be sent:
• Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C),
• Other Payer Group ID (992-MJ),
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU)
• And other Other Payer fields.
In addition, if any of the following three fields are sent:
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU),
• Other Payer Group ID (992-MJ),
then the Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C) must be sent.
Fields defined as Mandatory are required to be submitted when the segment is sent.
For Medicare Part D Eligibility Transactions returned by a CMS certified Eligibility Facilitator,
For Medicare Part D - If known, the Facilitator will return primary processor information and secondary processor information in the
Other Payer fields of the Response Coordination of Benefits/Other Payers Segment. The order of the Other Payer fields in the
Response Coordination of Benefits/Other Payers Segment are positional for a Medicare Part D Eligibility response—primary,
secondary, etc. must appear in that order. Additional information is returned in the Response Insurance Additional Information
Segment fields.
The Help Desk Phone Number (55Ø-8F) in the Response Status Segment will always reflect the CMS phone number.
6.4.3 TRANSMISSION REJECTED/TRANSACTION REJECTED
6.4.3.1 DIAGRAM FOR TRANSMISSION OF ELIGIBILITY VERIFICATION RESPONSE (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
Eligibility Verification transmission response
Header Response Status (5Ø1-F1) of "R" (Rejected) and
Transaction Response Status (112-AN) of "R" (Rejected)
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate Eligibility transaction.
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory
Group Separator
Segment Separator
Response Status Segment

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 58 -
6.4.3.2 ELIGIBILITY VERIFICATION RESPONSE SEGMENTS (TRANSMISSION REJECTED/TRANSACTION
REJECTED)
6.4.3.2.1 RESPONSE HEADER SEGMENT (ELIGIBILITY VERIFICATION) (TRANSMISSION REJECTED/TRANSACTION
REJECTED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on an Eligibility Verification Response:
The Response Header Segment is a mandatory, fixed length segment for Eligibility Verification response when the Header Response Status
(5Ø1-F1) of "R" (Rejected) and Transaction Response Status (112-AN) of "R" (Rejected). The “Situation” column is not applicable.
6.4.3.2.2 RESPONSE MESSAGE SEGMENT (ELIGIBILITY VERIFICATION) (TRANSMISSION REJECTED/TRANSACTION
REJECTED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory or
Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Eligibility Verification:
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on an Eligibility Verification Response:
The Response Message Segment is situational for Eligibility Verification response when the Header Response Status (5Ø1-F1) of "R"
(Rejected) and Transaction Response Status (112-AN) of "R" (Rejected). It is used when additional text information needs to be sent. Fields
defined as Mandatory are required to be submitted when the segment is sent.
6.4.3.2.3 RESPONSE STATUS SEGMENT (ELIGIBILITY VERIFICATION) (TRANSMISSION REJECTED/TRANSACTION
REJECTED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Eligibility Verification:
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT R Eligibility Verification:
Maximum count of 5.
Required.
511-FB REJECT CODE R***R*** Eligibility Verification:
Required.
546-4F REJECT FIELD OCCURRENCE INDICATOR Q***R*** Eligibility Verification:
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 59 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if a repeating field is in error, to identify repeating
field occurrence.
This field must be sent when relaying error information about a
repeating field or set. Note, if the Reject Code is not denoting a
repeating field or set, the Reject Field Occurrence Indicator
must not be sent.
547-5F APPROVED MESSAGE CODE COUNT N Eligibility Verification:
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Eligibility Verification:
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Eligibility Verification:
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Eligibility Verification:
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Eligibility Verification:
Required if additional text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single transaction
per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain transmission-
level text and Additional Message Information
(526-FQ) will contain transaction-level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Eligibility Verification:
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Eligibility Verification:
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER Q Eligibility Verification:
Required if needed to provide a support telephone number to
the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Eligibility Verification:
Not used.
993-A7 INTERNAL CONTROL NUMBER N Eligibility Verification:
Not used.
987-MA URL N Eligibility Verification:
Not used.
Notes on Response Status Segment on an Eligibility Verification Response:
The Response Status Segment is mandatory for an Eligibility Verification response when the Header Response Status (5Ø1-F1) of "R"
(Rejected) and Transaction Response Status (112-AN) of "R" (Rejected). The Response Status Segment is sent from the sender to the
receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 60 -
7. CLAIM BILLING OR ENCOUNTER INFORMATION
These messages include:
• Claim Billing (B1)
• Claim Reversal (B2)
• Claim Rebill (B3)
• Encounter (B1, see below)
Up to four transactions per transmission are permitted, except for compound billings. Only one transaction per transmission is allowed when
billing for a multiple ingredient prescription.
For Transaction Code of “B1” or “B2” or “B3”, in the Claim Segment or Response Claim Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “1” (Rx Billing).
Billings may be for products dispensed, DUR conflict resolution, or professional services rendered. Services may be correlated with a
dispensing event or may be separate and unrelated to any particular prescription. (See section “Service Billing (Professional Pharmacy
Service) Information”.
For Medicare Part D processing only one transaction per transmission is permitted because there is a need for the sequencing of the True Out
Of Pocket (TrOOP) update before the next claim is processed. The TrOOP should be updated before subsequent claims are processed.
See section “Response Processing Guidelines”, “Duplicate Transactions”.
7.1 CLAIM BILLING
These transactions are used by the Originator to request payment from the Processor for a specific patient for claims billed according to
appropriate plan parameters. The Transaction Code is “B1”.
Each claim submission request may contain up to four occurrences of claim/service data.
Depending upon the particular claim submission request, the Processor must provide one of the following general types of responses:
Captured - This occurs when the Processor acknowledges receipt of the claim, but is not making any judgment regarding eligibility
of the patient or payment for the claim at this time.
Duplicate of Captured - This occurs when the Processor has previously received the request and processed the transaction, but the
response did not return to the Originator. The Duplicate response contains the same information as returned in the Captured original
response.
Paid - This occurs when the Processor captures and processes the claim, and returns to the Originator the dollar amounts allowed
under the terms of the plan. The Paid response is not used in payer-to-payer transactions.
Duplicate of Paid - This occurs when the Processor has previously received the request and processed the transaction, but the
response did not return to the Originator. The Duplicate response contains the same information as returned in the Paid original
response. The Duplicate of Paid response is not used in payer-to-payer transactions.
Rejected - This occurs when the Processor has encountered an error in the transaction or processing.
7.2 ENCOUNTER
Encounter transactions are used to report health care product/services from the provider to the payer. This guide uses the definition stated in
the HIPAA regulations1:
“If there is no direct claim, because the reimbursement contract is based on a mechanism other than charges or reimbursement
rates for specific services, the transaction is the transmission of encounter information for the purpose of reporting health care.”
For example, a payer and provider have entered into a capitation agreement, i.e. $50/PMPM (per member per month). On an agreed upon
schedule, the encounter data will be reconciled with the capitated payments that have been made. The encounter data may support
adjustment (settlement) of the amount paid to the provider, based on actual experience and products/services provided, as well as incentives,
or other contract terms.
One method of distinguishing an encounter is the use of Submission Clarification Code (42Ø-DK) = 9 (Encounters). The Transaction Code is
“B1”.
An Encounter is not a payer-to-payer transaction.
Each encounter submission request may contain up to four occurrences of encounter data.
Depending upon the particular encounter submission request, the Processor must provide one of the following general types of responses:
1 45 CFR Parts 160 and 162 Subpart K—Health Care Claims or Equivalent Encounter Information § 162.1101 Health care claims or equivalent
encounter information transaction.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 61 -
Captured - This occurs when the Processor acknowledges receipt of the encounter, but is not making any judgment regarding
eligibility of the patient or payment for the encounter at this time.
Duplicate of Captured - This occurs when the Processor has previously received the request and processed the transaction, but the
response did not return to the Originator. The Duplicate response contains the same information as returned in the Captured original
response.
Paid - This occurs when the Processor captures and processes the encounter, and returns to the Originator the dollar amounts
allowed under the terms of the plan. The Paid response is not used in payer-to-payer transactions.
Duplicate of Paid - This occurs when the Processor has previously received the request and processed the transaction, but the
response did not return to the Originator. The Duplicate response contains the same information as returned in the Paid original
response. The Duplicate of Paid response is not used in payer-to-payer transactions.
Rejected - This occurs when the Processor has encountered an error in the transaction or processing.
7.2.1 ENCOUNTER DIAGRAMS
7.2.1.1 DIAGRAM FOR TRANSMISSION OF ONE, TWO, THREE, OR FOUR ENCOUNTER TRANSACTIONS
The diagrams for Claim Billing must be used for Encounters. The field situations will designate the Encounter usage with the tag “Encounter”.
7.2.1.2 DIAGRAM FOR TRANSMISSION OF ONE, TWO, THREE, OR FOUR ENCOUNTER RESPONSE
TRANSACTIONS
The diagrams for Claim Billing responses must be used for Encounter responses. The field situations will designate the Encounter usage with
the tag “Encounter”.
7.3 CLAIM BILLING OR ENCOUNTER REQUEST DIAGRAMS
7.3.1 DIAGRAM FOR TRANSMISSION OF ONE CLAIM BILLING OR ENCOUNTER
TRANSACTION
For a Claim Billing or Encounter the scenarios defined include
Claim Billing from a Sender to a Receiver
Claim Billing Paid/Captured/Rejected Transaction Response from a Sender to a Receiver
Standard Transmission Reject Response to a Claim Billing from a Sender to a Receiver
Mandatory
Transaction Header Segment
Segment Separator
Insurance Segment
Situational
Segment Separator
Patient Segment
Mandatory - first Claim/Encounter
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Coupon Segment
Segment Separator
Compound Segment
Segment Separator
Clinical Segment
Segment Separator
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 62 -
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
7.3.2 DIAGRAM FOR TRANSMISSION OF TWO CLAIM BILLING OR ENCOUNTER
TRANSACTIONS
Mandatory
Transaction Header Segment
Segment Separator
Insurance Segment
Situational
Segment Separator
Patient Segment
Mandatory - first Claim/Encounter
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Coupon Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
Mandatory - second Claim/Encounter
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Coupon Segment
Segment Separator
Clinical Segment
Segment Separator

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 63 -
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
7.3.3 DIAGRAM FOR TRANSMISSION OF THREE CLAIM BILLING OR ENCOUNTER
TRANSACTIONS
Mandatory
Transaction Header Segment
Segment Separator
Insurance Segment
Situational
Segment Separator
Patient Segment
Mandatory – first Claim/Encounter
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Coupon Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
Mandatory - second Claim/Encounter
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Coupon Segment
Segment Separator
Clinical Segment
Segment Separator
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 64 -
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
Mandatory - third Claim/Encounter
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Coupon Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
7.3.4 DIAGRAM FOR TRANSMISSION OF FOUR CLAIM BILLING OR ENCOUNTER
TRANSACTIONS
Mandatory
Transaction Header Segment
Segment Separator
Insurance Segment
Situational
Segment Separator
Patient Segment
Mandatory - first Claim/Encounter
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Coupon Segment
Segment Separator
Clinical Segment
Segment Separator
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 65 -
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
Mandatory - second Claim/Encounter
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Coupon Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
Mandatory - third Claim/Encounter
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Coupon Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
Mandatory - fourth Claim/Encounter
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 66 -
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Coupon Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
7.4 CLAIM BILLING OR ENCOUNTER REQUEST SEGMENTS
7.4.1 TRANSACTION HEADER SEGMENT (CLAIM BILLING OR ENCOUNTER)
TRANSACTION HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø1-A1 BIN NUMBER M
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M For Transaction Code of “B1”, in the Claim Segment, the
Prescription/Service Reference Number Qualifier (455-EM)
is “1” (Rx Billing).
1Ø4-A4 PROCESSOR CONTROL NUMBER M
1Ø9-A9 TRANSACTION COUNT M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
If the Date of Service contains the subsequent payer
coverage date, the Submission Clarification Code (42Ø-DK)
is required with value of “19” (Split Billing – indicates the
quantity dispensed is the remainder billed to a subsequent
payer when Medicare Part A expires. Used only in long-
term care settings) for individual unit of use medications.
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M
Notes on Transaction Header Segment on a Claim Billing or Encounter Request:
The Transaction Header Segment is a mandatory, fixed length segment for a Claim Billing or Encounter request. The “Situation” column is not
applicable.
7.4.2 INSURANCE SEGMENT (CLAIM BILLING OR ENCOUNTER)
INSURANCE SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø2-C2 CARDHOLDER ID M
312-CC CARDHOLDER FIRST NAME
Q Claim Billing/Encounter:
Required if necessary for state/federal/regulatory agency
programs when the cardholder has a first name.
313-CD CARDHOLDER LAST NAME
Q Claim Billing/Encounter:
Required if necessary for state/federal/regulatory agency
programs.
314-CE HOME PLAN
Q Claim Billing/Encounter:
Required if needed for receiver billing/encounter validation
and/or determination for Blue Cross or Blue Shield, if a
Patient has coverage under more than one plan, to
distinguish each plan.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 67 -
INSURANCE SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
524-FO PLAN ID
O Claim Billing/Encounter:
Optional.
3Ø9-C9 ELIGIBILITY CLARIFICATION CODE
Q Claim Billing/Encounter:
Required if needed for receiver inquiry validation and/or
determination, when eligibility is not maintained at the
dependent level. Required in special situations as defined
by the code to clarify the eligibility of an individual, which
may extend coverage.
3Ø1-C1 GROUP ID
Q Claim Billing/Encounter:
Required if necessary for state/federal/regulatory agency
programs.
Required if needed for pharmacy claim processing and
payment.
3Ø3-C3 PERSON CODE
Q Claim Billing/Encounter:
Required if needed to uniquely identify the family members
within the Cardholder ID.
3Ø6-C6 PATIENT RELATIONSHIP CODE
Q Claim Billing/Encounter:
Required if needed to uniquely identify the relationship of
the Patient to the Cardholder.
99Ø-MG OTHER PAYER BIN NUMBER N Claim Billing/Encounter:
Not used.
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER N Claim Billing/Encounter:
Not used.
356-NU OTHER PAYER CARDHOLDER ID N Claim Billing/Encounter:
Not used.
992-MJ OTHER PAYER GROUP ID N Claim Billing/Encounter:
Not used.
359-2A MEDIGAP ID Q Claim Billing/Encounter:
Required, if known, when patient has Medigap coverage.
36Ø-2B MEDICAID INDICATOR Q Claim Billing/Encounter:
Required, if known, when patient has Medicaid coverage.
361-2D PROVIDER ACCEPT ASSIGNMENT INDICATOR Q Claim Billing/Encounter:
Required if necessary for state/federal/regulatory agency
programs.
997-G2 CMS PART D DEFINED QUALIFIED FACILITY Q Claim Billing/Encounter:
Required if specified in trading partner agreement.
115-N5
MEDICAID ID NUMBER Q Claim Billing/Encounter:
Required, if known, when patient has Medicaid coverage.
Required when used for payer-to-payer coordination of
benefits to track the claim without regard to the “Service
Provider ID, Prescription Number, & Date of Service”.
116-N6
MEDICAID AGENCY NUMBER N Claim Billing/Encounter:
Not used.
Notes on Insurance Segment on a Claim Billing or Encounter Request:
The Insurance Segment is mandatory for a Claim Billing or Encounter request. Fields defined as Mandatory are required to be submitted
when the segment is sent.
7.4.2.1 INSURANCE SEGMENT (MEDICAID SUBROGATION CLAIM BILLING OR ENCOUNTER)
INSURANCE SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
115-N5
MEDICAID ID NUMBER QM Medicaid Subrogation - Claim Billing/Encounter:
Required to identify the member as uniquely known to
Medicaid.
116-N6
MEDICAID AGENCY NUMBER QM Medicaid Subrogation – Claim Billing/Encounter:
Required to identify the Medicaid agency.
See Medicaid Subrogation Implementation Guide.
Notes on Insurance Segment on a Medicaid Subrogation Claim Billing or Encounter Request:
The rules above for an “Insurance Segment (Claim Billing or Encounter)” are to be followed for Medicaid Subrogation. Specific fields
that are used differently in Medicaid Subrogation are noted in the table above.
7.4.3 PATIENT SEGMENT (CLAIM BILLING OR ENCOUNTER)
PATIENT SEGMENT
SITUATIONAL SEGMENT
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 68 -
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
331-CX PATIENT ID QUALIFIER
Q Claim Billing/Encounter:
Required if Patient ID (332-CY) is used.
332-CY PATIENT ID
Q Claim Billing/Encounter:
Required if necessary for state/federal/regulatory agency
programs to validate dual eligibility.
3Ø4-C4 DATE OF BIRTH
R Claim Billing/Encounter:
Required.
3Ø5-C5 PATIENT GENDER CODE
R Claim Billing/Encounter:
Required.
31Ø-CA PATIENT FIRST NAME
Q Claim Billing/Encounter:
Required when the patient has a first name.
311-CB PATIENT LAST NAME R Claim Billing/Encounter:
Required.
322-CM PATIENT STREET ADDRESS
O Claim Billing/Encounter:
Optional.
323-CN PATIENT CITY ADDRESS
O Claim Billing/Encounter:
Optional.
324-CO PATIENT STATE / PROVINCE ADDRESS
O Claim Billing/Encounter:
Optional.
325-CP PATIENT ZIP/POSTAL ZONE
O Claim Billing/Encounter:
Optional.
326-CQ PATIENT PHONE NUMBER
O Claim Billing/Encounter:
Optional.
3Ø7-C7 PLACE OF SERVICE
Q Claim Billing/Encounter:
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
333-CZ EMPLOYER ID
Q Claim Billing/Encounter:
Required if “required by law” as defined in the HIPAA final
Privacy regulations section 164.5Ø1 definitions (45 CFR
Parts 160 and 164 Standards for Privacy of Individually
Identifiable Health Information; Final Rule -
Thursday, December 28, 2000, page 82803 and following,
and Wednesday, August 14, 2002, page 53267 and
following.)
Required if needed for Workers’ Compensation billing.
334-1C SMOKER / NON-SMOKER CODE
N Claim Billing/Encounter:
Not used.
335-2C PREGNANCY INDICATOR
Q Claim Billing/Encounter:
Required if pregnancy could result in different coverage,
pricing, or patient financial responsibility.
Required if “required by law” as defined in the HIPAA final
Privacy regulations section 164.5Ø1 definitions (45 CFR
Parts 160 and 164 Standards for Privacy of Individually
Identifiable Health Information; Final Rule -
Thursday, December 28, 2000, page 82803 and following,
and Wednesday, August 14, 2002, page 53267 and
following.)
35Ø-HN PATIENT E-MAIL ADDRESS I Claim Billing/Encounter:
May be submitted for the receiver to relay patient health
care communications via the Internet when provided by the
patient.
This field is informational only.
384-4X PATIENT RESIDENCE Q Claim Billing/Encounter:
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
Notes on Patient Segment on a Claim Billing or Encounter Request:
The Patient Segment is situational for a Claim Billing or Encounter request. It is used when a receiver needs some of the patient demographic
information to perform eligibility and claim/encounter determination. The Patient Segment must be submitted when needed to differentiate
between the patient and the cardholder. If the cardholder and the patient are the same, then the Patient Segment is not submitted unless
additional information about the patient is needed to clarify the claim/encounter determination. The Segment is mandatory if required under
provider payer contract or mandatory on claims where this information is necessary for adjudication of the claim. Fields defined as Mandatory
are required to be submitted when the segment is sent.
7.4.3.1 PATIENT SEGMENT (MEDICAID SUBROGATION CLAIM BILLING OR ENCOUNTER)
PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 69 -
PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
31Ø-CA PATIENT FIRST NAME
QM Medicaid Subrogation - Claim Billing/Encounter:
Required to assist in identifying the cardholder when
specific eligibility cannot be established.
See Medicaid Subrogation Implementation Guide.
322-CM PATIENT STREET ADDRESS
QM Medicaid Subrogation - Claim Billing/Encounter:
Required to assist in identifying the cardholder when
specific eligibility cannot be established.
See Medicaid Subrogation Implementation Guide.
323-CN PATIENT CITY ADDRESS
QM Medicaid Subrogation - Claim Billing/Encounter:
Required to assist in identifying the cardholder when
specific eligibility cannot be established.
See Medicaid Subrogation Implementation Guide.
324-CO PATIENT STATE / PROVINCE ADDRESS
QM Medicaid Subrogation - Claim Billing/Encounter:
Required to assist in identifying the cardholder when
specific eligibility cannot be established.
See Medicaid Subrogation Implementation Guide.
325-CP PATIENT ZIP/POSTAL ZONE
QM Medicaid Subrogation - Claim Billing/Encounter:
Required to assist in identifying the cardholder when
specific eligibility cannot be established.
See Medicaid Subrogation Implementation Guide.
Notes on Patient Segment on a Medicaid Subrogation Claim Billing or Encounter Request:
The rules above for a “Patient Segment (Claim Billing or Encounter)” are to be followed for Medicaid Subrogation. Specific fields that
are used differently in Medicaid Subrogation are noted in the table above.
7.4.4 CLAIM SEGMENT (CLAIM BILLING OR ENCOUNTER)
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M For Transaction Code of “B1”, in the Claim Segment, the
Prescription/Service Reference Number Qualifier (455-EM)
is “1” (Rx Billing).
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
436-E1 PRODUCT/SERVICE ID QUALIFIER M Mandatory.
If billing for a multi-ingredient prescription, Product/Service
ID Qualifier (436-E1) is zero (“ØØ”).
4Ø7-D7 PRODUCT/SERVICE ID M Mandatory.
If billing for a multi-ingredient prescription, Product/Service
ID (4Ø7-D7) is zero. (Zero means “Ø”.)
456-EN ASSOCIATED PRESCRIPTION/SERVICE REFERENCE NUMBER Q Claim Billing/Encounter:
Required if the “completion” transaction in a partial fill
(Dispensing Status (343-HD) = “C” (Completed)).
See section “Specific Segment Discussion”, “Request
Segments”, Claim Segment” for more information.
Required if the Dispensing Status (343-HD) = “P” (Partial
Fill) and there are multiple occurrences of partial fills for this
prescription.
457-EP ASSOCIATED PRESCRIPTION/SERVICE DATE Q Claim Billing/Encounter:
Required if the “completion” transaction in a partial fill
(Dispensing Status (343-HD) = “C” (Completed)).
Required if Associated Prescription/Service Reference
Number (456-EN) is used.
See section “Specific Segment Discussion”, “Request
Segments”, Claim Segment” for more information.
Required if the Dispensing Status (343-HD) = “P” (Partial
Fill) and there are multiple occurrences of partial fills for this
prescription.
458-SE PROCEDURE MODIFIER CODE COUNT Q Claim Billing/Encounter:
Maximum count of 1Ø.
Required if Procedure Modifier Code (459-ER) is used.
459-ER PROCEDURE MODIFIER CODE Q***R*** Claim Billing/Encounter:
Required to define a further level of specificity if the
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 70 -
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Product/Service ID (4Ø7-D7) indicated a Procedure Code
was submitted.
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
Occurs the number of times identified in Procedure Modifier
Code Count (458-SE).
442-E7 QUANTITY DISPENSED R Claim Billing/Encounter:
Required.
4Ø3-D3 FILL NUMBER R Claim Billing/Encounter:
Required.
4Ø5-D5 DAYS SUPPLY R Claim Billing/Encounter:
Required.
4Ø6-D6 COMPOUND CODE R Claim Billing/Encounter:
Required.
4Ø8-D8 DISPENSE AS WRITTEN (DAW)/PRODUCT SELECTION CODE R Claim Billing/Encounter:
Required.
414-DE DATE PRESCRIPTION WRITTEN R Claim Billing/Encounter:
Required.
415-DF NUMBER OF REFILLS AUTHORIZED Q Claim Billing/Encounter:
Required if necessary for plan benefit administration.
419-DJ PRESCRIPTION ORIGIN CODE Q Claim Billing/Encounter:
Required if necessary for plan benefit administration.
354-NX SUBMISSION CLARIFICATION CODE COUNT Q Claim Billing/Encounter:
Maximum count of 3.
Required if Submission Clarification Code (42Ø-DK) is
used.
42Ø-DK SUBMISSION CLARIFICATION CODE Q***R*** Claim Billing/Encounter:
Required if clarification is needed and value submitted is
greater than zero (Ø).
Occurs the number of times identified in Submission
Clarification Code Count (354-NX).
If the Date of Service (4Ø1-D1) contains the subsequent
payer coverage date, the Submission Clarification Code
(42Ø-DK) is required with value of “19” (Split Billing –
indicates the quantity dispensed is the remainder billed to a
subsequent payer when Medicare Part A expires. Used
only in long-term care settings) for individual unit of use
medications.
46∅-ET QUANTITY PRESCRIBED N Claim Billing/Encounter:
Not used.
3Ø8-C8 OTHER COVERAGE CODE S Claim Billing/Encounter:
Required if needed by receiver, to communicate a
summation of other coverage information that has been
collected from other payers.
Required for Coordination of Benefits.
See section “Specific Segment Discussion”, “Request
Segments”, “Claim Segment”, “Other Coverage Code (3Ø8-
C8).
429-DT SPECIAL PACKAGING INDICATOR Q Claim Billing/Encounter:
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
453-EJ ORIGINALLY PRESCRIBED PRODUCT/SERVICE ID QUALIFIER Q Claim Billing/Encounter:
Required if Originally Prescribed Product/Service Code
(455-EA) is used.
445-EA ORIGINALLY PRESCRIBED PRODUCT/SERVICE CODE Q Claim Billing/Encounter:
Required if the receiver requests association to a
therapeutic, or a preferred product substitution, or when a
DUR alert has been resolved by changing medications, or
an alternative service than what was originally prescribed.
446-EB ORIGINALLY PRESCRIBED QUANTITY Q Claim Billing/Encounter:
Required if the receiver requests reporting for quantity
changes due to a therapeutic substitution that has occurred
or a preferred product/service substitution that has
occurred, or when a DUR alert has been resolved by
changing quantities.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 71 -
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
33Ø-CW ALTERNATE ID N Claim Billing/Encounter:
Not used.
454-EK SCHEDULED PRESCRIPTION ID NUMBER N Claim Billing/Encounter:
Not used.
6ØØ-28 UNIT OF MEASURE Q Claim Billing/Encounter:
Required if necessary for state/federal/regulatory agency
programs.
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
418-DI LEVEL OF SERVICE Q Claim Billing/Encounter:
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
461-EU PRIOR AUTHORIZATION TYPE CODE Q Claim Billing/Encounter:
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
462-EV PRIOR AUTHORIZATION NUMBER SUBMITTED Q Claim Billing/Encounter:
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
463-EW INTERMEDIARY AUTHORIZATION TYPE ID Q Claim Billing/Encounter:
Required for overriding an authorized intermediary system
edit when the pharmacy participates with an intermediary.
Required if Intermediary Authorization ID (464-EX) is used.
Not used for payer-to-payer transactions.
464-EX INTERMEDIARY AUTHORIZATION ID Q Claim Billing/Encounter:
Required for overriding an authorized intermediary system
edit when the pharmacy participates with an intermediary.
Not used for payer-to-payer transactions.
343-HD DISPENSING STATUS Q Claim Billing/Encounter:
Required for the partial fill or the completion fill of a
prescription.
344-HF QUANTITY INTENDED TO BE DISPENSED Q Claim Billing/Encounter:
Required for the partial fill or the completion fill of a
prescription.
345-HG DAYS SUPPLY INTENDED TO BE DISPENSED Q Claim Billing/Encounter:
Required for the partial fill or the completion fill of a
prescription.
357-NV DELAY REASON CODE Q Claim Billing/Encounter:
Required when needed to specify the reason that
submission of the transaction has been delayed.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Claim Billing/Encounter:
Not used.
391-MT PATIENT ASSIGNMENT INDICATOR (DIRECT MEMBER
REIMBURSEMENT INDICATOR)
Q Claim Billing/Encounter:
Required when the claims adjudicator does not assume the
patient assigned his/her benefits to the provider or when
the claims adjudicator supports a patient determination of
whether he/she wants to assign or retain his/her benefits.
995-E2 ROUTE OF ADMINISTRATION Q Claim Billing/Encounter:
Required if specified in trading partner agreement.
996-G1 COMPOUND TYPE Q Claim Billing/Encounter:
Required if specified in trading partner agreement.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Claim Billing/Encounter:
Not used.
147-U7 PHARMACY SERVICE TYPE Q Claim Billing/Encounter:
Required when the submitter must clarify the type of
services being performed as a condition for proper
reimbursement by the payer.
Notes on Claim Segment on a Claim Billing or Encounter Request:
The Claim Segment is mandatory for a Claim Billing or Encounter Request. The Claim Segment defines the product dispensed, dispensing
information, reference information for tieback to an original prescription in the case of partial fillings, or authorization information. Fields defined
as Mandatory are required to be submitted when the segment is sent.
7.4.4.1 CLAIM SEGMENT (MEDICAID SUBROGATION CLAIM BILLING OR ENCOUNTER)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 72 -
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
RM Medicaid Subrogation - Claim Billing/Encounter:
Required. Contains the Medicaid unique claim
identification number (also referred to as the ICN or
TCN).
See Medicaid Subrogation Implementation Guide.
Notes on Claim Segment on a Medicaid Subrogation Claim Billing or Encounter Request:
The rules above for a “Claim Segment (Claim Billing or Encounter)” are to be followed for Medicaid Subrogation. Specific fields that
are used differently in Medicaid Subrogation are noted in the table above.
7.4.5 PRICING SEGMENT (CLAIM BILLING OR ENCOUNTER)
PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
4Ø9-D9 INGREDIENT COST SUBMITTED R Claim Billing/Encounter:
Required.
412-DC DISPENSING FEE SUBMITTED Q Claim Billing/Encounter:
Required if its value has an effect on the Gross Amount
Due (43Ø-DU) calculation.
Zero (Ø) is a valid value.
477-BE PROFESSIONAL SERVICE FEE SUBMITTED N Claim Billing/Encounter:
Not used.
433-DX PATIENT PAID AMOUNT SUBMITTED Q Claim Billing/Encounter:
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
Not used in coordination of benefit claim to pass patient
liability information to a downstream payer. See section
“Standard Conventions”, “Repetition and Multiple
Occurrences”, Repeating Data Elements”, “Request
Segments”, “Coordination of Benefits/Other Payments
Segment”.
438-E3 INCENTIVE AMOUNT SUBMITTED Q Claim Billing/Encounter:
Required if its value has an effect on the Gross Amount
Due (43Ø-DU) calculation.
Zero (Ø) is a valid value.
478-H7 OTHER AMOUNT CLAIMED SUBMITTED COUNT Q Claim Billing/Encounter:
Maximum count of 3.
Required if Other Amount Claimed Submitted Qualifier
(479-H8) is used.
479-H8 OTHER AMOUNT CLAIMED SUBMITTED QUALIFIER Q***R*** Claim Billing/Encounter:
Required if Other Amount Claimed Submitted (48Ø-H9) is
used.
48Ø-H9 OTHER AMOUNT CLAIMED SUBMITTED Q***R*** Claim Billing/Encounter:
Required if its value has an effect on the Gross Amount
Due (43Ø-DU) calculation.
Zero (Ø) is a valid value.
481-HA FLAT SALES TAX AMOUNT SUBMITTED Q Claim Billing/Encounter:
Required if its value has an effect on the Gross Amount
Due (43Ø-DU) calculation.
Zero (Ø) is a valid value.
482-GE PERCENTAGE SALES TAX AMOUNT SUBMITTED Q Claim Billing/Encounter:
Required if its value has an effect on the Gross Amount
Due (43Ø-DU) calculation.
Zero (Ø) is a valid value.
483-HE PERCENTAGE SALES TAX RATE SUBMITTED Q Claim Billing/Encounter:
Required if Percentage Sales Tax Amount Submitted (482-
GE) and Percentage Sales Tax Basis Submitted (484-JE)
are used.
Required if this field could result in different pricing.
Required if needed to calculate Percentage Sales Tax
Amount Paid (559-AX).
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 73 -
PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
484-JE PERCENTAGE SALES TAX BASIS SUBMITTED Q Claim Billing/Encounter:
Required if Percentage Sales Tax Amount Submitted (482-
GE) and Percentage Sales Tax Rate Submitted (483-HE)
are used.
Required if this field could result in different pricing.
Required if needed to calculate Percentage Sales Tax
Amount Paid (559-AX).
426-DQ USUAL AND CUSTOMARY CHARGE Q Claim Billing/Encounter:
Required if needed per trading partner agreement.
43Ø-DU GROSS AMOUNT DUE R Claim Billing/Encounter:
Required.
See Pricing Formula for fields used in calculation.
423-DN BASIS OF COST DETERMINATION Q Claim Billing/Encounter:
Required if needed for receiver claim/encounter
adjudication.
113-N3
MEDICAID PAID AMOUNT N Claim Billing/Encounter:
Not used.
Notes on Pricing Segment on a Claim Billing or Encounter Request:
The Pricing Segment is mandatory for a Claim Billing or Encounter Request. The Pricing Segment defines dollar amounts and basis of costs
for a Claim Billing or Encounter.
It is highly recommended that whenever possible, the individual dollar fields are requested of the sender by the receiver. On the response, the
sender should return the individual payment response fields to allow the receiver to reconcile against the requested payment fields. It is
recommended that for the dollar fields, if the field is not required or situational in the calculation, that the dollar fields are not sent.
See section “Response Processing Guidelines”, “Pricing Guidelines”.
Fields defined as Mandatory are required to be submitted when the segment is sent.
7.4.5.1 PRICING SEGMENT (MEDICAID SUBROGATION CLAIM BILLING OR ENCOUNTER)
PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
113-N3
MEDICAID PAID AMOUNT QM Medicaid Subrogation - Claim Billing/Encounter:
Required if affects pricing in Medicaid Subrogation
(contains the amount paid tothe pharmacy).
See Medicaid Subrogation Implementation Guide.
Notes on Pricing Segment on a Medicaid Subrogation Claim Billing or Encounter Request:
The rules above for a “Pricing Segment (Claim Billing or Encounter)” are to be followed for Medicaid Subrogation. Specific fields that
are used differently in Medicaid Subrogation are noted in the table above.
7.4.6 PHARMACY PROVIDER SEGMENT (CLAIM BILLING OR ENCOUNTER)
PHARMACY PROVIDER SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
465-EY PROVIDER ID QUALIFIER Q Claim Billing/Encounter:
Required if Provider ID (444-E9) is used.
444-E9 PROVIDER ID
Q Claim Billing/Encounter:
Required if necessary for state/federal/regulatory agency
programs.
Required if necessary to identify the individual responsible
for dispensing of the prescription.
Required if needed for reconciliation of encounter-reported
data or encounter reporting.
Notes on Pharmacy Provider Segment on a Claim Billing or Encounter Request:
The Pharmacy Provider Segment is situational for a Claim Billing or Encounter request. It is used when a receiver needs pharmacy provider
information to perform claim/encounter determination. Fields defined as Mandatory are required to be submitted when the segment is sent.
7.4.7 PRESCRIBER SEGMENT (CLAIM BILLING OR ENCOUNTER)

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 74 -
PRESCRIBER SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
466-EZ PRESCRIBER ID QUALIFIER Q Claim Billing/Encounter:
Required if Prescriber ID (411-DB) is used.
411-DB PRESCRIBER ID Q Claim Billing/Encounter:
Required if this field could result in different coverage or
patient financial responsibility.
Required if necessary for state/federal/regulatory agency
programs.
427-DR PRESCRIBER LAST NAME Q Claim Billing/Encounter:
Required when the Prescriber ID (411-DB) is not known.
Required if needed for Prescriber ID (411-DB)
validation/clarification.
498-PM PRESCRIBER PHONE NUMBER Q Encounter:
Required if needed for Prior Authorization process.
Claim Billing:
Required if needed for Workers’ Compensation.
Required if needed to assist in identifying the prescriber.
Required if needed for Prior Authorization process.
468-2E PRIMARY CARE PROVIDER ID QUALIFIER Q Claim Billing/Encounter:
Required if Primary Care Provider ID (421-DL) is used.
421-DL PRIMARY CARE PROVIDER ID Q Claim Billing/Encounter:
Required if needed for receiver claim/encounter
determination, if known and available.
Required if this field could result in different coverage or
patient financial responsibility.
Required if necessary for state/federal/regulatory agency
programs.
47Ø-4E PRIMARY CARE PROVIDER LAST NAME Q Claim Billing/Encounter:
Required if this field is used as an alternative for Primary
Care Provider ID (421-DL) when ID is not known.
Required if needed for Primary Care Provider ID (421-DL)
validation/clarification.
364-2J PRESCRIBER FIRST NAME Q Claim Billing/Encounter:
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
365-2K PRESCRIBER STREET ADDRESS Q Claim Billing/Encounter:
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
366-2M PRESCRIBER CITY ADDRESS Q Claim Billing/Encounter:
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
367-2N PRESCRIBER STATE/PROVINCE ADDRESS Q Claim Billing/Encounter:
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
368-2P PRESCRIBER ZIP/POSTAL ZONE Q Claim Billing/Encounter:
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
Notes on Prescriber Segment on a Claim Billing or Encounter Request:
The Prescriber Segment is situational for a Claim Billing or Encounter request. It is used when prescriber information is needed to perform
claim/encounter determination. The Segment is mandatory if required under provider payer contract or mandatory on claims where this
information is necessary for adjudication of the claim. Fields defined as Mandatory are required to be submitted when the segment is sent.
7.4.8 COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT (CLAIM BILLING OR
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 75 -
ENCOUNTER)
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
337-4C COORDINATION OF BENEFITS/OTHER PAYMENTS COUNT M Maximum count of 9.
338-5C OTHER PAYER COVERAGE TYPE M***R*** Mandatory.
Occurs with Coordination of Benefits/Other Payments
Count (337-4C).
Grouped with Other Payer ID Qualifier (339-6C), Other
Payer ID (34Ø-7C), Other Payer Date (443-E8), and either
Other Payer Amount Paid Count (341-HB) and its grouping,
or Other Payer Reject Count (471-5E) and its grouping.
339-6C OTHER PAYER ID QUALIFIER Q***R*** Claim Billing/Encounter:
Required if Other Payer ID (34Ø-7C) is used.
34Ø-7C OTHER PAYER ID Q***R*** Claim Billing/Encounter:
Required if identification of the Other Payer is necessary for
claim/encounter adjudication.
443-E8 OTHER PAYER DATE Q***R*** Claim Billing/Encounter:
Required if identification of the Other Payer Date is
necessary for claim/encounter adjudication.
993-A7 INTERNAL CONTROL NUMBER Q***R*** Claim Billing/Encounter:
Required when used for payer-to-payer coordination of
benefits to track the claim without regard to the “Service
provider id, Prescription Number, & Date of Service”.
341-HB OTHER PAYER AMOUNT PAID COUNT Q Claim Billing/Encounter:
Maximum count of 9.
Required if Other Payer Amount Paid Qualifier (342-HC) is
used.
342-HC OTHER PAYER AMOUNT PAID QUALIFIER Q***R*** Claim Billing/Encounter:
Required if Other Payer Amount Paid (431-DV) is used.
431-DV OTHER PAYER AMOUNT PAID Q***R*** Claim Billing/Encounter:
Required if other payer has approved payment for some/all
of the billing.
Zero (Ø) is a valid value.
Not used for patient financial responsibility only billing.
Not used for non-governmental agency programs if Other
Payer-Patient Responsibility Amount (352-NQ) is
submitted.
471-5E OTHER PAYER REJECT COUNT Q Claim Billing/Encounter:
Maximum count of 5.
Required if Other Payer Reject Code (472-6E) is used.
472-6E OTHER PAYER REJECT CODE
Q***R*** Claim Billing/Encounter:
Required when the other payer has denied the payment for
the billing, designated with Other Coverage Code (3Ø8-C8)
= 3 (Other Coverage Billed – claim not covered).
Note: This field must only contain the NCPDP Reject Code
(511-FB) values.
353-NR OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT COUNT Q Claim Billing/Encounter:
Maximum count of 25.
Required if Other Payer-Patient Responsibility Amount
Qualifier (351-NP) is used.
Note the occurrences are dependent upon the number of
component parts returned from a previous payer.
351-NP OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT QUALIFIER Q***R*** Claim Billing/Encounter:
Required if Other Payer-Patient Responsibility Amount
(352-NQ) is used.
352-NQ OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT Q***R*** Claim Billing/Encounter:
Required if necessary for patient financial responsibility
only billing.
Required if necessary for state/federal/regulatory agency
programs.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 76 -
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Not used for non-governmental agency programs if Other
Payer Amount Paid (431-DV) is submitted.
392-MU BENEFIT STAGE COUNT Q Claim Billing/Encounter:
Maximum count of 4.
Required if Benefit Stage Amount (394-MW) is used.
393-MV BENEFIT STAGE QUALIFIER Q***R*** Claim Billing/Encounter:
Required if Benefit Stage Amount (394-MW) is used.
Must only have one value per iteration - value must not be
repeated.
394-MW BENEFIT STAGE AMOUNT Q***R*** Claim Billing/Encounter:
Required if the previous payer has financial amounts that
apply to Medicare Part D beneficiary benefit stages. This
field is required when the plan is a participant in a Medicare
Part D program that requires reporting of benefit stage
specific financial amounts.
Required if necessary for state/federal/regulatory agency
programs.
Notes on Coordination of Benefits/Other Payments Segment on a Claim Billing or Encounter Request:
The Coordination of Benefits/Other Payments Segment is situational for a Claim Billing or Encounter request. It is used when a receiver needs
payment information from other receivers to perform claim/encounter determination. This may be in the case of primary, secondary, tertiary et
cetera health plan coverage for example.
The Coordination of Benefits/Other Payments Segment is mandatory for a Claim Billing or Encounter request to a downstream payer. It is
used to assist a downstream payer to uniquely identify a claim or encounter in case of duplicate processing. Sometimes processors have
difficulty determining duplicate logic because the same processor is involved in multiple coordination of benefit occurrences for the same
patient. They are involved for example as the primary and secondary payer, or primary and tertiary, or secondary and tertiary. The downstream
payer uses the fields involved in duplicate logic, including the Other Payer Coverage Type (338-5C) to differentiate which claim or encounter to
process. See section “Response Processing Guidelines”, “Duplicate Transactions”.
Note, the Other Payer Coverage Type (338-5C) occurrences do not have to appear in sequential order (primary, secondary, tertiary),
but can appear in any order.
The Coordination of Benefits/Other Payments Segment is not used for a Claim Billing or Encounter request to a primary payer.
A coupon is used to reduce the patient out of pocket prescription cost – by either reducing the cost of a CASH prescription or the patient
financial responsibility from a Third Party payer who allows coupon usage. The coupon processor is the LAST payer. (Note: Some Federal
and State programs do not allow the reduction of patient’s financial responsibility.)
The Segment is mandatory if required under provider payer contract or mandatory on claims where this information is necessary for
adjudication of the claim.
Fields defined as Mandatory are required to be submitted when the segment is sent.
7.4.9 WORKERS’ COMPENSATION SEGMENT (CLAIM BILLING OR ENCOUNTER)
WORKERS’ COMPENSATION SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
434-DY DATE OF INJURY M
315-CF EMPLOYER NAME Q Claim Billing/Encounter:
Required if needed to process a claim/encounter for a work
related injury or condition.
316-CG EMPLOYER STREET ADDRESS Q Claim Billing/Encounter:
Required if needed to process a claim/encounter for a work
related injury or condition.
317-CH EMPLOYER CITY ADDRESS Q Claim Billing/Encounter:
Required if needed to process a claim/encounter for a work
related injury or condition.
318-CI EMPLOYER STATE/PROVINCE ADDRESS Q Claim Billing/Encounter:
Required if needed to process a claim/encounter for a work
related injury or condition.
319-CJ EMPLOYER ZIP/POSTAL ZONE Q Claim Billing/Encounter:
Required if needed to process a claim/encounter for a work
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 77 -
WORKERS’ COMPENSATION SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
related injury or condition.
32Ø-CK EMPLOYER PHONE NUMBER Q Claim Billing/Encounter:
Required if needed to process a claim/encounter for a work
related injury or condition.
321-CL EMPLOYER CONTACT NAME Q Claim Billing/Encounter:
Required if needed to process a claim/encounter for a work
related injury or condition.
327-CR CARRIER ID Q Claim Billing/Encounter:
Required if needed to process a claim/encounter for a work
related injury or condition.
435-DZ CLAIM/REFERENCE ID Q Claim Billing/Encounter:
Required if needed to process a claim/encounter for a work
related injury or condition.
117-TR BILLING ENTITY TYPE INDICATOR R Claim Billing/Encounter:
Required.
118-TS PAY TO QUALIFIER Q Claim Billing/Encounter:
Required if Pay To ID (119-TT) is used.
119-TT PAY TO ID Q Claim Billing/Encounter:
Required if transaction is submitted by a provider or agent,
but paid to another party.
12Ø-TU PAY TO NAME Q Claim Billing/Encounter:
Required if transaction is submitted by a provider or agent,
but paid to another party.
121-TV PAY TO STREET ADDRESS Q Claim Billing/Encounter:
Required if transaction is submitted by a provider or agent,
but paid to another party.
122-TW PAY TO CITY ADDRESS Q Claim Billing/Encounter:
Required if transaction is submitted by a provider or agent,
but paid to another party.
123-TX PAY TO STATE/PROVINCE ADDRESS Q Claim Billing/Encounter:
Required if transaction is submitted by a provider or agent,
but paid to another party.
124-TY PAY TO ZIP/POSTAL ZONE Q Claim Billing/Encounter:
Required if transaction is submitted by a provider or agent,
but paid to another party.
125-TZ GENERIC EQUIVALENT PRODUCT ID QUALIFIER Q Claim Billing/Encounter:
Required if Generic Equivalent Product ID (126-UA) is
used.
126-UA GENERIC EQUIVALENT PRODUCT ID Q Claim Billing/Encounter:
Required if necessary for state/federal/regulatory agency
programs.
Notes on Workers’ Compensation Segment on a Claim Billing or Encounter Request:
The Workers’ Compensation Segment is situational for a Claim Billing or Encounter request. It is used when processing a Claim Billing or
Encounter for a work-related injury or condition. Fields defined as Mandatory are required to be submitted when the segment is sent.
7.4.10 DUR/PPS SEGMENT (CLAIM BILLING OR ENCOUNTER)
DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
473-7E DUR/PPS CODE COUNTER Q***R*** Claim Billing/Encounter:
Maximum of 9 occurrences.
Required if DUR/PPS Segment is used.
439-E4 REASON FOR SERVICE CODE Q***R*** Claim Billing/Encounter:
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug utilization
review outcome.
Required if this field affects payment for or documentation of
professional pharmacy service.
44Ø-E5 PROFESSIONAL SERVICE CODE Q***R*** Claim Billing/Encounter:
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug utilization
review outcome.
Required if this field affects payment for or documentation of
professional pharmacy service.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 78 -
DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
441-E6 RESULT OF SERVICE CODE Q***R*** Claim Billing/Encounter:
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug utilization
review outcome.
Required if this field affects payment for or documentation of
professional pharmacy service.
474-8E DUR/PPS LEVEL OF EFFORT Q***R*** Claim Billing/Encounter:
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug utilization
review outcome.
Required if this field affects payment for or documentation of
professional pharmacy service.
475-J9 DUR CO-AGENT ID QUALIFIER Q***R*** Claim Billing/Encounter:
Required if DUR Co-Agent ID (476-H6) is used.
476-H6 DUR CO-AGENT ID Q***R*** Claim Billing/Encounter:
Required if this field could result in different drug utilization
review outcome.
Required if this field affects payment for or documentation of
professional pharmacy service.
Notes on DUR/PPS Segment on a Claim Billing or Encounter Request:
The DUR/PPS Segment is situational for a Claim Billing or Encounter request. It is used when a sender notifies the receiver of drug utilization,
drug evaluations, or information on the appropriate selection to process the claim/encounter. The DUR/PPS information may be sent on the
initial submission or alternatively sent after a DUR/PPS rejection from a receiver. The Segment is mandatory if required under provider payer
contract or mandatory on claims where this information is necessary for adjudication of the claim. Fields defined as Mandatory are required to
be submitted when the segment is sent.
7.4.11 COUPON SEGMENT (CLAIM BILLING OR ENCOUNTER)
COUPON SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
485-KE COUPON TYPE M
486-ME COUPON NUMBER M
487-NE COUPON VALUE AMOUNT Q Claim Billing/Encounter:
Required if needed for receiver claim/encounter
determination when a coupon value is known.
Required if this field could result in different pricing and/or
patient financial responsibility.
Notes on Coupon Segment on a Claim Billing or Encounter Request:
The Coupon Segment is situational for a Claim Billing or Encounter request. It is used when the sender seeks reimbursement for a claim billing
which includes a fixed amount or percentage of total price reduction. It is used in situations where the coupon is applied to the transaction.
To bill a coupon processor using the Coupon Segment, the Coupon Type (485-KE) and Coupon Number (486-ME) are mandatory.
A coupon is used to reduce the patient out of pocket prescription cost – by either reducing the cost of a CASH prescription or the patient
financial responsibility from a Third Party payer who allows coupon usage. The coupon processor is the LAST payer. (Note: Some Federal
and State programs do not allow the reduction of patient’s financial responsibility.)
When a customer has a coupon, the field Usual And Customary Charge (426-DQ) is not reduced by the amount of the coupon.
The Segment is mandatory if required under provider payer contract or mandatory on claims where this information is necessary for
adjudication of the claim.
Fields defined as Mandatory are required to be submitted when the segment is sent.
7.4.12 COMPOUND SEGMENT (CLAIM BILLING OR ENCOUNTER)
COMPOUND SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 79 -
COMPOUND SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
45Ø-EF COMPOUND DOSAGE FORM DESCRIPTION CODE M
451-EG COMPOUND DISPENSING UNIT FORM INDICATOR M
447-EC COMPOUND INGREDIENT COMPONENT COUNT M Maximum count of 25 ingredients.
488-RE COMPOUND PRODUCT ID QUALIFIER M***R***
489-TE COMPOUND PRODUCT ID M***R***
448-ED COMPOUND INGREDIENT QUANTITY M***R***
449-EE COMPOUND INGREDIENT DRUG COST Q***R*** Claim Billing:
Required if needed for receiver claim determination when
multiple products are billed.
Encounter:
Required if needed for receiver encounter determination
when multiple products are reported.
49Ø-UE COMPOUND INGREDIENT BASIS OF COST DETERMINATION Q***R*** Claim Billing:
Required if needed for receiver claim determination when
multiple products are billed.
Encounter:
Required if needed for receiver encounter determination
when multiple products are reported.
362-2G COMPOUND INGREDIENT MODIFIER CODE COUNT Q Claim Billing/Encounter:
Required when Compound Ingredient Modifier Code (363-
2H) is sent.
Maximum count of 1Ø.
363-2H COMPOUND INGREDIENT MODIFIER CODE Q***R*** Claim Billing/Encounter:
Required if necessary for state/federal/regulatory agency
programs.
Notes on Compound Segment on a Claim Billing or Encounter Request:
The Compound Segment is situational for a Claim Billing or Encounter request. It is used for multi-ingredient prescriptions, when each
ingredient is reported. The Segment is mandatory if required under provider payer contract or mandatory on claims where this information is
necessary for adjudication of the claim. Fields defined as Mandatory are required to be submitted when the segment is sent.
7.4.13 CLINICAL SEGMENT (CLAIM BILLING OR ENCOUNTER)
CLINICAL SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
491-VE DIAGNOSIS CODE COUNT Q Claim Billing/Encounter:
Maximum count of 5.
Required if Diagnosis Code Qualifier (492-WE) and
Diagnosis Code (424-DO) are used.
492-WE DIAGNOSIS CODE QUALIFIER Q***R*** Claim Billing/Encounter:
Required if Diagnosis Code (424-DO) is used.
424-DO DIAGNOSIS CODE Q***R*** Claim Billing/Encounter:
The value for this field is obtained from the prescriber or
authorized representative.
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
Required if this field affects payment for professional
pharmacy service.
Required if this information can be used in place of prior
authorization.
Required if necessary for state/federal/regulatory agency
programs.
493-XE CLINICAL INFORMATION COUNTER Q***R*** Claim Billing/Encounter:
Maximum 5 occurrences supported.
Grouped with Measurement fields (Measurement Date
(494-ZE), Measurement Time (495-H1), Measurement
Dimension (496-H2), Measurement Unit (497-H3),
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 80 -
CLINICAL SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Measurement Value (499-H4).
494-ZE MEASUREMENT DATE Q***R*** Claim Billing/Encounter:
Required if necessary when this field could result in
different coverage and/or drug utilization review outcome.
495-H1 MEASUREMENT TIME Q***R*** Claim Billing/Encounter:
Required if Time is known or has impact on measurement.
Required if necessary when this field could result in
different coverage and/or drug utilization review outcome.
496-H2 MEASUREMENT DIMENSION Q***R*** Claim Billing/Encounter:
Required if Measurement Unit (497-H3) and Measurement
Value (499-H4) are used.
Required if necessary when this field could result in
different coverage and/or drug utilization review outcome.
Required if necessary for patient’s weight and height when
billing Medicare for a claim that includes a Certificate of
Medical Necessity (CMN).
497-H3 MEASUREMENT UNIT Q***R*** Claim Billing/Encounter:
Required if Measurement Dimension (496-H2) and
Measurement Value (499-H4) are used.
Required if necessary for patient’s weight and height when
billing Medicare for a claim that includes a Certificate of
Medical Necessity (CMN).
Required if necessary when this field could result in
different coverage and/or drug utilization review outcome.
499-H4 MEASUREMENT VALUE Q***R*** Claim Billing/Encounter:
Required if Measurement Dimension (496-H2) and
Measurement Unit (497-H3) are used.
Required if necessary for patient’s weight and height when
billing Medicare for a claim that includes a Certificate of
Medical Necessity (CMN).
Required if necessary when this field could result in
different coverage and/or drug utilization review outcome.
Notes on Clinical Segment on a Claim Billing or Encounter Request:
The Clinical Segment is situational for a Claim Billing or Encounter request. It is used to specify diagnosis information associated with the
Claim Billing or Encounter transaction. The Segment is mandatory if required under provider payer contract or mandatory on claims where this
information is necessary for adjudication of the claim. Fields defined as Mandatory are required to be submitted when the segment is sent.
7.4.14 ADDITIONAL DOCUMENTATION SEGMENT (CLAIM BILLING OR ENCOUNTER)
ADDITIONAL DOCUMENTATION SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
369-2Q ADDITIONAL DOCUMENTATION TYPE ID M
374-2V REQUEST PERIOD BEGIN DATE Q Claim Billing/Encounter:
Required if necessary for state/federal/regulatory agency
programs.
375-2W REQUEST PERIOD RECERT/REVISED DATE Q Claim Billing/Encounter:
Required if necessary for state/federal/regulatory agency
programs.
Required if the Request Status (373-2U) = “2” (Revision) or
“3” (Recertification).
373-2U REQUEST STATUS Q Claim Billing/Encounter:
Required if necessary for state/federal/regulatory agency
programs.
371-2S LENGTH OF NEED QUALIFIER Q Claim Billing/Encounter:
Required if Length of Need (37Ø-2R) is used.
37Ø-2R LENGTH OF NEED Q Claim Billing/Encounter:
Required if necessary for state/federal/regulatory agency
programs.
372-2T PRESCRIBER/SUPPLIER DATE SIGNED Q Claim Billing/Encounter:
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 81 -
ADDITIONAL DOCUMENTATION SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if necessary for state/federal/regulatory agency
programs.
376-2X SUPPORTING DOCUMENTATION Q Claim Billing/Encounter:
Required if necessary for state/federal/regulatory agency
programs (using Section C of Medicare’s CMN forms).
377-2Z QUESTION NUMBER/LETTER COUNT Q Claim Billing/Encounter:
Maximum count of 5Ø.
Required if needed to provide response to narratives.
378-4B QUESTION NUMBER/LETTER Q***R*** Claim Billing/Encounter:
Required if necessary for state/federal/regulatory agency
programs to respond to questions included on a Medicare
form.
Required if Question Number/Letter Count (377-2Z) is
greater than Ø.
379-4D QUESTION PERCENT RESPONSE Q***R*** Claim Billing/Encounter:
Required if necessary for state/federal/regulatory agency
programs to respond to questions included on a Medicare
form that requires a percent as the response. (At least one
response is required per question.)
38Ø-4G QUESTION DATE RESPONSE Q***R*** Claim Billing/Encounter:
Required if necessary for state/federal/regulatory agency
programs to respond to questions included on a Medicare
form that requires a date as the response. (At least one
response is required per question.)
381-4H QUESTION DOLLAR AMOUNT RESPONSE Q***R*** Claim Billing/Encounter:
Required if necessary for state/federal/regulatory agency
programs to respond to questions included on a Medicare
form that requires a dollar amount as the response. (At
least one response is required per question.)
382-4J QUESTION NUMERIC RESPONSE Q***R*** Claim Billing/Encounter:
Required if necessary for
State/federal/regulatory agency programs to respond to
questions included on a Medicare form that requires a
numeric as the response. (At least one response is
required per question.)
383-4K QUESTION ALPHANUMERIC RESPONSE Q***R*** Claim Billing/Encounter:
Required if necessary for state/federal/regulatory agency
programs to respond to questions included on a Medicare
form that requires an alphanumeric as the response. (At
least one response is required per question.)
Notes on Additional Documentation Segment on a Claim Billing or Encounter Request:
The Additional Documentation Segment is situational for Claim Billing or Encounter request. It is used to provide additional information on
Medicare forms. Fields defined as Mandatory are required to be submitted when the segment is sent.
7.4.15 FACILITY SEGMENT (CLAIM BILLING OR ENCOUNTER)
FACILITY SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
336-8C FACILITY ID Q Claim Billing/Encounter:
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
385-3Q FACILITY NAME Q Claim Billing/Encounter:
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
386-3U FACILITY STREET ADDRESS Q Claim Billing/Encounter:
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
388-5J FACILITY CITY ADDRESS Q Claim Billing/Encounter:
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
387-3V FACILITY STATE/PROVINCE ADDRESS Q Claim Billing/Encounter:
Required if this field could result in different coverage,
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 82 -
FACILITY SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
pricing, patient financial responsibility, and/or drug
utilization review outcome.
389-6D FACILITY ZIP/POSTAL ZONE Q Claim Billing/Encounter:
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
Notes on Facility Segment on a Claim Billing or Encounter Request:
The Facility Segment is situational for Claim Billing or Encounter request. It is used when these fields could result in different coverage,
pricing, patient financial responsibility, and/or drug utilization review outcome. Fields defined as Mandatory are required to be submitted when
the segment is sent.
7.4.16 NARRATIVE SEGMENT (CLAIM BILLING OR ENCOUNTER)
NARRATIVE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
39Ø-BM NARRATIVE MESSAGE Q Claim Billing/Encounter:
Required if necessary only to support exception handling of
pharmacy claims for Medicare Part B claim billing.
Notes on Narrative Segment on a Claim Billing or Encounter Request:
The Narrative Segment is situational for Claim Billing or Encounter request. It is used to support exception handling of pharmacy claims for
Medicare claim billing. Fields defined as Mandatory are required to be submitted when the segment is sent.
7.5 CLAIM BILLING OR ENCOUNTER RESPONSE DIAGRAMS AND SEGMENTS
7.5.1 TRANSMISSION ACCEPTED/TRANSACTION PAID
7.5.1.1 DIAGRAM FOR TRANSMISSION OF ONE CLAIM BILLING OR ENCOUNTER RESPONSE
(TRANSMISSION ACCEPTED/TRANSACTION PAID)
Claim Billing or Encounter transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid)
The Paid or Duplicate of Paid response is not used in payer-to-payer transactions.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
7.5.1.2 DIAGRAM FOR TRANSMISSION OF TWO CLAIM BILLING OR ENCOUNTER RESPONSES
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 83 -
(TRANSMISSION ACCEPTED/TRANSACTION PAID)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
7.5.1.3 DIAGRAM FOR TRANSMISSION OF THREE CLAIM BILLING OR ENCOUNTER RESPONSES
(TRANSMISSION ACCEPTED/TRANSACTION PAID)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory second response
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 84 -
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
7.5.1.4 DIAGRAM FOR TRANSMISSION OF FOUR CLAIM BILLING OR ENCOUNTER RESPONSES
(TRANSMISSION ACCEPTED/TRANSACTION PAID)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 85 -
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
7.5.1.5 CLAIM BILLING OR ENCOUNTER RESPONSE SEGMENTS (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
7.5.1.5.1 RESPONSE HEADER SEGMENT (CLAIM BILLING OR ENCOUNTER) (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M For Transaction Code of “B1”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “1” (Rx Billing).
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Claim Billing or Encounter Response:
The Response Header Segment is a mandatory, fixed length segment for Claim Billing or Encounter response when the Header Response
Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid). The “Situation” column
is not applicable.
7.5.1.5.2 RESPONSE MESSAGE SEGMENT (CLAIM BILLING OR ENCOUNTER) (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Claim Billing/Encounter:
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 86 -
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Claim Billing or Encounter Response:
The Response Message Segment is situational for Claim Billing or Encounter response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid). It is used when additional text information
needs to be sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
7.5.1.5.3 RESPONSE INSURANCE SEGMENT (CLAIM BILLING OR ENCOUNTER) (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø1-C1 GROUP ID
Q Claim Billing/Encounter:
Required if needed to identify the actual cardholder or
employer group, to identify appropriate group number,
when available.
Required to identify the actual group that was used when
multiple group coverages exist.
Note: This field may contain the Group ID echoed from the
request. May contain the actual Group ID if unknown to the
receiver.
524-FO PLAN ID
Q Claim Billing/Encounter:
Required if needed to identify the actual plan parameters,
benefit, or coverage criteria, when available.
Required to identify the actual plan ID that was used when
multiple group coverages exist.
Required if needed to contain the actual plan ID if unknown
to the receiver.
545-2F NETWORK REIMBURSEMENT ID
Q Claim Billing/Encounter:
Required if needed to identify the network for the covered
member.
Required if needed to identify the actual Network
Reimbursement ID, when applicable and/or available.
Required to identify the actual Network Reimbursement ID
that was used when multiple Network Reimbursement IDs
exist.
568-J7 PAYER ID QUALIFIER Q Claim Billing/Encounter:
Required if Payer ID (569-J8) is used.
569-J8 PAYER ID
Q Claim Billing/Encounter:
Required to identify the ID of the payer responding.
115-N5
MEDICAID ID NUMBER N Claim Billing/Encounter:
Not used.
116-N6
MEDICAID AGENCY NUMBER N Claim Billing/Encounter:
Not used.
3Ø2-C2
CARDHOLDER ID Q Claim Billing/Encounter:
Required if the identification to be used in future
transactions is different than what was submitted on the
request.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 87 -
Notes on Response Insurance Segment on a Claim Billing or Encounter Response:
The Response Insurance Segment is situational for Claim Billing or Encounter response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid). It is used when coverage or reimbursement
parameters or identifiers need to be sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
7.5.1.5.3.1 Response Insurance Segment (Medicaid Subrogation Claim Billing or Encounter)
(Transmission Accepted/Transaction Paid)
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
115-N5
MEDICAID ID NUMBER QM Medicaid Subrogation - Claim Billing/Encounter:
Required to identify the member as uniquely known to
Medicaid.
116-N6
MEDICAID AGENCY NUMBER QM Medicaid Subrogation - Claim Billing/Encounter:
Required to identify the Medicaid agency.
See Medicaid Subrogation Implementation Guide.
Notes on Response Insurance Segment on a Medicaid Subrogation Claim Billing or Encounter Response:
The rules above for a “Response Insurance Segment (Claim Billing or Encounter)” are to be followed for Medicaid Subrogation.
Specific fields that are used differently in Medicaid Subrogation are noted in the table above.
7.5.1.5.4 RESPONSE PATIENT SEGMENT (CLAIM BILLING OR ENCOUNTER) (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
RESPONSE PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
31Ø-CA PATIENT FIRST NAME Q Claim Billing/Encounter:
Required if known.
311-CB PATIENT LAST NAME Q Claim Billing/Encounter:
Required if known.
3Ø4-C4 DATE OF BIRTH Q Claim Billing/Encounter:
Required if known.
Notes on Response Patient Segment on a Claim Billing/Encounter Response:
The Response Patient Segment is situational for Claim Billing or Encounter transmission response Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid) when patient demographic information needs to
be sent from the sender to the receiver. Fields defined as Mandatory are required to be submitted when the segment is sent.
7.5.1.5.5 RESPONSE STATUS SEGMENT (CLAIM BILLING OR ENCOUNTER) (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Claim Billing/Encounter:
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT N Claim Billing/Encounter:
Not used.
511-FB REJECT CODE N***R*** Claim Billing/Encounter:
Not used.
546-4F REJECT FIELD OCCURRENCE INDICATOR N***R*** Claim Billing/Encounter:
Not used.
547-5F APPROVED MESSAGE CODE COUNT Q Claim Billing/Encounter:
Maximum count of 5.
Required if Approved Message Code (548-6F) is used.
548-6F APPROVED MESSAGE CODE Q***R*** Claim Billing/Encounter:
Required if Approved Message Code Count (547-5F) is
used and the sender needs to communicate additional
follow up for a potential opportunity.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Claim Billing/Encounter:
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 88 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Claim Billing/Encounter:
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Claim Billing/Encounter:
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Claim Billing/Encounter:
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Claim Billing/Encounter:
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Claim Billing/Encounter:
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Claim Billing/Encounter:
Not used.
993-A7 INTERNAL CONTROL NUMBER Q Claim Billing/Encounter:
Required when used for payer-to-payer coordination of
benefits to track the claim without regard to the “Service
Provider ID, Prescription Number, & Date of Service”.
987-MA URL N Claim Billing/Encounter:
Not used.
Notes on Response Status Segment on a Claim Billing or Encounter Response:
The Response Status Segment is mandatory for a Claim Billing or Encounter response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid). The Response Status Segment is sent from the
sender to the receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is
sent.
7.5.1.5.6 RESPONSE CLAIM SEGMENT (CLAIM BILLING OR ENCOUNTER) (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M For Transaction Code of “B1”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “1” (Rx Billing).
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT Q Claim Billing/Encounter:
Maximum count of 6.
Required if Preferred Product ID (553-AR) is used.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 89 -
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
552-AP PREFERRED PRODUCT ID QUALIFIER Q***R*** Claim Billing/Encounter:
Required if Preferred Product ID (553-AR) is used.
553-AR PREFERRED PRODUCT ID Q***R*** Claim Billing/Encounter:
Required if a product preference exists that needs to be
communicated to the receiver via an ID.
554-AS PREFERRED PRODUCT INCENTIVE Q***R*** Claim Billing/Encounter:
Required if there is a known incentive amount associated
with the Preferred Product ID (553-AR) and/or Preferred
Product Description (556-AU).
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE Q***R*** Claim Billing/Encounter:
Required if there is a known patient financial responsibility
incentive amount associated with the Preferred Product ID
(553-AR) and/or Preferred Product Description (556-AU).
556-AU PREFERRED PRODUCT DESCRIPTION Q***R*** Claim Billing/Encounter:
Required if a product preference exists that either cannot
be communicated by the Preferred Product ID (553-AR) or
to clarify the Preferred Product ID (553-AR).
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Claim Billing/Encounter:
Not used.
Notes on Response Claim Segment on a Claim Billing or Encounter Response:
The Response Claim Segment is mandatory for a Claim Billing or Encounter response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid). The Response Claim Segment is sent from the
sender to the receiver to identify therapeutic or alternate product recommendations. Fields defined as Mandatory are required to be submitted
when the segment is sent.
7.5.1.5.6.1 Response Claim Segment (Medicaid Subrogation Claim Billing or Encounter) (Transmission
Accepted/Transaction Paid)
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
QM Medicaid Subrogation - Claim Billing/Encounter:
Required to report back on the response the claim
number assigned by the Medicaid Agency.
Notes on Response Claim Segment on a Medicaid Subrogation Claim Billing or Encounter Response:
The rules above for a “Response Claim Segment (Claim Billing or Encounter)” are to be followed for Medicaid Subrogation. Specific
fields that are used differently in Medicaid Subrogation are noted in the table above.
7.5.1.5.7 RESPONSE PRICING SEGMENT (CLAIM BILLING OR ENCOUNTER) (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
RESPONSE PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø5-F5 PATIENT PAY AMOUNT R Claim Billing/Encounter:
Required.
5Ø6-F6 INGREDIENT COST PAID Q Claim Billing/Encounter:
Required if this value is used to arrive at the final
reimbursement.
5Ø7-F7 DISPENSING FEE PAID Q Claim Billing/Encounter:
Required if this value is used to arrive at the final
reimbursement.
557-AV TAX EXEMPT INDICATOR Q Claim Billing/Encounter:
Required if the sender (health plan) and/or patient is tax
exempt and exemption applies to this billing.
558-AW FLAT SALES TAX AMOUNT PAID Q Claim Billing/Encounter:
Required if Flat Sales Tax Amount Submitted (481-HA) is
greater than zero (Ø) or if Flat Sales Tax Amount Paid
(558-AW) is used to arrive at the final reimbursement.
Zero (Ø) is a valid value.
559-AX PERCENTAGE SALES TAX AMOUNT PAID Q Claim Billing/Encounter:
Required if this value is used to arrive at the final
reimbursement.
Required if Percentage Sales Tax Amount Submitted (482-
GE) is greater than zero (Ø).

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 90 -
RESPONSE PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Zero (Ø) is a valid value.
Required if Percentage Sales Tax Rate Paid (56Ø-AY) and
Percentage Sales Tax Basis Paid (561-AZ) are used.
56Ø-AY PERCENTAGE SALES TAX RATE PAID Q Claim Billing/Encounter:
Required if Percentage Sales Tax Amount Paid (559-AX) is
greater than zero (Ø).
561-AZ PERCENTAGE SALES TAX BASIS PAID Q Claim Billing/Encounter:
Required if Percentage Sales Tax Amount Paid (559-AX) is
greater than zero (Ø).
521-FL INCENTIVE AMOUNT PAID
Q Claim Billing/Encounter:
Required if this value is used to arrive at the final
reimbursement.
Required if Incentive Amount Submitted (438-E3) is greater
than zero (Ø).
Zero (Ø) is a valid value.
562-J1 PROFESSIONAL SERVICE FEE PAID N Claim Billing/Encounter:
Not used.
563-J2 OTHER AMOUNT PAID COUNT Q Claim Billing/Encounter:
Maximum count of 3.
Required if Other Amount Paid (565-J4) is used.
564-J3 OTHER AMOUNT PAID QUALIFIER Q***R*** Claim Billing/Encounter:
Required if Other Amount Paid (565-J4) is used.
565-J4 OTHER AMOUNT PAID
Q***R*** Claim Billing/Encounter:
Required if this value is used to arrive at the final
reimbursement.
Required if Other Amount Claimed Submitted (48Ø-H9) is
greater than zero (Ø).
Zero (Ø) is a valid value.
Must respond to each occurrence submitted.
566-J5 OTHER PAYER AMOUNT RECOGNIZED Q Claim Billing/Encounter:
Required if this value is used to arrive at the final
reimbursement.
Required if Other Payer Amount Paid (431-DV) is greater
than zero (Ø) and Coordination of Benefits/Other Payments
Segment is supported.
5Ø9-F9 TOTAL AMOUNT PAID R Claim Billing/Encounter:
Required. Zero (Ø) value is valid.
See Pricing Formula for fields used in calculation.
522-FM BASIS OF REIMBURSEMENT DETERMINATION Q Claim Billing/Encounter:
Required if Ingredient Cost Paid (5Ø6-F6) is greater than
zero (Ø).
Required if Basis of Cost Determination (432-DN) is
submitted on billing.
523-FN AMOUNT ATTRIBUTED TO SALES TAX Q Claim Billing/Encounter:
Required if Patient Pay Amount (5Ø5-F5) includes sales tax
that is the financial responsibility of the member but is not
also included in any of the other fields that add up to
Patient Pay Amount.
See section “Specific Segment Discussion”, “Response
Segments”, “Response Pricing Segment” for guidance.
512-FC ACCUMULATED DEDUCTIBLE AMOUNT I Claim Billing/Encounter:
Provided for informational purposes only.
513-FD REMAINING DEDUCTIBLE AMOUNT I Claim Billing/Encounter:
Provided for informational purposes only.
514-FE REMAINING BENEFIT AMOUNT I Claim Billing/Encounter:
The Remaining Benefit Amount must not be returned with
zeroes unless there are no benefit dollars remaining. The
default value of 999999999 from previous versions must
not be used as a default in this field.
Provided for informational purposes only.
517-FH AMOUNT APPLIED TO PERIODIC DEDUCTIBLE Q Claim Billing/Encounter:
Required if Patient Pay Amount (5Ø5-F5) includes

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 91 -
RESPONSE PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
deductible.
518-FI AMOUNT OF COPAY Q Claim Billing/Encounter:
Required if Patient Pay Amount (5Ø5-F5) includes copay
as patient financial responsibility.
52Ø-FK AMOUNT EXCEEDING PERIODIC BENEFIT MAXIMUM Q Claim Billing/Encounter:
Required if Patient Pay Amount (5Ø5-F5) includes amount
exceeding periodic benefit maximum.
346-HH BASIS OF CALCULATION—DISPENSING FEE Q Claim Billing/Encounter:
Required if Dispensing Status (343-HD) on submission is
“P” (Partial Fill) or “C” (Completion of Partial Fill).
347-HJ BASIS OF CALCULATION—COPAY Q Claim Billing/Encounter:
Required if Dispensing Status (343-HD) on submission is
“P” (Partial Fill) or “C” (Completion of Partial Fill).
348-HK BASIS OF CALCULATION—FLAT SALES TAX Q Claim Billing/Encounter:
Required if Dispensing Status (343-HD) on submission is
“P” (Partial Fill) or “C” (Completion of Partial Fill) and Flat
Sales Tax Amount Paid (558-AW) is greater than zero (Ø).
349-HM BASIS OF CALCULATION—PERCENTAGE SALES TAX Q Claim Billing/Encounter:
Required if Dispensing Status (343-HD) on submission is
“P” (Partial Fill) or “C” (Completion of Partial Fill) and
Percentage Sales Tax Amount Paid (559-AX) is greater
than zero (Ø).
571-NZ AMOUNT ATTRIBUTED TO PROCESSOR FEE Q Claim Billing/Encounter:
Required if the customer is responsible for 1ØØ% of the
prescription payment and when the provider net sale is less
than the amount the customer is expected to pay.
575-EQ PATIENT SALES TAX AMOUNT I Claim Billing/Encounter:
Used when necessary to identify the Patient’s portion of the
Sales Tax.
Provided for informational purposes only.
574-2Y PLAN SALES TAX AMOUNT I Claim Billing/Encounter:
Used when necessary to identify the Plan’s portion of the
Sales Tax.
Provided for informational purposes only.
572-4U AMOUNT OF COINSURANCE Q Claim Billing/Encounter:
Required if Patient Pay Amount (5Ø5-F5) includes
coinsurance as patient financial responsibility.
573-4V BASIS OF CALCULATION-COINSURANCE Q Claim Billing/Encounter:
Required if Dispensing Status (343-HD) on submission is
“P” (Partial Fill) or “C” (Completion of Partial Fill).
392-MU BENEFIT STAGE COUNT Q Claim Billing/Encounter:
Maximum count of 4.
Required if Benefit Stage Amount (394-MW) is used.
393-MV BENEFIT STAGE QUALIFIER Q***R*** Claim Billing/Encounter:
Required if Benefit Stage Amount (394-MW) is used.
Must only have one value per iteration - value must not be
repeated.
394-MW BENEFIT STAGE AMOUNT Q***R*** Claim Billing/Encounter:
Required when a Medicare Part D payer applies financial
amounts to Medicare Part D beneficiary benefit stages.
This field is required when the plan is a participant in a
Medicare Part D program that requires reporting of benefit
stage specific financial amounts.
Required if necessary for state/federal/regulatory agency
programs.
577-G3 ESTIMATED GENERIC SAVINGS I Claim Billing/Encounter:
This information should be provided when a patient
selected the brand drug and a generic form of the drug was
available. It will contain an estimate of the difference
between the cost of the brand drug and the generic drug,
when the brand drug is more expensive than the generic.
It is information that the provider should provide to the
patient.
128-UC SPENDING ACCOUNT AMOUNT REMAINING I Claim Billing/Encounter:
This dollar amount will be provided, if known, to the
receiver when the transaction had spending account dollars
reported as part of the patient pay amount.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 92 -
RESPONSE PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
This field is informational only. It is reported back to the
provider and the patient the amount remaining on the
spending account after the current claim updated the
spending account.
129-UD HEALTH PLAN-FUNDED ASSISTANCE AMOUNT Q Claim Billing/Encounter:
Required when the patient meets the plan-funded
assistance criteria, to reduce Patient Pay Amount (5Ø5-
F5). The resulting Patient Pay Amount (5Ø5-F5) must be
greater than or equal to zero.
This field is always a negative amount or zero.
133-UJ AMOUNT ATTRIBUTED TO PROVIDER NETWORK SELECTION Q Claim Billing/Encounter:
Required if Patient Pay Amount (5Ø5-F5) includes an
amount that is attributable to a cost share differential due to
the selection of one pharmacy over another.
134-UK AMOUNT ATTRIBUTED TO PRODUCT SELECTION/BRAND
DRUG
Q Claim Billing/Encounter:
Required if Patient Pay Amount (5Ø5-F5) includes an
amount that is attributable to a patient’s selection of a
Brand drug.
135-UM AMOUNT ATTRIBUTED TO PRODUCT SELECTION/NON-
PREFERRED FORMULARY SELECTION
Q Claim Billing/Encounter:
Required if Patient Pay Amount (5Ø5-F5) includes an
amount that is attributable to a patient’s selection of a non-
preferred formulary product.
136-UN AMOUNT ATTRIBUTED TO PRODUCT SELECTION/BRAND
NON-PREFERRED FORMULARY SELECTION
Q Claim Billing/Encounter:
Required if Patient Pay Amount (5Ø5-F5) includes an
amount that is attributable to a patient’s selection of a
Brand non-preferred formulary product.
137-UP AMOUNT ATTRIBUTED TO COVERAGE GAP Q Claim Billing/Encounter:
Required when the patient’s financial responsibility is due to
the coverage gap.
148-U8 INGREDIENT COST CONTRACTED/REIMBURSABLE AMOUNT I Claim Billing/Encounter:
Required when Basis of Reimbursement Determination
(522-FM) is “14” (Patient Responsibility Amount) or “15”
(Patient Pay Amount) unless prohibited by
state/federal/regulatory agency.
This field is informational only.
149-U9 DISPENSING FEE CONTRACTED/REIMBURSABLE AMOUNT I Claim Billing/Encounter:
Required when Basis of Reimbursement Determination
(522-FM) is “14” (Patient Responsibility Amount) or “15”
(Patient Pay Amount) unless prohibited by
state/federal/regulatory agency.
This field is informational only.
Notes on Response Pricing Segment on a Claim Billing or Encounter Response:
The Response Pricing Segment is mandatory for a Claim Billing or Encounter Response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) is “P” (Paid) or “D” (Duplicate of Paid).
It is highly recommended that whenever possible, the individual dollar fields are to be returned in the response. On the response, the sender
should return the individual payment response fields to allow the receiver to reconcile against the requested payment fields.
See section “Response Processing Guidelines”, “Pricing Guidelines”.
Fields defined as Mandatory are required to be submitted when the segment is sent.
7.5.1.5.8 RESPONSE DUR/PPS SEGMENT (CLAIM BILLING OR ENCOUNTER) (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
RESPONSE DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
567-J6 DUR/PPS RESPONSE CODE COUNTER Q***R*** Claim Billing/Encounter:
Maximum 9 occurrences supported.
Required if Reason For Service Code (439-E4) is used.
439-E4 REASON FOR SERVICE CODE Q***R*** Claim Billing/Encounter:
Required if utilization conflict is detected.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 93 -
RESPONSE DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
528-FS CLINICAL SIGNIFICANCE CODE Q***R*** Claim Billing/Encounter:
Required if needed to supply additional information for the
utilization conflict.
529-FT OTHER PHARMACY INDICATOR Q***R*** Claim Billing/Encounter:
Required if needed to supply additional information for the
utilization conflict.
53Ø-FU PREVIOUS DATE OF FILL Q***R*** Claim Billing/Encounter:
Required if needed to supply additional information for the
utilization conflict.
Required if Quantity of Previous Fill (531-FV) is used.
531-FV QUANTITY OF PREVIOUS FILL Q***R*** Claim Billing/Encounter:
Required if needed to supply additional information for the
utilization conflict.
Required if Previous Date Of Fill (53Ø-FU) is used.
532-FW DATABASE INDICATOR Q***R*** Claim Billing/Encounter:
Required if needed to supply additional information for the
utilization conflict.
533-FX OTHER PRESCRIBER INDICATOR Q***R*** Claim Billing/Encounter:
Required if needed to supply additional information for the
utilization conflict.
544-FY DUR FREE TEXT MESSAGE Q***R*** Claim Billing/Encounter:
Required if needed to supply additional information for the
utilization conflict.
Notes on Response DUR/PPS Segment on a Claim Billing or Encounter Response:
The Response DUR/PPS Segment is situational for a Claim Billing or Encounter Response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid). The segment is used to identify a drug
utilization review or professional pharmacy service event, opportunity, or information. Fields defined as Mandatory are required to be submitted
when the segment is sent.
7.5.1.5.9 RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS SEGMENT (CLAIM BILLING OR ENCOUNTER)
(TRANSMISSION ACCEPTED/TRANSACTION PAID)
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS
SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
355-NT OTHER PAYER ID COUNT M Claim Billing/Encounter:
Maximum count of 3.
338-5C OTHER PAYER COVERAGE TYPE M***R***
339-6C OTHER PAYER ID QUALIFIER Q***R*** Claim Billing/Encounter:
Required if Other Payer ID (34Ø-7C) is used.
34Ø-7C OTHER PAYER ID Q***R*** Claim Billing/Encounter:
Required if other insurance information is available for
coordination of benefits.
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER Q***R*** Claim Billing/Encounter:
Required if other insurance information is available for
coordination of benefits.
356-NU OTHER PAYER CARDHOLDER ID Q***R*** Claim Billing/Encounter:
Required if other insurance information is available for
coordination of benefits.
992-MJ OTHER PAYER GROUP ID Q***R*** Claim Billing/Encounter:
Required if other insurance information is available for
coordination of benefits.
142-UV OTHER PAYER PERSON CODE Q***R*** Claim Billing/Encounter:
Required if needed to uniquely identify the family members
within the Cardholder ID, as assigned by the other payer.
127-UB OTHER PAYER HELP DESK PHONE NUMBER Q***R*** Claim Billing/Encounter:
Required if needed to provide a support telephone number
of the other payer to the receiver.
143-UW OTHER PAYER PATIENT RELATIONSHIP CODE Q***R*** Claim Billing/Encounter:
Required if needed to uniquely identify the relationship of
the patient to the cardholder ID, as assigned by the other
payer.
144-UX OTHER PAYER BENEFIT EFFECTIVE DATE Q***R*** Claim Billing/Encounter:
Required when other coverage is known which is after the
Date of Service submitted.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 94 -
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS
SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
145-UY OTHER PAYER BENEFIT TERMINATION DATE Q***R*** Claim Billing/Encounter:
Required when other coverage is known which is after the
Date of Service submitted.
Notes on Response Coordination of Benefits/Other Payers Segment on a Claim Billing or Encounter Response:
The Response Coordination of Benefits/Other Payers Segment is situational for a Claim Billing or Encounter response when the Header
Response Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid) when other
insurance information is available for coordination of benefits.
If subsequent payer(s) for this patient is not known, the Other Payer information is not sent.
If subsequent payer(s) for this patient is known, the following may be sent:
• Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C),
• Other Payer Group ID (992-MJ),
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU)
• And other Other Payer fields.
In addition, if any of the following three fields are sent:
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU),
• Other Payer Group ID (992-MJ),
then the Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C) must be sent.
Fields defined as Mandatory are required to be submitted when the segment is sent.
7.5.2 TRANSMISSION ACCEPTED/TRANSACTION CAPTURED
7.5.2.1 DIAGRAM FOR TRANSMISSION OF ONE CLAIM BILLING OR ENCOUNTER RESPONSE
(TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
Claim Billing or Encounter transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured)
The Response Pricing Segment and Response DUR/PPS Segments are not used in payer-to-payer transactions.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Pricing Segment
7.5.2.2 DIAGRAM FOR TRANSMISSION OF TWO CLAIM BILLING OR ENCOUNTER RESPONSES
(TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 95 -
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Pricing Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Pricing Segment
7.5.2.3 DIAGRAM FOR TRANSMISSION OF THREE CLAIM BILLING OR ENCOUNTER RESPONSES
(TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Pricing Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 96 -
Response DUR/PPS Segment
Segment Separator
Response Pricing Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Pricing Segment
7.5.2.4 DIAGRAM FOR TRANSMISSION OF FOUR CLAIM BILLING OR ENCOUNTER RESPONSES
(TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Pricing Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Pricing Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Pricing Segment
Mandatory fourth response
Group Separator
Segment Separator
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 97 -
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Pricing Segment
7.5.2.5 CLAIM BILLING OR ENCOUNTER RESPONSE SEGMENTS (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
7.5.2.5.1 RESPONSE HEADER SEGMENT (CLAIM BILLING OR ENCOUNTER) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M For Transaction Code of “B1”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “1” (Rx Billing).
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Claim Billing or Encounter Response:
The Response Header Segment is a mandatory, fixed length segment for Claim Billing or Encounter response when the Header Response
Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). The
“Situation” column is not applicable.
7.5.2.5.2 RESPONSE MESSAGE SEGMENT (CLAIM BILLING OR ENCOUNTER) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Claim Billing/Encounter:
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Claim Billing or Encounter Response:
The Response Message Segment is situational for Claim Billing or Encounter response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). It is used when additional text
information needs to be sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
7.5.2.5.3 RESPONSE INSURANCE SEGMENT (CLAIM BILLING OR ENCOUNTER) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 98 -
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø1-C1 GROUP ID
Q Claim Billing/Encounter:
Required if needed to identify the actual cardholder or
employer group, to identify appropriate group number,
when available.
Required to identify the actual group that was used when
multiple group coverages exist.
Note: This field may contain the Group ID echoed from the
request. May contain the actual Group ID if unknown to the
receiver.
524-FO PLAN ID
Q Claim Billing/Encounter:
Required if needed to identify the actual plan parameters,
benefit, or coverage criteria, when available.
Required to identify the actual plan ID that was used when
multiple group coverages exist.
Required if needed to contain the actual plan ID if unknown
to the receiver.
545-2F NETWORK REIMBURSEMENT ID
N Claim Billing/Encounter:
Not used.
568-J7 PAYER ID QUALIFIER N Claim Billing/Encounter:
Not used.
569-J8 PAYER ID
N Claim Billing/Encounter:
Not used.
115-N5
MEDICAID ID NUMBER N Claim Billing/Encounter:
Not used.
116-N6
MEDICAID AGENCY NUMBER N Claim Billing/Encounter:
Not used.
3Ø2-C2
CARDHOLDER ID Q Claim Billing/Encounter:
Required if the identification to be used in future
transactions is different than what was submitted on the
request.
Notes on Response Insurance Segment on a Claim Billing or Encounter Response:
The Response Insurance Segment is situational for Claim Billing or Encounter response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). It is used when coverage information
may be sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
7.5.2.5.3.1 Response Insurance Segment (Medicaid Subrogation Claim Billing or Encounter)
(Transmission Accepted/Transaction Captured)
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
115-N5
MEDICAID ID NUMBER QM Medicaid Subrogation - Claim Billing/Encounter:
Required to identify the member as uniquely known to
Medicaid.
116-N6
MEDICAID AGENCY NUMBER QM Medicaid Subrogation - Claim Billing/Encounter:
Required to identify the Medicaid agency.
See Medicaid Subrogation Implementation Guide.
Notes on Response Insurance Segment on a Medicaid Subrogation Claim Billing or Encounter Response:
The rules above for a “Response Insurance Segment (Claim Billing or Encounter)” are to be followed for Medicaid Subrogation.
Specific fields that are used differently in Medicaid Subrogation are noted in the table above.
7.5.2.5.4 RESPONSE PATIENT SEGMENT (CLAIM BILLING OR ENCOUNTER) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
RESPONSE PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
31Ø-CA PATIENT FIRST NAME Q Claim Billing/Encounter:
Required if known.
311-CB PATIENT LAST NAME Q Claim Billing/Encounter:
Required if known.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 99 -
RESPONSE PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
3Ø4-C4 DATE OF BIRTH Q Claim Billing/Encounter:
Required if known.
Notes on Response Patient Segment on a Claim Billing/Encounter Response:
The Response Patient Segment is situational for Claim Billing or Encounter transmission response Claim Billing or Encounter response when
the Header Response Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of
Captured) when patient demographic information needs to be sent from the sender to the receiver. Fields defined as Mandatory are required
to be submitted when the segment is sent.
7.5.2.5.5 RESPONSE STATUS SEGMENT (CLAIM BILLING OR ENCOUNTER) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Claim Billing/Encounter:
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT N Claim Billing/Encounter:
Not used.
511-FB REJECT CODE N***R*** Claim Billing/Encounter:
Not used.
546-4F REJECT FIELD OCCURRENCE INDICATOR N***R*** Claim Billing/Encounter:
Not used.
547-5F APPROVED MESSAGE CODE COUNT N Claim Billing/Encounter:
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Claim Billing/Encounter:
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Claim Billing/Encounter:
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Claim Billing/Encounter:
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Claim Billing/Encounter:
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Claim Billing/Encounter:
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Claim Billing/Encounter:
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER Q Claim Billing/Encounter:
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 100 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Claim Billing/Encounter:
Not used.
993-A7 INTERNAL CONTROL NUMBER Q Claim Billing/Encounter:
Required when used for payer-to-payer coordination of
benefits to track the claim without regard to the “Service
Provider ID, Prescription Number, & Date of Service”.
987-MA URL N Claim Billing/Encounter:
Not used.
Notes on Response Status Segment on a Claim Billing or Encounter Response:
The Response Status Segment is mandatory for a Claim Billing or Encounter response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). The Response Status Segment is
sent from the sender to the receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the
segment is sent.
7.5.2.5.6 RESPONSE CLAIM SEGMENT (CLAIM BILLING OR ENCOUNTER) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M For Transaction Code of “B1”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “1” (Rx Billing).
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT Q Claim Billing/Encounter:
Maximum count of 6.
Required if Preferred Product ID (553-AR) is used.
Not used in payer-to-payer transactions.
552-AP PREFERRED PRODUCT ID QUALIFIER Q***R*** Claim Billing/Encounter:
Required if Preferred Product ID (553-AR) is used.
Not used in payer-to-payer transactions.
553-AR PREFERRED PRODUCT ID Q***R*** Claim Billing/Encounter:
Required if a product preference exists that needs to be
communicated to the receiver via an ID.
Not used in payer-to-payer transactions.
554-AS PREFERRED PRODUCT INCENTIVE Q***R*** Claim Billing/Encounter:
Required if there is a known incentive amount associated
with the Preferred Product ID (553-AR) and/or Preferred
Product Description (556-AU).
Not used in payer-to-payer transactions.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE Q***R*** Claim Billing/Encounter:
Required if there is a known patient financial responsibility
incentive amount associated with the Preferred Product ID
(553-AR) and/or Preferred Product Description (556-AU).
Not used in payer-to-payer transactions.
556-AU PREFERRED PRODUCT DESCRIPTION Q***R*** Claim Billing/Encounter:
Required if a product preference exists that either cannot
be communicated by the Preferred Product ID (553-AR) or
to clarify the Preferred Product ID (553-AR).
Not used in payer-to-payer transactions.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Claim Billing/Encounter:
Not used.
Notes on Response Claim Segment on a Claim Billing or Encounter Response:
The Response Claim Segment is mandatory for a Claim Billing or Encounter response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). The Response Claim Segment is

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 101 -
sent from the sender to the receiver to identify therapeutic or alternate product recommendations. Fields defined as Mandatory are required to
be submitted when the segment is sent.
7.5.2.5.7 RESPONSE PRICING SEGMENT (CLAIM BILLING OR ENCOUNTER) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
RESPONSE PRICING SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø5-F5 PATIENT PAY AMOUNT Q Claim Billing/Encounter:
Required if known. This field cannot be an estimated
amount. Zero is a valid amount.
5Ø6-F6 INGREDIENT COST PAID Q Claim Billing/Encounter:
Required if this value is used to arrive at the estimated
reimbursement. If reimbursement is not estimated, this field
contains the submitted value.
5Ø7-F7 DISPENSING FEE PAID Q Claim Billing/Encounter:
Required if this value is used to arrive at the estimated
reimbursement. If reimbursement is not estimated, this field
contains the submitted value.
557-AV TAX EXEMPT INDICATOR Q Claim Billing/Encounter:
Required if the sender (health plan) and/or patient is tax
exempt and exemption applies to this billing.
558-AW FLAT SALES TAX AMOUNT PAID Q Claim Billing/Encounter:
Required if Flat Sales Tax Amount Submitted (481-HA) is
greater than zero (Ø) or if Flat Sales Tax Amount Paid
(558-AW) is used to arrive at the estimated reimbursement.
Zero (Ø) is a valid value.
If reimbursement is not estimated, this field contains the
submitted value.
559-AX PERCENTAGE SALES TAX AMOUNT PAID Q Claim Billing/Encounter:
Required if this value is used to arrive at the estimated
reimbursement. If reimbursement is not estimated, this field
contains the submitted value.
Required if Percentage Sales Tax Amount Submitted (482-
GE) is greater than zero (Ø).
Zero (Ø) is a valid value.
Required if Percentage Sales Tax Rate Paid (56Ø-AY) and
Percentage Sales Tax Basis Paid (561-AZ) are used.
56Ø-AY PERCENTAGE SALES TAX RATE PAID S Claim Billing/Encounter:
Required if Percentage Sales Tax Amount Paid (559-AX) is
greater than zero (Ø).
561-AZ PERCENTAGE SALES TAX BASIS PAID S Claim Billing/Encounter:
Required if Percentage Sales Tax Amount Paid (559-AX) is
greater than zero (Ø).
521-FL INCENTIVE AMOUNT PAID
Q Claim Billing/Encounter:
Required if this value is used to arrive at the estimated
reimbursement. If reimbursement is not estimated, this field
contains the submitted value.
Required if Incentive Amount Submitted (438-E3) is greater
than zero (Ø).
Zero (Ø) is a valid value.
562-J1 PROFESSIONAL SERVICE FEE PAID N Claim Billing/Encounter:
Not used.
563-J2 OTHER AMOUNT PAID COUNT Q Claim Billing/Encounter:
Maximum count of 3.
Required if Other Amount Paid (565-J4) is used.
564-J3 OTHER AMOUNT PAID QUALIFIER Q***R*** Claim Billing/Encounter:
Required if Other Amount Paid (565-J4) is used.
565-J4 OTHER AMOUNT PAID
Q***R*** Claim Billing/Encounter:
Required if this value is used to arrive at the estimated
reimbursement. If reimbursement is not estimated, this field
contains the submitted value.
Required if Other Amount Claimed Submitted (48Ø-H9) is
greater than zero (Ø).
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 102 -
RESPONSE PRICING SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Zero (Ø) is a valid value.
Must respond to each occurrence submitted.
566-J5 OTHER PAYER AMOUNT RECOGNIZED Q Claim Billing/Encounter:
Required if this value is used to arrive at the estimated
reimbursement. If reimbursement is not estimated, this field
contains the submitted value.
Required if Other Payer Amount Paid (431-DV) is greater
than zero (Ø) and Coordination of Benefits/Other Payments
Segment is supported.
5Ø9-F9 TOTAL AMOUNT PAID R Claim Billing/Encounter:
Required. Zero (Ø) value is valid.
See Pricing Formula for fields used in calculation.
522-FM BASIS OF REIMBURSEMENT DETERMINATION Q Claim Billing/Encounter:
Required if Ingredient Cost Paid (5Ø6-F6) is greater than
zero (Ø).
Required if Basis of Cost Determination (432-DN) is
submitted on billing.
523-FN AMOUNT ATTRIBUTED TO SALES TAX Q Claim Billing/Encounter:
Required if Patient Pay Amount (5Ø5-F5) includes sales tax
that is the financial responsibility of the member but is not
also included in any of the other fields that add up to
Patient Pay Amount.
See section “Specific Segment Discussion”, “Response
Segments”, “Response Pricing Segment” for guidance.
512-FC ACCUMULATED DEDUCTIBLE AMOUNT I Claim Billing/Encounter:
Provided for informational purposes only.
513-FD REMAINING DEDUCTIBLE AMOUNT I Claim Billing/Encounter:
Provided for informational purposes only.
514-FE REMAINING BENEFIT AMOUNT I Claim Billing/Encounter:
The Remaining Benefit Amount must not be returned with
zeroes unless there are no benefit dollars remaining. The
default value of 999999999 from previous versions must
not be used as a default in this field.
Provided for informational purposes only.
517-FH AMOUNT APPLIED TO PERIODIC DEDUCTIBLE Q Claim Billing/Encounter:
Required if Patient Pay Amount (5Ø5-F5) includes
deductible.
518-FI AMOUNT OF COPAY Q Claim Billing/Encounter:
Required if Patient Pay Amount (5Ø5-F5) includes copay
as patient financial responsibility.
52Ø-FK AMOUNT EXCEEDING PERIODIC BENEFIT MAXIMUM Q Claim Billing/Encounter:
Required if Patient Pay Amount (5Ø5-F5) includes amount
exceeding periodic benefit maximum.
346-HH BASIS OF CALCULATION—DISPENSING FEE Q Claim Billing/Encounter:
Required if Dispensing Status (343-HD) on submission is
“P” (Partial Fill) or “C” (Completion of Partial Fill).
347-HJ BASIS OF CALCULATION—COPAY Q Claim Billing/Encounter:
Required if Dispensing Status (343-HD) on submission is
“P” (Partial Fill) or “C” (Completion of Partial Fill).
348-HK BASIS OF CALCULATION—FLAT SALES TAX Q Claim Billing/Encounter:
Required if Dispensing Status (343-HD) on submission is
“P” (Partial Fill) or “C” (Completion of Partial Fill) and Flat
Sales Tax Amount Paid (558-AW) is greater than zero (Ø).
349-HM BASIS OF CALCULATION—PERCENTAGE SALES TAX Q Claim Billing/Encounter:
Required if Dispensing Status (343-HD) on submission is
“P” (Partial Fill) or “C” (Completion of Partial Fill) and
Percentage Sales Tax Amount Paid (559-AX) is greater
than zero (Ø).
571-NZ AMOUNT ATTRIBUTED TO PROCESSOR FEE Q Claim Billing/Encounter:
Required if the customer is responsible for 1ØØ% of the
prescription payment and when the provider net sale is less
than the amount the customer is expected to pay.
575-EQ PATIENT SALES TAX AMOUNT I Claim Billing/Encounter:
Used when necessary to identify the Patient’s portion of the
Sales Tax.
Provided for informational purposes only.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 103 -
RESPONSE PRICING SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
574-2Y PLAN SALES TAX AMOUNT I Claim Billing/Encounter:
Used when necessary to identify the Plan’s portion of the
Sales Tax.
Provided for informational purposes only.
572-4U AMOUNT OF COINSURANCE Q Claim Billing/Encounter:
Required if Patient Pay Amount (5Ø5-F5) includes
coinsurance as patient financial responsibility.
573-4V BASIS OF CALCULATION-COINSURANCE Q Claim Billing/Encounter:
Required if Dispensing Status (343-HD) on submission is
“P” (Partial Fill) or “C” (Completion of Partial Fill).
392-MU BENEFIT STAGE COUNT N Claim Billing/Encounter:
Not used.
393-MV BENEFIT STAGE QUALIFIER N***R*** Claim Billing/Encounter:
Not used.
394-MW BENEFIT STAGE AMOUNT N***R*** Claim Billing/Encounter:
Not used.
577-G3 ESTIMATED GENERIC SAVINGS I Claim Billing/Encounter:
This information should be provided when a patient
selected the brand drug and a generic form of the drug was
available. It will contain an estimate of the difference
between the cost of the brand drug and the generic drug,
when the brand drug is more expensive than the generic. It
is information that the provider should provide to the
patient.
128-UC SPENDING ACCOUNT AMOUNT REMAINING N Claim Billing/Encounter:
Not used.
129-UD HEALTH PLAN-FUNDED ASSISTANCE AMOUNT M Claim Billing/Encounter:
Not used.
133-UJ AMOUNT ATTRIBUTED TO PROVIDER NETWORK SELECTION Q Claim Billing/Encounter:
Required if Patient Pay Amount (5Ø5-F5) includes an
amount that is attributable to a cost share differential due to
the selection of one pharmacy over another.
134-UK AMOUNT ATTRIBUTED TO PRODUCT SELECTION/BRAND
DRUG
Q Claim Billing/Encounter:
Required if Patient Pay Amount (5Ø5-F5) includes an
amount that is attributable to a patient’s selection of a
Brand drug.
135-UM AMOUNT ATTRIBUTED TO PRODUCT SELECTION/NON-
PREFERRED FORMULARY SELECTION
Q Claim Billing/Encounter:
Required if Patient Pay Amount (5Ø5-F5) includes an
amount that is attributable to a patient’s selection of a non-
preferred formulary product.
136-UN AMOUNT ATTRIBUTED TO PRODUCT SELECTION/BRAND
NON-PREFERRED FORMULARY SELECTION
Q Claim Billing/Encounter:
Required if Patient Pay Amount (5Ø5-F5) includes an
amount that is attributable to a patient’s selection of a
Brand non-preferred formulary product.
137-UP AMOUNT ATTRIBUTED TO COVERAGE GAP Q Claim Billing/Encounter:
Required when the patient’s financial responsibility is due to
the coverage gap.
148-U8 INGREDIENT COST CONTRACTED/REIMBURSABLE AMOUNT N Claim Billing/Encounter:
Not used.
149-U9 DISPENSING FEE CONTRACTED/REIMBURSABLE AMOUNT N Claim Billing/Encounter:
Not used.
Notes on Response Pricing Segment on a Claim Billing or Encounter Response:
The Response Pricing Segment is situational for a Claim Billing or Encounter Response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured).
The Response Pricing Segment is not used in payer-to-payer transactions.
All dollar fields except Patient Pay Amount (5Ø5-F5) are estimated amounts. If actual amounts are returned on fields other than Patient Pay
Amount (5Ø5-F5), the “P” (Paid) response must be used.
If the Transaction Response Status (112-AN) = “C” (Captured) or “Q” (Duplicate of Captured), dollar fields should be supplied in the response.
• If the response is a “true” Capture (i.e. replacement of batch billing, with no edits or pricing), then corresponding response fields
should be populated with values as submitted. Ideally, processor should provide “real” patient financial responsibility values on a
Capture. If this is not possible, provider must know (by trading partner agreement) the patient financial responsibility to charge and
factor that into their system so collection occurs.
• If the response is captured by an Intermediary who can provide better pricing criteria, the corresponding response fields should be
populated with the probable values and those values used to determine estimated pricing as noted above. Since the claim has not
been fully adjudicated, this should remain a capture response.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 104 -
It is highly recommended that whenever possible, the individual dollar fields are returned in the response. On the response, the sender should
return the individual payment response fields to allow the receiver to reconcile against the requested payment fields.
See section “Response Processing Guidelines”, “Pricing Guidelines”.
Fields defined as Mandatory are required to be submitted when the segment is sent.
7.5.2.5.8 RESPONSE DUR/PPS SEGMENT (CLAIM BILLING OR ENCOUNTER) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
RESPONSE DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
567-J6 DUR/PPS RESPONSE CODE COUNTER Q***R*** Claim Billing/Encounter:
Maximum 9 occurrences supported.
Required if Reason For Service Code (439-E4) is used.
439-E4 REASON FOR SERVICE CODE Q***R*** Claim Billing/Encounter:
Required if utilization conflict is detected.
528-FS CLINICAL SIGNIFICANCE CODE Q***R*** Claim Billing/Encounter:
Required if needed to supply additional information for the
utilization conflict.
529-FT OTHER PHARMACY INDICATOR Q***R*** Claim Billing/Encounter:
Required if needed to supply additional information for the
utilization conflict.
53Ø-FU PREVIOUS DATE OF FILL Q***R*** Claim Billing/Encounter:
Required if needed to supply additional information for the
utilization conflict.
Required if Quantity of Previous Fill (531-FV) is used.
531-FV QUANTITY OF PREVIOUS FILL Q***R*** Claim Billing/Encounter:
Required if needed to supply additional information for the
utilization conflict.
Required if Previous Date Of Fill (53Ø-FU) is used.
532-FW DATABASE INDICATOR Q***R*** Claim Billing/Encounter:
Required if needed to supply additional information for the
utilization conflict.
533-FX OTHER PRESCRIBER INDICATOR Q***R*** Claim Billing/Encounter:
Required if needed to supply additional information for the
utilization conflict.
544-FY DUR FREE TEXT MESSAGE Q***R*** Claim Billing/Encounter:
Required if needed to supply additional information for the
utilization conflict.
57Ø-NS DUR ADDITIONAL TEXT Q***R*** Claim Billing/Encounter:
Required if needed to supply additional information for the
utilization conflict.
Notes on Response DUR/PPS Segment on a Claim Billing or Encounter Response:
The Response DUR/PPS Segment is situational for a Claim Billing or Encounter Response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). The segment is used to identify a
drug utilization review or professional pharmacy service event, opportunity, or information.
The Response DUR/PPS Segment is not used in payer-to-payer transactions.
Fields defined as Mandatory are required to be submitted when the segment is sent.
7.5.3 TRANSMISSION ACCEPTED/TRANSACTION REJECTED
7.5.3.1 DIAGRAM FOR TRANSMISSION OF ONE CLAIM BILLING OR ENCOUNTER RESPONSE
(TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
Claim Billing or Encounter transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
And Transaction Response Status (112-AN) of “R” (Rejected)
The Response DUR/PPS Segment and Response Prior Authorization Segments are not used in payer-to-payer transactions. Therefore, in this
case, there are no transaction-level situational segments.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
Mandatory
Response Header Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 105 -
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Prior Authorization Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
7.5.3.2 DIAGRAM FOR TRANSMISSION OF TWO CLAIM BILLING OR ENCOUNTER RESPONSES
(TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Prior Authorization Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Prior Authorization Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
7.5.3.3 DIAGRAM FOR TRANSMISSION OF THREE CLAIM BILLING OR ENCOUNTER RESPONSES
(TRANSMISSION ACCEPTED/TRANSACTION REJECTED)

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 106 -
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Prior Authorization Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Prior Authorization Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Prior Authorization Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
7.5.3.4 DIAGRAM FOR TRANSMISSION OF FOUR CLAIM BILLING OR ENCOUNTER RESPONSES
(TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 107 -
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Prior Authorization Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Prior Authorization Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Prior Authorization Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Prior Authorization Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
7.5.3.5 CLAIM BILLING OR ENCOUNTER RESPONSE SEGMENTS (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
7.5.3.5.1 RESPONSE HEADER SEGMENT (CLAIM BILLING OR ENCOUNTER) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M For Transaction Code of “B1”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “1” (Rx Billing).

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 108 -
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Claim Billing or Encounter Response:
The Response Header Segment is a mandatory, fixed length segment for Claim Billing or Encounter response when the Header Response
Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). The “Situation” column is not applicable.
7.5.3.5.2 RESPONSE MESSAGE SEGMENT (CLAIM BILLING OR ENCOUNTER) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Claim Billing/Encounter:
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Claim Billing or Encounter Response:
The Response Message Segment is situational for Claim Billing or Encounter response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). It is used when additional text information needs to be sent. Fields
defined as Mandatory are required to be submitted when the segment is sent.
7.5.3.5.3 RESPONSE INSURANCE SEGMENT (CLAIM BILLING OR ENCOUNTER) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø1-C1 GROUP ID
Q Claim Billing/Encounter:
Required if needed to identify the actual cardholder or
employer group, to identify appropriate group number,
when available.
Required to identify the actual group that was used when
multiple group coverages exist.
Note: This field may contain the Group ID echoed from the
request. May contain the actual Group ID if unknown to the
receiver.
524-FO PLAN ID
Q Claim Billing/Encounter:
Required if needed to identify the actual plan parameters,
benefit, or coverage criteria, when available.
Required to identify the actual plan ID that was used when
multiple group coverages exist.
Required if needed to contain the actual plan ID if unknown
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 109 -
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
to the receiver.
545-2F NETWORK REIMBURSEMENT ID
Q Claim Billing/Encounter:
Required if needed to identify the network for the covered
member.
Required if needed to identify the actual Network
Reimbursement ID, when applicable and/or available.
Required to identify the actual Network Reimbursement ID
that was used when multiple Network Reimbursement IDs
exist.
568-J7 PAYER ID QUALIFIER Q Claim Billing/Encounter:
Required if Payer ID (569-J8) is used.
569-J8 PAYER ID
Q Claim Billing/Encounter:
Required to identify the ID of the payer responding.
115-N5
MEDICAID ID NUMBER N Claim Billing/Encounter:
Not used.
116-N6
MEDICAID AGENCY NUMBER N Claim Billing/Encounter:
Not used.
3Ø2-C2
CARDHOLDER ID Q Claim Billing/Encounter:
Required if the identification to be used in future
transactions is different than what was submitted on the
request.
Notes on Response Insurance Segment on a Claim Billing or Encounter Response:
The Response Insurance Segment is situational for Claim Billing or Encounter response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). It is used when coverage or reimbursement parameters or identifiers
need to be sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
7.5.3.5.3.1 Response Insurance Segment (Medicaid Subrogation Claim Billing or Encounter)
(Transmission Accepted/Transaction Rejected)
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
115-N5
MEDICAID ID NUMBER QM Medicaid Subrogation - Claim Billing/Encounter:
Required to identify the member as uniquely known to
Medicaid.
116-N6
MEDICAID AGENCY NUMBER QM Medicaid Subrogation - Claim Billing/Encounter:
Required to identify the Medicaid agency.
See Medicaid Subrogation Implementation Guide.
Notes on Response Insurance Segment on a Medicaid Subrogation Claim Billing or Encounter Response:
The rules above for a “Response Insurance Segment (Claim Billing or Encounter)” are to be followed for Medicaid Subrogation.
Specific fields that are used differently in Medicaid Subrogation are noted in the table above.
7.5.3.5.4 RESPONSE PATIENT SEGMENT (CLAIM BILLING OR ENCOUNTER) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
31Ø-CA PATIENT FIRST NAME Q Claim Billing/Encounter:
Required if known.
311-CB PATIENT LAST NAME Q Claim Billing/Encounter:
Required if known.
3Ø4-C4 DATE OF BIRTH Q Claim Billing/Encounter:
Required if known.
Notes on Response Patient Segment on a Claim Billing/Encounter Response:
The Response Patient Segment is situational for Claim Billing or Encounter transmission response Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “R” (Rejected) when patient demographic information needs to be sent from the
sender to the receiver. Fields defined as Mandatory are required to be submitted when the segment is sent.
7.5.3.5.5 RESPONSE STATUS SEGMENT (CLAIM BILLING OR ENCOUNTER) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 110 -
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Claim Billing/Encounter:
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT R Claim Billing/Encounter:
Maximum count of 5.
Required.
511-FB REJECT CODE R***R*** Claim Billing/Encounter:
Required.
546-4F REJECT FIELD OCCURRENCE INDICATOR Q***R*** Claim Billing/Encounter:
Required if a repeating field is in error, to identify repeating
field occurrence.
This field must be sent when relaying error information about
a repeating field or set. Note, if the Reject Code is not
denoting a repeating field or set, the Reject Field Occurrence
Indicator must not be sent.
547-5F APPROVED MESSAGE CODE COUNT N Claim Billing/Encounter:
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Claim Billing/Encounter:
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Claim Billing/Encounter:
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Claim Billing/Encounter:
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Claim Billing/Encounter:
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Claim Billing/Encounter:
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Claim Billing/Encounter:
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Claim Billing/Encounter:
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Claim Billing/Encounter:
Not used.
993-A7 INTERNAL CONTROL NUMBER N Claim Billing/Encounter:
Not used.
987-MA URL I Claim Billing/Encounter:
Provided for informational purposes only to relay health
care communications via the Internet.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 111 -
Notes on Response Status Segment on a Claim Billing or Encounter Response:
The Response Status Segment is mandatory for a Claim Billing or Encounter response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). The Response Status Segment is sent from the sender to the
receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.
7.5.3.5.6 RESPONSE CLAIM SEGMENT (CLAIM BILLING OR ENCOUNTER) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M For Transaction Code of “B1”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “1” (Rx Billing).
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT Q Claim Billing/Encounter:
Maximum count of 6.
Required if Preferred Product ID (553-AR) is used.
Not used in payer-to-payer transactions.
552-AP PREFERRED PRODUCT ID QUALIFIER Q***R*** Claim Billing/Encounter:
Required if Preferred Product ID (553-AR) is used.
Not used in payer-to-payer transactions.
553-AR PREFERRED PRODUCT ID Q***R*** Claim Billing/Encounter:
Required if a product preference exists that needs to be
communicated to the receiver via an ID.
Not used in payer-to-payer transactions.
554-AS PREFERRED PRODUCT INCENTIVE Q***R*** Claim Billing/Encounter:
Required if there is a known incentive amount associated
with the Preferred Product ID (553-AR) and/or Preferred
Product Description (556-AU).
Not used in payer-to-payer transactions.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE Q***R*** Claim Billing/Encounter:
Required if there is a known patient financial responsibility
incentive amount associated with the Preferred Product ID
(553-AR) and/or Preferred Product Description (556-AU).
Not used in payer-to-payer transactions.
556-AU PREFERRED PRODUCT DESCRIPTION Q***R*** Claim Billing/Encounter:
Required if a product preference exists that either cannot
be communicated by the Preferred Product ID (553-AR) or
to clarify the Preferred Product ID (553-AR).
Not used in payer-to-payer transactions.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Claim Billing/Encounter:
Not used.
Notes on Response Claim Segment on a Claim Billing or Encounter Response:
The Response Claim Segment is mandatory for a Claim Billing or Encounter response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). The Response Claim Segment is sent from the sender to the
receiver to identify therapeutic or alternate product recommendations. Fields defined as Mandatory are required to be submitted when the
segment is sent.
7.5.3.5.6.1 Response Claim Segment (Medicaid Subrogation Claim Billing or Encounter) (Transmission
Accepted/Transaction Rejected)
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
QM Medicaid Subrogation - Claim Billing/Encounter:
Required to report back on the response the claim
number assigned by the Medicaid Agency.
Notes on Response Claim Segment on a Medicaid Subrogation Claim Billing or Encounter Response:
The rules above for a “Response Claim Segment (Claim Billing or Encounter)” are to be followed for Medicaid Subrogation. Specific
fields that are used differently in Medicaid Subrogation are noted in the table above.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 112 -
7.5.3.5.7 RESPONSE DUR/PPS SEGMENT (CLAIM BILLING OR ENCOUNTER) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
567-J6 DUR/PPS RESPONSE CODE COUNTER Q***R*** Claim Billing/Encounter:
Maximum 9 occurrences supported.
Required if Reason For Service Code (439-E4) is used.
439-E4 REASON FOR SERVICE CODE Q***R*** Claim Billing/Encounter:
Required if utilization conflict is detected.
528-FS CLINICAL SIGNIFICANCE CODE Q***R*** Claim Billing/Encounter:
Required if needed to supply additional information for the
utilization conflict.
529-FT OTHER PHARMACY INDICATOR Q***R*** Claim Billing/Encounter:
Required if needed to supply additional information for the
utilization conflict.
53Ø-FU PREVIOUS DATE OF FILL Q***R*** Claim Billing/Encounter:
Required if needed to supply additional information for the
utilization conflict.
Required if Quantity of Previous Fill (531-FV) is used.
531-FV QUANTITY OF PREVIOUS FILL Q***R*** Claim Billing/Encounter:
Required if needed to supply additional information for the
utilization conflict.
Required if Previous Date Of Fill (53Ø-FU) is used.
532-FW DATABASE INDICATOR Q***R*** Claim Billing/Encounter:
Required if needed to supply additional information for the
utilization conflict.
533-FX OTHER PRESCRIBER INDICATOR Q***R*** Claim Billing/Encounter:
Required if needed to supply additional information for the
utilization conflict.
544-FY DUR FREE TEXT MESSAGE Q***R*** Claim Billing/Encounter:
Required if needed to supply additional information for the
utilization conflict.
57Ø-NS DUR ADDITIONAL TEXT Q***R*** Claim Billing/Encounter:
Required if needed to supply additional information for the
utilization conflict.
Notes on Response DUR/PPS Segment on a Claim Billing or Encounter Response:
The Response DUR/PPS Segment is situational for a Claim Billing or Encounter Response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). The segment is used to identify a drug utilization review or
professional pharmacy service event, opportunity, or information.
The Response DUR/PPS Segment is not used in payer-to-payer transactions.
Fields defined as Mandatory are required to be submitted when the segment is sent.
7.5.3.5.8 RESPONSE PRIOR AUTHORIZATION SEGMENT (CLAIM BILLING OR ENCOUNTER) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE PRIOR AUTHORIZATION SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
498-PR PRIOR AUTHORIZATION PROCESSED DATE N Claim Billing/Encounter:
Not used.
498-PS PRIOR AUTHORIZATION EFFECTIVE DATE N Claim Billing/Encounter:
Not used.
498-PT PRIOR AUTHORIZATION EXPIRATION DATE N Claim Billing/Encounter:
Not used.
498-RA PRIOR AUTHORIZATION QUANTITY N Claim Billing/Encounter:
Not used.
498-RB PRIOR AUTHORIZATION DOLLARS AUTHORIZED N Claim Billing/Encounter:
Not used.
498-PW PRIOR AUTHORIZATION NUMBER OF REFILLS AUTHORIZED N Claim Billing/Encounter:
Not used.
498-PX PRIOR AUTHORIZATION QUANTITY ACCUMULATED N Claim Billing/Encounter:
Not used.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 113 -
RESPONSE PRIOR AUTHORIZATION SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
498-PY PRIOR AUTHORIZATION NUMBER–ASSIGNED Q Claim Billing/Encounter:
Required when the receiver must submit this Prior
Authorization Number in order to receive payment for the
claim. (An example of a situation may include a Benefit
Transition Period that allows for payment of claims, for a
period of time that would normally reject.)
Notes on Response Prior Authorization Segment on a Claim Billing or Encounter Response:
The Response Prior Authorization Segment is situational for a Claim Billing or Encounter response when the Header Response Status (5Ø1-
F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). It is used to relay the Prior Authorization Number -
Assigned (498-PY) which is returned when a Reject Code (511-FB) denotes that a prior authorization code needs to be submitted on the
subsequent billing.
The Response Prior Authorization Segment is not used in payer-to-payer transactions.
Fields defined as Mandatory are required to be submitted when the segment is sent.
7.5.3.5.9 RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS SEGMENT (CLAIM BILLING OR ENCOUNTER)
(TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS
SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
355-NT OTHER PAYER ID COUNT M Claim Billing/Encounter:
Maximum count of 3.
338-5C OTHER PAYER COVERAGE TYPE M***R***
339-6C OTHER PAYER ID QUALIFIER Q***R*** Claim Billing/Encounter:
Required if Other Payer ID (34Ø-7C) is used.
34Ø-7C OTHER PAYER ID Q***R*** Claim Billing/Encounter:
Required if other insurance information is available for
coordination of benefits.
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER Q***R*** Claim Billing/Encounter:
Required if other insurance information is available for
coordination of benefits.
356-NU OTHER PAYER CARDHOLDER ID Q***R*** Claim Billing/Encounter:
Required if other insurance information is available for
coordination of benefits.
992-MJ OTHER PAYER GROUP ID Q***R*** Claim Billing/Encounter:
Required if other insurance information is available for
coordination of benefits.
142-UV OTHER PAYER PERSON CODE Q***R*** Claim Billing/Encounter:
Required if needed to uniquely identify the family members
within the Cardholder ID, as assigned by the other payer.
127-UB OTHER PAYER HELP DESK PHONE NUMBER Q***R*** Claim Billing/Encounter:
Required if needed to provide a support telephone number
of the other payer to the receiver.
143-UW OTHER PAYER PATIENT RELATIONSHIP CODE Q***R*** Claim Billing/Encounter:
Required if needed to uniquely identify the relationship of
the patient to the cardholder ID, as assigned by the other
payer.
144-UX OTHER PAYER BENEFIT EFFECTIVE DATE Q***R*** Claim Billing/Encounter:
Required when other coverage is known which is after the
Date of Service submitted.
145-UY OTHER PAYER BENEFIT TERMINATION DATE Q***R*** Claim Billing/Encounter:
Required when other coverage is known which is after the
Date of Service submitted.
Notes on Response Coordination of Benefits/Other Payers Segment on a Claim Billing or Encounter Response:
The Response Coordination of Benefits/Other Payers Segment is situational for a Claim Billing or Encounter response when the Header
Response Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “R” (Rejected) when other insurance information
is available for coordination of benefits.
1. If the identity of the patient is partially verified and the Claim Billing or Encounter is rejected due to a non-match of field
verification, then the Other Payer information is not sent.
2. If the claim is rejected because it should be submitted to other payer(s) first, that Other Payer information should be sent, if
known.
3. If the claim is rejected due to benefit design limitations, then subsequent Other Payer information should be sent, if known.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 114 -
If the claim rejects for other reasons than above, Other Payer information is not sent.
If additional payer(s) for this patient is not known, the Other Payer information is not sent.
If additional payer(s) for this patient is known, the following may be sent:
• Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C),
• Other Payer Group ID (992-MJ),
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU)
• And other Other Payer fields.
In addition, if any of the following three fields are sent:
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU),
• Other Payer Group ID (992-MJ),
then the Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C) must be sent.
Fields defined as Mandatory are required to be submitted when the segment is sent.
7.5.4 TRANSMISSION REJECTED/TRANSACTION REJECTED
7.5.4.1 DIAGRAM FOR TRANSMISSION OF ONE CLAIM BILLING OR ENCOUNTER RESPONSE
(TRANSMISSION REJECTED/TRANSACTION REJECTED)
Claim Billing or Encounter transmission response Header Response Status (5Ø1-F1) of "R" (Rejected)
and Transaction Response Status (112-AN) of “R” (Rejected)
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
7.5.4.2 DIAGRAM FOR TRANSMISSION OF TWO CLAIM BILLING OR ENCOUNTER RESPONSES
(TRANSMISSION REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
7.5.4.3 DIAGRAM FOR TRANSMISSION OF THREE CLAIM BILLING OR ENCOUNTER RESPONSES
(TRANSMISSION REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 115 -
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
7.5.4.4 DIAGRAM FOR TRANSMISSION OF FOUR CLAIM BILLING OR ENCOUNTER RESPONSES
(TRANSMISSION REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
7.5.4.5 CLAIM BILLING OR ENCOUNTER RESPONSE SEGMENTS (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
7.5.4.5.1 RESPONSE HEADER SEGMENT (CLAIM BILLING OR ENCOUNTER) (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Claim Billing or Encounter Response:
The Response Header Segment is a mandatory, fixed length segment for Claim Billing or Encounter response when the Header Response
Status (5Ø1-F1) of "R" (Rejected) and Transaction Response Status (112-AN) of “R” (Rejected). The “Situation” column is not applicable.
If either the entire transmission or the Header is in error, the Header Response Status (5Ø1-F1) = “R” (Rejected). Every identifiable transaction
within the transmission must be rejected with an “R”.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 116 -
If the transaction rejects for detail errors, the Header Response Status (5Ø1-F1) = “A” (Accepted) and the Transaction Response Status (112-
AN) will be “R”.
7.5.4.5.2 RESPONSE MESSAGE SEGMENT (CLAIM BILLING OR ENCOUNTER) (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Claim Billing/Encounter:
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Claim Billing or Encounter Response:
The Response Message Segment is situational for a Claim Billing or Encounter response when the Header Response Status (5Ø1-F1) of "R"
(Rejected) and Transaction Response Status (112-AN) of “R” (Rejected). It is used when additional text information needs to be sent. Fields
defined as Mandatory are required to be submitted when the segment is sent.
7.5.4.5.3 RESPONSE STATUS SEGMENT (CLAIM BILLING OR ENCOUNTER) (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER
Q Claim Billing/Encounter:
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT
R Claim Billing/Encounter:
Maximum count of 5.
Required.
511-FB REJECT CODE
R***R*** Claim Billing/Encounter:
Required.
546-4F REJECT FIELD OCCURRENCE INDICATOR
Q***R*** Claim Billing/Encounter:
Required if a repeating field is in error, to identify repeating
field occurrence.
This field must be sent when relaying error information about
a repeating field or set. Note, if the Reject Code is not
denoting a repeating field or set, the Reject Field Occurrence
Indicator must not be sent.
547-5F APPROVED MESSAGE CODE COUNT N Claim Billing/Encounter:
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Claim Billing/Encounter:
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Claim Billing/Encounter:
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Claim Billing/Encounter:
Required if Additional Message Information (526-FQ) is
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 117 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Claim Billing/Encounter:
Required if additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Claim Billing/Encounter:
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER
Q Claim Billing/Encounter:
Required if Help Desk Phone Number (55Ø-F8) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Claim Billing/Encounter:
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Claim Billing/Encounter:
Not used.
993-A7 INTERNAL CONTROL NUMBER N Claim Billing/Encounter:
Not used.
987-MA URL N Claim Billing/Encounter:
Not used.
Notes on Response Status Segment on a Claim Billing or Encounter Response:
The Response Status Segment is mandatory for a Claim Billing or Encounter Response when the Header Response Status (5Ø1-F1) = “R”
(Rejected) and Transaction Response Status (112-AN) of “R” (Rejected). The Response Status Segment is sent from the sender to the
receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 118 -
8. PREDETERMINATION OF BENEFITS INFORMATION
The Predetermination of Benefits inquiry transaction is used by the Originator to request the following:
1. To determine if the patient is eligible for prescription coverage,
2. To determine if the submitted product is covered,
3. To identify the patient financial responsibility at that point in time, and
4. To potentially identify clinically relevant information.
The Originator uses the Processor’s response to communicate with the patient and determine if a subsequent prescription claim request is
submitted. The Originator recognizes that the Processor’s response is based upon the following:
1. Submitted information in the Predetermination of Benefits inquiry,
2. Processor plan parameters,
3. Date of Service, and
4. Patient’s current prescription claim and financial profile at the processor.
The subsequent submission of the same Predetermination of Benefits Inquiry or a corresponding prescription claim request may result in a
different response if any of the identified components (i.e. Submitted Data, Processor plan parameters, Date of Service, or Patient’s current
prescription and or financial profile) change between the period of time between the submission of a Predetermination of Benefits inquiry
transaction and the associated Prescription Claim request.
Conversely, the Predetermination of Benefits transaction response is used by the Processor to communicate the following:
1. To identify if the patient is eligible for prescription coverage,
2. To identify if the submitted product is covered,
3. To identify the patient financial responsibility at that point in time, and
4. To potentially identify clinically relevant information that may influence the submission of a corresponding prescription claim
request.
The Processor recognizes that the Originator’s inquiry is a “what if” transaction that may not result in the submission of a corresponding
prescription claim request.
The Predetermination of Benefits transaction does not result in payment or application to the patient’s benefit.
The Predetermination of Benefits transaction is used on claim submission only. It is not valid for a service submission.
Each Predetermination of Benefits submission request may contain up to four occurrences of claim data.
Depending upon the particular claim submission request, the Processor can provide one of the following general types of responses:
Benefit - This occurs when the Processor processes the claim, and returns to the Originator a snapshot of the patient’s responsibility
at this point in time.
Rejected - This occurs when the Processor has encountered an error in the transaction or processing.
There is no need for a duplicate response due to the nature of the Predetermination of Benefits transaction. Each submission of the
transaction is processed with the response reflective of current information.
8.1 PREDETERMINATION OF BENEFITS REQUEST DIAGRAMS
For a Predetermination of Benefits the scenarios defined include
Predetermination of Benefits from a Sender to a Receiver
Benefit/Rejected Transaction Response from a Sender to a Receiver
Standard Transmission Reject Response to a Predetermination of Benefits from a Sender to a Receiver
8.1.1 DIAGRAM FOR TRANSMISSION OF ONE PREDETERMINATION OF BENEFITS
TRANSACTION
Mandatory
Transaction Header Segment
Segment Separator
Insurance Segment
Situational
Segment Separator
Patient Segment
Mandatory - first Predetermination of Benefits
Group Separator
Segment Separator
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 119 -
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Compound Segment
Segment Separator
Clinical Segment
Segment Separator
Facility Segment
8.1.2 DIAGRAM FOR TRANSMISSION OF TWO PREDETERMINATION OF BENEFITS
TRANSACTIONS
Mandatory
Transaction Header Segment
Segment Separator
Insurance Segment
Situational
Segment Separator
Patient Segment
Mandatory - first Predetermination of Benefits
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Clinical Segment
Segment Separator
Facility Segment
Mandatory - second Predetermination of Benefits
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Clinical Segment
Segment Separator
Facility Segment
8.1.3 DIAGRAM FOR TRANSMISSION OF THREE OR FOUR PREDETERMINATION OF
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 120 -
BENEFITS TRANSACTIONS
These transaction diagrams will follow the example in the section “Diagram For Transmission Of Two Predetermination of Benefits
Transactions”. For three or four transactions, the Mandatory and Situational Predetermination Of Benefits transaction segments will be
repeated for the third and fourth transactions.
8.2 PREDETERMINATION OF BENEFITS REQUEST SEGMENTS
All segments noted above in the Predetermination of Benefits diagram section must follow the Claim Billing diagrams and situations stated in
this document. The Predetermination of Benefits transaction has unique requirements for the segments noted below.
8.2.1 PRICING SEGMENT (PREDETERMINATION OF BENEFITS)
PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
4Ø9-D9 INGREDIENT COST SUBMITTED R Predetermination Of Benefits:
Required.
412-DC DISPENSING FEE SUBMITTED Q Predetermination Of Benefits:
Required if its value has an effect on the Gross Amount
Due (43Ø-DU) calculation.
Zero (Ø) is a valid value.
477-BE PROFESSIONAL SERVICE FEE SUBMITTED N Predetermination Of Benefits:
Not used.
433-DX PATIENT PAID AMOUNT SUBMITTED Q Predetermination Of Benefits:
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
438-E3 INCENTIVE AMOUNT SUBMITTED Q Predetermination Of Benefits:
Required if its value has an effect on the Gross Amount
Due (43Ø-DU) calculation.
Zero (Ø) is a valid value.
478-H7 OTHER AMOUNT CLAIMED SUBMITTED COUNT Q Predetermination Of Benefits:
Maximum count of 3.
Required if Other Amount Claimed Submitted Qualifier
(479-H8) is used.
479-H8 OTHER AMOUNT CLAIMED SUBMITTED QUALIFIER Q***R*** Predetermination Of Benefits:
Required if Other Amount Claimed Submitted (48Ø-H9) is
used.
48Ø-H9 OTHER AMOUNT CLAIMED SUBMITTED Q***R*** Predetermination Of Benefits:
Required if its value has an effect on the Gross Amount
Due (43Ø-DU) calculation.
Zero (Ø) is a valid value.
481-HA FLAT SALES TAX AMOUNT SUBMITTED Q Predetermination Of Benefits:
Required if its value has an effect on the Gross Amount
Due (43Ø-DU) calculation.
Zero (Ø) is a valid value.
482-GE PERCENTAGE SALES TAX AMOUNT SUBMITTED Q Predetermination Of Benefits:
Required if its value has an effect on the Gross Amount
Due (43Ø-DU) calculation.
Zero (Ø) is a valid value.
483-HE PERCENTAGE SALES TAX RATE SUBMITTED Q Predetermination Of Benefits:
Required if Percentage Sales Tax Amount Submitted (482-
GE) and Percentage Sales Tax Basis Submitted (484-JE)
are used.
Required if this field could result in different pricing.
Required if needed to calculate Percentage Sales Tax
Amount Paid (559-AX).
484-JE PERCENTAGE SALES TAX BASIS SUBMITTED Q Predetermination Of Benefits:
Required if Percentage Sales Tax Amount Submitted (482-
GE) and Percentage Sales Tax Rate Submitted (483-HE)
are used.
Required if this field could result in different pricing.
Required if needed to calculate Percentage Sales Tax
Amount Paid (559-AX).
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 121 -
PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
426-DQ USUAL AND CUSTOMARY CHARGE Q Predetermination Of Benefits:
Required if needed per trading partner agreement.
43Ø-DU GROSS AMOUNT DUE R Predetermination Of Benefits:
Required.
See Pricing Formula in Implementation Guide for fields
used in calculation.
423-DN BASIS OF COST DETERMINATION Q Predetermination Of Benefits:
Required if needed for receiver claim/encounter
adjudication.
113-N3
MEDICAID PAID AMOUNT N Predetermination Of Benefits:
Not used.
Notes on Pricing Segment on a Predetermination Of Benefit Request:
The Pricing Segment is mandatory for a Predetermination Of Benefit Request. The Pricing Segment defines the components of the Patient
Pay Amount (5Ø5-F5) field for a Predetermination Of Benefit. See section “Pricing Guidelines”. Fields defined as Mandatory are required to be
submitted when the segment is sent.
8.3 PREDETERMINATION OF BENEFITS RESPONSE DIAGRAMS AND SEGMENTS
8.3.1 TRANSMISSION ACCEPTED/TRANSACTION BENEFIT
8.3.1.1 DIAGRAM FOR TRANSMISSION OF ONE PREDETERMINATION OF BENEFIT RESPONSE
(TRANSMISSION ACCEPTED/TRANSACTION BENEFIT)
Predetermination Of Benefit transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “B” (Benefit)
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
8.3.1.2 DIAGRAM FOR TRANSMISSION OF TWO PREDETERMINATION OF BENEFIT RESPONSES
(TRANSMISSION ACCEPTED/TRANSACTION BENEFIT)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 122 -
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
8.3.1.3 DIAGRAM FOR TRANSMISSION OF THREE OR FOUR PREDETERMINATION OF BENEFIT
RESPONSES (TRANSMISSION ACCEPTED/TRANSACTION BENEFIT)
These transaction diagrams will follow the example in the section “Diagram For Transmission Of Two Predetermination of Benefit Responses
(Transmission Accepted/Transaction Benefit)”. For three or four transactions, the Mandatory and Situational Predetermination Of Benefits
transaction segments will be repeated for the third and fourth transactions.
8.3.1.4 PREDETERMINATION OF BENEFITS RESPONSE SEGMENTS (TRANSMISSION
ACCEPTED/TRANSACTION BENEFIT)
All segments noted above in the Predetermination of Benefits Response diagram section must follow the Claim Billing Response (Paid)
diagrams and situations stated in this document, with guidance in the section “Transmission Structure”. The Predetermination of Benefits
transaction has unique requirements for the segments noted below.
8.3.1.4.1 RESPONSE PRICING SEGMENT (PREDETERMINATION OF BENEFITS) (TRANSMISSION
ACCEPTED/TRANSACTION BENEFIT)
RESPONSE PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø5-F5 PATIENT PAY AMOUNT R Predetermination Of Benefits:
Required.
5Ø6-F6 INGREDIENT COST PAID N Predetermination Of Benefits:
Not used.
5Ø7-F7 DISPENSING FEE PAID N Predetermination Of Benefits:
Not used.
557-AV TAX EXEMPT INDICATOR N Predetermination Of Benefits:
Not used.
558-AW FLAT SALES TAX AMOUNT PAID N Predetermination Of Benefits:
Not used.
559-AX PERCENTAGE SALES TAX AMOUNT PAID N Predetermination Of Benefits:
Not used.
56Ø-AY PERCENTAGE SALES TAX RATE PAID N Predetermination Of Benefits:
Not used.
561-AZ PERCENTAGE SALES TAX BASIS PAID N Predetermination Of Benefits:
Not used.
521-FL INCENTIVE AMOUNT PAID
N Predetermination Of Benefits:
Not used.
562-J1 PROFESSIONAL SERVICE FEE PAID N Predetermination Of Benefits:
Not used.
563-J2 OTHER AMOUNT PAID COUNT N Predetermination Of Benefits:

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 123 -
RESPONSE PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Not used.
564-J3 OTHER AMOUNT PAID QUALIFIER N***R*** Predetermination Of Benefits:
Not used.
565-J4 OTHER AMOUNT PAID
N***R*** Predetermination Of Benefits:
Not used.
566-J5 OTHER PAYER AMOUNT RECOGNIZED N Predetermination Of Benefits:
Not used.
5Ø9-F9 TOTAL AMOUNT PAID N Predetermination Of Benefits:
Not used.
522-FM BASIS OF REIMBURSEMENT DETERMINATION N Predetermination Of Benefits:
Not used.
523-FN AMOUNT ATTRIBUTED TO SALES TAX Q Predetermination Of Benefits:
Required if Patient Pay Amount (5Ø5-F5) includes sales tax
that is the financial responsibility of the member but is not
also included in any of the other fields that add up to
Patient Pay Amount.
See section “Specific Segment Discussion”, subsection
“Response Segments”, subsection “Response Pricing
Segment” for guidance.
512-FC ACCUMULATED DEDUCTIBLE AMOUNT N Predetermination Of Benefits:
Not used.
513-FD REMAINING DEDUCTIBLE AMOUNT N Predetermination Of Benefits:
Not used.
514-FE REMAINING BENEFIT AMOUNT N Predetermination Of Benefits:
Not used.
517-FH AMOUNT APPLIED TO PERIODIC DEDUCTIBLE Q Predetermination Of Benefits:
Required if Patient Pay Amount (5Ø5-F5) includes
deductible.
518-FI AMOUNT OF COPAY Q Predetermination Of Benefits:
Required if Patient Pay Amount (5Ø5-F5) includes copay
as patient financial responsibility.
52Ø-FK AMOUNT EXCEEDING PERIODIC BENEFIT MAXIMUM Q Predetermination Of Benefits:
Required if Patient Pay Amount (5Ø5-F5) includes amount
exceeding periodic benefit maximum.
346-HH BASIS OF CALCULATION—DISPENSING FEE N Predetermination Of Benefits:
Not used.
347-HJ BASIS OF CALCULATION—COPAY N Predetermination Of Benefits:
Not used.
348-HK BASIS OF CALCULATION—FLAT SALES TAX N Predetermination Of Benefits:
Not used.
349-HM BASIS OF CALCULATION—PERCENTAGE SALES TAX N Predetermination Of Benefits:
Predetermination Of Benefits:
Not used.
571-NZ AMOUNT ATTRIBUTED TO PROCESSOR FEE Q Predetermination Of Benefits:
Required if the customer is responsible for 1ØØ% of the
prescription payment and when the provider net sale is less
than the amount the customer is expected to pay.
575-EQ PATIENT SALES TAX AMOUNT N Predetermination Of Benefits:
Not used.
574-2Y PLAN SALES TAX AMOUNT N Predetermination Of Benefits:
Not used.
572-4U AMOUNT OF COINSURANCE Q Predetermination Of Benefits:
Required if Patient Pay Amount (5Ø5-F5) includes
coinsurance as patient financial responsibility.
573-4V BASIS OF CALCULATION-COINSURANCE N Predetermination Of Benefits:
Not used.
392-MU BENEFIT STAGE COUNT Q Predetermination Of Benefits:
Not used.
393-MV BENEFIT STAGE QUALIFIER N***R*** Predetermination Of Benefits:
Not used.
394-MW BENEFIT STAGE AMOUNT N***R*** Predetermination Of Benefits:
Not used.
577-G3 ESTIMATED GENERIC SAVINGS N Predetermination Of Benefits:
Not used.
128-UC SPENDING ACCOUNT AMOUNT REMAINING N Predetermination Of Benefits:
Not used.
129-UD HEALTH PLAN-FUNDED ASSISTANCE AMOUNT Q Predetermination Of Benefits:
Required when the patient meets the plan-funded
assistance criteria, to reduce Patient Pay Amount (5Ø5-
F5). The resulting Patient Pay Amount (5Ø5-F5) must be
greater than or equal to zero.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 124 -
RESPONSE PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
This field is always a negative amount or zero.
133-UJ AMOUNT ATTRIBUTED TO PROVIDER NETWORK SELECTION Q Predetermination Of Benefits:
Required if Patient Pay Amount (5Ø5-F5) includes an
amount that is attributable to a cost share differential due to
the selection of one pharmacy over another.
134-UK AMOUNT ATTRIBUTED TO PRODUCT SELECTION/BRAND
DRUG
Q Predetermination Of Benefits:
Required if Patient Pay Amount (5Ø5-F5) includes an
amount that is attributable to a patient’s selection of a
Brand drug.
135-UM AMOUNT ATTRIBUTED TO PRODUCT SELECTION/NON-
PREFERRED FORMULARY SELECTION
Q Predetermination Of Benefits:
Required if Patient Pay Amount (5Ø5-F5) includes an
amount that is attributable to a patient’s selection of a non-
preferred formulary product.
136-UN AMOUNT ATTRIBUTED TO PRODUCT SELECTION/BRAND
NON-PREFERRED FORMULARY SELECTION
Q Predetermination Of Benefits:
Required if Patient Pay Amount (5Ø5-F5) includes an
amount that is attributable to a patient’s selection of a
Brand non-preferred formulary product.
137-UP AMOUNT ATTRIBUTED TO COVERAGE GAP Q Predetermination Of Benefits:
Required when the patient’s financial responsibility is due to
the coverage gap.
148-U8 INGREDIENT COST CONTRACTED/REIMBURSABLE AMOUNT N Predetermination Of Benefits:
Not used.
149-U9 DISPENSING FEE CONTRACTED/REIMBURSABLE AMOUNT N Predetermination Of Benefits:
Not used.
Notes on Response Pricing Segment on a Predetermination Of Benefits Response:
The Response Pricing Segment is mandatory for a Predetermination Of Benefits Response when the Header Response Status (5Ø1-F1) of
"A" (Accepted) and Transaction Response Status (112-AN) is “B” (Benefit). Fields defined as Mandatory are required to be submitted when the
segment is sent.
8.3.2 TRANSMISSION ACCEPTED/TRANSACTION REJECTED
8.3.2.1 DIAGRAM FOR TRANSMISSION OF ONE PREDETERMINATION OF BENEFITS RESPONSE
(TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
Predetermination Of Benefits transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
And Transaction Response Status (112-AN) of “R” (Rejected)
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
8.3.2.2 DIAGRAM FOR TRANSMISSION OF TWO PREDETERMINATION OF BENEFITS RESPONSES
(TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 125 -
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
8.3.2.3 DIAGRAM FOR TRANSMISSION OF THREE OR FOUR PREDETERMINATION OF BENEFIT
RESPONSES (TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
These transaction diagrams will follow the example in the section “Diagram For Transmission Of Two Predetermination of Benefit Responses
(Transmission Accepted/Transaction Rejected)”. For three or four transactions, the Mandatory and Situational Predetermination Of Benefits
transaction segments will be repeated for the third and fourth transactions.
8.3.2.4 PREDETERMINATION OF BENEFITS RESPONSE SEGMENTS (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
All segments noted above in the Predetermination of Benefits Response diagram section must follow the Claim Billing Response
(Transmission Accepted/Transaction Rejected) diagrams and situations stated in this document with guidance in the section “Transmission
Structure”. The Predetermination of Benefits transaction has no unique requirements for the segments. (Note the Response Prior Authorization
Segment is not used in the Predetermination Of Benefits response (Transmission Accepted/Transaction Rejected).
8.3.3 TRANSMISSION REJECTED/TRANSACTION REJECTED
8.3.3.1 DIAGRAM FOR TRANSMISSION OF ONE PREDETERMINATION OF BENEFITS RESPONSE
(TRANSMISSION REJECTED/TRANSACTION REJECTED)
Predetermination Of Benefits transmission response Header Response Status (5Ø1-F1) of "R" (Rejected)
and Transaction Response Status (112-AN) of “R” (Rejected)
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
8.3.3.2 DIAGRAM FOR TRANSMISSION OF TWO PREDETERMINATION OF BENEFITS RESPONSES
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 126 -
(TRANSMISSION REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
8.3.3.3 DIAGRAM FOR TRANSMISSION OF THREE OR FOUR PREDETERMINATION OF BENEFITS
RESPONSES (TRANSMISSION REJECTED/TRANSACTION REJECTED)
These transaction diagrams will follow the example in the section “Diagram For Transmission Of Two Predetermination of Benefit Responses
(Transmission Rejected/Transaction Rejected)”. For three or four transactions, the Mandatory and Situational Predetermination Of Benefits
transaction segments will be repeated for the third and fourth transactions.
8.3.3.4 PREDETERMINATION OF BENEFITS RESPONSE SEGMENTS (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
All segments noted above in the Predetermination of Benefits Response diagram section must follow the Claim Billing Response
(Transmission Rejected/Transaction Rejected) diagrams and situations stated in this document with guidance in the section “Transmission
Structure”. The Predetermination of Benefits transaction has no unique requirements for the segments.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 127 -
9. SERVICE BILLING (PROFESSIONAL PHARMACY SERVICE)
INFORMATION
These messages include:
• Service Billing (S1)
• Service Reversal (S2)
• Service Rebill (S3)
Up to four transactions per transmission are permitted.
For Transaction Code of “S1” “S2” or “S3”, in the Claim Segment or Response Claim Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “2” (Service Billing).
If the Product/Service ID Qualifier (436-E1) is “Ø6” (DUR/PPS), the DUR/PPS Segment is required.
Billings may be for professional services rendered. Services may be correlated with a dispensing event or may be separate and unrelated to
any particular prescription. Professional pharmacy services may include but are not limited to blood pressure monitoring, taking a patient
history for a new disease or diagnosis, referring patients to other health care providers and counseling and education beyond the simple act of
describing a medication’s use and side effects.
See section “Response Processing Guidelines”, “Duplicate Transactions”.
9.1 SERVICE BILLING
These transactions are used by the Originator to request payment from the Processor for a specific patient for services billed according to
appropriate plan parameters. The Transaction Code is “S1”.
Each service submission request may contain up to four occurrences of claim/service data.
Depending upon the particular service submission request, the Processor must provide one of the following general types of responses:
Captured - This occurs when the Processor acknowledges receipt of the service, but is not making any judgment regarding eligibility
of the patient or payment for the service at this time.
Duplicate of Captured - This occurs when the Processor has previously received the request and processed the transaction, but the
response did not return to the Originator. The Duplicate response contains the same information as returned in the Captured original
response.
Paid - This occurs when the Processor captures and processes the service, and returns to the Originator the dollar amounts allowed
under the terms of the plan. The Paid response is not used in payer-to-payer transactions.
Duplicate of Paid - This occurs when the Processor has previously received the request and processed the transaction, but the
response did not return to the Originator. The Duplicate response contains the same information as returned in the Paid original
response. The Duplicate of Paid response is not used in payer-to-payer transactions.
Rejected - This occurs when the Processor has encountered an error in the transaction or processing.
9.2 SERVICE BILLING REQUEST DIAGRAMS
9.2.1 DIAGRAM FOR TRANSMISSION OF ONE SERVICE BILLING TRANSACTION
Service Billing to a Receiver
Service Billing Paid/Captured/Rejected Transaction Response from a Sender
Standard Transmission Rejected Response from a Sender
If the Product/Service ID Qualifier (436-E1) is “Ø6” (DUR/PPS), the DUR/PPS Segment is required.
The Compound Segment and the Prior Authorization Segment are not used in Service Billing requests.
Mandatory
Transaction Header Segment
Segment Separator
Insurance Segment
Situational
Segment Separator
Patient Segment
Mandatory - first service
Group Separator
Segment Separator
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 128 -
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
9.2.2 DIAGRAM FOR TRANSMISSION OF TWO SERVICE BILLING TRANSACTIONS
Mandatory
Transaction Header Segment
Segment Separator
Insurance Segment
Situational
Segment Separator
Patient Segment
Mandatory - first service
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
Mandatory - second service
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 129 -
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
9.2.3 DIAGRAM FOR TRANSMISSION OF THREE SERVICE BILLING TRANSACTIONS
Mandatory
Transaction Header Segment
Segment Separator
Insurance Segment
Situational
Segment Separator
Patient Segment
Mandatory - first service
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
Mandatory - second service
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 130 -
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
Mandatory - third service
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
9.2.4 DIAGRAM FOR TRANSMISSION OF FOUR SERVICE BILLING TRANSACTIONS
Mandatory
Transaction Header Segment
Segment Separator
Insurance Segment
Situational
Segment Separator
Patient Segment
Mandatory - first service
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Clinical Segment
Segment Separator
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 131 -
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
Mandatory - second service
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
Mandatory - third service
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
Mandatory - fourth service
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 132 -
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
9.3 SERVICE BILLING REQUEST SEGMENTS
9.3.1 TRANSACTION HEADER SEGMENT (SERVICE BILLING)
TRANSACTION HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø1-A1 BIN NUMBER M
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M For Transaction Code of “S1”, in the Claim Segment, the
Prescription/Service Reference Number Qualifier (455-EM)
is “2” (Service Billing).
1Ø4-A4 PROCESSOR CONTROL NUMBER M
1Ø9-A9 TRANSACTION COUNT M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M
Notes on Transaction Header Segment on a Service Billing Request:
The Transaction Header Segment is a mandatory, fixed length segment for a Service Billing request. The “Situation” column is not applicable.
9.3.2 INSURANCE SEGMENT (SERVICE BILLING)
INSURANCE SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø2-C2 CARDHOLDER ID M
312-CC CARDHOLDER FIRST NAME
Q Service Billing:
Required if necessary for state/federal/regulatory agency
programs when the cardholder has a first name.
313-CD CARDHOLDER LAST NAME
Q Service Billing:
Required if necessary for state/federal/regulatory agency
programs.
314-CE HOME PLAN
Q Service Billing:
Required if needed for receiver billing validation and/or
determination for Blue Cross or Blue Shield, if a Patient has
coverage under more than one plan, to distinguish each
plan.
524-FO PLAN ID
O Service Billing:
Optional.
3Ø9-C9 ELIGIBILITY CLARIFICATION CODE
Q Service Billing:
Required if needed for receiver inquiry validation and/or
determination, when eligibility is not maintained at the
dependent level. Required in special situations as defined
by the code to clarify the eligibility of an individual, which
may extend coverage.
3Ø1-C1 GROUP ID
Q Service Billing:
Required if necessary for state/federal/regulatory agency
programs.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 133 -
INSURANCE SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if needed for pharmacy claim processing and
payment.
3Ø3-C3 PERSON CODE
Q Service Billing:
Required if needed to uniquely identify the family members
within the Cardholder ID.
3Ø6-C6 PATIENT RELATIONSHIP CODE
Q Service Billing:
Required if needed to uniquely identify the relationship of
the Patient to the Cardholder ID.
99Ø-MG OTHER PAYER BIN NUMBER N Service Billing:
Not used.
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER N Service Billing:
Not used.
356-NU OTHER PAYER CARDHOLDER ID N Service Billing:
Not used.
992-MJ OTHER PAYER GROUP ID N Service Billing:
Not used.
359-2A MEDIGAP ID Q Service Billing:
Required, if known, when patient has Medigap coverage.
36Ø-2B MEDICAID INDICATOR Q Service Billing:
Required, if known, when patient has Medicaid coverage.
361-2D PROVIDER ACCEPT ASSIGNMENT INDICATOR Q Service Billing:
Required if necessary for state/federal/regulatory agency
programs.
997-G2 CMS PART D DEFINED QUALIFIED FACILITY N Service Billing:
Not used.
115-N5
MEDICAID ID NUMBER N Service Billing:
Not used.
116-N6
MEDICAID AGENCY NUMBER N Service Billing:
Not used.
Notes on Insurance Segment on a Service Billing Request:
The Insurance Segment is mandatory for a Service Billing request. Fields defined as Mandatory are required to be submitted when the
segment is sent.
9.3.3 PATIENT SEGMENT (SERVICE BILLING)
PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
331-CX PATIENT ID QUALIFIER Q Service Billing:
Required if Patient ID (332-CY) is used.
332-CY PATIENT ID Q Service Billing:
Required if necessary for state/federal/regulatory agency
programs to validate dual eligibility.
3Ø4-C4 DATE OF BIRTH
R Service Billing:
Required.
3Ø5-C5 PATIENT GENDER CODE
R Service Billing:
Required.
31Ø-CA PATIENT FIRST NAME
Q Service Billing:
Required when the patient has a first name.
311-CB PATIENT LAST NAME
R Service Billing:
Required.
322-CM PATIENT STREET ADDRESS
O Service Billing:
Optional.
323-CN PATIENT CITY ADDRESS
O Service Billing:
Optional.
324-CO PATIENT STATE / PROVINCE ADDRESS
O Service Billing:
Optional.
325-CP PATIENT ZIP/POSTAL ZONE
O Service Billing:
Optional.
326-CQ PATIENT PHONE NUMBER
O Service Billing:
Optional.
3Ø7-C7 PLACE OF SERVICE
Q Service Billing:
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
333-CZ EMPLOYER ID
Q Service Billing:
Required if “required by law” as defined in the HIPAA final
Privacy regulations section 164.5Ø1 definitions (45 CFR
Parts 160 and 164 Standards for Privacy of Individually
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 134 -
PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Identifiable Health Information; Final Rule -
Thursday, December 28, 2000, page 82803 and following,
and Wednesday, August 14, 2002, page 53267 and
following.)
Required if needed for Workers’ Compensation billing.
334-1C SMOKER / NON-SMOKER CODE
S Service Billing:
Not used.
335-2C PREGNANCY INDICATOR
Q Service Billing:
Required if pregnancy could result in different coverage,
pricing, or patient financial responsibility.
Required if “required by law” as defined in the HIPAA final
Privacy regulations section 164.5Ø1 definitions (45 CFR
Parts 160 and 164 Standards for Privacy of Individually
Identifiable Health Information; Final Rule -
Thursday, December 28, 2000, page 82803 and following,
and Wednesday, August 14, 2002, page 53267 and
following.)
35Ø-HN PATIENT E-MAIL ADDRESS I Service Billing:
May be submitted for the receiver to relay patient health
care communications via the Internet when provided by the
patient.
This field is informational only.
384-4X PATIENT RESIDENCE Q Service Billing:
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
Notes on Patient Segment on a Service Billing Request:
The Patient Segment is situational for a Service Billing request. It is used when a receiver needs some of the patient demographic information
to perform eligibility and service billing determination. The Patient Segment must be submitted when needed to differentiate between the
patient and the cardholder. If the cardholder and the patient are the same, then the Patient Segment is not submitted unless additional
information about the patient is needed to clarify the Service Billing determination. The Segment is mandatory if required under provider payer
contract or mandatory on service billings where this information is necessary for adjudication of the service. Fields defined as Mandatory are
required to be submitted when the segment is sent.
9.3.4 CLAIM SEGMENT (SERVICE BILLING)
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M For Transaction Code of “S1”, in the Claim Segment, the
Prescription/Service Reference Number Qualifier (455-EM)
is “2” (Service Billing).
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
436-E1 PRODUCT/SERVICE ID QUALIFIER M Mandatory.
If the Product/Service ID Qualifier (436-E1) = “Ø6”
(DUR/PPS), the Product/Service ID (4Ø7-D7) is zero. (Zero
means “Ø”.)
4Ø7-D7 PRODUCT/SERVICE ID M Service Billing:
If the Product/Service ID Qualifier (436-E1) = “Ø6”
(DUR/PPS), the Product/Service ID (4Ø7-D7) is zero. (Zero
means “Ø”.) Populate the DUR/PPS segment as
appropriate.
If the Product/Service ID Qualifier (436-E1) = “Ø7” (CPT-4),
the Product Service ID (4Ø7-D7) is the actual CPT-4 value.
If the Product/Service ID Qualifier (436-E1) = “Ø9”
(HCPCS), the Product Service ID (4Ø7-D7) is the actual
HCPCS value.
If the Product/Service ID Qualifier (436-E1) = “99” (Other),
the Product Service ID (4Ø7-D7) is the business partner
agreed value.
456-EN ASSOCIATED PRESCRIPTION/SERVICE REFERENCE NUMBER
Q Service Billing:
Required if needed to associate multiple
prescriptions/services from the same sender to allow billing
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 135 -
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
of the current prescription/service.
457-EP ASSOCIATED PRESCRIPTION/SERVICE DATE
Q Service Billing:
Required if Associated Prescription/Service Reference
Number (456-EN) is used.
Required if needed to associate multiple
prescriptions/services from the same sender to allow billing
of the current prescription/service.
458-SE PROCEDURE MODIFIER CODE COUNT
Q Service Billing:
Maximum count of 1Ø.
Required if Procedure Modifier Code (459-ER) is used.
459-ER PROCEDURE MODIFIER CODE
Q***R*** Service Billing:
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
Occurs the number of times identified in Procedure Modifier
Code Count (458-SE).
Required to define a further level of specificity if the
Product/Service ID (4Ø7-D7) indicated a Procedure Code
was submitted.
442-E7 QUANTITY DISPENSED
Q Service Billing:
Required if value is greater than zero (Ø).
4Ø3-D3 FILL NUMBER Q Service Billing:
Required if necessary for plan benefit administration.
4Ø5-D5 DAYS SUPPLY Q Service Billing:
Required if necessary for plan benefit administration.
4Ø6-D6 COMPOUND CODE N Service Billing:
Not used.
4Ø8-D8 DISPENSE AS WRITTEN (DAW)/PRODUCT SELECTION CODE N Service Billing:
Not used.
414-DE DATE PRESCRIPTION WRITTEN
Q Service Billing:
Required if necessary for plan benefit administration.
415-DF NUMBER OF REFILLS AUTHORIZED Q Service Billing:
Required if necessary for plan benefit administration.
419-DJ PRESCRIPTION ORIGIN CODE N Service Billing:
Not used.
354-NX SUBMISSION CLARIFICATION CODE COUNT N Service Billing:
Not used.
42Ø-DK SUBMISSION CLARIFICATION CODE N***R*** Service Billing:
Not used.
46Ø-ET QUANTITY PRESCRIBED
Q Service Billing:
Required if the prescriber orders a specific number of
iterations of a service.
Not required if value is equal to 1.
3Ø8-C8 OTHER COVERAGE CODE
Q Service Billing:
Required if needed by receiver, to communicate a
summation of other coverage information that has been
collected from other payers.
Required for Coordination of Benefits.
See section “Specific Segment Discussion”, “Request
Segments”, “Claim Segment”, “Other Coverage Code (3Ø8-
C8).
429-DT SPECIAL PACKAGING INDICATOR N Service Billing:
Not used.
453-EJ ORIGINALLY PRESCRIBED PRODUCT/SERVICE ID QUALIFIER Q Service Billing:
Required if Originally Prescribed Product/Service Code
(445-EA) is used.
445-EA ORIGINALLY PRESCRIBED PRODUCT/SERVICE CODE
Q Service Billing:
Required if the receiver requests association to a
therapeutic, or a preferred product substitution, or when a
DUR alert has been resolved by changing medications, or
an alternative service than what was originally prescribed.
446-EB ORIGINALLY PRESCRIBED QUANTITY
Q Service Billing:
Required if the receiver requests reporting for quantity
changes due to a therapeutic substitution that has occurred
or a preferred product/service substitution that has
occurred, or when a DUR alert has been resolved by
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 136 -
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
changing quantities.
33Ø-CW ALTERNATE ID
N Service Billing:
Not used.
454-EK SCHEDULED PRESCRIPTION ID NUMBER N Service Billing:
Not used.
6ØØ-28 UNIT OF MEASURE N Service Billing:
Not used.
418-DI LEVEL OF SERVICE
Q Service Billing:
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
461-EU PRIOR AUTHORIZATION TYPE CODE
Q Service Billing:
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
462-EV PRIOR AUTHORIZATION NUMBER SUBMITTED
Q Service Billing:
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
463-EW INTERMEDIARY AUTHORIZATION TYPE ID
Q Service Billing:
Required for overriding an authorized intermediary system
edit when the pharmacy participates with an intermediary.
Required if Intermediary Authorization ID (464-EX) is used.
Not used for payer-to-payer transactions.
464-EX INTERMEDIARY AUTHORIZATION ID
Q Service Billing:
Required for overriding an authorized intermediary system
edit when the pharmacy participates with an intermediary.
Not used for payer-to-payer transactions.
343-HD DISPENSING STATUS N Service Billing:
Not used.
344-HF QUANTITY INTENDED TO BE DISPENSED N Service Billing:
Not used.
345-HG DAYS SUPPLY INTENDED TO BE DISPENSED N Service Billing:
Not used.
357-NV DELAY REASON CODE Q Service Billing:
Required when needed to specify the reason that
submission of the transaction has been delayed.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Service Billing:
Not used.
391-MT PATIENT ASSIGNMENT INDICATOR (DIRECT MEMBER
REIMBURSEMENT INDICATOR)
Q Service Billing:
Required when the claims adjudicator does not assume the
patient assigned his/her benefits to the provider or when
the claims adjudicator supports a patient determination of
whether he/she wants to assign or retain his/her benefits.
995-E2 ROUTE OF ADMINISTRATION N Service Billing:
Not used.
996-G1 COMPOUND TYPE N Service Billing:
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Service Billing:
Not used.
147-U7 PHARMACY SERVICE TYPE Q Service Billing:
Required when the submitter must clarify the type of
services being performed as a condition for proper
reimbursement by the payer.
Notes on Claim Segment on a Service Billing Request:
The Claim Segment is mandatory for a Service Billing request. The Claim Segment defines the service performed, reference information for
tieback to an original prescription or service, or authorization information.
If the Prescription/Service Reference Number Qualifier (455-EM) is “2” (Service Billing) and the Product/Service ID Qualifier (436-E1) is “Ø6”
(DUR/PPS), the DUR/PPS Segment is required.
Fields defined as Mandatory are required to be submitted when the segment is sent.
9.3.5 PRICING SEGMENT (SERVICE BILLING)
PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 137 -
PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
4Ø9-D9 INGREDIENT COST SUBMITTED
N Service Billing:
Not used.
412-DC DISPENSING FEE SUBMITTED
N Service Billing:
Not used.
477-BE PROFESSIONAL SERVICE FEE SUBMITTED
R Service Billing:
Required.
433-DX PATIENT PAID AMOUNT SUBMITTED
Q Service Billing:
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
Not used in coordination of benefit claim to pass patient
liability information to a downstream payer. See section
“Standard Conventions”, “Repetition and Multiple
Occurrences”, Repeating Data Elements”, “Request
Segments”, “Coordination of Benefits/Other Payments
Segment”.
438-E3 INCENTIVE AMOUNT SUBMITTED
N Service Billing:
Not used.
478-H7 OTHER AMOUNT CLAIMED SUBMITTED COUNT
Q Service Billing:
Maximum count of 3.
Required if Other Amount Claimed Submitted Qualifier (479-
H8) is used.
479-H8 OTHER AMOUNT CLAIMED SUBMITTED QUALIFIER
Q***R*** Service Billing:
Required if Other Amount Claimed Submitted (48Ø-H9) is
used.
48Ø-H9 OTHER AMOUNT CLAIMED SUBMITTED
Q***R*** Service Billing:
Required if its value has an effect on the Gross Amount Due
(43Ø-DU) calculation.
Zero (Ø) is a valid value.
481-HA FLAT SALES TAX AMOUNT SUBMITTED
Q Service Billing:
Required if its value has an effect on the Gross Amount Due
(43Ø-DU) calculation.
Zero (Ø) is a valid value.
482-GE PERCENTAGE SALES TAX AMOUNT SUBMITTED
Q Service Billing:
Required if its value has an effect on the Gross Amount Due
(43Ø-DU) calculation.
Zero (Ø) is a valid value.
483-HE PERCENTAGE SALES TAX RATE SUBMITTED
Q Service Billing:
Required if needed to calculate Percentage Sales Tax
Amount Paid (559-AX).
484-JE PERCENTAGE SALES TAX BASIS SUBMITTED
N Service Billing:
Not used. Code list is not applicable.
426-DQ USUAL AND CUSTOMARY CHARGE
Q Service Billing:
Required if needed per trading partner agreement.
43Ø-DU GROSS AMOUNT DUE
R Service Billing:
Required.
See Pricing Formula for fields used in calculation.
423-DN BASIS OF COST DETERMINATION N Service Billing:
Not used.
113-N3
MEDICAID PAID AMOUNT N Service Billing:
Not used.
Notes on Pricing Segment on a Service Billing Request:
The Pricing Segment is mandatory for a Service Billing request. The Pricing Segment defines dollar amounts for a Service Billing.
See the pricing formulae.
It is highly recommended that whenever possible, the individual dollar fields are requested of the sender by the receiver. On the response, the
sender should return the individual payment response fields to allow the receiver to reconcile against the requested payment fields. It is
recommended that for the dollar fields, if the field is not required or situational in the calculation, that the dollar fields are not sent.
See section “Response Processing Guidelines”, “Pricing Guidelines”.
Fields defined as Mandatory are required to be submitted when the segment is sent.
9.3.6 PHARMACY PROVIDER SEGMENT (SERVICE BILLING)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 138 -
PHARMACY PROVIDER SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
465-EY PROVIDER ID QUALIFIER Q Service Billing:
Required if Provider ID (444-E9) is used.
444-E9 PROVIDER ID
Q Service Billing:
Required if necessary for state/federal/regulatory agency
programs.
Required if necessary to determine if provider is credentialed
to perform this service.
Required if needed for reconciliation of encounter-reported
data or encounter reporting.
Notes on Pharmacy Provider Segment on a Service Billing Request:
The Pharmacy Provider Segment is situational for a Service Billing request if required under provider payer contract or situational on service
billings where this information is necessary for adjudication of the service. Fields defined as Mandatory are required to be submitted when the
segment is sent.
9.3.7 PRESCRIBER SEGMENT (SERVICE BILLING)
PRESCRIBER SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
466-EZ PRESCRIBER ID QUALIFIER Q Service Billing:
Required if Prescriber ID (411-DB) is used.
411-DB PRESCRIBER ID
Q Service Billing:
Required if this field could result in different coverage or
patient financial responsibility.
Required if necessary for state/federal/regulatory agency
programs.
427-DR PRESCRIBER LAST NAME
Q Service Billing:
Required when the Prescriber ID (411-DB) is not known.
Required if needed for Prescriber ID (411-DB)
validation/clarification.
498-PM PRESCRIBER PHONE NUMBER
Q Service Billing:
Required if needed to assist in identifying the prescriber.
Required if needed for Prior Authorization process.
468-2E PRIMARY CARE PROVIDER ID QUALIFIER
Q Service Billing:
Required if Primary Care Provider ID (421-DL) is used.
421-DL PRIMARY CARE PROVIDER ID
Q Service Billing:
Required if needed for receiver service billing
determination, if known and available.
Required if this field could result in different coverage or
patient financial responsibility.
Required if necessary for state/federal/regulatory agency
programs.
47Ø-4E PRIMARY CARE PROVIDER LAST NAME
Q Service Billing:
Required if this field is used as an alternative for Primary
Care Provider ID (421-DL) when ID is not known.
Required if needed for Primary Care Provider ID (421-DL)
validation/clarification.
364-2J PRESCRIBER FIRST NAME
Q Service Billing:
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
365-2K PRESCRIBER STREET ADDRESS Q Service Billing:
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
366-2M PRESCRIBER CITY ADDRESS Q Service Billing:
Required if needed to assist in identifying the prescriber.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 139 -
PRESCRIBER SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if necessary for state/federal/regulatory agency
programs.
367-2N PRESCRIBER STATE/PROVINCE ADDRESS Q Service Billing:
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
368-2P PRESCRIBER ZIP/POSTAL ZONE Q Service Billing:
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
Notes on Prescriber Segment on a Service Billing Request:
The Prescriber Segment is situational for a Service Billing request. It is used when prescriber information is needed to perform Service Billing
determination. The Segment is mandatory if required under provider payer contract or mandatory on Service Billing where this information is
necessary for adjudication of the service. Fields defined as Mandatory are required to be submitted when the segment is sent.
9.3.8 COORDINATION OF BENEFITS /OTHER PAYMENTS SEGMENT (SERVICE BILLING)
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
337-4C COORDINATION OF BENEFITS/OTHER PAYMENTS COUNT M Maximum count of 9.
338-5C OTHER PAYER COVERAGE TYPE M***R*** Mandatory.
Occurs with Coordination of Benefits/Other Payments Count
(337-4C).
Grouped with Other Payer ID Qualifier (339-6C), Other Payer
ID (34Ø-7C), Other Payer Date (443-E8), and either Other
Payer Amount Paid Count (341-HB) and its grouping, or
Other Payer Reject Count (471-5E) and its grouping.
339-6C OTHER PAYER ID QUALIFIER Q***R*** Service Billing:
Required if Other Payer ID (34Ø-7C) is used.
34Ø-7C OTHER PAYER ID
Q***R*** Service Billing:
Required if identification of the Other Payer is necessary for
service billing adjudication.
443-E8 OTHER PAYER DATE
Q***R*** Service Billing:
Required if identification of the Other Payer Date is
necessary for service billing adjudication.
993-A7 INTERNAL CONTROL NUMBER Q***R*** Service Billing:
Required when used for payer-to-payer coordination of
benefits to track the claim without regard to the “Service
Provider ID, Prescription Number, & Date of Service”.
341-HB OTHER PAYER AMOUNT PAID COUNT
Q Service Billing:
Maximum count of 9.
Required if Other Payer Amount Paid Qualifier (342-HC) is
used.
342-HC OTHER PAYER AMOUNT PAID QUALIFIER
Q***R*** Service Billing:
Required if Other Payer Amount Paid (431-DV) is used.
431-DV OTHER PAYER AMOUNT PAID
Q***R*** Service Billing:
Required if other payer has approved payment for some/all
of the billing.
Zero (Ø) is a valid value.
Not used for patient financial responsibility only billing.
Not used for non-governmental agency programs if Other
Payer-Patient Responsibility Amount (352-NQ) is submitted.
471-5E OTHER PAYER REJECT COUNT Q Service Billing:
Maximum count of 5.
Required if Other Payer Reject Code (472-6E) is used.
472-6E OTHER PAYER REJECT CODE
Q***R*** Service Billing:
Required when the other payer has denied the payment for
the billing, designated with Other Coverage Code (3Ø8-C8) =
3 (Other Coverage Billed – claim not covered).
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 140 -
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Note: This field must only contain the NCPDP Reject Code
(511-FB) values.
353-NR OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT COUNT Q Service Billing:
Maximum count of 25.
Required if Other Payer-Patient Responsibility Amount
Qualifier (351-NP) is used.
Note the occurrences are dependent upon the number of
component parts returned from a previous payer.
351-NP OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT QUALIFIER Q***R*** Service Billing:
Required if Other Payer-Patient Responsibility Amount (352-
NQ) is used.
352-NQ OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT Q***R*** Service Billing:
Required if necessary for patient financial responsibility only
billing.
Required if necessary for state/federal/regulatory agency
programs.
Not used for non-governmental agency programs if Other
Payer Amount Paid (431-DV) is submitted.
392-MU BENEFIT STAGE COUNT Q Service Billing:
Not used.
393-MV BENEFIT STAGE QUALIFIER Q***R*** Service Billing:
Not used.
394-MW BENEFIT STAGE AMOUNT Q***R*** Service Billing:
Not used.
Notes on Coordination of Benefits/Other Payments Segment on a Service Billing Request:
The Coordination of Benefits/Other Payments Segment is situational for a Service Billing request. It is used when a receiver needs payment
information from other receivers to perform service billing determination. This may be in the case of primary, secondary, tertiary et cetera
health plan coverage for example.
The Coordination of Benefits/Other Payments Segment is mandatory for a Service Billing request to a downstream payer. It is used to assist
a downstream payer to uniquely identify a service billing in case of duplicate processing. Sometimes processors have difficulty determining
duplicate logic because the same processor is involved in multiple coordination of benefit occurrences for the same patient. They are involved
for example as the primary and secondary payer, or primary and tertiary, or secondary and tertiary. The downstream payer uses the fields
involved in duplicate logic, including the Other Payer Coverage Type (338-5C) to differentiate which service billing to process. See section
“Response Processing Guidelines”, “Duplicate Transactions”.
Note, the Other Payer Coverage Type (338-5C) occurrences do not have to appear in sequential order (primary, secondary, tertiary),
but can appear in any order.
The Coordination of Benefits/Other Payments Segment is not used for a Service Billing request to a primary payer.
The Segment is mandatory if required under provider payer contract or mandatory on Service Billing where this information is necessary for
adjudication of the service.
Fields defined as Mandatory are required to be submitted when the segment is sent.
9.3.9 WORKERS’ COMPENSATION SEGMENT (SERVICE BILLING)
WORKERS’ COMPENSATION SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
434-DY DATE OF INJURY M
315-CF EMPLOYER NAME Q Service Billing:
Required if needed to process a service billing for a work
related injury or condition.
316-CG EMPLOYER STREET ADDRESS Q Service Billing:
Required if needed to process a service billing for a work
related injury or condition.
317-CH EMPLOYER CITY ADDRESS Q Service Billing:
Required if needed to process a service billing for a work
related injury or condition.
318-CI EMPLOYER STATE/PROVINCE ADDRESS Q Service Billing:
Required if needed to process a service billing for a work
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 141 -
WORKERS’ COMPENSATION SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
related injury or condition.
319-CJ EMPLOYER ZIP/POSTAL ZONE Q Service Billing:
Required if needed to process a service billing for a work
related injury or condition.
32Ø-CK EMPLOYER PHONE NUMBER Q Service Billing:
Required if needed to process a service billing for a work
related injury or condition.
321-CL EMPLOYER CONTACT NAME Q Service Billing:
Required if needed to process a service billing for a work
related injury or condition.
327-CR CARRIER ID Q Service Billing:
Required if needed to process a service billing for a work
related injury or condition.
435-DZ CLAIM/REFERENCE ID Q Service Billing:
Required if needed to process a service billing for a work
related injury or condition.
117-TR BILLING ENTITY TYPE INDICATOR R Service Billing:
Required.
118-TS PAY TO QUALIFIER Q Service Billing:
Required if Pay To ID (119-TT) is used.
119-TT PAY TO ID Q Service Billing:
Required if transaction is submitted by a provider or agent,
but paid to another party.
12Ø-TU PAY TO NAME Q Service Billing:
Required if transaction is submitted by a provider or agent,
but paid to another party.
121-TV PAY TO STREET ADDRESS Q Service Billing:
Required if transaction is submitted by a provider or agent,
but paid to another party.
122-TW PAY TO CITY ADDRESS Q Service Billing:
Required if transaction is submitted by a provider or agent,
but paid to another party.
123-TX PAY TO STATE/PROVINCE ADDRESS Q Service Billing:
Required if transaction is submitted by a provider or agent,
but paid to another party.
124-TY PAY TO ZIP/POSTAL ZONE Q Service Billing:
Required if transaction is submitted by a provider or agent,
but paid to another party.
125-TZ GENERIC EQUIVALENT PRODUCT ID QUALIFIER Q Service Billing:
Required if Generic Equivalent Product ID (126-UA) is
used.
126-UA GENERIC EQUIVALENT PRODUCT ID Q Service Billing:
Required if necessary for state/federal/regulatory agency
programs.
Notes on Workers’ Compensation Segment on a Service Billing Request:
The Workers’ Compensation Segment is situational for a Service Billing request. It is used when processing a Service Billing for a work-related
injury or condition. Fields defined as Mandatory are required to be submitted when the segment is sent.
9.3.10 DUR/PPS SEGMENT (SERVICE BILLING)
DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
473-7E DUR/PPS CODE COUNTER
Q***R*** Service Billing:
Maximum 9 occurrences.
Required if DUR/PPS Segment is used.
439-E4 REASON FOR SERVICE CODE
Q***R*** Service Billing:
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
Required if this field affects payment for or documentation
of professional pharmacy service.
44Ø-E5 PROFESSIONAL SERVICE CODE
Q***R*** Service Billing:
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 142 -
DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if this field affects payment for or documentation
of professional pharmacy service.
441-E6 RESULT OF SERVICE CODE
Q***R*** Service Billing:
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
Required if this field affects payment for or documentation
of professional pharmacy service.
474-8E DUR/PPS LEVEL OF EFFORT
Q***R*** Service Billing:
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
Required if this field affects payment for or documentation
of professional pharmacy service.
475-J9 DUR CO-AGENT ID QUALIFIER Q***R*** Service Billing:
Required if DUR Co-Agent ID (476-H6) is used.
476-H6 DUR CO-AGENT ID
Q***R*** Service Billing:
Required if this field could result in different drug utilization
review outcome.
Required if this field affects payment for or documentation
of professional pharmacy service.
Notes on DUR/PPS Segment on a Service Billing Request:
The DUR/PPS Segment is situational for a Service Billing request. It is used when a sender notifies the receiver of information on the
appropriate selection to process the Service Billing. The DUR/PPS information may be sent on the initial submission or alternatively sent after
a DUR/PPS rejection from a receiver. The Segment is mandatory if required under provider payer contract or mandatory on Service Billing
where this information is necessary for adjudication of the service. Fields defined as Mandatory are required to be submitted when the
segment is sent.
9.3.11 CLINICAL SEGMENT (SERVICE BILLING)
CLINICAL SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
491-VE DIAGNOSIS CODE COUNT
Q Service Billing:
Maximum count of 5.
Required if Diagnosis Code Qualifier (492-WE) and
Diagnosis Code (424-DO) are used.
492-WE DIAGNOSIS CODE QUALIFIER Q***R*** Service Billing:
Required if Diagnosis Code (424-DO) is used.
424-DO DIAGNOSIS CODE
Q***R*** Service Billing:
The value for this field is obtained from the prescriber or
authorized representative.
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
Required if this field affects payment for professional
pharmacy service.
Required if this information can be used in place of prior
authorization.
Required if necessary for state/federal/regulatory agency
programs.
493-XE CLINICAL INFORMATION COUNTER
Q***R*** Service Billing:
Maximum 5 occurrences supported.
Grouped with Measurement fields (Measurement Date
(494-ZE), Measurement Time (495-H1), Measurement
Dimension (496-H2), Measurement Unit (497-H3),
Measurement Value (499-H4).
494-ZE MEASUREMENT DATE Q***R*** Service Billing:
Required if necessary when this field could result in
different coverage and/or drug utilization review outcome.
495-H1 MEASUREMENT TIME Q***R*** Service Billing:

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 143 -
CLINICAL SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if Time is known or has impact on measurement.
Required if necessary when this field could result in
different coverage and/or drug utilization review outcome.
496-H2 MEASUREMENT DIMENSION Q***R*** Service Billing:
Required if Measurement Unit (497-H3) and Measurement
Value (499-H4) are used.
Required if necessary when this field could result in
different coverage and/or drug utilization review outcome.
Required if necessary for patient’s weight and height when
billing Medicare for a claim that includes a Certificate of
Medical Necessity (CMN).
497-H3 MEASUREMENT UNIT Q***R*** Service Billing:
Required if Measurement Dimension (496-H2) and
Measurement Value (499-H4) are used.
Required if necessary for patient’s weight and height when
billing Medicare for a claim that includes a Certificate of
Medical Necessity (CMN).
Required if necessary when this field could result in
different coverage and/or drug utilization review outcome.
499-H4 MEASUREMENT VALUE Q***R*** Service Billing:
Required if Measurement Dimension (496-H2) and
Measurement Unit (497-H3) are used.
Required if necessary for patient’s weight and height when
billing Medicare for a claim that includes a Certificate of
Medical Necessity (CMN).
Required if necessary when this field could result in
different coverage and/or drug utilization review outcome.
Notes on Clinical Segment on a Service Billing Request:
The Clinical Segment is situational for a Service Billing request. It is used to specify clinical measurements and/or diagnosis information
associated with the Service Billing transaction. The Segment is mandatory if required under provider payer contract or mandatory on Service
Billing where this information is necessary for adjudication of the service. Fields defined as Mandatory are required to be submitted when the
segment is sent.
9.3.12 ADDITIONAL DOCUMENTATION SEGMENT (SERVICE BILLING)
ADDITIONAL DOCUMENTATION SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
369-2Q ADDITIONAL DOCUMENTATION TYPE ID M
374-2V REQUEST PERIOD BEGIN DATE Q Service Billing:
Required if necessary for state/federal/regulatory agency
programs.
375-2W REQUEST PERIOD RECERT/REVISED DATE Q Service Billing:
Required if necessary for state/federal/regulatory agency
programs.
Required if the Request Status (373-2U) = “2” (Revision) or
“3” (Recertification).
373-2U REQUEST STATUS Q Service Billing:
Required if necessary for state/federal/regulatory agency
programs.
371-2S LENGTH OF NEED QUALIFIER Q Service Billing:
Required if Length of Need (37Ø-2R) is used.
37Ø-2R LENGTH OF NEED Q Service Billing:
Required if necessary for state/federal/regulatory agency
programs.
372-2T PRESCRIBER/SUPPLIER DATE SIGNED Q Service Billing:
Required if necessary for state/federal/regulatory agency
programs.
376-2X SUPPORTING DOCUMENTATION Q Service Billing:
Required if necessary for state/federal/regulatory agency
programs (using Section C of Medicare’s CMN forms).
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 144 -
ADDITIONAL DOCUMENTATION SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
377-2Z QUESTION NUMBER/LETTER COUNT Q Service Billing:
Maximum count of 5Ø.
Required if needed to provide response to narratives.
378-4B QUESTION NUMBER/LETTER Q***R*** Service Billing:
Required if necessary for state/federal/regulatory agency
programs to respond to questions included on a Medicare
form.
Required if Question Number/Letter
Count (377-2Z) is greater than Ø.
379-4D QUESTION PERCENT RESPONSE Q***R*** Service Billing:
Required if necessary for state/federal/regulatory agency
programs to respond to questions included on a Medicare
form that requires a percent as the response. (At least one
response is required per question.)
38Ø-4G QUESTION DATE RESPONSE Q***R*** Service Billing:
Required if necessary for state/federal/regulatory agency
programs to respond to questions included on a Medicare
form that requires a date as the response. (At least one
response is required per question.)
381-4H QUESTION DOLLAR AMOUNT RESPONSE Q***R*** Service Billing:
Required if necessary for state/federal/regulatory agency
programs to respond to questions included on a Medicare
form that requires a dollar amount as the response. (At
least one response is required per question.)
382-4J QUESTION NUMERIC RESPONSE Q***R*** Service Billing:
Required if necessary for
State/federal/regulatory agency programs to respond to
questions included on a Medicare form that requires a
numeric as the response. (At least one response is
required per question.)
383-4K QUESTION ALPHANUMERIC RESPONSE Q***R*** Service Billing:
Required if necessary for state/federal/regulatory agency
programs to respond to questions included on a Medicare
form that requires an alphanumeric as the response. (At
least one response is required per question.)
Notes on Additional Documentation Segment on a Service Billing:
The Additional Documentation Segment is situational for Service Billing request. It is used to provide additional information on Medicare forms.
Fields defined as Mandatory are required to be submitted when the segment is sent.
9.3.13 FACILITY SEGMENT (SERVICE BILLING)
FACILITY SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
336-8C FACILITY ID Q Service Billing:
Required if necessary for state/federal/regulatory agency
programs.
Required if “required by law” as defined in the HIPAA final
Privacy regulations section 164.5Ø1 definitions (45 CFR
Parts 160 and 164 Standards for Privacy of Individually
Identifiable Health Information; Final Rule -
Thursday, December 28, 2000, page 82803 and following,
and Wednesday, August 14, 2002, page 53267 and
following.)
385-3Q FACILITY NAME Q Service Billing:
Required if necessary for state/federal/regulatory agency
programs.
386-3U FACILITY STREET ADDRESS Q Service Billing:
Required if necessary for state/federal/regulatory agency
programs.
388-5J FACILITY CITY ADDRESS Q Service Billing:
Required if necessary for state/federal/regulatory agency
programs.
387-3V FACILITY STATE/PROVINCE ADDRESS Q Service Billing/:
Required if necessary for state/federal/regulatory agency
programs.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 145 -
FACILITY SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
389-6D FACILITY ZIP/POSTAL ZONE Q Service Billing:
Required if necessary for state/federal/regulatory agency
programs.
Notes on Facility Segment on a Service Billing Request:
The Facility Segment is situational for Service Billing request. It is used when these fields could result in different coverage, pricing, and/or
patient financial responsibility. Fields defined as Mandatory are required to be submitted when the segment is sent.
9.3.14 NARRATIVE SEGMENT (SERVICE BILLING)
NARRATIVE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
39Ø-BM NARRATIVE MESSAGE Q Service Billing:
Required if necessary only to support exception handling of
pharmacy claims for Medicare Part B claim billing.
Notes on Narrative Segment on a Service Billing Request:
The Narrative Segment is situational for Service Billing request. It is used to support exception handling for Medicare service billing. Fields
defined as Mandatory are required to be submitted when the segment is sent.
9.4 SERVICE BILLING RESPONSE DIAGRAMS AND SEGMENTS
9.4.1 TRANSMISSION ACCEPTED/TRANSACTION PAID
9.4.1.1 DIAGRAM FOR TRANSMISSION OF ONE SERVICE BILLING RESPONSE (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
Service Billing transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid)
The Paid or Duplicate of Paid response is not used in payer-to-payer transactions.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
9.4.1.2 DIAGRAM FOR TRANSMISSION OF TWO SERVICE BILLING RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
Mandatory
Response Header Segment
Situational
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 146 -
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
9.4.1.3 DIAGRAM FOR TRANSMISSION OF THREE SERVICE BILLING RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 147 -
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
9.4.1.4 DIAGRAM FOR TRANSMISSION OF FOUR SERVICE BILLING RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 148 -
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
9.4.1.5 SERVICE BILLING RESPONSE SEGMENTS (TRANSMISSION ACCEPTED/TRANSACTION PAID)
9.4.1.5.1 RESPONSE HEADER SEGMENT (SERVICE BILLING) (TRANSMISSION ACCEPTED/TRANSACTION PAID)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M For Transaction Code of “S1”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “2” (Service Billing).
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Service Billing Response:
The Response Header Segment is a mandatory, fixed length segment for Service Billing response when the Header Response Status (5Ø1-
F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid). The “Situation” column is not
applicable.
9.4.1.5.2 RESPONSE MESSAGE SEGMENT (SERVICE BILLING) (TRANSMISSION ACCEPTED/TRANSACTION PAID)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Service Billing:
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 149 -
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
transaction-level text.
Notes on Response Message Segment on a Service Billing Response:
The Response Message Segment is situational for Service Billing response when the Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid). It is used when additional text information needs to be
sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
9.4.1.5.3 RESPONSE INSURANCE SEGMENT (SERVICE BILLING) (TRANSMISSION ACCEPTED/TRANSACTION PAID)
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø1-C1 GROUP ID Q Service Billing:
Required if needed to identify the actual cardholder or
employer group, to identify appropriate group number,
when available.
Required to identify the actual group that was used when
multiple group coverages exist.
Note: This field may contain the Group ID echoed from the
request. May contain the actual Group ID if unknown to the
receiver.
524-FO PLAN ID
Q Service Billing:
Required if needed to identify the actual plan parameters,
benefit, or coverage criteria, when available.
Required to identify the actual plan ID that was used when
multiple group coverages exist.
Required if needed to contain the actual plan ID if unknown
to the receiver.
545-2F NETWORK REIMBURSEMENT ID Q Service Billing:
Required if needed to identify the network for the covered
member.
Required if needed to identify the actual Network
Reimbursement ID, when applicable and/or available.
Required to identify the actual Network Reimbursement ID
that was used when multiple Network Reimbursement IDs
exist.
568-J7 PAYER ID QUALIFIER Q Service Billing:
Required if Payer ID (569-J8) is used.
569-J8 PAYER ID
Q Service Billing:
Required to identify the ID of the payer responding.
115-N5
MEDICAID ID NUMBER N Service Billing:
Not used.
116-N6
MEDICAID AGENCY NUMBER N Service Billing:
Not used.
3Ø2-C2
CARDHOLDER ID Q Service Billing:
Required if the identification to be used in future
transactions is different than what was submitted on the
request.
Notes on Response Insurance Segment on a Service Billing Response:
The Response Insurance Segment is situational for Service Billing response when the Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid). It is used when coverage or reimbursement parameters or
identifiers need to be sent from the sender to the receiver. Fields defined as Mandatory are required to be submitted when the segment is
sent.
9.4.1.5.4 RESPONSE PATIENT SEGMENT (SERVICE BILLING) (TRANSMISSION ACCEPTED/TRANSACTION PAID)
RESPONSE PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
31Ø-CA PATIENT FIRST NAME Q Service Billing:
Required if known.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 150 -
RESPONSE PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
311-CB PATIENT LAST NAME Q Service Billing:
Required if known.
3Ø4-C4 DATE OF BIRTH Q Service Billing:
Required if known.
Notes on Response Patient Segment on a Service Billing Response:
The Response Patient Segment is situational for Service Billing transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid) when patient demographic information needs to be sent
from the sender to the receiver. Fields defined as Mandatory are required to be submitted when the segment is sent.
9.4.1.5.5 RESPONSE STATUS SEGMENT (SERVICE BILLING) (TRANSMISSION ACCEPTED/TRANSACTION PAID)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER
Q Service Billing:
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT N Service Billing:
Not used.
511-FB REJECT CODE
N**R*** Service Billing:
Not used.
546-4F REJECT FIELD OCCURRENCE INDICATOR
N***R*** Service Billing:
Not used.
547-5F APPROVED MESSAGE CODE COUNT
Q Service Billing:
Maximum count of 5.
Required if Approved Message Code (548-6F) is used.
548-6F APPROVED MESSAGE CODE
Q***R*** Service Billing:
Required if Approved Message Code Count (547-5F) is
used and the sender needs to communicate additional
follow up for a potential opportunity.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Service Billing:
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Service Billing:
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Service Billing:
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Service Billing:
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 151 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
549-7F HELP DESK PHONE NUMBER QUALIFIER
Q Service Billing:
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Service Billing:
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Service Billing:
Not used.
993-A7 INTERNAL CONTROL NUMBER Q Service Billing:
Required when used for payer-to-payer coordination of
benefits to track the claim without regard to the “Service
Provider ID, Prescription Number, & Date of Service”.
987-MA URL N Service Billing:
Not used.
Notes on Response Status Segment on a Service Billing Response:
The Response Status Segment is mandatory for a Service Billing Response for Header Response Status (5Ø1-F1) = “A” (Accepted) and
Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid).
The Response Status Segment is sent from the sender to the receiver to identify the outcome of the request.
If subsequent payer(s) for this patient is not known, the Other Payer information is not sent.
If subsequent payer(s) for this patient is known, the following may be sent:
• Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C),
• Other Payer Group ID (992-MJ),
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU).
In addition, if any of the following three fields are sent:
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU),
• Other Payer Group ID (992-MJ),
then the Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C) must be sent.
Fields defined as Mandatory are required to be submitted when the segment is sent.
9.4.1.5.6 RESPONSE CLAIM SEGMENT (SERVICE BILLING) (TRANSMISSION ACCEPTED/TRANSACTION PAID)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M For Transaction Code of “S1”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “2” (Service Billing).
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT
N Service Billing:
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER N***R*** Service Billing:
Not used.
553-AR PREFERRED PRODUCT ID
N***R*** Service Billing:
Not used.
554-AS PREFERRED PRODUCT INCENTIVE
N***R*** Service Billing:
Not used.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE N***R*** Service Billing:
Not used.
556-AU PREFERRED PRODUCT DESCRIPTION
N***R*** Service Billing:
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Service Billing:
Not used.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 152 -
Notes on Response Claim Segment on a Service Billing Response:
The Response Claim Segment is mandatory for a Service Billing Response when the Header Response Status (5Ø1-F1) is “A” (Accepted) and
Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid). The Response Claim Segment is sent from the sender to the
receiver to mirror back the Prescription/Service Reference Number (4Ø2-D2). Fields defined as Mandatory are required to be submitted when
the segment is sent.
9.4.1.5.7 RESPONSE PRICING SEGMENT (SERVICE BILLING) (TRANSMISSION ACCEPTED/TRANSACTION PAID)
RESPONSE PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø5-F5 PATIENT PAY AMOUNT
R Service Billing:
Required.
5Ø6-F6 INGREDIENT COST PAID
N Service Billing:
Not used.
5Ø7-F7 DISPENSING FEE PAID
N Service Billing:
Not used.
557-AV TAX EXEMPT INDICATOR
Q Service Billing:
Required if the sender (health plan) and/or patient is tax
exempt and exemption applies to this billing.
558-AW FLAT SALES TAX AMOUNT PAID
Q Service Billing:
Required if Flat Sales Tax Amount Submitted (481-HA) is
greater than zero (Ø) or if Flat Sales Tax Amount Paid
(558-AW) is used to arrive at the final reimbursement. Zero
(Ø) value is valid.
559-AX PERCENTAGE SALES TAX AMOUNT PAID
Q Service Billing:
Required if Percentage Sales Tax Amount Submitted (482-
GE) is greater than zero (Ø) or if Percentage Sales Tax
Amount Paid (559-AX) is used to arrive at the final
reimbursement. Zero (Ø) value is valid.
Required if Percentage Sales Tax Rate Paid (56Ø-AY) is
used.
56Ø-AY PERCENTAGE SALES TAX RATE PAID
Q Service Billing:
Required if Percentage Sales Tax Amount Paid (559-AX) is
greater than zero (Ø).
561-AZ PERCENTAGE SALES TAX BASIS PAID
N Service Billing:
Not used. Code list is not applicable.
521-FL INCENTIVE AMOUNT PAID
N Service Billing:
Not used. Not supported in Service Billing formula.
562-J1 PROFESSIONAL SERVICE FEE PAID
R Service Billing:
Required.
563-J2 OTHER AMOUNT PAID COUNT
Q Service Billing:
Maximum count of 3.
Required if Other Amount Paid (565-J4) is used.
564-J3 OTHER AMOUNT PAID QUALIFIER Q***R*** Service Billing:
Required if Other Amount Paid (565-J4) is used.
565-J4 OTHER AMOUNT PAID
Q***R*** Service Billing:
Required if Other Amount Claimed Submitted (48Ø-H9) is
greater than zero (Ø) or if Other Amount Paid (565-J4) is
used to arrive at the final reimbursement. This field may be
equal to zero (Ø). Must respond to each occurrence
submitted.
566-J5 OTHER PAYER AMOUNT RECOGNIZED
Q Service Billing:
Required if Other Payer Amount Paid (431-DV) is greater
than zero (Ø) or if this field is used to arrive at the final
reimbursement. This field may be equal to zero (Ø).
5Ø9-F9 TOTAL AMOUNT PAID
R Service Billing:
Required. Zero (Ø) value is valid.
See Pricing Formula for fields used in calculation.
522-FM BASIS OF REIMBURSEMENT DETERMINATION
N Service Billing:
Not used. Definition is not applicable.
523-FN AMOUNT ATTRIBUTED TO SALES TAX
Q Service Billing:
Required if Patient Pay Amount (5Ø5-F5) includes sales tax
that is the financial responsibility of the member but is not
also included in any of the other fields that add up to
Patient Pay Amount.
See section “Specific Segment Discussion”, “Response
Segments”, “Response Pricing Segment” for guidance.
512-FC ACCUMULATED DEDUCTIBLE AMOUNT I Service Billing:
Provided for informational purposes only.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 153 -
RESPONSE PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
513-FD REMAINING DEDUCTIBLE AMOUNT I Service Billing:
Provided for informational purposes only.
514-FE REMAINING BENEFIT AMOUNT
I Service Billing:
Provided for informational purposes only.
The Remaining Benefit Amount must not be returned with
zeroes unless there are no benefit dollars remaining. The
default value of 999999999 from previous versions must
not be used as a default in this field.
517-FH AMOUNT APPLIED TO PERIODIC DEDUCTIBLE
Q Service Billing:
Required if Patient Pay Amount (5Ø5-F5) includes
deductible.
518-FI AMOUNT OF COPAY
Q Service Billing:
Required if Patient Pay Amount (5Ø5-F5) includes copay
as patient financial responsibility.
52Ø-FK AMOUNT EXCEEDING PERIODIC BENEFIT MAXIMUM
Q Service Billing:
Required if Patient Pay Amount (5Ø5-F5) includes amount
exceeding periodic benefit maximum.
346-HH BASIS OF CALCULATION—DISPENSING FEE
N Service Billing:
Not used.
347-HJ BASIS OF CALCULATION—COPAY
N Service Billing:
Not used.
348-HK BASIS OF CALCULATION—FLAT SALES TAX
N Service Billing:
Not used.
349-HM BASIS OF CALCULATION—PERCENTAGE SALES TAX N Service Billing:
Not used.
571-NZ AMOUNT ATTRIBUTED TO PROCESSOR FEE Q Service Billing:
Required if the customer is responsible for 1ØØ% of the
service payment and when the provider net sale is less
than the amount the customer is expected to pay.
575-EQ PATIENT SALES TAX AMOUNT I Service Billing:
Used when necessary to identify the Patient’s portion of the
Sales Tax.
Provided for informational purposes only.
574-2Y PLAN SALES TAX AMOUNT I Service Billing:
Used when necessary to identify the Plan’s portion of the
Sales Tax.
Provided for informational purposes only.
572-4U AMOUNT OF COINSURANCE Q Service Billing:
Required if Patient Pay Amount (5Ø5-F5) includes
coinsurance as patient financial responsibility.
573-4V BASIS OF CALCULATION-COINSURANCE N Service Billing:
Not used.
392-MU BENEFIT STAGE COUNT Q Service Billing:
Maximum count of 4.
Required if Benefit Stage Amount (394-MW) is used.
393-MV BENEFIT STAGE QUALIFIER Q***R*** Service Billing:
Required if Benefit Stage Amount (394-MW) is used.
Must only have one value per iteration - value must not be
repeated.
394-MW BENEFIT STAGE AMOUNT Q***R*** Service Billing:
Required when a Medicare Part D payer applies financial
amounts to Medicare Part D beneficiary benefit stages.
This field is required when the plan is a participant in a
Medicare Part D program that requires reporting of benefit
stage specific financial amounts.
Required if necessary for state/federal/regulatory agency
programs.
577-G3 ESTIMATED GENERIC SAVINGS N Service Billing:
Not used.
128-UC SPENDING ACCOUNT AMOUNT REMAINING I Service Billing:
This dollar amount will be provided, if known, to the
receiver when the transaction had spending account dollars
reported as part of the patient pay amount.
This field is informational only. It is reported back to the
provider and the patient the amount remaining on the

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 154 -
RESPONSE PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
spending account after the current claim updated the
spending account.
129-UD HEALTH PLAN-FUNDED ASSISTANCE AMOUNT Q Service Billing:
Required when the patient meets the plan-funded
assistance criteria, to reduce Patient Pay Amount (5Ø5-
F5). The resulting Patient Pay Amount (5Ø5-F5) must be
greater than or equal to zero.
This field is always a negative amount or zero.
133-UJ AMOUNT ATTRIBUTED TO PROVIDER NETWORK SELECTION Q Service Billing:
Required if Patient Pay Amount (5Ø5-F5) includes an
amount that is attributable to a cost share differential due to
the selection of one pharmacy over another.
134-UK AMOUNT ATTRIBUTED TO PRODUCT SELECTION/BRAND
DRUG
N Service Billing:
Not used.
135-UM AMOUNT ATTRIBUTED TO PRODUCT SELECTION/NON-
PREFERRED FORMULARY SELECTION
N Service Billing:
Not used.
136-UN AMOUNT ATTRIBUTED TO PRODUCT SELECTION/BRAND
NON-PREFERRED FORMULARY SELECTION
N Service Billing:
Not used.
137-UP AMOUNT ATTRIBUTED TO COVERAGE GAP Q Service Billing:
Required when the patient’s financial responsibility is due to
the coverage gap.
148-U8 INGREDIENT COST CONTRACTED/REIMBURSABLE AMOUNT N Service Billing:
Not used.
149-U9 DISPENSING FEE CONTRACTED/REIMBURSABLE AMOUNT N Service Billing:
Not used.
Notes on Response Pricing Segment on a Service Billing Response:
The Response Pricing Segment is mandatory for a Service Billing Response when the Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) is “P” (Paid) or “D” (Duplicate of Paid).
It is highly recommended that whenever possible, the individual dollar fields are returned in the response. On the response the sender should
return the individual payment response fields to allow the receiver to reconcile against the requested payment fields.
See section “Response Processing Guidelines”, “Pricing Guidelines”.
Fields defined as Mandatory are required to be submitted when the segment is sent.
9.4.1.5.8 RESPONSE DUR/PPS SEGMENT (SERVICE BILLING) (TRANSMISSION ACCEPTED/TRANSACTION PAID)
RESPONSE DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
567-J6 DUR/PPS RESPONSE CODE COUNTER
Q***R*** Service Billing:
Maximum 9 occurrences.
Required if Reason For Service Code (439-E4) is used.
439-E4 REASON FOR SERVICE CODE Q***R*** Service Billing:
Required if professional service opportunity reason is
detected by the receiver that is different from the
professional service submitted.
528-FS CLINICAL SIGNIFICANCE CODE
Q***R*** Service Billing:
Required if needed to supply additional information for the
service.
529-FT OTHER PHARMACY INDICATOR
Q***R*** Service Billing:
Required if needed to supply additional information for the
service.
53Ø-FU PREVIOUS DATE OF FILL
Q***R*** Service Billing:
Required if needed to supply additional information for the
service.
Required if Quantity of Previous Fill (531-FV) is used.
531-FV QUANTITY OF PREVIOUS FILL
Q***R*** Service Billing:
Required if needed to supply additional information for the
service.
Required if Previous Date Of Fill (53Ø-FU) is used.
532-FW DATABASE INDICATOR
Q***R*** Service Billing:
Required if needed to supply additional information for the
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 155 -
RESPONSE DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
service.
533-FX OTHER PRESCRIBER INDICATOR
Q***R*** Service Billing:
Required if needed to supply additional information for the
service.
544-FY DUR FREE TEXT MESSAGE
Q***R*** Service Billing:
Required if needed to supply additional information for the
service.
57Ø-NS DUR ADDITIONAL TEXT Q***R*** Service Billing:
Required if needed to supply additional information for the
service.
Notes on Response DUR/PPS Segment on a Service Billing Response:
The Response DUR/PPS Segment is situational for a Service Billing Response when the Header Response Status (5Ø1-F1) is “A” (Accepted)
and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid). This would be used when a processor identifies an
additional professional pharmacy service billing opportunity. Fields defined as Mandatory are required to be submitted when the segment is
sent.
9.4.1.5.9 RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS SEGMENT (SERVICE BILLING) (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS
SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
355-NT OTHER PAYER ID COUNT M Service Billing:
Maximum count of 3.
338-5C OTHER PAYER COVERAGE TYPE M***R***
339-6C OTHER PAYER ID QUALIFIER Q***R*** Service Billing:
Required if Other Payer ID (34Ø-7C) is used.
34Ø-7C OTHER PAYER ID Q***R*** Service Billing:
Required if other insurance information is available for
coordination of benefits.
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER Q***R*** Service Billing:
Required if other insurance information is available for
coordination of benefits.
356-NU OTHER PAYER CARDHOLDER ID Q***R*** Service Billing:
Required if other insurance information is available for
coordination of benefits.
992-MJ OTHER PAYER GROUP ID Q***R*** Service Billing:
Required if other insurance information is available for
coordination of benefits.
142-UV OTHER PAYER PERSON CODE Q***R*** Service Billing:
Required if needed to uniquely identify the family members
within the Cardholder ID, as assigned by the other payer.
127-UB OTHER PAYER HELP DESK PHONE NUMBER Q***R*** Service Billing:
Required if needed to provide a support telephone number
of the other payer to the receiver.
143-UW OTHER PAYER PATIENT RELATIONSHIP CODE Q***R*** Service Billing:
Required if needed to uniquely identify the relationship of
the patient to the cardholder ID, as assigned by the other
payer.
144-UX OTHER PAYER BENEFIT EFFECTIVE DATE Q***R*** Service Billing:
Required when other coverage is known which is after the
Date of Service submitted.
145-UY OTHER PAYER BENEFIT TERMINATION DATE Q***R*** Service Billing:
Required when other coverage is known which is after the
Date of Service submitted.
Notes on Response Coordination of Benefits/Other Payers Segment on a Service Billing Response:
The Response Coordination of Benefits/Other Payers Segment is situational for a Service Billing response when the Header Response Status
(5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid) when other insurance
information is available for coordination of benefits.
If subsequent payer(s) for this patient is not known, the Other Payer information is not sent.
If subsequent payer(s) for this patient is known, the following may be sent:
• Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C),
• Other Payer Group ID (992-MJ),
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 156 -
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU)
• And other Other Payer fields.
In addition, if any of the following three fields are sent:
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU),
• Other Payer Group ID (992-MJ),
then the Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C) must be sent.
Fields defined as Mandatory are required to be submitted when the segment is sent.
9.4.2 TRANSMISSION ACCEPTED/TRANSACTION CAPTURED
9.4.2.1 DIAGRAM FOR TRANSMISSION OF ONE SERVICE BILLING RESPONSE (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
Service Billing transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured)
The Response Pricing Segment is not used in payer-to-payer transactions. Therefore, in this case, there are no situational transaction-level
segments.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Pricing Segment
9.4.2.2 DIAGRAM FOR TRANSMISSION OF TWO SERVICE BILLING RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Pricing Segment

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 157 -
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Pricing Segment
9.4.2.3 DIAGRAM FOR TRANSMISSION OF THREE SERVICE BILLING RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Pricing Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Pricing Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Pricing Segment
9.4.2.4 DIAGRAM FOR TRANSMISSION OF FOUR SERVICE BILLING RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 158 -
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Pricing Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Pricing Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Pricing Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Pricing Segment
9.4.2.5 SERVICE BILLING RESPONSE SEGMENTS (TRANSMISSION ACCEPTED/TRANSACTION
CAPTURED)
9.4.2.5.1 RESPONSE HEADER SEGMENT (SERVICE BILLING) (TRANSMISSION ACCEPTED/TRANSACTION
CAPTURED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M For Transaction Code of “S1”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “2” (Service Billing).
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Service Billing Response:
The Response Header Segment is a mandatory, fixed length segment for Service Billing response when the Header Response Status (5Ø1-
F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). The “Situation” column is
not applicable.
9.4.2.5.2 RESPONSE MESSAGE SEGMENT (SERVICE BILLING) (TRANSMISSION ACCEPTED/TRANSACTION
CAPTURED)

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 159 -
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Service Billing:
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Service Billing Response:
The Response Message Segment is situational for Service Billing response when the Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). It is used when additional text information needs
to be sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
9.4.2.5.3 RESPONSE INSURANCE SEGMENT (SERVICE BILLING) (TRANSMISSION ACCEPTED/TRANSACTION
CAPTURED)
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø1-C1 GROUP ID Q Service Billing:
Required if needed to identify the actual cardholder or
employer group, to identify appropriate group number,
when available.
Required to identify the actual group that was used when
multiple group coverages exist.
Note: This field may contain the Group ID echoed from the
request. May contain the actual Group ID if unknown to the
receiver.
524-FO PLAN ID
Q Service Billing:
Required if needed to identify the actual plan parameters,
benefit, or coverage criteria, when available.
Required to identify the actual plan ID that was used when
multiple group coverages exist.
Required if needed to contain the actual plan ID if unknown
to the receiver.
545-2F NETWORK REIMBURSEMENT ID N Service Billing:
Not used.
568-J7 PAYER ID QUALIFIER N Service Billing:
Not used.
569-J8 PAYER ID N Service Billing:
Not used.
115-N5
MEDICAID ID NUMBER N Service Billing:
Not used.
116-N6
MEDICAID AGENCY NUMBER N Service Billing:
Not used.
3Ø2-C2
CARDHOLDER ID Q Service Billing:
Required if the identification to be used in future
transactions is different than what was submitted on the
request.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 160 -
Notes on Response Insurance Segment on a Service Billing Response:
The Response Insurance Segment is situational for Service Billing response when the Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). It is used when coverage information may be
provided from the sender to the receiver. Fields defined as Mandatory are required to be submitted when the segment is sent.
9.4.2.5.4 RESPONSE PATIENT SEGMENT (SERVICE BILLING) (TRANSMISSION ACCEPTED/TRANSACTION
CAPTURED)
RESPONSE PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
31Ø-CA PATIENT FIRST NAME Q Service Billing:
Required if known.
311-CB PATIENT LAST NAME Q Service Billing:
Required if known.
3Ø4-C4 DATE OF BIRTH Q Service Billing:
Required if known.
Notes on Response Patient Segment on a Service Billing Response:
The Response Patient Segment is situational for Service Billing transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured) when patient demographic information needs to
be sent from the sender to the receiver. Fields defined as Mandatory are required to be submitted when the segment is sent.
9.4.2.5.5 RESPONSE STATUS SEGMENT (SERVICE BILLING) (TRANSMISSION ACCEPTED/TRANSACTION
CAPTURED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER
Q Service Billing:
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT N Service Billing:
Not used.
511-FB REJECT CODE
N***R*** Service Billing:
Not used.
546-4F REJECT FIELD OCCURRENCE INDICATOR
N***R*** Service Billing:
Not used.
547-5F APPROVED MESSAGE CODE COUNT
N Service Billing:
Not used.
548-6F APPROVED MESSAGE CODE
N***R*** Service Billing:
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Service Billing:
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Service Billing:
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Service Billing:
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 161 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Service Billing:
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER
Q Service Billing:
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Service Billing:
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Service Billing:
Not used.
993-A7 INTERNAL CONTROL NUMBER Q Service Billing:
Required when used for payer-to-payer coordination of
benefits to track the claim without regard to the “Service
Provider ID, Prescription Number, & Date of Service”.
987-MA URL N Service Billing:
Not used.
Notes on Response Status Segment on a Service Billing Response:
The Response Status Segment is mandatory for a Service Billing Response for Header Response Status (5Ø1-F1) = “A” (Accepted) and
Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). The Response Status Segment is sent from the
sender to the receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is
sent.
9.4.2.5.6 RESPONSE CLAIM SEGMENT (SERVICE BILLING) (TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M For Transaction Code of “S1”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “2” (Service Billing).
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT
N Service Billing:
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER N***R*** Service Billing:
Not used.
553-AR PREFERRED PRODUCT ID
N***R*** Service Billing:
Not used.
554-AS PREFERRED PRODUCT INCENTIVE
N***R*** Service Billing:
Not used.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE N***R*** Service Billing:
Not used.
556-AU PREFERRED PRODUCT DESCRIPTION
N***R*** Service Billing:
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Service Billing:
Not used.
Notes on Response Claim Segment on a Service Billing Response:
The Response Claim Segment is mandatory for a Service Billing Response when the Header Response Status (5Ø1-F1) is “A” (Accepted) and
Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). The Response Claim Segment is sent from the
sender to the receiver to mirror back the Prescription/Service Reference Number (4Ø2-D2). Fields defined as Mandatory are required to be
submitted when the segment is sent.
9.4.2.5.7 RESPONSE PRICING SEGMENT (SERVICE BILLING) (TRANSMISSION ACCEPTED/TRANSACTION
CAPTURED)
RESPONSE PRICING SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 162 -
RESPONSE PRICING SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø5-F5 PATIENT PAY AMOUNT
Q Service Billing:
Required if known. This field cannot be an estimated
amount. Zero is a valid amount.
5Ø6-F6 INGREDIENT COST PAID
N Service Billing:
Not used.
5Ø7-F7 DISPENSING FEE PAID
N Service Billing:
Not used.
557-AV TAX EXEMPT INDICATOR
Q Service Billing:
Required if the sender (health plan) and/or patient is tax
exempt and exemption applies to this billing.
558-AW FLAT SALES TAX AMOUNT PAID
Q Service Billing:
Required if Flat Sales Tax Amount Submitted (481-HA) is
greater than zero (Ø) or if Flat Sales Tax Amount Paid
(558-AW) is used to arrive at the estimated reimbursement.
Zero (Ø) value is valid. If reimbursement is not estimated,
this field contains the submitted value.
559-AX PERCENTAGE SALES TAX AMOUNT PAID
Q Service Billing:
Required if Percentage Sales Tax Amount Submitted (482-
GE) is greater than zero (Ø) or if Percentage Sales Tax
Amount Paid (559-AX) is used to arrive at the estimated
reimbursement. Zero (Ø) value is valid. If reimbursement is
not estimated, this field contains the submitted value.
Required if Percentage Sales Tax Rate Paid (56Ø-AY) and
Percentage Sales Tax Basis Paid (561-AZ) are used.
56Ø-AY PERCENTAGE SALES TAX RATE PAID
Q Service Billing:
Required if Percentage Sales Tax Amount Paid (559-AX) is
greater than zero (Ø).
561-AZ PERCENTAGE SALES TAX BASIS PAID
N Service Billing:
Not used. Code list is not applicable.
521-FL INCENTIVE AMOUNT PAID
N Service Billing:
Not used.
562-J1 PROFESSIONAL SERVICE FEE PAID
R Service Billing:
Required.
563-J2 OTHER AMOUNT PAID COUNT
Q Service Billing:
Maximum count of 3.
Required if Other Amount Paid (565-J4) is used.
564-J3 OTHER AMOUNT PAID QUALIFIER Q***R*** Service Billing:
Required if Other Amount Paid (565-J4) is used.
565-J4 OTHER AMOUNT PAID
Q***R*** Service Billing:
Required if this value is used to arrive at the estimated
reimbursement. If reimbursement is not estimated, this field
contains the submitted value.
Required if Other Amount Claimed Submitted (48Ø-H9) is
greater than zero (Ø).
Zero (Ø) is a valid value.
Must respond to each occurrence submitted.
566-J5 OTHER PAYER AMOUNT RECOGNIZED
Q Service Billing:
Required if Other Payer Amount Paid (431-DV) is greater
than zero (Ø) or if this field is used to arrive at the
estimated reimbursement.
Zero (Ø) value is valid.
If reimbursement is not estimated, this field contains the
submitted value.
5Ø9-F9 TOTAL AMOUNT PAID
R Service Billing:
Required.
Zero (Ø) value is valid.
See Pricing Formula for fields used in calculation.
522-FM BASIS OF REIMBURSEMENT DETERMINATION
N Service Billing:
Not used. Definition is not applicable.
523-FN AMOUNT ATTRIBUTED TO SALES TAX
Q Service Billing:
Required if Patient Pay Amount (5Ø5-F5) includes sales tax
that is the financial responsibility of the member but is not
also included in any of the other fields that add up to
Patient Pay Amount.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 163 -
RESPONSE PRICING SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
See section “Specific Segment Discussion”, “Response
Segments”, “Response Pricing Segment” for guidance.
512-FC ACCUMULATED DEDUCTIBLE AMOUNT N Service Billing:
Not used.
513-FD REMAINING DEDUCTIBLE AMOUNT N Service Billing:
Not used.
514-FE REMAINING BENEFIT AMOUNT N Service Billing:
Not used.
517-FH AMOUNT APPLIED TO PERIODIC DEDUCTIBLE
N Service Billing:
Not used.
518-FI AMOUNT OF COPAY
Q Service Billing:
Required if Patient Pay Amount (5Ø5-F5) includes copay
as patient financial responsibility.
52Ø-FK AMOUNT EXCEEDING PERIODIC BENEFIT MAXIMUM
N Service Billing:
Not used.
346-HH BASIS OF CALCULATION—DISPENSING FEE
N Service Billing:
Not used.
347-HJ BASIS OF CALCULATION—COPAY
N Service Billing:
Not used.
348-HK BASIS OF CALCULATION—FLAT SALES TAX
N Service Billing:
Not used.
349-HM BASIS OF CALCULATION—PERCENTAGE SALES TAX N Service Billing:
Not used.
571-NZ AMOUNT ATTRIBUTED TO PROCESSOR FEE Q Service Billing:
Required if the customer is responsible for 1ØØ% of the
service payment and when the provider net sale is less
than the amount the customer is expected to pay.
575-EQ PATIENT SALES TAX AMOUNT I Service Billing:
Used when necessary to identify the Patient’s portion of the
Sales Tax.
Provided for informational purposes only.
574-2Y PLAN SALES TAX AMOUNT I Service Billing:
Used when necessary to identify the Plan’s portion of the
Sales Tax.
Provided for informational purposes only.
572-4U AMOUNT OF COINSURANCE Q Service Billing:
Required if Patient Pay Amount (5Ø5-F5) includes
coinsurance as patient financial responsibility.
573-4V BASIS OF CALCULATION-COINSURANCE N Service Billing:
Not used.
392-MU BENEFIT STAGE COUNT N Service Billing:
Not used.
393-MV BENEFIT STAGE QUALIFIER N***R*** Service Billing:
Not used.
394-MW BENEFIT STAGE AMOUNT N***R*** Service Billing:
Not used.
577-G3 ESTIMATED GENERIC SAVINGS N Service Billing:
Not used.
128-UC SPENDING ACCOUNT AMOUNT REMAINING N Service Billing:
Not used.
129-UD HEALTH PLAN-FUNDED ASSISTANCE AMOUNT N Service Billing:
Not used.
133-UJ AMOUNT ATTRIBUTED TO PROVIDER NETWORK SELECTION N Service Billing:
Not used.
134-UK AMOUNT ATTRIBUTED TO PRODUCT SELECTION/BRAND
DRUG
N Service Billing:
Not used.
135-UM AMOUNT ATTRIBUTED TO PRODUCT SELECTION/NON-
PREFERRED FORMULARY SELECTION
N Service Billing:
Not used.
136-UN AMOUNT ATTRIBUTED TO PRODUCT SELECTION/BRAND
NON-PREFERRED FORMULARY SELECTION
N Service Billing:
Not used.
137-UP AMOUNT ATTRIBUTED TO COVERAGE GAP Q Service Billing:
Required when the patient’s financial responsibility is due to
the coverage gap.
148-U8 INGREDIENT COST CONTRACTED/REIMBURSABLE AMOUNT N Service Billing:
Not used.
149-U9 DISPENSING FEE CONTRACTED/REIMBURSABLE AMOUNT N Service Billing:
Not used.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 164 -
Notes on Response Pricing Segment on a Service Billing Response:
The Response Pricing Segment is situational for a Service Billing Response when the Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) is “C” (Captured) or “Q” (Duplicate of Captured).
The Response Pricing Segment is not used in payer-to-payer transactions.
All dollar fields except Patient Pay Amount (5Ø5-F5) are estimated amounts. If actual amounts are returned on fields other than Patient Pay
Amount (5Ø5-F5), the “P” (Paid) response must be used.
If the Transaction Response Status (112-AN) = C (Captured) or Q (Duplicate of Captured), dollar fields should be supplied in the response.
• If the response is a “true” Capture (i.e. replacement of batch billing, with no edits or pricing), then corresponding response fields
should be populated with values as submitted. Ideally, processor should provide “real” patient financial responsibility values on a
Capture. If this is not possible, provider must know (by trading partner agreement) the patient financial responsibility to charge and
factor that into their system so collection occurs.
• If the response is captured by an Intermediary who can provide better pricing criteria, the corresponding response fields should be
populated with the probable values and those values used to determine estimated pricing as noted above. Since the claim has not
been fully adjudicated, this should remain a capture response.
It is highly recommended that whenever possible, the individual dollar fields are returned in the response. On the response the sender should
return the individual payment response fields to allow the receiver to reconcile against the requested payment fields.
See section “Response Processing Guidelines”, “Pricing Guidelines”.
Fields defined as Mandatory are required to be submitted when the segment is sent.
9.4.3 TRANSMISSION ACCEPTED/TRANSACTION REJECTED
9.4.3.1 DIAGRAM FOR TRANSMISSION OF ONE SERVICE BILLING RESPONSE (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
Service Billing transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “R” (Rejected)
The Response Prior Authorization Segment is not used in payer-to-payer transactions. Therefore, in this case, there are no situational
transaction-level segments.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Prior Authorization Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
9.4.3.2 DIAGRAM FOR TRANSMISSION OF TWO SERVICE BILLING RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 165 -
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Prior Authorization Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Prior Authorization Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
9.4.3.3 DIAGRAM FOR TRANSMISSION OF THREE SERVICE BILLING RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Prior Authorization Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Prior Authorization Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory third response
Group Separator
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 166 -
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Prior Authorization Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
9.4.3.4 DIAGRAM FOR TRANSMISSION OF FOUR SERVICE BILLING RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Prior Authorization Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Prior Authorization Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Prior Authorization Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 167 -
Segment Separator
Response Prior Authorization Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
9.4.3.5 SERVICE BILLING RESPONSE SEGMENTS (TRANSMISSION ACCEPTED/TRANSACTION
REJECTED)
9.4.3.5.1 RESPONSE HEADER SEGMENT (SERVICE BILLING) (TRANSMISSION ACCEPTED/TRANSACTION
REJECTED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M For Transaction Code of “S1”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “2” (Service Billing).
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Service Billing Response:
The Response Header Segment is a mandatory, fixed length segment for Service Billing response when the Header Response Status (5Ø1-
F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). The “Situation” column is not applicable.
9.4.3.5.2 RESPONSE MESSAGE SEGMENT (SERVICE BILLING) (TRANSMISSION ACCEPTED/TRANSACTION
REJECTED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Service Billing:
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Service Billing Response:
The Response Message Segment is situational for Service Billing response when the Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “R” (Rejected). It is used when additional text information needs to be sent. Fields defined as
Mandatory are required to be submitted when the segment is sent.
9.4.3.5.3 RESPONSE INSURANCE SEGMENT (SERVICE BILLING) (TRANSMISSION ACCEPTED/TRANSACTION
REJECTED)
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø1-C1 GROUP ID Q Service Billing:
Required if needed to identify the actual cardholder or
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 168 -
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
employer group, to identify appropriate group number,
when available.
Required to identify the actual group that was used when
multiple group coverages exist.
Note: This field may contain the Group ID echoed from the
request. May contain the actual Group ID if unknown to the
receiver.
524-FO PLAN ID
Q Service Billing:
Required if needed to identify the actual plan parameters,
benefit, or coverage criteria, when available.
Required to identify the actual plan ID that was used when
multiple group coverages exist.
Required if needed to contain the actual plan ID if unknown
to the receiver.
545-2F NETWORK REIMBURSEMENT ID Q Service Billing:
Required if needed to identify the network for the covered
member.
Required if needed to identify the actual Network
Reimbursement ID, when applicable and/or available.
Required to identify the actual Network Reimbursement ID
that was used when multiple Network Reimbursement IDs
exist.
568-J7 PAYER ID QUALIFIER Q Service Billing:
Required if Payer ID (569-J8) is used.
569-J8 PAYER ID
Q Service Billing:
Required to identify the ID of the payer responding.
115-N5
MEDICAID ID NUMBER N Service Billing:
Not used.
116-N6
MEDICAID AGENCY NUMBER N Service Billing:
Not used.
3Ø2-C2
CARDHOLDER ID Q Service Billing:
Required if the identification to be used in future
transactions is different than what was submitted on the
request.
Notes on Response Insurance Segment on a Service Billing Response:
The Response Insurance Segment is situational for Service Billing response when the Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “R” (Rejected). It is used when coverage or reimbursement parameters or identifiers need to be
sent from the sender to the receiver. Fields defined as Mandatory are required to be submitted when the segment is sent.
9.4.3.5.4 RESPONSE PATIENT SEGMENT (SERVICE BILLING) (TRANSMISSION ACCEPTED/TRANSACTION
REJECTED)
RESPONSE PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
31Ø-CA PATIENT FIRST NAME Q Service Billing:
Required if known.
311-CB PATIENT LAST NAME Q Service Billing:
Required if known.
3Ø4-C4 DATE OF BIRTH Q Service Billing:
Required if known.
Notes on Response Patient Segment on a Service Billing Response:
The Response Patient Segment is situational for Service Billing transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “R” (Rejected) when patient demographic information needs to be sent from the sender to the
receiver. Fields defined as Mandatory are required to be submitted when the segment is sent.
9.4.3.5.5 RESPONSE STATUS SEGMENT (SERVICE BILLING) (TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 169 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER
Q Service Billing:
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT R Service Billing:
Maximum count of 5.
Required.
511-FB REJECT CODE
R***R*** Service Billing:
Required.
546-4F REJECT FIELD OCCURRENCE INDICATOR
Q***R*** Service Billing:
Required if a repeating field is in error, to identify repeating
field occurrence.
This field must be sent when relaying error information about
a repeating field or set. Note, if the Reject Code is not
denoting a repeating field or set, the Reject Field Occurrence
Indicator must not be sent.
547-5F APPROVED MESSAGE CODE COUNT
N Service Billing:
Not used.
548-6F APPROVED MESSAGE CODE
N***R*** Service Billing:
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Service Billing:
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Service Billing:
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Service Billing:
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Service Billing:
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER
Q Service Billing:
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Service Billing:
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Service Billing:
Not used.
993-A7 INTERNAL CONTROL NUMBER N Service Billing:
Not used.
987-MA URL I Service Billing:
Provided for informational purposes only to relay health
care communications via the Internet.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 170 -
Notes on Response Status Segment on a Service Billing Response:
The Response Status Segment is mandatory for a Service Billing Response for Header Response Status (5Ø1-F1) = “A” (Accepted) and
Transaction Response Status (112-AN) of “R” (Rejected). The Response Status Segment is sent from the sender to the receiver to identify the
outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.
9.4.3.5.6 RESPONSE CLAIM SEGMENT (SERVICE BILLING) (TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M For Transaction Code of “S1”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “2” (Service Billing).
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT
N Service Billing:
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER N***R*** Service Billing:
Not used.
553-AR PREFERRED PRODUCT ID
N***R*** Service Billing:
Not used.
554-AS PREFERRED PRODUCT INCENTIVE
N***R*** Service Billing:
Not used.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE N***R*** Service Billing:
Not used.
556-AU PREFERRED PRODUCT DESCRIPTION
N***R*** Service Billing:
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Service Billing:
Not used.
Notes on Response Claim Segment on a Service Billing Response:
The Response Claim Segment is mandatory for a Service Billing Response when the Header Response Status (5Ø1-F1) is “A” (Accepted) and
Transaction Response Status (112-AN) of “R” (Rejected). The Response Claim Segment is sent from the sender to the receiver to mirror back
the Prescription/Service Reference Number (4Ø2-D2). Fields defined as Mandatory are required to be submitted when the segment is sent.
9.4.3.5.7 RESPONSE PRIOR AUTHORIZATION SEGMENT (SERVICE BILLING) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE PRIOR AUTHORIZATION SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
498-PR PRIOR AUTHORIZATION PROCESSED DATE N Service Billing:
Not used.
498-PS PRIOR AUTHORIZATION EFFECTIVE DATE N Service Billing:
Not used.
498-PT PRIOR AUTHORIZATION EXPIRATION DATE N Service Billing:
Not used.
498-RA PRIOR AUTHORIZATION QUANTITY N Service Billing:
Not used.
498-RB PRIOR AUTHORIZATION DOLLARS AUTHORIZED N Service Billing:
Not used.
498-PW PRIOR AUTHORIZATION NUMBER OF REFILLS AUTHORIZED N Service Billing/:
Not used.
498-PX PRIOR AUTHORIZATION QUANTITY ACCUMULATED N Service Billing:
Not used.
498-PY PRIOR AUTHORIZATION NUMBER–ASSIGNED Q Service Billing:
Required when the receiver must submit this Prior
Authorization Number in order to receive payment for the
claim. (An example of a situation may include a Benefit
Transition Period that allows for payment of claims, for a
period of time that would normally reject.)
Notes on Response Prior Authorization Segment on a Service Billing:
The Response Prior Authorization Segment is situational for a Service Billing response when the Header Response Status (5Ø1-F1) is “A”
(Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). It is used to relay the Prior Authorization Number - Assigned (498-
PY) which is returned when a Reject Code (511-FB) denotes that a prior authorization code needs to be submitted on the subsequent billing.
The Response Prior Authorization Segment is not used in payer-to-payer transactions.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 171 -
Fields defined as Mandatory are required to be submitted when the segment is sent.
9.4.3.5.8 RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS SEGMENT (SERVICE BILLING) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS
SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
355-NT OTHER PAYER ID COUNT M Service Billing:
Maximum count of 3.
338-5C OTHER PAYER COVERAGE TYPE M***R***
339-6C OTHER PAYER ID QUALIFIER Q***R*** Service Billing:
Required if Other Payer ID (34Ø-7C) is used.
34Ø-7C OTHER PAYER ID Q***R*** Service Billing:
Required if other insurance information is available for
coordination of benefits.
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER Q***R*** Service Billing:
Required if other insurance information is available for
coordination of benefits.
356-NU OTHER PAYER CARDHOLDER ID Q***R*** Service Billing:
Required if other insurance information is available for
coordination of benefits.
992-MJ OTHER PAYER GROUP ID Q***R*** Service Billing:
Required if other insurance information is available for
coordination of benefits.
142-UV OTHER PAYER PERSON CODE Q***R*** Service Billing:
Required if needed to uniquely identify the family members
within the Cardholder ID, as assigned by the other payer.
127-UB OTHER PAYER HELP DESK PHONE NUMBER Q***R*** Service Billing:
Required if needed to provide a support telephone number
of the other payer to the receiver.
143-UW OTHER PAYER PATIENT RELATIONSHIP CODE Q***R*** Service Billing:
Required if needed to uniquely identify the relationship of
the patient to the cardholder ID, as assigned by the other
payer.
144-UX OTHER PAYER BENEFIT EFFECTIVE DATE Q***R*** Service Billing:
Required when other coverage is known which is after the
Date of Service submitted.
145-UY OTHER PAYER BENEFIT TERMINATION DATE Q***R*** Service Billing:
Required when other coverage is known which is after the
Date of Service submitted.
Notes on Response Coordination of Benefits/Other Payers Segment on a Service Billing Response:
The Response Coordination of Benefits/Other Payers Segment is situational for a Service Billing response when the Header Response Status
(5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “R” (Rejected) when other insurance information is available for
coordination of benefits.
1. If the identity of the patient is partially verified and the Service Billing is rejected due to a non-match of field verification, then the
Other Payer information is not sent.
2. If the service is rejected because it should be submitted to other payer(s) first, that Other Payer information should be sent, if known.
3. If the service is rejected due to benefit design limitations, then subsequent Other Payer information should be sent, if known.
If the service rejects for other reasons than above, Other Payer information is not sent.
If additional payer(s) for this patient is not known, the Other Payer information is not sent.
If additional payer(s) for this patient is known, the following may be sent:
• Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C),
• Other Payer Group ID (992-MJ),
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU)
• And other Other Payer fields.
In addition, if any of the following three fields are sent:
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU),
• Other Payer Group ID (992-MJ),
then the Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C) must be sent.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 172 -
Fields defined as Mandatory are required to be submitted when the segment is sent.
9.4.4 TRANSMISSION REJECTED/TRANSACTION REJECTED
Service Billing transmission response Header Response Status (5Ø1-F1) of "R" (Rejected)
and Transaction Response Status (112-AN) of “R” (Rejected)
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
9.4.4.1 DIAGRAM FOR TRANSMISSION OF ONE SERVICE BILLING RESPONSE (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
9.4.4.2 DIAGRAM FOR TRANSMISSION OF TWO SERVICE BILLING RESPONSES (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
9.4.4.3 DIAGRAM FOR TRANSMISSION OF THREE SERVICE BILLING RESPONSES (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
9.4.4.4 DIAGRAM FOR TRANSMISSION OF FOUR SERVICE BILLING RESPONSES (TRANSMISSION
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 173 -
REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
9.4.4.5 SERVICE BILLING RESPONSE SEGMENTS (TRANSMISSION REJECTED/TRANSACTION
REJECTED)
9.4.4.5.1 RESPONSE HEADER SEGMENT (SERVICE BILLING) (TRANSMISSION REJECTED/TRANSACTION
REJECTED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Service Billing Response:
The Response Header Segment is a mandatory, fixed length segment for Service Billing response when the Header Response Status (5Ø1-
F1) is “R” (Rejected) and Transaction Response Status (112-AN) of “R” (Rejected). The “Situation” column is not applicable.
If either the entire transmission or the Header is in error, the Header Response Status (5Ø1-F1) = “R” (Rejected). Every identifiable transaction
within the transmission must be rejected with an “R”.
If the transaction rejects for detail errors, the Header Response Status (5Ø1-F1) = “A” (Accepted) and the Transaction Response Status (112-
AN) will be “R” (Rejected).
9.4.4.5.2 RESPONSE MESSAGE SEGMENT (SERVICE BILLING) (TRANSMISSION REJECTED/TRANSACTION
REJECTED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Service Billing:
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 174 -
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Service Billing Response:
The Response Message Segment is situational for Service Billing response when the Header Response Status (5Ø1-F1) is “R” (Rejected) and
Transaction Response Status (112-AN) of “R” (Rejected). It is used when additional text information needs to be sent. Fields defined as
Mandatory are required to be submitted when the segment is sent.
9.4.4.5.3 RESPONSE STATUS SEGMENT (SERVICE BILLING) (TRANSMISSION REJECTED/TRANSACTION REJECTED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Service Billing:
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT R Service Billing:
Maximum count of 5.
Required.
511-FB REJECT CODE
R***R*** Service Billing:
Required.
546-4F REJECT FIELD OCCURRENCE INDICATOR
Q***R*** Service Billing:
Required if a repeating field is in error, to identify repeating
field occurrence.
This field must be sent when relaying error information about
a repeating field or set. Note, if the Reject Code is not
denoting a repeating field or set, the Reject Field Occurrence
Indicator must not be sent.
547-5F APPROVED MESSAGE CODE COUNT N Service Billing:
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Service Billing:
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Service Billing:
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Service Billing:
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION
Q***R*** Service Billing:
Required if additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 175 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Service Billing:
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER
Q Service Billing:
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Service Billing:
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Service Billing:
Not used.
993-A7 INTERNAL CONTROL NUMBER N Service Billing:
Not used.
987-MA URL N Service Billing:
Not used.
Notes on Response Status Segment on a Service Billing Response:
The Response Status Segment is mandatory for a Service Billing Response for Header Response Status (5Ø1-F1) = “R” (Rejected) and
Transaction Response Status (112-AN) of “R” (Rejected). The Response Status Segment is sent from the sender to the receiver to identify the
outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 176 -
10. REVERSAL INFORMATION
The reversal transaction is used to “back out” a previously captured or paid prescription, service billing, information or controlled substance
reporting. Up to four reversal transactions per transmission are permitted. Reversal Transaction Codes are “B2”, “S2”, “N2”, or “C2”.
To correctly build a multi-reversal transmission, the reversal transaction(s) in this transmission must be
• In the same format (Version/Release Number) and
• Sent to the same entity (processor or PBM using the BIN Number and Processor Control Number) and
• For the same pharmacy (Service Provider ID and Qualifier) and
• For the same date (Date of Service).
Situational segments such as the Insurance Segment may be supported. If a processor/PBM needs this information to process a reversal, this
segment can be used. Only one Insurance Segment must be submitted per transmission.
If a processor/PBM does not need the Insurance Segment, but the pharmacy wishes to send it, the processor/PBM must ignore the valid
optional and/or situational information.
Date of Service (4Ø1-D1) is defined as “identifies date the prescription was filled or professional service rendered”. Therefore, since the date is
in the Transaction Header segment that occurs once (at the transmission level), one to four transactions (at the transaction level) must be for
the same date.
Multiple claim or service reversal transactions in a transmission must be for the same patient.
The structure does support multiple claim or service reversals for the same processor/PBM, for the same pharmacy, for the same Date of
Service, but for multiple patients. However, it is recommended that a transmission containing multiple reversals for multiple patients
not be supported. Even though the structure supports reversals for multiple patients, the recommendation is that this not be supported.
If, during the transmission of a reversal, the communication or procedure is interrupted, a provider may not receive notification that the
processor has reversed the transaction. If the provider retransmits the reversal, the processor must not apply the reversal more than once for
a given transaction. A “Reversal” resubmission must prompt the processor to reply with the same information returned on the original reversal
response, and use an “S” (Duplicate of Approved) status. The message field may be used to inform the submitter of the reason for the
duplicate status, e.g. reversal previously accepted. See section “Response Processing Guidelines”, “Duplicate Transactions”.
It is recommended that provider software not allow a reversed prescription to be deleted from the pharmacy system without first receiving a
response from the processor related to the reversal.
10.1 CLAIM OR SERVICE REVERSAL
These transactions are used by the Originator to cancel a claim or service submitted that had been processed previously.
Each claim or service reversal request contains up to 4 occurrences of claim/service data. The Transaction Code is “B2” (Claim Reversal) or
“S2” (Service Reversal).
To correctly build a multi-reversal transmission, the reversal transaction(s) in this transmission must be
• In the same format (Version/Release Number) and
• Sent to the same entity (processor or PBM using the BIN Number and Processor Control Number) and
• For the same pharmacy (Service Provider ID and Qualifier) and
• For the same date (Date of Service).
The Insurance Segment is situational. If a processor/PBM needs this information to process a reversal, this segment can be used. Only one
Insurance Segment must be submitted per transmission, as this segment occurs at the transmission level.
If a processor/PBM does not need the Insurance Segment, but the pharmacy wishes to send it, the processor/PBM must ignore the valid
situational and/or optional information.
Other situation segments include DUR/PPS, Pricing Segment, and Coordination of Benefits Segments. These segments occur at the
transaction level and may occur one to four times as part of each reversal transaction. The Coordination of Benefits Segment is situational
only for reversals to downstream payers; otherwise it is not used.
Date of Service (4Ø1-D1) is defined as “identifies date the prescription was filled or professional service rendered”. Therefore, since the date is
in the Transaction Header segment that occurs once (at the transmission level), one to four transactions (at the transaction level) must be for
the same date.
Multiple claim or service reversal transactions in a transmission must be for the same patient.
The structure does support multiple claim or service reversals for the same processor/PBM, for the same pharmacy, for the same Date of
Service, but for multiple patients. However, it is recommended that a transmission containing multiple reversals for multiple patients
not be supported.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 177 -
For Medicare Part D processing only one transaction per transmission is permitted because there is a need for the sequencing of the True Out
Of Pocket (TrOOP) update before the next transaction is processed. The TrOOP should be updated before subsequent transactions are
processed.
Depending upon the particular claim or service reversal request, the Processor must provide one of the following general types of responses:
Approved - This occurs when the Processor acknowledges receipt of the claim or service reversal, and successfully processes the
backing out of the claim or service.
Duplicate of Approved - This occurs when the Processor has previously received the reversal request and processed the
transaction, but the response did not return to the Originator. The Duplicate response contains the same information as returned in
the original response of Approved.
Captured - This occurs when the Processor acknowledges receipt of the reversal, but is not processing the reversal at this time.
Duplicate of Captured - This occurs when the Processor has previously received the reversal request and processed the
transaction, but the response did not return to the Originator. The Duplicate response contains the same information as returned in
the original response of Captured.
Rejected - This occurs when the Processor has encountered an error in the transaction or processing.
10.2 CLAIM REVERSAL REQUEST DIAGRAMS
10.2.1 DIAGRAM FOR TRANSMISSION OF ONE CLAIM REVERSAL TRANSACTION
For a Claim Reversal, the scenarios defined include
Claim Reversal from a Sender to a Receiver
Claim Reversal Accepted/Transaction Approved Response from a Sender to a Receiver
Claim Reversal Accepted/Transaction Captured Response from a Sender to a Receiver
Standard Transmission Accepted/Transaction Rejected Response from a Sender to a Receiver
Standard Transmission Reject Response to a Claim Reversal from a Sender to a Receiver
For payer-to-payer transactions, the DUR/PPS Segment, Pricing Segment, and Coordination of Benefits/Other Payments Segment are not
used. Therefore, in this case, there are no situational transaction-level segments.
Mandatory
Transaction Header Segment
Situational
Segment Separator
Insurance Segment
Mandatory - first Claim Reversal transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
DUR/PPS Segment
Segment Separator
Pricing Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
10.2.2 DIAGRAM FOR TRANSMISSION OF TWO CLAIM REVERSAL TRANSACTIONS
Mandatory
Transaction Header Segment
Situational
Segment Separator
Insurance Segment
Mandatory - first Claim Reversal transaction
Group Separator
Segment Separator
Claim Segment
Situational
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 178 -
Segment Separator
DUR/PPS Segment
Segment Separator
Pricing Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Mandatory - second Claim Reversal transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
DUR/PPS Segment
Segment Separator
Pricing Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
10.2.3 DIAGRAM FOR TRANSMISSION OF THREE CLAIM REVERSAL TRANSACTIONS
Mandatory
Transaction Header Segment
Situational
Segment Separator
Insurance Segment
Mandatory - first Claim Reversal transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
DUR/PPS Segment
Segment Separator
Pricing Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Mandatory - second Claim Reversal transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
DUR/PPS Segment
Segment Separator
Pricing Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Mandatory - third Claim Reversal transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
DUR/PPS Segment
Segment Separator
Pricing Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
10.2.4 DIAGRAM FOR TRANSMISSION OF FOUR CLAIM REVERSAL TRANSACTIONS
Mandatory
Transaction Header Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 179 -
Situational
Segment Separator
Insurance Segment
Mandatory - first Claim Reversal transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
DUR/PPS Segment
Segment Separator
Pricing Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Mandatory - second Claim Reversal transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
DUR/PPS Segment
Segment Separator
Pricing Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Mandatory - third Claim Reversal transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
DUR/PPS Segment
Segment Separator
Pricing Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Mandatory - fourth Claim Reversal transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
DUR/PPS Segment
Segment Separator
Pricing Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
10.3 CLAIM REVERSAL REQUEST SEGMENTS
10.3.1 TRANSACTION HEADER SEGMENT (CLAIM REVERSAL)
TRANSACTION HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø1-A1 BIN NUMBER M
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M For Transaction Code of “B2”, in the Claim Segment, the
Prescription/Service Reference Number Qualifier (455-EM)
is “1” (Rx Billing).
1Ø4-A4 PROCESSOR CONTROL NUMBER M
1Ø9-A9 TRANSACTION COUNT M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 180 -
TRANSACTION HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M
Notes on Transaction Header Segment on a Claim Reversal Request:
The Transaction Header Segment is a mandatory, fixed length segment for a Claim Reversal request. The “Situation” column is not applicable.
10.3.2 INSURANCE SEGMENT (CLAIM REVERSAL)
INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø2-C2 CARDHOLDER ID M
312-CC CARDHOLDER FIRST NAME N Claim Reversal:
Not used.
313-CD CARDHOLDER LAST NAME N Claim Reversal:
Not used.
314-CE HOME PLAN N Claim Reversal:
Not used.
524-FO PLAN ID N Claim Reversal:
Not used.
3Ø9-C9 ELIGIBILITY CLARIFICATION CODE N Claim Reversal:
Not used.
3Ø1-C1 GROUP ID Q Claim Reversal:
Required if needed to match the reversal to the original
billing transaction.
3Ø3-C3 PERSON CODE N Claim Reversal:
Not used.
3Ø6-C6 PATIENT RELATIONSHIP CODE N Claim Reversal:
Not used.
99Ø-MG OTHER PAYER BIN NUMBER N Claim Reversal:
Not used.
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER N Claim Reversal:
Not used.
356-NU OTHER PAYER CARDHOLDER ID N Claim Reversal:
Not used.
992-MJ OTHER PAYER GROUP ID N Claim Reversal:
Not used.
359-2A MEDIGAP ID Q Claim Reversal:
Required, if known, when patient has Medigap coverage.
36Ø-2B MEDICAID INDICATOR N Claim Reversal:
Not used.
361-2D PROVIDER ACCEPT ASSIGNMENT INDICATOR N Claim Reversal:
Not used.
997-G2 CMS PART D DEFINED QUALIFIED FACILITY N Claim Reversal:
Not used.
115-N5
MEDICAID ID NUMBER N Claim Reversal:
Not used.
116-N6
MEDICAID AGENCY NUMBER N Claim Reversal:
Not used.
Notes on Insurance Segment on a Claim Reversal Request:
The Insurance Segment is situational for a Claim Reversal request. If the Cardholder ID field is not submitted, the Insurance Segment is not
used. The Segment is mandatory if required under provider payer contract or mandatory on claims where this information is necessary for
reversal of the claim. Fields defined as Mandatory are required to be submitted when the segment is sent.
10.3.2.1 INSURANCE SEGMENT (MEDICAID SUBROGATION CLAIM REVERSAL)
INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
3Ø1-C1 GROUP ID QM Medicaid Subrogation – Claim Reversal: Required if
needed to match reversal to original Medicaid
Subrogation billing transaction.
115-N5
MEDICAID ID NUMBER QM Medicaid Subrogation - Claim Reversal:
Required to identify the member as uniquely known to
Medicaid.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 181 -
INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
116-N6
MEDICAID AGENCY NUMBER QM Medicaid Subrogation - Claim Reversal:
Required to identify the Medicaid agency.
See Medicaid Subrogation Implementation Guide.
Notes on Insurance Segment on a Medicaid Subrogation Claim Reversal Request:
The rules above for an “Insurance Segment (Claim Reversal)” are to followed for Medicaid Subrogation. Specific fields that are used
differently in Medicaid Subrogation are noted in the table above.
10.3.3 CLAIM SEGMENT (CLAIM REVERSAL)
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M For Transaction Code of “B2”, in the Claim Segment, the
Prescription/Service Reference Number Qualifier (455-EM)
is “1” (Rx Billing).
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
436-E1 PRODUCT/SERVICE ID QUALIFIER M Mandatory.
Must contain the Product/Service ID Qualifier (436-E1)
value from original Billing.
4Ø7-D7 PRODUCT/SERVICE ID M Mandatory.
Must contain the Product/Service ID (436-E1) value from
original Billing.
456-EN ASSOCIATED PRESCRIPTION/SERVICE REFERENCE NUMBER N Claim Reversal:
Not used.
457-EP ASSOCIATED PRESCRIPTION/SERVICE DATE N Claim Reversal:
Not used.
458-SE PROCEDURE MODIFIER CODE COUNT N Claim Reversal:
Not used.
459-ER PROCEDURE MODIFIER CODE N***R*** Claim Reversal:
Not used.
442-E7 QUANTITY DISPENSED N Claim Reversal:
Not used.
4Ø3-D3 FILL NUMBER Q Claim Reversal:
Required if needed for reversals when multiple fills of the
same Prescription/Service Reference Number (4Ø2-D2)
occur on the same day.
4Ø5-D5 DAYS SUPPLY N Claim Reversal:
Not used.
4Ø6-D6 COMPOUND CODE N Claim Reversal:
Not used.
4Ø8-D8 DISPENSE AS WRITTEN (DAW)/PRODUCT SELECTION CODE N Claim Reversal:
Not used.
414-DE DATE PRESCRIPTION WRITTEN N Claim Reversal:
Not used.
415-DF NUMBER OF REFILLS AUTHORIZED N Claim Reversal:
Not used.
419-DJ PRESCRIPTION ORIGIN CODE N Claim Reversal:
Not used.
354-NX SUBMISSION CLARIFICATION CODE COUNT N Claim Reversal:
Not used.
42Ø-DK SUBMISSION CLARIFICATION CODE N***R*** Claim Reversal:
Not used.
46Ø-ET QUANTITY PRESCRIBED N Claim Reversal:
Not used.
3Ø8-C8 OTHER COVERAGE CODE Q Claim Reversal:
Required if needed by receiver to match the claim that is
being reversed.
See section “Specific Segment Discussion”, “Request
Segments”, “Claim Segment”, “Other Coverage Code (3Ø8-
C8).
429-DT SPECIAL PACKAGING INDICATOR N Claim Reversal:
Not used.
453-EJ ORIGINALLY PRESCRIBED PRODUCT/SERVICE ID QUALIFIER N Claim Reversal:
Not used.
445-EA ORIGINALLY PRESCRIBED PRODUCT/SERVICE CODE N Claim Reversal:
Not used.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 182 -
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
446-EB ORIGINALLY PRESCRIBED QUANTITY N Claim Reversal:
Not used.
33Ø-CW ALTERNATE ID N Claim Reversal:
Not used.
454-EK SCHEDULED PRESCRIPTION ID NUMBER N Claim Reversal:
Not used.
6ØØ-28 UNIT OF MEASURE N Claim Reversal:
Not used.
418-DI LEVEL OF SERVICE N Claim Reversal:
Not used.
461-EU PRIOR AUTHORIZATION TYPE CODE N Claim Reversal:
Not used.
462-EV PRIOR AUTHORIZATION NUMBER SUBMITTED N Claim Reversal:
Not used.
463-EW INTERMEDIARY AUTHORIZATION TYPE ID N Claim Reversal:
Not used.
464-EX INTERMEDIARY AUTHORIZATION ID N Claim Reversal:
Not used.
343-HD DISPENSING STATUS N Claim Reversal:
Not used.
344-HF QUANTITY INTENDED TO BE DISPENSED N Claim Reversal:
Not used.
345-HG DAYS SUPPLY INTENDED TO BE DISPENSED N Claim Reversal:
Not used.
357-NV DELAY REASON CODE N Claim Reversal:
Not used.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Claim Reversal:
Not used.
391-MT PATIENT ASSIGNMENT INDICATOR (DIRECT MEMBER
REIMBURSEMENT INDICATOR)
N Claim Reversal:
Not used.
995-E2 ROUTE OF ADMINISTRATION N Claim Reversal:
Not used.
996-G1 COMPOUND TYPE N Claim Reversal:
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Claim Reversal:
Not used.
147-U7 PHARMACY SERVICE TYPE Q Claim Reversal:
Required when the submitter must clarify the type of
services being performed as a condition for proper
reimbursement by the payer.
Notes on Claim Segment on a Claim Reversal Request:
The Claim Segment is mandatory for a Claim Reversal request. The Claim Segment defines the product dispensed and dispensing
information. Fields defined as Mandatory are required to be submitted when the segment is sent.
10.3.4 DUR/PPS SEGMENT (CLAIM REVERSAL)
DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
473-7E DUR/PPS CODE COUNTER
Q***R*** Claim Reversal:
Maximum 9 occurrences supported.
Required if DUR/PPS Segment is used.
439-E4 REASON FOR SERVICE CODE
Q***R*** Claim Reversal:
Required if this field is needed to report drug utilization
review outcome.
44Ø-E5 PROFESSIONAL SERVICE CODE
Q***R*** Claim Reversal:
Required if this field is needed to report drug utilization
review outcome.
441-E6 RESULT OF SERVICE CODE
Q***R*** Claim Reversal:
Required if this field is needed to report drug utilization
review outcome.
474-8E DUR/PPS LEVEL OF EFFORT
Q***R*** Claim Reversal:
Required if this field is needed to report drug utilization
review outcome.
475-J9 DUR CO-AGENT ID QUALIFIER N***R*** Claim Reversal:
Not used.
476-H6 DUR CO-AGENT ID N***R*** Claim Reversal:
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 183 -
DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Not used.
Notes on DUR/PPS Segment on a Reversal Request:
The DUR/PPS Segment is situational for a Claim Reversal request. It is used when a sender notifies the receiver of drug utilization review
outcome. The Segment is mandatory if required under provider payer contract or mandatory on reversals where this information is necessary
for reversal of the claim.
The DUR/PPS Segment is not used in payer-to-payer transactions.
The Reason for Service Code (439-E4) is sometimes reported for DUR processing, and sometimes based on payment agreements. See
section “Notes on Pricing Segment on a Reversal Request” below.
Fields defined as Mandatory are required to be submitted when the segment is sent.
10.3.5 PRICING SEGMENT (CLAIM REVERSAL)
PRICING SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
4Ø9-D9 INGREDIENT COST SUBMITTED N Claim Reversal:
Not used.
412-DC DISPENSING FEE SUBMITTED N Claim Reversal:
Not used.
477-BE PROFESSIONAL SERVICE FEE SUBMITTED N Claim Reversal:
Not used.
433-DX PATIENT PAID AMOUNT SUBMITTED N Claim Reversal:
Not used.
438-E3 INCENTIVE AMOUNT SUBMITTED Q Claim Reversal:
Required if this field could result in contractually agreed
upon payment.
478-H7 OTHER AMOUNT CLAIMED SUBMITTED COUNT N Claim Reversal:
Not used.
479-H8 OTHER AMOUNT CLAIMED SUBMITTED QUALIFIER Q***R*** Claim Reversal:
Not used.
48Ø-H9 OTHER AMOUNT CLAIMED SUBMITTED Q***R*** Claim Reversal:
Not used.
481-HA FLAT SALES TAX AMOUNT SUBMITTED N Claim Reversal:
Not used.
482-GE PERCENTAGE SALES TAX AMOUNT SUBMITTED N Claim Reversal:
Not used.
483-HE PERCENTAGE SALES TAX RATE SUBMITTED N Claim Reversal:
Not used.
484-JE PERCENTAGE SALES TAX BASIS SUBMITTED N Claim Reversal:
Not used.
426-DQ USUAL AND CUSTOMARY CHARGE N Claim Reversal:
Not used.
43Ø-DU GROSS AMOUNT DUE Q Claim Reversal:
Required if this field could result in contractually agreed
upon payment.
423-DN BASIS OF COST DETERMINATION N Claim Reversal:
Not used.
113-N3
MEDICAID PAID AMOUNT N Claim Reversal:
Not used.
Notes on Pricing Segment on a Claim Reversal Request:
The Pricing Segment is situational for a Claim Reversal request. The Pricing Segment defines contractually agreed upon payment fields for a
Reversal. See the pricing formulae. DUR may be reported with or without contractual pricing. Incentive Amount Submitted (438-E3) is used to
report the contractual pricing.
The Pricing Segment is not used in payer-to-payer transactions.
Fields defined as Mandatory are required to be submitted when the segment is sent.
The following examples are simplified to show only the fields needed for the example.
10.3.5.1 EXAMPLE 1: REPORTING A DUR EVENT ON A CLAIM REVERSAL WITHOUT ANY INCENTIVE
SUBMITTED

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 184 -
REVERSAL TRANSACTION
Field Field Name Value
DUR/PPS Segment
439-E4 Reason for Service Code AT
44Ø-E5 Professional Service Code PØ
441-E6 Result of Service Code 2A
474-8E DUR/PPS Level of Effort 12
No Request Pricing Segment submitted
Field Field Name Value
5Ø1-F1 Header Response Status A
112-AN Transaction Response Status A, S, C, Q
No Response Pricing Segment returned
10.3.5.2 EXAMPLE 2: NO INCENTIVE AMOUNT SUBMITTED (438-E3) FOR A CLAIM REVERSAL.
INCENTIVE PAID
An Incentive Fee will be paid. The original claim was “P” (Paid) claim that includes a DUR alert.
REVERSAL TRANSACTION
Field Field Name Value
DUR/PPS Segment
439-E4 Reason for Service Code AT
44Ø-E5 Professional Service Code PØ
441-E6 Result of Service Code 2A
474-8E DUR/PPS Level of Effort 12
Field Field Name Value
5Ø1-F1 Header Response Status A
112-AN Transaction Response Status A, S
Response Pricing Segment
521-FL Incentive Amount Paid 14Ø{
5Ø9-F9 Total Amount Paid 14Ø{
10.3.5.3 EXAMPLE 3: INCENTIVE AMOUNT SUBMITTED (438-E3) FOR A CLAIM REVERSAL
The original claim was “P” (Paid) claim that includes a DUR alert.
REVERSAL TRANSACTION
Field Field Name Value
DUR/PPS Segment
439-E4 Reason for Service Code AT
44Ø-E5 Professional Service Code PØ
441-E6 Result of Service Code 2A
474-8E DUR/PPS Level of Effort 12
Request Pricing Segment.
438-E3 Incentive Amount Submitted 14Ø{
43Ø-DU Gross Amount Due 14Ø{
Field Field Name Value
5Ø1-F1 Header Response Status A
112-AN Transaction Response Status A, S
Response Pricing Segment
521-FL Incentive Amount Paid 14Ø{
5Ø9-F9 Total Amount Paid 14Ø{
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 185 -
10.3.5.4 EXAMPLE 4: INCENTIVE AMOUNT SUBMITTED (438-E3) FOR A CLAIM REVERSAL
The original claim was “P” (Paid) claim (for example restocking).
REVERSAL TRANSACTION
Field Field Name Value
Request Pricing Segment
438-E3 Incentive Amount Submitted 14Ø{
Field Field Name Value
5Ø1-F1 Header Response Status A
112-AN Transaction Response Status A, S
Response Pricing Segment
521-FL Incentive Amount Paid 14Ø{
5Ø9-F9 Total Amount Paid 14Ø{
10.3.6 COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT (CLAIM REVERSAL)
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
337-4C COORDINATION OF BENEFITS/OTHER PAYMENTS COUNT M Maximum count of 9.
338-5C OTHER PAYER COVERAGE TYPE M***R*** Mandatory.
Occurs with Coordination of Benefits/Other Payments
Count (337-4C).
339-6C OTHER PAYER ID QUALIFIER N***R*** Claim Reversal:
Not used.
34Ø-7C OTHER PAYER ID N***R*** Claim Reversal:
Not used.
443-E8 OTHER PAYER DATE N***R*** Claim Reversal:
Not used.
993-A7 INTERNAL CONTROL NUMBER N***R*** Claim Reversal:
Not used.
341-HB OTHER PAYER AMOUNT PAID COUNT N Claim Reversal:
Not used.
342-HC OTHER PAYER AMOUNT PAID QUALIFIER N***R*** Claim Reversal:
Not used.
431-DV OTHER PAYER AMOUNT PAID N***R*** Claim Reversal:
Not used.
471-5E OTHER PAYER REJECT COUNT N Claim Reversal:
Not used.
472-6E OTHER PAYER REJECT CODE
N***R*** Claim Reversal:
Not used.
353-NR OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT COUNT N Claim Reversal:
Not used.
351-NP OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT QUALIFIER N***R*** Claim Reversal:
Not used.
352-NQ OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT N***R*** Claim Reversal:
Not used.
392-MU BENEFIT STAGE COUNT N Claim Reversal:
Not used.
393-MV BENEFIT STAGE QUALIFIER N***R*** Claim Reversal:
Not used.
394-MW BENEFIT STAGE AMOUNT N***R*** Claim Reversal:
Not used.
Notes on Coordination of Benefits/Other Payments Segment on a Claim Reversal Request:
The Coordination of Benefits/Other Payments Segment is mandatory for a Claim Reversal request to a downstream payer. It is used when a
downstream payer needs to use the Other Payer Coverage Type (338-5C) to differentiate which claim to reverse because the same processor
is involved in multiple coordination of benefit occurrences for the same patient. Sometimes processors have difficulty determining which claim
to reverse when they are involved for example as the primary and secondary payer, or primary and tertiary, or secondary and tertiary. On a
reversal involved in Coordination of Benefits, to clarify which reversal the pharmacy is requesting to be processed, the Coordination of
Benefits/Other Payments Segment is sent. The Coordination of Benefits/Other Payments Segment provides the pointer to specify which
reversal to back out. This does not change the order of reversing claims; it clarifies which claim to reverse. The pharmacy must reverse the
claim in the correct back out order (see section “Reversal Information”).
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 186 -
Note, the Other Payer Coverage Type (338-5C) occurrences do not have to appear in sequential order (primary, secondary, tertiary),
but can appear in any order.
The Coordination of Benefits/Other Payments Segment is not used for a Claim Reversal request to a primary payer.
See section “Response Processing Guidelines”, “Duplicate Transactions”.
Fields defined as Mandatory are required to be submitted when the segment is sent.
10.3.6.1 COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT USAGE IN CLAIM REVERSAL
10.3.6.1.1 EXCERPT EXAMPLE 1
In this example, the claim reversal is sent to a payer. The highest value of Other Payer Coverage Type (338-5C) is “Ø2” (Secondary). This
means the claim reversal is being sent to the tertiary payer. This may be a payer that is involved in multiple coordination of benefits
occurrences (for example primary and tertiary). The tertiary payer must interrogate the claim reversal and use the Other Payer Coverage Type
(338-5C) to determine that the tertiary claim must be reversed, since “Ø2” is the highest value.
Coordination of Benefits/Other Payments Segment
Field Field Name
111-AM SEGMENT IDENTIFICATION
337-4C COORDINATION OF BENEFITS/OTHER PAYMENTS COUNT 2
338-5C OTHER PAYER COVERAGE TYPE Ø1 Primary
338-5C OTHER PAYER COVERAGE TYPE Ø2 Secondary
10.3.6.1.2 EXCERPT EXAMPLE 2
In this example, the claim reversal is sent to a payer. The highest value of Other Payer Coverage Type (338-5C) is “Ø1” (Primary). This means
the claim reversal is being sent to the secondary payer. This may be a payer that is involved in multiple coordination of benefits occurrences
(for example primary and secondary). The secondary payer must interrogate the claim reversal and use the Other Payer Coverage Type (338-
5C) to determine that the secondary claim must be reversed, since “Ø1” is the highest value.
Coordination of Benefits/Other Payments Segment
Field Field Name
111-AM SEGMENT IDENTIFICATION
337-4C COORDINATION OF BENEFITS/OTHER PAYMENTS COUNT 1
338-5C OTHER PAYER COVERAGE TYPE Ø1 Primary
10.4 CLAIM REVERSAL RESPONSE DIAGRAMS AND SEGMENTS
10.4.1 TRANSMISSION ACCEPTED/TRANSACTION APPROVED
Claim Reversal transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved)
The Response Pricing Segment is not used in payer-to-payer transactions. Therefore, in this case, there are no situational transaction-level
segments.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
10.4.1.1 DIAGRAM FOR TRANSMISSION OF ONE CLAIM REVERSAL RESPONSE (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 187 -
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Pricing Segment
10.4.1.2 DIAGRAM FOR TRANSMISSION OF TWO CLAIM REVERSAL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Pricing Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Pricing Segment
10.4.1.3 DIAGRAM FOR TRANSMISSION OF THREE CLAIM REVERSAL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Pricing Segment
Mandatory second response
Group Separator

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 188 -
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Pricing Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Pricing Segment
10.4.1.4 DIAGRAM FOR TRANSMISSION OF FOUR CLAIM REVERSAL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Pricing Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Pricing Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Pricing Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Pricing Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 189 -
10.4.1.5 CLAIM REVERSAL RESPONSE SEGMENTS (TRANSMISSION ACCEPTED/TRANSACTION
APPROVED)
10.4.1.5.1 RESPONSE HEADER SEGMENT (CLAIM REVERSAL) (TRANSMISSION ACCEPTED/TRANSACTION
APPROVED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M For Transaction Code of “B2”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “1” (Rx Billing).
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Claim Reversal Response:
The Response Header Segment is a mandatory, fixed length segment for Claim Reversal response when the Header Response Status (5Ø1-
F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved). The “Situation” column is
not applicable.
10.4.1.5.2 RESPONSE MESSAGE SEGMENT (CLAIM REVERSAL) (TRANSMISSION ACCEPTED/TRANSACTION
APPROVED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Claim Reversal:
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Claim Reversal Response:
The Response Message Segment is situational for Claim Reversal response when the Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved). It is used when additional text information needs
to be sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
10.4.1.5.3 RESPONSE STATUS SEGMENT (CLAIM REVERSAL) (TRANSMISSION ACCEPTED/TRANSACTION
APPROVED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Claim Reversal:
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT N Claim Reversal:
Not used.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 190 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
511-FB REJECT CODE N***R*** Claim Reversal:
Not used.
546-4F REJECT FIELD OCCURRENCE INDICATOR N***R*** Claim Reversal:
Not used.
547-5F APPROVED MESSAGE CODE COUNT N Claim Reversal:
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Claim Reversal:
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Claim Reversal:
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Claim Reversal:
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Claim Reversal:
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Claim Reversal:
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Claim Reversal:
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Claim Reversal:
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Claim Reversal:
Not used.
993-A7 INTERNAL CONTROL NUMBER Q Claim Reversal:
Required when used for payer-to-payer coordination of
benefits to track the claim without regard to the “Service
Provider ID, Prescription Number, & Date of Service”.
987-MA URL N Claim Reversal:
Not used.
Notes on Response Status Segment on a Claim Reversal Response:
The Response Status Segment is mandatory for a Claim Reversal response for Header Response Status (5Ø1-F1) = “A” (Accepted) and
Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved). The Response Status Segment is sent from the
sender to the receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is
sent.
10.4.1.5.4 RESPONSE CLAIM SEGMENT (CLAIM REVERSAL) (TRANSMISSION ACCEPTED/TRANSACTION APPROVED)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 191 -
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M For Transaction Code of “B2”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “1” (Rx Billing).
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT N Claim Reversal:
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER N***R*** Claim Reversal:
Not used.
553-AR PREFERRED PRODUCT ID N***R*** Claim Reversal:
Not used.
554-AS PREFERRED PRODUCT INCENTIVE N***R*** Claim Reversal:
Not used.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE N***R*** Claim Reversal:
Not used.
556-AU PREFERRED PRODUCT DESCRIPTION N***R*** Claim Reversal:
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Claim Reversal:
Not used.
Notes on Response Claim Segment on a Claim Reversal Response:
The Response Claim Segment is mandatory for a Claim Reversal response to identify the prescription/service reference number when the
Header Response Status (5Ø1-F1) is “A” (Accepted) and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of
Approved). Fields defined as Mandatory are required to be submitted when the segment is sent.
10.4.1.5.4.1 Response Claim Segment (Medicaid Subrogation Claim Reversal) (Transmission
Accepted/Transaction Approved)
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
RM Medicaid Subrogation - Claim Reversal:
Required. Contains the Medicaid unique claim
identification number (also referred to as the ICN or
TCN).
See Medicaid Subrogation Implementation Guide.
Notes on Claim Segment on a Medicaid Subrogation Claim Reversal Request:
The rules above for a “Response Claim Segment (Claim Reversal)” are to be followed for Medicaid Subrogation. Specific fields that
are used differently in Medicaid Subrogation are noted in the table above.
10.4.1.5.5 RESPONSE PRICING SEGMENT (CLAIM REVERSAL) (TRANSMISSION ACCEPTED/TRANSACTION
APPROVED)
RESPONSE PRICING SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø5-F5 PATIENT PAY AMOUNT N Claim Reversal:
Not used.
5Ø6-F6 INGREDIENT COST PAID N Claim Reversal:
Not used.
5Ø7-F7 DISPENSING FEE PAID N Claim Reversal:
Not used.
557-AV TAX EXEMPT INDICATOR N Claim Reversal:
Not used.
558-AW FLAT SALES TAX AMOUNT PAID N Claim Reversal:
Not used.
559-AX PERCENTAGE SALES TAX AMOUNT PAID N Claim Reversal:
Not used.
56∅-AY PERCENTAGE SALES TAX RATE PAID N Claim Reversal:
Not used.
561-AZ PERCENTAGE SALES TAX BASIS PAID N Claim Reversal:
Not used.
521-FL INCENTIVE AMOUNT PAID Q Claim Reversal:
Required if this field is reporting a contractually agreed

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 192 -
RESPONSE PRICING SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
upon payment.
562-J1 PROFESSIONAL SERVICE FEE PAID N Claim Reversal:
Not used.
563-J2 OTHER AMOUNT PAID COUNT N Claim Reversal:
Not used.
564-J3 OTHER AMOUNT PAID QUALIFIER N***R*** Claim Reversal:
Not used.
565-J4 OTHER AMOUNT PAID N***R*** Claim Reversal:
Not used.
566-J5 OTHER PAYER AMOUNT RECOGNIZED N Claim Reversal:
Not used.
5Ø9-F9 TOTAL AMOUNT PAID Q Claim Reversal:
Required if any other payment fields sent by the sender.
522-FM BASIS OF REIMBURSEMENT DETERMINATION N Claim Reversal:
Not used.
523-FN AMOUNT ATTRIBUTED TO SALES TAX N Claim Reversal:
Not used.
512-FC ACCUMULATED DEDUCTIBLE AMOUNT N Claim Reversal:
Not used.
513-FD REMAINING DEDUCTIBLE AMOUNT N Claim Reversal:
Not used.
514-FE REMAINING BENEFIT AMOUNT N Claim Reversal:
Not used.
517-FH AMOUNT APPLIED TO PERIODIC DEDUCTIBLE N Claim Reversal:
Not used.
518-FI AMOUNT OF COPAY N Claim Reversal:
Not used.
52Ø-FK AMOUNT EXCEEDING PERIODIC BENEFIT MAXIMUM N Claim Reversal:
Not used.
346-HH BASIS OF CALCULATION—DISPENSING FEE N Claim Reversal:
Not used.
347-HJ BASIS OF CALCULATION—COPAY N Claim Reversal:
Not used.
348-HK BASIS OF CALCULATION—FLAT SALES TAX N Claim Reversal:
Not used.
349-HM BASIS OF CALCULATION—PERCENTAGE SALES TAX N Claim Reversal:
Not used.
571-NZ AMOUNT ATTRIBUTED TO PROCESSOR FEE N Claim Reversal:
Not used.
575-EQ PATIENT SALES TAX AMOUNT N Claim Reversal:
Not used.
574-2Y PLAN SALES TAX AMOUNT N Claim Reversal:
Not used.
572-4U AMOUNT OF COINSURANCE N Claim Reversal:
Not used.
573-4V BASIS OF CALCULATION-COINSURANCE N Claim Reversal:
Not used.
392-MU BENEFIT STAGE COUNT N Claim Reversal:
Not used.
393-MV BENEFIT STAGE QUALIFIER N***R*** Claim Reversal:
Not used.
394-MW BENEFIT STAGE AMOUNT N***R*** Claim Reversal:
Not used.
577-G3 ESTIMATED GENERIC SAVINGS N Claim Reversal:
Not used.
128-UC SPENDING ACCOUNT AMOUNT REMAINING N Claim Reversal:
Not used.
129-UD HEALTH PLAN-FUNDED ASSISTANCE AMOUNT N Claim Reversal:
Not used.
133-UJ AMOUNT ATTRIBUTED TO PROVIDER NETWORK SELECTION N Claim Reversal:
Not used.
134-UK AMOUNT ATTRIBUTED TO PRODUCT SELECTION/BRAND
DRUG
N Claim Reversal:
Not used.
135-UM AMOUNT ATTRIBUTED TO PRODUCT SELECTION/NON-
PREFERRED FORMULARY SELECTION
N Claim Reversal:
Not used.
136-UN AMOUNT ATTRIBUTED TO PRODUCT SELECTION/BRAND
NON-PREFERRED FORMULARY SELECTION
N Claim Reversal:
Not used.
137-UP AMOUNT ATTRIBUTED TO COVERAGE GAP N Claim Reversal:
Not used.
148-U8 INGREDIENT COST CONTRACTED/REIMBURSABLE AMOUNT N Claim Reversal:

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 193 -
RESPONSE PRICING SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Not used.
149-U9 DISPENSING FEE CONTRACTED/REIMBURSABLE AMOUNT N Claim Reversal:
Not used.
Notes on Response Pricing Segment on a Claim Reversal Response:
The Response Pricing Segment is situational for a Claim Reversal response when the Header Response Status (5Ø1-F1) is “A” (Accepted)
and Transaction Response Status (112-AN) is “A” (Approved) or “S” (Duplicate of Approved).
The Response Pricing Segment is not used in payer-to-payer transactions.
It is highly recommended that whenever possible, the individual dollar fields are returned in the response. On the response the sender should
return the individual payment response fields to allow the receiver to reconcile against the requested payment fields.
Fields defined as Mandatory are required to be submitted when the segment is sent.
10.4.2 TRANSMISSION ACCEPTED/TRANSACTION CAPTURED
Claim Reversal transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured)
There are no transaction-level situation segments for Claim Reversal transmission response Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured).
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
10.4.2.1 DIAGRAM FOR TRANSMISSION OF ONE CLAIM REVERSAL RESPONSE (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
10.4.2.2 DIAGRAM FOR TRANSMISSION OF TWO CLAIM REVERSAL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
10.4.2.3 DIAGRAM FOR TRANSMISSION OF THREE CLAIM REVERSAL RESPONSES (TRANSMISSION
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 194 -
ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
10.4.2.4 DIAGRAM FOR TRANSMISSION OF FOUR CLAIM REVERSAL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
10.4.2.5 CLAIM REVERSAL RESPONSE SEGMENTS (TRANSMISSION ACCEPTED/TRANSACTION
CAPTURED)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 195 -
10.4.2.5.1 RESPONSE HEADER SEGMENT (CLAIM REVERSAL) (TRANSMISSION ACCEPTED/TRANSACTION
CAPTURED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M For Transaction Code of “B2”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “1” (Rx Billing).
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Claim Reversal Response:
The Response Header Segment is a mandatory, fixed length segment for Claim Reversal response when the Header Response Status (5Ø1-
F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). The “Situation” column is
not applicable.
10.4.2.5.2 RESPONSE MESSAGE SEGMENT (CLAIM REVERSAL) (TRANSMISSION ACCEPTED/TRANSACTION
CAPTURED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Claim Reversal:
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Claim Reversal Response:
The Response Message Segment is situational for Claim Reversal response when the Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). It is used when additional text information needs
to be sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
10.4.2.5.3 RESPONSE STATUS SEGMENT (CLAIM REVERSAL) (TRANSMISSION ACCEPTED/TRANSACTION
CAPTURED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Claim Reversal:
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT N Claim Reversal:
Not used.
511-FB REJECT CODE N***R*** Claim Reversal:
Not used.
546-4F REJECT FIELD OCCURRENCE INDICATOR N***R*** Claim Reversal:
Not used.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 196 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
547-5F APPROVED MESSAGE CODE COUNT N Claim Reversal:
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Claim Reversal:
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Claim Reversal:
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Claim Reversal:
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Claim Reversal:
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q**R*** Claim Reversal:
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Claim Reversal:
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Claim Reversal:
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Claim Reversal:
Not used.
993-A7 INTERNAL CONTROL NUMBER Q Claim Reversal:
Required when used for payer-to-payer coordination of
benefits to track the claim without regard to the “Service
Provider ID, Prescription Number, & Date of Service”.
987-MA URL N Claim Reversal:
Not used.
Notes on Response Status Segment on a Claim Reversal Response:
The Response Status Segment is mandatory for a Claim Reversal response for Header Response Status (5Ø1-F1) = “A” (Accepted) and
Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). The Response Status Segment is sent from the
sender to the receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is
sent.
10.4.2.5.4 RESPONSE CLAIM SEGMENT (CLAIM REVERSAL) (TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M For Transaction Code of “B2”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “1” (Rx Billing).

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 197 -
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT N Claim Reversal:
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER N**R*** Claim Reversal:
Not used.
553-AR PREFERRED PRODUCT ID N**R*** Claim Reversal:
Not used.
554-AS PREFERRED PRODUCT INCENTIVE N**R*** Claim Reversal:
Not used.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE NR*** Claim Reversal:
Not used.
556-AU PREFERRED PRODUCT DESCRIPTION N**R*** Claim Reversal:
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Claim Reversal:
Not used.
Notes on Response Claim Segment on a Claim Reversal Response:
The Response Claim Segment is mandatory for a Claim Reversal response to identify the prescription/service reference number when the
Header Response Status (5Ø1-F1) is “A” (Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of
Captured). Fields defined as Mandatory are required to be submitted when the segment is sent.
10.4.3 TRANSMISSION ACCEPTED/TRANSACTION REJECTED
10.4.3.1 DIAGRAM FOR TRANSMISSION OF ONE CLAIM REVERSAL RESPONSE (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
Claim Reversal transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) “R” (Rejected)
There are no transaction-level situation segments for Claim Reversal transmission response Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) “R” (Rejected).
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
10.4.3.2 DIAGRAM FOR TRANSMISSION OF TWO CLAIM REVERSAL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 198 -
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
10.4.3.3 DIAGRAM FOR TRANSMISSION OF THREE CLAIM REVERSAL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
10.4.3.4 DIAGRAM FOR TRANSMISSION OF FOUR CLAIM REVERSAL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 199 -
Segment Separator
Response Claim Segment
10.4.3.5 CLAIM REVERSAL RESPONSE SEGMENTS (TRANSMISSION ACCEPTED/TRANSACTION
REJECTED)
10.4.3.5.1 RESPONSE HEADER SEGMENT (CLAIM REVERSAL) (TRANSMISSION ACCEPTED/TRANSACTION
REJECTED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M For Transaction Code of “B2”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “1” (Rx Billing).
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Claim Reversal Response:
The Response Header Segment is a mandatory, fixed length segment for Claim Reversal response when the Header Response Status (5Ø1-
F1) of "A" (Accepted) and Transaction Response Status (112-AN) “R” (Rejected). The “Situation” column is not applicable.
10.4.3.5.2 RESPONSE MESSAGE SEGMENT (CLAIM REVERSAL) (TRANSMISSION ACCEPTED/TRANSACTION
REJECTED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Claim Reversal:
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Claim Reversal Response:
The Response Message Segment is situational for Claim Reversal response when the Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) “R” (Rejected). It is used when additional text information needs to be sent. Fields defined as
Mandatory are required to be submitted when the segment is sent.
10.4.3.5.3 RESPONSE STATUS SEGMENT (CLAIM REVERSAL) (TRANSMISSION ACCEPTED/TRANSACTION
REJECTED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Claim Reversal:
Required if needed to identify the transaction.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 200 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
51Ø-FA REJECT COUNT R Claim Reversal:
Maximum count of 5.
Required.
511-FB REJECT CODE R**R*** Claim Reversal:
Required.
546-4F REJECT FIELD OCCURRENCE INDICATOR Q***R*** Claim Reversal:
Required if a repeating field is in error, to identify repeating
field occurrence.
This field must be sent when relaying error information about
a repeating field or set. Note, if the Reject Code is not
denoting a repeating field or set, the Reject Field Occurrence
Indicator must not be sent.
547-5F APPROVED MESSAGE CODE COUNT N Claim Reversal:
Not used.
548-6F APPROVED MESSAGE CODE N**R*** Claim Reversal:
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Claim Reversal:
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q**R*** Claim Reversal:
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Claim Reversal:
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q**R*** Claim Reversal:
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Claim Reversal:
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Claim Reversal:
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Claim Reversal:
Not used.
993-A7 INTERNAL CONTROL NUMBER N Claim Reversal:
Not used.
987-MA URL N Claim Reversal:
Not used.
Notes on Response Status Segment on a Claim Reversal Response:
The Response Status Segment is mandatory for a Claim Reversal response for Header Response Status (5Ø1-F1) = “A” (Accepted) and
Transaction Response Status (112-AN) “R” (Rejected). The Response Status Segment is sent from the sender to the receiver to identify the
outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.
10.4.3.5.4 RESPONSE CLAIM SEGMENT (CLAIM REVERSAL) (TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 201 -
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M For Transaction Code of “B2”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “1” (Rx Billing).
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT N Claim Reversal:
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER N**R*** Claim Reversal:
Not used.
553-AR PREFERRED PRODUCT ID N**R*** Claim Reversal:
Not used.
554-AS PREFERRED PRODUCT INCENTIVE N**R*** Claim Reversal:
Not used.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE N**R*** Claim Reversal:
Not used.
556-AU PREFERRED PRODUCT DESCRIPTION N**R*** Claim Reversal:
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Claim Reversal:
Not used.
Notes on Response Claim Segment on a Claim Reversal Response:
The Response Claim Segment is mandatory for a Claim Reversal response to identify the prescription/service reference number when the
Header Response Status (5Ø1-F1) is “A” (Accepted) and Transaction Response Status (112-AN) “R” (Rejected). Fields defined as Mandatory
are required to be submitted when the segment is sent.
10.4.4 TRANSMISSION REJECTED/TRANSACTION REJECTED
Claim Reversal transmission response Header Response Status (5Ø1-F1) of "R" (Rejected)
and Transaction Response Status (112-AN) of “R” (Rejected)
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
10.4.4.1 DIAGRAM FOR TRANSMISSION OF ONE CLAIM REVERSAL RESPONSE (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
10.4.4.2 DIAGRAM FOR TRANSMISSION OF TWO CLAIM REVERSAL RESPONSES (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 202 -
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
10.4.4.3 DIAGRAM FOR TRANSMISSION OF THREE CLAIM REVERSAL RESPONSES (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
10.4.4.4 DIAGRAM FOR TRANSMISSION OF FOUR CLAIM REVERSAL RESPONSES (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 203 -
10.4.4.5 CLAIM REVERSAL RESPONSE SEGMENTS (TRANSMISSION REJECTED/TRANSACTION
REJECTED)
10.4.4.5.1 RESPONSE HEADER SEGMENT (CLAIM REVERSAL) (TRANSMISSION REJECTED/TRANSACTION
REJECTED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Claim Reversal Response:
The Response Header Segment is a mandatory, fixed length segment for Claim Reversal response when the Header Response Status (5Ø1-
F1) of "R" (Rejected) and Transaction Response Status (112-AN) of “R” (Rejected). The “Situation” column is not applicable.
If either the entire transmission or the Header is in error, the Header Response Status (5Ø1-F1) = “R” (Rejected). Every identifiable transaction
within the transmission must be rejected with an “R”.
If the transaction rejects for detail errors, the Header Response Status (5Ø1-F1) = “A” (Accepted) and the Transaction Response Status (112-
AN) will be “R”.
10.4.4.5.2 RESPONSE MESSAGE SEGMENT (CLAIM REVERSAL) (TRANSMISSION REJECTED/TRANSACTION
REJECTED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Claim Reversal:
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Claim Reversal Response:
The Response Message Segment is situational for Claim Reversal response when the Header Response Status (5Ø1-F1) of "R" (Rejected)
and Transaction Response Status (112-AN) of “R” (Rejected). It is used when additional text information needs to be sent. Fields defined as
Mandatory are required to be submitted when the segment is sent.
10.4.4.5.3 RESPONSE STATUS SEGMENT (CLAIM REVERSAL) (TRANSMISSION REJECTED/TRANSACTION REJECTED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Claim Reversal:
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT R Claim Reversal:

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 204 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Maximum count of 5.
Required.
511-FB REJECT CODE R**R*** Claim Reversal:
Required.
546-4F REJECT FIELD OCCURRENCE INDICATOR Q**R*** Claim Reversal:
Required if a repeating field is in error, to identify repeating
field occurrence.
This field must be sent when relaying error information about
a repeating field or set. Note, if the Reject Code is not
denoting a repeating field or set, the Reject Field Occurrence
Indicator must not be sent.
547-5F APPROVED MESSAGE CODE COUNT N Claim Reversal:
Not used.
548-6F APPROVED MESSAGE CODE N*R*** Claim Reversal:
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Claim Reversal:
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q**R*** Claim Reversal:
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Claim Reversal:
Required if additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q**R*** Claim Reversal:
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Claim Reversal:
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Claim Reversal:
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Claim Reversal:
Not used.
993-A7 INTERNAL CONTROL NUMBER N Claim Reversal:
Not used.
987-MA URL N Claim Reversal:
Not used.
Notes on Response Status Segment on a Claim Reversal Response:
The Response Status Segment is mandatory for a Claim Reversal response for Header Response Status (5Ø1-F1) = “R” (Rejected) and
Transaction Response Status (112-AN) of “R” (Rejected). The Response Status Segment is sent from the sender to the receiver to identify the
outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 205 -
10.5 SERVICE REVERSAL REQUEST DIAGRAMS
10.5.1 DIAGRAM FOR TRANSMISSION OF ONE SERVICE REVERSAL TRANSACTION
For a Service Reversal, the scenarios defined include
Service Reversal from a Sender to a Receiver
Service Reversal Accepted/Transaction Approved Response from a Sender to a Receiver
Service Reversal Accepted/Transaction Captured Response from a Sender to a Receiver
Standard Transmission Accepted/Transaction Rejected Response from a Sender to a Receiver
Standard Transmission Reject Response to a Claim Reversal from a Sender to a Receiver
The Coordination of Benefits Segment is situational only for reversals to downstream payers; otherwise it is not used.T
Mandatory
Transaction Header Segment
Situational
Segment Separator
Insurance Segment
Mandatory - first Service Reversal transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
Coordination of Benefits/Other Payments Segment
10.5.2 DIAGRAM FOR TRANSMISSION OF TWO SERVICE REVERSAL TRANSACTIONS
Mandatory
Transaction Header Segment
Situational
Segment Separator
Insurance Segment
Mandatory - first Service Reversal transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
Coordination of Benefits/Other Payments Segment
Mandatory - second Service Reversal transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
Coordination of Benefits/Other Payments Segment
10.5.3 DIAGRAM FOR TRANSMISSION OF THREE SERVICE REVERSAL TRANSACTIONS
Mandatory
Transaction Header Segment
Situational
Segment Separator
Insurance Segment
Mandatory - first Service Reversal transaction
Group Separator
Segment Separator
Claim Segment
Situational

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 206 -
Segment Separator
Coordination of Benefits/Other Payments Segment
Mandatory - second Service Reversal transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
Coordination of Benefits/Other Payments Segment
Mandatory – third Service Reversal transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
Coordination of Benefits/Other Payments Segment
10.5.4 DIAGRAM FOR TRANSMISSION OF FOUR SERVICE REVERSAL TRANSACTIONS
Mandatory
Transaction Header Segment
Situational
Segment Separator
Insurance Segment
Mandatory - first Service Reversal transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
Coordination of Benefits/Other Payments Segment
Mandatory - second Service Reversal transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
Coordination of Benefits/Other Payments Segment
Mandatory – third Service Reversal transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
Coordination of Benefits/Other Payments Segment
Mandatory – fourth Service Reversal transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
Coordination of Benefits/Other Payments Segment
10.6 SERVICE REVERSAL REQUEST SEGMENTS
10.6.1 TRANSACTION HEADER SEGMENT (SERVICE REVERSAL)
TRANSACTION HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø1-A1 BIN NUMBER M

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 207 -
TRANSACTION HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M For Transaction Code of “S2”, in the Claim Segment, the
Prescription/Service Reference Number Qualifier (455-
EM) is “2” (Service Billing).
1Ø4-A4 PROCESSOR CONTROL NUMBER M
1Ø9-A9 TRANSACTION COUNT M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M
Notes on Transaction Header Segment on a Service Reversal Request:
The Transaction Header Segment is a mandatory, fixed length segment for a Service Reversal request. The “Situation” column is not
applicable.
10.6.2 INSURANCE SEGMENT (SERVICE REVERSAL)
INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø2-C2 CARDHOLDER ID M
312-CC CARDHOLDER FIRST NAME N Service Reversal:
Not used.
313-CD CARDHOLDER LAST NAME N Service Reversal:
Not used.
314-CE HOME PLAN N Service Reversal:
Not used.
524-FO PLAN ID N Service Reversal:
Not used.
3Ø9-C9 ELIGIBILITY CLARIFICATION CODE N Service Reversal:
Not used.
3Ø1-C1 GROUP ID Q Service Reversal:
Required if needed to match the reversal to the original
billing transaction.
3Ø3-C3 PERSON CODE N Service Reversal:
Not used.
3Ø6-C6 PATIENT RELATIONSHIP CODE N Service Reversal:
Not used.
99Ø-MG OTHER PAYER BIN NUMBER N Service Reversal:
Not used.
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER N Service Reversal:
Not used.
356-NU OTHER PAYER CARDHOLDER ID N Service Reversal:
Not used.
992-MJ OTHER PAYER GROUP ID N Service Reversal:
Not used.
359-2A MEDIGAP ID N Service Reversal:
Not used.
36Ø-2B MEDICAID INDICATOR N Service Reversal:
Not used.
361-2D PROVIDER ACCEPT ASSIGNMENT INDICATOR N Service Reversal:
Not used.
997-G2 CMS PART D DEFINED QUALIFIED FACILITY N Service Reversal:
Not used.
115-N5
MEDICAID ID NUMBER N Service Reversal:
Not used.
116-N6
MEDICAID AGENCY NUMBER N Service Reversal:
Not used.
Notes on Insurance Segment on a Service Reversal Request:
The Insurance Segment is situational for a Service Reversal request. If the Cardholder ID field is not submitted, the Insurance Segment is not
used. The Segment is mandatory if required under provider payer contract or mandatory on claims where this information is necessary for
reversal of the claim. Fields defined as Mandatory are required to be submitted when the segment is sent.
10.6.3 CLAIM SEGMENT (SERVICE REVERSAL)

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 208 -
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M For Transaction Code of “S2”, in the Claim Segment, the
Prescription/Service Reference Number Qualifier (455-EM)
is “2” (Service Billing).
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
436-E1 PRODUCT/SERVICE ID QUALIFIER M Mandatory.
Must contain the Product/Service ID Qualifier (436-E1)
value from original Billing.
4Ø7-D7 PRODUCT/SERVICE ID M Mandatory.
Must contain the Product/Service ID (436-E1) value from
original Billing.
456-EN ASSOCIATED PRESCRIPTION/SERVICE REFERENCE NUMBER N Service Reversal:
Not used.
457-EP ASSOCIATED PRESCRIPTION/SERVICE DATE N Service Reversal:
Not used.
458-SE PROCEDURE MODIFIER CODE COUNT N Service Reversal:
Not used.
459-ER PROCEDURE MODIFIER CODE N***R*** Service Reversal:
Not used.
442-E7 QUANTITY DISPENSED N Service Reversal:
Not used.
4Ø3-D3 FILL NUMBER Q Service Reversal:
Required if needed for reversals when multiple fills of the
same Prescription/Service Reference Number (4Ø2-D2)
occur on the same day.
4Ø5-D5 DAYS SUPPLY N Service Reversal:
Not used.
4Ø6-D6 COMPOUND CODE N Service Reversal:
Not used.
4Ø8-D8 DISPENSE AS WRITTEN (DAW)/PRODUCT SELECTION CODE N Service Reversal:
Not used.
414-DE DATE PRESCRIPTION WRITTEN N Service Reversal:
Not used.
415-DF NUMBER OF REFILLS AUTHORIZED N Service Reversal:
Not used.
419-DJ PRESCRIPTION ORIGIN CODE N Service Reversal:
Not used.
354-NX SUBMISSION CLARIFICATION CODE COUNT N Service Reversal:
Not used.
42Ø-DK SUBMISSION CLARIFICATION CODE N**R*** Service Reversal:
Not used.
46Ø-ET QUANTITY PRESCRIBED N Service Reversal:
Not used.
3Ø8-C8 OTHER COVERAGE CODE Q Service Reversal:
Required if needed by receiver to match the claim that is
being reversed.
See section “Specific Segment Discussion”, “Request
Segments”, “Claim Segment”, “Other Coverage Code (3Ø8-
C8).
429-DT SPECIAL PACKAGING INDICATOR N Service Reversal:
Not used.
453-EJ ORIGINALLY PRESCRIBED PRODUCT/SERVICE ID QUALIFIER N Service Reversal:
Not used.
445-EA ORIGINALLY PRESCRIBED PRODUCT/SERVICE CODE N Service Reversal:
Not used.
446-EB ORIGINALLY PRESCRIBED QUANTITY N Service Reversal:
Not used.
33Ø-CW ALTERNATE ID N Service Reversal:
Not used.
454-EK SCHEDULED PRESCRIPTION ID NUMBER N Service Reversal:
Not used.
6ØØ-28 UNIT OF MEASURE N Service Reversal:
Not used.
418-DI LEVEL OF SERVICE N Service Reversal:
Not used.
461-EU PRIOR AUTHORIZATION TYPE CODE N Service Reversal:
Not used.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 209 -
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
462-EV PRIOR AUTHORIZATION NUMBER SUBMITTED N Service Reversal:
Not used.
463-EW INTERMEDIARY AUTHORIZATION TYPE ID N Service Reversal:
Not used.
464-EX INTERMEDIARY AUTHORIZATION ID N Service Reversal:
Not used.
343-HD DISPENSING STATUS N Service Reversal:
Not used.
344-HF QUANTITY INTENDED TO BE DISPENSED N Service Reversal:
Not used.
345-HG DAYS SUPPLY INTENDED TO BE DISPENSED N Service Reversal:
Not used.
357-NV DELAY REASON CODE N Service Reversal:
Not used.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Service Reversal:
Not used.
391-MT PATIENT ASSIGNMENT INDICATOR (DIRECT MEMBER
REIMBURSEMENT INDICATOR)
N Service Reversal:
Not used.
995-E2 ROUTE OF ADMINISTRATION N Service Reversal:
Not used.
996-G1 COMPOUND TYPE N Service Reversal:
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Service Reversal:
Not used.
147-U7 PHARMACY SERVICE TYPE Q Service Reversal:
Required when the submitter must clarify the type of
services being performed as a condition for proper
reimbursement by the payer.
Notes on Claim Segment on a Service Reversal Request:
The Claim Segment is mandatory for a Service Reversal request. The Claim Segment defines the product dispensed and dispensing
information. Fields defined as Mandatory are required to be submitted when the segment is sent.
10.6.4 COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT (SERVICE
REVERSAL)
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
337-4C COORDINATION OF BENEFITS/OTHER PAYMENTS COUNT M Maximum count of 9.
338-5C OTHER PAYER COVERAGE TYPE M***R*** Mandatory.
Occurs with Coordination of Benefits/Other Payments
Count (337-4C).
339-6C OTHER PAYER ID QUALIFIER N***R*** Service Reversal:
Not used.
34Ø-7C OTHER PAYER ID N***R*** Service Reversal:
Not used.
443-E8 OTHER PAYER DATE N***R*** Service Reversal:
Not used.
993-A7 INTERNAL CONTROL NUMBER N***R*** Service Reversal:
Not used.
341-HB OTHER PAYER AMOUNT PAID COUNT N Service Reversal:
Not used.
342-HC OTHER PAYER AMOUNT PAID QUALIFIER N***R*** Service Reversal:
Not used.
431-DV OTHER PAYER AMOUNT PAID N***R*** Service Reversal:
Not used.
471-5E OTHER PAYER REJECT COUNT N Service Reversal:
Not used.
472-6E OTHER PAYER REJECT CODE
N***R*** Service Reversal:
Not used.
353-NR OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT COUNT N Service Reversal:
Not used.
351-NP OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT QUALIFIER N***R*** Service Reversal:
Not used.
352-NQ OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT N***R*** Service Reversal:
Not used.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 210 -
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
392-MU BENEFIT STAGE COUNT N Service Reversal:
Not used.
393-MV BENEFIT STAGE QUALIFIER N***R*** Service Reversal:
Not used.
394-MW BENEFIT STAGE AMOUNT N***R*** Service Reversal:
Not used.
Notes on Coordination of Benefits/Other Payments Segment on a Service Reversal Request:
The Coordination of Benefits/Other Payments Segment is mandatory for a Service Reversal request to a downstream payer. It is used when
a downstream payer needs to use the Other Payer Coverage Type (338-5C) to differentiate which service to reverse because the same
processor is involved in multiple coordination of benefit occurrences for the same patient. Sometimes processors have difficulty determining
which service to reverse when they are involved for example as the primary and secondary payer, or primary and tertiary, or secondary and
tertiary. The On a reversal involved in Coordination of Benefits, to clarify which reversal the pharmacy is requesting to be processed, the
Coordination of Benefits/Other Payments Segment is sent. The Coordination of Benefits/Other Payments Segment provides the pointer to
specify which reversal to back out. This does not change the order of reversing services; it clarifies which service to reverse. pharmacy must
reverse the service in the correct back out order (see section “Reversal Information”).
Note, the Other Payer Coverage Type (338-5C) occurrences do not have to appear in sequential order (primary, secondary, tertiary),
but can appear in any order.
The Coordination of Benefits/Other Payments Segment is not used for a Service Reversal request to a primary payer.
See section “Response Processing Guidelines”, “Duplicate Transactions”.
Fields defined as Mandatory are required to be submitted when the segment is sent.
10.6.4.1 COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT USAGE IN SERVICE REVERSAL
10.6.4.1.1 EXCERPT EXAMPLE 1
In this example, the service reversal is sent to a payer. The highest value of Other Payer Coverage Type (338-5C) is “Ø2” (Secondary). This
means the service reversal is being sent to the tertiary payer. This may be a payer that is involved in multiple coordination of benefits
occurrences (for example primary and tertiary). The tertiary payer must interrogate the service reversal and use the Other Payer Coverage
Type (338-5C) to determine that the tertiary service must be reversed, since “Ø2” is the highest value.
Coordination of Benefits/Other Payments Segment
Field Field Name
111-AM SEGMENT IDENTIFICATION
337-4C COORDINATION OF BENEFITS/OTHER PAYMENTS COUNT 2
338-5C OTHER PAYER COVERAGE TYPE Ø1 Primary
338-5C OTHER PAYER COVERAGE TYPE Ø2 Secondary
10.6.4.1.2 EXCERPT EXAMPLE 2
In this example, the service reversal is sent to a payer. The highest value of Other Payer Coverage Type (338-5C) is “Ø1” (Primary). This
means the service reversal is being sent to the secondary payer. This may be a payer that is involved in multiple coordination of benefits
occurrences (for example primary and secondary). The secondary payer must interrogate the service reversal and use the Other Payer
Coverage Type (338-5C) to determine that the secondary service must be reversed, since “Ø1” is the highest value.
Coordination of Benefits/Other Payments Segment
Field Field Name
111-AM SEGMENT IDENTIFICATION
337-4C COORDINATION OF BENEFITS/OTHER PAYMENTS COUNT 1
338-5C OTHER PAYER COVERAGE TYPE Ø1 Primary
10.7 SERVICE REVERSAL RESPONSE DIAGRAMS AND SEGMENTS
10.7.1 TRANSMISSION ACCEPTED/TRANSACTION APPROVED
Service Reversal transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved)
There are no situational transaction-level segments in the Service Reversal transmission response Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved).

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 211 -
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
10.7.1.1 DIAGRAM FOR TRANSMISSION OF ONE SERVICE REVERSAL RESPONSE (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
10.7.1.2 DIAGRAM FOR TRANSMISSION OF TWO SERVICE REVERSAL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
10.7.1.3 DIAGRAM FOR TRANSMISSION OF THREE SERVICE REVERSAL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory third response
Group Separator
Segment Separator

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 212 -
Response Status Segment
Segment Separator
Response Claim Segment
10.7.1.4 DIAGRAM FOR TRANSMISSION OF FOUR SERVICE REVERSAL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
10.7.1.5 SERVICE REVERSAL RESPONSE SEGMENTS (TRANSMISSION ACCEPTED/TRANSACTION
APPROVED)
10.7.1.5.1 RESPONSE HEADER SEGMENT (SERVICE REVERSAL) (TRANSMISSION ACCEPTED/TRANSACTION
APPROVED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M For Transaction Code of “S2”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “2” (Service Billing).
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Service Reversal Response:
The Response Header Segment is a mandatory, fixed length segment for Service Reversal response when the Header Response Status
(5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved). The “Situation”
column is not applicable.
10.7.1.5.2 RESPONSE MESSAGE SEGMENT (SERVICE REVERSAL) (TRANSMISSION ACCEPTED/TRANSACTION
APPROVED)

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 213 -
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Service Reversal:
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Service Reversal Response:
The Response Message Segment is situational for Service Reversal response when the Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved). It is used when additional text information needs
to be sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
10.7.1.5.3 RESPONSE STATUS SEGMENT (SERVICE REVERSAL) (TRANSMISSION ACCEPTED/TRANSACTION
APPROVED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Service Reversal:
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT N Service Reversal:
Not used.
511-FB REJECT CODE N***R*** Service Reversal:
Not used.
546-4F REJECT FIELD OCCURRENCE INDICATOR N**R*** Service Reversal:
Not used.
547-5F APPROVED MESSAGE CODE COUNT N Service Reversal:
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Service Reversal:
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Service Reversal:
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q**R*** Service Reversal:
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Service Reversal:
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 214 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q**R*** Service Reversal:
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Service Reversal:
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Service Reversal:
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Service Reversal:
Not used.
993-A7 INTERNAL CONTROL NUMBER Q Service Reversal:
Required when used for payer-to-payer coordination of
benefits to track the claim without regard to the “Service
Provider ID, Prescription Number, & Date of Service”.
987-MA URL N Service Reversal:
Not used.
Notes on Response Status Segment on a Service Reversal Response:
The Response Status Segment is mandatory for a Service Reversal response for Header Response Status (5Ø1-F1) = “A” (Accepted) and
Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved). The Response Status Segment is sent from the
sender to the receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is
sent.
10.7.1.5.4 RESPONSE CLAIM SEGMENT (SERVICE REVERSAL) (TRANSMISSION ACCEPTED/TRANSACTION
APPROVED)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M For Transaction Code of “S2”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “2” (Service Billing).
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT N Service Reversal:
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER N***R*** Service Reversal:
Not used.
553-AR PREFERRED PRODUCT ID N***R*** Service Reversal:
Not used.
554-AS PREFERRED PRODUCT INCENTIVE N***R*** Service Reversal:
Not used.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE N***R*** Service Reversal:
Not used.
556-AU PREFERRED PRODUCT DESCRIPTION N***R*** Service Reversal:
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Service Reversal:
Not used.
Notes on Response Claim Segment on a Service Reversal Response:
The Response Claim Segment is mandatory for a Service Reversal response to identify the prescription/service reference number when the
Header Response Status (5Ø1-F1) is “A” (Accepted) and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of
Approved). Fields defined as Mandatory are required to be submitted when the segment is sent.
10.7.2 TRANSMISSION ACCEPTED/TRANSACTION CAPTURED
Service Reversal transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 215 -
and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured)
There are no situational transaction-level segments in the Service Reversal transmission response Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured).
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
10.7.2.1 DIAGRAM FOR TRANSMISSION OF ONE SERVICE REVERSAL RESPONSE (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
10.7.2.2 DIAGRAM FOR TRANSMISSION OF TWO SERVICE REVERSAL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
10.7.2.3 DIAGRAM FOR TRANSMISSION OF THREE SERVICE REVERSAL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 216 -
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
10.7.2.4 DIAGRAM FOR TRANSMISSION OF FOUR SERVICE REVERSAL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
10.7.2.5 SERVICE REVERSAL RESPONSE SEGMENTS (TRANSMISSION ACCEPTED/TRANSACTION
CAPTURED)
10.7.2.5.1 RESPONSE HEADER SEGMENT (SERVICE REVERSAL) (TRANSMISSION ACCEPTED/TRANSACTION
CAPTURED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M For Transaction Code of “S2”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “2” (Service Billing).
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Service Reversal Response:
The Response Header Segment is a mandatory, fixed length segment for Service Reversal response when the Header Response Status
(5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). The “Situation”
column is not applicable.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 217 -
10.7.2.5.2 RESPONSE MESSAGE SEGMENT (SERVICE REVERSAL) (TRANSMISSION ACCEPTED/TRANSACTION
CAPTURED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Service Reversal:
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Service Reversal Response:
The Response Message Segment is situational for Service Reversal response when the Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). It is used when additional text information needs
to be sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
10.7.2.5.3 RESPONSE STATUS SEGMENT (SERVICE REVERSAL) (TRANSMISSION ACCEPTED/TRANSACTION
CAPTURED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Service Reversal:
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT N Service Reversal:
Not used.
511-FB REJECT CODE N**R*** Service Reversal:
Not used.
546-4F REJECT FIELD OCCURRENCE INDICATOR N***R*** Service Reversal:
Not used.
547-5F APPROVED MESSAGE CODE COUNT N Service Reversal:
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Service Reversal:
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Service Reversal:
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Service Reversal:
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Service Reversal:
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 218 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q**R*** Service Reversal:
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Service Reversal:
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Service Reversal:
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Service Reversal:
Not used.
993-A7 INTERNAL CONTROL NUMBER Q Service Reversal:
Required when used for payer-to-payer coordination of
benefits to track the claim without regard to the “Service
Provider ID, Prescription Number, & Date of Service”.
987-MA URL N Service Reversal:
Not used.
Notes on Response Status Segment on a Service Reversal Response:
The Response Status Segment is mandatory for a Service Reversal response for Header Response Status (5Ø1-F1) = “A” (Accepted) and
Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). The Response Status Segment is sent from the
sender to the receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is
sent.
10.7.2.5.4 RESPONSE CLAIM SEGMENT (SERVICE REVERSAL) (TRANSMISSION ACCEPTED/TRANSACTION
CAPTURED)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M For Transaction Code of “S2”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “2” (Service Billing).
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT N Service Reversal:
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER N***R*** Service Reversal:
Not used.
553-AR PREFERRED PRODUCT ID N***R*** Service Reversal:
Not used.
554-AS PREFERRED PRODUCT INCENTIVE N***R*** Service Reversal:
Not used.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE N***R*** Service Reversal:
Not used.
556-AU PREFERRED PRODUCT DESCRIPTION N***R*** Service Reversal:
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Service Reversal:
Not used.
Notes on Response Claim Segment on a Service Reversal Response:
The Response Claim Segment is mandatory for a Service Reversal response to identify the prescription/service reference number when the
Header Response Status (5Ø1-F1) is “A” (Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of
Captured). Fields defined as Mandatory are required to be submitted when the segment is sent.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 219 -
10.7.3 TRANSMISSION ACCEPTED/TRANSACTION REJECTED
Service Reversal transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) “R” (Rejected)
There are no situational transaction-level segments in the Service Reversal transmission response Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) “R” (Rejected).
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
10.7.3.1 DIAGRAM FOR TRANSMISSION OF ONE SERVICE REVERSAL RESPONSE (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
10.7.3.2 DIAGRAM FOR TRANSMISSION OF TWO SERVICE REVERSAL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
10.7.3.3 DIAGRAM FOR TRANSMISSION OF THREE SERVICE REVERSAL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 220 -
Segment Separator
Response Claim Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
10.7.3.4 DIAGRAM FOR TRANSMISSION OF FOUR SERVICE REVERSAL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
10.7.3.5 SERVICE REVERSAL RESPONSE SEGMENTS (TRANSMISSION ACCEPTED/TRANSACTION
REJECTED)
10.7.3.5.1 RESPONSE HEADER SEGMENT (SERVICE REVERSAL) (TRANSMISSION ACCEPTED/TRANSACTION
REJECTED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M For Transaction Code of “S2”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “2” (Service Billing).
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Service Reversal Response:
The Response Header Segment is a mandatory, fixed length segment for Service Reversal response when the Header Response Status
(5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) “R” (Rejected). The “Situation” column is not applicable.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 221 -
10.7.3.5.2 RESPONSE MESSAGE SEGMENT (SERVICE REVERSAL) (TRANSMISSION ACCEPTED/TRANSACTION
REJECTED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Service Reversal:
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Service Reversal Response:
The Response Message Segment is situational for Service Reversal response when the Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) “R” (Rejected). It is used when additional text information needs to be sent. Fields defined as
Mandatory are required to be submitted when the segment is sent.
10.7.3.5.3 RESPONSE STATUS SEGMENT (SERVICE REVERSAL) (TRANSMISSION ACCEPTED/TRANSACTION
REJECTED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Service Reversal:
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT R Service Reversal:
Maximum count of 5.
Required.
511-FB REJECT CODE R**R*** Service Reversal:
Required.
546-4F REJECT FIELD OCCURRENCE INDICATOR Q***R*** Service Reversal:
Required if a repeating field is in error, to identify repeating
field occurrence.
This field must be sent when relaying error information about
a repeating field or set. Note, if the Reject Code is not
denoting a repeating field or set, the Reject Field Occurrence
Indicator must not be sent.
547-5F APPROVED MESSAGE CODE COUNT N Service Reversal:
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Service Reversal:
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Service Reversal:
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q**R*** Service Reversal:
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Service Reversal:

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 222 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q**R*** Service Reversal:
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Service Reversal:
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Service Reversal:
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Service Reversal:
Not used.
993-A7 INTERNAL CONTROL NUMBER N Service Reversal:
Not used.
987-MA URL N Service Reversal:
Not used.
Notes on Response Status Segment on a Service Reversal Response:
The Response Status Segment is mandatory for a Service Reversal response for Header Response Status (5Ø1-F1) = “A” (Accepted) and
Transaction Response Status (112-AN) “R” (Rejected). The Response Status Segment is sent from the sender to the receiver to identify the
outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.
10.7.3.5.4 RESPONSE CLAIM SEGMENT (SERVICE REVERSAL) (TRANSMISSION ACCEPTED/TRANSACTION
REJECTED)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M For Transaction Code of “S2”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “2” (Service Billing).
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT N Service Reversal:
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER N***R*** Service Reversal:
Not used.
553-AR PREFERRED PRODUCT ID N***R*** Service Reversal:
Not used.
554-AS PREFERRED PRODUCT INCENTIVE N***R*** Service Reversal:
Not used.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE N***R*** Service Reversal:
Not used.
556-AU PREFERRED PRODUCT DESCRIPTION N***R*** Service Reversal:
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Service Reversal:
Not used.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 223 -
Notes on Response Claim Segment on a Service Reversal Response:
The Response Claim Segment is mandatory for a Service Reversal response to identify the prescription/service reference number when the
Header Response Status (5Ø1-F1) is “A” (Accepted) and Transaction Response Status (112-AN) “R” (Rejected). Fields defined as Mandatory
are required to be submitted when the segment is sent.
10.7.4 TRANSMISSION REJECTED/TRANSACTION REJECTED
Service Reversal transmission response Header Response Status (5Ø1-F1) of "R" (Rejected)
and Transaction Response Status (112-AN) of “R” (Rejected)
There are no situational transaction-level segments in the Service Reversal transmission response Header Response Status (5Ø1-F1) of "R"
(Rejected) and Transaction Response Status (112-AN) “R” (Rejected).
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
10.7.4.1 DIAGRAM FOR TRANSMISSION OF ONE SERVICE REVERSAL RESPONSE (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
10.7.4.2 DIAGRAM FOR TRANSMISSION OF TWO SERVICE REVERSAL RESPONSES (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
10.7.4.3 DIAGRAM FOR TRANSMISSION OF THREE SERVICE REVERSAL RESPONSES (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Mandatory third response
Group Separator
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 224 -
Segment Separator
Response Status Segment
10.7.4.4 DIAGRAM FOR TRANSMISSION OF FOUR SERVICE REVERSAL RESPONSES (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
10.7.4.5 SERVICE REVERSAL RESPONSE SEGMENTS (TRANSMISSION REJECTED/TRANSACTION
REJECTED)
10.7.4.5.1 RESPONSE HEADER SEGMENT (SERVICE REVERSAL) (TRANSMISSION REJECTED/TRANSACTION
REJECTED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Service Reversal Response:
The Response Header Segment is a mandatory, fixed length segment for Service Reversal response when the Header Response Status
(5Ø1-F1) of "R" (Rejected) and Transaction Response Status (112-AN) of “R” (Rejected). The “Situation” column is not applicable.
If either the entire transmission or the Header is in error, the Header Response Status (5Ø1-F1) = “R” (Rejected). Every identifiable transaction
within the transmission must be rejected with an “R”.
If the transaction rejects for detail errors, the Header Response Status (5Ø1-F1) = “A” (Accepted) and the Transaction Response Status (112-
AN) will be “R”.
10.7.4.5.2 RESPONSE MESSAGE SEGMENT (SERVICE REVERSAL) (TRANSMISSION REJECTED/TRANSACTION
REJECTED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Service Reversal:
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 225 -
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Service Reversal Response:
The Response Message Segment is situational for Service Reversal response when the Header Response Status (5Ø1-F1) of "R" (Rejected)
and Transaction Response Status (112-AN) of “R” (Rejected). It is used when additional text information needs to be sent. Fields defined as
Mandatory are required to be submitted when the segment is sent.
10.7.4.5.3 RESPONSE STATUS SEGMENT (SERVICE REVERSAL) (TRANSMISSION REJECTED/TRANSACTION
REJECTED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Service Reversal:
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT R Service Reversal:
Maximum count of 5.
Required.
511-FB REJECT CODE R**R*** Service Reversal:
Required.
546-4F REJECT FIELD OCCURRENCE INDICATOR Q***R*** Service Reversal:
Required if a repeating field is in error, to identify repeating
field occurrence.
This field must be sent when relaying error information about
a repeating field or set. Note, if the Reject Code is not
denoting a repeating field or set, the Reject Field Occurrence
Indicator must not be sent.
547-5F APPROVED MESSAGE CODE COUNT N Service Reversal:
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Service Reversal:
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Service Reversal:
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q**R*** Service Reversal:
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Service Reversal:
Required if additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 226 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q**R*** Service Reversal:
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Service Reversal:
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Service Reversal:
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Service Reversal:
Not used.
993-A7 INTERNAL CONTROL NUMBER N Service Reversal:
Not used.
987-MA URL N Service Reversal:
Not used.
Notes on Response Status Segment on a Service Reversal Response:
The Response Status Segment is mandatory for a Service Reversal response for Header Response Status (5Ø1-F1) = “R” (Rejected) and
Transaction Response Status (112-AN) of “R” (Rejected). The Response Status Segment is sent from the sender to the receiver to identify the
outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 227 -
11. REBILL INFORMATION
A Rebill transaction is a prescription product or service billing or information submission with an implied reversal of the same Service
Reference Number. A previously captured or adjudicated claim or service or information reporting is reversed and then the new claim, service
or information reporting is processed, using a two-step procedure. Each part of the process works independently of the other. Up to four
transactions must occur within one transmission.
The three types of rebill transactions are:
• Claim Rebill (B3)
• Service Rebill (S3)
• Information Reporting Rebill (N3)
• Controlled Substance Reporting Rebill (C3)
First, the previously captured or adjudicated transaction is reversed. If the reversal cannot be processed, reject code “87 “ (Reversal not
processed) must be returned in the reject response, as well as reject code “85 “ (Claim not processed).
If the reversal is processed successfully, the second step is to process the “New” transaction. If the “New” transaction processes successfully,
a “Paid” (B3, S3, N3), “Captured” (B3, S3, C3, N3) or “Approved” (N3, C3) response must be returned. If the “New” transaction is rejected,
appropriate reject codes must be returned.
Duplicate response logic must not be applied by the processor to a Rebill transaction. There is no need for a duplicate response due to the
nature of the rebill transaction and its implied reversal. Because the implied reversal would reverse the paid claim, a duplicate transaction
would not exist.
If a processor supported duplicate responses in rebills the submitter would not be able to modify a field that is not included in the duplicate
field check. See sections “Response Processing Guidelines”, “Duplicate Transactions” and “Duplicate Processing For All Rebill Transactions”
for more information.
11.1 CLAIM OR SERVICE REBILL
This transaction is a claim or service submission with an implied reversal. It is used by the Originator to cancel a claim or service submitted
that had been processed previously, and submit a new claim or service in the same transaction.
For claim or service reversal guidelines, see section “Reversal Information”, Claim or Service Reversal”. The Transaction Code is “B2” (Claim
Reversal) or “S2” (Service Reversal).
For Medicare Part D processing only one transaction per transmission is permitted because there is a need for the sequencing of the True Out
Of Pocket (TrOOP) update before the next claim is processed. The TrOOP should be updated before subsequent claims are processed.
Depending upon the particular claim or service rebill request, the Processor must provide one of the following general types of responses:
Captured - This occurs when the Processor acknowledges receipt of the claim or service rebill, but is not making any judgment
regarding eligibility of the patient, reversal of the claim or service, or payment for the claim or service.
Paid - This occurs when the Processor processes the reversal, then processes the claim or service, and returns to the Originator the
dollar amounts allowed under the terms of the plan. The Paid response is not used in payer-to-payer transactions.
Rejected - This occurs when the Processor has encountered an error in the transaction or processing.
Please see section “Response Processing Guidelines”, “Duplicate Transactions” and “Duplicate Processing For All Rebill Transactions” for
more information about why duplicate responses are not supported in Rebill transactions.
11.2 CLAIM REBILL REQUEST DIAGRAMS
11.2.1 DIAGRAM FOR TRANSMISSION OF ONE CLAIM REBILL TRANSACTION
For a Claim Rebill, the scenarios defined include
Claim Rebill from a Sender to a Receiver
Claim Rebill Paid/Captured/Rejected Transaction Response from a Sender to a Receiver
Standard Transmission Reject Response to a Claim Rebill from a Sender to a Receiver
Claim Rebill transactions use the same diagrams as the Billing transactions. Up to four (4) rebill transactions are allowed in one transmission.
Mandatory
Transaction Header Segment
Segment Separator
Insurance Segment
Situational
Segment Separator

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 228 -
Patient Segment
Mandatory - first Claim Rebill transaction
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Coupon Segment
Segment Separator
Compound Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
11.2.2 DIAGRAM FOR TRANSMISSION OF TWO CLAIM REBILL TRANSACTIONS
Mandatory
Transaction Header Segment
Segment Separator
Insurance Segment
Situational
Segment Separator
Patient Segment
Mandatory - first Claim Rebill transaction
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Coupon Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 229 -
Segment Separator
Narrative Segment
Mandatory - second Claim Rebill transaction
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Coupon Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
11.2.3 DIAGRAM FOR TRANSMISSION OF THREE CLAIM REBILL TRANSACTIONS
Mandatory
Transaction Header Segment
Segment Separator
Insurance Segment
Situational
Segment Separator
Patient Segment
Mandatory - first Claim Rebill transaction
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Coupon Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 230 -
Mandatory - second Claim Rebill transaction
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Coupon Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
Mandatory - third Claim Rebill transaction
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Coupon Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
11.2.4 DIAGRAM FOR TRANSMISSION OF FOUR CLAIM REBILL TRANSACTIONS
Mandatory
Transaction Header Segment
Segment Separator
Insurance Segment
Situational
Segment Separator
Patient Segment
Mandatory - first Claim Rebill transaction
Group Separator

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 231 -
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Coupon Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
Mandatory - second Claim Rebill transaction
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Coupon Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
Mandatory - third Claim Rebill transaction
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 232 -
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Coupon Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
Mandatory - fourth Claim Rebill transaction
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Coupon Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
11.3 CLAIM REBILL REQUEST SEGMENTS
11.3.1 TRANSACTION HEADER SEGMENT (CLAIM REBILL)
TRANSACTION HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø1-A1 BIN NUMBER M
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M For Transaction Code of “B3”, in the Claim Segment, the
Prescription/Service Reference Number Qualifier (455-EM)
is “1” (Rx Billing).
1Ø4-A4 PROCESSOR CONTROL NUMBER M
1Ø9-A9 TRANSACTION COUNT M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
If the Date of Service contains the subsequent payer
coverage date, the Submission Clarification Code (42Ø-DK)
is required with value of “19” (Split Billing – indicates the
quantity dispensed is the remainder billed to a subsequent
payer when Medicare Part A expires. Used only in long-
term care settings) for individual unit of use medications.
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M
Notes on Transaction Header Segment on a Claim Rebill Request:
The Transaction Header Segment is a mandatory, fixed length segment for a Claim Rebill request. The “Situation” column is not applicable.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 233 -
11.3.2 INSURANCE SEGMENT (CLAIM REBILL)
INSURANCE SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø2-C2 CARDHOLDER ID M
312-CC CARDHOLDER FIRST NAME
Q Claim Rebill:
Required if necessary for state/federal/regulatory agency
programs when the cardholder has a first name.
313-CD CARDHOLDER LAST NAME
Q Claim Rebill:
Required if necessary for state/federal/regulatory agency
programs.
314-CE HOME PLAN
Q Claim Rebill:
Required if needed for receiver billing/encounter validation
and/or determination for Blue Cross or Blue Shield, if a
Patient has coverage under more than one plan, to
distinguish each plan.
524-FO PLAN ID
O Claim Rebill:
Optional.
3Ø9-C9 ELIGIBILITY CLARIFICATION CODE
Q Claim Rebill:
Required if needed for receiver inquiry validation and/or
determination, when eligibility is not maintained at the
dependent level. Required in special situations as defined
by the code to clarify the eligibility of an individual, which
may extend coverage.
3Ø1-C1 GROUP ID
Q Claim Rebill:
Required if necessary for state/federal/regulatory agency
programs.
Required if needed for pharmacy claim processing and
payment.
Required if needed to match the reversal to the original
billing transaction.
3Ø3-C3 PERSON CODE
Q Claim Rebill:
Required if needed to uniquely identify the family members
within the Cardholder ID.
3Ø6-C6 PATIENT RELATIONSHIP CODE
Q Claim Rebill:
Required if needed to uniquely identify the relationship of
the Patient to the Cardholder.
99Ø-MG OTHER PAYER BIN NUMBER N Claim Rebill:
Not used.
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER N Claim Rebill:
Not used.
356-NU OTHER PAYER CARDHOLDER ID N Claim Rebill:
Not used.
992-MJ OTHER PAYER GROUP ID N Claim Rebill:
Not used.
359-2A MEDIGAP ID Q Claim Rebill:
Required, if known, when patient has Medigap coverage.
36Ø-2B MEDICAID INDICATOR Q Claim Rebill:
Required, if known, when patient has Medicaid coverage.
361-2D PROVIDER ACCEPT ASSIGNMENT INDICATOR Q Claim Rebill:
Required if necessary for state/federal/regulatory agency
programs.
997-G2 CMS PART D DEFINED QUALIFIED FACILITY Q Claim Rebill:
Required if specified in trading partner agreement.
115-N5
MEDICAID ID NUMBER Q Claim Rebill:
Required, if known, when patient has Medicaid coverage.
Required when used for payer-to-payer coordination of
benefits to track the claim without regard to the “Service
Provider ID, Prescription Number, & Date of Service”.
116-N6
MEDICAID AGENCY NUMBER N Claim Rebill:
Not used.
Notes on Insurance Segment on a Claim Rebill Request:
The Insurance Segment is mandatory for a Claim Rebill request. Fields defined as Mandatory are required to be submitted when the segment
is sent.
11.3.2.1 INSURANCE SEGMENT (MEDICAID SUBROGATION CLAIM REBILL)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 234 -
INSURANCE SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
115-N5
MEDICAID ID NUMBER QM Medicaid Subrogation - Claim Rebill:
Required to identify the member as uniquely known to
Medicaid.
116-N6
MEDICAID AGENCY NUMBER QM Medicaid Subrogation - Claim Rebill:
Required to identify the Medicaid agency.
See Medicaid Subrogation Implementation Guide.
Notes on Insurance Segment on a Medicaid Subrogation Claim Rebill Request:
The rules above for an “Insurance Segment (Claim Rebill)” are to be followed for Medicaid Subrogation. Specific fields that are used
differently in Medicaid Subrogation are noted in the table above.
11.3.3 PATIENT SEGMENT (CLAIM REBILL)
PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
331-CX PATIENT ID QUALIFIER
Q Claim Rebill:
Required if Patient ID (332-CY) is used.
332-CY PATIENT ID
Q Claim Rebill:
Required if necessary for state/federal/regulatory agency
programs to validate dual eligibility.
3Ø4-C4 DATE OF BIRTH
R Claim Rebill:
Required.
3Ø5-C5 PATIENT GENDER CODE
R Claim Rebill:
Required.
31Ø-CA PATIENT FIRST NAME
Q Claim Rebill:
Required when the patient has a first name.
311-CB PATIENT LAST NAME R Claim Rebill:
Required.
322-CM PATIENT STREET ADDRESS
O Claim Rebill:
Optional.
323-CN PATIENT CITY ADDRESS
O Claim Rebill:
Optional.
324-CO PATIENT STATE / PROVINCE ADDRESS
O Claim Rebill:
Optional.
325-CP PATIENT ZIP/POSTAL ZONE
O Claim Rebill:
Optional.
326-CQ PATIENT PHONE NUMBER
O Claim Rebill:
Optional.
3Ø7-C7 PLACE OF SERVICE
Q Claim Rebill:
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
333-CZ EMPLOYER ID
Q Claim Rebill:
Required if “required by law” as defined in the HIPAA final
Privacy regulations section 164.5Ø1 definitions (45 CFR
Parts 160 and 164 Standards for Privacy of Individually
Identifiable Health Information; Final Rule -
Thursday, December 28, 2000, page 82803 and following,
and Wednesday, August 14, 2002, page 53267 and
following.)
Required if needed for Workers’ Compensation billing.
334-1C SMOKER / NON-SMOKER CODE
N Claim Rebill:
Not used.
335-2C PREGNANCY INDICATOR
Q Claim Rebill:
Required if pregnancy could result in different coverage,
pricing, or patient financial responsibility.
Required if “required by law” as defined in the HIPAA final
Privacy regulations section 164.5Ø1 definitions (45 CFR
Parts 160 and 164 Standards for Privacy of Individually
Identifiable Health Information; Final Rule -
Thursday, December 28, 2000, page 82803 and following,
and Wednesday, August 14, 2002, page 53267 and
following.)
35Ø-HN PATIENT E-MAIL ADDRESS I Claim Rebill:
May be submitted for the receiver to relay patient health
care communications via the Internet when provided by the
patient.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 235 -
PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
This field is informational only.
384-4X PATIENT RESIDENCE Q Claim Rebill:
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
Notes on Patient Segment on a Claim Rebill Request:
The Patient Segment is situational for a Claim Rebill request. It is used when a receiver needs some of the patient demographic information to
perform eligibility and claim/encounter determination. The Patient Segment must be submitted when needed to differentiate between the
patient and the cardholder. If the cardholder and the patient are the same, then the Patient Segment is not submitted unless additional
information about the patient is needed to clarify the claim/encounter determination. The Segment is mandatory if required under provider
payer contract or mandatory on claims where this information is necessary for adjudication of the claim. Fields defined as Mandatory are
required to be submitted when the segment is sent.
11.3.3.1 PATIENT SEGMENT (MEDICAID SUBROGATION CLAIM REBILL)
PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
31Ø-CA PATIENT FIRST NAME
QM Medicaid Subrogation - Claim Rebill:
Required to assist in identifying the cardholder when
specific eligibility cannot be established.
See Medicaid Subrogation Implementation Guide.
322-CM PATIENT STREET ADDRESS
QM Medicaid Subrogation - Claim Rebill:
Required to assist in identifying the cardholder when
specific eligibility cannot be established.
See Medicaid Subrogation Implementation Guide.
323-CN PATIENT CITY ADDRESS
QM Medicaid Subrogation - Claim Rebill:
Required to assist in identifying the cardholder when
specific eligibility cannot be established.
See Medicaid Subrogation Implementation Guide.
324-CO PATIENT STATE / PROVINCE ADDRESS
QM Medicaid Subrogation - Claim Rebill:
Required to assist in identifying the cardholder when
specific eligibility cannot be established.
See Medicaid Subrogation Implementation Guide.
325-CP PATIENT ZIP/POSTAL ZONE
QM Medicaid Subrogation - Claim Rebill:
Required to assist in identifying the cardholder when
specific eligibility cannot be established.
See Medicaid Subrogation Implementation Guide.
Notes on Patient Segment on a Medicaid Subrogation Claim Rebill Request:
The rules above for a “Patient Segment (Claim Rebill)” are to be followed for Medicaid Subrogation. Specific fields that are used
differently in Medicaid Subrogation are noted in the table above.
11.3.4 CLAIM SEGMENT (CLAIM REBILL)
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M For Transaction Code of “B3”, in the Claim Segment, the
Prescription/Service Reference Number Qualifier (455-EM)
is “1” (Rx Billing).
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
436-E1 PRODUCT/SERVICE ID QUALIFIER M Mandatory.
If billing for a multi-ingredient prescription, Product/Service
ID Qualifier (436-E1) is zero (“ØØ”).
4Ø7-D7 PRODUCT/SERVICE ID M Mandatory.
If billing for a multi-ingredient prescription, Product/Service
ID (4Ø7-D7) is zero. (Zero means “Ø”.)
456-EN ASSOCIATED PRESCRIPTION/SERVICE REFERENCE NUMBER Q Claim Rebill:
Required if the “completion” transaction in a partial fill
(Dispensing Status (343-HD) = “C” (Completed)).
See section “Specific Segment Discussion”, “Request
Segments”, Claim Segment” for more information.
Required if the Dispensing Status (343-HD) = “P” (Partial
Fill) and there are multiple occurrences of partial fills for this
prescription.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 236 -
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
457-EP ASSOCIATED PRESCRIPTION/SERVICE DATE Q Claim Rebill:
Required if the “completion” transaction in a partial fill
(Dispensing Status (343-HD) = “C” (Completed)).
Required if Associated Prescription/Service Reference
Number (456-EN) is used.
See section “Specific Segment Discussion”, “Request
Segments”, Claim Segment” for more information.
Required if the Dispensing Status (343-HD) = “P” (Partial
Fill) and there are multiple occurrences of partial fills for this
prescription.
458-SE PROCEDURE MODIFIER CODE COUNT Q Claim Rebill:
Maximum count of 1Ø.
Required if Procedure Modifier Code (459-ER) is used.
459-ER PROCEDURE MODIFIER CODE Q***R*** Claim Rebill:
Required to define a further level of specificity if the
Product/Service ID (4Ø7-D7) indicated a Procedure Code
was submitted.
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
Occurs the number of times identified in Procedure Modifier
Code Count (458-SE).
442-E7 QUANTITY DISPENSED R Claim Rebill:
Required.
4Ø3-D3 FILL NUMBER R Claim Rebill:
Required. This field must match the Fill Number of the
original billing.
4Ø5-D5 DAYS SUPPLY R Claim Rebill:
Required.
4Ø6-D6 COMPOUND CODE R Claim Rebill:
Required.
4Ø8-D8 DISPENSE AS WRITTEN (DAW)/PRODUCT SELECTION CODE R Claim Rebill:
Required.
414-DE DATE PRESCRIPTION WRITTEN R Claim Rebill:
Required.
415-DF NUMBER OF REFILLS AUTHORIZED Q Claim Rebill:
Required if necessary for plan benefit administration.
419-DJ PRESCRIPTION ORIGIN CODE Q Claim Rebill:
Required if necessary for plan benefit administration.
354-NX SUBMISSION CLARIFICATION CODE COUNT Q Claim Rebill:
Maximum count of 3.
Required if Submission Clarification Code (42Ø-DK) is
used.
42Ø-DK SUBMISSION CLARIFICATION CODE Q***R*** Claim Rebill:
Required if clarification is needed and value submitted is
greater than zero (Ø).
Occurs the number of times identified in Submission
Clarification Code Count (354-NX).
If the Date of Service (4Ø1-D1) contains the subsequent
payer coverage date, the Submission Clarification Code
(42Ø-DK) is required with value of “19” (Split Billing –
indicates the quantity dispensed is the remainder billed to a
subsequent payer when Medicare Part A expires. Used
only in long-term care settings) for individual unit of use
medications.
46∅-ET QUANTITY PRESCRIBED N Claim Rebill:
Not used.
3Ø8-C8 OTHER COVERAGE CODE Q Claim Rebill:
Required if needed by receiver to match the claim that is
being reversed.
Required if needed by receiver, to communicate a
summation of other coverage information that has been
collected from other payers.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 237 -
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Required for Coordination of Benefits.
See section “Specific Segment Discussion”, “Request
Segments”, “Claim Segment”, “Other Coverage Code (3Ø8-
C8).
429-DT SPECIAL PACKAGING INDICATOR Q Claim Rebill:
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
453-EJ ORIGINALLY PRESCRIBED PRODUCT/SERVICE ID QUALIFIER Q Claim Rebill:
Required if Originally Prescribed Product/Service Code
(455-EA) is used.
445-EA ORIGINALLY PRESCRIBED PRODUCT/SERVICE CODE Q Claim Rebill:
Required if the receiver requests association to a
therapeutic, or a preferred product substitution, or when a
DUR alert has been resolved by changing medications, or
an alternative service than what was originally prescribed.
446-EB ORIGINALLY PRESCRIBED QUANTITY Q Claim Rebill:
Required if the receiver requests reporting for quantity
changes due to a therapeutic substitution that has occurred
or a preferred product/service substitution that has
occurred, or when a DUR alert has been resolved by
changing quantities.
33Ø-CW ALTERNATE ID N Claim Rebill:
Not used.
454-EK SCHEDULED PRESCRIPTION ID NUMBER N Claim Rebill:
Not used.
6ØØ-28 UNIT OF MEASURE Q Claim Rebill:
Required if necessary for state/federal/regulatory agency
programs.
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
418-DI LEVEL OF SERVICE Q Claim Rebill:
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
461-EU PRIOR AUTHORIZATION TYPE CODE Q Claim Rebill:
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
462-EV PRIOR AUTHORIZATION NUMBER SUBMITTED Q Claim Rebill:
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
463-EW INTERMEDIARY AUTHORIZATION TYPE ID Q Claim Rebill:
Required for overriding an authorized intermediary system
edit when the pharmacy participates with an intermediary.
Required if Intermediary Authorization ID (464-EX) is used.
Not used for payer-to-payer transactions.
464-EX INTERMEDIARY AUTHORIZATION ID Q Claim Rebill:
Required for overriding an authorized intermediary system
edit when the pharmacy participates with an intermediary.
Not used for payer-to-payer transactions.
343-HD DISPENSING STATUS Q Claim Rebill:
Required for the partial fill or the completion fill of a
prescription.
344-HF QUANTITY INTENDED TO BE DISPENSED Q Claim Rebill:
Required for the partial fill or the completion fill of a
prescription.
345-HG DAYS SUPPLY INTENDED TO BE DISPENSED Q Claim Rebill:
Required for the partial fill or the completion fill of a
prescription.
357-NV DELAY REASON CODE Q Claim Rebill:
Required when needed to specify the reason that
submission of the transaction has been delayed.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Claim Rebill:
Not used.
391-MT PATIENT ASSIGNMENT INDICATOR (DIRECT MEMBER
REIMBURSEMENT INDICATOR)
Q Claim Rebill:
Required when the claims adjudicator does not assume the
patient assigned his/her benefits to the provider or when
the claims adjudicator supports a patient determination of
whether he/she wants to assign or retain his/her benefits.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 238 -
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
995-E2 ROUTE OF ADMINISTRATION Q Claim Rebill:
Required if specified in trading partner agreement.
996-G1 COMPOUND TYPE Q Claim Rebill:
Required if specified in trading partner agreement.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Claim Rebill:
Not used.
147-U7 PHARMACY SERVICE TYPE Q Claim Rebill:
Required when the submitter must clarify the type of
services being performed as a condition for proper
reimbursement by the payer.
Notes on Claim Segment on a Claim Rebill Request:
The Claim Segment is mandatory for a Claim Rebill Request. The Claim Segment defines the product dispensed, dispensing information,
reference information for tieback to an original prescription in the case of partial fillings, or authorization information. Fields defined as
Mandatory are required to be submitted when the segment is sent.
11.3.4.1 CLAIM SEGMENT (MEDICAID SUBROGATION CLAIM REBILL)
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
RM Medicaid Subrogation - Claim Rebill:
Required. Contains the Medicaid unique claim
identification number (also referred to as the ICN or
TCN).
See Medicaid Subrogation Implementation Guide.
Notes on Claim Segment on a Medicaid Subrogation Claim Rebill Request:
The rules above for a “Claim Segment (Claim Rebill)” are to be followed for Medicaid Subrogation. Specific fields that are used
differently in Medicaid Subrogation are noted in the table above.
11.3.5 PRICING SEGMENT (CLAIM REBILL)
PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
4Ø9-D9 INGREDIENT COST SUBMITTED R Claim Rebill:
Required.
412-DC DISPENSING FEE SUBMITTED Q Claim Rebill:
Required if its value has an effect on the Gross Amount
Due (43Ø-DU) calculation.
Zero (Ø) is a valid value.
477-BE PROFESSIONAL SERVICE FEE SUBMITTED N Claim Rebill:
Not used.
433-DX PATIENT PAID AMOUNT SUBMITTED Q Claim Rebill:
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
Not used in coordination of benefit claim to pass patient
liability information to a downstream payer. See section
“Standard Conventions”, “Repetition and Multiple
Occurrences”, Repeating Data Elements”, “Request
Segments”, “Coordination of Benefits/Other Payments
Segment”.
438-E3 INCENTIVE AMOUNT SUBMITTED Q Claim Rebill:
Required if its value has an effect on the Gross Amount
Due (43Ø-DU) calculation.
Zero (Ø) is a valid value.
478-H7 OTHER AMOUNT CLAIMED SUBMITTED COUNT Q Claim Rebill:
Maximum count of 3.
Required if Other Amount Claimed Submitted Qualifier
(479-H8) is used.
479-H8 OTHER AMOUNT CLAIMED SUBMITTED QUALIFIER Q***R*** Claim Rebill:
Required if Other Amount Claimed Submitted (48Ø-H9) is
used.
48Ø-H9 OTHER AMOUNT CLAIMED SUBMITTED Q***R*** Claim Rebill:
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 239 -
PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if its value has an effect on the Gross Amount
Due (43Ø-DU) calculation.
Zero (Ø) is a valid value.
481-HA FLAT SALES TAX AMOUNT SUBMITTED Q Claim Rebill:
Required if its value has an effect on the Gross Amount
Due (43Ø-DU) calculation.
Zero (Ø) is a valid value.
482-GE PERCENTAGE SALES TAX AMOUNT SUBMITTED Q Claim Rebill:
Required if its value has an effect on the Gross Amount
Due (43Ø-DU) calculation.
Zero (Ø) is a valid value.
483-HE PERCENTAGE SALES TAX RATE SUBMITTED Q Claim Rebill:
Required if Percentage Sales Tax Amount Submitted (482-
GE) and Percentage Sales Tax Basis Submitted (484-JE)
are used.
Required if this field could result in different pricing.
Required if needed to calculate Percentage Sales Tax
Amount Paid (559-AX).
484-JE PERCENTAGE SALES TAX BASIS SUBMITTED Q Claim Rebill:
Required if Percentage Sales Tax Amount Submitted (482-
GE) and Percentage Sales Tax Rate Submitted (483-HE)
are used.
Required if this field could result in different pricing.
Required if needed to calculate Percentage Sales Tax
Amount Paid (559-AX).
426-DQ USUAL AND CUSTOMARY CHARGE Q Claim Rebill:
Required if needed per trading partner agreement.
43Ø-DU GROSS AMOUNT DUE R Claim Rebill:
Required.
See Pricing Formula for fields used in calculation.
423-DN BASIS OF COST DETERMINATION Q Claim Rebill:
Required if needed for receiver claim/encounter
adjudication.
113-N3
MEDICAID PAID AMOUNT N Claim Rebill:
Not used.
Notes on Pricing Segment on a Claim Rebill Request:
The Pricing Segment is mandatory for a Claim Rebill Request. The Pricing Segment defines dollar amounts and basis of costs for a Claim
Billing, Claim Rebill, or Encounter.
It is highly recommended that whenever possible, the individual dollar fields are requested of the sender by the receiver. On the response, the
sender should return the individual payment response fields to allow the receiver to reconcile against the requested payment fields. It is
recommended that for the dollar fields, if the field is not required or situational in the calculation, that the dollar fields are not sent.
See section “Response Processing Guidelines”, “Pricing Guidelines”.
Fields defined as Mandatory are required to be submitted when the segment is sent.
11.3.5.1 PRICING SEGMENT (MEDICAID SUBROGATION CLAIM REBILL)
PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
113-N3
MEDICAID PAID AMOUNT QM Medicaid Subrogation - Claim Rebill:
Required if affects pricing in Medicaid Subrogation.
(contains the amount paid to the pharmacy). See
Medicaid Subrogation Implementation Guide.
Notes on Pricing Segment on a Medicaid Subrogation Claim Rebill Request:
The rules above for a “Pricing Segment (Claim Rebill)” are to be followed for Medicaid Subrogation. Specific fields that are used
differently in Medicaid Subrogation are noted in the table above.
11.3.6 PHARMACY PROVIDER SEGMENT (CLAIM REBILL)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 240 -
PHARMACY PROVIDER SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
465-EY PROVIDER ID QUALIFIER Q Claim Rebill:
Required if Provider ID (444-E9) is used.
444-E9 PROVIDER ID
Q Claim Rebill:
Required if necessary for state/federal/regulatory agency
programs.
Required if necessary to identify the individual responsible
for dispensing of the prescription.
Required if needed for reconciliation of encounter-reported
data or encounter reporting.
Notes on Pharmacy Provider Segment on a Claim Rebill Request:
The Pharmacy Provider Segment is situational for a Claim Rebill request. It is used when a receiver needs pharmacy provider information to
perform claim/encounter determination. Fields defined as Mandatory are required to be submitted when the segment is sent.
11.3.7 PRESCRIBER SEGMENT (CLAIM REBILL)
PRESCRIBER SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
466-EZ PRESCRIBER ID QUALIFIER Q Claim Rebill:
Required if Prescriber ID (411-DB) is used.
411-DB PRESCRIBER ID Q Claim Rebill:
Required if this field could result in different coverage or
patient financial responsibility.
Required if necessary for state/federal/regulatory agency
programs.
427-DR PRESCRIBER LAST NAME Q Claim Rebill:
Required when the Prescriber ID (411-DB) is not known.
Required if needed for Prescriber ID (411-DB)
validation/clarification.
498-PM PRESCRIBER PHONE NUMBER Q Claim Rebill:
Required if needed for Workers’ Compensation.
Required if needed to assist in identifying the prescriber.
Required if needed for Prior Authorization process.
468-2E PRIMARY CARE PROVIDER ID QUALIFIER Q Claim Rebill:
Required if Primary Care Provider ID (421-DL) is used.
421-DL PRIMARY CARE PROVIDER ID Q Claim Rebill:
Required if needed for receiver claim/encounter
determination, if known and available.
Required if this field could result in different coverage or
patient financial responsibility.
Required if necessary for state/federal/regulatory agency
programs.
47Ø-4E PRIMARY CARE PROVIDER LAST NAME Q Claim Rebill:
Required if this field is used as an alternative for Primary
Care Provider ID (421-DL) when ID is not known.
Required if needed for Primary Care Provider ID (421-DL)
validation/clarification.
364-2J PRESCRIBER FIRST NAME Q Claim Rebill:
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
365-2K PRESCRIBER STREET ADDRESS Q Claim Rebill:
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
366-2M PRESCRIBER CITY ADDRESS Q Claim Rebill:
Required if needed to assist in identifying the prescriber.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 241 -
PRESCRIBER SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if necessary for state/federal/regulatory agency
programs.
367-2N PRESCRIBER STATE/PROVINCE ADDRESS Q Claim Rebill:
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
368-2P PRESCRIBER ZIP/POSTAL ZONE Q Claim Rebill:
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
Notes on Prescriber Segment on a Claim Rebill Request:
The Prescriber Segment is situational for a Claim Rebill request. It is used when prescriber information is needed to perform claim/encounter
determination. The Segment is mandatory if required under provider payer contract or mandatory on claims where this information is
necessary for adjudication of the claim. Fields defined as Mandatory are required to be submitted when the segment is sent.
11.3.8 COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT (CLAIM REBILL)
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
337-4C COORDINATION OF BENEFITS/OTHER PAYMENTS COUNT M Maximum count of 9.
338-5C OTHER PAYER COVERAGE TYPE M***R*** Mandatory.
Occurs with Coordination of Benefits/Other Payments
Count (337-4C).
Grouped with Other Payer ID Qualifier (339-6C), Other
Payer ID (34Ø-7C), Other Payer Date (443-E8), and either
Other Payer Amount Paid Count (341-HB) and its grouping,
or Other Payer Reject Count (471-5E) and its grouping.
339-6C OTHER PAYER ID QUALIFIER Q***R*** Claim Rebill:
Required if Other Payer ID (34Ø-7C) is used.
34Ø-7C OTHER PAYER ID Q***R*** Claim Rebill:
Required if identification of the Other Payer is necessary for
claim/encounter adjudication.
993-A7 INTERNAL CONTROL NUMBER Q***R*** Claim Rebill:
Required when used for payer-to-payer coordination of
benefits to track the claim without regard to the “Service
Provider ID, Prescription Number, & Date of Service”.
443-E8 OTHER PAYER DATE Q***R*** Claim Rebill:
Required if identification of the Other Payer Date is
necessary for claim/encounter adjudication.
341-HB OTHER PAYER AMOUNT PAID COUNT Q Claim Rebill:
Maximum count of 9.
Required if Other Payer Amount Paid Qualifier (342-HC) is
used.
342-HC OTHER PAYER AMOUNT PAID QUALIFIER Q***R*** Claim Rebill:
Required if Other Payer Amount Paid (431-DV) is used.
431-DV OTHER PAYER AMOUNT PAID Q***R*** Claim Rebill:
Required if other payer has approved payment for some/all
of the billing.
Zero (Ø) is a valid value.
Not used for patient financial responsibility only billing.
Not used for non-governmental agency programs if Other
Payer-Patient Responsibility Amount (352-NQ) is
submitted.
471-5E OTHER PAYER REJECT COUNT Q Claim Rebill:
Maximum count of 5.
Required if Other Payer Reject Code (472-6E) is used.
472-6E OTHER PAYER REJECT CODE
Q***R*** Claim Rebill:
Required when the other payer has denied the payment for
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 242 -
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
the billing, designated with Other Coverage Code (3Ø8-C8)
= 3 (Other Coverage Billed – claim not covered).
Note: This field must only contain the NCPDP Reject Code
(511-FB) values.
353-NR OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT COUNT Q Claim Rebill:
Maximum count of 25.
Required if Other Payer-Patient Responsibility Amount
Qualifier (351-NP) is used.
Note the occurrences are dependent upon the number of
component parts returned from a previous payer.
351-NP OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT QUALIFIER Q***R*** Claim Rebill:
Required if Other Payer-Patient Responsibility Amount
(352-NQ) is used.
352-NQ OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT Q**R*** Claim Rebill:
Required if necessary for patient financial responsibility
only billing.
Required if necessary for state/federal/regulatory agency
programs.
Not used for non-governmental agency programs if Other
Payer Amount Paid (431-DV) is submitted.
392-MU BENEFIT STAGE COUNT Q Claim Rebill:
Maximum count of 4.
Required if Benefit Stage Amount (394-MW) is used.
393-MV BENEFIT STAGE QUALIFIER Q***R*** Claim Rebill:
Required if Benefit Stage Amount (394-MW) is used.
Must only have one value per iteration - value must not be
repeated.
394-MW BENEFIT STAGE AMOUNT Q***R*** Claim Rebill:
Required if the previous payer has financial amounts that
apply to Medicare Part D beneficiary benefit stages. This
field is required when the plan is a participant in a Medicare
Part D program that requires reporting of benefit stage
specific financial amounts.
Required if necessary for state/federal/regulatory agency
programs.
Notes on Coordination of Benefits/Other Payments Segment on a Claim Rebill Request:
The Coordination of Benefits/Other Payments Segment is situational for a Claim Rebill request. It is used when a receiver needs payment
information from other receivers to perform claim/encounter determination. This may be in the case of primary, secondary, tertiary et cetera
health plan coverage for example.
The Coordination of Benefits/Other Payments Segment is mandatory for a Claim Rebill request to a downstream payer. It is used to assist a
downstream payer to uniquely identify a Claim Rebill in case of duplicate processing. Sometimes processors have difficulty determining
duplicate logic because the same processor is involved in multiple coordination of benefit occurrences for the same patient. They are involved
for example as the primary and secondary payer, or primary and tertiary, or secondary and tertiary. The downstream payer uses the fields
involved in duplicate logic, including the Other Payer Coverage Type (338-5C) to differentiate which Claim Rebill to process. See section
“Response Processing Guidelines”, “Duplicate Transactions”.
Note, the Other Payer Coverage Type (338-5C) occurrences do not have to appear in sequential order (primary, secondary, tertiary),
but can appear in any order.
The Coordination of Benefits/Other Payments Segment is not used for a Claim Billing or Encounter request to a primary payer.
A coupon is used to reduce the patient out of pocket prescription cost – by either reducing the cost of a CASH prescription or the patient
financial responsibility from a Third Party payer who allows coupon usage. The coupon processor is the LAST payer. (Note: Some Federal
and State programs do not allow the reduction of patient’s financial responsibility.)
The Segment is mandatory if required under provider payer contract or mandatory on claims where this information is necessary for
adjudication of the claim.
Fields defined as Mandatory are required to be submitted when the segment is sent.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 243 -
11.3.9 WORKERS’ COMPENSATION SEGMENT (CLAIM REBILL)
WORKERS’ COMPENSATION SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
434-DY DATE OF INJURY M
315-CF EMPLOYER NAME Q Claim Rebill:
Required if needed to process a claim/encounter for a work
related injury or condition.
316-CG EMPLOYER STREET ADDRESS Q Claim Rebill:
Required if needed to process a claim/encounter for a work
related injury or condition.
317-CH EMPLOYER CITY ADDRESS Q Claim Rebill:
Required if needed to process a claim/encounter for a work
related injury or condition.
318-CI EMPLOYER STATE/PROVINCE ADDRESS Q Claim Rebill:
Required if needed to process a claim/encounter for a work
related injury or condition.
319-CJ EMPLOYER ZIP/POSTAL ZONE Q Claim Rebill:
Required if needed to process a claim/encounter for a work
related injury or condition.
32Ø-CK EMPLOYER PHONE NUMBER Q Claim Rebill:
Required if needed to process a claim/encounter for a work
related injury or condition.
321-CL EMPLOYER CONTACT NAME Q Claim Rebill:
Required if needed to process a claim/encounter for a work
related injury or condition.
327-CR CARRIER ID Q Claim Rebill:
Required if needed to process a claim/encounter for a work
related injury or condition.
435-DZ CLAIM/REFERENCE ID Q Claim Rebill:
Required if needed to process a claim/encounter for a work
related injury or condition.
117-TR BILLING ENTITY TYPE INDICATOR R Claim Rebill:
Required.
118-TS PAY TO QUALIFIER Q Claim Rebill:
Required if Pay To ID (119-TT) is used.
119-TT PAY TO ID Q Claim Rebill:
Required if transaction is submitted by a provider or agent,
but paid to another party.
12Ø-TU PAY TO NAME Q Claim Rebill:
Required if transaction is submitted by a provider or agent,
but paid to another party.
121-TV PAY TO STREET ADDRESS Q Claim Rebill:
Required if transaction is submitted by a provider or agent,
but paid to another party.
122-TW PAY TO CITY ADDRESS Q Claim Rebill:
Required if transaction is submitted by a provider or agent,
but paid to another party.
123-TX PAY TO STATE/PROVINCE ADDRESS Q Claim Rebill:
Required if transaction is submitted by a provider or agent,
but paid to another party.
124-TY PAY TO ZIP/POSTAL ZONE Q Claim Rebill:
Required if transaction is submitted by a provider or agent,
but paid to another party.
125-TZ GENERIC EQUIVALENT PRODUCT ID QUALIFIER Q Claim Rebill:
Required if Generic Equivalent Product ID (126-UA) is
used.
126-UA GENERIC EQUIVALENT PRODUCT ID Q Claim Rebill:
Required if necessary for state/federal/regulatory agency
programs.
Notes on Workers’ Compensation Segment on a Claim Rebill Request:
The Workers’ Compensation Segment is situational for a Claim Rebill request. It is used when processing a Claim Billing, Claim Rebill, or
Encounter for a work-related injury or condition. Fields defined as Mandatory are required to be submitted when the segment is sent.
11.3.10 DUR/PPS SEGMENT (CLAIM REBILL)
DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 244 -
DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
473-7E DUR/PPS CODE COUNTER Q***R*** Claim Rebill:
Maximum of 9 occurrences.
Required if DUR/PPS Segment is used.
439-E4 REASON FOR SERVICE CODE Q**R*** Claim Rebill:
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
Required if this field affects payment for or documentation
of professional pharmacy service.
44Ø-E5 PROFESSIONAL SERVICE CODE Q***R*** Claim Rebill:
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
Required if this field affects payment for or documentation
of professional pharmacy service.
441-E6 RESULT OF SERVICE CODE Q***R*** Claim Rebill:
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
Required if this field affects payment for or documentation
of professional pharmacy service.
474-8E DUR/PPS LEVEL OF EFFORT Q***R*** Claim Rebill:
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
Required if this field affects payment for or documentation
of professional pharmacy service.
475-J9 DUR CO-AGENT ID QUALIFIER Q***R*** Claim Rebill:
Required if DUR Co-Agent ID (476-H6) is used.
476-H6 DUR CO-AGENT ID Q***R*** Claim Rebill:
Required if this field could result in different drug utilization
review outcome.
Required if this field affects payment for or documentation
of professional pharmacy service.
Notes on DUR/PPS Segment on a Claim Rebill Request:
The DUR/PPS Segment is situational for a Claim Rebill request. It is used when a sender notifies the receiver of drug utilization, drug
evaluations, or information on the appropriate selection to process the claim/encounter. The DUR/PPS information may be sent on the initial
submission or alternatively sent after a DUR/PPS rejection from a receiver. The Segment is mandatory if required under provider payer
contract or mandatory on claims where this information is necessary for adjudication of the claim. Fields defined as Mandatory are required to
be submitted when the segment is sent.
11.3.11 COUPON SEGMENT (CLAIM REBILL)
COUPON SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
485-KE COUPON TYPE M
486-ME COUPON NUMBER M
487-NE COUPON VALUE AMOUNT Q Claim Rebill:
Required if needed for receiver claim/encounter
determination when a coupon value is known.
Required if this field could result in different pricing and/or
patient financial responsibility.
Notes on Coupon Segment on a Claim Rebill Request:
The Coupon Segment is situational for a Claim Rebill request. It is used when the sender seeks reimbursement for a claim billing which
includes a fixed amount or percentage of total price reduction. It is used in situations where the coupon is applied to the transaction.
To bill a coupon processor using the Coupon Segment, the Coupon Type (485-KE) and Coupon Number (486-ME) are mandatory.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 245 -
A coupon is used to reduce the patient out of pocket prescription cost – by either reducing the cost of a CASH prescription or the patient
financial responsibility from a Third Party payer who allows coupon usage. The coupon processor is the LAST payer. (Note: Some Federal
and State programs do not allow the reduction of patient’s financial responsibility.)
When a customer has a coupon, the field Usual And Customary Charge (426-DQ) is not reduced by the amount of the coupon.
The Segment is mandatory if required under provider payer contract or mandatory on claims where this information is necessary for
adjudication of the claim.
Fields defined as Mandatory are required to be submitted when the segment is sent.
11.3.12 COMPOUND SEGMENT (CLAIM REBILL)
COMPOUND SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
45Ø-EF COMPOUND DOSAGE FORM DESCRIPTION CODE M
451-EG COMPOUND DISPENSING UNIT FORM INDICATOR M
447-EC COMPOUND INGREDIENT COMPONENT COUNT M Maximum count of 25 ingredients.
488-RE COMPOUND PRODUCT ID QUALIFIER M***R***
489-TE COMPOUND PRODUCT ID M***R***
448-ED COMPOUND INGREDIENT QUANTITY M***R***
449-EE COMPOUND INGREDIENT DRUG COST Q***R*** Claim Rebill:
Required if needed for receiver claim determination when
multiple products are billed.
49Ø-UE COMPOUND INGREDIENT BASIS OF COST DETERMINATION Q***R*** Claim Rebill:
Required if needed for receiver claim determination when
multiple products are billed.
362-2G COMPOUND INGREDIENT MODIFIER CODE COUNT Q Claim Rebill:
Required when Compound Ingredient Modifier Code (363-
2H) is sent.
Maximum count of 1Ø.
363-2H COMPOUND INGREDIENT MODIFIER CODE Q***R*** Claim Rebill:
Required if necessary for state/federal/regulatory agency
programs.
Notes on Compound Segment on a Claim Rebill Request:
The Compound Segment is situational for a Claim Rebill request. It is used for multi-ingredient prescriptions, when each ingredient is reported.
The Segment is mandatory if required under provider payer contract or mandatory on claims where this information is necessary for
adjudication of the claim. Fields defined as Mandatory are required to be submitted when the segment is sent.
11.3.13 CLINICAL SEGMENT (CLAIM REBILL)
CLINICAL SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
491-VE DIAGNOSIS CODE COUNT Q Claim Rebill:
Maximum count of 5.
Required if Diagnosis Code Qualifier (492-WE) and
Diagnosis Code (424-DO) are used.
492-WE DIAGNOSIS CODE QUALIFIER Q***R*** Claim Rebill:
Required if Diagnosis Code (424-DO) is used.
424-DO DIAGNOSIS CODE Q***R*** Claim Rebill:
The value for this field is obtained from the prescriber or
authorized representative.
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
Required if this field affects payment for professional
pharmacy service.
Required if this information can be used in place of prior
authorization.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 246 -
CLINICAL SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if necessary for state/federal/regulatory agency
programs.
493-XE CLINICAL INFORMATION COUNTER Q***R*** Claim Rebill:
Maximum 5 occurrences supported.
Grouped with Measurement fields (Measurement Date
(494-ZE), Measurement Time (495-H1), Measurement
Dimension (496-H2), Measurement Unit (497-H3),
Measurement Value (499-H4).
494-ZE MEASUREMENT DATE Q***R*** Claim Rebill:
Required if necessary when this field could result in
different coverage and/or drug utilization review outcome.
495-H1 MEASUREMENT TIME Q***R*** Claim Rebill:
Required if Time is known or has impact on measurement.
Required if necessary when this field could result in
different coverage and/or drug utilization review outcome.
496-H2 MEASUREMENT DIMENSION Q***R*** Claim Rebill:
Required if Measurement Unit (497-H3) and Measurement
Value (499-H4) are used.
Required if necessary when this field could result in
different coverage and/or drug utilization review outcome.
Required if necessary for patient’s weight and height when
billing Medicare for a claim that includes a Certificate of
Medical Necessity (CMN).
497-H3 MEASUREMENT UNIT Q***R*** Claim Rebill:
Required if Measurement Dimension (496-H2) and
Measurement Value (499-H4) are used.
Required if necessary for patient’s weight and height when
billing Medicare for a claim that includes a Certificate of
Medical Necessity (CMN).
Required if necessary when this field could result in
different coverage and/or drug utilization review outcome.
499-H4 MEASUREMENT VALUE Q***R*** Claim Rebill:
Required if Measurement Dimension (496-H2) and
Measurement Unit (497-H3) are used.
Required if necessary for patient’s weight and height when
billing Medicare for a claim that includes a Certificate of
Medical Necessity (CMN).
Required if necessary when this field could result in
different coverage and/or drug utilization review outcome.
Notes on Clinical Segment on a Claim Rebill Request:
The Clinical Segment is situational for a Claim Rebill request. It is used to specify diagnosis information associated with the Claim Billing,
Claim Rebill, or Encounter transaction. The Segment is mandatory if required under provider payer contract or mandatory on claims where this
information is necessary for adjudication of the claim. Fields defined as Mandatory are required to be submitted when the segment is sent.
11.3.14 ADDITIONAL DOCUMENTATION SEGMENT (CLAIM REBILL)
ADDITIONAL DOCUMENTATION SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
369-2Q ADDITIONAL DOCUMENTATION TYPE ID M
374-2V REQUEST PERIOD BEGIN DATE Q Claim Rebill:
Required if necessary for state/federal/regulatory agency
programs.
375-2W REQUEST PERIOD RECERT/REVISED DATE Q Claim Rebill:
Required if necessary for state/federal/regulatory agency
programs.
Required if the Request Status (373-2U) = “2” (Revision) or
“3” (Recertification).
373-2U REQUEST STATUS Q Claim Rebill:
Required if necessary for state/federal/regulatory agency
programs.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 247 -
ADDITIONAL DOCUMENTATION SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
371-2S LENGTH OF NEED QUALIFIER Q Claim Rebill:
Required if Length of Need (37Ø-2R) is used.
37Ø-2R LENGTH OF NEED Q Claim Rebill:
Required if necessary for state/federal/regulatory agency
programs.
372-2T PRESCRIBER/SUPPLIER DATE SIGNED Q Claim Rebill:
Required if necessary for state/federal/regulatory agency
programs.
376-2X SUPPORTING DOCUMENTATION Q Claim Rebill:
Required if necessary for state/federal/regulatory agency
programs (using Section C of Medicare’s CMN forms).
377-2Z QUESTION NUMBER/LETTER COUNT Q Claim Rebill:
Maximum count of 5Ø.
Required if needed to provide response to narratives.
378-4B QUESTION NUMBER/LETTER Q***R*** Claim Rebill:
Required if necessary for state/federal/regulatory agency
programs to respond to questions included on a Medicare
form.
Required if Question Number/Letter
Count (377-2Z) is greater than Ø.
379-4D QUESTION PERCENT RESPONSE Q***R*** Claim Rebill:
Required if necessary for state/federal/regulatory agency
programs to respond to questions included on a Medicare
form that requires a percent as the response. (At least one
response is required per question.)
38Ø-4G QUESTION DATE RESPONSE Q***R*** Claim Rebill:
Required if necessary for state/federal/regulatory agency
programs to respond to questions included on a Medicare
form that requires a date as the response. (At least one
response is required per question.)
381-4H QUESTION DOLLAR AMOUNT RESPONSE Q***R*** Claim Rebill:
Required if necessary for state/federal/regulatory agency
programs to respond to questions included on a Medicare
form that requires a dollar amount as the response. (At
least one response is required per question.)
382-4J QUESTION NUMERIC RESPONSE Q***R*** Claim Rebill:
Required if necessary for
State/federal/regulatory agency programs to respond to
questions included on a Medicare form that requires a
numeric as the response. (At least one response is
required per question.)
383-4K QUESTION ALPHANUMERIC RESPONSE Q***R*** Claim Rebill:
Required if necessary for state/federal/regulatory agency
programs to respond to questions included on a Medicare
form that requires an alphanumeric as the response. (At
least one response is required per question.)
Notes on Additional Documentation Segment on a Claim Rebill Request:
The Additional Documentation Segment is situational for Claim Rebill request. It is used to provide additional information on Medicare forms.
Fields defined as Mandatory are required to be submitted when the segment is sent.
11.3.15 FACILITY SEGMENT (CLAIM REBILL)
FACILITY SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
336-8C FACILITY ID Q Claim Rebill:
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
385-3Q FACILITY NAME Q Claim Rebill:
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
386-3U FACILITY STREET ADDRESS Q Claim Rebill:
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 248 -
FACILITY SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
388-5J FACILITY CITY ADDRESS Q Claim Rebill:
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
387-3V FACILITY STATE/PROVINCE ADDRESS Q Claim Rebill:
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
389-6D FACILITY ZIP/POSTAL ZONE Q Claim Rebill:
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
Notes on Facility Segment on a Claim Rebill Request:
The Facility Segment is situational for Claim Rebill request. It is used when these fields could result in different coverage, pricing, patient
financial responsibility, and/or drug utilization review outcome. Fields defined as Mandatory are required to be submitted when the segment is
sent.
11.3.16 NARRATIVE SEGMENT (CLAIM REBILL)
NARRATIVE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
39Ø-BM NARRATIVE MESSAGE Q Claim Rebill:
Required if necessary only to support exception handling of
pharmacy claims for Medicare Part B claim billing.
Notes on Narrative Segment on a Claim Rebill Request:
The Narrative Segment is situational for Claim Rebill request. It is used to support exception handling of pharmacy claims for Medicare claim
billing. Fields defined as Mandatory are required to be submitted when the segment is sent.
11.4 CLAIM REBILL RESPONSE DIAGRAMS AND SEGMENTS
11.4.1 TRANSMISSION ACCEPTED/TRANSACTION PAID
11.4.1.1 DIAGRAM FOR TRANSMISSION OF ONE CLAIM REBILL RESPONSE (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
Claim Rebill transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “P” (Paid)
The Paid response is not used in payer-to-payer transactions.
The duplicate response codes for the Claim Rebill transaction are not applicable.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 249 -
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
11.4.1.2 DIAGRAM FOR TRANSMISSION OF TWO CLAIM REBILL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
11.4.1.3 DIAGRAM FOR TRANSMISSION OF THREE CLAIM REBILL RESPONSES (TRANSMISSION
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 250 -
ACCEPTED/TRANSACTION PAID)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
11.4.1.4 DIAGRAM FOR TRANSMISSION OF FOUR CLAIM REBILL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 251 -
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
11.4.2 CLAIM REBILL RESPONSE SEGMENTS (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
11.4.2.1.1 RESPONSE HEADER SEGMENT (CLAIM REBILL) (TRANSMISSION ACCEPTED/TRANSACTION PAID)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M For Transaction Code of “B3”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “1” (Rx Billing).
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 252 -
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Claim Rebill Response:
The Response Header Segment is a mandatory, fixed length segment for Claim Rebill response when the Header Response Status (5Ø1-F1)
of "A" (Accepted) and Transaction Response Status (112-AN) of “P” (Paid). The “Situation” column is not applicable.
11.4.2.1.2 RESPONSE MESSAGE SEGMENT (CLAIM REBILL) (TRANSMISSION ACCEPTED/TRANSACTION PAID)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Claim Rebill:
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Claim Rebill Response:
The Response Message Segment is situational for Claim Rebill response when the Header Response Status (5Ø1-F1) of "A" (Accepted) and
Transaction Response Status (112-AN) of “P” (Paid). It is used when additional text information needs to be sent. Fields defined as Mandatory
are required to be submitted when the segment is sent.
11.4.2.1.3 RESPONSE INSURANCE SEGMENT (CLAIM REBILL) (TRANSMISSION ACCEPTED/TRANSACTION PAID)
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø1-C1 GROUP ID
Q Claim Rebill:
Required if needed to identify the actual cardholder or
employer group, to identify appropriate group number,
when available.
Required to identify the actual group that was used when
multiple group coverages exist.
Note: This field may contain the Group ID echoed from the
request. May contain the actual Group ID if unknown to the
receiver.
524-FO PLAN ID
Q Claim Rebill:
Required if needed to identify the actual plan parameters,
benefit, or coverage criteria, when available.
Required to identify the actual plan ID that was used when
multiple group coverages exist.
Required if needed to contain the actual plan ID if unknown
to the receiver.
545-2F NETWORK REIMBURSEMENT ID Q Claim Rebill:
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 253 -
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if needed to identify the network for the covered
member.
Required if needed to identify the actual Network
Reimbursement ID, when applicable and/or available.
Required to identify the actual Network Reimbursement ID
that was used when multiple Network Reimbursement IDs
exist.
568-J7 PAYER ID QUALIFIER Q Claim Rebill:
Required if Payer ID (569-J8) is used.
569-J8 PAYER ID
Q Claim Rebill:
Required to identify the ID of the payer responding.
115-N5
MEDICAID ID NUMBER N Claim Rebill:
Not used.
116-N6
MEDICAID AGENCY NUMBER N Claim Rebill:
Not used.
3Ø2-C2
CARDHOLDER ID Q Claim Rebill:
Required if the identification to be used in future
transactions is different than what was submitted on the
request.
Notes on Response Insurance Segment on a Claim Rebill Response:
The Response Insurance Segment is situational for Claim Rebill response when the Header Response Status (5Ø1-F1) of "A" (Accepted) and
Transaction Response Status (112-AN) of “P” (Paid). It is used when coverage or reimbursement parameters or identifiers need to be sent.
Fields defined as Mandatory are required to be submitted when the segment is sent.
11.4.2.1.3.1 Response Insurance Segment (Medicaid Subrogation Claim Rebill) (Transmission
Accepted/Transaction Paid)
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
115-N5
MEDICAID ID NUMBER QM Medicaid Subrogation - Claim Rebill:
Required to identify the member as uniquely known to
Medicaid.
See Medicaid Subrogation Implementation Guide.
116-N6
MEDICAID AGENCY NUMBER QM Medicaid Subrogation - Claim Rebill:
Required to identify the Medicaid agency.
See Medicaid Subrogation Implementation Guide.
Notes on Response Insurance Segment on a Medicaid Subrogation Claim Rebill Response:
The rules above for a “Response Insurance Segment (Claim Rebill)” are to be followed for Medicaid Subrogation. Specific fields that
are used differently in Medicaid Subrogation are noted in the table above.
11.4.2.1.4 RESPONSE PATIENT SEGMENT (CLAIM REBILL) (TRANSMISSION ACCEPTED/TRANSACTION PAID)
RESPONSE PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
31Ø-CA PATIENT FIRST NAME Q Claim Rebill:
Required if known.
311-CB PATIENT LAST NAME Q Claim Rebill:
Required if known.
3Ø4-C4 DATE OF BIRTH Q Claim Rebill:
Required if known.
Notes on Response Patient Segment on a Claim Rebill Response:
The Response Patient Segment is situational for Claim Rebill transmission response Header Response Status (5Ø1-F1) of "A" (Accepted) and
Transaction Response Status (112-AN) of “P” (Paid) when patient demographic information needs to be sent from the sender to the receiver.
Fields defined as Mandatory are required to be submitted when the segment is sent.
11.4.2.1.5 RESPONSE STATUS SEGMENT (CLAIM REBILL) (TRANSMISSION ACCEPTED/TRANSACTION PAID)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 254 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Claim Rebill:
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT N Claim Rebill:
Not used.
511-FB REJECT CODE N**R*** Claim Rebill:
Not used.
546-4F REJECT FIELD OCCURRENCE INDICATOR N***R*** Claim Rebill:
Not used.
547-5F APPROVED MESSAGE CODE COUNT Q Claim Rebill:
Maximum count of 5.
Required if Approved Message Code (548-6F) is used.
548-6F APPROVED MESSAGE CODE Q***R*** Claim Rebill:
Required if Approved Message Code Count (547-5F) is
used and the sender needs to communicate additional
follow up for a potential opportunity.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Claim Rebill:
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Claim Rebill:
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Claim Rebill:
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Claim Rebill:
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Claim Rebill:
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Claim Rebill:
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Claim Rebill:
Not used.
993-A7 INTERNAL CONTROL NUMBER Q Claim Rebill:
Required when used for payer-to-payer coordination of
benefits to track the claim without regard to the “Service
Provider ID, Prescription Number, & Date of Service”.
987-MA URL N Claim Rebill:
Not used.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 255 -
Notes on Response Status Segment on a Claim Rebill Response:
The Response Status Segment is mandatory for a Claim Rebill response when the Header Response Status (5Ø1-F1) of "A" (Accepted) and
Transaction Response Status (112-AN) of “P” (Paid). The Response Status Segment is sent from the sender to the receiver to identify the
outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.
11.4.2.1.6 RESPONSE CLAIM SEGMENT (CLAIM REBILL) (TRANSMISSION ACCEPTED/TRANSACTION PAID)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M For Transaction Code of “B3”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “1” (Rx Billing).
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT Q Claim Rebill:
Maximum count of 6.
Required if Preferred Product ID (553-AR) is used.
552-AP PREFERRED PRODUCT ID QUALIFIER Q***R*** Claim Rebill:
Required if Preferred Product ID (553-AR) is used.
553-AR PREFERRED PRODUCT ID Q***R*** Claim Rebill:
Required if a product preference exists that needs to be
communicated to the receiver via an ID.
554-AS PREFERRED PRODUCT INCENTIVE Q***R*** Claim Rebill:
Required if there is a known incentive amount associated
with the Preferred Product ID (553-AR) and/or Preferred
Product Description (556-AU).
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE Q***R*** Claim Rebill:
Required if there is a known patient financial responsibility
incentive amount associated with the Preferred Product ID
(553-AR) and/or Preferred Product Description (556-AU).
556-AU PREFERRED PRODUCT DESCRIPTION Q***R*** Claim Rebill:
Required if a product preference exists that either cannot
be communicated by the Preferred Product ID (553-AR) or
to clarify the Preferred Product ID (553-AR).
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Claim Rebill:
Not used.
Notes on Response Claim Segment on a Claim Rebill Response:
The Response Claim Segment is mandatory for a Claim Rebill response when the Header Response Status (5Ø1-F1) of "A" (Accepted) and
Transaction Response Status (112-AN) of “P” (Paid). The Response Claim Segment is sent from the sender to the receiver to identify
therapeutic or alternate product recommendations. Fields defined as Mandatory are required to be submitted when the segment is sent.
11.4.2.1.6.1 Response Claim Segment (Medicaid Subrogation Claim Rebill) (Transmission
Accepted/Transaction Paid)
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
QM Medicaid Subrogation - Claim Rebill:
Required to report back on the response the claim
number assigned by the Medicaid Agency.
Notes on Response Claim Segment on a Medicaid Subrogation Claim Rebill Response:
The rules above for a “Response Claim Segment (Claim Rebill)” are to be followed for Medicaid Subrogation. Specific fields that are
used differently in Medicaid Subrogation are noted in the table above.
11.4.2.1.7 RESPONSE PRICING SEGMENT (CLAIM REBILL) (TRANSMISSION ACCEPTED/TRANSACTION PAID)
RESPONSE PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø5-F5 PATIENT PAY AMOUNT R Claim Rebill:
Required.
5Ø6-F6 INGREDIENT COST PAID Q Claim Rebill:
Required if this value is used to arrive at the final
reimbursement.
5Ø7-F7 DISPENSING FEE PAID Q Claim Rebill:
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 256 -
RESPONSE PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if this value is used to arrive at the final
reimbursement.
557-AV TAX EXEMPT INDICATOR Q Claim Rebill:
Required if the sender (health plan) and/or patient is tax
exempt and exemption applies to this billing.
558-AW FLAT SALES TAX AMOUNT PAID Q Claim Rebill:
Required if Flat Sales Tax Amount Submitted (481-HA) is
greater than zero (Ø) or if Flat Sales Tax Amount Paid
(558-AW) is used to arrive at the final reimbursement.
Zero (Ø) is a valid value.
559-AX PERCENTAGE SALES TAX AMOUNT PAID Q Claim Rebill:
Required if this value is used to arrive at the final
reimbursement.
Required if Percentage Sales Tax Amount Submitted (482-
GE) is greater than zero (Ø).
Zero (Ø) is a valid value.
Required if Percentage Sales Tax Rate Paid (56Ø-AY) and
Percentage Sales Tax Basis Paid (561-AZ) are used.
56Ø-AY PERCENTAGE SALES TAX RATE PAID Q Claim Rebill:
Required if Percentage Sales Tax Amount Paid (559-AX) is
greater than zero (Ø).
561-AZ PERCENTAGE SALES TAX BASIS PAID Q Claim Rebill:
Required if Percentage Sales Tax Amount Paid (559-AX) is
greater than zero (Ø).
521-FL INCENTIVE AMOUNT PAID
Q Claim Rebill:
Required if this value is used to arrive at the final
reimbursement.
Required if Incentive Amount Submitted (438-E3) is greater
than zero (Ø).
Zero (Ø) is a valid value.
562-J1 PROFESSIONAL SERVICE FEE PAID N Claim Rebill:
Not used.
563-J2 OTHER AMOUNT PAID COUNT Q Claim Rebill:
Maximum count of 3.
Required if Other Amount Paid (565-J4) is used.
564-J3 OTHER AMOUNT PAID QUALIFIER Q***R*** Claim Rebill:
Required if Other Amount Paid (565-J4) is used.
565-J4 OTHER AMOUNT PAID
Q***R*** Claim Rebill:
Required if this value is used to arrive at the final
reimbursement.
Required if Other Amount Claimed Submitted (48Ø-H9) is
greater than zero (Ø).
Zero (Ø) is a valid value.
Must respond to each occurrence submitted.
566-J5 OTHER PAYER AMOUNT RECOGNIZED Q Claim Rebill:
Required if this value is used to arrive at the final
reimbursement.
Required if Other Payer Amount Paid (431-DV) is greater
than zero (Ø) and Coordination of Benefits/Other Payments
Segment is supported.
5Ø9-F9 TOTAL AMOUNT PAID R Claim Rebill:
Required.
Zero (Ø) value is valid.
See Pricing Formula for fields used in calculation.
522-FM BASIS OF REIMBURSEMENT DETERMINATION Q Claim Rebill:
Required if Ingredient Cost Paid (5Ø6-F6) is greater than
zero (Ø).
Required if Basis of Cost Determination (432-DN) is
submitted on billing.
523-FN AMOUNT ATTRIBUTED TO SALES TAX Q Claim Rebill:
Required if Patient Pay Amount (5Ø5-F5) includes sales tax

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 257 -
RESPONSE PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
that is the financial responsibility of the member but is not
also included in any of the other fields that add up to
Patient Pay Amount.
See section “Specific Segment Discussion”, “Response
Segments”, “Response Pricing Segment” for guidance.
512-FC ACCUMULATED DEDUCTIBLE AMOUNT I Claim Rebill:
Provided for informational purposes only.
513-FD REMAINING DEDUCTIBLE AMOUNT I Claim Rebill:
Provided for informational purposes only.
514-FE REMAINING BENEFIT AMOUNT I Claim Rebill:
The Remaining Benefit Amount must not be returned with
zeroes unless there are no benefit dollars remaining. The
default value of 999999999 from previous versions must
not be used as a default in this field.
Provided for informational purposes only.
517-FH AMOUNT APPLIED TO PERIODIC DEDUCTIBLE Q Claim Rebill:
Required if Patient Pay Amount (5Ø5-F5) includes
deductible.
518-FI AMOUNT OF COPAY Q Claim Rebill:
Required if Patient Pay Amount (5Ø5-F5) includes copay
as patient financial responsibility.
52Ø-FK AMOUNT EXCEEDING PERIODIC BENEFIT MAXIMUM Q Claim Rebill:
Required if Patient Pay Amount (5Ø5-F5) includes amount
exceeding periodic benefit maximum.
346-HH BASIS OF CALCULATION—DISPENSING FEE Q Claim Rebill:
Required if Dispensing Status (343-HD) on submission is
“P” (Partial Fill) or “C” (Completion of Partial Fill).
347-HJ BASIS OF CALCULATION—COPAY Q Claim Rebill:
Required if Dispensing Status (343-HD) on submission is
“P” (Partial Fill) or “C” (Completion of Partial Fill).
348-HK BASIS OF CALCULATION—FLAT SALES TAX Q Claim Rebill:
Required if Dispensing Status (343-HD) on submission is
“P” (Partial Fill) or “C” (Completion of Partial Fill) and Flat
Sales Tax Amount Paid (558-AW) is greater than zero (Ø).
349-HM BASIS OF CALCULATION—PERCENTAGE SALES TAX Q Claim Rebill:
Required if Dispensing Status (343-HD) on submission is
“P” (Partial Fill) or “C” (Completion of Partial Fill) and
Percentage Sales Tax Amount Paid (559-AX) is greater
than zero (Ø).
571-NZ AMOUNT ATTRIBUTED TO PROCESSOR FEE Q Claim Rebill:
Required if the customer is responsible for 1ØØ% of the
prescription payment and when the provider net sale is less
than the amount the customer is expected to pay.
575-EQ PATIENT SALES TAX AMOUNT I Claim Rebill:
Used when necessary to identify the Patient’s portion of the
Sales Tax.
Provided for informational purposes only.
574-2Y PLAN SALES TAX AMOUNT I Claim Rebill:
Used when necessary to identify the Plan’s portion of the
Sales Tax.
Provided for informational purposes only.
572-4U AMOUNT OF COINSURANCE Q Claim Rebill:
Required if Patient Pay Amount (5Ø5-F5) includes
coinsurance as patient financial responsibility.
573-4V BASIS OF CALCULATION-COINSURANCE Q Claim Rebill:
Required if Dispensing Status (343-HD) on submission is
“P” (Partial Fill) or “C” (Completion of Partial Fill).
392-MU BENEFIT STAGE COUNT Q Claim Rebill:
Maximum count of 4.
Required if Benefit Stage Amount (394-MW) is used.
393-MV BENEFIT STAGE QUALIFIER Q***R*** Claim Rebill:
Required if Benefit Stage Amount (394-MW) is used.
Must only have one value per iteration - value must not be
repeated.
394-MW BENEFIT STAGE AMOUNT Q***R*** Claim Rebill:
Required when a Medicare Part D payer applies financial
amounts to Medicare Part D beneficiary benefit stages.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 258 -
RESPONSE PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
This field is required when the plan is a participant in a
Medicare Part D program that requires reporting of benefit
stage specific financial amounts.
Required if necessary for state/federal/regulatory agency
programs.
577-G3 ESTIMATED GENERIC SAVINGS I Claim Rebill:
This information should be provided when a patient
selected the brand drug and a generic form of the drug was
available. It will contain an estimate of the difference
between the cost of the brand drug and the generic drug,
when the brand drug is more expensive than the generic. It
is information that the provider should provide to the
patient.
128-UC SPENDING ACCOUNT AMOUNT REMAINING I Claim Rebill:
This dollar amount will be provided, if known, to the
receiver when the transaction had spending account dollars
reported as part of the patient pay amount.
This field is informational only. It is reported back to the
provider and the patient the amount remaining on the
spending account after the current claim updated the
spending account.
129-UD HEALTH PLAN-FUNDED ASSISTANCE AMOUNT Q Claim Rebill:
Required when the patient meets the plan-funded
assistance criteria, to reduce Patient Pay Amount (5Ø5-
F5). The resulting Patient Pay Amount (5Ø5-F5) must be
greater than or equal to zero.
This field is always a negative amount or zero.
133-UJ AMOUNT ATTRIBUTED TO PROVIDER NETWORK SELECTION Q Claim Rebill:
Required if Patient Pay Amount (5Ø5-F5) includes an
amount that is attributable to a cost share differential due to
the selection of one pharmacy over another.
134-UK AMOUNT ATTRIBUTED TO PRODUCT SELECTION/BRAND
DRUG
Q Claim Rebill:
Required if Patient Pay Amount (5Ø5-F5) includes an
amount that is attributable to a patient’s selection of a
Brand drug.
135-UM AMOUNT ATTRIBUTED TO PRODUCT SELECTION/NON-
PREFERRED FORMULARY SELECTION
Q Claim Rebill:
Required if Patient Pay Amount (5Ø5-F5) includes an
amount that is attributable to a patient’s selection of a non-
preferred formulary product.
136-UN AMOUNT ATTRIBUTED TO PRODUCT SELECTION/BRAND
NON-PREFERRED FORMULARY SELECTION
Q Claim Rebill:
Required if Patient Pay Amount (5Ø5-F5) includes an
amount that is attributable to a patient’s selection of a
Brand non-preferred formulary product.
137-UP AMOUNT ATTRIBUTED TO COVERAGE GAP Q Claim Rebill:
Required when the patient’s financial responsibility is due to
the coverage gap.
148-U8 INGREDIENT COST CONTRACTED/REIMBURSABLE AMOUNT I Claim Rebill:
Required when Basis of Reimbursement Determination
(522-FM) is “14” (Patient Responsibility Amount) or “15”
(Patient Pay Amount) unless prohibited by
state/federal/regulatory agency.
This field is informational only.
149-U9 DISPENSING FEE CONTRACTED/REIMBURSABLE AMOUNT I Claim Rebill:
Required when Basis of Reimbursement Determination
(522-FM) is “14” (Patient Responsibility Amount) or “15”
(Patient Pay Amount) unless prohibited by
state/federal/regulatory agency.
This field is informational only.
Notes on Response Pricing Segment on a Claim Rebill Response:
The Response Pricing Segment is mandatory for a Claim Rebill Response when the Header Response Status (5Ø1-F1) of "A" (Accepted) and
Transaction Response Status (112-AN) is “P” (Paid).
It is highly recommended that whenever possible, the individual dollar fields are to be returned in the response. On the response, the sender
should return the individual payment response fields to allow the receiver to reconcile against the requested payment fields.
See section “Response Processing Guidelines”, “Pricing Guidelines”.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 259 -
Fields defined as Mandatory are required to be submitted when the segment is sent.
11.4.2.1.8 RESPONSE DUR/PPS SEGMENT (CLAIM REBILL) (TRANSMISSION ACCEPTED/TRANSACTION PAID)
RESPONSE DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
567-J6 DUR/PPS RESPONSE CODE COUNTER Q***R*** Claim Rebill:
Maximum 9 occurrences supported.
Required if Reason For Service Code (439-E4) is used.
439-E4 REASON FOR SERVICE CODE Q***R*** Claim Rebill:
Required if utilization conflict is detected.
528-FS CLINICAL SIGNIFICANCE CODE Q***R*** Claim Rebill:
Required if needed to supply additional information for the
utilization conflict.
529-FT OTHER PHARMACY INDICATOR Q***R*** Claim Rebill:
Required if needed to supply additional information for the
utilization conflict.
53Ø-FU PREVIOUS DATE OF FILL Q**R*** Claim Rebill:
Required if needed to supply additional information for the
utilization conflict.
Required if Quantity of Previous Fill (531-FV) is used.
531-FV QUANTITY OF PREVIOUS FILL Q**R*** Claim Rebill:
Required if needed to supply additional information for the
utilization conflict.
Required if Previous Date Of Fill (53Ø-FU) is used.
532-FW DATABASE INDICATOR Q**R*** Claim Rebill:
Required if needed to supply additional information for the
utilization conflict.
533-FX OTHER PRESCRIBER INDICATOR Q**R*** Claim Rebill:
Required if needed to supply additional information for the
utilization conflict.
544-FY DUR FREE TEXT MESSAGE Q**R*** Claim Rebill:
Required if needed to supply additional information for the
utilization conflict.
Notes on Response DUR/PPS Segment on a Claim Rebill Response:
The Response DUR/PPS Segment is situational for a Claim Rebill Response when the Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “P” (Paid). The segment is used to identify a drug utilization review or professional pharmacy
service event, opportunity, or information. Fields defined as Mandatory are required to be submitted when the segment is sent.
11.4.2.1.9 RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS SEGMENT (CLAIM REBILL) (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS
SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
355-NT OTHER PAYER ID COUNT M Claim Rebill:
Maximum count of 3.
338-5C OTHER PAYER COVERAGE TYPE M***R***
339-6C OTHER PAYER ID QUALIFIER Q**R*** Claim Rebill:
Required if Other Payer ID (34Ø-7C) is used.
34Ø-7C OTHER PAYER ID Q***R*** Claim Rebill:
Required if other insurance information is available for
coordination of benefits.
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER Q***R*** Claim Rebill:
Required if other insurance information is available for
coordination of benefits.
356-NU OTHER PAYER CARDHOLDER ID Q***R*** Claim Rebill:
Required if other insurance information is available for
coordination of benefits.
992-MJ OTHER PAYER GROUP ID Q***R*** Claim Rebill:
Required if other insurance information is available for
coordination of benefits.
142-UV OTHER PAYER PERSON CODE Q***R*** Claim Rebill:
Required if needed to uniquely identify the family members
within the Cardholder ID, as assigned by the other payer.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 260 -
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS
SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
127-UB OTHER PAYER HELP DESK PHONE NUMBER Q***R*** Claim Rebill:
Required if needed to provide a support telephone number
of the other payer to the receiver.
143-UW OTHER PAYER PATIENT RELATIONSHIP CODE Q***R*** Claim Rebill:
Required if needed to uniquely identify the relationship of
the patient to the cardholder ID, as assigned by the other
payer.
144-UX OTHER PAYER BENEFIT EFFECTIVE DATE Q***R*** Claim Rebill:
Required when other coverage is known which is after the
Date of Service submitted.
145-UY OTHER PAYER BENEFIT TERMINATION DATE Q***R*** Claim Rebill:
Required when other coverage is known which is after the
Date of Service submitted.
Notes on Response Coordination of Benefits/Other Payers Segment on a Claim Rebill Response:
The Response Coordination of Benefits/Other Payers Segment is situational for a Claim Rebill response when the Header Response Status
(5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “P” (Paid) when other insurance information is available for
coordination of benefits.
If subsequent payer(s) for this patient is not known, the Other Payer information is not sent.
If subsequent payer(s) for this patient is known, the following may be sent:
• Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C),
• Other Payer Group ID (992-MJ),
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU)
• And other Other Payer fields.
In addition, if any of the following three fields are sent:
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU),
• Other Payer Group ID (992-MJ),
then the Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C) must be sent.
Fields defined as Mandatory are required to be submitted when the segment is sent.
11.4.3 TRANSMISSION ACCEPTED/TRANSACTION CAPTURED
11.4.3.1 DIAGRAM FOR TRANSMISSION OF ONE CLAIM REBILL RESPONSE (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
Claim Rebill transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “C” (Captured)
The Response Pricing Segment and Response DUR/PPS Segments are not used in payer-to-payer transactions.
The duplicate response codes for the Claim Rebill transaction are not applicable.
Claim Rebill transactions - The “C” (Captured) event occurs after the reversal portion of the claim rebill is processed successfully and the claim
is captured for processing. If the claim rebill reversal is not processed successfully, a “R” (Rejected) response must be sent.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 261 -
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Pricing Segment
11.4.3.2 DIAGRAM FOR TRANSMISSION OF TWO CLAIM REBILL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Pricing Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Pricing Segment
11.4.3.3 DIAGRAM FOR TRANSMISSION OF THREE CLAIM REBILL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 262 -
Segment Separator
Response Pricing Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Pricing Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Pricing Segment
11.4.3.4 DIAGRAM FOR TRANSMISSION OF FOUR CLAIM REBILL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Pricing Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Pricing Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 263 -
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Pricing Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Pricing Segment
11.4.3.5 CLAIM REBILL RESPONSE SEGMENTS (TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
11.4.3.5.1 RESPONSE HEADER SEGMENT (CLAIM REBILL) (TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M For Transaction Code of “B3”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “1” (Rx Billing).
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Claim Rebill Response:
The Response Header Segment is a mandatory, fixed length segment for Claim Rebill response when the Header Response Status (5Ø1-F1)
of "A" (Accepted) and Transaction Response Status (112-AN) of “C” (Captured). The “Situation” column is not applicable.
11.4.3.5.2 RESPONSE MESSAGE SEGMENT (CLAIM REBILL) (TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Claim Rebill:
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Claim Rebill Response:
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 264 -
The Response Message Segment is situational for Claim Rebill response when the Header Response Status (5Ø1-F1) of "A" (Accepted) and
Transaction Response Status (112-AN) of “C” (Captured). It is used when additional text information needs to be sent. Fields defined as
Mandatory are required to be submitted when the segment is sent.
11.4.3.5.3 RESPONSE INSURANCE SEGMENT (CLAIM REBILL) (TRANSMISSION ACCEPTED/TRANSACTION
CAPTURED)
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø1-C1 GROUP ID
Q Claim Rebill:
Required if needed to identify the actual cardholder or
employer group, to identify appropriate group number, when
available.
Required to identify the actual group that was used when
multiple group coverages exist.
Note: This field may contain the Group ID echoed from the
request. May contain the actual Group ID if unknown to the
receiver.
524-FO PLAN ID
Q Claim Rebill:
Required if needed to identify the actual plan parameters,
benefit, or coverage criteria, when available.
Required to identify the actual plan ID that was used when
multiple group coverages exist.
Required if needed to contain the actual plan ID if unknown
to the receiver.
545-2F NETWORK REIMBURSEMENT ID
N Claim Rebill:
Not used.
568-J7 PAYER ID QUALIFIER N Claim Rebill:
Not used.
569-J8 PAYER ID
N Claim Rebill:
Not used.
115-N5
MEDICAID ID NUMBER N Claim Rebill:
Not used.
116-N6
MEDICAID AGENCY NUMBER N Claim Rebill:
Not used.
3Ø2-C2
CARDHOLDER ID Q Claim Rebill:
Required if the identification to be used in future transactions
is different than what was submitted on the request.
Notes on Response Insurance Segment on a Claim Rebill Response:
The Response Insurance Segment is situational for Claim Rebill response when the Header Response Status (5Ø1-F1) of "A" (Accepted) and
Transaction Response Status (112-AN) of “C” (Captured). It is used when coverage information may be sent. Fields defined as Mandatory are
required to be submitted when the segment is sent.
11.4.3.5.3.1 Response Insurance Segment (Medicaid Subrogation Claim Rebill) (Transmission
Accepted/Transaction Captured)
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
115-N5
MEDICAID ID NUMBER QM Medicaid Subrogation - Claim Rebill:
Required to identify the member as uniquely known to
Medicaid.
116-N6
MEDICAID AGENCY NUMBER QM Medicaid Subrogation - Claim Rebill:
Required to identify the Medicaid agency.
See Medicaid Subrogation Implementation Guide.
Notes on Response Insurance Segment on a Medicaid Subrogation Claim Rebill Response:
The rules above for a “Response Insurance Segment (Claim Rebill)” are to be followed for Medicaid Subrogation. Specific fields that
are used differently in Medicaid Subrogation are noted in the table above.
11.4.3.5.4 RESPONSE PATIENT SEGMENT (CLAIM REBILL) (TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
RESPONSE PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 265 -
RESPONSE PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
31Ø-CA PATIENT FIRST NAME Q Claim Rebill:
Required if known.
311-CB PATIENT LAST NAME Q Claim Rebill:
Required if known.
3Ø4-C4 DATE OF BIRTH Q Claim Rebill:
Required if known.
Notes on Response Patient Segment on a Claim Rebill Response:
The Response Patient Segment is situational for Claim Rebill transmission response Header Response Status (5Ø1-F1) of "A" (Accepted) and
Transaction Response Status (112-AN) of “C” (Captured) when patient demographic information needs to be sent from the sender to the
receiver. Fields defined as Mandatory are required to be submitted when the segment is sent.
11.4.3.5.5 RESPONSE STATUS SEGMENT (CLAIM REBILL) (TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Claim Rebill:
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT N Claim Rebill:
Not used.
511-FB REJECT CODE N***R*** Claim Rebill:
Not used.
546-4F REJECT FIELD OCCURRENCE INDICATOR N***R*** Claim Rebill:
Not used.
547-5F APPROVED MESSAGE CODE COUNT N Claim Rebill:
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Claim Rebill:
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Claim Rebill:
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Claim Rebill:
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Claim Rebill:
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Claim Rebill:
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 266 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Claim Rebill:
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Claim Rebill:
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Claim Rebill:
Not used.
993-A7 INTERNAL CONTROL NUMBER Q Claim Rebill:
Required when used for payer-to-payer coordination of
benefits to track the claim without regard to the “Service
Provider ID, Prescription Number, & Date of Service”.
987-MA URL N Claim Rebill:
Not used.
Notes on Response Status Segment on a Claim Rebill Response:
The Response Status Segment is mandatory for a Claim Rebill response when the Header Response Status (5Ø1-F1) of "A" (Accepted) and
Transaction Response Status (112-AN) of “C” (Captured). The Response Status Segment is sent from the sender to the receiver to identify the
outcome of the request.
Claim Rebill transactions - The “C” (Captured) event occurs after the reversal portion of the claim rebill is processed successfully and the claim
is captured for processing. If the claim rebill reversal is not processed successfully, a “R” (Rejected) response must be sent.
Fields defined as Mandatory are required to be submitted when the segment is sent.
11.4.3.5.6 RESPONSE CLAIM SEGMENT (CLAIM REBILL) (TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M For Transaction Code of “B3”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “1” (Rx Billing).
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT Q Claim Rebill:
Maximum count of 6.
Required if Preferred Product ID (553-AR) is used.
Not used in payer-to-payer transactions.
552-AP PREFERRED PRODUCT ID QUALIFIER Q***R*** Claim Rebill:
Required if Preferred Product ID (553-AR) is used.
Not used in payer-to-payer transactions.
553-AR PREFERRED PRODUCT ID Q***R*** Claim Rebill:
Required if a product preference exists that needs to be
communicated to the receiver via an ID.
Not used in payer-to-payer transactions.
554-AS PREFERRED PRODUCT INCENTIVE Q***R*** Claim Rebill:
Required if there is a known incentive amount associated
with the Preferred Product ID (553-AR) and/or Preferred
Product Description (556-AU).
Not used in payer-to-payer transactions.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE Q***R*** Claim Rebill:
Required if there is a known patient financial responsibility
incentive amount associated with the Preferred Product ID
(553-AR) and/or Preferred Product Description (556-AU).
556-AU PREFERRED PRODUCT DESCRIPTION Q***R*** Claim Rebill:
Required if a product preference exists that either cannot
be communicated by the Preferred Product ID (553-AR) or
to clarify the Preferred Product ID (553-AR).
Not used in payer-to-payer transactions.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Claim Rebill:
Not used.
Notes on Response Claim Segment on a Claim Rebill Response:
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 267 -
The Response Claim Segment is mandatory for a Claim Rebill response when the Header Response Status (5Ø1-F1) of "A" (Accepted) and
Transaction Response Status (112-AN) of “C” (Captured). The Response Claim Segment is sent from the sender to the receiver to identify
therapeutic or alternate product recommendations. Fields defined as Mandatory are required to be submitted when the segment is sent.
11.4.3.5.7 RESPONSE PRICING SEGMENT (CLAIM REBILL) (TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
RESPONSE PRICING SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø5-F5 PATIENT PAY AMOUNT Q Claim Rebill:
Required if known. This field cannot be an estimated
amount. Zero is a valid amount.
5Ø6-F6 INGREDIENT COST PAID Q Claim Rebill:
Required if this value is used to arrive at the estimated
reimbursement. If reimbursement is not estimated, this field
contains the submitted value.
5Ø7-F7 DISPENSING FEE PAID Q Claim Rebill:
Required if this value is used to arrive at the estimated
reimbursement. If reimbursement is not estimated, this field
contains the submitted value.
557-AV TAX EXEMPT INDICATOR Q Claim Rebill:
Required if the sender (health plan) and/or patient is tax
exempt and exemption applies to this billing.
558-AW FLAT SALES TAX AMOUNT PAID Q Claim Rebill:
Required if Flat Sales Tax Amount Submitted (481-HA) is
greater than zero (Ø) or if Flat Sales Tax Amount Paid
(558-AW) is used to arrive at the estimated reimbursement.
Zero (Ø) is a valid value.
If reimbursement is not estimated, this field contains the
submitted value.
559-AX PERCENTAGE SALES TAX AMOUNT PAID Q Claim Rebill:
Required if this value is used to arrive at the estimated
reimbursement. If reimbursement is not estimated, this field
contains the submitted value.
Required if Percentage Sales Tax Amount Submitted (482-
GE) is greater than zero (Ø).
Zero (Ø) is a valid value.
Required if Percentage Sales Tax Rate Paid (56Ø-AY) and
Percentage Sales Tax Basis Paid (561-AZ) are used.
56Ø-AY PERCENTAGE SALES TAX RATE PAID Q Claim Rebill:
Required if Percentage Sales Tax Amount Paid (559-AX) is
greater than zero (Ø).
561-AZ PERCENTAGE SALES TAX BASIS PAID Q Claim Rebill:
Required if Percentage Sales Tax Amount Paid (559-AX) is
greater than zero (Ø).
521-FL INCENTIVE AMOUNT PAID
Q Claim Rebill:
Required if this value is used to arrive at the estimated
reimbursement. If reimbursement is not estimated, this field
contains the submitted value.
Required if Incentive Amount Submitted (438-E3) is greater
than zero (Ø).
Zero (Ø) is a valid value.
562-J1 PROFESSIONAL SERVICE FEE PAID N Claim Rebill:
Not used.
563-J2 OTHER AMOUNT PAID COUNT Q Claim Rebill:
Maximum count of 3.
Required if Other Amount Paid (565-J4) is used.
564-J3 OTHER AMOUNT PAID QUALIFIER Q***R*** Claim Rebill:
Required if Other Amount Paid (565-J4) is used.
565-J4 OTHER AMOUNT PAID
Q***R*** Claim Rebill:
Required if this value is used to arrive at the estimated
reimbursement. If reimbursement is not estimated, this field
contains the submitted value.
Required if Other Amount Claimed Submitted (48Ø-H9) is
greater than zero (Ø).

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 268 -
RESPONSE PRICING SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Zero (Ø) is a valid value.
Must respond to each occurrence submitted.
566-J5 OTHER PAYER AMOUNT RECOGNIZED Q Claim Rebill:
Required if this value is used to arrive at the estimated
reimbursement. If reimbursement is not estimated, this field
contains the submitted value.
Required if Other Payer Amount Paid (431-DV) is greater
than zero (Ø) and Coordination of Benefits/Other Payments
Segment is supported.
5Ø9-F9 TOTAL AMOUNT PAID R Claim Rebill:
Required. Zero (Ø) value is valid.
See Pricing Formula for fields used in calculation.
522-FM BASIS OF REIMBURSEMENT DETERMINATION Q Claim Rebill:
Required if Ingredient Cost Paid (5Ø6-F6) is greater than
zero (Ø).
Required if Basis of Cost Determination (432-DN) is
submitted on billing.
523-FN AMOUNT ATTRIBUTED TO SALES TAX Q Claim Rebill:
Required if Patient Pay Amount (5Ø5-F5) includes sales tax
that is the financial responsibility of the member but is not
also included in any of the other fields that add up to
Patient Pay Amount.
See section “Specific Segment Discussion”, “Response
Segments”, “Response Pricing Segment” for guidance.
512-FC ACCUMULATED DEDUCTIBLE AMOUNT I Claim Rebill:
Provided for informational purposes only.
513-FD REMAINING DEDUCTIBLE AMOUNT I Claim Rebill:
Provided for informational purposes only.
514-FE REMAINING BENEFIT AMOUNT I Claim Rebill:
The Remaining Benefit Amount must not be returned with
zeroes unless there are no benefit dollars remaining. The
default value of 999999999 from previous versions must
not be used as a default in this field.
Provided for informational purposes only.
517-FH AMOUNT APPLIED TO PERIODIC DEDUCTIBLE Q Claim Rebill:
Required if Patient Pay Amount (5Ø5-F5) includes
deductible.
518-FI AMOUNT OF COPAY Q Claim Rebill:
Required if Patient Pay Amount (5Ø5-F5) includes copay
as patient financial responsibility.
52Ø-FK AMOUNT EXCEEDING PERIODIC BENEFIT MAXIMUM Q Claim Rebill:
Required if Patient Pay Amount (5Ø5-F5) includes amount
exceeding periodic benefit maximum.
346-HH BASIS OF CALCULATION—DISPENSING FEE Q Claim Rebill:
Required if Dispensing Status (343-HD) on submission is
“P” (Partial Fill) or “C” (Completion of Partial Fill).
347-HJ BASIS OF CALCULATION—COPAY Q Claim Rebill:
Required if Dispensing Status (343-HD) on submission is
“P” (Partial Fill) or “C” (Completion of Partial Fill).
348-HK BASIS OF CALCULATION—FLAT SALES TAX Q Claim Rebill:
Required if Dispensing Status (343-HD) on submission is
“P” (Partial Fill) or “C” (Completion of Partial Fill) and Flat
Sales Tax Amount Paid (558-AW) is greater than zero (Ø).
349-HM BASIS OF CALCULATION—PERCENTAGE SALES TAX Q Claim Rebill:
Required if Dispensing Status (343-HD) on submission is
“P” (Partial Fill) or “C” (Completion of Partial Fill) and
Percentage Sales Tax Amount Paid (559-AX) is greater
than zero (Ø).
571-NZ AMOUNT ATTRIBUTED TO PROCESSOR FEE Q Claim Rebill:
Required if the customer is responsible for 1ØØ% of the
prescription payment and when the provider net sale is less
than the amount the customer is expected to pay.
575-EQ PATIENT SALES TAX AMOUNT I Claim Rebill:
Used when necessary to identify the Patient’s portion of the
Sales Tax.
Provided for informational purposes only.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 269 -
RESPONSE PRICING SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
574-2Y PLAN SALES TAX AMOUNT I Claim Rebill:
Used when necessary to identify the Plan’s portion of the
Sales Tax.
Provided for informational purposes only.
572-4U AMOUNT OF COINSURANCE Q Claim Rebill:
Required if Patient Pay Amount (5Ø5-F5) includes
coinsurance as patient financial responsibility.
573-4V BASIS OF CALCULATION-COINSURANCE Q Claim Rebill:
Required if Dispensing Status (343-HD) on submission is
“P” (Partial Fill) or “C” (Completion of Partial Fill).
392-MU BENEFIT STAGE COUNT N Claim Rebill:
Not used.
393-MV BENEFIT STAGE QUALIFIER N***R*** Claim Rebill:
Not used.
394-MW BENEFIT STAGE AMOUNT N***R*** Claim Rebill:
Not used.
577-G3 ESTIMATED GENERIC SAVINGS I Claim Rebill:
This information should be provided when a patient
selected the brand drug and a generic form of the drug was
available. It will contain an estimate of the difference
between the cost of the brand drug and the generic drug,
when the brand drug is more expensive than the generic. It
is information that the provider should provide to the
patient.
128-UC SPENDING ACCOUNT AMOUNT REMAINING N Claim Rebill:
Not used.
129-UD HEALTH PLAN-FUNDED ASSISTANCE AMOUNT N Claim Rebill:
Not used.
133-UJ AMOUNT ATTRIBUTED TO PROVIDER NETWORK SELECTION Q Claim Rebill:
Required if Patient Pay Amount (5Ø5-F5) includes an
amount that is attributable to a cost share differential due to
the selection of one pharmacy over another.
134-UK AMOUNT ATTRIBUTED TO PRODUCT SELECTION/BRAND
DRUG
Q Claim Rebill:
Required if Patient Pay Amount (5Ø5-F5) includes an
amount that is attributable to a patient’s selection of a
Brand drug.
135-UM AMOUNT ATTRIBUTED TO PRODUCT SELECTION/NON-
PREFERRED FORMULARY SELECTION
Q Claim Rebill:
Required if Patient Pay Amount (5Ø5-F5) includes an
amount that is attributable to a patient’s selection of a non-
preferred formulary product.
136-UN AMOUNT ATTRIBUTED TO PRODUCT SELECTION/BRAND
NON-PREFERRED FORMULARY SELECTION
Q Claim Rebill:
Required if Patient Pay Amount (5Ø5-F5) includes an
amount that is attributable to a patient’s selection of a
Brand non-preferred formulary product.
137-UP AMOUNT ATTRIBUTED TO COVERAGE GAP Q Claim Rebill:
Required when the patient’s financial responsibility is due to
the coverage gap.
148-U8 INGREDIENT COST CONTRACTED/REIMBURSABLE AMOUNT N Claim Rebill:
Not used.
149-U9 DISPENSING FEE CONTRACTED/REIMBURSABLE AMOUNT N Claim Rebill:
Not used.
Notes on Response Pricing Segment on a Claim Rebill Response:
The Response Pricing Segment is situational for a Claim Rebill Response when the Header Response Status (5Ø1-F1) of "A" (Accepted) and
Transaction Response Status (112-AN) of “C” (Captured).
The Response Pricing Segment is not used in payer-to-payer transactions.
All dollar fields except Patient Pay Amount (5Ø5-F5) are estimated amounts. If actual amounts are returned on fields other than Patient Pay
Amount (5Ø5-F5), the “P” (Paid) response must be used.
If the Transaction Response Status (112-AN) = “C” (Captured) or “Q” (Duplicate of Captured), dollar fields should be supplied in the response.
• If the response is a “true” Capture (i.e. replacement of batch billing, with no edits or pricing), then corresponding response fields
should be populated with values as submitted. Ideally, processor should provide “real” patient financial responsibility values on a
Capture. If this is not possible, provider must know (by trading partner agreement) the patient financial responsibility to charge and
factor that into their system so collection occurs.
• If the response is captured by an Intermediary who can provide better pricing criteria, the corresponding response fields should be
populated with the probable values and those values used to determine estimated pricing as noted above. Since the claim has not
been fully adjudicated, this should remain a capture response.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 270 -
It is highly recommended that whenever possible, the individual dollar fields are returned in the response. On the response, the sender should
return the individual payment response fields to allow the receiver to reconcile against the requested payment fields.
See section “Response Processing Guidelines”, “Pricing Guidelines”.
Fields defined as Mandatory are required to be submitted when the segment is sent.
11.4.3.5.8 RESPONSE DUR/PPS SEGMENT (CLAIM REBILL) (TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
RESPONSE DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
567-J6 DUR/PPS RESPONSE CODE COUNTER Q***R*** Claim Rebill:
Maximum 9 occurrences supported.
Required if Reason For Service Code (439-E4) is used.
439-E4 REASON FOR SERVICE CODE Q***R*** Claim Rebill:
Required if utilization conflict is detected.
528-FS CLINICAL SIGNIFICANCE CODE Q***R*** Claim Rebill:
Required if needed to supply additional information for the
utilization conflict.
529-FT OTHER PHARMACY INDICATOR Q***R*** Claim Rebill:
Required if needed to supply additional information for the
utilization conflict.
53Ø-FU PREVIOUS DATE OF FILL Q***R*** Claim Rebill:
Required if needed to supply additional information for the
utilization conflict.
Required if Quantity of Previous Fill (531-FV) is used.
531-FV QUANTITY OF PREVIOUS FILL Q***R*** Claim Rebill:
Required if needed to supply additional information for the
utilization conflict.
Required if Previous Date Of Fill (53Ø-FU) is used.
532-FW DATABASE INDICATOR Q***R*** Claim Rebill:
Required if needed to supply additional information for the
utilization conflict.
533-FX OTHER PRESCRIBER INDICATOR Q***R*** Claim Rebill:
Required if needed to supply additional information for the
utilization conflict.
544-FY DUR FREE TEXT MESSAGE Q***R*** Claim Rebill:
Required if needed to supply additional information for the
utilization conflict.
57Ø-NS DUR ADDITIONAL TEXT Q***R*** Claim Rebill:
Required if needed to supply additional information for the
utilization conflict.
Notes on Response DUR/PPS Segment on a Claim Rebill Response:
The Response DUR/PPS Segment is situational for a Claim Rebill Response when the Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “C” (Captured). The segment is used to identify a drug utilization review or professional
pharmacy service event, opportunity, or information.
The Response DUR/PPS Segment is not used in payer-to-payer transactions.
Fields defined as Mandatory are required to be submitted when the segment is sent.
11.4.4 TRANSMISSION ACCEPTED/TRANSACTION REJECTED
11.4.4.1 DIAGRAM FOR TRANSMISSION OF ONE CLAIM REBILL RESPONSE (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
Claim Rebill transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “R” (Rejected)
Claim Rebill transactions - If the claim rebill reversal is not processed successfully, a “R” (Rejected) response must be sent.
The Response DUR/PPS Segment and Response Prior Authorization Segments are not used in payer-to-payer transactions.
The duplicate response codes for the Claim Rebill transaction are not applicable. Therefore, in this case, there are no transaction-level
situational segments.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 271 -
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Prior Authorization Segment
11.4.4.2 DIAGRAM FOR TRANSMISSION OF TWO CLAIM REBILL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Prior Authorization Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Prior Authorization Segment
11.4.4.3 DIAGRAM FOR TRANSMISSION OF THREE CLAIM REBILL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 272 -
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Prior Authorization Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Prior Authorization Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Prior Authorization Segment
11.4.4.4 DIAGRAM FOR TRANSMISSION OF FOUR CLAIM REBILL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Prior Authorization Segment
Mandatory second response
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 273 -
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Prior Authorization Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Prior Authorization Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Prior Authorization Segment
11.4.4.5 CLAIM REBILL RESPONSE SEGMENTS (TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
11.4.4.5.1 RESPONSE HEADER SEGMENT (CLAIM REBILL) (TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M For Transaction Code of “B3”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “1” (Rx Billing).
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Claim Rebill Response:
The Response Header Segment is a mandatory, fixed length segment for Claim Rebill response when the Header Response Status (5Ø1-F1)
of "A" (Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). The “Situation” column is not applicable.
11.4.4.5.2 RESPONSE MESSAGE SEGMENT (CLAIM REBILL) (TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Claim Rebill:
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 274 -
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Claim Rebill Response:
The Response Message Segment is situational for Claim Rebill response when the Header Response Status (5Ø1-F1) of "A" (Accepted) and
Transaction Response Status (112-AN) of “R” (Rejected). It is used when additional text information needs to be sent. Fields defined as
Mandatory are required to be submitted when the segment is sent.
11.4.4.5.3 RESPONSE INSURANCE SEGMENT (CLAIM REBILL) (TRANSMISSION ACCEPTED/TRANSACTION
REJECTED)
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø1-C1 GROUP ID
Q Claim Rebill:
Required if needed to identify the actual cardholder or
employer group, to identify appropriate group number,
when available.
Required to identify the actual group that was used when
multiple group coverages exist.
Note: This field may contain the Group ID echoed from the
request. May contain the actual Group ID if unknown to the
receiver.
524-FO PLAN ID
Q Claim Rebill:
Required if needed to identify the actual plan parameters,
benefit, or coverage criteria, when available.
Required to identify the actual plan ID that was used when
multiple group coverages exist.
Required if needed to contain the actual plan ID if unknown
to the receiver.
545-2F NETWORK REIMBURSEMENT ID
Q Claim Rebill:
Required if needed to identify the network for the covered
member.
Required if needed to identify the actual Network
Reimbursement ID, when applicable and/or available.
Required to identify the actual Network Reimbursement ID
that was used when multiple Network Reimbursement IDs
exist.
568-J7 PAYER ID QUALIFIER Q Claim Rebill:
Required if Payer ID (569-J8) is used.
569-J8 PAYER ID
Q Claim Rebill:
Required to identify the ID of the payer responding.
115-N5
MEDICAID ID NUMBER N Claim Rebill:
Not used.
116-N6
MEDICAID AGENCY NUMBER N Claim Rebill:
Not used.
3Ø2-C2
CARDHOLDER ID Q Claim Rebill:
Required if the identification to be used in future
transactions is different than what was submitted on the
request.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 275 -
Notes on Response Insurance Segment on a Claim Rebill Response:
The Response Insurance Segment is situational for Claim Rebill response when the Header Response Status (5Ø1-F1) of "A" (Accepted) and
Transaction Response Status (112-AN) of “R” (Rejected). It is used when coverage or reimbursement parameters or identifiers need to be
sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
11.4.4.5.3.1 Response Insurance Segment (Medicaid Subrogation Claim Rebill) (Transmission
Accepted/Transaction Rejected)
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
115-N5
MEDICAID ID NUMBER QM Medicaid Subrogation - Claim Rebill:
Required to identify the member as uniquely known to
Medicaid.
116-N6
MEDICAID AGENCY NUMBER QM Medicaid Subrogation - Claim Rebill:
Required to identify the Medicaid agency.
See Medicaid Subrogation Implementation Guide.
Notes on Response Insurance Segment on a Medicaid Subrogation Claim Rebill Response:
The rules above for a “Response Insurance Segment (Claim Rebill)” are to be followed for Medicaid Subrogation. Specific fields that
are used differently in Medicaid Subrogation are noted in the table above.
11.4.4.5.4 RESPONSE PATIENT SEGMENT (CLAIM REBILL) (TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
RESPONSE PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
31Ø-CA PATIENT FIRST NAME Q Claim Rebill:
Required if known.
311-CB PATIENT LAST NAME Q Claim Rebill:
Required if known.
3Ø4-C4 DATE OF BIRTH Q Claim Rebill:
Required if known.
Notes on Response Patient Segment on a Claim Rebill Response:
The Response Patient Segment is situational for Claim Rebill transmission response Header Response Status (5Ø1-F1) of "A" (Accepted) and
Transaction Response Status (112-AN) of “R” (Rejected) when patient demographic information needs to be sent from the sender to the
receiver. Fields defined as Mandatory are required to be submitted when the segment is sent.
11.4.4.5.5 RESPONSE STATUS SEGMENT (CLAIM REBILL) (TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Claim Rebill:
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT R Claim Rebill:
Maximum count of 5.
Required.
511-FB REJECT CODE R***R*** Claim Rebill:
Required.
546-4F REJECT FIELD OCCURRENCE INDICATOR Q***R*** Claim Rebill:
Required if a repeating field is in error, to identify repeating
field occurrence.
This field must be sent when relaying error information about
a repeating field or set. Note, if the Reject Code is not
denoting a repeating field or set, the Reject Field Occurrence
Indicator must not be sent.
547-5F APPROVED MESSAGE CODE COUNT N Claim Rebill:
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Claim Rebill:
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Claim Rebill:
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 276 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Claim Rebill:
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Claim Rebill:
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Claim Rebill:
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Claim Rebill:
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Claim Rebill:
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Claim Rebill:
Not used.
993-A7 INTERNAL CONTROL NUMBER N Claim Rebill:
Not used.
987-MA URL I Claim Rebill:
Provided for informational purposes only to relay health
care communications via the Internet.
Notes on Response Status Segment on a Claim Rebill Response:
The Response Status Segment is mandatory for a Claim Rebill response when the Header Response Status (5Ø1-F1) of "A" (Accepted) and
Transaction Response Status (112-AN) of “R” (Rejected). The Response Status Segment is sent from the sender to the receiver to identify the
outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.
11.4.4.5.6 RESPONSE CLAIM SEGMENT (CLAIM REBILL) (TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M For Transaction Code of “B3”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “1” (Rx Billing).
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT Q Claim Rebill:
Maximum count of 6.
Required if Preferred Product ID (553-AR) is used.
Not used in payer-to-payer transactions.
552-AP PREFERRED PRODUCT ID QUALIFIER Q***R*** Claim Rebill:
Required if Preferred Product ID (553-AR) is used.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 277 -
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Not used in payer-to-payer transactions.
553-AR PREFERRED PRODUCT ID Q***R*** Claim Rebill:
Required if a product preference exists that needs to be
communicated to the receiver via an ID.
Not used in payer-to-payer transactions.
554-AS PREFERRED PRODUCT INCENTIVE Q***R*** Claim Rebill:
Required if there is a known incentive amount associated
with the Preferred Product ID (553-AR) and/or Preferred
Product Description (556-AU).
Not used in payer-to-payer transactions.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE Q***R*** Claim Rebill:
Required if there is a known patient financial responsibility
incentive amount associated with the Preferred Product ID
(553-AR) and/or Preferred Product Description (556-AU).
Not used in payer-to-payer transactions.
556-AU PREFERRED PRODUCT DESCRIPTION Q***R*** Claim Rebill:
Required if a product preference exists that either cannot
be communicated by the Preferred Product ID (553-AR) or
to clarify the Preferred Product ID (553-AR).
Not used in payer-to-payer transactions.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Claim Rebill:
Not used.
Notes on Response Claim Segment on a Claim Rebill Response:
The Response Claim Segment is mandatory for a Claim Rebill response when the Header Response Status (5Ø1-F1) of "A" (Accepted) and
Transaction Response Status (112-AN) of “R” (Rejected). The Response Claim Segment is sent from the sender to the receiver to identify
therapeutic or alternate product recommendations. Fields defined as Mandatory are required to be submitted when the segment is sent.
11.4.4.5.7 RESPONSE DUR/PPS SEGMENT (CLAIM REBILL) (TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
RESPONSE DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
567-J6 DUR/PPS RESPONSE CODE COUNTER Q***R*** Claim Rebill:
Maximum 9 occurrences supported.
Required if Reason For Service Code (439-E4) is used.
439-E4 REASON FOR SERVICE CODE Q***R*** Claim Rebill:
Required if utilization conflict is detected.
528-FS CLINICAL SIGNIFICANCE CODE Q***R*** Claim Rebill:
Required if needed to supply additional information for the
utilization conflict.
529-FT OTHER PHARMACY INDICATOR Q***R*** Claim Rebill:
Required if needed to supply additional information for the
utilization conflict.
53Ø-FU PREVIOUS DATE OF FILL Q***R*** Claim Rebill:
Required if needed to supply additional information for the
utilization conflict.
Required if Quantity of Previous Fill (531-FV) is used.
531-FV QUANTITY OF PREVIOUS FILL Q***R*** Claim Rebill:
Required if needed to supply additional information for the
utilization conflict.
Required if Previous Date Of Fill (53Ø-FU) is used.
532-FW DATABASE INDICATOR Q***R*** Claim Rebill:
Required if needed to supply additional information for the
utilization conflict.
533-FX OTHER PRESCRIBER INDICATOR Q***R*** Claim Rebill:
Required if needed to supply additional information for the
utilization conflict.
544-FY DUR FREE TEXT MESSAGE Q***R*** Claim Rebill:
Required if needed to supply additional information for the
utilization conflict.
57Ø-NS DUR ADDITIONAL TEXT Q***R*** Claim Rebill:
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 278 -
RESPONSE DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if needed to supply additional information for the
utilization conflict.
Notes on Response DUR/PPS Segment on a Claim Rebill Response:
The Response DUR/PPS Segment is situational for a Claim Rebill Response when the Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “R” (Rejected). The segment is used to identify a drug utilization review or professional
pharmacy service event, opportunity, or information.
The Response DUR/PPS Segment is not used on payer-to-payer transactions.
Fields defined as Mandatory are required to be submitted when the segment is sent.
11.4.4.5.8 RESPONSE PRIOR AUTHORIZATION SEGMENT (CLAIM REBILL) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE PRIOR AUTHORIZATION SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
498-PR PRIOR AUTHORIZATION PROCESSED DATE N Claim Rebill:
Not used.
498-PS PRIOR AUTHORIZATION EFFECTIVE DATE N Claim Rebill:
Not used.
498-PT PRIOR AUTHORIZATION EXPIRATION DATE N Claim Rebill:
Not used.
498-RA PRIOR AUTHORIZATION QUANTITY N Claim Rebill:
Not used.
498-RB PRIOR AUTHORIZATION DOLLARS AUTHORIZED N Claim Rebill:
Not used.
498-PW PRIOR AUTHORIZATION NUMBER OF REFILLS AUTHORIZED N Claim Rebill:
Not used.
498-PX PRIOR AUTHORIZATION QUANTITY ACCUMULATED N Claim Rebill:
Not used.
498-PY PRIOR AUTHORIZATION NUMBER–ASSIGNED Q Claim Rebill:
Required when the receiver must submit this Prior
Authorization Number in order to receive payment for the
claim. (An example of a situation may include a Benefit
Transition Period that allows for payment of claims, for a
period of time that would normally reject.)
Notes on Response Prior Authorization Segment on a Claim Rebill Response:
The Response Prior Authorization Segment is situational for a Claim Rebill response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). It is used to relay the Prior Authorization Number - Assigned (498-
PY) which is returned when a Reject Code (511-FB) denotes that a prior authorization code needs to be submitted on the subsequent billing.
The Response Prior Authorization Segment is not used on payer-to-payer transactions.
Fields defined as Mandatory are required to be submitted when the segment is sent.
11.4.4.5.9 RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS SEGMENT (CLAIM REBILL) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS
SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
355-NT OTHER PAYER ID COUNT M Claim Rebill:
Maximum count of 3.
338-5C OTHER PAYER COVERAGE TYPE M***R***
339-6C OTHER PAYER ID QUALIFIER Q***R*** Claim Rebill:
Required if Other Payer ID (34Ø-7C) is used.
34Ø-7C OTHER PAYER ID Q***R*** Claim Rebill:
Required if other insurance information is available for
coordination of benefits.
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER Q***R*** Claim Rebill:
Required if other insurance information is available for
coordination of benefits.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 279 -
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS
SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
356-NU OTHER PAYER CARDHOLDER ID Q***R*** Claim Rebill:
Required if other insurance information is available for
coordination of benefits.
992-MJ OTHER PAYER GROUP ID Q***R*** Claim Rebill:
Required if other insurance information is available for
coordination of benefits.
142-UV OTHER PAYER PERSON CODE Q***R*** Claim Rebill:
Required if needed to uniquely identify the family members
within the Cardholder ID, as assigned by the other payer.
127-UB OTHER PAYER HELP DESK PHONE NUMBER Q***R*** Claim Rebill:
Required if needed to provide a support telephone number
of the other payer to the receiver.
143-UW OTHER PAYER PATIENT RELATIONSHIP CODE Q***R*** Claim Rebill:
Required if needed to uniquely identify the relationship of
the patient to the cardholder ID, as assigned by the other
payer.
144-UX OTHER PAYER BENEFIT EFFECTIVE DATE Q***R*** Claim Rebill:
Required when other coverage is known which is after the
Date of Service submitted.
145-UY OTHER PAYER BENEFIT TERMINATION DATE Q***R*** Claim Rebill:
Required when other coverage is known which is after the
Date of Service submitted.
Notes on Response Coordination of Benefits/Other Payers Segment on a Claim Rebill Response:
The Response Coordination of Benefits/Other Payers Segment is situational for a Claim Rebill response when the Header Response Status
(5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “R” (Rejected) when other insurance information is available for
coordination of benefits.
1. If the identity of the patient is partially verified and the Claim Billing or Encounter is rejected due to a non-match of field verification,
then the Other Payer information is not sent.
2. If the claim is rejected because it should be submitted to other payer(s) first, that Other Payer information should be sent, if known.
3. If the claim is rejected due to benefit design limitations, then subsequent Other Payer information should be sent, if known.
If the claim rejects for other reasons than above, Other Payer information is not sent.
If additional payer(s) for this patient is not known, the Other Payer information is not sent.
If additional payer(s) for this patient is known, the following may be sent:
• Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C),
• Other Payer Group ID (992-MJ),
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU)
• And other Other Payer fields.
In addition, if any of the following three fields are sent:
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU),
• Other Payer Group ID (992-MJ),
then the Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C) must be sent.
Fields defined as Mandatory are required to be submitted when the segment is sent.
11.4.5 TRANSMISSION REJECTED/TRANSACTION REJECTED
11.4.5.1 DIAGRAM FOR TRANSMISSION OF ONE CLAIM REBILL RESPONSE (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
Claim Rebill transmission response Header Response Status (5Ø1-F1) of "R" (Rejected)
and Transaction Response Status (112-AN) of “R” (Rejected)
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 280 -
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
11.4.5.2 DIAGRAM FOR TRANSMISSION OF TWO CLAIM REBILL RESPONSES (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
11.4.5.3 DIAGRAM FOR TRANSMISSION OF THREE CLAIM REBILL RESPONSES (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
11.4.5.4 DIAGRAM FOR TRANSMISSION OF FOUR CLAIM REBILL RESPONSES (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Mandatory second response
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 281 -
Group Separator
Segment Separator
Response Status Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
11.4.5.5 CLAIM REBILL RESPONSE SEGMENTS (TRANSMISSION REJECTED/TRANSACTION REJECTED)
11.4.5.5.1 RESPONSE HEADER SEGMENT (CLAIM REBILL) (TRANSMISSION REJECTED/TRANSACTION REJECTED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Claim Rebill Response:
The Response Header Segment is a mandatory, fixed length segment for Claim Rebill response when the Header Response Status (5Ø1-F1)
of "R" (Rejected) and Transaction Response Status (112-AN) of “R” (Rejected). The “Situation” column is not applicable.
If either the entire transmission or the Header is in error, the Header Response Status (5Ø1-F1) = “R” (Rejected). Every identifiable transaction
within the transmission must be rejected with an “R”.
If the transaction rejects for detail errors, the Header Response Status (5Ø1-F1) = “A” (Accepted) and the Transaction Response Status (112-
AN) will be “R”.
11.4.5.5.2 RESPONSE MESSAGE SEGMENT (CLAIM REBILL) (TRANSMISSION REJECTED/TRANSACTION REJECTED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Claim Rebill:
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Claim Rebill Response:
The Response Message Segment is situational for a Claim Rebill response when the Header Response Status (5Ø1-F1) of "R" (Rejected) and
Transaction Response Status (112-AN) of “R” (Rejected). It is used when additional text information needs to be sent. Fields defined as
Mandatory are required to be submitted when the segment is sent.
11.4.5.5.3 RESPONSE STATUS SEGMENT (CLAIM REBILL) (TRANSMISSION REJECTED/TRANSACTION REJECTED)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 282 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER
Q Claim Rebill:
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT
R Claim Rebill:
Maximum count of 5.
Required.
511-FB REJECT CODE
R***R*** Claim Rebill:
Required.
546-4F REJECT FIELD OCCURRENCE INDICATOR
Q***R*** Claim Rebill:
Required if a repeating field is in error, to identify repeating
field occurrence.
This field must be sent when relaying error information about
a repeating field or set. Note, if the Reject Code is not
denoting a repeating field or set, the Reject Field Occurrence
Indicator must not be sent.
547-5F APPROVED MESSAGE CODE COUNT N Claim Rebill:
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Claim Rebill:
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Claim Rebill:
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Claim Rebill:
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Claim Rebill:
Required if additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Claim Rebill:
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER
Q Claim Rebill:
Required if Help Desk Phone Number (55Ø-F8) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Claim Rebill:
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Claim Rebill:
Not used.
993-A7 INTERNAL CONTROL NUMBER N Claim Rebill:
Not used.
987-MA URL N Claim Rebill:
Not used.
Notes on Response Status Segment on a Claim Rebill Response:

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 283 -
The Response Status Segment is mandatory for a Claim Rebill Response when the Header Response Status (5Ø1-F1) = “R” (Rejected) and
Transaction Response Status (112-AN) of “R” (Rejected). The Response Status Segment is sent from the sender to the receiver to identify the
outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.
11.5 SERVICE REBILL REQUEST DIAGRAMS
For Transaction Code of “S3”, in the Claim Segment or Response Claim Segment, the Prescription/Service Reference Number Qualifier (455-
EM) is “2” (Service Billing).
If the Product/Service ID Qualifier (436-E1) is “Ø6” (DUR/PPS), the DUR/PPS Segment is required.
11.5.1.1 DIAGRAM FOR TRANSMISSION OF ONE SERVICE REBILL TRANSACTION
Service Rebill to a Receiver
Service Rebill Paid/Captured/Rejected Transaction Response from a Sender
Standard Transmission Rejected Response from a Sender
Up to four (4) rebill transactions are allowed in one transmission.
Mandatory
Transaction Header Segment
Segment Separator
Insurance Segment
Situational
Segment Separator
Patient Segment
Mandatory - first Service Rebill transaction
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
11.5.1.2 DIAGRAM FOR TRANSMISSION OF TWO SERVICE REBILL TRANSACTIONS
Mandatory
Transaction Header Segment
Segment Separator
Insurance Segment
Situational
Segment Separator
Patient Segment
Mandatory - first Service Rebill transaction
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 284 -
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
Mandatory - second Service Rebill transaction
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
11.5.1.3 DIAGRAM FOR TRANSMISSION OF THREE SERVICE REBILL TRANSACTIONS
Mandatory
Transaction Header Segment
Segment Separator
Insurance Segment
Situational
Segment Separator
Patient Segment
Mandatory - first Service Rebill transaction
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 285 -
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
Mandatory - second Service Rebill transaction
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
Mandatory - third Service Rebill transaction
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
11.5.1.4 DIAGRAM FOR TRANSMISSION OF FOUR SERVICE REBILL TRANSACTIONS
Mandatory
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 286 -
Transaction Header Segment
Segment Separator
Insurance Segment
Situational
Segment Separator
Patient Segment
Mandatory - first Service Rebill transaction
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
Mandatory - second Service Rebill transaction
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
Mandatory - third Service Rebill transaction
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 287 -
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
Mandatory - fourth Service Rebill transaction
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
11.6 SERVICE REBILL REQUEST SEGMENTS
11.6.1 TRANSACTION HEADER SEGMENT (SERVICE REBILL)
TRANSACTION HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø1-A1 BIN NUMBER M
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M For Transaction Code of “S3”, in the Claim Segment, the
Prescription/Service Reference Number Qualifier (455-EM)
is “2” (Service Billing).
1Ø4-A4 PROCESSOR CONTROL NUMBER M
1Ø9-A9 TRANSACTION COUNT M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M
Notes on Transaction Header Segment on a Service Rebill Request:
The Transaction Header Segment is a mandatory, fixed length segment for a Service Rebill request. The “Situation” column is not applicable.
11.6.2 INSURANCE SEGMENT (SERVICE REBILL)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 288 -
INSURANCE SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø2-C2 CARDHOLDER ID M
312-CC CARDHOLDER FIRST NAME
Q Service Rebill:
Required if necessary for state/federal/regulatory agency
programs when the cardholder has a first name.
313-CD CARDHOLDER LAST NAME
Q Service Rebill:
Required if necessary for state/federal/regulatory agency
programs.
314-CE HOME PLAN
Q Service Rebill:
Required if needed for receiver billing validation and/or
determination for Blue Cross or Blue Shield, if a Patient has
coverage under more than one plan, to distinguish each
plan.
524-FO PLAN ID
O Service Rebill:
Optional.
3Ø9-C9 ELIGIBILITY CLARIFICATION CODE
Q Service Rebill:
Required if needed for receiver inquiry validation and/or
determination, when eligibility is not maintained at the
dependent level. Required in special situations as defined
by the code to clarify the eligibility of an individual, which
may extend coverage.
3Ø1-C1 GROUP ID
Q Service Rebill:
Required if necessary for state/federal/regulatory agency
programs.
Required if needed for pharmacy claim processing and
payment.
Required if needed to match the reversal to the original
billing transaction.
3Ø3-C3 PERSON CODE
Q Service Rebill:
Required if needed to uniquely identify the family members
within the Cardholder ID.
3Ø6-C6 PATIENT RELATIONSHIP CODE
Q Service Rebill:
Required if needed to uniquely identify the relationship of
the Patient to the Cardholder ID.
99Ø-MG OTHER PAYER BIN NUMBER N Service Rebill:
Not used.
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER N Service Rebill:
Not used.
356-NU OTHER PAYER CARDHOLDER ID N Service Rebill:
Not used.
992-MJ OTHER PAYER GROUP ID N Service Rebill:
Not used.
359-2A MEDIGAP ID Q Service Rebill:
Required, if known, when patient has Medigap coverage.
36Ø-2B MEDICAID INDICATOR Q Service Rebill:
Required, if known, when patient has Medicaid coverage.
361-2D PROVIDER ACCEPT ASSIGNMENT INDICATOR Q Service Rebill:
Required if necessary for state/federal/regulatory agency
programs.
997-G2 CMS PART D DEFINED QUALIFIED FACILITY N Service Rebill:
Not used.
115-N5
MEDICAID ID NUMBER N Service Rebill:
Not used.
116-N6
MEDICAID AGENCY NUMBER N Service Rebill:
Not used.
Notes on Insurance Segment on a Service Rebill Request:
The Insurance Segment is mandatory for a Service Rebill request. Fields defined as Mandatory are required to be submitted when the
segment is sent.
11.6.3 PATIENT SEGMENT (SERVICE REBILL)
PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
331-CX PATIENT ID QUALIFIER Q Service Rebill:
Required if Patient ID (332-CY) is used.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 289 -
PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
332-CY PATIENT ID Q Service Rebill:
Required if necessary for state/federal/regulatory agency
programs to validate dual eligibility.
3Ø4-C4 DATE OF BIRTH
R Service Rebill:
Required.
3Ø5-C5 PATIENT GENDER CODE
R Service Rebill:
Required.
31Ø-CA PATIENT FIRST NAME
Q Service Rebill:
Required when the patient has a first name.
311-CB PATIENT LAST NAME
R Service Rebill:
Required.
322-CM PATIENT STREET ADDRESS
O Service Rebill:
Optional.
323-CN PATIENT CITY ADDRESS
O Service Rebill:
Optional.
324-CO PATIENT STATE / PROVINCE ADDRESS
O Service Rebill:
Optional.
325-CP PATIENT ZIP/POSTAL ZONE
O Service Rebill:
Optional.
326-CQ PATIENT PHONE NUMBER
O Service Rebill:
Optional.
3Ø7-C7 PLACE OF SERVICE
Q Service Rebill:
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
333-CZ EMPLOYER ID
Q Service Rebill:
Required if “required by law” as defined in the HIPAA final
Privacy regulations section 164.5Ø1 definitions (45 CFR
Parts 160 and 164 Standards for Privacy of Individually
Identifiable Health Information; Final Rule -
Thursday, December 28, 2000, page 82803 and following,
and Wednesday, August 14, 2002, page 53267 and
following.)
Required if needed for Workers’ Compensation billing.
334-1C SMOKER / NON-SMOKER CODE
N Service Rebill:
Not used.
335-2C PREGNANCY INDICATOR
Q Service Rebill:
Required if pregnancy could result in different coverage,
pricing, or patient financial responsibility.
Required if “required by law” as defined in the HIPAA final
Privacy regulations section 164.5Ø1 definitions (45 CFR
Parts 160 and 164 Standards for Privacy of Individually
Identifiable Health Information; Final Rule -
Thursday, December 28, 2000, page 82803 and following,
and Wednesday, August 14, 2002, page 53267 and
following.)
35Ø-HN PATIENT E-MAIL ADDRESS I Service Rebill:
May be submitted for the receiver to relay patient health
care communications via the Internet when provided by the
patient.
This field is informational only.
384-4X PATIENT RESIDENCE Q Service Rebill:
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
Notes on Patient Segment on a Service Rebill Request:
The Patient Segment is situational for a Service Rebill request. It is used when a receiver needs some of the patient demographic information
to perform eligibility and service billing determination. The Patient Segment must be submitted when needed to differentiate between the
patient and the cardholder. If the cardholder and the patient are the same, then the Patient Segment is not submitted unless additional
information about the patient is needed to clarify the Service Rebill determination. The Segment is mandatory if required under provider payer
contract or mandatory on service billings where this information is necessary for adjudication of the service. Fields defined as Mandatory are
required to be submitted when the segment is sent.
11.6.4 CLAIM SEGMENT (SERVICE REBILL)
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 290 -
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M For Transaction Code of “S3”, in the Claim Segment, the
Prescription/Service Reference Number Qualifier (455-EM)
is “2” (Service Billing).
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
436-E1 PRODUCT/SERVICE ID QUALIFIER M Mandatory.
If the Product/Service ID Qualifier (436-E1) = “Ø6”
(DUR/PPS), the Product/Service ID (4Ø7-D7) is zero. (Zero
means “Ø”.)
4Ø7-D7 PRODUCT/SERVICE ID M Service Rebill:
If the Product/Service ID Qualifier (436-E1) = “Ø6”
(DUR/PPS), the Product/Service ID (4Ø7-D7) is zero. (Zero
means “Ø”.) Populate the DUR/PPS segment as
appropriate.
If the Product/Service ID Qualifier (436-E1) = “Ø7” (CPT-4),
the Product Service ID (4Ø7-D7) is the actual CPT-4 value.
If the Product/Service ID Qualifier (436-E1) = “Ø9”
(HCPCS), the Product Service ID (4Ø7-D7) is the actual
HCPCS value.
If the Product/Service ID Qualifier (436-E1) = “99” (Other),
the Product Service ID (4Ø7-D7) is the business partner
agreed value.
456-EN ASSOCIATED PRESCRIPTION/SERVICE REFERENCE NUMBER
Q Service Rebill:
Required if needed to associate multiple
prescriptions/services from the same sender to allow billing
of the current prescription/service.
457-EP ASSOCIATED PRESCRIPTION/SERVICE DATE
Q Service Rebill:
Required if Associated Prescription/Service Reference
Number (456-EN) is used.
Required if needed to associate multiple
prescriptions/services from the same sender to allow billing
of the current prescription/service.
458-SE PROCEDURE MODIFIER CODE COUNT
Q Service Rebill:
Maximum count of 1Ø.
Required if Procedure Modifier Code (459-ER) is used.
459-ER PROCEDURE MODIFIER CODE
Q***R*** Service Rebill:
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
Occurs the number of times identified in Procedure Modifier
Code Count (458-SE).
Required to define a further level of specificity if the
Product/Service ID (4Ø7-D7) indicated a Procedure Code
was submitted.
442-E7 QUANTITY DISPENSED
Q Service Rebill:
Required if value is greater than zero (Ø).
4Ø3-D3 FILL NUMBER Q Service Rebill:
Required if necessary for plan benefit administration. This
field must match the Fill Number of the original billing.
4Ø5-D5 DAYS SUPPLY Q Service Rebill:
Required if necessary for plan benefit administration.
4Ø6-D6 COMPOUND CODE N Service Rebill:
Not used.
4Ø8-D8 DISPENSE AS WRITTEN (DAW)/PRODUCT SELECTION CODE N Service Rebill:
Not used.
414-DE DATE PRESCRIPTION WRITTEN
Q Service Rebill:
Required if necessary for plan benefit administration.
415-DF NUMBER OF REFILLS AUTHORIZED Q Service Rebill:
Required if necessary for plan benefit administration.
419-DJ PRESCRIPTION ORIGIN CODE N Service Rebill:
Not used.
354-NX SUBMISSION CLARIFICATION CODE COUNT N Service Rebill:
Not used.
42Ø-DK SUBMISSION CLARIFICATION CODE N***R*** Service Rebill:
Not used.
46Ø-ET QUANTITY PRESCRIBED
Q Service Rebill:
Required if the prescriber orders a specific number of

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 291 -
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
iterations of a service.
Not required if value is equal to 1.
3Ø8-C8 OTHER COVERAGE CODE
Q Service Rebill:
Required if needed by receiver to match the claim that is
being reversed.
Required if needed by receiver, to communicate a
summation of other coverage information that has been
collected from other payers.
Required for Coordination of Benefits.
See section “Specific Segment Discussion”, “Request
Segments”, “Claim Segment”, “Other Coverage Code (3Ø8-
C8).
429-DT SPECIAL PACKAGING INDICATOR N Service Rebill:
Not used.
453-EJ ORIGINALLY PRESCRIBED PRODUCT/SERVICE ID QUALIFIER Q Service Rebill:
Required if Originally Prescribed Product/Service Code
(445-EA) is used.
445-EA ORIGINALLY PRESCRIBED PRODUCT/SERVICE CODE
Q Service Rebill:
Required if the receiver requests association to a
therapeutic, or a preferred product substitution, or when a
DUR alert has been resolved by changing medications, or
an alternative service than what was originally prescribed.
446-EB ORIGINALLY PRESCRIBED QUANTITY
Q Service Rebill:
Required if the receiver requests reporting for quantity
changes due to a therapeutic substitution that has occurred
or a preferred product/service substitution that has
occurred, or when a DUR alert has been resolved by
changing quantities.
33Ø-CW ALTERNATE ID
N Service Rebill:
Not used.
454-EK SCHEDULED PRESCRIPTION ID NUMBER N Service Rebill:
Not used.
6ØØ-28 UNIT OF MEASURE N Service Rebill:
Not used.
418-DI LEVEL OF SERVICE
Q Service Rebill:
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
461-EU PRIOR AUTHORIZATION TYPE CODE
Q Service Rebill:
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
462-EV PRIOR AUTHORIZATION NUMBER SUBMITTED
Q Service Rebill:
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
463-EW INTERMEDIARY AUTHORIZATION TYPE ID
Q Service Rebill:
Required for overriding an authorized intermediary system
edit when the pharmacy participates with an intermediary.
Required if Intermediary Authorization ID (464-EX) is used.
Not used for payer-to-payer transactions.
464-EX INTERMEDIARY AUTHORIZATION ID
Q Service Rebill:
Required for overriding an authorized intermediary system
edit when the pharmacy participates with an intermediary.
Not used for payer-to-payer transactions.
343-HD DISPENSING STATUS N Service Rebill:
Not used.
344-HF QUANTITY INTENDED TO BE DISPENSED N Service Rebill:
Not used.
345-HG DAYS SUPPLY INTENDED TO BE DISPENSED N Service Rebill:
Not used.
357-NV DELAY REASON CODE Q Service Rebill:
Required when needed to specify the reason that
submission of the transaction has been delayed.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Service Rebill:
Not used.
391-MT PATIENT ASSIGNMENT INDICATOR (DIRECT MEMBER
REIMBURSEMENT INDICATOR)
Q Service Rebill:
Required when the claims adjudicator does not assume the
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 292 -
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
patient assigned his/her benefits to the provider or when
the claims adjudicator supports a patient determination of
whether he/she wants to assign or retain his/her benefits.
995-E2 ROUTE OF ADMINISTRATION N Service Rebill:
Not used.
996-G1 COMPOUND TYPE N Service Rebill:
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Service Rebill:
Not used.
147-U7 PHARMACY SERVICE TYPE Q Service Rebill:
Required when the submitter must clarify the type of
services being performed as a condition for proper
reimbursement by the payer.
Notes on Claim Segment on a Service Rebill Request:
The Claim Segment is mandatory for a Service Rebill request. The Claim Segment defines the service performed, reference information for
tieback to an original prescription or service, or authorization information.
If the Prescription/Service Reference Number Qualifier (455-EM) is “2” (Service Billing) and the Product/Service ID Qualifier (436-E1) is “Ø6”
(DUR/PPS), the DUR/PPS Segment is required.
Fields defined as Mandatory are required to be submitted when the segment is sent.
11.6.5 PRICING SEGMENT (SERVICE REBILL)
PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
4Ø9-D9 INGREDIENT COST SUBMITTED
N Service Rebill:
Not used.
412-DC DISPENSING FEE SUBMITTED
N Service Rebill:
Not used.
477-BE PROFESSIONAL SERVICE FEE SUBMITTED
R Service Rebill:
Required.
433-DX PATIENT PAID AMOUNT SUBMITTED
Q Service Rebill:
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
Not used in coordination of benefit claim to pass patient
liability information to a downstream payer. See section
“Standard Conventions”, “Repetition and Multiple
Occurrences”, Repeating Data Elements”, “Request
Segments”, “Coordination of Benefits/Other Payments
Segment”.
438-E3 INCENTIVE AMOUNT SUBMITTED
N Service Rebill:
Not used.
478-H7 OTHER AMOUNT CLAIMED SUBMITTED COUNT
Q Service Rebill:
Maximum count of 3.
Required if Other Amount Claimed Submitted Qualifier
(479-H8) is used.
479-H8 OTHER AMOUNT CLAIMED SUBMITTED QUALIFIER
Q***R*** Service Rebill:
Required if Other Amount Claimed Submitted (48Ø-H9) is
used.
48Ø-H9 OTHER AMOUNT CLAIMED SUBMITTED
Q***R*** Service Rebill:
Required if its value has an effect on the Gross Amount
Due (43Ø-DU) calculation.
Zero (Ø) is a valid value.
481-HA FLAT SALES TAX AMOUNT SUBMITTED
Q Service Rebill:
Required if its value has an effect on the Gross Amount
Due (43Ø-DU) calculation.
Zero (Ø) is a valid value.
482-GE PERCENTAGE SALES TAX AMOUNT SUBMITTED
Q Service Rebill:
Required if its value has an effect on the Gross Amount
Due (43Ø-DU) calculation.
Zero (Ø) is a valid value.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 293 -
PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
483-HE PERCENTAGE SALES TAX RATE SUBMITTED
Q Service Rebill:
Required if needed to calculate Percentage Sales Tax
Amount Paid (559-AX).
484-JE PERCENTAGE SALES TAX BASIS SUBMITTED
N Service Rebill:
Not used. Code list is not applicable.
426-DQ USUAL AND CUSTOMARY CHARGE
Q Service Rebill:
Required if needed per trading partner agreement.
43Ø-DU GROSS AMOUNT DUE
R Service Rebill:
Required.
See Pricing Formula for fields used in calculation.
423-DN BASIS OF COST DETERMINATION N Service Rebill:
Not used.
113-N3
MEDICAID PAID AMOUNT N Service Rebill:
Not used.
Notes on Pricing Segment on a Service Rebill Request:
The Pricing Segment is mandatory for a Service Rebill request. The Pricing Segment defines dollar amounts for a Service Rebill.
It is highly recommended that whenever possible, the individual dollar fields are to be requested of the sender by the receiver. On the
response, the sender should return the individual payment response fields to allow the receiver to reconcile against the requested payment
fields. It is recommended that for the dollar fields, if the field is not required or situational in the calculation, that the dollar fields are not sent.
See section “Response Processing Guidelines”, “Pricing Guidelines”.
Fields defined as Mandatory are required to be submitted when the segment is sent.
11.6.6 PHARMACY PROVIDER SEGMENT (SERVICE REBILL)
PHARMACY PROVIDER SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
465-EY PROVIDER ID QUALIFIER Q Service Rebill:
Required if Provider ID (444-E9) is used.
444-E9 PROVIDER ID
Q Service Rebill:
Required if necessary for state/federal/regulatory agency
programs.
Required if necessary to determine if provider is
credentialed to perform this service.
Required if needed for reconciliation of encounter-reported
data or encounter reporting.
Notes on Pharmacy Provider Segment on a Service Rebill Request:
The Pharmacy Provider Segment is situational for a Service Rebill request if required under provider payer contract or situational on service
billings where this information is necessary for adjudication of the service. Fields defined as Mandatory are required to be submitted when the
segment is sent.
11.6.7 PRESCRIBER SEGMENT (SERVICE REBILL)
PRESCRIBER SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
466-EZ PRESCRIBER ID QUALIFIER Q Service Rebill:
Required if Prescriber ID (411-DB) is used.
411-DB PRESCRIBER ID
Q Service Rebill:
Required if this field could result in different coverage or
patient financial responsibility.
Required if necessary for state/federal/regulatory agency
programs.
427-DR PRESCRIBER LAST NAME
Q Service Rebill:
Required when the Prescriber ID (411-DB) is not known.
Required if needed for Prescriber ID (411-DB)
validation/clarification.
498-PM PRESCRIBER PHONE NUMBER Q Service Rebill:

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 294 -
PRESCRIBER SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if needed to assist in identifying the prescriber.
Required if needed for Prior Authorization process.
468-2E PRIMARY CARE PROVIDER ID QUALIFIER
Q Service Rebill:
Required if Primary Care Provider ID (421-DL) is used.
421-DL PRIMARY CARE PROVIDER ID
Q Service Rebill:
Required if needed for receiver service billing
determination, if known and available.
Required if this field could result in different coverage or
patient financial responsibility.
Required if necessary for state/federal/regulatory agency
programs.
47Ø-4E PRIMARY CARE PROVIDER LAST NAME
Q Service Rebill:
Required if this field is used as an alternative for Primary
Care Provider ID (421-DL) when ID is not known.
Required if needed for Primary Care Provider ID (421-DL)
validation/clarification.
364-2J PRESCRIBER FIRST NAME
Q Service Rebill:
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
365-2K PRESCRIBER STREET ADDRESS Q Service Rebill:
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
366-2M PRESCRIBER CITY ADDRESS Q Service Rebill:
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
367-2N PRESCRIBER STATE/PROVINCE ADDRESS Q Service Rebill:
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
368-2P PRESCRIBER ZIP/POSTAL ZONE Q Service Rebill:
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
Notes on Prescriber Segment on a Service Rebill Request:
The Prescriber Segment is situational for a Service Rebill request. It is used when prescriber information is needed to perform Service Rebill
determination. The Segment is mandatory if required under provider payer contract or mandatory on Service Rebills where this information is
necessary for adjudication of the service. Fields defined as Mandatory are required to be submitted when the segment is sent.
11.6.8 COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT (SERVICE REBILL)
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
337-4C COORDINATION OF BENEFITS/OTHER PAYMENTS COUNT M Maximum count of 9.
338-5C OTHER PAYER COVERAGE TYPE M***R*** Mandatory.
Occurs with Coordination of Benefits/Other Payments
Count (337-4C).
Grouped with Other Payer ID Qualifier (339-6C), Other
Payer ID (34Ø-7C), Other Payer Date (443-E8), and either
Other Payer Amount Paid Count (341-HB) and its grouping,
or Other Payer Reject Count (471-5E) and its grouping.
339-6C OTHER PAYER ID QUALIFIER Q***R*** Service Rebill:
Required if Other Payer ID (34Ø-7C) is used.
34Ø-7C OTHER PAYER ID
Q***R*** Service Rebill:
Required if identification of the Other Payer is necessary for
service billing adjudication.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 295 -
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
443-E8 OTHER PAYER DATE
Q***R*** Service Rebill:
Required if identification of the Other Payer Date is
necessary for service billing adjudication.
993-A7 INTERNAL CONTROL NUMBER Q***R*** Service Rebill:
Required when used for payer-to-payer coordination of
benefits to track the claim without regard to the “Service
Provider ID, Prescription Number, & Date of Service”.
341-HB OTHER PAYER AMOUNT PAID COUNT
Q Service Rebill:
Maximum count of 9.
Required if Other Payer Amount Paid Qualifier (342-HC) is
used.
342-HC OTHER PAYER AMOUNT PAID QUALIFIER
Q***R*** Service Rebill:
Required if Other Payer Amount Paid (431-DV) is used.
431-DV OTHER PAYER AMOUNT PAID
Q***R*** Service Rebill:
Required if other payer has approved payment for some/all
of the billing.
Zero (Ø) is a valid value.
Not used for patient financial responsibility only billing.
Not used for non-governmental agency programs if Other
Payer-Patient Responsibility Amount (352-NQ) is
submitted.
471-5E OTHER PAYER REJECT COUNT Q Service Rebill:
Maximum count of 5.
Required if Other Payer Reject Code (472-6E) is used.
472-6E OTHER PAYER REJECT CODE
Q***R*** Service Rebill:
Required when the other payer has denied the payment for
the billing, designated with Other Coverage Code (3Ø8-C8)
= 3 (Other Coverage Billed – claim not covered).
Note: This field must only contain the NCPDP Reject Code
(511-FB) values.
353-NR OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT COUNT Q Service Rebill:
Maximum count of 25.
Required if Other Payer-Patient Responsibility Amount
Qualifier (351-NP) is used.
Note the occurrences are dependent upon the number of
component parts returned from a previous payer.
351-NP OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT QUALIFIER Q***R*** Service Rebill:
Required if Other Payer-Patient Responsibility Amount
(352-NQ) is used.
352-NQ OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT Q***R*** Service Rebill:
Required if necessary for patient financial responsibility
only billing.
Required if necessary for state/federal/regulatory agency
programs.
Not used for non-governmental agency programs if Other
Payer Amount Paid (431-DV) is submitted.
392-MU BENEFIT STAGE COUNT N Service Rebill:
Not used.
393-MV BENEFIT STAGE QUALIFIER N***R*** Service Rebill:
Not used.
394-MW BENEFIT STAGE AMOUNT N***R*** Service Rebill:
Not used.
Notes on Coordination of Benefits/Other Payments Segment on a Service Rebill Request:
The Coordination of Benefits/Other Payments Segment is situational for a Service Rebill request. It is used when a receiver needs payment
information from other receivers to perform service billing determination. This may be in the case of primary, secondary, tertiary et cetera
health plan coverage for example.
The Coordination of Benefits/Other Payments Segment is mandatory for a Service Rebill request to a downstream payer. It is used to assist
a downstream payer to uniquely identify a Service Rebill in case of duplicate processing. Sometimes processors have difficulty determining
duplicate logic because the same processor is involved in multiple coordination of benefit occurrences for the same patient. They are involved
for example as the primary and secondary payer, or primary and tertiary, or secondary and tertiary. The downstream payer uses the fields
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 296 -
involved in duplicate logic, including the Other Payer Coverage Type (338-5C) to differentiate which Service Rebill to process. See section
“Response Processing Guidelines”, “Duplicate Transactions”.
Note, the Other Payer Coverage Type (338-5C) occurrences do not have to appear in sequential order (primary, secondary, tertiary),
but can appear in any order.
The Coordination of Benefits/Other Payments Segment is not used for a Service Rebill request to a primary payer.
The Segment is mandatory if required under provider payer contract or mandatory on Service Rebills where this information is necessary for
adjudication of the service.
Fields defined as Mandatory are required to be submitted when the segment is sent.
11.6.9 WORKERS’ COMPENSATION SEGMENT (SERVICE REBILL)
WORKERS’ COMPENSATION SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
434-DY DATE OF INJURY M
315-CF EMPLOYER NAME Q Service Rebill:
Required if needed to process a service billing for a work
related injury or condition.
316-CG EMPLOYER STREET ADDRESS Q Service Rebill:
Required if needed to process a service billing for a work
related injury or condition.
317-CH EMPLOYER CITY ADDRESS Q Service Rebill:
Required if needed to process a service billing for a work
related injury or condition.
318-CI EMPLOYER STATE/PROVINCE ADDRESS Q Service Rebill:
Required if needed to process a service billing for a work
related injury or condition.
319-CJ EMPLOYER ZIP/POSTAL ZONE Q Service Rebill:
Required if needed to process a service billing for a work
related injury or condition.
32Ø-CK EMPLOYER PHONE NUMBER Q Service Rebill:
Required if needed to process a service billing for a work
related injury or condition.
321-CL EMPLOYER CONTACT NAME Q Service Rebill:
Required if needed to process a service billing for a work
related injury or condition.
327-CR CARRIER ID Q Service Rebill:
Required if needed to process a service billing for a work
related injury or condition.
435-DZ CLAIM/REFERENCE ID Q Service Rebill:
Required if needed to process a service billing for a work
related injury or condition.
117-TR BILLING ENTITY TYPE INDICATOR R Service Rebill:
Required.
118-TS PAY TO QUALIFIER Q Service Rebill:
Required if Pay To ID (119-TT) is used.
119-TT PAY TO ID Q Service Rebill:
Required if transaction is submitted by a provider or agent,
but paid to another party.
12Ø-TU PAY TO NAME Q Service Rebill:
Required if transaction is submitted by a provider or agent,
but paid to another party.
121-TV PAY TO STREET ADDRESS Q Service Rebill:
Required if transaction is submitted by a provider or agent,
but paid to another party.
122-TW PAY TO CITY ADDRESS Q Service Rebill:
Required if transaction is submitted by a provider or agent,
but paid to another party.
123-TX PAY TO STATE/PROVINCE ADDRESS Q Service Rebill:
Required if transaction is submitted by a provider or agent,
but paid to another party.
124-TY PAY TO ZIP/POSTAL ZONE Q Service Rebill:
Required if transaction is submitted by a provider or agent,
but paid to another party.
125-TZ GENERIC EQUIVALENT PRODUCT ID QUALIFIER Q Service Rebill:
Required if Generic Equivalent Product ID (126-UA) is
used.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 297 -
WORKERS’ COMPENSATION SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
126-UA GENERIC EQUIVALENT PRODUCT ID Q Service Rebill:
Required if necessary for state/federal/regulatory agency
programs.
Notes on Workers’ Compensation Segment on a Service Rebill Request:
The Workers’ Compensation Segment is situational for a Service Rebill request. It is used when processing a Service Rebill for a work-related
injury or condition. Fields defined as Mandatory are required to be submitted when the segment is sent.
11.6.10 DUR/PPS SEGMENT (SERVICE REBILL)
DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
473-7E DUR/PPS CODE COUNTER
Q***R*** Service Rebill:
Maximum 9 occurrences.
Required if DUR/PPS Segment is used.
439-E4 REASON FOR SERVICE CODE
Q***R*** Service Rebill:
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
Required if this field affects payment for or documentation
of professional pharmacy service.
44Ø-E5 PROFESSIONAL SERVICE CODE
Q***R*** Service Rebill:
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
Required if this field affects payment for or documentation
of professional pharmacy service.
441-E6 RESULT OF SERVICE CODE
Q***R*** Service Rebill:
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
Required if this field affects payment for or documentation
of professional pharmacy service.
474-8E DUR/PPS LEVEL OF EFFORT
Q***R*** Service Rebill:
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
Required if this field affects payment for or documentation
of professional pharmacy service.
475-J9 DUR CO-AGENT ID QUALIFIER Q***R*** Service Rebill:
Required if DUR Co-Agent ID (476-H6) is used.
476-H6 DUR CO-AGENT ID
Q***R*** Service Rebill:
Required if this field could result in different drug utilization
review outcome.
Required if this field affects payment for or documentation
of professional pharmacy service.
Notes on DUR/PPS Segment on a Service Rebill Request:
The DUR/PPS Segment is situational for a Service Rebill request. It is used when a sender notifies the receiver of information on the
appropriate selection to process the Service Rebill. The DUR/PPS information may be sent on the initial submission or alternatively sent after
a DUR/PPS rejection from a receiver. The Segment is mandatory if required under provider payer contract or mandatory on Service Rebills
where this information is necessary for adjudication of the service. Fields defined as Mandatory are required to be submitted when the
segment is sent.
11.6.11 CLINICAL SEGMENT (SERVICE REBILL)
CLINICAL SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
491-VE DIAGNOSIS CODE COUNT
Q Service Rebill:
Maximum count of 5.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 298 -
CLINICAL SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if Diagnosis Code Qualifier (492-WE) and
Diagnosis Code (424-DO) are used.
492-WE DIAGNOSIS CODE QUALIFIER Q***R*** Service Rebill:
Required if Diagnosis Code (424-DO) is used.
424-DO DIAGNOSIS CODE
Q***R*** Service Rebill:
The value for this field is obtained from the prescriber or
authorized representative.
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
Required if this field affects payment for professional
pharmacy service.
Required if this information can be used in place of prior
authorization.
Required if necessary for state/federal/regulatory agency
programs.
493-XE CLINICAL INFORMATION COUNTER
Q***R*** Service Rebill:
Maximum 5 occurrences supported.
Grouped with Measurement fields (Measurement Date
(494-ZE), Measurement Time (495-H1), Measurement
Dimension (496-H2), Measurement Unit (497-H3),
Measurement Value (499-H4).
494-ZE MEASUREMENT DATE Q***R*** Service Rebill:
Required if necessary when this field could result in
different coverage and/or drug utilization review outcome.
495-H1 MEASUREMENT TIME Q***R*** Service Rebill:
Required if Time is known or has impact on measurement.
Required if necessary when this field could result in
different coverage and/or drug utilization review outcome.
496-H2 MEASUREMENT DIMENSION Q***R*** Service Rebill:
Required if Measurement Unit (497-H3) and Measurement
Value (499-H4) are used.
Required if necessary when this field could result in
different coverage and/or drug utilization review outcome.
Required if necessary for patient’s weight and height when
billing Medicare for a claim that includes a Certificate of
Medical Necessity (CMN).
497-H3 MEASUREMENT UNIT Q***R*** Service Rebill:
Required if Measurement Dimension (496-H2) and
Measurement Value (499-H4) are used.
Required if necessary for patient’s weight and height when
billing Medicare for a claim that includes a Certificate of
Medical Necessity (CMN).
Required if necessary when this field could result in
different coverage and/or drug utilization review outcome.
499-H4 MEASUREMENT VALUE Q***R*** Service Rebill:
Required if Measurement Dimension (496-H2) and
Measurement Unit (497-H3) are used.
Required if necessary for patient’s weight and height when
billing Medicare for a claim that includes a Certificate of
Medical Necessity (CMN).
Required if necessary when this field could result in
different coverage and/or drug utilization review outcome.
Notes on Clinical Segment on a Service Rebill Request:
The Clinical Segment is situational for a Service Rebill request. It is used to specify clinical measurements and/or diagnosis information
associated with the Service Rebill transaction. The Segment is mandatory if required under provider payer contract or mandatory on Service
Rebills where this information is necessary for adjudication of the service. Fields defined as Mandatory are required to be submitted when the
segment is sent.
11.6.12 ADDITIONAL DOCUMENTATION SEGMENT (SERVICE REBILL)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 299 -
ADDITIONAL DOCUMENTATION SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
369-2Q ADDITIONAL DOCUMENTATION TYPE ID M
374-2V REQUEST PERIOD BEGIN DATE Q Service Rebill:
Required if necessary for state/federal/regulatory agency
programs.
375-2W REQUEST PERIOD RECERT/REVISED DATE Q Service Rebill:
Required if necessary for state/federal/regulatory agency
programs.
Required if the Request Status (373-2U) = “2” (Revision) or
“3” (Recertification).
373-2U REQUEST STATUS Q Service Rebill:
Required if necessary for state/federal/regulatory agency
programs.
371-2S LENGTH OF NEED QUALIFIER Q Service Rebill:
Required if Length of Need (37Ø-2R) is used.
37Ø-2R LENGTH OF NEED Q Service Rebill:
Required if necessary for state/federal/regulatory agency
programs.
372-2T PRESCRIBER/SUPPLIER DATE SIGNED Q Service Rebill:
Required if necessary for state/federal/regulatory agency
programs.
376-2X SUPPORTING DOCUMENTATION Q Service Rebill:
Required if necessary for state/federal/regulatory agency
programs (using Section C of Medicare’s CMN forms).
377-2Z QUESTION NUMBER/LETTER COUNT Q Service Rebill:
Maximum count of 5Ø.
Required if needed to provide response to narratives.
378-4B QUESTION NUMBER/LETTER Q***R*** Service Rebill:
Required if necessary for state/federal/regulatory agency
programs to respond to questions included on a Medicare
form.
Required if Question Number/Letter
Count (377-2Z) is greater than Ø.
379-4D QUESTION PERCENT RESPONSE Q***R*** Service Rebill:
Required if necessary for state/federal/regulatory agency
programs to respond to questions included on a Medicare
form that requires a percent as the response. (At least one
response is required per question.)
38Ø-4G QUESTION DATE RESPONSE Q***R*** Service Rebill:
Required if necessary for state/federal/regulatory agency
programs to respond to questions included on a Medicare
form that requires a date as the response. (At least one
response is required per question.)
381-4H QUESTION DOLLAR AMOUNT RESPONSE Q***R*** Service Rebill:
Required if necessary for state/federal/regulatory agency
programs to respond to questions included on a Medicare
form that requires a dollar amount as the response. (At
least one response is required per question.)
382-4J QUESTION NUMERIC RESPONSE Q***R*** Service Rebill:
Required if necessary for
State/federal/regulatory agency programs to respond to
questions included on a Medicare form that requires a
numeric as the response. (At least one response is
required per question.)
383-4K QUESTION ALPHANUMERIC RESPONSE Q***R*** Service Rebill:
Required if necessary for state/federal/regulatory agency
programs to respond to questions included on a Medicare
form that requires an alphanumeric as the response. (At
least one response is required per question.)
Notes on Additional Documentation Segment on a Service Rebill:
The Additional Documentation Segment is situational for Service Rebill request. It is used to provide additional information on Medicare forms.
Fields defined as Mandatory are required to be submitted when the segment is sent.
11.6.13 FACILITY SEGMENT (SERVICE REBILL)
FACILITY SEGMENT
SITUATIONAL SEGMENT
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 300 -
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
336-8C FACILITY ID Q Service Rebill:
Required if necessary for state/federal/regulatory agency
programs.
Required if “required by law” as defined in the HIPAA final
Privacy regulations section 164.5Ø1 definitions (45 CFR
Parts 160 and 164 Standards for Privacy of Individually
Identifiable Health Information; Final Rule -
Thursday, December 28, 2000, page 82803 and following,
and Wednesday, August 14, 2002, page 53267 and
following.)
385-3Q FACILITY NAME Q Service Rebill:
Required if necessary for state/federal/regulatory agency
programs.
386-3U FACILITY STREET ADDRESS Q Service Rebill:
Required if necessary for state/federal/regulatory agency
programs.
388-5J FACILITY CITY ADDRESS Q Service Rebill:
Required if necessary for state/federal/regulatory agency
programs.
387-3V FACILITY STATE/PROVINCE ADDRESS Q Service Rebill:
Required if necessary for state/federal/regulatory agency
programs.
389-6D FACILITY ZIP/POSTAL ZONE Q Service Rebill:
Required if necessary for state/federal/regulatory agency
programs.
Notes on Facility Segment on a Service Rebill Request:
The Facility Segment is situational for Service Rebill request. It is used when these fields could result in different coverage, pricing, and/or
patient financial responsibility. Fields defined as Mandatory are required to be submitted when the segment is sent.
11.6.14 NARRATIVE SEGMENT (SERVICE REBILL)
NARRATIVE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
39Ø-BM NARRATIVE MESSAGE Q Service Rebill:
Required if necessary only to support exception handling of
pharmacy claims for Medicare Part B claim billing.
Notes on Narrative Segment on a Service Rebill Request:
The Narrative Segment is situational for Service Rebill request. It is used to support exception handling for Medicare service billing. Fields
defined as Mandatory are required to be submitted when the segment is sent.
11.7 SERVICE REBILL RESPONSE DIAGRAMS AND SEGMENTS
11.7.1 TRANSMISSION ACCEPTED/TRANSACTION PAID
11.7.1.1 DIAGRAM FOR TRANSMISSION OF ONE SERVICE REBILL RESPONSE (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
Service Rebill transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “P” (Paid)
The Paid response is not used in payer-to-payer transactions.
The duplicate response codes for the Service Rebill transaction are not applicable.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 301 -
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
11.7.1.2 DIAGRAM FOR TRANSMISSION OF TWO SERVICE REBILL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
11.7.1.3 DIAGRAM FOR TRANSMISSION OF THREE SERVICE REBILL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 302 -
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
11.7.1.4 DIAGRAM FOR TRANSMISSION OF FOUR SERVICE REBILL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 303 -
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
11.7.1.5 SERVICE REBILL RESPONSE SEGMENTS (TRANSMISSION ACCEPTED/TRANSACTION PAID)
11.7.1.5.1 RESPONSE HEADER SEGMENT (SERVICE REBILL) (TRANSMISSION ACCEPTED/TRANSACTION PAID)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M For Transaction Code of “S3”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “2” (Service Billing).
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Service Rebill Response:
The Response Header Segment is a mandatory, fixed length segment for Service Rebill response when the Header Response Status (5Ø1-
F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “P” (Paid). The “Situation” column is not applicable.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 304 -
11.7.1.5.2 RESPONSE MESSAGE SEGMENT (SERVICE REBILL) (TRANSMISSION ACCEPTED/TRANSACTION PAID)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Service Rebill:
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Service Rebill Response:
The Response Message Segment is situational for Service Rebill response when the Header Response Status (5Ø1-F1) of "A" (Accepted) and
Transaction Response Status (112-AN) of “P” (Paid). It is used when additional text information needs to be sent. Fields defined as Mandatory
are required to be submitted when the segment is sent.
11.7.1.5.3 RESPONSE INSURANCE SEGMENT (SERVICE REBILL) (TRANSMISSION ACCEPTED/TRANSACTION PAID)
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø1-C1 GROUP ID Q Service Rebill:
Required if needed to identify the actual cardholder or
employer group, to identify appropriate group number,
when available.
Required to identify the actual group that was used when
multiple group coverages exist.
Note: This field may contain the Group ID echoed from the
request. May contain the actual Group ID if unknown to the
receiver.
524-FO PLAN ID
Q Service Rebill:
Required if needed to identify the actual plan parameters,
benefit, or coverage criteria, when available.
Required to identify the actual plan ID that was used when
multiple group coverages exist.
Required if needed to contain the actual plan ID if unknown
to the receiver.
545-2F NETWORK REIMBURSEMENT ID Q Service Rebill:
Required if needed to identify the network for the covered
member.
Required if needed to identify the actual Network
Reimbursement ID, when applicable and/or available.
Required to identify the actual Network Reimbursement ID
that was used when multiple Network Reimbursement IDs
exist.
568-J7 PAYER ID QUALIFIER Q Service Rebill:
Required if Payer ID (569-J8) is used.
569-J8 PAYER ID
Q Service Rebill:
Required to identify the ID of the payer responding.
115-N5
MEDICAID ID NUMBER N Service Rebill:
Not used.
116-N6
MEDICAID AGENCY NUMBER N Service Rebill:
Not used.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 305 -
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
3Ø2-C2
CARDHOLDER ID Q Service Rebill:
Required if the identification to be used in future
transactions is different than what was submitted on the
request.
Notes on Response Insurance Segment on a Service Rebill Response:
The Response Insurance Segment is situational for Service Rebill response when the Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “P” (Paid). It is used when coverage or reimbursement parameters or identifiers need to be sent
from the sender to the receiver. Fields defined as Mandatory are required to be submitted when the segment is sent.
11.7.1.5.4 RESPONSE PATIENT SEGMENT (SERVICE REBILL) (TRANSMISSION ACCEPTED/TRANSACTION PAID)
RESPONSE PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
31Ø-CA PATIENT FIRST NAME Q Service Rebill:
Required if known.
311-CB PATIENT LAST NAME Q Service Rebill:
Required if known.
3Ø4-C4 DATE OF BIRTH Q Service Rebill:
Required if known.
Notes on Response Patient Segment on a Service Rebill Response:
The Response Patient Segment is situational for Service Rebill transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “P” (Paid) when patient demographic information needs to be sent from the sender to the
receiver. Fields defined as Mandatory are required to be submitted when the segment is sent.
11.7.1.5.5 RESPONSE STATUS SEGMENT (SERVICE REBILL) (TRANSMISSION ACCEPTED/TRANSACTION PAID)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER
Q Service Rebill:
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT N Service Rebill:
Not used.
511-FB REJECT CODE
N***R*** Service Rebill:
Not used.
546-4F REJECT FIELD OCCURRENCE INDICATOR
N***R*** Service Rebill:
Not used.
547-5F APPROVED MESSAGE CODE COUNT
Q Service Rebill:
Maximum count of 5.
Required if Approved Message Code (548-6F) is used.
548-6F APPROVED MESSAGE CODE
Q***R*** Service Rebill:
Required if Approved Message Code Count (547-5F) is
used and the sender needs to communicate additional
follow up for a potential opportunity.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Service Rebill:
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Service Rebill:
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Service Rebill:
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 306 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Service Rebill:
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER
Q Service Rebill:
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Service Rebill:
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Service Rebill:
Not used.
993-A7 INTERNAL CONTROL NUMBER Q Service Rebill:
Required when used for payer-to-payer coordination of
benefits to track the claim without regard to the “Service
Provider ID, Prescription Number, & Date of Service”.
987-MA URL N Service Rebill:
Not used.
Notes on Response Status Segment on a Service Rebill Response:
The Response Status Segment is mandatory for a Service Rebill Response for Header Response Status (5Ø1-F1) = “A” (Accepted) and
Transaction Response Status (112-AN) of “P” (Paid). The Response Status Segment is sent from the sender to the receiver to identify the
outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.
11.7.1.5.6 RESPONSE CLAIM SEGMENT (SERVICE REBILL) (TRANSMISSION ACCEPTED/TRANSACTION PAID)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M For Transaction Code of “S3”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “2” (Service Billing).
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT
N Service Rebill:
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER N***R*** Service Rebill:
Not used.
553-AR PREFERRED PRODUCT ID
N***R*** Service Rebill:
Not used.
554-AS PREFERRED PRODUCT INCENTIVE
N***R*** Service Rebill:
Not used.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE N***R*** Service Rebill:
Not used.
556-AU PREFERRED PRODUCT DESCRIPTION
N***R*** Service Rebill:
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Service Rebill:
Not used.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 307 -
Notes on Response Claim Segment on a Service Rebill Response:
The Response Claim Segment is mandatory for a Service Rebill Response when the Header Response Status (5Ø1-F1) is “A” (Accepted) and
Transaction Response Status (112-AN) of “P” (Paid). The Response Claim Segment is sent from the sender to the receiver to mirror back the
Prescription/Service Reference Number (4Ø2-D2). Fields defined as Mandatory are required to be submitted when the segment is sent.
11.7.1.5.7 RESPONSE PRICING SEGMENT (SERVICE REBILL) (TRANSMISSION ACCEPTED/TRANSACTION PAID)
RESPONSE PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø5-F5 PATIENT PAY AMOUNT
R Service Rebill:
Required.
5Ø6-F6 INGREDIENT COST PAID
N Service Rebill:
Not used.
5Ø7-F7 DISPENSING FEE PAID
N Service Rebill:
Not used.
557-AV TAX EXEMPT INDICATOR
Q Service Rebill:
Required if the sender (health plan) and/or patient is tax
exempt and exemption applies to this billing.
558-AW FLAT SALES TAX AMOUNT PAID
Q Service Rebill:
Required if Flat Sales Tax Amount Submitted (481-HA) is
greater than zero (Ø) or if Flat Sales Tax Amount Paid
(558-AW) is used to arrive at the final reimbursement. Zero
(Ø) value is valid.
559-AX PERCENTAGE SALES TAX AMOUNT PAID
Q Service Rebill:
Required if Percentage Sales Tax Amount Submitted (482-
GE) is greater than zero (Ø) or if Percentage Sales Tax
Amount Paid (559-AX) is used to arrive at the final
reimbursement. Zero (Ø) value is valid.
Required if Percentage Sales Tax Rate Paid (56Ø-AY) is
used.
56Ø-AY PERCENTAGE SALES TAX RATE PAID
Q Service Rebill:
Required if Percentage Sales Tax Amount Paid (559-AX) is
greater than zero (Ø).
561-AZ PERCENTAGE SALES TAX BASIS PAID
N Service Rebill:
Not used. Code list is not applicable.
521-FL INCENTIVE AMOUNT PAID
N Service Rebill:
Not used. Not supported in Service Billing formula.
562-J1 PROFESSIONAL SERVICE FEE PAID
R Service Rebill:
Required.
563-J2 OTHER AMOUNT PAID COUNT
Q Service Rebill:
Maximum count of 3.
Required if Other Amount Paid (565-J4) is used.
564-J3 OTHER AMOUNT PAID QUALIFIER Q***R*** Service Rebill:
Required if Other Amount Paid (565-J4) is used.
565-J4 OTHER AMOUNT PAID
Q***R*** Service Rebill:
Required if Other Amount Claimed Submitted (48Ø-H9) is
greater than zero (Ø) or if Other Amount Paid (565-J4) is
used to arrive at the final reimbursement. This field may be
equal to zero (Ø). Must respond to each occurrence
submitted.
566-J5 OTHER PAYER AMOUNT RECOGNIZED
Q Service Rebill:
Required if Other Payer Amount Paid (431-DV) is greater
than zero (Ø) or if this field is used to arrive at the final
reimbursement. This field may be equal to zero (Ø).
5Ø9-F9 TOTAL AMOUNT PAID
R Service Rebill:
Required. Zero (Ø) value is valid.
See Pricing Formula for fields used in calculation.
522-FM BASIS OF REIMBURSEMENT DETERMINATION
N Service Rebill:
Not used. Definition is not applicable.
523-FN AMOUNT ATTRIBUTED TO SALES TAX
Q Service Rebill:
Required if Patient Pay Amount (5Ø5-F5) includes sales tax
that is the financial responsibility of the member but is not
also included in any of the other fields that add up to
Patient Pay Amount.
See section “Specific Segment Discussion”, “Response
Segments”, “Response Pricing Segment” for guidance.
512-FC ACCUMULATED DEDUCTIBLE AMOUNT I Service Rebill:
Provided for informational purposes only.
513-FD REMAINING DEDUCTIBLE AMOUNT I Service Rebill:
Provided for informational purposes only.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 308 -
RESPONSE PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
514-FE REMAINING BENEFIT AMOUNT
I Service Rebill:
Provided for informational purposes only.
The Remaining Benefit Amount must not be returned with
zeroes unless there are no benefit dollars remaining. The
default value of 999999999 from previous versions must
not be used as a default in this field.
517-FH AMOUNT APPLIED TO PERIODIC DEDUCTIBLE
Q Service Rebill:
Required if Patient Pay Amount (5Ø5-F5) includes
deductible.
518-FI AMOUNT OF COPAY
Q Service Rebill:
Required if Patient Pay Amount (5Ø5-F5) includes copay
as patient financial responsibility.
52Ø-FK AMOUNT EXCEEDING PERIODIC BENEFIT MAXIMUM
Q Service Rebill:
Required if Patient Pay Amount (5Ø5-F5) includes amount
exceeding periodic benefit maximum.
346-HH BASIS OF CALCULATION—DISPENSING FEE
N Service Rebill:
Not used.
347-HJ BASIS OF CALCULATION—COPAY
N Service Rebill:
Not used.
348-HK BASIS OF CALCULATION—FLAT SALES TAX
N Service Rebill:
Not used.
349-HM BASIS OF CALCULATION—PERCENTAGE SALES TAX N Service Rebill:
Not used.
571-NZ AMOUNT ATTRIBUTED TO PROCESSOR FEE Q Service Rebill:
Required if the customer is responsible for 1ØØ% of the
service payment and when the provider net sale is less
than the amount the customer is expected to pay.
575-EQ PATIENT SALES TAX AMOUNT I Service Rebill:
Used when necessary to identify the Patient’s portion of the
Sales Tax.
Provided for informational purposes only.
574-2Y PLAN SALES TAX AMOUNT I Service Rebill:
Used when necessary to identify the Plan’s portion of the
Sales Tax.
Provided for informational purposes only.
572-4U AMOUNT OF COINSURANCE Q Service Rebill:
Required if Patient Pay Amount (5Ø5-F5) includes
coinsurance as patient financial responsibility.
573-4V BASIS OF CALCULATION-COINSURANCE N Service Rebill:
Not used.
392-MU BENEFIT STAGE COUNT N Service Rebill:
Not used.
393-MV BENEFIT STAGE QUALIFIER N***R*** Service Rebill:
Not used.
394-MW BENEFIT STAGE AMOUNT N***R*** Service Rebill:
Not used.
577-G3 ESTIMATED GENERIC SAVINGS N Service Rebill:
Not used.
128-UC SPENDING ACCOUNT AMOUNT REMAINING I Service Rebill:
This dollar amount will be provided, if known, to the
receiver when the transaction had spending account dollars
reported as part of the patient pay amount.
This field is informational only. It is reported back to the
provider and the patient the amount remaining on the
spending account after the current claim updated the
spending account.
129-UD HEALTH PLAN-FUNDED ASSISTANCE AMOUNT Q Service Rebill:
Required when the patient meets the plan-funded
assistance criteria, to reduce Patient Pay Amount (5Ø5-
F5). The resulting Patient Pay Amount (5Ø5-F5) must be
greater than or equal to zero.
This field is always a negative amount or zero.
133-UJ AMOUNT ATTRIBUTED TO PROVIDER NETWORK SELECTION Q Service Rebill:
Required if Patient Pay Amount (5Ø5-F5) includes an
amount that is attributable to a cost share differential due to
the selection of one pharmacy over another.
134-UK AMOUNT ATTRIBUTED TO PRODUCT SELECTION/BRAND N Service Rebill:

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 309 -
RESPONSE PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
DRUG Not used.
135-UM AMOUNT ATTRIBUTED TO PRODUCT SELECTION/NON-
PREFERRED FORMULARY SELECTION
N Service Rebill:
Not used.
136-UN AMOUNT ATTRIBUTED TO PRODUCT SELECTION/BRAND
NON-PREFERRED FORMULARY SELECTION
N Service Rebill:
Not used.
137-UP AMOUNT ATTRIBUTED TO COVERAGE GAP Q Service Rebill:
Required when the patient’s financial responsibility is due to
the coverage gap.
148-U8 INGREDIENT COST CONTRACTED/REIMBURSABLE AMOUNT N Service Rebill:
Not used.
149-U9 DISPENSING FEE CONTRACTED/REIMBURSABLE AMOUNT N Service Rebill:
Not used.
Notes on Response Pricing Segment on a Service Rebill Response:
The Response Pricing Segment is mandatory for a Service Rebill Response when the Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) is “P” (Paid).
It is highly recommended that whenever possible, the individual dollar fields are returned in the response. On the response the sender should
return the individual payment response fields to allow the receiver to reconcile against the requested payment fields.
See section “Response Processing Guidelines”, “Pricing Guidelines”.
Fields defined as Mandatory are required to be submitted when the segment is sent.
11.7.1.5.8 RESPONSE DUR/PPS SEGMENT (SERVICE REBILL) (TRANSMISSION ACCEPTED/TRANSACTION PAID)
RESPONSE DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
567-J6 DUR/PPS RESPONSE CODE COUNTER
Q***R*** Service Rebill:
Maximum 9 occurrences.
Required if Reason For Service Code (439-E4) is used.
439-E4 REASON FOR SERVICE CODE Q***R*** Service Rebill:
Required if professional service opportunity reason is
detected by the receiver that is different from the
professional service submitted.
528-FS CLINICAL SIGNIFICANCE CODE
Q***R*** Service Rebill:
Required if needed to supply additional information for the
service.
529-FT OTHER PHARMACY INDICATOR
Q***R*** Service Rebill:
Required if needed to supply additional information for the
service.
53Ø-FU PREVIOUS DATE OF FILL
Q***R*** Service Rebill:
Required if needed to supply additional information for the
service.
Required if Quantity of Previous Fill (531-FV) is used.
531-FV QUANTITY OF PREVIOUS FILL
Q***R*** Service Rebill:
Required if needed to supply additional information for the
service.
Required if Previous Date Of Fill (53Ø-FU) is used.
532-FW DATABASE INDICATOR
Q***R*** Service Rebill:
Required if needed to supply additional information for the
service.
533-FX OTHER PRESCRIBER INDICATOR
Q***R*** Service Rebill:
Required if needed to supply additional information for the
service.
544-FY DUR FREE TEXT MESSAGE
Q***R*** Service Rebill:
Required if needed to supply additional information for the
service.
57Ø-NS DUR ADDITIONAL TEXT Q***R*** Service Rebill:
Required if needed to supply additional information for the
service.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 310 -
Notes on Response DUR/PPS Segment on a Service Rebill Response:
The Response DUR/PPS Segment is situational for a Service Rebill Response when the Header Response Status (5Ø1-F1) is “A” (Accepted)
and Transaction Response Status (112-AN) of “P” (Paid). This would be used when a processor identifies an additional professional pharmacy
service billing opportunity. Fields defined as Mandatory are required to be submitted when the segment is sent.
11.7.1.5.9 RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS SEGMENT (SERVICE REBILL) (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS
SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
355-NT OTHER PAYER ID COUNT M Service Rebill:
Maximum count of 3.
338-5C OTHER PAYER COVERAGE TYPE M***R***
339-6C OTHER PAYER ID QUALIFIER Q***R*** Service Rebill:
Required if Other Payer ID (34Ø-7C) is used.
34Ø-7C OTHER PAYER ID Q***R*** Service Rebill:
Required if other insurance information is available for
coordination of benefits.
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER Q***R*** Service Rebill:
Required if other insurance information is available for
coordination of benefits.
356-NU OTHER PAYER CARDHOLDER ID Q***R*** Service Rebill:
Required if other insurance information is available for
coordination of benefits.
992-MJ OTHER PAYER GROUP ID Q***R*** Service Rebill:
Required if other insurance information is available for
coordination of benefits.
142-UV OTHER PAYER PERSON CODE Q***R*** Service Rebill:
Required if needed to uniquely identify the family members
within the Cardholder ID, as assigned by the other payer.
127-UB OTHER PAYER HELP DESK PHONE NUMBER Q***R*** Service Rebill:
Required if needed to provide a support telephone number
of the other payer to the receiver.
143-UW OTHER PAYER PATIENT RELATIONSHIP CODE Q***R*** Service Rebill:
Required if needed to uniquely identify the relationship of
the patient to the cardholder ID, as assigned by the other
payer.
144-UX OTHER PAYER BENEFIT EFFECTIVE DATE Q***R*** Service Rebill:
Required when other coverage is known which is after the
Date of Service submitted.
145-UY OTHER PAYER BENEFIT TERMINATION DATE Q***R*** Service Rebill:
Required when other coverage is known which is after the
Date of Service submitted.
Notes on Response Coordination of Benefits/Other Payers Segment on a Service Rebill Response:
The Response Coordination of Benefits/Other Payers Segment is situational for a Service Rebill response when the Header Response Status
(5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “P” (Paid) when other insurance information is available for
coordination of benefits.
If subsequent payer(s) for this patient is not known, the Other Payer information is not sent.
If subsequent payer(s) for this patient is known, the following may be sent:
• Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C),
• Other Payer Group ID (992-MJ),
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU)
• And other Other Payer fields.
In addition, if any of the following three fields are sent:
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU),
• Other Payer Group ID (992-MJ),
then the Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C) must be sent.
Fields defined as Mandatory are required to be submitted when the segment is sent.
11.7.2 TRANSMISSION ACCEPTED/TRANSACTION CAPTURED
11.7.2.1 DIAGRAM FOR TRANSMISSION OF ONE SERVICE REBILL RESPONSE (TRANSMISSION
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 311 -
ACCEPTED/TRANSACTION CAPTURED)
Service Rebill transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “C” (Captured)
The Response Pricing Segment is not used in payer-to-payer transactions. Therefore, in this case, there are no situational transaction-level
segments.
The duplicate response codes for the Service Rebill transaction are not applicable. See section “Response Processing Guidelines”, “Duplicate
Transactions” for the handling of a duplicate transaction.
Service Rebill transactions - The “C” (Captured) event occurs after the reversal portion of the service rebill is processed successfully and the
service is captured for processing. If the service rebill reversal is not processed successfully, a “R” (Rejected) response must be sent.
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Pricing Segment
11.7.2.2 DIAGRAM FOR TRANSMISSION OF TWO SERVICE REBILL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Pricing Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Pricing Segment
11.7.2.3 DIAGRAM FOR TRANSMISSION OF THREE SERVICE REBILL RESPONSES (TRANSMISSION
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 312 -
ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Pricing Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Pricing Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Pricing Segment
11.7.2.4 DIAGRAM FOR TRANSMISSION OF FOUR SERVICE REBILL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Pricing Segment
Mandatory second response
Group Separator
Segment Separator
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 313 -
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Pricing Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Pricing Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Pricing Segment
11.7.2.5 SERVICE REBILL RESPONSE SEGMENTS (TRANSMISSION ACCEPTED/TRANSACTION
CAPTURED)
11.7.2.5.1 RESPONSE HEADER SEGMENT (SERVICE REBILL) (TRANSMISSION ACCEPTED/TRANSACTION
CAPTURED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M For Transaction Code of “S3”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “2” (Service Billing).
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Service Rebill Response:
The Response Header Segment is a mandatory, fixed length segment for Service Rebill response when the Header Response Status (5Ø1-
F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “C” (Captured). The “Situation” column is not applicable.
11.7.2.5.2 RESPONSE MESSAGE SEGMENT (SERVICE REBILL) (TRANSMISSION ACCEPTED/TRANSACTION
CAPTURED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Service Rebill:
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 314 -
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Service Rebill Response:
The Response Message Segment is situational for Service Rebill response when the Header Response Status (5Ø1-F1) of "A" (Accepted) and
Transaction Response Status (112-AN) of “C” (Captured). It is used when additional text information needs to be sent. Fields defined as
Mandatory are required to be submitted when the segment is sent.
11.7.2.5.3 RESPONSE INSURANCE SEGMENT (SERVICE REBILL) (TRANSMISSION ACCEPTED/TRANSACTION
CAPTURED)
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø1-C1 GROUP ID Q Service Rebill:
Required if needed to identify the actual cardholder or
employer group, to identify appropriate group number,
when available.
Required to identify the actual group that was used when
multiple group coverages exist.
Note: This field may contain the Group ID echoed from the
request. May contain the actual Group ID if unknown to the
receiver.
524-FO PLAN ID
Q Service Rebill:
Required if needed to identify the actual plan parameters,
benefit, or coverage criteria, when available.
Required to identify the actual plan ID that was used when
multiple group coverages exist.
Required if needed to contain the actual plan ID if unknown
to the receiver.
545-2F NETWORK REIMBURSEMENT ID N Service Rebill:
Not used.
568-J7 PAYER ID QUALIFIER N Service Rebill:
Not used.
569-J8 PAYER ID N Service Rebill:
Not used.
115-N5
MEDICAID ID NUMBER N Service Rebill:
Not used.
116-N6
MEDICAID AGENCY NUMBER N Service Rebill:
Not used.
3Ø2-C2
CARDHOLDER ID Q Service Rebill:
Required if the identification to be used in future
transactions is different than what was submitted on the
request.
Notes on Response Insurance Segment on a Service Rebill Response:
The Response Insurance Segment is situational for Service Rebill response when the Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “C” (Captured). It is used when coverage information may be provided from the sender to the
receiver. Fields defined as Mandatory are required to be submitted when the segment is sent.
11.7.2.5.4 RESPONSE PATIENT SEGMENT (SERVICE REBILL) (TRANSMISSION ACCEPTED/TRANSACTION
CAPTURED)
RESPONSE PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 315 -
RESPONSE PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
31Ø-CA PATIENT FIRST NAME Q Service Rebill:
Required if known.
311-CB PATIENT LAST NAME Q Service Rebill:
Required if known.
3Ø4-C4 DATE OF BIRTH Q Service Rebill:
Required if known.
Notes on Response Patient Segment on a Service Rebill Response:
The Response Patient Segment is situational for Service Rebill transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “C” (Captured) when patient demographic information needs to be sent from the sender to the
receiver. Fields defined as Mandatory are required to be submitted when the segment is sent.
11.7.2.5.5 RESPONSE STATUS SEGMENT (SERVICE REBILL) (TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER
Q Service Rebill:
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT N Service Rebill:
Not used.
511-FB REJECT CODE
N***R*** Service Rebill:
Not used.
546-4F REJECT FIELD OCCURRENCE INDICATOR
N***R*** Service Rebill:
Not used.
547-5F APPROVED MESSAGE CODE COUNT
N Service Rebill:
Not used.
548-6F APPROVED MESSAGE CODE
N***R*** Service Rebill:
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Service Rebill:
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Service Rebill:
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Service Rebill:
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Service Rebill:
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER
Q Service Rebill:
Required if Help Desk Phone Number (55Ø-8F) is used.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 316 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
55Ø-8F HELP DESK PHONE NUMBER
Q Service Rebill:
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Service Rebill:
Not used.
993-A7 INTERNAL CONTROL NUMBER Q Service Rebill:
Required when used for payer-to-payer coordination of
benefits to track the claim without regard to the “Service
Provider ID, Prescription Number, & Date of Service”.
987-MA URL N Service Rebill:
Not used.
Notes on Response Status Segment on a Service Rebill Response:
The Response Status Segment is mandatory for a Service Rebill Response for Header Response Status (5Ø1-F1) = “A” (Accepted) and
Transaction Response Status (112-AN) of “C” (Captured). The Response Status Segment is sent from the sender to the receiver to identify the
outcome of the request.
Service Rebill transactions - The “C” (Captured) event occurs after the reversal portion of the service rebill is processed successfully and the
service is captured for processing. If the service rebill reversal is not processed successfully, a “R” (Rejected) response must be sent.
Fields defined as Mandatory are required to be submitted when the segment is sent.
11.7.2.5.6 RESPONSE CLAIM SEGMENT (SERVICE REBILL) (TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M For Transaction Code of “S3”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “2” (Service Billing).
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT
N Service Rebill:
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER N***R*** Service Rebill:
Not used.
553-AR PREFERRED PRODUCT ID
N***R*** Service Rebill:
Not used.
554-AS PREFERRED PRODUCT INCENTIVE
N***R*** Service Rebill:
Not used.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE N***R*** Service Rebill:
Not used.
556-AU PREFERRED PRODUCT DESCRIPTION
N***R*** Service Rebill:
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Service Rebill:
Not used.
Notes on Response Claim Segment on a Service Rebill Response:
The Response Claim Segment is mandatory for a Service Rebill Response when the Header Response Status (5Ø1-F1) is “A” (Accepted) and
Transaction Response Status (112-AN) of “C” (Captured). The Response Claim Segment is sent from the sender to the receiver to mirror back
the Prescription/Service Reference Number (4Ø2-D2). Fields defined as Mandatory are required to be submitted when the segment is sent.
11.7.2.5.7 RESPONSE PRICING SEGMENT (SERVICE REBILL) (TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
RESPONSE PRICING SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø5-F5 PATIENT PAY AMOUNT
Q Service Rebill:
Required if known. This field cannot be an estimated
amount. Zero is a valid amount.
5Ø6-F6 INGREDIENT COST PAID
N Service Rebill:
Not used.
5Ø7-F7 DISPENSING FEE PAID
N Service Rebill:
Not used.
557-AV TAX EXEMPT INDICATOR Q Service Rebill:

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 317 -
RESPONSE PRICING SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if the sender (health plan) and/or patient is tax
exempt and exemption applies to this billing.
558-AW FLAT SALES TAX AMOUNT PAID
Q Service Rebill:
Required if Flat Sales Tax Amount Submitted (481-HA) is
greater than zero (Ø) or if Flat Sales Tax Amount Paid
(558-AW) is used to arrive at the estimated reimbursement.
Zero (Ø) value is valid. If reimbursement is not estimated,
this field contains the submitted value.
559-AX PERCENTAGE SALES TAX AMOUNT PAID
Q Service Rebill:
Required if Percentage Sales Tax Amount Submitted (482-
GE) is greater than zero (Ø) or if Percentage Sales Tax
Amount Paid (559-AX) is used to arrive at the estimated
reimbursement. Zero (Ø) value is valid. If reimbursement is
not estimated, this field contains the submitted value.
Required if Percentage Sales Tax Rate Paid (56Ø-AY) and
Percentage Sales Tax Basis Paid (561-AZ) are used.
56Ø-AY PERCENTAGE SALES TAX RATE PAID
Q Service Rebill:
Required if Percentage Sales Tax Amount Paid (559-AX) is
greater than zero (Ø).
561-AZ PERCENTAGE SALES TAX BASIS PAID
N Service Rebill:
Not used. Code list is not applicable.
521-FL INCENTIVE AMOUNT PAID
N Service Rebill:
Not used.
562-J1 PROFESSIONAL SERVICE FEE PAID
R Service Rebill:
Required.
563-J2 OTHER AMOUNT PAID COUNT
Q Service Rebill:
Maximum count of 3.
Required if Other Amount Paid (565-J4) is used.
564-J3 OTHER AMOUNT PAID QUALIFIER Q***R*** Service Rebill:
Required if Other Amount Paid (565-J4) is used.
565-J4 OTHER AMOUNT PAID
Q***R*** Service Rebill:
Required if this value is used to arrive at the estimated
reimbursement. If reimbursement is not estimated, this field
contains the submitted value.
Required if Other Amount Claimed Submitted (48Ø-H9) is
greater than zero (Ø).
Zero (Ø) is a valid value.
Must respond to each occurrence submitted.
566-J5 OTHER PAYER AMOUNT RECOGNIZED
Q Service Rebill:
Required if Other Payer Amount Paid (431-DV) is greater
than zero (Ø) or if this field is used to arrive at the
estimated reimbursement.
Zero (Ø) value is valid.
If reimbursement is not estimated, this field contains the
submitted value.
5Ø9-F9 TOTAL AMOUNT PAID
R Service Rebill:
Required.
Zero (Ø) value is valid.
See Pricing Formula for fields used in calculation.
522-FM BASIS OF REIMBURSEMENT DETERMINATION
N Service Rebill:
Not used. Definition is not applicable.
523-FN AMOUNT ATTRIBUTED TO SALES TAX
Q Service Rebill:
Required if Patient Pay Amount (5Ø5-F5) includes sales tax
that is the financial responsibility of the member but is not
also included in any of the other fields that add up to
Patient Pay Amount.
See section “Specific Segment Discussion”, “Response
Segments”, “Response Pricing Segment” for guidance.
512-FC ACCUMULATED DEDUCTIBLE AMOUNT N Service Rebill:
Not used.
513-FD REMAINING DEDUCTIBLE AMOUNT N Service Rebill:
Not used.
514-FE REMAINING BENEFIT AMOUNT N Service Rebill:
Not used.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 318 -
RESPONSE PRICING SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
517-FH AMOUNT APPLIED TO PERIODIC DEDUCTIBLE
N Service Rebill:
Not used.
518-FI AMOUNT OF COPAY
Q Service Rebill:
Required if Patient Pay Amount (5Ø5-F5) includes copay
as patient financial responsibility.
52Ø-FK AMOUNT EXCEEDING PERIODIC BENEFIT MAXIMUM
N Service Rebill:
Not used.
346-HH BASIS OF CALCULATION—DISPENSING FEE
N Service Rebill:
Not used.
347-HJ BASIS OF CALCULATION—COPAY
N Service Rebill:
Not used.
348-HK BASIS OF CALCULATION—FLAT SALES TAX
N Service Rebill:
Not used.
349-HM BASIS OF CALCULATION—PERCENTAGE SALES TAX N Service Rebill:
Not used.
571-NZ AMOUNT ATTRIBUTED TO PROCESSOR FEE Q Service Rebill:
Required if the customer is responsible for 1ØØ% of the
service payment and when the provider net sale is less
than the amount the customer is expected to pay.
575-EQ PATIENT SALES TAX AMOUNT I Service Rebill:
Used when necessary to identify the Patient’s portion of the
Sales Tax.
Provided for informational purposes only.
574-2Y PLAN SALES TAX AMOUNT I Service Rebill:
Used when necessary to identify the Plan’s portion of the
Sales Tax.
Provided for informational purposes only.
572-4U AMOUNT OF COINSURANCE Q Service Rebill:
Required if Patient Pay Amount (5Ø5-F5) includes
coinsurance as patient financial responsibility.
573-4V BASIS OF CALCULATION-COINSURANCE N Service Rebill:
Not used.
392-MU BENEFIT STAGE COUNT N Service Rebill:
Not used.
393-MV BENEFIT STAGE QUALIFIER N***R*** Service Rebill:
Not used.
394-MW BENEFIT STAGE AMOUNT N***R*** Service Rebill:
Not used.
577-G3 ESTIMATED GENERIC SAVINGS N Service Rebill:
Not used.
128-UC SPENDING ACCOUNT AMOUNT REMAINING N Service Rebill:
Not used.
129-UD HEALTH PLAN-FUNDED ASSISTANCE AMOUNT N Service Rebill:
Not used.
133-UJ AMOUNT ATTRIBUTED TO PROVIDER NETWORK SELECTION N Service Rebill:
Not used.
134-UK AMOUNT ATTRIBUTED TO PRODUCT SELECTION/BRAND
DRUG
N Service Rebill:
Not used.
135-UM AMOUNT ATTRIBUTED TO PRODUCT SELECTION/NON-
PREFERRED FORMULARY SELECTION
N Service Rebill:
Not used.
136-UN AMOUNT ATTRIBUTED TO PRODUCT SELECTION/BRAND
NON-PREFERRED FORMULARY SELECTION
N Service Rebill:
Not used.
137-UP AMOUNT ATTRIBUTED TO COVERAGE GAP Q Service Rebill:
Required when the patient’s financial responsibility is due to
the coverage gap.
148-U8 INGREDIENT COST CONTRACTED/REIMBURSABLE AMOUNT N Service Rebill:
Not used.
149-U9 DISPENSING FEE CONTRACTED/REIMBURSABLE AMOUNT N Service Rebill:
Not used.
Notes on Response Pricing Segment on a Service Rebill Response:
The Response Pricing Segment is situational for a Service Rebill Response when the Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) is “C” (Captured).
The Response Pricing Segment is not used in payer-to-payer transactions.
All dollar fields except Patient Pay Amount (5Ø5-F5) are estimated amounts. If actual amounts are returned on fields other than Patient Pay
Amount (5Ø5-F5), the “P” (Paid) response must be used.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 319 -
If the Transaction Response Status (112-AN) = C (Captured) or Q (Duplicate of Captured), dollar fields should be supplied in the response.
• If the response is a “true” Capture (i.e. replacement of batch billing, with no edits or pricing), then corresponding response fields
should be populated with values as submitted. Ideally, processor should provide “real” patient financial responsibility values on a
Capture. If this is not possible, provider must know (by trading partner agreement) the patient financial responsibility to charge and
factor that into their system so collection occurs.
• If the response is captured by an Intermediary who can provide better pricing criteria, the corresponding response fields should be
populated with the probable values and those values used to determine estimated pricing as noted above. Since the claim has not
been fully adjudicated, this should remain a capture response.
It is highly recommended that whenever possible, the individual dollar fields are to be returned in the response. On the response the sender
should return the individual payment response fields to allow the receiver to reconcile against the requested payment fields.
See section “Response Processing Guidelines”, “Pricing Guidelines”.
Fields defined as Mandatory are required to be submitted when the segment is sent.
11.7.3 TRANSMISSION ACCEPTED/TRANSACTION REJECTED
11.7.3.1 DIAGRAM FOR TRANSMISSION OF ONE SERVICE REBILL RESPONSE (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
Service Rebill transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “R” (Rejected)
Service Rebill transactions - If the service rebill reversal is not processed successfully, a “R” (Rejected) response must be sent.
The Response Prior Authorization Segment is not used in payer-to-payer transactions. Therefore, in this case, there are no situational
transaction-level segments.
The duplicate response codes for the Service Rebill transaction are not applicable. See section “Response Processing Guidelines”, “Duplicate
Transactions” for the handling of a duplicate transaction.
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Prior Authorization Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
11.7.3.2 DIAGRAM FOR TRANSMISSION OF TWO SERVICE REBILL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 320 -
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Prior Authorization Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Prior Authorization Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
11.7.3.3 DIAGRAM FOR TRANSMISSION OF THREE SERVICE REBILL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Prior Authorization Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Prior Authorization Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 321 -
Response Prior Authorization Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
11.7.3.4 DIAGRAM FOR TRANSMISSION OF FOUR SERVICE REBILL RESPONSES (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Prior Authorization Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Prior Authorization Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Prior Authorization Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Prior Authorization Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
11.7.3.5 SERVICE REBILL RESPONSE SEGMENTS (TRANSMISSION ACCEPTED/TRANSACTION
REJECTED)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 322 -
11.7.3.5.1 RESPONSE HEADER SEGMENT (SERVICE REBILL) (TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M For Transaction Code of “S3”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “2” (Service Billing).
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Service Rebill Response:
The Response Header Segment is a mandatory, fixed length segment for Service Rebill response when the Header Response Status (5Ø1-
F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). The “Situation” column is not applicable.
11.7.3.5.2 RESPONSE MESSAGE SEGMENT (SERVICE REBILL) (TRANSMISSION ACCEPTED/TRANSACTION
REJECTED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Service Rebill:
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Service Rebill Response:
The Response Message Segment is situational for Service Rebill response when the Header Response Status (5Ø1-F1) of "A" (Accepted) and
Transaction Response Status (112-AN) of “R” (Rejected). It is used when additional text information needs to be sent. Fields defined as
Mandatory are required to be submitted when the segment is sent.
11.7.3.5.3 RESPONSE INSURANCE SEGMENT (SERVICE REBILL) (TRANSMISSION ACCEPTED/TRANSACTION
REJECTED)
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø1-C1 GROUP ID Q Service Rebill:
Required if needed to identify the actual cardholder or
employer group, to identify appropriate group number,
when available.
Required to identify the actual group that was used when
multiple group coverages exist.
Note: This field may contain the Group ID echoed from the
request. May contain the actual Group ID if unknown to the
receiver.
524-FO PLAN ID
Q Service Rebill:
Required if needed to identify the actual plan parameters,
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 323 -
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
benefit, or coverage criteria, when available.
Required to identify the actual plan ID that was used when
multiple group coverages exist.
Required if needed to contain the actual plan ID if unknown
to the receiver.
545-2F NETWORK REIMBURSEMENT ID Q Service Rebill:
Required if needed to identify the network for the covered
member.
Required if needed to identify the actual Network
Reimbursement ID, when applicable and/or available.
Required to identify the actual Network Reimbursement ID
that was used when multiple Network Reimbursement IDs
exist.
568-J7 PAYER ID QUALIFIER Q Service Rebill:
Required if Payer ID (569-J8) is used.
569-J8 PAYER ID
Q Service Rebill:
Required to identify the ID of the payer responding.
115-N5
MEDICAID ID NUMBER N Service Rebill:
Not used.
116-N6
MEDICAID AGENCY NUMBER N Service Rebill:
Not used.
3Ø2-C2
CARDHOLDER ID Q Service Rebill:
Required if the identification to be used in future
transactions is different than what was submitted on the
request.
Notes on Response Insurance Segment on a Service Rebill Response:
The Response Insurance Segment is situational for Service Rebill response when the Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “R” (Rejected). It is used when coverage or reimbursement parameters or identifiers need to be
sent from the sender to the receiver. Fields defined as Mandatory are required to be submitted when the segment is sent.
11.7.3.5.4 RESPONSE PATIENT SEGMENT (SERVICE REBILL) (TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
RESPONSE PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
31Ø-CA PATIENT FIRST NAME Q Service Rebill:
Required if known.
311-CB PATIENT LAST NAME Q Service Rebill:
Required if known.
3Ø4-C4 DATE OF BIRTH Q Service Rebill:
Required if known.
Notes on Response Patient Segment on a Service Rebill Response:
The Response Patient Segment is situational for Service Rebill transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “R” (Rejected) when patient demographic information needs to be sent from the sender to the
receiver. Fields defined as Mandatory are required to be submitted when the segment is sent.
11.7.3.5.5 RESPONSE STATUS SEGMENT (SERVICE REBILL) (TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER
Q Service Rebill:
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT R Service Rebill:
Maximum count of 5.
Required.
511-FB REJECT CODE
R***R*** Service Rebill:
Required.
546-4F REJECT FIELD OCCURRENCE INDICATOR
Q***R*** Service Rebill:
Required if a repeating field is in error, to identify repeating
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 324 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
field occurrence.
This field must be sent when relaying error information about
a repeating field or set. Note, if the Reject Code is not
denoting a repeating field or set, the Reject Field Occurrence
Indicator must not be sent.
547-5F APPROVED MESSAGE CODE COUNT
N Service Rebill:
Not used.
548-6F APPROVED MESSAGE CODE
N***R*** Service Rebill:
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Service Rebill:
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Service Rebill:
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Service Rebill:
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Service Rebill:
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER
Q Service Rebill:
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Service Rebill:
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Service Rebill:
Not used.
993-A7 INTERNAL CONTROL NUMBER N Service Rebill:
Not used.
987-MA URL I Service Rebill:
Provided for informational purposes only to relay health
care communications via the Internet.
Notes on Response Status Segment on a Service Rebill Response:
The Response Status Segment is mandatory for a Service Rebill Response for Header Response Status (5Ø1-F1) = “A” (Accepted) and
Transaction Response Status (112-AN) of “R” (Rejected). The Response Status Segment is sent from the sender to the receiver to identify the
outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.
11.7.3.5.6 RESPONSE CLAIM SEGMENT (SERVICE REBILL) (TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 325 -
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M For Transaction Code of “S3”, in the Response Claim
Segment, the Prescription/Service Reference Number
Qualifier (455-EM) is “2” (Service Billing).
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT
N Service Rebill:
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER N***R*** Service Rebill:
Not used.
553-AR PREFERRED PRODUCT ID
N***R*** Service Rebill:
Not used.
554-AS PREFERRED PRODUCT INCENTIVE
N***R*** Service Rebill:
Not used.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE N***R*** Service Rebill:
Not used.
556-AU PREFERRED PRODUCT DESCRIPTION
N***R*** Service Rebill:
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Service Rebill:
Not used.
Notes on Response Claim Segment on a Service Rebill Response:
The Response Claim Segment is mandatory for a Service Rebill Response when the Header Response Status (5Ø1-F1) is “A” (Accepted) and
Transaction Response Status (112-AN) of “R” (Rejected). The Response Claim Segment is sent from the sender to the receiver to mirror back
the Prescription/Service Reference Number (4Ø2-D2). Fields defined as Mandatory are required to be submitted when the segment is sent.
11.7.3.5.7 RESPONSE PRIOR AUTHORIZATION SEGMENT (SERVICE REBILL) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE PRIOR AUTHORIZATION SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
498-PR PRIOR AUTHORIZATION PROCESSED DATE N Service Rebill:
Not used.
498-PS PRIOR AUTHORIZATION EFFECTIVE DATE N Service Rebill:
Not used.
498-PT PRIOR AUTHORIZATION EXPIRATION DATE N Service Rebill:
Not used.
498-RA PRIOR AUTHORIZATION QUANTITY N Service Rebill:
Not used.
498-RB PRIOR AUTHORIZATION DOLLARS AUTHORIZED N Service Rebill:
Not used.
498-PW PRIOR AUTHORIZATION NUMBER OF REFILLS AUTHORIZED N Service Rebill:
Not used.
498-PX PRIOR AUTHORIZATION QUANTITY ACCUMULATED N Service Rebill:
Not used.
498-PY PRIOR AUTHORIZATION NUMBER–ASSIGNED Q Service Rebill:
Required when the receiver must submit this Prior
Authorization Number in order to receive payment for the
claim. (An example of a situation may include a Benefit
Transition Period that allows for payment of claims, for a
period of time that would normally reject.)
Notes on Response Prior Authorization Segment on a Service Rebill Response:
The Response Prior Authorization Segment is situational for a Service Rebill response when the Header Response Status (5Ø1-F1) is “A”
(Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). It is used to relay the Prior Authorization Number - Assigned (498-
PY) which is returned when a Reject Code (511-FB) denotes that a prior authorization code needs to be submitted on the subsequent billing.
The Response Prior Authorization Segment is not used in payer-to-payer transactions.
Fields defined as Mandatory are required to be submitted when the segment is sent.
11.7.3.5.8 RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS SEGMENT (SERVICE REBILL) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 326 -
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS
SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
355-NT OTHER PAYER ID COUNT M Service Rebill:
Maximum count of 3.
338-5C OTHER PAYER COVERAGE TYPE M***R***
339-6C OTHER PAYER ID QUALIFIER Q***R*** Service Rebill:
Required if Other Payer ID (34Ø-7C) is used.
34Ø-7C OTHER PAYER ID Q***R*** Service Rebill:
Required if other insurance information is available for
coordination of benefits.
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER Q***R*** Service Rebill:
Required if other insurance information is available for
coordination of benefits.
356-NU OTHER PAYER CARDHOLDER ID Q***R*** Service Rebill:
Required if other insurance information is available for
coordination of benefits.
992-MJ OTHER PAYER GROUP ID Q***R*** Service Rebill:
Required if other insurance information is available for
coordination of benefits.
142-UV OTHER PAYER PERSON CODE Q***R*** Service Rebill:
Required if needed to uniquely identify the family members
within the Cardholder ID, as assigned by the other payer.
127-UB OTHER PAYER HELP DESK PHONE NUMBER Q***R*** Service Rebill:
Required if needed to provide a support telephone number
of the other payer to the receiver.
143-UW OTHER PAYER PATIENT RELATIONSHIP CODE Q***R*** Service Rebill:
Required if needed to uniquely identify the relationship of
the patient to the cardholder ID, as assigned by the other
payer.
144-UX OTHER PAYER BENEFIT EFFECTIVE DATE Q***R*** Service Rebill:
Required when other coverage is known which is after the
Date of Service submitted.
145-UY OTHER PAYER BENEFIT TERMINATION DATE Q***R*** Service Rebill:
Required when other coverage is known which is after the
Date of Service submitted.
Notes on Response Coordination of Benefits/Other Payers Segment on a Service Rebill Response:
The Response Coordination of Benefits/Other Payers Segment is situational for a Service Rebill response when the Header Response Status
(5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “R” (Rejected) when other insurance information is available for
coordination of benefits.
1. If the identity of the patient is partially verified and the Service Rebill is rejected due to a non-match of field verification, then the
Other Payer information is not sent.
2. If the service is rejected because it should be submitted to other payer(s) first, that Other Payer information should be sent, if known.
3. If the service is rejected due to benefit design limitations, then subsequent Other Payer information should be sent, if known.
If the service rejects for other reasons than above, Other Payer information is not sent.
If additional payer(s) for this patient is not known, the Other Payer information is not sent.
If additional payer(s) for this patient is known, the following may be sent:
• Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C),
• Other Payer Group ID (992-MJ),
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU)
• And other Other Payer fields.
In addition, if any of the following three fields are sent:
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU),
• Other Payer Group ID (992-MJ),
then the Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C) must be sent.
Fields defined as Mandatory are required to be submitted when the segment is sent.
11.7.4 TRANSMISSION REJECTED/TRANSACTION REJECTED
Service Rebill transmission response Header Response Status (5Ø1-F1) of "R" (Rejected)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 327 -
and Transaction Response Status (112-AN) of “R” (Rejected)
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
11.7.4.1 DIAGRAM FOR TRANSMISSION OF ONE SERVICE REBILL RESPONSE (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
11.7.4.2 DIAGRAM FOR TRANSMISSION OF TWO SERVICE REBILL RESPONSES (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
11.7.4.3 DIAGRAM FOR TRANSMISSION OF THREE SERVICE REBILL RESPONSES (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
11.7.4.4 DIAGRAM FOR TRANSMISSION OF FOUR SERVICE REBILL RESPONSES (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 328 -
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
11.7.4.5 SERVICE REBILL RESPONSE SEGMENTS (TRANSMISSION REJECTED/TRANSACTION
REJECTED)
11.7.4.5.1 RESPONSE HEADER SEGMENT (SERVICE REBILL) (TRANSMISSION REJECTED/TRANSACTION REJECTED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Service Rebill Response:
The Response Header Segment is a mandatory, fixed length segment for Service Rebill response when the Header Response Status (5Ø1-
F1) is “R” (Rejected) and Transaction Response Status (112-AN) of “R” (Rejected). The “Situation” column is not applicable.
If either the entire transmission or the Header is in error, the Header Response Status (5Ø1-F1) = “R” (Rejected). Every identifiable transaction
within the transmission must be rejected with an “R”.
If the transaction rejects for detail errors, the Header Response Status (5Ø1-F1) = “A” (Accepted) and the Transaction Response Status (112-
AN) will be “R” (Rejected).
11.7.4.5.2 RESPONSE MESSAGE SEGMENT (SERVICE REBILL) (TRANSMISSION REJECTED/TRANSACTION
REJECTED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Service Rebill:
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 329 -
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Service Rebill Response:
The Response Message Segment is situational for Service Rebill response when the Header Response Status (5Ø1-F1) is “R” (Rejected) and
Transaction Response Status (112-AN) of “R” (Rejected). It is used when additional text information needs to be sent. Fields defined as
Mandatory are required to be submitted when the segment is sent.
11.7.4.5.3 RESPONSE STATUS SEGMENT (SERVICE REBILL) (TRANSMISSION REJECTED/TRANSACTION REJECTED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Service Rebill:
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT R Service Rebill:
Maximum count of 5.
Required.
511-FB REJECT CODE
R***R*** Service Rebill:
Required.
546-4F REJECT FIELD OCCURRENCE INDICATOR
Q***R*** Service Rebill:
Required if a repeating field is in error, to identify repeating
field occurrence.
This field must be sent when relaying error information about
a repeating field or set. Note, if the Reject Code is not
denoting a repeating field or set, the Reject Field Occurrence
Indicator must not be sent.
547-5F APPROVED MESSAGE CODE COUNT N Service Rebill:
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Service Rebill:
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Service Rebill:
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Service Rebill:
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION
Q***R*** Service Rebill:
Required if additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Service Rebill:
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 330 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER
Q Service Rebill:
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Service Rebill:
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Service Rebill:
Not used.
993-A7 INTERNAL CONTROL NUMBER N Service Rebill:
Not used.
987-MA URL N Service Rebill:
Not used.
Notes on Response Status Segment on a Service Rebill Response:
The Response Status Segment is mandatory for a Service Rebill Response for Header Response Status (5Ø1-F1) = “R” (Rejected) and
Transaction Response Status (112-AN) of “R” (Rejected). The Response Status Segment is sent from the sender to the receiver to identify the
outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 331 -
12. PRIOR AUTHORIZATION INFORMATION
The Prior Authorization transactions allow a Processor to authorize, authorize and immediately adjudicate the claim or service, defer, or pend
the request for review.
The Prior Authorization transactions include:
• Prior Authorization Request and Billing
• Prior Authorization Reversal
• Prior Authorization Inquiry
• Prior Authorization Request Only
See the section “Transmission Structure” for required segments. Prior authorization transactions in Version D and above allow providers and
payers to electronically communicate the need for and approval to dispense special situation medications. Only one transaction per
transmission is permitted.
Prior Authorization reversals are used to back out the request for authorization, but not any claims submitted against the prior authorization. To
reverse a Prior Authorization Request and Billing, paid billings must be reversed before the prior authorization is reversed. The pharmacy must
submit a Claim or Service Reversal (Transaction Code = B2) before submitting a Prior Authorization Reversal request. If there are no Claims
or Services paid for the Prior Authorization in question, the processor must accept the Prior Authorization Reversal for the prior authorization
only.
Please see the section “Prior Authorization Transaction Discussion”.
The transactions are described below.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 332 -
13. PRIOR AUTHORIZATION REQUEST AND BILLING INFORMATION
This transaction allows the Originator to request simultaneous adjudication/capture of the transaction by the Processor upon approval of the
prior authorization. This transaction allows the prior authorization function and the adjudication/capture function to happen within one request.
Each prior authorization request and billing request contains one occurrence of claim/service data. The Transaction Code is “P1”.
The Processor must provide one of the following general types of responses:
Captured - The Processor acknowledges receipt of a prior authorization request and billing but is not making any judgment about
the request at this time.
Duplicate of Captured - This occurs when the Processor has previously received the request and processed the transaction, but the
response did not return to the Originator. The Duplicate response contains the same information as returned in the Captured original
response.
Deferred - The Processor notifies the Originator of a deferment of a prior authorization request and billing. If a duplicate request is
received, the original response must be returned.
Paid - The Processor approves the authorization and adjudicates the claim or service in the same request.
Duplicate of Paid - This occurs when the Processor has previously received the request and processed the transaction, but the
response did not return to the Originator. The Duplicate response contains the same information as returned in the Paid original
response.
Rejected - The Processor has encountered an error in the transaction or processing, or does not approve the prior authorization
request.
See section “Response Processing Guidelines”, “Duplicate Transactions”.
13.1 PRIOR AUTHORIZATION REQUEST AND BILLING REQUEST DIAGRAMS
13.1.1 DIAGRAM FOR TRANSMISSION OF ONE PRIOR AUTHORIZATION REQUEST AND
BILLING TRANSACTION
For a Prior Authorization Request And Billing, the scenarios defined include
Prior Authorization Request and Billing from a Sender to a Receiver
Prior Authorization Request and Billing Paid/Captured/Deferred Transaction Response from a Sender to a Receiver
Standard Transmission Accepted/Transaction Rejected Response from a Sender to a Receiver
Standard Transmission Reject Response to a Prior Authorization Request And Billing from a Sender to a Receiver
Each Prior Authorization Request And Billing request contains one occurrence of claim/service data.
The Compound Segment is not used in when the Prior Authorization Request And Billing is for a service (Prescription/Service Reference
Number Qualifier (455-EM) = “2” (Service Billing)).
Mandatory
Transaction Header Segment
Segment Separator
Insurance Segment
Situational
Segment Separator
Patient Segment
Mandatory - Prior Authorization Request and Billing
Group Separator
Segment Separator
Claim Segment
Segment Separator
Pricing Segment
Segment Separator
Prior Authorization Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Coordination of Benefits/Other Payments Segment

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 333 -
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Compound Segment
Segment Separator
Clinical Segment
Segment Separator
Additional Documentation Segment
Segment Separator
Facility Segment
Segment Separator
Narrative Segment
13.2 PRIOR AUTHORIZATION REQUEST AND BILLING REQUEST SEGMENTS
13.2.1 TRANSACTION HEADER SEGMENT (PRIOR AUTHORIZATION REQUEST AND
BILLING)
TRANSACTION HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø1-A1 BIN NUMBER M
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø4-A4 PROCESSOR CONTROL NUMBER M
1Ø9-A9 TRANSACTION COUNT M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M If the Date of Service (4Ø1-D1) contains the subsequent
payer coverage date, the Submission Clarification Code
(42Ø-DK) is required with value of “19” (Split Billing –
indicates the quantity dispensed is the remainder billed to a
s
ubsequent payer when Medicare Part A expires. Used only
in long-term care settings) for individual unit of use
medications.
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M
Notes on Transaction Header Segment on a Prior Authorization Request And Billing Request:
The Transaction Header Segment is a mandatory, fixed length segment for a Prior Authorization Request And Billing request. The “Situation”
column is not applicable.
13.2.2 INSURANCE SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING)
INSURANCE SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø2-C2 CARDHOLDER ID M
312-CC CARDHOLDER FIRST NAME
Q Prior Authorization Request And Billing (Claim/Service):
Required if the Patient is the Cardholder, and Date of Birth
(3Ø4-C4) is not available. (Note: Cardholder ID (3Ø2-C2) is
mandatory.)
Not used when Cardholder ID (3Ø2-C2), Date of Birth
(3Ø4-C4), and Person Code (3Ø3-C3) are present.
It is a recommendation that Cardholder ID (3Ø2-C2) and
Date of Birth (3Ø4-C4) are used.
Required if necessary for state/federal/regulatory agency or
Workers’ Compensation programs.
Required if multiple people have the same Cardholder ID.
313-CD CARDHOLDER LAST NAME
Q Prior Authorization Request And Billing (Claim/Service):
Required if the Patient is the Cardholder, and the Date of
Birth (3Ø4-C4) is not available.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 334 -
INSURANCE SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if contractually obligated between trading
partners.
Not used when Cardholder ID (3Ø2-C2), Date of Birth
(3Ø4-C4), and Person Code (3Ø3-C3) are present.
It is a recommendation that Cardholder ID (3Ø2-C2) and
Date of Birth (3Ø4-C4) are used.
Required if necessary for state/federal/regulatory agency or
Workers’ Compensation programs.
Required if multiple people have the same Cardholder ID.
314-CE HOME PLAN
.
Q Prior Authorization Request And Billing (Claim/Service):
Required if needed for receiver inquiry validation and/or
determination for Blue Cross or Blue Shield, if a Patient has
coverage under more than one plan, to distinguish each
plan.
524-FO PLAN ID
Q Prior Authorization Request And Billing (Claim/Service):
Required if needed for pharmacy claim processing and
payment.
3Ø9-C9 ELIGIBILITY CLARIFICATION CODE
Q Prior Authorization Request And Billing (Claim/Service):
Required if needed for receiver inquiry validation and/or
determination, when eligibility is not maintained at the
dependent level. Required in special situations as defined
by the code to clarify the eligibility of an individual, which
may extend coverage.
3Ø1-C1 GROUP ID
Q Prior Authorization Request And Billing (Claim/Service):
Required if necessary for state/federal/regulatory agency
programs.
Required if needed for pharmacy claim processing and
payment.
3Ø3-C3 PERSON CODE
Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to uniquely identify the family members
within the Cardholder ID.
3Ø6-C6 PATIENT RELATIONSHIP CODE
Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to uniquely identify the relationship of
the Patient to the Cardholder ID.
99Ø-MG OTHER PAYER BIN NUMBER N Prior Authorization Request And Billing (Claim/Service):
Not used.
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER N Prior Authorization Request And Billing (Claim/Service):
Not used.
356-NU OTHER PAYER CARDHOLDER ID N Prior Authorization Request And Billing (Claim/Service):
Not used.
992-MJ OTHER PAYER GROUP ID N Prior Authorization Request And Billing (Claim/Service):
Not used.
359-2A MEDIGAP ID Q Prior Authorization Request And Billing (Claim/Service):
Required, if known, when patient has Medigap coverage.
36Ø-2B MEDICAID INDICATOR Q Prior Authorization Request And Billing (Claim/Service):
Required, if known, when patient has Medicaid coverage.
361-2D PROVIDER ACCEPT ASSIGNMENT INDICATOR Q Prior Authorization Request And Billing (Claim/Service):
Required if necessary for state/federal/regulatory agency
programs.
997-G2 CMS PART D DEFINED QUALIFIED FACILITY Q
N
Prior Authorization Request And Billing (Claim):
Required if specified in trading partner agreement.
Service:
Not used.
115-N5
MEDICAID ID N Prior Authorization Request And Billing (Claim/Service):
Not used.
116-N6
MEDICAID AGENCY NUMBER N Prior Authorization Request And Billing (Claim/Service):
Not used.
Notes on Insurance Segment on a Prior Authorization Request And Billing Request:
The Insurance Segment is mandatory for a Prior Authorization Request And Billing request. Fields defined as Mandatory are required to be
submitted when the segment is sent.
13.2.3 PATIENT SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING)
PATIENT SEGMENT
SITUATIONAL SEGMENT

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 335 -
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
331-CX PATIENT ID QUALIFIER Q Prior Authorization Request And Billing (Claim/Service):
Required if Patient ID (332-CY) is used.
332-CY PATIENT ID
Q Prior Authorization Request And Billing (Claim/Service):
Required if necessary for state/federal/regulatory agency
programs to validate dual eligibility.
3Ø4-C4 DATE OF BIRTH
R Prior Authorization Request And Billing (Claim/Service):
Required.
3Ø5-C5 PATIENT GENDER CODE
R Prior Authorization Request And Billing (Claim/Service):
Required.
31Ø-CA PATIENT FIRST NAME
Q Prior Authorization Request And Billing (Claim/Service):
Required when the patient has a first name.
311-CB PATIENT LAST NAME
R Prior Authorization Request And Billing (Claim/Service):
Required.
322-CM PATIENT STREET ADDRESS
O Prior Authorization Request And Billing (Claim/Service):
Optional.
323-CN PATIENT CITY ADDRESS
O Prior Authorization Request And Billing (Claim/Service):
Optional.
324-CO PATIENT STATE / PROVINCE ADDRESS
O Prior Authorization Request And Billing (Claim/Service):
Optional.
325-CP PATIENT ZIP/POSTAL ZONE
O Prior Authorization Request And Billing (Claim/Service):
Optional.
326-CQ PATIENT PHONE NUMBER
O Prior Authorization Request And Billing (Claim/Service):
Optional.
3Ø7-C7 PLACE OF SERVICE
Q Prior Authorization Request And Billing (Claim/Service):
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
333-CZ EMPLOYER ID
.
Q Prior Authorization Request And Billing (Claim/Service):
Required if “required by law” as defined in the HIPAA final
Privacy regulations section 164.5Ø1 definitions (45 CFR
Parts 160 and 164 Standards for Privacy of Individually
Identifiable Health Information; Final Rule -
Thursday, December 28, 2000, page 82803 and following,
and Wednesday, August 14, 2002, page 53267 and
following.)
Required if needed for Workers’ Compensation billing.
334-1C SMOKER / NON-SMOKER CODE
Q Prior Authorization Request And Billing (Claim/Service):
Required if clinical determination is dependent upon
patient’s smoking condition.
335-2C PREGNANCY INDICATOR
Q Prior Authorization Request And Billing (Claim/Service):
Required if pregnancy could result in different coverage,
pricing, or patient financial responsibility.
35Ø-HN PATIENT E-MAIL ADDRESS N Prior Authorization Request And Billing (Claim/Service):
Not used.
384-4X PATIENT RESIDENCE Q Prior Authorization Request And Billing (Claim/Service):
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
Notes on Patient Segment on a Prior Authorization Request And Billing Request:
The Patient Segment is situational for a Prior Authorization Request And Billing request. The Patient Segment must be submitted when
needed to differentiate between the patient and the cardholder. If the cardholder and the patient are the same, then the Patient Segment is not
submitted unless additional information about the patient is needed to clarify the Prior Authorization Request And Billing. The Segment is
mandatory if required under provider payer contract or mandatory on Prior Authorization Request And Billing where this information is
necessary for processing a prior authorization and/or adjudication of the claim. Fields defined as Mandatory are required to be submitted when
the segment is sent.
13.2.4 CLAIM SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING)
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
436-E1 PRODUCT/SERVICE ID QUALIFIER M Mandatory.
If billing for a multi-ingredient prescription, Product/Service
ID Qualifier (436-E1) is zero (Zero means “ØØ”).
If the Product/Service ID Qualifier (436-E1) = “Ø6”
(DUR/PPS), the Product/Service ID (4Ø7-D7) is zero. (Zero

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 336 -
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
means “Ø”.)
4Ø7-D7 PRODUCT/SERVICE ID M Mandatory.
If billing for a multi-ingredient prescription, Product/Service
ID (4Ø7-D7) is zero. (Zero means “Ø”.)
If the Product/Service ID Qualifier (436-E1) = “Ø6”
(DUR/PPS), the Product/Service ID (4Ø7-D7) is zero. (Zero
means “Ø”.) Populate the DUR/PPS segment as
appropriate.
If the Product/Service ID Qualifier (436-E1) = “Ø7” (CPT-4),
the Product Service ID (4Ø7-D7) is the actual CPT-4 value.
If the Product/Service ID Qualifier (436-E1) = “Ø9”
(HCPCS), the Product Service ID (4Ø7-D7) is the actual
HCPCS value.
If the Product/Service ID Qualifier (436-E1) = “99” (Other),
the Product Service ID (4Ø7-D7) is the business partner
agreed value.
456-EN ASSOCIATED PRESCRIPTION/SERVICE REFERENCE NUMBER Q Prior Authorization Request And Billing (Claim):
Required if the “completion” transaction in a partial fill
(Dispensing Status (343-HD) = “C” (Completed)).
See section “Specific Segment Discussion”, “Request
Segments”, Claim Segment” for more information.
Required if the Dispensing Status (343-HD) = “P” (Partial
Fill) and there are multiple occurrences of partial fills for this
prescription.
Service:
Required to associate the service to the product.
Contains the Prescription/Service Reference Number (4Ø2-
D2) of the prescription or service that prompted the service.
Required if Associated Prescription/Service Date (457-EP)
is used.
Required if needed to associate multiple
prescriptions/services from the same sender to allow billing
of the current prescription/service.
457-EP ASSOCIATED PRESCRIPTION/SERVICE DATE Q Prior Authorization Request And Billing (Claim):
Required if the “completion” transaction in a partial fill
(Dispensing Status (343-HD) = “C” (Completed).
Required if Associated Prescription/Service Reference
Number (456-EN) is used.
See section “Specific Segment Discussion”, “Request
Segments”, Claim Segment” for more information.
Required if needed to associate multiple prescriptions
within the same sender.
Required if the Dispensing Status (343-HD) = “P” (Partial
Fill) and there are multiple occurrences of partial fills for this
prescription.
Service:
Required to associate the service to the product.
Contains the service date of the prescription or service that
prompted the service.
Required if Associated Prescription/Service Reference
Number (456-EN) is used.
Required if needed to associate multiple
prescriptions/services from the same sender to allow billing
of the current prescription/service.
458-SE PROCEDURE MODIFIER CODE COUNT
Q Prior Authorization Request And Billing (Claim):
Maximum count of 1Ø.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 337 -
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if Procedure Modifier Code (459-ER) is used.
Service:
Maximum count of 1Ø.
Required if Procedure Modifier Code (459-ER) is used.
459-ER PROCEDURE MODIFIER CODE Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required to define a further level of specificity if the
Product/Service ID (4Ø7-D7) indicated a Procedure Code
was submitted.
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
Occurs the number of times identified in Procedure Modifier
Code Count (458-SE).
442-E7 QUANTITY DISPENSED R
Q
Prior Authorization Request And Billing (Claim):
Required.
Service:
Required if value is greater than zero (Ø).
4Ø3-D3 FILL NUMBER R
Q
Prior Authorization Request And Billing (Claim):
Required.
Service:
Required if necessary for plan benefit administration.
4Ø5-D5 DAYS SUPPLY R
Q
Prior Authorization Request And Billing (Claim):
Required.
Service:
Required if necessary for plan benefit administration.
4Ø6-D6 COMPOUND CODE R
N
Prior Authorization Request And Billing (Claim):
Required.
Service:
Not used.
4Ø8-D8 DISPENSE AS WRITTEN (DAW)/PRODUCT SELECTION CODE R
N
Prior Authorization Request And Billing (Claim):
Required.
Service:
Not used.
414-DE DATE PRESCRIPTION WRITTEN R
Q
Prior Authorization Request And Billing (Claim/Service):
Required.
Service:
Required if necessary for plan benefit administration.
415-DF NUMBER OF REFILLS AUTHORIZED Q Prior Authorization Request And Billing (Claim/Service):
Required if necessary for plan benefit administration.
419-DJ PRESCRIPTION ORIGIN CODE Q
N
Prior Authorization Request And Billing (Claim):
Required if necessary for plan benefit administration.
Service:
Not used.
354-NX SUBMISSION CLARIFICATION CODE COUNT Q
N
Prior Authorization Request And Billing (Claim):
Maximum count of 3.
Required if Submission Clarification Code (42Ø-DK) is
used.
Service:
Not used.
42Ø-DK SUBMISSION CLARIFICATION CODE Q***R***
Prior Authorization Request And Billing (Claim):
Required if clarification is known and values greater than
zero (Ø).
Occurs the number of times identified in Submission
Clarification Code Count (354-NX).
If the Date of Service (4Ø1-D1) contains the subsequent
payer coverage date, the Submission Clarification Code
(42Ø-DK) is required with value of “19” (Split Billing –
indicates the quantity dispensed is the remainder billed to a

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 338 -
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
N
subsequent payer when Medicare Part A expires. Used
only in long-term care settings) for individual unit of use
medications.
Service:
Not used.
46∅-ET QUANTITY PRESCRIBED N
Q
Prior Authorization Request And Billing (Claim):
Not used.
Service:
Required if the prescriber orders a specific number of
iterations of a service.
Not required if value is equal to 1.
3Ø8-C8 OTHER COVERAGE CODE Q Prior Authorization Request And Billing (Claim/Service):
Required if needed by receiver, to communicate a
summation of other coverage information that has been
collected from other payers.
Required for Coordination of Benefits.
See section “Specific Segment Discussion”, “Request
Segments”, “Claim Segment”, “Other Coverage Code (3Ø8-
C8).
429-DT SPECIAL PACKAGING INDICATOR Q
N
Prior Authorization Request And Billing (Claim):
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
Service:
Not used.
453-EJ ORIGINALLY PRESCRIBED PRODUCT/SERVICE ID QUALIFIER Q Prior Authorization Request And Billing (Claim/Service):
Required if Originally Prescribed Product/Service Code
(445-EA) is used.
445-EA ORIGINALLY PRESCRIBED PRODUCT/SERVICE CODE Q Prior Authorization Request And Billing (Claim/Service):
Required if the receiver requests association to a
therapeutic, or a preferred product substitution, or when a
DUR alert has been resolved by changing medications, or
an alternative service than what was originally prescribed.
446-EB ORIGINALLY PRESCRIBED QUANTITY Q Prior Authorization Request And Billing (Claim/Service):
Required if the receiver requests reporting for quantity
changes due to a therapeutic substitution that has occurred
or a preferred product/service substitution that has
occurred, or when a DUR alert has been resolved by
changing quantities.
33Ø-CW ALTERNATE ID N Prior Authorization Request And Billing (Claim/Service):
Not used.
454-EK SCHEDULED PRESCRIPTION ID NUMBER N Prior Authorization Request And Billing (Claim/Service):
Not used.
6ØØ-28 UNIT OF MEASURE Q
N
Prior Authorization Request And Billing (Claim):
Required if necessary for state/federal/regulatory agency
programs.
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
Service:
Not used.
418-DI LEVEL OF SERVICE Q Prior Authorization Request And Billing (Claim/Service):
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
461-EU PRIOR AUTHORIZATION TYPE CODE N Prior Authorization Request And Billing (Claim/Service):
Not used.
462-EV PRIOR AUTHORIZATION NUMBER SUBMITTED N Prior Authorization Request And Billing (Claim/Service):
Not used.
463-EW INTERMEDIARY AUTHORIZATION TYPE ID
Q Prior Authorization Request And Billing (Claim/Service):
Required for overriding an authorized intermediary system
edit when the pharmacy participates with an intermediary.
Required if Intermediary Authorization ID (464-EX) is used.
464-EX INTERMEDIARY AUTHORIZATION ID Q Prior Authorization Request And Billing (Claim/Service):
Required for overriding an authorized intermediary system
edit when the pharmacy participates with an intermediary.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 339 -
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
343-HD DISPENSING STATUS Q
N
Prior Authorization Request And Billing (Claim):
Required for the partial fill or the completion fill of a
prescription.
Service:
Not used.
344-HF QUANTITY INTENDED TO BE DISPENSED Q
N
Prior Authorization Request And Billing (Claim):
Required for the partial fill or the completion fill of a
prescription.
Service:
Not used.
345-HG DAYS SUPPLY INTENDED TO BE DISPENSED Q
N
Prior Authorization Request And Billing (Claim):
Required for the partial fill or completion fill of a
prescription.
Service:
Not used.
357-NV DELAY REASON CODE Q Prior Authorization Request And Billing (Claim/Service):
Required when needed to specify the reason that
submission of the transaction has been delayed.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Prior Authorization Request And Billing (Claim/Service):
Not used.
391-MT PATIENT ASSIGNMENT INDICATOR (DIRECT MEMBER
REIMBURSEMENT INDICATOR)
Q Prior Authorization Request And Billing (Claim/Service):
Required when the claims adjudicator does not assume the
patient assigned his/her benefits to the provider or when
the claims adjudicator supports a patient determination of
whether he/she wants to assign or retain his/her benefits.
995-E2 ROUTE OF ADMINISTRATION Q
N
Prior Authorization Request And Billing (Claim):
Required if an override to the “default” route of
administration is specified for the product For a multi-
ingredient compound, it is the route of the complete
compound mixture.
Service:
Not used.
996-G1 COMPOUND TYPE Q
N
Prior Authorization Request And Billing (Claim):
Required if specified in trading partner agreements
involving IV therapy delineate separate reimbursement
structures for different therapy types.
Service:
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Prior Authorization Request And Billing (Claim/Service):
Not used.
147-U7 PHARMACY SERVICE TYPE Q Prior Authorization Request And Billing (Claim/Service):
Required when the submitter must clarify the type of
services being performed as a condition for proper
reimbursement by the payer.
Notes on Claim Segment on a Prior Authorization Request And Billing Request:
The Claim Segment is mandatory for a Prior Authorization Request And Billing request. The Claim Segment defines the product dispensed,
dispensing information, reference information for tieback to an original prescription in the case of partial fillings, or authorization information.
Fields defined as Mandatory are required to be submitted when the segment is sent.
13.2.5 PRICING SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING)
PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
4Ø9-D9 INGREDIENT COST SUBMITTED R
N
Prior Authorization Request And Billing (Claim):
Required.
Service:
Not used.
412-DC DISPENSING FEE SUBMITTED Q
Prior Authorization Request And Billing (Claim):
Required if its value has an effect on the Gross Amount
Due (43Ø-DU) calculation.
Zero (Ø) is a valid value.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 340 -
PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
N
Service:
Not used.
477-BE PROFESSIONAL SERVICE FEE SUBMITTED N
R
Prior Authorization Request And Billing (Claim):
Not used.
Service:
Required.
433-DX PATIENT PAID AMOUNT SUBMITTED Q Prior Authorization Request And Billing (Claim/Service):
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
Not used in coordination of benefit claim to pass patient
liability information to a downstream payer. See section
“Standard Conventions”, “Repetition and Multiple
Occurrences”, Repeating Data Elements”, “Request
Segments”, “Coordination of Benefits/Other Payments
Segment”.
438-E3 INCENTIVE AMOUNT SUBMITTED Q
N
Prior Authorization Request And Billing (Claim):
Required if its value has an effect on the Gross Amount
Due (43Ø-DU) calculation.
Zero (Ø) is a valid value.
Service:
Not used.
478-H7 OTHER AMOUNT CLAIMED SUBMITTED COUNT
Q Prior Authorization Request And Billing (Claim/Service):
Maximum count of 3.
Required if Other Amount Claimed Submitted Qualifier
(479-H8) is used.
479-H8 OTHER AMOUNT CLAIMED SUBMITTED QUALIFIER
Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if Other Amount Claimed Submitted (48Ø-H9) is
used.
48Ø-H9 OTHER AMOUNT CLAIMED SUBMITTED
Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if its value has an effect on the Gross Amount
Due (43Ø-DU) calculation.
Zero (Ø) is a valid value.
481-HA FLAT SALES TAX AMOUNT SUBMITTED
Q Prior Authorization Request And Billing (Claim/Service):
Required if its value has an effect on the Gross Amount
Due (43Ø-DU) calculation.
Zero (Ø) is a valid value.
482-GE PERCENTAGE SALES TAX AMOUNT SUBMITTED
Q Prior Authorization Request And Billing (Claim/Service):
Required if its value has an effect on the Gross Amount
Due (43Ø-DU) calculation.
Zero (Ø) is a valid value.
483-HE PERCENTAGE SALES TAX RATE SUBMITTED
Q Prior Authorization Request And Billing (Claim):
Required if this field could result in different pricing.
Required if Percentage Sales Tax Rate Submitted (483-HE)
and Percentage Sales Tax Basis Submitted (484-JE) are
used.
Required if needed to calculate Percentage Sales Tax
Amount Paid (559-AX).
Service:
Required if needed to calculate Percentage Sales Tax
Amount Paid (559-AX).
484-JE PERCENTAGE SALES TAX BASIS SUBMITTED
Q
N
Prior Authorization Request And Billing (Claim):
Required if Percentage Sales Tax Amount Submitted (482-
GE) and Percentage Sales Tax Rate Submitted (483-HE)
are used.
Required if this field could result in different pricing.
Required if needed to calculate Percentage Sales Tax
Amount Paid (559-AX).
Service:
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 341 -
PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Not used. Code list is not applicable.
426-DQ USUAL AND CUSTOMARY CHARGE
Q Prior Authorization Request And Billing (Claim/Service):
Required if needed per trading partner agreement.
43Ø-DU GROSS AMOUNT DUE R Prior Authorization Request And Billing (Claim/Service):
Required.
See Pricing Formula for fields used in calculation.
423-DN BASIS OF COST DETERMINATION
Q
N
Prior Authorization Request And Billing (Claim):
Required if needed for receiver claim/encounter
adjudication.
Service:
Not used.
113-N3
MEDICAID PAID AMOUNT N Prior Authorization Request And Billing (Claim/Service):
Not used.
Notes on Pricing Segment on a Prior Authorization Request And Billing Request:
The Pricing Segment is mandatory for a Prior Authorization Request And Billing request. The Pricing Segment defines dollar amounts and
basis of costs for a Prior Authorization Request And Billing.
It is highly recommended that whenever possible, the individual dollar fields are to be requested of the sender by the receiver. In the response,
the receiver should return the individual payment response fields to allow the sender to reconcile against the requested payment fields. It is
recommended that for the dollar fields, if the field is not required or situational in the calculation, that the dollar fields are not sent.
See section “Response Processing Guidelines”, “Pricing Guidelines”.
Fields defined as Mandatory are required to be submitted when the segment is sent.
13.2.6 PRIOR AUTHORIZATION SEGMENT (PRIOR AUTHORIZATION REQUEST AND
BILLING)
PRIOR AUTHORIZATION SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
498-PA REQUEST TYPE M
498-PB REQUEST PERIOD DATE-BEGIN M
498-PC REQUEST PERIOD DATE-END M
498-PD BASIS OF REQUEST M
498-PE AUTHORIZED REPRESENTATIVE FIRST NAME Q Prior Authorization Request And Billing (Claim/Service):
Required if needed for receiver claim/encounter or prior
authorization determination.
498-PF AUTHORIZED REPRESENTATIVE LAST NAME Q Prior Authorization Request And Billing (Claim/Service):
Required if needed for receiver claim/encounter or prior
authorization determination.
498-PG AUTHORIZED REPRESENTATIVE STREET ADDRESS Q Prior Authorization Request And Billing (Claim/Service):
Required if needed for receiver claim/encounter or prior
authorization determination.
498-PH AUTHORIZED REPRESENTATIVE CITY ADDRESS Q Prior Authorization Request And Billing (Claim/Service):
Required if needed for receiver claim/encounter or prior
authorization determination.
498-PJ AUTHORIZED REPRESENTATIVE STATE/PROVINCE ADDRESS Q Prior Authorization Request And Billing (Claim/Service):
Required if needed for receiver claim/encounter or prior
authorization determination.
498-PK AUTHORIZED REPRESENTATIVE ZIP/POSTAL ZONE Q Prior Authorization Request And Billing (Claim/Service):
Required if needed for receiver claim/encounter or prior
authorization determination.
498-PY PRIOR AUTHORIZATION NUMBER-ASSIGNED Q Prior Authorization Request And Billing (Claim/Service):
Required if the Request Type (498-PA) = 2
(Reauthorization)
5Ø3-F3 AUTHORIZATION NUMBER Q Prior Authorization Request And Billing (Claim/Service):
Required if needed for receiver claim/encounter
determination.
498-PP PRIOR AUTHORIZATION SUPPORTING DOCUMENTATION Q Prior Authorization Request And Billing (Claim/Service):
Required if needed for receiver claim/encounter or prior
authorization determination.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 342 -
Notes on Prior Authorization Segment on a Prior Authorization Request And Billing Request:
The Prior Authorization Segment is mandatory for a Prior Authorization Request And Billing request. It is used when the sender submits a
billing to the receiver that includes the prior authorization approval information. The Segment is mandatory if required under provider payer
contract or mandatory on claims where this information is necessary for processing prior authorization and/or adjudication of the claim. Fields
defined as Mandatory are required to be submitted when the segment is sent.
13.2.7 PHARMACY PROVIDER SEGMENT (PRIOR AUTHORIZATION REQUEST AND
BILLING)
PHARMACY PROVIDER SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
465-EY PROVIDER ID QUALIFIER Q Prior Authorization Request And Billing (Claim/Service):
Required if Provider ID (444-E9) is used.
444-E9 PROVIDER ID
Q Prior Authorization Request And Billing (Claim):
Required if necessary for state/federal/regulatory agency
programs.
Required if necessary to identify the individual responsible
for dispensing of the prescription.
Service:
Required if necessary for state/federal/regulatory agency
programs.
Required if necessary to determine if provider is
credentialed to perform this service.
Notes on Pharmacy Provider Segment on a Prior Authorization Request And Billing Request:
The Pharmacy Provider Segment is situational for a Prior Authorization Request And Billing request if required under provider payer contract
or mandatory on claims where this information is necessary for processing prior authorization and/or adjudication of the claim. Fields defined
as Mandatory are required to be submitted when the segment is sent.
13.2.8 PRESCRIBER SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING)
PRESCRIBER SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
466-EZ PRESCRIBER ID QUALIFIER Q Prior Authorization Request And Billing (Claim/Service):
Required if Prescriber ID (411-DB) is used.
411-DB PRESCRIBER ID
Q Prior Authorization Request And Billing (Claim/Service):
Required if this field could result in different coverage or
patient financial responsibility.
Required if necessary for state/federal/regulatory agency
programs.
427-DR PRESCRIBER LAST NAME
Q Prior Authorization Request And Billing (Claim/Service):
Required when the Prescriber ID (411-DB) is not known.
Required if needed for Prescriber ID (411-DB)
validation/clarification.
498-PM PRESCRIBER PHONE NUMBER Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to assist in identifying the prescriber.
Required if needed for Prior Authorization process.
468-2E PRIMARY CARE PROVIDER ID QUALIFIER Q Prior Authorization Request And Billing (Claim/Service):
Required if Primary Care Provider ID (421-DL) is used.
421-DL PRIMARY CARE PROVIDER ID
Q Prior Authorization Request And Billing (Claim/Service):
Required if needed for receiver claim/encounter or prior
authorization request and billing determination, if known
and available.
Required if this field could result in different coverage or
patient financial responsibility.
Required if necessary for state/federal/regulatory agency
programs.
47Ø-4E PRIMARY CARE PROVIDER LAST NAME
.
Q Prior Authorization Request And Billing (Claim/Service):
Required if this field is used as an alternative for Primary
Care Provider ID (421-DL) when ID is not known.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 343 -
PRESCRIBER SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if needed for Primary Care Provider ID (421-DL)
validation/clarification.
364-2J PRESCRIBER FIRST NAME Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
365-2K PRESCRIBER STREET ADDRESS Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
366-2M PRESCRIBER CITY ADDRESS Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
367-2N PRESCRIBER STATE/PROVINCE ADDRESS Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
368-2P PRESCRIBER ZIP/POSTAL ZONE Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
Notes on Prescriber Segment on a Prior Authorization Request And Billing Request:
The Prescriber Segment is situational for a Prior Authorization Request And Billing request. It is used when prescriber information is needed to
process a Prior Authorization Request and Billing. The Segment is mandatory if required under provider payer contract or mandatory on claims
where this information is necessary for prior authorization and/or adjudication of the claim. Fields defined as Mandatory are required to be
submitted when the segment is sent.
13.2.9 COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT (PRIOR
AUTHORIZATION REQUEST AND BILLING)
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
337-4C COORDINATION OF BENEFITS/OTHER PAYMENTS COUNT M Maximum count of 9.
338-5C OTHER PAYER COVERAGE TYPE M***R*** Mandatory.
Occurs with Coordination of Benefits/Other Payments
Count (337-4C).
Grouped with Other Payer ID Qualifier (339-6C), Other
Payer ID (34Ø-7C), Other Payer Date (443-E8), and either
Other Payer Amount Paid Count (341-HB) and its grouping,
or Other Payer Reject Count (471-5E) and its grouping.
339-6C OTHER PAYER ID QUALIFIER Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if Other Payer ID (34Ø-7C) is used.
34Ø-7C OTHER PAYER ID
Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if identification of the Other Payer is necessary for
prior authorization billing adjudication.
443-E8 OTHER PAYER DATE
Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if identification of the Other Payer Date is
necessary for prior authorization billing adjudication.
993-A7 INTERNAL CONTROL NUMBER Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required when used for payer-to-payer coordination of
benefits to track the claim without regard to the “Service
Provider ID, Prescription Number, & Date of Service”.
341-HB OTHER PAYER AMOUNT PAID COUNT Q Prior Authorization Request And Billing (Claim/Service):
Maximum count of 9.
Required if Other Payer Amount Paid Qualifier (342-HC) is
used.
342-HC OTHER PAYER AMOUNT PAID QUALIFIER
Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if Other Payer Amount Paid (431-DV) is used.
431-DV OTHER PAYER AMOUNT PAID Q***R*** Prior Authorization Request And Billing (Claim/Service):
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 344 -
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if other payer has approved payment for some/all
of the billing.
Zero (Ø) is a valid value.
Not used for patient financial responsibility only billing.
Not used for non-governmental agency programs if Other
Payer-Patient Responsibility Amount (352-NQ) is
submitted.
471-5E OTHER PAYER REJECT COUNT Q Prior Authorization Request And Billing (Claim/Service):
Maximum count of 5.
Required if Other Payer Reject Code (472-6E) is used.
472-6E OTHER PAYER REJECT CODE Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required when the other payer has denied the payment for
the billing, designated with Other Coverage Code (3Ø8-C8)
= 3 (Other Coverage Billed – claim not covered).
Note: This field must only contain the NCPDP Reject Code
(511-FB) values.
353-NR OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT COUNT Q Prior Authorization Request And Billing (Claim/Service):
Maximum count of 25.
Required if Other Payer-Patient Responsibility Amount
Qualifier (351-NP) is used.
Note the occurrences are dependent upon the number of
component parts returned from a previous payer.
351-NP OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT QUALIFIER Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if Other Payer-Patient Responsibility Amount
(352-NQ) is used.
352-NQ OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if necessary for patient financial responsibility
only billing.
Required if necessary for state/federal/regulatory agency
programs.
Not used for non-governmental agency programs if Other
Payer Amount Paid (431-DV) is submitted.
392-MU BENEFIT STAGE COUNT Q Prior Authorization Request And Billing (Claim/Service):
Maximum count of 4.
Required if Benefit Stage Amount (394-MW) is used.
393-MV BENEFIT STAGE QUALIFIER Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if Benefit Stage Amount (394-MW) is used.
Must only have one value per iteration - value must not be
repeated.
394-MW BENEFIT STAGE AMOUNT Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if the previous payer has financial amounts that
apply to Medicare Part D beneficiary benefit stages. This
field is required when the plan is a participant in a Medicare
Part D program that requires reporting of benefit stage
specific financial amounts.
Required if necessary for state/federal/regulatory agency
programs.
Notes on Coordination of Benefits/Other Payments Segment on a Prior Authorization Request And Billing Request:
The Coordination of Benefits/Other Payments Segment is situational for a Prior Authorization Request And Billing request. It is used when a
receiver needs other payment information for coordination of benefits to process a Prior Authorization Request And Billing. This may be in the
case of primary, secondary, tertiary et cetera health plan coverage for example. Fields defined as Mandatory are required to be submitted
when the segment is sent.
13.2.10 WORKERS’ COMPENSATION SEGMENT (PRIOR AUTHORIZATION REQUEST AND
BILLING)
WORKERS’ COMPENSATION SEGMENT
SITUATIONAL SEGMENT
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 345 -
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
434-DY DATE OF INJURY M
315-CF EMPLOYER NAME Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to process a work related injury or
condition.
316-CG EMPLOYER STREET ADDRESS Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to process a work related injury or
condition.
317-CH EMPLOYER CITY ADDRESS Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to process a work related injury or
condition.
318-CI EMPLOYER STATE/PROVINCE ADDRESS Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to process a work related injury or
condition.
319-CJ EMPLOYER ZIP/POSTAL ZONE Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to process a work related injury or
condition.
32Ø-CK EMPLOYER PHONE NUMBER Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to process a work related injury or
condition.
321-CL EMPLOYER CONTACT NAME Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to process a work related injury or
condition.
327-CR CARRIER ID Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to process a work related injury or
condition.
435-DZ CLAIM/REFERENCE ID Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to process a work related injury or
condition.
117-TR BILLING ENTITY TYPE INDICATOR R Prior Authorization Request And Billing (Claim/Service):
Required.
118-TS PAY TO QUALIFIER Q Prior Authorization Request And Billing (Claim/Service):
Required if Pay To ID (119-TT) is used.
119-TT PAY TO ID Q Prior Authorization Request And Billing (Claim/Service):
Required if transaction is submitted by a provider or agent,
but paid to another party.
12Ø-TU PAY TO NAME Q Prior Authorization Request And Billing (Claim/Service):
Required if transaction is submitted by a provider or agent,
but paid to another party.
121-TV PAY TO STREET ADDRESS Q Prior Authorization Request And Billing (Claim/Service):
Required if transaction is submitted by a provider or agent,
but paid to another party.
122-TW PAY TO CITY ADDRESS Q Prior Authorization Request And Billing (Claim/Service):
Required if transaction is submitted by a provider or agent,
but paid to another party.
123-TX PAY TO STATE/PROVINCE ADDRESS Q Prior Authorization Request And Billing (Claim/Service):
Required if transaction is submitted by a provider or agent,
but paid to another party.
124-TY PAY TO ZIP/POSTAL ZONE Q Prior Authorization Request And Billing (Claim/Service):
Required if transaction is submitted by a provider or agent,
but paid to another party.
125-TZ GENERIC EQUIVALENT PRODUCT ID QUALIFIER Q Prior Authorization Request And Billing (Claim/Service):
Required if Generic Equivalent Product ID (126-UA) is
used.
126-UA GENERIC EQUIVALENT PRODUCT ID Q Prior Authorization Request And Billing (Claim/Service):
Required if necessary for state/federal/regulatory agency
programs.
Notes on Workers’ Compensation Segment on a Prior Authorization Request And Billing Request:
The Workers’ Compensation Segment is situational for a Prior Authorization Request And Billing request. It is used when processing a Prior
Authorization Request And Billing for a work-related injury or condition. Fields defined as Mandatory are required to be submitted when the
segment is sent.
13.2.11 DUR/PPS SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING)
DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
473-7E DUR/PPS CODE COUNTER
Q***R*** Prior Authorization Request And Billing (Claim/Service):
Maximum of 9 occurrences.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 346 -
DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if DUR/PPS Segment is used.
439-E4 REASON FOR SERVICE CODE
Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
Required if this field affects payment for or documentation
of professional pharmacy service.
44Ø-E5 PROFESSIONAL SERVICE CODE
Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
Required if this field affects payment for or documentation
of professional pharmacy service.
441-E6 RESULT OF SERVICE CODE
Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
Required if this field affects payment for or documentation
of professional pharmacy service.
474-8E DUR/PPS LEVEL OF EFFORT
Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
Required if this field affects payment for or documentation
of professional pharmacy service.
475-J9 DUR CO-AGENT ID QUALIFIER Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if DUR Co-Agent ID (476-H6) is used.
476-H6 DUR CO-AGENT ID
Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
Required if this field affects payment for or documentation
of professional pharmacy service.
Notes on DUR/PPS Segment on a Prior Authorization Request And Billing Request:
The DUR/PPS Segment is situational for a Prior Authorization Request And Billing request. It is used when a sender notifies the receiver of
drug utilization, drug evaluations, or information on the appropriate selection to process a Prior Authorization Request And Billing.
If the Prescription/Service Reference Number Qualifier (455-EM) is "2" (Service Billing) and the Product/Service ID Qualifier (436-E1) is "Ø6"
(DUR/PPS), the DUR/PPS Segment is required. For the other Product/Service ID Qualifiers, the DUR/PPS segment may help further explain
or define the service provided.
The Segment is mandatory if required under provider payer contract or mandatory on claims where this information is necessary for
processing prior authorization and/or adjudication of the claim.
Fields defined as Mandatory are required to be submitted when the segment is sent.
13.2.12 COMPOUND SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING)
COMPOUND SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
45Ø-EF COMPOUND DOSAGE FORM DESCRIPTION CODE M
451-EG COMPOUND DISPENSING UNIT FORM INDICATOR M
447-EC COMPOUND INGREDIENT COMPONENT COUNT M Maximum count of 25 ingredients.
488-RE COMPOUND PRODUCT ID QUALIFIER M***R***
489-TE COMPOUND PRODUCT ID M***R***
448-ED COMPOUND INGREDIENT QUANTITY M***R***
449-EE COMPOUND INGREDIENT DRUG COST Q***R*** Prior Authorization Request And Billing (Claim):
Required if needed when multiple products are reported for
receiver claim/encounter or prior authorization
determination.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 347 -
COMPOUND SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
49Ø-UE COMPOUND INGREDIENT BASIS OF COST DETERMINATION Q***R*** Prior Authorization Request And Billing (Claim):
Required if needed when multiple products are reported for
receiver claim/encounter or prior authorization
determination.
362-2G COMPOUND INGREDIENT MODIFIER CODE COUNT Q Prior Authorization Request And Billing (Claim):
Required when Compound Ingredient Modifier Code (363-
2H) is sent.
Maximum count of 1Ø.
363-2H COMPOUND INGREDIENT MODIFIER CODE Q***R*** Prior Authorization Request And Billing (Claim/):
Required if necessary for state/federal/regulatory agency
programs.
Notes on Compound Segment on a Prior Authorization Request And Billing Request:
The Compound Segment is situational for a Prior Authorization Request And Billing request. It is used for multi-ingredient prescriptions, when
each ingredient is reported in a Prior Authorization Request And Billing. The Segment is mandatory if required under provider payer contract or
mandatory on claims where this information is necessary for processing a prior authorization and/or adjudication of the claim.
The Compound Segment is not used in when the Prior Authorization Request And Billing is for a service (Prescription/Service Reference
Number Qualifier (455-EM) = “2” (Service Billing).
Fields defined as Mandatory are required to be submitted when the segment is sent.
13.2.13 CLINICAL SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING)
CLINICAL SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
491-VE DIAGNOSIS CODE COUNT Q Prior Authorization Request And Billing (Claim/Service):
Maximum count of 5.
Required if Diagnosis Code Qualifier (492-WE) and
Diagnosis Code (424-DO) are used.
492-WE DIAGNOSIS CODE QUALIFIER Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if Diagnosis Code (424-DO) is used.
424-DO DIAGNOSIS CODE
Q***R*** Prior Authorization Request And Billing (Claim/Service):
The value for this field is obtained from the prescriber or
authorized representative.
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug utilization
review outcome.
Required if this field affects payment for professional
pharmacy service.
Required if this information can be used in place of prior
authorization.
Required if necessary for state/federal/regulatory agency
programs.
493-XE CLINICAL INFORMATION COUNTER Q***R*** Prior Authorization Request And Billing (Claim/Service):
Maximum 5 occurrences supported.
Grouped with Measurement fields (Measurement Date (494-
ZE), Measurement Time (495-H1), Measurement Dimension
(496-H2), Measurement Unit (497-H3), Measurement Value
(499-H4).
494-ZE MEASUREMENT DATE Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if necessary when this field could result in different
coverage and/or drug utilization review outcome.
495-H1 MEASUREMENT TIME Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if Time is known or has impact on measurement.
Required if necessary when this field could result in different
coverage and/or drug utilization review outcome and is a
requirement for payment or authorization.
496-H2 MEASUREMENT DIMENSION Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if Measurement Unit (497-H3) and Measurement
Value (499-H4) are used.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 348 -
CLINICAL SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if necessary when this field could result in different
coverage and/or drug utilization review outcome and is a
requirement for payment or authorization.
Required if necessary for patient’s weight and height when
billing Medicare for a claim that includes a Certificate of
Medical Necessity (CMN).
497-H3 MEASUREMENT UNIT Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if Measurement Dimension (496-H2) and
Measurement Value (499-H4) are used.
Required if necessary for patient’s weight and height when
billing Medicare for a claim that includes a Certificate of
Medical Necessity (CMN).
Required if necessary when this field could result in different
coverage and/or drug utilization review outcome and is a
requirement for payment or authorization.
499-H4 MEASUREMENT VALUE Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if Measurement Dimension (496-H2) and
Measurement Unit (497-H3) are used.
Required if necessary for patient’s weight and height when
billing Medicare for a claim that includes a Certificate of
Medical Necessity (CMN).
Required if necessary when this field could result in different
coverage and/or drug utilization review outcome.
Notes on Clinical Segment on a Prior Authorization Request And Billing Request:
The Clinical Segment is situational for a Prior Authorization Request And Billing request. It is used to specify clinical measurements and/or
diagnosis information associated with the Claim Billing or Service Billing transaction. The Segment is mandatory if required under provider
payer contract or mandatory on claims where this information is necessary for processing a prior authorization and/or adjudication of the claim.
Fields defined as Mandatory are required to be submitted when the segment is sent.
13.2.14 ADDITIONAL DOCUMENTATION SEGMENT (PRIOR AUTHORIZATION REQUEST
AND BILLING)
ADDITIONAL DOCUMENTATION SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
369-2Q ADDITIONAL DOCUMENTATION TYPE ID M
374-2V REQUEST PERIOD BEGIN DATE Q Prior Authorization Request And Billing (Claim/Service):
Required if necessary for state/federal/regulatory agency
programs.
375-2W REQUEST PERIOD RECERT/REVISED DATE Q Prior Authorization Request And Billing (Claim/Service):
Required if necessary for state/federal/regulatory agency
programs.
Required if the Request Status (373-2U) = “2” (Revision) or
“3” (Recertification).
373-2U REQUEST STATUS Q Prior Authorization Request And Billing (Claim/Service):
Required if necessary for state/federal/regulatory agency
programs.
371-2S LENGTH OF NEED QUALIFIER Q Prior Authorization Request And Billing (Claim/Service):
Required if Length of Need (37Ø-2R) is used.
37Ø-2R LENGTH OF NEED Q Prior Authorization Request And Billing (Claim):
Required if the physician orders an item for a specified
length of time.
Service:
Required if the physician orders an item for a specified
length of time.
372-2T PRESCRIBER/SUPPLIER DATE SIGNED Q Prior Authorization Request And Billing (Claim/Service):
Required if necessary for state/federal/regulatory agency
programs.
376-2X SUPPORTING DOCUMENTATION Q Prior Authorization Request And Billing (Claim/Service):
Required if using Section C of Medicare’s CMN forms or

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 349 -
ADDITIONAL DOCUMENTATION SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
required if necessary for state/federal/regulatory agency
programs.
377-2Z QUESTION NUMBER/LETTER COUNT Q Prior Authorization Request And Billing (Claim/Service):
Maximum count of 5Ø.
Required if needed to provide response to narratives.
378-4B QUESTION NUMBER/LETTER Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if necessary for state/federal/regulatory agency
programs to respond to questions included on a form.
Required if Question Number/Letter
Count (377-2Z) is greater than Ø.
379-4D QUESTION PERCENT RESPONSE Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if necessary for state/federal/regulatory agency
programs to respond to questions included on a form that
requires a percent as the response. (At least one response
is required per question.)
38Ø-4G QUESTION DATE RESPONSE Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if necessary for state/federal/regulatory agency
programs to respond to questions included on a form that
requires a date as the response. (At least one response is
required per question.)
381-4H QUESTION DOLLAR AMOUNT RESPONSE Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if necessary for state/federal/regulatory agency
programs to respond to questions included on a form that
requires a dollar amount as the response. (At least one
response is required per question.)
382-4J QUESTION NUMERIC RESPONSE Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if necessary for
State/federal/regulatory agency programs to respond to
questions included on a form that requires a numeric as the
response. (At least one response is required per question.)
383-4K QUESTION ALPHANUMERIC RESPONSE Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if necessary for state/federal/regulatory agency
programs to respond to questions included on a form that
requires an alphanumeric as the response. (At least one
response is required per question.)
Notes on Additional Documentation Segment on a Prior Authorization Request And Billing Request:
It is used when using Section C of Medicare’s CMN forms or a state/federal/regulatory agency program has a form that requires multiple
answers to specific questions for the claim. Fields defined as Mandatory are required to be submitted when the segment is sent.
13.2.15 FACILITY SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING)
FACILITY SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
336-8C FACILITY ID Q Prior Authorization Request And Billing (Claim/Service):
Required if needed for receiver inquiry validation and/or
determination.
Required if necessary for state/federal/regulatory agency
programs.
385-3Q FACILITY NAME Q Prior Authorization Request And Billing (Claim/Service):
Required if necessary for state/federal/regulatory agency
programs.
386-3U FACILITY STREET ADDRESS Q Prior Authorization Request And Billing (Claim/Service):
Required if necessary for state/federal/regulatory agency
programs.
388-5J FACILITY CITY ADDRESS Q Prior Authorization Request And Billing (Claim/Service):
Required if necessary for state/federal/regulatory agency
programs.
387-3V FACILITY STATE/PROVINCE ADDRESS Q Prior Authorization Request And Billing (Claim/Service):
Required if necessary for state/federal/regulatory agency
programs.
389-6D FACILITY ZIP/POSTAL ZONE Q Prior Authorization Request And Billing (Claim/Service):
Required if necessary for state/federal/regulatory agency
programs.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 350 -
Notes on Facility Segment on a Prior Authorization Request And Billing Request:
The Facility Segment is situational for Prior Authorization Request And Billing request. It is used when a state/federal/regulatory agency
program requires the information on a claim. Fields defined as Mandatory are required to be submitted when the segment is sent.
13.2.16 NARRATIVE SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING)
NARRATIVE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
39Ø-BM NARRATIVE MESSAGE Q Prior Authorization Request And Billing (Claim/Service):
Required if necessary for state/federal/regulatory agency
programs to provide additional information.
Required if necessary only to support exception handling of
pharmacy claims for Medicare Part B claim billing.
Notes on Narrative Segment on a Prior Authorization Request And Billing Request:
The Narrative Segment is situational for Prior Authorization Request And Billing request. It is used when a state/federal/regulatory agency
program requires the information contained in the segment on a claim. Fields defined as Mandatory are required to be submitted when the
segment is sent.
13.3 PRIOR AUTHORIZATION REQUEST AND BILLING RESPONSE DIAGRAMS AND
SEGMENTS
13.3.1 TRANSMISSION ACCEPTED/TRANSACTION PAID
Prior Authorization Request And Billing transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
And Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid)
Each prior authorization request and billing request contains one occurrence of claim/service data.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
13.3.1.1 DIAGRAM FOR TRANSMISSION OF ONE PRIOR AUTHORIZATION REQUEST AND BILLING
RESPONSE (TRANSMISSION ACCEPTED/TRANSACTION PAID)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Segment Separator
Response Prior Authorization Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
13.3.1.2 PRIOR AUTHORIZATION REQUEST AND BILLING RESPONSE SEGMENTS (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
13.3.1.2.1 RESPONSE HEADER SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING) (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 351 -
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Prior Authorization Request And Billing Response:
The Response Header Segment is a mandatory, fixed length segment for a Prior Authorization Request And Billing response when the Header
Response Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid). The
“Situation” column is not applicable.
13.3.1.2.2 RESPONSE MESSAGE SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING) (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Prior Authorization Request And Billing (Claim/Service):
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Prior Authorization Request And Billing Response:
The Response Message Segment is situational for a Prior Authorization Request And Billing response when the Header Response Status
(5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid). It is used when additional text
information needs to be sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
13.3.1.2.3 RESPONSE INSURANCE SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING) (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø1-C1 GROUP ID
Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to identify the cardholder or employer
group, to identify appropriate group number for billing.
524-FO PLAN ID
Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to identify the actual plan parameters,
benefit, or coverage criteria, when available.
Required to identify the actual plan ID that was used when
multiple group coverages exist.
Required if needed to contain the actual plan ID if unknown
to the receiver.
545-2F NETWORK REIMBURSEMENT ID
Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to identify the network for the covered
member.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 352 -
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if needed to identify the actual Network
Reimbursement ID, when applicable and/or available.
Required to identify the actual Network Reimbursement ID
that was used when multiple Network Reimbursement IDs
exist.
568-J7 PAYER ID QUALIFIER Q Prior Authorization Request And Billing (Claim/Service):
Required if Payer ID (569-J8) is used.
569-J8 PAYER ID
Q Prior Authorization Request And Billing (Claim/Service):
Required to identify the ID of the payer responding.
115-N5
MEDICAID ID NUMBER N Prior Authorization Request And Billing (Claim/Service):
Not used.
116-N6
MEDICAID AGENCY NUMBER N Prior Authorization Request And Billing (Claim/Service):
Not used.
3Ø2-C2
CARDHOLDER ID Q Prior Authorization Request And Billing (Claim/Service):
Required if the identification to be used in future transactions
is different than what was submitted on the request.
Notes on Response Insurance Segment on a Prior Authorization Request And Billing Response:
The Response Insurance Segment is situational for a Prior Authorization Request And Billing response when the Header Response Status
(5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid). It is used when coverage or
reimbursement parameters or identifiers need to be sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
13.3.1.2.4 RESPONSE PATIENT SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING) (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
RESPONSE PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
31Ø-CA PATIENT FIRST NAME Q Prior Authorization Request And Billing (Claim/Service):
Required if known.
311-CB PATIENT LAST NAME Q Prior Authorization Request And Billing (Claim/Service):
Required if known.
3Ø4-C4 DATE OF BIRTH Q Prior Authorization Request And Billing (Claim/Service):
Required if known.
Notes on Response Patient Segment on a Prior Authorization Request And Billing Response:
The Response Patient Segment is situational for Prior Authorization Request And Billing transmission response Header Response Status
(5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid) when patient demographic
information needs to be sent from the sender to the receiver. Fields defined as Mandatory are required to be submitted when the segment is
sent.
13.3.1.2.5 RESPONSE STATUS SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING) (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT N Prior Authorization Request And Billing (Claim/Service):
Not used.
511-FB REJECT CODE N***R*** Prior Authorization Request And Billing (Claim/Service):
Not used.
546-4F REJECT FIELD OCCURRENCE INDICATOR N***R*** Prior Authorization Request And Billing (Claim/Service):
Not used.
547-5F APPROVED MESSAGE CODE COUNT Q Prior Authorization Request And Billing (Claim/Service):
Maximum count of 5.
Required if Approved Message Code (548-6F) is used.
548-6F APPROVED MESSAGE CODE
Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if Approved Message Code Count is used and the
sender needs to communicate additional follow up for a
potential opportunity.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 353 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Prior Authorization Request And Billing (Claim/Service):
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Prior Authorization Request And Billing (Claim/Service):
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Prior Authorization Request And Billing (Claim/Service):
Not used.
993-A7 INTERNAL CONTROL NUMBER Q Prior Authorization Request And Billing (Claim/Service):
Required when used for payer-to-payer coordination of
benefits to track the claim without regard to the “Service
Provider ID, Prescription Number, & Date of Service”.
987-MA URL N Prior Authorization Request And Billing (Claim/Service):
Not used.
Notes on Response Status Segment on a Prior Authorization Request And Billing Response:
The Response Status Segment is mandatory for a Prior Authorization Request And Billing response for Header Response Status (5Ø1-F1) =
“A” (Accepted) and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid). The Response Status Segment is sent from
the sender to the receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the segment
is sent.
13.3.1.2.6 RESPONSE CLAIM SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING) (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 354 -
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
551-9F PREFERRED PRODUCT COUNT Q
N
Prior Authorization Request And Billing (Claim):
Maximum count of 6.
Required if Preferred Product ID (553-AR) is used.
Service:
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER Q***R***
N
Prior Authorization Request And Billing (Claim):
Required if Preferred Product ID (553-AR) is used.
Service:
Not used.
553-AR PREFERRED PRODUCT ID
.
Q***R***
N
Prior Authorization Request And Billing (Claim):
Required if a product preference exists that needs to be
communicated to the receiver via an ID.
Service:
Not used.
554-AS PREFERRED PRODUCT INCENTIVE
Q***R***
N
Prior Authorization Request And Billing (Claim):
Required if there is a known incentive amount associated
with the Preferred Product ID (553-AR) and/or Preferred
Product Description (556-AU).
Service:
Not used.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE Q***R***
N
Prior Authorization Request And Billing (Claim):
Required if there is a known patient financial responsibility
incentive amount associated with the Preferred Product ID
(553-AR) and/or Preferred Product Description (556-AU).
Service:
Not used.
556-AU PREFERRED PRODUCT DESCRIPTION Q***R***
N
Prior Authorization Request And Billing (Claim):
Required if a product preference exists that either cannot
be communicated by the Preferred Product ID (553-AR) or
to clarify the Preferred Product ID (553-AR).
Service:
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Prior Authorization Request And Billing (Claim/Service):
Not used.
Notes on Response Claim Segment on a Prior Authorization Request And Billing Response:
The Response Claim Segment is mandatory for a Prior Authorization Request And Billing response when the Header Response Status (5Ø1-
F1) is “A” (Accepted) and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid). The Response Claim Segment is
sent from the sender to the receiver to identify therapeutic or alternate product recommendations. The Response Claim Segment is sent from
the sender to the receiver to mirror back the Prescription/Service Reference Number (4Ø2-D2). Fields defined as Mandatory are required to be
submitted when the segment is sent.
13.3.1.2.7 RESPONSE PRICING SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING) (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
RESPONSE PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø5-F5 PATIENT PAY AMOUNT R Prior Authorization Request And Billing (Claim/Service):
Required.
5Ø6-F6 INGREDIENT COST PAID Q
N
Prior Authorization Request And Billing (Claim):
Required if this value is used to arrive at the final
reimbursement.
Service:
Not used.
5Ø7-F7 DISPENSING FEE PAID Q
N
Prior Authorization Request And Billing (Claim):
Required if this value is used to arrive at the final
reimbursement.
Service:
Not used.
557-AV TAX EXEMPT INDICATOR Q Prior Authorization Request And Billing (Claim/Service):

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 355 -
RESPONSE PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if the sender (health plan) and/or patient is tax
exempt and exemption applies to this billing.
558-AW FLAT SALES TAX AMOUNT PAID Q Prior Authorization Request And Billing (Claim/Service):
Required if this value is used to arrive at the final
reimbursement.
Required if Flat Sales Tax Amount Submitted (481-HA) is
greater than zero (Ø). Zero (Ø) value is valid.
559-AX PERCENTAGE SALES TAX AMOUNT PAID Q Prior Authorization Request And Billing (Claim):
Required if this value is used to arrive at the final
reimbursement.
Required if Percentage Sales Tax Amount Submitted (482-
GE) is greater than zero (Ø). Zero (Ø) value is valid.
Required if Percentage Sales Tax Rate Paid (56Ø-AY) and
Percentage Sales Tax Basis Paid (561-AZ) are used.
Service:
Required if Percentage Sales Tax Amount Submitted (482-
GE) is greater than zero (Ø) or if Percentage Sales Tax
Amount Paid (559-AX) is used to arrive at the final
reimbursement. Zero (Ø) value is valid.
Required if Percentage Sales Tax Rate Paid (56Ø-AY) is
used.
56∅-AY PERCENTAGE SALES TAX RATE PAID Q Prior Authorization Request And Billing (Claim/Service):
Required if Percentage Sales Tax Amount Paid (559-AX) is
greater than zero (Ø).
561-AZ PERCENTAGE SALES TAX BASIS PAID Q
N
Prior Authorization Request And Billing (Claim):
Required if Percentage Sales Tax Amount Paid (559-AX) is
greater than zero (Ø).
Service:
Not used. Code list is not applicable.
521-FL INCENTIVE AMOUNT PAID Q
N
Prior Authorization Request And Billing (Claim):
Required if this value is used to arrive at the final
reimbursement.
Required if Incentive Amount Submitted (438-E3) is greater
than zero (Ø). Zero (Ø) value is valid.
Service:
Not used. Not supported in Service Billing formula.
562-J1 PROFESSIONAL SERVICE FEE PAID N
R
Prior Authorization Request And Billing (Claim):
Not used.
Service:
Required.
563-J2 OTHER AMOUNT PAID COUNT
Q Prior Authorization Request And Billing (Claim/Service):
Maximum count of 3.
Required if Other Amount Paid (565-J4) is used.
564-J3 OTHER AMOUNT PAID QUALIFIER Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if Other Amount Paid (565-J4) is used.
565-J4 OTHER AMOUNT PAID
Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if this value is used to arrive at the final
reimbursement.
Required if Other Amount Claimed Submitted (48Ø-H9) is
greater than zero (Ø). Zero (Ø) value is valid. Must respond
to each occurrence submitted.
566-J5 OTHER PAYER AMOUNT RECOGNIZED
Q Prior Authorization Request And Billing (Claim):
Required if this value is used to arrive at the final
reimbursement.
Required if Other Payer Amount Paid (431-DV) is greater
than zero (Ø) and Coordination of Benefits/Other Payments
Segment is supported.
Service:
Required if Other Payer Amount Paid (431-DV) is greater
than zero (Ø) or if this field is used to arrive at the final

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 356 -
RESPONSE PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
reimbursement. This field may be equal to zero (Ø).
5Ø9-F9 TOTAL AMOUNT PAID
R Prior Authorization Request And Billing (Claim/Service):
Required. Zero (Ø) value is valid.
See Pricing Formula for fields used in calculation.
522-FM BASIS OF REIMBURSEMENT DETERMINATION Q
N
Prior Authorization Request And Billing (Claim):
Required if Ingredient Cost Paid (5Ø6-F6) is greater than
zero (Ø).
Required if Basis of Cost Determination (432-DN) is
submitted on billing.
Service:
Not used.
523-FN AMOUNT ATTRIBUTED TO SALES TAX Q Prior Authorization Request And Billing (Claim/Service):
Required if Patient Pay Amount (5Ø5-F5) includes sales tax
that is the financial responsibility of the member but is not
also included in any of the other fields that add up to
Patient Pay Amount.
See section “Specific Segment Discussion”, “Response
Segments”, “Response Pricing Segment” for guidance.
512-FC ACCUMULATED DEDUCTIBLE AMOUNT I Prior Authorization Request And Billing (Claim/Service):
Provided for informational purposes only.
513-FD REMAINING DEDUCTIBLE AMOUNT I Prior Authorization Request And Billing (Claim/Service):
Provided for informational purposes only.
514-FE REMAINING BENEFIT AMOUNT I Prior Authorization Request And Billing (Claim/Service):
The Remaining Benefit Amount must not be returned with
zeroes unless there are no benefit dollars remaining. The
default value of 999999999 from previous versions must
not be used as a default in this field.
Provided for informational purposes only.
517-FH AMOUNT APPLIED TO PERIODIC DEDUCTIBLE Q Prior Authorization Request And Billing (Claim/Service):
Required if Patient Pay Amount (5Ø5-F5) includes
deductible.
518-FI AMOUNT OF COPAY Q Prior Authorization Request And Billing (Claim/Service):
Required if Patient Pay Amount (5Ø5-F5) includes copay
as patient financial responsibility.
52Ø-FK AMOUNT EXCEEDING PERIODIC BENEFIT MAXIMUM Q Prior Authorization Request And Billing (Claim/Service):
Required if Patient Pay Amount (5Ø5-F5) includes amount
exceeding periodic benefit maximum.
346-HH BASIS OF CALCULATION—DISPENSING FEE Q
N
Prior Authorization Request And Billing (Claim):
Required if Dispensing Status (343-HD) on submission is
“P” (Partial Fill) or “C” (Completion of Partial Fill).
Service:
Not used.
347-HJ BASIS OF CALCULATION—COPAY Q
N
Prior Authorization Request And Billing (Claim):
Required if Dispensing Status (343-HD) on submission is
“P” (Partial Fill) or “C” (Completion of Partial Fill).
Service:
Not used.
348-HK BASIS OF CALCULATION—FLAT SALES TAX Q
N
Prior Authorization Request And Billing (Claim):
Required if Dispensing Status (343-HD) on submission is
“P” (Partial Fill) or “C” (Completion of Partial Fill) and Flat
Sales Tax Amount Paid (558-AW) is greater than zero (Ø).
Service:
Not used.
349-HM BASIS OF CALCULATION—PERCENTAGE SALES TAX Q
N
Prior Authorization Request And Billing (Claim):
Required if Dispensing Status (343-HD) on submission is
“P” (Partial Fill) or “C” (Completion of Partial Fill and
Percentage Sales Tax Amount Paid (559-AX) is greater
than zero (Ø).
Service:
Not used.
571-NZ AMOUNT ATTRIBUTED TO PROCESSOR FEE Q Prior Authorization Request And Billing (Claim):
Required if the customer is responsible for 1ØØ% of the
prescription payment and when the provider net sale is less
than the amount the customer is expected to pay.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 357 -
RESPONSE PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Service:
Required if the customer is responsible for 1ØØ% of the
service payment and when the provider net sale is less
than the amount the customer is expected to pay.
575-EQ PATIENT SALES TAX AMOUNT I Prior Authorization Request And Billing (Claim/Service):
Used when necessary to identify the Patient’s portion of the
Sales Tax.
Provided for informational purposes only.
574-2Y PLAN SALES TAX AMOUNT I Prior Authorization Request And Billing (Claim/Service):
Used when necessary to identify the Plan’s portion of the
Sales Tax.
Provided for informational purposes only.
572-4U AMOUNT OF COINSURANCE Q Prior Authorization Request And Billing (Claim/Service):
Required if Patient Pay Amount (5Ø5-F5) includes
coinsurance as patient financial responsibility.
573-4V BASIS OF CALCULATION-COINSURANCE Q
N
Prior Authorization Request And Billing (Claim):
Required if Dispensing Status (343-HD) on submission is
“P” (Partial Fill) or “C” (Completion of Partial Fill).
Service:
Not used.
392-MU BENEFIT STAGE COUNT Q Prior Authorization Request And Billing (Claim/Service):
Maximum count of 4.
Required if Benefit Stage Amount (394-MW) is used.
393-MV BENEFIT STAGE QUALIFIER Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if Benefit Stage Amount (394-MW) is used.
Must only have one value per iteration - value must not be
repeated.
394-MW BENEFIT STAGE AMOUNT Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required when a Medicare Part D payer applies financial
amounts to Medicare Part D beneficiary benefit stages.
This field is required when the plan is a participant in a
Medicare Part D program that requires reporting of benefit
stage specific financial amounts.
Required if necessary for state/federal/regulatory agency
programs.
577-G3 ESTIMATED GENERIC SAVINGS Q
N
Prior Authorization Request And Billing (Claim):
This information should be provided when a patient
selected the brand drug and a generic form of the drug was
available. It will contain an estimate of the difference
between the cost of the brand drug and the generic drug,
when the brand drug is more expensive than the generic. It
is information that the provider should provide to the
patient.
Service:
Not used.
128-UC SPENDING ACCOUNT AMOUNT REMAINING I Prior Authorization Request And Billing (Claim/Service):
This dollar amount will be provided, if known, to the
receiver when the transaction had spending account dollars
reported as part of the patient pay amount.
This field is informational only. It is reported back to the
provider and the patient the amount remaining on the
spending account after the current claim updated the
spending account.
129-UD HEALTH PLAN-FUNDED ASSISTANCE AMOUNT Q Prior Authorization Request And Billing (Claim/Service):
Required when the patient meets the plan-funded
assistance criteria, to reduce Patient Pay Amount (5Ø5-
F5). The resulting Patient Pay Amount (5Ø5-F5) must be
greater than or equal to zero.
This field is always a negative amount or zero.
133-UJ AMOUNT ATTRIBUTED TO PROVIDER NETWORK SELECTION Q Prior Authorization Request And Billing (Claim/Service):
Required if Patient Pay Amount (5Ø5-F5) includes an
amount that is attributable to a cost share differential due to
the selection of one pharmacy over another.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 358 -
RESPONSE PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
134-UK AMOUNT ATTRIBUTED TO PRODUCT SELECTION/BRAND
DRUG
Q
N
Prior Authorization Request And Billing (Claim):
Required if Patient Pay Amount (5Ø5-F5) includes an
amount that is attributable to a patient’s selection of a
Brand drug.
Service:
Not used.
135-UM AMOUNT ATTRIBUTED TO PRODUCT SELECTION/NON-
PREFERRED FORMULARY SELECTION
Q
N
Prior Authorization Request And Billing (Claim):
Required if Patient Pay Amount (5Ø5-F5) includes an
amount that is attributable to a patient’s selection of a non-
preferred formulary product.
Service:
Not used.
136-UN AMOUNT ATTRIBUTED TO PRODUCT SELECTION/BRAND
NON-PREFERRED FORMULARY SELECTION
Q
N
Prior Authorization Request And Billing (Claim):
Required if Patient Pay Amount (5Ø5-F5) includes an
amount that is attributable to a patient’s selection of a
Brand non-preferred formulary product.
Service:
Not used.
137-UP AMOUNT ATTRIBUTED TO COVERAGE GAP Q Prior Authorization Request And Billing (Claim/Service):
Required when the patient’s financial responsibility is due to
the coverage gap.
148-U8 INGREDIENT COST CONTRACTED/REIMBURSABLE AMOUNT I
N
Prior Authorization Request And Billing (Claim):
Required when Basis of Reimbursement Determination
(522-FM) is “14” (Patient Responsibility Amount) or “15”
(Patient Pay Amount) unless prohibited by
state/federal/regulatory agency.
This field is informational only.
Service:
Not used.
149-U9 DISPENSING FEE CONTRACTED/REIMBURSABLE AMOUNT I
N
Prior Authorization Request And Billing (Claim):
Required when Basis of Reimbursement Determination
(522-FM) is “14” (Patient Responsibility Amount) or “15”
(Patient Pay Amount) unless prohibited by
state/federal/regulatory agency.
This field is informational only.
Service:
Not used.
Notes on Response Pricing Segment on a Prior Authorization Request And Billing Response:
The Response Pricing Segment is mandatory for a Prior Authorization Request And Billing response when the Header Response Status (5Ø1-
F1) of "A" (Accepted) and Transaction Response Status (112-AN) is “P” (Paid) or “D” (Duplicate of Paid).
It is highly recommended that whenever possible, the individual dollar fields are returned in the response. In the response, the sender should
return the individual payment response fields to allow the receiver to reconcile against the requested payment fields.
See section “Response Processing Guidelines”, “Pricing Guidelines”.
Fields defined as Mandatory are required to be submitted when the segment is sent.
13.3.1.2.8 RESPONSE PRIOR AUTHORIZATION SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING)
(TRANSMISSION ACCEPTED/TRANSACTION PAID)
RESPONSE PRIOR AUTHORIZATION SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
498-PR PRIOR AUTHORIZATION PROCESSED DATE R Prior Authorization Request And Billing (Claim/Service):
Required.
498-PS PRIOR AUTHORIZATION EFFECTIVE DATE Q Prior Authorization Request And Billing (Claim/Service):
Required if the prior authorization has an effective date.
498-PT PRIOR AUTHORIZATION EXPIRATION DATE Q Prior Authorization Request And Billing (Claim/Service):
Required if the prior authorization has an expiration date.
498-RA PRIOR AUTHORIZATION QUANTITY Q Prior Authorization Request And Billing (Claim/Service):
Required if the total quantity authorized is greater than
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 359 -
RESPONSE PRIOR AUTHORIZATION SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
zero.
498-RB PRIOR AUTHORIZATION DOLLARS AUTHORIZED Q Prior Authorization Request And Billing (Claim/Service):
Required if the total dollars authorized is greater than zero.
498-PW PRIOR AUTHORIZATION NUMBER OF REFILLS AUTHORIZED Q Prior Authorization Request And Billing (Claim/Service):
Required if a specific number of refills is authorized.
498-PX PRIOR AUTHORIZATION QUANTITY ACCUMULATED Q Prior Authorization Request And Billing (Claim/Service):
Required if the Prior Authorization Quantity (498-RA) is
greater than zero. The field must equal the total of the
quantities from all claims processed.
498-PY PRIOR AUTHORIZATION NUMBER–ASSIGNED R Prior Authorization Request And Billing (Claim/Service):
Required.
Notes on Response Prior Authorization Segment on a Prior Authorization Request And Billing Response:
The Response Prior Authorization Segment is mandatory for a Prior Authorization Request And Billing response when the Header Response
Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid). It is used to relay the
prior authorization periods, limitations, contracted amounts, as well as a Prior Authorization Number–Assigned (498-PY) which is to be used
for subsequent Claim or Service Billings. Fields defined as Mandatory are required to be submitted when the segment is sent.
13.3.1.2.9 RESPONSE DUR/PPS SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING) (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
RESPONSE DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
567-J6 DUR/PPS RESPONSE CODE COUNTER Q***R*** Prior Authorization Request And Billing (Claim/Service):
Maximum 9 occurrences supported.
Required if Reason For Service Code (439-E4) is used.
439-E4 REASON FOR SERVICE CODE Q***R*** Prior Authorization Request And Billing (Claim):
Required if detecting utilization conflict.
Service:
Required if professional service opportunity reason is
detected by the receiver that is different from the
professional service submitted.
528-FS CLINICAL SIGNIFICANCE CODE
Q***R*** Prior Authorization Request And Billing (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
529-FT OTHER PHARMACY INDICATOR
Q***R*** Prior Authorization Request And Billing (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
53Ø-FU PREVIOUS DATE OF FILL
Q***R*** Prior Authorization Request And Billing (Claim):
Required if needed to supply additional information for the
utilization conflict.
Required if Quantity of Previous Fill (531-FV) is used.
Service:
Required if needed to supply additional information for the
service.
531-FV QUANTITY OF PREVIOUS FILL
Q***R*** Prior Authorization Request And Billing (Claim):
Required if Previous Date Of Fill (53Ø-FU) is used.
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
532-FW DATABASE INDICATOR
Q***R*** Prior Authorization Request And Billing
Required if needed to supply additional information for the
utilization conflict.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 360 -
RESPONSE DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Service:
Required if needed to supply additional information for the
service.
533-FX OTHER PRESCRIBER INDICATOR
Q***R*** Prior Authorization Request And Billing (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
544-FY DUR FREE TEXT MESSAGE
Q***R*** Prior Authorization Request And Billing (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
57Ø-NS DUR ADDITIONAL TEXT Q***R*** Prior Authorization Request And Billing (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
Notes on Response DUR/PPS Segment on a Prior Authorization Request And Billing Response:
The Response DUR/PPS Segment is situational for a Prior Authorization Request And Billing response when the Header Response Status
(5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid), to identify a drug utilization
review or professional pharmacy service event, opportunity, or information. The Segment is mandatory if required under provider payer
contract or mandatory on claims where this information is necessary for adjudication of the claim. Fields defined as Mandatory are required to
be submitted when the segment is sent.
13.3.1.2.10 RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS SEGMENT (PRIOR AUTHORIZATION REQUEST
AND BILLING) (TRANSMISSION ACCEPTED/TRANSACTION PAID)
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS
SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
355-NT OTHER PAYER ID COUNT M Prior Authorization Request And Billing (Claim/Service):
Maximum count of 3.
338-5C OTHER PAYER COVERAGE TYPE M***R***
339-6C OTHER PAYER ID QUALIFIER Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if Other Payer ID (34Ø-7C) is used.
34Ø-7C OTHER PAYER ID Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if other insurance information is available for
coordination of benefits.
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if other insurance information is available for
coordination of benefits.
356-NU OTHER PAYER CARDHOLDER ID Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if other insurance information is available for
coordination of benefits.
992-MJ OTHER PAYER GROUP ID Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if other insurance information is available for
coordination of benefits.
142-UV OTHER PAYER PERSON CODE Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if needed to uniquely identify the family members
within the Cardholder ID, as assigned by the other payer.
127-UB OTHER PAYER HELP DESK PHONE NUMBER Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if needed to provide a support telephone number
of the other payer to the receiver.
143-UW OTHER PAYER PATIENT RELATIONSHIP CODE Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if needed to uniquely identify the relationship of
the patient to the cardholder ID, as assigned by the other
payer.
144-UX OTHER PAYER BENEFIT EFFECTIVE DATE Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required when other coverage is known which is after the
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 361 -
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS
SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Date of Service submitted.
145-UY OTHER PAYER BENEFIT TERMINATION DATE Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required when other coverage is known which is after the
Date of Service submitted.
Notes on Response Coordination of Benefits/Other Payers Segment on a Prior Authorization Request And Billing Response:
The Response Coordination of Benefits/Other Payers Segment is situational for a Prior Authorization Request And Billing response when the
Header Response Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid) when
other insurance information is available for coordination of benefits.
If subsequent payer(s) for this patient is not known, the Other Payer information is not sent.
If subsequent payer(s) for this patient is known, the following may be sent:
• Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C),
• Other Payer Group ID (992-MJ),
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU)
• And other Other Payer fields.
In addition, if any of the following three fields are sent:
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU),
• Other Payer Group ID (992-MJ),
then the Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C) must be sent.
Fields defined as Mandatory are required to be submitted when the segment is sent.
13.3.2 TRANSMISSION ACCEPTED/TRANSACTION CAPTURED
Prior Authorization Request And Billing transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
And Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured)
In a “C” (Captured) or “Q” (Duplicate of Captured) response, since the prior authorization has not been processed, the billing cannot
proceed, and therefore the Response Pricing Segment must not be returned.
Each prior authorization request and billing request contains one occurrence of claim/service data.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
13.3.2.1 DIAGRAM FOR TRANSMISSION OF ONE PRIOR AUTHORIZATION REQUEST AND BILLING
RESPONSE (TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
13.3.2.2 PRIOR AUTHORIZATION REQUEST AND BILLING RESPONSE SEGMENTS (TRANSMISSION
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 362 -
ACCEPTED/TRANSACTION CAPTURED)
13.3.2.2.1 RESPONSE HEADER SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Prior Authorization Request And Billing Response:
The Response Header Segment is a mandatory, fixed length segment for a Prior Authorization Request And Billing response when the Header
Response Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). The
“Situation” column is not applicable.
13.3.2.2.2 RESPONSE MESSAGE SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Prior Authorization Request And Billing (Claim/Service):
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Prior Authorization Request And Billing Response:
The Response Message Segment is situational for a Prior Authorization Request And Billing response when the Header Response Status
(5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). It is used when
additional text information needs to be sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
13.3.2.2.3 RESPONSE INSURANCE SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø1-C1 GROUP ID
Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to identify the actual cardholder or
employer group, to identify appropriate group number,
when available.
Required to identify the actual group that was used when
multiple group coverages exist.
Note: This field may contain the Group ID echoed from the
request. May contain the actual Group ID if unknown to the
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 363 -
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
receiver.
524-FO PLAN ID
Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to identify the actual plan parameters,
benefit, or coverage criteria, when available.
Required to identify the actual plan ID that was used when
multiple group coverages exist.
Required if needed to contain the actual plan ID if unknown
to the receiver.
545-2F NETWORK REIMBURSEMENT ID
N Prior Authorization Request And Billing (Claim/Service):
Not used.
568-J7 PAYER ID QUALIFIER N Prior Authorization Request And Billing (Claim/Service):
Not used.
569-J8 PAYER ID
N Prior Authorization Request And Billing (Claim/Service):
Not used.
115-N5
MEDICAID ID NUMBER N Prior Authorization Request And Billing (Claim/Service):
Not used.
116-N6
MEDICAID AGENCY NUMBER N Prior Authorization Request And Billing (Claim/Service):
Not used.
3Ø2-C2
CARDHOLDER ID Q Prior Authorization Request And Billing (Claim/Service):
Required if the identification to be used in future
transactions is different than what was submitted on the
request.
Notes on Response Insurance Segment on a Prior Authorization Request And Billing Response:
The Response Insurance Segment is situational for a Prior Authorization Request And Billing response when the Header Response Status
(5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). It is used when
coverage or reimbursement parameters or identifiers need to be sent. Fields defined as Mandatory are required to be submitted when the
segment is sent.
13.3.2.2.4 RESPONSE PATIENT SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
RESPONSE PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
31Ø-CA PATIENT FIRST NAME Q Prior Authorization Request And Billing (Claim/Service):
Required if known.
311-CB PATIENT LAST NAME Q Prior Authorization Request And Billing (Claim/Service):
Required if known.
3Ø4-C4 DATE OF BIRTH Q Prior Authorization Request And Billing (Claim/Service):
Required if known.
Notes on Response Patient Segment on a Prior Authorization Request And Billing Response:
The Response Patient Segment is situational for Prior Authorization Request And Billing transmission response Header Response Status
(5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured) when patient
demographic information needs to be sent from the sender to the receiver. Fields defined as Mandatory are required to be submitted when the
segment is sent.
13.3.2.2.5 RESPONSE STATUS SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER R Prior Authorization Request And Billing (Claim/Service):
Required.
51Ø-FA REJECT COUNT N Prior Authorization Request And Billing (Claim/Service):
Not used.
511-FB REJECT CODE N***R*** Prior Authorization Request And Billing (Claim/Service):
Not used.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 364 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
546-4F REJECT FIELD OCCURRENCE INDICATOR N***R*** Prior Authorization Request And Billing (Claim/Service):
Not used.
547-5F APPROVED MESSAGE CODE COUNT N Prior Authorization Request And Billing (Claim/Service):
Not used.
548-6F APPROVED MESSAGE CODE
N***R*** Prior Authorization Request And Billing (Claim/Service):
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Prior Authorization Request And Billing (Claim/Service):
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Prior Authorization Request And Billing (Claim/Service):
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Prior Authorization Request And Billing (Claim/Service):
Not used.
993-A7 INTERNAL CONTROL NUMBER Q Prior Authorization Request And Billing (Claim/Service):
Required when used for payer-to-payer coordination of
benefits to track the claim without regard to the “Service
Provider ID, Prescription Number, & Date of Service”.
987-MA URL N Prior Authorization Request And Billing (Claim/Service):
Not used.
Notes on Response Status Segment on a Prior Authorization Request And Billing Response:
The Response Status Segment is mandatory for a Prior Authorization Request And Billing response for Header Response Status (5Ø1-F1) =
“A” (Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). The Response Status Segment is
sent from the sender to the receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the
segment is sent.
13.3.2.2.6 RESPONSE CLAIM SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 365 -
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT Q
N
Prior Authorization Request And Billing (Claim):
Maximum count of 6.
Required if Preferred Product ID (553-AR) is used.
Service:
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER Q***R***
N
Prior Authorization Request And Billing (Claim):
Required if Preferred Product ID (553-AR) is used.
Service:
Not used.
553-AR PREFERRED PRODUCT ID
.
Q***R***
N
Prior Authorization Request And Billing (Claim):
Required if a product preference exists that needs to be
communicated to the receiver via an ID.
Service:
Not used.
554-AS PREFERRED PRODUCT INCENTIVE
Q***R***
N
Prior Authorization Request And Billing (Claim):
Required if there is a known incentive amount associated
with the Preferred Product ID (553-AR) and/or Preferred
Product Description (556-AU).
Service:
Not used.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE
Q***R***
N
Prior Authorization Request And Billing (Claim):
Required if there is a known patient financial responsibility
incentive amount associated with the Preferred Product ID
(553-AR) and/or Preferred Product Description (556-AU).
Service:
Not used.
556-AU PREFERRED PRODUCT DESCRIPTION Q***R***
N
Prior Authorization Request And Billing (Claim):
Required if a product preference exists that either cannot
be communicated by the Preferred Product ID (553-AR) or
to clarify the Preferred Product ID (553-AR).
Service:
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Prior Authorization Request And Billing (Claim/Service):
Not used.
Notes on Response Claim Segment on a Prior Authorization Request And Billing Response:
The Response Claim Segment is mandatory for a Prior Authorization Request And Billing response when the Header Response Status (5Ø1-
F1) is “A” (Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured).
The Response Claim Segment (Prior Authorization Request And Billing – Claim) is sent from the sender to the receiver to identify therapeutic
or alternate product recommendations.
The Response Claim Segment (Prior Authorization Request And Billing – Service) is sent from the sender to the receiver to mirror back the
Prescription/Service Reference Number (4Ø2-D2).
Fields defined as Mandatory are required to be submitted when the segment is sent.
13.3.2.2.7 RESPONSE DUR/PPS SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 366 -
RESPONSE DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
567-J6 DUR/PPS RESPONSE CODE COUNTER Q***R*** Prior Authorization Request And Billing (Claim/Service):
Maximum 9 occurrences supported.
Required if Reason For Service Code (439-E4) is used.
439-E4 REASON FOR SERVICE CODE Q***R*** Prior Authorization Request And Billing (Claim):
Required if utilization conflict is detected.
Service:
Required if professional service opportunity reason is
detected by the receiver. Should be different than the
original transmission.
528-FS CLINICAL SIGNIFICANCE CODE
Q***R*** Prior Authorization Request And Billing (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
529-FT OTHER PHARMACY INDICATOR
Q***R*** Prior Authorization Request And Billing (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
53Ø-FU PREVIOUS DATE OF FILL
Q***R*** Prior Authorization Request And Billing (Claim):
Required if needed to supply additional information for the
utilization conflict.
Required if Quantity of Previous Fill (531-FV) is used.
Service:
Required if needed to supply additional information for the
service.
531-FV QUANTITY OF PREVIOUS FILL
Q***R*** Prior Authorization Request And Billing (Claim):
Required if Previous Date Of Fill (53Ø-FU) is used.
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
532-FW DATABASE INDICATOR
Q***R*** Prior Authorization Request And Billing (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
533-FX OTHER PRESCRIBER INDICATOR
Q***R*** Prior Authorization Request And Billing (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
544-FY DUR FREE TEXT MESSAGE
Q***R*** Prior Authorization Request And Billing (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
57Ø-NS DUR ADDITIONAL TEXT Q***R*** Prior Authorization Request And Billing (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 367 -
Notes on Response DUR/PPS Segment on a Prior Authorization Request And Billing Response:
The Response DUR/PPS Segment is situational for a Prior Authorization Request And Billing response when the Header Response Status
(5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured), to identify a drug
utilization review or professional pharmacy service event, opportunity, or information. The Segment is mandatory if required under provider
payer contract or mandatory on claims where this information is necessary for adjudication of the claim. Fields defined as Mandatory are
required to be submitted when the segment is sent.
13.3.3 TRANSMISSION ACCEPTED/TRANSACTION DEFERRED
Response Header Response Status (5Ø1-F1) of "A" (Accepted) Prior Authorization Request And Billing transmission
and Transaction Response Status (112-AN) of “F” (Deferred)
Each prior authorization request and billing request contains one occurrence of claim/service data.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
Final determination of the Prior Authorization request cannot be made until additional medical information is obtained. The message (5Ø4-F4)
and/or Additional Message Information (526-FQ) will contain what additional information is needed. Each processor governs the submission
of additional information and the pharmacy should consult the appropriate provider billing manual. Typically, if the additional information is not
received within a specific timeframe, the prior authorization will be denied.
13.3.3.1 DIAGRAM FOR TRANSMISSION OF ONE PRIOR AUTHORIZATION REQUEST AND BILLING
RESPONSE (TRANSMISSION ACCEPTED/TRANSACTION DEFERRED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Prior Authorization Segment
13.3.3.2 PRIOR AUTHORIZATION REQUEST AND BILLING RESPONSE SEGMENTS (TRANSMISSION
ACCEPTED/TRANSACTION DEFERRED)
13.3.3.2.1 RESPONSE HEADER SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING) (TRANSMISSION
ACCEPTED/TRANSACTION DEFERRED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Prior Authorization Request And Billing Response:
The Response Header Segment is a mandatory, fixed length segment for a Prior Authorization Request And Billing response when the Header
Response Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “F” (Deferred). The “Situation” column is not
applicable.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 368 -
13.3.3.2.2 RESPONSE MESSAGE SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING) (TRANSMISSION
ACCEPTED/TRANSACTION DEFERRED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Prior Authorization Request And Billing (Claim/Service):
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Prior Authorization Request And Billing Response:
The Response Message Segment is situational for a Prior Authorization Request And Billing response when the Header Response Status
(5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “F” (Deferred). It is used when additional text information needs to
be sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
13.3.3.2.3 RESPONSE INSURANCE SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING) (TRANSMISSION
ACCEPTED/TRANSACTION DEFERRED)
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø1-C1 GROUP ID
Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to identify the actual cardholder or
employer group, to identify appropriate group number,
when available.
Required to identify the actual group that was used when
multiple group coverages exist.
Note: This field may contain the Group ID echoed from the
request. May contain the actual Group ID if unknown to the
receiver.
524-FO PLAN ID
Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to identify the actual plan parameters,
benefit, or coverage criteria, when available.
Required to identify the actual plan ID that was used when
multiple group coverages exist.
Required if needed to contain the actual plan ID if unknown
to the receiver.
545-2F NETWORK REIMBURSEMENT ID
N Prior Authorization Request And Billing (Claim/Service):
Not used.
568-J7 PAYER ID QUALIFIER N Prior Authorization Request And Billing (Claim/Service):
Not used.
569-J8 PAYER ID
N Prior Authorization Request And Billing (Claim/Service):
Not used.
115-N5
MEDICAID ID NUMBER N Prior Authorization Request And Billing (Claim/Service):
Not used.
116-N6
MEDICAID AGENCY NUMBER N Prior Authorization Request And Billing (Claim/Service):
Not used.
3Ø2-C2
CARDHOLDER ID Q Prior Authorization Request And Billing (Claim/Service):
Required if the identification to be used in future
transactions is different than what was submitted on the
request.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 369 -
Notes on Response Insurance Segment on a Prior Authorization Request And Billing Response:
The Response Insurance Segment is situational for a Prior Authorization Request And Billing response when the Header Response Status
(5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “F” (Deferred). It is used when coverage or reimbursement
parameters or identifiers need to be sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
13.3.3.2.4 RESPONSE PATIENT SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING) (TRANSMISSION
ACCEPTED/TRANSACTION DEFERRED)
RESPONSE PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
31Ø-CA PATIENT FIRST NAME Q Prior Authorization Request And Billing (Claim/Service):
Required if known.
311-CB PATIENT LAST NAME Q Prior Authorization Request And Billing (Claim/Service):
Required if known.
3Ø4-C4 DATE OF BIRTH Q Prior Authorization Request And Billing (Claim/Service):
Required if known.
Notes on Response Patient Segment on a Prior Authorization Request And Billing Response:
The Response Patient Segment is situational for Prior Authorization Request And Billing transmission response Header Response Status
(5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “F” (Deferred) when patient demographic information needs to be
sent from the sender to the receiver. Fields defined as Mandatory are required to be submitted when the segment is sent.
13.3.3.2.5 RESPONSE STATUS SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING) (TRANSMISSION
ACCEPTED/TRANSACTION DEFERRED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Prior Authorization Request And Billing (Claim/Service):
Required if Prior Authorization Number-Assigned (498-PY)
not sent.
51Ø-FA REJECT COUNT N Prior Authorization Request And Billing (Claim/Service):
Not used.
511-FB REJECT CODE N***R*** Prior Authorization Request And Billing (Claim/Service):
Not used.
546-4F REJECT FIELD OCCURRENCE INDICATOR N***R*** Prior Authorization Request And Billing (Claim/Service):
Not used.
547-5F APPROVED MESSAGE CODE COUNT N Prior Authorization Request And Billing (Claim/Service):
Not used.
548-6F APPROVED MESSAGE CODE
N***R*** Prior Authorization Request And Billing (Claim/Service):
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Prior Authorization Request And Billing (Claim/Service):
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 370 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Prior Authorization Request And Billing (Claim/Service):
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Prior Authorization Request And Billing (Claim/Service):
Not used.
993-A7 INTERNAL CONTROL NUMBER N Prior Authorization Request And Billing (Claim/Service):
Not used.
987-MA URL N Prior Authorization Request And Billing (Claim/Service):
Not used.
Notes on Response Status Segment on a Prior Authorization Request And Billing Response:
The Response Status Segment is mandatory for a Prior Authorization Request And Billing response for Header Response Status (5Ø1-F1) =
“A” (Accepted) and Transaction Response Status (112-AN) of “F” (Deferred). The Response Status Segment is sent from the sender to the
receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.
13.3.3.2.6 RESPONSE CLAIM SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING) (TRANSMISSION
ACCEPTED/TRANSACTION DEFERRED)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT Q
N
Prior Authorization Request And Billing (Claim):
Maximum count of 6.
Required if Preferred Product ID (553-AR) is used.
Service:
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER Q***R***
N
Prior Authorization Request And Billing (Claim):
Required if Preferred Product ID (553-AR) is used.
Service:
Not used.
553-AR PREFERRED PRODUCT ID
.
Q***R***
N
Prior Authorization Request And Billing (Claim):
Required if a product preference exists that needs to be
communicated to the receiver via an ID.
Service:
Not used.
554-AS PREFERRED PRODUCT INCENTIVE
Q***R***
N
Prior Authorization Request And Billing (Claim):
Required if there is a known incentive amount associated
with the Preferred Product ID (553-AR) and/or Preferred
Product Description (556-AU).
Service:
Not used.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE
Q***R***
Prior Authorization Request And Billing (Claim):
Required if there is a known patient financial responsibility
incentive amount associated with the Preferred Product ID
(553-AR) and/or Preferred Product Description (556-AU).
Service:
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 371 -
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
N Not used.
556-AU PREFERRED PRODUCT DESCRIPTION Q***R***
N
Prior Authorization Request And Billing (Claim):
Required if a product preference exists that either cannot
be communicated by the Preferred Product ID (553-AR) or
to clarify the Preferred Product ID (553-AR).
Service:
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Prior Authorization Request And Billing (Claim/Service):
Not used.
Notes on Response Claim Segment on a Prior Authorization Request And Billing Response:
The Response Claim Segment is mandatory for a Prior Authorization Request And Billing response when the Header Response Status (5Ø1-
F1) is “A” (Accepted) and Transaction Response Status (112-AN) of “F” (Deferred).
The Response Claim Segment (Prior Authorization Request And Billing - Claim) is sent from the sender to the receiver to identify therapeutic
or alternate product recommendations.
The Response Claim Segment (Prior Authorization Request And Billing – Service) is sent from the sender to the receiver to mirror back the
Prescription/Service Reference Number (4Ø2-D2).
Fields defined as Mandatory are required to be submitted when the segment is sent.
13.3.3.2.7 RESPONSE PRIOR AUTHORIZATION SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING)
(TRANSMISSION ACCEPTED/TRANSACTION DEFERRED)
RESPONSE PRIOR AUTHORIZATION SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
498-PR PRIOR AUTHORIZATION PROCESSED DATE R Prior Authorization Request And Billing (Claim/Service):
Required.
498-PS PRIOR AUTHORIZATION EFFECTIVE DATE N Prior Authorization Request And Billing (Claim/Service):
Not used.
498-PT PRIOR AUTHORIZATION EXPIRATION DATE N Prior Authorization Request And Billing (Claim/Service):
Not used.
498-RA PRIOR AUTHORIZATION QUANTITY N Prior Authorization Request And Billing (Claim/Service):
Not used.
498-RB PRIOR AUTHORIZATION DOLLARS AUTHORIZED N Prior Authorization Request And Billing (Claim/Service):
Not used.
498-PW PRIOR AUTHORIZATION NUMBER OF REFILLS AUTHORIZED N Prior Authorization Request And Billing (Claim/Service):
Not used.
498-PX PRIOR AUTHORIZATION QUANTITY ACCUMULATED N Prior Authorization Request And Billing (Claim/Service):
Not used.
498-PY PRIOR AUTHORIZATION NUMBER–ASSIGNED Q Prior Authorization Request And Billing (Claim/Service):
Required if Authorization Number (5Ø3-F3) not sent.
Notes on Response Prior Authorization Segment on a Prior Authorization Request And Billing Response:
The Response Prior Authorization Segment is situational for a Prior Authorization Request And Billing response when the Header Response
Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “F” (Deferred). The sender should consult the receiver’s
provider manual for further information. Fields defined as Mandatory are required to be submitted when the segment is sent.
13.3.3.2.8 RESPONSE DUR/PPS SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING) (TRANSMISSION
ACCEPTED/TRANSACTION DEFERRED)
RESPONSE DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
567-J6 DUR/PPS RESPONSE CODE COUNTER Q***R*** Prior Authorization Request And Billing (Claim/Service):
Maximum 9 occurrences supported.
Required if Reason For Service Code (439-E4) is used.
439-E4 REASON FOR SERVICE CODE Q***R*** Prior Authorization Request And Billing (Claim):
Required if utilization conflict is detected.
Service:
Required if professional service opportunity reason is

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 372 -
RESPONSE DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
detected by the receiver. Should be different than the
original transmission.
528-FS CLINICAL SIGNIFICANCE CODE
Q***R*** Prior Authorization Request And Billing (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
529-FT OTHER PHARMACY INDICATOR
Q***R*** Prior Authorization Request And Billing (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
53Ø-FU PREVIOUS DATE OF FILL
Q***R*** Prior Authorization Request And Billing (Claim):
Required if Quantity of Previous Fill (531-FV) is used.
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
531-FV QUANTITY OF PREVIOUS FILL
Q***R*** Prior Authorization Request And Billing (Claim):
Required if Previous Date Of Fill (53Ø-FU) is used.
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
532-FW DATABASE INDICATOR
Q***R*** Prior Authorization Request And Billing (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
533-FX OTHER PRESCRIBER INDICATOR
Q***R*** Prior Authorization Request And Billing (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
544-FY DUR FREE TEXT MESSAGE
Q***R*** Prior Authorization Request And Billing (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
57Ø-NS DUR ADDITIONAL TEXT Q***R*** Prior Authorization Request And Billing (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
Notes on Response DUR/PPS Segment on a Prior Authorization Request And Billing Response:
The Response DUR/PPS Segment is situational for a Prior Authorization Request And Billing response when the Header Response Status
(5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “F” (Deferred), to identify a drug utilization review or professional
pharmacy service event, opportunity, or information. The Segment is mandatory if required under provider payer contract or mandatory on
claims where this information is necessary for adjudication of the claim. Fields defined as Mandatory are required to be submitted when the
segment is sent.
13.3.4 TRANSMISSION ACCEPTED/TRANSACTION REJECTED RESPONSE
Prior Authorization Request And Billing transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 373 -
and Transaction Response Status (112-AN) of “R” (Rejected)
Each prior authorization request and billing request contains one occurrence of claim/service data.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
13.3.4.1 DIAGRAM FOR TRANSMISSION OF ONE PRIOR AUTHORIZATION REQUEST AND BILLING
RESPONSE (TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
13.3.4.2 PRIOR AUTHORIZATION REQUEST AND BILLING RESPONSE SEGMENTS (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
13.3.4.2.1 RESPONSE HEADER SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Prior Authorization Request And Billing Response:
The Response Header Segment is a mandatory, fixed length segment for a Prior Authorization Request And Billing response when the Header
Response Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). The “Situation” column is not
applicable.
If the transaction rejects for detail errors, the Header Response Status (5Ø1-F1) = “A” (Accepted) and the Transaction Response Status (112-
AN) will be “R”.
13.3.4.2.2 RESPONSE MESSAGE SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Prior Authorization Request And Billing (Claim/Service):
Required if text is needed for clarification or detail.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 374 -
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Prior Authorization Request And Billing Response:
The Response Message Segment is situational segment for a Prior Authorization Request And Billing response when the Header Response
Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). It is used when additional text information
needs to be sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
13.3.4.2.3 RESPONSE INSURANCE SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø1-C1 GROUP ID
Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to identify the actual cardholder or
employer group, to identify appropriate group number,
when available.
Required to identify the actual group that was used when
multiple group coverages exist.
Note: This field may contain the Group ID echoed from the
request. May contain the actual Group ID if unknown to the
receiver.
524-FO PLAN ID
Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to identify the actual plan parameters,
benefit, or coverage criteria, when available.
Required to identify the actual plan ID that was used when
multiple group coverages exist.
Required if needed to contain the actual plan ID if unknown
to the receiver.
545-2F NETWORK REIMBURSEMENT ID
Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to identify the network for the covered
member.
Required if needed to identify the actual Network
Reimbursement ID, when applicable and/or available.
Required to identify the actual Network Reimbursement ID
that was used when multiple Network Reimbursement IDs
exist.
568-J7 PAYER ID QUALIFIER N Prior Authorization Request And Billing (Claim/Service):
Not used.
569-J8 PAYER ID N Prior Authorization Request And Billing (Claim/Service):
Not used.
115-N5
MEDICAID ID NUMBER N Prior Authorization Request And Billing (Claim/Service):
Not used.
116-N6
MEDICAID AGENCY NUMBER N Prior Authorization Request And Billing (Claim/Service):
Not used.
3Ø2-C2
CARDHOLDER ID Q Prior Authorization Request And Billing (Claim/Service):
Required if the identification to be used in future
transactions is different than what was submitted on the
request.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 375 -
Notes on Response Insurance Segment on a Prior Authorization Request And Billing Response:
The Response Insurance Segment is situational segment for a Prior Authorization Request And Billing response when the Header Response
Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). It is used when coverage or reimbursement
parameters or identifiers need to be sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
13.3.4.2.4 RESPONSE PATIENT SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
31Ø-CA PATIENT FIRST NAME Q Prior Authorization Request And Billing (Claim/Service):
Required if known.
311-CB PATIENT LAST NAME Q Prior Authorization Request And Billing (Claim/Service):
Required if known.
3Ø4-C4 DATE OF BIRTH Q Prior Authorization Request And Billing (Claim/Service):
Required if known.
Notes on Response Patient Segment on a Prior Authorization Request And Billing Response:
The Response Patient Segment is situational for Prior Authorization Request And Billing transmission response Header Response Status
(5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “R” (Rejected) when patient demographic information needs to be
sent from the sender to the receiver. Fields defined as Mandatory are required to be submitted when the segment is sent.
13.3.4.2.5 RESPONSE STATUS SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT R Prior Authorization Request And Billing (Claim/Service):
Maximum count of 5.
Required.
511-FB REJECT CODE R***R*** Prior Authorization Request And Billing (Claim/Service):
Required.
546-4F REJECT FIELD OCCURRENCE INDICATOR Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if a repeating field is in error, to identify repeating
field occurrence.
This field must be sent when relaying error information about
a repeating field or set. Note, if the Reject Code is not
denoting a repeating field or set, the Reject Field Occurrence
Indicator must not be sent.
547-5F APPROVED MESSAGE CODE COUNT N Prior Authorization Request And Billing (Claim/Service):
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Prior Authorization Request And Billing (Claim/Service):
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Prior Authorization Request And Billing (Claim/Service):
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 376 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Prior Authorization Request And Billing (Claim/Service):
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Prior Authorization Request And Billing (Claim/Service):
Not used.
993-A7 INTERNAL CONTROL NUMBER N Prior Authorization Request And Billing (Claim/Service):
Not used.
987-MA URL N Prior Authorization Request And Billing (Claim/Service):
Not used.
Notes on Response Status Segment on a Prior Authorization Request And Billing Response:
The Response Status Segment is mandatory for a Prior Authorization Request And Billing response for Header Response Status (5Ø1-F1) =
“A” (Accepted) and Transaction Response Status (112-AN) = “R” (Rejected). The Response Status Segment is sent from the sender to the
receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.
13.3.4.2.6 RESPONSE CLAIM SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT Q
N
Prior Authorization Request And Billing (Claim):
Maximum count of 6.
Required if Preferred Product ID (553-AR) is used.
Service:
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER Q***R***
N
Prior Authorization Request And Billing (Claim):
Required if Preferred Product ID (553-AR) is used.
Service:
Not used.
553-AR PREFERRED PRODUCT ID
Q***R***
N
Prior Authorization Request And Billing (Claim):
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
Service:
Not used.
554-AS PREFERRED PRODUCT INCENTIVE
Q***R***
N
Prior Authorization Request And Billing (Claim):
Required if Preferred Product ID (553-AR) is used and
there is an incentive amount associated with the Preferred
Product ID (553-AR).
Service:
Not used.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 377 -
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE Q***R***
N
Prior Authorization Request And Billing (Claim):
Required if Preferred Product ID (553-AR) is used and
there is a patient financial responsibility incentive amount
associated with the Preferred Product ID (553-AR).
Service:
Not used.
556-AU PREFERRED PRODUCT DESCRIPTION Q***R***
N
Prior Authorization Request And Billing (Claim):
Required if preferred product description needs to be sent,
either as explanation to Preferred Product ID Qualifier (552-
AP) and Preferred Product ID (553-AR), or when a
Preferred Product ID (553-AR) and Qualifier (552-AP) are
not known.
Service:
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Prior Authorization Request And Billing (Claim/Service):
Not used.
Notes on Response Claim Segment on a Prior Authorization Request And Billing Response:
The Response Claim Segment is mandatory for a Prior Authorization Request And Billing response when the Header Response Status (5Ø1-
F1) is “A” (Accepted) and Transaction Response Status (112-AN) of “R” (Rejected).
The Response Claim Segment (Prior Authorization Request And Billing – Claim) is sent from the sender to the receiver to identify therapeutic
or alternate product recommendations.
The Response Claim Segment (Prior Authorization Request And Billing – Service) is sent from the sender to the receiver to mirror back the
Prescription/Service Reference Number (4Ø2-D2).
1. If the identity of the patient is partially verified and the Prior Authorization Request And Billing is rejected due to a non-match of field
verification, then the Other Payer information is not sent.
2. If the Prior Authorization Request And Billing is rejected because it should be submitted to other payer(s) first, that Other Payer
information should be sent, if known.
3. If the Prior Authorization Request And Billing is rejected due to benefit design limitations, then subsequent Other Payer information
should be sent, if known.
If the Prior Authorization Request And Billing rejects for other reasons than above, Other Payer information is not sent.
If additional payer(s) for this patient is not known, the Other Payer information is not sent.
If additional payer(s) for this patient is known, the following may be sent:
• Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C),
• Other Payer Group ID (992-MJ),
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU).
In addition, if any of the following three fields are sent:
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU),
• Other Payer Group ID (992-MJ),
then the Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C) must be sent.
Fields defined as Mandatory are required to be submitted when the segment is sent.
13.3.4.2.7 RESPONSE DUR/PPS SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
567-J6 DUR/PPS RESPONSE CODE COUNTER Q***R*** Prior Authorization Request And Billing (Claim/Service):
Maximum 9 occurrences supported.
Required if Reason For Service Code (439-E4) is used.
439-E4 REASON FOR SERVICE CODE Q***R*** Prior Authorization Request And Billing (Claim):
Required if utilization conflict is detected.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 378 -
RESPONSE DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Service:
Required if professional service opportunity reason is
detected by the receiver. Should be different than the
original transmission.
528-FS CLINICAL SIGNIFICANCE CODE
Q***R*** Prior Authorization Request And Billing (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
529-FT OTHER PHARMACY INDICATOR
Q***R*** Prior Authorization Request And Billing (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
53Ø-FU PREVIOUS DATE OF FILL
Q***R*** Prior Authorization Request And Billing (Claim):
Required if needed to supply additional information for the
utilization conflict.
Required if Quantity of Previous Fill (531-FV) is used.
Service:
Required if needed to supply additional information for the
service.
531-FV QUANTITY OF PREVIOUS FILL
Q***R*** Prior Authorization Request And Billing (Claim):
Required if Previous Date Of Fill (53Ø-FU) is used.
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
532-FW DATABASE INDICATOR
Q***R*** Prior Authorization Request And Billing (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
533-FX OTHER PRESCRIBER INDICATOR
Q***R*** Prior Authorization Request And Billing (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
544-FY DUR FREE TEXT MESSAGE
Q***R*** Prior Authorization Request And Billing (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
57Ø-NS DUR ADDITIONAL TEXT Q***R*** Prior Authorization Request And Billing (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
Notes on Response DUR/PPS Segment on a Prior Authorization Request And Billing Response:
The Response DUR/PPS Segment is situational for a Prior Authorization Request And Billing response when the Header Response Status
(5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “R” (Rejected), to identify a drug utilization review or professional
pharmacy service event, opportunity, or information. The Segment is mandatory if required under provider payer contract or mandatory on
claims where this information is necessary for adjudication of the claim. Fields defined as Mandatory are required to be submitted when the
segment is sent.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 379 -
13.3.4.2.8 RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS SEGMENT (PRIOR AUTHORIZATION REQUEST
AND BILLING) (TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS
SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
355-NT OTHER PAYER ID COUNT M Prior Authorization Request And Billing (Claim/Service):
Maximum count of 3.
338-5C OTHER PAYER COVERAGE TYPE M***R***
339-6C OTHER PAYER ID QUALIFIER Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if Other Payer ID (34Ø-7C) is used.
34Ø-7C OTHER PAYER ID Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if other insurance information is available for
coordination of benefits.
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if other insurance information is available for
coordination of benefits.
356-NU OTHER PAYER CARDHOLDER ID Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if other insurance information is available for
coordination of benefits.
992-MJ OTHER PAYER GROUP ID Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if other insurance information is available for
coordination of benefits.
142-UV OTHER PAYER PERSON CODE Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if needed to uniquely identify the family members
within the Cardholder ID, as assigned by the other payer.
127-UB OTHER PAYER HELP DESK PHONE NUMBER Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if needed to provide a support telephone number
of the other payer to the receiver.
143-UW OTHER PAYER PATIENT RELATIONSHIP CODE Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if needed to uniquely identify the relationship of
the patient to the cardholder ID, as assigned by the other
payer.
144-UX OTHER PAYER BENEFIT EFFECTIVE DATE Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required when other coverage is known which is after the
Date of Service submitted.
145-UY OTHER PAYER BENEFIT TERMINATION DATE Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required when other coverage is known which is after the
Date of Service submitted.
Notes on Response Coordination of Benefits/Other Payers Segment on a Prior Authorization Request And Billing Response:
The Response Coordination of Benefits/Other Payers Segment is situational for a Prior Authorization Request And Billing response when the
Header Response Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “R” (Rejected) when other insurance
information is available for coordination of benefits.
1. If the identity of the patient is partially verified and the Prior Authorization Request And Billing is rejected due to a non-match of field
verification, then the Other Payer information is not sent.
2. If the Prior Authorization Request And Billing is rejected because it should be submitted to other payer(s) first, that Other Payer
information should be sent, if known.
3. If the Prior Authorization Request And Billing is rejected due to benefit design limitations, then subsequent Other Payer information
should be sent, if known.
If the Prior Authorization Request And Billing rejects for other reasons than above, Other Payer information is not sent.
If additional payer(s) for this patient is not known, the Other Payer information is not sent.
If additional payer(s) for this patient is known, the following may be sent:
• Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C),
• Other Payer Group ID (992-MJ),
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU)
• And other Other Payer fields.
In addition, if any of the following three fields are sent:
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU),
• Other Payer Group ID (992-MJ),
then the Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C) must be sent.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 380 -
Fields defined as Mandatory are required to be submitted when the segment is sent.
13.3.5 TRANSMISSION REJECTED/TRANSACTION REJECTED
Prior Authorization Request And Billing transmission response Header Response Status (5Ø1-F1) of "R" (Rejected)
and Transaction Response Status (112-AN) of “R” (Rejected)
Each prior authorization request and billing request contains one occurrence of claim/service data.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
13.3.5.1 DIAGRAM FOR TRANSMISSION OF ONE PRIOR AUTHORIZATION REQUEST AND BILLING
RESPONSE (TRANSMISSION REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
13.3.5.2 PRIOR AUTHORIZATION REQUEST AND BILLING RESPONSE SEGMENTS (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
13.3.5.2.1 RESPONSE HEADER SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING) (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Prior Authorization Request And Billing Response:
The Response Header Segment is a mandatory, fixed length segment for a Prior Authorization Request And Billing response when the Header
Response Status (5Ø1-F1) of "R" (Rejected) and Transaction Response Status (112-AN) of “R” (Rejected). The “Situation” column is not
applicable.
If either the entire transmission or the Header is in error, the Header Response Status (5Ø1-F1) = “R” (Rejected). Every identifiable transaction
within the transmission must be rejected with an “R”.
13.3.5.2.2 RESPONSE MESSAGE SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING) (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Prior Authorization Request And Billing (Claim/Service):
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 381 -
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Prior Authorization Request And Billing Response:
The Response Message Segment is situational for a Prior Authorization Request And Billing response when the Header Response Status
(5Ø1-F1) of "R" (Rejected) and Transaction Response Status (112-AN) of “R” (Rejected). It is used when additional text information needs to
be sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
13.3.5.2.3 RESPONSE STATUS SEGMENT (PRIOR AUTHORIZATION REQUEST AND BILLING) (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT R Prior Authorization Request And Billing (Claim/Service):
Maximum count of 5.
Required.
511-FB REJECT CODE R***R*** Prior Authorization Request And Billing (Claim/Service):
Required.
546-4F REJECT FIELD OCCURRENCE INDICATOR Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if a repeating field is in error, to identify repeating
field occurrence.
This field must be sent when relaying error information about
a repeating field or set. Note, if the Reject Code is not
denoting a repeating field or set, the Reject Field Occurrence
Indicator must not be sent.
547-5F APPROVED MESSAGE CODE COUNT N Prior Authorization Request And Billing (Claim/Service):
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Prior Authorization Request And Billing (Claim/Service):
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Prior Authorization Request And Billing (Claim/Service):
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 382 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Prior Authorization Request And Billing (Claim/Service):
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Prior Authorization Request And Billing (Claim/Service):
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Prior Authorization Request And Billing (Claim/Service):
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Prior Authorization Request And Billing (Claim/Service):
Not used.
993-A7 INTERNAL CONTROL NUMBER N Prior Authorization Request And Billing (Claim/Service):
Not used.
987-MA URL N Prior Authorization Request And Billing (Claim/Service):
Not used.
Notes on Response Status Segment on a Prior Authorization Request And Billing Response:
The Response Status Segment is mandatory for a Prior Authorization Request And Billing response for Header Response Status (5Ø1-F1) =
“R” (Rejected) and Transaction Response Status (112-AN) of “R” (Rejected). The Response Status Segment is sent from the sender to the
receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 383 -
14. PRIOR AUTHORIZATION REVERSAL INFORMATION
This transaction allows the Originator to request the Processor to cancel a previously approved prior authorization request.
Prior Authorization reversals are used to back out the request for authorization, but not any claims submitted against the prior authorization. To
reverse a Prior Authorization Request and Billing, paid billings must be reversed before the prior authorization is reversed. The pharmacy must
submit a Claim or Service Reversal (Transaction Code = “B2”) before submitting a Prior Authorization Reversal request. If there are no Claims
or Services paid for the Prior Authorization in question, the processor must accept the Prior Authorization Reversal for the prior authorization
only.
Each prior authorization claim or service reversal request contains one occurrence of claim/service data. The Transaction Code is “P2”.
Depending upon the particular prior authorization claim or service reversal request, the Processor must provide one of the following general
types of responses:
Approved - This occurs when the Processor acknowledges receipt of the prior authorization claim or service reversal, and
successfully processes the backing out of the prior authorization request.
Duplicate of Approved - This occurs when the Processor has previously received the request and processed the transaction, but the
response did not return to the Originator. The Duplicate response contains the same information as returned in the Approved
scenario.
Captured - This occurs when the Processor acknowledges receipt of the prior authorization claim or service reversal, but does not
immediately process the reversal.
Duplicate of Captured - This occurs when the Processor has previously received the request and processed the transaction, but the
response did not return to the Originator. The Duplicate response contains the same information as returned in the Captured
scenario.
Rejected - This occurs when the Processor has encountered an error in the transaction or processing.
See section “Response Processing Guidelines”, “Duplicate Transactions”.
14.1 PRIOR AUTHORIZATION REVERSAL REQUEST DIAGRAMS
14.1.1 DIAGRAM FOR TRANSMISSION OF ONE PRIOR AUTHORIZATION REVERSAL
TRANSACTION
For a Prior Authorization Reversal, the scenarios defined include
Prior Authorization Reversal from a Sender to a Receiver
Prior Authorization Reversal Transaction Response from a Sender to a Receiver
Standard Transmission Accepted/Transaction Captured/Approved/Rejected Response from a Sender to a
Receiver
Standard Transmission Reject Response to a Prior Authorization Reversal from a Sender to a Receiver
Each prior authorization claim or service reversal request contains one occurrence of claim/service data.
There are no mandatory transaction-level segments.
Mandatory
Transaction Header Segment
Situational
Segment Separator
Insurance Segment
Mandatory - Prior Authorization Reversal
Group Separator
Situational
Segment Separator
Prior Authorization Segment
14.2 PRIOR AUTHORIZATION REVERSAL REQUEST SEGMENTS
14.2.1 TRANSACTION HEADER SEGMENT (PRIOR AUTHORIZATION REVERSAL)
TRANSACTION HEADER SEGMENT
MANDATORY SEGMENT

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 384 -
Field Field Name Mandatory
or Situational Situation
1Ø1-A1 BIN NUMBER M
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø4-A4 PROCESSOR CONTROL NUMBER M
1Ø9-A9 TRANSACTION COUNT M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M
Notes on Transaction Header Segment on a Prior Authorization Reversal Request:
The Transaction Header Segment is a mandatory, fixed length segment for a Prior Authorization Reversal request. The “Situation” column is
not applicable.
14.2.2 INSURANCE SEGMENT (PRIOR AUTHORIZATION REVERSAL)
INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø2-C2 CARDHOLDER ID M
312-CC CARDHOLDER FIRST NAME N Prior Authorization Reversal (Claim/Service):
Not used.
313-CD CARDHOLDER LAST NAME N Prior Authorization Reversal (Claim/Service):
Not used.
314-CE HOME PLAN N Prior Authorization Reversal (Claim/Service):
Not used.
524-FO PLAN ID N Prior Authorization Reversal: (Claim/Service)
Not used.
3Ø9-C9 ELIGIBILITY CLARIFICATION CODE N Prior Authorization Reversal (Claim/Service):
Not used.
3Ø1-C1 GROUP ID N Prior Authorization Reversal (Claim/Service):
Not used.
3Ø3-C3 PERSON CODE N Prior Authorization Reversal (Claim/Service):
Not used.
3Ø6-C6 PATIENT RELATIONSHIP CODE N Prior Authorization Reversal (Claim/Service):
Not used.
99Ø-MG OTHER PAYER BIN NUMBER N Prior Authorization Reversal (Claim/Service):
Not used.
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER N Prior Authorization Reversal (Claim/Service):
Not used.
356-NU OTHER PAYER CARDHOLDER ID N Prior Authorization Reversal (Claim/Service):
Not used.
992-MJ OTHER PAYER GROUP ID N Prior Authorization Reversal (Claim/Service):
Not used.
359-2A MEDIGAP ID N Prior Authorization Reversal (Claim/Service):
Not used.
36Ø-2B MEDICAID INDICATOR N Prior Authorization Reversal (Claim/Service):
Not used.
361-2D PROVIDER ACCEPT ASSIGNMENT INDICATOR N Prior Authorization Reversal (Claim/Service):
Not used.
997-G2 CMS PART D DEFINED QUALIFIED FACILITY N Prior Authorization Reversal (Claim/Service):
Not used.
115-N5
MEDICAID ID NUMBER N Prior Authorization Reversal (Claim/Service):
Not used.
116-N6
MEDICAID AGENCY NUMBER N Prior Authorization Reversal (Claim/Service):
Not used.
Notes on Insurance Segment on a Prior Authorization Reversal Request:
The Insurance Segment is situational for a Prior Authorization Reversal request. Fields defined as Mandatory are required to be submitted
when the segment is sent.
14.2.3 PRIOR AUTHORIZATION SEGMENT (PRIOR AUTHORIZATION REVERSAL)
PRIOR AUTHORIZATION SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 385 -
PRIOR AUTHORIZATION SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
498-PA REQUEST TYPE M
498-PB REQUEST PERIOD DATE-BEGIN M
498-PC REQUEST PERIOD DATE-END M
498-PD BASIS OF REQUEST M
498-PE AUTHORIZED REPRESENTATIVE FIRST NAME N Prior Authorization Reversal (Claim/Service):
Not used.
498-PF AUTHORIZED REPRESENTATIVE LAST NAME N Prior Authorization Reversal (Claim/Service):
Not used.
498-PG AUTHORIZED REPRESENTATIVE STREET ADDRESS N Prior Authorization Reversal (Claim/Service):
Not used.
498-PH AUTHORIZED REPRESENTATIVE CITY ADDRESS N Prior Authorization Reversal (Claim/Service):
Not used.
498-PJ AUTHORIZED REPRESENTATIVE STATE/PROVINCE ADDRESS N Prior Authorization Reversal (Claim/Service):
Not used.
498-PK AUTHORIZED REPRESENTATIVE ZIP/POSTAL ZONE N Prior Authorization Reversal (Claim/Service):
Not used.
498-PY PRIOR AUTHORIZATION NUMBER-ASSIGNED Q Prior Authorization Reversal (Claim/Service):
Required if known to sender; otherwise send Authorization
Number (5Ø3-F3).
5Ø3-F3 AUTHORIZATION NUMBER Q Prior Authorization Reversal (Claim/Service):
Required if Prior Authorization Number-Assigned (498-PY)
is not known.
498-PP PRIOR AUTHORIZATION SUPPORTING DOCUMENTATION N Prior Authorization Reversal (Claim/Service):
Not used.
Notes on Prior Authorization Segment on a Prior Authorization Reversal Request:
The Prior Authorization Segment is situational for a Prior Authorization Reversal request. It is used when the sender wishes to back out a
previous submitted prior authorization. Fields defined as Mandatory are required to be submitted when the segment is sent.
14.2.4 PRIOR AUTHORIZATION REVERSAL RESPONSE DIAGRAMS AND SEGMENTS
14.2.5 TRANSMISSION ACCEPTED/TRANSACTION APPROVED
Prior Authorization Reversal transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved)
Each prior authorization claim or service reversal request contains one occurrence of claim/service data.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
14.2.5.1 DIAGRAM FOR TRANSMISSION OF ONE PRIOR AUTHORIZATION REVERSAL RESPONSE
(TRANSMISSION ACCEPTED/TRANSACTION APPROVED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
14.2.5.2 PRIOR AUTHORIZATION REVERSAL RESPONSE SEGMENTS (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
14.2.5.2.1 RESPONSE HEADER SEGMENT (PRIOR AUTHORIZATION REVERSAL) (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 386 -
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Prior Authorization Reversal Response:
The Response Header Segment is a mandatory, fixed length segment for Prior Authorization Reversal response when the Header Response
Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved). The
“Situation” column is not applicable.
14.2.5.2.2 RESPONSE MESSAGE SEGMENT (PRIOR AUTHORIZATION REVERSAL) (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Prior Authorization Reversal (Claim/Service):
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Prior Authorization Reversal Response:
The Response Message Segment is situational for Prior Authorization Reversal response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved). It is used when additional text
information needs to be sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
14.2.5.2.3 RESPONSE STATUS SEGMENT (PRIOR AUTHORIZATION REVERSAL) (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Prior Authorization Reversal (Claim/Service):
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT N Prior Authorization Reversal (Claim/Service):
Not used.
511-FB REJECT CODE N***R*** Prior Authorization Reversal (Claim/Service):
Not used.
546-4F REJECT FIELD OCCURRENCE INDICATOR N***R*** Prior Authorization Reversal (Claim/Service):
Not used.
547-5F APPROVED MESSAGE CODE COUNT N Prior Authorization Reversal (Claim/Service):
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Prior Authorization Reversal (Claim/Service):
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Prior Authorization Reversal (Claim/Service):
Maximum count of 25.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 387 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Prior Authorization Reversal (Claim/Service):
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Prior Authorization Reversal (Claim/Service):
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Prior Authorization Reversal (Claim/Service):
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Prior Authorization Reversal (Claim/Service):
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Prior Authorization Reversal (Claim/Service):
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Prior Authorization Reversal (Claim/Service):
Not used.
993-A7 INTERNAL CONTROL NUMBER N Prior Authorization Reversal (Claim/Service):
Not used.
987-MA URL N Prior Authorization Reversal (Claim/Service):
Not used.
Notes on Response Status Segment on a Prior Authorization Reversal Response:
The Response Status Segment is mandatory for a Prior Authorization Reversal response for Header Response Status (5Ø1-F1) = “A”
(Accepted) and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved). The Response Status Segment is
sent from the sender to the receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the
segment is sent.
14.2.6 TRANSMISSION ACCEPTED/TRANSACTION CAPTURED
Prior Authorization Reversal transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured)
Each prior authorization claim or service reversal request contains one occurrence of claim/service data.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
14.2.6.1 DIAGRAM FOR TRANSMISSION OF ONE PRIOR AUTHORIZATION REVERSAL RESPONSE
(TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 388 -
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
14.2.6.2 PRIOR AUTHORIZATION REVERSAL RESPONSE SEGMENTS (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
14.2.6.2.1 RESPONSE HEADER SEGMENT (PRIOR AUTHORIZATION REVERSAL) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Prior Authorization Reversal Response:
The Response Header Segment is a mandatory, fixed length segment for Prior Authorization Reversal response when the Header Response
Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). The
“Situation” column is not applicable.
14.2.6.2.2 RESPONSE MESSAGE SEGMENT (PRIOR AUTHORIZATION REVERSAL) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Prior Authorization Reversal (Claim/Service):
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Prior Authorization Reversal Response:
The Response Message Segment is situational for Prior Authorization Reversal response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). It is used when additional text
information needs to be sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
14.2.6.2.3 RESPONSE STATUS SEGMENT (PRIOR AUTHORIZATION REVERSAL) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 389 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Prior Authorization Reversal (Claim/Service):
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT N Prior Authorization Reversal (Claim/Service):
Not used.
511-FB REJECT CODE N***R*** Prior Authorization Reversal (Claim/Service):
Not used.
546-4F REJECT FIELD OCCURRENCE INDICATOR N***R*** Prior Authorization Reversal (Claim/Service):
Not used.
547-5F APPROVED MESSAGE CODE COUNT N Prior Authorization Reversal (Claim/Service):
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Prior Authorization Reversal (Claim/Service):
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Prior Authorization Reversal (Claim/Service):
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Prior Authorization Reversal (Claim/Service):
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Prior Authorization Reversal (Claim/Service):
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Prior Authorization Reversal (Claim/Service):
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Prior Authorization Reversal (Claim/Service):
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Prior Authorization Reversal (Claim/Service):
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Prior Authorization Reversal (Claim/Service):
Not used.
993-A7 INTERNAL CONTROL NUMBER N Prior Authorization Reversal (Claim/Service):
Not used.
987-MA URL N Prior Authorization Reversal (Claim/Service):
Not used.
Notes on Response Status Segment on a Prior Authorization Reversal Response:
The Response Status Segment is mandatory for a Prior Authorization Reversal response for Header Response Status (5Ø1-F1) = “A”
(Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). The Response Status Segment is
sent from the sender to the receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the
segment is sent.
14.2.7 TRANSMISSION ACCEPTED/TRANSACTION REJECTED
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 390 -
Prior Authorization Reversal transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “R” (Rejected)
Each prior authorization claim or service reversal request contains one occurrence of claim/service data.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
14.2.7.1 DIAGRAM FOR TRANSMISSION OF ONE PRIOR AUTHORIZATION REVERSAL RESPONSE
(TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
14.2.7.2 PRIOR AUTHORIZATION REVERSAL RESPONSE SEGMENTS (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
14.2.7.2.1 RESPONSE HEADER SEGMENT (PRIOR AUTHORIZATION REVERSAL) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Prior Authorization Reversal Response:
The Response Header Segment is a mandatory, fixed length segment for Prior Authorization Reversal response when the Header Response
Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). The “Situation” column is not applicable.
14.2.7.2.2 RESPONSE MESSAGE SEGMENT (PRIOR AUTHORIZATION REVERSAL) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Prior Authorization Reversal (Claim/Service):
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 391 -
Notes on Response Message Segment on a Prior Authorization Reversal Response:
The Response Message Segment is situational for Prior Authorization Reversal response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). It is used when additional text information needs to be sent. Fields
defined as Mandatory are required to be submitted when the segment is sent.
14.2.7.2.3 RESPONSE STATUS SEGMENT (PRIOR AUTHORIZATION REVERSAL) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Prior Authorization Reversal (Claim/Service):
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT R Prior Authorization Reversal (Claim/Service):
Maximum count of 5.
Required.
511-FB REJECT CODE R***R*** Prior Authorization Reversal (Claim/Service):
Required.
546-4F REJECT FIELD OCCURRENCE INDICATOR Q***R*** Prior Authorization Reversal (Claim/Service):
Required if a repeating field is in error, to identify repeating
field occurrence.
This field must be sent when relaying error information about
a repeating field or set. Note, if the Reject Code is not
denoting a repeating field or set, the Reject Field Occurrence
Indicator must not be sent.
547-5F APPROVED MESSAGE CODE COUNT N Prior Authorization Reversal (Claim/Service):
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Prior Authorization Reversal (Claim/Service):
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Prior Authorization Reversal (Claim/Service):
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Prior Authorization Reversal (Claim/Service):
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Prior Authorization Reversal (Claim/Service):
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Prior Authorization Reversal (Claim/Service):
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Prior Authorization Reversal (Claim/Service):
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Prior Authorization Reversal (Claim/Service):
Required if needed to provide a support telephone number
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 392 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Prior Authorization Reversal (Claim/Service):
Not used.
993-A7 INTERNAL CONTROL NUMBER N Prior Authorization Reversal (Claim/Service):
Not used.
987-MA URL N Prior Authorization Reversal (Claim/Service):
Not used.
Notes on Response Status Segment on a Prior Authorization Reversal Response:
The Response Status Segment is mandatory for a Prior Authorization Reversal response for Header Response Status (5Ø1-F1) = “A”
(Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). The Response Status Segment is sent from the sender to the
receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.
14.2.8 TRANSMISSION REJECTED/TRANSACTION REJECTED
Prior Authorization Reversal transmission response Header Response Status (5Ø1-F1) of "R" (Rejected)
and Transaction Response Status (112-AN) of “R” (Rejected)
Each prior authorization claim or service reversal request contains one occurrence of claim/service data.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
14.2.8.1 DIAGRAM FOR TRANSMISSION OF ONE PRIOR AUTHORIZATION REVERSAL RESPONSE
(TRANSMISSION REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
14.2.8.2 PRIOR AUTHORIZATION REVERSAL RESPONSE SEGMENTS (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
14.2.8.2.1 RESPONSE HEADER SEGMENT (PRIOR AUTHORIZATION REVERSAL) (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Prior Authorization Reversal Response:
The Response Header Segment is a mandatory, fixed length segment for Prior Authorization Reversal when the Header Response Status
(5Ø1-F1) of "R" (Rejected) and Transaction Response Status (112-AN) of “R” (Rejected). The “Situation” column is not applicable.
If either the entire transmission or the Header is in error, the Header Response Status (5Ø1-F1) = “R” (Rejected). Every identifiable transaction
within the transmission must be rejected with an “R”.
14.2.8.2.2 RESPONSE MESSAGE SEGMENT (PRIOR AUTHORIZATION REVERSAL) (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 393 -
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Prior Authorization Reversal (Claim/Service):
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Prior Authorization Reversal Response:
The Response Message Segment is situational for Prior Authorization Reversal when the Header Response Status (5Ø1-F1) of "R" (Rejected)
and Transaction Response Status (112-AN) of “R” (Rejected). It is used when additional text information needs to be sent. Fields defined as
Mandatory are required to be submitted when the segment is sent.
14.2.8.2.3 RESPONSE STATUS SEGMENT (PRIOR AUTHORIZATION REVERSAL) (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Prior Authorization Reversal (Claim/Service):
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT R Prior Authorization Reversal (Claim/Service):
Maximum count of 5.
Required.
511-FB REJECT CODE R***R*** Prior Authorization Reversal (Claim/Service):
Required.
546-4F REJECT FIELD OCCURRENCE INDICATOR Q***R*** Prior Authorization Reversal (Claim/Service):
Required if a repeating field is in error, to identify repeating
field occurrence.
This field must be sent when relaying error information about
a repeating field or set. Note, if the Reject Code is not
denoting a repeating field or set, the Reject Field Occurrence
Indicator must not be sent.
547-5F APPROVED MESSAGE CODE COUNT N Prior Authorization Reversal (Claim/Service):
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Prior Authorization Reversal (Claim/Service):
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Prior Authorization Reversal (Claim/Service):
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Prior Authorization Reversal (Claim/Service):
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Prior Authorization Reversal (Claim/Service):
Required if additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 394 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Prior Authorization Reversal (Claim/Service):
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Prior Authorization Reversal (Claim/Service):
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Prior Authorization Reversal (Claim/Service):
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Prior Authorization Reversal (Claim/Service):
Not used.
993-A7 INTERNAL CONTROL NUMBER N Prior Authorization Reversal (Claim/Service):
Not used.
987-MA URL N Prior Authorization Reversal (Claim/Service):
Not used.
Notes on Response Status Segment on a Prior Authorization Reversal Response:
The Response Status Segment is mandatory for a Prior Authorization Reversal response for Header Response Status (5Ø1-F1) = “R”
(Rejected) and Transaction Response Status (112-AN) of “R” (Rejected). The Response Status Segment is sent from the sender to the
receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 395 -
15. PRIOR AUTHORIZATION INQUIRY INFORMATION
This transaction allows the Originator to request from the Processor to provide the status of a previously transmitted prior authorization request
that was pended by the Processor. The Originator is inquiring as to what, if anything has occurred.
Each submission request contains one occurrence of claim/service data. The Transaction Code is “P3”.
The Processor must provide one of the following general types of responses:
Approved - The Processor has approved the prior authorization. If a duplicate request is received, the original approved response
must be returned.
Captured - The Processor returns the status of the prior authorization originally submitted. The prior authorization was captured, but
no judgment has been made.
Duplicate of Captured - The Processor has previously received the request and processed the transaction, but the response did not
return to the Originator. The Duplicate response contains the same information as returned in the Captured original response.
Deferred - The Processor notifies the Originator that the status of a prior authorization request is that the request has been deferred.
If a duplicate request is received, the original response must be returned.
Paid - The Processor has approved the authorization and has adjudicated the claim or service.
Duplicate of Paid - The Processor has previously received the request and processed the transaction, but the response did not
return to the Originator. The Duplicate response contains the same information as returned in the Paid original response.
Rejected - The Processor has encountered an error in the transaction or processing, or does not approve the prior authorization
request.
See section “Response Processing Guidelines”, “Duplicate Transactions”.
15.1 PRIOR AUTHORIZATION INQUIRY REQUEST DIAGRAMS
15.1.1 DIAGRAM FOR TRANSMISSION OF ONE PRIOR AUTHORIZATION INQUIRY
TRANSACTION
The sender submits a Prior Authorization Inquiry to receive a status on a previously submitted Prior Authorization Request And Billing or a
previously submitted Prior Authorization Request Only.
This transaction is for use by sender of a prior authorization who wishes to determine the status of a previously submitted prior authorization
request. For instance, the sender received a “capture” response on the original request and more time is needed by the receiver to make a
determination for approval or rejection of a prior authorization request.
The intent is to:
• Determine the status of the request and/or
• Communicate the actual assigned number
It is not to be used to find a prior authorization by any party other than the sender of the prior authorization request.
For a Prior Authorization Inquiry, the scenarios defined include
Prior Authorization Inquiry from a Sender to a Receiver
Prior Authorization Inquiry Paid/Captured/Deferred/Approved Transaction Response from a Sender to a Receiver
Standard Transmission Accepted/Transaction Rejected Response from a Sender to a Receiver
Standard Transmission Reject Response to a Prior Authorization Inquiry from a Sender to a Receiver
There are no situational transaction-level segments.
Each submission request contains one occurrence of claim/service data.
The information contained in the Prior Authorization Inquiry segments must be the same as the information submitted on the original Prior
Authorization Request Only or Prior Authorization Request and Billing.
Mandatory
Transaction Header Segment
Segment Separator
Insurance Segment
Mandatory - Prior Authorization Inquiry
Group Separator
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 396 -
Segment Separator
Prior Authorization Segment
15.2 PRIOR AUTHORIZATION INQUIRY REQUEST SEGMENTS
15.2.1 TRANSACTION HEADER SEGMENT (PRIOR AUTHORIZATION INQUIRY)
TRANSACTION HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø1-A1 BIN NUMBER M
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø4-A4 PROCESSOR CONTROL NUMBER M
1Ø9-A9 TRANSACTION COUNT M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M
Notes on Transaction Header Segment on a Prior Authorization Inquiry Request:
The Transaction Header Segment is a mandatory, fixed length segment for a Prior Authorization Inquiry request. The “Situation” column is not
applicable.
15.2.2 INSURANCE SEGMENT (PRIOR AUTHORIZATION INQUIRY)
INSURANCE SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø2-C2 CARDHOLDER ID M
312-CC CARDHOLDER FIRST NAME N Prior Authorization Inquiry (Claim/Service):
Not used.
313-CD CARDHOLDER LAST NAME N Prior Authorization Inquiry (Claim/Service):
Not used.
314-CE HOME PLAN N Prior Authorization Inquiry (Claim/Service):
Not used.
524-FO PLAN ID
N Prior Authorization Inquiry (Claim/Service):
Not used.
3Ø9-C9 ELIGIBILITY CLARIFICATION CODE N Prior Authorization Inquiry (Claim/Service):
Not used.
3Ø1-C1 GROUP ID
N Prior Authorization Inquiry (Claim/Service):
Not used.
3Ø3-C3 PERSON CODE
N Prior Authorization Inquiry (Claim/Service):
Not used.
3Ø6-C6 PATIENT RELATIONSHIP CODE N Prior Authorization Inquiry (Claim/Service):
Not used.
99Ø-MG OTHER PAYER BIN NUMBER N Prior Authorization Inquiry (Claim/Service):
Not used.
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER N Prior Authorization Inquiry (Claim/Service):
Not used.
356-NU OTHER PAYER CARDHOLDER ID N Prior Authorization Inquiry (Claim/Service):
Not used.
992-MJ OTHER PAYER GROUP ID N Prior Authorization Inquiry (Claim/Service):
Not used.
359-2A MEDIGAP ID N Prior Authorization Inquiry (Claim/Service):
Not used.
36Ø-2B MEDICAID INDICATOR N Prior Authorization Inquiry (Claim/Service):
Not used.
361-2D PROVIDER ACCEPT ASSIGNMENT INDICATOR N Prior Authorization Inquiry (Claim/Service):
Not used.
997-G2 CMS PART D DEFINED QUALIFIED FACILITY N Prior Authorization Inquiry (Claim/Service):
Not used.
115-N5
MEDICAID ID NUMBER N Prior Authorization Inquiry (Claim/Service):
Not used.
116-N6
MEDICAID AGENCY NUMBER N Prior Authorization Inquiry (Claim/Service):
Not used.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 397 -
Notes on Insurance Segment on a Prior Authorization Inquiry Request:
The Insurance Segment is mandatory for a Prior Authorization Inquiry request. The Insurance Segment is submitted to identify the cardholder.
Fields defined as Mandatory are required to be submitted when the segment is sent.
15.2.3 PRIOR AUTHORIZATION SEGMENT (PRIOR AUTHORIZATION INQUIRY)
PRIOR AUTHORIZATION SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
498-PA REQUEST TYPE M
498-PB REQUEST PERIOD DATE-BEGIN M
498-PC REQUEST PERIOD DATE-END M
498-PD BASIS OF REQUEST M
498-PE AUTHORIZED REPRESENTATIVE FIRST NAME N Prior Authorization Inquiry (Claim/Service):
Not used.
498-PF AUTHORIZED REPRESENTATIVE LAST NAME N Prior Authorization Inquiry (Claim/Service):
Not used.
498-PG AUTHORIZED REPRESENTATIVE STREET ADDRESS N Prior Authorization Inquiry (Claim/Service):
Not used.
498-PH AUTHORIZED REPRESENTATIVE CITY ADDRESS N Prior Authorization Inquiry (Claim/Service):
Not used.
498-PJ AUTHORIZED REPRESENTATIVE STATE/PROVINCE ADDRESS N Prior Authorization Inquiry (Claim/Service):
Not used.
498-PK AUTHORIZED REPRESENTATIVE ZIP/POSTAL ZONE N Prior Authorization Inquiry (Claim/Service):
Not used.
498-PY PRIOR AUTHORIZATION NUMBER-ASSIGNED Q Prior Authorization Inquiry (Claim/Service):
Required if known to sender; otherwise send Authorization
Number (5Ø3-F3).
5Ø3-F3 AUTHORIZATION NUMBER Q Prior Authorization Inquiry (Claim/Service):
Required if Prior Authorization Number-Assigned (498-PY)
is not known.
498-PP PRIOR AUTHORIZATION SUPPORTING DOCUMENTATION N Prior Authorization Inquiry (Claim/Service):
Not used.
Notes on Prior Authorization Segment on a Prior Authorization Inquiry Request:
The Prior Authorization Segment is mandatory for a Prior Authorization Inquiry request. It is used when the sender submits a request for the
status of a previously submitted prior authorization request. Fields defined as Mandatory are required to be submitted when the segment is
sent.
15.3 PRIOR AUTHORIZATION INQUIRY RESPONSE DIAGRAMS AND SEGMENTS
15.3.1 TRANSMISSION ACCEPTED/TRANSACTION PAID
Prior Authorization Inquiry transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
And Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid)
Each response contains one occurrence of claim/service data.
A Prior Authorization Inquiry is submitted for a previously submitted Prior Authorization Request And Billing that was “C” (Captured). The Prior
Authorization Inquiry transaction supports multiple responses, but the responses are actually tied back to the originally requested transaction.
If the initial request was a Prior Authorization Request And Billing that was not “P” (Paid) or “R” (Rejected) initially (meaning follow up was
required) or a time out situation occurred, the subsequent Prior Authorization Inquiry would receive a response that was acceptable for the
initial Prior Authorization Request & Billing - “P” (Paid), “C” (Captured), “F” (Deferred), or “R” (Rejected). In this section, “P” (Paid) is shown.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
15.3.1.1 DIAGRAM FOR TRANSMISSION OF ONE PRIOR AUTHORIZATION INQUIRY RESPONSE
(TRANSMISSION ACCEPTED/TRANSACTION PAID)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 398 -
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Pricing Segment
Segment Separator
Response Prior Authorization Segment
Situational
Segment Separator
Response DUR/PPS Segment
Segment Separator
Response Coordination of Benefits/Other Payers Segment
15.3.1.2 PRIOR AUTHORIZATION INQUIRY RESPONSE SEGMENTS (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
15.3.1.2.1 RESPONSE HEADER SEGMENT (PRIOR AUTHORIZATION INQUIRY) (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Prior Authorization Inquiry Response:
The Response Header Segment is a mandatory, fixed length segment for a Prior Authorization Inquiry response when the Header Response
Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid). The “Situation” column
is not applicable.
15.3.1.2.2 RESPONSE MESSAGE SEGMENT (PRIOR AUTHORIZATION INQUIRY) (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Prior Authorization Inquiry (Claim/Service):
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 399 -
Notes on Response Message Segment on a Prior Authorization Inquiry Response:
The Response Message Segment is situational for a Prior Authorization Inquiry response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid). It is used when additional text information
needs to be sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
15.3.1.2.3 RESPONSE INSURANCE SEGMENT (PRIOR AUTHORIZATION INQUIRY) (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø1-C1 GROUP ID Q Prior Authorization Inquiry (Claim/Service):
Required if needed to identify the actual cardholder or
employer group, to identify appropriate group number,
when available.
Required to identify the actual group that was used when
multiple group coverages exist.
Note: This field may contain the Group ID echoed from the
request. May contain the actual Group ID if unknown to the
receiver.
524-FO PLAN ID Q Prior Authorization Inquiry (Claim/Service):
Required if needed to identify the actual plan parameters,
benefit, or coverage criteria, when available.
Required to identify the actual plan ID that was used when
multiple group coverages exist.
Required if needed to contain the actual plan ID if unknown
to the receiver.
545-2F NETWORK REIMBURSEMENT ID Q Prior Authorization Inquiry (Claim/Service):
Required if needed to identify the network for the covered
member.
Required if needed to identify the actual Network
Reimbursement ID, when applicable and/or available.
Required to identify the actual Network Reimbursement ID
that was used when multiple Network Reimbursement IDs
exist.
568-J7 PAYER ID QUALIFIER Q Prior Authorization Inquiry (Claim/Service):
Required if Payer ID (569-J8) is used.
569-J8 PAYER ID Q Prior Authorization Inquiry (Claim/Service):
Required to identify the ID of the payer responding.
115-N5
MEDICAID ID NUMBER N Prior Authorization Inquiry (Claim/Service):
Not used.
116-N6
MEDICAID AGENCY NUMBER N Prior Authorization Inquiry (Claim/Service):
Not used.
3Ø2-C2
CARDHOLDER ID Q Prior Authorization Inquiry (Claim/Service):
Required if the identification to be used in future
transactions is different than what was submitted on the
request.
Notes on Response Insurance Segment on a Prior Authorization Inquiry Response:
The Response Insurance Segment is situational for a Prior Authorization Inquiry response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid). It is used when coverage or reimbursement
parameters or identifiers need to be sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
15.3.1.2.4 RESPONSE PATIENT SEGMENT (PRIOR AUTHORIZATION INQUIRY) (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
RESPONSE PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
31Ø-CA PATIENT FIRST NAME Q Prior Authorization Inquiry (Claim/Service):
Required if known.
311-CB PATIENT LAST NAME Q Prior Authorization Inquiry (Claim/Service):
Required if known.
3Ø4-C4 DATE OF BIRTH Q Prior Authorization Inquiry (Claim/Service):
Required if known.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 400 -
Notes on Response Patient Segment on a Prior Authorization Inquiry Response:
The Response Patient Segment is situational for Prior Authorization Inquiry transmission response Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid) when patient demographic information needs to
be sent from the sender to the receiver. Fields defined as Mandatory are required to be submitted when the segment is sent.
15.3.1.2.5 RESPONSE STATUS SEGMENT (PRIOR AUTHORIZATION INQUIRY) (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Prior Authorization Inquiry (Claim/Service):
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT N Prior Authorization Inquiry (Claim/Service):
Not used.
511-FB REJECT CODE N***R*** Prior Authorization Inquiry (Claim/Service):
Not used.
546-4F REJECT FIELD OCCURRENCE INDICATOR N***R*** Prior Authorization Inquiry (Claim/Service):
Not used.
547-5F APPROVED MESSAGE CODE COUNT Q Prior Authorization Inquiry (Claim/Service):
Maximum count of 5.
Required if Approved Message Code (548-6F) is used.
548-6F APPROVED MESSAGE CODE Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if the Approved Message Code Count (547-5F) is
used and the sender needs to communicate additional
follow up for a potential opportunity.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Prior Authorization Inquiry (Claim/Service):
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Prior Authorization Inquiry (Claim/Service):
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Prior Authorization Inquiry (Claim/Service):
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Prior Authorization Inquiry (Claim/Service):
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Prior Authorization Inquiry (Claim/Service):
Not used.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 401 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
993-A7 INTERNAL CONTROL NUMBER Q Prior Authorization Inquiry (Claim/Service):
Required when used for payer-to-payer coordination of
benefits to track the claim without regard to the “Service
Provider ID, Prescription Number, & Date of Service”.
987-MA URL N Prior Authorization Inquiry (Claim/Service):
Not used.
Notes on Response Status Segment on a Prior Authorization Inquiry Response:
The Response Status Segment is mandatory for a Prior Authorization Inquiry response for Header Response Status (5Ø1-F1) = “A”
(Accepted) and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid). The Response Status Segment is sent from the
sender to the receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is
sent.
15.3.1.2.6 RESPONSE CLAIM SEGMENT (PRIOR AUTHORIZATION INQUIRY) (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT Q
N
Prior Authorization Inquiry (Claim):
Maximum count of 6.
Required if Preferred Product ID (553-AR) is used.
Service:
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER Q***R***
N
Prior Authorization Inquiry (Claim):
Required if Preferred Product ID (553-AR) is used.
Service:
Not used.
553-AR PREFERRED PRODUCT ID Q***R***
N
Prior Authorization Inquiry (Claim):
Required if a product preference exists that needs to be
communicated to the receiver via an ID.
Service:
Not used.
554-AS PREFERRED PRODUCT INCENTIVE Q***R***
N
Prior Authorization Inquiry (Claim):
Required if there is a known incentive amount associated
with the Preferred Product ID (553-AR) and/or Preferred
Product Description (556-AU).
Service:
Not used.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE Q***R***
N
Prior Authorization Inquiry (Claim):
Required if there is a known patient financial responsibility
incentive amount associated with the Preferred Product ID
(553-AR) and/or Preferred Product Description (556-AU).
Service:
Not used.
556-AU PREFERRED PRODUCT DESCRIPTION Q***R***
N
Prior Authorization Inquiry (Claim):
Required if a product preference exists that either cannot
be communicated by the Preferred Product ID (553-AR) or
to clarify the Preferred Product ID (553-AR).
Service:
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Prior Authorization Inquiry (Claim/Service):
Not used.
Notes on Response Claim Segment on a Prior Authorization Inquiry Response:
The Response Claim Segment is mandatory for a Prior Authorization Inquiry response when the Header Response Status (5Ø1-F1) is “A”
(Accepted) and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid).
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 402 -
The Response Claim Segment (Prior Authorization Inquiry – Claim) is sent from the sender to the receiver to identify therapeutic or alternate
product recommendations.
The Response Claim Segment (Prior Authorization Inquiry – Service) is sent from the sender to the receiver to mirror back the
Prescription/Service Reference Number (4Ø2-D2).
Fields defined as Mandatory are required to be submitted when the segment is sent.
15.3.1.2.7 RESPONSE PRICING SEGMENT (PRIOR AUTHORIZATION INQUIRY) (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
RESPONSE PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø5-F5 PATIENT PAY AMOUNT R Prior Authorization Inquiry (Claim/Service):
Required.
5Ø6-F6 INGREDIENT COST PAID Q
N
Prior Authorization Inquiry (Claim):
Required if this value is used to arrive at the final
reimbursement.
Service:
Not used.
5Ø7-F7 DISPENSING FEE PAID Q
N
Prior Authorization Inquiry (Claim):
Required if this value is used to arrive at the final
reimbursement.
Service:
Not used.
557-AV TAX EXEMPT INDICATOR Q Prior Authorization Inquiry (Claim/Service):
Required if the sender (health plan) and/or patient is tax
exempt and exemption applies to this billing.
558-AW FLAT SALES TAX AMOUNT PAID Q Prior Authorization Inquiry (Claim/Service):
Required if this value is used to arrive at the final
reimbursement.
Required if Flat Sales Tax Amount Submitted (481-HA) is
greater than zero (Ø). Zero (Ø) value is valid.
559-AX PERCENTAGE SALES TAX AMOUNT PAID Q Prior Authorization Inquiry (Claim):
Required if this value is used to arrive at the final
reimbursement.
Required if Percentage Sales Tax Amount Submitted (482-
GE) is greater than zero (Ø). Zero (Ø) value is valid.
Required if Percentage Sales Tax Rate Paid (56Ø-AY) and
Percentage Sales Tax Basis Paid (561-AZ) are used.
Service:
Required if Percentage Sales Tax Amount Submitted (482-
GE) is greater than zero (Ø) or if Percentage Sales Tax
Amount Paid (559-AX) is used to arrive at the final
reimbursement. Zero (Ø) value is valid.
Required if Percentage Sales Tax Rate Paid (56Ø-AY) is
used.
56∅-AY PERCENTAGE SALES TAX RATE PAID Q Prior Authorization Inquiry (Claim/Service):
Required if Percentage Sales Tax Amount Paid (559-AX) is
greater than zero (Ø).
561-AZ PERCENTAGE SALES TAX BASIS PAID Q
N
Prior Authorization Inquiry (Claim):
Required if Percentage Sales Tax Amount Paid (559-AX) is
greater than zero (Ø).
Service:
Not used. Code list is not applicable.
521-FL INCENTIVE AMOUNT PAID Q
N
Prior Authorization Inquiry (Claim):
Required if this value is used to arrive at the final
reimbursement.
Required if Incentive Amount Submitted (438-E3) is greater
than zero (Ø). Zero (Ø) value is valid.
Service:
Not used. Not supported in Service Billing formula.
562-J1 PROFESSIONAL SERVICE FEE PAID N Prior Authorization Inquiry (Claim):

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 403 -
RESPONSE PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
R
Not used.
Service:
Required.
563-J2 OTHER AMOUNT PAID COUNT
Q Prior Authorization Inquiry (Claim/Service):
Maximum count of 3.
Required if Other Amount Paid (565-J4) is used.
564-J3 OTHER AMOUNT PAID QUALIFIER Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if Other Amount Paid (565-J4) is used.
565-J4 OTHER AMOUNT PAID
Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if this value is used to arrive at the final
reimbursement.
Required if Other Amount Claimed Submitted (48Ø-H9) is
greater than zero (Ø). Zero (Ø) value is valid. Must respond
to each occurrence submitted.
566-J5 OTHER PAYER AMOUNT RECOGNIZED
Q Prior Authorization Inquiry (Claim):
Required if this value is used to arrive at the final
reimbursement.
Required if Other Payer Amount Paid (431-DV) is greater
than zero (Ø) and Coordination of Benefits/Other Payments
Segment is supported.
Service:
Required if Other Payer Amount Paid (431-DV) is greater
than zero (Ø) or if this field is used to arrive at the final
reimbursement. This field may be equal to zero (Ø).
5Ø9-F9 TOTAL AMOUNT PAID
R Prior Authorization Inquiry (Claim/Service):
Required. Zero (Ø) value is valid.
See Pricing Formula for fields used in calculation.
522-FM BASIS OF REIMBURSEMENT DETERMINATION Q
N
Prior Authorization Inquiry (Claim):
Required if Ingredient Cost Paid (5Ø6-F6) is greater than
zero (Ø).
Required if Basis of Cost Determination (432-DN) is
submitted on billing.
Service:
Not used.
523-FN AMOUNT ATTRIBUTED TO SALES TAX Q Prior Authorization Inquiry (Claim/Service):
Required if Patient Pay Amount (5Ø5-F5) includes sales tax
that is the financial responsibility of the member but is not
also included in any of the other fields that add up to
Patient Pay Amount.
See section “Specific Segment Discussion”, “Response
Segments”, “Response Pricing Segment” for guidance.
512-FC ACCUMULATED DEDUCTIBLE AMOUNT I Prior Authorization Inquiry (Claim/Service):
Provided for informational purposes only.
513-FD REMAINING DEDUCTIBLE AMOUNT I Prior Authorization Inquiry (Claim/Service):
Provided for informational purposes only.
514-FE REMAINING BENEFIT AMOUNT I Prior Authorization Inquiry (Claim/Service):
The Remaining Benefit Amount must not be returned with
zeroes unless there are no benefit dollars remaining. The
default value of 999999999 from previous versions must
not be used as a default in this field.
Provided for informational purposes only.
517-FH AMOUNT APPLIED TO PERIODIC DEDUCTIBLE Q Prior Authorization Inquiry (Claim/Service):
Required if Patient Pay Amount (5Ø5-F5) includes
deductible.
518-FI AMOUNT OF COPAY Q Prior Authorization Inquiry (Claim/Service):
Required if Patient Pay Amount (5Ø5-F5) includes copay
as patient financial responsibility.
52Ø-FK AMOUNT EXCEEDING PERIODIC BENEFIT MAXIMUM Q Prior Authorization Inquiry (Claim/Service):
Required if Patient Pay Amount (5Ø5-F5) includes amount
exceeding periodic benefit maximum.
346-HH BASIS OF CALCULATION—DISPENSING FEE Q
Prior Authorization Inquiry (Claim):
Required if Dispensing Status (343-HD) on submission is
“P” (Partial Fill) or “C” (Completion of Partial Fill).
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 404 -
RESPONSE PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
N Service:
Not used.
347-HJ BASIS OF CALCULATION—COPAY Q
N
Prior Authorization Inquiry (Claim):
Required if Dispensing Status (343-HD) on submission is
“P” (Partial Fill) or “C” (Completion of Partial Fill).
Service:
Not used.
348-HK BASIS OF CALCULATION—FLAT SALES TAX Q
N
Prior Authorization Inquiry (Claim):
Required if Dispensing Status (343-HD) on submission is
“P” (Partial Fill) or “C” (Completion of Partial Fill) and Flat
Sales Tax Amount Paid (558-AW) is greater than zero (Ø).
Service:
Not used.
349-HM BASIS OF CALCULATION—PERCENTAGE SALES TAX Q
N
Prior Authorization Inquiry (Claim):
Required if Dispensing Status (343-HD) on submission is
“P” (Partial Fill) or “C” (Completion of Partial Fill) and
Percentage Sales Tax Amount Paid (559-AX) is greater
than zero (Ø).
Service:
Not used.
571-NZ AMOUNT ATTRIBUTED TO PROCESSOR FEE Q Prior Authorization Inquiry (Claim):
Required if the customer is responsible for 1ØØ% of the
prescription payment and when the provider net sale is less
than the amount the customer is expected to pay.
Service:
Required if the customer is responsible for 1ØØ% of the
service payment and when the provider net sale is less
than the amount the customer is expected to pay.
575-EQ PATIENT SALES TAX AMOUNT I Prior Authorization Inquiry (Claim/Service):
Used when necessary to identify the Patient’s portion of the
Sales Tax.
Provided for informational purposes only.
574-2Y PLAN SALES TAX AMOUNT I Prior Authorization Inquiry (Claim/Service):
Used when necessary to identify the Plan’s portion of the
Sales Tax.
Provided for informational purposes only.
572-4U AMOUNT OF COINSURANCE Q Prior Authorization Inquiry (Claim/Service):
Required if Patient Pay Amount (5Ø5-F5) includes
coinsurance as patient financial responsibility.
573-4V BASIS OF CALCULATION-COINSURANCE Q
N
Prior Authorization Inquiry (Claim):
Required if Dispensing Status (343-HD) on submission is
“P” (Partial Fill) or “C” (Completion of Partial Fill).
Service:
Not used.
392-MU BENEFIT STAGE COUNT Q Prior Authorization Inquiry (Claim/Service):
Maximum count of 4.
Required if Benefit Stage Amount (394-MW) is used.
393-MV BENEFIT STAGE QUALIFIER Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if Benefit Stage Amount (394-MW) is used.
Must only have one value per iteration - value must not be
repeated.
394-MW BENEFIT STAGE AMOUNT Q***R*** Prior Authorization Inquiry (Claim/Service):
Required when a Medicare Part D payer applies financial
amounts to Medicare Part D beneficiary benefit stages.
This field is required when the plan is a participant in a
Medicare Part D program that requires reporting of benefit
stage specific financial amounts.
Required if necessary for state/federal/regulatory agency
programs.
577-G3 ESTIMATED GENERIC SAVINGS Q
Prior Authorization Inquiry (Claim):
This information should be provided when a patient
selected the brand drug and a generic form of the drug was
available. It will contain an estimate of the difference
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 405 -
RESPONSE PRICING SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
N
between the cost of the brand drug and the generic drug,
when the brand drug is more expensive than the generic. It
is information that the provider should provide to the
patient.
Service:
Not used.
128-UC SPENDING ACCOUNT AMOUNT REMAINING I Prior Authorization Inquiry (Claim/Service):
This dollar amount will be provided, if known, to the
receiver when the transaction had spending account dollars
reported as part of the patient pay amount.
This field is informational only. It is reported back to the
provider and the patient the amount remaining on the
spending account after the current claim updated the
spending account.
129-UD HEALTH PLAN-FUNDED ASSISTANCE AMOUNT Q Prior Authorization Inquiry (Claim/Service):
Required when the patient meets the plan-funded
assistance criteria, to reduce Patient Pay Amount (5Ø5-
F5). The resulting Patient Pay Amount (5Ø5-F5) must be
greater than or equal to zero.
This field is always a negative amount or zero.
133-UJ AMOUNT ATTRIBUTED TO PROVIDER NETWORK SELECTION Q Prior Authorization Inquiry (Claim/Service):
Required if Patient Pay Amount (5Ø5-F5) includes an
amount that is attributable to a cost share differential due to
the selection of one pharmacy over another.
134-UK AMOUNT ATTRIBUTED TO PRODUCT SELECTION/BRAND
DRUG
Q
N
Prior Authorization Inquiry (Claim):
Required if Patient Pay Amount (5Ø5-F5) includes an
amount that is attributable to a patient’s selection of a
Brand drug.
Service:
Not used.
135-UM AMOUNT ATTRIBUTED TO PRODUCT SELECTION/NON-
PREFERRED FORMULARY SELECTION
Q
N
Prior Authorization Inquiry (Claim):
Required if Patient Pay Amount (5Ø5-F5) includes an
amount that is attributable to a patient’s selection of a non-
preferred formulary product.
Service:
Not used.
136-UN AMOUNT ATTRIBUTED TO PRODUCT SELECTION/BRAND
NON-PREFERRED FORMULARY SELECTION
Q
N
Prior Authorization Inquiry (Claim):
Required if Patient Pay Amount (5Ø5-F5) includes an
amount that is attributable to a patient’s selection of a
Brand non-preferred formulary product.
Service:
Not used.
137-UP AMOUNT ATTRIBUTED TO COVERAGE GAP Q Prior Authorization Inquiry (Claim/Service):
Required when the patient’s financial responsibility is due to
the coverage gap.
148-U8 INGREDIENT COST CONTRACTED/REIMBURSABLE AMOUNT I
N
Prior Authorization Inquiry (Claim):
Required when Basis of Reimbursement Determination
(522-FM) is “14” (Patient Responsibility Amount) or “15”
(Patient Pay Amount) unless prohibited by
state/federal/regulatory agency.
This field is informational only.
Service:
Not used.
149-U9 DISPENSING FEE CONTRACTED/REIMBURSABLE AMOUNT I
N
Prior Authorization Inquiry (Claim):
Required when Basis of Reimbursement Determination
(522-FM) is “14” (Patient Responsibility Amount) or “15”
(Patient Pay Amount) unless prohibited by
state/federal/regulatory agency.
This field is informational only.
Service:
Not used.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 406 -
Notes on Response Pricing Segment on a Prior Authorization Inquiry Response:
The Response Pricing Segment is mandatory for a Prior Authorization Inquiry response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) is “P” (Paid) or “D” (Duplicate of Paid).
It is highly recommended that whenever possible, the individual dollar fields are to be returned in the response. In the response, the sender
should return the individual payment response fields to allow the receiver to reconcile against the requested payment fields.
See section “Response Processing Guidelines”, “Pricing Guidelines”.
Fields defined as Mandatory are required to be submitted when the segment is sent.
15.3.1.2.8 RESPONSE PRIOR AUTHORIZATION SEGMENT (PRIOR AUTHORIZATION INQUIRY) (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
RESPONSE PRIOR AUTHORIZATION SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
498-PR PRIOR AUTHORIZATION PROCESSED DATE R Prior Authorization Inquiry (Claim/Service):
Required.
498-PS PRIOR AUTHORIZATION EFFECTIVE DATE Q Prior Authorization Inquiry (Claim/Service):
Required if the prior authorization has an effective date.
498-PT PRIOR AUTHORIZATION EXPIRATION DATE Q Prior Authorization Inquiry (Claim/Service):
Required if the prior authorization has an expiration date.
498-RA PRIOR AUTHORIZATION QUANTITY Q Prior Authorization Inquiry (Claim/Service):
Required if the total quantity authorized is greater than
zero.
498-RB PRIOR AUTHORIZATION DOLLARS AUTHORIZED Q Prior Authorization Inquiry (Claim/Service):
Required if the total dollars authorized is greater than zero.
498-PW PRIOR AUTHORIZATION NUMBER OF REFILLS AUTHORIZED Q Prior Authorization Inquiry (Claim/Service):
Required if a specific number of refills is authorized.
498-PX PRIOR AUTHORIZATION QUANTITY ACCUMULATED Q Prior Authorization Inquiry (Claim/Service):
Required if the Prior Authorization Quantity (498-RA) is
greater than zero. The field must equal the total of the
quantities from all claims processed.
498-PY PRIOR AUTHORIZATION NUMBER–ASSIGNED R Prior Authorization Inquiry (Claim/Service):
Required.
Notes on Response Prior Authorization Segment on a Prior Authorization Inquiry Response:
The Response Prior Authorization Segment is mandatory for Prior Authorization Inquiry response when the Header Response Status (5Ø1-F1)
of "A" (Accepted) and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid). It is used to relay the prior authorization
periods, limitations, contracted amounts, as well as a Prior Authorization Number–Assigned (498-PY) which is to be used for subsequent
Claim or Service Billings. Fields defined as Mandatory are required to be submitted when the segment is sent.
15.3.1.2.9 RESPONSE DUR/PPS SEGMENT (PRIOR AUTHORIZATION INQUIRY) (TRANSMISSION
ACCEPTED/TRANSACTION PAID)
RESPONSE DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
567-J6 DUR/PPS RESPONSE CODE COUNTER Q***R*** Prior Authorization Inquiry (Claim/Service):
Maximum 9 occurrences supported.
Required if Reason For Service Code (439-E4) is used.
439-E4 REASON FOR SERVICE CODE Q***R*** Prior Authorization Inquiry (Claim):
Required if detecting utilization conflict.
Service:
Required if professional service opportunity reason is
detected by the receiver that is different from the
professional service submitted.
528-FS CLINICAL SIGNIFICANCE CODE
Q***R*** Prior Authorization Inquiry (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
529-FT OTHER PHARMACY INDICATOR
Q***R*** Prior Authorization Inquiry (Claim):
Required if needed to supply additional information for the
utilization conflict.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 407 -
RESPONSE DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Service:
Required if needed to supply additional information for the
service.
53Ø-FU PREVIOUS DATE OF FILL
Q***R*** Prior Authorization Inquiry (Claim):
Required if needed to supply additional information for the
utilization conflict.
Required if Quantity of Previous Fill (531-FV) is used.
Service:
Required if needed to supply additional information for the
service.
531-FV QUANTITY OF PREVIOUS FILL
Q***R*** Prior Authorization Inquiry (Claim):
Required if needed to supply additional information for the
utilization conflict.
Required if Previous Date Of Fill (53Ø-FU) is used.
Service:
Required if needed to supply additional information for the
service.
532-FW DATABASE INDICATOR
Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if needed to supply additional information for the
Reason for Service Code (439-E4).
533-FX OTHER PRESCRIBER INDICATOR
Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if needed to supply additional information for the
Reason for Service Code (439-E4).
544-FY DUR FREE TEXT MESSAGE
Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if needed to supply additional information for the
Reason for Service Code (439-E4).
57Ø-NS DUR ADDITIONAL TEXT Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if needed to supply additional information.
Notes on Response DUR/PPS Segment on a Prior Authorization Inquiry Response:
The Response DUR/PPS Segment is situational for a Prior Authorization Inquiry response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid). The Response DUR/PPS Segment identifies a
drug utilization review or professional pharmacy service event, opportunity, or information. Fields defined as Mandatory are required to be
submitted when the segment is sent.
15.3.1.2.10 RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS SEGMENT (PRIOR AUTHORIZATION INQUIRY)
(TRANSMISSION ACCEPTED/TRANSACTION PAID)
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS
SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
355-NT OTHER PAYER ID COUNT M Prior Authorization Inquiry (Claim/Service):
Maximum count of 3.
338-5C OTHER PAYER COVERAGE TYPE M***R***
339-6C OTHER PAYER ID QUALIFIER Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if Other Payer ID (34Ø-7C) is used.
34Ø-7C OTHER PAYER ID Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if other insurance information is available for
coordination of benefits.
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if other insurance information is available for
coordination of benefits.
356-NU OTHER PAYER CARDHOLDER ID Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if other insurance information is available for
coordination of benefits.
992-MJ OTHER PAYER GROUP ID Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if other insurance information is available for
coordination of benefits.
142-UV OTHER PAYER PERSON CODE Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if needed to uniquely identify the family members
within the Cardholder ID, as assigned by the other payer.
127-UB OTHER PAYER HELP DESK PHONE NUMBER Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if needed to provide a support telephone number
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 408 -
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS
SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
of the other payer to the receiver.
143-UW OTHER PAYER PATIENT RELATIONSHIP CODE Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if needed to uniquely identify the relationship of
the patient to the cardholder ID, as assigned by the other
payer.
144-UX OTHER PAYER BENEFIT EFFECTIVE DATE Q***R*** Prior Authorization Inquiry (Claim/Service):
Required when other coverage is known which is after the
Date of Service submitted.
145-UY OTHER PAYER BENEFIT TERMINATION DATE Q***R*** Prior Authorization Inquiry (Claim/Service):
Required when other coverage is known which is after the
Date of Service submitted.
Notes on Response Coordination of Benefits/Other Payers Segment on a Prior Authorization Inquiry Response:
The Response Coordination of Benefits/Other Payers Segment is situational for a Prior Authorization Inquiry response when the Header
Response Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “P” (Paid) or “D” (Duplicate of Paid) when other
insurance information is available for coordination of benefits.
If subsequent payer(s) for this patient is not known, the Other Payer information is not sent.
If subsequent payer(s) for this patient is known, the following may be sent:
• Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C),
• Other Payer Group ID (992-MJ),
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU)
• And other Other Payer fields.
In addition, if any of the following three fields are sent:
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU),
• Other Payer Group ID (992-MJ),
then the Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C) must be sent.
Fields defined as Mandatory are required to be submitted when the segment is sent.
15.3.2 TRANSMISSION ACCEPTED/TRANSACTION CAPTURED
Prior Authorization Inquiry transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured)
A Prior Authorization Inquiry is submitted for a previously submitted Prior Authorization Request And Billing or Prior Authorization Request
Only that was “C” (Captured). It is possible that the receiver has not completed processing of the Prior Authorization Request And Billing or
Prior Authorization Request Only, and will respond that the request is still pending, using the “C” (Captured) or “Q” (Duplicate of Captured).
Each response contains one occurrence of claim/service data.
There are no situational transaction-level segments in the Prior Authorization Inquiry transmission response Header Response Status (5Ø1-
F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured).
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
15.3.2.1 DIAGRAM FOR TRANSMISSION OF ONE PRIOR AUTHORIZATION INQUIRY RESPONSE
(TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
15.3.2.2 PRIOR AUTHORIZATION INQUIRY RESPONSE SEGMENTS (TRANSMISSION
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 409 -
ACCEPTED/TRANSACTION CAPTURED)
15.3.2.2.1 RESPONSE HEADER SEGMENT (PRIOR AUTHORIZATION INQUIRY) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Prior Authorization Inquiry Response:
The Response Header Segment is a mandatory, fixed length segment for a Prior Authorization Inquiry response when the Header Response
Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). The
“Situation” column is not applicable.
15.3.2.2.2 RESPONSE MESSAGE SEGMENT (PRIOR AUTHORIZATION INQUIRY) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Prior Authorization Inquiry (Claim/Service):
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Prior Authorization Inquiry Response:
The Response Message Segment is situational for a Prior Authorization Inquiry response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). It is used when additional text
information needs to be sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
15.3.2.2.3 RESPONSE STATUS SEGMENT (PRIOR AUTHORIZATION INQUIRY) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Prior Authorization Inquiry (Claim/Service):
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT N Prior Authorization Inquiry (Claim/Service):
Not used.
511-FB REJECT CODE N***R*** Prior Authorization Inquiry (Claim/Service):
Not used.
546-4F REJECT FIELD OCCURRENCE INDICATOR N***R*** Prior Authorization Inquiry (Claim/Service):
Not used.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 410 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
547-5F APPROVED MESSAGE CODE COUNT N Prior Authorization Inquiry (Claim/Service):
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Prior Authorization Inquiry (Claim/Service):
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Prior Authorization Inquiry (Claim/Service):
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Prior Authorization Inquiry (Claim/Service):
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Prior Authorization Inquiry (Claim/Service):
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Prior Authorization Inquiry (Claim/Service):
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Prior Authorization Inquiry (Claim/Service):
Not used.
993-A7 INTERNAL CONTROL NUMBER Q Prior Authorization Inquiry (Claim/Service):
Required when used for payer-to-payer coordination of
benefits to track the claim without regard to the “Service
Provider ID, Prescription Number, & Date of Service”.
987-MA URL N Prior Authorization Inquiry (Claim/Service):
Not used.
Notes on Response Status Segment on a Prior Authorization Inquiry Response:
The Response Status Segment is mandatory for a Prior Authorization Inquiry response for Header Response Status (5Ø1-F1) = “A”
(Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). The Response Status Segment is
sent from the sender to the receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the
segment is sent.
15.3.3 TRANSMISSION ACCEPTED/TRANSACTION APPROVED
Prior Authorization Inquiry transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved)
If the initial request was a Prior Authorization Request Only that was not approved or rejected initially (meaning follow up was required) or a
time out situation occurred, the subsequent Prior Authorization Inquiry would receive a response that was acceptable for the initial Prior
Authorization Request Only - “A” (Approved), “C” (Captured), “F” (Deferred), or “R” (Rejected). In this section, “A” (Approved) is shown.
Each response contains one occurrence of claim/service data.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 411 -
There are no situational transaction-level segments for Prior Authorization Inquiry transmission response Header Response Status (5Ø1-F1)
of "A" (Accepted) and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved).
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
15.3.3.1 DIAGRAM FOR TRANSMISSION OF ONE PRIOR AUTHORIZATION INQUIRY RESPONSE
(TRANSMISSION ACCEPTED/TRANSACTION APPROVED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Prior Authorization Segment
15.3.3.2 PRIOR AUTHORIZATION INQUIRY RESPONSE SEGMENTS (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
15.3.3.2.1 RESPONSE HEADER SEGMENT (PRIOR AUTHORIZATION INQUIRY) (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Prior Authorization Inquiry Response:
The Response Header Segment is a mandatory, fixed length segment for a Prior Authorization Inquiry response when the Header Response
Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved). The
“Situation” column is not applicable.
15.3.3.2.2 RESPONSE MESSAGE SEGMENT (PRIOR AUTHORIZATION INQUIRY) (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Prior Authorization Inquiry (Claim/Service):
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 412 -
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Prior Authorization Inquiry Response:
The Response Message Segment is situational for a Prior Authorization Inquiry response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved). It is used when additional text
information needs to be sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
15.3.3.2.3 RESPONSE STATUS SEGMENT (PRIOR AUTHORIZATION INQUIRY) (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Prior Authorization Inquiry (Claim/Service):
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT N Prior Authorization Inquiry (Claim/Service):
Not used.
511-FB REJECT CODE N***R*** Prior Authorization Inquiry (Claim/Service):
Not used.
546-4F REJECT FIELD OCCURRENCE INDICATOR N***R*** Prior Authorization Inquiry (Claim/Service):
Not used.
547-5F APPROVED MESSAGE CODE COUNT Q Prior Authorization Inquiry (Claim/Service):
Maximum count of 5.
Required if Approved Message Code (548-6F) is used.
548-6F APPROVED MESSAGE CODE Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if Approved Message Code Count (547-5F) is
used and the sender needs to communicate additional
follow up for a potential opportunity.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Prior Authorization Inquiry (Claim/Service):
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Prior Authorization Inquiry (Claim/Service):
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 413 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Prior Authorization Inquiry (Claim/Service):
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Prior Authorization Inquiry (Claim/Service):
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Prior Authorization Inquiry (Claim/Service):
Not used.
993-A7 INTERNAL CONTROL NUMBER Q Prior Authorization Inquiry (Claim/Service):
Required when used for payer-to-payer coordination of
benefits to track the claim without regard to the “Service
Provider ID, Prescription Number, & Date of Service”.
987-MA URL N Prior Authorization Inquiry (Claim/Service):
Not used.
Notes on Response Status Segment on a Prior Authorization Inquiry Response:
The Response Status Segment is mandatory for a Prior Authorization Inquiry response for Header Response Status (5Ø1-F1) = “A”
(Accepted) and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved). The Response Status Segment is
sent from the sender to the receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the
segment is sent.
15.3.3.2.4 RESPONSE CLAIM SEGMENT (PRIOR AUTHORIZATION INQUIRY) (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT Q
N
Prior Authorization Inquiry (Claim):
Maximum count of 6.
Required if Preferred Product ID (553-AR) is used.
Service:
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER Q***R***
N
Prior Authorization Inquiry (Claim):
Required if Preferred Product ID (553-AR) is used.
Service:
Not used.
553-AR PREFERRED PRODUCT ID Q***R***
N
Prior Authorization Inquiry (Claim):
Required if a product preference exists that needs to be
communicated to the receiver via an ID.
Service:
Not used.
554-AS PREFERRED PRODUCT INCENTIVE N***R*** Prior Authorization Inquiry (Claim/Service):
Not used.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE N***R*** Prior Authorization Inquiry (Claim/Service):
Not used.
556-AU PREFERRED PRODUCT DESCRIPTION Q***R***
N
Prior Authorization Inquiry (Claim):
Required if a product preference exists that either cannot
be communicated by the Preferred Product ID (553-AR) or
to clarify the Preferred Product ID (553-AR).
Service:
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Prior Authorization Inquiry (Claim/Service):
Not used.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 414 -
Notes on Response Claim Segment on a Prior Authorization Inquiry Response:
The Response Claim Segment is mandatory for a Prior Authorization Inquiry response when the Header Response Status (5Ø1-F1) is “A”
(Accepted) and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved).
The Response Claim Segment (Prior Authorization Inquiry – Claim) is sent from the sender to the receiver to identify therapeutic or alternate
product recommendations.
The Response Claim Segment (Prior Authorization Inquiry – Service) is sent from the sender to the receiver to mirror back the
Prescription/Service Reference Number (4Ø2-D2).
Fields defined as Mandatory are required to be submitted when the segment is sent.
15.3.3.2.5 RESPONSE PRIOR AUTHORIZATION SEGMENT (PRIOR AUTHORIZATION INQUIRY) (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
RESPONSE PRIOR AUTHORIZATION SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
498-PR PRIOR AUTHORIZATION PROCESSED DATE R Prior Authorization Inquiry (Claim/Service):
Required.
498-PS PRIOR AUTHORIZATION EFFECTIVE DATE Q Prior Authorization Inquiry:
(Claim/Service):
Required if the prior authorization has an effective date.
498-PT PRIOR AUTHORIZATION EXPIRATION DATE Q Prior Authorization Inquiry:
(Claim/Service):
Required if the prior authorization has an expiration date.
498-RA PRIOR AUTHORIZATION QUANTITY Q Prior Authorization Inquiry:
(Claim/Service):
Required if the total quantity authorized is greater than
zero.
498-RB PRIOR AUTHORIZATION DOLLARS AUTHORIZED Q Prior Authorization Inquiry:
(Claim/Service):
Required if the total dollars authorized is greater than zero.
498-PW PRIOR AUTHORIZATION NUMBER OF REFILLS AUTHORIZED Q Prior Authorization Inquiry:
(Claim/Service):
Required if a specific number of refills is authorized.
498-PX PRIOR AUTHORIZATION QUANTITY ACCUMULATED Q Prior Authorization Inquiry:
(Claim/Service):
Required if the Prior Authorization Quantity (498-RA) is
greater than zero. The field must equal the total of the
quantities from all claims processed.
498-PY PRIOR AUTHORIZATION NUMBER–ASSIGNED R Prior Authorization Inquiry:
(Claim/Service):
Required.
Notes on Response Prior Authorization Segment on a Prior Authorization Inquiry Response:
The Response Prior Authorization Segment is mandatory for Prior Authorization Inquiry transmission response Header Response Status (5Ø1-
F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved). It is used to relay the prior
authorization periods, limitations, contracted amounts, as well as a Prior Authorization Number–Assigned (498-PY) which is to be used for
subsequent Claim or Service Billings. Fields defined as Mandatory are required to be submitted when the segment is sent.
15.3.4 TRANSMISSION ACCEPTED/TRANSACTION DEFERRED
Prior Authorization Inquiry transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “F” (Deferred)
If the initial request was a Prior Authorization Request And Billing or Prior Authorization Request Only that was not approved or rejected
initially (meaning follow up was required) or a time out situation occurred, the subsequent Prior Authorization Inquiry would receive a response
that was acceptable for the initial Prior Authorization Request And Billing or Prior Authorization Request Only - “A” (Approved), “C” (Captured),
“F” (Deferred), or “R” (Rejected). In this section, “F” (Deferred) is shown.
Each response contains one occurrence of claim/service data.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
15.3.4.1 DIAGRAM FOR TRANSMISSION OF ONE PRIOR AUTHORIZATION INQUIRY RESPONSE
(TRANSMISSION ACCEPTED/TRANSACTION DEFERRED)
Mandatory
Response Header Segment
Situational

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 415 -
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Prior Authorization Segment
15.3.4.2 PRIOR AUTHORIZATION INQUIRY RESPONSE SEGMENTS (TRANSMISSION
ACCEPTED/TRANSACTION DEFERRED)
15.3.4.2.1 RESPONSE HEADER SEGMENT (PRIOR AUTHORIZATION INQUIRY) (TRANSMISSION
ACCEPTED/TRANSACTION DEFERRED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Prior Authorization Inquiry Response:
The Response Header Segment is a mandatory, fixed length segment for a Prior Authorization Inquiry response when the Header Response
Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “F” (Deferred). The “Situation” column is not applicable.
15.3.4.2.2 RESPONSE MESSAGE SEGMENT (PRIOR AUTHORIZATION INQUIRY) (TRANSMISSION
ACCEPTED/TRANSACTION DEFERRED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Prior Authorization Inquiry (Claim/Service):
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Prior Authorization Inquiry Response:
The Response Message Segment is situational for a Prior Authorization Inquiry response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “F” (Deferred). It is used when additional text information needs to be sent. Fields
defined as Mandatory are required to be submitted when the segment is sent.
15.3.4.2.3 RESPONSE STATUS SEGMENT (PRIOR AUTHORIZATION INQUIRY) (TRANSMISSION
ACCEPTED/TRANSACTION DEFERRED)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 416 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Prior Authorization Inquiry (Claim/Service):
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT N Prior Authorization Inquiry (Claim/Service):
Not used.
511-FB REJECT CODE N***R*** Prior Authorization Inquiry (Claim/Service):
Not used.
546-4F REJECT FIELD OCCURRENCE INDICATOR N***R*** Prior Authorization Inquiry (Claim/Service):
Not used.
547-5F APPROVED MESSAGE CODE COUNT N Prior Authorization Inquiry (Claim/Service):
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Prior Authorization Inquiry (Claim/Service):
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Prior Authorization Inquiry (Claim/Service):
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Prior Authorization Inquiry (Claim/Service):
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Prior Authorization Inquiry (Claim/Service):
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Prior Authorization Inquiry (Claim/Service):
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Prior Authorization Inquiry (Claim/Service):
Not used.
993-A7 INTERNAL CONTROL NUMBER N Prior Authorization Inquiry (Claim/Service):
Not used.
987-MA URL N Prior Authorization Inquiry (Claim/Service):
Not used.
Notes on Response Status Segment on a Prior Authorization Inquiry Response:
The Response Status Segment is mandatory for a Prior Authorization Inquiry response for Header Response Status (5Ø1-F1) = “A”
(Accepted) "A" (Accepted) and Transaction Response Status (112-AN) of “F” (Deferred). The Response Status Segment is sent from the
sender to the receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is
sent.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 417 -
15.3.4.2.4 RESPONSE CLAIM SEGMENT (PRIOR AUTHORIZATION INQUIRY) (TRANSMISSION
ACCEPTED/TRANSACTION DEFERRED)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT Q
N
Prior Authorization Inquiry (Claim):
Maximum count of 6.
Required if Preferred Product ID (553-AR) is used.
Service:
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER Q***R***
N
Prior Authorization Inquiry (Claim):
Required if Preferred Product ID (553-AR) is used.
Service:
Not used.
553-AR PREFERRED PRODUCT ID Q***R***
N
Prior Authorization Inquiry (Claim):
Required if a product preference exists that needs to be
communicated to the receiver via an ID
Service:
Not used.
554-AS PREFERRED PRODUCT INCENTIVE N***R*** Prior Authorization Inquiry (Claim/Service):
Not used.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE N***R*** Prior Authorization Inquiry (Claim/Service):
Not used.
556-AU PREFERRED PRODUCT DESCRIPTION Q***R***
N
Prior Authorization Inquiry (Claim):
Required if preferred product description Required if a
product preference exists that either cannot be
communicated by the Preferred Product ID (553-AR) or to
clarify the Preferred Product ID (553-AR).
Service:
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Prior Authorization Inquiry (Claim/Service):
Not used.
Notes on Response Claim Segment on a Prior Authorization Inquiry Response:
The Response Claim Segment is mandatory for a Prior Authorization Inquiry response when the Header Response Status (5Ø1-F1) is “A”
(Accepted) and Transaction Response Status (112-AN) of “F” (Deferred).
The Response Claim Segment (Prior Authorization Inquiry – Claim) is sent from the sender to the receiver to identify therapeutic or alternate
product recommendations.
The Response Claim Segment (Prior Authorization Inquiry – Service) is sent from the sender to the receiver to mirror back the
Prescription/Service Reference Number (4Ø2-D2).
Fields defined as Mandatory are required to be submitted when the segment is sent.
15.3.4.2.5 RESPONSE PRIOR AUTHORIZATION SEGMENT (PRIOR AUTHORIZATION INQUIRY) (TRANSMISSION
ACCEPTED/TRANSACTION DEFERRED)
RESPONSE PRIOR AUTHORIZATION SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
498-PR PRIOR AUTHORIZATION PROCESSED DATE R Prior Authorization Inquiry (Claim/Service):
Required.
498-PS PRIOR AUTHORIZATION EFFECTIVE DATE N Prior Authorization Inquiry (Claim/Service):
Not used.
498-PT PRIOR AUTHORIZATION EXPIRATION DATE N Prior Authorization Inquiry (Claim/Service):
Not used.
498-RA PRIOR AUTHORIZATION QUANTITY N Prior Authorization Inquiry (Claim/Service):
Not used.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 418 -
RESPONSE PRIOR AUTHORIZATION SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
498-RB PRIOR AUTHORIZATION DOLLARS AUTHORIZED N Prior Authorization Inquiry (Claim/Service):
Not used.
498-PW PRIOR AUTHORIZATION NUMBER OF REFILLS AUTHORIZED N Prior Authorization Inquiry (Claim/Service):
Not used.
498-PX PRIOR AUTHORIZATION QUANTITY ACCUMULATED N Prior Authorization Inquiry (Claim/Service):
Not used.
498-PY PRIOR AUTHORIZATION NUMBER–ASSIGNED Q Prior Authorization Inquiry (Claim/Service):
Required if the receiver’s system assigns this number.
Notes on Response Prior Authorization Segment on a Prior Authorization Inquiry Response:
The Response Prior Authorization Segment is situational for Prior Authorization Inquiry response for Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “F” (Deferred). It is used to relay the prior authorization periods, limitations,
contracted amounts, as well as a Prior Authorization Number–Assigned (498-PY) which is to be used for subsequent Claim or Service Billings.
Fields defined as Mandatory are required to be submitted when the segment is sent.
15.3.5 TRANSMISSION ACCEPTED/TRANSACTION REJECTED
Prior Authorization Inquiry transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “R” (Rejected)
If the initial request was a Prior Authorization Request And Billing or Prior Authorization Request Only that was not approved or rejected
initially (meaning follow up was required) or a time out situation occurred, the subsequent Prior Authorization Inquiry would receive a response
that was acceptable for the initial Prior Authorization Request And Billing or Prior Authorization Request Only - “A” (Approved), “C” (Captured),
“F” (Deferred), or “R” (Rejected). In this section, “R” (Rejected) is shown.
Each response contains one occurrence of claim/service data.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
15.3.5.1 DIAGRAM FOR TRANSMISSION OF ONE PRIOR AUTHORIZATION INQUIRY RESPONSE
(TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Coordination of Benefits/Other Payers Segment
15.3.5.2 PRIOR AUTHORIZATION INQUIRY RESPONSE SEGMENTS (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
15.3.5.2.1 RESPONSE HEADER SEGMENT (PRIOR AUTHORIZATION INQUIRY) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 419 -
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Prior Authorization Inquiry Response:
The Response Header Segment is a mandatory, fixed length segment for a Prior Authorization Inquiry response when the Header Response
Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). The “Situation” column is not applicable.
If the transaction rejects for detail errors, the Header Response Status (5Ø1-F1) = “A” (Accepted) and the Transaction Response Status (112-
AN) will be “R”.
15.3.5.2.2 RESPONSE MESSAGE SEGMENT (PRIOR AUTHORIZATION INQUIRY) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Prior Authorization Inquiry (Claim/Service):
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Prior Authorization Inquiry Response:
The Response Message Segment is situational for a Prior Authorization Inquiry response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). It is used when additional text information needs to be sent.
Fields defined as Mandatory are required to be submitted when the segment is sent.
15.3.5.2.3 RESPONSE STATUS SEGMENT (PRIOR AUTHORIZATION INQUIRY) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Prior Authorization Inquiry (Claim/Service):
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT R Prior Authorization Inquiry (Claim/Service):
Maximum count of 5.
Required.
511-FB REJECT CODE R***R*** Prior Authorization Inquiry (Claim/Service):
Required.
546-4F REJECT FIELD OCCURRENCE INDICATOR Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if a repeating field is in error, to identify repeating
field occurrence.
This field must be sent when relaying error information about
a repeating field or set. Note, if the Reject Code is not
denoting a repeating field or set, the Reject Field Occurrence
Indicator must not be sent.
547-5F APPROVED MESSAGE CODE COUNT N Prior Authorization Inquiry (Claim/Service):
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Prior Authorization Inquiry (Claim/Service):

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 420 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Prior Authorization Inquiry (Claim/Service):
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Prior Authorization Inquiry (Claim/Service):
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Prior Authorization Inquiry (Claim/Service):
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Prior Authorization Inquiry (Claim/Service):
Not used.
993-A7 INTERNAL CONTROL NUMBER N Prior Authorization Inquiry (Claim/Service):
Not used.
987-MA URL N Prior Authorization Inquiry (Claim/Service):
Not used.
Notes on Response Status Segment on a Prior Authorization Inquiry Response:
The Response Status Segment is mandatory for a Prior Authorization Inquiry response for Header Response Status (5Ø1-F1) = “A”
(Accepted) and Transaction Response Status = “R” (Rejected). The Response Status Segment is sent from the sender to the receiver to
identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.
15.3.5.2.4 RESPONSE CLAIM SEGMENT (PRIOR AUTHORIZATION INQUIRY) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT Q
Prior Authorization Inquiry (Claim):
Maximum count of 6.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 421 -
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
N
Required if Preferred Product ID (553-AR) is used.
Service:
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER Q***R***
N
Prior Authorization Inquiry (Claim):
Required if Preferred Product ID (553-AR) is used.
Service:
Not used.
553-AR PREFERRED PRODUCT ID
Q***R***
N
Prior Authorization Inquiry (Claim):
Required if this field could result in Required if a product
preference exists that needs to be communicated to the
receiver via an ID.
Service:
Not used.
554-AS PREFERRED PRODUCT INCENTIVE N***R*** Prior Authorization Inquiry (Claim/Service):
Not used.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE N***R*** Prior Authorization Inquiry (Claim/Service):
Not used.
556-AU PREFERRED PRODUCT DESCRIPTION Q***R***
N
Prior Authorization Inquiry (Claim):
Required if a product preference exists that either cannot
be communicated by the Preferred Product ID (553-AR) or
to clarify the Preferred Product ID (553-AR).
Service:
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Prior Authorization Inquiry (Claim/Service):
Not used.
Notes on Response Claim Segment on a Prior Authorization Inquiry Response:
The Response Claim Segment is mandatory for a Prior Authorization Inquiry response when the Header Response Status (5Ø1-F1) is “A”
(Accepted) and Transaction Response Status = “R” (Rejected). The Response Claim Segment (Prior Authorization Inquiry – Claim) is sent
from the sender to the receiver to identify therapeutic or alternate product recommendations. Fields defined as Mandatory are required to be
submitted when the segment is sent.
15.3.5.2.5 RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS SEGMENT (PRIOR AUTHORIZATION INQUIRY)
(TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS
SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
355-NT OTHER PAYER ID COUNT M Prior Authorization Inquiry (Claim/Service):
Maximum count of 3.
338-5C OTHER PAYER COVERAGE TYPE M***R***
339-6C OTHER PAYER ID QUALIFIER Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if Other Payer ID (34Ø-7C) is used.
34Ø-7C OTHER PAYER ID Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if other insurance information is available for
coordination of benefits.
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if other insurance information is available for
coordination of benefits.
356-NU OTHER PAYER CARDHOLDER ID Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if other insurance information is available for
coordination of benefits.
992-MJ OTHER PAYER GROUP ID Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if other insurance information is available for
coordination of benefits.
142-UV OTHER PAYER PERSON CODE Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if needed to uniquely identify the family members
within the Cardholder ID, as assigned by the other payer.
127-UB OTHER PAYER HELP DESK PHONE NUMBER Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if needed to provide a support telephone number
of the other payer to the receiver.
143-UW OTHER PAYER PATIENT RELATIONSHIP CODE Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if needed to uniquely identify the relationship of

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 422 -
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS
SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
the patient to the cardholder ID, as assigned by the other
payer.
144-UX OTHER PAYER BENEFIT EFFECTIVE DATE Q***R*** Prior Authorization Inquiry (Claim/Service):
Required when other coverage is known which is after the
Date of Service submitted.
145-UY OTHER PAYER BENEFIT TERMINATION DATE Q***R*** Prior Authorization Inquiry (Claim/Service):
Required when other coverage is known which is after the
Date of Service submitted.
Notes on Response Coordination of Benefits/Other Payers Segment on a Prior Authorization Inquiry Response:
The Response Coordination of Benefits/Other Payers Segment is situational for a Prior Authorization Inquiry response when the Header
Response Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status = “R” (Rejected) when other insurance information is available
for coordination of benefits.
1. If the identity of the patient is partially verified and the Prior Authorization Inquiry is rejected due to a non-match of field verification,
then the Other Payer information is not sent.
2. If the Prior Authorization Inquiry is rejected because it should be submitted to other payer(s) first, that Other Payer information
should be sent, if known.
3. If the Prior Authorization Inquiry is rejected due to benefit design limitations, then subsequent Other Payer information should be
sent, if known.
If the Prior Authorization Inquiry rejects for other reasons than above, Other Payer information is not sent.
If additional payer(s) for this patient is not known, the Other Payer information is not sent.
If additional payer(s) for this patient is known, the following may be sent:
• Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C),
• Other Payer Group ID (992-MJ),
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU)
• And other Other Payer fields.
In addition, if any of the following three fields are sent:
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU),
• Other Payer Group ID (992-MJ),
then the Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C) must be sent.
Fields defined as Mandatory are required to be submitted when the segment is sent.
15.3.6 TRANSMISSION REJECTED/TRANSACTION REJECTED
Prior Authorization Inquiry transmission response Header Response Status (5Ø1-F1) of "R" (Rejected)
and Transaction Response Status (112-AN) of “R” (Rejected)
Each response contains one occurrence of claim/service data.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
15.3.6.1 DIAGRAM FOR TRANSMISSION OF ONE PRIOR AUTHORIZATION INQUIRY RESPONSE
(TRANSMISSION REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
15.3.6.2 PRIOR AUTHORIZATION INQUIRY RESPONSE SEGMENTS (TRANSMISSION
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 423 -
REJECTED/TRANSACTION REJECTED)
15.3.6.2.1 RESPONSE HEADER SEGMENT (PRIOR AUTHORIZATION INQUIRY) (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Prior Authorization Inquiry Response:
The Response Header Segment is a mandatory, fixed length segment for a Prior Authorization Inquiry response when the Header Response
Status (5Ø1-F1) of "R" (Rejected) and Transaction Response Status (112-AN) of “R” (Rejected). The “Situation” column is not applicable.
If either the entire transmission or the Header is in error, the Header Response Status (5Ø1-F1) = “R” (Rejected). Every identifiable transaction
within the transmission must be rejected with an “R”.
15.3.6.2.2 RESPONSE MESSAGE SEGMENT (PRIOR AUTHORIZATION INQUIRY) (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Prior Authorization Inquiry (Claim/Service):
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Prior Authorization Inquiry Response:
The Response Message Segment is situational segment for a Prior Authorization Inquiry response when the Header Response Status (5Ø1-
F1) of "R" (Rejected) and Transaction Response Status (112-AN) of “R” (Rejected). It is used when additional text information needs to be
sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
15.3.6.2.3 RESPONSE STATUS SEGMENT (PRIOR AUTHORIZATION INQUIRY) (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Prior Authorization Inquiry (Claim/Service):
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT R Prior Authorization Inquiry (Claim/Service):
Maximum count of 5.
Required.
511-FB REJECT CODE R***R*** Prior Authorization Inquiry (Claim/Service):

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 424 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Required.
546-4F REJECT FIELD OCCURRENCE INDICATOR Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if a repeating field is in error, to identify repeating
field occurrence.
This field must be sent when relaying error information about
a repeating field or set. Note, if the Reject Code is not
denoting a repeating field or set, the Reject Field Occurrence
Indicator must not be sent.
547-5F APPROVED MESSAGE CODE COUNT N Prior Authorization Inquiry (Claim/Service):
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Prior Authorization Inquiry (Claim/Service):
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Prior Authorization Inquiry (Claim/Service):
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Prior Authorization Inquiry (Claim/Service):
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Prior Authorization Inquiry (Claim/Service):
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Prior Authorization Inquiry (Claim/Service):
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Prior Authorization Inquiry (Claim/Service):
Not used.
993-A7 INTERNAL CONTROL NUMBER N Prior Authorization Inquiry (Claim/Service):
Not used.
987-MA URL N Prior Authorization Inquiry (Claim/Service):
Not used.
Notes on Response Status Segment on a Prior Authorization Inquiry Response:
The Response Status Segment is mandatory for a Prior Authorization Inquiry response when the Header Response Status (5Ø1-F1) of "R"
(Rejected) and Transaction Response Status (112-AN) of “R” (Rejected). The Response Status Segment is sent from the sender to the
receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 425 -
16. PRIOR AUTHORIZATION REQUEST ONLY INFORMATION
This transaction allows the Originator to request of the Processor a prior authorization only and exclude the processing of the claim or service.
Each submission request contains one occurrence of claim/service data. The Transaction Code is “P4”.
The Processor must provide one of the following general types of responses:
Approved - The Processor has approved the prior authorization.
Duplicate of Approved - The Processor has previously received the request and processed the transaction, but the response did not
return to the Originator. The Duplicate response contains the same information as returned in the Approved original response.
Captured - The Processor returns the status of the prior authorization originally submitted. The prior authorization was captured, but
no judgment has been made.
Duplicate of Captured - The Processor has previously received the request and processed the transaction, but the response did not
return to the Originator. The Duplicate response contains the same information as returned in the Captured original response.
Deferred - The Processor notifies the Originator that the status of a prior authorization request is that the request has been deferred.
If a duplicate request is received, the original response must be returned.
Rejected - The Processor has encountered an error in the transaction or processing, or does not approve the prior authorization
request.
See section “Response Processing Guidelines”, “Duplicate Transactions”.
16.1 PRIOR AUTHORIZATION REQUEST ONLY REQUEST DIAGRAMS
16.1.1 DIAGRAM FOR TRANSMISSION OF ONE PRIOR AUTHORIZATION REQUEST ONLY
(CLAIM) TRANSACTION
For a Prior Authorization Request Only (Claim), the scenarios defined include
Prior Authorization Request Only (Claim) from a Sender to a Receiver
Prior Authorization Request Accepted – Approved/Captured/Deferred/Rejected Transaction Response from a Sender to a
Receiver
Standard Transmission Reject Response to a Prior Authorization Request Only from a Sender to a Receiver
Each submission request contains one occurrence of claim/service data.
Mandatory
Transaction Header Segment
Segment Separator
Insurance Segment
Situational
Segment Separator
Patient Segment
Mandatory - Prior Authorization Request Only (Claim)
Group Separator
Segment Separator
Claim Segment
Segment Separator
Prior Authorization Segment
Situational
Segment Separator
Prescriber Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Compound Segment
Segment Separator
Clinical Segment
16.1.2 DIAGRAM FOR TRANSMISSION OF ONE PRIOR AUTHORIZATION REQUEST ONLY
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 426 -
(SERVICE) TRANSACTION
For a Prior Authorization Request Only (Service), the scenarios defined include
Prior Authorization Request Only (Service) from a Sender to a Receiver
Prior Authorization Request Accepted – Approved/Captured/Deferred/Rejected Transaction Response from a Sender to a
Receiver
Standard Transmission Reject Response to a Prior Authorization Request Only from a Sender to a Receiver
Each submission request contains one occurrence of claim/service data.
The Compound Segment is not used for a Prior Authorization Request Only (Service).
Mandatory
Transaction Header Segment
Segment Separator
Insurance Segment
Situational
Segment Separator
Patient Segment
Mandatory - Prior Authorization Request Only (Service)
Group Separator
Segment Separator
Claim Segment
Segment Separator
Prior Authorization Segment
Situational
Segment Separator
Prescriber Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Clinical Segment
16.2 PRIOR AUTHORIZATION REQUEST ONLY REQUEST SEGMENTS
16.2.1 TRANSACTION HEADER SEGMENT (PRIOR AUTHORIZATION REQUEST ONLY)
TRANSACTION HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø1-A1 BIN NUMBER M
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø4-A4 PROCESSOR CONTROL NUMBER M
1Ø9-A9 TRANSACTION COUNT M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M
Notes on Transaction Header Segment on a Prior Authorization Request Only Request:
The Transaction Header Segment is a mandatory, fixed length segment for a Prior Authorization Request Only request. The “Situation” column
is not applicable.
16.2.2 INSURANCE SEGMENT (PRIOR AUTHORIZATION REQUEST ONLY)
INSURANCE SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø2-C2 CARDHOLDER ID M

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 427 -
INSURANCE SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
312-CC CARDHOLDER FIRST NAME
Q Prior Authorization Request Only (Claim/Service):
Required if the Patient is the Cardholder, and Date of Birth
(3Ø4-C4) is not available. (Note: Cardholder ID (3Ø2-C2) is
mandatory.)
Not used when Cardholder ID (3Ø2-C2), Date of Birth
(3Ø4-C4), and Person Code (3Ø3-C3) are present.
It is a recommendation that Cardholder ID (3Ø2-C2) and
Date of Birth (3Ø4-C4) are used.
Required if necessary for state/federal/regulatory agency or
Workers’ Compensation programs.
Required if multiple people have the same Cardholder ID.
Required if additional verification of the submitted eligibility
information is needed.
313-CD CARDHOLDER LAST NAME
Q Prior Authorization Request Only (Claim/Service):
Required if the Patient is the Cardholder, and the Date of
Birth (3Ø4-C4) is not available.
Required if Service Bureau when acting as an agent of
sender.
Required for presumptive eligibility.
Required for coupon/sample/trial dose programs when
there is no unique Cardholder ID.
Required if contractually obligated between trading
partners.
Not used when Cardholder ID (3Ø2-C2), Date of Birth
(3Ø4-C4), and Person Code (3Ø3-C3) are present.
It is a recommendation that Cardholder ID (3Ø2-C2) and
Date of Birth (3Ø4-C4) are used.
Required if necessary for state/federal/regulatory agency or
Workers’ Compensation programs.
Required if multiple people have the same Cardholder ID.
Required if additional verification of the submitted eligibility
information is needed.
314-CE HOME PLAN
.
Q Prior Authorization Request Only (Claim/Service):
Required if needed for receiver inquiry validation and/or
determination for Blue Cross or Blue Shield, if a Patient has
coverage under more than one plan, to distinguish each
plan.
524-FO PLAN ID
Q Prior Authorization Request Only (Claim/Service):
Required if needed for pharmacy claim processing and
payment.
3Ø9-C9 ELIGIBILITY CLARIFICATION CODE
Q Prior Authorization Request Only (Claim/Service):
Required if needed for receiver inquiry validation and/or
determination, when eligibility is not maintained at the
dependent level. Required in special situations as defined
by the code to clarify the eligibility of an individual, which
may extend coverage.
3Ø1-C1 GROUP ID
Q Prior Authorization Request Only (Claim/Service):
Required if necessary for state/federal/regulatory agency
programs.
Required if needed for pharmacy claim processing and
payment.
3Ø3-C3 PERSON CODE
Q Prior Authorization Request Only (Claim/Service):
Required if needed to uniquely identify the family members
within the Cardholder ID.
3Ø6-C6 PATIENT RELATIONSHIP CODE
Q Prior Authorization Request Only (Claim/Service):
Required if needed to uniquely identify the relationship of
the Patient to the Cardholder ID.
99Ø-MG OTHER PAYER BIN NUMBER N Prior Authorization Request Only (Claim/Service):

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 428 -
INSURANCE SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Not used.
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER N Prior Authorization Request Only (Claim/Service):
Not used.
356-NU OTHER PAYER CARDHOLDER ID N Prior Authorization Request Only (Claim/Service):
Not used.
992-MJ OTHER PAYER GROUP ID N Prior Authorization Request Only (Claim/Service):
Not used.
359-2A MEDIGAP ID Q Prior Authorization Request Only (Claim/Service):
Required, if known, when patient has Medigap coverage.
36Ø-2B MEDICAID INDICATOR Q Prior Authorization Request Only (Claim/Service):
Required, if known, when patient has Medicaid coverage.
361-2D PROVIDER ACCEPT ASSIGNMENT INDICATOR N Prior Authorization Request Only (Claim/Service):
Not used.
997-G2 CMS PART D DEFINED QUALIFIED FACILITY N Prior Authorization Request Only (Claim/Service):
Not used.
115-N5
MEDICAID ID NUMBER N Prior Authorization Request Only (Claim/Service):
Not used.
116-N6
MEDICAID AGENCY NUMBER N Prior Authorization Request Only (Claim/Service):
Not used.
Notes on Insurance Segment on a Prior Authorization Request Only Request:
The Insurance Segment is mandatory for a Prior Authorization Request Only request. Fields defined as Mandatory are required to be
submitted when the segment is sent.
16.2.3 PATIENT SEGMENT (PRIOR AUTHORIZATION REQUEST ONLY)
PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
331-CX PATIENT ID QUALIFIER Q Prior Authorization Request Only (Claim/Service):
Required if Patient ID (332-CY) is used.
332-CY PATIENT ID
Q Prior Authorization Request Only (Claim/Service):
Required if necessary for state/federal/regulatory agency
programs to validate dual eligibility.
3Ø4-C4 DATE OF BIRTH
Q Prior Authorization Request Only (Claim/Service):
Required if necessary for state/federal/regulatory agency
programs.
3Ø5-C5 PATIENT GENDER CODE
Q Prior Authorization Request Only (Claim/Service):
Required if additional verification of the submitted eligibility
information is needed.
Required if needed to assist in identifying the patient when
specific eligibility cannot be established.
31Ø-CA PATIENT FIRST NAME
Q Prior Authorization Request Only (Claim):
Required if the patient is not the cardholder and needed to
file the prior authorization request.
Required if the Patient is not the Cardholder and Date of
Birth (3Ø4-C4) is not available.
Required if necessary for state/federal/regulatory agency
programs.
Service:
Required if the patient is not the cardholder and needed to
file the prior authorization request.
Required if the Patient is not the Cardholder, and Date of
Birth (3Ø4-C4) is not available.
Required if necessary for state/federal/regulatory agency
programs.
311-CB PATIENT LAST NAME
Q Prior Authorization Request Only (Claim/Service):
Required if the patient is not the cardholder and needed to
file the prior authorization.
322-CM PATIENT STREET ADDRESS
Q Prior Authorization Request Only (Claim/Service):
Required if the patient is not the cardholder and needed to
file the prior authorization.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 429 -
PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if needed to assist in identifying the patient when
specific eligibility cannot be established.
Required if necessary for state/federal/regulatory agency
programs.
Required if the patient is not the cardholder and needed to
file the claim/encounter.
323-CN PATIENT CITY ADDRESS
Q Prior Authorization Request Only (Claim/Service):
Required if the patient is not the cardholder and needed to
file the prior authorization.
Required if needed to assist in identifying the patient when
specific eligibility cannot be established.
Required if necessary for state/federal/regulatory agency
programs
Required if the patient is not the cardholder and needed to
file the claim/encounter.
324-CO PATIENT STATE / PROVINCE ADDRESS
Q Prior Authorization Request Only (Claim/Service):
Required if the patient is not the cardholder and needed to
file the prior authorization.
Required if needed to assist in identifying the patient when
specific eligibility cannot be established.
Required if necessary for state/federal/regulatory agency
programs.
Required if the patient is not the cardholder and needed to
file the claim/encounter.
325-CP PATIENT ZIP/POSTAL ZONE
Q Prior Authorization Request Only (Claim/Service):
Required if known and if needed to adjudicate a workers’
compensation prior authorization.
Required if necessary for state/federal/regulatory agency
programs.
Required if the patient is not the cardholder and needed to
file the prior authorization.
Required if needed to assist in identifying the patient when
specific eligibility cannot be established. .
326-CQ PATIENT PHONE NUMBER
Q Prior Authorization Request Only (Claim/Service):
Required if known and if needed to adjudicate a workers’
compensation prior authorization.
Required if necessary for state/federal/regulatory agency
programs.
Required if known and if needed to adjudicate a workers’
compensation prior authorization.
3Ø7-C7 PLACE OF SERVICE
Q Prior Authorization Request Only (Claim/Service):
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
333-CZ EMPLOYER ID
.
Q Prior Authorization Request Only (Claim/Service):
Required if needed to file the prior authorization for receiver
claim determination such as Workers’ Compensation.
Required if necessary for state/federal/regulatory agency
programs.
Required if needed to file the prior authorization for receiver
claim determination such as Workers’ Compensation.
334-1C SMOKER / NON-SMOKER CODE
Q Prior Authorization Request Only (Claim/Service):
Required if clinical determination is dependent upon
patient’s smoking condition.
335-2C PREGNANCY INDICATOR
Q Prior Authorization Request Only (Claim/Service):
Required if clinical determination is dependent upon
patient’s pregnancy condition. Submitted until it is known
the patient is no longer pregnant.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 430 -
PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if pregnancy could result in different coverage,
pricing, or patient financial responsibility.
35Ø-HN PATIENT E-MAIL ADDRESS N Prior Authorization Request Only (Claim/Service):
Not used.
384-4X PATIENT RESIDENCE Q Prior Authorization Request Only (Claim/Service):
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
Notes on Patient Segment on a Prior Authorization Request Only Request:
The Patient Segment is situational for a Prior Authorization Request Only request. The Patient Segment must be submitted when needed to
differentiate between the patient and the cardholder. If the cardholder and the patient are the same, then the Patient Segment is not submitted
unless additional information about the patient is needed to clarify the Prior Authorization Request Only. The Segment is mandatory if required
under provider payer contract or mandatory on Prior Authorization Request Only where this information is necessary for processing a prior
authorization. Fields defined as Mandatory are required to be submitted when the segment is sent.
16.2.4 CLAIM SEGMENT (PRIOR AUTHORIZATION REQUEST ONLY)
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M If Prescription/Service Reference Number (4Ø2-D2) is
unknown, default to zeroes.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
436-E1 PRODUCT/SERVICE ID QUALIFIER M Mandatory.
If billing for a multi-ingredient prescription, Product/Service
ID Qualifier (436-E1) is zero (Zero means “ØØ”).
If the Product/Service ID Qualifier (436-E1) = “Ø6”
(DUR/PPS), the Product/Service ID (4Ø7-D7) is zero. (Zero
means “Ø”.)
4Ø7-D7 PRODUCT/SERVICE ID M Mandatory.
If billing for a multi-ingredient prescription, Product/Service
ID (4Ø7-D7) is zero. (Zero means “Ø”.)
If the Product/Service ID Qualifier (436-E1) = “Ø6”
(DUR/PPS), the Product/Service ID (4Ø7-D7) is zero. (Zero
means “Ø”.) Populate the DUR/PPS segment as
appropriate.
If the Product/Service ID Qualifier (436-E1) = “Ø7” (CPT-4),
the Product Service ID (4Ø7-D7) is the actual CPT-4 value.
If the Product/Service ID Qualifier (436-E1) = “Ø9”
(HCPCS), the Product Service ID (4Ø7-D7) is the actual
HCPCS value.
If the Product/Service ID Qualifier (436-E1) = “99” (Other),
the Product Service ID (4Ø7-D7) is the business partner
agreed value.
456-EN ASSOCIATED PRESCRIPTION/SERVICE REFERENCE NUMBER N Prior Authorization Request Only (Claim/Service):
Not used.
457-EP ASSOCIATED PRESCRIPTION/SERVICE DATE N Prior Authorization Request Only (Claim/Service):
Not used.
458-SE PROCEDURE MODIFIER CODE COUNT
Q Prior Authorization Request Only (Claim/Service):
Maximum count of 1Ø.
Required if Procedure Modifier Code (459-ER) is used.
459-ER PROCEDURE MODIFIER CODE Q***R*** Prior Authorization Request Only (Claim/Service):
Required to define a further level of specificity if the
Product/Service ID (4Ø7-D7) indicated a Procedure Code
was submitted.
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
Occurs the number of times identified in Procedure Modifier
Code Count (458-SE).

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 431 -
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
442-E7 QUANTITY DISPENSED R
Q
Prior Authorization Request Only (Claim):
Required.
Service:
Required if value is greater than zero (Ø).
4Ø3-D3 FILL NUMBER N Prior Authorization Request Only (Claim/Service):
Not used.
4Ø5-D5 DAYS SUPPLY R
Q
Prior Authorization Request Only (Claim):
Required.
Service:
Required if necessary for plan benefit administration.
4Ø6-D6 COMPOUND CODE Q
N
Prior Authorization Request Only (Claim):
Required if requesting a prior authorization for a compound
(Compound Code (4Ø6-D6) = 2).
Service:
Not used.
4Ø8-D8 DISPENSE AS WRITTEN (DAW)/PRODUCT SELECTION CODE Q Prior Authorization Request Only (Claim/Service):
Required if this field results in different coverage.
414-DE DATE PRESCRIPTION WRITTEN N Prior Authorization Request Only (Claim/Service):
Not used.
415-DF NUMBER OF REFILLS AUTHORIZED R Prior Authorization Request Only (Claim/Service):
Required.
419-DJ PRESCRIPTION ORIGIN CODE N Prior Authorization Request Only (Claim/Service):
Not used.
354-NX SUBMISSION CLARIFICATION CODE COUNT N Prior Authorization Request Only (Claim/Service):
Not used.
42Ø-DK SUBMISSION CLARIFICATION CODE N***R*** Prior Authorization Request Only (Claim/Service):
Not used.
46∅-ET QUANTITY PRESCRIBED N
Q
Prior Authorization Request Only (Claim):
Not used.
Service:
Required if the prescriber orders a specific number of
iterations of a service.
Not required if value is equal to 1.
3Ø8-C8 OTHER COVERAGE CODE N Prior Authorization Request Only (Claim/Service):
Not used.
429-DT SPECIAL PACKAGING INDICATOR Q
N
Prior Authorization Request Only (Claim):
Required if this field could result in different coverage,
pricing, or patient financial responsibility.
Service:
Not used.
453-EJ ORIGINALLY PRESCRIBED PRODUCT/SERVICE ID QUALIFIER Q Prior Authorization Request Only (Claim/Service):
Required if Originally Prescribed Product/Service Code
(445-EA) is used.
445-EA ORIGINALLY PRESCRIBED PRODUCT/SERVICE CODE Q Prior Authorization Request Only (Claim/Service):
Required if the receiver requests association to a
therapeutic, or a preferred product substitution, or when a
DUR alert has been resolved by changing medications, or
an alternative service than what was originally prescribed.
446-EB ORIGINALLY PRESCRIBED QUANTITY Q Prior Authorization Request Only (Claim/Service):
Required if the receiver requests reporting for quantity
changes due to a therapeutic substitution that has occurred
or a preferred product/service substitution that has
occurred, or when a DUR alert has been resolved by
changing quantities.
33Ø-CW ALTERNATE ID N Prior Authorization Request Only (Claim/Service):
Not used.
454-EK SCHEDULED PRESCRIPTION ID NUMBER N Prior Authorization Request Only (Claim/Service):
Not used.
6ØØ-28 UNIT OF MEASURE N Prior Authorization Request Only (Claim/Service):
Not used.
418-DI LEVEL OF SERVICE Q Prior Authorization Request Only (Claim/Service):
Required if prior authorization is needed for emergency
situation (value =3 Emergency).
461-EU PRIOR AUTHORIZATION TYPE CODE N Prior Authorization Request Only (Claim/Service):
Not used.
462-EV PRIOR AUTHORIZATION NUMBER SUBMITTED N Prior Authorization Request Only (Claim/Service):

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 432 -
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Not used.
463-EW INTERMEDIARY AUTHORIZATION TYPE ID N Prior Authorization Request Only (Claim/Service):
Not used.
464-EX INTERMEDIARY AUTHORIZATION ID N Prior Authorization Request Only (Claim/Service):
Not used.
343-HD DISPENSING STATUS N Prior Authorization Request Only (Claim/Service):
Not used.
344-HF QUANTITY INTENDED TO BE DISPENSED N Prior Authorization Request Only (Claim/Service):
Not used.
345-HG DAYS SUPPLY INTENDED TO BE DISPENSED N Prior Authorization Request Only (Claim/Service):
Not used.
357-NV DELAY REASON CODE Q Prior Authorization Request Only (Claim/Service):
Required when needed to specify the reason that
submission of the transaction has been delayed.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Prior Authorization Request Only (Claim/Service):
Not used.
391-MT PATIENT ASSIGNMENT INDICATOR (DIRECT MEMBER
REIMBURSEMENT INDICATOR)
N Prior Authorization Request Only (Claim/Service):
Not used.
995-E2 ROUTE OF ADMINISTRATION Q
N
Prior Authorization Request Only (Claim):
Required if an override to the “default” route of
administration is specified for the product For a multi-
ingredient compound, it is the route of the complete
compound mixture.
Service:
Not used.
996-G1 COMPOUND TYPE N Prior Authorization Request Only (Claim/Service):
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Prior Authorization Request Only (Claim/Service):
Not used.
147-U7 PHARMACY SERVICE TYPE N Prior Authorization Request Only (Claim/Service):
Not used.
Notes on Claim Segment on a Prior Authorization Request Only Request:
The Claim Segment is mandatory for a Prior Authorization Request Only request. The Claim Segment defines the prescribing information.
Fields defined as Mandatory are required to be submitted when the segment is sent.
16.2.5 PRIOR AUTHORIZATION SEGMENT (PRIOR AUTHORIZATION REQUEST ONLY)
PRIOR AUTHORIZATION SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
498-PA REQUEST TYPE M
498-PB REQUEST PERIOD DATE-BEGIN M
498-PC REQUEST PERIOD DATE-END M
498-PD BASIS OF REQUEST M
498-PE AUTHORIZED REPRESENTATIVE FIRST NAME Q Prior Authorization Request Only (Claim/Service):
Required if needed for prior authorization determination.
498-PF AUTHORIZED REPRESENTATIVE LAST NAME Q Prior Authorization Request Only (Claim/Service):
Required if needed for prior authorization determination.
498-PG AUTHORIZED REPRESENTATIVE STREET ADDRESS Q Prior Authorization Request Only (Claim/Service):
Required if needed for prior authorization determination.
498-PH AUTHORIZED REPRESENTATIVE CITY ADDRESS Q Prior Authorization Request Only (Claim/Service):
Required if needed for prior authorization determination.
498-PJ AUTHORIZED REPRESENTATIVE STATE/PROVINCE ADDRESS Q Prior Authorization Request Only (Claim/Service):
Required if needed for prior authorization determination.
498-PK AUTHORIZED REPRESENTATIVE ZIP/POSTAL ZONE Q Prior Authorization Request Only (Claim/Service):
Required if needed for prior authorization determination.
498-PY PRIOR AUTHORIZATION NUMBER-ASSIGNED Q Prior Authorization Request Only (Claim/Service):
Required if the Request Type (498-PA) = 2
(Reauthorization).
5Ø3-F3 AUTHORIZATION NUMBER N Prior Authorization Request Only (Claim/Service):
Not used.
498-PP PRIOR AUTHORIZATION SUPPORTING DOCUMENTATION Q Prior Authorization Request Only (Claim/Service):
Required if additional information is needed for prior
authorization determination.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 433 -
Notes on Prior Authorization Segment on a Prior Authorization Request Only Request:
The Prior Authorization Segment is mandatory for a Prior Authorization Request Only request. It is used when the sender submits a request for
the prior authorization approval. Fields defined as Mandatory are required to be submitted when the segment is sent.
16.2.6 PRESCRIBER SEGMENT (PRIOR AUTHORIZATION REQUEST ONLY)
PRESCRIBER SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
466-EZ PRESCRIBER ID QUALIFIER Q Prior Authorization Request Only (Claim/Service):
Required if Prescriber ID (411-DB) is used.
411-DB PRESCRIBER ID
Q Prior Authorization Request Only (Claim/Service):
Required if this field could result in different coverage or
patient financial responsibility.
Required if necessary for state/federal/regulatory agency
programs.
427-DR PRESCRIBER LAST NAME
Q Prior Authorization Request Only (Claim/Service):
Required when the Prescriber ID (411-DB) is not known.
Required if needed for Prescriber ID (411-DB)
validation/clarification.
498-PM PRESCRIBER PHONE NUMBER Q Prior Authorization Request Only (Claim/Service):
Required if needed to assist in identifying the prescriber.
Required if needed for Prior Authorization process.
468-2E PRIMARY CARE PROVIDER ID QUALIFIER Q Prior Authorization Request Only (Claim/Service):
Required if Primary Care Provider ID (421-DL) is used.
421-DL PRIMARY CARE PROVIDER ID Q Prior Authorization Request Only (Claim/Service):
Required if needed for receiver claim/encounter or prior
authorization request and billing determination, if known
and available.
Required if this field could result in different coverage or
patient financial responsibility.
Required if necessary for state/federal/regulatory agency
programs.
47Ø-4E PRIMARY CARE PROVIDER LAST NAME Q Prior Authorization Request Only (Claim/Service):
Required if this field is used as an alternative for Primary
Care Provider ID (421-DL) when ID is not known.
Required if needed for Primary Care Provider ID (421-DL)
validation/clarification.
364-2J PRESCRIBER FIRST NAME Q Prior Authorization Request Only (Claim/Service):
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
365-2K PRESCRIBER STREET ADDRESS Q Prior Authorization Request Only (Claim/Service):
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
366-2M PRESCRIBER CITY ADDRESS Q Prior Authorization Request Only (Claim/Service):
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
367-2N PRESCRIBER STATE/PROVINCE ADDRESS Q Prior Authorization Request Only (Claim/Service):
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
368-2P PRESCRIBER ZIP/POSTAL ZONE Q Prior Authorization Request Only (Claim/Service):
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 434 -
Notes on Prescriber Segment on a Prior Authorization Request Only Request:
The Prescriber Segment is situational for a Prior Authorization Request Only request. It is used when prescriber information is needed to
process a Prior Authorization Request Only. Fields defined as Mandatory are required to be submitted when the segment is sent.
16.2.7 WORKERS’ COMPENSATION SEGMENT (PRIOR AUTHORIZATION REQUEST ONLY)
WORKERS’ COMPENSATION SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
434-DY DATE OF INJURY M
315-CF EMPLOYER NAME N Prior Authorization Request Only (Claim/Service):
Not used.
316-CG EMPLOYER STREET ADDRESS N Prior Authorization Request Only (Claim/Service):
Not used.
317-CH EMPLOYER CITY ADDRESS N Prior Authorization Request Only (Claim/Service):
Not used.
318-CI EMPLOYER STATE/PROVINCE ADDRESS N Prior Authorization Request Only (Claim/Service):
Not used.
319-CJ EMPLOYER ZIP/POSTAL ZONE N Prior Authorization Request Only (Claim/Service):
Not used.
32Ø-CK EMPLOYER PHONE NUMBER N Prior Authorization Request Only (Claim/Service):
Not used.
321-CL EMPLOYER CONTACT NAME N Prior Authorization Request Only (Claim/Service):
Not used.
327-CR CARRIER ID N Prior Authorization Request Only (Claim/Service):
Not used.
435-DZ CLAIM/REFERENCE ID Q Prior Authorization Request Only (Claim/Service):
Required if needed and has been assigned, to process a
prior authorization request for a work related injury or
condition.
117-TR BILLING ENTITY TYPE INDICATOR N Prior Authorization Request Only (Claim/Service):
Not used.
118-TS PAY TO QUALIFIER N Prior Authorization Request Only (Claim/Service):
Not used.
119-TT PAY TO ID N Prior Authorization Request Only (Claim/Service):
Not used.
12Ø-TU PAY TO NAME N Prior Authorization Request Only (Claim/Service):
Not used.
121-TV PAY TO STREET ADDRESS N Prior Authorization Request Only (Claim/Service):
Not used.
122-TW PAY TO CITY ADDRESS N Prior Authorization Request Only (Claim/Service):
Not used.
123-TX PAY TO STATE/PROVINCE ADDRESS N Prior Authorization Request Only (Claim/Service):
Not used.
124-TY PAY TO ZIP/POSTAL ZONE N Prior Authorization Request Only (Claim/Service):
Not used.
125-TZ GENERIC EQUIVALENT PRODUCT ID QUALIFIER N Prior Authorization Request Only (Claim/Service):
Not used.
126-UA GENERIC EQUIVALENT PRODUCT ID N Prior Authorization Request Only (Claim/Service):
Not used.
Notes on Workers’ Compensation Segment on a Prior Authorization Request Only Request:
The Workers’ Compensation Segment is situational for a Prior Authorization Request Only request. It is used when processing a Prior
Authorization Request Only for a work-related injury or condition. Fields defined as Mandatory are required to be submitted when the segment
is sent.
16.2.8 DUR/PPS SEGMENT (PRIOR AUTHORIZATION REQUEST ONLY)
DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
473-7E DUR/PPS CODE COUNTER
Q***R*** Prior Authorization Request Only (Claim/Service):
Maximum of 9 occurrences.
Required if DUR/PPS Segment is used.
439-E4 REASON FOR SERVICE CODE
Q***R*** Prior Authorization Request Only (Claim/Service):
Required if needed to obtain prior authorization for clinical
services or drug utilization review overrides.
44Ø-E5 PROFESSIONAL SERVICE CODE
Q***R*** Prior Authorization Request Only (Claim/Service):
Required if needed to obtain prior authorization for clinical

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 435 -
DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
services or drug utilization review overrides.
441-E6 RESULT OF SERVICE CODE
Q***R*** Prior Authorization Request Only (Claim/Service):
Required if needed to obtain prior authorization for clinical
services or drug utilization review overrides.
474-8E DUR/PPS LEVEL OF EFFORT
Q***R*** Prior Authorization Request Only (Claim/Service):
Required if needed to obtain prior authorization for clinical
services or drug utilization review overrides.
475-J9 DUR CO-AGENT ID QUALIFIER Q***R*** Prior Authorization Request Only (Claim/Service):
Required if DUR Co-Agent ID (476-H6) is used.
476-H6 DUR CO-AGENT ID Q***R*** Prior Authorization Request Only (Claim/Service):
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
Required if this field affects payment for or documentation
of professional pharmacy service.
Notes on DUR/PPS Segment on a Prior Authorization Request Only Request:
The DUR/PPS Segment is situational for a Prior Authorization Request Only request. It is used when a sender notifies the receiver of clinical
services or drug utilization review overrides necessary to process a Prior Authorization Request Only. Fields defined as Mandatory are
required to be submitted when the segment is sent.
16.2.9 COMPOUND SEGMENT (PRIOR AUTHORIZATION REQUEST ONLY)
COMPOUND SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
45Ø-EF COMPOUND DOSAGE FORM DESCRIPTION CODE M
451-EG COMPOUND DISPENSING UNIT FORM INDICATOR M
447-EC COMPOUND INGREDIENT COMPONENT COUNT M Maximum count of 25 ingredients.
488-RE COMPOUND PRODUCT ID QUALIFIER M***R***
489-TE COMPOUND PRODUCT ID M***R***
448-ED COMPOUND INGREDIENT QUANTITY M***R***
449-EE COMPOUND INGREDIENT DRUG COST N***R*** Prior Authorization Request Only (Claim):
Not used.
49Ø-UE COMPOUND INGREDIENT BASIS OF COST DETERMINATION N***R*** Prior Authorization Request Only (Claim):
Not used.
362-2G COMPOUND INGREDIENT MODIFIER CODE COUNT Q Prior Authorization Request Only (Claim):
Required when Compound Ingredient Modifier Code (363-
2H) is sent.
Maximum count of 1Ø.
363-2H COMPOUND INGREDIENT MODIFIER CODE Q***R*** Prior Authorization Request Only (Claim):
Required if necessary for state/federal/regulatory agency
programs.
Notes on Compound Segment on a Prior Authorization Request Only Request:
The Compound Segment is situational for a Prior Authorization Request Only request. It is used for multi-ingredient prescriptions, when each
ingredient is reported in a Prior Authorization Request Only.
The Compound Segment is not used in when the Prior Authorization Request is for a service (Prescription/Service Reference Number
Qualifier (455-EM) = “2” (Service Billing)).
Fields defined as Mandatory are required to be submitted when the segment is sent.
16.2.10 CLINICAL SEGMENT (PRIOR AUTHORIZATION REQUEST ONLY)
CLINICAL SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
491-VE DIAGNOSIS CODE COUNT Q Prior Authorization Request Only (Claim/Service):
Maximum count of 5.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 436 -
CLINICAL SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if Diagnosis Code Qualifier (492-WE) and
Diagnosis Code (424-DO) are used.
492-WE DIAGNOSIS CODE QUALIFIER Q***R*** Prior Authorization Request Only (Claim/Service):
Required if Diagnosis Code (424-DO) is used.
424-DO DIAGNOSIS CODE
Q***R*** Prior Authorization Request Only (Claim/Service):
The value for this field is obtained from the prescriber or
authorized representative.
Required if this field could result in different coverage,
pricing, patient financial responsibility, and/or drug
utilization review outcome.
Required if this field affects payment for professional
pharmacy service.
Required if this information can be used in place of prior
authorization.
Required if necessary for state/federal/regulatory agency
programs.
493-XE CLINICAL INFORMATION COUNTER Q***R*** Prior Authorization Request Only (Claim/Service):
Maximum of 5 occurrences supported.
Grouped with Measurement fields (Measurement Date
(494-ZE), Measurement Time (495-H1), Measurement
Dimension (496-H2), Measurement Unit (497-H3),
Measurement Value (499-H4).
494-ZE MEASUREMENT DATE Q***R*** Prior Authorization Request Only (Claim/Service):
Required if necessary when this field could result in
different coverage and/or drug utilization review outcome.
495-H1 MEASUREMENT TIME Q***R*** Prior Authorization Request Only (Claim/Service):
Required if Time is known or has impact on measurement.
Required if necessary when this field could result in
different coverage and/or drug utilization review outcome
and is a requirement for authorization.
496-H2 MEASUREMENT DIMENSION Q***R*** Prior Authorization Request Only (Claim/Service):
Required if Measurement Unit (497-H3) and Measurement
Value (499-H4) are used.
Required if necessary when this field could result in
different coverage and/or drug utilization review outcome
and is a requirement for authorization.
Required if necessary for patient’s weight and height when
billing Medicare for a claim that includes a Certificate of
Medical Necessity (CMN).
497-H3 MEASUREMENT UNIT Q***R*** Prior Authorization Request Only (Claim/Service):
Required if Measurement Dimension (496-H2) and
Measurement Value (499-H4) are used.
Required if necessary for patient’s weight and height when
billing Medicare for a claim that includes a Certificate of
Medical Necessity (CMN).
Required if necessary when this field could result in
different coverage and/or drug utilization review outcome
and is a requirement for authorization.
499-H4 MEASUREMENT VALUE Q***R*** Prior Authorization Request Only (Claim/Service):
Required if Measurement Dimension (496-H2) and
Measurement Unit (497-H3) are used.
Required if necessary for patient’s weight and height when
billing Medicare for a claim that includes a Certificate of
Medical Necessity (CMN).
Required if necessary when this field could result in
different coverage and/or drug utilization review outcome
and is a requirement for authorization.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 437 -
Notes on Clinical Segment on a Prior Authorization Request Only Request:
The Clinical Segment is situational for a Prior Authorization Request Only request. It is used to specify clinical measurements and/or diagnosis
information associated with the Prior Authorization Request Only. Fields defined as Mandatory are required to be submitted when the segment
is sent.
16.3 PRIOR AUTHORIZATION REQUEST ONLY RESPONSE DIAGRAMS AND
SEGMENTS
16.3.1 TRANSMISSION ACCEPTED/TRANSACTION APPROVED
Prior Authorization Request Only transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved)
A sender’s Prior Authorization Request Only that is “A” (Approved) or “S” (Duplicate of Approved) must receive a response that includes a
Prior Authorization Number-Assigned (498-PY) and other information in the Response Prior Authorization Segment. The sender will not
receive any payment information.
Each response contains one occurrence of claim/service data.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
16.3.1.1 DIAGRAM FOR TRANSMISSION OF ONE PRIOR AUTHORIZATION REQUEST ONLY RESPONSE
(TRANSMISSION ACCEPTED/TRANSACTION APPROVED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Segment Separator
Response Prior Authorization Segment
Situational
Segment Separator
Response Coordination of Benefits/Other Payers Segment
16.3.1.2 PRIOR AUTHORIZATION REQUEST ONLY RESPONSE SEGMENTS (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
16.3.1.2.1 RESPONSE HEADER SEGMENT (PRIOR AUTHORIZATION REQUEST ONLY) (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Prior Authorization Request Only Response:
The Response Header Segment is a mandatory, fixed length segment for a Prior Authorization Request Only response when the Header
Response Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved).
The “Situation” column is not applicable.
16.3.1.2.2 RESPONSE MESSAGE SEGMENT (PRIOR AUTHORIZATION REQUEST ONLY) (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 438 -
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Prior Authorization Request Only (Claim/Service):
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Prior Authorization Request Only Response:
The Response Message Segment is situational for a Prior Authorization Request Only response when the Header Response Status (5Ø1-F1)
of "A" (Accepted) and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved). It is used when additional text
information needs to be sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
16.3.1.2.3 RESPONSE STATUS SEGMENT (PRIOR AUTHORIZATION REQUEST ONLY) (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER N Prior Authorization Request Only (Claim/Service):
Not used.
51Ø-FA REJECT COUNT N Prior Authorization Request Only (Claim/Service):
Not used.
511-FB REJECT CODE N***R*** Prior Authorization Request Only (Claim/Service):
Not used.
546-4F REJECT FIELD OCCURRENCE INDICATOR N***R*** Prior Authorization Request Only (Claim/Service):
Not used.
547-5F APPROVED MESSAGE CODE COUNT Q Prior Authorization Request Only (Claim/Service):
Maximum count of 5.
Required if Approved Message Code (548-6F) is used.
548-6F APPROVED MESSAGE CODE Q***R*** Prior Authorization Request Only (Claim/Service):
Required if Approved Message Code Count (547-5F) is
used and the sender needs to communicate additional
follow up for a potential opportunity.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Prior Authorization Request Only (Claim/Service):
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Prior Authorization Request Only (Claim/Service):
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Prior Authorization Request Only (Claim/Service):
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 439 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Prior Authorization Request Only (Claim/Service):
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Prior Authorization Request Only (Claim/Service):
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Prior Authorization Request Only (Claim/Service):
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Prior Authorization Request Only (Claim/Service):
Not used.
993-A7 INTERNAL CONTROL NUMBER N Prior Authorization Request Only (Claim/Service):
Not used.
987-MA URL N Prior Authorization Request Only (Claim/Service):
Not used.
Notes on Response Status Segment on a Prior Authorization Request Only Response:
The Response Status Segment is mandatory for a Prior Authorization Request Only response for Header Response Status (5Ø1-F1) = “A”
(Accepted) and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved). The Response Status Segment is
sent from the sender to the receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the
segment is sent.
16.3.1.2.4 RESPONSE CLAIM SEGMENT (PRIOR AUTHORIZATION REQUEST ONLY) (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT Q
N
Prior Authorization Request Only (Claim):
Maximum count of 6.
Required if Preferred Product ID (553-AR) is used.
Service:
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER Q***R***
N
Prior Authorization Request Only (Claim):
Required if Preferred Product ID (553-AR) is used.
Service:
Not used.
553-AR PREFERRED PRODUCT ID
Q***R***
N
Prior Authorization Request Only (Claim):
Required if a product preference exists that needs to be
communicated to the receiver via an ID.
Service:
Not used.
554-AS PREFERRED PRODUCT INCENTIVE N***R*** Prior Authorization Request Only (Claim/Service):
Not used.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE N***R*** Prior Authorization Request Only (Claim/Service):
Not used.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 440 -
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
556-AU PREFERRED PRODUCT DESCRIPTION Q***R***
N
Prior Authorization Request Only (Claim):
Required if a product preference exists that either cannot
be communicated by the Preferred Product ID (553-AR) or
to clarify the Preferred Product ID (553-AR).
Service:
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Prior Authorization Request Only (Claim/Service):
Not used.
Notes on Response Claim Segment on a Prior Authorization Request Only Response:
The Response Claim Segment is mandatory for a Prior Authorization Request Only response when the Header Response Status (5Ø1-F1) is
“A” (Accepted) and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved).
The Response Claim Segment (Prior Authorization Request Only – Claim) is sent from the sender to the receiver to identify therapeutic or
alternate product recommendations.
The Response Claim Segment (Prior Authorization Request Only – Service) is sent from the sender to the receiver to mirror back the
Prescription/Service Reference Number (4Ø2-D2).
Fields defined as Mandatory are required to be submitted when the segment is sent.
16.3.1.2.5 RESPONSE PRIOR AUTHORIZATION SEGMENT (PRIOR AUTHORIZATION REQUEST ONLY) (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
RESPONSE PRIOR AUTHORIZATION SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
498-PR PRIOR AUTHORIZATION PROCESSED DATE R Prior Authorization Request Only (Claim/Service):
Required.
498-PS PRIOR AUTHORIZATION EFFECTIVE DATE Q Prior Authorization Request Only (Claim/Service):
Required if the prior authorization has an effective date.
498-PT PRIOR AUTHORIZATION EXPIRATION DATE Q Prior Authorization Request Only (Claim/Service):
Required if the prior authorization has an expiration date.
498-RA PRIOR AUTHORIZATION QUANTITY Q Prior Authorization Request Only (Claim/Service):
Required if the total quantity authorized is greater than
zero.
498-RB PRIOR AUTHORIZATION DOLLARS AUTHORIZED Q Prior Authorization Request Only (Claim/Service):
Required if the total dollars authorized is greater than zero.
498-PW PRIOR AUTHORIZATION NUMBER OF REFILLS AUTHORIZED Q Prior Authorization Request Only (Claim/Service):
Required if a specific number of refills is authorized.
498-PX PRIOR AUTHORIZATION QUANTITY ACCUMULATED Q Prior Authorization Request Only (Claim/Service):
Required if the Prior Authorization Quantity (498-RA) is
greater than zero. The field must equal the total of the
quantities from all claims processed.
498-PY PRIOR AUTHORIZATION NUMBER–ASSIGNED R Prior Authorization Request Only (Claim/Service):
Required.
Notes on Response Prior Authorization Segment on a Prior Authorization Request Only Response:
The Response Prior Authorization Segment is mandatory when the Header Response (5Ø1-F1) is "A" (Accepted) and Transaction Response
Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved). It is used to relay the prior authorization periods, limitations, contracted
amounts, as well as a Prior Authorization Number–Assigned (498-PY) which is, when used, for subsequent Claim or Service Billings when the
Transaction Response Status (112-AN) = “A” (Approved) or “S” (Duplicate of Approved). Fields defined as Mandatory are required to be
submitted when the segment is sent.
16.3.1.2.6 RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS SEGMENT (PRIOR AUTHORIZATION REQUEST
ONLY) (TRANSMISSION ACCEPTED/TRANSACTION APPROVED)
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS
SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
355-NT OTHER PAYER ID COUNT M Prior Authorization Request Only (Claim/Service):
Maximum count of 3.
338-5C OTHER PAYER COVERAGE TYPE M***R***

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 441 -
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS
SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
339-6C OTHER PAYER ID QUALIFIER Q***R*** Prior Authorization Request Only (Claim/Service):
Required if Other Payer ID (34Ø-7C) is used.
34Ø-7C OTHER PAYER ID Q***R*** Prior Authorization Request Only (Claim/Service):
Required if other insurance information is available for
coordination of benefits.
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER Q***R*** Prior Authorization Request Only (Claim/Service):
Required if other insurance information is available for
coordination of benefits.
356-NU OTHER PAYER CARDHOLDER ID Q***R*** Prior Authorization Request Only (Claim/Service):
Required if other insurance information is available for
coordination of benefits.
992-MJ OTHER PAYER GROUP ID Q***R*** Prior Authorization Request Only (Claim/Service):
Required if other insurance information is available for
coordination of benefits.
142-UV OTHER PAYER PERSON CODE Q***R*** Prior Authorization Request Only (Claim/Service):
Required if needed to uniquely identify the family members
within the Cardholder ID, as assigned by the other payer.
127-UB OTHER PAYER HELP DESK PHONE NUMBER Q***R*** Prior Authorization Request Only (Claim/Service):
Required if needed to provide a support telephone number
of the other payer to the receiver.
143-UW OTHER PAYER PATIENT RELATIONSHIP CODE Q***R*** Prior Authorization Request Only (Claim/Service):
Required if needed to uniquely identify the relationship of
the patient to the cardholder ID, as assigned by the other
payer.
144-UX OTHER PAYER BENEFIT EFFECTIVE DATE Q***R*** Prior Authorization Request Only (Claim/Service):
Required when other coverage is known which is after the
Date of Service submitted.
145-UY OTHER PAYER BENEFIT TERMINATION DATE Q***R*** Prior Authorization Request Only (Claim/Service):
Required when other coverage is known which is after the
Date of Service submitted.
Notes on Response Coordination of Benefits/Other Payers Segment on a Prior Authorization Request Only Response:
The Response Coordination of Benefits/Other Payers Segment is situational for a Prior Authorization Request Only response when the Header
Response Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved)
when other insurance information is available for coordination of benefits.
If subsequent payer(s) for this patient is not known, the Other Payer information is not sent.
If subsequent payer(s) for this patient is known, the following may be sent:
• Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C),
• Other Payer Group ID (992-MJ),
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU)
• And other Other Payer fields.
In addition, if any of the following three fields are sent:
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU),
• Other Payer Group ID (992-MJ),
then the Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C) must be sent.
Fields defined as Mandatory are required to be submitted when the segment is sent.
16.3.2 TRANSMISSION ACCEPTED/TRANSACTION CAPTURED
Prior Authorization Request Only transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured)
It is possible that the receiver has not completed processing of the Prior Authorization Request Only, and will respond that the request is still
pending, using the “C” (Captured) or “Q” (Duplicate of Captured).
Each response contains one occurrence of claim/service data.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
16.3.2.1 DIAGRAM FOR TRANSMISSION OF ONE PRIOR AUTHORIZATION REQUEST ONLY RESPONSE
(TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 442 -
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
16.3.2.2 PRIOR AUTHORIZATION REQUEST ONLY RESPONSE SEGMENTS (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
16.3.2.2.1 RESPONSE HEADER SEGMENT (PRIOR AUTHORIZATION REQUEST ONLY) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Prior Authorization Request Only Response:
The Response Header Segment is a mandatory, fixed length segment for Prior Authorization Request Only response when the Header
Response Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). The
“Situation” column is not applicable.
16.3.2.2.2 RESPONSE MESSAGE SEGMENT (PRIOR AUTHORIZATION REQUEST ONLY) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Prior Authorization Request Only (Claim/Service):
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Prior Authorization Request Only Response:
The Response Message Segment is situational for Prior Authorization Request Only response when the Header Response Status (5Ø1-F1) of
"A" (Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). It is used when additional text
information needs to be sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 443 -
16.3.2.2.3 RESPONSE STATUS SEGMENT (PRIOR AUTHORIZATION REQUEST ONLY) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER R Prior Authorization Request Only (Claim/Service):
Required.
51Ø-FA REJECT COUNT N Prior Authorization Request Only (Claim/Service):
Not used.
511-FB REJECT CODE N***R*** Prior Authorization Request Only (Claim/Service):
Not used.
546-4F REJECT FIELD OCCURRENCE INDICATOR N***R*** Prior Authorization Request Only (Claim/Service):
Not used.
547-5F APPROVED MESSAGE CODE COUNT N Prior Authorization Request Only (Claim/Service):
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Prior Authorization Request Only (Claim/Service):
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Prior Authorization Request Only (Claim/Service):
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Prior Authorization Request Only (Claim/Service):
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Prior Authorization Request Only (Claim/Service):
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Prior Authorization Request Only (Claim/Service):
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Prior Authorization Request Only (Claim/Service):
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Prior Authorization Request Only (Claim/Service):
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Prior Authorization Request Only (Claim/Service):
Not used.
993-A7 INTERNAL CONTROL NUMBER N Prior Authorization Request Only (Claim/Service):
Not used.
987-MA URL N Prior Authorization Request Only (Claim/Service):
Not used.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 444 -
Notes on Response Status Segment on a Prior Authorization Request Only Response:
The Response Status Segment is mandatory for a Prior Authorization Request Only response for Header Response Status (5Ø1-F1) = “A”
(Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). The Response Status Segment is
sent from the sender to the receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the
segment is sent.
16.3.2.2.4 RESPONSE CLAIM SEGMENT (PRIOR AUTHORIZATION REQUEST ONLY) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT Q
N
Prior Authorization Request Only (Claim):
Maximum count of 6.
Required if Preferred Product ID (553-AR) is used.
Service:
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER Q***R***
N
Prior Authorization Request Only (Claim):
Required if Preferred Product ID (553-AR) is used.
Service:
Not used.
553-AR PREFERRED PRODUCT ID
Q***R***
N
Prior Authorization Request Only (Claim):
Required if a product preference exists that needs to be
communicated to the receiver via an ID.
Service:
Not used.
554-AS PREFERRED PRODUCT INCENTIVE N***R*** Prior Authorization Request Only (Claim/Service):
Not used.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE N***R*** Prior Authorization Request Only (Claim/Service):
Not used.
556-AU PREFERRED PRODUCT DESCRIPTION Q***R***
N
Prior Authorization Request Only (Claim):
Required if a product preference exists that either cannot
be communicated by the Preferred Product ID (553-AR) or
to clarify the Preferred Product ID (553-AR).
Service:
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Prior Authorization Request Only (Claim/Service):
Not used.
Notes on Response Claim Segment on a Prior Authorization Request Only Response:
The Response Claim Segment is mandatory for a Prior Authorization Request Only response when the Header Response Status (5Ø1-F1) is
“A” (Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured).
The Response Claim Segment (Prior Authorization Request Only – Claim) is sent from the sender to the receiver to identify therapeutic or
alternate product recommendations.
The Response Claim Segment (Prior Authorization Request Only – Service) is sent from the sender to the receiver to mirror back the
Prescription/Service Reference Number (4Ø2-D2).
Fields defined as Mandatory are required to be submitted when the segment is sent.
16.3.3 TRANSMISSION ACCEPTED/TRANSACTION DEFERRED
Prior Authorization Request Only transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “F” (Deferred)
Each response contains one occurrence of claim/service data.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
16.3.3.1 DIAGRAM FOR TRANSMISSION OF ONE PRIOR AUTHORIZATION REQUEST ONLY RESPONSE
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 445 -
(TRANSMISSION ACCEPTED/TRANSACTION DEFERRED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Prior Authorization Segment
16.3.3.2 PRIOR AUTHORIZATION REQUEST ONLY RESPONSE SEGMENTS (TRANSMISSION
ACCEPTED/TRANSACTION DEFERRED)
16.3.3.2.1 RESPONSE HEADER SEGMENT (PRIOR AUTHORIZATION REQUEST ONLY) (TRANSMISSION
ACCEPTED/TRANSACTION DEFERRED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Prior Authorization Request Only Response:
The Response Header Segment is a mandatory, fixed length segment for Prior Authorization Request Only response when the Header
Response Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “F” (Deferred). The “Situation” column is not
applicable.
16.3.3.2.2 RESPONSE MESSAGE SEGMENT (PRIOR AUTHORIZATION REQUEST ONLY) (TRANSMISSION
ACCEPTED/TRANSACTION DEFERRED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Prior Authorization Request Only (Claim/Service):
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 446 -
Notes on Response Message Segment on a Prior Authorization Request Only Response:
The Response Message Segment is situational for Prior Authorization Request Only response when the Header Response Status (5Ø1-F1) of
"A" (Accepted) and Transaction Response Status (112-AN) of “F” (Deferred). It is used when additional text information needs to be sent.
Fields defined as Mandatory are required to be submitted when the segment is sent.
16.3.3.2.3 RESPONSE STATUS SEGMENT (PRIOR AUTHORIZATION REQUEST ONLY) (TRANSMISSION
ACCEPTED/TRANSACTION DEFERRED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Prior Authorization Request Only (Claim/Service):
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT N Prior Authorization Request Only (Claim/Service):
Not used.
511-FB REJECT CODE N***R*** Prior Authorization Request Only (Claim/Service):
Not used.
546-4F REJECT FIELD OCCURRENCE INDICATOR N***R*** Prior Authorization Request Only (Claim/Service):
Not used.
547-5F APPROVED MESSAGE CODE COUNT N Prior Authorization Request Only (Claim/Service):
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Prior Authorization Request Only (Claim/Service):
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Prior Authorization Request Only (Claim/Service):
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Prior Authorization Request Only (Claim/Service):
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Prior Authorization Request Only (Claim/Service):
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Prior Authorization Request Only (Claim/Service):
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Prior Authorization Request Only (Claim/Service):
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Prior Authorization Request Only (Claim/Service):
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Prior Authorization Request Only (Claim/Service):
Not used.
993-A7 INTERNAL CONTROL NUMBER N Prior Authorization Request Only (Claim/Service):
Not used.
987-MA URL N Prior Authorization Request Only (Claim/Service):
Not used.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 447 -
Notes on Response Status Segment on a Prior Authorization Request Only Response:
The Response Status Segment is mandatory for a Prior Authorization Request Only response for Header Response Status (5Ø1-F1) = “A”
(Accepted) and Transaction Response Status (112-AN) of “F” (Deferred). The Response Status Segment is sent from the sender to the
receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.
16.3.3.2.4 RESPONSE CLAIM SEGMENT (PRIOR AUTHORIZATION REQUEST ONLY) (TRANSMISSION
ACCEPTED/TRANSACTION DEFERRED)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT Q
N
Prior Authorization Request Only (Claim):
Maximum count of 6.
Required if Preferred Product ID (553-AR) is used.
Service:
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER Q***R***
N
Prior Authorization Request Only (Claim):
Required if Preferred Product ID (553-AR) is used.
Service:
Not used.
553-AR PREFERRED PRODUCT ID
Q***R***
N
Prior Authorization Request Only (Claim):
Required if a product preference exists that needs to be
communicated to the receiver via an ID.
Service:
Not used.
554-AS PREFERRED PRODUCT INCENTIVE N***R*** Prior Authorization Request Only (Claim/Service):
Not used.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE N***R*** Prior Authorization Request Only (Claim/Service):
Not used.
556-AU PREFERRED PRODUCT DESCRIPTION Q***R***
N
Prior Authorization Request Only (Claim):
Required if a product preference exists that either cannot
be communicated by the Preferred Product ID (553-AR) or
to clarify the Preferred Product ID (553-AR).
Service:
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Prior Authorization Request Only (Claim/Service):
Not used.
Notes on Response Claim Segment on a Prior Authorization Request Only Response:
The Response Claim Segment is mandatory for a Prior Authorization Request Only response when the Header Response Status (5Ø1-F1) is
“A” (Accepted) and Transaction Response Status (112-AN) of “F” (Deferred).
The Response Claim Segment (Prior Authorization Request Only – Claim) is sent from the sender to the receiver to identify therapeutic or
alternate product recommendations.
The Response Claim Segment (Prior Authorization Request Only – Service) is sent from the sender to the receiver to mirror back the
Prescription/Service Reference Number (4Ø2-D2).
Fields defined as Mandatory are required to be submitted when the segment is sent.
16.3.3.2.5 RESPONSE PRIOR AUTHORIZATION SEGMENT (PRIOR AUTHORIZATION REQUEST ONLY) (TRANSMISSION
ACCEPTED/TRANSACTION DEFERRED)
RESPONSE PRIOR AUTHORIZATION SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
498-PR PRIOR AUTHORIZATION PROCESSED DATE Q Prior Authorization Request Only (Claim/Service):
Required if the receiver’s system assigns the number.
498-PS PRIOR AUTHORIZATION EFFECTIVE DATE N Prior Authorization Request Only (Claim/Service):
Not used.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 448 -
RESPONSE PRIOR AUTHORIZATION SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
498-PT PRIOR AUTHORIZATION EXPIRATION DATE N Prior Authorization Request Only (Claim/Service):
Not used.
498-RA PRIOR AUTHORIZATION QUANTITY N Prior Authorization Request Only (Claim/Service):
Not used.
498-RB PRIOR AUTHORIZATION DOLLARS AUTHORIZED N Prior Authorization Request Only (Claim/Service):
Not used.
498-PW PRIOR AUTHORIZATION NUMBER OF REFILLS AUTHORIZED N
Prior Authorization Request Only (Claim/Service):
Not used.
498-PX PRIOR AUTHORIZATION QUANTITY ACCUMULATED N Prior Authorization Request Only (Claim/Service):
Not used.
498-PY PRIOR AUTHORIZATION NUMBER–ASSIGNED Q Prior Authorization Request Only (Claim/Service):
Required if the receiver’s system assigns the number.
Notes on Response Prior Authorization Segment on a Prior Authorization Request Only Response:
The Response Prior Authorization Segment is situational on a Prior Authorization Request only response when the Header Response Status
(5Ø1-F1) is “A” (Accepted) and the Transaction Response Status (112-AN) is “F” (Deferred). Fields defined as Mandatory are required to be
submitted when the segment is sent.
16.3.4 TRANSMISSION ACCEPTED/TRANSACTION REJECTED
Prior Authorization Request Only transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “R” (Rejected)
Each response contains one occurrence of claim/service data.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
16.3.4.1 DIAGRAM FOR TRANSMISSION OF ONE PRIOR AUTHORIZATION REQUEST ONLY RESPONSE
(TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response Coordination of Benefits/Other Payers Segment
16.3.4.2 PRIOR AUTHORIZATION REQUEST ONLY RESPONSE SEGMENTS (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
16.3.4.2.1 RESPONSE HEADER SEGMENT (PRIOR AUTHORIZATION REQUEST ONLY) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 449 -
Notes on Response Header Segment on a Prior Authorization Request Only Response:
The Response Header Segment is a mandatory, fixed length segment for Prior Authorization Request Only when the Header Response Status
(5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). The “Situation” column is not applicable.
16.3.4.2.2 RESPONSE MESSAGE SEGMENT (PRIOR AUTHORIZATION REQUEST ONLY) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Prior Authorization Request Only (Claim/Service):
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Prior Authorization Request Only Response:
The Response Message Segment is situational for Prior Authorization Request Only when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). It is used when additional text information needs to be sent. Fields
defined as Mandatory are required to be submitted when the segment is sent.
16.3.4.2.3 RESPONSE STATUS SEGMENT (PRIOR AUTHORIZATION REQUEST ONLY) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Prior Authorization Request Only (Claim/Service):
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT R Prior Authorization Request Only (Claim/Service):
Maximum count of 5.
Required.
511-FB REJECT CODE R***R*** Prior Authorization Request Only (Claim/Service):
Required.
546-4F REJECT FIELD OCCURRENCE INDICATOR Q***R*** Prior Authorization Request Only (Claim/Service):
Required if a repeating field is in error, to identify repeating
field occurrence.
This field must be sent when relaying error information about
a repeating field or set. Note, if the Reject Code is not
denoting a repeating field or set, the Reject Field Occurrence
Indicator must not be sent.
547-5F APPROVED MESSAGE CODE COUNT N Prior Authorization Request Only (Claim/Service):
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Prior Authorization Request Only (Claim/Service):
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Prior Authorization Request Only (Claim/Service):
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Prior Authorization Request Only (Claim/Service):

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 450 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Prior Authorization Request Only (Claim/Service):
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Prior Authorization Request Only (Claim/Service):
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Prior Authorization Request Only (Claim/Service):
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Prior Authorization Request Only (Claim/Service):
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Prior Authorization Request Only (Claim/Service):
Not used.
993-A7 INTERNAL CONTROL NUMBER N Prior Authorization Request Only (Claim/Service):
Not used.
987-MA URL N Prior Authorization Request Only (Claim/Service):
Not used.
Notes on Response Status Segment on a Prior Authorization Request Only Response:
The Response Status Segment is mandatory for a Prior Authorization Request Only response for Header Response Status (5Ø1-F1) = “A”
(Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). The Response Status Segment is sent from the sender to the
receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.
16.3.4.2.4 RESPONSE CLAIM SEGMENT (PRIOR AUTHORIZATION REQUEST ONLY) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT Q
N
Prior Authorization Request Only (Claim):
Maximum count of 6.
Required if Preferred Product ID (553-AR) is used.
Service:
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER Q***R***
N
Prior Authorization Request Only (Claim):
Required if Preferred Product ID (553-AR) is used.
Service:
Not used.
553-AR PREFERRED PRODUCT ID
Q***R***
Prior Authorization Request Only (Claim):
Required if a product preference exists that needs to be
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 451 -
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
N
communicated to the receiver via an ID.
Service:
Not used.
554-AS PREFERRED PRODUCT INCENTIVE N***R*** Prior Authorization Request Only (Claim/Service):
Not used.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE N***R*** Prior Authorization Request Only (Claim/Service):
Not used.
556-AU PREFERRED PRODUCT DESCRIPTION Q***R***
N
Prior Authorization Request Only (Claim):
Required if a product preference exists that either cannot
be communicated by the Preferred Product ID (553-AR) or
to clarify the Preferred Product ID (553-AR).
Service:
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Prior Authorization Request Only (Claim/Service):
Not used.
Notes on Response Claim Segment on a Prior Authorization Request Only Response:
The Response Claim Segment is mandatory for a Prior Authorization Request Only response when the Header Response Status (5Ø1-F1) is
“A” (Accepted) and Transaction Response Status (112-AN) of “R” (Rejected).
The Response Claim Segment (Prior Authorization Request Only – Claim) is sent from the sender to the receiver to identify therapeutic or
alternate product recommendations.
The Response Claim Segment (Prior Authorization Request Only – Service) is sent from the sender to the receiver to mirror back the
Prescription/Service Reference Number (4Ø2-D2).
Fields defined as Mandatory are required to be submitted when the segment is sent.
16.3.4.2.5 RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS SEGMENT (PRIOR AUTHORIZATION REQUEST
ONLY) (TRANSMISSION ACCEPTED/TRANSACTION APPROVED)
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS
SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
355-NT OTHER PAYER ID COUNT M Prior Authorization Request Only (Claim/Service):
Maximum count of 3.
338-5C OTHER PAYER COVERAGE TYPE M***R***
339-6C OTHER PAYER ID QUALIFIER Q***R*** Prior Authorization Request Only (Claim/Service):
Required if Other Payer ID (34Ø-7C) is used.
34Ø-7C OTHER PAYER ID Q***R*** Prior Authorization Request Only (Claim/Service):
Required if other insurance information is available for
coordination of benefits.
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER Q***R*** Prior Authorization Request Only (Claim/Service):
Required if other insurance information is available for
coordination of benefits.
356-NU OTHER PAYER CARDHOLDER ID Q***R*** Prior Authorization Request Only (Claim/Service):
Required if other insurance information is available for
coordination of benefits.
992-MJ OTHER PAYER GROUP ID Q***R*** Prior Authorization Request Only (Claim/Service):
Required if other insurance information is available for
coordination of benefits.
142-UV OTHER PAYER PERSON CODE Q***R*** Prior Authorization Request Only (Claim/Service):
Required if needed to uniquely identify the family members
within the Cardholder ID, as assigned by the other payer.
127-UB OTHER PAYER HELP DESK PHONE NUMBER Q***R*** Prior Authorization Request Only (Claim/Service):
Required if needed to provide a support telephone number
of the other payer to the receiver.
143-UW OTHER PAYER PATIENT RELATIONSHIP CODE Q***R*** Prior Authorization Request Only (Claim/Service):
Required if needed to uniquely identify the relationship of
the patient to the cardholder ID, as assigned by the other
payer.
144-UX OTHER PAYER BENEFIT EFFECTIVE DATE Q***R*** Prior Authorization Request Only (Claim/Service):
Required when other coverage is known which is after the
Date of Service submitted.
145-UY OTHER PAYER BENEFIT TERMINATION DATE Q***R*** Prior Authorization Request Only (Claim/Service):

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 452 -
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS
SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Required when other coverage is known which is after the
Date of Service submitted.
Notes on Response Coordination of Benefits/Other Payers Segment on a Prior Authorization Request Only Response:
The Response Coordination of Benefits/Other Payers Segment is situational for a Prior Authorization Request Only response when the Header
Response Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “R” (Rejected) when other insurance information
is available for coordination of benefits.
1. If the identity of the patient is partially verified and the Prior Authorization Request Only is rejected due to a non-match of field
verification, then the Other Payer information is not sent.
2. If the Prior Authorization Request Only is rejected because it should be submitted to other payer(s) first, that Other Payer information
should be sent, if known.
3. If the Prior Authorization Request Only is rejected due to benefit design limitations, then subsequent Other Payer information should
be sent, if known.
If the Prior Authorization Request Only rejects for other reasons than above, Other Payer information is not sent.
If subsequent payer(s) for this patient is not known, the Other Payer information is not sent.
If subsequent payer(s) for this patient is known, the following may be sent:
• Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C),
• Other Payer Group ID (992-MJ),
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU)
• And other Other Payer fields.
In addition, if any of the following three fields are sent:
• Other Payer Processor Control Number (991-MH),
• Other Payer Cardholder ID (356-NU),
• Other Payer Group ID (992-MJ),
then the Other Payer ID (34Ø-7C) and Other Payer ID Qualifier (339-6C) must be sent.
Fields defined as Mandatory are required to be submitted when the segment is sent.
16.3.5 TRANSMISSION REJECTED/TRANSACTION REJECTED RESPONSE
Prior Authorization Request Only transmission response Header Response Status (5Ø1-F1) of "R" (Rejected)
and Transaction Response Status (112-AN) of “R” (Rejected)
Each response contains one occurrence of claim/service data.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
16.3.5.1 DIAGRAM FOR TRANSMISSION OF ONE PRIOR AUTHORIZATION REQUEST ONLY RESPONSE
(TRANSMISSION REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
16.3.5.2 PRIOR AUTHORIZATION REQUEST ONLY RESPONSE SEGMENTS (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
16.3.5.2.1 RESPONSE HEADER SEGMENT (PRIOR AUTHORIZATION REQUEST ONLY) (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 453 -
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on a Prior Authorization Request Only Response:
The Response Header Segment is a mandatory, fixed length segment for Prior Authorization Request Only when the Header Response Status
(5Ø1-F1) of "R" (Rejected) and Transaction Response Status (112-AN) of “R” (Rejected). The “Situation” column is not applicable.
If either the entire transmission or the Header is in error, the Header Response Status (5Ø1-F1) = “R” (Rejected). Every identifiable transaction
within the transmission must be rejected with an “R”.
16.3.5.2.2 RESPONSE MESSAGE SEGMENT (PRIOR AUTHORIZATION REQUEST ONLY) (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Prior Authorization Request Only (Claim/Service):
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on a Prior Authorization Request Only Response:
The Response Message Segment is situational for Prior Authorization Request Only when the Header Response Status (5Ø1-F1) of "R"
(Rejected) and Transaction Response Status (112-AN) of “R” (Rejected). It is used when additional text information needs to be sent. Fields
defined as Mandatory are required to be submitted when the segment is sent.
16.3.5.2.3 RESPONSE STATUS SEGMENT (PRIOR AUTHORIZATION REQUEST ONLY) (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Prior Authorization Request Only (Claim/Service):
Required if to identify the transaction.
51Ø-FA REJECT COUNT R Prior Authorization Request Only (Claim/Service):
Maximum count of 5.
Required.
511-FB REJECT CODE R***R*** Prior Authorization Request Only (Claim/Service):
Required.
546-4F REJECT FIELD OCCURRENCE INDICATOR Q***R*** Prior Authorization Request Only (Claim/Service):
Required if a repeating field is in error, to identify repeating
field occurrence.
This field must be sent when relaying error information about
a repeating field or set. Note, if the Reject Code is not

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 454 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
denoting a repeating field or set, the Reject Field Occurrence
Indicator must not be sent.
547-5F APPROVED MESSAGE CODE COUNT N Prior Authorization Request Only (Claim/Service):
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Prior Authorization Request Only (Claim/Service):
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Prior Authorization Request Only (Claim/Service):
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Prior Authorization Request Only (Claim/Service):
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Prior Authorization Request Only (Claim/Service):
Required if additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Prior Authorization Request Only (Claim/Service):
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Prior Authorization Request Only (Claim/Service):
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Prior Authorization Request Only (Claim/Service):
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Prior Authorization Request Only (Claim/Service):
Not used.
993-A7 INTERNAL CONTROL NUMBER N Prior Authorization Request Only (Claim/Service):
Not used.
987-MA URL N Prior Authorization Request Only (Claim/Service):
Not used.
Notes on Response Status Segment on a Prior Authorization Request Only Response:
The Response Status Segment is mandatory for Prior Authorization Request Only when the Header Response Status (5Ø1-F1) of "R"
(Rejected) and Transaction Response Status (112-AN) of “R” (Rejected). The Response Status Segment is sent from the sender to the
receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 455 -
17. PRIOR AUTHORIZATION TRANSACTION DISCUSSION
The Prior Authorization transactions have been created to allow a Processor to authorize, authorize and immediately adjudicate the claim or
service, defer, or pend the request for review.
Prior Authorization before dispensing prescriptions may be required for (but not limited to) medical exceptions, drug overrides or limitations, or
dosage limitations.
17.1 TRANSACTION USAGE
17.1.1 PRIOR AUTHORIZATION REQUEST AND BILLING
The pharmacy submits a Prior Authorization Request And Billing to receive approval for the Prior Authorization and to receive payment
information. If the processor responds that the Prior Authorization Request and Billing is “P” (Paid) or “D” (Duplicate of Paid), the response will
include a Prior Authorization Number-Assigned (498-PY), other pertinent information in the Response Prior Authorization Segment, and
payment information in the Response Pricing Segment.
When a Prior Authorization Request And Billing receives a “C” (Captured) or “Q” (Duplicate of Capture) response the pharmacy system will
not receive a Prior Authorization Number-Assigned (498-PY). The pharmacy must receive an Authorization Number (5Ø3-F3) in the
Response Status Segment to a “C” (Captured) or “Q” (Duplicate of Capture).
The pharmacy system may receive a Prior Authorization Number-Assigned (498-PY) with an “F” (Deferred) response, depending on the
processor’s requirements. The pharmacy may receive an Authorization Number (5Ø3-F3) with an "F" (Deferred) response, depending on the
processor’s requirements. On an “F” (Deferred), if the processor does not send a Prior Authorization Number-Assigned (498-PY), the
pharmacy will receive an Authorization Number (5Ø3-F3) in the response. Later, when the pharmacy inquires about the prior authorization by
using a Prior Authorization Inquiry, the value from the original transaction (Response Status Segment Authorization Number (5Ø3-F3)) would
be placed in the request field Authorization Number (5Ø3-F3) in the Prior Authorization Segment.
Chart 1 Prior Authorization Request And Billing
Response Prior Authorization
Number-Assigned
(498-PY)
Authorization
Number (5Ø3-F3) Response Prior
Authorization Segment (Prior
Authorization Information)
Response Pricing Segment
(Payment Information)
P-Paid
D- Duplicate Paid
Yes
Yes-if needed to
identify the
transaction
Yes
Yes
C-Captured
Q-Duplicate Captured
No
Yes
No
No
F-Deferred Yes-if Authorization
Number (5Ø3-F3) not
sent
Yes-if Prior
Authorization
Number-Assigned
(498-PY) not sent
Yes-if the Prior Authorization
Number-Assigned (498-PY) is
sent
No
R-Rejected No Processor Defined** No No
**Note: A processor may choose to return an Authorization Number (5Ø3-F3) on a Rejected response to track the transaction for
troubleshooting, customer service reasons. This use of the Authorization Number (5Ø3-F3) has no effect on the Prior Authorization, but is
simply a way to track a transaction.
17.1.2 PRIOR AUTHORIZATION REQUEST ONLY
The pharmacy submits a Prior Authorization Request Only to receive approval for a prior authorization, without any payment information.
A pharmacy’s Prior Authorization Request Only that is “A” (Approved) or “S” (Duplicate of Approved) must receive a response that includes a
Prior Authorization Number-Assigned (498-PY) and other information in the Response Prior Authorization Segment. The pharmacy will not
receive any payment information. When/If the pharmacy submits a Claim or Service Billing, the value of the field Prior Authorization Number-
Assigned (498-PY) returned from the processor is placed in the Prior Authorization Number Submitted (462-EV) on the Claim or Service Billing
transaction submission.
When a Prior Authorization Request Only receives a “C” (Captured) or “Q” (Duplicate of Capture) response, the pharmacy system will not
receive a Prior Authorization Number-Assigned (498-PY) as the Response Prior Authorization Segment is not used. The pharmacy must
receive an Authorization Number (5Ø3-F3) in the Response Status Segment to a “C” (Captured) or “Q” (Duplicate of Capture).
The pharmacy system may receive a Prior Authorization Number-Assigned (498-PY) with an “F” (Deferred) response, depending on the
processor’s requirements. On an “F” (Deferred) response, if the processor does not send a Prior Authorization Number-Assigned (498-PY), the
pharmacy will receive an Authorization Number (5Ø3-F3) in the response. Later, when the pharmacy inquires about the prior authorization by
using a Prior Authorization Inquiry, the value from the original transaction (Response Status Segment, Authorization Number (5Ø3-F3)) would
be placed in the request field Authorization Number (5Ø3-F3) in the Prior Authorization Segment.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 456 -
Chart 2 Prior Authorization Request Only
Response PA Number-
Assigned (498-PY) Authorization
Number (5Ø3-F3) Response Prior
Authorization Segment
(Prior Authorization
Information)
Response Pricing Segment
(Payment Information)
A-Approved
S- Duplicate Approved
Yes
No
Yes
No
C-Captured
Q-Duplicate Captured
No
Yes
No
No
F-Deferred Processor Defined Yes-if Prior
Authorization
Number-Assigned
(498-PY) not sent
Yes No
R-Rejected No Processor Defined** No No
**Note: A processor may choose to return an Authorization Number (5Ø3-F3) on a Rejected response to track the transaction for
troubleshooting, customer service reasons. This use of the Authorization Number (5Ø3-F3) has no effect on the Prior Authorization, but is
simply a way to track a transaction.
17.1.3 PRIOR AUTHORIZATION INQUIRY
The pharmacy submits a Prior Authorization Inquiry to receive a status on a previously submitted Prior Authorization Request And Billing or a
previously submitted Prior Authorization Request Only. A Prior Authorization Inquiry is submitted for a previously submitted Prior Authorization
Request And Billing or Prior Authorization Request Only that was “C” (Captured).
The Prior Authorization Inquiry transaction supports multiple responses, but the responses are actually tied back to the originally requested
transaction. The originally requested transaction is either a Prior Authorization Request And Billing or a Prior Authorization Request Only. The
valid responses are the values applicable to either of those transactions.
If the initial request was a Prior Authorization Request And Billing that was not “P” (Paid) or “R” (Rejected) initially (meaning follow up was
required) or a time out situation occurred, the subsequent Prior Authorization Inquiry must receive a response that was acceptable for the
initial Prior Authorization Request & Billing - “P” (Paid), “C” (Captured), “F” (Deferred), or “R” (Rejected).
Chart 3 Fields Sent By a Pharmacy in a Prior Authorization Inquiry
Based on the Response to the original Prior Authorization Request And Billing
Original Response on the Prior
Authorization Request And Billing Prior Authorization Number-Assigned
(498-PY) in Prior Authorization Segment Authorization Number (5Ø3-F3) in Prior
Authorization Segment
P-Paid Yes Yes-if sent by processor
C-Captured No Yes
F-Deferred Yes-if sent by processor Yes-if sent by processor
R-Rejected Not applicable. There is no inquiry on a rejected PA Request and Billing
Chart 4
Response to Chart 3. Fields Returned by the Processor in a Prior Authorization Inquiry Response
Based on the original Prior Authorization Request And Billing
Processor Response Prior Authorization Number-Assigned
(498-PY)
Authorization Number (5Ø3-F3)
P-Paid or D-Duplicate of Paid Yes Yes—if needed to identify the transaction
C-Captured or
Q-Duplicate of Capture
No-unless the status of the original request
has changed. Please see response according
to result of adjudication of original request
Yes
F-Deferred Processor Defined Yes-if Prior Authorization Number-Assigned (498-
PY) not sent
R- Reject No Processor Defined**
**Note: A processor may choose to return an Authorization Number (5Ø3-F3) on a Rejected response to track the transaction for
troubleshooting, customer service reasons. This use of the Authorization Number (5Ø3-F3) has no effect on the Prior Authorization, but is
simply a way to track a transaction.
If the initial request was a Prior Authorization Request Only that was not approved or rejected initially (meaning follow up was required) or a
time out situation occurred, the subsequent Prior Authorization Inquiry receives a response that was acceptable for the initial Prior
Authorization Request Only - “A” (Approved), “C” (Captured), “F” (Deferred), or “R” (Rejected).

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 457 -
Chart 5 Fields Sent in by Pharmacy in a Prior Authorization Inquiry
Based on the Response to an original Prior Authorization Request Only
Original Response on the Prior
Authorization Request Only Prior Authorization Number-Assigned
(498-PY) Authorization Number (5Ø3-F3)
A-Approved Yes No
C-Captured No Yes
F-Deferred Yes-if sent by processor Yes-if sent by processor
R-Rejected Not applicable. There is no inquiry on a rejected PA Request and Billing
Chart 6
Response to Chart 5. Fields Returned by the Processor in a Prior Authorization Inquiry Response
Based on the original Prior Authorization Request Only
Processor Response Prior Authorization Number-Assigned
(498-PY)
Authorization Number (5Ø3-F3)
A-Approved Yes No
C-Captured No-unless the status of the original request
has changed. Please see response according
to result of adjudication of original request
Yes
F-Deferred Processor Defined Yes-if Prior Authorization Number-Assigned (498-
PY) not sent
R-Rejected No Processor defined**
**Note: A processor may choose to return an Authorization Number (5Ø3-F3) on a Rejected response to track the transaction for
troubleshooting, customer service reasons. This use of the Authorization Number (5Ø3-F3) has no effect on the Prior Authorization, but is
simply a way to track a transaction.
17.1.4 PRIOR AUTHORIZATION REVERSAL
The Prior Authorization Reversal is used to back out the request for authorization, but not any claims submitted against the prior
authorization. To reverse a Prior Authorization Request And Billing, paid billings are to be reversed before the prior authorization is reversed.
The pharmacy must submit a Claim or Service Reversal (Transaction Code = “B2” or “S2”) before submitting a Prior Authorization Reversal
request. If there are no Claims or Services paid for the Prior Authorization in question, the processor must accept the Prior Authorization
Reversal for the prior authorization only.
The pharmacy would submit the Prior Authorization Number-Assigned (498-PY) in the Prior Authorization Reversal for those transactions with
original responses of “P” (Paid) or “A” (Approved) and the Authorization Number (5Ø3-F3) for those transactions with an original response of
“C” (Captured).
17.2 FIELD CLARIFICATION
17.2.1 PRIOR AUTHORIZATION FIELDS
The Prior Authorization Type Code (461-EU) defines the type of authorization being requested.
The Prior Authorization Number Submitted (462-EV) contains the value assigned to the authorization. Note: When/If the pharmacy submits a
Claim or Service Billing, the value of the field Prior Authorization Number-Assigned (498-PY) from the processor’s response is placed in
the Prior Authorization Number Submitted (462-EV) on the Claim or Service Billing transaction submission.
The Prior Authorization Number-Assigned (498-PY) is used to communicate to the provider the Prior Authorization number assigned by the
processor. This field is returned as part of the Prior Authorization Response Segment.
In addition, when performing a Prior Authorization Reversal (Transaction Code P2), this field contains the Prior Authorization Number the
provider is reversing. This field would be populated when reversing transaction with original responses of “P” (Paid) or “A” (Approved).
17.2.2 PRIOR AUTHORIZATION NUMBER-ASSIGNED (498-PY) IN RESPONSE PRIOR
AUTHORIZATION SEGMENT) AND AUTHORIZATION NUMBER (5Ø3-F3) IN RESPONSE
STATUS SEGMENT
This section explains the usage of Prior Authorization Number-Assigned (498-PY) in the response returned by the processor, in a prior
authorization situation.
For a Prior Authorization Request And Billing
The processor must return a Prior Authorization Number-Assigned (498-PY) in a “P” (Paid) or “D” (Duplicate of Paid) response.
For a Prior Authorization Request Only
The processor must return a Prior Authorization Number-Assigned (498-PY) in an “A” (Approved) or “S” (Duplicate of Approved) response.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 458 -
For a Prior Authorization Request And Billing AND a Prior Authorization Request Only
The processor must return an Authorization Number (5Ø3-F3) in a “C” (Capture) or “Q” (Duplicate of Capture) response and not return a Prior
Authorization Number-Assigned (498-PY). (The Response Prior Authorization Segment is not used in a Prior Authorization Request Only
transaction.) The Authorization Number (5Ø3-F3) is used in a Prior Authorization Inquiry transaction to ask for the status of the prior
authorization.
Some processors may return a Prior Authorization Number-Assigned (498-PY) in an “F” (Deferred) response. If Prior Authorization Number-
Assigned (498-PY) is not returned, then Authorization Number (5Ø3-F3) must be returned.
Note: When/If the pharmacy submits a subsequent claim or service billing, the value of the field Prior Authorization Number-Assigned (498-
PY) is placed in the Prior Authorization Number Submitted (462-EV) on the Claim or Service Billing transaction.
For a Prior Authorization Inquiry Only
Use the guidelines above depending on whether the initial transaction was a Prior Authorization Request And Billing, or a Prior Authorization
Request Only.
A Prior Authorization Inquiry must be sent with a Prior Authorization Number-Assigned (498-PY) or Authorization Number (5Ø3-F3).
17.2.3 AUTHORIZATION NUMBER (5Ø3-F3) IN PRIOR AUTHORIZATION SEGMENT
This section explains the usage of Authorization Number (5Ø3-F3) in the request submitted by the pharmacy, in a prior authorization situation.
For a Prior Authorization Request And Billing AND a Prior Authorization Request Only
The Authorization Number (5Ø3-F3) is not used for submission of a Prior Authorization Request And Billing OR a Prior Authorization Request
Only.
For a Prior Authorization Inquiry Only
The Authorization Number (5Ø3-F3) would be submitted in a Prior Authorization Inquiry Only when the pharmacy was seeking a status for a
previously sent Prior Authorization Request And Billing or Prior Authorization Request Only that received a “C” (Capture) or “Q” (Duplicate of
Capture) response or a "F" (Deferred) response where the Prior Authorization Number-Assigned (498-PY) was not returned.
For a Prior Authorization Reversal
The Authorization Number (5Ø3-F3) is supported in a submission of a Prior Authorization Reversal for "C" (Capture) responses only.
17.2.4 PRIOR AUTHORIZATION NUMBER SUBMITTED (462-EV) IN CLAIM SEGMENT
This field is used only in transaction activities for claims and services associated with an approved Prior Authorization request. It is NOT used
in a Prior Authorization Request And Billing or a Prior Authorization Request Only since the pharmacy is only seeking an approval.
When the pharmacy submits a Claim or Service Billing for which a Prior Authorization Number-Assigned (498-PY) was returned, the Prior
Authorization Number Submitted (462-EV) must be submitted with the transaction in the Claim Segment if the processor requires the Prior
Authorization Number to be submitted.
The Prior Authorization Number Submitted (462-EV) on the claim or service billing must contain the value from the Prior Authorization
Number-Assigned (498-PY) in the Response Prior Authorization Segment that was returned from the processor in the Prior Authorization
Request And Billing OR the Prior Authorization Request Only. The Prior Authorization Number-Assigned (498-PY) would have been returned
with a “P” (Paid) or “D” (Duplicate of Paid) response or with an “A” (Approved) or “S” (Duplicate of Approved) response.
17.3 SCENARIO EXAMPLES
The following illustrates a couple of the transaction scenarios discussed above, shown in tabular format. Treat each as a completely separate
case.
17.3.1 PRIOR AUTHORIZATION REQUEST AND BILLING RESPONSES
The pharmacy requests a Prior Authorization Request And Billing (seeking approval and payment information). The following choice of
responses would be sent by the processor.
• The processor responds with a “P” (Paid) or “D” (Duplicate of Paid) response. The payment information is included in the Response
Pricing Segment. The Prior Authorization Number-Assigned (498-PY) and pertinent prior authorization information is returned in the
Response Prior Authorization Segment.
Or
• The processor responds with a “C” (Captured) or “Q” (Duplicate of Capture) response. The processor is still evaluating the prior
authorization. The processor includes an Authorization Number (5Ø3-F3) in the response. The pharmacy will later submit a Prior
Authorization Inquiry with the Authorization Number (5Ø3-F3) in the Prior Authorization Segment.
Or
• The processor responds with a “F” (Deferred) response that includes a Prior Authorization Number-Assigned (498-PY) or an
Authorization Number (5Ø3-F3). The pharmacy should consult the processor’s provider manual for further information.
Or

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 459 -
• The processor responds with a “R” (Rejected) response, the pharmacy must examine the reject codes and messages. The
transaction may include missing/invalid information, or the processor may be denying the Prior Authorization Request And Billing.
17.3.1.1 SCENARIOS FOR PRIOR AUTHORIZATION REQUEST AND BILLING
1. The pharmacy requests a Prior Authorization Request And Billing (seeking approval and payment information).
The processor responds with a “C” (Captured) or “Q” (Duplicate of Capture) response that includes an Authorization
Number (5Ø3-F3).
The pharmacy later submits a Prior Authorization Inquiry with the Authorization Number (5Ø3-F3) in the Prior
Authorization Segment.
The processor has completed its evaluation of the original request and responds with a “P” (Paid) or “D”
(Duplicate of Paid) response. The payment information is included in the Response Pricing Segment. The Prior
Authorization Number-Assigned (498-PY) and pertinent prior authorization information are returned in the
Response Prior Authorization Segment.
Or
The processor responds with a “C” (Captured) or “Q” (Duplicate of Capture) response. The processor is still evaluating the
prior authorization. The pharmacy will later submit another Prior Authorization Inquiry with the Authorization Number (5Ø3-
F3) in the Prior Authorization Segment.
If the processor responds with another “C” (Captured) (or “Q” (Duplicate of Capture)) response, the same
Authorization Number (5Ø3-F3) as the original would be returned to the pharmacy. The processor must not
return a new Authorization Number (5Ø3-F3).
Or
The processor has completed its evaluation of the original request and responds with an “F” (Deferred) response that
includes a Prior Authorization Number-Assigned (498-PY) or an Authorization Number (5Ø3-F3). The pharmacy should
consult the processor’s provider manual for further information.
Or
The processor has completed its evaluation of the original request and responds with an “R” (Rejected) response. The
pharmacy must examine the reject codes and messages. The transaction may include missing/invalid information, or the
processor may be denying the original Prior Authorization Request And Billing.
2. The pharmacy submits a Prior Authorization Request And Billing (seeking approval and payment information.)
The processor responds with a “P” (Paid) or “D” (Duplicate of Paid). The payment information is included in the Response
Pricing Segment. The Prior Authorization Number-Assigned (498-PY) and pertinent prior authorization information is
returned in the Response Prior Authorization Segment.
To reverse the claim or service billing, the pharmacy submits a Claim or Service Reversal.
The processor responds with an “A” (Approved) or “S” (Duplicate of Approved) and backs out the
payment.
To reverse the prior authorization, the pharmacy submits a Prior Authorization Reversal
with the Prior Authorization Number Submitted (462-EV) in the Claim Segment.
The processor responds with an “A” (Approved) or “S” (Duplicate of Approved)
and backs out the authorization only.
*Note if claim reversal has not been initiated by the pharmacy, the Prior Authorization Reversal request would
receive an “R” (Rejected) response by the processor. The pharmacy must reverse the paid billings before
requesting a prior authorization reversal.
3. The pharmacy submits a Prior Authorization Request And Billing (seeking approval and payment information.)
The processor responds with a “P” (Paid). The payment information is included in the Response Pricing Segment. The
Prior Authorization Number-Assigned (498-PY) and pertinent prior authorization information is returned in the Response
Prior Authorization Segment. However, a timeout occurs and the pharmacy does not receive the prior
authorization/payment response.
The pharmacy must submit the same Prior Authorization Request And Billing transaction. (The pharmacy did
not receive an Authorization Number (5Ø3-F3) since there was a timeout and therefore cannot send a Prior
Authorization Inquiry to learn the status.)
17.3.2 PRIOR AUTHORIZATION REQUEST ONLY RESPONSES
The pharmacy requests a Prior Authorization Request Only (seeking approval, no payment information). The following choice of responses
would be sent by the processor.
• The processor responds with an “A” (Approved) or “S” (Duplicate of Approved) response, with a Prior Authorization Number-
Assigned (498-PY) given.
Note: When/If the pharmacy submits a claim or service billing, the value of the field Prior Authorization Number-Assigned
(498-PY) returned from the processor is placed in the Prior Authorization Number Submitted (462-EV) on the claim or
service billing transaction submission.
Or
• The processor responds with a “C” (Captured) or “Q” (Duplicate of Capture) response. Note, the Prior Authorization Number-
Assigned (498-PY) is not returned (this field is not applicable in a capture). The Authorization Number (5Ø3-F3) is returned by the
processor.
Or
• The processor responds with a “F” (Deferred) response that includes a Prior Authorization Number-Assigned (498-PY) or an
Authorization Number (5Ø3-F3). The pharmacy should consult the processor’s provider manual for further information.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 460 -
Or
• The processor responds with a “R” (Rejected) response, the pharmacy must examine the reject codes and messages. The
transaction may include missing/invalid information, or the processor may be denying the Prior Authorization Request Only.
17.3.2.1 SCENARIOS FOR PRIOR AUTHORIZATION REQUEST ONLY
1. The pharmacy submits a Prior Authorization Request Only (only seeking approval, not payment information).
The processor responds with an “A” (Approved) or “S” (Duplicate of Approved) response, with a Prior Authorization
Number-Assigned (498-PY) given. However, a timeout occurs and the pharmacy does not receive the prior authorization
response.
The pharmacy must submit the same Prior Authorization Request Only transaction. (The pharmacy did not
receive an Authorization Number (5Ø3-F3) since there was a timeout and therefore cannot send a Prior
Authorization Inquiry to learn the status.)
2. The pharmacy submits a Prior Authorization Request Only (only seeking approval, not payment information).
The processor responds with a “C” (Captured) or “Q” (Duplicate of Capture) response. Note, the Prior Authorization
Number-Assigned (498-PY) is not returned (this field is not applicable in a capture). The Authorization Number (5Ø3-F3) is
returned.
The pharmacy later submits a Prior Authorization Inquiry with the Authorization Number (5Ø3-F3).
The processor has completed its evaluation of the original request and responds with an “A”
(Approved) or “S” (Duplicate of Approved) response. The Prior Authorization Number-Assigned (498-
PY) along with other important information is returned.
Or
The processor responds with a “C” (Captured) or “Q” (Duplicate of Capture) response. The processor
is still evaluating the prior authorization. The pharmacy will later submit another Prior Authorization
Inquiry with the Authorization Number (5Ø3-F3). The same Authorization Number as the original
would be returned to the pharmacy. The processor must not return a new Authorization Number
(5Ø3-F3).
Or
The processor has completed its evaluation of the original request and responds with an “F”
(Deferred) response that includes a Prior Authorization Number-Assigned (498-PY) or an
Authorization Number (5Ø3-F3). The pharmacy should consult the processor’s provider manual for
further information.
Or
The processor has completed its evaluation of the original request and responds with an “R”
(Rejected) response. The pharmacy must examine the reject codes and messages. The transaction
may include missing/invalid information, or the processor may be denying the original Prior
Authorization Request Only.
3. The pharmacy submits a Prior Authorization Request Only (only seeking approval, not payment information).
The processor responds with a “C” (Captured) or “Q” (Duplicate of Capture) response. Note, the Prior Authorization
Number-Assigned (498-PY) is not returned (this field is not applicable in a capture). The Authorization Number (5Ø3-F3) is
returned.
To reverse the prior authorization, the pharmacy submits a Prior Authorization Reversal with the Authorization
Number (5Ø3-F3). This is to reverse the prior authorization only no paid billings have been made.
The processor responds with an “A” (Approved) or “S” (Duplicate of Approved) and backs out the
Prior Authorization Request Only.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 461 -
18. CONTROLLED SUBSTANCE REPORTING INFORMATION
Controlled substance reporting transactions allow Processors or Reporting Entities to collect information about prescribing, dispensing, and
consumption of dangerous or abusable drugs. These transactions include:
• Controlled Substance Reporting (C1)
• Controlled Substance Reporting Reversal (C2)
• Controlled Substance Reporting Rebill (C3)
It is assumed DUR screening and the performance of professional pharmacy services will occur on original service or product billings.
Therefore, processors should not apply DUR algorithms or request Professional Pharmacy Services on controlled substance reporting
transactions unless trading partners agree to this activity.
At this time, the business cases for this transaction are not fully defined. The transaction is designated as optional usage. The transactions
may be used. Trading partners are asked to bring their situations to NCPDP so that the situations may be defined when the industry begins using
this transaction.
Duplicate response logic must not be applied by the processor to Controlled Substance Reporting Rebill transactions. There is no need for a
duplicate response due to the nature of the rebill transaction and its implied reversal. Because the implied reversal would reverse the paid
claim, a duplicate transaction would not exist.
If a processor supported duplicate responses in rebills the submitter would not be able to modify a field that is not included in the duplicate
field check. See sections “Response Processing Guidelines”, “Duplicate Transactions” and “Duplicate Processing For All Rebill Transactions”
for more information.
These transactions are described below.
18.1 CONTROLLED SUBSTANCE REPORTING
This transaction is used to notify the Processor or Reporting Entity of a dispensing activity for a controlled substance.
Each submission message contains up to four occurrences of claim/service data. The Transaction Code is “C1”.
The Processor must provide one of the following general types of responses:
Approved - The Processor acknowledges receipt and successfully processes the transaction.
Duplicate of Approved - This occurs when the Processor has previously received the request and processed the transaction, but the
response did not return to the Originator. The Duplicate response contains the same information as returned in the original
Approved scenario.
Captured - This occurs when the Processor acknowledges receipt of the request for reporting purposes only.
Duplicate of Captured - This occurs when the Processor has previously received the request and processed the transaction, but the
response did not return to the Originator. The Duplicate response contains the same information as returned in the original Captured
scenario.
Rejected - This occurs when the Processor has encountered an error in the transaction or processing, or does not approve of the
transaction.
See section “Response Processing Guidelines”, “Duplicate Transactions”.
18.2 CONTROLLED SUBSTANCE REPORTING REQUEST DIAGRAMS
18.2.1 DIAGRAM FOR TRANSMISSION OF ONE CONTROLLED SUBSTANCE REPORTING
TRANSACTION
At this time, the business cases for these transactions are not fully defined. These transactions are designated as optional usage. Trading
partners must bring their situations to NCPDP so that the situations are defined before the industry begins using these transactions.
1. Currently a non-NCPDP batch format is being used by a majority of the industry.
2. A Controlled Substance Reporting Transaction has not been included in the HIPAA mandate.
3. If at a later time, an entity opts to use the transaction (or if its use is mandated), the data elements should be reviewed in light of the
situations existing at that time.
4. It is strongly suggested that individuals proposing to use the standard confer with the pharmacy experts in the industry by contacting
NCPDP.
For Controlled Substance Reporting, the scenarios defined include
Controlled Substance Reporting from a Sender to a Receiver
Controlled Substance Reporting Accepted - Captured/Approved/Rejected Transaction Response from a Sender to a
Receiver
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 462 -
Standard Transmission Reject Response to a Controlled Substance Reporting from a Sender to a Receiver
Mandatory
Transaction Header Segment
Segment Separator
Patient Segment
Mandatory - first Controlled Substance Reporting transaction
Group Separator
Segment Separator
Claim Segment
Optional
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
18.2.2 DIAGRAM FOR TRANSMISSION OF TWO CONTROLLED SUBSTANCE REPORTING
TRANSACTIONS
Mandatory
Transaction Header Segment
Segment Separator
Patient Segment
Mandatory - first Controlled Substance Reporting transaction
Group Separator
Segment Separator
Claim Segment
Optional
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Mandatory - second Controlled Substance Reporting
transaction
Group Separator
Segment Separator
Claim Segment
Optional
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
18.2.3 DIAGRAM FOR TRANSMISSION OF THREE OR FOUR CONTROLLED SUBSTANCE
REPORTING TRANSACTIONS
These transaction diagrams will follow the example in the section “Diagram For Transmission Of Two Controlled Substance Reporting
Transactions”. For three or four transactions, the Mandatory and Optional controlled substance reporting transaction segments will be
repeated for the third and fourth transactions.
18.3 CONTROLLED SUBSTANCE REPORTING RESPONSE DIAGRAMS
18.3.1 TRANSMISSION ACCEPTED/TRANSACTION CAPTURED, APPROVED, REJECTED
Controlled Substance Reporting transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured)
or Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved)
or Transaction Response Status (112-AN) of “R” (Rejected)
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
18.3.1.1 DIAGRAM FOR TRANSMISSION OF ONE CONTROLLED SUBSTANCE REPORTING RESPONSES
(TRANSMISSION ACCEPTED/TRANSACTION CAPTURED, APPROVED, REJECTED)
Mandatory
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 463 -
Response Header Segment
Optional
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
18.3.1.2 DIAGRAM FOR TRANSMISSION OF TWO CONTROLLED SUBSTANCE REPORTING RESPONSES
(TRANSMISSION ACCEPTED/TRANSACTION CAPTURED, APPROVED, REJECTED)
Mandatory
Response Header Segment
Optional
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
18.3.1.3 DIAGRAM FOR TRANSMISSION OF THREE OR FOUR CONTROLLED SUBSTANCE REPORTING
RESPONSES (TRANSMISSION ACCEPTED/TRANSACTION CAPTURED, APPROVED, REJECTED)
These transaction diagrams will follow the example in the section “Diagram For Transmission Of Two Controlled Substance Reporting
Responses (Transmission Accepted/Transaction Captured, Approved, Rejected)”. For three or four transactions, the Mandatory and Optional
controlled substance reporting transaction segments will be repeated for the third and fourth transactions.
18.3.2 TRANSMISSION REJECTED/TRANSACTION REJECTED
Controlled Substance Reporting transmission response Header Response Status (5Ø1-F1) of "R" (Rejected)
and Transaction Response Status (112-AN) of “R” (Rejected)
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
18.3.2.1 DIAGRAM FOR TRANSMISSION OF ONE CONTROLLED SUBSTANCE REPORTING RESPONSE
(TRANSMISSION REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Optional
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
18.3.2.2 DIAGRAM FOR TRANSMISSION OF TWO CONTROLLED SUBSTANCE REPORTING RESPONSES
(TRANSMISSION REJECTED/TRANSACTION REJECTED)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 464 -
Mandatory
Response Header Segment
Optional
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
18.3.2.3 DIAGRAM FOR TRANSMISSION OF THREE OR FOUR CONTROLLED SUBSTANCE REPORTING
RESPONSES (TRANSMISSION REJECTED/TRANSACTION REJECTED)
These transaction diagrams will follow the example in the section “Diagram For Transmission Of Two Controlled Substance Reporting
Responses (Transmission Rejected/Transaction Rejected)”. For three or four transactions, the Mandatory and Optional controlled substance
reporting transaction segments will be repeated for the third and fourth transactions.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 465 -
19. CONTROLLED SUBSTANCE REPORTING REVERSAL INFORMATION
19.1 CONTROLLED SUBSTANCE REPORTING REVERSAL
This transaction is used to reverse a previously submitted Controlled Substance Reporting transaction. It is requesting the Processor or
Reporting Entity to back out the previously reported information.
Each submission message contains up to four occurrences of claim/service Data. The Transaction Code is “C2”.
At this time, the business cases for this transaction are not fully defined. The transaction is designated as optional usage. The transactions
may be used. Trading partners are asked to bring their situations to NCPDP so that the situations may be defined as the industry begins using
this transaction.
The Transaction Header Segment is required, which contains the routing and identification information – BIN Number, Version/Release
Number, Transaction Code, Processor Control Number, Transaction Count, Service Provider ID and Qualifier, Date of Service.
Therefore, following the rules to correctly build a multi-reversal transmission, the reversal transaction(s) in this transmission must be
• in the same format (Version/Release Number) and
• sent to the same entity (processor or PBM using the BIN Number and Processor Control Number) and
• for the same pharmacy (Service Provider ID and Qualifier) and
• for the same date (Date of Service).
The Patient Segment is mandatory in a controlled substance reporting reversal. The Pharmacy Provider Segment and the Prescriber
Segment are optional. If a processor/PBM needs this information to process a reversal, these segments can be used. The Patient segment
must occur only once as this segment occurs at the transmission level.
If a processor/PBM does not need the Pharmacy Provider Segment and the Prescriber segment information, but the pharmacy wishes to send
it, the processor/PBM must ignore the valid optional and/or situational information. These segments occur at the transaction level and may
occur one to four times as part of each reversal transaction.
Date of Service (4Ø1-D1) is defined as “identifies date the prescription was filled or professional service rendered”. Therefore, since the date is
in the Transaction Header segment that occurs once (at the transmission level), one to four transactions (at the transaction level) must be for
the same date.
Multiple controlled substance reporting reversal transactions in a transmission must be for the same patient since the Patient Segment is
mandatory and must occur only once in a transmission.
The Processor must provide one of the following general types of responses:
Approved - The Processor acknowledges receipt of the reversal and backs out the previously submitted reporting transaction.
Duplicate of Approved - This occurs when the Processor has previously received the request and processed the transaction, but the
response did not return to the Originator. The Duplicate response contains the same information as returned in the original
Approved scenario.
Captured - This occurs when the Processor acknowledges receipt of the request for reporting purposes only, and is not making any
judgment regarding backing out the reporting.
Duplicate of Captured - This occurs when the Processor has previously received the request and processed the transaction, but the
response did not return to the Originator. The Duplicate response contains the same information as returned in the original Captured
scenario.
Rejected - This occurs when the Processor has encountered an error in the transaction or processing, or does not approve the
reversal.
See section “Response Processing Guidelines”, “Duplicate Transactions”.
19.2 CONTROLLED SUBSTANCE REPORTING REVERSAL REQUEST DIAGRAMS
19.2.1 DIAGRAM FOR TRANSMISSION OF ONE CONTROLLED SUBSTANCE REPORTING
REVERSAL TRANSACTION
For a Controlled Substance Reporting Reversal, the scenarios defined include
Controlled Substance Reporting Reversal from a Sender to a Receiver
Controlled Substance Reporting Reversal Transaction Response from a Sender to a Receiver
Standard Transmission Accepted/Transaction Captured/Approved/Rejected Response from a Sender to a
Receiver
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 466 -
Standard Transmission Reject Response to a Controlled Substance Reporting Reversal from a Sender to a
Receiver
Mandatory
Transaction Header Segment
Segment Separator
Patient Segment
Mandatory - first Controlled Substance Reporting Reversal
Group Separator
Segment Separator
Claim Segment
Optional
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
19.2.2 DIAGRAM FOR TRANSMISSION OF TWO CONTROLLED SUBSTANCE REPORTING
REVERSAL TRANSACTIONS
Mandatory
Transaction Header Segment
Segment Separator
Patient Segment
Mandatory - first Controlled Substance Reporting Reversal
Group Separator
Segment Separator
Claim Segment
Optional
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Mandatory - second Controlled Substance Reporting Reversal
Group Separator
Segment Separator
Claim Segment
Optional
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
19.2.3 DIAGRAM FOR TRANSMISSION OF THREE OR FOUR CONTROLLED SUBSTANCE
REPORTING REVERSAL TRANSACTIONS
These transaction diagrams will follow the example in the section “Diagram For Transmission Of Two Controlled Substance Reporting
Reversal Transactions”. For three or four transactions, the Mandatory and Optional Controlled Substance Reporting segments will be repeated
for the third and fourth transactions.
19.3 CONTROLLED SUBSTANCE REPORTING REVERSAL RESPONSE DIAGRAMS
19.3.1 TRANSMISSION ACCEPTED/TRANSACTION APPROVED, CAPTURED, REJECTED
Controlled Substance Reporting Reversal transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved)
or Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured)
or Transaction Response Status (112-AN) of “R” (Rejected)
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
19.3.1.1 DIAGRAM FOR TRANSMISSION OF ONE CONTROLLED SUBSTANCE REPORTING REVERSAL
RESPONSE (TRANSMISSION ACCEPTED/TRANSACTION APPROVED, CAPTURED, REJECTED)
Mandatory
Response Header Segment

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 467 -
Optional
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
19.3.1.2 DIAGRAM FOR TRANSMISSION OF TWO CONTROLLED SUBSTANCE REPORTING REVERSAL
RESPONSES (TRANSMISSION ACCEPTED/TRANSACTION APPROVED, CAPTURED, REJECTED)
Mandatory
Response Header Segment
Optional
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
19.3.1.3 DIAGRAM FOR TRANSMISSION OF THREE OR FOUR CONTROLLED SUBSTANCE REPORTING
REVERSAL RESPONSES (TRANSMISSION ACCEPTED/TRANSACTION APPROVED, CAPTURED,
REJECTED)
These transaction diagrams will follow the example in the section “Diagram For Transmission Of Two Controlled Substance Reporting
Reversal Responses (Transmission Accepted/Transaction Approved, Captured, Rejected)”. For three or four transactions, the Mandatory and
Optional Controlled Substance Reporting segments will be repeated for the third and fourth transactions.
19.3.2 TRANSMISSION REJECTED/TRANSACTION REJECTED
Controlled Substance Reporting Reversal transmission response Header Response Status (5Ø1-F1) of "R" (Rejected)
and Transaction Response Status (112-AN) of “R” (Rejected)
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
19.3.2.1 DIAGRAM FOR TRANSMISSION OF ONE CONTROLLED SUBSTANCE REPORTING REVERSAL
RESPONSE (TRANSMISSION REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Optional
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
19.3.2.2 DIAGRAM FOR TRANSMISSION OF TWO CONTROLLED SUBSTANCE REPORTING REVERSAL
RESPONSES (TRANSMISSION REJECTED/TRANSACTION REJECTED)

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 468 -
Mandatory
Response Header Segment
Optional
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
19.3.2.3 DIAGRAM FOR TRANSMISSION OF THREE OR FOUR CONTROLLED SUBSTANCE REPORTING
REVERSAL RESPONSES (TRANSMISSION REJECTED/TRANSACTION REJECTED)
These transaction diagrams will follow the example in the section “Diagram For Transmission Of Two Controlled Substance Reporting
Reversal Responses (Transmission Rejected/Transaction Rejected)”. For three or four transactions, the Mandatory and Optional Controlled
Substance Reporting segments will be repeated for the third and fourth transactions.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 469 -
20. CONTROLLED SUBSTANCE REPORTING REBILL INFORMATION
20.1 CONTROLLED SUBSTANCE REPORTING REBILL
This transaction is a controlled substance reporting transaction with an implied reversal. It is used by the Originator to cancel a previously
submitted controlled substance reporting transaction, and submit a new controlled substance reporting in the same transaction.
For controlled substance reversal guidelines, see section “Controlled Substance Reporting Reversal Information”. The Transaction Code is
“C3”.
At this time, the business cases for this transaction are not fully defined. The transaction is designated as optional usage. The transactions
may be used. Trading partners are asked to bring their situations to NCPDP so that the situations may be defined as the industry begins using
this transaction.
The Processor must provide one of the following general types of responses:
Approved - The Processor acknowledges receipt and successfully processes the reversal and new reporting transaction.
Captured - This occurs when the Processor acknowledges receipt of the request for a reversal and resubmission for reporting
purposes only, but is not making any judgment regarding the processing of the rebill.
Rejected - This occurs when the Processor has encountered an error in the transaction or processing, or does not approve of the
transaction.
Please see section “Response Processing Guidelines”, “Duplicate Transactions” and “Duplicate Processing For All Rebill Transactions” for
more information about why duplicate responses are not supported in Controlled Substance Reporting Rebill transactions.
20.2 CONTROLLED SUBSTANCE REPORTING REBILL REQUEST DIAGRAMS
20.2.1 DIAGRAM FOR TRANSMISSION OF ONE CONTROLLED SUBSTANCE REPORTING
REBILL TRANSACTION
At this time, the business cases for these transactions are not fully defined. These transactions are designated as optional usage. Trading
partners must bring their situations to NCPDP so that the situations are defined before the industry begins using these transactions.
1. Currently a non-NCPDP batch format is being used by a majority of the industry.
2. A Controlled Substance Reporting Rebill Transaction has not been included in the HIPAA mandate.
3. If at a later time, an entity opts to use the transaction (or if its use is mandated), the data elements should be reviewed in light of the
situations existing at that time.
4. It is strongly suggested that individuals proposing to use the standard confer with the pharmacy experts in the industry by contacting
NCPDP.
For Controlled Substance Reporting Rebill, the scenarios defined include
Controlled Substance Reporting Rebill from a Sender to a Receiver
Controlled Substance Reporting Rebill Accepted - Captured/Approved/Rejected Transaction Response from a Sender to a
Receiver
Standard Transmission Reject Response to a Controlled Substance Reporting Rebill from a Sender to a Receiver
Mandatory
Transaction Header Segment
Segment Separator
Patient Segment
Mandatory - first Controlled Substance Reporting Rebill
transaction
Group Separator
Segment Separator
Claim Segment
Optional
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
20.2.2 DIAGRAM FOR TRANSMISSION OF TWO CONTROLLED SUBSTANCE REPORTING
REBILL TRANSACTIONS
Mandatory
Transaction Header Segment
Segment Separator
Patient Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 470 -
Mandatory - first Controlled Substance Reporting Rebill
transaction
Group Separator
Segment Separator
Claim Segment
Optional
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Mandatory - second Controlled Substance Reporting Rebill
transaction
Group Separator
Segment Separator
Claim Segment
Optional
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
20.2.3 DIAGRAM FOR TRANSMISSION OF THREE OR FOUR CONTROLLED SUBSTANCE
REPORTING REBILL TRANSACTIONS
These transaction diagrams will follow the example in the section “Diagram For Transmission Of Two Controlled Substance Reporting Rebill
Transactions”. For three or four transactions, the Mandatory and Optional controlled substance rebill transaction section will be repeated for
the third and fourth transactions.
20.3 CONTROLLED SUBSTANCE REPORTING REBILL RESPONSE DIAGRAMS
20.3.1 TRANSMISSION ACCEPTED/TRANSACTION CAPTURED, APPROVED, REJECTED
Controlled Substance Reporting Rebill transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “C” (Captured)
or Transaction Response Status (112-AN) of “A” (Approved)
or Transaction Response Status (112-AN) of “R” (Rejected)
Controlled Substance Reporting Rebill transactions - The “C” (Captured) event occurs after the reversal portion of the controlled substance
reporting rebill is processed successfully and the controlled substance reporting is captured for processing. If the controlled substance
reporting reversal is not processed successfully, a “R” (Rejected) response must be sent.
The duplicate response codes for the Controlled Substance Rebill transaction are not applicable.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
20.3.1.1 DIAGRAM FOR TRANSMISSION OF ONE CONTROLLED SUBSTANCE REPORTING REBILL
RESPONSE (TRANSMISSION ACCEPTED/TRANSACTION CAPTURED, APPROVED, REJECTED)
Mandatory
Response Header Segment
Optional
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
20.3.1.2 DIAGRAM FOR TRANSMISSION OF TWO CONTROLLED SUBSTANCE REPORTING REBILL
RESPONSES (TRANSMISSION ACCEPTED/TRANSACTION CAPTURED, APPROVED, REJECTED)
Mandatory
Response Header Segment

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 471 -
Optional
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
20.3.1.3 DIAGRAM FOR TRANSMISSION OF THREE OR FOUR CONTROLLED SUBSTANCE REPORTING
REBILL RESPONSES (TRANSMISSION ACCEPTED/TRANSACTION CAPTURED, APPROVED, REJECTED)
These transaction diagrams will follow the example in the section “Diagram For Transmission Of Two Controlled Substance Reporting Rebill
Responses (Transmission Accepted/Transaction Captured, Approved, Rejected)”. For three or four transactions, the Mandatory and Optional
controlled substance rebill transaction section will be repeated for the third and fourth transactions.
20.3.2 TRANSMISSION REJECTED/TRANSACTION REJECTED
Controlled Substance Reporting Rebill transmission response Header Response Status (5Ø1-F1) of "R" (Rejected)
and Transaction Response Status (112-AN) of “R” (Rejected)
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
20.3.2.1 DIAGRAM FOR TRANSMISSION OF ONE CONTROLLED SUBSTANCE REPORTING REBILL
RESPONSE (TRANSMISSION REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Optional
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
20.3.2.2 DIAGRAM FOR TRANSMISSION OF TWO CONTROLLED SUBSTANCE REPORTING REBILL
RESPONSES (TRANSMISSION REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Optional
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
20.3.2.3 DIAGRAM FOR TRANSMISSION OF THREE OR FOUR CONTROLLED SUBSTANCE REPORTING
REBILL RESPONSES (TRANSMISSION REJECTED/TRANSACTION REJECTED)
These transaction diagrams will follow the example in the section “Diagram For Transmission Of Two Controlled Substance Reporting Rebill
Responses (Transmission Rejected/Transaction Rejected)”. For three or four transactions, the Mandatory and Optional controlled substance
reporting rebill transaction segments will be repeated for the third and fourth transactions.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 472 -
21. INFORMATION REPORTING INFORMATION
Information reporting transactions allow Processors or Reporting Entities to collect information about clinical and professional services
unrelated to a dispensing event. Examples might include allergy status, or purchase of non-covered medications that have significance to the
effectiveness of on-line prospective DUR (Drug Use Review). For use of Information Reporting functionality for Medicare Part D Processing,
see Appendix “Use Of Information Reporting (N1, N2, N3) Functionality For Medicare Part D Processing”. The Pricing Segment only supports
the field Patient Paid Amount Submitted (433-DX) that is used in Medicare Part D payer-to-payer facilitation. Otherwise, the Pricing Segment is
not used.
See section “Appendix F. ORDUR (Online Real-time Drug Utilization Review)” for DUR guidance.
These transactions include:
• Information Reporting
• Information Reporting Reversal
• Information Reporting Rebill
21.1 INFORMATION REPORTING
This transaction is used to report an event to the Processor or Reporting entity.
Each Information Reporting submission request contains up to four occurrences of Claim/Service Data. The Transaction Code is “N1”.
For Medicare Part D processing only one transaction per transmission is permitted because there is a need for the sequencing of the True Out
Of Pocket (TrOOP) update before the next transaction is processed. The TrOOP should be updated before subsequent transactions are
processed.
Depending upon the particular claim or service submission request, the Processor must provide one of the following general types of
responses:
Approved - This occurs when the Processor acknowledges the receipt of the information only transaction and successfully
processes the transaction. For Medicare Part D, this means that the PDP has updated the beneficiary's TrOOP to reflect the
transaction being reported.
Duplicate of Approved - This occurs when the Processor has previously received the request and processed the transaction, but the
response did not return to the Originator. The Duplicate response contains the same information as returned in the original
Approved scenario.
Captured - This occurs when the Processor acknowledges receipt of the information reporting transaction, but no judgment is made
about the processing of the transaction. For Medicare Part D, this means that the PDP has not yet updated the beneficiary's TrOOP
to reflect the transaction being reported.
Duplicate of Captured - This occurs when the Processor has previously received the request and processed the transaction, but the
response did not return to the Originator. The Duplicate response contains the same information as returned in the original Captured
scenario.
Rejected - This occurs when the Processor has encountered an error in the transaction or processing, or does not approve the
information only transaction.
A captured response means that the transaction was valid, but the downstream system but makes no judgment about the processing of the
transaction. An approved response means the system actually processed the data (stored the DUR, processed TrOOP, etc).
An example of the difference can be found in Medicare Part D processing. An Information Reporting transaction would require that the payer
receives a Claim/Service Billing transaction sometime before the Information Reporting transaction. For this program, the payer must capture
the Information Reporting transaction when the Claim/Service Billing transaction does not exist. Normally the payer would reject the
Information Reporting. A second example is when there is not enough other payer data on the Information Reporting transaction to determine
if the dollars are TrOOP eligible and therefore the process must crosswalk the other payer data on the Information Reporting transaction to the
other payer data received from CMS via the Coordination of Benefits (COB) file. If CMS was not aware of the other payer, the processor
cannot crosswalk. In that scenario, the payer must also capture the request.
See section “Response Processing Guidelines”, “Duplicate Transactions”.
21.2 INFORMATION REPORTING REQUEST DIAGRAMS
21.2.1 DIAGRAM FOR TRANSMISSION OF ONE INFORMATION REPORTING TRANSACTION
For an Information Reporting, the scenarios defined include
Information Reporting from a Sender to a Receiver
Information Reporting Accepted – Captured/Approved/Rejected Transaction Response from a Sender to a Receiver
Standard Transmission Reject Response to an Information Reporting from a Sender to a Receiver
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 473 -
The Pricing Segment only supports the field Patient Paid Amount Submitted (433-DX) that is used in Medicare Part D payer-to-payer
facilitation. Otherwise, the Pricing Segment is not used.
Mandatory
Transaction Header Segment
Segment Separator
Insurance Segment
Situational
Segment Separator
Patient Segment
Mandatory - first Information Reporting transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Pricing Segment
Segment Separator
Clinical Segment
21.2.2 DIAGRAM FOR TRANSMISSION OF TWO INFORMATION REPORTING
TRANSACTIONS
Mandatory
Transaction Header Segment
Segment Separator
Insurance Segment
Situational
Segment Separator
Patient Segment
Mandatory - first Information Reporting transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Pricing Segment
Segment Separator
Clinical Segment
Mandatory - second Information Reporting transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 474 -
Prescriber Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Pricing Segment
Segment Separator
Clinical Segment
21.2.3 DIAGRAM FOR TRANSMISSION OF THREE INFORMATION REPORTING
TRANSACTIONS
Mandatory
Transaction Header Segment
Segment Separator
Insurance Segment
Situational
Segment Separator
Patient Segment
Mandatory - first Information Reporting transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Pricing Segment
Segment Separator
Clinical Segment
Mandatory - second Information Reporting transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Pricing Segment
Segment Separator
Clinical Segment
Mandatory – third Information Reporting transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 475 -
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Pricing Segment
Segment Separator
Clinical Segment
21.2.4 DIAGRAM FOR TRANSMISSION OF FOUR INFORMATION REPORTING
TRANSACTIONS
Mandatory
Transaction Header Segment
Segment Separator
Insurance Segment
Situational
Segment Separator
Patient Segment
Mandatory - first Information Reporting transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Pricing Segment
Segment Separator
Clinical Segment
Mandatory - second Information Reporting transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Pricing Segment
Segment Separator
Clinical Segment
Mandatory – third Information Reporting transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 476 -
DUR/PPS Segment
Segment Separator
Pricing Segment
Segment Separator
Clinical Segment
Mandatory – fourth Information Reporting transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Pricing Segment
Segment Separator
Clinical Segment
21.3 INFORMATION REPORTING REQUEST SEGMENTS
21.3.1 TRANSACTION HEADER SEGMENT (INFORMATION REPORTING)
TRANSACTION HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø1-A1 BIN NUMBER M
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø4-A4 PROCESSOR CONTROL NUMBER M
1Ø9-A9 TRANSACTION COUNT M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M
Notes on Transaction Header Segment on an Information Reporting Request:
The Transaction Header Segment is a mandatory, fixed length segment for an Information Reporting request. The “Situation” column is not
applicable.
21.3.2 INSURANCE SEGMENT (INFORMATION REPORTING)
INSURANCE SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø2-C2 CARDHOLDER ID M
312-CC CARDHOLDER FIRST NAME
Q
Information Reporting (Claim/Service):
Required if necessary for state/federal/regulatory agency
programs when the cardholder has a first name.
313-CD CARDHOLDER LAST NAME
Q Information Reporting (Claim/Service):
Required if necessary for state/federal/regulatory agency
programs.
314-CE HOME PLAN
Q Information Reporting (Claim/Service):
Required if needed for receiver reporting validation and/or
determination for Blue Cross or Blue Shield, if a Patient has
coverage under more than one plan, to distinguish each
plan.
524-FO PLAN ID
Q Information Reporting (Claim/Service):
Required if needed to identify a set of parameters, benefit,
or coverage criteria.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 477 -
INSURANCE SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
3Ø9-C9 ELIGIBILITY CLARIFICATION CODE
Q
Information Reporting (Claim/Service):
Required if needed for receiver inquiry validation and/or
determination, when eligibility is not maintained at the
dependent level.
Required in special situations as defined by the code to
clarify the eligibility of an individual, which may extend
coverage.
3Ø1-C1 GROUP ID
Q Information Reporting (Claim/Service):
Required if needed to identify the actual cardholder or
employer group, to identify appropriate group number,
when available.
3Ø3-C3 PERSON CODE
Q Information Reporting (Claim/Service):
Required if needed to uniquely identify the family members
within the Cardholder ID.
3Ø6-C6 PATIENT RELATIONSHIP CODE Q Information Reporting (Claim/Service):
Required if needed to uniquely identify the relationship of
the Patient to the Cardholder ID.
99Ø-MG OTHER PAYER BIN NUMBER Q
N
Information Reporting (Claim):
Required for Medicare Part D payer-to-payer facilitation
when necessary to match the information reporting reversal
transaction to the original information reporting transaction.
Service:
Not used.
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER Q
N
Information Reporting (Claim):
Required for Medicare Part D payer-to-payer facilitation
when necessary to match the information reporting reversal
transaction to the original information reporting transaction.
Service:
Not used.
356-NU OTHER PAYER CARDHOLDER ID Q
N
Information Reporting (Claim):
Required for Medicare Part D payer-to-payer facilitation
when necessary to match the information reporting reversal
transaction to the original information reporting transaction.
Service:
Not used.
992-MJ OTHER PAYER GROUP ID Q
N
Information Reporting (Claim):
Required for Medicare Part D payer-to-payer facilitation
when necessary to match the information reporting reversal
transaction to the original information reporting transaction.
Service:
Not used.
359-2A MEDIGAP ID Q Information Reporting (Claim/Service):
Required, if known, when patient has Medigap coverage.
36Ø-2B MEDICAID INDICATOR Q Information Reporting (Claim/Service):
Required, if known, when patient has Medicaid coverage.
361-2D PROVIDER ACCEPT ASSIGNMENT INDICATOR N Information Reporting (Claim/Service):
Not used.
997-G2 CMS PART D DEFINED QUALIFIED FACILITY Q
N
Information Reporting (Claim):
Required if specified in trading partner agreement.
Service:
Not used.
115-N5
MEDICAID ID NUMBER N Information Reporting (Claim/Service):
Not used.
116-N6
MEDICAID AGENCY NUMBER N Information Reporting (Claim/Service):
Not used.
Notes on Insurance Segment on an Information Reporting Request:
The Insurance Segment is mandatory for an Information Reporting Request. Fields defined as Mandatory are required to be submitted when
the segment is sent.
21.3.3 PATIENT SEGMENT (INFORMATION REPORTING)
PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 478 -
PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
331-CX PATIENT ID QUALIFIER Q Information Reporting (Claim/Service):
Required if Patient ID (332-CY) is used.
332-CY PATIENT ID
Q Information Reporting (Claim):
Required if necessary for state/federal/regulatory agency
programs to validate dual eligibility.
3Ø4-C4 DATE OF BIRTH
R Information Reporting (Claim/Service):
Required.
3Ø5-C5 PATIENT GENDER CODE
Q Information Reporting (Claim/Service):
Required if additional verification of the submitted eligibility
information is needed.
31Ø-CA PATIENT FIRST NAME
Q Information Reporting (Claim/Service):
Required when the patient has a first name.
311-CB PATIENT LAST NAME
Q Information Reporting (Claim/Service):
Required when the patient last name is known.
322-CM PATIENT STREET ADDRESS
Q Information Reporting (Claim/Service):
Required if needed to assist in identifying the patient when
specific eligibility cannot be established.
Required if necessary for state/federal/regulatory agency
programs.
323-CN PATIENT CITY ADDRESS
Q Information Reporting (Claim/Service):
Required if needed to assist in identifying the patient when
specific eligibility cannot be established.
Required if necessary for state/federal/regulatory agency
programs.
324-CO PATIENT STATE / PROVINCE ADDRESS
Q Information Reporting (Claim/Service):
Required if needed to assist in identifying the patient when
specific eligibility cannot be established.
Required if necessary for state/federal/regulatory agency
programs.
325-CP PATIENT ZIP/POSTAL ZONE
Q Information Reporting (Claim/Service):
Required if needed to assist in identifying the patient when
specific eligibility cannot be established.
Required if necessary for state/federal/regulatory agency
programs.
326-CQ PATIENT PHONE NUMBER
Q Information Reporting (Claim/Service):
Required if needed per trading partner agreement.
Required if necessary for state/federal/regulatory agency
programs.
3Ø7-C7 PLACE OF SERVICE
Q Information Reporting (Claim/Service):
Required if needed per trading partner agreement.
333-CZ EMPLOYER ID
Q Information Reporting (Claim/Service):
Required if necessary for state/federal/regulatory agency
programs.
Required if needed for Workers’ Compensation reporting.
334-1C SMOKER / NON-SMOKER CODE
Q Information Reporting (Claim/Service):
Required if clinical determination is dependent upon
patient’s smoking condition.
335-2C PREGNANCY INDICATOR
Q
Information Reporting (Claim/Service):
Required if clinical determination is dependent upon
patient’s pregnancy condition. Submitted until it is known
the patient is no longer pregnant.
35Ø-HN PATIENT E-MAIL ADDRESS I Information Reporting (Claim/Service):
May be submitted for the receiver to relay patient health
care communications via the Internet when provided by the
patient.
This field is informational only.
384-4X PATIENT RESIDENCE Q Information Reporting (Claim/Service):
Required if needed per trading partner agreement.
Notes on Patient Segment on an Information Reporting Request:
The Patient Segment is situational. It is used when a receiver needs some of the patient demographic information to perform Information
Reporting requirements. The Patient Segment must be submitted when needed to differentiate between the patient and the cardholder. If the
cardholder and the patient are the same, then the Patient Segment is not submitted unless additional information about the patient is needed
to clarify the Information Reporting transaction. The Segment is mandatory if required under provider payer contract or mandatory on

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 479 -
Information Reporting where this information is necessary for reporting. Fields defined as Mandatory are required to be submitted when the
segment is sent.
21.3.4 CLAIM SEGMENT (INFORMATION REPORTING)
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
436-E1 PRODUCT/SERVICE ID QUALIFIER M Mandatory.
If reporting for a multi-ingredient prescription,
Product/Service ID Qualifier (436-E1) is zero (Zero means
“ØØ”).
If the Product/Service ID Qualifier (436-E1) = “Ø6”
(DUR/PPS), the Product/Service ID (4Ø7-D7) is zero. (Zero
means “Ø”.)
4Ø7-D7 PRODUCT/SERVICE ID M Mandatory.
If reporting for a multi-ingredient prescription,
Product/Service ID (4Ø7-D7) is zero. (Zero means “Ø”.)
If the Product/Service ID Qualifier (436-E1) = “Ø6”
(DUR/PPS), the Product/Service ID (4Ø7-D7) is zero. (Zero
means “Ø”.) Populate the DUR/PPS segment as
appropriate.
If the Product/Service ID Qualifier (436-E1) = “Ø7” (CPT-4),
the Product Service ID (4Ø7-D7) is the actual CPT-4 value.
If the Product/Service ID Qualifier (436-E1) = “Ø9”
(HCPCS), the Product Service ID (4Ø7-D7) is the actual
HCPCS value.
If the Product/Service ID Qualifier (436-E1) = “99” (Other),
the Product Service ID (4Ø7-D7) is the business partner
agreed value.
456-EN ASSOCIATED PRESCRIPTION/SERVICE REFERENCE NUMBER Q Information Reporting (Claim):
Required if the “completion” transaction in a partial fill
(Dispensing Status (343-HD) = “C” (Completed)).
See section “Specific Segment Discussion”, “Request
Segments”, Claim Segment” for more information.
Required if the Dispensing Status (343-HD) = “P” (Partial
Fill) and there are multiple occurrences of partial fills for this
prescription.
Service:
Required in order to associate the service to the product.
Contains the Prescription/Service Reference Number (4Ø2-
D2) of the prescription or service that prompted the service.
Required if Associated Prescription/Service Date (457-EP)
is used.
Required if needed to associate multiple
prescriptions/services from the same sender to allow
reporting of the current prescription/service.
457-EP ASSOCIATED PRESCRIPTION/SERVICE DATE Q Information Reporting (Claim):
Required if the “completion” transaction in a partial fill
(Dispensing Status (343-HD) = “C” (Completed)).
See section “Specific Segment Discussion”, “Request
Segments”, Claim Segment” for more information.
Required if Associated Prescription/Service Reference Date
(457-EP) is used.
Required if the Dispensing Status (343-HD) = “P” (Partial
Fill) and there are multiple occurrences of partial fills for this
prescription.
Service:

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 480 -
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Required in order to associate the service to the product.
Contains the Prescription/Service Reference Number (4Ø2-
D2) of the prescription or service that prompted the service.
Required if Associated Prescription/Service Date (457-EP)
is used.
Required if needed to associate multiple
prescriptions/services from the same sender to allow
reporting of the current prescription/service.
458-SE PROCEDURE MODIFIER CODE COUNT
Q Information Reporting (Claim/Service):
Maximum count of 1Ø.
Required if Procedure Modifier Code (459-ER) is used.
459-ER PROCEDURE MODIFIER CODE Q***R*** Information Reporting (Claim/Service):
Required to define a further level of specificity if the
Product/Service ID (4Ø7-D7) indicated a Procedure Code
was submitted.
Occurs the number of times identified in Procedure Modifier
Code Count (458-SE).
Required to define a further level of specificity if the
Product/Service ID (4Ø7-D7) indicated a Procedure Code
was submitted.
442-E7 QUANTITY DISPENSED Q
Q
Information Reporting (Claim):
Required if necessary for plan benefit administration.
Service:
Required if the value is greater than zero (Ø).
4Ø3-D3 FILL NUMBER Q
Q
Information Reporting (Claim):
Required for Medicare Part D payer-to-payer facilitation.
Information Reporting (Service):
Required if necessary for plan benefit administration.
4Ø5-D5 DAYS SUPPLY Q
Q
Information Reporting (Claim):
Required if necessary for plan benefit administration.
Service:
Required if necessary for plan benefit administration.
4Ø6-D6 COMPOUND CODE Q
N
Information Reporting (Claim):
Required if necessary for plan benefit administration.
Service:
Not used.
4Ø8-D8 DISPENSE AS WRITTEN (DAW)/PRODUCT SELECTION CODE Q
N
Information Reporting (Claim):
Required if necessary for plan benefit administration.
Service:
Not used.
414-DE DATE PRESCRIPTION WRITTEN Q Information Reporting (Claim/Service):
Required if necessary for plan benefit administration.
415-DF NUMBER OF REFILLS AUTHORIZED Q Information Reporting (Claim/Service):
Required if necessary for plan benefit administration.
419-DJ PRESCRIPTION ORIGIN CODE Q
N
Information Reporting (Claim):
Required if necessary for plan benefit administration.
Service:
Not used.
354-NX SUBMISSION CLARIFICATION CODE COUNT Q
N
Information Reporting (Claim):
Maximum count of 3.
Required if Submission Clarification Code (42Ø-DK) is
used.
Service:
Not used.
42Ø-DK SUBMISSION CLARIFICATION CODE Q***R***
Information Reporting (Claim):
Required if clarification is known and values greater than
zero (Ø).
Occurs the number of times identified in Submission
Clarification Code Count (354-NX).

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 481 -
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
N
Service:
Not used.
46Ø-ET QUANTITY PRESCRIBED N
Q
Information Reporting (Claim):
Not used.
Service:
Required if the prescriber orders a specific number of
iterations of a service.
Required for values greater than one (1).
3Ø8-C8 OTHER COVERAGE CODE Q Information Reporting (Claim/Service):
Required if needed by receiver, to communicate a
summation of other coverage information that has been
collected from other payers.
Required for Coordination of Benefits.
See section “Specific Segment Discussion”, “Request
Segments”, “Claim Segment”, “Other Coverage Code (3Ø8-
C8).
429-DT SPECIAL PACKAGING INDICATOR Q
N
Information Reporting (Claim):
Required if needed per trading partner agreement.
Service:
Not used.
453-EJ ORIGINALLY PRESCRIBED PRODUCT/SERVICE ID QUALIFIER Q Information Reporting (Claim/Service):
Required if Originally Prescribed Product/Service Code
(445-EA) is used.
445-EA ORIGINALLY PRESCRIBED PRODUCT/SERVICE CODE Q Information Reporting (Claim/Service):
Required if the receiver requests association to a
therapeutic, or a preferred product substitution, or when a
DUR alert has been resolved by changing medications, or
an alternative service than what was originally prescribed.
446-EB ORIGINALLY PRESCRIBED QUANTITY Q Information Reporting (Claim/Service):
Required if the receiver requests reporting for quantity
changes due to a therapeutic substitution that has occurred
or a preferred product/service substitution that has
occurred, or when a DUR alert has been resolved by
changing quantities.
33Ø-CW ALTERNATE ID N Information Reporting (Claim/Service):
Not used.
454-EK SCHEDULED PRESCRIPTION ID NUMBER N Information Reporting (Claim/Service):
Not used.
6ØØ-28 UNIT OF MEASURE Q
N
Information Reporting (Claim):
Required if needed per trading partner agreement.
Required if necessary for state/federal/regulatory agency
programs.
Service:
Not used.
418-DI LEVEL OF SERVICE Q Information Reporting (Claim/Service):
Required if needed per trading partner agreement.
461-EU PRIOR AUTHORIZATION TYPE CODE Q Information Reporting (Claim/Service):
Required if needed per trading partner agreement.
462-EV PRIOR AUTHORIZATION NUMBER SUBMITTED Q Information Reporting (Claim/Service):
Required if needed per trading partner agreement.
463-EW INTERMEDIARY AUTHORIZATION TYPE ID Q Information Reporting (Claim/Service):
Required for overriding an authorized intermediary system
edit when the pharmacy participates with an intermediary.
Required if Intermediary Authorization ID (464-EX) is used.
464-EX INTERMEDIARY AUTHORIZATION ID Q Information Reporting (Claim/Service):
Required for overriding an authorized intermediary system
edit when the pharmacy participates with an intermediary.
343-HD DISPENSING STATUS Q
N
Information Reporting (Claim):
Required for the partial fill or the completion fill of a
prescription.
Service:
Not used.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 482 -
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
344-HF QUANTITY INTENDED TO BE DISPENSED Q
N
Information Reporting (Claim):
Required for the partial fill or the completion fill of a
prescription.
Service:
Not used.
345-HG DAYS SUPPLY INTENDED TO BE DISPENSED Q
N
Information Reporting (Claim):
Required for the partial fill or completion fill of a
prescription.
Service:
Not used.
357-NV DELAY REASON CODE Q Information Reporting (Claim/Service):
Required if needed per trading partner agreement.
88Ø-K5 TRANSACTION REFERENCE NUMBER Q
N
Information Reporting (Claim):
Required for Medicare Part D payer-to-payer facilitation to
match the transaction response to the transaction.
Service:
Not used.
391-MT PATIENT ASSIGNMENT INDICATOR (DIRECT MEMBER
REIMBURSEMENT INDICATOR)
Q Information Reporting (Claim/Service):
Required if needed per trading partner agreement.
995-E2 ROUTE OF ADMINISTRATION Q
N
Information Reporting(Claim):
Required if specified in trading partner agreement.
Service:
Not used.
996-G1 COMPOUND TYPE Q
N
Information Reporting (Claim):
Required if specified in trading partner agreement.
Service:
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Information Reporting (Claim/Service):
Not used.
147-U7 PHARMACY SERVICE TYPE N Information Reporting (Claim/Service):
Not used.
Notes on Claim Segment on an Information Reporting Request:
The Claim Segment is mandatory for an Information Reporting Request. The Claim Segment defines the product dispensed, dispensing
information, reference information for tieback to an original prescription in the case of partial fillings. Fields defined as Mandatory are required
to be submitted when the segment is sent.
21.3.5 PHARMACY PROVIDER SEGMENT (INFORMATION REPORTING)
PHARMACY PROVIDER SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
465-EY PROVIDER ID QUALIFIER Q Information Reporting (Claim/Service):
Required if Provider ID (444-E9) is used.
444-E9 PROVIDER ID
Q Information Reporting (Claim):
Required if necessary for state/federal/regulatory agency
programs.
Required if necessary to identify the individual responsible
for dispensing of the prescription.
Information Reporting (Service):
Required if necessary for state/federal/regulatory agency
programs.
Required if necessary to identify the individual responsible
for provision of the service.
Notes on Pharmacy Provider Segment on an Information Reporting Request:
The Pharmacy Provider Segment is situational for an Information Reporting Request, if required under provider payer contract or where this
information is necessary to perform or meet Information Reporting requirements. Fields defined as Mandatory are required to be submitted
when the segment is sent.
21.3.6 PRESCRIBER SEGMENT (INFORMATION REPORTING)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 483 -
PRESCRIBER SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
466-EZ PRESCRIBER ID QUALIFIER Q Information Reporting (Claim/Service):
Required if Prescriber ID (411-DB) is used.
411-DB PRESCRIBER ID
Q Information Reporting (Claim/Service):
Required if this field could result in different coverage or
patient financial responsibility.
Required if necessary for state/federal/regulatory agency
programs.
427-DR PRESCRIBER LAST NAME
Q Information Reporting (Claim/Service):
Required when the Prescriber ID (411-DB) is not known.
Required if needed for Prescriber ID (411-DB)
validation/clarification.
498-PM PRESCRIBER PHONE NUMBER Q Information Reporting (Claim/Service):
Required if needed to assist in identifying the prescriber.
Required if needed for Prior Authorization process.
468-2E PRIMARY CARE PROVIDER ID QUALIFIER Q Information Reporting (Claim/Service):
Required if Primary Care Provider ID (421-DL) is used.
421-DL PRIMARY CARE PROVIDER ID
Q Information Reporting (Claim/Service):
Required if needed per trading partner agreement.
Required if necessary for state/federal/regulatory agency
programs.
47Ø-4E PRIMARY CARE PROVIDER LAST NAME
Q Information Reporting (Claim/Service):
Required if this field is used as an alternative for Primary
Care Provider ID (421-DL) when ID is not known.
Required if needed for Primary Care Provider ID (421-DL)
validation/clarification.
364-2J PRESCRIBER FIRST NAME Q Information Reporting (Claim/Service):
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
365-2K PRESCRIBER STREET ADDRESS Q Information Reporting (Claim/Service):
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
366-2M PRESCRIBER CITY ADDRESS Q Information Reporting (Claim/Service):
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
367-2N PRESCRIBER STATE/PROVINCE ADDRESS Q Information Reporting (Claim/Service):
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
368-2P PRESCRIBER ZIP/POSTAL ZONE Q Information Reporting (Claim/Service):
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
Notes on Prescriber Segment on an Information Reporting Request:
The Prescriber Segment is situational for an Information Reporting Request. It is used when prescriber information is needed to perform or
meet Information Reporting requirements. The Segment is mandatory if required under provider payer contract or where this information is
necessary for reporting. Fields defined as Mandatory are required to be submitted when the segment is sent.
21.3.7 WORKERS’ COMPENSATION SEGMENT (INFORMATION REPORTING)
WORKERS’ COMPENSATION SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
434-DY DATE OF INJURY M
315-CF EMPLOYER NAME Q Information Reporting (Claim/Service):
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 484 -
WORKERS’ COMPENSATION SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if needed to process an information reporting
transaction for a work related injury or condition.
316-CG EMPLOYER STREET ADDRESS Q Information Reporting (Claim/Service):
Required if needed to process an information reporting
transaction for a work related injury or condition.
317-CH EMPLOYER CITY ADDRESS Q Information Reporting (Claim/Service):
Required if needed to process an information reporting
transaction for a work related injury or condition.
318-CI EMPLOYER STATE/PROVINCE ADDRESS Q Information Reporting (Claim/Service):
Required if needed to process an information reporting
transaction for a work related injury or condition.
319-CJ EMPLOYER ZIP/POSTAL ZONE Q Information Reporting (Claim/Service):
Required if needed to process an information reporting
transaction for a work related injury or condition.
32Ø-CK EMPLOYER PHONE NUMBER Q Information Reporting (Claim/Service):
Required if needed to process an information reporting
transaction for a work related injury or condition.
321-CL EMPLOYER CONTACT NAME Q Information Reporting (Claim/Service):
Required if needed to process an information reporting
transaction for a work related injury or condition.
327-CR CARRIER ID Q Information Reporting (Claim/Service):
Required if needed to process an information reporting
transaction for a work related injury or condition.
435-DZ CLAIM/REFERENCE ID Q Information Reporting (Claim/Service):
Required if needed to process an information reporting
transaction for a work related injury or condition.
117-TR BILLING ENTITY TYPE INDICATOR N Information Reporting (Claim/Service):
Not used.
118-TS PAY TO QUALIFIER N Information Reporting (Claim/Service):
Not used.
119-TT PAY TO ID N Information Reporting (Claim/Service):
Not used.
12Ø-TU PAY TO NAME N Information Reporting (Claim/Service):
Not used.
121-TV PAY TO STREET ADDRESS N Information Reporting (Claim/Service):
Not used.
122-TW PAY TO CITY ADDRESS N Information Reporting (Claim/Service):
Not used.
123-TX PAY TO STATE/PROVINCE ADDRESS N Information Reporting (Claim/Service):
Not used.
124-TY PAY TO ZIP/POSTAL ZONE N Information Reporting (Claim/Service):
Not used.
125-TZ GENERIC EQUIVALENT PRODUCT ID QUALIFIER N Information Reporting (Claim/Service):
Not used.
126-UA GENERIC EQUIVALENT PRODUCT ID N Information Reporting (Claim/Service):
Not used.
Notes on Workers’ Compensation Segment on an Information Reporting Request:
The Workers’ Compensation Segment is situational for an Information Reporting request. It is used when processing an Information Reporting
request for a work-related injury or condition. Fields defined as Mandatory are required to be submitted when the segment is sent.
21.3.8 DUR/PPS SEGMENT (INFORMATION REPORTING)
DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
473-7E DUR/PPS CODE COUNTER
Q***R*** Information Reporting (Claim/Service):
Maximum of 9 occurrences.
Required if DUR/PPS Segment is used.
439-E4 REASON FOR SERVICE CODE
Q***R*** Information Reporting (Claim):
Required if this field could result in different drug utilization
review outcome.
Service:
Required if this field affects documentation of professional
pharmacy service.
44Ø-E5 PROFESSIONAL SERVICE CODE
Q***R*** Information Reporting (Claim):
Required if this field could result in different drug utilization
review outcome.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 485 -
DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Service:
Required if this field affects documentation of professional
pharmacy service.
441-E6 RESULT OF SERVICE CODE
Q***R*** Information Reporting (Claim):
Required if this field could result in different drug utilization
review outcome.
Service:
Required if this field affects documentation of professional
pharmacy service.
474-8E DUR/PPS LEVEL OF EFFORT
Q***R*** Information Reporting (Claim):
Required if this field could result in different drug utilization
review outcome.
Service:
Required if this field affects documentation of professional
pharmacy service.
475-J9 DUR CO-AGENT ID QUALIFIER Q***R*** Information Reporting (Claim/Service):
Required if DUR Co-Agent ID Qualifier (475-J9) is used.
476-H6 DUR CO-AGENT ID
Q***R*** Information Reporting (Claim):
Required if this field could result in different drug utilization
review outcome.
Service:
Required if this field affects documentation of professional
pharmacy service.
Notes on DUR/PPS Segment on an Information Reporting Request:
The DUR/PPS Segment is situational for an Information Reporting request. It is used when a sender notifies the receiver of drug utilization,
drug evaluations, or information on the appropriate selection to process Information Reporting. The DUR/PPS information may be sent on the
initial submission or alternatively sent after a DUR/PPS rejection from a receiver. The Segment is mandatory if required under provider payer
contract or where this information is necessary for processing the reporting. Fields defined as Mandatory are required to be submitted when
the segment is sent.
21.3.9 PRICING SEGMENT (INFORMATION REPORTING)
PRICING SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
4Ø9-D9 INGREDIENT COST SUBMITTED N Information Reporting (Claim/Service):
Not used.
412-DC DISPENSING FEE SUBMITTED N Information Reporting (Claim/Service):
Not used.
477-BE PROFESSIONAL SERVICE FEE SUBMITTED N Information Reporting (Claim/Service):
Not used.
433-DX PATIENT PAID AMOUNT SUBMITTED Q Information Reporting (Claim/Service):
Required for Medicare Part D payer-to-payer facilitation.
438-E3 INCENTIVE AMOUNT SUBMITTED N Information Reporting (Claim/Service):
Not used.
478-H7 OTHER AMOUNT CLAIMED SUBMITTED COUNT
N Information Reporting (Claim/Service):
Not used.
479-H8 OTHER AMOUNT CLAIMED SUBMITTED QUALIFIER
N***R*** Information Reporting (Claim/Service):
Not used.
48Ø-H9 OTHER AMOUNT CLAIMED SUBMITTED
N***R*** Information Reporting (Claim/Service):
Not used.
481-HA FLAT SALES TAX AMOUNT SUBMITTED
N Information Reporting (Claim/Service):
Not used.
482-GE PERCENTAGE SALES TAX AMOUNT SUBMITTED
N Information Reporting (Claim/Service):
Not used.
483-HE PERCENTAGE SALES TAX RATE SUBMITTED
N Information Reporting (Claim/Service):
Not used.
484-JE PERCENTAGE SALES TAX BASIS SUBMITTED
N Information Reporting (Claim/Service):
Not used.
426-DQ USUAL AND CUSTOMARY CHARGE
N Information Reporting (Claim/Service):
Not used.
43Ø-DU GROSS AMOUNT DUE N Information Reporting (Claim/Service):
Not used.
423-DN BASIS OF COST DETERMINATION N Information Reporting (Claim/Service):
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 486 -
PRICING SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Not used.
113-N3
MEDICAID PAID AMOUNT N Information Reporting (Claim/Service):
Not used.
Notes on Pricing Segment on an Information Reporting Request:
The Pricing Segment is situational for an Information Reporting Request. The Pricing Segment only supports the field Patient Paid Amount
Submitted (433-DX) that is used in Medicare Part D payer-to-payer facilitation. Otherwise, the Pricing Segment is not used. Fields defined as
Mandatory are required to be submitted when the segment is sent.
21.3.10 CLINICAL SEGMENT (INFORMATION REPORTING)
CLINICAL SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
491-VE DIAGNOSIS CODE COUNT Q Information Reporting (Claim/Service):
Maximum count of 5.
Required if Diagnosis Code Qualifier (492-WE) and
Diagnosis Code (424-DO) are used.
492-WE DIAGNOSIS CODE QUALIFIER Q***R*** Information Reporting (Claim/Service):
Required if Diagnosis Code (424-DO) is used.
424-DO DIAGNOSIS CODE
Q***R*** Information Reporting (Claim/Service):
The value for this field is obtained from the prescriber or
authorized representative.
Required if this field was reported on the original
adjudicated transaction.
Required if this field could result in different drug utilization
review outcome.
Required if this information can be used in place of prior
authorization.
Required if necessary for state/federal/regulatory agency
programs.
493-XE CLINICAL INFORMATION COUNTER Q***R*** Information Reporting (Claim/Service):
Maximum 5 occurrences supported.
Grouped with Measurement fields (Measurement Date
(494-ZE), Measurement Time (495-H1), Measurement
Dimension (496-H2), Measurement Unit (497-H3),
Measurement Value (499-H4).
494-ZE MEASUREMENT DATE Q***R*** Information Reporting (Claim/Service):
Required if necessary when this field could result in
different drug utilization review outcome.
495-H1 MEASUREMENT TIME Q***R*** Information Reporting (Claim/Service):
Required if Time is known or has impact on measurement.
Required if necessary when this field could result in drug
utilization review outcome.
496-H2 MEASUREMENT DIMENSION Q***R*** Information Reporting (Claim/Service):
Required if Measurement Unit (497-H3) and Measurement
Value (499-H4) are used.
Required if necessary when this field could result in
different drug utilization review outcome.
497-H3 MEASUREMENT UNIT Q***R*** Information Reporting (Claim/Service):
Required if Measurement Dimension (496-H2) and
Measurement Value (499-H4) are used.
Required if necessary when this field could result in
different drug utilization review outcome.
499-H4 MEASUREMENT VALUE Q***R*** Information Reporting (Claim/Service):
Required if Measurement Dimension (496-H2) and
Measurement Unit (497-H3) are used.
Required if necessary when this field could result in
different drug utilization review outcome.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 487 -
Notes on Clinical Segment on an Information Reporting Request:
The Clinical Segment is situational on an Information Reporting request. It is used to specify clinical measurements and/or diagnosis
information associated with the Information Reporting transaction. The Segment is mandatory if required under provider payer contract or
where this information is necessary for reporting. Fields defined as Mandatory are required to be submitted when the segment is sent.
21.4 INFORMATION REPORTING RESPONSE DIAGRAMS AND SEGMENTS
21.4.1 TRANSMISSION ACCEPTED/TRANSACTION CAPTURED
Information Reporting transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured)
The captured response is applicable when the receiver acknowledges receipt, but does not fully process the Information Reporting transaction.
In Medicare Part D payer-to-payer facilitation, no TrOOP is updated on a captured response.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
21.4.1.1 DIAGRAM FOR TRANSMISSION OF ONE INFORMATION REPORTING RESPONSE
(TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
21.4.1.2 DIAGRAM FOR TRANSMISSION OF TWO INFORMATION REPORTING RESPONSES
(TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 488 -
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
21.4.1.3 DIAGRAM FOR TRANSMISSION OF THREE INFORMATION REPORTING RESPONSES
(TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
21.4.1.4 DIAGRAM FOR TRANSMISSION OF FOUR INFORMATION REPORTING RESPONSES
(TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 489 -
Situational
Segment Separator
Response DUR/PPS Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
21.4.1.5 INFORMATION REPORTING RESPONSE SEGMENTS (TRANSMISSION ACCEPTED/TRANSACTION
CAPTURED)
21.4.1.5.1 RESPONSE HEADER SEGMENT (INFORMATION REPORTING) (TRANSMISSION ACCEPTED/TRANSACTION
CAPTURED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on an Information Reporting Response:
The Response Header Segment is a mandatory, fixed length segment for an Information Reporting response when the Header Response
Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). The
“Situation” column is not applicable.
21.4.1.5.2 RESPONSE MESSAGE SEGMENT (INFORMATION REPORTING) (TRANSMISSION ACCEPTED/TRANSACTION
CAPTURED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Information Reporting (Claim/Service):
Required if text is needed for clarification or detail.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 490 -
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on an Information Reporting Response:
The Response Message Segment is situational for an Information Reporting response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). It is used when additional text
information needs to be sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
21.4.1.5.3 RESPONSE INSURANCE SEGMENT (INFORMATION REPORTING) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø1-C1 GROUP ID
Q Information Reporting (Claim/Service):
Required if needed to identify the cardholder or employer
group, to identify appropriate group number for reporting.
Required to identify the actual group that was used when
multiple group coverages exist.
Note: This field may contain the Group ID echoed from the
request. May contain the actual Group ID if unknown to the
receiver.
524-FO PLAN ID
Q Information Reporting (Claim/Service):
Required to identify the actual plan ID that was used when
multiple group coverages exist.
Required if needed to contain the actual plan ID if unknown
to the receiver.
545-2F NETWORK REIMBURSEMENT ID
N Information Reporting (Claim/Service):
Not used.
568-J7 PAYER ID QUALIFIER N Information Reporting (Claim/Service):
Not used.
569-J8 PAYER ID
N Information Reporting (Claim/Service):
Not used.
115-N5
MEDICAID ID NUMBER N Information Reporting (Claim/Service):
Not used.
116-N6
MEDICAID AGENCY NUMBER N Information Reporting (Claim/Service):
Not used.
3Ø2-C2
CARDHOLDER ID N Information Reporting (Claim/Service):
Not used.
Notes on Response Insurance Segment on an Information Reporting Response:
The Response Insurance Segment is situational for an Information Reporting response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). It is used when coverage information
may be provided from the sender to the receiver. Fields defined as Mandatory are required to be submitted when the segment is sent.
21.4.1.5.4 RESPONSE PATIENT SEGMENT (INFORMATION REPORTING) (TRANSMISSION ACCEPTED/TRANSACTION
CAPTURED)
RESPONSE PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 491 -
RESPONSE PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
31Ø-CA PATIENT FIRST NAME Q Information Reporting (Claim/Service):
Required if known.
311-CB PATIENT LAST NAME Q Information Reporting (Claim/Service):
Required if known.
3Ø4-C4 DATE OF BIRTH Q Information Reporting (Claim/Service):
Required if known.
Notes on Response Patient Segment on an Information Reporting Response:
The Response Patient Segment is situational for Information Reporting transmission response Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured) when patient demographic information
needs to be sent from the sender to the receiver. Fields defined as Mandatory are required to be submitted when the segment is sent.
21.4.1.5.5 RESPONSE STATUS SEGMENT (INFORMATION REPORTING) (TRANSMISSION ACCEPTED/TRANSACTION
CAPTURED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Information Reporting (Claim/Service):
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT N Information Reporting (Claim/Service):
Not used.
511-FB REJECT CODE N***R*** Information Reporting (Claim/Service):
Not used.
546-4F REJECT FIELD OCCURRENCE INDICATOR N***R*** Information Reporting (Claim/Service):
Not used.
547-5F APPROVED MESSAGE CODE COUNT N Information Reporting (Claim/Service):
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Information Reporting (Claim/Service):
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Information Reporting (Claim/Service):
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Information Reporting (Claim/Service):
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Information Reporting (Claim/Service):
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Information Reporting (Claim/Service):
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 492 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Information Reporting (Claim/Service):
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Information Reporting (Claim/Service):
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER Q Information Reporting (Claim):
Required for Medicare Part D payer-to-payer facilitation to
match the transaction response to the transaction.
Service:
Not used.
993-A7 INTERNAL CONTROL NUMBER N Information Reporting (Claim/Service):
Not used.
987-MA URL N Information Reporting (Claim/Service):
Not used.
Notes on Response Status Segment on an Information Reporting Response:
The Response Status Segment is mandatory for an Information Reporting response for Header Response Status (5Ø1-F1) = “A” (Accepted)
and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). The Response Status Segment is sent from the
sender to the receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is
sent.
21.4.1.5.6 RESPONSE CLAIM SEGMENT (INFORMATION REPORTING) (TRANSMISSION ACCEPTED/TRANSACTION
CAPTURED)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT N Information Reporting (Claim/Service):
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER N***R*** Information Reporting (Claim/Service):
Not used.
553-AR PREFERRED PRODUCT ID
N***R*** Information Reporting (Claim/Service):
Not used.
554-AS PREFERRED PRODUCT INCENTIVE
N***R*** Information Reporting (Claim/Service):
Not used.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE
N***R*** Information Reporting (Claim/Service):
Not used.
556-AU PREFERRED PRODUCT DESCRIPTION N***R*** Information Reporting (Claim/Service):
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Information Reporting (Claim/Service):
Not used.
Notes on Response Claim Segment on an Information Reporting Response:
The Response Claim Segment is mandatory for an Information Reporting response when the Header Response Status (5Ø1-F1) is “A”
(Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured).
The Response Claim Segment (Information Reporting – Service) is sent from the sender to the receiver to mirror back the Prescription/Service
Reference Number (4Ø2-D2).
Fields defined as Mandatory are required to be submitted when the segment is sent.
21.4.1.5.7 RESPONSE DUR/PPS SEGMENT (INFORMATION REPORTING) (TRANSMISSION ACCEPTED/TRANSACTION
CAPTURED)
RESPONSE DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
567-J6 DUR/PPS RESPONSE CODE COUNTER Q***R*** Information Reporting (Claim/Service):
Maximum 9 occurrences supported.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 493 -
RESPONSE DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if Reason For Service Code (439-E4) is used.
439-E4 REASON FOR SERVICE CODE
Q***R*** Information Reporting (Claim):
Required if utilization conflict is detected.
Service:
Required if professional service opportunity reason is
detected by the receiver. Should be different than the
original transmission.
528-FS CLINICAL SIGNIFICANCE CODE
Q***R*** Information Reporting (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
529-FT OTHER PHARMACY INDICATOR
Q***R*** Information Reporting (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
53Ø-FU PREVIOUS DATE OF FILL
Q***R*** Information Reporting (Claim):
Required if needed to supply additional information for the
utilization conflict.
Required if Quantity of Previous Fill (531-FV) is used.
Service:
Required if needed to supply additional information for the
service.
Required if Quantity of Previous Fill (531-FV) is used.
531-FV QUANTITY OF PREVIOUS FILL
Q***R*** Information Reporting (Claim):
Required if Previous Date Of Fill (53Ø-FU) is used.
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
Required if Previous Date Of Fill (53Ø-FU) is used.
532-FW DATABASE INDICATOR
Q***R*** Information Reporting (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
533-FX OTHER PRESCRIBER INDICATOR
Q***R*** Information Reporting (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
544-FY DUR FREE TEXT MESSAGE
Q***R*** Information Reporting (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
57Ø-NS DUR ADDITIONAL TEXT Q***R*** Information Reporting (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 494 -
Notes on Response DUR/PPS Segment on an Information Reporting Response:
The Response DUR/PPS Segment is situational for an Information Reporting response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). The Response DUR/PPS Segment is
used to identify a drug utilization review or professional pharmacy service event, opportunity, or information.Fields defined as Mandatory are
required to be submitted when the segment is sent.
21.4.2 TRANSMISSION ACCEPTED/TRANSACTION APPROVED
Information Reporting transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved)
21.4.2.1 DIAGRAM FOR TRANSMISSION OF ONE INFORMATION REPORTING RESPONSE
(TRANSMISSION ACCEPTED/TRANSACTION APPROVED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
21.4.2.2 DIAGRAM FOR TRANSMISSION OF TWO INFORMATION REPORTING RESPONSES
(TRANSMISSION ACCEPTED/TRANSACTION APPROVED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 495 -
21.4.2.3 DIAGRAM FOR TRANSMISSION OF THREE INFORMATION REPORTING RESPONSES
(TRANSMISSION ACCEPTED/TRANSACTION APPROVED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
21.4.2.4 DIAGRAM FOR TRANSMISSION OF FOUR INFORMATION REPORTING RESPONSES
(TRANSMISSION ACCEPTED/TRANSACTION APPROVED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Mandatory second response
Group Separator
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 496 -
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
21.4.2.5 INFORMATION REPORTING RESPONSE SEGMENTS (TRANSMISSION ACCEPTED/TRANSACTION
APPROVED)
21.4.2.5.1 RESPONSE HEADER SEGMENT (INFORMATION REPORTING) (TRANSMISSION ACCEPTED/TRANSACTION
APPROVED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on an Information Reporting Response:
The Response Header Segment is a mandatory, fixed length segment for an Information Reporting response when the Header Response
Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved). The
“Situation” column is not applicable.
21.4.2.5.2 RESPONSE MESSAGE SEGMENT (INFORMATION REPORTING) (TRANSMISSION ACCEPTED/TRANSACTION
APPROVED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Information Reporting (Claim/Service):
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 497 -
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on an Information Reporting Response:
The Response Message Segment is situational for an Information Reporting response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved). It is used when additional text
information needs to be sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
21.4.2.5.3 RESPONSE INSURANCE SEGMENT (INFORMATION REPORTING) (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø1-C1 GROUP ID
Q Information Reporting (Claim/Service):
Required if needed to identify the cardholder or employer
group, to identify appropriate group number for reporting.
Required to identify the actual group that was used when
multiple group coverages exist.
Note: This field may contain the Group ID echoed from the
request. May contain the actual Group ID if unknown to the
receiver.
524-FO PLAN ID
Q Information Reporting (Claim/Service):
Required to identify the actual plan ID that was used when
multiple group coverages exist.
Required if needed to contain the actual plan ID if unknown
to the receiver.
545-2F NETWORK REIMBURSEMENT ID
S Information Reporting (Claim/Service):
Not used.
568-J7 PAYER ID QUALIFIER S Information Reporting (Claim/Service):
Not used.
569-J8 PAYER ID
S Information Reporting (Claim/Service):
Not used.
115-N5
MEDICAID ID NUMBER S Information Reporting (Claim/Service):
Not used.
116-N6
MEDICAID AGENCY NUMBER S Information Reporting (Claim/Service):
Not used.
3Ø2-C2
CARDHOLDER ID S Information Reporting (Claim/Service):
Required if the identification to be used in future
transactions is different than what was submitted on the
request.
Notes on Response Insurance Segment on an Information Reporting or Response:
The Response Insurance Segment is situational for an Information Reporting response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved). It is used when coverage information
may be provided from the sender to the receiver. Fields defined as Mandatory are required to be submitted when the segment is sent.
21.4.2.5.4 RESPONSE PATIENT SEGMENT (INFORMATION REPORTING) (TRANSMISSION ACCEPTED/TRANSACTION
APPROVED)
RESPONSE PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
31Ø-CA PATIENT FIRST NAME Q Information Reporting (Claim/Service):
Required if known.
311-CB PATIENT LAST NAME Q Information Reporting (Claim/Service):
Required if known.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 498 -
RESPONSE PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
3Ø4-C4 DATE OF BIRTH Q Information Reporting (Claim/Service):
Required if known.
Notes on Response Patient Segment on an Information Reporting Response:
The Response Patient Segment is situational for Information Reporting transmission response Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved) when patient demographic
information needs to be sent from the sender to the receiver. Fields defined as Mandatory are required to be submitted when the segment is
sent.
21.4.2.5.5 RESPONSE STATUS SEGMENT (INFORMATION REPORTING) (TRANSMISSION ACCEPTED/TRANSACTION
APPROVED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Information Reporting (Claim/Service):
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT N Information Reporting (Claim/Service):
Not used.
511-FB REJECT CODE N***R*** Information Reporting (Claim/Service):
Not used.
546-4F REJECT FIELD OCCURRENCE INDICATOR N***R*** Information Reporting (Claim/Service):
Not used.
547-5F APPROVED MESSAGE CODE COUNT N Information Reporting (Claim/Service):
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Information Reporting (Claim/Service):
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Information Reporting (Claim/Service):
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Information Reporting (Claim/Service):
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Information Reporting (Claim/Service):
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Information Reporting (Claim/Service):
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Information Reporting (Claim/Service):
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER Q Information Reporting (Claim/Service):

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 499 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER Q
N
Information Reporting (Claim):
Required for Medicare Part D payer-to-payer facilitation to
match the transaction response to the transaction.
Service:
Not used.
993-A7 INTERNAL CONTROL NUMBER N Information Reporting (Claim/Service):
Not used.
987-MA URL N Information Reporting (Claim/Service):
Not used.
Notes on Response Status Segment on an Information Reporting Response:
The Response Status Segment is mandatory for an Information Reporting response for Header Response Status (5Ø1-F1) = “A” (Accepted)
and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved). The Response Status Segment is sent from the
sender to the receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is
sent.
21.4.2.5.6 RESPONSE CLAIM SEGMENT (INFORMATION REPORTING) (TRANSMISSION ACCEPTED/TRANSACTION
APPROVED)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT N Information Reporting (Claim/Service):
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER N***R*** Information Reporting (Claim/Service):
Not used.
553-AR PREFERRED PRODUCT ID
N***R*** Information Reporting (Claim/Service):
Not used.
554-AS PREFERRED PRODUCT INCENTIVE
N***R*** Information Reporting (Claim/Service):
Not used.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE
N***R*** Information Reporting (Claim/Service):
Not used.
556-AU PREFERRED PRODUCT DESCRIPTION N***R*** Information Reporting (Claim/Service):
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN) N Information Reporting (Claim/Service):
Not used.
Notes on Response Claim Segment on an Information Reporting Response:
The Response Claim Segment is mandatory for an Information Reporting response when the Header Response Status (5Ø1-F1) is “A”
(Accepted) and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved).
The Response Claim Segment (Information Reporting – Service) is sent from the sender to the receiver to mirror back the Prescription/Service
Reference Number (4Ø2-D2).
Fields defined as Mandatory are required to be submitted when the segment is sent.
21.4.2.5.7 RESPONSE DUR/PPS SEGMENT (INFORMATION REPORTING) (TRANSMISSION ACCEPTED/TRANSACTION
APPROVED)
RESPONSE DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
567-J6 DUR/PPS RESPONSE CODE COUNTER Q***R*** Information Reporting (Claim/Service):
Maximum 9 occurrences supported.
Required if Reason For Service Code (439-E4) is used.
439-E4 REASON FOR SERVICE CODE
Q***R*** Information Reporting (Claim):
Required if utilization conflict is detected.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 500 -
RESPONSE DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Service:
Required if professional service opportunity reason is
detected by the receiver. Should be different than the
original transmission.
528-FS CLINICAL SIGNIFICANCE CODE
Q***R*** Information Reporting (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
529-FT OTHER PHARMACY INDICATOR
Q***R*** Information Reporting (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
53Ø-FU PREVIOUS DATE OF FILL
Q***R*** Information Reporting (Claim):
Required if needed to supply additional information for the
utilization conflict.
Required if Quantity of Previous Fill (531-FV) is used.
Service:
Required if needed to supply additional information for the
service.
Required if Quantity of Previous Fill (531-FV) is used.
531-FV QUANTITY OF PREVIOUS FILL
Q***R*** Information Reporting (Claim):
Required if Previous Date Of Fill (53Ø-FU) is used.
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
Required if Previous Date Of Fill (53Ø-FU) is used.
532-FW DATABASE INDICATOR
Q***R*** Information Reporting (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
533-FX OTHER PRESCRIBER INDICATOR
Q***R*** Information Reporting (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
544-FY DUR FREE TEXT MESSAGE
Q***R*** Information Reporting (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
57Ø-NS DUR ADDITIONAL TEXT Q***R*** Information Reporting (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
Notes on Response DUR/PPS Segment on an Information Reporting Response:
The Response DUR/PPS Segment is situational for an Information Reporting response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved). The Response DUR/PPS Segment is
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 501 -
used to identify a drug utilization review or professional pharmacy service event, opportunity, or information. Fields defined as Mandatory are
required to be submitted when the segment is sent.
21.4.3 TRANSMISSION ACCEPTED/TRANSACTION REJECTED
Information Reporting transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “R” (Rejected)
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
21.4.3.1 DIAGRAM FOR TRANSMISSION OF ONE INFORMATION REPORTING RESPONSE
(TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
There are no situational transaction-level segments for Information Reporting transmission response Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “R” (Rejected).
21.4.3.2 DIAGRAM FOR TRANSMISSION OF TWO INFORMATION REPORTING RESPONSES
(TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
21.4.3.3 DIAGRAM FOR TRANSMISSION OF THREE INFORMATION REPORTING RESPONSES
(TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 502 -
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
21.4.3.4 DIAGRAM FOR TRANSMISSION OF FOUR INFORMATION REPORTING RESPONSES
(TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
21.4.3.5 INFORMATION REPORTING RESPONSE SEGMENTS (TRANSMISSION ACCEPTED/TRANSACTION
REJECTED)
21.4.3.5.1 RESPONSE HEADER SEGMENT (INFORMATION REPORTING) (TRANSMISSION ACCEPTED/TRANSACTION
REJECTED)

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 503 -
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on an Information Reporting Response:
The Response Header Segment is a mandatory, fixed length segment for an Information Reporting response when the Header Response
Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). The “Situation” column is not applicable.
21.4.3.5.2 RESPONSE MESSAGE SEGMENT (INFORMATION REPORTING) (TRANSMISSION ACCEPTED/TRANSACTION
REJECTED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Information Reporting (Claim/Service):
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on an Information Reporting Response:
The Response Message Segment is situational for an Information Reporting response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). It is used when additional text information needs to be sent. Fields
defined as Mandatory are required to be submitted when the segment is sent.
21.4.3.5.3 RESPONSE INSURANCE SEGMENT (INFORMATION REPORTING) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø1-C1 GROUP ID
Q Information Reporting (Claim/Service):
Required if needed to identify the cardholder or employer
group, to identify appropriate group number for reporting.
Required to identify the actual group that was used when
multiple group coverages exist.
Note: This field may contain the Group ID echoed from the
request. May contain the actual Group ID if unknown to the
receiver.
524-FO PLAN ID
Q Information Reporting (Claim/Service):
Required if needed to identify the actual plan parameters,
benefit, or coverage criteria, when available.
Required to identify the actual plan ID that was used when
multiple group coverages exist.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 504 -
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if needed to contain the actual plan ID if unknown
to the receiver.
545-2F NETWORK REIMBURSEMENT ID
N Information Reporting (Claim/Service):
Not used.
568-J7 PAYER ID QUALIFIER Q Information Reporting (Claim/Service):
Required if Payer ID (569-J8) is used.
569-J8 PAYER ID
Q Information Reporting (Claim/Service):
Required to identify the ID of the payer responding.
115-N5
MEDICAID ID NUMBER N Information Reporting (Claim/Service):
Not used.
116-N6
MEDICAID AGENCY NUMBER N Information Reporting (Claim/Service):
Not used.
3Ø2-C2
CARDHOLDER ID Q Information Reporting (Claim/Service):
Required if the identification to be used in future
transactions is different than what was submitted on the
request.
Notes on Response Insurance Segment on an Information Reporting Response:
The Response Insurance Segment is situational for an Information Reporting or response when the Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). It is used when coverage or reimbursement parameters or identifiers
need to be sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
21.4.3.5.4 RESPONSE PATIENT SEGMENT (INFORMATION REPORTING) (TRANSMISSION ACCEPTED/TRANSACTION
REJECTED)
RESPONSE PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
31Ø-CA PATIENT FIRST NAME Q Information Reporting (Claim/Service):
Required if known.
311-CB PATIENT LAST NAME Q Information Reporting (Claim/Service):
Required if known.
3Ø4-C4 DATE OF BIRTH Q Information Reporting (Claim/Service):
Required if known.
Notes on Response Patient Segment on an Information Reporting Response:
The Response Patient Segment is situational for Information Reporting transmission response Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “R” (Rejected) when patient demographic information needs to be sent from the
sender to the receiver. Fields defined as Mandatory are required to be submitted when the segment is sent.
21.4.3.5.5 RESPONSE STATUS SEGMENT (INFORMATION REPORTING) (TRANSMISSION ACCEPTED/TRANSACTION
REJECTED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Information Reporting (Claim/Service):
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT R Information Reporting (Claim/Service):
Maximum count of 5.
Required.
511-FB REJECT CODE R***R*** Information Reporting (Claim/Service):
Required.
546-4F REJECT FIELD OCCURRENCE INDICATOR Q***R*** Information Reporting (Claim/Service):
Required if a repeating field is in error, to identify repeating
field occurrence.
This field must be sent when relaying error information about
a repeating field or set. Note, if the Reject Code is not
denoting a repeating field or set, the Reject Field Occurrence
Indicator must not be sent.
547-5F APPROVED MESSAGE CODE COUNT N Information Reporting (Claim/Service):
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Information Reporting (Claim/Service):

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 505 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Information Reporting (Claim/Service):
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Information Reporting (Claim/Service):
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Information Reporting (Claim/Service):
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Information Reporting (Claim/Service):
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Information Reporting (Claim/Service):
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Information Reporting (Claim/Service):
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER Q
N
Information Reporting (Claim):
Required for Medicare Part D payer-to-payer facilitation to
match the transaction response to the transaction.
Service:
Not used.
993-A7 INTERNAL CONTROL NUMBER N Information Reporting (Claim/Service):
Not used.
987-MA URL N Information Reporting (Claim/Service):
Not used.
Notes on Response Status Segment on an Information Reporting Response:
The Response Status Segment is mandatory for an Information Reporting response for Header Response Status (5Ø1-F1) = “A” (Accepted)
and Transaction Response Status (112-AN) of “R” (Rejected). The Response Status Segment is sent from the sender to the receiver to
identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.
21.4.3.5.6 RESPONSE CLAIM SEGMENT (INFORMATION REPORTING) (TRANSMISSION ACCEPTED/TRANSACTION
REJECTED)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 506 -
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT N Information Reporting (Claim/Service):
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER N***R*** Information Reporting (Claim/Service):
Not used.
553-AR PREFERRED PRODUCT ID
N***R*** I Information Reporting (Claim/Service):
Not used.
554-AS PREFERRED PRODUCT INCENTIVE
N***R*** Information Reporting (Claim/Service):
Not used.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE N***R*** Information Reporting (Claim/Service):
Not used.
556-AU PREFERRED PRODUCT DESCRIPTION N***R*** Information Reporting (Claim/Service):
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Information Reporting (Claim/Service):
Not used.
Notes on Response Claim Segment on an Information Reporting Response:
The Response Claim Segment is mandatory for an Information Reporting response when the Header Response Status (5Ø1-F1) is “A”
(Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). Fields defined as Mandatory are required to be submitted when the
segment is sent.
21.4.4 TRANSMISSION REJECTED/TRANSACTION REJECTED
Information Reporting transmission response Header Response Status (5Ø1-F1) of "R" (Rejected)
and Transaction Response Status (112-AN) of “R” (Rejected)
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
21.4.4.1 DIAGRAM FOR TRANSMISSION OF ONE INFORMATION REPORTING RESPONSE
(TRANSMISSION REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
There are no situational transaction-level segments for Information Reporting transmission response Header Response Status (5Ø1-F1) of "R"
(Rejected) and Transaction Response Status (112-AN) of “R” (Rejected).
21.4.4.2 DIAGRAM FOR TRANSMISSION OF TWO INFORMATION REPORTING RESPONSES
(TRANSMISSION REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
21.4.4.3 DIAGRAM FOR TRANSMISSION OF THREE INFORMATION REPORTING RESPONSES
(TRANSMISSION REJECTED/TRANSACTION REJECTED)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 507 -
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
21.4.4.4 DIAGRAM FOR TRANSMISSION OF FOUR INFORMATION REPORTING RESPONSES
(TRANSMISSION REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
21.4.4.5 INFORMATION REPORTING RESPONSE SEGMENTS (TRANSMISSION REJECTED/TRANSACTION
REJECTED)
21.4.4.5.1 IN RESPONSE HEADER SEGMENT (INFORMATION REPORTING) (TRANSMISSION REJECTED/TRANSACTION
REJECTED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 508 -
Notes on Response Header Segment on an Information Reporting Response:
The Response Header Segment is a mandatory, fixed length segment for an Information Reporting response when the Header Response
Status (5Ø1-F1) of "R" (Rejected) and Transaction Response Status (112-AN) of “R” (Rejected). The “Situation” column is not applicable.
If either the entire transmission or the Header is in error, the Header Response Status (5Ø1-F1) = “R” (Rejected). Every identifiable transaction
within the transmission must be rejected with an “R”.
If the transaction rejects for detail errors, the Header Response Status (5Ø1-F1) = “A” (Accepted) and the Transaction Response Status (112-
AN) will be “R”.
21.4.4.5.2 RESPONSE MESSAGE SEGMENT (INFORMATION REPORTING) (TRANSMISSION REJECTED/TRANSACTION
REJECTED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Information Reporting (Claim/Service):
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on an Information Reporting Response:
The Response Message Segment is situational for an Information Reporting or response when the Header Response Status (5Ø1-F1) of "R"
(Rejected) and Transaction Response Status (112-AN) of “R” (Rejected). It is used when additional text information needs to be sent. Fields
defined as Mandatory are required to be submitted when the segment is sent.
21.4.4.5.3 RESPONSE STATUS SEGMENT (INFORMATION REPORTING) (TRANSMISSION REJECTED/TRANSACTION
REJECTED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Information Reporting (Claim/Service):
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT R Information Reporting (Claim/Service):
Maximum count of 5.
Required.
511-FB REJECT CODE R***R*** Information Reporting (Claim/Service):
Required.
546-4F REJECT FIELD OCCURRENCE INDICATOR Q***R*** Information Reporting (Claim/Service):
Required if a repeating field is in error, to identify repeating
field occurrence.
This field must be sent when relaying error information about
a repeating field or set. Note, if the Reject Code is not
denoting a repeating field or set, the Reject Field Occurrence
Indicator must not be sent.
547-5F APPROVED MESSAGE CODE COUNT N Information Reporting (Claim/Service):
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Information Reporting (Claim/Service):
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Information Reporting (Claim/Service):
Maximum count of 25.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 509 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Information Reporting (Claim/Service):
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Information Reporting (Claim/Service):
Required if additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Information Reporting (Claim/Service):
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Information Reporting (Claim/Service):
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Information Reporting (Claim/Service):
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER Q
N
Information Reporting (Claim):
Required for Medicare Part D payer-to-payer facilitation to
match the transaction response to the transaction.
Service:
Not used.
993-A7 INTERNAL CONTROL NUMBER N Information Reporting (Claim/Service):
Not used.
987-MA URL N Information Reporting (Claim/Service):
Not used.
Notes on Response Status Segment on an Information Reporting Response:
The Response Status Segment is mandatory for an Information Reporting response when the Header Response Status (5Ø1-F1) of "R"
(Rejected) and Transaction Response Status (112-AN) of “R” (Rejected). The Response Status Segment is sent from the sender to the
receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 510 -
22. INFORMATION REPORTING REVERSAL INFORMATION
22.1 INFORMATION REPORTING REVERSAL
This transaction is used to reverse a previously submitted Information Reporting transaction. It is requesting the Processor or Reporting Entity
to back out the previously reported information. For use of Information Reporting functionality for Medicare Part D Processing, see Appendix
“Use Of Information Reporting (N1, N2, N3) Functionality For Medicare Part D Processing”.
Each submission request contains up to four occurrences of Claim Data. The Transaction Code is “N2”.
The following the rules to correctly build a multi-reversal transmission, the reversal transaction(s) in this transmission must be
• in the same format (Version/Release Number) and
• sent to the same entity (processor or PBM using the BIN Number and Processor Control Number) and
• for the same pharmacy (Service Provider ID and Qualifier) and
• for the same date (Date of Service).
Situational segments such as the Insurance Segment may be supported. If a processor/PBM needs this information to process a reversal, this
segment can be used. Only one Insurance Segment must be submitted per transmission, as this segment occurs at the transmission level.
If a processor/PBM does not need the Insurance Segment, but the pharmacy wishes to send it, the processor/PBM must ignore the valid
optional and/or situational information.
Date of Service (4Ø1-D1) is defined as “identifies date the prescription was filled or professional service rendered”. Therefore, since the date is
in the Transaction Header segment that occurs once (at the transmission level), one to four transactions (at the transaction level) must be for
the same date.
Multiple information reporting reversal transactions in a transmission must be for the same patient.
The structure does support multiple information reporting reversals for the same processor/PBM, for the same pharmacy, for the same Date of
Service, but for multiple patients. However, it is recommended that a transmission containing multiple information reporting reversal
transactions for multiple patients not be supported.
The Reject Code (511-FB) value “RV“ (Multiple Reversals Per Transmission Not Supported) can be used for Claim/Service Billing Reversals,
Rebill transmissions, Controlled Substance Reporting Reversals, and Information Reporting Reversals if the processor does not support
multiple reversal transactions within a transmission.
For Medicare Part D processing only one transaction per transmission is permitted because there is a need for the sequencing of the True Out
Of Pocket (TrOOP) update before the next transaction is processed. The TrOOP should be updated before subsequent transactions are
processed.
The Processor must provide one of the following general types of responses:
Approved - The Processor acknowledges receipt of the reversal and backs out the previously submitted reporting transaction. For
Medicare Part D, this means that the PDP has updated the beneficiary's TrOOP to reflect the transaction being reported.
Duplicate of Approved - This occurs when the Processor has previously received the request and processed the transaction, but the
response did not return to the Originator. The Duplicate response contains the same information as returned in the original
Approved scenario.
Captured - This occurs when the Processor acknowledges receipt of the reversal for reporting, but is not making any judgment
regarding the backing out of the reporting. For Medicare Part D, this means that the PDP has not yet updated the beneficiary's
TrOOP to reflect the transaction being reported.
Duplicate of Captured - This occurs when the Processor has previously received the request and processed the transaction, but the
response did not return to the Originator. The Duplicate response contains the same information as returned in the original Captured
scenario.
Rejected - This occurs when the Processor has encountered an error in the transaction or processing, or is unable to process the
reversal.
See section “Response Processing Guidelines”, “Duplicate Transactions”.
22.2 INFORMATION REPORTING REVERSAL (CLAIM) REQUEST DIAGRAMS
22.2.1 DIAGRAM FOR TRANSMISSION OF ONE INFORMATION REPORTING REVERSAL
(CLAIM) TRANSACTION
For an Information Reporting Reversal (Claim), the scenarios defined include
Information Reporting Reversal (Claim) from a Sender to a Receiver

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 511 -
Information Reporting Reversal Transaction Response from a Sender to a Receiver
Standard Transmission Accepted/Transaction Captured/Approved/Rejected Response from a Sender to a
Receiver
Standard Transmission Reject Response to an Information Reporting Reversal from a Sender to a Receiver
There are no situational transaction-level segments in an Information Reporting Reversal (Claim).
Mandatory
Transaction Header Segment
Situational
Segment Separator
Insurance Segment
Mandatory - first Information Reporting Reversal (Claim)
Group Separator
Segment Separator
Claim Segment
22.2.2 DIAGRAM FOR TRANSMISSION OF TWO INFORMATION REPORTING REVERSAL
(CLAIM) TRANSACTIONS
Mandatory
Transaction Header Segment
Situational
Segment Separator
Patient Segment
Segment Separator
Insurance Segment
Mandatory - first Information Reporting Reversal (Claim)
Group Separator
Segment Separator
Claim Segment
Mandatory - second Information Reporting Reversal (Claim)
Group Separator
Segment Separator
Claim Segment
22.2.3 DIAGRAM FOR TRANSMISSION OF THREE INFORMATION REPORTING REVERSAL
(CLAIM) TRANSACTIONS
Mandatory
Transaction Header Segment
Situational
Segment Separator
Patient Segment
Segment Separator
Insurance Segment
Mandatory - first Information Reporting Reversal (Claim)
Group Separator
Segment Separator
Claim Segment
Mandatory - second Information Reporting Reversal (Claim)
Group Separator
Segment Separator
Claim Segment
Mandatory - third Information Reporting Reversal (Claim)
Group Separator
Segment Separator
Claim Segment
22.2.4 DIAGRAM FOR TRANSMISSION OF FOUR INFORMATION REPORTING REVERSAL
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 512 -
(CLAIM) TRANSACTIONS
Mandatory
Transaction Header Segment
Situational
Segment Separator
Patient Segment
Segment Separator
Insurance Segment
Mandatory - first Information Reporting Reversal (Claim)
Group Separator
Segment Separator
Claim Segment
Mandatory - second Information Reporting Reversal (Claim)
Group Separator
Segment Separator
Claim Segment
Mandatory - third Information Reporting Reversal (Claim)
Group Separator
Segment Separator
Claim Segment
Mandatory - fourth Information Reporting Reversal (Claim)
Group Separator
Segment Separator
Claim Segment
22.3 INFORMATION REPORTING REVERSAL (CLAIM) REQUEST SEGMENTS
22.3.1 TRANSACTION HEADER SEGMENT (INFORMATION REPORTING REVERSAL
(CLAIM))
TRANSACTION HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø1-A1 BIN NUMBER M
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø4-A4 PROCESSOR CONTROL NUMBER M
1Ø9-A9 TRANSACTION COUNT M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M
Notes on Transaction Header Segment on an Information Reporting Reversal Request:
The Transaction Header Segment is a mandatory, fixed length segment for an Information Reporting Reversal (Claim) request. The “Situation”
column is not applicable.
22.3.2 INSURANCE SEGMENT (INFORMATION REPORTING REVERSAL (CLAIM))
INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø2-C2 CARDHOLDER ID M
312-CC CARDHOLDER FIRST NAME
N Information Reporting Reversal (Claim):
Not used.
313-CD CARDHOLDER LAST NAME
N Information Reporting Reversal (Claim):
Not used.
314-CE HOME PLAN
N Information Reporting Reversal (Claim):
Not used.
524-FO PLAN ID
N Information Reporting Reversal (Claim):
Not used.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 513 -
INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
3Ø9-C9 ELIGIBILITY CLARIFICATION CODE
N Information Reporting Reversal (Claim):
Not used.
3Ø1-C1 GROUP ID
Q Information Reporting Reversal (Claim):
Required if needed to match the reversal to the original
information reporting transaction.
3Ø3-C3 PERSON CODE
N Information Reporting Reversal (Claim):
Not used.
3Ø6-C6 PATIENT RELATIONSHIP CODE
N Information Reporting Reversal (Claim):
Not used.
99Ø-MG OTHER PAYER BIN NUMBER Q Information Reporting Reversal (Claim):
Required for Medicare Part D payer-to-payer facilitation
when necessary to match the information reporting reversal
transaction to the original information reporting transaction.
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER Q Information Reporting Reversal (Claim):
Required for Medicare Part D payer-to-payer facilitation
when necessary to match the information reporting reversal
transaction to the original information reporting transaction.
356-NU OTHER PAYER CARDHOLDER ID Q Information Reporting Reversal (Claim):
Required for Medicare Part D payer-to-payer facilitation
when necessary to match the information reporting reversal
transaction to the original information reporting transaction.
992-MJ OTHER PAYER GROUP ID Q Information Reporting Reversal (Claim):
Required for Medicare Part D payer-to-payer facilitation
when necessary to match the information reporting reversal
transaction to the original information reporting transaction.
359-2A MEDIGAP ID N Information Reporting Reversal (Claim):
Not used.
36Ø-2B MEDICAID INDICATOR N Information Reporting Reversal (Claim):
Not used.
361-2D PROVIDER ACCEPT ASSIGNMENT INDICATOR N Information Reporting Reversal (Claim):
Not used.
997-G2 CMS PART D DEFINED QUALIFIED FACILITY N Information Reporting Reversal (Claim):
Not used.
115-N5
MEDICAID ID NUMBER N Information Reporting Reversal (Claim):
Not used.
116-N6
MEDICAID AGENCY NUMBER N Information Reporting Reversal (Claim):
Not used.
Notes on Insurance Segment on an Information Reporting Reversal Request:
The Insurance Segment is situational for an Information Reporting Reversal (Claim) request. If the Cardholder ID field is not submitted, the
Insurance Segment is not used. The Segment is mandatory if required under provider payer contract or mandatory on claims where this
information is necessary for reversal of the claim. Fields defined as Mandatory are required to be submitted when the segment is sent.
22.3.3 CLAIM SEGMENT (INFORMATION REPORTING REVERSAL (CLAIM))
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
436-E1 PRODUCT/SERVICE ID QUALIFIER M Mandatory.
Must contain the Product/Service ID Qualifier (436-E1)
value from original Information Reporting.
4Ø7-D7 PRODUCT/SERVICE ID M Mandatory.
Must contain the Product/Service ID (4Ø7-D7) value from
original Information Reporting.
456-EN ASSOCIATED PRESCRIPTION/SERVICE REFERENCE NUMBER N Information Reporting Reversal (Claim):
Not used.
457-EP ASSOCIATED PRESCRIPTION/SERVICE DATE N Information Reporting Reversal (Claim):
Not used.
458-SE PROCEDURE MODIFIER CODE COUNT N Information Reporting Reversal (Claim):
Not used.
459-ER PROCEDURE MODIFIER CODE N***R*** Information Reporting Reversal (Claim):
Not used.
442-E7 QUANTITY DISPENSED N Information Reporting Reversal (Claim):
Not used.
4Ø3-D3 FILL NUMBER Q Information Reporting Reversal (Claim):
Required if needed for reversals when multiple fills of the

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 514 -
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
same Prescription/Service Reference Number occur on the
same day.
4Ø5-D5 DAYS SUPPLY N Information Reporting Reversal (Claim):
Not used.
4Ø6-D6 COMPOUND CODE N Information Reporting Reversal (Claim):
Not used.
4Ø8-D8 DISPENSE AS WRITTEN (DAW)/PRODUCT SELECTION CODE N Information Reporting Reversal (Claim):
Not used.
414-DE DATE PRESCRIPTION WRITTEN N Information Reporting Reversal (Claim):
Not used.
415-DF NUMBER OF REFILLS AUTHORIZED N Information Reporting Reversal (Claim):
Not used.
419-DJ PRESCRIPTION ORIGIN CODE N Information Reporting Reversal (Claim):
Not used.
354-NX SUBMISSION CLARIFICATION CODE COUNT N Information Reporting Reversal (Claim):
Not used.
42Ø-DK SUBMISSION CLARIFICATION CODE N***R*** Information Reporting Reversal (Claim):
Not used.
46Ø-ET QUANTITY PRESCRIBED N Information Reporting Reversal (Claim):
Not used.
3Ø8-C8 OTHER COVERAGE CODE N Information Reporting Reversal (Claim):
Not used.
429-DT SPECIAL PACKAGING INDICATOR N Information Reporting Reversal (Claim):
Not used.
453-EJ ORIGINALLY PRESCRIBED PRODUCT/SERVICE ID QUALIFIER N Information Reporting Reversal (Claim):
Not used.
445-EA ORIGINALLY PRESCRIBED PRODUCT/SERVICE CODE N Information Reporting Reversal (Claim):
Not used.
446-EB ORIGINALLY PRESCRIBED QUANTITY N Information Reporting Reversal (Claim):
Not used.
33∅-CW ALTERNATE ID N Information Reporting Reversal (Claim):
Not used.
454-EK SCHEDULED PRESCRIPTION ID NUMBER N Information Reporting Reversal (Claim):
Not used.
6ØØ-28 UNIT OF MEASURE N Information Reporting Reversal (Claim):
Not used.
418-DI LEVEL OF SERVICE N Information Reporting Reversal (Claim):
Not used.
461-EU PRIOR AUTHORIZATION TYPE CODE N Information Reporting Reversal (Claim):
Not used.
462-EV PRIOR AUTHORIZATION NUMBER SUBMITTED N Information Reporting Reversal (Claim):
Not used.
463-EW INTERMEDIARY AUTHORIZATION TYPE ID N Information Reporting Reversal (Claim):
Not used.
464-EX INTERMEDIARY AUTHORIZATION ID N Information Reporting Reversal (Claim):
Not used.
343-HD DISPENSING STATUS N Information Reporting Reversal (Claim):
Not used.
344-HF QUANTITY INTENDED TO BE DISPENSED N Information Reporting Reversal (Claim):
Not used.
345-HG DAYS SUPPLY INTENDED TO BE DISPENSED N Information Reporting Reversal (Claim):
Not used.
357-NV DELAY REASON CODE N Information Reporting Reversal (Claim):
Not used.
88Ø-K5 TRANSACTION REFERENCE NUMBER Q Information Reporting Reversal (Claim):
Required for Medicare Part D payer-to-payer facilitation to
match the transaction response to the transaction.
391-MT PATIENT ASSIGNMENT INDICATOR (DIRECT MEMBER
REIMBURSEMENT INDICATOR)
N Information Reporting Reversal (Claim):
Not used.
995-E2 ROUTE OF ADMINISTRATION N Information Reporting Reversal (Claim):
Not used.
996-G1 COMPOUND TYPE N Information Reporting Reversal (Claim):
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Information Reporting Reversal (Claim):
Not used.
147-U7 PHARMACY SERVICE TYPE N Information Reporting Reversal (Claim/Service):
Not used.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 515 -
Notes on Claim Segment on an Information Reporting Reversal Request:
The Claim Segment is mandatory for an Information Reporting Reversal (Claim) request. The Claim Segment defines the product dispensed,
dispensing information, reference information for tieback to an original prescription in the case of partial fillings. Fields defined as Mandatory
are required to be submitted when the segment is sent.
22.4 INFORMATION REPORTING REVERSAL (SERVICE) REQUEST DIAGRAMS
22.4.1 DIAGRAM FOR TRANSMISSION OF ONE INFORMATION REPORTING REVERSAL
(SERVICE) TRANSACTION
For an Information Reporting Reversal (Service), the scenarios defined include
Information Reporting Reversal (Service) from a Sender to a Receiver
Information Reporting Reversal Transaction Response from a Sender to a Receiver
Standard Transmission Accepted/Transaction Captured/Approved/Rejected Response from a Sender to a
Receiver
Standard Transmission Reject Response to an Information Reporting Reversal from a Sender to a Receiver
There are no situational transaction-level segments on an Information Reporting Reversal (Service).
Mandatory
Transaction Header Segment
Segment Separator
Situational
Segment Separator
Insurance Segment
Mandatory - Information Reporting Reversal (Service)
Group Separator
Segment Separator
Claim Segment
22.4.2 DIAGRAM FOR TRANSMISSION OF TWO INFORMATION REPORTING REVERSAL
(SERVICE) TRANSACTIONS
Mandatory
Transaction Header Segment
Segment Separator
Situational
Segment Separator
Insurance Segment
Mandatory - first Information Reporting Reversal (Service)
Group Separator
Segment Separator
Claim Segment
Mandatory - second Information Reporting Reversal (Service)
Group Separator
Segment Separator
Claim Segment
22.4.3 DIAGRAM FOR TRANSMISSION OF THREE INFORMATION REPORTING REVERSAL
(SERVICE) TRANSACTIONS
Mandatory
Transaction Header Segment
Segment Separator
Situational
Segment Separator
Insurance Segment
Mandatory - first Information Reporting Reversal (Service)
Group Separator
Segment Separator
Claim Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 516 -
Mandatory - second Information Reporting Reversal (Service)
Group Separator
Segment Separator
Claim Segment
Mandatory - third Information Reporting Reversal (Service)
Group Separator
Segment Separator
Claim Segment
22.4.4 DIAGRAM FOR TRANSMISSION OF FOUR INFORMATION REPORTING REVERSAL
(SERVICE) TRANSACTIONS
Mandatory
Transaction Header Segment
Segment Separator
Situational
Segment Separator
Insurance Segment
Mandatory - first Information Reporting Reversal (Service)
Group Separator
Segment Separator
Claim Segment
Mandatory - second Information Reporting Reversal (Service)
Group Separator
Segment Separator
Claim Segment
Mandatory - third Information Reporting Reversal (Service)
Group Separator
Segment Separator
Claim Segment
Mandatory - fourth Information Reporting Reversal (Service)
Group Separator
Segment Separator
Claim Segment
22.5 INFORMATION REPORTING REVERSAL (SERVICE) SEGMENTS
22.5.1 TRANSACTION HEADER SEGMENT (INFORMATION REPORTING REVERSAL
(SERVICE))
TRANSACTION HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø1-A1 BIN NUMBER M
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø4-A4 PROCESSOR CONTROL NUMBER M
1Ø9-A9 TRANSACTION COUNT M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M
Notes on Transaction Header Segment on an Information Reporting Reversal Request:
The Transaction Header Segment is a mandatory, fixed length segment for an Information Reporting Reversal (Service) request. The
“Situation” column is not applicable.
22.5.2 INSURANCE SEGMENT (INFORMATION REPORTING REVERSAL (SERVICE))
INSURANCE SEGMENT
SITUATIONAL SEGMENT
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 517 -
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø2-C2 CARDHOLDER ID M
312-CC CARDHOLDER FIRST NAME
N Information Reporting Reversal (Service):
Not used.
313-CD CARDHOLDER LAST NAME
N Information Reporting Reversal (Service):
Not used.
314-CE HOME PLAN
N Information Reporting Reversal (Service):
Not used.
524-FO PLAN ID
N Information Reporting Reversal (Service):
Not used.
3Ø9-C9 ELIGIBILITY CLARIFICATION CODE
N Information Reporting Reversal (Service):
Not used.
3Ø1-C1 GROUP ID
Q Information Reporting Reversal (Service):
Required if needed to match the reversal to the original
reporting transaction.
3Ø3-C3 PERSON CODE
N Information Reporting Reversal (Service):
Not used.
3Ø6-C6 PATIENT RELATIONSHIP CODE
N Information Reporting Reversal (Service):
Not used.
99Ø-MG OTHER PAYER BIN NUMBER N Information Reporting Reversal (Service):
Not used.
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER N Information Reporting Reversal (Service):
Not used.
356-NU OTHER PAYER CARDHOLDER ID N Information Reporting Reversal (Service):
Not used.
992-MJ OTHER PAYER GROUP ID N Information Reporting Reversal (Service):
Not used.
359-2A MEDIGAP ID N Information Reporting Reversal (Service):
Not used.
36Ø-2B MEDICAID INDICATOR N Information Reporting Reversal (Service):
Not used.
361-2D PROVIDER ACCEPT ASSIGNMENT INDICATOR N Information Reporting Reversal (Service):
Not used.
997-G2 CMS PART D DEFINED QUALIFIED FACILITY N Information Reporting Reversal (Service):
Not used.
115-N5
MEDICAID ID NUMBER N Information Reporting Reversal (Service):
Not used.
116-N6
MEDICAID AGENCY NUMBER N Information Reporting Reversal (Service):
Not used.
Notes on Insurance Segment on an Information Reporting Reversal Request:
The Insurance Segment is situational for an Information Reporting Reversal (Service) request. If the Cardholder ID field is not submitted, the
Insurance Segment is not used. The Segment is mandatory if required under provider payer contract or mandatory on claims where this
information is necessary for reversal of the claim. Fields defined as Mandatory are required to be submitted when the segment is sent.
22.5.3 CLAIM SEGMENT (INFORMATION REPORTING REVERSAL (SERVICE))
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
436-E1 PRODUCT/SERVICE ID QUALIFIER M Mandatory.
Must contain the Product/Service ID Qualifier (436-E1)
value from original Information Reporting.
4Ø7-D7 PRODUCT/SERVICE ID M Mandatory.
Must contain the Product/Service ID (4Ø7-D7) value from
original Information Reporting.
456-EN ASSOCIATED PRESCRIPTION/SERVICE REFERENCE NUMBER N Information Reporting Reversal (Service):
Not used.
457-EP ASSOCIATED PRESCRIPTION/SERVICE DATE N Information Reporting Reversal (Service):
Not used.
458-SE PROCEDURE MODIFIER CODE COUNT N Information Reporting Reversal (Service):
Not used.
459-ER PROCEDURE MODIFIER CODE N***R*** Information Reporting Reversal (Service):
Not used.
442-E7 QUANTITY DISPENSED N Information Reporting Reversal (Service):
Not used.
4Ø3-D3 FILL NUMBER Q Information Reporting Reversal (Service):

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 518 -
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if needed for reversals when multiple fills of the
same Prescription/Service Reference Number occur on the
same day.
4Ø5-D5 DAYS SUPPLY N Information Reporting Reversal (Service):
Not used.
4Ø6-D6 COMPOUND CODE N Information Reporting Reversal (Service):
Not used.
4Ø8-D8 DISPENSE AS WRITTEN (DAW)/PRODUCT SELECTION CODE N Information Reporting Reversal (Service):
Not used.
414-DE DATE PRESCRIPTION WRITTEN N Information Reporting Reversal (Service):
Not used.
415-DF NUMBER OF REFILLS AUTHORIZED N Information Reporting Reversal (Service):
Not used.
419-DJ PRESCRIPTION ORIGIN CODE N Information Reporting Reversal (Service):
Not used.
354-NX SUBMISSION CLARIFICATION CODE COUNT N Information Reporting Reversal (Service):
Not used.
42Ø-DK SUBMISSION CLARIFICATION CODE N***R*** Information Reporting Reversal (Service):
Not used.
46Ø-ET QUANTITY PRESCRIBED N Information Reporting Reversal (Service):
Not used.
3Ø8-C8 OTHER COVERAGE CODE N Information Reporting Reversal (Service):
Not used.
429-DT SPECIAL PACKAGING INDICATOR N Information Reporting Reversal (Service):
Not used.
453-EJ ORIGINALLY PRESCRIBED PRODUCT/SERVICE ID QUALIFIER N Information Reporting Reversal (Service):
Not used.
445-EA ORIGINALLY PRESCRIBED PRODUCT/SERVICE CODE N Information Reporting Reversal (Service):
Not used.
446-EB ORIGINALLY PRESCRIBED QUANTITY N Information Reporting Reversal (Service):
Not used.
33∅-CW ALTERNATE ID N Information Reporting Reversal (Service):
Not used.
454-EK SCHEDULED PRESCRIPTION ID NUMBER N Information Reporting Reversal (Service):
Not used.
6ØØ-28 UNIT OF MEASURE N Information Reporting Reversal (Service):
Not used.
418-DI LEVEL OF SERVICE N Information Reporting Reversal (Service):
Not used.
461-EU PRIOR AUTHORIZATION TYPE CODE N Information Reporting Reversal (Service):
Not used.
462-EV PRIOR AUTHORIZATION NUMBER SUBMITTED N Information Reporting Reversal (Service):
Not used.
463-EW INTERMEDIARY AUTHORIZATION TYPE ID N Information Reporting Reversal (Service):
Not used.
464-EX INTERMEDIARY AUTHORIZATION ID N Information Reporting Reversal (Service):
Not used.
343-HD DISPENSING STATUS N Information Reporting Reversal (Service):
Not used.
344-HF QUANTITY INTENDED TO BE DISPENSED N Information Reporting Reversal (Service):
Not used.
345-HG DAYS SUPPLY INTENDED TO BE DISPENSED N Information Reporting Reversal (Service):
Not used.
357-NV DELAY REASON CODE N Information Reporting Reversal (Service):
Not used.
88Ø-K5 TRANSACTION REFERENCE NUMBER N Information Reporting Reversal (Service):
Not used.
391-MT PATIENT ASSIGNMENT INDICATOR (DIRECT MEMBER
REIMBURSEMENT INDICATOR)
N Information Reporting Reversal (Service):
Not used.
995-E2 ROUTE OF ADMINISTRATION N Information Reporting Reversal (Service):
Not used.
996-G1 COMPOUND TYPE N Information Reporting Reversal (Service):
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Information Reporting Reversal (Service):
Not used.
147-U7 PHARMACY SERVICE TYPE N Information Reporting Reversal (Service):
Not used.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 519 -
Notes on Claim Segment on an Information Reporting Reversal Request:
The Claim Segment is mandatory for an Information Reporting Reversal (Service) request. The Claim Segment defines the product dispensed,
dispensing information, reference information for tieback to an original prescription in the case of partial fillings. Fields defined as Mandatory
are required to be submitted when the segment is sent.
22.6 INFORMATION REPORTING REVERSAL (CLAIM/SERVICE) RESPONSE
DIAGRAMS
Since there is very little difference in situations for an Information Reporting Reversal (Claim) versus an Information Reporting Reversal
(Service), the response sections are listed together.
22.6.1 TRANSMISSION ACCEPTED/TRANSACTION APPROVED
Information Reporting Reversal (Claim/Service) transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved)
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
There are no situational transaction-level segments for Information Reporting Reversal (Claim/Service) transmission response Header
Response Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved)
22.6.1.1 DIAGRAM FOR TRANSMISSION OF ONE INFORMATION REPORTING REVERSAL RESPONSE
(CLAIM/SERVICE) (TRANSMISSION ACCEPTED/TRANSACTION APPROVED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
22.6.1.2 DIAGRAM FOR TRANSMISSION OF TWO INFORMATION REPORTING REVERSAL RESPONSES
(CLAIM/SERVICE) (TRANSMISSION ACCEPTED/TRANSACTION APPROVED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
22.6.1.3 DIAGRAM FOR TRANSMISSION OF THREE INFORMATION REPORTING REVERSAL RESPONSES
(CLAIM/SERVICE) (TRANSMISSION ACCEPTED/TRANSACTION APPROVED)
Mandatory
Response Header Segment
Situational
Segment Separator
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 520 -
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
22.6.1.4 DIAGRAM FOR TRANSMISSION OF FOUR INFORMATION REPORTING REVERSAL RESPONSES
(CLAIM/SERVICE) (TRANSMISSION ACCEPTED/TRANSACTION APPROVED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
22.6.1.5 INFORMATION REPORTING REVERSAL RESPONSE SEGMENTS (CLAIM/SERVICE)
(TRANSMISSION ACCEPTED/TRANSACTION APPROVED)
22.6.1.5.1 RESPONSE HEADER SEGMENT (INFORMATION REPORTING REVERSAL (CLAIM/SERVICE))
(TRANSMISSION ACCEPTED/TRANSACTION APPROVED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 521 -
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on an Information Reporting Reversal Response:
The Response Header Segment is a mandatory, fixed length segment for an Information Reporting Reversal request when the Header
Response Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved).
The “Situation” column is not applicable.
22.6.1.5.2 RESPONSE MESSAGE SEGMENT (INFORMATION REPORTING REVERSAL (CLAIM/SERVICE))
(TRANSMISSION ACCEPTED/TRANSACTION APPROVED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Information Reporting Reversal (Claim/Service):
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on an Information Reporting Reversal Response:
The Response Message Segment is situational for an Information Reporting Reversal request when the Header Response Status (5Ø1-F1) of
"A" (Accepted) and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved). It is used when additional text
information needs to be sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
22.6.1.5.3 RESPONSE STATUS SEGMENT (INFORMATION REPORTING REVERSAL (CLAIM/SERVICE)) (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Information Reporting Reversal (Claim/Service):
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT N Information Reporting Reversal (Claim/Service):
Not used.
511-FB REJECT CODE N***R*** Information Reporting Reversal (Claim/Service):
Not used.
546-4F REJECT FIELD OCCURRENCE INDICATOR N***R*** Information Reporting Reversal (Claim/Service):
Not used.
547-5F APPROVED MESSAGE CODE COUNT N Information Reporting Reversal (Claim/Service):
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Information Reporting Reversal (Claim/Service):
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Information Reporting Reversal (Claim/Service):
Maximum count of 25.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 522 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Information Reporting Reversal (Claim/Service):
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Information Reporting Reversal (Claim/Service):
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Information Reporting Reversal (Claim/Service):
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Information Reporting Reversal (Claim/Service):
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Information Reporting Reversal (Claim/Service):
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER Q
N
Information Reporting Reversal (Claim):
Required for Medicare Part D payer-to-payer facilitation to
match the transaction response to the transaction.
Service:
Not used.
993-A7 INTERNAL CONTROL NUMBER N Information Reporting Reversal (Claim/Service):
Not used.
987-MA URL N Information Reporting Reversal (Claim/Service):
Not used.
Notes on Response Status Segment on an Information Reporting Reversal Response:
The Response Status Segment is mandatory for an Information Reporting Reversal response for Header Response Status (5Ø1-F1) = “A”
(Accepted) and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved). The Response Status Segment is
sent from the sender to the receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the
segment is sent.
22.6.1.5.4 RESPONSE CLAIM SEGMENT (INFORMATION REPORTING REVERSAL (CLAIM/SERVICE)) (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 523 -
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
551-9F PREFERRED PRODUCT COUNT N Information Reporting Reversal (Claim/Service):
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER N***R*** Information Reporting Reversal (Claim/Service):
Not used.
553-AR PREFERRED PRODUCT ID N***R*** Information Reporting Reversal (Claim/Service):
Not used.
554-AS PREFERRED PRODUCT INCENTIVE N***R*** Information Reporting Reversal (Claim/Service):
Not used.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE N***R*** Information Reporting Reversal (Claim/Service):
Not used.
556-AU PREFERRED PRODUCT DESCRIPTION N***R*** Information Reporting Reversal (Claim/Service):
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Information Reporting Reversal (Claim/Service):
Not used.
Notes on Response Claim Segment on an Information Reporting Reversal Response:
The Response Claim Segment is mandatory for an Information Reporting Reversal response when the Header Response Status (5Ø1-F1) is
“A” (Accepted) and Transaction Response Status (112-AN) of “A” (Approved) or “S” (Duplicate of Approved). The Response Claim Segment is
sent from the sender to the receiver to identify therapeutic or alternate product recommendations. Fields defined as Mandatory are required to
be submitted when the segment is sent.
22.6.2 TRANSMISSION ACCEPTED/TRANSACTION CAPTURED
Information Reporting Reversal transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured)
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
There are no situational transaction-level segments.
22.6.2.1 DIAGRAM FOR TRANSMISSION OF ONE INFORMATION REPORTING REVERSAL RESPONSE
(CLAIM/SERVICE) (TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
22.6.2.2 DIAGRAM FOR TRANSMISSION OF TWO INFORMATION REPORTING REVERSAL RESPONSES
(CLAIM/SERVICE) (TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 524 -
Response Claim Segment
22.6.2.3 DIAGRAM FOR TRANSMISSION OF THREE INFORMATION REPORTING REVERSAL RESPONSES
(CLAIM/SERVICE) (TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
22.6.2.4 DIAGRAM FOR TRANSMISSION OF FOUR INFORMATION REPORTING REVERSAL RESPONSES
(CLAIM/SERVICE) (TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 525 -
22.6.2.5 INFORMATION REPORTING REVERSAL RESPONSE SEGMENTS (CLAIM/SERVICE)
(TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
22.6.2.5.1 RESPONSE HEADER SEGMENT (INFORMATION REPORTING REVERSAL (CLAIM/SERVICE))
(TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on an Information Reporting Reversal Response:
The Response Header Segment is a mandatory, fixed length segment for an Information Reporting Reversal request when the Header
Response Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). The
“Situation” column is not applicable.
22.6.2.5.2 RESPONSE MESSAGE SEGMENT (INFORMATION REPORTING REVERSAL (CLAIM/SERVICE))
(TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Information Reporting Reversal (Claim/Service):
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on an Information Reporting Reversal Response:
The Response Message Segment is situational for an Information Reporting Reversal request when the Header Response Status (5Ø1-F1) of
"A" (Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). It is used when additional text
information needs to be sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
22.6.2.5.3 RESPONSE STATUS SEGMENT (INFORMATION REPORTING REVERSAL (CLAIM/SERVICE)) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Information Reporting Reversal (Claim/Service):
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT N Information Reporting Reversal (Claim/Service):
Not used.
511-FB REJECT CODE N***R*** Information Reporting Reversal (Claim/Service):
Not used.
546-4F REJECT FIELD OCCURRENCE INDICATOR N***R*** Information Reporting Reversal (Claim/Service):
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 526 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Not used.
547-5F APPROVED MESSAGE CODE COUNT N Information Reporting Reversal (Claim/Service):
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Information Reporting Reversal (Claim/Service):
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Information Reporting Reversal (Claim/Service):
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Information Reporting Reversal (Claim/Service):
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Information Reporting Reversal (Claim/Service):
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Information Reporting Reversal (Claim/Service):
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Information Reporting Reversal (Claim/Service):
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Information Reporting Reversal (Claim/Service):
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER Q
N
Information Reporting Reversal (Claim):
Required for Medicare Part D payer-to-payer facilitation to
match the transaction response to the transaction.
Service:
Not used.
993-A7 INTERNAL CONTROL NUMBER N Information Reporting Reversal (Claim/Service):
Not used.
987-MA URL N Information Reporting Reversal (Claim/Service):
Not used.
Notes on Response Status Segment on an Information Reporting Reversal Response:
The Response Status Segment is mandatory for an Information Reporting Reversal response for Header Response Status (5Ø1-F1) = “A”
(Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). The Response Status Segment is
sent from the sender to the receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the
segment is sent.
22.6.2.5.4 RESPONSE CLAIM SEGMENT (INFORMATION REPORTING REVERSAL (CLAIM/SERVICE)) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 527 -
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT N Information Reporting Reversal (Claim/Service):
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER N***R*** Information Reporting Reversal (Claim/Service):
Not used.
553-AR PREFERRED PRODUCT ID N***R*** Information Reporting Reversal (Claim/Service):
Not used.
554-AS PREFERRED PRODUCT INCENTIVE N***R*** Information Reporting Reversal (Claim/Service):
Not used.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE N***R*** Information Reporting Reversal (Claim/Service):
Not used.
556-AU PREFERRED PRODUCT DESCRIPTION N***R*** Information Reporting Reversal (Claim/Service):
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Information Reporting Reversal (Claim/Service):
Not used.
Notes on Response Claim Segment on an Information Reporting Reversal Response:
The Response Claim Segment is mandatory for an Information Reporting Reversal response when the Header Response Status (5Ø1-F1) is
“A” (Accepted) and Transaction Response Status (112-AN) of “C” (Captured) or “Q” (Duplicate of Captured). The Response Claim Segment is
sent from the sender to the receiver to identify therapeutic or alternate product recommendations. Fields defined as Mandatory are required to
be submitted when the segment is sent.
22.6.3 TRANSMISSION ACCEPTED/TRANSACTION REJECTED
Information Reporting Reversal transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “R” (Rejected)
There are no situational transaction-level segments for Information Reporting Reversal transmission response Header Response Status (5Ø1-
F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “R” (Rejected).
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
22.6.3.1 DIAGRAM FOR TRANSMISSION OF ONE INFORMATION REPORTING REVERSAL RESPONSE
(CLAIM/SERVICE) (TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
22.6.3.2 DIAGRAM FOR TRANSMISSION OF TWO INFORMATION REPORTING REVERSAL RESPONSES
(CLAIM/SERVICE) (TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 528 -
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
22.6.3.3 DIAGRAM FOR TRANSMISSION OF THREE INFORMATION REPORTING REVERSAL RESPONSES
(CLAIM/SERVICE) (TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
22.6.3.4 DIAGRAM FOR TRANSMISSION OF FOUR INFORMATION REPORTING REVERSAL RESPONSES
(CLAIM/SERVICE) (TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory fourth response
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 529 -
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
22.6.3.5 INFORMATION REPORTING REVERSAL RESPONSE SEGMENTS (CLAIM/SERVICE)
(TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
22.6.3.5.1 RESPONSE HEADER SEGMENT (INFORMATION REPORTING REVERSAL (CLAIM/SERVICE))
(TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on an Information Reporting Reversal Response:
The Response Header Segment is a mandatory, fixed length segment for an Information Reporting Reversal request when the Header
Response Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). The “Situation” column is not
applicable.
22.6.3.5.2 RESPONSE MESSAGE SEGMENT (INFORMATION REPORTING REVERSAL (CLAIM/SERVICE))
(TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Information Reporting Reversal (Claim/Service):
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on an Information Reporting Reversal Response:
The Response Message Segment is situational for an Information Reporting Reversal request when the Header Response Status (5Ø1-F1) of
"A" (Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). It is used when additional text information needs to be sent.
Fields defined as Mandatory are required to be submitted when the segment is sent.
22.6.3.5.3 RESPONSE STATUS SEGMENT (INFORMATION REPORTING REVERSAL (CLAIM/SERVICE)) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 530 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
5Ø3-F3 AUTHORIZATION NUMBER Q Information Reporting Reversal (Claim/Service):
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT R Information Reporting Reversal (Claim/Service):
Maximum count of 5.
Required.
511-FB REJECT CODE R***R*** Information Reporting Reversal (Claim/Service):
Required.
546-4F REJECT FIELD OCCURRENCE INDICATOR Q***R*** Information Reporting Reversal (Claim/Service):
Required if a repeating field is in error, to identify repeating
field occurrence.
This field must be sent when relaying error information about
a repeating field or set. Note, if the Reject Code is not
denoting a repeating field or set, the Reject Field Occurrence
Indicator must not be sent.
547-5F APPROVED MESSAGE CODE COUNT N Information Reporting Reversal (Claim/Service):
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Information Reporting Reversal (Claim/Service):
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Information Reporting Reversal (Claim/Service):
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Information Reporting Reversal (Claim/Service):
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Information Reporting Reversal (Claim/Service):
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Information Reporting Reversal (Claim/Service):
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Information Reporting Reversal (Claim/Service):
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Information Reporting Reversal (Claim/Service):
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER Q
N
Information Reporting Reversal (Claim):
Required for Medicare Part D payer-to-payer facilitation to
match the transaction response to the transaction.
Service:
Not used.
993-A7 INTERNAL CONTROL NUMBER N Information Reporting Reversal (Claim/Service):
Not used.
987-MA URL N Information Reporting Reversal (Claim/Service):
Not used.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 531 -
Notes on Response Status Segment on an Information Reporting Reversal Response:
The Response Status Segment is mandatory for an Information Reporting Reversal response for Header Response Status (5Ø1-F1) = “A”
(Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). The Response Status Segment is sent from the sender to the
receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.
22.6.3.5.4 RESPONSE CLAIM SEGMENT (INFORMATION REPORTING REVERSAL (CLAIM/SERVICE)) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT N Information Reporting Reversal (Claim/Service):
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER N***R*** Information Reporting Reversal (Claim/Service):
Not used.
553-AR PREFERRED PRODUCT ID N***R*** Information Reporting Reversal (Claim/Service):
Not used.
554-AS PREFERRED PRODUCT INCENTIVE N***R*** Information Reporting Reversal (Claim/Service):
Not used.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE N***R*** Information Reporting Reversal (Claim/Service):
Not used.
556-AU PREFERRED PRODUCT DESCRIPTION N***R*** Information Reporting Reversal (Claim/Service):
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Information Reporting Reversal (Claim/Service):
Not used.
Notes on Response Claim Segment on an Information Reporting Reversal Response:
The Response Claim Segment is mandatory for an Information Reporting Reversal response when the Header Response Status (5Ø1-F1) is
“A” (Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). The Response Claim Segment is sent from the sender to the
receiver to identify therapeutic or alternate product recommendations. Fields defined as Mandatory are required to be submitted when the
segment is sent.
22.6.4 TRANSMISSION REJECTED/TRANSACTION REJECTED
Information Reporting Reversal transmission response Header Response Status (5Ø1-F1) of "R" (Rejected)
and Transaction Response Status (112-AN) of “R” (Rejected)
There are no situational transaction-level segments.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
22.6.4.1 DIAGRAM FOR TRANSMISSION OF ONE INFORMATION REPORTING REVERSAL RESPONSE
(CLAIM/SERVICE) (TRANSMISSION REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
22.6.4.2 DIAGRAM FOR TRANSMISSION OF TWO INFORMATION REPORTING REVERSAL RESPONSES
(CLAIM/SERVICE) (TRANSMISSION REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 532 -
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
22.6.4.3 DIAGRAM FOR TRANSMISSION OF THREE INFORMATION REPORTING REVERSAL RESPONSES
(CLAIM/SERVICE) (TRANSMISSION REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
22.6.4.4 DIAGRAM FOR TRANSMISSION OF FOUR INFORMATION REPORTING REVERSAL RESPONSES
(CLAIM/SERVICE) (TRANSMISSION REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
22.6.4.5 INFORMATION REPORTING REVERSAL RESPONSE SEGMENTS (CLAIM/SERVICE)
(TRANSMISSION REJECTED/TRANSACTION REJECTED)
22.6.4.5.1 RESPONSE HEADER SEGMENT (INFORMATION REPORTING REVERSAL (CLAIM/SERVICE))
(TRANSMISSION REJECTED/TRANSACTION REJECTED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 533 -
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on an Information Reporting Reversal Response:
The Response Header Segment is a mandatory, fixed length segment for an Information Reporting Reversal response when the Header
Response Status (5Ø1-F1) of "R" (Rejected) and Transaction Response Status (112-AN) of “R” (Rejected). The “Situation” column is not
applicable.
If either the entire transmission or the Header is in error, the Header Response Status (5Ø1-F1) = “R” (Rejected). Every identifiable transaction
within the transmission must be rejected with an “R”.
If the transaction rejects for detail errors, the Header Response Status (5Ø1-F1) = “A” (Accepted) and the Transaction Response Status (112-
AN) will be “R”.
22.6.4.5.2 RESPONSE MESSAGE SEGMENT (INFORMATION REPORTING REVERSAL (CLAIM/SERVICE))
(TRANSMISSION REJECTED/TRANSACTION REJECTED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Information Reporting Reversal (Claim/Service):
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on an Information Reporting Reversal Response:
The Response Message Segment is situational for an Information Reporting Reversal response when the Header Response Status (5Ø1-F1)
of "R" (Rejected) and Transaction Response Status (112-AN) of “R” (Rejected). It is used when additional text information needs to be sent.
Fields defined as Mandatory are required to be submitted when the segment is sent.
22.6.4.5.3 RESPONSE STATUS SEGMENT (INFORMATION REPORTING REVERSAL (CLAIM/SERVICE)) (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Information Reporting Reversal (Claim/Service):
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT R Information Reporting Reversal (Claim/Service):
Maximum count of 5.
Required.
511-FB REJECT CODE R***R*** Information Reporting Reversal (Claim/Service):
Required.
546-4F REJECT FIELD OCCURRENCE INDICATOR Q***R*** Information Reporting Reversal (Claim/Service):
Required if a repeating field is in error, to identify repeating
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 534 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
field occurrence.
This field must be sent when relaying error information about
a repeating field or set. Note, if the Reject Code is not
denoting a repeating field or set, the Reject Field Occurrence
Indicator must not be sent.
547-5F APPROVED MESSAGE CODE COUNT N Information Reporting Reversal (Claim/Service):
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Information Reporting Reversal (Claim/Service):
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Information Reporting Reversal (Claim/Service):
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Information Reporting Reversal (Claim/Service):
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Information Reporting Reversal (Claim/Service):
Required if additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Information Reporting Reversal (Claim/Service):
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Information Reporting Reversal (Claim/Service):
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Information Reporting Reversal (Claim/Service):
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER Q
N
Information Reporting Reversal (Claim):
Required for Medicare Part D payer-to-payer facilitation to
match the transaction response to the transaction.
Service:
Not used.
993-A7 INTERNAL CONTROL NUMBER N Information Reporting Reversal (Claim/Service):
Not used.
987-MA URL N Information Reporting Reversal (Claim/Service):
Not used.
Notes on Response Status Segment on an Information Reporting Reversal Response:
The Response Status Segment is mandatory for an Information Reporting Reversal response for Header Response Status (5Ø1-F1) = “R”
(Rejected) and Transaction Response Status (112-AN) of “R” (Rejected). The Response Status Segment is sent from the sender to the
receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 535 -
23. INFORMATION REPORTING REBILL INFORMATION
23.1 INFORMATION REPORTING REBILL
This transaction is an information reporting submission with an implied reversal. It is used by the Originator to cancel an information reporting
submitted that had been processed previously, and submit a new information reporting in the same transaction. For use of Information
Reporting functionality for Medicare Part D Processing, see Appendix “Use Of Information Reporting (N1, N2, N3) Functionality For Medicare
Part D Processing”.
For information reporting reversal guidelines, see section “Information Reporting Reversal Information”. The Transaction Code is “N3”.
For Medicare Part D processing only one transaction per transmission is permitted because there is a need for the sequencing of the True Out
Of Pocket (TrOOP) update before the next transaction is processed. The TrOOP should be updated before subsequent transactions are
processed.
Depending upon the particular claim or service submission request, the Processor must provide one of the following general types of
responses:
Approved - This occurs when the Processor acknowledges the receipt of the information only transaction and successfully
processes the reversal and new information transaction. For Medicare Part D, this means that the PDP has updated the
beneficiary's TrOOP to reflect the transaction being reported.
Captured - This occurs when the Processor acknowledges receipt of the information reporting transaction, but no judgment is made
about the processing of the transaction. For Medicare Part D, this means that the PDP has not yet updated the beneficiary's TrOOP
to reflect the transaction being reported.
Rejected - This occurs when the Processor has encountered an error in the transaction or processing, or does not approve the
information only rebill transaction.
Duplicate response logic must not be applied by the processor to Information Reporting Rebill transactions. There is no need for a duplicate
response due to the nature of the rebill transaction and its implied reversal. Please see section “Response Processing Guidelines”, Duplicate
Transactions” and “Duplicate Processing For All Rebill Transactions” for more information about why duplicate responses are not supported in
Information Reporting Rebill transactions.
These transactions are described below.
23.2 INFORMATION REPORTING REBILL (CLAIM/SERVICE) REQUEST DIAGRAMS
23.2.1 DIAGRAM FOR TRANSMISSION OF ONE INFORMATION REPORTING REBILL
TRANSACTION
For an Information Reporting Rebill, the scenarios defined include
Information Reporting Rebill from a Sender to a Receiver
Information Reporting Accepted – Captured/Approved/Rejected Transaction Response from a Sender to a Receiver
Standard Transmission Reject Response to an Information Reporting Rebill from a Sender to a Receiver
The Pricing Segment only supports the field Patient Paid Amount Submitted (433-DX) that is used in Medicare Part D payer-to-payer
facilitation. Otherwise, the Pricing Segment is not used.
Mandatory
Transaction Header Segment
Segment Separator
Insurance Segment
Situational
Segment Separator
Patient Segment
Mandatory - first Information Reporting Rebill transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Workers’ Compensation Segment

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 536 -
Segment Separator
DUR/PPS Segment
Segment Separator
Pricing Segment
Segment Separator
Clinical Segment
23.2.2 DIAGRAM FOR TRANSMISSION OF TWO INFORMATION REPORTING REBILL
TRANSACTIONS
Mandatory
Transaction Header Segment
Segment Separator
Insurance Segment
Situational
Segment Separator
Patient Segment
Mandatory - first Information Reporting Rebill transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Pricing Segment
Segment Separator
Clinical Segment
Mandatory - second Information Reporting Rebill transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Pricing Segment
Segment Separator
Clinical Segment
23.2.3 DIAGRAM FOR TRANSMISSION OF THREE INFORMATION REPORTING REBILL
TRANSACTIONS
Mandatory
Transaction Header Segment
Segment Separator
Insurance Segment
Situational
Segment Separator
Patient Segment
Mandatory - first Information Reporting Rebill transaction
Group Separator
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 537 -
Segment Separator
Claim Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Pricing Segment
Segment Separator
Clinical Segment
Mandatory - second Information Reporting Rebill transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Pricing Segment
Segment Separator
Clinical Segment
Mandatory – third Information Reporting Rebill transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Pricing Segment
Segment Separator
Clinical Segment
23.2.4 DIAGRAM FOR TRANSMISSION OF FOUR INFORMATION REPORTING
TRANSACTIONS
Mandatory
Transaction Header Segment
Segment Separator
Insurance Segment
Situational
Segment Separator
Patient Segment
Mandatory - first Information Reporting Rebill transaction
Group Separator
Segment Separator
Claim Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 538 -
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Pricing Segment
Segment Separator
Clinical Segment
Mandatory - second Information Reporting Rebill transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Pricing Segment
Segment Separator
Clinical Segment
Mandatory – third Information Reporting Rebill transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Pricing Segment
Segment Separator
Clinical Segment
Mandatory – fourth Information Reporting Rebill transaction
Group Separator
Segment Separator
Claim Segment
Situational
Segment Separator
Pharmacy Provider Segment
Segment Separator
Prescriber Segment
Segment Separator
Workers’ Compensation Segment
Segment Separator
DUR/PPS Segment
Segment Separator
Pricing Segment
Segment Separator
Clinical Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 539 -
23.3 INFORMATION REPORTING REBILL REQUEST SEGMENTS
23.3.1 TRANSACTION HEADER SEGMENT (INFORMATION REPORTING REBILL)
TRANSACTION HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø1-A1 BIN NUMBER M
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø4-A4 PROCESSOR CONTROL NUMBER M
1Ø9-A9 TRANSACTION COUNT M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M
Notes on Transaction Header Segment on an Information Reporting Rebill Request:
The Transaction Header Segment is a mandatory, fixed length segment for an Information Reporting Rebill request. The “Situation” column is
not applicable.
23.3.2 INSURANCE SEGMENT (INFORMATION REPORTING REBILL)
INSURANCE SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø2-C2 CARDHOLDER ID M
312-CC CARDHOLDER FIRST NAME
Q Information Reporting Rebill (Claim/Service):
Required if necessary for state/federal/regulatory agency
programs when the cardholder has a first name.
313-CD CARDHOLDER LAST NAME
Q Information Reporting Rebill (Claim/Service):
Required if necessary for state/federal/regulatory agency
programs.
314-CE HOME PLAN
Q Information Reporting Rebill (Claim/Service):
Required if needed for receiver reporting validation and/or
determination for Blue Cross or Blue Shield, if a Patient has
coverage under more than one plan, to distinguish each
plan.
524-FO PLAN ID
Q Information Reporting Rebill (Claim/Service):
Required if needed to identify a set of parameters, benefit,
or coverage criteria.
3Ø9-C9 ELIGIBILITY CLARIFICATION CODE
Q Information Reporting Rebill (Claim/Service):
Required if needed for receiver inquiry validation and/or
determination, when eligibility is not maintained at the
dependent level.
Required in special situations as defined by the code to
clarify the eligibility of an individual, which may extend
coverage.
3Ø1-C1 GROUP ID
Q Information Reporting Rebill (Claim/Service):
Required if necessary for state/federal/regulatory agency
programs.
Required if needed for pharmacy information reporting
processing.
Required if needed to match the reversal to the original
information reporting transaction.
3Ø3-C3 PERSON CODE
Q Information Reporting Rebill (Claim/Service):
Required if needed to uniquely identify the family members
within the Cardholder ID.
3Ø6-C6 PATIENT RELATIONSHIP CODE Q Information Reporting Rebill (Claim/Service):
Required if needed to uniquely identify the relationship of
the Patient to the Cardholder ID.
99Ø-MG OTHER PAYER BIN NUMBER Q
Information Reporting Rebill (Claim):
Required for Medicare Part D payer-to-payer facilitation
when necessary to match the information reporting reversal
transaction to the original information reporting transaction.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 540 -
INSURANCE SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
N
Service:
Not used.
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER Q
N
Information Reporting Rebill (Claim):
Required for Medicare Part D payer-to-payer facilitation
when necessary to match the information reporting reversal
transaction to the original information reporting transaction.
Service:
Not used.
356-NU OTHER PAYER CARDHOLDER ID Q
N
Information Reporting Rebill (Claim):
Required for Medicare Part D payer-to-payer facilitation
when necessary to match the information reporting reversal
transaction to the original information reporting transaction.
Service:
Not used.
992-MJ OTHER PAYER GROUP ID Q
N
Information Reporting Rebill (Claim):
Required for Medicare Part D payer-to-payer facilitation
when necessary to match the information reporting reversal
transaction to the original information reporting transaction.
Service:
Not used.
359-2A MEDIGAP ID Q Information Reporting Rebill (Claim/Service):
Required, if known, when patient has Medigap coverage.
36Ø-2B MEDICAID INDICATOR Q Information Reporting Rebill (Claim/Service):
Required, if known, when patient has Medicaid coverage.
361-2D PROVIDER ACCEPT ASSIGNMENT INDICATOR N Information Reporting Rebill (Claim/Service):
Not used.
997-G2 CMS PART D DEFINED QUALIFIED FACILITY Q
N
Information Reporting Rebill (Claim):
Required if specified in trading partner agreement.
Service:
Not used.
115-N5
MEDICAID ID NUMBER N Information Reporting Rebill (Claim/Service):
Not used.
116-N6
MEDICAID AGENCY NUMBER N Information Reporting Rebill (Claim/Service):
Not used.
Notes on Insurance Segment on an Information Reporting Rebill Request:
The Insurance Segment is mandatory for an Information Reporting Rebill Request. Fields defined as Mandatory are required to be submitted
when the segment is sent.
23.3.3 PATIENT SEGMENT (INFORMATION REPORTING REBILL)
PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
331-CX PATIENT ID QUALIFIER Q Information Reporting Rebill (Claim/Service):
Required if Patient ID (332-CY) is used.
332-CY PATIENT ID
Q Information Reporting Rebill (Claim):
Required if necessary for state/federal/regulatory agency
programs to validate dual eligibility.
3Ø4-C4 DATE OF BIRTH
R Information Reporting Rebill (Claim/Service):
Required.
3Ø5-C5 PATIENT GENDER CODE
Q Information Reporting Rebill (Claim/Service):
Required if additional verification of the submitted eligibility
information is needed.
31Ø-CA PATIENT FIRST NAME
Q Information Reporting Rebill (Claim/Service):
Required when the patient has a first name.
311-CB PATIENT LAST NAME
Q Information Reporting Rebill (Claim/Service):
Required when the patient last name is known.
322-CM PATIENT STREET ADDRESS
Q Information Reporting Rebill (Claim/Service):
Required if needed to assist in identifying the patient when
specific eligibility cannot be established.
Required if necessary for state/federal/regulatory agency
programs.
323-CN PATIENT CITY ADDRESS
Q Information Reporting Rebill (Claim/Service):
Required if needed to assist in identifying the patient when
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 541 -
PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
specific eligibility cannot be established.
Required if necessary for state/federal/regulatory agency
programs.
324-CO PATIENT STATE / PROVINCE ADDRESS
Q Information Reporting Rebill (Claim/Service):
Required if needed to assist in identifying the patient when
specific eligibility cannot be established.
Required if necessary for state/federal/regulatory agency
programs.
325-CP PATIENT ZIP/POSTAL ZONE
Q Information Reporting Rebill (Claim/Service):
Required if needed to assist in identifying the patient when
specific eligibility cannot be established.
Required if necessary for state/federal/regulatory agency
programs.
326-CQ PATIENT PHONE NUMBER
Q Information Reporting Rebill (Claim/Service):
Required if needed per trading partner agreement.
Required if necessary for state/federal/regulatory agency
programs.
3Ø7-C7 PLACE OF SERVICE
Q Information Reporting Rebill (Claim/Service):
Required if needed per trading partner agreement.
333-CZ EMPLOYER ID
Q Information Reporting Rebill (Claim/Service):
Required if necessary for state/federal/regulatory agency
programs.
Required if needed for Workers’ Compensation reporting.
334-1C SMOKER / NON-SMOKER CODE
Q Information Reporting Rebill (Claim/Service):
Required if clinical determination is dependent upon
patient’s smoking condition.
335-2C PREGNANCY INDICATOR
Q Information Reporting Rebill (Claim/Service):
Required if clinical determination is dependent upon
patient’s pregnancy condition. Submitted until it is known
the patient is no longer pregnant.
35Ø-HN PATIENT E-MAIL ADDRESS I Information Reporting Rebill (Claim/Service):
May be submitted for the receiver to relay patient health
care communications via the Internet when provided by the
patient.
This field is informational only.
384-4X PATIENT RESIDENCE Q Information Reporting Rebill (Claim/Service):
Required if needed per trading partner agreement.
Notes on Patient Segment on an Information Reporting Rebill Request:
The Patient Segment is situational. It is used when a receiver needs some of the patient demographic information to perform Information
Reporting Rebill requirements. The Patient Segment must be submitted when needed to differentiate between the patient and the cardholder.
If the cardholder and the patient are the same, then the Patient Segment is not submitted unless additional information about the patient is
needed to clarify the Information Reporting Rebill transaction. The Segment is mandatory if required under provider payer contract or
mandatory on Information Reporting Rebill where this information is necessary for reporting. Fields defined as Mandatory are required to be
submitted when the segment is sent.
23.3.4 CLAIM SEGMENT (INFORMATION REPORTING REBILL)
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
436-E1 PRODUCT/SERVICE ID QUALIFIER M Mandatory.
If reporting for a multi-ingredient prescription,
Product/Service ID Qualifier (436-E1) is zero (Zero means
“ØØ”).
If the Product/Service ID Qualifier (436-E1) = “Ø6”
(DUR/PPS), the Product/Service ID (4Ø7-D7) is zero. (Zero
means “Ø”.)
4Ø7-D7 PRODUCT/SERVICE ID M Mandatory.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 542 -
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
If reporting for a multi-ingredient prescription,
Product/Service ID (4Ø7-D7) is zero. (Zero means “Ø”.)
If the Product/Service ID Qualifier (436-E1) = “Ø6”
(DUR/PPS), the Product/Service ID (4Ø7-D7) is zero. (Zero
means “Ø”.) Populate the DUR/PPS segment as
appropriate.
If the Product/Service ID Qualifier (436-E1) = “Ø7” (CPT-4),
the Product Service ID (4Ø7-D7) is the actual CPT-4 value.
If the Product/Service ID Qualifier (436-E1) = “Ø9”
(HCPCS), the Product Service ID (4Ø7-D7) is the actual
HCPCS value.
If the Product/Service ID Qualifier (436-E1) = “99” (Other),
the Product Service ID (4Ø7-D7) is the business partner
agreed value.
456-EN ASSOCIATED PRESCRIPTION/SERVICE REFERENCE NUMBER Q Information Reporting Rebill (Claim):
Required if the “completion” transaction in a partial fill
(Dispensing Status (343-HD) = “C” (Completed)).
See section “Specific Segment Discussion”, “Request
Segments”, Claim Segment” for more information.
Required if the Dispensing Status (343-HD) = “P” (Partial
Fill) and there are multiple occurrences of partial fills for this
prescription.
Service:
Required in order to associate the service to the product.
Contains the Prescription/Service Reference Number (4Ø2-
D2) of the prescription or service that prompted the service.
Required if Associated Prescription/Service Date (457-EP)
is used.
Required if needed to associate multiple
prescriptions/services from the same sender to allow
reporting of the current prescription/service.
457-EP ASSOCIATED PRESCRIPTION/SERVICE DATE Q Information Reporting Rebill (Claim):
Required if the “completion” transaction in a partial fill
(Dispensing Status (343-HD) = “C” (Completed)).
See section “Specific Segment Discussion”, “Request
Segments”, Claim Segment” for more information.
Required if Associated Prescription/Service Reference Date
(457-EP) is used.
Required if the Dispensing Status (343-HD) = “P” (Partial
Fill) and there are multiple occurrences of partial fills for this
prescription.
Service:
Required in order to associate the service to the product.
Contains the Prescription/Service Reference Number (4Ø2-
D2) of the prescription or service that prompted the service.
Required if Associated Prescription/Service Date (457-EP)
is used.
Required if needed to associate multiple
prescriptions/services from the same sender to allow
reporting of the current prescription/service.
458-SE PROCEDURE MODIFIER CODE COUNT
Q Information Reporting Rebill (Claim/Service):
Maximum count of 1Ø.
Required if Procedure Modifier Code (459-ER) is used.
459-ER PROCEDURE MODIFIER CODE Q***R*** Information Reporting Rebill (Claim/Service):
Required to define a further level of specificity if the
Product/Service ID (4Ø7-D7) indicated a Procedure Code
was submitted.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 543 -
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Occurs the number of times identified in Procedure Modifier
Code Count (458-SE).
Required to define a further level of specificity if the
Product/Service ID (4Ø7-D7) indicated a Procedure Code
was submitted.
442-E7 QUANTITY DISPENSED Q
Q
Information Reporting Rebill (Claim):
Required if necessary for plan benefit administration.
Service:
Required if the value is greater than zero (Ø).
4Ø3-D3 FILL NUMBER Q
Q
Information Reporting Rebill (Claim):
Required for Medicare Part D payer-to-payer facilitation.
Information Reporting Rebill (Service):
Required if necessary for plan benefit administration.
4Ø5-D5 DAYS SUPPLY Q
Information Reporting Rebill (Claim/Service):
Required if necessary for plan benefit administration.
4Ø6-D6 COMPOUND CODE Q
N
Information Reporting Rebill (Claim):
Required if necessary for plan benefit administration.
Service:
Not used.
4Ø8-D8 DISPENSE AS WRITTEN (DAW)/PRODUCT SELECTION CODE Q
N
Information Reporting Rebill (Claim):
Required if necessary for plan benefit administration.
Service:
Not used.
414-DE DATE PRESCRIPTION WRITTEN R
N
Information Reporting Rebill (Claim):
Required if necessary for plan benefit administration.
Service:
Not used.
415-DF NUMBER OF REFILLS AUTHORIZED Q Information Reporting Rebill (Claim/Service):
Required if necessary for plan benefit administration.
419-DJ PRESCRIPTION ORIGIN CODE Q
N
Information Reporting Rebill (Claim):
Required if necessary for plan benefit administration.
Service:
Not used.
354-NX SUBMISSION CLARIFICATION CODE COUNT Q
N
Information Reporting Rebill (Claim):
Maximum count of 3.
Required if Submission Clarification Code (42Ø-DK) is
used.
Service:
Not used.
42Ø-DK SUBMISSION CLARIFICATION CODE Q***R***
N
Information Reporting Rebill (Claim):
Required if clarification is known and values greater than
zero (Ø).
Occurs the number of times identified in Submission
Clarification Code Count (354-NX).
Service:
Not used.
46∅-ET QUANTITY PRESCRIBED N
Q
Information Reporting Rebill (Claim):
Not used.
Service:
Required if the prescriber orders a specific number of
iterations of a service.
Required for values greater than one (1).
3Ø8-C8 OTHER COVERAGE CODE Q Information Reporting Rebill (Claim/Service):
Required if needed by receiver, to communicate a
summation of other coverage information that has been
collected from other payers.
Required for Coordination of Benefits.
See section “Specific Segment Discussion”, “Request

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 544 -
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Segments”, “Claim Segment”, “Other Coverage Code (3Ø8-
C8).
429-DT SPECIAL PACKAGING INDICATOR Q
N
Information Reporting Rebill (Claim):
Required if needed per trading partner agreement.
Service:
Not used.
453-EJ ORIGINALLY PRESCRIBED PRODUCT/SERVICE ID QUALIFIER Q Information Reporting Rebill (Claim/Service):
Required if Originally Prescribed Product/Service Code
(445-EA) is used.
445-EA ORIGINALLY PRESCRIBED PRODUCT/SERVICE CODE Q Information Reporting Rebill (Claim/Service):
Required if the receiver requests association to a
therapeutic, or a preferred product substitution, or when a
DUR alert has been resolved by changing medications, or
an alternative service than what was originally prescribed.
446-EB ORIGINALLY PRESCRIBED QUANTITY Q Information Reporting Rebill (Claim/Service):
Required if the receiver requests reporting for quantity
changes due to a therapeutic substitution that has occurred
or a preferred product/service substitution that has
occurred, or when a DUR alert has been resolved by
changing quantities.
33Ø-CW ALTERNATE ID N Information Reporting Rebill (Claim/Service):
Not used.
454-EK SCHEDULED PRESCRIPTION ID NUMBER N Information Reporting Rebill (Claim/Service):
Not used.
6ØØ-28 UNIT OF MEASURE Q
N
Information Reporting Rebill (Claim):
Required if needed per trading partner agreement.
Required if necessary for state/federal/regulatory agency
programs.
Service:
Not used.
418-DI LEVEL OF SERVICE Q Information Reporting Rebill (Claim/Service):
Required if needed per trading partner agreement.
461-EU PRIOR AUTHORIZATION TYPE CODE Q Information Reporting Rebill (Claim/Service):
Required if needed per trading partner agreement.
462-EV PRIOR AUTHORIZATION NUMBER SUBMITTED Q Information Reporting Rebill (Claim/Service):
Required if needed per trading partner agreement.
463-EW INTERMEDIARY AUTHORIZATION TYPE ID Q Information Reporting Rebill (Claim/Service):
Required for overriding an authorized intermediary system
edit when the pharmacy participates with an intermediary.
Required if Intermediary Authorization ID (464-EX) is used.
464-EX INTERMEDIARY AUTHORIZATION ID Q Information Reporting Rebill (Claim/Service):
Required for overriding an authorized intermediary system
edit when the pharmacy participates with an intermediary.
343-HD DISPENSING STATUS Q
N
Information Reporting Rebill (Claim):
Required for the partial fill or the completion fill of a
prescription.
Service:
Not used.
344-HF QUANTITY INTENDED TO BE DISPENSED Q
N
Information Reporting Rebill (Claim):
Required for the partial fill or the completion fill of a
prescription.
Service:
Not used.
345-HG DAYS SUPPLY INTENDED TO BE DISPENSED Q
N
Information Reporting Rebill (Claim):
Required for the partial fill or completion fill of a
prescription.
Service:
Not used.
357-NV DELAY REASON CODE Q Information Reporting Rebill (Claim/Service):
Required if needed per trading partner agreement.
88Ø-K5 TRANSACTION REFERENCE NUMBER Q
Information Reporting Rebill (Claim):
Required for Medicare Part D payer-to-payer facilitation to
match the transaction response to the transaction.
Service:
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 545 -
CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
N Not used.
391-MT PATIENT ASSIGNMENT INDICATOR (DIRECT MEMBER
REIMBURSEMENT INDICATOR)
Q Information Reporting Rebill (Claim/Service):
Required if needed per trading partner agreement.
995-E2 ROUTE OF ADMINISTRATION Q
N
Information Reporting Rebill (Claim/):
Required if specified in trading partner agreement.
Service:
Not used.
996-G1 COMPOUND TYPE Q
N
Information Reporting Rebill (Claim):
Required if specified in trading partner agreement.
Service:
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Information Reporting Rebill (Claim/Service):
Not used.
147-U7 PHARMACY SERVICE TYPE N Information Reporting (Claim/Service):
Not used.
Notes on Claim Segment on an Information Reporting Rebill Request:
The Claim Segment is mandatory for an Information Reporting Rebill Request. The Claim Segment defines the product dispensed, dispensing
information, reference information for tieback to an original prescription in the case of partial fillings. Fields defined as Mandatory are required
to be submitted when the segment is sent.
23.3.5 PHARMACY PROVIDER SEGMENT (INFORMATION REPORTING REBILL)
PHARMACY PROVIDER SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
465-EY PROVIDER ID QUALIFIER Q Information Reporting Rebill (Claim/Service):
Required if Provider ID (444-E9) is used.
444-E9 PROVIDER ID
Q Information Reporting Rebill (Claim):
Required if necessary for state/federal/regulatory agency
programs.
Required if necessary to identify the individual responsible
for dispensing of the prescription.
Information Reporting/Information Reporting Rebill
(Service):
Required if necessary for state/federal/regulatory agency
programs.
Required if necessary to identify the individual responsible
for provision of the service.
Notes on Pharmacy Provider Segment on an Information Reporting Rebill Request:
The Pharmacy Provider Segment is situational for an Information Reporting Rebill Request, if required under provider payer contract or where
this information is necessary to perform or meet Information Reporting or Information Reporting Rebill requirements. Fields defined as
Mandatory are required to be submitted when the segment is sent.
23.3.6 PRESCRIBER SEGMENT (INFORMATION REPORTING REBILL)
PRESCRIBER SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
466-EZ PRESCRIBER ID QUALIFIER Q Information Reporting Rebill (Claim/Service):
Required if Prescriber ID (411-DB) is used.
411-DB PRESCRIBER ID
Q Information Reporting Rebill (Claim/Service):
Required if this field could result in different coverage or
patient financial responsibility.
Required if necessary for state/federal/regulatory agency
programs.
427-DR PRESCRIBER LAST NAME
Q Information Reporting Rebill (Claim/Service):
Required when the Prescriber ID (411-DB) is not known.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 546 -
PRESCRIBER SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
Required if needed for Prescriber ID (411-DB)
validation/clarification.
498-PM PRESCRIBER PHONE NUMBER Q Information Reporting Rebill (Claim/Service):
Required if needed to assist in identifying the prescriber.
Required if needed for Prior Authorization process.
468-2E PRIMARY CARE PROVIDER ID QUALIFIER Q Information Reporting Rebill (Claim/Service):
Required if Primary Care Provider ID (421-DL) is used.
421-DL PRIMARY CARE PROVIDER ID
Q Information Reporting Rebill (Claim/Service):
Required if needed per trading partner agreement.
Required if necessary for state/federal/regulatory agency
programs.
47Ø-4E PRIMARY CARE PROVIDER LAST NAME
Q Information Reporting Rebill (Claim/Service):
Required if this field is used as an alternative for Primary
Care Provider ID (421-DL) when ID is not known.
Required if needed for Primary Care Provider ID (421-DL)
validation/clarification.
364-2J PRESCRIBER FIRST NAME Q Information Reporting Rebill (Claim/Service):
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
365-2K PRESCRIBER STREET ADDRESS Q Information Reporting Rebill (Claim/Service):
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
366-2M PRESCRIBER CITY ADDRESS Q Information Reporting Rebill (Claim/Service):
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
367-2N PRESCRIBER STATE/PROVINCE ADDRESS Q Information Reporting Rebill (Claim/Service):
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
368-2P PRESCRIBER ZIP/POSTAL ZONE Q Information Reporting Rebill (Claim/Service):
Required if needed to assist in identifying the prescriber.
Required if necessary for state/federal/regulatory agency
programs.
Notes on Prescriber Segment on an Information Reporting Rebill Request:
The Prescriber Segment is situational for an Information Reporting Rebill Request. It is used when prescriber information is needed to perform
or meet Information Reporting Rebill requirements. The Segment is mandatory if required under provider payer contract or where this
information is necessary for reporting. Fields defined as Mandatory are required to be submitted when the segment is sent.
23.3.7 WORKERS’ COMPENSATION SEGMENT (INFORMATION REPORTING REBILL)
WORKERS’ COMPENSATION SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
434-DY DATE OF INJURY M
315-CF EMPLOYER NAME Q Information Reporting Rebill (Claim/Service):
Required if needed to process an information reporting
transaction for a work related injury or condition.
316-CG EMPLOYER STREET ADDRESS Q Information Reporting Rebill (Claim/Service):
Required if needed to process an information reporting
transaction for a work related injury or condition.
317-CH EMPLOYER CITY ADDRESS Q Information Reporting Rebill (Claim/Service):
Required if needed to process an information reporting
transaction for a work related injury or condition.
318-CI EMPLOYER STATE/PROVINCE ADDRESS Q Information Reporting Rebill (Claim/Service):
Required if needed to process an information reporting
transaction for a work related injury or condition.
319-CJ EMPLOYER ZIP/POSTAL ZONE Q Information Reporting Rebill (Claim/Service):
Required if needed to process an information reporting

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 547 -
WORKERS’ COMPENSATION SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
transaction for a work related injury or condition.
32Ø-CK EMPLOYER PHONE NUMBER Q Information Reporting Rebill (Claim/Service):
Required if needed to process an information reporting
transaction for a work related injury or condition.
321-CL EMPLOYER CONTACT NAME Q Information Reporting Rebill (Claim/Service):
Required if needed to process an information reporting
transaction for a work related injury or condition.
327-CR CARRIER ID Q Information Reporting Rebill (Claim/Service):
Required if needed to process an information reporting
transaction for a work related injury or condition.
435-DZ CLAIM/REFERENCE ID Q Information Reporting Rebill (Claim/Service):
Required if needed to process an information reporting
transaction for a work related injury or condition.
117-TR BILLING ENTITY TYPE INDICATOR N Information Reporting Rebill (Claim/Service):
Not used.
118-TS PAY TO QUALIFIER N Information Reporting Rebill (Claim/Service):
Not used.
119-TT PAY TO ID N Information Reporting Rebill (Claim/Service):
Not used.
12Ø-TU PAY TO NAME N Information Reporting Rebill (Claim/Service):
Not used.
121-TV PAY TO STREET ADDRESS N Information Reporting Rebill (Claim/Service):
Not used.
122-TW PAY TO CITY ADDRESS N Information Reporting Rebill (Claim/Service):
Not used.
123-TX PAY TO STATE/PROVINCE ADDRESS N Information Reporting Rebill (Claim/Service):
Not used.
124-TY PAY TO ZIP/POSTAL ZONE N Information Reporting Rebill (Claim/Service):
Not used.
125-TZ GENERIC EQUIVALENT PRODUCT ID QUALIFIER N Information Reporting Rebill (Claim/Service):
Not used.
126-UA GENERIC EQUIVALENT PRODUCT ID N Information Reporting Rebill (Claim/Service):
Not used.
Notes on Workers’ Compensation Segment on an Information Reporting Rebill Request:
The Workers’ Compensation Segment is situational for an Information Reporting Rebill request. It is used when processing an Information
Reporting Rebill request for a work-related injury or condition. Fields defined as Mandatory are required to be submitted when the segment is
sent.
23.3.8 DUR/PPS SEGMENT (INFORMATION REPORTING REBILL)
DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
473-7E DUR/PPS CODE COUNTER
Q***R*** Information Reporting Rebill (Claim/Service):
Maximum of 9 occurrences.
Required if DUR/PPS Segment is used.
439-E4 REASON FOR SERVICE CODE
Q***R*** Information Reporting Rebill (Claim):
Required if this field could result in different drug utilization
review outcome.
Service:
Required if this field affects documentation of professional
pharmacy service.
44Ø-E5 PROFESSIONAL SERVICE CODE
Q***R*** Information Reporting Rebill (Claim):
Required if this field could result in different drug utilization
review outcome.
Service:
Required if this field affects documentation of professional
pharmacy service.
441-E6 RESULT OF SERVICE CODE
Q***R*** Information Reporting Rebill (Claim):
Required if this field could result in different drug utilization
review outcome.
Service:
Required if this field affects documentation of professional
pharmacy service.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 548 -
DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
474-8E DUR/PPS LEVEL OF EFFORT
Q***R*** Information Reporting Rebill (Claim):
Required if this field could result in different drug utilization
review outcome.
Service:
Required if this field affects documentation of professional
pharmacy service.
475-J9 DUR CO-AGENT ID QUALIFIER Q***R*** Information Reporting Rebill (Claim/Service):
Required if DUR Co-Agent ID Qualifier (475-J9) is used.
476-H6 DUR CO-AGENT ID
Q***R*** Information Reporting Rebill (Claim):
Required if this field could result in different drug utilization
review outcome.
Service:
Required if this field affects documentation of professional
pharmacy service.
Notes on DUR/PPS Segment on an Information Reporting Rebill Request:
The DUR/PPS Segment is situational for an Information Reporting Rebill request. It is used when a sender notifies the receiver of drug
utilization, drug evaluations, or information on the appropriate selection to process Information Reporting Rebill. The DUR/PPS information
may be sent on the initial submission or alternatively sent after a DUR/PPS rejection from a receiver. The Segment is mandatory if required
under provider payer contract or where this information is necessary for processing the reporting. Fields defined as Mandatory are required to
be submitted when the segment is sent.
23.3.9 PRICING SEGMENT (INFORMATION REPORTING REBILL)
PRICING SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
4Ø9-D9 INGREDIENT COST SUBMITTED N Information Reporting Rebill (Claim/Service):
Not used.
412-DC DISPENSING FEE SUBMITTED N Information Reporting Rebill (Claim/Service):
Not used.
477-BE PROFESSIONAL SERVICE FEE SUBMITTED N Information Reporting Rebill (Claim/Service):
Not used.
433-DX PATIENT PAID AMOUNT SUBMITTED Q Information Reporting Rebill (Claim/Service):
Required for Medicare Part D payer-to-payer facilitation.
438-E3 INCENTIVE AMOUNT SUBMITTED N Information Reporting Rebill (Claim/Service):
Not used.
478-H7 OTHER AMOUNT CLAIMED SUBMITTED COUNT
N Information Reporting Rebill (Claim/Service):
Not used.
479-H8 OTHER AMOUNT CLAIMED SUBMITTED QUALIFIER
N***R*** Information Reporting Rebill (Claim/Service):
Not used.
48Ø-H9 OTHER AMOUNT CLAIMED SUBMITTED
N***R*** Information Reporting Rebill (Claim/Service):
Not used.
481-HA FLAT SALES TAX AMOUNT SUBMITTED
N Information Reporting Rebill (Claim/Service):
Not used.
482-GE PERCENTAGE SALES TAX AMOUNT SUBMITTED
N Information Reporting Rebill (Claim/Service):
Not used.
483-HE PERCENTAGE SALES TAX RATE SUBMITTED
N Information Reporting Rebill (Claim/Service):
Not used.
484-JE PERCENTAGE SALES TAX BASIS SUBMITTED
N Information Reporting Rebill (Claim/Service):
Not used.
426-DQ USUAL AND CUSTOMARY CHARGE
N Information Reporting Rebill (Claim/Service):
Not used.
43Ø-DU GROSS AMOUNT DUE N Information Reporting Rebill (Claim/Service):
Not used.
423-DN BASIS OF COST DETERMINATION
N Information Reporting Rebill (Claim/Service):
Not used.
113-N3
MEDICAID PAID AMOUNT N Information Reporting Rebill (Claim/Service):
Not used.
Notes on Pricing Segment on an Information Reporting Rebill Request:
The Pricing Segment is situational for an Information Reporting Rebill Request. The Pricing Segment only supports the field Patient Paid
Amount Submitted (433-DX) that is used in Medicare Part D payer-to-payer facilitation. Otherwise, the Pricing Segment is not used. Fields
defined as Mandatory are required to be submitted when the segment is sent.
23.3.10 CLINICAL SEGMENT (INFORMATION REPORTING REBILL)

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 549 -
CLINICAL SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
491-VE DIAGNOSIS CODE COUNT Q Information Reporting Rebill (Claim/Service):
Maximum count of 5.
Required if Diagnosis Code Qualifier (492-WE) and
Diagnosis Code (424-DO) are used.
492-WE DIAGNOSIS CODE QUALIFIER Q***R*** Information Reporting Rebill (Claim/Service):
Required if Diagnosis Code (424-DO) is used.
424-DO DIAGNOSIS CODE
Q***R*** Information Reporting Rebill (Claim/Service):
The value for this field is obtained from the prescriber or
authorized representative.
Required if this field was reported on the original
adjudicated transaction.
Required if this field could result in different drug utilization
review outcome.
Required if this information can be used in place of prior
authorization.
Required if necessary for state/federal/regulatory agency
programs.
493-XE CLINICAL INFORMATION COUNTER Q***R*** Information Reporting Rebill (Claim/Service):
Maximum 5 occurrences supported.
Grouped with Measurement fields (Measurement Date
(494-ZE), Measurement Time (495-H1), Measurement
Dimension (496-H2), Measurement Unit (497-H3),
Measurement Value (499-H4).
494-ZE MEASUREMENT DATE Q***R*** Information Reporting Rebill (Claim/Service):
Required if necessary when this field could result in
different drug utilization review outcome.
495-H1 MEASUREMENT TIME Q***R*** Information Reporting Rebill (Claim/Service):
Required if Time is known or has impact on measurement.
Required if necessary when this field could result in drug
utilization review outcome.
496-H2 MEASUREMENT DIMENSION Q***R*** Information Reporting Rebill (Claim/Service):
Required if Measurement Unit (497-H3) and Measurement
Value (499-H4) are used.
Required if necessary when this field could result in
different drug utilization review outcome.
497-H3 MEASUREMENT UNIT Q***R*** Information Reporting Rebill (Claim/Service):
Required if Measurement Dimension (496-H2) and
Measurement Value (499-H4) are used.
Required if necessary when this field could result in
different drug utilization review outcome.
499-H4 MEASUREMENT VALUE Q***R*** Information Reporting Rebill (Claim/Service):
Required if Measurement Dimension (496-H2) and
Measurement Unit (497-H3) are used.
Required if necessary when this field could result in
different drug utilization review outcome.
Notes on Clinical Segment on an Information Reporting Rebill Request:
The Clinical Segment is situational on an Reporting Rebill request. It is used to specify clinical measurements and/or diagnosis information
associated with the Information Reporting Rebill transaction. The Segment is mandatory if required under provider payer contract or where this
information is necessary for reporting. Fields defined as Mandatory are required to be submitted when the segment is sent.
23.4 INFORMATION REPORTING REBILL RESPONSE DIAGRAMS AND SEGMENTS
23.4.1 TRANSMISSION ACCEPTED/TRANSACTION CAPTURED
Information Reporting Rebill transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “C” (Captured)
The captured response is applicable when the receiver acknowledges receipt, but does not fully process the Information Reporting transaction.
In Medicare Part D payer-to-payer facilitation, no TrOOP is updated on a captured response.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 550 -
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
23.4.1.1 DIAGRAM FOR TRANSMISSION OF ONE INFORMATION REPORTING REBILL RESPONSE
(TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
23.4.1.2 DIAGRAM FOR TRANSMISSION OF TWO INFORMATION REPORTING REBILL RESPONSES
(TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
23.4.1.3 DIAGRAM FOR TRANSMISSION OF THREE INFORMATION REPORTING REBILL RESPONSES
(TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 551 -
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
23.4.1.4 DIAGRAM FOR TRANSMISSION OF FOUR INFORMATION REPORTING REBILL RESPONSES
(TRANSMISSION ACCEPTED/TRANSACTION CAPTURED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 552 -
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
23.4.1.5 INFORMATION REPORTING RESPONSE REBILL RESPONSE SEGMENTS (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
23.4.1.5.1 RESPONSE HEADER SEGMENT (INFORMATION REPORTING REBILL) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on an Information Reporting Rebill Response:
The Response Header Segment is a mandatory, fixed length segment for an Information Reporting Rebill response when the Header
Response Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “C” (Captured). The “Situation” column is not
applicable.
23.4.1.5.2 RESPONSE MESSAGE SEGMENT (INFORMATION REPORTING REBILL) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Information Reporting Rebill (Claim/Service):
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 553 -
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
transaction-level text.
Notes on Response Message Segment on an Information Reporting Rebill Response:
The Response Message Segment is situational for an Information Reporting Rebill response when the Header Response Status (5Ø1-F1) of
"A" (Accepted) and Transaction Response Status (112-AN) of “C” (Captured). It is used when additional text information needs to be sent.
Fields defined as Mandatory are required to be submitted when the segment is sent.
23.4.1.5.3 RESPONSE INSURANCE SEGMENT (INFORMATION REPORTING REBILL) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø1-C1 GROUP ID
Q Information Reporting Rebill (Claim/Service):
Required if needed to identify the cardholder or employer
group, to identify appropriate group number for reporting.
Required to identify the actual group that was used when
multiple group coverages exist.
Note: This field may contain the Group ID echoed from the
request. May contain the actual Group ID if unknown to the
receiver.
524-FO PLAN ID
Q Information Reporting Rebill (Claim/Service):
Required to identify the actual plan ID that was used when
multiple group coverages exist.
Required if needed to contain the actual plan ID if unknown
to the receiver.
545-2F NETWORK REIMBURSEMENT ID
N Information Reporting Rebill (Claim/Service):
Not used.
568-J7 PAYER ID QUALIFIER N Information Reporting Rebill (Claim/Service):
Not used.
569-J8 PAYER ID
N Information Reporting Rebill (Claim/Service):
Not used.
115-N5
MEDICAID ID NUMBER N Information Reporting Rebill (Claim/Service):
Not used.
116-N6
MEDICAID AGENCY NUMBER N Information Reporting Rebill (Claim/Service):
Not used.
3Ø2-C2
CARDHOLDER ID N Information Reporting Rebill (Claim/Service):
Not used.
Notes on Response Insurance Segment on an Information Reporting Rebill Response:
The Response Insurance Segment is situational for an Information Reporting Rebill response when the Header Response Status (5Ø1-F1) of
"A" (Accepted) and Transaction Response Status (112-AN) of “C” (Captured). It is used when coverage information may be provided from the
sender to the receiver. Fields defined as Mandatory are required to be submitted when the segment is sent.
23.4.1.5.4 RESPONSE PATIENT SEGMENT (INFORMATION REPORTING REBILL) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
RESPONSE PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
31Ø-CA PATIENT FIRST NAME Q Information Reporting Rebill (Claim/Service):
Required if known.
311-CB PATIENT LAST NAME Q Information Reporting Rebill (Claim/Service):
Required if known.
3Ø4-C4 DATE OF BIRTH Q Information Reporting Rebill (Claim/Service):
Required if known.
Notes on Response Patient Segment on an Information Reporting Rebill Response:
The Response Patient Segment is situational for Information Reporting Rebill transmission response Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “C” (Captured) when patient demographic information needs to be sent from the
sender to the receiver. Fields defined as Mandatory are required to be submitted when the segment is sent.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 554 -
23.4.1.5.5 RESPONSE STATUS SEGMENT (INFORMATION REPORTING REBILL) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Information Reporting Rebill (Claim/Service):
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT N Information Reporting Rebill (Claim/Service):
Not used.
511-FB REJECT CODE N***R*** Information Reporting Rebill (Claim/Service):
Not used.
546-4F REJECT FIELD OCCURRENCE INDICATOR N***R*** Information Reporting Rebill (Claim/Service):
Not used.
547-5F APPROVED MESSAGE CODE COUNT N Information Reporting Rebill (Claim/Service):
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Information Reporting Rebill (Claim/Service):
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Information Reporting Rebill (Claim/Service):
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Information Reporting Rebill (Claim/Service):
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Information Reporting Rebill (Claim/Service):
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Information Reporting Rebill (Claim/Service):
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Information Reporting Rebill (Claim/Service):
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Information Reporting Rebill (Claim/Service):
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER Q
N
Information Reporting Rebill (Claim):
Required for Medicare Part D payer-to-payer facilitation to
match the transaction response to the transaction.
Service:
Not used.
993-A7 INTERNAL CONTROL NUMBER N Information Reporting Rebill (Claim/Service):
Not used.
987-MA URL N Information Reporting Rebill (Claim/Service):
Not used.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 555 -
Notes on Response Status Segment on an Information Reporting Rebill Response:
The Response Status Segment is mandatory for an Information Reporting Rebill response for Header Response Status (5Ø1-F1) = “A”
(Accepted) and Transaction Response Status (112-AN) of “C” (Captured). The Response Status Segment is sent from the sender to the
receiver to identify the outcome of the request.
Information Reporting Rebill transactions - The “C” (Captured) event occurs after the reversal portion of the information reporting rebill is
processed successfully and the information reporting is captured for processing. If the information reporting rebill reversal is not processed
successfully, a “R” (Rejected) response must be sent.
Fields defined as Mandatory are required to be submitted when the segment is sent.
23.4.1.5.6 RESPONSE CLAIM SEGMENT (INFORMATION REPORTING REBILL) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT N Information Reporting Rebill (Claim/Service):
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER N***R*** Information Reporting Rebill (Claim/Service):
Not used.
553-AR PREFERRED PRODUCT ID
N***R*** Information Reporting Rebill (Claim/Service):
Not used.
554-AS PREFERRED PRODUCT INCENTIVE
N***R*** Information Reporting Rebill (Claim/Service):
Not used.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE
N***R*** Information Reporting Rebill (Claim/Service):
Not used.
556-AU PREFERRED PRODUCT DESCRIPTION N***R*** Information Reporting Rebill (Claim/Service):
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Information Reporting Rebill (Claim/Service):
Not used.
Notes on Response Claim Segment on an Information Reporting Rebill Response:
The Response Claim Segment is mandatory for an Information Reporting Rebill response when the Header Response Status (5Ø1-F1) is “A”
(Accepted) and Transaction Response Status (112-AN) of “C” (Captured).
The Response Claim Segment (Information Reporting – Service) is sent from the sender to the receiver to mirror back the Prescription/Service
Reference Number (4Ø2-D2).
Fields defined as Mandatory are required to be submitted when the segment is sent.
23.4.1.5.7 RESPONSE DUR/PPS SEGMENT (INFORMATION REPORTING REBILL) (TRANSMISSION
ACCEPTED/TRANSACTION CAPTURED)
RESPONSE DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
567-J6 DUR/PPS RESPONSE CODE COUNTER Q***R*** Information Reporting Rebill (Claim/Service):
Maximum 9 occurrences supported.
Required if Reason For Service Code (439-E4) is used.
439-E4 REASON FOR SERVICE CODE
Q***R*** Information Reporting Rebill (Claim):
Required if utilization conflict is detected.
Service:
Required if professional service opportunity reason is
detected by the receiver. Should be different than the
original transmission.
528-FS CLINICAL SIGNIFICANCE CODE
Q***R*** Information Reporting Rebill (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 556 -
RESPONSE DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
529-FT OTHER PHARMACY INDICATOR
Q***R*** Information Reporting Rebill (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
53Ø-FU PREVIOUS DATE OF FILL
Q***R*** Information Reporting Rebill (Claim):
Required if needed to supply additional information for the
utilization conflict.
Required if Quantity of Previous Fill (531-FV) is used.
Service:
Required if needed to supply additional information for the
service.
Required if Quantity of Previous Fill (531-FV) is used.
531-FV QUANTITY OF PREVIOUS FILL
Q***R*** Information Reporting Rebill (Claim):
Required if Previous Date Of Fill (53Ø-FU) is used.
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
Required if Previous Date Of Fill (53Ø-FU) is used.
532-FW DATABASE INDICATOR
Q***R*** Information Reporting Rebill (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
533-FX OTHER PRESCRIBER INDICATOR
Q***R*** Information Reporting Rebill (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
544-FY DUR FREE TEXT MESSAGE
Q***R*** Information Reporting Rebill (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
57Ø-NS DUR ADDITIONAL TEXT Q***R*** Information Reporting Rebill (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
Notes on Response DUR/PPS Segment on an Information Reporting Rebill Response:
The Response DUR/PPS Segment is situational for an Information Reporting Rebill response when the Header Response Status (5Ø1-F1) of
"A" (Accepted) and Transaction Response Status (112-AN) of “C” (Captured). The Response DUR/PPS Segment is used to identify a drug
utilization review or professional pharmacy service event, opportunity, or information. Fields defined as Mandatory are required to be submitted
when the segment is sent.
23.4.2 TRANSMISSION ACCEPTED/TRANSACTION APPROVED
Information Reporting Rebill transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “A” (Approved)
The duplicate response codes for the Information Reporting Rebill transaction are not applicable.
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
23.4.2.1 DIAGRAM FOR TRANSMISSION OF ONE INFORMATION REPORTING REBILL RESPONSE
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 557 -
(TRANSMISSION ACCEPTED/TRANSACTION APPROVED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
23.4.2.2 DIAGRAM FOR TRANSMISSION OF TWO INFORMATION REPORTING REBILL RESPONSES
(TRANSMISSION ACCEPTED/TRANSACTION APPROVED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
23.4.2.3 DIAGRAM FOR TRANSMISSION OF THREE INFORMATION REPORTING REBILL RESPONSES
(TRANSMISSION ACCEPTED/TRANSACTION APPROVED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 558 -
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
23.4.2.4 DIAGRAM FOR TRANSMISSION OF FOUR INFORMATION REPORTING REBILL RESPONSES
(TRANSMISSION ACCEPTED/TRANSACTION APPROVED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Mandatory third response
Group Separator
Segment Separator
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 559 -
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Situational
Segment Separator
Response DUR/PPS Segment
23.4.2.5 INFORMATION REPORTING REBILL RESPONSE SEGMENTS (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
23.4.2.5.1 RESPONSE HEADER SEGMENT (INFORMATION REPORTING REBILL) (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on an Information Reporting Rebill Response:
The Response Header Segment is a mandatory, fixed length segment for an Information Reporting Rebill response when the Header
Response Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “A” (Approved). The “Situation” column is not
applicable.
23.4.2.5.2 RESPONSE MESSAGE SEGMENT (INFORMATION REPORTING REBILL) (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Information Reporting Rebill (Claim/Service):
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on an Information Reporting Rebill Response:
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 560 -
The Response Message Segment is situational for an Information Reporting Rebill response when the Header Response Status (5Ø1-F1) of
"A" (Accepted) and Transaction Response Status (112-AN) of “A” (Approved). It is used when additional text information needs to be sent.
Fields defined as Mandatory are required to be submitted when the segment is sent.
23.4.2.5.3 RESPONSE INSURANCE SEGMENT (INFORMATION REPORTING REBILL) (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø1-C1 GROUP ID
Q Information Reporting Rebill (Claim/Service):
Required if needed to identify the cardholder or employer
group, to identify appropriate group number for reporting.
Required to identify the actual group that was used when
multiple group coverages exist.
Note: This field may contain the Group ID echoed from the
request. May contain the actual Group ID if unknown to the
receiver.
524-FO PLAN ID
Q Information Reporting Rebill (Claim/Service):
Required to identify the actual plan ID that was used when
multiple group coverages exist.
Required if needed to contain the actual plan ID if unknown
to the receiver.
545-2F NETWORK REIMBURSEMENT ID
N Information Reporting Rebill (Claim/Service):
Not used.
568-J7 PAYER ID QUALIFIER N Information Reporting Rebill (Claim/Service):
Not used.
569-J8 PAYER ID
N Information Reporting Rebill (Claim/Service):
Not used.
115-N5
MEDICAID ID NUMBER N Information Reporting Rebill (Claim/Service):
Not used.
116-N6
MEDICAID AGENCY NUMBER N Information Reporting Rebill (Claim/Service):
Not used.
3Ø2-C2
CARDHOLDER ID Q Information Reporting Rebill (Claim/Service):
Required if the identification to be used in future
transactions is different than what was submitted on the
request.
Notes on Response Insurance Segment on an Information Reporting Rebill Response:
The Response Insurance Segment is situational for an Information Reporting Rebill response when the Header Response Status (5Ø1-F1) of
"A" (Accepted) and Transaction Response Status (112-AN) of “A” (Approved). It is used when coverage information may be provided from the
sender to the receiver. Fields defined as Mandatory are required to be submitted when the segment is sent.
23.4.2.5.4 RESPONSE PATIENT SEGMENT (INFORMATION REPORTING REBILL) (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
RESPONSE PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
31Ø-CA PATIENT FIRST NAME Q Information Reporting Rebill (Claim/Service):
Required if known.
311-CB PATIENT LAST NAME Q Information Reporting Rebill (Claim/Service):
Required if known.
3Ø4-C4 DATE OF BIRTH Q Information Reporting Rebill (Claim/Service):
Required if known.
Notes on Response Patient Segment on an Information Reporting Rebill Response:
The Response Patient Segment is situational for Information Reporting Rebill transmission response Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “A” (Approved) when patient demographic information needs to be sent from the
sender to the receiver. Fields defined as Mandatory are required to be submitted when the segment is sent.
23.4.2.5.5 RESPONSE STATUS SEGMENT (INFORMATION REPORTING REBILL) (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 561 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Information Reporting Rebill (Claim/Service):
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT N Information Reporting Rebill (Claim/Service):
Not used.
511-FB REJECT CODE N***R*** Information Reporting Rebill (Claim/Service):
Not used.
546-4F REJECT FIELD OCCURRENCE INDICATOR N***R*** Information Reporting Rebill (Claim/Service):
Not used.
547-5F APPROVED MESSAGE CODE COUNT N Information Reporting Rebill (Claim/Service):
Not used.
548-6F APPROVED MESSAGE CODE N**R*** Information Reporting Rebill (Claim/Service):
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Information Reporting Rebill (Claim/Service):
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Information Reporting Rebill (Claim/Service):
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Information Reporting Rebill (Claim/Service):
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Information Reporting Rebill (Claim/Service):
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Information Reporting Rebill (Claim/Service):
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Information Reporting Rebill (Claim/Service):
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER Q
N
Information Reporting Rebill (Claim):
Required for Medicare Part D payer-to-payer facilitation to
match the transaction response to the transaction.
Service:
Not used.
993-A7 INTERNAL CONTROL NUMBER N Information Reporting Rebill (Claim/Service):
Not used.
987-MA URL N Information Reporting Rebill (Claim/Service):
Not used.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 562 -
Notes on Response Status Segment on an Information Reporting Rebill Response:
The Response Status Segment is mandatory for an Information Reporting Rebill response for Header Response Status (5Ø1-F1) = “A”
(Accepted) and Transaction Response Status (112-AN) of “A” (Approved). The Response Status Segment is sent from the sender to the
receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.
23.4.2.5.6 RESPONSE CLAIM SEGMENT (INFORMATION REPORTING REBILL) (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT N Information Reporting Rebill (Claim/Service):
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER N**R*** Information Reporting Rebill (Claim/Service):
Not used.
553-AR PREFERRED PRODUCT ID
N***R*** Information Reporting Rebill (Claim/Service):
Not used.
554-AS PREFERRED PRODUCT INCENTIVE
N***R*** Information Reporting Rebill (Claim/Service):
Not used.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE
N***R*** Information Reporting Rebill (Claim/Service):
Not used.
556-AU PREFERRED PRODUCT DESCRIPTION N***R*** Information Reporting Rebill (Claim/Service):
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Information Reporting Rebill (Claim/Service):
Not used.
Notes on Response Claim Segment on an Information Reporting Rebill Response:
The Response Claim Segment is mandatory for an Information Reporting Rebill response when the Header Response Status (5Ø1-F1) is “A”
(Accepted) and Transaction Response Status (112-AN) of “A” (Approved).
The Response Claim Segment (Information Reporting – Service) is sent from the sender to the receiver to mirror back the Prescription/Service
Reference Number (4Ø2-D2).
Fields defined as Mandatory are required to be submitted when the segment is sent.
23.4.2.5.7 RESPONSE DUR/PPS SEGMENT (INFORMATION REPORTING REBILL) (TRANSMISSION
ACCEPTED/TRANSACTION APPROVED)
RESPONSE DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
567-J6 DUR/PPS RESPONSE CODE COUNTER Q**R*** Information Reporting Rebill (Claim/Service):
Maximum 9 occurrences supported.
Required if Reason For Service Code (439-E4) is used.
439-E4 REASON FOR SERVICE CODE
Q***R*** Information Reporting Rebill (Claim):
Required if utilization conflict is detected.
Service:
Required if professional service opportunity reason is
detected by the receiver. Should be different than the
original transmission.
528-FS CLINICAL SIGNIFICANCE CODE
Q***R*** Information Reporting Rebill (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
529-FT OTHER PHARMACY INDICATOR
Q***R*** Information Reporting Rebill (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 563 -
RESPONSE DUR/PPS SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
service.
53Ø-FU PREVIOUS DATE OF FILL
Q***R*** Information Reporting Rebill (Claim):
Required if needed to supply additional information for the
utilization conflict.
Required if Quantity of Previous Fill (531-FV) is used.
Service:
Required if needed to supply additional information for the
service.
Required if Quantity of Previous Fill (531-FV) is used.
531-FV QUANTITY OF PREVIOUS FILL
Q***R*** Information Reporting Rebill (Claim):
Required if Previous Date Of Fill (53Ø-FU) is used.
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
Required if Previous Date Of Fill (53Ø-FU) is used.
532-FW DATABASE INDICATOR
Q***R*** Information Reporting Rebill (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
533-FX OTHER PRESCRIBER INDICATOR
Q***R*** Information Reporting Rebill (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
544-FY DUR FREE TEXT MESSAGE
Q***R*** Information Reporting Rebill (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
57Ø-NS DUR ADDITIONAL TEXT Q***R*** Information Reporting Rebill (Claim):
Required if needed to supply additional information for the
utilization conflict.
Service:
Required if needed to supply additional information for the
service.
Notes on Response DUR/PPS Segment on an Information Reporting Rebill Response:
The Response DUR/PPS Segment is situational for an Information Reporting Rebill response when the Header Response Status (5Ø1-F1) of
"A" (Accepted) and Transaction Response Status (112-AN) of “A” (Approved). The Response DUR/PPS Segment is used to identify a drug
utilization review or professional pharmacy service event, opportunity, or information. Fields defined as Mandatory are required to be submitted
when the segment is sent.
23.4.3 TRANSMISSION ACCEPTED/TRANSACTION REJECTED
Information Reporting Rebill transmission response Header Response Status (5Ø1-F1) of "A" (Accepted)
and Transaction Response Status (112-AN) of “R” (Rejected)
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
23.4.3.1 DIAGRAM FOR TRANSMISSION OF ONE INFORMATION REPORTING REBILL RESPONSE
(TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 564 -
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
There are no situational transaction-level segments for Information Reporting Rebill transmission response Header Response Status (5Ø1-F1)
of "A" (Accepted) and Transaction Response Status (112-AN) of “R” (Rejected).
23.4.3.2 DIAGRAM FOR TRANSMISSION OF TWO INFORMATION REPORTING REBILL RESPONSES
(TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
23.4.3.3 DIAGRAM FOR TRANSMISSION OF THREE INFORMATION REPORTING REBILL RESPONSES
(TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 565 -
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
23.4.3.4 DIAGRAM FOR TRANSMISSION OF FOUR INFORMATION REPORTING REBILL RESPONSES
(TRANSMISSION ACCEPTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Segment Separator
Response Insurance Segment
Segment Separator
Response Patient Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
Segment Separator
Response Claim Segment
23.4.3.5 INFORMATION REPORTING REBILL RESPONSE SEGMENTS (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
23.4.3.5.1 RESPONSE HEADER SEGMENT (INFORMATION REPORTING REBILL) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 566 -
Notes on Response Header Segment on an Information Reporting Rebill Response:
The Response Header Segment is a mandatory, fixed length segment for an Information Reporting Rebill response when the Header
Response Status (5Ø1-F1) of "A" (Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). The “Situation” column is not
applicable.
23.4.3.5.2 RESPONSE MESSAGE SEGMENT (INFORMATION REPORTING REBILL) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Information Reporting Rebill (Claim/Service):
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on an Information Reporting Rebill Response:
The Response Message Segment is situational for an Information Reporting Rebill response when the Header Response Status (5Ø1-F1) of
"A" (Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). It is used when additional text information needs to be sent.
Fields defined as Mandatory are required to be submitted when the segment is sent.
23.4.3.5.3 RESPONSE INSURANCE SEGMENT (INFORMATION REPORTING REBILL) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
3Ø1-C1 GROUP ID
Q Information Reporting Rebill (Claim/Service):
Required if needed to identify the cardholder or employer
group, to identify appropriate group number for reporting.
Required to identify the actual group that was used when
multiple group coverages exist.
Note: This field may contain the Group ID echoed from the
request. May contain the actual Group ID if unknown to the
receiver.
524-FO PLAN ID
Q Information Reporting Rebill (Claim/Service):
Required if needed to identify the actual plan parameters,
benefit, or coverage criteria, when available.
Required to identify the actual plan ID that was used when
multiple group coverages exist.
Required if needed to contain the actual plan ID if unknown
to the receiver.
545-2F NETWORK REIMBURSEMENT ID
N Information Reporting Rebill (Claim/Service):
Not used.
568-J7 PAYER ID QUALIFIER Q Information Reporting Rebill (Claim/Service):
Required if Payer ID (569-J8) is used.
569-J8 PAYER ID
Q Information Reporting Rebill (Claim/Service):
Required to identify the ID of the payer responding.
115-N5
MEDICAID ID NUMBER N Information Reporting Rebill (Claim/Service):
Not used.
116-N6
MEDICAID AGENCY NUMBER N Information Reporting Rebill (Claim/Service):
Not used.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 567 -
RESPONSE INSURANCE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
3Ø2-C2
CARDHOLDER ID Q Information Reporting Rebill (Claim/Service):
Required if the identification to be used in future
transactions is different than what was submitted on the
request.
Notes on Response Insurance Segment on an Information Reporting Rebill Response:
The Response Insurance Segment is situational for an Information Reporting Rebill response when the Header Response Status (5Ø1-F1) of
"A" (Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). It is used when coverage or reimbursement parameters or
identifiers need to be sent. Fields defined as Mandatory are required to be submitted when the segment is sent.
23.4.3.5.4 RESPONSE PATIENT SEGMENT (INFORMATION REPORTING REBILL) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE PATIENT SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
31Ø-CA PATIENT FIRST NAME Q Information Reporting Rebill (Claim/Service):
Required if known.
311-CB PATIENT LAST NAME Q Information Reporting Rebill (Claim/Service):
Required if known.
3Ø4-C4 DATE OF BIRTH Q Information Reporting Rebill (Claim/Service):
Required if known.
Notes on Response Patient Segment on an Information Reporting Rebill Response:
The Response Patient Segment is situational for Information Reporting Rebill transmission response Header Response Status (5Ø1-F1) of "A"
(Accepted) and Transaction Response Status (112-AN) of “R” (Rejected) when patient demographic information needs to be sent from the
sender to the receiver. Fields defined as Mandatory are required to be submitted when the segment is sent.
23.4.3.5.5 RESPONSE STATUS SEGMENT (INFORMATION REPORTING REBILL) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Information Reporting Rebill (Claim/Service):
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT R Information Reporting Rebill (Claim/Service):
Maximum count of 5.
Required.
511-FB REJECT CODE R**R*** Information Reporting Rebill (Claim/Service):
Required.
546-4F REJECT FIELD OCCURRENCE INDICATOR Q**R*** Information Reporting Rebill (Claim/Service):
Required if a repeating field is in error, to identify repeating
field occurrence.
This field must be sent when relaying error information about
a repeating field or set. Note, if the Reject Code is not
denoting a repeating field or set, the Reject Field Occurrence
Indicator must not be sent.
547-5F APPROVED MESSAGE CODE COUNT N Information Reporting Rebill (Claim/Service):
Not used.
548-6F APPROVED MESSAGE CODE N**R*** Information Reporting Rebill (Claim/Service):
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Information Reporting Rebill (Claim/Service):
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q**R*** Information Reporting Rebill (Claim/Service):
Required if Additional Message Information (526-FQ) is
used.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 568 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Information Reporting Rebill (Claim/Service):
Required when additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Information Reporting Rebill (Claim/Service):
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Information Reporting Rebill (Claim/Service):
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Information Reporting Rebill (Claim/Service):
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER Q
N
Information Reporting Rebill (Claim):
Required for Medicare Part D payer-to-payer facilitation to
match the transaction response to the transaction.
Service:
Not used.
993-A7 INTERNAL CONTROL NUMBER N Information Reporting Rebill (Claim/Service):
Not used.
987-MA URL N Information Reporting Rebill (Claim/Service):
Not used.
Notes on Response Status Segment on an Information Reporting Rebill Response:
The Response Status Segment is mandatory for an Information Reporting Rebill response for Header Response Status (5Ø1-F1) = “A”
(Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). The Response Status Segment is sent from the sender to the
receiver to identify the outcome of the request. Fields defined as Mandatory are required to be submitted when the segment is sent.
23.4.3.5.6 RESPONSE CLAIM SEGMENT (INFORMATION REPORTING REBILL) (TRANSMISSION
ACCEPTED/TRANSACTION REJECTED)
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M Significant digits on submission must be returned on
response.
See section “Standard Conventions”, “Character Set
Designation Truncation”, “Numeric”, “Numeric Truncation”.
551-9F PREFERRED PRODUCT COUNT N Information Reporting Rebill (Claim/Service):
Not used.
552-AP PREFERRED PRODUCT ID QUALIFIER N**R*** Information Reporting Rebill (Claim/Service):
Not used.
553-AR PREFERRED PRODUCT ID
N**R*** Information Reporting Rebill (Claim/Service):
Not used.
554-AS PREFERRED PRODUCT INCENTIVE
N**R*** Information Reporting Rebill (Claim/Service):
Not used.
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE N**R*** Information Reporting Rebill (Claim/Service):
Not used.
556-AU PREFERRED PRODUCT DESCRIPTION N**R*** Information Reporting Rebill (Claim/Service):
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 569 -
RESPONSE CLAIM SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
Not used.
114-N4
MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
N Information Reporting Rebill (Claim/Service):
Not used.
Notes on Response Claim Segment on an Information Reporting Rebill Response:
The Response Claim Segment is mandatory for an Information Reporting Rebill response when the Header Response Status (5Ø1-F1) is “A”
(Accepted) and Transaction Response Status (112-AN) of “R” (Rejected). Fields defined as Mandatory are required to be submitted when the
segment is sent.
23.4.4 TRANSMISSION REJECTED/TRANSACTION REJECTED
Information Reporting Rebill transmission response Header Response Status (5Ø1-F1) of "R" (Rejected)
and Transaction Response Status (112-AN) of “R” (Rejected)
See section “Response Processing Guidelines”, “Duplicate Transactions” for the handling of a duplicate transaction.
23.4.4.1 DIAGRAM FOR TRANSMISSION OF ONE INFORMATION REPORTING REBILL RESPONSE
(TRANSMISSION REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
There are no situational transaction-level segments for Information Reporting Rebill transmission response Header Response Status (5Ø1-F1)
of "R" (Rejected) and Transaction Response Status (112-AN) of “R” (Rejected).
23.4.4.2 DIAGRAM FOR TRANSMISSION OF TWO INFORMATION REPORTING REBILL RESPONSES
(TRANSMISSION REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
23.4.4.3 DIAGRAM FOR TRANSMISSION OF THREE INFORMATION REPORTING REBILL RESPONSES
(TRANSMISSION REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Mandatory second response
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 570 -
Group Separator
Segment Separator
Response Status Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
23.4.4.4 DIAGRAM FOR TRANSMISSION OF FOUR INFORMATION REPORTING REBILL RESPONSES
(TRANSMISSION REJECTED/TRANSACTION REJECTED)
Mandatory
Response Header Segment
Situational
Segment Separator
Response Message Segment
Mandatory first response
Group Separator
Segment Separator
Response Status Segment
Mandatory second response
Group Separator
Segment Separator
Response Status Segment
Mandatory third response
Group Separator
Segment Separator
Response Status Segment
Mandatory fourth response
Group Separator
Segment Separator
Response Status Segment
23.4.4.5 INFORMATION REPORTING REBILL RESPONSES (TRANSMISSION REJECTED/TRANSACTION
REJECTED)
23.4.4.5.1 IN RESPONSE HEADER SEGMENT (INFORMATION REPORTING REBILL) (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
RESPONSE HEADER SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
Notes on Response Header Segment on an Information Reporting Rebill Response:
The Response Header Segment is a mandatory, fixed length segment for an Information Reporting Rebill response when the Header
Response Status (5Ø1-F1) of "R" (Rejected) and Transaction Response Status (112-AN) of “R” (Rejected). The “Situation” column is not
applicable.
If either the entire transmission or the Header is in error, the Header Response Status (5Ø1-F1) = “R” (Rejected). Every identifiable transaction
within the transmission must be rejected with an “R”.
If the transaction rejects for detail errors, the Header Response Status (5Ø1-F1) = “A” (Accepted) and the Transaction Response Status (112-
AN) will be “R”.
23.4.4.5.2 RESPONSE MESSAGE SEGMENT (INFORMATION REPORTING REBILL) (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 571 -
RESPONSE MESSAGE SEGMENT
SITUATIONAL SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE Q Information Reporting Rebill (Claim/Service):
Required if text is needed for clarification or detail.
When Transaction Count (1Ø9-A9) is = 1 (single
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
Notes on Response Message Segment on an Information Reporting Rebill Response:
The Response Message Segment is situational for an Information Reporting Rebill response when the Header Response Status (5Ø1-F1) of
"R" (Rejected) and Transaction Response Status (112-AN) of “R” (Rejected). It is used when additional text information needs to be sent.
Fields defined as Mandatory are required to be submitted when the segment is sent.
23.4.4.5.3 RESPONSE STATUS SEGMENT (INFORMATION REPORTING REBILL) (TRANSMISSION
REJECTED/TRANSACTION REJECTED)
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER Q Information Reporting Rebill (Claim/Service):
Required if needed to identify the transaction.
51Ø-FA REJECT COUNT R Information Reporting Rebill (Claim/Service):
Maximum count of 5.
Required.
511-FB REJECT CODE R**R*** Information Reporting Rebill (Claim/Service):
Required.
546-4F REJECT FIELD OCCURRENCE INDICATOR Q***R*** Information Reporting Rebill (Claim/Service):
Required if a repeating field is in error, to identify repeating
field occurrence.
This field must be sent when relaying error information about
a repeating field or set. Note, if the Reject Code is not
denoting a repeating field or set, the Reject Field Occurrence
Indicator must not be sent.
547-5F APPROVED MESSAGE CODE COUNT N Information Reporting Rebill (Claim/Service):
Not used.
548-6F APPROVED MESSAGE CODE N***R*** Information Reporting Rebill (Claim/Service):
Not used.
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT Q Information Reporting Rebill (Claim/Service):
Maximum count of 25.
Required if Additional Message Information (526-FQ) is
used.
Used to qualify the number of occurrences of the Additional
Message Information (526-FQ) that is included in the
Response Status Segment.
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER Q***R*** Information Reporting Rebill (Claim/Service):
Required if Additional Message Information (526-FQ) is
used.
526-FQ ADDITIONAL MESSAGE INFORMATION Q***R*** Information Reporting Rebill (Claim/Service):
Required if additional text is needed for clarification or
detail.
When Transaction Count (1Ø9-A9) is = 1 (single
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 572 -
RESPONSE STATUS SEGMENT
MANDATORY SEGMENT
Field Field Name Mandatory
or Situational Situation
transaction per transmission),
• The Additional Message Information (526-FQ)
may contain an extension of the Message (5Ø4-
F4), or
• The Message (5Ø4-F4) will contain
transmission-level text and Additional Message
Information (526-FQ) will contain transaction-
level text.
When Transaction Count (1Ø9-A9) is > 1 (multiple
transactions per transmission),
• The Message (5Ø4-F4) will only contain
transmission-level text, and Additional Message
Information (526-FQ) will only contain
transaction-level text.
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY Q***R*** Information Reporting Rebill (Claim/Service):
Required if and only if current repetition of Additional
Message Information (526-FQ) is used, another populated
repetition of Additional Message Information (526-FQ)
follows it, and the text of the following message is a
continuation of the current.
549-7F HELP DESK PHONE NUMBER QUALIFIER Q Information Reporting Rebill (Claim/Service):
Required if Help Desk Phone Number (55Ø-8F) is used.
55Ø-8F HELP DESK PHONE NUMBER
Q Information Reporting Rebill (Claim/Service):
Required if needed to provide a support telephone number
to the receiver.
88Ø-K5 TRANSACTION REFERENCE NUMBER Q
N
Information Reporting Rebill (Claim):
Required for Medicare Part D payer-to-payer facilitation to
match the transaction response to the transaction.
Service:
Not used.
993-A7 INTERNAL CONTROL NUMBER N Information Reporting Rebill (Claim/Service):
Not used.
987-MA URL N Information Reporting Rebill (Claim/Service):
Not used.
Notes on Response Status Segment on an Information Reporting Rebill Response:
The Response Status Segment is mandatory for an Information Reporting Rebill response for an Information Reporting or Information
Reporting Rebill response when the Header Response Status (5Ø1-F1) of "R" (Rejected) and Transaction Response Status (112-AN) of “R”
(Rejected). The Response Status Segment is sent from the sender to the receiver to identify the outcome of the request. Fields defined as
Mandatory are required to be submitted when the segment is sent.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 573 -
24. TRANSMISSION STRUCTURE
See section “Standard Conventions”, General Syntax Outline” for information about segment order.
24.1 REQUEST SEGMENT MATRICES BY FIELD WITHIN SEGMENT - LEGEND
DESIGNATION VALUE EXPLANATION
MANDATORY M
The Segment is mandatory for the Transaction
or
The Field is mandatory for the Segment for the Transaction.
Mandatory elements have structural requirements.
Mandatory are bolded for clarity.
SITUATIONAL The Segment has been further designated for usage for the Transaction
or
The Field has been further designated for usage for the Transaction.
Required R The Field has been designated with the situation of "Required" for the Segment for the Transaction.
Required are bolded italicized for clarity.
Required for Medicaid Subrogation only RM The Field has been designated with the situation of "Required" for the Segment for the Transaction for Medicaid Subrogation
usage only.
Required are bolded italicized for clarity.
Qualified Requirement Q The situations designated have qualifications for usage ("Required if x", "Not required if y").
Qualified Requirement for Medicaid
Subrogation only QM The situations designated have qualifications for usage ("Required if x", "Not required if y") for Medicaid Subrogation.
INFORMATIONAL ONLY I The Field is for informational purposes only for the Transaction.
OPTIONAL O The Field has been designated as optional usage (situations were not intentionally defined).
NOT USED N The Segment is not used for the Transaction
or
The Field is not used for the Segment for the Transaction.
Not used are shaded for clarity.
New Field/Segment Since 5.1
Field Name Change Since 5.1
Red underline denotes a modification (to D.Ø)
from Telecommunication Standard Version C.4
usage

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 574 -
24.2 REQUEST SEGMENT MATRICES BY FIELD WITHIN SEGMENT
24.2.1 ELIGIBILITY/CLAIM BILLING/CLAIM REBILL/ENCOUNTER/SERVICE BILLING/SERVICE REBILL/CLAIM REVERSAL/SERVICE
REVERSAL MATRIX
. Eligibility Claim Billing/Claim
Rebill/Encounter Predetermination Of
Benefits (Claim) Service
Billing/Service Rebill Claim Reversal Service
Reversal
TRANSACTION HEADER SEGMENT
1Ø1-A1 BIN Number M M M M M M
1Ø2-A2 Version Release Numbe
r
M M M M M M
1Ø3-A3 Transaction Code M M M M M M
1Ø4-A4 Processor Control Number M M M M M M
1Ø9-A9 Transaction Count M M M M M M
2Ø2-B2 Service Provider ID Qualifier M M M M M M
2Ø1-B1 Service Provider ID M M M M M M
4Ø1-D1 Date of Service M M M M M M
11Ø-AK Software Vendor/Certification ID M M M M M M
INSURANCE SEGMENT
111-AM Segment Identification M M M M M M
3Ø2-C2 Cardholder ID M M M M M M
312-CC Cardholder First Name Q Q Q Q N N
313-CD Cardholder Last Name Q Q Q Q N N
314-CE Home Plan Q Q Q Q N N
524-FO Plan ID N O O O N N
3Ø9-C9 Eligibility Clarification Code Q Q Q Q N N
3Ø1-C1 Group ID Q Q Q Q Q, QM Q
3Ø3-C3 Person Code Q Q Q Q N N
3Ø6-C6 Patient Relationship Code Q Q Q Q N N
99Ø-MG Other Payer BIN Number N N N N N N
991-MH Other Payer Processor Control Number N N N N N N
356-NU Other Payer Cardholder ID N N N N N N
992-MJ Other Payer Group ID N N N N N N
359-2A Medigap ID N Q Q Q Q N
36Ø-2B Medicaid Indicator N Q Q Q N N
361-2D Provider Accept Assignment Indicator N Q Q Q N N
997-G2 CMS Part D Defined Qualified Facility N Q Q N N N
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 575 -
. Eligibility Claim Billing/Claim
Rebill/Encounter Predetermination Of
Benefits (Claim) Service
Billing/Service Rebill Claim Reversal Service
Reversal
115-N5 Medicaid ID Number N Q, QM Q N N, QM N
116-N6 Medicaid Agency Number N N, QM N N N, QM N
PATIENT SEGMENT
111-AM Segment Identification M M M M
331-CX Patient ID Qualifier N Q Q Q
332-CY Patient ID N Q Q Q
3Ø4-C4 Date of Birth Q R R R
3Ø5-C5 Patient Gender Code Q R R R
31Ø-CA Patient First Name Q Q, QM Q Q
311-CB Patient Last Name Q R R R
322-CM Patient Street Address Q O, QM O O
323-CN Patient City Q O, QM O O
324-CO Patient State or Province Q O, QM O O
325-CP Patient Zip/Postal Code Q O, QM O O
326-CQ Patient Phone number N O O O
3Ø7-C7 Place of Service Q Q Q Q
333-CZ Employer ID N Q Q Q
334-1C Smoker/Non-smoker Code N N N N
335-2C Pregnancy Indicator Q Q Q Q
35Ø-HN Patient E-Mail Address N I I I
384-4X Patient Residence Q Q Q Q
PHARMACY PROVIDER SEGMENT
111-AM Segment Identification M M M M
465-EY Provider ID Qualifier Q Q Q Q
444-E9 Provider ID Q Q Q Q
CLAIM SEGMENT
111-AM Segment Identification M M M M M
455-EM Prescription/Service Reference Number Qualifier M M M M M
4Ø2-D2 Prescription/Service Reference Number M M M M M
436-E1 Product/Service ID Qualifier M M M M M
4Ø7-D7 Product/Service ID M M M M M
456-EN
A
ssociated Prescription/Service Reference Numbe
r
Q Q Q N N
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 576 -
. Eligibility Claim Billing/Claim
Rebill/Encounter Predetermination Of
Benefits (Claim) Service
Billing/Service Rebill Claim Reversal Service
Reversal
457-EP
A
ssociated Prescription/Service Date Q Q Q N N
458-SE Procedure Modifier Code Count Q Q Q N N
459-ER Procedure Modifier Code Q Q Q N N
442-E7 Quantity Dispensed R R
Q N N
4Ø3-D3 Fill Number R R
Q Q Q
4Ø5-D5 Days Supply R R
Q N N
4Ø6-D6 Compound Code R R
N N N
4Ø8-D8 Dispense as Written/Product Selection Code R R
N N N
414-DE Date Prescription Written R R
Q N N
415-DF Number of Refills Authorized Q Q Q N N
419-DJ Prescription Origin Code Q Q N N N
354-NX Submission Clarification Code Count Q Q N N N
42Ø-DK Submission Clarification Code Q Q N N N
46Ø-ET Quantity Prescribed N N Q N N
3Ø8-C8 Other Coverage Code Q Q Q Q Q
429-DT Special Packaging Indicator Q Q N N N
453-EJ Originally Prescribed Product/Service ID Qualifier Q Q Q N N
445-EA Originally Prescribed Product/Service Code Q Q Q N N
446-EB Originally Prescribed Quantity Q Q Q N N
33Ø-CW
A
lternate ID N N N N N
454-EK Scheduled Prescription ID Number N N N N N
6ØØ-28 Unit of Measure Q Q N N N
418-DI Level of Service Q Q Q N N
461-EU Prior Authorization Type Code Q Q Q N N
462-EV Prior Authorization Number Submitted Q Q Q N N
463-EW Intermediary Authorization Type ID Q Q Q N N
464-EX Intermediary Authorization ID Q Q Q N N
343-HD Dispensing Status Q Q N N N
344-HF Quantity Intended to be Dispensed Q Q N N N
345-HG Days Supply Intended to be Dispensed Q Q N N N
357-NV Delay Reason Code Q Q Q N N
88Ø-K5 Transaction Reference Number N N N N N
391-MT Patient Assignment Indicator (Direct Member
Reimbursement Indicator)
Q Q Q N N
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 577 -
. Eligibility Claim Billing/Claim
Rebill/Encounter Predetermination Of
Benefits (Claim) Service
Billing/Service Rebill Claim Reversal Service
Reversal
995-E2 Route of Administration Q Q N N N
996-G1 Compound Type Q Q N N N
114-N4 Medicaid Subrogation Internal Control
Number/Transaction Control Number (ICN/TCN)
N,RM N N N N
147-U7 Pharmacy Service Type Q Q Q Q Q
PRESCRIBER SEGMENT
111-AM Segment Identification M M M M
466-EZ Prescriber ID Qualifier Q Q Q Q
411-DB Prescriber ID Q Q Q Q
427-DR Prescriber Last Name Q Q Q Q
498-PM Prescriber Phone Number N Q Q Q
468-2E Primary Care Provider ID Qualifier Q Q Q Q
421-DL Primary Care Provider ID Q Q Q Q
47Ø-4E Primary Care Provider Last Name Q Q Q Q
364-2J Prescriber First Name Q Q Q Q
365-2K Prescriber Street Address N Q Q Q
366-2M Prescriber City Address N Q Q Q
367-2N Prescriber State/Province Address N Q Q Q
368-2P Prescriber ZIP/Postal Zone N Q Q Q
COORDINATION OF BENEFITS/OTHER PAYMENTS
SEGMENT
111-AM Segment Identification M M M M
337-4C Coordination of Benefits/Other Payments Count M M M M
338-5C Other Payer Coverage Type M M M M
339-6C Other Payer ID Qualifier Q Q N N
34Ø-7C Other Payer ID Q Q N N
443-E8 Other Payer Date Q Q N N
993-A7 Internal Control Number Q Q N N
341-HB Other Payer Amount Paid Count Q Q N N
342-HC Other Payer Amount Paid Qualifier Q Q N N
431-DV Other Payer Amount Paid Q Q N N
471-5E Other Payer Reject Count Q Q N N
472-6E Other Payer Reject Code Q Q N N
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 578 -
. Eligibility Claim Billing/Claim
Rebill/Encounter Predetermination Of
Benefits (Claim) Service
Billing/Service Rebill Claim Reversal Service
Reversal
353-NR Other Payer-Patient Responsibility Amount Count Q Q N N
351-NP Other Payer-Patient Responsibility Amount Qualifier Q Q N N
352-NQ Other Payer-Patient Responsibility Amount Q Q N N
392-MU Benefit Stage Count Q N N N
393-MV Benefit Stage Qualifier Q N N N
394-MW Benefit Stage Amount Q N N N
WORKERS’ COMPENSATION SEGMENT
111-AM Segment Identification M M
434-DY Date of Injury M M
315-CF Employer Name Q Q
316-CG Employer Street Address Q Q
317-CH Employer City Address Q Q
318-CI Employer State/Province Address Q Q
319-CJ Employer Zip/Postal Code Q Q
32Ø-CK Employer Phone Number Q Q
321-CL Employer Contact Name Q Q
327-CR Carrier ID Q Q
435-DZ Claim/Reference ID Q Q
117-TR Billing Entity Type Indicator R R
118-TS Pay To Qualifier Q Q
119-TT Pay To ID Q Q
12Ø-TU Pay To Name Q Q
121-TV Pay To Street Address Q Q
122-TW Pay To City Address Q Q
123-TX Pay To State/Province Address Q Q
124-TY Pay To ZIP/Postal Zone Q Q
125-TZ Generic Equivalent Product ID Qualifier Q Q
126-UA Generic Equivalent Product ID Q Q
DUR/PPS SEGMENT
111-AM Segment Identification M M M M
473-7E DUR/PPS Code Counter Q Q Q Q
439-E4 Reason for Service Code Q Q Q Q
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 579 -
. Eligibility Claim Billing/Claim
Rebill/Encounter Predetermination Of
Benefits (Claim) Service
Billing/Service Rebill Claim Reversal Service
Reversal
44Ø-E5 Professional Service Code Q Q Q Q
441-E6 Result of Service Code Q Q Q Q
474-8E DUR/PPS Level of Effort Q Q Q Q
475-J9 DUR Co-Agent ID Qualifier Q Q Q N
476-H6 DUR Co-Agent ID Q Q Q N
PRICING SEGMENT
111-AM Segment Identification M M M M
4Ø9-D9 Ingredient Cost Submitted R R
N N
412-DC Dispensing Fee Submitted Q Q N N
477-BE Professional Service Fee Submitted N N R N
433-DX Patient Paid Amount Submitted Q Q Q N
438-E3 Incentive Amount Submitted Q Q N Q
478-H7 Other Amount Claimed Submitted Count Q Q Q N
479-H8 Other Amount Claimed Submitted Qualifier Q Q Q N
48Ø-H9 Other Amount Claimed Submitted Q Q Q N
481-HA Flat Sales Tax Amount Submitted Q Q Q N
482-GE Percentage Sales Tax Amount Submitted Q Q Q N
483-HE Percentage Sales Tax Rate Submitted Q Q Q N
484-JE Percentage Sales Tax Basis Submitted Q Q N N
426-DQ Usual and Customary Charge Q Q Q N
43Ø-DU Gross Amount Due R R R Q
423-DN Basis of Cost Determination Q Q N N
113-N3 Medicaid Paid Amount N, QM N N N
COUPON SEGMENT
111-AM Segment Identification M
485-KE Coupon Type M
486-ME Coupon Number M
487-NE Coupon Value Amount Q
COMPOUND SEGMENT
111-AM. Segment Identification M M
45Ø-EF Compound Dosage Form Description Code M M
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 580 -
. Eligibility Claim Billing/Claim
Rebill/Encounter Predetermination Of
Benefits (Claim) Service
Billing/Service Rebill Claim Reversal Service
Reversal
451-EG Compound Dispensing Unit Form Indicator M M
447-EC Compound Ingredient Component Count M M
488-RE Compound Product ID Qualifier M M
489-TE Compound Product ID M M
448-ED Compound Ingredient Quantity M M
449-EE Compound Ingredient Drug Cost Q Q
49Ø-UE Compound Ingredient Basis of Cost Determination Q Q
362-2G Compound Ingredient Modifier Code Count Q Q
363-2H Compound Ingredient Modifier Code Q Q
PRIOR AUTHORIZATION SEGMENT
111-AM Segment Identification
498-PA Request Type
498-PB Request Period Date - Begin
498-PC Request Period Date - End
498-PD Basis of Request
498-PE
A
uthorized Representative First Name
498-PF
A
uthorized Rep. Last Name
498-PG
A
uthorized Rep. Street Address
498-PH
A
uthorized Rep. Cit
y
498-PJ
A
uthorized Rep. State/Province
498-PK
A
uthorized Rep. Zip/Postal Code
498-PY Prior Authorization Number - Assigned
5Ø3-F3
A
uthorization Numbe
r
498-PP Prior Authorization Supporting Documentation
CLINICAL SEGMENT
111-AM Segment Identification M M M
491-VE Diagnosis Code Count Q Q Q
492-WE Diagnosis Code Qualifier Q Q Q
424-DO Diagnosis Code Q Q Q
493-XE Clinical Information Counter Q Q Q
494-ZE Measurement Date Q Q Q
495-H1 Measurement Time Q Q Q
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 581 -
. Eligibility Claim Billing/Claim
Rebill/Encounter Predetermination Of
Benefits (Claim) Service
Billing/Service Rebill Claim Reversal Service
Reversal
496-H2 Measurement Dimension Q Q Q
497-H3 Measurement Unit Q Q Q
499-H4 Measurement Value Q Q Q
ADDITIONAL DOCUMENTATION SEGMENT
111-AM Segment Identification M M M
369-2Q
A
dditional Documentation Type ID M M M
374-2V Request Period Begin Date Q Q Q
375-2W Request Period Recert/Revised Date Q Q Q
373-2U Request Status Q Q Q
371-2S Length Of Need Qualifier Q Q Q
37Ø-2R Length Of Need Q Q Q
372-2T Prescriber/Supplier Date Signed Q Q Q
376-2X Supporting Documentation Q Q Q
377-2Z Question Number/Letter Count Q Q Q
378-4B Question Number/Letter Q Q Q
379-4D Question Percent Response Q Q Q
38Ø-4G Question Date Response Q Q Q
381-4H Question Dollar Amount Response Q Q Q
382-4J Question Numeric Response Q Q Q
383-4K Question Alphanumeric Response Q Q Q
FACILITY SEGMENT
111-AM Segment Identification M M M
336-BC Facility ID Q Q Q
385-3Q Facility Name Q Q Q
386-3U Facility Street Address Q Q Q
388-5J Facility City Address Q Q Q
387-3V Facility State/Province Address Q Q Q
389-6D Facility ZIP/Postal Zone Q Q Q
NARRATIVE SEGMENT
111-AM Segment Identification M M
39Ø-BM Narrative Message Q Q
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 582 -
24.2.2 PRIOR AUTHORIZATION REQUEST AND BILLING (CLAIM/SERVICE)/PRIOR AUTHORIZATION REVERSAL/PRIOR
AUTHORIZATION INQUIRY/PRIOR AUTHORIZATION REQUEST ONLY (CLAIM/SERVICE) MATRIX
Prior Authorization
Request and Billing
(Claim)
Prior Authorization
Request and Billing
(Service)
Prior Authorization
Reversal
(Claim/Service)
Prior
Authorization
Inquiry
Prior
Authorization
Request Only
(Claim)
Prior
Authorization
Request Only
(Service)
TRANSACTION HEADER SEGMENT
1Ø1-A1 BIN Number M M M M M M
1Ø2-A2 Version Release Numbe
r
M M M M M M
1Ø3-A3 Transaction Code M M M M M M
1Ø4-A4 Processor Control Number M M M M M M
1Ø9-A9 Transaction Count M M M M M M
2Ø2-B2 Service Provider ID Qualifier M M M M M M
2Ø1-B1 Service Provider ID M M M M M M
4Ø1-D1 Date of Service M M M M M M
11Ø-AK Software Vendor/Certification ID M M M M M M
INSURANCE SEGMENT
111-AM Segment Identification M M M M M M
3Ø2-C2 Cardholder ID M M M M M M
312-CC Cardholder First Name Q Q N N Q Q
313-CD Cardholder Last Name Q Q N N Q Q
314-CE Home Plan Q Q N N Q Q
524-FO Plan ID Q Q N N Q Q
3Ø9-C9 Eligibility Clarification Code Q Q N N Q Q
3Ø1-C1 Group ID Q Q N N Q Q
3Ø3-C3 Person Code Q Q N N Q Q
3Ø6-C6 Patient Relationship Code Q Q N N Q Q
99Ø-MG Other Payer BIN Number N N N N N N
991-MH Other Payer Processor Control Number N N N N N N
356-NU Other Payer Cardholder ID N N N N N N
992-MJ Other Payer Group ID N N N N N N
359-2A Medigap ID Q Q N N Q Q
36Ø-2B Medicaid Indicator Q Q N N Q Q
361-2D Provider Accept Assignment Indicator Q Q N N N N
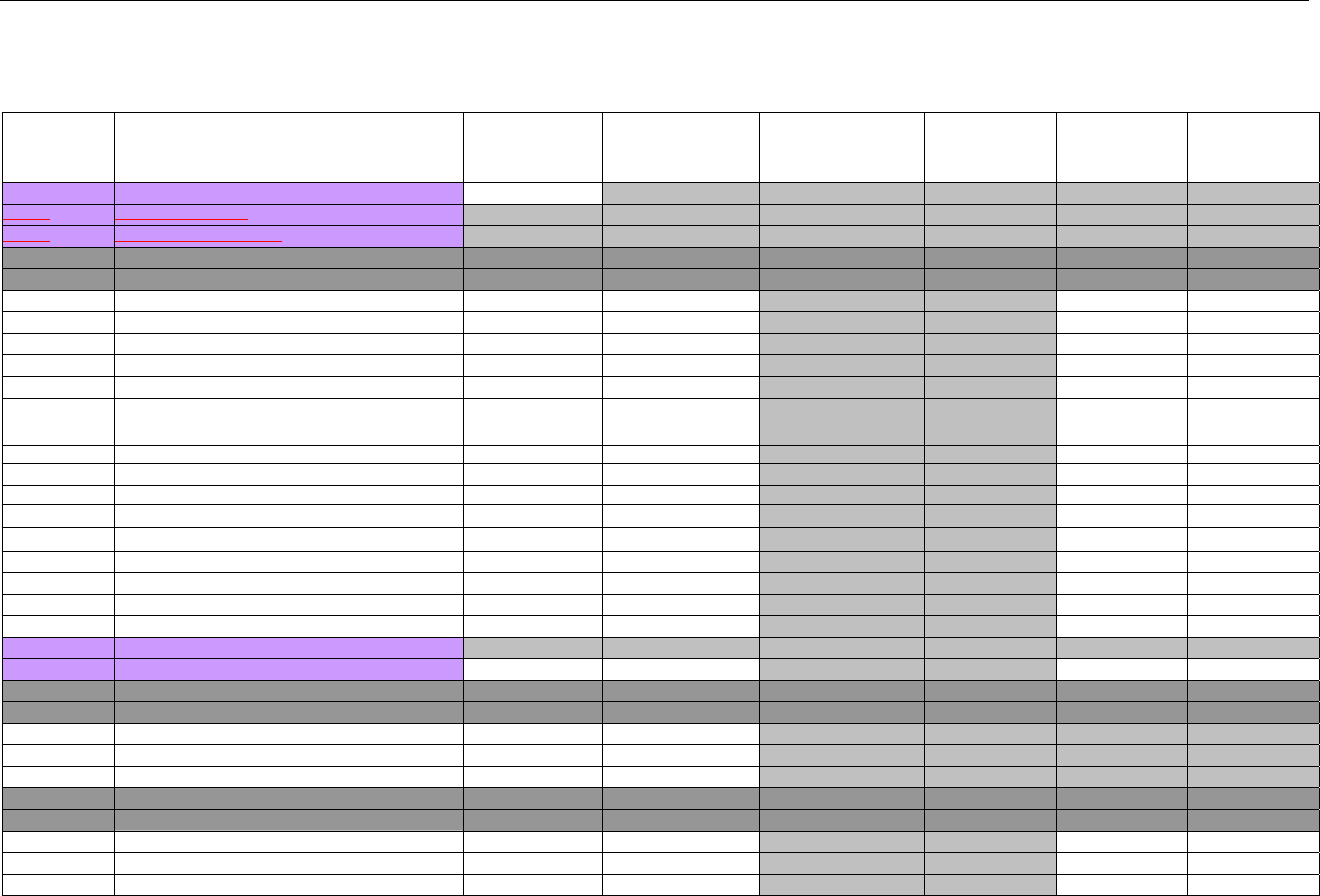
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 583 -
Prior Authorization
Request and Billing
(Claim)
Prior Authorization
Request and Billing
(Service)
Prior Authorization
Reversal
(Claim/Service)
Prior
Authorization
Inquiry
Prior
Authorization
Request Only
(Claim)
Prior
Authorization
Request Only
(Service)
997-G2 CMS Part D Defined Qualified Facility Q N N N N N
115-N5 Medicaid ID Number N N N N N N
116-N6 Medicaid Agency Number N N N N N N
PATIENT SEGMENT
111-AM Segment Identification M M M M
331-CX Patient ID Qualifier Q Q Q Q
332-CY Patient ID Q Q Q Q
3Ø4-C4 Date of Birth R R Q Q
3Ø5-C5 Patient Gender Code R R Q Q
31Ø-CA Patient First Name Q Q Q Q
311-CB Patient Last Name R R Q Q
322-CM Patient Street Address O O Q Q
323-CN Patient City O O Q Q
324-CO Patient State or Province O O Q Q
325-CP Patient Zip/Postal Code O O Q Q
326-CQ Patient Phone number O O Q Q
3Ø7-C7 Place of Service Q Q Q Q
333-CZ Employer ID Q Q Q Q
334-1C Smoker/Non-smoker Code Q Q Q Q
335-2C Pregnancy Indicator Q Q Q Q
35Ø-HN Patient E-Mail Address N N N N
384-4X Patient Residence Q Q Q Q
PHARMACY PROVIDER SEGMENT
111-AM Segment Identification M M
465-EY Provider ID Qualifier Q Q
444-E9 Provider ID Q Q
CLAIM SEGMENT
111-AM Segment Identification M M M M
455-EM Prescription/Service Reference Number Qualifier M M M M
4Ø2-D2 Prescription/Service Reference Number M M M M
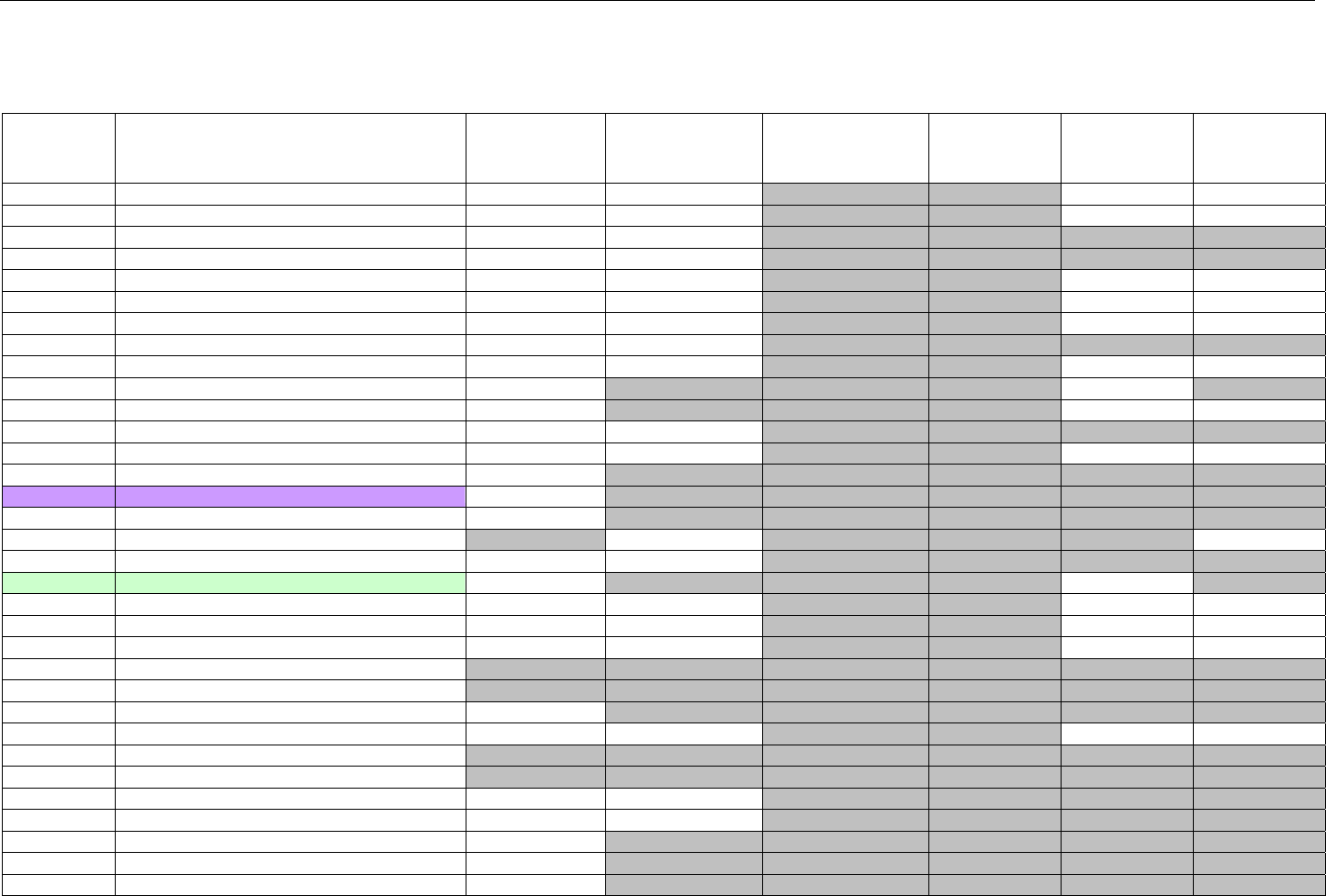
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 584 -
Prior Authorization
Request and Billing
(Claim)
Prior Authorization
Request and Billing
(Service)
Prior Authorization
Reversal
(Claim/Service)
Prior
Authorization
Inquiry
Prior
Authorization
Request Only
(Claim)
Prior
Authorization
Request Only
(Service)
436-E1 Product/Service ID Qualifier M M M M
4Ø7-D7 Product/Service ID M M M M
456-EN
A
ssociated Prescription/Service Reference Number Q Q N N
457-EP
A
ssociated Prescription/Service Date Q Q N N
458-SE Procedure Modifier Code Count Q Q Q Q
459-ER Procedure Modifier Code Q Q Q Q
442-E7 Quantity Dispensed R Q R Q
4Ø3-D3 Fill Number R Q N N
4Ø5-D5 Days Supply R Q R Q
4Ø6-D6 Compound Code R N Q N
4Ø8-D8 Dispense as Written/Product Selection Code R N Q Q
414-DE Date Prescription Written R Q N N
415-DF Number of Refills Authorized Q Q R R
419-DJ Prescription Origin Code Q N N N
354-NX Submission Clarification Code Count Q N N N
42Ø-DK Submission Clarification Code Q N N N
46Ø-ET Quantity Prescribed N Q N Q
3Ø8-C8 Other Coverage Code Q Q N N
429-DT Special Packaging Indicator Q N Q N
453-EJ Originally Prescribed Product/Service ID Qualifier Q Q Q Q
445-EA Originally Prescribed Product/Service Code Q Q Q Q
446-EB Originally Prescribed Quantity Q Q Q Q
33Ø-CW
A
lternate ID N N N N
454-EK Scheduled Prescription ID Number N N N N
6ØØ-28 Unit of Measure Q N N N
418-DI Level of Service Q Q Q Q
461-EU Prior Authorization Type Code N N N N
462-EV Prior Authorization Number Submitted N N N N
463-EW Intermediary Authorization Type ID Q Q N N
464-EX Intermediary Authorization ID Q Q N N
343-HD Dispensing Status Q N N N
344-HF Quantity Intended to be Dispensed Q N N N
345-HG Days Supply Intended to be Dispensed Q N N N
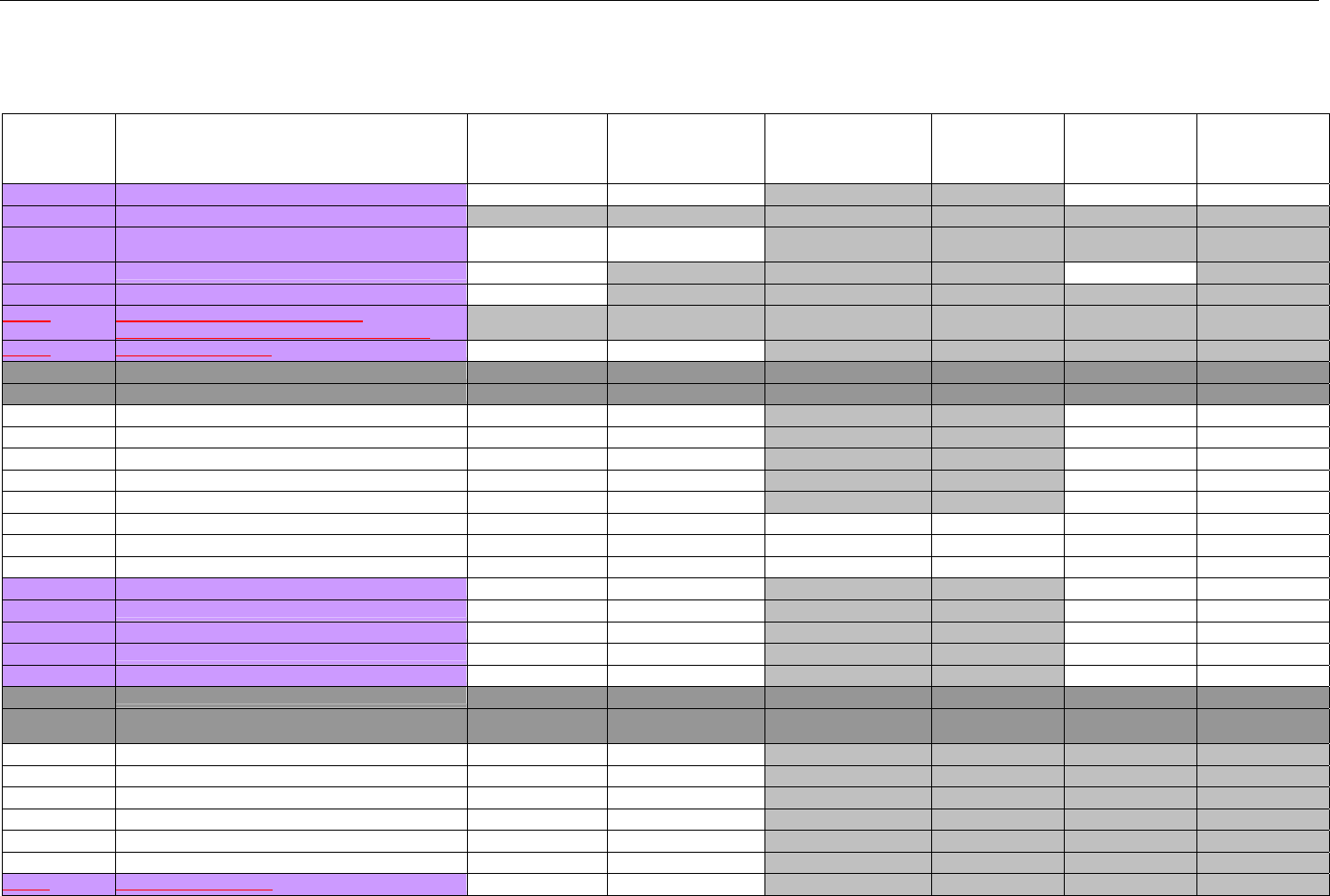
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 585 -
Prior Authorization
Request and Billing
(Claim)
Prior Authorization
Request and Billing
(Service)
Prior Authorization
Reversal
(Claim/Service)
Prior
Authorization
Inquiry
Prior
Authorization
Request Only
(Claim)
Prior
Authorization
Request Only
(Service)
357-NV Delay Reason Code Q Q Q Q
88Ø-K5 Transaction Reference Number N N N N
391-MT Patient Assignment Indicator (Direct Member
Reimbursement Indicator)
Q Q N N
995-E2 Route of Administration Q N Q N
996-G1 Compound Type Q N N N
114-N4 Medicaid Subrogation Internal Control
Number/Transaction Control Number (ICN/TCN)
N N N N
147-U7 Pharmacy Service Type Q Q N N
PRESCRIBER SEGMENT
111-AM Segment Identification M M M M
466-EZ Prescriber ID Qualifier Q Q Q Q
411-DB Prescriber ID Q Q Q Q
427-DR Prescriber Last Name Q Q Q Q
498-PM Prescriber Phone Number Q Q Q Q
468-2E Primary Care Provider ID Qualifier Q Q Q Q
421-DL Primary Care Provider ID Q Q Q Q
47Ø-4E Primary Care Provider Last Name Q Q Q Q
364-2J Prescriber First Name Q Q Q Q
365-2K Prescriber Street Address Q Q Q Q
366-2M Prescriber City Address Q Q Q Q
367-2N Prescriber State/Province Address Q Q Q Q
368-2P Prescriber ZIP/Postal Zone Q Q Q Q
COORDINATION OF BENEFITS/OTHER
PAYMENTS SEGMENT
111-AM Segment Identification M M
337-4C Coordination of Benefits/Other Payments Count M M
338-5C Other Payer Coverage Type M M
339-6C Other Payer ID Qualifier Q Q
34Ø-7C Other Payer ID Q Q
443-E8 Other Payer Date Q Q
993-A7 Internal Control Number Q Q
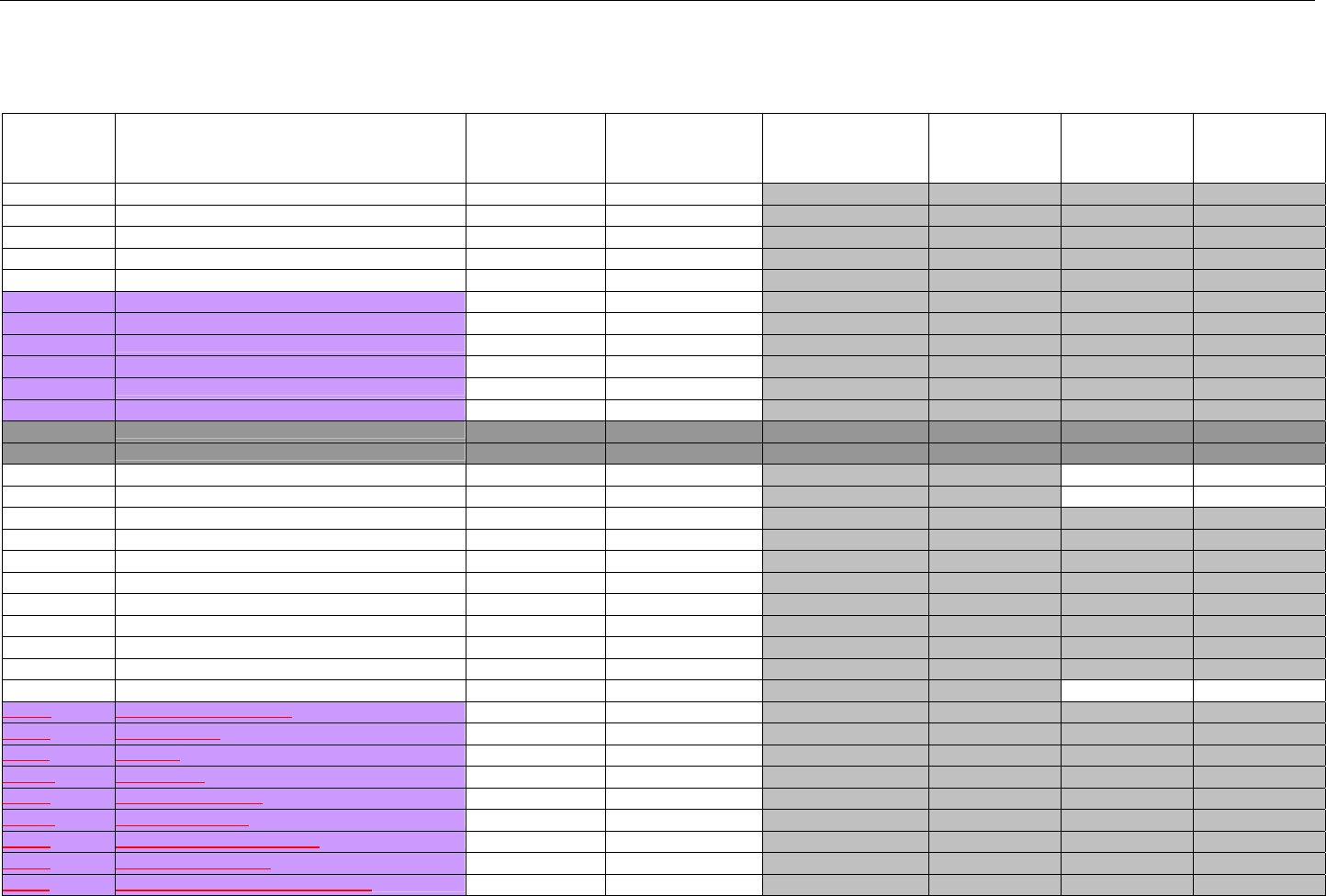
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 586 -
Prior Authorization
Request and Billing
(Claim)
Prior Authorization
Request and Billing
(Service)
Prior Authorization
Reversal
(Claim/Service)
Prior
Authorization
Inquiry
Prior
Authorization
Request Only
(Claim)
Prior
Authorization
Request Only
(Service)
341-HB Other Payer Amount Paid Count Q Q
342-HC Other Payer Amount Paid Qualifier Q Q
431-DV Other Payer Amount Paid Q Q
471-5E Other Payer Reject Count Q Q
472-6E Other Payer Reject Code Q Q
353-NR Other Payer-Patient Responsibility Amount Count Q Q
351-NP Other Payer-Patient Responsibility Amount Qualifier Q Q
352-NQ Other Payer-Patient Responsibility Amount Q Q
392-MU Benefit Stage Count Q Q
393-MV Benefit Stage Qualifier Q Q
394-MW Benefit Stage Amount Q Q
WORKERS’ COMPENSATION SEGMENT
111-AM Segment Identification M M M M
434-DY Date of Injury M M M M
315-CF Employer Name Q Q N N
316-CG Employer Street Address Q Q N N
317-CH Employer City Address Q Q N N
318-CI Employer State/Province Address Q Q N N
319-CJ Employer Zip/Postal Code Q Q N N
32Ø-CK Employer Phone Number Q Q N N
321-CL Employer Contact Name Q Q N N
327-CR Carrier ID Q Q N N
435-DZ Claim/Reference ID Q Q Q Q
117-TR Billing Entity Type Indicator R R N N
118-TS Pay To Qualifier Q Q N N
119-TT Pay To ID Q Q N N
12Ø-TU Pay To Name Q Q N N
121-TV Pay To Street Address Q Q N N
122-TW Pay To City Address Q Q N N
123-TX Pay To State/Province Address Q Q N N
124-TY Pay To ZIP/Postal Zone Q Q N N
125-TZ Generic Equivalent Product ID Qualifier Q Q N N
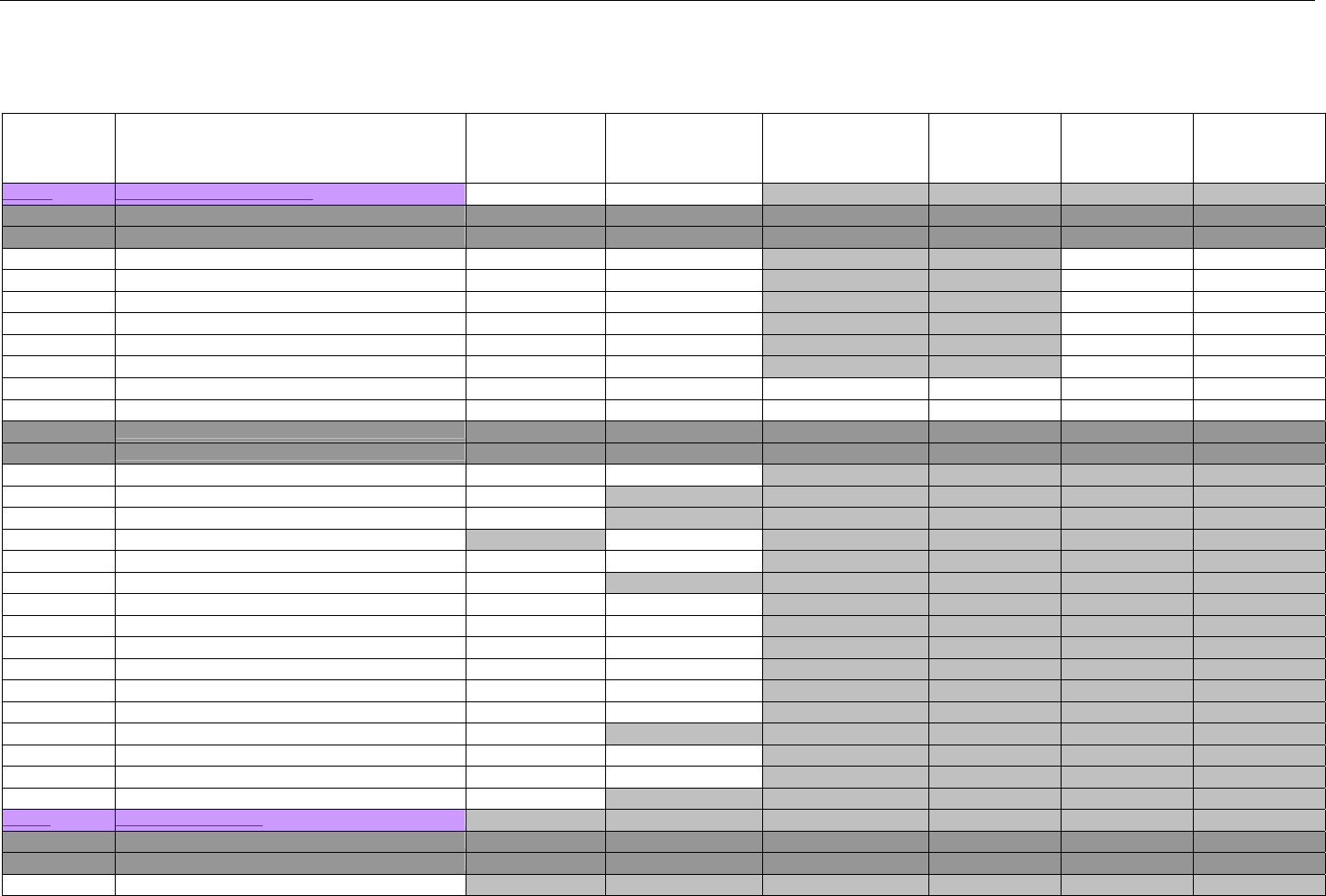
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 587 -
Prior Authorization
Request and Billing
(Claim)
Prior Authorization
Request and Billing
(Service)
Prior Authorization
Reversal
(Claim/Service)
Prior
Authorization
Inquiry
Prior
Authorization
Request Only
(Claim)
Prior
Authorization
Request Only
(Service)
126-UA Generic Equivalent Product ID Q Q N N
DUR/PPS SEGMENT
111-AM Segment Identification M M M M
473-7E DUR/PPS Code Counter Q Q Q Q
439-E4 Reason for Service Code Q Q Q Q
44Ø-E5 Professional Service Code Q Q Q Q
441-E6 Result of Service Code Q Q Q Q
474-8E DUR/PPS Level of Effort Q Q Q Q
475-J9 DUR Co-Agent ID Qualifier Q Q Q Q
476-H6 DUR Co-Agent ID Q Q Q Q
PRICING SEGMENT
111-AM Segment Identification M M
4Ø9-D9 Ingredient Cost Submitted R N
412-DC Dispensing Fee Submitted Q N
477-BE Professional Service Fee Submitted N R
433-DX Patient Paid Amount Submitted Q Q
438-E3 Incentive Amount Submitted Q N
478-H7 Other Amount Claimed Submitted Count Q Q
479-H8 Other Amount Claimed Submitted Qualifier Q Q
48Ø-H9 Other Amount Claimed Submitted Q Q
481-HA Flat Sales Tax Amount Submitted Q Q
482-GE Percentage Sales Tax Amount Submitted Q Q
483-HE Percentage Sales Tax Rate Submitted Q Q
484-JE Percentage Sales Tax Basis Submitted Q N
426-DQ Usual and Customary Charge Q Q
43Ø-DU Gross Amount Due R R
423-DN Basis of Cost Determination Q N
113-N3 Medicaid Paid Amount N N
COUPON SEGMENT
111-AM Segment Identification
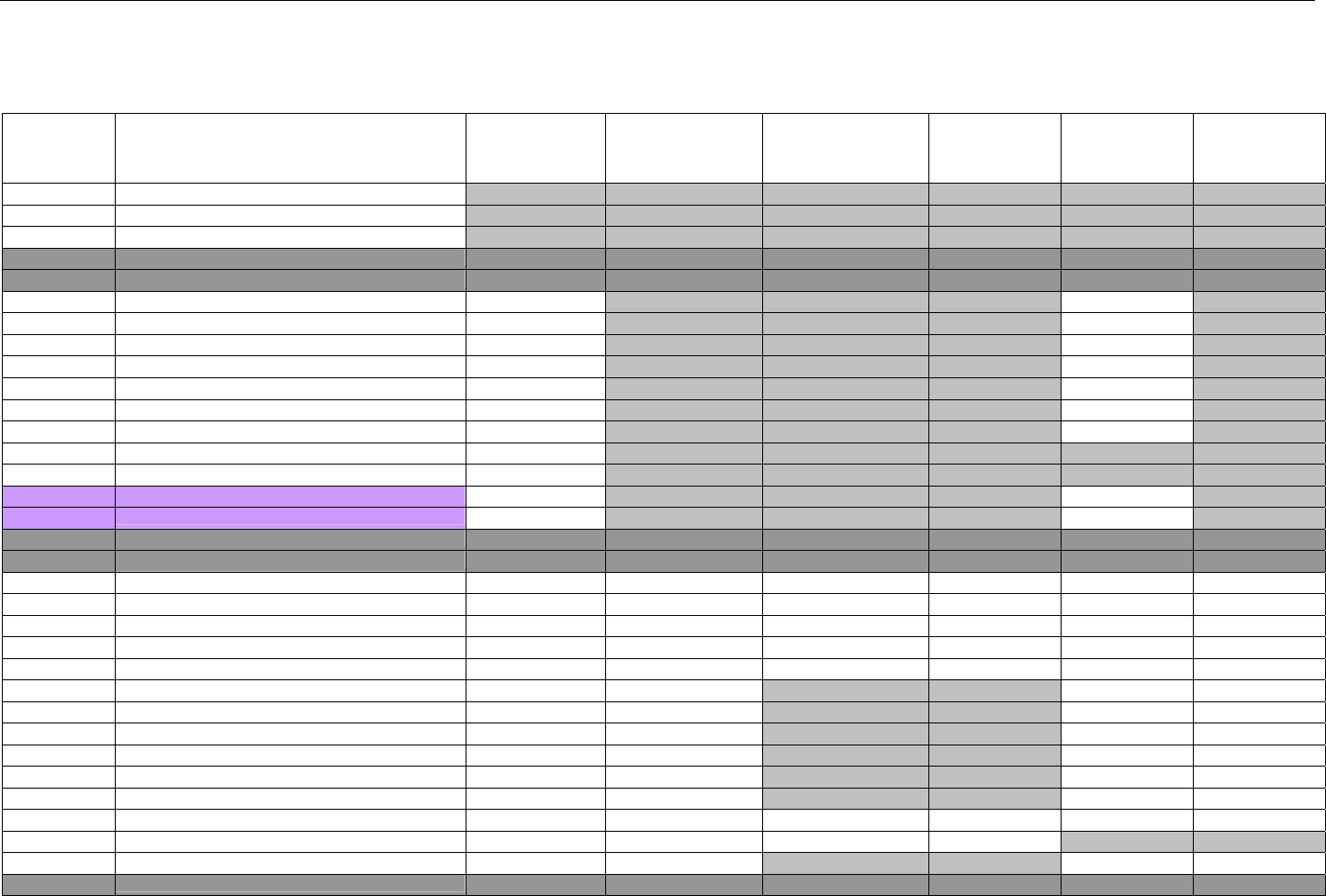
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 588 -
Prior Authorization
Request and Billing
(Claim)
Prior Authorization
Request and Billing
(Service)
Prior Authorization
Reversal
(Claim/Service)
Prior
Authorization
Inquiry
Prior
Authorization
Request Only
(Claim)
Prior
Authorization
Request Only
(Service)
485-KE Coupon Type
486-ME Coupon Number
487-NE Coupon Value Amount
COMPOUND SEGMENT
111-AM. Segment Identification M M
45Ø-EF Compound Dosage Form Description Code M M
451-EG Compound Dispensing Unit Form Indicator M M
447-EC Compound Ingredient Component Count M M
488-RE Compound Product ID Qualifier M M
489-TE Compound Product ID M M
448-ED Compound Ingredient Quantity M M
449-EE Compound Ingredient Drug Cost Q N
49Ø-UE Compound Ingredient Basis of Cost Determination Q N
362-2G Compound Ingredient Modifier Code Count Q Q
363-2H Compound Ingredient Modifier Code Q Q
PRIOR AUTHORIZATION SEGMENT
111-AM Segment Identification M M M M M M
498-PA Request Type M M M M M M
498-PB Request Period Date - Begin M M M M M M
498-PC Request Period Date - End M M M M M M
498-PD Basis of Request M M M M M M
498-PE
A
uthorized Representative First Name Q Q N N Q Q
498-PF
A
uthorized Rep. Last Name Q Q N N Q Q
498-PG
A
uthorized Rep. Street Address Q Q N N Q Q
498-PH
A
uthorized Rep. Cit
y
Q Q N N Q Q
498-PJ
A
uthorized Rep. State/Province Q Q N N Q Q
498-PK
A
uthorized Rep. Zip/Postal Code Q Q N N Q Q
498-PY Prior Authorization Number - Assigned Q Q Q Q Q Q
5Ø3-F3
A
uthorization Numbe
r
Q Q Q Q N N
498-PP Prior Authorization Supporting Documentation Q Q N N Q Q

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 589 -
Prior Authorization
Request and Billing
(Claim)
Prior Authorization
Request and Billing
(Service)
Prior Authorization
Reversal
(Claim/Service)
Prior
Authorization
Inquiry
Prior
Authorization
Request Only
(Claim)
Prior
Authorization
Request Only
(Service)
CLINICAL SEGMENT
111-AM Segment Identification M M M M
491-VE Diagnosis Code Count Q Q Q Q
492-WE Diagnosis Code Qualifier Q Q Q Q
424-DO Diagnosis Code Q Q Q Q
493-XE Clinical Information Counter Q Q Q Q
494-ZE Measurement Date Q Q Q Q
495-H1 Measurement Time Q Q Q Q
496-H2 Measurement Dimension Q Q Q Q
497-H3 Measurement Unit Q Q Q Q
499-H4 Measurement Value Q Q Q Q
ADDITIONAL DOCUMENTATION SEGMENT
111-AM Segment Identification M M
369-2Q
A
dditional Documentation Type ID M M
374-2V Request Period Begin Date Q Q
375-2W Request Period Recert/Revised Date Q Q
373-2U Request Status Q Q
371-2S Length Of Need Qualifier Q Q
37Ø-2R Length Of Need Q Q
372-2T Prescriber/Supplier Date Signed Q Q
376-2X Supporting Documentation Q Q
377-2Z Question Number/Letter Count Q Q
378-4B Question Number/Letter Q Q
379-4D Question Percent Response Q Q
38Ø-4G Question Date Response Q Q
381-4H Question Dollar Amount Response Q Q
382-4J Question Numeric Response Q Q
383-4K Question Alphanumeric Response Q Q
FACILITY SEGMENT
111-AM Segment Identification M M
336-BC Facility ID Q Q

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 590 -
Prior Authorization
Request and Billing
(Claim)
Prior Authorization
Request and Billing
(Service)
Prior Authorization
Reversal
(Claim/Service)
Prior
Authorization
Inquiry
Prior
Authorization
Request Only
(Claim)
Prior
Authorization
Request Only
(Service)
385-3Q Facility Name Q Q
386-3U Facility Street Address Q Q
388-5J Facility City Address Q Q
387-3V Facility State/Province Address Q Q
389-6D Facility ZIP/Postal Zone Q Q
NARRATIVE SEGMENT
111-AM Segment Identification M M
39Ø-BM Narrative Message Q Q
24.2.3 INFORMATION REPORTING (CLAIM/SERVICE)/INFORMATION REPORTING REBILL (CLAIM/SERVICE)/INFORMATION REPORTING
REVERSAL (CLAIM/SERVICE) MATRIX
Information
Reporting (Claim) Information Reporting
(Service) Information
Reporting Rebill
(Claim)
Information
Reporting Rebill
(Service)
Information
Reporting Reversal
(Claim)
Information
Reporting Reversal
(Service)
TRANSACTION HEADER SEGMENT
1Ø1-A1 BIN Number M M M M M M
1Ø2-A2 Version Release Numbe
r
M M M M M M
1Ø3-A3 Transaction Code M M M M M M
1Ø4-A4 Processor Control Number M M M M M M
1Ø9-A9 Transaction Count M M M M M M
2Ø2-B2 Service Provider ID Qualifier M M M M M M
2Ø1-B1 Service Provider ID M M M M M M
4Ø1-D1 Date of Service M M M M M M
11Ø-AK Software Vendor/Certification ID M M M M M M
INSURANCE SEGMENT
111-AM Segment Identification M M M M M M
3Ø2-C2 Cardholder ID M M M M M M
312-CC Cardholder First Name Q Q Q Q N N
313-CD Cardholder Last Name Q Q Q Q N N
314-CE Home Plan Q Q Q Q N N
524-FO Plan ID Q Q Q Q N N

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 591 -
Information
Reporting (Claim) Information Reporting
(Service) Information
Reporting Rebill
(Claim)
Information
Reporting Rebill
(Service)
Information
Reporting Reversal
(Claim)
Information
Reporting Reversal
(Service)
3Ø9-C9 Eligibility Clarification Code Q Q Q Q N N
3Ø1-C1 Group ID Q Q Q Q Q Q
3Ø3-C3 Person Code Q Q Q Q N N
3Ø6-C6 Patient Relationship Code Q Q Q Q N N
99Ø-MG Other Payer BIN Number Q N Q N Q N
991-MH Other Payer Processor Control Number Q N Q N Q N
356-NU Other Payer Cardholder ID Q N Q N Q N
992-MJ Other Payer Group ID Q N Q N Q N
359-2A Medigap ID Q Q Q Q N N
36Ø-2B Medicaid Indicator Q Q Q Q N N
361-2D Provider Accept Assignment Indicator N N N N N N
997-G2 CMS Part D Defined Qualified Facility Q N Q N N N
115-N5 Medicaid ID Number N N N N N N
116-N6 Medicaid Agency Number N N N N N N
PATIENT SEGMENT
111-AM Segment Identification M M M M
331-CX Patient ID Qualifier Q Q Q Q
332-CY Patient ID Q Q Q Q
3Ø4-C4 Date of Birth R R R R
3Ø5-C5 Patient Gender Code Q Q Q Q
31Ø-CA Patient First Name Q Q Q Q
311-CB Patient Last Name Q Q Q Q
322-CM Patient Street Address Q Q Q Q
323-CN Patient City Q Q Q Q
324-CO Patient State or Province Q Q Q Q
325-CP Patient Zip/Postal Code Q Q Q Q
326-CQ Patient Phone number Q Q Q Q
3Ø7-C7 Place of Service Q Q Q Q
333-CZ Employer ID Q Q Q Q
334-1C Smoker/Non-smoker Code Q Q Q Q
335-2C Pregnancy Indicator Q Q Q Q
35Ø-HN Patient E-Mail Address I I I I
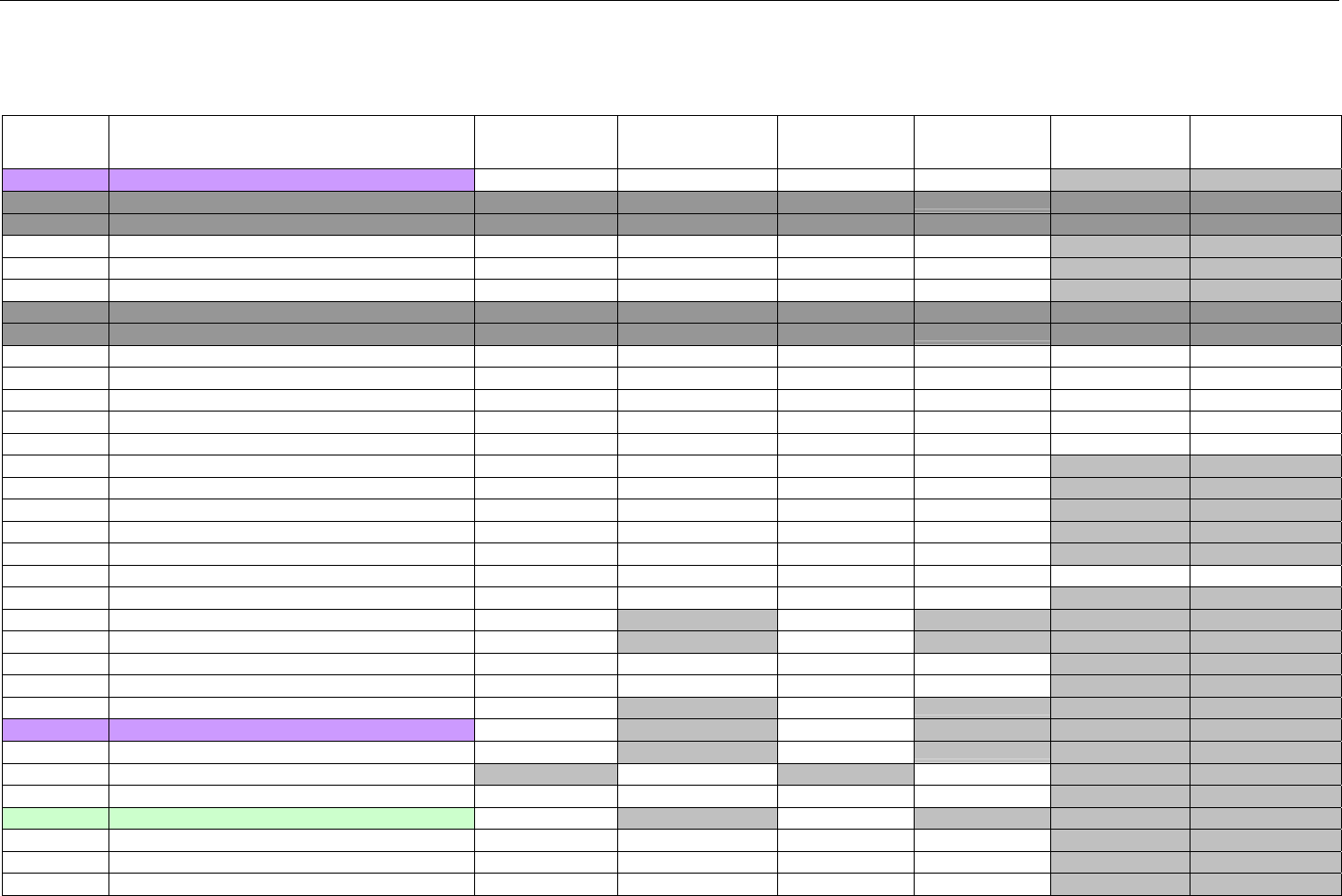
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 592 -
Information
Reporting (Claim) Information Reporting
(Service) Information
Reporting Rebill
(Claim)
Information
Reporting Rebill
(Service)
Information
Reporting Reversal
(Claim)
Information
Reporting Reversal
(Service)
384-4X Patient Residence Q Q Q Q
PHARMACY PROVIDER SEGMENT
111-AM Segment Identification M M M M
465-EY Provider ID Qualifier Q Q Q Q
444-E9 Provider ID Q Q Q Q
CLAIM SEGMENT
111-AM Segment Identification M M M M M M
455-EM Prescription/Service Reference Number Qualifier M M M M M M
4Ø2-D2 Prescription/Service Reference Number M M M M M M
436-E1 Product/Service ID Qualifier M M M M M M
4Ø7-D7 Product/Service ID M M M M M M
456-EN
A
ssociated Prescription/Service Reference Numbe
r
Q Q Q Q N N
457-EP
A
ssociated Prescription/Service Date Q Q Q Q N N
458-SE Procedure Modifier Code Count Q Q Q Q N N
459-ER Procedure Modifier Code Q Q Q Q N N
442-E7 Quantity Dispensed Q Q Q Q N N
4Ø3-D3 Fill Number Q Q Q Q Q Q
4Ø5-D5 Days Supply Q Q Q Q N N
4Ø6-D6 Compound Code Q N Q N N N
4Ø8-D8 Dispense as Written/Product Selection Code Q N Q N N N
414-DE Date Prescription Written Q Q Q Q N N
415-DF Number of Refills Authorized Q Q Q Q N N
419-DJ Prescription Origin Code Q N Q N N N
354-NX Submission Clarification Code Count Q N Q N N N
42Ø-DK Submission Clarification Code Q N Q N N N
46Ø-ET Quantity Prescribed N Q N Q N N
3Ø8-C8 Other Coverage Code Q Q Q Q N N
429-DT Special Packaging Indicator Q N Q N N N
453-EJ Originally Prescribed Product/Service ID Qualifier Q Q Q Q N N
445-EA Originally Prescribed Product/Service Code Q Q Q Q N N
446-EB Originally Prescribed Quantity Q Q Q Q N N
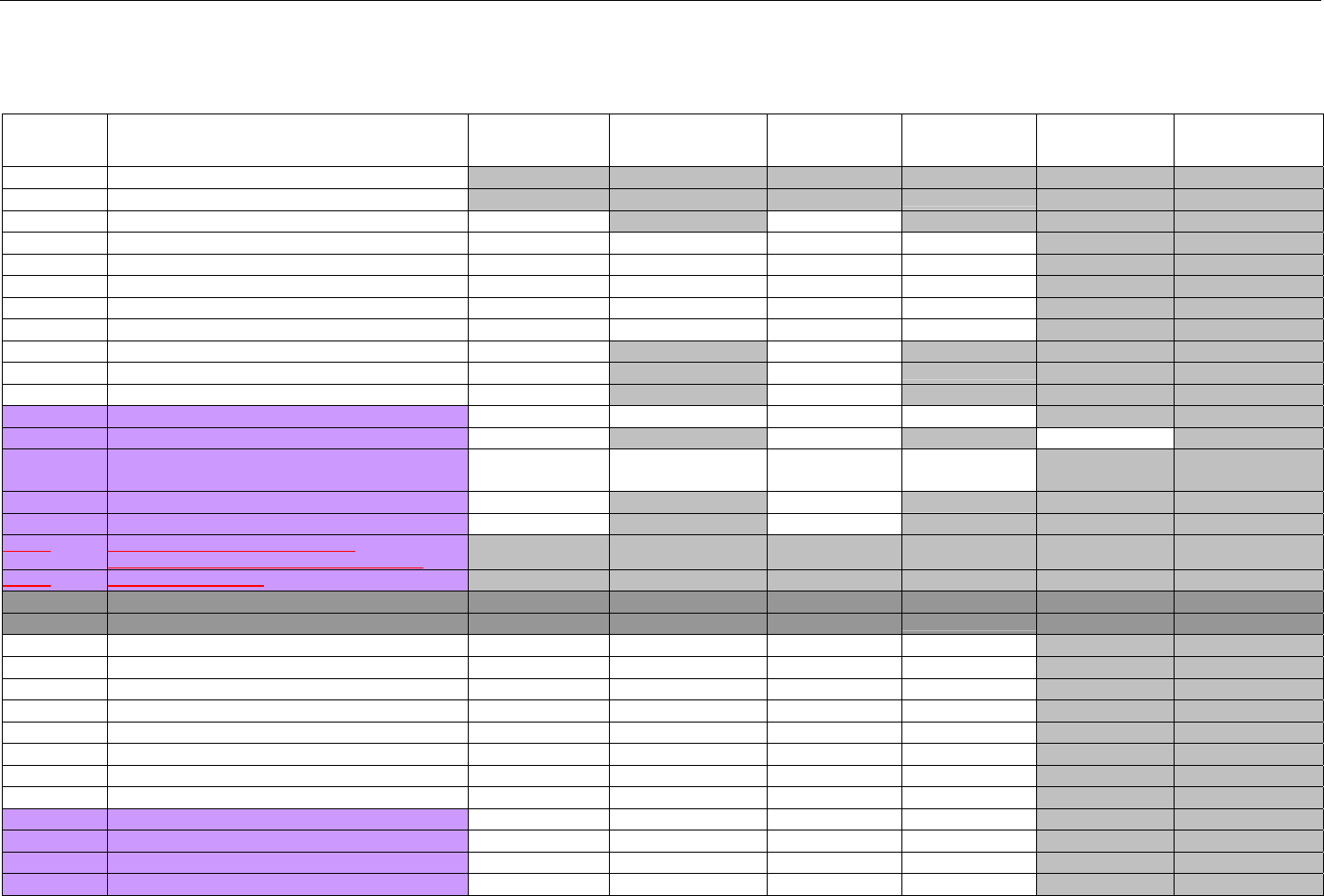
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 593 -
Information
Reporting (Claim) Information Reporting
(Service) Information
Reporting Rebill
(Claim)
Information
Reporting Rebill
(Service)
Information
Reporting Reversal
(Claim)
Information
Reporting Reversal
(Service)
33Ø-CW
A
lternate ID N N N N N N
454-EK Scheduled Prescription ID Number N N N N N N
6ØØ-28 Unit of Measure Q N Q N N N
418-DI Level of Service Q Q Q Q N N
461-EU Prior Authorization Type Code Q Q Q Q N N
462-EV Prior Authorization Number Submitted Q Q Q Q N N
463-EW Intermediary Authorization Type ID Q Q Q Q N N
464-EX Intermediary Authorization ID Q Q Q Q N N
343-HD Dispensing Status Q N Q N N N
344-HF Quantity Intended to be Dispensed Q N Q N N N
345-HG Days Supply Intended to be Dispensed Q N Q N N N
357-NV Delay Reason Code Q Q Q Q N N
88Ø-K5 Transaction Reference Number Q N Q N Q N
391-MT Patient Assignment Indicator (Direct Member
Reimbursement Indicator)
Q Q Q Q
N N
995-E2 Route of Administration Q N Q N N N
996-G1 Compound Type Q N Q N N N
114-N4 Medicaid Subrogation Internal Control
Number/Transaction Control Number (ICN/TCN)
N N N N N N
147-U7 Pharmacy Service Type N N N N N N
PRESCRIBER SEGMENT
111-AM Segment Identification M M M M
466-EZ Prescriber ID Qualifier Q Q Q Q
411-DB Prescriber ID Q Q Q Q
427-DR Prescriber Last Name Q Q Q Q
498-PM Prescriber Phone Number Q Q Q Q
468-2E Primary Care Provider ID Qualifier Q Q Q Q
421-DL Primary Care Provider ID Q Q Q Q
47Ø-4E Primary Care Provider Last Name Q Q Q Q
364-2J Prescriber First Name Q Q Q Q
365-2K Prescriber Street Address Q Q Q Q
366-2M Prescriber City Address Q Q Q Q
367-2N Prescriber State/Province Address Q Q Q Q
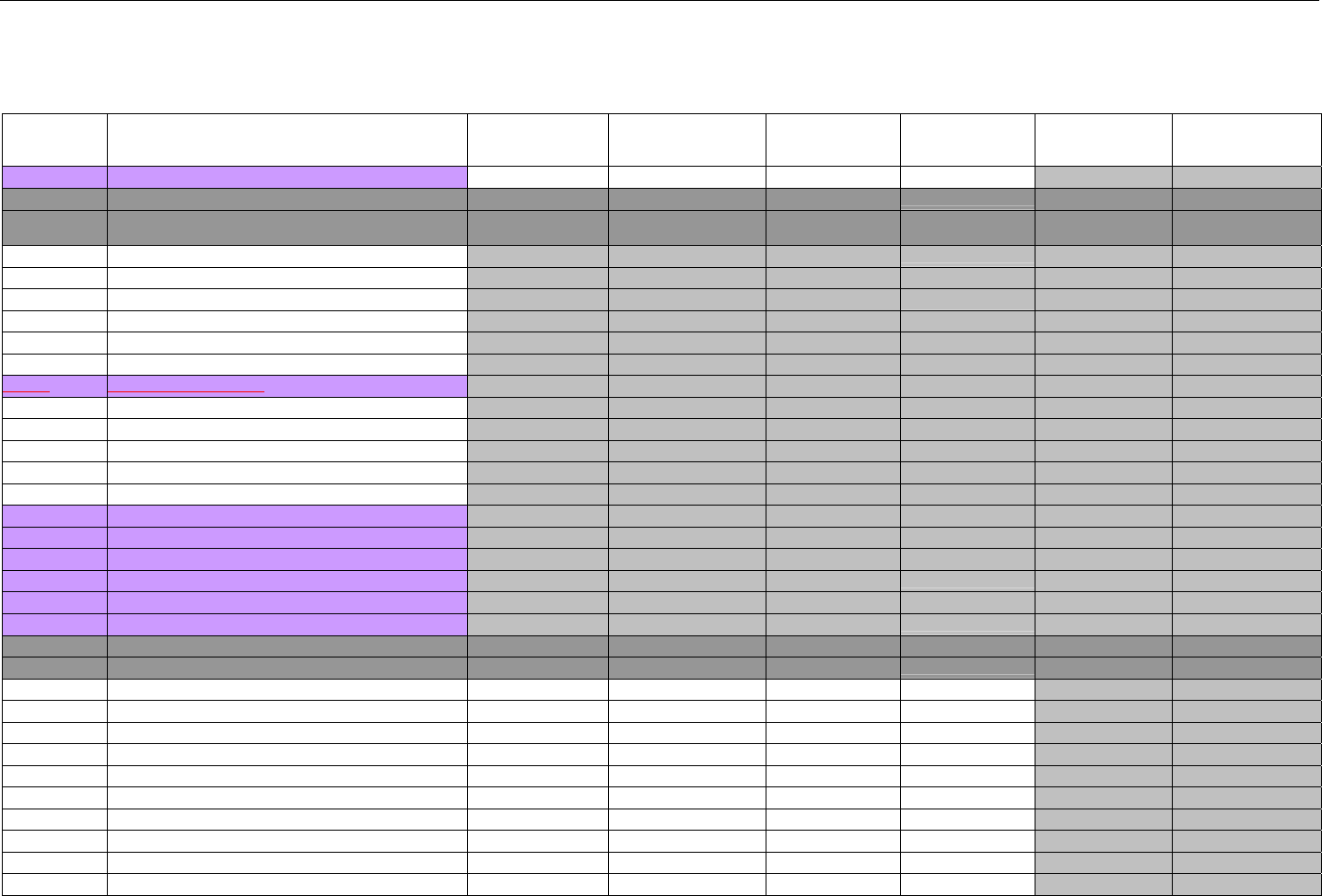
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 594 -
Information
Reporting (Claim) Information Reporting
(Service) Information
Reporting Rebill
(Claim)
Information
Reporting Rebill
(Service)
Information
Reporting Reversal
(Claim)
Information
Reporting Reversal
(Service)
368-2P Prescriber ZIP/Postal Zone Q Q Q Q
COORDINATION OF BENEFITS/OTHER PAYMENTS
SEGMENT
111-AM Segment Identification
337-4C Coordination of Benefits/Other Payments Count
338-5C Other Payer Coverage Type
339-6C Other Payer ID Qualifier
34Ø-7C Other Payer ID
443-E8 Other Payer Date
993-A7 Internal Control Number
341-HB Other Payer Amount Paid Count
342-HC Other Payer Amount Paid Qualifier
431-DV Other Payer Amount Paid
471-5E Other Payer Reject Count
472-6E Other Payer Reject Code
353-NR Other Payer-Patient Responsibility Amount Count
351-NP Other Payer-Patient Responsibility Amount Qualifier
352-NQ Other Payer-Patient Responsibility Amount
392-MU Benefit Stage Count
393-MV Benefit Stage Qualifier
394-MW Benefit Stage Amount
WORKERS’ COMPENSATION SEGMENT
111-AM Segment Identification M M M M
434-DY Date of Injury M M M M
315-CF Employer Name Q Q Q Q
316-CG Employer Street Address Q Q Q Q
317-CH Employer City Address Q Q Q Q
318-CI Employer State/Province Address Q Q Q Q
319-CJ Employer Zip/Postal Code Q Q Q Q
32Ø-CK Employer Phone Number Q Q Q Q
321-CL Employer Contact Name Q Q Q Q
327-CR Carrier ID Q Q Q Q
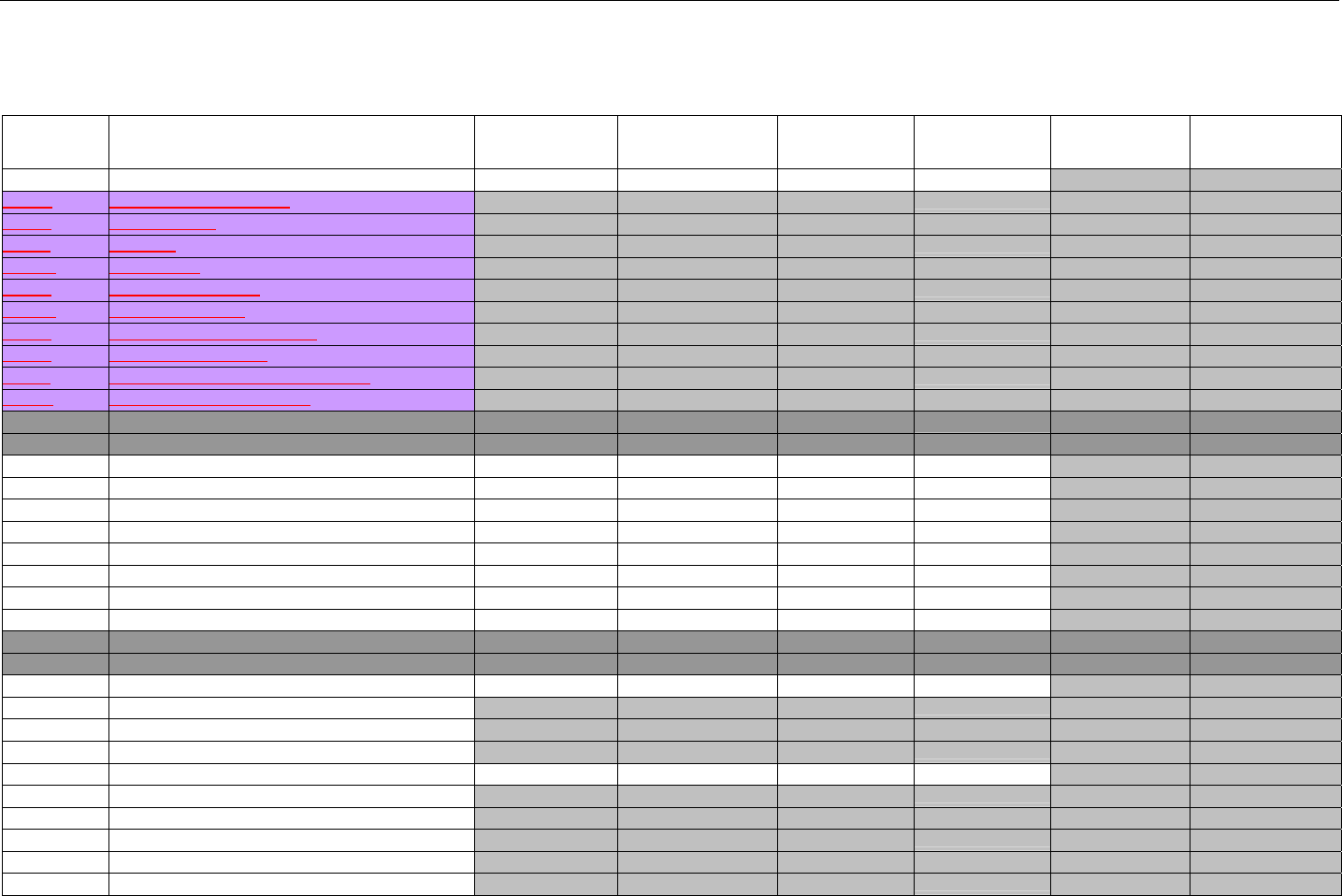
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 595 -
Information
Reporting (Claim) Information Reporting
(Service) Information
Reporting Rebill
(Claim)
Information
Reporting Rebill
(Service)
Information
Reporting Reversal
(Claim)
Information
Reporting Reversal
(Service)
435-DZ Claim/Reference ID Q Q Q Q
117-TR Billing Entity Type Indicator N N N N
118-TS Pay To Qualifier N N N N
119-TT Pay To ID N N N N
12Ø-TU Pay To Name N N N N
121-TV Pay To Street Address N N N N
122-TW Pay To City Address N N N N
123-TX Pay To State/Province Address N N N N
124-TY Pay To ZIP/Postal Zone N N N N
125-TZ Generic Equivalent Product ID Qualifier N N N N
126-UA Generic Equivalent Product ID N N N N
DUR/PPS SEGMENT
111-AM Segment Identification M M M M
473-7E DUR/PPS Code Counter Q Q Q Q
439-E4 Reason for Service Code Q Q Q Q
44Ø-E5 Professional Service Code Q Q Q Q
441-E6 Result of Service Code Q Q Q Q
474-8E DUR/PPS Level of Effort Q Q Q Q
475-J9 DUR Co-Agent ID Qualifier Q Q Q Q
476-H6 DUR Co-Agent ID Q Q Q Q
PRICING SEGMENT
111-AM Segment Identification M M M M
4Ø9-D9 Ingredient Cost Submitted N N N N
412-DC Dispensing Fee Submitted N N N N
477-BE Professional Service Fee Submitted N N N N
433-DX Patient Paid Amount Submitted Q Q Q Q
438-E3 Incentive Amount Submitted N N N N
478-H7 Other Amount Claimed Submitted Count N N N N
479-H8 Other Amount Claimed Submitted Qualifier N N N N
48Ø-H9 Other Amount Claimed Submitted N N N N
481-HA Flat Sales Tax Amount Submitted N N N N
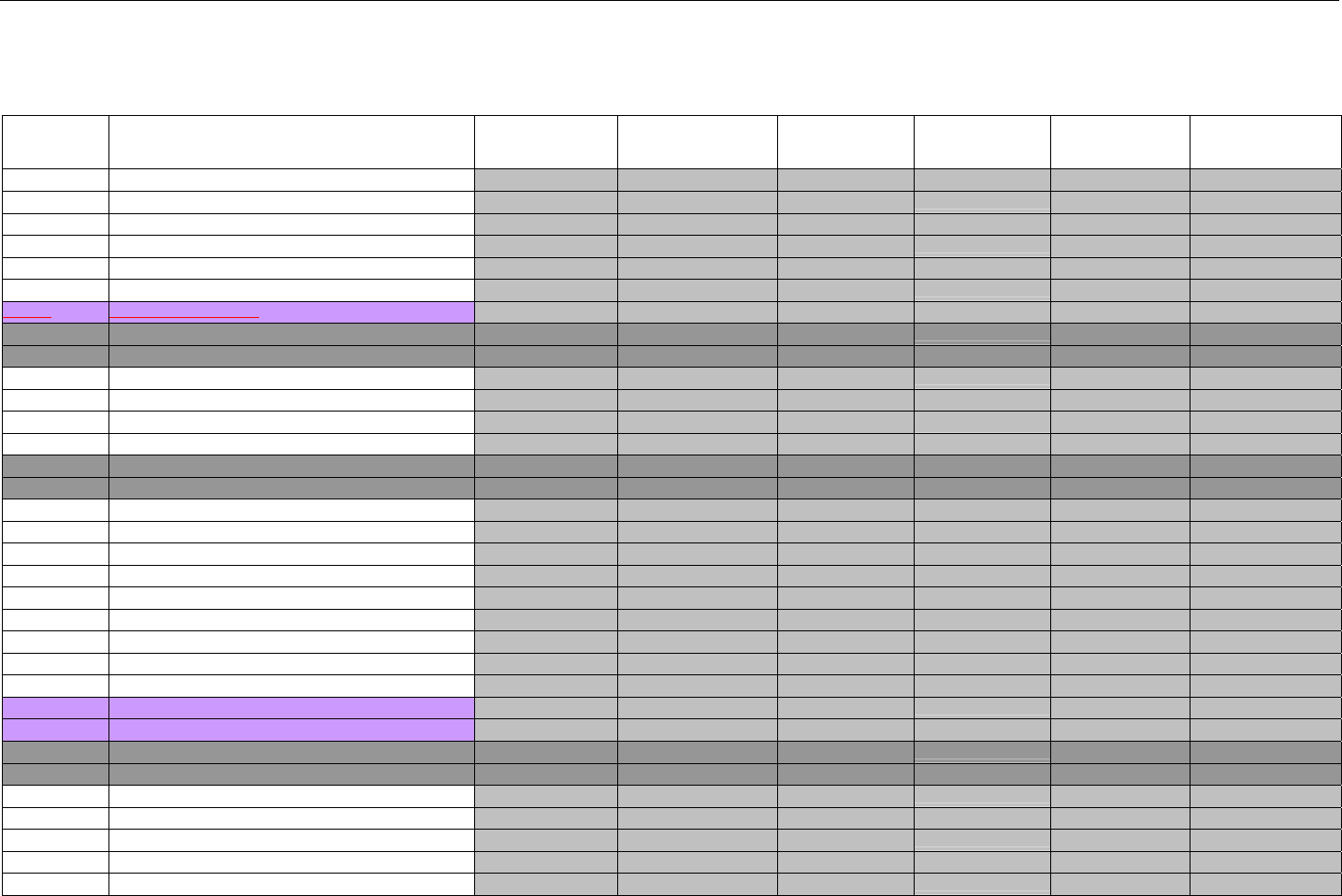
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 596 -
Information
Reporting (Claim) Information Reporting
(Service) Information
Reporting Rebill
(Claim)
Information
Reporting Rebill
(Service)
Information
Reporting Reversal
(Claim)
Information
Reporting Reversal
(Service)
482-GE Percentage Sales Tax Amount Submitted N N N N
483-HE Percentage Sales Tax Rate Submitted N N N N
484-JE Percentage Sales Tax Basis Submitted N N N N
426-DQ Usual and Customary Charge N N N N
43Ø-DU Gross Amount Due N N N N
423-DN Basis of Cost Determination N N N N
113-N3 Medicaid Paid Amount N N N N
COUPON SEGMENT
111-AM Segment Identification
485-KE Coupon Type
486-ME Coupon Number
487-NE Coupon Value Amount
COMPOUND SEGMENT
111-AM. Segment Identification
45Ø-EF Compound Dosage Form Description Code
451-EG Compound Dispensing Unit Form Indicator
447-EC Compound Ingredient Component Count
488-RE Compound Product ID Qualifier
489-TE Compound Product ID
448-ED Compound Ingredient Quantity
449-EE Compound Ingredient Drug Cost
49Ø-UE Compound Ingredient Basis of Cost Determination
362-2G Compound Ingredient Modifier Code Count
363-2H Compound Ingredient Modifier Code
PRIOR AUTHORIZATION SEGMENT
111-AM Segment Identification
498-PA Request Type
498-PB Request Period Date - Begin
498-PC Request Period Date - End
498-PD Basis of Request
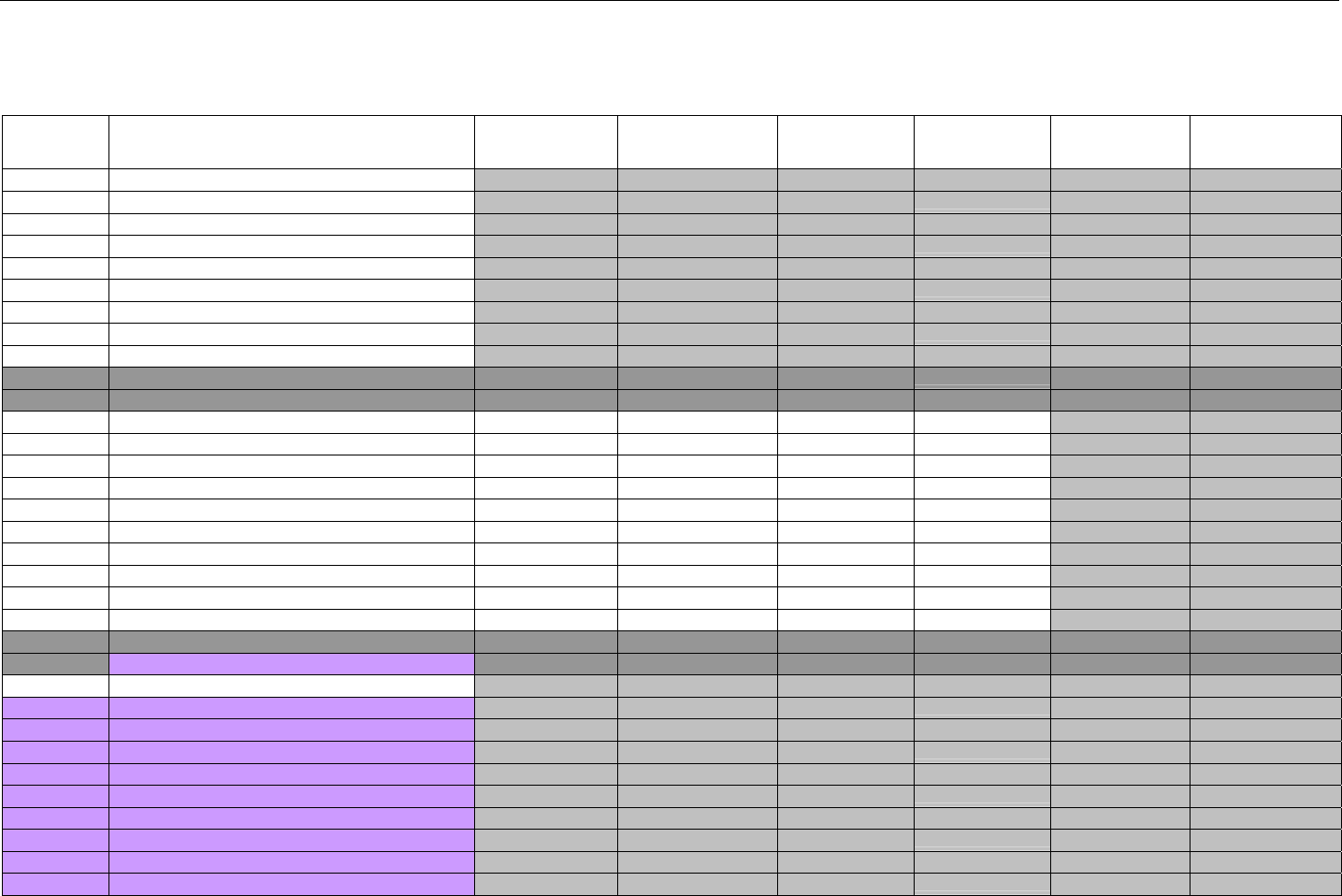
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 597 -
Information
Reporting (Claim) Information Reporting
(Service) Information
Reporting Rebill
(Claim)
Information
Reporting Rebill
(Service)
Information
Reporting Reversal
(Claim)
Information
Reporting Reversal
(Service)
498-PE
A
uthorized Representative First Name
498-PF
A
uthorized Rep. Last Name
498-PG
A
uthorized Rep. Street Address
498-PH
A
uthorized Rep. Cit
y
498-PJ
A
uthorized Rep. State/Province
498-PK
A
uthorized Rep. Zip/Postal Code
498-PY Prior Authorization Number - Assigned
5Ø3-F3
A
uthorization Numbe
r
498-PP Prior Authorization Supporting Documentation
CLINICAL SEGMENT
111-AM Segment Identification M M M M
491-VE Diagnosis Code Count Q Q Q Q
492-WE Diagnosis Code Qualifier Q Q Q Q
424-DO Diagnosis Code Q Q Q Q
493-XE Clinical Information Counter Q Q Q Q
494-ZE Measurement Date Q Q Q Q
495-H1 Measurement Time Q Q Q Q
496-H2 Measurement Dimension Q Q Q Q
497-H3 Measurement Unit Q Q Q Q
499-H4 Measurement Value Q Q Q Q
ADDITIONAL DOCUMENTATION SEGMENT
111-AM Segment Identification
369-2Q
A
dditional Documentation Type ID
374-2V Request Period Begin Date
375-2W Request Period Recert/Revised Date
373-2U Request Status
371-2S Length Of Need Qualifier
37Ø-2R Length Of Need
372-2T Prescriber/Supplier Date Signed
376-2X Supporting Documentation
377-2Z Question Number/Letter Count
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 598 -
Information
Reporting (Claim) Information Reporting
(Service) Information
Reporting Rebill
(Claim)
Information
Reporting Rebill
(Service)
Information
Reporting Reversal
(Claim)
Information
Reporting Reversal
(Service)
378-4B Question Number/Letter
379-4D Question Percent Response
38Ø-4G Question Date Response
381-4H Question Dollar Amount Response
382-4J Question Numeric Response
383-4K Question Alphanumeric Response
FACILITY SEGMENT
111-AM Segment Identification
336-BC Facility ID
385-3Q Facility Name
386-3U Facility Street Address
388-5J Facility City Address
387-3V Facility State/Province Address
389-6D Facility ZIP/Postal Zone
NARRATIVE SEGMENT
111-AM Segment Identification
39Ø-BM Narrative Message
24.2.4 CONTROLLED SUBSTANCE REPORTING/CONTROLLED SUBSTANCE REPORTING REBILL/CONTROLLED SUBSTANCE
REPORTING REVERSAL MATRIX
Controlled Substance Reporting
(Claim/Service)/ Controlled
Substance Reporting Rebill
Controlled Substance
Reporting Reversal
TRANSACTION HEADER SEGMENT
1Ø1-A1 BIN Number M M
1Ø2-A2 Version Release Numbe
r
M M
1Ø3-A3 Transaction Code M M
1Ø4-A4 Processor Control Number M M
1Ø9-A9 Transaction Count M M
2Ø2-B2 Service Provider ID Qualifier M M
2Ø1-B1 Service Provider ID M M
4Ø1-D1 Date of Service M M
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 599 -
Controlled Substance Reporting
(Claim/Service)/ Controlled
Substance Reporting Rebill
Controlled Substance
Reporting Reversal
11Ø-AK Software Vendor/Certification ID M M
INSURANCE SEGMENT
111-AM Segment Identification
3Ø2-C2 Cardholder ID
312-CC Cardholder First Name
313-CD Cardholder Last Name
314-CE Home Plan
524-FO Plan ID
3Ø9-C9 Eligibility Clarification Code
3Ø1-C1 Group ID
3Ø3-C3 Person Code
3Ø6-C6 Patient Relationship Code
99Ø-MG Other Payer BIN Number
991-MH Other Payer Processor Control Number
356-NU Other Payer Cardholder ID
992-MJ Other Payer Group ID
359-2A Medigap ID
36Ø-2B Medicaid Indicator
361-2D Provider Accept Assignment Indicator
997-G2 CMS Part D Defined Qualified Facility
115-N5 Medicaid ID Number
116-N6 Medicaid Agency Number
PATIENT SEGMENT
111-AM Segment Identification M M
331-CX Patient ID Qualifier Q Q
332-CY Patient ID O O
3Ø4-C4 Date of Birth O O
3Ø5-C5 Patient Gender Code O O
31Ø-CA Patient First Name O O
311-CB Patient Last Name O O
322-CM Patient Street Address O O
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 600 -
Controlled Substance Reporting
(Claim/Service)/ Controlled
Substance Reporting Rebill
Controlled Substance
Reporting Reversal
323-CN Patient City O O
324-CO Patient State or Province O O
325-CP Patient Zip/Postal Code O O
326-CQ Patient Phone number O O
3Ø7-C7 Place of Service O O
333-CZ Employer ID O O
334-1C Smoker/Non-smoker Code O O
335-2C Pregnancy Indicator O O
35Ø-HN Patient E-Mail Address O O
384-4X Patient Residence O O
PHARMACY PROVIDER SEGMENT
111-AM Segment Identification M M
465-EY Provider ID Qualifier Q Q
444-E9 Provider ID O O
CLAIM SEGMENT
111-AM Segment Identification M M
455-EM Prescription/Service Reference Number Qualifier M M
4Ø2-D2 Prescription/Service Reference Number M M
436-E1 Product/Service ID Qualifier M M
4Ø7-D7 Product/Service ID M M
456-EN
A
ssociated Prescription/Service Reference Numbe
r
O O
457-EP
A
ssociated Prescription/Service Date O O
458-SE Procedure Modifier Code Count O O
459-ER Procedure Modifier Code O O
442-E7 Quantity Dispensed O O
4Ø3-D3 Fill Number O O
4Ø5-D5 Days Supply O O
4Ø6-D6 Compound Code O O
4Ø8-D8 Dispense as Written/Product Selection Code O O
414-DE Date Prescription Written O O
415-DF Number of Refills Authorized O O
419-DJ Prescription Origin Code O O
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 601 -
Controlled Substance Reporting
(Claim/Service)/ Controlled
Substance Reporting Rebill
Controlled Substance
Reporting Reversal
354-NX Submission Clarification Code Count O O
42Ø-DK Submission Clarification Code O O
46Ø-ET Quantity Prescribed O O
3Ø8-C8 Other Coverage Code O O
429-DT Special Packaging Indicator O O
453-EJ Originally Prescribed Product/Service ID Qualifier Q Q
445-EA Originally Prescribed Product/Service Code O O
446-EB Originally Prescribed Quantity O O
33Ø-CW
A
lternate ID O O
454-EK Scheduled Prescription ID Number O O
6ØØ-28 Unit of Measure O O
418-DI Level of Service O O
461-EU Prior Authorization Type Code O O
462-EV Prior Authorization Number Submitted O O
463-EW Intermediary Authorization Type ID Q Q
464-EX Intermediary Authorization ID O O
343-HD Dispensing Status O O
344-HF Quantity Intended to be Dispensed O O
345-HG Days Supply Intended to be Dispensed O O
357-NV Delay Reason Code O O
88Ø-K5 Transaction Reference Number O O
391-MT Patient Assignment Indicator (Direct Member
Reimbursement Indicator)
O O
995-E2 Route of Administration O O
996-G1 Compound Type O O
114-N4 Medicaid Subrogation Internal Control
Number/Transaction Control Number (ICN/TCN)
O O
147-U7 Pharmacy Service Type O O
PRESCRIBER SEGMENT
111-AM Segment Identification M M
466-EZ Prescriber ID Qualifier Q Q
411-DB Prescriber ID O O
427-DR Prescriber Last Name O O
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 602 -
Controlled Substance Reporting
(Claim/Service)/ Controlled
Substance Reporting Rebill
Controlled Substance
Reporting Reversal
498-PM Prescriber Phone Number O O
468-2E Primary Care Provider ID Qualifier Q Q
421-DL Primary Care Provider ID O O
47Ø-4E Primary Care Provider Last Name O O
364-2J Prescriber First Name O O
365-2K Prescriber Street Address O O
366-2M Prescriber City Address O O
367-2N Prescriber State/Province Address O O
368-2P Prescriber ZIP/Postal Zone O O
COORDINATION OF BENEFITS/OTHER PAYMENTS
SEGMENT
111-AM Segment Identification
337-4C Coordination of Benefits/Other Payments Count
338-5C Other Payer Coverage Type
339-6C Other Payer ID Qualifier
34Ø-7C Other Payer ID
443-E8 Other Payer Date
993-A7 Internal Control Number
341-HB Other Payer Amount Paid Count
342-HC Other Payer Amount Paid Qualifier
431-DV Other Payer Amount Paid
471-5E Other Payer Reject Count
472-6E Other Payer Reject Code
353-NR Other Payer-Patient Responsibility Amount Count
351-NP Other Payer-Patient Responsibility Amount Qualifier
352-NQ Other Payer-Patient Responsibility Amount
392-MU Benefit Stage Count
393-MV Benefit Stage Qualifier
394-MW Benefit Stage Amount
WORKERS’ COMPENSATION SEGMENT
111-AM Segment Identification
434-DY Date of Injury
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 603 -
Controlled Substance Reporting
(Claim/Service)/ Controlled
Substance Reporting Rebill
Controlled Substance
Reporting Reversal
315-CF Employer Name
316-CG Employer Street Address
317-CH Employer City Address
318-CI Employer State/Province Address
319-CJ Employer Zip/Postal Code
32Ø-CK Employer Phone Number
321-CL Employer Contact Name
327-CR Carrier ID
435-DZ Claim/Reference ID
117-TR Billing Entity Type Indicator
118-TS Pay To Qualifier
119-TT Pay To ID
12Ø-TU Pay To Name
121-TV Pay To Street Address
122-TW Pay To City Address
123-TX Pay To State/Province Address
124-TY Pay To ZIP/Postal Zone
125-TZ Generic Equivalent Product ID Qualifier
126-UA Generic Equivalent Product ID
DUR/PPS SEGMENT
111-AM Segment Identification
473-7E DUR/PPS Code Counter
439-E4 Reason for Service Code
44Ø-E5 Professional Service Code
441-E6 Result of Service Code
474-8E DUR/PPS Level of Effort
475-J9 DUR Co-Agent ID Qualifier
476-H6 DUR Co-Agent ID
PRICING SEGMENT
111-AM Segment Identification
4Ø9-D9 Ingredient Cost Submitted
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 604 -
Controlled Substance Reporting
(Claim/Service)/ Controlled
Substance Reporting Rebill
Controlled Substance
Reporting Reversal
412-DC Dispensing Fee Submitted
477-BE Professional Service Fee Submitted
433-DX Patient Paid Amount Submitted
438-E3 Incentive Amount Submitted
478-H7 Other Amount Claimed Submitted Count
479-H8 Other Amount Claimed Submitted Qualifier
48Ø-H9 Other Amount Claimed Submitted
481-HA Flat Sales Tax Amount Submitted
482-GE Percentage Sales Tax Amount Submitted
483-HE Percentage Sales Tax Rate Submitted
484-JE Percentage Sales Tax Basis Submitted
426-DQ Usual and Customary Charge
43Ø-DU Gross Amount Due
423-DN Basis of Cost Determination
113-N3 Medicaid Paid Amount
COUPON SEGMENT
111-AM Segment Identification
485-KE Coupon Type
486-ME Coupon Number
487-NE Coupon Value Amount
COMPOUND SEGMENT
111-AM. Segment Identification
45Ø-EF Compound Dosage Form Description Code
451-EG Compound Dispensing Unit Form Indicator
447-EC Compound Ingredient Component Count
488-RE Compound Product ID Qualifier
489-TE Compound Product ID
448-ED Compound Ingredient Quantity
449-EE Compound Ingredient Drug Cost
49Ø-UE Compound Ingredient Basis of Cost Determination
362-2G Compound Ingredient Modifier Code Count

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 605 -
Controlled Substance Reporting
(Claim/Service)/ Controlled
Substance Reporting Rebill
Controlled Substance
Reporting Reversal
363-2H Compound Ingredient Modifier Code
PRIOR AUTHORIZATION SEGMENT
111-AM Segment Identification
498-PA Request Type
498-PB Request Period Date - Begin
498-PC Request Period Date - End
498-PD Basis of Request
498-PE
A
uthorized Representative First Name
498-PF
A
uthorized Rep. Last Name
498-PG
A
uthorized Rep. Street Address
498-PH
A
uthorized Rep. Cit
y
498-PJ
A
uthorized Rep. State/Province
498-PK
A
uthorized Rep. Zip/Postal Code
498-PY Prior Authorization Number - Assigned
5Ø3-F3
A
uthorization Numbe
r
498-PP Prior Authorization Supporting Documentation
CLINICAL SEGMENT
111-AM Segment Identification
491-VE Diagnosis Code Count
492-WE Diagnosis Code Qualifier
424-DO Diagnosis Code
493-XE Clinical Information Counter
494-ZE Measurement Date
495-H1 Measurement Time
496-H2 Measurement Dimension
497-H3 Measurement Unit
499-H4 Measurement Value
ADDITIONAL DOCUMENTATION SEGMENT
111-AM Segment Identification
369-2Q
A
dditional Documentation Type ID
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 606 -
Controlled Substance Reporting
(Claim/Service)/ Controlled
Substance Reporting Rebill
Controlled Substance
Reporting Reversal
374-2V Request Period Begin Date
375-2W Request Period Recert/Revised Date
373-2U Request Status
371-2S Length Of Need Qualifier
37Ø-2R Length Of Need
372-2T Prescriber/Supplier Date Signed
376-2X Supporting Documentation
377-2Z Question Number/Letter Count
378-4B Question Number/Letter
379-4D Question Percent Response
38Ø-4G Question Date Response
381-4H Question Dollar Amount Response
382-4J Question Numeric Response
383-4K Question Alphanumeric Response
FACILITY SEGMENT
111-AM Segment Identification
336-BC Facility ID
385-3Q Facility Name
386-3U Facility Street Address
388-5J Facility City Address
387-3V Facility State/Province Address
389-6D Facility ZIP/Postal Zone
NARRATIVE SEGMENT
111-AM Segment Identification
39Ø-BM Narrative Message
24.3 REQUEST SEGMENT MATRICES BY SEGMENT - LEGEND
LEGEND:
Categorization Explanation
M Mandatory
The Segment is Mandatory.
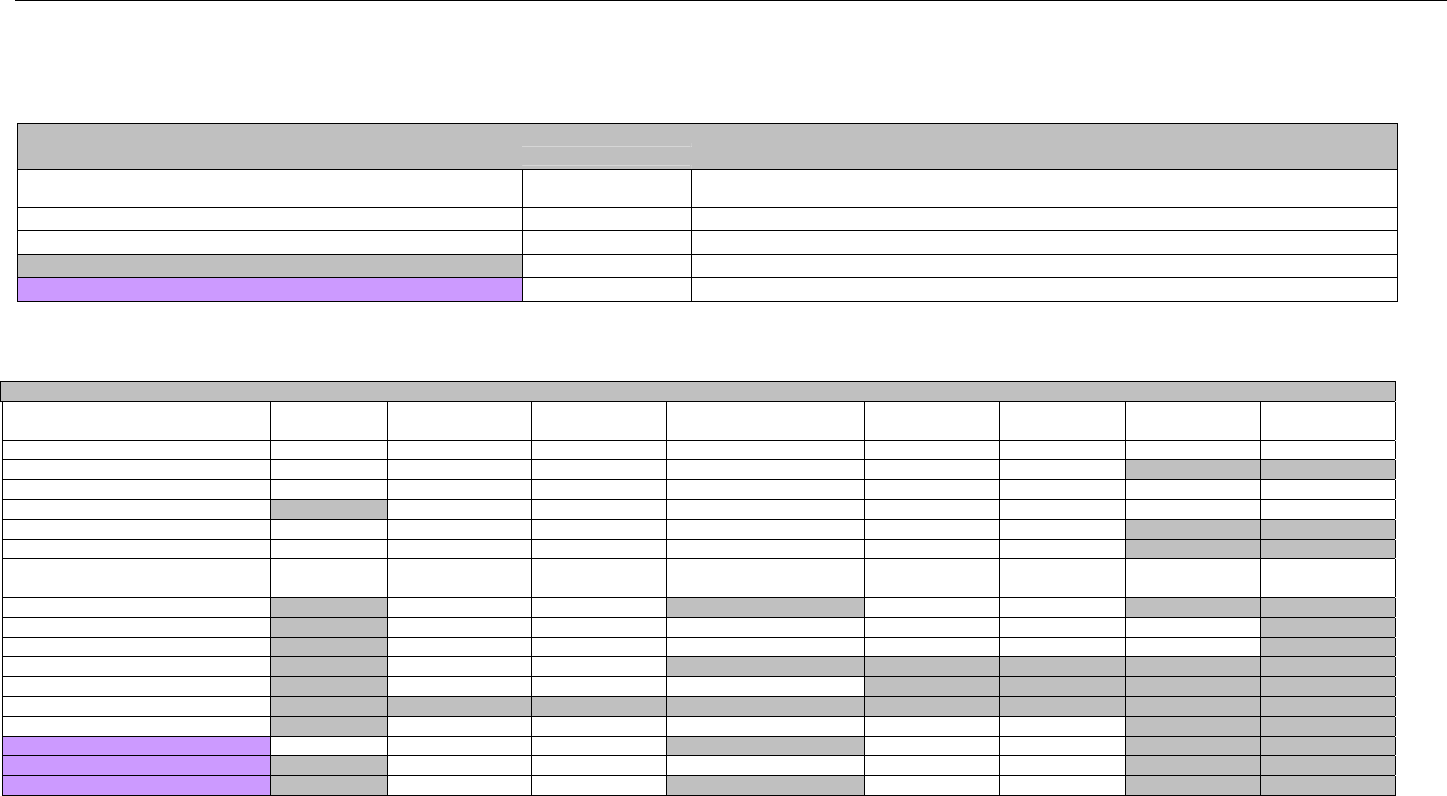
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 607 -
LEGEND:
Categorization Explanation
S Situational
The segment situations defined have qualifications for usage ("Required if x", "Not required if y") in
this Transaction.
O Optional
The segment has been defined as optional usage (situations were not defined) in this Tranasction.
N Not used
The segment is not used in this Transaction.
Row/Column Shaded The segment is not valid for this Transaction.
New Field/Segment Since 5.1
24.4 REQUEST SEGMENT MATRICES BY SEGMENT
24.4.1 ELIGIBILITY/BILLING/ENCOUNTER/REBILL/REVERSAL MATRIX
VERSION D AND ABOVE REQUEST SEGMENT USAGE MATRIX
SEGMENT Eligibility Billing (Claim) or
Encounter Rebill (Claim) Predetermination Of
Benefits (Claim) Billing (Service) Rebill (Service) Reversal (Claim) Reversal
(Service)
Header M M M M M M M M
Patient S S S S S S N N
Insurance M M M M M M S S
Claim N M M M M M M M
Pharmacy Provider S S S S S S N N
Prescriber S S S S S S N N
Coordination of Benefits/Other
Payments N S S N S S S S
Workers’ Compensation N S S N S S N N
DUR/PPS N S S S S S S N
Pricing N M M M M M
S N
Coupon N S S N N N N N
Compound N S S S N N N N
Prior Authorization N N N N N N N N
Clinical N S S S S S N N
Additional Documentation S S S N S S N N
Facility N S S S S S N N
Narrative N S S N S S N N
24.4.2 PRIOR AUTHORIZATION REQUEST AND BILLING/PRIOR AUTHORIZATION REVERSAL/PRIOR AUTHORIZATION INQUIRY/PRIOR
AUTHORIZATION REQUEST ONLY MATRIX
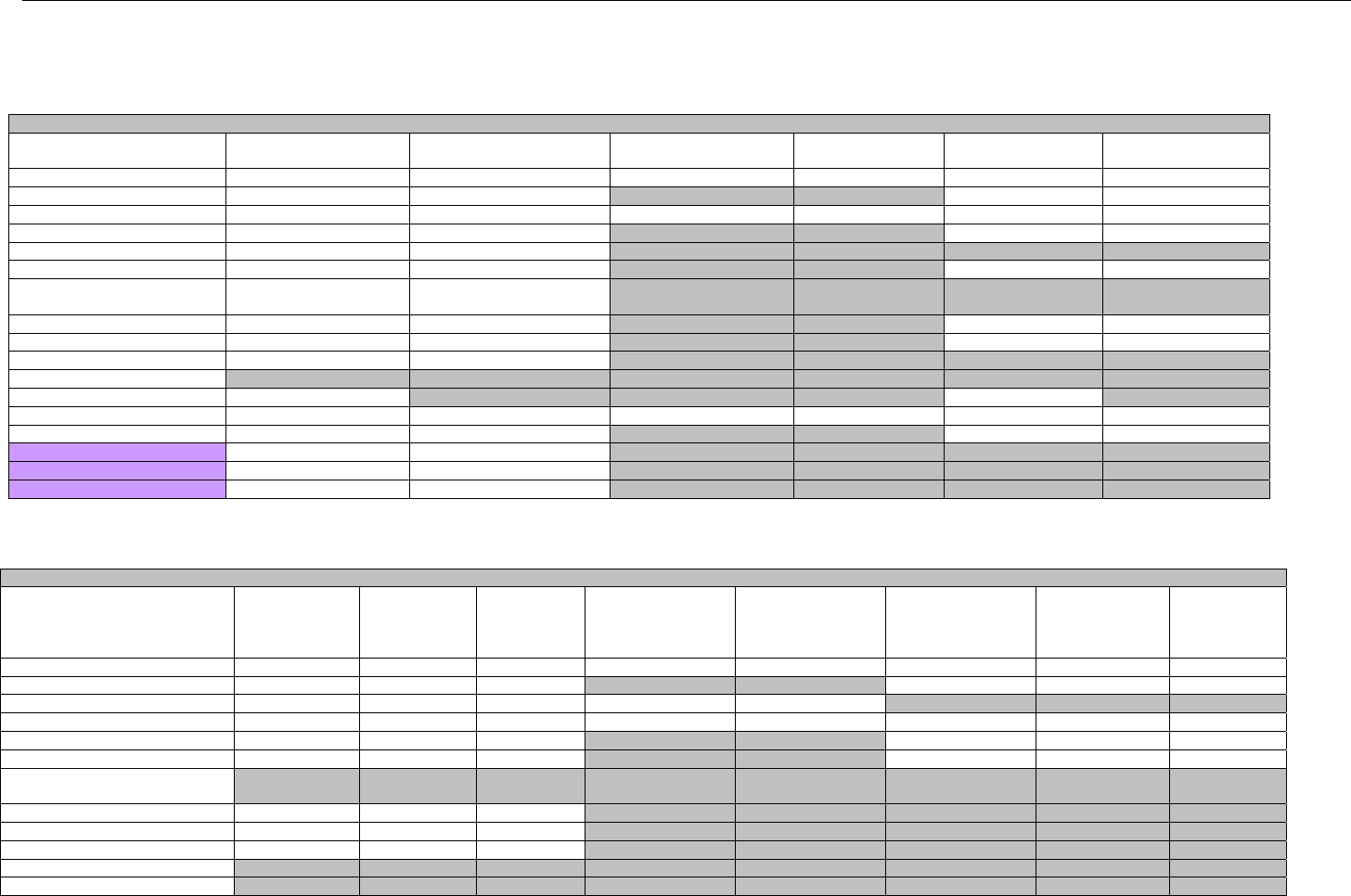
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 608 -
VERSION D AND ABOVE REQUEST SEGMENT USAGE MATRIX (Continued)
SEGMENT Prior Authorization
Request & Billing (Claim) Prior Authorization Request
& Billing (Service) Prior Authorization
Reversal (Claim/Service) Prior Authorization
Inquiry Prior Authorization
Request Only (Claim) Prior Authorization
Request Only (Service)
Header M M M M M M
Patient S S N N S S
Insurance M M
S S
M M
Claim M M
N N M M
Pharmacy Provider S S N N N N
Prescriber S S N N S S
Coordination of Benefits/Other
Payments S S N N N N
Workers’ Compensation S S N N S S
DUR/PPS S S N N S S
Pricing M M
N N N N
Coupon N N N N N N
Compound S N N N S N
Prior Authorization M M S M M M
Clinical S S N N S S
Additional Documentation S S N N N N
Facility S S N N N N
Narrative S S N N N N
24.4.3 INFORMATION REPORTING/INFORMATION REPORTING REVERSAL/INFORMATION REPORTING REBILL/CONTROLLED
SUBSTANCE REPORTING/CONTROLLED SUBSTANCE REVERSAL/CONTROLLED SUBSTANCE REBILL
VERSION D AND ABOVE REQUEST SEGMENT USAGE MATRIX (Continued)
SEGMENT Information
Reporting (Claim) Information
Reporting
(Service)
Information
Reporting
Rebill
Information
Reporting Reversal
(Claim)
Information
Reporting Reversal
(Service)
Controlled
Substance Reporting Controlled
Substance
Reporting
Reversal
Controlled
Substance
Reporting Rebill
Header M M M M M M M M
Patient S S S N N M M M
Insurance M M M
S S N N N
Claim M M M M M M M M
Pharmacy Provider S S S N N O O O
Prescriber S S S N N O O O
Coordination of Benefits/Other
Payments N N N N N N N N
Workers’ Compensation S S S N N N N N
DUR/PPS S S S N N N N N
Pricing S S S N N N N N
Coupon N N N N N N N N
Compound N N N N N N N N
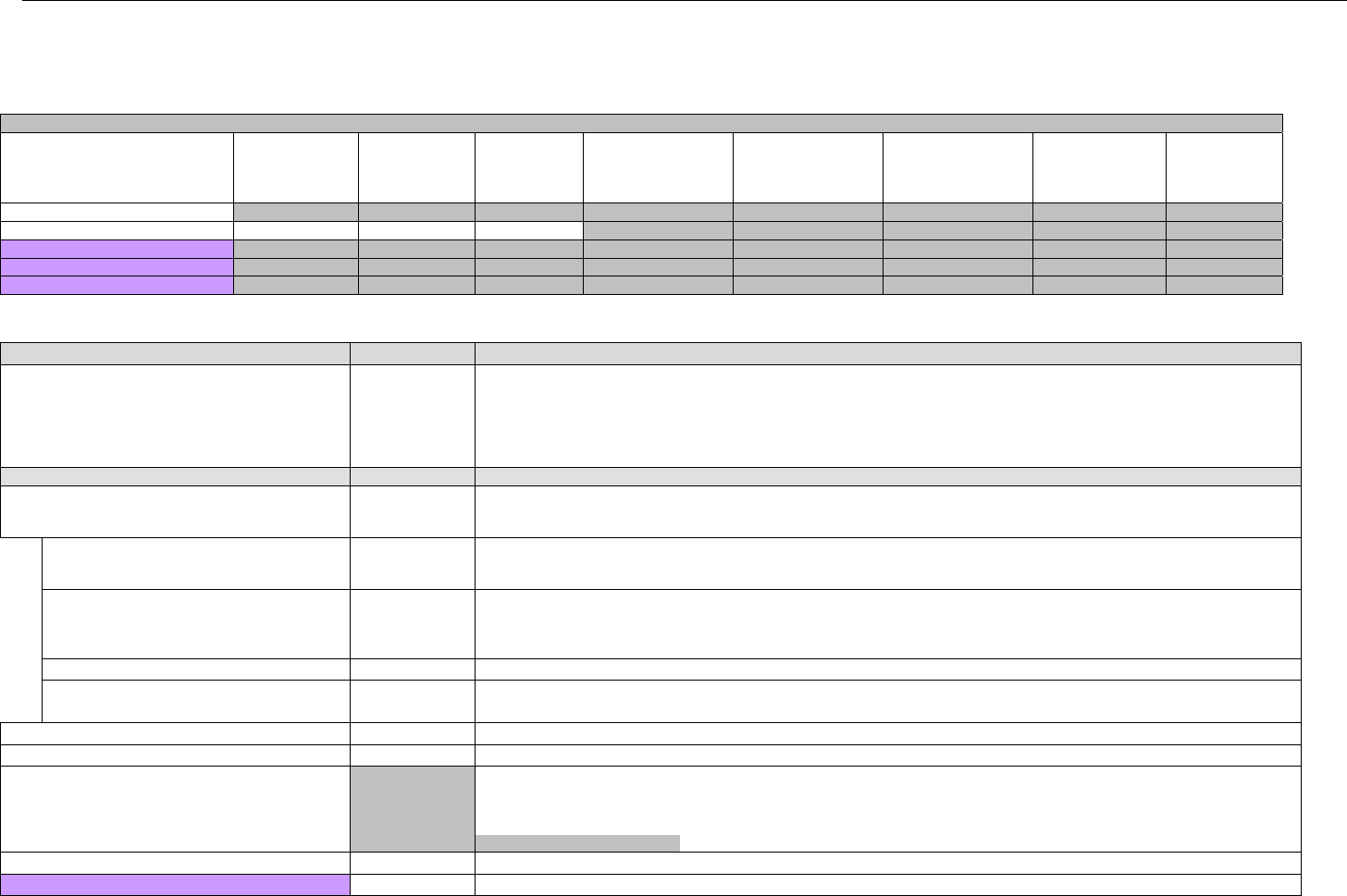
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 609 -
VERSION D AND ABOVE REQUEST SEGMENT USAGE MATRIX (Continued)
SEGMENT Information
Reporting (Claim) Information
Reporting
(Service)
Information
Reporting
Rebill
Information
Reporting Reversal
(Claim)
Information
Reporting Reversal
(Service)
Controlled
Substance Reporting Controlled
Substance
Reporting
Reversal
Controlled
Substance
Reporting Rebill
Prior Authorization N N N N N N N N
Clinical S S S N N N N N
Additional Documentation N N N N N N N N
Facility N N N N N N N N
Narrative N N N N N N N N
24.5 RESPONSE SEGMENT MATRICES BY FIELD WITHIN SEGMENT - LEGEND
DESIGNATION VALUE EXPLANATION
MANDATORY M
The Segment is mandatory for the Transaction
or
The Field is mandatory for the Segment for the Transaction.
Mandatory elements have structural requirements.
Mandatory are bolded for clarity.
SITUATIONAL The Segment has been further designated for usage for the Transaction
or
The Field has been further designated for usage for the Transaction.
Required R The Field has been designated with the situation of "Required" for the Segment for the Transaction.
Required are bolded italicized for clarity.
Required for Medicaid Subrogation only RM The Field has been designated with the situation of "Required" for the Segment for the Transaction for Medicaid Subrogation
usage only.
Required are bolded italicized for clarity.
Qualified Requirement Q The situations designated have qualifications for usage ("Required if x", "Not required if y").
Qualified Requirement for Medicaid
Subrogation only QM The situations designated have qualifications for usage ("Required if x", "Not required if y") for Medicaid Subrogation.
INFORMATIONAL ONLY I The Field is for informational purposes only for the Transaction.
OPTIONAL O The Field has been designated as optional usage (situations were not defined).
NOT USED N The Segment is not used for the Transaction
or
The Field is not used for the Segment for the Transaction.
Not used are shaded for clarity.
New Field/Segment Since 5.1
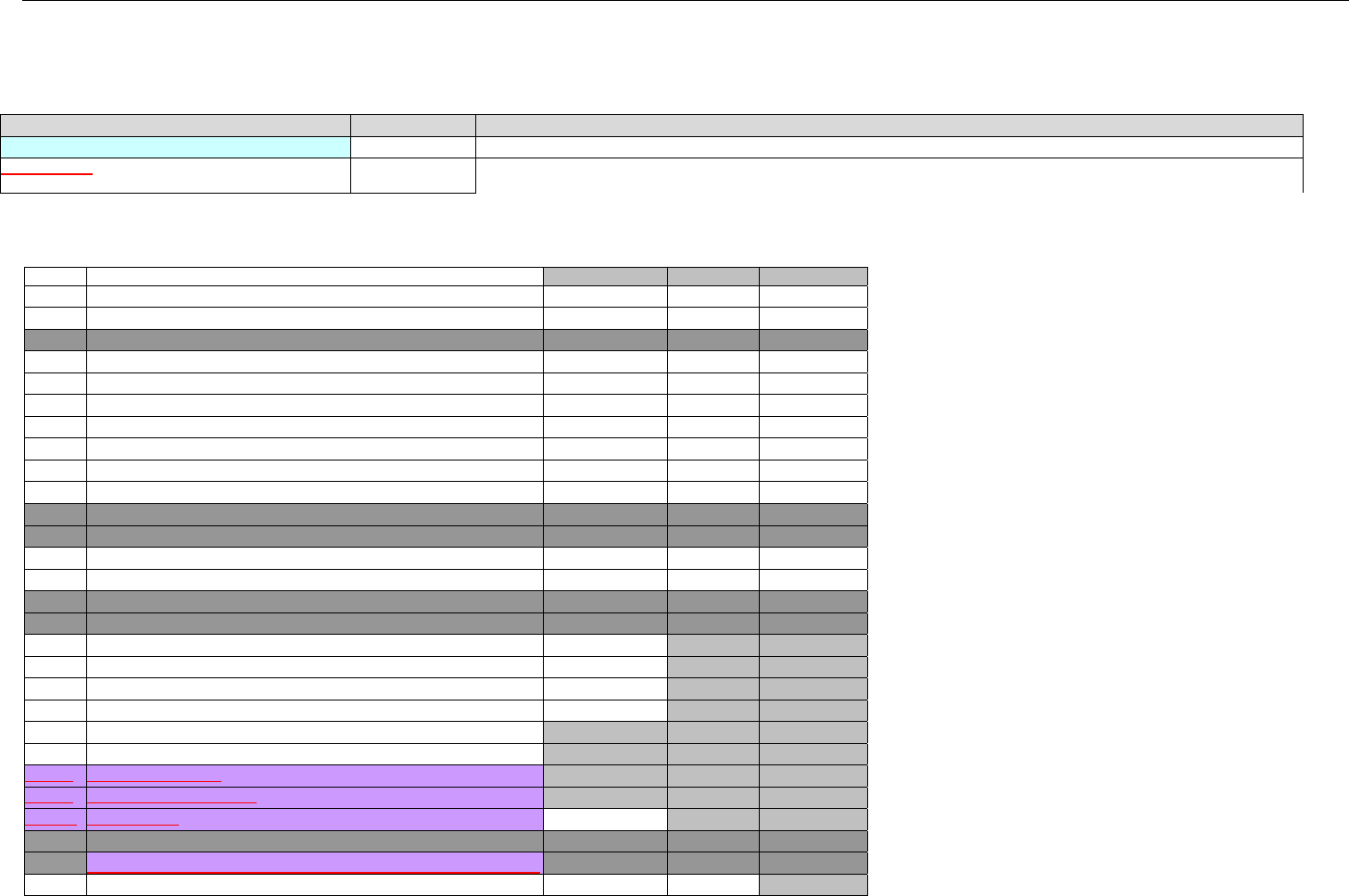
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 610 -
DESIGNATION VALUE EXPLANATION
Field Name Change Since 5.1
Red underline denotes a modification (to D.Ø) from
Telecommunication Standard Version C.4 usage
24.6 RESPONSE SEGMENT MATRICES BY FIELD WITHIN SEGMENT
24.6.1 ELIGIBILITY MATRIX
Eligibility
Header Response Status Accepted Accepted Rejected
Transaction Response Status Approved Rejected Rejected
RESPONSE HEADER SEGMENT
1Ø2-A2 Version Release Numbe
r
M M M
1Ø3-A3 Transaction Code M M M
1Ø9-A9 Transaction Count M M M
5Ø1-FI Header Response Status M M M
2Ø2-B2 Service Provider ID Qualifier M M M
2Ø1-B1 Service Provider ID M M M
4Ø1-D1 Date of Service M M M
RESPONSE MESSAGE SEGMENT
111-AM Segment Identification M M M
5Ø4-F4 Message Q Q Q
RESPONSE INSURANCE SEGMENT
111-AM Segment Identification M
3Ø1-C1 Group ID Q
524-FO Plan ID Q
545-2F Network Reimbursement ID Q
568-J7 Payer ID Qualifier N
569-J8 Payer ID N
115-N5 Medicaid ID Number N
116-N6 Medicaid Agency Number N
3Ø2-C2 Cardholder ID Q
RESPONSE INSURANCE ADDITIONAL INFORMATION SEGMENT
111-AM Segment Identification M M
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 611 -
Eligibility
Header Response Status Accepted Accepted Rejected
Transaction Response Status Approved Rejected Rejected
139-UR Medicare Part D Coverage Code Q N
138-UQ CMS Low Income Cost Sharing (LICS) Level Q N
24Ø-U1 Contract Number Q N
926-FF Formulary ID Q N
757-U6 Benefit ID Q N
14Ø-US Next Medicare Part D Effective Date Q Q
141-UT Next Medicare Part D Termination Date Q Q
RESPONSE PATIENT SEGMENT
111-AM Segment Identification M M
31Ø-CA Patient First Name Q Q
311-CB Patient Last Name Q Q
3Ø4-C4 Date Of Birth Q Q
RESPONSE STATUS SEGMENT
111-AM Segment Identification M M M
112-AN Transaction Response Status M M M
5Ø3-F3
A
uthorization Numbe
r
Q Q Q
51Ø-FA Reject Count N R R
511-FB Reject Code N R R
546-4F Reject Field Occurrence Indicator N Q Q
547-5F
A
pproved Message Code Count N N N
548-6F
A
pproved Message Code N N N
13Ø-UF
A
dditional Message Information Count Q Q Q
132-UH
A
dditional Message Information Qualifie
r
Q Q Q
526-FQ
A
dditional Message Information Q Q Q
131-UG
A
dditional Message Information Continuity Q Q Q
55Ø-7F Help Desk Phone Number Qualifier Q Q Q
55Ø-8F Help Desk Phone Number Q Q Q
88Ø-K5 Transaction Reference Number N N N
993-A7 Internal Control Number N N N
987-MA URL N i N
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 612 -
Eligibility
Header Response Status Accepted Accepted Rejected
Transaction Response Status Approved Rejected Rejected
RESPONSE CLAIM SEGMENT
111-AM Segment Identification
455-EM Prescription/Service Reference Number Qualifier
4Ø2-D2 Prescription/Service Reference Number
551-9F Preferred Product Count
552-AP Preferred Product ID Qualifier
553-AR Preferred Product ID
554-AS Preferred Product Incentive
555-AT Preferred Product Cost Share Incentive
551-9F Preferred Product Description
114-N4 Medicaid Subrogation Internal Control Number/Transaction Control
Number (ICN/TCN)
RESPONSE PRICING SEGMENT
111-AM Segment Identification
5Ø5-F5 Patient Pay Amount
5Ø6-F6 Ingredient Cost Paid
5Ø7-F7 Dispensing Fee Paid
557-AV Tax Exempt Indicator
558-AW Flat Sales Tax Amount Paid
559-AX Percentage Sales Tax Amount Paid
56Ø-AY Percentage Sales Tax Rate Paid
561-AZ Percentage Sales Tax Basis Paid
521-FL Incentive Amount Paid
562-J1 Professional Service Fee Paid
563-J2 Other Amount Paid Count
564-J3 Other Amount Paid Qualifier
565-J4 Other Amount Paid
566-J5 Other Payer Amount Recognized
5Ø9-F9 Total Amount Paid
522-FM Basis of Reimbursement Determination
523-FN
A
mount Attributed to Sales Tax
512-FC
A
ccumulated Deductible Amount
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 613 -
Eligibility
Header Response Status Accepted Accepted Rejected
Transaction Response Status Approved Rejected Rejected
513-FD Remaining Deductible Amount
514-FE Remaining Benefit Amount
517-FH
A
mount Applied to Periodic Deductible
518-F1
A
mount of Copa
y
52Ø-FK
A
mount Exceeding Periodic Benefit Maximum
346-HH Basis of Calculation – Dispensing Fee
347-HJ Basis of Calculation – Copay
348-HK Basis of Calculation – Flat Sales Tax
349-HM Basis of Calculation – Percentage Sales Tax
571-NZ
A
mount Attributed to Processor Fee
575-EQ Patient Sales Tax Amount
574-2Y Plan Sales Tax Amount
572-4U
A
mount of Coinsurance
573-4V Basis of Calculation-Coinsurance
392-MU Benefit Stage Count
393-MV Benefit Stage Qualifier
394-MW Benefit Stage Amount
577-G3 Estimated Generic Savings
128-UC Spending Account Amount Remaining
129-UD Health Plan-Funded Assistance Amount
133-UJ
A
mount Attributed to Provider Network Selection
134-UK
A
mount Attributed to Product Selection/Brand Drug
135-UM
A
mount Attributed to Product Selection/Non-Preferred Formulary
Selection
136-UN
A
mount Attributed to Product Selection/Brand Non-Preferred
Formulary Selection
137-UP
A
mount Attributed to Coverage Gap
148-U8 Ingredient Cost Contracted/Reimbursable Amount
149-U9 Dispensing Fee Contracted/Reimbursable Amount
RESPONSE DUR/PPS SEGMENT
111-AM Segment Identification
567-J6 DUR/PPS Response Code Counter
439-E4 Reason for Service Code
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 614 -
Eligibility
Header Response Status Accepted Accepted Rejected
Transaction Response Status Approved Rejected Rejected
528-FS Clinical Significance Code
529-FT Other Pharmacy Indicator
531-FV Quantity of Previous Fill
53Ø-FU Previous Date of Fill
532-FW Database Indicator
533-FX Other Prescriber Indicator
544-FY DUR Free Text Message
57Ø-NS DUR Additional Text
RESPONSE PRIOR AUTHORIZATION SEGMENT
111-AM Segment Identification
498-PR Prior Authorization Processed Date
498-PS Prior Authorization Effective Date
498-PT Prior Authorization Expiration Date
498-RA Prior Authorization Quantity
498-RB Prior Authorization Dollars Authorized
498-PW Prior Authorization Number of Refills Authorized
498-PX Prior Authorization Quantity Accumulated
498-PY Prior Authorization Number - Assigned
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS
SEGMENT
111-AM Segment Identification M M
355-NT Other Payer ID Count M M
338-5C Other Payer Coverage Type M M
339-6C Other Payer ID Qualifier Q Q
34Ø-7C Other Payer ID Q Q
991-MH Other Payer Processor Control Number Q Q
356-NU Other Payer Cardholder ID Q Q
992-MJ Other Payer Group ID Q Q
142-UV Other Payer Person Code Q Q
127-UB Other Payer Help Desk Phone Number Q Q
143-UW Other Payer Patient Relationship Code Q Q
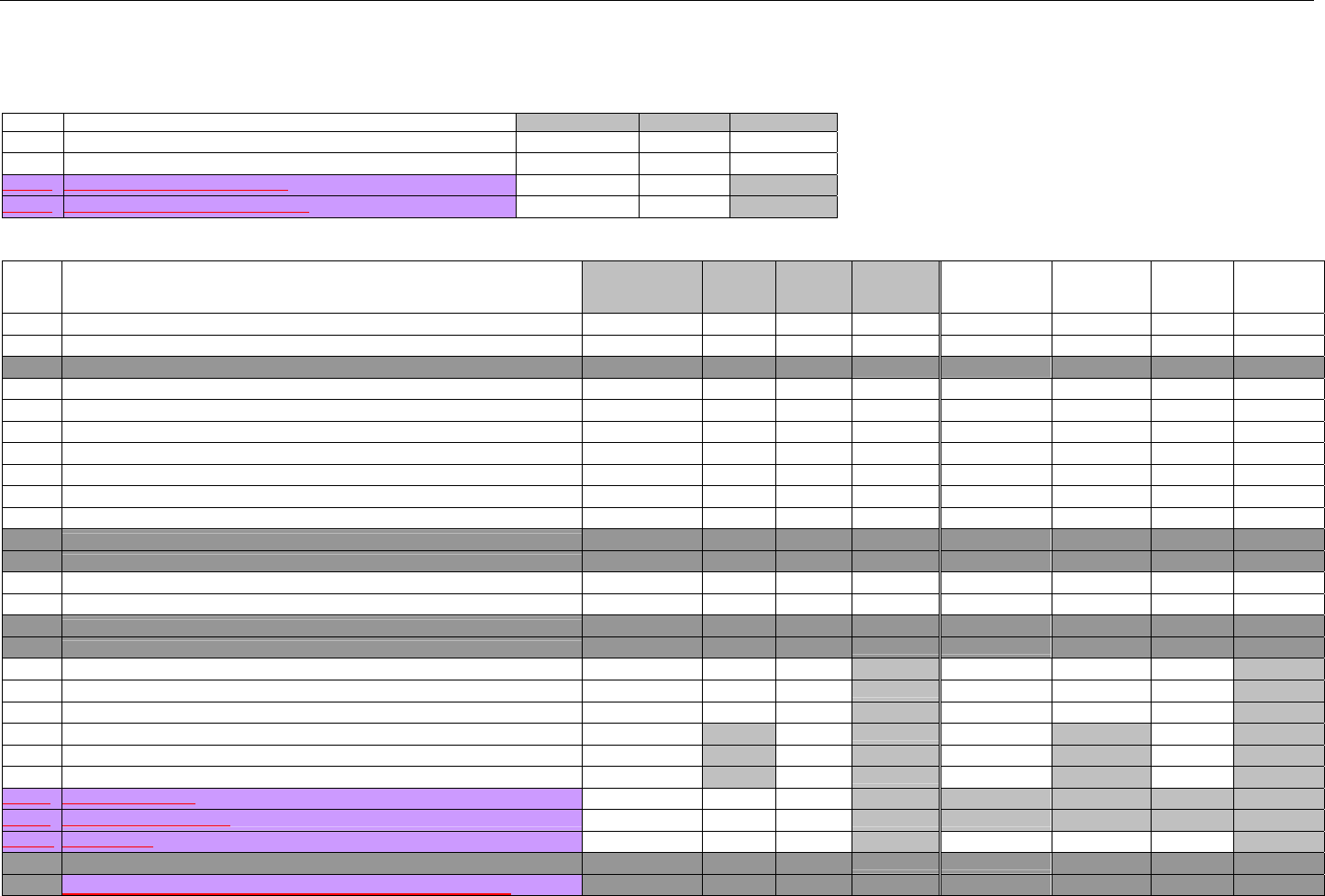
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 615 -
Eligibility
Header Response Status Accepted Accepted Rejected
Transaction Response Status Approved Rejected Rejected
144-UX Other Payer Benefit Effective Date Q Q
145-UY Other Payer Benefit Termination Date Q Q
24.6.2 CLAIM BILLING/CLAIM REBILL/ENCOUNTER/SERVICE BILLING/SERVICE REBILL MATRIX
Claim
Billing/Claim
Rebill/Encounter
Service
Billing/Service
Rebill
Header Response Status Accepted Accepted Accepted Rejected Accepted Accepted Accepted Rejected
Transaction Response Status Paid Captured Rejected Rejected Paid Captured Rejected Rejected
RESPONSE HEADER SEGMENT
1Ø2-A2 Version Release Numbe
r
M M M M M M M M
1Ø3-A3 Transaction Code M M M M M M M M
1Ø9-A9 Transaction Count M M M M M M M M
5Ø1-FI Header Response Status M M M M M M M M
2Ø2-B2 Service Provider ID Qualifier M M M M M M M M
2Ø1-B1 Service Provider ID M M M M M M M M
4Ø1-D1 Date of Service M M M M M M M M
RESPONSE MESSAGE SEGMENT
111-AM Segment Identification M M M M M M M M
5Ø4-F4 Message Q Q Q Q Q Q Q Q
RESPONSE INSURANCE SEGMENT
111-AM Segment Identification M M M
M M M
3Ø1-C1 Group ID Q Q Q
Q Q Q
524-FO Plan ID Q Q Q
Q Q Q
545-2F Network Reimbursement ID Q N Q Q N Q
568-J7 Payer ID Qualifier Q N Q Q N Q
569-J8 Payer ID Q N Q Q N Q
115-N5 Medicaid ID Number N, QM N, QM N, QM N N N
116-N6 Medicaid Agency Number N, QM N, QM N, QM N N N
3Ø2-C2 Cardholder ID Q Q Q Q Q Q
RESPONSE INSURANCE ADDITIONAL INFORMATION SEGMENT
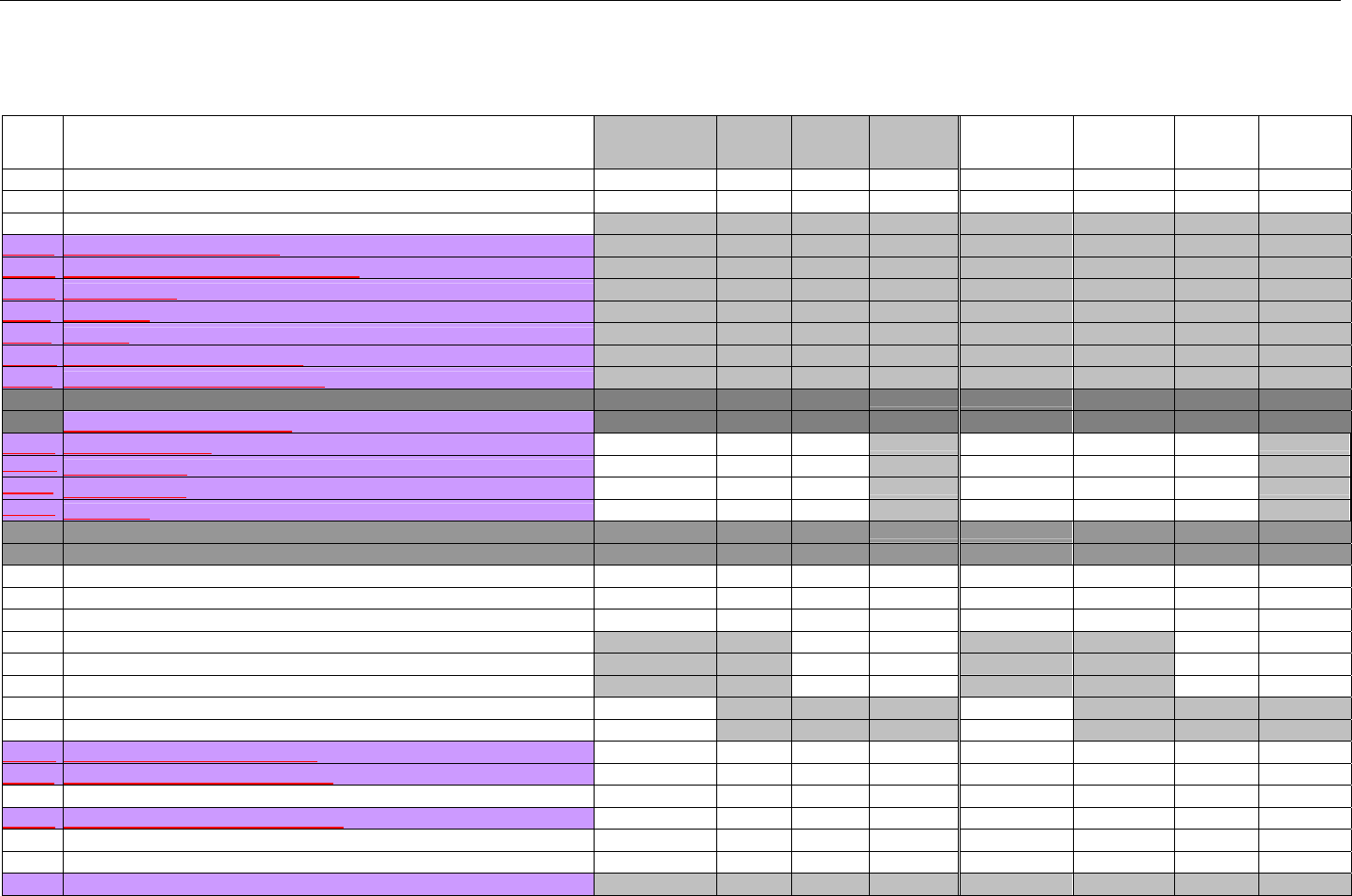
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 616 -
Claim
Billing/Claim
Rebill/Encounter
Service
Billing/Service
Rebill
Header Response Status Accepted Accepted Accepted Rejected Accepted Accepted Accepted Rejected
Transaction Response Status Paid Captured Rejected Rejected Paid Captured Rejected Rejected
111-AM Segment Identification
139-UR Medicare Part D Coverage Code
138-UQ CMS Low Income Cost Sharing (LICS) Level
24Ø-U1 Contract Number
926-FF Formulary ID
757-U6 Benefit ID
14Ø-US Next Medicare Part D Effective Date
141-UT Next Medicare Part D Termination Date
RESPONSE PATIENT SEGMENT
111-AM Segment Identification M M M
M M M
31Ø-CA Patient First Name Q Q Q
Q Q Q
311-CB Patient Last Name Q Q Q
Q Q Q
3Ø4-C4 Date Of Birth Q Q Q
Q Q Q
RESPONSE STATUS SEGMENT
111-AM Segment Identification M M M M M M M M
112-AN Transaction Response Status M M M M M M M M
5Ø3-F3
A
uthorization Numbe
r
Q Q Q Q Q Q Q Q
51Ø-FA Reject Count N N R R N N R R
511-FB Reject Code N N R R N N R R
546-4F Reject Field Occurrence Indicator N N Q Q N N Q Q
547-5F
A
pproved Message Code Count Q N N N Q N N N
548-6F
A
pproved Message Code Q N N N Q N N N
13Ø-UF
A
dditional Message Information Count Q Q Q Q Q Q Q Q
132-UH
A
dditional Message Information Qualifier Q Q Q Q Q Q Q Q
526-FQ
A
dditional Message Information Q Q Q Q Q Q Q Q
131-UG
A
dditional Message Information Continuit
y
Q Q Q Q Q Q Q Q
55Ø-7F Help Desk Phone Number Qualifier Q Q Q Q Q Q Q Q
55Ø-8F Help Desk Phone Number Q Q Q Q Q Q Q Q
88Ø-K5 Transaction Reference Number N N N N N N N N
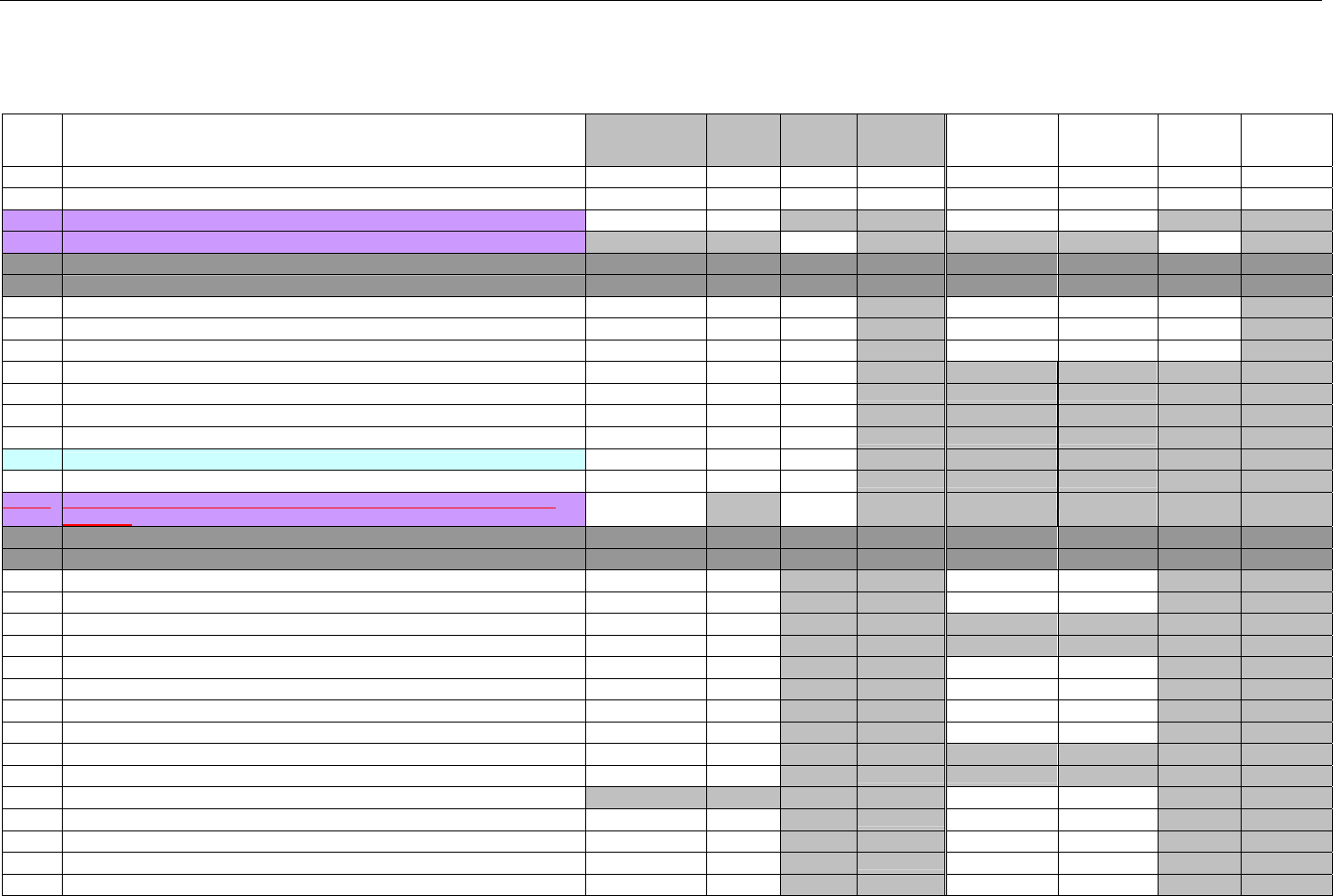
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 617 -
Claim
Billing/Claim
Rebill/Encounter
Service
Billing/Service
Rebill
Header Response Status Accepted Accepted Accepted Rejected Accepted Accepted Accepted Rejected
Transaction Response Status Paid Captured Rejected Rejected Paid Captured Rejected Rejected
993-A7 Internal Control Number Q Q N N Q Q N N
987-MA URL N N I N N N I N
RESPONSE CLAIM SEGMENT
111-AM Segment Identification M M M
M M M
455-EM Prescription/Service Reference Number Qualifier M M M
M M M
4Ø2-D2 Prescription/Service Reference Number M M M
M M M
551-9F Preferred Product Count Q Q Q
N N N
552-AP Preferred Product ID Qualifier Q Q Q
N N N
553-AR Preferred Product ID Q Q Q
N N N
554-AS Preferred Product Incentive Q Q Q
N N N
555-AT Preferred Product Cost Share Incentive Q Q Q
N N N
551-9F Preferred Product Description Q Q Q
N N N
114-N4 Medicaid Subrogation Internal Control Number/Transaction Control Number
(ICN/TCN)
N, QM N N, QM N N N
RESPONSE PRICING SEGMENT
111-AM Segment Identification M M
M M
5Ø5-F5 Patient Pay Amount R Q R Q
5Ø6-F6 Ingredient Cost Paid Q Q
N N
5Ø7-F7 Dispensing Fee Paid Q Q
N N
557-AV Tax Exempt Indicator Q Q
Q Q
558-AW Flat Sales Tax Amount Paid Q Q
Q Q
559-AX Percentage Sales Tax Amount Paid Q Q
Q Q
56Ø-AY Percentage Sales Tax Rate Paid Q Q
Q Q
561-AZ Percentage Sales Tax Basis Paid Q Q
N N
521-FL Incentive Amount Paid Q Q
N N
562-J1 Professional Service Fee Paid N N R R
563-J2 Other Amount Paid Count Q Q
Q Q
564-J3 Other Amount Paid Qualifier Q Q
Q Q
565-J4 Other Amount Paid Q Q
Q Q
566-J5 Other Payer Amount Recognized Q Q
Q Q
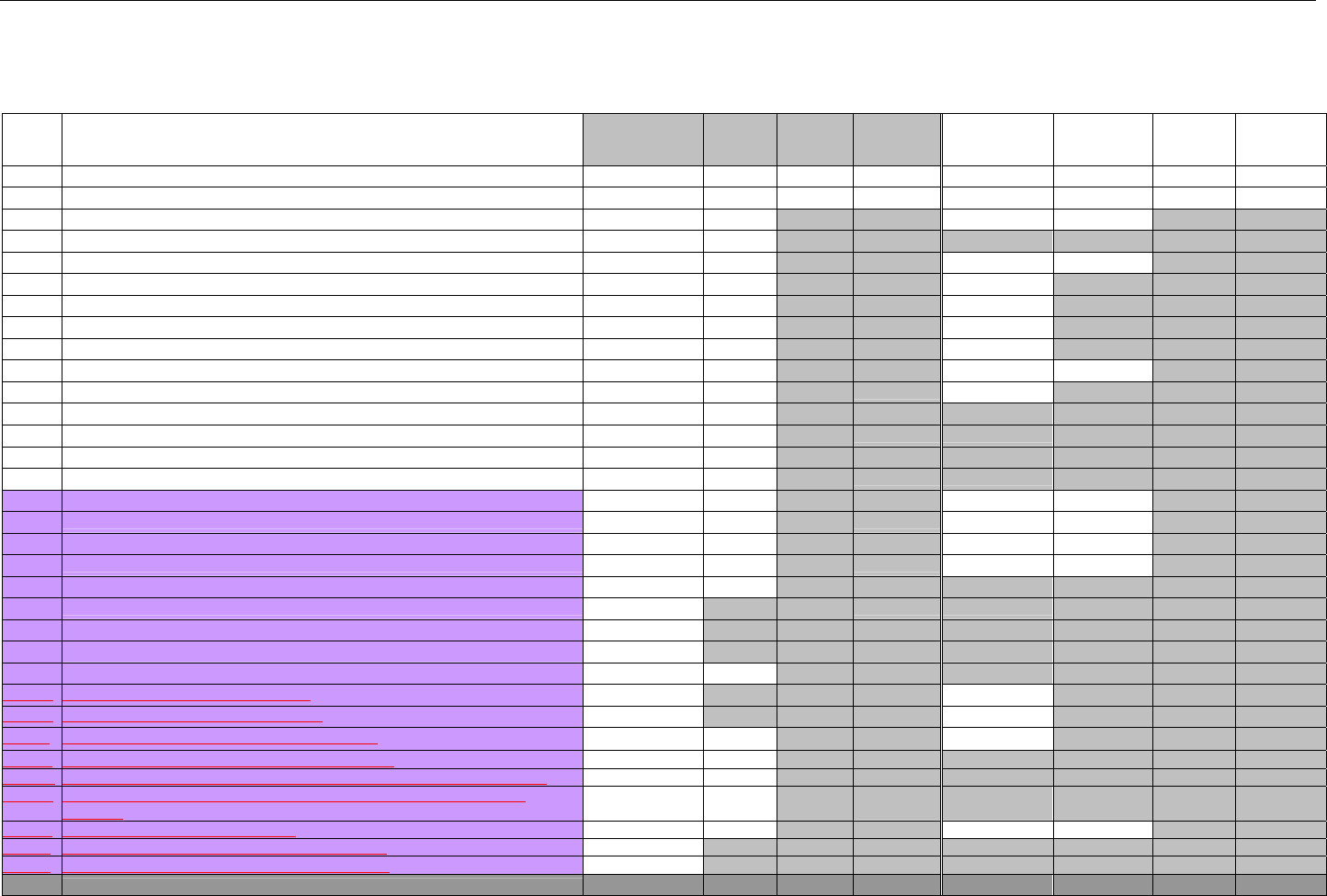
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 618 -
Claim
Billing/Claim
Rebill/Encounter
Service
Billing/Service
Rebill
Header Response Status Accepted Accepted Accepted Rejected Accepted Accepted Accepted Rejected
Transaction Response Status Paid Captured Rejected Rejected Paid Captured Rejected Rejected
5Ø9-F9 Total Amount Paid R R
R R
522-FM Basis of Reimbursement Determination Q Q
N N
523-FN
A
mount Attributed to Sales Tax Q Q
Q Q
512-FC
A
ccumulated Deductible Amount I I
I N
513-FD Remaining Deductible Amount I I
I N
514-FE Remaining Benefit Amount I I
I N
517-FH
A
mount Applied to Periodic Deductible Q Q
Q N
518-F1
A
mount of Copa
y
Q Q
Q Q
52Ø-FK
A
mount Exceeding Periodic Benefit Maximum Q Q
Q N
346-HH Basis of Calculation – Dispensing Fee Q Q
N N
347-HJ Basis of Calculation – Copay Q Q
N N
348-HK Basis of Calculation – Flat Sales Tax Q Q
N N
349-HM Basis of Calculation – Percentage Sales Tax Q Q
N N
571-NZ
A
mount Attributed to Processor Fee Q Q Q Q
575-EQ Patient Sales Tax Amount I I I I
574-2Y Plan Sales Tax Amount I I I I
572-4U
A
mount of Coinsurance Q Q Q Q
573-4V Basis of Calculation-Coinsurance Q Q N N
392-MU Benefit Stage Count Q N N N
393-MV Benefit Stage Qualifier Q N N N
394-MW Benefit Stage Amount Q N N N
577-G3 Estimated Generic Savings I I N N
128-UC Spending Account Amount Remaining I N I N
129-UD Health Plan-Funded Assistance Amount Q N Q N
133-UJ
A
mount Attributed to Provider Network Selection Q Q Q N
134-UK
A
mount Attributed to Product Selection/Brand Drug Q Q N N
135-UM
A
mount Attributed to Product Selection/Non-Preferred Formulary Selection Q Q N N
136-UN
A
mount Attributed to Product Selection/Brand Non-Preferred Formulary
Selection
Q Q
N N
137-UP
A
mount Attributed to Coverage Gap Q Q Q Q
148-U8 Ingredient Cost Contracted/Reimbursable Amount I N N N
149-U9 Dispensing Fee Contracted/Reimbursable Amount I N N N
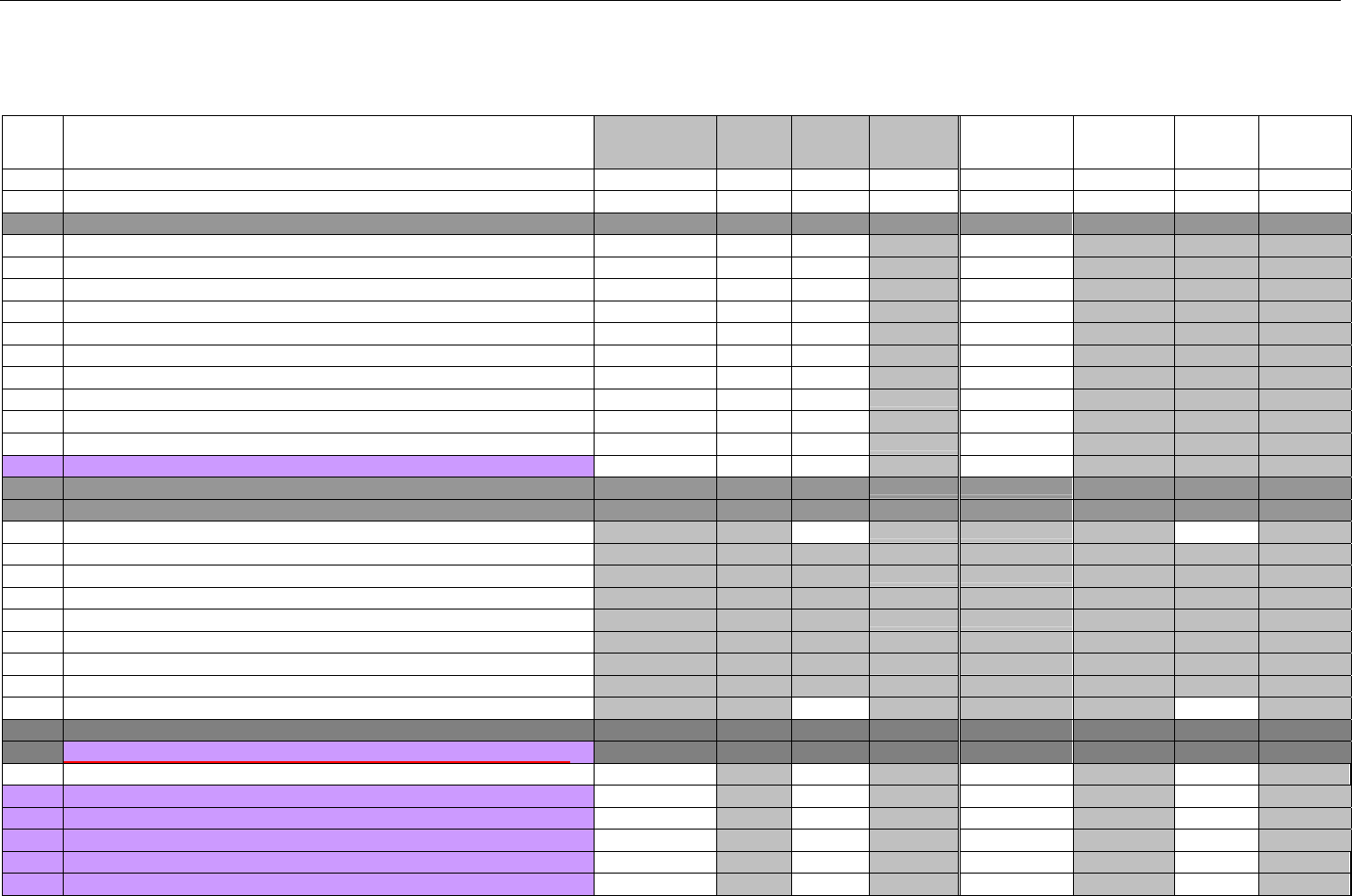
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 619 -
Claim
Billing/Claim
Rebill/Encounter
Service
Billing/Service
Rebill
Header Response Status Accepted Accepted Accepted Rejected Accepted Accepted Accepted Rejected
Transaction Response Status Paid Captured Rejected Rejected Paid Captured Rejected Rejected
RESPONSE DUR/PPS SEGMENT
111-AM Segment Identification M M M
M
567-J6 DUR/PPS Response Code Counter Q Q Q
Q
439-E4 Reason for Service Code Q Q Q
Q
528-FS Clinical Significance Code Q Q Q
Q
529-FT Other Pharmacy Indicator Q Q Q
Q
531-FV Quantity of Previous Fill Q Q Q
Q
53Ø-FU Previous Date of Fill Q Q Q
Q
532-FW Database Indicator Q Q Q
Q
533-FX Other Prescriber Indicator Q Q Q
Q
544-FY DUR Free Text Message Q Q Q
Q
57Ø-NS DUR Additional Text Q Q Q Q
RESPONSE PRIOR AUTHORIZATION SEGMENT
111-AM Segment Identification M M
498-PR Prior Authorization Processed Date N N
498-PS Prior Authorization Effective Date N N
498-PT Prior Authorization Expiration Date N N
498-RA Prior Authorization Quantity N N
498-RB Prior Authorization Dollars Authorized N N
498-PW Prior Authorization Number of Refills Authorized N N
498-PX Prior Authorization Quantity Accumulated N N
498-PY Prior Authorization Number - Assigned Q Q
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS SEGMENT
111-AM Segment Identification M M M M
355-NT Other Payer ID Count M M M M
338-5C Other Payer Coverage Type M M M M
339-6C Other Payer ID Qualifier Q Q Q Q
34Ø-7C Other Payer ID Q Q Q Q
991-MH Other Payer Processor Control Number Q Q Q Q
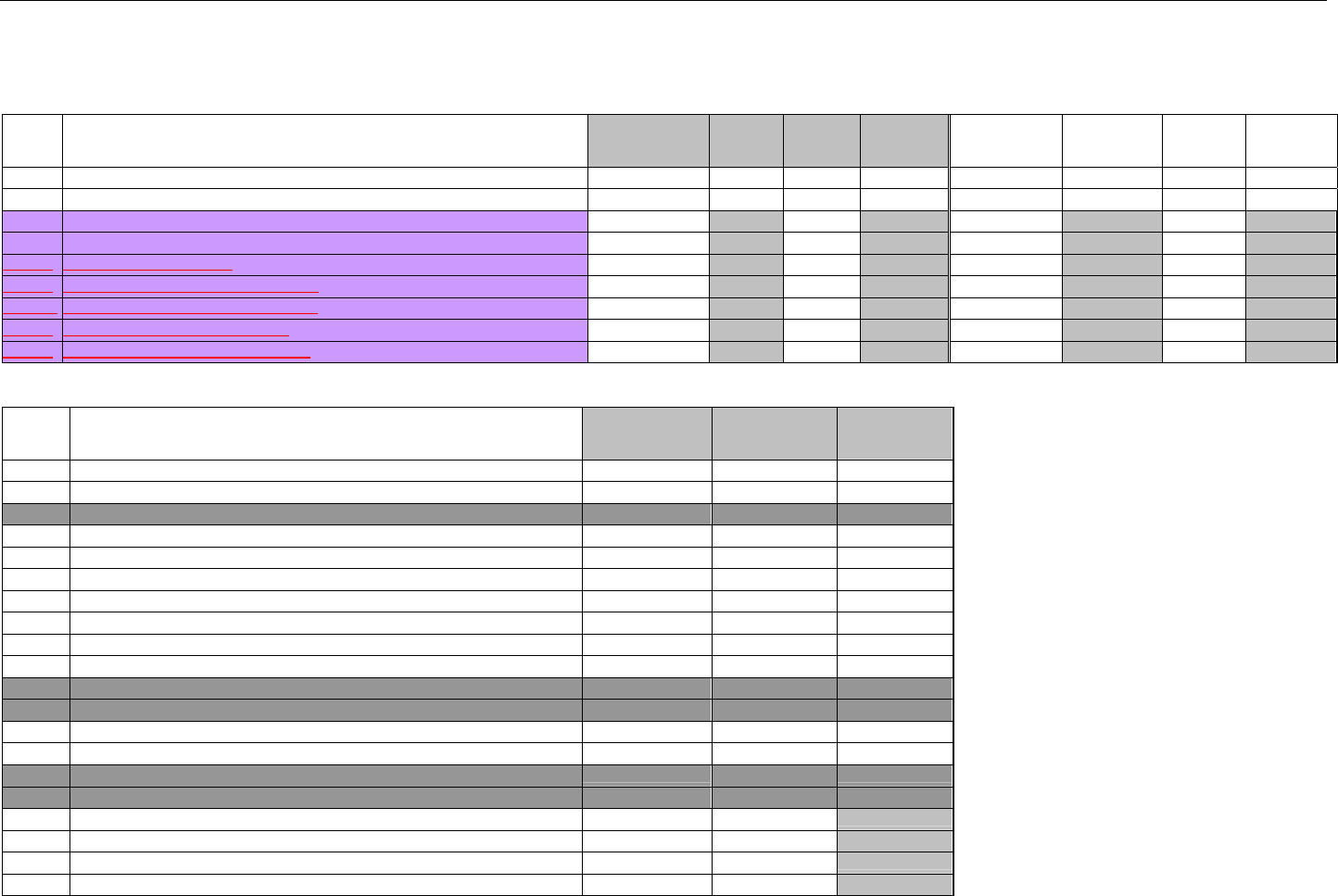
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 620 -
Claim
Billing/Claim
Rebill/Encounter
Service
Billing/Service
Rebill
Header Response Status Accepted Accepted Accepted Rejected Accepted Accepted Accepted Rejected
Transaction Response Status Paid Captured Rejected Rejected Paid Captured Rejected Rejected
356-NU Other Payer Cardholder ID Q Q Q Q
992-MJ Other Payer Group ID Q Q Q Q
142-UV Other Payer Person Code Q Q Q Q
127-UB Other Payer Help Desk Phone Number Q Q Q Q
143-UW Other Payer Patient Relationship Code Q Q Q Q
144-UX Other Payer Benefit Effective Date Q Q Q Q
145-UY Other Payer Benefit Termination Date Q Q Q Q
24.6.3 PREDETERMINATION OF BENEFITS (CLAIM) MATRIX
Predetermination
Of Benefits
(Claim)
Header Response Status Accepted Accepted Rejected
Transaction Response Status Benefit Rejected Rejected
RESPONSE HEADER SEGMENT
1Ø2-A2 Version Release Numbe
r
M M M
1Ø3-A3 Transaction Code M M M
1Ø9-A9 Transaction Count M M M
5Ø1-FI Header Response Status M M M
2Ø2-B2 Service Provider ID Qualifier M M M
2Ø1-B1 Service Provider ID M M M
4Ø1-D1 Date of Service M M M
RESPONSE MESSAGE SEGMENT
111-AM Segment Identification M M M
5Ø4-F4 Message Q Q Q
RESPONSE INSURANCE SEGMENT
111-AM Segment Identification M M
3Ø1-C1 Group ID Q Q
524-FO Plan ID Q Q
545-2F Network Reimbursement ID Q Q

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 621 -
Predetermination
Of Benefits
(Claim)
Header Response Status Accepted Accepted Rejected
Transaction Response Status Benefit Rejected Rejected
568-J7 Payer ID Qualifier Q Q
569-J8 Payer ID Q Q
115-N5 Medicaid ID Number N N
116-N6 Medicaid Agency Number N N
3Ø2-C2 Cardholder ID Q Q
RESPONSE INSURANCE ADDITIONAL INFORMATION SEGMENT
111-AM Segment Identification
139-UR Medicare Part D Coverage Code
138-UQ CMS Low Income Cost Sharing (LICS) Level
24Ø-U1 Contract Number
926-FF Formulary ID
757-U6 Benefit ID
14Ø-US Next Medicare Part D Effective Date
141-UT Next Medicare Part D Termination Date
RESPONSE PATIENT SEGMENT
111-AM Segment Identification M M
31Ø-CA Patient First Name Q Q
311-CB Patient Last Name Q Q
3Ø4-C4 Date Of Birth Q Q
Q Q
RESPONSE STATUS SEGMENT
111-AM Segment Identification M M M
112-AN Transaction Response Status M M M
5Ø3-F3
A
uthorization Number Q Q Q
51Ø-FA Reject Count N R R
511-FB Reject Code N R R
546-4F Reject Field Occurrence Indicator N Q Q
547-5F
A
pproved Message Code Count Q N N
548-6F
A
pproved Message Code Q N N
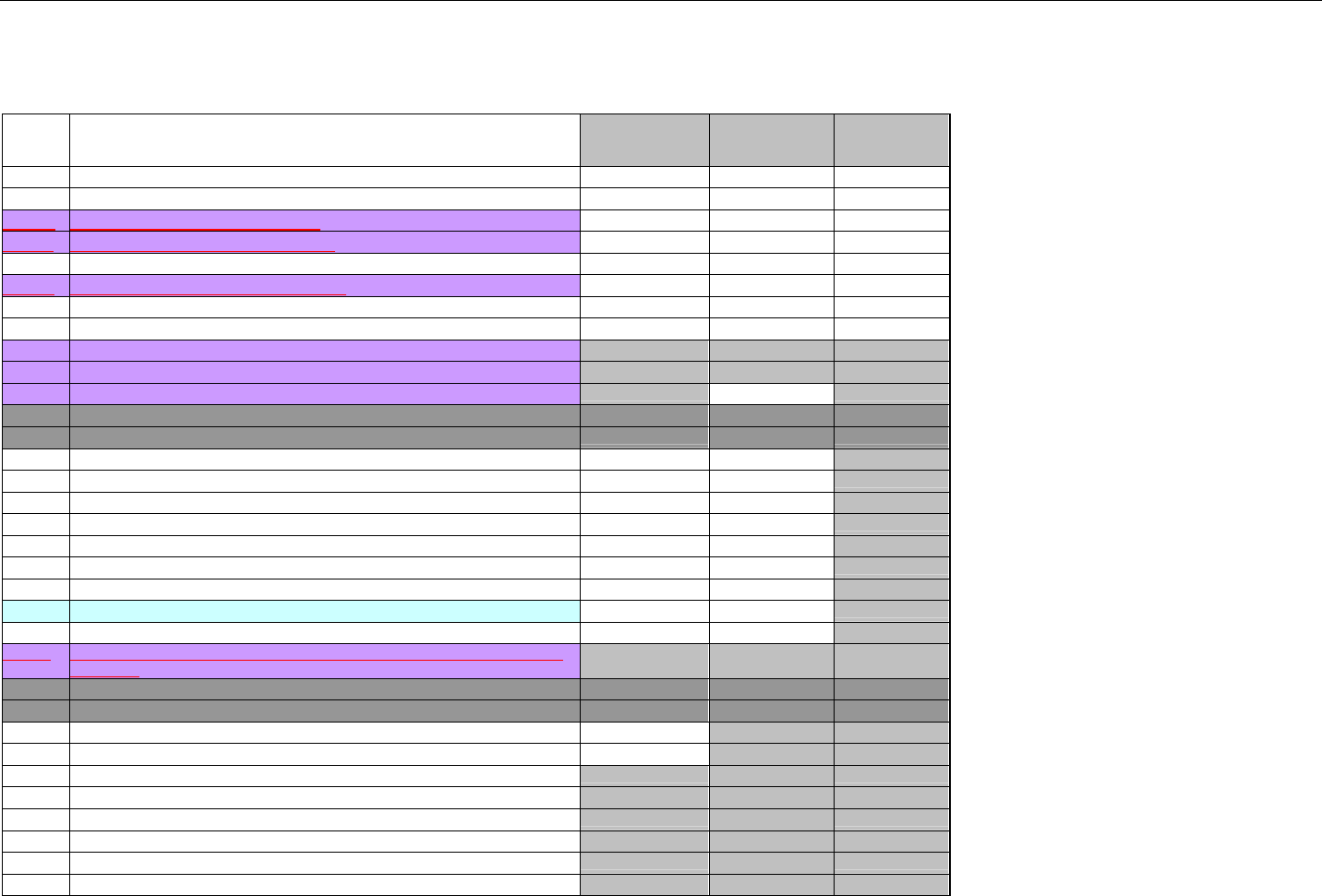
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 622 -
Predetermination
Of Benefits
(Claim)
Header Response Status Accepted Accepted Rejected
Transaction Response Status Benefit Rejected Rejected
13Ø-UF
A
dditional Message Information Count Q Q Q
132-UH
A
dditional Message Information Qualifier Q Q Q
526-FQ
A
dditional Message Information Q Q Q
131-UG
A
dditional Message Information Continuit
y
Q Q Q
55Ø-7F Help Desk Phone Number Qualifier Q Q Q
55Ø-8F Help Desk Phone Number Q Q Q
88Ø-K5 Transaction Reference Number N N N
993-A7 Internal Control Number N N N
987-MA URL N i N
RESPONSE CLAIM SEGMENT
111-AM Segment Identification M M
455-EM Prescription/Service Reference Number Qualifier M M
4Ø2-D2 Prescription/Service Reference Number M M
551-9F Preferred Product Count Q Q
552-AP Preferred Product ID Qualifier Q Q
553-AR Preferred Product ID Q Q
554-AS Preferred Product Incentive Q Q
555-AT Preferred Product Cost Share Incentive Q Q
551-9F Preferred Product Description Q Q
114-N4 Medicaid Subrogation Internal Control Number/Transaction Control Number
(ICN/TCN)
N N
RESPONSE PRICING SEGMENT
111-AM Segment Identification M
5Ø5-F5 Patient Pay Amount R
5Ø6-F6 Ingredient Cost Paid N
5Ø7-F7 Dispensing Fee Paid N
557-AV Tax Exempt Indicator N
558-AW Flat Sales Tax Amount Paid N
559-AX Percentage Sales Tax Amount Paid N
56Ø-AY Percentage Sales Tax Rate Paid N
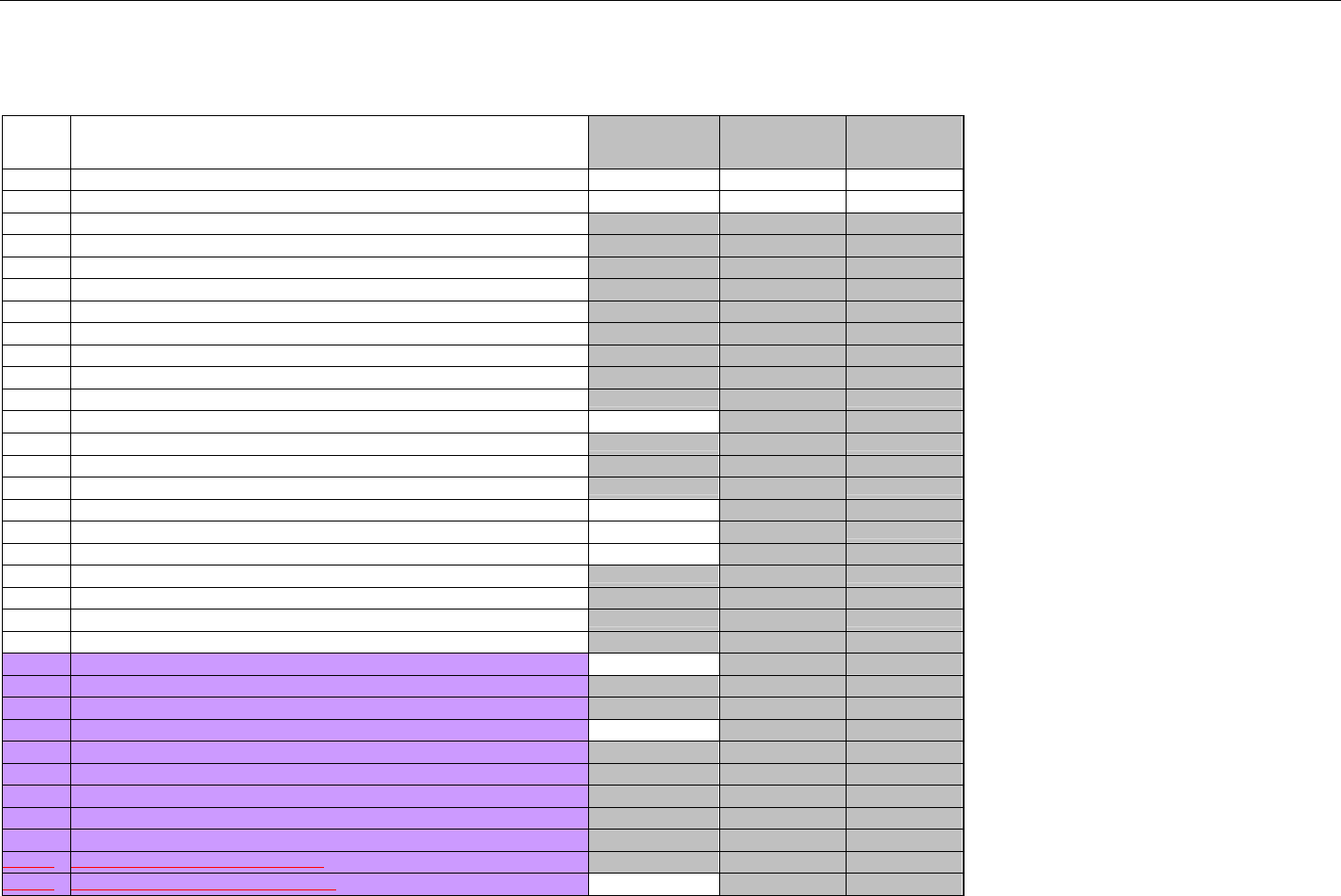
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 623 -
Predetermination
Of Benefits
(Claim)
Header Response Status Accepted Accepted Rejected
Transaction Response Status Benefit Rejected Rejected
561-AZ Percentage Sales Tax Basis Paid N
521-FL Incentive Amount Paid N
562-J1 Professional Service Fee Paid N
563-J2 Other Amount Paid Count N
564-J3 Other Amount Paid Qualifier N
565-J4 Other Amount Paid N
566-J5 Other Payer Amount Recognized N
5Ø9-F9 Total Amount Paid N
522-FM Basis of Reimbursement Determination N
523-FN
A
mount Attributed to Sales Tax Q
512-FC
A
ccumulated Deductible Amount N
513-FD Remaining Deductible Amount N
514-FE Remaining Benefit Amount N
517-FH
A
mount Applied to Periodic Deductible Q
518-F1
A
mount of Copa
y
Q
52Ø-FK
A
mount Exceeding Periodic Benefit Maximum Q
346-HH Basis of Calculation – Dispensing Fee N
347-HJ Basis of Calculation – Copay N
348-HK Basis of Calculation – Flat Sales Tax N
349-HM Basis of Calculation – Percentage Sales Tax N
571-NZ
A
mount Attributed to Processor Fee Q
575-EQ Patient Sales Tax Amount N
574-2Y Plan Sales Tax Amount N
572-4U
A
mount of Coinsurance Q
573-4V Basis of Calculation-Coinsurance N
392-MU Benefit Stage Count N
393-MV Benefit Stage Qualifier N
394-MW Benefit Stage Amount N
577-G3 Estimated Generic Savings N
128-UC Spending Account Amount Remaining N
129-UD Health Plan-Funded Assistance Amount Q
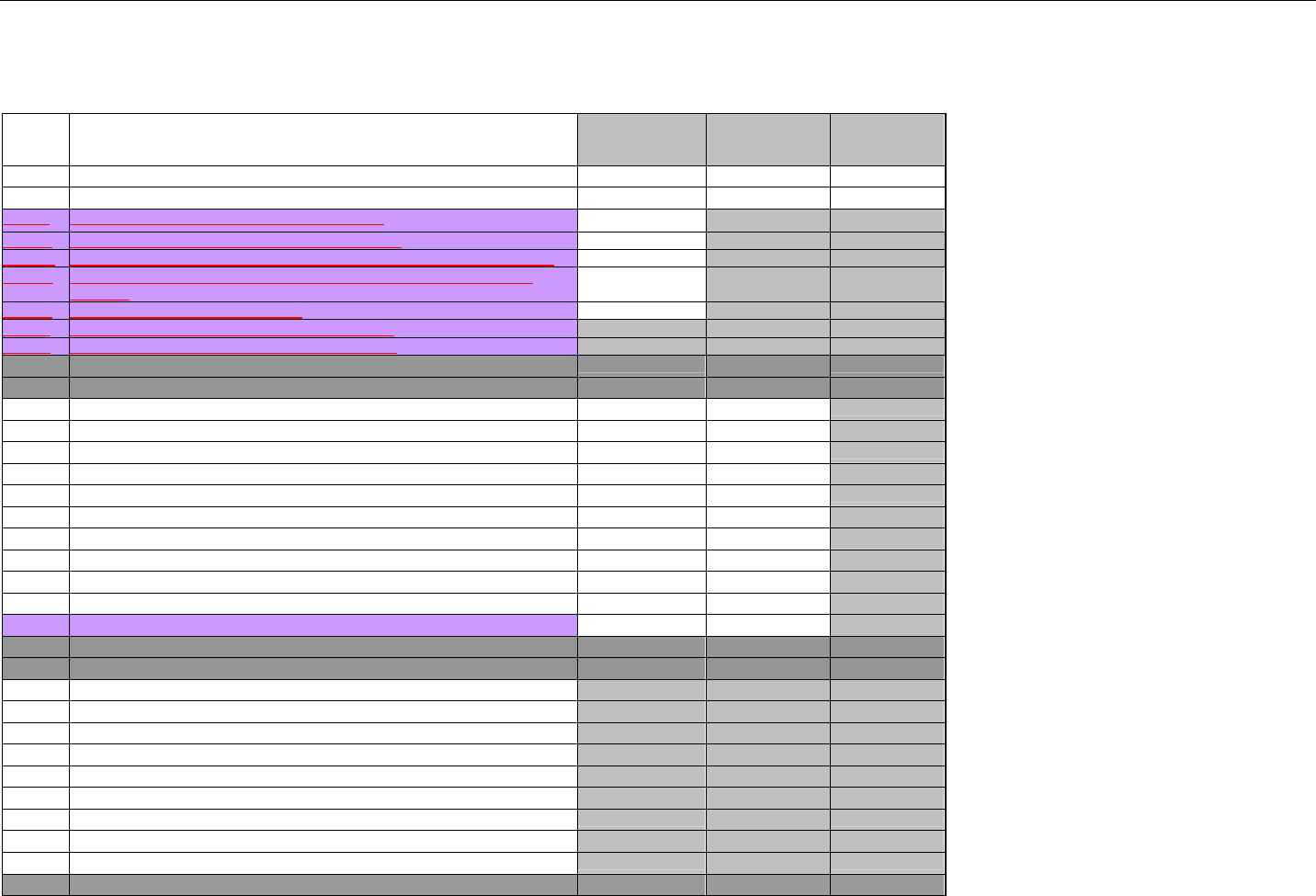
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 624 -
Predetermination
Of Benefits
(Claim)
Header Response Status Accepted Accepted Rejected
Transaction Response Status Benefit Rejected Rejected
133-UJ
A
mount Attributed to Provider Network Selection Q
134-UK
A
mount Attributed to Product Selection/Brand Drug Q
135-UM
A
mount Attributed to Product Selection/Non-Preferred Formulary Selection Q
136-UN
A
mount Attributed to Product Selection/Brand Non-Preferred Formulary
Selection
Q
137-UP
A
mount Attributed to Coverage Gap Q
148-U8 Ingredient Cost Contracted/Reimbursable Amount N
149-U9 Dispensing Fee Contracted/Reimbursable Amount N
RESPONSE DUR/PPS SEGMENT
111-AM Segment Identification M M
567-J6 DUR/PPS Response Code Counter Q Q
439-E4 Reason for Service Code Q Q
528-FS Clinical Significance Code Q Q
529-FT Other Pharmacy Indicator Q Q
531-FV Quantity of Previous Fill Q Q
53Ø-FU Previous Date of Fill Q Q
532-FW Database Indicator Q Q
533-FX Other Prescriber Indicator Q Q
544-FY DUR Free Text Message Q Q
57Ø-NS DUR Additional Text Q Q
RESPONSE PRIOR AUTHORIZATION SEGMENT
111-AM Segment Identification
498-PR Prior Authorization Processed Date
498-PS Prior Authorization Effective Date
498-PT Prior Authorization Expiration Date
498-RA Prior Authorization Quantity
498-RB Prior Authorization Dollars Authorized
498-PW Prior Authorization Number of Refills Authorized
498-PX Prior Authorization Quantity Accumulated
498-PY Prior Authorization Number - Assigned
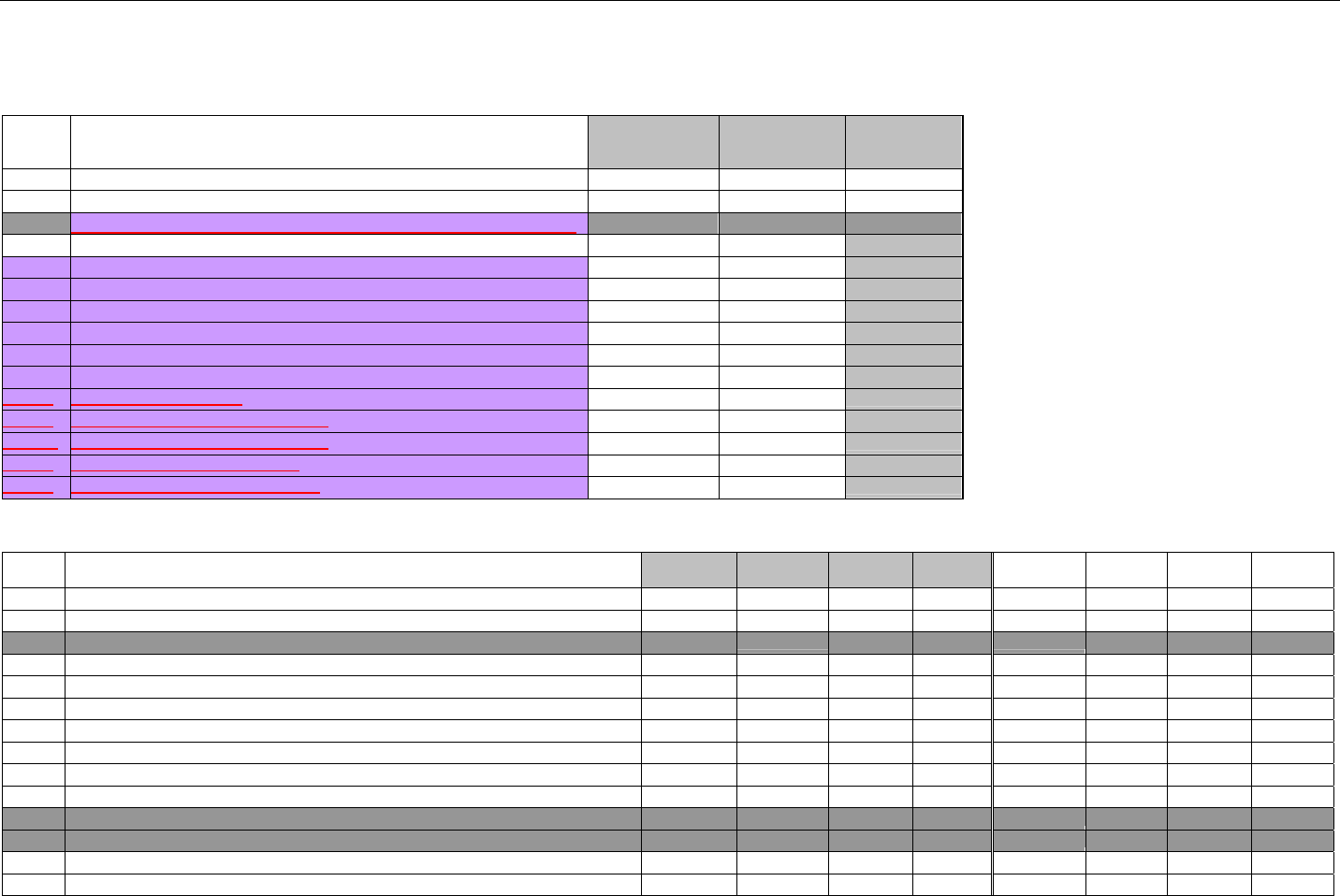
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 625 -
Predetermination
Of Benefits
(Claim)
Header Response Status Accepted Accepted Rejected
Transaction Response Status Benefit Rejected Rejected
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS SEGMENT
111-AM Segment Identification M M
355-NT Other Payer ID Count Q Q
339-6C Other Payer ID Qualifier Q Q
34Ø-7C Other Payer ID Q Q
991-MH Other Payer Processor Control Number Q Q
356-NU Other Payer Cardholder ID Q Q
992-MJ Other Payer Group ID Q Q
142-UV Other Payer Person Code Q Q
127-UB Other Payer Help Desk Phone Number Q Q
143-UW Other Payer Patient Relationship Code Q Q
144-UX Other Payer Benefit Effective Date Q Q
145-UY Other Payer Benefit Termination Date Q Q
24.6.4 CLAIM REVERSAL/SERVICE REVERSAL MATRIX
Claim
Reversal Service
Reversal
Header Response Status Accepted Accepted Accepted Rejected Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Rejected Rejected Approved Captured Rejected Rejected
RESPONSE HEADER SEGMENT
1Ø2-A2 Version Release Numbe
r
M M M M M M M M
1Ø3-A3 Transaction Code M M M M M M M M
1Ø9-A9 Transaction Count M M M M M M M M
5Ø1-FI Header Response Status M M M M M M M M
2Ø2-B2 Service Provider ID Qualifier M M M M M M M M
2Ø1-B1 Service Provider ID M M M M M M M M
4Ø1-D1 Date of Service M M M M M M M M
RESPONSE MESSAGE SEGMENT
111-AM Segment Identification M M M M M M M M
5Ø4-F4 Message Q Q Q Q Q Q Q Q
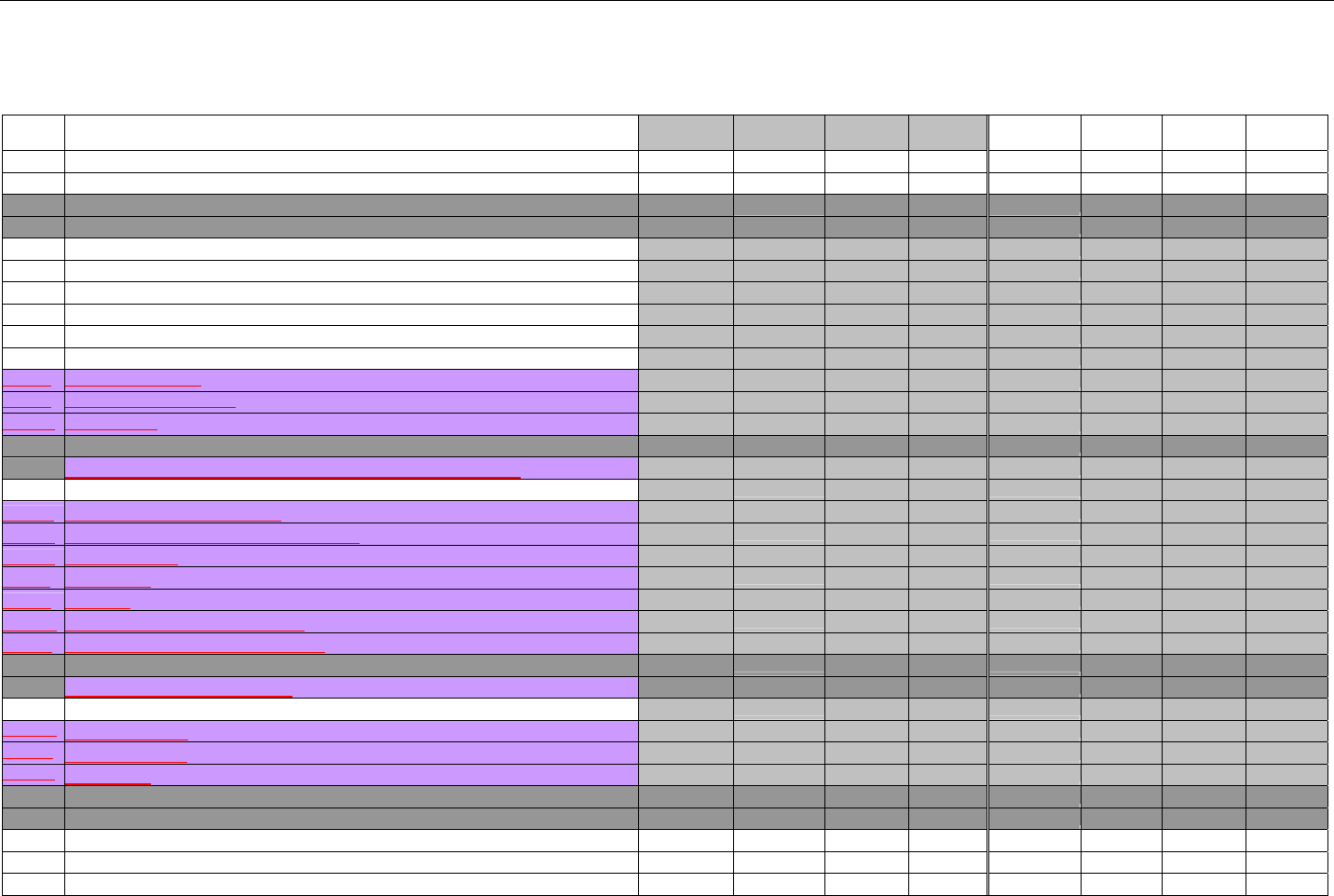
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 626 -
Claim
Reversal Service
Reversal
Header Response Status Accepted Accepted Accepted Rejected Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Rejected Rejected Approved Captured Rejected Rejected
RESPONSE INSURANCE SEGMENT
111-AM Segment Identification
3Ø1-C1 Group ID
524-FO Plan ID
545-2F Network Reimbursement ID
568-J7 Payer ID Qualifier
569-J8 Payer ID
115-N5 Medicaid ID Number
116-N6 Medicaid Agency Number
3Ø2-C2 Cardholder ID
RESPONSE INSURANCE ADDITIONAL INFORMATION SEGMENT
111-AM Segment Identification
139-UR Medicare Part D Coverage Code
138-UQ CMS Low Income Cost Sharing (LICS) Level
24Ø-U1 Contract Number
926-FF Formulary ID
757-U6 Benefit ID
14Ø-US Next Medicare Part D Effective Date
141-UT Next Medicare Part D Termination Date
RESPONSE PATIENT SEGMENT
111-AM Segment Identification
31Ø-CA Patient First Name
311-CB Patient Last Name
3Ø4-C4 Date Of Birth
RESPONSE STATUS SEGMENT
111-AM Segment Identification M M M M M M M M
112-AN Transaction Response Status M M M M M M M M
5Ø3-F3
A
uthorization Numbe
r
Q Q Q Q Q Q Q Q
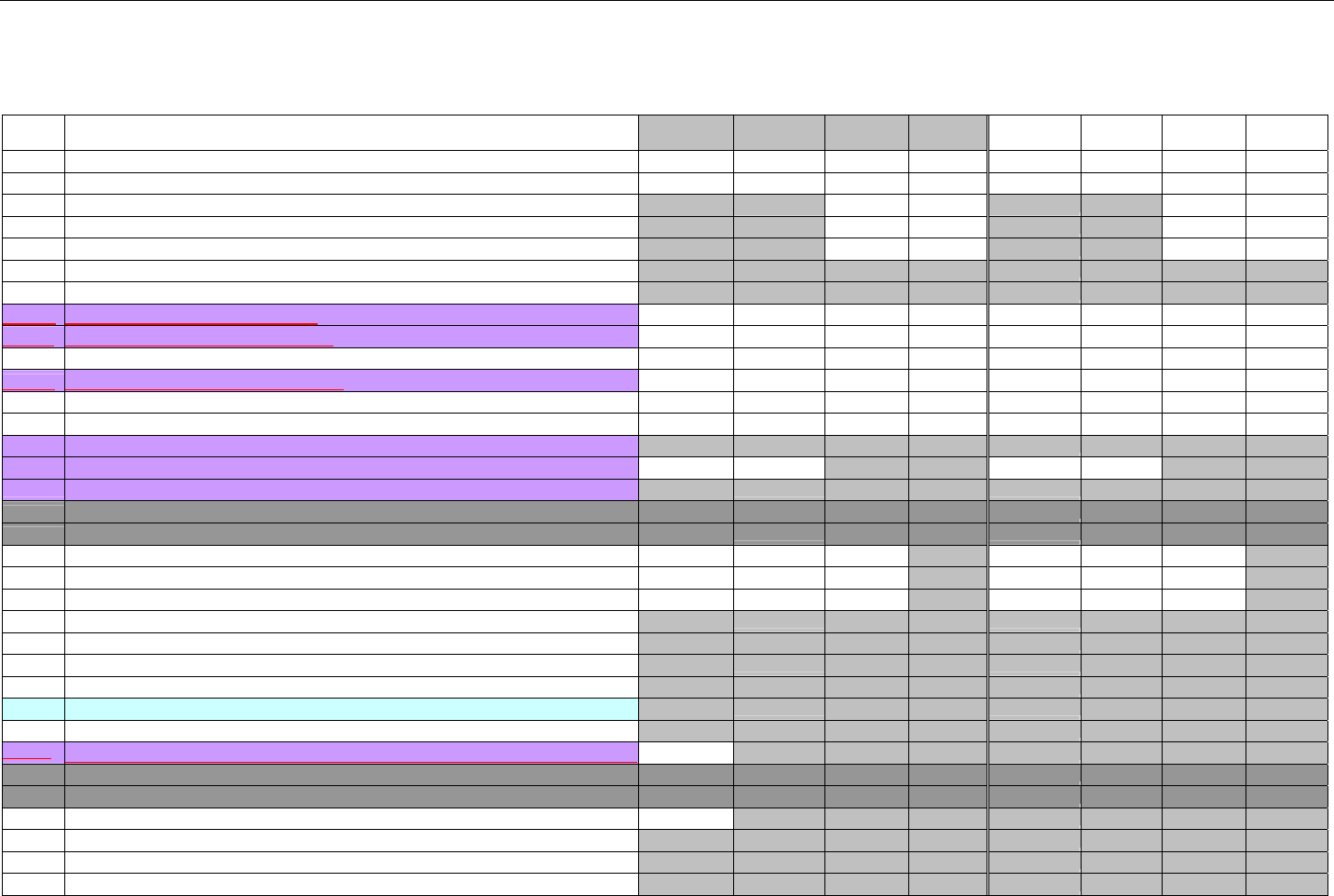
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 627 -
Claim
Reversal Service
Reversal
Header Response Status Accepted Accepted Accepted Rejected Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Rejected Rejected Approved Captured Rejected Rejected
51Ø-FA Reject Count N N R R N N R R
511-FB Reject Code N N R R N N R R
546-4F Reject Field Occurrence Indicator N N Q Q N N Q Q
547-5F
A
pproved Message Code Count N N N N N N N N
548-6F
A
pproved Message Code N N N N N N N N
13Ø-UF
A
dditional Message Information Count Q Q Q Q Q Q Q Q
132-UH
A
dditional Message Information Qualifie
r
Q Q Q Q Q Q Q Q
526-FQ
A
dditional Message Information Q Q Q Q Q Q Q Q
131-UG
A
dditional Message Information Continuit
y
Q Q Q Q Q Q Q Q
55Ø-7F Help Desk Phone Number Qualifier Q Q Q Q Q Q Q Q
55Ø-8F Help Desk Phone Number Q Q Q Q Q Q Q Q
88Ø-K5 Transaction Reference Number N N N N N N N N
993-A7 Internal Control Number Q Q N N Q Q N N
987-MA URL N N N N N N N N
RESPONSE CLAIM SEGMENT
111-AM Segment Identification M M M
M M M
455-EM Prescription/Service Reference Number Qualifier M M M
M M M
4Ø2-D2 Prescription/Service Reference Number M M M
M M M
551-9F Preferred Product Count N N N N N N
552-AP Preferred Product ID Qualifier N N N N N N
553-AR Preferred Product ID N N N N N N
554-AS Preferred Product Incentive N N N N N N
555-AT Preferred Product Cost Share Incentive N N N N N N
551-9F Preferred Product Description N N N N N N
114-N4 Medicaid Subrogation Internal Control Number/Transaction Control Number (ICN/TCN) N, RM N N N N N
RESPONSE PRICING SEGMENT
111-AM Segment Identification M
5Ø5-F5 Patient Pay Amount N
5Ø6-F6 Ingredient Cost Paid N
5Ø7-F7 Dispensing Fee Paid N
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 628 -
Claim
Reversal Service
Reversal
Header Response Status Accepted Accepted Accepted Rejected Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Rejected Rejected Approved Captured Rejected Rejected
557-AV Tax Exempt Indicator N
558-AW Flat Sales Tax Amount Paid N
559-AX Percentage Sales Tax Amount Paid N
56Ø-AY Percentage Sales Tax Rate Paid N
561-AZ Percentage Sales Tax Basis Paid N
521-FL Incentive Amount Paid Q
562-J1 Professional Service Fee Paid N
563-J2 Other Amount Paid Count N
564-J3 Other Amount Paid Qualifier N
565-J4 Other Amount Paid N
566-J5 Other Payer Amount Recognized N
5Ø9-F9 Total Amount Paid Q
522-FM Basis of Reimbursement Determination N
523-FN
A
mount Attributed to Sales Tax N
512-FC
A
ccumulated Deductible Amount N
513-FD Remaining Deductible Amount N
514-FE Remaining Benefit Amount N
517-FH
A
mount Applied to Periodic Deductible N
518-F1
A
mount of Copa
y
N
52Ø-FK
A
mount Exceeding Periodic Benefit Maximum N
346-HH Basis of Calculation – Dispensing Fee N
347-HJ Basis of Calculation – Copay N
348-HK Basis of Calculation – Flat Sales Tax N
349-HM Basis of Calculation – Percentage Sales Tax N
571-NZ
A
mount Attributed to Processor Fee N
575-EQ Patient Sales Tax Amount N
574-2Y Plan Sales Tax Amount N
572-4U
A
mount of Coinsurance N
573-4V Basis of Calculation-Coinsurance N
392-MU Benefit Stage Count N
393-MV Benefit Stage Qualifier N
394-MW Benefit Stage Amount N
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 629 -
Claim
Reversal Service
Reversal
Header Response Status Accepted Accepted Accepted Rejected Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Rejected Rejected Approved Captured Rejected Rejected
577-G3 Estimated Generic Savings N
128-UC Spending Account Amount Remaining N
129-UD Health Plan-Funded Assistance Amount N
133-UJ
A
mount Attributed to Provider Network Selection N
134-UK
A
mount Attributed to Product Selection/Brand Drug N
135-UM
A
mount Attributed to Product Selection/Non-Preferred Formulary Selection N
136-UN
A
mount Attributed to Product Selection/Brand Non-Preferred Formulary Selection N
137-UP
A
mount Attributed to Coverage Gap N
148-U8 Ingredient Cost Contracted/Reimbursable Amount N
149-U9 Dispensing Fee Contracted/Reimbursable Amount N
RESPONSE DUR/PPS SEGMENT
111-AM Segment Identification
567-J6 DUR/PPS Response Code Counter
439-E4 Reason for Service Code
528-FS Clinical Significance Code
529-FT Other Pharmacy Indicator
531-FV Quantity of Previous Fill
53Ø-FU Previous Date of Fill
532-FW Database Indicator
533-FX Other Prescriber Indicator
544-FY DUR Free Text Message
57Ø-NS DUR Additional Text
RESPONSE PRIOR AUTHORIZATION SEGMENT
111-AM Segment Identification
498-PR Prior Authorization Processed Date
498-PS Prior Authorization Effective Date
498-PT Prior Authorization Expiration Date
498-RA Prior Authorization Quantity
498-RB Prior Authorization Dollars Authorized
498-PW Prior Authorization Number of Refills Authorized
498-PX Prior Authorization Quantity Accumulated

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 630 -
Claim
Reversal Service
Reversal
Header Response Status Accepted Accepted Accepted Rejected Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Rejected Rejected Approved Captured Rejected Rejected
498-PY Prior Authorization Number - Assigned
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS SEGMENT
111-AM Segment Identification
355-NT Other Payer ID Count
338-5C Other Payer Coverage Type
339-6C Other Payer ID Qualifier
34Ø-7C Other Payer ID
991-MH Other Payer Processor Control Number
356-NU Other Payer Cardholder ID
992-MJ Other Payer Group ID
142-UV Other Payer Person Code
127-UB Other Payer Help Desk Phone Number
143-UW Other Payer Patient Relationship Code
144-UX Other Payer Benefit Effective Date
145-UY Other Payer Benefit Termination Date
24.6.5 PRIOR AUTHORIZATION REQUEST AND BILLING (CLAIM/SERVICE) MATRIX
Prior
A
uthorization
Request And
Billing
(Claim)
Prior
A
uthorization
Request And
Billing
(Service)
Header Response Status Accepted Accepted Accepted Accepted Rejected Accepted Accepted Accepted Accepted Rejected
Transaction Response Status Paid Captured Deferred Rejected Rejected Paid Captured Deferred Rejected Rejected
RESPONSE HEADER SEGMENT
1Ø2-A2 Version Release Numbe
r
M M M M M M M M M M
1Ø3-A3 Transaction Code M M M M M M M M M M
1Ø9-A9 Transaction Count M M M M M M M M M M
5Ø1-FI Header Response Status M M M M M M M M M M
2Ø2-B2 Service Provider ID Qualifier M M M M M M M M M M
2Ø1-B1 Service Provider ID M M M M M M M M M M
4Ø1-D1 Date of Service M M M M M M M M M M
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 631 -
Prior
A
uthorization
Request And
Billing
(Claim)
Prior
A
uthorization
Request And
Billing
(Service)
Header Response Status Accepted Accepted Accepted Accepted Rejected Accepted Accepted Accepted Accepted Rejected
Transaction Response Status Paid Captured Deferred Rejected Rejected Paid Captured Deferred Rejected Rejected
RESPONSE MESSAGE SEGMENT
111-AM Segment Identification M M M M M M M M M M
5Ø4-F4 Message Q Q Q Q Q Q Q Q Q Q
RESPONSE INSURANCE SEGMENT
111-AM Segment Identification M M M M M M M M
3Ø1-C1 Group ID Q Q Q Q Q Q Q Q
524-FO Plan ID Q Q Q Q Q Q Q Q
545-2F Network Reimbursement ID Q N N Q Q N N Q
568-J7 Payer ID Qualifier Q N N N Q N N N
569-J8 Payer ID Q N N N Q N N N
115-N5 Medicaid ID Number N N N N N N N N
116-N6 Medicaid Agency Number N N N N N N N N
3Ø2-C2 Cardholder ID Q Q Q Q Q Q Q Q
RESPONSE INSURANCE ADDITIONAL
INFORMATION SEGMENT
111-AM Segment Identification
139-UR Medicare Part D Coverage Code
138-UQ CMS Low Income Cost Sharing (LICS) Level
24Ø-U1 Contract Number
926-FF Formulary ID
757-U6 Benefit ID
14Ø-US Next Medicare Part D Effective Date
141-UT Next Medicare Part D Termination Date
RESPONSE PATIENT SEGMENT
111-AM Segment Identification M M M M M M M M
31Ø-CA Patient First Name Q Q Q Q Q Q Q Q
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 632 -
Prior
A
uthorization
Request And
Billing
(Claim)
Prior
A
uthorization
Request And
Billing
(Service)
Header Response Status Accepted Accepted Accepted Accepted Rejected Accepted Accepted Accepted Accepted Rejected
Transaction Response Status Paid Captured Deferred Rejected Rejected Paid Captured Deferred Rejected Rejected
311-CB Patient Last Name Q Q Q Q Q Q Q Q
3Ø4-C4 Date Of Birth Q Q Q Q Q Q Q Q
RESPONSE STATUS SEGMENT
111-AM Segment Identification M M M M M M M M M M
112-AN Transaction Response Status M M M M M M M M M M
5Ø3-F3
A
uthorization Numbe
r
Q R Q Q Q Q R Q Q Q
51Ø-FA Reject Count N N N R R N N N R R
511-FB Reject Code N N N R R N N N R R
546-4F Reject Field Occurrence Indicator N N N Q Q N N N Q Q
547-5F
A
pproved Message Code Count Q N N N N Q N N N N
548-6F
A
pproved Message Code Q N N N N Q N N N N
13Ø-UF
A
dditional Message Information Count Q Q Q Q Q Q Q Q Q Q
132-UH
A
dditional Message Information Qualifie
r
Q Q Q Q Q Q Q Q Q Q
526-FQ
A
dditional Message Information Q Q Q Q Q Q Q Q Q Q
131-UG
A
dditional Message Information Continuit
y
Q Q Q Q Q Q Q Q Q Q
55Ø-7F Help Desk Phone Number Qualifier Q Q Q Q Q Q Q Q Q Q
55Ø-8F Help Desk Phone Number Q Q Q Q Q Q Q Q Q Q
88Ø-K5 Transaction Reference Number N N N N N N N N N N
993-A7 Internal Control Number Q Q N N N Q Q N N N
987-MA URL N N N N N N N N N N
RESPONSE CLAIM SEGMENT
111-AM Segment Identification M M M M M M M M
455-EM Prescription/Service Reference Number Qualifier M M M M M M M M
4Ø2-D2 Prescription/Service Reference Number M M M M M M M M
551-9F Preferred Product Count Q Q Q Q N N N N
552-AP Preferred Product ID Qualifier Q Q Q Q N N N N
553-AR Preferred Product ID Q Q Q Q N N N N
554-AS Preferred Product Incentive Q Q Q Q N N N N
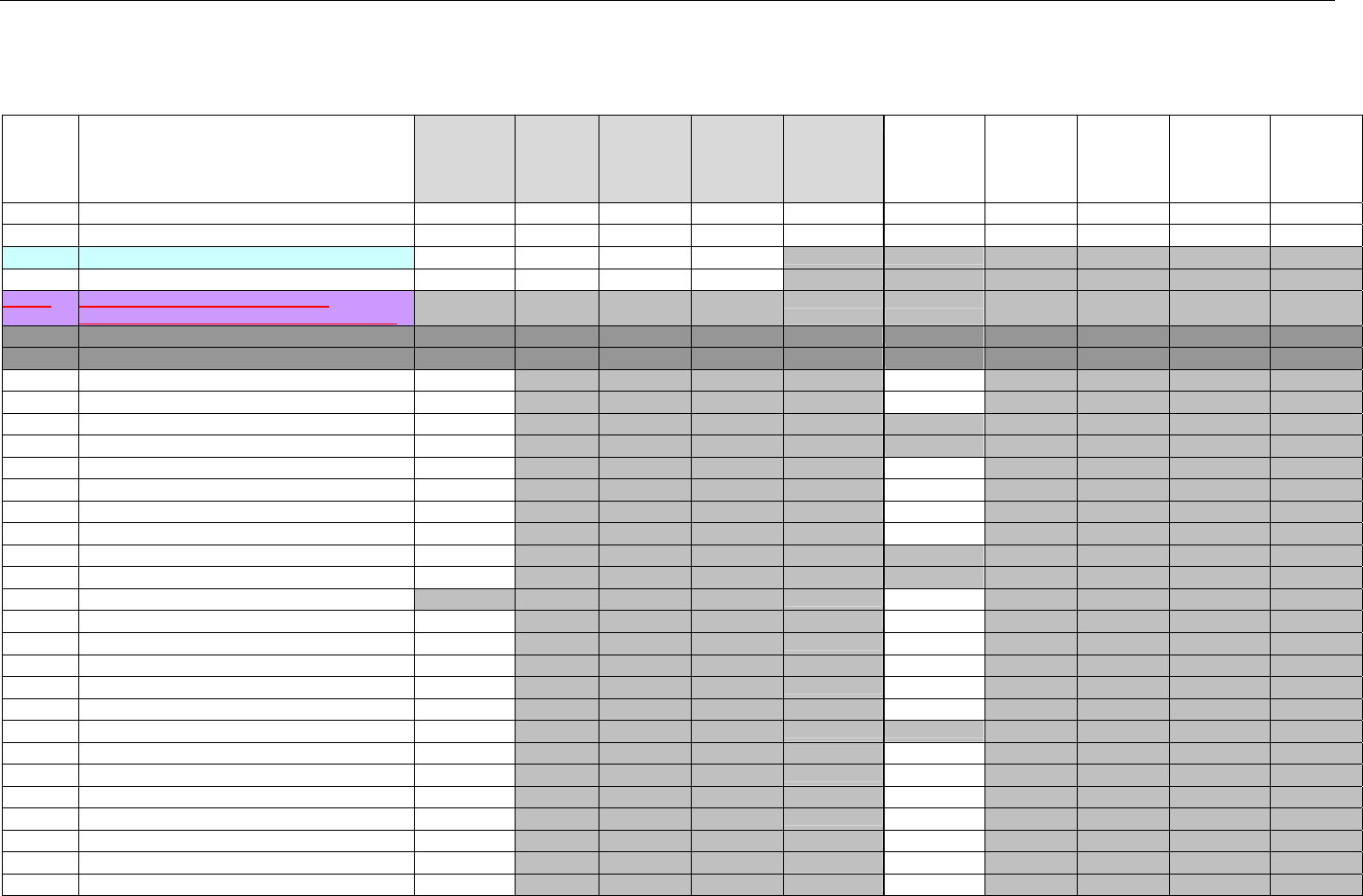
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 633 -
Prior
A
uthorization
Request And
Billing
(Claim)
Prior
A
uthorization
Request And
Billing
(Service)
Header Response Status Accepted Accepted Accepted Accepted Rejected Accepted Accepted Accepted Accepted Rejected
Transaction Response Status Paid Captured Deferred Rejected Rejected Paid Captured Deferred Rejected Rejected
555-AT Preferred Product Cost Share Incentive Q Q Q Q N N N N
551-9F Preferred Product Description Q Q Q Q N N N N
114-N4 Medicaid Subrogation Internal Control
Number/Transaction Control Number (ICN/TCN)
N N N N N N N N
RESPONSE PRICING SEGMENT
111-AM Segment Identification M M
5Ø5-F5 Patient Pay Amount R R
5Ø6-F6 Ingredient Cost Paid Q N
5Ø7-F7 Dispensing Fee Paid Q N
557-AV Tax Exempt Indicator Q Q
558-AW Flat Sales Tax Amount Paid Q Q
559-AX Percentage Sales Tax Amount Paid Q Q
56Ø-AY Percentage Sales Tax Rate Paid Q Q
561-AZ Percentage Sales Tax Basis Paid Q N
521-FL Incentive Amount Paid Q N
562-J1 Professional Service Fee Paid N R
563-J2 Other Amount Paid Count Q Q
564-J3 Other Amount Paid Qualifier Q Q
565-J4 Other Amount Paid Q Q
566-J5 Other Payer Amount Recognized Q Q
5Ø9-F9 Total Amount Paid R R
522-FM Basis of Reimbursement Determination Q N
523-FN
A
mount Attributed to Sales Tax Q Q
512-FC
A
ccumulated Deductible Amount I I
513-FD Remaining Deductible Amount I I
514-FE Remaining Benefit Amount I I
517-FH
A
mount Applied to Periodic Deductible Q Q
518-F1
A
mount of Copa
y
Q Q
52Ø-FK
A
mount Exceeding Periodic Benefit Maximum Q Q
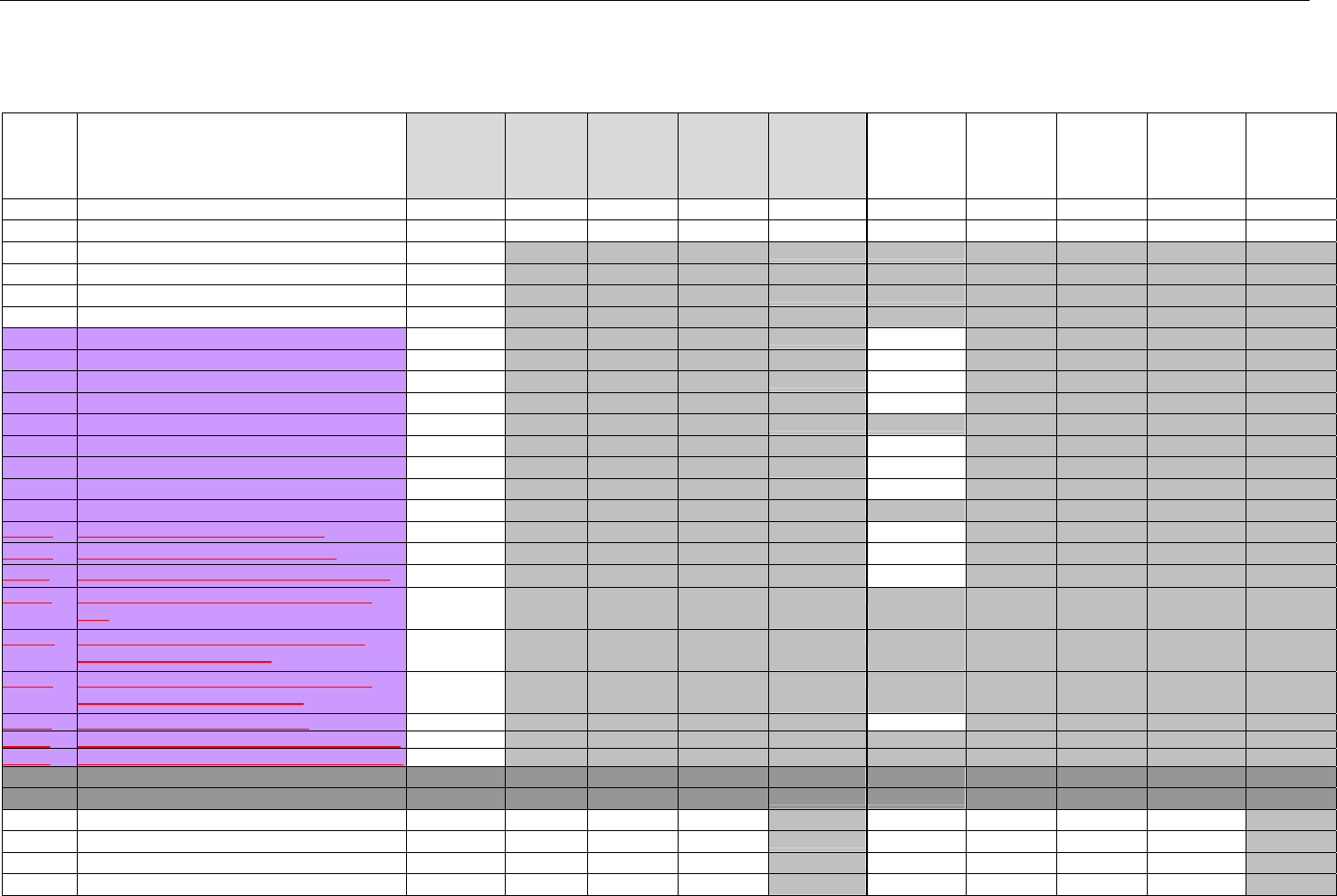
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 634 -
Prior
A
uthorization
Request And
Billing
(Claim)
Prior
A
uthorization
Request And
Billing
(Service)
Header Response Status Accepted Accepted Accepted Accepted Rejected Accepted Accepted Accepted Accepted Rejected
Transaction Response Status Paid Captured Deferred Rejected Rejected Paid Captured Deferred Rejected Rejected
346-HH Basis of Calculation – Dispensing Fee Q N
347-HJ Basis of Calculation – Copay Q N
348-HK Basis of Calculation – Flat Sales Tax Q N
349-HM Basis of Calculation – Percentage Sales Tax Q N
571-NZ
A
mount Attributed to Processor Fee Q Q
575-EQ Patient Sales Tax Amount I I
574-2Y Plan Sales Tax Amount I I
572-4U
A
mount of Coinsurance Q Q
573-4V Basis of Calculation-Coinsurance Q N
392-MU Benefit Stage Count Q Q
393-MV Benefit Stage Qualifier Q Q
394-MW Benefit Stage Amount Q Q
577-G3 Estimated Generic Savings Q N
128-UC Spending Account Amount Remaining I I
129-UD Health Plan-Funded Assistance Amount Q Q
133-UJ
A
mount Attributed to Provider Network Selection Q Q
134-UK
A
mount Attributed to Product Selection/Brand
Drug
Q N
135-UM
A
mount Attributed to Product Selection/Non-
Preferred Formulary Selection
Q N
136-UN
A
mount Attributed to Product Selection/Brand
Non-Preferred Formulary Selection
Q N
137-UP
A
mount Attributed to Coverage Gap Q Q
148-U8 Ingredient Cost Contracted/Reimbursable Amount I N
149-U9 Dispensing Fee Contracted/Reimbursable Amount I N
RESPONSE DUR/PPS SEGMENT
111-AM Segment Identification M M M M M M M M
567-J6 DUR/PPS Response Code Counter Q Q Q Q Q Q Q Q
439-E4 Reason for Service Code Q Q Q Q Q Q Q Q
528-FS Clinical Significance Code Q Q Q Q Q Q Q Q
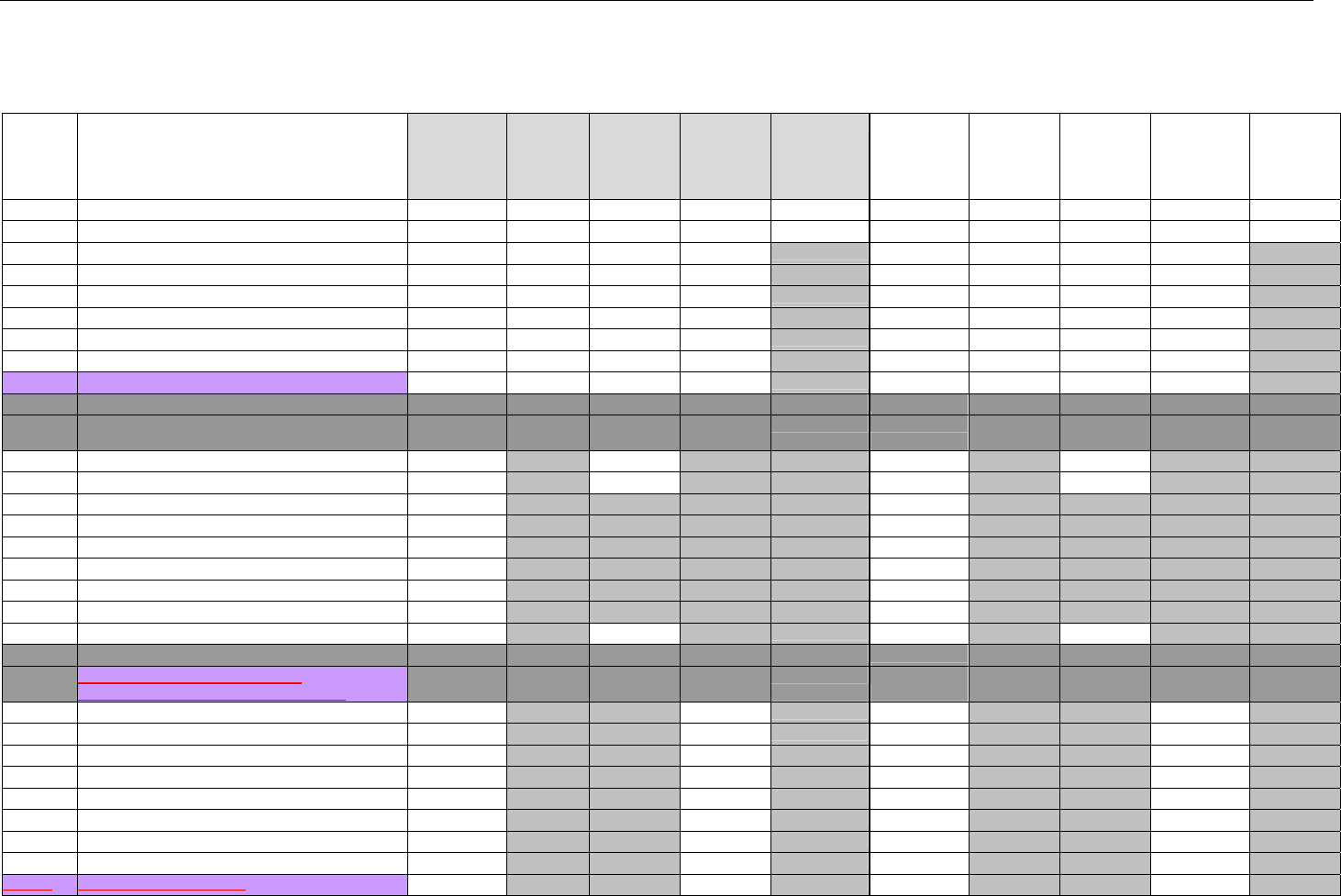
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 635 -
Prior
A
uthorization
Request And
Billing
(Claim)
Prior
A
uthorization
Request And
Billing
(Service)
Header Response Status Accepted Accepted Accepted Accepted Rejected Accepted Accepted Accepted Accepted Rejected
Transaction Response Status Paid Captured Deferred Rejected Rejected Paid Captured Deferred Rejected Rejected
529-FT Other Pharmacy Indicator Q Q Q Q Q Q Q Q
531-FV Quantity of Previous Fill Q Q Q Q Q Q Q Q
53Ø-FU Previous Date of Fill Q Q Q Q Q Q Q Q
532-FW Database Indicator Q Q Q Q Q Q Q Q
533-FX Other Prescriber Indicator Q Q Q Q Q Q Q Q
544-FY DUR Free Text Message Q Q Q Q Q Q Q Q
57Ø-NS DUR Additional Text Q Q Q Q Q Q Q Q
RESPONSE PRIOR AUTHORIZATION
SEGMENT
111-AM Segment Identification M M M M
498-PR Prior Authorization Processed Date R R R R
498-PS Prior Authorization Effective Date Q N Q N
498-PT Prior Authorization Expiration Date Q N Q N
498-RA Prior Authorization Quantity Q N Q N
498-RB Prior Authorization Dollars Authorized Q N Q N
498-PW Prior Authorization Number of Refills Authorized Q N Q N
498-PX Prior Authorization Quantity Accumulated Q N Q N
498-PY Prior Authorization Number - Assigned R Q R Q
RESPONSE COORDINATION OF
BENEFITS/OTHER PAYERS SEGMENT
111-AM Segment Identification M M M M
355-NT Other Payer ID Count M M M M
338-5C Other Payer Coverage Type M M M M
339-6C Other Payer ID Qualifier Q Q Q Q
34Ø-7C Other Payer ID Q Q Q Q
991-MH Other Payer Processor Control Number Q Q Q Q
356-NU Other Payer Cardholder ID Q Q Q Q
992-MJ Other Payer Group ID Q Q Q Q
142-UV Other Payer Person Code Q Q Q Q
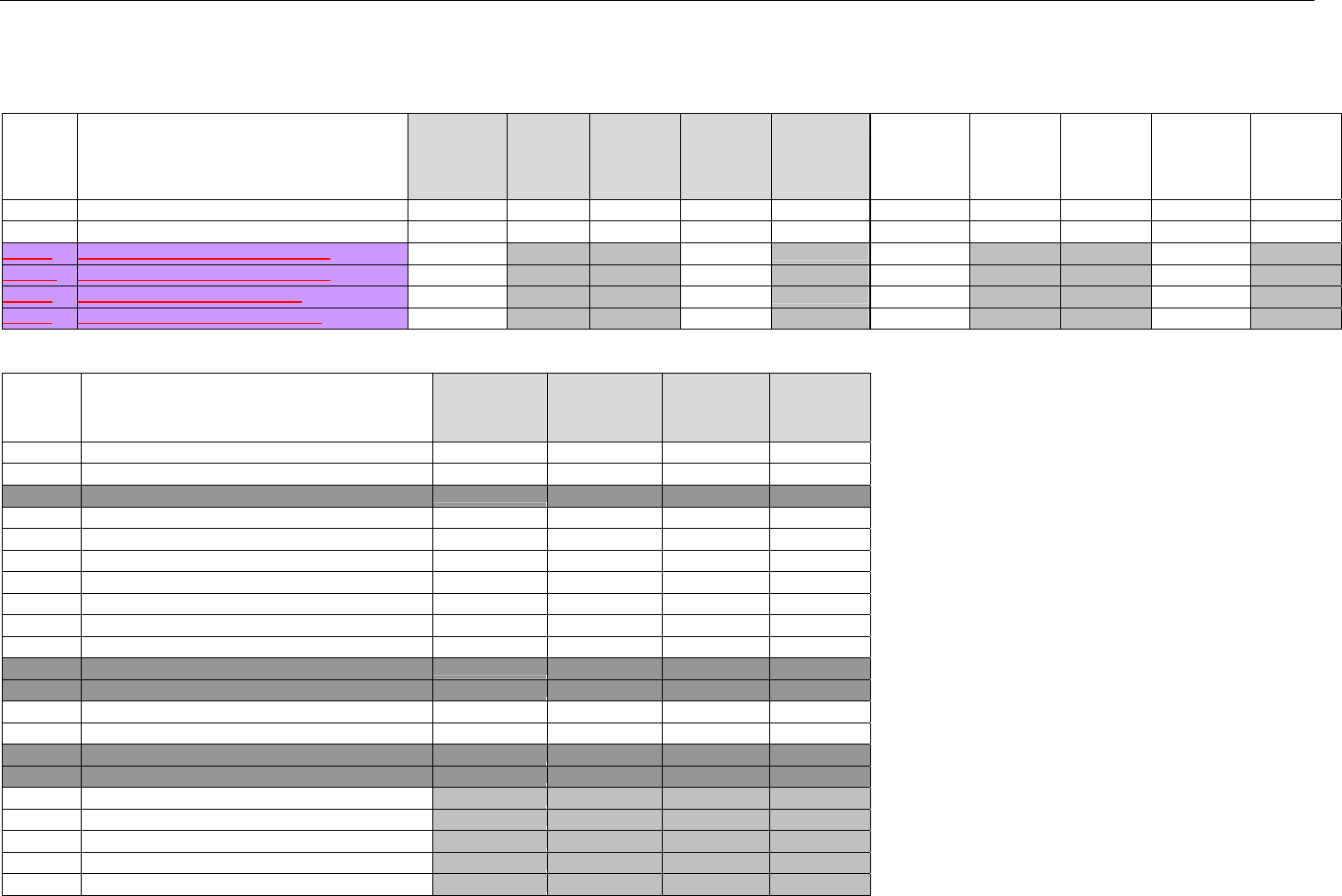
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 636 -
Prior
A
uthorization
Request And
Billing
(Claim)
Prior
A
uthorization
Request And
Billing
(Service)
Header Response Status Accepted Accepted Accepted Accepted Rejected Accepted Accepted Accepted Accepted Rejected
Transaction Response Status Paid Captured Deferred Rejected Rejected Paid Captured Deferred Rejected Rejected
127-UB Other Payer Help Desk Phone Number Q Q Q Q
143-UW Other Payer Patient Relationship Code Q Q Q Q
144-UX Other Payer Benefit Effective Date Q Q Q Q
145-UY Other Payer Benefit Termination Date Q Q Q Q
24.6.6 PRIOR AUTHORIZATION REVERSAL MATRIX
Prior
A
uthorization
Reversal
(Claim/Service)
Header Response Status Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Rejected Rejected
RESPONSE HEADER SEGMENT
1Ø2-A2 Version Release Numbe
r
M M M M
1Ø3-A3 Transaction Code M M M M
1Ø9-A9 Transaction Count M M M M
5Ø1-FI Header Response Status M M M M
2Ø2-B2 Service Provider ID Qualifier M M M M
2Ø1-B1 Service Provider ID M M M M
4Ø1-D1 Date of Service M M M M
RESPONSE MESSAGE SEGMENT
111-AM Segment Identification M M M M
5Ø4-F4 Message Q Q Q Q
RESPONSE INSURANCE SEGMENT
111-AM Segment Identification
3Ø1-C1 Group ID
524-FO Plan ID
545-2F Network Reimbursement ID
568-J7 Payer ID Qualifier
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 637 -
Prior
A
uthorization
Reversal
(Claim/Service)
Header Response Status Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Rejected Rejected
569-J8 Payer ID
115-N5 Medicaid ID Number
116-N6 Medicaid Agency Number
3Ø2-C2 Cardholder ID
RESPONSE INSURANCE ADDITIONAL
INFORMATION SEGMENT
111-AM Segment Identification
139-UR Medicare Part D Coverage Code
138-UQ CMS Low Income Cost Sharing (LICS) Level
24Ø-U1 Contract Number
926-FF Formulary ID
757-U6 Benefit ID
14Ø-US Next Medicare Part D Effective Date
141-UT Next Medicare Part D Termination Date
RESPONSE PATIENT SEGMENT
111-AM Segment Identification
31Ø-CA Patient First Name
311-CB Patient Last Name
3Ø4-C4 Date Of Birth
RESPONSE STATUS SEGMENT
111-AM Segment Identification M M M M
112-AN Transaction Response Status M M M M
5Ø3-F3
A
uthorization Numbe
r
Q Q Q Q
51Ø-FA Reject Count N N R R
511-FB Reject Code N N R R
546-4F Reject Field Occurrence Indicator N N Q Q
547-5F
A
pproved Message Code Count N N N N
548-6F
A
pproved Message Code N N N N
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 638 -
Prior
A
uthorization
Reversal
(Claim/Service)
Header Response Status Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Rejected Rejected
13Ø-UF
A
dditional Message Information Count Q Q Q Q
132-UH
A
dditional Message Information Qualifie
r
Q Q Q Q
526-FQ
A
dditional Message Information Q Q Q Q
131-UG
A
dditional Message Information Continuit
y
Q Q Q Q
55Ø-7F Help Desk Phone Number Qualifier Q Q Q Q
55Ø-8F Help Desk Phone Number Q Q Q Q
88Ø-K5 Transaction Reference Number N N N N
993-A7 Internal Control Number N N N N
987-MA URL N N N N
RESPONSE CLAIM SEGMENT
111-AM Segment Identification
455-EM Prescription/Service Reference Number Qualifier
4Ø2-D2 Prescription/Service Reference Number
551-9F Preferred Product Count
552-AP Preferred Product ID Qualifier
553-AR Preferred Product ID
554-AS Preferred Product Incentive
555-AT Preferred Product Cost Share Incentive
551-9F Preferred Product Description
114-N4 Medicaid Subrogation Internal Control
Number/Transaction Control Number (ICN/TCN)
RESPONSE PRICING SEGMENT
111-AM Segment Identification
5Ø5-F5 Patient Pay Amount
5Ø6-F6 Ingredient Cost Paid
5Ø7-F7 Dispensing Fee Paid
557-AV Tax Exempt Indicator
558-AW Flat Sales Tax Amount Paid
559-AX Percentage Sales Tax Amount Paid

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 639 -
Prior
A
uthorization
Reversal
(Claim/Service)
Header Response Status Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Rejected Rejected
56Ø-AY Percentage Sales Tax Rate Paid
561-AZ Percentage Sales Tax Basis Paid
521-FL Incentive Amount Paid
562-J1 Professional Service Fee Paid
563-J2 Other Amount Paid Count
564-J3 Other Amount Paid Qualifier
565-J4 Other Amount Paid
566-J5 Other Payer Amount Recognized
5Ø9-F9 Total Amount Paid
522-FM Basis of Reimbursement Determination
523-FN
A
mount Attributed to Sales Tax
512-FC
A
ccumulated Deductible Amount
513-FD Remaining Deductible Amount
514-FE Remaining Benefit Amount
517-FH
A
mount Applied to Pe
r
iodic Deductible
518-F1
A
mount of Copa
y
52Ø-FK
A
mount Exceeding Periodic Benefit Maximum
346-HH Basis of Calculation – Dispensing Fee
347-HJ Basis of Calculation – Copay
348-HK Basis of Calculation – Flat Sales Tax
349-HM Basis of Calculation – Percentage Sales Tax
571-NZ
A
mount Attributed to Processor Fee
575-EQ Patient Sales Tax Amount
574-2Y Plan Sales Tax Amount
572-4U
A
mount of Coinsurance
573-4V Basis of Calculation-Coinsurance
392-MU Benefit Stage Count
393-MV Benefit Stage Qualifier
394-MW Benefit Stage Amount
577-G3 Estimated Generic Savings
128-UC Spending Account Amount Remaining
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 640 -
Prior
A
uthorization
Reversal
(Claim/Service)
Header Response Status Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Rejected Rejected
129-UD Health Plan-Funded Assistance Amount
133-UJ
A
mount Attributed to Provider Network Selection
134-UK
A
mount Attributed to Product Selection/Brand Drug
135-UM
A
mount Attributed to Product Selection/Non-Preferred
Formulary Selection
136-UN
A
mount Attributed to Product Selection/Brand Non-
Preferred Formulary Selection
137-UP
A
mount Attributed to Coverage Gap
148-U8 Ingredient Cost Contracted/Reimbursable Amount
149-U9 Dispensing Fee Contracted/Reimbursable Amount
RESPONSE DUR/PPS SEGMENT
111-AM Segment Identification
567-J6 DUR/PPS Response Code Counter
439-E4 Reason for Service Code
528-FS Clinical Significance Code
529-FT Other Pharmacy Indicator
531-FV Quantity of Previous Fill
53Ø-FU Previous Date of Fill
532-FW Database Indicator
533-FX Other Prescriber Indicator
544-FY DUR Free Text Message
57Ø-NS DUR Additional Text
RESPONSE PRIOR AUTHORIZATION SEGMENT
111-AM Segment Identification
498-PR Prior Authorization Processed Date
498-PS Prior Authorization Effective Date
498-PT Prior Authorization Expiration Date
498-RA Prior Authorization Quantity
498-RB Prior Authorization Dollars Authorized
498-PW Prior Authorization Number of Refills Authorized
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 641 -
Prior
A
uthorization
Reversal
(Claim/Service)
Header Response Status Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Rejected Rejected
498-PX Prior Authorization Quantity Accumulated
498-PY Prior Authorization Number - Assigned
RESPONSE COORDINATION OF
BENEFITS/OTHER PAYERS SEGMENT
111-AM Segment Identification M M
355-NT Other Payer ID Count M M
338-5C Other Payer Coverage Type M M
339-6C Other Payer ID Qualifier Q Q
34Ø-7C Other Payer ID Q Q
991-MH Other Payer Processor Control Number Q Q
356-NU Other Payer Cardholder ID Q Q
992-MJ Other Payer Group ID Q Q
142-UV Other Payer Person Code Q Q
127-UB Other Payer Help Desk Phone Number Q Q
143-UW Other Payer Patient Relationship Code Q Q
144-UX Other Payer Benefit Effective Date Q Q
145-UY Other Payer Benefit Termination Date Q Q
24.6.7 PRIOR AUTHORIZATION INQUIRY (CLAIM/SERVICE) MATRIX
Prior
A
uthorization
Inquiry (Claim)
Prior
A
uthorization
Inquiry
(Service)
Header Response Status Accepted Accepted Accepted Accepted Accepted Accepted
Transaction Response Status Paid Captured Approved Paid Captured Approved
RESPONSE HEADER SEGMENT
1Ø2-A2 Version Release Numbe
r
M M M M M M
1Ø3-A3 Transaction Code M M M M M M
1Ø9-A9 Transaction Count M M M M M M
5Ø1-FI Header Response Status M M M M M M
2Ø2-B2 Service Provider ID Qualifier M M M M M M
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 642 -
Prior
A
uthorization
Inquiry (Claim)
Prior
A
uthorization
Inquiry
(Service)
Header Response Status Accepted Accepted Accepted Accepted Accepted Accepted
Transaction Response Status Paid Captured Approved Paid Captured Approved
2Ø1-B1 Service Provider ID M M M M M M
4Ø1-D1 Date of Service M M M M M M
RESPONSE MESSAGE SEGMENT
111-AM Segment Identification M M M M M M
5Ø4-F4 Message Q Q Q Q Q Q
RESPONSE INSURANCE SEGMENT
111-AM Segment Identification M M
3Ø1-C1 Group ID Q Q
524-FO Plan ID Q Q
545-2F Network Reimbursement ID Q Q
568-J7 Payer ID Qualifier Q Q
569-J8 Payer ID Q Q
115-N5 Medicaid ID Number N N
116-N6 Medicaid Agency Number N N
3Ø2-C2 Cardholder ID Q Q
RESPONSE INSURANCE ADDITIONAL
INFORMATION SEGMENT
111-AM Segment Identification
139-UR Medicare Part D Coverage Code
138-UQ CMS Low Income Cost Sharing (LICS) Level
24Ø-U1 Contract Number
926-FF Formulary ID
757-U6 Benefit ID
14Ø-US Next Medicare Part D Effective Date
141-UT Next Medicare Part D Termination Date
RESPONSE PATIENT SEGMENT M M
111-AM Segment Identification Q Q
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 643 -
Prior
A
uthorization
Inquiry (Claim)
Prior
A
uthorization
Inquiry
(Service)
Header Response Status Accepted Accepted Accepted Accepted Accepted Accepted
Transaction Response Status Paid Captured Approved Paid Captured Approved
31Ø-CA Patient First Name Q Q
311-CB Patient Last Name Q Q
3Ø4-C4 Date Of Birth Q Q
RESPONSE STATUS SEGMENT
111-AM Segment Identification M M M M M M
112-AN Transaction Response Status M M M M M M
5Ø3-F3
A
uthorization Numbe
r
Q Q Q Q Q Q
51Ø-FA Reject Count N N N N N N
511-FB Reject Code N N N N N N
546-4F Reject Field Occurrence Indicator N N N N N N
547-5F
A
pproved Message Code Count Q N Q Q N Q
548-6F
A
pproved Message Code Q N Q Q N Q
13Ø-UF
A
dditional Message Information Count Q Q Q Q Q Q
132-UH
A
dditional Message Information Qualifie
r
Q Q Q Q Q Q
526-FQ
A
dditional Message Information Q Q Q Q Q Q
131-UG
A
dditional Message Information Continuit
y
Q Q Q Q Q Q
55Ø-7F Help Desk Phone Number Qualifier Q Q Q Q Q Q
55Ø-8F Help Desk Phone Number Q Q Q Q Q Q
88Ø-K5 Transaction Reference Number N N N N N N
993-A7 Internal Control Number Q Q Q Q Q Q
987-MA URL N N N N N N
RESPONSE CLAIM SEGMENT
111-AM Segment Identification M M M M
455-EM Prescription/Service Reference Number Qualifier M M M M
4Ø2-D2 Prescription/Service Reference Number M M M M
551-9F Preferred Product Count Q Q N N
552-AP Preferred Product ID Qualifier Q Q N N
553-AR Preferred Product ID Q Q N N
554-AS Preferred Product Incentive Q N N N
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 644 -
Prior
A
uthorization
Inquiry (Claim)
Prior
A
uthorization
Inquiry
(Service)
Header Response Status Accepted Accepted Accepted Accepted Accepted Accepted
Transaction Response Status Paid Captured Approved Paid Captured Approved
555-AT Preferred Product Cost Share Incentive Q N N N
551-9F Preferred Product Description Q Q N N
114-N4 Medicaid Subrogation Internal Control
Number/Transaction Control Number (ICN/TCN)
N N N N
RESPONSE PRICING SEGMENT
111-AM Segment Identification M M
5Ø5-F5 Patient Pay Amount R R
5Ø6-F6 Ingredient Cost Paid Q N
5Ø7-F7 Dispensing Fee Paid Q N
557-AV Tax Exempt Indicator Q Q
558-AW Flat Sales Tax Amount Paid Q Q
559-AX Percentage Sales Tax Amount Paid Q Q
56Ø-AY Percentage Sales Tax Rate Paid Q Q
561-AZ Percentage Sales Tax Basis Paid Q N
521-FL Incentive Amount Paid Q N
562-J1 Professional Service Fee Paid N R
563-J2 Other Amount Paid Count Q Q
564-J3 Other Amount Paid Qualifier Q Q
565-J4 Other Amount Paid Q Q
566-J5 Other Payer Amount Recognized Q Q
5Ø9-F9 Total Amount Paid R R
522-FM Basis of Reimbursement Determination Q N
523-FN
A
mount Attributed to Sales Tax Q Q
512-FC
A
ccumulated Deductible Amount I I
513-FD Remaining Deductible Amount I I
514-FE Remaining Benefit Amount I I
517-FH
A
mount Applied to Periodic Deductible Q Q
518-F1
A
mount of Copay Q Q
52Ø-FK
A
mount Exceeding Periodic Benefit Maximum Q Q
346-HH Basis of Calculation – Dispensing Fee Q N
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 645 -
Prior
A
uthorization
Inquiry (Claim)
Prior
A
uthorization
Inquiry
(Service)
Header Response Status Accepted Accepted Accepted Accepted Accepted Accepted
Transaction Response Status Paid Captured Approved Paid Captured Approved
347-HJ Basis of Calculation – Copay Q N
348-HK Basis of Calculation – Flat Sales Tax Q N
349-HM Basis of Calculation – Percentage Sales Tax Q N
571-NZ
A
mount Attributed to Processor Fee Q Q
575-EQ Patient Sales Tax Amount I I
574-2Y Plan Sales Tax Amount I I
572-4U
A
mount of Coinsurance Q Q
573-4V Basis of Calculation-Coinsurance Q N
392-MU Benefit Stage Count Q Q
393-MV Benefit Stage Qualifier Q Q
394-MW Benefit Stage Amount Q Q
577-G3 Estimated Generic Savings Q N
128-UC Spending Account Amount Remaining I I
129-UD Health Plan-Funded Assistance Amount Q Q
133-UJ
A
mount Attributed to Provider Network Selection Q Q
134-UK
A
mount Attributed to Product Selection/Brand Drug Q N
135-UM
A
mount Attributed to Product Selection/Non-
Preferred Formulary Selection
Q N
136-UN
A
mount Attributed to Product Selection/Brand Non-
Preferred Formulary Selection
Q N
137-UP
A
mount Attributed to Coverage Gap Q Q
148-U8 Ingredient Cost Contracted/Reimbursable Amount I N
149-U9 Dispensing Fee Contracted/Reimbursable Amount I N
RESPONSE DUR/PPS SEGMENT
111-AM Segment Identification M M
567-J6 DUR/PPS Response Code Counter Q Q
439-E4 Reason for Service Code Q Q
528-FS Clinical Significance Code Q Q
529-FT Other Pharmacy Indicator Q Q
531-FV Quantity of Previous Fill Q Q
53Ø-FU Previous Date of Fill Q Q
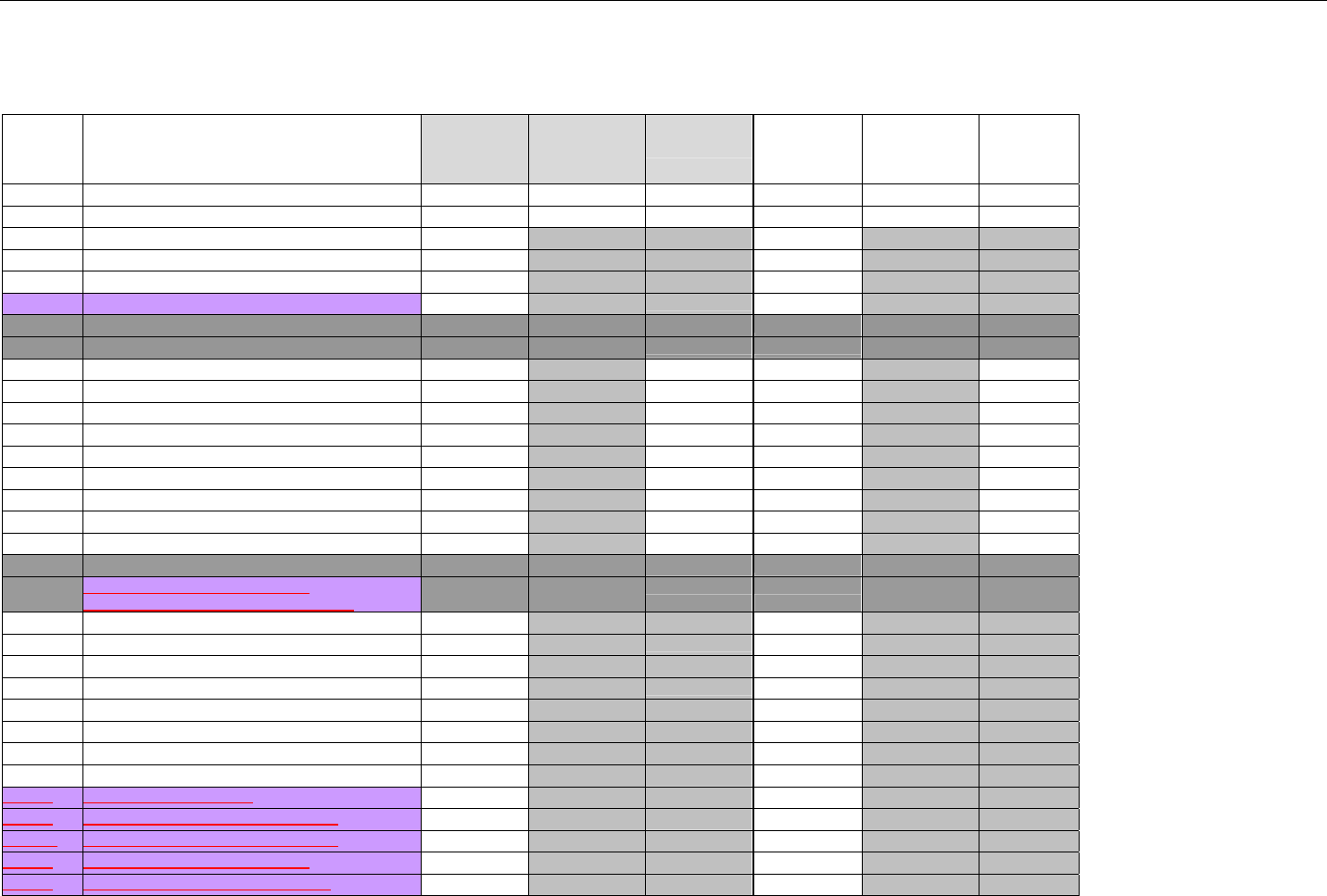
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 646 -
Prior
A
uthorization
Inquiry (Claim)
Prior
A
uthorization
Inquiry
(Service)
Header Response Status Accepted Accepted Accepted Accepted Accepted Accepted
Transaction Response Status Paid Captured Approved Paid Captured Approved
532-FW Database Indicator Q Q
533-FX Other Prescriber Indicator Q Q
544-FY DUR Free Text Message Q Q
57Ø-NS DUR Additional Text Q Q
RESPONSE PRIOR AUTHORIZATION SEGMENT
111-AM Segment Identification M M M M
498-PR Prior Authorization Processed Date R R R R
498-PS Prior Authorization Effective Date Q Q Q Q
498-PT Prior Authorization Expiration Date Q Q Q Q
498-RA Prior Authorization Quantity Q Q Q Q
498-RB Prior Authorization Dollars Authorized Q Q Q Q
498-PW Prior Authorization Number of Refills Authorized Q Q Q Q
498-PX Prior Authorization Quantity Accumulated Q Q Q Q
498-PY Prior Authorization Number - Assigned R R R R
RESPONSE COORDINATION OF
BENEFITS/OTHER PAYERS SEGMENT
111-AM Segment Identification M M
355-NT Other Payer ID Count M M
338-5C Other Payer Coverage Type M M
339-6C Other Payer ID Qualifier Q Q
34Ø-7C Other Payer ID Q Q
991-MH Other Payer Processor Control Number Q Q
356-NU Other Payer Cardholder ID Q Q
992-MJ Other Payer Group ID Q Q
142-UV Other Payer Person Code Q
Q
127-UB Other Payer Help Desk Phone Number Q
Q
143-UW Other Payer Patient Relationship Code Q
Q
144-UX Other Payer Benefit Effective Date Q
Q
145-UY Other Payer Benefit Termination Date Q
Q
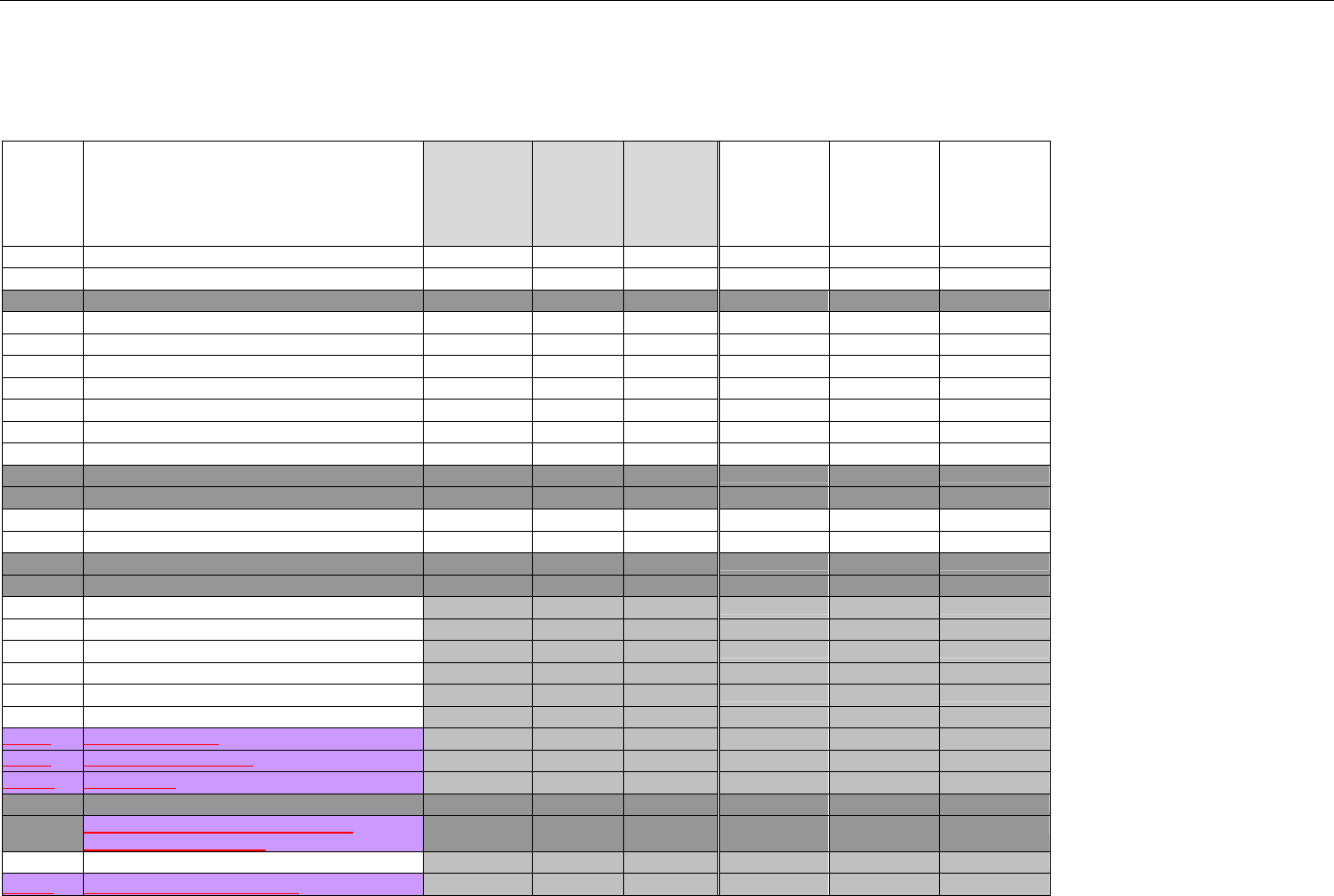
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 647 -
Prior
A
uthorization
Inquiry (Claim)
Prior
A
uthorization
Inquiry
(Service)
Header Response Status Accepted Accepted Rejected Accepted Accepted Rejected
Transaction Response Status Deferred Rejected Rejected Deferred Rejected Rejected
RESPONSE HEADER SEGMENT
1Ø2-A2 Version Release Numbe
r
M M M M M M
1Ø3-A3 Transaction Code M M M M M M
1Ø9-A9 Transaction Count M M M M M M
5Ø1-FI Header Response Status M M M M M M
2Ø2-B2 Service Provider ID Qualifier M M M M M M
2Ø1-B1 Service Provider ID M M M M M M
4Ø1-D1 Date of Service M M M M M M
RESPONSE MESSAGE SEGMENT
111-AM Segment Identification M M M M M M
5Ø4-F4 Message Q Q Q Q Q Q
RESPONSE INSURANCE SEGMENT
111-AM Segment Identification
3Ø1-C1 Group ID
524-FO Plan ID
545-2F Network Reimbursement ID
568-J7 Payer ID Qualifier
569-J8 Payer ID
115-N5 Medicaid ID Number
116-N6 Medicaid Agency Number
3Ø2-C2 Cardholder ID
RESPONSE INSURANCE ADDITIONAL
INFORMATION SEGMENT
111-AM Segment Identification
139-UR Medicare Part D Coverage Code
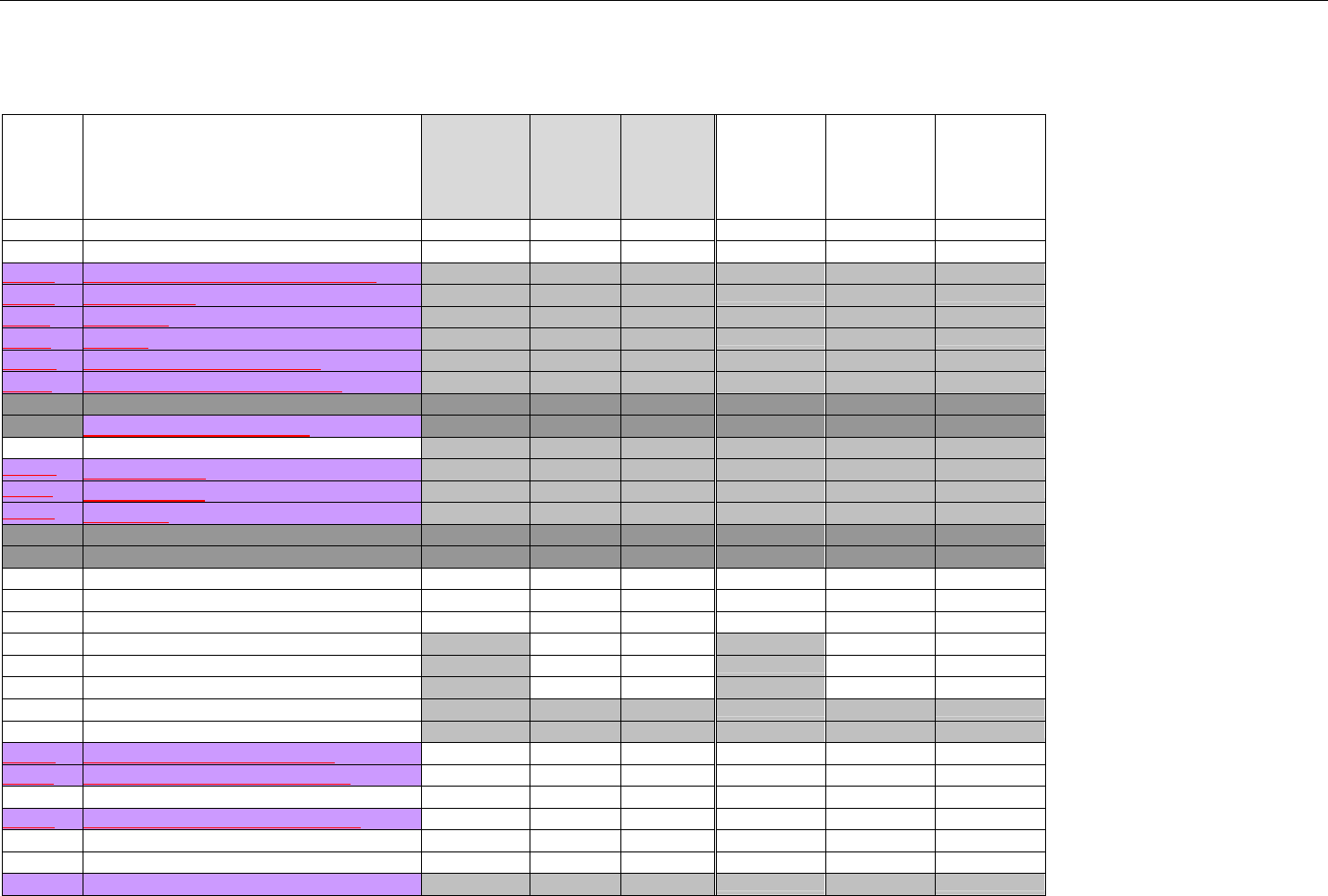
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 648 -
Prior
A
uthorization
Inquiry (Claim)
Prior
A
uthorization
Inquiry
(Service)
Header Response Status Accepted Accepted Rejected Accepted Accepted Rejected
Transaction Response Status Deferred Rejected Rejected Deferred Rejected Rejected
138-UQ CMS Low Income Cost Sharing (LICS) Level
24Ø-U1 Contract Number
926-FF Formulary ID
757-U6 Benefit ID
14Ø-US Next Medicare Part D Effective Date
141-UT Next Medicare Part D Termination Date
RESPONSE PATIENT SEGMENT
111-AM Segment Identification
31Ø-CA Patient First Name
311-CB Patient Last Name
3Ø4-C4 Date Of Birth
RESPONSE STATUS SEGMENT
111-AM Segment Identification M M M M M M
112-AN Transaction Response Status M M M M M M
5Ø3-F3
A
uthorization Numbe
r
Q Q Q Q Q Q
51Ø-FA Reject Count N R R N R R
511-FB Reject Code N R R N R R
546-4F Reject Field Occurrence Indicator N Q Q N Q Q
547-5F
A
pproved Message Code Count N N N N N N
548-6F
A
pproved Message Code N N N N N N
13Ø-UF
A
dditional Message Information Count Q Q Q Q Q Q
132-UH
A
dditional Message Information Qualifie
r
Q Q Q Q Q Q
526-FQ
A
dditional Message Information Q Q Q Q Q Q
131-UG
A
dditional Message Information Continuit
y
Q Q Q Q Q Q
55Ø-7F Help Desk Phone Number Qualifier Q Q Q Q Q Q
55Ø-8F Help Desk Phone Number Q Q Q Q Q Q
88Ø-K5 Transaction Reference Number N N N N N N
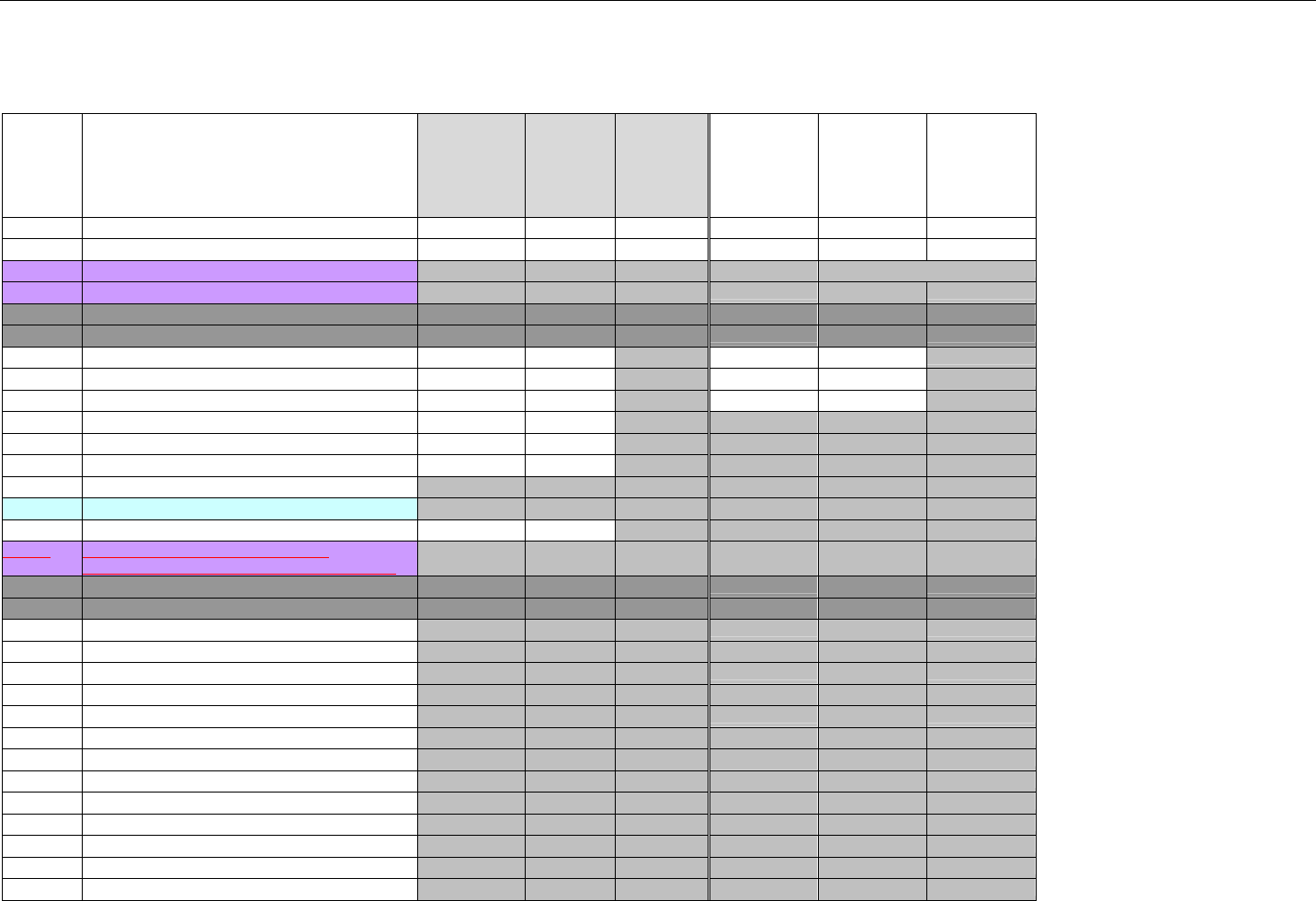
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 649 -
Prior
A
uthorization
Inquiry (Claim)
Prior
A
uthorization
Inquiry
(Service)
Header Response Status Accepted Accepted Rejected Accepted Accepted Rejected
Transaction Response Status Deferred Rejected Rejected Deferred Rejected Rejected
993-A7 Internal Control Number N N N N N N
987-MA URL N N N N N N
RESPONSE CLAIM SEGMENT
111-AM Segment Identification M M M M
455-EM Prescription/Service Reference Number Qualifier M M
M M
4Ø2-D2 Prescription/Service Reference Number M M
M M
551-9F Preferred Product Count Q Q
N N
552-AP Preferred Product ID Qualifier Q Q
N N
553-AR Preferred Product ID Q Q
N N
554-AS Preferred Product Incentive N N N N
555-AT Preferred Product Cost Share Incentive N N N N
551-9F Preferred Product Description Q Q
N N
114-N4 Medicaid Subrogation Internal Control
Number/Transaction Control Number (ICN/TCN)
N N N N
RESPONSE PRICING SEGMENT
111-AM Segment Identification
5Ø5-F5 Patient Pay Amount
5Ø6-F6 Ingredient Cost Paid
5Ø7-F7 Dispensing Fee Paid
557-AV Tax Exempt Indicator
558-AW Flat Sales Tax Amount Paid
559-AX Percentage Sales Tax Amount Paid
56Ø-AY Percentage Sales Tax Rate Paid
561-AZ Percentage Sales Tax Basis Paid
521-FL Incentive Amount Paid
562-J1 Professional Service Fee Paid
563-J2 Other Amount Paid Count
564-J3 Other Amount Paid Qualifier
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 650 -
Prior
A
uthorization
Inquiry (Claim)
Prior
A
uthorization
Inquiry
(Service)
Header Response Status Accepted Accepted Rejected Accepted Accepted Rejected
Transaction Response Status Deferred Rejected Rejected Deferred Rejected Rejected
565-J4 Other Amount Paid
566-J5 Other Payer Amount Recognized
5Ø9-F9 Total Amount Paid
522-FM Basis of Reimbursement Determination
523-FN
A
mount Attributed to Sales Tax
512-FC
A
ccumulated Deductible Amount
513-FD Remaining Deductible Amount
514-FE Remaining Benefit Amount
517-FH
A
mount Applied to Periodic Deductible
518-F1
A
mount of Copa
y
52Ø-FK
A
mount Exceeding Periodic Benefit Maximum
346-HH Basis of Calculation – Dispensing Fee
347-HJ Basis of Calculation – Copay
348-HK Basis of Calculation – Flat Sales Tax
349-HM Basis of Calculation – Percentage Sales Tax
571-NZ
A
mount Attributed to Processor Fee
575-EQ Patient Sales Tax Amount
574-2Y Plan Sales Tax Amount
572-4U
A
mount of Coinsurance
573-4V Basis of Calculation-Coinsurance
392-MU Benefit Stage Count
393-MV Benefit Stage Qualifier
394-MW Benefit Stage Amount
577-G3 Estimated Generic Savings
128-UC Spending Account Amount Remaining
129-UD Health Plan-Funded Assistance Amount
133-UJ
A
mount Attributed to Provider Network Selection
134-UK
A
mount Attributed to Product Selection/Brand Drug
135-UM
A
mount Attributed to Product Selection/Non-
Preferred Formulary Selection
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 651 -
Prior
A
uthorization
Inquiry (Claim)
Prior
A
uthorization
Inquiry
(Service)
Header Response Status Accepted Accepted Rejected Accepted Accepted Rejected
Transaction Response Status Deferred Rejected Rejected Deferred Rejected Rejected
136-UN
A
mount Attributed to Product Selection/Brand Non-
Preferred Formulary Selection
137-UP
A
mount Attributed to Coverage Gap
148-U8 Ingredient Cost Contracted/Reimbursable Amount
149-U9 Dispensing Fee Contracted/Reimbursable Amount
RESPONSE DUR/PPS SEGMENT
111-AM Segment Identification
567-J6 DUR/PPS Response Code Counter
439-E4 Reason for Service Code
528-FS Clinical Significance Code
529-FT Other Pharmacy Indicator
531-FV Quantity of Previous Fill
53Ø-FU Previous Date of Fill
532-FW Database Indicator
533-FX Other Prescriber Indicator
544-FY DUR Free Text Message
57Ø-NS DUR Additional Text
RESPONSE PRIOR AUTHORIZATION SEGMENT
111-AM Segment Identification M M
498-PR Prior Authorization Processed Date R R
498-PS Prior Authorization Effective Date N N
498-PT Prior Authorization Expiration Date N N
498-RA Prior Authorization Quantity N N
498-RB Prior Authorization Dollars Authorized N N
498-PW Prior Authorization Number of Refills Authorized N N
498-PX Prior Authorization Quantity Accumulated N N
498-PY Prior Authorization Number - Assigned Q Q

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 652 -
Prior
A
uthorization
Inquiry (Claim)
Prior
A
uthorization
Inquiry
(Service)
Header Response Status Accepted Accepted Rejected Accepted Accepted Rejected
Transaction Response Status Deferred Rejected Rejected Deferred Rejected Rejected
RESPONSE COORDINATION OF
BENEFITS/OTHER PAYERS SEGMENT
111-AM Segment Identification M M
355-NT Other Payer ID Count M M
338-5C Other Payer Coverage Type M M
339-6C Other Payer ID Qualifier Q Q
34Ø-7C Other Payer ID Q Q
991-MH Other Payer Processor Control Number Q Q
356-NU Other Payer Cardholder ID Q Q
992-MJ Other Payer Group ID Q Q
142-UV Other Payer Person Code Q Q
127-UB Other Payer Help Desk Phone Number Q Q
143-UW Other Payer Patient Relationship Code Q Q
144-UX Other Payer Benefit Effective Date Q Q
145-UY Other Payer Benefit Termination Date Q Q
24.6.8 PRIOR AUTHORIZATION REQUEST ONLY (CLAIM) MATRIX
Prior
A
uthorization
Request Only
(Claim)
Header Response Status Accepted Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Deferred Rejected Rejected
RESPONSE HEADER SEGMENT
1Ø2-A2 Version Release Numbe
r
M M M M M
1Ø3-A3 Transaction Code M M M M M
1Ø9-A9 Transaction Count M M M M M
5Ø1-FI Header Response Status M M M M M
2Ø2-B2 Service Provider ID Qualifier M M M M M
2Ø1-B1 Service Provider ID M M M M M
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 653 -
Prior
A
uthorization
Request Only
(Claim)
Header Response Status Accepted Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Deferred Rejected Rejected
4Ø1-D1 Date of Service M M M M M
RESPONSE MESSAGE SEGMENT
111-AM Segment Identification M M M M M
5Ø4-F4 Message Q Q Q Q Q
RESPONSE INSURANCE SEGMENT
111-AM Segment Identification
3Ø1-C1 Group ID
524-FO Plan ID
545-2F Network Reimbursement ID
568-J7 Payer ID Qualifier
569-J8 Payer ID
115-N5 Medicaid ID Number
116-N6 Medicaid Agency Number
3Ø2-C2 Cardholder ID
RESPONSE INSURANCE ADDITIONAL INFORMATION SEGMENT
111-AM Segment Identification
139-UR Medicare Part D Coverage Code
138-UQ CMS Low Income Cost Sharing (LICS) Level
24Ø-U1 Contract Number
926-FF Formulary ID
757-U6 Benefit ID
14Ø-US Next Medicare Part D Effective Date
141-UT Next Medicare Part D Termination Date
RESPONSE PATIENT SEGMENT
111-AM Segment Identification
31Ø-CA Patient First Name
311-CB Patient Last Name
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 654 -
Prior
A
uthorization
Request Only
(Claim)
Header Response Status Accepted Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Deferred Rejected Rejected
3Ø4-C4 Date Of Birth
RESPONSE STATUS SEGMENT
111-AM Segment Identification M M M M M
112-AN Transaction Response Status M M M M M
5Ø3-F3
A
uthorization Numbe
r
N R Q Q Q
51Ø-FA Reject Count N N N R R
511-FB Reject Code N N N R R
546-4F Reject Field Occurrence Indicator N N N Q Q
547-5F
A
pproved Message Code Count Q N N N N
548-6F
A
pproved Message Code Q N N N N
13Ø-UF
A
dditional Message Information Count Q Q Q Q Q
132-UH
A
dditional Message Information Qualifie
r
Q Q Q Q Q
526-FQ
A
dditional Message Information Q Q Q Q Q
131-UG
A
dditional Message Information Continuity Q Q Q Q Q
55Ø-7F Help Desk Phone Number Qualifier Q Q Q Q Q
55Ø-8F Help Desk Phone Number Q Q Q Q Q
88Ø-K5 Transaction Reference Number N N N N N
993-A7 Internal Control Number N N N N N
987-MA URL N N N N N
RESPONSE CLAIM SEGMENT
111-AM Segment Identification M M M M
455-EM Prescription/Service Reference Number Qualifier M M M M
4Ø2-D2 Prescription/Service Reference Number M M M M
551-9F Preferred Product Count Q Q Q Q
552-AP Preferred Product ID Qualifier Q Q Q Q
553-AR Preferred Product ID Q Q Q Q
554-AS Preferred Product Incentive N N N N
555-AT Preferred Product Cost Share Incentive N N N N
551-9F Preferred Product Description Q Q Q Q
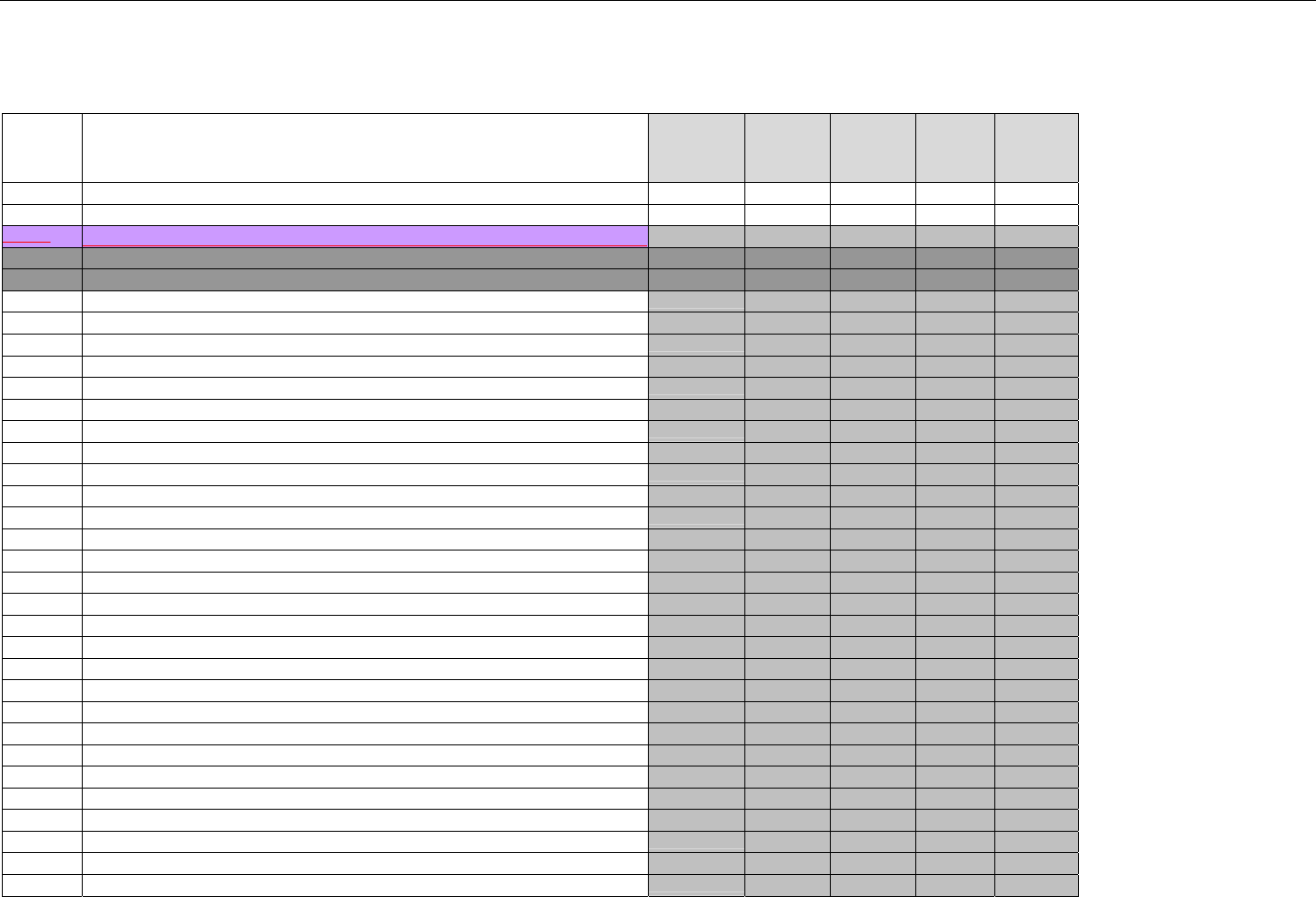
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 655 -
Prior
A
uthorization
Request Only
(Claim)
Header Response Status Accepted Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Deferred Rejected Rejected
114-N4 Medicaid Subrogation Internal Control Number/Transaction Control Number (ICN/TCN) N N N N
RESPONSE PRICING SEGMENT
111-AM Segment Identification
5Ø5-F5 Patient Pay Amount
5Ø6-F6 Ingredient Cost Paid
5Ø7-F7 Dispensing Fee Paid
557-AV Tax Exempt Indicator
558-AW Flat Sales Tax Amount Paid
559-AX Percentage Sales Tax Amount Paid
56Ø-AY Percentage Sales Tax Rate Paid
561-AZ Percentage Sales Tax Basis Paid
521-FL Incentive Amount Paid
562-J1 Professional Service Fee Paid
563-J2 Other Amount Paid Count
564-J3 Other Amount Paid Qualifier
565-J4 Other Amount Paid
566-J5 Other Payer Amount Recognized
5Ø9-F9 Total Amount Paid
522-FM Basis of Reimbursement Determination
523-FN
A
mount Attributed to Sales Tax
512-FC
A
ccumulated Deductible Amount
513-FD Remaining Deductible Amount
514-FE Remaining Benefit Amount
517-FH
A
mount Applied to Periodic Deductible
518-F1
A
mount of Copa
y
52Ø-FK
A
mount Exceeding Periodic Benefit Maximum
346-HH Basis of Calculation – Dispensing Fee
347-HJ Basis of Calculation – Copay
348-HK Basis of Calculation – Flat Sales Tax
349-HM Basis of Calculation – Percentage Sales Tax
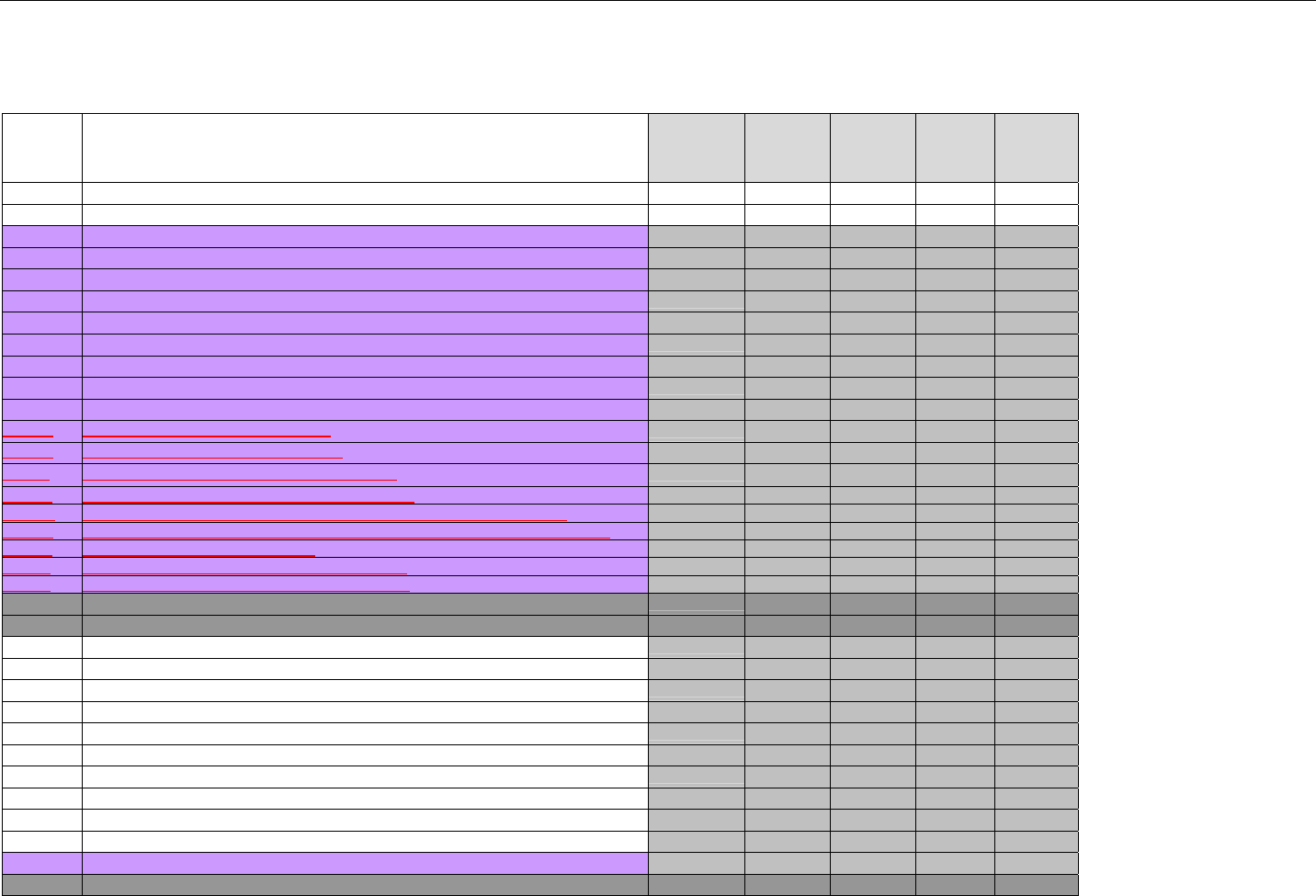
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 656 -
Prior
A
uthorization
Request Only
(Claim)
Header Response Status Accepted Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Deferred Rejected Rejected
571-NZ
A
mount Attributed to Processor Fee
575-EQ Patient Sales Tax Amount
574-2Y Plan Sales Tax Amount
572-4U
A
mount of Coinsurance
573-4V Basis of Calculation-Coinsurance
392-MU Benefit Stage Count
393-MV Benefit Stage Qualifier
394-MW Benefit Stage Amount
577-G3 Estimated Generic Savings
128-UC Spending Account Amount Remaining
129-UD Health Plan-Funded Assistance Amount
133-UJ
A
mount Attributed to Provider Network Selection
134-UK
A
mount Attributed to Product Selection/Brand Drug
135-UM
A
mount Attributed to Product Selection/Non-Preferred Formulary Selection
136-UN
A
mount Attributed to Product Selection/Brand Non-Preferred Formulary Selection
137-UP
A
mount Attributed to Coverage Gap
148-U8 Ingredient Cost Contracted/Reimbursable Amount
149-U9 Dispensing Fee Contracted/Reimbursable Amount
RESPONSE DUR/PPS SEGMENT
111-AM Segment Identification
567-J6 DUR/PPS Response Code Counter
439-E4 Reason for Service Code
528-FS Clinical Significance Code
529-FT Other Pharmacy Indicator
531-FV Quantity of Previous Fill
53Ø-FU Previous Date of Fill
532-FW Database Indicator
533-FX Other Prescriber Indicator
544-FY DUR Free Text Message
57Ø-NS DUR Additional Text
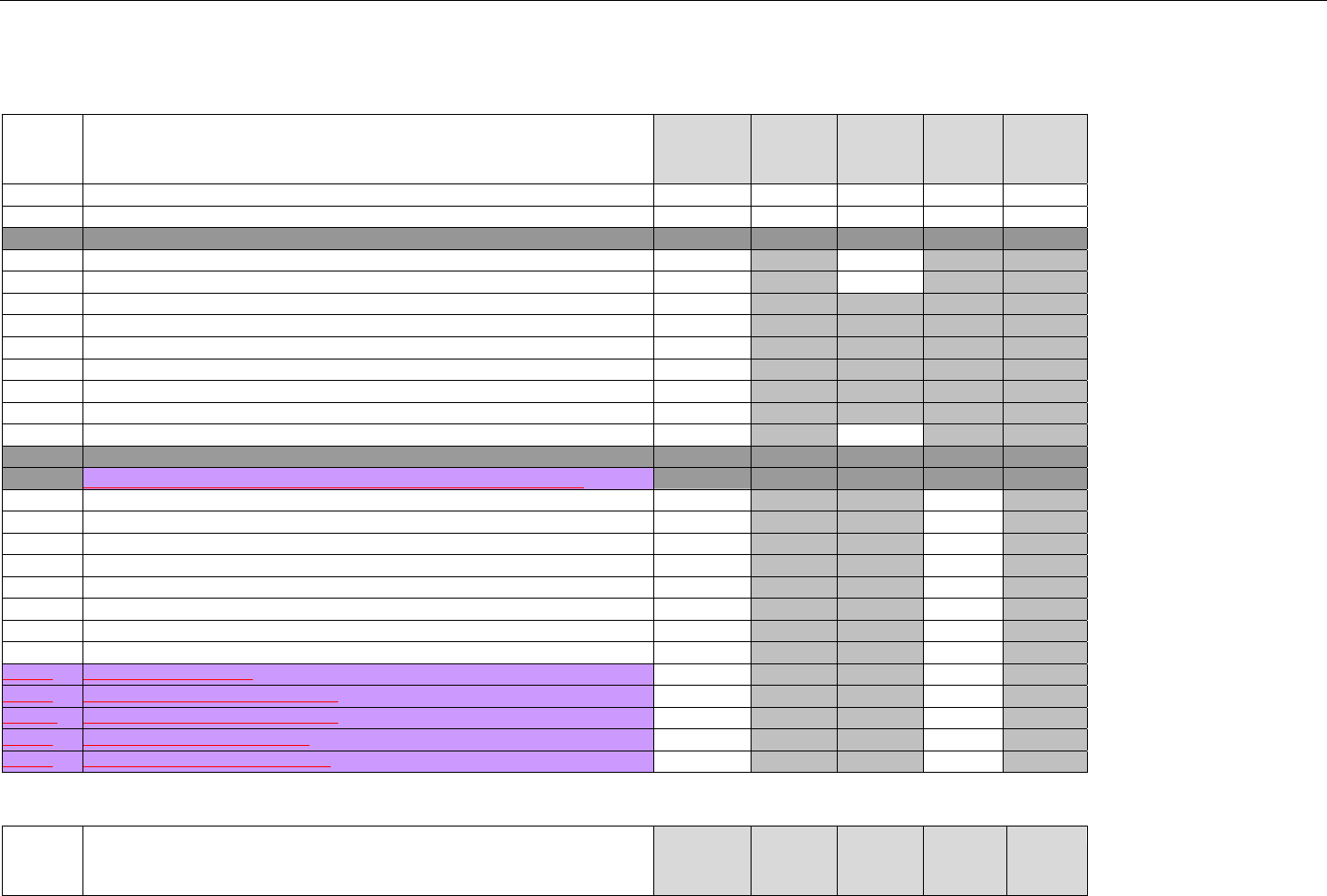
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 657 -
Prior
A
uthorization
Request Only
(Claim)
Header Response Status Accepted Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Deferred Rejected Rejected
RESPONSE PRIOR AUTHORIZATION SEGMENT
111-AM Segment Identification M M
498-PR Prior Authorization Processed Date R Q
498-PS Prior Authorization Effective Date Q N
498-PT Prior Authorization Expiration Date Q N
498-RA Prior Authorization Quantity Q N
498-RB Prior Authorization Dollars Authorized Q N
498-PW Prior Authorization Number of Refills Authorized Q N
498-PX Prior Authorization Quantity Accumulated Q N
498-PY Prior Authorization Number - Assigned R Q
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS SEGMENT
111-AM Segment Identification M M
355-NT Other Payer ID Count M M
338-5C Other Payer Coverage Type M M
339-6C Other Payer ID Qualifier Q Q
34Ø-7C Other Payer ID Q Q
991-MH Other Payer Processor Control Number Q Q
356-NU Other Payer Cardholder ID Q Q
992-MJ Other Payer Group ID Q Q
142-UV Other Payer Person Code Q Q
127-UB Other Payer Help Desk Phone Number Q Q
143-UW Other Payer Patient Relationship Code Q Q
144-UX Other Payer Benefit Effective Date Q Q
145-UY Other Payer Benefit Termination Date Q Q
24.6.9 PRIOR AUTHORIZATION REQUEST ONLY (SERVICE) MATRIX
Prior
A
uthorization
Request Only
(Service)
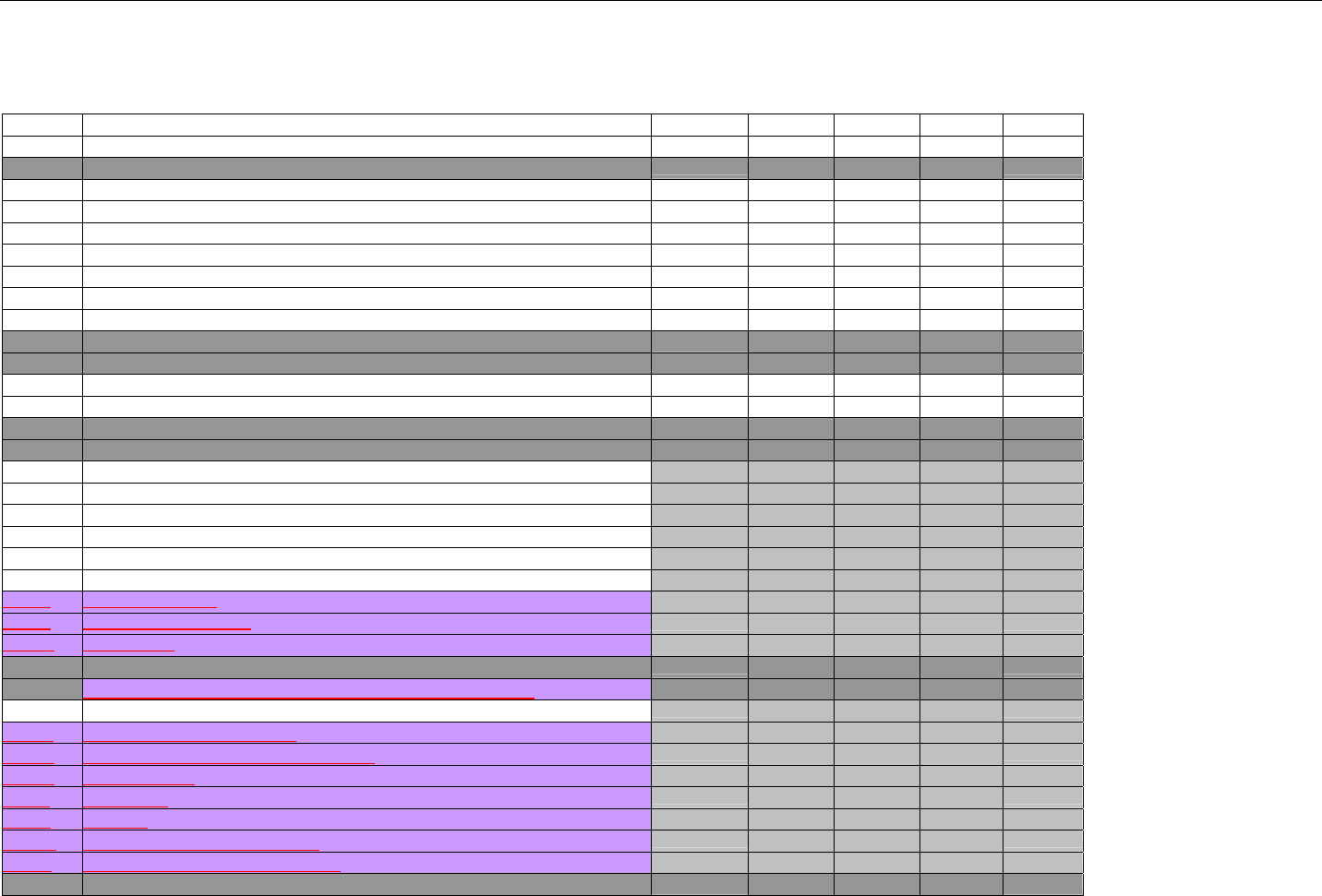
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 658 -
Header Response Status Accepted Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Deferred Rejected Rejected
RESPONSE HEADER SEGMENT
1Ø2-A2 Version Release Numbe
r
M M M M M
1Ø3-A3 Transaction Code M M M M M
1Ø9-A9 Transaction Count M M M M M
5Ø1-FI Header Response Status M M M M M
2Ø2-B2 Service Provider ID Qualifier M M M M M
2Ø1-B1 Service Provider ID M M M M M
4Ø1-D1 Date of Service M M M M M
RESPONSE MESSAGE SEGMENT
111-AM Segment Identification M M M M M
5Ø4-F4 Message Q Q Q Q Q
RESPONSE INSURANCE SEGMENT
111-AM Segment Identification
3Ø1-C1 Group ID
524-FO Plan ID
545-2F Network Reimbursement ID
568-J7 Payer ID Qualifier
569-J8 Payer ID
115-N5 Medicaid ID Number
116-N6 Medicaid Agency Number
3Ø2-C2 Cardholder ID
RESPONSE INSURANCE ADDITIONAL INFORMATION SEGMENT
111-AM Segment Identification
139-UR Medicare Part D Coverage Code
138-UQ CMS Low Income Cost Sharing (LICS) Level
24Ø-U1 Contract Number
926-FF Formulary ID
757-U6 Benefit ID
14Ø-US Next Medicare Part D Effective Date
141-UT Next Medicare Part D Termination Date
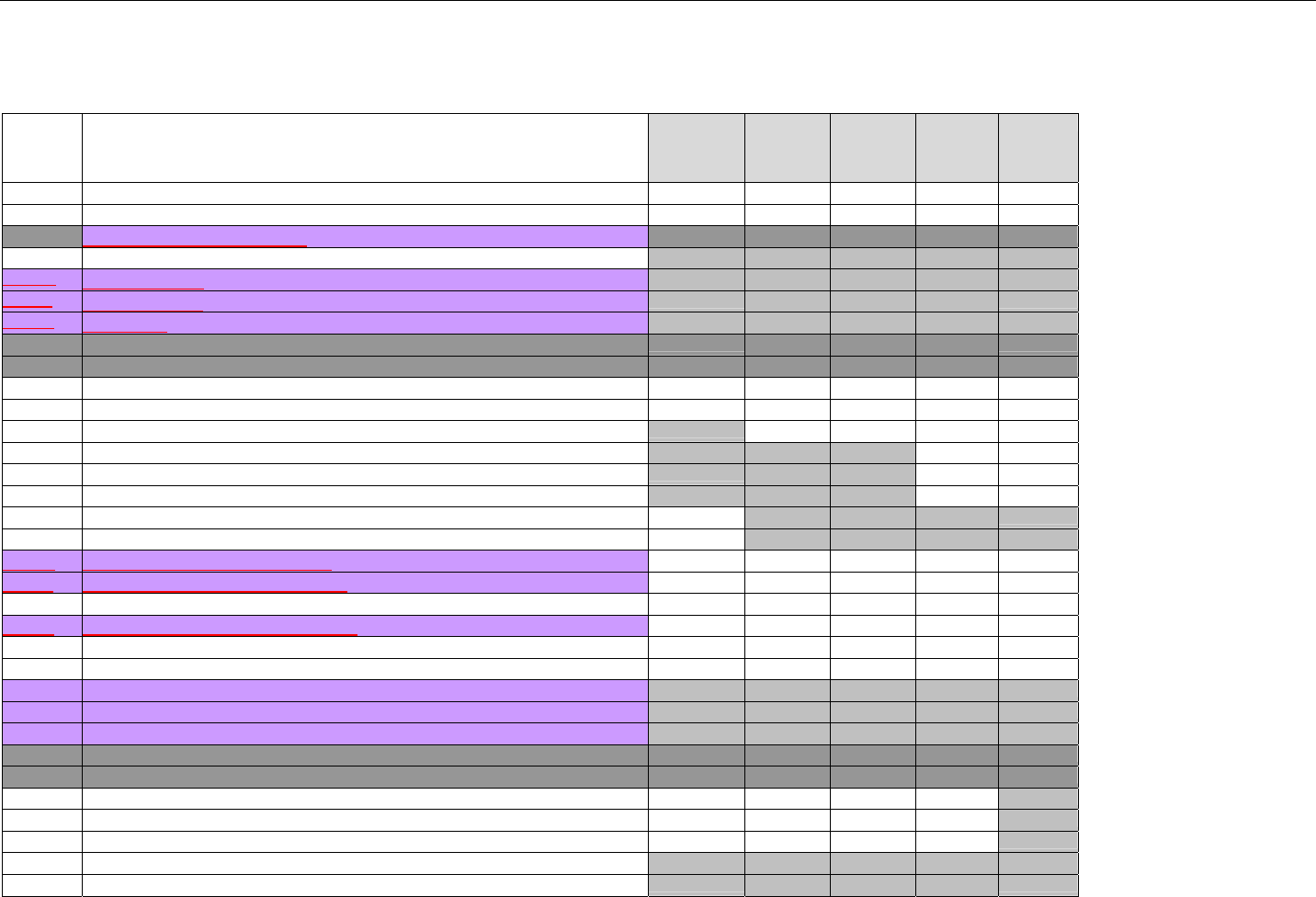
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 659 -
Prior
A
uthorization
Request Only
(Service)
Header Response Status Accepted Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Deferred Rejected Rejected
RESPONSE PATIENT SEGMENT
111-AM Segment Identification
31Ø-CA Patient First Name
311-CB Patient Last Name
3Ø4-C4 Date Of Birth
RESPONSE STATUS SEGMENT
111-AM Segment Identification M M M M M
112-AN Transaction Response Status M M M M M
5Ø3-F3
A
uthorization Numbe
r
N R Q Q Q
51Ø-FA Reject Count N N N R R
511-FB Reject Code N N N R R
546-4F Reject Field Occurrence Indicator N N N Q Q
547-5F
A
pproved Message Code Count Q N N N N
548-6F
A
pproved Message Code Q N N N N
13Ø-UF
A
dditional Message Information Count Q Q Q Q Q
132-UH
A
dditional Message Information Qualifie
r
Q Q Q Q Q
526-FQ
A
dditional Message Information Q Q Q Q Q
131-UG
A
dditional Message Information Continuit
y
Q Q Q Q Q
55Ø-7F Help Desk Phone Number Qualifier Q Q Q Q Q
55Ø-8F Help Desk Phone Number Q Q Q Q Q
88Ø-K5 Transaction Reference Number N N N N N
993-A7 Internal Control Number N N N N N
987-MA URL N N N N N
RESPONSE CLAIM SEGMENT
111-AM Segment Identification M M M M
455-EM Prescription/Service Reference Number Qualifier M M M M
4Ø2-D2 Prescription/Service Reference Number M M M M
551-9F Preferred Product Count N N N N
552-AP Preferred Product ID Qualifier N N N N
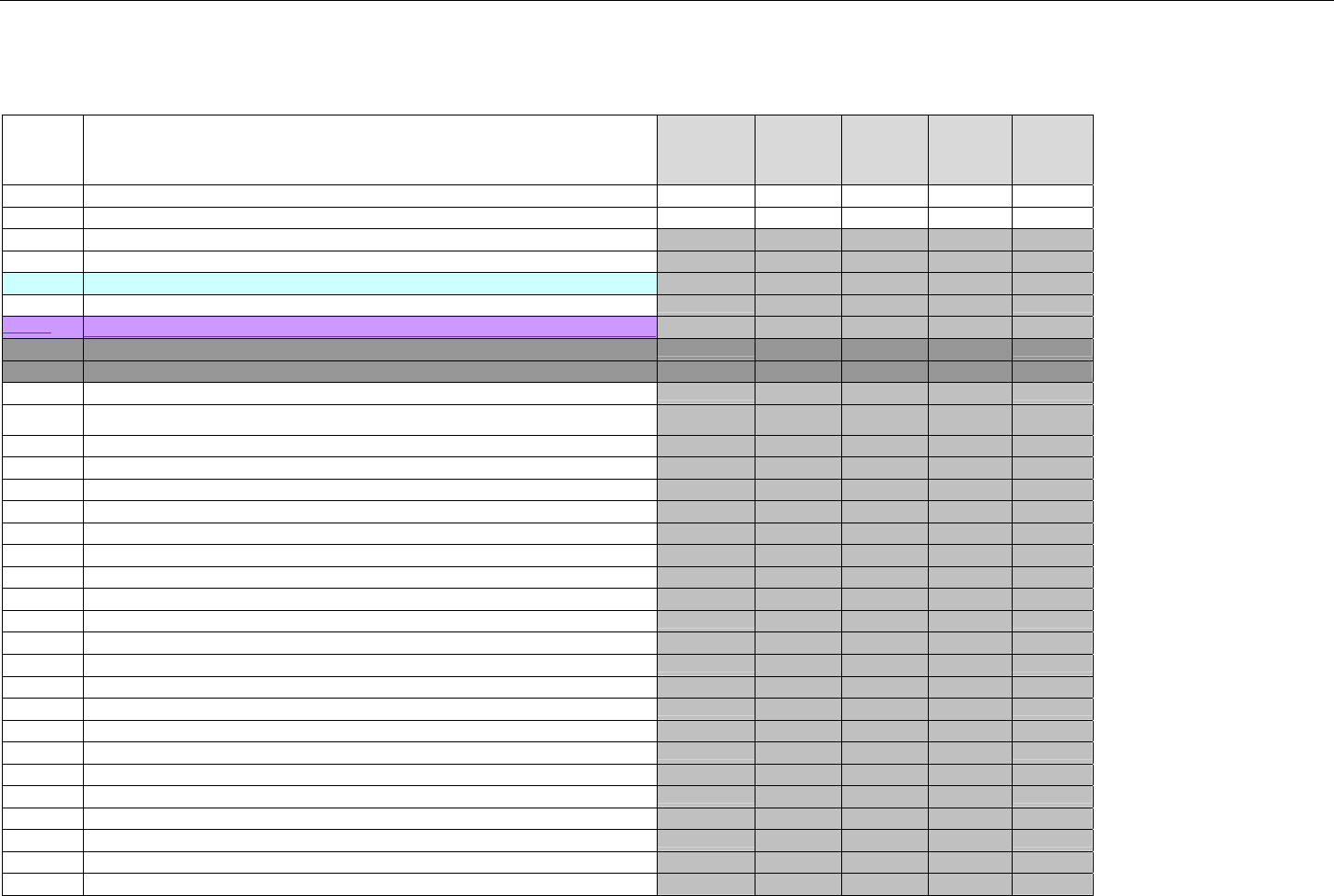
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 660 -
Prior
A
uthorization
Request Only
(Service)
Header Response Status Accepted Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Deferred Rejected Rejected
553-AR Preferred Product ID N N N N
554-AS Preferred Product Incentive N N N N
555-AT Preferred Product Cost Share Incentive N N N N
551-9F Preferred Product Description N N N N
114-N4 Medicaid Subrogation Internal Control Number/Transaction Control Number (ICN/TCN) N N N N
RESPONSE PRICING SEGMENT
111-AM Segment Identification
5Ø5-F5 Patient Pay Amount
5Ø6-F6 Ingredient Cost Paid
5Ø7-F7 Dispensing Fee Paid
557-AV Tax Exempt Indicator
558-AW Flat Sales Tax Amount Paid
559-AX Percentage Sales Tax Amount Paid
56Ø-AY Percentage Sales Tax Rate Paid
561-AZ Percentage Sales Tax Basis Paid
521-FL Incentive Amount Paid
562-J1 Professional Service Fee Paid
563-J2 Other Amount Paid Count
564-J3 Other Amount Paid Qualifier
565-J4 Other Amount Paid
566-J5 Other Payer Amount Recognized
5Ø9-F9 Total Amount Paid
522-FM Basis of Reimbursement Determination
523-FN
A
mount Attributed to Sales Tax
512-FC
A
ccumulated Deductible Amount
513-FD Remaining Deductible Amount
514-FE Remaining Benefit Amount
517-FH
A
mount Applied to Periodic Deductible
518-F1
A
mount of Copa
y
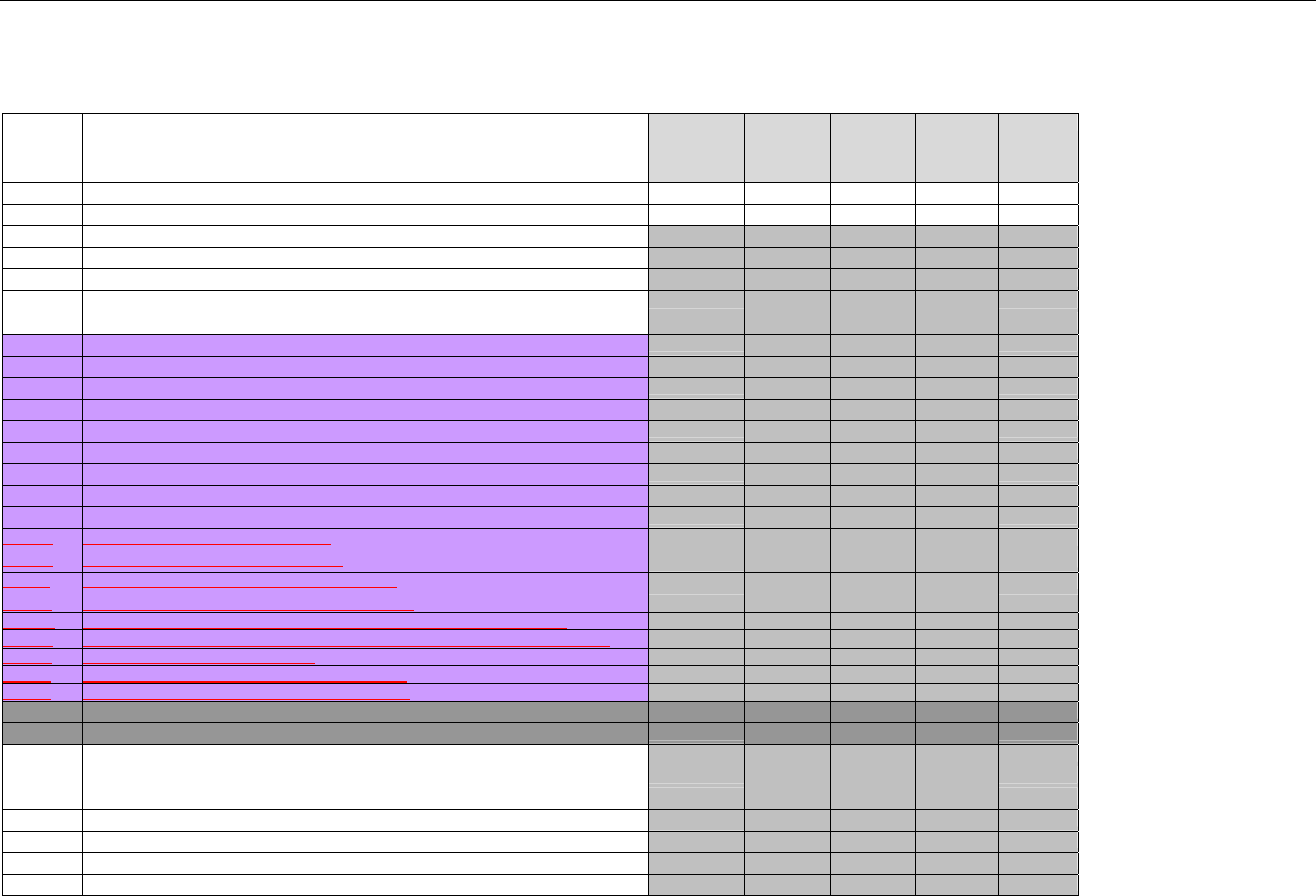
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 661 -
Prior
A
uthorization
Request Only
(Service)
Header Response Status Accepted Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Deferred Rejected Rejected
52Ø-FK
A
mount Exceeding Periodic Benefit Maximum
346-HH Basis of Calculation – Dispensing Fee
347-HJ Basis of Calculation – Copay
348-HK Basis of Calculation – Flat Sales Tax
349-HM Basis of Calculation – Percentage Sales Tax
571-NZ
A
mount Attributed to Processor Fee
575-EQ Patient Sales Tax Amount
574-2Y Plan Sales Tax Amount
572-4U
A
mount of Coinsurance
573-4V Basis of Calculation-Coinsurance
392-MU Benefit Stage Count
393-MV Benefit Stage Qualifier
394-MW Benefit Stage Amount
577-G3 Estimated Generic Savings
128-UC Spending Account Amount Remaining
129-UD Health Plan-Funded Assistance Amount
133-UJ
A
mount Attributed to Provider Network Selection
134-UK
A
mount Attributed to Product Selection/Brand Drug
135-UM
A
mount Attributed to Product Selection/Non-Preferred Formulary Selection
136-UN
A
mount Attributed to Product Selection/Brand Non-Preferred Formulary Selection
137-UP
A
mount Attributed to Coverage Gap
148-U8 Ingredient Cost Contracted/Reimbursable Amount
149-U9 Dispensing Fee Contracted/Reimbursable Amount
RESPONSE DUR/PPS SEGMENT
111-AM Segment Identification
567-J6 DUR/PPS Response Code Counter
439-E4 Reason for Service Code
528-FS Clinical Significance Code
529-FT Other Pharmacy Indicator
531-FV Quantity of Previous Fill
53Ø-FU Previous Date of Fill
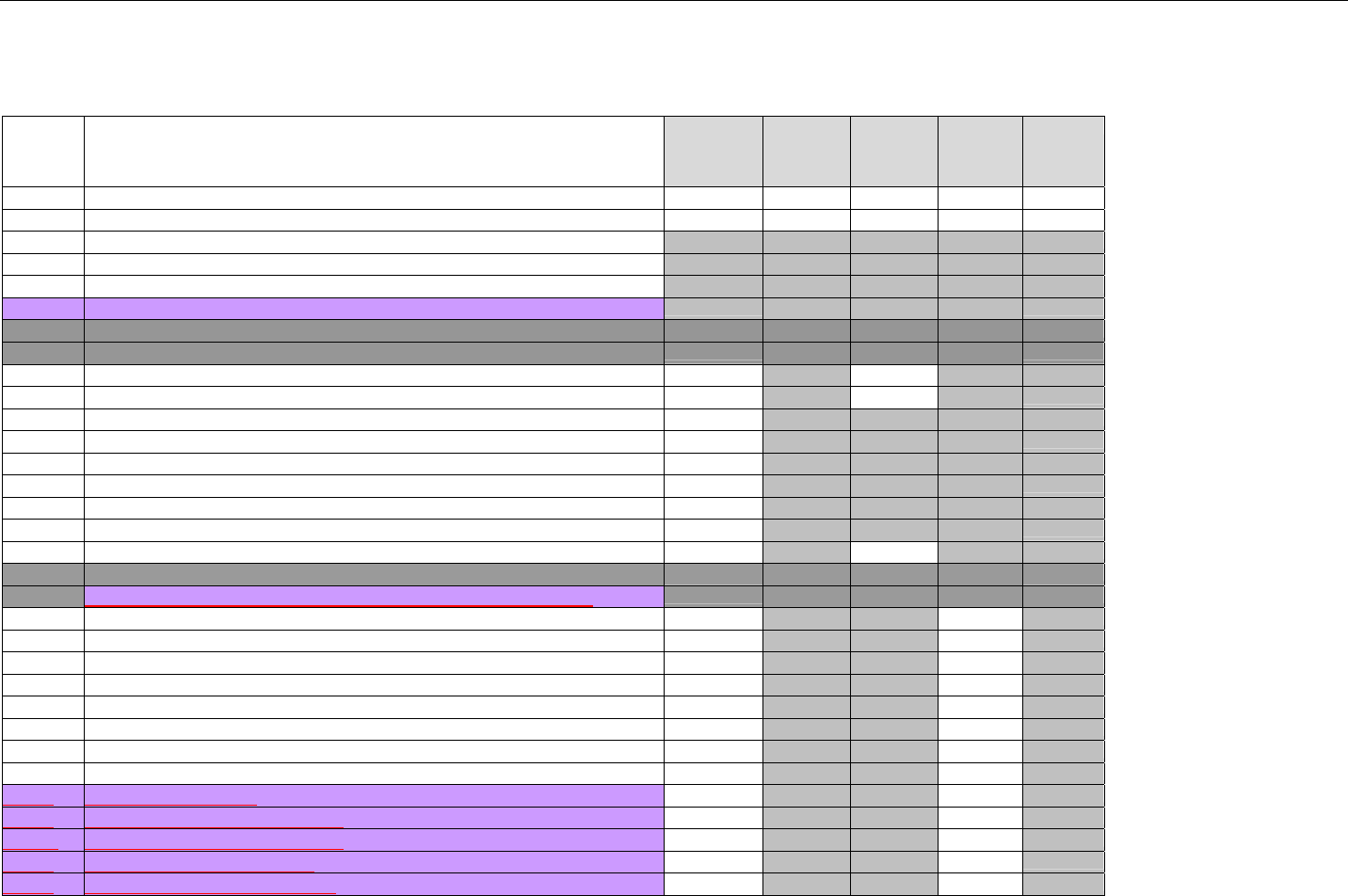
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 662 -
Prior
A
uthorization
Request Only
(Service)
Header Response Status Accepted Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Deferred Rejected Rejected
532-FW Database Indicator
533-FX Other Prescriber Indicator
544-FY DUR Free Text Message
57Ø-NS DUR Additional Text
RESPONSE PRIOR AUTHORIZATION SEGMENT
111-AM Segment Identification M M
498-PR Prior Authorization Processed Date R Q
498-PS Prior Authorization Effective Date Q N
498-PT Prior Authorization Expiration Date Q N
498-RA Prior Authorization Quantity Q N
498-RB Prior Authorization Dollars Authorized Q N
498-PW Prior Authorization Number of Refills Authorized Q N
498-PX Prior Authorization Quantity Accumulated Q N
498-PY Prior Authorization Number - Assigned R Q
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS SEGMENT
111-AM Segment Identification M M
355-NT Other Payer ID Count M M
338-5C Other Payer Coverage Type M M
339-6C Other Payer ID Qualifier Q Q
34Ø-7C Other Payer ID Q Q
991-MH Other Payer Processor Control Number Q Q
356-NU Other Payer Cardholder ID Q Q
992-MJ Other Payer Group ID Q Q
142-UV Other Payer Person Code Q Q
127-UB Other Payer Help Desk Phone Number Q Q
143-UW Other Payer Patient Relationship Code Q Q
144-UX Other Payer Benefit Effective Date Q Q
145-UY Other Payer Benefit Termination Date Q Q
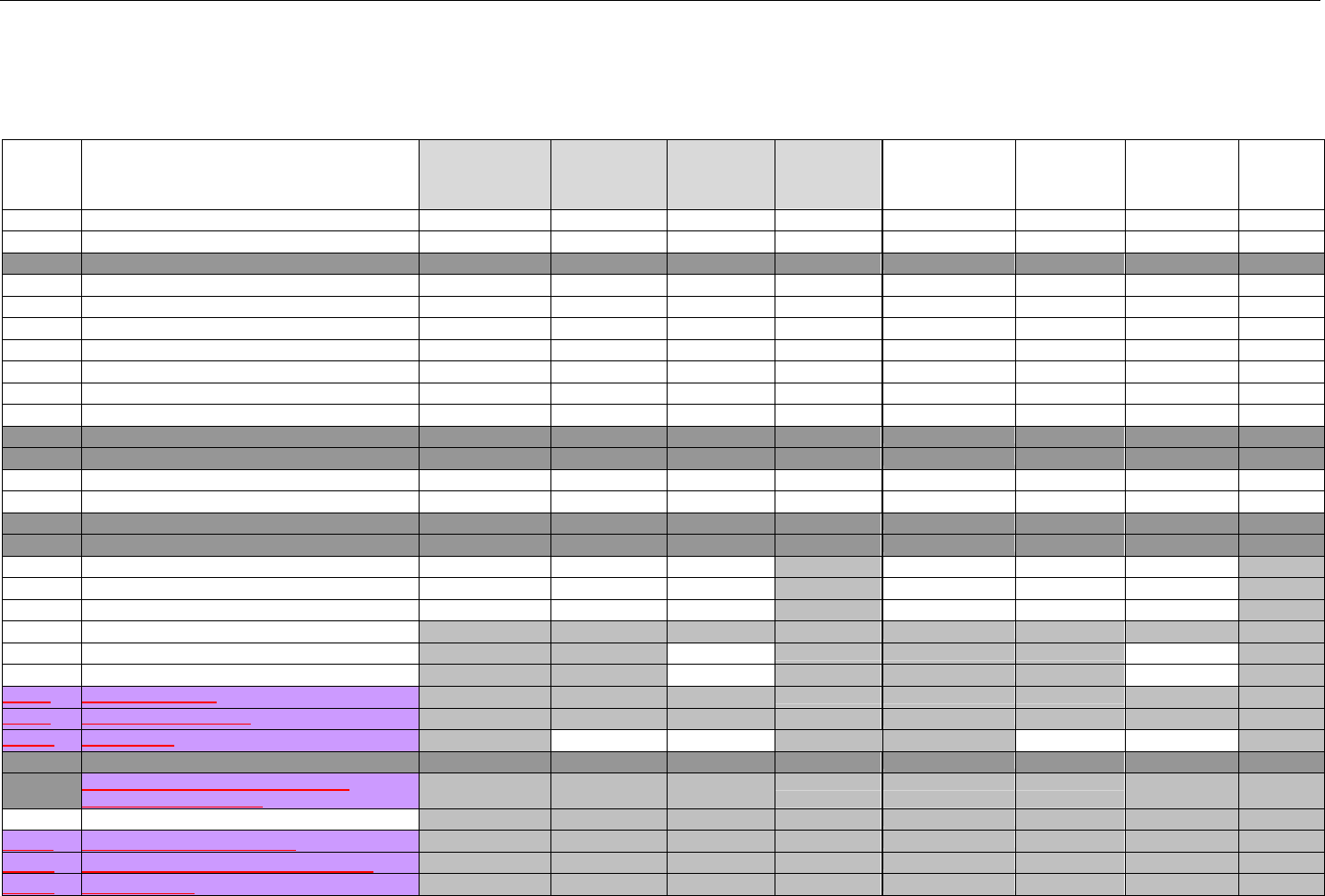
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 663 -
24.6.10 INFORMATION REPORTING/INFORMATION REPORTING REBILL (CLAIM/SERVICE) MATRIX
Information
Reporting/Informa
tion Reporting
Rebill (Claim)
Information
Reporting/Informa
tion Reporting
Rebill (Service)
Header Response Status Accepted Accepted Accepted Rejected Accepted Accepted Accepted Rejected
Transaction Response Status Captured Approved Rejected Rejected Captured Approved Rejected Rejected
RESPONSE HEADER SEGMENT
1Ø2-A2 Version Release Numbe
r
M M M M M M M M
1Ø3-A3 Transaction Code M M M M M M M M
1Ø9-A9 Transaction Count M M M M M M M M
5Ø1-FI Header Response Status M M M M M M M M
2Ø2-B2 Service Provider ID Qualifier M M M M M M M M
2Ø1-B1 Service Provider ID M M M M M M M M
4Ø1-D1 Date of Service M M M M M M M M
RESPONSE MESSAGE SEGMENT
111-AM Segment Identification M M M M M M M M
5Ø4-F4 Message Q Q Q Q Q Q Q Q
RESPONSE INSURANCE SEGMENT
111-AM Segment Identification M M M
M M M
3Ø1-C1 Group ID Q Q Q
Q Q Q
524-FO Plan ID Q Q Q
Q Q Q
545-2F Network Reimbursement ID N N N N N N
568-J7 Payer ID Qualifier N N Q N N Q
569-J8 Payer ID N N Q N N Q
115-N5 Medicaid ID Number N N N N N N
116-N6 Medicaid Agency Number N N N N N N
3Ø2-C2 Cardholder ID N Q Q N Q Q
RESPONSE INSURANCE ADDITIONAL
INFORMATION SEGMENT
111-AM Segment Identification
139-UR Medicare Part D Coverage Code
138-UQ CMS Low Income Cost Sharing (LICS) Level
24Ø-U1 Contract Number
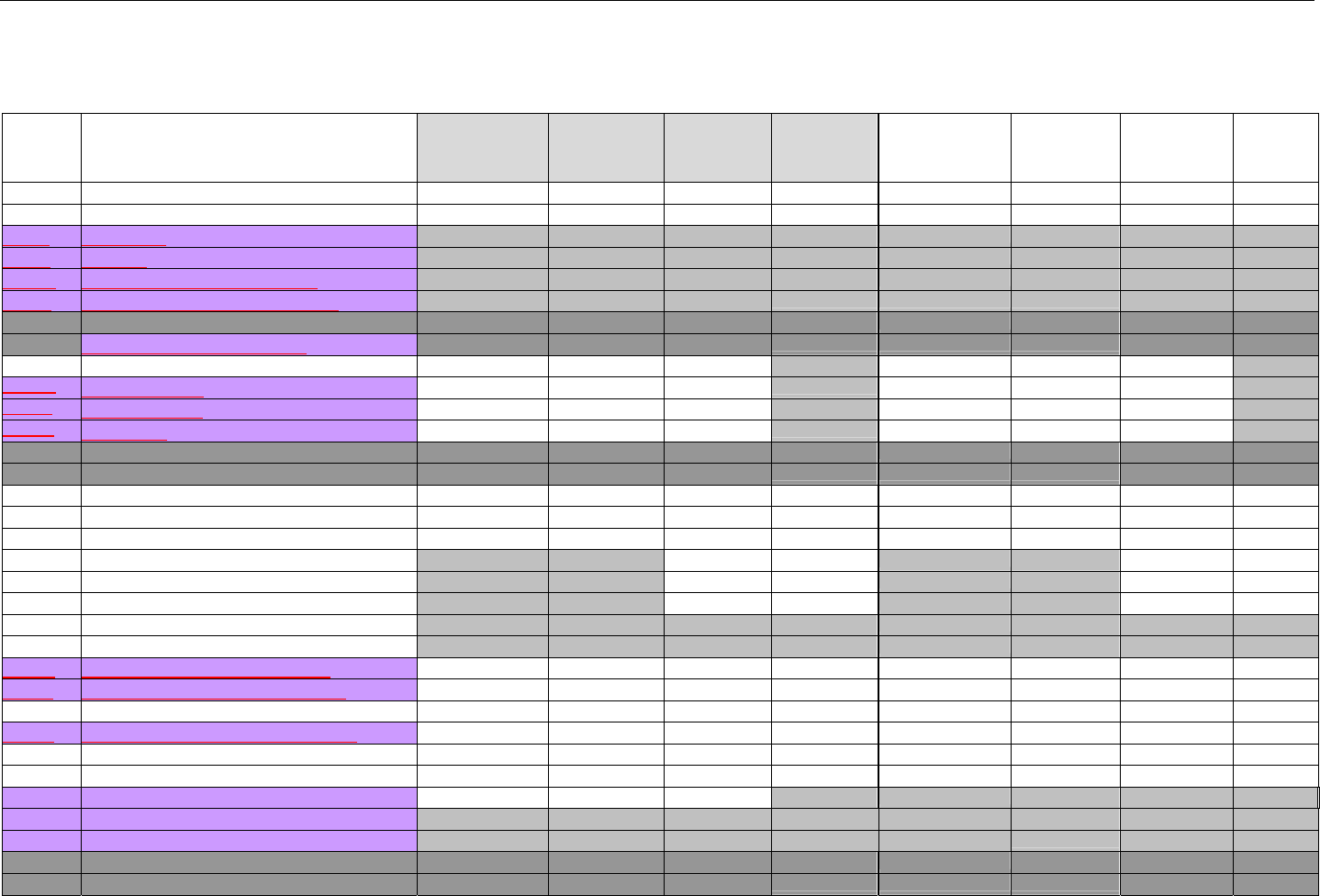
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 664 -
Information
Reporting/Informa
tion Reporting
Rebill (Claim)
Information
Reporting/Informa
tion Reporting
Rebill (Service)
Header Response Status Accepted Accepted Accepted Rejected Accepted Accepted Accepted Rejected
Transaction Response Status Captured Approved Rejected Rejected Captured Approved Rejected Rejected
926-FF Formulary ID
757-U6 Benefit ID
14Ø-US Next Medicare Part D Effective Date
141-UT Next Medicare Part D Termination Date
RESPONSE PATIENT SEGMENT
111-AM Segment Identification M M M
M M M
31Ø-CA Patient First Name Q Q Q
Q Q Q
311-CB Patient Last Name Q Q Q
Q Q Q
3Ø4-C4 Date Of Birth Q Q Q
Q Q Q
RESPONSE STATUS SEGMENT
111-AM Segment Identification M M M M M M M M
112-AN Transaction Response Status M M M M M M M M
5Ø3-F3
A
uthorization Numbe
r
Q Q Q Q Q Q Q Q
51Ø-FA Reject Count N N R R N N R R
511-FB Reject Code N N R R N N R R
546-4F Reject Field Occurrence Indicator N N Q Q N N Q Q
547-5F
A
pproved Message Code Count N N N N N N N N
548-6F
A
pproved Message Code N N N N N N N N
13Ø-UF
A
dditional Message Information Count Q Q Q Q Q Q Q Q
132-UH
A
dditional Message Information Qualifier Q Q Q Q Q Q Q Q
526-FQ
A
dditional Message Information Q Q Q Q Q Q Q Q
131-UG
A
dditional Message Information Continuit
y
Q Q Q Q Q Q Q Q
55Ø-7F Help Desk Phone Number Qualifier Q Q Q Q Q Q Q Q
55Ø-8F Help Desk Phone Number Q Q Q Q Q Q Q Q
88Ø-K5 Transaction Reference Number Q Q Q N N N N N
993-A7 Internal Control Number N N N N N N N N
987-MA URL N N N N N N N N
RESPONSE CLAIM SEGMENT
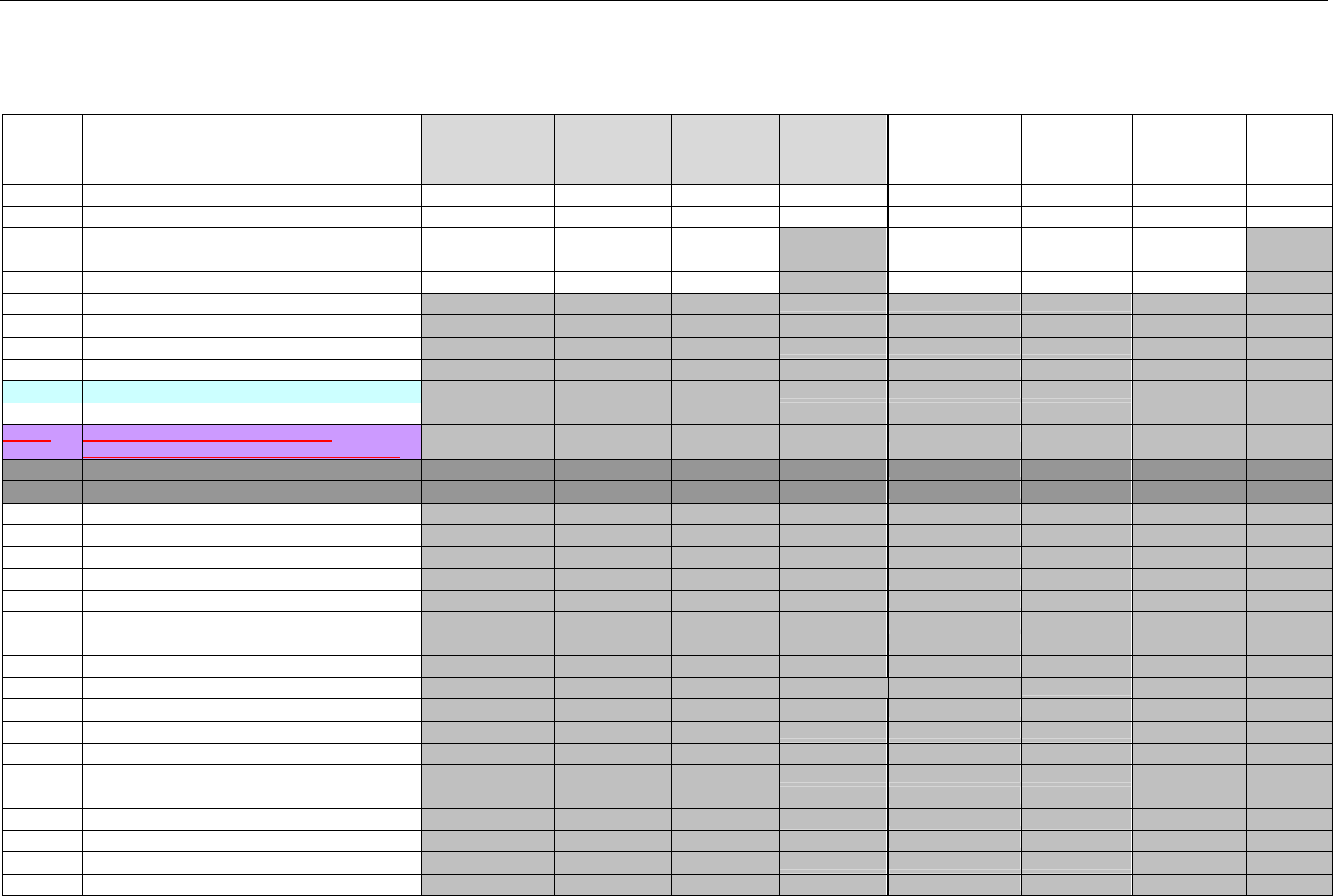
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 665 -
Information
Reporting/Informa
tion Reporting
Rebill (Claim)
Information
Reporting/Informa
tion Reporting
Rebill (Service)
Header Response Status Accepted Accepted Accepted Rejected Accepted Accepted Accepted Rejected
Transaction Response Status Captured Approved Rejected Rejected Captured Approved Rejected Rejected
111-AM Segment Identification M M M
M M M
455-EM Prescription/Service Reference Number Qualifier M M M
M M M
4Ø2-D2 Prescription/Service Reference Number M M M
M M M
551-9F Preferred Product Count N N N N N N
552-AP Preferred Product ID Qualifier N N N N N N
553-AR Preferred Product ID N N N N N N
554-AS Preferred Product Incentive N N N N N N
555-AT Preferred Product Cost Share Incentive N N N N N N
551-9F Preferred Product Description N N N N N N
114-N4 Medicaid Subrogation Internal Control
Number/Transaction Control Number (ICN/TCN)
N N N N N N
RESPONSE PRICING SEGMENT
111-AM Segment Identification
5Ø5-F5 Patient Pay Amount
5Ø6-F6 Ingredient Cost Paid
5Ø7-F7 Dispensing Fee Paid
557-AV Tax Exempt Indicator
558-AW Flat Sales Tax Amount Paid
559-AX Percentage Sales Tax Amount Paid
56Ø-AY Percentage Sales Tax Rate Paid
561-AZ Percentage Sales Tax Basis Paid
521-FL Incentive Amount Paid
562-J1 Professional Service Fee Paid
563-J2 Other Amount Paid Count
564-J3 Other Amount Paid Qualifier
565-J4 Other Amount Paid
566-J5 Other Payer Amount Recognized
5Ø9-F9 Total Amount Paid
522-FM Basis of Reimbursement Determination
523-FN
A
mount Attributed to Sales Tax
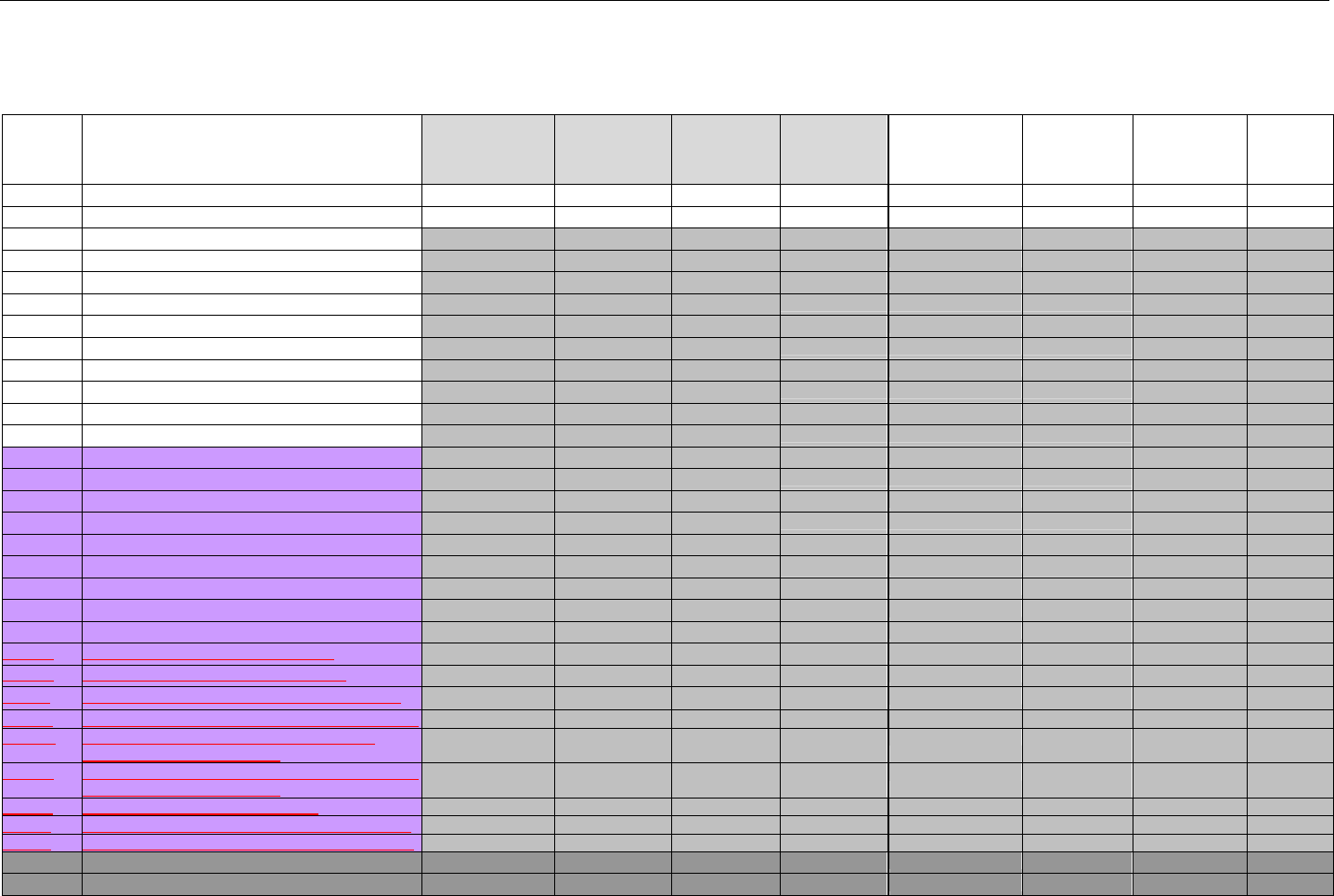
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 666 -
Information
Reporting/Informa
tion Reporting
Rebill (Claim)
Information
Reporting/Informa
tion Reporting
Rebill (Service)
Header Response Status Accepted Accepted Accepted Rejected Accepted Accepted Accepted Rejected
Transaction Response Status Captured Approved Rejected Rejected Captured Approved Rejected Rejected
512-FC
A
ccumulated Deductible Amount
513-FD Remaining Deductible Amount
514-FE Remaining Benefit Amount
517-FH
A
mount Applied to Periodic Deductible
518-F1
A
mount of Copa
y
52Ø-FK
A
mount Exceeding Periodic Benefit Maximum
346-HH Basis of Calculation – Dispensing Fee
347-HJ Basis of Calculation – Copay
348-HK Basis of Calculation – Flat Sales Tax
349-HM Basis of Calculation – Percentage Sales Tax
571-NZ
A
mount Attributed to Processor Fee
575-EQ Patient Sales Tax Amount
574-2Y Plan Sales Tax Amount
572-4U
A
mount of Coinsurance
573-4V Basis of Calculation-Coinsurance
392-MU Benefit Stage Count
393-MV Benefit Stage Qualifier
394-MW Benefit Stage Amount
577-G3 Estimated Generic Savings
128-UC Spending Account Amount Remaining
129-UD Health Plan-Funded Assistance Amount
133-UJ
A
mount Attributed to Provider Network Selection
134-UK
A
mount Attributed to Product Selection/Brand Drug
135-UM
A
mount Attributed to Product Selection/Non-
Preferred Formulary Selection
136-UN
A
mount Attributed to Product Selection/Brand Non-
Preferred Formulary Selection
137-UP
A
mount Attributed to Coverage Gap
148-U8 Ingredient Cost Contracted/Reimbursable Amount
149-U9 Dispensing Fee Contracted/Reimbursable Amount
RESPONSE DUR/PPS SEGMENT
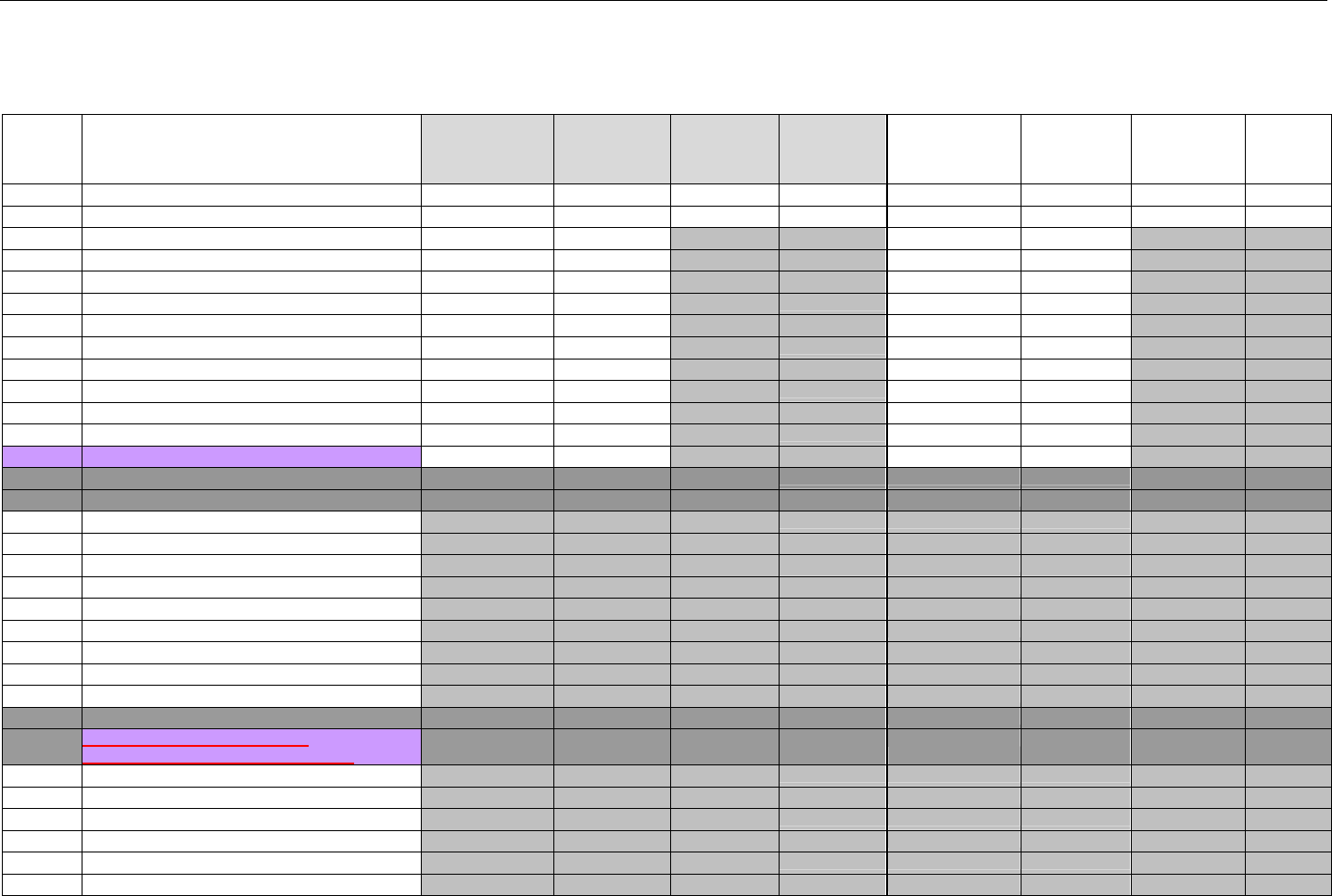
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 667 -
Information
Reporting/Informa
tion Reporting
Rebill (Claim)
Information
Reporting/Informa
tion Reporting
Rebill (Service)
Header Response Status Accepted Accepted Accepted Rejected Accepted Accepted Accepted Rejected
Transaction Response Status Captured Approved Rejected Rejected Captured Approved Rejected Rejected
111-AM Segment Identification M M
M M
567-J6 DUR/PPS Response Code Counter Q Q
Q Q
439-E4 Reason for Service Code Q Q
Q Q
528-FS Clinical Significance Code Q Q
Q Q
529-FT Other Pharmacy Indicator Q Q
Q Q
531-FV Quantity of Previous Fill Q Q
Q Q
53Ø-FU Previous Date of Fill Q Q
Q Q
532-FW Database Indicator Q Q
Q Q
533-FX Other Prescriber Indicator Q Q
Q Q
544-FY DUR Free Text Message Q Q
Q Q
57Ø-NS DUR Additional Text Q Q Q Q
RESPONSE PRIOR AUTHORIZATION SEGMENT
111-AM Segment Identification
498-PR Prior Authorization Processed Date
498-PS Prior Authorization Effective Date
498-PT Prior Authorization Expiration Date
498-RA Prior Authorization Quantity
498-RB Prior Authorization Dollars Authorized
498-PW Prior Authorization Number of Refills Authorized
498-PX Prior Authorization Quantity Accumulated
498-PY Prior Authorization Number - Assigned
RESPONSE COORDINATION OF
BENEFITS/OTHER PAYERS SEGMENT
111-AM Segment Identification
355-NT Other Payer ID Count
338-5C Other Payer Coverage Type
339-6C Other Payer ID Qualifier
34Ø-7C Other Payer ID
991-MH Other Payer Processor Control Number
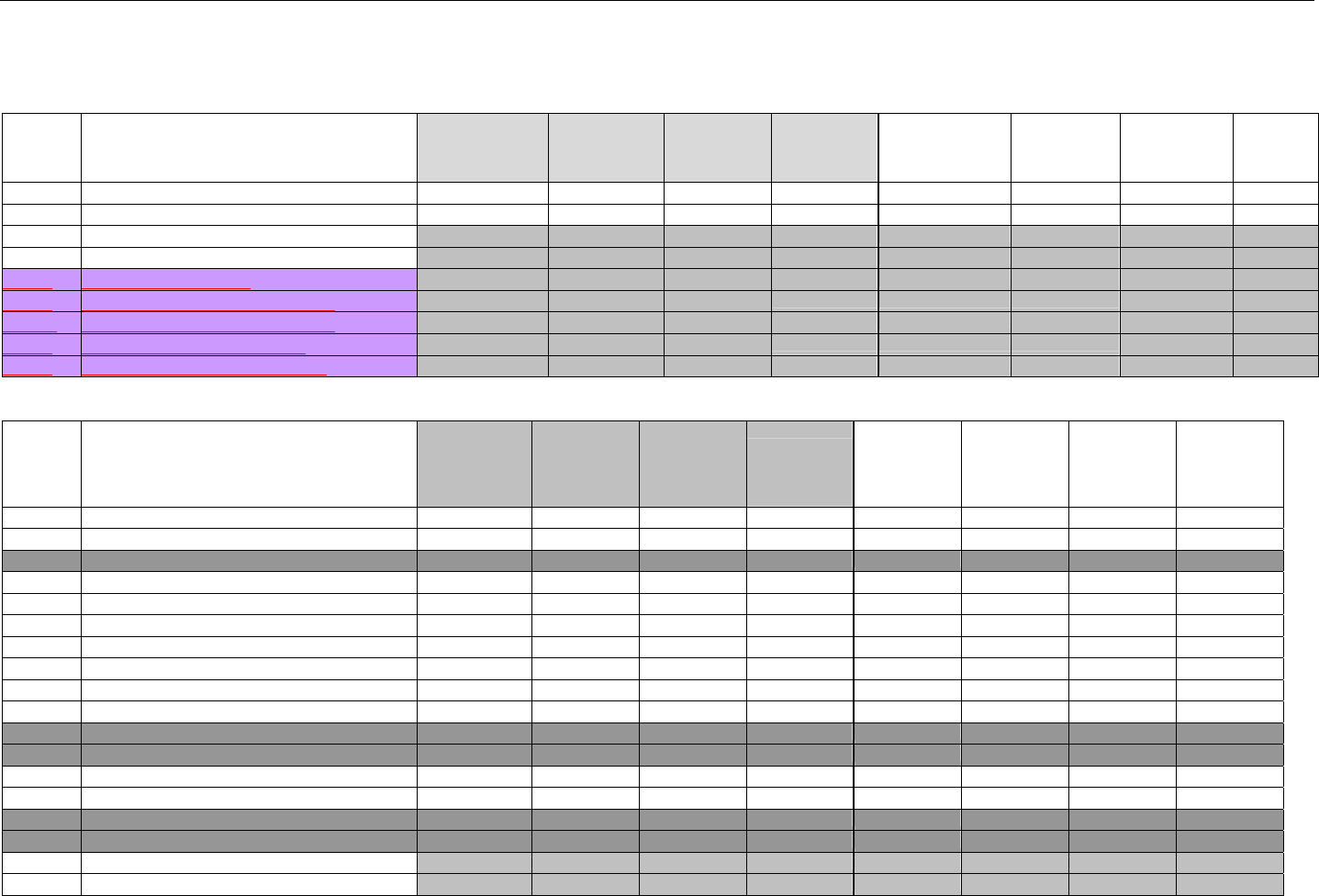
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 668 -
Information
Reporting/Informa
tion Reporting
Rebill (Claim)
Information
Reporting/Informa
tion Reporting
Rebill (Service)
Header Response Status Accepted Accepted Accepted Rejected Accepted Accepted Accepted Rejected
Transaction Response Status Captured Approved Rejected Rejected Captured Approved Rejected Rejected
356-NU Other Payer Cardholder ID
992-MJ Other Payer Group ID
142-UV Other Payer Person Code
127-UB Other Payer Help Desk Phone Number
143-UW Other Payer Patient Relationship Code
144-UX Other Payer Benefit Effective Date
145-UY Other Payer Benefit Termination Date
24.6.11 INFORMATION REPORTING REVERSAL (CLAIM/SERVICE) MATRIX
Information
Reporting
Reversal
(Claim)
Information
Reporting
Reversal
(Service)
Header Response Status Accepted Accepted Accepted Rejected Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Rejected Rejected Approved Captured Rejected Rejected
RESPONSE HEADER SEGMENT
1Ø2-A2 Version Release Numbe
r
M M M M M M M M
1Ø3-A3 Transaction Code M M M M M M M M
1Ø9-A9 Transaction Count M M M M M M M M
5Ø1-FI Header Response Status M M M M M M M M
2Ø2-B2 Service Provider ID Qualifier M M M M M M M M
2Ø1-B1 Service Provider ID M M M M M M M M
4Ø1-D1 Date of Service M M M M M M M M
RESPONSE MESSAGE SEGMENT
111-AM Segment Identification M M M M M M M M
5Ø4-F4 Message Q Q Q Q Q Q Q Q
RESPONSE INSURANCE SEGMENT
111-AM Segment Identification
3Ø1-C1 Group ID
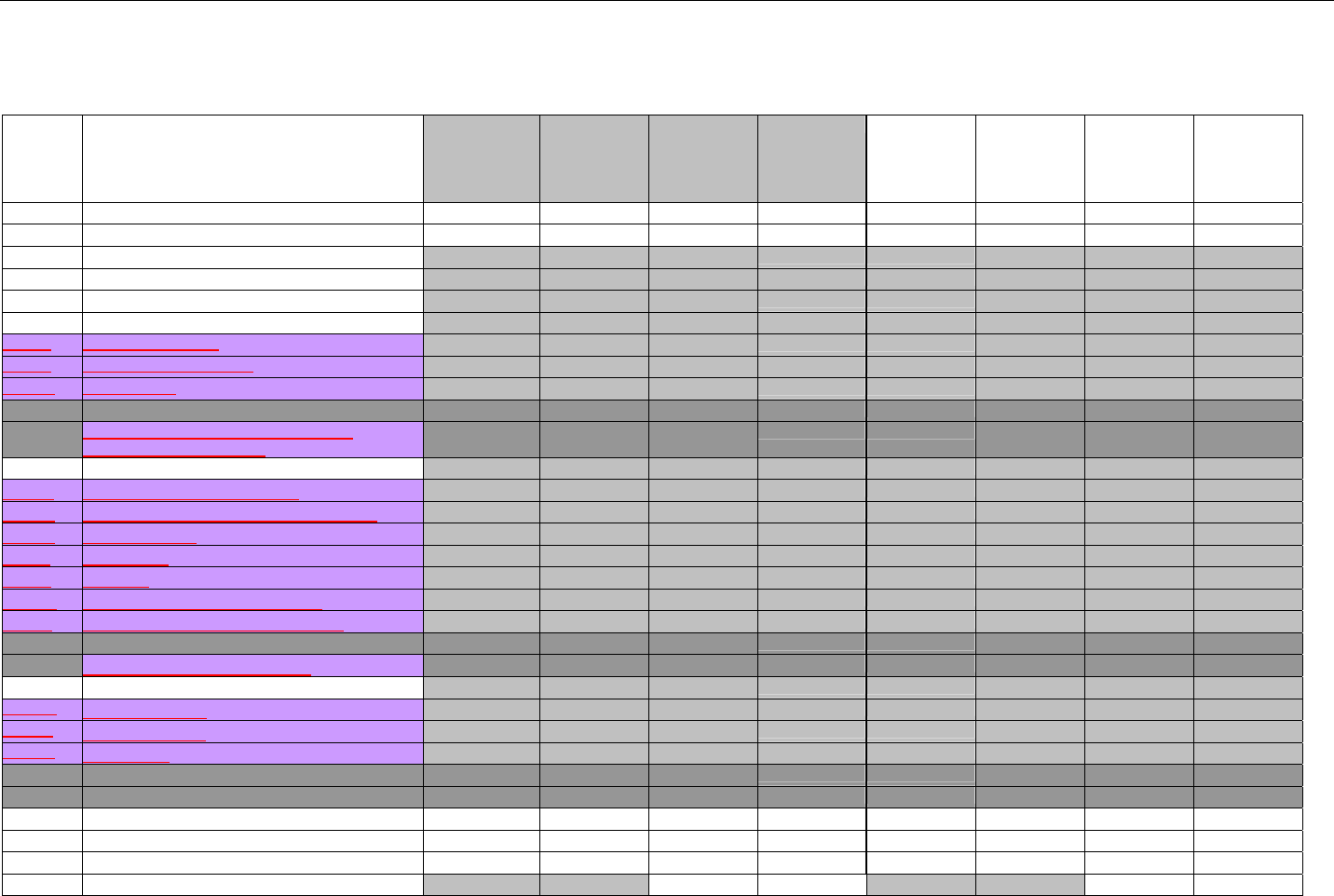
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 669 -
Information
Reporting
Reversal
(Claim)
Information
Reporting
Reversal
(Service)
Header Response Status Accepted Accepted Accepted Rejected Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Rejected Rejected Approved Captured Rejected Rejected
524-FO Plan ID
545-2F Network Reimbursement ID
568-J7 Payer ID Qualifier
569-J8 Payer ID
115-N5 Medicaid ID Number
116-N6 Medicaid Agency Number
3Ø2-C2 Cardholder ID
RESPONSE INSURANCE ADDITIONAL
INFORMATION SEGMENT
111-AM Segment Identification
139-UR Medicare Part D Coverage Code
138-UQ CMS Low Income Cost Sharing (LICS) Level
24Ø-U1 Contract Number
926-FF Formulary ID
757-U6 Benefit ID
14Ø-US Next Medicare Part D Effective Date
141-UT Next Medicare Part D Termination Date
RESPONSE PATIENT SEGMENT
111-AM Segment Identification
31Ø-CA Patient First Name
311-CB Patient Last Name
3Ø4-C4 Date Of Birth
RESPONSE STATUS SEGMENT
111-AM Segment Identification M M M M M M M M
112-AN Transaction Response Status M M M M M M M M
5Ø3-F3
A
uthorization Numbe
r
Q Q Q Q Q Q Q Q
51Ø-FA Reject Count N N R R N N R R
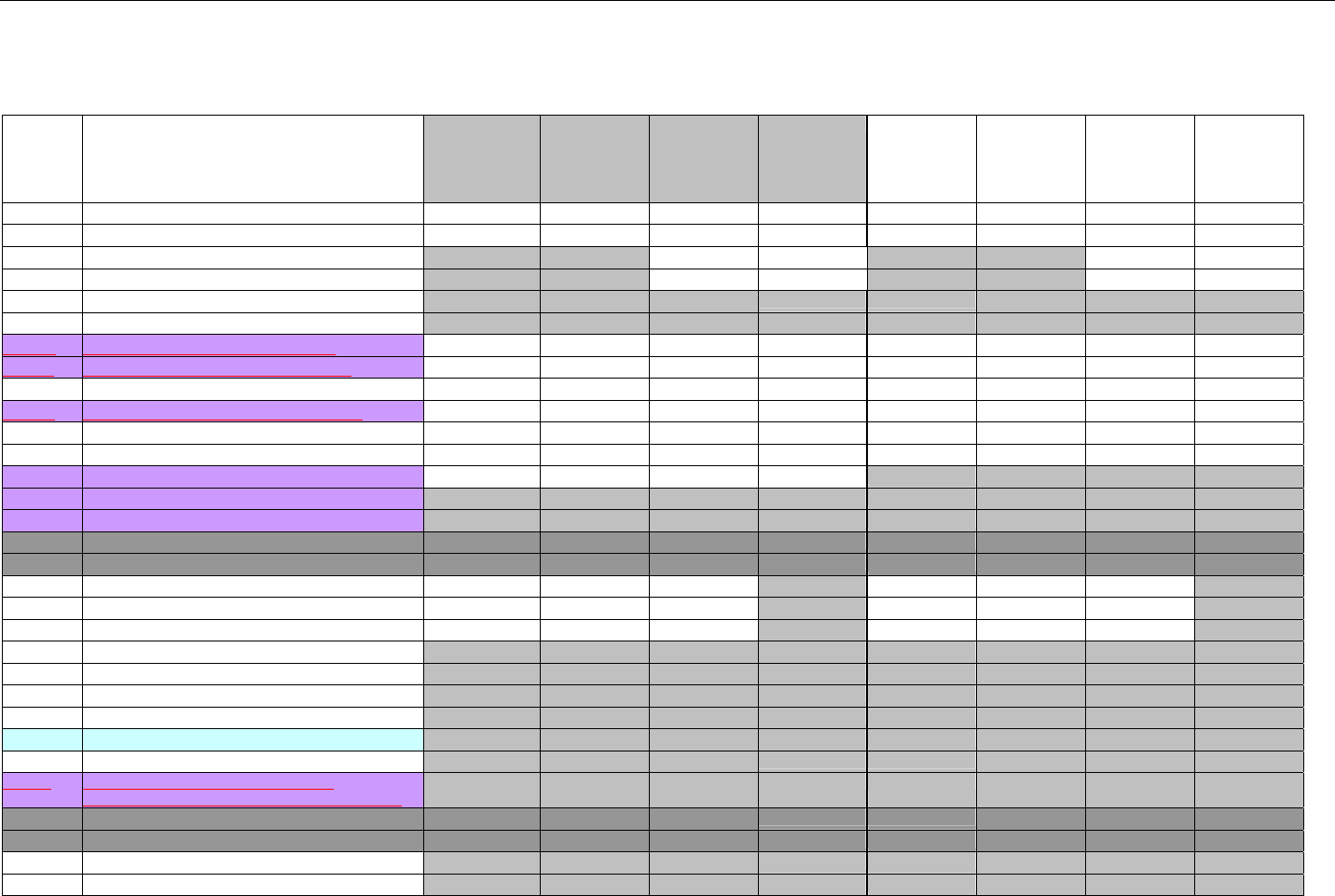
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 670 -
Information
Reporting
Reversal
(Claim)
Information
Reporting
Reversal
(Service)
Header Response Status Accepted Accepted Accepted Rejected Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Rejected Rejected Approved Captured Rejected Rejected
511-FB Reject Code N N R R N N R R
546-4F Reject Field Occurrence Indicator N N Q Q N N Q Q
547-5F
A
pproved Message Code Count N N N N N N N N
548-6F
A
pproved Message Code N N N N N N N N
13Ø-UF
A
dditional Message Information Count Q Q Q Q Q Q Q Q
132-UH
A
dditional Message Information Qualifie
r
Q Q Q Q Q Q Q Q
526-FQ
A
dditional Message Information Q Q Q Q Q Q Q Q
131-UG
A
dditional Message Information Continuit
y
Q Q Q Q Q Q Q Q
55Ø-7F Help Desk Phone Number Qualifier Q Q Q Q Q Q Q Q
55Ø-8F Help Desk Phone Number Q Q Q Q Q Q Q Q
88Ø-K5 Transaction Reference Number Q Q Q Q N N N N
993-A7 Internal Control Number N N N N N N N N
987-MA URL N N N N N N N N
RESPONSE CLAIM SEGMENT
111-AM Segment Identification M M M M M M
455-EM Prescription/Service Reference Number Qualifier M M M M M M
4Ø2-D2 Prescription/Service Reference Number M M M M M M
551-9F Preferred Product Count N N N N N N
552-AP Preferred Product ID Qualifier N N N N N N
553-AR Preferred Product ID N N N N N N
554-AS Preferred Product Incentive N N N N N N
555-AT Preferred Product Cost Share Incentive N N N N N N
551-9F Preferred Product Description N N N N N N
114-N4 Medicaid Subrogation Internal Control
Number/Transaction Control Number (ICN/TCN)
N N N N N N
RESPONSE PRICING SEGMENT
111-AM Segment Identification
5Ø5-F5 Patient Pay Amount
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 671 -
Information
Reporting
Reversal
(Claim)
Information
Reporting
Reversal
(Service)
Header Response Status Accepted Accepted Accepted Rejected Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Rejected Rejected Approved Captured Rejected Rejected
5Ø6-F6 Ingredient Cost Paid
5Ø7-F7 Dispensing Fee Paid
557-AV Tax Exempt Indicator
558-AW Flat Sales Tax Amount Paid
559-AX Percentage Sales Tax Amount Paid
56Ø-AY Percentage Sales Tax Rate Paid
561-AZ Percentage Sales Tax Basis Paid
521-FL Incentive Amount Paid
562-J1 Professional Service Fee Paid
563-J2 Other Amount Paid Count
564-J3 Other Amount Paid Qualifier
565-J4 Other Amount Paid
566-J5 Other Payer Amount Recognized
5Ø9-F9 Total Amount Paid
522-FM Basis of Reimbursement Determination
523-FN
A
mount Attributed to Sales Tax
512-FC
A
ccumulated Deductible Amount
513-FD Remaining Deductible Amount
514-FE Remaining Benefit Amount
517-FH
A
mount Applied to Periodic Deductible
518-F1
A
mount of Copa
y
52Ø-FK
A
mount Exceeding Periodic Benefit Maximum
346-HH Basis of Calculation – Dispensing Fee
347-HJ Basis of Calculation – Copay
348-HK Basis of Calculation – Flat Sales Tax
349-HM Basis of Calculation – Percentage Sales Tax
571-NZ
A
mount Attributed to Processor Fee
575-EQ Patient Sales Tax Amount
574-2Y Plan Sales Tax Amount
572-4U
A
mount of Coinsurance
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 672 -
Information
Reporting
Reversal
(Claim)
Information
Reporting
Reversal
(Service)
Header Response Status Accepted Accepted Accepted Rejected Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Rejected Rejected Approved Captured Rejected Rejected
573-4V Basis of Calculation-Coinsurance
392-MU Benefit Stage Count
393-MV Benefit Stage Qualifier
394-MW Benefit Stage Amount
577-G3 Estimated Generic Savings
128-UC Spending Account Amount Remaining
129-UD Health Plan-Funded Assistance Amount
133-UJ
A
mount Attributed to Provider Network Selection
134-UK
A
mount Attributed to Product Selection/Brand Drug
135-UM
A
mount Attributed to Product Selection/Non-
Preferred Formulary Selection
136-UN
A
mount Attributed to Product Selection/Brand Non-
Preferred Formulary Selection
137-UP
A
mount Attributed to Coverage Gap
148-U8 Ingredient Cost Contracted/Reimbursable Amount
149-U9 Dispensing Fee Contracted/Reimbursable Amount
RESPONSE DUR/PPS SEGMENT
111-AM Segment Identification
567-J6 DUR/PPS Response Code Counter
439-E4 Reason for Service Code
528-FS Clinical Significance Code
529-FT Other Pharmacy Indicator
531-FV Quantity of Previous Fill
53Ø-FU Previous Date of Fill
532-FW Database Indicator
533-FX Other Prescriber Indicator
544-FY DUR Free Text Message
57Ø-NS DUR Additional Text
RESPONSE PRIOR AUTHORIZATION SEGMENT

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 673 -
Information
Reporting
Reversal
(Claim)
Information
Reporting
Reversal
(Service)
Header Response Status Accepted Accepted Accepted Rejected Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Rejected Rejected Approved Captured Rejected Rejected
111-AM Segment Identification
498-PR Prior Authorization Processed Date
498-PS Prior Authorization Effective Date
498-PT Prior Authorization Expiration Date
498-RA Prior Authorization Quantity
498-RB Prior Authorization Dollars Authorized
498-PW Prior Authorization Number of Refills Authorized
498-PX Prior Authorization Quantity Accumulated
498-PY Prior Authorization Number - Assigned
RESPONSE COORDINATION OF
BENEFITS/OTHER PAYERS SEGMENT
111-AM Segment Identification
355-NT Other Payer ID Count
338-5C Other Payer Coverage Type
339-6C Other Payer ID Qualifier
34Ø-7C Other Payer ID
991-MH Other Payer Processor Control Number
356-NU Other Payer Cardholder ID
992-MJ Other Payer Group ID
142-UV Other Payer Person Code
127-UB Other Payer Help Desk Phone Number
143-UW Other Payer Patient Relationship Code
144-UX Other Payer Benefit Effective Date
145-UY Other Payer Benefit Termination Date
24.6.12 CONTROLLED SUBSTANCE REPORTING/CONTROLLED SUBSTANCE REPORTING REBILL MATRIX
Controlled
Substance
Reporting
Controlled
Substance
Reporting
Rebill

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 674 -
Header Response Status Accepted Accepted Accepted Rejected Accepted Accepted Accepted Rejected
Transaction Response Status Captured Approved Rejected Rejected Captured Approved Rejected Rejected
RESPONSE HEADER SEGMENT
1Ø2-A2 Version Release Numbe
r
M M M M M M M M
1Ø3-A3 Transaction Code M M M M M M M M
1Ø9-A9 Transaction Count M M M M M M M M
5Ø1-FI Header Response Status M M M M M M M M
2Ø2-B2 Service Provider ID Qualifier M M M M M M M M
2Ø1-B1 Service Provider ID M M M M M M M M
4Ø1-D1 Date of Service M M M M M M M M
RESPONSE MESSAGE SEGMENT
111-AM Segment Identification M M M M M M M M
5Ø4-F4 Message O O O O O O O O
RESPONSE INSURANCE SEGMENT
111-AM Segment Identification
3Ø1-C1 Group ID
524-FO Plan ID
545-2F Network Reimbursement ID
568-J7 Payer ID Qualifier
569-J8 Payer ID
115-N5 Medicaid ID Number
116-N6 Medicaid Agency Number
3Ø2-C2 Cardholder ID
RESPONSE INSURANCE ADDITIONAL
INFORMATION SEGMENT
111-AM Segment Identification
139-UR Medicare Part D Coverage Code
138-UQ CMS Low Income Cost Sharing (LICS) Level
24Ø-U1 Contract Number
926-FF Formulary ID
757-U6 Benefit ID
14Ø-US Next Medicare Part D Effective Date
141-UT Next Medicare Part D Termination Date

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 675 -
Controlled
Substance
Reporting
Controlled
Substance
Reporting
Rebill
Header Response Status Accepted Accepted Accepted Rejected Accepted Accepted Accepted Rejected
Transaction Response Status Captured Approved Rejected Rejected Captured Approved Rejected Rejected
RESPONSE PATIENT SEGMENT
111-AM Segment Identification
31Ø-CA Patient First Name
311-CB Patient Last Name
3Ø4-C4 Date Of Birth
RESPONSE STATUS SEGMENT
111-AM Segment Identification M M M M M M M M
112-AN Transaction Response Status M M M M M M M M
5Ø3-F3
A
uthorization Numbe
r
O O O O O O O O
51Ø-FA Reject Count N N R R N N R R
511-FB Reject Code N N R R N N R R
546-4F Reject Field Occurrence Indicator N N O O N N O O
547-5F
A
pproved Message Code Count N O N N N O N N
548-6F
A
pproved Message Code N O N N N O N N
13Ø-UF
A
dditional Message Information Count O O O O O O O O
132-UH
A
dditional Message Information Qualifie
r
O O O O O O O O
526-FQ
A
dditional Message Information O O O O O O O O
131-UG
A
dditional Message Information Continuit
y
O O O O O O O O
55Ø-7F Help Desk Phone Number Qualifier Q Q Q Q Q Q Q Q
55Ø-8F Help Desk Phone Number O O O O O O O O
88Ø-K5 Transaction Reference Number N N N N N N N N
993-A7 Internal Control Number N N N N N N N N
987-MA URL N N N N N N N N
RESPONSE CLAIM SEGMENT
111-AM Segment Identification M M M
M M M
455-EM Prescription/Service Reference Number Qualifier M M M
M M M
4Ø2-D2 Prescription/Service Reference Number M M M
M M M
551-9F Preferred Product Count O O O
O O O
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 676 -
Controlled
Substance
Reporting
Controlled
Substance
Reporting
Rebill
Header Response Status Accepted Accepted Accepted Rejected Accepted Accepted Accepted Rejected
Transaction Response Status Captured Approved Rejected Rejected Captured Approved Rejected Rejected
552-AP Preferred Product ID Qualifier Q Q Q
Q Q Q
553-AR Preferred Product ID O O O
O O O
554-AS Preferred Product Incentive O O O
O O O
555-AT Preferred Product Cost Share Incentive O O O
O O O
551-9F Preferred Product Description O O O
O O O
114-N4 Medicaid Subrogation Internal Control
Number/Transaction Control Number (ICN/TCN)
O O O
O O O
RESPONSE PRICING SEGMENT
111-AM Segment Identification
5Ø5-F5 Patient Pay Amount
5Ø6-F6 Ingredient Cost Paid
5Ø7-F7 Dispensing Fee Paid
557-AV Tax Exempt Indicator
558-AW Flat Sales Tax Amount Paid
559-AX Percentage Sales Tax Amount Paid
56Ø-AY Percentage Sales Tax Rate Paid
561-AZ Percentage Sales Tax Basis Paid
521-FL Incentive Amount Paid
562-J1 Professional Service Fee Paid
563-J2 Other Amount Paid Count
564-J3 Other Amount Paid Qualifier
565-J4 Other Amount Paid
566-J5 Other Payer Amount Recognized
5Ø9-F9 Total Amount Paid
522-FM Basis of Reimbursement Determination
523-FN
A
mount Attributed to Sales Tax
512-FC
A
ccumulated Deductible Amount
513-FD Remaining Deductible Amount
514-FE Remaining Benefit Amount
517-FH
A
mount Applied to Periodic Deductible

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 677 -
Controlled
Substance
Reporting
Controlled
Substance
Reporting
Rebill
Header Response Status Accepted Accepted Accepted Rejected Accepted Accepted Accepted Rejected
Transaction Response Status Captured Approved Rejected Rejected Captured Approved Rejected Rejected
518-F1
A
mount of Copa
y
52Ø-FK
A
mount Exceeding Periodic Benefit Maximum
346-HH Basis of Calculation – Dispensing Fee
347-HJ Basis of Calculation – Copay
348-HK Basis of Calculation – Flat Sales Tax
349-HM Basis of Calculation – Percentage Sales Tax
571-NZ
A
mount Attributed to Processor Fee
575-EQ Patient Sales Tax Amount
574-2Y Plan Sales Tax Amount
572-4U
A
mount of Coinsurance
573-4V Basis of Calculation-Coinsurance
392-MU Benefit Stage Count
393-MV Benefit Stage Qualifier
394-MW Benefit Stage Amount
577-G3 Estimated Generic Savings
128-UC Spending Account Amount Remaining
129-UD Health Plan-Funded Assistance Amount
133-UJ
A
mount Attributed to Provider Network Selection
134-UK
A
mount Attributed to Product Selection/Brand Drug
135-UM
A
mount Attributed to Product Selection/Non-
Preferred Formulary Selection
136-UN
A
mount Attributed to Product Selection/Brand Non-
Preferred Formulary Selection
137-UP
A
mount Attributed to Coverage Gap
148-U8 Ingredient Cost Contracted/Reimbursable Amount
149-U9 Dispensing Fee Contracted/Reimbursable Amount
RESPONSE DUR/PPS SEGMENT
111-AM Segment Identification
567-J6 DUR/PPS Response Code Counter
439-E4 Reason for Service Code
528-FS Clinical Significance Code
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 678 -
Controlled
Substance
Reporting
Controlled
Substance
Reporting
Rebill
Header Response Status Accepted Accepted Accepted Rejected Accepted Accepted Accepted Rejected
Transaction Response Status Captured Approved Rejected Rejected Captured Approved Rejected Rejected
529-FT Other Pharmacy Indicator
531-FV Quantity of Previous Fill
53Ø-FU Previous Date of Fill
532-FW Database Indicator
533-FX Other Prescriber Indicator
544-FY DUR Free Text Message
57Ø-NS DUR Additional Text
RESPONSE PRIOR AUTHORIZATION SEGMENT
111-AM Segment Identification
498-PR Prior Authorization Processed Date
498-PS Prior Authorization Effective Date
498-PT Prior Authorization Expiration Date
498-RA Prior Authorization Quantity
498-RB Prior Authorization Dollars Authorized
498-PW Prior Authorization Number of Refills Authorized
498-PX Prior Authorization Quantity Accumulated
498-PY Prior Authorization Number - Assigned
RESPONSE COORDINATION OF
BENEFITS/OTHER PAYERS SEGMENT
111-AM Segment Identification
355-NT Other Payer ID Count
338-5C Other Payer Coverage Type
339-6C Other Payer ID Qualifier
34Ø-7C Other Payer ID
991-MH Other Payer Processor Control Number
356-NU Other Payer Cardholder ID
992-MJ Other Payer Group ID
142-UV Other Payer Person Code
127-UB Other Payer Help Desk Phone Number

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 679 -
Controlled
Substance
Reporting
Controlled
Substance
Reporting
Rebill
Header Response Status Accepted Accepted Accepted Rejected Accepted Accepted Accepted Rejected
Transaction Response Status Captured Approved Rejected Rejected Captured Approved Rejected Rejected
143-UW Other Payer Patient Relationship Code
144-UX Other Payer Benefit Effective Date
145-UY Other Payer Benefit Termination Date
24.6.13 CONTROLLED SUBSTANCE REPORTING REVERSAL MATRIX
Controlled
Substance
Reporting
Reversal
Header Response Status Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Rejected Rejected
RESPONSE HEADER SEGMENT
1Ø2-A2 Version Release Numbe
r
M M M M
1Ø3-A3 Transaction Code M M M M
1Ø9-A9 Transaction Count M M M M
5Ø1-FI Header Response Status M M M M
2Ø2-B2 Service Provider ID Qualifier M M M M
2Ø1-B1 Service Provider ID M M M M
4Ø1-D1 Date of Service M M M M
RESPONSE MESSAGE SEGMENT
111-AM Segment Identification M M M M
5Ø4-F4 Message O O O O
RESPONSE INSURANCE SEGMENT
111-AM Segment Identification
3Ø1-C1 Group ID
524-FO Plan ID
545-2F Network Reimbursement ID
568-J7 Payer ID Qualifier
569-J8 Payer ID
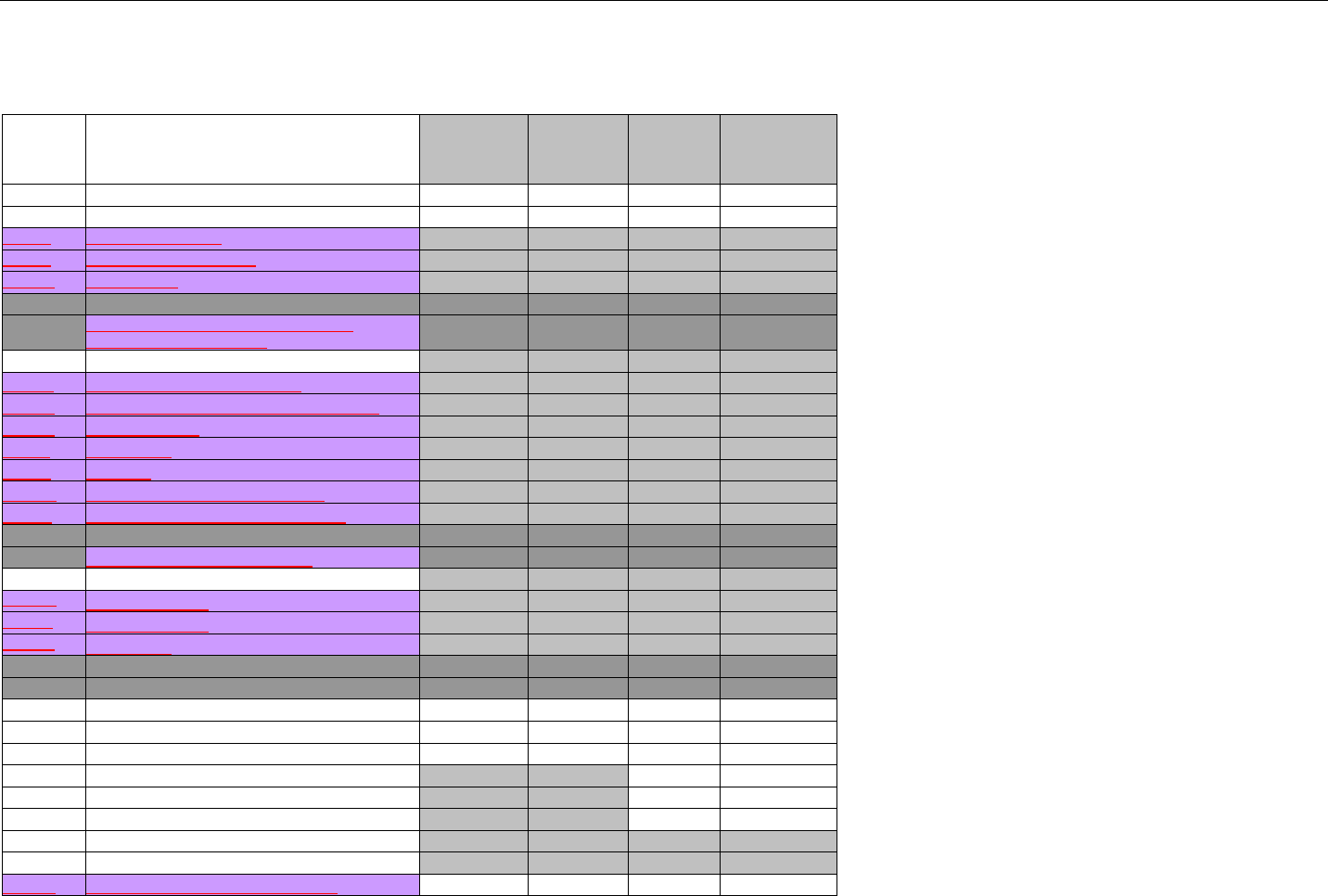
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 680 -
Controlled
Substance
Reporting
Reversal
Header Response Status Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Rejected Rejected
115-N5 Medicaid ID Number
116-N6 Medicaid Agency Number
3Ø2-C2 Cardholder ID
RESPONSE INSURANCE ADDITIONAL
INFORMATION SEGMENT
111-AM Segment Identification
139-UR Medicare Part D Coverage Code
138-UQ CMS Low Income Cost Sharing (LICS) Level
24Ø-U1 Contract Number
926-FF Formulary ID
757-U6 Benefit ID
14Ø-US Next Medicare Part D Effective Date
141-UT Next Medicare Part D Termination Date
RESPONSE PATIENT SEGMENT
111-AM Segment Identification
31Ø-CA Patient First Name
311-CB Patient Last Name
3Ø4-C4 Date Of Birth
RESPONSE STATUS SEGMENT
111-AM Segment Identification M M M M
112-AN Transaction Response Status M M M M
5Ø3-F3
A
uthorization Numbe
r
O O O O
51Ø-FA Reject Count N N R R
511-FB Reject Code N N R R
546-4F Reject Field Occurrence Indicator N N O O
547-5F
A
pproved Message Code Count N N N N
548-6F
A
pproved Message Code N N N N
13Ø-UF
A
dditional Message Information Count O O O O
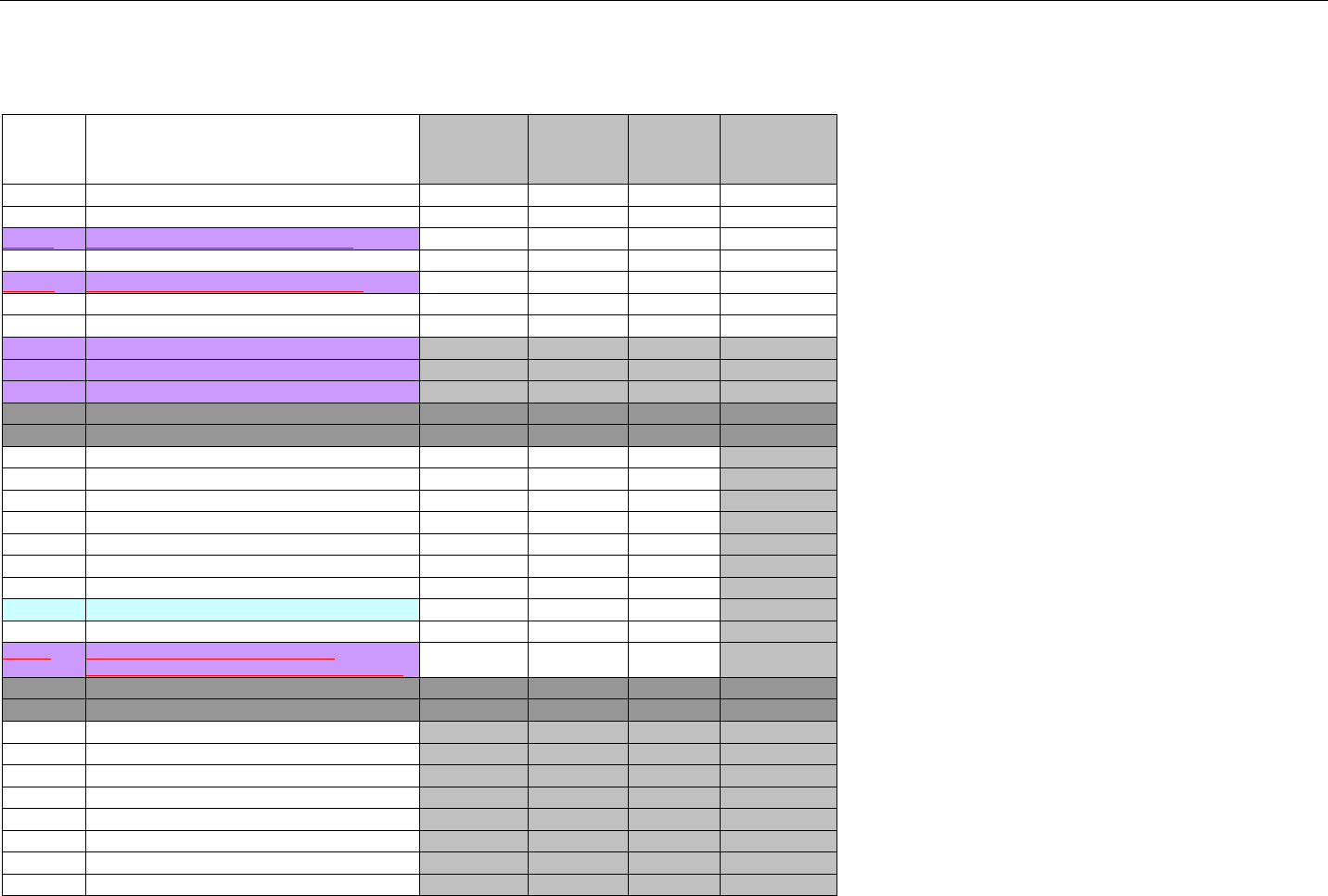
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 681 -
Controlled
Substance
Reporting
Reversal
Header Response Status Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Rejected Rejected
132-UH
A
dditional Message Information Qualifier O O O O
526-FQ
A
dditional Message Information O O O O
131-UG
A
dditional Message Information Continuit
y
O O O O
55Ø-7F Help Desk Phone Number Qualifier Q Q Q Q
55Ø-8F Help Desk Phone Number O O O O
88Ø-K5 Transaction Reference Number N N N N
993-A7 Internal Control Number N N N N
987-MA URL N N N N
RESPONSE CLAIM SEGMENT
111-AM Segment Identification M M M
455-EM Prescription/Service Reference Number Qualifier M M M
4Ø2-D2 Prescription/Service Reference Number M M M
551-9F Preferred Product Count O O O
552-AP Preferred Product ID Qualifier Q Q Q
553-AR Preferred Product ID O O O
554-AS Preferred Product Incentive O O O
555-AT Preferred Product Cost Share Incentive O O O
551-9F Preferred Product Description O O O
114-N4 Medicaid Subrogation Internal Control
Number/Transaction Control Number (ICN/TCN)
O O O
RESPONSE PRICING SEGMENT
111-AM Segment Identification
5Ø5-F5 Patient Pay Amount
5Ø6-F6 Ingredient Cost Paid
5Ø7-F7 Dispensing Fee Paid
557-AV Tax Exempt Indicator
558-AW Flat Sales Tax Amount Paid
559-AX Percentage Sales Tax Amount Paid
56Ø-AY Percentage Sales Tax Rate Paid
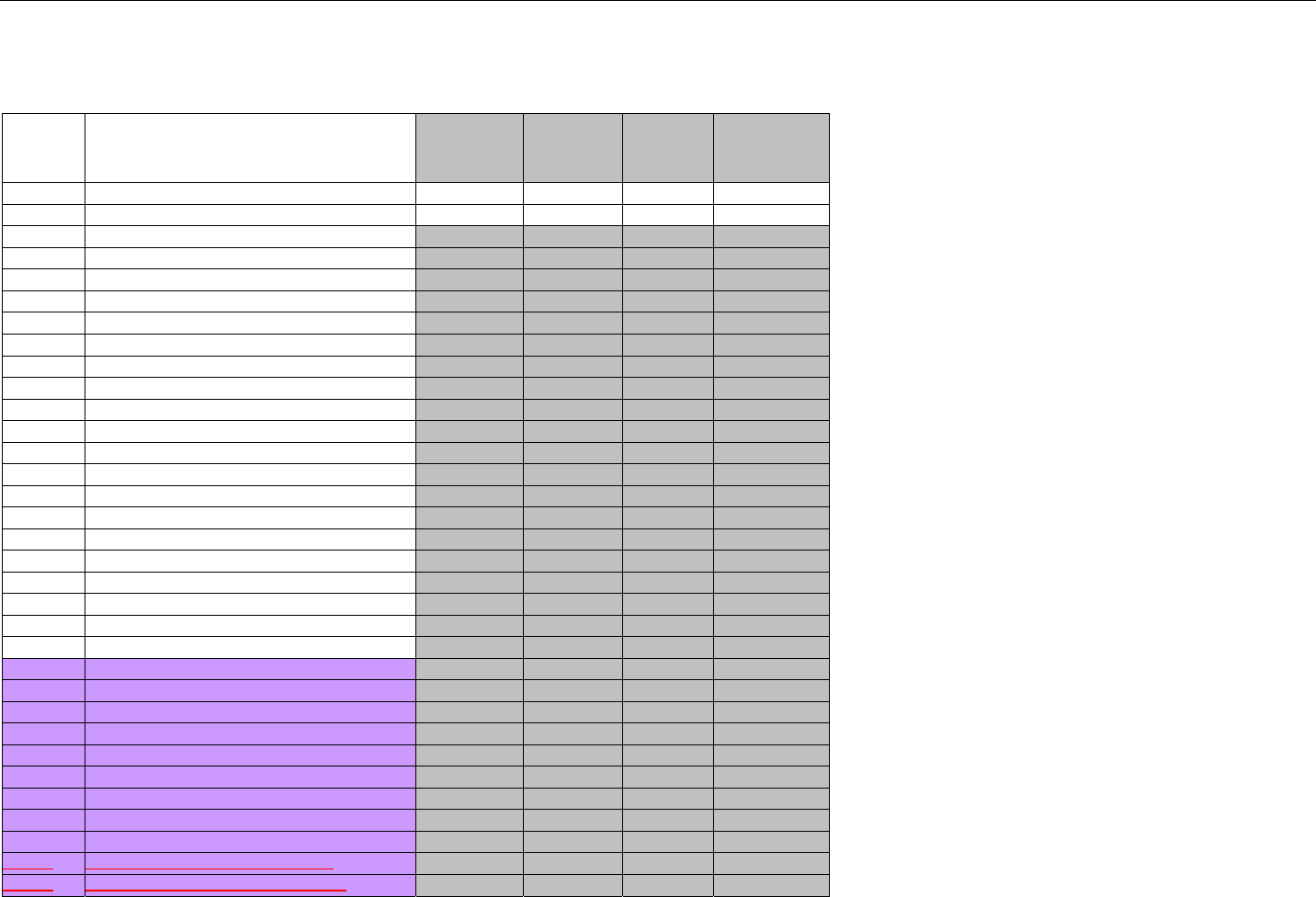
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 682 -
Controlled
Substance
Reporting
Reversal
Header Response Status Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Rejected Rejected
561-AZ Percentage Sales Tax Basis Paid
521-FL Incentive Amount Paid
562-J1 Professional Service Fee Paid
563-J2 Other Amount Paid Count
564-J3 Other Amount Paid Qualifier
565-J4 Other Amount Paid
566-J5 Other Payer Amount Recognized
5Ø9-F9 Total Amount Paid
522-FM Basis of Reimbursement Determination
523-FN
A
mount Attributed to Sales Tax
512-FC
A
ccumulated Deductible Amount
513-FD Remaining Deductible Amount
514-FE Remaining Benefit Amount
517-FH
A
mount Applied to Periodic Deductible
518-F1
A
mount of Copa
y
52Ø-FK
A
mount Exceeding Periodic Benefit Maximum
346-HH Basis of Calculation – Dispensing Fee
347-HJ Basis of Calculation – Copay
348-HK Basis of Calculation – Flat Sales Tax
349-HM Basis of Calculation – Percentage Sales Tax
571-NZ
A
mount Attributed to Processor Fee
575-EQ Patient Sales Tax Amount
574-2Y Plan Sales Tax Amount
572-4U
A
mount of Coinsurance
573-4V Basis of Calculation-Coinsurance
392-MU Benefit Stage Count
393-MV Benefit Stage Qualifier
394-MW Benefit Stage Amount
577-G3 Estimated Generic Savings
128-UC Spending Account Amount Remaining
129-UD Health Plan-Funded Assistance Amount
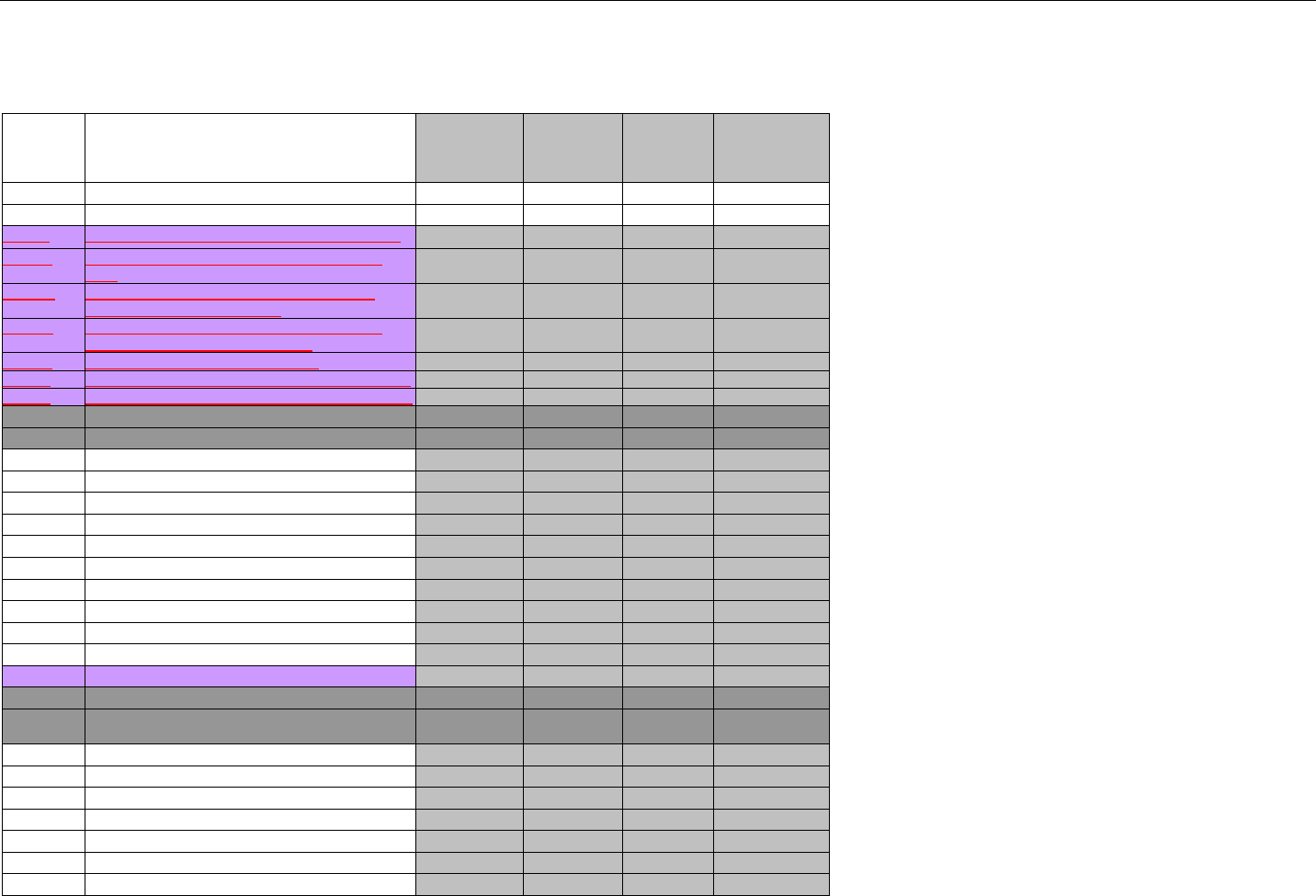
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 683 -
Controlled
Substance
Reporting
Reversal
Header Response Status Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Rejected Rejected
133-UJ
A
mount Attributed to Provider Network Selection
134-UK
A
mount Attributed to Product Selection/Brand
Drug
135-UM
A
mount Attributed to Product Selection/Non-
Preferred Formulary Selection
136-UN
A
mount Attributed to Product Selection/Brand
Non-Preferred Formulary Selection
137-UP
A
mount Attributed to Coverage Gap
148-U8 Ingredient Cost Contracted/Reimbursable Amount
149-U9 Dispensing Fee Contracted/Reimbursable Amount
RESPONSE DUR/PPS SEGMENT
111-AM Segment Identification
567-J6 DUR/PPS Response Code Counter
439-E4 Reason for Service Code
528-FS Clinical Significance Code
529-FT Other Pharmacy Indicator
531-FV Quantity of Previous Fill
53Ø-FU Previous Date of Fill
532-FW Database Indicator
533-FX Other Prescriber Indicator
544-FY DUR Free Text Message
57Ø-NS DUR Additional Text
RESPONSE PRIOR AUTHORIZATION
SEGMENT
111-AM Segment Identification
498-PR Prior Authorization Processed Date
498-PS Prior Authorization Effective Date
498-PT Prior Authorization Expiration Date
498-RA Prior Authorization Quantity
498-RB Prior Authorization Dollars Authorized
498-PW Prior Authorization Number of Refills Authorized
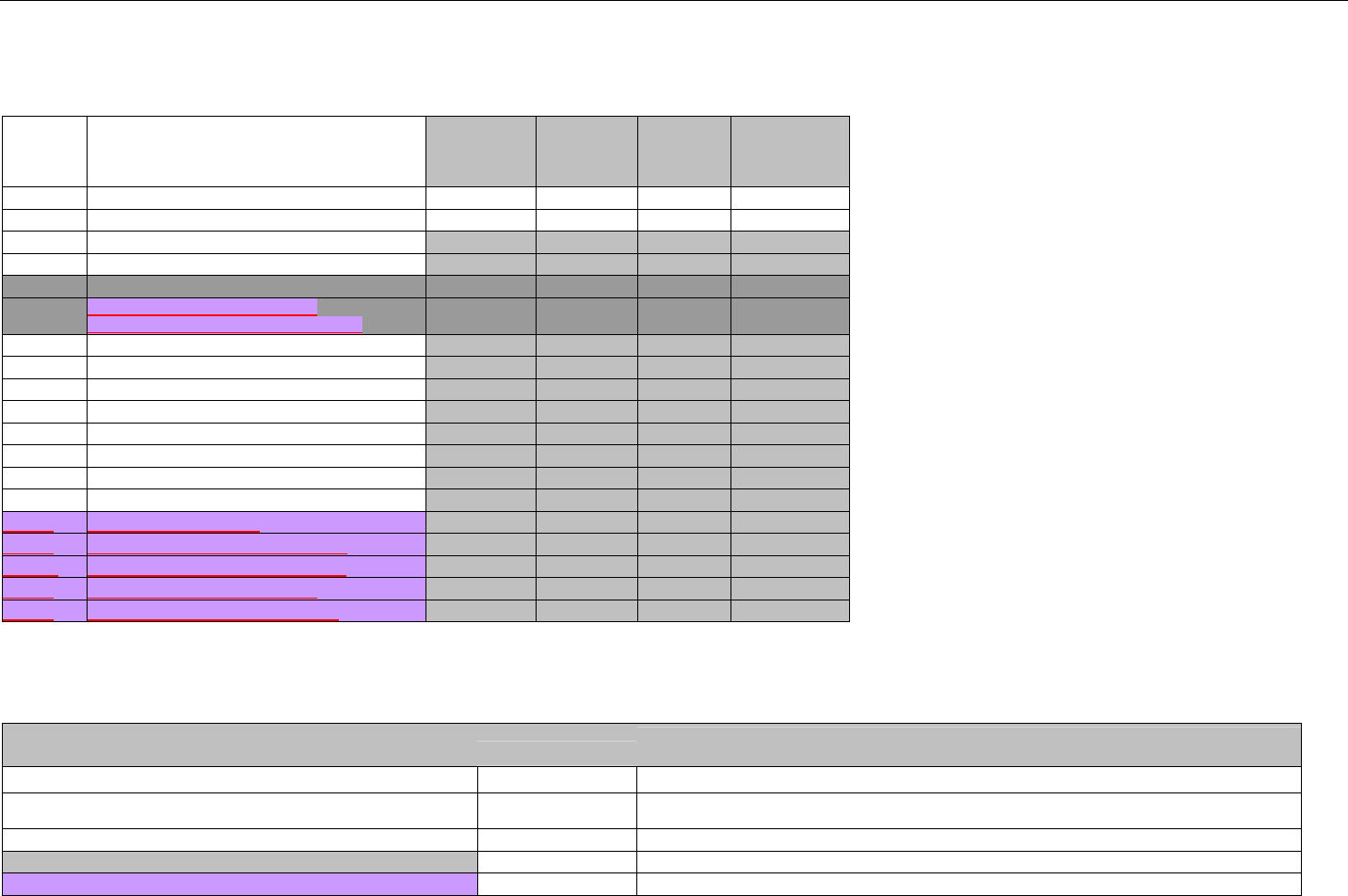
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 684 -
Controlled
Substance
Reporting
Reversal
Header Response Status Accepted Accepted Accepted Rejected
Transaction Response Status Approved Captured Rejected Rejected
498-PX Prior Authorization Quantity Accumulated
498-PY Prior Authorization Number - Assigned
RESPONSE COORDINATION OF
BENEFITS/OTHER PAYERS SEGMENT
111-AM Segment Identification
355-NT Other Payer ID Count
338-5C Other Payer Coverage Type
339-6C Other Payer ID Qualifier
34Ø-7C Other Payer ID
991-MH Other Payer Processor Control Number
356-NU Other Payer Cardholder ID
992-MJ Other Payer Group ID
142-UV Other Payer Person Code
127-UB Other Payer Help Desk Phone Number
143-UW Other Payer Patient Relationship Code
144-UX Other Payer Benefit Effective Date
145-UY Other Payer Benefit Termination Date
24.7 RESPONSE SEGMENT MATRICES BY SEGMENT – LEGEND
Submission and response requirements are shown for each segment as Mandatory (M), Situational (S), or Not Sent (N). Valid “values” are shown for each transaction type in the Header and Transaction
Response Status Fields (5Ø1-F1 and 112-AN).
LEGEND:
Categorization Explanation
M Mandatory
The Segment is Mandatory.
S Situational
The segment situations defined have qualifications for usage ("Required if x", "Not required if y") in
this Transaction.
N Not used
The segment is not used in this Transaction.
Row/Column Shaded The segment is not valid for this Transaction.
New Field/Segment Since 5.1
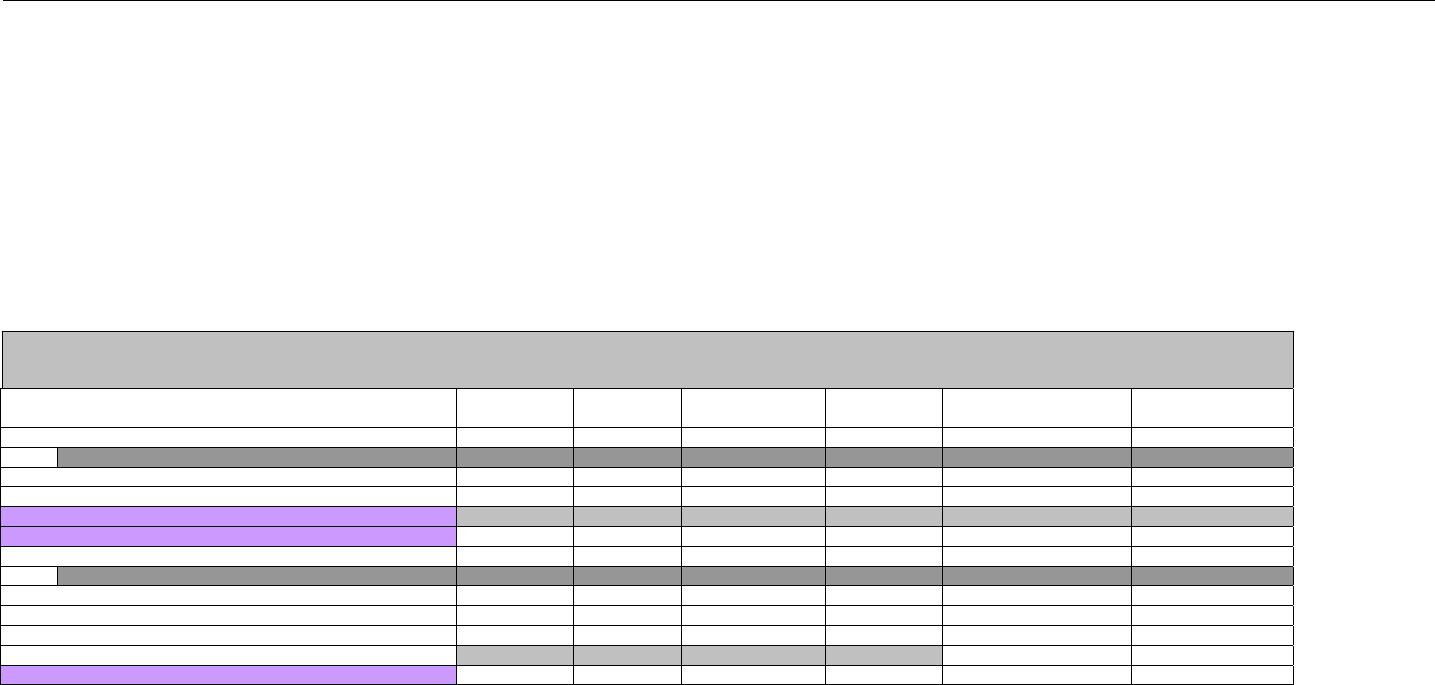
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 685 -
24.8 RESPONSE SEGMENT MATRICES BY SEGMENT
24.8.1 TRANSMISSION ACCEPTED; TRANSACTION PAID OR DUPLICATE OF PAID
Transmission
Header Response Header
Header Response Status (5Ø1-F1) = “A” Accepted
Transaction
Response Status
Transaction Response Status (112-AN) = “P” Paid or “D” Duplicate of Paid
The following transactions are supported in “P” Paid or “D” Duplicate of Paid Matrix:
VERSION D AND ABOVE TRANSMISSION ACCEPTED
TRANSACTION PAID OR DUPLICATE OF PAID
RESPONSE SEGMENT USAGE MATRIX
SEGMENT Billing (Claim)
or Encounter Rebill (Claim) Billing (Service) Rebill (Service) Prior Authorization
Request & Billing Prior Authorization
Inquiry
Response Header Segment M M M M M M
Header Response Status (5Ø1-F1) A A A A A A
Response Message Segment S S S S S S
Response Insurance Segment S S S S S S
Response Insurance Additional Information Segment N N N N N N
Response Patient Segment S S S S S S
Response Status Segment M M M M M M
Transaction Response Status (112-AN) P,D P P,D P P,D P,D
Response Claim Segment M M M M M M
Response Pricing Segment M M M M M M
Response DUR/PPS Segment S S S S S S
Response Prior Authorization Segment N N N N M M
Response Coordination of Benefits/Other Payers Segment S S S S S S
The following transactions do not support the “D” Duplicate of Paid response:
Rebill
Information Reporting Rebill
*Special Note:
Prior Authorization reversals are used to back out the request for authorization, but not any claims submitted against the prior authorization. To reverse a Prior Authorization Request and Billing, paid
billings must be reversed before the prior authorization is reversed. The pharmacy must submit a Claim or Service Reversal (Transaction Code = B2) before submitting a Prior Authorization Reversal
request. If there are no Claims or Services paid for the Prior Authorization in question, the processor must accept the Prior Authorization Reversal for the prior authorization only.
24.8.2 TRANSMISSION ACCEPTED; TRANSACTION BENEFIT MATRIX
Transmission
Header Response Header
Header Response Status (5Ø1-F1) = “A” Accepted
Transaction
Response Status
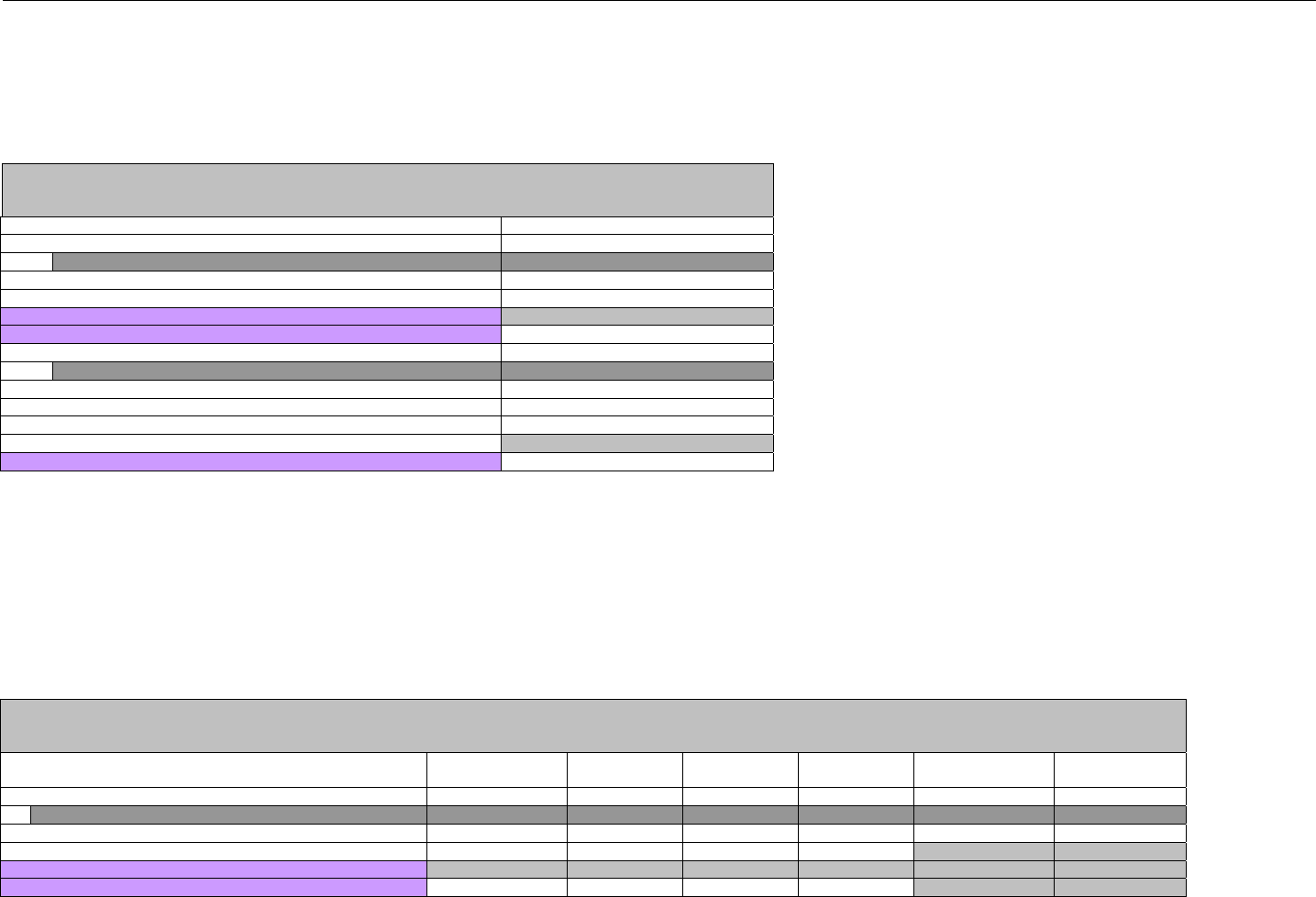
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 686 -
Transaction Response Status (112-AN) = “B” Benefit
The following transactions are supported in “B” Benefit Matrix:
VERSION D AND ABOVE TRANSMISSION ACCEPTED
TRANSACTION BENEFIT
RESPONSE SEGMENT USAGE MATRIX
SEGMENT Predetermination Of Benefits (Claim)
Response Header Segment M
Header Response Status (5Ø1-F1) A
Response Message Segment S
Response Insurance Segment S
Response Insurance Additional Information Segment N
Response Patient Segment S
Response Status Segment M
Transaction Response Status (112-AN) B
Response Claim Segment M
Response Pricing Segment M
Response DUR/PPS Segment S
Response Prior Authorization Segment N
Response Coordination of Benefits/Other Payers Segment S
The following transactions do not support the “D” Duplicate of Paid response:
Rebill
Information Reporting Rebill
24.8.3 TRANSMISSION ACCEPTED; TRANSACTION CAPTURED OR DUPLICATE OF CAPTURE MATRIX
Transmission
Response Header
Header Response Status (5Ø1-F1) = “A” Accepted
Transaction
Response Status
Transaction Response Status (112-AN) = “C” Captured or “Q” Duplicate of Captured
The following transactions are supported in “C” Captured or “Q” Duplicate of Captured Matrix:
VERSION D AND ABOVE TRANSMISSION ACCEPTED
TRANSACTION CAPTURED OR DUPLICATE OF CAPTURE
RESPONSE SEGMENT USAGE MATRIX
SEGMENT
Billing (Claim) or
Encounter Rebill (Claim) Billing (Service) Rebill (Service) Reversal (Claim) Reversal (Service)
Response Header Segment M M M M M M
Header Response Status (5Ø1-F1) A A A A A A
Response Message Segment S S S S S S
Response Insurance Segment S S S S N N
Response Insurance Additional Information Segment N N N N N N
Response Patient Segment S S S S N N
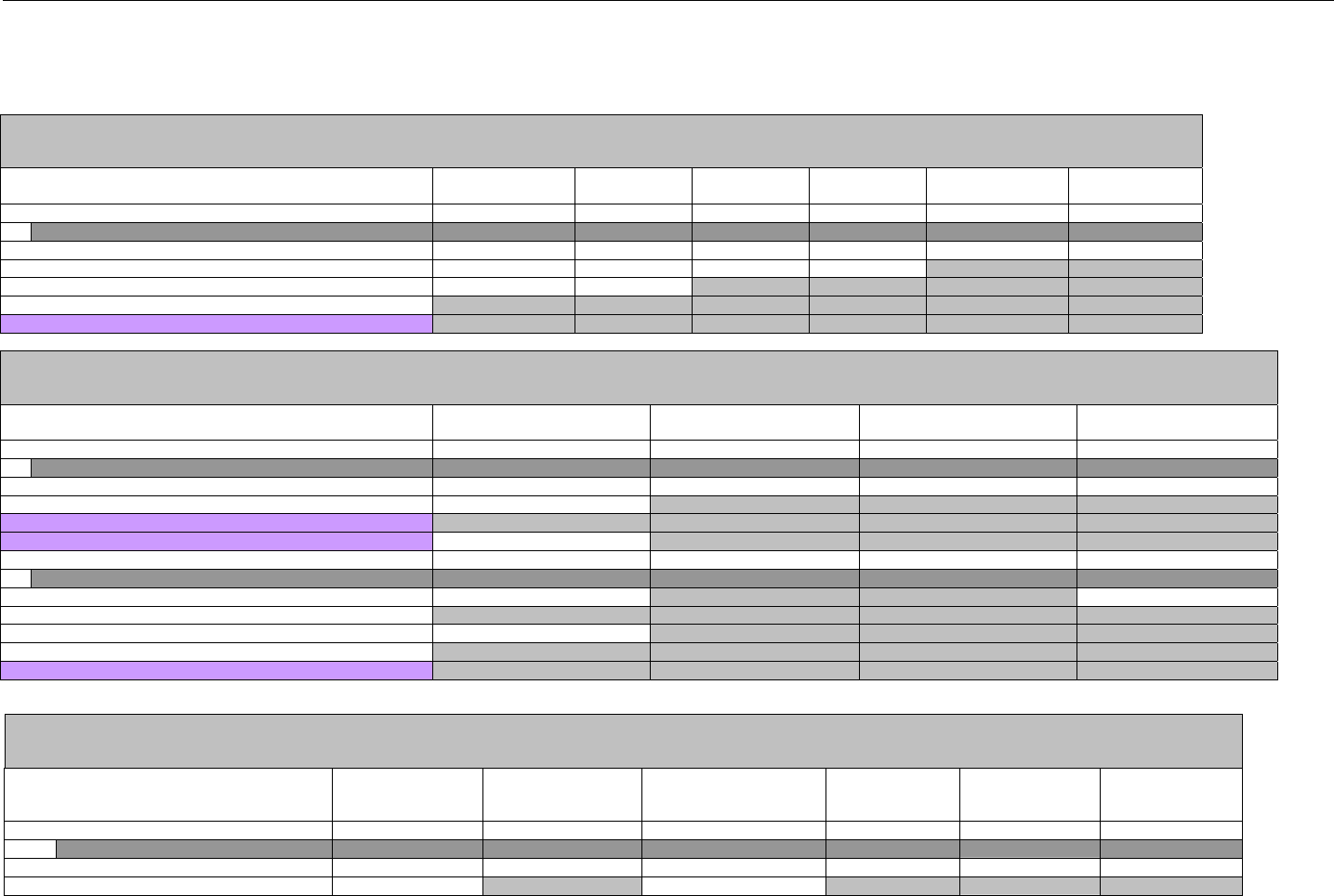
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 687 -
VERSION D AND ABOVE TRANSMISSION ACCEPTED
TRANSACTION CAPTURED OR DUPLICATE OF CAPTURE
RESPONSE SEGMENT USAGE MATRIX
SEGMENT
Billing (Claim) or
Encounter Rebill (Claim) Billing (Service) Rebill (Service) Reversal (Claim) Reversal (Service)
Response Status Segment M M M M M M
Transaction Response Status (112-AN) C,Q C C,Q C C,Q C,Q
Response Claim Segment M M M M M M
Response Pricing Segment S S S S N N
Response DUR/PPS Segment S S
N N N N
Response Prior Authorization Segment N N N N N N
Response Coordination of Benefits/Other Payers Segment N N N N N N
VERSION D AND ABOVE TRANSMISSION ACCEPTED
TRANSACTION CAPTURED OR DUPLICATE OF CAPTURE (Continued)
RESPONSE SEGMENT USAGE MATRIX
SEGMENT
Prior Authorization Request
And Billing (Claim/Service) Prior Authorization Reversal
(Claim/Service) Prior Authorization Inquiry
(Claim/Service) Prior Authorization Request
Only (Claim/Service)
Response Header Segment M M M M
Header Response Status (5Ø1-F1) A A A A
Response Message Segment S S S S
Response Insurance Segment S N N N
Response Insurance Additional Information Segment N N N N
Response Patient Segment S N N N
Response Status Segment M M M M
Transaction Response Status (112-AN) C,Q C,Q C,Q C,Q
Response Claim Segment M N N M
Response Pricing Segment N N N N
Response DUR/PPS Segment S N N N
Response Prior Authorization Segment N N N N
Response Coordination of Benefits/Other Payers Segment N N N N
VERSION D AND ABOVE TRANSMISSION ACCEPTED (Continued)
TRANSACTION CAPTURED OR DUPLICATE OF CAPTURE
RESPONSE SEGMENT USAGE MATRIX
SEGMENT
Information
Reporting
(Claim/Service)
Information Reporting
Reversal
(Claim/Service)
Information Reporting
Rebill (Claim/Service) Controlled
Substance
Reporting
Controlled
Substance
Reversal
Controlled
Substance Rebill
Response Header Segment M M M M M M
Header Response Status (5Ø1-F1) A A A A A A
Response Message Segment S S S O O O
Response Insurance Segment S N S N N N
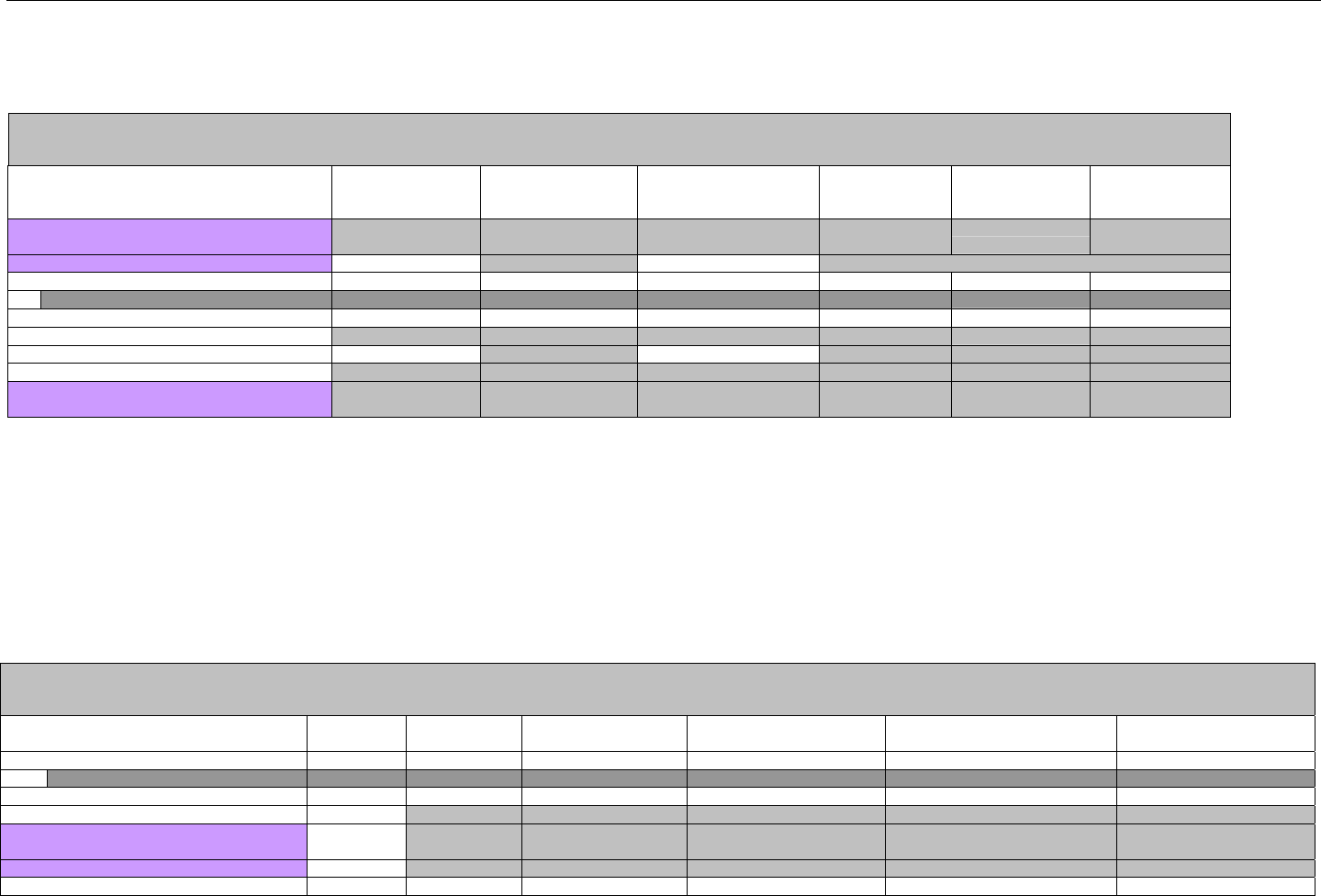
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 688 -
VERSION D AND ABOVE TRANSMISSION ACCEPTED (Continued)
TRANSACTION CAPTURED OR DUPLICATE OF CAPTURE
RESPONSE SEGMENT USAGE MATRIX
SEGMENT
Information
Reporting
(Claim/Service)
Information Reporting
Reversal
(Claim/Service)
Information Reporting
Rebill (Claim/Service) Controlled
Substance
Reporting
Controlled
Substance
Reversal
Controlled
Substance Rebill
Response Insurance Additional Information
Segment N N N N N N
Response Patient Segment S N S N N N
Response Status Segment M M M M M M
Transaction Response Status (112-AN) C,Q C,Q C C,Q C,Q C
Response Claim Segment M M M M M M
Response Pricing Segment N N N N N N
Response DUR/PPS Segment S N S N N N
Response Prior Authorization Segment N N N N N N
Response Coordination of Benefits/Other
Payers Segment N N N N N N
The following transactions do not support the “Q” Duplicate of Captured response:
Rebill
Information Reporting Rebill
Controlled Substance Reporting Rebill
24.8.4 TRANSMISSION ACCEPTED; TRANSACTION APPROVED OR DUPLICATE OF APPROVED MATRIX
Transmission
Response Header
Header Response Status (5Ø1-F1) = “A” Accepted
Transaction
Response Status
Transaction Response Status (112-AN) = “A” Approved, or “S” Duplicate of Approved
The following transactions are supported in “A” Approved, or “S” Duplicate of Approved Matrix:
VERSION D AND ABOVE TRANSMISSION ACCEPTED
TRANSACTION APPROVED OR DUPLICATE OF APPROVED
RESPONSE SEGMENT USAGE MATRIX
SEGMENT Eligibility Reversal (Claim) Reversal (Service) Prior Authorization Reversal
(Claim/Service) Prior Authorization Inquiry
(Claim/Service) Prior Authorization Request
Only (Claim/Service)
Response Header Segment M M M M M M
Header Response Status (5Ø1-F1) A A A A A A
Response Message Segment S S S S S S
Response Insurance Segment S N N N N N
Response Insurance Additional Information
Segment S N N N N N
Response Patient Segment S N N N N N
Response Status Segment M M M M M M
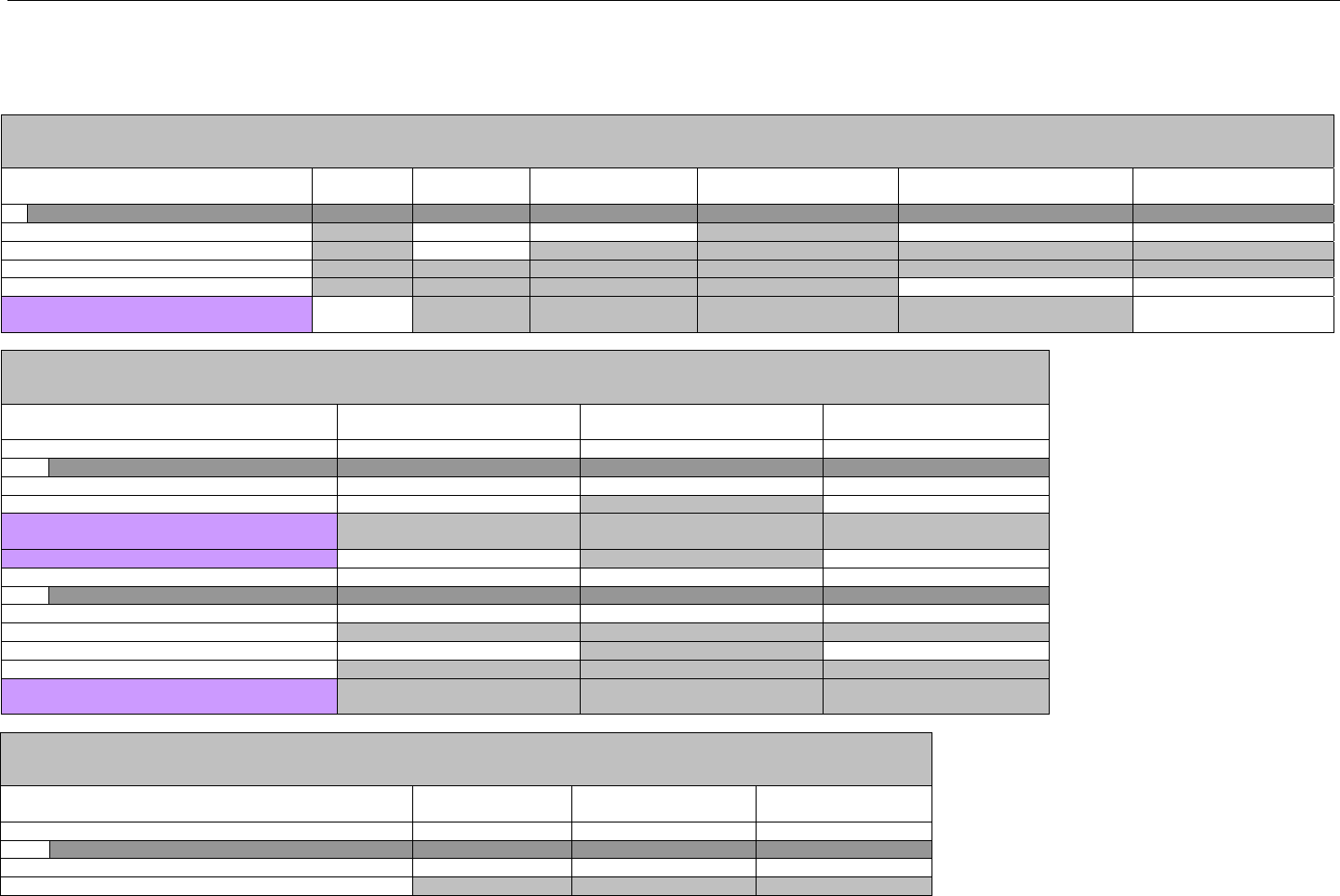
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 689 -
VERSION D AND ABOVE TRANSMISSION ACCEPTED
TRANSACTION APPROVED OR DUPLICATE OF APPROVED
RESPONSE SEGMENT USAGE MATRIX
SEGMENT Eligibility Reversal (Claim) Reversal (Service) Prior Authorization Reversal
(Claim/Service) Prior Authorization Inquiry
(Claim/Service) Prior Authorization Request
Only (Claim/Service)
Transaction Response Status (112-AN) A A,S A,S A,S A A,S
Response Claim Segment N M M N M M
Response Pricing Segment N S N N N N
Response DUR/PPS Segment N N N N N N
Response Prior Authorization Segment N N N N M M
Response Coordination of Benefits/Other
Payers Segment S N N N N S
VERSION D AND ABOVE TRANSMISSION ACCEPTED
TRANSACTION APPROVED OR DUPLICATE OF APPROVED (Continued)
RESPONSE SEGMENT USAGE MATRIX
SEGMENT Information Reporting
(Claim/Service) Information Reporting Reversal
(Claim/Service) Information Reporting Rebill
(Claim/Service)
Response Header Segment M M M
Header Response Status (5Ø1-F1) A A A
Response Message Segment S S S
Response Insurance Segment S N S
Response Insurance Additional Information
Segment N N N
Response Patient Segment S N S
Response Status Segment M M M
Transaction Response Status (112-AN) A,S A,S A
Response Claim Segment M M M
Response Pricing Segment N N N
Response DUR/PPS Segment S N S
Response Prior Authorization Segment N N N
Response Coordination of Benefits/Other
Payers Segment N N N
VERSION D AND ABOVE TRANSMISSION ACCEPTED (Continued)
TRANSACTION APPROVED OR DUPLICATE OF APPROVED
RESPONSE SEGMENT USAGE MATRIX
SEGMENT Controlled Substance
Reporting Controlled Substance
Reporting Reversal Controlled Substance
Reporting Rebill
Response Header Segment M M M
Header Response Status (5Ø1-F1) A A A
Response Message Segment O O O
Response Insurance Segment N N N
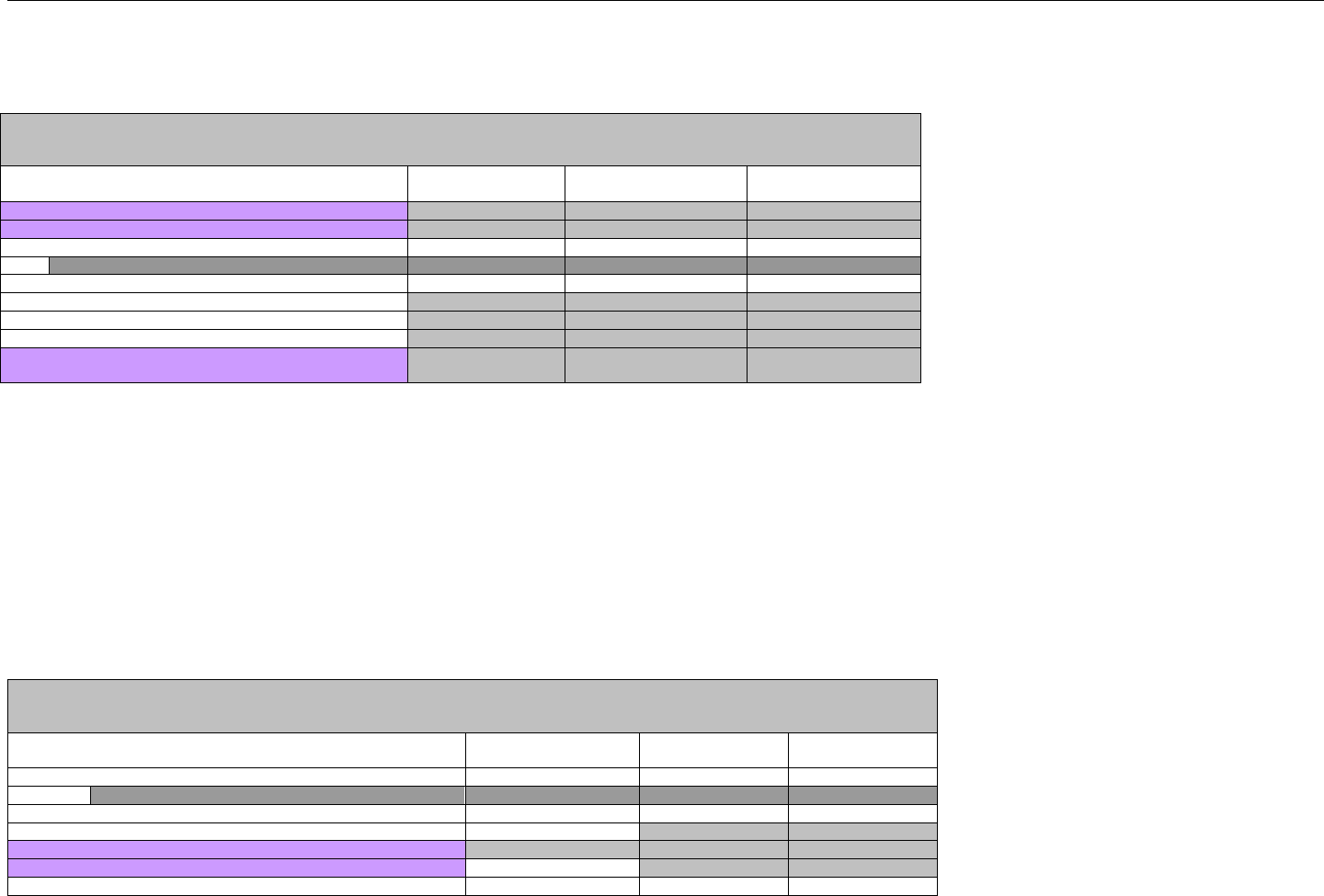
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 690 -
VERSION D AND ABOVE TRANSMISSION ACCEPTED (Continued)
TRANSACTION APPROVED OR DUPLICATE OF APPROVED
RESPONSE SEGMENT USAGE MATRIX
SEGMENT Controlled Substance
Reporting Controlled Substance
Reporting Reversal Controlled Substance
Reporting Rebill
Response Insurance Additional Information Segment N N N
Response Patient Segment N N N
Response Status Segment M M M
Transaction Response Status (112-AN) A,S A,S A
Response Claim Segment M M M
Response Pricing Segment N N N
Response DUR/PPS Segment N N N
Response Prior Authorization Segment N N N
Response Coordination of Benefits/Other Payers
Segment N N N
The following transactions do not support an “S” Duplicate of Approved response:
Eligibility
Prior Authorization Inquiry
Information Reporting Rebill
Controlled Substance Reporting Rebill
If an Eligibility or Prior Authorization Inquiry request is a duplicate, the Processor must return the original “A” Approved response a second time.
24.8.5 TRANSMISSION ACCEPTED; TRANSACTION DEFERRED MATRIX
Transmission
Response Header
Header Response Status (5Ø1-F1) = “A” Accepted
Transaction
Response Status
Transaction Response Status (112-AN) = “F” Deferred
The following transactions are supported in “F” Deferred Matrix:
VERSION D AND ABOVE TRANSMISSION ACCEPTED
TRANSACTION DEFERRED
RESPONSE SEGMENT USAGE MATRIX
SEGMENT Prior Authorization
Request & Billing Prior Authorization
Inquiry Prior Authorization
Request Only
Response Header Segment M M M
Header Response Status (5Ø1-F1) A A A
Response Message Segment S S S
Response Insurance Segment S N N
Response Insurance Additional Information Segment N N N
Response Patient Segment S N N
Response Status Segment M M M
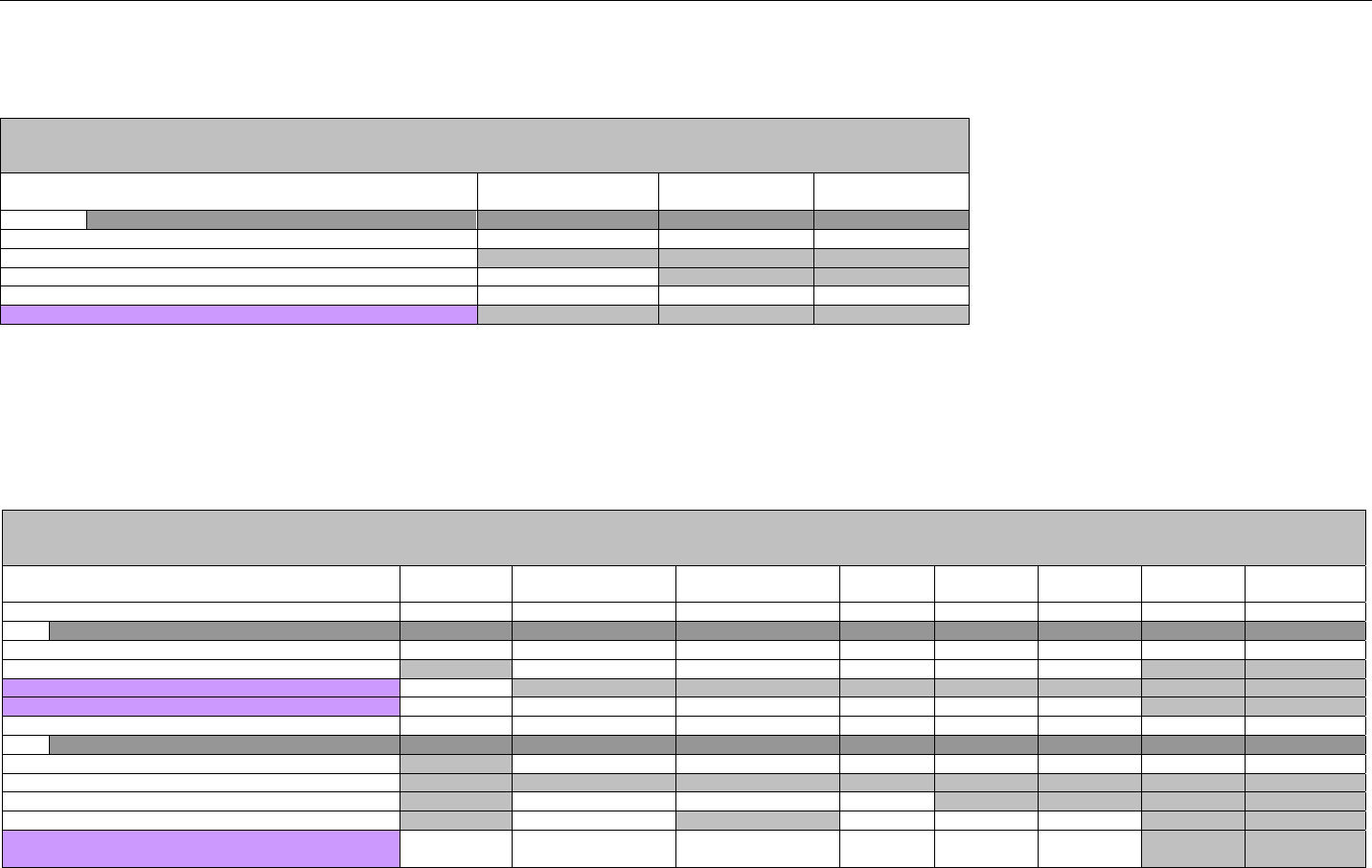
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 691 -
VERSION D AND ABOVE TRANSMISSION ACCEPTED
TRANSACTION DEFERRED
RESPONSE SEGMENT USAGE MATRIX
SEGMENT Prior Authorization
Request & Billing Prior Authorization
Inquiry Prior Authorization
Request Only
Transaction Response Status (112-AN) F F F
Response Claim Segment M M M
Response Pricing Segment N N N
Response DUR/PPS Segment S N N
Response Prior Authorization Segment S S S
Response Coordination of Benefits/Other Payers Segment N N N
24.8.6 TRANSMISSION ACCEPTED; TRANSACTION REJECTED MATRIX
Transmission
Response Header
Header Response Status (5Ø1-F1) = “A” Accepted
Transaction
Response Status
Transaction Response Status (112-AN) = “R” Rejected
The following transactions are supported in “A” Accepted/”R” Rejected Matrix:
VERSION D AND ABOVE TRANSMISSION ACCEPTED
TRANSACTION REJECTED
RESPONSE SEGMENT USAGE MATRIX
SEGMENT Eligibility Billing (Claim) or
Encounter Predetermination of
Benefits (Claim) Rebill
(Claim) Billing
(Service) Rebill
(Service) Reversal
(Claim) Reversal
(Service)
Response Header Segment M M M M M M M M
Header Response Status (5Ø1-F1) A A A A A A A A
Response Message Segment S S S S S S S S
Response Insurance Segment N S S S S S N N
Response Insurance Additional Information Segment S N N N N N N N
Response Patient Segment S S S S S S
N N
Response Status Segment M M M M M M M M
Transaction Response Status (112-AN)) R R R R R R R R
Response Claim Segment N M M M M M M M
Response Pricing Segment N N N N N N N N
Response DUR/PPS Segment N S S S N N N N
Response Prior Authorization Segment N S N S S S N N
Response Coordination of Benefits/Other Payers
Segment S S S S S S
N N

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 692 -
VERSION D AND ABOVE TRANSMISSION ACCEPTED (Continued)
TRANSACTION REJECTED
RESPONSE SEGMENT USAGE MATRIX
SEGMENT Prior Authorization
Request & Billing Prior Authorization
Reversal Prior Authorization
Inquiry Prior Authorization
Request Only Information
Reporting Information
Reporting Reversal Information
Reporting Rebill
Response Header Segment M M M M M M M
Header Response Status (5Ø1-F1) A A A A A A A
Response Message Segment S S S S S S S
Response Insurance Segment S N N N S N S
Response Insurance Additional Information Segment N N N N N N N
Response Patient Segment S N N N S N S
Response Status Segment M M M M M M M
Transaction Response Status (112-AN)) R R R R R R R
Response Claim Segment M N M M M M M
Response Pricing Segment N N N N N N N
Response DUR/PPS Segment S N N N N N N
Response Prior Authorization Segment N N N N N N N
Response Coordination of Benefits/Other Payers
Segment S N S S N N N
VERSION D AND ABOVE TRANSMISSION ACCEPTED (Continued)
TRANSACTION REJECTED
RESPONSE SEGMENT USAGE MATRIX
SEGMENT Controlled Substance Reporting Controlled Substance
Reporting Reversal Controlled Substance
Reporting Rebill
Response Header Segment M M M
Header Response Status (5Ø1-F1) A A A
Response Message Segment O O O
Response Insurance Segment N N N
Response Insurance Additional Information Segment N N N
Response Patient Segment N N N
Response Status Segment M M M
Transaction Response Status (112-AN)) R R R
Response Claim Segment M M M
Response Pricing Segment N N N
Response DUR/PPS Segment N N N
Response Prior Authorization Segment N N N
Response Coordination of Benefits/Other Payers
Segment N N N
24.8.7 TRANSMISSION REJECTED; TRANSACTION REJECTED MATRIX
Transmission

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 693 -
Response Header
Header Response Status (5Ø1-F1) = “R” Rejected
Transaction
Response Status
Transaction Response Status (112-AN) = “R” Rejected
The following transactions are supported in “R” Rejected/”R” Rejected Matrix:
VERSION D AND ABOVE TRANSMISSION REJECTED
TRANSACTION REJECTED
RESPONSE SEGMENT USAGE MATRIX
SEGMENT Eligibility Billing (Claim)
or Encounter Predetermination of
Benefits (Claim) Rebill (Claim) Billing (Service) Rebill (Service) Reversal (Claim) Reversal (Service)
Response Header Segment M M M M M M M M
Header Response Status (5Ø1-F1) R R R R R R R R
Response Message Segment S S S S S S S S
Response Insurance Segment N N N N N N N N
Response Insurance Additional Information
Segment N N N N N N N N
Response Patient Segment N N N N N N N N
Response Status Segment M M M M M M M M
Transaction Response Status (112-AN) R R R R R R R R
Response Claim Segment N N N N N N N N
Response Pricing Segment N N N N N N N N
Response DUR/PPS Segment N N N N N N N N
Response Prior Authorization Segment N N N N N N N N
Response Coordination of Benefits/Other
Payers Segment N N N N N N N N
VERSION D AND ABOVE TRANSMISSION REJECTED (Continued)
TRANSACTION REJECTED
RESPONSE SEGMENT USAGE MATRIX
SEGMENT Prior Authorization
Request & Billing Prior Authorization
Reversal Prior Authorization
Inquiry Prior Authorization
Request Only Information
Reporting Information
Reporting Reversal Information
Reporting Rebill
Response Header Segment M M M M M M M
Header Response Status (5Ø1-F1) R R R R R R R
Response Message Segment S S S S S S S
Response Insurance Segment N N N N N N N
Response Insurance Additional Information
Segment N N N N N N N
Response Patient Segment N N N N N N N
Response Status Segment M M M M M M M
Transaction Response Status (112-AN) R R R R R R R
Response Claim Segment N N N N N N N
Response Pricing Segment N N N N N N N
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 694 -
VERSION D AND ABOVE TRANSMISSION REJECTED (Continued)
TRANSACTION REJECTED
RESPONSE SEGMENT USAGE MATRIX
SEGMENT Prior Authorization
Request & Billing Prior Authorization
Reversal Prior Authorization
Inquiry Prior Authorization
Request Only Information
Reporting Information
Reporting Reversal Information
Reporting Rebill
Response DUR/PPS Segment N N N N N N N
Response Prior Authorization Segment N N N N N N N
Response Coordination of Benefits/Other
Payers Segment N N N N N N N
VERSION D AND ABOVE TRANSMISSION REJECTED (Continued)
TRANSACTION REJECTED
RESPONSE SEGMENT USAGE MATRIX
SEGMENT Controlled Substance
Reporting Controlled Substance
Reporting Reversal Controlled Substance
Reporting Rebill
Response Header Segment M M M
Header Response Status (5Ø1-F1) R R R
Response Message Segment O O O
Response Insurance Segment N N N
Response Insurance Additional Information Segment N N N
Response Patient Segment N N N
Response Status Segment M M M
Transaction Response Status (112-AN) R R R
Response Claim Segment N N N
Response Pricing Segment N N N
Response DUR/PPS Segment N N N
Response Prior Authorization Segment N N N
Response Coordination of Benefits/Other Payers
Segment N N N
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 695 -
25. RESPONSE OVERVIEW
25.1 RESPONSE STATUS BY TRANSACTION TYPE
For multiple transactions within a transmission, the Response Status segment is repeated for each transaction. An “Acceptable” transmission
response may contain paid, captured, approved, and rejected status codes for multiple transactions.
If all transactions are rejected then each transaction must contain status codes that have values. A status code response must be transmitted
for all submitted transactions whether approved, rejected for unacceptable header information, or rejected for unacceptable transaction
information.
If the status code indicates the header data is unacceptable, all detail items submitted are in error and the reject codes that are applicable are
present in the first transaction reject code list in addition to any reject codes that are specific to the first transaction. Any reject codes that are
applicable are present in the second and subsequent transaction, along with reject codes that are specific to the second or subsequent
transaction.
The following is a high level summary. Please refer to section “Transmission Structure”, “Response Segment Matrices By Segment”.
Response Status
Transaction Type Response Header
Segment - Header
Response Status
Response Status
Segment - Transaction
Response Status
Comment
Eligibility Verification A A Transmission Accepted.
Transaction Approved.
Duplicate approved eligibility must be
responded to with an “A”.
A R Transmission Accepted.
Transaction Rejected.
R R Transmission Rejected.
Transaction Rejected.
Claim Billing or Encounter,
Service Billing
A C, Q Transmission Accepted.
Transaction Captured, or Duplicate of
Captured.
A P, D Transmission Accepted.
Transaction Paid, or Duplicate of Paid.
A R Transmission Accepted.
Transaction Rejected.
R R Transmission Rejected.
Transaction Rejected.
Predetermination Of Benefits A B Transmission Accepted. Transaction
Benefit.
A R Transmission Accepted. Transaction
Rejected.
R R Transmission Rejected. Transaction
Rejected.
Claim or Service Reversal A A, S Transmission Accepted.
Transaction Approved, or Duplicate of
Approved.
A C, Q Transmission Accepted.
Transaction Captured, or Duplicate of
Captured.
A R Transmission Accepted.
Transaction Rejected.
R R Transmission Rejected.
Transaction Rejected.
Claim or Service Rebill A C Transmission Accepted.
Transaction Captured.
A P Transmission Accepted.
Transaction Paid.
A R Transmission Accepted.
Transaction Rejected.
R R Transmission Rejected.
Transaction Rejected.
Prior Authorization Request and
Billing (Claim/Service)
A C, Q Transmission Accepted.
Transaction Captured, or Duplicate of
Captured.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 696 -
Response Status
Transaction Type Response Header
Segment - Header
Response Status
Response Status
Segment - Transaction
Response Status
Comment
A P, D Transmission Accepted.
Transaction Paid, or Duplicate of Paid.
A F Transmission Accepted.
Transaction Deferred.
Duplicate Deferred Prior Authorization
Request and Billing must be responded to
with an “F”.
A R Transmission Accepted.
Transaction Rejected.
R R Transmission Rejected.
Transaction Rejected.
Prior Authorization Reversal A A, S Transmission Accepted.
Transaction Approved, or Duplicate of
Approved.
A C, Q Transmission Accepted.
Transaction Captured, or Duplicate of
Captured.
A R Transmission Accepted.
Transaction Rejected.
R R Transmission Rejected.
Transaction Rejected.
Prior Authorization Inquiry
(Claim/Service)
A A Transmission Accepted.
Transaction Approved. Duplicate approved
Prior Authorization Inquiry must be
responded to with an “A”.
A C, Q Transmission Accepted.
Transaction Captured, or Duplicate of
Captured.
A F Transmission Accepted.
Transaction Deferred.
Duplicate Prior Authorization Inquiry
deferred must be responded to with an “F”.
A P, D Transmission Accepted.
Transaction Paid, or Duplicate of Paid.
A R Transmission Accepted.
Transaction Rejected.
R R Transmission Rejected.
Transaction Rejected.
Prior Authorization Request Only
(Claim/Service)
A A, S Transmission Accepted.
Transaction Approved, or Duplicate of
Approved.
A C, Q Transmission Accepted.
Transaction Captured, or Duplicate of
Captured.
A F Transmission Accepted.
Transaction Deferred.
Duplicate Prior Authorization Request Only
deferred must be responded to with “F”.
A R Transmission Accepted.
Transaction Rejected.
R R Transmission Rejected.
Transaction Rejected.
Information Reporting
(Claim/Service)
A A, S Transmission Accepted.
Transaction Approved, or Duplicate of
Approved.
A C, Q Transmission Accepted.
Transaction Captured, or Duplicate of
Captured.
A P, D Transmission Accepted.
Transaction Paid, or Duplicate of Paid.
Not valid for Medicare Part D.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 697 -
Response Status
Transaction Type Response Header
Segment - Header
Response Status
Response Status
Segment - Transaction
Response Status
Comment
A R Transmission Accepted.
Transaction Rejected.
R R Transmission Rejected.
Transaction Rejected.
Information Reporting Reversal
(Claim/Service)
A A, S Transmission Accepted.
Transaction Approved, or Duplicate of
Approved.
A C, Q Transmission Accepted.
Transaction Captured, or Duplicate of
Captured.
A R Transmission Accepted.
Transaction Rejected.
R R Transmission Rejected.
Transaction Rejected.
Information Reporting Rebill
(Claim/Service)
A A Transmission Accepted.
Transaction Approved.
A C Transmission Accepted.
Transaction Captured.
A P Transmission Accepted.
Transaction Paid.
Not valid for Medicare Part D.
A R Transmission Accepted.
Transaction Rejected.
R R Transmission Rejected.
Transaction Rejected.
Controlled Substance Reporting A A, S Transmission Accepted.
Transaction Approved, or Duplicate of
Approved.
A C, Q Transmission Accepted.
Transaction Captured, or Duplicate of
Captured.
A R Transmission Accepted.
Transaction Rejected.
R R Transmission Rejected.
Transaction Rejected.
Controlled Substance Reporting
Reversal
A A, S Transmission Accepted.
Transaction Approved, or Duplicate of
Approved.
A C, Q Transmission Accepted.
Transaction Captured, or Duplicate of
Captured.
A R Transmission Accepted.
Transaction Rejected.
R R Transmission Rejected.
Transaction Rejected.
Controlled Substance Rebill A A Transmission Accepted.
Transaction Approved.
A C Transmission Accepted.
Transaction Captured.
A R Transmission Accepted.
Transaction Rejected.
R R Transmission Rejected.
Transaction Rejected.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 698 -
26. RESPONSE PROCESSING GUIDELINES
As with all transmissions, the number of response transactions must match the number of request transactions. The processor/PBM must
respond with the appropriate Transaction Response Status codes for the Transaction Count. For example if 3 reversal transactions are within
a transmission (Transaction Count = 3), the processor/PBM must respond with a Transaction Count = 3 with three transaction responses, one
for each reversal. There is one exception - when the transmission is rejected at the header level due to errors in invalid Version/Release
Number (1Ø2-A2) or Transaction Count (1Ø9-A9) - only one response must be returned.
The Response Status Segment will have response information to match up to each transaction in the request. Each transaction response will
contain its own Transaction Response Status and therefore, each transaction may receive a different response. For example, Transaction one
might be paid, Transaction two might be rejected, or Transaction one might be rejected, Transaction two might be captured, et cetera.
26.1 TRANSACTION RESPONSE STATUS (112-AN)
26.1.1 APPROVED
An Approved response is returned in Eligibility transactions when the patient or cardholder is eligible. In other transactions, the Approved
response is returned when the processor or reporting entity acknowledges and processes the request.
26.1.2 REJECT
Note: For syntax errors, the Reject Code (511-FB) of “R8 “ must be used whenever a specific reject code is not designated. Specific reject
codes must be returned whenever possible to assist in understanding the rejection.
26.1.3 DEFERRED
Final determination of the Prior Authorization request cannot be made until additional medical information is obtained. The message (5Ø4-F4)
and/or Additional Message Information (526-FQ) will contain what additional information is needed. Each processor governs the submission
of additional information and the pharmacy should consult the appropriate provider billing manual. Typically, if the additional information is not
received within a specific timeframe, the prior authorization will be denied.
26.1.4 BENEFIT
A Benefit response is returned to the provider when the Processor processes the claim, and returns a snapshot of the patient’s responsibility
at this point in time. See section “Predetermination Of Benefits Information”.
The Predetermination Of Benefits transaction is used on claim submission only. It is not valid for a service submission.
The component fields of Patient Pay Amount (5Ø5-F5) are returned in the Response Pricing Segment and the Patient Pay Amount Formula
must be adhered to. See section “Specific Segment Discussion”, “Response Segments”, “Patient Pay Amount (5Ø5-F5) Formula”. Of note, the
Total Amount Paid (5Ø9-F9) is not used in this transaction response. There is no need for a duplicate response due to the nature of the
predetermination of benefits transaction. Each submission of the transaction is processed with the response reflective of current information.
26.1.5 CAPTURED
A captured response is employed when the processor does not require on-line payment information. It is also used when information
transactions are sent and require nothing more than acknowledgment of their receipt at the processor or endpoint.
If a transaction has already been captured, but the response was not received by the submitter, upon receipt of a resubmitted transaction the
processor must return a duplicate response containing the original response information. See section “Transmission Structure” to determine
where duplicate responses apply.
26.1.5.1 BUSINESS FUNCTION OF CAPTURE
26.1.5.1.1 VALID USES
In Claim/Service Billing, a “C” (Capture) response is supported. The business of capture is to be used for:
1. Intermediary Services Two valid Intermediary services are:
1. Provider/Intermediary agreements to provide services such as additional editing, pricing, billing, and payment
reconciliation.
2. Payer/Intermediary agreements to provide some level of editing, pricing, and patient financial responsibility calculation,
with the ultimate payer having the option to perform additional edits.
2. Replacement of manual billing
The usage of this type of Capture should be used with caution, due to issues of:
• Inability for provider to be able to accurately determine patient financial responsibility for reasons of:
• Most plans today expect patient to pay some portion of product cost.
• Many plans vary patient financial responsibility based on brand/generic, formulary/non-formulary, etc.
• Drug Databases do not categorize drugs the same way.
• Some drugs/patients are excluded from patient financial responsibility.
• Coordinated Pro-DUR
This business function must take place within a “P” (Paid) or “R” (Rejected) response, however it may be allowed on a “C”
Capture used to replace manual billing when regulated for governmental agencies.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 699 -
If the Transaction Response Status (112-AN) = C (Captured) or Q (Duplicate of Captured), dollar fields should be supplied in the response.
• If the response is a “true” Capture (i.e. replacement of batch billing, with no edits or pricing), then corresponding response fields
should be populated with values as submitted. Ideally, processor will provide “real” copay or coinsurance values on a Capture. If
this is not possible, provider must know (by trading partner agreement) the copays or coinsurance to charge and factor that into
their system so collection occurs.
• If the response is captured by an Intermediary who provides better pricing criteria, the corresponding response fields are populated
with the probable values and those values used to determine estimated pricing as noted above. Since the claim has not been fully
adjudicated, this remains a capture response.
• When processor is doing maintenance, claims must be rejected. The recommendation is to use Reject Code (511-FB) = 96 -
Scheduled Downtime however; other 9x codes could be used if the maintenance was not scheduled. The reject code lets the
provider know to reprocess the claim at a later time.
To determine patient financial responsibility, providers can attempt to edit and calculate patient financial responsibility to submit this on the
original claim in the field Patient Paid Amount Submitted (433-DX), however,
• The resulting patient financial responsibility may be incorrect.
• This could be considered fraudulent if patient is overcharged.
Therefore, to support replacement of manual billing, the processor should
• Determine the patient financial responsibility and return it as a valid Patient Pay Amount (5Ø5-F5).
• Then calculate Total Amount Paid (5Ø9-F9) using the submitted fields and the determined patient financial responsibility amount.
• Since this result comes with a “C” (captured) response indication, provider should recognize that further editing and re-calculation
may occur which may result in different actual amounts reimbursed.
For example:
Ingredient Cost Submitted (4Ø9-D9) 35.ØØ Ingredient Cost Paid (5Ø6-F6) 35.ØØ
Dispensing Fee Submitted (412-DC) 3.ØØ Dispensing Fee Paid (5Ø7-F7) 3.ØØ
Incentive Amount Submitted (438-E3) 1.ØØ Incentive Amount Paid (521-FL) 1.ØØ
Flat Sales Tax Amount Submitted (481-HA) .25 Flat Sales Tax Amount Paid (558-AW) .25
Percentage Sales Tax Amount Submitted
(482-GE)
.75 Percentage Sales Tax Amount Paid (559-
AX)
.75
Other Amount Claimed Submitted (48Ø-H9) 1.ØØ Other Amount Paid (565-J4) 1.ØØ
Patient Pay Amount (5Ø5-F5) 1Ø.ØØ
Patient Sales Tax Amount (575-EQ) .5Ø
Plan Sales Tax Amount (574-2Y) .5Ø
Gross Amount Due (43Ø-DU) 41.ØØ Total Amount Paid (5Ø9-F9) 31.ØØ
Amount Attributed to Copay (518-FI) 1Ø.ØØ
In this example, Patient Pay Amount (5Ø5-F5) is “real” and Total Amount Paid (5Ø9-F9) is calculated using submitted fields and
“real” patient financial responsibility. The other amount fields contain the submitted value.
26.1.5.1.2 CAPTURE CONSISTENCY
The use of a “C” (Capture) response should be consistent within a BIN Number (1Ø1-A1)/Processor Control Number (1Ø2-A2) combination.
All claims at all times for this BIN/PCN combination should be handled the same way. If the processor would normally “P” (Paid) or “R”
(Rejected) this claim were it submitted at a different time, a Capture Response must not be used. With this consistency, providers should be
able to know by trading partner agreement when returned dollar amounts are parroted versus when they are estimated dollar amounts.
Rule of Thumb:
Submitted dollar amounts = Response Captured dollar amounts
Assume parroted values from submission returned
Submitted dollar amounts not = Response Captured dollar amounts
Assume estimated values returned
26.1.5.2 REVERSALS AND CAPTURE
If a processor routinely captures claims for products and/or services online, they must also support reversals of those claims online.
It is a recommended business practice that multiple claim or service reversal transactions in a transmission must be for the same patient.
The structure does support multiple claim or service reversals for the same processor/PBM, for the same pharmacy, for the same Date of
Service, but for multiple patients. However, it is recommended that a transmission containing multiple reversals for multiple patients
not be supported. Even though the structure supports reversals for multiple patients, the recommendation is that this not be supported.
The Reject Code (511-FB) value “RV“ (Multiple Reversals Per Transmission Not Supported) can be used for Claim/Service Billing Reversals,
Rebill transmissions, Controlled Substance Reporting Reversals, and Information Reporting Reversals if the processor does not support
multiple reversal transactions within a transmission.
The response of an approved Reversal must be supported in order to adjust actual payment and/or utilization data via remittance processing.
It is noted that the captured response is not supported in some transactions that support reversals.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 700 -
26.1.5.3 BUSINESS FUNCTIONS NOT SUPPORTED FOR CAPTURE
The following business functions for Capture are not supported:
• 1ØØ% patient financial responsibility – This is technically a payment and a “P” (Paid) response is to be returned.
• Maintenance Windows – this is a Reject. Suggest use of Reject Code (511-FB) = 96 – Scheduled Downtime; however any 9x error
code would supply provider with information to reprocess claim later.
• Coordinated Pro-DUR – this business function should take place within a “P” (Paid) or “R”(Rejected) response. See note above.
• Product Ordering – this is not a function of a Claim or Service Billing.
• Coupon processing – this business function should take place within a “P” (Paid) response. Until the payment information is returned
on the coupon, the sender is unable to determine the final charge for the product.
• Coordination of Benefits processing cannot proceed without final determination. Provider is unable to send appropriate claim to next
payer.
• A Capture response does not contain rejection information. The Reject Code and Count fields, which are specifically for reject
situations, are to be used when the Transaction Response Status = “R” (Rejected). These fields must not be returned for values
other than “R”.
26.1.6 PAID
A Paid response is returned to the provider when all telecommunication and plan requirements have been met. The transmission is accepted,
the transaction is accepted and the billing is in compliance with plan parameters.
26.2 PRICING GUIDELINES (CLAIM/SERVICE)
26.2.1 DEFINITIONS
These terms are used throughout the NCPDP documentation and defined as follows:
• Copay/Amount of Copay - “Amount of Copay” is defined as “Amount to be collected from the patient that is included in “Patient Pay
Amount” (5Ø5-F5) that is due to a per prescription copay.” “Copay” is a “form of cost sharing that holds the patient responsible for a
fixed dollar amount for each product/service received and regardless of the patient’s current benefit status, product selection or
network selection.
• Coinsurance/Amount of Coinsurance - “Amount of Coinsurance” (Amount to be collected from the patient that is included in
“Patient Pay Amount” (5Ø5-F5) that is due to a per prescription coinsurance. “Coinsurance” is a “form of cost sharing that holds the
patient responsible for a dollar amount based on a percentage for each product/service received and regardless of the patient’s
current benefit status, product selection or network selection.
• Patient Financial Responsibility – Patient Financial Responsibility refers to the amount of money a provider is to collect from a
patient or their representative for providing a product/service. Patient Financial Responsibility is alternatively known as the patient’s
“out-of-pocket expense or patient pay amount” and can include such components as Copay and Coinsurance.
26.2.2 OTHER PRICING INFORMATION
• The fields containing the values used to arrive at the final reimbursement must be detailed on the response record.
• If claim/service submission included the field with a value not equal to zero, then the corresponding response field must be returned
- even if the response value for that field = zeros.
The following fields are mandatory on all payment responses:
• Patient Pay Amount (5Ø5-F5)
• Total Amount Paid (5Ø9-F9)
It is the sum of these two fields that determines final provider reimbursement. With both fields present (even when zero) there is no ambiguity
regarding the final payment amount of the claim/service.
26.2.3 CLAIM
26.2.3.1 CORRESPONDING PRICING FIELDS (CLAIM)
This includes Claim Billing, Claim Rebill, and Prior Authorization Request And Billing (Claim).
Request Pricing Fields Corresponding Response Pricing Fields
4Ø9-D9 INGREDIENT COST SUBMITTED 5Ø6-F6 INGREDIENT COST PAID
423-DN BASIS OF COST DETERMINATION 522-FM BASIS OF REIMBURSEMENT DETERMINATION
412-DC DISPENSING FEE SUBMITTED 5Ø7-F7 DISPENSING FEE PAID
433-DX PATIENT PAID AMOUNT SUBMITTED Not applicable
438-E3 INCENTIVE AMOUNT SUBMITTED 521-FL INCENTIVE AMOUNT PAID
478-H7 OTHER AMOUNT CLAIMED
SUBMITTED COUNT
563-J2 OTHER AMOUNT PAID COUNT
479-H8 OTHER AMOUNT CLAIMED
SUBMITTED QUALIFIER
564-J3 OTHER AMOUNT PAID QUALIFIER
48Ø-H9 OTHER AMOUNT CLAIMED
SUBMITTED
565-J4 OTHER AMOUNT PAID
481-HA FLAT SALES TAX AMOUNT
SUBMITTED
558-AW FLAT SALES TAX AMOUNT PAID
482-GE PERCENTAGE SALES TAX AMOUNT
SUBMITTED
559-AX PERCENTAGE SALES TAX AMOUNT PAID
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 701 -
426-DQ USUAL AND CUSTOMARY CHARGE Not applicable
43Ø-DU GROSS AMOUNT DUE 5Ø9-F9 TOTAL AMOUNT PAID
5Ø5-F5 PATIENT PAY AMOUNT
Fields that are part of Patient Pay Amount:
523-FN AMOUNT ATTRIBUTED TO SALES TAX
518-FI AMOUNT OF COPAY
572-4U AMOUNT OF COINSURANCE
517-FH AMOUNT APPLIED TO PERIODIC
DEDUCTIBLE
52Ø-FK AMOUNT EXCEEDING PERIODIC
BENEFIT MAXIMUM
571-NZ AMOUNT ATTRIBUTED TO
PROCESSOR FEE
134-UK AMOUNT ATTRIBUTED TO PRODUCT
SELECTION/BRAND DRUG
135-UM AMOUNT ATTRIBUTED TO PRODUCT
SELECTION/NON-PREFERRED
FORMULARY SELECTION
136-UN AMOUNT ATTRIBUTED TO PRODUCT
SELECTION/BRAND NON-PREFERRED
FORMULARY SELECTION
26.2.4 PATIENT FINANCIAL RESPONSIBILITY (CLAIM)
When the patient is expected to pay 1ØØ% of processor determined amount as total claim reimbursement, the response must contain:
Patient Pay Amount (5Ø5-F5) plus any of the applicable Patient Responsibility fields included in this amount:
• Amount Applied To Periodic Deductible (517-FH)
• Amount Exceeding Periodic Benefit Maximum (52Ø-FK)
• Amount Of Copay (518-FI)
• Amount of Coinsurance (572-4U)
• Amount Attributed to Processor Fee (571-NZ)
• Amount Attributed To Sales Tax (523-FN)
• Amount Attributed to Provider Network Selection (133-UJ)
• Amount Attributed to Product Selection/Brand Drug (134-UK)
• Amount Attributed to Product Selection/Non-Preferred Formulary Selection (135-UM)
• Amount Attributed to Product Selection/Brand Non-Preferred Formulary Selection (136-UN)
If processor calculates 1ØØ% patient financial responsibility, populated in Patient Pay Amount (5Ø5-F5), which results in the customer paying
more than pharmacy will net for the claim, Total Amount Paid (5Ø9-F9) must be provided with a negative value so the sale can be booked
correctly.
26.2.5 SERVICE
26.2.5.1 CORRESPONDING PRICING FIELDS (SERVICE)
This includes Service Billing, Service Rebill, and Prior Authorization Request And Billing (Service).
Request Pricing Fields Corresponding Response Pricing Fields
433-DX PATIENT PAID AMOUNT SUBMITTED Not applicable
477-BE PROFESSIONAL SERVICE FEE
SUBMITTED
562-J2 PROFESSIONAL SERVICE FEE PAID
478-H7 OTHER AMOUNT CLAIMED
SUBMITTED COUNT
563-J2 OTHER AMOUNT PAID COUNT
479-H8 OTHER AMOUNT CLAIMED
SUBMITTED QUALIFIER
564-J3 OTHER AMOUNT PAID QUALIFIER
48Ø-H9 OTHER AMOUNT CLAIMED
SUBMITTED
565-J4 OTHER AMOUNT PAID
481-HA FLAT SALES TAX AMOUNT
SUBMITTED
558-AW FLAT SALES TAX AMOUNT PAID
482-GE PERCENTAGE SALES TAX AMOUNT
SUBMITTED
559-AX PERCENTAGE SALES TAX AMOUNT PAID
426-DQ USUAL AND CUSTOMARY CHARGE Not applicable
43Ø-DU GROSS AMOUNT DUE 5Ø9-F9 TOTAL AMOUNT PAID
5Ø5-F5 PATIENT PAY AMOUNT
Fields that are part of Patient Pay Amount:
523-FN AMOUNT ATTRIBUTED TO SALES TAX
518-FI AMOUNT OF COPAY
572-4U AMOUNT OF COINSURANCE

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 702 -
26.2.5.2 PATIENT FINANCIAL RESPONSIBILITY (SERVICE)
When the patient is expected to pay 1ØØ% of processor determined amount as total claim reimbursement, the response must contain:
Patient Pay Amount (5Ø5-F5) plus any of the applicable Patient Responsibility fields included in this amount:
• Amount Applied To Periodic Deductible (517-FH)
• Amount Exceeding Periodic Benefit Maximum (52Ø-FK)
• Amount Of Copay (518-FI)
• Amount of Coinsurance (572-4U)
• Amount Attributed to Processor Fee (571-NZ)
• Amount Attributed To Sales Tax (523-FN)
• Amount Attributed to Provider Network Selection (133-UJ)
If processor calculates 1ØØ% patient financial responsibility, populated in Patient Pay Amount (5Ø5-F5), which results in the customer paying
more than pharmacy will net for the claim, Total Amount Paid (5Ø9-F9) must be provided with a negative value so the sale can be booked
correctly.
26.3 DUPLICATE TRANSACTIONS
There are situations where the Originator sends the transaction request and the Processor receives the request and processes the
transaction. Then, due to communication problems or interruptions, the response is never received by the Originator. In these cases, the
Originator must resubmit the transaction request. The Processor must respond with the same information as the first conversation, but the
Transaction Response Status (112-AN) must contain the appropriate duplicate value. See section “Response Overview”, “Response Status By
Transaction Type” for more information. Any transaction that does not fit the “D” Duplicate criteria must result in “R” Reject.
A transmission request is considered a duplicate submission for these transactions
• Billing
• Reversal
• Prior Authorization Request and Billing
• Prior Authorization Reversal
• Prior Authorization Request Only
• Prior Authorization Inquiry
• Information Reporting
• Information Reporting Reversal
• Controlled Substance Reporting
• Controlled Substance Reporting Reversal
26.3.1 DUPLICATE TRANSMISSION FOR A PRIMARY PAYER
A duplicate transmission for a primary payer is based on the following criteria:
• Same patient/member
• Same Service Provider ID
• Same Date of Service
• Same Product/Service ID
• Same Prescription/Service Reference Number
• Same Fill Number (required if Claim Billing/Claim Rebill/Encounters; situational on Service Billing/Service Rebill)
26.3.2 DUPLICATE TRANSMISSION FOR A DOWNSTREAM PAYER
A duplicate transmission for a downstream payer is based on the following criteria:
• Same patient/member
• Same Service Provider ID
• Same Date of Service
• Same Product/Service ID
• Same Prescription/Service Reference Number
• Same Fill Number (required if Claim Billing/Claim Rebill/Encounters; situational on Service Billing/Service Rebill)
• Same Other Coverage Code
• Same Other Payer Coverage Type (the highest coverage type value)
The same processor may be involved in coordination of benefits for a patient for multiple benefit plans (multiple coordination of benefits
occurrences). Sometimes processors have difficulty determining a duplicate claim/service when they are involved for example as the primary
and secondary payer, or primary and tertiary, or secondary and tertiary. Communication timeouts may occur that cause a pharmacy to
resubmit a claim/service to obtain the response. To determine a duplicate claim/service involved in Coordination of Benefits, the Coordination
of Benefits/Other Payments Segment must be interrogated when the same processor is involved in multiple coordination of benefit
occurrences. The Coordination of Benefits/Other Payments Segment provides the pointer to clarify the duplicate.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 703 -
The Other Payer Coverage Type (338-5C) is in the Coordination of Benefits/Other Payments Segment. A downstream payer must interrogate
the Coordination of Benefits/Other Payments Segment, looking at the highest value of the Other Payer Coverage Type (338-5C) to determine
if the claim/service is a duplicate.
Note, the Other Payer Coverage Type (338-5C) occurrences do not have to appear in sequential order (primary, secondary, tertiary),
but can appear in any order.
26.3.2.1 EXCERPT EXAMPLE 1
In this excerpt, the highest value of Other Payer Coverage Type (338-5C) is “Ø2” (Secondary). This means the claim/service is being sent to a
tertiary payer. For this claim/service to be a duplicate, the tertiary payer must interrogate the duplicate fields cited above, with the Other Payer
Coverage Type (338-5C) of “Ø2”, since “Ø2” is the highest value.
Coordination of Benefits/Other Payments Segment
Field Field Name
111-AM SEGMENT IDENTIFICATION
337-4C COORDINATION OF BENEFITS/OTHER PAYMENTS COUNT 2
338-5C OTHER PAYER COVERAGE TYPE Ø1 Primary
339-6C OTHER PAYER ID QUALIFIER
34Ø-7C OTHER PAYER ID Medicare ID
443-E8 OTHER PAYER DATE 20061109
993-A7 INTERNAL CONTROL NUMBER AC22355
341-HB OTHER PAYER AMOUNT PAID COUNT 1
342-HC OTHER PAYER AMOUNT PAID QUALIFIER
431-DV OTHER PAYER AMOUNT PAID
471-5E OTHER PAYER REJECT COUNT
472-6E OTHER PAYER REJECT CODE
353-NR OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT COUNT
351-NP OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT QUALIFIER
352-NQ OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT
392-MU BENEFIT STAGE COUNT
393-MV BENEFIT STAGE QUALIFIER
394-MW BENEFIT STAGE AMOUNT
338-5C OTHER PAYER COVERAGE TYPE Ø2 Secondary
339-6C OTHER PAYER ID QUALIFIER
34Ø-7C OTHER PAYER ID Medicaid ID
443-E8 OTHER PAYER DATE 20061110
993-A7 INTERNAL CONTROL NUMBER 88993433
341-HB OTHER PAYER AMOUNT PAID COUNT 1
342-HC OTHER PAYER AMOUNT PAID QUALIFIER Etc
431-DV OTHER PAYER AMOUNT PAID
471-5E OTHER PAYER REJECT COUNT
472-6E OTHER PAYER REJECT CODE
353-NR OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT COUNT
351-NP OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT QUALIFIER
352-NQ OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT
392-MU BENEFIT STAGE COUNT
393-MV BENEFIT STAGE QUALIFIER
394-MW BENEFIT STAGE AMOUNT
26.3.2.2 EXCERPT EXAMPLE 2
In this excerpt, the highest value of Other Payer Coverage Type (338-5C) is “Ø1” (Primary). This means the claim/service is being sent to a
secondary payer. For this claim/service to be a duplicate, the secondary payer must interrogate the duplicate fields cited above, with the Other
Payer Coverage Type (338-5C) of “Ø1”, since “Ø1” is the highest value.
Coordination of Benefits/Other Payments Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 704 -
Field Field Name
111-AM SEGMENT IDENTIFICATION
337-4C COORDINATION OF BENEFITS/OTHER PAYMENTS COUNT 2
338-5C OTHER PAYER COVERAGE TYPE Ø1 Primary
339-6C OTHER PAYER ID QUALIFIER
34Ø-7C OTHER PAYER ID Medicare ID
443-E8 OTHER PAYER DATE 20061109
993-A7 INTERNAL CONTROL NUMBER AC22355
341-HB OTHER PAYER AMOUNT PAID COUNT 1
342-HC OTHER PAYER AMOUNT PAID QUALIFIER
431-DV OTHER PAYER AMOUNT PAID
471-5E OTHER PAYER REJECT COUNT
472-6E OTHER PAYER REJECT CODE
353-NR OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT COUNT
351-NP OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT QUALIFIER
352-NQ OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT
392-MU BENEFIT STAGE COUNT
393-MV BENEFIT STAGE QUALIFIER
394-MW BENEFIT STAGE AMOUNT
26.3.3 DUPLICATE TRANSMISSION FOR A REVERSAL FOR A PRIMARY PAYER
For Reversal transactions for a primary payer, the following criteria must be used to determine a duplicate request:
• Same Service Provider ID
• Same Date of Service
• Same Prescription/Service Reference Number
• Same Product/Service ID
• Same Fill Number (required if Claim Billing/Claim Rebill/Encounters; situational on Service Billing/Service Rebill)
26.3.4 DUPLICATE TRANSMISSION FOR A REVERSAL FOR A DOWNSTREAM PAYER
For Reversal transactions for a downstream payer, the following criteria must be used to determine a duplicate request:
• Same Service Provider ID
• Same Date of Service
• Same Prescription/Service Reference Number
• Same Product/Service ID
• Same Fill Number (required if Claim Billing/Claim Rebill/Encounters; situational on Service Billing/Service Rebill)
• Same Other Coverage Code
• Same Other Payer Coverage Type (the highest coverage type value)
The same processor may be involved in coordination of benefits for a patient for multiple benefit plans (multiple coordination of benefits
occurrences). Sometimes processors have difficulty determining a duplicate claim/service reversal when they are involved for example as the
primary and secondary payer, or primary and tertiary, or secondary and tertiary. Communication timeouts may occur that cause a pharmacy to
resubmit a claim/service reversal to obtain the response. On a reversal involved in Coordination of Benefits, to clarify which reversal the
pharmacy is requesting to be processed, the Coordination of Benefits/Other Payments Segment is sent. The Coordination of Benefits/Other
Payments Segment provides the pointer to specify which reversal to back out. This does not change the order of reversing claims/services; it
clarifies which claim/service to reverse. The pharmacy must reverse the claim/service in the correct back out order (see section “Reversal
Information”).
26.3.4.1 EXCERPT EXAMPLE 1
In this example, the claim/service reversal is sent to the payer. The highest value of Other Payer Coverage Type (338-5C) is “Ø2”
(Secondary). This means the claim/service reversal is being sent to a tertiary payer. For this claim/service reversal to be a duplicate, the
tertiary payer must interrogate the duplicate fields cited above, with the Other Payer Coverage Type (338-5C) of “Ø2”, since “Ø2” is the highest
value.
Coordination of Benefits/Other Payments Segment
Field Field Name
111-AM SEGMENT IDENTIFICATION
337-4C COORDINATION OF BENEFITS/OTHER PAYMENTS COUNT 2

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 705 -
338-5C OTHER PAYER COVERAGE TYPE Ø1 Primary
338-5C OTHER PAYER COVERAGE TYPE Ø2 Secondary
26.3.4.2 EXCERPT EXAMPLE 2
In this example, the claim/service reversal is sent to the payer. The highest value of Other Payer Coverage Type (338-5C) is “Ø1” (Primary).
This means the claim/service reversal is being sent to a secondary payer. For this claim/service reversal to be a duplicate, the secondary
payer must interrogate the duplicate fields cited above, with the Other Payer Coverage Type (338-5C) of “Ø1”, since “Ø1” is the highest value.
Coordination of Benefits/Other Payments Segment
Field Field Name
111-AM SEGMENT IDENTIFICATION
337-4C COORDINATION OF BENEFITS/OTHER PAYMENTS COUNT 1
338-5C OTHER PAYER COVERAGE TYPE Ø1 Primary
26.3.5 DUPLICATE INFORMATION FOR OTHER TRANSACTIONS
Eligibility Verification approval, Prior Authorization Inquiry approval, and Prior Authorization deferred responses for duplicate scenarios have
specific handling described in the appropriate transaction section. Transaction responses that do not fit the duplicate criteria will result in the
“R” (Reject) Transaction Response Status (112-AN).
Duplicate response logic must not be applied by the processor to:
• Rebill
• Information Reporting Rebill
• Controlled Substance Reporting Rebill
• Predetermination Of Benefits
There is no need for a duplicate response due to the nature of the predetermination of benefits transaction. Each submission of the transaction
is processed with the response reflective of current information.
There is no need for a duplicate response due to the nature of the rebill transaction and its implied reversal. Because the implied reversal
would reverse the paid claim, a duplicate transaction would not exist.
If a processor supported duplicate responses in rebills the submitter would not be able to modify a field that is not included in the duplicate
field check. See section “Response Processing Guidelines”, Duplicate Processing For All Rebill Transactions” for more information.
26.4 DUPLICATE PROCESSING FOR ALL REBILL TRANSACTIONS
In previous versions of the standard, the rebill transactions supported the duplicate Transaction Response Status (112-AN) values, as
appropriate. The rebill transactions are:
Transaction Code (1Ø3-A3) of
• B3 - Rebill (claim/service)
• N3 - Information Reporting Rebill
• C3 - Controlled Substance Reporting Rebill
Upon further review, the following discussion took place.
Per this document, a duplicate check is based on same Patient, Service Provider ID, Date of Service, Product/Service Reference
Number, Prescription/Service Reference Number, and Fill Number (see section “Response Processing Guidelines”, “Duplicate
Transactions”).
For a reversal, the duplicate check is based on the same Service Provider ID, Date of Service, Product/Service Reference Number,
Prescription/Service Reference Number, and Fill Number.
All rebill transactions have an implied reversal.
See Rebill section for each transaction, for example “Rebill Information” (for Claim or Service Rebills), “Information Reporting Rebill
Information”, “Controlled Substance Reporting Rebill Information”
Scenario:
Transaction 1 - A claim is submitted and paid by a processor.
Transaction 2 - The same claim is sent to the processor as a Rebill to correct the Prescriber ID. The processor receives the Rebill
and processes the reversal and pays the claim with the different Prescriber ID.
There is a communication-level drop and the provider does not receive the response.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 706 -
Transaction 3 - The provider sends the Rebill again. The processor applies the duplicate logic and returns a “D” (Duplicate of Paid)
response. So far, the process works.
Transaction 4 - The same day the provider realizes that he had entered the wrong days supply and resubmits a Rebill of the same
claim but with a corrected days supply.
The processor applies the duplicate logic and returns a “D” (Duplicate of Paid) response. The processor might not notice the days
supply changed, since the duplicate field check was applied first. It appears the only way to correct the day's supply is by submitting
two transactions.
Discussion:
The correction of fields not included in the duplicate check may be made using the rebill transaction. Because rebills have an
implied reversal, it appears that the Transaction Response Status (112-AN) values for duplicates do not apply to rebill transactions.
Since the same fields are used for a duplicate check and the implied reversal exists, the same problem occurs for Information and
Controlled Substance Reporting Rebills as well.
Every transaction has the chance of a communications drop, but in this case, the duplicate response is not needed for the
resubmission due to a communications drop.
Processing:
Therefore, based on discussions, the members determined that there is no business reason found for the duplicate responses for
the rebill transactions. By having duplicate responses in rebills the submitter is not able to modify a field that is not included in the
duplicate field check.
The duplicate Transaction Response Status (112-AN) of “D” (Duplicate of Paid) and “Q” (Duplicate of Captured) on Claim/Service
Rebill transactions (B3) are not needed.
The Transaction Response Status (112-AN) of “S” (Duplicate of Approved) and “Q” (Duplicate of Captured) for Controlled Substance
Reporting Rebill transactions (C3) are not needed.
The Transaction Response Status (112-AN) of “S” (Duplicate of Approved), “Q” (Duplicate of Captured), and “D” (Duplicate of Paid)
for Information Reporting Rebill transactions (N3) are not needed.
Therefore duplicate values have been removed for rebill transactions.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 707 -
27. STRUCTURE QUICK REFERENCE
See section “Transmission Structure” for specific information on segment and field usage per transaction.
The following conventions appear in the charts below.
M = Mandatory field
S = Situational field – which may be defined as situational, optional, or not used, per the segment and field usage in section “Transmission
Structure”.
***R*** = Repeating field
NOTE: Truncation within a Transaction Header Segment is not allowed.
NOTE: Special instructions for submitting repeating fields that are situational or optional can be found in section “Standard Conventions”,
“Repetition and Multiple Occurrences”.
NOTE: See section “General Syntax Outline” for information about segment order.
27.1 REQUEST SEGMENTS
27.1.1 TRANSMISSION LEVEL
Transaction Header Segment
Field Field Name Mandatory or
Situational
1Ø1-A1 BIN NUMBER M
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø4-A4 PROCESSOR CONTROL NUMBER M
1Ø9-A9 TRANSACTION COUNT M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M
The Transaction Header Segment is a fixed length segment of 56 bytes.
Patient Segment
Field Field Name Mandatory or
Situational
111-AM SEGMENT IDENTIFICATION M
331-CX PATIENT ID QUALIFIER S
332-CY PATIENT ID S
3Ø4-C4 DATE OF BIRTH S
3Ø5-C5 PATIENT GENDER CODE S
31Ø-CA PATIENT FIRST NAME S
311-CB PATIENT LAST NAME S
322-CM PATIENT STREET ADDRESS S
323-CN PATIENT CITY ADDRESS S
324-CO PATIENT STATE / PROVINCE ADDRESS S
325-CP PATIENT ZIP/POSTAL ZONE S
326-CQ PATIENT PHONE NUMBER S
3Ø7-C7 PLACE OF SERVICE S
333-CZ EMPLOYER ID S
334-1C SMOKER / NON-SMOKER CODE S
335-2C PREGNANCY INDICATOR S
35Ø-HN PATIENT E-MAIL ADDRESS S
384-4X PATIENT RESIDENCE S
This segment is variable length.
Insurance Segment

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 708 -
Field Field Name Mandatory or
Situational
111-AM SEGMENT IDENTIFICATION M
3Ø2-C2 CARDHOLDER ID M
312-CC CARDHOLDER FIRST NAME S
313-CD CARDHOLDER LAST NAME S
314-CE HOME PLAN S
524-FO PLAN ID S
3Ø9-C9 ELIGIBILITY CLARIFICATION CODE S
3Ø1-C1 GROUP ID S
3Ø3-C3 PERSON CODE S
3Ø6-C6 PATIENT RELATIONSHIP CODE S
99Ø-MG OTHER PAYER BIN NUMBER S
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER S
356-NU OTHER PAYER CARDHOLDER ID S
992-MJ OTHER PAYER GROUP ID S
359-2A MEDIGAP ID S
36Ø-2B MEDICAID INDICATOR S
361-2D PROVIDER ACCEPT ASSIGNMENT INDICATOR S
997-G2 CMS PART D DEFINED QUALIFIED FACILITY S
115-N5 MEDICAID ID NUMBER S
116-N6 MEDICAID AGENCY NUMBER S
This segment is variable length.
27.1.2 TRANSACTION LEVEL
Claim Segment
Field Field Name Mandatory or
Situational
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M
436-E1 PRODUCT/SERVICE ID QUALIFIER M
4Ø7-D7 PRODUCT/SERVICE ID M
456-EN ASSOCIATED PRESCRIPTION/SERVICE REFERENCE # S
457-EP ASSOCIATED PRESCRIPTION/SERVICE DATE S
458-SE PROCEDURE MODIFIER CODE COUNT S
459-ER PROCEDURE MODIFIER CODE S***R***
442-E7 QUANTITY DISPENSED S
4Ø3-D3 FILL NUMBER S
4Ø5-D5 DAYS SUPPLY S
4Ø6-D6 COMPOUND CODE S
4Ø8-D8 DISPENSE AS WRITTEN (DAW)/PRODUCT SELECTION CODE S
414-DE DATE PRESCRIPTION WRITTEN S
415-DF NUMBER OF REFILLS AUTHORIZED S
419-DJ PRESCRIPTION ORIGIN CODE S
354-NX SUBMISSION CLARIFICATION CODE COUNT S
42Ø-DK SUBMISSION CLARIFICATION CODE S***R***
46Ø-ET QUANTITY PRESCRIBED S
3Ø8-C8 OTHER COVERAGE CODE S
429-DT SPECIAL PACKAGING INDICATOR S
453-EJ ORIG PRESCRIBED PRODUCT/SERVICE ID QUALIFIER S
445-EA ORIGINALLY PRESCRIBED PRODUCT/SERVICE CODE S
446-EB ORIGINALLY PRESCRIBED QUANTITY S

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 709 -
33Ø-CW ALTERNATE ID S
454-EK SCHEDULED PRESCRIPTION ID NUMBER S
6ØØ-28 UNIT OF MEASURE S
418-DI LEVEL OF SERVICE S
461-EU PRIOR AUTHORIZATION TYPE CODE S
462-EV PRIOR AUTHORIZATION NUMBER SUBMITTED S
463-EW INTERMEDIARY AUTHORIZATION TYPE ID S
464-EX INTERMEDIARY AUTHORIZATION ID S
343-HD DISPENSING STATUS S
344-HF QUANTITY INTENDED TO BE DISPENSED S
345-HG DAYS SUPPLY INTENDED TO BE DISPENSED S
357-NV DELAY REASON CODE S
88Ø-K5 TRANSACTION REFERENCE NUMBER S
391-MT PATIENT ASSIGNMENT INDICATOR (DIRECT MEMBER
REIMBURSEMENT INDICATOR)
S
995-E2 ROUTE OF ADMINISTRATION S
996-G1 COMPOUND TYPE S
114-N4 MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
S
147-U7 PHARMACY SERVICE TYPE S
This segment is variable length.
Pharmacy Provider Segment
Field Field Name Mandatory or
Situational
111-AM SEGMENT IDENTIFICATION M
465-EY PROVIDER ID QUALIFIER S
444-E9 PROVIDER ID S
This segment is variable length.
Prescriber Segment
Field Field Name Mandatory or
Situational
111-AM SEGMENT IDENTIFICATION M
466-EZ PRESCRIBER ID QUALIFIER S
411-DB PRESCRIBER ID S
427-DR PRESCRIBER LAST NAME S
498-PM PRESCRIBER PHONE NUMBER S
468-2E PRIMARY CARE PROVIDER ID QUALIFIER S
421-DL PRIMARY CARE PROVIDER ID S
47Ø-4E PRIMARY CARE PROVIDER LAST NAME S
364-2J PRESCRIBER FIRST NAME S
365-2K PRESCRIBER STREET ADDRESS S
366-2M PRESCRIBER CITY ADDRESS S
367-2N PRESCRIBER STATE/PROVINCE ADDRESS S
368-2P PRESCRIBER ZIP/POSTAL ZONE S
This segment is variable length.
Coordination of Benefits/Other Payments Segment
Field Field Name Mandatory or
Situational
111-AM SEGMENT IDENTIFICATION M
337-4C COORDINATION OF BENEFITS/OTHER PAYMENTS COUNT M

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 710 -
338-5C OTHER PAYER COVERAGE TYPE M***R***
339-6C OTHER PAYER ID QUALIFIER S***R***
34Ø-7C OTHER PAYER ID S***R***
443-E8 OTHER PAYER DATE S***R***
993-A7 INTERNAL CONTROL NUMBER S***R***
341-HB OTHER PAYER AMOUNT PAID COUNT S
342-HC OTHER PAYER AMOUNT PAID QUALIFIER S***R***
431-DV OTHER PAYER AMOUNT PAID S***R***
471-5E OTHER PAYER REJECT COUNT S
472-6E OTHER PAYER REJECT CODE S***R***
353-NR OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT COUNT S
351-NP OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT QUALIFIER S***R***
352-NQ OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT S***R***
392-MU BENEFIT STAGE COUNT S
393-MV BENEFIT STAGE QUALIFIER S***R***
394-MW BENEFIT STAGE AMOUNT S***R***
This segment is variable length.
Workers’ Compensation Segment
Field Field Name Mandatory or
Situational
111-AM SEGMENT IDENTIFICATION M
434-DY DATE OF INJURY M
315-CF EMPLOYER NAME S
316-CG EMPLOYER STREET ADDRESS S
317-CH EMPLOYER CITY ADDRESS S
318-CI EMPLOYER STATE/PROVINCE ADDRESS S
319-CJ EMPLOYER ZIP/POSTAL ZONE S
32Ø-CK EMPLOYER PHONE NUMBER S
321-CL EMPLOYER CONTACT NAME S
327-CR CARRIER ID S
435-DZ CLAIM/REFERENCE ID S
117-TR BILLING ENTITY TYPE INDICATOR S
118-TS PAY TO QUALIFIER S
119-TT PAY TO ID S
12Ø-TU PAY TO NAME S
121-TV PAY TO STREET ADDRESS S
122-TW PAY TO CITY ADDRESS S
123-TX PAY TO STATE/PROVINCE ADDRESS S
124-TY PAY TO ZIP/POSTAL ZONE S
125-TZ GENERIC EQUIVALENT PRODUCT ID QUALIFIER S
126-UA GENERIC EQUIVALENT PRODUCT ID S
This segment is variable length.
DUR/PPS Segment
Field Field Name Mandatory or
Situational
111-AM SEGMENT IDENTIFICATION M
473-7E DUR/PPS CODE COUNTER S***R***
439-E4 REASON FOR SERVICE CODE S***R***
44Ø-E5 PROFESSIONAL SERVICE CODE S***R***
441-E6 RESULT OF SERVICE CODE S***R***

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 711 -
474-8E DUR/PPS LEVEL OF EFFORT S***R***
475-J9 DUR CO-AGENT ID QUALIFIER S***R***
476-H6 DUR CO-AGENT ID S***R***
This segment is variable length.
Pricing Segment
Field Field Name Mandatory or
Situational
111-AM SEGMENT IDENTIFICATION M
4Ø9-D9 INGREDIENT COST SUBMITTED S
412-DC DISPENSING FEE SUBMITTED S
477-BE PROFESSIONAL SERVICE FEE SUBMITTED S
433-DX PATIENT PAID AMOUNT SUBMITTED S
438-E3 INCENTIVE AMOUNT SUBMITTED S
478-H7 OTHER AMOUNT CLAIMED SUBMITTED COUNT S
479-H8 OTHER AMOUNT CLAIMED SUBMITTED QUALIFIER S***R***
48Ø-H9 OTHER AMOUNT CLAIMED SUBMITTED S***R***
481-HA FLAT SALES TAX AMOUNT SUBMITTED S
482-GE PERCENTAGE SALES TAX AMOUNT SUBMITTED S
483-HE PERCENTAGE SALES TAX RATE SUBMITTED S
484-JE PERCENTAGE SALES TAX BASIS SUBMITTED S
426-DQ USUAL AND CUSTOMARY CHARGE S
43Ø-DU GROSS AMOUNT DUE S
423-DN BASIS OF COST DETERMINATION S
113-N3 MEDICAID PAID AMOUNT S
This segment is variable length.
Coupon Segment
Field Field Name Mandatory or
Situational
111-AM SEGMENT IDENTIFICATION M
485-KE COUPON TYPE M
486-ME COUPON NUMBER M
487-NE COUPON VALUE AMOUNT S
This segment is variable length.
Compound Segment
Field Field Name Mandatory or
Situational
111-AM SEGMENT IDENTIFICATION M
45Ø-EF COMPOUND DOSAGE FORM DESCRIPTION CODE M
451-EG COMPOUND DISPENSING UNIT FORM INDICATOR M
447-EC COMPOUND INGREDIENT COMPONENT COUNT M
488-RE COMPOUND PRODUCT ID QUALIFIER M***R***
489-TE COMPOUND PRODUCT ID M***R***
448-ED COMPOUND INGREDIENT QUANTITY M***R***
449-EE COMPOUND INGREDIENT DRUG COST S***R***
49Ø-UE COMPOUND INGREDIENT BASIS OF COST DETERMINATION S***R***
362-2G COMPOUND INGREDIENT MODIFIER CODE COUNT S
363-2H COMPOUND INGREDIENT MODIFIER CODE S***R***
This segment is variable length.
Prior Authorization Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 712 -
Field Field Name Mandatory or
Situational
111-AM SEGMENT IDENTIFICATION M
498-PA REQUEST TYPE M
498-PB REQUEST PERIOD DATE-BEGIN M
498-PC REQUEST PERIOD DATE-END M
498-PD BASIS OF REQUEST M
498-PE AUTHORIZED REPRESENTATIVE FIRST NAME S
498-PF AUTHORIZED REPRESENTATIVE LAST NAME S
498-PG AUTHORIZED REPRESENTATIVE STREET ADDRESS S
498-PH AUTHORIZED REPRESENTATIVE CITY ADDRESS S
498-PJ AUTHORIZED REPRESENTATIVE STATE/PROVINCE ADDRESS S
498-PK AUTHORIZED REPRESENTATIVE ZIP/POSTAL ZONE S
498-PY PRIOR AUTHORIZATION NUMBER-ASSIGNED S
5Ø3-F3 AUTHORIZATION NUMBER S
498-PP PRIOR AUTHORIZATION SUPPORTING DOCUMENTATION S
This segment is variable length.
Clinical Segment
Field Field Name Mandatory or
Situational
111-AM SEGMENT IDENTIFICATION M
491-VE DIAGNOSIS CODE COUNT S
492-WE DIAGNOSIS CODE QUALIFIER S***R***
424-DO DIAGNOSIS CODE S***R***
493-XE CLINICAL INFORMATION COUNTER S***R***
494-ZE MEASUREMENT DATE S***R***
495-H1 MEASUREMENT TIME S***R***
496-H2 MEASUREMENT DIMENSION S***R***
497-H3 MEASUREMENT UNIT S***R***
499-H4 MEASUREMENT VALUE S***R***
This segment is variable length.
Additional Documentation Segment
Field Field Name Mandatory or
Situational
111-AM SEGMENT IDENTIFICATION M
369-2Q ADDITIONAL DOCUMENTATION TYPE ID M
374-2V REQUEST PERIOD BEGIN DATE S
375-2W REQUEST PERIOD RECERT/REVISED DATE S
373-2U REQUEST STATUS S
371-2S LENGTH OF NEED QUALIFIER S
37Ø-2R LENGTH OF NEED S
372-2T PRESCRIBER/SUPPLIER DATE SIGNED S
376-2X SUPPORTING DOCUMENTATION S
377-2Z QUESTION NUMBER/LETTER COUNT S
378-4B QUESTION NUMBER/LETTER S***R***
379-4D QUESTION PERCENT RESPONSE S***R***
38Ø-4G QUESTION DATE RESPONSE S***R***
381-4H QUESTION DOLLAR AMOUNT RESPONSE S***R***
382-4J QUESTION NUMERIC RESPONSE S***R***
383-4K QUESTION ALPHANUMERIC RESPONSE S***R***
This segment is variable length.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 713 -
Facility Segment
Field Field Name Mandatory or
Situational
111-AM SEGMENT IDENTIFICATION M
336-8C FACILITY ID S
385-3Q FACILITY NAME S
386-3U FACILITY STREET ADDRESS S
388-5J FACILITY CITY ADDRESS S
387-3V FACILITY STATE/PROVINCE ADDRESS S
389-6D FACILITY ZIP/POSTAL ZONE S
This segment is variable length.
Narrative Segment
Field Field Name Mandatory or
Situational
111-AM SEGMENT IDENTIFICATION M
39Ø-BM NARRATIVE MESSAGE M
This segment is variable length.
27.2 RESPONSE SEGMENTS
NOTE: Truncation is not allowed in Response Header Segment.
27.2.1 TRANSMISSION LEVEL
Response Header Segment
Field Field Name Mandatory or
Situational
1Ø2-A2 VERSION/RELEASE NUMBER M
1Ø3-A3 TRANSACTION CODE M
1Ø9-A9 TRANSACTION COUNT M
5Ø1-F1 HEADER RESPONSE STATUS M
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M
2Ø1-B1 SERVICE PROVIDER ID M
4Ø1-D1 DATE OF SERVICE M
The Response Header Segment is a fixed length segment of 31 bytes.
Response Message Segment Mandatory or
Situational
Field Field Name Mandatory or
Situational
111-AM SEGMENT IDENTIFICATION M
5Ø4-F4 MESSAGE S
This segment is variable length.
Response Insurance Segment
Field Field Name Mandatory or
Situational
111-AM SEGMENT IDENTIFICATION M
3Ø1-C1 GROUP ID S
524-FO PLAN ID S
545-2F NETWORK REIMBURSEMENT ID S
568-J7 PAYER ID QUALIFIER S
569-J8 PAYER ID S
115-N5 MEDICAID ID NUMBER S

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 714 -
116-N6 MEDICAID AGENCY NUMBER S
3Ø2-C2 CARDHOLDER ID S
This segment is variable length.
Response Insurance Additional Information Segment
Field Field Name Mandatory or
Situational
111-AM SEGMENT IDENTIFICATION M
139-UR MEDICARE PART D COVERAGE CODE M
138-UQ CMS LOW INCOME COST SHARING (LICS) LEVEL S
24Ø-U1 CONTRACT NUMBER S
926-FF FORMULARY ID S
757-U6 BENEFIT ID S
14Ø-US NEXT MEDICARE PART D EFFECTIVE DATE S
141-UT NEXT MEDICARE PART D TERMINATION DATE S
This segment is variable length.
Response Patient Segment
Field Field Name Mandatory or
Situational
111-AM SEGMENT IDENTIFICATION M
31Ø-CA PATIENT FIRST NAME S
311-CB PATIENT LAST NAME S
3Ø4-C4 DATE OF BIRTH S
This segment is variable length.
27.2.2 TRANSACTION LEVEL
Response Status Segment Mandatory or
Situational
Field Field Name Mandatory or
Situational
111-AM SEGMENT IDENTIFICATION M
112-AN TRANSACTION RESPONSE STATUS M
5Ø3-F3 AUTHORIZATION NUMBER S
51Ø-FA REJECT COUNT S
511-FB REJECT CODE S***R***
546-4F REJECT FIELD OCCURRENCE INDICATOR S***R***
547-5F APPROVED MESSAGE CODE COUNT S
548-6F APPROVED MESSAGE CODE S***R***
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT S
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER S***R***
526-FQ ADDITIONAL MESSAGE INFORMATION S***R***
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY S***R***
549-7F HELP DESK PHONE NUMBER QUALIFIER S
55Ø-8F HELP DESK PHONE NUMBER S
88Ø-K5 TRANSACTION REFERENCE NUMBER S
993-A7 INTERNAL CONTROL NUMBER S
987-MA URL S
This segment is variable length.
Response Claim Segment
Field Field Name Mandatory or
Situational

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 715 -
111-AM SEGMENT IDENTIFICATION M
455-EM PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER M
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE NUMBER M
551-9F PREFERRED PRODUCT COUNT S
552-AP PREFERRED PRODUCT ID QUALIFIER S***R***
553-AR PREFERRED PRODUCT ID S***R***
554-AS PREFERRED PRODUCT INCENTIVE S***R***
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE S***R***
556-AU PREFERRED PRODUCT DESCRIPTION S***R***
114-N4 MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
S
This segment is variable length.
Response Pricing Segment
Field Field Name Mandatory or
Situational
111-AM SEGMENT IDENTIFICATION M
5Ø5-F5 PATIENT PAY AMOUNT S
5Ø6-F6 INGREDIENT COST PAID S
5Ø7-F7 DISPENSING FEE PAID S
557-AV TAX EXEMPT INDICATOR S
558-AW FLAT SALES TAX AMOUNT PAID S
559-AX PERCENTAGE SALES TAX AMOUNT PAID S
56Ø-AY PERCENTAGE SALES TAX RATE PAID S
561-AZ PERCENTAGE SALES TAX BASIS PAID S
521-FL INCENTIVE AMOUNT PAID S
562-J1 PROFESSIONAL SERVICE FEE PAID S
563-J2 OTHER AMOUNT PAID COUNT S
564-J3 OTHER AMOUNT PAID QUALIFIER S***R***
565-J4 OTHER AMOUNT PAID S***R***
566-J5 OTHER PAYER AMOUNT RECOGNIZED S
5Ø9-F9 TOTAL AMOUNT PAID S
522-FM BASIS OF REIMBURSEMENT DETERMINATION S
523-FN AMOUNT ATTRIBUTED TO SALES TAX S
512-FC ACCUMULATED DEDUCTIBLE AMOUNT S
513-FD REMAINING DEDUCTIBLE AMOUNT S
514-FE REMAINING BENEFIT AMOUNT S
517-FH AMOUNT APPLIED TO PERIODIC DEDUCTIBLE S
518-FI AMOUNT OF COPAY S
52Ø-FK AMOUNT EXCEEDING PERIODIC BENEFIT MAXIMUM S
346-HH BASIS OF CALCULATION-DISPENSING FEE S
347-HJ BASIS OF CALCULATION-COPAY S
348-HK BASIS OF CALCULATION-FLAT SALES TAX S
349-HM BASIS OF CALCULATION-PERCENTAGE SALES TAX S
571-NZ AMOUNT ATTRIBUTED TO PROCESSOR FEE S
575-EQ PATIENT SALES TAX AMOUNT S
574-2Y PLAN SALES TAX AMOUNT S
572-4U AMOUNT OF COINSURANCE S
573-4V BASIS OF CALCULATION-COINSURANCE S
392-MU BENEFIT STAGE COUNT S
393-MV BENEFIT STAGE QUALIFIER S***R***
394-MW BENEFIT STAGE AMOUNT S***R***
577-G3 ESTIMATED GENERIC SAVINGS S

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 716 -
128-UC SPENDING ACCOUNT AMOUNT REMAINING S
129-UD HEALTH PLAN-FUNDED ASSISTANCE AMOUNT S
133-UJ AMOUNT ATTRIBUTED TO PROVIDER NETWORK SELECTION S
134-UK AMOUNT ATTRIBUTED TO PRODUCT SELECTION/BRAND DRUG S
135-UM AMOUNT ATTRIBUTED TO PRODUCT SELECTION/NON-PREFERRED
FORMULARY SELECTION
S
136-UN AMOUNT ATTRIBUTED TO PRODUCT SELECTION/BRAND NON-
PREFERRED FORMULARY SELECTION
S
137-UP AMOUNT ATTRIBUTED TO COVERAGE GAP S
148-U8 INGREDIENT COST CONTRACTED/REIMBURSABLE AMOUNT S
149-U9 DISPENSING FEE CONTRACTED/REIMBURSABLE AMOUNT S
This segment is variable length.
Response DUR/PPS Segment
Field Field Name Mandatory or
Situational
111-AM SEGMENT IDENTIFICATION M
567-J6 DUR/PPS RESPONSE CODE COUNTER S***R***
439-E4 REASON FOR SERVICE CODE S***R***
528-FS CLINICAL SIGNIFICANCE CODE S***R***
529-FT OTHER PHARMACY INDICATOR S***R***
53Ø-FU PREVIOUS DATE OF FILL S***R***
531-FV QUANTITY OF PREVIOUS FILL S***R***
532-FW DATABASE INDICATOR S***R***
533-FX OTHER PRESCRIBER INDICATOR S***R***
544-FY DUR FREE TEXT MESSAGE S***R***
57Ø-NS DUR ADDITIONAL TEXT S***R***
This segment is variable length.
Response Prior Authorization Segment
Field Field Name Mandatory or
Situational
111-AM SEGMENT IDENTIFICATION M
498-PR PRIOR AUTHORIZATION PROCESSED DATE S
498-PS PRIOR AUTHORIZATION EFFECTIVE DATE S
498-PT PRIOR AUTHORIZATION EXPIRATION DATE S
498-RA PRIOR AUTHORIZATION QUANTITY S
498-RB PRIOR AUTHORIZATION DOLLARS AUTHORIZED S
498-PW PRIOR AUTHORIZATION NUMBER OF REFILLS AUTHORIZED S
498-PX PRIOR AUTHORIZATION QUANTITY ACCUMULATED S
498-PY PRIOR AUTHORIZATION NUMBER-ASSIGNED S
This segment is variable length.
Response Coordination of Benefits/Other Payers Segment
Field Field Name Mandatory or
Situational
111-AM SEGMENT IDENTIFICATION M
355-NT OTHER PAYER ID COUNT M
338-5C OTHER PAYER COVERAGE TYPE M***R***
339-6C OTHER PAYER ID QUALIFIER S***R***
34Ø-7C OTHER PAYER ID S***R***
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER S***R***
356-NU OTHER PAYER CARDHOLDER ID S***R***
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 717 -
992-MJ OTHER PAYER GROUP ID S***R***
142-UV OTHER PAYER PERSON CODE S***R***
127-UB OTHER PAYER HELP DESK PHONE NUMBER S***R***
143-UW OTHER PAYER PATIENT RELATIONSHIP CODE S***R***
144-UX OTHER PAYER BENEFIT EFFECTIVE DATE S***R***
145-UY OTHER PAYER BENEFIT TERMINATION DATE S***R***
This segment is variable length.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 718 -
28. SPECIFIC SEGMENT DISCUSSION
28.1 REQUEST SEGMENTS
28.1.1 TRANSACTION HEADER SEGMENT
The Header Segment is required and must be first in the transmission. All fields are required positionally. When a field is not used, the field
must be filled with zeroes or spaces, as appropriate.
28.1.1.1 TRANSACTION COUNT
The Transaction Count (1Ø9-A9) is present on every transaction request and response. This count specifies the number of iterations to be
parsed and processed within each request and response. The count number submitted on the request must be echoed back and appropriately
responded to in the response. For every iteration in a request, there must be the same number of iterations in the response and the response
count must contain the same value. There is one exception - when the transmission is rejected at the header level due to errors in invalid
Version/Release Number (1Ø2-A2) or Transaction Count (1Ø9-A9) - only one response must be returned.
28.1.2 PATIENT SEGMENT
The Patient Segment must be submitted when needed to differentiate between the patient and the cardholder. If the cardholder and the patient
are the same, then the Patient Segment is not submitted unless additional information about the patient is needed to clarify the transaction
determination.
28.1.3 INSURANCE SEGMENT
If the cardholder and the patient are the same, then the Patient Segment need not be submitted unless additional information about the patient
is needed to clarify the transaction.
28.1.3.1 MEDICARE PART D INFORMATION REPORTING USAGE
For Medicare Part D Information Reporting transactions, when the Unique BIN/PCN is not used and the Secondary/Tertiary/etc Payer needs to
report updated patient pay information directly through the Facilitator to the PDP, the Secondary/Tertiary/etc Payer is required, in the
Insurance Segment:
• To put their Cardholder ID in Cardholder ID (3Ø2-C2) and in Other Payer Cardholder ID (356-NU),
• To put their BIN, PCN (if applicable), and Group ID (if applicable) in the Other Payer BIN Number (99Ø-MG), Other Payer Processor
Control Number (991-MH), and Other Payer Group ID (992-MJ).
28.1.4 PHARMACY PROVIDER SEGMENT
The Pharmacy Provider Segment refers to the pharmacist dispensing the medication, not the prescriber writing the prescription. It provides
information about the specific pharmacist involved in the transaction.
28.1.5 PRESCRIBER SEGMENT
When checking eligibility for a recipient under various restricted programs, the ordering provider (Prescriber ID (411-DB)) and referring
provider (Primary Care Provider ID (421-DL)) may be validated by the recipient eligibility check to verify that the recipient is eligible for
services.
28.1.6 COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
This segment contains situational fields to indicate other responsible parties to the non-primary payer as well as the date upon which payment
or denial was made. Payment must be sent in the fields for Other Payer Amount Paid Count (341-HB), Other Payer Amount Paid Qualifier
(342-HC), and Other Payer Amount Paid (431-DV). Reject information is sent in the fields of Other Payer Reject Count (471-5E), and Other
Payer Reject Code (472-6E).
When zeroes are sent in the Other Payer Amount Paid (431-DV), the pharmacy system is notifying the processor of no payment dollars
received. Pharmacy systems must be cautioned that this segment must not be sent unless needed and the Other Payer Amount Paid field
must not be defaulted (zero filled), as it would lead the processor to an incorrect conclusion of other payment paid.
In the situation where there are more than 9 coverages for a patient, each loop of coordination of benefits must show the payment or rejection
from the payer. After the 9th payer, the claim is handled manually to subsequent payers.
When supported, the Other Payer-Patient Responsibility fields communicate the patient’s financial responsibility as reported by the previous
payer(s) to the next payer, within the occurrences of the Coordination of Benefits/Other Payments Count (337-4C). If a patient’s financial
responsibility was returned from a primary and a secondary payer, both these occurrences can be reported to the tertiary payer.
The values of the Other Payer-Patient Responsibility Amount Qualifier and amounts reported in the Other Payer-Patient Responsibility Amount
depend upon whether the payer accepts the individual line item detail amounts for which the patient is responsible, or the total amount
responsible by the patient.
Note: The Other Payer-Patient Responsibility Amount Count, Qualifier, and Amount do not depend upon the Other Payer Amount Paid Count
fields. The pharmacy may relay that the other payer has paid some of the other charges (incentive, shipping, et cetera) and/or the patient has
shared in some of the financial responsibility. See table below.
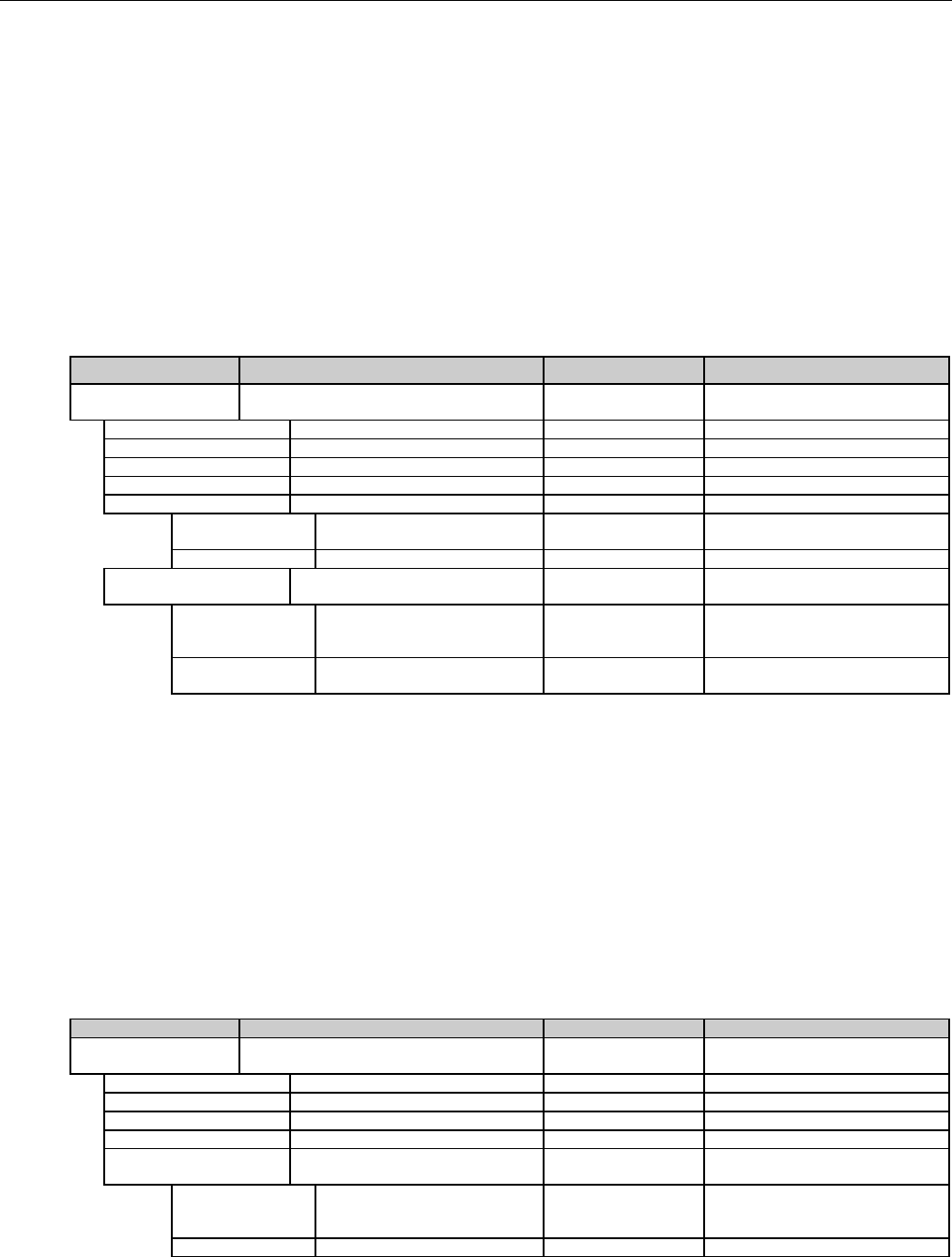
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 719 -
28.1.6.1 TO DENOTE A TOTAL AMOUNT OF PATIENT FINANCIAL RESPONSIBILITY AS REPORTED FROM
A PREVIOUS PAYER
The Other Payer-Patient Responsibility Amount Count (353-NR) must contain a value of 1 when the Other Payer-Patient
Responsibility Amount Qualifier (351-NP) contains a value of “Ø6” (Patient Pay Amount (5Ø5-F5) as Reported by the Previous Payer).
This qualifier denotes a total amount returned in the Patient Pay Amount, as reported from the previous payer in a previous claim or service
billing.
For example, in an original claim or service billing, the primary payer reports a Patient Pay Amount (5Ø5-F5). That amount would then be
reported to the secondary payer. In the claim or service billing to the secondary payer, the Other Payer-Patient Responsibility Amount would
contain the amount reported from the primary payer that was in the Patient Pay Amount in the original claim or service billing.
This is a total amount of the patient’s responsibility from the previous payer. The Other Payer-Patient Responsibility Amount Qualifier would
contain a value of “Ø6” to denote a total of the Patient Pay Amount as reported by the previous payer.
In the following excerpted example, the pharmacy is reporting to the secondary payer. In a previous claim or service billing, the primary payer
has paid an incentive fee. The patient has shared in the financial responsibility. The patient’s responsibility is shown as a total (patient pay
amount as reported from previous payer).
Field Field Name Value Comment
337-4C Coordination of Benefits/Other Payments
Count
1 One occurrence
338-5C Other Payer Coverage Type Ø1 Primary payer
339-6C Other Payer ID Qualifier Ø3 BIN
34Ø-7C Other Payer ID 123456
443-E8 Other Payer Date 2ØØØØ712 July 12, 2ØØØ
341-HB Other Payer Amount Paid Count 1 One occurrence
342-HC Other Payer Amount Paid
Qualifier
Ø5 Incentive
431-DV Other Payer Amount Paid 1Ø{ $1.ØØ
353-NR Other Payer-Patient Responsibility
Amount Count
1 One occurrence
351-NP Other Payer-Patient
Responsibility Amount
Qualifier
Ø6 Patient Pay Amount (5Ø5-F5) as
reported by previous payer
352-NQ Other Payer-Patient
Responsibility Amount
223{ $22.3Ø
28.1.6.2 TO DENOTE INDIVIDUAL AMOUNTS OF PATIENT FINANCIAL RESPONSIBILITY AS REPORTED
FROM A PREVIOUS PAYER
The Other Payer-Patient Responsibility Amount Qualifier (351-NP) will contain a value other than “Ø6” when the Other Payer-Patient
Responsibility Amount contains the individual amount(s) of the patient’s financial responsibility.
Values other than “Ø6” are used when some or all of the dollar fields of the Patient Pay Amount (5Ø5-F5) formula are returned in a previous
claim or service billing from the previous payer. (See section “Patient Pay Amount (5Ø5-F5) Formula”.)
For example, in an original claim or service billing, the primary payer returns amounts in the Amount Attributed to Product Selection/Brand
Drug (134-UK) and Amount Attributed to Sales Tax (523-FN). The pharmacy submits the claim or service billing to the secondary payer. The
amounts in these two fields are then reflected in two occurrences of the Other Payer-Patient Responsibility Amount, with the Qualifier
reflecting one occurrence with a value of “Ø2” (Amount Attributed to Product Selection/Brand Drug (134-UK) as reported by a previous payer)
and a second occurrence with a value of “ Ø5” (Amount of Copay (518-FI) as reported by previous payer).
In the following excerpted example, the pharmacy has received patient responsibility amounts from a primary payer. The pharmacy is reporting
to the secondary payer. The Other Payer-Patient Responsibility Amount Count contains a value of 2 to relay two individual amounts of the
patient’s financial responsibility – amount attributed to product selection/brand drug and amount of copay as reported from a previous payer.
Field Field Name Value Comment
337-4C Coordination of Benefits/Other Payments
Count
1 One occurrence
338-5C Other Payer Coverage Type Ø1 Primary payer
339-6C Other Payer ID Qualifier Ø3 BIN
34Ø-7C Other Payer ID 123456
443-E8 Other Payer Date 2ØØØØ712 July 12, 2ØØØ
353-NR Other Payer-Patient Responsibility
Amount Count
2 Two occurrences
351-NP Other Payer-Patient
Responsibility Amount
Qualifier
Ø2 Amount Attributed to Product
Selection/Brand Drug (134-UK)
as reported by previous payer
352-NQ Other Payer-Patient 122{ $12.2Ø
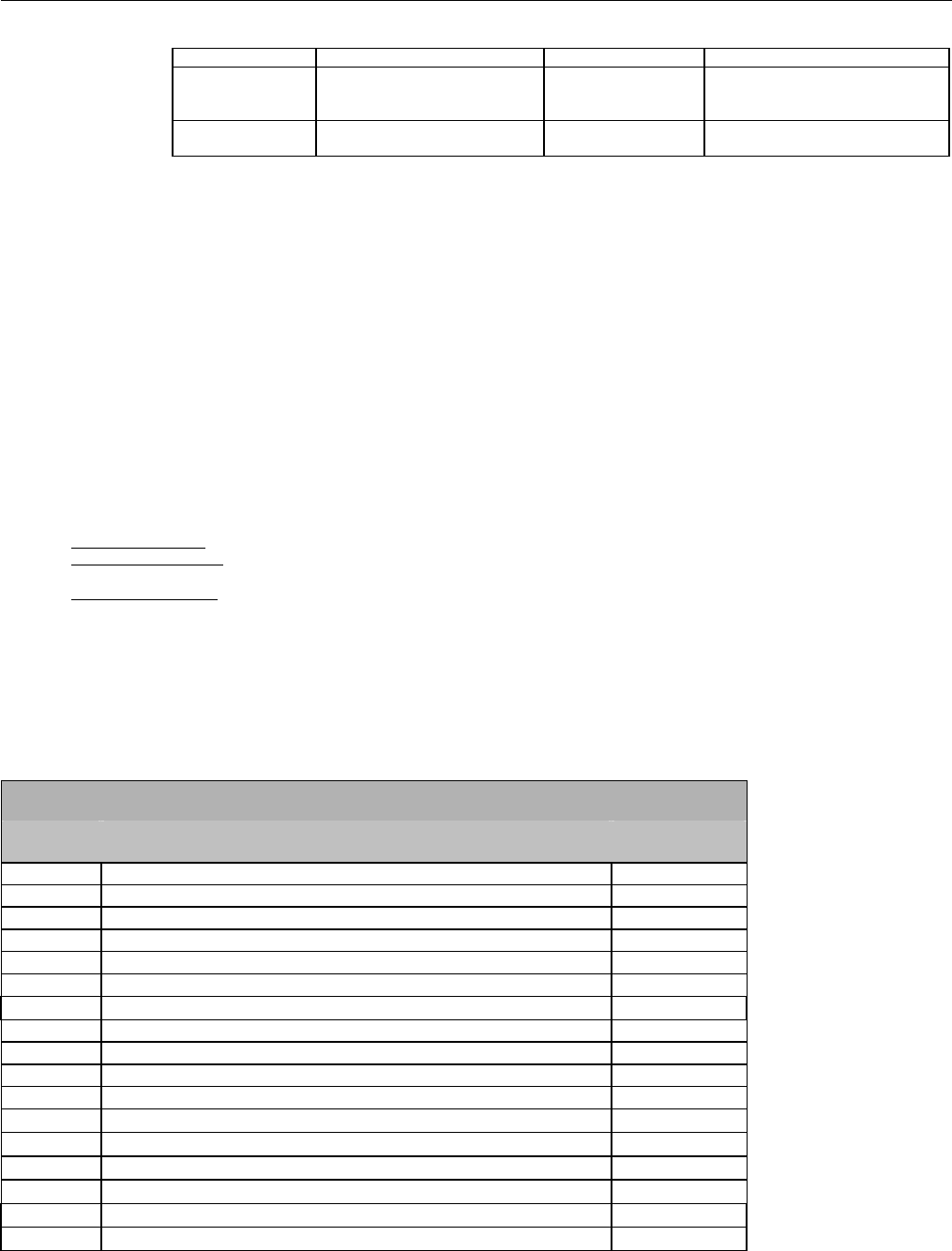
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 720 -
Responsibility Amount
351-NP Other Payer-Patient
Responsibility Amount
Qualifier
Ø5 Amount of Copay (518-FI) as
reported by previous payer
352-NQ Other Payer-Patient
Responsibility Amount
1ØØ{ $1Ø.ØØ
28.1.6.3 WHEN THE PREVIOUS PAYER HAS REJECTED THE SERVICE OR CLAIM
The fields Other Payer-Patient Responsibility Amount Count, Other Payer-Patient Responsibility Amount Qualifier, and Other Payer-Patient
Responsibility Amount would not appear if the previous payer rejected the service or claim submitted, as there would not be a patient’s share
of financial responsibility.
If the payer rejects the service or claim submitted, the payer would not have returned the amounts (Amount Applied to Periodic Deductible,
Amount Attributed to Product Selection fields, et cetera) that apply to the usage of Other Payer-Patient Responsibility Amount Qualifier.
In addition, Example “Billing – Transaction Code B1 – Coordination of Benefits Scenarios Pharmacy Bills To Insurance Designated By Patient”
and Example “Billing – Transaction Code B1 – Coordination of Benefits – Scenario 1: Pharmacy Bills Secondary Insurance” has been added
to show coordination of benefits scenarios.
28.1.6.4 MEDICARE PART D
For Medicare Part D Information Reporting processing, the Coordination of Benefits/Other Payments Segment is not used since the
information being reported is not to be used for payment of a claim. The Insurance Segment is used since the information transmitted provides
clarification on additional attributes of the patient (Other Payer BIN Number (99Ø-MG), Other Payer Processor Control Number (991-MH),
Other Payer Cardholder ID (356-NU), and Other Payer Group ID (992-MJ)) to facilitate the Information Reporting transaction.
These fields are required when the previous payer has financial amounts that apply to Medicare Part D beneficiary benefit stages. These fields
are required when the plan is a participant in a Medicare Part D program that requires reporting of benefit stage specific financial amounts.
Benefit Stage Count (392-MU)
Benefit Stage Qualifier (393-MV) – the value contained in the qualifier must only be used once in all the iterations of Benefit Stage
Count (392-MU) for the transaction.
Benefit Stage Amount (394-MW)
See section “Specific Segment Discussion”, “Response Segments”, “Response Pricing Segments”, “Medicare Part D” for more information.
28.1.6.5 PAYER-TO-PAYER USAGE OF INTERNAL CONTROL NUMBER (993-A7)
The Internal Control Number (993-A7) is only used in payer-to-payer situations for payers to relay their internal numbers to other downstream
payers. When there are multiple payers, the Internal Control Number occurs with the other payer information, inside the specific coordination
of benefits loop.
For example :
Medicare generates a transaction to Medicaid (next downstream payer). Medicare attaches their Internal Control Number to the transaction.
Coordination of Benefits/Other Payments Segment
Field Field Name
111-AM SEGMENT IDENTIFICATION
337-4C COORDINATION OF BENEFITS/OTHER PAYMENTS COUNT 1
338-5C OTHER PAYER COVERAGE TYPE Ø1
339-6C OTHER PAYER ID QUALIFIER
34Ø-7C OTHER PAYER ID Medicare ID
443-E8 OTHER PAYER DATE 20061109
993-A7 INTERNAL CONTROL NUMBER AC22355
341-HB OTHER PAYER AMOUNT PAID COUNT 1
342-HC OTHER PAYER AMOUNT PAID QUALIFIER
431-DV OTHER PAYER AMOUNT PAID Etc…
471-5E OTHER PAYER REJECT COUNT
472-6E OTHER PAYER REJECT CODE
353-NR OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT COUNT
351-NP OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT QUALIFIER
352-NQ OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT
392-MU BENEFIT STAGE COUNT
393-MV BENEFIT STAGE QUALIFIER

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 721 -
394-MW BENEFIT STAGE AMOUNT
Medicaid processes the claim and sends it to the next downstream payer (if one exists). Medicaid includes the Internal Control Number of the
previous payer (if given – in this case Medicare did assign an Internal Control Number). Medicaid can include their Internal Control Number, if
they choose.
Coordination of Benefits/Other Payments Segment
Field Field Name
111-AM SEGMENT IDENTIFICATION
337-4C COORDINATION OF BENEFITS/OTHER PAYMENTS COUNT 2
338-5C OTHER PAYER COVERAGE TYPE Ø1
339-6C OTHER PAYER ID QUALIFIER
34Ø-7C OTHER PAYER ID Medicare ID
443-E8 OTHER PAYER DATE 20061109
993-A7 INTERNAL CONTROL NUMBER AC22355
341-HB OTHER PAYER AMOUNT PAID COUNT 1
342-HC OTHER PAYER AMOUNT PAID QUALIFIER
431-DV OTHER PAYER AMOUNT PAID
471-5E OTHER PAYER REJECT COUNT
472-6E OTHER PAYER REJECT CODE
353-NR OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT COUNT
351-NP OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT QUALIFIER
352-NQ OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT
392-MU BENEFIT STAGE COUNT
393-MV BENEFIT STAGE QUALIFIER
394-MW BENEFIT STAGE AMOUNT
338-5C OTHER PAYER COVERAGE TYPE Ø2
339-6C OTHER PAYER ID QUALIFIER
34Ø-7C OTHER PAYER ID Medicaid ID
443-E8 OTHER PAYER DATE 20061110
993-A7 INTERNAL CONTROL NUMBER 88993433
341-HB OTHER PAYER AMOUNT PAID COUNT 1
342-HC OTHER PAYER AMOUNT PAID QUALIFIER Etc
431-DV OTHER PAYER AMOUNT PAID
471-5E OTHER PAYER REJECT COUNT
472-6E OTHER PAYER REJECT CODE
353-NR OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT COUNT
351-NP OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT QUALIFIER
352-NQ OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT
392-MU BENEFIT STAGE COUNT
393-MV BENEFIT STAGE QUALIFIER
394-MW BENEFIT STAGE AMOUNT
28.1.7 WORKERS’ COMPENSATION SEGMENT
Billing Entity Type Indicator (117-TR) - Code that identifies the entity submitting the billing transaction.
• If the transaction is submitted by the provider and paid to the provider, then the Service Provider ID Qualifier (2Ø2-B2) and Service
Provider ID (2Ø1-B1) govern communication and payment.
• If the transaction is submitted by an agent and paid to the agent, then the Service Provider ID Qualifier (2Ø2-B2) and Service
Provider ID (2Ø1-B1), relative to agent, govern communication and payment and dispensing pharmacy information is place in the
Facility Segment.
• If the transaction is submitted by a provider or agent, but paid to another party, then the Service Provider ID Qualifier (2Ø2-B2) and
Service Provider ID (2Ø1-B1), relative to submitting entity, govern communication, but the information for the party to be paid is
placed in the "Pay To” fields of this segment. If submitting entity is different than dispensing pharmacy, the pharmacy information is
placed in the Facility Segment.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 722 -
Generic Equivalent Product ID Qualifier (125-TZ) and Generic Equivalent Product ID (126-UA) - In some jurisdictions, generics are mandated
but an injured worker may pay the difference for a brand. In those cases information on the brand dispensed and its generic equivalent must
be collected.
28.1.8 DUR/PPS SEGMENT
28.1.8.1 TERMINOLOGY
Drug Use Review (DUR)
Review of appropriate use of medications has undergone significant changes over the past ten or so years. The original evaluations called
Drug Utilization Review, included retrospective review of patients’ charts in the hospital or paid claims from a PBM or processor to determine
the utilization patterns of drugs. Utilization meant how much, how many and at what cost, for the most part.
In the late 197Øs and early 8Øs, Drug Utilization Review transitioned to Drug Use Review and focused increasingly on the appropriate
selection of the medication. In 199Ø, with the passage of OBRA 9Ø (Omnibus Budget Reconciliation Act of 199Ø), the federal government
separated the terms Drug Utilization Review and Drug Use Review to signify retrospective and prospective review of medication regimens,
respectively.
Since that time, utilization review has matured into drug use evaluation, which encompasses much more than the volume or cost of the drug
employed in treatment. Drug Use Evaluation (DUE) is the current terminology that takes into account not only costs but also appropriate
selection based on specific patient parameters such as other drugs in the regimen, concomitant diseases, and competency of organ systems.
When DUR is indicated in this Guide, the implication is that DUE is occurring.
Professional Pharmacy Services (PPS)
Professional Pharmacy Services (PPS) refers to a variety of cognitive services performed by pharmacists. These include, but are not limited
to, the performance of administrative services, prospective DUR, disease management, and delivery of pharmaceutical care. It is oriented
toward preventing and/or solving health care-related problems and achieving positive health outcomes. Some of the problems that justify PPS
are listed in the Reason for Service Codes that describe professional activities that require pharmacists’ attention.
PPS also includes the process of performing, documenting, and receiving reimbursement for cognitive services. PPS begins when a either a
pharmacist or a processor identifies a patient-specific, health care-related problem and notifies the other party. After resolving the issue, the
pharmacist submits the documentation needed to explain the steps planned and the measures taken to resolve the problem. Unlike DUR,
which is always tied to a prescription drug claim, PPS may be completely unrelated to the dispensing of a prescription. Also unlike DUR,
which is completed within the submission of one claim, PPS is a dynamic process that may require multiple claim submissions over time to
document resolution of the patient-care issue.
28.1.8.2 SPECIFIC DISCUSSION – DUR
28.1.8.2.1 THE PROBLEM OF NOISE
The success of any DUR program depends on two factors, namely, the caliber of the criteria used to identify potential drug-related problems
and programming computers to utilize all available information to avoid false positive alerts.
The following are guidelines that computerized review systems can use to reduce the amount of unnecessary traffic (sometimes referred to as
DUR noise) but would still provide a high level of confidence to the client, administrator, processor and pharmacist.
1. Consideration should be given to making DUR alerts specific to the patient and they should be driven by the patient’s individual diagnosis
(reported or inferred), medication history, age, and gender.
2. Consideration should be given to establishing Gender and Age parameters, whenever appropriate.
3. Consideration should be given to the parameters that define a Drug-Pregnancy alert. At a minimum, gender edits should be applied.
Taking into account the patient’s age, reported diagnosis (ICD9), and other drugs the patient may be taking (e.g. oral contraceptive or
prenatal vitamins) should be part of a second-level review.
4. Consideration should be given to the parameters for Lactation/Nursing alerts. At a minimum, gender and age edits should be applied.
5. Consideration should be given to the parameters that define High Dose. Allowance should be given for the inclusion of a percentage
multiplier before displaying i.e., do not display High Dose alert unless the calculated dose is at least a predefined percentage greater than
the benchmark high dose. Alternatively, if the dosing calculation results in a fraction of the days supply, round down to the nearest whole
day.
6. Consideration should be given to the parameters that define Low Dose. Allowance should be given to incorporate a percentage multiplier
before displaying i.e., do not display Low Dose alert unless the calculated dose is at least a predefined percentage lower than the
benchmark low dose. Alternatively, do not alert on maintenance medications where the patient has a sustained refill history of a
predefined number of months or number of prescriptions and an appropriate clinical response.
7. Consideration should be given to Therapeutic Duplication alerts. Prescriptions being reviewed should be active (i.e. have days supply
remaining). Prescriptions should not represent a refill and consideration should be given to switching within a therapeutic category by
allowing a predefined number of days supply overlap within a therapeutic category.
8. Consideration should be given to Therapeutic Duplication alerts when the new prescription is a drug taken for a few days or on an “as
needed” basis (e.g. cough syrup containing codeine for 5 days or less and chronic pain medication containing a narcotic).
9. Consideration of the submission, by the pharmacist, of an appropriate Submission Clarification Code as override for Overuse/Early Refills
should be considered.
10. Consideration should be given to accommodate the patient behavior (e.g. convenience, weather, and transportation) for Overuse/Early
Refill edits. The calculation of days remaining should be rounded down. For example, 25% of 3Ø days = 7.5 days, round down to 7 days)
so Early Refill alerts are not applied on partial days.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 723 -
11. Consideration should be given to the clinical significance of Drug-Drug Interactions. Anything less significant than major interactions may
increase DUR noise without benefit to the patient. Documentation should be provided. Use of the DUR Additional Text field to provide
helpful clarification in a response is encouraged. Drug-drug interaction programming should allow for alerting on various severity levels
depending on the interaction involved, rather than all major or no minor interactions.
12. Consideration should be given to documenting the type and number of drug-drug interactions and other alerts encountered and periodic
reviews should be conducted to determine the validity of alerts. Follow up may be required to establish override mechanisms or improve
the quality of the criteria.
13. Consideration should be given to timeframes when performing DUR checks. Is 365 days too long a time to look back through a profile for
potential problems? It may be appropriate in some cases, but not in others. The same rule may not be appropriate for all types of
checking. All prescriptions in history should have remaining days supply before participating in DUR alerts.
14. Consideration should be given to information provided by the pharmacy on the in-bound transaction. Submission Clarification Codes,
DUR interventions and results, ICD9’s, etc. that indicate a pharmacy has identified and responded to an alert should provide a
mechanism to by-pass processor-generated alerts.
15. Consideration should be given when inferring patients’ medical conditions. Is the inferred diagnosis verified by any other contributing
data?
16. Consideration should be given to the response displayed to the pharmacist. Is all pertinent information provided on the response for the
pharmacist to make a proper decision, e.g. Clinical Significance, Other Pharmacy, Previous Fill Date, Quantity of Previous Fill, Database
Indicator, Other Prescriber, Free text, and DUR Additional Text, if applicable.
17. Consideration should be given to information on the patient’s profile. Outdated or inaccurate data should be removed or excluded to
prevent unwarranted and incorrect alerts from being generated. This could include information about allergies.
18. Consideration should be given to including the Help Desk number whenever an alert is given. At a minimum, it should be included
whenever a specific Call Help Desk (CH) alert is given. Is there a special Clinical Help Desk phone number that applies, instead of the
one used for general eligibility/plan coverage issues?
19. Consideration should be given to maximizing the inherent editing/parameter establishing capabilities that exist within your DUR system.
Understanding them and utilizing them to the fullest will assist in providing as “quiet” a system as possible, while maintaining the highest
level of professional and clinical awareness.
20. Consideration to DUR system maintenance is crucial. Application of clinical data supporting the DUR alert system should be updated
continuously. Delay in the application of the information can lead to outdated alerts, medical and pharmaceutical information and
documentation resulting in distrust of the alerts.
21. Suppress reversed transactions from DUR screening against new transactions. Assume that the claim was reversed because the patient
never took possession of the prescription.
22. Consideration should be given to minimizing DUR alerts especially in creating Reject Code “88 “ (DUR Reject Error) in batch transactions
as the patient most likely has already received the prescription and minimal pharmacist intervention would be possible.
The following chart illustrates alerts that contribute to DUR Noise
DUR/PPS Alert Category Inappropriate DUR Message (False Positive)
General (Applies to all alerts) - Repeat alerts on subsequent dispensings despite pharmacist override/reversal on previous fill of
same medication to same patient by same prescriber
- Alerting on retroactively billed claims when days supply has been exhausted at the time of billing
- Alerting despite in-store disease contraindication overrides
- Sending messages on rebills or resubmissions after reversals
Drug-Pregnancy (PG) - Alerting on claims for males or for females outside childbearing age or with current prescription
for oral contraceptives
- Alerting when claims database contains ICD9 for termination of pregnancy or procedures such as
tubal ligations or hysterectomies
- Alerting on claims that contain estrogens used for menopause
Therapeutic Duplication (TD) - Alerting on refills or if prescription number changes (alert is really ER)
- Alerting on same ingredient/different strength (alert is really ID)
- Alerting when both TD and ID apply (system may not differentiate identical ingredients within the
therapeutic class)
- Alerting even though the therapy is common medical practice (SSRI + trazodone; combinations
of anticonvulsants, insulins)
- Alerting when changing therapy within a therapeutic class (cimetidine to ranitidine; ibuprofen to
naproxen)
Early/Late Refill (ER/LR) - Alerting on titrated drugs (also impacts HD/LD)
High Dose (HD) - Alerting when quantity divided by days supply results in a fraction
- Alerting when literature value differs from standard medical practice (acetaminophen w/codeine
based on acetaminophen content nte 6/d yet directions call for up to 12/day (1-2 q4-6h)
- Alerting on pediatric claims using adult dosing parameters
Low Dose (LD) - Alerting on titrated drugs or those not considered chronic medications
Drug-Disease (DC) - Alerting when drugs infer multiple diseases
Drug Interactions (DD) - Alerting when ICD9 or procedure would render the interaction null and void (e.g. digoxin +
quinidine or verapamil with a pacemaker)
- Alerting when interaction is dose specific (i.e. when drug interaction occurs only with high doses
of either or both medications)
Drug Allergy (DA) - Alerting even though ICD9s refer to “adverse reactions” not necessarily allergies. Pharmacist
profiles represent a more accurate source of allergy information.
Drug-Gender (SX) - Alerting when the prescription is actually for a female
Formulary Issues - Alerting that drug is non-formulary without displaying preferred choice

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 724 -
28.1.8.2.2 DUR INPUTS
The information and support files for the DUR standard fall into four categories:
Member information
Prescription information; Medical information, if available
Prescriber information
Pharmacy information
Three primary files form the electronic claims management (ECM) system that supports ORDUR (On-line, Real-time Drug Use Review)
processing. These files are pertinent regardless whether the electronic claim is submitted via batch mode or real time.
Patient profiles (drug use history file; medical claims history file)
Complete drug master file (drug reference database)
Drug information files (dosing/conflict/interaction database)
28.1.8.2.3 ORDUR SCREENING
On-line DUR categorizes therapeutic conflicts commonly noted in drug therapy according to their mechanism of action. Each category or
"module" makes up a Drug Conflict Rules File or database. Standard codes identify the drug conflicts in each module. The pharmacy provider
and electronic claim ORDUR processor use the codes when exchanging structured electronic messages and responses.
28.1.8.2.4 DOSING/LIMITS
The following therapeutic problems fall into the Dosing/Limits Module:
• Low Dose (LD) detects drug doses that fall below the standard adult dosing range.
• High Dose (HD) detects drug doses that fall above the standard adult dosing range.
• Overuse (ER) detects prescription refills that occur before the days supply of the previous dispensing should have been
exhausted.
• Underuse (LR) detects prescription refills that occur after the days supply of the previous dispensing should have been
exhausted.
• Excessive Duration (MX) detects days supply that are longer than the maximal limit of therapy for the drug product based on
the product's common uses.
28.1.8.2.5 DRUG INTERACTIONS
Two therapeutic problems fall into the drug interaction module.
• Drug-Drug Interaction (DD) detects drug combinations in which the net pharmacologic response may be different from the
result expected when each drug is given separately.
• Drug Incompatibility (DI) identifies physical and chemical incompatibilities between two or more drugs.
28.1.8.2.6 DRUG CONFLICTS
Drug Conflicts consist of a number of drug therapy problems that arise as a result of a combination of the patient's characteristics and a
particular drug. The following therapeutic problems are included in Drug Conflicts:
• Drug-Allergy (DA) indicates that an adverse event may occur due to the patient's previously demonstrated heightened
response to the drug product in question. These responses are not necessary immunologically mediated; they can be
idiosyncratic reactions unrelated to true allergies.
• Prior Adverse Reaction (PR) identifies those drugs to which the patient has previously reacted in an atypical manner.
• Drug-Disease (Inferred) (DC) indicates that the use of the drug may be inappropriate in light of a specific medical condition that
the patient has. The existence of the specific medical condition is inferred from drugs in the patient's medication history.
• Drug-Disease (Reported) (MC) indicates that the use of the drug may be inappropriate in light of a specific medical condition
that the patient has. Information about the specific medical condition is provided by ICD9s, CPT-4s or other specified coding
schemes.
• Drug-Age (PA) detects drugs that are contraindicated for specific ages and apply to patient for whom the claim is submitted.
• Drug-Gender (SX) identifies contraindicated or inappropriate therapy in either males or females.
• Additive Toxicity (AT) detects drugs with similar side effects that could exhibit additive toxic potential.
• Drug-Pregnancy (PG) detects pregnancy-related drug problems. This information is intended to assist the healthcare
professional in weighing the therapeutic value of a drug against possible adverse effects on the mother or fetus.
• Iatrogenic Condition (IC) detects possibly inappropriate use of drugs that are designed to ameliorate complications caused by
another medication (e.g. polypharmacy).
• Side Effect (SE) reports possible major side effects of the prescribed drug.
28.1.8.2.7 DUPLICATE THERAPY
The following two therapeutic problems constitute duplicate therapy.
• Therapeutic Duplication (TD) detects simultaneous use of different chemical entities that have the same therapeutic or
pharmacologic effect.
• Ingredient Duplication (ID) detects simultaneous use of drug products containing one or more identical chemical entities.
28.1.8.2.8 PRECAUTIONARY SCREENINGS

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 725 -
The following therapeutic problems constitute precautionary screenings.
• Alcohol Conflict (OH) detects prescribed drugs that are contraindicated or conflict with the consumption of alcoholic beverages.
• Tobacco Use (DS) conflict detects when a prescribed drug is contraindicated or conflicts with the use of tobacco products.
• Drug-Lab Conflict (DL) indicates that laboratory values may be altered due to the use of the drug, or that the patient's response
to the drug may be altered due to a condition that is identified by a certain lab value.
• Drug-Food Interaction (DF) identifies interactions between a drug and certain foods.
• Call Help Desk (CH) informs the user to call the claims processor's help desk to obtain additional DUR information.
28.1.8.3 SPECIFIC DISCUSSION-PROFESSIONAL PHARMACY SERVICES
28.1.8.3.1 PPS PROCESSING
Like DUR, PPS screening depends upon information contained in many fields.
• Reason for Service Code
• Professional Service Code
• Result of Service Code
• DUR/PPS Level of Effort
• Measurement Date
• Measurement Time
• Measurement Dimension
• Measurement Unit
• Measurement Value
Depending on the source of the transmission, a code transmitted in the Reason for Service Code (439-E4) describes a problem or a request
for a professional pharmacy service identified or initiated by a processor (i.e., processor-to-pharmacist transmission), or the reason a
professional service was performed by a pharmacist (i.e., pharmacist-to-processor transmission).
These codes have been grouped into five areas for better understanding of their uses. The administrative codes are used for claims
processing or plan rules functions. Dosing limits, drug conflicts and disease management codes are used for clinical interventions.
Precautionary codes are used primarily for informational messaging.
ADMINISTRATIVE DOSING/LIMITS DRUG CONFLICT DISEASE MANAGEMENT PRECAUTIONARY
AN - PRESCRIPTION
AUTHENTICATION
ER - OVERUSE AT - ADDITIVE TOXICITY AD - ADDITIONAL DRUG
NEEDED
DF - DRUG-FOOD
INTERACTION
CH - CALL HELP DESK EX - EXCESSIVE
QUANTITY
DA - DRUG-ALLERGY AR - ADVERSE DRUG
REACTION
DL - DRUG-LAB CONFLICT
LK - LOCK IN RECIPIENT HD - HIGH DOSE DC - DRUG-DISEASE
(INFERRED)
CD - CHRONIC DISEASE
MANAGEMENT
DS - TOBACCO USE
MS - MISSING INFORMATION/
CLARIFICATION
LD - LOW DOSE DD - DRUG-DRUG
INTERACTION
CS - PATIENT COMPLAINT/
SYMPTOM
OH - ALCOHOL CONFLICT
NA - DRUG NOT AVAILABLE LR - UNDERUSE DI - DRUG INCOMPATIBILITY DM - APPARENT DRUG
MISUSE
RE - SUSPECTED
ENVIRON-MENTAL
RISK
NC - NON-COVERED DRUG
PURCHASE
MN - INSUFFICIENT
DURATION
IC - IATROGENIC
CONDITION
ED -PATIENT EDUCATION/
INSTRUCTION
SE - SIDE EFFECT
NF - NON-FORMULARY DRUG MX - EXCESSIVE
DURATION
ID - INGREDIENT
DUPLICATION
ND - NEW DISEASE/
DIAGNOSIS
NP - NEW PATIENT PROCESSING NS - INSUFFICIENT
QUANTITY
MC - DRUG-DISEASE
(REPORTED)
NN - UNNECESSARY DRUG
PP - PLAN PROTOCOL SF - SUBOPTIMAL
DOSAGE FORM
NR - LACTATION/NURSING
INTERACTION
PC - PATIENT
QUESTION/CONCERN
PS - PRODUCT SELECTION
OPPORTUNITY
SR - SUBOPTIMAL
REGIMEN
PA - DRUG-AGE PH - PREVENTIVE HEALTH
CARE
TP - PAYER/PROCESSOR
QUESTION
PG - DRUG-PREGNANCY PN - PRESCRIBER
CONSULTATION
PR - PRIOR ADVERSE
REACTION
RF - HEALTH PROVIDER
REFERRAL
SX - DRUG-GENDER SC - SUBOPTIMAL
COMPLIANCE
TD - THERAPEUTIC
DUPLICATION
SD - SUBOPTIMAL DRUG/
INDICATION
TN - LABORATORY TEST
NEEDED
The Professional Service Code (44Ø-E5) describes the professional service performed in responding to the problem identified or service
requested. These codes have been grouped into two areas for better understanding of their uses.
ADMINISTRATIVE PATIENT CARE
∅∅ - NO INTERVENTION AS - PATIENT ASSESSMENT
FE - FORMULARY ENFORCEMENT CC - COORDINATION OF CARE
GP - GENERIC PRODUCT SELECTION DE - DOSING EVALUATION/DETERMINATION
PH - PATIENT MEDICATION HISTORY M∅ - PRESCRIBER CONSULTED
SW - LITERATURE SEARCH/REVIEW MA - MEDICATION ADMINISTRATION
TC - PAYOR/PROCESSOR CONSULTED MR - MEDICATION REVIEW

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 726 -
ADMINISTRATIVE PATIENT CARE
TH - THERAPEUTIC PRODUCT INTERCHANGE P∅ - PATIENT CONSULTED
PE - PATIENT EDUCATION/INSTRUCTION
PM - PATIENT MONITORING
R∅ - PHARMACIST CONSULTED OTHER SOURCE
RT - RECOMMENDED LABORATORY TEST
SC – SELF-CARE CONSULTATION
The Result of Service Code (441-E6) consists primarily of process or procedural results of the professional service that was performed.
Outcome codes that begin with “1” indicate that a drug was dispensed or a professional service provided, “2” indicate that the drug was not
dispensed or the professional service was not provided, and “3” may or may not indicate that a drug was dispensed or a service was provided.
Separate from the Claim Segment Level of Service is the DUR/PPS Level of Effort (474-8E) field that is determined by the complexity of the
decision-making process or resources utilized by a pharmacist to perform a professional service. Following is an example of how the field
might be used:
LEVEL OF
EFFORT CODE EXAMPLE
Ø=Not Specified
11=Level 1
(Lowest)
Straightforward: Service involved minimal diagnosis or treatment options, minimal amount or complexity of data
considered, and minimal risk;
OR Counseling or coordination of care dominated the encounter and required LESS THAN 5 MINUTES of the
pharmacist’s time.
12=Level 2 Low Complexity: Service involved limited diagnosis or treatment options, limited amount or complexity of data
considered, and low risk;
OR Counseling or coordination of care dominated the encounter and required LESS THAN 15 MINUTES of the
pharmacist’s time.
13=Level 3 Moderate Complexity: Service involved moderate diagnosis or treatment options, moderate amount or complexity of
data considered, and moderate risk; OR Counseling or coordination of care dominated the encounter and required
LESS THAN 3Ø MINUTES of the pharmacist’s time.
14=Level 4 High Complexity: Service involved multiple diagnosis or treatment options, extensive amount or complexity of data
considered, and high risk;
OR Counseling or coordination of care dominated the encounter and required LESS THAN 1 HOUR of the
pharmacist’s time.
15=Level 5
(Highest)
Comprehensive: Service involved extensive diagnosis or treatment options, exceptional amount or complexity of
data considered, and very high risk; OR Counseling or coordination of care dominated the encounter and required
GREATER THAN 1 HOUR of the pharmacist’s time.
Five repeating groupings of measurement fields provide clinical information about a patient and assist processors in determining if DUR/PPS
messaging will offer additional advantages in providing optimal patient care. Self-explanatory fields include “Measurement Date” and
“Measurement Time”. Three other measurement fields, Dimension, Unit, and Value, describe the clinical information in specific detail. The
“Measurement Dimension” refers to the clinical domain of the observed value; e.g. blood pressure, temperature, height or weight. The
“Measurement Unit” field contains the metric or English units used for the clinical information; e.g. mmHg, Fahrenheit, inches or kilograms. The
“Measurement Value” field contains the actual value of the clinical information submitted; e.g. 12Ø/8Ø, 98.6, 67, or 7Ø.
28.1.8.4 SPECIAL CONSIDERATIONS
When submitting a service billing for a DUR conflict resolution or professional service provided, the Product/Service ID Qualifier (436-E1) in
the Claim Segment must contain “Ø6” DUR/PPS, the Product/Service ID field (4Ø7-D7) in the Claim Segment must contain zero (“Ø”), and the
appropriate DUR Reason for Service (439-E4) must be submitted in the DUR/PPS Segment, along with additional applicable fields related to
the service claim (an NDC in the Originally Prescribed Product/Service Code; the DUR Co-Agent ID field, etc.)
Further clarification,
If the Product/Service ID Qualifier (436-E1) = “Ø6” (DUR/PPS), the Product/Service ID (4Ø7-D7) is zero. (Zero means “Ø”.)
Populate the DUR/PPS segment as appropriate.
If the Product/Service ID Qualifier (436-E1) = “Ø7” (CPT-4), the Product Service ID (4Ø7-D7) is the actual CPT-4 value.
If the Product/Service ID Qualifier (436-E1) = “Ø9” (HCPCS), the Product Service ID (4Ø7-D7) is the actual HCPCS value.
If the Product/Service ID Qualifier (436-E1) = “99” (Other), the Product Service ID (4Ø7-D7) is the business partner agreed value.
If more than eight Reasons for Service occur, it is recommended the ninth repetition of DUR Reason for Service (439-E4) and all repeating
fields that follow be used to notify the provider to Call Help Desk (“CH”). See transaction Example “Billing w/Submitted DUR Override-
Transaction Code B1”.
DUR Reason for Service (439-E4)
Professional Service Code (44Ø-E5)
Result of Service (441-E6)
Drug Use Review codes and Professional Pharmacy Service codes have been combined to create Reason for Service, Professional Service,
and Result of Service codes.
Professional Service Fee Submitted (477-BE)
For Services Billings (Transaction Code = “S1”), the Professional Service Fee Submitted field in the Pricing Segment must be submitted on a
Prescription/Service Reference Number Qualifier of “2” (Service) and a Product/Service ID Qualifier of “Ø6” (DUR/PPS) in the Claim Segment.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 727 -
The Professional Service Fee Submitted field in the Pricing Segment must not be submitted on a Billing (Transaction Code = “B1”) with a
Prescription/Service Reference Number Qualifier “1” for claims with a Product/Service ID Qualifier of “Ø3” (NDC) in the Claim Segment.
DUR Co-Agent ID Qualifier (475-J9)
DUR Co-Agent ID (476-H6)
When the pharmacist detects and chooses to override a drug-drug interaction or contraindication of therapy involving the drug to be
dispensed, the DUR Co-Agent ID Qualifier and DUR Co-Agent ID should be populated on the claim submission. These fields allow the
processor to discern the drug or medical condition in conflict that the pharmacist is overriding and not return the same DUR conflict message.
However, the processor could still send DUR alerts on other therapeutic conflicts.
The very nature of professional services demands that the fields within the DUR/PPS Segment are not mandatory on a submission.
Professional services may or may not be a part of the reimbursable component of a patient’s pharmacy benefit; the professional service may
be separate and distinct from a product dispensing and, therefore, may or may not be recognized for reimbursement by the payer.
28.1.9 CLAIM SEGMENT
Prescription/Service Reference Number Qualifier (455-EM)
Product/Service ID Qualifier (436-E1)
Product/Service ID (4Ø7-D7)
For Service Billings with Product/Service ID Qualifier of DUR/PPS (Ø6), the Product/Service ID defaults to “Ø” (zero) and the DUR/PPS
Segment is required.
Associated Prescription/Service Reference Number (456-EN)
Associated Prescription/Service Date (457-EP)
A Service Billing may be associated with a prescription dispensed or professional service provided, either at the time of service provision or at
some earlier time. If used, the Associated Prescription/Service Reference Number must contain the Prescription/Service Reference Number
that prompted the service. The Associated Prescription/Service Reference Date must contain the service date of the prescription or service
that prompted the current billing for service. The combination of the Associated Prescription/Service Reference Number and Associated
Prescription/Service Date allows the processor’s system to search for the original item.
If the Prescription/Service Reference Number Qualifier is “2” (Service) billing, and the Product/Service ID Qualifier is “Ø6” DUR/PPS, the Claim
Segment fields must include the default Product/Service ID (“Ø”), and, if applicable, the Associated Prescription/Service Reference Number
and the Associated Prescription/Service Date. Also, for this transaction type, the DUR/PPS Segment is required.
In Version D.Ø and above, the Service Billings have their own Transaction Code (S1, S2, S3). The Transaction Code is at the transmission
level. Claim and service billings are associated (using the Associated Prescription/Service Reference Number (456-EN) and Associated
Prescription/Service Date (457-EP), but they must appear in separate transmissions. Drug product billings are designated by Transaction
Code = “B1” (Billing) and Prescription/Service Reference Number Qualifier = “1” (Rx Billing). Service billings are designated by Transaction
Code = “S1” (Service Billing) and Prescription/Service Reference Number Qualifier = “2” (Service Billing).
Note that in other Transaction Codes (Prior Authorizations, Information Reporting, and Controlled Substance Reporting), the differentiation of
claim versus service remains at the transaction level. For example, drug product transactions are designated by Transaction Code = “P1”
(Prior Authorization Request And Billing) and Prescription/Service Reference Number Qualifier = “1” (Rx Billing). Service billings are
designated by Transaction Code = “P1” (Prior Authorization Request And Billing) and Prescription/Service Reference Number Qualifier = “2”
(Service Billing).
CPT Use
CPT use wasn’t specifically illustrated in this guide. But CPT-4 or 5 are valid values in the Product/Service ID Qualifier field (436-E1).
Example: Transmit a CPT-based service claim not tied to a product by populating the Product/Service ID Qualifier with the value for CPT-4
(“Ø7”), and the Product/Service ID field with the actual CPT-4 value. If the need exists to tie the service claim to an actual billed product, also
populate the situational Associated Prescription/Service Reference Number (456-EN) and Associated Prescription/Service Date (457-EP)
fields.
Quantity Dispensed (442-E7)
Originally Prescribed Quantity (446-EB)
Quantity Prescribed (46Ø-ET)
Only dispensed quantities in the exact fractional amount including three decimal places are supported. Whole number quantities are submitted
as 9999999.ØØØ
Procedure Modifier Code Count (458-SE)
Procedure Modifier Code (459-ER)
Professional services that are related to CPT-4 or CPT5 codes will be submitted in these fields. If the Product/Service ID Qualifier is “Ø9”
(HCPCS), the Procedure Modifier Count and Procedure Modifier Code may be used.
The standard does not prohibit the reporting of procedure code modifier(s) with national drug codes.
Originally Prescribed Product/Service Code (445-EA)
Originally Prescribed Quantity (446-EB)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 728 -
The Originally Prescribed Product/Service Code and Originally Prescribed Quantity fields are used when therapeutic substitution has occurred
or when a DUR alert has been resolved by changing medications or quantities. These fields allow tracking of pharmacists’ interventions for
payers who require this information. The Originally Prescribed Product/Service Code (445-EA) and the Originally Prescribed Quantity (446-EB)
are used to provide necessary data to calculate the exact difference in cost between the prescribed product and the dispensed product. The
Originally Prescribed Quantity (446-EB) is for use with therapeutic interchange only.
Intermediary Authorization Fields (463-EW), (464-EX)
Providers should have access to the Intermediary Authorization Type ID and Intermediary Authorization ID, if applicable for trading partners.
Intermediaries may require providers to enter values in these fields in order to circumvent a system edit that could cause a transaction to
reject.
The Intermediary Authorization fields are also used for adjudication status information from a processor to a client to inform the receiving entity
what action was taken regarding the encounter by the Managed Care Plan. The first digit of the Intermediary Authorization ID (464-EX) will
support the values noted in the Data Dictionary. For this situation, the Intermediary Authorization Type ID (463-EW) will be 99 (Other
Override).
Transaction Reference Number (88Ø-K5)
This field has been added for use in the Medicare Part D Information Reporting Process. The transaction reference number is being used to
track all transactions related to a particular dispensing event. Whoever creates the Information Reporting Transaction is responsible for
creating this number. The entity receiving the Information Reporting Transaction is expected to include that number in their response.
Pharmacy Service Type (147-U7)
A pharmacy has multiple reimbursement contracts with a payer. When the pharmacy submits a transaction to the payer, they would indicate
what type of service they are performing so that the payer can apply the correct contract terms during the adjudication process. For example,
“Joe’s Pharmacy” normally fills prescriptions as an in-store retail provider under contract with “Acme PBM”, but can also receive prescription
orders via mail or Internet and would then fill and mail the prescription to the patient’s home under a separate mail service contractual
arrangement with same payer. “Joe’s Pharmacy” would then submit the claim using a Pharmacy Service Type (147-U7) value of “06” (Mail
Order). “Acme PBM” would then adjudicate the claim under the mail service contract terms.
For pharmacies which have only one contract with a payer, this field may not be sent.
28.1.9.1 PARTIAL FILL
Partial Fill Fields (Dispensing Status (343-HD), Associated Prescription/Service Date (457-EP), Associated Prescription/Service Reference
Number (456-EN), Quantity Intended To Be Dispensed (344-HF), Days Supply Intended To Be Dispensed (345-HG), Basis Of Calculation –
Dispensing Fee (346-HH), Basis of Calculation – Copay (347-HJ), Basis Of Calculation – Flat Sales Tax (348-HK), Basis Of Calculation –
Percentage Sales Tax (349-HM), Basis of Calculation-Coinsurance (573-4V))
On occasion, inventory shortages at the pharmacy prevent a pharmacist from filling a total quantity of prescribed medication. When this
occurs, the pharmacist has three choices:
1. Not fill the prescription that day and have the patient return at a later date to pick it up,
2. Send the patient to another pharmacy, or
3. Partially fill the prescription using the available quantity and have the patient return at a later date to pick up the balance of the
medication (or, alternatively, deliver or mail the remaining medication to the patient).
For several reasons, both pharmacist and patient generally favor option #3. This scenario, however, creates a potential problem because
most pharmacy practice management systems closely integrate the “dispensing” and “billing” functions.
In order to accommodate the need to fill a prescription partially on one day and complete the dispensing on a different date, the following fields
have been are in the Claim Segment:
Dispensing Status (343-HD)
The code in this field indicates that the quantity dispensed is an initial partial fill (P) or the completion of a partial fill (C) and is used only in
situations where inventory shortages do not allow the full quantity to be dispensed.
Associated Prescription/Service Date (457-EP)
Date of the initial transaction in a partial fill. Used when submitting the “completion” transaction.
Associated Prescription/Service Reference Number (456-EN)
The Prescription or Service Reference Number of the initial transaction in a partial fill. Used when submitting the “completion” transaction.
Quantity Intended to be Dispensed (344-HF)
The metric decimal quantity that would have been dispensed if adequate inventory were available. This field is used only in association with a
“P” or “C” in the Dispensing Status field. Note: If populating this field, an assumption is made that the “Days Supply Intended to be Dispensed”
is also sent.
Days Supply Intended to be Dispensed (345-HG)
Days supply for the metric decimal quantity that would have been dispensed on original dispensing if adequate inventory were available. This
field is used only in association with a “P” or “C” in the Dispensing Status field.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 729 -
28.1.9.1.1 PARTIAL FILL ASSUMPTIONS & RECOMMENDATIONS
Partial Fill transactions are limited to an initial “P” transaction indicating a “Partial” fill and a subsequent “C” transaction indicating the
“Completion” of the initial partial fill. Implementation assumptions and recommendations include the following:
Assumptions:
• “Partial” and “Completion” transactions originate from the same provider.
• Completion transactions are submitted to the same processor as the initial Partial transaction.
• See also section “Frequently Asked Questions” “Partial Fill And Change Of Coverage”
• Patient information (i.e. group and cardholder information) must be the same on a “Partial” and its companion “Completion”
transaction.
• A “Partial” transaction can exist without a companion “Completion” transaction. As an example, a “Partial” transaction is submitted
due to an inventory shortage. The patient never returns to pick up the quantity represented by the “Completion” transaction.
• If a pharmacy submits a claim with a value of “P” or “C” in the Dispensing Status field and the processor does not accept/utilize
partial fill logic, the processor will reject the claim and indicate that “Partial Fill” logic is not recognized/supported.
Recommendations:
• Multiple “P” transactions for a single dispensing are not recommended. However, by trading partner agreement, multiple “P”
transactions may be used.
• The Fill Number for both the “P” and “C” transactions are the same (i.e. the fill number is not incremented for the “C” transaction)
unless the prescription number changes.
• On a “C” transaction, the following fields may contain, but are not limited to, data that is different from the data submitted on the
initial “P” transaction: Date of Service, Prescription/Service Reference Number, Quantity Dispensed, Fill Number, Days Supply,
Product/Service ID (i.e., NDC), Ingredient Cost Submitted, Dispensing Fee Submitted, Sales Tax Amount Submitted, and Usual &
Customary Charge.
• “P” and “C” transactions must not be allowed with the same “Date of Service”. When it is necessary to submit a “Partial” and
“Completion” transaction on the same date, the provider must reverse the “Partial” and resubmit the claim with the total quantity.
• The “Associated Prescription/Service Reference Number” and “Associated Prescription/Service Date” fields are required on “C”
transactions. These fields are not required on “P” transactions, unless there are multiple occurrences of partial fills (“P”) for this
prescription.
• When a partial fill transaction is entered into the pharmacy practice management system, special care should be given to price the
initial partial prescription at the Usual & Customary rate which would apply if the full quantity were being dispensed (i.e. per unit price
of the full quantity). Likewise, the subsequent “Completion” transaction should be priced at the same per unit price used in the initial
partial fill transaction.
• In cases where the provider has submitted both a “P” AND “C” transaction but later needs to reverse BOTH transactions, the
transactions must be reversed in the following order:
1. Reverse the “C” transaction.
2. After the “C” transaction has been successfully reversed, reverse the “P” transaction.
28.1.9.2 OTHER COVERAGE CODE (3Ø8-C8)
This is a code representing a summation of other coverage information that has been collected from other payers. The
“Usage/Segment/Clarification” column provides rules for which values to use in summation.
Value Description Usage/Segment/Clarification
Ø Not specified by patient Coordination of Benefits/Other Payments Segment must not be sent.
Zero is the default value.
1 No other coverage This value must only be submitted AFTER the provider has exhausted all
means of determining pharmacy benefit coverage and no other coverage was
identified.
Coordination of Benefits/Other Payments Segment must not be sent.
This value must not be used as a default.
2 Other coverage exists/billed-payment collected Used when Total Amount Paid (5Ø9-F9) from a prior payer is greater than zero.
Coordination of Benefits/Other Payments Segment is required.
If multiple payers have been billed and at least one has paid with Total Amount
Paid (5Ø9-F9) greater than Ø, Other Coverage Code will be 2 regardless of
additional payer responses.
3 Other Coverage Billed – claim not covered Populated when claim is rejected.
Coordination of Benefits/Other Payments Segment is required.
Supporting Coordination of Benefits Reject Code(s) is required.
4 Other coverage exists/billed-payment not
collected
If multiple payers have been billed and none have returned Total Amount Paid
(5Ø9-F9) >Ø, but at least one has returned Total Amount Paid <= Ø, Other
Coverage Code will be 4 regardless of any additional payer rejections.
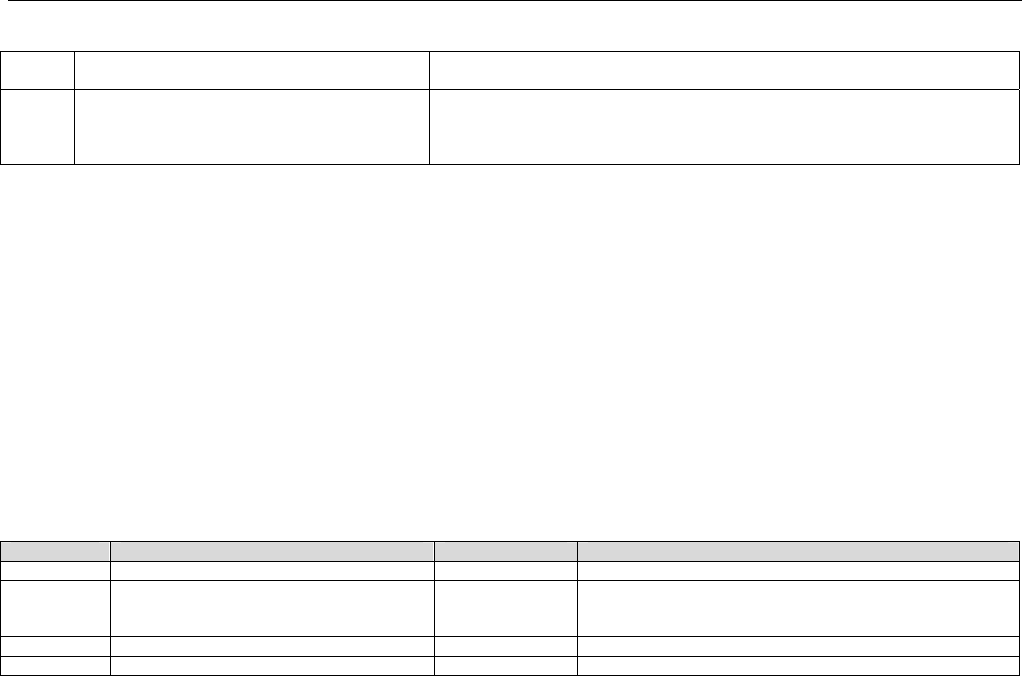
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 730 -
Coordination of Benefits/Other Payments Segment is required.
8 Claim is billing for patient financial
responsibility
Coordination of Benefits/Other Payments Segment is required. It is used to
provide Patient Responsibility detail fields as determined by payer sheet.
See section “Transmission Examples”, and also section “Response Processing Guidelines”, “Pricing Guidelines”, “Patient Financial
Responsibility”.
The Coordination of Benefits/Other Payments Segment/Other Payments Segment is used for secondary, tertiary, etc claims that have
successfully adjudicated with a “P” Paid or “D” Duplicate of Paid or “R” Rejected response from the previous payer(s). The Coordination of
Benefits/Other Payments Segment is not used when the primary payer “C” Captures the claim.
Usage for More than Nine Coverages in Coordination of Benefits:
In the situation where there are more than 9 coverages for a patient, each loop of the Coordination of Benefits/Other Payments Segment must
show the payment or rejection from the payer(s). After the 9th payer, the claim is handled manually to subsequent payers.
28.1.9.3 SPLIT BILLING IN LONG TERM CARE
A skilled nursing facility is reimbursed for Medicare Part A based on the MDS and RUGS score which is a per-diem reimbursement system
that focuses on time-and-motion of a nurse's attention to the resident. The medications that a patient receives during that stay are also paid
for using the same Medicare Part A funds. Part A reimbursement ceases as of the Part A benefit expiration date for the resident. When
applicable, the next covering business entity (insurance, PDP, family, estate) is billed for the rest of the medication days supply.
Scenario: A Medicare Part A resident is dispensed a 30 day supply of medications on September 6th. 11 days into that 30-day supply, the
resident's Part A benefit expires. Rather than return the unused medications to the pharmacy, and then redispense a fresh supply to the
resident, the resident keeps the medication. The 11 days supply is billed to the Part A stay. The 19 days supply are then billed to the next
payer using a date of service of September 17th for a 19 days supply of ingredient cost and no dispensing fee.
Field ID Field Value Comment
4Ø1-D1 Date of Service 2ØØ7Ø917 September 17, 2ØØ7
42Ø-DK Submission Clarification Code 19 Split Billing – indicates the quantity dispensed is the
remainder billed to a subsequent payer when Medicare
Part A expires. Used only in long-term care settings
4Ø5-D5 Days Supply 19
442-E7 Quantity Dispensed 19ØØØ 19.ØØØ
28.1.10 PRICING SEGMENT
To calculate the net amount due, apply one of these formulae.
28.1.10.1 PRESCRIPTION CLAIM REQUEST FORMULA
Ingredient Cost Submitted (4Ø9-D9)
+ Dispensing Fee Submitted (412-DC)
+ Incentive Amount Submitted (438-E3)
+ Other Amount Claimed Submitted (48Ø-H9)
+ Flat Sales Tax Amount Submitted (481-HA)
+ Percentage Sales Tax Amount Submitted (482-GE)
-------------------------------------------------------
= Gross Amount Due (43Ø-DU)
- Patient Paid Amount Submitted (433-DX)
- Other Payer Amount Paid (431-DV)
(Result is Net Amount Due)
Note: Net Amount Due as defined above is applicable to primary and COB claims in which Other Payer Amount Paid (431-DV) is
submitted. Net Amount Due for COB claim billings for Other Payer-Patient Responsibility Amount equals sum of the parts of other
payer-patient responsibility amount(s).
28.1.10.2 SERVICE CLAIM REQUEST FORMULA
Professional Service Fee Submitted (477-BE)
+ Flat Sales Tax Amount Submitted (481-HA)
+ Percentage Sales Tax Amount Submitted (482-GE)
+ Other Amount Claimed Submitted (48Ø-H9)
------------------------------------------------------------
= Gross Amount Due (43Ø-DU)
- Patient Paid Amount Submitted (433-DX)
- Other Payer Amount Paid (431-DV)
(Result is Net Amount Due)
Note: Net Amount Due as defined above is applicable to primary and COB services in which Other Payer Amount Paid (431-DV) is
submitted. Net Amount Due for COB service billings for Other Payer-Patient Responsibility Amount equals sum of the parts of other
payer-patient responsibility amount(s).

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 731 -
28.1.10.3 OTHER INFORMATION
Note: Other Payer Amount Paid is in the Coordination of Benefits/Other Payments Segment, not the Pricing Segment.
Processors and third party programs determine the rules for which fields are required or situational, in light of the situations defined in this
document. All other fields submitted would be ignored by the processor. If a pharmacy system chooses to send in more fields than are
required or situational by the processor, these fields would be ignored. It is recommended that especially for the dollar fields, if the field is not
required or situational in the calculation, that the dollar field not be sent.
The Usual and Customary Charge (426-DQ) represents the value that a pharmacist is willing to accept as their total reimbursement for
dispensing the product/service to a cash-paying customer. It does not include Other Amount Claimed Submitted (48Ø-H9), Dispensing Fee
Submitted (412-DC), Flat Sales Tax Amount Submitted (481-HA), Percentage Sales Tax Amount Submitted (482-GE), Professional Service
Fee Submitted (477-BE), or Incentive Amount Submitted (438-E3). Usual and Customary Charge (426-DQ) is independent of contracted
Dispensing Fee Submitted (412-DC) and Ingredient Cost Submitted (4Ø9-D9).
28.1.11 COUPON SEGMENT
Coupons may be fixed amounts or percentages of total price and may be reimbursed to the pharmacy by the coupon originator or third-party
payer. Transactions for coupon processing accommodate electronic conversations between the pharmacy and the coupon originator as well
as third-party payers.
To bill a coupon processor using the Coupon Segment, the Coupon Type (485-KE) and Coupon Number (486-ME) fields are mandatory. If
applicable, the value amount of the coupon is entered into the Coupon Value Amount (487-NE).
A coupon is used to reduce the patient out of pocket prescription cost – by either reducing the cost of a CASH prescription or the copay from a
Third Party payer who allows coupon usage. The coupon processor is the LAST payer. (Note: Some Federal and State programs do not
allow the reduction of copays.)
Patients are provided with product coupons from manufacturers and/or may also receive coupons distributed from their third party plan.
• A manufacturer coupon is typically for a specific product and may be found in a magazine, newspaper, etc. Some coupons are
provided by manufacturers to the physician – in place of providing free sample products. Regardless of how the patient
received the coupon, they must have a prescription for the coupon product. Use of coupons is encouraged for better patient
care as pharmacies are likely to have a more complete record of medications prescribed by ‘other’ physicians.
• Third party plans may provide coupons that are more generic in nature. For example, the patient will get a reduced copay for
this fill by switching to a formulary product or it may be more product specific as with the manufacturer coupon.
Programs providing coupons want to ‘track’ their usage. They do this via the coupon identifier only (if identifier is unique) or by coupon
identifier and patient identifiable information. When required, patient identifiable information is generally used to provide patient limitations (e.g.
one offer per customer). This often occurs in instances where the coupon identifier is not a unique number (e.g. newspaper or magazine
coupon).
Requirements for submission of Patient and Coupon criteria must be specified in the payer sheets or similar communications in order for the
submitter to know the patient information required and how the coupon is to be identified to the payer.
The Coupon Segment supports
1) Free Product - Patient is provided the product at no cost. Manufacturer coupons for a Free Product should be submitted as Primary
Billing.
2) Price Discount - Patient’s out of pocket cost is reduced by a designated coupon amount (e.g. $5.00 off). Please note state or federal
regulations may prohibit the use of coupons.
The Coupon Segment should NOT be used for replacement of inventory since the Telecommunication Standard was not designed to address
this. Only one coupon is allowed (one Coupon Segment) per transaction.
28.1.12 COMPOUND SEGMENT
This document supports compound prescription processing including up to 99 ingredients. It is recommended that not more than 25
ingredients be submitted at one time to prevent exceeding the normal buffer capacity and causing time-out situations between pharmacy and
processor. Only one transaction per transmission is allowed when billing for a multi-ingredient prescription.
A Compound is submitted using the Compound segment with multiple iterations of the Compound Product ID Qualifier, Compound Product ID
and other repeating fields – one iteration for each ingredient in the compound. This transaction allows the pharmacy to submit any/all of the
ingredients included in the preparation of the compound.
Each ingredient of a compound is contained within the iterations of the Compound Segment within a transaction. Each ingredient is not
allowed to be sent in separate transactions of a transmission.
The order of the compound ingredients does not make any difference when submitting a claim.
Advantages:
1. Ability to perform DUR.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 732 -
2. Ability to claim manufacturers rebates for all ingredients
3. Ability to minimize rebate disputes.
4. Ability to perform accurate pricing per ingredient.
28.1.12.1 CLAIM AND PRICING SEGMENT FIELDS
When billing for multiple ingredients, use the following Claim and Pricing Segment fields:
Product/Service ID (4Ø7-D7) – defaults to zero. (Zero means “Ø”.)
Product/Service ID Qualifier (436-E1) – defaults to “ØØ”
The Product/Service ID must contain a value of “Ø” and Product/Service ID Qualifier must contain a value of “ØØ” when used for
multi-ingredient compounds.
Quantity Dispensed (442-E7) – quantity of entire multi-ingredient product
Ingredient Cost Submitted (4Ø9-D9) – sum of all individual ingredient costs
Compound Code (4Ø6-D6) – must be “2”
Route of Administration (995-E2) – When used in multiple ingredient processing, this field contains the route of administration of the
complete compound mixture. The data in this field is used primarily for on-line real-time drug use review in order to avoid
unnecessary processing time and screening by the claims processor. This field can be used to selectively apply DUR modules to
compounds submitted on-line. For example, in general, topical preparations do not result in drug-drug interactions; thereby the
claims processor can bypass this DUR module.
28.1.12.2 DEFINITIONS
Compound Dosage Form Description Code (45Ø-EF)
Definition: Dosage form of the complete compound mixture.
Purpose: The data in this field is reported one time. When used in combination with Compound Dispensing Unit Form Indicator field 451-EG, a
complete description of the compound prescription dispensed is provided.
Compound Dispensing Unit Form Indicator (451-EG)
Definition: NCPDP standard product billing codes.
Purpose: The total compound metric decimal quantity expressed as Each, Gram, or Milliliter. When used in combination with Compound
Dosage Form Description Code field 45Ø-EF, a complete description of the compound prescription dispensed is provided.
Example: Describes the units’ form of the entire compound, such as 1Ø each, 3Ø grams, or 1ØØØ milliliters.
Compound Ingredient Component Count (447-EC)
Definition: Count of compound product IDs (both active and inactive) in the compound mixture submitted.
Purpose: Compound count number provides the total iterations of the ingredients submitted for reporting, billing, reimbursement and DUR.
Compound Product ID Qualifier (488-RE)
Definition: Code qualifying the type of product dispensed.
Purpose: Identifies what type of drug code is reported in the Compound Product ID. For example, is the product identifier an NDC or a UPC?
Compound Product ID (489-TE)
Definition: Product identification of an ingredient used in a compound.
Purpose: Identifies the code of the product being dispensed for which payment is being requested. For example, this could be the NDC or the
UPC that is unique to the product.
Compound Ingredient Quantity (448-ED)
Definition: Amount expressed in metric decimal units of the product included in the compound mixture.
Purpose: Data in this field reports the metric decimal quantity of the product used in the compound mixture and facilitates the calculation of the
reimbursement amount for this ingredient.
Compound Ingredient Drug Cost (449-EE)
Definition: Ingredient cost for the metric decimal quantity of the product included in the compound mixture indicated in “Compound Ingredient
Quantity” (448-ED).
Purpose: Facilitates the calculation of reimbursement for this ingredient.
Compound Ingredient Basis of Cost Determination (49Ø-UE)
Definition: Code indicating the method by which the drug cost of an ingredient used in a compound was calculated.
Purpose: Facilitates the calculation of reimbursement for the ingredient by specifying the method by which the drug cost was calculated.
Compound Ingredient Modifier Code Count (362-2G)
Definition: Code indicating the number of modifiers codes to follow.
Compound Ingredient Modifier Code (363-2H)
Definition: Identifies special circumstances related to the dispensing/payment of the product as identified in the Compound Product ID (498-
TE).
Submission Clarification Code Count (354-NX)
Definition: Code indicating the number of clarification codes to follow.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 733 -
Submission Clarification Code (42Ø-DK)
Definition: Code indicating that the pharmacist is clarifying the submission.
Value: 8 Process Compound for Approved Ingredients.
Purpose: If one or more ingredients is not covered, and a value of 8 is not submitted, the claim must be rejected. However, the pharmacist
may decide to accept payment excluding the non-covered ingredient(s). A value 8 is resubmitted on a rejected compound prescription when
the pharmacist decides to accept payment for all other ingredients, except those not covered by the plan.
28.1.12.3 USE OF COMPOUND FIELDS
The following fields pertain to the entire compound. Each field is preceded by its field identifier and is followed by a field separator. The
Compound Ingredient Component Count contains the total number of ingredient iterations that will be present.
Field # Field Name
45Ø-EF Compound Dosage Form Description Code
451-EG Compound Dispensing Unit Form Indicator
447-EC Compound Ingredient Component Count
The following fields pertain to each compound ingredient. These fields must be repeated as required, for each ingredient. Each field
is preceded by its field identifier and followed by a field separator.
Field # Field Name
488-RE Compound Product ID Qualifier
489-TE Compound Product ID
448-ED Compound Ingredient Quantity
449-EE Compound Ingredient Drug Cost
49Ø-UE Compound Ingredient Basis Of Cost Determination
362-2G Compound Ingredient Modifier Code Count
363-2H Compound Ingredient Modifier Code
There are situations where a modifier could be necessary for a payer to properly process a claim with an NDC code. For example, a KO
modifier could be required on nebulizer drugs. The Compound Ingredient Modifier Code is used to identify the modifier that is applicable to a
particular ingredient (NDC) within the compound.
Compound Ingredient Drug Cost (449-EE) and Compound Ingredient Basis of Cost Determination (49Ø-UE) must be sent, even if the
Compound Ingredient Drug Cost (449-EE) rounds to zero.
28.1.12.4 COMPOUND INGREDIENT CALCULATES TO BE LESS THAN $Ø.ØØ5
If an ingredient in a compound calculates to be less than $Ø.ØØ5 cent for the dosage being prescribed and is reported, the Compound
Ingredient Drug Cost (449-EE) and Compound Ingredient Basis of Cost Determination (49Ø-UE) must be sent for this drug in the compound
segment.
For example a compound contains 4 ingredients:
NDC Name Strength Pack
Size Cost Qty in
Compound Extended Cost
ØØ574-Ø421-25 Hydrocortisone
Acetate
25 $56.2Ø 1.5ØØ $3.372
ØØ395-1619-64 Menthol Crystals 12Ø $17.56 .Ø6Ø $Ø.ØØ87
ØØ395-Ø467-92 Camphor Spirits
Sol.
6Ø $Ø.97 .Ø6Ø $Ø.ØØØ97
6Ø432-Ø546-16 Lindane Lotion 1% 48Ø $47.Ø6 6Ø.ØØ $5.882
The Camphor Spirits has an extended cost of less than $Ø.ØØ1. If reported, these fields must be sent, even if the Compound Ingredient
Drug Cost (449-EE) rounds to zero.
28.1.12.5 SUPPORT OF A SINGLE INGREDIENT COMPOUND
The support of the Compound Segment must be used for one or more ingredients in a compound. The Count reflects the number of iterations
of product sent, whether one or more than one.
28.1.12.6 MULTI-INGREDIENT COMPOUND AND REJECTS
How do you indicate on the initial rejected response for a multi-ingredient compound transaction which ingredients will not be paid, so the
provider will understand which ingredients will be paid, if they decide to submit another transaction with a Submission Clarification Code of 8
(Process Compound For Approved Ingredients)?
In this compound question, the Missing/Invalid (M/I) reject code may not be specific enough and an appropriate drug-level reject code must be
used. In other rejection situations, the M/I reject codes are specific enough.
In the NCPDP Data Dictionary, the Appendix “Reject Codes”, the chart contains a column “Field Number Possibly In Error”. This column can
be used as guidance for identifying the field in error. For example, Reject Code “7Ø “ states that 4Ø7 (Product/Service ID) is possibly in error.
Whether billing for a single ingredient or multiple ingredient, reject codes exist to further explain the rejection. Therefore reject codes that refer
to similar fields in the Request Claim Segment or the Compound Segment can be used to explain the rejection.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 734 -
Either of the structures below could be used. In this example, the provider submits 5 ingredients to the processor. The processor sends back
3 rejects. Two rejects are related to compound ingredients and one is not. The processor rejects ingredients three and four.
Reject Codes related to compound ingredients:
Reject Code ”7Ø “ Product/Service Not Covered (ingredient 3)
Since this claim is a multi-ingredient compound claim, there is only one claim permitted in the transmission, and
the Compound Segment is present. The Product/Service Not Covered by default has to reference the
Compound Product ID, which by definition is a repeating field and eligible to use the Reject Field Occurrence
Indicator field. In this situation, the “possible field in error” is the Compound Product ID (489-TE).
Reject Code “21 “ M/I Product/Service ID – using Compound Product ID (489-TE) (ingredient 4)
Reject Code “56 “ is not related to compound ingredient rejects, but to another error in the transaction:
Reject Code”56 “ Non-matched Prescriber ID
Example 1:
111-AM SEGMENT IDENTIFICATION 21
112-AN TRANSACTION RESPONSE STATUS R
51Ø-FA REJECT COUNT 3
511-FB REJECT CODE 7Ø
546-4F REJECT FIELD OCCURRENCE INDICATOR 3
511-FB REJECT CODE 21
546-4F REJECT FIELD OCCURRENCE INDICATOR 4
511-FB REJECT CODE 56
Or Example 2:
111-AM SEGMENT IDENTIFICATION 21
112-AN TRANSACTION RESPONSE STATUS R
51Ø-FA REJECT COUNT 3
511-FB REJECT CODE 56
511-FB REJECT CODE 7Ø
546-4F REJECT FIELD OCCURRENCE INDICATOR 3
511-FB REJECT CODE 21
546-4F REJECT FIELD OCCURRENCE INDICATOR 4
In Example 1, the Reject Codes related to occurrences appear first (“7Ø “ and “21 “) and the Reject Code at the transaction level (“56 “) occurs
last. In Example 2, the Reject Code at the transaction level (“56 “) occurs first and then any Reject Codes related to occurrences follow (“7Ø “
and “21 “). Either method is permitted because parsing routines must interrogate the Reject Code, then look for the next field. If the next field is
the Reject Field Occurrence Indicator, the Reject Code is pointing to a field that has a relationship to an occurrence. If the next field is not the
Reject Field Occurrence Indicator, the Reject Code stands on its own (transaction level).
28.1.12.7 MULTI-INGREDIENT COMPOUNDS AND DUR REJECTS
The Response DUR/PPS Segment is not set up to “point” to given reject scenarios, so it must not be interpreted as such. The DUR
information cannot be syntactically “tied” to specific Reject Codes, or a specific Compound Ingredient count occurrence. A possible solution
uses the DUR Free Text (544-FY). See examples below.
When the DUR information is related to prescriptions previously sent by this same pharmacy, the Prescription/Service ID, would work to
provide more specific detail about the reasons for the DUR information; whereas the Product/Service ID and/or Drug Name is more helpful for
a different pharmacy.
28.1.12.7.1 SCENARIO ONE
DUR Rejections (Reject Code “88 “) for ingredients within a submitted compound claim can have the ingredient identified in the Reject Field
Occurrence Indicator (546-4F). The following example from this guide illustrates that a HIGH DOSE alert REJECTION is applicable. The
example showed Reject Code “88 “ at the transaction level, which is not incorrect, but is not specific enough. By modifying the example to
specifically illustrate that another Reject Field Occurrence Indicator (546-4F) immediately after the ”88 “ Reject Code is permitted and refers to
the “88 “ Reject Code, (occurring immediately above the first 546-4F field), provides greater clarity that the DUR Reject is related to the third
ingredient. The second 546-4F refers then to the “EE “ code, per the original example, also referencing the 3rd ingredient. Note that even
though both the “88 “ and the “EE “ refer to the 3rd ingredient; each reject code must have the 546-4F field to specify the ingredient number.
Compounded Rx Billing Rejected Response
Billing rejected for processor-identified DUR conflict.
RESPONSE HEADER SEGMENT
FIELD FIELD NAME VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER DØ D.Ø Transaction Standard
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 735 -
1Ø3-A3 TRANSACTION CODE B1 Billing
1Ø9-A9 TRANSACTION COUNT 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER Ø7 NCPDP Provider ID
2Ø1-B1 SERVICE PROVIDER ID 4563663bbbbbbbb
4Ø1-D1 DATE OF SERVICE 1997Ø92Ø September 2Ø, 1997
RESPONSE STATUS SEGMENT
FIELD FIELD NAME VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS R Rejected
5Ø3-F3 AUTHORIZATION NUMBER 123456789123456789
51Ø-FA REJECT COUNT 2 2 Reject Codes follow
511-FB REJECT CODE 88 DUR reject
546-4F REJECT FIELD OCCURRENCE INDICATOR 3 Ingred #3: Diphenhydramine
511-FB REJECT CODE EE M/I Compound Ingredient Drug Cost
546-4F REJECT FIELD OCCURRENCE INDICATOR 3 Ingred #3: Diphenhydramine
549-7F HELP DESK PHONE NUMBER QUALIFIER 3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER 6Ø2357Ø862
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION 22 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
1234567
RESPONSE DUR/PPS SEGMENT
FIELD FIELD NAME VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION 24 RESPONSE DUR/PPS SEGMENT
567-J6 DUR/PPS RESPONSE CODE COUNTER 1 1st DUR conflict follows
439-E4 REASON FOR SERVICE CODE HD High Dose alert
532-FW DATABASE INDICATOR 5 Other
544-FY DUR FREE TEXT MESSAGE MAX DOSE=6/DAY (Up to 3Ø bytes)
This example is accurate, but does not relay a complete picture. Please continue reading.
28.1.12.7.2 SCENARIO TWO
But, even just indicating the occurrence indicator (i.e., the ingredient number) may be difficult for the pharmacist to associate these reject
codes and occurrence indicators with the Response DUR/PPS Segment. For example, the Reject Field Occurrence Indicator in the above
example states that the DUR Rejection was with the third ingredient. The Response DUR/PPS Segment has the applicable DUR/PPS codes
as the FIRST DUR/PPS Segment loop.
28.1.12.7.3 SCENARIO THREE
DUR Alerts that are non-rejections (just a warning message via the Response DUR/PPS Segment) will not get a DUR Reject Code and
therefore no DUR Reject Field Occurrence Indicator since these are not rejections. The ingredient within the compound causing the DUR
message still needs to be identified for the pharmacist.
28.1.12.7.4 SCENARIO FOUR
DUR problems with a newly submitted non-compound claim (lovastatin) may exist with a previously filled multi-ingredient compound claim
(clarithromycin tablet in a compound that contains a vehicle and a flavoring agent—the patient cannot tolerate the manufacture’s suspension
product for some reason). In this case, the ingredient in the profiled compound claim has to be identified in the Response DUR/PPS Segment.
28.1.12.7.5 SCENARIO FIVE
An ingredient within a submitted multiple-ingredient compound claim interacts with an ingredient in another previously submitted and paid
multiple-ingredient compound claim. In this case, both ingredients (in the new and the old claims) need to be relayed to the pharmacist.
28.1.12.7.6 RECOMMENDATIONS
1) A possible solution is to use the DUR Free Text (544-FY) field contents in the event of a DUR alert with one of the ingredients of the
incoming compound claim. Insert the hard coded prefix “ING##”, where “##” is replaced with the count number of the applicable ingredient, in
front of the system-generated free text message. If the resultant message is longer than the 3Ø bytes maximum for the field, truncate trailing
characters to make 3Ø.
For example, if a high dose alert is generated with the fourth ingredient in the compound, the text field may be, “ING04 MAX DOSE = 6
UNITS/DAY” (28 characters long). If this is not a DUR Reject situation (the transaction is not rejected; no Reject Code 88 is generated or to
be returned to the pharmacy), the Reject Code and Reject Field Occurrence Indicator fields do not get populated.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 736 -
RESPONSE DUR/PPS SEGMENT
FIELD FIELD NAME VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION 24 RESPONSE DUR/PPS SEGMENT
567-J6 DUR/PPS RESPONSE CODE COUNTER 1 1st DUR conflict follows
439-E4 REASON FOR SERVICE CODE HD High Dose alert
532-FW DATABASE INDICATOR 5 Other
544-FY DUR FREE TEXT MESSAGE INGØ4 MAX DOSE=6/DAY The 4th ingredient in the compound is
potentially dosed too high.
2) If an incoming non-compound claim creates a DUR alert with a compound claim already on file, insert the hard coded prefix “CMPD:” before
the system-generated free text message. If the resultant message is longer than the 3Ø bytes maximum for the field, truncate trailing
characters to make 3Ø.
For example if a drug-drug interaction exists between a non-compound lovastatin claim with the first ingredient (clarithromycin tablet) in a
previously submitted compound, “CMPD:CLARITHROMYCIN”.
RESPONSE DUR/PPS SEGMENT
FIELD FIELD NAME VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION 24 RESPONSE DUR/PPS SEGMENT
567-J6 DUR/PPS RESPONSE CODE COUNTER 1 1st DUR conflict follows
439-E4 REASON FOR SERVICE CODE DD Drug-Drug Interaction
528-FS CLINICAL SIGNIFICANCE CODE 1 Severity Level 1
529-FT OTHER PHARMACY INDICATOR 3 Different pharmacy
53Ø-FU PREVIOUS DATE OF FILL 1997Ø9Ø1 September 1, 1997
531-FV QUANTITY OF PREVIOUS FILL 3Ø
532-FW DATABASE INDICATOR 5 Other
533-FX OTHER PRESCRIBER INDICATOR 1 Same prescriber
544-FY DUR FREE TEXT MESSAGE CMPD: CLARITHROMYCIN
TAB 5ØØMG The interaction is due to the Clarithromycin in
a previously filled multiple ingredient
compound claim.
3) If an ingredient in an incoming Multi-Ingredient Compound claim causes a DUR alert due to an ingredient in a profiled, previously-filled
compound, the free text message should be “ING## W/CMPD: DRUG NAME”. The ingredient number in the submitted claim is displayed first,
followed by the indicator that a profiled compound claim is also involved, followed by as much of the drug name, medical condition, or
whatever applicable text string as possible within the available 3Ø bytes.
For example, if the second ingredient (Morphine) in a submitted common compounded oral pain cocktail interacts with the 5th ingredient
(Gorillicillin) in a profiled, previously submitted multiple ingredient compound claim, the following is represented:
RESPONSE DUR/PPS SEGMENT
FIELD FIELD NAME VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION 24 RESPONSE DUR/PPS SEGMENT
567-J6 DUR/PPS RESPONSE CODE COUNTER 1 1st DUR conflict follows
439-E4 REASON FOR SERVICE CODE DD Drug-Drug Interaction
528-FS CLINICAL SIGNIFICANCE CODE 1 Severity Level 1
529-FT OTHER PHARMACY INDICATOR 3 Different pharmacy
53Ø-FU PREVIOUS DATE OF FILL 1997Ø9Ø1 September 1, 1997
531-FV QUANTITY OF PREVIOUS FILL 3Ø
532-FW DATABASE INDICATOR 5 Other
533-FX OTHER PRESCRIBER INDICATOR 1 Same prescriber
544-FY DUR FREE TEXT MESSAGE INGØ2 W/ CMPD:
GORILLICILLIN The second ingredient in the submitted
compound is in conflict with the Gorillicillin in a
previously filled multiple ingredient compound
claim.
Note: there is not enough room in the DUR Free Text field to adequately display information on both ingredients from each compound claim.
28.1.12.8 SHARED REJECT CODES
The Telecommunication Reject Codes listed in the NCPDP External Code List (ECL), offers guidance on which fields to review for potential
correction of rejections by providing, in a separate column, “Field Numbers Possibly in Error” for individual reject codes. Within the NCPDP
Telecommunication Standard there are like fields that are used within the processing of a compounded claim and a non-compound claim.
Since a Claim Billing using the Compound Segment must contain only one (1) transaction within a transmission, there would be no occasion
where like fields would be submitted within the same transmission. Therefore, reject codes, which apply to fields used in non-compounded
claim transactions can in most cases also apply to like fields used in compounded claim transactions.
For example, there are many reject codes that refer to like fields, Product/Service ID (4Ø7-D7) and Compound Product ID (489-TE) in the
“Field Numbers Possibly in Error” column. These fields qualify for shared usage of reject codes since the Product/Service ID (4Ø7-D7) field
has no significance in a compounded claim transaction. This same logic holds true for other fields within the Compound Segment, Compound
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 737 -
Ingredient Basis Of Cost Determination (49Ø-UE) and Compound Product ID Qualifier (488-RE) specifically. Their counterpart non-
compounded claim transaction fields, Basis Of Cost Determination (423-DN) and Product/Service ID Qualifier (436-E1) respectively are not
used within a compounded claim transaction. The reject codes that must be used with these like fields will be those reject codes that pertain to
the non-compounded claim fields. Guidance on compound prescription processing within this Guide provides the following information:
When billing for multiple ingredients, use the following Claim Segment fields:
Product/Service ID (4Ø7-D7) – defaults to zero. (Zero means “Ø”.)
Product/Service ID Qualifier (436-E1) – defaults to “ØØ”
Because default values have been provided, there is the possibility that bad data submitted for these fields could cause a claim rejection. It is
important to note that such rejections would be readily distinguished from rejections on fields Compound Ingredient Basis Of Cost
Determination (49Ø-UE) and Compound Product ID Qualifier (488-RE) since the Reject Field Occurrence Indicator (546-4F) would not be sent
as it would for rejections involving the repeating fields. Compound Ingredient Basis Of Cost Determination (49Ø-UE) and Compound Product
ID Qualifier (488-RE).
The remaining fields within the Compound Segment do not follow this same logic and fall into two categories.
• The first category of fields also has like counterpart non-compounded claim fields but those like fields have significance within the
processing of a compounded claim transaction and therefore cannot share reject codes. The counterpart fields for Compound Ingredient
Quantity (448-ED) and Compound Ingredient Drug Cost (449-EE) are Quantity Dispensed (442-E7) and Ingredient Cost Submitted (4Ø9-
D9) respectively. For compounded claim transactions, Quantity Dispensed is populated with the final quantity of the compounded drug
and Ingredient Cost Submitted with the total ingredient cost of all component ingredients within the compound.
• The second category of fields do not have like non-compounded claim counterpart fields since the information is inherent in other fields
submitted on Claim Billing transactions or are unique to compounded claim transactions. For example Compound Dosage Form
Description Code (45Ø-EF), and Compound Dispensing Unit Form Indicator (451-EG) do not have claim counterpart fields since the
information they supply on a compounded claim is gleaned from the National Drug Code (NDC) as it resides on a Formulary Data Base
for non-compounded claims. Compound Ingredient Component Count (447-EC) does not have a like non-compounded claim counterpart
field because of it’s uniqueness to the processing of compounds and the need to know how many ingredients exist within the compound.
This will not create confusion for the claim provider because as the creator of that transaction, the software system is aware of whether or not
the transaction is for a compound or a single ingredient. Additionally, processor/payer software systems will benefit from having a single set of
reject codes that apply whether it is for a single ingredient billing transaction or a compound billing transaction.
28.1.13 PRIOR AUTHORIZATION SEGMENT
Prior Authorization Supporting Documentation (498-PP) is used to supply information not included in other data fields that may be required to
process the prior authorization transaction.
When Request Type (498-PA) value of “2” (Reauthorization) is used, the Prior Authorization Number-Assigned (498-PY) is populated with the
prior authorization number from the original request.
See the NCPDP Data Dictionary for comments under each field for further clarification.
28.1.14 CLINICAL SEGMENT
The Clinical Segment includes the fields necessary to identify unique patient demographics, such as diagnoses, height, weight, and laboratory
measurements. The standard utilizes several new fields to accomplish the goal of describing patients’ current health status.
Diagnosis Code (424-DO)
All diagnosis code fields must adhere to the owner’s code set rules and formats.
Clinical Information Counter (493-XE) indicates the occurrence number of set/grouping of patient information that follows. The term “counter”
as used in the clinical information and DUR/PPS segments is synonymous with occurrence number. For example, in a repetition of four, the
first occurrence of the field or set/logical grouping would be preceded by a counter with a value of “1”. The second occurrence of that field or
set/logical grouping would be preceded by a counter with a value of “2”, the third occurrence would be preceded by a counter with a value of
“3” and so forth.
Measurement Date (494-ZE) is the date on which the submitted measurement was valid.
Measurement Time (495-H1) is the time at which the submitted measurement was valid entered as military time. For example, 24ØØ is
midnight on the date indicated; 2359 is 11:59p.m. or one minute before midnight on the date indicated.
Measurement Dimension (496-H2) represents the domain of the clinical information; e.g., Height, Weight, Theophylline Level, Blood Pressure
(BP), and Serum Creatinine (SCr).
Measurement Unit (497-H3) indicates the measuring system used in the Measurement Value field that follows; e.g., cm, lb, mg/dl, and mmHg.
Measurement Value (499-H4) is the numerical result of the clinical measurement; e.g. 173, 154, 15, 12Ø/7Ø.
28.1.15 ADDITIONAL DOCUMENTATION SEGMENT
The Additional Documentation Segment includes the fields necessary to identify unique data required for special processing needs related to
forms, i.e. Certificates of Medical Necessity.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 738 -
Additional Documentation Type ID (369-2Q) is used to identify the name or number of a proprietary form. Data elements within the Additional
Documentation Segment provide the responses to specific questions or data requests on a form. The payer/processor would indicate to the
provider which forms are supported and provide the form number(s) and questions needed for additional information.
Question Number/Letter Count (377-2Z) indicates the number of iterations of Question Number/Letter and the one response field (Question
Percent Response, Question Date Response, Question Dollar Amount Response, Question Numeric Response, Question Alphanumeric
Response) that will follow. If the Question Number/Letter Count (377-2Z) were contained 3, the fields would logically appear as:
Field Field Name
377-2Z Question Number/Letter Count
378-4B Question Number/Letter
38Ø-4G Question Date Response
378-4B Question Number/Letter
372-4J Question Numeric Response
378-4B Question Number/Letter
379-4D Question Percent Response
Each Number/Letter occurrence must be sequential and unique within the count. For example: 1, 2A, 2B, 3, 4, 5A, 5C must not be shown as 1,
2, 2, 3, 4, 5, 5. Hence, the order of the Response fields would depend on the order and type of questions on the form. The form could include
all date questions and would repeat the Question Date Response (38Ø-4G) according to the number of questions responded to. For example,
if the Question Number/Letter Count (377-2Z) contained 4, and the responses all dealt with date related questions, the fields would logically
appear as:
Field Field Name
377-2Z Question Number/Letter Count
378-4B Question Number/Letter
38Ø-4G Question Date Response
378-4B Question Number/Letter
38Ø-4G Question Date Response
378-4B Question Number/Letter
38Ø-4G Question Date Response
378-4B Question Number/Letter
38Ø-4G Question Date Response
28.1.16 FACILITY SEGMENT
The Facility Segment includes the fields necessary to identify the name and address of the Facility ID (336-8C). If the Facility ID (336-8C) is
submitted, then the Facility Segment may be used to provide the demographic information on the Facility. Facility information is used to
identify where the service was performed since some payers base payment on place of service. There is no standard link established between
this field and a patient, insurance, or prescriber. The Facility ID (336-8C) is typically used to identify long-term or rest home facility. Currently,
this is a trading partner issue on how it is used.
28.1.17 NARRATIVE SEGMENT
The Narrative Segment includes two fields: Segment Identification and Narrative Message (39Ø-BM). “Narrative Message” is used to
document the medical necessity of a prescription claim. Narrative documentation, otherwise called free-text information, is used to support
exception handling of pharmacy claims. The National Standard Format (NSF) and the ASC X12N 837 standards both support the
documentation of narrative information. The Narrative Message field duplicates this function in the NCPDP Telecommunication Standard for
Medicare Claim billing. An example includes either of the following: (1.) When a nebulizer medication is billed at a quantity higher than
typically allowed, supporting documentation must be provided to support a claim authorization. The physician’s narrative information
supporting the request is documented in this field. (2.) A payer will reject multiple claims for multiple drugs within the same therapeutic
category. To support exception handling of this type of condition, the physician’s narrative information supporting the request is documented
in this field.
28.2 RESPONSE SEGMENTS
28.2.1 RESPONSE HEADER SEGMENT
The Header Segment is required and must be first in the transmission. All fields are required positionally. When a field is not used, depending
upon trading partner needs, the field must be filled with zeroes or spaces, as appropriate.
Header Response Status (5Ø1-F1)
If either the entire transmission or the Header is in error, the Header Response Status in the Response Header will be “R “. When possible,
every transaction within the transmission should be rejected with an “R “.
If the transaction rejects for detail errors, the Header Response Status in the Response Header will be “A” and the Transaction Response
Status field in the Response Status Segment will be “R”. The appropriate reject code(s) must be displayed when transactions reject for detail
errors.
28.2.2 RESPONSE PATIENT SEGMENT

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 739 -
This segment is used for Medicare Part D Eligibility transactions to provide patient name and date of birth in order to provide additional patient
information. This information could assist in the verification that the eligibility information returned is indeed the patient for which the eligibility
request was intended.
This segment is returned only when the patient has had Medicare Part D eligibility at some point within the Facilitator’s files and
within the search parameters established. The data returned is based on information within the Facilitator’s files and not on
information sent on the Eligibility Request.
Patient First Name (31Ø-CA) – will contain the first name of the patient as known on the Facilitator’s files.
Patient Last Name (311-CB) - will contain the last name of the patient as known on the Facilitator’s files.
Date of Birth (3Ø4-C4) - will contain the birth date of the patient as known on the Facilitator’s files.
28.2.3 RESPONSE INSURANCE SEGMENT
In the event the processor receiving the original claim is not the primary payer, the Payer ID field can be used to identify the appropriate entity
to receive the transaction first.
28.2.4 RESPONSE INSURANCE ADDITIONAL INFORMATION SEGMENT
This segment is used solely for Medicare Part D Eligibility transactions to provide Medicare specific benefit information.
Next Medicare Part D Effective Date (14Ø-US)
Next Medicare Part D Termination Date (141-UT)
These fields are populated only when, based upon the Date of Service (4Ø1-D1), future Medicare Part D coverage exists. The future date
closest to the date of service requested will be returned should more than one future coverage exist.
Medicare Part D Coverage Code (139-UR) – will indicate if Medicare Part D is the primary insurer, secondary insurer, etc. for the patient.
CMS Low Income Cost Sharing (LICS) Level (138-UQ) – will provide the low-income subsidy copay level for a Part D patient.
Contract Number (24Ø-U1) = will contain the unique identifier of the Prescription Drug Plan (PDP) in which the patient is enrolled.
Benefit ID (757-U6) = will contain the plan benefit package identifier within the Prescription Drug Plan (PDP).
Formulary ID (926-FF) = will identify the formulary of the covered patient.
28.2.5 RESPONSE STATUS SEGMENT
28.2.5.1 REJECT FIELD OCCURRENCE INDICATOR (546-4F)
Due to the usage of repeating fields within segments, the Reject Field Occurrence Indicator is used to identify which repeating fields are in
error. If a processor wishes to report a particular ingredient within a compound that is in error, the appropriate reject code is utilized, and the
Reject Field Occurrence field indicates which repetition is in error. Likewise, if a particular repetition of a field is missing or invalid in syntax, the
particular field is indicated using both the reject code and the occurrence.
For example, if a field on a transmission request repeats three times and the second occurrence has an error, the Reject Code (Field 511-FB)
would contain the appropriate error code and the Reject Field Occurrence Indicator must contain the value “2” for the second occurrence in
error. If a field is designated as not repeating and this field has an error, the Reject Field Occurrence Indicator field must be omitted. See
Example “Compounded Rx Billing - Transaction Code B1 (Ø1)” and section “Standard Conventions”, “Repetition And Multiple Occurrences”,
“Repeating Data Elements”, “Reject Field Occurrence Indicator”.
28.2.5.2 SHARED REJECT CODES
See section above “Shared Reject Codes”.
28.2.5.3 ADDITIONAL MESSAGE INFORMATION FIELDS
The usage of the Additional Message Information (526-FQ) field has changed notably from versions prior to D.Ø. The Additional Message
Information (526-FQ) has been shortened to 4Ø bytes and it may repeat multiple times to relay free text messages and/or structured
messages. If a free text message is longer than 4Ø bytes (the maximum length of this field), one or more subsequent occurrences are to be
used for message completion (see section “Free Text Messages” below). This allows clearly sending multiple distinct free text messages.
Additional Message Information Qualifier (132-UH) values “Ø1”-“ Ø9” are defined for free text messages and qualify the previously unqualified
usage of Additional Message Information (526-FQ), (see section “Free Text Messages” below). Additional Message Information Qualifier
(132-UH) values must occur no more than once per transaction and the values must be ordered sequentially (numeric characters
precede alpha characters, i.e., Ø-9, then A-Z); note gaps may occur. While the Additional Message Information Qualifier (132-UH) is defined to
allow a maximum of 25 occurrences per transaction, there are only 9 qualifier values initially defined and each qualifier may only occur one
time per transaction, this results in a maximum count of 9 occurrences until more values are defined in the NCPDP External Code List (ECL).
Entities receiving the response transaction must allow for new values to be defined for Additional Message Information Qualifier (132-UH) in
future updates to the ECL. A receiving entity should allow for the receipt of such a new/unrecognized value such that a system error or
rejection of the response does not occur. The receiving entity can choose how to process (i.e. display or ignore) that qualifier and message.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 740 -
28.2.5.3.1 FREE TEXT MESSAGES
Up to 9 free text messages can be included in the response for each transaction. The first message occurrence in each transaction will be
qualified in Additional Message Information Qualifier (132-UH) with the first qualifier value (“Ø1”) and each following occurrence will be
assigned the next available qualifier value.
Instructions to the processor, sending the transaction response –
If a full message is longer than will fit in the 4Ø characters allowed in the Additional Message Information (526-FQ) field,
the message should be divided into occurrences of the Additional Message Information field, not exceeding the field size
limit. The Additional Message Information field should then be populated into the necessary number of occurrences and
the continuation of the message is indicated by including the Additional Message Information Continuity (131-UG) field
with each Additional Message Information field, except for the final occurrence.
Instructions to the provider, receiving the transaction response –
The Additional Message Information Continuity (131-UG) field following an Additional Message Information (526-FQ) is
used to denote that the free text continues in the next Additional Message Information (526-FQ) occurrence, allowing for
enhanced human viewing and/or facilitating a programmed system displaying the text for readability. A provider system
can use this continuity indicator in any manner it determines suitable to cleanly format the text for its display purposes. If
this free text message is not continued to the next Additional Message Information (526-FQ) occurrence, the Additional
Message Information Continuity (131-UG) field is omitted. Additional free text messages may follow using the same
approach, up to a maximum of 9 occurrences of the Additional Message Information (526-FQ) field.
28.2.5.3.2 STRUCTURED MESSAGES
There are no qualifiers defined for Structured Messages in the release of the NCPDP Telecommunication Standard Implementation Guide
Version D.Ø, however, the following defines the process for requesting and using qualifier values for Structured Messages.
If an entity wishes to implement and use a structured message, the structure should be brought forward to NCPDP to establish standardized
industry usage of the structure. An Additional Message Information Qualifier (132-UH) value will be assigned and added to the ECL upon
approval by the Maintenance & Control Work Group. Once the ECL is published, the new structured message may be implemented by trading
partners that are utilizing a compatible version of the Telecommunication Standard.
An example of a theoretical need to implement a structured message and the process to do it is as follows:
Genetically tuned variants of Gorillacillin are released to the market that require knowing a specific section of a patient’s DNA
sequence on chromosome 3 in order to properly dispense the appropriate version of the medication to the patient. If a payer
believes the patient to have the relevant DNA sequence AGTACAGAGT, but the pharmacy has submitted the Gorillacillin variant
appropriate for sequence ATGAGACATG, it would be beneficial for the processor to reply in a manner that supports the pharmacy’s
ability to use this information to resolve the discrepancy and either dispense the proper alternative therapy or update the information
on record with the payer. The simple rejection with Reject Code “7Ø ” could be problematic without additional qualification and
assisting information.
A request must be brought forward through a Data Element Request Form (DERF) for the new fields in a future Standard version
and a structured message to support a processor reply in the current version of the Standard. The ECL component of the request
could for example, take the form for these values to be returned by the processor as the one or two digit chromosome identifier, the
15 byte alphanumeric section identifier, and the relevant DNA sequence of up to 2Ø characters with a semicolon separating the
three values. In this example, it could appear in the Additional Message Information (526-FQ) field as “3;A98XC-94;
AGTACAGAGT”. The Maintenance & Control Work Group discusses the request and approves the request for the new structure . It
is assigned (for example) Additional Message Information Qualifier (132-UH) value “DN” for this structured message. Once the new
updated ECL document is approved by the Board of Trustees and published, trading partners may begin using the new structured
message.
28.2.5.3.3 EXAMPLE 1: ONE FREE TEXT MESSAGE IS SENT, LESS THAN 4Ø BYTES
The free text message is
“HELP DESK TO ASSIST WITH QUESTIONS”.
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P Paid
5Ø3-F3 AUTHORIZATION NUMBER Q 123456789123456789
13Ø-UF ADDITIONAL MESSAGE INFORMATION
COUNT
R 1 1 occurrence
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø1 Used for first line of free form text with no pre-
defined structure.
526-FQ ADDITIONAL MESSAGE INFORMATION Q HELP DESK TO ASSIST
WITH QUESTIONS Up to 4Ø Bytes
549-7F HELP DESK PHONE NUMBER QUALIFIER R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
28.2.5.3.4 EXAMPLE 2: ONE FREE TEXT MESSAGE IS SENT, LONGER THAN 4Ø BYTES; NO CONTINUATION NEEDED
One free text message is sent, greater than 4Ø bytes, no continuation character necessary because each occurrence stands on its own in 4Ø
bytes.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 741 -
The raw data free text message is
“HELP DESK TO ASSIST WITH QUESTIONS. ASK FOR SHELLY SMITH.”
The readable free text message is
“HELP DESK TO ASSIST WITH QUESTIONS. ASK FOR SHELLY SMITH.”
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P Paid
5Ø3-F3 AUTHORIZATION NUMBER Q 123456789123456789
13Ø-UF ADDITIONAL MESSAGE INFORMATION
COUNT
R 2 2 occurrences
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø1 Used for first line of free form text with no pre-
defined structure.
526-FQ ADDITIONAL MESSAGE INFORMATION Q HELP DESK TO ASSIST
WITH QUESTIONS. Up to 4Ø Bytes
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø2 Used for second line of free form text with no
pre-defined structure.
526-FQ ADDITIONAL MESSAGE INFORMATION Q ASK FOR SHELLY
SMITH. Up to 4Ø Bytes
549-7F HELP DESK PHONE NUMBER QUALIFIER R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
28.2.5.3.5 EXAMPLE 3: THREE FREE TEXT MESSAGES; CONTINUITY CHARACTER NEEDED
Three free text messages are sent with the continuation character necessary for readability/programmatic manipulation of the message.
The raw data free text message is
“PRIOR AUTHORIZATION EXPIRATION 12/31/2ØØ+7. FOR CONTINUATION OF SERVICE, CONTACT+ PRESCRIBER.”
The readable free text message is
“PRIOR AUTHORIZATION EXPIRATION 12/31/2ØØ7. FOR CONTINUATION OF SERVICE, CONTACT PRESCRIBER.”
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P Paid
5Ø3-F3 AUTHORIZATION NUMBER Q 123456789123456789
13Ø-UF ADDITIONAL MESSAGE INFORMATION
COUNT
R 3 3 occurrences
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø1 Used for first line of free form text with no pre-
defined structure.
526-FQ ADDITIONAL MESSAGE INFORMATION Q PRIOR AUTHORIZATION
EXPIRATION 12/31/2ØØ Up to 4Ø Bytes
131-UG ADDITIONAL MESSAGE INFORMATION
CONTINUITY
R + Continuation character
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø2 Used for second line of free form text with no
pre-defined structure.
526-FQ ADDITIONAL MESSAGE INFORMATION Q 7. FOR CONTINUATION
OF SERVICE, CONTACT Up to 4Ø Bytes
131-UG ADDITIONAL MESSAGE INFORMATION
CONTINUITY
R + Continuation character
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø3 Used for third line of free form text with no pre-
defined structure.
526-FQ ADDITIONAL MESSAGE INFORMATION Q PRESCRIBER. Up to 4Ø Bytes
549-7F HELP DESK PHONE NUMBER QUALIFIER R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
28.2.5.3.6 EXAMPLE 4: ONE FREE TEXT MESSAGE, LESS THAN 4Ø BYTES
One free text message is sent, less than 4Ø bytes.
The free text message is
“MINIMUM AGE = 12 YEARS”. RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M R Rejected
51Ø-FA REJECT COUNT R 1 1 Reject code follows
511-FB REJECT CODE R 6Ø Product/Service Not Covered for Patient Age
13Ø-UF ADDITIONAL MESSAGE INFORMATION
COUNT
R 1 1 occurrence
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø1 Used for first line of free form text with no pre-
defined structure.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 742 -
526-FQ ADDITIONAL MESSAGE INFORMATION Q MINIMUM AGE = 12
YEARS Up to 4Ø Bytes
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R 3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 8ØØ654321Ø
28.2.5.3.7 EXAMPLE 5: TWO FREE TEXT MESSAGES; CONTINUITY CHARACTER NEEDED
Two free text messages are sent with the continuation character necessary for readability/programmatic manipulation of the message.
The raw data free text message is
“NEXT AVAILABLE FILL DATE = 12/31/2ØØ7 WIT+H PRIOR AUTHORIZATION EXPIRING”
The readable free text message is
“NEXT AVAILABLE FILL DATE = 12/31/2ØØ7 WITH PRIOR AUTHORIZATION EXPIRING”
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M R Rejected
51Ø-FA REJECT COUNT R 1 1 Reject code follows
511-FB REJECT CODE R 79 Refill Too Soon
13Ø-UF ADDITIONAL MESSAGE INFORMATION
COUNT
R 2 2 occurrences
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø1 Used for first line of free form text with no pre-
defined structure.
526-FQ ADDITIONAL MESSAGE INFORMATION Q NEXT AVAILABLE FILL
DATE = 12/31/2ØØ7
WIT
Up to 4Ø Bytes
131-UG ADDITIONAL MESSAGE INFORMATION
CONTINUITY
R + Continuation character
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø2 Used for first line of free form text with no pre-
defined structure.
526-FQ ADDITIONAL MESSAGE INFORMATION Q H PRIOR
AUTHORIZATION
EXPIRING
Up to 4Ø Bytes
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R 3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 8ØØ654321Ø
See also secton “Standard Conventions”, “Repetition And Multiple Occurrences”, “Repeating Data Elements”, ”Response Status Segment”.
28.2.5.4 TRANSACTION REFERENCE NUMBER (88Ø-K5)
This field has been added for use in the Medicare Part D Information Reporting Process. The transaction reference number is being used to
track all transactions related to a particular dispensing event. Whoever creates the Information Reporting Transaction is responsible for
creating this number. The entity receiving the Information Reporting Transaction is expected to include that number in their response.
The Transaction Reference Number designated in the N1 is carried through in the N2.
28.2.6 RESPONSE PRICING SEGMENT
28.2.6.1 PRESCRIPTION RESPONSE FORMULA
Ingredient Cost Paid (5Ø6-F6)
+ Dispensing Fee Paid (5Ø7-F7)
+ Incentive Amount Paid (521-FL)
+ Other Amount Paid (565-J4)
+ Flat Sales Tax Amount Paid (558-AW)
+ Percentage Sales Tax Amount Paid (559-AX)
- Patient Pay Amount (5Ø5-F5)
- Other Payer Amount Recognized (566-J5)
-------------------------------------------------------
= Total Amount Paid (5Ø9-F9)
28.2.6.2 SERVICE RESPONSE FORMULA
Professional Service Fee Paid (562-J1)
+ Flat Sales Tax Amount Paid (558-AW)
+ Percentage Sales Tax Amount Paid (559-AX)
+ Other Amount Paid (565-J4)
- Patient Pay Amount (5Ø5-F5)
- Other Payer Amount Recognized (566-J5)
-------------------------------------------------------
= Total Amount Paid (5Ø9-F9)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 743 -
28.2.6.3 PATIENT PAY AMOUNT (5Ø5-F5) FORMULA
In order to balance the Patient Pay Amount (5Ø5-F5) the following formula must be adhered to:
Amount Applied to Periodic Deductible (517-FH)
+ Amount Exceeding Periodic Benefit Maximum (52Ø-FK)
+ Amount of Copay (518-FI)
+ Amount of Coinsurance (572-4U)
+ Amount Attributed to Processor Fee (571-NZ)
+ Amount Attributed to Sales Tax (523-FN)
+ Amount Attributed to Provider Network Selection (133-UJ)
+ Amount Attributed to Product Selection/Brand Drug (134-UK)
+ Amount Attributed to Product Selection/Non-Preferred Formulary Selection (135-UM)
+ Amount Attributed to Product Selection/Brand Non-Preferred Formulary Selection (136-UN)
+ Amount Attributed to Coverage Gap (137-UP)
+ Health Plan Funded Assistance Amount (129-UD) (this field is always negative or zero)
= Patient Pay Amount (5Ø5-F5)
The resulting Patient Pay Amount (5Ø5-F5) must be greater than or equal to zero.
The above formula must be followed and the component fields returned on a response if the Patient Pay Amount (5Ø5-F5) is other than zero.
Exception: The Amount Attributed to Sales Tax (523-FN) could contain an amount that is also represented in another field of the Patient Pay
Amount (5Ø5-F5) field components. For example, sales tax could apply to Amount Applied to Periodic Deductible (517-FH) or Amount of
Copay (518-FI). When this occurs the sales tax must be populated in the appropriate field(s) and the Amount Attributed to Sales Tax (523-FN)
populated as zero. In order to ascertain who is responsible for the amount of sales tax that is applied, the response must contain populated
sales tax amounts in either Patient Sales Tax Amount (575-EQ) or Plan Sales Tax Amount (574-2Y). When a proportionate share of the sales
tax exists, both fields must be populated. The formula for these two fields as they relate to the Flat Sales Tax Amount Paid (558-AW) and
Percentage Sales Tax Amount Paid (559-AX) represented in the Prescription Response Formula is:
Flat Sales Tax Amount Paid (558-AW)
+ Percentage Sales Tax Amount Paid (559-AX)
= Total of Patient Sales Tax Amount (575-EQ) + Plan Sales Tax Amount (574-2Y)
Examples of the relationship of these fields follow:
28.2.6.3.1 EXAMPLE #1
Patient Responsible for 1ØØ% of Sales Tax and included in Amount Applied to Periodic Deductible (517-FH)
ID Field Amount
5Ø6-F6 Ingredient Cost Paid 45.ØØ
5Ø7-F7 + Dispensing Fee Paid 2.5Ø
521-FL + Incentive Amount Paid Ø.ØØ
565-J4 + Other Amount Paid Ø.ØØ
558-AW + Flat Sales Tax Amount Paid Ø.ØØ
559-AX + Percentage Sales Tax Amount Paid 2.38
5Ø5-F5 - Patient Pay Amount 49.88
566-J5 - Other Payer Amount Recognized Ø.ØØ
5Ø9-F9 = Total Amount Paid Ø.ØØ
517-FH Amount Applied to Periodic Deductible 49.88
52Ø-FK + Amount Exceeding Periodic Benefit Maximum Ø.ØØ
518-FI + Amount of Copay Ø.ØØ
572-4U + Amount of Coinsurance Ø.ØØ
571-NZ + Amount Attributed to Processor Fee Ø.ØØ
523-FN + Amount Attributed to Sales Tax Ø.ØØ
5Ø5-F5 = Patient Pay Amount 49.88
575-EQ Patient Sales Tax Amount 2.38
574-2Y Plan Sales Tax Amount Ø.ØØ
(Note, the fields are not shown in the actual signed format.)
Notes:
• In this example, the patient is responsible for 1ØØ% of the calculated sales tax amount.
• The Patient Sales Tax Amount (575-EQ) (i.e. $2.38) plus the Plan Sales Tax Amount (574-2Y) (i.e. $Ø.ØØ) must equal the Flat Sales
Tax Amount Paid (558-AW) (i.e. $Ø.ØØ) and the Percentage Sales Tax Amount Paid (559-AX) (i.e. $2.38).
• This allows the pharmacy practice management system to always recognize the Patient Sales Tax Amount (575-EQ) when printing this
information on the prescription receipt.
28.2.6.3.2 EXAMPLE #2
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 744 -
Patient Responsible for 1ØØ% of Sales Tax and included in Amount of Copay (518-FI)
ID Field Amount
5Ø6-F6 Ingredient Cost Paid 45.ØØ
5Ø7-F7 + Dispensing Fee Paid 2.5Ø
521-FL + Incentive Amount Paid Ø.ØØ
565-J4 + Other Amount Paid Ø.ØØ
558-AW + Flat Sales Tax Amount Paid Ø.ØØ
559-AX + Percentage Sales Tax Amount Paid 2.38
5Ø5-F5 - Patient Pay Amount 49.88
566-J5 - Other Payer Amount Recognized Ø.ØØ
5Ø9-F9 = Total Amount Paid 29.88
517-FH Amount Applied to Periodic Deductible Ø.ØØ
52Ø-FK + Amount Exceeding Periodic Benefit Maximum Ø.ØØ
518-FI + Amount of Copay 2Ø.ØØ
572-4U + Amount of Coinsurance Ø.ØØ
571-NZ + Amount Attributed to Processor Fee Ø.ØØ
523-FN + Amount Attributed to Sales Tax Ø.ØØ
5Ø5-F5 = Patient Pay Amount 49.88
575-EQ Patient Sales Tax Amount 2.38
574-2Y Plan Sales Tax Amount Ø.ØØ
(Note, the fields are not shown in the actual signed format.)
Notes:
• In Example #2, the patient is again responsible for 1ØØ% of the calculated sales tax amount. However, in this example the sales tax is
recognized as a portion of the Amount of Copay (518-FI).
• The Patient Sales Tax Amount (575-EQ) (i.e. $2.38) plus the Plan Sales Tax Amount (574-2Y) (i.e. $Ø.ØØ) must equal the sum of the
Flat Sales Tax Amount Paid (558-AW) (i.e. $Ø.ØØ) and the Percentage Sales Tax Amount Paid (559-AX) (i.e. $2.38).
• This allows the pharmacy practice management system to always recognize the Patient Sales Tax Amount (575-EQ) when printing this
information on the prescription receipt.
28.2.6.3.3 EXAMPLE #3
Patient Responsible for Proportional Amount of the Sales Tax and included in Amount of Copay (518-FI).
ID Field Amount
5Ø6-F6 Ingredient Cost Paid 45.ØØ
5Ø7-F7 + Dispensing Fee Paid 2.5Ø
521-FL + Incentive Amount Paid Ø.ØØ
565-J4 + Other Amount Paid Ø.ØØ
558-AW + Flat Sales Tax Amount Paid Ø.ØØ
559-AX + Percentage Sales Tax Amount Paid 2.38
5Ø5-F5 - Patient Pay Amount 2Ø.ØØ
566-J5 - Other Payer Amount Recognized Ø.ØØ
5Ø9-F9 = Total Amount Paid 29.88
517-FH Amount Applied to Periodic Deductible Ø.ØØ
52Ø-FK + Amount Exceeding Periodic Benefit Maximum Ø.ØØ
518-FI + Amount of Copay 2Ø.ØØ
572-4U + Amount of Coinsurance Ø.ØØ
571-NZ + Amount Attributed to Processor Fee Ø.ØØ
523-FN + Amount Attributed to Sales Tax Ø.ØØ
5Ø5-F5 = Patient Pay Amount 2Ø.ØØ
575-EQ Patient Sales Tax Amount Ø.95
574-2Y Plan Sales Tax Amount 1.43
(Note, the fields are not shown in the actual signed format.)
Notes:
• In Example #3, the patient is responsible for a proportional amount of the calculated sales tax.
• The Patient Sales Tax Amount (575-EQ) (i.e. $Ø.95) plus the Plan Sales Tax Amount (574-2Y) (i.e. $1.43) must equal the sum of the Flat
Sales Tax Amount Paid (558-AW) (i.e. $Ø.ØØ) and the Percentage Sales Tax Amount Paid (559-AX) (i.e. $2.38).
• This allows the pharmacy practice management system to always recognize the Patient Sales Tax Amount (575-EQ) when printing this
information on the prescription receipt.
28.2.6.3.4 EXAMPLE #4
Patient Responsible for Proportional Amount of the Sales Tax and it is added to the other Patient Financial Responsibilities

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 745 -
ID Field Amount
5Ø6-F6 Ingredient Cost Paid 45.ØØ
5Ø7-F7 + Dispensing Fee Paid 2.5Ø
521-FL + Incentive Amount Paid Ø.ØØ
565-J4 + Other Amount Paid Ø.ØØ
558-AW + Flat Sales Tax Amount Paid Ø.ØØ
559-AX + Percentage Sales Tax Amount Paid 2.38
5Ø5-F5 - Patient Pay Amount 2Ø.95
566-J5 - Other Payer Amount Recognized Ø.ØØ
5Ø9-F9 = Total Amount Paid 28.93
517-FH Amount Applied to Periodic Deductible Ø.ØØ
52Ø-FK + Amount Exceeding Periodic Benefit Maximum Ø.ØØ
518-FI + Amount of Copay 2Ø.ØØ
572-4U + Amount of Coinsurance Ø.ØØ
571-NZ + Amount Attributed to Processor Fee Ø.ØØ
523-FN + Amount Attributed to Sales Tax Ø.95
5Ø5-F5 = Patient Pay Amount 2Ø.95
575-EQ Patient Sales Tax Amount Ø.95
574-2Y Plan Sales Tax Amount 1.43
(Note, the fields are not shown in the actual signed format.)
Notes:
• In Example #4, the patient is responsible for a proportional amount of the calculated sales tax.
• The Patient Sales Tax Amount (575-EQ) (i.e. $Ø.95) plus the Plan Sales Tax Amount (574-2Y) (i.e. $1.43) must equal the sum of the Flat
Sales Tax Amount Paid (558-AW) (i.e. $Ø.ØØ) and the Percentage Sales Tax Amount Paid (559-AX) (i.e. $2.38)
• This allows the pharmacy practice management system to always recognize the Patient Sales Tax Amount (575-EQ) when printing this
information on the prescription receipt.
Partial Fill Fields (Basis Of Calculation – Dispensing Fee (346-HH), Basis Of Calculation – Copay (347-HJ), Basis Of Calculation – Flat
Sales Tax (348-HK), Basis Of Calculation – Percentage Sales Tax (349-HM), Basis of Calculation-Coinsurance (573-4V))
Several fields are in the Response Pricing Segment to facilitate transmission of payment calculations for transactions that represent partial
fills.
Basis of Calculation-Dispensing Fee (346-HH)
This field informs the pharmacy of the processor’s method for determining the Dispensing Fee Paid (5Ø7-F7).
Basis of Calculation-Copay (347-HJ)
This field informs the pharmacy of the processor’s method for determining the copay portion of the Patient Pay Amount (5Ø5-F5).
Basis of Calculation-Coinsurance (573-4V)
This field informs the pharmacy of the processor’s method for determining the coinsurance portion of the Patient Pay Amount (5Ø5-F5).
Basis of Calculation-Flat Sales Tax (348-HK)
This field informs the pharmacy of the processor’s method for determining the Flat Sales Tax Amount Paid (558-AW).
Basis of Calculation-Percentage Sales Tax (349-HM)
This field informs the pharmacy of the processor’s method for determining the Percentage Sales Tax Amount Paid (559-AX).
Other Guidance
Remaining Benefit Amount (514-FE)
The Remaining Benefit Amount must not be returned with zeroes unless there are no benefit dollars remaining. This field must not be
defaulted (zero filled), as it would lead the pharmacy to an incorrect conclusion of no benefit dollars remaining. (Unlike Version 3.2, the value
of 999999999 must not be used as a default in this field.)
Spending Account Amount Remaining (128-UC)
This field will be returned on an approved transaction with a payable response, if known. This field is informational only. It is being requested
to report back to the provider and the patient the amount remaining on the spending account after the current claim updated the spending
account.
Health Plan-funded Assistance Amount (129-UD)
This field is part of the patient pay amount calculation and is used to report back to the provider and patient the portion of Patient Pay Amount
(5Ø5-F5) that was reduced due to this plan-funded assistance. In this transaction, the patient pays the value reported in Patient Pay Amount
(5Ø5-F5) however without this field the patient would have been required to pay a higher dollar amount. NOTE: There is no credit card
transaction involved in this type of Patient Spending Assistance, as in a Flexible Spending Account (FSA). This field will be sent back on a “P”
(Paid) or “D” (Duplicate of Paid) transaction when a patient meets the plan-funded assistance criteria, as part of Patient Pay Amount (5Ø5-F5)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 746 -
to indicate to the provider and patient that the patient’s financial responsibility would have been more if the plan-funded assistance was not
available to the patient. The value of this field will always be negative and is significant should billing to subsequent payers be required.
See section “Healthcare Reimbursement Account (HRA), Health Savings Accounts (HSAs), and Healthcare Flexible Spending Account (FSA)”
below.
28.2.6.4 MEDICARE PART D
These fields are required when the previous payer has financial amounts that apply to Medicare Part D beneficiary benefit stages. These fields
are required when the plan is a participant in a Medicare Part D program that requires reporting of benefit stage specific financial amounts.
Benefit Stage Count (392-MU)
Benefit Stage Qualifier (393-MV) – the value contained in the qualifier must only be used once in all the iterations of Benefit Stage
Count (392-MU) for the transaction.
If an individual is in an initial phase, the value for initial is to be used. If an individual is in a catastrophic phase, the value
for catastrophic is to be used. If new program stages emerge in the program, values can be added in the future. If there is
no gap, the initial benefit is returned until the patient moves into catastrophic. If a deductible does not apply, the initial
benefit is to be used.
Benefit Stage Amount (394-MW) –
The sum of all submitted Benefit Stage Amounts must equal the sum of Patient Pay Amount (5Ø5-F5) and Total Amount
Paid (5Ø9-F9).
(Calculation: Sum Benefit Stage Amount occurrences 1 through 4 = Patient Pay Amount (5Ø5-F5) + Total
Amount Paid (5Ø9-F9)).
28.2.6.4.1 EXCERPT EXAMPLES
28.2.6.4.1.1 Example 1 Brand Selection
There is $3ØØ left of initial coverage benefit for the beneficiary at the PDP. A claim is submitted for a brand drug that cost $1ØØ while the
generic costs $75. The claim adjudicates with a MAC penalty of $25, a copay amount of $1Ø.ØØ and a payment amount of $65.ØØ. (Note:
the Response provides the reason for the Amount Attributed To Product Selection/Brand Drug (134-UK))
Response from PDP for primary claim RESPONSE PRICING SEGMENT
FIELD FIELD NAME VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT 35Ø{ $35.ØØ
5Ø9-F9 TOTAL AMOUNT PAID 65Ø{ $65.ØØ
134-UK AMOUNT ATTRIBUTED TO PRODUCT
SELECTION/BRAND DRUG
25Ø{ $25.ØØ
518-FI AMOUNT OF COPAY 1ØØ{ $1Ø.ØØ
392-MU BENEFIT STAGE COUNT 1
393-MV BENEFIT STAGE QUALIFIER Ø2 Initial Benefit
394-MW BENEFIT STAGE AMOUNT 1ØØØ{ $1ØØ.ØØ - Full claim value applies to the
Part D benefit
Pharmacy then submits the amounts from the response to the secondary payer. In all cases, the provider should be made “whole” for the
product dispensed according to the Medicare PDP. The response must address the total of the amounts submitted.
When the Patient has a responsibility amount from prior payers that is due to Product Selection this payer must process via one of the
following methods:
1. Pay the claim including all the Product Selection dollars. Other appropriate patient pay amounts may be reimbursed by the payer.
2. Pay the claim. Plan reimburses for appropriate patient pay amounts. The remainder of the Product Selection dollars would be
charged to the patient and returned in the Patient Pay Amount (5Ø5-F5).
3. Reject the claim with indication that patient does not have the opportunity for product selection. Other appropriate rejections may
apply.
When the Patient has a responsibility amount from prior payers that is due to Patient Sales Tax (575-EQ), this payer must process via one of
the following methods:
1. Pay the claim including the patient responsibility Sales Tax dollars.
2. Pay the claim charging some or all of the Sales Tax dollars to the patient and paying the difference.
When the Amount Attributed to Processor Fee (571-NZ) is greater than zero resulting in a negative payment to the provider, the claim is
reversed and billed to the next payer as Primary using the appropriate Other Coverage Code (3Ø8-C8) value.
For other fields that are included in Patient Pay Amount (5Ø5-F5), see section “Specific Segment Discussion”, “Response Segments”,
“Response Pricing Segment”, Patient Pay Amount (5Ø5-F5) Formula”.
Request segment from pharmacy to secondary insurance
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 747 -
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
FIELD FIELD NAME VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION Ø5 COORDINATION OF BENEFITS/OTHER PAYMENTS
SEGMENT
337-4C COORDINATION OF BENEFITS/OTHER
PAYMENTS COUNT
1 One occurrence
338-5C OTHER PAYER COVERAGE TYPE Ø1 Primary
392-MU BENEFIT STAGE COUNT 1
393-MV BENEFIT STAGE QUALIFIER Ø2 Initial Benefit
394-MW BENEFIT STAGE AMOUNT 1ØØØ{ $1ØØ.ØØ Amount Applied to Benefit Stage
Amount as reported by previous payer
353-NR OTHER PAYER –PATIENT RESPONSIBILITY
AMOUNT COUNT
2 Two occurrences
351-NP OTHER PAYER-PATIENT RESPONSIBILITY
AMOUNT QUALIFIER
Ø2 Amount Attributed to Product Selection/Brand
Drug (134-UK) as reported by previous payer
352-NQ OTHER PAYER-PATIENT RESPONSIBILITY
AMOUNT
25Ø{ $25.ØØ
351-NP OTHER PAYER-PATIENT RESPONSIBILITY
AMOUNT QUALIFIER
Ø5 Amount Of Copay (518-FI) as reported by
previous payer.
352-NQ OTHER PAYER-PATIENT RESPONSIBILITY
AMOUNT
1ØØ{ $1Ø.ØØ
Response Pricing Segment from the Secondary Payer to the Pharmacy
RESPONSE PRICING SEGMENT
FIELD FIELD NAME VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT 25Ø{ $25.ØØ
5Ø9-F9 TOTAL AMOUNT PAID 1ØØ{ $1Ø.ØØ
134-UK AMOUNT ATTRIBUTED TO PRODUCT
SELECTION/BRAND DRUG
25Ø{ $25.ØØ
518-FI AMOUNT OF COPAY ØØ{ $ØØ.ØØ
Note: The secondary payer has picked up the $1Ø.ØØ copay on the patient’s behalf but has not reimbursed the pharmacy for the Amount
Attributed to Product Selection, which remains the responsibility of the patient.
28.2.6.4.1.2 Example 2 Deductible Not Met
In this example, the patient has not yet met their deductible. The full value of the claim is being applied to the deductible benefit stage. The
patient is responsible for the entire amount. Had the patient been willing to utilize a different product, they would have realized a cost savings.
RESPONSE PRICING SEGMENT
FIELD FIELD NAME VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT 1ØØØ{ $1ØØ.ØØ
5Ø9-F9 TOTAL AMOUNT PAID ØØ{ $ØØ.ØØ
134-UK AMOUNT ATTRIBUTED TO PRODUCT
SELECTION/BRAND DRUG
25Ø{ $25.ØØ
517-FH AMOUNT ATTRIBUTED TO PERIODIC
DEDUCTIBLE
75Ø{ $75.ØØ
392-MU BENEFIT STAGE COUNT 1
393-MV BENEFIT STAGE QUALIFIER Ø1 Deductible
394-MW BENEFIT STAGE AMOUNT 1ØØØ{ $1ØØ.ØØ - Full claim value applies to the
Part D benefit
Pharmacy then submits the amounts from the response to the secondary payer
Request segment from the Pharmacy to Secondary Payer
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
FIELD FIELD NAME VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION Ø5 COORDINATION OF BENEFITS/OTHER PAYMENTS
SEGMENT
337-4C COORDINATION OF BENEFITS/OTHER
PAYMENTS COUNT
1 One occurrence
338-5C OTHER PAYER COVERAGE TYPE Ø1 Primary
392-MU BENEFIT STAGE COUNT 1
393-MV BENEFIT STAGE QUALIFIER Ø1 Deductible
394-MW BENEFIT STAGE AMOUNT 1ØØØ{ $1ØØ.ØØ Amount Applied to Benefit Stage
Amount as reported by previous payer
353-NR OTHER PAYER –PATIENT RESPONSIBILITY
AMOUNT COUNT
2 Two occurrences

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 748 -
351-NP OTHER PAYER-PATIENT RESPONSIBILITY
AMOUNT QUALIFIER
Ø1 Amount Attributed to Periodic Deductible as
reported by previous payer
352-NQ OTHER PAYER-PATIENT RESPONSIBILITY
AMOUNT
75Ø{ $75.ØØ
351-NP OTHER PAYER-PATIENT RESPONSIBILITY
AMOUNT QUALIFIER
Ø2 Amount Attributed to Product Selection/Brand
Drug (134-UK) as reported by previous payer
352-NQ OTHER PAYER-PATIENT RESPONSIBILITY
AMOUNT
25Ø{ $25.ØØ
Response Pricing Segment from the Secondary Payer to the Pharmacy
RESPONSE PRICING SEGMENT
FIELD FIELD NAME VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT 25Ø{ $25.ØØ
5Ø9-F9 TOTAL AMOUNT PAID 75Ø{ $75.ØØ
134-UK AMOUNT ATTRIBUTED TO PRODUCT
SELECTION/BRAND DRUG
25Ø{ $25.ØØ
517-FH AMOUNT ATTRIBUTED TO PERIODIC
DEDUCTIBLE
ØØ{ $ØØ.ØØ
Note: The secondary payer has picked up the $75.ØØ of the deductible on the patient’s behalf but has not reimbursed the pharmacy for the
Amount Attributed to Product Selection/Brand Drug (134-UK), which remains the responsibility of the patient.
28.2.6.4.1.3 Example 3 Coverage Gap
Patient has fallen into the coverage gap (i.e. “donut hole”). Claim straddles Initial Benefit state and Coverage Gap stage. In this scenario
there was no penalty due to product selection.
While the payment to the pharmacy is split as
• Plan to pay $27
• Patient to pay a total of $53.ØØ ($8.ØØ copay and $45.ØØ coverage gap)
• Total provider reimbursement = $8Ø.ØØ
The “break out” for Medicare tallying purposes is as follows:
• Initial benefit = $35.ØØ
• Coverage Gap = $45.ØØ
Note: when part of a claim is in the coverage gap, the Patient Pay Amount (5Ø5-F5) will always be equal to or greater than the coverage gap
amount.
RESPONSE PRICING SEGMENT
FIELD FIELD NAME VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT 53Ø{ $53.ØØ
5Ø9-F9 TOTAL AMOUNT PAID 27Ø{ $27.ØØ
137-UP AMOUNT ATTRIBUTED TO COVERAGE GAP 45Ø{ $45.ØØ
518-FI AMOUNT OF COPAY 8Ø{ $8.ØØ
392-MU BENEFIT STAGE COUNT 2 Two occurrences
393-MV BENEFIT STAGE QUALIFIER Ø2 Initial Benefit
394-MW BENEFIT STAGE AMOUNT 35Ø{ $35.ØØ
393-MV BENEFIT STAGE QUALIFIER Ø3 Coverage Gap (donut hole)
394-MW BENEFIT STAGE AMOUNT 45Ø{ $45.ØØ
Provider then submits the amounts from the response to the secondary payer. This is a “straight” move of the data as supplied by the prior
payer.
Request segment from pharmacy to secondary insurance
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
FIELD FIELD NAME VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION Ø5 COORDINATION OF BENEFITS/OTHER PAYMENTS
SEGMENT
337-4C COORDINATION OF BENEFITS/OTHER
PAYMENTS COUNT
1 One occurrence
338-5C OTHER PAYER COVERAGE TYPE Ø1 Primary
392-MU BENEFIT STAGE COUNT 2 Two occurrences
393-MV BENEFIT STAGE QUALIFIER Ø2 Initial Benefit
394-MW BENEFIT STAGE AMOUNT 35Ø{ $35.ØØ Amount Applied to Benefit Stage
Amount as reported by previous payer
393-MV BENEFIT STAGE QUALIFIER Ø3 Coverage Gap (donut hole)
394-MW BENEFIT STAGE AMOUNT 45Ø{ $45.ØØ Amount Applied to Benefit Stage
Amount (394-MW) as reported by previous
payer
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 749 -
353-NR OTHER PAYER –PATIENT RESPONSIBILITY
AMOUNT COUNT
1 One occurrence
351-NP OTHER PAYER-PATIENT RESPONSIBILITY
AMOUNT QUALIFIER
Ø5 Amount Of Copay (518-FI) as reported by
previous payer.
352-NQ OTHER PAYER-PATIENT RESPONSIBILITY
AMOUNT
8Ø{ $8.ØØ
351-NP OTHER PAYER-PATIENT RESPONSIBILITY
AMOUNT QUALIFIER
12 Amount Attributed to Coverage Gap (137-UP)
as reported by previous payer.
352-NQ OTHER PAYER-PATIENT RESPONSIBILITY
AMOUNT
45Ø{ $45.ØØ
Response Pricing Segment from the Secondary Payer to the Pharmacy
RESPONSE PRICING SEGMENT
FIELD FIELD NAME VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT 8Ø{ $8.ØØ
5Ø9-F9 TOTAL AMOUNT PAID 45Ø{ $45.ØØ
518-FI AMOUNT OF COPAY 8Ø{ $8.ØØ
Note: The secondary payer has picked up the $45.ØØ due to coverage gap on the patient’s behalf but has not reimbursed the pharmacy for
the copay, which remains the responsibility of the patient.
28.2.6.4.1.4 Example 4 Non-preferred Formulary Selection
There is $3ØØ left of initial coverage benefit for the beneficiary at the PDP. A claim is submitted for a non-preferred formulary drug that
carries a $25.ØØ penalty. The claim adjudicates with a non-preferred formulary drug selection penalty of $25.ØØ, a copay amount of $1Ø.ØØ
and a payment amount of $65.ØØ. (Note: the Response provides the reason for the Amount Attributed To Product Selection/Non-Preferred
Formulary Selection (135-UM)).
Response from PDP for primary claim RESPONSE PRICING SEGMENT
FIELD FIELD NAME VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT 35Ø{ $35.ØØ
5Ø9-F9 TOTAL AMOUNT PAID 65Ø{ $65.ØØ
135-UM AMOUNT ATTRIBUTED TO PRODUCT
SELECTION/NON-PREFERRED FORMULARY
SELECTION
25Ø{ $25.ØØ
518-FI AMOUNT OF COPAY 1ØØ{ $1Ø.ØØ
392-MU BENEFIT STAGE COUNT 1
393-MV BENEFIT STAGE QUALIFIER Ø2 Initial Benefit
394-MW BENEFIT STAGE AMOUNT 1ØØØ{ $1ØØ.ØØ - Full claim value applies to the
Part D benefit
Pharmacy then submits the amounts from the response to the secondary payer.
Request segment from pharmacy to secondary insurance
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
FIELD FIELD NAME VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION Ø5 COORDINATION OF BENEFITS/OTHER PAYMENTS
SEGMENT
337-4C COORDINATION OF BENEFITS/OTHER
PAYMENTS COUNT
1 One occurrence
338-5C OTHER PAYER COVERAGE TYPE Ø1 Primary
392-MU BENEFIT STAGE COUNT 1
393-MV BENEFIT STAGE QUALIFIER Ø2 Initial Benefit
394-MW BENEFIT STAGE AMOUNT 1ØØØ{ $1ØØ.ØØ Amount Applied to Benefit Stage
Amount as reported by previous payer
353-NR OTHER PAYER –PATIENT RESPONSIBILITY
AMOUNT COUNT
2 Two occurrences
351-NP OTHER PAYER-PATIENT RESPONSIBILITY
AMOUNT QUALIFIER
Ø8 Amount Attributed to Product Selection/Non-
Preferred Formulary Selection (135-UM) as
reported by previous payer
352-NQ OTHER PAYER-PATIENT RESPONSIBILITY
AMOUNT
25Ø{ $25.ØØ
351-NP OTHER PAYER-PATIENT RESPONSIBILITY
AMOUNT QUALIFIER
Ø5 Amount Of Copay (518-FI) as reported by
previous payer.
352-NQ OTHER PAYER-PATIENT RESPONSIBILITY
AMOUNT
1ØØ{ $1Ø.ØØ
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 750 -
Response Pricing Segment from the Secondary Payer to the Pharmacy
RESPONSE PRICING SEGMENT
FIELD FIELD NAME VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT 25Ø{ $25.ØØ
5Ø9-F9 TOTAL AMOUNT PAID 1ØØ{ $1Ø.ØØ
135-UM AMOUNT ATTRIBUTED TO PRODUCT
SELECTION/NON-PREFERRED FORMULARY
SELECTION
25Ø{ $25.ØØ
518-FI AMOUNT OF COPAY ØØ{ $ØØ.ØØ
Note: The secondary payer has picked up the $1Ø.ØØ copay on the patient’s behalf but has not reimbursed the pharmacy for the Amount
Attributed to Product Selection/Non-Preferred Formulary Selection (135-UM), which remains the responsibility of the patient.
28.2.6.5 HEALTHCARE REIMBURSEMENT ACCOUNT (HRA), HEALTH SAVINGS ACCOUNTS (HSAS),
AND HEALTHCARE FLEXIBLE SPENDING ACCOUNT (FSA)
HRA accounts are funded by the Plan Sponsor and not the employee. HSA accounts can be funded by the employee and/or employer, and
FSA accounts are funded by the employee.
28.2.6.5.1 HEALTHCARE REIMBURSEMENT ACCOUNT (HRA) – BASED PLAN DESIGNS
These plan designs link a plan-sponsored spending account to the healthcare benefit. The spending account contains funds that can be used
by the member to offset out of pocket costs. An HRA is typically offered in combination with a high-deductible benefit, creating a “3-stage”
benefit design
The HRA can typically be used to fund 100% of employee and dependents’ healthcare expenses until the HRA is depleted. If the HRA funds
are depleted, the employee is typically responsible for a specified amount (similar to a deductible) until traditional health plan coverage takes
effect. If the HRA funds are not depleted by end of year, a plan may allow remaining dollars to roll over to the following year’s account balance
An HRA-based benefit can be offered:
• With medical and pharmacy integrated across high deductible plan
• For medical only, with “traditional” pharmacy benefit
• As a pharmacy-only HRA plan, with a “traditional” medical offering
28.2.6.5.2 HEALTH SAVINGS ACCOUNTS (HSAS) AND QUALIFYING HEALTH PLANS
The HSA provision of the Medicare Prescription Drug, Improvement, and Modernization Act of 2003 allows eligible individuals to establish an
HSA to pay for medical expenses.
What is an HSA?
• An income tax-exempt, interest-earning trust account that can be used by eligible individuals to pay for qualified healthcare
expenses
• Unused dollars rollover from year to year and are portable
Who can contribute to an HSA?
• Any eligible individual or a family member
• Eligible individual’s employer
• Annual contribution limits apply
28.2.6.5.3 HEALTHCARE FLEXIBLE SPENDING ACCOUNT (FSA)
Flexible spending accounts offer another option for employees to pay for eligible medical expenses on a pre-tax basis. When offered by an
employer, a Health Care FSA program allows employees to set aside their own money on a pre-tax basis to pay for healthcare expenses
incurred by the employee and his/her eligible dependents. When an employee incurs an eligible medical expense (e.g., a co-payment for a
prescription) the amount incurred by the employee is reimbursed by the FSA. Any funds set aside by the employee that are unused by the end
of year are forfeited.
Negative dollar amounts must be supported by payers involved in coordination of benefits.
28.2.6.5.4 PRIMARY PAYS THE CLAIM USING PLAN-FUNDED HEALTH REIMBURSEMENT ACCOUNT
HRA Account before prescription: $1,ØØØ
Normal Claim Reimbursement (Ingredient Cost + Dispensing Fee, etc.): $ 1ØØ
Plan to Pay: $ 65
Patient to Pay: $ 35
Because the claim is eligible for plan-assisted benefit, the Patient Pay Amount (5Ø5-F5) will be reduced to $15.ØØ due to Health Plan
Funded Assistance. By the time of the next fill, the assistance funds may be exhausted since these dollars may be used for other health
related patient costs. For this reason, plan would like patient to “see” when HRA dollars have been utilized and know what dollars remain for
that “moment in time”.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 751 -
Example Excerpt: RESPONSE PRICING SEGMENT
FIELD FIELD NAME VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT 15Ø{ $15.ØØ
5Ø6-F6 INGREDIENT COST PAID 95Ø{ $95.ØØ
5Ø7-F7 DISPENSING FEE PAID 5Ø{ $5.ØØ
5Ø9-F9 TOTAL AMOUNT PAID 85Ø{ $85.ØØ
522-FM BASIS OF REIMBURSEMENT DETERMINATION 1 Ingredient Cost Paid as Submitted
518-FI AMOUNT OF COPAY 1ØØ{ $1Ø.ØØ
517-FH AMOUNT APPLIED TO PERIODIC DEDUCTIBLE 2ØØ{ $2Ø.ØØ
135-UM AMOUNT ATTRIBUTED TO PRODUCT
SELECTION/NON-PREFERRED FORMULARY
SELECTION
5Ø{ $5.ØØ
129-UD HEALTH PLAN FUNDED ASSISTANCE AMOUNT 2ØØ} -$2Ø.ØØ Note this FIELD is ALWAYS a
negative amount.
128-UC SPENDING ACCOUNT AMOUNT REMAINING 98ØØ{ $98Ø.ØØ (Informational field)
Claim Balancing:
Ingredient Cost Paid 95.ØØ Patient Pay Amount 15.ØØ
Dispensing Fee Paid 5.ØØ Total Amount Paid 85.ØØ
Total 1ØØ.ØØ Total 1ØØ.ØØ
Patient Pay Amount 15.ØØ
= Amount of Copay 1Ø.ØØ
+ Amount Applied to Periodic Deductible 2Ø.ØØ
+ Amount Attributed to Product Selection 5.ØØ
+ Health Plan Funded Assistance Amount -2Ø.ØØ (this field is always negative or zero)
28.2.6.5.4.1 SCENARIO 1A: PHARMACY BILLS SECONDARY INSURANCE – HRA used in PRIMARY
PAYMENT
Submit claim indicating Other Payer Amount Paid
(no change from normal Coordination of Benefits processing)
Only pertinent fields to Coordination of Benefits submission are included in example.
CLAIM SEGMENT
FIELD FIELD NAME VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION Ø7 CLAIM SEGMENT
3Ø8-C8 OTHER COVERAGE CODE 2 Other coverage exists/billed-payment collected
PRICING SEGMENT
FIELD FIELD NAME VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED 1ØØØ{ $1ØØ.ØØ
412-DC DISPENSING FEE SUBMITTED 5Ø{ $5.ØØ
426-DQ USUAL AND CUSTOMARY CHARGE 11ØØ{ $11Ø.ØØ
43Ø-DU GROSS AMOUNT DUE 1Ø5Ø{ $1Ø5.ØØ
423-DN BASIS OF COST DETERMINATION Ø1 AWP
Billing for Contracted Rate of Secondary with Indication in Coordination of Benefits/Other Payments Segment of Amount that has been Paid.
* By definition, Gross Amt Due only allows for “the sum of” selected fields as presented in the Pricing Segment. It does NOT allow for the
“sum of” the fields minus Other Payer Amount Paid.
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
FIELD FIELD NAME VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION Ø5 COORDINATION OF BENEFITS/OTHER PAYMENTS
SEGMENT
337-4C COORDINATION OF BENEFITS/OTHER
PAYMENTS COUNT
1 One occurrence
338-5C OTHER PAYER COVERAGE TYPE Ø1 Primary
443-E8 OTHER PAYER DATE 2ØØ6Ø616 June 16, 2ØØ6
341-HB OTHER PAYER AMOUNT PAID COUNT 1 One occurrence
342-HC OTHER PAYER AMOUNT PAID QUALIFIER Ø7 Drug Benefit
431-DV OTHER PAYER AMOUNT PAID 85Ø{ $85.ØØ paid
Because plan is funding the HRA dollars, this is a normal Other Payer Amount Paid Coordination of Benefits claim.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 752 -
28.2.6.5.4.2 SCENARIO 1B: SECONDARY INSURANCE PAYS THE CLAIM
RESPONSE STATUS SEGMENT
FIELD FIELD NAME VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS P Paid
RESPONSE PRICING SEGMENT
FIELD FIELD NAME VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT 5Ø{ $5.ØØ
5Ø6-F6 INGREDIENT COST PAID 1ØØØ{ $1ØØ.ØØ
5Ø7-F7 DISPENSING FEE PAID 3Ø{ $3.ØØ
566-J5 OTHER PAYER AMOUNT RECOGNIZED 85Ø{ $85.ØØ paid by Primary
5Ø9-F9 TOTAL AMOUNT PAID 13Ø{ $13.ØØ
522-FM BASIS OF REIMBURSEMENT DETERMINATION 1 Ingredient Cost Paid as Submitted
518-FI AMOUNT OF COPAY 5Ø{ $5.ØØ
When processing “Other Payer Amount Paid” Coordination of Benefits claims, Coordination of Benefits payer should determine contracted rate
for the product billed, reduce that by Other Payer Amount Paid and then split this result between payer and patient. The submitted Other
Payer Amount Paid values used should be summarized and reported in Other Payer Amount Recognized; unless the subsequent payer has a
reimbursement formula that is lower than what was reported by previous payer(s).
Balancing:
Ingredient Cost Paid 1ØØ.ØØ Patient Pay Amount 5.ØØ
Dispensing Fee Paid 3.ØØ Total Amount Paid 13.ØØ
Other Payer Amount Recognized -85.ØØ
Total 18.ØØ Total 18.ØØ
28.2.6.5.4.3 SCENARIO 2A: PHARMACY BILLS SECONDARY INSURANCE – HRA used in PRIMARY
PAYMENT
Submit claim indicating PATIENT RESPONSIBILITY AMOUNT
Only pertinent fields to Coordination of Benefits submission are included in example.
CLAIM SEGMENT
FIELD FIELD NAME VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION Ø7 CLAIM SEGMENT
3Ø8-C8 OTHER COVERAGE CODE 8 Claim is a billing for patient financial
responsibility
PRICING SEGMENT
FIELD FIELD NAME VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED 1ØØØ{ $1ØØ.ØØ
412-DC DISPENSING FEE SUBMITTED 5Ø{ $5.ØØ
426-DQ USUAL AND CUSTOMARY CHARGE 11ØØ{ $11Ø.ØØ
43Ø-DU GROSS AMOUNT DUE 1Ø5Ø{ $1Ø5.ØØ
423-DN BASIS OF COST DETERMINATION Ø1 AWP
When Other Coverage Code > 8, the Coordination of Benefits/Other Payments Segment must be viewed to determine the Patient
Responsibility Amount from the prior payer. In coordination of benefits processing, the Pricing Segment appears as it would exist for a
PRIMARY CLAIM. Processor must use Coordination of Benefits/Other Payments Segment fields to determine billing amount.
28.2.6.5.4.4 SCENARIO 2A-1: BILLING FOR “LUMP SUM” PATIENT RESPONSIBILITY AMOUNT AS
REPORTED BY LAST PAYER
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
FIELD FIELD NAME VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION Ø5 COORDINATION OF BENEFITS/OTHER PAYMENTS
SEGMENT
337-4C COORDINATION OF BENEFITS/OTHER
PAYMENTS COUNT
1 One occurrence
338-5C OTHER PAYER COVERAGE TYPE Ø1 Primary
443-E8 OTHER PAYER DATE 2ØØ6Ø616 June 16, 2ØØ6
353-NR OTHER PAYER-PATIENT RESPONSIBILITY
AMOUNT COUNT
1 One occurrence
351-NP OTHER PAYER-PATIENT RESPONSIBILITY Ø6 Patient Pay Amount as reported
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 753 -
AMOUNT QUALIFIER by previous payer.
352-NQ OTHER PAYER-PATIENT RESPONSIBILITY
AMOUNT
15Ø{ $15.ØØ
28.2.6.5.4.5 SCENARIO 2A-2: SECONDARY INSURANCE PAYS THE CLAIM RESULTING IN REDUCED
PATIENT RESPONSIBILITY
Billing is for $15. Secondary payer in this scenario is paying that amount plus an additional Dispensing Fee via contract arrangement.
RESPONSE STATUS SEGMENT
FIELD FIELD NAME VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS P Paid
RESPONSE PRICING SEGMENT
FIELD FIELD NAME VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT 5Ø{ $5.ØØ
5Ø9-F9 TOTAL AMOUNT PAID 12Ø{ $12.ØØ
5Ø6-F6 INGREDIENT COST PAID 15Ø{ $15.ØØ
5Ø7-F7 DISPENSING FEE PAID 2Ø{ $2.ØØ
518-FI AMOUNT OF COPAY 5Ø{ $5.ØØ
522-FM BASIS OF REIMBURSEMENT DETERMINATION 14 Other Payer-Patient Responsibility Amount -
Indicates reimbursement was based on the
Other Payer Patient Responsibility Amount
(352-NQ),
148-U8 INGREDIENT COST CONTRACTED/
REIMBURSABLE AMOUNT
1Ø1Ø{ $1Ø1.ØØ
149-U9 DISPENSING FEE CONTRACTED/
REIMBURSABLE AMOUNT
4Ø{ $4.ØØ
NOTE: Incentives and other fees may be paid based on contractual agreements.
Ingredient Cost Paid 15.ØØ Patient Pay Amount 5.ØØ
Dispensing Fee Paid 2.ØØ Total Amount Paid 12.ØØ
Total 17.ØØ Total 17. ØØ
28.2.6.5.4.6 SCENARIO 2B-1: BILLING FOR “PARTS” OF PATIENT RESPONSIBILITY AMOUNT AS
REPORTED BY LAST PAYER.
Pricing Segment submitted is exactly the same as scenario 2A. Coordination of Benefits/Other Payments Segment differs.
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
FIELD FIELD NAME VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION Ø5 COORDINATION OF BENEFITS/OTHER PAYMENTS
SEGMENT
337-4C COORDINATION OF BENEFITS/OTHER
PAYMENTS COUNT
1 One occurrence
338-5C OTHER PAYER COVERAGE TYPE Ø1 Primary
443-E8 OTHER PAYER DATE 2ØØ6Ø616 June 16, 2ØØ6
353-NR OTHER PAYER-PATIENT RESPONSIBILITY
AMOUNT COUNT
4 Four occurrences
351-NP OTHER PAYER-PATIENT RESPONSIBILITY
AMOUNT QUALIFIER
Ø1 Amount Applied to Periodic Deductible as
reported by previous payer
352-NQ OTHER PAYER-PATIENT RESPONSIBILITY
AMOUNT
2ØØ{ $2Ø.ØØ
351-NP OTHER PAYER-PATIENT RESPONSIBILITY
AMOUNT QUALIFIER
Ø2 Amount attributed to Product Selection/Brand
Drug (134-UK) as reported by previous payer
352-NQ OTHER PAYER-PATIENT RESPONSIBILITY
AMOUNT
5Ø{ $5.ØØ
351-NP OTHER PAYER-PATIENT RESPONSIBILITY
AMOUNT QUALIFIER
Ø5 Amount of Co-pay as reported by previous
payer
352-NQ OTHER PAYER-PATIENT RESPONSIBILITY
AMOUNT
1ØØ{ $1Ø.ØØ
351-NP OTHER PAYER-PATIENT RESPONSIBILITY
AMOUNT QUALIFIER
Ø9 Amount attributed to Health Plan Assistance as
reported by previous payer
352-NQ OTHER PAYER-PATIENT RESPONSIBILITY
AMOUNT
2ØØ} $-2Ø.ØØ
NOTE: THIS IS A NEGATIVE AMOUNT. This

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 754 -
amount is coming out of HRA for patient
28.2.6.5.4.7 SCENARIO 2B-2: SECONDARY INSURANCE PAYS THE DETAILED PATIENT
RESPONSIBILITY CLAIM RESULTING IN REDUCED PATIENT RESPONSIBILITY
NOTE: In this example, the net reimbursement to provider is the same ($17.ØØ) regardless of whether Patient Pay Amount or “parts” of
Patient Pay Amount were billed. By contractual agreement, processor has agreed to pay a dispensing fee associated with the Coordination of
Benefits claim.
• If Coordination of Benefits payer chooses not to pay part of the Patient Responsibility submitted fields, these must be returned as
part of the new Patient Pay Amount so provider is made whole.
• If Coordination of Benefits payer cannot require patient payment, then claim must be rejected. This allows the patient the option to
pay the original Patient Pay Amount or to have the prescriber determine a product that will be covered by all payers.
RESPONSE PRICING SEGMENT
FIELD FIELD NAME VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT 8Ø{ $8.ØØ
5Ø9-F9 TOTAL AMOUNT PAID 9Ø{ $9.ØØ
5Ø6-F6 INGREDIENT COST PAID 15Ø{ $15.ØØ
5Ø7-F7 DISPENSING FEE PAID 2Ø{ $2.ØØ
522-FM BASIS OF REIMBURSEMENT DETERMINATION 14 Other Payer-Patient Responsibility Amount -
Indicates reimbursement was based on the
Other Payer Patient Responsibility Amount
(352-NQ)
518-FI AMOUNT OF COPAY 3Ø{ $3.ØØ
134-UK AMOUNT ATTRIBUTED TO PRODUCT
SELECTION/BRAND DRUG
5Ø{ $5.ØØ
148-U8 INGREDIENT COST CONTRACTED/
REIMBURSABLE AMOUNT
1Ø1Ø{ $1Ø1.ØØ
149-U9 DISPENSING FEE CONTRACTED/
REIMBURSABLE AMOUNT
4Ø{ $4.ØØ
Balancing:
Net Reimburse Patient Pay Parts Submitted Net Reimburse
Ingredient Cost Paid 15.ØØ Amount Attributed to Product Selection 5.ØØ Patient Pay Amount 8.ØØ
Dispensing Fee Paid 2.ØØ Amount Applied to Periodic Deductible 2Ø.ØØ Total Amount Paid 9.ØØ
Amount of Copay 1Ø.ØØ
Amount Attributed to Health Plan Assistance -2Ø.ØØ
Total 15.ØØ
Patient Pay Parts Paid
Amount Attributed to Product Selection 5.ØØ
Amount of Copay 3.ØØ
Total 17.ØØ Total 8.ØØ Total 17.ØØ
In this scenario, Plan has opted to return their “normal” $3.ØØ copay as well as the Product Selection cost that the Primary passed to the
patient ($5.ØØ) resulting in Patient Pay Amount of $8.ØØ.
28.2.7 RESPONSE CLAIM SEGMENT
The Response Claim Segment includes Preferred Product fields (551-9F, 552-AP, 553-AR, 554-AS, 555-AT, 556-AU) that facilitate informing
providers when therapeutic substitution is desired by the payer.
28.2.8 RESPONSE DUR/PPS SEGMENT
DUR Additional Text (57Ø-NS) was created for the processor/PBM to provide more information to the pharmacist about the DUR problem. For
example, drug interaction Onset and Documentation support enhanced drug-interaction reporting and medical conditions that could justify a
high dose alert may use this field to relay this information. Information that appears in the DUR Additional Text (57Ø-NS) is in addition to the
current data contents. This field is not used for continuing strings of text from the DUR Free Text Message (544-FY).
Some examples of usage cited:
1. For a drug interaction “Lanoxin Tab Ø.25mg – onset=DELAYED; documentation=ESTABLISHED.”
2. For a therapeutic duplication “ANTIHYPERTENSIVES – 4 duplications detected, only 3 are permitted.”
3. For a low dose “Min Dose = 2 per units/day – Liver Insufficiency may justify low dose.”
28.2.8.1 DUR/PPS AND MULTI-INGREDIENT COMPOUNDS
Please see section “Request Segments”, “Compound Segment”, “Multi-Ingredient Compounds And DUR Rejects” for more information and
sample examples.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 755 -
28.2.8.2 DUR/PPS CLAIMS DATA AND RESPONSES IN BATCH TRANSACTIONS
The NCPDP Batch Standard Version 1.1 supports the off-line file transmission of claims from the pharmacy to the processor and the relay of
the results back in a file to the pharmacy. The recommendations and discussions contained in this Implementation Guide apply to batch
transactions as well. The differences are that in a batch transaction:
• The user who establishes the rules of DUR responses needs to keep in mind that no real-time DUR responses are possible—the
patient has already received the prescription and has left the pharmacy
• Rejections for DUR and DUR message-only responses should be minimized, to avoid unnecessary and potentially noisy alerts that
would not affect the immediate outcome of drug therapy at the point of dispensing.
However, the batch submission of Professional Pharmacy Services using the NCPDP Batch Standard Version 1.1 appears to work quite well.
The only difference in these types of transactions is that the dispensing pharmacist cannot know real-time if their professional pharmacy
service claim is acceptable and reimbursable by and from the processor. Batch professional pharmacy service claims can still reference
online-transmitted product claims for the purpose of linking a dispensing event to a professional service (see the use of the Associated
Prescription/Service Reference Number (456-EN) and Associated Prescription/Service Date (457-EP) in this document.
28.2.9 RESPONSE PRIOR AUTHORIZATION SEGMENT
Please see the section “Prior Authorization Transaction Discussion”.
In some situations of a Claim Billing, a rejected response must be sent from the payer to the pharmacy that requires the pharmacy to submit a
Prior Authorization Number in order to receive payment for the claim. An example of a situation may include a Benefit Transition Period that
allows for payment of claims, for a period of time that would normally reject.
When a rejection of this nature is returned and a Reject Code (511-FB) of
• “N7 “ Use Prior Authorization Code Provided During Transition Period,
• “N8 ” Use Prior Authorization Code Provided For Emergency Fill
• “N9 ” Use Prior Authorization Code Provided For Level of Care Change
is returned, the Prior Authorization Number-Assigned (498-PY) field of the Response Prior Authorization Segment must also be returned. The
pharmacy will take the value from the Prior Authorization Number-Assigned (498-PY) of the response and place it in the field Prior
Authorization Number-Submitted (462-EV) of the Claim Segment. The pharmacy will then submit the claim.
28.2.10 RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS SEGMENT
Other Payer Coverage Type (355-NT) – will contain the other payer’s level of coverage for the patient, such as primary, secondary, tertiary,
etc.
Other Payer ID (34Ø-7C) - will contain the identifier of the payer(s). For Medicare Part D Eligibility Transaction this field must contain the BIN
(with appropriate Other Payer ID Qualifier (339-6C)).
Other Payer Processor Control Number (991-MH) - will contain the Processor Control Number (if used) of the payer(s).
Other Payer Group ID (992-MJ) - will contain the Group ID (if used) of the payer(s).
Other Payer Cardholder ID (356-NU) – will contain the Cardholder ID used by the payer(s).
Other Payer Benefit Effective Date (144-UX) = will contain the effective date of the enrollment. Note the situations defined for the Eligibility
Verification transactions are different than other transactions.
Other Payer Benefit Termination Date (145-UY) = will contain the last date of coverage. Note the situations defined for the Eligibility
Verification transactions are different than other transactions.
Other Payer Person Code (142-UV) = will contain the other payer’s code (if used) that specifies the person within a family.
Other Payer Patient Relationship Code (143-UW) = will contain the code to indicate the relationship of patient to cardholder, such as spouse,
child, etc.
Other Payer Help Desk Phone Number (127-UB) = will contain the phone number of the other payer’s help desk.
Other Payer ID Fields
In coordination of benefits or other payments situations, the Other Payer ID fields may be used by one payer to reject the claim or service
billing and show that other coverage exists. Other Payer ID Count (355-NT) designates the number of occurrences of other coverage the payer
is aware of. Other Payer ID Qualifier (339-6C), Other Payer ID (34Ø-7C), and Other Payer Cardholder ID (356-NU) may occur as one payer
has knowledge of other coverages. In addition, Example “Billing – Transaction Code B1 – Coordination of Benefits Scenarios Pharmacy Bills
To Insurance Designated By Patient” and Example “Billing – Transaction Code B1 – Coordination of Benefits – Scenario 1: Pharmacy Bills
Secondary Insurance” has been added to show coordination of benefits scenarios.
For Medicare Part D Eligibility transactions these fields are used by the Facilitator to provide Other Payer information to the provider. In
primary billing transactions for Medicare Part D, the PDP will return to the Pharmacy the secondary/tertiary/etc payer identifier information
(Other Payer ID (34Ø-7C), Other Payer Processor Control Number (991-MH), Other Payer Cardholder ID (356-NU), and Other Payer Group ID
(992-MJ), etc).

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 756 -
For Medicare Part D payment transactions, when the Pharmacy submits the secondary/tertiary claim for payment, the Facilitator then submits
an Information Reporting transaction to the PDP to update patient pay information from the secondary/tertiary/etc claim. The Facilitator
populates Other Payer ID (34Ø-7C), Other Payer Processor Control Number (991-MH), Other Payer Cardholder ID (356-NU), and Other Payer
Group ID (992-MJ) in the Insurance Segment on the Information Reporting request transaction. The data found in Other Payer ID (34Ø-7C)
from the Response Coordination of Benefits/Other Payers Segment is placed in the Other Payer BIN Number (99Ø-MG). Likewise the Other
Payer Processor Control Number (991-MN), Other Payer Cardholder ID (356-NU), and Other Payer Group ID (992-MJ), etc are populated.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 757 -
29. VERSION IDENTIFICATION SYSTEM
A Version/Release level reference scheme is in place for the NCPDP Telecommunication Standard Implementation Guide. The reference
scheme consists of a two-digit sequential enumerator.
The Version Identification changes may be: addition of new fields with or without values, addition/deletion/re-definition of values in an existing
field, redefinition of fields, changes in field size or format, and updated documentation or clarification of existing or new data elements. Such
changes must be accomplished through the ballot process.
Changes/addition/deletion of values that reside in the External Code List do not require the ballot process and do not have any impact on a
Standards Version enumeration.
Editorial changes within an Implementation Guide, additions of Frequently Asked Questions, and all modifications made to provide clarity to
the standard are considered publication changes. Publication changes do not impact a Standards Version enumeration. Publication changes
are so noted on the publication page of the standard. Any additions, deletions, or modifications to the Implementation Guide that makes a
substantive difference to the standard must be approved by process of a ballot.
NCPDP maintains and makes available the latest release from the last two (2) Master Versions of the NCPDP Telecommunication Standard
Implementation Guide.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 758 -
30. FRAMEWORK
A communication standard is intended for use within a specific framework. There are two aspects to this framework:
• Business Framework - defines the nature of the business transaction for which this communication standard is an essential element.
• Technical Framework - describes the essential features of the technology that will be used to implement the standard, and how the
standard affects the technology.
The Business Framework was described in a previous section. The Technical Framework for this standard is described below.
30.1 TECHNICAL FRAMEWORK
The International Standards Organization (ISO) has defined a framework for the definition of telecommunication standards. This standard,
known as the "Open Systems Interface" standard, defines a seven-layer hierarchy of functions within a telecommunication network:
• Applications Layer - provides all services that are directly "comprehensible" to the users' applications -- in other words, this is the
level that interfaces with the users' application. The Applications Layer identifies the users and sets an agreed upon level of security
and makes one user responsible for error recovery.
• Presentation Layer - restructures data into the required format.
• Session Layer - establishes, synchronizes and coordinates the interaction between the end-application processes.
• Transport Layer - provides end-to-end data integrity and error correction.
• Network Layer - switches and routes information between the appropriate nodes.
• Data Link Layer - responsible for managing the physical transfer of data between the nodes.
• Physical Layer - responsible for accessing the physical media.
This version of the NCPDP Telecommunication Standard Implementation Guide addresses the message formats that are used by a specific
application; it is principally a Presentation Layer standard.
All data should be treated in a "transparent" mode throughout the OSI ISO hierarchy to avoid a session termination in an SNA LU2
environment.
30.2 SCOPE
As defined by the Business and Technical Framework described above, the scope of this version of the NCPDP Telecommunication Standard
Implementation Guide is limited as follows:
• Defines the communication of data and the corresponding responses with respect to communications at the Presentation Layer.
• Discusses and recommends specific implementation at the Application, Session, and Transport Layers.
• Recognizes the implications of specific implementations at the Network, Data Link, and Physical Layers.
• Does not define or preclude from use any additional data elements whose intent is to assist the processor or its telecommunication
intermediary in fulfilling specific requirements of the Presentation, Session, Transport, Network, Data Link or Physical Layers of the
ISO OSI Standard to which this version of the standard adheres. Such information includes network logons, protocols and data
fields that are added as prefixes to the start of the application record.
30.3 TECHNICAL DEFINITIONS
This document facilitates the submission of a transaction by a Sender, and accommodates a specific response to that transaction submission
by a Processor or Reporting Entity. This communication is performed in an on-line, real-time environment. The essential features of this
environment are outlined below:
• On-Line - In the context of this version of this document, an "on-line environment" means a logically direct electronic connection
between two active participants. An "active participant" is any device with the capability to accept and act upon a data stream,
recognize the start and end of the data stream, and respond based upon the content of the data stream. This device can range from
a simple data capture terminal to a full-function, general-purpose computer. The participants are assumed to be from two
independent organizations. This standard is for use between organizations, not within an organization.
Within the Business Framework described above, the originator of the transaction is the Sender, and the "Receiver" is either the Processor or
Reporting Entity.
• Real-Time - In the context of this document, a "real-time" transaction is one that is functionally instantaneous. The Sender of the
transaction asks a question or makes a request of the Receiver, and the Sender does not proceed with its current task until it
receives a response. This is a single request/single response type of communication.
• Transmission - The highest level of data transfer is the transmission. The transmission contains information, which is global to the
entire data set. This includes routing information, identification, and information, which determine the parsing of the transactions
within. A transmission may contain one to four transactions, depending upon the transaction type.
• Transaction - Transactions occur within transmissions. Transactions are comprised of data segments of related data elements. One
to four transactions may occur within a transmission, depending upon the type of transaction.
30.4 CONNECTIVITY BETWEEN PARTICIPANTS
There are different connections that might exist between the Originator and the Receiver including:
• Dial-up directly from Originator to Receiver
• Leased-line directly from Originator to Receiver

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 759 -
• Dial-up from Originator to Switch, Leased-line from Switch to Receiver
• Leased-line from Originator to Switch, Leased-line from Switch to Receiver
• Dial-up from Originator to Switch, Leased-line from Switch to Intermediary, Leased-line from Switch to Receiver
• Leased-line from Originator to Switch, Leased-line from Switch to Intermediary, Leased-line from Switch to Receiver
These types of connections are illustrated in the following diagram.
Dedicated Line
Dial
Legend
Pharmacy 2
Pharmacy 1
Switch
Pharmacy
Headquarters
Processor
Processor
Reporting Entity
Reporting Entity
Pharmacy 3
Pharmacy 4
Pharmacy 5
Pharmacy 6
Intermediary
Switch
Figure 3. Connectivity between participants.
The type of connection will dictate the specific considerations applicable to a particular telecommunication implementation. The following
conventions should be followed whenever appropriate:
• The asynchronous communications protocol certified by VISA, USA, Inc. is recommended for any dial-up connection. This protocol
is recommended due to its wide usage and internal error detection features. The question/answer nature of the conversation
precludes any benefit from a multi-block protocol such as VISA-II.
• The ANSI BIN number is widely used as a network destination designator. The message formats described in this document are
consistent with this mechanism. All Processors should contact ANSI and obtain a BIN number to uniquely identify them. The
contact information for ANSI can be found in the Data Dictionary. If a BIN number cannot be obtained from ANSI, then
contact NCPDP for a unique processor number that will be assigned by the Council.
• The default field values are the same in all cases. This process should maximize the opportunity for data compression through the
elimination of redundant characters. This data compression typically occurs at either the Physical Layer or the Data Link Layer, and
depends on the specific connection.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 760 -
• Modem and transmission speed should be identified and should be appropriate to the specific connection. In a dial-up environment,
a modem that supports a commonly used format and line speed should be used. In a leased-line environment, the arrangements
are determined by the parties involved.
30.5 SOFTWARE/SYSTEM DEVELOPMENT
• Trading partners must be capable of transmitting and receiving transactions in full variable format.
• Trading partners must determine which transaction types are used.
• Trading partners must determine which fields in the Version D and above record are required, in accordance with the Version D and
above standard, to properly process a transaction.
• Trading partners must agree upon the acceptable number of transactions per transmission.
• Trading partners must determine whether the processor or switch requires a certification procedure before transmitting transactions.
• Processor software must be capable of generating, and Provider software must be capable of receiving, a response with the same
“Version/Release Number”, “Transaction Code”, and “Transaction Count” as the transaction transmitted.
30.6 RESPONSIBILITIES OF THE PARTICIPANTS
When using this standard, the Originator, Switch, and the Receiver are expected to perform specific technical functions, as outlined below:
30.6.1 RESPONSIBILITIES OF THE ORIGINATOR
At a high-level, the Originator is responsible for:
• Populating all mandatory fields for this request transmission.
• Populating all situational or optional fields for this request transmission, as determined by the rule of this guide and the trading
partner(s).
• Establishing the connection with the Switch or Processor, and initiating the telecommunication session.
• Formatting the request and sending it in the message envelope that is appropriate to the protocol being used.
• Interpreting and acting upon any response provided by the Processor. This will vary from Processor to Processor, plan to plan, and
from time to time, (i.e., during an equipment problem). This will also include the situation where no response is received (a timeout).
• Terminating the session and disconnecting the transmission.
30.6.2 RESPONSIBILITIES OF THE SWITCH
At a high-level, the Switch is responsible for:
• Establishing the connection with the Processor and delivering the request from the Originator.
• Interpreting the request submitted by the Originator and responding as needed to provide the maximum amount of information for
error correction and resolution when required.
• Providing the ability to convert versions of the standard as feasibly possible and needed based on trading partner agreements.
• Returning the response from the Processor to the Originator.
• Providing a high level of system availability and providing a viable fallback mechanism in the event of equipment failure.
30.6.3 RESPONSIBILITIES OF THE RECEIVER
At a high-level, the Receiver is responsible for:
• Interpreting requests submitted by the Originator and responding as needed to provide the maximum amount of information for error
correction and resolution when required.
• Populating all mandatory fields for this response transmission.
• Populating all situational or optional fields for this response transmission, as determined by the rule of this guide and the trading
partner(s).
• Formatting the response and sending it in the message envelope that is appropriate to the protocol being used.
• Ignoring irrelevant data that may be supplied by the Originator (i.e., the request may have data in fields not required for a particular
plan. This situation must not create an error).
• Recognizing and supporting multiple versions of the standard for a long enough period of time to allow the users to convert their
processing as new versions of the standard are developed and released.
• Providing a high level of system availability and providing a viable fallback mechanism in the event of equipment failure.
30.6.4 RESPONSIBILITIES OF THE FACILITATOR
At a high-level, the Facilitator in the Medicare Part D claims environment is responsible for:
• Processing Eligibility Inquiries
• Reporting supplemental claims to the Prescription Drug Plan (PDP) for True Out-Of-Pocket (TrOOP) calculation.
Facilitators utilize existing network connectivity to capture secondary, tertiary, etc. claims activities originating from the Pharmacy providers to
a Switch. The Facilitator receives a transmission from the Switch. Routing is accomplished through unique combination of
• BIN and PCN, or
• BIN and PCN and Group ID, or
• BIN and Group ID assignments.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 761 -
30.7 PROCESSOR IMPLEMENTATION
Processors accepting Version D and above transactions from providers may encounter the following special concerns.
30.7.1 TRANSMITTING A RESPONSE
Systems must relay complete information regarding the disposition of a transaction, including status, reason(s) for reject, and basis of
determination for payment, as applicable. If the claim or service is paid, payment amount determination must be returned. The following fields
will be utilized to accommodate this requirement.
• The “Basis of Reimbursement Determination” (522-FM) provides a code indicating the method of calculating the claim payment
amount.
• The component parts of the amount paid for the claim must be returned in the applicable fields. See section “Response Processing
Guidelines”, “Pricing Guidelines”.
• Refer to the section “Prescription And Service Pricing Formulae” in the “Frequently Asked Questions” section of this Guide.
• See section “Response Pricing Segment” for an in-depth discussion of pricing fields.
• The preferred product fields in the Response Claim Segment may be used to provide information regarding therapeutic substitution
opportunities. The fields repeat to accommodate multiple preferences of products to be dispensed.
30.7.2 OTHER CONSIDERATIONS
• Based on the value in the Transaction Count (1Ø9-A9), the same number of transaction responses must be returned. For example,
if Transaction Count is 2, there must be two transaction responses returned. If the Transaction Count is 4, there must be four
transaction responses returned. There is one exception - when the transmission is rejected at the header level due to errors in
invalid Version/Release Number (1Ø2-A2) or Transaction Count (1Ø9-A9) - only one response must be returned.
• The message fields are to be used to provide supplemental information regarding the payment of the claim or the reason for
rejection.
• The “Approved Message Code” (548-6F) may be used to indicate that an additional follow-up action is warranted; e.g., “Generic
Available,” “Non-formulary Drug,” “Maintenance Drug.”
• If a claim rejects at the claim header level, it is not necessary to return claim detail.
• Claim level reject detail must be provided if claim level detail caused the rejection.
30.8 SWITCH IMPLEMENTATION
A “Switch” may support the reception and transmission of all NCPDP format variations. Switches may offer to reformat (convert) transactions
from one format or version to another if trading partners require this feature for compatibility reasons.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 762 -
31. GENERAL STRUCTURAL OVERVIEW
31.1 OVERVIEW
31.1.1 TRANSMISSION
Transmission - The highest level of data transfer is the transmission. The transmission contains information, which is global to the entire data
set. This includes routing information, identification, and information, which determine the parsing of the transactions within. A transmission
may contain one to four transactions, depending upon the transaction type.
The Transaction Header and Response Header segments contain fixed length data elements. These segments do not use field separators to
separate data elements. In these segments, each data element is transmitted at its maximum length positionally. The Header Segment is
required and must be first in the transmission. When a field is not used, depending upon trading partner needs, the field must be filled with
zeroes or spaces, as appropriate.
At the Transmission request level, the Transaction Header Segment must appear first. The Patient Segment and Insurance Segment can be
submitted in either order, if both appear, regardless of whether they are mandatory, situational, or optional segments. At the Transaction
request level, the Group Separator occurs, and then the other segments may occur in any order. Note the Segments must occur only once and
according to the rules for that transaction.
At the Transmission response level, the Response Header Segment must appear first. The Response Message Segment and Response
Insurance Segment may occur in either order, if both appear, regardless of whether they are mandatory, situational, or optional segments. At
the Transaction response level, the Group Separator occurs, and then the Response Status Segment through Response Coordination of
Benefits/Other Payers Segment may occur in any order. Note the Segments must occur only once and according to the rules for that
transaction.
31.1.2 TRANSACTION
Transaction - Transactions occur within transmissions. Transactions are comprised of data segments of related data elements. One to four
transactions may occur within a transmission, depending upon the type of transaction.
Transactions are a collection of segments. Transactions are separated within a transmission with the use of a Group separator character.
31.1.2.1 SEGMENTS
Segments are a collection of data fields. Segments denote similar data elements or functions. Segments are separated with the use of a
Segment separator and a Segment identifier.
The receiver must not force an order of segments.
The other segments contain mandatory and situational or optional fields. All data fields within these segments are separated from one
another by the use of a field separator character. Data fields are identified with the use of a field identifier.
Mandatory data elements must occur first within the appropriate segment. Each mandatory field is preceded by the field separator and
the field's identifier. Mandatory fields may be truncated.
Situational or optional fields occur after the mandatory fields in a segment. Each situational or optional field is preceded by the field
separator and the field's identifier. Situational or optional fields may be truncated. Situational or optional fields may occur in any order in a
segment except for those designated with a qualifier or in a repeating group. Refer to section “Standard Conventions”, “Qualifiers” and
“Repetition And Multiple Occurrences” for information on qualifiers and repeating fields usage.
Segments must not occur multiple times within a transaction. However, segments may occur multiple times within a transmission. It is
recommended that the Segment Identification field not be submitted if no ensuing fields will be sent in that segment. However, if a
transmission contains a Segment Identification with no data elements following, a syntax rejection must not result, unless trading partners
have agreed that one or more data elements are necessary in this segment.
For all transaction types, if only the Segment ID field applies (i.e., no information will be submitted in any of the situational or optional fields),
the segment is not required. However, if the Segment ID is sent, even though no ensuing situational or optional fields are transmitted, the
transaction must not be rejected by the processor.
An Eligibility Verification transaction does not use a group separator. All other transactions use group separators whether one, two,
three, or four transactions occur within a transmission (according to that transaction’s rules).
31.2 TRANSMISSION LEVEL FOR A REQUEST
The following segments occur at the transmission level in a request. Refer to section “Transmission Structure” to determine which segments
are appropriate for each transaction code.
• Transaction Header Segment
• Patient Segment
• Insurance Segment

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 763 -
For every request, the following rules apply:
• The Transaction Header is mandatory and must appear first in the request.
• The transmission level segments follow (Insurance, Patient).
• Other request segments are mandatory, situational, optional, or not used according to the matrices published in this document.
• The Transaction Count on the request must match the number of transactions sent within the transmission.
31.2.1 RULES FOR 2, 3 OR 4 TRANSACTION FORMATS
1. The additional transactions must be for the same patient.
2. The additional transactions must be for the same date of service.
3. If the Insurance segment is used, the additional transactions must be for the same benefit program.
31.3 TRANSACTION LEVEL FOR A REQUEST
The following segments occur at the transaction level in a request. Refer to section “Transmission Structure” in this document to determine
which segments are appropriate for each transaction code.
• Pharmacy Provider Segment
• Prescriber Segment
• Coordination of Benefits/Other Payments Segment
• Workers’ Compensation Segment
• Claim Segment
• DUR/PPS Segment
• Coupon Segment
• Compound Segment
• Pricing Segment
• Prior Authorization Segment
• Clinical Segment
• Additional Documentation Segment
• Facility Segment
• Narrative Segment
31.4 TRANSMISSION LEVEL FOR A RESPONSE
The following segments occur at the transmission level in a response. Refer to section “Transmission Structure” to determine which segments
are appropriate for each transaction code.
• Response Header Segment
• Response Message Segment
• Response Insurance Segment
• Response Insurance Additional Information Segment
• Response Patient Segment
Response Header, field Header Response Status (5Ø1-F1) is limited to:
“A “ for transmission "accepted"
“R “ for transmission "rejected"
For every response, the following rules apply:
• The Response Header segment is mandatory and must appear first in the response.
• The Response Message segment follows, and is situational or optional.
• The Response Status segment is mandatory.
• Other response segments are mandatory, situational, optional, or not used according to the matrices published in this document.
• Based on the value in the Transaction Count (1Ø9-A9), the same number of transaction responses must be returned. There is one
exception - when the transmission is rejected at the header level due to errors in invalid Version/Release Number (1Ø2-A2) or
Transaction Count (1Ø9-A9) - only one response must be returned.
31.5 TRANSACTION LEVEL FOR A RESPONSE
The following segments occur at the transaction level in a response. Refer to section “Transmission Structure” to determine which segments
are appropriate for each transaction code.
• Response Status Segment
• Response Claim Segment
• Response Pricing Segment
• Response DUR/PPS Segment
• Response Prior Authorization Segment
• Response Coordination of Benefits/Other Payers Segment
Response Status Segment, field Transaction Response Status (112-AN) is limited to:
“A” for "Approved"
“B” for “Benefit”

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 764 -
“C“ for "Captured"
“D“ for "Duplicate of Paid"
“F” for "Prior Authorization Deferred"
“P“ for "Paid"
“Q” for “Duplicate of Captured”
“R “ for "Rejected"
“S” for “Duplicate of Approved”

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 765 -
32. NOTABLE CHANGES FROM PREVIOUS TELECOMMUNICATION
VERSIONS
General:
• The NCPDP Telecommunication Specification and the Telecommunication Standard Implementation Guide documents were
combined into one document that is now referred to as the NCPDP Telecommunication Standard Implementation Guide.
• An NCPDP External Code List (ECL) was created where values of data element fields reside. The values support the data elements
within the NCPDP approved standards.
• The NCPDP Professional Pharmacy Services Implementation Guide was incorporated into the NCPDP Telecommunication
Standard Implementation Guide so the reader would have one source.
• The NCPDP ORDUR (Online Real-time Drug Utilization Review) Implementation Guide was incorporated into the NCPDP
Telecommunication Standard Implementation Guide so the reader would have one source.
In This Document:
• Every transaction request and response now supports usage situations and matrices for consistent implementation of transactions,
segments, and fields. Due to HIPAA Privacy requirements concerning mandatory/situational data elements submitted between
covered entities, it was necessary to add situations and charts for usage. It is anticipated that these charts add clarification for
implementation.
• For Compounded Claim Processing – the two alternatives (Scenario A - Most expensive legend drug and Scenario B - Billing codes)
were removed and only one method of billing remains - the use of the Compound Segment with the Claim Segment.
• New fields and guidance have been added to this document for coordination of benefits processing.
• New fields and guidance have been added to this document for consistent use of pricing fields.
• The terms for “Copay” and “Coinsurance” were reviewed and redefined throughout this guide where appropriate. The term “1ØØ%
Copay” was modified to “Patient Financial Responsibility” throughout the document.
• The process flow was modified to include payer-to-payer and the introduction of new entities, “Facilitator” and “Participant”.
Guidance was added to this document to support these types of processing.
• An enhanced Eligibility response is included to support Medicare Part D. This effected modifications in response segments which
affect other transactions.
• Additions and modifications to segments, fields, and values can be found in section “Appendix A. History of Document Changes”, as
well as in the NCPDP Data Dictionary and the NCPDP External Code List.
• Additional Message Information (526-FQ) size has been modified and the field repeats with a count, a qualifier, and the ability to use
a continuation character.
• Duplicate logic has been enhanced for downstream payers. See section “Response Processing Guidelines”, “Duplicate
Transactions”.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 766 -
33. STANDARD CONVENTIONS
This section discusses the generally accepted practices used by the industry and provides guidelines for determining which variant(s) of the
standard to use.
33.1 VARIABLE USAGE GUIDELINES
The NCPDP Telecommunication Standard Implementation Guide (Version D and above) allows variable length transactions only.
• Sending only necessary data elements and truncating whenever possible assures minimal transmission time.
• Situational or optional fields and segments may be added to or deleted from the transmission as necessary to accommodate
changing needs.
• The segment usage matrix included with this Guide specifies required, situational, optional and not used segments for each
transaction type.
• Version D and above supports up to four transactions per transmission for transaction codes B1-B3 (except for compounds), S1-S3,
N1-N3, and C1-C3.
• Fields in the Version D and above record are defined as alphanumeric, numeric, or dollar fields.
• Dollar fields default to zeroes; however, dollar fields are always signed. The least significant digit of a dollar field must always be an
Overpunch Sign, not a digit.
• Reject Code (511-FB) guidance can be found in the NCPDP External Code List section “Appendix A – Reject Codes”.
33.2 GENERAL SYNTAX OUTLINE
Data elements have been grouped into segments to assist in usage of similar information.
33.2.1 HEADER SEGMENT
The first segment of every transmission (request or response) is the Header Segment. This is the only segment that does not have a Segment
Identifier since it is a fixed field and length segment. After the Header Segment, other segments are included, according to the particular
transaction type (see section “Transmission Structure”). Every other segment has an identifier to denote the particular segment for parsing.
Segments may appear in any order after the Header Segment, according to whether the segment occurs at the transmission or transaction
level. Segments are not allowed to repeat within a transaction. Segments must occur more than once only in a multiple transaction
transmission.
In the Header Segment, all fields are required positionally and filled to their maximum designation. This is a fixed segment. If a required field is
not used in the Header Segment, it must be filled with spaces or zeroes, as appropriate. The fields within the Header Segment do not use field
separators.
33.2.2 OTHER SEGMENTS
Other segments may have both required and situational or optional fields. Situational or optional fields in a segment are submitted after the
required fields. Both types of fields must be preceded by a field separator and the field’s identifier. Situational or optional fields may appear in
any order except for those designated with a qualifier or in a repeating group. The required, situational, and optional fields may be truncated to
the actual size used. Refer to the “Standard Conventions”, “Qualifiers” and “Repetition And Maximum Occurrences” sections that follow.
It is recommended that the Segment ID field not be submitted if no ensuing fields will be sent. If the Segment is situational or optional for that
transaction and there are no Mandatory fields within that Segment, the Segment Identification (111-AM) can be sent without an error
generated. This is not recommended, but is possible.
The key is that the Segment must be situational or optional for that transaction and there must not be any Mandatory fields within that
Segment. If the Segment contains Mandatory fields, failure to send the mandatory fields is an error.
If the Segment is not used for that transaction, it is an error to send a Segment that is not defined for that transaction.
Parsing is accomplished with the use of separators. Version D and above uses three separators.
• Segment separator Hex 1E (Dec 3Ø)
• Group separator Hex 1D (Dec 29)
• Field separator Hex 1C (Dec 28)
33.2.3 A TRANSMISSION
A transmission includes the total request or response being sent. A transmission consists of the Header Segment followed by situational or
optional Segments relating to the entire transmission.
A transmission consists of one or more transactions separated by group separators. With one exception, the Eligibility Verification
transmission, which does not use a group separator, all other transmissions, whether for one, two, three, or four transactions, use group
separators to denote the start of a transaction.
33.2.4 A TRANSACTION
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 767 -
Within a transaction, appropriate segments are included. Segments are delineated with the usage of Segment separators. Segments are also
identified with the usage of a Segment Identifier in the first position of each segment. One to many segments may be included in each
transaction. Field separators are used to delineate fields in the segments.
The general syntax of a transmission request and response will appear as follows:
Header Segment
Header Segment Fields
Segment Separator
Mandatory Fields within Segment as appropriate, with field separators
Situational or Optional Segment Fields with field separators
Segment Separator
Mandatory Fields within Segment as appropriate, with field separators
Situational or Optional Segment Fields with field separators
Group Separator
Segment Separator
Mandatory Fields within Segment as appropriate, with field separators
Situational or Optional Segment Fields with field separators
Segment Separator
Mandatory Fields within Segment as appropriate, with field separators
Situational or Optional Segment Fields with field separators
33.2.5 ORDER OF SEGMENTS
At the Transmission request level, the Transaction Header Segment must appear first. The Patient Segment and Insurance Segment can be
submitted in either order, if both appear, regardless of whether they are mandatory, situational, or optional segments. At the Transaction
request level, the Group Separator occurs, and then the other segments may occur in any order. Note the Segments must occur only once and
according to the rules for that transaction.
At the Transmission response level, the Response Header Segment must appear first. The Response Message Segment and Response
Insurance Segment may occur in either order, if both appear, regardless of whether they are mandatory, situational, or optional segments. At
the Transaction response level, the Group Separator occurs, and then the Response Status Segment through Response Coordination of
Benefits/Other Payers Segment may occur in any order. Note the Segments must occur only once and according to the rules for that
transaction.
The receiver must not force an order of segments.
The general structure of a request, for most transactions, will appear as follows (recognizing that some segments may not be used for a given
transaction):
Transmission
Header Segment
Patient Segment
Insurance Segment
Transactions (up to four per transmission)
Claim Segment
Pharmacy Provider Segment
Prescriber Segment
Coordination of Benefits/Other Payments Segment
Workers’ Compensation Segment
DUR/PPS Segment
Pricing Segment
Coupon Segment
Compound Segment
Prior Authorization Segment
Clinical Segment
Additional Documentation Segment
Facility Segment
Narrative Segment
The general structure of a response, for most transactions, will appear as follows (recognizing that some segments may not be used for a
given transaction):
Response
Response Header Segment
Response Message Segment
Response Insurance Segment
Transaction Response (up to four per transmission)
Response Status Segment
Response Claim Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 768 -
Response Pricing Segment
Response DUR/PPS Segment
Response Prior Authorization Segment
Response Coordination of Benefits/Other Payers Segment
33.3 EXPLANATION OF SEGMENT AND FIELD DESIGNATION
Categorization Explanation
MANDATORY Segment:
The Segment is mandatory for the Transaction.
Field:
The Field is mandatory for the Segment for the Transaction.
Mandatory field elements must occur first in the Segment, in the order specified.
SITUATIONAL Segment:
The Segment has been further designated for usage for the Transaction.
See section “Order of Segments” above.
Field:
The Field has been further designated for usage for the Transaction. See indention below fo
r
specific guidance.
Situational fields may occur in any order, as long as the qualifier rule and count or counte
r
rules are followed. Qualifier fields must be submitted first, followed by the field qualified. I
f
the field is not needed in the transaction type, both the qualifier and the field qualified are
eliminated.
Required Field:
The Field has been designated with the situation of "Required" for the Segment for the
Transaction.
Required for Medicaid Subrogation
only The Field has been designated with the situation of "Required" for the Segment for the
Transaction for Medicaid Subrogation usage only.
Qualified Requirement The situations designated have qualifications for usage ("Required if x", "Not required if y")
for the Segment for the Transaction.
Qualified Requirement for Medicaid
Subrogation only The situations designated have qualifications for usage ("Required if x", "Not required if y")
for Medicaid Subrogation.
INFORMATIONAL ONLY The Field is for informational purposes only for the Segment for the Transaction.
OPTIONAL The Field has been designated as optional usage (situations were not intentionally defined)
for the Segment for the Transaction.
NOT USED The Segment is not used for the Transaction
or
The Field is not used for this Segment for the Transaction.
33.4 SEPARATOR CHARACTERS
Level of Separator Decimal Representation Hex Representation Comment
Segment Ø3Ø 1E Separates segments from each other.
Group Ø29 1D Separates groups from each other.
Field Ø28 1C Separates fields from each other.
For example, in a sample transmission, shown with very simplified syntax below, the following data stream might appear. Please note this
uses the hex values represented above as <1E>, <1D>, <1C>.
This example represents a Billing request transmission with two prescriptions. Please refer to the NCPDP Data Dictionary for field and
segment cross-reference.
Note: the presence of a string of b’s (bbbb) in the Content column designates a field that must be padded out to spaces.
Field # ID Name Content Comment
1Ø1 BIN Number 61122Ø
1Ø2 Version/Release Number 53
1Ø3 Transaction Code B1
1Ø4 Processor Control Number 123456789Ø
1Ø9 Transaction Count 2 Two billing transactions
2Ø2 Service Provider ID Qualifier Ø7
2Ø1 Service Provider ID 4563663bbbbbbbb
4Ø1 Date of Service 2ØØ8Ø1Ø2
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 769 -
11Ø Software Vendor/Certification ID 98765bbbbb
SS Segment Separator <1E>
FS AM <1C>AM
111 Segment Identification Ø1 Patient segment
FS C4 <1C>C4
3Ø4 Date of Birth 1962Ø615
FS C5 <1C>C5
3Ø5 Patient Gender Code 1
FS CA <1C>CA
31Ø Patient First Name JOSEPH
FS CB <1C>CB
311 Patient Last Name SMITH
SS Segment Separator <1E>
FS AM <1C>AM
111 Segment Identification Ø4 Insurance segment
FS C2 <1C>C2
3Ø2 Cardholder ID 987654321
FS C6 <1C>C6
3Ø6 Patient Relationship Code 1
GS Group Separator <1D>
SS Segment Separator <1E>
FS AM <1C>AM
111 Segment Identification Ø7 Claim Segment
FS EM <1C>EM
455 Prescription/Service Reference Number
Qualifier
1
FS D2 <1C>D2
4Ø2 Prescription/Service Reference Number 1234567
FS E1 <1C>E1
436 Product/Service ID Qualifier Ø3 NDC
FS D7 <1C>D7
4Ø7 Product/Service ID ØØØØ6Ø94228 NDC number
FS E7 <1C>E7
442 Quantity Dispensed 3ØØØØ 3Ø.ØØØ
FS D3 <1C>D3
4Ø3 Fill Number Ø
FS D5 <1C>D5
4Ø5 Days Supply 3Ø
FS D6 <1C>D6
4Ø6 Compound Code 1
FS DE <1C>DE
414 Date Prescription Written 2ØØ8Ø1Ø2
FS DF <1C>DF
415 Number of Refills Authorized 5
SS Segment Separator <1E>
FS AM <1C>AM
111 Segment Identification Ø3 Prescriber Segment
FS EZ <1C>EZ
466 Prescriber Identification Qualifier 8
FS DB <1C>DB
411 Prescriber Identification ØØG2345
SS Segment Separator <1E>
FS AM <1C>AM
111 Segment Identification 11 Pricing Segment
FS D9 <1C>D9
4Ø9 Ingredient Cost Submitted 557{ 55.7Ø
FS DC <1C>DC
412 Dispensing Fee Submitted 1ØØ{ 1Ø.ØØ
FS DX <1C>DX
433 Patient Paid Amount Submitted 1ØØ{ 1Ø.ØØ
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 770 -
FS DQ <1C>DQ
426 Usual and Customary Charge 7ØØ{ 7Ø.ØØ
FS DU <1C>DU
43Ø Gross Amount Due 657{ 65.7Ø
GS Group Separator <1D>
SS Segment Separator <1E>
FS AM <1C>AM
111 Segment Identification Ø7 Claim Segment
FS EM <1C>EM
455 Prescription/Service Reference Number
Qualifier
1
FS D2 <1C>D2
4Ø2 Prescription/Service Reference Number 1233456
FS E1 <1C>E1
436 Product/Service ID Qualifier Ø3 NDC
FS D7 <1C>D7
4Ø7 Product/Service ID 17236Ø569Ø1 NDC number
FS E7 <1C>E7
442 Quantity Dispensed 15ØØØ 15.ØØØ
FS D3 <1C>D3
4Ø3 Fill Number Ø
FS D5 <1C>D5
4Ø5 Days Supply 15
FS D6 <1C>D6
4Ø6 Compound Code 1
FS DE <1C>DE
414 Date Prescription Written 2ØØ8Ø1Ø2
FS DF <1C>DF
415 Number of Refills Authorized Ø
SS Segment Separator <1E>
FS AM <1C>AM
111 Segment Identification Ø3 Prescriber Segment
FS EZ <1C>EZ
466 Prescriber Identification Qualifier 8
FS DB <1C>DB
411 Prescriber Identification ØH22345
SS Segment Separator <1E>
FS AM <1C>AM
111 Segment Identification 11 Pricing Segment
FS D9 <1C>D9
4Ø9 Ingredient Cost Submitted 3ØØ{ 3Ø.ØØ
FS DC <1C>DC
412 Dispensing Fee Submitted 1ØØ{ 1Ø.ØØ
FS DX <1C>DX
433 Patient Paid Amount Submitted 1ØØ{ 1Ø.ØØ
FS DQ <1C>DQ
426 Usual and Customary Charge 45Ø{ 45.ØØ
FS DU <1C>DU
43Ø Gross Amount Due 4ØØ{ 4Ø.ØØ
33.4.1 SEPARATOR CHARACTER RULES
The software that creates transactions according to the rules of this document must ensure that the segment, group and field separator
characters do not appear as data in any field.
33.5 FIELD DEFINITIONS AND VALUES
A definition of each data element and appropriate values is provided in the NCPDP Data Dictionary. The NCPDP Data Dictionary identifies
and defines the information that is specified in the NCPDP Standard Formats. Each data element that is presented in a transaction data set is
identified in the NCPDP Data Dictionary.
Every effort has been made to keep references to the names of data elements in the standard consistent with the NCPDP Data Dictionary. To
facilitate presentation and readability within this document, customarily acceptable abbreviations may be used (e.g., "#" for number, "RX" for
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 771 -
prescription, "DAW" for Dispense As Written, etc.). Please refer to the Data Dictionary for the complete names and definitions of the data
elements.
The NCPDP External Code List defines the valid values for the data elements.
33.6 CHARACTER SETS DESIGNATION
N Unsigned Numeric, always right justified, zero filled.
Example:
9(7)v999 is represented as 9999999999
D Signed Numeric, sign is internal and trailing. Zeroes are always positive, always right justified.
Dollar-cents amount with 2 positions to the right of the implied decimal point. All other positions to
the left of the implied decimal point.
Example:
D field of length 8 is represented $$$$$$cc
A/N Alphanumeric, always left justified, space filled. A-Z, Ø-9, and printable characters.
33.7 CHARACTER SET DESIGNATION TRUNCATION
The following field format values are supported and are subject to truncation described in previous sections.
33.7.1 OVERVIEW
Version D and above allows for variable length transactions. "Variable" implementation allows the sender and receiver the option of
compressing or eliminating “situational” or "optional" data elements to reduce message length where these data elements are not required by
Processor or Reporting Entity.
The request and response contain "Mandatory" segments. The Transaction Header and Response Header segments contain fixed length data
elements. These segments do not use field separators to separate data elements. In these segments, each data element is transmitted at its
maximum length positionally. Data elements not needed for a particular transmission are to be zero or space filled as appropriate. See
“Transmission Structure” section for more detail.
For other segments, situational or optional fields which are identified by field separators and field identifiers are utilized. “Situational” or
"optional" data elements may be present for both the header and transaction sections of a request or response. See “General Syntax
Structure” section above for more information.
Situational or optional data elements that are not mandatory may be eliminated or truncated. In the truncated method, data compression of
leading zeros in numeric (“N” & “D”) fields and trailing spaces in the alphanumeric (“A/N”) fields may be suppressed to decrease transmission
time. Processors must indicate the extent of their ability to accept variable transactions in their user documentation if this capability is desired.
Note: Processors must be prepared to ignore situational or optional fields submitted by providers that are not used. These fields may not be of
importance to the processor, but may be required of the originating pharmacy system.
When transmitting a Version D and above record, truncating trailing blanks and leading zeroes within fields in the variable portions of the
record is recommended. If a field in one of the variable portions is empty, omit the field entirely (including the Field Separator and Field
Identifier). Do not truncate or eliminate any fields in the required header segments.
33.7.2 NUMERIC
"N" = Unsigned Numeric, always right justified, zero filled.
Example: 9(7)v999 represents 9999999999
Truncation: ØØØØØØØ4ØØ becomes 4ØØ
Remove leading zeros
Numeric fields default to zeroes.
33.7.2.1 NUMERIC TRUNCATION
When numeric fields are in a mandatory fixed length segment, such as the Transaction Header Segment or Response Header Segment, the
numeric fields must be padded with zeroes to the maximum length of the numeric field.
For all other numeric fields used in the NCPDP Telecommunication Standard Implementation Guide, sending the leading zero(es) is
permissible (but not recommended), or truncating the leading zero(es) is permissible (and recommended). For a situational or optional numeric
field, a value of Ø1 is the same as 1 and either is permitted. A value of ØØ15 is the same as 15 and either is permitted.
33.7.3 DOLLAR
"D" = Signed Numeric, sign is internal and trailing (see section “Internal Representation of Overpunch Signs” below), zero
always positive, always right justified, zero filled dollar-cents amount with 2 positions to the right of the implied decimal point, all other positions
to the left of the implied decimal point.
Example: "D" fields of length 8 represent $$$$$$cc
Truncation: ØØØØØ21Ø{ becomes 21Ø{
Remove leading zeros

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 772 -
33.7.3.1 DOLLAR TRUNCATION
When a dollar field is supported, a value must always be returned, whether zero or higher or lower. The only time a dollar field is not returned
is when it is not supported or its value cannot be determined. If a dollar field is sent on the request, the response-paired field must be returned
if supported, so that balancing can occur.
See section “Response Processing Guidelines”, “Pricing Guidelines”.
33.7.4 ALPHANUMERIC
"A/N" = Alphanumeric, upper case when alpha, always left justified, space filled, upper case, printable characters.
Truncation: “1234ABC44bbbbb“ becomes “1234ABC44”
Remove trailing spaces
The NCPDP Telecommunication Standard Implementation Guide allows the use of
<space>
Ø123456789
ABCDEFGHIJKLMNOPQRSTUVWXYZ
~`!@#$%^&*()_-=+\|{[]}:,<.>/?;'"
Alphanumeric fields default to spaces, not null characters, when empty.
The use of lower case letters ASCII 97 - 122 (61 - 7A hex) is not allowed in the NCPDP Telecommunication Standard Implementation Guide.
33.7.4.1 ALPHANUMERIC TRUNCATION
For situational or optional alphanumeric fields used in the NCPDP Telecommunication Standard Implementation Guide, sending the trailing
space(s) is permissible (but not recommended), or truncating the trailing space(s) is permissible (and recommended). For a situational or
optional alphanumeric field, a value of "1 " is the same as "1" and either are permitted. A value of "ØØ1 " is the same as "ØØ1" and either
are permitted.
When alphanumeric fields are in a mandatory fixed length segment, such as the Transaction Header Segment or Response Header Segment,
the alphanumeric fields must be padded with spaces to the maximum length of the alphanumeric field.
An alphanumeric field may contain a space or spaces anywhere within the field. For example (where b is a space)
“ABCbDE” or
“bbABCbDE”
are valid uses of a field with spaces. They are technically different values.
Trailing spaces may be truncated. For example,
“ABC” and
“ABCbbb“
are the same value when the trailing spaces are truncated.
Spaces at the beginning of the field must not be truncated. For example,
“ABC” and
“bbABC”
represent technically different values for the same field.
However, while leading spaces are technically valid, leading spaces are not recommended as one must consider the individual who will enter
or view the data in question. For example, spaces at the beginning of a Cardholder ID or Group ID appear to be “white” space on the ID card
so it is unlikely that it will be known that a leading space exists.
For example, Person Code (3Ø3-C3) is defined as a format of alphanumeric 3. In an alphanumeric field, every digit has significance, with
trailing spaces allowed to be truncated. The value “6Ø” in a three-byte alphanumeric field is actually “6Ø “ (six-zero-blank) and is not the same
value as “Ø6Ø” (zero-six-zero).
33.8 DEFAULT VALUES
The NCPDP Data Dictionary defines values and default values for the fields contained in this document. In general, unless otherwise
specified by the Data Dictionary,
• Alphanumeric ("A/N") fields have default values of spaces
• Numeric ("N") and Signed Numeric ("D"), used for dollar fields, have default values of zeros.
33.9 INTERNAL REPRESENTATION OF OVERPUNCH SIGNS
The purpose of using Overpunch signs in dollar fields is to allow the representation of positive and negative dollar amounts without expanding
the size of the field (i.e., to hold the plus or minus character).
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 773 -
The Overpunch sign replaces the right most character in a dollar field. The signed value designates the positive or negative status of the
numeric value. The dollar field of $99.95 would be represented as 999E with truncation. A negative dollar amount of $2.5Ø would be
represented as 25} with truncation.
UNITS SIGNED POSITIVE SIGNED NEGATIVE
Digit Graphics Oct Dec Hex Graphics Oct Dec Hex
Ø { 173 123 7B } 175 125 7D
1 A 1Ø1 65 41 J 112 74 4A
2 B 1Ø2 66 42 K 113 75 4B
3 C 1Ø3 67 43 L 114 76 4C
4 D 1Ø4 68 44 M 115 77 4D
5 E 1Ø5 69 45 N 116 78 4E
6 F 1Ø6 7Ø 46 O 117 79 4F
7 G 1Ø7 71 47 P 12Ø 8Ø 5Ø
8 H 11Ø 72 48 Q 121 81 51
9 I 111 73 49 R 122 82 52
Table shows ASCII values
33.10 DATE FORMAT
All dates are in the format "CCYYMMDD". A 4-digit year is used to minimize software conversion at the change of the century, and to properly
handle situations such as when patients are older than 1ØØ years.
33.10.1 DEFAULT DATE FORMAT
Fields defined as Date format (CCYYMMDD) must not be defaulted to ØØØØØØØØ.
A date field must not default to zeroes, as this is an invalid date. If a pharmacy submits a date of zeroes, the processor must reject it as an
invalid date, even if the processor ignores/does not use this field in their processing, but must store this field as part of the original transaction
data. In databases that store this field as “date”, write routines would fail with a write exception for the invalid date.
A processor that returns a date field is held to the same valid date rule.
33.11 IMPLIED DECIMAL POINTS
Decimal points in dollar fields are implied. Diagnosis code fields must adhere to the owner’s code set rules and formats.
33.12 EXPLICIT HYPHENS
In the Version D and above standards, only the Employer ID (333-CZ) field will contain an explicit hyphen. All other hyphens are implied.
33.13 QUALIFIERS
Some data elements are further defined with the use of qualifiers. Qualifier fields must be submitted first, followed by the field qualified. If the
field is not needed in the transaction type, both the qualifier and the field qualified are eliminated.
33.14 REPETITION AND MULTIPLE OCCURRENCES
Version D and above includes the ability to repeat certain fields and groups of fields. This document has detailed information and examples.
33.14.1 MULTIPLE OCCURRENCES OF SEGMENTS
A segment must appear only once in a transaction. Segments do not repeat or have multiple occurrences. However, since up to four (4)
transactions can be sent within a transmission (for certain transaction codes), there may be multiple occurrences of segments in a
transmission.
An example is a Billing transaction, where multiple claims or services are submitted. In this example,
Mandatory at the transmission level:
• Transaction Header Segment
• Insurance Segment
Situational or optional at the transmission level:
• Patient Segment
Mandatory at the transaction level:
• Claim Segment
• Pricing Segment
Situational or optional at the transaction level:
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 774 -
• Pharmacy Provider Segment
• Prescriber Segment
• Coordination of Benefits/Other Payments Segment
• Workers’ Compensation Segment
• DUR/PPS Segment
• Coupon Segment
• Clinical Segment
• Additional Documentation Segment
• Facility Segment
• Narrative Segment
The transaction segments (mandatory, situational, and optional) are within group separators. The group separators separate the actual
multiple billings. The mandatory, situational, and optional transaction segments must occur only once within the group separator. In a second
instance of the multiple billing, within another group separator, these mandatory, situational, and optional transaction segments might appear
again. This is the only situation where segments are allowed to repeat. See diagrams in each transaction section for more information.
33.14.2 REPEATING DATA ELEMENTS
Data elements are allowed to be repeated according to rules described in this document. Repeating fields are always preceded with a count or
counter field.
Repeating fields may also be part of a set of common or similar fields, which means fields have a natural occurrence within a group that
repeats. These sets/logical groupings are outlined in this document.
When multiple repeating fields are part of a set, the fields are situational or optional. If the field is not needed for this set, the field identification
and data must be eliminated.
Version D and above contains repeating fields that are formatted to accommodate a greater number of occurrences than might be practical for
real-time transmissions. Every occurrence sent or received should be displayed by the software formatting the transaction. However, if more
repetitions occur than can reasonably be displayed on the pharmacy terminal, the following recommendations for maximum number to
display are offered. These are recommendations only; trading partner requirements will determine the final number of occurrences displayed.
33.14.2.1 COUNT FIELDS
Certain fields are used as count field. A count field indicates the number of repetitions that follow. It is the total number of repetitions that
follow.
For example, a count field containing 4 means that four occurrences of the field or set/logical grouping will follow.
A count is the total number of repetitions that follow. To denote count usage in this section, the table is indented to show the Count field offset
from the fields that occur with the Count. The Count field occurs once and the fields occur the number of times denoted in the Count. For
example:
Field Field Name
YYY-YY Count field that contains the total number of repetitions
456-BB Field A that occurs repetition 1
789-CC Field B that occurs repetition 1
456-BB Field A that occurs repetition 2
789-CC Field B that occurs repetition 2
456-BB Et cetera
In the Response Status Segment, the Reject Count (51Ø-FA) would contain the value 4, with Reject Code (511-FB) following 4 times with each
reject code value. The following is for illustration only.
For illustration only.
Field # ID Field Name Value Comment
111 AM Segment Identification 21 Response Status Segment
112 AN Transaction Response Status R
51Ø FA Reject Count 4 Total number of occurrences = Four
511 FB Reject Code Ø1 M/I BIN
511 FB Reject Code Ø4 M/I Processor Control Number
511 FB Reject Code Ø5 M/I Service Provider ID
511 FB Reject Code Ø6 M/I Group Number
An example of the usage of “count” follows. Italics denote the counts. Coordination of Benefits/Other Payments Count has a value of 2, and
two repetitions follow (Other Payer Coverage Type = “Ø2” and “Ø1”). Other Payer Reject Count has a value of 1, and one repetition follows.
Other Payer Amount Paid Count has a value of 2, and two repetitions follow of Other Payer Amount Paid Qualifier and Amount Paid.
Field Field Name Value
337-4C Coordination of Benefits/Other Payments Count 2
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 775 -
338-5C Other Payer Coverage Type Ø2
339-6C Other Payer ID Qualifier Ø1
34Ø-7C Other Payer ID 123456789Ø
443-E8 Other Payer Date 2ØØ8Ø1Ø2
471-5E Other Payer Reject Count 1
472-6E Other Payer Reject Code 7Ø
338-5C Other Payer Coverage Type Ø1
339-6C Other Payer ID Qualifier Ø3
34Ø-7C Other Payer ID 234567
443-E8 Other Payer Date 2ØØ8Ø1Ø2
341-HB Other Payer Amount Paid Count 2
342-HC Other Payer Amount Paid
Qualifier
Ø5
431-DV Other Payer Amount Paid 1Ø{
342-HC Other Payer Amount Paid
Qualifier
Ø7
431-DV Other Payer Amount Paid 15Ø{
33.14.2.2 COUNTER FIELDS
The term “counter” as used in this standard, is synonymous with occurrence number. A counter field may occur multiple times. A counter field
indicates which loop of the repetition. A counter field will be followed by fields in a set or logical grouping. Each repetition of the set/logical
grouping must use the counter field, in sequential, ascending order (repetition 1, then 2, then 3, et cetera). A counter field is used when all
fields in the repetition set/logical grouping are situational or optional.
Note not all fields within a set/logical grouping must be present in each repetition. The fields needed within each set/logical grouping will be
determined by what is being reported for each counter repetition.
For example, in a repetition of four, the first occurrence of the field or set/logical grouping would be preceded by a counter with a
value of 1. The second occurrence of that field or set/logical grouping would be preceded by a counter with a value of 2. The third
occurrence would be preceded by a value of 3 and the fourth by a counter with a value of 4.
A counter field identifies a specific loop in a series of loops, in sequential order. To denote counter usage in this section, the table is indented
to show the Counter field column lined up with the fields that occur with each repetition of the Counter field. For example:
Field Field Name
YYY-YY Counter field that increments for each occurrence
123-AA Field that occurs with each counter occurrence
222-BB Field that occurs with each counter occurrence
333-CC Field that occurs with each counter occurrence
YYY-YY Counter field that increments for each occurrence
123-AA Field that occurs with each counter occurrence
222-BB Field that occurs with each counter occurrence
333-CC Field that occurs with each counter occurrence
For example, in the Clinical Segment, the Clinical Information Counter (493-X3) would contain 1 with any/all of the Measurement fields
following for this repetition. The Clinical Information Counter would then repeat and contain 2 with any/all of the Measurement fields following
for this repetition. The Clinical Information Counter would then repeat and contain 3 with any/all of the Measurement fields following for this
repetition. The Clinical Information Counter would then repeat and contain 4 with any/all of the Measurement fields following for this repetition.
Below for each repetition of Clinical Information Counter, the Measurement fields are situational or optional within the set/logical grouping.
Each repetition may have different combinations of the Measurement fields, depending on what is being reported. This chart also shows a
count example of Diagnosis Code.
For illustration only.
Field # ID Field Name Value Comment
111 AM Segment Identification 13 Clinical Segment
491 VE Diagnosis Code Count 2 Two occurrences total
492 WE Diagnosis Code Qualifier First diagnosis qualifier
424 DO Diagnosis Code First diagnosis code
492 WE Diagnosis Code Qualifier Second diagnosis qualifier
424 DO Diagnosis Code Second diagnosis code
493 XE Clinical Information Counter 1 First repetition
494 ZE Measurement Date
495 H1 Measurement Time
496 H2 Measurement Dimension
497 H3 Measurement Unit
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 776 -
499 H4 Measurement Value
493 XE Clinical Information Counter 2 Second repetition
495 H1 Measurement Time
496 H2 Measurement Dimension
497 H3 Measurement Unit
499 H4 Measurement Value
493 XE Clinical Information Counter 3 Third repetition
494 ZE Measurement Date
495 H1 Measurement Time
496 H2 Measurement Dimension
497 H3 Measurement Unit
499 H4 Measurement Value
493 XE Clinical Information Counter 4 Fourth repetition
495 H1 Measurement Time
496 H2 Measurement Dimension
497 H3 Measurement Unit
499 H4 Measurement Value
An example of the usage of “counter” follows. The DUR/PPS Code Counter occurs in sequential order, for three occurrences. Italics denote
the counters.
Field Field Name Value
473-7E DUR/PPS Code Counter 1
439-E4 Reason For Service Code DA
44Ø-E5 Professional Service Code MØ
441-E6 Result of Service Code 1B
474-8E DUR/PPS Level of Effort 11
473-7E DUR/PPS Code Counter 2
439-E4 Reason For Service Code LR
44Ø-E5 Professional Service Code PØ
441-E6 Result of Service Code 1B
474-8E DUR/PPS Level of Effort 11
473-7E DUR/PPS Code Counter 3
439-E4 Reason For Service Code TD
44Ø-E5 Professional Service Code MØ
441-E6 Result of Service Code 1B
474-8E DUR/PPS Level of Effort 11
475-J9 DUR Co-Agent ID Qualifier Ø1
476-H6 DUR Co-Agent ID 17236Ø569Ø1
33.14.2.3 USAGE
The following counter fields are submitted by the provider:
Clinical Information Counter (493-XE) – maximum 5 occurrences supported
DUR/PPS Code Counter (473-7E) – maximum 9 occurrences supported
The following count fields are submitted by the provider:
Coordination of Benefits/Other Payments Count (337-4C) – maximum count of 9
Procedure Modifier Code Count (458-SE) – maximum count of 1Ø
Diagnosis Code Count (491-VE) – maximum count of 5
Compound Ingredient Component Count (447-EC) – maximum count of 25 ingredients
Compound Ingredient Modifier Code Count (362-2G) – maximum count of 1Ø
Other Amount Claimed Submitted Count (478-H7) – maximum count of 3
Other Payer Reject Count (471-5E) – maximum count of 5
Other Payer Amount Paid Count (341-HB) – maximum count of 9
Other Payer-Patient Responsibility Amount Count (353-NR) – maximum count of 25. Note the occurrences are dependent upon
the number of component parts returned from a previous payer.
Submission Clarification Code Count (354-NX) – maximum count of 3
Question Number/Letter Count (377-2Z) – maximum count of 5Ø
Benefit Stage Count (392-MU) – maximum count of 4
The following counter fields are returned by the processor:
DUR/PPS Response Code Counter (567-J6) – maximum 9 occurrences
The following count fields are returned by the processor:
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 777 -
Reject Count (51Ø-FA) – maximum count of 5
Approved Message Code Count (547-5F) – maximum count of 5
Additional Message Information Count (13Ø-UF) – maximum count of 25
Preferred Product Count (551-9F) – maximum count of 6
Other Amount Paid Count (563-J2) – maximum count of 3
Other Payer ID Count (355-NT) – maximum count of 3
Benefit Stage Count (392-MU) – maximum count of 4
Section “Structure Quick Reference” of this guide lists mandatory, situational, and optional fields within each segment. In addition, some
repeating fields contain logical groupings that facilitate parsing. Logical groupings include:
33.14.2.4 REQUEST SEGMENTS
33.14.2.4.1 COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
33.14.2.4.1.1 In Payment Scenarios
In the following charts, the previous payer returned payment information. This payment information is then sent on to the next payer in the
“Other Payer” fields.
The Coordination of Benefits/Other Payments Segment may be represented multiple ways in payment scenarios. The requirements will be
determined by business need.
33.14.2.4.1.1.1 1. Other Payer Amount Paid Repetitions Only
The processor has a business need to know information reported from previous payers, which includes the other payer amounts (shipping,
delivery, incentive, cognitive service, et cetera) only. The chart representation would be as follows. In this scenario, only the Other Payer
Amount Paid Count repetitions would be present.
Field Field Name
337-4C Coordination of Benefits/Other Payments Count
338-5C Other Payer Coverage Type
339-6C Other Payer ID Qualifier
34Ø-7C Other Payer ID
443-E8 Other Payer Date
341-HB Other Payer Amount Paid Count
342-HC Other Payer Amount Paid Qualifier
431-DV Other Payer Amount Paid
If the previous payer has financial amounts that apply to Medicare Part D
beneficiary benefit stages, the following fields are required when a
state/federal/regulatory agency program requires reporting of benefit stage
specific financial amounts:
392-MU Benefit Stage Count
393-MV Benefit Stage Qualifier
394-MW Benefit Stage Amount
33.14.2.4.1.1.2 2. Other Payer-Patient Responsibility Amount Repetitions Only
The processor has a business need to know information from previous payers, which includes the patient’s responsibility amounts only. The
chart representation would be as follows. In this scenario, the Other Payer-Patient Responsibility Amount Count repetitions would be present.
Field Field Name
337-4C Coordination of Benefits/Other Payments Count
338-5C Other Payer Coverage Type
339-6C Other Payer ID Qualifier
34Ø-7C Other Payer ID
443-E8 Other Payer Date
353-NR Other Payer-Patient Responsibility Amount Count
351-NP Other Payer-Patient Responsibility Amount Qualifier
352-NQ Other Payer-Patient Responsibility Amount
If the previous payer has financial amounts that apply to Medicare Part D
beneficiary benefit stages, the following fields are required when a
state/federal/regulatory agency program requires reporting of benefit stage
specific financial amounts:
392-MU Benefit Stage Count
393-MV Benefit Stage Qualifier
394-MW Benefit Stage Amount
33.14.2.4.1.1.3 3. Other Payer Amount Paid, Other Payer-Patient Responsibility Amount, and Benefit Stage
Repetitions Present (Government Programs)

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 778 -
The processor has a business need to know information reported from previous payers, which includes both the other payer amounts
(shipping, delivery, incentive, cognitive service, et cetera), and the patient’s responsibility amounts. This is represented in the following chart.
In this scenario, both the Other Payer Amount Paid Count repetitions and the Other Payer-Patient Responsibility Amount Count repetitions
would be present.
Field Field Name
337-4C Coordination of Benefits/Other Payments Count
338-5C Other Payer Coverage Type
339-6C Other Payer ID Qualifier
34Ø-7C Other Payer ID
443-E8 Other Payer Date
341-HB Other Payer Amount Paid Count
342-HC Other Payer Amount Paid Qualifier
431-DV Other Payer Amount Paid
353-NR Other Payer-Patient Responsibility Amount Count
351-NP Other Payer-Patient Responsibility Amount Qualifier
352-NQ Other Payer-Patient Responsibility Amount
If the previous payer has financial amounts that apply to Medicare Part D
beneficiary benefit stages, the following fields are required when a
state/federal/regulatory agency program requires reporting of benefit stage
specific financial amounts:
392-MU Benefit Stage Count
393-MV Benefit Stage Qualifier
394-MW Benefit Stage Amount
33.14.2.4.1.2 General Information
From the above information, the field Coordination of Benefits/Other Payments Count (337-4C) when supported will contain a maximum count
of 9. The Count will contain a value between 1 and 9 when used and the indented fields below (Other Payer Coverage Type, Other Payer ID
Qualifier, et cetera) will repeat the number of times the Count specifies, with mandatory/situational/optional requirements as defined in the
section “Structure Quick Reference”.
The field Other Payer Amount Paid Count (341-HB) when supported will contain a maximum count of 9. The Count will contain a value
between 1 and 9 when used and the indented fields (Other Payer Amount Paid Qualifier and Other Payer Amount Paid) will repeat the number
of times the Count specifies, with mandatory/situational/optional requirements as defined in the section “Structure Quick Reference”. When
Other Payer Amount Paid Count (341-HB) is supported, Other Payer Amount Paid Qualifier (342-HC) and Other Payer Amount Paid (431-DV)
must be supported.
The field Other Payer-Patient Responsibility Amount Count (353-NR) when supported will contain a maximum count of 25. The Count will
contain a value between 1 and 25 when used. The indented fields (Other Payer-Patient Responsibility Amount Qualifier (351-NP) and Other
Payer-Patient Responsibility Amount (352-NQ)) will repeat the number of times the Count specifies with mandatory/situational/optional
requirements as defined in the section “Structure Quick Reference”. Note the occurrences are dependent upon the number of component parts
returned from a previous payer.
The field Benefit Stage Count (392-MU) when supported will contain a maximum count of 4. The Count will contain a value between 1 and 4
when used. The indented fields (Benefit Stage Qualifier (393-MV) and Benefit Stage Amount (394-MW)) will repeat the number of times the
Count specifies with mandatory/situational/optional requirements as defined in the section “Structure Quick Reference”.
Please see the section in this document called “Specific Segment Discussion”, “Request Segments”, “Coordination of Benefits/Other
Payments Segment”. This section defines important rules for usage of the field Other Payer-Patient Responsibility Amount Count (353-NR)
depending upon the value in the field Other Payer-Patient Responsibility Amount Qualifier (351-NP).
33.14.2.4.1.3 In Reject Scenarios
From the above information, the field Coordination of Benefits/Other Payments Count (337-4C) when supported will contain a maximum count
of 9. The Count will contain a value between 1 and 9 when used and the indented fields below (Other Payer Coverage Type, Other Payer ID
Qualifier, et cetera) will repeat the number of times the Count specifies, with mandatory/situational/optional requirements as defined in the
section “Structure Quick Reference”.
33.14.2.4.1.3.1 Other Payer Reject Fields
In the next chart, the previous payer returned rejection information. This rejection information is then sent in a separate transmission to the
next payer in the Other Payer Reject fields.
The field Other Payer Reject Count (471-5E) when supported will contain a maximum count of 5. The Count will contain a value between 1
and 5 when used and Other Payer Reject Code will repeat the number of times the Count specifies.
Field Field Name
337-4C Coordination of Benefits/Other Payments Count
338-5C Other Payer Coverage Type
339-6C Other Payer ID Qualifier
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 779 -
34Ø-7C Other Payer ID
443-E8 Other Payer Date
471-5E Other Payer Reject Count
472-6E Other Payer Reject Code
Either “Other Payer Amount Paid” or “Other Payer Reject Count and Code” will occur, depending on the outcome of a previous claim or
service submitted.
The next chart indicates the previous payer returned multiple reject codes. These multiple reject codes are sent to the next payer.
In this scenario example, the Coordination of Benefits/Other Payments Count contains a value of 1. Other Payer Coverage Type, Other Payer
ID Qualifier, Other Payer ID, Other Payer Date, and Other Payer Reject Count would each occur once, with mandatory/situational/optional
requirements as defined in the section “Structure Quick Reference”.
The field Other Payer Reject Count (471-5E) in this scenario example would contain a value of 3. Other Payer Reject Code (472-6E) would
occur 3 times, with 3 unique values, to denote the 3 reject codes as specified by the Count.
Field Field Name
337-4C Coordination of Benefits/Other Payments Count
338-5C Other Payer Coverage Type
339-6C Other Payer ID Qualifier
34Ø-7C Other Payer ID
443-E8 Other Payer Date
471-5E Other Payer Reject Count
472-6E Other Payer Reject Code
472-6E Other Payer Reject Code
472-6E Other Payer Reject Code
33.14.2.4.2 CLAIM SEGMENT
33.14.2.4.2.1 Procedure Modifier Code Count
Field Field Name
458-SE Procedure Modifier Code Count
459-ER Procedure Modifier Code
From the above information, the field Procedure Modifier Code Count (458-SE) when supported will contain a maximum count of 1Ø. The
Count will contain a value between 1 to 1Ø when used and Procedure Modifier Code will repeat the number of times the Count specifies.
33.14.2.4.2.2 Submission Clarification Code Count
Field Field Name
354-NX Submission Clarification Code Count
42Ø-DK Submission Clarification Code
From the above information, the field Submission Clarification Code Count (354-NX) when supported will contain a maximum count of 3. The
Count will contain a value between 1 to 3 when used and the Submission Clarification Code will repeat the number of times the Count
specifies.
33.14.2.4.3 DUR/PPS SEGMENT
33.14.2.4.3.1 DUR/PPS Code Counter
Field Field Name
473-7E DUR/PPS Code Counter
439-E4 Reason For Service Code
44Ø-E5 Professional Service Code
441-E6 Result of Service Code
474-8E DUR/PPS Level of Effort
475-J9 DUR Co-Agent ID Qualifier
476-H6 DUR Co-Agent ID
From the above information, the field DUR/PPS Code Counter (473-7E) when supported will repeat a maximum of 9 occurrences. The counter
field indicates which sequential loop of the repetition. For each repetition of the DUR/PPS Code Counter (1, 2, 3, et cetera) the fields Reason
for Service Code, Professional Service Code, Result of Service Code, et cetera will occur, with mandatory/situational/optional requirements as
defined in the section “Structure Quick Reference”.
33.14.2.4.4 COMPOUND SEGMENT
33.14.2.4.4.1 Compound Ingredient Component Count
Field Field Name
447-EC Compound Ingredient Component Count
488-RE Compound Product ID Qualifier
489-TE Compound Product ID
448-ED Compound Ingredient Quantity
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 780 -
449-EE Compound Ingredient Drug Cost
49Ø-UE Compound Ingredient Basis of Cost Determination
362-2G Compound Ingredient Modifier Code Count
363-2H Compound Ingredient Modifier Code
From the above information, the field Compound Ingredient Component Count (447-EC) when supported will contain a maximum count of 25
ingredients. The Count will contain a value between 1 to 25 when used and the indented fields (Compound Product ID Qualifier, Compound
Product ID, Compound Ingredient Quantity, et cetera) will repeat the number of times the Count specifies, with mandatory/situational/optional
requirements as defined in the section “Structure Quick Reference”.
The Compound Ingredient Modifier Code Count (362-2G) when supported will contain a maximum count of 1Ø. The Count will contain a value
from 1 to 1Ø when used and the indented field, Compound Ingredient Modifier Code, will repeat the number of times the Count specifies.
33.14.2.4.5 PRICING SEGMENT
33.14.2.4.5.1 Other Amount Claimed Submitted Count
Field Field Name
478-H7 Other Amount Claimed Submitted Count
479-H8 Other Amount Claimed Submitted Qualifier
48Ø-H9 Other Amount Claimed Submitted
From the above information, the field Other Amount Claimed Submitted Count (478-H7) when supported will contain a maximum count of 3.
The Count will contain a value between 1 to 3 when used and the fields Other Amount Claimed Submitted Qualifier and Other Amount
Claimed Submitted will repeat the number of times the Count specifies, with mandatory/situational/optional requirements as defined in the
section “Structure Quick Reference”.
33.14.2.4.6 CLINICAL SEGMENT
33.14.2.4.6.1 Diagnosis Code Count
Field Field Name
491-VE Diagnosis Code Count
492-WE Diagnosis Code Qualifier
424-DO Diagnosis Code
493-XE Clinical Information Counter
494-ZE Measurement Date
495-H1 Measurement Time
496-H2 Measurement Dimension
497-H3 Measurement Unit
499-H4 Measurement Value
From the above information, the field Diagnosis Code Count (491-VE) when supported will contain a maximum count of 5. The Count will
contain a value between 1 to 5 when used and the fields Diagnosis Code Qualifier and Diagnosis Code will repeat the number of times the
Count specifies, with mandatory/situational/optional requirements as defined in the section “Structure Quick Reference”.
33.14.2.4.6.2 Clinical Information Counter
From the above information, the field Clinical Information Counter (493-XE) when supported will repeat a maximum of 5 occurrences. The
counter field indicates which loop of the repetition, in sequential order. For each repetition of the Clinical Information Counter (1, 2, 3, et
cetera..), the fields Measurement Date, Measurement Time, et cetera will occur, with mandatory/situational/optional requirements as defined in
the section “Structure Quick Reference”.
33.14.2.4.7 ADDITIONAL DOCUMENTATION SEGMENT
33.14.2.4.7.1 Question Number/Letter Count
Field Field Name
377-2Z Question Number/Letter Count
378-4B Question Number/Letter
379-4D Question Percent Response
38Ø-4G Question Date Response
381-4H Question Dollar Amount Response
382-4J Question Numeric Response
383-4K Question Alphanumeric Response
From the above information, the field Question Number/Letter Count (377-2Z) when supported will contain a maximum count of 5Ø. The Count
will contain a value between 1 to 5Ø when used, and will indicate the number of times Question Number/Letter (378-4B) will occur. Question
Number/Letter (378-4B) is required when Question Number/Letter Count (377-2Z) is submitted and will indicate the question number of the
one field in the logical grouping (Question Percent Response, Question Date Response, Question Dollar Amount Response, Question
Numeric Response, and Question Alphanumeric Response) that follows. (See section “Specific Segment Discussion”, “Request Segments”,
“Additional Documentation Segment”).
33.14.2.5 RESPONSE SEGMENTS
33.14.2.5.1 RESPONSE STATUS SEGMENT
33.14.2.5.1.1 Approved Message Code Count

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 781 -
The following denotes an accepted response.
From the above information, the field Approved Message Code Count (547-5F) when supported will contain a maximum count of 5. The Count
will contain a value between 1 to 5 when used and the field Approved Message Code will repeat the number of times the Count specifies.
Field Field Name
547-5F Approved Message Code Count
548-6F Approved Message Code
33.14.2.5.1.2 Reject Count
The following denotes a rejected response.
From the above information, the field Reject Count (51Ø-FA) when supported will contain a maximum count of 5. The Count will contain a
value between 1 to 5 when used and the fields Reject Code and Reject Field Occurrence Indicator will repeat the number of times the Count
specifies, with mandatory/situational/optional requirements as defined in the section “Structure Quick Reference”.
Either the reject or approved fields will appear, but not both, based on the response. If the field rejected is not a repeating field, the “Reject
Field Occurrence Indicator” must be eliminated.
33.14.2.5.1.3 Additional Message Information Count
The Additional Message Information loop may appear on an accepted or a rejected response.
From the above information, the field Additional Message Information Count (13Ø-UF) when supported will contain a maximum count of 25.
The Count will contain a value between 1 to 25 when used and the fields Additional Message Information Qualifier (132-UH), Additional
Message Information (526-FQ), and Additional Message Information Continuity (131-UG) will repeat the number of times the Count specifies.
Note, Additional Message Information Continuity (131-UG) will only occur for each count if the applicable situation stated is satisfied.
Field Field Name
13Ø-UF Additional Message Information Count
132-UH Additional Message Information Qualifier
526-FQ Additional Message Information
131-UG Additional Message Information Continuity
33.14.2.5.2 RESPONSE CLAIM SEGMENT
33.14.2.5.2.1 Preferred Product Count
Field Field Name
551-9F Preferred Product Count
552-AP Preferred Product ID Qualifier
553-AR Preferred Product ID
554-AS Preferred Product Incentive
555-AT Preferred Product Cost Share Incentive
556-AU Preferred Product Description
NOTE: If the Preferred Product Count is sent, the Preferred Product ID Qualifier must precede each occurrence of the Preferred Product ID.
From the above information, the field Preferred Product Count (551-9F) when supported will contain a maximum count of 6. The Count will
contain a value between 1 to 6 when used and the indented fields (Preferred Product ID Qualifier, Preferred Product ID, et cetera) will repeat
the number of times the Count specifies, with mandatory/situational/optional requirements as defined in the section “Structure Quick
Reference”.
33.14.2.5.3 RESPONSE PRICING SEGMENT
33.14.2.5.3.1 Other Amount Paid Repetitions Only
Field Field Name
563-J2 Other Amount Paid Count
564-J3 Other Amount Paid Qualifier
565-J4 Other Amount Paid
From the above information, the field Other Amount Paid Count (563-J2) when supported will contain a maximum count of 3. The Count will
contain a value between 1 to 3 when used and the fields Other Amount Paid Qualifier and Other Amount Paid will repeat the number of times
the Count specifies, with mandatory/situational/optional requirements as defined in the section “Structure Quick Reference”.
33.14.2.5.3.2 Benefit Stage Repetitions Only
Field Field Name
51Ø-FA Reject Count
511-FB Reject Code
546-4F Reject Field Occurrence Indicator
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 782 -
The previous payer has financial amounts that apply to Medicare Part D beneficiary benefit stages. These fields are required when the plan is
a participant in a Medicare Part D program that requires reporting of benefit stage specific financial amounts. The chart representation would
be as follows. In this scenario, the Benefit Stage Count repetitions would be present.
Field Field Name
392-MU Benefit Stage Count
393-MV Benefit Stage Qualifier
394-MW Benefit Stage Amount
33.14.2.5.4 RESPONSE DUR/PPS SEGMENT
33.14.2.5.4.1 DUR/PPS Response Code Counter
Field Field Name
567-J6 DUR/PPS Response Code Counter
439-E4 Reason for Service Code
528-FS Clinical Significance Code
529-FT Other Pharmacy Indicator
53Ø-FU Previous Date of Fill
531-FV Quantity of Previous Fill
532-FW Database Indicator
533-FX Other Prescriber Indicator
544-FY DUR Free Text Message
57Ø-NS DUR Additional Text
From the above information, the field DUR/PPS Response Code Counter (567-J6) when supported will repeat a maximum of 9 occurrences.
The counter field indicates which loop of the repetition, in sequential order. For each repetition of the DUR/PPS Response Code Counter (1, 2,
3, et cetera..), the fields Reason for Service Code, Clinical Significance Code, et cetera will occur, with mandatory/situational/optional
requirements as defined in the section “Structure Quick Reference”.
33.14.2.5.5 RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS SEGMENT
33.14.2.5.5.1 Other Payer ID Count
Field Field Name
355-NT Other Payer ID Count
338-5C Other Payer Coverage Type
339-6C Other Payer ID Qualifier
34Ø-7C Other Payer ID
991-MH Other Payer Processor Control Number
356-NU Other Payer Cardholder ID
992-MJ Other Payer Group ID
142-UV Other Payer Person Code
127-UB Other Payer Help Desk Phone Number
143-UW Other Payer Patient Relationship Code
144-UX Other Payer Benefit Effective Date
145-UY Other Payer Benefit Termination Date
NOTE: If the Other Payer ID Count and the Other Payer Coverage Type (338-5C) are sent, the Other Payer ID Qualifier must precede each
occurrence of the Other Payer ID.
From the above information, the field Other Payer ID Count (355-NT) when supported will contain a maximum count of 3. The Count will
contain a value between 1 to 3 when used and the fields Other Payer Coverage Type, Other Payer ID Qualifier, Other Payer ID, and the Other
Payer fields will repeat the number of times the Count specifies. Other Payer ID Qualifier and Other Payer ID will occur, but Other Payer
Processor Control Number, Other Payer Cardholder ID, and the rest of the Other Payer fields will only occur if supported.
33.14.3 REJECT FIELD OCCURRENCE INDICATOR
When an error condition arises on fields that are repeatable, the Reject Field Occurrence Indicator (546-4F) is used to denote which
occurrence of the field or set in question has been rejected. See section “Structure Quick Reference” for a list of the repeating fields or sets.
When a repeating field or set is in error, a Reject Code (511-FB) must denote the missing/invalid field or set, and the Reject Field Occurrence
Indicator (546-4F) denote which occurrence is in error. For example, if an occurrence of the Reason For Service Code (439-E4) is in error, one
Reject Code (511-FB) is “E4” to denote “Missing/Invalid Reason For Service Code”, and the Reject Field Occurrence Indicator (546-4F) must
specify which iteration is in error (for example, “1” or “2”). By denoting the missing/invalid field in error, and which occurrence, the transaction
may be interrogated to determine which field is in error. A partial view of the Response Status Segment follows:
Field # ID Field Name Value Comment
111 AM Segment Identification 21
112 AN Transaction Response Status R
51Ø FA Reject Count 1 One occurrence

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 783 -
511 FB Reject Code E4 M/I Reason for Service Code
546 4F Reject Field Occurrence Indicator 1 First iteration of Reason for Service Code is in
error
Another example is a high dosage alert for an ingredient within a compound. A Reject Code (511-FB) should denote a Missing/Invalid
Product/Service ID. The Reject Field Occurrence Indicator (546-4F) must denote which ingredient is in error within the compound occurrences.
Other Reject Codes are included which further explain the error condition, and in this instance, the Response DUR/PPS Segment may denote
additional pertinent information. An example follows:
Field # ID Field Name Value Comment
111 AM Segment Identification 21
112 AN Transaction Response Status R
51Ø FA Reject Count 2 Two occurrences
511 FB Reject Code 88 DUR Reject Error
511 FB Reject Code 21 M/I Product /Service ID
546 4F Reject Field Occurrence Indicator 3 Third iteration (ingredient) of Compound
Product ID
The Reject Field Occurrence Indicator (546-4F) must directly follow the Reject Code (511-FB) when signifying a repeating field or set is in
error. The Reject Code must denote the repeating field that is in error
Note, the Reject Field Occurrence Indicator is a situational or optional field, and therefore, if the Reject Code is not denoting a repeating field,
the Reject Field Occurrence Indicator must not be sent. It must only be sent when relaying error information about a repeating field.
33.14.3.1 REJECT FIELD OCCURRENCE INDICATOR USE FOR MULTI INGREDIENT COMPOUND
TRANSACTION
When an error condition arises on fields, which are repeatable, the Reject Field Occurrence Indicator (546-4F) is used to denote which
occurrence of the field or set in question has been rejected.
When returning a rejected response for a Multi-Ingredient Compound Segment submission and when a repeating field is in error, Reject Code
(511-FB) is populated with the a reject code that provides the clearest reason for the reject and the Reject Field Occurrence Indicator (546-4F)
denotes which occurrence is in error.
The second occurrence of Compound Product ID (489-TE) is in error for Product Not Covered. Reject Code (511-FB) will be populated with
the most appropriate reject code to denote “Product/Service Not Covered” (Reject “7Ø “). The Reject Field Occurrence Indicator (546-4F) will
specify which iteration is in error (for example,“2”). By returning the reject code and the reject field occurrence indicator, the transaction
response may be interrogated. A partial view of the Response Status Segment follows:
Field # ID Field Name Value Comment
111 AM Segment Identification 21 Response Status Segment
112 AN Transaction Response Status R Rejected
51Ø FA Reject Count 1 One occurrence
511 FB Reject Code 7Ø Product Service Not covered
546 4F Reject Field Occurrence Indicator 2 Second iteration of Compound Product ID is
not covered
Another example is a drug-to-drug interaction rejection for an ingredient within a compound. Reject Code (511-FB) should denote an “88 ” if
the transaction is rejected. The Reject Field Occurrence Indicator (546-4F) must denote which ingredient is in error within the compound
occurrences. Other Reject Codes can be included which further explain the error condition, and in this instance, the Response DUR/PPS
Segment may denote additional pertinent information. An example follows:
Field # ID Field Name Value Comment
111 AM Segment Identification 21 Response Status Segment
112 AN Transaction Response Status R Rejected
51Ø FA Reject Count 1 One occurrence
511 FB Reject Code 88 DUR Reject Error
546 4F Reject Field Occurrence Indicator 1 First iteration (ingredient) of Compound
Product ID
Field # ID Field Name Value Comment
111 AM Segment Identification 24 Response DUR/PPS Segment
567 J6 DUR/PPS Response Code Counter 1 One occurrence
439 E4 Reason for Service Code DD Drug-Drug Interaction
528 FS Clinical Significance Code 1 Major
529 FT Other Pharmacy Indicator 1 Your Pharmacy
53Ø FU Previous Date of Fill 2ØØ8Ø1Ø2 CCYYMMDD
531 FV Quantity of Previous Fill 3Ø
532 FW Database Indicator 2 Medi-Span Product Line
533 FX Other Prescriber Indicator 1 Same Prescriber
544 FY DUR Free Text INGØ1: The first ingredient in this compound interacts

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 784 -
WARFARIN
TAB 1 MG
with Warfarin.
The Reject Field Occurrence Indicator (546-4F) must directly follow the Reject Code (511-FB) when signifying a repeating field or set is in
error. The Reject Code must denote the repeating field that is in error.
Note, the Reject Field Occurrence Indicator is a situational or optional field, and therefore, if the Reject Code is not denoting a repeating field,
the Reject Field Occurrence Indicator must not be sent. It must only be sent when relaying error information about a repeating field.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 785 -
34. TRANSMISSION EXAMPLES
This section contains examples of transaction requests and responses. All fields are shown with only the significant example data in them.
Each example is to be taken in its context. For example, based on the particular business example, pricing fields may be required or
situational or not used.
The NCPDP Telecommunication Standard Implementation Guide (Version D and above) allows only variable length transactions. Variable
implementation in Version D and above offers the option of truncating or eliminating situational or optional data elements and reducing overall
message length.
The Version D and above format contains two Mandatory fixed segments, the “Transaction Header” and “Response Header” segments. These
two segments do not use field separators or field identifiers. All other segments use a Field Separator (hex character 1C) to separate each
field. Each field has a unique identifier code that, when used in conjunction with the Field Separator, shows the start of a new field in the
record (for example, FB refers to Field 511-FB, Reject Code).
All Version D and above examples show field truncation and also omit situational or optional fields when no values are given or required. It is
recommended that trading partners be able to send and receive truncated fields and be capable of recognizing when situational or optional
fields have been eliminated.
34.1 EXAMPLE CONVENTIONS
The examples are shown with mandatory fields followed by situational or optional fields that provide additional information for the provider.
Situational or optional segments and fields may or may not be transmitted, depending upon situational segment and field rules, and trading
partner needs.
Segments will appear with the Header first (as required).
Transmission level Segments (in any order) appear next.
Transaction level Segments (in any order per Transaction) appear next.
Mandatory fields always appear first, and are in the order designated.
Required, situational, and optional fields appear in any order (but must follow the qualifier rule and count or counter rules).
Formatting conventions: In the examples that follow, “bbbbb…” denotes blanks and are included to populate required fixed length fields in the
header segments.
For errors, the “VALUE” shown in bold type emphasizes the data in error.
34.1.1 RAW DATA STREAMS
Some examples show the raw data streams immediately after the charts. An example
121212DØE123232323bb1Ø14563663bbbbbbbb2ØØ7Ø91598765bbbbb<1E><1C>AMØ1<1C>C41962Ø615<1C>C51<1C>CAJO
SEPH<1C>CBSMITH<1C>CM123 MAIN STREET<1C>CNMY TOWN<1C>COCO<1C>CP34567<1C>C71<1E><1C>AMØ4<1C>C21234
56789
Not all examples show the raw data streams as there is redundancy in the examples. To show a new transaction type, a new segment, or
occurrence of a field, for example, the raw data stream is shown.
34.1.2 CATEGORY (CAT) COLUMN
The CAT (Category) column:
LEGEND:
Categorization Explanation
M Mandatory Field has been defined as mandatory for the Segment for the Transaction, structural
requirements.
R Required The situational field has been defined with the situation of "Required" for the Segment for the
Transaction.
Q Qualified Requirement
The situations defined have qualifications for usage ("Required if x", "Not required if y").
In examples, if a requirement is met for the field, the categorization of the field will be
“R” (Required).
For example, if Basis of Reimbursement Determination (522-FM) has a situation of
“Required if Ingredient Cost Paid (5Ø6-F6) is greater than zero (Ø)” and the
Ingredient Cost Paid in the example is greater than Ø, the categorization will be “R”.
Or a qualifier is dependent upon the qualified field. With the presence of the qualified
field in the example, the qualifier becomes required.
Or a count and a qualifier are dependent upon the qualified field. With the presence
of the qualified field in the example, the count and the qualifier become required.
Not all business cases can be represented, so where fields are categorized as “Q”, if they are
present in the example, it is assumed they meet the business requirements to satisfy the
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 786 -
LEGEND:
Categorization Explanation
situation(s).
O Optional Field has been defined as optional usage (situations were not defined).
I Informational
The situational usage for the field is for informational purposes only.
34.1.3 “MANDATORY” CATEGORIZATION EXAMPLES
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 121212
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M E1 Eligibility verification
1Ø4-A4 PROCESSOR CONTROL NUMBER M 23232323bb
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbb
bb
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø1Ø2 January 2, 2ØØ8
11Ø-AK SOFTWARE VENDOR/CERTIFICATION
ID
M 98765bbbbb
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 123456789 Cardholder ID
34.1.4 “REQUIRED” CATEGORIZATION EXAMPLES
For a Claim Billing,
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
442-E7 QUANTITY DISPENSED R 3ØØØØ 3Ø.ØØØ tablets
4Ø3-D3 FILL NUMBER R Ø Original dispensing for RX#
4Ø5-D5 DAYS SUPPLY R 3Ø 3Ø Days supply
4Ø6-D6 COMPOUND CODE R 1 Not a compound
4Ø8-D8 DISPENSE AS WRITTEN
(DAW)/PRODUCT SELECTION CODE
R Ø No product selection indicated
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ7Ø915 September 15, 2ØØ7
415-DF NUMBER OF REFILLS AUTHORIZED Q 5 5 Refills
419-DJ PRESCRIPTION ORIGIN CODE Q 1 Written prescription
354-NX SUBMISSION CLARIFICATION CODE
COUNT
R 1 One occurrence
42Ø-DK SUBMISSION CLARIFICATION CODE Q 4 Lost Prescription
3Ø8-C8 OTHER COVERAGE CODE Q 1 No other coverage
429-DT SPECIAL PACKAGING INDICATOR Q 1 Not unit dose
6ØØ-28 UNIT OF MEASURE Q EA Each
Note, the “R” (Required) Categorizations marked in bold (R) have situations of “Required.” Submission Clarification Code Count (354-NX)
while marked “R” is required due to a situation qualification. It is a “Q” (Qualified Requirement) which has met the requirement. See below.
For a Claim Billing, PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 557{ $55.7Ø
412-DC DISPENSING FEE SUBMITTED Q 1ØØ{ $1Ø.ØØ
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 787 -
478-H7 OTHER AMOUNT CLAIMED
SUBMITTED COUNT
R 1 One occurrence
479-H8 OTHER AMOUNT CLAIMED
SUBMITTED QUALIFIER
R Ø1 Delivery cost
48Ø-H9 OTHER AMOUNT CLAIMED
SUBMITTED
Q 15Ø{ $15.ØØ
426-DQ USUAL AND CUSTOMARY CHARGE Q 867{ $86.7Ø
43Ø-DU GROSS AMOUNT DUE R 8Ø7{ $8Ø.7Ø
423-DN BASIS OF COST DETERMINATION Q Ø3 Direct
Gross Amount Due (43Ø-DU) is “Required”. Other Amount Claimed Submitted Count (478-H7) and Other Amount Claimed Submitted Qualifier
(479-H8) are “Q” (Qualified Requirement) which have met the requirements. See below.
For a Claim Billing with Transaction Response Status (112-AN) of “P” (Paid),
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 1ØØ{ $1Ø.ØØ
5Ø6-F6 INGREDIENT COST PAID R 557{ $55.7Ø
5Ø7-F7 DISPENSING FEE PAID R 8Ø{ $8.ØØ
557-AV TAX EXEMPT INDICATOR Q 1 Payer/Plan is Tax Exempt (The Payer/Plan is
not responsible for tax. The patient may be
charged tax.)
563-J2 OTHER AMOUNT PAID COUNT R 1 One occurrence
564-J3 OTHER AMOUNT PAID QUALIFIER R Ø1 Delivery
565-J4 OTHER AMOUNT PAID Q 15Ø{ $15.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 7Ø7{ $7Ø.7Ø
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 1 Ingredient cost paid as submitted
523-FN AMOUNT ATTRIBUTED TO SALES TAX Q 2Ø{ $2.ØØ
518-FI AMOUNT OF COPAY Q 8Ø{ $8.ØØ
558-AW FLAT SALES TAX AMOUNT PAID Q 2Ø{ $2.ØØ
575-EQ PATIENT SALES TAX AMOUNT Q 2Ø{ $2.ØØ
Patient Pay Amount (5Ø5-F5) and Total Amount Paid (5Ø9-F9) are “R” (Required). The other fields marked “R” meet the situational
requirements, and are shown below.
34.1.5 “QUALIFIED REQUIREMENT” CATEGORIZATION EXAMPLES
If the Transaction Response Status (112-AN) = “R” (Rejected), the Reject Count (51Ø-FA) and Reject Code(s) (511-FB) are required.
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M R Rejected
51Ø-FA REJECT COUNT R Ø1 1 Reject code follows
511-FB REJECT CODE R P6 Date Of Service Prior To Date Of Birth
For a Claim Billing,
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
442-E7 QUANTITY DISPENSED R 3ØØØØ 3Ø.ØØØ tablets
4Ø3-D3 FILL NUMBER R Ø Original dispensing for RX#
4Ø5-D5 DAYS SUPPLY R 3Ø 3Ø Days supply
4Ø6-D6 COMPOUND CODE R 1 Not a compound
4Ø8-D8 DISPENSE AS WRITTEN
(DAW)/PRODUCT SELECTION CODE
R Ø No product selection indicated
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ7Ø915 September 15, 2ØØ7
415-DF NUMBER OF REFILLS AUTHORIZED Q 5 5 Refills
419-DJ PRESCRIPTION ORIGIN CODE Q 1 Written prescription
354-NX SUBMISSION CLARIFICATION CODE R 1 One occurrence
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 788 -
COUNT
42Ø-DK SUBMISSION CLARIFICATION CODE Q 4 Lost Prescription
3Ø8-C8 OTHER COVERAGE CODE Q 1 No other coverage
429-DT SPECIAL PACKAGING INDICATOR Q 1 Not unit dose
6ØØ-28 UNIT OF MEASURE Q EA Each
Submission Clarification Code Count (354-NX) is marked “R” (Required) due to a situation qualification. It is a “Q” (Qualified Requirement)
which has met the requirement.
For a Claim Billing, PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 557{ $55.7Ø
412-DC DISPENSING FEE SUBMITTED Q 1ØØ{ $1Ø.ØØ
478-H7 OTHER AMOUNT CLAIMED
SUBMITTED COUNT
R 1 One occurrence
479-H8 OTHER AMOUNT CLAIMED
SUBMITTED QUALIFIER
R Ø1 Delivery cost
48Ø-H9 OTHER AMOUNT CLAIMED
SUBMITTED
Q 15Ø{ $15.ØØ
426-DQ USUAL AND CUSTOMARY CHARGE Q 867{ $86.7Ø
43Ø-DU GROSS AMOUNT DUE R 8Ø7{ $8Ø.7Ø
423-DN BASIS OF COST DETERMINATION Q Ø3 Direct
Other Amount Claimed Submitted Count (478-H7) and Other Amount Claimed Submitted Qualifier (479-H8) are “Q” (Qualified Requirement)
which have met the requirements.
For a Claim Billing with Transaction Response Status (112-AN) of “P” (Paid),
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 1ØØ{ $1Ø.ØØ
5Ø6-F6 INGREDIENT COST PAID R 557{ $55.7Ø
5Ø7-F7 DISPENSING FEE PAID R 8Ø{ $8.ØØ
557-AV TAX EXEMPT INDICATOR Q 1 Payer/Plan is Tax Exempt (The Payer/Plan is
not responsible for tax. The patient may be
charged tax.)
563-J2 OTHER AMOUNT PAID COUNT R 1 One occurrence
564-J3 OTHER AMOUNT PAID QUALIFIER R Ø1 Delivery
565-J4 OTHER AMOUNT PAID Q 15Ø{ $15.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 7Ø7{ $7Ø.7Ø
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 1 Ingredient cost paid as submitted
523-FN AMOUNT ATTRIBUTED TO SALES TAX Q 2Ø{ $2.ØØ
518-FI AMOUNT OF COPAY Q 8Ø{ $8.ØØ
558-AW FLAT SALES TAX AMOUNT PAID Q 2Ø{ $2.ØØ
575-EQ PATIENT SALES TAX AMOUNT Q 2Ø{ $2.ØØ
Ingredient Cost Paid (5Ø6-F6), Dispensing Fee Paid (5Ø7-F7), Other Amount Paid Count (563-J2), Other Amount Paid Qualifier (564-J3), and
Basis of Reimbursement Determination (522-FM) are “Q” (Qualified Requirement) which has met the requirements.
34.1.6 “OPTIONAL” CATEGORIZATION EXAMPLES
For a Claim Billing, INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 123456789
312-CC CARDHOLDER FIRST NAME Q JOHN
313-CD CARDHOLDER LAST NAME Q SMITH
314-CE HOME PLAN Q 6Ø2 BC/BS Plan Number
524-FO PLAN ID O 5678
3Ø9-C9 ELIGIBILITY CLARIFICATION CODE Q 4 Disabled dependent
3Ø1-C1 GROUP ID Q 987654321
3Ø3-C3 PERSON CODE Q 3 Place in family
3Ø6-C6 PATIENT RELATIONSHIP CODE Q 3 Child
For a Claim Billing,
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 789 -
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø1 PATIENT SEGMENT
3Ø4-C4 DATE OF BIRTH R 1962Ø615 Born June 15, 1962
3Ø5-C5 PATIENT GENDER CODE R 1 Male
31Ø-CA PATIENT FIRST NAME R JOSEPH
311-CB PATIENT LAST NAME R SMITH
322-CM PATIENT STREET ADDRESS O 123 MAIN STREET
323-CN PATIENT CITY ADDRESS O MY TOWN
324-CO PATIENT STATE/PROVINCE ADDRESS O CO
325-CP PATIENT ZIP/POSTAL ZONE O 34567
3Ø7-C7 PLACE OF SERVICE Q 1 Pharmacy
34.1.7 “INFORMATIONAL” CATEGORIZATION EXAMPLES
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M R Rejected
51Ø-FA REJECT COUNT R Ø1 1 Reject code follows
511-FB REJECT CODE R P6 Date Of Service Prior To Date Of Birth
987-MA URL I www.health.com
34.2 ELIGIBILITY VERIFICATION - TRANSACTION CODE E1
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 121212
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M E1 Eligibility verification
1Ø4-A4 PROCESSOR CONTROL NUMBER M 23232323bb
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE VENDOR/CERTIFICATION
ID
M 98765bbbbb
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø1 PATIENT SEGMENT
3Ø4-C4 DATE OF BIRTH Q 1962Ø615 Born June 15, 1962
3Ø5-C5 PATIENT GENDER CODE Q 1 Male
31Ø-CA PATIENT FIRST NAME Q JOSEPH
311-CB PATIENT LAST NAME Q SMITH
322-CM PATIENT STREET ADDRESS Q 123 MAIN STREET
323-CN PATIENT CITY ADDRESS Q MY TOWN
324-CO PATIENT STATE/PROVINCE ADDRESS Q CO
325-CP PATIENT ZIP/POSTAL ZONE Q 34567
3Ø7-C7 PLACE OF SERVICE Q 1 Pharmacy
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 123456789 Cardholder ID
121212DØE123232323bb1Ø14563663bbbbbbbb2ØØ7Ø91598765bbbbb<1E><1C>AMØ1<1C>C41962Ø615<1C>C51<1C>CAJOSEPH<1C>
CBSMITH<1C>CM123 MAIN STREET<1C>CNMY TOWN<1C>COCO<1C>CP34567<1C>C71<1E><1C>AMØ4<1C>C2123456789
34.2.1 ELIGIBILITY VERIFICATION ACCEPTED RESPONSE
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M E1 Eligibility Verification
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 790 -
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M A Approved
DØE11AØ14563663bbbbbbbb2ØØ7Ø915<1D><1E><1C>AM21<1C>ANA
34.3 ELIGIBILITY VERIFICATION - TRANSMISSION REJECTED
Eligibility Request with incorrect Date of Service (Bold type).
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M E1 Eligibility verification
1Ø4-A4 PROCESSOR CONTROL NUMBER M 23232323bb
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 1957Ø915 September 15, 1957
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 123456789 Cardholder ID
DØE123232323bb1Ø14563663bbbbbbbb1957Ø915<1E><1C>AMØ4<1C>C2123456789
34.3.1 ELIGIBILITY VERIFICATION TRANSMISSION REJECTED RESPONSE
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M E1 Eligibility Verification
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M R Rejected
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 1957Ø915 September 15, 1957
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M R Rejected
51Ø-FA REJECT COUNT R Ø1 1 Reject code follows
511-FB REJECT CODE R P6 Date Of Service Prior To Date Of Birth
DØE11RØ14563663bbbbbbbb1957Ø915<1D><1E><1C>AM21<1C>ANR<1C>FAØ1<1C>FBP6
34.4 ELIGIBILITY VERIFICATION TRANSACTION REJECTED
Eligibility Request for Patient Not Covered. TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 121212
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M E1 Eligibility transaction
1Ø4-A4 PROCESSOR CONTROL NUMBER M 23232323bb
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
INSURANCE SEGMENT
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 791 -
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID R 123456789 Cardholder ID
121212DØE123232323bb1Ø14563663bbbbbbbb2ØØ7Ø915<1E><1C>AMØ4<1C>C2123456789
34.4.1 ELIGIBILITY VERIFICATION TRANSACTION REJECTED RESPONSE
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M E1 Eligibility Verification
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M R Rejected
51Ø-FA REJECT COUNT R 1 1 Reject code follows
511-FB REJECT CODE R 65 Patient is not covered
13Ø-UF ADDITIONAL MESSAGE INFORMATION
COUNT
R 1 1 occurrence
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø1 Used for first line of free form text with no pre-
defined structure.
526-FQ ADDITIONAL MESSAGE INFORMATION Q TRANSACTION
MESSAGE TEXT For illustrative purposes only. Up to 4Ø Bytes
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R 3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 8ØØ654321Ø
DØE11AØ14563663bbbbbbbb2ØØ7Ø915<1D><1E><1C>AM21<1C>ANR<1C>FAØ1<1C>FB65<1C>UF1<1C>UHØ1<1C>FQTRANSACTION ME
SSAGE TEXT<1C>7F3<1C>8F8ØØ654321Ø
34.5 BILLING - TRANSACTION CODE B1
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE VENDOR/CERTIFICATION
ID
M 98765bbbbb
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø1 PATIENT SEGMENT
3Ø4-C4 DATE OF BIRTH R 1962Ø615 Born June 15, 1962
3Ø5-C5 PATIENT GENDER CODE R 1 Male
31Ø-CA PATIENT FIRST NAME R JOSEPH
311-CB PATIENT LAST NAME R SMITH
322-CM PATIENT STREET ADDRESS O 123 MAIN STREET
323-CN PATIENT CITY ADDRESS O MY TOWN
324-CO PATIENT STATE/PROVINCE ADDRESS O CO
325-CP PATIENT ZIP/POSTAL ZONE O 34567
326-CQ PATIENT PHONE NUMBER O 2Ø14658923
35Ø-HN PATIENT E-MAIL ADDRESS I JSMITH@NCPDP.ORG Patient’s E-MAIL Address
INSURANCE SEGMENT
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 792 -
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 987654321 Cardholder ID
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
442-E7 QUANTITY DISPENSED R 3ØØØØ 3Ø.ØØØ tablets
4Ø3-D3 FILL NUMBER R Ø Original dispensing for RX#
4Ø5-D5 DAYS SUPPLY R 3Ø 3Ø Days supply
4Ø6-D6 COMPOUND CODE R 1 Not a compound
4Ø8-D8 DISPENSE AS WRITTEN
(DAW)/PRODUCT SELECTION CODE
R Ø No product selection indicated
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ7Ø915 September 15, 2ØØ7
415-DF NUMBER OF REFILLS AUTHORIZED Q 5 5 Refills
419-DJ PRESCRIPTION ORIGIN CODE Q 1 Written prescription
354-NX SUBMISSION CLARIFICATION CODE
COUNT
R 1 One occurrence
42Ø-DK SUBMISSION CLARIFICATION CODE Q 4 Lost Prescription
3Ø8-C8 OTHER COVERAGE CODE Q 1 No other coverage
429-DT SPECIAL PACKAGING INDICATOR Q 1 Not unit dose
6ØØ-28 UNIT OF MEASURE Q EA Each
PHARMACY PROVIDER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø2 PHARMACY PROVIDER SEGMENT
465-EY PROVIDER ID QUALIFIER R Ø5 National Provider ID
444-E9 PROVIDER ID Q 3935933111
PRESCRIBER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø3 PRESCRIBER SEGMENT
466-EZ PRESCRIBER ID QUALIFIER R Ø8 State license
411-DB PRESCRIBER ID Q ØØG2345
427-DR PRESCRIBER LAST NAME Q JONES
498-PM PRESCRIBER TELEPHONE NUMBER Q 2Ø13639572
468-2E PRIMARY CARE PROVIDER ID
QUALIFIER
R Ø1 National Provider ID
421-DL PRIMARY CARE PROVIDER ID Q 1234566111
47Ø-4E PRIMARY CARE PROVIDER LAST
NAME
Q WRIGHT
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 557{ $55.7Ø
412-DC DISPENSING FEE SUBMITTED Q 1ØØ{ $1Ø.ØØ
478-H7 OTHER AMOUNT CLAIMED
SUBMITTED COUNT
R 1 One occurrence
479-H8 OTHER AMOUNT CLAIMED
SUBMITTED QUALIFIER
R Ø1 Delivery cost
48Ø-H9 OTHER AMOUNT CLAIMED
SUBMITTED
Q 15Ø{ $15.ØØ
426-DQ USUAL AND CUSTOMARY CHARGE Q 867{ $86.7Ø
43Ø-DU GROSS AMOUNT DUE R 8Ø7{ $8Ø.7Ø
423-DN BASIS OF COST DETERMINATION Q Ø3 Direct
61ØØ66DØB1123456789Ø1Ø14563663bbbbbbbb2ØØ7Ø91598765bbbbb<1E><1C>AMØ1<1C>C41962Ø615<1C>C51<1C>CAJOSEPH<1C>
CBSMITH<1C>CM123 MAIN STREET<1C>CNMY TOWN<1C>COCO<1C>CP34567<1C>CQ2Ø14658923<1C>HNJSMITH@NCPDP.ORG<1E><1C
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 793 -
>AMØ4<1C>C2987654321<1D><1E><1C>AMØ7<1C>EM1<1C>D21234567<1C>E1Ø3<1C>D7ØØØØ6Ø94268<1C>E73ØØØØ<1C>D3Ø<1C>D5
3Ø<1C>D61<1C>D8Ø<1C>DE2ØØ7Ø915<1C>DF5<1C>DJ1<1C>NX1<1C>DK4<1C>C81<1C>DT1<1C>28EA<1E><1C>AMØ2<1C>EYØ5<1C>E
93935933<1E><1C>AMØ3<1C>EZØ8<1C>DBØØG2345<1C>DRJONES<1C>PM2Ø13639572<1C>2EØ1<1C>DL1234566<1C>4EWRIGHT<1E>
<1C>AM11<1C>D9557{<1C>DC1ØØ{<1C>H71<1C>H8Ø1<1C>H915Ø{<1C>DQ867{<1C>DU8Ø7{<1C>DNØ3
34.5.1 BILLING WITH INTERMEDIARY PROCESSING OVERRIDE CODES - TRANSACTION
B1 TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 484848
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M 56789Ø1234
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE VENDOR/CERTIFICATION
ID
M 98765bbbbb
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 987654321 Cardholder ID
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø1 PATIENT SEGMENT
3Ø4-C4 DATE OF BIRTH R 1962Ø615 Born June 15, 1962
3Ø5-C5 PATIENT GENDER CODE R 1 Male
31Ø-CA PATIENT FIRST NAME R JOSEPH
311-CB PATIENT LAST NAME R SMITH
322-CM PATIENT STREET ADDRESS O 123 MAIN STREET
323-CN PATIENT CITY ADDRESS O MY TOWN
324-CO PATIENT STATE/PROVINCE ADDRESS O CO
325-CP PATIENT ZIP/POSTAL ZONE O 34567
35Ø-HN PATIENT E-MAIL ADDRESS I JSMITH@NCPDP.ORG Patient’s E-MAIL Address
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
442-E7 QUANTITY DISPENSED R 3ØØØØ 3Ø.ØØØ tablets
4Ø3-D3 FILL NUMBER R Ø Original dispensing for RX#
4Ø5-D5 DAYS SUPPLY R 3Ø 3Ø Days supply
4Ø6-D6 COMPOUND CODE R 1 Not a compound
4Ø8-D8 DISPENSE AS WRITTEN
(DAW)/PRODUCT SELECTION CODE
R Ø No product selection indicated
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ7Ø915 September 15, 2ØØ7
415-DF NUMBER OF REFILLS AUTHORIZED Q 5 5 Refills
419-DJ PRESCRIPTION ORIGIN CODE Q 1 Written prescription
464-EX INTERMEDIARY AUTHORIZATION ID R 4689 “4689” Intermediary Override
463-EW INTERMEDIARY AUTH. TYPE ID Q 1 Intermediary Authorization
PRESCRIBER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø3 PRESCRIBER SEGMENT
466-EZ PRESCRIBER ID QUALIFIER R Ø8 State license
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 794 -
411-DB PRESCRIBER ID Q ØØG2345
427-DR PRESCRIBER LAST NAME Q JONES
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 557{ $55.7Ø
412-DC DISPENSING FEE SUBMITTED Q 5Ø{ $5.ØØ
426-DQ USUAL AND CUSTOMARY CHARGE Q 587{ $58.7Ø
43Ø-DU GROSS AMOUNT DUE R 6Ø7{ $6Ø.7Ø
423-DN BASIS OF COST DETERMINATION Q Ø3 Direct
484848DØB156789Ø12341Ø14563663bbbbbbbb2ØØ7Ø91598765bbbbb<1E>1C>AMØ4<1C>C2987654321<1E><1C>AMØ1<1C>C41962Ø
615<1C>C51<1C>CAJOSEPH<1C>CBSMITH<1C>CM123 MAIN STREET<1C>CNMY TOWN<1C>COCO<1C>CP24567<1C>HNJSMITH@NCPDP.
ORG<1D><1E><1C>AMØ7<1C>EM1<1C>D21234567<1C>E1Ø3<1C>D7ØØØØ6Ø94268<1C>E73ØØØØ<1C>D3Ø<1C>D53Ø<1C>D61<1C>D8Ø<
1C>DE2ØØ7Ø915<1C>DF5<1C>DJ1>1C>EX4689<1C>EW1<1E><1C>AMØ3<1C>EZØ8<1C>DBØØG2345<1C>DRJONES<1E><1C>AM11<1C>D
9557{<1C>DC5Ø{<1C>DQ587{<1C>DU6Ø7{<1C>DNØ3
34.5.2 BILLING ACCEPTED RESPONSE- PAID (DUPLICATE OF PAID)
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE MESSAGE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 2Ø RESPONSE MESSAGE SEGMENT
5Ø4-F4 MESSAGE Q TRANSMISSION
MESSAGE TEXT For illustrative purposes only. Up to 2ØØ
Bytes
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P or D Paid or Duplicate of Paid
5Ø3-F3 AUTHORIZATION NUMBER Q 123456789123456789
13Ø-UF ADDITIONAL MESSAGE INFORMATION
COUNT
R 1 1 occurrence
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø1 Used for first line of free form text with no pre-
defined structure.
526-FQ ADDITIONAL MESSAGE INFORMATION Q TRANSACTION
MESSAGE TEXT For illustrative purposes only. Up to 4Ø Bytes
549-7F HELP DESK PHONE NUMBER QUALIFIER R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
551-9F PREFERRED PRODUCT COUNT R 1 1 Preferred product identified
552-AP PREFERRED PRODUCT ID QUALIFIER R Ø3 NDC
553-AR PREFERRED PRODUCT ID Q 17236Ø569Ø1 Ibuprofen 6ØØmg tablet
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 1ØØ{ $1Ø.ØØ
5Ø6-F6 INGREDIENT COST PAID R 557{ $55.7Ø
5Ø7-F7 DISPENSING FEE PAID R 8Ø{ $8.ØØ
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 795 -
557-AV TAX EXEMPT INDICATOR Q 1 Payer/Plan is Tax Exempt (The Payer/Plan is
not responsible for tax. The patient may be
charged tax.)
563-J2 OTHER AMOUNT PAID COUNT R 1 One occurrence
564-J3 OTHER AMOUNT PAID QUALIFIER R Ø1 Delivery
565-J4 OTHER AMOUNT PAID Q 15Ø{ $15.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 7Ø7{ $7Ø.7Ø
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 1 Ingredient cost paid as submitted
523-FN AMOUNT ATTRIBUTED TO SALES TAX Q 2Ø{ $2.ØØ
518-FI AMOUNT OF COPAY Q 8Ø{ $8.ØØ
558-AW FLAT SALES TAX AMOUNT PAID Q 2Ø{ $2.ØØ
575-EQ PATIENT SALES TAX AMOUNT Q 2Ø{ $2.ØØ
Example with Paid Response
DØB11AØ14563663bbbbbbbb2ØØ7Ø915<1E><1C>AM2Ø<1C>F4TRANSMISSION MESSAGE TEXT<1D><1E><1C>AM21<1C>ANP<1C>F312
3456789123456789<1C>UF1<1C>UHØ1<1C>FQTRANSACTION MESSAGE TEXT<1C>7FØ3<1C>8F6Ø2357Ø862<1E><1C>AM22<1C>EM1<
1C>D21234567<1C>9F1<1C>APØ3<1C>AR17236Ø569Ø1<1E><1C>AM23<1C>F51ØØ{<1C>F6557{<1C>F71ØØ{<1C>AV1<1C>J21<1C>J
3Ø1<1C>J415Ø{<1C>F97Ø7{<1C>FM1<1C>FN2Ø{<1C>FI8Ø{<1C>AW2Ø{<1C>EQ2Ø{
34.5.3 BILLING ACCEPTED RESPONSE-CAPTURED
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE MESSAGE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 2Ø RESPONSE MESSAGE SEGMENT
5Ø4-F4 MESSAGE Q TRANSMISSION
MESSAGE TEXT For illustrative purposes only. Up to 2ØØ Bytes
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M C Captured
5Ø3-F3 AUTHORIZATION NUMBER Q 123456789123456789
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
551-9F PREFERRED PRODUCT COUNT R 1 1 Preferred product identified
552-AP PREFERRED PRODUCT ID QUALIFIER R Ø3 NDC
553-AR PREFERRED PRODUCT ID Q 17236Ø569Ø1 Ibuprofen 6ØØmg tablet
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT Q 15Ø{ $15.ØØ
5Ø6-F6 INGREDIENT COST PAID R 557{ $55.7Ø
5Ø7-F7 DISPENSING FEE PAID Q 1ØØ{ $1Ø.ØØ
557-AV TAX EXEMPT INDICATOR Q 1 Payer/Plan is Tax Exempt (The Payer/Plan is not
responsible for tax. The patient may be charged
tax.)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 796 -
5Ø9-F9 TOTAL AMOUNT PAID R 7Ø7{ $7Ø.7Ø
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 1 Ingredient cost paid as submitted
518-FI AMOUNT OF COPAY Q 15Ø{ $15.ØØ
DØB11AØ14563663bbbbbbbb2ØØ7Ø915<1E><1C>AM2Ø<1C>F4TRANSMISSION MESSAGE TEXT<1D><1E><1C>AM21<1C>ANC<1C>F312
3456789123456789<1C>7FØ3<1C>8F6Ø2357Ø862<1D><1E><1C>AM22<1C>EM1<1C>D21234567<1C>9F1<1C>APØ3<1C>AR17236Ø56
9Ø1<1E><1C>AM23<1C>F515Ø{<1C>F6557{<1C>F71ØØ{<1C>AV1<1C>F97Ø7{<1C>FM1<1C>FI15Ø{
34.5.4 BILLING ACCEPTED RESPONSE WITH APPROVED MESSAGE CODES
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE MESSAGE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 2Ø RESPONSE MESSAGE SEGMENT
5Ø4-F4 MESSAGE Q TRANSMISSION
MESSAGE TEXT For illustrative purposes only. Up to 2ØØ Bytes
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P or D Paid or Duplicate of Paid
5Ø3-F3 AUTHORIZATION NUMBER Q 123456789123456789
13Ø-UF ADDITIONAL MESSAGE INFORMATION
COUNT
R 1 1 occurrence
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø1 Used for first line of free form text with no pre-
defined structure.
526-FQ ADDITIONAL MESSAGE INFORMATION Q USE NAPROXEN Up to 4Ø Bytes
547-5F APPROVED MESSAGE CODE COUNT R 2 2 occurrences
548-6F APPROVED MESSAGE CODE R ØØ2 Non-Formulary Drug
548-6F APPROVED MESSAGE CODE Q ØØ3 Maintenance Drug
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 1ØØ{ $1Ø.ØØ
5Ø6-F6 INGREDIENT COST PAID Q 557{ $55.7Ø
5Ø7-F7 DISPENSING FEE PAID Q 5Ø{ $5.ØØ
557-AV TAX EXEMPT INDICATOR Q 1 Payer/Plan is Tax Exempt (The Payer/Plan is not
responsible for tax. The patient may be charged
tax.)
5Ø9-F9 TOTAL AMOUNT PAID R 5Ø7{ $5Ø.7Ø
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 1 Ingredient cost paid as submitted
DØB11AØ14563663bbbbbbbb2ØØ7Ø915<1E><1C>AM2Ø<1C>F4TRANSMISSION MESSAGE TEXT<1D><1E><1C>AM21<1C>ANP<1C>F312
3456789123456789<1C>UF1<1C>UHØ1<1C>FQUSE NAPROXEN<1C>5F2<1C>6FØØ2<1C>6FØØ3<1C>7FØ3<1C>8F6Ø2357Ø862<1E><1C
>AM22<1C>EM1<1C>D21234567<1E><1C>AM23<1C>F51ØØ{<1C>F6557{<1C>F75Ø{<1C>AV1<1C>F95Ø7{<1C>FM1
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 797 -
34.5.5 BILLING TRANSMISSION REJECTED RESPONSE
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M R Rejected
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
TRANSACTION RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M R Rejected
51Ø-FA REJECT COUNT R 2 2 Reject Codes follow
511-FB REJECT CODE R Ø1 M/I BIN Number
511-FB REJECT CODE Q Ø4 M/I Processor Control Number
13Ø-UF ADDITIONAL MESSAGE INFORMATION
COUNT
R 1 1 occurrence
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø1 Used for first line of free form text with no pre-
defined structure.
526-FQ ADDITIONAL MESSAGE INFORMATION Q TRANSACTION
MESSAGE TEXT For illustrative purposes only. Up to 4Ø Bytes
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
DØB11RØ14563663bbbbbbbb2ØØ7Ø915<1D><1E><1C>AM21<1C>ANR<1C>FA2<1C>FBØ1<1C>FBØ4<1C>UF1<1C>UHØ1<1C>FQTRANSAC
TION MESSAGE TEXT<1C>7FØ3<1C>8F6Ø2357Ø862
34.5.6 BILLING TRANSACTION REJECTED RESPONSE
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE MESSAGE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 2Ø RESPONSE MESSAGE SEGMENT
5Ø4-F4 MESSAGE Q TRANSMISSION
MESSAGE TEXT For illustrative purposes only. Up to 2ØØ Bytes
TRANSACTION RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M R Rejected
51Ø-FA REJECT COUNT R 1 1 Reject Code follows
511-FB REJECT CODE R 7Ø Product/Service not covered
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 798 -
551-9F PREFERRED PRODUCT COUNT R 1 One preferred product identified
552-AP PREFERRED PRODUCT ID QUALIFIER R Ø3 NDC
553-AR PREFERRED PRODUCT ID R 17236Ø569Ø1 Ibuprofen 6ØØmg tablet
554-AS PREFERRED PRODUCT INCENTIVE Q 1Ø{ $1.ØØ
DØB11AØ14563663bbbbbbbb2ØØ7Ø915<1E><1C>AM2Ø<1C>F4TRANSMISSION MESSAGE TEXT<1D><1E><1C>AM21<1C>ANR<1C>FA1<
1C>FB7Ø<1C>7FØ3<1C>8F6Ø2357Ø862<1E><1C>AM22<1C>EM1<1C>D21234567<1C>9F1<1C>APØ3<1C>AR17236Ø569Ø1<1C>AS1Ø
34.6 BILLING – TRANSACTION CODE B1 – COORDINATION OF BENEFITS
SCENARIOS PHARMACY BILLS TO INSURANCE DESIGNATED BY PATIENT
See the next suite of examples for continuation of Coordination of Benefits scenarios.
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE VENDOR/CERTIFICATION
ID
M bbbbbbbbbb
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 987654321 Cardholder ID
3Ø1-C1 GROUP ID Q 1234
3Ø3-C3 PERSON CODE Q 3 Place in family
3Ø6-C6 PATIENT RELATIONSHIP CODE Q 3 Child
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
4Ø8-D8 DISPENSE AS WRITTEN/PRODUCT
SELECTION CODE
R 2 Patient has requested Brand
442-E7 QUANTITY DISPENSED R 3ØØØØ 3Ø.ØØØ tablets
4Ø3-D3 FILL NUMBER R Ø Original dispensing for RX#
4Ø5-D5 DAYS SUPPLY R 3Ø 3Ø Days supply
4Ø6-D6 COMPOUND CODE R 1 Not a compound
442-E7 QUANTITY DISPENSED R 3ØØØØ 3Ø.ØØØ tablets
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ7Ø915 September 15, 2ØØ7
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 557{ $55.7Ø
412-DC DISPENSING FEE SUBMITTED Q 5Ø{ $5.ØØ
426-DQ USUAL AND CUSTOMARY CHARGE Q 7Ø7{ $7Ø.7Ø
43Ø-DU GROSS AMOUNT DUE R 6Ø7{ $6Ø.7Ø
423-DN BASIS OF COST DETERMINATION Q Ø3 Direct
34.6.1 BILLING ACCEPTED RESPONSE – PAYER REJECTS INDICATING OTHER
COVERAGE EXISTS
Payer provides some information about other Payers.
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 799 -
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M R Rejected
51Ø-FA REJECT COUNT R 1 One occurrence
511-FB REJECT CODE R 41 Submit Bill to Other Payer or Primary Payer
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R Ø3 Help desk number of Processor/PBM of this
transaction
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 28 RESPONSE COORDINATION OF BENEFITS/OTHER
PAYERS SEGMENT
355-NR OTHER PAYER ID COUNT M 1 One occurrence
338-5C OTHER PAYER COVERAGE TYPE M Ø1 Primary
339-6C OTHER PAYER ID QUALIFIER R Ø3 BIN Number
34Ø-7C OTHER PAYER ID Q 999999 Payer’s ID
356-NU OTHER PAYER CARDHOLDER ID Q 998877665 Known ID for Cardholder for the above payer.
For purposes of this document example, only one payer is on file as noted above.
If processor has MORE than one other payer on file the data would be reported as follows. In this second example, the Cardholder ID is
available for the first payer on file but not available for the second payer.
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 28 RESPONSE COORDINATION OF BENEFITS/OTHER
PAYERS SEGMENT
355-NT OTHER PAYER ID COUNT M 2 Two occurrences
338-5C OTHER PAYER COVERAGE TYPE M Ø1 Primary
339-6C OTHER PAYER ID QUALIFIER R Ø3 BIN Number for first occurrence
34Ø-7C OTHER PAYER ID R 999999 Payer’s ID
356-NU OTHER PAYER CARDHOLDER ID Q 998877665 Known ID for Cardholder for the above payer.
338-5C OTHER PAYER COVERAGE TYPE M Ø2 Secondary
339-6C OTHER PAYER ID QUALIFIER R Ø1 National Payer ID for second occurrence
34Ø-7C OTHER PAYER ID Q 123456 Payer’s ID
34.6.2 BILLING – TRANSACTION CODE B1 – PHARMACY BILLS TO OTHER INSURANCE
This occurs after pharmacy gets data from patient.
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 999999
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M XYZbbbbbbb
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE VENDOR/CERTIFICATION
ID
M bbbbbbbbbb
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 998877665 Cardholder ID
3Ø1-C1 GROUP ID Q 3451
3Ø3-C3 PERSON CODE Q 4 Place in family
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 800 -
3Ø6-C6 PATIENT RELATIONSHIP CODE Q 3 Child
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M
Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
4Ø8-D8 DISPENSE AS WRITTEN/PRODUCT
SELECTION CODE
R 2 Patient has requested Brand
442-E7 QUANTITY DISPENSED R 3ØØØØ 3Ø.ØØØ tablets
4Ø3-D3 FILL NUMBER R Ø Original dispensing for RX#
4Ø5-D5 DAYS SUPPLY R 3Ø 3Ø Days supply
4Ø6-D6 COMPOUND CODE R 1 Not a compound
442-E7 QUANTITY DISPENSED R 3ØØØØ 3Ø.ØØØ tablets
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ7Ø915 September 15, 2ØØ7
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 567{ $56.7Ø
412-DC DISPENSING FEE SUBMITTED Q 45{ $4.5Ø
426-DQ USUAL AND CUSTOMARY CHARGE Q 7Ø7{ $7Ø.7Ø
43Ø-DU GROSS AMOUNT DUE R 612{ $61.2Ø
423-DN BASIS OF COST DETERMINATION Q Ø1 AWP
Pricing fields submitted per rate for THIS payer.
34.6.2.1 BILLING ACCEPTED RESPONSE – PAID - PRIMARY INSURANCE PAYS THE CLAIM
Included in the Patient Pay Amount (5Ø5-F5) of $2Ø.ØØ is a deductible amount, a standard copay and a product selection amount.
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE INSURANCE SEGMENT
FIELD FIELD NAME CAT Value COMMENTS
111-AM SEGMENT IDENTIFICATION M 25 RESPONSE INSURANCE SEGMENT
524-FO PLAN ID Q 2316
568-J7 PAYER ID QUALIFIER R 1 National Payer ID of Processor/PBM of this
transaction
569-J8 PAYER ID Q 2223345678
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P Paid
5Ø3-F3 AUTHORIZATION NUMBER Q 123456789123456789
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R 3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 8ØØ9986222
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE M 1234567
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 801 -
NUMBER
551-9F PREFERRED PRODUCT COUNT R 1 1 Preferred product identified
552-AP PREFERRED PRODUCT ID QUALIFIER R Ø3 NDC
553-AR PREFERRED PRODUCT ID Q 17236Ø569Ø1 Ibuprofen 6ØØmg tablet
In this example, patient has requested the Brand Product (Dispense As Written (DAW)/Product Selection Code = 2). This request will result in
the processor adding the cost difference between the preferred and brand products to the Patient Pay Amount. Using the above fields, the
processor provides information about the preferred alternative if customer wishes to change their mind.
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 2ØØ{ $2Ø.ØØ
5Ø6-F6 INGREDIENT COST PAID Q 567{ $56.7Ø
5Ø7-F7 DISPENSING FEE PAID Q 45{ $4.5Ø
5Ø9-F9 TOTAL AMOUNT PAID R 412{ $41.2Ø
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 1 Ingredient Cost Paid as Submitted
517-FH AMOUNT APPLIED TO PERIODIC
DEDUCTIBLE
R 55{ $5.5Ø
518-FI AMOUNT OF COPAY R 12Ø{ $12. ØØ
134-UK AMOUNT ATTRIBUTED TO PRODUCT
SELECTION/BRAND DRUG
R 25{ $2.5Ø
Patient Pay Amount (5Ø5-F5) and Amount Applied to Periodic Deductible (517-FH):
Examples: A patient has a $5Ø.ØØ deductible to meet. The patient’s first prescription costs $95.ØØ. The amount applied to the periodic
deductible would reflect $5Ø.ØØ. This field would reflect: 5ØØ{.
A patient has a $1ØØ.ØØ deductible to meet. The patient has previously met $8Ø.ØØ of the deductible. The next prescription purchased
costs $42.ØØ. The amount applied to the periodic deductible would reflect $2Ø.ØØ. This field would reflect: 2ØØ{.
Amount of Copay (518-FI):
Examples: If the patient’s copay is $5.ØØ, but they have also met a deductible in the same transaction, this field may not be the same as the
amount in field 5Ø5-F5. This field would reflect: 5Ø{.
Amount Attributed to Product Selection/Brand Drug (134-UK):
Examples: The patient chooses a brand drug instead of the generic. The plan design for the patient’s benefit package requires that the patient
must pay for the difference between the prescribed drug price and the preferred drug price. If the difference is $17.54, this field would reflect:
175D.
34.6.3 BILLING – TRANSACTION CODE B1 – COORDINATION OF BENEFITS – SCENARIO
1: PHARMACY BILLS SECONDARY INSURANCE
Submit claim indicating Other Payer Amount Paid.
See also previous example for Coordination of Benefits.
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE VENDOR/CERTIFICATION
ID
M bbbbbbbbbb
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 987654321 Cardholder ID
3Ø1-C1 GROUP ID Q 1234
3Ø3-C3 PERSON CODE Q 3 Place in family
3Ø6-C6 PATIENT RELATIONSHIP CODE Q 3 Child
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
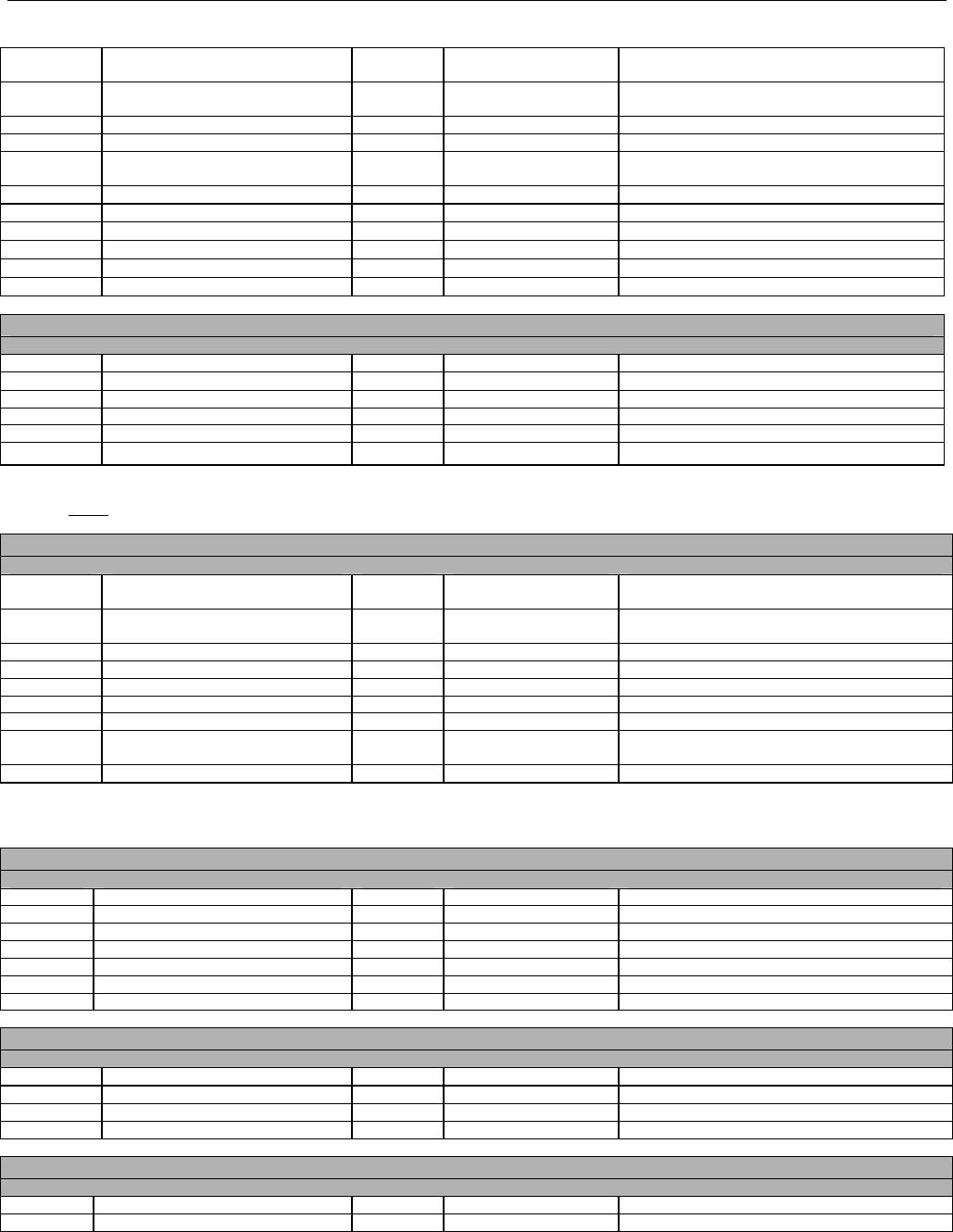
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 802 -
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
3Ø8-C8 OTHER COVERAGE CODE R 2 Other coverage exists/billed-payment
collected
442-E7 QUANTITY DISPENSED R 3ØØØØ 3Ø.ØØØ tablets
4Ø3-D3 FILL NUMBER R Ø Original dispensing for RX#
4Ø5-D5 DAYS SUPPLY R 3Ø 3Ø Days supply
4Ø6-D6 COMPOUND CODE R 1 Not a compound
442-E7 QUANTITY DISPENSED R 3ØØØØ 3Ø.ØØØ tablets
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ7Ø915 September 15, 2ØØ7
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 557{ $55.7Ø
412-DC DISPENSING FEE SUBMITTED Q 5Ø{ $5.ØØ
426-DQ USUAL AND CUSTOMARY CHARGE Q 7Ø7{ $7Ø.7Ø
43Ø-DU GROSS AMOUNT DUE* R 6Ø7{ $6Ø.7Ø*
423-DN BASIS OF COST DETERMINATION Q Ø3 Direct
Billing for Contracted Rate of Secondary with Indication of Amount that has been paid.
* Definition of Gross Amount Due only allows for “the sum of” selected fields as presented in the Pricing Segment. It does NOT allow for the
“sum of” minus Other Payer Amount Paid.
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø5 COORDINATION OF BENEFITS/OTHER PAYMENTS
SEGMENT
337-4C COORDINATION OF BENEFITS/OTHER
PAYMENTS COUNT
M 1 One occurrence
338-5C OTHER PAYER COVERAGE TYPE M Ø1 Primary
339-6C OTHER PAYER ID QUALIFIER R Ø3 BIN #
34Ø-7C OTHER PAYER ID Q 999999 ID assigned to payer
443-E8 OTHER PAYER DATE Q 2ØØ7Ø915 September 15, 2ØØ7
341-HB OTHER PAYER AMOUNT PAID COUNT R 1 One occurrence
342-HC OTHER PAYER AMOUNT PAID
QUALIFIER
R Ø7 Drug Benefit
431-DV OTHER PAYER AMOUNT PAID Q 412{ $41.2Ø paid
34.6.3.1 SCENARIO 1 RESPONSE: SECONDARY INSURANCE PAYS THE CLAIM SUBMITTED WITH
AMOUNT PAID BY OTHER PAYER
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE INSURANCE SEGMENT
FIELD FIELD NAME CAT Value COMMENTS
111-AM SEGMENT IDENTIFICATION M 25 RESPONSE INSURANCE SEGMENT
524-FO PLAN ID Q 9988
568-J7 PAYER ID QUALIFIER R 1 National Payer ID
569-J8 PAYER ID Q 12121212
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P Paid
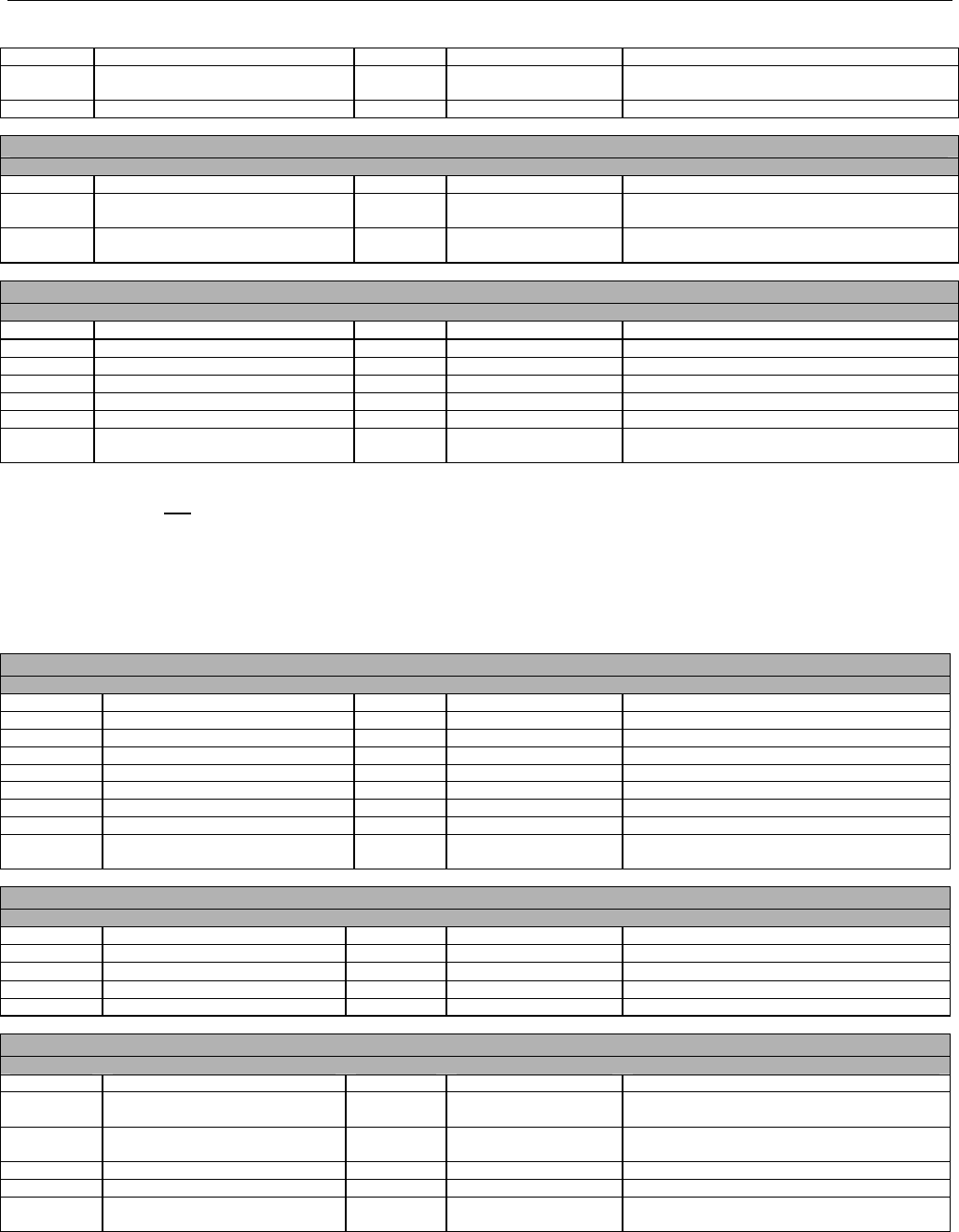
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 803 -
5Ø3-F3 AUTHORIZATION NUMBER Q 11122233345678
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R 3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 5{ $ØØ.5Ø
5Ø6-F6 INGREDIENT COST PAID R 557{ $55.7Ø
5Ø7-F7 DISPENSING FEE PAID Q 5Ø{ $5.ØØ
566-J5 OTHER PAYER AMOUNT RECOGNIZED R 412{ $41.2Ø
5Ø9-F9 TOTAL AMOUNT PAID R 19Ø{ $19.ØØ
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 1 Ingredient Cost Paid as Submitted
TOTAL AMOUNT PAID represents a sum of “Ingredient Cost Paid” (5Ø6-F6), “Dispensing Fee Paid” (5Ø7-F7), “Flat Sales Tax Amount Paid”
(558-AW), “Percentage Sales Tax Amount Paid” (559-AX), “Incentive Amount Paid” (521-FL), “Professional Service Fee Paid” (562-J1), “Other
Amount Paid” (565-J4) less “Patient Pay Amount” (5Ø5-F5) and “Other Payer Amount Recognized” (566-J5).
In above example, secondary payer’s contracted rate is less than that of the primary ($19.5Ø vs. $2Ø.ØØ). They have returned a $Ø.5Ø
copay and the agreement to pay $19.ØØ.
34.6.4 BILLING – TRANSACTION CODE B1 – COORDINATION OF BENEFITS – SCENARIO
2: PHARMACY BILLS SECONDARY INSURANCE
Submit Other Payer Patient Responsibility Amount.
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE VENDOR/CERTIFICATION
ID
M bbbbbbbbbb
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 987654321 Cardholder ID
3Ø1-C1 GROUP ID Q 1234
3Ø3-C3 PERSON CODE Q 3 Place in family
3Ø6-C6 PATIENT RELATIONSHIP CODE Q 3 Child
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
3Ø8-C8 OTHER COVERAGE CODE R 8 Claim is a billing for patient financial
responsibility
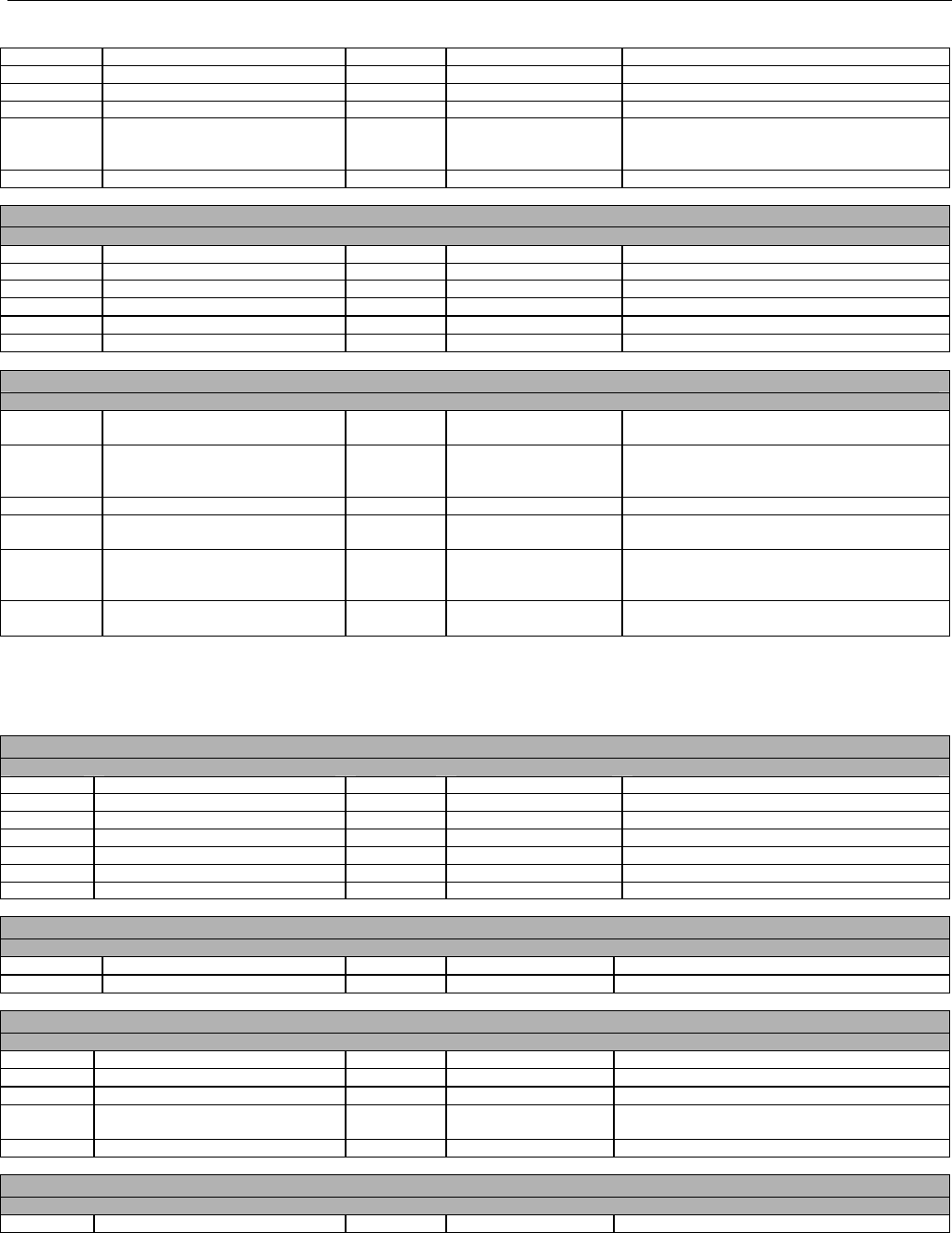
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 804 -
442-E7 QUANTITY DISPENSED R 6Ø
4Ø3-D3 FILL NUMBER R Ø Original Fill
4Ø5-D5 DAYS SUPPLY R 3Ø
4Ø6-D6 COMPOUND CODE R 1 Not a Compound
4Ø8-D8 DISPENSE AS WRITTEN
(DAW)/PRODUCT SELECTION
CODE
R 2 Patient has requested Brand
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ7Ø915 September 15, 2ØØ7
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 557{ $55.7Ø
412-DC DISPENSING FEE SUBMITTED Q 5Ø{ $5.ØØ
43Ø-DU GROSS AMOUNT DUE R 6Ø7{ $6Ø.7Ø
426-DQ USUAL AND CUSTOMARY CHARGE Q 7Ø7{ $7Ø.7Ø
423-DN BASIS OF COST DETERMINATION Q Ø3 Direct
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø5 COORDINATION OF BENEFITS/OTHER PAYMENTS
SEGMENT
337-4C COORDINATION OF
BENEFITS/OTHER PAYMENTS
COUNT
M 1 One occurrence
338-5C OTHER PAYER COVERAGE TYPE M Ø1 Primary
353-NR OTHER PAYER –PATIENT
RESPONSIBILITY AMOUNT COUNT
R 1 One occurrence
351-NP OTHER PAYER-PATIENT
RESPONSIBILITY AMOUNT
QUALIFIER
R Ø6 Patient Pay Amount (5Ø5-F5) as reported by
previous payer.
352-NQ OTHER PAYER-PATIENT
RESPONSIBILITY AMOUNT
R 2ØØ{ $2Ø.ØØ
Note: The Other Payer ID fields do not need to be sent in every case. In some business cases, it is not necessary to denote the previous
payer(s).
34.6.4.1 SCENARIO 2 RESPONSE: SECONDARY INSURANCE PAYS THE CLAIM SUBMITTED WITH NET
OTHER PAYER PATIENT RESPONSIBILITY AMOUNT
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 25 RESPONSE INSURANCE SEGMENT
524-FO PLAN ID Q 9988
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P Paid
5Ø3-F3 AUTHORIZATION NUMBER Q 11122233345678
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R 3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
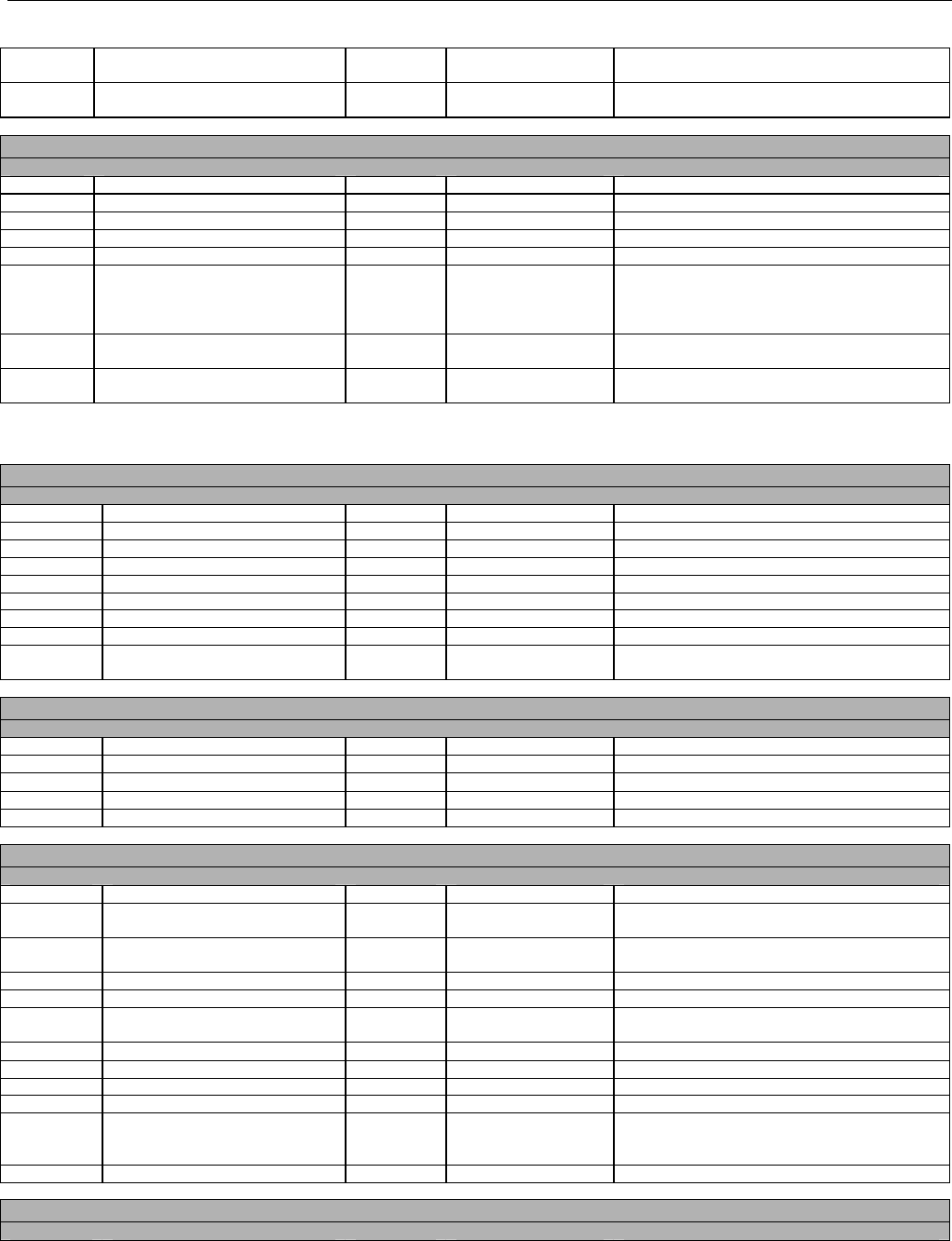
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 805 -
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 5{ $ØØ.5Ø
5Ø6-F6 INGREDIENT COST PAID R 557{ $2Ø.ØØ
5Ø7-F7 DISPENSING FEE PAID Q 2ØØ{ $Ø.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 195{ $19.5Ø
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 14 Other Payer-Patient Responsibility Amount -
Indicates reimbursement was based on the
Other Payer Patient Responsibility Amount
(352-NQ)
148-U8 INGREDIENT COST CONTRACTED/
REIMBURSABLE AMOUNT
I 557{ $55.7Ø
149-U9 DISPENSING FEE CONTRACTED/
REIMBURSABLE AMOUNT
I 5Ø{ $5.ØØ
34.6.5 SCENARIO 3: PHARMACY BILLS SECONDARY INSURANCE
Submit “pieces” that make up OTHER PAYER PATIENT RESPONSIBILTY AMOUNT.
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID
M bbbbbbbbbb
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 987654321 Cardholder ID
3Ø1-C1 GROUP ID Q 1234
3Ø3-C3 PERSON CODE Q 3 Place in family
3Ø6-C6 PATIENT RELATIONSHIP CODE Q 3 Child
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
3Ø8-C8 OTHER COVERAGE CODE R 8 Claim is a billing for patient financial
responsibility
442-E7 QUANTITY DISPENSED R 6Ø
4Ø3-D3 FILL NUMBER R Ø Original Fill
4Ø5-D5 DAYS SUPPLY R 3Ø
4Ø6-D6 COMPOUND CODE R 1 Not a Compound
4Ø8-D8 DISPENSE AS WRITTEN
(DAW)/PRODUCT SELECTION
CODE
R 2 Patient has requested Brand
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ7Ø915 September 15, 2ØØ7
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 806 -
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 557{ $55.7Ø
412-DC DISPENSING FEE SUBMITTED Q 5Ø{ $5.ØØ
43Ø-DU GROSS AMOUNT DUE R 6Ø7{ $6Ø.7Ø
426-DQ USUAL AND CUSTOMARY CHARGE Q 7Ø7{ $7Ø.7Ø
423-DN BASIS OF COST DETERMINATION Q Ø3 Direct
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø5 COORDINATION OF BENEFITS/OTHER PAYMENTS
SEGMENT
337-4C COORDINATION OF
BENEFITS/OTHER PAYMENTS
COUNT
M 1 One occurrence
338-5C OTHER PAYER COVERAGE TYPE M Ø1 Primary
353-NR OTHER PAYER –PATIENT
RESPONSIBILITY AMOUNT COUNT
R 3 Three occurrences
351-NP OTHER PAYER-PATIENT
RESPONSIBILITY AMOUNT
QUALIFIER
R Ø1 Amount Applied to Periodic Deductible (517-
FH) as reported by previous payer
352-NQ OTHER PAYER-PATIENT
RESPONSIBILITY AMOUNT
Q 55{ $5.5Ø
351-NP OTHER PAYER-PATIENT
RESPONSIBILITY AMOUNT
QUALIFIER
R Ø2 Amount Attributed to Product Selection/Brand
Drug (134-UK) as reported by previous payer
352-NQ OTHER PAYER-PATIENT
RESPONSIBILITY AMOUNT
Q 25{ $2.5Ø
351-NP OTHER PAYER-PATIENT
RESPONSIBILITY AMOUNT
QUALIFIER
R Ø5 Amount Of Copay (518-FI) as reported by
previous payer.
352-NQ OTHER PAYER-PATIENT
RESPONSIBILITY AMOUNT
Q 12Ø{ $12. ØØ
Note: The Other Payer ID fields do not need to be sent in every case. In some business cases, it is not necessary to denote the previous
payer(s).
34.6.5.1 SCENARIO 3 RESPONSE: SECONDARY INSURANCE PAYS THE CLAIM SUBMITTED WITH THE
“PIECES” OF OTHER PAYER-PATIENT RESPONSIBILITY AMOUNT
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 25 RESPONSE INSURANCE SEGMENT
524-FO PLAN ID Q 9988
568-J7 PAYER ID QUALIFIER R 1 National Payer ID
569-J8 PAYER ID Q 12121212
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P Paid
5Ø3-F3 AUTHORIZATION NUMBER Q 11122233345678
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R 3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 807 -
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 3Ø{ $3.ØØ
5Ø6-F6 INGREDIENT COST PAID R 557{ $2Ø.ØØ
5Ø7-F7 DISPENSING FEE PAID Q 2ØØ{ $Ø.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 17Ø{ $17. ØØ
134-UK AMOUNT ATTRIBUTED TO PRODUCT
SELECTION/BRAND DRUG
Q 25{ $2.5Ø
518-FI AMOUNT OF COPAY Q 5{ $ØØ.5Ø
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 14 Other Payer-Patient Responsibility Amount -
Indicates reimbursement was based on the
Other Payer Patient Responsibility Amount
(352-NQ)
148-U8 INGREDIENT COST CONTRACTED/
REIMBURSABLE AMOUNT
I 557{ $55.7Ø
149-U9 DISPENSING FEE CONTRACTED/
REIMBURSABLE AMOUNT
I 5Ø{ $5.ØØ
Secondary payer determines that they will pay some of the Patient Responsibility amounts; however, the patient WILL have some, but lesser
financial responsibility. In example:
Plan to pay Deductible 5.5Ø
Plan to pay portion of Amount of Copay (518-FI) 11.5Ø of submitted 12.ØØ
Total Amount Paid 17.ØØ
Patient to pay portion of Amount of Copay (518-FI) Ø.5Ø
Patient to pay all of Product Selection 2.5Ø
Patient Pay Amount 3.ØØ
• When the “pieces” that make up Patient Pay Amount are submitted, if secondary payer is not going to reimburse one or all of these, these
amounts are to be included in Patient Pay Amount to be charged to the customer and detail information provided as was provided by on
the submission of this claim.
• If Coordination of benefit claim is reimbursed based on Other Payer Patient Responsibility Amount (Basis of Reimbursement Code 14),
the sum of Total Amount Paid and Patient Pay Amount must be equal to or greater than the net other payer patient responsibility amount
submitted. If coordinated benefit does not allow for coverage for specific pieces of the other payer patient responsibility amount, the
claim must be rejected.
34.7 BILLING W/SUBMITTED DUR OVERRIDE - TRANSACTION CODE B1
Pharmacist submits resolved DUR conflicts on initial transaction.
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID
M 98765bbbbb
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 123456789 Cardholder ID
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M
Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 808 -
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
436-E1 PRODUCT/SERVICE CODE
QUALIFIER
M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
442-E7 QUANTITY DISPENSED R 3ØØØØ 3Ø.ØØØ tablets
4Ø3-D3 FILL NUMBER R Ø Original dispensing for RX#
4Ø5-D5 DAYS SUPPLY R 3Ø 3Ø Days supply
4Ø6-D6 COMPOUND CODE R 1 Not a compound
4Ø8-D8 DISPENSE AS WRITTEN
(DAW)/PRODUCT SELECTION
CODE
R Ø No product selection indicated
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ7Ø915 September 15, 2ØØ7
415-DF NUMBER OF REFILLS AUTHORIZED R 5 5 Refills
419-DJ PRESCRIPTION ORIGIN CODE Q 1 Written prescription
DUR/PPS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø8 DUR/PPS Segment
473-7E DUR/PPS CODE COUNTER R 1 1st DUR action
439-E4 REASON FOR SERVICE CODE Q DA Drug-Allergy alert
44Ø-E5 PROFESSIONAL SERVICE CODE Q MØ Prescriber consulted
441-E6 RESULT OF SERVICE CODE Q 1B Rx filled as is
474-8E DUR/PPS LEVEL OF EFFORT Q 11 Lowest level of complexity
473-7E DUR/PPS CODE COUNTER R 2 2nd DUR action
439-E4 REASON FOR SERVICE CODE Q LR Underutilization
44Ø-E5 PROFESSIONAL SERVICE CODE Q PØ Patient consulted
441-E6 RESULT OF SERVICE CODE Q 1B Rx filled as is
474-8E DUR/PPS LEVEL OF EFFORT Q 11 Lowest level of complexity
473-7E DUR/PPS CODE COUNTER R 3 3rd DUR action
439-E4 REASON FOR SERVICE CODE Q TD Therapeutic duplication
44Ø-E5 PROFESSIONAL SERVICE CODE Q MØ Prescriber consulted
441-E6 RESULT OF SERVICE CODE Q 1B Rx filled as is
474-8E DUR/PPS LEVEL OF EFFORT Q 11 Lowest level of complexity
475-J9 DUR CO-AGENT ID QUALIFIER R Ø3 NDC
476-H6 DUR CO-AGENT ID Q 17236Ø569Ø1 Ibuprofen 6ØØmg tablet
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 557{ $55.7Ø
412-DC DISPENSING FEE SUBMITTED R 1ØØ{ $1Ø.ØØ
478-H7 OTHER AMOUNT CLAIMED
SUBMITTED COUNT
R 1 One occurrence
479-H8 OTHER AMOUNT CLAIMED
SUBMITTED QUALIFIER
R Ø1 Delivery cost
48Ø-H9 OTHER AMOUNT CLAIMED
SUBMITTED
Q 15Ø{ $15.ØØ
426-DQ USUAL AND CUSTOMARY CHARGE Q 716E $71.65
43Ø-DU GROSS AMOUNT DUE R 8Ø7{ $8Ø.7Ø
423-DN BASIS OF COST DETERMINATION Q Ø3 Direct
61ØØ66DØB1123456789Ø1Ø14563663bbbbbbbb2ØØ7Ø91598765bbbbb<1E><1C>AMØ4<1C>C2123456789<1D><1E><1C>AMØ7<1C>EM
1<1C>D21234567<1C>E1Ø3<1C>D7ØØØØ6Ø94268<1C>E73ØØØØ<1C>D3Ø<1C>D53Ø<1C>D61<1C>D8Ø<1C>DE2ØØ7Ø915<1C>DF5<1C>D
J1<1E><1C>AMØ8<1C>7E1<1C>E4DA<1C>E5MØ<1C>E61B<1C>8E11<1C>7E2<1C>E4LR<1C>E5PØ<1C>E61B<1C>8E11<1C>7E3<1C>E4
TD<1C>E5MØ<1C>E61B<1C>8E11<1C>J9Ø3<1C>H617236Ø569Ø1<1E><1C>AM11<1C>D9557{<1C>DC1ØØ{<1C>H71<1C>H8Ø1<1C>H91
5Ø{<1C>DQ716E<1C>DU8Ø7{<1C>DNØ3
34.7.1 BILLING W/SUBMITTED DUR OVERRIDE ACCEPTED RESPONSE- PAID
Processor accepts pharmacist’s DUR submission. RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 809 -
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P Paid
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 1ØØ{ $1Ø.ØØ
5Ø6-F6 INGREDIENT COST PAID Q 557{ $55.7Ø
5Ø7-F7 DISPENSING FEE PAID Q 1ØØ{ $1Ø.ØØ
557-AV TAX EXEMPT INDICATOR Q 1 Payer/Plan is Tax Exempt (The Payer/Plan is not
responsible for tax. The patient may be charged
tax.)
563-J2 OTHER AMOUNT PAID COUNT R 1 One occurrence
564-J3 OTHER AMOUNT PAID QUALIFIER R Ø1 Delivery
565-J4 OTHER AMOUNT PAID Q 15Ø{ $15.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 7Ø7{ $7Ø.7Ø
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 1 Ingredient cost paid as submitted
DØB11AØ14563663bbbbbbbb2ØØ7Ø915<1D><1E><1C>AM21<1C>ANP<1E><1C>AM22<1C>EM1<1C>D21234567<1E><1C>AM23<1C>F51
ØØ{<1C>F6557{<1C>F71ØØ{<1C>AV1<1C>J21<1C>J3Ø1<1C>J415Ø{<1C>F97Ø7{<1C>FM1
34.7.2 BILLING W/SUBMITTED DUR OVERRIDE REJECTED RESPONSE
Processor identifies the same DUR conflicts AND identifies additional conflicts.
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M R Rejected
51Ø-FA REJECT COUNT R 1 1 Reject code follows
511-FB REJECT CODE R 88 DUR Reject
5Ø3-F3 AUTHORIZATION NUMBER Q 1234567891234567
89
13Ø-UF ADDITIONAL MESSAGE INFORMATION
COUNT
R 1 1 occurrence
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø1 Used for first line of free form text with no pre-
defined structure.
526-FQ ADDITIONAL MESSAGE INFORMATION Q TRANSACTION
MESSAGE TEXT For illustrative purposes only. Up to 4Ø Bytes.
Submitted DUR accepted; additional conflicts
identified.
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT Value COMMENTS
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 810 -
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
RESPONSE DUR/PPS SEGMENT
FIELD FIELD NAME CAT Value COMMENTS
111-AM SEGMENT IDENTIFICATION M 24 RESPONSE DUR/PPS SEGMENT
567-J6 DUR/PPS RESPONSE CODE
COUNTER
R 1 1st DUR conflict
439-E4 REASON FOR SERVICE CODE Q LD Low Dose alert
532-FW DATABASE INDICATOR Q 5 Other
544-FY DUR FREE TEXT Q MIN DAILY DOSE=2
EA/DAY
57Ø-NS DUR ADDITIONAL TEXT Q RENAL
IMPAIRMENT MAY
JUSTIFY LOW
DOSE
Additional Text if needed
567-J6 DUR/PPS RESPONSE CODE
COUNTER
R 2 2nd DUR conflict
439-E4 REASON FOR SERVICE CODE Q MC Drug-Disease Alert-Reported
528-FS CLINICAL SIGNIFICANCE CODE Q 3 Severity Level 3
532-FW DATABASE INDICATOR Q 5 Other
544-FY DUR FREE TEXT Q BRONCHIAL
ASTHMA
567-J6 DUR/PPS RESPONSE CODE
COUNTER
R 3 3rd DUR conflict
439-E4 REASON FOR SERVICE CODE Q ER Overutilization
529-FT OTHER PHARMACY INDICATOR Q 3 Different pharmacy
53Ø-FU PREVIOUS DATE OF FILL Q 2ØØ7Ø9Ø1 September 1, 2ØØ7
531-FV QUANTITY OF PREVIOUS FILL Q 3Ø
533-FX OTHER PRESCRIBER INDICATOR Q 1 Same prescriber
544-FY DUR FREE TEXT Q RX IS 1Ø DAYS
EARLY
567-J6 DUR/PPS RESPONSE CODE
COUNTER
R 4 4th DUR conflict
439-E4 REASON FOR SERVICE CODE Q TD Therapeutic Duplication
529-FT OTHER PHARMACY INDICATOR Q 3 Different pharmacy
53Ø-FU PREVIOUS DATE OF FILL Q 2ØØ7Ø913 September 13, 2ØØ7
531-FV QUANTITY OF PREVIOUS FILL Q 9Ø
532-FW DATABASE INDICATOR Q 5 Other
533-FX OTHER PRESCRIBER INDICATOR Q 2 Different prescriber
544-FY DUR FREE TEXT Q IBUPROFEN
DØB11AØ14563663bbbbbbbb2ØØ7Ø915<1D><1E><1C>AM21<1C>ANR<1C>FA1<1C>FB88<1C>F3123456789123456789<1C>UF1<1C>U
HØ1<1C>FQTRANSACTION MESSAGE TEXT<1C>7FØ3<1C>8F6Ø2357Ø862<1E><1C>AM22<1C>EM1<1C>D21234567<1E><1C>AM24<1C>
J61<1C>E4LD<1C>FW5<1C>FYMIN DAILY DOSE=2 EA/DAY<1C>NSRENAL IMPAIRMENT MAY JUSTIFY LOW DOSE<1C>J62<1C>E4MC
<1C>FS3<1C>FW5<1C>FYBRONCHIAL ASTHMA<1C>J63<1C>E4ER<1C>FT3<1C>FU2ØØ7Ø9Ø1<1C>FV3Ø<1C>FX1<1C>FYRX IS 1Ø DAY
S EARLY<1C>J64<1C>E4TD<1C>FT3<1C>FU2ØØ7Ø913<1C>FV9Ø<1C>FW5<1C>FX2<1C>FYIBUPROFEN
34.8 BILLING W/DUR CONFLICTS - TRANSACTION CODE B1
Pharmacist submits claim that will generate DUR alert. Processor identifies DUR conflict and responds to pharmacist.
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 45636663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE VENDOR/CERTIFICATION
ID
M bbbbbbbbbb
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 811 -
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 123456789
312-CC CARDHOLDER FIRST NAME O JOHN
313-CD CARDHOLDER LAST NAME O SMITH
314-CE HOME PLAN Q 6Ø2 BC/BS Plan Number
524-FO PLAN ID O 5678
3Ø9-C9 ELIGIBILITY CLARIFICATION CODE Q 4 Disabled dependent
3Ø1-C1 GROUP ID Q 987654321
3Ø3-C3 PERSON CODE Q 3 Place in family
3Ø6-C6 PATIENT RELATIONSHIP CODE Q 3 Child
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØ56Ø1747Ø Coumadin 1Ømg tab
442-E7 QUANTITY DISPENSED R 6ØØØØ 6Ø.ØØØ (High dose)
4Ø3-D3 FILL NUMBER R 3 Third dispensing for Rx#
4Ø5-D5 DAYS SUPPLY R 3Ø 3Ø Days supply
4Ø6-D6 COMPOUND CODE R 1 Not a compound
4Ø8-D8 DISPENSE AS WRITTEN
(DAW)/PRODUCT SELECTION CODE
R 1 Substitution Not Allowed by Prescriber
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ7Ø72Ø July 2Ø, 2ØØ7
415-DF NUMBER OF REFILLS AUTHORIZED R 5 5 Refills
419-DJ PRESCRIPTION ORIGIN CODE Q 1 Written prescription
429-DT SPECIAL PACKAGING INDICATOR Q 1 Not unit dose
6ØØ-28 UNIT OF MEASURE Q EA Each
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 657{ $65.7Ø
412-DC DISPENSING FEE SUBMITTED Q 1ØØ{ $1Ø.ØØ
433-DX PATIENT PAID AMOUNT SUBMITTED Q 1ØØ{ $1Ø.ØØ
478-H7 OTHER AMOUNT CLAIMED
SUBMITTED COUNT
R 1 One occurrence
479-H8 OTHER AMOUNT CLAIMED
SUBMITTED QUALIFIER
R Ø1 Delivery cost
48Ø-H9 OTHER AMOUNT CLAIMED
SUBMITTED
Q 15Ø{ $15.ØØ
426-DQ USUAL AND CUSTOMARY CHARGE Q 7Ø7{ $7Ø.7Ø
43Ø-DU GROSS AMOUNT DUE R 9Ø7{ $9Ø.7Ø
423-DN BASIS OF COST DETERMINATION Q Ø3 Direct
FACILITY SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 15 Facility Segment
336-8C FACILITY ID Q 6579Ø1
34.8.1 BILLING W/INFORMATION DUR ACCEPTED RESPONSE- PAID
Processor returns information-only DUR conflicts with notice of paid claim.
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE STATUS SEGMENT
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 812 -
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P Paid
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 75{ $7.5Ø
5Ø6-F6 INGREDIENT COST PAID Q 7Ø7{ $7Ø.7Ø
557-AV TAX EXEMPT INDICATOR Q 1 Payer/Plan is Tax Exempt (The Payer/Plan is not
responsible for tax. The patient may be charged
tax.)
563-J2 OTHER AMOUNT PAID COUNT R 1 One occurrence
564-J3 OTHER AMOUNT PAID QUALIFIER R Ø1 Delivery
565-J4 OTHER AMOUNT PAID Q 15Ø{ $15.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 782{ $78.2Ø
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 4 U&C paid as submitted
In this Example, provider submitted U&C along with a Contractual submitted amount based on direct pricing. There is also a delivery charge
included.
Provider has opted to pay the U&C as submitted for the drug/qty but ALSO is paying the Delivery Charge so the net to the pharmacy is
$7Ø.7Ø (U&C) + $15.ØØ Delivery or $85.7Ø. This then is split between the Patient and Payer as $7.5Ø Patient Pay and $78.2Ø Payer Pay.
RESPONSE DUR/PPS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 24 RESPONSE DUR/PPS SEGMENT
567-J6 DUR/PPS RESPONSE CODE
COUNTER
R 1 1st DUR conflict
439-E4 REASON FOR SERVICE CODE Q HD High Dose alert
532-FW DATABASE INDICATOR Q 5 Other
544-FY DUR FREE TEXT Q MAX DAILY DOSE =
1EX/DAY
567-J6 DUR/PPS RESPONSE CODE
COUNTER
R 2 2nd DUR conflict
439-E4 REASON FOR SERVICE CODE Q MC Drug-Disease Alert-Reported
528-FS CLINICAL SIGNIFICANCE CODE Q 1 Severity Level 1
544-FY DUR FREE TEXT Q HEMOPHILIA
567-J6 DUR/PPS RESPONSE CODE
COUNTER
R 3 3rd DUR conflict
439-E4 REASON FOR SERVICE CODE Q DD Drug Interaction Alert
528-FS CLINICAL SIGNIFICANCE CODE Q 1 Severity Level 1
529-FT OTHER PHARMACY INDICATOR Q 3 Different pharmacy
53Ø-FU PREVIOUS DATE OF FILL Q 2ØØ7Ø915 September 15, 2ØØ7
531-FV QUANTITY OF PREVIOUS FILL Q 6Ø
532-FW DATABASE INDICATOR Q 5 Other
533-FX OTHER PRESCRIBER INDICATOR Q 1 Same prescriber
544-FY DUR FREE TEXT Q GLIPIZIDE
57Ø-NS DUR ADDITIONAL TEXT Q INCREASED
HYPOGLYCEMIC
EFFECT
PROBABLE
Additional text as needed.
567-J6 DUR/PPS RESPONSE CODE
COUNTER
R 4 4th DUR conflict
439-E4 REASON FOR SERVICE CODE Q ER Overutilization
529-FT OTHER PHARMACY INDICATOR Q 1 Same pharmacy
53Ø-FU PREVIOUS DATE OF FILL Q 2ØØ7Ø9Ø1 September 1, 2ØØ7
531-FV QUANTITY OF PREVIOUS FILL Q 3Ø
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 813 -
533-FX OTHER PRESCRIBER INDICATOR Q 1 Same prescriber
544-FY DUR FREE TEXT Q RX IS 1Ø DAYS
EARLY
567-J6 DUR/PPS RESPONSE CODE
COUNTER
R 5 5th DUR conflict
439-E4 REASON FOR SERVICE CODE Q DD Drug Interaction
528-FS CLINICAL SIGNIFICANCE CODE Q 1 Severity Level 1
529-FT OTHER PHARMACY INDICATOR Q 1 Same pharmacy
53Ø-FU PREVIOUS DATE OF FILL Q 2ØØ7Ø913 September 13, 2ØØ7
531-FV QUANTITY OF PREVIOUS FILL Q 3Ø
532-FW DATABASE INDICATOR Q 5 Other
533-FX OTHER PRESCRIBER INDICATOR Q 1 Same prescriber
544-FY DUR FREE TEXT Q ASPIRIN
567-J6 DUR/PPS RESPONSE CODE
COUNTER
R 6 6th DUR conflict
439-E4 REASON FOR SERVICE CODE Q ETC. 1 additional DUR conflict
528-FS CLINICAL SIGNIFICANCE CODE Q
529-FT OTHER PHARMACY INDICATOR Q
53Ø-FU PREVIOUS DATE OF FILL Q
531-FV QUANTITY OF PREVIOUS FILL Q
532-FW DATABASE INDICATOR Q
533-FX OTHER PRESCRIBER INDICATOR Q
544-FY DUR FREE TEXT Q
567-J6 DUR/PPS RESPONSE CODE
COUNTER
R 7 7th DUR conflict
439-E4 REASON FOR SERVICE CODE Q ETC. 1 additional DUR conflict
528-FS CLINICAL SIGNIFICANCE CODE Q
529-FT OTHER PHARMACY INDICATOR Q
53Ø-FU PREVIOUS DATE OF FILL Q
531-FV QUANTITY OF PREVIOUS FILL Q
532-FW DATABASE INDICATOR Q
533-FX OTHER PRESCRIBER INDICATOR Q
544-FY DUR FREE TEXT Q
567-J6 DUR/PPS RESPONSE CODE
COUNTER
R 8 8th DUR conflict
439-E4 REASON FOR SERVICE CODE Q ETC. 1 additional DUR conflict
528-FS CLINICAL SIGNIFICANCE CODE Q
529-FT OTHER PHARMACY INDICATOR Q
53Ø-FU PREVIOUS DATE OF FILL Q
531-FV QUANTITY OF PREVIOUS FILL Q
532-FW DATABASE INDICATOR Q
533-FX OTHER PRESCRIBER INDICATOR Q
544-FY DUR FREE TEXT Q
567-J6 DUR/PPS RESPONSE CODE
COUNTER
R 9 9th DUR conflict
439-E4 REASON FOR SERVICE CODE Q CH Call Help Desk
544-FY DUR FREE TEXT Q 1 DUR CONFLICT 1 Add’l DUR conflict identified
34.8.2 BILLING W/DUR CONFLICTS REJECTED RESPONSE
Processor returns DUR conflicts to pharmacist with rejected claim.
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M R Rejected
51Ø-FA REJECT COUNT R 1 1 Reject code follows
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 814 -
511-FB REJECT CODE R 88 DUR Reject
5Ø3-F3 AUTHORIZATION CODE Q 1234567891234567
89
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
RESPONSE DUR/PPS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 24 Response DUR/PPS Segment
567-J6 DUR/PPS RESPONSE CODE
COUNTER
R 1 1st DUR conflict
439-E4 REASON FOR SERVICE CODE Q HD High Dose Alert
532-FW DATABASE INDICATOR Q 5 Other
544-FY DUR FREE TEXT Q MAX DOSE=2 EA/DAY
34.9 SERVICE BILLING - TRANSACTION CODE S1 (Ø1/Ø2)
Pharmacist submits claim for two professional services unrelated to a dispensing event.
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M S1 Service Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 2 Two occurrences
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID
M bbbbbbbbbb
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø1 PATIENT SEGMENT
331-CX PATIENT ID QUALIFIER R Ø1 Social Security Number
332-CY PATIENT ID Q ØØ5492368 Patient’s SSN
3Ø4-C4 DATE OF BIRTH R 1962Ø615 Born June 15, 1962
3Ø5-C5 PATIENT GENDER CODE R 1 Male
31Ø-CA PATIENT FIRST NAME R JOSEPH
311-CB PATIENT LAST NAME R SMITH
322-CM PATIENT STREET ADDRESS O 123 MAIN STREET
323-CN PATIENT CITY ADDRESS O MY TOWN
324-CO PATIENT STATE/PROVINCE
ADDRESS
O CO
325-CP PATIENT ZIP/POSTAL ZONE O 34567
326-CQ PATIENT PHONE NUMBER O 2Ø14658923
333-CZ EMPLOYER ID Q XYZ123
35Ø-HN PATIENT E-MAIL ADDRESS I JSMITH@NCPDP.O
RG Patient’s E-Mail Address
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 123456789 Cardholder ID
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 2 Service billing

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 815 -
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 7654321
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø6 DUR/PPS
4Ø7-D7 PRODUCT/SERVICE ID M Ø
DUR/PPS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø8 DUR/PPS Segment
473-7E DUR/PPS CODE COUNTER R 1 1st DUR activity
439-E4 REASON FOR SERVICE CODE Q PN Prescriber consultation
44Ø-E5 PROFESSIONAL SERVICE CODE Q RT Recommend lab test
441-E6 RESULT OF SERVICE CODE Q 3A Recommendation accepted
474-8E DUR/PPS LEVEL OF EFFORT Q 11 Lowest level of complexity
473-7E DUR/PPS CODE COUNTER R 2 2nd DUR activity
439-E4 REASON FOR SERVICE CODE Q TN Laboratory test needed
44Ø-E5 PROFESSIONAL SERVICE CODE Q PT Perform laboratory test
441-E6 RESULT OF SERVICE CODE Q 3A Recommendation accepted
474-8E DUR/PPS LEVEL OF EFFORT Q 12 Service with medium complexity
PRICING SEGMENT
FIELD FIELD NAME CAT Value COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
477-BE PROFESSIONAL SERVICE FEE
SUBMITTED
R 5Ø{ $5.ØØ
426-DQ USUAL AND CUSTOMARY CHARGE Q 5Ø{ $5.ØØ
43Ø-DU GROSS AMOUNT DUE R 5Ø{ $5.ØØ
CLINICAL SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 13 CLINICAL SEGMENT
493-XE CLINICAL INFORMATION COUNTER R 1 1st occurrence
494-ZE MEASUREMENT DATE Q 2ØØ7Ø915 September 15, 2ØØ7
495-H1 MEASUREMENT TIME Q 143Ø Measured at 2:3Øpm
496-H2 MEASUREMENT DIMENSION Q Ø1 Blood Pressure (BP)
497-H3 MEASUREMENT UNIT Q 1Ø Millimeters of mercury (mmHg)
499-H4 MEASUREMENT VALUE Q 15Ø/95 Pt is hypertensive
493-XE CLINICAL INFORMATION COUNTER R 2 2nd occurrence
494-ZE MEASUREMENT DATE Q 2ØØ7Ø915 September 15, 2ØØ7
495-H1 MEASUREMENT TIME Q 143Ø Measured at 2:3Øpm
496-H2 MEASUREMENT DIMENSION Q Ø2 Blood Glucose
497-H3 MEASUREMENT UNIT Q Ø8 Milligrams per deciliter (mg/dl)
499-H4 MEASUREMENT VALUE Q 24Ø Pt is hyperglycemic
493-XE CLINICAL INFORMATION COUNTER R 3 3rd occurrence
494-ZE MEASUREMENT DATE Q 2ØØ7Ø915 September 15, 2ØØ7
495-H1 MEASUREMENT TIME Q 113Ø Measured at 11:3Øam
496-H2 MEASUREMENT DIMENSION Q 14 Weight
497-H3 MEASUREMENT UNIT Q Ø3 Pounds (lb)
499-H4 MEASUREMENT VALUE Q 21Ø Pt weighs 21Ø pounds
493-XE CLINICAL INFORMATION COUNTER R 4 4th occurrence
494-ZE MEASUREMENT DATE Q 2ØØ7Ø915 September 15, 2ØØ7
495-H1 MEASUREMENT TIME Q Ø8ØØ Measured at 8:ØØam
496-H2 MEASUREMENT DIMENSION Q 12 Theophylline
497-H3 MEASUREMENT UNIT Q Ø8 Milligrams per deciliter (mg/dl)
499-H4 MEASUREMENT VALUE Q 15 Drug level is therapeutic
493-XE CLINICAL INFORMATION COUNTER R 5 5th occurrence
494-ZE MEASUREMENT DATE Q 2ØØ7Ø915 September 15, 2ØØ7
495-H1 MEASUREMENT TIME Q 153Ø Measured at 3:3Øpm
496-H2 MEASUREMENT DIMENSION Q 17 Creatinine Clearance (CrCl)
497-H3 MEASUREMENT UNIT Q Ø8 Milligrams per deciliter (mg/dl)
499-H4 MEASUREMENT VALUE Q 3.2 Pt has renal failure
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 2 Service billing
4Ø2-D2 PRESCRIPTION/SERVICE M 7654322
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 816 -
REFERENCE NUMBER
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø6 DUR/PPS
4Ø7-D7 PRODUCT/SERVICE ID M Ø
DUR/PPS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø8 DUR/PPS Segment
473-7E DUR/PPS CODE COUNTER R 1 1st DUR/PPS activity follows
439-E4 REASON FOR SERVICE CODE Q TN Laboratory test needed
44Ø-E5 PROFESSIONAL SERVICE CODE Q PT Perform laboratory test
441-E6 RESULT OF SERVICE CODE Q 3E Therapy changed
474-8E DUR/PPS LEVEL OF EFFORT Q 14 High level of complexity
PRICING SEGMENT
FIELD FIELD NAME CAT Value COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
477-BE PROFESSIONAL SERVICE FEE
SUBMITTED
R 2ØØ{ $2Ø.ØØ
426-DQ USUAL AND CUSTOMARY CHARGE Q 2ØØ{ $2Ø.ØØ
43Ø-DU GROSS AMOUNT DUE R 2ØØ{ $2Ø.ØØ
34.9.1 SERVICE BILLING ACCEPTED RESPONSE- PAID (DUPLICATE OF PAID)
Processor accepts billing and pays pharmacist for professional service however the contracted rate is different from that submitted. The
processor does pay the incentive as submitted. RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M S1 Service Billing
1Ø9-A9 TRANSACTION COUNT M 2 Two occurrences
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P OR D Paid or Duplicate of Paid
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 2 Service Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M Ø
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R { $Ø
562-J1 PROFESSIONAL SERVICE FEE PAID R 5Ø{ $5.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 5Ø{ $5.ØØ
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P Paid
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 2 Service Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE M 7654322
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 817 -
NUMBER
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R { $Ø
562-J1 PROFESSIONAL SERVICE FEE PAID R 2ØØ{ $2Ø.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 2ØØ{ $2Ø.ØØ
34.9.2 SERVICE BILLING TRANSMISSION REJECTED RESPONSE
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M S1 Service Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M R Rejected
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbb
bb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M R Rejected
51Ø-FA REJECT COUNT R 1 1 Reject Code follows
511-FB REJECT CODE R Ø1 M/I BIN Number
13Ø-UF ADDITIONAL MESSAGE INFORMATION
COUNT
R 1 1 occurrence
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø1 Used for first line of free form text with no pre-
defined structure.
526-FQ ADDITIONAL MESSAGE INFORMATION Q TRANSACTION
MESSAGE
TEXT
For illustrative purposes only. Up to 4Ø Bytes
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R 3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
34.9.3 SERVICE BILLING TRANSMISSION – ONE REJECTED, ONE PAID RESPONSE
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M S1 Service Billing
1Ø9-A9 TRANSACTION COUNT M 2 Two occurrences
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbb
bb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE MESSAGE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 2Ø RESPONSE MESSAGE SEGMENT
5Ø4-F4 MESSAGE Q TRANSMISSION
MESSAGE
TEXT
For illustrative purposes only. Up to 2ØØ Bytes
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M R Rejected
51Ø-FA REJECT COUNT R 1 1 Reject Code follows
511-FB REJECT CODE R 7Ø Product/Service not covered
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 818 -
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R 3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 2 Service Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 7654321
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P Paid
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 2 Service Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 7654322
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R { $Ø
562-J1 PROFESSIONAL SERVICE FEE PAID R 2ØØ{ $2Ø.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 2ØØ{ $2Ø.ØØ
34.10 COMPOUNDED RX BILLING - TRANSACTION CODE B1 (Ø1)
Billing for Product with DUR.
For this example, the first occurrence of the Compound Ingredient Drug Cost (449-EE) is intentionally missing. This correlates to the rejected
response example that shows a “M/I Compound Ingredient Drug Cost” occurrence 1.
For the Captured/Paid response, assume the $1.2Ø was actually submitted in the Compound Ingredient Drug Cost (449-EE) first occurrence.
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbb
bb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID
M bbbbbbbbbb
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 123456789 Cardholder ID
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE M 1234567
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 819 -
REFERENCE NUMBER
436-E1 PRODUCT/SERVICE ID QUALIFIER M ØØ Default for multi-ingredient compounds
4Ø7-D7 PRODUCT/SERVICE ID M Ø Default for multi-ingredient compounds
442-E7 QUANTITY DISPENSED R 12ØØØØ 12Ø.ØØØml
4Ø3-D3 FILL NUMBER R 1 First dispensing for Rx#
4Ø5-D5 DAYS SUPPLY R 3 3 Days supply
4Ø6-D6 COMPOUND CODE R 2 Compounded Rx
4Ø8-D8 DISPENSE AS WRITTEN
(DAW)/PRODUCT SELECTION
CODE
R Ø No product selection indicated
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ7Ø915 September 15, 2ØØ7
415-DF NUMBER OF REFILLS AUTHORIZED Q 5 5 Refills
419-DJ PRESCRIPTION ORIGIN CODE Q 1 Written prescription
3Ø8-C8 OTHER COVERAGE CODE Q 1 No other coverage
429-DT SPECIAL PACKAGING INDICATOR Q 1 Not unit dose
6ØØ-28 UNIT OF MEASURE Q ML Milliliters
995-E2 ROUTE OF ADMINISTRATION Q 11 Oral
DUR/PPS SEGMENT
FIELD FIELD NAME CAT Value COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø8 DUR/PPS Segment
473-7E DUR/PPS CODE COUNTER R 1 1st DUR action
439-E4 REASON FOR SERVICE CODE Q DD Drug Interaction
44Ø-E5 PROFESSIONAL SERVICE CODE Q RØ Consulted other source
441-E6 RESULT OF SERVICE CODE Q 1B Filled Rx, as is
474-8E DUR/PPS LEVEL OF EFFORT Q 11 Lowest level of complexity
475-J9 DUR CO-AGENT ID QUALIFIER R Ø3 NDC
476-H6 DUR CO-AGENT ID Q Ø4ØØØØØØ216 Ferrous Sulfate 325mg tab
COMPOUND SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 1Ø COMPOUND SEGMENT
45Ø-EF COMPOUND DOSAGE FORM
DESCRIPTION CODE
M 11 Solution
451-EG COMPOUND DISPENSING UNIT
FORM INDICATOR
M 3 Milliliters
447-EC COMPOUND INGREDIENT
COMPONENT COUNT
M Ø3 3 Ingredients
488-RE COMPOUND PRODUCT ID
QUALIFIER
M Ø3 NDC
489-TE COMPOUND PRODUCT ID M 11845Ø139Ø1 Tetracycline 5ØØmg cap
448-ED COMPOUND INGREDIENT
QUANTITY
M 12ØØØ 12 capsules
449-EE COMPOUND INGREDIENT DRUG
COST
Q ($1.2Ø – intentionally left off for rejected response
example to designate an error)
49Ø-UE COMPOUND INGREDIENT BASIS OF
COST DETERMINATION
Q Ø1 AWP
488-RE COMPOUND PRODUCT ID
QUALIFIER
M Ø3 NDC
489-TE COMPOUND PRODUCT ID M ØØ6Ø3148Ø49 Nystatin 1ØØØØØu/ml Susp
448-ED COMPOUND INGREDIENT
QUANTITY
M 12ØØØØ 12Ø.ØØØml
449-EE COMPOUND INGREDIENT DRUG
COST
Q 84{ $8.4Ø
49Ø-UE COMPOUND INGREDIENT BASIS OF
COST DETERMINATION
Q Ø1 AWP
488-RE COMPOUND PRODUCT ID
QUALIFIER
M Ø3 NDC
489-TE COMPOUND PRODUCT ID M 6Ø8Ø9Ø31Ø55 Diphenhydramine 5Ømg cap
448-ED COMPOUND INGREDIENT
QUANTITY
M 24ØØØ 24 capsules
449-EE COMPOUND INGREDIENT DRUG
COST
Q 46{ $4.6Ø
49Ø-UE COMPOUND INGREDIENT BASIS OF
COST DETERMINATION
Q Ø1 AWP
PRICING SEGMENT
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 820 -
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 142{ $14.2Ø
412-DC DISPENSING FEE SUBMITTED Q 15Ø{ $15.ØØ
478-H7 OTHER AMOUNT CLAIMED
SUBMITTED COUNT
R 1 One occurrence
479-H8 OTHER AMOUNT CLAIMED
SUBMITTED QUALIFIER
R Ø1 Delivery Cost
48Ø-H9 OTHER AMOUNT CLAIMED
SUBMITTED
Q 5Ø{ $5.ØØ
426-DQ USUAL AND CUSTOMARY CHARGE Q 311E $31.15
43Ø-DU GROSS AMOUNT DUE R 342{ $34.2Ø
423-DN BASIS OF COST DETERMINATION Q Ø1 AWP
Situational Field 449-EE intentionally not listed
61ØØ66DØB1123456789Ø1Ø14563663bbbbbbbb2ØØ7Ø915bbbbbbbbbb<1E><1C>AMØ4<1C>C2123456789<1C>C96<1D><1E><1C>AMØ
7<1C>EM1<1C>D21234567<1C>E1ØØ<1C>D7Ø<1C>E712ØØØØ<1C>D31<1C>D53<1C>D62<1C>D8Ø<1C>DE2ØØ7Ø915<1C>DF5<1C>DJ1<
1C>NX1<1C>DKØ<1C>C81<1C>DT1<1C>28ML<1C>E211<1E><1C>AMØ8<1C>7E1<1C>E4DD<1C>E5RØ<1C>E61B<1C>8E11<1C>J9Ø3<1C
>H6Ø4ØØØØØØ216<1E><1C>AM1Ø<1C>EF11<1C>EG3<1C>ECØ3<1C>REØ3<1C>TE11845Ø139Ø1<1C>ED12ØØØ<1C>UEØ1<1C>REØ3<1C>
TEØØ6Ø3148Ø49<1C>ED12ØØØØ<1C>EE84{<1C>UEØ1<1C>REØ3<1C>TE6Ø8Ø9Ø31Ø55<1C>ED24ØØØ<1C>EE46{<1C>UEØ1<1E><1C>AM
11<1C>D9142{<1C>DC15Ø{<1C>H71<1C>H8Ø1<1C>H95Ø{<1C>DQ311E<1C>DU342{<1C>DNØ1
34.10.1 COMPOUNDED RX BILLING ACCEPTED RESPONSE- PAID (DUPLICATE OF PAID)
Note: Assume in this example that the $1.2Ø Compound Ingredient Drug Cost was submitted and this is the payment or captured response.
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbb
bb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P OR D Paid (or Duplicate of Paid)
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 5Ø{ $5.ØØ
5Ø6-F6 INGREDIENT COST PAID Q 142{ $14.2Ø
5Ø7-F7 DISPENSING FEE PAID Q 15Ø{ $15.ØØ
557-AV TAX EXEMPT INDICATOR Q 1 Payer/Plan is Tax Exempt (The Payer/Plan is not
responsible for tax. The patient may be charged tax.)
563-J2 OTHER AMOUNT PAID COUNT R 1 One occurrence
564-J3 OTHER AMOUNT PAID QUALIFIER R Ø1 Delivery
565-J4 OTHER AMOUNT PAID Q 5Ø{ $5.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 292{ $29.2Ø
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 1 Ingredient Cost Paid as Submitted
Note: Assume in this example that the $1.2Ø Compound Ingredient Drug Cost was submitted and this is the payment response.
Example with Paid Response
DØB11AØ14563663bbbbbbbb2ØØ7Ø915<1D><1E><1C>AM21<1C>ANP<1E><1C>AM22<1C>EM1<1C>D21234567<1E><1C>AM23<1C>F55
Ø{<1C>F6142{<1C>F715Ø{<1C>AV1<1C>J21<1C>J3Ø1<1C>J45Ø{<1C>F9292{<1C>FM1
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 821 -
34.10.2 COMPOUNDED RX BILLING REJECTED RESPONSE
Billing rejected for processor-identified DUR conflict.
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbb
bb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M R Rejected
51Ø-FA REJECT COUNT R 2 2 Reject Codes follow
511-FB REJECT CODE R 88 DUR reject
546-4F REJECT FIELD OCCURRENCE
INDICATOR
R 3 Ingred #3: Diphenhydramine
511-FB REJECT CODE R EE M/I Compound Ingredient Drug Cost
546-4F REJECT FIELD OCCURRENCE
INDICATOR
R 3 Ingred #3: Diphenhydramine
5Ø3-F3 AUTHORIZATION NUMBER Q 1234567891234
56789
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
RESPONSE DUR/PPS SEGMENT
FIELD FIELD NAME CAT Value COMMENTS
111-AM SEGMENT IDENTIFICATION M 24 RESPONSE DUR/PPS SEGMENT
567-J6 DUR/PPS RESPONSE CODE
COUNTER
R 1 1st DUR conflict follows
439-E4 REASON FOR SERVICE CODE Q HD High Dose alert
532-FW DATABASE INDICATOR Q 5 Other
544-FY DUR FREE TEXT Q MAX
DOSE=6/DAY (Up to 3Ø bytes)
Note: Assume in this example that the Compound Ingredient Drug Cost of $1.2Ø was not included in the submission (as shown above). The
rejected response correlates with that missing field.
DØB11AØ14563663bbbbbbbb2ØØ7Ø915<1D><1E><1C>AM21<1C>ANR<1C>FA2<1C>FB88<1C>4F3<1C>FBEE<1C>4F3<1C>F312345678
9123456789<1C>7FØ3<1C>8F6Ø2357Ø862<1E><1C>AM22<1C>EM1<1C>D21234567<1E><1C>AM24<1C>J61<1C>E4HD<1C>FW5<1C>F
YMAXDOSE=6/DAY
34.10.3 BILLING RESUBMISSION W/DUR RESOLUTION
Pharmacist reduces dose of diphenhydramine and resubmits claim.
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbb
bb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 822 -
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID
M 98765bbbbb
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 123456789
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID QUALIFIER M ØØ Default for multi-ingredient compounds
4Ø7-D7 PRODUCT/SERVICE ID M Ø Default for multi-ingredient compounds
442-E7 QUANTITY DISPENSED R 12ØØØØ 12Ø.ØØØml
4Ø3-D3 FILL NUMBER R 1 First dispensing for Rx#
4Ø5-D5 DAYS SUPPLY R 3 3 Days supply
4Ø6-D6 COMPOUND CODE R 2 Compounded Rx
4Ø8-D8 DISPENSE AS WRITTEN
(DAW)/PRODUCT SELECTION
CODE
R Ø No product selection indicated
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ7Ø915 September 15, 2ØØ7
995-E2 ROUTE OF ADMINISTRATION Q 11 Oral
DUR/PPS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø8 DUR/PPS Segment
473-7E DUR/PPS CODE COUNTER R 1 1st DUR action
439-E4 REASON FOR SERVICE CODE Q HD High Dose alert
44Ø-E5 PROFESSIONAL SERVICE CODE Q MØ Prescriber consulted
441-E6 RESULT OF SERVICE CODE Q 1C Filled with different dose
474-8E DUR/PPS LEVEL OF EFFORT Q 11 Lowest level of complexity
COMPOUND SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 1Ø COMPOUND SEGMENT
45Ø-EF COMPOUND DOSAGE FORM
DESCRIPTION CODE
M 11 Solution
451-EG COMPOUND DISPENSING UNIT
FORM INDICATOR
M 3 Milliliters
447-EC COMPOUND INGREDIENT
COMPONENT COUNT
M Ø3 3 Ingredients
488-RE COMPOUND PRODUCT ID
QUALIFIER
M Ø3 NDC
489-TE COMPOUND PRODUCT ID M 11845Ø139Ø1 Tetracycline 5ØØmg cap
448-ED COMPOUND INGREDIENT
QUANTITY
M 12ØØØ 12 capsules
449-EE COMPOUND INGREDIENT DRUG
COST
Q 12{ $1.2Ø
49Ø-UE COMPOUND INGREDIENT BASIS OF
COST DETERMINATION
Q Ø1 AWP
488-RE COMPOUND PRODUCT ID
QUALIFIER
M Ø3 NDC
489-TE COMPOUND PRODUCT ID M ØØ6Ø3148Ø49 Nystatin 1ØØØØØu/ml Susp
448-ED COMPOUND INGREDIENT
QUANTITY
M 12ØØØØ 12Ø.ØØØml
449-EE COMPOUND INGREDIENT DRUG
COST
Q 84{ $8.4Ø
49Ø-UE COMPOUND INGREDIENT BASIS OF
COST DETERMINATION
Q Ø1 AWP
488-RE COMPOUND PRODUCT ID
QUALIFIER
M Ø3 NDC
489-TE COMPOUND PRODUCT ID M 6Ø8Ø9Ø31Ø55 Diphenhydramine 5Ømg cap
448-ED COMPOUND INGREDIENT M 12ØØØ 12 capsules
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 823 -
QUANTITY
449-EE COMPOUND INGREDIENT DRUG
COST
Q 23{ $2.3Ø
49Ø-UE COMPOUND INGREDIENT BASIS OF
COST DETERMINATION
Q Ø1 AWP
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 119{ $11.9Ø
412-DC DISPENSING FEE SUBMITTED Q 15Ø{ $15.ØØ
478-H7 OTHER AMOUNT CLAIMED
SUBMITTED COUNT
R 1 One occurrence
479-H8 OTHER AMOUNT CLAIMED
SUBMITTED QUALIFIER
Q Ø1 Delivery Cost
48Ø-H9 OTHER AMOUNT CLAIMED
SUBMITTED
Q 5Ø{ $5.ØØ
426-DQ USUAL AND CUSTOMARY CHARGE Q 288E $28.85
43Ø-DU GROSS AMOUNT DUE R 269{ $26.9Ø
423-DN BASIS OF COST DETERMINATION Q Ø1 AWP
34.10.4 BILLING RESUBMISSION W/DUR ACCEPTED RESPONSE- PAID (DUPLICATE OF
PAID) RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 2 Two occurrences
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbb
bb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P OR D Paid or Duplicate of Paid
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 5Ø{ $5.ØØ
5Ø6-F6 INGREDIENT COST PAID Q 119{ $11.9Ø
5Ø7-F7 DISPENSING FEE PAID Q 15Ø{ $15.ØØ
557-AV TAX EXEMPT INDICATOR Q 1 Payer/Plan is Tax Exempt (The Payer/Plan is not
responsible for tax. The patient may be charged tax.)
563-J2 OTHER AMOUNT PAID COUNT R 1 One occurrence
564-J3 OTHER AMOUNT PAID QUALIFIER R Ø1 Delivery
565-J4 OTHER AMOUNT PAID Q 5Ø{ $5.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 269{ $26.9Ø
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 1 Ingredient Cost Paid as Submitted
34.11 BILLING, PARTIAL FILL-INITIAL - TRANSACTION CODE B1
TRANSACTION HEADER SEGMENT
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 824 -
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbb
b
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID
M 98765bbbbb
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø1 PATIENT SEGMENT
331-CX PATIENT ID QUALIFIER R Ø1 Social Security Number
332-CY PATIENT ID Q 123456789 Patient’s SSN
3Ø4-C4 DATE OF BIRTH R 1962Ø615 Born June 15, 1962
3Ø5-C5 PATIENT GENDER CODE R 1 Male
31Ø-CA PATIENT FIRST NAME R JOSEPH
311-CB PATIENT LAST NAME R SMITH
322-CM PATIENT STREET ADDRESS O 123 MAIN
STREET
323-CN PATIENT CITY ADDRESS O MY TOWN
324-CO PATIENT STATE/PROVINCE
ADDRESS
O CO
325-CP PATIENT ZIP/POSTAL ZONE O 34567
326-CQ PATIENT PHONE NUMBER O 2Ø14658923
3Ø7-C7 PLACE OF SERVICE Q 1 Pharmacy
333-CZ EMPLOYER ID Q 5ØZ123
35Ø-HN PATIENT’S E-MAIL ADDRESS I JSMITH@NCPDP
.ORG Patient’s E-Mail Address
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 987654321 Cardholder ID
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
442-E7 QUANTITY DISPENSED R 15ØØØ 15.ØØØ tablets
4Ø3-D3 FILL NUMBER R Ø Original dispensing for RX#
4Ø5-D5 DAYS SUPPLY R 15 15 Days supply
4Ø6-D6 COMPOUND CODE R 1 Not a compound
4Ø8-D8 DISPENSE AS WRITTEN
(DAW)/PRODUCT SELECTION
CODE
R Ø No product selection indicated
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ7Ø915 September 15, 2ØØ7
415-DF NUMBER OF REFILLS AUTHORIZED Q 5 5 Refills
419-DJ PRESCRIPTION ORIGIN CODE Q 1 Written prescription
3Ø8-C8 OTHER COVERAGE CODE Q 1 No other coverage
429-DT SPECIAL PACKAGING INDICATOR Q 1 Not unit dose
6ØØ-28 UNIT OF MEASURE Q EA Each
343-HD DISPENSING STATUS R P Partial Fill
344-HF QUANTITY INTENDED TO BE
DISPENSED
R 3ØØØØ 3Ø.ØØØ tablets
345-HG DAYS SUPPLY INTENDED TO BE
DISPENSED
R 3Ø 3Ø days
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 825 -
PHARMACY PROVIDER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø2 PHARMACY PROVIDER SEGMENT
465-EY PROVIDER ID QUALIFIER R Ø5 National Provider ID
444-E9 PROVIDER ID Q 3935933111
PRESCRIBER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø3 PRESCRIBER SEGMENT
466-EZ PRESCRIBER ID QUALIFIER R Ø1 National Provider ID
411-DB PRESCRIBER ID Q ØØ12345
427-DR PRESCRIBER LAST NAME Q JONES
498-PM PRESCRIBER TELEPHONE
NUMBER
Q 2Ø13639572
468-2E PRIMARY CARE PROVIDER ID
QUALIFIER
R Ø1 National Provider ID
421-DL PRIMARY CARE PROVIDER ID Q 1234566111
47Ø-4E PRIMARY CARE PROVIDER LAST
NAME
Q WRIGHT
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 278E $27.85
412-DC DISPENSING FEE SUBMITTED Q 5Ø{ $5.ØØ
481-HA FLAT SALES TAX AMOUNT
SUBMITTED
Q 1Ø{ $1.ØØ
426-DQ USUAL AND CUSTOMARY CHARGE Q 376E $37.65
43Ø-DU GROSS AMOUNT DUE R 338E $33.85
423-DN BASIS OF COST DETERMINATION Q Ø3 Direct
34.11.1 BILLING, INITIAL PARTIAL FILL ACCEPTED RESPONSE- PAID (DUPLICATE OF
PAID) RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbb
b
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE MESSAGE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 2Ø RESPONSE MESSAGE SEGMENT
5Ø4-F4 MESSAGE Q TRANSMISSION
MESSAGE TEXT For illustrative purposes only. Up to 2ØØ Bytes
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P or D Paid or Duplicate of Paid
5Ø3-F3 AUTHORIZATION NUMBER Q 12345678912345
6789
13Ø-UF ADDITIONAL MESSAGE
INFORMATION COUNT
R 1 1 occurrence
132-UH ADDITIONAL MESSAGE
INFORMATION QUALIFIER
R Ø1 Used for first line of free form text with no pre-
defined structure.
526-FQ ADDITIONAL MESSAGE
INFORMATION
Q TRANSACTION
MESSAGE TEXT For illustrative purposes only. Up to 4Ø Bytes
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 826 -
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
551-9F PREFERRED PRODUCT COUNT M 1 1 Preferred product identified
552-AP PREFERRED PRODUCT ID
QUALIFIER
M Ø3 NDC
553-AR PREFERRED PRODUCT ID M 17236Ø569Ø1 Ibuprofen 6ØØmg tablet
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 5Ø{ $5.ØØ
5Ø6-F6 INGREDIENT COST PAID Q 278E $27.85
5Ø7-F7 DISPENSING FEE PAID Q 5Ø{ $5.ØØ
557-AV TAX EXEMPT INDICATOR Q 3 Patient is tax exempt (The patient cannot be
charged tax.)
558-AW FLAT SALES TAX AMOUNT PAID Q 1Ø{ $1.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 288E $28.85
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 1 Ingredient cost paid as submitted
518-FI AMOUNT OF COPAY Q 5Ø{ $5.ØØ
346-HH BASIS OF CALCULATION-
DISPENSING FEE
R Ø3 U&C-prorated
347-HJ BASIS OF CALCULATION-COPAY R Ø1 Quantity dispensed
348-HK BASIS OF CALCULATION-FLAT
SALES TAX
R Ø1 Quantity dispensed
574-2Y PLAN SALES TAX AMOUNT Q 1Ø{ $1.ØØ
34.12 BILLING, PARTIAL FILL-COMPLETION - TRANSACTION CODE B1
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbb
b
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID
M 98765bbbbb
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø1 PATIENT SEGMENT
331-CX PATIENT ID QUALIFIER R Ø1 Social Security Number
332-CY PATIENT ID Q 123456789 Patient’s SSN
3Ø4-C4 DATE OF BIRTH R 1962Ø615 Born June 15, 1962
3Ø5-C5 PATIENT GENDER CODE R 1 Male
31Ø-CA PATIENT FIRST NAME R JOSEPH
311-CB PATIENT LAST NAME R SMITH
322-CM PATIENT STREET ADDRESS O 123 MAIN
STREET
323-CN PATIENT CITY ADDRESS O MY TOWN
324-CO PATIENT STATE/PROVINCE
ADDRESS
O CO
325-CP PATIENT ZIP/POSTAL ZONE O 34567
326-CQ PATIENT PHONE NUMBER O 2Ø14658923
3Ø7-C7 PLACE OF SERVICE Q 1 Pharmacy
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 827 -
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 987654321 Cardholder ID
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234568
436-E1 PRODUCT/SERVICE ID
QUALIFIER
M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
457-EP ASSOCIATED
PRESCRIPTION/SERVICE DATE
R 2ØØ7Ø914 September 14, 2ØØ7
456-EN ASSOCIATED
PRESCRIPTION/SERVICE
REFERENCE NUMBER
R 1234567 Rx # for “P” transaction
442-E7 QUANTITY DISPENSED R 15ØØØ 15.ØØØ tablets
4Ø3-D3 FILL NUMBER R Ø Original dispensing for RX#
4Ø5-D5 DAYS SUPPLY R 15 15 Days supply
4Ø6-D6 COMPOUND CODE R 1 Not a compound
4Ø8-D8 DISPENSE AS WRITTEN
(DAW)/PRODUCT SELECTION
CODE
R Ø No product selection indicated
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ7Ø915 September 15, 2ØØ7
415-DF NUMBER OF REFILLS
AUTHORIZED
Q 5 5 Refills
419-DJ PRESCRIPTION ORIGIN CODE Q 1 Written prescription
3Ø8-C8 OTHER COVERAGE CODE Q 1 No other coverage
429-DT SPECIAL PACKAGING INDICATOR Q 1 Not unit dose
6ØØ-28 UNIT OF MEASURE Q EA Each
343-HD DISPENSING STATUS R C Completion of partial fill
344-HF QUANTITY INTENDED TO BE
DISPENSED
R 3ØØØØ 3Ø.ØØØ tablets
345-HG DAYS SUPPLY INTENDED TO BE
DISPENSED
R 3Ø 3Ø days
PHARMACY PROVIDER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø2 PHARMACY PROVIDER SEGMENT
465-EY PROVIDER ID QUALIFIER R Ø5 National Provider ID
444-E9 PROVIDER ID Q 3935933111
PRESCRIBER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø3 PRESCRIBER SEGMENT
466-EZ PRESCRIBER ID QUALIFIER R Ø1 National Provider ID
411-DB PRESCRIBER ID Q ØØ12345111
427-DR PRESCRIBER LAST NAME Q JONES
498-PM PRESCRIBER TELEPHONE
NUMBER
Q 2Ø13639572
468-2E PRIMARY CARE PROVIDER ID
QUALIFIER
R Ø1 National Provider ID
421-DL PRIMARY CARE PROVIDER ID Q 1234566111
47Ø-4E PRIMARY CARE PROVIDER LAST
NAME
Q WRIGHT
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 278E $27.85
412-DC DISPENSING FEE SUBMITTED Q 5Ø{ $5.ØØ
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 828 -
433-DX PATIENT PAID AMOUNT
SUBMITTED
Q 5Ø{ $5.ØØ
481-HA FLAT SALES TAX AMOUNT
SUBMITTED
Q 1Ø{ $1.ØØ
426-DQ USUAL AND CUSTOMARY
CHARGE
Q 376E $37.65
43Ø-DU GROSS AMOUNT DUE R 338E $33.85
423-DN BASIS OF COST DETERMINATION Q Ø3 Direct
34.12.1 BILLING, COMPLETION PARTIAL FILL ACCEPTED RESPONSE- PAID
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbb
b
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE MESSAGE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 2Ø RESPONSE MESSAGE SEGMENT
5Ø4-F4 MESSAGE 1 TRANSMISSION
MESSAGE TEXT For illustrative purposes only. Up to 2ØØ Bytes
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P or D Paid or Duplicate of Paid
5Ø3-F3 AUTHORIZATION NUMBER Q 12345678912345
6789
13Ø-UF ADDITIONAL MESSAGE
INFORMATION COUNT
R 1 1 occurrence
132-UH ADDITIONAL MESSAGE
INFORMATION QUALIFIER
R Ø1 Used for first line of free form text with no pre-
defined structure.
526-FQ ADDITIONAL MESSAGE
INFORMATION
Q TRANSACTION
MESSAGE TEXT For illustrative purposes only. Up to 4Ø Bytes
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234568
551-9F PREFERRED PRODUCT COUNT M 1 1 Preferred product identified
552-AP PREFERRED PRODUCT ID
QUALIFIER
M Ø3 NDC
553-AR PREFERRED PRODUCT ID M 17236Ø569Ø1 Ibuprofen 6ØØmg tablet
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 5Ø{ $5.ØØ
5Ø6-F6 INGREDIENT COST PAID Q 278E $27.85
5Ø7-F7 DISPENSING FEE PAID Q 5Ø{ $5.ØØ
557-AV TAX EXEMPT INDICATOR Q 3 Patient is tax exempt (The patient cannot be
charged tax.)
558-AW FLAT SALES TAX AMOUNT PAID Q 1Ø{ $1.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 288E $28.85
522-FM BASIS OF REIMBURSEMENT R 1 Ingredient cost paid as submitted

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 829 -
DETERMINATION
518-FI AMOUNT OF COPAY Q 5Ø{ $5.ØØ
346-HH BASIS OF CALCULATION-
DISPENSING FEE
R Ø3 U&C-prorated
347-HJ BASIS OF CALCULATION-COPAY R Ø1 Quantity dispensed
348-HK BASIS OF CALCULATION-FLAT
SALES TAX
R Ø1 Quantity dispensed
574-2Y PLAN SALES TAX AMOUNT Q 1Ø{ $1.ØØ
34.13 REVERSAL – PARTIAL FILL TRANSACTIONS
If both “P” and “C” transactions have been accepted by the processor, always reverse the “C” transaction before reversing the “P” transaction.
34.14 WORKERS’ COMPENSATION BILLING - TRANSACTION CODE B1
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø7 NCPDP Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663bbbbbbb
b
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID
M bbbbbbbbbb
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 123456789 Cardholder ID
WORKERS’ COMPENSATION SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø6 WORKERS’ COMPENSATION SEGMENT
434-DY DATE OF INJURY M 2ØØ7Ø9Ø1 September 1, 2ØØ7
315-CF EMPLOYER NAME Q MA BELL
316-CG EMPLOYER STREET ADDRESS Q 1234 CAPITOL
AVENUE
317-CH EMPLOYER CITY ADDRESS Q BELLTOWN
318-CI EMPLOYER STATE/PROVINCE
ADD
Q UT
319-CJ EMPLOYER ZIP/POSTAL ZONE Q 88888
32Ø-CK EMPLOYER PHONE NUMBER Q 8Ø49786421
327-CR CARRIER ID Q 9Ø87654321
435-DZ CLAIM/REFERENCE ID Q AA181114
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID
QUALIFIER
M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
442-E7 QUANTITY DISPENSED R 3ØØØØ 3Ø.ØØØ
4Ø3-D3 FILL NUMBER R Ø Original dispensing for RX#
4Ø5-D5 DAYS SUPPLY R 3Ø 3Ø Days supply
4Ø6-D6 COMPOUND CODE R 1 Not a compound
4Ø8-D8 DISPENSE AS WRITTEN
(DAW)/PRODUCT SELECTION
CODE
R Ø No product selection indicated
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 830 -
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ7Ø915 September 15, 2ØØ7
415-DF NUMBER OF REFILLS
AUTHORIZED
Q 5 5 Refills
419-DJ PRESCRIPTION ORIGIN CODE Q 1 Written prescription
429-DT SPECIAL PACKAGING INDICATOR Q 1 Not unit dose
6ØØ-28 UNIT OF MEASURE Q EA Each
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 557{ $55.7Ø
412-DC DISPENSING FEE SUBMITTED Q 1ØØØ{ $1Ø.ØØ
478-H7 OTHER AMOUNT CLAIMED
SUBMITTED COUNT
R 1 One occurrence
479-H8 OTHER AMOUNT CLAIMED
SUBMITTED QUALIFIER
R Ø4 Administrative Charge
48Ø-H9 OTHER AMOUNT CLAIMED
SUBMITTED
Q 15Ø{ $15.ØØ
426-DQ USUAL AND CUSTOMARY
CHARGE
Q 8Ø7{ $8Ø.7Ø
43Ø-DU GROSS AMOUNT DUE R 8Ø7{ $8Ø.7Ø
423-DN BASIS OF COST DETERMINATION Q Ø3 Direct
34.14.1 WORKERS’ COMPENSATION BILLING ACCEPTED RESPONSE- PAID (DUPLICATE
OF PAID) RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø7 NCPDP Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663bbbbbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P OR D Paid or Duplicate of Paid
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
551-9F PREFERRED PRODUCT COUNT M 1 1 Preferred product identified
552-AP PREFERRED PRODUCT ID
QUALIFIER
M Ø3 NDC
553-AR PREFERRED PRODUCT ID M 17236Ø569Ø1 Ibuprofen 6ØØmg tablet
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 24 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R Ø{ $Ø.ØØ
5Ø6-F6 INGREDIENT COST PAID Q 557{ $55.7Ø
5Ø7-F7 DISPENSING FEE PAID Q 1ØØ{ $1Ø.ØØ
557-AV TAX EXEMPT INDICATOR Q 1 Payer/Plan is Tax Exempt (The Payer/Plan is not
responsible for tax. The patient may be charged
tax.)
563-J2 OTHER AMOUNT PAID COUNT R 1 One occurrence
564-J3 OTHER AMOUNT PAID QUALIFIER R Ø4 Administrative
565-J4 OTHER AMOUNT PAID Q 15Ø{ $15.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 8Ø7{ $8Ø.7Ø
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 831 -
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 5 MAC pricing ingredient cost paid
34.15 BILLING W/COUPON (FREE PRODUCT) - TRANSACTION CODE B1-BILLING
TO COUPON PROCESSOR
In which the coupon number is in the Coupon Segment, and includes a Patient Segment. This is an example of a manufacturer’s coupon. One
coupon is to be used per member or per family for which cardholder and patient information is required in processing of the benefit. There is a
generic coupon number assigned, for example from a magazine, and the coupon is for a Free Product (Coupon Type Qualifier).
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ6Ø22Ø February 2Ø, 2ØØ6
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID
M 98765bbbbb
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 123456789 ID as required by coupon processor
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø1 PATIENT SEGMENT
3Ø4-C4 DATE OF BIRTH R 1962Ø615 Born June 15, 1962
3Ø5-C5 PATIENT GENDER CODE R 1 Male
31Ø-CA PATIENT FIRST NAME R JOSEPH
311-CB PATIENT LAST NAME R SMITH
322-CM PATIENT STREET ADDRESS O 123 MAIN
STREET
323-CN PATIENT CITY ADDRESS O MY TOWN
324-CO PATIENT STATE/PROVINCE
ADDRESS
O CO
325-CP PATIENT ZIP/POSTAL ZONE O 34567
326-CQ PATIENT PHONE NUMBER O 1962Ø615 Born June 15, 1962
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID
QUALIFIER
M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
Any other applicable claim
fields
COUPON SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø9 COUPON SEGMENT
485-KE COUPON TYPE QUALIFIER M Ø2 Free product
486-ME COUPON NUMBER M 123451234512345
In the case of a Free Product, the Usual And Customary of the fill and/or contract rate should be used to determine payment to provider. The
coupon generally will have no stated value so in this example we have NOT included a Coupon Value Amount. If the Coupon Value was
submitted for a free product is it assumed that the value matches the Usual And Customary value.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 832 -
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 587{ $58.7Ø
426-DQ USUAL AND CUSTOMARY
CHARGE
Q 587{ $58.7Ø
43Ø-DU GROSS AMOUNT DUE R 587{ $58.7Ø
423-DN BASIS OF COST DETERMINATION Q Ø7 Usual and Customary
Billing is for Usual And Customary so dispensing fee is not submitted.
34.15.1 BILLING W/COUPON (FREE PRODUCT) ACCEPTED RESPONSE- PAID (DUPLICATE
OF PAID) RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ6Ø22Ø February 2Ø, 2ØØ6
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P or D Paid or Duplicate of Paid
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R ØØØ{ $ØØ.ØØ
5Ø6-F6 INGREDIENT COST PAID Q 587{ $58.7Ø
5Ø9-F9 TOTAL AMOUNT PAID R 587{ $58.7Ø
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 4 Usual And Customary Paid as Submitted
In above payment response, provider is paid the Usual And Customary as submitted. Dispensing Fee or other Fees may be made to provider
depending on contractual agreements.
34.16 BILLING TO A COUPON PROCESSOR TO REDUCE A PATIENT
RESPONSIBILITY AMOUNT
Billing has occurred to a Third Party which returned Patient Pay Amount. If allowed, the coupon can be used to reduce a patient’s
responsibility amount.
Payment from Prior ‘primary’ billing was as follows:
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 357{ $35.7Ø
5Ø6-F6 INGREDIENT COST PAID Q 587{ $58.7Ø
5Ø7-F7 DISPENSING FEE PAID Q 2Ø{ $2.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 25Ø{ $25.ØØ
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 1 Ingredient Cost Paid as Submitted
517-FH AMOUNT APPLIED TO PERIODIC
DEDUCTIBLE
Q 11Ø{ $11.ØØ
518-FI AMOUNT OF COPAY Q 1ØØ{ $1Ø.ØØ
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 833 -
134-UK AMOUNT ATTRIBUTED TO
PRODUCT SELECTION/BRAND
DRUG
Q 147{ $14.7Ø
Balancing Data:
Ingredient Cost Paid 58.7Ø Patient Pay Amount 35.7Ø Deductible 11.ØØ
Dispensing Fee Paid 2.ØØ Total Amount Paid 25.ØØ Copay 1Ø.ØØ
Net 6Ø.7Ø Net 6Ø.7Ø Product Selection 14.7Ø
Patient Pay Amount 35.7Ø
34.16.1 BILL “PATIENT RESPONSIBILITY AMOUNT” TO COUPON PROCESSOR USING THE
PATIENT PAY AMOUNT (5Ø5-F5) AS RETURNED BY PRIOR PAYER
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 75Ø267
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Rx Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbb
b
4Ø1-D1 DATE OF SERVICE M 2ØØ6Ø22Ø February 2Ø, 2ØØ6
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID
M 98765bbbbb
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 123456789A11 ID as required by Coupon Processor
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø1 PATIENT SEGMENT
3Ø4-C4 DATE OF BIRTH R 1962Ø615 Born June 15, 1962
3Ø5-C5 PATIENT GENDER CODE R 1 Male
31Ø-CA PATIENT FIRST NAME R JOSEPH
311-CB PATIENT LAST NAME R SMITH
322-CM PATIENT STREET ADDRESS O 123 MAIN
STREET
323-CN PATIENT CITY ADDRESS O MY TOWN
324-CO PATIENT STATE/PROVINCE
ADDRESS
O CO
325-CP PATIENT ZIP/POSTAL ZONE O 34567
326-CQ PATIENT PHONE NUMBER O 1962Ø615 Born June 15, 1962
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID
QUALIFIER
M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
442-E7 QUANTITY DISPENSED R 3ØØØØ 3Ø.ØØØ tablets
4Ø3-D3 FILL NUMBER R Ø Original dispensing for RX#
4Ø5-D5 DAYS SUPPLY R 3Ø 3Ø Days supply
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 834 -
4Ø6-D6 COMPOUND CODE R 1 Not a compound
4Ø8-D8 DISPENSE AS WRITTEN
(DAW)/PRODUCT SELECTION
CODE
R Ø No product selection indicated
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ6Ø22Ø February 2Ø, 2ØØ6
46Ø-ET QUANTITY PRESCRIBED Q 3ØØØØ 3Ø.ØØØ
3Ø8-C8 OTHER COVERAGE CODE Q 8 Claim is billing for patient financial responsibility
NOTE: Inclusion of Other Coverage Code requires processor to look to Coordination of Benefits/Other Payments Segment for further
information regarding payment by a prior payer.
COUPON SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø9 COUPON SEGMENT
485-KE COUPON TYPE QUALIFIER M Ø1 Price Discount
486-ME COUPON NUMBER M 123451234512345
487-NE COUPON VALUE AMOUNT Q 1ØØ{ $1Ø.ØØ
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 587{ $58.7Ø
426-DQ USUAL AND CUSTOMARY
CHARGE
Q 587{ $58.7Ø
43Ø-DU GROSS AMOUNT DUE R 587{ $58.7Ø
423-DN BASIS OF COST DETERMINATION Q Ø7 Usual And Customary
NOTE: When billing as Coordination of Benefits – Pricing Segment appears as this would be IF the claim were primary. Due to inclusion of
Other Coverage Code in the Claim Segment, the Coordination of Benefits/Other Payments Segment must be used to determine result of prior
claim billing.
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø5 COORDINATION OF BENEFITS/OTHER
PAYMENTS SEGMENT
337-4C COORDINATION OF
BENEFITS/OTHER PAYMENTS
COUNT
M 1 One occurrence
338-5C OTHER PAYER COVERAGE TYPE M Ø1 Primary
339-6C OTHER PAYER ID QUALIFIER R Ø3 BIN #
34Ø-7C OTHER PAYER ID Q 123456
443-E8 OTHER PAYER DATE Q 2ØØ6Ø22Ø February 2Ø, 2ØØ6
353-NR OTHER PAYER-PATIENT
RESPONSIBILITY AMOUNT COUNT
Q 1 One occurrence
351-NP OTHER PAYER-PATIENT
RESPONSIBILITY AMOUNT
QUALIFIER
Q Ø6 Patient pay amount as reported
by previous payer.
352-NQ OTHER PAYER-PATIENT
RESPONSIBILITY AMOUNT
Q 357{ $35.7Ø
NOTE: For COB Patient Responsibility reporting, it is recommended that providers should always send component parts of Patient Pay
Amount unless the prior payer has not provided component details that summarize to 5Ø5-F5 – Patient Pay Amount.
However COUPON processing, while generally using the COB Patient Responsibility Only methodology, is not a ‘coordinated benefit’. For
this type of processing, the suggested method of billing is to report Patient Pay Amount as reported by Previous Payer.
34.16.2 BILLING W/COUPON ACCEPTED RESPONSE—PAID
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 835 -
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ6Ø22Ø February 2Ø, 2ØØ6
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P or D Paid or Duplicate of Paid
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 257{ $25.7Ø ($1Ø less than total Patient Pay Amount
submitted)
5Ø6-F6 INGREDIENT COST PAID Q 357{ $35.7Ø
5Ø7-F7 DISPENSING FEE PAID Q ØØ{ $Ø.ØØ Fee may be paid per trading partner
agreement. In this example no fee applies.
5Ø9-F9 TOTAL AMOUNT PAID R 1ØØ{ $1Ø.ØØ Coupon + Fee
518-FI AMOUNT OF COPAY Q 257{ $25.7Ø
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 16 Coupon Payment
For a Patient Responsibility Only claim, coupon processors are not required to return the below fields that ARE required when this method is
used for true Coordination of Benefit processing:
148-U8 INGREDIENT COST CONTRACTED/
REIMBURSABLE AMOUNT
I Not used
149-U9 DISPENSING FEE CONTRACTED/
REIMBURSABLE AMOUNT
I Not used
Balancing Data:
Ingredient Cost Paid 35.7Ø Patient Pay
Amount
25.7Ø Ingredient Cost Paid 35.7Ø
Dispensing Fee Paid Ø.ØØ Total Amount Paid 1Ø.ØØ Dispensing Fee Paid Ø.ØØ
MINUS
Patient Pay Amount
-25.7Ø
Total 35.7Ø Total 35.7Ø Total Amount Paid 1Ø.ØØ
34.17 REVERSAL - TRANSACTION CODE B2
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B2 Reversal
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbb
b
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID
M 98765bbbbb
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 836 -
436-E1 PRODUCT/SERVICE ID
QUALIFIER
M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
61ØØ66DØB2123456789Ø1Ø14563663bbbbbbbb2ØØ7Ø91598765bbbbb<1D><1E><1C>AMØ7<1C>EM1<1C>D21234567<1C>E1Ø3<1C>D
7ØØØØ6Ø94268
34.17.1 REVERSAL WITH SITUATIONAL FIELDS SUBMITTED - TRANSACTION CODE B2
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B2 Reversal
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbb
b
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID
M 98765bbbbb
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 123456789 Cardholder ID
3Ø1-C1 GROUP ID Q MX468 Group ID
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID
QUALIFIER
M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
DUR/PPS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø8 DUR/PPS Segment
473-7E DUR/PPS CODE COUNTER R 1 1st DUR action
439-E4 REASON FOR SERVICE CODE Q MS Missing Information / Clarification
44Ø-E5 PROFESSIONAL SERVICE CODE Q MØ Prescriber consulted
441-E6 RESULT OF SERVICE CODE Q 2A Prescription Not Filled
474-8E DUR/PPS LEVEL OF EFFORT Q 11 Lowest level of complexity
61ØØ66DØB2123456789Ø1Ø14563663bbbbbbbb2ØØ7Ø91598765bbbbb<1E><1C>AMØ4<1C>C2123456789<1C>C1MX468<1D><1E><1C
>AMØ7<1C>EM1<1C>D21234567<1C>E1Ø3<1C>D7ØØØØ6Ø94268<1E><1C>AMØ8<1C>7E1<1C>E4MS<1C>E5MØ<1C>E62A<1C>8E11
34.17.2 REVERSAL ACCEPTED RESPONSE-CAPTURED, APPROVED
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B2 Reversal
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbb
b
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE MESSAGE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 2Ø RESPONSE MESSAGE SEGMENT
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 837 -
5Ø4-F4 MESSAGE Q TRANSMISSION
MESSAGE TEXT For illustrative purposes only. Up to 2ØØ Bytes
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M A or C Approved or Captured
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
Examples shows Captured response
DØB21AØ14563663bbbbbbbb2ØØ7Ø915<1E><1C>AM2Ø<1C>F4TRANSMISSION MESSAGE TEXT<1D><1E><1C>AM21<1C>ANC<1E><1C>
AM22<1C>EM1<1C>D21234567
Approved Response might contain: RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
521-FL INCENTIVE AMOUNT PAID R 3Ø{ $3.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 3Ø{ $3.ØØ
34.17.3 REVERSAL ACCEPTED RESPONSE-DUPLICATE OF APPROVED
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B2 Reversal
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbb
b
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE MESSAGE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 2Ø RESPONSE MESSAGE SEGMENT
5Ø4-F4 MESSAGE Q TRANSMISSION
MESSAGE TEXT For illustrative purposes only. Up to 2ØØ Bytes
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M S Duplicate of Approved
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
DØB21AØ14563663bbbbbbbb2ØØ7Ø915<1E><1C>AM2Ø<1C>F4TRANSMISSION MESSAGE TEXT<1D><1E><1C>AM21<1C>ANS<1E><1C>
AM22<1C>EM1<1C>D21234567
34.17.4 REVERSAL REJECTED RESPONSE
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B2 Reversal
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 838 -
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbb
b
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE MESSAGE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 2Ø RESPONSE MESSAGE SEGMENT
5Ø4-F4 MESSAGE Q TRANSMISSION
MESSAGE TEXT For illustrative purposes only. Up to 2ØØ Bytes
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M R Rejected
51Ø-FA REJECT COUNT R 1 1 Reject Code follows
511-FB REJECT CODE R 87 Reversal not processed
13Ø-UF ADDITIONAL MESSAGE
INFORMATION COUNT
R 1 1 occurrence
132-UH ADDITIONAL MESSAGE
INFORMATION QUALIFIER
R Ø1 Used for first line of free form text with no pre-
defined structure.
526-FQ ADDITIONAL MESSAGE
INFORMATION
Q TRANSACTION
MESSAGE TEXT For illustrative purposes only. Up to 4Ø Bytes
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
DØB21AØ14563663bbbbbbbb2ØØ7Ø915<1E><1C>AM2Ø<1C>F4TRANSMISSION MESSAGE TEXT<1D><1E><1C>AM21<1C>ANR<1C>FA1<
1C>FB87<1C>UF1<1C>UHØ1<1C>FQTRANSACTION MESSAGE TEXT<1C>7FØ3<1C>8F6Ø2357Ø862<1E><1C>AM22<1C>EM1<1C>D21234
567
34.18 CLAIM REBILL - TRANSACTION CODE B3
Contains the reversal and claim in one transmission. There are no repeating segments.
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B3 Rebill
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbb
b
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID
M bbbbbbbbbb
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 123456789 Cardholder ID
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 839 -
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID
QUALIFIER
M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø341782
1 Ketoprofen 75mg capsule
442-E7 QUANTITY DISPENSED R 3ØØØØ 3Ø.ØØØ
4Ø3-D3 FILL NUMBER R Ø Original dispensing for RX#
4Ø5-D5 DAYS SUPPLY R 3Ø 3Ø Days supply
4Ø6-D6 COMPOUND CODE R 1 Not a compound
4Ø8-D8 DISPENSE AS WRITTEN
(DAW)/PRODUCT SELECTION
CODE
R Ø No product selection indicated
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ7Ø915 September 15, 2ØØ7
415-DF NUMBER OF REFILLS
AUTHORIZED
Q 5 5 Refills
419-DJ PRESCRIPTION ORIGIN CODE Q 1 Written prescription
3Ø8-C8 OTHER COVERAGE CODE Q 1 No other coverage
429-DT SPECIAL PACKAGING INDICATOR Q 1 Not unit dose
6ØØ-28 UNIT OF MEASURE Q EA Each
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 357F $35.76
412-DC DISPENSING FEE SUBMITTED Q 8Ø{ $8.ØØ
433-DX PATIENT PAID AMOUNT
SUBMITTED
Q 5Ø{ $5.ØØ
426-DQ USUAL AND CUSTOMARY
CHARGE
Q 528E $52.85
43Ø-DU GROSS AMOUNT DUE R 437F $43.76
423-DN BASIS OF COST DETERMINATION Q Ø3 Direct
61ØØ66DØB3123456789Ø1Ø14563663bbbbbbbb2ØØ7Ø915bbbbbbbbbb<1E><1C>AMØ4<1C>C2123456789<1D><1E><1C>AMØ7<1C>EM
1<1C>D21234567<1C>E1Ø3<1C>D7ØØØØ6Ø3417821<1C>E73ØØØØ<1C>D3Ø<1C>D53Ø<1C>D61<1C>D8Ø<1C>DE2ØØ7Ø915<1C>DF5<1C
>DJ1<1C>C81<1C>DT1<1C>28EA<1E><1C>AM11<1C>D9357F<1C>DC8Ø{<1C>DX5Ø{<1C>DQ528E<1C>DU437F<1C>DNØ3
34.18.1 REBILL ACCEPTED RESPONSE-CAPTURED
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B3 Rebill
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbb
b
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE MESSAGE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 2Ø RESPONSE MESSAGE SEGMENT
5Ø4-F4 MESSAGE Q TRANSMISSION
MESSAGE TEXT For illustrative purposes only. Up to 2ØØ Bytes
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M C Captured
5Ø3-F3 AUTHORIZATION NUMBER Q 12345678912345
6789
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 840 -
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT Q 5Ø{ $5.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 5Ø{ $5.ØØ
DØB31AØ14563663bbbbbbbb2ØØ7Ø915<1E><1C>AM2Ø<1C>F4TRANSMISSION MESSAGE TEXT<1D><1E><1C>AM21<1C>ANC<1C>F312
3456789123456789<1C>7FØ3<1C>8F6Ø2357Ø862<1E><1C>AM22<1C>EM1<1C>D21234567<1E><1C>AM23<1C>F55Ø{<1C>F95Ø{
34.18.2 REBILL ACCEPTED RESPONSE-PAID
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B3 Rebill
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbb
b
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE MESSAGE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 2Ø RESPONSE MESSAGE SEGMENT
5Ø4-F4 MESSAGE Q TRANSMISSION
MESSAGE TEXT For illustrative purposes only. Up to 2ØØ Bytes
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P Paid
5Ø3-F3 AUTHORIZATION NUMBER Q 12345678912345
6789
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
R 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
Q 1234567
551-9F PREFERRED PRODUCT COUNT R 1 1 Preferred product identified
552-AP PREFERRED PRODUCT ID
QUALIFIER
R Ø3 NDC
553-AR PREFERRED PRODUCT ID Q 17236Ø569Ø1 Ibuprofen 6ØØmg tablet
554-AS PREFERRED PRODUCT INCENTIVE Q 25{ $2.5Ø
555-AT PREFERRED PRODUCT COST
SHARE INCENTIVE
Q 3Ø{ $3.ØØ
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 5Ø{ $5.ØØ
5Ø6-F6 INGREDIENT COST PAID Q 357F $35.76
5Ø7-F7 DISPENSING FEE PAID Q 8Ø{ $8.ØØ
557-AV TAX EXEMPT INDICATOR Q 1 Payer/Plan is Tax Exempt (The Payer/Plan is not
responsible for tax. The patient may be charged tax.)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 841 -
5Ø9-F9 TOTAL AMOUNT PAID R 387F $38.76
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 1 Ingredient cost paid as submitted
DØB31AØ14563663bbbbbbbb2ØØ7Ø915<1E><1C>AM2Ø<1C>F4TRANSMISSION MESSAGE TEXT<1D><1E><1C>AM21<1C>ANP<1C>F312
3456789123456789<1C>7FØ3<1C>8F6Ø2357Ø862<1E><1C>AM22<1C>EM1<1C>D21234567<1C>9F1<1C>APØ3<1C>AR17236Ø569Ø1<
1C>AS25{<1C>AT3Ø{<1E><1C>AM23<1C>F55Ø{<1C>F6357F<1C>F78Ø{<1C>AV1<1C>F9387F<1C>FM1
34.18.3 REBILL REJECTED RESPONSE
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B3 Rebill
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbb
b
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE MESSAGE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 2Ø RESPONSE MESSAGE SEGMENT
5Ø4-F4 MESSAGE Q TRANSMISSION
MESSAGE TEXT For illustrative purposes only. Up to 2ØØ Bytes
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE
STATUS
M R Rejected
51Ø-FA REJECT COUNT R 3 3 Reject codes follow
511-FB REJECT CODE R 85 Claim not processed
511-FB REJECT CODE Q 87 Reversal not processed
511-FB REJECT CODE Q 78 Cost exceeds maximum
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
DØB31AØ14563663bbbbbbbb2ØØ7Ø915<1E><1C>AM2Ø<1C>F4TRANSMISSION MESSAGE TEXT<1D><1E><1C>AM21<1C>ANR<1C>FA3<
1C>FB85<1C>FB87<1C>FB78<1C>7FØ3<1C>8F6Ø2357Ø862<1E<1C>AM22<1C>EM1<1C>D21234567
34.19 PRIOR AUTHORIZATION REQUEST AND BILLING (CLAIM) - TRANSACTION
CODE P1
This is an initial request for prior authorization approval with payment information. Prior Authorization Segment contains the requested period
dates. TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M P1 Prior Authorization Request And billing
1Ø4-A4 PROCESSOR CONTROL
NUMBER
M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbb
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 842 -
b
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID
M bbbbbbbbbb
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 123456789 Cardholder ID
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER
QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID
QUALIFIER
M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
442-E7 QUANTITY DISPENSED R 3ØØØØ 3Ø.ØØØ
4Ø3-D3 FILL NUMBER R Ø Original dispensing for RX#
4Ø5-D5 DAYS SUPPLY R 3Ø 3Ø Days supply
4Ø6-D6 COMPOUND CODE R 1 Not a compound
4Ø8-D8 DISPENSE AS WRITTEN
(DAW)/PRODUCT SELECTION
CODE
R 1 Substitution Not Allowed by Prescriber
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ7Ø915 September 15, 2ØØ7
415-DF NUMBER OF REFILLS
AUTHORIZED
Q 5 5 Refills
419-DJ PRESCRIPTION ORIGIN CODE Q 1 Written prescription
3Ø8-C8 OTHER COVERAGE CODE Q 1 No other coverage
429-DT SPECIAL PACKAGING
INDICATOR
Q 1 Not unit dose
6ØØ-28 UNIT OF MEASURE Q EA Each
PRESCRIBER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø3 PRESCRIBER SEGMENT
466-EZ PRESCRIBER ID QUALIFIER R Ø1 National Provider ID
411-DB PRESCRIBER ID Q ØØ12345111
427-DR PRESCRIBER LAST NAME Q JONES
498-PM PRESCRIBER TELEPHONE
NUMBER
Q 2Ø13639572
468-2E PRIMARY CARE PROVIDER ID
QUALIFIER
R Ø1 National Provider Identifier
421-DL PRIMARY CARE PROVIDER ID Q 1234577111
47Ø-4E PRIMARY CARE PROVIDER LAST
NAME
Q HARRIS
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 557{ $55.7Ø
412-DC DISPENSING FEE SUBMITTED Q 1ØØ{ $1Ø.ØØ
433-DX PATIENT PAID AMOUNT
SUBMITTED
Q 1ØØ{ $1Ø.ØØ
426-DQ USUAL AND CUSTOMARY
CHARGE
Q 725{ $72.5Ø
43Ø-DU GROSS AMOUNT DUE R 657{ $65.7Ø
423-DN BASIS OF COST
DETERMINATION
Q Ø3 Direct
PRIOR AUTHORIZATION SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 843 -
111-AM SEGMENT IDENTIFICATION M 12 PRIOR AUTHORIZATION SEGMENT
498-PA REQUEST TYPE M 1 Initial
498-PB REQUEST PERIOD DATE -
BEGIN
M 2ØØ7Ø915 September 15, 2ØØ7
498-PC REQUEST PERIOD DATE - END M 2ØØ8Ø914 September 14, 2ØØ8
498-PD BASIS OF REQUEST M ME Medical exception
498-PE AUTHORIZED REP FIRST NAME Q CAROLYN
498-PF AUTHORIZED REP LAST NAME Q MILLER
498-PG AUTHORIZED REP ADDRESS Q 1234 WALNUT
AVENUE
498-PH AUTHORIZED REP CITY Q DOVER
498-PJ AUTHORIZED REP
STATE/PROVINCE
Q DE
498-PK AUTHORIZED REP ZIP/POSTAL
ZONE
Q 21234
498-PP PRIOR AUTHORIZATION
SUPPORTING DOCUMENTATION
Q Up to 5ØØ bytes
If the parameters upon which the authorization was approved change, it may be necessary to submit a Prior Authorization Reversal to back
out the original Prior Authorization. A subsequent claim Reversal to back out any billings that were submitted may be required by the
processor.
61ØØ66DØP1123456789Ø1Ø14563663bbbbbbbb2ØØ7Ø915bbbbbbbbbb<1E><1C>AMØ4<1C>C2123456789<1D><1E><1C>AMØ7<1C>EM
1<1C>D21234567<1C>E1Ø3<1C>D7ØØØØ6Ø94268<1C>E73ØØØØ<1C>D3Ø<1C>D53Ø<1C>D61<1C>D81<1C>DE2ØØ7Ø915<1C>DF5<1C>D
J1<1C>C81<1C>DT1<1C>28EA<1E><1C>AMØ3<1C>EZØ8<1C>D8ØØG2345<1C>1E1Ø<1C>DRJONES<1C>PM2Ø13639572<1C>2E1<1C>DL
1234577<1C>H51Ø1<1C>4EHARRIS<1C>AM11<1C>D9557{<1C>DC1ØØ{<1C>DX1ØØ{<1C>DQ725{<1C>DU657{<1C>DNØ3<1E><1C>AM1
2<1C>PA1<1C>PB2ØØ915<1C>PC2ØØ8Ø914<1C>PDME<1C>PECAROLYN<1C>PFMILLER<1C>PG1234 WALNUT AVENUE<1C>PHDOVER<1C
>PJDE<1C>PK21234<1C>PPPRIOR AUTHORIZATION SUPPORTING DOCUMENTATION
34.19.1 PRIOR AUTHORIZATION REQUEST AND BILLING ACCEPTED RESPONSE-
CAPTURED RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M P1 Prior Authorization Request And Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbb
b
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE
STATUS
M C Captured
5Ø3-F3 AUTHORIZATION NUMBER Q 67891234567
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
DØP11AØ14563663bbbbbbbb2ØØ7Ø915<1D><1E><1C>AM21<1C>ANC<1C>F367891234567<1E><1C>AM22<1C>EM1<1C>D21234567
34.19.2 PRIOR AUTHORIZATION REQUEST AND BILLING ACCEPTED RESPONSE-PAID
The pharmacy receives prior authorization and payment information in the response.
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M P1 Prior Authorization Request And Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 844 -
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbb
b
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE STATUS SEGMENT
FIELD FIELD NAME VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE
STATUS
M P Paid
5Ø3-F3 AUTHORIZATION NUMBER Q 67891234567
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 1ØØ{ $1Ø.ØØ
5Ø6-F6 INGREDIENT COST PAID Q 725{ $72.5Ø
557-AV TAX EXEMPT INDICATOR Q 1 Payer/Plan is Tax Exempt (The Payer/Plan is not
responsible for tax. The patient may be charged tax.)
5Ø9-F9 TOTAL AMOUNT PAID R 625{ $62.5Ø
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 4 U&C paid as submitted
RESPONSE PRIOR AUTHORIZATION SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 26 RESPONSE PRIOR AUTHORIZATION SEGMENT
498-PR PRIOR AUTHORIZATION
PROCESSED DATE
R 2ØØ7Ø915 September 15, 2ØØ7
498-PS PRIOR AUTHORIZATION
EFFECTIVE DATE
Q 2ØØ7Ø915 September 15, 2ØØ7
498-PT PRIOR AUTHORIZATION
EXPIRATION DATE
Q 2ØØ8Ø914 September 14, 2ØØ8
498-RA PRIOR AUTHORIZATION
QUANTITY
Q 15ØØØØ 15Ø tablets
498-RB PRIOR AUTHORIZATION DOLLARS
AUTHORIZED
Q 2785{ $278.5Ø
498-PW PRIOR AUTHORIZATION NUMBER
OF REFILLS AUTHORIZED
Q 5 5 refills
498-PX PRIOR AUTHORIZATION
QUANTITY ACCUMULATED
Q 3ØØØØ 3Ø tablets dispensed
498-PY PRIOR AUTHORIZATION
NUMBER-ASSIGNED
R 54321543215
DØP11AØ14563663bbbbbbbb2ØØ7Ø915<1D><1E><1C>AM21<1C>ANP<1C>F367891234567<1E><1C>AM22<1C>EM1<1C>D21234567<1
E><1C>AM23<1C>F51ØØ{<1C>F6725{<1C>AV1<1C>F9625{<1C>FM4<1E><1C>AM26<1C>PR2ØØ7Ø915<1C>PS2ØØ8Ø914<1C>PT1998Ø
919<1C>RA15ØØØØ<1C>RB2785{<1C>PW5<1C>PX3ØØØØ<1C>PY54321543215
34.19.3 PRIOR AUTHORIZATION REQUEST AND BILLING REJECTED RESPONSE
The pharmacy receives the response from the processor that the product or service is not covered. The preferred product information is
returned. A Help Desk Number is available for follow up questions.
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M P1 Prior Authorization Request And Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø1 National Provider ID
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 845 -
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbb
b
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE
STATUS
M R Rejected
51Ø-FA REJECT COUNT R 1 1 Reject Code follows
511-FB REJECT CODE R 7Ø Product/Service not covered
5Ø3-F3 AUTHORIZATION NUMBER Q 12345678912345
6789
13Ø-UF ADDITIONAL MESSAGE
INFORMATION COUNT
R 1 1 occurrence
132-UH ADDITIONAL MESSAGE
INFORMATION QUALIFIER
R Ø1 Used for first line of free form text with no pre-
defined structure.
526-FQ ADDITIONAL MESSAGE
INFORMATION
Q TRANSACTION
MESSAGE TEXT For illustrative purposes only. Up to 4Ø Bytes
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
551-9F PREFERRED PRODUCT COUNT R 1 1 Preferred Product Identified
552-AP PREFERRED PRODUCT ID
QUALIFIER
R Ø3 NDC
553-AR PREFERRED PRODUCT ID Q 17236Ø569Ø1 Ibuprofen 6ØØmg tablet
DØP11AØ14563663bbbbbbbb2ØØ7Ø915<1D><1E><1C>AM21<1C>ANR<1C>FA1<1C>FB7Ø<1C>F3123456789123456789<1C>UF1<1C>U
HØ1<1C>FQTRANSACTION MESSAGE TEXT<1C>7FØ3<1C>8F6Ø2357Ø862<1E><1C>AM22<1C>EM1<1C>D21234567<1C>9F1<1C>APØ3<
1C>AR17236Ø569Ø1
34.19.4 PRIOR AUTHORIZATION REQUEST AND BILLING DUPLICATE OF PAID RESPONSE
The pharmacy receives a duplicate paid response. The information is the same as above.
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M P1 Prior Authorization Request And Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbb
b
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE
STATUS
M D Duplicate of Paid
5Ø3-F3 AUTHORIZATION NUMBER Q 67891234567
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 846 -
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 1ØØ{ $1Ø.ØØ
5Ø6-F6 INGREDIENT COST PAID Q 825{ $72.5Ø
557-AV TAX EXEMPT INDICATOR Q 1 Payer/Plan is Tax Exempt (The Payer/Plan is not
responsible for tax. The patient may be charged tax.)
5Ø9-F9 TOTAL AMOUNT PAID R 625{ $62.5Ø
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 4 U&C paid as submitted
RESPONSE PRIOR AUTHORIZATION SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 26 RESPONSE PRIOR AUTHORIZATION SEGMENT
498-PR PRIOR AUTHORIZATION
PROCESSED DATE
R 2ØØ7Ø915 September 15, 2ØØ7
498-PS PRIOR AUTHORIZATION
EFFECTIVE DATE
Q 2ØØ7Ø915 September 15, 2ØØ7
498-PT PRIOR AUTHORIZATION
EXPIRATION DATE
Q 2ØØ8Ø914 September 14, 2ØØ8
498-RA PRIOR AUTHORIZATION
QUANTITY
Q 15ØØØØ 15Ø tablets
498-RB PRIOR AUTHORIZATION DOLLARS
AUTHORIZED
Q 2785{ $278.5Ø
498-PW PRIOR AUTHORIZATION NUMBER
OF REFILLS AUTHORIZED
Q 5 5 refills
498-PX PRIOR AUTHORIZATION
QUANTITY ACCUMULATED
Q 3ØØØØ 3Ø tablets dispensed
498-PY PRIOR AUTHORIZATION
NUMBER-ASSIGNED
R 54321543215
34.20 PRIOR AUTHORIZATION REVERSAL - TRANSACTION CODE P2
The pharmacy wishes to reverse the prior authorization that was previously processed. This is a request to reverse just the prior authorization.
If claim or service billings were billed with this prior authorization, the claim or service billings would need to be reversed first; then the prior
authorization reversed. TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M P2 Prior Authorization Reversal
1Ø4-A4 PROCESSOR CONTROL
NUMBER
M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbb
bb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID
M bbbbbbbbbb
PRIOR AUTHORIZATION SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 12 PRIOR AUTHORIZATION SEGMENT
498-PA REQUEST TYPE M 1 Initial
498-PB REQUEST PERIOD DATE -
BEGIN
M 2ØØ7Ø915 September 15, 2ØØ7
498-PC REQUEST PERIOD DATE - END M 2ØØ7Ø914 September 14, 2ØØ8
498-PD BASIS OF REQUEST M ME Medical exception
498-PY PRIOR AUTHORIZATION
NUMBER-ASSIGNED
Q 54321543215
61ØØ66DØP2123456789Ø1Ø14563663bbbbbbbb2ØØ7Ø915bbbbbbbbbb<1E><1C>AM12<1C>PA1<1C>PB2ØØ7Ø915<1C>PC2ØØ8Ø914<1
C>PDME<1C>PY54321543215
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 847 -
34.20.1 PRIOR AUTHORIZATION REVERSAL ACCEPTED RESPONSE-CAPTURED,
APPROVED RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M P2 Prior Authorization Reversal
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbb
b
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE MESSAGE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 2Ø RESPONSE MESSAGE SEGMENT
5Ø4-F4 MESSAGE Q TRANSMISSION
MESSAGE TEXT For illustrative purposes only. Up to 2ØØ Bytes
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE
STATUS
M A OR C Approved or Captured
DØP21AØ14563663bbbbbbbb2ØØ7Ø915<1E><1C>AM2Ø<1C>F4TRANSMISSION MESSAGE TEXT<1D><1E><1C>AM21<1C>ANA
34.21 PRIOR AUTHORIZATION INQUIRY - TRANSACTION CODE P3
New scenario. The pharmacy has submitted a PA Request And Billing sometime in the past, and received a captured response. The
pharmacy is now submitting a PA Inquiry to determine the outcome, using the Authorization Number ((5Ø3-F3) received during the PA
Request And Billing original conversation. TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M P3 Prior Authorization Inquiry
1Ø4-A4 PROCESSOR CONTROL
NUMBER
M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbb
b
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID
M bbbbbbbbbb
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 123456789 Cardholder ID
PRIOR AUTHORIZATION SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 12 PRIOR AUTHORIZATION SEGMENT
498-PA REQUEST TYPE M 1 Initial
498-PB REQUEST PERIOD DATE –
BEGIN
M 2ØØ7Ø915 September 15, 2ØØ7
498-PC REQUEST PERIOD DATE – END M 2ØØ8Ø914 September 14, 2ØØ8
498-PD BASIS OF REQUEST M ME Medical Exception
5Ø3-F3 AUTHORIZATION NUMBER Q 9876545678
61ØØ66DØP3123456789Ø1Ø14563663bbbbbbbb2ØØ7Ø915bbbbbbbbbb<1E><1C>AMØ4<1C>C2123456789<1D><1E><1C>AMØ7<1C>EM
1<1C>D21234567<1C>E1Ø3<1C>D7ØØØØ6Ø94268<1E><1C>AM12<1C>PA1<1C>PB2ØØ7Ø915<1C>PC2ØØ8Ø914<1C>PDME<1C>F398765
45678
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 848 -
34.21.1 PRIOR AUTHORIZATION INQUIRY ACCEPTED RESPONSE-PAID
The processor is responding that the original PA Request And Billing has been approved and payment information is included. The processor
assigns an Authorization Number to conversation. The processor returns payment, as well as prior authorization information, including a Prior
Authorization Number–Assigned (498-PY). RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M P3 Prior Authorization Inquiry
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE
STATUS
M P Paid
5Ø3-F3 AUTHORIZATION NUMBER Q 67891234567
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 1ØØ{ $1Ø.ØØ
5Ø6-F6 INGREDIENT COST PAID Q 557{ $55.7Ø
5Ø7-F7 DISPENSING FEE PAID Q 1ØØ{ $1Ø.ØØ
557-AV TAX EXEMPT INDICATOR Q 1 Payer/Plan is Tax Exempt (The Payer/Plan is not
responsible for tax. The patient may be charged tax.)
5Ø9-F9 TOTAL AMOUNT PAID R 557{ $55.7Ø
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 1 Ingredient cost paid as submitted
RESPONSE PRIOR AUTHORIZATION SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 26 RESPONSE PRIOR AUTHORIZATION SEGMENT
498-PR PRIOR AUTHORIZATION
PROCESSED DATE
R 2ØØ7Ø915 September 15, 2ØØ7
498-PS PRIOR AUTHORIZATION
EFFECTIVE DATE
Q 2ØØ7Ø915 September 15, 2ØØ7
498-PT PRIOR AUTHORIZATION
EXPIRATION DATE
Q 2ØØ8Ø914 September 14, 2ØØ8
498-RA PRIOR AUTHORIZATION
QUANTITY
Q 15ØØØØ 15Ø tablets
498-RB PRIOR AUTHORIZATION DOLLARS
AUTHORIZED
Q 2785{ $278.5Ø
498-PW PRIOR AUTHORIZATION NUMBER
OF REFILLS AUTHORIZED
Q 5 5 refills
498-PX PRIOR AUTHORIZATION
QUANTITY ACCUMULATED
Q 3ØØØØ 3Ø tablets dispensed
498-PY PRIOR AUTHORIZATION
NUMBER-ASSIGNED
R 54321543215
DØP31AØ14563663bbbbbbbb2ØØ7Ø915<1D><1E><1C>AM21<1C>ANP<1C>F367891234567<1E><1C>AM22<1C>EM1<1C>D21234567<1
E><1C>AM23<1C>F51ØØ{<1C>F6557{<1C>F71ØØ{<1C>AV1<1C>F9557{<1C>FM1<1E><1C>AM26<1C>PR2ØØ7Ø915<1C>PS2ØØ8Ø914<
1C>PT1998Ø919<1C>RA15ØØØØ<1C>RB2785{<1C>PW5<1C>PX3ØØØØ<1C>PY54321543215
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 849 -
34.22 PRIOR AUTHORIZATION REQUEST ONLY (CLAIM) - TRANSACTION CODE P4
New scenario. The pharmacy is requesting a prior authorization approval only (no payment). The Prior Authorization Segment includes the
prior authorization period date and other information.
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M P4 Prior Authorization Request Only
1Ø4-A4 PROCESSOR CONTROL
NUMBER
M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID
M bbbbbbbbbb
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 123456789 Cardholder ID
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER
QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID
QUALIFIER
M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
442-E7 QUANTITY DISPENSED R 3ØØØØ 3Ø.ØØØ
4Ø5-D5 DAYS SUPPLY R 3Ø 3Ø Days supply
4Ø6-D6 COMPOUND CODE Q 1 Not a compound
4Ø8-D8 DISPENSE AS WRITTEN
(DAW)/PRODUCT SELECTION
CODE
Q 1 Substitution Not Allowed by Prescriber
415-DF NUMBER OF REFILLS
AUTHORIZED
R 5 5 Refills
46Ø-ET QUANTITY PRESCRIBED R 3ØØØØ 3Ø.ØØØ
429-DT SPECIAL PACKAGING
INDICATOR
Q 1 Not unit dose
PRIOR AUTHORIZATION SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 12 PRIOR AUTHORIZATION SEGMENT
498-PA REQUEST TYPE M 1 Initial
498-PB REQUEST PERIOD DATE -
BEGIN
M 2ØØ7Ø915 September 15, 2ØØ7
498-PC REQUEST PERIOD DATE - END M 2ØØ8Ø914 September 14, 2ØØ8
498-PD BASIS OF REQUEST M ME Medical exception
61ØØ66DØP4123456789Ø1Ø14563663bbbbbbbb2ØØ7Ø915bbbbbbbbbb<1E><1C>AMØ4<1C>123456789<1D><1E><1C>AMØ7<1C>EM1<
1C>D21234567<1C>E1Ø3<1C>D7ØØØØ6Ø94268<1C>E73ØØØØ<1C>D53Ø<1C>D61<1C>D81<1C>DF5<1C>ET3ØØØØ<1C>DT1<1E><1C>AM
12<1C>PA1<1C>PB2ØØ7Ø915<1C>PC2ØØ8Ø914<1C>PDME
34.22.1 PRIOR AUTHORIZATION REQUEST ONLY ACCEPTED RESPONSE-APPROVED
The processor responds that the request for prior authorization has been approved, with appropriate prior authorization information.
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M P4 Prior Authorization Request Only
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 850 -
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE
STATUS
M A Approved
5Ø3-F3 AUTHORIZATION NUMBER Q 123456789123456
789
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
RESPONSE PRIOR AUTHORIZATION SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 26 RESPONSE PRIOR AUTHORIZATION SEGMENT
498-PR PRIOR AUTHORIZATION
PROCESSED DATE
R 2ØØ7Ø915 September 15, 2ØØ7
498-PS PRIOR AUTHORIZATION
EFFECTIVE DATE
Q 2ØØ7Ø915 September 15, 2ØØ7
498-PT PRIOR AUTHORIZATION
EXPIRATION DATE
Q 2ØØ8Ø914 September 14, 2ØØ8
498-RA PRIOR AUTHORIZATION
QUANTITY
Q 15ØØØØ 15Ø tablets
498-RB PRIOR AUTHORIZATION DOLLARS
AUTHORIZED
Q 2785{ $278.5Ø
498-PW PRIOR AUTHORIZATION NUMBER
OF REFILLS AUTHORIZED
Q 5 5 refills
498-PX PRIOR AUTHORIZATION
QUANTITY ACCUMULATED
Q 3ØØØØ 3Ø tablets dispensed
498-PY PRIOR AUTHORIZATION
NUMBER-ASSIGNED
R 54321543215
DØP41AØ14563663bbbbbbbb2ØØ7Ø915<1D><1E><1C>AM21<1C>ANA<1C>F3123456789123456789<1E><1C>AM22<1C>EM1<1C>D212
34567<1E><1C>AM26<1C>PR2ØØ7Ø915<1C>PS2ØØ7Ø915<1C>PT2ØØ8Ø914<1C>RA15ØØØØ<1C>RB2785{<1C>PW5<1C>PX3ØØØØ<1C>P
Y54321543215
34.22.2 PRIOR AUTHORIZATION REQUEST ONLY REJECTED RESPONSE
The processor is not approving the request for a prior authorization, as the product is not covered.
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M P4 Prior Authorization Request Only
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE
STATUS
M R Rejected
51Ø-FA REJECT COUNT R 1 1 Reject Code follows
511-FB REJECT CODE R 7Ø Product/Service not covered
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 851 -
5Ø3-F3 AUTHORIZATION NUMBER Q 123456789123456
789
13Ø-UF ADDITIONAL MESSAGE
INFORMATION COUNT
R 1 1 occurrence
132-UH ADDITIONAL MESSAGE
INFORMATION QUALIFIER
R Ø1 Used for first line of free form text with no pre-
defined structure.
526-FQ ADDITIONAL MESSAGE
INFORMATION
Q TRANSACTION
MESSAGE TEXT For illustrative purposes only. Up to 4Ø Bytes
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
34.23 INFORMATION REPORTING (SERVICE – DUR/PPS) - TRANSACTION CODE
N1
Pharmacist submits information of value to processor/payer.
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M N1 Information reporting
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø7 NCPDP Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663bbbbbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID
M 98765bbbbb
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø1 PATIENT SEGMENT
331-CX PATIENT ID QUALIFIER R Ø1 Social Security Number
332-CY PATIENT ID Q ØØ5492368 Patient’s SSN
3Ø4-C4 DATE OF BIRTH R 1962Ø615 Born June 15, 1962
3Ø5-C5 PATIENT GENDER CODE Q 1 Male
31Ø-CA PATIENT FIRST NAME R JOSEPH
311-CB PATIENT LAST NAME R SMITH
322-CM PATIENT STREET ADDRESS Q 123 MAIN STREET
323-CN PATIENT CITY ADDRESS Q MY TOWN
324-CO PATIENT STATE/PROVINCE
ADDRESS
Q CO
325-CP PATIENT ZIP/POSTAL ZONE Q 34567
326-CQ PATIENT PHONE NUMBER Q 2Ø14658923
334-1C SMOKER/NON-SMOKER CODE Q 2 Smoker
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 123456789 Cardholder ID
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER
QUALIFIER
M 2 Service billing
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 852 -
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 7654321
436-E1 PRODUCT/SERVICE ID
QUALIFIER
M Ø6 DUR/PPS
4Ø7-D7 PRODUCT/SERVICE ID M Ø
DUR/PPS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø8 DUR/PPS Segment
473-7E DUR/PPS CODE COUNTER R 1 1st PPS action
439-E4 REASON FOR SERVICE CODE Q DA Drug-Allergy conflict
44Ø-E5 PROFESSIONAL SERVICE CODE Q PØ Patient consulted
441-E6 RESULT OF SERVICE CODE Q 3A Recommendation accepted
474-8E DUR/PPS LEVEL OF EFFORT Q 11 Lowest level of complexity
475-J9 DUR CO-AGENT ID QUALIFIER R Ø7 ICD9
476-H6 DUR CO-AGENT ID Q E93ØØØ Allergic to penicillins
473-7E DUR/PPS CODE COUNTER R 2 2nd PPS action
439-E4 REASON FOR SERVICE CODE Q NC Non-covered drug purchase
44Ø-E5 PROFESSIONAL SERVICE CODE Q SC Self-care consultation
441-E6 RESULT OF SERVICE CODE Q 3A Recommendation accepted
474-8E DUR/PPS LEVEL OF EFFORT Q 11 Lowest level of complexity
475-J9 DUR CO-AGENT ID QUALIFIER R Ø3 NDC
476-H6 DUR CO-AGENT ID Q 17236Ø378Ø1 Aspirin 325mg tab
Note: Diagnosis Code (424-DO) - For example purposes only, and may not be billable. Refer to owner’s code set rules and formats.
CLINICAL SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 13 CLINICAL SEGMENT
491-VE DIAGNOSIS CODE COUNT R 4 4 Diagnoses follow
492-WE DIAGNOSIS CODE QUALIFIER R Ø1 ICD9
424-DO DIAGNOSIS CODE Q 7169Ø Osteoarthritis
492-WE DIAGNOSIS CODE QUALIFIER R Ø1 ICD9
424-DO DIAGNOSIS CODE Q 4Ø19 Hypertension
492-WE DIAGNOSIS CODE QUALIFIER R Ø1 ICD9
424-DO DIAGNOSIS CODE Q 5939 Renal failure
492-WE DIAGNOSIS CODE QUALIFIER R Ø1 ICD9
424-DO DIAGNOSIS CODE Q 493ØØ Asthma
493-XE CLINICAL INFORMATION
COUNTER
R 1 1st occurrence
494-ZE MEASUREMENT DATE Q 2ØØ7Ø915 September 15, 2ØØ7
495-H1 MEASUREMENT TIME Q 143Ø Measured at 2:3Øpm
496-H2 MEASUREMENT DIMENSION Q Ø1 Blood Pressure (BP)
497-H3 MEASUREMENT UNIT Q 1Ø Millimeters of mercury (mmHg)
499-H4 MEASUREMENT VALUE Q 15Ø/9Ø Pt is hypertensive
493-XE CLINICAL INFORMATION
COUNTER
R 2 2nd occurrence
494-ZE MEASUREMENT DATE Q 2ØØ7Ø915 September 15, 2ØØ7
495-H1 MEASUREMENT TIME Q 143Ø Measured at 2:3Øpm
496-H2 MEASUREMENT DIMENSION Q Ø2 Blood Glucose
497-H3 MEASUREMENT UNIT Q Ø8 Milligrams per deciliter (mg/dl)
499-H4 MEASUREMENT VALUE Q 24Ø Pt is hyperglycemic
493-XE CLINICAL INFORMATION
COUNTER
R 3 3rd occurrence
494-ZE MEASUREMENT DATE Q 2ØØ7Ø715 July 15, 2ØØ7
496-H2 MEASUREMENT DIMENSION Q 14 Weight
497-H3 MEASUREMENT UNIT Q Ø3 Pounds (lb)
499-H4 MEASUREMENT VALUE Q 21Ø Pt weighs 21Ø pounds
493-XE CLINICAL INFORMATION
COUNTER
R 4 4th occurrence
494-ZE MEASUREMENT DATE Q 2ØØ7Ø815 August 15, 2ØØ7
495-H1 MEASUREMENT TIME Q Ø93Ø Measured at 9:3Øam
496-H2 MEASUREMENT DIMENSION Q 12 Theophylline
497-H3 MEASUREMENT UNIT Q Ø8 Milligrams per deciliter (mg/dl)
499-H4 MEASUREMENT VALUE Q 15 Drug level is therapeutic
493-XE CLINICAL INFORMATION
COUNTER
R 5 5th occurrence
494-ZE MEASUREMENT DATE Q 2ØØ7Ø915 September 15, 2ØØ7
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 853 -
496-H2 MEASUREMENT DIMENSION Q Ø4 Serum Creatinine (SCr)
497-H3 MEASUREMENT UNIT Q Ø8 Milligrams per deciliter (mg/dl)
499-H4 MEASUREMENT VALUE Q 3.2 Pt has renal failure
Note: Diagnosis Code (424-DO) - For example purposes only, and may not be billable. Refer to owner’s code set rules and formats.
61ØØ66DØN1123456789Ø1Ø74563663bbbbbbbb2ØØ7Ø91598765bbbbb<1E><1C>AMØ1<1C>CXØ1<1C>CYØØ5492368<1C>C41962Ø615
<1C>C51<1C>CAJOSEPH<1C>CBSMITH<1C>CM123 MAIN STREET<1C>CNMY TOWN<1C>COCO<1C>CP34567<1C>CQ2Ø14658923<1C>1C
2<1E<1C>AMØ4<1C>C2123456789<1D><1E><1C>AMØ7<1C>EM2<1C>D27654321<1C>E1Ø6<1C>D7Ø<1E><1C>AMØ8<1C>7E1<1C>E4DA
<1C>E5PØ<1C>E63A<1C>8E11<1C>J9Ø7<1C>H6E93ØØØ<1C>7E2<1C>E4NC<1C>E5SC<1C>E63A<1C>8E11<1C>J9Ø3<1C>H617236Ø37
8Ø1<1E><1C>AM13 (situational fields not listed)
34.23.1 INFORMATION REPORTING ACCEPTED RESPONSE-CAPTURED, APPROVED
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M P4 Prior Authorization Request Only
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø7 NCPDP Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663bbbbbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE
STATUS
M A OR C Approved or Captured
5Ø3-F3 AUTHORIZATION NUMBER Q 123456789123456
789
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 7654321
DØP41AØ74563663bbbbbbbb2ØØ7Ø915<1D><1E><1C>AM21<1C>ANA<1C>F3123456789123456789<1E><1C>AM22<1C>EM1<1C>D276
54321
34.24 INFORMATION REPORTING REVERSAL - TRANSACTION CODE N2
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M N2 Information Reporting Reversal
1Ø4-A4 PROCESSOR CONTROL
NUMBER
M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø7 NCPDP Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663bbbbbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID
M bbbbbbbbbb
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER
QUALIFIER
M 2 Service billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 7654321
436-E1 PRODUCT/SERVICE ID M Ø6 DUR/PPS
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 854 -
QUALIFIER
4Ø7-D7 PRODUCT/SERVICE ID M Ø
61ØØ66DØN2123456789Ø1Ø74563663bbbbbbbb2ØØ7Ø915bbbbbbbbbb<1D><1E><1C>AMØ7<1C>EM2<1C>D27654321<1C>E1Ø6<1C>D
7Ø
34.24.1 INFORMATION REPORTING REVERSAL ACCEPTED RESPONSE—CAPTURED OR
APPROVED (OR DUPLICATE)
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M N2 Information Reporting Reversal
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø7 NCPDP Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663bbbbbbb
b
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE
STATUS
M C or Q, or A or S Captured or Duplicate of Captured or Approved or
Duplicate of Approved
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER
QUALIFIER
M 2 Service billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 7654321
34.24.2 INFORMATION REPORTING REVERSAL REJECTED RESPONSE
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M N2 Reversal
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø7 NCPDP Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663bbbbbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE
STATUS
M R Rejected
51Ø-FA REJECT COUNT R 1 1 Reject Code follows
511-FB REJECT CODE R 9Ø Host Hung Up
13Ø-UF ADDITIONAL MESSAGE
INFORMATION COUNT
R 1 1 occurrence
132-UH ADDITIONAL MESSAGE
INFORMATION QUALIFIER
R Ø1 Used for first line of free form text with no pre-defined
structure.
526-FQ ADDITIONAL MESSAGE
INFORMATION
Q TRANSACTION
MESSAGE TEXT For illustrative purposes only. Up to 4Ø Bytes
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
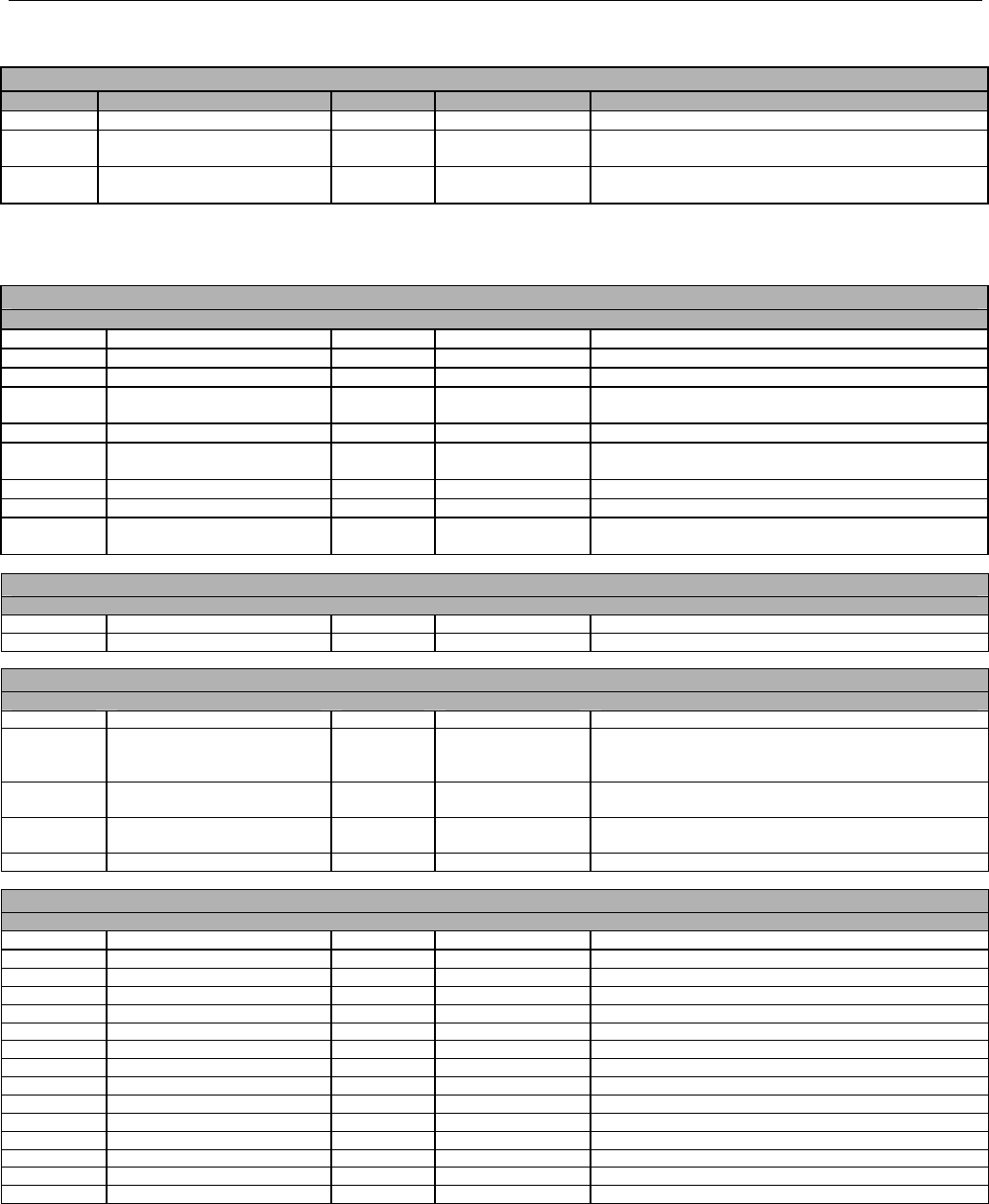
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 855 -
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 2 Service billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 7654321
34.25 INFORMATION REPORTING REBILL (SERVICE – DUR/PPS) - TRANSACTION
CODE N3 TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M N3 Information Rebill
1Ø4-A4 PROCESSOR CONTROL
NUMBER
M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø7 NCPDP Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663bbbbbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID
M bbbbbbbbbb
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 123456789 Cardholder ID
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER
QUALIFIER
M 2 Service billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 7654321
436-E1 PRODUCT/SERVICE ID
QUALIFIER
M Ø6 DUR/PPS
4Ø7-D7 PRODUCT/SERVICE ID M Ø
DUR/PPS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø8 DUR/PPS Segment
473-7E DUR/PPS CODE COUNTER R 1 1st PPS action
439-E4 REASON FOR SERVICE CODE Q DA Drug-Allergy conflict
44Ø-E5 PROFESSIONAL SERVICE CODE Q PØ Patient consulted
441-E6 RESULT OF SERVICE CODE Q 3A Recommendation accepted
474-8E DUR/PPS LEVEL OF EFFORT Q 11 Lowest level of complexity
475-J9 DUR CO-AGENT ID QUALIFIER R 15 ICD9
476-H6 DUR CO-AGENT IDENTIFIER Q E9353Ø Allergic to salicylates
473-7E DUR/PPS CODE COUNTER R 2 2nd PPS action
439-E4 REASON FOR SERVICE CODE Q NC Non-covered drug purchase
44Ø-E5 PROFESSIONAL SERVICE CODE Q SC Self-care consultation
441-E6 RESULT OF SERVICE CODE Q 3A Recommendation accepted
474-8E DUR/PPS LEVEL OF EFFORT Q 11 Lowest level of complexity
475-J9 DUR CO-AGENT ID QUALIFIER R Ø3 NDC
476-H6 DUR CO-AGENT IDENTIFIER Q 17236Ø378Ø1 Aspirin 325mg tab
Note: Diagnosis Code (424-DO) - For example purposes only, and may not be billable. Refer to owner’s code set rules and formats.
34.25.1 INFORMATION REPORTING REBILL ACCEPTED RESPONSE-CAPTURED
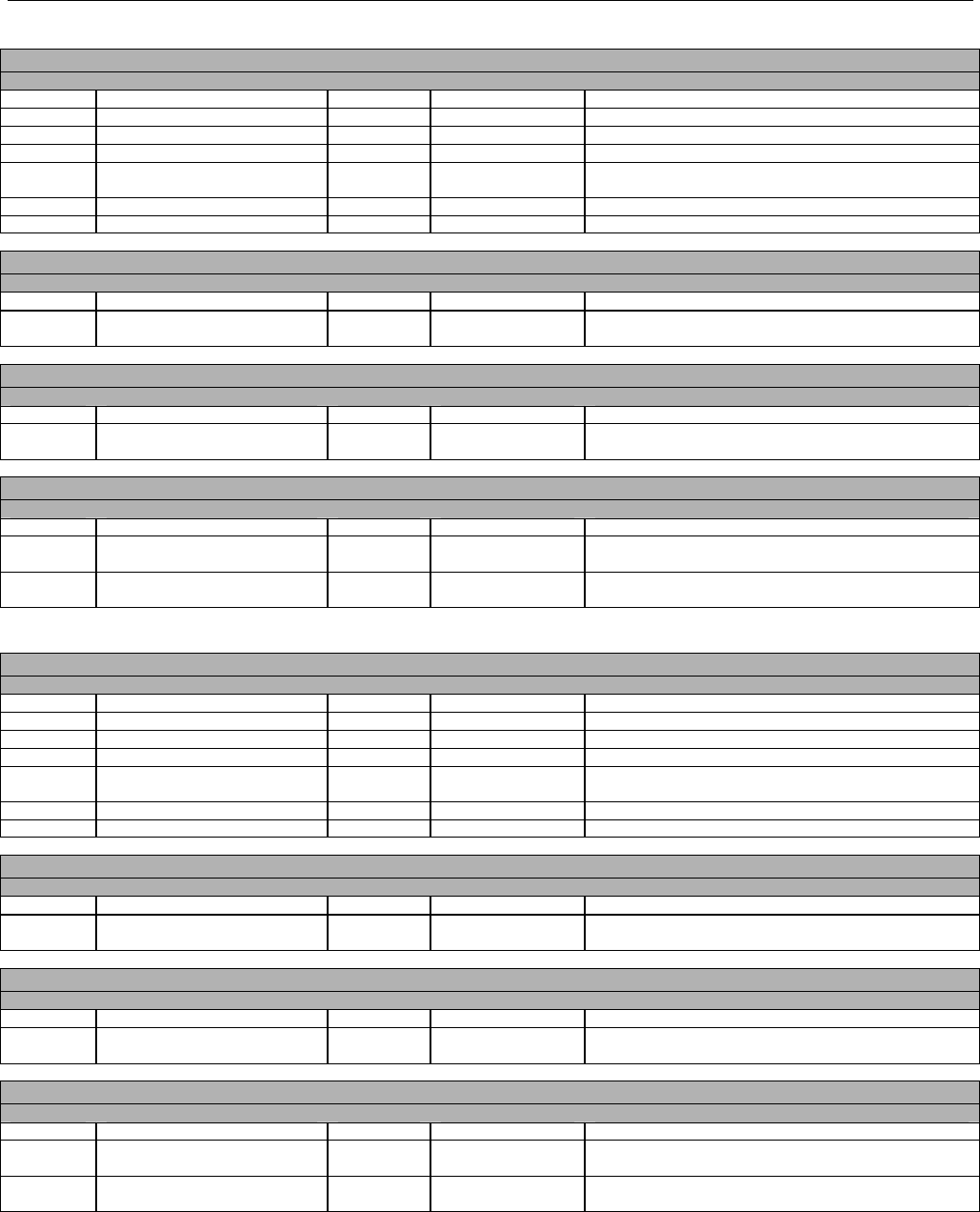
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 856 -
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M N3 Information reporting Rebill
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø7 NCPDP Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663bbbbbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE MESSAGE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 2Ø RESPONSE MESSAGE SEGMENT
5Ø4-F4 MESSAGE Q TRANSMISSION
MESSAGE TEXT For illustrative purposes only. Up to 2ØØ Bytes
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE
STATUS
M C Captured
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 7654321
34.25.2 INFORMATION REPORTING REBILL ACCEPTED RESPONSE-CAPTURED
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M N3 Information reporting Rebill
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø7 NCPDP Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663bbbbbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE MESSAGE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 2Ø RESPONSE MESSAGE SEGMENT
5Ø4-F4 MESSAGE Q TRANSMISSION
MESSAGE TEXT For illustrative purposes only. Up to 2ØØ Bytes
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE
STATUS
M A Approved
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 7654321
34.25.3 INFORMATION REPORTING REBILL REJECTED RESPONSE
Refer to Example “Rebill-Transaction Code B3”, “Rebill Rejected Response”, for illustration.
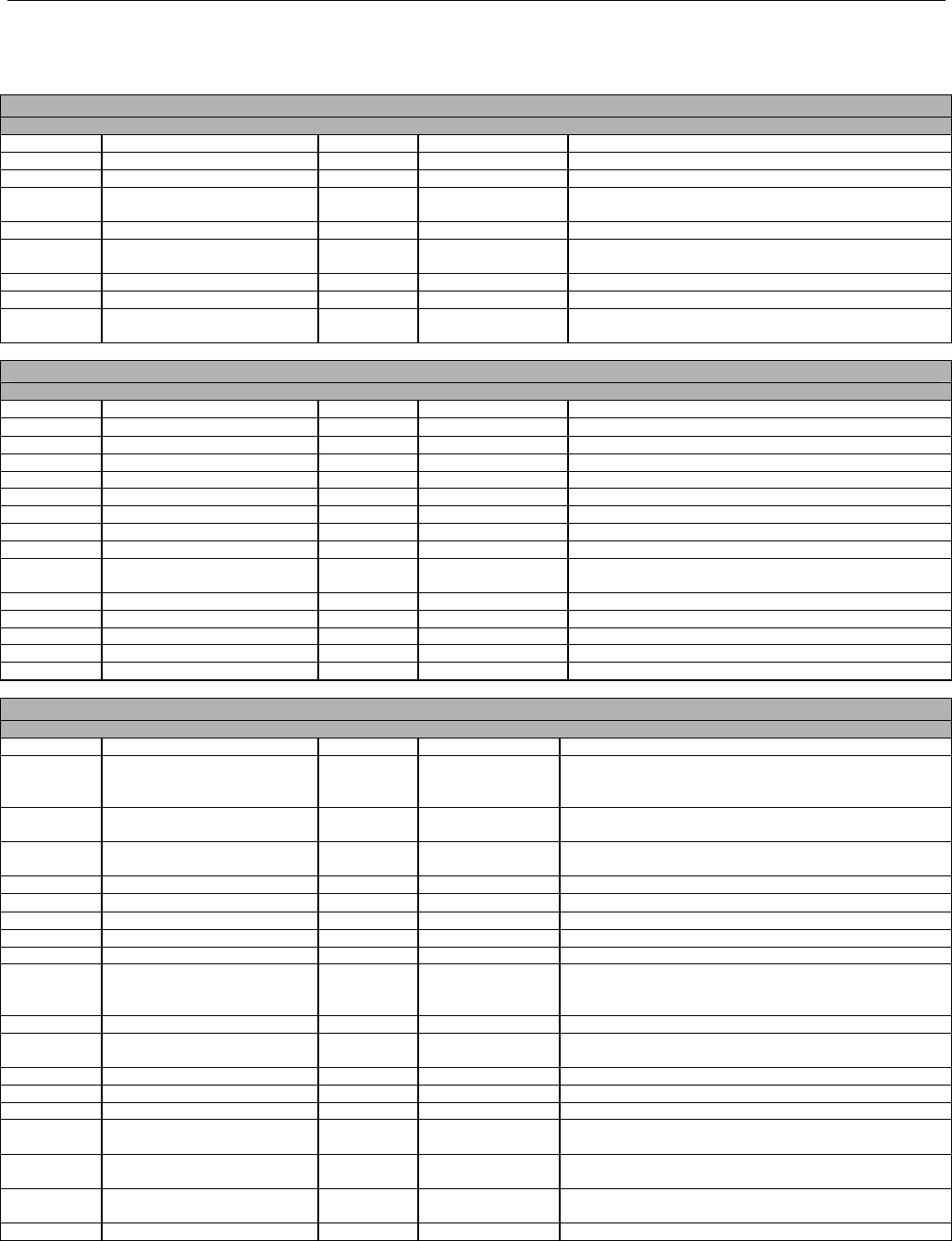
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 857 -
34.26 CONTROLLED SUBSTANCE REPORTING - TRANSACTION CODE C1
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M C1 Controlled Substance Reporting
1Ø4-A4 PROCESSOR CONTROL
NUMBER
M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø7 NCPDP Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663bbbbbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID
M 98765bbbbb
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø1 PATIENT SEGMENT
331-CX PATIENT ID QUALIFIER R Ø1 Social Security Number
332-CY PATIENT ID O 123456789 Patient’s SSN
3Ø4-C4 DATE OF BIRTH O 1962Ø615 Born June 15, 1962
3Ø5-C5 PATIENT GENDER CODE O 1 Male
31Ø-CA PATIENT FIRST NAME O JOSEPH
311-CB PATIENT LAST NAME O SMITH
322-CM PATIENT STREET ADDRESS O 123 MAIN STREET
323-CN PATIENT CITY ADDRESS O MY TOWN
324-CO PATIENT STATE/PROVINCE
ADDRESS
O CO
325-CP PATIENT ZIP/POSTAL ZONE O 34567
326-CQ PATIENT PHONE NUMBER O 2Ø14658923
3Ø7-C7 PLACE OF SERVICE O 1 Pharmacy
333-CZ EMPLOYER ID O XYZ123
334-1C SMOKER/NON-SMOKER CODE O 2 Smoker
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER
QUALIFIER
M 2 Service billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID
QUALIFIER
M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M 6Ø999Ø1211Ø Morphine sulf 3Ømg tab
442-E7 QUANTITY DISPENSED O 12ØØØØ 12Ø.ØØØ
4Ø3-D3 FILL NUMBER O Ø Original dispensing for RX#
4Ø5-D5 DAYS SUPPLY O 3Ø 3Ø Days supply
4Ø6-D6 COMPOUND CODE O 1 Not a compound
4Ø8-D8 DISPENSE AS WRITTEN
(DAW)/PRODUCT SELECTION
CODE
O Ø No product selection indicated
414-DE DATE PRESCRIPTION WRITTEN O 2ØØ7Ø915 September 15, 2ØØ7
415-DF NUMBER OF REFILLS
AUTHORIZED
O Ø No refills authorized
419-DJ PRESCRIPTION ORIGIN CODE O 1 Written prescription
46Ø-ET QUANTITY PRESCRIBED O 12ØØØØ 12Ø.ØØØ
3Ø8-C8 OTHER COVERAGE CODE O 1 No other coverage
429-DT SPECIAL PACKAGING
INDICATOR
O 1 Not unit dose
33Ø-CW ALTERNATE ID O MARIANNE
EVANS Person receiving Scheduled Rx
454-EK SCHEDULED PRESCRIPTION ID
NUMBER
O 6789Ø6789Ø67
6ØØ-28 UNIT OF MEASURE O EA Each
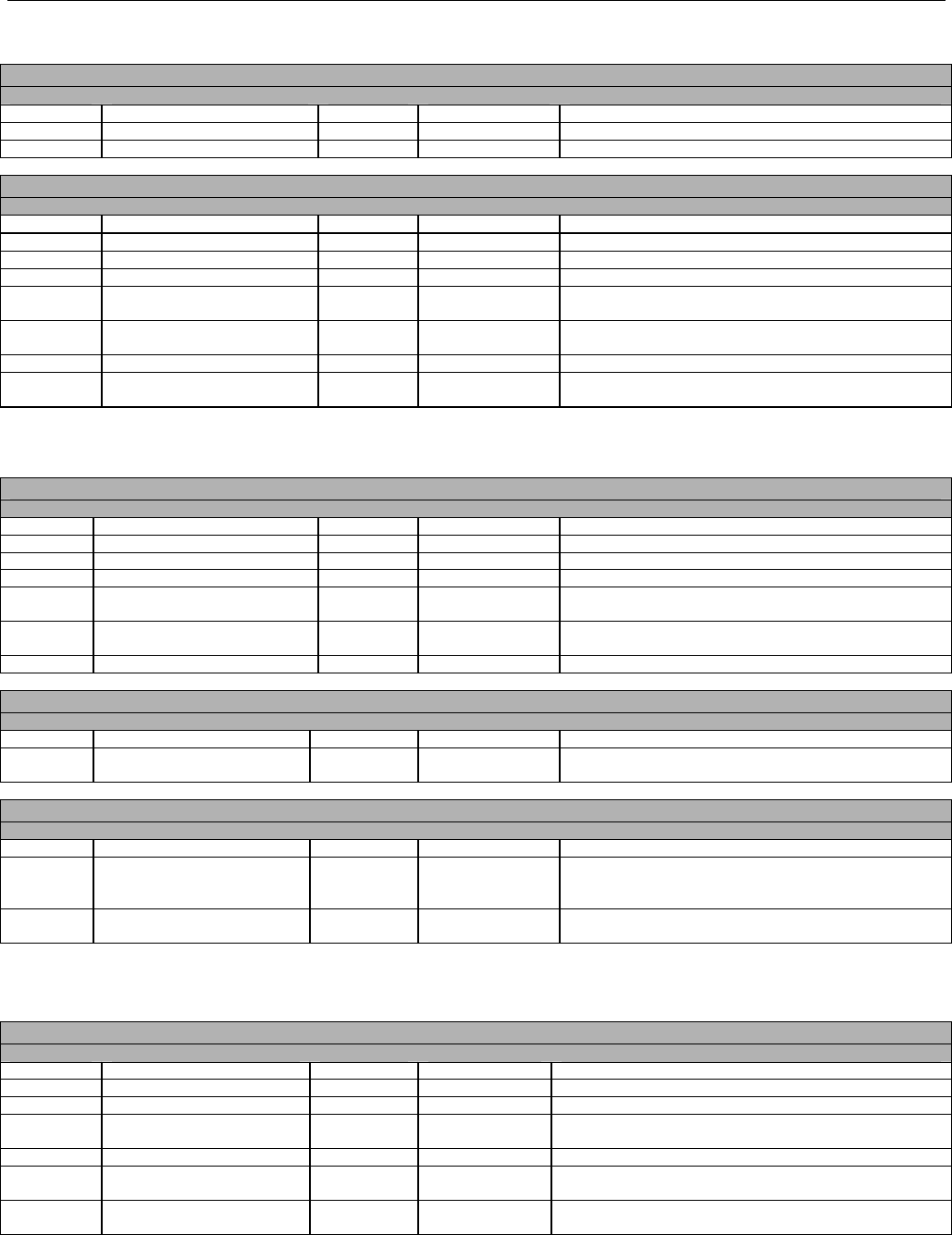
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 858 -
PHARMACY PROVIDER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø2 PROVIDER SEGMENT
465-EY PROVIDER ID QUALIFIER R Ø2 License number
444-E9 PROVIDER ID O 39359
PRESCRIBER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø3 PRESCRIBER SEGMENT
466-EZ PRESCRIBER ID QUALIFIER R Ø8 State license
411-DB PRESCRIBER ID O ØØG2345
427-DR PRESCRIBER LAST NAME O JONES
498-PM PRESCRIBER TELEPHONE
NUMBER
O 2Ø13639572
468-2E PRIMARY CARE PROVIDER ID
QUALIFIER
R 2 Blue Cross
421-DL PRIMARY CARE PROVIDER ID O 123456
47Ø-4E PRIMARY CARE PROVIDER LAST
NAME
O JONES
34.26.1 CONTROLLED SUBSTANCE REPORTING ACCEPTED RESPONSE-CAPTURED,
APPROVED RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M C1 Controlled Substance Reporting
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø7 NCPDP Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663bbbbbbb
b
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE
STATUS
M A OR C Approved or Captured
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER
QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
34.27 CONTROLLED SUBSTANCE REPORTING REVERSAL - TRANSACTION CODE
C2 TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M C2 Controlled Substance Reporting Reversal
1Ø4-A4 PROCESSOR CONTROL
NUMBER
M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø7 NCPDP Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663bbbbbb
bb
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 859 -
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID
M 98765bbbbb
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø1 PATIENT SEGMENT
331-CX PATIENT ID QUALIFIER R Ø1 Social Security Number
332-CY PATIENT ID O 123456789 Patient’s SSN
3Ø4-C4 DATE OF BIRTH O 1962Ø615 Born June 15, 1962
3Ø5-C5 PATIENT GENDER CODE O 1 Male
31Ø-CA PATIENT FIRST NAME O JOSEPH
311-CB PATIENT LAST NAME O SMITH
322-CM PATIENT STREET ADDRESS O 123 MAIN
STREET
323-CN PATIENT CITY ADDRESS O MY TOWN
324-CO PATIENT STATE/PROVINCE
ADDRESS
O CO
325-CP PATIENT ZIP/POSTAL ZONE O 34567
326-CQ PATIENT PHONE NUMBER O 2Ø14658923
3Ø7-C7 PLACE OF SERVICE O 1 Pharmacy
333-CZ EMPLOYER ID O XYZ123
334-1C SMOKER/NON-SMOKER CODE O 2 Smoker
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER
QUALIFIER
M 2 Service billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID
QUALIFIER
M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M 6Ø999Ø1211Ø Morphine sulf 3Ømg tab
34.27.1 CONTROLLED SUBSTANCE REPORTING REVERSAL ACCEPTED RESPONSE-
CAPTURED, APPROVED
Refer to Examples “Reversal-Transaction Code B2”, “Reversal Accepted Response”, for illustration.
34.28 CONTROLLED SUBSTANCE REPORTING REBILL - TRANSACTION CODE C3
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M C3 Controlled Substance Rebill
1Ø4-A4 PROCESSOR CONTROL
NUMBER
M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø7 NCPDP Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663bbbbbb
bb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID
M bbbbbbbbbb
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø1 PATIENT SEGMENT
331-CX PATIENT ID QUALIFIER R Ø1 Social Security Number
332-CY PATIENT ID O 123456789 Patient’s SSN
3Ø4-C4 DATE OF BIRTH O 1962Ø615 Born June 15, 1962
3Ø5-C5 PATIENT GENDER CODE O 1 Male
31Ø-CA PATIENT FIRST NAME O JOSEPH

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 860 -
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
311-CB PATIENT LAST NAME O SMITH
322-CM PATIENT STREET ADDRESS O 123 MAIN
STREET
323-CN PATIENT CITY ADDRESS O MY TOWN
324-CO PATIENT STATE/PROVINCE
ADDRESS
O CO
325-CP PATIENT ZIP/POSTAL ZONE O 34567
326-CQ PATIENT PHONE NUMBER O 2Ø14658923
3Ø7-C7 PLACE OF SERVICE O 1 Pharmacy
333-CZ EMPLOYER ID O XYZ123
334-1C SMOKER/NON-SMOKER CODE O
1 Smoker
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER
QUALIFIER
M 2 Service billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234568
436-E1 PRODUCT/SERVICE ID
QUALIFIER
M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ2255Ø
Ø2 Morphine sulfate15mg tab
442-E7 QUANTITY DISPENSED O 9ØØØØ 9Ø.ØØØ
4Ø3-D3 FILL NUMBER O Ø Original dispensing for RX#
4Ø5-D5 DAYS SUPPLY O 3Ø 3Ø Days supply
4Ø6-D6 COMPOUND CODE O 1 Not a compound
4Ø8-D8 DISPENSE AS WRITTEN
(DAW)/PRODUCT SELECTION
CODE
O Ø No product selection indicated
414-DE DATE PRESCRIPTION
WRITTEN
O 2ØØ7Ø915 September 15, 2ØØ7
415-DF NUMBER OF REFILLS
AUTHORIZED
O Ø No refills authorized
419-DJ PRESCRIPTION ORIGIN CODE O 1 Written prescription
46Ø-ET QUANTITY PRESCRIBED O 9ØØØØ 9Ø.ØØØ
3Ø8-C8 OTHER COVERAGE CODE O 1 No other coverage
429-DT SPECIAL PACKAGING
INDICATOR
O 1 Not unit dose
33Ø-CW ALTERNATE ID O MARIANNE
EVANS Person receiving Scheduled Rx
454-EK SCHEDULED RX ID NUMBER O 6789Ø6789Ø
68
6ØØ-28 UNIT OF MEASURE O EA Each
34.28.1 CONTROLLED SUBSTANCE REPORTING REBILL ACCEPTED RESPONSE-
CAPTURED, APPROVED
Refer to Example “Rebill-Transaction Code B3”, “Rebill Accepted Response”, for illustration. Note the examples differ in that the Pricing
Segment will not be present for any Controlled Substance Reporting transactions.
34.28.2 CONTROLLED SUBSTANCE REPORTING REBILL REJECTED RESPONSE
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M C3 Controlled Substance Rebill
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø7 NCPDP Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663bbbb
bbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 861 -
RESPONSE MESSAGE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 2Ø RESPONSE MESSAGE SEGMENT
5Ø4-F4 MESSAGE O TRANSMISSI
ON
MESSAGE
TEXT
For illustrative purposes only. Up to 2ØØ Bytes
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE
STATUS
M R Rejected
51Ø-FA REJECT COUNT R 3 3 Reject codes follow
511-FB REJECT CODE R 85 Claim not processed
511-FB REJECT CODE O 87 Reversal not processed
511-FB REJECT CODE O CY M/I Patient ID
5Ø3-F3 AUTHORIZATION NUMBER O 12345678912
3456789
13Ø-UF ADDITIONAL MESSAGE
INFORMATION COUNT
R 1 1 occurrence
132-UH ADDITIONAL MESSAGE
INFORMATION QUALIFIER
R Ø1 Used for first line of free form text with no pre-defined
structure.
526-FQ ADDITIONAL MESSAGE
INFORMATION
Q TRANSACTIO
N MESSAGE
TEXT
For illustrative purposes only. Up to 4Ø Bytes
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER O 6Ø2357Ø862
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER
QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234568
34.29 BILLING WITH DUR SEGMENT USING CO-AGENT FIELDS - TRANSACTION
CODE B1 (Ø1/Ø2)
Pharmacist submits resolved DUR conflicts on initial transaction.
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø4-A4 PROCESSOR CONTROL
NUMBER
M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111b
bbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID
M 98765bbbbb
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 123456789 Cardholder ID
CLAIM SEGMENT
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 862 -
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER
QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
436-E1 PRODUCT/SERVICE CODE
QUALIFIER
M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø942
68 Clinoril 2ØØmg
442-E7 QUANTITY DISPENSED R 3ØØØØ 3Ø.ØØØ tablets
4Ø3-D3 FILL NUMBER R Ø Original dispensing for RX#
4Ø5-D5 DAYS SUPPLY R 3Ø 3Ø Days supply
4Ø6-D6 COMPOUND CODE R 1 Not a compound
4Ø8-D8 DISPENSE AS WRITTEN
(DAW)/PRODUCT SELECTION
CODE
R Ø No product selection indicated
414-DE DATE PRESCRIPTION
WRITTEN
R 2ØØ7Ø915 September 15, 2ØØ7
415-DF NUMBER OF REFILLS
AUTHORIZED
Q 5 5 Refills
419-DJ PRESCRIPTION ORIGIN CODE Q 1 Written prescription
DUR/PPS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø8 DUR/PPS Segment
473-7E DUR/PPS CODE COUNTER R 1 1st DUR action
439-E4 REASON FOR SERVICE CODE Q DA Drug-Allergy alert
44Ø-E5 PROFESSIONAL SERVICE
CODE
Q MØ Prescriber consulted
441-E6 RESULT OF SERVICE CODE Q 1B Rx filled as is
474-8E DUR/PPS LEVEL OF EFFORT Q 11 Lowest level of complexity
473-7E DUR/PPS CODE COUNTER R 2 2nd DUR action
439-E4 REASON FOR SERVICE CODE Q LR Underutilization
44Ø-E5 PROFESSIONAL SERVICE
CODE
Q PØ Patient consulted
441-E6 RESULT OF SERVICE CODE Q 1B Rx filled as is
474-8E DUR/PPS LEVEL OF EFFORT Q 11 Lowest level of complexity
473-7E DUR/PPS CODE COUNTER R 3 3rd DUR action
439-E4 REASON FOR SERVICE CODE Q TD Therapeutic duplication
44Ø-E5 PROFESSIONAL SERVICE
CODE
Q MØ Prescriber consulted
441-E6 RESULT OF SERVICE CODE Q 1B Rx filled as is
474-8E DUR/PPS LEVEL OF EFFORT Q 11 Lowest level of complexity
475-J9 DUR CO-AGENT ID
QUALIFIER
R Ø3 NDC
476-H6 DUR CO-AGENT ID Q 17236Ø569Ø
1 Ibuprofen 6ØØmg tablet
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 557{ $55.7Ø
412-DC DISPENSING FEE SUBMITTED Q 1ØØ{ $1Ø.ØØ
433-DX PATIENT PAID AMOUNT
SUBMITTED
Q 1ØØ{ $1Ø.ØØ
478-H7 OTHER AMOUNT CLAIMED
SUBMITTED COUNT
R 1 One occurrence
479-H8 OTHER AMOUNT CLAIMED
SUBMITTED QUALIFIER
R Ø1 Delivery cost
48Ø-H9 OTHER AMOUNT CLAIMED
SUBMITTED
Q 15Ø{ $15.ØØ
426-DQ USUAL AND CUSTOMARY
CHARGE
Q 716{ $71.65
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 863 -
43Ø-DU GROSS AMOUNT DUE R 8Ø7{ $8Ø.7Ø
423-DN BASIS OF COST
DETERMINATION
Q Ø3 Direct
34.29.1 BILLING WITH DUR SEGMENT USING CO-AGENT FIELDS —PAID (DUPLICATE
OF PAID)
Processor accepts pharmacist’s DUR submission. The processor system detected the same LR (Underutilization) and TD (Therapeutic
Duplication) with a previously filled ibuprofen prescription, but suppresses these DUR Alerts since the pharmacist told the processor about
them and his resultant activities in the claim submission.
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111b
bbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE
STATUS
M P OR D Paid or Duplicate of Paid
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER
QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION /SERVICE
REFERENCE NUMBER
M 1234567
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 1ØØ{ $1Ø.ØØ
5Ø6-F6 INGREDIENT COST PAID Q 557{ $55.7Ø
5Ø7-F7 DISPENSING FEE PAID Q 1ØØ{ $1Ø.ØØ
557-AV TAX EXEMPT INDICATOR Q 1 Payer/Plan is Tax Exempt (The Payer/Plan is not
responsible for tax. The patient may be charged tax.)
563-J2 OTHER AMOUNT PAID COUNT R 1 One occurrence
564-J3 OTHER AMOUNT PAID
QUALIFIER
R Ø1 Delivery cost
565-J4 OTHER AMOUNT PAID Q 15Ø{ $15.ØØ
5Ø9-F9 TOTAL AMOUNT PAID Q 7Ø7{ $7Ø.7Ø
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 1 Ingredient cost paid as submitted
34.29.2 BILLING WITH DUR SEGMENT USING CO-AGENT FIELDS —PAID, BUT WITH A
DIFFERENT DUR MESSAGE REPORTED
Processor accepts pharmacist’s DUR submission in example above, suppresses similar DUR Alerts based on Co-Agent fields, but returns an
additional Therapeutic Duplication DUR message due to a different profiled drug than was submitted by the pharmacist.
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 864 -
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111b
bbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE
STATUS
M P Paid
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER
QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
RESPONSE DUR/PPS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 24 RESPONSE DUR/PPS SEGMENT
567-J6 DUR/PPS RESPONSE CODE
COUNTER
R 1 1st DUR conflict
439-E4 REASON FOR SERVICE CODE Q TD Therapeutic Duplication
529-FT OTHER PHARMACY INDICATOR Q 3 Different pharmacy
53Ø-FU PREVIOUS DATE OF FILL Q 2ØØ7Ø913 September 13, 2ØØ7
531-FV QUANTITY OF PREVIOUS FILL Q 9Ø
532-FW DATABASE INDICATOR Q 5 Other
533-FX OTHER PRESCRIBER
INDICATOR
Q 2 Different prescriber
544-FY DUR FREE TEXT Q NALFON
6ØØMG TAB
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 1ØØ{ $1Ø.ØØ
5Ø6-F6 INGREDIENT COST PAID Q 557{ $55.7Ø
5Ø7-F7 DISPENSING FEE PAID Q 1ØØ{ $1Ø.ØØ
557-AV TAX EXEMPT INDICATOR Q 1 Payer/Plan is Tax Exempt (The Payer/Plan is not
responsible for tax. The patient may be charged tax.)
563-J2 OTHER AMOUNT PAID COUNT R 1 One occurrence
564-J3 OTHER AMOUNT PAID
QUALIFIER
R Ø1 Delivery cost
565-J4 OTHER AMOUNT PAID Q 15Ø{ $15.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 7Ø7{ $7Ø.7Ø
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 1 Ingredient cost paid as submitted
34.30 BILLING PAID RESPONSE USING DUR ADDITIONAL TEXT – TRANSACTION
CODE B1 (Ø1/Ø2)
Paid Claim Response with a DUR Message. Note that no corresponding Submission example exists.
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø1 National Provider ID
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 865 -
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bb
bbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE
STATUS
M P Paid
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER
QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
RESPONSE DUR/PPS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 24 RESPONSE DUR/PPS SEGMENT
567-J6 DUR/PPS RESPONSE CODE
COUNTER
R 1 1st DUR conflict
439-E4 REASON FOR SERVICE CODE Q DD Drug-Drug Interaction
528-FS CLINICAL SIGNIFICANCE CODE Q 1 Severity Level 1
529-FT OTHER PHARMACY INDICATOR Q 3 Different pharmacy
53Ø-FU PREVIOUS DATE OF FILL Q 2ØØ7Ø815 August 15, 2ØØ7
531-FV QUANTITY OF PREVIOUS FILL Q 9Ø
532-FW DATABASE INDICATOR Q 5 Other
533-FX OTHER PRESCRIBER
INDICATOR
Q 2 Different prescriber
544-FY DUR FREE TEXT Q ASPIRIN
325MG TAB
57Ø –NS DUR ADDITIONAL TEXT Q ONSET=RAPID
;
DOCUMENTAT
ION=ESTABLI
SHED
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 1ØØ{ $1Ø.ØØ
5Ø6-F6 INGREDIENT COST PAID Q 557{ $55.7Ø
5Ø7-F7 DISPENSING FEE PAID Q 1ØØ{ $1Ø.ØØ
557-AV TAX EXEMPT INDICATOR Q 1 Payer/Plan is Tax Exempt (The Payer/Plan is not
responsible for tax. The patient may be charged tax.)
563-J2 OTHER AMOUNT PAID COUNT R 1 One occurrence
564-J3 OTHER AMOUNT PAID
QUALIFIER
R Ø1 Delivery
565-J4 OTHER AMOUNT PAID Q 15Ø{ $15.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 7Ø7{ $7Ø.7Ø
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 1 Ingredient cost paid as submitted
34.31 BILLING - TRANSACTION CODE B1 WITH ADDITIONAL DOCUMENTATION
SEGMENT
This example illustrates how a pharmacy can electronically submit a Medicare form for an immunosuppressive drug required for a Medicare
claim by answering questions using the Additional Documentation Segment.
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 866 -
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø4-A4 PROCESSOR CONTROL
NUMBER
M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø4 Medicare Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663bbbbbbb
b
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID
M 98765bbbbb
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø1 PATIENT SEGMENT
3Ø4-C4 DATE OF BIRTH R 1962Ø615 Born June 15, 1962
3Ø5-C5 PATIENT GENDER CODE R 1 Male
31Ø-CA PATIENT FIRST NAME R JOSEPH
311-CB PATIENT LAST NAME R SMITH
322-CM PATIENT STREET ADDRESS O 123 MAIN STREET
323-CN PATIENT CITY ADDRESS O MY TOWN
324-CO PATIENT STATE/PROVINCE
ADDRESS
O CO
325-CP PATIENT ZIP/POSTAL ZONE O 34567
326-CQ PATIENT PHONE NUMBER O 2Ø14658923
384-4X PATIENT RESIDENCE Q 1 Home
333-CZ EMPLOYER ID Q 5ØZ123
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 987654321A Medicare Cardholder ID
312-CC CARDHOLDER FIRST NAME Q JOSEPH
313-CD CARDHOLDER LAST NAME Q SMITH
359-2A MEDIGAP ID Q TXMEDICAID Designation for Medicare that this is a Texas Medicaid
client
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER
QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID
QUALIFIER
M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ9Ø11312 Injection, Methylprednisolone Sodium Succinate, Up to
4ØMG
442-E7 QUANTITY DISPENSED R 1
4Ø3-D3 FILL NUMBER R Ø Original dispensing for RX#
4Ø5-D5 DAYS SUPPLY R 3Ø 3Ø Days supply
4Ø6-D6 COMPOUND CODE R 1 Not a compound
4Ø8-D8 DISPENSE AS WRITTEN
(DAW)/PRODUCT SELECTION
CODE
R Ø No product selection indicated
414-DE DATE PRESCRIPTION
WRITTEN
R 2ØØ7Ø915 September 15, 2ØØ7
415-DF NUMBER OF REFILLS
AUTHORIZED
Q 5 5 Refills
419-DJ PRESCRIPTION ORIGIN CODE Q 1 Written prescription
354-NX SUBMISSION CLARIFICATION
CODE COUNT
R 1 One occurrence
42Ø-DK SUBMISSION CLARIFICATION Q 11 Certification on File- The supplier's guarantee that a
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 867 -
CODE copy of the paper certification, signed and dated by the
physician, is on file at the supplier's office
3Ø8-C8 OTHER COVERAGE CODE Q 1 No other coverage
429-DT SPECIAL PACKAGING
INDICATOR
Q 1 Not unit dose
6ØØ-28 UNIT OF MEASURE Q EA Each
PRESCRIBER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø3 PRESCRIBER SEGMENT
466-EZ PRESCRIBER ID QUALIFIER R Ø1 National Provider ID
411-DB PRESCRIBER ID Q 1123456111
427-DR PRESCRIBER LAST NAME Q JONES
498-PM PRESCRIBER TELEPHONE
NUMBER
Q 2Ø13639572
468-2E PRIMARY CARE PROVIDER ID
QUALIFIER
R Ø1 National Provider ID
421-DL PRIMARY CARE PROVIDER ID Q 1234566111
47Ø-4E PRIMARY CARE PROVIDER
LAST NAME
Q WRIGHT
364-2J PRESCRIBER FIRST NAME Q SALLY
365-2K PRESCRIBER STREET
ADDRESS
Q 345 NOPLACE RD
366-2M PRESCRIBER CITY ADDRESS Q ANYTOWN
367-2N PRESCRIBER
STATE/PROVINCE ADDRESS
Q CO
368-2P PRESCRIBER ZIP/POSTAL
ZONE
Q 123456789
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 557{ $55.7Ø
412-DC DISPENSING FEE SUBMITTED Q 1ØØ{ $1Ø.ØØ
433-DX PATIENT PAID AMOUNT
SUBMITTED
Q 1ØØ{ $1Ø.ØØ
478-H7 OTHER AMOUNT CLAIMED
SUBMITTED COUNT
R 1 One occurrence
479-H8 OTHER AMOUNT CLAIMED
SUBMITTED QUALIFIER
R Ø1 Delivery cost
48Ø-H9 OTHER AMOUNT CLAIMED
SUBMITTED
Q 15Ø{ $15.ØØ
426-DQ USUAL AND CUSTOMARY
CHARGE
Q 7ØØ{ $7Ø.ØØ
43Ø-DU GROSS AMOUNT DUE R 8Ø7{ $8Ø.7Ø
423-DN BASIS OF COST
DETERMINATION
Q Ø3 Direct
ADDITIONAL DOCUMENTATION SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 14 ADDITIONAL DOCUMENTATION SEGMENT
369-2Q ADDITIONAL DOCUMENTATION
TYPE ID
M Ø11 Medicare Ø8.Ø2
Immunosuppressive Drugs
374-2V REQUEST PERIOD BEGIN
DATE
Q 2ØØ7Ø915 September 15, 2ØØ7
373-2U REQUEST STATUS Q 1 Initial
371-2S LENGTH OF NEED QUALIFIER R 4 Months
37Ø-2R LENGTH OF NEED Q 6
372-2T PRESCRIBER/SUPPLIER DATE
SIGNED
Q 2ØØ7Ø915 September 15, 2ØØ7
377-2Z QUESTION NUMBER/LETTER
COUNT
R 11
378-4B QUESTION NUMBER/LETTER R 1A What drugs are prescribed (HCPCS)
383-4K QUESTION ALPHANUMERIC
RESPONSE
Q J292Ø
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 868 -
378-4B QUESTION NUMBER/LETTER R 1B What drugs are prescribed (dosage)
382-4J QUESTION NUMERIC
RESPONSE
Q 4Ø 4Ø MG
378-4B QUESTION NUMBER/LETTER R 1C What drugs are prescribed (frequency per day)
382-4J QUESTION NUMERIC
RESPONSE
Q 1 Once per day
378-4B QUESTION NUMBER/LETTER R 4 Has patient had an organ transplant…
383-4K QUESTION ALPHANUMERIC
RESPONSE
Q Y Patient had an organ transplant covered
378-4B QUESTION NUMBER/LETTER R 5A Which organs
383-4K QUESTION ALPHANUMERIC
RESPONSE
Q 1 Heart
378-4B QUESTION NUMBER/LETTER R 5B Which organs
383-4K QUESTION ALPHANUMERIC
RESPONSE
Q 3 Kidney
378-4B QUESTION NUMBER/LETTER R 8 Name of facility
383-4K QUESTION ALPHANUMERIC
RESPONSE
Q HEART INSTITUTE
378-4B QUESTION NUMBER/LETTER R 9 City where facility…
383-4K QUESTION ALPHANUMERIC
RESPONSE
Q HEARTSVILLE
378-4B QUESTION NUMBER/LETTER R 1Ø State where facility…
383-4K QUESTION ALPHANUMERIC
RESPONSE
Q MO
378-4B QUESTION NUMBER/LETTER R 11 Discharge Date
38Ø-4G QUESTION DATE RESPONSE Q 2ØØ7Ø911 September 11, 2ØØ7
378-4B QUESTION NUMBER/LETTER R 12 Any prior transplant failure of same…
383-4K QUESTION ALPHANUMERIC
RESPONSE
Q N No
61ØØ66DØB1123456789Ø1Ø44563663bbbbbbbb2ØØ7Ø915198765bbbbb<1E><1C>Ø1<1C>C41962Ø615<1C>C51<1C>JOSEPH<1C>SMI
TH<1C>CM123 MAIN STREET<1C>CNMY TOWN<1C>COCO<1C>CP34567<1C>CQ2Ø14658923<1C>4X1<1C>CZ5ØZ123<1E><1C>AMØ4<1C
>C2987654321A<1C>CCJOSEPH<1C>CDSMITH<1C>2ATXMEDICAID<1C>AMØ7<1C>EM1<1C>D21234567<1C>E1Ø3<1C>D7ØØØØ9Ø11312
<1C>E71<1C>D3Ø<1C>D53Ø<1C>D61<1C>D8Ø<1C>DE2ØØ3Ø5Ø1<1C>DF5<1C>DJ1<1C>NX1<1C>DK11<1C>C82<1C>DT1<1C>28EA<1E>
<1C>AMØ3<1C>EZØ1<1C>D81123456<1C>DRJONES<1C>PM2Ø13639572<1C>2E1<1C>DL1234566<1C>H51Ø1<1C>4EWRIGHT<1C>2JSA
LLY<1C>2K345 NOPLACE RD<1C>2MANYTOWN<1C>2NCO<1C>2P123456789<1E><1C>AM11<1C>D9557{<1C>DC1ØØ{<1C>DX1ØØ{<1C>
H71<1C>H8Ø1<1C>H915Ø{<1C>DQ7ØØ{<1C>DU8Ø7{<1C>DNØ3<1E><1C>AM14<1C>2QØ11<1C>2V2ØØ7Ø915<1C>2U1<1C>2S4<1C>2R6
<1C>2T2ØØ7Ø915<1C>2Z11<1C>4B1A<1C>4KJ292Ø<1C>4B1B<1C>4J4O<1C>4B1C<1C>4J1<1C>4B4<1C>4KY<1C>4B5A<1C>4K1<1C>
4B5B<1C>4K3<1C>4B8<1C>4KHEART INSTITUTE<1C>4B9<1C>4KHEARTSVILLE<1C>4b1Ø<1C>4KMO<1C>4B11<1C>4G2ØØ7Ø911<1C>
4B12<1C>4KN
34.31.1 BILLING ACCEPTED RESPONSE- PAID
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø4 Medicare Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663bbbbbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
RESPONSE MESSAGE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 2Ø RESPONSE MESSAGE SEGMENT
5Ø4-F4 MESSAGE Q TRANSMISSION
MESSAGE TEXT For illustrative purposes only. Up to 2ØØ Bytes
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE
STATUS
M P Paid
5Ø3-F3 AUTHORIZATION NUMBER Q 1234567891234567
89
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 869 -
13Ø-UF ADDITIONAL MESSAGE
INFORMATION COUNT
R 1 1 occurrence
132-UH ADDITIONAL MESSAGE
INFORMATION QUALIFIER
R Ø1 Used for first line of free form text with no pre-defined
structure.
526-FQ ADDITIONAL MESSAGE
INFORMATION
Q TRANSACTION
MESSAGE TEXT For illustrative purposes only. Up to 4Ø Bytes
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 1ØØ{ $1Ø.ØØ
5Ø6-F6 INGREDIENT COST PAID Q 557{ $55.7Ø
5Ø7-F7 DISPENSING FEE PAID Q 1ØØ{ $1Ø.ØØ
557-AV TAX EXEMPT INDICATOR Q 1 Payer/Plan is Tax Exempt (The Payer/Plan is not
responsible for tax. The patient may be charged tax.)
563-J2 OTHER AMOUNT PAID COUNT R 1 One occurrence
564-J3 OTHER AMOUNT PAID QUALIFIER R Ø1 Delivery
565-J4 OTHER AMOUNT PAID Q 15Ø{ $15.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 7Ø7{ $7Ø.7Ø
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 1 Ingredient cost paid as submitted
523-FN AMOUNT ATTRIBUTED TO SALES
TAX
Q 2Ø{ $2.ØØ
518-FI AMOUNT OF COPAY Q 8Ø{ $8.ØØ
34.32 BILLING - TRANSACTION CODE B1 WITH FACILITY INFORMATION
The example displays the request portion only.
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø4 Medicare Provider ID
2Ø1-B1 SERVICE PROVIDER ID M Ø12347ØØØ1bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID
M 98765bbbbb
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø1 PATIENT SEGMENT
3Ø4-C4 DATE OF BIRTH R 1962Ø615 Born June 15, 1962
3Ø5-C5 PATIENT GENDER CODE R 1 Male
31Ø-CA PATIENT FIRST NAME R JOSEPH
311-CB PATIENT LAST NAME R SMITH
322-CM PATIENT STREET ADDRESS O 123 MAIN STREET
322-CN PATIENT CITY ADDRESS O MY TOWN
324-CO PATIENT STATE/PROVINCE
ADDRESS O CO
325-CP PATIENT ZIP/POSTAL ZONE O 34567
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 870 -
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
326-CQ PATIENT PHONE NUMBER O 2Ø14658923
384-4X PATIENT RESIDENCE Q 11 Hospice
333-CZ EMPLOYER ID Q 5ØZ123
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 987654321A Medicare Cardholder ID
312-CC CARDHOLDER FIRST NAME Q JOSEPH
313-CD CARDHOLDER LAST NAME Q SMITH
359-2A MEDIGAP ID Q TXMEDICAID Designation for Medicare that this is a Texas
Medicaid client
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER
QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID
QUALIFIER
M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94228 Clinoril 2ØØmg
442-E7 QUANTITY DISPENSED R 3ØØØØ 3Ø.ØØØ tablets
4Ø3-D3 FILL NUMBER R Ø Original dispensing for RX#
4Ø5-D5 DAYS SUPPLY R 3Ø 3Ø Days supply
4Ø6-D6 COMPOUND CODE R 1 Not a compound
4Ø8-D8 DISPENSE AS WRITTEN
(DAW)/PRODUCT SELECTION
CODE
R Ø No product selection indicated
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ7Ø915 September 15, 2ØØ7
415-DF NUMBER OF REFILLS
AUTHORIZED
Q 5 5 Refills
3Ø8-C8 OTHER COVERAGE CODE Q 1 No other coverage
429-DT SPECIAL PACKAGING
INDICATOR
Q 1 Not unit dose
6ØØ-28 UNIT OF MEASURE Q EA Each
PRESCRIBER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø3 PRESCRIBER SEGMENT
466-EZ PRESCRIBER ID QUALIFIER R Ø1 National Provider ID
411-DB PRESCRIBER ID Q 1123456111
427-DR PRESCRIBER LAST NAME Q JONES
498-PM PRESCRIBER TELEPHONE
NUMBER
Q 2Ø13639572
364-2J PRESCRIBER FIRST NAME Q SALLY
365-2K PRESCRIBER STREET ADDRESS Q 345 NOPLACE RD
366-2M PRESCRIBER CITY ADDRESS Q ANYTOWN
367-2N PRESCRIBER STATE/PROVINCE
ADDRESS Q CO
368-2P PRESCRIBER ZIP/POSTAL ZONE Q 123456789
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 557{ $55.7Ø
412-DC DISPENSING FEE SUBMITTED Q 1ØØ{ $1Ø.ØØ
433-DX PATIENT PAID AMOUNT
SUBMITTED
Q 1ØØ{ $1Ø.ØØ
478-H7 OTHER AMOUNT CLAIMED R 1 One occurrence
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 871 -
SUBMITTED COUNT
479-H8 OTHER AMOUNT CLAIMED
SUBMITTED QUALIFIER
R Ø1 Delivery cost
48Ø-H9 OTHER AMOUNT CLAIMED
SUBMITTED
Q 15Ø{ $15.ØØ
426-DQ USUAL AND CUSTOMARY
CHARGE
Q 7ØØ{ $7Ø.ØØ
43Ø-DU GROSS AMOUNT DUE R 8Ø7{ $8Ø.7Ø
423-DN BASIS OF COST DETERMINATION Q Ø3 Direct
FACILITY SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 15 FACILITY SEGMENT
336-8C FACILITY ID Q 123456789Ø
385-3Q FACILITY NAME Q RONALD
MCDONALD
HOUSE
386-3U FACILITY STREET ADDRESS Q 789 HOSPICE RD
388-5J FACILITY CITY ADDRESS Q ANYTOWN
387-3V FACILITY STATE/PROVINCE
ADDRESS Q CO
389-6D FACILITY ZIP/POSTAL ZONE Q 123456789
34.33 BILLING - TRANSACTION CODE B1 WITH ADDITIONAL DOCUMENTATION AND
FACILITY INFORMATION
The example displays the request portion only. This example illustrates how a pharmacy can electronically submit answers to a Medicare form
using the Additional Documentation Segment and Facility Segment.
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ TRANSACTION FORMAT
1Ø3-A3 TRANSACTION CODE M B1 BILLING
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø4 Medicare Provider ID
2Ø1-B1 SERVICE PROVIDER ID M Ø12347ØØØ1bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE/VENDOR
CERTIFICATION ID M 98765bbbbb
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø1 Patient Segment
3Ø4-C4 DATE OF BIRTH R 1962Ø615 Born June 15, 1962
3Ø5-C5 PATIENT GENDER CODE R 1 Male
31Ø-CA PATIENT FIRST NAME R JOSEPH
311-CB PATIENT LAST NAME R SMITH
322-CM PATIENT STREET ADDRESS O 123 MAIN STREET
322-CN PATIENT CITY ADDRESS O MY TOWN
324-CO PATIENT STATE/PROVINCE
ADDRESS O CO
325-CP PATIENT ZIP/POSTAL ZONE O 34567
326-CQ PATIENT PHONE NUMBER O 2Ø14658923
384-4X PATIENT RESIDENCE Q 11 Hospice
333-CZ EMPLOYER ID Q 5ØZ123
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 Insurance Segment
3Ø2-C2 CARDHOLDER ID M 987654321A Medicare Cardholder ID
312-CC CARDHOLDER FIRST NAME Q JOSEPH
313-CD CARDHOLDER LAST NAME Q SMITH
359-2A MEDIGAP ID Q TXMEDICAID Designation for Medicare that this is a Texas
Medicaid client
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 872 -
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 Claim Segment
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID
QUALIFIER
M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ9Ø11312 INJECTION, METHYLPREDNISOLONE SODIUM
SUCCINATE, UP TO 4ØMG
442-E7 QUANTITY DISPENSED R 1 4ØMG
4Ø3-D3 FILL NUMBER R Ø Original dispensing for RX#
4Ø5-D5 DAYS SUPPLY R 3Ø 3Ø days supply
4Ø6-D6 COMPOUND CODE R 1 Not a compound
4Ø8-D8 DISPENSE AS WRITTEN
(DAW)/PRODUCT SELECTION
CODE
R Ø No product selection indicated
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ7Ø915 September 15, 2ØØ7
415-DF NUMBER OF REFILLS
AUTHORIZED
Q 5 5 Refills
419-DJ PRESCRIPTION ORIGIN CODE Q 1 Written prescription
354-NX SUBMISSION CLARIFICATION
CODE COUNT
R 1
42Ø-DK SUBMISSION CLARIFICATION
CODE
Q 11 Certification on File- The supplier's guarantee that
a copy of the paper certification, signed and dated
by the physician, is on file at the supplier's office
3Ø8-C8 OTHER COVERAGE CODE Q 1 No other coverage
429-DT SPECIAL PACKAGING
INDICATOR
Q 1 Not Unit Dose
6ØØ-28 UNIT OF MEASURE Q EA Each
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 Pricing Segment
4Ø9-D9 INGREDIENT COST SUBMITTED R 557{ $55.7Ø
412-DC DISPENSING FEE SUBMITTED Q 1ØØ{ $1Ø.ØØ
433-DX PATIENT PAID AMOUNT
SUBMITTED
Q 1ØØ{ $1Ø.ØØ
478-H7 OTHER AMOUNT CLAIMED
SUBMITTED COUNT
R 1 One occurrence
479-H8 OTHER AMOUNT CLAIMED
SUBMITTED QUALIFIER
R Ø1 Delivery Cost
48Ø-H9 OTHER AMOUNT CLAIMED
SUBMITTED
Q 15Ø{ $15.ØØ
426-DQ USUAL AND CUSTOMARY CHARGE Q 7ØØ{ $7Ø.ØØ
43Ø-DU GROSS AMOUNT DUE R 8Ø7{ $8Ø.7Ø
423-DN BASIS OF COST DETERMINATION Q Ø3 Direct
PRESCRIBER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø3 Prescriber Segment
466-EZ PRESCRIBER ID QUALIFIER R Ø1 National Provider ID
411-DB PRESCRIBER ID Q 1123451111
427-DR PRESCRIBER LAST NAME Q JONES
498-PM PRESCRIBER TELEPHONE NUMBER Q 2Ø13639572
364-2J PRESCRIBER FIRST NAME Q SALLY
365-2K PRESCRIBER STREET ADDRESS Q 345 NOPLACE RD
366-2M PRESCRIBER CITY ADDRESS Q ANYTOWN
367-2N PRESCRIBER STATE/PROVINCE
ADDRESS Q CO
368-2P PRESCRIBER ZIP/POSTAL ZONE Q 123456789
ADDITIONAL DOCUMENTATION SEGMENT
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 873 -
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 14 Additional Documentation Segment
369-2Q ADDITIONAL DOCUMENTATION
TYPE ID
M Ø11 DMERC INFORMATION FORM –
IMMUNOSUPPRESSIVE DRUGS
374-2V REQUEST PERIOD BEGIN DATE Q 2ØØ7Ø915 September 15, 2ØØ7
373-2U REQUEST STATUS Q 1 1 = INITIAL
371-2S LENGTH OF NEED QUALIFIER R 4 4 = MONTHS
37Ø-2R LENGTH OF NEED Q 6 6 MONTHS
372-2T PRESCRIBER/SUPPLIER DATE
SIGNED
Q 2ØØ7Ø915 September 15, 2ØØ7
377-2Z QUESTION NUMBER/LETTER
COUNT
R 11
378-4B QUESTION NUMBER/LETTER R 1A What drugs are prescribed (HCPCS)
383-4K QUESTION ALPHANUMERIC
RESPONSE
Q J292Ø
378-4B QUESTION NUMBER/LETTER R 1B What drugs are prescribed (Dosage)
382-4J QUESTION NUMERIC/RESPONSE Q 4Ø 4Ø MG
378-4B QUESTION NUMBER/LETTER R 1C What drugs are prescribed (Frequency per day)
382-4J QUESTION NUMERIC/RESPONSE Q 1 Once per day
378-4B QUESTION NUMBER/LETTER R 4 Has patient had an organ transplant
383-4K QUESTION ALPHANUMERIC
RESPONSE
Q Y Patient had an organ transplant covered…
378-4B QUESTION NUMBER/LETTER R 5A Which organ(s)
383-4K QUESTION ALPHANUMERIC
RESPONSE
Q 1 1 = HEART
378-4B QUESTION NUMBER/LETTER R 5B Which organ(s)
383-4K QUESTION ALPHANUMERIC
RESPONSE
Q 3 3 = KIDNEY
378-4B QUESTION NUMBER/LETTER R 8 Name of Facility
383-4K QUESTION ALPHANUMERIC
RESPONSE
Q HEART INSTITUTE
378-4B QUESTION NUMBER/LETTER R 9 City where facility…
383-4K QUESTION ALPHANUMERIC
RESPONSE
Q HEARTSVILLE
378-4B QUESTION NUMBER/LETTER R 1Ø State where facility…
383-4K QUESTION ALPHANUMERIC
RESPONSE
Q MO
378-4B QUESTION NUMBER/LETTER R 11 Discharge Date
38Ø-4G QUESTION DATE RESPONSE Q 2ØØ7Ø911 September 11, 2ØØ7
378-4B QUESTION NUMBER/LETTER R 12 Any prior transplant failure of same
383-4K QUESTION ALPHANUMERIC
RESPONSE
Q N No
FACILITY SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 15 Facility Segment
336-8C FACILITY ID Q 123456789Ø
385-3Q FACILITY NAME Q RONALD
MCDONALD
HOUSE
386-3U FACILITY STREET ADDRESS Q 789 HOSPICE RD
388-5J FACILITY CITY ADDRESS Q ANYTOWN
387-3V FACILITY STATE/PROVINCE
ADDRESS Q CO
389-6D FACILITY ZIP/POSTAL ZONE Q 123456789
34.34 BILLING - TRANSACTION CODE B1 WITH NARRATIVE INFORMATION
The example displays the request portion only. The Narrative Segment was submitted to provide information necessary for claim payment.
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 874 -
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø4 Medicare Provider ID
2Ø1-B1 SERVICE PROVIDER ID M Ø12347ØØØ1bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID M 98765bbbbb
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø1 Patient Segment
3Ø4-C4 DATE OF BIRTH R 1962Ø615 Born June 15, 1962
3Ø5-C5 PATIENT GENDER CODE R 1 Male
31Ø-CA PATIENT FIRST NAME R JOSEPH
311-CB PATIENT LAST NAME R SMITH
322-CM PATIENT STREET ADDRESS O 123 MAIN STREET
322-CN PATIENT CITY ADDRESS O MY TOWN
324-CO PATIENT STATE/PROVINCE
ADDRESS O CO
325-CP PATIENT ZIP/POSTAL ZONE O 34567
326-CQ PATIENT PHONE NUMBER O 2Ø14658923
384-4X PATIENT RESIDENCE Q 1 Home
333-CZ EMPLOYER ID Q 5ØZ123
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 Insurance Segment
3Ø2-C2 CARDHOLDER ID M 987654321A Medicare Cardholder ID
312-CC CARDHOLDER FIRST NAME Q JOSEPH
313-CD CARDHOLDER LAST NAME Q SMITH
359-2A MEDIGAP ID Q TXMEDICAID Designation for Medicare that this is a Texas
Medicaid client
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 Claim Segment
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER M 1 Rx Billing
4Ø2-D2 PRESCRIBER/SERVICE
REFERENCE NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID
QUALIFIER
M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØ548Ø6311 ALBUTEROL SULFATE
442-E7 QUANTITY DISPENSED R 15ØØØØ 15Ø.ØØØ ML
4Ø3-D3 FILL NUMBER R Ø Original dispensing for Rx#
4Ø5-D5 DAYS SUPPLY R 3Ø 3Ø Days supply
4Ø6-D6 COMPOUND CODE R 1 Not a Compound
4Ø8-D8 DISPENSE AS WRITTEN
(DAW)/PRODUCT SELECTION
CODE
R Ø No Product Selection Indicated
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ7Ø915 September 15, 2ØØ7
415-DF NUMBER OF REFILLS
AUTHORIZED
Q 5 5 Refills
419-DJ PRESCRIPTION ORIGIN CODE Q 1 Written Prescription
3Ø8-C8 OTHER COVERAGE CODE Q 1 No other coverage
429-DT SPECIAL PACKAGING
INDICATOR
Q 1 Not Unit Dose
6ØØ-28 UNIT OF MEASURE Q ML Milliliters
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 Pricing Segment
4Ø9-D9 INGREDIENT COST SUBMITTED R 557{ $55.7Ø
412-DC DISPENSING FEE SUBMITTED Q 1ØØ{ $1Ø.ØØ
433-DX PATIENT PAID AMOUNT
SUBMITTED
Q 1ØØ{ $1Ø.ØØ
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 875 -
478-H7 OTHER AMOUNT CLAIMED
SUBMITTED COUNT
R 1 One occurrence
479-H8 OTHER AMOUNT CLAIMED
SUBMITTED QUALIFIER
R Ø1 Delivery Cost
48Ø-H9 OTHER AMOUNT CLAIMED
SUBMITTED
Q 15Ø{ $15.ØØ
426-DQ USUAL AND CUSTOMARY CHARGE Q 7ØØ{ $7Ø.ØØ
43Ø-DU GROSS AMOUNT DUE R 8Ø7{ $8Ø.7Ø
423-DN BASIS OF COST DETERMINATION Q Ø3 Direct
PRESCRIBER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø3 Prescriber Segment
466-EZ PRESCRIBER ID QUALIFIER R Ø1 National Provider ID
411-DB PRESCRIBER ID Q 1123451111
427-DR PRESCRIBER LAST NAME Q JONES
498-PM PRESCRIBER TELEPHONE NUMBER Q 2Ø13639572
364-2J PRESCRIBER FIRST NAME Q SALLY
365-2K PRESCRIBER STREET ADDRESS Q 345 NOPLACE RD
366-2M PRESCRIBER CITY ADDRESS Q ANYTOWN
367-2N PRESCRIBER STATE/PROVINCE
ADDRESS Q CO
368-2P PRESCRIBER ZIP/POSTAL ZONE Q 123456789
NARRATIVE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 16 Narrative Segment
39Ø-BM NARRATIVE MESSAGE Q XOPENEX 125MG IS
SUBJECT TO A
MANUFACTURER
REBATE OF UP TO
415 OF LIST
34.35 BILLING - TRANSACTION CODE B1 WITH FACILITY INFORMATION AND
NARRATIVE INFORMATION
The example displays the request portion only.
In this example the patient location/place of residence is not provided at the home and therefore Medicare requires the facility information.
The claim is for blood glucose test strips in a quantity that exceeds the normal Medicare allowed and therefore the narrative segment indicates
the patient has “uncontrollable BS” Blood Sugar requiring more frequent testing.
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID
QUALIFIER
M Ø4 Medicare Provider ID
2Ø1-B1 SERVICE PROVIDER ID M Ø12347ØØØ1bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID M 98765bbbbb
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø1 Patient Segment
3Ø4-C4 DATE OF BIRTH R 1962Ø615 Born June 15, 1962
3Ø5-C5 PATIENT GENDER CODE R 1 Male
31Ø-CA PATIENT FIRST NAME R JOSEPH
311-CB PATIENT LAST NAME R SMITH
322-CM PATIENT STREET ADDRESS O 123 MAIN STREET
322-CN PATIENT CITY ADDRESS O MY TOWN
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 876 -
324-CO PATIENT STATE/PROVINCE
ADDRESS O CO
325-CP PATIENT ZIP/POSTAL ZONE O 34567
326-CQ PATIENT PHONE NUMBER O 2Ø14658923
384-4X PATIENT RESIDENCE Q 11 Hospice
333-CZ EMPLOYER ID Q 5ØZ123
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 Insurance Segment
3Ø2-C2 CARDHOLDER ID M 987654321A Medicare Cardholder ID
312-CC CARDHOLDER FIRST NAME Q JOSEPH
313-CD CARDHOLDER LAST NAME Q SMITH
3Ø1-C1 GROUP ID Q TXMEDICAID Designation for Medicare that this is a Texas
Medicaid client
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 Claim Segment
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID
QUALIFIER
M Ø9 HCPCS
4Ø7-D7 PRODUCT/SERVICE ID M A4253 BLOOD GLUCOSE TEST STRIPS
442-E7 QUANTITY DISPENSED R 6ØØØ 6.ØØØ EA
4Ø3-D3 FILL NUMBER R Ø Original Dispensing for Rx#
4Ø5-D5 DAYS SUPPLY R 3Ø 3Ø Days Supply
4Ø6-D6 COMPOUND CODE R 1 Not a Compound
4Ø8-D8 DISPENSE AS WRITTEN
(DAW)/PRODUCT SELECTION
CODE
R Ø No Product Selection Indicated
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ7Ø915 September 15, 2ØØ7
415-DF NUMBER OF REFILLS
AUTHORIZED
Q 5 5 Refills
419-DJ PRESCRIPTION ORIGIN CODE Q 1 Written Prescription
3Ø8-C8 OTHER COVERAGE CODE Q 1 No other coverage
429-DT SPECIAL PACKAGING
INDICATOR
Q 1 Not Unit Dose
6ØØ-28 UNIT OF MEASURE Q EA Each
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 Pricing Segment
4Ø9-D9 INGREDIENT COST SUBMITTED R 557{ $55.7Ø
412-DC DISPENSING FEE SUBMITTED Q 1ØØ{ $1Ø.ØØ
433-DX PATIENT PAID AMOUNT
SUBMITTED
Q 1ØØ{ $1Ø.ØØ
478-H7 OTHER AMOUNT CLAIMED
SUBMITTED COUNT
R 1 One occurrence
479-H8 OTHER AMOUNT CLAIMED
SUBMITTED QUALIFIER
R Ø1 Delivery Cost
48Ø-H9 OTHER AMOUNT CLAIMED
SUBMITTED
Q 15Ø{ $15.ØØ
426-DQ USUAL AND CUSTOMARY CHARGE Q 7ØØ{ $7Ø.ØØ
43Ø-DU GROSS AMOUNT DUE R 8Ø7{ $8Ø.7Ø
423-DN BASIS OF COST DETERMINATION Q Ø3 Direct
PRESCRIBER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø3 Prescriber Segment
466-EZ PRESCRIBER ID QUALIFIER R Ø1 National Provider ID
411-DB PRESCRIBER ID Q 1123451111
427-DR PRESCRIBER LAST NAME Q JONES
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 877 -
498-PM PRESCRIBER TELEPHONE NUMBER Q 2Ø13639572
364-2J PRESCRIBER FIRST NAME Q SALLY
365-2K PRESCRIBER STREET ADDRESS Q 345 NOPLACE RD
366-2M PRESCRIBER CITY ADDRESS Q ANYTOWN
367-2N PRESCRIBER STATE/PROVINCE
ADDRESS Q CO
368-2P PRESCRIBER ZIP/POSTAL ZONE Q 123456789
FACILITY SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 15 Facility Segment
336-8C FACILITY ID Q 123456789Ø
385-3Q FACILITY NAME Q RONALD MCDONALD
HOUSE
386-3U FACILITY STREET ADDRESS Q 789 HOSPICE RD
388-5J FACILITY CITY ADDRESS Q ANYTOWN
387-3V FACILITY STATE/PROVINCE
ADDRESS Q CO
389-6D FACILITY ZIP/POSTAL ZONE Q 123456789
NARRATIVE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 16 Narrative Segment
39Ø-BM NARRATIVE MESSAGE Q UNCONTROLLED BS
34.36 BILLING - TRANSACTION CODE B1 WITH ADDITIONAL DOCUMENTATION AND
NARRATIVE INFORMATION
The example displays the request portion only. TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M Ø12347ØØØ1bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø915 September 15, 2ØØ7
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID M 98765bbbbb
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø1 Patient Segment
3Ø4-C4 DATE OF BIRTH R 1962Ø615 Born June 15, 1962
3Ø5-C5 PATIENT GENDER CODE R 1 Male
31Ø-CA PATIENT FIRST NAME R JOSEPH
311-CB PATIENT LAST NAME R SMITH
322-CM PATIENT STREET ADDRESS O 123 MAIN STREET
322-CN PATIENT CITY ADDRESS O MY TOWN
324-CO PATIENT STATE/PROVINCE
ADDRESS O CO
325-CP PATIENT ZIP/POSTAL ZONE O 34567
326-CQ PATIENT PHONE NUMBER O 2Ø14658923
384-4X PATIENT RESIDENCE Q 1 Home
333-CZ EMPLOYER ID Q 5ØZ123
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 Insurance Segment
3Ø2-C2 CARDHOLDER ID M 987654321A Medicare Cardholder ID
312-CC CARDHOLDER FIRST NAME Q JOSEPH
313-CD CARDHOLDER LAST NAME Q SMITH

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 878 -
3Ø1-C1 GROUP ID Q TXMEDICAID Designation for Medicare that this is a Texas
Medicaid client
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 Claim Segment
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID
QUALIFIER
M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ9Ø11312 INJECTION, METHYLPREDNISOLONE
SODIUM SUCCINATE, UP TO 4ØMG
442-E7 QUANTITY DISPENSED R 1 4ØMG
4Ø3-D3 FILL NUMBER R Ø Original dispensing for Rx#
4Ø5-D5 DAYS SUPPLY R 3Ø 3Ø Days Supply
4Ø6-D6 COMPOUND CODE R 1 Not A Compound
4Ø8-D8 DISPENSE AS WRITTEN
(DAW)/PRODUCT SELECTION
CODE
R Ø No Product Selection Indicated
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ7Ø915 September 15, 2ØØ7
415-DF NUMBER OF REFILLS
AUTHORIZED
Q 5 5 Refills
419-DJ PRESCRIPTION ORIGIN CODE Q 1 Written Prescription
354-NX SUBMISSION CLARIFICATION
CODE COUNT
R 1
42Ø-DK SUBMISSION CLARIFICATION
CODE
Q 11 The supplier's guarantee that a copy of the
paper certification, signed and dated by the
physician, is on file at the supplier's office
3Ø8-C8 OTHER COVERAGE CODE Q 1 No Other Coverage
429-DT SPECIAL PACKAGING
INDICATOR
Q 1 Not Unit Dose
6ØØ-28 UNIT OF MEASURE Q EA Each
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 Pricing Segment
4Ø9-D9 INGREDIENT COST SUBMITTED R 557{ $55.7Ø
412-DC DISPENSING FEE SUBMITTED Q 1ØØ{ $1Ø.ØØ
433-DX PATIENT PAID AMOUNT
SUBMITTED
Q 1ØØ{ $1Ø.ØØ
478-H7 OTHER AMOUNT CLAIMED
SUBMITTED COUNT
R 1 One occurrence
479-H8 OTHER AMOUNT CLAIMED
SUBMITTED QUALIFIER
R Ø1 Delivery cost
48Ø-H9 OTHER AMOUNT CLAIMED
SUBMITTED
Q 15Ø{ $15.ØØ
426-DQ USUAL AND CUSTOMARY CHARGE Q 7ØØ{ $7Ø.ØØ
43Ø-DU GROSS AMOUNT DUE R 8Ø7{ $8Ø.7Ø
423-DN BASIS OF COST DETERMINATION Q Ø3 Direct
PRESCRIBER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø3 Prescriber Segment
466-EZ PRESCRIBER ID QUALIFIER R Ø1 National Provider ID
411-DB PRESCRIBER ID Q 1123451111
427-DR PRESCRIBER LAST NAME Q JONES
498-PM PRESCRIBER TELEPHONE NUMBER Q 2Ø13639572
364-2J PRESCRIBER FIRST NAME Q SALLY
365-2K PRESCRIBER STREET ADDRESS Q 345 NOPLACE RD
366-2M PRESCRIBER CITY ADDRESS Q ANYTOWN
367-2N PRESCRIBER STATE/PROVINCE
ADDRESS Q CO
368-2P PRESCRIBER ZIP/POSTAL ZONE q 123456789
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 879 -
ADDITIONAL DOCUMENTATION SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 14 Additional Documentation Segment
369-2Q ADDITIONAL DOCUMENTATION TYPE
ID
M Ø11 DMERC INFORMATION FORM – Ø8.Ø2
IMMUNOSUPPRESSIVE DRUGS
374-2V REQUEST PERIOD BEGIN DATE Q 2ØØ7Ø915 September 15, 2ØØ7
373-2U REQUEST STATUS Q 1 1 = INITIAL CMN
371-2S LENGTH OF NEED QUALIFIER R 4 4 = MONTHS
37Ø-2R LENGTH OF NEED Q 6 6 MONTHS
372-2T PRESCRIBER/SUPPLIER DATE
SIGNED
Q 2ØØ7Ø915 September 15, 2ØØ7
377-2Z QUESTION NUMBER/LETTER COUNT R 11
378-4B QUESTION NUMBER/LETTER R 1A What drugs are prescribed (HCPCS)
383-4K QUESTION ALPHANUMERIC
RESPONSE
Q J292Ø
378-4B QUESTION NUMBER/LETTER R 1B What drugs are prescribed (dosage)
382-4J QUESTION NUMERIC RESPONSE Q 4Ø 4Ø MG
378-4B QUESTION NUMBER/LETTER R 1C What drugs are prescribed (frequency per day)
382-4J QUESTION NUMERIC RESPONSE Q 1 Once per day
378-4B QUESTION NUMBER/LETTER R 4 Had patient had an organ transplant
383-4K QUESTION ALPHANUMERIC
RESPONSE
Q Y Patient had an organ transplant covered.
378-4B QUESTION NUMBER/LETTER R 5A Which Organ(s)
383-4K QUESTION ALPHANUMERIC
RESPONSE
Q 1 1 = HEART
378-4B QUESTION NUMBER/LETTER R 5B Which organ(s)
383-4K QUESTION ALPHANUMERIC
RESPONSE
Q 3 3 = KIDNEY
378-4B QUESTION NUMBER/LETTER R 8 Name of Facility
383-4K QUESTION ALPHANUMERIC
RESPONSE
Q HEART INSTITUTE
378-4B QUESTION NUMBER/LETTER R 9 City where facility…
383-4K QUESTION ALPHANUMERIC
RESPONSE
Q HEARTSVILLE
378-4B QUESTION NUMBER/LETTER R 1Ø State where facility…
383-4K QUESTION ALPHANUMERIC
RESPONSE
Q MO
378-4B QUESTION NUMBER/LETTER R 11 Discharge Date
38Ø-4G QUESTION DATE RESPONSE Q 2ØØ7Ø911 September 11, 2ØØ7
378-4B QUESTION NUMBER/LETTER R 12 Any prior transplant failure of same
383-4K QUESTION ALPHANUMERIC
RESPONSE
Q N No
NARRATIVE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 16 Narrative Segment
39Ø-BM NARRATIVE MESSAGE 1 PATIENT
TRANSFERRED FROM
MEDICARE HMO
Ø6Ø4Ø3
34.37 PRIMARY CLAIM FROM PHARMACY TO PDP
The following examples illustrate the use of the Telecommunication Standard to support specific data routing needs for Medicare Part D.
Billing - Transaction Code B1 TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66 PDP BIN
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø PDP PCN
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 880 -
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
11Ø-AK SOFTWARE VENDOR/CERTIFICATION
ID
M 98765bbbbb
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø1 PATIENT SEGMENT
3Ø4-C4 DATE OF BIRTH R 1962Ø615 Born June 15, 1962
3Ø5-C5 PATIENT GENDER CODE R 1 Male
31Ø-CA PATIENT FIRST NAME R JOSEPH
311-CB PATIENT LAST NAME R SMITH
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 987654321 Cardholder ID
3Ø1-C1 GROUP ID Q PARTD
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
442-E7 QUANTITY DISPENSED R 3ØØØØ 3Ø.ØØØ tablets
4Ø3-D3 FILL NUMBER R Ø Original dispensing for RX#
4Ø5-D5 DAYS SUPPLY R 3Ø 3Ø Days supply
4Ø6-D6 COMPOUND CODE R 1 Not a compound
4Ø8-D8 DISPENSE AS WRITTEN
(DAW)/PRODUCT SELECTION CODE
R Ø No product selection indicated
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ8Ø312 March 12, 2ØØ8
415-DF NUMBER OF REFILLS AUTHORIZED Q 5 5 Refills
429-DT SPECIAL PACKAGING INDICATOR Q 1 Not unit dose
6ØØ-28 UNIT OF MEASURE Q EA Each
PRESCRIBER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø3 PRESCRIBER SEGMENT
466-EZ PRESCRIBER ID QUALIFIER R Ø8 State license
411-DB PRESCRIBER ID Q ØØG2345
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 762{ $76.2Ø
412-DC DISPENSING FEE SUBMITTED Q 45{ $4.5Ø
426-DQ USUAL AND CUSTOMARY CHARGE Q 9ØØ{ $9Ø.ØØ
43Ø-DU GROSS AMOUNT DUE R 8Ø7{ $8Ø.7Ø
423-DN BASIS OF COST DETERMINATION Q Ø1 AWP
34.37.1 RESPONSE FROM PDP TO PHARMACY ON PRIMARY CLAIM
Billing Accepted Response- Paid RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 881 -
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
RESPONSE MESSAGE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 2Ø RESPONSE MESSAGE SEGMENT
5Ø4-F4 MESSAGE Q TRANSMISSION
MESSAGE TEXT For illustrative purposes only. Up to 2ØØ
Bytes
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P Paid
5Ø3-F3 AUTHORIZATION NUMBER Q 123456789123456789
13Ø-UF ADDITIONAL MESSAGE INFORMATION
COUNT
R 1 1 occurrence
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø1 Used for first line of free form text with no
pre-defined structure.
526-FQ ADDITIONAL MESSAGE INFORMATION Q TRANSACTION
MESSAGE TEXT For illustrative purposes only. Up to 4Ø Bytes
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 5ØØ{ $5Ø.ØØ
5Ø6-F6 INGREDIENT COST PAID Q 762{ $76.2Ø
5Ø7-F7 DISPENSING FEE PAID Q 45{ $4.5Ø
5Ø9-F9 TOTAL AMOUNT PAID R 3Ø7{ $3Ø.7Ø
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 1 Ingredient Cost Paid as Submitted
517-FH AMOUNT APPLIED TO PERIODIC
DEDUCTIBLE
Q 4ØØ{ $4Ø.ØØ
518-FI AMOUNT OF COPAY Q 1ØØ{ $1Ø.ØØ
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 28 Response Coordination of Benefits/Other
Payers Segment
355-NT OTHER PAYER ID COUNT M 2 Two occurrences
338-5C OTHER PAYER COVERAGE TYPE M Ø2 Secondary
339-6C OTHER PAYER ID QUALIFIER R Ø3 BIN
34Ø-7C OTHER PAYER ID Q 283749 Secondary Payer’s BIN
991-MH OTHER PAYER PROCESSOR CONTROL
NUMBER
Q 29348bbbbb Secondary Payer’s PCN
356-NU OTHER PAYER CARDHOLDER ID Q 3827493 Cardholder ID for Secondary Payer
992-MJ OTHER PAYER GROUP ID Q MDP348 Secondary Payer’s Group ID
338-5C OTHER PAYER COVERAGE TYPE M Ø3 Tertiary
339-6C OTHER PAYER ID QUALIFIER R Ø3 BIN
34Ø-7C OTHER PAYER ID Q 283499 Tertiary Payer’s BIN
991-MH OTHER PAYER PROCESSOR CONTROL
NUMBER
Q 293A38BNDI Tertiary Payer’s PCN
356-NU OTHER PAYER CARDHOLDER ID Q 38473KJ Cardholder ID for Tertiary Payer
992-MJ OTHER PAYER GROUP ID Q COSTATE Tertiary Payer’s Group ID
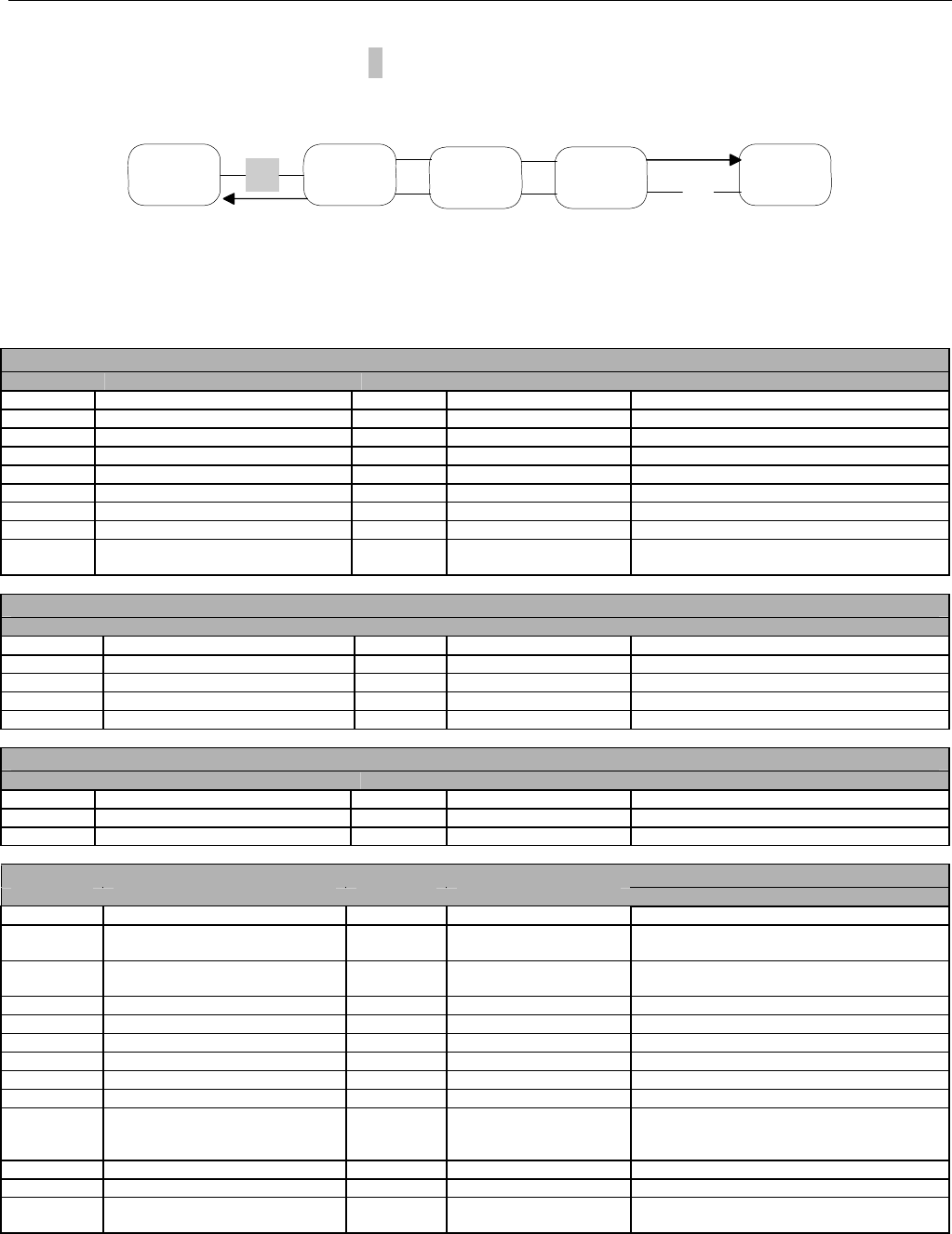
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 882 -
34.38 MEDICARE PART D - 1- CLAIM SUBMITTED TO SECONDARY PAYER FROM
PHARMACY
Pharmacy Switch Facilitator Switch Secondary
Payer
1
2
Additional Insurance Information received from the PDP:
BIN Number: 283749
Processor Control Number: 29348
Group ID: MDP348
Cardholder ID: 3827493
Help Desk Phone: 8ØØ-123-4567
Billing - Transaction Code B1 TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 283749 Secondary payer’s BIN
1Ø2-A2 VERSION/RELEASE NUMBER M DØ DØTransaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M 29348bbbbb Secondary payer’s PCN
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
11Ø-AK SOFTWARE VENDOR/CERTIFICATION
ID
M 6ØØ2387bbb
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø1 PATIENT SEGMENT
3Ø4-C4 DATE OF BIRTH R 1962Ø615 Born June 15, 1962
3Ø5-C5 PATIENT GENDER CODE R 1 Male
31Ø-CA PATIENT FIRST NAME R JOSEPH
311-CB PATIENT LAST NAME R SMITH
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 3827493 Cardholder ID
3Ø1-C1 GROUP ID Q MDP348
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
442-E7 QUANTITY DISPENSED R 3ØØØØ 3Ø.ØØØ tablets
4Ø3-D3 FILL NUMBER R Ø Original dispensing for RX#
4Ø5-D5 DAYS SUPPLY R 3Ø 3Ø Days supply
4Ø6-D6 COMPOUND CODE R 1 Not a compound
4Ø8-D8 DISPENSE AS WRITTEN
(DAW)/PRODUCT SELECTION
CODE
R Ø No product selection indicated
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ8Ø312 March 12, 2ØØ8
415-DF NUMBER OF REFILLS AUTHORIZED Q 5 5 Refills
3Ø8-C8 OTHER COVERAGE CODE Q 2 Other coverage exists/billed-payment
collected
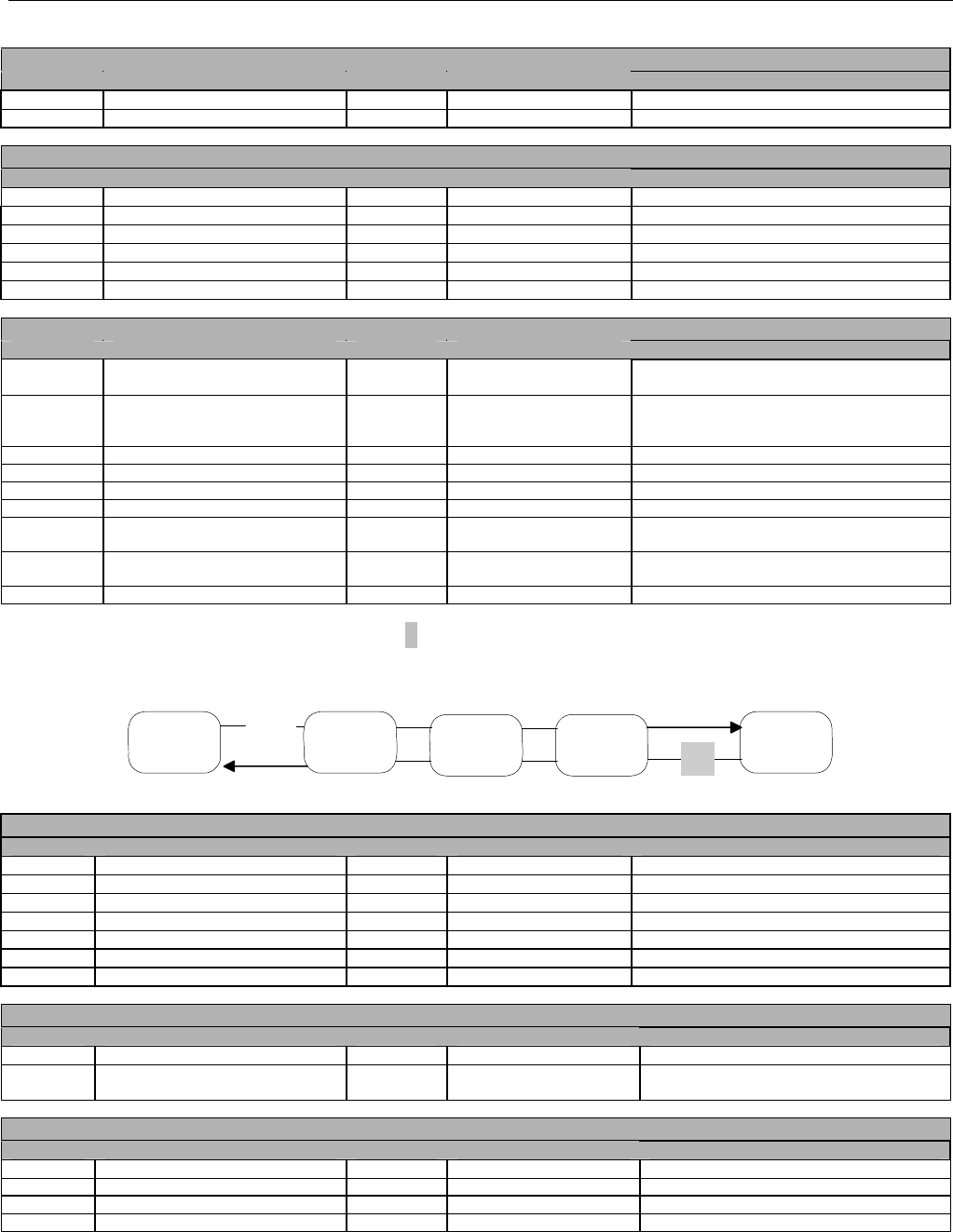
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 883 -
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
429-DT SPECIAL PACKAGING INDICATOR Q 1 Not unit dose
6ØØ-28 UNIT OF MEASURE Q EA Each
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 557{ $55.7Ø
412-DC DISPENSING FEE SUBMITTED Q 5Ø{ $5.ØØ
426-DQ USUAL AND CUSTOMARY CHARGE Q 8Ø7{ $8Ø.7Ø
43Ø-DU GROSS AMOUNT DUE R 6Ø7{ $6Ø.7Ø
423-DN BASIS OF COST DETERMINATION Q Ø3 Direct
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø5 COORDINATION OF BENEFITS/OTHER
PAYMENTS SEGMENT
337-4C COORDINATION OF
BENEFITS/OTHER PAYMENTS
COUNT
M 1 One occurrence
338-5C OTHER PAYER COVERAGE TYPE M Ø1 Primary
339-6C OTHER PAYER ID QUALIFIER R Ø3 BIN #
34Ø-7C OTHER PAYER ID Q 61ØØ66 ID assigned to payer
443-E8 OTHER PAYER DATE Q 2ØØ8Ø313 March 13, 2ØØ8
341-HB OTHER PAYER AMOUNT PAID
COUNT
R 1 One occurrence
342-HC OTHER PAYER AMOUNT PAID
QUALIFIER
R Ø7 Drug Benefit
431-DV OTHER PAYER AMOUNT PAID Q 3Ø7{ $3Ø.7Ø paid
34.38.1 MEDICARE PART D - 2 – RESPONSE FROM SECONDARY PAYER TO PHARMACY
FOR SECONDARY CLAIM
Pharmacy Switch Facilitator Switch Secondary
Payer
1
2
Billing Accepted Response- Paid RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
RESPONSE MESSAGE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 2Ø RESPONSE MESSAGE SEGMENT
5Ø4-F4 MESSAGE Q TRANSMISSION
MESSAGE TEXT For illustrative purposes only. Up to 2ØØ
Bytes
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P Paid
5Ø3-F3 AUTHORIZATION NUMBER Q 384732938745
13Ø-UF ADDITIONAL MESSAGE INFORMATION R 1 1 occurrence
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 884 -
COUNT
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø1 Used for first line of free form text with no
pre-defined structure.
526-FQ ADDITIONAL MESSAGE INFORMATION Q TRANSACTION
MESSAGE TEXT For illustrative purposes only. Up to 4Ø
Bytes
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 8Ø43827877
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 8Ø{ $8.ØØ
5Ø6-F6 INGREDIENT COST PAID Q 557{ $55.7Ø
5Ø7-F7 DISPENSING FEE PAID Q 5Ø{ $5.ØØ
566-J5 OTHER PAYER AMOUNT RECOGNIZED R 3Ø7{ $3Ø.7Ø
5Ø9-F9 TOTAL AMOUNT PAID R 22Ø{ $22.ØØ
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 1 Ingredient Cost Paid as Submitted
34.39 MEDICARE PART D - 3 – INFORMATION REPORTING (N1) FROM
FACILITATOR TO PDP FOR SECONDARY CLAIM
Switch
Facilitator
PDP
3
4
Additional Insurance Information originally received from the PDP by pharmacy, populated on Secondary Claim from pharmacy and now
appearing in the Insurance Segment to identify the Secondary Payer to the PDP:
BIN Number: 283749
Processor Control Number: 29348
Group ID: MDP348
Cardholder ID: 3827493
Help Desk Phone: 8ØØ-123-4567
The Facilitator generates the Transaction Reference Number. It is echoed back by the PDP in the response.
Information Reporting - Transaction Code N1 TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66 PDP BIN
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M N1 Information Reporting
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø PDP PCN
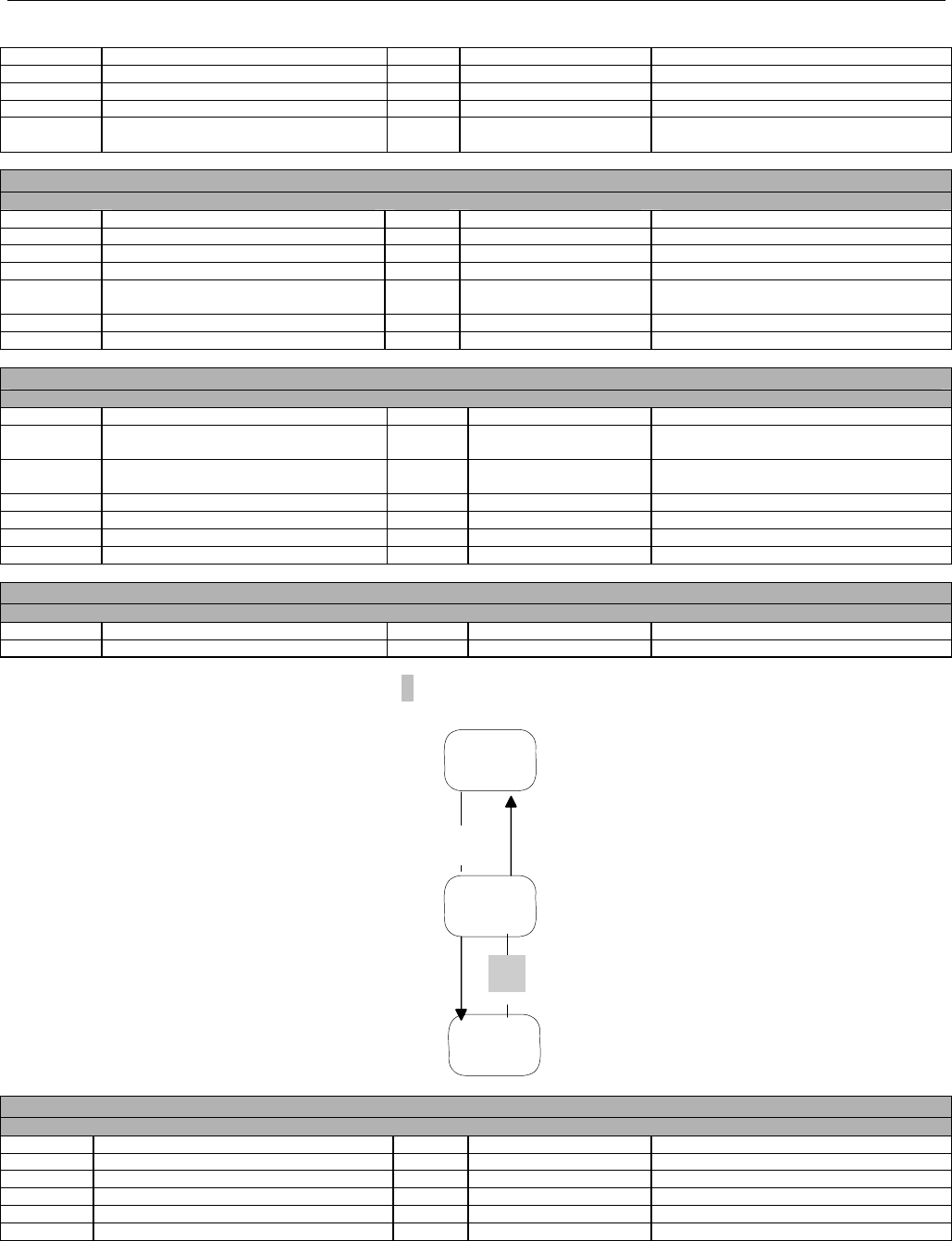
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 885 -
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M TF28374387 Facilitator-assigned source of software
being used (Example format only)
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 987654321 PDP Cardholder ID
3Ø1-C1 GROUP ID Q PARTD PDP Group ID
99Ø-MG OTHER PAYER BIN NUMBER R 283749 Secondary Payer’s BIN
991-MH OTHER PAYER PROCESSOR CONTROL
NUMBER
R 29348bbbbb Secondary Payer’s PCN
356-NU OTHER PAYER CARDHOLDER ID R 3827493 Cardholder ID for Secondary Payer
992-MJ OTHER PAYER GROUP ID R MDP348 Secondary Payer’s Group ID
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
4Ø3-D3 FILL NUMBER R Ø Original dispensing for RX#
88Ø-K5 TRANSACTION REFERENCE NUMBER R 2383838377
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
433-DX PATIENT PAID AMOUNT SUBMITTED R 8Ø{ $8.ØØ TrOOP update
34.39.1 MEDICARE PART D - 4 – RESPONSE FROM PDP TO FACILITATOR FOR
INFORMATION REPORTING (N1)
Switch
Facilitator
PDP
3
4
Accepted Response- Approved RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M N1 Information Reporting
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
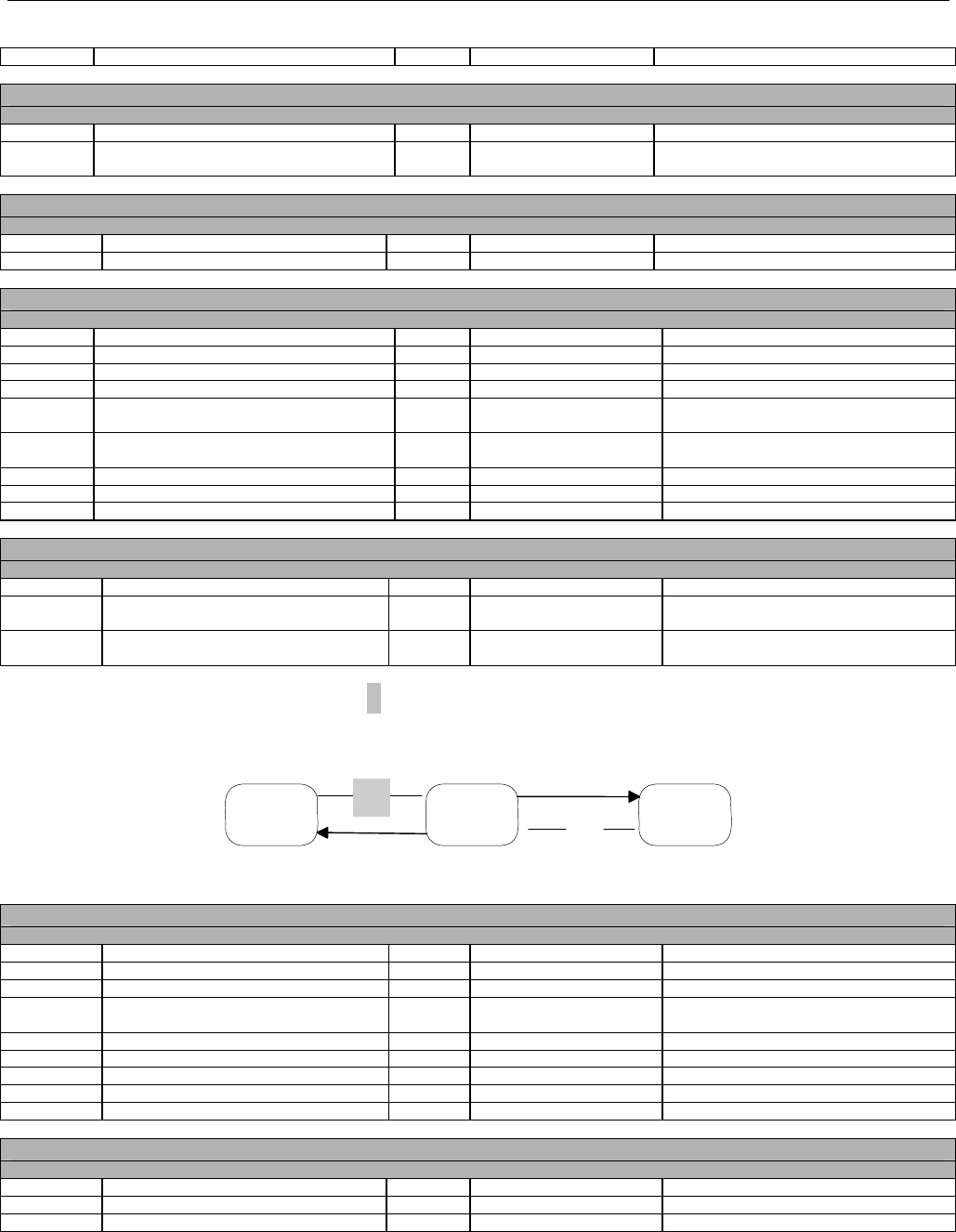
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 886 -
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
RESPONSE MESSAGE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 2Ø RESPONSE MESSAGE SEGMENT
5Ø4-F4 MESSAGE Q TRANSMISSION
MESSAGE TEXT For illustrative purposes only. Up to 2ØØ
Bytes
RESPONSE INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 25 INSURANCE SEGMENT
3Ø1-C1 GROUP ID Q PARTD PDP Group ID
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M A Approved
5Ø3-F3 AUTHORIZATION NUMBER Q 28379993748
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT R 1 1 occurrence
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø1 Used for first line of free form text with no
pre-defined structure.
526-FQ ADDITIONAL MESSAGE INFORMATION Q TRANSACTION
MESSAGE TEXT For illustrative purposes only. Up to 4Ø
Bytes
549-7F HELP DESK PHONE NUMBER QUALIFIER R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 8Ø49382998
88Ø-K5 TRANSACTION REFERENCE NUMBER R 2383838377
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
34.40 MEDICARE PART D - 5 – CLAIM SUBMITTED FROM PHARMACY TO
TERTIARY PAYER WITHOUT UNIQUE BIN/PCN COMBINATION
SwitchPharmacy Tertiary
Payer
5
6
Claim does not route through Facilitator because the pharmacy was not provided with a unique BIN/PCN combination from the Primary Payer.
Update becomes the responsibility of the Tertiary Payer to submit TrOOP update to the Primary Payer.
Billing - Transaction Code B1 TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 283499 Tertiary payer’s BIN
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M bbbbbbbbbb Unique BIN/PCN combination not
submitted
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M 14 Plan Specific
2Ø1-B1 SERVICE PROVIDER ID M AF13487Kbbbbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M bbbbbbbbbb
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø1 PATIENT SEGMENT
3Ø4-C4 DATE OF BIRTH R 1962Ø615 Born June 15, 1962
3Ø5-C5 PATIENT GENDER CODE R 1 Male
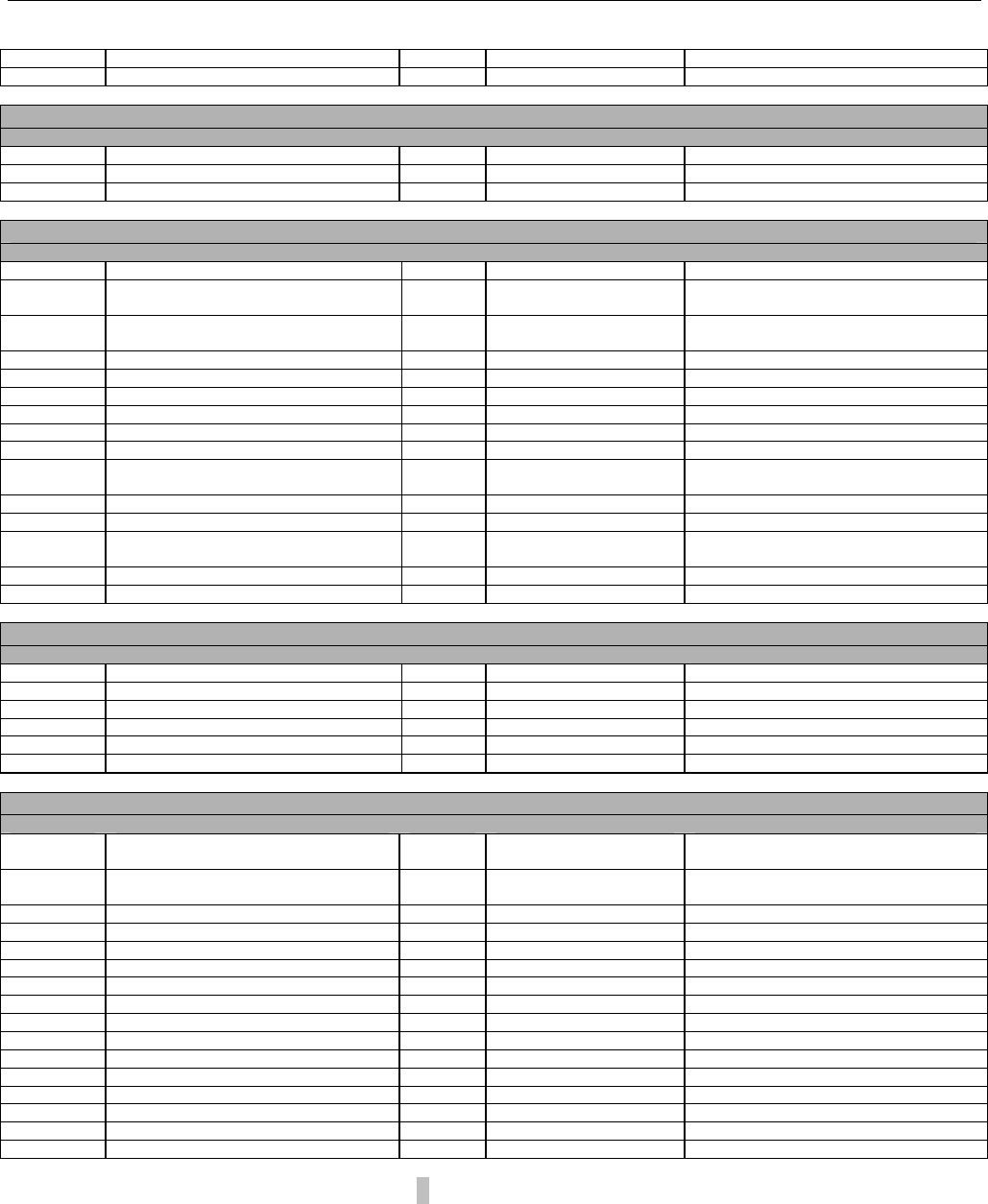
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 887 -
31Ø-CA PATIENT FIRST NAME R JOSEPH
311-CB PATIENT LAST NAME R SMITH
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 38473KJ Cardholder ID
3Ø1-C1 GROUP ID Q COSTATE
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
442-E7 QUANTITY DISPENSED R 3ØØØØ 3Ø.ØØØ tablets
4Ø3-D3 FILL NUMBER R Ø Original dispensing for RX#
4Ø5-D5 DAYS SUPPLY R 3Ø 3Ø Days supply
4Ø6-D6 COMPOUND CODE R 1 Not a compound
4Ø8-D8 DISPENSE AS WRITTEN (DAW)/PRODUCT
SELECTION CODE
R Ø No product selection indicated
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ8Ø313 March 13, 2ØØ8
415-DF NUMBER OF REFILLS AUTHORIZED Q 5 5 Refills
3Ø8-C8 OTHER COVERAGE CODE Q 2 Other coverage exists/billed-payment
collected
429-DT SPECIAL PACKAGING INDICATOR Q 1 Not unit dose
6ØØ-28 UNIT OF MEASURE Q EA Each
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 557{ $55.7Ø
412-DC DISPENSING FEE SUBMITTED Q 8Ø{ $8.ØØ
426-DQ USUAL AND CUSTOMARY CHARGE Q 8Ø7{ $8Ø.7Ø
43Ø-DU GROSS AMOUNT DUE R 637{ $63.7Ø
423-DN BASIS OF COST DETERMINATION Q Ø3 Direct
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø5 COORDINATION OF BENEFITS/OTHER
PAYMENTS SEGMENT
337-4C COORDINATION OF BENEFITS/OTHER
PAYMENTS COUNT
M 2 Two occurrences
338-5C OTHER PAYER COVERAGE TYPE M Ø1 Primary
339-6C OTHER PAYER ID QUALIFIER R Ø3 BIN #
34Ø-7C OTHER PAYER ID Q 61ØØ66 ID assigned to payer
443-E8 OTHER PAYER DATE Q 2ØØ8Ø313 March 13, 2ØØ8
341-HB OTHER PAYER AMOUNT PAID COUNT R 1 One occurrence
342-HC OTHER PAYER AMOUNT PAID QUALIFIER R Ø7 Drug Benefit
431-DV OTHER PAYER AMOUNT PAID Q 3Ø7{ $3Ø.7Ø paid
338-5C OTHER PAYER COVERAGE TYPE M Ø2 Secondary
339-6C OTHER PAYER ID QUALIFIER R Ø3 BIN #
34Ø-7C OTHER PAYER ID Q 283749 ID assigned to payer
443-E8 OTHER PAYER DATE Q 2ØØ8Ø313 March 13, 2ØØ8
341-HB OTHER PAYER AMOUNT PAID COUNT R 1 One occurrence
342-HC OTHER PAYER AMOUNT PAID QUALIFIER R Ø7 Drug Benefit
431-DV OTHER PAYER AMOUNT PAID Q 22Ø{ $22.ØØ paid
34.40.1 MEDICARE PART D - 6 – RESPONSE FROM TERTIARY PAYER TO PHARMACY
FOR TERTIARY CLAIM
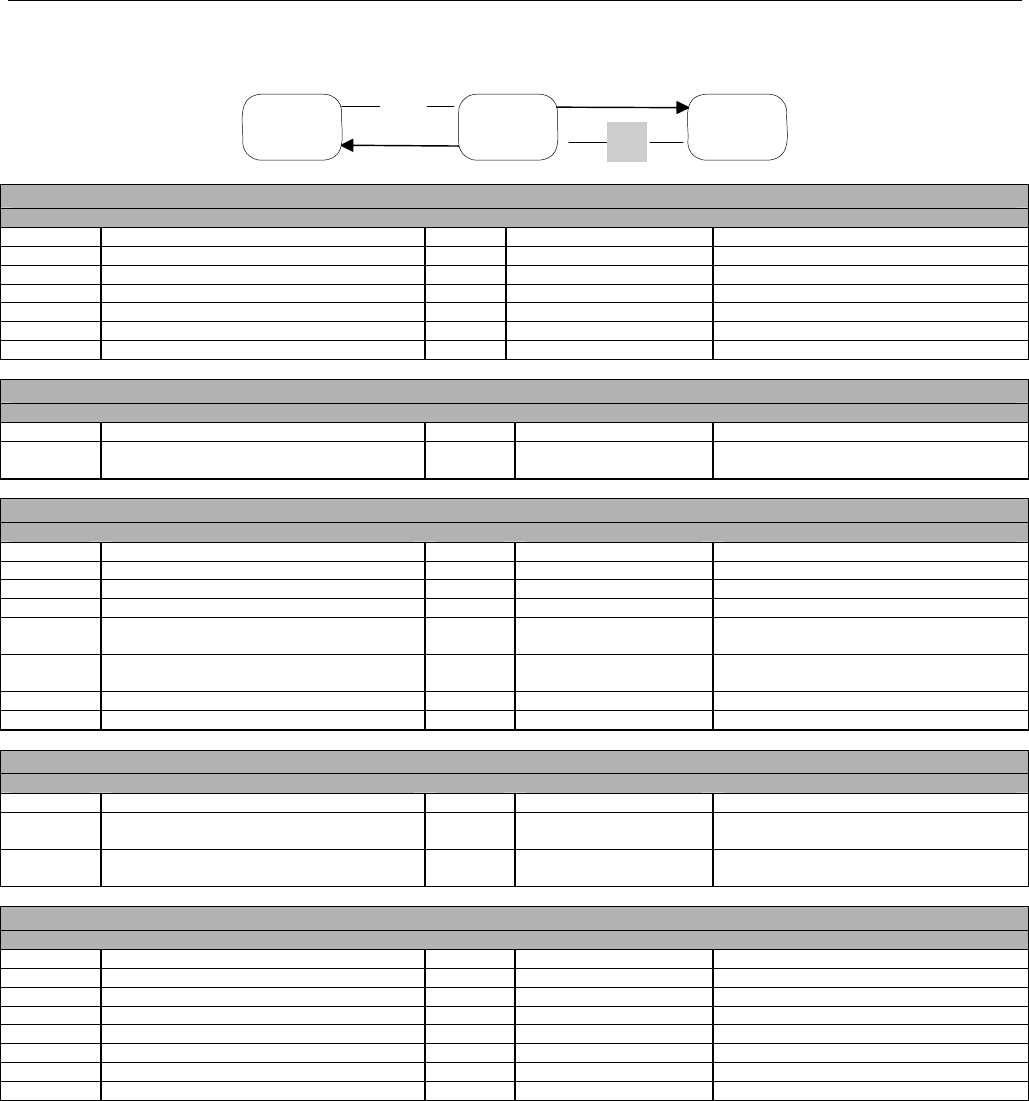
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 888 -
SwitchPharmacy Tertiary
Payer
5
6
Billing Accepted Response- Paid RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M 14 Plan Specific
2Ø1-B1 SERVICE PROVIDER ID M AF13487Kbbbbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
RESPONSE MESSAGE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 2Ø RESPONSE MESSAGE SEGMENT
5Ø4-F4 MESSAGE Q TRANSMISSION
MESSAGE TEXT For illustrative purposes only. Up to 2ØØ
Bytes
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P Paid
5Ø3-F3 AUTHORIZATION NUMBER Q 384732938745
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT R 1 1 occurrence
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø1 Used for first line of free form text with no
pre-defined structure.
526-FQ ADDITIONAL MESSAGE INFORMATION Q TRANSACTION
MESSAGE TEXT For illustrative purposes only. Up to 4Ø
Bytes
549-7F HELP DESK PHONE NUMBER QUALIFIER R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 8ØØ3339999
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 1Ø{ $1.ØØ
5Ø6-F6 INGREDIENT COST PAID Q 557{ $55.7Ø
5Ø7-F7 DISPENSING FEE PAID Q 8Ø{ $8.ØØ
566-J5 OTHER PAYER AMOUNT RECOGNIZED R 527{ $52.7Ø
5Ø9-F9 TOTAL AMOUNT PAID R 1ØØ{ $ 1Ø.ØØ
522-FM BASIS OF REIMBURSEMENT DETERMINATION R 1 Ingredient Cost Paid as Submitted
518-FI AMOUNT OF COPAY Q 1Ø{ $1.ØØ
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 889 -
34.41 MEDICARE PART D – 7 – INFORMATION REPORTING TRANSACTION
SUBMITTED FROM TERTIARY PAYER TO FACILITATOR
Switch
Facilitator
Tertiary
Payer
7
The Tertiary Payer sends to the Facilitator their Cardholder ID. The Facilitator uses that information to look up the patient’s primary BIN, PCN,
Group ID, and Cardholder ID.
When the Secondary/Tertiary/etc Payer needs to report updated patient pay information directly through the Facilitator to the PDP, the
Secondary/Tertiary/etc Payer is required, in the Insurance Segment:
• to put their Cardholder ID in Cardholder ID (3Ø2-C2) and in Other Payer Cardholder ID (356-NU),
• to put their BIN, PCN (if applicable), and Group ID (if applicable) in the Other Payer BIN Number (99Ø-MG), Other Payer Processor
Control Number (991-MH), and Other Payer Group ID (992-MJ).
Information Reporting - Transaction Code N1 TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 773356 Facilitator BIN
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M N1 Information Reporting
1Ø4-A4 PROCESSOR CONTROL NUMBER M 7733566ØØ2 Facilitator PCN
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M 14 Plan Specific
2Ø1-B1 SERVICE PROVIDER ID M AF13487Kbbbbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M QU3827298b Facilitator-assigned source of software
being used of tertiary (Example format
only)
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 38473KJ Cardholder ID
99Ø-MG OTHER PAYER BIN NUMBER R 283499 Tertiary Payer BIN
991-MH OTHER PAYER PROCESSOR CONTROL
NUMBER
R 293A38BNDI Tertiary Payer PCN
356-NU OTHER PAYER CARDHOLDER ID R 38473KJ Cardholder ID for Tertiary Payer
992-MJ OTHER PAYER GROUP ID R COSTATE Tertiary Payer’s Group ID
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 890 -
4Ø3-D3 FILL NUMBER R Ø Original dispensing for RX#
88Ø-K5 TRANSACTION REFERENCE NUMBER R ABC12445
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
433-DX PATIENT PAID AMOUNT SUBMITTED R 1Ø{ $1.ØØ TrOOP update
34.41.1 MEDICARE PART D - 8 – INFORMATION REPORTING TRANSACTION SUBMITTED
FROM FACILITATOR TO PDP WITH TERTIARY TROOP UPDATE
Switch
PDP
Facilitator
8
This is the same request in Flow 7, but the Facilitator has now replaced the Transaction Header Segment information and the Cardholder ID
and Group ID in the Insurance Segment with the Primary PDP values.
Information Reporting - Transaction Code N1 TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66 PDP BIN
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M N1 Information Reporting
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø PDP PCN
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M PY28374387 Identifies Payer as source of software
being used (Example format only)
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 987654321 PDP Cardholder ID
3Ø1-C1 GROUP ID Q PARTD PDP Group ID
99Ø-MG OTHER PAYER BIN NUMBER R 283499 Tertiary Payer BIN
991-MH OTHER PAYER PROCESSOR CONTROL
NUMBER
R 293A38BNDI Tertiary Payer PCN
356-NU OTHER PAYER CARDHOLDER ID R 38473KJ Cardholder ID for Tertiary Payer
992-MJ OTHER PAYER GROUP ID R COSTATE Tertiary Payer’s Group ID
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø3 NDC
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 891 -
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
4Ø3-D3 FILL NUMBER R Ø Original dispensing for RX#
88Ø-K5 TRANSACTION REFERENCE NUMBER R 2937438293
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
433-DX PATIENT PAID AMOUNT SUBMITTED R 1Ø{ $1.ØØ TrOOP update
34.41.2 MEDICARE PART D - 9 – RESPONSE FOR INFORMATION REPORTING
TRANSACTION FROM PDP TO FACILITATOR
Switch
PDP
Facilitator
9
Accepted Response- Approved RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M N1 Information Reporting
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
RESPONSE MESSAGE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 2Ø RESPONSE MESSAGE SEGMENT
5Ø4-F4 MESSAGE Q TRANSMISSION
MESSAGE TEXT For illustrative purposes only. Up to
2ØØ Bytes
RESPONSE INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 25 INSURANCE SEGMENT
3Ø1-C1 GROUP ID Q PARTD PDP Group ID
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M A Approved
5Ø3-F3 AUTHORIZATION NUMBER Q 738429999
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT R 1 1 occurrence
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø1 Used for first line of free form text with
no pre-defined structure.
526-FQ ADDITIONAL MESSAGE INFORMATION Q TRANSACTION
MESSAGE TEXT For illustrative purposes only. Up to 4Ø
Bytes
549-7F HELP DESK PHONE NUMBER QUALIFIER R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 9193847388
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 892 -
88Ø-K5 TRANSACTION REFERENCE NUMBER R 2937438293
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
34.41.3 MEDICARE PART D - 10 – RESPONSE FOR INFORMATION REPORTING
TRANSACTION FROM FACILITATOR TO TERTIARY PAYER
Switch
Facilitator
Tertiary
Payer
10
Accepted Response- Approved RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M N1 Information Reporting
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M 14 Plan Specific
2Ø1-B1 SERVICE PROVIDER ID M AF13487Kbbbbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
RESPONSE MESSAGE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 2Ø RESPONSE MESSAGE SEGMENT
5Ø4-F4 MESSAGE Q TRANSMISSION
MESSAGE TEXT For illustrative purposes only. Up to
2ØØ Bytes
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M A Approved
5Ø3-F3 AUTHORIZATION NUMBER Q 738429999
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT R 1 1 occurrence
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø1 Used for first line of free form text with
no pre-defined structure.
526-FQ ADDITIONAL MESSAGE INFORMATION Q TRANSACTION
MESSAGE TEXT For illustrative purposes only. Up to 4Ø
Bytes
549-7F HELP DESK PHONE NUMBER QUALIFIER R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 9193847388
88Ø-K5 TRANSACTION REFERENCE NUMBER R ABC12445
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 893 -
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
34.42 MEDICARE PART D - 11 – B2 TRANSACTION REVERSAL FROM PHARMACY
TO TERTIARY PAYER WITHOUT UNIQUE BIN/PCN COMBINATION
SwitchPharmacy Tertiary
Payer
11
12
Reversal does not route through Facilitator because the Pharmacy was not provided with a unique BIN/PCN combination from the Primary
Payer. Update becomes the responsibility of the Tertiary Payer to submit TrOOP update to the Primary Payer.
Reversal - Transaction Code B2 TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 283499 Tertiary payer’s BIN
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B2 Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M bbbbbbbbbb Unique BIN/PCN combination not
submitted
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M 14 Plan Specific
2Ø1-B1 SERVICE PROVIDER ID M AF13487Kbbbbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M bbbbbbbbbb
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
3Ø8-C8 OTHER COVERAGE CODE Q 2 Other coverage exists/billed-payment
collected
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø5 COORDINATION OF BENEFITS/OTHER
PAYMENTS SEGMENT
337-4C COORDINATION OF BENEFITS/OTHER
PAYMENTS COUNT
M 2 Two occurrences
338-5C OTHER PAYER COVERAGE TYPE M Ø1 Primary
338-5C OTHER PAYER COVERAGE TYPE M Ø2 Secondary
34.42.1 MEDICARE PART D - 12 – RESPONSE FROM TERTIARY PAYER TO PHARMACY
FOR TERTIARY REVERSAL
SwitchPharmacy Tertiary
Payer
11
12
Reversal Response- Approved RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B2 Billing
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 894 -
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M 14 Plan Specific
2Ø1-B1 SERVICE PROVIDER ID M AF13487Kbbbbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
RESPONSE MESSAGE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 2Ø RESPONSE MESSAGE SEGMENT
5Ø4-F4 MESSAGE Q TRANSMISSION
MESSAGE TEXT For illustrative purposes only. Up to
2ØØ Bytes
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M A Approved
5Ø3-F3 AUTHORIZATION NUMBER Q 384728374996
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT R 1 1 occurrence
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø1 Used for first line of free form text with
no pre-defined structure.
526-FQ ADDITIONAL MESSAGE INFORMATION Q TRANSACTION
MESSAGE TEXT For illustrative purposes only. Up to 4Ø
Bytes
549-7F HELP DESK PHONE NUMBER QUALIFIER R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 8ØØ3339999
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
34.43 MEDICARE PART D -13 – INFORMATION REPORTING REVERSAL SUBMITTED
FROM TERTIARY PAYER TO FACILITATOR
Switch
Facilitator
Tertiary
Payer
13
The next examples that follow show the order of reversals that must occur should a transaction be reversed from the tertiary and secondary
payers. The last payer must be reversed first.
When the Secondary/Tertiary/etc Payer needs to report updated patient pay information directly through the Facilitator to the PDP, the
Secondary/Tertiary/etc Payer is required, in the Insurance Segment:
• to put their Cardholder ID in Cardholder ID (3Ø2-C2) and in Other Payer Cardholder ID (356-NU),
• to put their BIN, PCN (if applicable), and Group ID (if applicable) in the Other Payer BIN Number (99Ø-MG), Other Payer Processor
Control Number (991-MH ), and Other Payer Group ID (992-MJ).
Information Reporting Reversal- Transaction Code N2

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 895 -
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 773356 Facilitator BIN
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M N2 Information Reporting Reversal
1Ø4-A4 PROCESSOR CONTROL NUMBER M 7733566ØØ2 Facilitator PCN
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M 14 Plan Specific
2Ø1-B1 SERVICE PROVIDER ID M AF13487Kbbbbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M QU3827298b Facilitator-assigned source of software
being used of tertiary (Example format
only)
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 38473KJ
99Ø-MG OTHER PAYER BIN NUMBER R 283499 Tertiary Payer BIN
991-MH OTHER PAYER PROCESSOR CONTROL
NUMBER
Q 293A38BNDI Tertiary Payer PCN
356-NU OTHER PAYER CARDHOLDER ID R 38473KJ Cardholder ID for Tertiary Payer
992-MJ OTHER PAYER GROUP ID Q COSTATE Tertiary Payer’s Group ID
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
88Ø-K5 TRANSACTION REFERENCE NUMBER R 54X17Y
34.43.1 MEDICARE PART D - 14 – INFORMATION REPORTING REVERSAL SUBMITTED
FROM FACILITATOR TO PDP FOR REVERSAL OF TERTIARY CLAIM
Switch
PDP
Facilitator
14
This is the same request in Flow 13, but the Facilitator has now replaced the Transaction Header Segment information and the Cardholder ID
and Group ID in the Insurance Segment with the Primary PDP values.
Information Reporting Reversal- Transaction Code N2
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66 PDP BIN
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 896 -
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø3-A3 TRANSACTION CODE M N2 Information Reporting Reversal
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø PDP PCN
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø7 NCPDP Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663bbbbbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M PY28374381 Identifies Payer as source of software
being sent. Example format only.
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 987654321 PDP Cardholder ID
3Ø1-C1 GROUP ID Q PARTD PDP Group ID
99Ø-MG OTHER PAYER BIN NUMBER R 283499 Tertiary Payer BIN
991-MH OTHER PAYER PROCESSOR CONTROL
NUMBER
Q 293A38BNDI Tertiary Payer PCN
356-NU OTHER PAYER CARDHOLDER ID R 38473KJ Cardholder ID for Tertiary Payer
992-MJ OTHER PAYER GROUP ID Q COSTATE Tertiary Payer’s Group ID
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
88Ø-K5 TRANSACTION REFERENCE NUMBER R 2937438293
34.43.2 MEDICARE PART D - 15 – RESPONSE FOR INFORMATION REPORTING REVERSAL
FROM PDP TO FACILITATOR FOR TERTIARY CLAIM
Switch
PDP
Facilitator
15
Information Reporting Reversal Response- Approved
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M N2 Information Reporting Reversal
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø7 NCPDP Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663bbbbbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 897 -
RESPONSE MESSAGE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 2Ø RESPONSE MESSAGE SEGMENT
5Ø4-F4 MESSAGE Q TRANSMISSION
MESSAGE TEXT For illustrative purposes only. Up to
2ØØ Bytes
RESPONSE INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 25 RESPONSE INSURANCE SEGMENT
3Ø1-C1 GROUP ID Q PARTD PDP Group ID
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M A Approved
5Ø3-F3 AUTHORIZATION NUMBER Q 73843ØØØØ
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT R 1 1 occurrence
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø1 Used for first line of free form text with
no pre-defined structure.
526-FQ ADDITIONAL MESSAGE INFORMATION Q TRANSACTION
MESSAGE TEXT For illustrative purposes only. Up to
4Ø Bytes
549-7F HELP DESK PHONE NUMBER QUALIFIER R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 9193847388
88Ø-K5 TRANSACTION REFERENCE NUMBER R 2937438293
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
34.43.3 MEDICARE PART D - 16 – RESPONSE FOR INFORMATION REPORTING REVERSAL
FROM FACILITATOR TO TERTIARY PAYER OF TERTIARY CLAIM
Switch
Facilitator
Tertiary
Payer
16
Information Reporting Reversal Response- Approved
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M N2 Information Reporting Reversal
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M 14 Plan Specific
2Ø1-B1 SERVICE PROVIDER ID M AF13487Kbbbbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
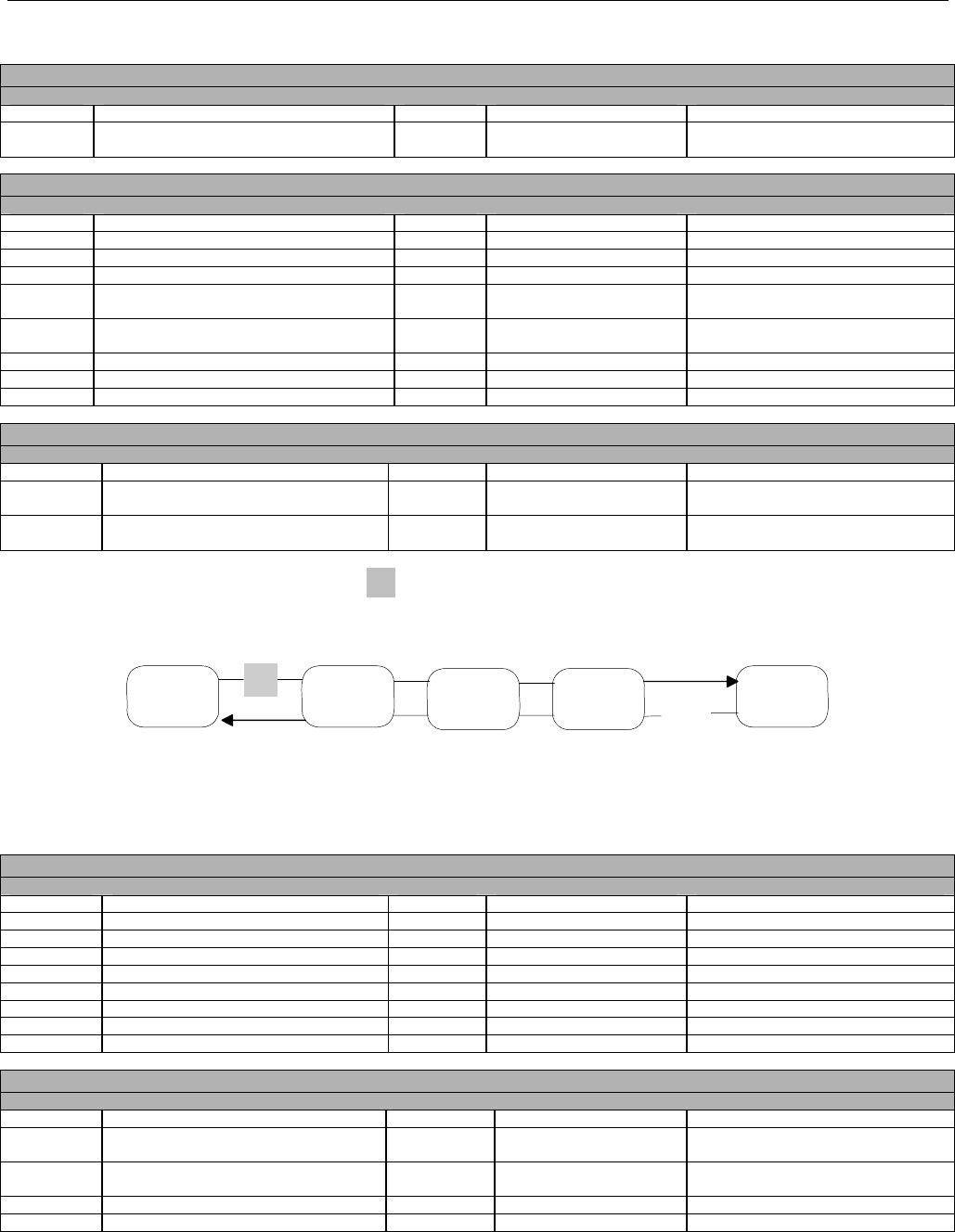
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 898 -
RESPONSE MESSAGE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 2Ø RESPONSE MESSAGE SEGMENT
5Ø4-F4 MESSAGE Q TRANSMISSION
MESSAGE TEXT For illustrative purposes only. Up to
2ØØ Bytes
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M A Approved
5Ø3-F3 AUTHORIZATION NUMBER Q 73843ØØØØ
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT R 1 1 occurrence
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø1 Used for first line of free form text
with no pre-defined structure.
526-FQ ADDITIONAL MESSAGE INFORMATION Q TRANSACTION
MESSAGE TEXT For illustrative purposes only. Up to
4Ø Bytes
549-7F HELP DESK PHONE NUMBER QUALIFIER R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 9193847388
88Ø-K5 TRANSACTION REFERENCE NUMBER R 54X17Y
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
34.44 MEDICARE PART D - 17 – REVERSAL SUBMITTED FROM PHARMACY TO
SECONDARY PAYER
Pharmacy Switch Facilitator Switch Secondary
Payer
17
18
Additional Insurance Information received from the PDP:
BIN Number: 283749
Processor Control Number: 29348
Group ID: MDP348
Cardholder ID: 3827493
Help Desk Phone: 8ØØ-123-4567
Reversal - Transaction Code B2 TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 283749 Secondary payer’s BIN
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B2 Reversal
1Ø4-A4 PROCESSOR CONTROL NUMBER M 29348bbbb Secondary payer’s PCN
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø7 NCPDP Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663bbbbbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M 6ØØ2384bbb
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
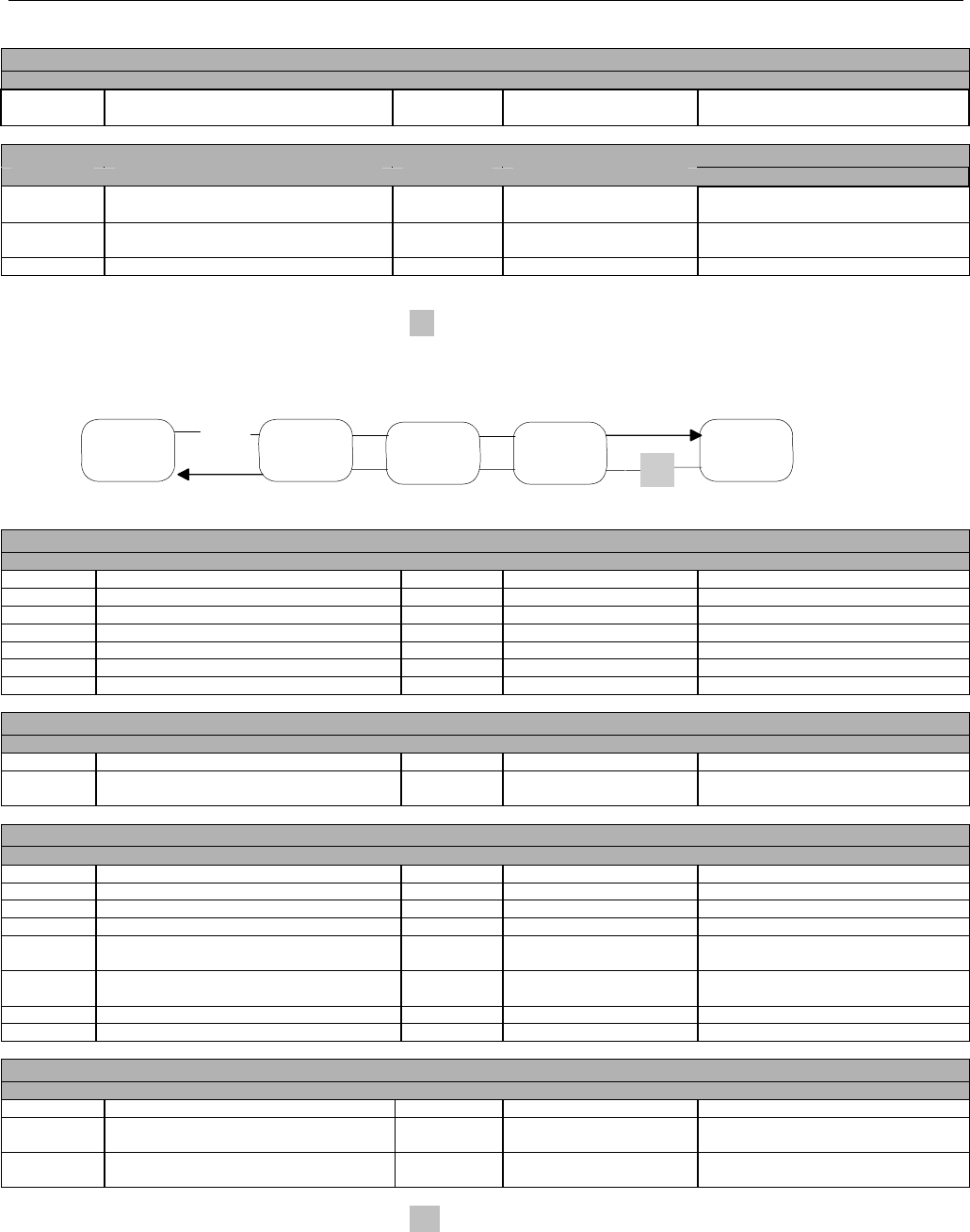
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 899 -
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
3Ø8-C8 OTHER COVERAGE CODE Q 2 Other coverage exists/billed-payment
collected
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø5 COORDINATION OF BENEFITS/OTHER
PAYMENTS SEGMENT
337-4C COORDINATION OF BENEFITS/OTHER
PAYMENTS COUNT
M 1 One occurrence
338-5C OTHER PAYER COVERAGE TYPE M Ø1 Primary
34.44.1 MEDICARE PART D - 18 – RESPONSE FROM SECONDARY PAYER TO PHARMACY
FOR REVERSAL OF SECONDARY CLAIM
Pharmacy Switch Facilitator Switch Secondary
Payer
17
18
Reversal Response- Approved RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B2 Reversal
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø7 NCPDP Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663bbbbbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
RESPONSE MESSAGE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 2Ø RESPONSE MESSAGE SEGMENT
5Ø4-F4 MESSAGE Q TRANSMISSION
MESSAGE TEXT For illustrative purposes only. Up to
2ØØ Bytes
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M A Approved
5Ø3-F3 AUTHORIZATION NUMBER Q 38473293875Ø
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT R 1 1 occurrence
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø1 Used for first line of free form text
with no pre-defined structure.
526-FQ ADDITIONAL MESSAGE INFORMATION Q TRANSACTION
MESSAGE TEXT For illustrative purposes only. Up to
4Ø Bytes
549-7F HELP DESK PHONE NUMBER QUALIFIER R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 8Ø43827877
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
34.44.2 MEDICARE PART D - 19 – INFORMATION REPORTING REVERSAL SUBMITTED
FROM FACILITATOR TO PDP FOR REVERSAL OF SECONDARY CLAIM
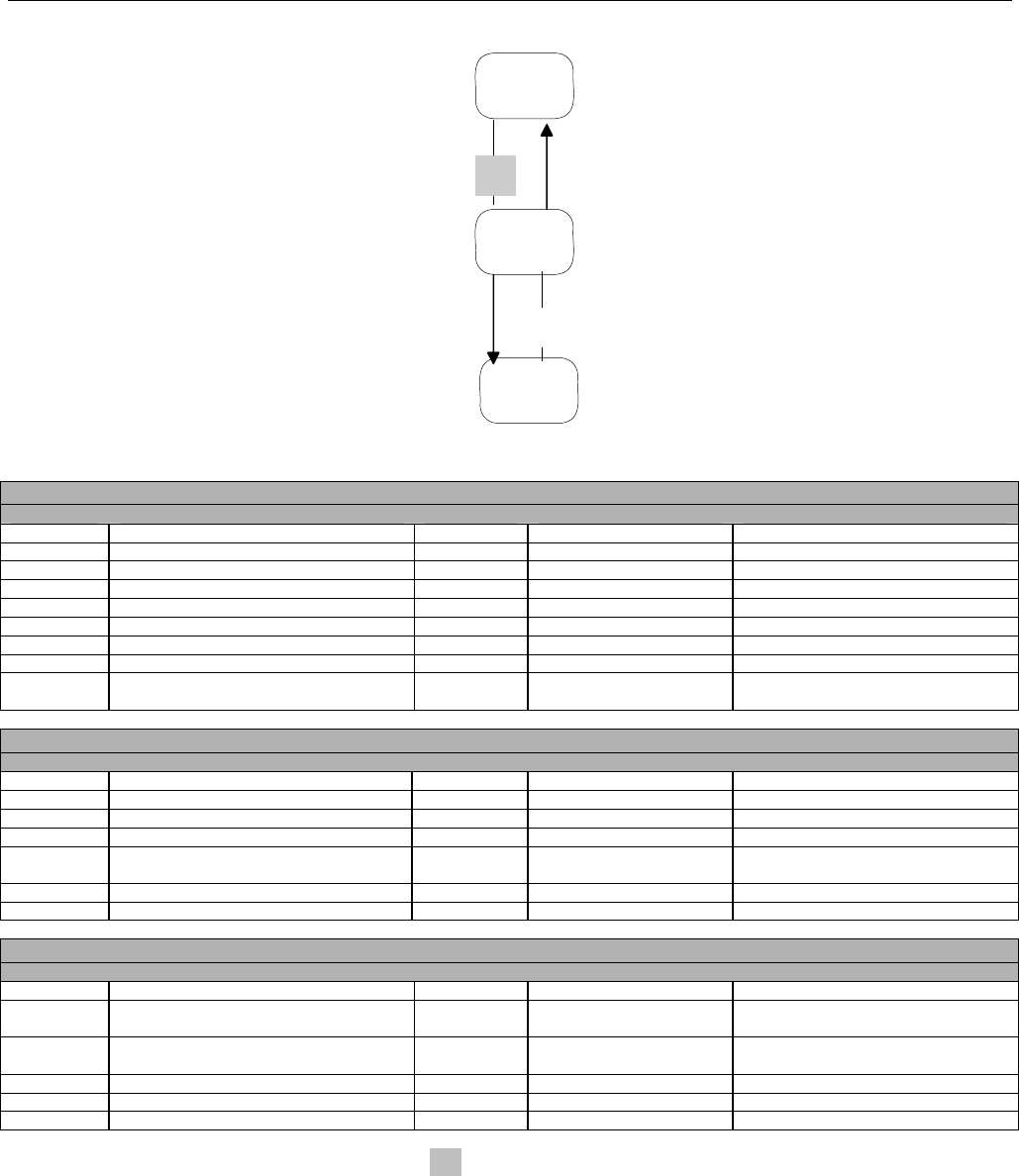
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 900 -
Switch
Facilitator
PDP
19
20
This is the same request in Flow 18, but the Facilitator has now replaced the Transaction Header Segment information and the Cardholder ID
and Group ID in the Insurance Segment with the Primary PDP values
Information Reporting Reversal - Transaction Code N2
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66 PDP BIN
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M N2 Information Reporting Reversal
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø PDP PCN
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø7 NCPDP Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663bbbbbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M TF28374387 Identifies Payer as source of software
being sent. Example format only.
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 987654321 PDP Cardholder ID
3Ø1-C1 GROUP ID Q PARTD PDP Group ID
99Ø-MG OTHER PAYER BIN NUMBER R 283749 Secondary Payer BIN
991-MH OTHER PAYER PROCESSOR CONTROL
NUMBER
Q 29348bbbbb Secondary Payer PCN
356-NU OTHER PAYER CARDHOLDER ID R 3827493 Cardholder ID for Secondary Payer
992-MJ OTHER PAYER GROUP ID Q MDP348 Secondary Payer’s Group ID
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
88Ø-K5 TRANSACTION REFERENCE NUMBER R 2383838377
34.44.3 Medicare Part D – 2Ø – RESPONSE FOR INFORMATION REPORTING
REVERSAL FROM PDP TO FACILITATOR FOR SECONDARY CLAIM
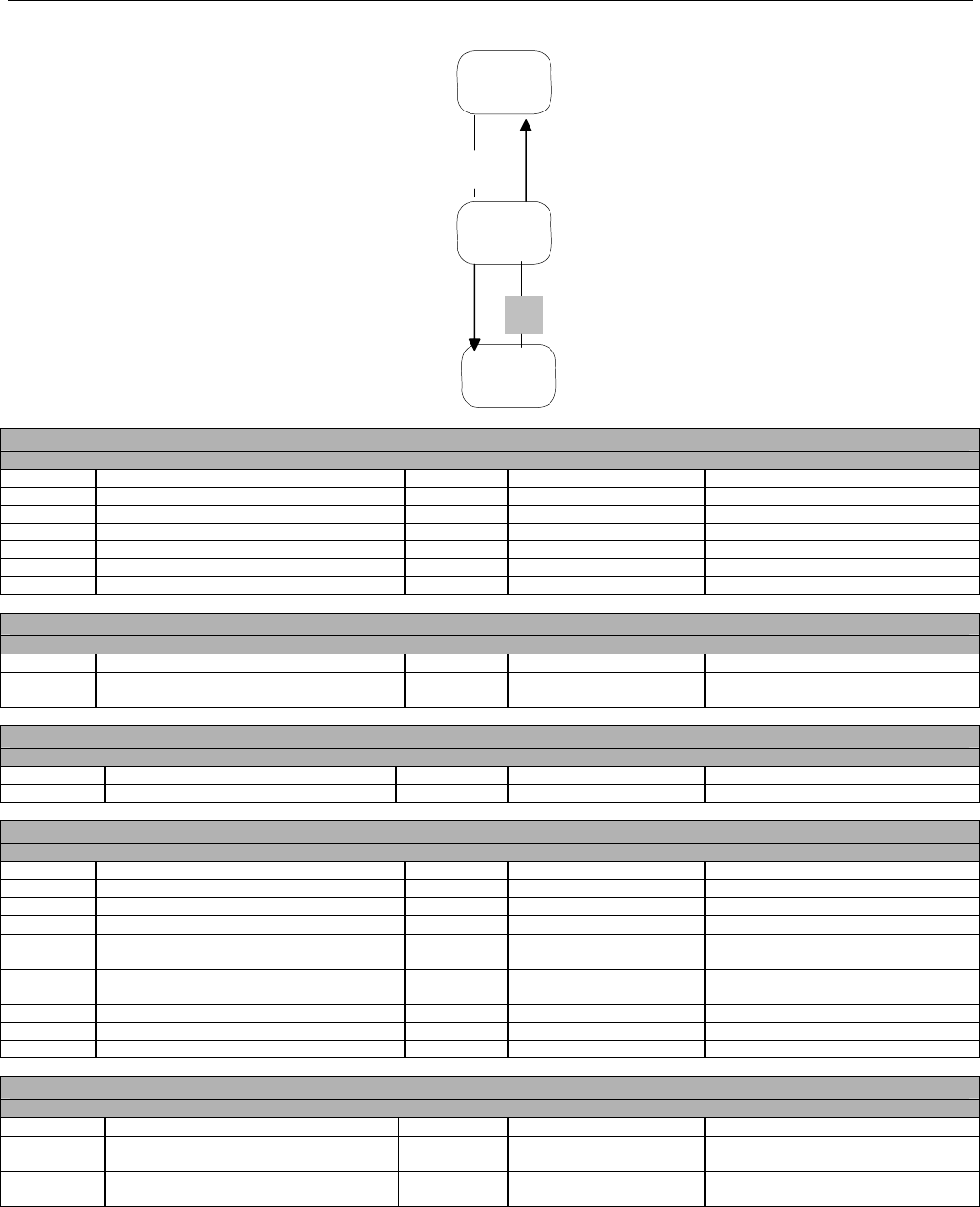
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 901 -
Switch
Facilitator
PDP
19
20
Information Reporting Reversal Response- Approved
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M N2 Information Reporting Reversal
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø7 NCPDP Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663bbbbbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
RESPONSE MESSAGE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 2Ø RESPONSE MESSAGE SEGMENT
5Ø4-F4 MESSAGE Q TRANSMISSION
MESSAGE TEXT For illustrative purposes only. Up to
2ØØ Bytes
RESPONSE INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 25 RESPONSE INSURANCE SEGMENT
3Ø1-C1 GROUP ID Q PARTD PDP Group ID
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M A Approved
5Ø3-F3 AUTHORIZATION NUMBER Q 738429384
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT R 1 1 occurrence
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø1 Used for first line of free form text
with no pre-defined structure.
526-FQ ADDITIONAL MESSAGE INFORMATION Q TRANSACTION
MESSAGE TEXT For illustrative purposes only. Up to
4Ø Bytes
549-7F HELP DESK PHONE NUMBER QUALIFIER R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 9193847388
88Ø-K5 TRANSACTION REFERENCE NUMBER R 2383838377
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 902 -
34.45 COMPOUNDED RX BILLING - TRANSACTION CODE B1 (Ø1) –
COORDINATION OF BENEFITS SCENARIO
Multi-ingredient compound claim with two payers. TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M bbbbbbbbbb
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 123456789 Cardholder ID
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID QUALIFIER M ØØ Default for multi-ingredient
compounds
4Ø7-D7 PRODUCT/SERVICE ID M Ø Default for multi-ingredient
compounds
442-E7 QUANTITY DISPENSED R 12ØØØØ 12Ø.ØØØml
4Ø3-D3 FILL NUMBER R 1 First dispensing for Rx#
4Ø5-D5 DAYS SUPPLY R 3 3 Days supply
4Ø6-D6 COMPOUND CODE R 2 Compounded Rx
4Ø8-D8 DISPENSE AS WRITTEN (DAW)/PRODUCT
SELECTION CODE
R Ø No product selection indicated
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ8Ø313 March 13, 2ØØ8
415-DF NUMBER OF REFILLS AUTHORIZED Q 5 5 Refills
419-DJ PRESCRIPTION ORIGIN CODE Q 1 Written prescription
354-NX SUBMISSION CLARIFICATION CODE COUNT R 1 One occurrence
42Ø-DK SUBMISSION CLARIFICATION CODE Q 8 Process Compound For Approved
Ingredients
429-DT SPECIAL PACKAGING INDICATOR Q 1 Not unit dose
6ØØ-28 UNIT OF MEASURE Q ML Milliliters
995-E2 ROUTE OF ADMINISTRATION Q 11 Oral
COMPOUND SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 1Ø COMPOUND SEGMENT
45Ø-EF COMPOUND DOSAGE FORM DESCRIPTION
CODE
M 11 Solution
451-EG COMPOUND DISPENSING UNIT FORM
INDICATOR
M 3 Milliliters
447-EC COMPOUND INGREDIENT COMPONENT
COUNT
M Ø3 3 Ingredients
488-RE COMPOUND PRODUCT ID QUALIFIER M Ø3 NDC
489-TE COMPOUND PRODUCT ID M 11845Ø139Ø1 Tetracycline 5ØØmg cap
448-ED COMPOUND INGREDIENT QUANTITY M 12ØØØ 12 capsules
449-EE COMPOUND INGREDIENT DRUG COST Q 12{ $1.2Ø
49Ø-UE COMPOUND INGREDIENT BASIS OF COST
DETERMINATION
Q Ø1 AWP
488-RE COMPOUND PRODUCT ID QUALIFIER M Ø3 NDC
489-TE COMPOUND PRODUCT ID M ØØ6Ø3148Ø49 Nystatin 1ØØØØØu/ml Susp
448-ED COMPOUND INGREDIENT QUANTITY M 12ØØØØ 12Ø.ØØØml
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 903 -
COMPOUND SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
449-EE COMPOUND INGREDIENT DRUG COST Q 84{ $8.4Ø
49Ø-UE COMPOUND INGREDIENT BASIS OF COST
DETERMINATION
Q Ø1 AWP
488-RE COMPOUND PRODUCT ID QUALIFIER M Ø3 NDC
489-TE COMPOUND PRODUCT ID M 6Ø8Ø9Ø31Ø55 Diphenhydramine 5Ømg cap
448-ED COMPOUND INGREDIENT QUANTITY M 24ØØØ 24 capsules
449-EE COMPOUND INGREDIENT DRUG COST Q 46{ $4.6Ø
49Ø-UE COMPOUND INGREDIENT BASIS OF COST
DETERMINATION
Q Ø1 AWP
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 25Ø{ $25.ØØ
412-DC DISPENSING FEE SUBMITTED Q 15Ø{ $15.ØØ
478-H7 OTHER AMOUNT CLAIMED SUBMITTED
COUNT
R 1 One occurrence
479-H8 OTHER AMOUNT CLAIMED SUBMITTED
QUALIFIER
R Ø1 Delivery Cost
48Ø-H9 OTHER AMOUNT CLAIMED SUBMITTED Q 5Ø{ $5.ØØ
426-DQ USUAL AND CUSTOMARY CHARGE Q 4ØØ{ $4Ø.ØØ
43Ø-DU GROSS AMOUNT DUE R 45Ø{ $45.ØØ
423-DN BASIS OF COST DETERMINATION Q Ø1 AWP
DUR/PPS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø8 DUR/PPS Segment
473-7E DUR/PPS CODE COUNTER R 1 1st DUR action
474-8E DUR/PPS LEVEL OF EFFORT Q 15 Highest level of complexity
34.45.1 COMPOUNDED RX BILLING ACCEPTED RESPONSE- PAID
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P Paid
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 5Ø{ $5.ØØ
5Ø6-F6 INGREDIENT COST PAID Q 2ØØ{ $2Ø.ØØ
5Ø7-F7 DISPENSING FEE PAID Q 15Ø{ $15.ØØ
557-AV TAX EXEMPT INDICATOR Q 1 Payer/Plan is Tax Exempt (The
Payer/Plan is not responsible for tax.
The patient may be charged tax.)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 904 -
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
563-J2 OTHER AMOUNT PAID COUNT R 1 One occurrence
564-J3 OTHER AMOUNT PAID QUALIFIER R Ø1 Delivery
565-J4 OTHER AMOUNT PAID Q 5Ø{ $5.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 35Ø{ $35.ØØ
522-FM BASIS OF REIMBURSEMENT DETERMINATION R 3 Ingredient Cost Reduced to AWP
Less X% Pricing
34.45.2 BILLING – TRANSACTION CODE B1 – COMPOUND – COORDINATION OF
BENEFITS –PHARMACY BILLS SECONDARY INSURANCE
Submit claim indicating Other Payer Amount Paid.
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ44
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M Bbbbbbbbbb
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M bbbbbbbbbb
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 987654321 Cardholder ID
3Ø1-C1 GROUP ID Q 1234
3Ø3-C3 PERSON CODE Q 3 Place in family
3Ø6-C6 PATIENT RELATIONSHIP CODE Q 4 Other
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID QUALIFIER M ØØ Default for multi-ingredient
compounds
4Ø7-D7 PRODUCT/SERVICE ID M Ø Default for multi-ingredient
compounds
442-E7 QUANTITY DISPENSED R 12ØØØØ 12Ø.ØØØml
4Ø3-D3 FILL NUMBER R 1 First dispensing for Rx#
4Ø5-D5 DAYS SUPPLY R 3 3 Days supply
4Ø6-D6 COMPOUND CODE R 2 Compounded Rx
4Ø8-D8 DISPENSE AS WRITTEN (DAW)/PRODUCT
SELECTION CODE
R Ø No product selection indicated
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ8Ø313 March 13, 2ØØ8
415-DF NUMBER OF REFILLS AUTHORIZED Q 5 5 Refills
419-DJ PRESCRIPTION ORIGIN CODE Q 1 Written prescription
354-NX SUBMISSION CLARIFICATION CODE COUNT R 1 One occurrence
42Ø-DK SUBMISSION CLARIFICATION CODE Q 8 Process Compound For Approved
Ingredients
3Ø8-C8 OTHER COVERAGE CODE R 2 Other coverage exists/billed-payment
collected
429-DT SPECIAL PACKAGING INDICATOR Q 1 Not unit dose
6ØØ-28 UNIT OF MEASURE Q ML Milliliters
995-E2 ROUTE OF ADMINISTRATION Q 11 Oral
COMPOUND SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 1Ø COMPOUND SEGMENT
45Ø-EF COMPOUND DOSAGE FORM DESCRIPTION M 11 Solution
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 905 -
COMPOUND SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
CODE
451-EG COMPOUND DISPENSING UNIT FORM
INDICATOR
M 3 Milliliters
447-EC COMPOUND INGREDIENT COMPONENT
COUNT
M Ø3 3 Ingredients
488-RE COMPOUND PRODUCT ID QUALIFIER M Ø3 NDC
489-TE COMPOUND PRODUCT ID M 11845Ø139Ø1 Tetracycline 5ØØmg cap
448-ED COMPOUND INGREDIENT QUANTITY M 12ØØØ 12 capsules
449-EE COMPOUND INGREDIENT DRUG COST Q 12{ $12.ØØ
49Ø-UE COMPOUND INGREDIENT BASIS OF COST
DETERMINATION
Q Ø1 AWP
488-RE COMPOUND PRODUCT ID QUALIFIER M Ø3 NDC
489-TE COMPOUND PRODUCT ID M ØØ6Ø3148Ø49 Nystatin 1ØØØØØu/ml Susp
448-ED COMPOUND INGREDIENT QUANTITY M 12ØØØØ 12Ø.ØØØml
449-EE COMPOUND INGREDIENT DRUG COST Q 84{ $8.4Ø
49Ø-UE COMPOUND INGREDIENT BASIS OF COST
DETERMINATION
Q Ø1 AWP
488-RE COMPOUND PRODUCT ID QUALIFIER M Ø3 NDC
489-TE COMPOUND PRODUCT ID M 6Ø8Ø9Ø31Ø55 Diphenhydramine 5Ømg cap
448-ED COMPOUND INGREDIENT QUANTITY M 24ØØØ 24 capsules
449-EE COMPOUND INGREDIENT DRUG COST Q 46{ $4.6Ø
49Ø-UE COMPOUND INGREDIENT BASIS OF COST
DETERMINATION
Q Ø1 AWP
DUR/PPS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø8 DUR/PPS Segment
473-7E DUR/PPS CODE COUNTER R 1 1st DUR action
474-8E DUR/PPS LEVEL OF EFFORT Q 15 Highest level of complexity
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 25Ø{ $25.ØØ
412-DC DISPENSING FEE SUBMITTED Q 15Ø{ $15.ØØ
478-H7 OTHER AMOUNT CLAIMED SUBMITTED
COUNT
R 1 One occurrence
479-H8 OTHER AMOUNT CLAIMED SUBMITTED
QUALIFIER
R Ø1 Delivery Cost
48Ø-H9 OTHER AMOUNT CLAIMED SUBMITTED Q 5Ø{ $5.ØØ
426-DQ USUAL AND CUSTOMARY CHARGE Q 4ØØ{ $4Ø.ØØ
43Ø-DU GROSS AMOUNT DUE R 45Ø{ $45.ØØ
423-DN BASIS OF COST DETERMINATION Q Ø1 AWP
* Definition of Gross Amount Due only allows for “the sum of” selected fields as presented in the Pricing Segment. It does NOT allow for the
“sum of” minus Other Payer Amount Paid.
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø5 COORDINATION OF BENEFITS/OTHER
PAYMENTS SEGMENT
337-4C COORDINATION OF BENEFITS/OTHER
PAYMENTS COUNT
M 1 One occurrence
338-5C OTHER PAYER COVERAGE TYPE M Ø1 Primary
339-6C OTHER PAYER ID QUALIFIER R Ø3 BIN #
34Ø-7C OTHER PAYER ID Q 61ØØ66 ID assigned to payer
443-E8 OTHER PAYER DATE Q 2ØØ8Ø313 March 13, 2ØØ8
341-HB OTHER PAYER AMOUNT PAID COUNT R 2 Two occurrences
342-HC OTHER PAYER AMOUNT PAID QUALIFIER R Ø7 Drug Benefit
431-DV OTHER PAYER AMOUNT PAID Q 3ØØ{ $3Ø.ØØ paid
342-HC OTHER PAYER AMOUNT PAID QUALIFIER R Ø1 Delivery Cost
431-DV OTHER PAYER AMOUNT PAID Q 5Ø{ $5.ØØ paid
34.45.2.1 SECONDARY INSURANCE PAYS THE CLAIM SUBMITTED WITH AMOUNT PAID BY

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 906 -
OTHER PAYER RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
RESPONSE INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 25 RESPONSE INSURANCE SEGMENT
524-FO PLAN ID Q 9988
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P Paid
5Ø3-F3 AUTHORIZATION NUMBER Q 11122233345678
549-7F HELP DESK PHONE NUMBER QUALIFIER R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 5{ $ØØ.5Ø
5Ø6-F6 INGREDIENT COST PAID Q 2ØØ{ $2Ø.ØØ
5Ø7-F7 DISPENSING FEE PAID Q 15Ø{ $15.ØØ
563-J2 OTHER AMOUNT PAID COUNT R 1 One occurrence
564-J3 OTHER AMOUNT PAID QUALIFIER R Ø1 Delivery cost
565-J4 OTHER AMOUNT PAID R { $Ø Delivery charge “recognized” but
not paid
566-J5 OTHER PAYER AMOUNT RECOGNIZED R 3ØØ{ $3Ø.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 45{ $4.5Ø
522-FM BASIS OF REIMBURSEMENT DETERMINATION R 1 Ingredient Cost Reduced to AWP Less
X% Pricing
34.46 PREDETERMINATION OF BENEFITS - TRANSACTION CODE D1
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M D1 Predetermination of Benefits
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M 98765bbbbb
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø1 PATIENT SEGMENT
3Ø4-C4 DATE OF BIRTH R 1962Ø615 Born June 15, 1962
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 907 -
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
3Ø5-C5 PATIENT GENDER CODE R 1 Male
31Ø-CA PATIENT FIRST NAME R JOSEPH
311-CB PATIENT LAST NAME R SMITH
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 3827493 Cardholder ID
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
442-E7 QUANTITY DISPENSED R 3ØØØØ 3Ø.ØØØ tablets
4Ø3-D3 FILL NUMBER R Ø Original dispensing for RX#
4Ø5-D5 DAYS SUPPLY R 3Ø 3Ø Days supply
4Ø6-D6 COMPOUND CODE R 1 Not a compound
4Ø8-D8 DISPENSE AS WRITTEN (DAW)/PRODUCT
SELECTION CODE
R Ø No product selection indicated
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ8Ø313 March 13, 2ØØ8
415-DF NUMBER OF REFILLS AUTHORIZED Q 5 5 Refills
3Ø8-C8 OTHER COVERAGE CODE Q 2 Other coverage exists/billed-payment
collected
429-DT SPECIAL PACKAGING INDICATOR Q 1 Not unit dose
6ØØ-28 UNIT OF MEASURE Q EA Each
PHARMACY PROVIDER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø2 PROVIDER SEGMENT
465-EY PROVIDER ID QUALIFIER R Ø2 License number
444-E9 PROVIDER ID O 39359
PRESCRIBER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø3 PRESCRIBER SEGMENT
466-EZ PRESCRIBER ID QUALIFIER R Ø8 State license
411-DB PRESCRIBER ID O ØØG2345
427-DR PRESCRIBER LAST NAME O JONES
498-PM PRESCRIBER TELEPHONE NUMBER O 2Ø13639572
468-2E PRIMARY CARE PROVIDER ID QUALIFIER R Ø2 Blue Cross
421-DL PRIMARY CARE PROVIDER ID O 123456
47Ø-4E PRIMARY CARE PROVIDER LAST NAME O WRIGHT
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 557{ $55.7Ø
412-DC DISPENSING FEE SUBMITTED Q 1ØØ{ $1Ø.ØØ
426-DQ USUAL AND CUSTOMARY CHARGE Q 867{ $86.7Ø
43Ø-DU GROSS AMOUNT DUE R 657{ $65.7Ø
423-DN BASIS OF COST DETERMINATION Q Ø3 Direct
34.46.1 PREDETERMINATION ACCEPTED RESPONSE - BENEFIT
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 908 -
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø3-A3 TRANSACTION CODE M D1 Predetermination of Benefits
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
RESPONSE MESSAGE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 2Ø RESPONSE MESSAGE SEGMENT
5Ø4-F4 MESSAGE Q TRANSMISSION
MESSAGE TEXT For illustrative purposes only. Up to
2ØØ Bytes
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M B Benefit
5Ø3-F3 AUTHORIZATION NUMBER Q 123456789123456789
13Ø-UF ADDITIONAL MESSAGE INFORMATION
COUNT
R 1 1 occurrence
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø1 Used for first line of free form text
with no pre-defined structure.
526-FQ ADDITIONAL MESSAGE INFORMATION Q TRANSACTION
MESSAGE TEXT For illustrative purposes only. Up to
4Ø Bytes
549-7F HELP DESK PHONE NUMBER QUALIFIER R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
551-9F PREFERRED PRODUCT COUNT R 1 1 Preferred Product Identified
552-AP PREFERRED PRODUCT ID QUALIFIER R Ø3 NDC
553-AR PREFERRED PRODUCT ID Q 17236Ø569Ø1 Ibuprofen 6ØØmg tablet
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 1ØØ{ $1Ø.ØØ
523-FN AMOUNT ATTRIBUTED TO SALES TAX Q 2Ø{ $2.ØØ
518-FI AMOUNT OF COPAY Q 8Ø{ $8.ØØ
34.46.2 PREDETERMINATION OF BENEFITS TRANSMISSION REJECTED RESPONSE
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M D1 Predetermination of Benefits
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M R Rejected
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
RESPONSE STATUS SEGMENT

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 909 -
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M R Rejected
51Ø-FA REJECT COUNT R 1 1 Reject Code follows
511-FB REJECT CODE R Ø1 M/I BIN Number
13Ø-UF ADDITIONAL MESSAGE INFORMATION
COUNT
R 1 1 occurrence
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø1 Used for first line of free form text with
no pre-defined structure.
526-FQ ADDITIONAL MESSAGE INFORMATION Q TRANSACTION
MESSAGE TEXT For illustrative purposes only. Up to
4Ø Bytes
549-7F HELP DESK PHONE NUMBER QUALIFIER R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
34.46.3 PREDETERMINATION OF BENEFITS TRANSACTION REJECTED RESPONSE
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M D1 Predetermination of Benefits
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M R Rejected
51Ø-FA REJECT COUNT R 1 1 Reject Code follows
511-FB REJECT CODE R 7Ø Product/Service not covered
13Ø-UF ADDITIONAL MESSAGE INFORMATION
COUNT
R 1 1 occurrence
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø1 Used for first line of free form text with
no pre-defined structure.
526-FQ ADDITIONAL MESSAGE INFORMATION Q TRANSACTION
MESSAGE TEXT For illustrative purposes only. Up to
4Ø Bytes
549-7F HELP DESK PHONE NUMBER QUALIFIER R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
551-9F PREFERRED PRODUCT COUNT R 1 1 Preferred Product Identified
552-AP PREFERRED PRODUCT ID QUALIFIER R Ø3 NDC
553-AR PREFERRED PRODUCT ID Q 17236Ø569Ø1 Ibuprofen 6ØØmg tablet
554-AS PREFERRED PRODUCT INCENTIVE Q 1Ø{ $1.ØØ
34.47 ELIGIBILITY MEDICARE PART D TO FACILITATOR – REQUEST
34.47.1 SCENARIO 1 – COULD NOT FIND THIS MEMBER
Member never had Medicare Part D coverage in the past, does not have current Part D coverage, and has no future Part D Coverage (Could
not find this member.)
Date of Request: 1Ø-1-2ØØ6
Date of Service: 8-1-2ØØ6
Response: Rejected Response
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 910 -
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M Ø11727
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M E1 Eligibility verification
1Ø4-A4 PROCESSOR CONTROL NUMBER M 2222222222
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ6Ø8Ø1 August 1, 2ØØ6
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M 98765bbbbb
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø1 PATIENT SEGMENT
3Ø4-C4 DATE OF BIRTH Q 1962Ø615 Born June 15, 1962
3Ø5-C5 PATIENT GENDER CODE Q 1 Male
31Ø-CA PATIENT FIRST NAME Q SAMUEL
311-CB PATIENT LAST NAME Q JONES
322-CM PATIENT STREET ADDRESS Q 123 MAIN STREET
323-CN PATIENT CITY ADDRESS Q MY TOWN
324-CO PATIENT STATE/PROVINCE ADDRESS Q CO
325-CP PATIENT ZIP/POSTAL ZONE Q 34567
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 123456789 The HICN (Health Insurance Claim
Number, Part A, B, or C)
34.48 ELIGIBILITY MEDICARE PART D TO FACILITATOR – REJECT RESPONSE
34.48.1 SCENARIO 1 – COULD NOT FIND THIS MEMBER
Scenario 1 - Eligibility Rejected Response – Patient could not be found
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M E1 Eligibility Verification
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ6Ø8Ø1 August 1, 2ØØ6
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M R Rejected
51Ø-FA REJECT COUNT R 1
511-FB REJECT CODE R N1 No Patient Match Found
13Ø-UF ADDITIONAL MESSAGE INFORMATION
COUNT
R 1 1 occurrence
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø1 Used for first line of free form text with
no pre-defined structure.
526-FQ ADDITIONAL MESSAGE INFORMATION Q TRANSACTION
MESSAGE TEXT For illustrative purposes only. Up to
4Ø Bytes
34.49 ELIGIBILITY MEDICARE PART D TO FACILITATOR – REQUEST
34.49.1 SCENARIO 2 – FOUND MEMBER BUT NO COVERAGE
Member had Medicare Part D Coverage in the past but does not have current Part D coverage. (Found member but no coverage)
Date of Request: 1Ø-1-2ØØ6
Date of Service: 8-1-2ØØ6
Member has no Medicare Part D coverage as of date of service.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 911 -
Response: Rejected Response- does not meet criteria of having current or future Part D coverage
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M Ø11727
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M E1 Eligibility verification
1Ø4-A4 PROCESSOR CONTROL NUMBER M 2222222222
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ6Ø8Ø1 August 1, 2ØØ6
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M 98765bbbbb
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø1 PATIENT SEGMENT
3Ø4-C4 DATE OF BIRTH Q 1962Ø615 Born June 15, 1962
3Ø5-C5 PATIENT GENDER CODE Q 1 Male
31Ø-CA PATIENT FIRST NAME Q SAMUEL
311-CB PATIENT LAST NAME Q JONES
322-CM PATIENT STREET ADDRESS Q 123 MAIN STREET
323-CN PATIENT CITY ADDRESS Q MY TOWN
324-CO PATIENT STATE/PROVINCE ADDRESS Q CO
325-CP PATIENT ZIP/POSTAL ZONE Q 34567
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 123456789 The HICN (Health Insurance Claim
Number, Part A, B, or C)
34.50 ELIGIBILITY MEDICARE PART D TO FACILITATOR – REJECT RESPONSE
34.50.1 SCENARIO 2 – FOUND MEMBER BUT NO COVERAGE
Scenario 2 - Eligibility Rejected Response – Patient found but no Part D Coverage for Date of Service
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M E1 Eligibility Verification
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ6Ø8Ø1 August 1, 2ØØ6
RESPONSE PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 29 RESPONSE PATIENT SEGMENT
31Ø-CA PATIENT FIRST NAME M SAMUEL
311-CB PATIENT LAST NAME Q JONES
3Ø4- C4 DATE OF BIRTH Q 1962Ø615 Born June 15, 1962
Note: This Patient data is from the Facilitator’s system. It is not echoed back from the submission information.
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M R Rejected
51Ø-FA REJECT COUNT R 1
511-FB REJECT CODE R 65 Patient is Not Covered
13Ø-UF ADDITIONAL MESSAGE INFORMATION
COUNT
R 1 1 occurrence
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø1 Used for first line of free form text with
no pre-defined structure.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 912 -
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
526-FQ ADDITIONAL MESSAGE INFORMATION Q TRANSACTION
MESSAGE TEXT For illustrative purposes only. Up to
4Ø Bytes
34.51 ELIGIBILITY MEDICARE PART D TO FACILITATOR – REQUEST
34.51.1 SCENARIO 3 - MEMBER HAS CURRENT MEDICARE PART D COVERAGE AND NO
OTHER COVERAGE
Date of Request: 1Ø-1-2ØØ6
Date of Service: 8-1-2ØØ6
Member is effective as of date of service with Medicare Part D as primary (1-1-2ØØ6 through 9-3Ø-2ØØ6)
Response: Approved TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M Ø11727
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M E1 Eligibility verification
1Ø4-A4 PROCESSOR CONTROL NUMBER M 2222222222
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ6Ø8Ø1 August 1, 2ØØ6
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M 98765bbbbb
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø1 PATIENT SEGMENT
3Ø4-C4 DATE OF BIRTH Q 1962Ø615 Born June 15, 1962
3Ø5-C5 PATIENT GENDER CODE Q 1 Male
31Ø-CA PATIENT FIRST NAME Q SAMUEL
311-CB PATIENT LAST NAME Q JONES
322-CM PATIENT STREET ADDRESS Q 123 MAIN STREET
323-CN PATIENT CITY ADDRESS Q MY TOWN
324-CO PATIENT STATE/PROVINCE ADDRESS Q CO
325-CP PATIENT ZIP/POSTAL ZONE Q 34567
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 123456789 The HICN (Health Insurance Claim
Number, Part A, B, or C)
34.52 ELIGIBILITY MEDICARE PART D TO FACILITATOR – APPROVED RESPONSE
34.52.1 SCENARIO 3 - MEMBER HAS CURRENT MEDICARE PART D COVERAGE AND NO
OTHER COVERAGE
Scenario 3 - Eligibility Approved Response
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M E1 Eligibility Verification
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ6Ø8Ø1 August 1, 2ØØ6
RESPONSE INSURANCE ADDITIONAL INFORMATION SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 27 RESPONSE INSURANCE ADDITIONAL
INFORMATION SEGMENT
139-UR MEDICARE PART D COVERAGE CODE M 1 Primary
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 913 -
RESPONSE INSURANCE ADDITIONAL INFORMATION SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
138-UQ CMS LOW INCOME COST SHARING (LICS)
LEVEL
Q Y Yes
24Ø-U1 CONTRACT NUMBER Q ABCXUX333
926-FF FORMULARY ID Q F33H12XU
757-U6 BENEFIT ID Q 123
RESPONSE PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 29 RESPONSE PATIENT SEGMENT
31Ø-CA PATIENT FIRST NAME M SAM
311-CB PATIENT LAST NAME Q JONES
3Ø4- C4 DATE OF BIRTH Q 1962Ø615 Born June 15, 1962
Note: This Patient data is from the Facilitator’s system. It is not echoed back from the submission information.
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M A Approved
5Ø3-F3 AUTHORIZATION NUMBER Q 123456789123456789
13Ø-UF ADDITIONAL MESSAGE INFORMATION
COUNT
R 1 1 occurrence
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø1 Used for first line of free form text with
no pre-defined structure.
526-FQ ADDITIONAL MESSAGE INFORMATION Q TRANSACTION
MESSAGE TEXT For illustrative purposes only. Up to
4Ø Bytes
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 28 RESPONSE COORDINATION OF
BENEFITS/OTHER PAYERS SEGMENT
355-NT OTHER PAYER ID COUNT M 1 One occurrence
338-5C OTHER PAYER COVERAGE TYPE M Ø1 Primary
339-6C OTHER PAYER ID QUALIFIER R Ø3 BIN
34Ø-7C OTHER PAYER ID Q 123456
991-MH OTHER PAYER PROCESSOR CONTROL
NUMBER
Q 987654321Ø
356-NU OTHER PAYER CARDHOLDER ID Q 456789123
992-MJ OTHER PAYER GROUP ID Q 789123
142-UV OTHER PAYER PERSON CODE Q Ø1 Ø1 = Other Payer assigned person
code
143-UW OTHER PAYER PATIENT RELATIONSHIP
CODE
Q 1
127-UB OTHER PAYER HELP DESK NUMBER Q 5556861111 Primary Payer listed Help Desk Phone
Number - in this instance is the Part D
help desk
144-UX OTHER PAYER BENEFIT EFFECTIVE DATE Q 2ØØ6Ø1Ø1 January 1, 2ØØ6
145-UY OTHER PAYER BENEFIT TERMINATION DATE Q 2ØØ6Ø93Ø September 3Ø, 2ØØ6
34.53 ELIGIBILITY MEDICARE PART D TO FACILITATOR – REQUEST
34.53.1 SCENARIO 4 – MEMBER HAS CURRENT MEDICARE PART D COVERAGE
(PRIMARY) AND CURRENT OTHER COVERAGE
Date of Request: 1Ø-1-2ØØ6
Date of Service: 8-1-2ØØ6
Member is effective as of date of service with Medicare Part D as primary (1-1-2ØØ6 through 9-3Ø-2ØØ6)
Member is effective as of date of service with Other Payer “A” as secondary (2-1-2ØØ6 through 11-3Ø-2ØØ6)
Member is effective as of date of service with Other Payer “B” as tertiary (8-1-2ØØ6 through12-31-2ØØ6)
Response: Approved
Loops of Coordination of Benefits/Other Payments Segment show three Other Coverages in the order shown above.
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M Ø11727
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 914 -
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M E1 Eligibility verification
1Ø4-A4 PROCESSOR CONTROL NUMBER M 2222222222
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ6Ø8Ø1 August 1, 2ØØ6
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M 98765bbbbb
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø1 PATIENT SEGMENT
3Ø4-C4 DATE OF BIRTH Q 1962Ø615 Born June 15, 1962
3Ø5-C5 PATIENT GENDER CODE Q 1 Male
31Ø-CA PATIENT FIRST NAME Q SAMUEL
311-CB PATIENT LAST NAME Q JONES
322-CM PATIENT STREET ADDRESS Q 123 MAIN STREET
323-CN PATIENT CITY ADDRESS Q MY TOWN
324-CO PATIENT STATE/PROVINCE ADDRESS Q CO
325-CP PATIENT ZIP/POSTAL ZONE Q 34567
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 123456789 The HICN (Health Insurance Claim
Number, Part A, B, or C)
34.54 ELIGIBILITY MEDICARE PART D TO FACILITATOR – APPROVED RESPONSE
34.54.1 SCENARIO 4 – MEMBER HAS CURRENT MEDICARE PART D COVERAGE
(PRIMARY) AND CURRENT OTHER COVERAGE
Scenario 4 - Eligibility Approved Response With More Than Two Payers
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M E1 Eligibility Verification
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ6Ø8Ø1 August 1, 2ØØ6
RESPONSE INSURANCE ADDITIONAL INFORMATION SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 27 RESPONSE INSURANCE ADDITIONAL
INFORMATION SEGMENT
139-UR MEDICARE PART D COVERAGE CODE M 1 Primary
138-UQ CMS LOW INCOME COST SHARING (LICS)
LEVEL
Q Y Yes
24Ø-U1 CONTRACT NUMBER Q ABCXUX333
926-FF FORMULARY ID Q F33H12XU
757-U6 BENEFIT ID Q 123
RESPONSE PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 29 RESPONSE PATIENT SEGMENT
31Ø-CA PATIENT FIRST NAME M SAM
311-CB PATIENT LAST NAME Q JONES
3Ø4- C4 DATE OF BIRTH Q 1962Ø615 Born June 15, 1962
Note: This Patient data is from the Facilitator’s system. It is not echoed back from the submission information.
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 915 -
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M A Approved
5Ø3-F3 AUTHORIZATION NUMBER Q 123456789123456789
13Ø-UF ADDITIONAL MESSAGE INFORMATION
COUNT
R 1 1 occurrence
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø1 Used for first line of free form text with
no pre-defined structure.
526-FQ ADDITIONAL MESSAGE INFORMATION Q TRANSACTION
MESSAGE TEXT For illustrative purposes only. Up to
4Ø Bytes
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 28 RESPONSE COORDINATION OF
BENEFITS/OTHER PAYERS SEGMENT
355-NT OTHER PAYER ID COUNT M 3 3 occurrences
338-5C OTHER PAYER COVERAGE TYPE M Ø1 Primary
339-6C OTHER PAYER ID QUALIFIER R Ø3 BIN
34Ø-7C OTHER PAYER ID Q 123456 This is the Medicare Part D payer
based on the Medicare Part D
Coverage Code (139-UR) = 1
(Primary)
991-MH OTHER PAYER PROCESSOR CONTROL
NUMBER
Q 987654321Ø
356-NU OTHER PAYER CARDHOLDER ID Q 456789123
992-MJ OTHER PAYER GROUP ID Q 789123
142-UV OTHER PAYER PERSON CODE Q Ø1Ø Ø1 = Other Payer assigned person
code.
143-UW OTHER PAYER PATIENT RELATIONSHIP
CODE
Q 1 Cardholder
127-UB OTHER PAYER HELP DESK NUMBER Q 5556861111 Primary Payer listed Help Desk Phone
Number - in this instance is the Part D
help desk
144-UX OTHER PAYER BENEFIT EFFECTIVE DATE Q 2ØØ6Ø1Ø1 January 1, 2ØØ6
145-UY OTHER PAYER BENEFIT TERMINATION DATE Q 2ØØ6Ø93Ø September 3Ø, 2ØØ6
338-5C OTHER PAYER COVERAGE TYPE M Ø2 Secondary
339-6C OTHER PAYER ID QUALIFIER R Ø3 BIN
34Ø-7C OTHER PAYER ID Q 888555 Other Payer A
991-MH OTHER PAYER PROCESSOR CONTROL
NUMBER
Q 8522542311
356-NU OTHER PAYER CARDHOLDER ID Q 23456789
992-MJ OTHER PAYER GROUP ID Q 888222
142-UV OTHER PAYER PERSON CODE Q ØØ ØØ = Other Payer assigned person
code
143-UW OTHER PAYER PATIENT RELATIONSHIP
CODE
Q 1 Cardholder
127-UB OTHER PAYER HELP DESK NUMBER Q 5558884444 Other Payer A Help Desk Phone
Number
144-UX OTHER PAYER BENEFIT EFFECTIVE DATE Q 2ØØ6Ø2Ø1 February 1, 2ØØ6
145-UY OTHER PAYER BENEFIT TERMINATION DATE Q 2ØØ6113Ø November 3Ø, 2ØØ6
338-5C OTHER PAYER COVERAGE TYPE M Ø3 Tertiary
339-6C OTHER PAYER ID QUALIFIER R Ø3 BIN
34Ø-7C OTHER PAYER ID Q 552233 Other Payer B
991-MH OTHER PAYER PROCESSOR CONTROL
NUMBER
Q Ø987654321
356-NU OTHER PAYER CARDHOLDER ID Q 553322123
992-MJ OTHER PAYER GROUP ID Q 123456
142-UV OTHER PAYER PERSON CODE Q ØØ ØØ = Other Payer assigned person
code
143-UW OTHER PAYER PATIENT RELATIONSHIP
CODE
Q 1 Cardholder
127-UB OTHER PAYER HELP DESK NUMBER Q 5558885555 Other Payer B Help Desk Phone
Number
144-UX OTHER PAYER BENEFIT EFFECTIVE DATE Q 2ØØ6Ø8Ø1 August 1, 2ØØ6
145-UY OTHER PAYER BENEFIT TERMINATION DATE Q 2ØØ61231 December 31, 2ØØ6
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 916 -
34.55 ELIGIBILITY MEDICARE PART D TO FACILITATOR – REQUEST
34.55.1 SCENARIO 5 – FUTURE EFFECTIVE WITH MEDICARE PART D
Member is not currently effective with Medicare Part D, but has a future effective date with Medicare Part D as primary and has other coverage
not currently effective but is effective in the future. (Note: Eligibility will only return Medicare Part D future effective date)
Date of Request: 1Ø-1-2ØØ6
Date of Service: 8-1-2ØØ6
Member is not effective as of date of service with Other Payer “A”, but will be effective in the future (9-1-2ØØ6 through 12-31-2ØØ6) as
primary
Member is not effective as of date of service with Other Payer “B”, but will be in the future (1Ø-1-2ØØ6 through 12-31-2ØØ6)
Member is not effective as of date of service with Medicare Part D, but will be in the future (11-1-2ØØ6 through 12-31-2ØØ6)
Response: Rejected
Fields NEXT MEDICARE PART D EFFECTIVE DATE (14Ø-US) AND NEXT MEDICARE PART D TERMINATION DATE (141-UT) will be
populated in the Response Insurance Additional Information Segment for the Part D coverage starting in 11-1-2ØØ6 and ending 12-31-2ØØ6.
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M Ø11727
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M E1 Eligibility verification
1Ø4-A4 PROCESSOR CONTROL NUMBER M 2222222222
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ6Ø8Ø1 August 1, 2ØØ6
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M 98765bbbbb
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø1 PATIENT SEGMENT
3Ø4-C4 DATE OF BIRTH Q 1962Ø615 Born June 15, 1962
3Ø5-C5 PATIENT GENDER CODE Q 1 Male
31Ø-CA PATIENT FIRST NAME Q SAMUEL
311-CB PATIENT LAST NAME Q JONES
322-CM PATIENT STREET ADDRESS Q 123 MAIN STREET
323-CN PATIENT CITY ADDRESS Q MY TOWN
324-CO PATIENT STATE/PROVINCE ADDRESS Q CO
325-CP PATIENT ZIP/POSTAL ZONE Q 34567
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 123456789 The HICN (Health Insurance Claim
Number, Part A, B, or C)
34.56 ELIGIBILITY MEDICARE PART D TO FACILITATOR – REJECTED RESPONSE
34.56.1 SCENARIO 5 – FUTURE EFFECTIVE WITH MEDICARE PART D
Scenario 5 - Eligibility Rejected Response
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M E1 Eligibility Verification
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ6Ø8Ø1 August 1, 2ØØ6
RESPONSE INSURANCE ADDITIONAL INFORMATION SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 27 RESPONSE INSURANCE ADDITIONAL
INFORMATION SEGMENT
14Ø-US NEXT MEDICARE PART D EFFECTIVE DATE Q 2ØØ611Ø1 November 1, 2ØØ6
141-UT NEXT MEDICARE PART D TERMINATION Q 2ØØ61231 December 31, 2ØØ6
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 917 -
DATE
RESPONSE PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 29 RESPONSE PATIENT SEGMENT
31Ø-CA PATIENT FIRST NAME M SAMUEL
311-CB PATIENT LAST NAME Q JONES
3Ø4- C4 DATE OF BIRTH Q 1962Ø615 Born June 15, 1962
Note: This Patient data is from the Facilitator’s system. It is not echoed back from the submission information.
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M R Rejected
51Ø-FA REJECT COUNT R 1
511-FB REJECT CODE R 65 Patient is not covered
34.57 ELIGIBILITY MEDICARE PART D TO FACILITATOR – REQUEST
34.57.1 SCENARIO 6 – ADJUSTED REQUEST TO SCENARIO 5
Eligibility request was submitted as in Scenario 5. Requester submits a second request based on information returned in Scenario 5(rejected
with Future dates for Medicare Part D) New submission has a date of service in the future.
Date of Request: 1Ø-1-2ØØ6
Date of Service: 11-1-2ØØ6
Member is effective with Other Payer A as of the date of service, (9-1-2ØØ6 through 12-31-2ØØ6)
Member is effective with Other Payer B as secondary as of 1Ø-1-2ØØ6 through 12-31-2ØØ6
Member is effective with Medicare Part D as tertiary as of 11-1- 2ØØ6 through 12-21-2ØØ6
Response: Approved Response TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M Ø11727
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M E1 Eligibility verification
1Ø4-A4 PROCESSOR CONTROL NUMBER M 2222222222
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ6Ø8Ø1 August 1, 2ØØ6
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID M 98765bbbbb
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø1 PATIENT SEGMENT
3Ø4-C4 DATE OF BIRTH Q 1962Ø615 Born June 15, 1962
3Ø5-C5 PATIENT GENDER CODE Q 1 Male
31Ø-CA PATIENT FIRST NAME Q SAMUEL
311-CB PATIENT LAST NAME Q JONES
322-CM PATIENT STREET ADDRESS Q 123 MAIN STREET
323-CN PATIENT CITY ADDRESS Q MY TOWN
324-CO PATIENT STATE/PROVINCE ADDRESS Q CO
325-CP PATIENT ZIP/POSTAL ZONE Q 34567
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 123456789 The HICN (Health Insurance Claim
Number, Part A, B, or C)
34.58 ELIGIBILITY MEDICARE PART D TO FACILITATOR – APPROVED RESPONSE
34.58.1 SCENARIO 6 – ADJUSTED REQUEST TO SCENARIO 5
Scenario 6 - Eligibility Accepted Response
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 918 -
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M E1 Eligibility Verification
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ6Ø8Ø1 August 1, 2ØØ6
RESPONSE INSURANCE ADDITIONAL INFORMATION SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 27 RESPONSE INSURANCE ADDITIONAL
INFORMATION SEGMENT
139-UR MEDICARE PART D COVERAGE CODE M 3 Tertiary
138-UQ CMS LOW INCOME COST SHARING (LICS)
LEVEL
Q N No
24Ø-U1 CONTRACT NUMBER Q ABCXUX333
926-FF FORMULARY ID Q F33H12XU
757-U6 BENEFIT ID Q 123
RESPONSE PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 29 RESPONSE PATIENT SEGMENT
31Ø-CA PATIENT FIRST NAME M SAMUEL
311-CB PATIENT LAST NAME Q JONES
3Ø4- C4 DATE OF BIRTH Q 1962Ø615 Born June 15, 1962
Note: This Patient data is from the Facilitator’s system. It is not echoed back from the submission information.
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M A Approved
5Ø3-F3 AUTHORIZATION NUMBER Q 123456789123456789
13Ø-UF ADDITIONAL MESSAGE INFORMATION
COUNT
R 1 1 occurrence
132-UH ADDITIONAL MESSAGE INFORMATION
QUALIFIER
R Ø1 Used for first line of free form text with
no pre-defined structure.
526-FQ ADDITIONAL MESSAGE INFORMATION Q TRANSACTION
MESSAGE TEXT For illustrative purposes only. Up to
4Ø Bytes
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 28 RESPONSE COORDINATION OF
BENEFITS/OTHER PAYERS SEGMENT
355-NT OTHER PAYER ID COUNT M 3 3 occurrences
338-5C OTHER PAYER COVERAGE TYPE M Ø1 Primary
339-6C OTHER PAYER ID QUALIFIER R Ø3 BIN
34Ø-7C OTHER PAYER ID Q 888555 Other Payer A
991-MH OTHER PAYER PROCESSOR CONTROL
NUMBER
Q 8522542311
356-NU OTHER PAYER CARDHOLDER ID Q 23456789
992-MJ OTHER PAYER GROUP ID Q 888222
142-UV OTHER PAYER PERSON CODE Q ØØ ØØ = Other Payer assigned person
code
143-UW OTHER PAYER PATIENT RELATIONSHIP
CODE
Q 1 Cardholder
127-UB OTHER PAYER HELP DESK NUMBER Q 5558884444 Primary Payer listed Help Desk Phone
Number - in this instance is the Other
Payer A help desk
144-UX OTHER PAYER BENEFIT EFFECTIVE DATE Q 2ØØ6Ø9Ø1 September Ø1, 2ØØ6
145-UY OTHER PAYER BENEFIT TERMINATION DATE Q 2ØØ61231 December 31, 2ØØ6
338-5C OTHER PAYER COVERAGE TYPE M Ø2 Secondary
339-6C OTHER PAYER ID QUALIFIER R Ø3 BIN
34Ø-7C OTHER PAYER ID Q 552233 Payer B
991-MH OTHER PAYER PROCESSOR CONTROL Q Ø987654321
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 919 -
RESPONSE COORDINATION OF BENEFITS/OTHER PAYERS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
NUMBER
356-NU OTHER PAYER CARDHOLDER ID Q 553322123
992-MJ OTHER PAYER GROUP ID Q 123456
142-UV OTHER PAYER PERSON CODE Q ØØ ØØ = Other Payer assigned person
code
143-UW OTHER PAYER PATIENT RELATIONSHIP
CODE
Q 1 Cardholder
127-UB OTHER PAYER HELP DESK NUMBER Q 5558885555 Other Payer B Help Desk Phone
Number
144-UX OTHER PAYER BENEFIT EFFECTIVE DATE Q 2ØØ61ØØ1 October Ø1, 2ØØ6
145-UY OTHER PAYER BENEFIT TERMINATION DATE Q 2ØØ61231 December 31, 2ØØ6
338-5C OTHER PAYER COVERAGE TYPE M Ø3 Tertiary - Medicare Part D
339-6C OTHER PAYER ID QUALIFIER R Ø3 BIN
34Ø-7C OTHER PAYER ID Q 123456 This is the Medicare Part D payer
based on the Medicare Part D
Coverage Code (139-UR) = 3
(Tertiary)
991-MH OTHER PAYER PROCESSOR CONTROL
NUMBER
Q 987654321Ø
356-NU OTHER PAYER CARDHOLDER ID Q 456789123
992-MJ OTHER PAYER GROUP ID Q 789123
142-UV OTHER PAYER PERSON CODE Q Ø1 Ø1 = Other Payer assigned person
code
143-UW OTHER PAYER PATIENT RELATIONSHIP
CODE
Q 1 Cardholder
127-UB OTHER PAYER HELP DESK NUMBER Q 5556861111 Medicare Part D help desk number
144-UX OTHER PAYER BENEFIT EFFECTIVE DATE Q 2ØØ611Ø1 November Ø1, 2ØØ6
145-UY OTHER PAYER BENEFIT TERMINATION DATE Q 2ØØ61231 December 31, 2ØØ6
34.59 BILLING - TRANSACTION CODE B1 - COB SCENARIO - PHARMACY BILLS
REPORTING AMOUNT PAID BY PREVIOUS PAYER ONLY
Excerpt response from Primary Payer RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
518-FI AMOUNT OF COPAY Q 35Ø{ $35.ØØ
5Ø5-F5 PATIENT PAY AMOUNT R 35Ø{ $35.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 4ØØ{ $4Ø.ØØ
5Ø6-F6 INGREDIENT COST PAID R 7ØØ{ $7Ø.ØØ
5Ø7-F7 DISPENSING FEE PAID Q 5Ø{ $5.ØØ
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 3 Ingredient Cost Reduced to AWP Less X%
Pricing
Balancing Data Primary Response:
Ingredient Cost Paid $7Ø.ØØ Patient Pay Amount $35.ØØ Patient Pay Amount $35.ØØ
Dispensing Fee Paid $5.ØØ Total Amount Paid $4Ø.ØØ
Net: $75.ØØ Net $75.ØØ
34.59.1 PHARMACY BILLS SECONDARY INSURANCE
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø313 March 13, 2ØØ7
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID
M bbbbbbbbbb
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 920 -
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 987654321 Cardholder ID
3Ø1-C1 GROUP ID Q 1234
3Ø3-C3 PERSON CODE Q 3 Place in family
3Ø6-C6 PATIENT RELATIONSHIP CODE Q 3 Child
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
3Ø8-C8 OTHER COVERAGE CODE R 2 Other coverage exists/billed-payment collected
442-E7 QUANTITY DISPENSED R 6Ø
4Ø3-D3 FILL NUMBER R Ø Original Fill
4Ø5-D5 DAYS SUPPLY R 3Ø
4Ø6-D6 COMPOUND CODE R 1 Not a Compound
4Ø8-D8 DISPENSE AS WRITTEN
(DAW)/PRODUCT SELECTION
CODE
R Ø No product selection indicated
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ7Ø313 March 13, 2ØØ7
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 785{ $78.5Ø
412-DC DISPENSING FEE SUBMITTED Q 25{ $2.5Ø
43Ø-DU GROSS AMOUNT DUE R 81Ø{ $81.ØØ
426-DQ USUAL AND CUSTOMARY CHARGE Q 819I $81.99
423-DN BASIS OF COST DETERMINATION Q Ø1 AWP
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø5 COORDINATION OF BENEFITS/OTHER PAYMENTS
SEGMENT
337-4C COORDINATION OF
BENEFITS/OTHER PAYMENTS
COUNT
M 1 One occurrence
338-5C OTHER PAYER COVERAGE TYPE M Ø1 Primary
353-NR OTHER PAYER –PATIENT
RESPONSIBILITY AMOUNT COUNT
R 1 One occurrence
339-6C OTHER PAYER ID QUALIFIER R Ø3 BIN #
34Ø-7C OTHER PAYER ID Q 123456 ID assigned to payer
443-E8 OTHER PAYER DATE Q 2ØØ7Ø313 March 13, 2ØØ7
341-HB OTHER PAYER AMOUNT PAID
COUNT
R 1 One occurrence
342-HC OTHER PAYER AMOUNT PAID
QUALIFIER
R Ø7 Drug Benefit
431-DV OTHER PAYER AMOUNT PAID Q 4ØØ{ $4Ø.ØØ
34.59.1.1 SECONDARY RESPONSE - PAID
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø313 March 13, 2ØØ7
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 921 -
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P Paid
5Ø3-F3 AUTHORIZATION NUMBER Q 11122233345678
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R 3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 3Ø{ $3.ØØ
5Ø6-F6 INGREDIENT COST PAID R 69Ø{ $69.ØØ
5Ø7-F7 DISPENSING FEE PAID R 3Ø{ $3.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 29Ø{ $29.ØØ
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 14 Other Payer-Patient Responsibility Amount -
Indicates reimbursement was based on the
Other Payer Patient Responsibility Amount (352-
NQ)
518-FI AMOUNT OF COPAY Q 3Ø{ $3.ØØ
566-J5 OTHER PAYER AMOUNT
RECOGNIZED
R 4ØØ{ $40.ØØ
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 3 Ingredient Cost Reduced to AWP Less X%
Pricing
Balancing Data Secondary Response:
Ingredient Cost Paid $69.ØØ Patient Pay Amount $3.ØØ Copay $3.ØØ
Dispensing Fee Paid $3.ØØ Total Amount Paid $29.ØØ
Other Payer Amount
Recognized
$4Ø.ØØ
Net $72.ØØ Net $72.ØØ Patient Pay Amount $3.ØØ
34.60 BILLING – TRANSACTION CODE B1 – COORDINATION OF BENEFITS
• Billing to Secondary - Patient Responsibility Only
o Payer Requirement to Report Patient Pay Amount as Received from Prior Payer)
o Reimbursement Based on the Other Payer Patient Responsibility Amount (352-NQ)
Excerpt response from Primary Payer RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 35Ø{ $35.ØØ
5Ø6-F6 INGREDIENT COST PAID R 7ØØ{ $7Ø.ØØ
5Ø7-F7 DISPENSING FEE PAID Q 5Ø{ $5.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 53Ø{ $53.ØØ
521-FL INCENTIVE AMOUNT PAID R 3Ø{ $3.ØØ
563-J2 OTHER AMOUNT PAID COUNT R 1 One occurrence
564-J3 OTHER AMOUNT PAID QUALIFIER R Ø1 Delivery
565-J4 OTHER AMOUNT PAID Q 8Ø{ $8.ØØ
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 1 Ingredient cost paid as submitted
518-FI AMOUNT OF COPAY 1ØØ{ $1Ø.ØØ
517-FH AMOUNT APPLIED TO PERIODIC
DEDUCTIBLE
25Ø{ $25.ØØ
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 922 -
Balancing Data Primary Response:
Ingredient Cost Paid $7Ø.ØØ Patient Pay Amount $35.ØØ Patient Pay Amount $35.ØØ
Dispensing Fee Paid $5.ØØ Total Amount Paid $53.ØØ = Copay $1Ø.ØØ
Incentive Amt Paid $8.ØØ Net $88.ØØ + Deductible $25.ØØ
Other Amount Paid $5.ØØ
Net: $88.ØØ
34.60.1 PHARMACY BILLS SECONDARY INSURANCE
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø313 March 13, 2ØØ7
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID
M bbbbbbbbbb
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 987654321 Cardholder ID
3Ø1-C1 GROUP ID Q 1234
3Ø3-C3 PERSON CODE Q 3 Place in family
3Ø6-C6 PATIENT RELATIONSHIP CODE Q 3 Child
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
3Ø8-C8 OTHER COVERAGE CODE R 8 Claim is a billing for patient financial
responsibility
442-E7 QUANTITY DISPENSED R 6Ø
4Ø3-D3 FILL NUMBER R Ø Original Fill
4Ø5-D5 DAYS SUPPLY R 3Ø
4Ø6-D6 COMPOUND CODE R 1 Not a Compound
4Ø8-D8 DISPENSE AS WRITTEN
(DAW)/PRODUCT SELECTION
CODE
R Ø No product selection indicated
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ7Ø313 March 13, 2ØØ7
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 8ØØ{ $8Ø.ØØ
412-DC DISPENSING FEE SUBMITTED Q 25{ $2.5Ø
43Ø-DU GROSS AMOUNT DUE R 825{ $82.5Ø
426-DQ USUAL AND CUSTOMARY CHARGE Q 859I $85.99
423-DN BASIS OF COST DETERMINATION Q Ø1 AWP
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø5 COORDINATION OF BENEFITS/OTHER PAYMENTS
SEGMENT
337-4C COORDINATION OF
BENEFITS/OTHER PAYMENTS
M 1 One occurrence
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 923 -
COUNT
338-5C OTHER PAYER COVERAGE TYPE M Ø1 Primary
353-NR OTHER PAYER –PATIENT
RESPONSIBILITY AMOUNT COUNT
R 1 One occurrence
339-6C OTHER PAYER ID QUALIFIER R Ø3 BIN #
34Ø-7C OTHER PAYER ID Q 123456 ID assigned to payer
443-E8 OTHER PAYER DATE Q 2ØØ7Ø313 March 13, 2ØØ7
351-NP OTHER PAYER-PATIENT
RESPONSIBILITY AMOUNT
QUALIFIER
R Ø6 Patient Pay Amount (5Ø5-F5) as reported by
previous payer
352-NQ OTHER PAYER-PATIENT
RESPONSIBILITY AMOUNT
Q 35Ø{ $35.ØØ
34.60.1.1 SECONDARY RESPONSE - PAID
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø313 March 13, 2ØØ7
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P Paid
5Ø3-F3 AUTHORIZATION NUMBER Q 11122233345678
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R 3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 5Ø{ $5.ØØ
5Ø6-F6 INGREDIENT COST PAID R 35Ø{ $35.ØØ
5Ø7-F7 DISPENSING FEE PAID R ØØ{ $Ø.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 3ØØ{ $3Ø.ØØ
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 14 Other Payer-Patient Responsibility Amount -
Indicates reimbursement was based on the
Other Payer Patient Responsibility Amount (352-
NQ)
518-FI AMOUNT OF COPAY Q 5Ø{ $5.ØØ
148-U8 INGREDIENT COST CONTRACTED/
REIMBURSABLE AMOUNT
I 75Ø{ $75.ØØ
149-U9 DISPENSING FEE CONTRACTED/
REIMBURSABLE AMOUNT
I 25{ $2.5Ø
Balancing Data Secondary Response:
Ingredient Cost Paid $35.ØØ Patient Pay Amount $5.ØØ Copay $5.ØØ
Dispensing Fee Paid $Ø.ØØ Total Amount Paid $3Ø.ØØ Patient Pay Amount $5.ØØ
Net $35.ØØ Net $35.ØØ
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 924 -
34.61 BILLING – TRANSACTION CODE B1 – COORDINATION OF BENEFITS –
REIMBURSEMENT BASED ON THE OTHER PAYER PATIENT RESPONSIBILITY
AMOUNT (352-NQ) AND PATIENT REQUEST OF BRAND
Excerpt response from Primary Payer RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 9ØØ{ $9Ø.ØØ
5Ø6-F6 INGREDIENT COST PAID R 85Ø{ $85.ØØ
5Ø7-F7 DISPENSING FEE PAID Q 5Ø{ $5.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 15Ø{ $15.ØØ
563-J2 OTHER AMOUNT PAID COUNT R 1 One occurrence
564-J3 OTHER AMOUNT PAID QUALIFIER R Ø1 Delivery
565-J4 OTHER AMOUNT PAID Q 15Ø{ $15.ØØ
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 2 Ingredient Cost Reduced to AWP Pricing
517-FH AMOUNT APPLIED TO PERIODIC
DEDUCTIBLE
R 3ØØ{ $3Ø.ØØ
136-UN AMOUNT ATTRIBUTED TO PRODUCT
SELECTION/BRAND NON-
PREFERRED FORMULARY SELECTION
Q 6ØØ{ $6Ø.ØØ
Balancing Data Primary Response:
Ingredient Cost Paid $85.ØØ Patient Pay Amount $9Ø.ØØ Product Selection $6Ø.ØØ
Dispensing Fee Paid $5.ØØ Total Amount Paid $15.ØØ Deductible $3Ø.ØØ
Other Amount Paid $15.ØØ Net $1Ø5.ØØ
Patient Pay
Amount $9Ø.ØØ
Net $1Ø5.ØØ
34.61.1 PHARMACY BILLS SECONDARY INSURANCE
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø313 March 13, 2ØØ7
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID
M bbbbbbbbbb
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 987654321 Cardholder ID
3Ø1-C1 GROUP ID Q 1234
3Ø3-C3 PERSON CODE Q 3 Place in family
3Ø6-C6 PATIENT RELATIONSHIP CODE Q 3 Child
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
3Ø8-C8 OTHER COVERAGE CODE R 8 Claim is a billing for patient financial
responsibility
442-E7 QUANTITY DISPENSED R 6Ø
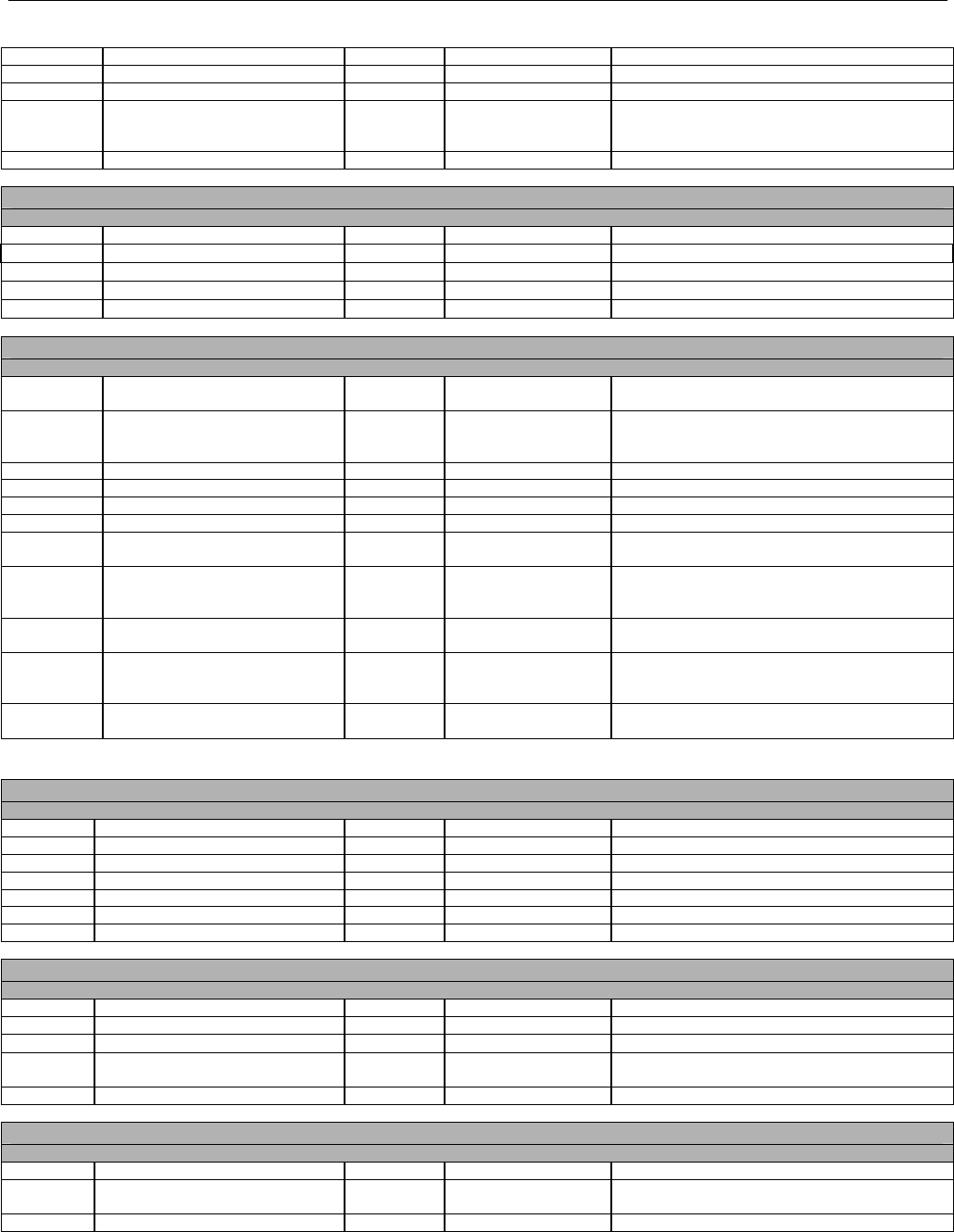
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 925 -
4Ø3-D3 FILL NUMBER R Ø Original Fill
4Ø5-D5 DAYS SUPPLY R 3Ø
4Ø6-D6 COMPOUND CODE R 1 Not a Compound
4Ø8-D8 DISPENSE AS WRITTEN
(DAW)/PRODUCT SELECTION
CODE
R 2 Patient has requested Brand
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ7Ø313 March 13, 2ØØ7
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 969I $96.99
43Ø-DU GROSS AMOUNT DUE R 969I $96.99
426-DQ USUAL AND CUSTOMARY CHARGE Q 969I $96.99
423-DN BASIS OF COST DETERMINATION Q Ø7 Usual And Customary
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø5 COORDINATION OF BENEFITS/OTHER PAYMENTS
SEGMENT
337-4C COORDINATION OF
BENEFITS/OTHER PAYMENTS
COUNT
M 1 One occurrence
338-5C OTHER PAYER COVERAGE TYPE M Ø1 Primary
339-6C OTHER PAYER ID QUALIFIER R Ø3 BIN #
34Ø-7C OTHER PAYER ID Q 123456 ID assigned to payer
443-E8 OTHER PAYER DATE Q 2ØØ7Ø313 March 13, 2ØØ7
353-NR OTHER PAYER –PATIENT
RESPONSIBILITY AMOUNT COUNT
R 2 Two occurrences
351-NP OTHER PAYER-PATIENT
RESPONSIBILITY AMOUNT
QUALIFIER
R Ø1 Amount Applied to Periodic Deductible (517-FH)
as reported by previous payer
352-NQ OTHER PAYER-PATIENT
RESPONSIBILITY AMOUNT
Q 3ØØ{ $3Ø.ØØ
351-NP OTHER PAYER-PATIENT
RESPONSIBILITY AMOUNT
QUALIFIER
R Ø7 Amount of Coinsurance (572-4U) as reported by
previous payer
352-NQ OTHER PAYER-PATIENT
RESPONSIBILITY AMOUNT
Q 6ØØ{ $6Ø.ØØ
34.61.1.1 SECONDARY RESPONSE - PAID
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø313 March 13, 2ØØ7
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P Paid
5Ø3-F3 AUTHORIZATION NUMBER Q 11122233345678
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R 3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE M 1234567
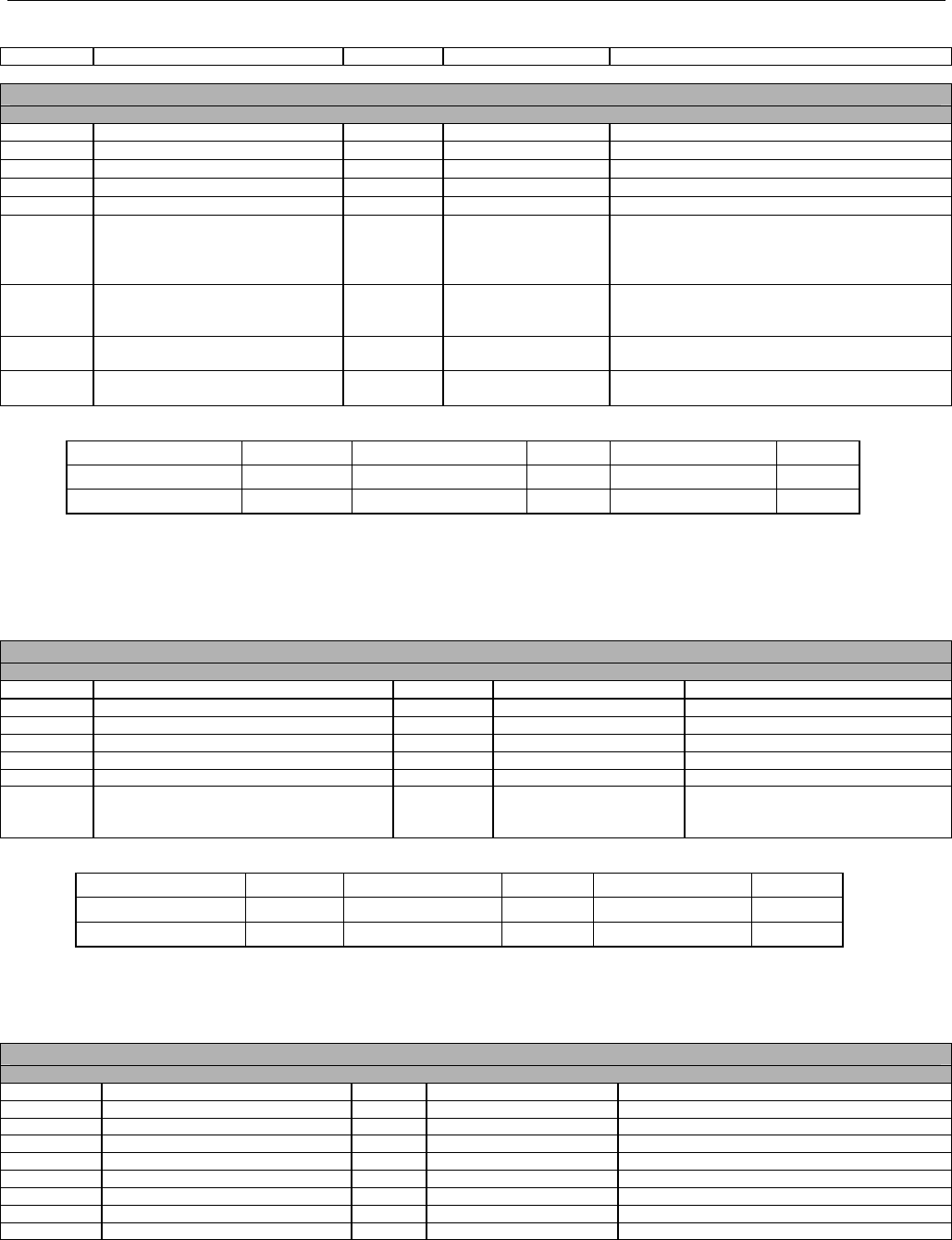
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 926 -
NUMBER
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 6ØØ{ $6Ø.ØØ
5Ø6-F6 INGREDIENT COST PAID R 9ØØ{ $9Ø.ØØ
5Ø7-F7 DISPENSING FEE PAID Q ØØ{ $Ø.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 3ØØ{ $3Ø.ØØ
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 14 Other Payer-Patient Responsibility Amount -
Indicates reimbursement was based on the
Other Payer Patient Responsibility Amount (352-
NQ)
136-UN AMOUNT ATTRIBUTED TO PRODUCT
SELECTION/BRAND NON-
PREFERRED FORMULARY SELECTION
Q 6ØØ{ $6Ø.ØØ
148-U8 INGREDIENT COST CONTRACTED/
REIMBURSABLE AMOUNT
I 38Ø{ $38.ØØ
149-U9 DISPENSING FEE CONTRACTED/
REIMBURSABLE AMOUNT
I 2Ø{ $2.ØØ
Balancing Data Secondary Response:
Ingredient Cost Paid $9Ø.ØØ Patient Pay Amount $6Ø.ØØ Product Selection $6Ø.ØØ
Dispensing Fee Paid $Ø.ØØ Total Amount Paid $3Ø.ØØ Patient Pay Amount $6Ø.ØØ
Net $9Ø.ØØ Net $9Ø.ØØ
34.62 BILLING – TRANSACTION CODE B1 – COORDINATION OF BENEFITS
SCENARIO PHARMACY BILLS TO SECONDARY WHICH MEETS DESIGNATION AS
GOVERNMENT PAYER , PATIENT REQUESTS BRAND
Excerpt of response from Primary Payer. RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 55Ø{ $55.ØØ
5Ø6-F6 INGREDIENT COST PAID Q 125Ø{ $125.ØØ
5Ø7-F7 DISPENSING FEE PAID Q 5Ø{ $5.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 75Ø{ $75.ØØ
518-FI AMOUNT OF COPAY Q 3ØØ{ $3Ø.ØØ
136-UN AMOUNT ATTRIBUTED TO PRODUCT
SELECTION/BRAND NON-PREFERRED
FORMULARY SELECTION
Q 25Ø{ $25.ØØ
Balancing Data Primary Response:
Ingredient Cost Paid $125.ØØ Patient Pay Amount $55.ØØ Copay $3Ø.ØØ
Dispensing Fee Paid $5.ØØ Total Amount Paid $75.ØØ Product Selection $25.ØØ
Net $13Ø.ØØ Net $13Ø.ØØ Patient Pay Amount $55.ØØ
34.62.1 BILLING – TRANSACTION CODE B1 – COORDINATION OF BENEFITS SCENARIO,
PHARMACY BILLS TO SECONDARY WHICH MEETS DESIGNATION AS GOVERNMENT
PAYER TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 999999
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M XYZbbbbbbb
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø313 March 13, 2ØØ7
11Ø-AK SOFTWARE VENDOR/CERTIFICATION M bbbbbbbbbb
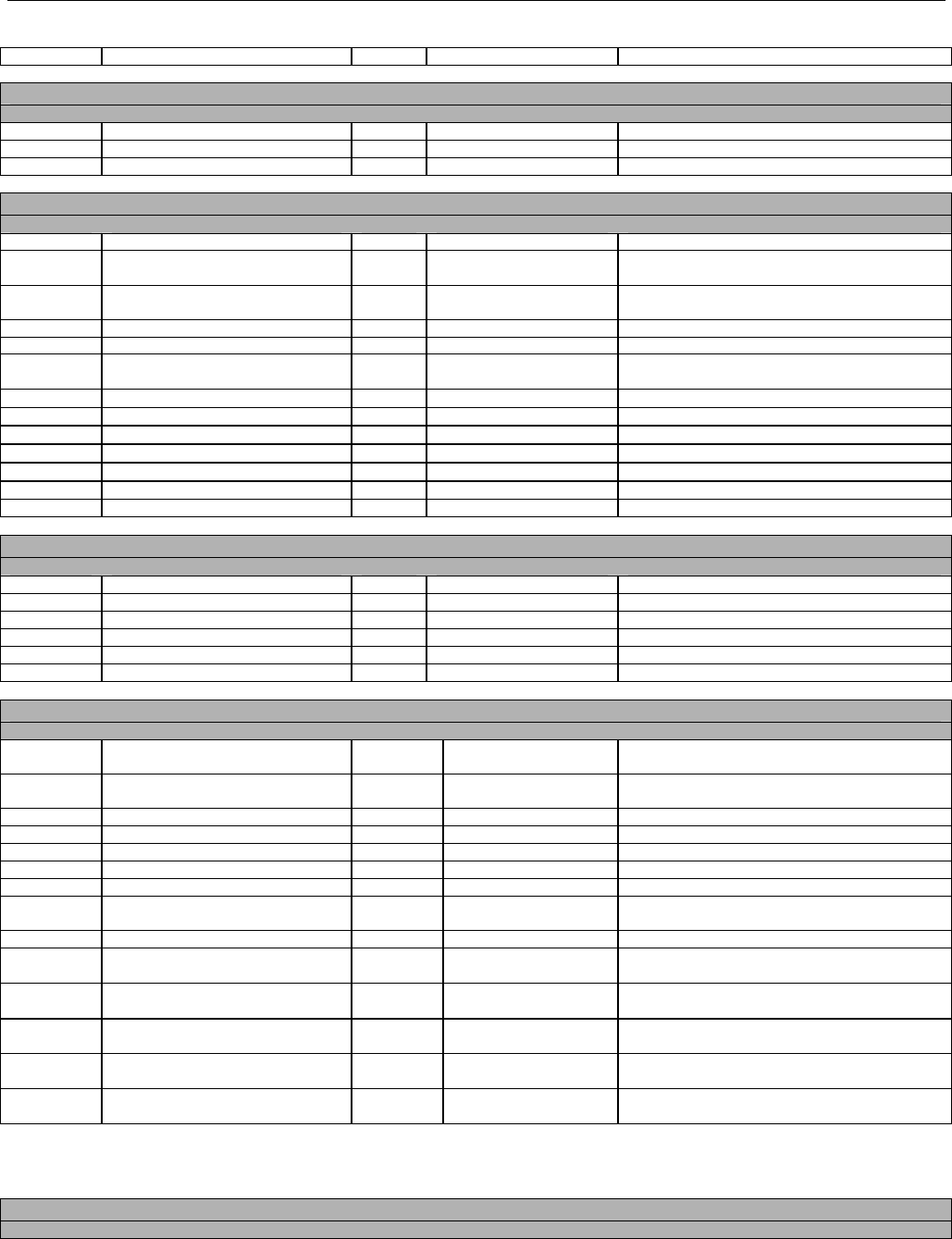
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 927 -
ID
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 998877665 Cardholder ID
3Ø1-C1 GROUP ID Q 3451
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M
Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
4Ø8-D8 DISPENSE AS WRITTEN/PRODUCT
SELECTION CODE
R 2 Patient has requested Brand
442-E7 QUANTITY DISPENSED R 3ØØØØ 3Ø.ØØØ tablets
4Ø3-D3 FILL NUMBER R Ø Original dispensing for RX#
4Ø5-D5 DAYS SUPPLY R 3Ø 3Ø Days supply
4Ø6-D6 COMPOUND CODE R 1 Not a compound
442-E7 QUANTITY DISPENSED R 3ØØØØ 3Ø.ØØØ tablets
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ7Ø313 March 13, 2ØØ7
3Ø8-C8 OTHER COVERAGE CODE Q 2 Other coverage exists/billed-payment collected
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 13ØØ{ $13Ø.ØØ
412-DC DISPENSING FEE SUBMITTED Q 5Ø{ $5.ØØ
426-DQ USUAL AND CUSTOMARY CHARGE Q 14ØØ{ $14Ø.ØØ
43Ø-DU GROSS AMOUNT DUE R 135Ø{ $135.ØØ
423-DN BASIS OF COST DETERMINATION Q Ø1 AWP
COORDINATION OF BENEFITS/OTHER PAYMENTS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø5 COORDINATION OF BENEFITS/OTHER PAYMENTS
SEGMENT
337-4C COORDINATION OF BENEFITS/OTHER
PAYMENTS COUNT
M 1 One occurrence
338-5C OTHER PAYER COVERAGE TYPE M Ø1 Primary
339-6C OTHER PAYER ID QUALIFIER R Ø3 BIN #
34Ø-7C OTHER PAYER ID Q 999999 ID assigned to payer
443-E8 OTHER PAYER DATE Q 2ØØ7Ø313 March 13, 2ØØ7
341-HB OTHER PAYER AMOUNT PAID COUNT R 1 One occurrence
342-HC OTHER PAYER AMOUNT PAID
QUALIFIER
R Ø7 Drug Benefit
431-DV OTHER PAYER AMOUNT PAID Q 75Ø{ $75.ØØ
353-NR OTHER PAYER –PATIENT
RESPONSIBILITY AMOUNT COUNT
R 2 Two occurrences
351-NP OTHER PAYER-PATIENT
RESPONSIBILITY AMOUNT QUALIFIER
R Ø1 Amount Applied to Periodic Deductible (517-
FH) as reported by previous payer
352-NQ OTHER PAYER-PATIENT
RESPONSIBILITY AMOUNT
R 3ØØ{ $3Ø.ØØ
351-NP OTHER PAYER-PATIENT
RESPONSIBILITY AMOUNT QUALIFIER
R Ø7 Amount of Coinsurance (572-4U) as reported
by previous payer.
352-NQ OTHER PAYER-PATIENT
RESPONSIBILITY AMOUNT
R 25Ø{ $25.ØØ
34.62.1.1 RESPONSE FROM SECONDARY PAYER– PAID
Note: any secondary payer can respond this way; the COB limitation of a downstream payer which meets government designation applies to
claim submission requiring full payment disclosure. RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
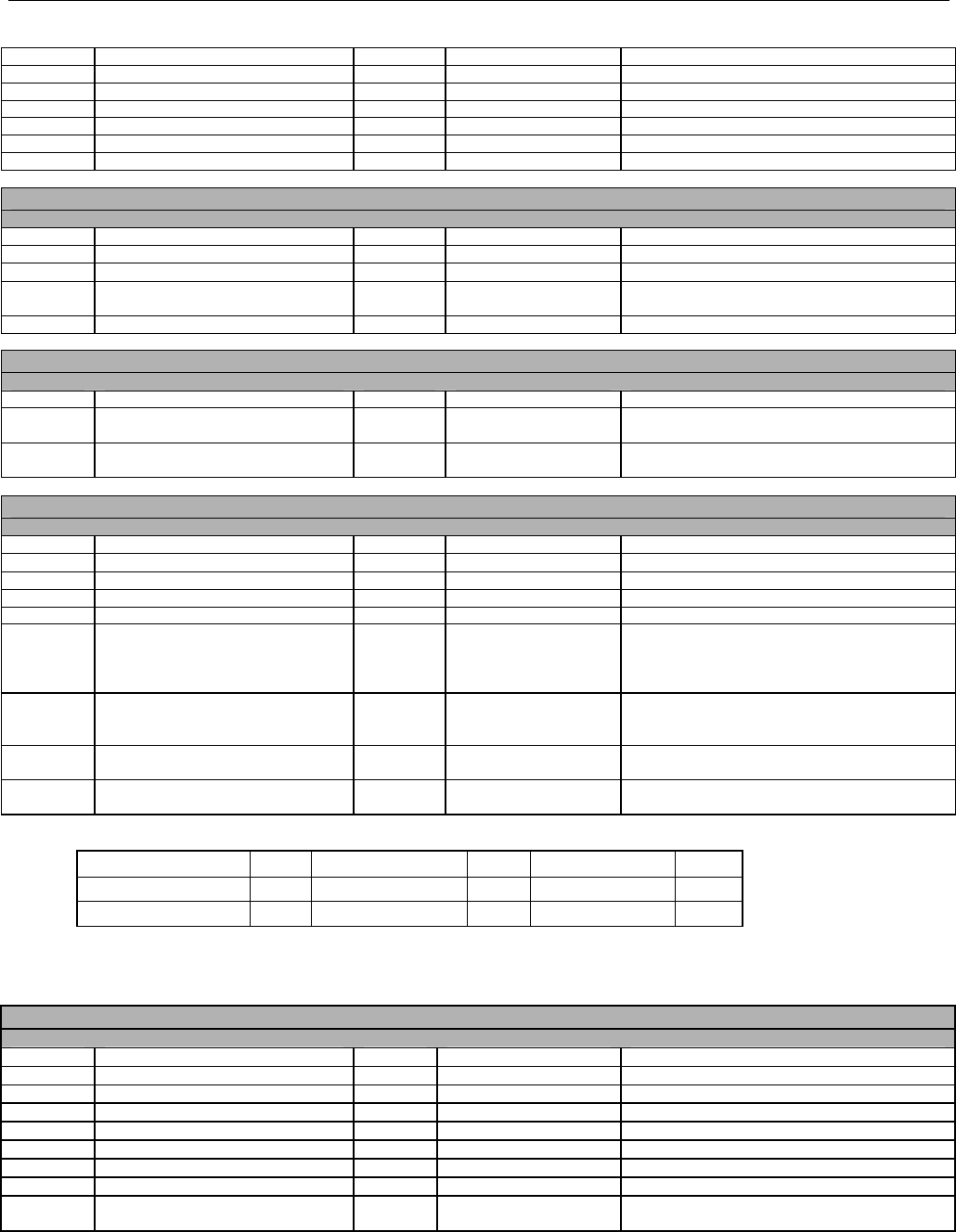
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 928 -
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø313 March 13, 2ØØ7
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P Paid
5Ø3-F3 AUTHORIZATION NUMBER Q 11122233345678
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R 3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 25Ø{ $25.ØØ
5Ø6-F6 INGREDIENT COST PAID R 55Ø{ $55.ØØ
5Ø7-F7 DISPENSING FEE PAID Q ØØ{ $Ø.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 3ØØ{ $3Ø.ØØ
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 14 Other Payer-Patient Responsibility Amount -
Indicates reimbursement was based on the
Other Payer Patient Responsibility Amount
(352-NQ)
136-UN AMOUNT ATTRIBUTED TO PRODUCT
SELECTION/BRAND NON-PREFERRED
FORMULARY SELECTION
Q 25Ø{ $25.ØØ
148-U8 INGREDIENT COST CONTRACTED/
REIMBURSABLE AMOUNT
I 13ØØ{ $13Ø.ØØ
149-U9 DISPENSING FEE CONTRACTED/
REIMBURSABLE AMOUNT
I 5Ø{ $5.ØØ
Balancing Data Secondary Response:
Ingredient Cost Paid 55.ØØ Patient Pay Amount 25.ØØ Product Selection 25.ØØ
Dispensing Fee Paid Ø.ØØ Total Amount Paid 3Ø.ØØ Patient Pay Amount 25.ØØ
Net 55.ØØ Net 55.ØØ
34.63 BILLING - TRANSACTION CODE B1 - REIMBURSEMENT BASED ON PATIENT
PAY AMOUNT (5Ø5-F5) TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø313 March 13, 2ØØ7
11Ø-AK SOFTWARE VENDOR/CERTIFICATION
ID
M 98765bbbbb

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 929 -
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø1 PATIENT SEGMENT
3Ø4-C4 DATE OF BIRTH R 1962Ø615 Born June 15, 1962
3Ø5-C5 PATIENT GENDER CODE R 1 Male
31Ø-CA PATIENT FIRST NAME R JOSEPH
311-CB PATIENT LAST NAME R SMITH
322-CM PATIENT STREET ADDRESS O 123 MAIN STREET
323-CN PATIENT CITY ADDRESS O MY TOWN
324-CO PATIENT STATE/PROVINCE ADDRESS O CO
325-CP PATIENT ZIP/POSTAL ZONE O 34567
326-CQ PATIENT PHONE NUMBER O 2Ø14658923
35Ø-HN PATIENT E-MAIL ADDRESS I JSMITH@NCPDP.ORG Patient’s E-MAIL Address
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 987654321 Cardholder ID
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø3 NDC
4Ø7-D7 PRODUCT/SERVICE ID M ØØØØ6Ø94268 Clinoril 2ØØmg
442-E7 QUANTITY DISPENSED R 3ØØØØ 3Ø.ØØØ tablets
4Ø3-D3 FILL NUMBER R Ø Original dispensing for RX#
4Ø5-D5 DAYS SUPPLY R 3Ø 3Ø Days supply
4Ø6-D6 COMPOUND CODE R 1 Not a compound
4Ø8-D8 DISPENSE AS WRITTEN
(DAW)/PRODUCT SELECTION CODE
R Ø No product selection indicated
414-DE DATE PRESCRIPTION WRITTEN R 2ØØ7Ø313 March 13, 2ØØ7
415-DF NUMBER OF REFILLS AUTHORIZED Q 5 5 Refills
419-DJ PRESCRIPTION ORIGIN CODE Q 1 Written prescription
354-NX SUBMISSION CLARIFICATION CODE
COUNT
R 1 One occurrence
42Ø-DK SUBMISSION CLARIFICATION CODE Q 4 Lost Prescription
3Ø8-C8 OTHER COVERAGE CODE Q 1 No other coverage
429-DT SPECIAL PACKAGING INDICATOR Q 1 Not unit dose
6ØØ-28 UNIT OF MEASURE Q EA Each
PHARMACY PROVIDER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø2 PHARMACY PROVIDER SEGMENT
465-EY PROVIDER ID QUALIFIER R Ø1 National Provider ID
444-E9 PROVIDER ID Q 3935933111
PRESCRIBER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø3 PRESCRIBER SEGMENT
466-EZ PRESCRIBER ID QUALIFIER R Ø8 State license
411-DB PRESCRIBER ID Q ØØG2345
427-DR PRESCRIBER LAST NAME Q JONES
498-PM PRESCRIBER TELEPHONE NUMBER Q 2Ø13639572
468-2E PRIMARY CARE PROVIDER ID
QUALIFIER
R Ø1 National Provider ID
421-DL PRIMARY CARE PROVIDER ID Q 1234566111
47Ø-4E PRIMARY CARE PROVIDER LAST
NAME
Q WRIGHT

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 930 -
PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
4Ø9-D9 INGREDIENT COST SUBMITTED R 129I $12.99
426-DQ USUAL AND CUSTOMARY CHARGE Q 129I $12.99
43Ø-DU GROSS AMOUNT DUE R 129I $12.99
423-DN BASIS OF COST DETERMINATION Q Ø7 Usual And Customary
34.63.1 BILLING - ACCEPTED RESPONSE- PAID (DUPLICATE OF PAID)
RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M B1 Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663111bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ7Ø313 March 13, 2ØØ7
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P or D Paid or Duplicate of Paid
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 1 Rx Billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 1ØØ{ $1Ø.ØØ
5Ø6-F6 INGREDIENT COST PAID Q 72E $7.25
5Ø7-F7 DISPENSING FEE PAID Q 27E $2.75
5Ø9-F9 TOTAL AMOUNT PAID R Ø{ $.ØØ
522-FM BASIS OF REIMBURSEMENT
DETERMINATION
R 15 Patient Pay Amount - Indicates reimbursement was
based on the Patient Pay Amount (5Ø5-F5)
518-FI AMOUNT OF COPAY Q 1ØØ{ $1Ø.ØØ
148-U8 INGREDIENT COST CONTRACTED/
REIMBURSABLE AMOUNT
I 32E $3.25
149-U9 DISPENSING FEE CONTRACTED/
REIMBURSABLE AMOUNT
I 27E $2.75
Balancing Data Primary Response:
Ingredient Cost Paid $7.25 Patient Pay Amount $1Ø.ØØ Copay $1Ø.ØØ
Dispensing Fee Paid $2.75 Total Amount Paid $Ø.ØØ Patient Pay Amount $1Ø.ØØ
Net $1Ø.ØØ Net $1Ø.ØØ
34.64 SERVICE BILLING – TRANSACTION CODE S1 WITH CPT CODES
Examples of Service Billing transactions without a medication.
34.64.1 SCENARIO USING CPT CODES
Mary Simmons is a 77 year-old female who lives at home and takes seven medications on a regular basis, with doses administered at four
different times throughout the day. Her four major diagnoses are diabetes, arthritis, angina and osteoporosis. Because of the complexity of her
regimen, she frequently misses doses of her medication. Her daughter is especially concerned and asks the physician for assistance. The
patient’s physician refers Ms. Simmons to a geriatric pharmacist for evaluation and assistance.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 931 -
The pharmacist sees the patient and daughter in his private office. The office visit was 45 minutes in length, with 30 minutes face-to-face. He
reviews the drug regimen and recommends changes to the prescriber to simplify the regimen. He also prepares a schedule and instructions
for the patient to follow to assist adherence to the regimen, and arranges for the medications to be provided in special packaging to enhance
compliance.
In this example, CPT4 codes are used. As an alternative, this example could be sent as two transmissions containing one transaction for each
15 minute increment billed. TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M S1 Service Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 2 Two occurrences
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663556bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID
M 98765bbbbb
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø1 PATIENT SEGMENT
3Ø4-C4 DATE OF BIRTH R 193ØØ615 Born June 15, 193Ø
3Ø5-C5 PATIENT GENDER CODE R 2 Female
31Ø-CA PATIENT FIRST NAME R MARY
311-CB PATIENT LAST NAME R SIMMONS
384-4X PATIENT RESIDENCE Q 1 Home
INSURANCE SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 987654321 Cardholder ID
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 2 Service billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234567 Service Reference Number
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø7 CPT4
4Ø7-D7 PRODUCT/SERVICE ID M Ø115T 15 minutes of initial visit face-to-face consultation
PRESCRIBER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø3 PRESCRIBER SEGMENT
466-EZ PRESCRIBER ID QUALIFIER R Ø1 National Provider ID
411-DB PRESCRIBER ID Q 1177882556
PRICING SEGMENT
FIELD FIELD NAME CAT Value COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
477-BE PROFESSIONAL SERVICE FEE
SUBMITTED
R 15Ø{ $15.ØØ
426-DQ USUAL AND CUSTOMARY CHARGE Q 15Ø{ $15.ØØ
43Ø-DU GROSS AMOUNT DUE R 15Ø{ $15.ØØ
CLINICAL SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 13 CLINICAL SEGMENT
491-VE DIAGNOSIS CODE COUNT R 4 Four occurrences
492-WE DIAGNOSIS CODE QUALIFIER Q Ø1 International Classification of Diseases (ICD9)
424-DO DIAGNOSIS CODE Q 25Ø.ØØ Diabetes
492-WE DIAGNOSIS CODE QUALIFIER Q Ø1 International Classification of Diseases (ICD9)
424-DO DIAGNOSIS CODE Q 715 Arthritis
492-WE DIAGNOSIS CODE QUALIFIER Q Ø1 International Classification of Diseases (ICD9)
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 932 -
424-DO DIAGNOSIS CODE Q 413.9Ø Angina
492-WE DIAGNOSIS CODE QUALIFIER Q Ø1 International Classification of Diseases (ICD9)
424-DO DIAGNOSIS CODE Q 733.ØØ Osteoporosis
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 2 Service billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 1234568 Service Reference Number
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø7 CPT4
4Ø7-D7 PRODUCT/SERVICE ID M Ø117T 15 add-on minutes of face-to-face consultation
PRESCRIBER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø3 PRESCRIBER SEGMENT
466-EZ PRESCRIBER ID QUALIFIER R Ø1 National Provider ID
411-DB PRESCRIBER ID Q 1177882556
PRICING SEGMENT
FIELD FIELD NAME CAT Value COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
477-BE PROFESSIONAL SERVICE FEE
SUBMITTED
R 15Ø{ $15.ØØ
426-DQ USUAL AND CUSTOMARY CHARGE Q 15Ø{ $15.ØØ
43Ø-DU GROSS AMOUNT DUE R 15Ø{ $15.ØØ
CLINICAL SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 13 CLINICAL SEGMENT
491-VE DIAGNOSIS CODE COUNT R 4 Four occurrences
492-WE DIAGNOSIS CODE QUALIFIER Q Ø1 International Classification of Diseases (ICD9)
424-DO DIAGNOSIS CODE Q 25Ø.ØØ Diabetes
492-WE DIAGNOSIS CODE QUALIFIER Q Ø1 International Classification of Diseases (ICD9)
424-DO DIAGNOSIS CODE Q 715 Arthritis
492-WE DIAGNOSIS CODE QUALIFIER Q Ø1 International Classification of Diseases (ICD9)
424-DO DIAGNOSIS CODE Q 413.9Ø Angina
492-WE DIAGNOSIS CODE QUALIFIER Q Ø1 International Classification of Diseases (ICD9)
424-DO DIAGNOSIS CODE Q 733.ØØ Osteoporosis
34.64.1.1 PAID RESPONSE RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M S1 Service Billing
1Ø9-A9 TRANSACTION COUNT M 2 Two occurrences
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663556bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P Paid
5Ø3-F3 AUTHORIZATION NUMBER Q 123456789123456
789
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 933 -
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 2 Service Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234567 Service Reference Number
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R { $Ø
562-J1 PROFESSIONAL SERVICE FEE PAID R 15Ø{ $15.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 15Ø{ $15.ØØ
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P Paid
5Ø3-F3 AUTHORIZATION NUMBER Q 123456789123456
79Ø
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 2 Service Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 1234568 Service Reference Number
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
562-J1 PROFESSIONAL SERVICE FEE PAID R 15Ø{ $15.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 15Ø{ $15.ØØ
34.64.2 SCENARIO USING CPT CODES WITH DUR/PPS SEGMENT
Pearl Johnson is an 83 year-old female who is moving in to an assisted living community. During the initial assessment by the nurse, Ms.
Johnson reports that she has experienced several falls in recent weeks. Fortunately, serious injury has not yet resulted. Because she takes
nine regularly scheduled medications, Ms. Johnson’s physician refers her to a geriatric pharmacist for a consultation.
The pharmacist interviews the patient face to face at the assisted living facility for 15 minutes and reviews the drug regimen for medications
that may increase the risk of falls. The pharmacist makes recommendations to the prescriber for medication changes to decrease the risk of
falls, and suggests that the patient change one of her medications to bedtime instead of morning administration.
This example uses CPT codes with the DUR/PPS Segment.
TRANSACTION HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø1-A1 BIN NUMBER M 61ØØ66
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M S1 Service Billing
1Ø4-A4 PROCESSOR CONTROL NUMBER M 123456789Ø
1Ø9-A9 TRANSACTION COUNT M 2 Two occurrences
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663556bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
11Ø-AK SOFTWARE
VENDOR/CERTIFICATION ID
M 98765bbbbb
PATIENT SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø1 PATIENT SEGMENT
3Ø4-C4 DATE OF BIRTH R 1924Ø615 Born June 15, 1924
3Ø5-C5 PATIENT GENDER CODE R 2 Female
31Ø-CA PATIENT FIRST NAME R PEARL
311-CB PATIENT LAST NAME R JOHNSON
384-4X PATIENT RESIDENCE Q 4 Assisted Living Facility
INSURANCE SEGMENT
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 934 -
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø4 INSURANCE SEGMENT
3Ø2-C2 CARDHOLDER ID M 223345611 Cardholder ID
CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø7 CLAIM SEGMENT
455-EM PRESCRIPTION/SERVICE
REFERENCE NUMBER QUALIFIER
M 2 Service billing
4Ø2-D2 PRESCRIPTION/SERVICE
REFERENCE NUMBER
M 2233227 Service Reference Number
436-E1 PRODUCT/SERVICE ID QUALIFIER M Ø7 CPT4
4Ø7-D7 PRODUCT/SERVICE ID M Ø115T Initial 15 minutes
PRESCRIBER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø3 PRESCRIBER SEGMENT
466-EZ PRESCRIBER ID QUALIFIER R Ø1 National Provider ID
411-DB PRESCRIBER ID Q 1177882556
DUR/PPS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M Ø8 DUR/PPS Segment
473-7E DUR/PPS CODE COUNTER R
1 1st PPS activity
439-E4 REASON FOR SERVICE CODE Q
PN Prescriber consultation
44Ø-E5 PROFESSIONAL SERVICE CODE Q
RT Recommend lab test
441-E6 RESULT OF SERVICE CODE Q
3A Recommendation accepted
474-8E DUR/PPS LEVEL OF EFFORT R
11 Lowest level of complexity
473-7E DUR/PPS CODE COUNTER Q
2 2nd PPS activity
439-E4 REASON FOR SERVICE CODE Q
TN Laboratory test needed
44Ø-E5 PROFESSIONAL SERVICE CODE Q
PT Perform laboratory test
441-E6 RESULT OF SERVICE CODE Q
3A Recommendation accepted
474-8E DUR/PPS LEVEL OF EFFORT R
12 Service with medium complexity
PRICING SEGMENT
FIELD FIELD NAME CAT Value COMMENTS
111-AM SEGMENT IDENTIFICATION M 11 PRICING SEGMENT
477-BE PROFESSIONAL SERVICE FEE
SUBMITTED
R 15Ø{ $15.ØØ
426-DQ USUAL AND CUSTOMARY CHARGE Q 15Ø{ $15.ØØ
43Ø-DU GROSS AMOUNT DUE R 15Ø{ $15.ØØ
34.64.2.1 PAID RESPONSE RESPONSE HEADER SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
1Ø2-A2 VERSION/RELEASE NUMBER M DØ Transaction Format
1Ø3-A3 TRANSACTION CODE M S1 Service Billing
1Ø9-A9 TRANSACTION COUNT M 1 One occurrence
5Ø1-F1 HEADER RESPONSE STATUS M A Accepted
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER M Ø1 National Provider ID
2Ø1-B1 SERVICE PROVIDER ID M 4563663556bbbbb
4Ø1-D1 DATE OF SERVICE M 2ØØ8Ø313 March 13, 2ØØ8
RESPONSE STATUS SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 21 RESPONSE STATUS SEGMENT
112-AN TRANSACTION RESPONSE STATUS M P Paid
5Ø3-F3 AUTHORIZATION NUMBER Q 123456789123456
789
549-7F HELP DESK PHONE NUMBER
QUALIFIER
R Ø3 Processor/PBM
55Ø-8F HELP DESK PHONE NUMBER Q 6Ø2357Ø862
RESPONSE CLAIM SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 22 RESPONSE CLAIM SEGMENT

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 935 -
455-EM PRESCRIPTION/SERVICE REFERENCE
NUMBER QUALIFIER
M 2 Service Billing
4Ø2-D2 PRESCRIPTION/SERVICE REFERENCE
NUMBER
M 2233227 Service Reference Number
RESPONSE PRICING SEGMENT
FIELD FIELD NAME CAT VALUE COMMENTS
111-AM SEGMENT IDENTIFICATION M 23 RESPONSE PRICING SEGMENT
5Ø5-F5 PATIENT PAY AMOUNT R 2Ø{ $2.ØØ
562-J1 PROFESSIONAL SERVICE FEE PAID R 15Ø{ $15.ØØ
5Ø9-F9 TOTAL AMOUNT PAID R 13Ø{ $13.ØØ

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 936 -
35. FREQUENTLY ASKED QUESTIONS
Technical support for this document is available through the Council office. Answers to frequently asked questions follow.
35.1 NOTABLE CHANGES FROM VERSION 5.1 TO VERSION D.Ø
Question: What Are My Sources For Finding Notable Changes From Version 5.1 to Version D.Ø?
Response: See sections “Notable Changes From Previous Telecommunication Versions” and “Appendix A. History of Document Changes”.
35.2 UNUSUAL PACKAGE SIZE
Question: How Do I Handle Transactions For Unusual Package Size?
Response: Refer to the NCPDP Billing Unit Standard.
35.3 COMPOUNDED PRESCRIPTIONS
Question: How Do I Handle Compounded Prescriptions?
Response: Refer to section “Specific Segment Discussion”, “Request Segments”, “Compound Segment” in this guide for specific usage of
fields within the Compound Segment.
In previous versions, there was one recommended method of billing for compounds (multi-ingredients reported using the Claim and
Compound Segments). There were two alternative methods (most expensive legend drugs or use of billing codes). The two alternative
methods are no longer supported. Billing for multiple ingredients by using the Claim and Compound Segments is the only method supported.
Only one compound Billing transaction for multiple ingredients is allowed per transmission.
35.4 COMPOUND INGREDIENTS IN SEPARATE TRANSACTIONS
Question: Can Each Ingredient of a Compound Be Submitted in Separate Transactions?
Response: No. Each ingredient of a compound is contained within the iterations of the Compound Segment within a transaction. Each
ingredient is not allowed to be sent in separate transactions of a transmission.
35.5 NON-COVERED INGREDIENTS IN A COMPOUND
Question: How Do I Handle Non-Covered Ingredients Within A Compounded, Multiple Ingredient Prescription?
Response: Processor will identify individual ingredients not covered by returning a Reject Code (511-FB) and Reject Field Occurrence
Indicator (546-4F). Resubmission of the claim with the value “Ø8” in Submission Clarification Code (42Ø-DK) will indicate the pharmacist’s
acceptance of payment for covered ingredients only.
35.6 ELIGIBILITY CHECK
Question: How Do I Check Eligibility?
Response: Submit an Eligibility Verification (Transaction Code E1) to ascertain eligibility status. Refer to the section “Eligibility Verification
Information” and see transaction Example “Eligibility Verification”.
35.7 BILLING FOR PARTIAL FILLS
Question: How Do I Bill For Partial Fills Of Prescriptions?
Response: See sections “Specific Segment Discussion”, “Claim Segment” , “Partial Fill”, and section “Response Pricing Segment” and section
“Transmission Examples”, “Billing, Partial Fill-Initial-Transaction Code B1” and Example “Billing, Partial Fill-Completion-Transaction Code B1”
for a complete discussion of partial fills. These sections illustrate the following considerations:
• The solution addresses the legal requirements associated with reporting the actual quantity and date of dispensing for the product
• The solution requires the remainder of the partial fill quantity to be billed as a separate transaction and not as an inclusion on a
subsequent refill
• This solution allows, as an option, the inclusion of the Dispensing Status field on a reversal transaction
When dispensing a partial fill, the Dispensing Status code is submitted to indicate the transaction is for an “initial” partial fill. When the
“outstanding” quantity is dispensed, the transaction 1) indicates the Dispensing Status code is for the “completion” of the partial fill; 2) identifies
the Associated Prescription/Service Reference Number; and 3) identifies the Associated Prescription/Service Date.
35.8 PRESCRIPTION AND SERVICE PRICING FORMULAE
Question: What Are The Prescription And Service Pricing Formulae?
Response:
Prescription Formula Claim Request:
Ingredient Cost Submitted (4Ø9-D9)
+ Dispensing Fee Submitted (412-DC)
+ Incentive Amount Submitted (438-E3)
+ Other Amount Claimed Submitted (48Ø-H9)
+ Flat Sales Tax Amount Submitted (481-HA)
+ Percentage Sales Tax Amount Submitted (482-GE)

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 937 -
-------------------------------------------------------
= Gross Amount Due (43Ø-DU)
- Patient Paid Amount Submitted (433-DX)
- Other Payer Amount Paid (431-DV)
(Result is Net Amount Due)
Note: Net Amount Due as defined above is applicable to primary and COB claims in which Other Payer Amount Paid (431-DV) is
submitted. Net Amount Due for COB claim billings for Other Payer-Patient Responsibility Amount equals sum of the parts of other
payer-patient responsibility amount(s).
Prescription Formula Response:
Ingredient Cost Paid (5Ø6-F6)
+ Dispensing Fee Paid (5Ø7-F7)
+ Incentive Amount Paid (521-FL)
+ Other Amount Paid (565-J4)
+ Flat Sales Tax Amount Paid (558-AW)
+ Percentage Sales Tax Amount Paid (559-AX)
- Patient Pay Amount (5Ø5-F5)
- Other Payer Amount Recognized (566-J5)
-------------------------------------------------------
= Total Amount Paid (5Ø9-F9)
Service Claim Request Formula:
Professional Service Fee Submitted (477-BE)
+ Flat Sales Tax Amount Submitted (481-HA)
+ Percentage Sales Tax Amount Submitted (482-GE)
+ Other Amount Claimed Submitted (48Ø-H9)
------------------------------------------------------------
= Gross Amount Due (43Ø-DU)
- Patient Paid Amount Submitted (433-DX)
- Other Payer Amount Paid (431-DV)
(Result is Net Amount Due)
Note: Net Amount Due as defined above is applicable to primary and COB services in which Other Payer Amount Paid (431-DV) is
submitted. Net Amount Due for COB service billings for Other Payer-Patient Responsibility Amount equals sum of the parts of other
payer-patient responsibility amount(s).
Service Response Formula:
Professional Service Fee Paid (562-J1)
+ Flat Sales Tax Amount Paid (558-AW)
+ Percentage Sales Tax Amount Paid (559-AX)
+ Other Amount Paid (565-J4)
- Patient Pay Amount (5Ø5-F5)
- Other Payer Amount Recognized (566-J5)
-------------------------------------------------------
= Total Amount Paid (5Ø9-F9)
35.9 CALCULATE NET AMOUNT DUE
Question: How Do I Calculate The Net Amount Due On A Billing?
Response: Although the net amount due is not an actual data field in the preceding formulae, it can be derived by subtracting the Patient Paid
Amount Submitted and the Other Payer Amount Paid, if these apply to the billing, from the Gross Amount Due.
Net Amount Due as defined above is applicable to primary and COB claims/services in which Other Payer Amount Paid (431-DV) is submitted.
Net Amount Due for COB claim/service billings for Other Payer-Patient Responsibility Amount equals sum of the parts of other payer-patient
responsibility amount(s).
35.10 DUPLICATE TRANSACTIONS
Question: How Do I Handle Duplicate Transactions?
Response: The status code “D” for duplicate is used when a provider transmits a transaction that has been previously accepted by a
processor.
Normally, information is transmitted a second time only when the pharmacy has some reason to believe that the processor did not receive the
first attempt. The situation may arise due to human or telecommunications errors. Alternately, the response from the processor may have been
interrupted and never received by the pharmacy.
Upon receiving a duplicate transaction, the processor must reply to the pharmacy with the same values shown in the initial response except
the transaction status code will be “D” for duplicate.
For all billings, processors should return the same values shown on the initial response with the transaction status code “D” for duplicate
instead of “P” for paid.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 938 -
When a duplicate eligibility transaction is encountered, the processor must return the original approved response again.
See section “Response Pricing Guidelines”, subsection “Duplicate Transactions”.
35.11 PRESCRIPTION AND SERVICE BILLINGS IN ONE TRANSACTION
Question: Can I Submit DUR/PPS Codes And Service Billings With A Claim For Product?
Response: No, in Version D.Ø and above, the Service Billings have their own Transaction Code (S1, S2, S3). The Transaction Code is at the
transmission level. Claim and service billings are associated (using the Associated Prescription/Service Reference Number (456-EN) and
Associated Prescription/Service Date (457-EP), but they must appear in separate transmissions. Drug product billings are designated by
Transaction Code = “B1” (Billing) and Prescription/Service Reference Number Qualifier = “1” (Rx Billing). Service billings are designated by
Transaction Code = “S1” (Service Billing) and Prescription/Service Reference Number Qualifier = “2” (Service Billing).
Note that in other Transaction Codes (Prior Authorizations, Information Reporting, and Controlled Substance Reporting), the differentiation of
claim versus service remains at the transaction level. For example, drug product transactions are designated by Transaction Code = “P1”
(Prior Authorization Request And Billing) and Prescription/Service Reference Number Qualifier = “1” (Rx Billing). Service billings are
designated by Transaction Code = “P1” (Prior Authorization Request And Billing) and Prescription/Service Reference Number Qualifier = “2”
(Service Billing).
35.12 REVERSING PRIOR AUTHORIZATION REQUEST AND BILLING TRANSACTIONS
Question: How Do I Reverse Prior Authorization Requests And Billings?
Response: Prior Authorization reversals are used to back out the request for authorization, but not any claims submitted against the prior
authorization. To reverse a Prior Authorization Request and Billing, paid billings must be reversed before the prior authorization is reversed.
The pharmacy must submit a Claim or Service Reversal (Transaction Code = “B2” or “S2”) before submitting a Prior Authorization Reversal
request. If there are no Claims or Services paid for the Prior Authorization in question, the processor must accept the Prior Authorization
Reversal for the prior authorization only.
35.13 PRIOR AUTHORIZATION NUMBER-ASSIGNED (462-EV)
Question: What Do I Do With The Prior Authorization Number-Assigned?
Response: When using the Prior Authorization Transactions (P1 and P4) to request a Prior Authorization number, the processor will return the
assigned number in the Prior Authorization Number-Assigned (498-PY). When submitting a Claim or Service Billing that requires a Prior
Authorization, place the number returned in the Prior Authorization Number Submitted (462-EV).
See section “Prior Authorization Transaction Discussion”.
35.14 TRUNCATION IN THE HEADER SEGMENTS
Question: Can I Truncate Fields In The Header?
Response: No. Neither the Request Header nor the Response Header fields may be truncated. See section “Standard Conventions”,
“Character Set Designation Truncation” for additional information.
35.15 SITUATIONAL/OPTIONAL FIELD POSITIONING
Question: Are There Rules For Positioning Situational or Optional Fields Within A Segment?
Response: Yes. See section “Standard Conventions”, “Repetition And Multiple Occurrences” for a discussion of repeating field rules that
affect situational or optional fields.
35.16 SYNTAX ERRORS
Question: How Do I Handle Syntax Errors?
Response: The NCPDP Data Dictionary contains reject codes for many syntax situations (Reject Code 511-FB). These reject codes must be
used whenever possible. If a particular reject reason relating to syntax is not defined, the Reject Code “R8 ” (Syntax Error) must be returned.
The message fields are to be used for additional comments to clarify and point to the error.
35.17 USE OF COUNTERS
Question: Please Explain The Use Of Counters
Response: The term “counter” as used in the clinical information and DUR/PPS segments is synonymous with occurrence number. For
example, in a repetition of four, the first occurrence of the field or set/logical grouping would be preceded by a counter with a value of “1”. The
second occurrence of that field or set/logical grouping would be preceded by a counter with a value of “2”, the third occurrence would be
preceded by a counter with a value of “3” and so forth.
See section “Standard Conventions”, “Repetition and Multiple Occurrences”, “Repeating Data Elements”, “Count Fields” and “Counter Fields”
for important information.
35.18 PARTIAL FILL AND CHANGE OF COVERAGE
Question: How Do I Handle Partial Fill Completion Transactions When A Change In Coverage Or Plan Processors Has Occurred?

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 939 -
Response: Providers will submit the completion transaction to the same processor to whom the initial partial fill transaction was sent. In the
event a change of coverage or plan processor between the initial and completion transactions, results and/or handling may differ depending
upon arrangements between the processor and payer. It may be necessary to communicate with the processor’s Help Desk to resolve any
outstanding issues.
35.19 ZERO DOLLAR AMOUNTS
Question: How Should Zero Dollar Amounts Be Handled In A Variable Transaction?
Response: The NCPDP Telecommunication Standard Implementation Guide (Version D and above) provides the ability to only send/receive
the data necessary to fulfill a business requirement.
In the past, the Version 3.2 formats allowed the fixed transaction formats of 3A, 3B, and 3C. Due to the fixed formats, fields that were not
needed in the business case still had to be defaulted (zero or space filled) to retain the position in the fixed format.
The fixed formats supported in older versions are no longer supported in this version. By adhering to the rules of which segments are required,
which fields are mandatory, and only sending/receiving the dollar fields that are situationally or optionally needed for the business case, fields
that are not needed, must not be sent.
Dollar amounts must not be sent unless needed in the business case. If it is necessary to relay a dollar field that contains zeroes, the field
must be sent. It is not recommended to relay a dollar field of zeroes to retain a position in a segment.
See section “Standard Conventions” and section “Response Pricing Guidelines”.
35.20 IDENTIFIER OF AN INGREDIENT
Question: How Do I Enter An Ingredient In A Compound That Does Not Have An Identifier (For Example Water)?
Response: Identifying each ingredient in a compound is important in order for the ingredients to support the sum total of the quantity.
The Compound Product ID Qualifier has many values (i.e., NDC, UPC) that must be used when possible. If not, trading partners need to agree
on usage. When an ingredient does not have an identifier, it is possible to use the value of “99” (Other) in the qualifier and an agreed upon
value for the product.
35.21 BILLING FOR PARTIAL FILL COMPOUND
Question: How Do I Bill For A Partial Fill Of A Compound?
Response: The partial fill of a compound is to be handled the same as a partial fill of any other prescription.
35.22 RESPONSE PRICING SEGMENT IN CAPTURED RESPONSE
Question: Why Would The Response Pricing Segment Be Used (situational) In A Billing Transaction (Or Other Transaction) When A
Processor Returns A “C” (Captured) Response?
Response: A “C”(Captured) response is used when the Processor/PBM accepts the receipt of the transaction but does not render a judgment
regarding eligibility or payment, for example. The Processor/PBM may return copay and/or coinsurance information. The response copay and
coinsurance fields are found in the Response Pricing Segment.
35.23 PRIOR AUTHORIZATION INQUIRY AND CAPTURED RESPONSE
Question: Will Each Different “C” Captured Response Of A “Prior Authorization Inquiry” Transaction Come Back With A Unique Authorization
Number (5Ø3-F3) Or Does It Come Back With The Same One Each Time Regardless Of How Many Times You Submit The “Prior
Authorization Inquiry” Transaction And Receive Responses?
Another way of asking this question is:
Do you use the original Authorization Number from the first “C” Captured response from the “Request and Billing” transaction over and over
again if you keep sending “Prior Authorization Inquiry” transactions, or would you send an Authorization Number from the most recent “Prior
Authorization Inquiry” transaction response on the “Prior Authorization Inquiry” transactions?
Response: The processor must return the same Authorization Number (5Ø3-F3) in a Capture situation. The pharmacy must submit the same
Authorization Number (5Ø3-F3) on each Prior Authorization Inquiry for that Captured transaction.
35.24 RESPONSE HEADER SEGMENT FIELDS
Question: Should The Fields Submitted In The Transaction Header Segment On A Request Be Returned Without Modification On The
Response Header Segment? (Should They Be Mirrored?)
Response: Yes. The Response Header Segment contains the field Version/Release Number, Transaction Code, Transaction Count, Service
Provider ID Qualifier, Service Provider ID, and Date of Service that are also used in the Transaction Header Segment. The intent of these
fields within the Response Header Segment was that the values submitted in these fields on the request from the provider to the payer would
be returned without change in the response from the payer to the provider. These fields in the Response Header Segment are used by the
software system to offer a level of verification at the transmission level that the response is paired to the request. (The Prescription/Service
Reference Number in the Response Claim Segment, when applicable, may be used to match as well.)
For example, (b denotes a space or blank)
Transaction Header Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 940 -
Field Field Name Value
1Ø1-A1 BIN NUMBER 999999
1Ø2-A2 VERSION/RELEASE NUMBER DØ
1Ø3-A3 TRANSACTION CODE B1
1Ø4-A4 PROCESSOR CONTROL NUMBER bbbbbbbbbb
1Ø9-A9 TRANSACTION COUNT Ø1
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER Ø7
2Ø1-B1 SERVICE PROVIDER ID 4563663bbbbbbbb
4Ø1-D1 DATE OF SERVICE 2ØØ2Ø811
11Ø-AK SOFTWARE VENDOR/CERTIFICATION ID bbbbbbbbbb
Response Header Segment
Field Field Name Value
1Ø2-A2 VERSION/RELEASE NUMBER DØ
1Ø3-A3 TRANSACTION CODE B1
1Ø9-A9 TRANSACTION COUNT Ø1
5Ø1-F1 HEADER RESPONSE STATUS A
2Ø2-B2 SERVICE PROVIDER ID QUALIFIER Ø7
2Ø1-B1 SERVICE PROVIDER ID 4563663bbbbbbbb
4Ø1-D1 DATE OF SERVICE 2ØØ2Ø811
35.25 ACCEPTED AND REJECTED INFORMATION IN ONE RESPONSE
Question: Can A Response Transaction Contain Accepted And Rejected Information?
For example, on an Claim Billing (B1), could the response be returned with a Transaction Response Status of “P” (Paid) and in the Response
Status Segment, Reject Code and Count fields be included to relay information? Or in another example, could a Reversal (B2) response be
“A” (Approved) and Reject Code and Count fields be included?
Response: No. The Reject Code and Count fields, which are specifically for reject situations, are to be used when the Transaction Response
Status = “R” (Rejected). These fields must not be returned for values other than “R”. See each transaction section (such as “Claim Billing Or
Encounter Information”) which detail each field within each segment within each transaction response, with situations for valid use of the field.
35.26 DUR IN A COMPOUND
Question: On Compounded Claims, Does DUR "Hit" Each Drug Within The Compound?
Response: The standard does allow it. Whether each ingredient is interrogated in the DUR process is at the discretion of the payer/processor.
35.27 AN ORDER TO COMPOUND INGREDIENTS
Question: Should Compound Ingredients Be Put In Highest Usage Amount Order? (i.e., Product A 8Ø%, Product B 1Ø%, Product C 1Ø%).
Response: The order of the compound ingredients does not make any difference when submitting a claim.
35.28 FORMAT OF PERCENTAGE SALES TAX FIELDS
Question: How Is The Format Of Percentage Sales Tax Rate Submitted (483-HE) And Percentage Sales Tax Rate Paid (56Ø-AY)
Expressed?
Response: These fields are defined as s9(3)v4 allowing values of .ØØØ1% through 1ØØ.ØØØØ%.
Examples:
A rate of: Spelled out: Would be expressed as
(without truncation): Would be expressed
as (with truncation):
.ØØØ1% one ten thousandth of a percent ØØØØØØA A
7% seven percent ØØ7ØØØ{ 7ØØØ{
.5% five tenths of a percent ØØØ5ØØ{ 5ØØ{
25.75% twenty five and seventy five one
hundredths of a percent
Ø2575Ø{ 2575Ø{
1ØØ% One hundred percent 1ØØØØØ{ 1ØØØØØ{
Seven percent (7%) would not be represented as 7Ø{ (.Ø7Ø{).
Note the difference between the expression of .ØØØ1% and 1ØØ%. They are very different expressions and must not be confused.
35.29 ELIGIBILITY TRANSACTION AND THE GROUP SEPARATOR
Question: In the Telecommunication Standard Implementation Guide 5.1 it states that "A transmission consists of one or more transactions
separated by group separators. With one exception, the Eligibility Verification transmission, which does not use a group separator.."
Response: The transmission of the Eligibility request does not have a Group Separator. The transmission of the Eligibility response does
have a Group Separator, so that all response transmissions are parsed the same way (with the Response Status Segment coming after the
Group Separator). The members discussed putting the Group Separator in the Eligibility request, but determined it was extraneous since the

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 941 -
only “transaction level” segments were the Patient Segment, Pharmacy Provider Segment, and the Prescriber Segment and as situational,
may not be sent. The Group Separator was therefore not supported in the Eligibility Verification request.
35.30 REJECTING FOR INVALID HEADER FIELDS
Question: How Should A Clearinghouse Or Payer Handle Rejecting A Transaction Sent From A Provider With An Invalid Version/Release
Number (1Ø2-A2), Transaction Code (1Ø3-A3), Or Transaction Count (1Ø9-A9)?
Response: The recommendation is that when the Transaction Count (1Ø9-A9) is invalid, the processor system must generate a Transmission
Rejected/Transaction Rejected format. The processor system must generate a response with a Transaction Count (1Ø9-A9) of 1 and
appropriate Reject Codes (511-FB).
It is possible that the processor system may not respond to this invalid transaction, or may respond with only a string or text message, not in
NCPDP format. This would then appear as a timeout to the provider system.
If the Version/Release Number (1Ø2-A2) is garbage (not a valid value, or values for example of “??” or “**”), the processor cannot build an
appropriate response. In this case, a timeout at the provider system is appropriate.
If the Transaction Code (1Ø3-A3) is garbage (not a valid value, or values for example of “??” or “**”), the processor system does not know how
to build an appropriately formatted response.
If the Transaction Count (1Ø9-A9) is not a valid value (but the Version/Release and Transaction Code are appropriate), it is recommended the
Transaction Count contain a value of 1 with the appropriate Response Status Segment containing Reject Codes (511-FB) signifying the invalid
Transaction Count field.
35.31 PRIOR AUTHORIZATION REQUEST AND BILLING – PRIOR AUTHORIZATION
NOT REQUIRED
Question: If a pharmacy submits a Prior Authorization Request and Billing transaction and the processor determines that the billing part of the
transaction doesn't require a prior authorization, what response should the processor return? If the processor returns a paid response, it is
required to have the prior authorization assigned number and pertinent prior authorization information. If the billing didn't require a PA, how
can they return the PA assigned number and pertinent information?
Response: The Prior Authorization Request and Billing must be rejected in this scenario. For the processor to return a “P” (Paid) response
would mean the pertinent PA information is not returned (nor should it be) and this could cause confusion for the pharmacy system. Reject
Code “3R ” (Prior Authorization Not Required) and “85 “ (Claim Not Processed) as well as any other pertinent reject codes should be
considered.
35.32 PAYMENT AMOUNT BASED ON DISPENSED OR INTENDED
Question: Do NCPDP standards require the payment amount to be based on the amount actually dispensed, or can the intended amount be
used instead?
Response: No, the standards do not require the payer to pay either way. The determination of the whether the payer will pay based on
quantity dispensed or quantity intended to be dispensed is a trading partner decision.
35.33 COORDINATION OF BENEFITS AND PARTIAL FILLS
Question: How Should Partial Fills Be Handled For A Coordination Of Benefits (Coordination of Benefits) Billing? How does the reject of
“Partial Fill Transaction Not Supported” affect this processing?
Response: Since there are many combinations (Primary accepts/does not accept Partial Fills/Primary does/does not do online Coordination of
Benefits, Secondary accepts/does not accept Partial Fills/Secondary does/does not do online Coordination of Benefits), it is recommended
that Coordination of Benefits billing to the secondary (or downstream payer) should not occur until the pharmacy has determined the final
resolution of the claim.
35.34 NATIONAL DRUG CODES (NDCS) AND PROCEDURE CODE MODIFIERS
Question: From A Standards Perspective Is It Valid To Require The Reporting Of Procedure Code Modifier(s) With National Drug Codes?
Response: The standard does not prohibit the reporting of procedure code modifier(s) with National Drug Codes (NDC).
35.35 INVALID PRESCRIPTION/SERVICE REFERENCE NUMBER QUALIFIER (455-
EM)
Question: A payer receives a “B1” (Billing) or “S1” (Service Billing) transaction but the Prescription/Service Reference Number Qualifier (455-
EM) is sent with the Field ID only and no value. What should be in the Prescription/Service Reference Number Qualifier field (if the incoming
request contained no value)?
Response: Spaces are not allowed as the value in the mandatory field of Prescription/Service Reference Number Qualifier (455-EM). When
the Prescription/Service Reference Number Qualifier (455-EM) is missing or invalid, the processor system must generate a Transmission
Accepted/Transaction Rejected response.
If the transaction is a B1 (Billing), the processor system must generate a response with a Prescription/Service Reference Number Qualifier
(455-EM) of “1”. Prescription/Service Number (4Ø2-D2) must contain a value of Ø (a single zero). If the transaction is a S1 (Service Billing),

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 942 -
the processor system must generate a response with a Prescription/Service Reference Number Qualifier (455-EM) of “2”. Prescription/Service
Number (4Ø2-D2) must contain a value of Ø (a single zero).
The appropriate Reject Codes (511-FB) must be returned for a missing/invalid field. The Reject Code of “R8 “ (Syntax Error) may also be
used. A Transmission Rejected/Transaction Rejected (header reject noted in the question) does not apply because invalid information in the
Claim Segment causes a transaction reject, not a transmission reject.
35.36 PREDETERMINATION OF BENEFITS DIFFERENCE TO CLAIM
Question: What Is The Business Difference Between A Predetermination Of Benefits Transaction And A Claim Or Service Billing?
Response: The Predetermination of Benefits transaction does not actually modify the patient’s benefit or create a receivable or generate a
payment.
35.37 COUPONS NOT SUBMITTED AFTER BILLING PRIMARY INSURANCE
Question: Are there circumstances under which coupons may not be submitted after billing the primary insurance?
Response: State or federal regulations may prohibit the use of coupons. Please check business trading partner agreements.
35.38 FREE PRODUCT DEFINITION
Question: Define “Free Product”.
Response: A Free Product is a product, which is dispensed to a patient at no cost. An example of this is the billing to a coupon processor that
returns a $Ø.ØØ copay. This is NOT synonymous with the replacement of inventory (or consignment programs) to a provider at no charge.
Free product can be billed with or without the Coupon Segment as determined by the processor or third party payer.
35.39 COUPONS AND REPLACEMENT OF INVENTORY
Question: Should the Coupon Segment be used for the replacement of inventory?
Response: No. Replacement programs, such as consignment, do not result in claim billings. NCPDP Telecommunication Standard was not
designed to address the replacement of inventory at no cost to a provider.
35.40 MANUFACTURER CARDS AND COUPONS
Question: Are manufacturer cards the same as coupons?
Response: No, manufacturer cards are viewed as discount cards or similar to a third party insurer for cash patients. A claim billing is
submitted to a manufacturer card processor without the Coupon Segment.
35.41 COUPONS AND PATIENT IDENTIFICATION
Question: How will coupon processing accommodate the handling of patient identifiable information?
Response: Some manufacturer programs track by coupon identifier while others require coupon identifier and patient identifiable information.
Patient information is often required in order to provide limitations (e.g. 1 free product per patient). These requirements must be specified in
the payer sheets in order for the submitter to determine when to send patient identifiable information with the Coupon Segment.
35.42 PROCESS COUPONS WITHOUT COUPON SEGMENT
Question: Can you process a coupon without using the Coupon Segment?
Response: Yes, when the processor is responsible for payment of the discount or for payment of the entire prescription. The discount is
usually presented in the form of a coupon or an ID card. In either form, the NCPDP ID card data elements are printed on the coupon, i.e.
RxBIN, RxGRP, RxPCN, Member ID, and/or a Prior Authorization Number. The Coupon Segment is not necessary, as the processor will
adjudicate the discount in real time using the submitted data elements. The payment for the discount or the entire prescription will be included
on the provider’s remittance advice.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 943 -
36. UPDATES AND CORRECTIONS TO STANDARDS
The Data Element Request Form (DERF) provides the mechanism for changing NCPDP standards and using or requesting new data elements
and new code set values in business functions. To request a change in NCPDP standards, complete an NCPDP Data Element Request
Form, available at www.ncpdp.org (under “Request Changes”). Appropriate NCPDP Work Groups and Committees consider information
submitted on the DERF. The Data Element Request Form process makes it possible for NCPDP working committees to adequately address
these concerns before accepting or approving new information requests into a standard. The final acceptance of new requests into the
standard is made by NCPDP at the suggestion or recommendation of the Work Group or Committee, and must be approved by consensus or
consensus ballot of the membership.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 944 -
37. APPENDIX A. HISTORY OF DOCUMENT CHANGES
It is recognized that section references might no longer be valid, as the document has been updated past the reference.
37.1 VERSION 5.1
Changes for Version 5.1 included additional values added to fields Reason For Service Code (439-E4) and Result of Service Code (441-E6).
37.2 VERSION 5.2
Changes for Version 5.2 included new data element Patient Email Address (35Ø-HN) and additional values added to Measurement Dimension
(496-H2) and Measurement Unit (497-H3).
37.3 VERSION 5.3
Changes for Version 5.3 included a new value of “4” (Custom Repackaging) in the Unit Dose Indicator field (429-DT).
Updates to the Version 5.3 Implementation Guide include:
• Zero Dollar Amounts clarifications in Sections 4.2.6, 4.2.9, 4.4.4, & 8.25
• Count & Counters Section Rewritten in Section 2.4
• Response Claim Pricing Examples corrected - #’s 7.3.1, 7.7.1, 7.8.1, 7.9.1, & 7.12.1
• Miscellaneous NDC Product/Service ID Qualifier examples corrected
37.4 VERSION 5.4
Section 3.7 “Revision Information” - Removed the table rows for 5.1, 5.2, 5.3 since the same information is contained in this Appendix.
A new value “Ø8” (Disproportionate Share Pricing/Public Health Service) was added to “Basis of Cost Determination”.
37.5 VERSION 5.5
Changes for Version 5.5 include the new data elements of Other Payer-Patient Responsibility Amount Qualifier (351-NP), Other Payer-Patient
Responsibility Amount (352-NQ), and Other Payer-Patient Responsibility Amount Count (353-NR).
A new value of 9 was added to the Clinical Significance Code (528-FS) to support a possible interaction with variable or unknown severity.
The table in section 8.2.2.2 “Counter Fields” was changed. In the table showing count and counter usage, the Diagnosis Code Count was
incorrectly represented. An additional row with Diagnosis Code Qualifier was added. For a count field repetition, the Diagnosis Code Qualifier
and Diagnosis Code repeat the number of times the Count specifies.
Section 2.4 “Repeating Fields – Maximum Occurrences” was updated to reflect the recommendations for the new Other Payer-Patient
Responsibility Amount Count field.
Section 2.4 “Repeating Fields – Maximum Occurrences” subsection ”Coordination of Benefits/Other Payments Segment” section was updated
to reflect the support of the new Other Payer-Patient Responsibility Amount fields.
In section “Transmission Examples” 7.4 and 7.4.1 were created
“Billing Secondary Payer – Notification of Other Payer-Patient Responsibilities”
“Billing Secondary Payer – Notification of Other Payer-Patient Responsibilities – Captured, Paid”
Section 12.6 “General Information For Transmission Accepted/Transaction Rejected Response” was slightly modified. In previous releases,
the Response Insurance, Response Pricing, and Response Prior Authorization were listed as not used. This section now correctly notes the
Response Pricing and Response Prior Authorization as the two segments not used. This has been modified to match the Response Segment
Matrices in the Implementation Guide.
37.6 VERSION 5.6
Changes to Version 5.6 include editorial changes to remove the references to the Compound Implementation Guide and the Prior
Authorization Implementation Guide, which were supported in previous versions. Text from these guides was incorporated into the
implementation guide as appropriate.
The diagrams in sections “Diagram for Two Billing Transactions”, “Diagram for Three Billing Transactions”, “Diagram for Four Billing
Transactions”, and “Diagram for Two Rebill Transactions” have been modified to remove the Compound Segment. This was an error. The
Compound Segment may only be sent in one billing transaction or one rebill transaction transmissions.
Verbiage for the support of multiple reversal transactions in a transmission has been added to the documents. This is to offer more clarification
to the support of multiple reversals for Claim or Service Reversals, Controlled Substance Reporting Reversals, Information Reporting
Reversals, and Rebill transactions.
A new field DUR Additional Text (57Ø-NS) has also been added to the Response DUR/PPS Segment.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 945 -
Text from prior versions of the Compound Implementation Guide and Prior Authorization Implementation Guide was incorporated into this
document as appropriate. In the section “Request Transaction Segments and Fields”, subsections “DUR/PPS Segment”, “Compound
Segment”, and “Prior Authorization Segment” have incorporated the pertinent information formerly found in the implementation guide.
In the section “Response Transaction Segments and Fields”, the subsection “Response Prior Authorization Segment” has incorporated the
pertinent information formerly found in the implementation guides.
Example 7.16.1 “Prior Authorization Request and Billing Accepted Response – Captured” and 7.18.1 “Prior Authorization Inquiry Accepted
Response – Captured” have been modified to remove the Response Prior Authorization Segment. This segment is not used in Captured
responses.
In the Specification, the diagrams in sections “Diagram for Two Billing Transactions”, “Diagram for Three Billing Transactions”, “Diagram for
Four Billing Transactions”, and “Diagram for Two Rebill Transactions” have been modified to remove the Compound Segment. The Compound
Segment may only be sent in one billing transaction or one rebill transaction transmissions.
In this guide, two new frequently asked questions related to compounds were added (“How Do I Enter An Ingredient In A Compound That
Does Not Have An Identifier (For Example Water)?” and How Do I Bill For A Partial Fill Of A Compound?”).
Verbiage for the support of multiple reversal transactions in a transmission has been added to this document. This is to offer more clarification
to the support of multiple reversals for Claim or Service Reversals, Controlled Substance Reporting Reversals, Information Reporting
Reversals, and Rebill transactions.
Two frequently asked questions related to multiple reversal transactions in a transmission were added (“What Are The Recommended
Guidelines For Supporting Multiple Claim Or Service Reversal (B2) Transactions Within A Transmission?” and “What Are The Recommended
Guidelines For Supporting Multiple Rebill (B3, N3, C3) Transactions Within A Transmission?”).
A new Reject Code (511-FB) value of “RV” was added for “Multiple Reversals Per Transmission Not Supported”.
Version 5.6 also added a new field DUR Additional Text (57Ø-NS) to the Response DUR/PPS Segment. Section “Repeating Fields –
Maximum Occurrences” was updated with this field, as well as the “Response Segment” matrices section. The section “Response DUR/PPS
Segment” was added with verbiage about this field.
Example 7.5.2 “Billing w/Submitted DUR Override Rejected Response” includes the DUR Additional Text. Example 7.6.1 “Billing w/Information
DUR Accepted Response-Captured, Paid” also includes the usage of DUR Additional Text.
Example 7.3 and 7.5 added the Quantity, Days Supply, DAW and other fields in the situational fields for the Claim Segment for clarification.
In examples 7.8 “Compounded Rx Billing – Transaction Code B1 (Ø1)” and 7.8.3 “Billing Resubmission w/DUR Resolution” the
Product/Service ID Qualifier in the Claim Segment has been changed from Ø3 to ØØ. For a multi-ingredient compound, the Product/Service ID
and Qualifier in the Claim Segment defaults to zeroes.
Text within the document that specifically stated “Version 5.6” was changed to “Version 5” where appropriate. Examples that show specific
field values were updated to the value of “56”.
In section 4.1.3 “Reversal (Transaction Codes B2, N2, C2)”, the paragraph beginning “If, during the transmission of a reversal…” has been
modified to correctly state “and use a “S” status.” (The statement originally said “and use a “D” status.” This was incorrect.) Example 7.14.3
“Reversal Accepted Response – Duplicate” incorrectly displayed a Transaction Response Status of “D”. This has been changed to “S”
(duplicate of reversal).
37.7 VERSION 6.Ø
In Version 6.Ø, references in the Specification and Implementation Guide have been changed to “Version 5 and above” to encompass version
6 and futures.
The length of fields Prescription/Service Reference Number (4Ø2-D2) and Associated Prescription/Service Reference Number (456-EN) has
been increased to 9 bytes. These fields are numeric with a length of 9.
Added the question “Why would the Response Pricing segment be used (situational) in a Billing transaction (or other transaction) when a
processor returns a “C”aptured response?” to the “Frequently Asked Questions” section.
Version 6.Ø also corrected various typographical errors in the Implementation Guide. A list of the changes follows. The changes have been
corrected.
In some examples, Number of Refills Authorized (415-DF) was displayed as two (2) digits. The field is numeric and zero suppressed. The
examples were changed to display the field as one (1) digit.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 946 -
In some examples, the qualifier fields (Patient ID Qualifier, Prescriber ID Qualifier, Other Payer ID Qualifier, DUR Co-Agent ID Qualifier) and
Compound Ingredient Basis of Cost Determination were displayed as one (1) digit. Since these fields are alphanumeric two (2) digits, both
positions should be displayed. The examples were changed to display the field as two (2) digits.
In some examples, Patient City Address (323-CN) was incorrectly labeled as (322-CN).
In some examples, Provider ID (444-E9) was labeled as (449-E9).
In some examples, Provider ID Qualifier (465-EY) was labeled as (466-EZ).
In some examples, Cardholder Last Name (313-CD) was labeled as (313-DC
In some examples, DUR/PPS Response Code Counter (567-J6) was labeled as (473-7E).
In some examples, DUR/PPS Code Counter (473-7E) was labeled as (567-J6).
In example 7.8.2 “Compounded Rx Billing – Rejected Response”, Reject Occurrence (546-4F) should have displayed 1 (not 3), since the
Compound Ingredient Drug Cost was missing on occurrence 1.
In example 7.9.1 “Billing, Initial Partial Fill Accepted Response – Captured, Paid”, Flat Sales Tax Amount Paid was designated as 558AW.
This has been corrected to 558-AW.
In example 7.12 “Billing w/Coupon – Transaction Code B1 – Billing to Coupon Processor”, the value of 1ØØ{ was added to the Value column.
In an example, Preferred Product Description (556-AU) was labeled as (557-AU).
In an example, Measurement Date (494-ZE) was labeled as (949-ZE).
In an example, Professional Service Fee Paid (562-J1) was labeled as (477-BE).
In an example, Help Desk Phone Number Qualifier (549-7F) was labeled as (459-7F).
In example 7.15.1 “Rebill Accepted Response – Captured”, the Response Pricing Segment to only have copay fields.
In some Rejected examples, the Reject Count (51Ø-FA) and Code (511-FB) fields are now displayed in “The Following Fields, though
Situational, are Mandatory for Reject Response:” section. These examples include:
7.5.2 “Billing w/Submitted DUR Override Rejected Response”
7.6.2 “Billing w/DUR Conflicts Rejected Response”
7.14.4 “Reversal Rejected Response”
7.25.2 “Controlled Substance Reporting Rebill Rejected Response”
In some examples, the Authorization Number (5Ø3-F3) is now displayed in the “The Following Fields are Situational:” section. These examples
include:
7.3.2 “Billing w/Insurance and Coordination of Benefits Rejected Response”
7.5.2 “Billing w/Submitted DUR Override Rejected Response”
7.6.2 “Billing w/DUR Conflicts Rejected Response”
7.8.2 “Compounded Rx Billing Rejected Response”
7.16.1 “Prior Authorization Request & Billing Accepted Response – Captured”
7.16.2 “Prior Authorization Request & Billing Accepted Response – Paid”
7.16.3 “Prior Authorization Request & Billing Rejected Response”
7.16.4 “Prior Authorization Request & Billing Duplicate Response”
7.18.1 “Prior Authorization Inquiry Accepted Response – Captured”
7.18.2 “Prior Authorization Inquiry Accepted Response – Paid”
7.19.2 “Prior Authorization Request Only Rejected Response”
In example 7.8.2 “Compounded Rx Billing Rejected Response”, the Reject Field Occurrence Indicator (546-4F) is designated as a field though
situational, is mandatory for Reject Response and mandatory when used to designate the occurrence of a repeating field.
In example 7.8 “Compounded Rx Billing – Transaction Code B1 (Ø1)”, notation was added to clarify the intentionally missing field of
Compound Ingredient Drug Cost (449-EE).
In example 7.8 “Compounded Rx Billing – Transaction Code B1 (Ø1)” and 7.8.3 “Billing Resubmission w/DUR Resolution”, a mandatory and
situational field note was added to designate the field usage in a Compound Segment. For example, Compound Ingredient Drug Cost and
Compound Ingredient Basis of Cost Determination are designated as situational fields in each occurrence of ingredient.
37.8 VERSION 7.Ø
The new DERF form has been included in this document.
Version 7.Ø adds the functionality of “adjudication status”. Adjudication status is to inform the receiving entity what action was taken regarding
the encounter by the Managed Care Plan. To relay this information, the Implementation Guide was modified with a comment for the usage of
Intermediary Authorization ID and Type ID. For this purpose, the first digit of Intermediary Authorization ID (464-EX) will be defined as
“C” Capitated
“P” Paid
“D” Denied
and the Intermediary Authorization Type ID (463-EW) will contain 99 (Other Override).

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 947 -
Submission Clarification Code (42Ø-DK) now supports a new value of 1Ø for “Meets Plan Limitations”. This is used in some programs as
“Code 1” certification, where the pharmacy certifies that the transaction is in compliance with the program’s policies and rules that are specific
to the particular product being billed. Submission Clarification Code also now repeats with the addition of Submission Clarification Code Count
(354-NX). The maximum repetitions are 3. Modifications to the document occurred in
section 2.4 “Repeating Fields – Maximum Occurrences”, subsection “Usage”,
section 2.4 “Repeating Fields – Maximum Occurrences”, subsection “Claim Segment”,
section 5.1 “Request Segments” – to add the field to the chart,
section 7 “Transactions Examples” – to add the Submission Clarification Code Count whenever Submission Clarification Code was used in an
example.
“Appendix K” in the Data Dictionary has been modified to clarify the acronyms used and to modify the usage of “Micromedex” and “Medical
Economics” to “Micromedex/Medical Economics”. The term “Medi-Span” has also been modified to “First DataBank”.
The definition of Prior Authorization Type Code (461-EU) has been modified to “Code clarifying the Prior Authorization Number Submitted
(462-EV) or benefit/plan exemption.” The Prior Authorization Type Code can now be used when a Prior Authorization Number Submitted is not
required (i.e. 4 = Exemption from Copay).
The Prior Authorization Segment has been modified to “Not Used” in the Billing and Rebill transactions. These sections in the Specification
have been modified:
8.2.1 “Multiple Occurrences of Segments”
1Ø.3 “Diagram for One Billing Transaction”
1Ø.4 “Diagram for Two Billing Transactions”
1Ø.5 “Diagram for Three Billing Transactions”
1Ø.6 “Diagram for Four Billing Transactions”
1Ø.11 “Diagram for One Rebill Transaction”
1Ø.12 “Diagram for Two Rebill Transactions”
Additional functionality has been added to the business function Coordination of Benefits. Two new fields, Other Payer ID Count (355-NT) and
Other Payer Cardholder ID (356-NU) have been added. These two fields, along with Other Payer ID Qualifier (339-6C) and Other Payer ID
(34Ø-7C) have been added to the Response Status Segment.
The old Example 7.3 and 7.4 have been replaced with “Billing – Transaction Code B1 – Coordination of Benefits Scenarios Pharmacy Bills To
Insurance Designated By Patient” and “Billing – Transaction Code B1 – Coordination of Benefits – Scenario 1: Pharmacy Bills Secondary
Insurance” to show coordination of benefits scenarios. Modifications to the document occurred in the following:
section 2.4 “Repeating Fields – Maximum Occurrences”, subsection “Usage”
section 2.4 “Repeating Fields – Maximum Occurrences”, subsection “Response Status Segment”
section 4.4.3 “Response Status Segment”
section 5.2 “Response Segments” – added fields to the chart
37.9 VERSION 7.1
One new field has been added to Version 7.1. Delay Reason Code (357-NV) has been added with codes to specify the reason that
submission of the transaction has been delayed. This field has been added to the Claim Segment.
Extensive clarification has been added to the Implementation Guide for Prior Authorization.
Section 4.1.6 “Prior Authorization Fields” has been replaced. Section 4.1.7 “Response Prior Authorization Segment” has been replaced.
Section 4.2.12 “Prior Authorization Segment” includes information for Request Type (498-PA) = “2” (Reauthorization).
A new section 4.5 “Prior Authorization Transaction Discussion” has been added. Three new Frequently Asked Questions have been added:
• “The initial transaction is a Prior Authorization Request Only. The pharmacy submits a Prior Authorization Inquiry ..”
• “Once the Prior Authorization Number is assigned, on subsequent refills, can you just submit the Prior Authorization in the Prior
Authorization Number Submitted field in the Claim Segment, or …”
• “Will each different “C” Captured response of a “Prior Authorization Inquiry” transaction come back with a unique Authorization
Number (5Ø3-F3) or …”.
The following examples were modified to present correct models for Prior Authorization transactions.
7.16 “PA Request and Billing – Transaction Code P1”
Added blurb:
This is an initial request for prior authorization approval with payment information. Prior Authorization Segment contains the requested period
dates.
Removed Prior Authorization Number-Assigned (498-PY) from table.
7.16.2 “Prior Authorization Request & Billing Accepted Response – Paid”
Added blurb:
The pharmacy receives prior authorization and payment information in the response.
7.16.3 “Prior Authorization Request & Billing Rejected Response”
Added blurb:

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 948 -
The pharmacy receives the response from the processor that the product or service is not covered. The preferred product information is
returned. A Help Desk number is available for follow up questions.
7.16.4 “Prior Authorization Request & Billing Duplicate Response”
Added blurb:
The pharmacy receives a duplicate paid response. The information is the same as 7.16.2.
7.17 “Prior Authorization Reversal – Transaction Code P2”
Added blurb:
The pharmacy wishes to reverse the prior authorization that was previously processed. This is a request to reverse just the prior authorization.
If claim or service billings were billed with this prior authorization, the claim or service billings would need to be reversed first; then the prior
authorization reversed.
Removed the Claim Segment because the claims are to be reversed separately.
7.17.1 “Prior Authorization Reversal Accepted Response – Captured, Approved”
Removed the Response Claim Segment because the claims are to be reversed separately.
7.18 “Prior Authorization Inquiry – Transaction Code P3”
Added blurb:
New scenario. The pharmacy has submitted a PA Request And Billing sometime in the past, and received a captured response. The
pharmacy is now submitting a PA Inquiry to determine the outcome, using the Authorization Number (5Ø3-F3) received during the PA Request
And Billing conversation.
Removed all fields from PA Segment except Segment ID, Request Type, Request Period Begin and End, and Auth Number.
7.18.1 “Prior Authorization Inquiry Accepted Response – Captured”
Added blurb:
The original PA Request And Billing received a “C” Captured response. The pharmacy submits an inquiry as to the status. The processor is
still evaluating the original PA Request And Billing and sends a “C” Captured response back to the pharmacy.
Also, the Authorization Number (5Ø3-F3) returned on the Captured response is the same as submitted (9876545678) per section 4.5.3.1.1
“Scenarios for Prior Authorization Request And Billing”.
7.18.2 “Prior Authorization Inquiry Accepted Response – Paid”
The processor is responding that the original PA Request And Billing has been approved and payment information is included. The processor
assigns an Authorization Number to conversation. The processor returns payment, as well as prior authorization information, including a Prior
Authorization Number-Assigned (498-PY).
7.19 “Prior Authorization Request Only – Transaction Code P4”
Added blurb:
New scenario. The pharmacy is requesting a prior authorization approval only (no payment). The Prior Authorization Segment includes the
prior authorization period date and other information.
7.19.1 “Prior Authorization Request Only Accepted Response –Approved”
Added blurb:
The processor responds that the request for prior authorization has been approved, with appropriate prior authorization information.
Removed Capture from the heading and the Transaction Response Status and Note. Keep 498-PY.
7.19.2 “Prior Authorization Request Only Rejected Response”
Added blurb:
The processor is not approving the request for a prior authorization, as the product is not covered.
37.10 VERSION 8.Ø
Based on discussion, it was determined that the Transaction Response Status (112-AN) duplicate values for the Rebill, Information Reporting
Rebill, and Controlled Substance Reporting Rebill transactions were not needed. There is no business reason found for the duplicate
responses for the rebill transactions.
A new subsection “Duplicate Processing for all Rebill Transactions” in section 11 “Transmission Response Discussion” has been added that
discusses the removal of the duplicate response codes for the rebill transactions. Sections “Duplicate Transactions”, “Rebill”, “Controlled
Substance Reporting Rebill”, “Information Reporting Rebill” and “Response Status By Transaction Type” have been modified with the removal
of the Transaction Response Status (112-AN) duplicate values.
Section 3.2 “Response Segment Matrices” was modified to note that Rebill, Information Reporting Rebill, and Controlled Substance Reporting
Rebill transactions do not support the duplicate Transaction Response Status (112-AN) codes. The duplicate values were removed from the
matrices.
The section 4.6 “Duplicate Processing for all Rebill Transactions” was added. Section 4.1 “Request Transactions” was updated to point to the
new section. Section 4.3.2 “Duplicate” was updated for rebill transactions. The following examples for rebill transactions did not change 7.15
“Rebill – Transaction Code B3”, 7.22 “Information Reporting Rebill – Transaction Code N3”, and 7.25 “Controlled Substance Reporting Rebill –
Transaction Code C3”.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 949 -
Frequently Asked Question 8.29 “What are the recommended guidelines for supporting multiple rebill (B3, N3, C3) transactions in a
transmission?” was updated to point to the duplicate sections.
New frequently asked questions were added to the Telecommunication Standard Implementation Guide:
• Should the fields submitted in the Transaction Header Segment on a request be returned without modification on the Response
Header Segment? (Should they be mirrored?)
• Can a response transaction contain accepted and rejected information? For example, on an Rx Billing (B1), could the response be
returned with a Transaction Response Status of “P” (Paid) and in the Response Status Segment, Reject Code and Count fields be
included to relay information? Or in another example, could a Reversal (B2) response be “A” (Approved) and Reject Code and
Count fields be included?
• On compounded claims, does DUR "hit" each drug within the compound?
• Should compound ingredients be put in highest usage amount order? (i.e., product A 8Ø%, product B 1Ø%, product C 1Ø%).
• By business partner agreement, a pharmacy wishes to submit Workers’ Compensation claims to its billing services provider using
the NCPDP Telecom v7.Ø Standard. (This should not need to comply with HIPAA regulations for transaction and code sets.) This
is an update to the existing process that currently utilizes RTDS 3B of the NCPDP Telecom v3.2 Standard. The current process
uses NDC Number (field 4Ø7-D7) to carry UPC and HRI codes in an 11-digit format. The v5.Ø and subsequent Standard releases
have renamed and restructured the field (now Product/Service ID, 11 digits to 19 characters) and include a qualifier for the field,
Product/Service ID Qualifier (436-E1). Now that the qualifier is available, should the UPC and HRI values be sent in their native
format instead of being reformatted to an 11-digit value?
In the section “Transmission Examples”, the Product/Service ID (4Ø7-D7) Clinoril 2ØØmg was corrected from ØØØØ6Ø94228 to
ØØØØ6Ø94268.
37.11 VERSION 8.1
In the “Truncation” section, subsections were added for “Numeric Truncation” and “Alphanumeric Truncation”. In section “Field Format Values”,
a note was made about explicit hyphens.
In the section “Diagram For Eligibility Verification Response”, information was added about the use of the group separator in an eligibility
transaction.
A comment column was added to “Appendix K Product/Service Qualifier” of the Data Dictionary. Comments were added to Employer ID (333-
CZ) in the Data Dictionary.
In the following examples, the Response Message Segment was added to the responses. Although the Response Message Segment is
situational or optional, the segment is shown to provide guidance.
• Reversal – Transaction Code B2
• Rebill – Transaction Code B3
• Prior Authorization Reversal – Transaction Code P2
• Information Reporting Rebill – Transaction Code N3
• Controlled Substance Reporting Rebill – Transaction Code C3
New frequently asked questions were added to the Telecommunication Standard Implementation Guide:
• How is the format of Percentage Sales Tax Rate Submitted (483-HE) and Percentage Sales Tax Rate Paid (56Ø-AY) expressed?
• Eligibility Transaction and the Group Separator.
• Fill Number (4Ø3-D3) – Default?
• How should a clearinghouse or payer handle rejecting a transaction sent from a provider with an invalid Version/Release Number
(1Ø2-A2), Transaction Code (1Ø3-A3), or Transaction Count (1Ø9-A9)?
• Prior Authorization Request And Billing – PA Not Required
• Prior Authorization Request And Billing – Deferred
• Product/Service ID field (4Ø7-D7) and Compounds in Reversals.
• Can a cardholder ID contain symbols such as hyphens and apostrophes? Also includes information on printable characters.
37.12 VERSION 8.2
A new value of “12” (End Stage Renal Disease Treatment Facility) has been added to Patient Location (3Ø7-C7). A new field Amount
Attributed to Processor Fee (571-NZ) has been added to the Response Pricing Segment.
New frequently asked questions were added to the Telecommunication Standard Implementation Guide:
• 1ØØ% Copay and Negative Amounts
• Other Coverage Code (3Ø8-C8) And Coordination of Benefits
• Payment Amount Based on Dispensed or Intended?
• Reject Code for Incorrect Other Payer Amount Paid Count (341-HB)

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 950 -
37.13 VERSION 8.3
Version 8.3 added Reject Codes (511-FB) for Count fields that were mistakenly left out of Data Dictionary. The values are “SF” Other Payer
Amount Paid Count Does Not Match Number Of Repetitions, “SG” Submission Clarification Code Count Does Not Match Number of
Repetitions, and “SH” Other Payer-Patient Responsibility Amount Count Does Not Match Number of Repetitions. To provide guidance for how
to handle these missing values in the Version 5 world, new Frequently Asked Questions were added to the Telecommunication Standard
Implementation Guide. (See also new “Frequently Asked Questions” added in Version 8.2 above for Other Payer Amount Paid Count (341-
HB).
• What Reject Code (511-FB) should be used when the Submission Clarification Code (42Ø-DK) doesn’t match the number submitted
in the Submission Clarification Code Count (354-NX)?
• What Reject Code (511-FB) should be used when the Other Payer-Patient Responsibility Amount (352-NQ) and Qualifier (351-NP)
doesn’t match the number submitted in the Other Payer-Patient Responsibility Amount Count (353-NR)?
37.14 VERSION 9.Ø
Version 9.Ø modified the value “21” in Appendix K. Product/Service Qualifier of the Data Dictionary from “International Classification of
Diseases (ICD1Ø)” to “International Classification of Diseases-1Ø-Clinical Modifications (ICD-1Ø-CM)”. Value “27” has been added for
“International Classification of Diseases-1Ø-Procedure Coding System (ICD-1Ø-PCS).
Diagnosis Code Qualifier (492-WE) value “Ø2” has changed from “International Classification of Diseases (ICD1Ø)” to “International
Classification of Diseases-1Ø-Clinical Modifications (ICD-1Ø-CM)”.
Based on Designated Standards Maintenance Organizations (DSMO) Change Request System (CRS) 763 that was approved to add more
repetitions for Procedure Modifiers, the Procedure Modifier Code Count (458-SE) has increased in size and the number of repetitions. The
Procedure Modifier Code Count was “maximum count of 9; recommend support count ≤ 4” to “maximum count of 99; recommend support
count ≤ 1Ø”. This change is reflected in section 2.4 “Repeating Fields - Maximum Occurrences”.
Further clarification was made in section “Compound Segment”
When billing for multiple ingredients, use the following Claim and Pricing Segment fields:
Product/Service ID (Field 4Ø7-D7) – defaults to zero (Zero means “Ø”.)
Product/Service ID Qualifier (Field 436-E1) – defaults to “ØØ”
The Product/Service ID must contain a value of “Ø” and Product/Service ID Qualifier must contain a value of “ØØ” when used for
multi-ingredient compounds.
In section “Compound Segment”, subsection “Use of Fields In A Variable Format”, a statement was added for guidance to rounding of
Compound Ingredient Drug Cost (449-EE) and Compound Ingredient Basis of Cost Determination (49Ø-UE).
In section “Response Transactions”, a subsection of “Pricing Guidelines” was added. Under subsection “Captured or Deferred”, a subsection
was added of “Business Function of Capture”.
New Frequently Asked Questions were added to the Telecommunication Standard Implementation Guide.
• Does Usual And Customary Charge (426-DQ) include a dispensing fee?
This response was also included in section “Pricing Segment”.
• Transaction Fee Charge
• Facility ID Usage
• How should Partial Fills be handled for a Coordination of Benefits (Coordination of Benefits) billing?
• From a standards perspective is it valid to require the reporting of procedure code modifier(s) with national drug codes?
This response was also included in section “Claim Segment”.
• Quantity Dispensed (442-E7) and Compounds
• Truncation of Dollar Fields
This response was also included in section “Truncation”, subsection “Dollar Truncation”.
• When the value 99=composite is used in the Other Payer Coverage Type, what is placed in the Other Payer ID? Is it not sent?
This response was also included in section “Coordination of Benefits /Other Payments Segment”.
• Should the Product/Service ID Qualifier be Ø3/NDC or is blank or ØØ/Unspecified acceptable?
This response was also included in section “Compound Segment”.
• Compound Ingredient Calculates To Be Less Than $Ø.ØØ5
This response was also included in section “Compound Segment”.
• Explicit Decimal Points in Diagnosis Code (424-DO)
A statement was added to section “Implied Decimal Points” and “Clinical Segment”.
In the examples, Patient E-Mail Address (35Ø-HN) was corrected to display uppercase letters, per the character set.
37.15 VERSION A.Ø
In Version A.Ø, the Prescriber Segment in the Eligibility Transaction has changed from “Not Used” to “Optional”. In section “Segment Usage
Matrices” subsection “Request Segment Matrix”, the Prescriber Segment changed from “N” to “O” in the Eligibility transaction column of the
diagram. “Frequently Asked Questions” “Eligibility Transaction and the Group Separator” has been modified to note the Patient Segment and
the Prescriber Segment are optional as well as the Pharmacy Provider Segment. In section “Request Transaction Segments and Fields”,
subsection “Prescriber Segment”, information as been added to note the use of this segment for validation under various restricted programs.
The section “Diagram For Eligibility Verification” has been modified to include the Prescriber Segment. The section “Diagram For Eligibility

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 951 -
Verification Response“ has been modified to note the Patient Segment and the Prescriber Segment are optional as well as the Pharmacy
Provider Segment.
Clarification was made to the Telecommunication Standard Implementation Guide for the decimal point in Diagnosis Code (424-DO). In
section “Data Conventions”, subsection “Implied Decimal Points”, the paragraph was modified to clarify that diagnosis code fields must adhere
to the owner’s code set rules and formats. In section “Request Transactions Segment and Fields”, subsection “Clinical Segment”, the verbiage
under Diagnosis Code (424-DO) was modified to clarify that diagnosis code fields must adhere to the owner’s code set rules and formats.
“Frequently Asked Questions” “Explicit Decimal Points in Diagnosis Code (424-DO)” was deleted.
In Example “Information Reporting - Transaction Code N1”, the Diagnosis Code (424-DO) fields were modified.
• Change the 1st Diagnosis Code from 716.9Ø to 7169Ø
• Change the 2nd Diagnosis Code from 4Ø1.9 to 4Ø19
• Change the 3rd Diagnosis Code from 593.9Ø to 5939
• Change the 4th Diagnosis Code from 493. to 493ØØ
The following note was added to the example: “Note: Diagnosis Code (424-DO) - For example purposes only, and may not be billable. Refer to
owner’s code set rules and formats.”
In section “Document Conventions” subsection “Overview”, paragraphs were added that discuss whether there is an order to how segments
appear in a transmission. A note was added to refer to this section in the “Transmission Request Diagrams” and “Transmission Response
Diagrams” sections.
A note was added to section “Date Format” to see the Telecommunication Standard Implementation Guide for a frequently asked question on
date default values.
New Frequently Asked Questions were added to the Telecommunication Standard Implementation Guide.
• “Default Date Format” - Fields defined as Date format – what is the default? Can Date fields be defaulted to ØØØØØØØØ?
• A reference to the question was made in “Character Sets Designation”.
• How are compounded pills submitted?
• Is a Person Code (3Ø3-C3) of “Ø6Ø” the same as “6Ø”?
• Can a Segment Identification (111-AM) be sent without any fields in that segment and not be in error?
• Must the mandatory data elements be sent in the order that they are listed in the implementation guide?
• Is there an order to the way segments must appear in a transmission?
• Paragraphs were added to “General Syntax Outline”.
• Section “Segment Usage Matrices” includes a note to see “General Syntax Outline” for information on segment order. This same
note was added to section “Request and Response Segment Quick Reference”.
• Please clarify the definition of the Patient Location (3Ø7-C7) Field.
A correction to a field name was made in section “Prior Authorization Number-Assigned (462-EV) in Claim Segment” – 462-EV is Prior
Authorization Number Submitted.
37.16 VERSION A.1
Section “Reject Field Occurrence Indicator Use for Multi-Ingredient Compound Transaction” was added.
New Frequently Asked Questions were added.
• Multi-Ingredient Compounds And Rejects
• Multi-Ingredient Compounds And DUR Rejects
Modification was also made to example “Compounded Rx Billing - Transaction Code B1 (Ø1)”,” Compounded Rx Billing
Rejected Response”
A reference was also added to these questions in section “Compound Segment”.
• Alphanumeric Field And Leading Spaces
The following Frequently Asked Question was modified.
• Please clarify the definition of the Patient Location (3Ø7-C7) Field.
In the sentence “In the future, a Data Element Request Form (DERF) may be submitted to clarify the place where the patient resides versus
the place the patient receives the product or service.” was modified from “(DERF) will” to ”(DERF may)”.
The Telecommunication Specification and the Telecommunication Standard Implementation Guide were combined into one document, using
the new standard template. Section or example modifications referenced in previous version/releases cited above in this section refer to the
section or example as of that version/release. In this version and going forward, sections and examples are now cited by name rather than by
number.
The DERF was removed from the actual document, but guidance included about obtaining a copy.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 952 -
37.17 VERSION B.Ø
The values in Patient Location (3Ø7-C7) were a mixture of patient locations and places of service. Patient Location was renamed to Place of
Service (3Ø7-C7) with new values assigned. A new field Patient Residence (384-4X) was added. The following sections were modified for
these fields
• “Structure Quick Reference”
• Section “Transmission Examples” listing Patient Location (all contained value ØØ (Unspecified) were changed to Place Of Service
value 91 (Pharmacy = A duly licensed entity that delivers pharmaceutical goods or services for sale to or use by the final consumer).
• Section FAQ “Please Clarify The Definition Of The Patient Location (3Ø7-C7) Field.” was modified to explain the one field prior to
B.Ø and the two fields in B.Ø and above.
New Reject Codes (511-FB) were introduced in this version. Some Reject Codes were retired. Some Reject Codes modified the reference
fields. See the NCPDP External Code List for specifics.
In section “Standards Conventions”, subsection “Repetition And Multiple Occurrences”, subsection “Repeating Data Elements”,
subsection “Reject Field Occurrence Indicator”, examples that use the Reject Code of “TE “ were modified to “21”.
FAQ “Multi-Ingredient Compounds And Rejects”, examples that use the Reject Code of “TE “ were modified to “21”.
A new subsection “Shared Reject Codes” was added to section “Specific Segment Discussion”, subsection “Compound Segment” to
provide guidance on using the same Reject Codes for claim-level fields as compound-level fields, when appropriate. A note was
added in “Response Status Segment” to see “Shared Reject Codes” section.
In section “Transmission Examples”, “Billing – Transaction Code B1 – Coordination of Benefits – Scenario 2: Pharmacy Bills Secondary
Insurance”, and “Scenario 3: Pharmacy Bills Secondary Insurance”, a typographical error was found that Other Payer-Patient Responsibility
Amount Qualifier was labeled 352-NP. It has been corrected to 351-NP.
The Professional Pharmacy Services Implementation Guide was incorporated into this document. The following sections were moved from the
Professional Pharmacy Services Implementation Guide into this document.
• Section “Controlled Substance Reporting Information” added the paragraph “It is assumed DUR screening…”
• Section “Specific Segment Discussion”, subsection “DUR/PPS Segment”, subsection “Terminology” added a paragraph heading of
“Drug Use Review (DUR)”. The section “Professional Pharmacy Services” was added. Subsection “Specific Discussion” was
renamed to “Specific Discussion – DUR” and subsections “The Problem of Noise”, “DUR Inputs”, “ORDUR Screening”,
“Dosing/Limits”, “Drug Interactions”, “Drug Conflicts”, “Duplicate Therapy”, “Precautionary Screening” were added. Subsection
“Specific Discussion-Professional Pharmacy Services” was added. A heading of “Special Considerations” and the paragraph “The
very nature of professional services…” were added. Subsection “Response DUR/PPS Segment” added the subsection “DUR/PPS
Claims Data And Responses In Batch Transactions”.
• Section “Transmission Examples”, “Billing With DUR Segment Using Co-Agent Fields - Transaction Code B1 (Ø1/Ø2)” was added.
Example “Billing Paid Response Using DUR Additional Text - Transaction Code B1 (Ø1/Ø2)” was added.
• Section “Transmission Examples”, “Service Billing Transmission Rejected Response”, “Service Billing Transmission – One Rejected,
One Paid Response”, “Information Reporting Reversal Accepted Response—Duplicate of Captured or Approved”, and “Information
Reporting Reversal Rejected Response” were added to their respective already existing request examples.
• Section “Frequently Asked Questions” added “DUR Additional Text (57Ø-NS) Field”.
37.18 VERSION C.Ø
In section “Structure Quick Reference”, the fixed length of the Request and Response Headers were included. A note was added to the
variable segments that they do not have a fixed length.
The document was updated to support Certificate of Medical Necessity (CMN) needs. Section “Standard Conventions”, subsection “General
Syntax Outline” added the
• Additional Documentation Segment
• Facility Segment
• Narrative Segment
Subsection “Repetition and Multiple Occurrences” subsection “Multiple Occurrence of Segments” also added these three segments.
Subsection “Usage” added the new count field of Question Number/Letter Count (377-2Z). The subsection “Additional Documentation
Segment” was added.
Section “Transmission Structure”, subsection “Request Segment Matrix” added the Facility, Narrative, and Additional Documentation
Segments. Section “Specific Segment Discussion” added subsections for the Facility, Narrative, and Additional Documentation Segments.
Section “General Structural Overview” subsection “Transaction Level For A Request” added the three segments. Section “Structure Quick
Reference” Facility ID (336-8C) was moved from the Insurance Segment to the Facility Segment.
In the Prescriber Segment, the following fields were added:
Prescriber Segment

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 953 -
Field Field Name Mandatory or
Optional
364-2J PRESCRIBER FIRST NAME O
365-2K PRESCRIBER STREET ADDRESS O
366-2M PRESCRIBER CITY ADDRESS O
367-2N PRESCRIBER STATE/PROVINCE ADDRESS O
368-2P PRESCRIBER ZIP/POSTAL ZONE O
The following segments were added with the following fields:
Additional Documentation Segment
Field Field Name Mandatory or
Optional
111-AM SEGMENT IDENTIFICATION M
369-2Q ADDITIONAL DOCUMENTATION TYPE ID M
374-2V REQUEST PERIOD BEGIN DATE O
375-2W REQUEST PERIOD RECERT/REVISED DATE O
373-2U REQUEST STATUS O
371-2S LENGTH OF NEED QUALIFIER O
37Ø-2R LENGTH OF NEED O
372-2T PRESCRIBER/SUPPLIER DATE SIGNED O
376-2X SUPPORTING DOCUMENTATION O
377-2Z QUESTION NUMBER/LETTER COUNT O
378-4B QUESTION NUMBER/LETTER O***R***
379-4D QUESTION PERCENT RESPONSE O***R***
38Ø-4G QUESTION DATE RESPONSE O***R***
381-4H QUESTION DOLLAR AMOUNT RESPONSE O***R***
382-4J QUESTION NUMERIC RESPONSE O***R***
383-4K QUESTION ALPHANUMERIC RESPONSE O***R***
Facility Segment
Field Field Name Mandatory or
Optional
111-AM SEGMENT IDENTIFICATION M
336-8C FACILITY ID (MOVED FROM THE INSURANCE SEGMENT) O
385-3Q FACILITY NAME O
386-3U FACILITY STREET ADDRESS O
388-5J FACILITY CITY ADDRESS O
387-3V FACILITY STATE/PROVINCE ADDRESS O
389-6D FACILITY ZIP/POSTAL ZONE O
Narrative Segment
Field Field Name Mandatory or
Optional
111-AM SEGMENT IDENTIFICATION M
39Ø-BM NARRATIVE MESSAGE M
Section “Transmission Examples”, subsection “Billing w/DUR Conflicts - Transaction Code B1”, the Facility ID was moved from the Insurance
Segment to the Facility Segment. Section “Frequently Asked Questions”, “Facility ID Usage”, a note was added that the Facility ID was moved
to the Facility Segment in this version.
Examples were added to section “Transmission Examples” of
• Billing - Transaction Code B1 With Additional Documentation Segment
• Billing – Transaction Code B1 With Facility Information
• Billing – Transaction Code B1 With Additional Documentation And Facility Information
• Billing – Transaction Code B1 With Narrative Information

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 954 -
• Billing – Transaction Code B1 With Facility Information And Narrative Information
• Billing – Transaction Code B1 with Additional Documentation And Narrative Information
These examples contain verbiage that refers to the NCPDP Version 5 Editorial document. The CMN requested changes were approved, but
not the Medicare claims modifications. DERF 7ØØ was removed from a ballot. Medigap ID was included. Since there is a need to relay this
information, but the modifications were not approved, the NCPDP Version 5 Editorial document addresses a solution that puts a designation in
the Group ID. Please see this document for more information.
Section “Eligibility Verification Information” diagram added the Additional Documentation Segment.
Section “Claim and Service Billing Information”, “Rebill Information”, “Prior Authorization Request And Billing”, and “Prior Authorization Inquiry”
diagrams added the Additional Documentation Segment, Facility Segment, and Narrative Segment.
New values have been added to Submission Clarification Code (42Ø-DK).
• 11 “Certification on File- The supplier's guarantee that a copy of the paper certification, signed and dated by the physician, is on file
at the supplier's office”
• 12 “DME Replacement Indicator- Indicator that this certification is for a DME item replacing a previously purchased DME item”
The following were added to the section “Frequently Asked Questions”.
• Invalid Prescription/Service Reference Number Qualifier (455-EM)
• Should Other Payer Amount Recognized (556-J5) be included in the response from a secondary (or downstream) payer?
“Appendix D. What is the 11-digit Format for an NDC, UPC, or HRI?” was added.
37.19 VERSION C.1
Section “Document Scope” was updated to the Billing Unit Standard Implementation Guide Version 2.Ø.
Section “Version Identification System” was updated to move to a sequential enumerator.
Modifications have been brought forward to support claims processing functions under the Medicare Modernization Act (MMA).
Section “Business Environment” has been updated to include the Facilitator function in MMA.
“Figure 1. Participants” has been updated to include the Facilitator function in MMA.
Sections “Information Reporting Information”, “Information Reporting Reversal Information” and “Information Reporting Rebill
Information” reference a new appendix “Use Of Information Reporting (N1, N2, N3) Functionality For Medicare Part D Processing”.
Section “Transmission Structure”, subsections “Transmission Accepted; Transaction Captured Or Duplicate Of Capture”, and
“Transmission Accepted; Transaction Approved or Duplicate of Approved” the Response Insurance Segment was changed to
Optional for the Information Reporting Reversal transmission.
Section “Structure Quick Reference”, the following fields were added:
Insurance Segment
Field Field Name Mandatory or
Optional
99Ø-MG OTHER PAYER BIN NUMBER O
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER O
356-NU OTHER PAYER CARDHOLDER ID O
992-MJ OTHER PAYER GROUP ID O
Claim Segment
Field Field Name Mandatory or
Optional
88Ø-K5 TRANSACTION REFERENCE NUMBER O
Response Status Segment
Field Field Name Mandatory or
Optional
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER O***R***
992-MJ OTHER PAYER GROUP ID O***R***
88Ø-K5 TRANSACTION REFERENCE NUMBER O
In section “Specific Segment Discussion”, information on Medicare Part D processing was added to “Insurance Segment”,
“Coordination of Benefits /Other Payments Segment”, “Claim Segment”, and “Response Status Segment”.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 955 -
Section “Framework” added the responsibilities of the Facilitator.
Section “Standard Conventions”, subsection “Usage”, the Other Payer Processor Control Number (991-MH) and Other Payer Group
ID (992-MJ) were added to the Response Status Segment chart.
Section “Examples” - examples were added to show Medicare Part D transaction processing.
37.20 VERSION C.2
Section “Structure Quick Reference”, the following fields were added:
Insurance Segment
Field Field Name Mandatory or
Optional
359-2A MEDIGAP ID O
36Ø-2B MEDICAID INDICATOR O
361-2D PROVIDER ACCEPT ASSIGNMENT INDICATOR O
The following sections were updated to support these changes:
• “Transmission Examples”
Examples now use the Medigap ID (359-2A) instead of the Group ID (3Ø1-C1) to relay the designation for Medicare that this is a
Medicaid patient.
Compound Segment
Field Field Name Mandatory or
Optional
362-2G COMPOUND INGREDIENT MODIFIER CODE COUNT O
363-2H COMPOUND INGREDIENT MODIFIER CODE O***R***
The following sections were updated to support these changes:
• “Compound Segment”
• “Usage”
Response Pricing Segment
Field Field Name Mandatory or
Optional
575-EQ PATIENT SALES TAX AMOUNT O
574-2Y PLAN SALES TAX AMOUNT O
Updates were made to the following sections to support Patient Sales Tax Amount (575-EQ) and Plan Sales Tax Amount (574-2Y) fields:
• “Response Processing Guidelines”, “Captured Or Deferred”, “Business Function Of Capture”,
Valid Uses”
• “Pricing Guidelines”, “1ØØ% Copay”
• “Specific Segment Discussion”, “Response Segments”, “Response Pricing Segment”
Amount Of Copay/Coinsurance (518-FI) was split up into two fields Amount Of Copay (518-FI) and Amount Of Coinsurance (572-4U) and a
new field added.
Response Pricing Segment
Field Field Name Mandatory or
Optional
572-4U AMOUNT OF COINSURANCE O
573-4V BASIS OF CALCULATION-COINSURANCE O
The following sections were updated to support these changes:
• “Coordination of Benefits /Other Payments Segment”
Where denoted “values 1 to 5” was modified to “values 1 to 5 or 7”.
• “Claim Segment” – Partial Fill Fields updated to list Basis of Calculation – Coinsurance (573-4V)
• “Response Pricing Segment”
• “Transmission Examples”
• “FAQ”, “Should Other Payer Amount Recognized (556-J5) Be Included In The Response From A Secondary (Or Downstream)
Payer?”
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 956 -
Data Dictionary or External Code List changes include the above, as well as
• The addition of a value to Other Payer ID Qualifier (339-6C) for Medicare carrier number.
• Other Payer-Patient Responsibility Amount Qualifier (351-NP) value modifications to support the split of Amount of
Copay/Coinsurance into two fields.
• New Reject Codes (511-FB) were introduced. See the NCPDP External Code List for specifics.
Version C.2 also adds the updated ORDUR manual to this document as an appendix.
In Version B.Ø, the values in Patient Location (3Ø7-C7) were a mixture of patient locations and places of service. Patient Location was
renamed to Place of Service (3Ø7-C7) with new values assigned. A new field Patient Residence (384-4X) was added. The following sections
were modified for these fields
• Section “Transmission Examples” listing Patient Location (all contained value ØØ (Unspecified) were changed to Place Of Service
value 91 (Pharmacy = A duly licensed entity that delivers pharmaceutical goods or services for sale to or use by the final consumer).
• Since publication, CMS assigned a different value of Ø1, so the examples were modified in Version C.2.
• Section FAQ “Please Clarify The Definition Of The Patient Location (3Ø7-C7) Field.” was modified to explain the one field prior to
B.Ø and the two fields in B.Ø and above.
• This question was removed in Version C.2
37.21 VERSION C.3
Section “Structure Quick Reference”, the following fields were added:
Claim Segment
Field Field Name Mandatory or
Optional
391-MT PATIENT ASSIGNMENT INDICATOR (DIRECT MEMBER
REIMBURSEMENT INDICATOR)
O
Coordination of Benefits/Other Payments Segment
Field Field Name Mandatory or
Optional
392-MU BENEFIT STAGE COUNT O
393-MV BENEFIT STAGE QUALIFIER O***R***
394-MW BENEFIT STAGE AMOUNT O***R***
Response Pricing Segment
Field Field Name Mandatory or
Optional
392-MU BENEFIT STAGE COUNT O
393-MV BENEFIT STAGE QUALIFIER O***R***
394-MW BENEFIT STAGE AMOUNT O***R***
576-MQ AMOUNT ATTRIBUTED TO PRODUCT SELECTION QUALIFIER O
Note: Amount Attributed To Product Selection Qualifier (576-MQ) was added above Amount Attributed To Product Selection (519-FJ) on the
Response Pricing Segment chart so the qualifier appears with the field it qualifies.
The following sections were updated to support the Benefit Stage fields.
• “Specific Segment Discussion”, “Request Segments”, “Coordination of Benefits/Other Payments Segment”, “Medicare Part D”.
• “Specific Segment Discussion”, “Response Segments”, “Response Pricing Segment”, “Medicare Part D”. This section offers much
guidance for the use of the Benefit Stage fields and processing of transactions, including example excerpts of different
situations. Information about using initial benefit and catastrophic values in Benefit Stage Qualifier (393-MV) was also
added.
• “Repeating Fields – Maximum Occurrences”, subsection “Usage”.
Data Dictionary or External Code List changes include the above, as well as
• Reject Codes (511-FB) to support the new fields.
• Modifications to Other Payer-Patient Responsibility Amount Qualifier (351-NP).
o Value added to support
“Amount Attributed to Product Selection (519-FJ) for Non-preferred Formulary as reported by previous payer.
o Value modified to
“Amount Attributed to Product Selection (519-FJ) for Brand as reported by previous payer.”

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 957 -
Examples that supported the Amount Attributed To Product Selection (519-FJ) field now support the Amount Attributed To Product Selection
Qualifier (576-MQ). Examples that used values of Other Payer-Patient Responsibility Amount Qualifier (351-NP) that have changed were
modified to show the new value definition.
Version C.3 adds the guidance of the original “Two-Way Communication to Increase the Value of On-Line Messaging” document to this
document as an appendix. This document was modified to remove references to version 5.1 as it applies to any applicable version of the
NCPDP Telecommunication Standard Implementation Guide.
It was noted that in some of the examples, the Dispensing Fee is included in the Usual & Customary Charge, including some of the Workers’
Compensation examples. The examples and others were reviewed and corrected.
• “Standard Conventions”, “Separator Characters” excerpt example Usual And Customary Charge (426-DQ) was modified to $7Ø.ØØ
and $45.ØØ.
• “Billing – Transaction Code B1” Patient Paid Amount Submitted (433-DX) was removed and Usual And Customary Charge (426-DQ)
was modified to $86.7Ø.
• “Billing w/Submitted DUR Override - Transaction Code B1” Patient Paid Amount Submitted (433-DX) was removed and Usual And
Customary Charge (426-DQ) was modified to $71.65.
• “Billing w/DUR Conflicts - Transaction Code B1” Ingredient Cost Submitted (4Ø9-D9) was modified to $65.7Ø and Gross Amount
Due (43Ø-DU) was modified to $9Ø.7Ø. On the response, Patient Pay Amount (5Ø5-F5) was modified to $7.5Ø, Ingredient Cost
Paid (5Ø6-F6) was modified to $7Ø.7Ø, Dispensing Fee Paid (5Ø7-F7) was removed, and Total Amount Paid (5Ø9-F9) was
modified to $78.2Ø.
• “Service Billing - Transaction Code B1 (Ø1/Ø2)” Incentive Amount Submitted (438-E3) was added, Gross Amount Due (43Ø-DU)
was modified to $28.ØØ, and Basis of Cost Determination (423-DN) was removed. On the response, Incentive Amount Paid (421-
FL) was added and Total Amount Paid (5Ø9-F9) was modified to $28.ØØ. In the “Payment Reduced” response, Professional
Service Fee Paid (562-J1) and Total Amount Paid (5Ø9-F9) were modified to $15.ØØ.
• “Compounded Rx Billing - Transaction Code B1 (Ø1)” Compound Ingredient Basis of Cost Determination (49Ø-UE) and Basis of
Cost Determination (423-DN) were modified to Ø1 (AWP), Patient Paid Amount Submitted (433-DX) was removed, Usual And
Customary Charge (426-DQ) was modified to $31.15. On the response, Basis of Reimbursement Determination (522-FM) was
modified to 1 (Ingredient Cost Paid as Submitted). On “Billing Resubmission w/DUR Resolution” Compound Ingredient Basis of Cost
Determination (49Ø-UE) and Basis of Cost Determination (423-DN) were modified to Ø1 (AWP), Patient Paid Amount Submitted
(433-DX) was removed, and Usual And Customary Charge (426-DQ) was modified to $28.85. On the response, Basis of
Reimbursement Determination (522-FM) was modified to 1 (Ingredient Cost Paid as Submitted).
• “Billing, Partial Fill-Initial - Transaction Code B1” Patient Paid Amount Submitted (433-DX) was removed, and Usual And Customary
Charge (426-DQ) was modified to $37.65.
• “Billing, Partial Fill-Completion - Transaction Code B1” Usual And Customary Charge (426-DQ) was modified to $37.65.
• “Workers’ Compensation Billing - Transaction Code B1” Usual And Customary Charge (426-DQ) was modified to $66.5Ø.
• “Rebill - Transaction Code B3” Usual And Customary Charge (426-DQ) was modified to $52.85.
• “Prior Authorization Request And Billing - Transaction Code P1” Usual And Customary Charge (426-DQ) was modified to $72.5Ø.
On the response, Ingredient Cost Paid (5Ø6-F6) was modified to $72.5Ø, Dispensing Fee Paid (5Ø7-F7) was removed, and Total
Amount Paid (5Ø9-F9) was modified to $62.5Ø. On the duplicate response, Ingredient Cost Paid (5Ø6-F6) was modified to $72.5Ø,
Dispensing Fee Paid (5Ø7-F7) was removed, and Total Amount Paid (5Ø9-F9) was modified to $62.5Ø.
• “Prior Authorization Request Only - Transaction Code P4” Usual And Customary Charge (426-DQ) was modified to $72.5Ø.
• “Billing With DUR Segment Using Co-Agent Fields - Transaction Code B1 (Ø1/Ø2)” Usual And Customary Charge (426-DQ) was
modified to $71.65.
• “Billing - Transaction Code B1 With Additional Documentation Segment” Usual And Customary Charge (426-DQ) was modified to
$7Ø.ØØ.
• “Billing - Transaction Code B1 With Facility Information” Usual And Customary Charge (426-DQ) was modified to $7Ø.ØØ.
• “Billing - Transaction Code B1 With Additional Documentation and Facility Information” Usual And Customary Charge (426-DQ) was
modified to $7Ø.ØØ.
• “Billing - Transaction Code B1 With Narrative Information” Usual And Customary Charge (426-DQ) was modified to $7Ø.ØØ.
• “Billing - Transaction Code B1 With Facility Information And Narrative Information” Usual And Customary Charge (426-DQ) was
modified to $7Ø.ØØ.
• “Billing - Transaction Code B1 With Additional Documentation And Narrative Information” Usual And Customary Charge (426-DQ)
was modified to $7Ø.ØØ.
• “Primary Claim From Pharmacy To PDP” Usual And Customary Charge (426-DQ) was modified to $9Ø.ØØ.
37.22 VERSION C.4
Section “Specific Segment Discussion”, subsection “Request Segments”, subsection “Claim Segment” includes “CPT Use” with guidance for
reporting CPT codes in billing.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 958 -
“Specific Segment Discussion”, “Request Segments”, “Coordination of Benefits/Other Payments Segment” removed the verbiage that forced a
composite when there were more than 4 payers. When there are more than 9 payers (rarely if ever), the claim to the subsequent payers
should be handled manually.
Section FAQ “Other Coverage Code (3Ø8-C8) And Coordination Of Benefits” was modified to remove the chart that showed a composite
example and includes information on when there are more than 9 payers.
Section “Repeating Fields – Maximum Occurrences”, subsection “Usage” was modified from a recommended support count of ≤ 3 to support
of 9 for the Coordination of Benefits/Other Payments Count (337-4C).
The Data Dictionary includes new values for Other Payer Coverage Type (338-5C).
Ø4 = Quaternary
Ø5 = Quinary
Ø6 = Senary
Ø7 = Septenary
Ø8 = Octonary
Ø9 = Nonary
Section “Structure Quick Reference”, the following fields were added:
Insurance Segment
Field Field Name Mandatory or
Optional
997-G2 CMS PART D DEFINED QUALIFIED FACILITY O
Claim Segment
Field Field Name Mandatory or
Optional
995-E2 ROUTE OF ADMINISTRATION O
Appendix “Route of Administration Mapping” was added to assist in transition from the NCPDP code values of this field to the SNOMED
values.
(See Compound Segment – Compound Route of Administration (452-EH) below.)
A new value of “Intravenous” was added to Route of Administration (995-E2).
Section “Structure Quick Reference”, the following field has been renamed:
Was: Unit Dose Indicator (429-DT)
Claim Segment
Field Field Name Mandatory or
Optional
429-DT SPECIAL PACKAGING INDICATOR O
A new value of “Multi-drug compliance packaging is packaging that may contain drugs from multiple manufacturers combined to ensure
compliance and safe administration” for Special Packaging Indicator (429-DT) was added. Reject Code (511-FB) values were adjusted for the
new field name.
New values for Submission Clarification Code (42Ø-DK) were added.
• 13 = Long Term Care Leave of Absence-The pharmacist is indicating that the cardholder requires a short-fill of a prescription due to
a leave of absence from the Long Term Care (LTC) facility.
• 14 = Long Term Care Replacement Medication - Medication has been contaminated during administration in a Long Term Care
setting.
• 15 = Long Term Care Emergency box (kit) or automated dispensing machine – Indicates that the transaction is a replacement
supply for doses previously dispensed to the patient after hours.
• 16 = Long Term Care Emergency supply remainder-Indicates that the transaction is for the remainder of the drug originally begun
from an Emergency Kit.
• 17 = Long Term Care Patient Admit/Readmit Indicator=Indicates that the transaction is for a new dispensing of medication due to
the patient’s admission or readmission status.
Section “Structure Quick Reference”, the following field is no longer supported:
Compound Segment

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 959 -
Field Field Name Mandatory or
Optional
452-EH COMPOUND ROUTE OF ADMINISTRATION M
The External Code List adjusts Reject Code (511-FB) values assigned to the Compound Route of Administration to Route of Administration
(995-E2).
Compound usage has been modified to provide guidance on the use of Route of Administration (995-E2). References to Compound Route of
Administration have been modified to Route of Administration in verbiage and in examples:
• “Compounded Rx Billing - Transaction Code B1 (Ø1)”
• “Billing Resubmission w/DUR Resolution”
Section “Structure Quick Reference”, the following fields were added:
Claim Segment
Field Field Name Mandatory or
Optional
996-G1 COMPOUND TYPE O
Coordination of Benefits/Other Payments Segment
Field Field Name Mandatory or
Optional
993-A7 INTERNAL CONTROL NUMBER O
Response Pricing Segment
Field Field Name Mandatory or
Optional
577-G3 ESTIMATED GENERIC SAVINGS O
Response Status Segment
Field Field Name Mandatory or
Optional
993-A7 INTERNAL CONTROL NUMBER O
987-MA URL O
Section “Appendix G. Two-Way Communication to Increase the Value of On-Line Messaging” added guidance for two new Reject Codes (511-
FB) that add further clarification when Reject Code (511-FB) = “75 “ (Prior Authorization Required).
“G4 “ Physician must contact plan
“G5 “ Pharmacist must contact plan
Guidance was also added that the field URL (987-MA) if available could be sent to provide an electronic address for additional prior
authorization information.
Also in section “Appendix G. Two-Way Communication to Increase the Value of On-Line Messaging” guidance was added for two new Reject
Codes (511-FB) that add further clarification when Reject Code (511-FB) = “4Ø ” (Pharmacy Not Contracted with Plan on Date of Service).
“G6 “ Pharmacy Not Contracted in Specialty Network
“G7 “ Pharmacy Not Contracted in Home Infusion Network
“G8 “ Pharmacy Not Contracted in Long Term Care Network
“G9 “ Pharmacy Not Contracted in 9Ø Day Retail Network (this message would be used when the pharmacy is not contracted to
provide a 9Ø days supply of drugs)
Also in section “Appendix G. Two-Way Communication to Increase the Value of On-Line Messaging” other existing Reject Codes (511-FB)
correlate to “76 “ (Plan Limitations Exceeded).
“7Ø “ Product/Service Not Covered “Specific Plan Exclusion”
“6Ø “ Product/Service Not Covered for Patient Age Maximum (or Minimum) Age = NN Years
“61 “ Product/Service Not Covered for Patient Gender
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 960 -
“AG “ Days Supply Limitations for Product/Service Maximum Days Supply = XXX Days
“M4 “ Prescription/Service Reference Number/Time Limit Exceeded Define the number of prescriptions allowed within a
given time period
“RN “ Plan Limits Exceeded on Intended Partial Fill Values Maximum Days Supply = XXX Days
“66 “ Patient Age Exceeds Maximum Age Maximum Patient Age = XX Years
As a guide to implementers, raw data streams were added to certain examples.
37.23 VERSION D.Ø
37.23.1 AUGUST 2ØØ6 DERF APPROVALS
Definitions for “Copay/Amount of Copay”, “Coinsurance/Amount of Coinsurance”, and “Patient Financial Responsibility” were added to section
“Response Processing Guidelines”, “Pricing Guidelines”, “Definitions”. The term “1ØØ% Copay” was modified to “Patient Financial
Responsibility” throughout the document. Where appropriate, the term “copay” added “coinsurance” to offer clarification. Sections included
“Response Processing Guidelines”, “Pricing Guidelines”, “Business Function of Capture”, and “Business Functions Not Supported For
Capture”. Also modified for copay/coinsurance clarification was sections “Specific Segment Discussion”, “Response Segments”, “Response
Pricing Segment”. The new field Amount Attributed to Provider Network Selection (133-UJ) was added to the section “Patient Pay Amount
(5Ø5-F5) Formula”. The section of “Example #2” was modified and the section “Partial Fill Fields” was modified.
The section “Transmission Examples” was modified from references of copay to patient financial responsibility.
• “Billing – Transaction Code B1 – Coordination of Benefits – Scenario 2: Pharmacy Bills Secondary Insurance”
• “Scenario 2 Response: Secondary Insurance Pays The Claim Submitted With Total Patient Pay Amount”
• “Scenario 3: Pharmacy Bills Secondary Insurance”
• “Scenario 3 Response: Secondary Insurance Pays The Claim Submitted With The “Pieces” Of Patient Pay Amount”
The following “Frequently Asked Questions” were modified from references of copay to patient financial responsibility.
• “How Is The Pregnancy Indicator (335-2C) Used In The Processor’s System?”
• “Why Would The Response Pricing Segment Be Used (Situational) In A Billing Transaction (Or Other Transaction) When A
Processor Returns A “C”aptured Response?”
• “Patient Financial Responsibility And Negative Amounts”
• “Other Coverage Code (3Ø8-C8) And Coordination Of Benefits”
• “Transaction Fee Charge”
• “Truncation Of Dollar Fields”
• “Should Other Payer Amount Recognized (556-J5) Be Included In The Response From A Secondary (Or Downstream) Payer?”
The field Patient Pay Amount (5Ø5-F5) definition was modified to include coinsurance - “Amount that is calculated by the processor and
returned to the pharmacy as the TOTAL amount to be paid by the patient to the pharmacy; the patient’s total cost share, including copayment,
coinsurance, amounts applied to deductible, over maximum amounts, penalties, etc.”
The value of Prior Authorization Type Code (461-EU) “4” was modified to “Exemption from copay and/or coinsurance”.
Section “Structure Quick Reference”, the following field was modified:
Preferred Product Copay Incentive (555-AT) field name was modified to Preferred Product Cost Share Incentive.
Response Claim Segment
Field Field Name Mandatory or
Situational
555-AT PREFERRED PRODUCT COST SHARE INCENTIVE S***R***
Section “Structure Quick Reference”, the following fields were added:
Response Pricing Segment
Field Field Name Mandatory or
Situational
128-UC SPENDING ACCOUNT AMOUNT REMAINING S
129-UD HEALTH PLAN-FUNDED ASSISTANCE AMOUNT S
Section Specific Segment Discussion”, “Response Segments”, “Response Pricing Segment” added guidance for Spending Account Amount
Remaining (128-UC) and Health Plan-Funded Assistance Amount (129-UD).
Basis of Cost Determination (423-DN) and Basis of Reimbursement Determination (522-FM) support the situation of “Not used in reporting
patient financial responsibility billing.”
Note: After the November 2ØØ6 pricing and qualifier modifications were made, it was determined that Basis of Cost Determination
(423-DN) and Basis of Reimbursement Determination (522-FM) needed to be available in patient financial responsibility billing so
the situation of “Not used in reporting patient financial responsibility billing” was removed.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 961 -
Other Payer-Patient Responsibility Amount Qualifier (351-NP) added a value for “Amount attributed to Health Plan Assistance Amount (129-
UD) as reported by previous payer”.
A section has been added to section Specific Segment Discussion”, “Response Segments”, “Response Pricing Segment” for “Healthcare
Reimbursement Account (HRA), Health Savings Accounts (HSAs), and Healthcare Flexible Spending Account (FSA)”. “Patient Pay Amount
(5Ø5-F5) Formula” has been updated to include Health Plan-Funded Assistance Amount (129-UD).
Section “Structure Quick Reference”, the following fields were added:
Response Pricing Segment
Field Field Name Mandatory or
Situational
133-UJ AMOUNT ATTRIBUTED TO PROVIDER NETWORK SELECTION S
134-UK AMOUNT ATTRIBUTED TO PRODUCT SELECTION/BRAND DRUG S
135-UM AMOUNT ATTRIBUTED TO PRODUCT SELECTION/NON-PREFERRED
FORMULARY SELECTION
S
136-UN AMOUNT ATTRIBUTED TO PRODUCT SELECTION/BRAND NON-
PREFERRED FORMULARY SELECTION
S
These fields were added to the section “Patient Pay Amount (5Ø5-F5) Formula” found under “Specific Segment Discussion”, “Response
Segments”, “Response Pricing Segment”.
Other Payer-Patient Responsibility Amount Count (353-NR) has been increased in size from 1 to 2 numeric. The max count has been
increased from 5 to 25 to allow for more occurrences of the detailed patient responsibility fields from a previous payer. Other Payer-Patient
Responsibility Amount Qualifier (351-NP) added additional values, which caused the field size to be increased from A/N 1 to A/N 2. Values 1-9
added a preceding Ø (e.g. Ø1, Ø2, Ø3). The qualifier supports new values for “1Ø” (Amount Attributed to Provider Network Selection (133-UJ))
and “11” (Amount Attributed to Product Selection/Brand Non-Preferred Formulary Selection (136-UN)). The existing values of “Ø2” and “Ø7”
were changed. “Ø2” (Amount Attributed to Product Selection/Brand Drug (134-UK)), “Ø7” (Amount Attributed to Product Selection/Non-
Preferred Formulary Selection (135-UM)).
Section “Structure Quick Reference”, the following fields were deleted due to the specific fields added above:
Response Pricing Segment
Field Field Name Mandatory or
Situational
576-MQ AMOUNT ATTRIBUTED TO PRODUCT SELECTION QUALIFIER S
519-FJ AMOUNT ATTRIBUTED TO PRODUCT SELECTION S
All references to these two fields have been removed, with the specific new field added.
The section “Long Term Care Transition, Emergency Fill and Change in level of Care Messaging for Rejected and Paid Claims” was added to
“Appendix G. Two-Way Communication to Increase the Value of On-Line Messaging”. New Reject Codes (511-FB) and new Approved
Message Codes (548-6F) were added to support this transition processing. The Response Prior Authorization Segment is optional for rejected
Billing and Rebill transactions (see section “Response Segment Usage Matrix”, “Transaction Rejected”).
In section “Specific Segment Discussion”, “Response Segments”, “Response Prior Authorization Segment” guidance was added about the
need for a prior authorization in transition processing.
Compound Processing – the two alternatives (Scenario A (Most expensive legend drug) and Scenario B (Billing codes)) are no longer
supported. When Telecom Version 5.Ø was created, the intent for processing compounds was to use one method – Option 1 (preferred) - the
use of the Compound Segment with the Claim Segment. Two alternatives (Scenario A (Most expensive legend drug) and Scenario B (Billing
codes)) were inadvertently left in the implementation guide in a Frequently Asked Question. The support of multiple methods was never the
intention. Multiple methods of billing compounds create problems in the coordination of benefits process when one payer requires the
compound claim submitted using one method and downstream payers use another method. The preferred method will be the only method
allowed. The following sections have been modified.
• “Specific Segment Discussion”, “Request Segments”, “Compound Segment”
• Also in this section “Two Options To Designate A Compound” has been removed
• “Frequently Asked Questions”, “Quantity Dispensed (442-E7) And Compounds” – this question has been removed since it dealt
with alternate options for compounds
• “APPENDIX F. ORDUR (Online Real-time Drug Utilization Review)”, “Information Categories”, “Prescription Information” –
removed reference to legend drug
• A new example “Compounded Rx Billing - Transaction Code B1 (Ø1) – Coordination of Benefits Scenario” was added
A new value was added to Patient ID Qualifier (331-CX) – “Ø6” (Medicaid ID).
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 962 -
The definition of Other Payer Amount Paid (431-DV) was modified to “Amount of any payment known by the pharmacy from other sources”
(removed coupon reference).
The definition of Other Payer Amount Recognized (566-J5) was modified to “Total amount recognized by the processor of any payment from
another source” (removed coupon reference).
Section “Structure Quick Reference”, the following fields were deleted because the National Provider ID (NPR) rule did not name a location
field and these fields were specifically added in anticipation of the NPI location:
Prescriber Segment
Field Field Name Mandatory or
Situational
467-1E PRESCRIBER LOCATION CODE S
469-H5 PRIMARY CARE PROVIDER LOCATION CODE S
Section “Business Environments”, “Participants” has added verbiage about payer-to-payer usage of this document. Figure 1 has been
renamed “Provider/Adjudicator Participants”. “Figure 2. Between Adjudicator Participants” has been added. Original Figure 2 has been
renamed “Figure 3. Connectivity between participants”.
Other Coverage Code (3Ø8-C8) had the following changes:
• Delete value of 5 (Managed care plan denial)
• Delete value of 6 (Other coverage denied-not participating provider),
• Delete value of 7 (Other coverage exists-not in effect on Date of Service)
as they are duplicates to value 3 (Other coverage billed – claim not covered).
• Change description of value 3 from (Other coverage billed – claim rejected) to (Other coverage billed – claim not covered)
• Change description of value 8 from (Claim is billing for copay) to (Claim is billing for patient financial responsibility only)
• Change description of value Ø from (Not specified) to (Not specified by patient)
• Guidance was added to section “Specific Segment Discussion”, “Request Segments”, “Claim Segment”, with section added
“Other Coverage Code (3Ø8-C8).
• “Transmission Examples” have been modified.
• “Frequently Asked Question”, “Other Coverage Code (3Ø8-C8) And Coordination Of Benefits” was removed. Applicable
information was moved into “Specific Segment Discussion”, “Request Segments”, “Claim Segment”, “Other Coverage Code
(3Ø8-C8).
Dispense As Written/Product Selection Code (4Ø8-D8) modified value “9” (Other – Reserved and not in use) to (Substitution Allowed By
Prescriber but Plan Requests Brand - Patient's Plan Requested Brand Product To Be Dispensed - This value is used when the prescriber has
indicated, in a manner specified by prevailing law, that generic substitution is permitted, but the plan's formulary requests the brand product.
This situation can occur when the prescriber writes the prescription using either the brand or generic name and the product is available from
multiple sources).
The following fields added a qualifier for the HCIdea Number:
• Prescriber ID Qualifier (466-EZ)
• Primary Care Provider ID Qualifier (468-2E)
• Service Provider ID Qualifier (2Ø2-B2)
The Date of Service (4Ø1-D1) definition was modified to “Identifies date the prescription (was filled) or (professional service rendered) or
(subsequent payer began coverage following Part A expiration in a long-term care setting only).”
The Submission Clarification Code (42Ø-DK) added a value of “19” (Split Billing – indicates the quantity dispensed is the remainder billed to a
subsequent payer when Medicare Part A expires. Used only in long-term care settings.) Guidance for “Split Billing in Long Term Care” was
added to “Specific Segment Discussion”, “Request Segments”, “Claim Segment”.
Also in this section, Originally Prescribed Product/Service Code (445-EA) and Originally Prescribed Quantity (446-EB) added verbiage
removed from the Data Dictionary (“The Originally Prescribed Product/Service Code (445-EA) and the Originally Prescribed Quantity (446-EB)
are used to provide necessary data to calculate the exact difference in cost between the prescribed product and the dispensed product. The
Originally Prescribed Quantity (446-EB) is for use with therapeutic interchange only.”)
Section “Document Scope” modified the reference to the Billing Unit Standard to remove the specific version. Like the Data Dictionary and the
External Code List, the most recent version is to be used.
37.23.2 NOVEMBER 2ØØ6 APPROVALS
The business transaction of Predetermination Of Benefits has been add to all appropriate sections, with an explanation section
“Predetermination Of Benefits Information”. An example has been added. A frequently asked question has been added.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 963 -
In preparation for the use of the National Provider ID (NPI), some examples within “Transmission Examples” have been modified.
The length of fields Prescription/Service Reference Number (4Ø2-D2) and Associated Prescription/Service Reference Number (456-EN) has
been increased to 12 bytes. These fields are numeric with a length of 12.
Claim Billing, Claim Rebill, Reversal, Information Reporting, Information Reporting Reversal, and Information Reporting Rebill transactions for
Medicare Part D are limited to one transaction per transmission.
Additional logic has been added to duplicate processing. See section “Response Processing Guidelines”, “Duplicate Transactions.” The
Coordination of Benefits/Other Payments Segment has been added to the Claim Reversal and Service Reversal transactions. Additional
guidance has been added to the Coordination of Benefits/Other Payments Segment sections in Claim Billing or Encounter, Claim Rebill,
Service Billing, and Service Rebill transactions.
Additional Message Information (526-FQ) size has been modified and the field repeats with a count, a qualifier, and the ability to use a
continuation character. See section “Specific Segment Discussion”, “Response Segments”, “Response Status Segment”, “Additional Message
Information Fields”.
In Version D.Ø and above, the Service Billings have their own Transaction Code (S1, S2, S3). The Transaction Code is at the transmission
level. Claim and service billings are associated (using the Associated Prescription/Service Reference Number (456-EN) and Associated
Prescription/Service Date (457-EP), but they must appear in separate transmissions. Drug product billings are designated by Transaction
Code = B1 (Billing) and Prescription/Service Reference Number Qualifier = 1 (Rx Billing). Service billings are designated by Transaction Code
= S1 (Service Billing) and Prescription/Service Reference Number Qualifier = 2 (Service Billing).
Note that in other Transaction Codes (Prior Authorizations, Information Reporting, and Controlled Substance Reporting), the differentiation of
claim versus service remains at the transaction level. For example, drug product transactions are designated by Transaction Code = “P1”
(Prior Authorization Request And Billing) and Prescription/Service Reference Number Qualifier = 1 (Rx Billing). Service billings are designated
by Transaction Code = P1 (Prior Authorization Request And Billing) and Prescription/Service Reference Number Qualifier = 2 (Service Billing).
Clarification has been added to the document to support these changes.
Dispense As Written/Product Selection Code (4Ø8-D8) clarified value “1” (Substitution Not Allowed by Prescriber).
Prior Authorization Type Code (461-EU) changed value 4 (Exemption from Copay) to (Exemption from Copay and/or Coinsurance) and added
value 9 (Emergency Preparedness - Code used to override claim edits during an emergency situation.)
Other Payer-Patient Responsibility Amount Qualifier (351-NP) supports new values for “12” (Amount Attributed to Coverage Gap (137-UP) that
was collected from the patient due to a coverage gap). A new value of “13” (Amount Attributed to Processor Fee (571-NZ)) has been added.
Basis of Reimbursement Determination (522-FM) supports new values for 14 (Other Payer-Patient Responsibility Amount - Indicates
reimbursement was based on the Other Payer Patient Responsibility Amount (352-NQ)), 15 (Patient Pay Amount - Indicates reimbursement
was based on the Patient Pay Amount (5Ø5-F5)), 16 (Coupon Payment – Indicates reimbursement was based on the Coupon Value Amount
(487-NE) submitted or the coupon amount determined by the processor).
Percentage Sales Tax Basis Submitted (484-JE) and Percentage Sales Tax Basis Paid (561-AZ) no longer support value “Ø1” (Gross Amount
Due).
Tax Exempt Indicator (557-AV)
• no longer supports value “2” (Not Tax Exempt)
• value “1” was modified to “Payer/Plan is Tax Exempt (The Payer/Plan is not responsible for tax. The patient may be charged
tax.)”
• value “3” was added “Patient is Tax Exempt (The patient cannot be charged tax.)”
• value “4” was added “Payer/Plan and Patient are Tax Exempt (Neither the payer/plan nor the patient can be charged tax.)”
Certain fields with Ø or blank values or value of 99 or values signifying “Not Specified” were modified in the NCPDP “External Code List”,
especially for Telecommunication Standard Implementation Guide usage. Please see the NCPDP “External Code List” document. Examples
were modified to correspond with these changes.
The NCPDP Data Dictionary and External Code List were updated to provide definitions to values as more clarification for their usage. See
these documents for more information.
Section “Structure Quick Reference”, the following fields were added:
Insurance Segment
Field Field Name Mandatory or
Situational
115-N5 MEDICAID ID NUMBER S
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 964 -
116-N6 MEDICAID AGENCY NUMBER S
Claim Segment
Field Field Name Mandatory or
Situational
114-N4 MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
S
147-U7 PHARMACY SERVICE TYPE S
Pricing Segment
Field Field Name Mandatory or
Situational
113-N3 MEDICAID PAID AMOUNT S
Workers’ Compensation Segment
Field Field Name Mandatory or
Situational
117-TR BILLING ENTITY TYPE INDICATOR S
118-TS PAY TO QUALIFIER S
119-TT PAY TO ID S
12Ø-TU PAY TO NAME S
121-TV PAY TO STREET ADDRESS S
122-TW PAY TO CITY ADDRESS S
123-TX PAY TO STATE/PROVINCE ADDRESS S
124-TY PAY TO ZIP/POSTAL ZONE S
125-TZ GENERIC EQUIVALENT PRODUCT ID QUALIFIER S
126-UA GENERIC EQUIVALENT PRODUCT ID S
Section “Specific Segment Discussion” added a subsection for the Workers’ Compensation Segment.
Response Insurance Segment
Field Field Name Mandatory or
Situational
115-N5 MEDICAID ID NUMBER S
116-N6 MEDICAID AGENCY NUMBER S
3Ø2-C2 CARDHOLDER ID S
The definition for Cardholder ID (3Ø2-C2) was modified. See the NCPDP “Data Dictionary”.
Response Patient Segment
Field Field Name Mandatory or
Situational
111-AM SEGMENT IDENTIFICATION M
31Ø-CA PATIENT FIRST NAME S
311-CB PATIENT LAST NAME S
3Ø4-C4 DATE OF BIRTH S
This segment is variable length.
To support enhancements for eligibility checking, specifically for Medicare Part D usage, the following segment and fields have been added.
Guidance has been added to the section “Eligibility Verification Information”. In addition subsections have been added for the segment in the
section “Specific Segment Discussion”.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 965 -
Response Insurance Additional Information Segment
Field Field Name Mandatory or
Situational
111-AM SEGMENT IDENTIFICATION M
139-UR MEDICARE PART D COVERAGE CODE M
138-UQ CMS LOW INCOME COST SHARING (LICS) LEVEL S
24Ø-U1 CONTRACT NUMBER S
926-FF FORMULARY ID S
757-U6 BENEFIT ID S
14Ø-US NEXT MEDICARE PART D EFFECTIVE DATE S
141-UT NEXT MEDICARE PART D TERMINATION DATE S
This segment is variable length.
Additional Message Information (526-FQ) size has been modified and the field repeats with a count, a qualifier, and the ability to use a
continuation character. Additional guidance can be found in section “Specific Segment Discussion”, “Response Segments”, “Response Status
Segment”.
Response Status Segment
Field Field Name Mandatory or
Situational
13Ø-UF ADDITIONAL MESSAGE INFORMATION COUNT S
132-UH ADDITIONAL MESSAGE INFORMATION QUALIFIER S***R***
526-FQ ADDITIONAL MESSAGE INFORMATION (existing field) S***R***
131-UG ADDITIONAL MESSAGE INFORMATION CONTINUITY S***R***
Response Claim Segment
Field Field Name Mandatory or
Situational
114-N4 MEDICAID SUBROGATION INTERNAL CONTROL
NUMBER/TRANSACTION CONTROL NUMBER (ICN/TCN)
S
Response Pricing Segment
Field Field Name Mandatory or
Situational
137-UP AMOUNT ATTRIBUTED TO COVERAGE GAP S
148-U8 INGREDIENT COST CONTRACTED/REIMBURSABLE AMOUNT S
149-U9 DISPENSING FEE CONTRACTED/REIMBURSABLE AMOUNT S
The following fields have been moved from the Response Status Segment to a new Response Coordination of Benefits/Other Payers
Segment. Subsections have been added for each segment in the section “Specific Segment Discussion”.
Response Status Segment
Field Field Name Mandatory or
Situational
355-NT OTHER PAYER ID COUNT S
339-6C OTHER PAYER ID QUALIFIER S***R***
34Ø-7C OTHER PAYER ID S***R***
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER S***R***
356-NU OTHER PAYER CARDHOLDER ID S***R***
992-MJ OTHER PAYER GROUP ID S***R***
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 966 -
Response Coordination of Benefits/Other Payers Segment
Field Field Name Mandatory or
Situational
111-AM SEGMENT IDENTIFICATION M
355-NT OTHER PAYER ID COUNT M
338-5C OTHER PAYER COVERAGE TYPE M***R***
339-6C OTHER PAYER ID QUALIFIER S***R***
34Ø-7C OTHER PAYER ID S***R***
991-MH OTHER PAYER PROCESSOR CONTROL NUMBER S***R***
356-NU OTHER PAYER CARDHOLDER ID S***R***
992-MJ OTHER PAYER GROUP ID S***R***
142-UV OTHER PAYER PERSON CODE S***R***
127-UB OTHER PAYER HELP DESK PHONE NUMBER S***R***
143-UW OTHER PAYER PATIENT RELATIONSHIP CODE S***R***
144-UX OTHER PAYER BENEFIT EFFECTIVE DATE S***R***
145-UY OTHER PAYER BENEFIT TERMINATION DATE S***R***
This segment is variable length.
Clarifications to Coupon processing were added to section “Specific Segment Discussion”, “Request Segments”, “Coupon Segment”.
Additional coupon questions were added to section “Frequently Asked Questions”. Example “Billing w/Coupon - Transaction Code B1—
Primary Billing to Coupon Processor” was deleted. The remaining coupon examples in section “Transmission Examples” were clarified and a
new example added for reducing copay.
In Version C.4, Internal Control Number was added. Upon review, it was determined the field was in an incorrect position in the segment. It is a
repeating field. This has been corrected. Clarification has been added to section “Specific Segment Discussion”, “Request Segments”,
“Coordination of Benefits/Other Payments Segment”.
Coordination of Benefits/Other Payments Segment
Field Field Name Mandatory or
Situational
993-A7 INTERNAL CONTROL NUMBER S***R***
Other Payer Coverage Type (338-5C) no longer supports value “98” (Coupon) and “99” (Composite).
Other Payer ID Qualifier (339-6C) no longer supports value “Ø9” (Coupon).
Other Payer Amount Paid Qualifier (342-HC) no longer supports values “Ø8” (Sum of all reimbursements), “98” (Coupon), and “99” (Other).
Errant references to section “Segment Quick Reference” were modified to correctly state section “Structure Quick Reference”.
Protocol Document Related Changes:
The work of the past years on the Protocol Document, to define the Segments and the Field Situations for use has been incorporated into this
version.
• The document has been reviewed for verbiage of “may” “might”, “could”, and other less specific language. Where appropriate,
the verbiage has been modified to “must”, “will”, etc.
• In each of the transaction sections “Eligibility Information”, “Reversal Information”, “Rebill Information”, etc, the diagrams have
been reviewed according to Protocol Document decisions. Segments have been added or removed as applicable to the
specific transaction. Where appropriate, the “claim” versus the “service” has been split out into separate diagrams for clarity.
• The section “Claim and Service Billing Information” has been modified to “Claim, Encounter, and Service Billing Information”.
Encounter information has been added to this section.
• In each of the transaction information sections (e.g. “Claim or Service Billing Information (Professional Pharmacy Service)”),
response diagrams have been added as appropriate.
• Information Reporting and Information Reporting Rebill transactions no longer support a “P” (Paid) or “D” (Duplicate of Paid)
response since no business need was brought forward.
• In section “Business Functions”, the last paragraph has been added (some sentences brought from the Protocol Document).
• In section “Business Functions”, a business function was added to the list of Medicaid Subrogation. A subsection was added
“Medicaid Subrogation” to explain the situational charts for this function brought from the Protocol Document.
• In section “Prior Authorization Transaction Discussion”, the charts were updated to match the situations on the fields.
• In section “Controlled Substance Reporting Information”, “Controlled Substance Reporting Reversal Information”, and
“Controlled Substance Reporting Rebill Information” a paragraph has been added that at this time, the business cases for this
transaction are not fully defined.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 967 -
• The section “Appendix F. ORDUR (Online Real-time Drug Utilization Review)” guidance on Information Reporting transactions
has been aligned with sections “Information Reporting Information”, for current known business usages.
• In sections “Controlled Substance Reporting Information”, “Controlled Substance Reporting Reversal Information”, and
“Controlled Substance Reporting Rebill Information” a paragraph has been added that at this time, the business cases for this
transaction are not fully defined.
• The section “Terminology Used Throughout” has been added from the Protocol Document.
• In section “Specific Segment Discussion”, “Request Segments”, “Claim Segment”, “Partial Fill” the section “Recommendations”
was clarified further.
• The section “Repeating Fields – Maximum Occurrences”, subsection “Usage” has been updated with the maximum
occurrences of count and counter fields to match the situations described in the fields.
• In section “Response Pricing Guidelines”, the subsections of “Pricing Guidelines” and “Captured”, “Business Function of
Capture” additional guidance has been added. The subsections of “Captured” and “Deferred” have been split.
• In section “Standard Conventions”, the subsection “Explanation of Segment And Field Designations” has been added.
• In section “Transmission Examples”, the subsection “Example Conventions” has been added. Examples were reviewed and
aligned with situations defined in each transaction.
Modifications to section “Frequently Asked Questions” due for version D.Ø applicability
All questions have been given a topic title.
Deleted FAQs:
• “When Using Version 5 And Above, Which Segments Are Used?”
• “When Using Version 5 And Above, Which Fields Are Used?”
• “How Do I Handle Sets Of Repeating Fields?”
• “How Do I Use The ‘Software Vendor/Certification ID’ Field?”
• “How Do I Format The PA/MC Fields?”
• “How Do I Return Additional Information?”
• “How Do I Indicate That A Patient Is Pregnant?”
• “How Is The Pregnancy Indicator (335-2C) Used In The Processor’s System?”
• “What Are The Recommended Guidelines For Supporting Multiple Claim Or Service Reversal (B2) Transactions Within A
Transmission?”
• “What Are The Recommended Guidelines For Supporting Multiple Rebill (B3, N3, C3) Transactions Within A Transmission?”
• “The Initial Transaction Is A Prior Authorization Request Only. The Pharmacy Submits A Prior Authorization Inquiry For A Status.
What Is The Difference Between A Prior Authorization Inquiry Response Of “C” (Capture) And “A” (Approved)?”
• “Once The Prior Authorization Number Is Assigned, On Subsequent Refills, Can You Just Submit The Prior Authorization In The
Prior Authorization Number Submitted (462-EV) Field In The Claim Segment, Or Do You Need To Keep Sending The Prior
Authorization Segment With The Prior Authorization Value In The Prior Authorization Number Assigned Field?”
• “Now That The Qualifier Is Available, Should The UPC And HRI Values Be Sent In Their Native Format Instead Of Being
Reformatted To An 11-Digit Value?”
• “Fill Number (4Ø3-D3) – Default?”
• “Product/Service ID Field (4Ø7-D7) And Compounds In Reversals”
• “Can A Cardholder ID Contain Symbols Such As Hyphens And Apostrophes?”
• “1ØØ% Patient Financial Responsibility And Negative Amounts”
• “Reject Code For Incorrect Other Payer Amount Paid Count (341-HB)”
• “Reject Code For Incorrect Submission Clarification Code Count (354-NX)?”
• “Reject Code For Incorrect Other Payer-Patient Responsibility Amount Code (352-NQ) And Qualifier (351-NP)?”
• “Does Usual And Customary Charge (426-DQ) Include A Dispensing Fee?”
• “Transaction Fee Charge”
• “Truncation Of Dollar Fields”
• “Should The Product/Service ID Qualifier Be Ø3/NDC Or Is Blank Or ØØ/Unspecified Acceptable?”
• “How Are Compounded Pills Submitted?”
• “Must The Mandatory Data Elements Be Sent In The Order That They Are Listed In The Implementation Guide?”
• “Is There An Order To The Way Segments Must Appear In A Transmission?”
• “DUR Additional Text (57Ø-NS) Field”
• “Prior Authorization Request And Billing – Deferred”
• “Should Other Payer Amount Recognized (556-J5) Be Included In The Response From A Secondary (Or Downstream) Payer?”
• “When The Value 99=Composite Is Used In The Other Payer Coverage Type, What Is Placed In The Other Payer ID? Is It Not
Sent? “
Modified Questions:

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 968 -
• “When Using Version 5 And Above, Which Segments Are Used?” to “What Are My Sources For Finding Notable Changes From
Version 5.1 to Version D.Ø?”
• “Invalid Prescription/Service Reference Number Qualifier (455-EM)”
Clarified Response:
• “How Do I Reverse Prior Authorization Requests And Billings?”
• “How Do I Handle Syntax Errors?”
• “Can A Response Transaction Contain Accepted And Rejected Information?”
• “On Compounded Claims, Does DUR "Hit" Each Drug Within The Compound?”
Modified Response:
• “Prescription And Service Billings In One Transaction”
o Due to the creation of a separate Transaction Code (1Ø3-A3) for Service Billing transactions, a claim and a service cannot
appear in the same transmission structurally. They still can be associated, but must be within separate transmissions.
• “Identifier Of An Ingredient”
o The response originally noted the use of value ØØ could be used when an ingredient does not have an identifier. Due to
the changes of the default values in the Data Dictionary and External Code List, this response has been modified for the
possible use of the value “99” (Other).
Moved Verbiage into sections of the Implementation Guide proper:
• “Facility ID Usage”
• “Compound Ingredient Calculates To Be Less Than $Ø.ØØ5”
• “Default Date Format”
• “Is A Person Code (3Ø3-C3) Of “Ø6Ø” The Same As “6Ø”?”
• “Can A Segment Identification (111-AM) Be Sent Without Any Fields In That Segment And Not Be In Error?”
• “Multi-Ingredient Compound And Rejects”
• “Multi-Ingredient Compounds and DUR Rejects”
• “Alphanumeric Field and Leading Spaces”
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 969 -
37.23.3 REQUEST SEGMENT MATRICES MODIFICATIONS
37.23.3.1 REQUEST SEGMENT MATRICES BY SEGMENT
It was requested to show which segments had designation modifications from previous versions. The charts below show only the changes. For example, the chart below shows that the
Patient Segment changed from Optional (O) to Not Used (N) in the Reversal transactions. These charts do not show that the Optional Segments were changed to Situational Segments,
as all changed except Controlled Substance Reporting transactions.
VERSION D AND ABOVE REQUEST SEGMENT USAGE MATRIX
SEGMENT Eligibility Billing (Claim) or
Encounter Rebill
(Claim) Predetermination Of
Benefits (Claim) Billing
(Service) Rebill
(Service) Reversal
(Claim) Reversal
(Service)
Patient Segment
O to N O to N
37.23.3.1.1 ELIGIBILITY/BILLING/ENCOUNTER/REBILL/REVERSAL MATRIX
VERSION D AND ABOVE REQUEST SEGMENT USAGE MATRIX
SEGMENT Eligibility Billing (Claim) or
Encounter Rebill
(Claim) Predetermination
Of Benefits (Claim) Billing
(Service) Rebill
(Service) Reversal
(Claim) Reversal
(Service)
Transaction Header
Segment
Patient Segment
O to N O to N
Insurance Segment
Claim Segment
Pharmacy Provider
Segment
Prescriber Segment
Coordination of
Benefits/Other Payments
Segment
N to S N to S
Workers’ Compensation
Segment
DUR/PPS Segment
O to N
Pricing Segment
O to N
Coupon Segment
O to N O to N
Compound Segment
Prior Authorization
Segment
Clinical Segment
Additional Documentation
Segment
Facility Segment
Narrative Segment
37.23.3.1.2 PRIOR AUTHORIZATION REQUEST AND BILLING/PRIOR AUTHORIZATION REVERSAL/PRIOR AUTHORIZATION INQUIRY/PRIOR AUTHORIZATION
REQUEST ONLY MATRIX
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 970 -
VERSION D AND ABOVE REQUEST SEGMENT USAGE MATRIX (Continued)
SEGMENT Prior Authorization
Request & Billing (Claim) Prior Authorization Request &
Billing (Service) Prior Authorization
Reversal Prior
Authorization
Inquiry
Prior Authorization
Request Only (Claim) Prior Authorization
Request Only (Service)
Transaction Header Segment
Patient Segment
O to N O to N
Insurance Segment
Claim Segment M to N M to N
Pharmacy Provider Segment
O to N O to N O to N O to N
Prescriber Segment
O to N O to N
Coordination of
Benefits/Other Payments
Segment
O to N O to N O to N
Workers’ Compensation
Segment
O to N O to N
DUR/PPS Segment
O to N O to N
Pricing Segment O to N O to N O to N O to N
Coupon Segment O to N O to N O to N O to N O to N O to N
Compound Segment O to N O to N O to N O to N
Prior Authorization Segment
Clinical Segment
Additional Documentation
Segment
O to N
Facility Segment
O to N
Narrative Segment
O to N
37.23.3.1.3 INFORMATION REPORTING/INFORMATION REPORTING REVERSAL/INFORMATION REPORTING REBILL/CONTROLLED SUBSTANCE
REPORTING/CONTROLLED SUBSTANCE REVERSAL/CONTROLLED SUBSTANCE REBILL
VERSION D AND ABOVE REQUEST SEGMENT USAGE MATRIX (Continued)
SEGMENT Information Reporting
(Claim) Information Reporting
(Service) Information Reporting
Rebill Information Reporting
Reversal (Claim) Information Reporting
Reversal (Service) Controlled
Substance
Reporting
Controlled
Substance
Reporting
Reversal
Controlled
Substance
Reporting
Rebill
Transaction Header
Segment
Patient Segment
O to N O to N
Insurance Segment
Claim Segment
Pharmacy Provider
Segment
Prescriber Segment
Coordination of
Benefits/Other Payments
Segment
O to N O to N O to N
Workers’ Compensation
Segment

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 971 -
VERSION D AND ABOVE REQUEST SEGMENT USAGE MATRIX (Continued)
SEGMENT Information Reporting
(Claim) Information Reporting
(Service) Information Reporting
Rebill Information Reporting
Reversal (Claim) Information Reporting
Reversal (Service) Controlled
Substance
Reporting
Controlled
Substance
Reporting
Reversal
Controlled
Substance
Reporting
Rebill
DUR/PPS Segment
Pricing Segment
O to N O to N
Coupon Segment
Compound Segment
Prior Authorization
Segment
Clinical
Additional Documentation
Segment
Facility Segment
Narrative Segment
37.23.4 RESPONSE SEGMENT MATRICES MODIFICATIONS
It was requested to show which segments had designation modifications from previous versions. The charts below show only the changes. These charts do not show that the Optional
Segments were changed to Situational Segments, as all changed except Controlled Substance Reporting transactions.
37.23.4.1 RESPONSE SEGMENT MATRICES BY SEGMENT
37.23.4.1.1 TRANSMISSION ACCEPTED; TRANSACTION PAID OR DUPLICATE OF PAID, OR BENEFIT MATRIX
Transmission
Header Response Header
Header Response Status (5Ø1-F1) = “A” Accepted
Transaction
Response Status
Transaction Response Status (112-AN) = “P” Paid or “D” Duplicate of Paid or “B” Benefit
The following transactions are supported in “P” Paid or “D” Duplicate of Paid or “B” Benefit Matrix:
VERSION D AND ABOVE TRANSMISSION ACCEPTED
TRANSACTION PAID OR DUPLICATE OF PAID
RESPONSE SEGMENT USAGE MATRIX
SEGMENT Billing (Claim) or
Encounter Predetermination
Of Benefits (Claim) Rebill (Claim) Billing
(Service) Rebill (Service) Prior Authorization
Request & Billing Prior
Authorization
Inquiry
Response Header Segment
Header Response Status (5Ø1-F1) A A A A A A A
Response Message Segment
Response Insurance Segment
Response Insurance Additional Information
Segment
Response Patient Segment
Response Status Segment
Transaction Response Status (112-AN) P,D B P P,D P P,D P,D
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 972 -
VERSION D AND ABOVE TRANSMISSION ACCEPTED
TRANSACTION PAID OR DUPLICATE OF PAID
RESPONSE SEGMENT USAGE MATRIX
SEGMENT Billing (Claim) or
Encounter Predetermination
Of Benefits (Claim) Rebill (Claim) Billing
(Service) Rebill (Service) Prior Authorization
Request & Billing Prior
Authorization
Inquiry
Response Claim Segment
Response Pricing Segment
Response DUR/PPS Segment
Response Prior Authorization Segment
Response Coordination of Benefits/Other
Payers Segment
The following transactions do not support the “D” Duplicate of Paid response:
Rebill
Information Reporting Rebill
37.23.4.1.2 TRANSMISSION ACCEPTED; TRANSACTION CAPTURED OR DUPLICATE OF CAPTURE MATRIX
Transmission
Response Header
Header Response Status (5Ø1-F1) = “A” Accepted
Transaction
Response Status
Transaction Response Status (112-AN) = “C” Captured or “Q” Duplicate of Captured
The following transactions are supported in “C” Captured or “Q” Duplicate of Captured Matrix:
VERSION D AND ABOVE TRANSMISSION ACCEPTED
TRANSACTION CAPTURED OR DUPLICATE OF CAPTURE
RESPONSE SEGMENT USAGE MATRIX
SEGMENT
Billing (Claim)
or Encounter Rebill
(Claim) Billing
(Service) Rebill
(Service) Reversal
(Claim) Reversal
(Service) Prior Authorization
Request & Billing Prior
A
uthorization
Reversal
Prior
Authorization
Inquiry
Prior
Authorization
Request Only
Response Header Segment
Header Response Status (5Ø1-F1) A A A A A A A A A A
Response Message Segment
Response Insurance Segment
O to N O to N
Response Insurance Additional Information
Segment
Response Patient Segment
Response Status Segment
Transaction Response Status (112-AN)
C,Q C C,Q C C,Q C,Q C,Q C,Q C,Q C,Q
Response Claim Segment M to N M to N
Response Pricing Segment
O to N O to N O to N O to N O to N
Response DUR/PPS Segment
O to N O to N
Response Prior Authorization Segment M to N O to N M to N
Response Coordination of Benefits/Other
Payers Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 973 -
VERSION D AND ABOVE TRANSMISSION ACCEPTED (Continued)
TRANSACTION CAPTURED OR DUPLICATE OF CAPTURE
RESPONSE SEGMENT USAGE MATRIX
SEGMENT
Information
Reporting Information Reporting
Reversal Information Reporting
Rebill Controlled Substance
Reporting Controlled Substance
Reversal Controlled Substance
Rebill
Response Header Segment
Header Response Status (5Ø1-F1) A A A A A A
Response Message Segment
Response Insurance Segment
Response Insurance Additional Information
Segment
Response Patient Segment
Response Status Segment
Transaction Response Status (112-AN) C,Q C,Q C C,Q C,Q C
Response Claim Segment
Response Pricing Segment S to N S to N S to N
Response DUR/PPS Segment
Response Prior Authorization Segment
Response Coordination of Benefits/Other
Payers Segment
The following transactions do not support the “Q” Duplicate of Captured response:
Rebill
Information Reporting Rebill
Controlled Substance Reporting Rebill
37.23.4.1.3 TRANSMISSION ACCEPTED; TRANSACTION APPROVED OR DUPLICATE OF APPROVED MATRIX
Transmission
Response Header
Header Response Status (5Ø1-F1) = “A” Accepted
Transaction
Response Status
Transaction Response Status (112-AN) = “A” Approved, or “S” Duplicate of Approved
The following transactions are supported in “A” Approved, or “S” Duplicate of Approved Matrix:
VERSION D AND ABOVE TRANSMISSION ACCEPTED
TRANSACTION APPROVED OR DUPLICATE OF APPROVED
RESPONSE SEGMENT USAGE MATRIX
SEGMENT Eligibility Reversal
(Claim) Reversal
(Service) Prior Authorization
Reversal Prior Authorization
Inquiry Prior Authorization
Request Only Information
Reporting Information
Reporting Reversal Information
Reporting Rebill
Response Header Segment
Header Response Status
(5Ø1-F1) A A A A A A A A A
Response Message Segment
Response Insurance Segment O to N O to N O to N
Response Insurance Additional
Information Segment
Response Patient Segment
Response Status Segment
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 974 -
VERSION D AND ABOVE TRANSMISSION ACCEPTED
TRANSACTION APPROVED OR DUPLICATE OF APPROVED
RESPONSE SEGMENT USAGE MATRIX
SEGMENT Eligibility Reversal
(Claim) Reversal
(Service) Prior Authorization
Reversal Prior Authorization
Inquiry Prior Authorization
Request Only Information
Reporting Information
Reporting Reversal Information
Reporting Rebill
Transaction Response Status
(112-AN) A A,S A,S A,S A A,S A,S A,S A
Response Claim Segment M to N
Response Pricing Segment
O to N O to N O to N O to N O to N
Response DUR/PPS Segment O to N O to N
Response Prior Authorization
Segment
Response Coordination of
Benefits/Other Payers Segment
VERSION D AND ABOVE TRANSMISSION ACCEPTED (Continued)
TRANSACTION APPROVED OR DUPLICATE OF APPROVED
RESPONSE SEGMENT USAGE MATRIX
SEGMENT Controlled Substance
Reporting Controlled Substance
Reporting Reversal Controlled Substance
Reporting Rebill
Response Header Segment
Header Response Status (5Ø1-F1) A A A
Response Message Segment
Response Insurance Segment
Response Insurance Additional Information
Segment
Response Patient Segment
Response Status Segment
Transaction Response Status (112-AN) A,S A,S A
Response Claim Segment
Response Pricing Segment
Response DUR/PPS Segment
Response Prior Authorization Segment
Response Coordination of Benefits/Other
Payers Segment
The following transactions do not support an “S” Duplicate of Approved response:
Eligibility
Prior Authorization Inquiry
Information Reporting Rebill
Controlled Substance Reporting Rebill
If an Eligibility or Prior Authorization Inquiry request is a duplicate, the Processor must return the original “A” Approved response a second time.
37.23.4.1.4 TRANSMISSION ACCEPTED; TRANSACTION DEFERRED MATRIX
Transmission
Response Header
Header Response Status (5Ø1-F1) = “A” Accepted
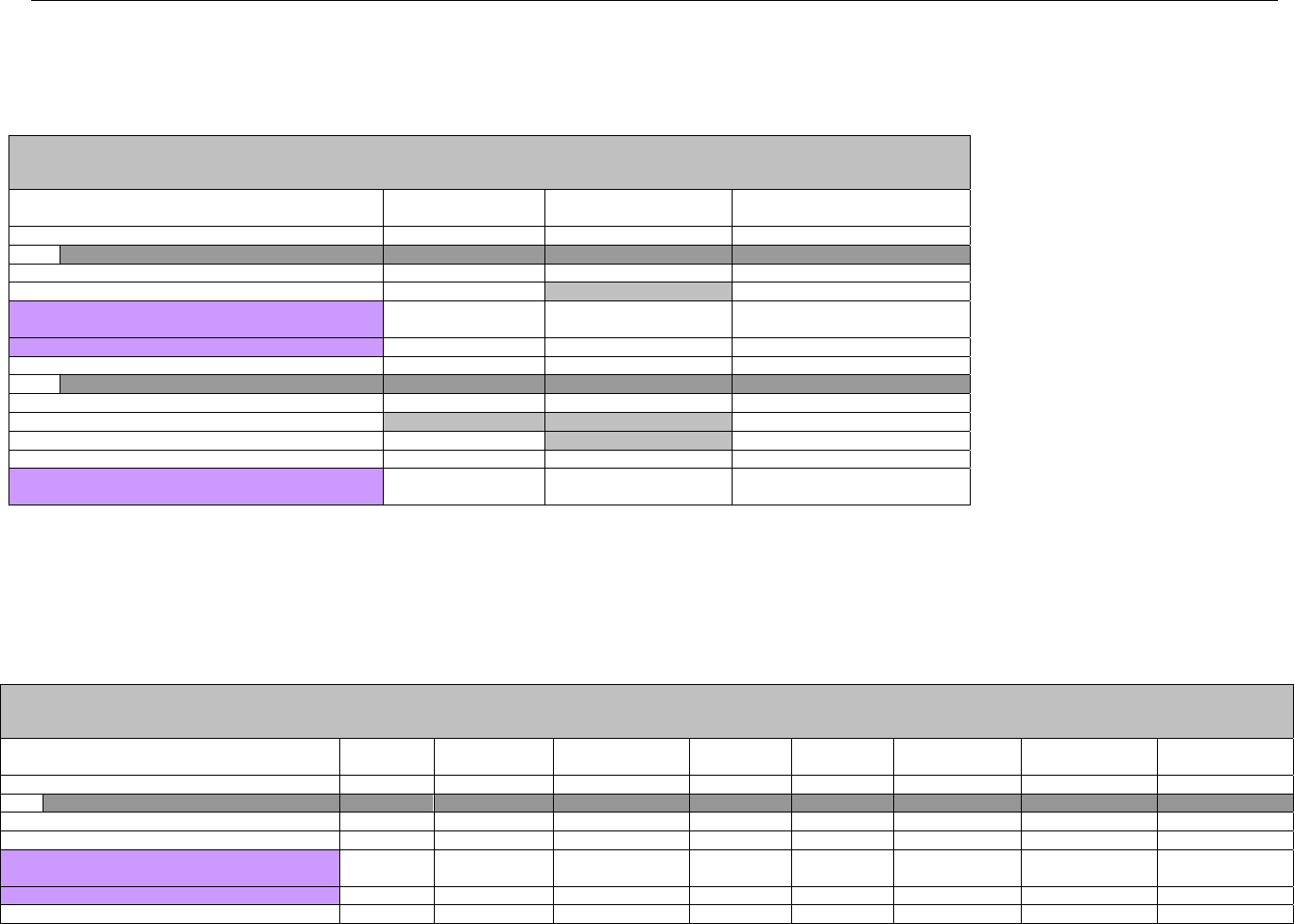
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 975 -
Transaction
Response Status
Transaction Response Status (112-AN) = “F” Deferred
The following transactions are supported in “F” Deferred Matrix:
VERSION D AND ABOVE TRANSMISSION ACCEPTED
TRANSACTION DEFERRED
RESPONSE SEGMENT USAGE MATRIX
SEGMENT Prior Authorization
Request & Billing Prior Authorization
Inquiry Prior Authorization Request Only
Response Header Segment
Header Response Status (5Ø1-F1) A A A
Response Message Segment
Response Insurance Segment O to N
Response Insurance Additional Information
Segment
Response Patient Segment
Response Status Segment
Transaction Response Status (112-AN) F F F
Response Claim Segment
Response Pricing Segment O to N O to N
Response DUR/PPS Segment O to N
Response Prior Authorization Segment M to S S M to S
Response Coordination of Benefits/Other Payers
Segment
37.23.4.1.5 TRANSMISSION ACCEPTED; TRANSACTION REJECTED MATRIX
Transmission
Response Header
Header Response Status (5Ø1-F1) = “A” Accepted
Transaction
Response Status
Transaction Response Status (112-AN) = “R” Rejected
The following transactions are supported in “A” Accepted/”R” Rejected Matrix:
VERSION D AND ABOVE TRANSMISSION ACCEPTED
TRANSACTION REJECTED
RESPONSE SEGMENT USAGE MATRIX
SEGMENT Eligibility Billing (Claim)
or Encounter Predetermination
of Benefits (Claim) Rebill (Claim) Billing
(Service) Rebill (Service) Reversal (Claim) Reversal (Service)
Response Header Segment
Header Response Status (5Ø1-F1) A A A A A A A A
Response Message Segment
Response Insurance Segment
Response Insurance Additional Information
Segment
Response Patient Segment
Response Status Segment
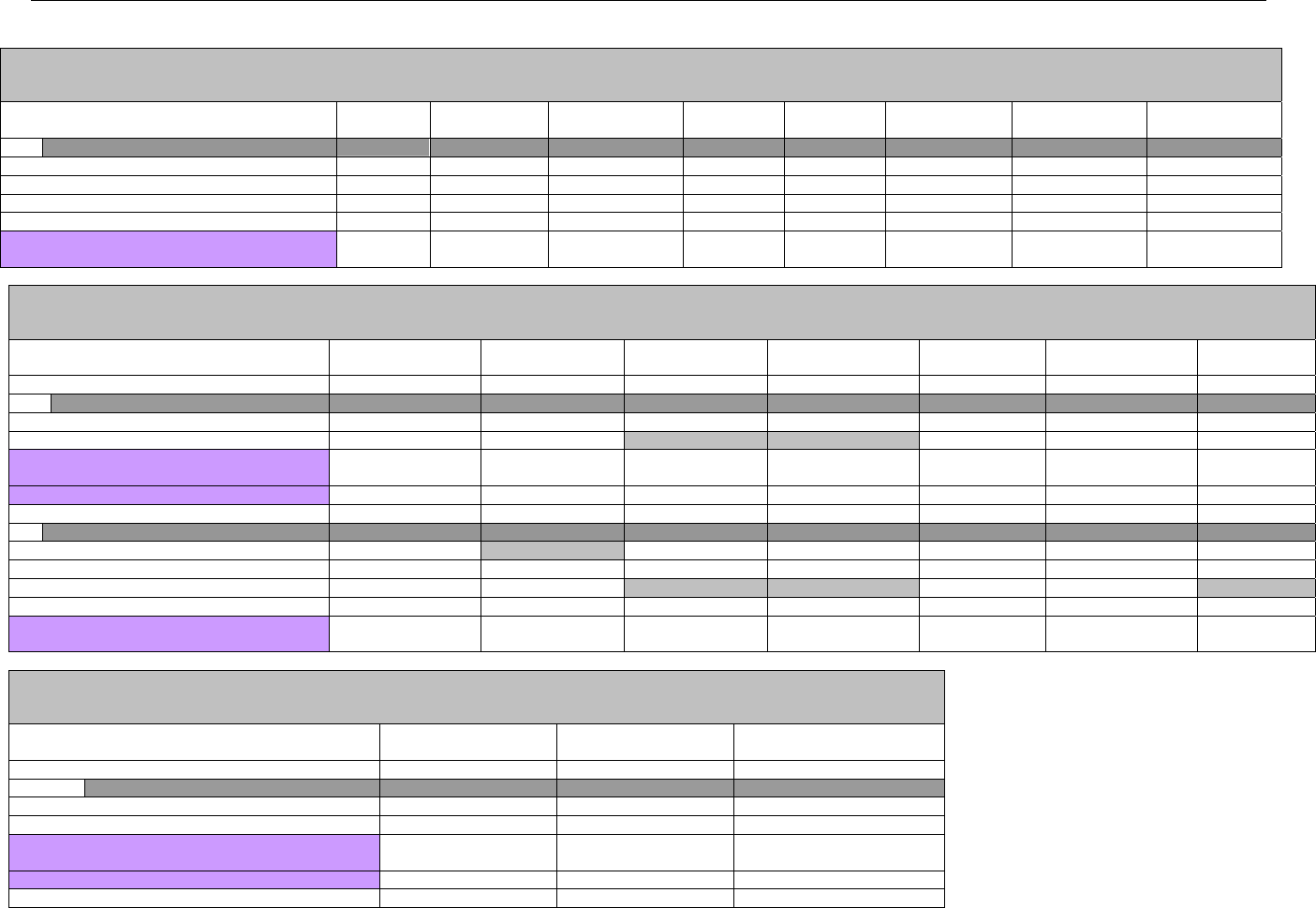
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 976 -
VERSION D AND ABOVE TRANSMISSION ACCEPTED
TRANSACTION REJECTED
RESPONSE SEGMENT USAGE MATRIX
SEGMENT Eligibility Billing (Claim)
or Encounter Predetermination
of Benefits (Claim) Rebill (Claim) Billing
(Service) Rebill (Service) Reversal (Claim) Reversal (Service)
Transaction Response Status (112-AN)) R R R R R R R R
Response Claim Segment
Response Pricing Segment
Response DUR/PPS Segment
Response Prior Authorization Segment N to S N to S N to S N to S
Response Coordination of Benefits/Other
Payers Segment
VERSION D AND ABOVE TRANSMISSION ACCEPTED (Continued)
TRANSACTION REJECTED
RESPONSE SEGMENT USAGE MATRIX
SEGMENT Prior Authorization
Request & Billing Prior Authorization
Reversal Prior Authorization
Inquiry Prior Authorization
Request Only Information
Reporting Information
Reporting Reversal Information
Reporting Rebill
Response Header Segment
Header Response Status (5Ø1-F1) A A A A A A A
Response Message Segment
Response Insurance Segment
O to N O to N
Response Insurance Additional Information
Segment
Response Patient Segment
Response Status Segment
Transaction Response Status (112-AN)) R R R R R R R
Response Claim Segment M to N M M M M M
Response Pricing Segment
Response DUR/PPS Segment
O to N O to N O to N
Response Prior Authorization Segment
Response Coordination of Benefits/Other
Payers Segment
VERSION D AND ABOVE TRANSMISSION ACCEPTED (Continued)
TRANSACTION REJECTED
RESPONSE SEGMENT USAGE MATRIX
SEGMENT Controlled Substance
Reporting Controlled Substance
Reporting Reversal Controlled Substance
Reporting Rebill
Response Header Segment
Header Response Status (5Ø1-F1) A A A
Response Message Segment
Response Insurance Segment
Response Insurance Additional Information
Segment
Response Patient Segment
Response Status Segment
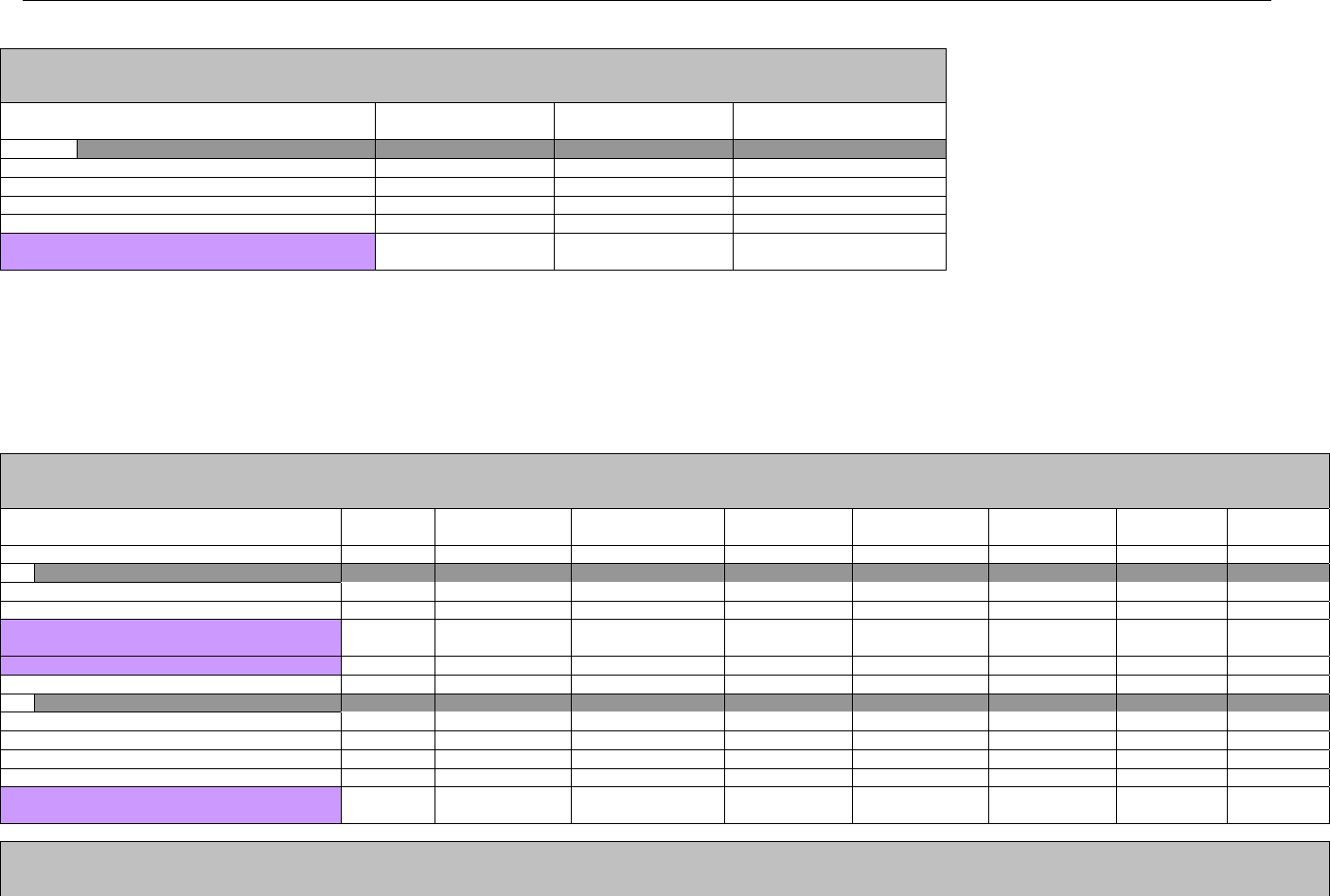
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 977 -
VERSION D AND ABOVE TRANSMISSION ACCEPTED (Continued)
TRANSACTION REJECTED
RESPONSE SEGMENT USAGE MATRIX
SEGMENT Controlled Substance
Reporting Controlled Substance
Reporting Reversal Controlled Substance
Reporting Rebill
Transaction Response Status (112-AN)) R R R
Response Claim Segment
Response Pricing Segment
Response DUR/PPS Segment
Response Prior Authorization Segment
Response Coordination of Benefits/Other Payers
Segment
37.23.4.1.6 TRANSMISSION REJECTED; TRANSACTION REJECTED MATRIX
Transmission
Response Header
Header Response Status (5Ø1-F1) = “R” Rejected
Transaction
Response Status
Transaction Response Status (112-AN) = “R” Rejected
The following transactions are supported in “R” Rejected/”R” Rejected Matrix:
VERSION D AND ABOVE TRANSMISSION REJECTED
TRANSACTION REJECTED
RESPONSE SEGMENT USAGE MATRIX
SEGMENT Eligibility Billing (Claim) or
Encounter Predetermination of
Benefits (Claim) Rebill (Claim) Billing (Service) Rebill (Service) Reversal
(Claim) Reversal
(Service)
Response Header Segment
Header Response Status (5Ø1-F1) R R R R R R R R
Response Message Segment
Response Insurance Segment
Response Insurance Additional Information
Segment
Response Patient Segment
Response Status Segment
Transaction Response Status (112-AN) R R R R R R R R
Response Claim Segment
Response Pricing Segment
Response DUR/PPS Segment
Response Prior Authorization Segment
Response Coordination of Benefits/Other
Payers Segment
VERSION D AND ABOVE TRANSMISSION REJECTED (Continued)
TRANSACTION REJECTED
RESPONSE SEGMENT USAGE MATRIX
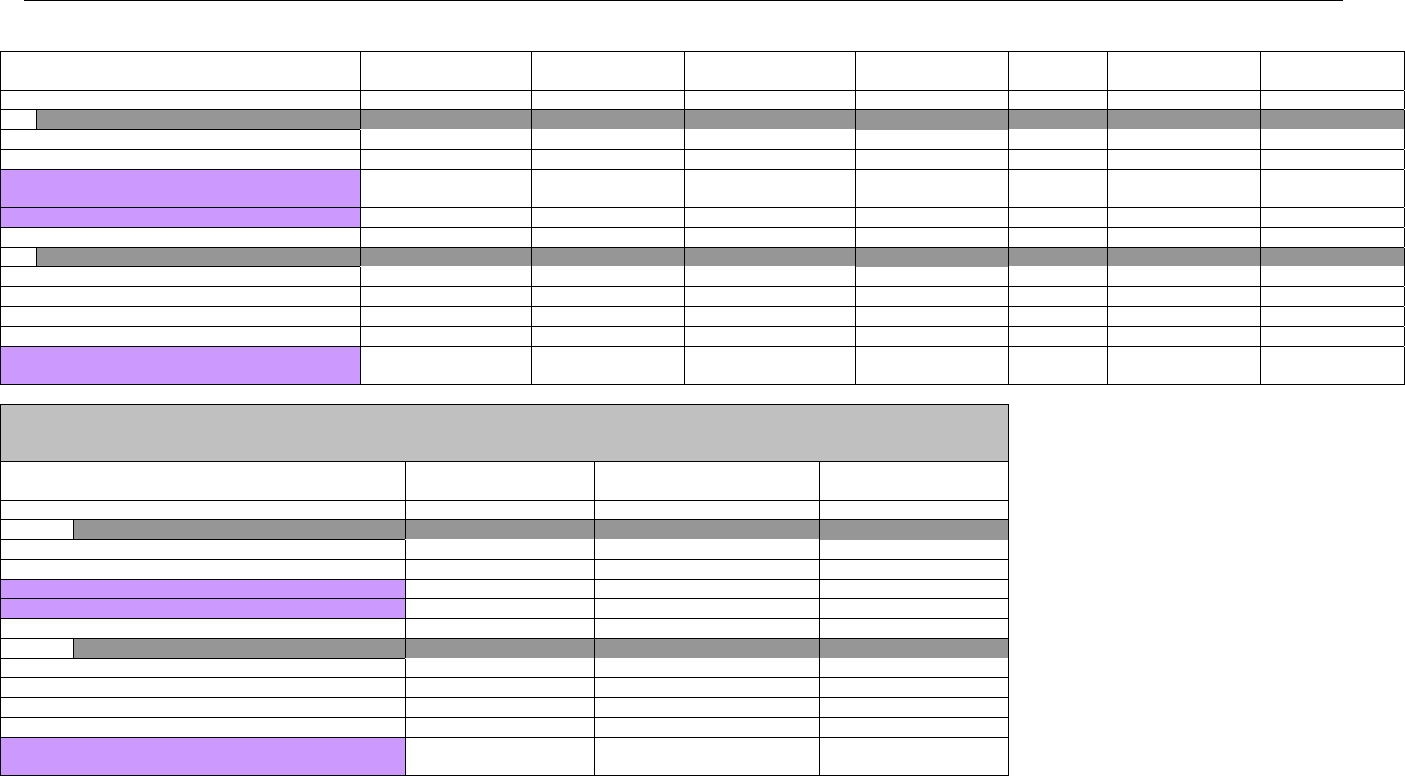
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 978 -
SEGMENT Prior Authorization
Request & Billing Prior Authorization
Reversal Prior Authorization
Inquiry Prior Authorization
Request Only Information
Reporting Information
Reporting Reversal Information
Reporting Rebill
Response Header Segment
Header Response Status (5Ø1-F1) R R R R R R R
Response Message Segment
Response Insurance Segment
Response Insurance Additional Information
Segment
Response Patient Segment
Response Status Segment
Transaction Response Status (112-AN) R R R R R R R
Response Claim Segment
Response Pricing Segment
Response DUR/PPS Segment
Response Prior Authorization Segment
Response Coordination of Benefits/Other
Payers Segment
VERSION D AND ABOVE TRANSMISSION REJECTED (Continued)
TRANSACTION REJECTED
RESPONSE SEGMENT USAGE MATRIX
SEGMENT Controlled Substance
Reporting Controlled Substance
Reporting Reversal Controlled Substance
Reporting Rebill
Response Header Segment
Header Response Status (5Ø1-F1) R R R
Response Message Segment
Response Insurance Segment
Response Insurance Additional Information Segment
Response Patient Segment
Response Status Segment
Transaction Response Status (112-AN) R R R
Response Claim Segment
Response Pricing Segment
Response DUR/PPS Segment
Response Prior Authorization Segment
Response Coordination of Benefits/Other Payers
Segment
37.23.5 AUGUST 2ØØ7 APPROVALS
In section “Transmission Examples”, section “Service Billing – Transaction Code S1 With CPT Codes” has been added.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 979 -
38. APPENDIX B. REVISION INFORMATION
NCPDP has developed and released several updated versions of the NCPDP Telecommunication Standard Implementation Guide, including:
Version/Release Date Comment
Ø1 Ø9/Ø1/1988
• Initial telecommunication specification.
• Utilized formats with fixed fields only.
Ø2 Not Released • Not released due to the early development of Version 3.Ø.
• Version Ø2 only has corrections to typographical errors found in Version Ø1.
3.Ø 12/Ø1/1989 • Variable (hybrid) format. Released on a limited basis pending final approval by
NCPDP membership. Modified by next release prior to formal approval by the
Council.
3.1 Ø2/Ø5/1991
• General release followed approval by the NCPDP Board of Trustees and by
membership vote at the February 1991 NCPDP Annual Meeting.
• Version 3.Ø and 3.1 reduced the number of fixed fields and added the mechanism to
append fields to both the transaction header and claim information in what was called
the optional data area.
3.2 Ø2/11/1992
• General release of Version #3 Release #2 followed approval of the NCPDP Board of
Trustees and by membership at the February 1992 NCPDP Annual Meeting. A "Pre-
Publication Annual Convention Release" was distributed in limited quantities at the
annual meeting. Some limited typographical errors were still evident in this document
labeled "Pre-Publication". The fully corrected document labeled "OFFICIAL
RELEASE" was distributed to the entire membership in early March 1992.
• Version 3.2 supported both fixed and variable transaction sets, utilizing both fixed as
well as optional fields within the transaction header and claim information.
3.3 Ø2/1996 • Encompassed specification upgrade for compound drug transactions.
• RTDS transaction sets no longer supported.
3.4 Ø6/1996
• Prior Authorization transaction sets supported.
3.5 1Ø/1996
• New values to existing data elements.
4.Ø 1Ø/1996
• New values, name, and definition changes to existing data elements.
4.1 Ø7/1997
• New values, definitions change to existing data elements.
4.2 Ø3/1998
• Addition of new data elements.
5.Ø Ø6/1999
• Fully variable transaction sets.
• Data elements modeled into usage segments.
5.1 Ø9/1999
• Named in HIPAA (August 2ØØØ).
5.2-C.4 2ØØØ –2ØØ6
• Addition of new data elements to clarify coordination of benefits, pricing, Medicare Part
D needs, etc. These changes were in preparation for the next version of
Telecommunication Standard Implementation Guide to be named to HIPAA.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 980 -
39. APPENDIX C. DATA DICTIONARY FIELD DELETIONS
The following fields are not supported in Version D and above.
NON-SUPPORTED FIELDS
FIELD # FIELD NAME SEGMENT
519-FJ Amount Attributed To Product Selection Response Pricing Segment
576-MQ Amount Attributed To Product Selection Qualifier Response Pricing Segment
467-1E Prescriber Location Code Prescriber Segment
469-H5 Primary Care Provider Location Code Prescriber Segment
452-EH Compound Route of Administration Compound Segment
For definition, value, format, or other field-level changes, please see the NCPDP Data Dictionary and the NCPDP External Code List.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 981 -
40. APPENDIX D. WHAT IS THE 11-DIGIT FORMAT FOR AN NDC, UPC, OR
HRI?
Drug products and drug administration items are most commonly identified by National Drug Codes (NDC), National Health Related Items
Codes (NHRIC or HRI) and by Universal Product Codes (UPC). Confusion exists as to the structure of these codes and the manner in which
they are formatted for use within NCPDP standards. A business need was recognized requiring consistent representation of these numbers in
telecommunication standards. A methodology to represent these codes as 11 digits was established.
40.1 NATIONAL DRUG CODES (NDC)
National Drug Codes are used to identify drug products. “Each drug product listed under Section 51Ø of the Federal Food, Drug, and Cosmetic
Act is assigned a unique 1Ø-digit, 3-segment number. This number, known as the National Drug Code (NDC), identifies the labeler/vendor,
product, and trade package size. The first segment, the labeler code, is assigned by the FDA. A labeler is any firm that manufactures, repacks
or distributes a drug product. The second segment, the product code, identifies a specific strength, dosage form, and formulation for a
particular firm. The third segment, the package code identifies package sizes. Both the product and package codes are assigned by the firm.
The NDC will be in one of the following configurations: 4-4-2, 5-3-2, or 5-4-1. Information on the proper use of the NDC is available from the
FDA in the Drug Registration and Listing Instruction Booklet”
(Source http://www.fda.gov/cder/ndc/database/default.htm).
These 1Ø digit numbers can be formatted into 11 digit numbers for use in NCPDP standards. This formatting allows the NDC to be
represented in a consistent manner where the distributor/manufacturer is always represented by five digits, the product by four digits and the
packaging by two digits.
Below are examples of the three NDC formats and the methods for formatting them into 11 digits for use in NCPDP standards by the
placement of a zero in the proper position. In a 4-4-2 format the zero is placed in the first position, in a 5-3-2 format the zero is placed in the
sixth position, in a 5-4-1 format the zero is placed in the tenth position.
NDC FORMATS TO NCPDP STANDARD 11-DIGIT NDC
4-4-2
(9999-9999-99)
Ø9999999999
5-3-2
(99999-999-99)
99999Ø99999
5-4-1
(99999-9999-9)
999999999Ø9
40.2 UNIVERSAL PRODUCT CODES (UPC)
The UPC is a generic term that refers to the UCC-12 data structure encoded in a UPC-A or UPC-E Bar Code Symbol, a standard for the
identification of products that is defined by the Uniform Code Council (UCC). The 1Ø-digit NDC can be represented within the UCC standards
for Universal Product Codes. Most non-prescription healthcare products are not assigned an NDC code. These items are most often
represented by the UPC. UPC codes are often represented by a bar code on product packaging. UPCs may be represented by a bar code, but
not all bar codes represent true UPCs as defined by the standards of the Uniform Code Council. A true UPC can only be assigned by a
manufacturer/distributor that is a member of the Uniform Code Council and adheres to their standards for product identification. Information on
the UCC and UPCs can be found at www.uc-council.org.
The UPC most commonly seen on drug products currently consists of 12 digits. The first digit defines the type of product, the next 5 digits
define the distributor/manufacturer and are assigned by the UCC (except for pharmaceutical labelers where the FDA assigns the labeler code),
the next 5 digits define the product and its packaging and is assigned by the distributor/manufacturer, the last digit is a check digit. NCPDP
standards use only the 1Ø digits representing the manufacturer and product. This number is then formatted into 11 digits by the addition of a
zero in the sixth position.
UPCs as represented in the NCPDP standards are not true representations of a UPC. The UCC states that a UPC number should not be
truncated or changed in any way to represent a product. Within NCPDP standards the UPC can be represented as 11 digits in a manner
similar to NDCs.
Below is an example of the proper formatting of a UPC into an 11-digit number for use within NCPDP standards. The zero is placed in the sixth
position.
1Ø-DIGIT UPC FORMATS TO 11-DIGIT UPC
5-5
(99999-99999)
99999Ø99999
Please note that the UCC recognizes the 1Ø-digit NDC within the UPC standard. .

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 982 -
40.3 NATIONAL HEALTH RELATED ITEM CODES (NHRIC OR HRI)
“The National Health Related Items Code (NHRIC) is a system for identification and numbering of marketed
device packages that is compatible with other numbering systems such as the National Drug Code (NDC) or
Universal Product Code (UPC). In the early 197Ø's, the Drug Listing Branch of FDA set aside a block of numbers
that could be assigned to medical device manufacturers and distributors. Those manufacturers who desire to use
the NHRIC number for unique product identification may apply to FDA for a labeler code. This labeler code is the
first segment in the two segment NHRIC system. Participating manufacturers and distributors then complete the
code by identifying their devices with a sequential number. The manufacturer or distributor assumes
responsibility for maintaining this number.” (Source: http://www.fda;/gov/cdrh/nhric/nhric.html)
The first four digits are assigned by the FDA and represent the manufacturer/distributor. The last six digits are assigned by the
manufacturer/distributor for the product. HRIs have a format of four-digits for the labeler and six-digits for the product. To convert a 1Ø-digit
HRI into the 11-digit NCPDP format, insert a zero in the first position.
Below is an example of the proper formatting of an HRI into an 11-digit number for use within NCPDP standards.
1Ø-DIGIT HRI FORMATS TO 11-DIGIT HRI
4-6
(9999-999999)
Ø9999999999
40.4 NON STANDARD PRODUCT CODES
It must be noted that some manufacturer/distributors assign product codes that are neither NDC, UPC, nor HRI numbers. These product
codes do not adhere to these standards and may be the cause of confusion when used in the healthcare industry. It should be emphasized
that a product containing a bar coded number does not necessarily comply with standards for the NDC, UPC, or HRI.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 983 -
41. APPENDIX E. USE OF INFORMATION REPORTING (N1, N2, N3)
FUNCTIONALITY FOR MEDICARE PART D PROCESSING
41.1 BACKGROUND
In December 2ØØ3, Congress passed the Medicare Prescription Drug Benefit, Improvement and Modernization Act (MMA), allowing Medicare
payment to Medicare Advantage organizations, Prescription Drug Plan (PDP) sponsors, Programs for the All-Inclusive Care of the Elderly
(PACE) plans, and Cost Plans (Part D plans) offering coverage beginning January 2ØØ6 of prescription drugs under the new Medicare Part D
benefit. The Notice of Proposed Rulemaking (NPRM) was August 3, 2ØØ4 and CMS proposed to collect a limited set of data elements for
1ØØ percent of prescription drug claims or events from plans offering Part D coverage. Some comments received on the NPRM voiced
concerns over how to track spending and sources of drug claims payments in order to effectively coordinate True Out-Of-Pocket (TrOOP)
beneficiary costs. An established TrOOP threshold triggers the beneficiary’s catastrophic drug coverage protection. Interested parties met over
a period of several months to establish the communication flow from the point of sale for a Medicare Part D transaction to the notification to the
PDP of other insurance payments.
41.2 INFORMATION REPORTING
The establishment of a new entity, Facilitator, to route payment information from payer-to-payer was identified and its functions defined. The
Facilitator process is documented within this implementation guide and is triggered by the submission of a transaction by a pharmacy to a
secondary payer. Payment information routing from the Facilitator to the PDP utilizes the Information Reporting transactions, N1, N2, and N3.
Process:
• This process begins after a Pharmacy has submitted a claim for a Part D Medicare Beneficiary to a Prescription Drug Plan
(PDP). The response from the PDP provides other payer information to the Pharmacy, when available.
• Pharmacy submits prescription claim to Secondary Payer based upon the information received from the Primary Payer via a
Switch and is routed to a Facilitator.
• Secondary Payer adjudicates claim.
• Secondary Payer responds to the Pharmacy via the Facilitator and Switch.
Then
• Facilitator transmits Information Reporting transaction containing secondary patient pay amount information to PDP to update
TrOOP calculations, etc.
• PDP transmits response to Facilitator.
A similar process would occur for Reversals from the Pharmacy to the Secondary/Tertiary Payer, which would result in Information Reporting
Reversals from the Facilitator to the PDP.
For correct Facilitator routing environment, a unique BIN/PCN will be assigned to a Secondary, Tertiary, etc. Payer’s health plan. This will
allow the Switch to determine the appropriate routing for a Medicare patient’s transactions. This will also trigger the Facilitator to create the
N1/N2/N3 transaction to the PDP. The unique BIN/PCN will be sent to the PDP so they may identify the correct Secondary/Tertiary Payer’s
health plan.
If a unique BIN/PCN is not assigned to the Secondary/Tertiary Payer’s health plan, then the Switch will not know to route the transaction to the
Facilitator. It is then the responsibility of the Secondary/Tertiary Payer to send the appropriate Information Reporting transaction to the
Facilitator. The Information Reporting transaction is created according to the following rules –
• If the Secondary/Tertiary Payer’s health plan is identified by a unique BIN/PCN and it is not sent by the Pharmacy, the Information
Reporting transaction must be formatted with the unique BIN/PCN and sent to the Facilitator.
• If the Secondary/Tertiary Payer’s health plan does not have a unique BIN/PCN, it must be able to be uniquely identified by a
combination of BIN/Group or BIN/PCN/Group. The Information Reporting transaction must be formatted with the unique
combination and sent to the Facilitator.
In order to facilitate this reporting and the effective update of TrOOP calculations, new fields were added and an existing field modified within
the Information Reporting Transaction process. The new fields are:
Other Payer BIN Number (99Ø-MG) – will provide the PDP with the BIN number of the payer reporting the patient pay amount
Other Payer Processor Control Number (991-MH)- will provide the PDP with the Processor Control Number (if used) of the payer reporting the
patient pay amount
Other Payer Group ID (992-MJ) - will provide the PDP with the Group ID of the secondary, tertiary, etc. payer
Changes to existing fields are:
Transaction Reference Number (88Ø-K5) – was added for use within the Telecommunication Standard.
The transaction reference number is being used to track all transactions related to a particular dispensing event. Whoever creates the
Information Reporting Transaction is responsible for creating this number. The entity receiving the Information Reporting Transaction is
expected to include that number in their response.
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 984 -
This field can be used by the Facilitator to enable them to match all claims and reversals related to a particular dispensing event. The
Facilitator originates this number in the Information Reporting transactions to the PDP. The Transaction Reference Number designated in the
N1 is carried through in the N2.
Other Payer Cardholder ID (356-NU) – was modified to be included in the Request Insurance Segment of the Telecommunication Standard.
Depending upon the particular submission request, the PDP must provide one of the following general types of responses to the Facilitator:
Approved - This occurs when the PDP acknowledges the receipt of the information only transaction and successfully processes the
transaction. For Medicare Part D, this means that the PDP has updated the beneficiary's TrOOP to reflect the transaction being
reported.
Duplicate of Approved - This occurs when the PDP has previously received the request and processed the transaction, but the
response did not return to the Originator. The Duplicate response contains the same information as returned in the original
Approved scenario.
Captured - This occurs when the PDP acknowledges receipt of the information reporting transaction, but no judgment is made about
the processing of the transaction. For Medicare Part D, this means that the PDP has not yet updated the beneficiary's TrOOP to
reflect the transaction being reported.
Duplicate of Captured - This occurs when the PDP has previously received the request and processed the transaction, but the
response did not return to the Originator. The Duplicate response contains the same information as returned in the original Captured
scenario.
Paid - This response type must not be used for Medicare Part D Information Reporting (N1) or Information Reporting Rebill (N3)
transactions.
Duplicate of Paid - This response type must not be used for Medicare Part D Information Reporting (N1) or Information Reporting
Rebill (N3) transactions.
Rejected - This occurs when the PDP has encountered an error in the transaction or processing, or does not approve the
information only transaction.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 985 -
42. APPENDIX F. ORDUR (ONLINE REAL-TIME DRUG UTILIZATION
REVIEW)
42.1 INTRODUCTION
Inappropriate drug therapy can cause patient injury leading to the provision of additional health care services resulting in increased total health
care expenditures. Research indicates that an estimated three to five percent of hospital admissions result from medication toxicities.
In an attempt to solve this problem, the U.S. Congress enacted federal legislation in 199Ø that requires pharmacy providers that participate in
state Medicaid programs to perform prospective drug utilization review (DUR) and to provide patient counseling before each Medicaid
prescription is dispensed. Presumably, prospective DUR can identify and prevent drug therapy problems, using various drug, patient and
provider databases that make up the DUR system. The Omnibus Budget Reconciliation Act (OBRA '9Ø) required that outpatient prospective
DUR be performed for all Medicaid patients by January 1, 1993. Under OBRA '9Ø and Centers for Medicare and Medicaid Services (CMS)
guidelines, prospective DUR can be performed manually by the dispensing pharmacist or physician, as a component of his store's
computerized drug delivery and screening software, or through an online, real-time drug utilization review (ORDUR) programs administered via
a data modem by a third party claims processor.
The National Council for Prescription Drug Programs (NCPDP) responded to this legal mandate by developing an ORDUR component in its
existing NCPDP Telecommunications Standard Version 3.2. Subsequent adoption by CMS of NCPDP Telecommunication Standard Version
5.1 continues to accommodate ORDUR messaging.
In 1991 the NCPDP established the DUR Work Group as a task force of the organization's Standardization Committee. This 65-member,
industry-wide task force was convened to develop a standard format for the transmission of DUR conflict messages and responses in an
online real-time environment. This task force had representation from chain, independent, and mail-service pharmacy providers; software
vendors; Electronic Claim Management (ECM) processor organizations; software database companies; national pharmacy trade associations,
and plan sponsors. In 1992 NCPDP's membership ratified NCPDP Telecommunication Standard Version 3.2, adding capability for ORDUR to
the claims administration process.
The NCPDP standard for ORDUR processing will also help assure that implementation of DUR messages from multiple ECM processors will
be administratively uniform from the pharmacist's perspective. This will help pharmacy computer system vendors in developing optimum
system support for pharmacist DUR activity. This means that the resulting DUR activity will help the pharmacist identify and prevent improper
drug therapy, but will not excessively impact the pharmacist's operational capacity, cost, or efficiency.
NCPDP standards are widely used by private sector ECM processors. (An ECM system connects the community pharmacy provider with a
third party payer's drug benefit sponsor's ECM processing representative.) The purpose of this manual is to facilitate the performance of
ORDUR as a component of an ECM system.
In addition, NCPDP has established a process that will allow changes in its ORDUR processing and telecommunication standards that
involves consensus-based evaluation of requested changes. A Data Element Request form (DERF) is the method to use in requesting
consideration of changes in any NCPDP standard. Users of this appendix or any NCPDP standard are encouraged to become involved in the
NCPDP Work Group process.
42.2 CHAPTER 1. ORDUR PROCESSING DESIGN AND IMPLEMENTATION
NCPDP has identified the information and support files necessary for DUR processing on an ECM system. This chapter describes these data
elements and support files and discusses their practical and effective use.
Fundamental system design, implementation and ongoing operational issues are considered. This information will assist management and
users in understanding the challenges of conducting ORDUR within their drug programs. Data processing professionals will obtain an
understanding for the use and interaction of various input, reference, and output information resources needed by the system.
42.2.1 INFORMATION CATEGORIES
The information and support files for the ORDUR standard fall into four categories:
Member information
Prescription information
Prescriber identification
Pharmacy identification
ORDUR system managers and designers can draw the information that is to be used in the design of the ORDUR component of the ECM
system from the ECM system itself, the pharmacist's prescription filling system, and the commercial drug reference files.
They must evaluate the level of detail necessary, the accuracy of various sources, and the most efficient alternatives for obtaining the required
elements (that is, commercial drug databases, the pharmacy, or the ECM processor).
42.2.1.1 MEMBER INFORMATION
During any form of DUR processing, accurate patient identification is imperative to ensure that the system draws information from the correct
patient drug history profile.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 986 -
In an ORDUR system, where the volume of information exchange and interaction with the pharmacist is limited, a unique patient
identifier/other unique number is the preferred method for identifying a patient. Such a unique identifier for each individual prevents any
ambiguity that might occur with other methods of member identification occasionally used in claims processing systems.
Total reliance on patient first name, birth date, or sex in selecting among family members will cause obvious ambiguities in certain common
instances of child naming and multiple sibling births. However, the use of a unique identifying number in combination with multiple items such
as patient's birth date, unique person code number, first name, and relationship code, can provide another way to identify a patient accurately.
42.2.1.2 PRESCRIPTION INFORMATION
Prescription information is necessary input to the DUR system, and only the dispensing pharmacy can provide this information. Data items
that identify and describe a particular dispensing event that might be considered for inclusion in a DUR standard for ORDUR systems are
available immediately in an online real-time processing system. Most of these common data items that describe a dispensing event are also
used for benefit claims processing. You can easily obtain items such as the prescription number, National Drug Code (NDC), dispensed
quantity, and pharmacist estimated days supply for DUR or claims processing, if you follow industry standards. The NCPDP
Telecommunication Standard supports transfer of all these common data items from the pharmacy to the ECM system.
Diagnosis information availability to pharmacists and ORDUR processors improves DUR processing. The NCPDP Telecommunication format
accommodates diagnosis information in the form of International Classification of Diseases (ICD-9) codes. But, this information generally is
not available to the pharmacist at the time of dispensing. The patient's medical record is one source of diagnosis or indication information.
Yet this can be difficult for the pharmacist to obtain.
Implied diagnosis created by proxy from prescription drug claims history can be used in DUR, although problems with accuracy in this method
exist. The importance of accurate and timely diagnostic information cannot be overstated. ORDUR systems currently use a combination of
various means to capture diagnostic information. As additional diagnostic information is available, the processor can update the patient's
diagnostic file.
Conducting DUR on every drug that the patient encounters is important. This applies to compounded prescriptions as well. Since one NDC is
assigned to these combination drug products, or in the case of compounded prescriptions, each ingredient can be identified. DUR processors
can interrogate the drug conflict potential of each individual drug component. Commercial drug reference databases make this activity
possible.
The collection of information on drug products not covered by the particular benefit plan is also important in effective ORDUR. Over the
counter drugs often fall into this category, as do other legend prescription drugs under certain circumstances. The transmission of non-
covered drug product information for ORDUR purposes is possible through "information only" transactions in the NCPDP Telecommunication
Standard. Although technically possible, the transmission of non-covered drug activity may be impractical until appropriate compensation for
such services are resolved.
42.2.1.3 PRESCRIBER IDENTIFICATION
Effective and appropriate DUR also depends upon accurate identification of prescribers. Without this information, DUR efforts and educational
intervention is impossible. Many drug claim processors use the prescribers' National Provider ID (NPI), Drug Enforcement Administration
(DEA) number, Universal Physician Identification Number (UPIN), state medical license number, or a processor-assigned identification
scheme to identify prescribers.
42.2.1.4 PHARMACY IDENTIFICATION
NCPDP and the National Association of Boards of Pharmacy (NABP) have defined a unique pharmacy identification numbering system for use
in prescription processing systems. The NCPDP number is an efficient way for ECM processors to identify pharmacy providers and dispensing
locations throughout the nation. In 2ØØ6 the National Provider ID (NPI) will be used for covered entities under HIPAA.
The DUR Work Group considered more finite levels of pharmacy provider identification that might be considered when designing an ORDUR
system. Pharmacy setting is one of the many important factors when applying DUR conflict parameters. The NCPDP pharmacy file contains
the pharmacy setting as part of the profile for pharmacy setting (that is, retail, hospital, mail service, nursing home).
42.2.2 DUR SYSTEM SUPPORT FILES
Three primary files form the ECM system support ORDUR processing.
Patient profiles (drug use history file)
Complete drug master file (drug reference database)
Drug conflict (conflict/interaction database)
You can design the DUR support files in numerous formats. The first file is the continually changing longitudinal view of a patient's drug use.
The next two files or databases are related to the drug product itself, independent of patient utilization. The Master File contains the
information that describes the drug and its characteristics that may have an effect on DUR processing. The Drug Conflict Database describes
clinical use variables and relationships with other drug products at varying degrees of detail. The following descriptions provide the conceptual
purpose and contrast the functionality that is required for each file type. Together all these files contain the information needed for benefit
claims processing, reporting, and drug utilization review.
Patient Profiles
Some DUR modules, such as therapeutic duplication and drug interaction, depend upon the availability of historical patient prescription use
information. Prior use history may be necessary for various time periods depending on the system's design and DUR module's purpose. We

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 987 -
do not make a definitive recommendation for how much drug use history is necessary for ORDUR processing in this manual. Each ECM
processor and clinician associated with ORDUR design and implementation must carefully consider the appropriate volume and time frames of
history required.
Drug Data Files
Drug Reference and Drug Conflict Rules databases are the keystones to performing interactive monitoring and screening functions of a DUR
program. Along with actual drug use contained in the patient profiles, appropriately maintained reference and conflict rules drug databases
are the most critical elements in a successful ORDUR processing system. Careful attention to the detailed characteristics of these files and
the design of interfaces and applications that use them is essential in ORDUR system design.
Complete Drug Master Files
Complete drug master files are available from several commercial vendors. These sources supply automated reference file implementation
and maintenance subscriptions to ECM processors interested in prescription benefit processing. Information is accessed through the National
Drug Code (NDC) number key associated with the detail items for each drug package. Examples of the types of information available from a
complete drug master file include:
Drug name and strength
Dosage form
Therapeutic classification
Generic name
With this type of information maintained in these files, the pharmacist does not need to transfer detailed drug information with each claim. For
example, pharmacist transmission of drug name and NDC would be redundant and would add unnecessary overhead to the claims
transmissions. Similarly, ECM processors conducting ORDUR processing could gather information, such as therapeutic classification, from
their internal reference files rather than incoming claims transmissions.
Drug Conflict Rules Files
Drug conflict rules files provide specific clinical use information items and rule sets used to identify problem drug therapy. These rules files
provide the data element drivers and general processing logic needed to identify drug conflicts.
ECM processors implementing ORDUR systems must recognize that most pharmacy management computer systems operating in the
provider pharmacy already conduct some level of drug interaction screening. This is often accomplished using the same commercial vendor
drug conflict rules database products that vendors supply to the processing industry.
It is possible that ORDUR processing systems can enhance the pharmacy-based drug interaction systems, as the ECM processor may have a
more complete description of a patient's drug use profile than an individual pharmacy. However, plan administrators must carefully consider
the level of clinical significance that address online messages transmitted from the ORDUR system. Excessive identification of insignificant
clinical events may desensitize pharmacy providers to other, more significant DUR messages and events.
The NCPDP DUR Work Group categorizes therapeutic conflicts commonly noted in drug therapy according to their mechanism of action.
Each category or "module" makes up a Drug Conflict Rules File or database. Standard codes identify the drug conflicts in each module. The
pharmacy provider and ECM ORDUR processor use the codes when exchanging structured electronic messages and responses. See chapter
two for detailed descriptions and information on using these codes.
Dosing/Limits Module
The following therapeutic problems fall into the Dosing/Limits Module.
Low Dose (LD) detects drug doses that fall below the standard dosing range.
High Dose (HD) detects drug doses that fall above the standard dosing range.
Overuse (ER) detects prescription refills that occur before the days supply of the previous filling should have been exhausted.
Underuse (LR) detects prescription refills that occur after the days supply of the previous filling should have been exhausted.
Insufficient Duration (MN) detects regimens that are shorter than the minimal limit of therapy for the drug product based on the
product's common uses.
Excessive Duration (MX) detects regimens that are longer than the maximal limit of therapy for the drug product based on the
product's common uses.
Incorrect drug dosing can significantly impact the quality of patient care. Many adverse drug reactions and therapy failures can be traced to
low and high-dose problems in drug therapy. The complexity of proper drug dosing creates the need for thorough professional evaluation of
drug therapy. The dose and use information that either can be calculated or that is readily available in claims data lends itself to automated
detection of potential therapy problems. Indeed, intervention in potential dose-related problems in drug therapy through real-time processing
systems is considered a key area for ORDUR system focus.
ORDUR processing systems hold great hope for the prevention of many dose-related problems. Certain areas of drug use where patients are
prone to non-compliance could be favorably affected by appropriately designed ORDUR processing systems. In addition, simple errors in
dispensing and prescribing may be detected more often if ECM administrators provide pharmacists with information about possible dose-
related problems. Payers may also need to consider or weigh claims rejections verses information only. In either case, the ORDUR system
should still permit the pharmacist to override the rejection or warning to account for extenuating circumstances associated with a dispensing
event.
We must recognize professionals in contact with the patient as potential sources of additional relevant information surrounding the prescription
dispensing event that are not detectable in standardized automated information exchanges. DUR system designers must design the ORDUR
processing system to permit the pharmacist to use this "professional prerogative" when determining the significance of ECM administrator-

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 988 -
detected potential dosing problems. We have designed the NCPDP Telecommunication Standard specifically to permit pharmacist
communication of interventions that might require override of dose limit alerts and rejections.
Therapy duration non-compliance is another significant drug use problem. When a patient discontinues use of hypertensive therapy or
antibiotics too soon or uses oral anti-diabetic agents erratically, serious health care problems can result.
Therapy that extends needlessly beyond effective recommended time periods or at doses exceeding recommended maintenance levels can
also impact desired outcomes. Recognizing when a product is beyond the acute treatment phase and the clinical measurements that should
dictate a reduction in dose for longer-term use is an area where ORDUR programs might be useful. The ORDUR processing system can
remind clinicians delivering therapy at higher doses beyond the usual acute phase to re-evaluate the need for acute dose levels.
Drug Interaction Module
Two therapeutic problems fall into the drug interaction module.
Drug-Drug Interaction (DD) detects drug combinations in which the net pharmacologic response may be different from the result
expected when each drug is given separately.
Drug Incompatibility (DI) identifies physical and chemical incompatibilities between two or more drugs.
Adverse effects of drug interactions are usually preventable. ORDUR systems can assist the pharmacy provider in identifying these conflicts.
Although many professionals find the frequency and severity of interactions to be relatively small, drug interactions can impede of optimum
health care. Preventing adverse effects in even a few cases can improve quality of care and save health care dollars.
Drug Conflict Module
The Drug Conflict Module consists of a number of drug therapy problems that arise as a result of an interaction between the individual patient's
characteristics and a particular drug. The following therapeutic problems are in this module.
Drug-Allergy (DA) indicates that an adverse immune event may occur due to the patient's previously demonstrated heightened
allergic response to the drug product in question.
Prior Adverse Reaction (PR) identifies those drugs that the patient has previously reacted in an atypical manner.
Drug-Disease (Inferred) (DC) indicates that the use of the drug may be inappropriate in light of a specific medical condition that the
patient has. The existence of the specific medical condition is inferred from drugs in the patient's medication history.
Drug-Disease (Reported) (MC) indicates that the use of the drug may be inappropriate in light of a specific medical condition that the
patient has. Information about the specific medical condition was provided by the prescriber, patient, or pharmacist.
Drug-Age (PA) detects age-dependent drug problems.
Drug-Gender (SX) identifies contraindicated or inappropriate therapy in either males or females.
Additive Toxicity (AT) detects drugs with similar side effects that could exhibit additive toxic potential.
Drug-Pregnancy (PG) detects pregnancy-related drug problems. This information is intended to assist the healthcare professional in
weighing the therapeutic value of a drug against possible adverse effects on the fetus.
Iatrogenic Condition (IC) detects possibly inappropriate use of drugs that are designed to ameliorate complications caused by
another medication.
Side Effect (SE) reports possible major side effects of the prescribed drug.
Adverse effects of drugs include both side effects and allergic reactions. Adverse reactions are not always predictable; however, once a
patient has experienced an adverse reaction, it is highly likely that a similar reaction will occur if the same drug or similar drug product is again
prescribed for that patient. Reactions of this nature are preventable through ORDUR system messages and alerts.
Prior adverse reaction monitoring requires a complete patient profile assembled from all pharmacies and physicians the patient has used. The
transmission of information regarding a patient's allergies could be conveyed to the pharmacist using the NCPDP DUR Free Text Message or
DUR Additional Text . However, no method currently exists to transmit allergic information from the pharmacist to the ECM. Pharmacists may
be in the best position to detect drug-allergy and prior adverse reaction alerts. Common types or groupings of adverse reactions include:
skin rash-hives
shock
unconsciousness
asthma
shortness of breath
nausea-vomiting
anemia and other blood disorders
To be effective and meaningful, prior adverse reaction information transferred through the ORDUR system should identify the severity of the
interaction, the "onset profile" and duration of the reaction. Similarly, the level of documentation, frequency and scope of individual encounters
with the reaction should be described. These items all assist the ECM ORDUR processor in determining how to weigh the significance of
various interaction messages that might be sent to the pharmacy provider.
Drug-disease analysis and warnings might be useful additions to an ECM ORDUR processing system. A drug can affect disease conditions.
It might improve the disease, make it worse, or create a second disease. Existing patient conditions might contraindicate the use of a newly
prescribed drug. A newly identified disease or medical condition might contraindicate the use of drugs the patient is currently taking. Drug-
disease screening should detect these situations.
Even single drug therapies can cause other disease states. These drug-produced disease states are called iatrogenic disease conditions.
Drug-disease monitoring can also be used to detect iatrogenic disease states. For example, the additional use of a cough suppressant in a
patient taking an ACE-inhibitor may indicate a side effect to the ACE-inhibitor. By monitoring the sequence of drug use and diagnosis proxies

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 989 -
created by comparing expected reasons for a particular drug's use, links can be made to the prescribing of certain drugs used to combat side
effects caused by previously administered therapy.
The NCPDP Work Group also identified the need to distinguish between drug-disease interactions based on inferred diseases from drug proxy
and those diseases that might have been available because of their report and capture in a medical claims system.
Drug-disease screening can be conducted in a concurrent time frame, that is, while the patient is still receiving the therapy under review. In
this way, alterations in drug therapy can be made while the patient is actively being treated for a disease condition. Early notification enables
prescriber and pharmacist to confer before the pharmacist fills the patient's prescription. ORDUR processing of drug-disease DUR messages
enables the pharmacist to evaluate a patient's complete profile, including prescriptions from multiple pharmacies and physicians.
Other drug conflicts in this module include precautions about drug use in certain patient demographic situations. For example, common age
and sex restrictions for some drug products can be compared to proposed drug use to identify possible errors in dispensing or prescribing.
We added major side effects warnings as a valid Reason for Service Code for the standard DUR message as part of this module.
Duplicate Therapy Module
The following two therapeutic problems fall into the duplicate therapy module.
Therapeutic Duplication (TD) detects simultaneous use of different primary generic chemical entities that have the same therapeutic
effect.
Ingredient Duplication (ID) detects simultaneous use of drug products containing one or more identical generic chemical entities.
Both therapeutic and ingredient duplication can lead to excessive drug therapy cost, therapeutic failures, adverse drug reactions, and serious
health consequences. ORDUR processing systems can alert pharmacy providers of this duplication to assure that it is not unintentional. It
might not be necessary to report the difference between these two types of duplication, but it certainly is important to detect both types.
Precautionary Module
The following therapeutic problems fall into the precautionary module.
Alcohol Conflict (OH) detects when a prescribed drug is contraindicated or might conflict with the use of alcoholic beverages.
Tobacco Use (DS) conflict detects when a prescribed drug is contraindicated or might conflict with the use of tobacco products.
Drug-Lab Conflict (DL) indicates that laboratory values may be altered due to the use of the drug, or that the patient's response to
the drug may be altered due to a condition that is identified by a certain lab value.
Drug-Food Interaction (DF) informs the user of interactions between a drug and certain foods.
Call Help Desk (CH) informs the user to call the claims processor's help desk to obtain additional DUR information.
The pharmacy's dispensing review at the store level delivers the DUR conflicts contained in the precautionary module. Many local pharmacy
management systems make use of data available on commercial drug conflict rule databases related to these precautions. The pharmacist's
knowledge of the particular relevance of any of these potential conflicts for a particular patient is very important. These precautions are
currently best handled in the local store system. However, in the future, when medical claims systems are fully integrated with ORDUR
processing, some ECM processors might be able to effectively include standard DUR precautionary messages. The NCPDP has established
DUR Reason for Service Codes for these precautions so that the pharmacist can document the DUR activities of local pharmacy systems by
submitting these Reason for Service Codes on original claims.
42.2.3 DESIGN DISCUSSION SUMMARY
The extent to which any of the DUR modules can be effectively implemented depends on the availability and reliability of patient-specific
information. Therefore, we urge system designers and implementers to assure that utilization history and other patient demographic
information is accurate. The design must also take into account the pharmacy provider's operating environment so that the appropriateness of
various warning or rejection design decisions can be carefully evaluated. Finally, ECM processors must be aware of the cost issues that must
be considered when designing, implementing, and operating the ORDUR system.
The NCPDP Telecommunication Standard provides a framework to support the implementation of ORDUR programs. It supports the
interactive communication of standard DUR Response Data and standard DUR Action Codes between ECM processors and pharmacy
providers. The design of the NCPDP Telecommunication Standard provides for standard format outgoing messages from ECM ORDUR
processing systems in the various paid, captured, and denied response formats, and for standard-format pharmacist action codes on incoming
claim, reversal, and information only transactions.
The standard layout of the NCPDP Telecommunication Standard DUR segment permits pharmacy management system vendors to design
interfaces to handle the required pharmacist intervention and record-keeping associated with the receipt of standard DUR messages. Without
standard formats, ECM processors would have to use free form areas of the response formats which would be impossible for computer
vendors to leverage into smooth operating warning and record keeping systems. Disruptions to pharmacy operating flow in the dispensing
process can be costly and lead to decreases in access to needed drug therapy. ECM processors are encouraged to create ORDUR
processing within the NCPDP standards functionality. Processors that wish to develop new processing capabilities or functionalities are
encouraged to make use of the NCPDP standard development and maintenance process using the consensus method and due process for all
requests via an array of volunteer Work Groups. For more information on NCPDP Work Group activities and maintenance procedures contact
the NCPDP office.
42.3 CHAPTER 2. ORDUR MESSAGE FORMATS
42.3.1 STANDARD DUR MESSAGE

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 990 -
When an ECM system processes a claim and an ORDUR component of the system identifies conflicts, the system can return standard DUR
messages to the pharmacist. The payer and processor must decide whether the DUR conflict will result in a claim rejection for DUR purposes
or whether the claim will be accepted with the issuance of DUR conflict advisory warnings
Reason for Service Code - (439-E4) identifies the conflict module into which the detected conflict falls. This code should be generated and
sent back to the pharmacy when ORDUR processing detects this type of conflict. Valid values are listed in the External Code List (ECL).
Clinical Significance Code - (528-FS) indicates the significance of the detected conflict. The system pulls the clinical significance code from
the originating drug reference database. We recommend that processors developing unique databases use the following clinical significance
codes.
blank = Not specified
1 = Major
2 = Moderate
3 = Minor
ECM processors must prioritize ORDUR message transmissions to the pharmacies in order of severity. In other words, processors must
transmit standard DUR messages that are considered major before those considered less important. In addition, the processors must develop
a hierarchy of concern that ranks each Reason for Service Code module. In this way, messages from one module would have a higher or
lower priority than messages from a different module, but with the same clinical significance code.
For example, if drug allergies are determined to be more significant than drug-drug interactions, then allergy messages with clinical
significance level of "one" would be transmitted before drug-drug interactions with clinical significance level of "one". ECM processor
implementation specifications must define the hierarchy plan.
Other Pharmacy Indicator - (529-FT) indicates the dispensing location or source of the previous prescription or condition that is in conflict
with the prescription being submitted. This information might be useful to the pharmacist as he evaluates how to proceed with investigating or
intervening in the potential conflict noted.
Previous Date of Fill - (53Ø-FU) identifies the date (YYYYMMDD) of the previous prescription that triggered the conflict with the submitted
prescription. It might help the pharmacist, physician, and patient evaluate the relevance of potential conflicts.
Quantity of Previous Fill - (531-FV) indicates the quantity of the conflicting agent that was previously dispensed.
Database Indicator - (532-FW) identifies the source of drug reference file used to detect the potential DUR conflict. The valid drug reference
database codes are found in the NCPDP External Code List (ECL).
Other Prescriber Indicator - (533-FX) indicates whether the same or a different prescriber was responsible for the previously filled conflicting
prescription.
Dur Free Text Message- (544-FY) and DUR Additional Text (57Ø-NS) transmits additional information (that is, drug name, disease name)
that highlights the detected DUR conflict situation, along with some detail (for example, min-dose = X units/day) when the DUR alert code does
not include enough information.
Free Text Message Formatting Considerations
When entering this information, ECM processors should recognize certain style issues that may help the pharmacy evaluate and act on these
free form messages more consistently across all third party plans. We recommend that processors use the following guidelines to create
consistent, easily interpreted standard DUR message free text.
Write the message clearly and concisely. Enough information must be in the text to convey the problem, but excessive information
will be ignored.
Abbreviate only when necessary and use standard medical abbreviations.
When examples of content are included in the ORDUR Standard DUR Message, use those messages when possible.
Use NCPDP Standards to report product quantities in the message. Designate quantities in metric units not apothecary
equivalents. For example, use 325 MG, not 5 Grains.
Identify the dosage form when possible or at a minimum use the NCPDP Standard for Unit Type billing units. For example, EACH,
ML and GM.
When identifying dosage form or dose unit strength, use common industry abbreviations such as ML for milliliters, GM for grams,
MG for milligrams, etc.
When reporting DUR problems involving an ingredient in a profiled compound drug, identify the causitive agent in the Free Text
field, prefaced by "CMPD: "
Identify the date in a format of YYYYMMDD. For example, 1992Ø425 for April 25, 1992.
When returning drug name for interactions, use the trade drug name.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 991 -
When returning the drug name for such things as Additive Toxicity and Duplicate Therapy, include the dosage strength.
For age limitations, list the age for which incompatibility occurs. For example "CONTRAIND. UNDER 12 YEARS"
For laboratory test incompatibilities, specify the laboratory test the alert refers to.
Reason for Service Code CODE MESSAGE (FREE TEXT)
Low Dose LD Min Dose = X units/day
High Dose HD Max Dose = X units/day
Overuse ER "Processor Free Text"
Underuse LR "Processor Free Text"
Insufficient Duration MN Min Days Therapy = XXX
Excessive Duration MX Max Days Therapy = XXX
DRUG INTERACTION MODULE
Reason for Service Code CODE MESSAGE (FREE TEXT)
Drug-Drug Interaction DD Drug Trade Name
Drug Incompatibility DI Incompatible Agent
DRUG CONFLICT MODULE
Reason for Service Code CODE MESSAGE (FREE TEXT)
Drug-Allergy DA Name of Allergen
Prior Adverse Drug Reaction PR Drug Name
Drug-Disease (Inferred) DC Name of Drug or Inferred Condition
Drug-Disease (Reported) MC Name of Drug or Reported Condition
Drug-Age PA "Processor Free Text"
Drug-Gender SX "Processor Free Text"
Additive Toxicity AT Drug Trade Name
Drug-Pregnancy PG Pregnancy Contraindication
Iatrogenic Condition IC Drug Trade Name of previous drug
Side Effect SE "Processor Free Text"
DUPLICATE THERAPY MODULE
Reason for Service Code CODE MESSAGE (FREE TEXT)
Therapeutic Duplication TD Drug Name
Ingredient Duplication ID Drug Name
PRECAUTIONARY MODULE
Reason for Service Code CODE MESSAGE (FREE TEXT)
Alcohol OH Alcohol Precaution
Tobacco Use DS Tobacco Precaution
Drug-Lab Conflict DL Deferred
Drug-Food Interaction DF Food Name, "Food" or "Processor Free Text"

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 992 -
Call Help Desk CH Help Desk Phone Number
Notes on use of CH (Call Help Desk Reason for Service Code): Use this code with caution. Overuse can cause serious problems for both
the pharmacy and the processor. (For example, not enough phone lines to the processor or not enough processor agents to answer "Call
Help Desk" calls from the pharmacy.) If at all possible, assign the problem another standard DUR Reason for Service Code and include
additional information in the claim's free text field to further explain the DUR conflict.
42.3.2 DUR ACTION CODE MESSAGES
DUR Action Code
When a processor sends a pharmacy a standard DUR message alert in the online claim response, the pharmacist must act upon the
message. That pharmacist's action can be an intervention or the decision to ignore the message. If the DUR conflict message received by
the pharmacist is considered relevant, the pharmacist's resulting actions are called "interventions" in the NCPDP ORDUR standard model.
The pharmacist translates his or her actions that result from a DUR conflict alert received from the ECM processor or from the pharmacy
system's independent capabilities into DUR action codes that define the conflict, intervention, and outcome. The pharmacist can transmit
these standard action codes to the ECM processor in all pharmacist-to-processor claim transaction formats.
The NCPDP Telecommunication Standard defines standard codes for the pharmacist to use in documenting these interventions. The
pharmacist can document the intervention whether it occurred as a result of an ECM ORDUR processor's DUR alert message or as a result of
the pharmacist's independent determination that DUR conflict has occurred. Under either circumstance, the pharmacist can tie the
intervention type to one standard Reason for Service Code. For example, a code exists to let the pharmacist indicate he has contacted the
physician regarding a drug interaction conflict noted.
In addition to documenting interventions associated with various conflicts, the NCPDP standard lets the pharmacist tie the conflict and
intervention to an "outcome" of the dispensing process. For example, the outcome of the dispensing process might be changing the
prescription that generated the conflict, filling the prescription unchanged, or not filling the prescription at all.
NCPDP developed relatively simple, standard intervention codes to encourage their use. In producing the standard, an overly complex coding
scheme could result in limited use or misuse of codes. Similarly, the outcome code list is currently limited to the consensus agreement
reached between processors and providers who were members of the NCPDP DUR Work Group.
By receiving transactions from the pharmacist that contain the intervention action taken and the outcome result of the intervention, ECM
ORDUR processors and payer can evaluate the relevance of their standard DUR messages to improved health status and direct cost savings.
Over time, if a large number of DUR conflict and intervention/outcome response events are studied in relationship to other healthcare data, it
may be possible to measure the indirect value of ORDUR processing on patient health and cost and quality of medical care.
42.3.3 DUR INFORMATION ENTRY
The NCPDP Telecommunication Standard provides for:
Claim transmission
Claim reversal
DUR informational response from store (with DUR action & outcome codes)
Reversal response (with DUR action & outcome codes)
The following sections discuss the various stages of the dispensing and claims billing processes where DUR information can be entered. We
also discuss the functional capabilities of the NCPDP Telecommunication Standard transactions as they relate to DUR information transfer at
each stage of the process.
Transactions
Drug Utilization Review information may be entered at various stages of the drug benefit delivery and claims billing process. Stage 1 DUR
information entered on transmission of claims from the pharmacy provider to the ECM processor or claims "transactions" is the first point
where information related to DUR may be entered.
Original Claim
Most pharmacy systems can perform many of the DUR checks that are available to processors. When the pharmacist enters a prescription
into the pharmacy system, a DUR conflict might be detected by that system before the pharmacist sends a claim transaction to the processor.
This type of DUR screening, before claims are transmitted to the ECM processor, must be considered as it might affect and interact with the
ORDUR processing goals of the processor.
The pharmacist should submit these messages on the original claim so that the processor can record any DUR conflicts that the pharmacist
has already overridden.
Claim Reversal
The pharmacist uses claim reversal transaction formats primarily to adjust, or reverse out, successfully adjudicated paid claim responses that
were received for a prescription that for some reason the patient never obtained.
With DUR functionality in the NCPDP Telecommunication Standard, reversals might occur as a result of standard DUR messages the ECM
processor sent and DUR interventions the pharmacist made. In the following two examples, the pharmacist transmits a DUR action code
message with a reversal to document an action taken resulting from the standard DUR message the processor sent.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 993 -
In the case of a prescription reversed as a result of DUR, even though the pharmacist will no longer be paid for dispensing the original
prescription, the pharmacist might be eligible for reimbursement for delivering the cognitive services that resulted in the reversal decision. The
NCPDP Telecommunication Standard supports the functionality of the pharmacist to reverse originally approved prescriptions, provide DUR
action code messages, and request reimbursement in the reversal transaction.
In another case, the pharmacist might note a conflict, intervene in some way, and as a result fill the prescription with a different drug. The
pharmacist would then submit a reversal for the original claim, indicating that a new claim was imminent using Result of Service code IE "Filled
with different drug."
Information Reporting Transaction
The pharmacist can send transactions with DUR relevant information to the processor for informational purposes only. The submission can
include therapy for drugs not normally covered or submitted to the processor. The transaction does not have to include DUR action codes; its
purpose is only to update the processor about over-the-counter or other drug therapy that has potential relevance to future ORDUR processing
for the patient.
Various situations create the need for an information-only transaction.
First, uncaptured prescription claims data at the point-of-sale can create situations where a patient is vulnerable to therapeutic conflicts without
detection. This lost data can compromise even the best ORDUR processing programs. Data can be lost for various reasons.
• Most claims processors and sponsors do not currently suggest that the pharmacist submit a claim for non-covered medication.
• The trend toward higher cost sharing prevents the pharmacist from transmitting many claims (for example, where the copayment
exceeds the cost of the drug product).
• Patients sometimes switch the prescription to an over-the-counter drug (some of which cause significant drug interactions) for which
the pharmacist sends no transaction.
Second, claims that a processor denies are usually not subject to DUR processing. However, although dispensed to a member for cash
payment, the drug might still be relevant to future ORDUR processing. When a processor denies an original claim, the pharmacist can submit
an information-only transaction with or without DUR action code messages. Then, any relevant information will be available to the processor
for future ORDUR processing.
The pharmacist submits an Information Reporting claim and DUR messaging to document the cognitive service performed and outcome
results on the subject prescription. These transactions are significantly flexible and powerful tools that help the payer and ECM processor to
determine which types of ORDUR processing are most cost effective and which modes of administrative processing result in cost effective
capture of relevant DUR information.
We offer in summary the following guidelines as to when a pharmacy provider should use them to transmit DUR information.
The original prescription claim is denied or not covered and submitting the information will assist in future ORDUR processing
accuracy.
The original prescription is denied with a DUR conflict reported and the pharmacist wants to document DUR intervention and
outcome actions associated with the message on the denied claim by submitting a DUR action code message.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 994 -
43. APPENDIX G. TWO-WAY COMMUNICATION TO INCREASE THE VALUE
OF ON-LINE MESSAGING
43.1 BACKGROUND
Information on the incidence of claim rejections by reject code across the industry is not readily available. The following table illustrates the
most frequent reject codes from one claim processor’s data. Nothing is implied regarding a patient population, date range, or any other criteria
for inclusion or exclusion of claims from that which generated this table. Data from another processor, during a specified time frame, and/or
for a specific set of client parameters may yield different results. This information is simply representative and to be used in the context of the
examples and recommendations within this document. Several of these rejections contain reject codes that can and should be further
explained through the use of the Additional Message Information field (526-FQ).
Table 1: Distribution of Rejected Claims by NCPDP Code, © NDCHealth, 2ØØ3
% of Total
Rejected
Claims
NCPDP Reject
Code
NCPDP Reject Code Translation
15% “76 “ Plan Limitations Exceeded
12% “79 “ Refill Too Soon
12% “52 “ Non-Matched Cardholder ID
1Ø% “69 “ Filled After Coverage Terminated
7% “68 “ Filled After Coverage Expired
7% “7Ø “ Product/Service Not Covered
6% “Ø6 “ M/I Group ID
5% “19 “ M/I Days Supply
4% “88 “ DUR Reject Error
4% “65 “ Patient Is Not Covered
3% “Ø7 “ M/I Cardholder ID
3% “54 “ Non-Matched Product/Service ID Number
3% “75 “ Prior Authorization Required
2% “Ø9 “ M/I Date of Birth
2% “51 “ Non-Matched Group ID
2% “92 “ System Unavailable/Host Unavailable
Instances of messages that are not clear and effective can occur at various times during and at different points in the processing of
prescription drug claims. For example, pharmacy systems do not always translate the NCPDP Reject Codes into the specified NCPDP reject
messages. This can lead to confusion in interpreting the displayed reject message, because the message that is presented is not the same
message specified by the processor. In addition, claims processing systems sometimes populate free text fields with text that duplicates the
reject code translations, resulting in redundant information. Redundancy may also occur when DUR information is placed in the claim
message fields in addition to the information in the DUR Segment.
Plan rejections and supplementary messages can often be incomplete, leaving the pharmacist without a recommended course of action. For
example, an NCPDP Reject Code of “76 “ (Plan Limitations Exceeded) without an accompanying free text message explaining the limitation
does not provide enough information for the pharmacist to take action. Likewise, “Refill Too Soon” rejects sometime do not inform the
pharmacist of the next available fill date, while “Drug Not Covered” rejections do not always supply the names of the covered alternatives.
Eight of the reject codes listed in Table 1 (“52 “, “69 “, “68 “, “Ø6 “, “65 “, “Ø7 “, “Ø9 “, “51 “) are caused by eligibility problems, and collectively
have a high degree of occurrence. These errors are primarily due to incomplete or inaccurate eligibility data that the health plan/employer
supplied to the claims processor. When a processor cannot find a match in their eligibility files using the submitted claim information, the
exact data element that is causing the problem is not always known. Therefore, the processor will often send multiple reject codes to the
pharmacy, even though there may be only one error on the claim. For example, if the Cardholder ID field were submitted with an error, the
processor would be unable to identify the patient, but may also be unable to identify the specific cause of the error, because errors in any of
several fields (i.e. Group Number, BIN number) can cause misidentified/unidentified cardholders. As a result, the processor should send an
error message for each field that may have erroneous data. Some processors utilize other submitted data elements, like Date of Birth and
Gender Code to find a “best two out of three” match. Transmittal of such information from pharmacy to processor is subject to covered entity
interpretation under the HIPAA regulations.
43.2 SPECIFIC DATA FIELD USE RECOMMENDATIONS
Claims processors:
Be more specific in the information relayed to the pharmacist. Pharmacists want messages to be relevant and actionable.
Use the most specific reject code(s) possible.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 995 -
Eliminate unnecessary free text messages.
Populate the Help Desk Phone Number field (55Ø-8F) with the applicable phone number for the pharmacist to call for additional
assistance. If multiple phone numbers exist for different issue types (technical claim support versus clinical prior authorization
support), return the most appropriate phone number for the situation at hand.
Use only standard abbreviations in the Additional Message Information field (526-FQ). Keep the messages succinct. The NCPDP
Reject Code Translations must not be placed in this field.
Target DUR inter-pharmacy conflicts (rather than intra-pharmacy conflicts) and all DUR messages should remain in the Response
DUR/PPS Segment.
Use the Additional Message Information field (526-FQ) to explain sudden changes in coverage issues, such as an increase in
copayment for a non-preferred drug product.
The URL field (987-MA) should be populated in the response transaction whenever possible to provide electronic address for
additional prior authorization information.
Software vendors:
Display the entire Additional Message Information field (526-FQ).
Show the standard definitions for the NCPDP Reject Codes.
New fields and new values for old fields are introduced in NCPDP Telecommunication Standard Implementation Guide. Some specific uses of
these fields are highlighted in the table below, along with possible alternative Reject Codes and recommended supplemental messages that
may be transmitted in the Additional Message Information field (526-FQ). In the table, the Reject Code in question is listed in the first row, but
the definition is not repeated. Supplemental messages, if any, which should be used with the Reject Code, are listed in the last column.
However, a supplemental message is not always needed, as the standard definition of the Reject Code may be self-explanatory.
These recommendations are intended as guidelines, not mandates, for use in pharmacy and claim processing systems to increase the value
of messaging. Their use is highly recommended, but not required. Additional operational and system improvements are beyond the scope of
this document, and are not discussed here.
43.2.1 BENEFIT- OR PLAN-GENERATED REJECTIONS
43.2.1.1 REJECT CODE “76 “ (PLAN LIMITATIONS EXCEEDED)
Related Reject
Codes NCPDP Reject Code Definition Supplementary Message/Notes
“76 “ Plan Limitations Exceeded Define the specific limit that caused the
rejection.
“73 “ Refills Are Not Covered “New Prescription Required” or “No Further
Claims for This Product Are Allowed”
“78 “ Cost Exceeds Maximum “Maximum Amount = $XXX”
“Submit Date > NN Days from Fill Date” “81 “ Claim Too Old
“Fill date > NNN Days from Written Date”
“AG “ Days Supply Limitations for Product/Service “Maximum Days Supply = XXX Days.”
“M4 “ Prescription/Service Reference Number/Time
Limit Exceeded
Define the number of Rxs allowed within a
given time period
“RN “ Plan Limits Exceeded on Intended Partial Fill
Values
“Maximum Days Supply = XXX Days”
“7Ø “ Product/Service Not Covered “Specific Plan Exclusion”
“6Ø “ Product/Service Not Covered for Patient Age Maximum (or Minimum) Age = NN Years
“61 “ Product/Service Not Covered for Patient Gender
“AG “ Days Supply Limitations for Product/Service Maximum Days Supply = XXX Days
“M4 “ Prescription/Service Reference Number/Time
Limit Exceeded
Define the number of prescriptions allowed
within a given time period
“RN “ Plan Limits Exceeded on Intended Partial Fill
Values
Maximum Days Supply = XXX Days
“66 “ Patient Age Exceeds Maximum Age Maximum Patient Age = XX Years
43.2.1.2 REJECT CODE “79 “ (REFILL TOO SOON)
Related Reject
Codes NCPDP Reject Code Definition Supplementary Message/Notes
“79 “ Refill Too Soon “Next Available Fill Date = MM/DD/CCYY”
43.2.1.3 REJECT CODE “52 “ (NON-MATCHED CARDHOLDER ID)
Related Reject
Codes NCPDP Reject Code Definition Supplementary Message/Notes
“52 “ Non-Matched Cardholder ID Since this code is often transmitted with
other reject codes, an example of a
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 996 -
supplementary message is: “One or more of
these reasons may apply.”
43.2.1.4 REJECT CODE “69 “ (FILLED AFTER COVERAGE TERMINATED)
Related Reject
Codes NCPDP Reject Code Definition Supplementary Message/Notes
“69 “ Filled After Coverage Terminated “Terminated MM/DD/CCYY.”
43.2.1.5 REJECT CODE “68 “ (FILLED AFTER COVERAGE EXPIRED)
Related Reject
Codes NCPDP Reject Code Definition Supplementary Message/Notes
“68 “ Filled After Coverage Expired “Coverage Expired MM/DD/CCYY.”
“66 “ Patient Age Exceeds Maximum Age “Maximum Patient Age = XX Years.”
43.2.1.6 REJECT CODE “7Ø “ (PRODUCT/SERVICE NOT COVERED)
Related Reject
Codes NCPDP Reject Code Definition Supplementary Message/Notes
“Specific Plan Exclusion.” “7Ø “ Product/Service Not Covered
“Non-Formulary Product.” The Preferred
Product fields should be populated, and the
pharmacy system should display them. *
“6Ø “ Product/Service Not Covered for Patient Age “Maximum (or Minimum) Age = NN Years”
“61 “ Product/Service Not Covered for Patient Gender
“63 “ Institutionalized Patient Product/Service ID Not
Covered
“73 “ Refills Are Not Covered
“AC “ Product Not Covered Non-Participating
Manufacturer
Identify the covered manufacturer(s).
“AH “ Unit Dose Packaging Only Payable For Nursing
Home Recipients
“AJ “ Generic Drug Required
* Use the Response Claim Segment to provide the Preferred Product ID (553-AR), its Qualifier (552-AP), Description (556-AU), Incentive
(554-AS) and Copay/Coinsurance Incentive (555-AT) whenever applicable, with the Preferred Product Count (551-9F). If multiple preferred
products are possible, use the Preferred Product Count (551-9F) field, populating it with the correct number of products and repeat the above
fields as needed.
43.2.1.7 REJECT CODE “Ø6 “ (M/I GROUP ID)
Related Reject
Codes NCPDP Reject Code Definition Supplementary Message/Notes
“Ø6 “ M/I Group ID Since this code is often transmitted with
other reject codes, an example of a
supplementary message is: “One or more of
these reasons may apply.”
“RD “ Mismatched Cardholder/Group ID-Partial to
Completion
43.2.1.8 REJECT CODE “19 “ (M/I DAYS SUPPLY)
Related Reject
Codes NCPDP Reject Code Definition Supplementary Message/Notes
“19 “ M/I Days Supply Do not use this reject code for claims that
exceed a days supply limitation – use Code
76 and indicate the maximum days supply in
the Message fields.
43.2.1.9 REJECT CODE “88 “ (DUR REJECT ERROR)
Related Reject
Codes NCPDP Reject Code Definition Supplementary Message/Notes
“88 “ DUR Reject Error Note that the Response DUR/PPS Segment
does not indicate which of the potentially
multiple DUR Alerts caused the rejection—
one or more of the other Alerts may just be
informational warning messages. Consider
indicating in the Additional Message
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 997 -
Related Reject
Codes NCPDP Reject Code Definition Supplementary Message/Notes
Information field (526-FQ) the DUR Alert
number(s) that caused the rejection, for
example, “DUR Alerts 1 and 2 are Rejection
Alerts.”
43.2.1.10 REJECT CODE “65 “ (PATIENT IS NOT COVERED)
Related Reject
Codes NCPDP Reject Code Definition Supplementary Message/Notes
“Maximum Patient Age for this drug is XX
years.”
“6Ø “ Product/Service Not Covered for Patient Age
“Minimum Patient Age for this drug is XX
years.”
“61 “ Product/Service Not Covered for Patient Gender
“63 “ Institutionalized Patient Product/Service ID Not
Covered
“66 “ Patient Age Exceeds Maximum Age “Maximum Patient Age for this drug is XX
years.”
43.2.1.11 REJECT CODE “Ø7 “ (M/I CARDHOLDER ID)
Related Reject
Codes NCPDP Reject Code Definition Supplementary Message/Notes
“Ø7 “ M/I Cardholder ID Since this code is often transmitted with
other reject codes, an example of a
supplementary message is: “One or more of
these reasons may apply.”
“RD “ Mismatched Cardholder/Group ID-Partial to
Completion
43.2.1.12 REJECT CODE “54 “ (NON-MATCHED PRODUCT/SERVICE ID NUMBER)
Related Reject
Codes NCPDP Reject Code Definition Supplementary Message/Notes
“54 “ Non-Matched Product/Service ID Number “No Active NDC number found.”
“55 “ Non-Matched Product Package Size
“77 “ Discontinued Product/Service ID Number If a replacement NDC and date are known,
“Superceded by NNNNN-NNNN-NN on
MM/DD/YY.”
43.2.1.13 REJECT CODE “75 “ (PRIOR AUTHORIZATION REQUIRED)
Related Reject
Codes NCPDP Reject Code Definition Supplementary Message/Notes
“75 “ Prior Authorization Required Processors should populate the Help Desk
Phone Number field (55Ø-8F) and system
vendors should display the contents of this
field when this reject code appears.
The URL field (987-MA) should be populated
in the response transaction whenever
possible.
“3W “ Prior Authorization in Process “Requested on MM/DD/CCYY.” Processors
should populate the Help Desk Phone
Number field (55Ø-8F) and system vendors
should display the contents of this field when
this reject code appears.
The URL field (987-MA) should be populated
in the response transaction whenever
possible.
“3X “ Authorization Number Not Found Processors should populate the Help Desk
Phone Number field (55Ø-8F) and system
vendors should display the contents of this
field when this reject code appears.
The URL field (987-MA) should be populated
in the response transaction whenever
possible.
“3Y “ Prior Authorization Denied Processors should populate the Help Desk
Phone Number field (55Ø-8F) and system

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 998 -
Related Reject
Codes NCPDP Reject Code Definition Supplementary Message/Notes
vendors should display the contents of this
field when this reject code appears.
The URL field (987-MA) should be populated
in the response transaction whenever
possible.
“G4 “ Physician must contact plan Processors should populate the Help Desk
Phone Number field (55Ø-8F) and system
vendors should display the contents of this
field when this reject code appears.
The URL field (987-MA) should be populated
in the response transaction whenever
possible.
“G5 “ Pharmacist must contact plan Processors should populate the Help Desk
Phone Number field (55Ø-8F) and system
vendors should display the contents of this
field when this reject code appears.
The URL field (987-MA) should be populated
in the response transaction whenever
possible.
43.2.1.14 REJECT CODE “Ø9 “ (M/I DATE OF BIRTH)
Related Reject
Codes NCPDP Reject Code Definition Supplementary Message/Notes
“Ø9 “ M/I Date Of Birth Since this code is often transmitted with
other reject codes, an example of a
supplementary message is: “One or more of
these reasons may apply.”
43.2.1.15 REJECT CODE “51 “ (NON-MATCHED GROUP ID)
Related Reject
Codes NCPDP Reject Code Definition Supplementary Message/Notes
“51 “ Non-Matched Group ID Since this code is often transmitted with
other reject codes, an example of a
supplementary message is: “One or more of
these reasons may apply.”
43.2.1.16 REJECT CODE “92 “ (SYSTEM UNAVAILABLE/HOST UNAVAILABLE)
Related Reject
Codes NCPDP Reject Code Definition Supplementary Message/Notes
“92 “ System Unavailable/Host Unavailable
“9Ø “ Host Hung Up
“91 “ Host Response Error
“95 “ Time Out
“96 “ Scheduled Downtime “Expected to resume at HH:MM EST” or
CST, MST, PST, EDT, CDT, MDT, PDT as
appropriate based on the processor’s
location.
“97 “ Payer Unavailable
“98 “ Connection to Payer Is Down “Expected to resume at HH:MM EST” or
CST, MST, PST, EDT, CDT, MDT, PDT as
appropriate based on the processor’s
location.
“99 “ Host Processing Error
43.2.2 OTHER NOTABLE REJECT CODES
43.2.2.1 REJECT CODE “83 “ (DUPLICATE PAID/CAPTURED CLAIM)
Related Reject
Codes NCPDP Reject Code Definition Supplementary Message/Notes
“83 “ Duplicate Paid/Captured Claim “Change fill number if multiple fills are
requested on same day.”
43.2.2.2 REJECT CODE “53 “ (NON-MATCHED PERSON CODE)
Related Reject NCPDP Reject Code Definition Supplementary Message/Notes
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 999 -
Codes
“53 “ Non-Matched Person Code Since this code is often transmitted with other
reject codes, an example of a supplementary
message is: “One or more of these reasons
may apply.”
43.2.2.3 REJECT CODE “4Ø “ (PHARMACY NOT CONTRACTED WITH PLAN)
These additional Reject Codes would provide the type of pharmacy network through which the drug would be covered.
Related Reject
Codes NCPDP Reject Code Definition Supplementary Message/Notes
“G6 “ Pharmacy Not Contracted in Specialty Network Processors should populate the Help Desk
Phone Number field (55Ø-8F) and system
vendors should display the contents of this
field when this reject code appears.
“G7 “ Pharmacy Not Contracted in Home Infusion
Network
Processors should populate the Help Desk
Phone Number field (55Ø-8F) and system
vendors should display the contents of this
field when this reject code appears.
“G8 “ Pharmacy Not Contracted in Long Term Care
Network
Processors should populate the Help Desk
Phone Number field (55Ø-8F) and system
vendors should display the contents of this
field when this reject code appears.
“G9 “ Pharmacy Not Contracted in 9Ø Day Retail
Network (this message would be used when the
pharmacy is not contracted to provide a 9Ø days
supply of drugs)
Processors should populate the Help Desk
Phone Number field (55Ø-8F) and system
vendors should display the contents of this
field when this reject code appears.
If a given processor’s Payer Sheet indicates a specific field is required for the claim to process and that field is not submitted, the appropriate
field “M/I” Reject Code must be returned in the Reject Response and its appropriate Explanation/Definition displayed for the pharmacist.
43.3 DUR-GENERATED REJECTIONS
Claims processing systems should develop methods to provide a different set of criteria for DUR Alerts detected when the claims for the
interacting drugs originate from the same pharmacy versus DUR Alerts detected due to claims where the interacting drugs are dispensed at
different pharmacies. In some instances, DUR Alerts are based on information on a claims processor’s patient profile—data possibly collected
from multiple sources. Responses for same-pharmacy DUR Alerts (that is, the information was obtained from the same pharmacy submitting
the claim) should be significantly downgraded as compared to Alerts generated due to other pharmacy-submitted or other profiled data. One
possible answer is the development of three-tier DUR responses:
• Tier 1 = Other Pharmacy Response
• Tier 2 = Same Pharmacy without use of claim Professional Pharmacy Service (PPS) fields
• Tier 3 = Same Pharmacy when claim PPS fields are transmitted to processor
Alternatively, processors and pharmacists may determine the most appropriate process for identifying the origin of the Drug Use Evaluation
(DUE) alert.
Processors should utilize four levels of DUR Responses:
• Hard Reject: This level should be used for a small subset of Alerts. Only a processor-assigned Prior Authorization can override
“Hard Rejections”. Recommendations should be followed to define this subset of Alerts, but the decision of which Alerts actually fall
into this category should be left to the plan sponsors, due to processor liability concerns.
• Soft Reject: This level should be used for other severe or major DUR Alerts. Processor-assigned Prior Authorizations and
pharmacist-submitted NCPDP PPS codes can override these rejections. Enhancements must be made to in-house and processor
software systems to facilitate this NCPDP standard-facilitated activity (see below).
• Message Only Alerts: This level should be used for the remaining DUR Alerts that are deemed necessary to warn the pharmacist of
potential patient harm. Claims are not rejected, but the processor provides information to make the pharmacist aware of potential
problems and allow the pharmacist to make an informed decision whether or not to continue with the claim. Lack of any additional
claim activity (i.e. Claim Reversal) assumes the pharmacist has judged that the warning(s) is/are of no significance for the patient.
• No Alerts: At this level, DUR Alerts are generated by the processor, but not returned to the pharmacy. This allows retrospective
analysis of DUR Alerts, where the processor has determined that the immediate patient risk is minimal (low severity Alerts). This
level can also be used for those Alerts otherwise downgraded due to same pharmacy detection or the transmission of applicable
PPS codes with the claim.
It is prudent to note that even a statistically insignificant drug-drug interaction can be significant in any given patient. Patients do experience
low incidence and minor severity problems, and when this occurs, it is significant to them. There are always outliers in any study that attempts
to categorize DUR Alerts based on statistical probabilities. There are degrees of occurrence for every drug-drug interaction, and a problem
does not "always happen" or "never happens" in every patient. All stakeholders must recognize the need to balance the risk of suppression of
DUR messages in the interest of reducing noise to the risk of individual patient significance and harm of even low risk DUR Alerts.
Information fields in the Claim Submission should be used whenever possible when a pharmacist’s in-house system detects a drug-drug
interaction, but in the pharmacist’s professional judgment, it is decided that the interaction is of minimal risk to the patient and the product is

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 1000 -
dispensed. Some systems provide the capability for the pharmacist to document this decision internally. These documentations usually
include the description of the problem, the identity of the person making the decision, and the result of the decision. The NCPDP
Telecommunication Standard Implementation Guide contains the following fields in its Response DUR/PPS Segment that should map to these
documentations: Reason for Service Code (439-E4), Professional Service Code (44Ø-E5), Result of Service Code (441-E6), and DUR Co-
Agent ID (476-H6).
In situations in which the pharmacist decides to transmit a claim that he/she knows will trigger a DUR Alert, the PPS fields should be populated
with the correct codes and transmitted to the processor with the claim. If the Claims Processing system has functionality built around these
fields and codes, it then searches the claims and clinical databases, plus the patient demographic information on file to determine if DUR
problems exist. Then the processor should compare these submitted codes to criteria on the claims processing system to determine if the
defined DUR Alert response should be reduced or suppressed entirely.
The NCPDP Telecommunication Standard Implementation Guide does not differentiate between a Hard Reject and a Soft Reject. Both
situations simply generate a Reject Code of “88 “ (DUR Reject Error) and claims processors should populate the DUR Segment with the
appropriate values. In the event that a DUR Reject is transmitted to the pharmacy and the pharmacist desires to override the rejection, the
pharmacist should use the four PPS fields above and retransmit the claim. If the rejection was a “Soft Reject,” then this action may override
the rejection. If it will not override the rejection, the pharmacist can always call the phone number in the Help Desk Phone Number field (55Ø-
8F) and obtain a prior authorization or information that will override the rejection. The pharmacist should first attempt a second transaction
using the PPS codes—it may avert the need to call the Help Desk. Pharmacy systems can be built to facilitate the population of these PPS
fields. All DUR Alerts have the Reason for Service Code (439-E4) populated for each Alert. The value from the processor automatically
should be placed in this same field when building the claim re-submittal transaction. Then the pharmacist should be presented a list of values
for the Professional Service Code (44Ø-E5) and Result of Service Code (441-E6) fields to transmit. If the system programmer wants to further
enhance the system, the available values in these latter two fields can be reduced to only those codes that apply for a given Reason for
Service Code, thereby minimizing the long list of codes from which a pharmacist must choose.2
Implementation of these recommendations is voluntary. There is value in streamlining the on-line message functionality that exists within the
NCPDP Telecommunication Standard Implementation Guide. Selected benefits of more meaningful and actionable messages include
improved patient quality of care and saved time by all parties in researching and interpreting such messages.
43.4 PARTICIPATING ORGANIZATIONS
NCPDP would like to thank the following organizations that provided input and comments in the original writing of this appendix. The
organizations listed below should not be considered as endorsers for the content but rather contributors to information contained within the
appendix.
America’s Health Insurance Plans (AHIP)
Academy of Managed Care Pharmacy (AMCP)
American Pharmacists Association (APhA)
Blue Cross Blue Shield Association (BCBSA)
Council for Affordable Quality Healthcare (CAQH)
National Association of Chain Drug Stores (NACDS)
National Community Pharmacists Association (NCPA)
National Council for Prescription Drug Programs (NCPDP)
Pharmaceutical Care Management Association (PCMA)
43.5 LONG TERM CARE TRANSITION, EMERGENCY FILL AND CHANGE IN LEVEL
OF CARE MESSAGING FOR REJECTED AND PAID CLAIMS
43.5.1 BACKGROUND
There is a current need for an industry wide methodology for response messaging for claims that meet the transition period/emergency
fill/change in level of care criteria.
In the 2ØØ7 guidance CMS states: "In addition, we strongly encourage point-of-sale notification of enrollees about transition supplies by
pharmacists"
In this document we are recommending two possible methods that meet CMS’ notification criteria.
1) Deny the claim and provide messaging with a Prior Authorization Number Submitted(462-EV) (PA #) that allows the pharmacy to
override the denial at point of sale and indicates why the override is being allowed. Since the claim is being rejected the pharmacy
receives the reject code, which also indicates why the claim would not be paid outside the transition/emergency fill/change in level of
care.
2) Pay the claim and notify the pharmacy why the claim would have rejected if the claim would have been outside the
transition/emergency fill/change in level of care.
CMS Transition Guidance in Summary
CMS TRANSITION PERIOD REQUIREMENT
Non-LTC:” The minimum transition process standards described in Section I will apply to beneficiaries obtaining their drugs in a
retail setting (or via home infusion, safety-net, or I/T/U pharmacies). However, we clarify that, in the retail setting, the one-time,
temporary supply of non-formulary Part D drugs – including Part D drugs that are on a plan’s formulary but require prior
2 See NCPDP Data Dictionary and External Code List for list of values.

Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 1001 -
authorization or step therapy under a plan’s utilization management rules – must be for at least 3Ø days of medication, unless the
prescription is written by a prescriber for less than 3Ø days. Plans should note that, outside the long term care setting, such a
temporary fill may be a one-time fill only.”
LTC: “The minimum transition process standards described in Section I will apply to beneficiaries obtaining their drugs in a long-
term care setting. The temporary supply of non-formulary Part D drugs – including Part D drugs that are on a plan's formulary but
require prior authorization or step therapy under a plan's utilization management rules – for a new enrollee in a LTC facility must be
for at least 31 days (unless the prescription is written for less than 31 days). We are requiring a 31-day transition supply given that
many LTC pharmacies and facilities dispense medications in 31-day increments. However, unlike in the retail setting, plans must
honor multiple fills of non-formulary Part D drugs, including Part D drugs that are on a plan’s formulary but require prior authorization
or step therapy under a plan’s utilization management rules, as necessary during the entire length of the 9Ø-day transition period.”
CMS EMERGENCY FILL REQUIREMENT
Non-LTC: No CMS requirement (however plans may choose to offer this for non-LTC claims)
LTC: “Since, as a matter of general practice, LTC facility residents must receive their medications as ordered without delay, Part D
plans must cover an emergency supply of non-formulary Part D drugs for LTC facility residents as part of their transition process.
During the first 9Ø days after a beneficiary's enrollment, he or she will receive a transition supply via the process described above.
However, to the extent that an enrollee in a LTC setting is outside his or her 9Ø-day transition period, the plan must still provide an
emergency supply of non-formulary Part D drugs – including Part D drugs that are on a plan's formulary but require prior
authorization or step therapy under a plan's utilization management rules – while an exception is being processed. These
emergency supplies of non-formulary Part D drugs – including Part D drugs that are on a plan’s formulary but require prior
authorization or step therapy under a plan’s utilization management rules – must be for at least 31 days of medication, unless the
prescription is written by a prescriber for less than 31 days. We are requiring a 31-day emergency supply given that many LTC
pharmacies and facilities dispense medications in 31-day increments.”
CMS CHANGE IN LEVEL OF CARE REQUIREMENT
“In addition to circumstances impacting new enrollees who may enter a plan with a medication list that contains non-formulary Part D
drugs, other circumstances exist in which unplanned transitions for current enrollees could arise and in which prescribed drug
regimens may not be on plan formularies. These circumstances usually involve level of care changes in which a beneficiary is
changing from one treatment setting to another. For example, beneficiaries who enter LTC facilities from hospitals are sometimes
accompanied by a discharge list of medications from the hospital formulary, with very short term planning taken into account (often
under 8 hours). Similar situations may exist, for example, for beneficiaries who are discharged from a hospital to a home; for
beneficiaries who end their skilled nursing facility Medicare Part A stay (where payments include all pharmacy charges) and who
need to revert to their Part D plan formulary; for beneficiaries who give up hospice status to revert to standard Medicare Part A and
B benefits; for beneficiaries who end a long-term care facility stay and return to the community; and for beneficiaries who are
discharged from psychiatric hospitals with medication regimens that are highly individualized. For these unplanned transitions,
beneficiaries and providers must clearly avail themselves of plan exceptions and appeals processes. We have streamlined the
grievance, coverage determination, and appeals process requirements in order to ensure that beneficiaries receive quick
determinations regarding the medications they need. In all cases, we make it clear that a Part D plan sponsor is required to make
coverage determinations and re-determinations as expeditiously as the enrollee’s health condition requires. In addition, and as
described above, current enrollees entering LTC settings from other care settings will be provided emergency supplies of non-
formulary drugs – including Part D drugs that are on a plan’s formulary but require prior authorization or step therapy under a plan’s
utilization management rules”.
43.5.2 REJECTED CLAIM OPTION
Reject Codes NCPDP Reject Code Definition Supplementary Message/Notes
“N7 “ Use Prior Authorization Code Provided
During Transition Period
“N8 ” Use Prior Authorization Code Provided For
Emergency Fill
“N9 ” Use Prior Authorization Code Provided For
Level of Care Change
Used when a processor rejects a claim and
requires the claim to be resubmitted with a
prior authorization code to allow it to process.
“RL ” Transitional Benefit/Resubmit Claim
“TN ” Emergency Fill/Resubmit Claim
“TP ” Level of Care Change/Resubmit Claim
Used when processor automatically creates
and applies a prior auth to a claim previously
submitted in order to allow the resubmitted
claim to process.
43.5.2.1 WHEN PRIOR AUTHORIZATION NUMBER (498-PY) REQUIRED
In some situations of a Claim Billing, a rejected response may be sent from the payer to the pharmacy that requires the pharmacy to submit a
Prior Authorization Number in order to receive payment for the claim. An example of a situation may include a Benefit Transition Period that
allows for payment of claims, for a period of time that would normally reject.
When a rejection of this nature is returned and a Reject Code (511-FB) of
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 1002 -
• “N7 ” Use Prior Authorization Code Provided During Transition Period,
• “N8 ” Use Prior Authorization Code Provided For Emergency Fill
• “N9 ” Use Prior Authorization Code Provided For Level of Care Change
is returned, the Prior Authorization Number-Assigned (498-PY) field of the Response Prior Authorization Segment must also be returned. The
pharmacy will take the value from the Prior Authorization Number-Assigned (498-PY) of the response and place it in the field Prior
Authorization Number-Submitted (462-EV) of the Claim Segment. The pharmacy will then resubmit the claim.
43.5.2.2 TRANSITION AND SAFETY-RELATED REJECTS
From CMS:
“We note that although Part D plans may implement quantity limits for safety purposes or drug utilization edits that are based upon
approved product labeling during a beneficiary’s transition period, to the extent that the prescription is dispensed for less than the
written amount due to a plan edit, plans must provide refills for that transition supply (up to a 3Ø-day supply in a retail setting and a
9Ø-day supply in a long-term care setting). For example, if a beneficiary presents at a retail pharmacy with a prescription for one
tablet per day for 3Ø days and a plan has a quantity limit edit in place that limits the days supply to 14 per prescription for safety
purposes, the beneficiary would receive a 14-day supply (consistent with the safety edit). At the conclusion of the 14-day supply, the
beneficiary should be entitled to another 14-day supply while he/she continues to pursue an exception with the Part D plan, or a
switch to a therapeutic alternative that is on the plan’s formulary.”
Reject Codes NCPDP Reject Code Definition Supplementary Message/Notes
“TQ “ Dosage Exceeds Product Labeling Limit Fields Possibly in Error 442-E7, 4Ø5-D5
When this reject code is returned in the response, a prior authorization number will not be returned in the prior authorization segment as CMS
has stated that safety related rejects are not required to be overridden during transition.
43.5.3 CLAIMS PAID DUE TO CMS INITIAL ELIGIBILITY TRANSITION PERIOD
43.5.3.1 APPROVED MESSAGE CODE “ØØ4” (FILLED DURING TRANSITION BENEFIT)
If during the transition period a claim is not rejected (claim is paid) and the processor paid the claim by setting errors to soft or has the ability to
tell you why this claim would have rejected, use the following codes.
Approved Message
Codes (548-6F) NCPDP Approved Message Code
Definition Supplementary Message/Notes
“ØØ5“ Filled During Transition Benefit/Prior
Authorization Required
Mapped when Reject Code
“75 “ (Prior Authorization Required) is
overridden
“ØØ6“ Filled During Transition Benefit /Non-
Formulary
Mapped when Reject Code
“61 “ (Product/Service Not Covered For
Patient Gender),
“6Ø “ (Product/Service Not Covered For
Patient Age),
“7Ø “ (Product/Service Not Covered)
is overridden
“ØØ7“ Filled During Transition Benefit /Other
Rejection (e.g. Step Therapy, Benefit
Maximum, Generic First Requirement, and
Non- safety related DUR)
Mapped when Reject Code
“76 “ (Plan Limitations Exceeded),
“78 “ (Cost Exceeds Maximum),
“8Ø “ (Drug-Diagnosis Mismatch),
“88 “ (DUR Reject Error)
is overridden
43.5.4 CLAIMS PAID DUE TO CMS EMERGENCY FILL REQUIREMENT
43.5.4.1 APPROVED MESSAGE CODE “ØØ8” (EMERGENCY FILL SITUATION)
If a claim that meets emergency fill criteria is not rejected (claim is paid) and the processor paid the claim by setting errors to soft or has the
ability to tell you why this claim would have rejected, use the following codes.
Approved Message
Codes (548-6F) NCPDP Approved Message Code
Definition Supplementary Message/Notes
“ØØ9“ Emergency Fill Situation/ Prior Authorization
Required
Mapped when Reject Code
“75 “ (Prior Authorization Required)
is overridden
“Ø1Ø“ Emergency Fill Situation/ /Non-Formulary Mapped when Reject Code
“61 “ (Product/Service Not Covered For
Patient Gender),
“6Ø “ (Product/Service Not Covered For
Patient Age),
“7Ø “ (Product/Service Not Covered)
is overridden
“Ø11“ Emergency Fill Situation/Other rejection
(e.g. Step Therapy, Benefit Maximum,
Generic First Requirement, and Non- safety
related DUR)
Mapped when Reject Code
“76 “ (Plan Limitations Exceeded),
“78 “ (Cost Exceeds Maximum),
“8Ø “ (Drug-Diagnosis Mismatch),
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 1003 -
“88 “ (DUR Reject Error)
is overridden
43.5.5 CLAIMS PAID DUE TO CMS CHANGE IN LEVEL OF CARE REQUIREMENT
43.5.5.1 APPROVED MESSAGE CODE “Ø12” (LEVEL OF CARE CHANGE)
If a claim that meets level of care change criteria is not rejected (claim is paid) and the processor and the processor paid the claim by setting
errors to soft or has the ability to tell you why this claim would have rejected, use the following codes.
Approved Message
Codes (548-6F) NCPDP Approved Message Code
Definition Supplementary Message/Notes
“Ø13“ Level Of Care Change/ Prior Authorization
Required
Mapped when Reject Code
“75 “ (Prior Authorization Required)
is overridden
“Ø14“ Level Of Care Change/Non-Formulary Mapped when Reject Code
“61 “ (Product/Service Not Covered For
Patient Gender),
“6Ø “ (Product/Service Not Covered For
Patient Age),
“7Ø “ (Product/Service Not Covered)
is overridden
“Ø15“ Level Of Care Change/Other rejection (e.g.
Step Therapy, Benefit Maximum, Generic
First Requirement, and Non- safety related
DUR)
Mapped when Reject Code
“76 “ (Plan Limitations Exceeded),
“78 “ (Cost Exceeds Maximum),
“8Ø “ (Drug-Diagnosis Mismatch),
“88 “ (DUR Reject Error)
is overridden
Telecommunication Standard Implementation Guide Version D.Ø
Version D.Ø August 2ØØ7
***OFFICIAL RELEASE***
©National Council for Prescription Drug Programs, Inc.
Confidential Material - Not for Distribution Without Permission of Authors
- 1004 -
44. APPENDIX H. ROUTE OF ADMINISTRATION TRANSITION
This appendix was added to assist in transition from the NCPDP code values formerly found in Compound Route of Administration (452-EH) in
the Compound Segment to the Route of Administration (995-E2) in the Claim Segment, which only uses Systematized Nomenclature of
Medicine Clinical Terms® (SNOMED CT) available at http://www.snomed.org/.
Prior to Version C.4, Compound Route of Administration was used. In Version C.4, Compound Route of Administration was sunsetted. Route
of Administration, supported in Version C.4 and above, uses the SNOMED values – column “High Level”.
NCPDP Description High level high level description
1 Buccal 54471007 Buccal route (qualifier value)
2 Dental 372449004 Dental route (qualifier value)
2 Dental 372449004 Dental route (qualifier value)
3 Inhalation 112239003 By inhalation (route) (qualifier value)
4 Injection 385218009 By injection (route) qualifier value)
5 Intraperitoneal 38239002 Intraperitoneal route (qualifier value)
6 Irrigation 47056001 By irrigation (route) (qualifier value)
7 Mouth/Throat 26643008 Oral route (qualifier value)
8 Mucous Membrane 419874009 Submucosal route (qualifier value)
9 Nasal 46713006 Nasal route (qualifier value)
1Ø Ophthalmic 54485002 Ophthalmic route (qualifier value)
11 Oral 26643006 Oral route (qualifier value)
12 Other/Miscellaneous NA
13 Otic 10547007 Otic route (qualifier value)
14 Perfusion C444364 By infusion (route) qualifier value)
15 Rectal 37161004 Per rectum (route) (qualifier value)
16 Sublingual 37839007 Sublingual route (qualifier value)
17 Topical 419464001 Iontophoresis route (qualifier value)
18 Transdermal 372464004 Intradermal route (qualifier value)
19 Translingual 37839007 Sublingual route (qualifier value)
21 Vaginal 16857009 Per vagina (route) (qualifier value)
22 Enteral 417985001 Enteral route (qualifier value)
1Ø Ophthalmic 54485002 Ophthalmic route (qualifier value)
2Ø Urethral 90028008 Urethral route (qualifier value)