The ACS Style Guide Effective Communication Of Scientific Information 3rd
The%20ACS%20Style%20Guide_%20Effective%20Communication%20of%20Scientific%20Information-3rd
User Manual:
Open the PDF directly: View PDF ![]() .
.
Page Count: 445 [warning: Documents this large are best viewed by clicking the View PDF Link!]
- Cover Page
- Title Page
- ISBN 978-0841239999
- Contents
- Foreword
- Preface
- Contributors
- Part 1 Scientific Communication
- Part 2 Style Guidelines
- CHAPTER 9 Grammar, Punctuation, and Spelling
- CHAPTER 10 Editorial Style
- CHAPTER 11 Numbers, Mathematics, and Units of Measure
- CHAPTER 12 Names and Numbers for Chemical Compounds
- CHAPTER 13 Conventions in Chemistry
- CHAPTER 14 References
- CHAPTER 15 Figures
- CHAPTER 16 Tables
- CHAPTER 17 Chemical Structures
- CHAPTER 18 Selected Bibliography
- Index

➤ ➤ ➤ ➤ ➤
The ACS
Style Guide

➤ ➤ ➤ ➤ ➤ THIRD EDITION
The ACS
Style Guide
Effective Communication
of Scientific Information
Anne M. Coghill
Lorrin R. Garson
Editors
AMERICAN CHEMICAL SOCIETY Washington, DC
OXFORD UNIVERSITY PRESS New York Oxford
2006
Oxford University Press
Oxford New York
Athens Auckland Bangkok Bogotá Buenos Aires Calcutta
Cape Town Chennai Dar es Salaam Delhi Florence Hong Kong Istanbul
Karachi Kuala Lumpur Madrid Melbourne Mexico City Mumbai
Nairobi Paris São Paulo Singapore Taipei Tokyo Toronto Warsaw
and associated companies in
Berlin Idaban
Copyright © 2006 by the American Chemical Society, Washington, DC
Developed and distributed in partnership by the
American Chemical Society and Oxford University Press
Published by Oxford University Press, Inc.
198 Madison Avenue, New York, NY 10016
Oxford is a registered trademark of Oxford University Press
All rights reserved. No part of this publication may be reproduced, stored in a retrieval
system, or transmitted, in any form or by any means, electronic, mechanical, photocopying,
recording, or otherwise, without the prior permission of the American Chemical Society.
Library of Congress Cataloging-in-Publication Data
The ACS style guide : effective communication of scientific information.—3rd ed. /
Anne M. Coghill [and] Lorrin R. Garson, editors.
p. cm.
Includes bibliographical references and index.
ISBN-13: 978-0-8412-3999-9 (cloth : alk. paper)
1. Chemical literature—Authorship—Handbooks, manuals, etc. 2. Scientific literature—
Authorship—Handbooks, manuals, etc. 3. English language—Style—Handbooks, manuals,
etc. 4. Authorship—Style manuals.
I. Coghill, Anne M. II. Garson, Lorrin R. III. American Chemical Society
QD8.5.A25 2006
808'.06654—dc22 2006040668
1 3 5 7 9 8 6 4 2
Printed in the United States of America
on acid-free paper
v
➤ ➤ ➤ ➤ ➤
Contents
Foreword. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . vii
Madeleine Jacobs
Preface. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .ix
Contributors. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .xiii
Part 1. Scientific Communication
1. Ethics in Scientific Communication . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Gordon G. Hammes
appendix 1-1: Ethical Guidelines to Publication of Chemical Research. . . . . . .11
2. Scientific Papers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
3. The Editorial Process. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27
appendix 3-1: Proofreaders’ Marks . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .36
4. Writing Style and Word Usage . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 41
5. Electronic Submission of Manuscripts Using Web-Based Systems. . . . . . . 59
Sarah C. Blendermann
appendix 5-1: Online Submission at Selected Scientific Publishers
and Research Grant Agencies . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .65
appendix 5-2: Key Features of Selected Online Submission Systems. . . . . . . . .68
6. Peer Review . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 71
Barbara A. Booth
7. Copyright Basics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 77
Karen S. Buehler, C. Arleen Courtney, and Eric S. Slater
vi ➤ The ACS Style Guide
8. Markup Languages and the Datument . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 87
Peter Murray-Rust and Henry S. Rzepa
appendix 8-1: The IUPAC International Chemical Identifier, InChI. . . . . . . .101
Stephen R. Heller and Alan D. McNaught
Part 2. Style Guidelines
9. Grammar, Punctuation, and Spelling . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .105
appendix 9-1: Recommended Spelling List . . . . . . . . . . . . . . . . . . . . . . . . . . . .129
10. Editorial Style . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .135
appendix 10-1: Computer and Internet Terms . . . . . . . . . . . . . . . . . . . . . . . . .163
appendix 10-2: Abbreviations, Acronyms, and Symbols. . . . . . . . . . . . . . . . . .169
11. Numbers, Mathematics, and Units of Measure . . . . . . . . . . . . . . . . . . . . . . . . 203
appendix 11-1: The International System of Units (SI) . . . . . . . . . . . . . . . . . . .228
12. Names and Numbers for Chemical Compounds . . . . . . . . . . . . . . . . . . . . . .233
appendix 12-1: End-of-Line Hyphenation of Chemical Names . . . . . . . . . . . .247
appendix 12-2: Representation of Combinatorial Chemistry . . . . . . . . . . . . .250
Derek Maclean
appendix 12-3: CAS Registry Numbers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .253
13. Conventions in Chemistry . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .255
appendix 13-1: Symbols for Commonly Used Physical Quantities . . . . . . . . .277
appendix 13-2: The Crystallographic Information File. . . . . . . . . . . . . . . . . . .284
Frank H. Allen
14. References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .287
Janet S. Dodd, Leah Solla, and Paula M. Bérard
appendix 14-1: CASSI Abbreviations for the 1000+ Most Commonly
Cited Journals. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .328
appendix 14-2: A Sample CASSI Entry . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .340
15. Figures. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .343
Betsy Kulamer
16. Tables. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 369
Betsy Kulamer
17. Chemical Structures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 375
Antony Williams
18. Selected Bibliography . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .385
Index. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .389
vii
I
fell in love with chemistry when I was 13. I fell in love with
writing at the age of four when I learned to read. Indeed, my
love of writing, and of writing well, was inspired by my love of reading. Perhaps
that is true for all writers.
Fortunately for me, I have been able to combine my love of chemistry with
my love of reading and writing in a long career as a science communicator and
journalist. Most recently, I served for eight and a half years as editor-in-chief
of Chemical & Engineering News, the flagship newsmagazine of the American
Chemical Society. This gave me ample opportunity to read all of the stories in
C&EN every week, not once but twice and sometimes three times; write weekly
editorials and occasionally longer stories; and indulge my love of chemistry
vicariously, as I read the scientific papers we highlighted in C&EN.
But writing is not as easy as reading. Writing and communicating take a great
deal of skill and effort. One of my favorite quotations on the subject of writing
comes from the novelist John Irving, who observed in The World According to
Garp that a writer never reads for fun. It’s true for me. When I read a sentence
that is well crafted or even better, a scientific paper that is full of well-crafted
sentences, I am always trying to figure out how the author managed to express a
complicated idea with such ease and grace.
The goal of The ACS Style Guide is to help authors and editors achieve that
ease and grace in all of their communications. To my mind, there’s no reason
why scientific papers should not be as easy to read as a good novel. That’s a tall
order, I realize, but if you read through this style guide, you will have all the tools
➤ ➤ ➤ ➤ ➤
Foreword
Copyright 2006 American Chemical Society
viii ➤ The ACS Style Guide
you need to help you achieve that goal. It’s a wonderful reference book that I
keep on my bookshelf and refer to often. I hope you will as well.
Madeleine Jacobs
Executive Director and Chief Executive Officer
American Chemical Society
ix
Since publication of the second edition of The ACS Style
Guide in 1997, much has changed in the world of scientific
communication—and yet, many things remain the same.
During the past eight years, electronic dissemination of scientific, technical,
and medical (STM) information has come to fruition. In chemistry, both the
American Chemical Society and the Royal Society of Chemistry have made their
scientific journals available on the World Wide Web and have digitized their
respective publications back to the 19th century. Commercial publishers, who
publish most of the world’s chemical information, have likewise made their pub-
lications available on the Web. Publications in other scientific disciplines, engi-
neering, and medicine have also taken this digital pathway. Whereas traditional
journals continue to be printed and used, electronic delivery has greatly expanded
the availability and reading of STM information far beyond what could have ever
been envisioned with paper journals. Most manuscripts are now written with de
facto standard word-processing software and adhere to formats developed for
electronic creation and processing. Most manuscripts are submitted electroni-
cally, principally via the Internet on the Web. Communications among editors,
reviewers, and authors are now largely electronic, as is communication between
editors and production facilities and printers.
Regardless of the mode of information creation and delivery, the necessity for
accurate information communicated in a clear, unambiguous manner, coupled
with the ethical behavior of all participants, remains the same. As Janet Dodd
wrote in the preface to the second edition, “In the midst of all this change, the
comforting thought is that one goal of authors and editors has not changed: to
communicate information in the most understandable and expedient fashion in
➤ ➤ ➤ ➤ ➤
Preface
Copyright 2006 American Chemical Society
x ➤ The ACS Style Guide
publications of the highest quality. To accomplish that goal, we need guidelines.
This book is intended to guide and answer questions for authors and editors, to
save them time, and to ensure clarity and consistency.”
Third Edition
The third edition aims to continue such guidance while broadening the scope
of the book to accommodate changes in technology and the homogenization of
international scientific publishing. New topics in the third edition include chap-
ters on
• ethics in scientific communication;
• submitting manuscripts via the Web;
• preparing and submitting publisher-ready figures, tables, and chemical
structures, including information about various software programs to
create artwork;
• formatting manuscript references to electronic resources and informa-
tion on reference-management software; and
• markup languages, in anticipation of the classification and capture of sci-
entific information in yet-to-be-defined structures.
The chapters on peer review, copyright, the editorial process, and writing
style and word choice have been extensively rewritten. Although language cer-
tainly evolves with time, there have not been substantial changes in English dur-
ing the past seven years. The chapters on grammar, punctuation, spelling, and
conventions in chemistry remain largely the same as in the second edition. The
use of typefaces, superscripts and subscripts, Greek letters, special symbols, num-
bers, mathematics, units of measure, and names and numbers for chemical com-
pounds are generally unchanged, although some of the existing rules have been
clarified. Some new rules and examples have been added to reflect new fields in
chemistry, such as combinatorial chemistry and chemical biology. In all chap-
ters, errors have been corrected (and almost certainly new errors inadvertently
introduced!), and some changes have been made to reflect changes in practice,
particularly as related to electronic issues.
Several features have been added to the third edition to improve the readers’
ease of use:
• The contents are reorganized into two sections. The first section, “Scien-
tific Communication”, contains chapters giving readers information on
broad topics such as ethics in scientific communication, writing style and
word usage, and submission of manuscripts using a Web-based system.
The second section, “Style Guidelines”, contains chapters that give specific
rules and examples. For instance, in these chapters readers will find infor-

mation on such topics as grammar, punctuation, and spelling; format-
ting numbers and specialized chemical conventions; when to use special
typefaces; how to format references; and how to create figures, tables, and
chemical structures.
• Throughout the book, the arrowlike icon (➤) precedes rules. These rules
may refer to grammar, word usage, or punctuation rules. Also, the icon
may precede rules for creating publisher-ready artwork, rules about styl-
ing chemical terms, or rules about formatting names and chemical com-
pounds. Examples are given under the rule to further illustrate it.
• Attention is drawn to particularly important topics by the use of remind-
ers and boxes. Reminders are bounded by horizontal rules and are identi-
fied with a small pencil icon (✐); they contain a brief note on a single
topic. Boxes are numbered sequentially within each chapter and contain
more extensive information on a specific topic. Reminders and boxes that
contain ACS-specific information are identified by a small ACS phoenix
icon ( ). We believe that identification of these key issues in this man-
ner will be helpful to readers.
Because of the desire on the part of the publisher to increase the use of the
third edition of The ACS Style Guide, it is being made available on the World
Wide Web. It is expected that periodic updates will be made to the electronic edi-
tion, which would not be feasible for the printed version. Additionally, if readers
would like to request clarification of rules, they may do so by contacting the pub-
lisher at styleguide@acs.org or by addressing correspondence to The ACS Style
Guide, Books Department, American Chemical Society, 1155 Sixteenth Street,
NW, Washington, DC 20036.
Although The ACS Style Guide is written with an emphasis on chemistry and,
to some extent, a focus on ACS journals, we believe that it has wide applicabil-
ity to the sciences, engineering, medicine, and other disciplines. Chemistry is a
mature science that cuts across virtually all basic and applied sciences.
Science in its broadest sense has always been an international activity. How-
ever, there is an increasing trend toward internationalization of scientific com-
munication. For example, for the past several years, the majority of authors pub-
lishing in ACS journals reside outside North America. English has become the
lingua franca of science in the same way that French once was the international
language of diplomacy and commerce. The venerated Beilsteins Handbuch der
Organischen Chemie has been published in English for a number of years. The
prestigious journal Angewandte Chemie: International Edition in English conveys
internationalization and the English language merely by its title. The premier
publications Science and Nature, both published in English, have broad inter-
national authorship and readership. We believe that The ACS Style Guide will
be a useful tool for the international scientific community using this common
language.
Preface ➤ xi
xii ➤ The ACS Style Guide
Acknowledgments
The editors would like to thank all the chapter authors and reviewers who con-
tributed to this project. In particular, we would like to thank our colleagues in
Columbus who provided assistance with all the style guidelines in the book,
namely, Toddmichael Janiszewski, Diane Needham, “Ram” Ramaswami Ravi,
Teresa Schleifer, and Joe Yurvati. A special thank you goes to Betsy Kulamer and
Paula M. Bérard for their skilled editorial efforts. We certainly could not have
completed this project without their capable assistance. We want to thank Sue
Nedrow, who prepared an in-depth index that we think will be very useful to the
readers. We also wish to express our appreciation to Bob Hauserman at the ACS
for his suggestions and help.
Finally, we would like to express our indebtedness to Janet S. Dodd, who
edited the first and second editions of The ACS Style Guide. Janet was more than
the editor; she wrote much of the first two editions. Her contributions persist in
the third edition.
Anne M. Coghill
Lorrin R. Garson
April 2006
xiii
➤ ➤ ➤ ➤ ➤
Contributors
Frank H. Allen
Cambridge Crystallographic Data Centre
Paula M. Bérard
Chattanooga, Tennessee
Sarah C. Blendermann
ACS Publications Division, Office of Journal
Support Services
American Chemical Society
Barbara A. Booth
Department of Civil and Environmental
Engineering
University of Iowa
Karen S. Buehler
ACS Publications Division, Copyright Office
American Chemical Society
C. Arleen Courtney
ACS Publications Division, Copyright Office
American Chemical Society
Janet S. Dodd
Chemical & Engineering News
American Chemical Society
Gordon G. Hammes
Department of Biochemistry
Duke University
Stephen R. Heller
Division of Chemical Nomenclature and
Structure Representation
International Union of Pure and Applied
Chemistry
Betsy Kulamer
Kulamer Publishing Services
Derek Maclean
KAI Pharmaceuticals
Alan D. McNaught
Division of Chemical Nomenclature and
Structure Representation
International Union of Pure and Applied
Chemistry
Peter Murray-Rust
Unilever Centre for Molecular Informatics
Department of Chemistry
University of Cambridge
Copyright 2006 American Chemical Society
xiv ➤ The ACS Style Guide
Henry S. Rzepa
Department of Chemistry
Imperial College London
Eric S. Slater
ACS Publications Division, Copyright Office
American Chemical Society
Leah Solla
Physical Sciences Library
Cornell University
Antony Williams
Advanced Chemistry Development, Inc.
➤ ➤ ➤ ➤ ➤
Part 1
Scientific Communication
3
➤ ➤ ➤ ➤ ➤ CHAPTER 1
Ethics in Scientific
Publication
Gordon G. Hammes
The principles that govern the ethics of scientific publica-
tion are no different than for any other endeavor: complete
and accurate reporting and appropriate attribution to the contributions of oth-
ers. However, as always, “the devil is in the details.” The ethical responsibilities
of authors and reviewers are sufficiently important and complex that the editors
of the American Chemical Society journals have developed a detailed document
outlining these responsibilities. (This document, “Ethical Guidelines to Pub-
lication of Chemical Research” is presented in Appendix 1-1.) The purpose of
this chapter is not to duplicate this document, but rather to discuss some of the
important underlying principles and situations that often arise.
Scientific research, perhaps more than most professions, crucially depends
on the integrity of the investigators. Most research consists of a series of com-
plex experiments or theoretical calculations that cannot (or will not) be dupli-
cated easily elsewhere. Moreover, it is usually extremely difficult to determine
in detail if the results are correct and can be trusted. Published results generally
are accepted at face value. Very often related work eventually may be done by
others that tests the results, so that checks and balances exist within the system.
This is usually a long process, however, and the advance of science may be sig-
nificantly delayed if published results are not correct. The bottom line is that we
depend on the integrity of the investigators reporting the results. We assume that
the description of the work is accurate and honest unless proven otherwise. This
places a considerable burden on the authors to ensure that the system works.
Research is by its nature exploratory, and honest mistakes may occur. Errors
due to human fallibility are unfortunate, but not unethical. Research inevitably
Copyright 2006 American Chemical Society

4 ➤ The ACS Style Guide
pushes the boundaries of existing methodology and theory, so that errors in
judgment and interpretation are bound to occur. This is a normal part of the sci-
entific establishment. An often-quoted adage is that the only way never to make
a mistake in print is never to publish. Errors due to carelessness or haste are poor
science; they represent irresponsible, but not unethical, behavior.
Errors due to fabrication and falsification clearly are unethical and cannot be
tolerated under any circumstances. Breakdowns in the system that are not honest
mistakes have occurred; some examples are published by the Office of Research
Integrity of the U.S. Department of Health and Human Services at http://ori.
dhhs.gov. Fortunately, these breakdowns seem to be relatively few.
It is the responsibility of each author to ensure the quality and integrity of the
research that is reported. The ethical principles governing the conduct of science
should be well understood by all participants. This chapter considers only some
aspects of this subject. An excellent introductory publication is available online
from the National Academy of Sciences; see “On Being a Scientist: Responsible
Conduct in Research” at http://www.nap.edu/readingroom/books/obas/.
When To Publish: Significance and Timeliness
When is it time to publish? Research is open-ended, so the answer to this ques-
tion is not always obvious and requires authors to balance significance and time-
liness to arrive at a high-quality manuscript.
✐Reminder: Research should be published in a timely manner when
enough work has been done to yield significant results.
Researchers must decide when enough work has been done to make a signifi-
cant contribution to a field. “Significant” is in the eye of the beholder, and some-
times reviewers and authors will differ markedly with regard to this judgment.
The give and take between authors and reviewers is part of the normal process of
science and undoubtedly improves the quality of published work. Clearly neither
science nor scientific publishing are enhanced by a continual stream of short,
incomplete descriptions of a research project. A publication should describe a
project that is complete unto itself and represents a true advance in the field.
(An exception to this rule occurs when a very unusual result is obtained that is
of great interest and significance—in this case, publication as a preliminary note
may be justified.)
Scientists also have an obligation to publish their research results in a timely
manner. Unpublished research results constitute research not done in the eyes of
other scientists. Unnecessary delays can result in duplication of efforts and may
hinder the advancement of science. Under no circumstances should a manuscript
Chapter 1: Ethics in Scientific Publication ➤ 5
be submitted and then held up in the revision or page proof stage for reasons not
directly related to the research—for example, because of patent considerations.
Given the “publish or perish” mentality that sometimes exists, researchers
may be tempted to maximize their number of publications by publishing many
short, somewhat repetitive research reports. This practice serves no useful pur-
pose for science or the investigator. In truth, the reputation of an investigator is
ultimately determined by the quality of research done over an extended time.
Beginning independent investigators are often told that a research reputation
can be thought of as a product of quantity times quality of published work. If
only one publication appears every 10 years, they may be advised, it had better
be a good one. On the other hand, a large number of low-quality publications is
not of benefit to the individual or the profession.
Investigators may be tempted to publish the same material, or material only
slightly different, multiple times. This practice is unethical. The manuscript should
clearly describe prior work that has been done by the authors. It is the obligation
of the corresponding author to inform the journal editor of any related manu-
scripts that have been submitted and/or published elsewhere, including prelimi-
nary communications and symposium volumes. There are no exceptions. More-
over, although the review process can be lengthy, under no circumstances should a
manuscript be submitted simultaneously to multiple journals.
What To Publish: Full Disclosure
Unfortunately, because of space limitations, the trend in publishing research
results is to provide less and less detail. Although brevity is admirable, it is impor-
tant that the results be described fully and accurately. Moreover, all of the results
should be reported, not just those supporting the underlying hypotheses of the
research. If necessary, most journals allow the possibility of submitting support-
ing documentation as supplementary information. Although this material does
not appear in the printed version, it is readily available online. The rule of thumb
is that sufficient information should be provided so that other investigators could
repeat the experiments if they so desired. The necessity for providing sufficient
detail has to be balanced with the need to conserve publication space. As might
be expected, considerable variation exists in practice as to what this entails. The
manuscript review process plays a tempering role, balancing these two factors.
Representative data and/or calculations are an important part of any scien-
tific presentation. Obviously, not all of the data, derivations, and calculations can
be presented. It is acceptable for the “typical data and/or calculations” that are
presented to be among the best, but all the data should be included in the analy-
ses. The reproducibility of the results is an implicit assumption for published
work. However, first-rate research often involves difficult measurements at the
edge of existing methodology, and the difference between signal and noise may
6 ➤ The ACS Style Guide
be hard to distinguish. It is acceptable to report results for which this is the case,
as long as the appropriate qualifications are clearly stated. A critical assessment
of the research should be made by the investigator, including an error analysis.
No one should be more critical of the research that is reported than the authors.
Who Are Authors?
Generally speaking, all authors of a publication should have made significant and
substantial intellectual contributions to the work being reported. Unfortunately,
this principle is often breached, as evidenced by manuscripts with tens, even hun-
dreds, of authors. Some laboratories put the names of everyone in the laboratory
on the published work, and some individuals put their names on every publica-
tion coming out of a laboratory, even if their participation was only nominal.
If a colleague prepared buffers or did routine computer programming, these
contributions should be acknowledged, but they are not sufficient contributions
for authorship. General discussion with colleagues or within research groups is
rarely sufficient for inclusion in authorship. Despite some arbitrariness in defin-
ing what constitutes a significant intellectual contribution, the guiding ethical
principle is clear and should be adhered to. Usually the question of authorship
can be decided by discussion among the participants in the research. Occasion-
ally, a third party may be required to adjudicate this issue. In any event, this mat-
ter should be fully resolved before submission of a manuscript.
A question that often arises concerns the order of the authors’ names. This
is not really an ethical issue, and practice varies from place to place. Most often
the first author is assumed to have made the major contribution to the work, and
the senior and/or corresponding author is listed last. However, many variations
to this theme exist, such as putting the authors in alphabetical order. In some
cases, the specific contributions of each author are described. Ideally, the order
of authorship should be decided amicably among the authors, but perceptions
sometimes differ between the individuals involved. Authors should not become
obsessed with this matter. Ultimately, a researcher’s scientific reputation rests on
the totality of publications and the significance of contributions to the field.
It is often said that all authors are responsible for the entire content of a
manuscript. This is a meritorious ideal, but unrealistic. Most manuscripts have
multiple authors, and very often, a single author is responsible for only a portion
of the work being presented. For example, the manuscript may contain a crystal
structure, determined by an expert crystallographer; spectral data, determined
by an expert spectroscopist; kinetic data, determined by an expert kineticist; etc.
In cases such as this, a single author cannot be held responsible for all of the
results presented. A more realistic assessment of what authorship implies is that
each author should have read the manuscript carefully and understood the find-
ings, but the technical responsibility is only for the area in which a given author

Chapter 1: Ethics in Scientific Publication ➤ 7
has the appropriate expertise. The responsibility of the corresponding author is
to ensure that all authors have approved the manuscript before submission and
for all subsequent revisions.
What Went Before: Attribution and Context
Every scientific publication must include the proper attribution of the contribu-
tions of others by appropriate referencing and the placement of results within
the context of the research field.
Referencing is a complex subject (see Chapter 14 of this volume). Every ref-
erence in the field cannot be cited, or the reference list would become intoler-
ably long. However, important ideas and experiments must be cited. The intro-
duction and discussion sections of a manuscript should be absolutely clear as
to what the work of others has contributed to the research being reported. If
data are presented that have been previously published, this should be clearly
indicated. Direct quotations of more than a few words should be indicated by
quotation marks and referenced. Paraphrases of quotations also should be refer-
enced. Plagiarism—taking the writings or ideas of another and passing them off
as one’s own—of any type represents unethical conduct.
Occasionally, the attribution of an idea or fact may be to a “private commu-
nication” of a colleague or fellow scientist. In such cases, permission must be
obtained from the individual in question before the citation is made. Reference
to unpublished material should be avoided if possible because it generally will
not be available to interested readers.
✐Reminder: Every manuscript must reference the contributions of others
and place results in the context of the research field.
The results and conclusions sections of a manuscript should be placed within
the context of the research area. What was known before the research being pre-
sented? What has this research contributed that is new and significant? It should
also be clear what conclusions are based on the work presented and which are
speculations. It is appropriate to speculate—in fact, this is a stimulus to the
field—as long as speculations are labeled as such. In this regard, the values and
judgments of the authors and current thinking appropriately come into play.
Not all attributions to previous work cite supportive data. In some cases,
results under discussion may differ from previous work, or authors may make
critical comments about earlier research. Differences between the work reported
and previous results must be discussed and reconciled. Criticism of previous
work should be presented carefully and objectively, in terms of the facts only.
This is part of normal scientific discourse. Criticism should never be directed at
8 ➤ The ACS Style Guide
individuals or laboratories; it is essential to consider only the facts that have been
presented.
Acknowledgments should be made to people who have assisted in the project,
but not sufficiently for authorship, and to sponsoring agencies. It is also impera-
tive to acknowledge potential conflicts of interest that may exist. For example,
if the research being reported concerns drug XYZ and one of the authors has a
substantial financial interest in a company that makes drug XYZ or is conducting
clinical trials with drug XYZ, these facts should be explicitly stated.
What Next: After Publication
An author’s obligations do not stop with publication. If errors are found in the
published work, they should be corrected with the publication of errata. If other
investigators request more information or more complete data, the requests
should be fulfilled without delay.
A trickier issue concerns the distribution of special materials used in the
research. The rule of thumb is that the authors should be willing to provide others
with a reasonable supply of special materials that have been used in the research.
However, some common sense should be applied to this rule. For example, if
two years have been spent cloning a specific protein and it will be used in future
research, it is unreasonable to expect researchers to give this clone to competi-
tors who are planning similar experiments. Similarly, if a complex substance has
been synthesized and only a small supply is available, it would be unreasonable
to expect the material to be given away. However, the publication should provide
sufficient detail so that other researchers can develop the clone themselves or
synthesize the compound in question. Although ethical behavior in this area is
not always clear, the general rule is that all aspects of the research should be fully
disclosed and reasonable assistance should be given to other researchers. Prog-
ress in science depends greatly on open communication and cooperation.
Obligations of a Reviewer
Scientific discourse depends on critical review of manuscripts before publica-
tion. (Peer review—including ethical considerations—is discussed in greater
detail in Chapter 6 of this volume.) The primary obligation of reviewers is to
provide a rational, objective review of the science. This requires a careful reading
of the manuscript and a careful preparation of the review. The review process is
anonymous for most journals, but this does not mean that the reviewer has free
rein to criticize. Any criticism must be logically and objectively delineated, and it
should never be directed at the authors personally. Reviewers also should place
the work within the context of the field: is it a major contribution, minor contri-
Chapter 1: Ethics in Scientific Publication ➤ 9
bution, or an insufficient contribution to merit publication? Promptness in car-
rying out reviews is important and an ethical issue. Delaying a publication could
be costly to an author, especially in a competitive field. The usual golden rule
applies: review with the care and speed you expect for your own manuscripts. If
a reviewer cannot meet a deadline, he or she should inform the publisher as soon
as possible.
Manuscripts sent to reviewers are confidential documents. Unfortunately,
a significant number of reviewers interpret the word “confidential” incorrectly.
Confidential does not mean that reviewers can expand the scope of confidential-
ity, for example, within their research groups, by including a few colleagues, and
so on. Confidential documents should not be shared or discussed with anybody
without the explicit consent of the journal editor, the editorial board member
handling the manuscript, or both. For example, senior investigators sometimes
have graduate students or postdoctorals review manuscripts. This is accept-
able only if the permission of the editor or editorial board member has been
obtained. In some cases, a reviewer may discuss the results with a colleague; this
also is forbidden if permission has not been obtained. Although breaches of con-
fidentiality do not usually do any harm and are not intended to do so, they are
unethical and should be avoided.
If reviewers have conflicts of interest with regard to a given manuscript, the
manuscript should be returned as quickly as possible to the editor. Conflicts of
interest vary. Perhaps similar research is being carried out in the reviewer’s labo-
ratory, or the reviewer may be privy to confidential information that conflicts
with the results reported. Conflicts of interest can be more personal in nature:
perhaps a reviewer has had personal difficulties with or is a close friend of one
of the authors. When in doubt, the usual rule is not to review or read the manu-
script. If you are unsure, ask the editor handling the manuscript. The editor may
want your expert opinion even if some level of apparent conflict exists.
Finally, the results in a manuscript under review cannot be quoted or incor-
porated into a reviewer’s own research program. After the work is published, a
reviewer may use the ideas and data presented (with proper attribution), but the
reviewer should not do so based on the review process. Such behavior is akin to
insider trading in the purchase of stocks. Although a prison term is unlikely for
this breach of conduct, the ethical principle is quite clear.
Obligations as a Reader
Not all errors are found before publication by authors and reviewers; some are
discovered by readers. If the errors involve serious misinterpretation or mis-
quotation of the literature, the most straightforward procedure is to contact the
author(s) directly. If this is awkward, the editor can be informed. It is not worth-
while, however, to create a fuss for nonsubstantive errors. Self-serving com-
10 ➤ The ACS Style Guide
plaints, such as not quoting the reader’s own work enough, seldom have much
credibility.
In rare situations, a scientist may have evidence that published material con-
tains falsification, fabrication, or plagiarism. It is the obligation of every scientist
to report such cases immediately to the editor of the journal. Institutions receiv-
ing financial support from the National Institutes of Health and the National
Science Foundation are required to have mechanisms in place to investigate such
occurrences, and direct reporting to the appropriate institutional office may be
more expedient. Accusations must be supported by fact, not suspicions, because
academic misconduct is a serious matter with career-threatening implications.
Unpleasant as this situation may be, it should not be ignored.
For the Health of Research
This chapter has emphasized the global ethics of the publication process. Eth-
ics are not complicated, and the practices and rules are mainly common sense.
Adherence to ethical standards in research and publication is not optional; rather,
it is essential for the health of scientific research.

11
➤ ➤ ➤ ➤ ➤
APPENDIX 1-1
Ethical Guidelines to Publication
of Chemical Research
The guidelines embodied in this document were revised by the Editors of the
Publications Division of the American Chemical Society in January 2000.
Preface
The American Chemical Society serves the chemistry profession and society at
large in many ways, among them by publishing journals which present the results
of scientific and engineering research. Every editor of a Society journal has the
responsibility to establish and maintain guidelines for selecting and accepting
papers submitted to that journal. In the main, these guidelines derive from the
Society’s definition of the scope of the journal and from the editor’s perception
of standards of quality for scientific work and its presentation.
An essential feature of a profession is the acceptance by its members of a code
that outlines desirable behavior and specifies obligations of members to each
other and to the public. Such a code derives from a desire to maximize perceived
benefits to society and to the profession as a whole and to limit actions that
might serve the narrow self-interests of individuals. The advancement of science
requires the sharing of knowledge between individuals, even though doing so
may sometimes entail forgoing some immediate personal advantage.
With these thoughts in mind, the editors of journals published by the American
Chemical Society now present a set of ethical guidelines for persons engaged in the
publication of chemical research, specifically, for editors, authors, and manuscript
reviewers. These guidelines are offered not in the sense that there is any immediate
crisis in ethical behavior, but rather from a conviction that the observance of high
ethical standards is so vital to the whole scientific enterprise that a definition of
those standards should be brought to the attention of all concerned.
We believe that most of the guidelines now offered are already understood
and subscribed to by the majority of experienced research chemists. They may,
however, be of substantial help to those who are relatively new to research. Even
The ethical guidelines are also available in their most recent version on the Web at https://
paragon.acs.org.
12 ➤ The ACS Style Guide
well-established scientists may appreciate an opportunity to review matters so
significant to the practice of science.
Guidelines
A. Ethical Obligations of Editors of Scientific Journals
1. An editor should give unbiased consideration to all manuscripts offered
for publication, judging each on its merits without regard to race, religion,
nationality, sex, seniority, or institutional affiliation of the author(s). An edi-
tor may, however, take into account relationships of a manuscript immedi-
ately under consideration to others previously or concurrently offered by the
same author(s).
2. An editor should consider manuscripts submitted for publication with all
reasonable speed.
3. The sole responsibility for acceptance or rejection of a manuscript rests with
the editor. Responsible and prudent exercise of this duty normally requires
that the editor seek advice from reviewers, chosen for their expertise and
good judgment, as to the quality and reliability of manuscripts submitted for
publication. However, manuscripts may be rejected without review if consid-
ered inappropriate for the journal.
4. The editor and members of the editor’s staff should not disclose any infor-
mation about a manuscript under consideration to anyone other than those
from whom professional advice is sought. (However, an editor who solicits,
or otherwise arranges beforehand, the submission of manuscripts may need
to disclose to a prospective author the fact that a relevant manuscript by
another author has been received or is in preparation.) After a decision has
been made about a manuscript, the editor and members of the editor’s staff
may disclose or publish manuscript titles and authors’ names of papers that
have been accepted for publication, but no more than that unless the author’s
permission has been obtained.
5. An editor should respect the intellectual independence of authors.
6. Editorial responsibility and authority for any manuscript authored by an edi-
tor and submitted to the editor’s journal should be delegated to some other
qualified person, such as another editor of that journal or a member of its
Editorial Advisory Board. Editorial consideration of the manuscript in any
way or form by the author-editor would constitute a conflict of interest, and
is therefore improper.
7. Unpublished information, arguments, or interpretations disclosed in a sub-
mitted manuscript should not be used in an editor’s own research except
with the consent of the author. However, if such information indicates that
some of the editor’s own research is unlikely to be profitable, the editor could
Chapter 1: Ethics in Scientific Publication ➤ 13
ethically discontinue the work. When a manuscript is so closely related to
the current or past research of an editor as to create a conflict of interest,
the editor should arrange for some other qualified person to take editorial
responsibility for that manuscript. In some cases, it may be appropriate to tell
an author about the editor’s research and plans in that area.
8. If an editor is presented with convincing evidence that the main substance
or conclusions of a report published in an editor’s journal are erroneous, the
editor should facilitate publication of an appropriate report pointing out the
error and, if possible, correcting it. The report may be written by the person
who discovered the error or by an original author.
9. An author may request that the editor not use certain reviewers in consider-
ation of a manuscript. However, the editor may decide to use one or more of
these reviewers, if the editor feels their opinions are important in the fair con-
sideration of a manuscript. This might be the case, for example, when a man-
uscript seriously disagrees with the previous work of a potential reviewer.
B. Ethical Obligations of Authors
1. An author’s central obligation is to present an accurate account of the
research performed as well as an objective discussion of its significance.
2. An author should recognize that journal space is a precious resource created
at considerable cost. An author therefore has an obligation to use it wisely
and economically.
3. A primary research report should contain sufficient detail and reference to
public sources of information to permit the author’s peers to repeat the work.
When requested, the authors should make a reasonable effort to provide sam-
ples of unusual materials unavailable elsewhere, such as clones, microorgan-
ism strains, antibodies, etc., to other researchers, with appropriate material
transfer agreements to restrict the field of use of the materials so as to protect
the legitimate interests of the authors.
4. An author should cite those publications that have been influential in deter-
mining the nature of the reported work and that will guide the reader quickly
to the earlier work that is essential for understanding the present investiga-
tion. Except in a review, citation of work that will not be referred to in the
reported research should be minimized. An author is obligated to perform a
literature search to find, and then cite, the original publications that describe
closely related work. For critical materials used in the work, proper citation
to sources should also be made when these were supplied by a nonauthor.
5. Any unusual hazards inherent in the chemicals, equipment, or procedures
used in an investigation should be clearly identified in a manuscript report-
ing the work.
6. Fragmentation of research reports should be avoided. A scientist who has
done extensive work on a system or group of related systems should organize
14 ➤ The ACS Style Guide
publication so that each report gives a well-rounded account of a particu-
lar aspect of the general study. Fragmentation consumes journal space exces-
sively and unduly complicates literature searches. The convenience of readers
is served if reports on related studies are published in the same journal, or in
a small number of journals.
7. In submitting a manuscript for publication, an author should inform the edi-
tor of related manuscripts that the author has under editorial consideration or
in press. Copies of those manuscripts should be supplied to the editor, and the
relationships of such manuscripts to the one submitted should be indicated.
8. It is improper for an author to submit manuscripts describing essentially the
same research to more than one journal of primary publication, unless it is
a resubmission of a manuscript rejected for or withdrawn from publication.
It is generally permissible to submit a manuscript for a full paper expanding
on a previously published brief preliminary account (a “communication” or
“letter”) of the same work. However, at the time of submission, the editor
should be made aware of the earlier communication, and the preliminary
communication should be cited in the manuscript.
9. An author should identify the source of all information quoted or offered,
except that which is common knowledge. Information obtained privately, as
in conversation, correspondence, or discussion with third parties, should not
be used or reported in the author’s work without explicit permission from the
investigator with whom the information originated. Information obtained in
the course of confidential services, such as refereeing manuscripts or grant
applications, should be treated similarly.
10. An experimental or theoretical study may sometimes justify criticism, even
severe criticism, of the work of another scientist. When appropriate, such
criticism may be offered in published papers. However, in no case is personal
criticism considered to be appropriate.
11. The coauthors of a paper should be all those persons who have made sig-
nificant scientific contributions to the work reported and who share respon-
sibility and accountability for the results. Other contributions should be
indicated in a footnote or an “Acknowledgments” section. An administra-
tive relationship to the investigation does not of itself qualify a person for
coauthorship (but occasionally it may be appropriate to acknowledge major
administrative assistance). Deceased persons who meet the criterion for
inclusion as coauthors should be so included, with a footnote reporting date
of death. No fictitious name should be listed as an author or coauthor. The
author who submits a manuscript for publication accepts the responsibility
of having included as coauthors all persons appropriate and none inappro-
priate. The submitting author should have sent each living coauthor a draft
copy of the manuscript and have obtained the coauthor’s assent to coauthor-
ship of it.
Chapter 1: Ethics in Scientific Publication ➤ 15
12. The authors should reveal to the editor any potential conflict of interest, e.g.,
a consulting or financial interest in a company, that might be affected by pub-
lication of the results contained in a manuscript. The authors should ensure
that no contractual relations or proprietary considerations exist that would
affect the publication of information in a submitted manuscript.
C. Ethical Obligations of Reviewers of Manuscripts
1. Inasmuch as the reviewing of manuscripts is an essential step in the publica-
tion process, and therefore in the operation of the scientific method, every
scientist has an obligation to do a fair share of reviewing.
2. A chosen reviewer who feels inadequately qualified to judge the research
reported in a manuscript should return it promptly to the editor.
3. A reviewer (or referee) of a manuscript should judge objectively the quality
of the manuscript, of its experimental and theoretical work, of its interpreta-
tions and its exposition, with due regard to the maintenance of high scientific
and literary standards. A reviewer should respect the intellectual indepen-
dence of the authors.
4. A reviewer should be sensitive to the appearance of a conflict of interest
when the manuscript under review is closely related to the reviewer’s work
in progress or published. If in doubt, the reviewer should return the manu-
script promptly without review, advising the editor of the conflict of interest
or bias. Alternatively, the reviewer may wish to furnish a signed review stat-
ing the reviewer’s interest in the work, with the understanding that it may, at
the editor’s discretion, be transmitted to the author.
5. A reviewer should not evaluate a manuscript authored or coauthored by a
person with whom the reviewer has a personal or professional connection if
the relationship would bias judgment of the manuscript.
6. A reviewer should treat a manuscript sent for review as a confidential docu-
ment. It should neither be shown to nor discussed with others except, in spe-
cial cases, to persons from whom specific advice may be sought; in that event,
the identities of those consulted should be disclosed to the editor.
7. Reviewers should explain and support their judgments adequately so that
editors and authors may understand the basis of their comments. Any state-
ment that an observation, derivation, or argument had been previously
reported should be accompanied by the relevant citation. Unsupported asser-
tions by reviewers (or by authors in rebuttal) are of little value and should be
avoided.
8. A reviewer should be alert to failure of authors to cite relevant work by other
scientists, bearing in mind that complaints that the reviewer’s own research
was insufficiently cited may seem self-serving. A reviewer should call to the
editor’s attention any substantial similarity between the manuscript under
16 ➤ The ACS Style Guide
consideration and any published paper or any manuscript submitted concur-
rently to another journal.
9. A reviewer should act promptly, submitting a report in a timely manner.
Should a reviewer receive a manuscript at a time when circumstances pre-
clude prompt attention to it, the unreviewed manuscript should be returned
immediately to the editor. Alternatively, the reviewer might notify the editor
of probable delays and propose a revised review date.
10. Reviewers should not use or disclose unpublished information, arguments,
or interpretations contained in a manuscript under consideration, except
with the consent of the author. If this information indicates that some of
the reviewer’s work is unlikely to be profitable, the reviewer, however, could
ethically discontinue the work. In some cases, it may be appropriate for the
reviewer to write the author, with copy to the editor, about the reviewer’s
research and plans in that area.
11. The review of a submitted manuscript may sometimes justify criticism, even
severe criticism, from a reviewer. When appropriate, such criticism may be
offered in published papers. However, in no case is personal criticism of the
author considered to be appropriate.
D. Ethical Obligations of Scientists Publishing
outside the Scientific Literature
1. A scientist publishing in the popular literature has the same basic obligation
to be accurate in reporting observations and unbiased in interpreting them
as when publishing in a scientific journal.
2. Inasmuch as laymen may not understand scientific terminology, the scientist
may find it necessary to use common words of lesser precision to increase
public comprehension. In view of the importance of scientists’ communi-
cating with the general public, some loss of accuracy in that sense can be
condoned. The scientist should, however, strive to keep public writing,
remarks, and interviews as accurate as possible consistent with effective com-
munication.
3. A scientist should not proclaim a discovery to the public unless the experi-
mental, statistical, or theoretical support for it is of strength sufficient to war-
rant publication in the scientific literature. An account of the experimental
work and results that support a public pronouncement should be submitted
as quickly as possible for publication in a scientific journal. Scientists should,
however, be aware that disclosure of research results in the public press or in
an electronic database or bulletin board might be considered by a journal edi-
tor as equivalent to a preliminary communication in the scientific literature.
17
➤ ➤ ➤ ➤ ➤ CHAPTER 2
Scientific Papers
The chemistry community, like other scientific communi-
ties, depends on the communication of scientific results.
Scientists communicate in a variety of ways, but much of the communication is
through publication in books and journals. In this chapter, the different types of
book and journal presentations are described, along with the components of the
standard format for reporting original research.
Types of Books
Books for the professional scientific community fall into one of three categories:
proceedings volumes, monographs, and handbooks.
Proceedings Volumes
Books based on meetings are called proceedings volumes. These are multiau-
thored volumes. The chapters in proceedings volumes may be accounts of origi-
nal research or literature reviews. Generally, the chapters are developed and
expanded from presentations given at symposia, but additional chapters may be
written especially for the book to make sure that the coverage of the topic is
complete. Proceedings volumes should contain at least one chapter that reviews
the subject and also provides an overview of the book to unify the chapters into a
coherent treatment of the subject. In a longer book that is divided into sections,
each section may need a short overview chapter.
Copyright 2006 American Chemical Society
18 ➤ The ACS Style Guide
Monographs
Monographs are books that examine a single topic in detail. They are written by
one author or collaboratively by more than one author. Each chapter treats one
subdivision of the broader topic.
Handbooks
Handbooks are large, multiauthored volumes that discuss a field in depth. Gen-
erally, the individual submissions are short, about three or four pages. Each sub-
mission is written by one or two authors and provides a detailed discussion of a
narrow topic within the scope of the book.
Journal Presentations
There are four general types of presentations published in journals: articles,
notes, communications, and reviews.
Articles
Articles, also called full papers, are definitive accounts of significant, original
studies. They present important new data or provide a fresh approach to an
established subject. The organization and length of an article should be deter-
mined by the amount of new information to be presented and by space restric-
tions within the publication.
Notes
Notes are concise accounts of original research of a limited scope. They may also
be preliminary reports of special significance. The material reported must be
definitive and may not be published again later. Appropriate subjects for notes
include improved procedures of wide applicability or interest, accounts of novel
observations or of compounds of special interest, and development of new tech-
niques. Notes are subject to the same editorial appraisal as full-length articles.
Communications
Communications, called “letters” or “correspondence” in some publications, are
usually preliminary reports of special significance and urgency that are given
expedited publication. They are accepted if the editor believes that their rapid
publication will be a service to the scientific community. Communications are
generally subject to strict length limitations; they must contain specific results to
support their conclusions, but they may not contain nonessential experimental
details.

Chapter 2: Scientific Papers ➤ 19
The same rigorous standards of acceptance that apply to full-length articles
also apply to communications. Like all types of presentations in journals, com-
munications are submitted to review. In many cases, authors are expected to
publish complete details (not necessarily in the same journal) after their com-
munications have been published. Acceptance of a communication, however,
does not guarantee acceptance of the detailed manuscript.
Reviews
Reviews integrate, correlate, and evaluate results from published literature on
a particular subject. They seldom report new experimental findings. Effective
review articles have a well-defined theme, are usually critical, and may present
novel theoretical interpretations. Ordinarily, reviews do not give experimental
details, but in special cases (as when a technique is of central interest), experi-
mental procedures may be included. An important function of reviews is to serve
as a guide to the original literature; for this reason, accuracy and completeness of
references cited are essential.
Standard Format for Reporting Original Research
The main text of scientific papers presenting original research is generally orga-
nized into a standard format: abstract, introduction, experimental details or
theoretical basis, results, discussion, and conclusions, although not necessarily
in this order. This format has become standard because it is suitable for most
reports of original research, it is basically logical, and it is easy to use. The reason
it accommodates most reports of original research is that it parallels the scientific
method of deductive reasoning: define the problem, create a hypothesis, devise
an experiment to test the hypothesis, conduct the experiment, and draw conclu-
sions. Furthermore, this format enables the reader to understand quickly what is
being presented and to find specific information easily. This ability is crucial now
more than ever because scientists, if not all professionals, must read much more
material than in the past.
✐Reminder: Journal articles and proceedings chapters are usually orga-
nized with an abstract, introduction, experimental details or theoretical
basis, results, discussion, and conclusions.
Use the standard form for reports of original research whether the report is
published in a journal or proceedings volume. Even if the information is more
suited to one of the shorter types of presentations, the logic of the standard
format applies, although some headings or sections may be omitted or other
sections and subsections added. Manuscripts for monographs, handbooks,
20 ➤ The ACS Style Guide
literature reviews, or theoretical papers generally do not follow the standard
form. Consult author guidelines for information on how to organize these
types of presentations or look at previously published work. Regardless of the
type of presentation, be sure to present all parts of the paper as concisely as
possible.
An extremely important step is to check the specific requirements of the
publication targeted and follow them. Some publishers provide templates that
help authors produce manuscripts in the requested format. Templates are also
useful in making sure that the manuscript is not too long. Most editors require
revisions of manuscripts that are not in their requested format. Thus, not fol-
lowing a publication’s requirements can delay publication and make more work
for authors.
Title
The best time to determine the title is after the text is written, so that the title will
reflect the paper’s content and emphasis accurately and clearly. The title must
be brief and grammatically correct but accurate and complete enough to stand
alone. A two- or three-word title may be too vague, but a 14- or 15-word title
is unnecessarily long. If the title is too long, consider breaking it into title and
subtitle.
The title serves two main purposes: to attract the potential audience and to
aid retrieval and indexing. Therefore, include several keywords. The title should
provide the maximum information for a computerized title search.
➤ Choose terms that are as specific as the text permits, e.g., “a vanadium–iron
alloy” rather than “a magnetic alloy”. Avoid phrases such as “on the”, “a study
of”, “research on”, “report on”, “regarding”, and “use of”. In most cases, omit “the”
at the beginning of the title. Avoid nonquantitative, meaningless words such as
“rapid” and “new”.
➤ Spell out all terms in the title, and avoid jargon, symbols, formulas, and abbre-
viations. Whenever possible, use words rather than expressions containing super-
scripts, subscripts, or other special notations. Do not cite company names, spe-
cific trademarks, or brand names of chemicals, drugs, materials, or instruments.
➤ Series titles are of little value. Some publications do not permit them at all.
If consecutive papers in a series are published simultaneously, a series title may
be relevant, but in a long series, paper 42 probably bears so limited a relation-
ship to paper 1 that they do not warrant a common title. In addition, an editor
or reviewer seeing the same title repeatedly may reject it on the grounds that it is
only one more publication on a general topic that has already been discussed at
length.

Chapter 2: Scientific Papers ➤ 21
Byline and Affiliation
Include in the byline all those, and only those, who made substantial contri-
butions to the work, even if the paper was actually written by only one person.
Chapter 1 and Appendix 1-1 in this book are more explicit on this topic.
➤ Many ACS publications specifically request at least one full given name for
each author, rather than only initials. Use your first name, initial, and surname
(e.g., John R. Smith) or your first initial, second name, and surname (e.g., J. Rob-
ert Smith). Whatever byline is used, be consistent. Papers by John R. Smith, Jr., J.
Smith, J. R. Smith, Jack Smith, and J. R. Smith, Jr., will not be indexed in the same
manner; the bibliographic citations may be listed in five different locations, and
ascribing the work to a single author will therefore be difficult if not impossible.
➤ Do not include professional, religious, or official titles or academic degrees.
➤ The affiliation is the institution (or institutions) at which the work was
conducted. If the author has moved to another institution since the work was
done, many publications include a footnote giving the current address. Contact
the editor about this.
➤ If there is more than one author, use an asterisk or superscript (check the
specific publication’s style) to indicate the author or authors to whom corre-
spondence should be addressed. Clarify all corresponding authors’ addresses by
accompanying footnotes if they are not apparent from the affiliation line. E-mail
addresses may be included in corresponding author footnotes.
Abstract
Most publications require an informative abstract for every paper, even if they
do not publish abstracts. For a research paper, briefly state the problem or the
purpose of the research, indicate the theoretical or experimental plan used, sum-
marize the principal findings, and point out major conclusions. Include chemical
safety information when applicable. Do not supplement or evaluate the conclu-
sions in the abstract. For a review paper, the abstract describes the topic, scope,
sources reviewed, and conclusions. Write the abstract last to be sure that it accu-
rately reflects the content of the paper.
✐Reminder: The abstract allows the reader to determine the nature and
scope of the paper and helps technical editors identify key features for
indexing and retrieval.
➤ Although an abstract is not a substitute for the article itself, it must be con-
cise, self-contained, and complete enough to appear separately in abstract pub-
lications. Often, authors’ abstracts are used with little change in abstract pub-
22 ➤ The ACS Style Guide
lications. The optimal length is one paragraph, but it could be as short as two
sentences. The length of the abstract depends on the subject matter and the
length of the paper. Between 80 and 200 words is usually adequate.
➤ Do not cite references, tables, figures, or sections of the paper in the abstract.
Do not include equations, schemes, or structures that require display on a line
separate from the text.
➤ Use abbreviations and acronyms only when it is necessary to prevent awk-
ward construction or needless repetition. Define abbreviations at first use in the
abstract (and again at first use in the text).
Introduction
A good introduction is a clear statement of the problem or project and the rea-
sons for studying it. This information should be contained in the first few sen-
tences. Give a concise and appropriate background discussion of the problem
and the significance, scope, and limits of the work. Outline what has been done
before by citing truly pertinent literature, but do not include a general survey of
semirelevant literature. State how your work differs from or is related to work
previously published. Demonstrate the continuity from the previous work to
yours. The introduction can be one or two paragraphs long. Often, the head-
ing “Introduction” is not used because it is superfluous; opening paragraphs are
usually introductory.
Experimental Details or Theoretical Basis
In research reports, this section can also be called “Experimental Methods”,
“Experimental Section”, or “Materials and Methods”. Be sure to check the specific
publication for the correct title of this section. For experimental work, give suf-
ficient detail about the materials and methods so that other experienced work-
ers can repeat the work and obtain comparable results. When using a standard
method, cite the appropriate literature and give only the details needed.
➤ Identify the materials used and give information on the degree of and criteria
for purity, but do not reference standard laboratory reagents. Give the chemical
names of all compounds and the chemical formulas of compounds that are new or
uncommon. Use meaningful nomenclature; that is, use standard systematic nomen-
clature where specificity and complexity require, or use trivial nomenclature where
it will adequately and unambiguously define a well-established compound.
➤ Describe apparatus only if it is not standard or not commercially available.
Giving a company name and model number in parentheses is nondistracting
and adequate to identify standard equipment.
Chapter 2: Scientific Papers ➤ 23
➤ Avoid using trademarks and brand names of equipment and reagents. Use
generic names; include the trademark in parentheses after the generic name only
if the material or product used is somehow different from others. Remember
that trademarks often are recognized and available as such only in the country of
origin. In ACS publications, do not use trademark (™) and registered trademark
(®) symbols.
➤ Describe the procedures used, unless they are established and standard.
➤ Note and emphasize any hazards, such as explosive or pyrophoric tendencies
and toxicity, in a separate paragraph introduced by the heading “Caution:”.
Include precautionary handling procedures, special waste disposal procedures,
and any other safety considerations in adequate detail so that workers repeating
the experiments can take appropriate safety measures. Some ACS journals also
indicate hazards as footnotes on their contents pages.
In theoretical reports, this section is called, for example, “Theoretical Basis”
or “Theoretical Calculations” instead of “Experimental Details” and includes suf-
ficient mathematical detail to enable other researchers to reproduce derivations
and verify numerical results. Include all background data, equations, and for-
mulas necessary to the arguments, but lengthy derivations are best presented as
supporting information.
Results
Summarize the data collected and their statistical treatment. Include only rel-
evant data, but give sufficient detail to justify the conclusions. Use equations,
figures, and tables only where necessary for clarity and brevity. Extensive but rel-
evant data should be included in supporting information.
Discussion
The purpose of the discussion is to interpret and compare the results. Be objec-
tive; point out the features and limitations of the work. Relate your results to cur-
rent knowledge in the field and to the original purpose in undertaking the project:
Was the problem resolved? What has been contributed? Briefly state the logical
implications of the results. Suggest further study or applications if warranted.
Present the results and discussion either as two separate sections or as one
combined section if it is more logical to do so. Do not repeat information given
elsewhere in the manuscript.
Conclusions
The purpose of the conclusions section is to put the interpretation into the con-
text of the original problem. Do not repeat discussion points or include irrele-
vant material. Conclusions should be based on the evidence presented.

24 ➤ The ACS Style Guide
Summary
A summary is unnecessary in most papers. In long papers, a summary of the
main points can be helpful, but be sure to stick to the main points. If the sum-
mary itself is too long, its purpose is defeated.
Acknowledgments
Generally, the last paragraph of the paper is the place to acknowledge people,
organizations, and financing. As simply as possible, thank those persons, other
than coauthors, who added substantially to the work, provided advice or techni-
cal assistance, or aided materially by providing equipment or supplies. Do not
include their titles. If applicable, state grant numbers and sponsors here, as well
as auspices under which the work was done, including permission to publish if
appropriate.
Follow the publication’s guidelines on what to include in the acknowledg-
ments section. Some journals permit financial aid to be mentioned in acknowl-
edgments, but not meeting references. Some journals put financial aid and meet-
ing references together, but not in the acknowledgments section.
References
In many books and journals, references are placed at the end of the article or
chapter; in others, they are treated as footnotes. In any case, place the list of refer-
ences at the end of the manuscript.
In ACS books and most journals, the style and content of references are stan-
dard regardless of where they are located. Follow the reference style presented in
Chapter 14.
The accuracy of the references is the author’s responsibility. Errors in refer-
ences are one of the most common errors found in scientific publications and
are a source of frustration to readers. Increasingly, hypertext links are automati-
cally generated in Web-based publications, but this cannot be done for references
containing errors. If citations are copied from another source, check the original
reference for accuracy and appropriate content.
✐Reminder: The accuracy of the references is the author’s responsibility.
Special Sections
This discussion on format applies to most manuscripts, but it is not a set of
rigid rules and headings. If the paper is well organized, scientifically sound,
and appropriate to the publication, adding other sections and subsections
may be helpful to readers. For example, an appendix contains material that
Chapter 2: Scientific Papers ➤ 25
is not critical to understanding the text but provides important background
information.
Supporting Information
Material that may be essential to the specialized reader but not require elab-
oration in the paper itself is published as supporting information, usually on
the journal’s Web page. Examples of supporting information include large tables,
extensive figures, lengthy experimental procedures, mathematical derivations,
analytical and spectral characterization data, biological test data for a series,
molecular modeling coordinates, modeling programs, crystallographic informa-
tion files, instrument and circuit diagrams, and expanded discussions of periph-
eral findings.
More journals are encouraging this type of publishing to keep printed papers
shorter. For ACS journals, supporting information is available immediately by
linking to it from the citing paper on the Web. For example, for the article “Vana-
dium-Based, Extended Catalytic Lifetime Catechol Dioxygenases: Evidence for
a Common Catalyst” by Cindy-Xing Yin and Richard G. Finke in The Journal
of the American Chemical Society 2005, 127, 9003–9013, the supporting infor-
mation consists of two files, ja051594esi20050517_053152.pdf (453 K) and
ja051594erom20050320_064528.cif (24 K).
When including supporting information, place a statement to that effect at
the end of the paper, using the format specified in the author instructions for the
specific journal. For complete instructions on how to prepare this material for
publication, check the author instructions for the publication.
Web-Enhanced Objects
Some publishers, including ACS, have started exploring various Web-based tech-
nologies to enhance the way that information in a research article is conveyed.
Selected papers in Web editions may contain Web-enhanced objects (WEOs)
to supplement a reader’s understanding of the research being reported. These
types of files include color figures (including three-dimensional, rotatable fig-
ures), chemical structures, animations, spectra, video, and sound files. Links to
WEOs will appear in the Web edition of the paper. These objects, although not
essential to the understanding of the science, should help to augment a read-
er’s understanding of the research being reported. The types of objects suitable
for this form of publication should be viewable with commonly available plug-
ins (e.g., Chime) or helper applications (e.g., WebLab Viewer, RasMol), which
allow viewing and manipulating these objects within the HTML file itself or in
a separate window. For example, a figure in the journal article “Orientation and
Phase Transitions of Fat Crystals under Shear” by Gianfranco Mazzanti, Sarah
E. Guthrie, Eric B. Sirota, Alejandro G. Marangoni, and Stefan H. J. Idziak, in
26 ➤ The ACS Style Guide
Crystal Growth & Design 2003, 3, 721–725, is supplemented by a movie WEO (in
.mov format) depicting the time sequence of synchrotron X-ray diffraction pat-
terns for the crystallization of cocoa butter in chocolate (see http://pubs.acs.org/
isubscribe/journals/cgdefu/asap/objects/cg034048a/Mazzantivideouip.mov).
As with other types of special information, authors should check the author
guidelines for the publication for instructions on how to prepare and submit
WEOs.
27
➤ ➤ ➤ ➤ ➤ CHAPTER 3
The Editorial
Process
Publishing a manuscript, whether intended for a journal or
a book, is a process. It has four stages: the draft manuscript,
manuscript review, the final manuscript, and processing of accepted manuscripts.
Along the way, responsibility for the different stages passes from the author, to
the journal or book editor, back to the author, and finally to the technical editor.
This chapter provides an overview of each of these stages as they evolve in scien-
tific, technical, and medical (STM) publishing.
The Draft Manuscript
Getting Started
Before beginning to write, authors should review the ethical principles of scien-
tific publication (see Chapter 1). The editorial process is supported by the ethi-
cal obligations of authors, editors, reviewers, and readers. Author integrity and
adherence to the principles that guide scientific publications—such as deciding
when it is the appropriate time to publish, determining who should author the
manuscript, and providing the proper attribution and context for the research—
are as integral to the success of scientific publication as providing science that is
sound and of high quality.
Although there is no fixed set of “writing rules” to be followed like a cook-
book recipe or an experimental procedure, some guidelines can be helpful. Start
by considering the questions in Box 3-1; answering these questions will clarify
your goals and make it easier to write the manuscript with the proper amount of
detail. It will also make it easier for the book or journal editor to determine the
Copyright 2006 American Chemical Society

28 ➤ The ACS Style Guide
manuscript’s suitability for the publication. Writing is like so many other things:
once the goal is identified, the details fall into place.
After you have determined the function of the manuscript and identified
the audience, review your material for completeness or excess. Reports of origi-
nal research, whether intended for a journal or a book, can be organized in the
standard format: abstract, introduction, experimental details or theoretical basis,
results, discussion, and conclusions. These sections are discussed in Chapter 2.
Keep in mind that scientific writing is not literary writing. Scientific writ-
ing serves a purpose completely different from that of literary writing, and it
must therefore be precise and unambiguous. You and your colleagues probably
have been discussing the project for months, so the words seem familiar, com-
mon, and clear to you. However, the readers will not have been part of these
discussions. Many words are clear when speaking because you can amplify the
meaning with gestures, expressions, and vocal inflections—but when these same
words are written, they may be clear only to you. Chapter 4 presents strategies
on how to write clearly and concisely as well as to select words that convey the
meaning intended.
If English is not your first language, ask an English-speaking colleague—if
possible, a native English speaker—for help with grammar and diction.
Publishers’ Requirements
An extremely important step is to check the specific requirements of the publica-
tion and to follow them. Journals often specify a format, the number of pages,
Box 3-1. Questions for Drafting Your Manuscript
What is the function or purpose of this manuscript? Are you describing
original and significant research results? Are you reviewing the liter-
ature? Are you providing an overview of the topic? Something else?
Who is the audience? Why would they want to read your manuscript?
What will you need to tell them to help them understand your work?
How is your work different from that described in other reports on the
same subject? (Unless you are writing a review, be sure that your manu-
script will make an original contribution. Most STM publishers, including
ACS, do not publish previously published material.)
What is the best format for publishing this manuscript—as a journal
article, book, or book chapter? If you choose a journal article, which
journal is most appropriate? (Links to ACS journals can be found at
http://pubs.acs.org/about.html.)
➤ ➤ ➤ ➤ ➤

Chapter 3: The Editorial Process ➤ 29
what software packages or file formats are acceptable, how to cite references, and
many other aspects of manuscript preparation. Requirements can vary from
journal to journal even if the same publisher publishes them. Author guidelines
for journals are generally posted on the Web at the journal’s Web site, and they
are also typically published in the first issue of each year. Book publishers also
have author guidelines that need to be followed to expedite publication. Under-
standing the requirements for the manuscript cannot be overemphasized.
Publishing with ACS: The author guidelines for ACS journals can be
seen at http://paragon.acs.org/paragon/index.jsp (see “Author Informa-
tion”). The author guidelines for ACS books can be found at http://pubs.
acs.org/books (see “Info for Authors”).
Some publishers provide templates for authors to use when preparing their
manuscripts. Use of a template makes it easier for authors to control margins,
fonts, and paragraph styles, as well as the length of the manuscript. It also facili-
tates peer review by placing tabular and graphical material near the discussion in
the text and providing journal and book editors with a single file to work with.
Templates are generally available for Windows and Macintosh platforms, and
they can be downloaded from a publisher’s or journal’s Web page.
Publishing with ACS: For ACS journals, templates can be accessed at
http://paragon.acs.org/paragon/index.jsp (see “Download Manuscript
Templates”). For ACS proceedings books, templates are available at
http://pubs.acs.org/books/authorinfo.shtml (see “Request instructions
on how to prepare your camera-ready manuscript”).
Artwork
As you write your draft manuscript, consider where structures, schemes, figures,
and tables could be used appropriately to illustrate or support the material. Well-
placed and well-designed artwork communicates information effectively, but too
much artwork can be distracting.
Few scientists have access to graphic arts professionals. Consequently, chemi-
cal professionals need to know how to prepare art for manuscripts. Fortunately,
software packages are available that can be easily mastered to produce good-
looking graphs, charts, schemes, and structures. Chapters 15, 16, and 17 provide
guidelines on when to use artwork and how to create figures, tables, or chemical
structures and schemes that publishers can use effortlessly. These chapters also
describe how to number figures, tables, structures, and schemes.
Sometimes you may wish to use artwork that has been previously published,
whether from your own publications or from those of other authors. To use pre-

30 ➤ The ACS Style Guide
viously published artwork, you must get permission from the copyright holder,
which is generally the publisher, even if you wrote the manuscript. Because it can
take some time to secure reprint permission, it is a good idea to start obtaining
permissions as you prepare your draft manuscript. If you wait until your manu-
script is accepted for publication to initiate any permissions correspondence, pub-
lication of your manuscript may be delayed because publishers generally will not
begin working on a manuscript when permissions are missing. Chapter 7 discusses
how to get permission to reprint figures that have been previously published. Pub-
lishers’ policies, and forms if required, are generally posted on their Web sites.
Publishing with ACS: Authors can reprint artwork previously published
in ACS books and journals in other ACS publications without permission,
provided that ACS is the original copyright holder. ACS’s copyright policy
and procedures can be found at http://pubs.acs.org/copyright_info.html.
Journals vary in their requirements about where tables and figures are placed
in the manuscript. Some journals permit tables and figures to be inserted into
the text for the draft but require that the tables and figures be submitted sepa-
rately in the final manuscript. Other journals request that the tables and figures
be embedded in the text. Some publishers accept figures prepared in a wide range
of software packages, whereas others specify use of certain drawing programs.
Check the specific requirements of the publication targeted before submitting
the draft manuscript.
Publishing with ACS: Placement of artwork submitted to ACS journals
depends on whether the manuscript is submitted through Paragon or
the Paragon Plus environment. Be sure to check the author guidelines for
the specific journal.
References
References are an important component of every scholarly manuscript. Having
complete and accurate references is the author’s responsibility. Errors in refer-
ences are one of the most common mistakes authors make. Although correct
citations have always been important, the increasing number of hypertext links
in Web-based publications makes correct citations more important than ever.
Given the volume of manuscripts that publishers produce yearly, technical edi-
tors cannot verify each reference in each manuscript.
The citation of references in text is a subject that varies widely from journal
to journal and publisher to publisher. There are three ways to cite references in
text in ACS publications: superscript numbers, italic numbers in parentheses,
or author name and year of publication. Authors are encouraged to check the

Chapter 3: The Editorial Process ➤ 31
author guidelines for a specific publication to find information on citing refer-
ences. Chapter 14 explains how to cite references in ACS publications and how
to format references from a variety of publications, in both print and electronic
formats.
✐Reminder: Although correct citations have always been important, the
increasing number of hypertext links in Web-based publications makes
correct citations more important than ever.
Revising the Draft Manuscript
Once you have written your initial draft, the next step is a careful revision
with an eye to organization, content, and editorial style, beginning with the
questions in Box 3-2. Several chapters in this book are designed to help you
communicate clearly. Chapter 9 reviews grammar, punctuation, and spelling.
Chapter 10 provides guidelines on stylistic and editorial conventions, such as
hyphenation and capitalization. Chapter 10 also includes a large appendix with
abbreviations, acronyms, and symbols. Guidelines for using numbers, mathe-
matics, and units of measure are given in Chapter 11. Two other chapters focus
on more specific issues related to chemistry. Chapter 12 examines the use of
proper chemical nomenclature. It provides rules for general chemistry nomen-
clature, as well as nomenclature in several specialized areas, such as polymer
chemistry, biological chemistry, and combinatorial chemistry. Chapter 13
presents a quick reference guide for the use of typefaces, Greek letters, super-
scripts and subscripts, and special symbols that are commonly used in chem-
istry. Chapter 13 also includes an appendix containing symbols for commonly
used physical quantities.
Manuscript Review
When your draft manuscript is complete, check the journal or book author
guidelines again for information on how and where to submit your draft. Some
editors request that authors suggest possible reviewers. Some journals require
that multiple copies of a draft manuscript be submitted and only accept manu-
scripts through the mail. Other journals request that the manuscript be submit-
ted electronically via e-mail. Still others, like ACS, are using a Web-based system
where authors submit a word-processing file or a PDF. For more information on
submitting manuscripts using a Web-based system, see Chapter 5.
Once the editor has reviewed the manuscript and determined that it is appro-
priate for the publication, the peer-review process begins. Chapter 6 describes
peer review and the responsibilities of reviewers and authors.

32 ➤ The ACS Style Guide
The Final Manuscript
If your manuscript is accepted, the editor of the book or journal will return the
peer-reviewed manuscript with a cover letter synthesizing the reviewers’ com-
ments and indicating what changes must be made for the final manuscript to
be accepted. You, the author, then revise the manuscript accordingly. When you
submit your final manuscript, include a cover letter indicating what changes
you made. If you decide not to make some of the requested changes, you should
write a rebuttal and send it with your final manuscript. For more information,
see Chapter 6.
Authors are encouraged to submit all the paperwork with the final, revised
manuscript. This includes all necessary permissions correspondence and, if
required, a signed form transferring copyright from the author to the publisher.
Chapter 7 gives a general introduction to copyright. If the manuscript is transmit-
ted electronically, mail the forms separately.
Box 3-2. Questions for Revising Your Manuscript
Does your manuscript as it is written perform the function—new research,
literature review, or topic overview—that you identified before you
began your draft? Do you still think the format you selected—journal
article, book, book chapter—is the best choice?
Have you explained terms, concepts, and procedures in a way that is ap-
propriate to the audience you identified at the start?
Is your material presented in a logical fashion, so that a reader can easily
follow your reasoning?
Is the manuscript too long? If so, what sections could be eliminated or
possibly used as supporting information?
Do some sections need to be expanded to further clarify the material?
Are the sentences clear and unambiguous?
Are all the words spelled correctly and technical terms used appropriately?
Did you follow generally accepted conventions—such as those in this
book—for communicating math and chemistry?
Could you use another opinion? You may find it helpful to ask a col-
league, preferably one who is not closely involved with the research
on which the manuscript is based, and preferably a native English
speaker, to read and comment on your draft.
➤ ➤ ➤ ➤ ➤

Chapter 3: The Editorial Process ➤ 33
✐Reminder: When you submit your final manuscript, you should include
the final versions of text, tables, and illustrations, as well as any neces-
sary permissions correspondence and a signed copyright transfer form.
Finally, keep a copy of the revised manuscript and all permissions correspon-
dence. You will need the revised manuscript to check against the proofs that your
publisher sends. Copies of the permissions correspondence can save you time
and effort if the permissions correspondence gets lost or separated from your
manuscript.
Processing of Accepted Manuscripts
Journal editors and multiauthored book editors send accepted manuscripts
directly to the publisher. Authors of monographs interact directly with the
publisher, generally through an acquisitions editor. Accepted manuscripts go
through three phases before publication: technical editing, proofing and review
by the author, and correction by the publisher.
Technical Editing
During the process of creating a book or journal issue, authors’ electronic word-
processing files are manipulated in a variety of ways. Files are tagged to iden-
tify data elements for print production and links for online products. Artwork
is prepared for both publication media. The manuscript is copyedited to ensure
consistency, clarity, and grammatical accuracy; changes are introduced to ensure
the use of standard chemical conventions, graphics presentation, and tabular
format. Copy editors often contact authors or query them at the proof stage for
clarification of material.
Author’s Proof
One author, generally the author to whom correspondence should be addressed,
receives a proof of the manuscript for final approval before publication. Papers
are not generally released for printing until the author’s proof or other approval
has been received. Hence, proofs should be checked and returned promptly
according to individual journal or book instructions.
Publishing with ACS: ACS journals request that proofs be returned
within 48 hours of receipt.
Authors should check proofs very carefully and submit all of the corrections
at one time; see Box 3-3 and Appendix 3-1 for information about reviewing

34 ➤ The ACS Style Guide
proofs. Only corrections and necessary changes can be made to proofs. Although
all authors may look at the proofs, only the corresponding author should submit
corrections. Extensive changes may require editorial approval, which delays pub-
lication. Printer’s errors are corrected at no cost to authors, but some publishers
charge authors the cost of extensive production work made necessary by their
own alterations.
Publication
After you return your corrected proofs, the technical editor will review them and
ensure that the corrections are made properly.
ASAP (As Soon As Publishable) Articles
Many STM publishers, including ACS, publish journal articles on the Web before
publishing them in print. Papers accepted for publication in ACS journals will be
posted on the “Articles ASAP” page on the journal Web site as soon as they are
ready for publication; that is, when the proofs are corrected and all author con-
cerns are resolved. Publication on the Web usually occurs within four working
days of receipt of proof corrections; this can be any time from three to six weeks
before the date of the printed issue. Once a paper appears on the Web, ACS and
the scientific community consider it published.
Box 3-3. Tips for Checking Proofs
➤ If you are instructed to return changes via the Web, list all corrections,
revisions, and additions and clearly identify their location.
➤ If you are instructed to return changes in hard copy (paper print-
outs), mark corrections legibly in the margins of the proofs as instructed
by the publisher. Do not erase or obliterate type; instead, strike one line
through copy to be deleted and write the change in the margin.
➤ Clarify complicated corrections by rewriting the entire phrase or sen-
tence.
➤ Check all text, data, and references against the original manuscript.
Pay particular attention to equations; formulas; tables; captions; spelling
of proper names; and numbering of equations, illustrations, tables and
references.
➤ Answer explicitly all queries made by the technical editor.
Proofreader’s marks and a sample of marked manuscript are given in
Appendix 3-1.
➤ ➤ ➤ ➤ ➤
Chapter 3: The Editorial Process ➤ 35
Article Reprints and Complimentary Copies
Generally, authors receive a form for reprint orders with the author proof.
Authors should follow the instructions on the form. Some publishers provide
electronic reprints, as well as paper reprints. Customarily, there is a charge for
paper reprints, and reprints with color artwork cost more.
Book authors sometimes also receive complimentary copies of the volume in
which their chapter appears. On contributions with more than one author, the
number of complimentary copies is generally limited; that is, not all authors will
receive complimentary copies.
Corrections to Published Manuscripts
Corrections of consequence to a paper that has already been published should
be sent to the editor. Most journals publish corrections soon after they have been
received. Some journals have a specific format for additions or corrections; check
the author guidelines. In books, errata sheets will be printed and included in
every book, and the book itself will be corrected before reprinting. However,
additions and corrections generally reflect poorly on the authors, and careful
manuscript preparation and attention to detail in the entire publication process
can prevent the necessity for subsequent corrections.
36
➤ ➤ ➤ ➤ ➤
APPENDIX 3-1
Proofreaders’ Marks
Publishers long ago established conventions for marking changes to manuscripts
and proofs. These conventions, known as proofreaders’ marks, evolved as an eco-
nomical and precise shorthand for indicating on paper various types of changes.
Changes to double-spaced manuscript are marked one way (Figure 3A-1).
Authors may be called on to interpret these proofreaders’ marks if the publisher
has copyediting done by hand, on paper. Common proofreaders’ marks for man-
uscripts are presented in Figure 3A-2. Note that corrections to manuscripts are
made in place (not in the margins) and usually need no additional explanation.
Changes to typeset proofs are marked somewhat differently (Figure 3A-3).
Authors may be called on to use this proofreading method if the publisher sup-
plies hard-copy (paper) proofs. Common proofreaders’ marks for proofs (also
called galleys or page proofs) are presented in Figure 3A-4. Note that corrections
to proofs are made in two places: a minimal mark is made in the typeset text,
to indicate where a change is being made, and an explanatory mark is made in
the margin to describe the exact change. For example, a carat mark (^) in the
typeset text indicates where new words are to be inserted; the words themselves
are written in the margin. If there is more than one change to a typeset line, the
changes in the margin are separated by slashes. (If you want to insert a slash, you
should write out the word “slash” and circle it in the margin.) Two slashes in a
row indicate that the first correction should be repeated. Try not to black out or
obliterate the typeset characters. Avoid using arrows and lines to indicate where
corrections go because more than one or two on a page breed confusion.
Chapter 3: The Editorial Process ➤ 37
Figure 3A-1. Sample of a manuscript copyedited by hand.

38 ➤ The ACS Style Guide
Strike through to delete a word or words.
Triple underline to capitalize the “w”.
Slash to make the “w” lowercase.
Transpose two letters.
Transpose two words.
Double underline to make “ord” small capitals
Draw a wavy line to indicate bold face.
Underline to indicate italic type.
Draw an inverted carat to indicate superscript.
Draw a carat to indicate subscript.
Put dots or short dashes under copy that you wish to retain as it originally
appeared.
Figure 3A-2. Common proofreaders’ marks for copyediting manuscripts.
Figure 3A-3. Sample of a marked proof.

Chapter 3: The Editorial Process ➤ 39
Operational Signs Typographical Signs
Delete Lowercase a capital letter
Close up; delete space Capitalize a lowercase letter
Delete and close up Set in small capitals
Insert space Set in italic type
Begin new paragraph Set in roman type
Run paragraphs together Set in boldface type
One em space Wrong font; set in correct type
Move right Superscript
Move left Subscript
Center Punctuation Marks
Move up Insert comma
Move down Insert apostrophe (or single
quotation mark)
Align horizontally Insert quotation marks
Align vertically Insert period
Transpose Insert question mark
Spell out Insert semicolon
Let it stand Insert colon
Flush left Insert hyphen
Flush right Insert em dash
Center Insert en dash
Figure 3A-4. Common proofreaders’ marks for marking proofs.
41
➤ ➤ ➤ ➤ ➤ CHAPTER 4
Writing Style and
Word Usage
Every writer has a personal style, but all good writing tends
to observe guidelines and conventions that communicate
meaning clearly and exactly to readers. Scientific writing, in particular, must be
precise and unambiguous to be effective.
This chapter presents guidelines for correct sentence structure and word
usage. Other chapters of this book present topics also related to good writing
style. Chapter 2 discusses the parts of a scientific paper; Chapter 3 presents an
overview of the editorial process. Chapters in Part 2 address more specialized
rules for usage; see, especially, Chapter 9 on grammar, punctuation, and spelling
and Chapter 10 on editorial style.
Correct Sentence Structure
Good organization (see Chapter 2) and sentence structure are an author’s pri-
mary tools for conveying information in a logical, persuasive manner. When the
words in a sentence are placed so that the reader follows easily from one fact
or point to the next, then the reader is best able to comprehend the author’s
intended meaning. Poorly structured or ordered sentences create confusion for
readers, who are then unable to understand accurately the author’s meaning.
Short, simple declarative sentences—that is, sentences that make statements,
rather than pose questions, issue commands, or exclaim—are the easiest to write
and the easiest to read. They are also usually clear. However, too many short sen-
tences in a row can sound abrupt or monotonous. They also can place too heavy
a burden on the reader to connect the ideas from one sentence to the next. To
Copyright 2006 American Chemical Society
42 ➤ The ACS Style Guide
add sentence variety and to enhance the flow of ideas, it is better to start with
simple declarative sentences and then combine some of them, rather than to start
with long rambling sentences and then try to shorten them. Two or more simple
sentences (sentences with one independent clause and no subordinate clauses)
can be combined to form a compound sentence. A complex sentence is created by
adding one or more subordinate clauses to a simple sentence. A clause is a group
of words that has a subject and a verb. If a clause can stand all by itself, it is an
independent clause. If a clause cannot stand alone, it is a dependent or subordinate
clause.
Verbs
Voice
A sentence is said to be in active voice when the subject of the sentence is the
doer of the action indicated by the verb. The subject of an active verb is doing
the action of the verb. In passive voice, the subject is the receiver of the action
indicated by the verb.
➤ Use the active voice when it is less wordy and more direct than the passive.
poor
The fact that such processes are under strict stereoelectronic control is demon-
strated by our work in this area.
better
Our work in this area demonstrates that such processes are under strict stereo-
electronic control.
➤ Use the passive voice when the doer of the action is unknown or not impor-
tant or when you would prefer not to specify the doer of the action.
The solution is shaken until the precipitate forms.
Melting points and boiling points have been approximated.
Identity specifications and tests are not included in the monographs for reagent
chemicals.
Tense
Using the appropriate verb tense helps to orient the reader as to the nature of the
information.
➤ Simple past tense is correct for stating what was done, either by others or
by you.
The solutions were heated to boiling.
Chapter 4: Writing Style and Word Usage ➤ 43
Jones reviewed the literature and gathered much of this information.
We found that relativistic effects enhance the bond strength.
The structures were determined by neutron diffraction methods.
➤ Present tense is correct for statements of fact.
Absolute rate constants for a wide variety of reactions are available.
Hyperbranched compounds are macromolecular compounds that contain a
branching point in each structural repeat unit.
➤ Present and simple past tenses may both be correct for results, discussion,
and conclusions.
The characteristics of the voltammetric wave indicate that electron transfer
occurs spontaneously.
The absence of substitution was confirmed by preparative-scale electrolysis.
IR spectroscopy shows that nitrates are adsorbed and are not removed by wash-
ing with distilled water.
However, the use of present or simple past tense for results, discussion, and con-
clusions should be consistent within a paper.
Other Forms
➤ It is acceptable to use split infinitives to avoid awkwardness or ambiguity.
awkward
The program is designed to assist financially the student who is considering a
career in chemistry.
better
The program is designed to financially assist the student who is considering a
career in chemistry.
ambiguous
The bonded phases allowed us to investigate fully permanent gases.
better
The bonded phases allowed us to fully investigate permanent gases.
Subjects and Subject–Verb Agreement
➤ Use first person when it helps to keep your meaning clear and to express a
purpose or a decision.
Jones reported xyz, but I (or we) found ….
44 ➤ The ACS Style Guide
I (or we) present here a detailed study ….
My (or our) recent work demonstrated ….
To determine the effects of structure on photophysics, I (or we) ….
However, avoid clauses such as “we believe”, “we feel”, and “we can see”, as well as
personal opinions.
➤ Subjects and verbs must agree in person and number; this important point is
discussed in detail in Chapter 9.
Sentence Modifiers
Modifiers made up of phrases or dependent clauses can be added to sim-
ple sentences to indicate, for example, cause and effect, or time sequence, or
comparison.
➤ A restrictive phrase or clause is one that is essential to the meaning of the sen-
tence. Restrictive modifiers are not set off by commas.
Only doctoral students who have completed their coursework may apply for this
grant.
Several systems that take advantage of this catalysis can be used to create new pal-
ladium compounds.
➤ A nonrestrictive phrase or clause is one that adds meaning to the sentence but
is not essential; in other words, the meaning of the basic sentence would be the
same without it. Nonrestrictive modifiers are set off by commas.
Doctoral students, who often have completed their coursework, apply for this
teaching fellowship.
Several systems, which will be discussed below, take advantage of this catalytic
reaction.
➤ A misplaced modifier is one that is placed next to the wrong word in the sen-
tence, so it inadvertently misrepresents the author’s intended meaning.
incorrect
We commenced a new round of experiments unable to point to meaningful con-
clusions.
correct
Unable to point to meaningful conclusions, we commenced a new round of
experiments.
Chapter 4: Writing Style and Word Usage ➤ 45
➤ A dangling modifier is one that lacks a word in the sentence to modify in a
logical or sensible way. It should not be confused with an absolute construction,
which modifies an entire sentence. (See also the discussion of dangling modifiers
in Chapter 9.)
incorrect
Adding 2 mL of indicator solution, the end point for the titration was reached.
correct
Adding 2 mL of indicator solution, we reached the end point for the titration.
When we added 2 mL of indicator solution, the end point for the titration was
reached.
Sentence Construction and Word Order
➤ Use an affirmative sentence rather than a double negative.
instead of consider using
This reaction is not uncommon. This reaction is common.
This reaction is not rare.
This reaction occurs about 40%
of the time.
This transition was not unexpected. This transition was expected.
We knew that such transitions
were possible.
This strategy is not infrequently used. This strategy is frequently used.
This strategy is occasionally used.
This result is not unlikely to occur. This result is likely to occur.
This result is possible.
➤ Watch the placement of the word “only”. It has different meanings in differ-
ent places in the sentence.
Only the largest group was injected with the test compound. (Meaning: and no
other group)
The largest group was only injected with the test compound. (Meaning: and not
given the compound in any other way)
The largest group was injected with only the test compound. (Meaning: and no
other compounds)
The largest group was injected with the only test compound. (Meaning: there
were no other test compounds)
➤ Be sure that the antecedents of pronouns are clear; in other words, when you
use a pronoun (for example, “he”, “she”, “it”, or “they”), the noun to which the
pronoun refers should be obvious (for example, “Isaac Newton”, “Marie Curie”,
46 ➤ The ACS Style Guide
“the compound”, or “the research team”). This is particularly true for the pro-
nouns “this” and “that”. If there is a chance of ambiguity, use a noun to clarify
your meaning.
ambiguous
The photochemistry of transition-metal carbonyl complexes has been the focus
of many investigations. This is due to the central role that metal carbonyl com-
plexes play in various reactions.
unambiguous
The photochemistry of transition-metal carbonyl complexes has been the focus
of many investigations. This interest is due to the central role that metal carbonyl
complexes play in various reactions.
➤ Use the proper subordinating conjunctions. (Conjunctions join parts of a sen-
tence; subordinating conjunctions join subordinate clauses to the main sentence.)
“While” and “since” have strong connotations of time. Do not use them where
you mean “although”, “because”, or “whereas”.
poor
Since solvent reorganization is a potential contributor, the selection of data is
very important.
better
Because solvent reorganization is a potential contributor, the selection of data is
very important.
poor
While the reactions of the anion were solvent-dependent, the corresponding
reactions of the substituted derivatives were not.
better
Although the reactions of the anion were solvent-dependent, the corresponding
reactions of the substituted derivatives were not.
The reactions of the anion were solvent-dependent, but (or whereas) the corre-
sponding reactions of the substituted derivatives were not.
Parallelism
Parallelism, or parallel construction, is the use of words or groups of words of
equal grammatical rank. Equal grammatical rank means that words are con-
nected only to words, phrases only to phrases, subordinate clauses only to other
subordinate clauses, and sentences only to other sentences. Establish parallel
construction by using coordinating conjunctions, correlative conjunctions, and
correlative constructions.
Chapter 4: Writing Style and Word Usage ➤ 47
➤ A coordinating conjunction is a single word, such as “and”, “but”, “or”, “nor”,
“yet”, “for”, and sometimes “so”.
incorrect
Compound 12 was prepared analogously and by Lee’s method (5).
correct
Compound 12 was prepared in an analogous manner and by Lee’s method (5).
➤ A correlative conjunction is a pairing of words, such as “either … or”; “neither
… nor”; “both … and”; “not only … but also”; and “not … but”.
incorrect
The product was washed either with alcohol or acetone.
correct
The product was washed with either alcohol or acetone.
The product was washed either with alcohol or with acetone.
incorrect
It is best to use alternative methods both because of the condensation reaction
and because the amount of water in the solvent increases with time.
correct
It is best to use alternative methods both because of the condensation reaction
and because of the increase in the amount of water in the solvent with time.
incorrect
Not only was the NiH functionality active toward the C-donor derivatives but
also toward the N donors.
correct
The NiH functionality was active not only toward the C-donor derivatives but
also toward the N donors.
The NiH functionality was not only active toward the C-donor derivatives but
also active toward the N donors.
Not only was the NiH functionality active toward the C-donor derivatives, but it
was also active toward the N donors.
➤ A correlative construction is a sentence structure that uses “as … as” (for
example, “as well as”).
He performed the experiment as well as I could have done it.
48 ➤ The ACS Style Guide
➤ Do not try to use parallel construction around the word “but” when it is not
used as a coordinating conjunction.
Increasing the number of fluorine atoms on the adjacent boron atom decreases
the chemical shift, but only by a small amount.
The reaction proceeded readily, but with some decomposition of the product.
➤ Use parallel constructions in series and lists, including section headings and
subheadings in text and tables and listings in figure captions.
Comparisons
➤ Introductory phrases that imply comparisons should refer to the subject of
the sentence and be followed by a comma.
incorrect
Unlike alkali-metal or alkaline-earth-metal cations, hydrolysis of trivalent lan-
thanides proceeds significantly at this pH.
correct
Unlike that of alkali-metal or alkaline-earth-metal cations, hydrolysis of trivalent
lanthanides proceeds significantly at this pH.
Unlike alkali-metal or alkaline-earth-metal cations, trivalent lanthanides hydro-
lyze significantly at this pH.
incorrect
In contrast to the bromide anion, there is strong distortion of the free fluoride
anion on the vibrational spectroscopy time scale.
correct
In contrast to the bromide anion, the free fluoride anion is strongly distorted on
the vibrational spectroscopy time scale.
➤ Use the verb “compare” followed by the preposition “to” when similarities are
being noted. Use “compare” followed by the preposition “with” when differences
are being noted. Only things of the same class should be compared.
Compared to compound 3, compound 4 shows an NMR spectrum with cor-
responding peaks.
Compared with compound 3, compound 4 shows a more complex NMR spectrum.
➤ Do not omit words needed to complete comparisons, and do not use con-
fusing word order. The subordinating conjunction “than” is often used to intro-
duce the second element in a comparison, following an adjective or adverb in the
comparative degree.
Chapter 4: Writing Style and Word Usage ➤ 49
incorrect
The alkyne stretching bands for the complexes are all lower than the uncoordi-
nated alkyne ligands.
correct
The alkyne stretching bands for the complexes are all lower than those for the
uncoordinated alkyne ligands.
The alkyne stretching bands are all lower for the complexes than for the uncoor-
dinated alkyne ligands.
incorrect
The decrease in isomer shift for compound 1 is greater in a given pressure incre-
ment than for compound 2.
correct
The decrease in isomer shift for compound 1 is greater in a given pressure incre-
ment than that for compound 2.
The decrease in isomer shift in a given pressure increment is greater for com-
pound 1 than for compound 2.
➤ Idioms often used in comparisons are “different from”, “similar to”, “identical
to”, and “identical with”. Generally these idioms should not be split.
incorrect
The complex shows a significantly different NMR resonance from that of com-
pound 1.
correct
The complex shows an NMR resonance significantly different from that of com-
pound 1.
incorrect
Compound 5 does not catalyze hydrogenation under similar conditions to com-
pound 6.
correct
Compound 5 does not catalyze hydrogenation under conditions similar to those
for compound 6.
exception These idioms can be split if an intervening prepositional phrase
modifies the first word in the idiom.
The single crystals are all similar in structure to the crystals of compound 7.
Solution A is identical in appearance with solution B.
50 ➤ The ACS Style Guide
➤ Phrases such as “relative to”, “as compared to”, and “as compared with” and
words such as “versus” are also used to introduce the second element in a com-
parison. The things being compared must be parallel.
The greater acidity of nitric acid relative to nitrous acid is due to the initial-state
charge distribution in the molecules.
The lowering of the vibronic coupling constants for Ni as compared with Cu is
due to configuration interaction.
This behavior is analogous to the reduced Wittig-like reactivity in thiolate versus
phenoxide complexes.
Correct Word Usage
The words chosen by a writer are one of the defining characteristics of that
author’s style; however, word choice is not governed by style alone. The audience
for a paper (as discussed in Chapter 3) must influence a writer’s choice of words
so that the writer can select words that are likely to be known to the audience
and define the words that are not. The type of document also may influence a
writer’s word choices because some documents, such as scientific papers, journal
articles, and books, tend to more formal word usage, whereas other documents,
such as e-mails, allow less formality.
The choice of the correct word to express meaning begins with a good dic-
tionary, but it also extends to understanding small differences in meaning
between two words or phrases that are almost synonymous or that are spelled
similarly but have significant differences in meaning. It is best to use words in
their primary meanings and to avoid using a word to express a thought if such
usage is uncommon, informal, or primarily literary. Many words are clear when
you are speaking because you can amplify your meaning with gestures, expres-
sions, and vocal inflections—but when these same words are written, they may
be clear only to you.
This chapter presents only a few words and phrases that are commonly mis-
used in scientific writing; consult a good reference on word usage for more com-
prehensive assistance. (Several such references are listed under the heading “Ref-
erences on Scientific Communication” in Chapter 18.)
Grouping and Comparison Words
➤ Use “respectively” to relate two or more sequences in the same sentence.
The excitation and emission were measured at 360 and 440 nm, respectively.
(That is, the excitation was measured at 360 nm, and the emission was measured
at 440 nm.)
Chapter 4: Writing Style and Word Usage ➤ 51
➤ Use the more accurate terms “greater than” or “more than” rather than the
imprecise “over” or “in excess of”.
greater than 50% (not in excess of 50%)
more than 100 samples (not over 100 samples)
more than 25 mg (not in excess of 25 mg, not over 25 mg)
➤ Use “fewer” to refer to number; use “less” to refer to quantity.
fewer than 50 animals
fewer than 100 samples
less product
less time
less work
➤ However, use “less” with number and unit of measure combinations because
they are regarded as singular.
less than 5 mg
less than 3 days
➤ Use “between” with two named objects; use “among” with three or more
named or implied objects.
Communication between scientists and the public is essential.
Communication among scientists, educators, and the public is essential.
Communication among scientists is essential.
Commonly Confused Words and Phrases
➤ Choose “myself” and “me” depending on your meaning. “Myself” is a reflex-
ive pronoun that is used only in sentences in which “I” is the subject, whereas
“me” is used as a direct or indirect object or the object of a preposition. “Myself”
is never a substitute for “me”.
Please give a copy of the agenda to Anne and me. (not to Anne and myself)
I myself checked the agenda.
Cheryl and I checked the agenda. (not Cheryl and myself)
The agenda was checked by Barbara and me. (not by Barbara and myself)
➤ Choose “due to”, which means “attributable to”, only to modify a noun or
pronoun directly preceding it in the sentence or following a form of the verb
“to be”.
Cutbacks due to decreased funding have left us without basic reference books.
The accuracy of the prediction is due to a superior computer program.
52 ➤ The ACS Style Guide
➤ Choose “based on” and “on the basis of” depending on your meaning.
Phrases starting with “based on” must modify a noun or pronoun that usually
immediately precedes or follows the phrase. Use phrases starting with “on the
basis of” to modify a verb.
The doctors’ new methods in brain surgery were based on Ben Carson’s work.
On the basis of the molecular orbital calculations, we propose a mechanism that
can account for all the major features of alkali and alkaline earth catalyzed gasifi-
cation reactions. (not Based on …)
➤ Choose “assure”, “ensure”, and “insure” depending on your meaning. To
assure is to affirm; to ensure is to make certain; to insure is to indemnify for
money.
He assured me that the work had been completed.
The procedure ensures that clear guidelines have been established.
You cannot get a mortgage unless you insure your home.
➤ Choose “affect”, “effect”, and “impact” depending on your meaning. When
“affect” is used as a verb, it means to influence, modify, or change. When “effect”
is used as a verb, it means to bring about, but as a noun it means consequence,
outcome, or result. “Impact” is a noun meaning a significant effect.
The increased use of pesticides affects agricultural productivity.
The use of polychlorinated benzenes has an effect on the cancer rate.
The effect of the added acid was negligible.
The new procedure effected a 50% increase in yield.
The impact of pesticide use on health is felt throughout the world.
The acid did not have a great impact on the reaction rate.
➤ Use “whether” to introduce at least two alternatives, either stated or implied.
I am not sure whether I should repeat the experiment.
I am not sure whether I should repeat the experiment or use a different statistical
treatment.
I am going to repeat the experiment whether the results are positive or negative.
Use “whether or not” to mean “regardless of whether”.
incorrect
I am not sure whether or not to repeat the experiment.
Chapter 4: Writing Style and Word Usage ➤ 53
correct
I am not sure whether to repeat the experiment.
Whether or not the results are positive, I will repeat the experiment.
Whether or not I repeat the experiment, I will probably leave the laboratory late
tonight.
➤ Use “to comprise” to mean “to contain” or “to consist of”; it is not a synonym
for “to compose”. The whole comprises the parts, or the whole is composed of the
parts, but the whole is not comprised of the parts. Never use “is comprised of”.
incorrect
A book is comprised of chapters.
correct
A book comprises chapters.
A book is composed of chapters.
incorrect
Our research was comprised of three stages.
correct
Our research comprised three stages.
Use of “A” and “An”
➤ Choose the articles “a” and “an” according to the pronunciation of the words
or abbreviations they precede. See pp 257 and 264 for the use of “a” and “an”
with chemical elements and isotopes.
a nuclear magnetic resonance spectrometer
an NMR spectrometer
➤ Use “a” before an aspirated “h”; use “an” before the vowel sounds of a, e, i, o,
“soft” or “short” u, and y.
a house, a history (but an hour, an honor)
a union, a U-14C (but an ultimate)
a yard (but an ylide, an yttrium compound)
➤ Choose the proper article to precede B.A., B.S., M.A., M.S., and Ph.D., accord-
ing to pronunciation of the first letter.
a B.S. degree
an M.S. degree
a Ph.D.
54 ➤ The ACS Style Guide
Words and Phrases To Avoid
➤ Avoid slang and jargon.
➤ Be brief. Wordiness obscures your message and annoys your readers.
➤ Omit empty phrases such as
As already stated
It has been found that
It has long been known that
It is interesting to note that
It is worth mentioning at this point
It may be said that
It was demonstrated that
➤ Omit excess words.
instead of consider using
It is a procedure that is often used. This procedure is often used.
There are seven steps that must be completed. Seven steps must be completed.
This is a problem that is …. This problem is ….
These results are preliminary in nature. These results are preliminary.
➤ Write economically (and usually more precisely) by using single words
instead of phrases.
instead of consider using
a number of many, several
a small number of a few
are found to be are
are in agreement agree
are known to be are
at present now
at the present time now
based on the fact that because
by means of by
despite the fact that although
due to the fact that because
during that time while
fewer in number fewer
for the reason that because
has been shown to be is
if it is assumed that if
in color, e.g., red in color just state the color, e.g., red
in consequence of this fact therefore, consequently
in length long
in order to to
in shape, e.g., round in shape just state the shape, e.g., round
in size, e.g., small in size just state the size, e.g., small
Chapter 4: Writing Style and Word Usage ➤ 55
instead of consider using
in spite of the fact that although
in the case of … in …, for …
in the near future soon
in view of the fact that because
is known to be is
it appears that apparently
it is clear that clearly
it is likely that likely
it is possible that possibly
it would appear that apparently
of great importance important
on the order of about
owing to the fact that because
prior to before
reported in the literature reported
subsequent to after
➤ Do not use contractions in scientific papers.
incorrect
The identification wasn’t confirmed by mass spectrometry.
correct
The identification was not confirmed by mass spectrometry.
➤ Do not use the word “plus” or the plus sign as a synonym for “and”.
incorrect
Two bacterial enzymes were used in a linked-enzyme assay for heroin plus metab-
olites.
correct
Two bacterial enzymes were used in a linked-enzyme assay for heroin and its
metabolites.
➤ Do not use “respectively” when you mean “separately” or “independently”.
incorrect
The electrochemical oxidations of chromium and tungsten tricarbonyl com-
plexes, respectively, were studied.
correct
The electrochemical oxidations of chromium and tungsten tricarbonyl com-
plexes were studied separately.
56 ➤ The ACS Style Guide
➤ Avoid misuse of prepositional phrases introduced by “with”.
poor
Nine deaths from leukemia occurred, with six expected.
better
Nine deaths from leukemia occurred, and six had been expected.
poor
Of the 20 compounds tested, 12 gave positive reactions, with three being greater
than 75%.
better
Of the 20 compounds tested, 12 gave positive reactions; three of these were
greater than 75%.
poor
Two weeks later, six more animals died, with the total rising to 25.
better
Two weeks later, six more animals died, and the total was then 25.
➤ Do not use a slash to mean “and” or “or”.
incorrect
Hot/cold extremes will damage the samples.
correct
Hot and cold extremes will damage the samples.
➤ Replace “and/or” with either “and” or “or”, depending on your meaning.
incorrect
Our goal was to confirm the presence of the alkaloid in the leaves and/or roots.
correct
Our goal was to confirm the presence of the alkaloid in the leaves and roots.
Our goal was to confirm the presence of the alkaloid in either the leaves or the
roots.
Our goal was to confirm the presence of the alkaloid in the leaves, the roots, or
both.
➤ If you have already presented your results at a symposium or other meeting
and are now writing the paper for publication in a book or journal, delete all
references to the meeting or symposium, such as “Good afternoon, ladies and
gentlemen”, “This morning we heard”, “in this symposium”, “at this meeting”, and
Chapter 4: Writing Style and Word Usage ➤ 57
“I am pleased to be here”. Such phrases would be appropriate only if you were
asked to provide an exact transcript of a speech.
➤ Avoid using the word “recently”. Your article or book may be available for a
long time. This word will make it look dated in little time.
poor
It was recently found that these effects enhance the bond strength.
better
Harris and Harris (2006) found that these effects enhance the bond strength.
Gender-Neutral Language
The U.S. government and many publishers have gone to great effort to encourage
the use of gender-neutral language in their publications. Gender-neutral language
is also a goal of many chemists. Recent style guides and writing guides urge copy
editors and writers to choose terms that do not reinforce outdated sex roles. Gen-
der-neutral language can be accurate and unbiased and not necessarily awkward.
The most problematic words are the noun “man” and the pronouns “he” and
“his”, but there are usually several satisfactory gender-neutral alternatives for these
words. Choose an alternative carefully and keep it consistent with the context.
➤ Instead of “man”, use “people”, “humans”, “human beings”, or “human spe-
cies”, depending on your meaning.
outdated
The effects of compounds I–X were studied in rats and man.
gender-neutral
The effects of compounds I–X were studied in rats and humans.
outdated
Men working in hazardous environments are often unaware of their rights and
responsibilities.
gender-neutral
People working in hazardous environments are often unaware of their rights and
responsibilities.
outdated
Man’s search for beauty and truth has resulted in some of his greatest accom-
plishments.
gender-neutral
The search for beauty and truth has resulted in some of our greatest accomplish-
ments.
58 ➤ The ACS Style Guide
➤ Instead of “manpower”, use “workers”, “staff”, “work force”, “labor”, “crew”,
“employees”, or “personnel”, depending on your meaning.
➤ Instead of “man-made”, use “synthetic”, “artificial”, “built”, “constructed”,
“manufactured”, or even “factory-made”.
➤ Instead of “he” and “his”, change the construction to a plural form (“they”
and “theirs”) or first person (“we”, “us”, and “ours”). Alternatively, delete “his”
and replace it with “a”, “the”, or nothing at all. “His or her”, if not overused, is also
acceptable. Using passive voice or second person (“you”, “your”, and “yours”) also
works sometimes.
outdated
The principal investigator should place an asterisk after his name.
gender-neutral
Principal investigators should place asterisks after their names.
If you are the principal investigator, place an asterisk after your name.
The name of the principal investigator should be followed by an asterisk.
➤ Do not use a plural pronoun with a singular antecedent.
incorrect
The principal investigator should place an asterisk after their name.
correct
The principal investigators should place asterisks after their names.
➤ Instead of “wife”, use “family” or “spouse” where appropriate.
outdated
The work of professionals such as chemists and doctors is often so time-consum-
ing that their wives are neglected.
gender-neutral
The work of professionals such as chemists and doctors is often so time-consum-
ing that their families are neglected.
outdated
the society member and his wife
gender-neutral
the society member and spouse

59
➤ ➤ ➤ ➤ ➤ CHAPTER 5
Electronic Submission
of Manuscripts Using
Web-Based Systems
Sarah C. Blendermann
Electronic submission of manuscripts to journals is under-
going significant change as publishers respond to mount-
ing pressure to publish faster, better, and more efficiently. The use of the Web and
e-mail enables the peer-review process to move more rapidly, speeding review
and decision cycles. And although scientific research has always been an interna-
tional activity, journal publishing is increasingly global, with authors, reviewers,
and editors contributing from numerous countries and all participants benefit-
ing from electronic communications.
✐Reminder: Good practices and appropriate file creation start early in
the manuscript preparation process. If author source files are not of
adequate quality for production, then the publication process will be
delayed. Authors should be mindful of publishers’ requirements early in
the writing process.
This chapter covers the major systems used by dominant commercial pub-
lishers and professional societies to manage the submission, review, and accep-
tance of scholarly manuscripts, and it endeavors to guide authors through the
routine tasks associated with submitting a manuscript online. It is important
to acknowledge that publishers will revise their systems and add new features
quickly, so the information that follows will likely age rapidly. However, authors
can rely on this chapter to guide them through the general process of online
submission.
Proprietary and commercial editorial systems are frequently designed to
accommodate both electronic and paper publication processes. Capabilities vary,
Copyright 2006 American Chemical Society
60 ➤ The ACS Style Guide
but many systems have become similar as publishers establish parallel mecha-
nisms for managing the peer-review process. Leading commercial software
packages currently include Bench>Press from HighWire Press, Editorial Man-
ager from Aries Systems, EJPress from eJournal Press, ScholarOne Manuscript
Central, and Rapid Review from Cadmus Systems. Other Web-based commer-
cial products include EdiKit from Berkeley Electronic Press; myICAAP, EPRESS,
ESPERE, Fontisworks, and Open Journal Systems from University of British
Columbia; PublishASAP; Temple Peer Review Manager; and Xpress Track. Each
package provides authors, reviewers, and editors with submission acknowledg-
ments, decision letters, and review documents transmitted using e-mail; no
paper correspondence is needed.
Several publishers have opted to develop proprietary programs rather than
purchase services from a third party. Examples include the American Chemi-
cal Society’s ACS Paragon System and the American Institute of Physics’ Peer
X-Press. These packages offer features and workflow similar to those contained
in commercial editorial packages.
Appendix 5-1 matches selected scientific publishers and research grant agen-
cies with the manuscript submission software they use and manuscript submis-
sion sites.
Preparing Materials
The author guidelines for each journal contain generic and journal-specific
instructions concerning manuscript preparation. They indicate the types of
components that are required for online submissions, such as the cover letter,
abstract, manuscript document, supporting information, figures, and proposed
reviewers. Publishers require these items to be submitted in common word-pro-
cessing or graphics formats, and author guidelines provide the technical details
for preparing these manuscript components. Assembling and organizing elec-
tronic components of a manuscript in advance will streamline the process and
reduce the possibility of errors in submission.
The author guidelines will indicate if there are word-processing templates
available for authors to download and will specify if there are requirements
about their use. Template files contain all the necessary formatting for a particu-
lar journal and are provided by the publisher from its Web site. Templates make
it easier to format submissions to meet the publisher’s specifications.
It is important to understand why authors are asked to provide manuscripts
as both word-processing and PDF files. These versions of the manuscript are
used in different ways; the PDF version of a manuscript is more suitable for peer
review because it is easily transferred among authors, editors, and reviewers and
is readable with the ubiquitous free Adobe Acrobat Reader. PDF files are portable

Chapter 5: Electronic Submission of Manuscripts ➤ 61
across computer platforms, whereas original word-processing files may not be
compatible with the system of an editor or reviewer. Publishers may require that
authors submit a PDF file, or they may automatically generate one as part of the
online submission process. A PDF file assists in maintaining control of versions
of the manuscript because editors, authors, and reviewers can insert comments
and notes for correction without altering the original text. Most publishers will
require word-processing (or TeX or its derivatives) files for production purposes.
These versions of the manuscript are manipulated during the final electronic
creation of a journal for publication online or in print.
✐Reminder: Most publishers require both PDF and word-processing
versions of a manuscript to be used for peer review and publication
production, respectively.
Another critical element of your submission is the figures associated with the
manuscript. Graphics should be high-resolution and of good quality to ensure
clarity and accuracy in the final published copy and to facilitate any needed
reduction required in the print publication (see Chapter 15 for more detail on
preparing illustrations). Many software programs—including PowerPoint, Word,
Excel, and WordPerfect—do not create high-resolution images suitable for pub-
lication. For that reason, it is recommended that authors create graphics using
applications that can prepare figures in TIFF or EPS formats. Figures should
have clear, sharp lines, should be clearly labeled, and should be at a high enough
resolution that they can be used to compose the print journal.
Appendix 5-2 presents the text and image formats accepted by seven major
manuscript peer-review and submission systems.
Beginning Your Submission
Depending on the publisher, authors may be asked to e-mail the prepared manu-
script to the editor, to upload it via an FTP server, or to upload files through the
publisher’s Web browser to a secure Web site using HTTP protocol. It is extremely
important to submit manuscripts in the method designated for that particular
journal because submission mechanisms may vary, even within one publisher’s
journals. If the journal requires online manuscript submission, submit all files
to that Web site. Similarly, if the journal uses FTP or e-mail, follow the instruc-
tions carefully and send all manuscript components via the same route, unless
indicated otherwise.
When authors are asked to e-mail the manuscript directly to an editor, pay
particular attention to the type of files authors are asked to provide, and note

62 ➤ The ACS Style Guide
if the manuscript should be provided in the body of the e-mail or as an attach-
ment. Publishers offering an FTP server for author use will have detailed instruc-
tions on how to access the FTP server. Authors may be asked to compress files
when transmitting manuscripts by e-mail, FTP, or as part of an online submis-
sion system. If so, the publisher’s Web site will detail the acceptable formats for
compressed files. These may include zipped, tarred, uuencoded, or BinHex files.
Under some circumstances, Web uploads will automatically invoke a compres-
sion process.
Leading online submission packages Bench>Press, Editorial Manager, EJPress,
Manuscript Central, and Rapid Review, as well as the ACS Paragon System and
the American Institute of Physics’ Peer X-Press all use the author home page.
This simple online profile contains the author’s contact details and establishes
a user name and password. The e-mail address entered here must be correct; a
typo could delay important e-mail notifications about acceptance or requests for
revision. If the author has already established an account, future submissions can
begin with the author login.
Several publishers have adopted the concept of a submitting agent or a second
author if someone other than the author is submitting the manuscript on the
author’s behalf. This person is responsible for the tasks associated with submis-
sion, but correspondence and requests for further changes are made to the party
designated as the corresponding or primary author of the manuscript. Submitting
agent accounts are created similarly to author accounts, but they require informa-
tion about the primary author also to be added to the user account profile.
Passwords to either author accounts or submitting agent accounts should not
be shared. Doing so leaves the account holder at risk of incomplete submissions
being changed or new submissions being created under his or her name.
The Author Home Page
After logging in, the author is presented with his or her home page. Typically,
the author home page is divided into several areas; from this location, authors
can begin a new submission, check the status of a previous submission, continue
a submission begun earlier, or submit a revised manuscript. The author home
page also shows the progress of accepted manuscripts through the production
cycle to publication. As publishers increasingly recognize the value of this author
home page, new features are likely to be added. In the case of the Cadmus Rapid
Review software, the author home page has already evolved to include a scien-
tist’s activities as both a referee and an author.
✐Reminder: If an author home page is available, use it to submit new
articles and to track the status of current submissions.
Chapter 5: Electronic Submission of Manuscripts ➤ 63
Submitting Your Manuscript
Preparing materials in advance allows the authors either to complete their full
submissions in one sitting or to partially complete the process, depending on
individual preferences and the requirements of the publisher. E-mail and FTP
submissions require the submission to be completed at one time. However, more
publishers with online submission sites allow authors to interrupt their online
submissions and to complete them later. During a partial submission, each step
must be completed for the information pertaining to that submission step to be
saved. The author can complete the submission by logging into the journal site
and accessing his or her author home page. After selecting the link for that man-
uscript, the submission process can be resumed, beginning with the first incom-
plete step. Before the manuscript is submitted, authors are asked to review all the
component parts and make any final changes.
Supplemental information should also be reviewed and validated. Crystallo-
graphic information files (CIFs) in particular can be verified using the free Check-
CIF utility, which is available from the International Union of Crystallography.
Usually in the cases of e-mail, FTP, or online submission, no additional changes
to the manuscript or associated documents are permitted after submission unless
an editor requests a revision or the publisher contacts the author to fix a problem.
Submitted Manuscripts
After the submission is complete, the author typically receives an e-mail acknowl-
edging the submission from either the editor or the submission system. If the pub-
lisher offers an author home page, the status of the manuscript can be tracked from
that site. These sites also notify authors by e-mail as the status of the manuscript
changes. If the publisher makes use of an online system for submission, the editors
for that publisher may be able to use features available only to them to shepherd
the manuscript through the peer-review process. In these cases, the system notifies
the editor of newly arrived manuscripts, allows the editor to view all associated
files and details of the submission, and allows for the manuscript to be assigned to
the appropriate editors and reviewers. Many systems generate the correspondence
that accompanies the notification of editors, reviewers, and authors. As decisions
are made and correspondence is sent, the corresponding status changes for the
manuscript are displayed on the author home page. Additional details about the
peer-review process can be found in Chapter 6.
Stops Along the Way (Revisions)
When a revision of the paper is requested, an e-mail from the editor will detail
the necessary changes. Where FTP or direct-to-editor e-mail is the preferred sub-
64 ➤ The ACS Style Guide
mission mechanism, the author will send revised files directly to the indicated
e-mail address or FTP location. For publishers with author home pages, authors
can view the status of submitted manuscripts and determine that a revision has
been requested. From the author home page, authors access the submission and
upload revised manuscript documents, images, and associated files. During
the submission of the revision, authors may also provide rebuttal information
or revision details to clarify how the manuscript has been altered. If an author
determines that a revision is necessary before a request is made by the editor,
authors using Web-based submission systems must contact the appropriate edi-
tor and request that the status of the manuscript be changed within the system
to indicate that a revision is needed. This step will allow the author to adjust
and replace files and then to submit a revision. Once the revision is received,
an acknowledgment is sent by e-mail, and the author will not be able to make
further changes to the paper unless the editor requests another revision to the
manuscript.
Acceptance
Authors can expect to be notified of their manuscripts’ acceptance by e-mail.
Accepted manuscripts are copyedited and formatted according to specific jour-
nal style, using the original word-processing files from the author’s online sub-
mission. As the manuscript progresses through the various stages of production,
the status on the author home page changes to reflect each new step.

65
➤ ➤ ➤ ➤ ➤
APPENDIX 5-1
Online Submission at Selected
Scientific Publishers and
Research Grant Agencies
This appendix contains a list of scientific publishers and research grant agen-
cies, along with the software they use for online manuscript submission and the
manuscript submission site, if there is one.
Table 5A-1. Scientific Publishers
Publisher Software URL
American Academy of
Forensic Science
Information and
forms only
No electronic submission
American Association
for Clinical Chemistry
Bench>Press http://submit.clinchem.org/?ctst=y
American Association
of Pharmaceutical
Scientists
Editorial Manager Pharmaceutical Research submissions at
https://www.editorialmanager.com/
pharmres/
American Chemical
Society
ACS Paragon System https://paragon.acs.org/paragon/index.jsp
American Geophysical
Union
Geophysical Elec-
tronic Manuscript
System
http://gcubed-submit.agu.org/
American Institute for
Chemical Engineers
Rapid Review https://www.rapidreview.com/AIChE2/
CALogon.jsp
American Institute of
Physics
Some journals use
Peer X-Press
http://www.aipservices.org/peerxpress/
index.html
After acceptance, you will be asked to use
their FTP or e-mail site at http://www.aip.
org/epub/submittext.html.
American Mathematical
Society
Information and
forms only
No electronic submission
American Peptide
Society
ScholarOne Manu-
script Central
http://bip-pep-wiley.manuscriptcentral.com/
American Pharmacists
Association
ScholarOne Manu-
script Central
Journal of Pharmaceutical Sciences sub-
missions at http://jpharmsci-wiley.
manuscriptcentral.com/
American Physical
Society
— http://publish.aps.org/ESUB/
American Society for
Biochemistry and
Molecular Biology
—The Journal of Biological Chemistry submis-
sions at http://osrs.jbc.org/asbmb/osrs.
nsf/StartSubmission?OpenForm
Continued on next page

66 ➤ The ACS Style Guide
Table 5A-1. Scientific Publishers—Continued
Publisher Software URL
American Society for
Cell Biology
EJPress http://www.mbcpapers.org/
American Society for
Mass Spectrometry
Elsevier author
gateway
http://authors.elsevier.com/JournalDetail.
html?PubID=505727&Precis=DESC
American Society for
Microbiology
Rapid Review https://www.rapidreview.com/ASM2/
CALogon.jsp
Biophysical Society Bench>Press http://submit.biophysj.org/?ctst=y
Blackwell Publishing Information and
forms only
No electronic submission
Cambridge University
Press
Information and
forms only
No electronic submission
Electrochemical Society Peer X-Press http://jes.peerx-press.org/cgi-bin/main.plex
Elsevier Elsevier author
gateway
Each journal has its own instructions for
submitting work. Go to the home page of
your journal of interest at http://authors.
elsevier.com/.
Institute of Electrical &
Electronics Engineers
(IEEE)
— Each journal has its own instructions for
submitting work. Go to the home page of
your journal of interest at http://www.ieee.
org/organizations/pubs/guide.html.
Institute of Food
Technologists
ScholarOne Manu-
script Central
http://ift.manuscriptcentral.com/
Materials Research
Society
ScholarOne Manu-
script Central
http://jmr.manuscriptcentral.com/
Nature EJPress http://npg.nature.com/npg/servlet/
Content?data=xml/05_sub.
xml&style=xml/05_sub.xsl
Oxford University Press Some journals use
ScholarOne Manu-
script Central
Each journal has its own instructions for sub-
mitting work. Go to the home page of your
journal of interest at http://www3.oup.
co.uk/jnls/.
Royal Society of
Chemistry
ReSourCe http://chemistry.rsc.org/Publishing/
ReSourCe/
Science Submit to Science http://www.submit2science.org/ws/menu.
asp
Society of Plastics
Engineers
Information and
forms only
No electronic submission
Society of Toxicology ScholarOne Manu-
script Central
http://toxsci.manuscriptcentral.com/
Taylor & Francis Information and
forms only
No electronic submission
Wiley-VCH Some journals use
ManuscriptXpress
Each journal has its own instructions for sub-
mitting work. Go to the home page of your
journal of interest.
Wolters Kluwer/Springer ScholarOne Manu-
script Central
Each journal has its own instructions for
submitting work. Go to the home page
of your journal of interest at http://www.
kluweronline.com.
World Scientific Each journal has its own instructions for
submitting work. Go to the home page
of your journal of interest at http://www.
worldscinet.com/subject.shtml.

Chapter 5: Electronic Submission of Manuscripts ➤ 67
Table 5A-2. Research Grant Agencies
Research Grant Agency Software URL
Alexander von Humboldt
Stiftung
Information and forms
only
No electronic submission
http://www.humboldt-foundation.de/
en/programme/bewerbung.htm
American Chemical Society
Petroleum Research Fund
— Electronic submission only.
http://www.chemistry.org/prf
American Heart Association — Electronic submission only.
http://www.americanheart.org/
presenter.jhtml?identifier=270
https://home.heart.org/resch/
Australian Research Council Grant Application
Management System
(GAMS)
Electronic submission only.
https://gams.arc.gov.au/
Camille and Henry Dreyfus
Foundation
Limited online
submission
http://www.dreyfus.org/index.shtml
Ford Foundation Diversity
Fellowships
NRCOnline http://nrc58.nas.edu/nrconline/ford/
login/Login.asp
NASA NASA Solicitation and
Proposal Integrated
Review and Evalua-
tion System (NSPIRES)
http://nspires.nasaprs.com/external/
The National Academies,
Research Associateship
Programs
NRC WebRAP http://nrc58.nas.edu/nrcwebrap/rap/
login/Register.asp
National Institutes of Health,
Office of Extramural
Research
Information and forms
only
No electronic submission
http://grants1.nih.gov/grants/oer.htm
National Science Foundation FastLane https://www.fastlane.nsf.gov/fastlane.
jsp
Office of Naval Research Information only No electronic submission
http://www.onr.navy.mil/02/how_
to.asp
proposalCENTRAL Research and Manage-
ment System (RAMS)
https://v2.ramscompany.com/
U.S. Air Force Office of Scien-
tific Research
Information and forms
only
No electronic submission
http://www.afosr.af.mil/
U.S. Department of Energy,
Office of Science
PureEdge http://www.science.doe.gov/grants/
Welch Foundation Information and forms
only
No electronic submission
http://www.welch1.org/
Wellcome Trust eGrants http://www.wellcome.ac.uk/node2110.
html or http://www.wellcome.ac.uk/
doc_WTD004053.html
68
➤ ➤ ➤ ➤ ➤
APPENDIX 5-2
Key Features of Selected
Online Submission Systems
The following systems are entirely Internet-based and incorporate all elements of
submission, review, and manuscript management, unless otherwise noted. They
operate with standard browsers and require that authors, editors, and review-
ers have Adobe Acrobat Reader to view PDF documents. Authors, reviewers, and
editors are offered a secure, password-protected login. Each system incorporates
an author home page for the submission, revision, and tracking of manuscripts.
Similarly, each system allows referees to submit reviews of manuscripts online.
Publishers modify these systems in varying degrees to reflect different work-
flows. For this reason, journals using the same software may have slightly differ-
ent features. This list indicates acceptable submission file types for each system
and highlights the system’s distinctive features. As technology changes and new
features become available, publishers will amend submission sites and systems.
Therefore, authors should check regularly for current requirements with the
journal before beginning a manuscript submission.
Table 5A-3 catalogs a variety of scientific publishers and the systems they cur-
rently use to accept manuscripts online. This table is not all-encompassing, but
it is intended to be representative of publishing organizations that contribute to
the peer-review literature used by ACS members.

Chapter 5: Electronic Submission of Manuscripts ➤ 69
Table 5A-3. Text and Image Formats Acceptable to Different Web-Based
Manuscript Submission Systems
Format
ACS
Paragon
System
Bench>
Press
Editorial
Manager EJPress
Manu-
script
Central
Peer
X-Press
Rapid
Review
Text Formats
MS Word (.DOC) Yes Yes Yes Yes Yes Yes —
WordPerfect (.WPD) — Yes Yes Yes Yes Yes —
Encapsulated PostScript
(.EPS) Yes — Yes Yes Yes Yes —
PostScript (.PS) Yes — Yes Yes Yes Yes —
Text—ASCII (.TXT) — — Yes — Yes — —
Rich text format (.RTF) Yes — Yes Yes Yes Yes —
TeX Yes — Yes — Yes — —
LaTeX Yes — Yes — Yes — —
Portable document format
(.PDF) Yes Yes Yes Yes Yes Yes —
Image Formats
Graphics interchange for-
mat (.GIF) Yes Yes Yes Yes Yes Yes —
Joint Photographic
Experts Group (.JPEG) Yes Yes Yes Yes Yes Yes —
Tagged image file (.TIF) Yes Yes Yes Yes Yes Yes Yes
Encapsulated PostScript
(.EPS) — Yes Yes Yes Yes Yes Yes
PostScript (.PS) — — — Yes Yes Yes —
MS PowerPoint (.PPT) — — — — — — Yes
Other Acceptable Formats a b b a b a b
Chemical markup lan-
guage (.CML) Yes Yes Yes — Yes — Yes
Chemical structures,
such as those created
by ChemDraw, Chem-
Sketch, ISIS/Draw Yes Yes Yes — Yes — Yes
DrawIt (formerly
Chemwindow) Yes Yes Yes — Yes — Yes
Crystallographic informa-
tion file (.CIF) Yes Yes Yes — Yes — Yes
Executable (.EXE) Yes Yes Yes — Yes — Yes
MS Excel (.XLS) Yes Yes Yes — Yes — Yes
MOL Yes Yes Yes — Yes — Yes
Video files, such as Quick-
Time (.AVI, .MPEG) Yes Yes Yes — Yes — Yes
Protein Data Bank (.PDB) Yes Yes Yes — Yes — Yes
Windows metafile (.WMF) Yes Yes Yes — Yes — Yes
Winzip file (.ZIP) Yes Yes Yes — Yes — Yes
Note: The systems and their developers are as follows: ACS Paragon System, American Chemical Society;
Bench>Press, HighWire Press; Editorial Manager, Aries Systems; EJPress, eJournal Press; Manuscript Central,
ScholarOne; Peer X-Press, American Institute of Physics; and Rapid Review, Cadmus Systems.
aThese systems allow a variety of designated file types to be uploaded as supplemental information.
bThese systems allow any file type to be uploaded as supplemental information.
71
➤ ➤ ➤ ➤ ➤ CHAPTER 6
Peer Review
Barbara A. Booth
Peer review is a process used by scientific publications to
assist editors in evaluating manuscripts, particularly for
scientific merit. It is not the only system used to evaluate manuscripts, and it is
not perfect. Editors of peer-reviewed books and journals send manuscripts to
several reviewers and request their opinions on originality and scientific impor-
tance of the topic, the quality of the work performed, and the appropriateness
for the specific journal. Reviewers may also comment on language usage, clarity
of figures and tables, manuscript length, and anything that they find relevant to
effective communication. Although not every editor uses peer review in the same
manner, this chapter presents a summary of many common attributes of the
peer-review process. (For a broader discussion of peer review, see “Peer Review
and the Acceptance of New Scientific Ideas” at http://www.senseaboutscience.
org.uk/PDF/peerReview.pdf.)
Purpose of Peer Review
When a manuscript is submitted for consideration, peer review provides the edi-
tor with advice on whether to accept the manuscript for publication. Reviewers
also provide suggestions for improving the manuscript. The decision on whether
to accept the manuscript for publication rests solely with the editor. Reviewers
provide additional expertise and have perspectives that may complement that of
the editor. Customarily, peer review is anonymous: the identities of reviewers are
not revealed by editors. Some journals also hold authors’ names and affiliations
in confidence, a double-blind review approach. Occasionally, reviewers request
Copyright 2006 American Chemical Society
72 ➤ The ACS Style Guide
that the editor disclose their names to authors; this is allowed at the discretion of
the editor, based on the policy of the individual publication. In one variation of
the peer-review process, review is not anonymous; all reviewers are identified.
Peer review is also intended to help authors. External review can help improve
the presentation and interpretation of data alike, and ultimately, the research.
Clearly and succinctly describing a scientific study is challenging, and review-
ers provide valuable feedback. Few manuscripts are so well written that they are
accepted without revision. Data and interpretation that seem clear to authors
are not always comprehensible to readers. Scientific research is both competitive
and cooperative; at its best, peer review is a part of the cooperative process. For
example, after evaluating data, a reviewer may suggest an alternative explanation
or additional experiments that trigger ideas for further research. Also, a reviewer
pointing out an error can save an author the embarrassment of subsequently
publishing a correction.
The Peer-Review Process
When the editor receives a manuscript, he or she examines it and determines
whether the manuscript fits within the scope of the journal, whether it meets
the specific requirements of the journal, and whether it is of sufficient scien-
tific merit for consideration. Not all manuscripts are transmitted to reviewers.
In some cases, the editor decides to reject a manuscript without review, or rarely,
to accept it for publication. Manuscripts are rejected without review for various
reasons, including the following: the topic is inappropriate for the journal; the
concept or the data are not novel; the format is incorrect; the writing is so poor
that the manuscript is unreadable; or the authors have previously published
overly similar papers. If a manuscript is rejected without review, the editor will
usually briefly inform the author of the reason. Some editors offer suggestions to
help authors with future submissions.
If the editor decides to send the manuscript for peer review, customarily
two to four individuals with appropriate expertise—training or research experi-
ence—are asked to review the manuscript. The editor may identify reviewers in
a number of ways. Many editors ask authors to recommend reviewers; some do
not. Author-recommended reviewers may or may not be used. Most editors will
not send manuscripts to specific reviewers if an author so requests. Other poten-
tial reviewers may be authors cited in the manuscript, acknowledged experts
in the field, or other active researchers in the field. Editors often use scientific
search services (such as SciFinder or SciFinder Scholar for chemists) to identify
qualified potential reviewers. Reviewers may or may not be known personally to
the editor. Most journals maintain records on thousands of reviewers, including
their expertise, manuscripts they have reviewed, performance, and so on.

Chapter 6: Peer Review ➤ 73
Usually, reviewers are asked whether they are willing to review a manuscript.
If they agree, the manuscript is provided in either hard copy or electronically,
often with an accompanying review form. Editors generally ask reviewers to sub-
mit their reviews in two or three weeks. When a review is overdue, the editor
usually sends a reminder to the reviewer.
Once reviews are returned, the editor reads the reviews in conjunction with the
relevant manuscript, evaluating both the manuscript and the reviews, and then
makes a decision whether to accept the manuscript, request revisions, reject the
manuscript, or send it for additional review. In some cases of conflicting advice or
opinions of reviewers, editors may seek advice from others. Editors are not obli-
gated to follow the recommendations of reviewers. Reviewer ratings are not aver-
aged; often, a single cogent negative review leads to rejection of a manuscript.
Responsibilities of Reviewers
Peer review is a critical component of formal scientific communication, and
every scientist has an obligation to do a fair share of reviewing.
✐Reminder: Manuscripts should be reviewed in a timely and balanced
manner and should be kept confidential until publication.
When a manuscript is under review, it is a confidential document and it
should not be discussed or shown to others. After reading a manuscript, a
reviewer may conclude that a better review could be accomplished with assis-
tance from a colleague. In this circumstance, the reviewer should inform the edi-
tor before engaging the colleague. Reviewers are expected to provide reviews that
are thorough and unbiased. A reviewer in direct competition with the authors of
a manuscript should inform the editor that there is a potential conflict of interest
and discuss the issue with the editor. In addition, if reviewers are asked to review
the work of someone at the same institution or the work of a previous student or
co-worker, the reviewer should inform the editor. In some cases, the editor will
excuse the reviewer from doing the review; in other cases, the editor will con-
sider the relationship when evaluating the review.
Reviewers are expected to submit their reviews on time. Most reviewers are
also authors and expect reviews of their manuscripts to be handled expedi-
tiously. If circumstances arise that prevent or delay a review, the editor should be
informed as soon as possible.
The entire manuscript should be read carefully and critically. Most reviewers
read a manuscript more than once. Manuscripts should be rated on technical
quality, significance of the work, importance to the research field, and adequacy
of expression. Often a standard form is provided for this portion of the review.

74 ➤ The ACS Style Guide
Reviewers should feel free to comment on the suitability of the manuscript for
the particular publication. Sometimes first-rate manuscripts are submitted to an
inappropriate publication. In addition to the actual review, some editors allow
reviewers to submit confidential comments about the manuscript. These are not
forwarded to the author. If suspicions of abuse, plagiarism, or fraud arise, the
editor should be informed immediately.
Many reviewers divide their reviews into general comments and specific,
detailed comments. In the general section, reviewers should draw attention to
both the strong and the weak points of the manuscript, the concepts, the objec-
tives, and the methods. Like an author writing a manuscript, reviewers should
write reviews in a comprehensive but concise manner, addressing the questions
presented in Box 6-1.
Reviews should be written in a helpful, tactful manner. Editors generally edit
or do not pass discourteous comments to authors. Abusive reviews lack credibil-
ity and also reflect poorly on the reviewer.
Box 6-1. Suggested Topics for a Peer Review
Are the methods (experimental section) adequately described and ref-
erenced?
Are there any unsupported conclusions?
Is there anything that is confusing or ambiguous?
Do figures and tables appropriately illustrate the data?
Is the introduction clear and informative?
Is either the introduction or discussion longer than necessary, and do
they make sense in relation to the subject and the data?
Although the discussion is the appropriate place for speculation, is it
excessive?
Are the appropriate references cited? Are the references accurate?
Is English usage and grammar adequate? Some reviewers may be inclined
to edit or annotate the manuscript. However, reviews are more valu-
able to editors if reviewers mention that there are problems with the
English and concentrate on evaluating the data and its interpretation.
Is the length of the manuscript unwarranted? Suggestions on how a
manuscript can be shortened are appreciated by editors.
Is the use of color warranted? Printing color is a significant expense for
the publisher.
➤ ➤ ➤ ➤ ➤

Chapter 6: Peer Review ➤ 75
Responsibilities of Authors
Authors should read the current instructions to authors for the publication to
which they intend to submit a manuscript. For journals, these instructions are
typically published in the first issue of the year and can often be found on the
Web. Author guidelines for books are available from the publisher, and some-
times they are posted on the publisher’s Web site. Many submissions are rejected
without review because the manuscript does not conform to the publication
guidelines.
✐Reminder: Authors should read the current instructions to authors for
any publication to which they intend to submit a manuscript.
If English is not the author’s native language, it is a good idea to ask a native
English speaker to edit the manuscript before submission. Alternatively, authors
can employ a technical editing service. Such editing services can be found
through recommendations from colleagues, by searching the Web, or advertised
in scientific publications. However, it is the author’s responsibility to ensure that
the writing is accurate.
In suggesting potential reviewers, authors should not recommend members
of their own institution or current or recent collaborators (including students or
postdoctoral associates). Many authors suggest the names of eminent scientists
in their fields. These individuals are frequently asked to review a large number
of manuscripts and, constrained by time, may not be available to review addi-
tional manuscripts. Therefore, it is more helpful to suggest the names of highly
qualified, less well known researchers. Many book and journal editors also allow
authors to request that certain individuals, such as those in direct competition,
not be selected to review. Editors usually comply with these requests.
Authors should read reviews carefully and dispassionately, with the expecta-
tion that reviewers’ comments will improve their manuscripts. When an edi-
tor requests a revision, authors should respond to all comments and answer all
questions. Editors generally reject perfunctory revisions. When it is apparent
that a reviewer has misread or misinterpreted the text, authors should provide
a direct but tactful reply. Revisions should be submitted in a timely manner.
Generally, editors expect revisions within a few weeks, not months. If there is
long delay in submitting a revision, some editors will consider the revision to be
a new manuscript. Sometimes an editor or reviewer will ask authors to perform
additional experiments or to reanalyze data, necessitating a delay in submitting
a revision. Authors should ask the editor for an extension, if necessary, provid-
ing an explanation.
When a manuscript is rejected, authors should read the editor’s letter and
reviews carefully to determine the reasons for the rejection. Rejection usually

76 ➤ The ACS Style Guide
means that another publication should be considered for submission of the
manuscript. Some manuscripts should be abandoned. Although some editors
will consider an appeal of a rejection, successful appeals are rare. Only if authors
are confident that with the help of the reviews, the research and its presenta-
tion can be improved, should they resubmit the manuscript to the same pub-
lication. A cover letter accompanying the revision of the rejected manuscript
should describe all changes to the manuscript, reply to all reviewer comments,
and respond to all questions. Ultimately, whether the manuscript is submitted to
a different publication, revised and resubmitted to the same publication, or fur-
ther experiments are performed to broaden the scope of the investigation, criti-
cal assessment from reviewers can aid both research and its communication.
Encouragement to New Investigators
The peer-review process may seem daunting and at times unfair to new investi-
gators. On occasion, reviewers will be unduly critical and unhelpful—and infre-
quently, outright nasty and rude. Sometimes reviewers can be exasperatingly
slow. Good manuscripts are rejected, in particular by prestigious journals, which
receive many more high-quality manuscripts than they can publish. However,
many reviewers and editors will be solicitous and encouraging to investigators in
the early stages of their careers. Despite its shortcomings, peer review is regarded
by the scientific community as an essential component to high-quality, effective
communication that further advances science.
✐Reminder: Frequently consult the “Ethical Guidelines to Publication of
Chemical Research” (Appendix 1-1 in this book, or on the Web at https://
paragon.acs.org). The guidelines are reviewed regularly to ensure their
clarity.

77
➤ ➤ ➤ ➤ ➤ CHAPTER 7
Copyright Basics
Karen S. Buehler, C. Arleen Courtney,
and Eric S. Slater
Copyright law is a cornerstone of intellectual and scientific
exchange. This chapter is intended to introduce a complex
and constantly changing legal area; we do not intend to provide legal advice. The
first section is an overview of U.S. copyright law: what materials are subject to
copyright, who owns copyright, and when copyrighted materials may be used by
others. The second part of the chapter presents the methods of obtaining per-
mission to use someone else’s copyrighted work. The third part briefly explains
how copyright is transferred from the author to the publisher.
U.S. Copyright Law
What Can Be Copyrighted
Copyright is a doctrine of federal law (Title 17, U.S. Code) and is defined as a
form of intellectual property law that protects original works of authorship fixed
in a tangible medium of expression for a specified period of time. A tangible
medium of expression is something that can be seen, touched, or heard (see the
examples below). Copyright applies to all media of expression, including print,
the Internet, CD-ROM, and videotape. Copyright allows authors and creators
certain rights to protect their original works. Copyright law protects both pub-
lished and unpublished works.
The authors wish to thank Barbara F. Polansky and William J. Cook for their time in reviewing
and commenting on this chapter.
Copyright 2006 American Chemical Society
78 ➤ The ACS Style Guide
Copyright applies to a variety of works including the following:
• literary works (including scientific works and computer programs);
• musical works (including lyrics);
• dramatic works (including accompanying music);
• pantomimes and choreographic works;
• pictorial, graphic, and sculptural works;
• motion pictures and other audiovisual works;
• sound recordings;
• architectural works; and
• compilations and databases to the extent that they reflect originality in the
selection and arrangement of elements.
What Cannot Be Copyrighted
Copyright does not protect the following:
• Works not fixed in a tangible form of expression. Copyright does not pro-
tect ideas, only the fixed expression of ideas. Thus a thought, not written
down in any way, is not protected.
• Titles, names, short phrases, slogans, familiar symbols, or designs. (These
items may be protected under trademark or service mark laws.)
• Lists of ingredients, contents, or facts.
• Ideas, procedures, methods, systems, processes, concepts, principles, dis-
coveries, and devices. (These items may be protected under patent law.)
• Standard calendars, rulers, lists, or tables taken from the public domain
and other works containing no original authorship.
Who Owns the Copyright
The copyright owner is the author or creator of the original work. An original
work can be in the form of an article, photograph, illustration, figure, table, etc.
Copyright does not protect ideas, only the actual expression of the ideas. There-
fore, the copyright owner is the person who drew a figure or table on a computer
and saved it, or took a photograph (the subject of the picture is not the copyright
owner), or wrote a narrative on a piece of paper.
There are two exceptions when the author or creator of the original work is
not the copyright owner: (1) when the copyright was transferred in writing to
another person or entity (usually via a copyright status form) and (2) when the
work was created as a work-made-for-hire. In a work-made-for-hire situation,
employees create the work within the scope of their employment; therefore, the
copyright owner is the employer. The employer may be an individual, corpo-
ration, or university. The above two examples represent typical scenarios when
publishing scientific or scholarly works. Work-made-for-hire can also cover,
among other specified categories, independent contractor situations under cer-
tain conditions and if the parties have agreed in writing.

Chapter 7: Copyright Basics ➤ 79
Rights of the Copyright Owner
The copyright owner can be described as controlling a “bundle” of stated rights.
The copyright owner (whether an individual, a corporation, or a publisher)
retains certain exclusive rights in the work. The bundle of rights includes
• reproducing copies of the work;
• distributing copies of the work to the public;
• creating derivative works based on the work;
• performing the work publicly (for certain types of works) and, in the case
of sound recordings, by digital audio transmission; and
• displaying the work publicly (for certain types of works).
Most scientific and scholarly publishers require authors (or their employers)
to transfer copyright as part of their publication agreement. The ACS Publica-
tions Division requires authors (or their employers) to transfer copyright to the
American Chemical Society, although some rights are transferred back to the
authors (or their employers). For specific information regarding transferring
copyright to ACS and authors’ rights, see http://pubs.acs.org/copyright_info.
html or contact the ACS Copyright Office (see Box 7-1).
Copyright Notice
A work is protected by copyright even if it does not contain a formal copyright
notice (the word “copyright”, abbreviation “copr”, or symbol © with the year of
first publication and name of copyright owner). It is strongly recommended to
place a copyright notice in or on a work, thereby giving notice that someone con-
trols the rights to that work. Registration of a work with the U.S. Copyright Office
is not necessary to obtain copyright protection, although there are substantial
Box 7-1. Copyright at ACS
Each division of the ACS is responsible for copyright issues related
to material that appears in its publications. The ACS Copyright Of-
fice can assist ACS authors and editors with copyright issues per-
taining to materials published by the ACS Publications Division.
The e-mail address is copyright@acs.org. The mailing address is
American Chemical Society, Copyright Office, 1155 Sixteenth Street,
NW, Washington, DC 20036. The fax number is 202-776-8112, and
the telephone number is 202-872-4368 or 4367. Contact other ACS
divisions directly for permission to use their material. For specific
advice regarding copyright matters, it is best to seek legal counsel.
80 ➤ The ACS Style Guide
benefits to doing so. For more information about copyright registration, contact
the U.S. Copyright Office at 202-707-5959, or at http://www.copyright.gov/.
The U.S. copyright law also provides remedies for infringement. Authors
should contact the appropriate book or journal editor in cases where they believe
their work has been infringed upon. If a copyright owner (in the case of an ACS
publication, the ACS) believes that his or her work has been infringed upon, it is
the responsibility of the copyright owner to bring action.
Fair Use
The U.S. copyright law contains limitations on rights granted to the copyright
owner. Probably one of most misunderstood of these limitations is fair use.
Essentially, fair use permits certain actions that might otherwise infringe upon
an exclusive right of the copyright owner. It has been our experience that the
interpretation of what constitutes fair use can vary widely. For instance, a copy-
right owner’s interpretation may be very narrow; a party looking to take advan-
tage of fair use will interpret it broadly.
Using excerpts from works for the following purposes may qualify as fair use:
• criticism or comment;
• news reporting;
• teaching (but only in a spontaneous situation. If one knows in advance
that copyrighted material will be used in a class, permission must be
obtained.); and
• scholarship or research. (Private researchers and nonprofit employees may
make one photocopy of an article for their own research purposes only.)
These uses may not constitute copyright infringement. The determination of
whether a use is fair depends on the facts in each case. Even if the facts are virtu-
ally identical, one degree of minutiae can cut against fair use.
The U.S. copyright law provides four factors that must be considered in
determining whether any particular use is fair use. Courts weigh the four factors
against each other, and no one factor is determinative in every case. The four fac-
tors are the following:
1. the purpose and character of the use, including whether it is of a commercial
nature or for nonprofit educational purposes;
2. the nature of the work;
3. the amount and substantiality of the portion used in relation to the work as a
whole; and
4. the effect of the use in question on the potential market for or value of the
work.
There are no numerical guidelines or percentages that can be used for each
situation where fair use might be involved. Even one percent of a work used in a

Chapter 7: Copyright Basics ➤ 81
manuscript may be determined to be against fair use if the material is considered
the most important part or the heart of that work. Fair use is a defense, and a
strong argument must be made that the use is actually fair. Never assume that a
use is a fair use; it is always best to seek permission to avoid potential and some-
times costly problems.
✐Reminder: Never assume that a use is a fair use; it is always best to seek
permission to avoid potential problems.
Public Domain and U.S. Government Works
Works that are in the public domain are not protected by copyright. The follow-
ing are situations in which a work can have public domain status:
1. works published before 1923 whose copyright term has expired (see the sec-
tion called “Duration of Copyright”), or works for which copyright was not
renewed;
2. works published before 1978 without a proper copyright notice;
3. works dedicated to the public domain by their creator, e.g., so-called share-
ware; and
4. works authored by U.S. government employees within the scope of their
employment.
Generally, it is not necessary to seek anyone’s permission to use public domain
works. However, when using material that appeared in a U.S. government publi-
cation, check the caption or reference section to see if credit is given to a source
for which permission is required.
✐Reminder: Even though material in a U.S. government publication
generally does not require permission to reproduce it, sometimes gov-
ernment publications include material that is protected by copyright.
Always check the caption or reference section to determine whether
you need to obtain permission to reproduce it.
Common Misconceptions about Copyright
One misconception about copyright status involves material found on Internet
Web sites. Someone does own copyright to the material on a Web site, and it is
necessary to obtain permission to reuse it. The lack of a copyright notice does
not mean that the work is not protected, nor does it mean that a work is in the
public domain or that the author of the work has waived his or her rights.
Other misconceptions involve photographs. The individuals appearing in
photographs are not the copyright owners. The photographer, or employer if
82 ➤ The ACS Style Guide
the photo is a work-made-for-hire, is the copyright owner, and permission to
reuse a photograph must come from the copyright owner. (Permission of people
appearing in photographs may also be required by a publisher, but this is not a
copyright issue. It is a privacy/right of publicity concern.)
Another problem involves the use of photographs of old works of art. It may
be true that the art itself is in the public domain (i.e., a van Gogh or Michelan-
gelo painting). However, the photograph of the artwork is protected by copyright.
In these cases, the copyright owner may not be an individual photographer but a
museum or a historical society.
Duration of Copyright
Under current U.S. copyright law, length of copyright is life of the author plus 70
years. For works-made-for-hire, length of copyright is 95 years from the date of
first publication, or 120 years from the date of creation, whichever is shorter.
Copyright in the Electronic Age
Several recent court cases have involved copyright infringement in electronic
media. The legal disputes centered on which party controlled specific rights
under the contractual arrangements authors had with publishers. A common
thread to these cases is whether a publisher controls all rights to publish content
in any format.
Publishers and authors need to be aware of the rights they hold, either explic-
itly under the U.S. copyright law, or via a licensing arrangement. ACS requires
complete transfer of copyright in all formats, thus allowing ACS to publish mate-
rial in different formats.
In cases where authors do not transfer copyright, publishers generally require
a nonexclusive licensing arrangement. Here, the author retains copyright and
grants certain rights to the publisher. Placing material on the Internet without
authorization from the copyright owner is a copyright infringement, even if the
publisher had permission to use the material in print.
It is important to have a working knowledge of copyright law, and it is help-
ful to have some familiarity with contracts. Publishers and authors need to read
contracts and copyright transfer forms thoroughly before signing and be certain
that they understand what they are agreeing to.
There are numerous Web sites containing copyright information. We recom-
mend the following Web sites for accurate and up-to-date information, although
this is by no means a complete list:
• U.S. Copyright Office, http://www.copyright.gov/
• Association of American Publishers, http://www.publishers.org/
• American Chemical Society Copyright Learning Module, http://pubs.acs.
org/copyright/learning_module/module.html

Chapter 7: Copyright Basics ➤ 83
Obtaining Permission To Reproduce Material
Whenever an author wishes to include a figure, a table, or a substantial portion
of text that has already been published elsewhere (in print or on the Internet),
the author must obtain permission from the copyright holder to reproduce the
material. It is the author’s responsibility to
1. identify material in a paper or manuscript that has been previously published
in print or on the Internet;
2. determine whether that material is subject to copyright protection;
3. if so, identify the copyright owner and request permission in writing; and
4. ensure that permission is granted and forwarded to the author’s publisher.
The first two points are discussed at the beginning of this chapter. The second
two points will be considered next in a general way applicable to requesting per-
mission from any scientific or scholarly publisher. For information on how to
reproduce materials from books, journals, or magazines published by the ACS
Publications Division, see Box 7-2.
✐Reminder: Material published on an Internet site is not necessarily in
the public domain, even if the site owner does not have a copyright
notice on a Web page. If you download a photograph, illustration, chart,
table, or text from a Web site, you must obtain permission from the
copyright owner to use it in another publication. Bear in mind that the
Web page publisher may not be the copyright owner.
Writing To Request Permission
Once an author has identified the tables, figures, and text that require permis-
sion, the next step is for the author to write to the copyright owner to request
permission. This is not a difficult task, but it requires some organization and
attention to detail, and it may take several months for permission to be granted.
Fortunately, the Internet has made the task less burdensome than it used to be
because most publishers post their permission policy, forms, and contact infor-
mation on the Web. Some publishers now have Web-based permission systems.
✐Reminder: Bear in mind that a copyright owner is not necessarily a pub-
lisher. For example, images such as a company logo or a still shot from a
movie or television commercial require permission from the corporate
office or the motion picture studio or the advertising agency.
➤ Begin by organizing your materials requiring permission according to pub-
lisher and source. This way, you can list all materials from a single publisher and
source on one form, reducing paperwork for yourself and the publisher.
84 ➤ The ACS Style Guide
➤ Determine whether the publisher has permission-request information on the
Web and, if so, download or print out the appropriate request forms. (Even if the
publisher has a Web-based permission system, you might want to print out the
blank form, so you can be sure that you have collected the necessary information
when you fill it out online.)
➤ If the publisher does not have forms on the Web, then draft a letter request-
ing permission. Be sure to include the elements listed in Box 7-3.
➤ List all the material for which you need permission from a given publisher on
one form or on the form with an attachment. Unless the publisher specifically
directs differently, avoid submitting one form for each item requiring permission.
➤ Include your specific deadline date by which you wish to have the permission
granted.
Box 7-2. Permissions at ACS
Permission is needed to reproduce tables, figures, or text from
books, journals, or magazines published by the ACS Publications
Division. Permission is also needed if adapting or using part of a
figure or table. Permission is not needed if using data from the text
to create an original figure or table. When using a figure that ap-
peared in an ACS journal and ACS owns copyright to that figure,
permission is not needed to use it in a paper that will appear in the
same or another ACS journal, although a credit line is required.
The ACS permission policy and forms for ACS journals and mag-
azines are available at http://pubs.acs.org/copyright_info.html and
for ACS books, at http://pubs.acs.org/books/forms.shtml. An inter-
active form can be found at http://pubs.acs.org/cgi-bin/display-
copyright?bichaw.
Permission requests to reproduce materials from the books,
journals, and magazines of the ACS Publications Division should
be directed to the ACS Copyright Office. All permission requests
must be in writing. The ACS Copyright Office accepts permission
requests via e-mail, mail, and fax. Permission is granted only by fax
or by mail, so it is important to include a fax number.
The Copyright Office e-mail address is copyright@acs.org. The
mailing address is American Chemical Society, Copyright Office,
1155 Sixteenth Street, NW, Washington DC 20036. The fax number is
202-776-8112, and the telephone number is 202-872-4368 or 4367.

Chapter 7: Copyright Basics ➤ 85
➤ Include complete contact information for yourself, including a mailing
address, e-mail address, and telephone and fax numbers.
➤ If you do not have a response from the copyright owner in a reasonable
amount of time, contact the publisher again and try to determine the reasons for
the delay; perhaps your request was not received or was missing information. Do
Box 7-3. Components of a Permission Request
Your request for permission to reproduce material will be processed
more quickly by a publisher if you provide all the necessary informa-
tion on the publisher’s form or in a request letter. A permission request
should provide the following information:
1. A list of the original figure or table numbers for each figure or table
that you wish to reproduce from a given source, along with a com-
plete reference citation for the source.
• The reference citation for a journal article should include the
name(s) of the author(s); the name of the journal; the month, day,
and year of publication; volume and issue numbers; and inclusive
page numbers for the article.
• The reference citation for a book should include the title of the
book (and series name and number if applicable); the name(s) of
the author(s) or editor(s); the year of publication; and the page
numbers on which the original figures or tables appear. If appro-
priate, also list the title of the chapter, the name(s) of the chapter
author(s), and the inclusive page numbers for that chapter.
2. A description of where the material will be published. Include the title
of the forthcoming publication, the type of publication (e.g., journal,
book, magazine, or Web journal), and the name of your publisher.
3. A list of the formats in which the requested material will appear,
such as print, online, CD-ROM, or proceedings. Unless all rights are
requested, only print rights will be granted.
4. Your complete mailing address, e-mail address, and telephone and
fax numbers.
5. A specific deadline (calendar date).
Many permission request letters also include a space for the original
publisher to specify the publisher’s preferred credit line.
➤ ➤ ➤ ➤ ➤
86 ➤ The ACS Style Guide
not assume that you have permission unless you have heard explicitly from the
publisher, even if you set a deadline.
What Sort of Credit Line Is Appropriate?
➤ Once the publisher has granted permission to reproduce materials, incor-
porate the required copyright credit line into your manuscript. This credit line
notifies readers that the publisher owns copyright to the material. Each publisher
has its own style for credit lines. For example, the credit line required by the ACS
is shown below:
Reprinted with permission from REFERENCE CITATION. Copyright YEAR
American Chemical Society. (Insert the appropriate information in place of the
capitalized words.)
➤ Include the credit line on the first page where copyrighted text appears and
under each copyrighted table or figure.
➤ If you are adapting or modifying copyrighted material or if parts of copy-
righted material are being used, the words “Adapted” or “Reprinted in part”
should replace “Reprinted” in the credit line.
Transferring Copyright
Most scientific and scholarly publishers require the transfer of copyright owner-
ship as part of the publication agreement between the publisher and authors.
When the publisher owns copyright, it removes the burden from authors to grant
permission and it assures them that only legitimate requests for their material are
approved. Similarly, if the publisher owns copyright, it is easier for authors who
are seeking permission to come to one source that handles requests from mul-
tiple references and processes them quickly. If individual authors owned copy-
right, it might be difficult and cumbersome to contact authors for permission to
use their works or portions of their works. If you are in doubt about a publisher’s
requirements, contact the publisher or seek legal advice.
Each publisher has forms for transferring copyright to the publisher, and
the submission of a completed copyright transfer form is nearly always neces-
sary before a publisher will schedule publication of or begin production work
on a manuscript. When authors publish in an ACS publication, they assign
copyright interest via the ACS Copyright Status Form. This form is available on
the ACS Publications Division Web site at http://pubs.acs.org/cgi-bin/display-
copyright?bichaw and in the January issues of ACS journals. Authors publishing
books with ACS will find a form at http://pubs.acs.org/books/forms.shtml. Com-
pleted Copyright Status Forms should be submitted to the appropriate book or
journal editor, not the ACS Copyright Office.
87
➤ ➤ ➤ ➤ ➤ CHAPTER 8
Markup Languages
and the Datument
Peter Murray-Rust and Henry S. Rzepa
A
style guide is presumed in its basic objective as guiding
authors in the task of producing a document describing
their work. In a conventional sense, style is traditionally presentational in objec-
tive and is aimed at producing a (visually) homogeneous form suitable for aggre-
gation into a journal issue or a book. At the outset of this chapter, we should pin
our flag firmly to a rather different mast. This chapter is less about the applica-
tion of style to the presentation of a document and more about the general prin-
ciples involved in its application to data to achieve a homogeneous form capable
of being reused. It is not so much about how to create a document, but rather
about how to create a more data-focused entity we call a datument (1), that is, a
container for data and its associated descriptions. The datument can have con-
ventional attributes of authorship, affiliation, and other familiar structural com-
ponents, such as sections, tables, figures, and bibliography, but it extends this by
also carrying data. This differs from, for instance, tabulated or quoted data typi-
cal of that found in most chemical documents. To illustrate this most important
difference, consider the following assertion:
The melting point of aspirin is 135°, and its molecular ion has the formula
C9H8O4+·.
As a human trained in chemistry, you probably understand much of the seman-
tics, but consider how much potential ambiguity and implied meaning is con-
tained in this admittedly concise statement.
• You understand what is meant by the term melting point, but you might
have to seek a librarian’s help to locate a relevant dictionary of chemical
terms where you can check a more precise definition should the need arise.
Copyright 2006 American Chemical Society
88 ➤ The ACS Style Guide
• After a little thinking, you conclude that the glyph ° indicates that the
number preceding it is a temperature expressed in Celsius units. You prob-
ably also recognize that this number is probably only accurate to ± 1 °C,
only because you have made such measurements yourself. At the back of
your mind is probably the knowledge that a value of 135° is reasonable
for an organic compound (and that 1135° would not be), that this implies
that this substance is a solid at room temperature and that this value may
be used as an approximate indicator of chemical purity.
• You certainly recognize the term aspirin as a trivial, unsystematic but
commonly used description of a nevertheless well-defined molecule (the
structure, or more accurately the connection table, of which you may need
to ascertain).
• The term molecular ion is a term that tends to be used in a particular
branch of spectroscopy known as mass spectrometry.
• You have little difficulty in recognizing the molecular formula as by con-
vention listing the number of carbon atoms as the subscript to the initial
C, then followed by the number of hydrogen and then other atoms in
alphabetical order. The final suffix indicates the charge on the system and
reconciles with the use of the term ion, and you will notice that the suffix
reveals the presence of an unpaired electron.
• You probably are aware of rules that would allow you to check the validity
of this formula (i.e., are the + and the • consistent with each other; is the
formula physically possible within the constraints of valence theory, etc.).
• You might want to derive a molecular mass from the formula, in which
case you need to know about atomic weights, isotopes, and other concepts.
• You might want to relate the formula to a two-dimensional structural rep-
resentation, indicating perhaps where the charge might reside, or how the
ion might fragment, or a three-dimensional representation for molecular
modeling.
• As a human, you will infer properties such as aromaticity or the probable
presence of a hydrogen bond.
A knowledgeable chemist could probably make quite a few more inferences
from the above, but the purpose here is to illustrate how much implicit (i.e., unde-
clared) information there is in such a brief statement (all well-defined chemical
terms are shown above in italics to emphasize this). The purpose in writing it all
down is to reinforce the idea that although such a statement (as might be found
in a document) contains much data, only a human can really make significant
use of it. Well, that would be true only if the human were to give undivided atten-
tion to the contents of such a document; the human certainly could not cope well
with more than a few such documents, and not at all with, say, millions of docu-
ments. A normal response to such issues of scale would be to say that the essential
data and semantics of the above phrase need to be abstracted (with inevitable loss
Chapter 8: Markup Languages and the Datument ➤ 89
of some data and information) into a database representation and then suitably
queried if needed; after all, agencies such as Chemical Abstracts Service exist to
provide such a service. However, consider that the process of converting even a
brief statement such as the above into a true chemical abstract requires an expert
(and it must be said, error-prone) human. Even then, the knowledge required to
construct the list above would have to be acquired from other sources.
Could instead much, perhaps all, of this work be handled by a computer? The
answer is a clear “yes”, but only if the ground is well prepared for such a task.
The purpose of this chapter is to outline some of the basic principles (but not
the technical details) of how this could be done and to set out the grander vision
that creating an infrastructure that adopts such principles would be the first step
toward what has been described as a semantic web of information and knowledge.
Not only humans but machines could roam, on vast scales if need be, on such a
web, and by doing so discover connections between data and concepts which in
days past might have been described as the art of scientific serendipity (2).
Markup Languages and the World Wide Web
The World Wide Web arose from the need for high-energy physicists at Conseil
Européen pour la Recherche Nucléaire (CERN) to communicate and exchange
data and information within a large dispersed community. The basic design,
which has been well documented, involved the creation of structured documents
containing identifiable information components linked by uniform resource
identifiers using markup tags that could be recognized by machines and used as
formatting or styling instructions rather than being part of the actual content.
Using the HTML version of the previous example:
<p>The melting point of aspirin is 135°, and its
molecular ion has the formula C<sub>9</sub>H<sub>8
</sub>O<sub>4</sub><sup>+.</sup>.</p>
The “tags” in the angle brackets are recognized by the processor as markup
and are used as instructions rather than content to produce the previously ren-
dered sentence. Although such examples will be familiar to many readers, we
emphasize it here because it illustrates the critical importance of separating con-
tent from style (or form). The <p> tags precisely define a paragraph, a unit for
structuring the document. A machine could now easily count the paragraphs in
a document and the number of characters (but not individual words, which are
separated not by tags, but by spaces) in each. HTML provides a flexible (perhaps
rather too flexible) document structure for text (paragraphs, headers, tables, lists),
embedded images (and other multimedia objects), human interactivity (through
forms), programs (through scripts, applets, and plug-ins), styling (some degree
of formatting and screen layout), and metadata (essentially descriptions of data).
90 ➤ The ACS Style Guide
Whereas this is a substantial list, its success has generated many problems, which
HTML in its original form cannot solve:
• HTML can only support a fixed tagset (for example 59 in the latest speci-
fication for XHTML 2.0), and even this number is regarded as close to
unmanageable (no software yet implements this full set consistently, accu-
rately, and completely). Any other tags that might be present (e.g., <mol-
ecule>) are simply ignored (strictly speaking, they should be marked as
invalid HTML, although they may be valid for other languages).
• Much of the behavior (semantics) is undefined. This lack has led to spe-
cific disciplines creating their proprietary methods of supporting func-
tionality (e.g., through scripting languages, plug-ins, applets, and other
software).
• HTML was designed to be error-tolerant, in recognition that it would be
authored (and viewed) mostly by humans. Browsers may try to recover
from nonconforming documents and may do so in different ways.
Humans are good at recognizing and often correcting errors in HTML
(missing links, broken formatting, incomplete text). Machines cannot
normally manage broken HTML other than in a “fuzzy” manner.
• Author-provided metadata is often entirely absent. If present, it will likely
adhere to a general form (the so-called Dublin Core schema, http://dublincore.
org/), of limited utility in scientific, technical, and medical (STM) areas.
• The emphasis on presentation in many of the original tags (such as fonts,
colors, and layout) muddled the separation of content from style. The
World Wide Web Consortium soon developed technologies (CSS, or
cascading style sheets, and XSL, or extensible stylesheet language; more
information on this and other XML issues is available at http://www.w3c.
org/) to help overcome this problem, but as with HTML, CSS is variably
implemented in most browsers. Most commercial tools for authoring
HTML emphasize presentation or interactivity (to capture the reader’s
attention), and in such HTML, the content is subservient to the style.
Examples of XML and of Chemical Markup Language
These conventional markup approaches (HTML, CSS, and XSL) are inadequate
for datuments because there is usually no domain-specific support. XML, or
extensible markup language, was introduced as a solution to this problem. XML
was designed to be simple, easy to use, and small; it is a fully conforming subset
of the older SGML (essentially “SGML lite”). It allows new markup languages to
be defined through an XML schema formalism (see http://www.w3c.org/XML/
Schema). A schema specifies a set of rules (syntax, structure, and vocabulary) to
which a document must conform; those that do are said to be “valid”. Schemas
allow more precise constraints, allow the definition of datatypes, and enhance
the potential for machine processing.
Chapter 8: Markup Languages and the Datument ➤ 91
We start by explaining the terms used in the XML language, illustrated with
a small and simple example (kept brief for simplicity and hence not relating to a
real molecule) (Scheme 8-1).
<molecule>
<identifier convention=”CAS-RN” >150-78-2 </identifier>
<identifier version=”1.0” convention=”InChI” >1.0/C9H8O4/c1-6(10)
13-8-5-3-2-4-7(8)9(11)12/...</identifier>
<atomArray>
<atom id=”a1” elementType=”C” x2=”-5.4753” y2=”5.0867”/>
<atom id=”a2” elementType=”C” x2=”-5.4753” y2=”3.5466”/>
</atomArray>
<bondArray>
<bond atomRefs2=”a1 a2” order=”2”/>
</bondArray>
</molecule>
Scheme 8-1. The basic features of an XML document.
The core of the language consists of a set of data containers, or more formally
elements (not to be confused with the chemical elements), the enumeration of
which is ideally defined by a schema. In this example, the elements are <mol-
ecule>, <identifier>, <atomArray>, <atom>, <bondArray>, and <bond>.
These have a clearly defined relationship to one another (illustrated above by
indentation of the text). Thus the element <atomArray> is said to be the parent
of a child element termed <atom>, and both are children of the top-level element
<molecule>, which can also be called the document root element. This hierar-
chy among elements is precisely defined and must carry no ambiguity.
Elements can specify data or information in two ways. First, data can be con-
tained between the start and end of any particular element, such as <identifier>
and </identifier> in the example. Such content can of course be other (child)
elements, but it can also be character or numeric data, as in the example above,
which uses both the CAS Registry Number, a unique identifier assigned to chemi-
cal structures by CAS (see Appendix 12-3), and a unique canonical molecule iden-
tifier known as InChI (International Chemical Identifier) (for more on InChI, see
Appendix 8-1).
Second, data can also occur as the value of an attribute to the element. In the
example above, the <identifier> element has two attributes, version=“1.0”
and convention=“InChI”. Both the name of the attribute and its value can be
enumerated if needed by the schema; if the attribute is unknown, or its value is
outside defined limits, the entire document or datument can be flagged as invalid
by suitable software. Thus <atom> has attributes elementType=“C” and x2=
“-5.4753”. For the former, a value of “C” is allowed (because it is recognized as
the standard symbol for the chemical element carbon), but a value of say “CX”
would not be allowed. The second attribute is defined (in the schema) as the x
coordinate of a set of two-dimensional molecular coordinates. As such, its pres-
ence implies that it should be paired with a y2 coordinate. One can specify in the
92 ➤ The ACS Style Guide
schema what kind of behavior to impose if, say, y2 were to be missing. One might
decide that its presence would be inferred and that its value should be y2=“0.0”,
although in practice that would be a dangerous assumption, and it would be
better to flag its absence as an error. Decisions also have to be made regarding
the value of this attribute. With two-dimensional coordinates, no assumptions
can really be made about the units in which the coordinates are specified, and it
would be up to any software to process the values in a sensible manner. Whereas
a human might think that, e.g., x2=“-54753.0” looks unreasonable, it may still
be internally consistent with the other coordinates. Such software would prob-
ably also be expected to trap conditions such as two atoms with identical coor-
dinates, or truly unreasonable values. However, one can be a little more specific
about, e.g., x3=“-5.4753”. This would be interpreted as the x coordinate of a
three-dimensional set, and as such a reasonable implicit behavior would be to
treat this value as corresponding to Angstrom units unless otherwise specified.
It is also worth noting that elements which specify data in the form of attributes
need not enclose any further data; thus <atom /> in this case represents both the
start and the end of the element (in other words it is an empty container).
The preceding discussion has been fairly precise and meticulous, if only to
illustrate how XML can be used to impose well-defined structures and relation-
ships on data. We emphasize, however, that it would not normally be a human
who has to cope with such levels of detail and precision; the design is such that
in fact software will carry almost all of the burden of producing the XML in the
first place and then validating and using it subsequently. The preceding argu-
ment served only to illustrate how such software can be made to safely operate
without the need for human intervention in the process.
The second example (Scheme 8-2) is an elaboration of the first fragment,
but formalized below as CML (chemical markup language) (3). We empha-
size that this chapter is not meant to be an instructional manual for any given
markup language, with CML here serving only to illustrate the general principles
involved. Many other scientific applications of XML have been developed (3, 4),
and syntactically, either of these examples could be replaced by other such mod-
ularized markup languages.
This more extensive example illustrates how a wider range of properties
can be defined and also contains a new feature called a namespace. The pur-
pose of this namespace is to enable this entire XML fragment to be combined
or aggregated with other XML languages so that no conflict between the names
used for the elements can arise. This aggregation is achieved by prefacing each
element with a unique (to the document) short string: <cml:molecule>. An
attribute xmlns:cml is now used to define what is called a URI (uniform
resource identifier), which stamps a globally unique identifier on the meaning
of the cml: prefix. This uniqueness will allow this datument to coexist with
other XML languages without conflict (an example of which is described later
in this chapter).
Chapter 8: Markup Languages and the Datument ➤ 93
<cml:molecule id=”m01” title=”aspirin” xmlns:cml=”http://www.
xml-cml.org/schema/CML2/Core”>
<cml:metadata name=”dc:identifier” content=”InChI”/>
<cml:identifier version=”1.0” convention=”InChI”>
1.0/C9H8O4/c1-6(10)13-8-5-3-2-4-7(8)9(11)12/h1H3,2-
5H,(H,11,12)
</cml:identifier>
<cml:metadata name=”dc:identifier” content=”CAS-RN”/>
<cml:identifier convention=”CAS-RN”>150-78-2</cml:identifier>
<cml:atomArray atomID=”a1 a2 a3 a4 a5 a6 a7 a8 a9 a10
a11 a12 a13” elementType=”C C C C C C C C C O O O O”
formalCharge=”0 0 0 0 0 0 0 0 0 0 0 0 0” hydrogenCount=”1 1 1
1 0 0 0 0 3 0 1 0 0”
x2=”-5.475336 -5.475336 -4.141667 -2.807998 -4.141667 -
4.141667 -2.807998 -1.075737 -1.075737 -5.629193 -2.807988 -
1.474318 0.464263”
y2=”5.086684 3.546650 2.776633 3.546650 5.856700 7.396700
5.086684 7.344209 8.884209 7.795282 8.166700 5.856684 7.344209”
/>
<cml:bondArray atomRef1=”a5 a1 a2 a3 a4 a5 a5 a6 a6 a7 a12
a8 a8” atomRef2=”a1 a2 a3 a4 a7 a7 a6 a11 a10 a12 a8 a13 a9”
order=”1 2 1 2 1 2 1 1 2 1 1 2 1” />
<cml:propertyList>
<cml:property dictRef=”chem:mpt” title=”melting point”
xmlns:chem=”http://www.xml-cml.org/dict/core” >
<cml:scalar dataType=”xsd:decimal” errorValue=”1.0”
dictRef=”chem:mpt” units=”unit:c”>136</cml:scalar>
</cml:property>
</cml:propertyList>
</cml:molecule>
Scheme 8-2. A CML datument describing a property of aspirin.
The molecule element in this example contains five child elements: cml:meta-
data, cml:identifier, cml:atomArray, cml:bondArray, and cml:prop-
ertyList. Of these, cml:metadata, cml:atomArray, and cml:bondArray
have no children and are empty containers, defining only attribute/value pairs,
whereas cml:propertyList has one child, cml:property. The latter itself has
a child: cml:scalar. As well as the namespace, the cml:molecule element itself
has two other attributes, id and title.
The </cml:property> and </cml:scalar> elements reference namespaces
other than CML. This is done to facilitate aggregation with other XML compo-
nents, such as dictionaries, and by this means to reduce what has been called
“tag soup”. For example, <cml:property dictRef=“chem:mpt”/> defines a
namespace for a dictionary reference called chem:mpt. Any processing software
that might need to process a melting point property would be directed to this
dictionary for further information on the semantics of this term. Similarly, the
94 ➤ The ACS Style Guide
attribute units=“unit:c” would handle the conversion of scientific units, and
dataType=“xsd:decimal” would handle the basic datatype (i.e., the defini-
tion of a decimal number) itself. This mechanism avoids overburdening CML
itself with the need to specify such semantics. No other elements or attributes
in this example have XML-defined semantics; all other semantics are imposed
by CML itself. Thus, the CML schema defines an enumeration (list) of allowed
elementTypes and defines their meaning, use, and boundaries. These aspects are
discussed in more detail below.
The Use of Identifiers
The examples of XML shown in Schemes 8-1 and 8-2 illustrate the use of two
types of identifiers. The identifier attributes seen in, e.g., <cml:atomArray
atomID=“a1 a2 a3 a4 a5 ...”> are used internally to enable specifica-
tion of, e.g., <cml:bondArray atomRef1=“a5 a1 a2 ...” atomRef2=“a1
a2 a3 ...”> and should be unique within the datument (but not necessar-
ily globally) to ensure that this XML document is well-formed and valid. The
second type is an element containing identifiers. Various identifiers could be
used, such as SMILES, CAS-RN, and the InChI canonical identifier (as shown
here), precisely derived from the molecule connection table and used to establish
molecular global uniqueness. Any two datuments that contain the same InChI
identifier (in this example, C9H8O4/c1-6(10)13-8-5-3-2-4-7(8)9(11)12/
h1H3,2-5H,(H,11,12)) should be presumed to refer to the same molecule (in
the sense of a connection table, but not necessarily other properties, such as 3-D
coordinates, for example). The whole aspect of identifiable data is pivotal to the
concepts used here. The CAS-RN is widely used and offers comprehensive cover-
age from simple molecules to polymers to Markush structures. The InChI can
be derived from the structure, but it is still a fairly new standard. It does not yet
have wide support and does not yet offer complete coverage of all materials.
Display of XML and Specific XML Languages
The default way of “displaying” or “browsing” any XML-compliant language,
such as CML, is as a so-called tree view, outlining the structure of the document
(Figure 8-1) but to which no style has been applied. Most modern Web browsers
will support this feature (we recommend Firefox).
Of more utility is to associate a specific style or transform with this datu-
ment. A technology known as XSLT is essentially a specification of how an XML-
based datument might be transformed into a different representation (or subset)
of the data. Four examples of how this might be used to transform this datument
are listed below:
• Extraction of atom two- or three-dimensional coordinates and rewrapping
with appropriate syntax for interactive display on screen using appropriate
Chapter 8: Markup Languages and the Datument ➤ 95
(A)
+<cml:molecule id=”m01” title=”aspirin”></cml:molecule>
(B)
-<cml:molecule id=”m01” title=”aspirin”>
<cml:metadata name=”dc:identifer” content=”InChI”/>
+ <cml:identifier version=”1.0” convention=”InChI”></cml:identifier>
<cml:metadata name=”dc:identifier” content=”CAS-RN”/>
<cml:identifier convention=”CAS-RN”>150-78-2</cml:identifier>
<cml:atomArray atomID=”a1 a2 a3 a4 a5 a6 a7 a8 a9 a10 a11 a12 a13”
elementType=”C C C C C C C C C O O O O” formalCharge=”0 0 0 0 0 0
0 0 0 0 0 0 0” hydrogenCount=”1 1 1 1 0 0 0 0 3 0 1 0 0”
x2=”-5.475336 -5.475336 -4.141667 -2.807998 -4.141667 -4.141667
-2.807988 -1.075737 -1.075737 -5.629193 -2.807988 -1.474318
0.464263” y2=”5.086684 3.546650 2.776633 3.546650 5.856700
7.396700 5.086684 7.344209 8.884209 7.795282 8.166700 5.856684
7.344209/>
<cml:bondArray atomRef1=”a5 a1 a2 a3 a4 a5 a5 a6 a6 a7 a12 a8 a8”
atomRef2=”a1 a2 a3 a4 a7 a7 a6 a11 a10 a12 a8 a13 a9”
order=”1 2 1 2 1 2 1 1 2 1 1 2 1”/>
+ <cml:propertyList></cml:propertyList>
</cml:molecule>
(C)
-<cml:molecule id=”m01” title=”aspirin”>
<cml:metadata name=”dc:identifer” content=”InChI”/>
+ <cml:identifier version=”1.0” convention=”InChI”>
1.0/c9h8O4/c1-6(10)13-8-5-3-2-4-7(8)9(11)12/h1H3, 2-5H,(H,11,12)
</cml:identifier>
<cml:metadata name=”dc:identifier” content=”CAS-RN”/>
<cml:identifier convention=”CAS-RN”>150-78-2</cml:identifier>
<cml:atomArray atomID=”a1 a2 a3 a4 a5 a6 a7 a8 a9 a10 a11 a12 a13”
elementType=”C C C C C C C C C O O O O” formalCharge=”0 0 0 0 0 0
0 0 0 0 0 0 0” hydrogenCount=”1 1 1 1 0 0 0 0 3 0 1 0 0”
x2=”-5.475336 -5.475336 -4.141667 -2.807998 -4.141667 -4.141667
-2.807988 -1.075737 -1.075737 -5.629193 -2.807988 -1.474318
0.464263” y2=”5.086684 3.546650 2.776633 3.546650 5.856700
7.396700 5.086684 7.344209 8.884209 7.795282 8.166700 5.856684
7.344209/>
<cml:bondArray atomRef1=”a5 a1 a2 a3 a4 a5 a5 a6 a6 a7 a12 a8 a8”
atomRef2=”a1 a2 a3 a4 a7 a7 a6 a11 a10 a12 a8 a13 a9”
order=”1 2 1 2 1 2 1 1 2 1 1 2 1”/>
- <cml:propertyList>
<cml:property dictRef=”chem:mpt” title=”melting point”
<cml:scalar dataType=”xsd:decimal” errorValue=”1.0”
dictRef=”chem:mpt” units=”unit:c”>136</cml:scalar>
</cml:property>
</cml:propertyList>
</cml:molecule>
Figure 8-1. Viewing the XML datument described in Scheme 8-2 in a Web
browser, shown as (A) a collapsed tree view, (B) partially expanded, and (C)
fully expanded.
96 ➤ The ACS Style Guide
software. This use was first demonstrated in an unusual series of articles
about themselves, in which the theme was to demonstrate how they could
be dynamically transformed into other representations of the data con-
tained within (5–7).
• Use of the atom array to calculate a molecular formula and weight.
• Calculation of molecular properties via invocation of molecular model-
ing algorithms using, e.g., Web services.
• Use as a database query (“Is aspirin in this database?”).
Only the first of these uses really corresponds to the conventional sense of
a document (whether it be an HTML page on the Web or a journal article in
PDF format). The last three uses would be true applications of the datument as
part of a semantic web. Another difference is that the semantics in such a datu-
ment cannot be unambiguously deduced from inspecting such examples but
must be formally defined (e.g., in an XML schema or similar tool). Thus, CML2
defines that, e.g., the atomRef1 and atomRef2 attributes (of the element bond-
Array) contains references to id attributes on atomArray elements and that the
order=“1” attribute relates to a single bond. How a single bond is handled must
in turn be specified by the software; a display program may render the bond in
a particular width, color, taper, etc., whereas a database query may handle this
information quite differently, or indeed not need it at all.
Datument Validation
Publishers often provide human-readable guidelines for authors (also known as
style guides) for document preparation, which sometimes can extend to entire
books. Humans are also quite prone to noncompliance or imperfect compliance
because they are often busy or perhaps simply readily bored. Guidelines for data
preparation, if they exist, are often to be found in optional categories, such as
supporting information. If an author deposits data in such a form, how does the
publisher know that it is correct? A key aspect of XML is that documents (and of
course datuments) can be validated. For publishing purposes, validation implies
a contract between the author and the publisher, which is machine-enforceable.
A schema formalizes the syntax, vocabulary, document structure, and some of
the semantics. It comprises a set of machine-based rules to which a datument
must conform. If it does not, it is the author’s responsibility to edit it until it
does. If it conforms, it is assumed that the author has complied with the publish-
er’s requirements.
Validation guarantees that the datument conforms to rules. The more power-
ful the rules, the more invalid data can be detected. Thus, schemas can allow the
detection of some disallowed data, particularly with a controlled vocabulary. An
atom in CML is not allowed an elementType of “CO” (arising perhaps as a mis-
Chapter 8: Markup Languages and the Datument ➤ 97
print for the element “Co”), or a hydrogenCount of –1. It is, however, allowed a
formalCharge of “+10”. This might be corrupted data or a legitimate description
of a highly ionized atom. Individual schema-based rules (e.g., for different jour-
nals) could allow discrimination between these possibilities.
Datument Vocabularies
The construction of a schema immediately emphasizes the need for a communal
vocabulary. An element such as <molecule> must be processed in the same con-
sistent manner regardless of the author, the reader, or the processing software.
We emphasize “processing”; the implementor must adhere to the same software
specifications, and the software must behave in a predictable manner. For many
scientists, this implementation will require a change in their thinking, and we
emphasize the consequences here.
In its strictest form, this attitude is a controlled vocabulary. Only certain terms
may be used, and their meaning is specified by a trusted authority. An example is
IUPAC “gold book” vocabulary of chemical terms and definitions (at http://www.
chemsoc.org/chembytes/goldbook/). Controlled vocabularies are widely used to
enforce the mapping of a discipline such as chemistry onto a generally agreed or
mandated vocabulary. They often require substantial formal guidelines or train-
ing sessions to ensure consistency of interpretation. Markup languages require
us to use absolute precision in syntax and structure. It is highly desirable to have
additional precision in semantics (the meaning and behavior of documents). The
attachment of semantics to documents is not generally appreciated but is a critical
process, and we must have a formal means of attaching semantics to every XML
element and attribute and their content. At present, this can be achieved in four
principal ways, ordered below in terms of machine-understandable rigor.
A Human-Readable Prose Description
This description can be as simple as a definition in a dictionary, which may or
may not give an indication as to how it might be used. An example from the CIF
(crystallographic) dictionary is _chemical_compound_source, which is defined
as “description of the source of the compound under study, or of the parent mol-
ecule if a simple derivative is studied. This includes the place of discovery for min-
erals or the actual source of a natural product.” This description formalizes the
concept but (deliberately) gives wide latitude in its implementation and content.
A Human-Readable Set of Instructions for Machine Implementation
Another CIF entry (abbreviated) for _atom_site_U_iso_or_equiv might
specify carefully how the concept must be implemented and indicate constraints,
such as datatype, enumeration range, and units. The constraints are all machine-
processable, and the definition includes an implementable algorithmic constraint.
98 ➤ The ACS Style Guide
Because CIF predates XML, this definition is not machine-processable (i.e., it acts
as a specification for a human programmer, but it cannot be used to generate
software automatically). XML schemas provide mechanisms to overcome this.
Definitions by Software
Many elements of a controlled vocabulary are effectively defined by software
implementation. Thus, the description of the HTML language requires certain
elements to have specified behavior. For example, <img> supports the display of
raster images, but the precise look may vary between implementations and file
types. Implementation through software is useful and powerful where authors,
publishers, readers, and processors all use the same system. Because STM pub-
lishing is increasingly multidisciplinary, this implementation becomes problem-
atic. Often a reader may have to download specialist software that is idiosyncratic
and that may not have enough functionality, especially the export of semantically
rich data. Moreover, the semantic rules are often buried deep in the software and
difficult to understand precisely.
Formal Semantics
We believe that the chemical community should move toward the adoption of
formal rules for expressing semantics and ontology (semantics is the branch of
semiotics, the philosophy or study of signs, that deals with meaning. Ontology
is defined as a description, such as a formal specification of a program, of the
concepts and relationships that can exist for an agent or a community of agents).
Our central message is that we need carefully constructed and curated machine-
processable ontologies. We believe that scientific and scholarly organizations have
a major role to play and that openness and free access to ontologies is critical.
Authoring and Editing Tools
At present, most chemical publications are created by authors in a publisher-spe-
cific manner. Each publisher requires a particular document structure, often a
particular technology (e.g., formats of text, images, references, and domain-spe-
cific data) and a particular (usually implicit) ontology. The author has to change
each of these according to the publisher’s requirements and independently of the
content. The publisher (or author) then has to make significant technical edits,
often as a result of author noncompliance. Original data are transformed into
text-oriented formatting languages for rendering to human-readable output,
either paper or e-paper, and during this process the machine-processability is
lost. Supporting data are often prepared in a variety of (ill-suited) formats; thus,
spectral information is frequently merely a scanned image corresponding to the
original printed spectrum (itself formatted for human convenience rather than
processability).
Chapter 8: Markup Languages and the Datument ➤ 99
XML has the potential to revolutionize this situation. With agreed XML-
based markup languages, authors can have a single environment independent
of the publishers’ requirements. Publishers can transform the XML into their
in-house systems, but quite independently other “reusers” can do so to their own
(possibly quite different) requirements. The original datument, which contains
all the “supporting data”, can be archived along with the semantics and ontology,
all in XML. To achieve all of this consistency, a major change will be required in
authoring tools. Instead of proprietary text-based tools, with little useful sup-
port for semantics of either text or data, we will require XML-based tools with
domain-specific XML components.
An XML editor display environment contains generic mechanisms to manage
any domain-specific schema and therefore ensures that a resulting datument is
valid. It will also contain mechanisms for supporting domain-specific software,
such as editors and browsers (e.g., for molecules, spectra, etc.). Much high-qual-
ity chemistry software capable of being used in this context is already available.
One commercial example based on XML, which encapsulates this concept, has
already appeared. The Publicon tool, for example, provides a comprehensive
technical authoring environment capable of handling mathematics and express-
ing the result in XML; interestingly, its target audience includes chemists and
bioscientists. Because all XML-conforming markup languages have essentially
the same kinds of structures, syntax, and rules, it becomes much easier to write
appropriate, often generic, software to handle it.
The Datument as a Component
of a Scientific Grid
The use of XML has the potential to create savings in certain areas (time for
authors and staff costs for technical editors), but a major benefit is that the col-
lected XML datuments, together with the ontologies, would effectively create a
machine-processable knowledge base for chemistry. At present, primary publica-
tions do not create knowledge without a lot of additional human actions to max-
imize the knowledge created by primary publications: abstracting, collating, and
validating are needed. If this knowledge could be captured and tagged within
the primary publication itself, without simply transferring that human action to
a different point in the process, a significant proportion of the knowledge base
could be extracted by machine. If the metadata, structure, datatypes, ontology,
semantics, and processing behavior of a piece of information are determined, it
essentially becomes a self-describing information component. These information
components—which might be implemented by a mixture of XML protocols and
object-oriented code—can be regarded as stand-alone, self-describing parts of a
knowledge base. Protocols such as XML Query are able to search a heterogeneous
aggregate of such components, and RDF (resource description framework, a way
100 ➤ The ACS Style Guide
to use metadata so that, for example, search engines can locate it) will be able
to make deductions from their metadata. By combination of different markup
languages, all information, even at a fine level, can be captured without loss. Any
part of it can be retrieved, and hence a collection of marked up XML publica-
tions would constitute a knowledge base.
If each datument has sufficient high-quality metadata, there may be no essen-
tial need for a knowledge base to be centralized. By collecting those publications
of interest, any reader or group can create their own personal base. In turn, such
metadata can be exported to a wider community using new mechanisms such
as RDF or RSS (RDF Site Summary or Rich Site Summary, a way of collecting
metadata of interest to an individual reader).
With such exciting technologies in the offing, authors, funders, editors, pub-
lishers, and readers have a unique opportunity to start experimenting with cre-
ation and dissemination of machine-understandable information and data as an
integral part of the process of scientific publishing.
References
1. Murray-Rust, P.; Rzepa, H. S. J. Digital Inf. 2004, 5, article 248, 2004-03-18.
2. Murray-Rust, P.; Rzepa, H. S.; Tyrrell, S. M.; Zhang, Y. Y. Org. Biomol. Chem. 2004, 2,
3192–3203.
3. See Murray-Rust, P.; Rzepa, H. S. J. Chem. Inf. Comput. Sci. 2003, 43, 757–772 and refer-
ences cited therein. For online information, including schemas, see http://cml.sourceforge.
net/.
4. A full review and listing of all scientifically based XML languages is beyond the scope of
this short chapter. Other than CML, the best documented is ThermoML, which is used for
thermophysical and thermochemical data. Frenkel, M.; Chirico, R. D.; Diky, V. V.; Marsh,
K. N.; Dymond, J. H.; Wakeham, W. A. J. Chem. Eng. Data 2004, 49, 381–393 and refer-
ences cited therein.
5. Murray-Rust, P.; Rzepa, H. S.; Wright, M.; Zara, S. Chem. Commun. (Cambridge, U.K.)
2000, 1471–1472.
6. Murray-Rust, P.; Rzepa, H. S.; Wright, M. New J. Chem. 2001, 618–634.
7. Gkoutos, G. K.; Murray-Rust, P.; Rzepa, H. S.; Wright, M. J. Chem. Inf. Comput. Sci. 2001,
41, 1124–1130.
101
➤ ➤ ➤ ➤ ➤
APPENDIX 8-1
The IUPAC International
Chemical Identifier, InChI
Stephen R. Heller and Alan D. McNaught
The ability to represent uniquely a chemical compound is a fundamental require-
ment for storage or transmission of chemical information. We define compounds
by their molecular structure, as shown in two-dimensional diagrams or stored in
computers. Pronounceable names have been developed for oral and written com-
munication, ranging from the trivial, containing no structural information, to
completely systematic names, which can be decoded to yield the original structure.
However, the application of systematic nomenclature to complicated structures
requires expert knowledge of elaborate systems of nomenclature rules. The use of
systematic nomenclature to convey information about the increasingly complex
molecular systems handled by today’s chemists is both laborious and inefficient.
Over the past decade, with ever-increasing reliance by chemists on computer
processing, the International Union of Pure and Applied Chemistry (IUPAC)
recognized a need to develop methods of nomenclature that can be interpreted
by computers, or more precisely, by computer algorithms. A new program was
initiated, aimed at creating a method to generate a freely available, nonpropri-
etary identifier for chemical substances that could be used in printed and elec-
tronic data sources. The technical development was carried out primarily at the
U.S. National Institute of Standards and Technology, and the product is referred
to as the IUPAC International Chemical Identifier (InChI).
InChI is not a registry system. It does not depend on the existence of a data-
base of unique substance records to establish the next available sequence num-
ber for any new chemical substance being assigned an identifier. Instead, InChI
transforms the chemical structure of a compound into a string of characters that
uniquely identify that compound. This conversion of a graphical representation
of a chemical substance into the unique InChI label can be carried out automati-
cally by any organization, and the facility can be built into any chemical struc-
ture drawing program. InChI labels are completely transferable and can be cre-
ated from existing collections of chemical structures.
Whereas the theory needed for conversion of a structure to a unique string
of characters has been known for a long time, when work on InChI began there
were no freely available unique representations for compound identification, nor
102 ➤ The ACS Style Guide
was their development being actively discussed. Thus, before active development
could proceed, a precise specification of requirements was wanted, and the fol-
lowing five characteristics were specified as needed for such an identifier:
1. The structure of the compound can be drawn using common conventions.
2. The identifier is derived directly from the structure by an algorithm.
3. Exactly one identifier is associated with a given structure, that is, different
structures give different identifiers.
4. The identifier works for a large fraction of all “drawable” chemical substances.
5. The identifier must be openly available.
To be as precise and broadly applicable as desired, InChI uses a layered for-
mat to represent all available structural information relevant to compound iden-
tity. Each layer in an InChI representation contains a specific type of structural
information. These layers, automatically extracted from the input structure, are
designed so that each successive layer adds additional detail. The specific lay-
ers generated depend on the level of structural detail available and whether tau-
tomerism is allowed. Any ambiguities or uncertainties in the original structure
will remain in the InChI. The InChI layers are formula (standard Hill sorted);
connectivity (no formal bond orders), including disconnected and connected
metals; isotopes; stereochemistry, including double bond (Z/E) and tetrahedral
(sp3); and tautomers (on or off). Charges are not part of the basic InChI, but
rather are added at the end of the InChI string.
An example of an InChI representation is given in Figure 8A-1. The acronym
InChI and version number are regarded as part of the InChI string (InChI=1 in
this case). It is important to recognize, however, that InChI strings are intended
for use by computers, and end users need not understand any of their details. In
fact, the open nature of InChI and its flexibility of representation, after imple-
mentation into software systems, may allow chemists to be even less concerned
with the details of structure representation by computers. Source code and an
executable version of the structure-to-InChI conversion algorithm are freely
available from the IUPAC InChI Web site at http://www.iupac.org/inchi.
(A) (B) (C)
InChI=1/C5H5N5O/c6-5-9-3-2(4(11)10-5)7-1-8-3/h1H,(H4,6,7,8,9,10,11)/f/h8,10H,6H2
Figure 8A-1. InChI for guanine: (A) input structure; (B) mobile H canonical
numbering, with the attachment points of four mobile H and changeable
bonds indicated in bold; and (C) with fixed H canonical numbering.
➤ ➤ ➤ ➤ ➤
Part 2
Style Guidelines
105
➤ ➤ ➤ ➤ ➤ CHAPTER 9
Grammar,
Punctuation,
and Spelling
This chapter presents grammatical points that cover most
situations. It does not attempt to discuss all the rules of
grammar; many excellent grammar texts are available for that purpose, such
as those given in the selected bibliography, Chapter 18. Writing style and word
usage are discussed in Chapter 4. Punctuation, spelling, and word usage are also
discussed in Chapter 11 with respect to numbers, mathematics, and units of
measure and in Chapter 12 with respect to chemical names.
Grammar
Subject–Verb Agreement
Everyone knows that a subject and its verb must agree in number. Nevertheless,
errors in subject–verb agreement are quite common. The primary cause is con-
fusion about the number of the subject.
➤ The number of the subject can be obscured when one or more prepositional
phrases come between the subject and the verb.
Application of this technique to studies on the phytoplankton biomass and its
environments is described. (The subject is “application”, which is singular.)
➤ The number of the subject can be obscured when the sentence is constructed
in the order prepositional phrase, verb, subject.
To the mixture were added KCl, HEPES, and water.
To the solution was added the parent compound.
Copyright 2006 American Chemical Society
106 ➤ The ACS Style Guide
➤ Two singular subjects joined by “and” require a plural verb.
Growth and isolation of M13 virus were described.
exception A subject that is plural in form but singular in effect takes a sin-
gular verb. Here a compound subject functions as a single entity.
Research and development is attracting a growing number of young scientists.
Its inventor and chief practitioner is a native son of Boston, Robert Coles.
Much inconsistency and confusion exists with technical documentation.
➤ When two or more subjects are joined by “or”, the verb takes the number of
the closer or closest subject.
All of the pH values or the median pH value was used.
The median pH value or all of the pH values were used.
➤ Collective nouns take a singular verb when the group as a whole is meant;
in that case, they are often preceded by the word “the”. Collective nouns take a
plural verb when individuals of the group are meant; in that case, they are often
preceded by the word “a”.
contents majority range
couple number series
dozen pair variety
group
The number of metal amides synthesized was the largest to date. (Refers to the
number as a unit.)
A number of metal amides were synthesized. (Refers to each amide.)
The series of compounds was prepared to test the hypothesis. (Refers to the series
as a unit.)
A series of compounds were tested. (Refers to each compound.)
The variety of materials tested was sufficient for comparative analysis. (Refers to
variety as a unit.)
A variety of materials were tested for selective removal of 90Sr from nuclear waste
solutions. (Refers to the materials individually.)
This group of workers is well aware of its responsibilities. (Refers to the group as
a unit.)
This group of workers are willing to sign their names. (Refers to the individuals.)
➤ “Data” can be a singular or plural noun.
After the data is printed and distributed, we can meet to discuss it. (Refers to the
whole collection of data as one unit.)
Experimental data that we obtained are compared with previously reported
results. (Refers to the data as individual results.)
Chapter 9: Grammar, Punctuation, and Spelling ➤ 107
➤ Units of measure are treated as collective nouns that take a singular verb.
The mixture was stirred, and 5 mL of diluent was added.
Five grams of NaCl was added to the solution.
Three weeks is needed to complete the experiment.
To the mixture was added 5 g of compound B.
Under high pressure, 5 volumes of solution A was added.
➤ Nouns ending in “ics” and denoting a scientific discipline are usually singular.
dynamics mechanics
kinetics physics
mathematics thermodynamics
Mechanics involves the application of Newton’s three laws of motion.
The kinetics of electron transfer to and from photogenerated radicals was exam-
ined by laser flash photolysis.
The thermodynamics is governed by the positions of the valence and conduction
bands.
➤ Compound subjects containing the words “each”, “every”, and “everybody”
take singular verbs.
Each flask and each holder was sterilized before use.
Every rat injected and every rat dosed orally was included.
Everybody in the group and every visitor is assigned a different journal each
month.
➤ Sometimes, one of these words is implicit; such cases take a singular verb.
Each name and address is entered into the database.
➤ If both components of the compound subject do not contain, explicitly or
implicitly, one of the words “each”, “every”, or “everybody”, the verb must be plural.
Each student and all the professors were invited.
➤ Indefinite pronouns themselves (or adjectives combined with the indefinite
pronoun “one”) can be the subject of the sentence.
• Those that take a singular verb are “each”, “either”, “neither”, “no one”,
“every one”, “anyone”, “someone”, “everyone”, “anybody”, “somebody”, and
“everybody”.
Each was evaluated for its effect on metabolism.
Neither disrupts the cell membrane.
108 ➤ The ACS Style Guide
Regarding compounds 1–10, every one reacts with the control agent.
Someone measures the volume every day.
• Those that take a plural verb are “several”, “few”, “both”, and “many”.
Several were evaluated for their effects on metabolism.
Few disrupt the cell membrane.
Regarding compounds 1 and 2, both react with the control agent.
Many were chosen to be part of the study.
• Those that take either a singular or a plural verb, depending on context,
are “some”, “any”, “none”, “all”, and “most”. The number of the object of the
preposition determines the number of the indefinite pronoun related to it.
All of the money was stolen.
Most of the books were lost.
Not all the disks are here; some were lost.
➤ When a fraction is the subject of the sentence, the number of the attendant
object of the preposition determines the number of the subject.
One-third of the precipitate was dissolved.
One-fourth of the electrons were excited.
➤ When a subject and its predicate noun disagree in number, the verb takes the
number of the subject. (A predicate noun is the “complement” of a form of the
verb “to be”; it refers to the same person or thing as the subject.)
The preparation and structure determination [plural subject] of these three com-
pounds are the topic [singular predicate noun] of this paper.
The topic of this paper [singular subject] is the preparation and structure deter-
mination [plural predicate noun] of these three compounds.
Awkward Omissions of Verbs and Auxiliary Verbs
➤ Each subject in a compound sentence must have the proper verb and auxil-
iary verb.
incorrect
The eluant was added to the column, and the samples collected in 10 mL incre-
ments.
correct
The eluant was added to the column, and the samples were collected in 10 mL
increments.
Chapter 9: Grammar, Punctuation, and Spelling ➤ 109
Restrictive and Nonrestrictive Expressions
➤ A phrase or clause is restrictive when it is necessary to the sense of the sen-
tence; that is, the sentence would become pointless without the phrase or clause.
Restrictive clauses are best introduced by “that”, not “which”.
It was necessary to find a blocking group that would react with the amino group
but not with the hydroxyl group.
Comparison will be restricted to acetylene compounds that have the same func-
tional end groups.
If the clauses beginning with “that” were deleted, the sentences would not convey
the information intended. Therefore, the clauses are restrictive.
Phrases can also be restrictive.
Reactions leading to the desired products are shown in Scheme 1.
If the phrase “leading to the desired products” were deleted, the sentence would
not convey the information intended.
➤ A phrase or clause is nonrestrictive if it adds information but is not essential;
that is, the sentence does not lose its meaning if the phrase or clause is deleted.
Nonrestrictive phrases and clauses are set off by commas. Nonrestrictive clauses
may be introduced by “who” or “which” but not by “that”.
Squalene, a precursor of cholesterol, is a 30-carbon isoprenoid.
This highly readable book, written in nontechnical language, surveys the field of
chemistry by describing the contributions of chemistry to everyday life.
Moore, working at the Rockefeller Institute, developed methods for the quantita-
tive determination of amino acids.
The current–voltage curves, which are shown in Figure 6, clearly demonstrate the
reversibility of all four processes.
Several hazardous waste disposal sites are located along the shores of the Niagara
River, which is a major water source.
Melvin Calvin, who won the Nobel Prize in 1961, elucidated the biochemical
pathways in photosynthesis.
James Aberdeen, professor emeritus of Central State University, which has pro-
vided significant scholarship support to minority students over the years, made a
generous contribution to the school’s building fund.
Dangling Modifiers
A dangling modifier is a modifying word or phrase that does not clearly and
logically modify another word in the sentence. In scientific writing, the passive
voice is often necessary (“the solutions were heated”; “melting points were deter-
mined”), but its use can lead to dangling modifiers.
110 ➤ The ACS Style Guide
➤ If a modifier precedes the subject of a sentence, it must modify that subject
and be separated from it by a comma. Otherwise, it is a dangling modifier.
incorrect
Splitting the atom, many new elements were discovered by Seaborg.
correct
Splitting the atom, Seaborg discovered many new elements.
incorrect
Upon splitting the atom, many new elements were discovered by Seaborg.
correct
Upon splitting the atom, Seaborg discovered many new elements.
incorrect
When confronted with these limitations, the experiments were discontinued.
correct
When confronted with these limitations, we discontinued the experiments.
In light of these limitations, the experiments were discontinued.
incorrect
Understanding the effect of substituents on the parent molecules, the ortho
hydrogens could be assigned to the high-frequency peak.
correct
Understanding the effect of substituents on the parent molecules, we could assign
the ortho hydrogens to the high-frequency peak.
incorrect
Using the procedure described previously, the partition function can be evalu-
ated.
correct
Using the procedure described previously, we can evaluate the partition func-
tion.
➤ In some cases, the passive voice can be used to correct a dangling modifier.
incorrect
After combining the reactants, the reaction mixture was stirred at room tempera-
ture for 3 h.
correct
After the reactants were combined, the reaction mixture was stirred at room tem-
perature for 3 h.
Chapter 9: Grammar, Punctuation, and Spelling ➤ 111
incorrect
After stirring the mixture, 5 mg of compound 2 was added.
correct
After the mixture was stirred, 5 mg of compound 2 was added.
➤ Phrases starting with “based on” must modify a noun or pronoun that usu-
ally immediately precedes or follows the phrase. Use phrases starting with “on
the basis of” to modify a verb.
incorrect
Based on resonance enhancement and frequency shifts, changes in the inter-ring
separation were calculated.
correct
On the basis of resonance enhancement and frequency shifts, changes in the
inter-ring separation were calculated.
incorrect
Based on extensive study, this genetic deficiency was attributed to the loss of one
isozyme. (“Based on extensive study” modifies the noun “deficiency”, but this is
not the meaning.)
correct
On the basis of extensive study, this genetic deficiency was attributed to the
loss of one isozyme. (“On the basis of extensive study” modifies the verb “was
attributed”.)
Style guidelines based on authoritative sources are included in this book. (“Based
on authoritative sources” modifies the noun “guidelines”.)
➤ “Due to” means “attributable to”; use it only to modify a noun or pronoun
directly preceding it in the sentence or following a form of the verb “to be”.
incorrect
Delays resulted due to equipment failure.
correct
Delays due to equipment failure resulted.
The delays were due to equipment failure.
incorrect
This high value resulted due to the high conversion efficiencies of the enzymatic
reactor.
112 ➤ The ACS Style Guide
correct
This high value is due to the high conversion efficiencies of the enzymatic reactor.
This high value resulted from the high conversion efficiencies of the enzymatic
reactor.
incorrect
Due to exposure to low levels of lead, children can be at risk for developmental
problems.
correct
Because of exposure to low levels of lead, children can be at risk for developmen-
tal problems.
Children can be at risk for developmental problems because of exposure to low
levels of lead.
➤ Absolute constructions are words, phrases, or clauses that are grammatically
unconnected with the rest of the sentence in which they appear. They are some-
times called “sentence modifiers” because they qualify the rest of the sentence.
They may occur anywhere in the sentence, and they are always set off by com-
mas. They are not dangling modifiers.
Contrary to the excited-state situation, metal–metal bonding interactions in the
ground states are weak.
The conclusions were premature, considering the lack of available data.
Judging from the spectral changes, exhaustive photolysis of compound 4 had
occurred.
The conformations about the Re–Re bond, in addition, are different for all three
complexes.
When necessary, the solutions were deaerated by bubbling nitrogen.
Clearly, alternative synthetic methods are possible.
The instructor having made her point, the discussion continued.
Absolute constructions often begin with one of the following words:
concerning judging
considering provided
failing providing
given regarding
• In mathematical papers, absolute phrases beginning with the words
“assuming” and “taking” are often used as sentence modifiers.
Assuming that distance d is induced by the norm, M is a symmetrical and pos-
itively defined matrix.
Taking this value as an upper limit, the two shortest distances are sometimes too
long for incipient hydrogen bonds.
Chapter 9: Grammar, Punctuation, and Spelling ➤ 113
• A subordinate or elliptical clause may be used as a sentence modifier.
The compound is stable in air, as we concluded from the experimental evidence.
The Mo 5s orbitals, as expected, interact strongly with the ligands.
• An introductory infinitive or infinitive phrase may be a sentence modifier.
To prepare compound 2, the method of Garner was followed.
Reflexive Pronouns
➤ Use the reflexive pronouns “myself”, “yourself”, “himself”, “herself”, “itself”,
“ourselves”, and “themselves” only to refer back to a noun or another pronoun in
the same sentence.
incorrect
Please send your manuscript to the associate editor or myself.
correct
Please send your manuscript to the associate editor or me.
The associate editor herself will review your manuscript.
incorrect
My collaborators and myself will evaluate the results.
correct
My collaborators and I will evaluate the results.
I will evaluate the results myself.
I myself will evaluate the results.
Punctuation
Comma
➤ Use a comma before, but not after, the coordinating conjunctions “and”, “or”,
“nor”, “but”, “yet”, “for”, and “so” connecting two or more main clauses (complete
thoughts).
Toluene and hexane were purified by standard procedures, and benzene was
redistilled from calcium hydride.
The role of organic templates in zeolite synthesis has been studied extensively,
but no general principles have been delineated.
Supported metals are among the most important industrial catalysts, yet only a
few have been studied thoroughly.
No dielectric constants are available for concentrated acids, so it is difficult to
give a quantitative explanation for the results.
114 ➤ The ACS Style Guide
➤ Use a comma after a subordinate clause that precedes the main clause in a
complex sentence.
Although 40 different P450 enzymes have been identified, only six are respon-
sible for the processing of carcinogens.
Since the institute opened, plant breeders have developed three new prototypes.
Because the gene and the molecular marker are so close on the chromosome, they
segregate together in the progeny.
➤ Use a comma after most introductory words and phrases.
However, the public is being inundated with stories about cancer-causing chemicals.
Therefore, the type of organic solvent used is an important factor in lipase-
catalyzed enzymatic synthesis.
After 3 months, the plants grown under phosphorus-deficient conditions were
evaluated.
Thus, their motion is the result of the rotation of ferromagnetic domains.
On cooling, a crystalline phase may develop in coexistence with an amorphous
phase.
➤ Use a comma before the coordinating conjunction in a series of words,
phrases, or clauses of equal rank containing three or more items. (This comma is
called the serial comma.)
Water, sodium hydroxide, and ammonia were the solvents.
The red needles were collected, washed with toluene, and dried in a vacuum
desiccator.
The compound does not add bromine, undergo polymerization by the Diels–
Alder reaction, or react with electrophiles.
➤ In compound sentences containing coordinating conjunctions, the clause
following the conjunction is punctuated as if it were alone.
The reaction proceeds smoothly, and by use of appropriate reagents, the yields
will be enhanced.
The compounds were separated, and after the filters had been washed, the experi-
ments were completed.
➤ Do not use a comma to separate a verb from its subject, its object, or its pred-
icate noun.
incorrect
The addition of substituted silanes to carbon–carbon double bonds, has been
studied extensively.
Chapter 9: Grammar, Punctuation, and Spelling ➤ 115
correct
The addition of substituted silanes to carbon–carbon double bonds has been
studied extensively.
incorrect
The disciplines described in the brochure include, materials science, biotechnol-
ogy, and environmental chemistry.
correct
The disciplines described in the brochure include materials science, biotechnol-
ogy, and environmental chemistry.
incorrect
The solvents used in this study were, cyclohexane, methanol, n-pentane, and
toluene.
correct
The solvents used in this study were cyclohexane, methanol, n-pentane, and
toluene.
➤ Do not use a comma before the conjunction joining a compound predicate
consisting of only two parts.
incorrect
The product distribution results were obtained in sodium hydroxide, and are
listed in Table 10.
correct
The product distribution results were obtained in sodium hydroxide and are
listed in Table 10.
➤ Use commas to separate items in a series that contains another series in
parentheses already separated by commas.
The structure was confirmed with spectroscopy (1H NMR, UV, and IR), high-
resolution mass spectrometry, and elemental analysis.
➤ Use a comma between two or more adjectives preceding a noun only if you
can reverse the order of the adjectives without losing meaning. If you can insert
the word “and”, the comma is correct.
The intense, broad signals of the two groups confirmed their location.
The broad, intense signals of the two groups confirmed their location.
Sample preparation is a repetitious, labor-intensive task.
Sample preparation is a labor-intensive, repetitious task.
A powerful, versatile tool for particle sizing is quasi-elastic light scattering.
A versatile, powerful tool for particle sizing is quasi-elastic light scattering.
116 ➤ The ACS Style Guide
But:
Polyethylene is an important industrial polymer.
The rapid intramolecular reaction course leads to ring formation.
The backbone dihedral angles were characterized by J couplings.
The local structural environment of the Mn cluster was determined.
➤ Use a comma before, but not after, the subordinating conjunction in a nonre-
strictive clause.
incorrect
The bryopyran ring system is a unique requirement for anticancer activity
whereas, the ester substituents influence the degree of cytotoxicity.
correct
The bryopyran ring system is a unique requirement for anticancer activity,
whereas the ester substituents influence the degree of cytotoxicity.
➤ Use commas to set off nonrestrictive phrases or clauses.
The products, which were produced at high temperatures, were unstable.
➤ Phrases introduced by “such as” or “including” can be restrictive (and thus
not set off by commas) or nonrestrictive (and thus set off by commas).
Potassium compounds such as KCl are strong electrolytes; other potassium com-
pounds are weak electrolytes.
Previously, we described a mathematical model including a description of chlo-
rophyll degradation in foods.
Divalent metal ions, such as magnesium(II) and zinc(II), are located in the cata-
lytic active sites of the enzymes.
Hydrogen-bonded complexes, including proton-bound dimers, are well-known
species.
In the first two sentences, the phrases are restrictive because the sentences do
not make their points without the phrases. In the third and fourth sentences, the
phrases are nonrestrictive because the sentences can make their points without
the phrases.
➤ An appositive is a noun that follows another noun and identifies or explains
the meaning of the first noun.
My wife, Jeanne, is a biochemist at the National Institutes of Health.
My son James plays baseball, and my son John plays soccer.
An appositive is nonrestrictive (and therefore set off by commas) when it names
the only possibility. In the first sentence, Jeanne is a nonrestrictive appositive. An
Chapter 9: Grammar, Punctuation, and Spelling ➤ 117
appositive is restrictive (and therefore not set off by commas) when it points out
one of two or more possibilities. In the second sentence, the names of the two
sons are restrictive appositives.
➤ Use commas to set off the words “that is”, “namely”, and “for example” when
they are followed by a word or list of words and not a clause. Also use a comma
after the item or items being named. Use a comma after “i.e.” and “e.g.” in paren-
thetical expressions.
The new derivatives obtained with the simpler procedure, that is, reaction with
organocuprates, were evaluated for antitumor activity.
Alkali metal derivatives of organic compounds exist as aggregates of ion pairs,
namely, dimers, trimers, and tetramers, in solvents of low polarity.
Many antibiotics, for example, penicillins, cephalosporins, and vancomycin,
interfere with bacterial peptidoglycan construction.
These oxides are more stable in organic solvents (e.g., ketones, esters, and ethers)
than previously believed.
➤ Use commas to separate two reference citation numbers, but use an en dash
(–) to express a range of three or more in sequence, whether they are superscripts
or are on the line in parentheses. When they are superscripts, do not use a space
after the comma.
Experimental investigations10,14,18–25 concerned the relative importance of field
and electronegativity effects.
Certain complexes of cobalt were reported (10, 11) to have catalytic effects on
hydrolysis reactions.
Flash photolysis studies (3–7) demonstrated the formation of transient inter-
mediate products such as triplet states.
➤ Use a comma before Jr. and Sr., but treat II and III according to the person’s
preference. Within a sentence, always use a comma after Jr. and Sr., but use a
comma after II and III only if they are preceded by a comma.
William M. Delaney, Jr.
Charles J. Smith, III
John J. Alden II
William M. Delaney, Jr., was elected to the governing board.
Charles J. Smith, III, received a majority of the votes.
John J. Alden II did not run for office this year.
➤ Do not use a comma preceding “et al.” unless commas are needed for other
reasons.
118 ➤ The ACS Style Guide
Saltzman et al.
Saltzman, M. J., et al.
Saltzman, Brown, et al.
➤ In dates, use a comma after the day, but not after the month when the day is
not given.
June 15, 1996
June 1996
When giving a complete date within a sentence, use a comma after the year as
well.
On August 18, 1984, an extraordinary person was born.
➤ When a geographical location is named within a sentence and the name
includes a comma, use a comma at the end of the name as well.
Iona College, in New Rochelle, New York, is the CEO’s alma mater.
The lead researcher, who obtained her education at the University of Calgary,
Alberta, Canada, addressed the reporters’ questions.
➤ Use a comma to introduce quotations.
In the words of Pasteur, “Chance favors the prepared mind.”
Pasteur said, “Chance favors the prepared mind.”
➤ Do not use a comma after a quotation that is the subject of the sentence.
“Chance favors the prepared mind” is a translation from the French. (The quota-
tion is the subject of the sentence.)
Period
➤ Use a period at the end of a declarative sentence, but never in combination
with any other punctuation marks.
He said, “Watch out!”
She asked, “May I go?”
➤ Do not use periods after most abbreviated units of measure, except when the
abbreviation could be confused with a word (in. for inches, at. for atomic, no. for
number).
➤ If a sentence ends with an abbreviation that includes a period, do not add
another period.
She will return at 3 a.m.
Chapter 9: Grammar, Punctuation, and Spelling ➤ 119
➤ Use periods and spaces after initials in persons’ names.
J.-L. Gay Lussac J. E. Lennard-Jones M. S. Newman
exception Use periods but no spaces when referring to authors of a paper
in the acknowledgment paragraph of the paper.
R.C.McD. and C.R. thank Dr. Rose Allan for carefully reading the manuscript.
C.-C.Y., L.B.-P., N.-h.X., and S.Zh.O. are grateful for generous support from the
university.
➤ Do not use periods in abbreviations or acronyms of institution or organiza-
tion names.
ACS CNRS NASA NIH
Semicolon
➤ Use a semicolon to separate independent clauses that are not joined by a con-
junction.
All solvents were distilled from an appropriate drying agent; tetrahydrofuran and
diethyl ether were also pretreated with activity I alumina.
➤ Use semicolons between items in a series of words, phrases, or data strings if
one or more of the items already contain commas.
We thank Zachary Axelrod, University of Michigan, for spectral data; Caroline
Fleissner, Harvard University, for helpful discussions; and the National Science
Foundation for financial support (Grant XYZ 123456).
The product was dried under vacuum to give compound 2: yield 68%; IR 1991
m, 1896 s, sh, 1865 s cm–1; 1H NMR 0.36 ppm; 13C NMR 221.3, 8.1 ppm.
Figure 1. Cyclic voltammograms in dichloromethane: (a) compound 1, 23 °C; (b)
compound 2, –40 °C; (c) compound 4, 23 °C.
Figure 6. Ru–H stretches in the IR spectrum of compound 5: ×, 298 K; +, 90 K.
This rule holds even if the only group containing the commas is the last in the
series.
The compounds studied were methyl ethyl ketone; sodium benzoate; and acetic,
benzoic, and cinnamic acids.
➤ Use a semicolon between independent clauses joined by conjunctive adverbs
or transitional phrases such as “that is”, “however”, “therefore”, “hence”, “indeed”,
“accordingly”, “besides”, and “thus”.
The rate at which bleaching occurred was dependent on cluster size; that is, the
degradation of the mononuclear cluster was about 5 times faster than that of the
tetranuclear cluster.
120 ➤ The ACS Style Guide
Many kinetic models have been investigated; however, the first-order reactions
were studied most extensively.
The proposed intermediate is not easily accessible; therefore, the final product is
observed initially.
The restriction of the rotational motions of the tert-butyl group gives rise to large
entropy changes for the association reaction; hence, the covalent form is rela-
tively easy to identify.
The efficiency of the cross-coupling depends on the nature of X in RX; thus, the
reaction is performed at room temperature by slow addition of the ester.
➤ Do not use a semicolon between dependent and independent clauses.
incorrect
The activity on bromopyruvate was decreased; whereas, the activity on pyruvate
was enhanced.
correct
The activity on bromopyruvate was decreased, whereas the activity on pyruvate
was enhanced.
Colon
➤ Use a colon to introduce a word, a phrase, a complete sentence, or several
complete sentences that illustrate, clarify, or expand the information that pre-
cedes it. Capitalize the first word after a colon only if the colon introduces more
than one complete sentence, a quotation, or a formal statement.
The electron density was studied for the ground state of three groups of mol-
ecules: (1) methane–methanol–carbon dioxide, (2) water–hydrogen peroxide,
and (3) ferrous oxide–ferric oxide.
We now report a preliminary finding: no chemical shift changes were detected in
the concentration range 0.1–10 M.
The following are our conclusions: Large-angle X-ray scattering studies give us
an accurate picture of structures up to 9 Å. They do not allow the specification of
defects, such as random ruptures of the chains. The structural models defined are
strongly supported by magnetic measurements.
➤ In figure captions, use a colon to introduce explanations of symbols or other
aspects of the figure.
Figure 1. Variable-temperature 1H NMR spectra of compound 12: top, 403 K;
middle, 353 K; bottom, 298 K.
Figure 3. Brønsted-type plots for aminolysis in 1 M KCl at 25 °C: , 2-nitrophenyl
acetate; , 3-chlorobenzoic acid; , 2,6-dinitrobenzoic acid.
Chapter 9: Grammar, Punctuation, and Spelling ➤ 121
➤ Do not use a colon (or any punctuation) between a verb and its object or
complement or between a preposition and its object.
incorrect
The rate constants for the reaction in increasing concentrations of sodium
hydroxide are: 3.9, 4.1, 4.4, 4.6, and 4.9.
correct
The rate constants for the reaction in increasing concentrations of sodium
hydroxide are 3.9, 4.1, 4.4, 4.6, and 4.9.
incorrect
The thermal decomposition was investigated with: gas chromatography, BET
surface areas, and X-ray powder diffraction.
correct
The thermal decomposition was investigated with gas chromatography, BET sur-
face areas, and X-ray powder diffraction.
incorrect
Transition-metal nitrides have many properties that make them suitable for
industrial applications, including: high wear resistance, high decomposition tem-
perature, and high microhardness.
correct
Transition-metal nitrides have many properties that make them suitable for
industrial applications, including high wear resistance, high decomposition tem-
perature, and high microhardness.
➤ Use either a colon or a slash to represent a ratio, but not an en dash. Use
either a slash or an en dash between components of a mixture, but not a colon.
dissolved in 5:1 glycerin/water
dissolved in 5:1 glycerin–water
the metal/ligand (1:1) reaction mixture
the metal–ligand (1:1) reaction mixture
the metal–ligand (1/1) reaction mixture
the methane/oxygen/argon (1/50/450) matrix
the methane/oxygen/argon (1:50:450) matrix
Quotation Marks
Location of closing quotation marks with respect to other punctuation is a style
point in which ACS differs from other authorities. In 1978, ACS questioned the
traditional practice and recommended a deviation: logical placement. Thus, if
the punctuation is part of the quotation, then it should be within the quotation
122 ➤ The ACS Style Guide
marks; if the punctuation is not part of the quotation, the writer should not mis-
lead the reader by implying that it is.
➤ Place closing quotation marks before all punctuation that is not part of the
original quotation. Place them after all punctuation that is part of the quotation.
The sample solution was stirred briefly with a magnetic “flea”.
Ralph Waldo Emerson said, “The reward of a thing well done is to have done it.”
➤ Use quotation marks around words used in a new sense or words not used
literally, but only the first time they appear in text.
Plastocyanin is a soluble “blue” copper protein.
The integrated intensity of each diagonal in the spectrum is proportional to a
“mixing coefficient”.
The “electron-deficient” cations are, in fact, well-established intermediates.
➤ Use quotation marks to enclose the titles of uniquely named parts and sec-
tions of a book or a paper.
A complete description of the oils is given in the section “Flavonoids in Citrus
Peel Oils”, and other references are listed in the bibliography.
But:
The preface describes the complexity of the problem.
➤ Use quotation marks to enclose short direct quotations (up to three sentences).
In the book Megatrends, Naisbitt concludes, “We are moving from the specialist
who is soon obsolete to the generalist who can adapt.”
➤ Use a narrower column width (that is, indented on both sides) for longer
quotations (extracts) of 50 words or more. Do not use quotation marks.
Everything is made of atoms. That is the key hypothesis. The most impor-
tant hypothesis in all of biology, for example, is that everything that ani-
mals do, atoms do. In other words, there is nothing that living things do
that cannot be understood from the point of view that they are made of
atoms acting according to the laws of physics.
—Richard Phillips Feynman
However, this convention does not apply in an article quoting someone who has
been interviewed. In such cases, quoted text need not be differentiated by col-
umn width, and quotation marks should be used.
➤ Use single quotation marks only when they are within double quotation marks.
He said, “You should read the article ‘Fullerenes Gain Nobel Stature’ in the Janu-
ary 6, 1997, issue of Chemical & Engineering News.”
Chapter 9: Grammar, Punctuation, and Spelling ➤ 123
Parentheses
Parenthetical expressions contain information that is subsidiary to the point
of the sentence. The sentence does not depend on the information within the
parentheses.
➤ Use parentheses for parenthetical expressions that clarify, identify, or illus-
trate and that direct the reader.
The total amount (10 mg) was recovered by modification of the procedure.
The final step (washing) also was performed under a hood.
The curve (Figure 2) obeys the Beer–Lambert law.
The results (Table 1) were consistently positive.
Only 15 samples (or 20%) were analyzed.
➤ Punctuate after, not before, parenthetical expressions.
incorrect
Compound 1, (7 mg) obtained by typical workup methods, was used without
further purification.
correct
Compound 1 (7 mg), obtained by typical workup methods, was used without
further purification.
➤ If a parenthetical sentence is within another sentence, do not use a final
period within the closing parenthesis, and do not start the parenthetical sentence
with a capital letter.
Our results (the spectra are shown in Figure 5) justified our conclusions.
Our results justified our conclusions (the spectra are shown in Figure 5).
➤ If a parenthetical sentence is not within another sentence, use a final period
inside the closing parenthesis, and start the parenthetical sentence with a capital
letter.
A mechanism involving loss of a CH radical followed by rearrangement was pro-
posed. (The reactions are shown in Scheme 1.)
➤ Use parentheses to enclose numerals in a list. Always use parentheses in
pairs, not singly.
Three applications of this reaction are possible: (1) isomerization of sterically
hindered aryl radicals, (2) enol–keto transformation, and (3) sigmatropic hydro-
gen shift.
124 ➤ The ACS Style Guide
➤ Use parentheses to identify the manufacturer of reagents and equipment.
cobalt chloride (Mallinckrodt)
a pH meter with a glass electrode (Corning)
➤ Do not use parentheses when citing a reference number in narrative text. In
such a case, the reference number is the point of the sentence, not subsidiary
information, and thus not parenthetical.
incorrect
in ref (12), in (12)
correct
in ref 12
➤ Use parentheses in mathematical expressions as discussed in Chapter 11 and
in chemical nomenclature and notation as discussed in Chapters 12 and 13.
Square Brackets
➤ Use square brackets within quotation marks to indicate material that is not
part of a direct quote.
In the words of Sir William Lawrence Bragg, “The important thing in science is
not so much to obtain new facts as to discover new ways [italics added] of think-
ing about them.”
➤ Use square brackets to indicate concentration: [Ca+].
➤ Use square brackets in mathematical expressions as discussed in Chapter 11
and in chemical nomenclature and notation as discussed in Chapters 12 and 13.
Dashes
The shortest dash is the hyphen (-); the en dash (–) is longer; and the em dash
(—) is the longest. Hyphens are discussed in the section on hyphenation in
Chapter 10, starting on p 135.
En Dash
➤ Use an en dash to mean the equivalent of “and”, “to”, or “versus” in multiword
concepts where the words are of equal weight.
acid–base titration
bromine–olefin complex
carbon–oxygen bond
cis–trans isomerization
cost–benefit analysis
dose–response relationship
ethanol–ether mixture
freeze–pump–thaw degassed
helix–coil transition
host–guest complexation
Chapter 9: Grammar, Punctuation, and Spelling ➤ 125
log–normal function
metal–ligand complex
metal–metal bonding
nickel–cadmium battery
oxidation–reduction potential
producer–user communication
pump–probe technique
red–black dichroic crystals
structure–activity relationship
structure–property relationship
temperature–time curve
vapor–liquid equilibrium
winter–fall maxima
0–t–∞ sequence
exception Use a hyphen for color combinations such as blue-green. See
Chapter 10, page 140.
➤ Use an en dash to mean “to” or “through” with a span of three or more
numerals or other types of ranges.
12–20 months Figures 1–4 5–50 kg
sections 1b–1f parts C–E compounds A–I
Lyon and co-workers (23–26) Lyon and co-workers23a–d
exception 1 When either one or both numbers are negative or include a
symbol that modifies the number, use the word “to” or “through”, not the en
dash.
–20 to +120 K –145 to –30 °C ≈50 to 60
10 to >600 mL <5 to 15 mg
exception 2 Do not use an en dash when the word “from” or “between” is
used.
from 500 to 600 mL (not from 500–600 mL)
between 7 and 10 days (not between 7–10 days)
➤ Use an en dash to link the names of two or more persons of equal impor-
tance used as a modifier.
Bednorz–Müller theory
Beer–Lambert law
Bose–Einstein statistics
Debye–Hückel theory
Diels–Alder reaction
Fermi–Dirac statistics
Fischer–Tropsch effect
Fisher–Johns hypothesis
Flory–Huggins interaction
Franck–Condon factor
Friedel–Crafts reaction
Geiger–Müller effect
Henderson–Hasselbalch equation
Jahn–Teller effect
Lee–Yang–Parr method
Lineweaver–Burk method
Mark–Houwink plot
Meerwein–Ponndorf theory
Michaelis–Menten kinetics
Stern–Volmer plot
van’t Hoff–Le Bel theory
Wolff–Kishner theory
Young–Laplace equation
Ziegler–Natta-type catalyst
Treatment of double surnames is covered in Chapter 10 (p 139).
126 ➤ The ACS Style Guide
➤ Use an en dash between components of a mixed solvent. (A slash can also be
used.)
The melting point was unchanged after four crystallizations from hexane–benzene.
Em Dash
➤ Use em dashes to set off words that would be misunderstood without them.
incorrect
All three experimental parameters, temperature, time, and concentration, were
strictly followed.
correct
All three experimental parameters—temperature, time, and concentration—were
strictly followed.
➤ Do not use em dashes to separate phrases or nonrestrictive clauses if another
form of punctuation can be used.
incorrect
Knauth—not Stevens—obtained good correlation of results and calculations.
correct
Knauth, not Stevens, obtained good correlation of results and calculations.
incorrect
The singly charged complexes—which constituted bands 1 and 3—liberated
maleate anion upon decomposition.
correct
The singly charged complexes, which constituted bands 1 and 3, liberated male-
ate anion upon decomposition.
Ellipsis Points
➤ Within a quotation, use three periods (points of ellipsis) to indicate deleted
words or phrases. These three periods are in addition to other needed punctua-
tion. Thus, if a period is already there, the result will be four periods.
No science is immune to the infection of politics and the corruption of
power…. The time has come to consider how we might bring about a
separation, as complete as possible, between Science and Government in
all countries.
—Jacob Bronowski
➤ Do not begin or end a quotation with ellipsis points.
Chapter 9: Grammar, Punctuation, and Spelling ➤ 127
➤ Use ellipsis points where part of a series is omitted, when the pattern of the
series is unambiguous.
a = 1, 2, 3, …
n = 2, 4, 6, …
x = 1, 3, 5, …, 15
Spelling
Consult a dictionary to resolve spelling questions. Merriam-Webster’s Collegiate
Dictionary and Webster’s New World College Dictionary are the desk dictionar-
ies used by the ACS technical editing staff. ACS staff members also use the
unabridged Webster’s Third New International Dictionary. However, whatever
your dictionary, choose the first spelling of a word. Use American spellings,
except in proper names and direct quotations (including titles). Appendix 9-1
contains a list of the recommended spellings for words that have two or more
acceptable spellings.
➤ For the correct spelling and styling of company names, search the Internet
for the company and look for the company name on its “contact” page. Do not
rely on either a company’s home page or the presence of a particular spelling in
a search engine, because the former may be informal and the latter common but
also incorrect.
Tricky Possessives
➤ Form the possessive of a joint owner by adding an apostrophe and an “s”
after the last name only.
Celapino and Marshall’s results
Bausch and Lomb’s equipment
➤ Form the possessive of plural nouns that do not end in “s” by adding an apos-
trophe and an “s”. Form the possessive of plural nouns that end in “s” by adding
an apostrophe only.
people’s rights
children’s books
compounds’ structures
➤ Form the possessive of a proper name ending in “s” by adding an apostrophe
and an “s”.
Jacobs’s laboratory
Mathers’s reception
128 ➤ The ACS Style Guide
Tricky Plurals
Sometimes, the plural form is so familiar that it is used erroneously instead of
the singular, usually with Latin and other non-English words. The following list
shows the correct singulars and plurals. The preferred forms are given first.
singular plural
alga algae
apparatus apparatus, apparatuses
appendix appendixes, appendices
bacterium bacteria
basis bases
criterion criteria, criterions
erratum errata
focus focuses, foci
formula formulas, formulae
fungus fungi, funguses
genus genera, genuses
helix helixes, helices
hypothesis hypotheses
index indexes
index indices (mathematical)
latex latices, latexes
locus loci
matrix matrices (mathematical)
matrix matrixes (media)
maximum maximums, maxima
medium media, mediums
minimum minimums, minima
phenomenon phenomena, phenomenons
polyhedron polyhedrons, polyhedra
sequela sequelae
spectrum spectra, spectrums
stratum strata
symposium symposia, symposiums
vertex vertexes, vertices
vortex vortexes, vortices
129
➤ ➤ ➤ ➤ ➤
APPENDIX 9-1
Recommended Spelling List
Many words in regular usage, as well as many technical terms, have two or more
acceptable spellings. The following list gives recommended spellings and capi-
talizations, where appropriate, for some terms not found in easily accessible dic-
tionaries, words often misspelled, common expressions, and words for which the
ACS preference may not match your dictionary’s.
absorbance
absorbency
absorbent
accommodate
acknowledgment
adsorbent
aerobic
aging
aglycon
air-dry (verb)
ambiguous
amine (RNH2)
ammine (NH3 complex)
amphiphile
ampule
analog (computer)
analogue (structural derivative)
analyte
analyze
annelation
annulation
antioxidant
appendixes
aqua regia
Arrhenius
artifact
asymmetry
audio frequency
autoxidation
auxiliary
Avogadro
bacitracin
back-bonding
back-donation
back-titrate (verb)
backscatter
backscattering
backward
band gap
bandwidth
baseline
Beckmann (thermometer, rearrangement)
Beer’s law
Beilstein
bit
black box
blackbody
blender
Boltzmann
borderline
Bragg scattering
break-seal
break up (verb)
breakup (noun)
bremsstrahlung
bridgehead
broad band (noun)
broad-band (adjective)
Brønsted
Büchner
build up (verb)
buildup (noun)
buret
butanol, 1-butanol (not n-butanol)
130 ➤ The ACS Style Guide
n-butyl alcohol
tert-butylation
byline
bypass
byproduct
byte
canceled
canister
cannot
Cartesian
catalog
chloramine
chloro amine
chlorophyll a
clean up (verb)
cleanup (noun)
clear-cut
close up (verb)
close-up (noun)
co-ion
co-occurrence
co-worker
coauthor
collinear
colorimetric
complexometric
concomitant
condensable
conductometric
conrotatory
constantan
coordination
Coulombic
counter electrode
counteranion
counterion
coverslip
cross-coupling
cross-link
cross over (verb)
cross-react
cross-reaction
cross section (noun)
cross-sectional (adjective)
crossover (noun, adjective)
cuboctahedron
cut off (verb)
cutoff (noun)
cuvette
cytochrome c
Darzens
database
deamino (not desamino)
deoxy (not desoxy)
dependent
desamine (amino acid names only)
desiccator
deuterioxide
deuteroporphyrin
Dewar benzene
Dewar flask
dialogue
diffractometer
disc (electrophoresis, compact disc)
discernible
disk (anatomy, computers)
disrotatory
dissymmetric
distill
dry ice
drybox
dyad
e-mail
ebullioscopic
eigenfunction
eigenvalue
electroless
electron microscope
electronvolt
electrooptic
electropositive
eluant
eluate
eluent
Elvehjem
end point
enzymatic
enzymic
Erlenmeyer (flask)
exchangeable
fall off (verb)
falloff (noun, adjective)
Chapter 9: Grammar, Punctuation, and Spelling ➤ 131
far-infrared
faradic (referring to current, not the
person)
fax (noun, verb, adjective)
feedback
fiber-optic (adjective)
fiber optics (noun)
filterable
firebrick
flavin
flow sheet
fluoborate
fluoramine
fluoro amine
focused
follow up (verb)
follow-up (noun, adjective)
forbear (verb, to refrain)
forebear (noun, ancestor)
forego (verb, to go before)
foreword (part of a book)
forgo (verb, to do without)
formulas
forward (direction)
freeze-dry (verb)
fulfill
γ ray
gauge
Gaussian
gegenion
glovebag
glovebox
Gouy
graduated cylinder
gram
Gram-negative
Gram-positive
gray
Grignard
groundwater
half-ester
half-life
half-width
halfway
Hamiltonian
Hantzsch
hazmat
heat-treat (verb)
hemoglobin
hemolysate
heterogeneous
Hoffmann degradation
homogeneous
homologue
Hunsdiecker
hydrindan
hydriodic
hydriodide
hydrolysate
hydrolyzed
ice-cold
ice–water bath (use en dash, see p 124)
inasmuch
indan
indexes (book parts, catalog)
indices (mathematical)
indispensable
inflection
infrared
innocuous
inoculate
insofar
inter-ring
intra-ring
iodometric
iodometry
isooctane
isopiestic
isopropyl alcohol (not isopropanol)
isosbestic
judgment
Karl Fischer
kayser
Kekulé
Kjeldahl
Kramers
Kugelrohr
labeled
laser
leukocyte
leveling
levorotatory
132 ➤ The ACS Style Guide
lifetime
ligancy
ligate
ligated
line shape
line width
liquefy
liter
lumiflavin
luster
lysate
lysed
make up (verb)
makeup (noun)
Markovnikov
matrices (mathematical)
matrixes (media)
megohm
Mendeleev
mesoporphyrin
metalate
metalation
metallization
metallize
metalloenzyme
meter
micro-Kjeldahl
mid-infrared
midpoint
minuscule
mixture melting point
monochromator
Mössbauer
naphthyl
near-ultraviolet
neopentyl
Nernstian
nuclide
occurred
occurrence
occurring
ortho ester
orthoformate
orthohydrogen
orthopositronium
outgas
outgassing
overall
parametrization
path length
percent
Petri
pharmacopeia
phenolphthalein
phlorin
phosphomonoester
phosphorous (as in phosphorous acid)
phosphorus (element)
phthalic
pipet
pipetted
plaster of Paris
point source
porphine
porphyrin
portland cement
programmed
2-propanol (not isopropanol)
pseudo-first-order
pyrolysate
quantitation
radio frequency
radioelement
radioiodine
radionuclide
re-form (to form again)
reform (to amend)
repellent
riboflavin
ring-expand (verb)
rotamer
scale up (verb)
scale-up (noun)
scavengeable
Schwarzkopf
seawater
self-consistent
selfsame
Sephadex
set up (verb)
setup (noun)
side arm (noun)
Chapter 9: Grammar, Punctuation, and Spelling ➤ 133
side chain
sideband
siphon
Soxhlet
spin-label (noun)
spin–lattice (use en dash, see p 124)
spin–orbit (use en dash, see p 124)
steam bath
steam-distill (verb)
stepwise
stereocenter
stereopair
stereoptically
Student’s t test or the Student t test
sulfolane
sulfur
superacid
superhigh frequency
supernatant (adjective)
supernate (noun)
syndet
synthase
synthetase
test tube
θ solvent
thiamin
thioacid
thioester
thioether
thioketone
toward
transmetalation
tropin
Ubbelohde
ultrahigh vacuum
un-ionized
uni-univalent
upfield
urethane
van der Waals
VandenHeuvel
van’t Hoff–Le Bel (use en dash, see p 124)
Vigreux
vis-à-vis
voltameter (measures voltaic electricity)
voltammeter (measures ranges of volts
and amperes)
voltmeter (measures cell potential)
wastewater
wave function
waveform
wavelength
wavenumber
well-known
work up (verb)
workup (noun)
X-irradiation
X-ray
ylide
zerovalent
zigzag
zinc blende
135
➤ ➤ ➤ ➤ ➤ CHAPTER 10
Editorial
Style
This chapter presents recommended stylistic and editorial
conventions, mainly but not solely for ACS publications.
The style recommended by ACS is, for the most part, taken from established
authoritative sources, such as The Chicago Manual of Style, Words into Type, and
the United States Government Printing Office Style Manual.
Other points of style are discussed in Chapter 11, “Numbers, Mathematics,
and Units of Measure”; Chapter 12, “Names and Numbers for Chemical Com-
pounds”; and Chapter 13, “Conventions in Chemistry”.
Hyphenation
Consult a dictionary to resolve hyphenation questions. Merriam-Webster’s Col-
legiate Dictionary and Webster’s New World College Dictionary are the desk dic-
tionaries used by the ACS technical editing staff. ACS staff also use the unabridged
Webster’s Third New International Dictionary.
Prefixes
➤ Most prefixes are not hyphenated. Do not hyphenate the following prefixes
when added to words that are not proper nouns.
after
ante
anti
auto
bi
bio
by
co
counter
cyber
de
di
down
electro
extra
hetero
homo
hyper
hypo
in
Copyright 2006 American Chemical Society
136 ➤ The ACS Style Guide
infra
inter
intra
intro
iso
macro
mega
meso
meta
metalla
metallo
micro
mid
mini
mis
mono
multi
nano
neo
non
over
peri
photo
physico
poly
post
pre
pro
pseudo
re
retro
semi
stereo
sub
super
supra
techno
tele
thermo
trans
tri
ultra
un
under
uni
up
video
visco
examples
antibacterial
cooperation
cyberspace
extranuclear
interelectrode
isospin
microorganism
multicolored
nonpolar
photoredox
precooled
pseudomorph
superacid
transactinide
viscoelastic
exceptions Hyphens are sometimes used (1) when letters are doubled, (2)
when more than one prefix is present, or (3) when the unhyphenated form
does not convey the intended meaning.
anti-infective
anti-inflammatory
bi-univalent
co-ion
co-worker
inter-ring
intra-ring
mid-infrared
non-native
non-nuclear
post-reorganization
post-translational
pre-equilibrium
sub-bandwidth
un-ionize
➤ Some prefixes may be hyphenated or not, depending on meaning.
recollect or re-collect
recover or re-cover
reform or re-form
retreat or re-treat
rare exceptions
autoxidation
counter electrode
hetero group
homo nucleoside
➤ Do not hyphenate multiplying prefixes.
hemi, mono, di, tri, tetra, penta, hexa, hepta, octa, ennea, nona, deca, deka,
undeca, dodeca, etc.
semi, uni, sesqui, bi, ter, quadri, quater, quinque, sexi, septi, octi, novi, deci, etc.
bis, tris, tetrakis, pentakis, hexakis, heptakis, octakis, nonakis, decakis, etc.
Chapter 10: Editorial Style ➤ 137
examples
2,2′-bipyridine
1,4-bis(3-bromo-1-oxopropyl)piperazine
divalent
hemihydrate
heptacoordinate tetrahedron
hexachlorobenzene
1,1′:3′,1″:3″,1′′′-quaterphenyl
tetrakis(hydroxymethyl)methane
triatomic
triethyl phosphate
tris(ethylenediamine)cadmium dihydroxide
➤ Hyphenate a prefix to a two-word compound.
multi-million-dollar lawsuit
non-diffusion-controlled system
non-English-speaking colleagues
non-radiation-caused effects
non-tumor-bearing organ
pre-steady-state condition
pseudo-first-order reaction
➤ Hyphenate prefixes to chemical terms.
non-alkane
non-phenyl atoms
➤ Hyphenate a prefix to a numeral.
pre-1900s
➤ Hyphenate prefixes to proper nouns and adjectives, and retain the capital letter.
anti-Markovnikov
non-Coulombic
non-Gaussian
non-Newtonian
oxy-Cope
Suffixes
➤ Most suffixes are not hyphenated. Do not hyphenate the following suffixes
when added to words that are not proper nouns.
able
fold
ful
less
like
ment
ship
wide
wise
138 ➤ The ACS Style Guide
examples
clockwise
fellowship
lifelike
multifold
rodlike
spoonful
statewide
worldwide
exceptions
bell-like
gel-like
shell-like
➤ Hyphenate the suffixes “like” and “wide” when they are added to words of
three or more syllables.
bacteria-like
computer-like
radical-like
resonance-like
university-wide
➤ Hyphenate the suffix “like” in two-word compounds used as unit modifiers.
first-order-like
ion-exchange-like
rare-earth-like
transition-metal-like
➤ Hyphenate the suffix “like” to chemical names.
adamantane-like
cycloalkane-like
morphine-like
olefin-like
➤ Hyphenate a numeral and a suffix.
10-fold
25-fold
➤ Hyphenate suffixes to proper nouns, and retain the capital letter.
Asia-wide
Claisen-like
Kennedy-like
Michaelis–Menten-like
Compound Words
Compound words are two or more terms used to express a single idea. Com-
pound words in common usage are listed in most dictionaries. Many are hyphen-
ated, but many are not.
back-reaction
cross hairs
cross-link
crosshatch
half-life
self-consistent
Chapter 10: Editorial Style ➤ 139
➤ Hyphenate spelled-out fractions.
one-half
one-ninth
three-fourths
two-thirds
➤ Hyphenate two-word verbs.
air-dry
flame-seal
freeze-dry
jump-start
ring-expand
vacuum-dry
➤ Do not hyphenate phrasal verbs. As unit modifiers or nouns, these words are
often hyphenated or closed up; check a dictionary.
break down
build up
grow up
hand out
line up
mix up
scale up
set off
set up
slow down
stand by
take off
warm up
wear out
➤ Do not hyphenate foreign phrases used as unit modifiers.
ab initio calculation
ad hoc committee
in situ evaluation
in vivo reactions
exception Some foreign phrases are hyphenated in the original language,
for example, laissez-faire.
➤ People who have double surnames may choose to hyphenate them or use a
space between them. When they are hyphenated, use a hyphen, not an en dash,
between the two surnames in a person’s name. Some combinations of two given
names are also hyphenated.
Robert Baden-Powell
David Ben-Gurion
Cecil Day-Lewis
Chen-Chou Fu
Joseph-Louis Gay-Lussac
Irene Joliot-Curie
Jackie Joyner-Kersee
John Edward Lennard-Jones
Unit Modifiers
Unit modifiers are two words that together describe a noun; they are almost
always hyphenated. Most unit modifiers consist of
• a noun and an adjective (e.g., time-dependent reaction, radiation-sensi-
tive compound, water-soluble polymer, halogen-free oscillator);
• an adjective and a noun (e.g., high-frequency transition, small-volume
method, first-order reaction, outer-sphere redox couple);
140 ➤ The ACS Style Guide
• an adjective and a participle (e.g., slow-growing tree, broad-based sup-
port, far-reaching influence);
• a noun and a participle (e.g., time-consuming project, earth-shaking
news, silver-coated electrode);
• an adverb and an adjective (e.g., above-average results, still-unproven
technique); or
• two nouns (e.g., ion-exchange resin, liquid-crystal polymers, transition-
state modeling, charge-transfer reaction, gas-phase hydrolysis).
The following is a short list (by no means complete) of unit modifiers commonly
seen in ACS publications. These should be hyphenated when modifying a noun.
air-dried
air-equilibrated
back-bonding
14C-labeled
charge-transfer
cost-effective
diffusion-controlled
double-bond
electron-diffraction
electron-transfer
energy-transfer
excited-state
first-order
flame-ionization
fluorescence-quenching
free-energy
free-radical
gas-phase
gel-filtration
Gram-negative
Gram-positive
halogen-free
high-energy
high-frequency
high-performance
high-pressure
high-resolution
high-temperature
inner-sphere
ion-exchange
ion-promoted
ion-selective
large-volume
laser-induced
least-squares
light-catalyzed
long-chain
long-lived
low-energy
low-frequency
low-pressure
low-resolution
low-temperature
moisture-sensitive
nearest-neighbor
oil-soluble
outer-sphere
radiation-caused
radiation-produced
radiation-sensitive
rate-limiting
reversed-phase
room-temperature
round-bottom
rubber-lined
second-harmonic
second-order
short-chain
side-chain
size-dependent
small-volume
solid-phase
solid-state
species-specific
steady-state
structure-specific
temperature-dependent
thin-layer
three-dimensional
three-phase
time-dependent
transition-metal
transition-state
two-dimensional
vapor-phase
water-soluble
weak-field
wild-type
exceptions
particle size distribution
water gas shift
➤ Hyphenate combinations of color terms used as unit modifiers.
blue-green solution
bluish-purple solid
red-black precipitate
silver-gray body
Chapter 10: Editorial Style ➤ 141
➤ Do not hyphenate unit modifiers if the first word is an adverb ending in “ly”.
accurately measured values
carefully planned experiment
poorly written report
recently developed procedure
➤ Hyphenate unit modifiers containing the adverbs “well”, “still”, “ever”, “ill”,
and “little”.
ever-present danger
ever-rising costs
ill-fitting stopper
little-known hypothesis
still-new equipment
well-known scientist
well-trained assistants
exception Do not hyphenate unit modifiers containing the adverbs “well”,
“still”, “ever”, “ill”, and “little” if they are modified by another adverb.
most ill advised investment
very high density lipoprotein
very well studied hypothesis
➤ Hyphenate unit modifiers containing a comparative or superlative if the
meaning could be different without the hyphen.
best-known processes
best-loved advisor
higher-temperature values
least-squares analysis
lowest-frequency wavelengths
nearest-neighbor interaction
➤ Do not hyphenate a number and a unit of time or measure used as a unit
modifier.
1.2 × 10–4 cm–1 peak
25 K increments
10 mg sample
a 0.1 mol dm–3 solution
20 mL aliquot
12˚ angle
➤ When two or more unit modifiers with the same ending base modify one
noun, use a hyphen after each element, and do not repeat the ending base.
first- and second-order reactions
high-, medium-, and low-frequency measurements
➤ Do not hyphenate unit modifiers that are chemical names.
acetic anhydride concentration
amino acid level
barium sulfate precipitate
sodium hydroxide solution
142 ➤ The ACS Style Guide
➤ Hyphenate unit modifiers made up of a single letter or number and a noun
or adjective.
α-helix structure
13C-enriched proteins
14C-labeling patterns
d-configuration settings
γ-ray spectrometer
1-isomer profile
O-ring suppliers
π-electron system
3-position substitution
s-orbital diagrams
t-test analysis
U-band transmitter
x-axis labels
X-band radar
➤ Do not hyphenate unit modifiers if one of the words is a proper name.
Fourier transform technique
Lewis acid catalysis
Schiff base measurement
➤ Hyphenate unit modifiers that contain spelled-out numbers.
five-coordinate complex
one-electron transfer
seven-membered ring
three-dimensional model
three-neck flask
three-stage sampler
two-compartment model
two-phase system
➤ Hyphenate unit modifiers that contain a present or past participle.
air-equilibrated samples
English-speaking colleagues
fluorescence-quenching solution
hydrogen-bonding group
immobilized-phase method
ion-promoted reaction
laser-induced species
methyl-substituted intermediate
photon-induced conversion
rate-limiting step
research-related discussion
steam-distilled sample
caution Watch for cases where the participle forms a unit with the noun
that follows: for example, “ligand binding site” should not be hyphenated.
➤ Hyphenate unit modifiers of three or more words.
head-to-head placement
high-molecular-weight compound
nine-membered-ring species
out-of-plane distance
root-mean-square analysis
signal-to-noise ratio
tried-and-true approach
voltage-to-frequency converter
➤ Hyphenate unit modifiers containing three words when similar two-word
modifiers are hyphenated.
acid-catalyzed reaction
general-acid-catalyzed reaction
metal-promoted reaction
transition-metal-promoted reaction
Chapter 10: Editorial Style ➤ 143
exception Do not hyphenate unit modifiers containing three or more
words, even if similar two-word modifiers are hyphenated, when doing so
would break other rules. For example, do not hyphenate unit modifiers if one
of the words is a proper name. Do not hyphenate unit modifiers that are two-
word chemical names.
acid-catalyzed reactions (but Lewis acid catalyzed reactions)
copper-to-iron ratio (but sodium chloride to iron ratio)
➤ Hyphenate unit modifiers used as predicate adjectives. (Predicate adjectives
are usually used with the verb “to be”; they are adjectives that modify the subject
but come after the verb.) Usually, only unit modifiers that consist of nouns and
adjectives or nouns and participles can be used as predicate adjectives.
All compounds were light-sensitive and were stored in the dark.
In these cluster reactions, dehydrogenation is size-dependent.
The antibody is species-specific.
The complex is square-planar.
The movie was thought-provoking.
The reaction is first-order.
➤ Hyphenate phrases also containing en dashes (see pp 124–126) when they are
used as unit modifiers.
alkyl–heavy-metal complexes
high-spin–low-spin transition
metal–metal-bonded complexes
Michaelis–Menten-like kinetics
retro-Diels–Alder reaction
transition-metal–chalcogen complexes
➤ Hyphenate phrases containing parenthetical expressions when they are used
as unit modifiers.
element (silicon or tin)-centered radicals
Capitalization
In Text
Generally, in text keep all words lowercase, including chemical names and terms,
except proper nouns and adjectives. However, there are many exceptions.
➤ Capitalize the words “figure”, “table”, “chart”, and “scheme” only when they
refer to a specific numbered item.
Chart 4
Figure 1
Schemes 4–7
Table II
144 ➤ The ACS Style Guide
➤ Do not capitalize the “r” in “X-ray” at the beginning of a sentence or in a title.
➤ Capitalize parts of a book when they refer to a specific titled and numbered
part.
Appendix I
Chapter 3
Section 4.2
But
the appendix
the chapter
the contents
the preface
➤ Capitalize only the name of an eponym, not the accompanying noun.
Avogadro’s number
Boltzmann constant
Einstein’s theory
Graham’s law
Hodgkin’s disease
Lewis acid
nuclear Overhauser effects
Raman spectroscopy
Schiff base
exceptions
Nobel Peace Prize
Nobel Prize
➤ Capitalize adjectives formed from proper names.
Boolean
Cartesian
Copernican
Coulombic
Darwinian
Einsteinian
Freudian
Gaussian
Hamiltonian
Laplacian
Lorentzian
Mendelian
Newtonian
➤ Capitalize the first word after a colon if the colon introduces more than one
complete sentence, a quotation, or a formal statement.
Chemists find enzymes attractive as potentially useful synthetic tools for many
reasons: Enzymes catalyze reactions with high regio- and stereoselectivity. They
cause tremendous rate accelerations under mild reaction conditions. They reduce
the need for protecting groups and give enantiomerically pure products.
An emulsion is a thermodynamically unstable system: it has a tendency to sepa-
rate into two phases.
Two types of asymmetric reactions were conducted: synthesis of styrene oxide
and reduction of olefinic ketones.
The editor wishes to make the following point: No papers will receive preferential
treatment on the basis of artwork.
Chapter 10: Editorial Style ➤ 145
➤ Do not capitalize lowercase chemical descriptors hyphenated to chemical
names when they are at the beginning of a sentence.
cis-4-Chloro-3-buten-2-one was obtained in 74% yield.
o-Dichlorobenzene was the solvent.
➤ When the first word of a sentence is a roman chemical descriptor that is not
part of a chemical name, capitalize it.
Cis and trans isomers are used in pharmaceuticals and agrochemicals.
Erythro diols were obtained in good yield.
Syn hydroxylation of cycloalkenes was attempted.
Trans hydroxyl groups are oxidized biochemically.
➤ Do not capitalize chemical names or nonproprietary drug names unless they
are at the beginning of a sentence or are in a title or heading. In such cases, capi-
talize the first letter of the English word, not the locant, stereoisomer descriptor,
or positional prefix. (See Chapter 12, “Names and Numbers for Chemical Com-
pounds”.)
➤ Some reaction names are preceded by element symbols; they may be used as
nouns or adjectives. When they are the first word of a sentence or appear in titles
and headings, the first letter of the word is capitalized.
N-Oxidation of the starting compounds yielded compounds 3–10.
N-Benzoylated amines undergo hydroxylation when incubated with yeast.
Preparation of S-Methylated Derivatives
O-Substituted Structural and Functional Analogues
➤ When a sentence begins with a symbol that is not hyphenated to the follow-
ing word, the word is not capitalized.
π-Electron contributions are evident.
π electrons make significant contributions in this system.
σ values were calculated from eq 3.
➤ Always capitalize kingdom, phylum, class, order, family, and genus taxonomic
names, as well as names of cultivars. Subclassifications follow the same presenta-
tion as the main category.
Animalia, Planta (kingdom)
Chordata (phylum)
Vertebrata (subphylum)
Mammalia, Reptilia (class)
Primates, Testudines (order)
146 ➤ The ACS Style Guide
Hominidae, Apiaceae (family)
Homo, Drosophila (genus)
Lycopersicon esculentum Mill. cv. Jennita (cultivar)
➤ Use lowercase for species, subspecies, and varieties, even in titles.
Escherichia coli
Achromobacter haemolyticus subsp. alcaligenes
Zea mays var. rugosa (variety)
Three New Dihydroisocoumarins from the Greek Endemic Species Scorzonera
cretica
➤ Do not capitalize the abbreviation for species, singular or plural (sp. or spp.,
respectively), subspecies (subsp.), variety (var.), or cultivar (cv.).
Salmonella sp.
Polygonum spp.
Petroselinum crispum Mill. subsp. tuberosum
Zea mays var. rugosa
Lycopersicon esculentum Mill. cv. Jennita
➤ Do not capitalize genus names used as common nouns except at the begin-
ning of a sentence or in a title or heading.
bacillus
gorilla, a member of the genus Gorilla
hippopotamus, a member of the genus Hippopotamus
klebsiella
pseudomonad, a member of the genus Pseudomonas
streptococcus
➤ Do not capitalize the adjectival or plural form of a genus name unless it is at
the beginning of a sentence or in a title or heading.
bacilli
pneumococcal
streptococcal
➤ In text, do not capitalize polymer names that contain the names of the
polymerizing species in parentheses following the prefix “poly”. At the beginning
of a sentence, capitalize only the “P” in “poly”.
Poly(vinyl chloride) is a less useful polymer than poly(ethylene glycol).
➤ Capitalize trademarks; use them as adjectives with the appropriate nouns.
Ficoll
Novocain (but novocaine)
Plexiglas (but plexiglass)
Pyrex
Sephadex
Styrofoam
Teflon
Triton
Tween
Chapter 10: Editorial Style ➤ 147
➤ Do not capitalize the word “model” with a number or code.
γ counter (Beckman model 5500B)
mass spectrometer (PerkinElmer model 240C)
multichannel spectrometer (Otsuka model MCPD-1000)
spectrometer (Varian model XL-200)
Waters model 660 gradient controller
➤ Do not capitalize the common names of equipment.
dynamic mechanical analyzer
electron-diffraction chamber
flame-ionization detector
gas chromatograph
mass spectrometer
mercury lamp
spectrophotometer
temperature controller unit
➤ Use only an initial capital letter, not all capitals, for company names that are
not acronyms, because company names are not trademarks and are not protected
by law.
Valspar Corporation
Xerox Corporation
But
EMD Chemicals Inc.
IBM Corporation
➤ Capitalize the names of specific organizations or entities, including ACS local
sections, committees, and governing bodies, but not the general terms for them.
ACS Board of Directors the board
ACS Committee on Analytical Reagents an ACS committee
ACS Division of Fuel Chemistry the division
American Chemical Society the society
Clean Water Act the act
Environmental Protection Agency the agency
the Milwaukee Section a local section
University of Michigan the university
➤ Capitalize the names of specific titles when they appear with a person’s name,
but not the general terms for them.
the professor
the general
the mayor
Professor Perry Key
General James Shore
Mayor Ralph Estes
Walter Baldwin, Professor of Chemistry
148 ➤ The ACS Style Guide
The well-known professor Perry Key will give a tutorial.
James Shore, a general in the U.S. Army, will teach a graduate course.
Our speaker will be the retired general James Shore.
Isaac Bickford is an assistant professor.
Ralph Estes is the mayor of a small town in upstate New York.
➤ Capitalize the names of special events but not the general terms for them.
229th ACS National Meeting
39th ACS Western Regional Meeting
79th ACS Colloid and Surface Science Symposium
the regional meeting
the spring national meeting
the symposium
➤ Capitalize sections of the country but not the corresponding adjectives.
the Midwest, but midwestern
the Northeast, but northeastern
➤ Do not capitalize the names of the four seasons: summer, fall, autumn, win-
ter, spring.
➤ Capitalize Earth, Sun, and Moon only when used in an astronomical sense.
Venus and Mars are the closest planets to Earth.
The Earth rotates on its axis and revolves around the Sun.
The Moon is the only body that orbits the Earth.
But
Water bodies on the earth’s surface contain a variety of chromophoric sub-
stances.
Pollution occurs to some extent everywhere on earth.
The sun is the primary source of radiation that can cause chemical transfor-
mations.
The next full moon will be on Thursday.
In Titles and Headings
These guidelines apply to titles and headings at all levels; that is, they apply to
subtitles and subheadings. They also apply to table, scheme, and chart titles.
➤ In titles and headings that are typeset in capital and lowercase letters, capital-
ize the main words, which are nouns, pronouns, verbs, adjectives, adverbs, and
Chapter 10: Editorial Style ➤ 149
subordinating conjunctions, regardless of the number of letters. Do not capital-
ize coordinating conjunctions (“and”, “but”, “or”, “nor”, “yet”, “so”), articles (“a”,
“an”, “the”), or prepositions. Do capitalize the “to” in infinitives. Do capitalize the
first and last words of a title or heading, regardless of part of speech, unless the
word is mandated to be lowercase (e.g., pH, d Orbital).
Changes in the Electronic Properties of a Molecule When It Is Wired into a Circuit
Derivatives from a Chiral Borane–Amine Adduct
In Situ Nutrient Analyzer
In Vitro and in Vivo Antiestrogenic Effects of Polycyclic Musks in Zebrafish
Nickel-Catalyzed Addition of Grignard Reagents: Ring-Opening Reactions with
Nucleophiles
Phosphonolipids with and without Purified Hydrophobic Lung Surfactant
Proteins
Properties of Organometallic Fragments in the Gas Phase
Reactions of Catalyst Precursors with Hydrogen and Deuterium
Scope of the Investigations: The First Phase
The Computer as a Tool To Improve Chemistry Teaching
Vibrations in Situ
exception 1 In titles and headings, capitalize small words that are parts of
phrasal verbs.
Break Down
Build Up
Set Off
Set Up
Slow Down
Wear Out
exception 2 In titles and headings, capitalize small words that are parts of
phrasal adjectives.
End-On Bonding
In-Plane Atoms
Side-On Bonding
(but Out-of-Plane Vibrations [only the first preposition is capitalized])
➤ In titles and headings, capitalize “as” when it is used as a subordinating con-
junction but not when it is used as a preposition.
Alumina as a Catalyst Support
Kinetics of Cyanocobalamin As Determined by Binding Capacity
➤ Do not capitalize the “r” in “X-ray” in titles and headings. Do capitalize the
“r” in “γ ray” and the “p” in “α particle” and “β particle” in titles and headings.
150 ➤ The ACS Style Guide
➤ Do not capitalize lowercase chemical descriptors in titles and headings, but
do capitalize the first letter of the English word.
Reaction of trans-4-(Phenylsulfonyl)-3-buten-2-one
➤ When abbreviated units are acceptable in titles and headings, do not cap-
italize those that are ordinarily lowercase.
Analysis of Milligram Amounts
Determination of N-Nitrosodimethylamine at Concentrations <7 ng/L
➤ Always capitalize genus names, but never capitalize species names, in titles
and headings.
Active-Site Nucleophile of Bacillus circulans Xylanase
Novel Metabolites of Siphonaria pectinata
➤ In titles and headings, capitalize all main words in a unit modifier.
Base-Catalyzed Cyclization
Cross-Linked Polymer
Deuterium-Labeling Experiment
High-Temperature System
Non-Hydrogen-Bonding Molecules
Thyrotropin-Releasing Hormone
➤ In titles and headings, capitalize each component of compound words if the
component would be capitalized when standing alone.
Cross-Link
Half-Life
Quasi-Elastic
➤ Do not capitalize hyphenated suffixes.
Synthesis of Cubane-like Clusters
➤ In titles and headings, capitalize only the first letter (“P”) of polymer names
that contain the names of the polymerizing species in parentheses following the
prefix “poly”.
IR Spectroscopic Analysis of Poly(1H,1H-fluoroalkyl α-fluoroacrylate)
Light-Scattering Studies of Poly(ethylene-co-butylene)
New Uses for Poly(ethylene terephthalate)
Polystyrene-block-poly(2-cinnamoylethyl methacrylate) Adsorption
Reactions of Poly(methyl methacrylate)
Synthesis and Characterization of Poly(isobutylene-b-methyl vinyl ether)
Chapter 10: Editorial Style ➤ 151
➤ Capitalize only the first letter in a chemical name containing complex sub-
stituents in parentheses or brackets.
2-[3-[2-[(2S)-2-Cyano-1-pyrrolidinyl]-2-oxoethylamino]-3-methyl-1-oxobutyl]-
1,2,3,4-tetrahydroisoquinoline: A Potent, Selective, and Orally Bioavailable Dipep-
tide-Derived Inhibitor of Dipeptidyl Peptidase IV
Preparations of (Methyl isocyanide)iron Compounds
Structures of Tetrakis(methyl isocyanide)iron Complexes
➤ Capitalize parenthetical phrases in titles and headings as if they were not par-
enthetical.
Versatile Organic (Fullerene)–Inorganic (CdTe Nanoparticle) Nanoensembles
Surnames
Capitalization
Although a current trend is to lowercase the surnames of persons when these
names are used as modifiers and have become very familiar, many are still capi-
talized. The following is a list (by no means complete) of names that should be
capitalized.
Avogadro
Beckmann
Beilstein
Boltzmann
Bragg
Brønsted
Büchner
Bunsen
Claisen
Dewar benzene, flask
Dreiding
Erlenmeyer
Gram
Kekulé
Kjeldahl
Mahalanobis
Markovnikov
Mössbauer
Petri
Poiseuille
Poisson
Priestley
Scatchard
VandenHeuvel
exceptions
de Broglie
van der Waals
van’t Hoff
➤ Surnames that are used as units of measure are lowercase.
ampere
angstrom
coulomb
curie
dalton
darcy
debye
einstein
faraday
gauss
gilbert
gray
hartree
henry
hertz
joule
kelvin
langmuir
newton
ohm
pascal
poise
siemens
sievert
stokes
tesla
watt
weber
152 ➤ The ACS Style Guide
In the temperature–current curves, temperature is given in kelvins and current is
shown in amperes.
NMR coupling constants are reported in hertz.
Hyphenation
➤ Hyphenation of double surnames is discussed on p 139.
➤ Hyphenate prefixes and suffixes to proper names as nouns and adjectives,
and retain the capital letter.
anti-Markovnikov
hetero-Diels–Alder
Kennedy-like
Michaelis–Menten-like
non-Coulombic
non-Gaussian
non-Newtonian
oxy-Cope
retro-Diels–Alder
Foreign Surnames
Some foreign surnames follow a format different from the American system. The
Chinese use their surnames first, followed by their given names. For example,
Sun Yat-sen’s surname is Sun. However, the problem of identifying surnames
extends to many other cultures. This multiplicity of usage can create problems
in bibliographic indexes and in reference citations. A reference citation in a bib-
liography should always list the surname first, followed by given name or initials.
In a byline, the author names should be presented in standard American format
(given names first and surnames last) to ensure consistency of citation practice.
If a footnote would clarify the situation or eliminate any perceived confusion,
use a footnote.
In most cultures, the surname is the family name, but it may not be the for-
mal name, that is, the name or shortest string of names that are properly used
following a title (Mr., Dr., Professor, etc.). Presented here are some cases in which
different customs are used for the order of surnames, given names, and formal
names. This list is by no means complete, but at least it will help you to be aware
of these differences.
arabic Often many names; the position of the surname is highly variable.
The formal name often consists of two or three names, including articles that
can be joined. Examples: Ibn Saud, Abd al-Qadir.
chinese Two or three names; the surname is first. Examples: Chiang Kai-
shek is Dr. Chiang; Chou En-lai is Dr. Chou.
hebrew Two or three names; the surname is the last one or two and is the
formal name. Examples: David Ben-Gurion is Dr. Ben-Gurion; Moshe Bar-
Even is Dr. Bar-Even.
Chapter 10: Editorial Style ➤ 153
hungarian Two names; the surname is first, and it is the formal name.
However, the second name is accepted as formal internationally.
japanese Two names; the surname is the formal name. The surname is first
in Japanese. However, when the names are translated into non-Asian lan-
guages, surnames appear last. Example: Taro Yamada is Dr. Yamada.
korean Usually three names; the surname is first and is the formal name. In
North Korean names, all three parts start with a capital letter. Examples: Kim
Il Sung is Dr. Kim. In South Korean names, the two parts of the given name
are hyphenated, and the second part is lowercase. Example: Kim Young-sum
is Dr. Kim.
spanish Frequently three or more names; the last two are surnames, some-
times connected by “y”. The second surname is often dropped or abbrevi-
ated. The formal name begins with the first surname and includes the sec-
ond surname only in very formal usage. Example: Juan Perez Avelar is Dr.
Perez or Dr. Perez Avelar, but never Dr. Avelar. The two surnames may also
be hyphenated. Example: José Gregorio Angulo-Vivas is Dr. Angulo or Dr.
Angulo-Vivas, but never Dr. Vivas.
thai Two names; the surname is last, but the formal name is first.
vietnamese Two or three names; the first is the surname and formal name.
initials Some foreign names are abbreviated with two-letter initials that
reflect transliteration from a non-Latin alphabet: Ch., Kh., Ph., Sh., Th., Ts.,
Ya., Ye., Yu., and Zh.
Some foreign names are abbreviated with hyphenated initials: C.-C. Yu.
Special Typefaces
Special typefaces help the reader quickly distinguish certain letters, words, or
phrases from the rest of the text.
Italic Type
Chapter 11 describes the use of italic type in mathematical material, and Chap-
ters 12 and 13 give guidelines for the use of italic type in chemical names and
conventions in chemistry.
➤ Use italic type sparingly to emphasize a word or phrase. Do not use italics for
long passages.
154 ➤ The ACS Style Guide
➤ Use italic type for a word being defined or for a newly introduced term the
first time it appears in text.
In an outer-sphere transfer, an electron moves from reductant to oxidant with no
chemical alteration of the primary coordination spheres.
We call these materials microcapsules.
➤ Use italic type for the titles and abbreviations of periodicals, books, and
newspapers. If “the” is the first word of the title, italicize and capitalize it.
An article on a promising cholesterol biosynthesis inhibitor appeared in The
Journal of Organic Chemistry this month.
Enough for One Lifetime is the biography of Wallace Carothers.
I read three articles on that new chiral compound in the Journal of the American
Chemical Society.
The Washington Post did a feature story on the president’s daughter.
➤ Do not use italic type for common Latin terms and abbreviations.
a priori
ab initio
ad hoc
ca.
de novo
e.g.
et al.
etc.
i.e.
in situ
in vitro
in vivo
status quo
vs
➤ Use italic type for genus, species, subspecies, and variety names of all ani-
mals, plants, and microorganisms, but not when these names are used as singu-
lar or plural common nouns or when they are adjectival.
a bacterium of the genus Salmonella
Bacillus coagulans and Bacillus dysenteriae are two species of bacilli.
Staphylococcus aureus is the bacterium that causes staphylococcal infection.
Streptococcus pneumoniae (genus and species)
The red rhododendron, Rhododendron arboreum, needs bright sun.
Achromobacter haemolyticus subsp. alcaligenes (subspecies)
Zea mays var. rugosa (variety)
➤ Use italic type for genotypes (representations of genes) and roman type for
phenotypes (representations of proteins).
The vanA gene that encodes one such inducible resistance protein is designated
VanA.
E. coli DH5α ∆(lacZYA–argF)U169 deoR recA1 endA1 hsdR17(rK+, mK+) supE44
λ– thi-1 gyrA96 relA1 F′ proAB+ lac1qZ ∆ M15 ssf::Tn5 [Kmr]
Chapter 10: Editorial Style ➤ 155
➤ Names of restriction endonucleases should follow the typeface conventions
of the names from which they were derived: use italic type for the three-letter
portion derived from the genus and species name; use roman type for additional
strain designators (letters and/or arabic numerals) and for the roman numeral
identifiers.
BamHI
EcoRI
HaeII
HaeIII
HindIII
Sau3AI
SmaI
XhoI
exception Use roman type for abbreviations of general enzyme types.
Exo III for exonuclease III
Pol α for DNA polymerase α
Topo II for topoisomerase II
➤ Do not use italic type for “pH”; “p” is always lowercase, and “H” is always
capitalized.
➤ Do not use italic type for M (molar) or N (normal). Do use italic type for m
(molal).
Greek Letters
➤ Use Greek letters, not the spelled-out words, for chemical and physical terms.
Do not italicize Greek letters.
α helix (not alpha helix)
β particle (not beta particle)
β sheet (not beta sheet)
γ radiation (not gamma radiation)
NFκB (nuclear factor κB) (not NF kappa B)
exceptions
delta opioid receptor
mu opioid receptor
Computer-Related Usage
➤ Capitalize the first letter of the names of computer languages.
AP
Basic
Cobol
Eiffel
Fortran
Java
Logo
Pascal
Perl
Python
Smalltalk
156 ➤ The ACS Style Guide
➤ Capitalize the first letter of the names of programs, and follow the man-
ufacturer’s or creator’s usage within the name.
Acrobat
Alchemy
ChemDraw
ChemIntosh
ChemPlus
EasyPlot
EndNote
FileMaker Pro
Freehand
HyperChem
ISIS/Draw
KaleidaGraph
LaTeX
Mathematica
MathType
Microsoft Excel
Microsoft PowerPoint
Microsoft Word
Molecular Presentation Graphics
MULTAN78
Oracle
Photoshop
ProCite
SigmaPlot
SIMI4A
Symphony
TK Solver
Un-Plot-It
UniVersions
WordPerfect
➤ Use lowercase letters for the spelled-out forms of protocols, except as the first
word of a sentence and in titles and headings.
network news-transfer protocol (NNTP)
Appendix 10-1 contains a list of some common computer and Internet terms.
Uniform Resource Locators
A typical uniform resource locator (URL), which is an address on the World
Wide Web, takes the following forms:
http://www.domain.zone
http://www.domain.zone/name1/~name2/
http://domain.zone/name1/~name2/name3.html
The number of names varies. For example,
http://www.chemistry.org
http://nobelprize.org/chemistry/index.html
These examples are short, but URLs can be quite long, and in narrative text they
often will need to be broken at the end of a line. If the URL does not fit on one
line, it can be broken according to the following guidelines:
➤ Break after an ampersand, a slash, or a period, but keep two slashes together.
➤ Do not add a hyphen to the end of the line.
➤ Do not break after a hyphen to avoid confusion as to the hyphen’s purpose.
Chapter 10: Editorial Style ➤ 157
E-Mail Addresses
A typical e-mail address usually takes one of these forms:
personname@companyname.zone
initial_surname@companyname.zone
surnameinitial@companyname.zone
All kinds of variations on the person’s name and initials are possible, and besides the
underscore, other types of punctuation are used. Long names are often truncated.
➤ Break e-mail addresses in text after the @ or a period. Do not insert a hyphen
or any other character.
Chapter 14, “References”, presents the editorial style for electronic sources listed
in reference lists and bibliographies.
Trademarks
A trademark is an adjective that describes a material or product (e.g., Teflon
resin, Kleenex tissue). The term “brand name” is a synonym for trademark. In
ACS publications, do not use the trademark symbols ™ and ® or the service mark
symbol SM. They are not necessary to ensure legal protection for the trademark.
➤ Capitalize trademarks; use them as adjectives with the appropriate nouns. Do
not use them in titles.
Ficoll
Novocain (but novocaine)
Plexiglas (but plexiglass)
Pyrex
Sephadex
Styrofoam
Teflon
Triton
Tween
➤ In general, however, use generic names rather than trade names.
cross-linked dextran polymer beads (not Sephadex)
diatomaceous earth (not Celite)
4,4′-isopropylidenediphenol (not Bisphenol A)
2-methoxyethanol (not Methyl Cellosolve)
mineral oil (not Nujol)
paclitaxel (not Taxol)
petroleum jelly (not Vaseline)
photocopy (not Xerox)
poly(ethylene glycol) (not Carbowax)
tensile testing machine (not Instron tester)
➤ Use trademarks as adjectives only, never as nouns or verbs. Because trade-
marks are adjectives, they do not have plural forms.
158 ➤ The ACS Style Guide
Abbreviations and Acronyms
An abbreviation is a short form of a word; often the individual letters are pro-
nounced. In an acronym, the letters always form a pronounceable word. ACS is
an abbreviation; CASSI is an acronym.
A list of ACS-recommended abbreviations, acronyms, and symbols is given
in Appendix 10-2. Check the list to find an abbreviation. If no abbreviation is
listed for the term you are using, you may devise an abbreviation provided that
(1) it is not identical to an abbreviation of a unit of measure, (2) it will not be
confused with the symbol of an element or a group, (3) it does not hamper the
reader’s understanding, and (4) you do not use the same abbreviation for more
than one spelled-out form.
➤ If a very long name or term is repeated many times throughout a paper, an
abbreviation is warranted. Place the abbreviation in parentheses following the
spelled-out form the first time it appears in the text. If it is used in the abstract,
define it in the abstract and again in the text. After defining the abbreviation in
the text, you may use it throughout the paper.
exceptions The following list shows abbreviations that never need to be
defined. Refer to Appendix 10-2 for all other abbreviations.
a.m. before noon (Latin ante meridiem)
anal. analysis
at. wt atomic weight
bp boiling point
ca. about (Latin circa)
cf. compare (Latin confer)
CP chemically pure
DNA deoxyribonucleic acid
e.g. for example (Latin exempli gratia)
ed., eds. edition, editions
Ed., Eds. Editor, Editors
eq(s) equation(s) [with number(s)]
equiv equivalent(s) [with number(s)]
equiv wt equivalent weight
et al. and others (Latin et alii)
etc. and so forth (Latin et cetera)
fp freezing point
GLC gas–liquid chromatography
i.d. inside diameter
i.e. that is (Latin id est)
in. inch, inches
IR infrared
m molal
M molar
Chapter 10: Editorial Style ➤ 159
mmp mixture melting point
mp melting point
Mr relative molecular mass (molecular weight)
N normal
NMR nuclear magnetic resonance
no., nos. number, numbers
o.d. outside diameter
p, pp page, pages
p.m. after noon (Latin post meridiem)
P.O. Post Office (with Box and number)
ref(s) reference(s) [with number(s)]
RNA ribonucleic acid
sp., spp. species, singular and plural
sp gr specific gravity
sp ht specific heat
sp vol specific volume
U.K. United Kingdom
U.S. United States
USP United States Pharmacopeia
UV ultraviolet
v/v volume per volume
vol volume
vs versus
w/v weight per volume
w/w weight per weight
wt weight
➤ Avoid abbreviations in the title of a paper.
➤ For some, but not all, abbreviations, case is important; that is, if they are
capitalized, they must never be made lowercase; if they are lowercase, they must
never be capitalized. This guideline applies to abbreviations that would lose
their meanings or change meanings if their forms were changed, such as units
of measure (e.g., mg cannot be changed to Mg, min cannot be changed to Min),
mathematical symbols (e.g., pH cannot be changed to PH or ph), and chemical
symbols (e.g., o for ortho cannot be changed to O).
However, if the meaning would not be affected, some abbreviations can be
capitalized at the beginning of a sentence and in titles and headings, especially if
they are so common that they are more like words than abbreviations. For exam-
ple, you could use “e-mail” in text and “E-mail” at the beginning of a sentence.
➤ Symbols for the chemical elements are not treated as abbreviations. They
need not be defined, and they are typeset in roman type. (See Table 13-1 on
p 270 f.)
160 ➤ The ACS Style Guide
➤ Abbreviate units of measure and do not define them when they follow a
number. Without a number, spell them out.
9 V/s or 9 V· s–1 (but measured in volts per second)
For exceptions, see p 225.
➤ Abbreviations that are common to a specific field may be permitted without
identification in books and journals in that field only, at the discretion of the editor.
➤ For genus and species names, spell out the full genus name in the title, in the
abstract, and the first time it appears in text. Abbreviate it thereafter with the
same species name, but spell it out again with each different species name. Form
the abbreviation with the initial of the genus name. If the paper contains more
than one genus name that starts with the same initial letter, devise abbreviations
that distinguish them. Use italic type for all names and abbreviations.
first time subsequently
Bacillus stearothermophilus B. stearothermophilus
Bacillus subtilis B. subtilis
Escherichia coli E. coli
Salmonella typhimurium S. typhimurium
Staphylococcus aureus Staph. aureus
➤ Use “e.g.”, “i.e.”, “vs”, and “etc.” only in figure captions, in tables, and in paren-
theses in text. Elsewhere, spell out “for example”, “that is”, “versus”, and “and so
forth”.
➤ Do not confuse abbreviations and mathematical symbols. An abbreviation
is usually two or more letters; a mathematical symbol should generally be only
one letter, possibly with a subscript or superscript. An abbreviation may be used
in narrative text but seldom appears in equations; a mathematical symbol is pre-
ferred in equations and may also be used in text. For example, in text with no
equations, PE may be used for potential energy, but in mathematical text and
equations, Ep is preferred. Abbreviations are typeset in roman type; most math-
ematical symbols are typeset in italic type.
➤ In text, spell out all months with or without a specific day.
On August 3, 1996, we completed the second phase of the experiment.
The final results will be available in January 1997.
➤ Use the following abbreviations (with no periods) or spelled-out forms for
months with a day or with a day and year in footnotes, tables, figure captions,
bibliographies, and lists of literature cited.
Chapter 10: Editorial Style ➤ 161
Jan
Feb
March
April
May
June
July
Aug
Sept
Oct
Nov
Dec
➤ Use the abbreviations U.S. and U.K. as adjectives only; spell out United States
and United Kingdom as the noun forms in text. Either United Kingdom or U.K.
may be used in addresses.
U.K. educational system
educational system in the United Kingdom
U.S. science policy
chemical industry in the United States
➤ Form the plurals of multiletter, all-capital abbreviations and abbreviations
ending in a capital letter by adding a lowercase “s” only, with no apostrophe.
HOMOs
JPEGs
PAHs
PCBs
PCs
pHs
➤ To avoid ambiguity or poor appearance, add an apostrophe and a lowercase
“s” to form the plurals of lowercase abbreviations, single-capital-letter abbrevia-
tions, abbreviations ending in a subscript or superscript, and abbreviations end-
ing in an italic letter.
cmc’s
O’s (or oxygens; Os is the symbol for osmium)
pK’s
pKa’s
Tg’s
➤ Use two-letter abbreviations for U.S. state and territory names and the Dis-
trict of Columbia and for Canadian province and territory names on all letters
going through the U.S. Postal Service and most express delivery services. Use
them after the name of a city in text, footnotes, and references.
u.s. states, territories, and the district of columbia
Alabama AL
Alaska AK
American Samoa AS
Arizona AZ
Arkansas AR
California CA
Colorado CO
Connecticut CT
Delaware DE
District of Columbia DC
Federated States of Micronesia FM
Florida FL
162 ➤ The ACS Style Guide
Georgia GA
Guam GU
Hawaii HI
Idaho ID
Illinois IL
Indiana IN
Iowa IA
Kansas KS
Kentucky KY
Louisiana LA
Maine ME
Marshall Islands MH
Maryland MD
Massachusetts MA
Michigan MI
Minnesota MN
Mississippi MS
Missouri MO
Montana MT
Nebraska NE
Nevada NV
New Hampshire NH
New Jersey NJ
New Mexico NM
New York NY
North Carolina NC
North Dakota ND
Northern Mariana Islands MP
Ohio OH
Oklahoma OK
Oregon OR
Palau PW
Pennsylvania PA
Puerto Rico PR
Rhode Island RI
South Carolina SC
South Dakota SD
Tennessee TN
Texas TX
Utah UT
Vermont VT
Virgin Islands VI
Virginia VA
Washington WA
West Virginia WV
Wisconsin WI
Wyoming WY
canadian provinces and territories
Alberta AB
British Columbia BC
Manitoba MB
New Brunswick NB
Newfoundland and Labrador NL
Northwest Territories NT
Nova Scotia NS
Nunavut NU
Ontario ON
Prince Edward Island PE
Quebec QC
Saskatchewan SK
Yukon Territory YT
➤ Spell out and capitalize “company” and “corporation” as part of company
names when they appear in an author’s affiliation. Abbreviate them elsewhere
in text. After the first mention, drop Co. and Corp. and use only the company
name.
163
➤ ➤ ➤ ➤ ➤
APPENDIX 10-1
Computer and Internet Terms
This appendix lists the spelling, capitalization, and abbreviations of some com-
mon computer and Internet terms. This list is not intended to be exclusive. Alter-
native choices, in many cases, are acceptable. Proscribed usages are specifically
indicated.
An excellent source for definitions of these terms may be found at http://
www.google.com/. In the search statement, use the syntax “define:xyz” where xyz
is the term for which a definition is sought.
active matrix
ADSL (asymmetric digital subscriber line)
AI (artificial intelligence)
anonymous FTP
applet
application
archive
ASCII (American Standard Code for
Information Interchange)
ASP (application service provider)
asynchronous
AVI (audio video interleaved)
back up (verb)
backup (noun, adjective)
bandwidth
Base 64
batch processing
baud
baud rate
BBS (bulletin board system)
BinHex (binary hexadecimal)
BIOS (basic input/output system)
bit
bitmap
Bitnet (Because It’s There NETwork)
bitstream
blog, blogger
bookmark
boot
bounce (e-mail)
bps (bits per second)
broadband
browser
bulletin board
byte
C (programming language)
C++ (programming language)
cable modem
cache
CAD (computer-assisted design)
CAD/CAM (computer-assisted design
and manufacturing)
CCD (charge-coupled device)
CD (compact disc)
CD key
CD-R (compact disc read-only)
CD-ROM (compact disc with read-only
memory)
CD-RW (compact disc read–write)
CDMA (code division multiple access)
CGI (common gateway interface)
CGM (computer graphics metafile)
CIF (crystallographic information file)
codec
compact disc (CD)
compiler
CPU (central processing unit)
CRT (cathode ray tube)
164 ➤ The ACS Style Guide
CSS (cascading style sheet)
cursor
CVC (color video controller)
cyberspace
daemon
data domain
data log
data parse, data parsing
data processing
data set
database
DBMS (database management system)
debug (verb)
default
defragment
desktop
DHCP (dynamic host configuration
protocol)
DHTML (dynamic hypertext markup
language)
dialog box
digital signal
directory
disc (compact disc only)
disk (floppy or hard disk)
disk drive
disk space
diskette
DNS (domain name system or server)
domain name
DOI (digital object identifier)
DOS (disk operating system)
double-click (as verb)
download
dpi (dots per inch)
drag and drop
DSL (digital subscriber line)
DTD (document-type definition)
duplex
DVD (digital video disc)
DVD-R (digital video disc read-only)
DVD-RW (digital video disc read–write)
e-book
e-journal
e-mail (electronic mail)
e-money
e-publish
e-zine (electronic magazine)
EBCDIC (Extended Binary-Coded
Decimal Interchange Code)
emoticons
encryption
end user (noun)
EPS (encapsulated PostScript)
Ethernet (but an ethernet)
extranet
FAQ (frequently asked question)
FDDI (fiber distributed data interface)
fiber optics
file compression
file name
filter
finger
Firefox
firewall
FireWire
flash memory
floppy disk
flowchart
format, formatted, formatting
Fortran
FreeNet (but a freenet)
freeware
front end
FTP (file transfer protocol)
gateway
GB (gigabyte, equal to 1024 megabytes;
always a space between number and
GB)
GDDM (graphical data display manager)
GDI (graphics device interface)
GIF (graphics interchange format)
4GL (fourth-generation language)
glyph
Google
GPIB (general purpose interface bus)
graphic (noun)
graphical interface
Chapter 10: Editorial Style ➤ 165
graphics (adjective)
graphics conversion
graphics files
graphics terminal emulation
GUI (graphical user interface)
hard disk
hard disk drive
hardware
hardwired
high-level-language compiler
home directory
home page (lowercase, but capitalized
when part of a specific name, e.g.,
ACS Home Page)
hot key
hotline
HTML (hypertext markup language)
HTTP (hypertext transfer protocol)
HTTPS (hypertext transfer protocol
secure)
hyperlink
hypermedia
hypertext
IBM-compatible
icon
iconization
iconize
IM (instant messaging)
IMAP (Internet message access protocol)
information superhighway
input
integrated circuit
interdomain conversion
Internet
intranet
I/O (input/output)
IP (Internet protocol)
IP address
IRC (Internet relay chat)
ISDN (integrated services digital network)
ISP (Internet service provider)
Java
Javascript
JDK (Java Development Kit)
joystick
JPEG (Joint Photographic Experts Group)
K (kilobyte, equal to 1024 bytes; always
closed up to number; as in 8K or 16K
disk drive; kB is preferred)
kB (kilobyte, equal to 1024 bytes; always a
space between number and kB)
KB (kilobyte; kB is preferred)
kbps (kilobits per second)
kBps (kilobytes per second)
keyboard
keypad
keystroke
kilobit
kilobyte
LAN (local area network)
laptop
LaTeX (pronounced “lahtek”)
LCD (liquid-crystal display)
Lexis
LexisNexis
list-administration software
list-management software
list owner
list server
Listserv (software)
local area network (LAN)
log in, logging in (verb)
log off, logging off (verb)
log on, logging on (verb)
log out, logging out (verb)
login name
logon name
Macintosh, Macintoshes
macro, macros
mainframe
math coprocessor
MB (megabyte, equal to 1024 kilobytes;
always a space between number and
MB)
megapixel
meta-list
166 ➤ The ACS Style Guide
metadata
microchip
microcomputer, microcomputing
microprocessor
Microsoft Excel
Microsoft PowerPoint
Microsoft Windows
Microsoft Word
MIDI (musical instrument digital inter-
face)
MIME (multipurpose Internet mail
extension)
minicomputer
minifloppy disk
modem
monitor (noun)
motherboard
motif
mouse (plural: mouse devices)
Mozilla
MP3 (MPEG audio layer 3)
MPEG (Motion Picture Experts Group)
MS-DOS (Microsoft disk operating sys-
tem, always hyphenated)
MTA (mail-transfer agent)
MUA (mail-user agent)
multitasking
NCP (network control program)
NCSA (National Center for Super-
computing Applications)
Net (when referring to the Internet; lower-
case when referring to any network)
netiquette
netizen
Netscape
netware
network
newsgroup
NIC (Network Information Center)
NNTP (network news transfer protocol)
node, nodes
NREN (National Research and Education
Network)
OCR (optical character recognition)
ODBC (open database connectivity)
off-site (always hyphenated)
offline (one word in computer context)
on-site (always hyphenated)
online (one word in computer context)
open source
OS (operating system)
OSX (Macintosh Operating System X)
output
PageMaker
PAM (pulse amplitude modulation)
parallel port
parser
password
path
PC (personal computer)
PCMCIA (Personal Computer Memory
Card International Association)
pdb (Protein Data Bank) format
PDF (portable document format)
Perl (programming language)
PHP (personal home page)
PIF (picture interchange format)
pixel
plaintext
plug and play
plug-in
PNG (portable network graphics)
POP (post office protocol)
popup
PostScript
PPP (point-to-point protocol)
primary domain
print queue
programmer, programming
PROM (programmable read-only memory)
protocol
proxy server
PSTN (public switched telephone network)
pull-down (adjective)
QuarkXPress
queue
QuickTime
RAM (random-access memory)
raster, rasterize
Chapter 10: Editorial Style ➤ 167
RDBMS (relational database man-
agement system)
read/write permission
real time (noun)
real-time (unit modifier)
reboot
RFC (request for comments)
rich text
RJE (remote job entry)
ROM (read-only memory)
router
RPG (report program generator)
RSS (rich site summary)
RTF (rich text format)
run time (noun)
run-time (adjective)
Safari
scale up (verb)
scanner
screen dump
script
SCSI (small computer system interface,
pronounced “skuzzy”)
search engine
security certificate
serial communication
serial port
server
servlet
set up (verb)
setup (noun)
SGML (standard generalized markup
language)
shared user
shareware
shortcut
shut down (verb)
shutdown (noun, adjective)
sign off (verb)
sign-off (noun, adjective)
simplex
SLIP (serial-line Internet protocol)
SMB (server message block)
SMDS (switched multimegabit data
service)
smiley (the :) or symbol)
SMTP (simple mail transfer protocol)
SNMP (simple network management
protocol)
SOAP (simple object access protocol)
software
source code
spam
spelling checker
spreadsheet
spyware
SQL (structured query language)
SSL (secure sockets layer)
stand-alone (always hyphenated)
start up (verb)
startup (noun)
strikethrough
submenu
SVGA (super video graphics adapter)
systems programs
T-1, T-3
TB (terabyte)
Tcl (programming language, pronounced
“tickle”)
TCP (transmission control protocol)
TCP/IP (transmission control protocol/
Internet protocol)
telecommute
Telnet
terminal emulation program
terminal server
TeX (pronounced “tek”)
TFT (thin-film transistor)
throughput
TIFF (tagged image file format)
time-sharing (always hyphenated)
TLD (top-level domain)
toolbar
toolbox
trackball
Trojan horse
TTL (transistor–transistor logic)
TTY (teletype)
UDP (user datagram protocol)
UGA (ultra graphics accelerator)
Unicode
168 ➤ The ACS Style Guide
Unify
Unix
upload
UPS (uninterruptible power source)
URC (uniform resource characteristic)
URI (uniform resource identifier)
URL (uniform resource locator)
URN (uniform resource name)
USB (universal serial bus)
Usenet
user id, user ids
utility program
uuencode (Unix-to-Unix encoding)
vector, vectorize
VGA (video graphics adapter)
video adapter
Visual Basic
VoIP (voice over Internet protocol)
VPN (virtual private network)
VRML (virtual reality modeling lan-
guage)
W3C (World Wide Web Consortium)
WAIS (wide-area information service or
server)
WAN (wide-area network)
the Web
Web browser
Web page
Web server
Web site
Webmaster
Webzine
Wi-Fi (wireless fidelity)
window (general term, not specific pro-
gram)
Windows (Microsoft program)
WinZIP
word-processing software
word processor
WordPerfect
wordwrap
workstation
World Wide Web (three words, no
hyphens)
World Wide Web Consortium (W3C)
worm
WORM (write once, read many times)
WWW (World Wide Web)
WYSIWYG (what you see is what you
get)
Xbase
Xenix
XML (extensible markup language)
ZIP archive
169
α fine structure constant
optical rotation
stereochemical descriptor
[α]t
D specific rotation at temperature t and wavelength of sodium d line
[α]t
λ specific rotation at temperature t and wavelength λ
a antisymmetric
are (unit of area, 100 m2)
atto (10–18)
axial [use 2(a)-methyl in names]
a a axis
absorptivity
axial chirality [as in (aR)-6,6′-dinitrodiphenic acid]
a0 Bohr radius (0.52917 Å)
A adenosine
alanine
ampere
ring (italic in steroid names)
Å angstrom
A absorbance [as in A = log(l/T)]
anticlockwise (chirality symbol)
Helmholtz energy
mass number
A.D. anno Domini
a.m. ante meridiem
AAS atomic absorption spectroscopy
abs absolute
ac alternating current
ac anticlinal
➤ ➤ ➤ ➤ ➤
APPENDIX 10-2
Abbreviations, Acronyms,
and Symbols
This list is not intended to be exclusive. Alternative choices, in many cases, are
acceptable. Proscribed usages are specifically indicated. If no abbreviation is
listed for the term you are using, you may devise an abbreviation provided that
(1) it is not identical to an abbreviation of a unit of measure, (2) it will not be
confused with the symbol of an element or a group, (3) it does not hamper the
reader’s understanding, and (4) you do not use the same abbreviation for more
than one spelled-out form.
170 ➤ The ACS Style Guide
Ac acetyl
actinium
acac acetylacetonato (ligand)
acam acetamide (ligand)
AcCh acetylcholine
AcChE acetylcholinesterase
AcO acetate
ACTH adrenocorticotropin; adrenocorticotropic hormone
Ade adenine
Ado adenosine
ADP adenosine 5′-diphosphate
AEM analytical electron microscopy
AES atomic emission spectroscopy
Auger electron spectroscopy
af audio frequency
AFM atomic force microscopy
AFS atomic fluorescence spectroscopy
AGU anhydroglucose unit
ala alanyl in genetics
Ala alanyl, alanine
alt alternating, as in poly(A-alt-B)
AM amplitude modulation
AMP adenosine 5′-monophosphate, adenosine 5′-phosphate
amu atomic mass unit [amu, reference to oxygen, is deprecated; u (refer-
ence to mass of 12C) should be used]
anal. analysis (Anal. in combustion analysis presentations)
anhyd anhydrous
ANN artificial neural network
ANOVA analysis of variance
Ans ansyl
ansyl 8-anilino-1-naphthalenesulfonyl
antilog antilogarithm
AO atomic orbital
ap antiperiplanar
AP appearance potential
APIMS atmospheric pressure ionization mass spectrometry
APS appearance potential spectroscopy
aq aqueous
Ar relative atomic mass (atomic weight)
Ar aryl
AR analytical reagent (e.g., AR grade)
Ara arabinose
ara-A adenosine, with arabinose rather than ribose (arabinoadenosine, also
ara-A, araA)
ara-C cytidine, with arabinose rather than ribose (arabinocytidine, also
ara-C, araC)
arb unit arbitrary unit (clinical)
Chapter 10: Editorial Style ➤ 171
Arg arginyl, arginine
ARPES angle-resolved photoelectron spectroscopy
ARPS angle-resolved photoelectron spectroscopy
as asymmetrical
AS absorption spectroscopy
Asa β-carboxyaspartic acid
ASIS aromatic solvent-induced shift
Asn asparaginyl, asparagine
Asp aspartyl, aspartic acid
Asx “Asn or Asp”
asym asymmetrical
at. wt atomic weight
ATCC American Type Culture Collection
atm atmosphere
atom % atom percent
ATP adenosine 5′-triphosphate
ATPase adenosinetriphosphatase
ATR attenuated total reflection
au atomic unit
AU absorbance unit
astronomical unit (length)
AUFS absorbance units at full scale
av average
β stereochemical descriptor
b barn (neutron capture area, 10–24 cm2)
bohr (unit of length)
broad or broadened (spectra)
b b axis
block, as in poly(A-b-B)
B “aspartic acid or asparagine”
bel
buckingham (10–26 esu cm2)
ring (italic in steroid names)
B boat (conformation)
B.C. before Christ
B.C.E. before the common era
b.i.d. twice a day
bar unit of pressure; unit and abbreviation are the same
bbl barrel
bcc body-centered cubic (crystal structure)
bccub body-centered cubic (crystal structure)
BCD binary coded decimal
Bd baud
BEHP bis(2-ethylhexyl) phthalate
BET Brunauer–Emmett–Teller (adsorption isotherm)
BeV billion electronvolts
172 ➤ The ACS Style Guide
bGH bovine growth hormone
Bi biot
binap 2,2′-bis(diphenylphosphino)-1,1′-binaphthyl (ligand)
binol 1,1′-bi-2-naphthol (ligand)
biol biological(ly)
bipy 2,2′-bipyridine, 2,2′-bipyridyl (bpy preferred)
4,4′-bipyridine, 4,4′-bipyridyl (bpy preferred)
bis-Tris [bis(2-hydroxyethyl)amino]tris(hydroxymethyl)methane (also
bistris, Bis-Tris, bis-tris)
bit binary digit
BL bioluminescence
BM Bohr magneton (use µB)
Bn benzyl (also Bzl)
BN bond number
BO Born–Oppenheimer
BOD biological oxygen demand
bp base pair
boiling point
bps bits per second
Bps bytes per second
bpy 2,2′-bipyridine, 2,2′-bipyridyl
4,4′-bipyridine, 4,4′-bipyridyl
BPY bipyramidal (coordination compounds)
Bq becquerel
br broad or broadened (spectra)
BSA bovine serum albumin
BSSE basis set superposition error
Btu British thermal unit
bu bushel
Bu butyl
BWR Benedict–Webb–Rubin (equation)
Bz benzoyl
Bzac benzoylacetone
Bzl benzyl (also Bn)
χ magnetic susceptibility
c candle
centered (crystal structure)
centi (10–2)
cyclo [as in c-C6H11, c-Hx (cyclohexyl)]
c c axis
concentration, for rotation, e.g., [α]20
489 +25 (c 0.13, CHCl3)
cyclo [as in c-S6 (cyclo-hexasulfur)]
specific cytochrome (i.e., cytochrome c)
C Celsius (use °C as unit abbreviation)
coulomb
cysteine
Chapter 10: Editorial Style ➤ 173
C cytidine
ring (italic in steroid names)
C chair (conformation)
clockwise (chirality symbol)
C.E. common era
c/m2 candles per square meter
13C NMR carbon nuclear magnetic resonance
ca. circa, about [used before an approximate date or figure (ca. 1960)]
CAD computer-assisted design
cal calorie
calIT International Table calorie
calcd calculated
CAM computer-assisted manufacturing
cAMP adenosine cyclic 3′,5′-phosphate
adenosine 3′,5′-cyclic phosphate
CAN ceric ammonium nitrate
CARS coherent anti-Stokes Raman spectroscopy
CAT computed axial tomography
computer-averaged transients
cB conjugate base, counterbase (also CB)
Cbz carbobenzoxy, carbobenzyloxy, (benzyloxy)carbonyl, benzyl-
oxycarbonyl (also Z)
cc cubic centimeter (do not use; use cm3 or mL)
CCD charge-coupled device
CCGC capillary column gas chromatography
ccp cubic close-packed (crystal structure)
cd candela
current density
CD circular dichroism
CDH ceramide dihexoside [Cer(Hex)2]
cDNA complementary DNA
CDP cytidine 5′-diphosphate
CE Cotton effect
CE–MS capillary electrophoresis–mass spectrometry
cf. compare
CFC chlorofluorocarbon
cfm cubic feet per minute
CFSE crystal field stabilization energy (also cfse)
cfu colony-forming units (bacterial inocula)
cgs centimeter–gram–second (as in cgs system)
cgsu centimeter–gram–second unit(s)
ChE cholinesterase
CHF coupled Hartree–Fock
Ci curie
CI chemical ionization
configuration interaction
CIDEP chemically induced dynamic electron polarization
174 ➤ The ACS Style Guide
CIDNP chemically induced dynamic nuclear polarization
CIMS chemical ionization mass spectrometry
CL cathodoluminescence
chemiluminescence
CM carboxymethyl (as in CM-cellulose)
cmc critical micelle concentration
CMH ceramide monohexoside [Cer(Hex)]
CMO canonical molecular orbital
CMP cytidine 5′-monophosphate, cytidine 5′-phosphate
cmr carbon magnetic resonance (do not use; use 13C NMR)
CMR carbon magnetic resonance (do not use; use 13C NMR)
CN coordination number
CNDO complete neglect of differential overlap
CNS central nervous system
co copoly (as in A-co-B)
CoA coenzyme A
cod 1,5-cyclooctadiene (ligand)
COD chemical oxygen demand
coeff coefficient
colog cologarithm
compd compound
con conrotatory (may be italic)
concd concentrated
concn concentration
const constant
cor corrected
cos cosine
cosh hyperbolic cosine
COSY correlation spectroscopy
cot cotangent
1,3,5,7-cyclooctatetraene (ligand)
coth hyperbolic cotangent
counts/s counts per second
Cp heat capacity at constant pressure
cp candlepower
cP centipoise
Cp cyclopentadienyl
CP chemically pure
cross-polarization
CP/MAS cross-polarization/magic-angle spinning (also CP-MAS, CP–MAS,
CPMAS, CP MAS)
cpd contact potential difference
CPE controlled-potential electrolysis
CPK Corey–Pauling–Koltun (molecular models)
creatine phosphokinase
CPL circular polarization of luminescence
cpm counts per minute
Chapter 10: Editorial Style ➤ 175
cps counts per second (use counts/s)
cycles per second (use Hz or s–1)
CRAMPS combined rotation and multiple-pulse spectroscopy
CRIMS chemical reaction interface mass spectrometry
crit critical
cRNA complementary RNA
CRT cathode ray tube
CRU constitutional repeating unit
cryst crystalline
csc cosecant
csch hyperbolic cosecant
CT charge transfer
CTEM conventional transmission electron microscopy
CTH ceramide trihexoside [Cer(Hex)3]
CTP cytidine 5′-triphosphate
CU-8 cubic, coordination number 8
cub cubic (crystal structure)
Cv heat capacity at constant volume
CV coefficient of variation
cyclic voltammetry
CVD chemical vapor deposition
CW constant width
continuous wave (as in CW ESR)
cwt hundredweight
Cy cyclohexyl
cyclam 1,4,8,11-tetraazacyclotetradecane
Cyd cytidine
Cys cysteinyl, cysteine
cyt cytochrome
Cyt cytosine
cytRNA cytoplasmic RNA
CZE capillary zone electrophoresis
δ NMR chemical shift in parts per million downfield from a standard
∂ partial differential
d day (spelled-out form is preferred)
deci (10–1)
deoxy
deuteron
differential (mathematical)
diffuse
doublet (spectra)
d. diameter, with i. and o. (inside and outside)
d density
dextrorotatory
distance
spacing (X-ray)
176 ➤ The ACS Style Guide
d absolute configuration
D aspartic acid
debye
deuterium
ring (italic in steroid names)
D diffusion coefficient
symmetry group [e.g., D3; also used in names, such as (+)-D3-tris-
homocubane]
2-D two-dimensional (also 2D)
3-D three-dimensional (also 3D)
da deca or deka (10)
Da dalton
daf dry ash free
dAMP 2′-deoxyadenosine 5′-monophosphate or phosphate (the A can be
replaced with C, G, U, etc.)
dansyl 5-(dimethylamino)-1-naphthalenesulfonyl
dB decibel
dc direct current
DD-8 dodecahedral, coordination number 8
DD NMR dipolar decoupling NMR
DDT 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane
de diastereomeric excess
DEAE (diethylamino)ethyl (as in DEAE-cellulose)
dec decomposition
decomp decompose
DEFT driven equilibrium Fourier transform
deg degree (use °B, degrees Baumé; °C, °F, but K)
DEG diethylene glycol
DEHP bis(2-ethylhexyl) phthalate (BEHP is preferred)
DES diethylstilbestrol
det determinant
df degrees of freedom
DF degrees of freedom
DFT density functional theory
diam diameter
dil dilute
dis disrotatory (may be italic)
distd distilled
DLVO Derjaguin–Landau–Verwey–Overbeek
DMA dynamic mechanical analyzer
DMBA 9,10-dimethylbenz[a]anthracene
DME 1,2-dimethoxyethane
dropping mercury electrode
DMEM Dulbecco’s modified Eagle’s medium
DMF dimethylformamide
DMN diaminomaleonitrile
dmr deuterium magnetic resonance (do not use; use 2H NMR)
Chapter 10: Editorial Style ➤ 177
DMR deuterium magnetic resonance (do not use; use 2H NMR)
DMSO dimethyl sulfoxide (also Me2SO)
DMTA dynamic mechanical thermal analyzer
DNA deoxyribonucleic acid
DNase deoxyribonuclease
DNMR dynamic nuclear magnetic resonance
DNP deoxynucleoprotein
dynamic nuclear polarization (NMR)
DNPH (2,4-dinitrophenyl)hydrazine
Dns dansyl
Dopa 3-(3,4-dihydroxyphenyl)alanine (also DOPA)
DP degree of polymerization (also dp)
dpm disintegrations per minute
DPPH 2,2-diphenyl-1-picrylhydrazyl
dps disintegrations per second
Dq crystal field splittings
DQF double quantum filtered
DRIFT diffuse reflectance Fourier transform
Ds crystal field splittings
DSC differential scanning calorimetry
dT thymidine
Dt crystal field splittings
DTA differential thermal analysis
DTC depolarization thermocurrent
differential thermal calorimetry
dTDP thymidine 5′-diphosphate
DTE dithioerythritol
dThd thymidine
dTMP thymidine 5′-monophosphate, thymidine 5′-phosphate
DTT dithiothreitol
dTTP thymidine 5′-triphosphate
dyn dyne
ε dielectric constant
molar absorptivity
ε∗ complex permittivity
e base of natural logarithm
electron
equatorial [in names, e.g., 2(e)-methyl]
eaq– hydrated electron
e–(aq) hydrated electron
es– solvated electron
e–(s) solvated electron
e electronic charge
E exa (1018)
glutamic acid
178 ➤ The ACS Style Guide
E electromotive force
energy
entgegen (configuration)
envelope (conformation)
potential energy
specific extinction coefficient (E280nm1%,1cm)
Young’s modulus
E° standard electrode potential
standard electromotive force
E1/2 half-wave potential
E1 first-order elimination
E2 second-order elimination
e.g. for example
Ea, EA Arrhenius or activation energy
ea0 electronic charge in electrostatic units × Bohr radius or atomic units
for dipole moment
EC exclusion chromatography
ECD electron-capture detector, detection
ECE electrochemical, chemical, electrochemical (mechanisms)
ECG electrocardiogram
ecl electrochemical luminescence
ECL electrochemical luminescence
ECP effective core potential
ed. edition, edited
Ed. editor
ED effective dose
ED50 dose that is effective in 50% of test subjects (also ED50)
edda ethylenediaminediacetato (ligand)
eds. editions
Eds. editors
EDS energy-dispersive system (or spectrometry)
edta ethylenediaminetetraacetato (ligand)
EDTA ethylenediaminetetraacetic acid, ethylenediaminetetraacetate
EDXS energy-dispersive X-ray spectrometry
ee enantiomeric excess
EEG electroencephalogram
EELS electron energy loss spectroscopy
EFG electric field gradient
EGA evolved gas analysis
EGD evolved gas detection
EGR exhaust gas recirculation
Eh hartree (unit); Hartree energy
EH extended Hückel
EI electron impact
electron ionization
EIA enzyme immunoassay
Ek kinetic energy
Chapter 10: Editorial Style ➤ 179
EKC electrokinetic chromatography
EKG electrocardiogram
EL electroluminescence
ELISA enzyme-linked immunosorbent (immunoadsorbent) assay
e/m ratio of electron charge to mass
EM electron microscopy
e-mail electronic mail
EMC equilibrium moisture content
emf electromotive force
EMIS electromagnetic isotope separation
emu electromagnetic unit
en ethylenediamine (ligand)
ENDOR electron–nuclear double resonance
ent reversal of stereocenters
Ep potential energy
EPA ether–isopentane–ethanol (solvent system)
epi inversion of normal configuration (italic with a number, as in 15-epi-
prostaglandin A)
EPMA electron probe microanalysis
EPR electron paramagnetic resonance
EPXMA electron probe X-ray microanalysis
eq equation
equiv equivalent
equiv wt equivalent weight
erf error function
erfc error function complement
erfc–1 inverse error function complement
ESCA electron spectroscopy for chemical analysis
esd estimated standard deviation
ESE electron spin echo
ESEEM electron spin echo envelope modulation
ESI electrospray ionization
ESIMS electrospray ionization mass spectrometry
ESP elimination of solvation procedure
ESR electron spin resonance
esu electrostatic unit
Et ethyl
et al. and others
etc. and so forth
eu entropy unit
EU enzyme unit
eV electronvolt
EXAFS extended X-ray absorption fine structure
exch exchangeable (spectra)
exp exponential
expt experiment
exptl experimental
180 ➤ The ACS Style Guide
f and page following (as in p 457 f)
femto (10–15)
fermi (unit of length, also fm)
fine (spectral)
f focal length
frequency (in statistics)
function [as in f(x)]
furanose form
F Fahrenheit (use °F as unit abbreviation)
farad
formal (use judiciously; M is preferred)
phenylalanine
F Faraday constant
variance ratio (in statistics)
FAAS flame atomic absorption spectroscopy
FABMS fast atom bombardment mass spectrometry
fac facial
FAD flavin adenine dinucleotide
FAES flame atomic emission spectroscopy
FAFS flame atomic fluorescence spectroscopy
FAS flame absorption spectroscopy
fcc face-centered cubic (crystal structure)
FCC fluid catalytic cracking
Fd ferredoxin
FEM field emission microscopy or spectroscopy
FES field emission spectroscopy
flame emission spectrometry
ff and pages following (as in p 457 ff)
FFEM freeze–fracture electron microscopy
FFF field flow fractionation
FFS flame fluorescence spectroscopy
FFT fast Fourier transform
FHT Fisher–Hirschfelder–Taylor (space-filling models)
FI field ionization
FIA flow-injection analysis
fluorescence immunoassay
fid free induction decay (in Fourier transform work)
FID flame ionization detector, detection
free induction decay (in Fourier transform work)
FIK field ionization kinetics
FIR far-infrared
FLC ferroelectric liquid crystal
fm femtometer
fermi (unit of length, also f)
FM frequency modulation
FMN flavin mononucleotide
FMO frontier molecular orbital
Chapter 10: Editorial Style ➤ 181
FOPPA first-order polarization propagator approach
fp freezing point
FPC fixed partial charge
FPT finite perturbation theory
Fr franklin
Fr Froude number
Fru fructose
FSGO floating spherical Gaussian orbital
FSH follicle-stimulating hormone
ft foot
FT Fourier transform
ft-c foot-candle
ft-lb foot-pound
ft-lbf foot-pound-force
FTICR Fourier transform ion cyclotron resonance
FTIR Fourier transform infrared (also FT/IR, FT-IR, and FT IR)
FTIRS Fourier transform infrared spectroscopy
FTP file transfer protocol
FTS Fourier transform spectroscopy
Fuc fucose
fw formula weight
fwhh full width at half-height
fwhm full width at half-maximum
γ microgram (use µg)
photon
surface tension
Γ surface concentration
g gas [as in H2O(g)]
gram
g acceleration due to gravity (closed up to number preceding)
splitting factor (ESR and NMR spectroscopy)
G gauss
generally labeled
giga (109)
glycine
guanosine
G Gibbs energy
gravitational constant
g-atom gram-atom (use mol)
Ga Galileo number
gal gallon
Gal galactose
galileo (unit of acceleration)
GalNAc N-acetylgalactosamine
GC gas chromatography
GDC gas displacement chromatography
182 ➤ The ACS Style Guide
GDMS glow discharge mass spectrometry
GDP guanosine 5′-diphosphate
gem geminal
GFAAS graphite furnace atomic absorption spectroscopy
GFC gas frontal chromatography
gfw gram formula weight
GH growth hormone (somatotropin)
GHz gigahertz
Gi gilbert
GIAO gauge-invariant atomic orbital
Glc glucose
GLC gas–liquid chromatography
GlcNAc N-acetylglucosamine
Gln glutaminyl, glutamine
GLPC gas–liquid partition chromatography
Glu glutamyl, glutamic acid
Glx “Gln or Glu”
gly glycine (ligand)
Gly glycyl, glycine
GMP guanosine 5′-monophosphate, guanosine 5′-phosphate
GPC gel permeation chromatography
gr grain (unit of weight)
GSC gas–solid chromatography
GSH reduced glutathione
GSL glycosphingolipid
GSSG oxidized glutathione
GTP guanosine 5′-triphosphate
Gua guanine
Guo guanosine
Gy gray (international unit of absorbed dose)
η hapto
viscosity
h hecto (102)
helion
hour
h crystallographic index (hkl)
Planck’s constant
Planck’s constant divided by 2π
H henry
histidine
H enthalpy
half-chair (conformation)
Hamiltonian
Hamiltonian
1H NMR proton nuclear magnetic resonance
Chapter 10: Editorial Style ➤ 183
2H NMR deuterium nuclear magnetic resonance
H0 magnetic field (ESR and NMR spectroscopy)
ha hectare
Hb hemoglobin
Hbg biguanide
HCG human chorionic gonadotropin
hcp hexagonal close-packed (crystal structure)
HCP hexachlorophene
HCS hazard communication standard
HDPE high-density polyethylene
Hedta ethylenediaminetetraacetate(3–) (-ato as ligand in full name)
H2edta ethylenediaminetetraacetate(2–) (-ato as ligand in full name)
H3edta ethylenediaminetetraacetate(1–) (-ato as ligand in full name)
H4edta ethylenediaminetetraacetic acid
HEEDTA N-(2-hydroxyethyl)ethylenediaminetriacetate
Hepes N-(2-hydroxyethyl)piperazine-N′-ethanesulfonic acid (also HEPES,
hepes)
Hepps N-(2-hydroxyethyl)piperazine-N′-propanesulfonic acid (also HEPPS,
hepps)
hex hexagonal (crystal structure)
HF Hartree–Fock
hfs hyperfine splitting
hfsc hyperfine splitting constant
hGH human growth hormone
HIPS high-impact polystyrene
His histidyl, histidine
HIV human immunodeficiency virus
hkl crystallographic index
HMDS hexamethyldisilane
hexamethyldisiloxane
HMO Hückel molecular orbital
HMPA hexamethylphosphoramide
HMPT hexamethylphosphoric triamide
hnRNA heterogeneous nuclear RNA
hν indicates light; h is Planck’s constant, and ν is the photon frequency
HOHAHA homonuclear Hartmann–Hahn
HOMO highest occupied molecular orbital
H2ox oxalic acid
hp horsepower
HPCE high-performance capillary electrophoresis
HPLC high-performance liquid chromatography
high-pressure liquid chromatography
HREELS high-resolution electron energy loss spectroscopy
HREM high-resolution electron microscopy
HRMS high-resolution mass spectrometry
HSP heat shock protein
184 ➤ The ACS Style Guide
Hyl hydroxylysyl, hydroxylysine
Hyp hydroxyprolyl, hydroxyproline
hypoxanthine
Hz hertz
i (–1)1/2
i iso (as in i-Pr; do not use i-propyl)
I inosine
isoleucine
I electric current (also i)
ionic strength
moment of inertia
spin quantum number (ESR and NMR spectroscopy)
i.d. inside diameter
i.e. that is
I/O input–output
ibid. in the same place (in the reference cited; use is discouraged)
ic intracerebrally
IC integrated circuit
ion chromatography
ICP inductively coupled plasma
ICR ion cyclotron resonance
ics internal chemical shift
ICSH interstitial-cell-stimulating hormone
ICT International Critical Tables
id diffusion current
ID infective dose
ID50 dose that is infective in 50% of test subjects (also ID50)
IDAS isotope dilution α spectrometry
IDMS isotope dilution mass spectrometry
IDP inosine 5′-diphosphate
IE ionization energy
IEC ion-exchange chromatography
IEF isoelectric focusing
IEP isoelectric point
IETS inelastic electron-tunneling spectroscopy
IFQ interfacial fluorescence quenching
IKES ion kinetic energy spectroscopy
Ile isoleucyl, isoleucine
ILS increased life span
im intramuscularly
IMMA ion microprobe mass analysis
IMP inosine 5′-monophosphate, inosine 5′-phosphate
in. inch
INDO intermediate neglect of differential overlap
INDOR internal nuclear double resonance
internucleus (nucleus–nucleus) double resonance
Chapter 10: Editorial Style ➤ 185
INH inhibitor
isonicotinic acid hydrazide
Ino inosine
INO iterative natural orbital
insol insoluble
ip intraperitoneally
IP ionization potential
ips iron pipe size
IR infrared
IRDO intermediate retention of differential overlap
IRMA immunoradiometric assay
IRMS isotopic ratio mass spectrometry
IRP internal reflection photolysis
IRRAS infrared reflection–absorption spectroscopy
IRS internal reflection spectroscopy
isc intersystem crossing
ISCA ionization spectroscopy for chemical analysis
ISE ion-selective electrode
iso inversion of normal chirality (not as in isopropyl, but in uses such as
8-iso-prostaglandin E1; generally italic with a number)
ISS ion-scattering spectroscopy
ITP inosine 5′-triphosphate
isotachophoresis
IU international unit
iv intravenous, intravenously
J joule
J coupling constant (NMR and ESR spectroscopy)
JT Jahn–Teller
k kilo (103)
k Boltzmann constant (also kB)
crystallographic index (hkl)
rate constant
K 1000 (as in 60K protein)
kayser (use cm–1)
kelvin (do not use °K)
kilobyte (kB is preferred)
lysine
K equilibrium constant
Kα spectral line
kat katal (unit of enzyme catalytic activity)
Kβ spectral line
kB Boltzmann constant
kb kilobase
kilobit
186 ➤ The ACS Style Guide
kB kilobel
kilobyte
kbar kilobar
kbp kilobase pair
kD kilodebye
kDa kilodalton
KE kinetic energy
kg kilogram
kgf kilogram-force
kHz kilohertz
Km Michaelis constant
Koc carbon-referenced sediment partition coefficient
organic chemicals partition coefficient
Kow octanol–water partition coefficient
KSP solubility product constant
Kw autoionization constant
λ absolute activity
microliter (use µL)
wavelength
λex excitation wavelength
λmax wavelength of maximum absorption
l liquid [as in NH3(l)]
l crystallographic index (hkl)
levorotatory
l absolute configuration
L leucine
ligand
liter
LI spectral line
LII spectral line
LIII spectral line
Lac lactose
LAMMA laser microprobe mass spectrometry
lat latitude
lb pound
lbf pound-force
LC liquid chromatography
LCAO linear combination of atomic orbitals
LCD liquid-crystal display
LCICD liquid-crystal-induced circular dichroism
LCVAO linear combination of virtual atomic orbitals
LD lethal dose
LD50 dose that is lethal to 50% of test subjects (also LD50)
LDH lactic dehydrogenase
LDMS laser desorption mass spectrometry
LE locally excited
Chapter 10: Editorial Style ➤ 187
LED light-emitting diode
LEED low-energy electron diffraction
LEEDS low-energy electron diffraction spectroscopy
LEISS low-energy ion-scattering spectroscopy
LEMF local effective mole fraction
Leu leucyl, leucine
LFER linear free-energy relationship
LH luteinizing hormone
LIF laser-induced fluorescence
lim limit
LIMS laboratory information management system
LIS lanthanide-induced shift
lit. literature
LJ, L-J Lennard-Jones
LLC liquid–liquid chromatography
lm lumen
LMCT ligand-to-metal charge transfer
ln natural logarithm
LNDO local neglect of differential overlap
log logarithm to the base 10
Log principal logarithm
long. longitude
Lp Lorentz–polarization (effect)
Lp Lorentz factor × polarization factor
LSC liquid–solid chromatography
LSD lysergic acid diethylamide
LSR lanthanide shift reagent
LUMO lowest unoccupied molecular orbital
lut lutidine (ligand)
Lut lutidine
lx lux
LYP Lee–Yang–Parr
Lys lysyl, lysine
µ chemical potential
dipole moment
electrophoretic mobility
micro (10–6)
micron (do not use; use µm or micrometer)
µ± muon
µB Bohr magneton
µN nuclear magneton
µW Weiss magneton
m medium (spectra)
meter
mile (in mpg and mph; otherwise mi)
milli (10–3)
188 ➤ The ACS Style Guide
m multiplet (spectra)
m isotopic mass
magnetic quantum number (ESR and NMR spectroscopy)
meta
molal (mol kg–1)
M mega (106)
mesomeric
metal (do not use Me)
methionine
molar (mol dm–3, mol L–1)
M minus (left-handed helix)
[M] molecular rotation
m/e mass-to-charge ratio (m/z is preferred)
mAb monoclonal antibody (also Mab, MAb)
Mal maltose
MALDI matrix-assisted laser desorption ionization
MALDI-TOFMS matrix-assisted laser desorption ionization time-of-flight mass
spectrometry (also MALDI-TOF MS)
Man mannose
MAO monoamine oxidase
MAS magic-angle spinning
MASS magic-angle sample spinning
max maximum
Mb myoglobin
MBE molecular beam epitaxy
MCD magnetic circular dichroism
mCi millicurie
MCT mercury cadmium telluride
MD molecular dynamics
me electron rest mass
Me methyl (not metal)
MED mean effective dose
MEKC micellar electrokinetic capillary chromatography
MEM minimum Eagle’s essential medium
mequiv milliequivalent
mer polymer notation (as in 16-mer)
mer meridional
Mes mesityl (2,4,6-trimethylphenyl), 2-morpholinoethanesulfonic acid,
2-morpholinoethanesulfonate (also MES)
Met methionyl, methionine
MetHb methemoglobin
MetMb metmyoglobin
MeV million electronvolts
mho reciprocal ohm (Ω–1 is preferred)
MHz megahertz
mi mile
Chapter 10: Editorial Style ➤ 189
min minimum
minute
MINDO modified intermediate neglect of differential overlap
MIR mid-infrared
MIRS multiple internal reflection spectroscopy
ML monolayer
MLCT metal-to-ligand charge transfer
MLR multiple linear regression
mmHg millimeters of mercury (measure of pressure)
mmp mixture melting point
mmu millimass unit
mn neutron rest mass
Mn number-average molecular weight
MO molecular orbital
mol mole
mol % mole percent
molar equiv molar equivalent
mol wt molecular weight (Mr is preferred)
MOM methoxymethyl
mon monoclinic (crystal structure)
mp proton rest mass
mp melting point
MP Møller–Plesset
MP2 second-order Møller–Plesset perturbation theory
mpg miles per gallon
mph miles per hour
MPI multiphoton ionization
MPV Meerwein–Ponndorf–Verley
MQ ENDOR multiple-quantum electron nuclear double resonance
Mr relative molecular mass (molecular weight)
MR molecular refraction
MRI magnetic resonance imaging
mRNA messenger RNA
Ms mesyl (methylsulfonyl)
MS mass spectrometry
mass spectrum
microwave spectroscopy
MSDS manufacturer’s safety data sheet
material safety data sheet
MSG monosodium glutamate
MSH melanocyte-stimulating hormone, melanotropin
Mt megaton
MTD mean therapeutic dose
mtDNA mitochondrial DNA
mtRNA mitochondrial RNA
mu mass unit
190 ➤ The ACS Style Guide
MVA mevalonic acid
MVS multiple-variable storage
Mw weight-average molecular weight
MW molecular weight (Mr is preferred)
MWD molecular weight distribution
Mx maxwell
Mz z-average molecular weight
m/z mass-to-charge ratio
ν frequency
ν
∼ wavenumber
ν1/2 full width at half-maximum height (NMR spectra)
νe neutrino
νmax frequency of maximum absorption
n nano (10–9)
neutron
n normal (as in n-butyl, n-Bu)
refractive index (n20
D, at 20 °C, Na D line)
total number of individuals
N asparagine
newton
normal (concentration)
unspecified nucleoside
N.B. nota bene (note well)
NA Avogadro’s number
NAA neutron activation analysis
[Na]ATPase sodium ion activated ATPase (also Na-ATPase, NaATPase)
NAD nicotinamide adenine dinucleotide
NADH reduced nicotinamide adenine dinucleotide
NADP nicotinamide adenine dinucleotide phosphate
NADPH reduced nicotinamide adenine dinucleotide phosphate
[Na,K]ATPase sodium and potassium ion activated ATPase (also Na,K-ATPase)
NBS N-bromosuccinimide
NDA New Drug Application
NDDO neglect of diatomic differential overlap
nDNA nuclear DNA
NEMO nonempirical molecular orbital
neut equiv neutralization equivalent
NHE normal hydrogen electrode
NIR near-infrared
Nle norleucyl, norleucine
NLO nonlinear optical (optics)
nm nanometer
NM nuclear magneton (use µN)
NMN nicotinamide mononucleotide
NMR nuclear magnetic resonance (do not use nmr)
no. number
Chapter 10: Editorial Style ➤ 191
NO natural orbital (as in CNDO/2-NO)
NOCOR neglect of core orbitals
NOE nuclear Overhauser effect
NOESY nuclear Overhauser enhancement spectroscopy
NOx nitrogen oxides
Np neper
NPR net protein retention
NQR nuclear quadrupole resonance
nRNA nuclear RNA
NRTL nonrandom two-liquid
NSOM near-field scanning optical microscopy
NTP normal temperature and pressure
unspecified nucleoside 5′-triphosphate
Nuc nucleoside (unspecified)
Nva norvalyl, norvaline
ω angular frequency
Ω ohm
o ortho
O orotidine
o.d. outside diameter
o-rh orthorhombic (crystal structure)
o/w oil in water (emulsion)
O/W oil in water (emulsion)
OAc acetate
obsd observed
OC-6 octahedral, coordination number 6
OCR optical character recognition
OD optical density
ODMR optically detected magnetic resonance
ODU optical density unit
Oe oersted
OES optical emission spectroscopy
OFDR off-frequency decoupling resonance
OMVPE organometallic vapor-phase epitaxy
Ord orotidine
ORD optical rotary dispersion
Orn ornithyl, ornithine
Oro orotic acid
ORTEP Oak Ridge thermal ellipsoid plot
osm osmolar (also osM, Osm)
OTTLE optically transparent thin-layer electrode
ox oxalato (ligand)
oxidized or oxidation (in subscripts and superscripts)
oxidn oxidation
oz ounce
192 ➤ The ACS Style Guide
% percent
‰ per thousand (parts per thousand)
π pros (near) in NMR measurements (as in Nπ of histidine)
type of orbital, electron
π± pion
π0 pion
ψ pseudouridine
ψrd pseudouridine
p negative logarithm (as in pH)
page
pico (10–12)
proton
p angular momentum (ESR and NMR spectroscopy)
para
probability (in statistics)
pyranose form
P peta (1015)
poise
proline
P plus (right-handed helix)
probability (in statistics)
p.m. post meridiem
P450 specific cytochrome designation (i.e., cytochrome P450)
P-450 specific cytochrome designation (i.e., cytochrome P-450)
P450 specific cytochrome designation (i.e., cytochrome P450)
31P NMR phosphorus-31 nuclear magnetic resonance
Pa pascal
PAC perturbed angular correlation
PAD perturbed angular distribution
PAGE polyacrylamide gel electrophoresis
paH negative logarithm of hydrogen ion activity
PAH polycyclic aromatic hydrocarbon
PAN polyacrylonitrile
PBS phosphate-buffered saline
pc parsec (unit of length)
PC paper chromatography
personal computer
planar chromatography
PCB polychlorobiphenyl, polychlorinated biphenyl
PCDD polychlorinated dibenzo-p-dioxin
polychlorodibenzo-p-dioxin
PCDF polychlorodibenzofuran
PCILO perturbed configuration interaction with localized orbitals
PCP pentachlorophenol
PCR polymerase chain reaction
PCTFE poly(chlorotrifluoroethylene)
Chapter 10: Editorial Style ➤ 193
PDL pumped dye laser
PDMS plasma desorption mass spectrometry
PE polyethylene
potential energy
PEG poly(ethylene glycol)
PEL permissible exposure limit
PEO poly(ethylene oxide)
PES photoelectron spectroscopy
PET positron emission tomography
PETP poly(ethylene terephthalate)
PFU plaque-forming unit
PG prostaglandin
pH negative logarithm of hydrogen ion concentration
Ph phenyl (for C6H5 only)
Phe phenylalanyl, phenylalanine
phen 1,10-phenanthroline, o-phenanthroline
phr parts per hundred parts of resin (or rubber)
Pi inorganic phosphate
PIB polyisobutylene
PIXE proton-induced X-ray emission
pK negative logarithm of equilibrium constant
pKa pK for association
PL photoluminescence
PLOT porous-layer open-tubular
PMMA poly(methyl methacrylate)
PMO perturbational molecular orbital
PMR phosphorus magnetic resonance (do not use; use 31P NMR)
polymerization of monomeric reactants
proton magnetic resonance (do not use; use 1H NMR)
PNA polynuclear aromatic hydrocarbon
PNDO partial neglect of differential overlap
po per os (orally)
POM poly(oxymethylene), polyformaldehyde
POPOP 1,4-bis(5-phenyl-2-oxazolyl)benzene
pp pages
PP polypropene
ppb parts per billion
ppbv parts per billion by volume
PPi inorganic pyrophosphate, phosphoric acid
ppm parts per million
ppmv parts per million by volume
PPO 2,5-diphenyloxazole
PPP Pariser–Parr–Pople
PPS photophoretic spectroscopy
ppt parts per trillion
precipitate
194 ➤ The ACS Style Guide
pptv parts per trillion by volume
Pr propyl
PRDDO partial retention of diatomic differential overlap
prepn preparation
PRFT partially relaxed Fourier transform
Pro prolyl, proline
pro-R stereochemical descriptor (also pro-R)
pro-S stereochemical descriptor (also pro-S)
PRT platinum resistance thermometer
Ps positronium
PS polystyrene
psi pounds per square inch
psia pounds per square inch absolute
psig pounds per square inch gauge
pt pint
point
PTC phase-transfer catalysis
PTFE poly(tetrafluoroethylene)
PTH parathyroid hormone
phenylthiohydantoin
PTV programmed-temperature vaporizer
PU polyurethane
PVA poly(vinyl alcohol)
PVAC poly(vinyl acetate)
PVAL poly(vinyl alcohol)
PVC poly(vinyl chloride)
PVDC poly(vinylidene dichloride)
PVDF poly(vinylidene difluoride)
PVE poly(vinyl ether)
PVF poly(vinyl fluoride)
PXRD powder X-ray diffraction
py pyridine (ligand)
Py pyridine
PY pyramidal (coordination compounds)
Py–GC–MS pyrolysis–gas chromatography–mass spectrometry
pyr pyrazine (ligand)
pyrr pyrrolidine (ligand)
pz pyrazole (ligand)
q quartet (spectra)
q heat, electric charge (also Q)
Q glutamine
Q heat, electric charge (also q)
QCPE Quantum Chemistry Program Exchange
QELS quasi-elastic light scattering
QSAR quantitative structure–activity relationship
qt quart
Chapter 10: Editorial Style ➤ 195
ρ density
r correlation coefficient
R arginine
Rankine (temperature scale, use °R as unit abbreviation)
roentgen
R gas constant
rectus (configurational)
regression coefficient
resistance
rac racemic
rad radian
unit of radiation
RBS Rutherford backscattering spectrometry
rd rad
RDE rotating disk electrode
re electron radius
re stereochemical descriptor (as in the re face)
recryst recrystallized
red reduced or reduction (in subscripts and superscripts)
redn reduction
redox reduction–oxidation
ref reference
rel relative
rel relative (stereochemical descriptor)
REL recommended exposure limit
rem roentgen equivalent man
REM rapid eye movement
rep roentgen equivalent physical
rf radio frequency
Rf retention factor (ratio of distance traveled by the center of a zone to
the distance simultaneously traveled by the mobile phase)
RFC request for comments
RH relative humidity
Rha rhamnose
RI refractive index
RIA radioimmunoassay
Rib ribose
RIMS resonance ionization mass spectrometry
RIS resonance ionization spectrometry
rms root mean square
RNA ribonucleic acid
RNase ribonuclease
ROA Raman optical activity
RPLC reversed-phase liquid chromatography
rpm revolutions per minute
RQ respiratory quotient
RRDE rotating ring-disk electrode
196 ➤ The ACS Style Guide
RRKM Rice–Ramsperger–Kassel–Marcus
rRNA ribosomal RNA
RRS resonance Raman spectroscopy
RRT relative retention time
RS Raman spectroscopy
RSD relative standard deviation
risk-specific dose
Ry rydberg
σ standard deviation
surface charge density
surface tension
tensile strength
type of orbital, electron
Σ summation
s second
single bond [as in s-cis (italic in compound names)]
singlet (spectra)
solid [as in NaCl(s)]
strong (spectra)
s secondary (as in s-Bu; but sec-butyl)
sedimentation coefficient
standard deviation (analytical)
symmetrical
s020,w sedimentation coefficient measured at 20 °C in water and
extrapolated to 0 °C
s2 sample variance
S serine
siemens
S entropy
sinister (configurational)
skew (conformation)
S/N signal-to-noise ratio
SAM self-assembled monolayer
SANS small-angle neutron scattering
SAPR-8 square antiprismatic, coordination number 8
sar sarcosine (N-methylglycine) (ligand)
Sar sarcosyl, sarcosine (N-methylglycine)
SAR structure–activity relationship
SARISA surface analysis by resonance ionization of sputtered atoms
SAXS small-angle X-ray scattering (or spectroscopy)
sc subcutaneously
sc synclinal
sccm standard cubic centimeters per minute
SCE saturated calomel electrode
SCF self-consistent field
SCF–HF self-consistent field, Hartree–Fock
Chapter 10: Editorial Style ➤ 197
scfh standard cubic feet per hour
SCOT support-coated open-tubular
SD standard deviation
SDS sodium dodecyl sulfate
SE standard error
SE2 second-order electrophilic substitution
sec secant
sec secondary (as in sec-butyl; but s-Bu)
SEC size exclusion chromatography
sech hyperbolic secant
SECM scanning electrochemical microscopy
SECS simulation and evaluation of chemical synthesis
SEM scanning electron microscopy
standard error of the mean
Ser seryl, serine
SERS surface-enhanced Raman spectroscopy (or scattering)
SEW surface electromagnetic wave
Sex exciplex substitution
SFC supercritical-fluid chromatography
sh sharp (spectra)
shoulder (spectra)
Sh Sherwood number
SHC shape and Hamiltonian constant
SHE standard hydrogen electrode
si stereochemical descriptor (as in the si face)
SI International System of Units (Système International)
secondary ion (as in SIMS)
SIM selected-ion monitoring
SIMS secondary-ion mass spectrometry
sin sine
sinh hyperbolic sine
SLR spin–lattice relaxation
SMOSS surface Mössbauer
SMSI strong metal support interaction
sn stereospecific numbering
SN separation number
SN1 first-order nucleophilic substitution
SN2 second-order nucleophilic substitution
SNi internal nucleophilic substitution
SNO semiempirical natural orbital
sol solid
soln solution
sp specific
sp. species (singular)
sp synperiplanar
SP-4 square planar, coordination number 4
sp gr specific gravity
198 ➤ The ACS Style Guide
sp ht specific heat
sp vol specific volume
SPECT single-photon-emission computed tomography
spp. species (plural)
SPR stroboscopic pulse radiolysis
SPY-5 square pyramidal, coordination number 5
sq square
SQF single quantum filtered
SQUID superconducting quantum interference device
sr steradian
SRN1 first-order nucleophilic substitution triggered by electron transfer
SRS stimulated Raman scattering
SSC standard saline citrate (NaCl–citrate)
St stokes
std standard
STEM scanning transmission electron microscopy
STM scanning tunneling microscopy
STO Slater-type orbital
STO-3G Slater-type orbital, three Gaussian
STP standard temperature and pressure
subsp. subspecies
Suc sucrose
Sv sievert
svedberg
SVL single vibrational level
swg standard wire gauge
sym symmetrical
τ tele (far) in NMR measurements (as in Nτ of histidine)
θ angle
[θ] ORD measurement, deg cm2/dmol
Θ temperature (e.g., in Curie–Weiss expressions)
t metric ton
triplet (spectra)
triton
t Student distribution (the Student t test in statistics)
temperature (in degrees Celsius)
tertiary (as in t-Bu; but tert-butyl)
time
t1/2 half-life
T ribosylthymine
tautomeric
tera (1012)
tesla
threonine
tritium
Chapter 10: Editorial Style ➤ 199
T temperature (in kelvins)
twist (conformation)
T-4 tetrahedral, coordination number 4
T/C treated vs cured
tan tangent
tan δ mechanical loss factor
tanh hyperbolic tangent
TBP tri-n-butyl phosphate
TBPY-5 trigonal bipyramidal, coordination number 5
TCA tricarboxylic acid cycle (citric acid cycle, Krebs cycle)
trichloroacetic acid
TCD thermal conductivity detector
TCP/IP transmission control protocol/Internet protocol
TDS total dissolved solids
TEA tetraethylammonium
transversely excited atmospheric
TEAE triethylaminoethyl (as in TEAE-cellulose)
TEM transmission electron microscopy
temp temperature
tert tertiary (as in tert-butyl; but t-Bu)
tetr tetragonal (crystal structure)
TFA trifluoroacetyl
Tg glass-transition temperature
TGA thermogravimetric analysis
Tham tris(hydroxymethyl)aminomethane (also Tris)
THC tetrahydrocannabinol
Thd ribosylthymine
theor theoretical
THF tetrahydrofuran
Thr threonyl, threonine
Thy thymine
TIMS thermal ionization mass spectrometry
TIP temperature-independent paramagnetism
TL triboluminescence
TLC thin-layer chromatography
TMA thermomechanical analysis
TMS tetramethylsilane
trimethylsilyl
TMV tobacco mosaic virus
TnL tunnel luminescence
TOC total organic carbon
TOD total oxygen demand
TOFMS time-of-flight mass spectrometry (also TOF MS)
tol tolyl (also Tol)
TOM transmitted optical microscopy
Torr torr
200 ➤ The ACS Style Guide
tosyl 4-toluenesulfonyl (also Ts)
TPD temperature-programmed desorption
TPDE temperature-programmed decomposition
TPR temperature-programmed reduction
TPR-6 trigonal prismatic, coordination number 6
TQMS triple-quadrupole mass spectrometry
tR retention time
tr trace
Tr trace
tric triclinic (crystal structure)
triflate trifluoromethanesulfonate
trig trigonal (crystal structure)
TRIR time-resolved infrared
Tris tris(hydroxymethyl)aminomethane (also Tham)
tRNA transfer RNA
Trp tryptophyl, tryptophan
Ts tosyl (4-toluenesulfonyl)
TSC thermal stimulated current
TSH thyroid-stimulating hormone
tu thiourea (ligand)
TVA thermal volatilization analysis
Tyr tyrosyl, tyrosine
u unified atomic mass unit
U uniformly labeled
uridine
U internal energy
UCST upper critical solution temperature
UDP uridine 5′-diphosphate
uhf ultrahigh frequency
UHF ultrahigh frequency
unrestricted Hartree–Fock
UHV ultrahigh vacuum
ULSI ultra-large-scale integration
UMP uridine 5′-monophosphate, uridine 5′-phosphate
uncor uncorrected
uns unsymmetrical
UPS ultraviolet photoelectron spectroscopy
ur urea (ligand)
Ura uracil
Urd uridine
USP United States Pharmacopeial Convention
UTP uridine 5′-triphosphate
UV ultraviolet
UV PES ultraviolet photoelectron spectroscopy
UV–vis ultraviolet–visible
Chapter 10: Editorial Style ➤ 201
v vendeko (10–30)
v scan rate
velocity
V valine
vendeca (1030)
volt
v/v volume per volume
Val valyl, valine
VASS variable-angle sample spinning
VB valence bond
VCD vibrational circular dichroism
VDT video display terminal
VEELS vibrational electron energy loss spectroscopy
VESCF variable electronegativity self-consistent field
vhf very high frequency
VHF very high frequency
vic vicinal
vis visible
viz. namely
VLE vapor–liquid equilibrium
VLSI very large scale integration
VOA vibrational optical activity
VOC volatile organic compound
vol volume
vol % volume percent
vp vapor pressure
VPC vapor-phase chromatography
VPO vapor pressure osmometry
VRML virtual reality modeling language
vs versus (v in legal expressions)
very strong (spectra)
VSIP valence-state ionization potential
VUV vacuum ultraviolet
VVk Van Vleck
vw very weak (spectra)
w weak (spectra)
w weighting factor
work
W tryptophan
watt
W work
w/v weight per volume
w/w weight per weight
WAN wide-area network
WAXS wide-angle X-ray scattering
202 ➤ The ACS Style Guide
Wb weber
WCOT wall-coated open-tubular
WDS wavelength-dispersive spectroscopy
WHSV weight-hourly space velocity
WLF Williams–Landel–Ferry (molecular models)
wt weight
wt % weight percent
x xenno (10–27)
x x axis
X xanthosine (use N for unknown nucleoside)
xenna (1027)
Xan xanthine
XANES X-ray absorption near-edge spectroscopy
X-ray absorption near-edge structure
Xao xanthosine
XEDS X-ray energy-dispersive spectrometry
XES X-ray emission spectroscopy
XMP xanthosine 5′-monophosphate, xanthosine 5′-phosphate
XPS X-ray photoelectron spectroscopy
XRD X-ray diffraction
XRDF X-ray radial distance function
XRF X-ray fluorescence
Xyl xylose
y yocto (10–24)
y y axis
Y tyrosine
yotta (1024)
z zepto (10–21)
z charge number of an ion
z axis
Z benzyloxycarbonyl (also Cbz)
“glutamic acid or glutamine”
zetta (1021)
Z atomic number
zusammen (configurational)
zfs zero-field splitting
zfsc zero-field-splitting constant
203
➤ ➤ ➤ ➤ ➤ CHAPTER 11
Numbers,
Mathematics, and
Units of Measure
Numbers
Both numerals and words can be used to express numbers. The usage and style
conventions for numerals and words are different for technical and nontechnical
material.
Numeral and Word Usage
➤ Use numerals with units of time or measure, and use a space between the
numeral and the unit, except %, $, ° (angular degrees), ′ (angular minutes), and
″ (angular seconds).
6 min 25 mL 125 V/s
0.30 g 50% $250
273 K 47°8′23″ 180° (but 180 °C)
90 °F 50 µg of compound/dL of water
exception Spell out numbers with units of measure used in a nontechni-
cal sense.
If you take five minutes to read this article, you’ll be surprised.
➤ With items other than units of time or measure, use words for cardinal
numbers less than 10; use numerals for 10 and above. Spell out ordinals “first”
through “ninth”; use numerals for 10th or greater.
three flasks 30 flasks
Copyright 2006 American Chemical Society
204 ➤ The ACS Style Guide
third flask 12th flask
seven trees 10 trees
eighth example 33rd example
first century 21st century
sixfold 20-fold
exception 1 Use all numerals in a series or range containing numbers 10
or greater, even in nontechnical text.
5, 8, and 12 experiments
2nd and 20th samples
5–15 repetitions
exception 2 Use all numerals for numbers modifying nouns in parallel
construction in the same sentence if one of the numbers is 10 or greater.
Activity was reduced in 2 pairs, not significantly changed in 11 pairs, and
increased in 6 pairs.
We present new results pertaining to 12 phenanthrolines and 3 porphyrins.
exception 3 For very large numbers used in a nontechnical sense, use a
combination of numerals and words.
1 billion tons
180 million people
2 million pounds (not lb)
4.5 billion years
$15 million (not 15 million dollars)
➤ When a sentence starts with a specific quantity, spell out the number as well
as the unit of measure.
Twelve species were evaluated in this study.
Twenty slides of each blood sample were prepared.
Fifteen milliliters of supernate was added to the reaction vessel.
Twenty-five milliliters of acetone was added, and the mixture was centrifuged.
However, if possible, recast the sentence.
Acetone (25 mL) was added, and the mixture was centrifuged.
A 25 mL portion of acetone was added, and the mixture was centrifuged.
➤ Even when a sentence starts with a spelled-out quantity, use numerals when
appropriate in the rest of the sentence.
Twenty-five milliliters of acetone and 5 mL of HCl were added.
Three micrograms of sample was dissolved in 20 mL of acid.
Fifty samples were collected, but only 22 were tested.
Chapter 11: Numbers, Mathematics, and Units of Measure ➤ 205
➤ Use numerals for expressions used in a mathematical sense.
The incidence of disease increased by a factor of 4.
The yield of product was decreased by 6 orders of magnitude.
The efficiency of the reaction was increased 2-fold.
After 2 half-lives, the daughter product could be measured.
The control group had 3 times the risk for colon cancer.
The values are determined with 5 degrees of freedom.
➤ When the suffix “fold” is used in a nonmathematical sense, spell out the
accompanying number if it is less than 10.
The purpose of this discussion is twofold.
➤ When the word “times” is used in a nonmathematical sense, spell out the
accompanying number if it is less than 10.
The beaker was rinsed four times.
➤ Use numerals in ratios.
a ratio of 1:10
a ratio of 1/10
a 1:1 (v/v) mixture
a 1/1 (v/v) mixture
➤ In dates, use numerals without ordinal endings.
January 3, Jan 3 (not January 3rd, Jan 3rd)
September 5, Sept 5 (not September 5th, Sept 5th)
➤ Use numerals for decades, and form their plurals by adding an “s”. Do not use
apostrophes in any position.
the 1960s (not the 1960’s, not the ’60s)
values in the 90s (not the 90’s)
She is in her 20s. (not her 20’s)
➤ Use numerals with a.m. and p.m.
12:15 a.m. 4:00 p.m.
➤ Spell out and hyphenate fractions whose terms are both less than 10. If one
of the terms is 10 or greater, use a piece fraction.
one-quarter of the experiments
two-thirds of the results
¹⁄₂₀ of the subjects
¹⁄₁₂ of the volume
206 ➤ The ACS Style Guide
➤ Use numerals to label figures, tables, schemes, structures, charts, equations,
and references. Number sequentially; do not skip numbers or number out of
sequence. Use arabic numerals for references, but for the other items, the use of
arabic and roman numerals varies among ACS publications. Consult a recent
issue or author instructions to determine what system is preferred.
➤ In journal articles and book chapters, instead of repeating chemical names
over and over, use numerals in boldface (not italic) type to identify chemical spe-
cies. Use these identifiers only in text, not in article or chapter titles, and number
consecutively.
This paper describes the syntheses, structures, and stereodynamic behavior of the
novel hexacoordinate silicon complexes 1–4.
The cyclization of 1,3,5-hexatriene (6) to 1,3-cyclohexadiene (7) is predicted to
proceed more rapidly in an electrostatic field.
Complexes 8–12, in the presence of monoamine oxidase, produce active catalysts
for propylene polymerization.
Primary amines 2–5, 7, and 9 gave the same Cotton effect signs, depending on
the configuration.
Monomer III reacts with the initiator (I, Ar = 2,6-diisopropylphenyl) via a ring-
opening metathesis polymerization mechanism.
➤ Numerals may be used to name members of a series.
Sample 1 contained a high level of contamination, but samples 2 and 3 were rela-
tively pure.
Methods 1 and 2 were used for water-soluble compounds, and methods 3 and 4
were used for oil-soluble compounds.
➤ When numerals are used as names and not enumerators, form their plurals
by adding an apostrophe and “s” to avoid confusion with mathematical expres-
sions and to make it clear that the “s” is not part of the name.
The athlete received five 9’s from the judges.
Boeing 747’s are among the largest airplanes.
➤ Arabic numerals in parentheses may be used to enumerate a list of phrases or
sentences in text. Always use an opening and a closing parenthesis, not one alone.
Some advantages of these materials are (1) their electrical properties after pyrol-
ysis, (2) their ability to be modified chemically before pyrolysis, and (3) their
abundance and low cost.
The major conclusions are the following: (1) We have further validated the utility
of molecular mechanical methods in simulating the kinetics of these reactions.
Chapter 11: Numbers, Mathematics, and Units of Measure ➤ 207
(2) A comparison of the calculated structures with available X-ray structures
revealed satisfactory agreement. (3) The combined use of different theoretical
approaches permitted characterization of the properties of a new isomer.
➤ Arabic numerals followed by periods or enclosed in parentheses may be used
to enumerate a displayed list of sentences or to number paragraphs. Here are two
acceptable ways to format a list.
These results suggest the following:
1. Ketones are more acidic than esters.
2. Cyclic carboxylic acids are more acidic than their acyclic analogues.
3. Alkylation of the active methylene carbon reduces the acidity.
These results suggest the following:
(1) Ketones are more acidic than esters.
(2) Cyclic carboxylic acids are more acidic than their acyclic analogues.
(3) Alkylation of the active methylene carbon reduces the acidity.
Style for Numbers
➤ For very large numbers with units of measure, use scientific notation or
choose an appropriate multiplying prefix for the unit to avoid numbers of more
than four digits.
1.2 × 106 s
3.0 × 104 kg
5.8 × 10–5 M or 58 µM
42.3 L (not 42,300 mL or 42 300 mL)
exception 1 In tables, use the same unit and multiplying prefix for all entries
in a column, even if some entries therefore require four or more digits.
exception 2 Use the preferred unit of a discipline, even when the numbers
require four or more digits:
g/L for mass density of fluids
kg/m3 for mass density of solids
GPa for modulus of elasticity
kPa for fluid pressure
MPa for stress
➤ In four-digit numbers, use no commas or spaces.
exception Spaces or commas are inserted in four-digit numbers when
alignment is needed in a column containing numbers of five or more digits.
➤ When a long number cannot be written in scientific notation, the digits must
be grouped. For grouping of digits in long numbers (five digits or greater), check
the publication in which the manuscript will appear. Two styles are possible.
208 ➤ The ACS Style Guide
style 1 In some publications, for numbers with five or more digits, the dig-
its are grouped with commas placed between groups of three counting to the
left of the decimal point.
4837
10,000
930,582
6,398,210
85,798.62578
style 2 In some publications (including ACS journals), for numbers with
five or more digits, the digits are grouped with a thin space between groups
of three, counting both to the left and to the right of the decimal point.
9319.4
74 183.0629
0.508 27
501 736.293 810 4
exceptions
• U.S. monetary values are always written with commas: $5,000.
• U.S. patent numbers are always written with commas: U.S. Patent
6,555,655. The patent numbers of other countries should be presented as
on the original patent document.
• Page numbers in reference citations are always printed solid: p 11597.
➤ Use the period as the decimal point, never a comma.
➤ Use numerals before and after a decimal point.
0.25 (not .25)
78.0 or 78 (not 78.)
➤ Use a decimal and a zero following a numeral only when such usage truly repre-
sents the precision of the measurement: 27.0 °C and 27 °C are not interchangeable.
➤ Use decimals rather than fractions with units of time or measure, except
when doing so would imply an unwarranted accuracy.
3.5 h (not 3½ h)
5.25 g (not 5¼ g)
➤ Standard deviation, standard error, or degree of accuracy can be given in two
ways:
• with only the deviation in the least significant digit(s) placed in parenthe-
ses following the main number and closed up to it or
• with all digits preceded by a ± and following the main number. Spaces are
left on each side of the ±.
Chapter 11: Numbers, Mathematics, and Units of Measure ➤ 209
2.0089(1) means 2.0089 ± 0.0001
1.4793(23) means 1.4793 ± 0.0023
The shorter version is better in tables. Always specify which measure (e.g., stand-
ard deviation or standard error) of uncertainty is being used.
➤ When two numbered items are cited in narrative, use “and”.
Figures 1 and 2
refs 23 and 24
compounds I and II
➤ Use a comma between two reference callouts in parentheses or as superscripts.
Lewis (12, 13) found
Lewis12,13 found
When the reference numbers are on the line, the comma is followed by a space;
when the numbers are superscripts, the comma is not followed by a space.
➤ Use an en dash in ranges or series of three or more numbered items, whether
on the line or in a superscript.
43–49
325–372
2005–2008
Tables 1–4
temperatures of 100–125 °C
refs 3–5
aliquots of 50–100 mL
eqs 6–9
samples 5–10
past results (27–31)
past results27–31
pp 165–172
exception 1 Do not use an en dash in expressions with the words “from …
to” or “between … and”.
from 20 to 80 (not from 20–80)
between 50 and 100 mL (not between 50–100 mL)
exception 2 When either one or both numbers are negative or include a
symbol that modifies the number, use the word “to” or “through”, not the en
dash.
–20 to +120 K
–145 to –30 °C
≈50 to 60
10 to >600 mL
<5 to 15 mg
➤ For ranges in scientific notation, retain all parts of all numbers or avoid
ambiguity by use of parentheses or other enclosing marks.
9.2 × 10–3 to 12.6 × 10–3 or (9.2–12.6) × 10–3 (not 9.2 to 12.6 × 10–3)
210 ➤ The ACS Style Guide
➤ For very large numbers in ranges, retain all parts of all numbers.
26 million to 35 million
➤ Do not use e or E to mean “multiplied by the power of 10”.
3.7 × 105 (not 3.7e5, 3.7E+5)
Mathematics
Mathematical Concepts
variable A variable is a quantity that changes in value, substance, or
amount, such as V for volume, m for mass, and t for time.
constant A constant is a quantity that has a fixed value, such as h for the
Planck constant and F for the Faraday constant.
function The function f(x) = y represents a rule that assigns a unique
value of y to every x. The argument of the function is x.
operator An operator is a symbol, such as a function (d, derivative; ln,
natural logarithm; and , the Hamiltonian operator) or an arithmetic sign
(+, –, =, ÷, and ×), denoting an operation to be performed.
physical quantity A physical quantity is a product of a numerical value
(a pure number) and a unit. Physical quantities may be scalars or vectors,
variables or constants.
scalar A scalar is an ordinary number without direction, such as length,
temperature, or mass. Any quantity that is not a vector quantity is a scalar
quantity.
vector A vector is a quantity with both magnitude and direction, such as
force or velocity. For the vector V = [a, b] (also denoted as V
j= [a, b]), a and
b are the components of the vector.
tensor A tensor represents a generalized vector with more than two com-
ponents.
matrix A matrix is represented by a rectangular array of elements; an array
consists of rows and columns. The elements of matrix U are u11, u12, etc.
U=
u u
u u
n
n nn
11 1
1

Chapter 11: Numbers, Mathematics, and Units of Measure ➤ 211
determinant The determinant of a matrix is a function that assigns a
number to a matrix. For example, the determinant of the n × n matrix B is
represented by
det B=
b b
b b
n
n nn
11 1
1
index An index is a subscript or superscript character in an element of a
matrix, vector, or tensor; indices usually represent numbers. For example, i
and j are indices in bij.
Do not confuse abbreviations and mathematical symbols. An abbreviation is usu-
ally two or more letters; a mathematical symbol is generally only one letter, pos-
sibly with a subscript or superscript. An abbreviation is used in narrative text but
seldom appears in equations; a mathematical symbol is preferred in equations and
may also be used in text. For example, in text with no equations, PE for potential
energy is acceptable, but in mathematical text and equations, Ep is preferred.
Usage and Style for Symbols
➤ Define all symbols for mathematical constants, variables, and unknown
quantities the first time you use them in the text. If you use them in the abstract,
define them there and then again at their first appearance in text. Do not define
standard mathematical constants such as π, i, and e.
➤ Form the plurals of mathematical symbols by adding an apostrophe and “s”
if you cannot use a word such as “values” or “levels”.
large r values is better than large r’s
➤ Do not use an equal sign as an abbreviation for the word “is” or the word
“equals” in narrative text.
PV = nRT, where P is pressure (not where P = pressure)
when the temperature is 50 °C (not when the temperature = 50 °C)
➤ Do not use a plus sign as an abbreviation for the word “and” in narrative text.
a mixture of A and B (not a mixture of A + B)
➤ Do not use an asterisk to indicate multiplication except in computer language
expressions.
212 ➤ The ACS Style Guide
Italic Type
➤ Use italic type for
• variables: T for temperature, x for mole fraction, r for rate
• axes: the y axis
• planes: plane P
• components of vectors and tensors: a1 + b1
• elements of determinants and matrices: gn
• constants: kB, the Boltzmann constant; g, the acceleration due to gravity
• functions that describe variables: f(x)
➤ Even when you use mathematical constants, variables, and unknown quanti-
ties in adjective combinations, retain the italic type.
In this equation, Vi is the frequency of the ith mode.
In eq 4, n is the number of extractions and M is the mass remaining after the nth
extraction.
➤ Use italic type for two-letter variables defining transport properties.
Al Alfvén number
Bi Biot number
Co Cowling number
Da Damkohler number
Eu Euler number
Fo Fourier number
Fr Froude number
Ga Galileo number
Gr Grashof number
Ha Hartmann number
Kn Knudsen number
Le Lewis number
Ma Mach number
Nu Nusselt number
Pe Péclet number
Pr Prandtl number
Ra Rayleigh number
Re Reynolds number
Sc Schmidt number
Sh Sherwood number
Sr Strouhal number
St Stanton number
We Weber number
Wi Weissenberg number
Roman Type
➤ Use roman type for
• numerals;
• punctuation and enclosing marks such as square brackets, parentheses,
and braces;
• most operators;
• units of measure and time: mg, milligram; K, kelvin; Pa, pascal; mmHg,
millimeters of mercury;
• nonmathematical quantities or symbols: R, radical in chemical nomen-
clature; S1, molecular state; s, atomic orbital;

Chapter 11: Numbers, Mathematics, and Units of Measure ➤ 213
• multiple-letter abbreviations for variables: IP, ionization potential; cmc,
critical micelle concentration;
• mathematical constants:
e, the base of the natural logarithm, 2.71828…
i, the imaginary number, (–1)1/2
π, 3.14159…
• transposes of matrices: AT (T is the transpose of matrix A);
• points and lines: point A, line AB;
• determinants: det A is the determinant of matrix A; and
• trigonometric and other functions:
Ad adjoint
Ai Airy function
arg argument
Bd bound
cl closure
Coker cokernel
cos cosine
cosh hyperbolic cosine
cot cotangent
coth hyperbolic cotangent
csc cosecant
csch hyperbolic cosecant
det determinant
dim dimension
div divergence
erf error function
erfc complement of error function
exp exponential
GL general linear
glb greater lower bound
grad gradient
hom homology
Im imaginary
inf inferior
int interior
ker kernel
lim limit
lim inf limit inferior
lim sup limit superior
ln natural logarithm (base e)
log logarithm (base 10)
Log principal logarithm
lub least upper bound
max maximum
min minimum
mod modulus
P property
Re real
sec secant
sech hyperbolic secant
sign, sgn sign
sin sine
sinh hyperbolic sine
SL special linear
sp spin
Sp symplectic
sup superior
Sz(g) Suzuki group
tan tangent
tanh hyperbolic tangent
tr trace
wr wreath
Boldface Type
➤ Use boldface type for
• vectors;
• tensors;
• matrices; and
• multidimensional physical quantities: H, magnetic field strength.
214 ➤ The ACS Style Guide
Greek Letters
Greek letters (lightface or boldface) can be used for variables, constants, and vec-
tors and anywhere a Latin letter can be used.
name uppercase lowercase
Alpha Α α
Beta Β β
Gamma Γ γ
Delta ∆ δ, ∂
Epsilon Ε , ε
Zeta Ζ ζ
Eta Η η
Theta Θ θ, ϑ
Iota Ι ι
Kappa Κ κ
Lambda Λ λ
Mu Μ µ
Nu Ν ν
Xi Ξ ξ
Omicron Ο ο
Pi Π π
Rho Ρ ρ
Sigma Σ σ
Tau Τ τ
Upsilon Υ υ
Phi Φ ϕ, φ
Chi Χ χ
Psi Ψ ψ
Omega Ω ω
Script and Open-Faced Letters
➤ Script () and open-faced (; also known as blackboard boldface) letters are
available but should not be used routinely. For open-faced letters, only uppercase
is available.
Spacing
➤ Leave a space before and after functions set in roman type, unless the argu-
ment is enclosed in parentheses, brackets, or braces.
log 2
–log x
4 sin θ
tan2 y
exp(–x)
cosh(βe0φ)
4 tan(2y)
erfc(y)
Chapter 11: Numbers, Mathematics, and Units of Measure ➤ 215
➤ Leave a space before and after mathematical operators that function as verbs
or conjunctions; that is, they have numbers on both sides or a symbol for a vari-
able on one side and a number on the other.
20 ± 2%
3.24 ± 0.01
4 × 5 cm
8 × 10–4
k ≥ 420 s–1
p < 0.01
Tg = 176 °C
n = 25
1 in. = 2.54 cm
exception 1 Leave no space around mathematical operators in subscripts
and superscripts.
∆Hn–1
Eλ>353
M(x+y)+
exception 2 Leave no space around a slash (a/b), a ratio colon (1:10), or a
centered dot (PM·V).
➤ Leave no space between simple variables being multiplied: xy. Do not use a
centered dot (·) or the times sign (×) with single-letter scalar variables.
➤ In multiplication involving the two-letter symbols for transport properties,
use a space, enclose them in parentheses, or use the times sign. When super-
scripts or subscripts are present, the symbols can be closed up.
Re Nu
(Re)(Nu)
Re × Nu
RexNuy
➤ Use a space for simple multiplication of functions of the type f(x) (one-dimen-
sional) or g(y, z) (multidimensional). Close up multipliers to such functions where
applicable. You may also use additional enclosing marks instead of spaces.
W = 2f(x) g(y, z)
W = 2[f(x)][g(y, z)]
➤ When mathematical symbols are used as adjectives, that is, with one number
that is not part of a mathematical operation, do not leave a space between the
symbol and the number.
–12 °C
25 g (±1%)
at 400× magnification
a conversion of >50%
a probability of <0.01
The level can vary from –15 to +25 m.
216 ➤ The ACS Style Guide
Enclosing Marks
➤ Use enclosing marks (parentheses, brackets, and braces, also called fences)
in accordance with the rules of mathematics. Enclose parentheses within square
brackets, and square brackets within braces: {[( )]}.
➤ Use enclosing marks around arguments when necessary for clarity.
sin(x + 1)
sin[2π(x – y)/n]
log[–V(r)/kT]
➤ Do not use square brackets, parentheses, or braces around the symbol for a
quantity to make it represent any other quantity.
incorrect
where V is volume and (V) is volume at equilibrium
correct
where V is volume and Ve is volume at equilibrium
Subscripts and Superscripts
➤ Use italic type for subscripts and superscripts that are themselves symbols for
physical quantities or numbers. Use roman type for subscripts and superscripts
that are abbreviations and not symbols.
Cp for heat capacity at constant pressure
CB for heat capacity of substance B
Cg where g is gas
Ei for energy of the ith level, where i is a number
gn where n is normal
µr where r is relative
Ek where k is kinetic
ξe where e is electric
➤ In most cases, staggered subscripts and superscripts are preferred. Exponents
should follow subscripts.
x12
Cx 1/2
T2m–1
∆H1‡
Eads°
exceptions
λ+
∞σp
+B2
exptl
Chapter 11: Numbers, Mathematics, and Units of Measure ➤ 217
➤ Use a slash (/) in all subscript and superscript fractions, with no space on
either side.
t1/2 x1/2 M2/3 fa/b
➤ Leave no space around operators in subscripts and superscripts.
M(2–n)+ ET+θ
➤ Leave no space around other expressions in subscripts and superscripts,
unless confusion or misreading would result.
Qn-Bu(750°C) βzero level E365nm
➤ The terms ea and exp a have the same meaning and can be interchanged.
When an exponent to the base e is very long or complicated, replace the e with
exp and place the exponent on line and in enclosing marks. Leave no space
between exp and the opening enclosing mark.
exp(∫y dt) (not e∫ydt)
exp{½kT[Y(a + b) – Z]} (not e½kT[Y(a+b)–Z])
➤ In running text, do not use the radical sign (√–) with long terms. Use enclos-
ing marks around the term and a superscript 1/2, 1/3, 1/4 (etc.) for square, cube,
fourth root (etc.), respectively.
(x – y2)1/3
[sinh2 u + (cosh u – 1)2]1/2
Abbreviations and Symbols
➤ Certain abbreviations are used only in the context of mathematical equa-
tions. Define all of these the first time they are used.
lhs left-hand side (of an equation)
rhs right-hand side (of an equation)
ODE ordinary differential equation
rms root mean square
rmsd root-mean-square deviation
s.t. subject to
wrt, WRT with respect to
➤ Some standard usages and symbols for mathematical operations and con-
stants need never be defined. They include the following:
e natural base (approximately 2.7183)
exp x, ex exponential of x
i
−1
ln x natural logarithm of x

218 ➤ The ACS Style Guide
log x logarithm to the base 10 of x
loga x logarithm to the base a of x
≈ approximately equal to
asymptotically equal to
∝, ∼ proportional to
→ approaches (tends to)
≡ identically equal to
∞ infinity
Σ summation
Π product
union
integral
line integral around a closed path
∇ del (or nabla) operator, gradient
∇2 Laplacian operator
< less than
≤ less than or equal to
much less than
> greater than
≥ greater than or equal to
much greater than
≠ not equal to
|| parallel to
⊥ perpendicular to
|a| absolute magnitude of a
a1/2,
a
square root of a
a1/n,
a
n
nth root of a
a, 〈a〉 mean value of a
∆x finite increment of x
∂x partial differential, infinitesimal increment of x
dx total differential of x
f(x) function of x
y dx integral of y with respect to x
y x
a
b
∫d
integral of y from x = a to x = b
A vector of magnitude A
A·B scalar product of A and B
A × B, AB vector product of A and B
AB length of line from A to B
Equations
Mathematical equations can be presented within running text or displayed on
lines by themselves. Follow the guidelines for style and usage just described
under “Usage and Style for Symbols” (starting on p 211).

Chapter 11: Numbers, Mathematics, and Units of Measure ➤ 219
➤ Leave a space
• before and after mathematical signs used as operators (=, ≠, ≡, ∼, ≈, ≅, >,
<, +, –, ×, ÷, , , , , etc., but not slash (/), ratio colon, or centered
dot), except when they appear in superscripts or subscripts
• before trigonometric and other functions set in roman type
• after trigonometric and other functions set in roman type when their
arguments are not in enclosing marks
• before and after derivatives: f(x) dx f(y) dy or x ∂x
• between built-up (display) fractions as components of products:
a
b
c
d
or write on one line and clarify with enclosing marks and no space:
(a/b)(c/d)
• between functions as components of products: W = 2f(x) g(y, z)
➤ Leave no space
• between single-item variables being multiplied
• in any part of a superscript or subscript, unless confusion or misreading
would result
• between any character and its own superscript, prime, or subscript
• on either side of a colon used for a ratio
• on either side of a centered dot
• on either side of a slash (/)
• after mathematical operators used as adjectives: –10
• after functions when the argument is in parentheses: tanh(λ/2)
• between an opening parenthesis, bracket, or brace and the next character:
(2x)y
• between a closing parenthesis, bracket, or brace and the previous charac-
ter: 2(xy)
• between back-to-back parentheses, brackets, and braces, e.g., ](
• between nested parentheses, brackets, and braces, e.g., [(
• in any part of limits to summations, products, and integrals
• in any part of lower limits to min, max, lim, and inf
➤ Use or do not use spaces around ellipses, depending on the treatment of
other items in the series.
no spaces: anan+1an+2…an+36
spaces: an + an+1 + an+2 + … + an+36
space before: a, b, …, x

220 ➤ The ACS Style Guide
➤ Use enclosing marks in accordance with the rules of mathematics. If the slash
(/) is used in division and if there is any doubt where the numerator ends or
where the denominator starts, use enclosing marks for one or the other or both.
(x + y)/(3x – y)
(a/b)/c, or a/(b/c) but never a/b/c
x y z
+=
2
would be better as (x + y)/2 = z
x y
za
++2
would be better as [(x + y)/z] + 2a
➤ If an equation is very short and will not be referred to again, you may run it
into the text.
A fluid is said to be Newtonian when it obeys Newton’s law of viscosity, given by
τ = ηγ, where τ is the shear stress, η is the fluid dynamic constant, and γ is the
shear rate.
➤ You may use mathematical expressions as part of a sentence when the sub-
ject, verb, and object are all part of the mathematical expression.
When V = 12, eq 15 is valid.
(V is the subject, = is the verb, and 12 is the object.)
➤ When an equation is too long to fit on one line, break it after an operator
that is not within an enclosing mark (parentheses, brackets, or braces) or break
it between sets of enclosing marks. Do not break equations after integral, prod-
uct, and summation signs; after trigonometric and other functions set in roman
type; or before derivatives.
➤ Number displayed equations by using any consistent system of sequencing.
1, 2, 3, …
1a, 1b, 2, …
I, II, III, …
A, B, C, …
A-1, A-2, A-3, …
B.1, B.2, B.3, …
C1, C2, C3, …
➤ Use equation identifiers in the proper sequence according to appearance in
text. Do not skip numbers or letters in the sequence.
➤ Place identifiers in parentheses, flush right on the same line as the equation.
V = 64πkTγ2 exp(–κh) (3)
Chapter 11: Numbers, Mathematics, and Units of Measure ➤ 221
➤ Do not use any closing punctuation on the line with displayed equations.
➤ When introducing a displayed equation, do not automatically use a colon;
in most cases a colon is incorrect because the equation finishes a phrase or sen-
tence.
An ideal gas law analogy is
πA = nRT
If the principal radii are R1 and R2, then
∆ = R1 – R2
The area per adsorbed molecule can be calculated from
a = NAΓS
The attractive energy can be approximated by
VA = Ar(12H)–1
The simplest method is to use a mapping potential of the form
εm = (1 – λm)εA + λmεB
Marshall developed an equation for rapid coagulation:
n = 1 + SπDrt
➤ Following a displayed equation that is part of a sentence, punctuate the text
as if it were a continuation of a sentence including the equation but do not punc-
tuate at the end of the equation. Note the absence of a comma at the end of the
equation in the example. Punctuation that would normally be present at the end
of an equation in text is absent but implicit at the end of a displayed equation.
The capillary pressure P depends significantly on the wetting contact angle
according to
P = 2γ cos(θ/r)
where γ is the surface tension, θ is the wetting contact angle, and r is the radius
of the capillary.
➤ To cite an equation in text, use the abbreviation “eq” if it is not the first word
of the sentence. Spell out “equation” when it is the first word of a sentence or
when it is not accompanied by a number. The plural of “eq” is “eqs”.
The number of independent points can be calculated from eq 3.
The number of independent points can be calculated from eqs 3 and 4.
Equation 1 is not accurate for distances greater than 10 µm.
Equations 1 and 2 are not accurate for distances greater than 10 µm.
222 ➤ The ACS Style Guide
➤ Some notations differ in text and in display:
In display In text
i
N
=
∑
1
ΣN
i=1 or Σi=1N
k
n
=
∏
2
Πn
k=2 or Πk=2n
lim
i=1
limi=1
max
j=2
maxj=2
min
,j k n=
minj=k,n
Ratio and Mixture Notation
➤ Use either a colon or a slash (/) to represent a ratio, but not an en dash. Use
either a slash or an en dash between components of a mixture, but not a colon.
dissolved in 5:1 glycerin/water
dissolved in 5:1 glycerin–water
the metal/ligand (1:1) reaction mixture
the metal–ligand (1:1) reaction mixture
the metal–ligand (1/1) reaction mixture
the methane/oxygen/argon (1/50/450) matrix
the methane/oxygen/argon (1:50:450) matrix
Set Notation
The following symbols are used in set notation. Leave a space before and after all
operators, but not before and after braces.
A = {a, b} set A; A is italic; braces are used
A B union of sets A and B
A B intersection of sets A and B
A B A is a member (element) of B
A B A is not a member (element) of B
A B A is contained in B
A B A is not contained in B
A B A contains B
A B A does not contain B
∀ A for all (every) A
∃ there exists
∋ such that
∴ therefore

Chapter 11: Numbers, Mathematics, and Units of Measure ➤ 223
Geometric Notation
➤ Leave no spaces around geometric notation. Use italic type for planes and
axes and roman type for points and lines.
X⊥Y X is perpendicular to Y
X||Y X is parallel to Y
AB the angle between A and B
AB length of line from A to B
Statistics
Certain statistical symbols are standard.
CV coefficient of variation
df, DF degrees of freedom
f frequency
F variance ratio
n, N total number of individuals or random variables
p, P probability
r correlation coefficient
R regression coefficient
RSD relative standard deviation
σ, SD standard deviation
Σ summation
s2 sample variance
SE standard error
SEM standard error of the mean
t Student distribution (the Student t test)
x arithmetic mean
A common statistical measurement is the Student t test or Student’s t test. Stu-
dent was the pseudonym of W. Gossett, an eminent mathematician.
Units of Measure
➤ Where possible, use metric and SI units (discussed in Appendix 11-1) in all
technical documents. The following conventions apply to all units of measure:
• Abbreviate units of measure when they accompany numbers.
• Leave a space between a number and its unit of measure.
• Do not use a period after an abbreviated unit of measure (exception: in.
for inch).
224 ➤ The ACS Style Guide
• Do not define units of measure.
500 mL
3 min
4 Å
9 V/s
9 V s–1
9 V·s–1
200 mV
4.14 × 10–9 m2/(V s)
2.6 × 104 J
3 min interval
2 µm droplet
500 mL flask
exception Do not leave a space between a number and the percent, angu-
lar degree, angular minute, or angular second symbols.
50%
90°
75′
18″
➤ Use °C with a space after a number, but no space between the degree symbol
and the capital C: 15 °C.
➤ Do not add an “s” to make the plural of any abbreviated units of measure.
The abbreviations are used as both singular and plural.
50 mg (not 50 mgs)
3 mol (not 3 mols)
➤ Write abbreviated compound units with a centered dot or a space between
the units to indicate multiplication and a slash (/) or negative exponent for divi-
sion. Enclose compound units following a slash in parentheses. Usage should be
consistent within a paper.
watt per meter-kelvin is W·m–1·K–1 or
W/(m·K) or
W m–1 K–1 or
W (m K)–1 or
W/(m K)
cubic decimeter per mole-second is dm3·mol–1·s–1 or
dm3/(mol·s) or
dm3 mol–1 s–1 or
dm3 (mol s)–1 or
dm3/(mol s)
joule per mole-kelvin is J·mol–1·K–1 or
J/(mol·K) or
J mol–1 K–1 or
J (mol K)–1 or
J/(mol K)
Chapter 11: Numbers, Mathematics, and Units of Measure ➤ 225
➤ Spell out units of measure that do not follow a number. Do not capitalize
them unless they are at the beginning of a sentence or in a title.
several milligrams (not several mg)
a few milliliters (not a few mL)
degrees Celsius
reciprocal seconds
milligrams per kilogram
volts per square meter
exception 1 Abbreviate units of measure in parentheses after the defini-
tions of variables directly following an equation.
L = D/PO
where L is the distance between particles (cm), D is the particle density (g/cm3),
and PO is the partial pressure of oxygen (kPa).
exception 2 Certain units of measure have no abbreviations: bar, darcy,
einstein, erg, faraday, and langmuir. The symbol for the unit torr is Torr. The
unit rad is abbreviated rd; the unit radian is abbreviated rad.
exception 3 In column headings of tables and in axis labels of figures,
abbreviate units of measure, even without numbers.
➤ Add an “s” to form the plural of spelled-out units: milligrams, poises, kelvins,
amperes, watts, newtons, and so on.
exceptions bar, hertz, lux, stokes, siemens, and torr remain unchanged;
darcy becomes darcies; henry becomes henries.
➤ Do not capitalize surnames that are used as units of measure.
ampere
angstrom
coulomb
curie
dalton
darcy
debye
einstein
erg
faraday
franklin
gauss
gilbert
gray
hartree
henry
hertz
joule
kelvin
langmuir
newton
ohm
pascal
poise
siemens
sievert
stokes
tesla
watt
weber
Celsius and Fahrenheit are always capitalized. They are not themselves units;
they are the names of temperature scales.
➤ Do not use a slash (/) in spelled-out units of measure. Use the word “per”.
Results are reported in meters per second.
The fluid density is given in kilograms per cubic meter.
226 ➤ The ACS Style Guide
➤ Do not mix abbreviations and spelled-out units within units of measure.
newtons per meter (not N per meter)
100 F/m (not 100 farad/m)
exception in more complex situations
50 mL of water and 20 mg of NaOH per gram of compound
➤ Use a slash (/), not the word “per”, before the abbreviation for a unit in com-
plex expressions.
50 µg of peptide/mL
25 mg of drug/kg of body weight
➤ When the first part of a unit of measure is a word that is not itself a unit of
measure, use a slash (/) before the final abbreviated unit.
10 counts/s
12 domains/cm3
2 × 103 ions/min
125 conversions/mm2
➤ When the last part of a unit of measure is a word that is not itself a unit of meas-
ure, use either a slash (/) or the word “per” before the word that is not a unit.
0.8 keV/channel
0.8 keV per channel
7 µB/boron
7 µB per boron
➤ Leave no space between the multiplying prefix and the unit, whether abbrevi-
ated or spelled out.
kilojoule or kJ
milligram or mg
microampere or µA
➤ Use only one multiplicative prefix per unit.
nm (not mµm)
➤ In ranges and series, retain only the final unit of measure.
10–12 mg
5, 10, and 20 kV
60–90°
between 25 and 50 mL
from 10 to 15 min
Chapter 11: Numbers, Mathematics, and Units of Measure ➤ 227
➤ Do not use the degree symbol with kelvin: 115 K.
➤ In titles and headings, do not capitalize abbreviated units of measure that are
ordinarily lowercase.
Analysis of 2 mg Samples
A 50 kDa Protein To Modulate Guanine Nucleotide Binding
228
➤ ➤ ➤ ➤ ➤
APPENDIX 11-1
The International
System of Units (SI)
Before the 1960s, four systems of units were commonly used in the scientific
literature: the English system (centuries old, using yard and pound), the met-
ric system (dating from the 18th century, using meter and kilogram as standard
units), the CGS system (based on the metric system, using centimeter, gram, and
second as base units), and the MKSA or Giorgi system (using meter, kilogram,
second, and ampere as base units).
The International System of Units (SI, Système International d’Unités) is the
most recent effort to develop a coherent system of units. It is coherent because
there is only one unit for each base physical quantity, and units for all other
quantities are derived from these base units by simple equations. It has been
adopted as a universal system to simplify communication of numerical data and
to restrict proliferation of systems. SI units are used by the National Institute
of Standards and Technology (NIST). More information on SI can be found at
http://www.physics.nist.gov/cuu/index.html.
The SI is constructed from seven base units for independent quantities
(ampere, candela, kelvin, kilogram, meter, mole, and second) plus two sup-
plementary units for plane and solid angles (radian and steradian). Most physi-
cochemical measurements can be expressed in terms of these units.
Certain units not part of the SI are so widely used that it is impractical to
abandon them (e.g., liter, minute, and hour) or are so well established that the
International Committee on Weights and Measures has authorized their contin-
ued use (e.g., bar, curie, and angstrom). In addition, quantities that are expressed
in terms of the fundamental constants of nature, such as elementary charge, pro-
ton mass, Bohr magneton, speed of light, and Planck constant, are also accept-
able. However, broad terms such as “atomic units” are not acceptable, although
atomic mass unit, u, is acceptable and relevant to chemistry.
Follow all usage conventions given for units of measure. Use the abbre-
viations for SI units with capital and lowercase letters exactly as they appear in
Tables 11A-1 to 11A-6.

Chapter 11: Numbers, Mathematics, and Units of Measure ➤ 229
Table 11A-1. SI Units
Name Symbol Physical Quantity
Base units
ampere A electric current
candela cd luminous intensity
kelvin K thermodynamic temperature
kilogram kg mass
meter m length
mole mol amount of substance
second s time
Supplementary units
radian rad plane angle
steradian sr solid angle
Table 11A-2. Multiplying Prefixes
Factor Prefix Symbol Factor Prefix Symbol
10–24 yocto y 101deka da
10–21 zepto z 102hecto h
10–18 atto a 103kilo k
10–15 femto f 106mega M
10–12 pico p 109giga G
10–9 nano n 1012 tera T
10–6 micro µ1015 peta P
10–3 milli m 1018 exa E
10–2 centi c 1021 zetta Z
10–1 deci d 1024 yotta Y
Note: Any of these prefixes may be combined with any of the symbols permitted within the SI. Thus, kPa and
GPa will both be common combinations in measurements of pressure, as will mL and cm for measurements of
volume and length, respectively. As a general rule, however, the prefix chosen should be 10 raised to that mul-
tiple of 3 that will bring the numerical value of the quantity to a positive value less than 1000.
Table 11A-3. SI-Derived Units
Name Symbol Quantity
In Terms of
Other Units
In Terms of
SI Base Units
becquerel Bq activity (of a radionuclide) — s–1
coulomb C quantity of electricity, electric charge — s·A
farad F capacitance C/V m–2·kg–1·s4·A2
gray Gy absorbed dose, kerma, specific energy
imparted
J/kg m2·s–2
henry H inductance Wb/A m2·kg·s–2·A–2
hertz Hz frequency — s–1
joule J energy, work, quantity of heat N·m m2·kg·s–2
lumen lm luminous flux cd·sr m2·m–2·cd = cd
lux lx illuminance lm/m2 m2·m–4·cd = m–2·cd
newton N force — m·kg·s–2
ohm Ωelectric resistance V/A m2·kg·s–3·A–2
pascal Pa pressure, stress N/m2m–1·kg·s–2
siemens S conductance A/V m–2·kg–1·s3·A2
sievert Sv dose equivalent J/kg m2·s–2
tesla T magnetic flux density Wb/m2kg·s–2·A–1
volt V electric potential, potential difference,
electromotive force
W/A m2·kg·s–3·A–1
watt W power, radiant flux J/s m2·kg·s–3
weber Wb magnetic flux V·s m2·kg·s–2·A–1

230 ➤ The ACS Style Guide
Table 11A-4. SI-Derived Compound Units
Name Symbol Quantity
In Terms of
Other Units
ampere per meter A/m magnetic field strength —
ampere per square meter A/m2current density —
candela per square meter cd/m2luminance —
coulomb per cubic meter C/m3electric charge density m–3·s·A
coulomb per kilogram C/kg exposure (X-rays and γ rays) —
coulomb per square meter C/m2electric flux density m–2·s·A
cubic meter m3volume —
cubic meter per kilogram m3/kg specific volume —
farad per meter F/m permittivity m–3·kg–1·s4·A2
henry per meter H/m permeability m·kg·s–2·A–2
joule per cubic meter J/m3energy density m–1·kg·s–2
joule per kelvin J/K heat capacity, entropy m2·kg·s–2·K–1
joule per kilogram J/kg specific energy m2·s–2
joule per kilogram kelvin J/(kg K) specific heat capacity, specific
entropy
m2·s–2·K–1
joule per mole J/mol molar energy m2·kg·s–2·mol–1
joule per mole kelvin J/(mol K) molar entropy, molar heat
capacity
m2·kg·s–2·K–1·mol–1
kilogram per cubic meter kg/m3density, mass density —
meter per second m/s speed, velocity —
meter per second squared m/s2acceleration —
mole per cubic meteramol/m3concentration (amount of
substance per volume)
—
newton-meter N·m moment of force m2·kg·s–2
newton per meter N/m surface tension kg·s–2
pascal second Pa·s dynamic viscosity m–1·kg·s–1
radian per second rad/s angular velocity —
radian per second squared rad/s2angular acceleration —
reciprocal meter m–1 wavenumber —
reciprocal second s–1 frequency —
square meter m2area —
square meter per second m2/s kinematic viscosity —
volt per meter V/m electric field strength m·kg·s–3·A–1
watt per meter kelvin W/(m K) thermal conductivity m·kg·s–3·K–1
watt per square meter W/m2heat flux density, irradiance kg·s–3
watt per square meter steradian W/(m2 sr) radiance —
watt per steradian W/sr radiant intensity —
aLiter (L) is a special name for cubic decimeter. The symbol M is not an SI unit, but expressions such as 0.1 M,
meaning a solution with concentration of 0.1 mol/L, are acceptable.

Chapter 11: Numbers, Mathematics, and Units of Measure ➤ 231
Table 11A-5. Other Units
Name Symbol Quantity Value in SI Units
angstrom Å distance 1 Å = 10–10 m = 0.1 nm
bar bar pressure 1 bar = 105 Pa = 100 kPa = 0.1 MPa
barn b area, cross section 1 b = 10–28 m2 = 100 fm2
bohr b, a0length 1 b ≈ 5.291 77 × 10–11 m
curieaCi activity 1 Ci = 3.7 × 1010 Bq
dalton Da atomic mass 1 Da = 1.660 540 × 10–27 kg
darcybdarcy permeability —
day day time 1 day = 24 h = 86 400 s
debyecD electric dipole moment —
degree ° plane angle 1° = (π/180) rad
degree Celsiusd°C temperature —
dyneedyn force —
einsteinfeinstein light energy —
electronvoltgeV — 1 eV = 1.602 19 × 10–19 J
ergherg energy or work —
faraday faraday electric charge 1 faraday = 96 485.31 C
fermi f length 1 f = 10–15 m
franklin Fr electric charge 1 Fr = 3.335 64 × 10–10 C
galileo Gal acceleration 1 Gal = 10–2 m s–2
gauss G magnetic induction 1 G = 10–4 Wb/m2
gilbertiGi magnetomotive force —
hartree hartree, Ehenergy 1 hartree = 4.359 75 × 10–18 J
hectare ha area 1 ha = 1 hm2 = 104 m2
hour h time 1 h = 60 min = 3600 s
liter L volume 1 L = 1 dm3 = 10–3 m3
metric ton t mass 1 t = 103 kg
minute min time 1 min = 60 s
minute ′plane angle 1′ = (1/60)° = (π/10 800) rad
parsec pc length 1 pc ≈ 3.085 68 × 1016 m
poisejP dynamic viscosity —
rad rad, rdkabsorbed dose 1 rad = 0.01 Gy = 1 cGy = 100 erg·g–1
roentgen R exposure 1 R = 2.58 × 10–4 C·kg–1
roentgen equivalent
manl
rem weighted absorbed
dose
1 rem = 0.01 Sv
second ″plane angle 1″ + (1/60)′ = (π/648 000) rad
stokesmSt kinematic viscosity —
svedberg Sv time 1 Sv = 10–13 s
unified atomic mass
unit n
u — 1 u = 1.660 540 × 10–27 kg
a1 Ci = 2.2 × 1012 disintegrations per minute.
b1 darcy is the permeation achieved by the passage of 1 mL of fluid of 1 cP viscosity flowing in 1 s under a pres-
sure of 1 atm (101 kPa) through a porous medium that has a cross-sectional area of 1 cm2 and a length of 1 cm.
c1 D = 10–18 Fr cm.
dTemperature intervals in kelvins and degrees Celsius are identical; however, temperature in kelvins equals tem-
perature in degrees Celsius plus 273.15.
e1 dyn is equal to the force that imparts an acceleration of 1 cm/s2 to a 1 g mass.
f1 einstein equals Avogadro’s number times the energy of one photon of light at the frequency in question.
gThe electronvolt is the kinetic energy acquired by an electron in passing through a potential difference of 1 V
in vacuum.
h1 erg is the work done by a 1 dyn force when the point at which the force is applied is displaced by 1 cm in the
direction of the force.
i1 Gi is the magnetomotive force of a closed loop of one turn in which there is a current of (1/4π) × 10 A.
j1 P is the dynamic viscosity of a fluid in which there is a tangential force of 1 dyn/cm2 resisting the flow of two
parallel fluid layers past each other when their differential velocity is 1 cm/s per centimeter of separation.
kWhen there is a possibility of confusion with the symbol for radian, rd may be used as the symbol for rad.
l1 rem has the same biological effect as 1 rad of X-rays.
m1 St is the kinematic viscosity of a fluid with a dynamic viscosity of 1 P and a density of 1 g/cm3.
nThe unified atomic mass unit is equal to 1
/12 of the mass of an atom of the nuclide 12C.
232 ➤ The ACS Style Guide
Table 11A-6. Non-SI Units That Are Discouraged
Discouraged Unit Value in SI Units
calorie (thermochemical) 4.184 J
conventional millimeter of mercury 133.322 Pa
grad 2π/400 rad
kilogram-force 9.806 65 N
metric carat 0.2 g
metric horsepower 735.499 W
mho 1 S
micron 1 µm
standard atmosphere 101.325 kPa
technical atmosphere 98.066 5 kPa
torr 133.322 Pa
233
➤ ➤ ➤ ➤ ➤ CHAPTER 12
Names and Numbers
for Chemical
Compounds
The use of proper chemical nomenclature is essential for
effective scientific communication. More than one million
new substances are reported each year, each of which must be identified clearly,
unambiguously, and completely in the primary literature. Chemical compounds
are named according to the rules established by the International Union of Pure
and Applied Chemistry (IUPAC), the International Union of Biochemistry and
Molecular Biology (IUBMB) [formerly the International Union of Biochemistry
(IUB)], the Chemical Abstracts Service (CAS), the Committee on Nomenclature,
Terminology, and Symbols of the American Chemical Society, and other authori-
ties as appropriate. For more information on naming chemical compounds, refer
to the bibliography in Chapter 18. This chapter gives the editorial conventions
and style points for chemical compound names.
Components of Chemical Names
The names of chemical compounds may consist of one or more words, and
they may include locants, descriptors, and syllabic portions. Locants and
descriptors can be numerals, element symbols, small capital letters, Greek let-
ters, Latin letters, italic words and letters, and combinations of these. Treat the
word or syllabic portions of chemical names just like other common nouns:
use roman type, keep them lowercase in text, capitalize them at the beginnings
of sentences and in titles, and hyphenate them only when they do not fit com-
pletely on one line.
Copyright 2006 American Chemical Society

234 ➤ The ACS Style Guide
Locants and Descriptors
➤ Numerals used as locants can occur at the beginning of or within a chemical
name. They are set off with hyphens. (See Box 12-1.)
6-aminobenzothiazole
di-2-propenylcyanamide
4a,8a-dihydronaphthalene
5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one
6-hydroxy-2-naphthalenesulfonic acid
3′-methylphthalanilic acid
➤ Use italic type for chemical element symbols that denote attachment to an
atom or a site of ligation.
B,B′-di-3-pinanyldiborane
bis[(ethylthio)acetato-O,S]platinum
glycinato-N
N-acetyl group
N-ethylaniline
N,N′-bis(3-aminopropyl)-1,4-butanediamine
O,O,S-triethyl phosphorodithioate
P-phenylphosphinimidic acid
S-methyl benzenethiosulfonate
➤ When element symbols are used with a type of reaction as a noun or adjec-
tive, use roman type for the symbol and hyphenate it to the word that follows it.
N-acetylated
N-acetylation
N-oxidation
N-oxidized
O-substituted
O-substitution
S-methylated
S-methylation
Box 12-1. Correct Forms for Alcohols
Correct forms Incorrect forms
1-butanol, butyl alcohol
2-butanol, sec-butyl alcohol
2-methyl-1-propanol, isobutyl alcohol isobutanol
2-methyl-2-propanol, tert-butyl alcohol
1-propanol, propyl alcohol
2-propanol, isopropyl alcohol isopropanol
any combination of sec
or tert with the words
butanol or propanol
➤ ➤ ➤ ➤ ➤
Chapter 12: Names and Numbers for Chemical Compounds ➤ 235
➤ Use italic type for the capital H that denotes indicated or added hydrogen.
1H-1,3-diazepine
3H-fluorene
2H-indene
phosphinin-2(1H)-one
2H-pyran-3(4H)-thione
➤ Use Greek letters, not the spelled-out forms, in chemical names to denote posi-
tion or stereochemistry. Use a hyphen to separate them from the chemical name.
α-amino acid (not alpha amino acid)
β-naphthol (not beta naphthol)
5α,10β,15α,20α-tetraphenylporphyrin
Use the Greek letters eta (η) to indicate hapticity and kappa (κ) to designate the
ligating atom in complicated formulas.
bis(η6-benzene)-chromium
bis(η-cyclopentadienyl) iron
[2-(diphenylphosphino-κP)phenyl-κC1]hydrido(triphenylphosphine-κP)-
nickel(II)
N,N′-bis(2-amino-κN-ethyl)ethane-1,2-diamine-κN]chloroplatinum(II)
➤ Use italic type for positional, stereochemical, configurational, and descriptive
structural prefixes when they appear with the chemical name or formula. Use a
hyphen to separate them from the chemical name. Accepted prefixes include the
following:
abeo
ac
altro
amphi
anti
antiprismo
ar
arachno
as
asym
c
catena
cis
cisoid
closo
cyclo
d
dodecahedro
E
endo
erythro
exo
facgem
hexahedro
hexaprismo
hypho
icosahedro
klado
l
m
M
mer
meso
n
nido
o
octahedro
p
P
pentaprismo
quadro
r
R
R∗
rel
retro
ribo
s
S
S∗
sec
sn
sym
syn
t
tert
tetrahedro
threo
trans
transoid
triangulo
triprismo
uns
vic
xylo
Z
➤ Do not capitalize prefixes that are shown here as lowercase, even at the begin-
ning of a sentence or in a title (see Tables 12-1 and 12-2), and never use lower-
236 ➤ The ACS Style Guide
case for those that are written in capital letters. Enclose the prefixes E, R, R∗, S,
S∗, and Z in parentheses.
anti-bicyclo[3.2.1]octan-8-amine
ar-chlorotoluene
as-trichlorobenzene
catena-triphosphoric acid
cis-diamminedichloroplatinum
cis-[PtCl2(NH3)2]
cyclo-hexasulfur, c-S6
(E,E)-2,4-hexadienoic acid
(E,Z)-1,3-di-1-propenylnaphthalene
erythro-2,3-dibromosuccinic acid
exo-chloro-p-menthane
m-ethylpropylbenzene
meso-tartaric acid
o-dibromobenzene
p-aminoacetanilide
s-triazine
(S)-2,3-dihydroxypropanoic acid
5-sec-butylnonane
sym-dibromoethane
tert-pentyl bromide, t-C5H11Br
threo-2,3-dihydroxy-1,4-
dimercaptobutane
trans-2,3-dimethylacrylic acid
uns-dichloroacetone
vic-triazine
➤ Use small capital letters d and l to indicate absolute configuration with
amino acids and carbohydrates.
2-(difluoromethyl)-dl-ornithine
l-galactosamine
2-O-β-d-glucopyranosyl-α-d-glucose
➤ Use plus and minus signs enclosed in parentheses as stereochemical descriptors.
(±)-2-allylcyclohexanone
(+)-dihydrocinchonine
(–)-3-(3,4-dihydroxyphenyl)-l-alanine
➤ When the structural prefixes cyclo, iso, neo, and spiro are integral parts of
chemical names, close them up to the rest of the name (without hyphens) and
do not italicize them.
cyclohexane
isopropyl alcohol
neopentane
However, italicize and hyphenate cyclo as a nonintegral structural descriptor.
cyclo-octasulfur
cyclo-triphosphoric acid
➤ Use numerals separated by periods within square brackets in names of
bridged and spiro alicyclic compounds.
bicyclo[3.2.0]heptane
bicyclo[4.4.0]decane
1-methylspiro[3.5]non-5-ene
spiro[4.5]decane

Chapter 12: Names and Numbers for Chemical Compounds ➤ 237
Table 12-1. Examples of Multiword Chemical Names
In Text At Beginning of Sentence In Titles and Headings
Acids
benzoic acid Benzoic acid Benzoic Acid
ethanethioic S-acid Ethanethioic S-acid Ethanethioic S-Acid
hydrochloric acid Hydrochloric acid Hydrochloric Acid
Alcohols
ethyl alcohol Ethyl alcohol Ethyl Alcohol
ethylene glycol Ethylene glycol Ethylene Glycol
Ketones
di-2-naphthyl ketone Di-2-naphthyl ketone Di-2-naphthyl Ketone
methyl phenyl ketone Methyl phenyl ketone Methyl Phenyl Ketone
Ethers
di-sec-butyl ether Di-sec-butyl ether Di-sec-butyl Ether
methyl propyl ether Methyl propyl ether Methyl Propyl Ether
Anhydrides
acetic anhydride Acetic anhydride Acetic Anhydride
phthalic anhydride Phthalic anhydride Phthalic Anhydride
Esters
methyl acetate Methyl acetate Methyl Acetate
phenyl thiocyanate Phenyl thiocyanate Phenyl Thiocyanate
propyl benzoate Propyl benzoate Propyl Benzoate
Polymer Names
1,2-polybutadiene 1,2-Polybutadiene 1,2-Polybutadiene
poly(butyl methacrylate) Poly(butyl methacrylate) Poly(butyl methacrylate)
poly(ethylene glycol) Poly(ethylene glycol) Poly(ethylene glycol)
poly(N,N-dimethylacrylamide) Poly(N,N-dimethylacrylamide) Poly(N,N-dimethylacrylamide)
Other Organic Compounds
aniline hydrochloride Aniline hydrochloride Aniline Hydrochloride
benzyl hydroperoxide Benzyl hydroperoxide Benzyl Hydroperoxide
butyl chloride Butyl chloride Butyl Chloride
dicyclohexyl peroxide Dicyclohexyl peroxide Dicyclohexyl Peroxide
diethyl sulfide Diethyl sulfide Diethyl Sulfide
methyl iodide Methyl iodide Methyl Iodide
2-naphthoyl bromide 2-Naphthoyl bromide 2-Naphthoyl Bromide
sodium S-phenyl thiosulfite Sodium S-phenyl thiosulfite Sodium S-Phenyl Thiosulfite
tert-butyl fluoride tert-Butyl fluoride tert-Butyl Fluoride
Inorganic and Coordination Compounds
ammonium hydroxide Ammonium hydroxide Ammonium Hydroxide
bis(diethyl phosphato)zinc Bis(diethyl phosphato)zinc Bis(diethyl phosphato)zinc
calcium sulfate Calcium sulfate Calcium Sulfate
(dimethyl sulfoxide)-cadmium
sulfate
(Dimethyl sulfoxide)-cadmium
sulfate
(Dimethyl sulfoxide)-cadmium
Sulfate
magnesium oxide Magnesium oxide Magnesium Oxide
sodium cyanide Sodium cyanide Sodium Cyanide
sulfur dioxide Sulfur dioxide Sulfur Dioxide

238 ➤ The ACS Style Guide
Table 12-2. Locants and Descriptors in Chemical Names
In Text At Beginning of Sentence In Titles and Headings
Numeral Locants
adenosine 5′-triphosphate Adenosine 5′-triphosphate Adenosine 5′-Triphosphate
1,3-bis(bromomethyl)-
benzene
1,3-Bis(bromomethyl)-benzene 1,3-Bis(bromomethyl)-benzene
2-benzoylbenzoic acid 2-Benzoylbenzoic acid 2-Benzoylbenzoic Acid
1-bromo-3-chloropropane 1-Bromo-3-chloropropane 1-Bromo-3-chloropropane
2-(2-chloroethyl)pentanoic
acid
2-(2-Chloroethyl)pentanoic
acid
2-(2-Chloroethyl)pentanoic
Acid
7-(4-chlorophenyl)-1-
naphthol
7-(4-Chlorophenyl)-1-naphthol 7-(4-Chlorophenyl)-1-naphthol
1,2-dicyanobutane 1,2-Dicyanobutane 1,2-Dicyanobutane
4a,8a-dihydronaphthalene 4a,8a-Dihydronaphthalene 4a,8a-Dihydronaphthalene
Element Symbol Locants
(2,3-butanedione dioximato-
O,O′)copper
(2,3-Butanedione dioximato-
O,O′)copper
(2,3-Butanedione dioximato-
O,O′)copper
N-ethylaniline N-Ethylaniline N-Ethylaniline
N,2-dihydroxybenzamide N,2-Dihydroxybenzamide N,2-Dihydroxybenzamide
N,N′-dimethylurea N,N′-Dimethylurea N,N′-Dimethylurea
3H-fluorene 3H-Fluorene 3H-Fluorene
O,S,S-triethyl
phosphorodithioate
O,S,S-Triethyl
phosphorodithioate
O,S,S-Triethyl
Phosphorodithioate
S-methyl
benzenethiosulfonate
S-Methyl benzenethiosulfonate S-Methyl Benzenethiosulfonate
Greek Letter Locants and Descriptors
α-hydroxy-β-aminobutyric
acid
α-Hydroxy-β-aminobutyric
acid
α-Hydroxy-β-aminobutyric Acid
17α-hydroxy-5β-pregnane 17α-Hydroxy-5β-pregnane 17α-Hydroxy-5β-pregnane
1α-hydroxycholecalciferol 1α-Hydroxycholecalciferol 1α-Hydroxycholecalciferol
α-methylbenzeneacetic acid α-Methylbenzeneacetic acid α-Methylbenzeneacetic Acid
α1-sitosterol α1-Sitosterol α1-Sitosterol
β-chloro-1-
naphthalenebutanol
β-Chloro-1-
naphthalenebutanol
β-Chloro-1-naphthalenebutanol
β,4-dichlorocyclohexane-
propionic acid
β,4-Dichlorocyclohexane-
propionic acid
β,4-Dichlorocyclohexane-
propionic Acid
β-endorphin β-Endorphin β-Endorphin
ω,ω′-dibromopolybutadiene ω,ω′-Dibromopolybutadiene ω,ω′-Dibromopolybutadiene
tris(β-chloroethyl)amine Tris(β-chloroethyl)amine Tris(β-chloroethyl)amine
Small Capital Letter Descriptors
β-D-arabinose β-D-Arabinose β-D-Arabinose
D-1,2,4-butanetriol D-1,2,4-Butanetriol D-1,2,4-Butanetriol
D-serine D-Serine D-Serine
DL-alanine DL-Alanine DL-Alanine
DS-threonine DS-Threonine DS-Threonine
L-methionine L-Methionine L-Methionine
Positional and Structural Descriptors
7-bromo-p-cymene 7-Bromo-p-cymene 7-Bromo-p-cymene
4-chloro-m-cresol 4-Chloro-m-cresol 4-Chloro-m-cresol
m-hydroxybenzyl alcohol m-Hydroxybenzyl alcohol m-Hydroxybenzyl Alcohol
n-butyl iodide n-Butyl iodide n-Butyl Iodide
Continued on next page

Chapter 12: Names and Numbers for Chemical Compounds ➤ 239
Table 12-2. Locants and Descriptors in Chemical Names—Continued
In Text At Beginning of Sentence In Titles and Headings
Positional and Structural Descriptors—Continued
2-(o-chlorophenyl)-1-
naphthol
2-(o-Chlorophenyl)-1-naphthol 2-(o-Chlorophenyl)-1-naphthol
o-dibromobenzene o-Dibromobenzene o-Dibromobenzene
p-benzenediacetic acid p-Benzenediacetic acid p-Benzenediacetic Acid
p-tert-butylphenol p-tert-Butylphenol p-tert-Butylphenol
s-triazine s-Triazine s-Triazine
sec-butyl alcohol sec-Butyl alcohol sec-Butyl Alcohol
sym-dibromoethane sym-Dibromoethane sym-Dibromoethane
tert-pentyl isovalerate tert-Pentyl isovalerate tert-Pentyl Isovalerate
1-(trans-1-propenyl)-3-(cis-1-
propenyl)naphthalene
1-(trans-1-Propenyl)-3-(cis-1-
propenyl)naphthalene
1-(trans-1-Propenyl)-3-(cis-1-
propenyl)naphthalene
Stereochemical Descriptors
anti-bicyclo[3.2.1]octan-8-
amine
anti-Bicyclo[3.2.1]octan-8-
amine
anti-Bicyclo[3.2.1]octan-8-
amine
cis-1,2-dichloroethene cis-1,2-Dichloroethene cis-1,2-Dichloroethene
d-camphor d-Camphor d-Camphor
dl-2-aminopropanoic acid dl-2-Aminopropanoic acid dl-2-Aminopropanoic Acid
(E)-diphenyldiazene (E)-Diphenyldiazene (E)-Diphenyldiazene
endo-2-chlorobicyclo[2.2.1]-
heptane
endo-2-Chlorobicyclo[2.2.1]-
heptane
endo-2-Chlorobicyclo[2.2.1]-
heptane
(+)-erythro-2-amino-3-
methylpentanoic acid
(+)-erythro-2-Amino-3-
methylpentanoic acid
(+)-erythro-2-Amino-3-
methylpentanoic Acid
erythro-β-hydroxyaspartic
acid
erythro-β-Hydroxyaspartic acid erythro-β-Hydroxyaspartic Acid
exo-bicyclo[2.2.2]oct-5-en-
2-ol
exo-Bicyclo[2.2.2]oct-5-en-2-ol exo-Bicyclo[2.2.2]oct-5-en-2-ol
exo-5,6-dimethyl-endo-
bicyclo[2.2.2]octan-2-ol
exo-5,6-Dimethyl-endo-
bicyclo[2.2.2]octan-2-ol
exo-5,6-Dimethyl-endo-
bicyclo[2.2.2]octan-2-ol
L-threo-2,3-dichlorobutyric
acid
L -threo-2,3-Dichlorobutyric
acid
L -threo-2,3-Dichlorobutyric
Acid
meso-tartaric acid meso-Tartaric acid meso-Tartaric Acid
(1R*,3S*)-1-bromo-3-
chlorocyclohexane
(1R*,3S*)-1-Bromo-3-
chlorocyclohexane
(1R*,3S*)-1-Bromo-3-
chlorocyclohexane
rel-(1R,3R)-1-bromo-3-
chlorocyclohexane
rel-(1R,3R)-1-Bromo-3-
chlorocyclohexane
rel-(1R,3R)-1-Bromo-3-
chlorocyclohexane
(S)-2,3-dihydroxypropanoic
acid
(S)-2,3-Dihydroxypropanoic
acid
(S)-2,3-Dihydroxypropanoic
Acid
sn-glycerol 1-(dihydrogen
phosphate)
sn-Glycerol 1-(dihydrogen
phosphate)
sn-Glycerol 1-(Dihydrogen
phosphate)
syn-7-methylbicyclo[2.2.1]-
heptene
syn-7-Methylbicyclo[2.2.1]-
heptene
syn-7-Methylbicyclo[2.2.1]-
heptene
trans-cisoid-trans-
perhydrophenanthrene
trans-cisoid-trans-
Perhydrophenanthrene
trans-cisoid-trans-
Perhydrophenanthrene
(Z)-5-chloro-4-pentenoic acid (Z)-5-Chloro-4-pentenoic acid (Z)-5-Chloro-4-pentenoic Acid
(1Z,4E)-1,2,4,5-tetrachloro-
1,4-pentadiene
(1Z,4E)-1,2,4,5-Tetrachloro-1,4-
pentadiene
(1Z,4E)-1,2,4,5-Tetrachloro-1,4-
pentadiene
240 ➤ The ACS Style Guide
➤ Use italic letters within square brackets in names of polycyclic aromatic
compounds.
dibenz[a,j]anthracene
dibenzo[c,g]phenanthrene
dicyclobuta[de,ij]naphthalene
1H-benzo[de]naphthacene
indeno[1,2-a]indene
Syllabic Portion of Chemical Names
Multiplying affixes are integral parts of the chemical name; they are set in roman
type and are always closed up to the rest of the name (without hyphens). Use
hyphens only to set off intervening locants or descriptors. Use enclosing marks
(parentheses, brackets, or braces) to ensure clarity or to observe other recom-
mended nomenclature conventions. Multiplying prefixes include the following:
• hemi, mono, di, tri, tetra, penta, hexa, hepta, octa, ennea, nona, deca,
deka, undeca, dodeca, etc.
• semi, uni, sesqui, bi, ter, quadri, quater, quinque, sexi, septi, octi, novi,
deci, etc.
• bis, tris, tetrakis, pentakis, hexakis, heptakis, octakis, nonakis, decakis, etc.
3,4′-bi-2-naphthol
2,2′-bipyridine
bis(benzene)chromium(0)
1,4-bis(3-bromo-1-
oxopropyl)piperazine
1,3-bis(diethylamino)propane
di-tert-butyl malonate
dichloride
1,2-ethanediol
hemihydrate
hexachlorobenzene
2,4,6,8-nonanetetrone
pentachloroethane
3,4,5,6-tetrabromo-o-cresol
tetrakis(hydroxymethyl)methane
tri-sec-butylamine
triamine
triethyl phosphate
tris(amine)
2,3,5-tris(aziridin-1-yl)-p-
benzoquinone
tris(ethylenediamine)cadmium
dihydroxide
Capitalization of Chemical Names
Chemical names are not capitalized unless they are the first word of a sentence or
are part of a title or heading. Then, the first letter of the syllabic portion is capi-
talized, not the locant, stereoisomer descriptor, or positional prefix. Table 12-1
presents examples of simple chemical names and their capitalization. Table 12-2
presents chemical names that include locants and descriptors.
➤ Some reaction names are preceded by element symbols; they may be used as
nouns or adjectives. When they are the first word of a sentence or appear in titles
Chapter 12: Names and Numbers for Chemical Compounds ➤ 241
and headings, capitalize the first letter of the word. Do not italicize the element
symbol.
N-Oxidation of the starting compounds yielded compounds 3–10.
N-Benzoylated amines undergo hydroxylation when incubated with yeast.
Preparation of S-Methylated Derivatives
O-Substituted Structural and Functional Analogues
Punctuation in Chemical Names
➤ Use commas between numeral locants, chemical element symbol locants, and
Greek locants, with no space after the comma. When a single locant consists of a
numeral and a Greek letter together with no space or punctuation, the numeral
precedes the Greek letter. When the Greek letter precedes the numeral, they indi-
cate two different locants and should be separated by a comma. For example, α,2
denotes two locants; 1α is viewed as one locant.
(6α,11β,16α)-6-fluoro-16-methylpregna-1,4-diene
β,4-dichlorocyclohexanepropionic acid
1,2-dinitrobutane
N,N-dimethylacetamide
2,3,3a,4-tetrahydro-1H-indole
➤ Use hyphens to separate locants and configurational descriptors from each
other and from the syllabic portion of the name.
α-ketoglutaric acid
2-benzoylbenzoic acid
1,4-bis(2-ethylhexyl) sulfosuccinate
3-chloro-4-methylbenzoic acid
cis-dichloroethylene
d-arabinose
(1,4-dioxaspiro[4.5]dec-2-ylmethyl) guanidine
(E)-2-(3,7-dimethyl-2,6-octadienyl)-1,4-benzenediol
N-hydroxy-N-nitrosobenzeneamine
N-methylmethanamine
4-O-β-d-galactopyranosyl-d-fructose
tetrahydro-3,4-dipiperonyl-2-furanol
trans-2-bromocyclopentanol
➤ Do not use hyphens to separate the syllables of a chemical name unless the
name is too long to fit on one line. Appendix 12-1 is a list of prefixes, suffixes,
roots, and some complete words hyphenated as they would be at the end of a
line.
242 ➤ The ACS Style Guide
Specialized Groups of Chemicals
Polymers
Polymer names are often one or two words in parentheses following the prefix
“poly”. “Poly” is a syllabic prefix, not a descriptor, and thus is set in roman type.
Here is a short list of correctly formatted names of frequently cited polymers.
(These names are not necessarily IUPAC or CA index preferences.)
nylon-6
nylon-6,6
polyacrylamide
poly(acrylic acid)
polyacrylonitrile
polyamide
poly(aryl sulfone)
polybutadiene
1,2-polybutadiene
1,4-polybutadiene
poly(butyl acrylate)
poly(butyl methacrylate)
poly(n-butyl methacrylate)
poly(butylene terephthalate)
polycarbonate
polychloroprene
poly(N,N-dimethylacrylamide)
poly(dimethylsiloxane)
polyester
polyether
poly(ether imide)
poly(ether ketone)
poly(ether sulfone)
poly(ethyl acrylate)
poly(ethyl methacrylate)
polyethylene
poly(ethylene adipate)
poly(ethylene glycol)
poly(ethylene oxide)
poly(ethylene terephthalate)
polyformaldehyde
polyimidazole
polyimide
poly(isobutyl methacrylate)
polyisobutylene
polyisoprene
poly(methacrylic acid)
poly(methyl acrylate)
poly(methyl methacrylate)
poly(methylene)
poly(N,N′-hexamethyleneadipamide)
poly(oxy-1,4-phenylene)
poly(oxyethylene)
poly(oxymethylene)
poly(phenylene ether)
poly(phenylene oxide)
poly(phenylene sulfide)
polypropylene
poly(propylene glycol)
polystyrene
polysulfide
polysulfone
poly(tetrafluoroethylene)
poly(tetramethylene oxide)
polythiazole
poly(thiocarbonate)
polyurethane
poly(vinyl acetate)
poly(vinyl alcohol)
poly(vinyl butyral)
poly(vinyl chloride)
poly(vinyl ether)
poly(vinyl trichloroacetate)
poly(vinylidene chloride)
poly(vinylpyrrolidone)
povidone
➤ In text, keep polymer names lowercase. As the first word of a sentence and in
titles or headings, capitalize only the first letter of the polymer name.
New Uses for Poly(ethylene terephthalate)
Chapter 12: Names and Numbers for Chemical Compounds ➤ 243
Poly(vinyl chloride) is a less useful polymer than poly(ethylene glycol).
Reactions of Poly(methyl methacrylate)
➤ In copolymer nomenclature, descriptive lowercase italic infixes may be used.
These include alt, blend, block (or b), co, cross, graft (or g), inter, per, stat, and ran.
polybutadiene-graft-[polystyrene:poly(methyl methacrylate)]
poly(cross-butadiene)
poly[cross-(ethyl acrylate)]-inter-polybutadiene
poly(ethylene-alt-carbon monoxide)
polyisoprene-blend-polystyrene
poly[(methyl methacrylate)-b-(styrene-co-butadiene)]
poly[(methyl methacrylate)-co-styrene]
polystyrene-block-polybutadiene
poly(styrene-co-butadiene)
poly(styrene-g-acrylonitrile)
poly(vinyl trichloroacetate)-cross-polystyrene
Saccharides
Abbreviations for the major monosaccharides are presented in Table 12-3.
➤ Designation of the bond between two monosaccharides should specify the
first residue, its anomeric configuration (α or β), the position of attachment on
both sugar residues, and the second residue. Additional locants and configura-
tional descriptors may be present but are not required. A number of variations
may be used.
Galβ1→4Glc Galβ1–4Glc Galβ1,4Glc
Galβ(1→4)Glc Galβ(1–4)Glc Galβ(1,4)Glc
Table 12-3. Abbreviations for Major Monosaccharides
Saccharide Abbreviation
arabinose Ara
fucose Fuc
galactose Gal
N-acetylgalactosamine GalNAc
glucose Glc
N-acetylglucosamine GlcNAc
glucuronic acid GlcA
mannose Man
N-acetylneuraminic acid Neu5Ac
N-glycoloylneuraminic acid Neu5Gc
rhamnose Rha
xylose Xyl
244 ➤ The ACS Style Guide
➤ For larger oligosaccharides, use parentheses or brackets for branched residues:
Manα1→6(Manα1→3)Manβ1→4GlcNAcβ1→4(±Fucα1→6)GlcNAc
Manα(1–6)[Manα(1–3)]Manβ(1–4)GlcNAcβ(1–4)[±Fucα(1–6)]GlcNAc
Nucleic Acids
Table 12-4 presents the standard abbreviations for nucleic acids.
➤ By convention, numbering of the bases is unprimed and numbering of the
sugars is primed. A nucleic acid polymer typically has the phosphate group
attached to the 5′ position of the first nucleotide (the 5′ end), and the other end
terminates in a hydroxyl group at the 3′ position of the sugar of the last nucleo-
tide (the 3′ end). By convention, nucleotide sequences are almost always read
and presented in the 5′ to 3′ direction. The sequence may be presented in unbro-
ken form or in evenly spaced blocks.
5′-TAGCTAACCCGTTTTAGCGTCGTC-3′
5′-TAGCT AACCC GTTTT AGCGT CGTC-3′
➤ Complementary base pairs are joined by hydrogen bonds and are best repre-
sented by a centered dot: dA·dT and dG·dC are the canonical pairings in double-
stranded DNA, although alternate pairings, DNA·RNA hybrids, triplexes, and
other variations may occur.
Table 12-4. Abbreviations for Nucleic Acids
Base Sugar
Nucleosidea
(base + sugar)
Abbreviation for
Nucleoside
Monophosphateb
Nucleoside
Diphosphate
Nucleoside
Triphosphate
Naturally occurring in RNA, ribonucleic acid
adenine ribose adenosine, A AMP ADP ATP
cytosine ribose cytidine, C CMP CDP CTP
guanine ribose guanosine, G GMP GDP GTP
uracil ribose uridine, U UMP UDP UTP
Naturally occurring in DNA, deoxyribonucleic acid
adenine deoxyribose deoxyadenosine, dA dAMP dADP dATP
cytosine deoxyribose deoxycytidine, dC dCMP dCDP dCTP
guanine deoxyribose deoxyguanosine, dG dGMP dGDP dGTP
thymine deoxyribose thymidine, dTcdTMP dTDP dTTP
aOther nucleosides may occur (e.g., inosine, I, or xanthosine, X), but those listed are the canonical building
blocks of nucleic acids and need never be defined. N may be used for an unspecified nucleoside or a mixture of
all four (i.e., dNTPs were added to the reaction mixture).
bA nucleoside monophosphate is also known as a nucleotide.
cThymidine consists of thymine + deoxyribose by definition and is abbreviated dT by definition; deoxythymi-
dine would imply a different compound lacking another oxygen.

Chapter 12: Names and Numbers for Chemical Compounds ➤ 245
Amino Acids
➤ The abbreviations of the essential 20 amino acids are listed in Table 12-5;
these abbreviations do not need to be defined. In analytical situations, undif-
ferentiated mixtures of aspartic acid/asparagine (Asx, B) or glutamic acid/gluta-
mine (Glx, Z) may occur.
➤ For amino acids other than the essential 20, three-letter abbreviations may be
used and defined at their first appearance.
homocysteine Hcy
hydroxyproline Hyp
norvaline Nva
ornithine Orn
Three-letter amino acid abbreviations may be preceded by a configurational des-
ignator, d or l, set in small capital letters.
➤ Always capitalize the three-letter and one-letter abbreviations for amino acids.
➤ In sequences of amino acids, separate the three-letter abbreviations with
hyphens.
Pro-Gln-Ile-Ala
Table 12-5. Abbreviations for Amino Acids
Amino Acid Three-Letter Abbreviation One-Letter Abbreviation
alanine Ala A
arginine Arg R
asparagine Asn N
aspartic acid Asp D
cysteine Cys C
glutamic acid Glu E
glutamine Gln Q
glycine Gly G
histidine His H
isoleucine Ile I
leucine Leu L
lysine Lys K
methionine Met M
phenylalanine Phe F
proline Pro P
serine Ser S
threonine Thr T
tryptophan Trp W
tyrosine Tyr Y
valine Val V
246 ➤ The ACS Style Guide
➤ Do not use abbreviations for individual amino acids in running text.
Selective labeling of arginine and serine made it possible to monitor the kinetics
of folding of the individual residues.
➤ Position numbers may follow one- or three-letter abbreviations or spelled-
out names and may be closed up, hyphenated, spaced, or superscripted. Designa-
tion of a mutation includes the original amino acid, the position number, and
the new amino acid:
A134V
Ala134Val
alanine-134 → valine
Combinatorial Compounds
➤ Combinatorial libraries are described by generic representations, which con-
sist of a generic structure together with one or more lists of substituents. The
position of substituents is indicated by superatoms in the generic structure.
➤ Superatoms are designated by roman letters: R for any set of substituents or
residues, for example, or Ar for a list of aromatic substituents. Superatoms may
be distinguished by the addition of designation digits, typically a subscript fol-
lowing the superatom: RA, RB, and RC could specify the residue of reagents A, B,
and C.
➤ Subscript numbers are generally used to indicate the order in which residues
were introduced to the reaction scheme: R1, R2, R3, and so on.
➤ The Journal of Combinatorial Chemistry recommends the use of ChemSet
notation, in which a structure number is followed by the reagent sets associated
with it. The structure number is typeset in bold, and the reagent sets are set in
italics and enclosed in curly brackets.
1{1–5} + 2{1–6} + 4{1–4} → 5{1–5,1–4,1–6}
4{1–5,1–4,1–6}
➤ The composition of a final library can be described in terms of mixtures or
separate products. An uppercase X is used to indicate products mixed; an upper-
case O is used to indicate products separate.
4{X,X,O}
4{O,O,O}
4{X 1–5,X 1–4,O 1–6}
4{O 1–5,O 1–4,O 1–6}
Additional information on the representation of combinatorial chemistry is
given in Appendix 12-2.
247
ace-naph-tho
ace-tal
acet-al-de-hyde
acet-amide
acet-ami-do
acet-amin-o-phen
acet-an-i-lide
ace-tate
ac-et-azol-amide
ace-tic
ace-to
ace-to-ace-tic
ace-tone
ace-to-ni-trile
ace-tyl
acet-y-late
acet-y-lene
acro-le-in
ac-ryl-am-ide
ac-ry-late
acryl-ic
ac-ry-lo
ad-i-po-yl
al-kyl
al-lyl
ami-di-no
amide
ami-do
amine
ami-no
am-mine
am-mo-nio
am-mo-ni-um
an-thra
an-thra-cene
an-thra-ce-no
an-thryl
ar-se-nate
ar-si-no
aryl
az-i-do
azi-no
azo
benz-ami-do
ben-zene
benz-hy-dryl
ben-zo-yl
ben-zyl
ben-zyl-i-dene
bi-cy-clo
bo-ryl
bro-mide
bro-mo
bu-tane
bu-ten-yl
bu-tyl
bu-tyl-ene
bu-tyl-i-dene
car-ba-mate
car-bam-ic
car-ba-mide
carb-an-ion
car-ba-ryl
➤ ➤ ➤ ➤ ➤
APPENDIX 12-1
End-of-Line Hyphenation
of Chemical Names
This appendix contains a list of prefixes, suffixes, roots, and some complete
words hyphenated as they would be at the end of a line. To hyphenate a chemical
name such as
5-(2-chloroethyl)-9-(diaminomethyl)-2-anthracenol
look up each syllable that is to be hyphenated in the list. Also, chemical names
can be broken after hyphens that are integral in their names. Follow other stand-
ard rules for hyphenation of regular words; for example, try to leave at least three
characters on each line. Thus, the example given could be hyphenated as fol-
lows:
5-(2-chlo-ro-eth-yl)-9-(di-ami-no-meth-yl)-2-anth-ra-cenol
Most desk dictionaries contain the names of common chemicals; they also give
end-of-line hyphenation.
248 ➤ The ACS Style Guide
car-ba-zole
car-bi-nol
car-bol-ic
car-bon-ate
car-bon-ic
car-bo-ni-um
car-bon-yl
car-box-ami-do
car-boxy
car-box-yl
car-byl-a-mi-no
chlo-ride
chlo-ro
chlo-ro-syl
chlo-ryl
cu-mene
cy-a-nate
cy-a-nide
cy-a-na-to
cy-a-no
cy-clo
cy-clo-hex-ane
cy-clo-hex-yl
di-azo
di-bo-ran-yl
di-car-bon-yl
di-im-ino
di-oxy
di-oyl
diyl
do-de-cyl
ep-oxy
eth-ane
eth-a-no
eth-a-nol
eth-a-no-yl
eth-en-yl
eth-yl
eth-yl-ene
eth-yl-i-dene
eth-yn-yl
fluo-res-cence
fluo-ride
fluo-ro
form-al-de-hyde
form-ami-do
for-mic
form-imi-do-yl
for-myl
fu-ran
ger-myl
gua-ni-di-no
gua-nyl
halo
hep-tane
hep-tyl
hex-ane
hex-yl
hy-dra-zide
hy-dra-zine
hy-dra-zi-no
hy-dra-zo
hy-dra-zo-ic
hy-dric
hy-dride
hy-dri-od-ic
hy-dro
hy-dro-chlo-ric
hy-dro-chlo-ride
hy-dro-chlo-ro
hy-drox-ide
hy-droxy
hy-drox-yl
imi-da-zole
imide
imi-do
imi-do-yl
imi-no
in-da-mine
in-da-zole
in-dene
in-de-no
in-dole
io-date
io-dide
iodo
io-do-syl
io-dyl
iso-cy-a-na-to
iso-cy-a-nate
iso-cy-a-nide
iso-pro-pen-yl
iso-pro-pyl
mer-cap-to
mer-cu-ric
meth-an-ami-do
meth-ane
meth-ano
meth-yl
meth-yl-ate
meth-yl-ene
meth-yl-i-dene
mono
mono-ac-id
mono-amine
naph-tha-lene
naph-tho
naph-thyl
neo-pen-tyl
ni-trate
ni-tric
ni-trile
ni-trilo
ni-trite
ni-tro
ni-troso
oc-tane
oc-tyl
ox-idase
ox-ide
ox-ido
ox-ime
oxo
ox-o-nio
oxy
palm-i-toyl
pen-tane
pen-tyl
pen-tyl-i-dene
per-chlo-rate
per-chlo-ride
per-chlo-ryl
per-man-ga-nate
per-ox-idase
per-ox-ide
per-oxy
phen-ac-e-tin
phen-an-threne
Chapter 12: Names and Numbers for Chemical Compounds ➤ 249
phen-an-thro
phen-an-thryl
phen-a-zine
phe-no
phe-nol
phe-none
phen-ox-ide
phen-oxy
phen-yl
phen-yl-ene
phos-phate
phos-phide
phos-phine
phos-phi-no
phos-phin-yl
phos-phite
phos-pho
phos-pho-nio
phos-pho-no
phos-phor-anyl
phos-pho-li-pase
phos-pho-lip-id
phos-pho-ni-um
phos-pho-ric
phos-pho-rus
phos-pho-ryl
plum-byl
pro-pane
pro-pen-yl
pro-pen-yl-ene
pro-pyl
pro-pyl-ene
pro-pyl-i-dene
pu-rine
py-ran
pyr-a-zine
pyr-a-zole
pyr-i-dine
pyr-id-a-zine
pyr-role
quin-o-line
qui-none
sel-e-nate
se-le-nic
sel-e-nide
sel-e-nite
se-le-no
si-lane
sil-anyl
sil-ox-anyl
sil-ox-yl
si-lyl
spi-ro
stan-nic
stan-nite
stan-nous
stan-nyl
stib-ino
sty-rene
sty-ryl
sul-fa-mo-yl
sul-fate
sul-fe-no
sul-fe-nyl
sul-fide
sul-fi-do
sul-fi-no
sul-fi-nyl
sul-fite
sul-fo
sul-fon-ami-do
sul-fo-nate
sul-fone
sul-fon-ic
sul-fo-nio
sul-fo-nyl
sulf-ox-ide
sul-fu-ric
sul-fu-rous
sul-fu-ryl
tet-ra
thio
thio-nyl
thio-phene
thi-oxo
thi-oyl
tol-u-ene
tol-u-ide
tol-yl
tri-a-zine
tri-a-zole
tri-yl
urea
ure-ide
ure-ido
uric
vi-nyl
vi-nyl-i-dene
xan-thene
xan-tho
xy-lene
xy-li-dine
xy-lyl
xy-li-din-yl
yl-i-dene
250
➤ ➤ ➤ ➤ ➤
APPENDIX 12-2
Representation of
Combinatorial Chemistry
Derek Maclean
Combinatorial chemistry entails the reaction of sets of reagents to produce sets
or libraries of products in numbers up to the size of each reagent set multiplied
together. Thus 5 reagents A, plus 4 reagents B, plus 6 reagents C could prepare
5 × 4 × 6, or 120 products ABC. The unique feature of this strategy is the ability
to efficiently prepare very large numbers of compounds—a so-called combinato-
rial library. The challenges in describing combinatorial chemistry consist of con-
cisely but accurately reporting the constituents and form of such a compound
collection.
The individual products of combinatorial chemistry are called members. The
distinction between a member and a compound is important and is based on the
respective level of characterization. A compound will meet the typical standards
for reporting new chemical structures; a member will fall short of that standard.
In fact, a member may, in principle, simply be expected to be present in a library,
especially for large libraries, and those that consist of mixtures rather than dis-
crete samples of members.
The structural representation of combinatorial chemistry consists of a generic
structure plus a list of substituents that may be present in that structure.
The generic structure is very similar to a typical structural formula with the
addition of special notation to indicate the potential for variable substitution at
certain parts of the molecule.
The position of variable substituents on a generic structure is indicated by
superatoms, such as the R designation. In a combinatorial library, R need not
simply designate an alkyl radical but is conventionally used to represent any
set of substituents or residues. The residues R typically define those portions of
reagents or building blocks that are found in the final product of the synthesis
and that vary among library members. Particular superatoms may more precisely
define the composition of a library, such as Ar for a list of aromatic substituents.
To distinguish superatoms within a generic structure, it is typical to use
additional designation digits. Thus RA, RB, and RC could specify the residue of
reagents A, B, and C in our example. The position of the additional digit has
Chapter 12: Names and Numbers for Chemical Compounds ➤ 251
not been standardized but may be typically added as a subscript following the
superatom.
The designation digit may usefully convey additional information beyond
the simple differentiation of superatoms. For instance, R1, R2, R3, and so on
may indicate the order in which these residues were introduced to the reaction
scheme. Alternatively, RX or RO make use of the pool designations X and O (see
the section “ChemSet Notation”) to concisely describe library composition in a
generic structure.
It is often possible to draw multiple generic structures for a combinatorial
library. Care should be taken in the choice of the appropriate structure, taking into
account the purpose for the structure. Often the maximum common substruc-
ture is a good choice, but other structures may better illustrate structure–activity
relationships or the synthetic potential of a combinatorial reaction strategy. The
relationship between residue and reagent or product should be defined. This may
be conveniently shown in the generic reaction scheme; see Figure 12A-1.
A typical feature of combinatorial synthesis is the use of techniques that facil-
itate the isolation of products and intermediates. The most common example is
the attachment to a solid support, such as a polymer bead, to allow isolation
by simple filtration (solid-phase chemistry). A variety of solid and soluble sup-
ports have been developed. These may be attached to either the products or the
reagents of a library.
Such supports can be dealt with in generic reaction schemes as a special type
of superatom. Often, a pictorial representation is used. A polymer bead may be
conveniently represented by an s orbital in common chemistry software pack-
ages. Few standards have been developed for the representation of other sup-
ports, but a filled structure designates a solid support and an open structure may
be used for a soluble support.
ChemSet Notation
A convenient descriptive notation for combinatorial libraries (recommended by
the Journal of Combinatorial Chemistry) is the ChemSet terminology.
A ChemSet is denoted by a structure number followed by the reagent sets
associated with that structure. Thus the combinatorial library with 120 products
could be described as follows, where the numbers in curly brackets define the
reagents which were used to prepare that library; see Figure 12A-1.
5{1–5,1–6,1–4}
The synthetic scheme to prepare such a library may also be described in terms of
ChemSets:
1{1–5} + 2{1–6} + 4{1–4} → 5{1–5,1–6,1–4}
The composition of the final library may consist of a mixture of all members, or
as a collection of discrete product samples, or as a set of smaller pools. An exten-
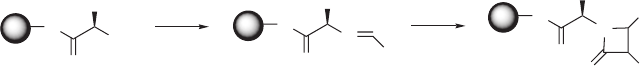
252 ➤ The ACS Style Guide
sion to the ChemSet notation allows these situations to be precisely defined. The
useful designations X and O indicate respectively, “products mixed” and “prod-
ucts separate”. Thus, the library 5{X 1–5,X 1–6,O 1–4} is a set of 6 pools, each
with 20 members deriving from a single reagent from set 4, since the O indicates
that the six individual product mixtures were kept separate from one another
after adding reagents 4. In contrast, 5{O 1–5,O 1–4,O 1–6} represents 120 dis-
crete product samples, also called parallel synthesis, since the O for each set indi-
cates that all products were kept separate at each stage.
If all of a given set of reagents have been used in a given pool, then the
number for that set may be omitted. Thus, 5{X,X,O} and 5{O,O,O} accurately
describe the composition of the above libraries. However, if a subset of possible
reagents have been used, this must be indicated using the numerical list format,
e.g., 5{X 1–6,O 1–2,3}.
O
O
NH2
R1
O
O
N
R1
R2
O
O
N
R1
O
R2
R3
1{1–5 }
R2-CHO R3-COCl
2{1–6 }4{1–4 }
3{1–5,1–6 }5{1–5,1–6,1–4 }
Figure 12A-1. Combinatorial reaction scheme and ChemSet notation.
Source: Reprinted from J. Am. Chem. Soc. 1996, 118, 253–254. Copyright 1996 American Chemical Society.
253
➤ ➤ ➤ ➤ ➤
APPENDIX 12-3
CAS Registry Numbers
Chemical substances, their syntheses, the determination of their properties, and
their applications are the core of chemistry and the main occupation of chemists.
In their communications, chemists represent chemical substances by structural
diagrams, names, molecular formulas, codes, and identification numbers. One
of the most frequently used identification numbers is the CAS Registry Number.
Today, CAS Registry Numbers are often used to identify chemical substances in
handbooks, indexes, databases, and inventories, and even on many commercial
product labels.
The CAS Chemical Registry System is a computer-based system that uniquely
identifies chemical substances on the basis of their molecular structures. Begun
originally in 1965 to support indexing for Chemical Abstracts (CA), the CAS
Chemical Registry System now serves not only as a support system for identify-
ing substances within Chemical Abstracts Service operations but also as an inter-
national resource for chemical substance identification by scientists, industry,
and regulatory bodies. The CAS Registry provides a means of bridging the many
differences in systematic, generic, proprietary, and trivial substance names that
may be used to identify a single substance.
The CAS Chemical Registry System database is the largest collection of infor-
mation on naturally occurring and synthetic chemical substances in the world,
including organic compounds, inorganic compounds, organometallics, metals,
polymers, coordination compounds, alloys, elements, isotopes, nuclear particles,
proteins, nucleic acids, and minerals. By the end of 2005, the CAS Chemical Reg-
istry System contained records for more than 27 million organic and inorganic
substances, with new records added at the rate of some 5000 per day. A running
total of registered substances can be found on the CAS Web site at http://www.
cas.org/cgi-bin/regreport.pl.
The database contains CAS Registry Numbers, structures, and names for
substances reported in the chemical literature covered in CA, in addition to sub-
stances registered from special collections, for governmental and industrial orga-
nizations, and for individual requesters. CAS Registry Numbers are also assigned
to sequences such as DNA and proteins.
CAS Registry Numbers are assigned in sequential order as substances are
entered into the CAS Chemical Registry System database for the first time; the
numbers have no chemical significance. CAS Registry Numbers link the molecu-
lar structure diagram, systematic CA index name, synonyms, molecular formula,
254 ➤ The ACS Style Guide
and other identifying information for each substance. Because CAS Registry
Numbers are independent of the many different systems of chemical nomen-
clature, they can bridge these systems and link often unrecognized synonymous
names.
A format was developed using hyphens to make the numbers easier to read
and to recognize. A CAS Registry Number includes up to nine digits that are
separated into three parts by hyphens. The first part, starting from the left, has
up to six digits, the second part has two digits, and the final part is a single check
digit to verify the validity of the total number (e.g., 7732-18-5 for water).
Within the registry system, each substance is assigned a separate CAS Regis-
try Number. For example, each salt of an acid receives a distinct number, and an
ion receives a number different from that of the neutral compound.
CAS Registry Numbers are included in the printed Chemical Abstracts chemi-
cal substance and formula indexes and in the CAS databases. The full set of CAS
Chemical Registry System database information—structures, names, formulas,
and ring data—is available for search and display through STN International,
SciFinder, and other CAS search services. CAS Registry information is also avail-
able in CAS databases offered by other online system vendors.
In addition to their inclusion in the CAS databases, CAS Registry Num-
bers are used in many public and private databases. Many handbooks, guides,
and other reference works include CAS Registry Numbers and provide special
indexes that allow the reader to find the proper place in the text without first
having to identify the full name of the substance. The reader benefits because the
full name may differ from handbook to handbook.
CAS Registry Numbers are also widely used as standard identifiers for chemi-
cal substances in many of the commercial chemical inventories of governmental
regulatory agencies, such as the Toxic Substances Control Act (TSCA) Inventory
in the United States, the European Inventory of Existing Commercial Chemi-
cal Substances (EINECS), and the Canadian Domestic and Non-Domestic Sub-
stance Lists (DSL/NDSL).
Whenever a chemical substance is sold, transported, imported, exported,
reported to a regulatory agency, or disposed of, a CAS Registry Number is prob-
ably involved.
255
➤ ➤ ➤ ➤ ➤ CHAPTER 13
Conventions
in Chemistry
This chapter presents a quick reference guide for the use of
typefaces (roman, italic, and bold), Greek letters, super-
scripts and subscripts, and special symbols that are commonly used in chemistry.
Appendix 13-1 presents the symbols for commonly used physical quantities.
Detailed recommendations from the International Union of Pure and
Applied Chemistry (IUPAC, http://www.iupac.org) are given in the book titled
Quantities, Units and Symbols in Physical Chemistry, 2nd edition, nicknamed the
“green book”, published by Blackwell Science, Oxford, U.K., 1993. Updates are
published as articles in the journal Pure and Applied Chemistry.
Detailed recommendations from the International Organization for Stan-
dardization (ISO, http://www.iso.org) are given in the ISO Standards Handbook
2, Quantities and Units, published by ISO, Geneva, Switzerland, 1993. Some indi-
vidual standards have been amended, and their updates are available at http://
www.iso.org/iso/en/prods-service/ISOstore/store.html. The National Institute
of Standards (NIST) Special Publication 330, 2001 Edition, available at http://
physics.nist.gov/Pubs/SP330/sp330.pdf, is the U.S. updated edition of the Eng-
lish version of the Bureau International des Poids et Mesures. The booklet The
International System of Units (SI), 7th ed., published by the International Bureau
of Weights and Measures (BIPM), Sèvres, France, 1998, is the definitive reference
on SI units.
Some books and journals follow IUPAC recommendations for representa-
tions of various chemical conventions. Some specify the use of ISO standards.
Others are less stringent as long as the manuscript is consistent in usage within
itself. Always consult the author guidelines.
Copyright 2006 American Chemical Society
256 ➤ The ACS Style Guide
Subatomic Particles and Quanta
➤ Use lowercase Latin or Greek letters for abbreviations for subatomic particles.
alpha particle α
beta particle β
deuteron d
electron e
helion h
muon µ±
neutrino νe
neutron n
photon γ
pion π
proton p
triton t
➤ Indicate electric charges with the appropriate superscript (+, –, or 0).
n0
e+
e–
π±
If the symbols p and e are used without indication of charge, they refer to posi-
tive proton and negative electron, respectively.
Electronic Configuration
➤ Denote electron shells with the uppercase roman letters K, L, M, and N.
➤ Name electron subshells and atomic orbitals with the lowercase roman letters
s, p, d, and f. Write principal energy levels 1–7 on the line and to the left of the
letter; give the number of electrons in the orbital as a superscript to the right of
the letter. Specify orbital axes with italic subscripts.
7s electron
5f2 ions
5f orbital
6d orbital
sp3 hybrid orbital
fn–3ds2 configuration
3d44s4p2 configuration
pxpypz
dxzdyzdxy
dz2
dx2–y2
The ground state of boron is 1s22s22px12py02pz0.
The valence-shell configuration of nitrogen is 2s22px12py12pz1.
The electronic configuration of potassium is 1s22s22p63s23p64s1.
The valence-electron configuration is described by 5d106s1.
➤ Use Greek letters for some bonding orbitals and the bonds they generate.
π bond
σ orbital
σ∗ orbital
Chapter 13: Conventions in Chemistry ➤ 257
➤ Name the electronic states of atoms with the uppercase roman letters S, P, D, F,
G, H, I, and K, corresponding to quantum numbers l = 0–7. Use the correspond-
ing lowercase letters to indicate the orbital angular momentum of a single elec-
tron. The left superscript is the spin multiplicity; the right subscript is the total
angular momentum quantum number J.
2S0
2s0
8F1/2
8f1/2
4P1/2
4p1/2
8G1/2
8g1/2
7F0
7f0
2P3/2
2p3/2
7D1
7d1
➤ Name the electronic states of molecules with the uppercase roman letters A, B,
E, and T; the ground state is X. Use the corresponding lowercase letters for one-
electron orbitals. A tilde (~) is added for polyatomic molecules. The subscripts
describe the symmetry of the orbital.
Ã
2A1g
A2g
ã
2a1g
a2g
3B1
3b1
Eg
E2g
eg
e2g
T2g
t2g
Chemical Elements and Formulas
➤ Write the names of the chemical elements in roman type and treat them as
common nouns.
calcium
californium
carbon
einsteinium
francium
helium
hydrogen
oxygen
seaborgium
uranium
➤ Write the symbols for the chemical elements in roman type with an initial
capital letter.
Ca
Cf
C
Es
Fr
He
H
O
Sg
U
The complete list of chemical elements and symbols is given in Table 13-1.
➤ Even when symbols are used, the element’s name is pronounced. Therefore,
choose the article (a or an) preceding the element symbol to accommodate the
pronunciation of the element name. (This usage does not apply to isotopes, as
described in the section on isotopes.)
a Au electrode (pronounced “a gold electrode”)
a N-containing compound (pronounced “a nitrogen-containing compound”)
a He–Ne laser (pronounced “a helium–neon laser”)

258 ➤ The ACS Style Guide
Name Sym. No. Atomic Wt. Notes
Actinium Ac 89 *
Aluminum Al 13 26.981538(2)
Americium Am 95 *
Antimony Sb 51 121.760(1) g
Argon Ar 18 39.948(1) g, r
Arsenic As 33 74.92160(2)
Astatine At 85 *
Barium Ba 56 137.327(7)
Berkelium Bk 97 *
Beryllium Be 4 9.012182(3)
Bismuth Bi 83 208.98038(2)
Bohrium Bh 107 *
Boron B 5 10.811(7) g, m, r
Bromine Br 35 79.904(1)
Cadmium Cd 48 112.411(8) g
Calcium Ca 20 40.078(4) g
Californium Cf 98 *
Carbon C 6 12.0107(8) g, r
Cerium Ce 58 140.116(1) g
Cesium Cs 55 132.90545(2)
Chlorine Cl 17 35.453(2) g, m, r
Chromium Cr 24 51.9961(6)
Cobalt Co 27 58.933200(9)
Copper Cu 29 63.546(3) r
Curium Cm 96 *
Dubnium Db 105 *
Dysprosium Dy 66 162.500(1) g
Einsteinium Es 99 *
Erbium Er 68 167.259(3) g
Europium Eu 63 151.964(1) g
Fermium Fm 100 *
Fluorine F 9 18.9984032(5)
Francium Fr 87 *
Gadolinium Gd 64 157.25(3) g
Gallium Ga 31 69.723(1)
Germanium Ge 32 72.64(1)
Gold Au 79 196.96655(2)
Hafnium Hf 72 178.49(2)
Hassium Hs 108 *
Helium He 2 4.002602(2) g, m
Holmium Ho 67 164.93032(2)
Hydrogen H 1 1.00794(7) g, m, r
Indium In 49 114.818(3)
Iodine I 53 126.90447(3)
Iridium Ir 77 192.217(3)
Iron Fe 26 55.845(2)
Krypton Kr 36 83.798(2) g, m
Lanthanum La 57 138.9055(2) g
Name Sym. No. Atomic Wt. Notes
Lawrencium Lr 103 *
Lead Pb 82 207.2(1) g, r
Lithium Li 3 6.941(2)† g, m, r
Lutetium Lu 71 174.967(1) g
Magnesium Mg 12 24.3050(6)
Manganese Mn 25 54.938049(9)
Meitnerium Mt 109 *
Mendelevium Md 101 *
Mercury Hg 80 200.59(2)
Molybdenum Mo 42 95.94(2) g
Neodymium Nd 60 144.24(3) g
Neon Ne 10 20.1797(6) g, m
Neptunium Np 93 *
Nickel Ni 28 58.6934(2)
Niobium Nb 41 92.90638(2)
Nitrogen N 7 14.0067(2) g, r
Nobelium No 102 *
Osmium Os 76 190.23(3) g
Oxygen O 8 15.9994(3) g, r
Palladium Pd 46 106.42(1) g
Phosphorus P 15 30.973761(2)
Platinum Pt 78 195.078(2)
Plutonium Pu 94 *
Polonium Po 84 *
Potassium K 19 39.0983(1)
Praseodymium Pr 59 140.90765(2)
Promethium Pm 61 *
Protactinium Pa 91 231.03588(2)*
Radium Ra 88 *
Radon Rn 86 *
Rhenium Re 75 186.207(1)
Rhodium Rh 45 102.90550(2)
Rubidium Rb 37 85.4678(3) g
Ruthenium Ru 44 101.07(2) g
Rutherfordium Rf 104 *
Samarium Sm 62 150.36(3) g
Scandium Sc 21 44.955910(8)
Seaborgium Sg 106 *
Selenium Se 34 78.96(3) r
Silicon Si 14 28.0855(3) r
Silver Ag 47 107.8682(2) g
Sodium Na 11 22.989770(2)
Strontium Sr 38 87.62(1) g, r
Sulfur S 16 32.065(5) g, r
Tantalum Ta 73 180.9479(1)
Technetium Tc 43 *
Tellurium Te 52 127.60(3) g
Continued on next page
Table 13-1. Atomic Weights of the Elements 2001
Chapter 13: Conventions in Chemistry ➤ 259
➤ Write the names of chemical compounds in roman type and treat them
as common nouns. (Names for chemical compounds are discussed further in
Chapter 12.)
benzaldehyde
calcium carbonate
chlorobenzene
ethanol
hydrochloric acid
iron(III) nitrate
isopropyl iodide
magnesium sulfate
mercuric sulfate
methyl salicylate
phenol
sodium hydroxide
➤ Use roman type for the symbols for chemical compounds.
BaSO4
C2H5OH
C6H5Cl
C6H5OH
CaCO3
CH3COOH
Fe(NO3)3
H3PO4
HCl
HgSO4
NaOH
Ni3P2O8
P2S5
VF5
Zn(C2H3O2)2
Name Sym. No. Atomic Wt. Notes
Terbium Tb 65 158.92534(2)
Thallium Tl 81 204.3833(2)
Thorium Th 90 232.0381(1)* g
Thulium Tm 69 168.93421(2)
Tin Sn 50 118.710(7) g
Titanium Ti 22 47.867(1)
Tungsten W 74 183.84(1)
Ununbium Uub 112 *
Ununhexium Uuh 116 *
Ununnilium Uun 110 *
Name Sym. No. Atomic Wt. Notes
Ununquadium Uuq 114 *
Unununium Uuu 111 *
Uranium U 92 238.02891(3)* g, m
Vanadium V 23 50.9415(1)
Xenon Xe 54 131.293(6) g, m
Ytterbium Yb 70 173.04(3) g
Yttrium Y 39 88.90585(2)
Zinc Zn 30 65.409(4)
Zirconium Zr 40 91.224(2) g
Notes: Scaled to the relative atomic mass, Ar(12C) = 12, where 12C is a neutral atom in its nuclear and electronic
ground state.
The atomic weights of many elements are not invariant but depend on the origin and treatment of the
material. The standard values of Ar(E) and the uncertainties (in parentheses following the last significant figure
to which they are attributed) apply to elements of natural terrestrial origin. The footnotes to this table elabo-
rate the types of variation that may occur for individual elements and that may be larger than the listed uncer-
tainties of values of Ar(E). Names of elements with atomic numbers 110 to 116 are provisional.
*Element has no stable nuclides. However, three such elements (Pa, Th, and U) do have a characteristic terres-
trial isotopic composition, and for these an atomic weight is tabulated.
†Commercially available Li materials have atomic weights that range between 6.939 and 6.996; if a more accu-
rate value is required, it must be determined for the specific material.
g Geological specimens are known in which the element has an isotopic composition outside the limits for
normal material. The difference between the atomic weight of the element in such specimens and that given in
the table may exceed the stated uncertainty.
m Modified isotopic compositions may be found in commercially available material because it has been sub-
jected to an undisclosed or inadvertent isotopic fractionation. Substantial deviations in atomic weight of the
element from that given in the table can occur.
r Range in isotopic composition of normal terrestrial material prevents a more precise Ar(E) being given; the
tabulated Ar(E) value should be applicable to any normal material.
Source: Reprinted with permission from IUPAC, 2003. Copyright 2003 IUPAC.
Table 13-1. Atomic Weights of the Elements 2001—Continued
260 ➤ The ACS Style Guide
➤ You may use both chemical symbols and element names in text, but it is best to
use one or the other consistently. Do not mix symbols and words within a name.
NaCl or sodium chloride, not Na chloride
➤ Unnamed elements may be designated by using the atomic number (for
example, element 125). They may also be designated by using the systematic
name or symbol devised by IUPAC for elements of atomic number greater than
100 that have not yet received trivial names. In this system, an element name
consists of a series of numerical roots corresponding to the numerals in the
atomic number of the element, followed by “ium”. The roots are as follows:
0 nil
1 un
2 bi
3 tri
4 quad
5 pent
6 hex
7 sept
8 oct
9 enn
The symbols consist of the first letters of the numerical roots.
examples
element 146 unquadhexium Uqh
element 187 unoctseptium Uos
element 209 binilennium Bne
element 290 biennilium Ben
element 501 pentnilunium Pnu
element 502 pentnilbium Pnb
element 503 pentniltrium Pnt
element 900 ennilnilium Enn
Drop the final “n” in “enn” when it is followed by “nil” (see elements 290 and
900) and the final “i” in “bi” and “tri” when they are followed by “ium” (see ele-
ments 502 and 503).
➤ You may use common abbreviations for organic groups in formulas and
structures, but not in text. These (and only these) abbreviations need not be
defined.
Ac acetyl
Ar aryl
Bu butyl
i-Bu isobutyl
sec-Bu sec-butyl
t-Bu tert-butyl
Bz benzoyl
Bn, Bzl benzyl
Et ethyl
Me methyl
Ph phenyl
Pr propyl
i-Pr isopropyl
R, R′ alkyl
➤ Use square brackets in formulas for coordination entities.
[Cr(C6H6)2]
K[PtCl3(C2H4)]
Chapter 13: Conventions in Chemistry ➤ 261
➤ In the formula for an addition compound, use a centered dot, closed up on
each side. (Although the IUPAC books show a space on each side, this spacing
would wreak havoc with many typesetting systems.)
BH3·NH3
Ni(NO3)2·2Ni(OH)2
Water of hydration follows a centered dot, closed up on each side.
Na2SO4·10H2O
Zn(NO3)2·H2O
➤ Use either a slash or an en dash between components of a mixture, but not a
colon.
dissolved in 5:1 glycerin/water
dissolved in 5:1 glycerin–water
the metal/ligand (1:1) reaction mixture
the metal–ligand (1:1) reaction mixture
the metal–ligand (1/1) reaction mixture
the methane/oxygen/argon (1/50/450) matrix
the methane/oxygen/argon (1:50:450) matrix
Reference to the Periodic Table
➤ Always use lowercase for the word “group”, even with a specific number.
group 15 elements
group IVB elements
➤ Always use lowercase for the words “periodic table”.
The elements in group 8 of the periodic table include Fe, Ru, and Os.
Atoms and Molecules
Nuclide descriptors are specified with superscripts and subscripts to the element
symbol, as follows.
Use the Left Superscript for Mass Number
➤ The mass number of an atom is usually shown only for isotopes or in dis-
cussions of isotopes.
12C
35Cl
32S
262 ➤ The ACS Style Guide
Use the Left Subscript for Atomic Number
➤ The atomic number of an atom is usually used only in discussions of nuclear
chemistry.
6C
16S
Use the Right Superscript for Ionic Charge
➤ The charge number is followed by the sign of the ionic charge. When the
charge number is 1, only the sign is used.
Ca2+
Na+
NO3–
➤ Stagger the subscript and superscript; do not align them. The subscript comes
first with ionic charge.
PO43–
➤ Do not use multiple plus or minus signs, and do not circle the charge.
Hg2+ (not Hg++)
Use the Right Asterisk for Excited Electronic State
He∗
NO∗
Use the Right Superscript for Oxidation Number
➤ You may use superscript roman numerals for oxidation numbers. In for-
mulas, do not use numbers on the line to avoid confusion with the symbols for
iodine or vanadium.
CoIII
FeIICl2
MnIII/IV
MnIII–MnIV
MnIII/MnIV
MnIVO2
(NH3)2PtII
Ni0
O–II
PbIVO2
RuII–RuIII
RuII/RuIII
➤ Stagger the subscript and superscript; do not align them. The subscript fol-
lows the superscript with oxidation number.
PbII2
➤ You may also write oxidation numbers on the line in parentheses closed up
to the element name or symbol.
Chapter 13: Conventions in Chemistry ➤ 263
cobalt(III) or Co(III)
copper(II) or Cu(II)
diammineplatinum(II)
ferrate(VI) ion
iron(II) or Fe(II)
iron(II) chloride
manganese(IV) oxide
Mn(III)–Mn(IV) complex
Mn(III)/Mn(IV) complex
potassium tetracyanonickelate(0)
Use the Right Subscript for Number of Atoms
➤ With an element symbol, use a subscript to indicate the number of atoms,
whether in formulas or in narrative text.
Al2O3
C6
C6H5CH3
(CH3)4C
Fe3
FeSi2
H2S
NH4
The C60 fullerene molecule is shaped like a soccer ball.
➤ With an element name, follow the usual conventions for numbers in text.
Molecules composed of 60 carbon atoms are shaped like soccer balls.
In this reaction, three hydrogen atoms are lost.
Atom in a Specific Position
➤ Use either words or symbols and numbers on the line to refer to an atom in a
specific position.
at the carbon in the 6-position or at C6 or at C-6
the atom in the β-position or the β atom
Isotopes
➤ Specify the isotope of an element by a mass number written as a left super-
script to the element symbol.
13C
15N
32S
29Si
235U
264 ➤ The ACS Style Guide
➤ Alternatively, indicate an isotope by using the spelled-out element name
hyphenated to its mass number.
carbon-14
uranium-235
➤ In either case, the isotope name or symbol is pronounced first, then the num-
ber. Thus, 14C is pronounced “c fourteen”. Consequently, choose the article (a
or an) preceding the isotope to accommodate the pronunciation of the element
name or symbol, not the number.
a carbon-14 isotope a 14C isotope (pronounced “c fourteen”)
a hydrogen-3 isotope an 3H isotope (pronounced “aitch three”)
a nitrogen-15 isotope an 15N isotope (pronounced “en fifteen”)
➤ Use the symbols 2H or D for deuterium and 3H or T for tritium when no
other nuclides are present.
D2O
CD2H2
3H2
CH3O3H
(T2N)2CO
HDSO4
2H2S
CH2TOH
3H2
An isotopically unmodified compound is one whose isotopic nuclides are
present in the proportions that occur in nature. An isotopically modified com-
pound has a nuclide composition that deviates measurably from that occurring
in nature.
An isotopically substituted compound has a composition such that all of the
molecules of the compound have only the indicated nuclides at the designated
positions. To indicate isotopic substitution in formulas, the nuclide symbols are
incorporated into the formulas. To indicate isotopic substitution in spelled-out
compound names, the number and symbol (and locants if needed) are placed in
parentheses closed up to the name.
N15NF2
24NaCl
H2N14CONH2
14CH4
238UCl3
32PO43–
Mo(12CO)6
Na235S
(15N)ammonia
(14C6)glucose
(1,3-3H2)benzene
1-chloro(2-3H)benzene
An isotopically labeled compound is a mixture of an isotopically unmodified
compound with an analogous isotopically substituted compound or compounds.
Isotopically labeled compounds may be specifically labeled or selectively labeled.
To indicate isotopic labeling, the number and symbol (and locants if needed) are
enclosed in square brackets closed up to the compound name or formula.
specifically labeled:
[14C]H4
CH2[2H2]
CH3CH2[18O]H
Chapter 13: Conventions in Chemistry ➤ 265
selectively labeled:
[2H]CH4
[2H]PH3
[36Cl]SOCl2
[6,7-15N]adenosine
[15N]alanine
[15N]ammonium chloride
[1,3-3H2]benzene
[57Co]cyanocobalamin
2,4-diamino[18O]phenol
[2,8-3H]inosine
[2-14C]leucine
➤ When the isotope position is specified by a group name that is part of the
parent compound, italicize the group name.
[methyl-14C]toluene
➤ Isotopically labeled compounds may also be described by inserting the sym-
bol in brackets into the name of the compound.
hydrogen [36Cl]chloride
[35S]sulfuric [2H]acid
➤ Do not use the left superscript within an abbreviation.
[32P]CMP (not CM32P)
➤ To indicate general labeling, use the symbol G in the names of selectively
labeled compounds in which all positions of the designated element are labeled,
but not necessarily in the same isotopic ratio.
d-[G-14C]glucose
➤ To indicate uniform labeling, use the symbol U in the names of selectively
labeled compounds in which all positions of the designated element are labeled
in the same isotopic ratio.
d-[U-14C]glucose
➤ When it is unknown or irrelevant whether the compound is isotopically
labeled or isotopically substituted, simply hyphenate the isotope symbol to the
compound name and do not use square brackets or parentheses.
14C-glucose
3H-benzene
15N-adenosine
The Boughton system, used in Chemical Abstracts, does not distinguish
between labeling and substitution. The isotopic variation is shown by the sym-
bol for the isotope (with a subscript numeral to indicate the number of isotopic
atoms) placed after the name or relevant portion of the name; locants are cited
266 ➤ The ACS Style Guide
if necessary. The locants and symbols are in italics, except subscripts and Greek
letters, and hyphens are used to separate them.
acetamide-1-13C-15N
acetic-17O2 acid
benzeneacetic-carboxy,α-14C2 acid
benzoic-18O acid
4-(2-propenyl-3-13C-oxy)benzoic acid
toluene-methyl-14C
➤ In this system, deuterium and tritium are represented by italic lowercase let-
ters d and t, respectively.
acetic-t3 acid-t
alanine-N,N,1-d3
ammonia-d-t
ethane-1-d-2-t
1-(ethyl-2,2,2-d3)-4-(methyl-d3)benzene
methan-t-ol
methane-d4
tri(silyl-d3)phosphine
urea-t4
Radicals
➤ In the formula of a free radical, indicate the unshared electron by a super-
script or centered dot closed up to the element symbol or formula. The super-
script dot comes after the symbol or formula; centered dots come before or after
the symbol or formula.
Br•
Br•
•CH3
C6H5•
H•
HO•
•NH2
•SH
(SiH3)•
•SnH3
➤ Charged radical cations and anions are often indicated by the symbol, for-
mula, or structure with a superscript dot followed by a plus or minus sign. How-
ever, in mass spectrometry, the reverse is used. Therefore, use the order of dots
and signs for charges that is appropriate for the context.
(Ag2)•+
C6H5NO•3–
HCO•+
R•–
R2•+
R(•)(2–)
(SO2) • –
mass spectrometry
C3H6+•
R+•
Chapter 13: Conventions in Chemistry ➤ 267
Bonds
➤ For linear formulas in text, do not show single bonds unless the bonds are the
subject of the discussion.
C6H5CH3
C6H5COOCOCH3
CH3CHOHCH3
CH3COOH
H2SO4
➤ When necessary for the discussion, indicate bonds by en dashes.
the –CH2– segment
the C–H distances
the C–C–C angle
(–CH2–CH(CH3)–O–)n
➤ When necessary, show double and triple bonds in linear formulas.
CH3C≡CH
CH2=CH2
R–C–OH is better as RCOOH or RCO2H or RC(=O)OH
||
O
➤ Use three centered dots to indicate association of an unspecified type (e.g.,
hydrogen bonding, bond formation, or bond breaking).
CPt
FH–NH3
H2Oπ aromatic hydrogen bonding
Mg2+O–
NiAl
Crystallography
Planes and Directions in Crystals
➤ Miller indices of a crystal face or a single net plane are enclosed in parenthe-
ses. (123) or (hkl) is a plane or set of planes that describe crystal faces; (h1h2h3) is
a single net plane.
➤ Laue indices are not enclosed. 123 or hkl is the Bragg reflection from the set
of net planes (123) or (hkl), respectively.

268 ➤ The ACS Style Guide
➤ Indices of a set of all symmetrically equivalent crystal faces or net planes are
enclosed in braces. {hkl} is a form.
➤ Indices of a zone axis or lattice direction are enclosed in square brackets.
[123] or [uvw] is a direction.
➤ Indices of a set of symmetrically equivalent lattice directions are enclosed in
angle brackets. <uvw> represents all crystallographically equivalent directions of
the type [uvw].
120
1,10,1
11,0,1
1,–2,0 reflections
the (111) face
the (120) face
the [001] axis
the [010] direction
the [101] direction
h00 diffraction lines
the hk0 zone
the 002 reflection
the 00l class of reflections
➤ When indices are used with spelled-out element names, separate the name of
the element and the index with a space.
copper (111)
rhenium (010)
a gold (111) substrate
on silicon (111) surfaces
the silver (110) surface
➤ However, when indices are used with element symbols, close up the element
symbol to the index.
Au(210)
CdTe(100)
Cu(111)
GaAs(100)
Rh(010)
Si(400)
an iodine-modified Ag(111) electrode
the Ag(110) surface

Chapter 13: Conventions in Chemistry ➤ 269
Types of Crystal Lattices
bcc body-centered cubic
ccp cubic close-packed
fcc face-centered cubic
hcp hexagonal close-packed
Symmetry Operations and Structural Point Groups
➤ Use italic type for the letters in symmetry operations and structural point
groups. The symbols (Schoenflies) are as follows: E, identity; C, cyclic; D, dihe-
dral; T, tetrahedral; O, octahedral; I, icosahedral; S, rotation–reflection; and σ,
mirror plane. Align subscripts and superscripts.
C1
Ci
Cs
C2
C2
3
C2
4
C∞
C2v
C3v
C4v
C∞v
C2h
C3h
D2d (Vd)
D3d
D4d
D2h (Vh)
D3h
D4h
D∞h
Ih
Oh (Kh)
S3
S4
2S6
S8
σ
2σv
3σv
4σv
3σd
σh
Td
Crystallographic Point Groups
➤ Use arabic numerals or combinations of numerals and the italic letter m to
designate the 32 crystallographic point groups (Hermann–Mauguin). The num-
ber is the degree of the rotation, and m stands for mirror plane. Use an overbar
to indicate rotation inversion.
1
m
2/m
mm2
mmm
4mm
32
622
6/mmm
43m
m3m
Space Groups
➤ Designate space groups by a combination of unit cell type and point group
symbol, modified to include screw axes and glide planes (Hermann–Mauguin);
230 space groups are possible. Use italic type for conventional types of unit cells
(or Bravais lattices): P, primitive; I, body-centered; A, A-face-centered; B, B-face-
centered; C, C-face-centered; F, all faces centered; and R, rhombohedral.

270 ➤ The ACS Style Guide
Pnma
C2/c
Pbcn
I41/a
Fd3m
Pnn2
P43212
Fm3m
R3m
Cmc21
P1
Fdd2
Aba2
I4/mmm
Crystallographic Information File
A description of the Crystallographic Information File, CIF, is included in
Appendix 13-2.
Chirality
➤ Use italic type for certain chirality symbols and symmetry site terms.
A anticlockwise
C clockwise
CU cube
DD dodecahedron
OC octahedron
TP trigonal phase
TPR trigonal prism
TPY trigonal pyramid
These symbols are often combined with coordination numbers and position
designations for stereochemical descriptors (e.g., OC-6-11′).
➤ In chemical names, use (R) and (S), with designated locants when applicable,
as prefixes to designate absolute configuration.
(R)-hydroxyphenylacetic acid
(S)-2,3-dihydroxypropanoic acid
(1S,2S,4R)-trichloro-1,2,4-trimethylcyclohexane
➤ Indicate optical rotation by plus and minus signs in parentheses and hyphen-
ate them to the chemical name.
(±)-4-(2-aminopropyl)phenol
(+)-glucose
(–)-tartaric acid
➤ Use small capital letters d and l for absolute configuration with amino acids
and carbohydrates.
β-d-cellotetraose
d-allothreonine
d-glucose
d-valine
dl-leucine
hydroxy-dl-glutamic acid
5-hydroxy-l-lysine
hydroxy-l-proline
l-alanine
l-alloisoleucine
l-ascorbic acid
l-phenylalanine
Chapter 13: Conventions in Chemistry ➤ 271
➤ Use a hyphen between (+) or (–) and d or l.
(–)-d-fructose
(–)-d-glyceraldehyde
(+)-l-phosphoglycerol
Concentration
➤ Use square brackets enclosing an element symbol or formula to indicate its
concentration in reactions and equations, but not in narrative text.
correct
[Mg2+] = 3×10–2 M
The Mg concentration decreased with repeated washings.
incorrect
The [Mg] was found to be greater in the unwashed samples.
➤ Do not use square brackets to indicate concentration with a spelled-out
name.
[Ca2+] (not [calcium])
[NaCl] (not [sodium chloride])
➤ Do not use italic type for the chemical concentration unit M (molar, moles
per cubic decimeter, moles per liter) or the unit N (normal). Use italic type for
the unit m (molal, moles per kilogram). Use a space between the number and
these abbreviations, that is, on each side of these abbreviations.
8 M urea
1 mM EDTA
6 N HCl
2.0 m NaOH
➤ When concentration is given as percentage, use the percent sign closed up to
the number.
20% H2SO4
90% acetonitrile/10% water
➤ Generally, the negative logarithm of the hydrogen ion concentration is
denoted by pH; the negative logarithm of the hydroxide ion concentration
is denoted by pOH. Use a space to separate pH or pOH and the number. Use
272 ➤ The ACS Style Guide
roman type for pH and pOH; always use lowercase for “p”; always capitalize “H”
and “OH”.
Solutions were titrated to pH >11.
The UV spectra were measured at pH 6.
A pOH of <12 was acceptable.
Chemical Reactions
➤ Short chemical reactions may be run into text or they may be displayed and
numbered, if numbering is needed. Long chemical reactions should be displayed
separately from the text. The sequential numbering system used may integrate
both chemical and mathematical equations, or separate sequences using differ-
ent notations may be used for different types of equations (e.g., eqs 1–3 could be
used for a set of chemical reactions and eqs I–III could be used for a set of math-
ematical equations). The use of lettering, rather than numbering, sequences is
also acceptable.
Cr(CO)4 + CO → Cr(CO)5 (1)
NH3 + HCOOH → NH2CHO + H2O (2)
(C6H5)2P–P(C6H5)2 → 2(C6H5)2P• (3)
Fe(CO)5 + OCH3– → Fe(CO)4(CO2CH3)– (4)
➤ Many kinds and combinations of arrows can be used. For example, two full
arrows in opposite directions (O) indicate a reaction that is proceeding in both
directions. Two arrows with half heads in opposite directions (s) indicate a
reaction in equilibrium. A single arrow with heads on both sides (↔) indicates
resonance structures, not a reaction.
➤ Specify the number of each species (molecules, atoms, ions, etc.) of reactants
and products by a numeral written on the line and closed up to the symbol.
2Al + 6NaOH → 2Na3AlO3 + 3H2 (5)
➤ To indicate the aqueous, solid, liquid, or gas state, use the appropriate abbre-
viations on the line, in parentheses, and with no space preceding them.
Ag(s) + H+(aq) + Cl–(aq) → AgCl(s) + ½H2(g) (6)
4FeS(s) + 7O2(g) → 2Fe2O3(s) + 4SO2(g) (7)
➤ Indicate reaction conditions and catalysts over and under the arrow in a
smaller type size. The Greek capital letter delta indicates heat; hν indicates light,
where h is Planck’s constant and the Greek letter nu is the photon frequency.
Chapter 13: Conventions in Chemistry ➤ 273
PhS PhS e
C H g O g CO g H O g
RC N H P
− −
→ +
+ → +
≡ +
hνi
3 8 2 2 2
2
5 3 4
2
( ) ( ) ( ) ( )
∆
tt or Pd
3
KMnO
H SO
RCH NH
H O CH C CH =C CH CH4
→
+ →
2 2
2 3 3 3
22 4
( ) ( ) ,∆
+
+ →
+
°
2 2
2
3 3 2
2 5 2 3 125 2 5 2
CH C =O CH H
C H C=O CH OH C H C O
H
C
( )
( ) ( ) ( CCH H O
3 2 2
)+
➤ Specify nuclear reactions according to the following scheme:
initial nuclide
incoming outgoing
particle(s) , particle(s)
or quuanta or quanta
final nuclide
examples
14N(α,p)17O
59Co(n,γ)60Co
23Na(γ,3n)20Na
31P(γ,pn)29Si
➤ Treat chemical equations that include structures with rings as illustrations.
They are discussed in Chapter 17.
➤ Abbreviate reaction types with capital roman letters and arabic numerals.
SN1 SN2 first- and second-order nucleophilic substitution, respectively
E1 E2 first- and second-order elimination, respectively
SRN1 SRN2 first- and second-order radical nucleophilic substitution,
respectively
➤ Subscripts denote a chemical process or reaction.
ads adsorption
at atomization
c combustion
dil dilution
dpl displacement
f formation
fus fusion
imm immersion
mix mixing
r reaction in general
sol solution
sub sublimation
trs transition
vap vaporization
274 ➤ The ACS Style Guide
➤ Certain superscripts are recommended.
‡ activated complex, transition state
′ apparent
E excess quantity
id ideal
∞ infinite dilution
∗ pure substance
°, & standard state
Reporting Analytical Data
There is no best way to present data. A presentation that is suitable for one paper
or publication may be unsuitable for another. The following are examples of
acceptable presentations of analytical data. These are not necessarily real exam-
ples; they may be combinations of data from two or more samples, intended to
show various style possibilities. You need not define the abbreviations and sym-
bols in the paper.
Melting and Boiling Points
mp 175.5 °C (lit.25 mp 175–176 °C)
mp 225 °C dec
bp 127 °C
Abbreviations: mp, melting point; bp, boiling point; lit., literature value; and dec,
decomposition. A full space is used between the number and the unit °C; the
degree symbol is closed up to the C. A superscript number after “lit.” denotes the
number of the reference.
Specific Rotation
[α]D
20 + 25.4 (c 1.00, CHCl3)
Abbreviations: α, specific rotation; D, the sodium D line or wavelength of light
used for the determination; and the superscript number, temperature (°C) at
which the determination was made. In parentheses: c stands for concentration;
the number following c is the concentration in grams per 100 mL of solution;
and last is the solvent name or formula.
NMR Spectroscopy
1H NMR (400 MHz, CD3OD, δ): 8.73 (s, 3H, –OCH3), 7.50 (s, 1H, CH), 7.15 (d, J
= 8.2 Hz, 1H, Ar H), 6–3 (br s, 5H, NH and NH2).
Compound 5: 1H NMR (500 MHz, CDCl3, δ) 1.12 (t, J = 7.1 Hz, –CH2CH3, 3H),
3.34 (q, J = 7.1 Hz, –CH2CH3, 2H), 3.38 (t, J = 6.0 Hz, –CH2CH2OH, 2H), 3.72 (t,
J = 6.0 Hz, –CH2CH2OH, 2H), 6.57 (dd, J = 8.7 Hz, Ar H, 2H).
13C NMR (DMSO-d6, δ): 175.4 (C=O), 156.5 (C4), 147.4 (C6), 138.3 (C2), 110.5
(d, J = 11.3 Hz, C5), 52.3 (CH3), 28.4 and 28.8 (C7).

Chapter 13: Conventions in Chemistry ➤ 275
13C NMR (DMSO-d6, δ): 0.43 (2C), 27.56 (4C), 131.8 (1C), 161.9 (2C).
13C NMR (CDCl3, 75.4 MHz): δ 213.50 (s, C-21), 178.27 (s, C-2), 168.69 (s, C-8),
164.61 (d, C-10), 119.67 (d, C-7), 52.45 (t, C-22), 38.95 (q, C-25).
13C NMR (C6D12, δ): 6.51 (s, C5Me5), 14.41 (d, J = 157 Hz, PMe3), 28.68 (s, Me),
105.1 (t, J = 3.7 Hz, C5Me5), 128.52 (s), 135.19 (br s), 212.56 (C=O).
If the experimental conditions have already been described elsewhere in the
paper, they need not be repeated.
NMR: 3.81, 2.56, and 2.12 ppm.
Compound 27: NMR 5.14, 3.90, 2.67, and 1.88 ppm.
Abbreviations: δ, chemical shift in parts per million (ppm) downfield from the
standard; J, coupling constant in hertz; and the multiplicities s, singlet; d, dou-
blet; t, triplet; q, quartet; and br, broadened. Italicized elements or groups are
those that are responsible for the shifts.
IR Spectroscopy
IR (KBr) νmax: 967 (Ti=O), 3270 cm–1 (NH).
IR (KBr, thin film) νmax (cm–1): 3017, 2953 (s, OH), 2855 (s), 2192, 1512, 1360,
1082, 887.
IR (dry film) νmax: 3324 (OH), 2973–2872 (CH, aliphatic), 1706 (C=O, ketone),
1595, 1437, 1289, 1184, 1048, 870, 756, 677 cm–1.
IR: 2000, 2030, 2040, 2050 cm–1.
IR (cm–1): 3130, 3066, 2964, 1654, 1500, 1371.
Compound 6: IR 2910, 2487, 1972, 1564, 1190 cm–1.
GC–FTIR νmax (cm–1): 2979 (w), 1400 (m), 1264 (s), 827 (vs).
Abbreviations: νmax is the wavenumber of maximum absorption peaks in recip-
rocal centimeters, and the absorptions are w, weak; m, medium; s, strong; vw,
very weak; vs, very strong; and br, broad.
Mass Spectrometry
MS m/z (relative intensity): 238.2058 (44.8%), 195.1487 (100%), 153.1034
(21.2%).
GC–MS m/z (% relative intensity, ion): 202 (9, M + 4), 200 (32, M + 2), 198 (23,
M+), 142 (35, M – 2CO), 321 (95, M – Me), 415 (M+ – Cl).
HRMS–FAB (m/z): [M + H]+ calcd for C21H38N4O6S, 475.259; found, 475.256.
EIMS (70 eV) m/z: M+ 420 (15), 241 (15), 201 (59), 135 (14), 69 (23).
Abbreviations: m/z is the mass-to-charge ratio, M is the molecular weight of the
molecule itself, M+ is the molecular ion, HRMS is high-resolution mass spec-
trometry, FAB is fast atom bombardment, and EIMS is electron-impact mass
spectrometry.

276 ➤ The ACS Style Guide
UV–Visible Spectroscopy
UV (hexanes) λmax, nm (ε): 250 (1070).
UV (CH3OH) λmax (log ε) 210 (3.33), 242 (sh, 3.02), 288 (sh, 2.21), 421 nm (3.16).
Abbreviations: λmax is the wavelength of maximum absorption in nanometers; ε
is the molar absorption coefficient or molar absorptivity; and sh is the shoulder.
The wavenumber, ν, in reciprocal micrometers, might also be given.
Quantitative Analysis
Anal. Calcd for C45H28N4O7: C, 62.47; H, 3.41; N, 6.78. Found: C, 61.80; H, 3.55;
N, 6.56.
All values are given as percentages.
X-ray Diffraction Spectroscopy
➤ Separate the element and its spectral line by a space.
Au LI
Au LIII
Cr K
Cu Kα
Cu Kβ
Mo Kα
Cu Kα radiation
the Co K absorption edge
the Ni K edge
Citing ASTM, ANSI, and ISO Standards
ASTM International, originally known as the American Society for Testing and
Materials; ANSI, the American National Standards Institute; and ISO, the Inter-
national Organization for Standardization, are organizations that set standards
in a variety of areas.
➤ For ASTM standards, separate the letter and the number of the standard by a
space. For ANSI standards, close up the letter and the number.
astm
D 3137
D 573
D 130
D 1660
DS 4B
DS 64
DS 48A
E 380-93
STP 1249
STP 169C
STP 315I
as described in ASTM Standard D 1223
ansi
Z358.1-1990 Z88.2-1992
as noted in ANSI Standard H35.1M-1993
iso
14020 9000 71.040
ISO/DIS 14010
according to ISO Standard 11634
277
➤ ➤ ➤ ➤ ➤
APPENDIX 13-1
Symbols for Commonly
Used Physical Quantities
Atoms and Molecules
name symbol si unit
Atomic mass ma kg
Atomic mass constant mu kg
Atomic number Z dimensionless
Decay constant λ s–1
Electron rest mass me kg
Electronic term Te m–1
Elementary charge (of a proton) e C
g factor, g value g dimensionless
Ionization energy Ei, I J
Magnetogyric ratio γ s–1·T–1
Mass number A dimensionless
Neutron number N dimensionless
Nucleon number A dimensionless
Planck constant h J·s
Planck constant/2π J·s
Proton number Z dimensionless
Proton rest mass mp kg
Rotational constants A, B, C m–1
Rotational term F m–1
Total angular momentum component mj, mJ dimensionless
Total term T m–1
Unified atomic mass unit mu kg
Vibrational quantum number v dimensionless
Vibrational term G m–1
Chemical Kinetics
name symbol si unit
Boltzmann constant k, kB J/K, J·K–1
Energy of activation; Arrhenius or
activation energy E, Ea, EA J/mol, J·mol–1
Half-life t1/2 s
Photochemical yield φ dimensionless
Quantum yield φ dimensionless
278 ➤ The ACS Style Guide
Chemical Kinetics—Continued
name symbol si unit
Rate constant, first order k s–1
Rate constant, second order k mol–1·s–1
Rate of concentration change of
substance B rB mol/(m3·s), mol·m–3·s–1
Rate of conversion ζ
· mol/s, mol·s–1
Rate of reaction v mol/(m3·s), mol·m–3·s–1
Relaxation time τ s
Scattering angle θ rad
Standard enthalpy of activation ∆‡H°, ∆H‡ J/mol, J·mol–1
Standard entropy of activation ∆‡S°, ∆S‡ J/(mol·K), J·mol–1·K–1
Standard Gibbs energy of activation ∆‡G°, ∆G‡ J/mol, J·mol–1
Standard internal energy of
activation ∆‡U°, ∆U‡ J/mol, J·mol–1
Temperature, absolute T K
Thermal energy kT J
Volume of activation ∆‡V, ∆V‡ J/mol, J·mol–1
Electricity and Magnetism
name symbol si unit
Capacitance C C/V, C·V–1, F
Charge density ρ C/m3, C·m–3
Conductance G S
Conductivity κ S/m, S·m–1
Dielectric polarization P C/m2, C·m–2
Electric charge Q C
Electric current I A
Electric current density j A/m2, A·m–2
Electric dipole moment p, µ C·m
Electric displacement D C/m2, C·m–2
Electric field strength E V/m, V·m–1
Electric potential V, φ V, J/C, J·C–1
Electric potential difference U, ∆V, ∆φ V
Electric resistance R Ω
Electric susceptibility χe dimensionless
Electromagnetic moment µ, m A·m2, J/T, J·T–1
Impedance Z Ω
Inductance H A/m, A·m–1, H
Magnetic field strength H A/m, A·m–1, H
Magnetic flux Φ Wb
Magnetic flux density B T
Magnetic induction B T
Magnetic moment M A/m, A·m–1
Magnetic susceptibility χ dimensionless
Chapter 13: Conventions in Chemistry ➤ 279
Electricity and Magnetism—Continued
name symbol si unit
Magnetization M A/m, A·m–1
Permeability µ H/m, H·m–1
Permittivity ε F/m, F·m–1
Polarization (of a particle) α m2·C·V–1
Relative permeability µr dimensionless
Relative permittivity εr dimensionless
Resistance R Ω
Resistivity ρ Ω·m
Self-inductance L H
Voltage U, ∆V, ∆φ V
Electrochemistry
name symbol si unit
Charge number of an ion z dimensionless
Conductivity κ S/m, S·m–1
Diffusion rate constant kd m/s, m·s–1
Electric current I A
Electric current density j A/m2, A·m–2
Electric mobility u m2/(V·s), m2·V–1·s–1
Electrode potential E V
Electrolytic conductivity κ S/m, S·m–1
Electromotive force (emf) E V
Elementary charge e C
Faraday constant F C/mol, C·mol–1
Half-wave potential E1/2 V
Ionic strength
concentration basis Ic, I mol/m3, mol·m–3
molality basis Im, I mol/kg, mol·kg–1
Mass-transfer coefficient kd m/s, m·s–1
Molar conductivity Λ S·m2/mol, S·m2·mol–1
Reduction potential E° V
Standard electrode potential E° V
Standard electromotive force (emf) E° V
Surface charge density σ C/m2, C·m–2
Transport number t dimensionless
General Chemistry
name symbol si unit
Amount concentration c mol/m3, mol·m–3
Amount of substance n mol
Atomic weight Ar dimensionless
Concentration c mol/m3, mol·m–3
Degree of dissociation α dimensionless
280 ➤ The ACS Style Guide
General Chemistry—Continued
name symbol si unit
Extent of reaction ζ mol
Mass fraction w dimensionless
Molality m, b mol/kg, mol·kg–1
Molar mass M kg/mol, kg·mol–1
Molar volume Vm m3/mol, m3·mol–1
Molarity M mol/L, mol·L–1
Mole fraction x dimensionless
Molecular weight Mr dimensionless
Number concentration C, n m–3
Number density of entities C, n m–3
Number of entities N dimensionless
Partial pressure of substance B pB Pa
Relative atomic mass Ar dimensionless
Relative molecular mass Mr dimensionless
Stoichiometric coefficient ν dimensionless
Surface concentration Γ mol/m2, mol·m–2
Volume fraction φ dimensionless
Mechanics
name symbol si unit
Angular momentum L J·s
Density ρ kg/m3, kg·m–3
Energy E J
Force F N
Gravitational constant G N·m2/kg2, N·m2·kg–2
Hamiltonian function H J
Kinetic energy Ek, K J
Lagrange function L J
Mass m kg
Moment of force M N·m
Moment of inertia I kg·m2
Momentum p kg·m/s, kg·m·s–1
Potential energy Ep, V J
Power P W
Pressure P, p Pa, N/m2, N·m–2
Reduced mass µ kg
Relative density d dimensionless
Specific volume v m3/kg, m3·kg–1
Surface tension γ N/m, N·m–1, J/m2, J·m–2
Torque T N·m
Weight G, W N
Work w, W J
Chapter 13: Conventions in Chemistry ➤ 281
NMR Spectroscopy
name symbol si unit
Bohr magneton µB, β J/T, J·T–1
Bohr radius a0 m
Chemical shift, δ scale δ dimensionless
Coupling constant
(indirect) spin–spin JAB Hz
direct (dipolar) DAB Hz
reduced spin–spin KAB T2·J–1, N·A–2·m–3
Delay time τ s
Electron spin quantum component ms, mS dimensionless
Electron spin quantum number s, S dimensionless
Hyperfine coupling constant a, A, T Hz
Larmor angular frequency ωL s–1
Larmor frequency νL Hz
Magnetogyric ratio γ s–1·T–1
Nuclear magneton µN J/T, J·T–1
Nuclear spin quantum component MI dimensionless
Nuclear spin quantum number I dimensionless
Orbital quantum number l, L dimensionless
Orbital quantum number component ml, mL dimensionless
Principal quantum number n dimensionless
Quadrupole moment Q, Θ C·m2
Relaxation time
longitudinal T1 s
transverse T2 s
Shielding constant σ dimensionless
Polymer Chemistry
name symbol si unit
Bulk modulus K Pa
Complex permittivity ε∗ F/m, F·m–1
Crack-tip radius ρc m
Electrophoretic mobility µ m2·V–1·s–1
Flory–Huggins interaction parameter χ
Fracture strain γf, εf dimensionless
Fracture stress σf Pa
Glass-transition temperature Tg K
Modulus of elasticity E Pa
Tensile strength σ Pa
Viscosity ν Pa·s
Volume fraction Vf dimensionless
Yield stress σy Pa
Young’s modulus E Pa

282 ➤ The ACS Style Guide
Radiation
name symbol si unit
Absorbance A dimensionless
Absorption factor α dimensionless
Angle of optical rotation α dimensionless, rad
Angular frequency ω s–1, rad/s, rad·s–1
Emissivity, emittance ε dimensionless
Frequency ν Hz
Linear decadic absorption coefficient a m–1
Molar decadic absorption coefficient ε m2/mol, m2·mol–1
Molar refraction Rm m3/mol, m3·mol–1
Radiant energy Q, W J
Radiant intensity I W/sr, W·sr–1
Radiant power P W
Refractive index n dimensionless
Speed of light c m/s, m·s–1
Stefan–Boltzmann constant σ W/(m2·K4), W·m–2·K–4
Transmittance T dimensionless
Wavelength λ m
Wavenumber (in a vacuum) ν m–1
Space and Time
name symbol si unit
Acceleration a m/s2, m·s–2
Area A, S, AS m2
Cartesian space coordinates x, y, z m
Characteristic time interval τ s
Circular frequency ω s–1, rad/s, rad·s–1
Diameter d m
Frequency ν, f Hz
Height h m
Length l m
Position vector r m
Radius r m
Speed v, u, w, c m/s, m·s–1
Thickness, distance d, δ m
Time t s
Time constant τ s
Velocity v, u, w, c m/s, m·s–1
Volume V m3
Chapter 13: Conventions in Chemistry ➤ 283
Thermodynamics
name symbol si unit
Absolute activity λ dimensionless
Affinity of a reaction A J/mol, J·mol–1
Chemical potential µ J/mol, J·mol–1
Cubic expansion coefficient α K–1
Energy E J
Enthalpy H J
Entropy S J/K, J·K–1
Fugacity f Pa
Gas constant R J/(K·mol), J·K–1·mol–1
Gibbs energy G J
Heat q, Q J
Heat capacity, molar Cm J/(K·mol), J·K–1·mol–1
Heat capacity at constant pressure Cp J/K, J·K–1
Heat capacity at constant volume Cv J/K, J·K–1
Helmholtz energy A J
Internal energy U J
Isothermal compressibility κ Pa–1
Joule–Thomson coefficient µ K/Pa, K·Pa–1
Pressure, osmotic Π Pa
Pressure coefficient β Pa/K, Pa·K–1
Specific heat capacity c J/(K·kg), J·K–1·kg–1
Surface tension γ, σ J/m2, J·m–2, N/m, N·m–1
Temperature
Celsius t, θ °C
thermodynamic T K
Viscosity η Pa·s
Work w, W J
Transport Properties
name symbol si unit
Coefficient of heat transfer h W/(m2·K), W·m–2·K–1
Diffusion coefficient D m2/s, m2·s–1
Flux of a quantity x Jx, J varies
Heat flow rate φ W
Kinematic viscosity ν m2/s, m2·s–1
Mass flow rate qm kg/s, kg·s–1
Mass-transfer coefficient kd m/s, m·s–1
Thermal conductivity λ, k W/(m·K), W·m–1·K–1
Thermal diffusion coefficient DT m2/s, m2·s–1
Thermal diffusivity a m2/s, m2·s–1
Viscosity η Pa·s
Volume flow rate qv, V m3/s, m3·s–1
284
➤ ➤ ➤ ➤ ➤
APPENDIX 13-2
The Crystallographic
Information File
Frank H. Allen
The Crystallographic Information File (CIF) is the internationally agreed on
standard file format for the exchange of crystallographic information. Most
major journals now require that crystal structure data for small molecules and
inorganic compounds, obtained by single-crystal and powder diffraction analy-
ses, be deposited as electronic CIFs as part of the manuscript submission pro-
cess. A related format, the macromolecular CIF, or mmCIF, has been developed
for data deposition with the Protein Data Bank. The CIF (and mmCIF) standard
is maintained by the International Union of Crystallography (IUCr), and full
information and leading references can be found at www.iucr.org/iucr-top/cif/.
Important sources of three-dimensional crystal structure data, including data
in CIF or mmCIF format, are the following:
• The Cambridge Crystallographic Data Centre produces the Cambridge
Structural Database (http://www.ccdc.cam.ac.uk/products/csd/), which
covers organic and metal-organic small-molecule crystal structures.
• CRYSTMET (http://www.tothcanada.com/) contains data for metals,
intermetallics, and alloys.
• FIZ Karlsruhe produces the ICSD (http://www.fiz-informationsdienste.
de/en/FG/Kristall/index.html), which contains information on inorganic
crystal structures.
• The Nucleic Acid Database (http://ndbserver.rutgers.edu/) contains data
on oligonucleotides.
• The Protein Data Bank (http://www.rcsb.org/pdb/) contains biological
macromolecular structure data.
CIF principles are simple. Every data item is represented by a unique data name
followed by its associated data value. Data items are described in an electronic
dictionary that defines meaning, usage, and (where appropriate) permitted val-
ues or ranges of values. Data names start with an underscore (underline) charac-
ter, and data values can be any type of string (text, numeric, or mixed), ranging
from a single character to many lines of text. Data values are delimited by spaces,
double or single quotes, or pairs of lines beginning with a semicolon (to delimit
Chapter 13: Conventions in Chemistry ➤ 285
multiline data items). Related data items, such as those that relate to an indi-
vidual crystal structure, are grouped together in a data block. The start of a block
is designated by the string data_ prefixing the name of the block, and the end
of a block is recognized by another data_ record introducing a new block, or by
the end of the file. A complete CIF may contain any number of data blocks, each
reporting an individual crystal structure, and these may be preceded by a data
block containing items (such as author names and contact details) that are com-
mon for all structures in the complete CIF.
These basic principles, together with the data names in the CIF standard,
make the CIF human readable, as illustrated below:
data_structure_1
_cell_length_a 5.959(1)
_chemical_formula_moiety ‘C23 H36 O7’
_publ_contact_author
;
Dr J. Smith
Department of Chemistry
University of Nowhere
Nowhere
Anystate 20761
USA
;
If a data name is preceded by loop_, then a series of values can be associated
with that name, e.g., _symmetry_equiv_pos_as_xyz. A series of data values
can also be grouped together under different data names using the loop_ con-
struct, as in the example below, which shows a loop containing the atom labels
and x,y,z-coordinates for four atoms. Here, the data names can be regarded as
the column headings in a conventional printed table.
loop_
_atom_site_label
_atom_site_fract_x
_atom_site_fract_y
_atom_site_fract_z
I1 0.26639(7) 0.61557(3) 0.94292(3)
I2 0.64548(7) 0.364889(3) 0.56299(3)
P3 0.0438(2) 0.27607(11) 0.74432(13)
O1 -0.0989(7) 0.2488(3) 0.6619(4)
The crystallographic part of a CIF is generated automatically by the software
package used for structure refinement. However, the software can only output
data items that it knows about, i.e., those items that are input to or are generated
by the refinement process, or which can be generated reliably from these data.
This is seldom sufficient for journal submission, where additional data, such
as author names and contact details, and other chemical or crystal property
286 ➤ The ACS Style Guide
information are almost always required. Because these additional requirements
will vary from journal to journal, authors should consult the author guidelines
for the journal of their choice to ensure that they submit files that conform to
the publisher’s requirements.
Although the CIF can be edited using standard text editors, this is not rec-
ommended because the strict syntax, if broken in any small way, renders the
file unreadable by application software. To solve the editing issue, a number of
stand-alone CIF editors have been written. For example, the enCIFer program is
available for free download from the Cambridge Crystallographic Data Centre
Web site (http://www.ccdc.cam.ac.uk). The program will handle single-block or
multiblock CIFs and has a graphical interface that permits the following:
• location, display, and correction of syntax or format violations using the
current CIF dictionary;
• spreadsheet representation of looped data items;
• editing and/or addition of individual or looped data items;
• addition of certain standard information (basic bibliographic and/or
property information) via two data entry wizards; and
• three-dimensional visualization of the structure(s) contained in the CIF.
The IUCr Web site (http://www.iucr.org/iucr-top/cif/) lists other software
that can be used to manipulate or visualize CIF content. Most importantly, they
maintain a Web-based service that provides a classified validation report on any
format-compliant CIF. This service can be accessed at http://checkcif.iucr.org/.
CheckCIF reports can be lengthy, but it is wise to scan them carefully, particu-
larly the most serious Class A alerts. Journal editors and their referees will often
indicate the types of alerts that they would normally expect to be fixed by the
authors before publication.
287
➤ ➤ ➤ ➤ ➤ CHAPTER 14
References
Janet S. Dodd, Leah Solla,
and Paula M. Bérard
This chapter presents style conventions for citing references
within a manuscript and for listing complete reference
citations. Many of the references in the examples were created to illustrate a style
point under discussion; they may not be real references.
Citing References in Text
In ACS publications, you may cite references in text in three ways:
1. By superscript numbers, which appear outside the punctuation if the citation
applies to a whole sentence or clause.
Oscillation in the reaction of benzaldehyde with oxygen was reported previously.3
2. By italic numbers in parentheses on the line of text and inside the punctu-
ation.
The mineralization of TCE by a pure culture of a methane-oxidizing organism
has been reported (6).
3. By author name and year of publication in parentheses inside the punctua-
tion (known as author–date).
The primary structure of this enzyme has also been determined (Finnegan et al.,
2004).
In ACS books, all three of these systems are used, depending on the subject mat-
ter and series. Table 14-1 lists the referencing systems used by the ACS journals
currently in print.
Copyright 2006 American Chemical Society

288 ➤ The ACS Style Guide
Table 14-1. ACS Periodicals, with Referencing Style, CASSI Abbreviation, and
2006 Volume Number
Name as Registered in the U.S. Patent and
Trademark Office
Referencing
StyleaCASSI Abbreviation 2006 Vol.
Accounts of Chemical Research 1Acc. Chem. Res. 39
ACS Chemical Biology 2ACS Chem. Biol. 1
Analytical Chemistry 1Anal. Chem. 78
review issues 2
Biochemistry 2Biochemistry 45
Bioconjugate Chemistry 2Bioconjugate Chem. 17
Biomacromolecules 1Biomacromolecules 7
Biotechnology Progress 2Biotechnol. Prog. 22
Chemical & Engineering News Chem. Eng. News 84
Chemical Research in Toxicology 2Chem. Res. Toxicol. 19
Chemical Reviews 1Chem. Rev. 106
Chemistry of Materials 1Chem. Mater. 18
Crystal Growth & Design 1Cryst. Growth Des. 6
Energy & Fuels 1Energy Fuels 20
Environmental Science & Technology 2Environ. Sci. Technol. 40
Industrial & Engineering Chemistry Research 1Ind. Eng. Chem. Res. 45
Inorganic Chemistry 1Inorg. Chem. 45
Journal of Agricultural and Food Chemistry 2J. Agric. Food Chem. 54
Journal of the American Chemical Society 1J. Am. Chem. Soc. 128
Journal of Chemical and Engineering Data 1J. Chem. Eng. Data 51
Journal of Chemical Information and Modeling 1J. Chem. Inf. Model. 46
Journal of Chemical Theory and Computation 1J. Chem. Theory Comput. 2
Journal of Combinatorial Chemistry 1J. Comb. Chem. 8
Journal of Medicinal Chemistry 1J. Med. Chem. 49
Journal of Natural Products 1J. Nat. Prod. 69
The Journal of Organic Chemistry 1J. Org. Chem. 71
The Journal of Physical Chemistry A 1J. Phys. Chem. A 110
The Journal of Physical Chemistry B 1J. Phys. Chem. B 110
Journal of Proteome Research 1J. Proteome Res. 5
Langmuir 1Langmuir 22
Macromolecules 1Macromolecules 39
Molecular Pharmaceutics 1Mol. Pharm. 3
Nano Letters 1Nano Lett. 6
Organic Letters 1Org. Lett. 8
Organic Process Research & Development 1Org. Process Res. Dev. 10
Organometallics 1Organometallics 25
aReference style 1 uses superscript numbers, and 2 uses italic numbers in parentheses on the line of the text.
Chapter 14: References ➤ 289
➤ In all three systems, the author’s name may be made part of the sentence. In
such cases, in the author–date system, place only the year in parentheses.
The syntheses described by Fraser8 take advantage of carbohydrate topology.
Jensen (3) reported oscillation in the reaction of benzaldehyde with oxygen.
According to Harris (2003), drug release is controlled by varying the hydrolytic
stability of the ester bond.
➤ With numerical reference citations, start with 1 and number consecutively
throughout the paper, including references in text and those in tables, figures,
and other nontext components. If a reference is repeated, do not give it a new
number; use the original reference number.
➤ Whenever authors are named, if a reference has two authors, give both names
joined by the word “and”. If a reference has more than two authors, give only the
first name listed, followed by “et al.” Do not use a comma before et al.; always use
a period after al.
Allison and Perez12
Johnson et al. (12)
(O’Brien and Alenno, 2005)
(Bachrach et al., 2004)
➤ To cite more than one reference by the same principal author and various
coauthors in one of the numerical citation systems, use the principal author’s
name followed by “and co-workers” or “and colleagues”.
Pauling and co-workers10,11
Cram and colleagues (27–29)
➤ When citing more than one reference at one place by number in one of the
numerical systems, list the numbers in ascending order and separate them by
commas (without spaces as superscripts, with spaces on line), or if they are part
of a consecutive series, use an en dash to indicate a range of three or more.
in the literature2,5,8
were reported3–5,10
in the literature (2, 5, 8)
were reported (3–5, 10)
➤ When citing more than one reference at one place by the author–date sys-
tem, list them alphabetically according to the first author’s name, followed by a
comma and the year. Use a semicolon to separate individual references.
(Axelrod, 2003; Cobbs and Stolman, 2005; Gerson et al., 2001)
290 ➤ The ACS Style Guide
➤ When citing more than one reference by the same author at one place by the
author–date system, do not repeat the name. List the name followed by the year
of each of the references in ascending order; separate the years by commas. If
an author has more than one reference in the same year, add lowercase letters to
the years to differentiate them. Add letters to all of the years, for example, 2005a,
2005b, etc., not 2005, 2005a, etc. (The references in the list will need to be listed
the same way, for example, 2005a, 2005b.
(Trapani, 2003, 2005; Zillman, 2004)
(Knauth, 2005a, 2005b)
(Fordham, 2004; Fordham and Rizzo, 2004)
➤ Cite the reference in a logical place in the sentence.
recent investigations (cite)
other developments (cite)
was reported (cite)
as described previously (cite)
previous results (cite)
were demonstrated (cite)
a molecular mechanics study (cite)
Marshall and Levitt’s approach (cite)
the procedure of Lucas et al. (cite)
Style for Reference Lists
Authors are responsible for the accuracy and completeness of all references.
Authors should check all parts of each reference listing against the original
document.
A reference must include certain minimum data:
• Periodical references must include the author names, abbreviated jour-
nal title, year of publication, volume number (if any), and initial page of
cited article (the complete span is better).
• Book references must include the author or editor names, book title, pub-
lisher, city of publication, and year of publication.
• For material other than books and journals, sufficient information must
be provided so that the source can be identified and located.
In lists, references always end with a period.
Table 14-2 provides sample references for common reference types.
Chapter 14: References ➤ 291
Periodicals
recommended formats
Author 1; Author 2; Author 3; etc. Title of Article. Journal Abbreviation Year, Vol-
ume, Inclusive Pagination.
Author 1; Author 2; Author 3; etc. Journal Abbreviation Year, Volume, Inclusive
Pagination.
The journal Biochemistry is an exception. Consult this journal’s instructions to
authors for the correct format.
Author Name Field
Include all author names in a reference citation. With multiple authors, separate
the names from one another by semicolons. Always end the author field with a
period (exception: Biochemistry). List the names in inverted form: surname first,
then first initial, middle initial, and qualifiers (Jr., II). Some publications list the
first 10 authors followed by a semicolon and et al.; check the guidelines.
Cotton, F. A.
Basconi, J.; Lin, P. B.
Chandler, J. P., III; Levine, S. M.
Schafer, F. W., Jr.
Fishman, W., II.
Farhataziz. (a single name is uncommon, but does occur; no period in Bio-
chemistry)
Inderjit; Fontana, M. J. (the first author has a single name)
Article Title Field
Article titles are not essential in reference citations, but they are considered
desirable to highlight the contents of a paper and facilitate location in reference
libraries. Some ACS publications include the article title in journal references,
and some do not; check the publication itself. Article titles are set in roman type
without quotation marks and end with a period (or a question mark if that is
part of the title). In ACS journals, capitalization follows that of the original pub-
lication; in other publications, the main words are capitalized.
Caruso, R. A.; Susha, A.; Caruso, F. Multilayered Titania, Silica, and Laponite
Nanoparticle Coatings on Polystyrene Colloidal Templates and Resulting
Inorganic Hollow Spheres. Chem. Mater. 2001, 13, 400–409.
Journal Abbreviation Field
The journal name is an essential component of a periodical reference citation.
Abbreviate the name according to the Chemical Abstracts Service Source Index

292 ➤ The ACS Style Guide
Table 14-2. Common Types of References with Examples
Reference Type See Pages Example
Print Sources
Journal article
with article title 291 Klingler, J. Influence of Pretreatment on Sodium Powder. Chem.
Mater. 2005, 17, 2755–2768.
without article title 291 Klingler, J. Chem. Mater. 2005, 17, 2755–2768.
Nonscientific magazines
and newspapers
299 Squires, S. Falling Short on Nutrients. The Washington Post, Oct
4, 2005, p H1.
Books
without editors 300 Le Couteur, P.; Burreson, J. Napoleon’s Buttons: How 17 Mol-
ecules Changed History; Jeremy P. Tarcher/Putnam: New
York, 2003; pp 32–47.
with editors 300 Almlof, J.; Gropen, O. Relativistic Effects in Chemistry. In
Reviews in Computational Chemistry; Lipkowitz, K. B., Boyd,
D. B., Eds.; VCH: New York, 1996; Vol. 8, pp 206–210.
Series publication
cited as a book 306 Puls, J.; Saake, B. Industrially Isolated Hemicelluloses. In Hemi-
celluloses: Science and Technology; Gatenholm, P., Tenkanen,
M., Eds.; ACS Symposium Series 864; American Chemical
Society: Washington, DC, 2004; pp 24–37.
cited as a journal 306 Puls, J.; Saake, B. ACS Symp. Ser. 2004, 864, 24–37.
Meeting or conference,
full citation
307–308 Garrone, E.; Ugliengo, P. In Structure and Reactivity of Surfaces,
Proceedings of the European Conference, Trieste, Italy,
Sept 13–20, 1988; Zecchina, A., Cost, G., Morterra, C., Eds.;
Elsevier: Amsterdam, 1988.
Theses 309–310 Mäckel, H. Capturing the Spectra of Silicon Solar Cells. Ph.D.
Thesis, The Australian National University, December 2004.
Patents 310–311 Sheem, S. K. Low-Cost Fiber Optic Pressure Sensor. U.S. Patent
6,738,537, May 18, 2004.
Government
publications, U.S.
311–314 Agriculture Fact Book 2000; U.S. Department of Agriculture, U.S.
Government Printing Office: Washington, DC, 2000.
Technical reports and
bulletins
314 Tschantz, B. A.; Moran, B. M. Modeling of the Hydrologic Trans-
port of Mercury in the Upper East Fork Poplar Creek (UEFPC)
Watershed; Technical Report for Lockheed Martin Energy
Systems: Bethesda, MD, September 2004.
Material Safety Data
Sheets
315 Titanium Dioxide; MSDS No. T3627; Mallinckrodt Baker: Phil-
lipsburg, NJ, Nov 12, 2003.
Personal
communications
315–316 Henscher, L. X. University of Minnesota, Minneapolis, MN. Per-
sonal communication, 2001.
Online Periodicals
Based on print editions 318 Fine, L. Einstein Revisited. J. Chem. Educ. [Online] 2005,
82, 1601 ff. http://jchemed.chem.wisc.edu/Journal/
Issues/2005/Nov/abs1601.html (accessed Oct 15, 2005).
Published in advance of
print issue
318–319 Pratt, D. A.; van der Donk, W. A. Theoretical Investigations into the
Intermediacy of Chlorinated Vinylcobalamins in the Reduc-
tive Dehalogenation of Chlorinated Ethylenes. J. Am. Chem.
Soc. [Online early access]. DOI: 10.1021/ja047915o. Published
Online: Dec 8, 2004. http://pubs.acs.org/cgi-bin/asap.cgi/
jacsat/asap/html/ja047915o.html (accessed Dec 8, 2004).
Continued on next page

Chapter 14: References ➤ 293
Table 14-2. Common Types of References with Examples—Continued
Reference Type See Pages Example
Retrieved from a
database provider
318 Hallet, V. Scanning the Globe for Organic Chemistry. U.S.
News and World Report [Online], April 19, 2004, p 59. Busi-
ness Source Premier. http://www.epnet.com/academic/
bussourceprem.asp (accessed April 24, 2005).
Published only
electronically
319 Zloh, M.; Esposito, D.; Gibbons, W. A. Helical Net Plots and Lipid
Favourable Surface Mapping of Transmembrane Helices
of Integral Membrane Proteins: Aids to Structure Deter-
mination of Integral Membrane Proteins. Internet J. Chem.
[Online] 2003, 6, Article 2. http://www.ijc.com/articles/
2003v6/2/ (accessed Oct 13, 2004).
From preprint servers 319 Ward, D. W.; Nelson, K. A. Finite Difference Time Domain (FDTD)
Simulations of Electromagnetic Wave Propagation Using a
Spreadsheet. 2004, arXiv:physics/0402096. arXiv.org e-Print
archive. http://arxiv.org/abs/physics/0402096 (accessed
Oct 13, 2004).
Online books
without editors 319–320 Tour, J. M. Molecular Electronics: Commercial Insights, Chemis-
try, Devices, Architecture and Programming [Online]; World
Scientific: River Edge, NJ, 2003; pp 177–180. http://legacy.
netlibrary.com/ebook_info.asp?product_id=91422&piclist
=19799,20141,20153 (accessed Nov 7, 2004).
with editors 320 Oleksyn, B. J.; Stadnicka, K.; Sliwinski, J. Structural Chemistry
of Enamines: A Statistical Approach. In The Chemistry
of Enamines [Online]; Rappoport, Z., Ed.; The Chem-
istry of Functional Groups; Patai, S., Rappoport, Z.,
Series Eds.; Wiley & Sons: New York, 1994; Chapter 2, pp
87–218. http://www3.interscience.wiley.com/cgi-bin/
summary/109560980/SUMMARY (accessed April 24, 2005).
Online encyclopedias 320 Alkanolamines from Nitro Alcohols. Kirk-Othmer Encyclopedia
of Chemical Technology [Online]; Wiley & Sons, Posted March
14, 2003. http://www.mrw.interscience.wiley.com/kirk/
articles/alkaboll.a01/frame.html (accessed Nov 7, 2004).
Other Online Sources
General Web sites 320–321 ACS Publications Division Home Page. http://pubs.acs.org
(accessed Nov 7, 2004).
Electronic lists and
newsgroups
322 Chemical Information List Server, CHMINF-L@iubvm.ucs.
indiana.edu (accessed Oct 13, 2004).
Computational Chemistry List, solvent discussion in archived
messages of September 2003, chemistry@ccl.net (accessed
Nov 10, 2004).
Electronic mail
messages
322 Solla, L. R. Cornell University, Ithaca, NY. Personal communica-
tion, 2005.
CD-ROMs and DVDs
periodicals 322 Fleming, S. A.; Jensen, A. W. Substitutent Effects on the Pho-
tocleavage of Benzyl–Sulfur Bonds. Observation of the
“Meta” Effect. J. Org. Chem. [CD-ROM] 1996, 61, 7044.
books 322–323 Green Chemistry: Meeting Global Challenges [DVD]; American
Chemical Society: Washington, DC, 2003.
294 ➤ The ACS Style Guide
(CASSI), and italicize it. One-word journal names are not abbreviated (e.g., Bio-
chemistry, Macromolecules, Nature, Science). No punctuation is added to end this
field; thus, a period will be there with an abbreviation but not with a spelled-out
word.
CASSI and its quarterly supplements provide an extensive list of recom-
mended journal abbreviations. Appendix 14-1 lists CASSI abbreviations for
more than 1000 of the most commonly cited journals. ACS publication names,
their abbreviations, and their volume numbers for 2006 are given in Table 14-1.
Note that, in some cases, the word “the” is part of the title.
Sometimes journal names change. Authors should use the abbreviation of
the journal title that was in use at the time the article was published. CASSI lists
the journal titles and the range of years during which the title was being used.
Information Found in CASSI
Entries are arranged in CASSI alphabetically according to the abbreviated form
of the title. Abbreviations are based on the standards of the International Orga-
nization for Standardization (ISO). Recommended abbreviations are indicated
in boldface type. See Appendix 14-2 for a sample CASSI entry with a description
of each element in an entry.
Using CASSI Abbreviations
➤ The boldface components of the publication title form the abbreviated title.
Use a period after each abbreviation, and maintain the punctuation shown in
CASSI.
Journal of Polymer Science, Part A: Polymer Science
J. Polym. Sci., Part A: Polym. Sci.
➤ Maintain the word spacing shown in CASSI, except for D.C., N.Y., U.K., U.S.,
and U.S.A.
Analyst (Cambridge, U.K.)
Anesth. Analg. (Hagerstown, MD, U.S.)
Ann. N.Y. Acad. Sci.
Proc. Natl. Acad. Sci. U.S.A.
Science (Washington, DC, U.S.)
➤ Use a terminal period only if the last word of the periodical title is abbreviated.
International Journal of Nanoscience
Int. J. Nanosci. (last word is abbreviated; period is used)
Journal of Controlled Release
J. Controlled Release (last word is not abbreviated; no period is used)
Chapter 14: References ➤ 295
➤ If the periodical abbreviation in CASSI shows a hyphen with spaces on both
sides, change the hyphen to an em dash closed up on each side.
Annual Technical Conference - Society of Plastics Engineers
Annu. Tech. Conf.—Soc. Plast. Eng.
➤ If a boldface n precedes the volume number in CASSI, use the abbreviation
“No.” before the volume number in italics in the entry.
British Medical Journal … n6372 1983
Br. Med. J. 1983, No. 6372.
Include all the information shown for volume in italics, especially for references
to government publications and reports.
Los Alamos National Laboratory, [Report] LA (United States) … LA-14240-SR 2005
Los Alamos Natl. Lab., [Rep.] LA (U.S.) 2005, LA-14240-SR.
Exceptions to the Rules of CASSI Abbreviations
➤ Strict rules for CASSI abbreviations can be modified for periodicals whose
titles include multiple parts, sections, and series.
abbreviation
Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 2005, 61, 99–102.
acceptable variation The section title need not be named:
Acta Crystallogr., Sect. C 2005, 61, 99–102.
acceptable variation The section can be indicated by the volume
number:
Acta Crystallogr. 2005, C61, 99–102.
➤ For some periodicals whose CASSI abbreviation includes a place of pub-
lication, you need not add the place of publication unless its omission would
create ambiguity. If CASSI lists only one journal with a given main title, there is
no ambiguity in omitting the place of publication.
use not necessarily
Clin. Chem. Clin. Chem. (Washington, DC, U.S.)
Nature Nature (London, U.K.)
Science Science (Washington, DC, U.S.)
In contrast, omission of the place of publication would create ambiguity for dif-
ferent journals having the same main title.
Transition Met. Chem. (Dordrecht, Neth.)
Transition Met. Chem. (N.Y.)
296 ➤ The ACS Style Guide
Year of Publication Field
The year of publication is essential information in a periodical citation. The year
is set in boldface type, followed by a comma in boldface type.
Publication Volume Field
The volume number is important information and is recommended for all peri-
odical citations; it is essential for publications having more than one volume per
year (such as the Journal of Chemical Physics). The volume number is set in italic
type and is separated from the pagination information by a comma, which is set
in italic type.
➤ For periodicals in which each issue begins with page 1, include issue infor-
mation (either the number or the date) in the publication volume field. Issue
information is set in roman type, enclosed in parentheses, and spaced from the
volume number, which it directly follows.
issue number
Mullin, R. Chem. Eng. News 2005, 83 (42), 7.
date of issue
Mullin, R. Chem. Eng. News 2005, 83 (Oct 17), 7.
➤ For publications that have supplements, the following form is recom-
mended.
Taylor, C. W.; Kumar, S. Eur. J. Cancer 2005, 40 (Suppl. 1), 781.
Eur. J. Anaesthesiol. 2005, 22 (Suppl. S36), 1–35.
➤ For journals that have no volume numbers, include issue numbers, especially
when the pagination of each issue begins with page 1. Use the following form.
Note that the issue number is not italicized.
Wills, M. R.; Savory, J. Lancet 1983, No. 2, 29.
Pagination Field
Pagination is an essential element of a reference citation. The complete page
range is preferable, but initial page numbers are acceptable.
➤ In page spans, use all digits, closed up, with no commas or spaces.
2–15
44–49
103–107
1376–1382
2022–2134
11771–11779
Chapter 14: References ➤ 297
➤ You may also indicate pagination in reference citations by “f” or “ff”, which
mean “and following” page or pages, respectively. The f or ff is set in roman type
and is spaced from the preceding number:
60 f (indicates page 60 and the page following—pages 60 and 61)
60 ff (indicates page 60 and pages following)
58–60 ff (indicates pages 58 through 60 and pages following—essentially the
same as 58 ff except that the three pages enumerated contain the most pertinent
information and other relevant information is scattered on the rest of the pages)
➤ The pagination field may also include terms such as “and references therein”
and similar expressions (especially in references to review articles). This phrase
follows the page numbers and is not separated by a comma.
Puskas, J. E.; Chan, S. W. P.; McAuley, K. B.; Shaikh, S.; Kaszas, G. J. Polym. Sci.,
Part A: Polym. Chem. 2005, 43, 5394–5413 and references therein.
➤ Some publications use article numbering, rather than page numbering, where
each article starts on page 1. Use the article number in the pagination field.
Brosset, C. Ark. Kemi, Mineral. Geol. 1945, 20A, No. 7.
Use of Punctuation To Indicate Repeating Fields of Information
The choice of what punctuation to use to indicate repeating fields of informa-
tion depends on whether the publication will appear strictly in print or on the
Web. For publications that will appear in both print and on the Web, use the
rules for Web publications.
➤ In references that will appear only in print publications, use a semicolon, a
comma, or a period to indicate repeating information.
1. Same authors in multiple publications:
Chauvin, Y.; Gilbert, B.; Guibard, I. Vib. Spectrosc. 1991, 1, 299–304; J. Chem. Soc.,
Chem. Commun. 1990, 1715–1716.
2. Same authors in multiple publications, but with letters to separate the refer-
ences (the semicolon from the previous example is changed to a period):
(a) Schrock, R. R. Chem. Commun. 2003, 2389. (b) J. Mol. Catal. A 2004, 213,
21–30.
3. Same authors of multiple articles in the same journal:
Lu, Y.; Pignatello, J. J. Environ. Sci. Technol. 2002, 36, 4553–4561; 2004, 38, 5853–
5862.
When the year and volume are the same:
Clay, S. A.; Koskinen, W. C. Weed Sci. 1990, 38, 74–80, 262–266.
298 ➤ The ACS Style Guide
When the year is the same but the volumes are different:
Badyal, R.; Fleissner, A. Chem. Phys. 2005, 317, 73–86; 2005, 316, 201–215.
➤ In references that will appear only in Web publications, provide complete
references so that the references can be properly linked. If two or more refer-
ences with the same authors are cited, it is not acceptable to combine them into a
single reference.
1. Same authors in multiple publications:
Chauvin, Y.; Gilbert, B.; Guibard, I. Vib. Spectrosc. 1991, 1, 299–304; Chauvin, Y.;
Gilbert, B.; Guibard, I. J. Chem. Soc., Chem. Commun. 1990, 1715–1716.
2. Same authors in multiple publications, but with letters to separate the refer-
ences:
(a) Schrock, R. R. Chem. Commun. 2003, 2389. (b) Schrock, R. R. J. Mol. Catal. A
2004, 213, 21–30.
3. Same authors of multiple articles in the same journal:
Lu, Y.; Pignatello, J. J. Environ. Sci. Technol. 2002, 36, 4553–4561; Lu, Y.; Pignatello,
J. J. Environ. Sci. Technol. 2004, 38, 5853–5862.
The same principle holds no matter what information is being repeated: provide
each reference in its entirety. Do not use the Latin terms ibid. (in the same place)
or idem (the same).
References to Chemical Abstracts
Use a semicolon to separate the periodical citation from a reference to its abstract
(Chemical Abstracts).
Mohamed, A. M.; Hawata, A.; El-Torgoman, M.; El-Kousy, S. M.; Ismail, A. E.;
Øgaard Madsen, J.; Søtofte, I.; Senning, A. Eur. J. Org. Chem. 2002, 2039–
2045; Chem. Abstr. 2003, 138, 4195.
Mloston, G.; Majchrazak, A.; Senning, A.; Søtofte, I. J. Org. Chem. 2002, 67,
5690–5695; Chem. Abstr. 2002, 137, 201289.
Chemical Abstracts routinely contains more than one abstract per page. The
method of distinguishing which abstract was being cited has changed over the
years. Three variations are worth noting.
1. The column (two columns per page) in which the abstract occurs followed
by a superscript number:
Chem. Abstr. 1946, 40, 44638. (This is the eighth abstract in column 4463.)
2. The column (two columns per page) in which the abstract occurs followed
by a letter, either on the line or superscript (generally italic):
Chapter 14: References ➤ 299
Chem. Abstr. 1953, 47, 1167f. (This is abstract f in column 1167.)
Chem. Abstr. 1947, 41, 571d. (This is abstract d in column 571.)
3. The abstract number itself followed by an online letter (roman), often a com-
puter check character:
Chem. Abstr. 1989, 110, 8215j. (This is abstract number 8215.)
Special Situations
➤ You may treat Beilstein references as periodical references.
Beilstein, 4th ed. 1950, 12, 237.
➤ Cite journals published in a foreign language either by the actual non-Eng-
lish title or by a translated form.
Nippon Ishikai Zasshi or J. Jpn. Med. Assoc.
Nouv. J. Chim. or New J. Chem.
➤ When citing an article printed in the English translation of a foreign-lan-
guage journal, include reference to the original article, if possible, and use a
semicolon to separate the two citations.
Tarasov, Y. I.; Kochikov, I. V.; Kovtun, D. M.; Vogt, N.; Novosadov, B. K.; Saakyan,
A. S. J. Struct. Chem. (Engl. Transl.) 2004, 45 (5), 778–785; Zh. Strukt. Khim.
2004, 45 (5), 822–829.
➤ Separate two or more companion publications with a semicolon.
Clear, J. M.; Kelly, J. M.; O’Connell, C. M.; Vos, J. G. J. Chem. Res., Miniprint 2005,
3038; J. Chem. Res., Synop. 2005, 260.
Nonscientific Magazines and Newspapers
recommended format
Author 1; Author 2; Author 3; etc. Title of Article. Title of Periodical, Complete
Date, Pagination.
For nonscientific magazines and other periodicals that are not abstracted by
Chemical Abstracts Service, give the authors’ names in inverted form ending
with a period, the article title in roman type with main words capitalized and
ending with a period, the full magazine title in italic type followed by a comma
in italic type, the complete date of the issue (see pp 160–161 about dates) ending
with a comma, and the pagination.
Squires, S. Falling Short on Nutrients. The Washington Post, Oct 4, 2005, p H1.
300 ➤ The ACS Style Guide
Books
Some ACS publications include the chapter title in book references, and some do
not; check the publication itself. Also, consult the instructions to authors in Bio-
chemistry for exceptions to the format presented here and elsewhere in this chapter.
recommended formats for books without editors
Author 1; Author 2; Author 3; etc. Chapter Title. Book Title, Edition Number;
Series Information (if any); Publisher: Place of Publication, Year; Volume
Number, Pagination.
Author 1; Author 2; Author 3; etc. Book Title; Series Information (if any); Pub-
lisher: Place of Publication, Year; Volume Number, Pagination.
When a book has authors and no editors, it means either that the entire book
was written by one author or that two or more authors collaborated on the entire
book.
Le Couteur, P.; Burreson, J. Napoleon’s Buttons: How 17 Molecules Changed His-
tory; Jeremy P. Tarcher/Putnam: New York, 2003; pp 32–47.
Morris, R. The Last Sorcerers: The Path from Alchemy to the Periodic Table; Joseph
Henry Press: Washington, DC, 2003; pp 145–158.
recommended formats for books with editors
Author 1; Author 2; Author 3; etc. Chapter Title. In Book Title, Edition Number;
Editor 1, Editor 2, etc., Eds.; Series Information (if any); Publisher: Place of
Publication, Year; Volume Number, Pagination.
Author 1; Author 2; Author 3; etc. In Book Title, Edition Number; Editor 1, Editor
2, etc., Eds.; Series Information (if any); Publisher: Place of Publication, Year;
Volume Number, Pagination.
When a book has editors, it means that different authors wrote various parts of the
book independently of each other. The word “In” before the book title indicates
that the authors mentioned wrote only a part of the book, not the entire book.
Holbrey, J. D.; Chen, J.; Turner, M. B.; Swatloski, R. P.; Spear, S. K.; Rogers, R.
D. Applying Ionic Liquids for Controlled Processing of Polymer Materials.
In Ionic Liquids in Polymer Systems: Solvents, Additives, and Novel Applica-
tions; Brazel, C. S., Rogers, R. D., Eds.; ACS Symposium Series 913; American
Chemical Society: Washington, DC, 2005; pp 71–88.
Almlof, J.; Gropen, O. Relativistic Effects in Chemistry. In Reviews in Compu-
tational Chemistry; Lipkowitz, K. B., Boyd, D. B., Eds.; VCH: New York, 1996;
Vol. 8, pp 206–210.
If the book as a whole is being referenced, the author names might not appear.
Ionic Liquids in Polymer Systems: Solvents, Additives, and Novel Applications; Bra-
zel, C. S., Rogers, R. D., Eds.; ACS Symposium Series 913; American Chemical
Society: Washington, DC, 2005.
Chapter 14: References ➤ 301
Advances in Inorganic Chemistry and Radiochemistry; Emeléus, H. J., Sharpe, A.
G., Eds.; Academic: New York, 2001.
Author Name Field
➤ Separate the names of multiple authors by semicolons, and always end the
author field with a period (except in Biochemistry). List names in inverted form:
surname first, then first initial, middle initial, and qualifiers (Jr., II).
➤ If a book has no primary authors because each chapter was written by a dif-
ferent author, you may place the editor names in the author name field (espe-
cially for lists in alphabetical order). Separate editor names by commas, and in
this case, the period after the abbreviation Ed. or Eds. terminates the field.
Stocker, J. H., Ed. Chemistry and Science Fiction; American Chemical Society:
Washington, DC, 1998.
➤ A book might have no named authors because it was compiled by a com-
mittee or organization. These books are discussed under the section “Works
Written by an Organization or a Committee”, p 307.
Chapter Title Field
Chapter titles are not essential, but they are considered desirable components in
reference citations because they highlight the contents of a paper and facilitate
its location in reference libraries. Chapter titles are set in roman type and end
with a period.
Puls, J.; Saake, B. Industrially Isolated Hemicelluloses. In Hemicelluloses: Science
and Technology; Gatenholm, P., Tenkanen, M., Eds.; ACS Symposium Series
864; American Chemical Society: Washington, DC, 2004; pp 24–37.
Book Title Field
Book titles are essential elements in book reference citations. In general, book
titles should not be abbreviated. They are set in italic type and are separated from
the next field of the reference by a semicolon, which is set in italic type.
➤ The edition number (in ordinal form) and the abbreviation “ed.” follow the
book title, set off by an italic comma; they are set in roman type. The edition
information is separated from the next field of the reference by a semicolon.
Reagent Chemicals, 10th ed.;
➤ When both authors and editors are given, use the word “In” (set in roman
type) immediately before the title of the book to indicate that the cited authors
wrote only part of the book.
302 ➤ The ACS Style Guide
Hillman, L. W. In Dye Laser Principles with Applications; Duarte, F. J., Hillman, L.
W., Eds.; Academic: New York, 1990; Chapter 2.
Editor Name Field
For books with editors, list the names of the editors, after title and edition infor-
mation, in inverted form as described in the section “Author Name Field”, sepa-
rated from one another by commas. The names are denoted as editors by includ-
ing the abbreviation “Eds.” or “Ed.” after the final name. The editor field is set
in roman type and ends with a semicolon (unless it is used in the author field
location).
Lignocellulose Biodegradation; Saha, B. C., Hayashi, K., Eds.; ACS Symposium
Series 889; American Chemical Society: Washington, DC, 2004.
The Chemistry of the Atmosphere: Oxidants and Oxidation in the Earth’s Atmo-
sphere; Bandy, A. R., Ed.; Royal Society of Chemistry: Cambridge, U.K., 1995.
In books that have no primary authors, the names of the editors may appear in
either the author name field (especially for lists in alphabetical order) or the edi-
tor name field. When the editor names appear in the author name field, they are
separated by commas and the field ends with a period.
Saha, B. C., Hayashi, K., Eds.; Lignocellulose Biodegradation; ACS Symposium
Series 889; American Chemical Society: Washington, DC, 2004.
Bandy, A. R., Ed. The Chemistry of the Atmosphere: Oxidants and Oxidation in the
Earth’s Atmosphere; Royal Society of Chemistry: Cambridge, U.K., 1995.
Publication Information Field
The name of the publisher, place of publication, and year of publication are
essential elements in a book reference.
Name of Publisher
Check the title page, front and back, for the publisher’s name and location.
Names and addresses of publishers are also listed in Chemical Abstracts Service
Source Index, 1907–2004 Cumulative, pp 21I–39I.
➤ Generally, do not abbreviate publishers’ names.
American Chemical Society, not Am. Chem. Soc. or ACS
American Ceramic Society, not Am. Ceram. Soc.
exception You may use well-known acronyms or abbreviations created by
the publishers themselves.
AIChE or American Institute of Chemical Engineers
ASTM or American Society for Testing and Materials
IUPAC or International Union of Pure and Applied Chemistry
Chapter 14: References ➤ 303
➤ In some publisher’s names, words such as Co., Inc., Publisher, and Press are
not essential.
Academic Press: New York or Academic: New York
➤ Expanded names are also not essential.
John Wiley & Sons or John Wiley or Wiley
➤ It is not necessary to repeat the publisher’s name for a book compiled by the
organization that published it.
CRC Handbook of Chemistry and Physics, 85th ed.; Boca Raton, FL, 2004.
Place of Publication
For the place of publication, give the city and state for U.S. cities or the city and
country for all others. The country or state is not needed if the city is considered
a major city in the world and could not be confused easily with other cities of
the same name (e.g., London, Paris, New York, and Rome). Use the two-letter
postal abbreviations (listed in Chapter 10) for states. Spell out names of coun-
tries unless they have standard abbreviations, such as U.K. for United Kingdom.
Birmingham, U.K.
Boca Raton, FL
Cambridge, MA
Cambridge, U.K.
Chichester, U.K.
Dordrecht, Netherlands
Elmsford, NY
Englewood Cliffs, NJ
London
New York
Princeton, NJ
Springfield, IL
Springfield, MA
Washington, DC
Year of Publication
In book references, the year is set in lightface (not bold) roman type, following
the place of publication. Terminate the field with a period or with a semicolon if
further information is given.
Gould, S. J. The Structure of Evolutionary Theory; Belknap Press: Cambridge, MA,
2002.
Kline, R. B. Principles and Practice of Structural Equation Modeling, 2nd ed.; Guil-
ford Press: New York, 2004.
Volume and Pagination Field
Volume Information
➤ The volume field contains specific information, such as volume number and
chapter number. Use the following abbreviations and spelled-out forms with the
capitalization, spelling, and punctuation shown:
Abstract
Chapter
304 ➤ The ACS Style Guide
No.
Paper
Part
Vol. (for specific volumes, Vol. 4; Vols. 1, 2; Vols. 1 and 2; Vols. 3–5)
vols. (for a number of volumes, 4 vols.)
Annual Review of Physical Chemistry; Leone, S. R., McDermott, A. E., Paul, A.,
Eds.; Annual Reviews: Palo Alto, CA, 2005; Vol. 56.
➤ If a volume or part number refers to the volume or part of an entire series of
books, this information is placed where a series number would normally appear
and not in the volume field for the specific book being cited.
Wiberg, K. In Investigations of Rates and Mechanisms of Reactions; Lewis, E. S.,
Ed.; Techniques of Chemistry, Vol. VI, Part I; Wiley & Sons: New York, 1974;
p 764.
➤ If the book or set of books as a whole is the reference, do not include indi-
vidual volume information.
McGraw-Hill Encyclopedia of Science and Technology, 9th ed.; McGraw-Hill: New
York, 2002; 20 vols.
Pagination Information
➤ If you are citing a chapter, the complete page range is best, but initial page
numbers are acceptable. Pagination may also be indicated by “f” or “ff” notation
(meaning “and following” page or pages, respectively). The f or ff is set in roman
type and is spaced from the preceding number. These points are illustrated under
the “Pagination Field” heading for periodicals.
➤ Pagination information is set in roman type and ends with a period, except
when miscellaneous information follows it, in which case it should end with a
semicolon (see the next section). Use the abbreviations “p” and “pp” to indicate
single and multiple pages, respectively.
p 57
p 93 f
pp 48–51
pp 30, 52, 76
pp 30, 52, 76 ff
pp 30, 52, and 76
pp 562–569
pp 562–9 (acceptable in journals)
2005; Vol. 2, p 35.
2004; pp 55–61.
➤ If the book as a whole is the reference, page numbers need not be given.
Chapter 14: References ➤ 305
Miscellaneous Information
If you wish to include additional information about a book that is important for
the reader to know, you may add it at the end of the reference with or without
parentheses, append it to the title in parentheses before the semicolon, or place it
between the title and the publisher.
AOCS. Official Methods and Recommended Practices of the American Oil Chemists’
Society; Link, W. E., Ed.; Champaign, IL, 1958 (revised 1973).
Brown, H. C. The Nonclassical Ion Problem; Plenum: New York, 1977; Chapter 5
(with comments by P. v. R. Schleyer).
Otsu, T.; Kinoshita, M. Experimental Methods of Polymer Synthesis (in Japanese);
Kagakudojin: Kyoto, Japan, 1972; p 72.
Tessier, J. Structure, Synthesis and Physical–Chemical Properties of Deltamethrin.
In Deltamethrin Monograph; Tessier, J., Ed.; Roussel-Uclaf: Paris, 1982; pp
37–66; translated by B. V. d. G. Walden.
Tessier, J. Structure, Synthesis and Physical–Chemical Properties of Deltamethrin.
In Deltamethrin Monograph; Tessier, J., Ed.; Walden, B. V. d. G., Translator;
Roussel-Uclaf: Paris, 1982.
Volatile Compounds in Foods and Beverages; Maarse, H., Ed.; Marcel Dekker: New
York, 1991; see also references therein.
Special Situations
➤ Organic Syntheses collective volumes should be treated as books.
Organic Syntheses; Wiley & Sons: New York, Year; Collect. Vol. No., Pagination.
year collective volume no.
1941 I
1943 II
1955 III
1963 IV
1973 V
1988 VI
1990 VII
1993 VIII
1998 IX
2004 X
Organic Syntheses, Cumulative Indices for Collective Volumes I–VIII was published
in 1995. Beginning with Volume 82, each volume of Organic Syntheses is planned
to be published online on orgsyn.org in installments about every three months,
with printed volumes appearing annually.
➤ For references to the Kirk-Othmer Encyclopedia, include the article title fol-
lowed by a period, similar to the citation of a chapter title.
306 ➤ The ACS Style Guide
Chloramines and Bromamines. Kirk-Othmer Encyclopedia of Chemical Technol-
ogy, 4th ed.; Wiley & Sons: New York, 1993; Vol. 4, pp 931–932.
Series Publications
Publications such as book series that are periodical in nature but are not journals
may be styled as either books or journals. CASSI lists every document abstracted
and indexed by the Chemical Abstracts Service; hence, book titles are included
and abbreviated. Key words to look for with these types of publications include
“Advances”, “Methods”, “Progress”, and “Series”.
recommended format for citation as a book
Author 1; Author 2; Author 3; etc. In Title; Editor 1, Editor 2, Eds.; Series Title
and Number; Publisher: Place of Publication, Year; Pagination.
➤ In book format, use the regular citation format for a book reference, but
include information pertaining to the series. The series title is spelled out and set
in roman type.
Kebarle, P. In Techniques for the Study of Ion–Molecule Reactions; Saunders, W.,
Farrar, J. M., Eds.; Techniques of Chemistry Series 20; Wiley & Sons: New
York, 1988; p 125.
Lignocellulose Biodegradation; Saha, B. C., Hayashi, K., Eds.; ACS Symposium
Series 889; American Chemical Society: Washington, DC, 2004.
➤ If a volume or part number is given for a series of books instead of a series
number, cite this information where a series number would normally appear.
Wiberg, K. In Investigations of Rates and Mechanisms of Reactions; Lewis, E. S., Ed.;
Techniques of Chemistry, Vol. VI, Part I; Wiley & Sons: New York, 1974; p 764.
➤ As for any book, you may cite specific chapters.
Puls, J.; Saake, B. Industrially Isolated Hemicelluloses. In Hemicelluloses: Science
and Technology; Gatenholm, P., Tenkanen, M., Eds.; ACS Symposium Series
864; American Chemical Society: Washington, DC, 2004; pp 24–37.
➤ In journal format, the series title is used as a journal title, abbreviated accord-
ing to CASSI and italicized, and the series number is used as a journal volume
number.
recommended format for citation as a journal
Author 1; Author 2; Author 3; etc. Abbreviation Year, Volume, Pagination.
Puls, J.; Saake, B. ACS Symp. Ser., 2004, 864, 24–37.
Kebarle, P. Tech. Chem. (N.Y.) 1988, 20, 125.
Chapter 14: References ➤ 307
Works Written by an Organization or a Committee
An organization or a committee may be the author of a book or periodical arti-
cle. Acronyms for very well known organizations may be used. It is not neces-
sary to repeat the publisher’s name for a work compiled by the organization that
published it.
book format
American Chemical Society, Committee on Analytical Reagents. Reagent Chemi-
cals: Specifications and Procedures, 10th ed.; Washington, DC, 2006.
World Health Organization. Pathology and Genetics of Tumours of the Head and
Neck; Albany, NY, 2002; Vol. 9.
periodical format
International Union of Pure and Applied Chemistry, Physical Chemistry Divi-
sion, Commission on Molecular Structure and Spectroscopy. Presentation
of Molecular Parameter Values for Infrared and Raman Intensity Measure-
ments. Pure Appl. Chem. 1988, 60, 1385–1388.
IUPAC. Molecular Absorption Spectroscopy, Ultraviolet and Visible (UV/VIS).
Pure Appl. Chem. 1988, 60, 1449–1460.
Meetings and Conferences
References to work presented at conferences and meetings must be treated on a
case-by-case basis. At least three types of citations are possible:
1. Full citations of published abstracts and proceedings. The format resembles
that of a book citation.
2. CASSI citations of published abstracts and proceedings. The format is that of
a periodical citation.
3. References to oral presentations, posters, or demonstrations at technical
meetings, possibly accompanied by handouts or brochures. These references
contain no publication information.
Full Citations
recommended format
Author 1; Author 2; Author 3; etc. Title of Presentation. In Title of the Collected
Work, Proceedings of the Name of the Meeting, Location of Meeting, Date of
Meeting; Editor 1, Editor 2, etc., Eds.; Publisher: Place of Publication, Year;
Abstract Number, Pagination.
The format resembles that of a book citation. The title field, however, includes
additional information on the meeting title, location, and dates. The actual title
308 ➤ The ACS Style Guide
of the book (collected work) is set in italic type and is separated from the meet-
ing information by a comma. The information on meeting location is set in
roman type, but it is not repeated if it is included in the book title. The entire
field ends with a semicolon.
Garrone, E.; Ugliengo, P. In Structure and Reactivity of Surfaces, Proceedings of
the European Conference, Trieste, Italy, Sept 13–20, 1988; Zecchina, A., Cost,
G., Morterra, C., Eds.; Elsevier: Amsterdam, 1988.
Abstracts are slightly different in that they usually do not have editors. The word
“in” is not used before the book title.
Prasad, A.; Jackson, P. Abstracts of Papers, Part 2, 212th National Meeting of the
American Chemical Society, Orlando, FL, Aug 25–29, 1996; American Chem-
ical Society: Washington, DC, 1996; PMSE 189.
When the phrase “Proceedings of” is part of the reference, include the pub-
lisher and place of publication. When a society sponsors a meeting, the society
is assumed to be the publisher. If the place of the meeting and the place of publi-
cation are the same, additional publisher and place information is not required.
However, many organizations such as the ACS sponsor meetings in various cities.
Harwood, J. S. Direct Detection of Volatile Metabolites Produced by Micro-
organisms. Proceedings of the 36th ASMS Conference on Mass Spectrometry
and Allied Topics, San Francisco, CA, June 5–10, 1988.
CASSI Citations
Proceedings and abstracts of meetings and conferences are indexed in CASSI.
The reference format follows that for periodicals.
Abstr. Pap.—Am. Chem. Soc. 1989, 198.
CASSI gives the number of a meeting in ordinal form. Convert this number to
an italic cardinal number, and use it as the volume number in the citation, unless
CASSI has already indicated another volume number.
Journal format can be used for references to preprint papers.
Jones, J.; Oferdahl, K. Natl. Meet.—Am. Chem. Soc., Div. Environ. Chem. 1989, 29
(2), ENVR 22 (or Paper 22).
Material That Has No Publication Information
recommended formats
Author 1; Author 2; Author 3; etc. Title of Presentation (if any). Presented at
Conference Title, Place, Date; Paper Number.
List the data concerning the conference (name, place, and date) separated by
commas and followed by a semicolon and the paper number (if any). The entire
citation is set in roman type.
Chapter 14: References ➤ 309
Zientek, K. D.; Eyler, J. R. Presented at the 51st ASMS Conference on Mass Spec-
trometry and Allied Topics, Montreal, Canada, June 8–12, 2003.
Dizman, B.; Elasri, M. O.; Mathias, L. J. Presented at the 227th National Meeting
of the American Chemical Society, Anaheim, CA, March 28–April 1, 2004;
Paper POLY 229.
Theses
recommended formats
Author. Title of Thesis. Level of Thesis, Degree-Granting University, Location of
University, Date of Completion.
References to theses should be as specific as practical, including, at a minimum,
the degree-granting institution and date.
Chandrakanth, J. S. Effects of Ozone on the Colloidal Stability of Particles Coated
with Natural Organic Matter. Ph.D. Dissertation, University of Colorado,
Boulder, CO, 1994.
Mäckel, H. Capturing the Spectra of Silicon Solar Cells. Ph.D. Thesis, The Austra-
lian National University, December 2004.
Kulamer, T. M.S. Thesis, Princeton University, 2004.
Author Name Field
Cite the name in inverted form: surname first, then first initial, middle initial,
and qualifiers (Jr., II). End the field with a period.
Title Field
Thesis titles are not essential, but they are informative. They are set in roman
type and end with a period.
Erickson, T. A. Development and Application of Geostatistical Methods to Mod-
eling Spatial Variation in Snowpack Properties, Front Range, Colorado. Ph.D.
Dissertation, University of Colorado, Boulder, CO, 2004.
Moore, S. Synthesis and Pharmacology of Potential Site-Directed Therapeutic
Agents for Cocaine Abuse. Ph.D. Thesis, Georgia Institute of Technology,
Atlanta, GA, 2004.
Thesis Level Field
Work done at a master’s level is often called a thesis. Work toward the Ph.D.
(doctor of philosophy) may be called a thesis or a dissertation, depending on
the policy of the degree-granting institution. The following abbreviations are
standard for U.S. degrees. Many variations exist for degrees from institutions of
other countries.
310 ➤ The ACS Style Guide
A.B., B.A., B.S.
A.M., M.A., M.S., M.B.A.
Ph.D., M.D.
Coghill, S. M.S. Thesis, Northwestern University, Evanston, IL, 2004.
Breton, J. C. Intégrales Multiples Stochastiques Poissonniennes. Ph.D. Disserta-
tion, University of Lille, France, 2001.
University Name and Location Field
The name of the degree-granting university is the minimum requirement for an
acceptable citation. You should also include the city and state or city and coun-
try. Use the two-letter postal abbreviations for states. Spell out names of coun-
tries unless they have standard abbreviations, such as U.K. for United Kingdom.
Blättler, T. M. Covalent Immobilization of Poly(l-lysine)-g-poly(ethylene glycol)
onto Aldehyde Plasma Polymer Coated Surfaces. Diploma Thesis, University
of South Australia, 2004.
Mohamed, M. Waterjet Cutting Up to 900 MPa. Ph.D. Thesis, University of Han-
nover, Germany, 2004.
Date of Completion Field
Indicate the date the thesis was completed by year only; month and year; or
month, day, and year.
Fleissner, C. Ph.D. Thesis, New York University, March 2003.
Marshall, M. Ph.D. Thesis, University of California, San Francisco, CA, 2005.
Stover, J. Ph.D. Dissertation, Harvard University, May 24, 2001.
Patents
recommended format
Patent Owner 1; Patent Owner 2; etc. Title of Patent. Patent Number, Date.
The minimum data required for an acceptable citation are the name(s) of the
patent owner(s), the patent number, and the date. Ensure that the patent stage
(Patent, Patent Application, etc.) is indicated and that the pattern of the number
(e.g., spaces, commas, dashes) follows that of the original patent document. If
possible, include the title and the Chemical Abstracts reference (preceded by a
semicolon) as well.
Sheem, S. K. Low-Cost Fiber Optic Pressure Sensor. U.S. Patent 6,738,537, May
18, 2004.
Lenssen, K. C.; Jantscheff, P.; Kiedrowski, G.; Massing, U. Cationic Lipids with Ser-
ine Backbone for Transfecting Biological Molecules. Eur. Pat. Appl. 1457483,
2004.
Chapter 14: References ➤ 311
Petrovick, P. R.; Carlini, E. Antiulcerogenic Preparation from Maytenus ilicifolia
and Obtaintion Process. Br. Patent PI 994502, March 6, 1999.
Langhals, H.; Wetzel, F. Perylene Pigments with Metallic Effects. Ger. Offen. DE
10357978.8, Dec 11, 2003; Chem. Abstr. 2005, 143, 134834.
Shimizu, Y.; Kajiyama, H. (Kanebo, Ltd., Japan; Kanebo Synthetic Fibers, Ltd.).
Jpn. Kokai Tokkyo Koho JP 2004176197 A2 20040624, 2004.
Government Publications
Publications of the U.S. government and those of state and local governments can
be pamphlets, brochures, books, maps, journals, or almost anything else that can
be printed. They may have authors or editors, who may be individuals, offices, or
committees, or the author may not be identified. They are published by specific
agencies, but they are usually (though not always) available through the Govern-
ment Printing Office rather than the issuing agency. To enable others to find the
publication, the American Library Association suggests that you include as much
information as possible in the citation. The following are examples of the most
commonly cited types of references.
Publications of Federal Government Agencies
recommended format
Author 1; Author 2; etc. Chapter Title. Document Title; Government Publication
Number; Publishing Agency: Place of Publication, Year; Pagination.
The format resembles that of a serial publication in book format. Include as
much information as possible.
Gebhardt, S. E.; Thomas, R. G. Nutritive Value of Foods; Home and Garden Bulle-
tin No. 72; U.S. Department of Agriculture, U.S. Government Printing Office:
Washington, DC, 2002.
Agriculture Fact Book 2000; U.S. Department of Agriculture, U.S. Government
Printing Office: Washington, DC, 2000.
Dey, A. N.; Bloom, B. Summary Health Statistics for United States Children:
National Health Interview Survey, 2003; DHHS Publication PHS 2005-1551;
Department of Health and Human Services, Centers for Disease Control and
Prevention, National Center for Health Statistics, U.S. Government Printing
Office: Washington, DC, 2005.
ISCORS Assessment of Radioactivity in Sewage Sludge: Modeling To Assess Radia-
tion Doses; NUREG-1783; EPA 832-R-03-002A; DOE/EH-0670; ISCORS
Technical Report 2004-03; Interagency Steering Committee on Radiation
Standards, Sewage Sludge Subcommittee, U.S. Government Printing Office:
Washington, DC, 2005.
312 ➤ The ACS Style Guide
Author Name Field
Include all author names. With multiple authors, separate the names from one
another by semicolons. Always end the author field with a period. List the names
in inverted form: surname first, then first initial, middle initial, and qualifiers
(Jr., II). Some publications list the first 10 authors followed by a semicolon and
“et al.”
Chapter Title Field
Chapter titles are set in roman type and end with a period.
Document Title Field
Treat the formal title of the document as the title of a book. These titles are set
in italic type and are separated from the next component of the reference by a
semicolon, which is set in italic type.
Government Publication Number Field
The government publication number, also called an agency report number, is
important because it is unique to the publication and because some indexing
services provide access by these numbers. These numbers (or number–letter
combinations) are usually printed somewhere on the cover or title page of the
document and are sometimes identified as a “report/accession number”. Treat a
report number the same as a series number; that is, it follows the book title, ends
with a semicolon, and is set in roman type.
Publishing Agency Field
The publishing agency field may take on added complexity in government publi-
cations. Often, the office or agency issuing the report as well as the Government
Printing Office must be cited. The order is department or agency, administration
or office, and finally U.S. Government Printing Office, all separated by commas
and set in roman type. The field ends with a colon.
Place of Publication Field
For the U.S. Government Printing Office, it is always Washington, DC. The field
ends with a comma preceding the date of publication.
Year of Publication Field
The year of publication is set in roman type and ends with a semicolon if it is
followed by pagination information. It ends with a period if it is the last field.
Pagination Field
The page numbers are set in roman type and end with a period, unless mis-
cellaneous material is appended to the reference.
Chapter 14: References ➤ 313
Alternative Format
Government agency references can also be given with CASSI abbreviations. In
that case, the format is the same as for periodicals.
Thompson, C. R.; Van Atta, G. R.; Bickoff, E. M.; Walter, E. D.; Livingston, A. L.;
Guggloz, J. Tech. Bull.—U.S. Dep. Agric. 1957, No. 1161, 63–70.
Other Federal Publications
Federal Register
The Federal Register is a periodical and is treated as such in citations.
Agency for Toxic Substances and Disease Registry. Update on the Status of the
Superfund Substance-Specific Applied Research Program. Fed Regist. 2002,
67, 4836–4854.
U.S. Food and Drug Administration. Food Labeling: Health Claims and Label
Statements for Dietary Supplements. Fed Regist. 1999, 65 (195), 59855–59857.
Code of Federal Regulations
Licensing of Government Owned Inventions. Code of Federal Regulations, Part
404, Title 37, 2005.
Labeling Requirements for Pesticides and Devices. Code of Federal Regulations,
Part 156, Title 40, 1998; Fed. Regist. 1998, 15, 7.
U.S. Code
The Public Health and Welfare. U.S. Code, Section 1396a, Title 42, 2000.
U.S. Laws
Treat the name of the law as a chapter title (roman, terminated with a period).
No publisher name is needed. The number and date of the law are separated
by a comma. If additional publication information is given, it is preceded by a
semicolon.
Domestic Quarantine Notices. Code of Federal Regulations, Section 301.10, Title
7, Vol. 5, 2005.
Federal Insecticide, Fungicide, and Rodenticide Act. Public Law 92-516, 1972;
Code of Federal Regulations, Section 136, Title 7, 1990.
State and Local Government Publications
recommended format
Author 1; Author 2; etc. Chapter Title. Document Title; Publication Number or
Type; Publishing Agency: Place of Publication, Date; Pagination.
Annual Report 2004: Moving Forward; Santa Barbara County Air Pollution Con-
trol District: Santa Barbara, CA, 2005.
314 ➤ The ACS Style Guide
Turner, B.; Powell, S.; Miller, N.; Melvin, J. A Field Study of Fog and Dry Depo-
sition as Sources of Inadvertent Pesticide Residues on Row Crops; Report of the
Environmental Hazard Assessment Program; California Department of Food
and Agriculture: Sacramento, CA, November 1989.
Technical Reports and Bulletins
Technical reports and bulletins come in many forms. Examples of some of these
have already been presented. Many are in-house publications, and some are gov-
ernment publications. Others are reports of work in progress. The publication
itself may include a phrase alluding to its status as a technical report or techni-
cal bulletin, but it may also simply be called a report or bulletin. Include what-
ever information is available, following the format shown for the word “Report”,
“Report No.”, etc. Document titles are set in italic type.
recommended format
Author 1; Author 2; etc. Title of Report or Bulletin; Technical Report or Bulletin
Number; Publisher: Place of Publication, Date; Pagination.
Tschantz, B. A.; Moran, B. M. Modeling of the Hydrologic Transport of Mercury in
the Upper East Fork Poplar Creek (UEFPC) Watershed; Technical Report for
Lockheed Martin Energy Systems: Bethesda, MD, September 2004.
Fourth DELOS Workshop. Evaluation of Digital Libraries; Final Report to the
National Science Foundation on Grant IIS-225626; Hungarian Academy of
Sciences: Budapest, 2002.
Data Sets
Data sets are compilations of data, such as spectra or property tables. These
data sets are often published serially as loose-leaf services, but the content is not
always organized in chapters as in other serial publications. The citation of a
serial data set should contain the title of the data set, the publisher, the place of
publication, the date of the volume, the data entry number (as opposed to the
data value), and the name of the figure or other identifying information. The
page number can be included in the citation if page numbers are used in the
index of the data set.
References to data retrieved from a stand-alone database should cite the source
as a computer program (for example, MDL CrossFire Commander, see p 323 f) or
as an online reference book (for example, the Kirk-Othmer Encyclopedia of Chemi-
cal Technology, see p 305 f), with the data entry number or other identifying infor-
mation included at the end of the citation. Data retrieved from an Internet-based
database should cite the source as a Web site (see pp 316 ff). If the data retrieved are
calculated data, also cite the software used for calculation (for example, ACD/Labs).
Chapter 14: References ➤ 315
recommended format for printed data sets
Title; Publisher: Place of Publication, Date; Data Entry Number, Figure Title or
other identifying information.
The Sadtler Standard Spectra: 300 MHz Proton NMR Standards; Bio-Rad, Sadtler
Division: Philadelphia, PA, 1994; No. 7640 (1-Chloropentane).
Material Safety Data Sheets
Material Safety Data Sheets (MSDSs) are published by the company that manu-
factures the material covered on the sheet. Citations should include the title of
the data sheet, which is the name of the material; the MSDS number; the man-
ufacturing company; the location of the company; and the date on which the
document was released. If the online version was used, the designation “Online”
is included in brackets after the MSDS number, and the URL and date accessed
are included at the end of the citation.
recommended formats
Title; MSDS Number; Manufacturing Company: Location of Company, Date.
Title; MSDS Number [Online]; Manufacturing Company: Location of Company,
Date. URL (accessed Month Day, Year).
Titanium Dioxide; MSDS No. T3627; Mallinckrodt Baker: Phillipsburg, NJ, Nov
12, 2003.
Acetic Anhydride; MSDS No. A0338 [Online]; Mallinckrodt Baker: Phillipsburg, NJ,
Feb 18, 2003. http://www.jtbaker.com/msds/englishhtml/a0338.htm (accessed
Nov 10, 2004).
Unpublished Materials
Material in any stage preceding actual publication falls under this general classi-
fication, as do personal communications and work not destined for publication.
recommended format for material intended for publication
Author 1; Author 2; etc. Title of Unpublished Work. Journal Abbreviation, phrase
indicating stage of publication.
Various phrases indicating the stage of publication are acceptable in these references.
➤ For material accepted for publication, use the phrase “in press”.
Tang, D.; Rupe, R.; Small, G. J.; Tiede, D. M. Chem. Phys., in press.
➤ For material intended for publication but not yet accepted, use “unpublished
work”, “submitted for publication”, or “to be submitted for publication”.
316 ➤ The ACS Style Guide
Chatterjee, K.; Visconti, A.; Mirocha, C. J. Deepoxy T-2 Tetraol: A Metabolite of
T-2 Toxin Found in Cow Urine. J. Agric. Food Chem., submitted for publica-
tion, 2004.
Nokinara, K. Duke University, Durham, NC. Unpublished work, 2003.
As Gordon G. Hammes says in Chapter 1 of this book,
Occasionally, the attribution of an idea or fact may be to a “private com-
munication” of a colleague or fellow scientist. In such cases, permission
must be obtained from the individual in question before the citation is
made. Reference to unpublished material should be avoided if possible
because it generally will not be available to interested readers.
recommended format for material not intended
for publication
Author. Affiliation, City, State. Phrase describing the material, Year.
Henscher, L. X. University of Minnesota, Minneapolis, MN. Personal com-
munication, 2001.
Heltman, L. R. DuPont. Private communication, 2003.
Wagner, R. L. University of Utah, Salt Lake City, UT. Unpublished work, 2004.
Messages sent by electronic mail are considered personal communications and
are referred to as such.
Electronic Sources
Electronic media continue to develop rapidly in content, organization, and presen-
tation of information. The conventions for citing electronic resources are evolving
to reflect these changes, but the basic principles of citation remain the same: present
enough documentation with enough clarity to establish the identity and authority
of the source and direction for locating the reference. The guidelines stress consis-
tency both in presentation of information and in reasons for exceptions.
To date, much of the material available in electronic media corresponds to
and/or is modeled after the traditional print-based sources discussed earlier in
this chapter and should be cited according to those guidelines as appropriate.
However, given the transient nature of electronic sources, it is important to pro-
vide additional documentation about the format or online location and the date
the source was accessed.
Internet Sources
Internet sources include online editions of traditional sources such as periodicals
and books available through Web technology; new collective sites of informa-
tion, including Internet-based databases using Web, file transfer protocol (FTP),
Chapter 14: References ➤ 317
and Telnet technologies; and electronic mailing lists and mail messages that may
or may not use Web interfaces. Each source has an electronic address; for sources
using the World Wide Web, this address is called the uniform resource locator
(URL). As Web interfaces and supporting technologies evolve, direct addresses of
items will often change to reflect new structures. Changing addresses can disrupt
access to information sources that may still be available but at new locations and
in modified formats. This issue can be resolved locally through use of persistent
URLs. A persistent URL remains constant, but the actual location of a source is
tracked through a local database that can be updated without disrupting the URL.
Information sources can also be tracked globally through coordinated efforts
such as the Digital Object Identifier (DOI) system. Information providers reg-
ister their sources, which are assigned unique and persistent DOIs. Each DOI is
similar to a barcode that manages a complex profile of multiple pieces, formats,
locations, ownership rights, and interoperability features. The identity of and
access to an electronic information source is maintained through its DOI regard-
less of changes in location, format, or publisher. The use of DOIs is spreading
among publishers as an efficient system to manage journal articles and other
types of intellectual property on the Web. Further information about the DOI
system can be found at http://www.doi.org (accessed April 13, 2005).
CrossRef is an application of the DOI system that links online citations across
publishers. The unique identification and persistent location information in the
DOI is packaged into an open URL that publishers and libraries can use to link
to subscribed full-text content from reference lists. These links appear with cita-
tions in the reference lists of online articles and databases from several partici-
pating publishers.
For the purposes of citation, reference style conventions continue to use the
URL as the most direct route to the location of a source. DOIs are sometimes
used by publishers in place of page numbers or article numbers and should be
included in citations in this context.
➤ URLs can be long and complicated, and there are conventions for splitting an
address between multiple lines; see Chapter 10 (pp 156–157) for guidelines on
breaking URLs and e-mail addresses.
Online Periodicals
There are several types of periodicals online, including those based on print
editions, electronic copies retrieved from databases, articles released online in
advance of a full print issue, periodicals published only in electronic format, and
article preprints posted in preprint servers. The reference styles for periodicals
apply, with additional information concerning online location and accession
date assigned as needed. As for print periodicals, article titles are desirable but
not included in all ACS publications; check the publication itself.
318 ➤ The ACS Style Guide
recommended format for online periodicals
based on print editions
Author 1; Author 2; Author 3; etc. Title of Article. Journal Abbreviation [Online]
Year, Volume, Inclusive pagination or other identifying information. URL
(accessed Month Day, Year).
Currently, the majority of the articles retrieved from online publications are based
on corresponding print versions. For these articles, the basic periodical reference
style is used, but if the article has been viewed only in its electronic form, the des-
ignation “Online” is included in brackets after the journal abbreviation.
Fine, L. Einstein Revisited. J. Chem. Educ. [Online] 2005, 82, 1601 ff. http://jchemed.
chem.wisc.edu/Journal/Issues/2005/Nov/abs1601.html (accessed Oct 15, 2005).
recommended formats for electronic copies of articles
retrieved from a database provider
Author 1; Author 2; Author 3; etc. Title of Article. Journal Abbreviation [Online]
Year, Volume, Article Number or other identifying information. Database
Provider. URL of top page (accessed Month Day, Year).
Author 1; Author 2; Author 3; etc. Title of Article. Title of Periodical [Online],
Complete Date, Pagination. Database Provider. URL of top page (accessed
Month Day, Year).
Electronic copies of periodicals, nonscientific magazines, or newspapers retrieved
from subscription database services often provide only the original text but not
the original formatting or the figures. For online articles provided as content in
a subscription database, use the reference style for periodicals or nonscientific
magazines as appropriate, and include the name of the database provider, the
URL of the top page, and the date accessed.
Hallet, V. Scanning the Globe for Organic Chemistry. U.S. News and World Report
[Online], April 19, 2004, p 59. Business Source Premier. http://www.epnet.
com/academic/bussourceprem.asp (accessed April 24, 2005).
recommended format for articles published online
in advance of print issues
Author 1; Author 2; Author 3; etc. Title of Article. Journal Abbreviation [Online
early access]. DOI or other identifying information. Published Online: Month
Day, Year. URL (accessed Month Day, Year).
Often, articles are ready for publication in advance of a full issue of a periodi-
cal. Several publishers offer these articles online up to weeks in advance of the
print issue. They are identical to the corresponding print articles except that
page numbers are often not yet available. Publishers market this service under
different names; the ACS Publications Division labels them As Soon As Publish-
able (ASAP). For citation purposes, use the designation “Online early access” in
brackets after the journal abbreviation in place of the publisher-specific term.
Chapter 14: References ➤ 319
Also include the DOI or other identifying information, the online publication
date, the URL, and the date accessed.
Pratt, D. A.; van der Donk, W. A. Theoretical Investigations into the Intermediacy
of Chlorinated Vinylcobalamins in the Reductive Dehalogenation of Chlo-
rinated Ethylenes. J. Am. Chem. Soc. [Online early access]. DOI: 10.1021/
ja047915o. Published Online: Dec 8, 2004. http://pubs.acs.org/cgi-bin/asap.
cgi/jacsat/asap/html/ja047915o.html (accessed Dec 8, 2004).
recommended format for periodicals published only
in electronic format
Author 1; Author 2; Author 3; etc. Title of Article. Journal Abbreviation [Online]
Year, Volume, Article Number or other identifying information. URL
(accessed Month Day, Year).
A periodical published only in electronic format may include additional elec-
tronic features, data, or commentaries. Use the reference style for periodicals,
and include the direct URL of the article as well as the date accessed. Volume
and page numbers are often not relevant. If they are not used, include the article
number, DOI, or other identifying information.
Zloh, M.; Esposito, D.; Gibbons, W. A. Helical Net Plots and Lipid Favourable
Surface Mapping of Transmembrane Helices of Integral Membrane Proteins:
Aids to Structure Determination of Integral Membrane Proteins. Internet J.
Chem. [Online] 2003, 6, Article 2. http://www.ijc.com/articles/2003v6/2/
(accessed Oct 13, 2004).
recommended format for articles retrieved from
preprint servers
Author 1; Author 2; Author 3; etc. Title of Article. Year, Article Number. Name of
Repository. URL (accessed Month Day, Year).
Ward, D. W.; Nelson, K. A. Finite Difference Time Domain (FDTD) Simulations
of Electromagnetic Wave Propagation Using a Spreadsheet. 2004, arXiv:phys-
ics/0402096. arXiv.org e-Print archive. http://arxiv.org/abs/physics/0402096
(accessed Oct 13, 2004).
Online Books
Books published online generally correspond to printed versions, and the refer-
ence styles are similar. Online location and access date should always be included
when citing online books. Reference works published online are often updated
with new content, and the dates on which sections were posted or updated
should also be included.
recommended format for online books without editors
Author 1; Author 2; Author 3; etc. Book Title [Online]; Series Information (if
any); Publisher: Place of Publication, Year; Volume Number, Pagination. URL
(accessed Month Day, Year).
320 ➤ The ACS Style Guide
Tour, J. M. Molecular Electronics: Commercial Insights, Chemistry, Devices, Archi-
tecture and Programming [Online]; World Scientific: River Edge, NJ, 2003; pp
177–180. http://legacy.netlibrary.com/ebook_info.asp?product_id=91422&
piclist=19799,20141,20153 (accessed Nov 7, 2004).
recommended format for online books with editors
Author 1; Author 2; Author 3; etc. Chapter Title. In Book Title [Online]; Editor 1,
Editor 2, etc., Eds.; Series Information (if any); Publisher: Place of Publica-
tion, Year; Volume Number, Pagination. URL (accessed Month Day, Year).
Oleksyn, B. J.; Stadnicka, K.; Sliwinski, J. Structural Chemistry of Enamines: A
Statistical Approach. In The Chemistry of Enamines [Online]; Rappoport, Z.,
Ed.; The Chemistry of Functional Groups; Patai, S., Rappoport, Z., Series
Eds.; Wiley & Sons: New York, 1994; Chapter 2, pp 87–218. http://www3.
interscience.wiley.com/cgi-bin/summary/109560980/SUMMARY (accessed
April 24, 2005).
recommended format for online encyclopedias
Article Title. Encyclopedia Title, edition [Online]; Publisher, Posted Online Post-
ing Date. URL (accessed Month Day, Year).
Alkanolamines from Nitro Alcohols. Kirk-Othmer Encyclopedia of Chemical Tech-
nology [Online]; Wiley & Sons, Posted March 14, 2003. http://www.mrw.
interscience.wiley.com/kirk/articles/alkaboll.a01/frame.html (accessed Nov
7, 2004).
Web Sites
Aside from online periodicals and books, general Web sites containing a wide
variety of information might need to be cited. Some sites are accessible by any-
one, but many are accessible only by subscription. Reference styles for FTP and
Telnet sites are similar to those for Web sites. Specific examples are given here for
general Web sites and databases, stand-alone documents, unpublished confer-
ence proceedings, and electronic theses.
recommended format for general web sites
Author (if any). Title of Site. URL (accessed Month Day, Year), other identifying
information (if any).
Use the title found on the Web site itself; add the words “Home Page” for clarifica-
tion when needed. Data retrieved from Internet-based databases should include a
data entry number. Stand-alone databases should be cited as computer programs
(see p 323).
ACS Publications Division Home Page. http://pubs.acs.org (accessed Nov 7, 2004).
Chemical Abstracts Service. STN on the Web. http://stnweb.cas.org (accessed
Nov 7, 2004).
International Union of Pure and Applied Chemistry Home Page. http://www.
iupac.org/dhtml_home.html (accessed April 24, 2005).
Chapter 14: References ➤ 321
Library of Congress Home Page. http://www.loc.gov (accessed April 24, 2005).
Northern Illinois University. Department of Chemistry and Biochemistry Home
Page. http://www.chembio.niu.edu (accessed Nov 7, 2004).
Sheffield Chemistry Software Archive (Macintosh). ftp://ftp.shef.ac.uk/pub/uni/
academic/A-C/chem (accessed April 24, 2005).
U.S. Environmental Protection Agency. http://www.epa.gov (accessed Nov 7, 2004).
recommended format for documents retrieved from
institutional or agency web sites
Author 1; Author 2; Author 3; etc. Title of Document, Year. Title of Site. URL
(accessed Month Day, Year).
If an article is contained within a large and complex Web site, such as that for a
university or a government agency, the host organization and the relevant pro-
gram or department should be identified before giving the direct URL of the
article and accession date.
Chou, L.; McClintock, R.; Moretti, F.; Nix, D. H. Technology and education: New
wine in new bottles: Choosing pasts and imagining educational futures, 1993.
Columbia University Institute for Learning Technologies Web site. http://www.
ilt.columbia.edu/publications/papers/newwine1.html (accessed Aug 24, 2000).
recommended format for online unpublished
conference presentations
Author 1; Author 2; etc. Title of Presentation. Presented at Conference Title
[Online], Place, Date; Paper Number. Title of Site. URL (accessed Month
Day, Year).
Works presented at conferences or meetings can be cited in several formats, as
discussed earlier in this chapter. Generally, published abstracts or proceedings
can be cited as online books or as online periodicals. Materials from oral presen-
tations, posters, or demonstrations that do not contain publication information
should be cited as follows.
Manly, S. Collective flow with PHOBOS. Presented at the 20th Winter Workshop
on Nuclear Dynamics [Online], Trelawny Beach, Jamaica, March 15–20, 2004.
University of Rochester, DSpace Web site. http://hdl.handle.net/1802/228
(accessed Oct 13, 2004).
recommended format for electronic theses
Author. Title of Thesis. Level of Thesis [Online], Degree-Granting University,
Location of University, Date of Completion. URL (accessed Month Day, Year).
Lozano, P. C. Studies on the Ion-Droplet Mixed Regime in Colloid Thrusters.
Ph.D. Thesis [Online], Massachusetts Institute of Technology, Cambridge,
MA, January 2003. http://theses.mit.edu/Dienst/UI/2.0/Describe/0018.mit.
etheses%2f2003-1 (accessed Nov 7, 2004).
322 ➤ The ACS Style Guide
Electronic Lists and Newsgroups
recommended format for electronic lists and newsgroups
Mailing List or Newsgroup Name, other information, electronic address (accessed
Month Day, Year).
Chemical Information List Server, CHMINF-L@iubvm.ucs.indiana.edu (accessed
Oct 13, 2004).
Computational Chemistry List, solvent discussion in archived messages of Sep-
tember 2003, chemistry@ccl.net (accessed Nov 10, 2004).
Molecular Diversity for Basic Research & Drug Discovery, mol-diversity@listserv.
arizona.edu (accessed Nov 10, 2004).
Electronic Mail Messages
Whether the message was personal and sent only to you or whether it was posted
in a newsgroup, it is not published. E-mail messages should be cited the same as
any other personal communication. Include the year and the professional affili-
ation of the author.
recommended format for electronic mail messages
Author. Affiliation, City, State. Personal communication, Year.
Solla, L. R. Cornell University, Ithaca, NY. Personal communication, 2005.
CD-ROMs and DVDs
The reference style for information published in CD-ROM or DVD format fol-
lows that for periodicals and books as appropriate, and the designation “CD-
ROM” or “DVD” is included in brackets.
recommended format for cd-rom and dvd periodicals
Author 1; Author 2; Author 3; etc. Title of Article. Journal Abbreviation [CD-
ROM or DVD] Year, Volume, pagination or other identifying information.
Fleming, S. A.; Jensen, A. W. Substituent Effects on the Photocleavage of Benzyl–
Sulfur Bonds. Observation of the “Meta” Effect. J. Org. Chem. [CD-ROM]
1996, 61, 7044.
recommended formats for cd-rom and dvd books
Author 1; Author 2; etc. Chapter Title. In Book Title, Edition Number [CD-ROM
or DVD]; Editor 1, Editor 2, etc., Eds.; Publisher: Place of Publication, Year;
Volume Number.
Author 1; Author 2; etc. Chapter Title. Book Title, Edition Number [CD-ROM or
DVD]; Publisher: Place of Publication, Year; Volume Number.
Vining, W. J.; Kotz, J.; Harman, P.; Vining, W.; McDonald, A.; Ward, J. General
Chemistry, 3rd ed. [CD-ROM]; Thomson Brooks/Cole: Florence, KY, 2002.
Rowley, D.; Ramaker, D. Standard Deviants Chemistry DVD Pack [DVD]; Goldhil
Educational: Camarillo, CA, 2000.
Chapter 14: References ➤ 323
Many books in CD-ROM or DVD format are reference works, so they have no
authors, editors, or chapter titles.
The Merck Index, 13.4 [CD-ROM]; Wiley: New York, 2005.
Green Chemistry: Meeting Global Challenges [DVD]; American Chemical Soci-
ety: Washington, DC, 2003.
recommended format for conference proceedings
on cd-rom or dvd
Author 1; Author 2; etc. Title of Presentation. In Title of Conference, Location of
Meeting, Date of Meeting [CD-ROM or DVD]; Publisher: Place of Publica-
tion, Year; other identifying information.
Vasaru, G. Sources of Tritium. In Proceedings of the 2nd International Conference
on Nuclear Science and Technology in Iran, Shiraz, Iran, April 27–30, 2004
[CD-ROM]; Conference Permanent Committee, Ed.; Shiraz University: Shi-
raz, Iran, 2004.
Computer Programs
References to computer programs must be treated on a case-by-case basis. Five
common presentations of computer programs are possible:
1. book format, with the name of the program as the title
2. technical report format
3. CASSI format
4. free style, as a simple listing of program title and author of program
5. thesis style
Book Format
recommended format
Author 1; Author 2; etc. Program Title, version or edition; Publisher: Place of
Publication, Year.
The recommended format is the same as that for a book citation, except that
there are no chapters or pages. The name of the computer program, with any
descriptors, is considered the title and is set in italic type. If you wish to include
additional information about a program that is important for the reader to
know, you may add it at the end of the reference with or without parentheses or
append it to the title in parentheses before the semicolon.
Binkley, J. S. GAUSSIAN82; Department of Chemistry, Carnegie Mellon Uni-
versity: Pittsburgh, PA, 1982.
Main, P. MULTAN 80: A System of Computer Programs for the Automated Solu-
tion of Crystal Structures from X-ray Diffraction Data; Universities of York and
Louvain: York, England, and Louvain, Belgium, 1980.
324 ➤ The ACS Style Guide
recommended format for commercial software and databases
Program Title, version or edition; comments; Publisher: Place of Publication,
Year.
References to data should include the data entry number or other identifying
information at the end of the citation. The date of access can also be included if
the database is updated frequently. If the data retrieved are calculated data, also
cite the software used for the calculation (for example, ACD/Labs).
Mathematica, version 5.1; software for technical computation; Wolfram Research:
Champaign, IL 2004.
MDL CrossFire Commander, version 7; Elsevier MDL: San Leandro, CA, 2004;
BRN 635994.
Scifinder Scholar, version 2004.2; Chemical Abstracts Service: Columbus, OH,
2004; RN 107-21-1 (accessed Dec 20, 2005); calculated using ACD/Labs soft-
ware, version 8.14; ACD/Labs 1994–2006.
Technical Report Format
recommended format
Author. Title of Report; Technical Report Number; Publisher: Place of Publication,
Year; Pagination (if any).
In a citation to a computer program as a technical report, a report or technical
report number is included. As with book format, the name of the computer pro-
gram is considered the title of the technical report.
Beurskens, P. T.; Bossman, W. P.; Doesburg, H. M.; Gould, R. O.; van der Hark,
Th. E. M.; Prick, P. A. J. DIRDIF: Direct Methods for Difference Structures;
Technical Report 1980/1; Crystallographic Laboratory: Toernooiveld, Neth-
erlands, 1980.
Johnson, C. K. ORTEP-II: A Fortran Thermal Ellipsoid Plot Program for Crystal
Structure Illustrations; Report ORNL-5138; National Technical Information
Service, U.S. Department of Commerce: Springfield, VA, 1976.
CASSI Format
Because of the broad base from which Chemical Abstracts indexes work, com-
puter programs, in the form of technical reports, may be referenced. In such
cases, CASSI format would be appropriate.
Johnson, C. K. Oak Ridge Natl. Lab., [Rep.] ORNL (U.S.) 1978, ORNL-5348.
Free Style
When only minimal information (e.g., author and program name) is available,
present the information as simply as possible.
Chapter 14: References ➤ 325
Programs used in this study included local modifications of Jacobson’s ALLS,
Zalkins’s FORDAP, Busing and Levy’s ORFEE, and Johnson’s ORTEP2.
Lozos, G.; Hoffman, B.; Franz, C. SIMI4A, Chemistry Department, Northwestern
University.
Thesis Style
Sheldrick, G. M. SHELX-76: Program for Crystal Structure Determination. Cam-
bridge University, 1976.
Collating References
Collate all references at the end of the manuscript in numerical order if cited
by number and in alphabetical order if cited by author. Do not include items in
the reference list that are not cited in the manuscript. Check the publication for
which you are writing. Some publications do not allow multiple references to be
listed as one numbered entry; they prefer that each numbered entry include only
one unique reference.
To collate references according to the author–date style, use the following
format.
1. Alphabetize in order of the first authors’ surnames.
2. When the same first author is common to multiple references,
• Group the single-author references first. List them chronologically. To
distinguish among references having the same year, add a lowercase letter
(a, b, c, etc.) to the year.
• Group the two-author references next. List them chronologically. To dis-
tinguish among references having the same year, add a lowercase letter (a,
b, c, etc.) to the year.
• Group all multiple-author (three or more) references last. List them
chronologically. To distinguish among references having the same year,
add a lowercase letter (a, b, c, etc.) to the year.
Hamilton, F. J. Biochemistry 2003, 42, 78–86.
Hamilton, F. J. J. Agric. Food Chem. 2004a, 52, 1622–1633.
Hamilton, F. J. J. Org. Chem. 2004b, 69, 298–306.
Hamilton, F. J.; Salvo, P. A. J. Agric. Food Chem. 2005, 53, 918–924.
Hurd, R. J. Magn. Reson. 1999, 87, 422.
Mills, M. S.; Thurman, E. M. Anal. Chem. 2001, 73, 1985–1990.
O’Connor, D. J. Environ. Eng. ASCE 2002, 114, 507–522.
Rahwan, R. G., Witiak, D. T., Eds. Calcium Regulation by Calcium Antagonists;
ACS Symposium Series 201; American Chemical Society: Washington, DC,
2004.
Scarponi, T. M.; Moreno, S. P. Biochemistry 2002, 41, 345–360.
326 ➤ The ACS Style Guide
Scarponi, T. M.; Adams, J. S. J. Pharm. Sci. 2003, 92, 703–712.
Serpone, N.; Pellizetti, E. Photocatalysis: Fundamentals and Applications; Wiley &
Sons: New York, 2004.
Tewey, L. P.; Rodriguez, R. E.; Jennes, A. C. J. Agric. Food Chem. 2001, 49, 1879–
1886.
Tewey, L. P.; Rodriguez, R. E.; Fortunato, B. D.; Jennes, A. C. Ind. Eng. Chem. Res.
2002a, 41, 465–472.
Tewey, L. P.; Hiroshi, C. Y.; Allen, P. R.; Lowe, D. L. Biochemistry 2002b, 41, 11689–
11699.
Tewey, L. P.; Rolland, H. J.; Harwood, C. C. J. Org. Chem. 2002c, 67, 3548– 3556.
Tewey, L. P.; Allen, P. R.; Levy, M. S. J. Am. Chem. Soc. 2003, 125, 2520.
Do not use the Latin terms ibid. (in the same place) or idem (the same) because
the actual reference source cannot be searched on electronic databases.
Reference/Citation Managers
Software programs are available to assist with the process of collecting and col-
lating references. With such programs, researchers can create personal electronic
collections or libraries of references and tailor the formatting to any number of
uses and publishing guidelines. Citations are parsed into searchable databases of
component fields, and formatting templates draw on the data to produce refer-
ence lists in a variety of reference styles. The process is further enhanced by filters
designed to correctly interpret the variety of incoming reference formats. Filters,
fields, and templates are customizable to accommodate additional sources and
styles.
Additional features have been developed to improve the convenience of these
tools, including connection scripts for hundreds of public-access and subscrip-
tion-based bibliographic databases and increased variety of field types to accom-
modate figures, cross-linking, personal annotation, etc. Plug-ins are available for
word-processing packages to format citations within the text, reference lists, and
lists of figures as authors write. There are also networking options for coopera-
tive reference building and linking to full-text versions of references.
Researchers can search literature databases either directly or through a refer-
ence manager interface; import text, images, and figures from journal articles,
Web sites, and other reference managers; arrange reference lists in numerous col-
lections or libraries; search and retrieve records by any field; format footnotes,
endnotes, and stand-alone bibliographies; share and co-edit these lists with col-
leagues; and export citations in hundreds of publication-specific styles in several
languages. These software packages assist the research process from initial litera-
ture searching to writing and editing final publications.
Leading reference management programs include EndNote, Reference Man-
ager, ProCite, RefWorks, and Biblioscape. EndNote, Reference Manager, and
Chapter 14: References ➤ 327
ProCite are currently all owned by Thomson Scientific and are available as stand-
alone software packages for both Windows and Macintosh platforms. RefWorks
is a Web-based program with individual accounts that can be accessed across
platforms from any point of Internet access. Biblioscape is available in a vari-
ety of stand-alone and Web-based options. For the most part, these programs
cover the gamut of research disciplines and are fairly well populated with filters
and templates specific to the chemistry literature. Reviews of these and other
bibliographic management software tools are regularly available in the library
literature.
The Thomson Scientific products were developed independently and still
retain distinctive characteristics in their functionality. EndNote focuses on the
reference input and output needs of the individual researcher, with hundreds
of connection scripts and filters and more than 1000 citation style templates.
EndNote is updated regularly and has a growing number of enhanced features
available. Reference Manager has traditionally targeted collaborative reference
sharing between colleagues, with networking options that allow multiple users
to work on the same reference list for a project. Some of these features are now
becoming available in EndNote as well. Reference Manager is only available for
the Windows platform. ProCite has focused on managing reference collections
with larger numbers of fields and reference types and more advanced grouping
and searching techniques. ProCite has not been updated since version 5 in 2001.
RefWorks is published by RefWorks.com and emphasizes the convenience
and collaborative nature of Web-based software. The program and updates are
provided on the RefWorks server, and users’ bibliographic data are stored there
as well. Multiuser accounts are available for collaborative work. The options for
filters, fields, reference types, and templates are less developed in RefWorks than
in the other tools discussed here and do not include filters or templates for ACS
journal styles.
Biblioscape is published by CG Information, founded by scientists specifi-
cally to manage scientific and electronic information. Biblioscape is a suite of
products with different sets of features designed for a variety of users, includ-
ing undergraduate and graduate students, researchers, and librarians. Options
include Web access, intranet, and freeware editions. More than 1000 output
styles are available, including the ACS journal styles.

328
ACS Symp. Ser.
Acta Crystallogr., Sect. C: Cryst. Struct.
Commun.
Acta Crystallogr., Sect. D: Biol. Crystallogr.
Acta Crystallogr., Sect. E: Struct. Rep.
Online
Acta Hortic.
Acta Mater.
Acta Pharmacol. Sin.
Acta Phys. Pol., B
Adv. Exp. Med. Biol.
Adv. Mass Spectrom.
Adv. Mater. (Weinheim, Ger.)
Adv. Sci. Technol. (Faenza, Italy)
Adv. Space Res.
Adv. Synth. Catal.
AIChE J.
AIDS (London, U.K.)
AIDS Res. Hum. Retroviruses
AIP Conf. Proc.
Alcohol.: Clin. Exp. Res.
Aliment. Pharmacol. Ther.
Am. Heart J.
Am. J. Cardiol.
Am. J. Clin. Nutr.
Am. J. Hum. Genet.
Am. J. Obstet. Gynecol.
Am. J. Pathol.
Am. J. Physiol.
Am. J. Respir. Cell Mol. Biol.
Am. J. Vet. Res.
Am. Mineral.
Anal. Bioanal. Chem.
Anal. Biochem.
Anal. Chem.
Anal. Chim. Acta
Anal. Lett.
Anal. Sci.
Analyst (Cambridge, U.K.)
Anesth. Analg. (Hagerstown, MD, U.S.)
Anesthesiology
Angew. Chem., Int. Ed.
Anim. Genet.
Ann. N.Y. Acad. Sci.
Ann. Neurol.
Annu. Rep.—Conf. Electr. Insul. Dielectr.
Phenom.
Annu. Tech. Conf.—Soc. Plast. Eng.
Anti-Cancer Drugs
Anticancer Res.
Antimicrob. Agents Chemother.
Antioxid. Redox Signaling
➤ ➤ ➤ ➤ ➤
APPENDIX 14-1
CASSI Abbreviations for
the 1000+ Most Commonly
Cited Journals
This appendix lists the Chemical Abstracts Service Source Index, or CASSI, abbre-
viations for more than 1000 of the most commonly cited journals. Note that
some journals of the same name are published in more than one city. Authors
should check the journal name carefully and include the city to prevent misun-
derstanding.
Reprinted from CASSI Annual Collective Issue for January–December 2004; American Chemi-
cal Society: Washington, DC, 2004; pp 42I–45I. Copyright 2004 American Chemical Society.
Chapter 14: References ➤ 329
Appl. Biochem. Biotechnol.
Appl. Catal., A
Appl. Catal., B
Appl. Environ. Microbiol.
Appl. Geochem.
Appl. Microbiol. Biotechnol.
Appl. Opt.
Appl. Organomet. Chem.
Appl. Phys. A: Mater. Sci. Process.
Appl. Phys. B: Lasers Opt.
Appl. Phys. Lett.
Appl. Radiat. Isot.
Appl. Spectrosc.
Appl. Surf. Sci.
Aquaculture
Aquat. Toxicol.
Arch. Biochem. Biophys.
Arch. Environ. Contam. Toxicol.
Arch. Pharmacal Res.
Arch. Virol.
ARKIVOC (Gainesville, FL, U.S.)
Arterioscler., Thromb., Vasc. Biol.
Arthritis Rheum.
Asian–Australas. J. Anim. Sci.
Asian J. Chem.
Astron. Astrophys.
Astron. J.
Astron. Soc. Pac. Conf. Ser.
Astrophys. J.
Astrophys. J., Suppl. Ser.
Atherosclerosis (Amsterdam, Neth.)
Atmos. Chem. Phys.
Atmos. Environ.
Aust. J. Chem.
Azerb. Khim. Zh.
Bandaoti Xuebao
Behav. Brain Res.
Biochem. Biophys. Res. Commun.
Biochem. Eng. J.
Biochem. J.
Biochem. Pharmacol.
Biochem. Soc. Trans.
Biochem. Syst. Ecol.
Biochemistry
Biochemistry (Moscow, Russ. Fed.)
Biochim. Biophys. Acta
Bioconjugate Chem.
Bioinformatics
Biol. Chem.
Biol. Pharm. Bull.
Biol. Psychiatry
Biol. Reprod.
Biol. Trace Elem. Res.
Biomacromolecules
Biomaterials
Bioorg. Med. Chem.
Bioorg. Med. Chem. Lett.
Biophys. Chem.
Biophys. J.
Biopolymers
Bioresour. Technol.
Biosci., Biotechnol., Biochem.
Biosens. Bioelectron.
BioTechniques
Biotechnol. Bioeng.
Biotechnol. Lett.
Biotechnol. Prog.
Blood
BMC Bioinf.
Bone (San Diego, CA, U.S.)
Bone Marrow Transplant.
Br. J. Anaesth.
Br. J. Cancer
Br. J. Clin. Pharmacol.
Br. J. Haematol.
Br. J. Nutr.
Br. J. Pharmacol.
Brain Res.
Breast Cancer Res. Treat.
Bul. Stiint. Univ. “Politeh.” Timisoara
Rom., Ser. Chim. Ing. Mediului
Bull. Chem. Soc. Jpn.
Bull. Environ. Contam. Toxicol.
Bull. Exp. Biol. Med.
Bull. Korean Chem. Soc.
Bunseki Kagaku
C. R. Chim.
Cailiao Kexue Yu Gongcheng Xuebao
Can. J. Chem.
Cancer (New York, NY, U.S.)
Cancer Biol. Ther.
Cancer Cell
330 ➤ The ACS Style Guide
Cancer Chemother. Pharmacol.
Cancer Epidemiol., Biomarkers Prev.
Cancer Genet. Cytogenet.
Cancer Lett. (Amsterdam, Neth.)
Cancer Res.
Cancer Sci.
Carbon
Carbohydr. Polym.
Carbohydr. Res.
Carcinogenesis
Cardiovasc. Res.
Catal. Commun.
Catal. Lett.
Catal. Today
Cell (Cambridge, MA, U.S.)
Cell Biol. Int.
Cell Cycle
Cell Death Differ.
Cell. Mol. Life Sci.
Cell. Signalling
Cem. Concr. Compos.
Cem. Concr. Res.
Ceram. Eng. Sci. Proc.
Ceram. Int.
Ceram. Trans.
Cereal Chem.
Chem. Biol.
Chem. Commun. (Cambridge, U.K.)
Chem. Eng. J. (Amsterdam, Neth.)
Chem. Eng. News
Chem. Eng. Process.
Chem. Eng. Res. Des.
Chem. Eng. Sci.
Chem.—Eur. J.
Chem. Geol.
Chem. Heterocycl. Compd. (New York,
NY, U.S.)
Chem. Ing. Tech.
Chem. Lett.
Chem. Mater.
Chem. Nat. Compd.
Chem. Pet. Eng.
Chem. Pharm. Bull.
Chem. Phys.
Chem. Phys. Lett.
Chem. Res. Chin. Univ.
Chem. Res. Toxicol.
Chem. Rev. (Washington, DC, U.S.)
Chem. Sens.
ChemBioChem
Chemosphere
ChemPhysChem
Chest
Chin. Chem. Lett.
Chin. J. Chem.
Chin. Med. J. (Beijing, China, Engl. Ed.)
Chin. Sci. Bull.
Chromatographia
Circ. Res.
Circulation
Clin. Biochem.
Clin. Cancer Res.
Clin. Chem. (Washington, DC, U.S.)
Clin. Chem. Lab. Med.
Clin. Chim. Acta
Clin. Diagn. Lab. Immunol.
Clin. Endocrinol. (Oxford, U.K.)
Clin. Exp. Allergy
Clin. Exp. Immunol.
Clin. Exp. Pharmacol. Physiol.
Clin. Immunol. (San Diego, CA, U.S.)
Clin. Infect. Dis.
Clin. Sci.
Collect. Czech. Chem. Commun.
Colloid Polym. Sci.
Colloids Surf., A
Colloids Surf., B
Combust. Flame
Commun. Soil Sci. Plant Anal.
Comp. Biochem. Physiol., Part A: Mol.
Integr. Physiol.
Comp. Biochem. Physiol., Part B:
Biochem. Mol. Biol.
Compos. Sci. Technol.
Comput. Chem. Eng.
Congr. Anu.—Assoc. Bras. Metal. Mater.
Corros. Sci.
Crit. Care Med.
Cryst. Growth Des.
Cuihua Xuebao
Curr. Biol.
Curr. Med. Chem.
Chapter 14: References ➤ 331
Curr. Microbiol.
Curr. Pharm. Des.
Curr. Sci.
Cytogenet. Genome Res.
Cytokine+
Czech. J. Phys.
Dalton Trans.
Desalination
Dev. Biol. (San Diego, CA, U.S.)
Dev. Brain Res.
Dev. Cell
Dev. Dyn.
Development (Cambridge, U.K.)
Di-San Junyi Daxue Xuebao
Diabetes
Diabetologia
Diamond Relat. Mater.
Dianchi
Dianyuan Jishu
Dier Junyi Daxue Xuebao
Diffus. Defect Data, Pt. B
Dig. Dis. Sci.
Disi Junyi Daxue Xuebao
Diyi Junyi Daxue Xuebao
DNA Repair
Dokl. Biol. Sci.
Dokl. Bulg. Akad. Nauk.
Dokl. Earth Sci.
Dopov. Nats. Akad. Nauk Ukr.
Drug Metab. Dispos.
Dyes Pigm.
EAAP Publ.
Earth Planet. Sci. Lett.
Ecotoxicol. Environ. Saf.
Electroanalysis
Electrochem. Commun.
Electrochem. Solid-State Lett.
Electrochemistry (Tokyo, Jpn.)
Electrochim. Acta
Electron. Lett.
Electrophoresis
EMBO J.
EMBO Rep.
Endocrinology
Energy Fuels
Environ. Health Perspect.
Environ. Pollut. (Oxford, U.K.)
Environ. Sci. Technol.
Environ. Technol.
Environ. Toxicol. Chem.
Enzyme Microb. Technol.
Eukaryotic Cell
Eur. Food Res. Technol.
Eur. J. Biochem.
Eur. J. Cancer
Eur. J. Clin. Nutr.
Eur. J. Endocrinol.
Eur. J. Hum. Genet.
Eur. J. Immunol.
Eur. J. Inorg. Chem.
Eur. J. Org. Chem.
Eur. J. Pharm. Biopharm.
Eur. J. Pharm. Sci.
Eur. J. Pharmacol.
Eur. Phys. J. A
Eur. Phys. J. B
Eur. Phys. J. C
Eur. Phys. J. D
Eur. Polym. J.
Eur. Space Agency, [Spec. Publ.] SP
Europhys. Lett.
Exp. Biol. Med. (Maywood, NJ, U.S.)
Exp. Cell Res.
Exp. Eye Res.
Exp. Gerontol.
Exp. Hematol. (New York, NY, U.S.)
Exp. Neurol.
Expert Opin. Invest. Drugs
Expert Opin. Pharmacother.
Farmaco
FASEB J.
FEBS Lett.
Fed. Regist.
FEMS Immunol. Med. Microbiol.
FEMS Microbiol. Lett.
Fenxi Huaxue
Fenxi Kexue Xuebao
Fenxi Shiyanshi
Ferroelectrics
Fish Physiol. Biochem.
Fiz. Khim. Tverd. Tila
Fluid Phase Equilib.
332 ➤ The ACS Style Guide
Food Addit. Contam.
Food Chem.
Food Chem. Toxicol.
Food Hydrocolloids
Forensic Sci. Int.
Free Radical Biol. Med.
Free Radical Res.
Fresenius Environ. Bull.
Front. Biosci.
Front. Sci. Ser.
Fuel
Fuel Process. Technol.
Fusion Energy
Fusion Eng. Des.
Gangtie
Gaodeng Xuexiao Huaxue Xuebao
Gaofenzi Cailiao Kexue Yu Gongcheng
Gaofenzi Xuebao
Gaoneng Wuli Yu Hewuli
Gaoxiao Huaxue Gongcheng Xuebao
Gastroenterology
Gen. Comp. Endocrinol.
Gendai Iryo
Gene
Gene Expression Patterns
Gene Ther.
Genes Dev.
Genetics
Genome Res.
Genomics
Geochim. Cosmochim. Acta
Geophys. Res. Lett.
Gongcheng Suliao Yingyong
Gongneng Cailiao
Gongye Cuihua
Green Chem.
Guangpu Shiyanshi
Guangpuxue Yu Guangpu Fenxi
Guangzi Xuebao
Guisuanyan Xuebao
Gut
Gynecol. Oncol.
Haematologica
Handb. Exp. Pharmacol.
Han’guk Hwankyong Uisaeng Hakhoechi
Han’guk Sikp’um Yongyang Kwahak
Hoechi
Hecheng Huaxue (1000)
Hecheng Xiangjiao Gongye
Helv. Chim. Acta
Hepatology (Philadelphia, PA, U.S.)
Heterocycles
Horm. Metab. Res.
Huagong Shikan
Huagong Xuebao (Chin. Ed.)
Huanjing Kexue Xuebao
Huanjing Wuran Zhili Jishu Yu Shebei
Huaxue Tongbao
Huaxue Xuebao
Huaxue Yanjiu Yu Yingyong
Hum. Mol. Genet.
Hum. Mutat.
Hum. Pathol.
Hum. Reprod.
Hydrobiologia
Hyomen Gijutsu
Hyperfine Interact.
Hypertension
IEEE Electron Device Lett.
IEEE J. Quantum Electron.
IEEE Trans. Electron Devices
IEEE Trans. Magn.
IEEE Trans. Nucl. Sci.
Igaku no Ayumi
Immunol. Lett.
Immunology
Ind. Eng. Chem. Res.
Indian J. Chem., Sect. A: Inorg., Bio-
inorg., Phys., Theor. Anal. Chem.
Indian J. Chem., Sect. B: Org. Chem. Incl.
Med. Chem.
Indian J. Environ. Prot.
Indian J. Pharm. Sci.
Infect. Immun.
Inflammation Res.
Inorg. Chem.
Inorg. Chem. Commun.
Inorg. Chim. Acta
Inorg. Mater.
Insect Biochem. Mol. Biol.
Inst. Phys. Conf. Ser.
Int. Conf. Thermoelectr.
Int. Congr. Ser.
Int. DATA Ser., Sel. Data Mixtures, Ser. A
Chapter 14: References ➤ 333
Int. Immunol.
Int. Immunopharmacol.
Int. J. Antimicrob. Agents
Int. J. Biochem. Cell Biol.
Int. J. Cancer
Int. J. Food Microbiol.
Int. J. Heat Mass Transfer
Int. J. Hydrogen Energy
Int. J. Mass Spectrom.
Int. J. Mod. Phys. B
Int. J. Mol. Med.
Int. J. Nanosci.
Int. J. Oncol.
Int. J. Parasitol.
Int. J. Pharm.
Int. J. Quantum Chem.
Int. J. Syst. Evol. Microbiol.
Integr. Ferroelectr.
Intermetallics
IP.com J.
ISIJ Int.
Izv. Akad. Nauk, Ser. Fiz.
Izv. Vyssh. Uchebn. Zaved., Khim. Khim.
Tekhnol.
J. Agric. Food Chem.
J. Allergy Clin. Immunol.
J. Alloys Compd.
J. Am. Ceram. Soc.
J. Am. Chem. Soc.
J. Am. Coll. Cardiol.
J. Am. Oil Chem. Soc.
J. Am. Soc. Mass Spectrom.
J. Am. Soc. Nephrol.
J. Anal. Appl. Pyrolysis
J. Anal. At. Spectrom.
J. Anal. Chem.
J. Anim. Sci. (Savoy, IL, U.S.)
J. Antimicrob. Chemother.
J. AOAC Int.
J. Appl. Crystallogr.
J. Appl. Electrochem.
J. Appl. Microbiol.
J. Appl. Phys.
J. Appl. Physiol.
J. Appl. Polym. Sci.
J. Appl. Spectrosc.
J. Bacteriol.
J. Biochem. (Tokyo, Jpn.)
J. Biol. Chem.
J. Biomed. Mater. Res., Part A
J. Biomed. Mater. Res., Part B
J. Biomol. NMR
J. Biosci. Bioeng.
J. Biotechnol.
J. Bone Miner. Res.
J. Cardiovasc. Pharmacol.
J. Catal.
J. Cell. Biochem.
J. Cell Biol.
J. Cell. Physiol.
J. Cell Sci.
J. Ceram. Soc. Jpn.
J. Chem. Ecol.
J. Chem. Educ.
J. Chem. Eng. Data
J. Chem. Eng. Jpn.
J. Chem. Inf. Comput. Sci.
J. Chem. Phys.
J. Chem. Res., Synop.
J. Chem. Technol. Biotechnol.
J. Chin. Chem. Soc. (Taipei, Taiwan)
J. Chromatogr., A
J. Chromatogr., B: Anal. Technol. Biomed.
Life Sci.
J. Clin. Endocrinol. Metab.
J. Clin. Invest.
J. Clin. Microbiol.
J. Colloid Interface Sci.
J. Comp. Neurol.
J. Comput. Chem.
J. Comput. Electron.
J. Controlled Release
J. Coord. Chem.
J. Cryst. Growth
J. Dairy Sci.
J. Electroanal. Chem.
J. Electrochem. Soc.
J. Electron. Mater.
J. Endocrinol.
J. Environ. Eng. (Reston, VA, U.S.)
J. Environ. Monit.
J. Environ. Qual.
334 ➤ The ACS Style Guide
J. Environ. Radioact.
J. Environ. Sci. Health, Part A: Toxic/
Hazard. Subst. Environ. Eng.
J. Essent. Oil Res.
J. Eur. Ceram. Soc.
J. Exp. Biol.
J. Exp. Bot.
J. Exp. Med.
J. Exp. Theor. Phys.
J. Fluorine Chem.
J. Food Prot.
J. Food Sci.
J. Gen. Virol.
J. Geophys. Res., [Atmos.]
J. Hazard. Mater.
J. Hepatol.
J. Heterocycl. Chem.
J. Histochem. Cytochem.
J. Hypertens.
J. Immunol.
J. Immunol. Methods
J. Inclusion Phenom. Macrocyclic Chem.
J. Indian Chem. Soc.
J. Infect. Dis.
J. Inorg. Biochem.
J. Invest. Dermatol.
J. Korean Ceram. Soc.
J. Korean Phys. Soc.
J. Leukocyte Biol.
J. Lipid Res.
J. Liq. Chromatogr. Relat. Technol.
J. Low Temp. Phys.
J. Lumin.
J. Magn. Magn. Mater.
J. Magn. Reson.
J. Mass Spectrom.
J. Mater. Chem.
J. Mater. Process. Technol.
J. Mater. Res.
J. Mater. Sci.
J. Mater. Sci. Lett.
J. Mater. Sci.: Mater. Electron.
J. Mater. Sci.: Mater. Med.
J. Mater. Sci. Technol. (Shenyang, China)
J. Med. Chem.
J. Med. Genet.
J. Med. Virol.
J. Membr. Sci.
J. Metastable Nanocryst. Mater.
J. Microbiol. Biotechnol.
J. Microbiol. Methods
J. Mol. Biol.
J. Mol. Catal. A: Chem.
J. Mol. Catal. B: Enzym.
J. Mol. Cell. Cardiol.
J. Mol. Evol.
J. Mol. Liq.
J. Mol. Spectrosc.
J. Mol. Struct.
J. Nat. Prod.
J. Natl. Cancer Inst.
J. Neurochem.
J. Neuroimmunol.
J. Neurophysiol.
J. Neurosci.
J. Neurosci. Res.
J. Non-Cryst. Solids
J. Nucl. Mater.
J. Nutr.
J. Opt. Soc. Am. B
J. Optoelectron. Adv. Mater.
J. Org. Chem.
J. Organomet. Chem.
J. Pathol.
J. Pharm. Biomed. Anal.
J. Pharm. Pharmacol.
J. Pharm. Sci.
J. Pharmacol. Exp. Ther.
J. Pharmacol. Sci. (Tokyo, Jpn.)
J. Photochem. Photobiol., A
J. Phys. A: Math. Gen.
J. Phys. B: At., Mol. Opt. Phys.
J. Phys. Chem. A
J. Phys. Chem. B
J. Phys. Chem. Solids
J. Phys.: Condens. Matter
J. Phys. D: Appl. Phys.
J. Phys. G: Nucl. Part. Phys.
J. Phys. IV
J. Phys. Org. Chem.
J. Phys. Soc. Jpn.
J. Physiol. (Oxford, U.K.)
Chapter 14: References ➤ 335
J. Plant Physiol.
J. Polym. Sci., Part A: Polym. Chem.
J. Polym. Sci., Part B: Polym. Phys.
J. Power Sources
J. Quant. Spectrosc. Radiat. Transfer
J. Radioanal. Nucl. Chem.
J. Raman Spectrosc.
J. Rheumatol.
J. Sci. Food Agric.
J. Sep. Sci.
J. Solid State Chem.
J. Steroid Biochem. Mol. Biol.
J. Surg. Res.
J. Therm. Anal. Calorim.
J. Thromb. Haemostasis
J. Univ. Chem. Technol. Metall.
J. Urol. (Hagerstown, MD, U.S.)
J. Vac. Sci. Technol., A
J. Vac. Sci. Technol., B: Microelectron.
Nanometer Struct.—Process., Meas.,
Phenom.
J. Vet. Pharmacol. Ther.
J. Virol.
J. Virol. Methods
JAERI—Conf
JETP Lett.
Jiegou Huaxue
Jikken Igaku
Jingxi Huagong
Jingxi Huagong Zhongjianti
Jinshu Xuebao
Jisuanji Yu Yingyong Huaxue
Jixie Gongcheng Cailiao
Jpn. J. Appl. Phys., Part 1
Jpn. J. Appl. Phys., Part 2
Kagaku to Kogyo (Tokyo, Jpn.)
Kagaku to Kyoiku
Kagaku to Seibutsu
KEK Proc.
Key Eng. Mater.
Kidney Int.
Kogyo Zairyo
Kongop Hwahak
Korean J. Chem. Eng.
Lab. Invest.
Lancet
Langmuir
Lect. Notes Phys.
Leuk. Lymphoma
Leuk. Res.
Leukemia
Life Sci.
Liq. Cryst.
Low Temp. Phys.
Lung Biol. Health Dis.
Macromol. Chem. Phys.
Macromol. Rapid Commun.
Macromol. Symp.
Macromolecules
Magn. Reson. Chem.
Mar. Pollut. Bull.
Mater. Chem. Phys.
Mater. Lett.
Mater. Res. Bull.
Mater. Res. Soc. Symp. Proc.
Mater. Sci. Eng., A
Mater. Sci. Eng., B
Mater. Sci. Eng., C
Mater. Sci. Forum
Mater. Sci. Technol.
Mater. Trans.
Meas. Sci. Technol.
Meat Sci.
Mech. Dev.
Med. Hypotheses
Meded.—Fac. Landbouwkd. Toegepaste
Biol. Wet. (Univ. Gent)
Metab., Clin. Exp.
Metall. Mater. Trans. A
Meteorit. Planet. Sci.
Methods Enzymol.
Methods Mol. Biol. (Totowa, NJ, U.S.)
Methods Mol. Med.
Microbes Infect.
Microbiology (Reading, U.K.)
Microchim. Acta
Microelectron. Eng.
Microelectron. Reliab.
Microporous Mesoporous Mater.
Miner. Eng.
Mod. Phys. Lett. A
Mol. Biochem. Parasitol.
336 ➤ The ACS Style Guide
Mol. Biol. Cell
Mol. Biol. Evol.
Mol. Brain Res.
Mol. Cancer Ther.
Mol. Cell
Mol. Cell. Biochem.
Mol. Cell. Biol.
Mol. Cell. Endocrinol.
Mol. Cell. Neurosci.
Mol. Cryst. Liq. Cryst.
Mol. Ecol.
Mol. Ecol. Notes
Mol. Endocrinol.
Mol. Genet. Genomics
Mol. Genet. Metab.
Mol. Immunol.
Mol. Med. (Tokyo, Jpn.)
Mol. Microbiol.
Mol. Pharmacol.
Mol. Phylogenet. Evol.
Mol. Phys.
Mol. Plant–Microbe Interact.
Mol. Psychiatry
Mol. Reprod. Dev.
Mol. Ther.
Mon. Not. R. Astron. Soc.
Monatsh. Chem.
Mutat. Res.
N. Engl. J. Med.
Nano Lett.
Nanotechnology
NASA Conf. Publ.
Nat. Biotechnol.
Nat. Cell Biol.
Nat. Genet.
Nat. Immunol.
Nat. Mater.
Nat. Med. (New York, NY, U.S.)
NATO Sci. Ser., II
NATO Sci. Ser., IV
NATO Sci. Ser., Ser. I
Nature (London, U.K.)
Naunyn-Schmiedeberg’s Arch. Pharmacol.
Nephrol., Dial., Transplant.
Neuron
Neurobiol. Aging
Neurobiol. Dis.
Neurochem. Int.
Neurochem. Res.
Neurology
Neuropharmacology
Neuropsychopharmacology
NeuroReport
Neurosci. Lett.
Neuroscience (Oxford, U.K.)
New J. Chem.
New Phytol.
Nippon Kessho Seicho Gakkaishi
Nippon Kikai Gakkai Ronbunshu, B-hen
Nongyao
Nongye Huanjing Kexue Xuebao
Nucl. Eng. Des.
Nucl. Fusion
Nucl. Instrum. Methods Phys. Res., Sect. A
Nucl. Instrum. Methods Phys. Res., Sect. B
Nucl. Phys. A
Nucl. Phys. B
Nucl. Phys. B, Proc. Suppl.
Nucleic Acids Res.
Nucleosides, Nucleotides Nucleic Acids
Oncogene
Oncol. Rep.
Opt. Commun.
Opt. Lett.
Opt. Mater. (Amsterdam, Neth.)
Opt. Spectrosc.
Org. Biomol. Chem.
Org. Lett.
Org. Process Res. Dev.
Organohalogen Compd.
Organometallics
Orient. J. Chem.
Oxford Monogr. Med. Genet.
Pain
Pediatr. Res.
Peptides (New York, NY, U.S.)
Pfluegers Arch.
Pharm. Chem. J.
Pharm. Res.
Pharmacol., Biochem. Behav.
Pharmacol. Res.
Pharmazie
Chapter 14: References ➤ 337
Philos. Mag.
Phosphorus, Sulfur Silicon Relat. Elem.
Photochem. Photobiol.
Photochem. Photobiol. Sci.
Phys. At. Nucl.
Phys. Chem. Chem. Phys.
Phys. Fluids
Phys. Lett. A
Phys. Lett. B
Phys. Plasmas
Phys. Rev. A: At., Mol., Opt. Phys.
Phys. Rev. B: Condens. Matter Mater. Phys.
Phys. Rev. C: Nucl. Phys.
Phys. Rev. D: Part. Fields
Phys. Rev. E: Stat., Nonlinear, Soft Matter
Phys.
Phys. Rev. Lett.
Phys. Solid State
Phys. Status Solidi A
Phys. Status Solidi B
Phys. Status Solidi C
Physica B (Amsterdam, Neth.)
Physica C (Amsterdam, Neth.)
Physica E (Amsterdam, Neth.)
Physiol. Behav.
Physiol. Genomics
Physiol. Plant.
Phytochemistry (Elsevier)
Planta
Plant Cell
Plant Cell Physiol.
Plant J.
Plant Mol. Biol.
Plant Physiol.
Plant Sci. (Amsterdam, Neth.)
Plant Soil
Planta Med.
Plasma Phys. Controlled Fusion
Plast. Massy
Pol. J. Chem.
Polyhedron
Polym. Degrad. Stab.
Polym. Eng. Sci.
Polym. Int.
Polym. J. (Tokyo, Jpn.)
Polym. Mater. Sci. Eng.
Polym. Prepr. (Am. Chem. Soc., Div.
Polym. Chem.)
Polymer
Poult. Sci.
Poverkhnost
Powder Technol.
Pramana
Prepr.—Am. Chem. Soc., Div. Pet. Chem.
Prepr. Ext. Abstr. ACS Natl. Meet., Am.
Chem. Soc., Div. Environ. Chem.
Prepr. Symp.—Am. Chem. Soc., Div. Fuel
Chem.
Proc.—Annu. Conf., Am. Water Works
Assoc.
Proc.—Electrochem. Soc.
Proc. Natl. Acad. Sci. U.S.A.
Proc. SPIE—Int. Soc. Opt. Eng.
Proc.—Water Qual. Technol. Conf.
Process Biochem. (Oxford, U.K.)
Prog. Org. Coat.
Prostaglandins, Leukotrienes Essent. Fatty
Acids
Prostate (New York, NY, U.S.)
Protein Expression Purif.
Protein Sci.
Proteins: Struct., Funct., Bioinf.
Proteomics
Psychopharmacology (Berlin, Ger.)
Publ. Australas. Inst. Min. Metall.
Pure Appl. Chem.
Quim. Nova
Radiat. Phys. Chem.
Radiat. Prot. Dosim.
Ranliao Huaxue Xuebao
Rapid Commun. Mass Spectrom.
React. Kinet. Catal. Lett.
Recents Prog. Genie Procedes
Regul. Pept.
Rengong Jingti Xuebao
Reproduction (Bristol, U.K.)
Res. Discl.
Rev. Chim. (Bucharest, Rom.)
Rev. Mex. Astron. Astrofis., Ser. Conf.
Rev. Roum. Chim.
Rev. Sci. Instrum.
RILEM Proc.
338 ➤ The ACS Style Guide
Rinsho Men’eki
RNA
Russ. Chem. Bull.
Russ. J. Appl. Chem.
Russ. J. Coord. Chem.
Russ. J. Electrochem.
Russ. J. Gen. Chem.
Russ. J. Genet.
Russ. J. Org. Chem.
Saibo Kogaku
Sci. Total Environ.
Science (Washington, DC, U.S.)
Scr. Mater.
Sekitan Kagaku Kaigi Happyo Ronbunshu
Semicond. Sci. Technol.
Semiconductors
Sens. Actuators, A
Sens. Actuators, B
Sep. Purif. Technol.
Sep. Sci. Technol.
Sepu
Shandong Daxue Xuebao, Yixueban
Shengwu Yixue Gongchengxue Zazhi
Shijie Huaren Xiaohua Zazhi
Shipin Kexue (Beijing, China)
Shiyou Huagong
Shiyou Lianzhi Yu Huagong
Shock
Soc. Automot. Eng., [Spec. Publ.] SP
Soil Biol. Biochem.
Soil Sci. Soc. Am. J.
Sol. Energy Mater. Sol. Cells
Solid State Commun.
Solid-State Electron.
Solid State Ionics
Solid State Sci.
Spec. Publ.—R. Soc. Chem.
Spectrochim. Acta, Part A
Spectrochim. Acta, Part B
Steroids
Stroke
Structure (Cambridge, MA, U.S.)
Stud. Surf. Sci. Catal.
Supercond. Sci. Technol.
Surf. Coat. Technol.
Surf. Interface Anal.
Surf. Sci.
Symp.—Int. Astron. Union
Synlett
Synth. Commun.
Synth. Met.
Synthesis
Talanta
Tanpakushitsu Kakusan Koso
Tech. Phys.
Tech. Phys. Lett.
Tetrahedron
Tetrahedron: Asymmetry
Tetrahedron Lett.
Tetsu to Hagane
Text. Res. J.
Tezhong Zhuzao Ji Youse Hejin
THEOCHEM
Theor. Appl. Genet.
Theriogenology
Thermochim. Acta
Thin Solid Films
Thromb. Haemostasis
Thromb. Res.
Tissue Antigens
Tissue Eng.
Tokyo Daigaku Genshiryoku Kenkyu Sogo
Senta Shinpojumu
Top. Catal.
Toxicol. Appl. Pharmacol.
Toxicol. Lett.
Toxicol. Sci.
Toxicology
Toxicon
Trans. Am. Foundry Soc.
Trans. Nonferrous Met. Soc. China
Transition Met. Chem. (Dordrecht, Neth.)
Transplant. Proc.
Transplantation
Trends Opt. Photonics
Trends Pharmacol. Sci.
Tsvetn. Met. (Moscow, Russ. Fed.)
Ukr. Khim. Zh. (Russ. Ed.)
Vaccine
Vacuum
VDI—Ber.
Vet. Microbiol.
Chapter 14: References ➤ 339
Virology
Virus Res.
Vysokomol. Soedin., Ser. A Ser. B
Water, Air, Soil Pollut.
Water Res.
Water Sci. Technol.
Wear
World J. Gastroenterol.
Wuji Cailiao Xuebao
Wuji Huaxue Xuebao
Wuli Huaxue Xuebao
Wuli Xuebao
Xibao Yu Fenzi Mianyixue Zazhi
Xiyou Jinshu
Xiyou Jinshu Cailiao Yu Gongcheng
Yaoxue Xuebao
Yingyong Huaxue
Yingyong Shengtai Xuebao
Youji Huaxue
Z. Anorg. Allg. Chem.
Z. Kristallogr.—New Cryst. Struct.
Z. Metallkd.
Z. Naturforsch., B: Chem. Sci.
Z. Naturforsch., C: J. Biosci.
Zairyo
Zavod. Lab., Diagn. Mater.
Zh. Fiz. Khim.
Zh. Neorg. Khim.
Zhengzhou Daxue Xuebao, Yixueban
Zhongcaoyao
Zhongguo Bingli Shengli Zazhi
Zhongguo Dongmai Yinghua Zazhi
Zhongguo Gonggong Weisheng
Zhongguo Jiguang
Zhongguo Jishui Paishui
Zhongguo Shenghua Yaowu Zazhi
Zhongguo Shengwu Gongcheng Zazhi
Zhongguo Shengwu Huaxue Yu Fenzi
Shengwu Xuebao
Zhongguo Shouyi Xuebao
Zhongguo Suliao
Zhongguo Xinyao Zazhi
Zhongguo Xitu Xuebao
Zhongguo Yaolixue Tongbao
Zhongguo Yaoxue Zazhi (Beijing, China)
Zhongguo Yiyao Gongye Zazhi
Zhongguo Yiyuan Yaoxue Zazhi
Zhongguo Youse Jinshu Xuebao
Zhonghua Yixue Yichuanxue Zazhi
Zhongliu Fangzhi Zazhi
340
➤ ➤ ➤ ➤ ➤
APPENDIX 14-2
A Sample CASSI Entry
Journal of the American Chemical Society. JACSAT. ISSN 0002–7863 (Absorbed
Am. Chem. J.). In English; English sum. History: v1 1879+. w 126 2004. ACS
Journals or Maruzen.
american chemical society. journal. washington, d. c.
Doc. Supplier: CAS.
AAP; AB 1905+; ABSR; ARaS; ATVA; AU–M 1893–1918,1920–1926,1928+;
AkU 1879–1906,1919+; ArU; ArU–M 1923+; AzTeS; AzU 1889+; C; CL; CLSU;
CLSU–M 1895–1897,1905,1908+; CLU–M; CLU–P; CMenSR 1916+; CPT; CSf;
CSt; CSt–L; CU; CU–A; CU–I 1920+; CU–M; CU–Riv 1907+; CU–RivA; CU–
RivP; CU–S; CU–SB; [etc.]
In this example,
• Journal of the American Chemical Society is the complete publication title
with its abbreviated form indicated by boldface type (J. Am. Chem. Soc.).
• JACSAT is the CODEN, a six-character, unique title abbreviation used
to represent titles in manual or machine-based information systems.
The CODEN source is the International CODEN Directory, administered
by Chemical Abstracts Service. The sixth character of each CODEN is a
computer-calculated check character that ensures the reliability of the
CODEN in computer-based systems.
• ISSN 0002–7863 is the International Standard Serial Number (ISSN),
assigned by the Library of Congress.
• Absorbed Am. Chem. J. is a reference to former titles and to any variant
forms of the selected title.
• In English; English sum. is the language of the publication, summaries,
and tables of contents.
• History: v1 1879+ is the history of the publication. Volume 1 began in
1879. The + following the year indicates that the publication is still in
existence under that title.
• w means weekly. The frequency of publication could also be a for annually,
ba for biennially (every two years), bm for bimonthly (every two months),
bw for biweekly (every two weeks), d for daily, m for monthly, q for quar-
terly, sa for semiannually (two times per year), sm for semimonthly (two
times per month), or sw for semiweekly (two times per week).
Chapter 14: References ➤ 341
• 126 2004 is the volume–year correlation (i.e., the first volume number
of that year, which is the most recent covered by that edition of CASSI;
volume 126 was the first volume number of 2004).
• ACS Journals or Maruzen is the publisher or source address or abbrevia-
tion.
• AMERICAN CHEMICAL SOCIETY. JOURNAL. WASHINGTON, D. C. is the AACR entry.
This is the abbreviated entry as catalogued according to the Anglo-Ameri-
can Cataloguing Rules (2nd ed.). It is included here because of its pre-
dominance in library collection records.
• Doc. Supplier: CAS means that articles are available through the CAS
Document Delivery Service.
• AAP; AB 1905+; ABSR; etc., is the library holdings information. Libraries
are identified by their National Union Catalog symbols, and holdings are
shown by inclusive years.
343
➤ ➤ ➤ ➤ ➤ CHAPTER 15
Figures
Betsy Kulamer
This chapter discusses methods of preparing and submit-
ting the figures and other illustrations that accompany a
scientific paper for publication. The past 10 years have seen a radical techno-
logical shift in the way figures are created by authors and handled by publishers.
Whereas authors previously submitted figures that were camera-ready—that is,
ready for the printer’s camera to photograph before making printing plates—
today many authors create figures using computer software, and most publish-
ers encourage electronic versions of figures. This chapter presents guidelines for
working with figures using the computer tools currently in widespread use; it
also includes recommendations regarding the use of color, fonts, and scanners,
as well as good style for citing and captioning figures.
Technical requirements and certain style points differ from publisher to pub-
lisher and from journal to journal, so bear in mind that this chapter presents
general guidelines. ACS journals have some specific requirements that may differ
from the general guidelines presented here, and these are noted throughout the
chapter. As always, before you finalize figures for your manuscript, you should
consult your publisher’s guidelines for submitting artwork. For ACS journals,
consult recent issues as well as the Guide, Notes, or Instructions for Authors
that appear in each journal’s first issue of the year or at https://paragon.acs.org/
paragon/index.jsp (see “Author Information”). For ACS books, consult the bro-
chure “How To Prepare Your Manuscript for the ACS Symposium Series” or see
“Info for Authors” at https://pubs.acs.org/books.
Copyright 2006 American Chemical Society

344 ➤ The ACS Style Guide
When To Use Figures
A figure is an illustration used in scientific or scholarly publishing. Figures can be
graphs of data, photographs, sketches, flow charts, and so on. Figures can play a
major role in highlighting, clarifying, and summarizing data and results and can
substantially increase the readers’ comprehension of the text by communicating
visually. For example, line graphs show trends. Bar graphs compare magnitudes.
Pie charts show relative portions of a whole. Photographs can provide absolute
proof of findings. In general, figures should be used when the picture really is
worth a thousand (or so) words.
However, figures can decrease a reader’s comprehension, and they can cause
outright confusion if they are poorly rendered or cluttered, if they do little more
than repeat data already presented in text, or, worse, if they present information
at odds with the text. An excessive number of figures can dilute the value of any
individual figure: when presented with too many figures, a reader may look care-
fully at none of them. Figures should not be used to present data that would be
better presented in a table. See Box 15-1 and Chapter 16, “Tables.”
The cost of publishing figures has decreased substantially over the past two
decades, partly because authors are able to prepare better quality figures (so the
publisher does not have to) and partly because of the shift to electronic methods
of Web and print publishing that are less cumbersome than earlier technologies,
which required a high-quality, pristine paper original of all art. The cost of pub-
lishing color figures has also decreased substantially because most color photo-
graphs can now be provided in electronic form and no longer require expensive
color separations before being put on a printing press. The cost of using color in
Web publications is inconsequential (although color should still be used care-
fully to enhance your meaning, not just to grab attention). See Box 15-2.
Box 15-1. Do I Need a Figure or a Table?
Do I want the basic point to be communicated at a glance?
Use a figure.
Do I want the reader to see trends and relationships?
Use a figure.
Do I want the reader to see exact numbers?
Use a table.
Do I want to communicate a lot of information with words?
Use a table.
➤ ➤ ➤ ➤ ➤

Chapter 15: Figures ➤ 345
How To Cite Figures
All figures must be “called out”, that is, mentioned or discussed by name and
number in the text. (If you cannot find a graceful, logical place to put the callout,
then you might consider deleting the figure.) Follow these guidelines in citing
figures.
➤ Capitalize the word “Figure” when it is followed by the figure number.
➤ Number figures sequentially with arabic numerals in order of discussion in
the text (Figure 1, Figure 2, etc.).
➤ Designate parts of a figure by using a combination of the arabic numeral and
a sequence of consistent labels, usually (but not always) letters: Figure 1a, Figure
1b; Figure 1A, Figure 1B; Figure 1-I, Figure 1-II. Do not cite, for example, Figure
4 and Figure 4A.
examples of figure callouts in text
The block copolymers may contain a small but detectable fraction of impurities,
as shown in Figures 1 and 2.
Figures 3–5 show the production of acid-reactive substances in three different oils.
The deuterium-labeled substrate gave rise to the partial 1H NMR spectra shown
in Figure 2a,b.
Box 15-2. Do I Need To Use Color?
Do I need color to make the picture comprehensible to the reader?
Color should be used only when it is essential to understand the chemi-
cal nature of the material in the picture. Otherwise the picture should be
prepared in black and white.
Do I need color in my line graph, bar graph, or pie chart?
Color is rarely required in these figures. Lines with varying dash styles in
line graphs and distinct grayscale shades in bar graphs or pie charts work
as well.
Do I need color to catch the reader’s eye?
No.
Do I need color to organize related information for the reader?
No. Judicious use of fonts and careful placement of words and objects
usually does just as well.
➤ ➤ ➤ ➤ ➤
346 ➤ The ACS Style Guide
As seen in Figure 3b–d, the catalytic wave shows a small but distinct decrease
upon addition of the nucleophile.
Parts a and b of Figure 4 illustrate that the voltammetric plateau current depends
on the number of enzyme monolayers.
Curves c–e of Figure 5 were obtained for various methyl groups in the protein.
poor examples of figure callouts in text
Figure 2c and Figure 2D show… should read Figure 2c,d shows…
Figure 3 and Figure 4 show… should read Figures 3 and 4 show…
Chemical structures and schemes should not be numbered as figures; they should
be labeled according to separate sequences. See Chapter 17, Chemical Structures.
How To Prepare Figures
At one time, almost all publishers required that figures be submitted as camera-
ready artwork. Today, most publishers accept, many encourage, and a few require
that figures be submitted in electronic format. Most scientists store their data on
computers and create their figures using computer software, and good-quality
document scanners are in common use. Thus, the submission of electronic files
is simpler than ever before and has the advantage of keeping original data and
photographs in the author’s own hands. Satisfactory results in the final publica-
tion are not guaranteed, however, and the burden rests on authors to prepare
figures to suit the requirements of the medium in which they will be published.
Publication Medium
The traditional method for publishing books, journals, and other documents is
by print publishing, that is, the application of ink on paper. Newer avenues include
publishing on the Internet, usually called Web publishing, as well as other formats
that are viewed on a computer monitor, such as CD-ROMs. The requirements of
ink-on-paper and pixels-on-screen publishing are quite different and may affect
your choices regarding figure creation and final electronic format. For printed
materials to be satisfactory, the figure files must have much detail and therefore
must be prepared at a higher resolution, that is, a higher number of pixels per
inch (ppi). Paper printers also use a scale called dots per inch or dpi, which you
may need to use sometimes. Materials viewed on a computer monitor do not
require the same level of detail but—on the Web especially—they must down-
load quickly, so they are prepared at a low resolution. Only for printed materi-
als is color significantly more expensive than black-and-white, so in print, color
is used conservatively. Conversely, on a computer monitor, color costs nothing
additional and is used widely.

Chapter 15: Figures ➤ 347
Publishing with ACS: When preparing figures for ACS journals—which
are published in print and on the Web—prepare your figures to the
higher standard required for print. ACS routinely prepares the Web
figures from files provided for the print figures.
Types of Figure Artwork
The two broad categories of figure artwork are line art and continuous-tone art.
Line art consists of black markings on a white background, with no additional
colors and no shades of gray (see Figure 15-1A). Line art contains lines, solid
shapes, and type (letters or words). Examples of line art include line graphs, flow
charts, and scatter plots. (Structures and schemes are usually line art as well;
see Chapter 17.) Continuous-tone art (also called halftone art) contains shades
of gray (or color) (see Figure 15-1B). Examples of continuous-tone art include
photographs (whether black-and-white or color) and drawings or sketches with
midtones.
In print publishing, line art is straightforwardly rendered by ink on paper.
To print continuous-tone art, the shading must be converted into dots through
a process called screening. The size and spacing of the dots produces the illu-
sion of shading to the reader’s eye. Halftones do not reproduce type or fine lines
well because screening distorts the edges of the letters and lines. On a computer
monitor, all text and images are converted to uniformly sized pixels.
Until recently, publishers strongly encouraged authors to submit figures as
line art, with halftones reserved for photographs. Currently available software
and the prevalence of electronic file submission is bringing a third category of
art into more common use. Grayscale line art is black-and-white art that con-
tains gray shading alongside fine lines and type (see Figure 15-1C). Grayscale
line art may be used for pie charts, bar graphs, and area graphs, where different
sections are distinguished by different shades of gray.
A fourth category of art is a combination of a photograph with some type, or a
combo (see Figure 15-1D). An example might be a photomicrograph with a unit-
of-measure legend in one corner; another might be a photograph with callouts
identifying parts of an image, such as a piece of equipment. If there is only a small
amount of type (as in the first example), then a combo can be handled like other
halftones. When there is more type (as in the second example), then a combo
requires special handling, because screened type loses legibility very quickly.
Publishing with ACS: For ACS journals, line art should be created and
saved at 1200 ppi. Grayscale art, including grayscale line art and combos,
should be created and saved at 600 ppi. Halftones, whether black-and-
white or color, should be created and saved at 300 ppi.
348 ➤ The ACS Style Guide
Figure 15-1. Examples of categories of figure art: (A) line art, (B) halftone or
continuous-tone art, (C) grayscale line art, and (D) a combo.
Sources: (A) Reprinted from Stevens, L. L.; Haycraft, J. J.; Eckhardt, C. J. Cryst. Growth Des. 2005, 5, 2060–2065. Copy-
right 2005 American Chemical Society. (B) Photograph courtesy of Raj Mehta. Reprinted from Reagent Chemi-
cals, 10th ed. Copyright 2006 American Chemical Society. (C) Reprinted from Watanabe, K.; Niwa, S.; Mori, Y. H. J.
Chem. Eng. Data 2005, 50, 1672–1676. Copyright 2005 American Chemical Society. (D) Adapted from Odelius, K.;
Plikk, P.; Albertsson, A.-C. Biomacromolecules 2005, 6, 2718–2725. Copyright 2005 American Chemical Society.
A
B
Chapter 15: Figures ➤ 349
Figure 15-1. Continued.
C
D

350 ➤ The ACS Style Guide
Use of Color
The use of color in figures depends partly on the medium and partly on a par-
ticular publisher’s preferences. As mentioned earlier, color can be used in Web
publishing at virtually no cost, so the only restrictions have to do with scientific
effectiveness and good taste. Color in print publishing adds to the cost, so before
preparing figures in color, you should ascertain a publisher’s policies on color.
Publishing with ACS: ACS journals do not require authors to pay the cost
of color illustrations. Authors who publish color figures in ACS books are
asked to pay half the cost.
In publications, color is produced by one of three modes:
• Black-and-white, or B&W: For print publications, where only one color
of ink (usually black) is used.
• Cyan–magenta–yellow–black, or CMYK (also called four-color): For print
publications, where all shades of color are produced by layering screened
dots of four standardized inks.
• Red–green–blue, or RGB: For Web publications and others viewed on
computer monitors, where all shades of color are produced by overlap-
ping red, green, and blue light.
Most publishers can switch electronically from CMYK to RGB, although it is
always better to use the publisher’s preferred format. Switching from one of the
color formats to B&W may produce unsatisfactory results, however. If the gray
tones that result are hard to distinguish from each other, a figure that was intel-
ligible in color can become meaningless in B&W. For this reason, it is always bet-
ter to prepare art that will be reproduced in black and white as B&W.
Electronic File Formats
Many software packages are available today for drawing, scanning, and manipu-
lating figures. When a figure is saved in a format that can be read or reopened
only by the software that produced it, the file is called an application file. Exam-
ples include files with names that end with the suffix .cdx, which can be opened
only in Cambridgesoft ChemDraw, or files with names that end in the suffix
.psd, which can be opened only in Adobe Photoshop. Application files often pose
problems in both Web and print publishing because the publisher (or reader)
might not have the necessary software to open or view the file. Many publishers
will not accept application files for figures.
There are a handful of file formats, however, that can be opened in many dif-
ferent software packages. Drawing and scanning programs generally provide the
option of saving to one or more of these formats. For example, ChemDraw and

Chapter 15: Figures ➤ 351
Photoshop will both export figures as TIFFs, a format frequently used for print
publications. The most universally accepted of these formats are the following:
For Print
• Encapsulated PostScript, or EPS. PostScript is a programming language
developed by Adobe to describe pages, graphics, and fonts; an EPS file is
coded to be embedded in a large PostScript file.
• Tagged Image File Format, or TIFF. TIFFs describe an image by dividing
it into a grid of pixels and assigning a value to each square. The quality
of a TIFF image depends on its resolution (in ppi), that is, the size of the
original grid.
For Web
• Graphics Interchange Format, or GIF. GIF is a pixel-based format like
TIFF, except the resolution is preset at 72 ppi, which is appropriate for the
Web but seldom acceptable for print.
• Joint Photographic Experts Group, or JPEG. JPEG is another pixel-based
format like TIFF but capable of higher resolutions than GIFs. JPEGs,
however, are actually compressed files, and they lose small amounts of
digital information every time they are opened, recompressed, and saved,
so JPEG images tend to degrade with repeated processing. Only “fresh”,
high-quality JPEGs are suitable for print publication.
For Print and Web
Many publishers now accept properly created Portable Document Files, or PDFs.
A PDF document is created when an application file produced by any of a num-
ber of software programs is “distilled”, using software such as Adobe’s Acrobat
Distiller. The PDF document can be viewed on any platform using viewing soft-
ware such as Adobe’s Acrobat Reader, regardless of whether the viewer has the
original application software.
Publishing with ACS: For ACS journals, figure art that will be submitted
in PDF format should be distilled using Adobe Acrobat Distiller’s “Press
Quality” setting.
With so many different software packages and file formats, there is no single “right”
way to prepare figures for publication. A few common paths are listed here:
• A line-art figure is drawn in an application such as Adobe Illustrator, Macro-
media Freehand, or Corel Draw and exported as an EPS or a TIFF file.
• A B&W photograph is scanned with the scanner’s software and saved as a
grayscale TIFF.

352 ➤ The ACS Style Guide
• A color digital photograph cropped in Photoshop is saved as a 72 ppi
RGB GIF file for the Web or a 300 ppi CMYK TIFF file for print.
• Grayscale line art is drawn in an application, such as Illustrator or Free-
hand, and saved as an EPS file.
Table 15-1 lists image file formats commonly accepted by publishers and their
suitability for different publishing media and types of artwork.
Several software packages that are in common use can produce satisfactory
GIFs for Web publishing but do not export images in a form that is acceptable
for the more rigorous requirements of print. Corel WordPerfect and Micro-
soft Word, Excel, and PowerPoint cannot save figures as valid PostScript files or
export directly to TIFFs. Box 15-3 offers suggestions for preparing publishable
figures in that program.
Sizing Artwork
Always determine the size at which your figures will be printed and prepare the
final electronic version to those dimensions. Consult guidelines from the pub-
lisher first. If there are no guidelines, use the following:
• For a Web publication, size your artwork so that it is visually appealing
and all parts of the graphic are easily readable on your monitor at 100%.
Table 15-1. Image File Formats and Their Suitability by Publication Medium
and Type of Figurea
Publication Medium Best Used To Represent
File Format
Optimal
ppibWeb
B&W
Printing
Color
Printing Line Art
Photo-
graphs
Grayscale
Line Art Combos
For Web
GIF 72 H X X R R R R
RGB JPEG 72 R X X R R R R
For Print
B&W JPEG 300 X R X X R R R
CMYK JPEG 300 X X R X R R R
600–1200 X X R R X X X
Bitmap TIFF 1200 X H X H X X X
Grayscale TIFF 600 X H X X H R R
CMYK TIFF 300 X X H X H R R
EPS N/A X R H HcX Hc Hc
aH indicates highly recommended; R indicates recommended; X indicates not recommended.
bThe optimal number of pixels per inch (ppi) for art prepared to the size at which it will be published. It is always
permissible to supply art with a higher ppi than what is listed here.
cEPS files are the best way to submit grayscale line art and combos, but the fonts used must be embedded or
converted to outlines.

Chapter 15: Figures ➤ 353
Box 15-3. Tips for Submitting
Figures Created in Excel
Microsoft Excel and PowerPoint are widely used to create graphs and
other figures, but they are unable to export to TIFF or any PostScript file
format. Therefore, if figures prepared with these software programs are
destined for print publication, they must be printed on paper first and
then scanned. (It may also work to submit the figure as a PDF.) Moreover,
the default settings of these programs will not produce satisfactory fig-
ures. The following tips help you get the best results from Excel.
• Do not use color to differentiate areas of the figure. Use black only
for a color, and use gray tones to differentiate areas of the figure.
• Be sure that the weight of all lines is at least 0.5 point. Some lines in
Excel default to 0.12 point, which is too thin for scanning. If the figure
will be printed large and then reduced, calculate the width of lines
accordingly (see the chart below).
• Be sure that the type and symbols also will scale down accordingly.
• It is better for tick marks to extend into the figure, rather than extend
outside.
• Print the figure on clean, opaque, white paper, using a fresh ink or
toner cartridge. Do not use a dot matrix printer. Set the laser or inkjet
printer to a resolution of at least 600 ppi.
• Scan the figure to the correct size, and save it as a 600 ppi bitmap
TIFF.
If your publisher requests the Excel application file, be sure that the
source data are embedded (not linked) in the file you send.
Line width for a typical figure created in Excel, printed landscape on 8.5
× 11 in. paper, and destined to be printed at a width of 20 or 27 picas:
20 picas
(single-column journal)
27 picas
(6 x 9 in. book)
Approximate reduction 38% 50%
Type size in Excel 14 pt 14 pt
Type size after reduction 5 pt 7 pt
Line width in Excel 1.5 pt 1 pt
Line width after reduction 0.5 pt 0.5 pt
➤ ➤ ➤ ➤ ➤
354 ➤ The ACS Style Guide
• For a printed publication, you can determine the column width and page
length by measuring a sample copy of the journal or a similar book from
the same publisher.
• For ACS journals, try to design figures to fit the width of one column. Table
15-2 gives the column widths and page lengths of many ACS publications.
• Do not put unnecessary frames or boxes around your figures.
• Remember that the length that you measure or take from Table 15-2 is
the maximum space available for the figure plus captions or notes. There-
fore, the art must be small enough to leave space for the caption.
• Finally, the width and depth of a figure should not exceed the needs of
that figure. Be economical; do not waste space.
Working with Line Art
If you are creating line-art figures with a computer program, save them in an
appropriate format (see Table 15-1) or print them on white, high-quality,
smooth, laser-printer paper and scan them. If line-art figures are being drawn by
hand, use black ink on white, high-quality, smooth, opaque paper, and then scan
them. Box 15-4 provides tips for successful scanning.
Here are some ways to improve the quality of the line-art figures you submit:
➤ Select software that will yield the best results for the line art you wish to
create.
Table 15-2. Column Dimensions Most Common in ACS Publications
Column Width Page Length
Publication picas inches
centi-
meters picas inches
centi-
meters
Books, trim size
6 × 9 in. 27 4½ 11 42 7 17½
7 × 10 in. 33 5½ 13½ 51 8½ 21½
8 × 11 in. 56 9 23
single column 20 3¼ 8¼
double column 42 7 17½
Journals and magazines,
two-column format
60 10 25½
single column 20 3¼ 8¼
double column 42 7 17½
Magazines, three-column format 60 10 25½
single column 13 2 5
double column 27½ 4½ 11
triple column 42 7 17½

Chapter 15: Figures ➤ 355
➤ Create the line art to the exact size at which it will be published.
➤ Keep line-art figures clear and simple. Keep words to a minimum. Lengthy
explanations should go in the caption or a note accompanying the caption.
➤ Scale the length, width, type, symbols, and lines of the art proportionally;
keep the symbols, lines, and type at uniform density, or darkness.
• Make the lines at least 0.5 point wide and usually not more than 1.5 points
wide.
Box 15-4. Tips for Successful Scanning
• If possible, use a flatbed scanner, where the art rests flat on a glass
surface and is scanned by a moving scanner’s eye, rather than a
sheet-fed scanner, where the art is rolled past the scanner’s eye. Flat-
bed scanners are less likely to distort your figure.
• Use your scanner’s optical (that is, actual) resolution capability, not
the interpolated (that is, calculated) one.
• Scan photographs at a minimum resolution of 300 ppi.
• Scan line art at a resolution of minimum 600 ppi, but 800, 1000, or 1200
ppi is even better. (Line art for ACS publications must be 1200 ppi.)
• If possible, determine the final size of your figure in print and calcu-
late the percentage of expansion or reduction. Then scan the photo-
graph at that percentage.
• If you are not sure about the final size, scan your photograph at a
larger size (say, 150% with 300 ppi) or higher resolution (say, 100%
with 450 ppi). Scan your line art at 150% with 600 ppi or at 100%
with 1000 ppi. It is better to submit image files with too much infor-
mation than too little.
• Do not adjust contrast or color according to what you see on your
monitor. Instead, use the automatic adjustments that come with the
scanning software or use the calibration curves that come with im-
age-editing software, such as Photoshop.
• Do not apply any screening in the scanning or editing software, even
if you know the figure will be screened before it is published. Let
the publisher and the publisher’s printer determine the appropriate
screening.
➤ ➤ ➤ ➤ ➤

356 ➤ The ACS Style Guide
• Select a type size of 7–10 points; for ACS journals, select a type size of 5–6
points.
• Make the symbols at least the size of a lowercase letter “oh” (about 2 mm)
(see Box 15-5).
• Use a clear type font, preferably Helvetica or Times Roman (see Box 15-6).
• Disparities between the size of the type and the symbols make for illegible
or unattractive art; if the type is the right size, the symbols will appear too
small or too large, and vice versa. Likewise, the proportion between the
overall size of the figure and the type and symbols should be appropriate
(see Figure 15-2).
➤ Use simple, common symbols that would not be confused with each other
and would be readily available in any publishing house, for example, , , , ,
, , , , , , +, ×, , and . (Even if you provide a legend within the
figure, you may wish to refer to the symbols in your text.)
Box 15-5. Type Size and Font
In publishing, type is measured in points; space is measured in picas.
There are 72 points to an inch; there are 6 picas to an inch. The size of
the type you are reading on this page is 10 points. The column is 28 picas
wide, and the text page is 44 picas long.
You can use a pica ruler to measure space, but it is hard to find a type
gauge for measuring type; you must compare it to known type sizes. For
example, in 10 point type, no character actually is 10 points high.
The font (or typeface) is the style or design of the letters. There are
literally hundreds of fonts, but plain, simple fonts such as Helvetica or
Times Roman are best for scientific art.
Type also comes in different weights. Most of the type you are read-
ing is lightface; this is boldface type. Type may be italic or roman. Gen-
erally, you should use lightface, roman type for figures.
This is 12 point Times Roman.
14C6H6 shows subscripts and superscripts.
This is 12 point Helvetica.
14C6H6 shows subscripts and superscripts.
This is 14 point Times Roman.
14C6H6 shows subscripts and superscripts.
This is 14 point Helvetica.
14C6H6 shows subscripts and superscripts.
➤ ➤ ➤ ➤ ➤

Chapter 15: Figures ➤ 357
Box 15-6. Tips Regarding Fonts
The fonts used to create some types of electronic figure files occasional-
ly cause problems for publishers, in both print and Web publications. The
problems can include character substitution, distorted spacing between
letters, and copyright infringement.
The software behind a font is covered by copyright law. Whereas
some copyright holders allow open access to their fonts, others exercise
some sort of limit. Publishers respect the copyright on fonts and will not
use a font without proper permission.
Only some electronic files embed font software in the files.
• GIFs, TIFFs, JPEGs, and other pixel-based files embed only the images
of letters, not the font software itself, in their files. Type in these files
can be erased, but it is not truly editable.
• EPS and PDF files must have font software fully embedded to be us-
able. Type in these files is fully editable.
TrueType is a font format designed by Microsoft for maximum leg-
ibility on a computer monitor, and it prints well on dot-matrix, inkjet, and
laser printers. Microsoft permits free use of the fonts it provides (such
as Times New Roman and Arial), but other companies that provide True-
Type fonts may have use restrictions. TrueType fonts perform well in Web
publishing, but they often pose technical problems in print publishing.
TrueType fonts are fine for producing GIFs, TIFFs, and JPEGs, as well as
PDFs intended for the Web.
PostScript is a font format designed for high-end graphic arts. These
fonts are designed for maximum legibility on paper and for maximum
compatibility with high-quality, high-output printing equipment. Post-
Script fonts are generally covered by copyright and have limitations on
their use. They seldom pose technical problems in print publishing, but
the copyright issues may limit their usefulness in Web publishing. Post-
Script fonts are best for EPS files and PDFs intended for print.
OpenType fonts are a relatively new format intended to combine the
virtues of both TrueType and PostScript fonts.
STIX (scientific and technical information exchange) fonts were de-
veloped by a group of six scientific publishers to establish a compre-
hensive set of fonts that contains essentially every character that might
be needed to publish a technical article in any scientific discipline. STIX
fonts are available as a free download, under license, at www.stixfonts.
org. A white paper by Tim Ingolsby on the STIX fonts project is available
at www.aipservices.org/newsroom/white_papers/pdf/STIX-fonts.pdf.
➤ ➤ ➤ ➤ ➤
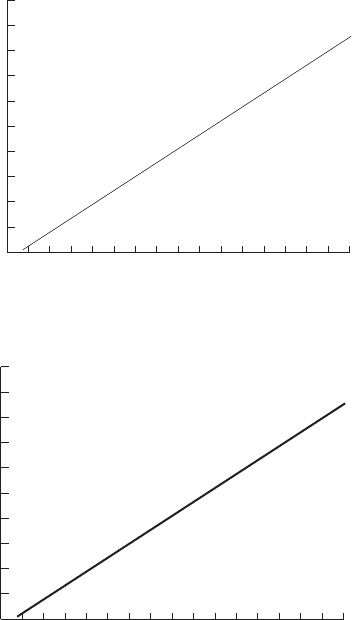
358 ➤ The ACS Style Guide
➤ Lines may be differentiated by the use of varying line styles, such as solid,
dashed, dotted, and various weights (but never less than 0.5 point wide).
➤ You may combine curves plotted on the same set of axes, but do not put more
than five curves in one figure. (If you have more than five curves, consider split-
ting the figure into two parts or two separate figures.) Label all curves clearly.
Leave sufficient space between curves; they should not overlap so much that they
become indistinguishable.
–0.2 –0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4
–2.5
–2.0
–1.5
–1.0
–0.5
0.0
0.5
1.0
1.5
2.0
2.5
Log k
Br
Cl
OTs
F
σ∗
–0.2 –0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4
–2.5
–2.0
–1.5
–1.0
–0.5
0.0
0.5
1.0
1.5
2.0
2.5
Log k
Br
Cl
OTs
F
σ∗
Figure 15-2. (A) Example of a poorly rendered line graph, with lines that are
too thin, a type font that is too ornate, and type and symbols that are sized
disproportionally to the figure. (B) Example of the same line graph properly
rendered.
Source: Adapted from Alunni, S.; De Angelis, F.; Ottavi, L.; Papavasileiou, M.; Tarantelli, F. J. Am. Chem. Soc. 2005,
127, 15151–15160. Copyright 2005 American Chemical Society.
A
B
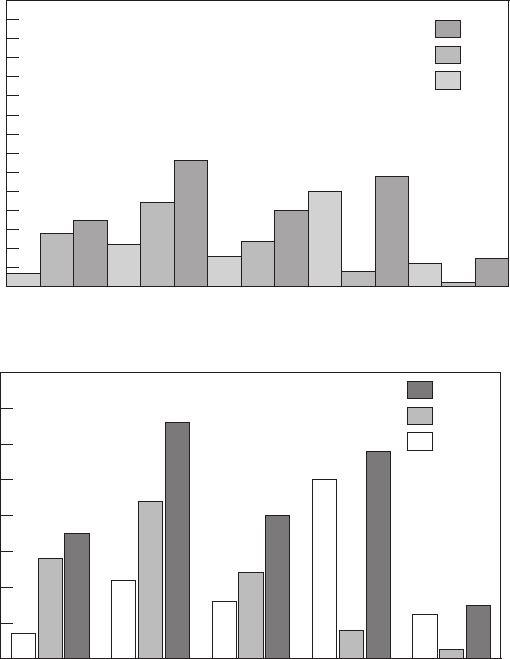
Chapter 15: Figures ➤ 359
➤ Keep line-art figures compact; draw axes only long enough to define the con-
tents. For example, if the highest data point on the curve is 14, then the scale should
extend no longer than 15. Furthermore, the origin or lowest point on the axes does
not have to be zero. For example, if the lowest data point on the curve is 4, then the
axis can start at 3. Put grid marks on the axes to indicate the scale divisions.
➤ Use complete and consistent axis labels (see Figure 15-3).
• Label each axis with the parameter or variable being measured and the
units of measure in parentheses.
0
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
total
wet
dry
SUPERIORMICHIGANLAKE HURONLAKE ERIEONTARIO
LAKES
DEPOSITION
0
1
2
3
4
5
6
7
8
Total
Wet
Dry
SuperiorMichiganHuronErieOntario
Lakes
Deposition (kg/yr)
Figure 15-3. (A) Example of a poorly rendered bar graph, with excess
white space at the top, shading that is difficult to distinguish, and poor and
incomplete labels. (B) Example of the same bar graph properly rendered.
Source: Adapted from Ma, J.; Venkatesh, S.; Li, Y.-F.; Daggupaty, S. Environ. Sci. Technol. 2005, 39, 8123–8131. Copy-
right 2005 American Chemical Society.
A
B
360 ➤ The ACS Style Guide
• Use initial capital letters only, not all capitals: Time (min), Reaction Tem-
perature (°C), Thickness (µm).
• Place all labels outside and parallel to the axes. Numbers and letters on
the abscissa and ordinate should read from left to right and from bottom
to top, respectively.
• Do not place arrowheads on the ends of the axis lines.
• Label the tick marks on an axis in type that is one or two points smaller
than the axis labels (but not smaller than 7 points in general, 5 points for
ACS journals).
➤ Do not draw a box around line-art figures.
➤ For insets, labels, or legends within the figure, use initial capital letters and
use the same size type as for the axis labels.
➤ Save electronic figure files with a minimum of white space around the edges.
➤ If you are printing line-art figures for subsequent scanning, always check that
the toner or ink cartridge is fresh, and never use a dot-matrix printer.
➤ If you print a hard copy to submit with the electronic file, print it with one
figure per page and be sure that the figure number appears on the page.
Working with Photographs and
Other Halftone Figures
Good reproduction of photographs begins with good photographs. When you
are selecting photographs for figures to accompany your text, consider the fol-
lowing points:
➤ As much as possible, the photograph should show what is important to
your text, and only what is important. Clutter and unrelated objects should be
cropped out. (This is easy to do with electronic images.)
➤ Photographs always lose some detail in reproduction, so be sure to take the
photograph in good focus and, for digital photographs, with sufficient resolu-
tion. See Figure 15-4.
➤ Photographs in print will tend to lose contrast and become muddy or gray,
so take a few steps to ensure that your photograph has good contrast and tonal
definition:
• Adequately light objects that you are photographing.
• Place objects on light or dark backgrounds that are plain or solid.
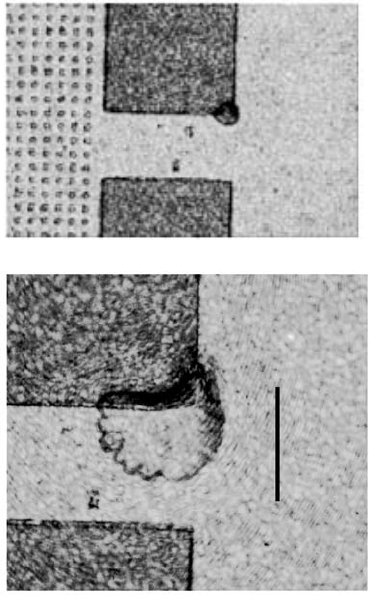
Chapter 15: Figures ➤ 361
• Take several photographs at different settings, so that you can select the
one with the best contrast and tonal definition.
➤ Photographs can have tricky copyright and permissions issues. Be sure you
understand whether permission is needed to publish the photograph. (See the
section called “Common Misconceptions about Copyright” in Chapter 7.)
➤ If you are scanning photographic prints, see Box 15-4.
➤ If you are using a digital camera, select a setting that collects enough digital
information. The lowest setting might be sufficient for GIFs, but the higher set-
tings will be necessary for print publication.
Figure 15-4. (A) Example of a photograph with a resolution that is too low
for print, so the pixels show as squares, instead of blending to show tones. (B)
A similar photograph with a true resolution of 300 ppi.
Source: Adapted from Balss, K. M.; Avedisian, C. T.; Cavicchi, R. E.; Tarlov, M. J. Langmuir 2005, 21, 10459–10467.
Copyright 2005 American Chemical Society.
A
B
362 ➤ The ACS Style Guide
When you are working with the electronic files of photographs and other
halftones, keep the following in mind:
➤ Do not adjust the ppi upward without adjusting the overall dimensions
downward. Simply changing the ppi setting from 72 to 300, even if your software
allows it, does not put digital information in that was not there before. The file
might indicate that the resolution is 300 ppi, but the art will still print as fuzzily
as a 72 ppi picture.
➤ You can adjust the ppi downward without changing the overall dimensions.
This throws away information that is not necessary and makes a file more por-
table if, for instance, you scanned at a higher resolution because you were not
sure how much you would crop it.
➤ You can switch from RGB to CMYK color modes and vice versa.
➤ If you switch from either RGB or CMYK to B&W, you will probably need to
adjust the contrast of your photograph to get the black areas black and the white
areas white.
If you are providing photographic prints instead of electronic files, the fol-
lowing points are important to remember:
➤ Submit a good-quality photographic print, preferably not the only original
you have. Some publishers try to return original art, but many do not.
➤ Label photographs by writing on an adhesive label (such as a mailing label)
first and then applying the label to the back of the photograph, or by writing on
a separate sheet of paper and taping it to the back. Writing directly on the back
of the photographic print might damage the front: ballpoint pens can break the
smooth surface of the photograph, and felt tip pens can bleed through.
➤ Handle photographs with care. Most flaws on a photographic print will be
reproduced in the published photograph. You can damage photographs by paper
clipping, folding, stapling, or using tape on them. Depending on the amount and
location of the damage, you might have to reprint the photograph.
➤ Do not submit prescreened illustrations, that is, illustrations that were scanned
from a print source (which is already screened) or scanned using a screen setting.
➤ Do not submit color prints to be reproduced in black and white. Use a pro-
fessional photo lab to prepare B&W prints from color originals because photo
labs use special paper to make B&W prints from color originals.
Chapter 15: Figures ➤ 363
Working with Grayscale Line Art
Grayscale line art for print publication of such figures as pie charts, bar graphs,
and area graphs should be submitted only in an electronic format, preferably EPS.
EPS files use mathematical formulas to produce lines and shading, so lines and
type are never screened, and gray areas are screened only when the printing plates
are made. (Rescreening screened art creates a distortion called moiré.) As a result,
the lines in EPS files remain crisp even when the gray tones have been screened.
➤ Always fully embed the fonts or the font outlines in an EPS file. Otherwise,
a different font may be substituted at the publisher’s, with unsatisfactory results.
Be sure that you have rights to embed the font—you will be safest if you use
Times Roman or Helvetica.
➤ Use screen shadings that are different by at least 20 percentage points. For
example, you could fill in the sections of a bar graph with 10%, 30%, 50%, 70%,
and 100% black. (0%, 20%, 40%, 60%, 80%, and 100% also work well.)
➤ If you choose to screen lines within a line graph (never the axes), assign the
lines a 1.5 or 2 point weight, and do not use symbols to mark points on the lines.
Working with Combinations
of Type and Photographs
As mentioned in the earlier section, “Types of Figure Artwork”, a photograph that
has a simple measuring scale or a few single-letter labels can be adequately handled
like other halftones. However, if the photograph (or other halftone artwork) must
be accompanied by text to convey meaning, then you should prepare a combo.
One way to do this is to prepare the photograph as a grayscale or color TIFF
and then import the photographic image file into a drawing program, such as
Illustrator or Freehand. Use the drawing program to add type, lines, axes, tick
marks, and so on, and save the file as an EPS with an embedded TIFF.
An alternative is to create the combo in a word-processing program, such as
Word or WordPerfect, and then export the page as a PDF file. Simply assembling the
type and photograph in the word-processing program and submitting the applica-
tion (.doc or .wpd) file is seldom acceptable to publishers other than ACS journals.
How To Submit Illustrations
If you follow the recommendations presented in this chapter, you should be well
on your way to trouble-free submission of your figure files. Keep the following
points in mind for submitting to publishers other than ACS journals.
364 ➤ The ACS Style Guide
Publishing with ACS: For manuscripts submitted to ACS journals
through Paragon, illustrations should always be embedded in the text
document before submission—that is, illustrations should not be sub-
mitted as separate files from the text. Illustrations submitted through
the Paragon Plus environment can be embedded in the text or supplied
separately. Before being embedded, however, the illustrations should be
prepared outside the word-processing program to the correct size and
resolution as recommended in this chapter. Each illustration should be
“inserted” (not “pasted”) into the text document so that it maintains its
original resolution, and it should not be adjusted for size after insertion.
Graphics taken from Web sites should not be used because, at 72 ppi,
their resolution is not adequate for print publication.
For All Figures
➤ Submit a clean photocopy or printout of all figures to accompany your man-
uscript.
➤ Unless a publisher instructs otherwise, do not put the figures in the midst of
text; put them on separate pages at the end.
➤ Put one figure on each page, and label the page with the figure number only.
Submit captions as part of your text.
For Electronic Files
➤ Be sure that the art is oriented correctly in the file.
➤ The file name should be brief and consistent, preferably something like
Smith_fig02.tiff or Jones_chap5_fig10.eps.
➤ Unless the publisher requests that you submit via a Web site, write the files
to a CD, DVD, Zip disk, or 3.5 in. floppy disk. Label the disk with the author
name, manuscript title, and general contents, such as manuscript number, fig-
ures, tables, and so on.
For Camera-Ready Art
➤ Note the figure number and the first author’s surname either on the back or
on the front of each piece of artwork, about 1 in. (2.5 cm) clear of the image area.
Do not write on the front or back of the image area; write only in the margins.
➤ Indicate the top of the illustration with the word “top” if the correct orienta-
tion is not obvious.
Chapter 15: Figures ➤ 365
➤ Do not fold or roll artwork or photographs; protect art with cardboard or
heavy paper for transport. Do not staple, clip, or punch holes in photographs or
any artwork.
How To Prepare Figure Captions
Every figure must have a caption that includes the figure number and a brief,
informative description, preferably in nonsentence format.
good examples of figure captions
Figure 2. Mass spectrum obtained when laboratory ambient air containing 2.5
ppm of 1 was introduced into the MS system.
Figure 4. Change in carotenoid contents during maturation of three varieties of
grapes: (A) Concord grapes; (B) Thompson seedless; and (C) Chilean red.
Figure 6. Variable-temperature NMR spectra of 3d in CD2Cl2 solution at 500
MHz.
Figure 7. Reaction rate constants as a function of proton affinity for the reactions
shown in eqs 5–7: kexp, experimental; kc, calculated.
Figure 1. Specificity of bovine muscle LDH antibodies in a sandwich ELISA. Data
represent the averages of three replicates.
If more information is necessary, use complete sentences and standard punctua-
tion. The caption should be understandable without reference to the text (this
is essential in Web publishing because the figure may open in a separate frame)
and should not include material that is at odds with the text. Use similar wording
for captions of related figures.
If a figure contains symbols that require long explanations or has more than
four or five symbols, then the key to the symbols will be large and give the artwork
a cluttered appearance. In this case, put the key to the symbols in the caption.
Make sure that the symbols and abbreviations in the caption agree with those in
the figure itself and in the text and that the symbols are typographically available.
Submit the figure captions separately from the artwork, typed double-spaced
on a page at the end of the text. Verify that the numbers in the caption agree with
the numbers on the figures and in the electronic file names. Bear in mind that
figures and captions are handled differently in both Web and print production;
captions are usually typeset in a font and size that follow the style of the publica-
tion, whereas figures are used as is. If you place the caption on the art, the editors
usually will delete it and have the caption typeset according to the publication’s
style. At best, this creates additional work and an opportunity for errors to occur;
at worst, the presence of captions with the text and on the art may cause confu-
sion if they are inconsistent.
366 ➤ The ACS Style Guide
Reproducing Previously Published Materials
To reproduce a figure, photograph, or table that has been published elsewhere,
you (the author) must obtain permission in writing from the copyright owner
(usually the publisher), and you must submit the written permissions along with
your final manuscript. Even if you were the author of the previously published
figure or table, you still need written permission from the copyright owner. The
only exception is for a work of the U.S. government. See Box 15-7 for examples
of credit lines. See also Chapter 7, “Copyright Basics”, for further information.
➤ If you construct a figure or a table from data that were previously published
as text or use data from a table to create an original figure, you do not need per-
mission, but you should reference the source of the data (e.g., “Data are from ref
7.”). However, if you are using a portion of a table that has been previously pub-
lished, even very small portions such as a few data points, permission is needed
from the copyright holder.
➤ If you adapt or use only part of a figure or table, permission is still needed.
The credit line for adapted material is similar to the credit lines in Box 15-7
except “reprinted” is replaced with “adapted”.
Adapted with permission from ref XX. Copyright Year Copyright Owner’s
Name.
Adapted with permission from Author Names (Year of Publication). Copyright
Year Copyright Owner’s Name.
➤ If you are thinking about using a previously published figure or table, con-
sider carefully whether citing it as a reference would be adequate.

Chapter 15: Figures ➤ 367
Box 15-7. Credit Lines in ACS Publications
In ACS publications, credit lines for art reproduced from previously
published work appear at the end of the caption in parentheses in
one of two formats and follow three possible wordings, depending
on the original source:
Format 1
Most publishers: Reprinted with permission from ref XX. Copyright Year
Copyright Owner’s Name.
Reprinted with permission from ref 10. Copyright 2003 American
Pharmaceutical Association.
Published by ACS: Reprinted from ref XX. Copyright Year American Chem-
ical Society.
Reprinted from ref 12. Copyright 2005 American Chemical Society.
Published by the U.S. government: Reprinted from ref XX.
Reprinted from ref 23.
Format 2
Most publishers: Reprinted with permission from Author Names (Year of
Publication). Copyright Year Copyright Owner’s Name.
Reprinted with permission from Camiola and Altieri (2006). Copyright
2006 American Institute of Physics.
Published by ACS: Reprinted from Author Names (Year of Publication).
Copyright Year American Chemical Society.
Reprinted from Fitzgerald and Cheng (2004). Copyright 2004 Ameri-
can Chemical Society.
Published by the U.S. government: Reprinted from Author Names (Year
of Publication).
Reprinted from Takanishi and Schmidt (2006).
Be sure to check the author guidelines for your publication.
369
➤ ➤ ➤ ➤ ➤ CHAPTER 16
Tables
Betsy Kulamer
This chapter presents guidelines for preparing the tables that
accompany a scientific paper for publication. Tables are
handled in many ways like figures, so this chapter focuses on the ways in which
tables are different and briefly discusses the preparation of tables using word-
processing programs.
When To Use Tables
Use tables when the data cannot be presented clearly as narrative, when many
precise numbers must be presented, or when meaningful interrelationships can
be better conveyed by the tabular format. Tables should supplement, not dupli-
cate, text and figures. (If you are not sure whether you need a table or figure,
see Box 15-1.) Examples of material that is best handled as narrative in text are
results of IR absorption and NMR chemical shift studies, unless they are major
topics of discussion. In many instances, one table with representative data, rather
than several tables, is all that is needed to convey an idea.
How To Cite Tables
Like figures, all tables must be called out, that is, mentioned or discussed by
name and number in the text.
➤ Capitalize the word “Table” when it is followed by the table number.
➤ Number tables sequentially with arabic or roman numerals, depending on
the publication’s style, in order of discussion in the text: Table 2 or Table IV.
Copyright 2006 American Chemical Society
370 ➤ The ACS Style Guide
➤ Discuss tables sequentially, so that Table 1 is discussed before Table 2, Table 2
before Table 3, and so on.
For good examples of a callout in text, see Chapter 15, Figures, pp 345–346.
How To Prepare Tables
There are two kinds of tables: informal (or in-text) and formal. An informal table
consists of three to five lines and is no more than four columns wide; it cannot
exceed the width of a text column. Informal tables may be placed in text follow-
ing an introductory sentence, and each column should have a heading. They are
not given titles or numbers, nor do they contain footnotes.
A formal table should consist of at least three interrelated columns and three
rows. If you have only two columns, try writing the material as narrative. If you
have three columns, but they do not relate to each other, perhaps the material is
really a list of items and not a table at all (see the discussion of lists at the end
of this chapter). If your table has unusual alignment and positioning require-
ments, perhaps it should really be a figure. It is important to understand these
differences because tables are more expensive to produce than text; the larger
the table, the higher the cost. A well-constructed, meaningful table is worth the
expense, but anything else is wasteful and does not enhance your paper.
Tables should be simple and concise; arrange all data for optimal use of space.
If you have many small tables, consider combining some. Combining is usually
possible when the same column is repeated in separate tables or when the same
type of material is presented in several small tables. Use consistent wording for
all elements of similar or related tables. Be consistent with symbols and abbre-
viations among tables and between tables and text.
➤ The table width will depend on the widths of its individual columns.
• Generally, tables having up to 6 columns will fit in a single journal col-
umn; tables having up to 13 columns will fit in the double-column spread.
Tables that exceed the double-column spread will be rotated 90° and set
lengthwise on the page.
• In books, tables having up to 8 columns can fit in the page width; tables
having 9–12 columns will be set lengthwise on the page. Larger tables can
span two pages.
• In all publications, extremely wide tables can cause composition difficulties.
In such cases, consider presenting the material as two or more smaller tables.
➤ The style for the individual parts of tables (i.e., the use of capital and low-
ercase letters and whether the entries are centered or flush left) varies among
publications. Consult a recent edition of the journal or the journal’s instructions
for authors.
Chapter 16: Tables ➤ 371
➤ Keep sections of multipart tables at similar widths. Widely divergent section
widths within a table waste space and detract from general appearance.
Effective tables are well-designed, so think carefully, first, about the data you need
to present and, second, about the best way to present it visually on a page. Some-
times, what looks fine on a letter-size sheet of paper is not practicable for a journal
or book page. Sometimes, what you originally conceived as the column headings
works better as the row headings. (In general, you should have more row headings
than column headings.) Understanding the parts of a table will help you design
your tables effectively; they are identified in Figure 16-1.
Title
➤ Give every formal table a brief, informative title that describes its contents
in nonsentence format. The title should be complete enough to be understood
without referring to the text. Place details in table footnotes, not in the title.
➤ Begin the table title with the word “Table” and its number, and then continue
with the title.
Column Headings
Every column must have a heading that describes the material below it. A col-
umn heading should not apply to the entire table; information that describes all
of the columns belongs in a general table footnote. If a column heading applies
to more than one column, use a rule below it that spans the columns to which
it applies; this is called a straddle rule. Below the rule, give the specific headings
for each column. A unit of measure alone is not an acceptable column heading,
unless the column heading appears under a straddle rule.
➤ Be as succinct as possible, keep column headings to two lines if possible, and
use abbreviations and symbols whenever practical.
➤ Be consistent with the text and with other column headings.
➤ Define nonstandard abbreviations in table footnotes. Name the variable being
measured, and indicate the unit of measure after a comma or slash or within
enclosing marks. Use the same style within and among all tables.
Column Entries
In many tables, the leftmost column is the stub or reading column. Usually, all
other columns refer back to it. Stub entries should be consistent with the text as
well as logical and grammatically parallel. Main stub entries may also have sub-
entries, which should be indented.
372 ➤ The ACS Style Guide
➤ Material in columns can be aligned in various ways; use only one type of
alignment per column. Words are usually aligned on the left, and numbers are
usually aligned on the decimals, unless they do not have the same units, in which
case they are aligned on the left. Use numbers on both sides of a decimal point;
numbers less than 1 should have a zero to the left of the decimal point. Columns
that are made up of numbers and words together or columns that contain a vari-
ety of sizes or types of information might call for alignment on the left, right, or
center, depending on the publication’s style.
➤ Do not use ditto marks or the word “ditto” to indicate the same entry in a
column; repeat the entry.
➤ Define nonstandard abbreviations in table footnotes.
➤ Try to keep all entries at similar lengths by placing any explanatory material
in table footnotes. If you use a dash as a column entry, explain it in a footnote the
first time it is used (e.g., “—, too low to be measured.”).
➤ Make sure that all of the columns are really necessary. If there are no entries
in most of a column, it probably should be deleted and replaced with a general
Figure 16-1. Parts of a table.
Source: Adapted from Chen, D.; Yang, C. Q.; Qiu, X. Ind. Eng. Chem. Res. 2005, 44, 7921–7927. Copyright 2005
American Chemical Society.
Table
title
Column
headings
Straddle headStub column
Table text
Table
footnote
Table 2. Conditioned WRA and Mechanical Strength of Plain-Weave Cotton
Fabric Treated with Different Cross-Linking Agentsa
Cross-Linker
Concentration
Catalyst
Concentration
Curing
Condition
WRA (deg, w + f) No.
of Laundering Cycles Flex Abrasion Retention
(no. of cycles, warp)1 5 10 20
Unmodified
8% PMA
Dried 2% NaH2PO2180 ˚C,
1.5 min
273 261 261 260 309
Soaked 3% NaH2PO2182 ˚C,
1.3 min
274 260 260 262 310
6% BTCA 4% NaH2PO2 180 ˚C,
1.5 min
287 276 273 270 148
Modified
10.5% modified
DMDHEU
precatalyzed 165 ˚C,
1.5 min
278 273 269 264 68
control 180 ˚C,
1.5 min
190 868
aThe concentrations of PMA, BTCA, and NaH2PO2 are calculated on the basis of 100% active ingre-
dient; the concentration of DMDHEU is based on the weight of the commercial product, which con-
tains 55% solid. The wet pickup of the treated fabric is approximately 105–110%.
Chapter 16: Tables ➤ 373
table footnote. Alternatively, if the entries in the entire column are the same, the
column should be replaced with an appropriate table footnote, such as “In all
cases, the value was x.”
Footnotes
Table footnotes include explanatory material referring to the whole table and to
specific entries. Examples of information that should be placed in general foot-
notes referring to the whole table are the following: units of measure that apply
to all entries in the table, explanations of abbreviations and symbols used fre-
quently throughout the table, details of experimental conditions if not already
described in the text or if different from the text, general sources of data, and
other literature citations.
Information that should be placed in specific footnotes includes units of meas-
ure that are too long to fit in the column headings, explanations of abbreviations
and symbols used with only one or two entries, statistical significance of entries,
experimental details that apply to specific entries, and different sources of data.
In some publications, such as books, general footnotes and sources are not
cited with superscripts; they are labeled “Note” and “Source”, respectively. Spe-
cific footnotes are cited with superscripts. In other publications, all footnotes are
cited with superscripts. Check the directions for the publication to which you are
submitting your paper.
➤ Where superscripts are needed, use superscript lowercase italic letters in alpha-
betical order, starting from the top of the table and proceeding from left to right.
➤ Write footnotes as narrative and use standard punctuation. Short phrases
such as “ND, not determined.” and “x = 23.” are acceptable.
➤ Label each footnote with its superscript letter and group the footnotes together
at the end of the table. All footnotes must have a callout in the table title or text.
Using Word-Processing Software
When you prepare your tables using word-processing software, a few techniques
ensure a smoother transition to either Web or print publication.
➤ In Microsoft Word or WordPerfect, use the software’s table feature, rather than
aligning columns using the tab key. Entries arranged with the table feature are more
likely to be properly aligned in publication than entries that have been tabbed.
➤ Set up the table in 10 or 12 point type, although 8 point type can be used if
necessary. If you need to use type smaller than 8 points to fit your table on a let-
ter-size page, it probably will not fit comfortably on a book or journal page.
➤ Double-space the text in the table.

374 ➤ The ACS Style Guide
➤ When you use the table feature, put only one row of entries in each row of
the table. Do not put multiple entries in a single cell by using the hard return.
➤ Avoid using hard returns to add space between rows of the table. If you wish
to show more space than is apparent with double-spacing, use the line format-
ting feature of the word-processing program instead.
How To Submit Tables
If you follow the recommendations presented in this chapter, you should experi-
ence trouble-free submission of your tables. Keep the following points in mind:
➤ Place formal tables after the references at the end of the text file, each on its
own page.
➤ Place informal tables in place within the text.
➤ Submit a printout of tables along with the printout of text if the publisher
requests one.
➤ If a table must contain structures or other art or special symbols, or if a table
has special alignment and positioning requirements, be sure that these are evi-
dent on the printout.
Publishing with ACS: In manuscripts submitted to ACS journals through
Paragon, tables should always be embedded in the text document before
submission, that is, tables should not be submitted as separate files from
the text. Tables in manuscripts submitted through the Paragon Plus envi-
ronment can be embedded in the text or supplied separately.
When To Use Lists
Sometimes you may need to give numerous examples of items, such as chemical
names. In such cases, if there are too many to run into the text, they can be set
as a list in some publications. Put the entries in alphabetical order, unless there
is a reason to do otherwise. A list of names is not truly a figure and not really a
table. Give the list an unnumbered title. In ACS journals, lists may be handled as
informal tables or even as charts.
potentially carcinogenic medicines
azacitidine cyclophosphamide methotrexate
azathioprine cytarabine nitrofurazone
chloramphenicol dacarbazine phenacetin
chlornaphazine fluorouracil phenoxybenzamine
375
➤ ➤ ➤ ➤ ➤ CHAPTER 17
Chemical
Structures
Antony Williams
The communication of chemical structures is inherently
visual, so the language of both chemistry and biochemis-
try would be sparse without the ability to accurately represent chemistry in a
graphical format. Scientific manuscripts today benefit from the visually appeal-
ing output of software used to generate structure representations conveying the
details of molecular connectivity as well as the details associated with chemical
structures, reactions, and schemes. This chapter discusses the use of chemical
structures; the related reactions, charts, and schemes, in a scientific manuscript;
and methods of preparing and submitting structures for publication. The chap-
ter presents general guidelines; authors should consult with their publishers for
specific instructions.
When To Use Structures
A chemical structure is a pictorial representation of the bonding of atoms in a
molecule. Chemical structures appear within text at the point at which they are
discussed. Structures are numbered sequentially with either arabic or roman
numerals; consult the publisher’s guidelines to determine if one is preferred over
the other. Generally, there is no need to provide graphical representations of
structures for materials that can be accurately represented on one line or in the
form of text. For scientific publications, structures should be included for clarity
of communication only.
For example, papers describing previously unreported syntheses or reaction
sequences, structure–activity relationships, or newly discovered chemical com-
pounds make good use of chemical structure images. Simple chemical struc-
Copyright 2006 American Chemical Society
376 ➤ The ACS Style Guide
tures can often be represented by a line formula, such as C6H5COOH or 1,2,4-
Br3C6H3, or by the systematic name of the compound, such as benzoic acid or
1,2,4-tribromobenzene, but complex chemical structures depend on structural
representations exhibiting the atom–atom connectivity, including the order and
stereochemistry of the bonds. Even though a systematic name can accurately
describe the chemical structure of paclitaxel, specifically, (2aR,4S,4aS,6R,9S,
11S,12S,12aR,12bS)-6,12b-diacetoxy-9-{[(2R,3S)-3-(benzoylamino)-2-hydroxy-
3-phenylpropanoyl]oxy}-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-2a,3,4,4a,
5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-
b]oxet-12-yl benzoate), most chemists would prefer to visualize the structure
(shown in Figure 17-1).
✐Reminder: Simple chemical structures can often be represented by a line
formula or by the systematic name of the compound, but complex chemical
structures depend on structural representations exhibiting the atom–atom
connectivity, including the order and stereochemistry of the bonds.
A chemical reaction is a pictorial representation of a change or process. Reac-
tions can be represented with either line formulas or structures; they are placed
in text and numbered much like mathematical equations. See pp 272–274 of
Chapter 13 for information on how to format chemical reactions.
Groups of structures are called charts. Charts contain representations of mul-
tiple structures to facilitate discussion of them. Groups of reactions are called
schemes; as shown in Figure 17-2, several reactions can be presented together to
show, for example, a step-by-step process. Schemes show action; charts do not.
How To Cite Structures
➤ Structures are identified in text with boldface numerals (either arabic or
roman), boldface alphabet letters (capital or lowercase), or a combination of
NH
O
O
O
O
OO
O
O
O
OH O
O
OH
OH
H
Figure 17-1. Example of a chemical structure.
Chapter 17: Chemical Structures ➤ 377
these. (These numbers should also appear as labels on the structures themselves.)
For several structures or for a series of structures, use a consistent sequence of
labels. Typical series may include the following:
1, 2, 3
I, II, III
A, B, C
a, b, c
1a, 1b, 1c
Ia, Ib, Ic
1A, 1B, 1C
IA, IB, IC
If a compound is mentioned several times within the text, refer to it only by its
label (e.g., “as shown by the reaction with 9”). Do not include or label structures
that are not discussed in the text.
➤ Chemical reactions set as equations should be labeled with lightface roman
letters, numerals, or combinations in parentheses in the right margin (see Chap-
ter 13). Depending on the text, it may be appropriate to use one set of labels
for both chemical reactions and mathematical equations, or two separate and
distinct numbering sequences (for instance, eqs 1, 2, 3, … and reactions I, II, III,
…). The numbering sequences for structures and for reactions should always
remain separate.
✐Reminder: If a compound is mentioned several times within the text,
refer to it only by its label.
➤ Charts are numbered consecutively as Chart 1, Chart 2, and so on, with either
arabic or roman numerals. Charts should be labeled with “Chart” and a number;
they also may have brief titles and footnotes.
H
Ph Ph
O
Ph
H Ph
O
Sn (Closed form) S0
* (EZZ, ZEZ, EEZ)
hν
<2 ps
∆
∆
∆
≈ 20 ps
OPh
Ph
Closed form
Ph
Ph
O
H
EZZ (sub ns)
EEZ (48 µs)
Major isomer
ZEZ (730 ns)
∆
hν
EZE, ZEE, EEE
(>1 ms)
Scheme 1
Figure 17-2. Example of a scheme representing a group of reactions.
Representing work reported by Kodama, Y.; Nakabayashi, T.; Segawa, K.; Hattori, E.; Sakuragi, M.; Nishi, N.; Sakuragi,
H. J. Phys. Chem. A 2000, 104, 11478.
378 ➤ The ACS Style Guide
➤ Schemes are numbered consecutively as Scheme 1, Scheme 2, and so on, with
either arabic or roman numerals. Schemes should be labeled with “Scheme” and
a number; they also may have brief titles and footnotes.
How To Prepare Structures
The quality and size requirements for structures (and, by extension, reactions,
schemes, and charts) are similar to those for other illustrations (see Chapter 15).
The requirements associated with the representation of structures are supported
by most of the software programs for drawing chemical structures (described in
Box 17-1). The following guidelines should be used when creating the graphics
for chemical structures.
➤ Arrange structures in horizontal rows within the width of a column.
➤ Make the size of the rings and the size of the type proportional. The pub-
lished size of six-membered rings should be approximately ¼ in. (6.35 mm) in
diameter; the published size of five-membered rings should be slightly smaller.
The type size should be 5–8 points.
➤ Keep oddly shaped rings and the shapes of bicyclic structures consistent
throughout a manuscript. In multiring structures such as steroids, use partial
structures that show only the pertinent points.
➤ In three-dimensional drawings, use dashed lines for lines in the background
that are crossed by lines in the foreground to give a greater three-dimensional
effect. Make lines in the foreground heavier, as shown below.
➤ Center the compound labels (either numbers or letters) just below the struc-
tures. If a series of related compounds are being discussed, draw only one parent
structure, use a general designation (e.g., R or Ar) at the position(s) where the
substituents differ, and specify modifications below the structure.
✐Reminder: Take care that the structure graphic inserted into your text
file is of sufficient resolution, that is, 300 ppi minimum, with 600 or 1200
ppi even better.
➤ For structures mentioned in tables, provide labeled structures as separate art-
work (in text or charts), and use only their labels in the table.
Chapter 17: Chemical Structures ➤ 379
➤ Center the reaction arrows vertically on the midline of the structure height
and align the centers of all structures. The midline for all structures is the center
of the “tallest” structure on the same line.
An example of a correctly drawn reaction is represented below. As shown, the
arrow and the one-line structure are centered, top to bottom, in the height of
the full structure. The reaction arrow and the plus sign have an equal amount of
space on both sides.
R4
O
O
N
OH NH2R' R4
O
N
N
H
R'
+
➤ Do not waste space, either vertical or horizontal. Use the full column width
before starting a new line. A compact presentation is most effective. Avoid using
vertical arrows unless it is necessary to portray a cyclic or “square” reaction
scheme. Schemes generally read from left to right. As long as the proper sequence
is maintained, it does not matter on which line any given structure appears. If
a reaction continues to the next line, keep the arrow or other operator on the
top line. Refrain from the use of double-column-width charts or schemes unless
absolutely necessary because these charts and schemes consume a significant
amount of space.
➤ Do not place circles around plus or minus signs.
➤ For most chemical structure drawing programs, it works best to use the
copy-and-paste feature to insert the structure drawing into a Microsoft Word
document. However, you should take care that the structure graphic inserted
into your text file is of sufficient resolution, that is, 300 ppi minimum, with 600
or 1200 ppi even better. The author bears the responsibility to ensure the appro-
priate insertion.
Software for Creating Chemical Structures
A number of chemical structure drawing and rendering programs are available
to create structure images of excellent quality and accuracy for inclusion in sci-
entific publications. The most popular commercial structure-drawing packages
today include ISIS/Draw, ChemDraw, and ChemSketch (see Box 17-1). Other
commercial packages include ChemWindow (now known as DrawIt) and Chem-
istry 4-D Draw. At this time, Web-based applets do not support the production
of graphical output of sufficient quality for most books and journals; other soft-
ware, such as JMol, should be considered as structure-rendering engines only.
Box 17-2 contains parameter settings for ACS publications.
380 ➤ The ACS Style Guide
The cost of these software packages can vary considerably based on academic
or industrial usage. Fortunately, the cost should not be a barrier to the inclu-
sion of appropriate renderings of chemical structures because several freeware
structure-drawing packages are now available for download from the World
Wide Web. These include freeware versions of both ISIS/Draw and ChemSketch.
A detailed comparison of capabilities for both the freeware and commercial
versions of the packages has been made by Tamas Gunda (http://dragon.klte.
hu/~gundat/rajzprogramok/dprog.html). This review is an objective compari-
son, updated on an almost annual basis, of the capabilities of five popular draw-
ing packages [ISIS/Draw, ChemDraw, DrawIt (ChemWindow), ChemSketch,
and Chemistry 4-D Draw].
✐Reminder: The cost should not be a barrier to the inclusion of appropri-
ate renderings of chemical structures because several freeware structure-
drawing packages are now available for download.
It is preferable to use one of the drawing packages that offer journal-based
templates containing the appropriate bond widths, bond lengths, fonts, and
Box 17-1. Software for Drawing Chemical
Structures
The following is a selection of commercially available software for draw-
ing chemical structures suitable for publication.
ChemDraw: Cambridgesoft, 100 CambridgePark Drive, Cambridge, MA
02140. Commercial: http://www.chemdraw.com.
Chemistry 4-D Draw: Cheminnovation, 7966 Arjons Dr, #A-201, San Diego,
CA 92126. Commercial: http://www.cheminnovation.com/products/
chem4d.asp.
ChemSketch: Advanced Chemistry Development, 110 Yonge Street,
14th Floor, Toronto, Ontario M5C 1T4, Canada. Commercial: http://
www.acdlabs.com/products/chem_dsn_lab/chemsketch/. Freeware:
http://www.freechemsketch.com.
DrawIt (formerly ChemWindow): Bio-Rad, Informatics, Sadtler Group,
3316 Spring Garden Street, Philadelphia, PA 19104-2596. Commercial:
http://www.knowitall.com/.
ISIS/Draw: Elsevier MDL, 14600 Catalina Street, San Leandro, CA 94577. Com-
mercial: http://www.mdli.com/products/framework/isis_draw/index.jsp.
Freeware: http://www.mdli.com/downloads/downloadable/index.jsp.
MDL will ultimately discontinue ISIS/Draw and replace it with Mol Draw.
➤ ➤ ➤ ➤ ➤
Chapter 17: Chemical Structures ➤ 381
other settings recommended by a particular journal. Using these templates aids
in the production of a chemical structure drawing acceptable to the publishers.
How To Submit Chemical Structures
Many common graphical formats can be generated using structure-drawing
packages, including TIFF, GIF, and BMP; follow the guidelines for submitting
figures presented in Chapter 15. Also, because file formats continue to develop
and the preferences of a given publisher can change, authors should check with
the publisher’s or journal’s author guidelines for acceptable software and file for-
mats. Box 17-3 shows guidelines for submitting structures to ACS journals.
Box 17-2. Parameter Settings for
ACS Publications
At present, ACS journals request that chemical structures be
prepared according to the guidelines below. (The guidelines are
also available at https://paragon.acs.org/paragon/index.jsp. Click
on “Read Author Information”, and then select the appropriate jour-
nal for specific information.) The parameters below are specifically
for ChemDraw, using the ACS 1996 document settings; authors
using other drawing packages should adapt these parameters
to their systems. Most commercial and freeware packages allow
these settings to be reproduced either by manual settings or by
some sort of journal template feature.
Drawing Settings chain angle 120 degrees
bond spacing 18% of width
fixed length 14.4 pt (0.2 in.)
bold width 2.0 pt (0.0278 in.)
line width 0.6 pt (0.0083 in.)
margin width 1.6 pt (0.0222 in.)
hash spacing 2.5 pt (0.0345 in.)
Text Settings page setup US/Letter/Paper
scale 100%
font Helvetica (Mac), Arial (PC)
size 10 pt
Preferences units points
tolerances 3 pixels
382 ➤ The ACS Style Guide
The visual appeal of a structure or reaction can differ from one software
package to another, so authors may wish to interchange data between pro-
grams to obtain the best graphical display required for publication. Such
an interchange is normally performed using a specific format of the atom–
atom connection table known as a molfile. (Information on the MDL molfile
format is available at http://www.mdl.com/downloads/public/ctfile/ctfile.pdf.)
Such collections may, for example, describe molecules, molecular fragments,
substructures, substituent groups, polymers, alloys, formulations and mixtures,
and unconnected atoms. Most chemical structure drawing software packages
available today allow both import and export of a molfile. An alternative generic
file format is CML, the chemical markup language, which takes advantage of
the strength of Web-based technologies and is discussed in Chapter 8.
The Future of Representing Chemical Structures
Despite the vast array of tools available today for structure drawing and repre-
sentation, each tool is lacking in some way for the representation of complete
structure space. Small organic molecules have been well supported, but the needs
Box 17-3. Guidelines for Submitting Chemical
Structures to ACS Journals
• In manuscripts submitted to ACS journals through Paragon,
structures should be embedded in text documents. In manu-
scripts submitted through the Paragon Plus environment,
structures can be embedded in the text or supplied separately.
• Submit graphical images of chemical structures to ACS jour-
nals as embedded TIFF files, using a resolution of 300 pixels per
inch (ppi) for color and 1200 ppi for black-and-white images.
• ACS journals accept application files (native formats) for several
common software programs, including .skc files from ISIS/Draw
and .cdx files from ChemDraw.
• Information on submitting chemical structure connection tables
is available at https://paragon.acs.org/paragon/application?
pageid=content&mid=preferredsoftware.html&parentid=
authorchecklist&headername=Preferred%20Software.
• CML is accepted by the ACS Paragon System for submission of
supporting information.
Chapter 17: Chemical Structures ➤ 383
of the inorganic, organometallic, and polymer chemist have commonly lagged
behind. Chemical structure drawing programs will continue to develop version
by version as software vendors deliver an ongoing array of functionality for their
customers.
The need to introduce additional flexibility for structure representation
will be ongoing, specifically as standards are set for preferred drawing styles by
organizations such as IUPAC. A scoping exercise initiated by the IUPAC com-
mission has defined the preferred drawing styles for structure representation to
ensure accurate communication of chemical structures. (See http://www.iupac.
org/projects/2003/2003-045-3-800.html. The stereo part of this project is now
available as provisional recommendations at http://www.iupac.org/reports/
provisional/abstract05/brecher_310705.html.) It is likely that publishers will
embrace the opportunity to standardize the structure representations within
their publications to try to achieve greater homogeneity.
Whereas chemical structures will always be the primary vehicle for visual
communication, it is possible that journal articles will be reporting a unique label
representing a chemical structure in the near future, especially because this label
will already allow the searching of chemical structures contained in publications
indexed using Web-based search engines. For more on this, see Appendix 8-1.
385
➤ ➤ ➤ ➤ ➤ CHAPTER 18
Selected
Bibliography
References on Scientific Communication
Technical Writing
Barrass, R. Scientists Must Write: A Guide to Better Writing for Scientists, Engineers, and
Students, 2nd ed.; Routledge: London, 2002.
Cook, C. K. Line by Line: How To Edit Your Own Writing; Houghton Mifflin: Boston, MA,
1986.
Day, R. A. The Development of Research Writing. Scholarly Publishing January 1989, pp
107–115.
Day, R. A. Scientific English: A Guide for Scientists and Other Professionals, 2nd ed.; Oryx
Press: Phoenix, AZ, 1995.
Eisenberg, A. Writing Well for the Technical Professions; Harper & Row: New York, 1989.
King, L. S. Why Not Say It Clearly: A Guide to Scientific Writing, 2nd ed.; Little, Brown:
Boston, MA, 1991.
O’Connor, M. Writing Successfully in Science; Chapman & Hall: New York, 1992.
Rathbone, R. R. Communicating Technical Information: A New Guide to Current Uses and
Abuses in Scientific and Engineering Writing, 2nd ed.; Addison-Wesley: Reading, MA,
1985.
Schoenfeld, R. The Chemist’s English, 3rd ed.; VCH: Weinheim, Germany, 1990.
Shaw, H. Errors in English and Ways To Correct Them, 4th ed.; HarperPerennial: New York,
1993.
Strunk, W., Jr.; White, E. B. The Elements of Style, 3rd ed.; Allyn & Bacon: New York,
1995.
Zinsser, W. On Writing Well: The Classic Guide to Writing Nonfiction, 6th ed.; Harper-
Perennial: New York, 1998.
Zinsser, W. Writing To Learn; Collins: New York, 1993.
Copyright 2006 American Chemical Society
386 ➤ The ACS Style Guide
Style and Usage
Bernstein, T. M. The Careful Writer: A Modern Guide to English Usage; The Free Press: New
York, 1998.
Berry, T. E. The Most Common Mistakes in English Usage; McGraw-Hill: New York, 1971.
Copperud, R. H. American Usage and Style: The Consensus; Van Nostrand-Reinhold: New
York, 1980.
Flesch, R. F. The ABC of Style: A Guide to Plain English; HarperCollins: New York, 1980.
Hodges, J. C.; Horner, W. B.; Webb, S. S.; Miller, R. K. Harbrace College Handbook, 13th ed.;
Harcourt, Brace, Jovanovich: New York, 1998.
Merriam-Webster’s Collegiate Dictionary, 11th ed.; Merriam-Webster Inc.: Springfield, MA,
2003. (Available as a package with a CD and online; see http://www.m-w./com for infor-
mation on the online product.)
The New York Public Library Writer’s Guide to Style and Usage; Sutcliffe, A., Ed.; Harper-
Collins: New York, 1994.
van Leunen, M.-C. A Handbook for Scholars, 2nd ed.; Oxford University Press: New York,
1992.
Webster’s Third New International Dictionary, Unabridged; Merriam-Webster, Inc.: Spring-
field, MA, 2002. (Available as a package with a CD and online; see http://www.m-w./
com for information on the online product.)
Webster’s New World Dictionary of the American Language, 2nd ed.; Simon & Schuster:
New York, 1980.
Style Manuals
AIP Style Manual, 4th ed.; American Institute of Physics: New York, 1990.
American Medical Association Manual of Style, 9th ed.; Flanagin, A., et al., Eds.; Lippincott
Williams & Wilkins: Baltimore, MD, 1997.
ASM Style Manual for Journals and Books; American Society for Microbiology: Wash-
ington, DC, 1992.
The Chicago Manual of Style, 15th ed.; University of Chicago Press: Chicago, IL, 2003.
Merriam-Webster’s Standard American Style Manual; Merriam-Webster: Springfield, MA,
1994.
The Microsoft Manual of Style for Technical Publications, 3rd ed.; Microsoft Press: Red-
mond, WA, 2003.
Publication Manual of the American Psychological Association, 5th ed.; American Psy-
chological Association: Washington, DC, 2001.
Scientific Style and Format: The CBE Manual for Authors, Editors, and Publishers, 6th ed.;
Cambridge University Press: New York, 1994.
Skillin, M. E.; Gay, R. M. Words into Type, 3rd ed.; Prentice-Hall: Englewood Cliffs, NJ, 1974.
U.S. Government Printing Office Style Manual 2000, 29th ed.; Government Printing Office:
Washington, DC, 2000.
Wired Style: Principles of English Usage in the Digital Age; Hale, C., Ed.; HardWired: San
Francisco, CA, 1997.
Mathematics and Numbers
Swanson, E.; O’Sean, A.; Schleyer, A. Mathematics into Type: Copy Editing and Proofreading
of Mathematics for Editorial Assistants and Authors; American Mathematical Society:
Providence, RI, 1999.
Chapter 18: Selected Bibliography ➤ 387
Taylor, B. N. Guide for the Use of the International System of Units (SI); National Institute
of Standards and Technology: Gaithersburg, MD, 1995.
Preparing Illustrations
Briscoe, M. H. Preparing Scientific Illustrations: A Guide to Better Posters, Presentations,
and Publications, 2nd ed.; Springer Verlag: New York, 1996.
Pocket Pal: A Graphic Arts Production Handbook, 18th ed.; Graphic Arts Technical Founda-
tion: Alexandria, VA, 2000.
References on Chemistry
Conventions in Chemistry
National Institute of Standards (NIST) Special Publication 330, 2001 Edition, Engl. version
of The International System of Units (SI), 7th ed.; Bureau International des Poids et
Mesures: Sèvres, France, 1998. http://physics.nist.gov/Pubs/SP330/sp330.pdf.
Quantities and Units; International Organization for Standardization: Geneva, Switzer-
land, 1993. http://www.iso.org/iso/en/prods-service/ISOstore/store.html.
Quantities, Units and Symbols in Physical Chemistry, 2nd ed.; Blackwell Science, Oxford,
U.K., 1993 (commonly known as the “green book”). http://www.iupac.org.
Biochemistry
Biochemical Nomenclature and Related Documents, 2nd ed.; Liébecq, C., Ed.; Portland
Press: London, 1992 (commonly referred to as the “white book”). http://www.chem.
qmw.ac.uk/iupac/bibliog/white.html.
Enzyme Nomenclature; Academic: New York, 1992. http://www.chem.qmw.ac.uk/iubmb/
enzyme/.
Chemical Abstracts
Advice on Chemical Abstracts nomenclature is available from the Manager of Nomen-
clature Services, Department 64, Chemical Abstracts Service, P.O. Box 3012, Colum-
bus, OH 43210. A name-generation service is available through CAS Client Services,
Chemical Abstracts Service, P.O. Box 3343, Columbus, OH 43210; e-mail address,
answers@cas.org; and URL, http://www.cas.org/Support/client.html.
Combinatorial Chemistry
Maclean, D.; Martin, E. J. On the Representation of Combinatorial Libraries (A Perspec-
tive) J. Comb. Chem. 2004, 6, 1–11.
Maclean, D.; Baldwin, J. J.; Ivanov, V. T.; Kato, Y.; Shaw, A.; Schneider, P.; Gordon, E. M.
Glossary of Terms Used in Combinatorial Chemistry (IUPAC Technical Report) Pure
Appl. Chem. 1999, 71, 2349–2365; Reprinted J. Comb. Chem. 2000, 2, 562–578; Trans-
lated to German Angew. Chem. 2002, 114, 893–906.
Drug Names
The USP Dictionary of USAN and International Drug Names; U.S. Pharmacopeial Con-
vention: Rockville, MD, 2005 (updated annually).
388 ➤ The ACS Style Guide
General Chemistry
Chemical Abstracts Index Guide; American Chemical Society: Columbus, OH; Appendix
IV (updated periodically).
Compendium of Chemical Terminology, 2nd ed.; McNaught, A. D., Wilkinson, A., Eds.;
Blackwell Science: Oxford, U.K., 1997.
CRC Handbook of Chemistry and Physics, 86th ed.; Lide, D. R., Ed.; CRC Press: Boca Raton,
FL, 2005.
Kirk-Othmer Encyclopedia of Chemical Technology, concise, 4th ed.; Wiley & Sons: New
York, 2003.
The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 13th ed.; O’Neil,
M. J., Smith, A., Heckelman, P. E., Budavari, S., Merck, Eds.; John Wiley & Sons: New
York, 2001.
Inorganic Chemistry
Nomenclature of Inorganic Chemistry, Recommendations 2005; Connelly, N. G., Hartshorn,
R. M., Damhus, T., Hutton, A. T., Eds.; Royal Society of Chemistry: Cambridge, U.K.,
2005.
Block, B. P.; Powell, W. H.; Fernelius, W. C. Inorganic Chemical Nomenclature: Principles
and Practice; American Chemical Society: Washington, DC, 1990.
Nomenclature
The International Union of Pure and Applied Chemistry (IUPAC) Nomenclature Doc-
uments Home Page on the World Wide Web can be found at http://www.chem.qmw.
ac.uk/iupac/. This site contains information on IUPAC itself, the names and publishers
of references on chemical nomenclature, and many of the recommendations.
Organic Chemistry
IUPAC. Nomenclature of Organic Chemistry: Sections A, B, C, D, E, F, and H; Rigaudy, J.,
Klesney, S. P., Eds.; Pergamon: Elmsford, NY, 1979 (commonly referred to as the “blue
book”). http://www.acdlabs.com/iupac/nomenclature.
A Guide to IUPAC Nomenclature of Organic Compounds, Recommendations 1993; Panico,
R., Powell, W. H., Richer, C., Eds.; Blackwell Scientific Publications: Oxford, U.K.,
1994. http://www.acdlabs.com/iupac/nomenclature.
Ring Systems Handbook; American Chemical Society: Columbus, OH, 2003 (and supple-
ments).
Fox, R. B.; Powell, W. H. Nomenclature of Organic Compounds: Principles and Practice, 2nd
ed.; American Chemical Society: New York, 2001.
Polymer Chemistry
IUPAC. Compendium of Macromolecular Nomenclature; Blackwell Scientific Publications:
Oxford, U.K., 1991. http://www.iupac.org/publications/books/author/metanomski.
html.
389
➤ ➤ ➤ ➤ ➤
Index
In page references, b indicates boxes, f indicates
figures, s indicates schemes, and t indicates
tables.
“a”
before collective nouns, 106
as gender-neutral alternative, 58
in titles, 149
See also Articles (part of speech)
@ in e-mail addresses, 157
“a” and “b” references
in reference lists, 297–298, 325
in text citations, 290
“a” vs “an”
before element symbols, 257
general usage, 53
before isotopes, 257, 264
Abbreviations
“a” vs “an” before, 53
in abstracts, 22, 158, 160
academic degrees, 310
acronyms differentiated from, 158
for amino acids, 245t–246
Canadian provinces and territories, 162
capitalization, 146, 150, 156, 159, 227, 228,
272
case sensitivity, 159
chemical reactions, 272, 273
commonly used in chemistry, 169–202
computer and Internet terms, 163–168
defining, 22, 158, 160, 245, 371, 372, 373
devising your own, 158, 169
editorial style overview, 158–162
Abbreviations—continued
at editor’s discretion, 158, 169
in equations, 160, 211, 217
in figures, 225, 365
in foreign names, 153
genus name repetition, 160
in government agency references, 313
isotopic labeling, 265
in mathematical copy, 213, 217
mathematical symbols differentiated from,
160, 211
for monosaccharides, 243t
for months, 160–161
not needing definition, 158–159, 217–218,
260
for nucleic acids, 244t
for organic groups, 260
period use, 118, 119, 223, 294
plural forms, 161
for publisher names, 302
reporting analytical data, 274
roman type use, 154–155, 159–160, 212–
213, 216, 271–272
for saccharides, 243t–244
in tables, 160, 225, 370, 371, 372, 373
in titles, 20, 150, 159, 160
U.S. states and territories, 162
for volume information in references,
303–304
See also Acronyms, Chemical Abstracts
Service Source Index (CASSI)
abbreviations, Symbols, Units of
measure
Copyright 2006 American Chemical Society
390 ➤ The ACS Style Guide
Absolute configuration
D and L forms, 236, 270–271
R and S forms, 270
Absolute constructions, dangling modifiers
compared with, 45, 112
“Abstract”, capitalization in references, 303
Abstracts
abbreviations defined in, 22, 158, 160
abstract number in references, 298–299
datuments as alternative, 88–89
mathematical symbols defined in, 211
references to meetings and conferences,
307–308, 321
scientific paper format, 21–22
Abuse, ethical considerations, 74
Academic degrees
“a” vs “an” before, 53
abbreviations, 310
in bylines, 21
thesis levels, 309–310
Acceptance of submissions
communications (journal presentations), 19
manuscripts, 12, 28
notification of, 64
peer-review role, 71
Accepted manuscripts, processing, 33–35
See also Editorial process, Electronic sub-
mission of manuscripts
Accession dates, Internet sources, 315, 317–
321, 324
Accession numbers, U.S. government publica-
tions, 312
Acids, capitalization examples, 237t
Acknowledgments
ethical obligations, 8, 14
scientific paper format, 24
Acrobat Distiller (software), 351
Acrobat Reader (software), 60, 68, 351
Acronyms
abbreviations differentiated from, 158
in abstracts, 22
commonly used in chemistry, 169–202
computer terms, 156, 163–168
editorial style overview, 158–162
period use, 119
for publisher names, 302, 307
See also Abbreviations, Symbols
ACS books
author guidelines, 29, 343
column dimensions, 354t
copyright policy, 30
figure costs, 350
reference format, 24, 287–290
template downloads, 29
See also Copyright, Editorial process, Peer
review
ACS committees, capitalization guidelines, 147
ACS Copyright Learning Module Web site, 82
ACS Copyright Office
contact information, 79b, 84b
Copyright Status Form, 86
requesting permission from, 84b
ACS copyright policy, 30, 79b, 82
ACS Copyright Status Form, 86
ACS desk references, 127, 135
ACS governing bodies, capitalization guide-
lines, 147
ACS journals and magazines
ASAP articles, 34
author guidelines, 29, 343, 381b, 382b
CASSI abbreviations, 288t
column dimensions, 354t
copyright policy, 30
ethical guidelines, 11–16
figure costs, 350
figure guidelines, 343, 347, 351, 364
hazard footnotes in, 23
home pages, 28b
numbers style, 208
parameter settings, 381b
reference format, 24, 287–290, 291
reference management programs, 327
structure guidelines, 381b, 382b
supporting information availability, 25
table guidelines, 374
template downloads, 29
type size, 356
See also Copyright, Editorial process, Peer
review
ACS local sections, capitalization guidelines,
147
ACS magazines, See ACS journals and maga-
zines
ACS Paragon Plus System, 30, 364, 374, 382b
ACS Paragon System
electronic submission of manuscripts, 60,
62, 69t
submission of artwork, 30, 364, 374, 382b
ACS periodicals, See ACS journals and maga-
zines
ACS Permission Request Form, 84b
ACS permission request guidelines, 83–86
ACS publications
ASAP articles, 34, 318–319
author guidelines, 29, 343, 381b, 382b
column dimensions, 354t
copyright policy, 30
credit lines in, 367b
figure costs, 350
figure guidelines, 343, 346, 364
numeral and word usage, 206
parameter settings, 381b
Index ➤ 391
ACS publications—continued
reference format, 24, 287–290, 291, 300, 317
template downloads, 29
trademark symbols not used in, 23
type size, 356
See also ACS journals and magazines, Copy-
right, Editorial process, Peer review
ACS Publications Division
ASAP articles, 318
copyright owner rights, 78
Copyright Status Form, 86
requesting permission from, 79b, 83, 84b
Web site, 86
ACS quotation marks policy, 121–122
ACS spelling preferences, 129–133
Active voice, writing style, 42, 54
Adaptation of artwork, 84b, 366
Addition compounds, centered dot use, 261
Addresses, computer, See Electronic mail
(e-mail) addresses, Uniform resource
locator, World Wide Web sites
Adjectives
comma use, 115–116
mathematical operators used as, 219
mathematical symbols used as, 215
phrasal, 149
predicate, 143
proper, 137, 143, 152
trademarks as, 146, 157
as unit modifier element, 139–140, 143
See also Unit modifiers
Adobe software
electronic submission of manuscripts, 61, 68
figure preparation, 350–352, 355b, 363
Advanced Chemistry Development software,
379–380, 380b
“Advances” as CASSI keyword, 306
Adverbs
hyphenation guidelines, 141
semicolon use, 119–120
as unit modifier element, 140
“Affect” vs “effect” or “impact”, 52
Affiliation
company name styling, 162
scientific paper format, 21
Affirmative sentences vs double negatives, 45
Agents, submitting, 62
Agreement, subject–verb, 43–44, 105–108
Alcohols
capitalization examples, 237t
forms for, 234b
Alicyclic compounds, nomenclature, 236
Alignment
chemical structures, 379
columnar material in tables, 370, 372, 373,
374
Alignment—continued
columns containing numbers, 207, 372
superscripts and subscripts (stacking), 269
“All”, subject–verb agreement, 108
“Alpha (α) particle”, capitalization of “p”, 149
“Alpha” vs α, 155
Alphabet, Greek, 214
See also Greek letters
Alphabetizing
CASSI abbreviations, 294
reference citations, 289
reference lists, 325
unnumbered lists, 374
alt infix in copolymer names, 243
“Although” instead of “while”, 46
“a.m.”, 205
American Institute of Physics, 60, 62, 69t
American Library Association, 311
American National Standards Institute
(ANSI), 276
American Society for Testing and Materials,
276
Amino acids
abbreviations, 245t–246
small capital letter use, 236, 245, 270
“Among” vs “between”, 51
Ampersand in Web addresses, 156
“an” vs “a”, See “a” vs “an”
Analytical data
reporting, 274–276
as supporting information, 25
See also Data, Physical quantities, SI units,
Units of measure
“And”
vs “and/or”, 56
with author citations in text, 289
as coordinating conjunction, 47, 113, 149
vs en dash, 124–125
number of subject, 106
between numbered items, 209
vs plus sign, 55, 211
slash misused for, 56
vs “with” constructions, 56
“And/or”, 56
“And so forth”, 160
Angle brackets, with crystallographic indices,
268
Angular degree or time symbols, 203, 224
Angular vs linear formulas, 267, 376
Anhydrides, capitalization examples, 237t
Animations, Web-enhanced objects, 25
Anions, charged, 266
Anomeric configuration of saccharides, 243
Anonymity of peer reviewers, 8, 71–72
ANSI, See American National Standards Insti-
tute
392 ➤ The ACS Style Guide
Antecedents of pronouns, 45–46, 58
“Any”, subject–verb agreement, 108
Apostrophes
plurals
of abbreviations, 161
of decades, 205
of mathematical symbols, 211
of numerals used as names, 206
possessives, 127
Apparatus, “Experimental” section contents, 22
“Appendix”, capitalization, 144
Appendixes in scientific papers, 24–25
Application files for artwork
definition, 350
Excel file tips, 353b
PDF file creation, 351
See also Computer file formats, specific
applications
Appositives, restrictive and nonrestrictive,
116–117
Aqueous state, indicating in reactions, 272
Ar designation for aromatic substituents, 246,
250
Arabic numerals, See Numerals
Arabic surnames, 152
Area graphs
types of artwork, 347
working with, 363
Arguments (mathematical)
definition, 210
enclosing marks for clarity, 216
spacing considerations, 214, 219
Arial typeface, 357b
Aries Systems, editorial systems, 60, 69t
Aromatic compounds, 240
Aromatic substituents, 246, 250
Arrays, mathematical concept, 210
Arrows in reactions, 272–273, 379
Article numbering in references, 297
Article reprints, 35
See also Permissions
Article titles in periodical citations, 291, 292t
Articles (journal), See Journal articles
Articles (part of speech)
“a” vs “an”, 53, 257, 264
at beginning of titles, 20, 154
capitalization in titles and headings, 149
before collective nouns, 106
before element symbols, 257
in foreign names, 152–153
as gender-neutral alternative, 58
before isotopes, 257, 264
“Articles ASAP” page, 34
Artwork
ACS copyright policy, 30
adapted, 84b, 366
Artwork—continued
article reprint costs, 35
editorial process, 29–30, 363–365
embedding requirement, 30, 364, 374
handling tips, 362, 364–365
numbering of, 206, 345, 365
permission requirements, 30, 85b, 366
sizing, 352–354, 355, 370, 373
types of, 29, 347–349
See also Charts, Figures, Illustrations,
Photographs, Schemes, Structures,
Tables
“As”, capitalization in titles and headings, 149
“As compared to” and “as compared with”, 50
As Soon As Publishable (ASAP) articles, 34,
318–319
“As well as”, 47
Asian surnames, 152–153
Aspartic acid/asparagine mixtures, 245
Assignment of copyright by ACS, See ACS
copyright policy
Association of American Publishers, 82
“Assuming” as sentence modifier, 112
“Assure” vs “ensure” or “insure”, 52
Asterisk
for corresponding author information, 21
for excited electronic state, 262
for multiplication in computer languages,
211
ASTM International, citing standards, 276
Astronomical terms, capitalization, 149
“at.”, unit of measure, 118
At symbol (@) in e-mail addresses, 157
Atomic numbers
left subscript use, 262
list of elements, 258t–259t
for unnamed elements, 260
Atomic orbitals, chemical conventions, 256
Atomic substituents or residues, designating,
246, 250–251
Atomic weights, list of elements, 258t–259t
Atoms
datument validation, 96
electronic configuration, 256–257
isotopic, 265
markup languages and the WWW, 91,
92–94
nuclide descriptors, 261–263
symbols and SI units, 277
Attributes (markup language component),
91–94, 96, 97
Attribution, ethical considerations for, 7
Audience considerations
for draft manuscripts, 28b
in popular science, 16
word choice, 50
Index ➤ 393
Author–date citations
multiple references by same author,
290
reference collation, 290, 325
reference format, 30, 287, 289–290
Author guidelines
ACS publications, 29, 343, 381b, 382b
additions or corrections, 35
CIF format, 286
conventions in chemistry, 255
datument validation, 96
electronic submission of manuscripts,
30, 60, 63
figure submission, 343, 363–365
numeral and word usage, 206
peer-review process, 75
reference format, 31, 291
references to books, 300
standard format, 20, 25, 26, 28–29
structure submission, 381
table preparation, 370
Author home page, 62, 63, 64, 68
Author names in references
books, 300–301
e-mail messages, 322
editorial process overview, 30
organization or committee as author, 301,
307, 308, 312, 321
periodicals, 291, 297–299
reference collation, 290, 325–326
theses, 309
U.S. government publications, 312
Author names in text
byline format, 21, 152
citation guidelines, 30, 287, 289–290
period use and spacing, 119
Author responsibility
checking proofs, 33–35, 36
datument potential, 98–99
datument validation, 96
ethical considerations, 3–8, 13–15, 16, 27
graphics insertion, 379
paperwork submission, 32, 33
peer-review process, 75–76
reference accuracy, 24, 30, 31, 290
See also Author guidelines, Copyright
entries, Permissions
Author’s complimentary copy, 35
Author’s proof, 33–35, 36
Authorship
copyright considerations, 79, 81, 86
determining, 6–7, 14, 21
order of names as issue, 6
See also Coauthors, Multiple authors
Auxiliary verbs, in compound sentences,
108
Axes
crystallographic notation, 268, 269
mathematical use and italic type, 212, 223
orbital, 256
scaling and labeling, 225, 359–360
b infix in copolymer names, 243
Bar graphs
color use, 345b
examples, 359f
types of artwork, 347
when to use, 344
working with, 363
Base pairs, representation of, 244
“Based on” vs “on the basis of”, 52, 111
Bases, numbering in nucleotide sequences, 244t
“Because” instead of “since”, 46
“Because of” instead of “due to”, 112
Behavioral guidelines, See Ethical guidelines
Beilstein references, 299
Bench>Press (software), 60, 62, 69t
Berkeley Electronic Press, editorial systems, 60
“Beta (β) particle”, capitalization of “p”, 149
“Beta” vs β, 155
“Between” vs “among”, 51
“Between … and”, 125, 209
“bi” in element names, 260
Bias
bias-free language, 57–58
and peer reviews, 9, 15, 75
Bibliographic citations, See Reference entries
Bibliographic indexes, foreign surname
format, 152
Bibliographies, abbreviation use in, 160
See also Reference entries
Biblioscape (software), 326–327
Billions, 204
BinHex files, 62
Binomial terms, See Taxonomic terms
Bio-Rad software, 379–380, 380b
Biochemistry, reference format exceptions, 291,
300, 301
Biological structure data sources, 284
Biological test data as supporting informa-
tion, 25
“Bis” as multiplying prefix, 235
Black-and-white (B&W) mode, 350, 351, 352t,
362
Blackboard boldface letters, 214
blend infix in copolymer names, 243
block infix in copolymer names, 243
BMP format, 381
Boiling points, reporting analytical data, 274
Boldface type
with CASSI abbreviations, 295
chemical name numbering, 206
394 ➤ The ACS Style Guide
Boldface type—continued
chemical structure identification, 274,
376–377
combinatorial chemistry representation, 246
for Greek letters, 214
in mathematical copy, 213
references to periodicals, 296
superatom representation, 246
Bonding orbitals, chemical conventions, 256
Bonds
punctuation, 244, 267
symbols, 243, 244
Book as reference
author names, 300–301
book title and editions, 301–302, 306
books with editors, 292t, 300–301, 320
books without editors, 292t, 300, 319–320
chapter title, 301, 306
computer program citation format,
323–324
editor names, 300–302, 320
examples, 292t–293t
meetings and conferences, 307–308
miscellaneous information, 305
online books, 293t, 314, 319–320
organization or committee as author, 300,
307
pagination information, 304
place of publication, 303
publisher name, 302–303
series publications, 292t, 304, 305–306
special situations, 305–306
volume information, 303–304, 306
year of publication, 303
See also References
Book titles
in book citations, 301–302, 306
italic type for, 154
in presentation citations, 307–308
U.S. government publications, 312
Books (in general)
artwork sizing, 354, 370, 373
table footnotes, 373
types of, 17–18
word choice, 50
See also ACS books, Manuscript entries,
Scientific papers
Borrowed material, See Adaptation of artwork,
Copyright, Permissions
“Both”, subject–verb agreement, 108
“Both … and”, 47
Boughton system, isotopic naming, 265–266
Braces
with crystallographic indices, 268
in mathematical copy, 212, 214, 216
in mathematical equations, 219–220
Braces—continued
nesting order, 216
in set notation, 222
with syllabic portion of chemical names, 240
Brackets, See Angle brackets, Curly brackets,
Square brackets
Bragg reflection, crystallography, 267
Branched residues of saccharides, 244
Brand names
editorial style, 157
scientific paper format, 23
in titles, 20
See also Trademarks
Bravais lattices, 269
Breaks at end of line, See Line breaks
Bridged compounds, nomenclature, 236
Browsers, See Web browsers
Bulletins, technical, 292t, 314, 324
Bureau International des Poids et Mesures, 255
“But”
as coordinating conjunction, 113
parallel construction, 47, 48
in titles and headings, 149
B&W mode, 350, 351, 352, 362
Bylines
foreign surname format, 152
scientific paper format, 21
See also Authorship
Cadmus Systems, editorial systems, 60, 62, 69t
Callouts, See Citations in text
Cambridge Crystallographic Data Centre, 284,
286
Cambridge Structural Database, 284
Cambridgesoft software, 350–351, 379–380,
380b, 381b, 382b
Camera-ready artwork
definition, 343
preparation guidelines, 346
submission guidelines, 364–365
See also Hard copy
Canada, province and territory abbreviations,
162
Canadian Domestic and Non-Domestic Sub-
stance Lists, 254
Canonical building blocks of nucleic acids, 244
Capitalization
abbreviations, 146, 150, 156, 159, 227, 228,
272
amino acid abbreviations, 245
axis labels on figures, 360
case-sensitive abbreviations and symbols,
159
chemical element symbols, 145, 240–241, 257
chemical names, 143, 145, 151, 233, 235–
241, 237t–239t
Index ➤ 395
Capitalization—continued
chemical reaction types, 273
after colons, 120, 144
company and organizational names, 147
computer and Internet terms, 156, 163–168
computer languages, 155
computer protocols, 156
computer software, 156
event names, 148
“figure”, “table”, “chart”, and “scheme”, 143,
345, 369
parenthetical sentences, 123
parts of a book, 144
planet names, 149
polymer names, 146, 150, 237t, 242–243
professional titles, 147
references to periodicals, 291, 299
regions of the country, 148
SI units, 228
small capital letters, 233, 236, 238t, 245,
270–271
spelling recommendations list, 129–133
surnames, 151–152, 153, 225
taxonomic names, 145–146, 150
in text, 143–148
in titles and headings, 148–151, 227,
237t–239t
trademarks, 147, 157
units of measure, 150, 225, 227
words preceded by element symbols, 145
Captions
abbreviation use, 160
colon use, 120
as copyright ownership indicator, 81
manuscript submission, 364
parallelism, 48
preparing, 355, 365
sizing artwork, 354
symbol keys, 365
Carbohydrates, small capital letter use, 236, 270
Cardinal numbers, 203–204, 308
See also Numbers
CAS, See Chemical Abstracts Service
Cascading Style Sheets (CSS), 90
Case sensitivity for abbreviations, 159
Cases regarding copyright, 80, 82
CASSI, See Chemical Abstracts Service Source
Index
Catalysts, indicating in reactions, 272
Cations, charged, 266
“Caution:”, “Experimental” section contents,
23
CD-ROM publications
cited as references, 293t, 322–323
illustration preparation, 346
Celsius, capitalization, 225
Centered dot
for bond formation or breaking, 267
in chemical formulas, 260
with compound units of measure, 224
in mathematics, 215, 219
nucleic acid representation, 244
spacing guidelines, 215, 219, 261
Centering of text and artwork, See Alignment
CG Information software, 327
CGS system, background of, 228
“Chapter”, capitalization, 144, 303
Chapter number in book references, 303
Chapter titles
in book references, 301, 306
in U.S. government publications, 312
Charges
electric, 256
ionic, 262, 266
“Chart”, capitalization, 143
Charts
citing, 143
editorial process, 29
groups of structures as, 376, 377, 379
numbering, 206, 377
permissions, 83
titles, 148
types of artwork, 347
when to use, 344
working with, 363
See also Artwork
Check-CIF utility, 63, 286
Checking proofs, 33–35, 36
Chemaxon software, 380, 380b
ChemDraw (software), 350–351, 379–380,
380b, 381b, 382b
Chemical Abstracts (CA)
Boughton system for isotopes, 265
and CAS Registry Numbers, 253, 254
citing references in text, 298–299
datuments as alternative, 89
patent citation format, 310
Chemical Abstracts Service (CAS)
chemical nomenclature, 233
Chemical Registry System, 91, 94, 253–254
Chemical Abstracts Service Source Index (CASSI)
computer program citations, 324
meeting and conference presentation cita-
tions, 308
publisher names and addresses, 302
sample entry, 340–341
series publications, 306
Chemical Abstracts Service Source Index (CASSI)
abbreviations
of ACS periodicals, 288t
commonly cited journals list, 328–339
government agency references, 313
396 ➤ The ACS Style Guide
Chemical Abstracts Service Source Index (CASSI)
abbreviations—continued
sample entry, 340–341
series titles, 306
use guidelines, 291, 292–293
Chemical apparatus, describing, 22
Chemical descriptors
capitalization, 145, 238t–239t, 240
style guidelines, 234–236, 240, 241, 270–271
in titles and headings, 150
types of, 233
See also Stereochemical descriptors
Chemical elements
chemical conventions, 257–261, 264
crystallographic indices, 268
isotopes, 263–266
list, 258t–259t
nuclide descriptors, 262–263
unnamed, 260
See also Element symbols
Chemical formulas
bonds, 267
Chemical Abstracts index of, 254
chemical conventions, 257–261
concentrations, 271
“Experimental” section contents, 22, 23
isotopic nuclides, 264
linear vs angular forms, 267, 376
nuclide descriptors, 262–263
prefix treatment, 235
roman numerals in, 262
in titles, 20
Chemical inventories, commercial, 254
Chemical kinetics, symbols and SI units,
277–278
Chemical literature, popular, 16
Chemical markup language, See CML
Chemical names
boldface numbering sequence, 206
capitalization, 143, 145, 151, 233, 235–241,
237t–239t
CAS Registry Numbers, 253–254
components of, 233–240
“Experimental” section contents, 22
hyphenation at end of line, 233, 241,
247–249
hyphenation of prefixes and suffixes, 138,
235–236, 270
isotopic labeling, 264–266
locant and descriptor portion of, 234–240,
238t–239t
multiword examples, 237t
numeral and word usage, 206
punctuation in, 241
specialized groups of chemicals, 242–246
syllabic portion of, 233, 240, 241
Chemical names—continued
as unit modifiers, 141, 143
See also Chemical descriptors, Chemical
nomenclature, Locants
Chemical nomenclature
authoritative sources, 233
and CAS Registry Numbers, 254
published updates, 255
punctuation, 124
roman type use, 212
systematic vs trivial, 22
See also Chemical names
Chemical purity, degree of and criteria for, 22
Chemical reactions
abbreviation use, 272, 273
arrows in, 272–273, 379
concentrations in, 271
displayed vs run in, 272, 376
grouped as schemes, 376, 377f
numbering, 272, 377
preparing, 378–381
spacing guidelines, 272, 379
submitting, 381–382
See also Schemes
Chemical Registry System, CAS, 253–254
Chemical research, ethical guidelines, 11–16
Chemical safety information
in abstracts, 21
ethical guidelines, 13
“Experimental” section contents, 23
Chemical structures, See Structures
Chemical symbols
case sensitivity, 159
element names used with, 260
list, 169–202, 258t–259t
published updates, 255
roman type use, 212, 259
See also Abbreviations, Element symbols,
SI units, Symbols, Units of measure,
specific symbols
Chemical terms
capitalization, 143
datument vocabularies, 97
Greek letter use, 155
hyphenation of prefixes, 137
See also Chemical descriptors, Chemical
names, Chemical nomenclature
Cheminnovation (software), 379–380, 380b
Chemistry 4-D Draw (software), 379–380,
380b
ChemSet notation, 246, 251–252
ChemSketch (software), 379–380, 380b
ChemWindow (software), 379–380, 380b
Child elements (markup language compo-
nent), 91, 93
Chime (plug-in), 25
Index ➤ 397
Chinese surnames, 152
Chirality, 270–271
CIF, See Crystallographic information files
Circling of charges, 262
Circuit diagrams as supporting information, 25
“Cis”, italicization and capitalization, 145
Citation manager programs, 326–327
Citations in text
in abstracts, 22
of equations, 221
of figures, 143, 345–346
of footnotes, 373
of numbered items, 209
of structures and schemes, 143, 376–378
of tables, 143, 369–370
See also Reference entries
City and state names for place of publication,
294, 295, 303, 310, 328
Class names (taxonomic), capitalization,
145–146
Classroom use of copyrighted materials, 80
Clause, definition, 42
CML (chemical markup language)
datument validation, 96–97
display of, 94, 96
examples of, 92–94, 93s
structure submission, 382
CMYK color mode, 350, 352, 362
“Co.”, 162, 303
co infix in copolymer names, 243
“Co-workers” in textual citations, 289
Coauthors
ethical obligations, 14
reference citation wording, 289
See also Authorship, Multiple authors
Code of Federal Regulations, cited as reference,
313
CODEN abbreviation, 340
Collating references, 290, 325–326
“Colleagues” in textual citations, 289
Collective nouns, 106–107
Colon
capitalization after, 120, 144
with display equations, 221
in figure captions, 120
general use, 120
misuse with components of mixture, 121,
261
in ratios, 121, 215, 222
references to U.S. government publications,
312
between verb or preposition and object, 121
Color artwork, and reprint costs, 35
Color combinations, hyphen use, 125, 140
Color modes, 350–352, 362
Color photographs, 362
Color use in figures, 344, 345b, 346, 350, 353b
Column number in references, 298
Column widths for ACS publications, 354t
Columns in tables, See Tables
Combinatorial chemistry, representation of,
246, 250–252
Combinatorial libraries, 246, 250–252
Combos (artwork)
definition, 347
example, 349f
file formats, 352t
working with, 347, 363
Comma
between adjectives, 115–116
with appositives, 116–117
in chemical names, 241
with compound predicates, 115
with coordinating conjunctions, 113, 114,
115
with dates, 118
and display equations, 221
with “et al.”, 117–118, 289
with geographical locations, 118
with introductory words and phrases, 48,
114
with “Jr.” and “Sr.”, 117
between modifier and subject, 110
with nonrestrictive clauses, 44, 109, 116
in numbers with five or more digits, 207–208
in numbers with four digits, 207
with quotations, 118
reference citation guidelines, 289–290
with reference citation numbers, 117, 209,
289
references to books, 301–302
references to meetings and conferences,
308–309
references to periodicals, 296–299
references to U.S. government publications,
312–313
serial, 114, 115, 119
with subordinate clauses, 114
with subordinating conjunctions, 116
with two or more adjectives, 115–116
with units of measure in tables, 371
verb separated from subject, object, or
predicate noun by, 114–115
Commercial chemical inventories of regula-
tory agencies, 254
Committee on Nomenclature, Terminology,
and Symbols of the American Chemical
Society, 233
Committees as authors, 301, 307
Communications (journal presentation type),
18–19
See also Personal communications
398 ➤ The ACS Style Guide
Companion publications, cited as reference, 299
“Company”, when to abbreviate and when to
spell out, 162
Company logos, copyright basics, 83
Company names
capitalization, 147
MSDS citation, 315
parentheses use, 22, 124
spelling and styling, 127
in titles, 20
when to use, 162
Comparatives, hyphenation in unit modifiers,
141
“Compared to” vs “compared with”, 48, 50
Comparisons
sentence structure, 48–50
word choice, 50–51
Complementary base pairs, representation, 244
Complex sentences, definition, 42
Complimentary copies, 35
Components of mixtures, punctuation, 121,
126, 222, 261
Components of vectors and tensors
italic type use, 212
as mathematical concept, 210
“Composed of” vs “comprised”, 53
Compound predicates, 115
Compound sentences, definition, 42
Compound subjects, 106–107
Compound surnames, hyphenation, 139,
152–153
Compound units of measure, 224, 226, 230t
Compound words
capitalization, 150
definition, 138
hyphenation, 138–139
See also Phrasal verbs, Unit modifiers
Compression of computer files, 62, 351
“Comprised” vs “composed of”, 53
Computer addresses, See Electronic mail
(e-mail) addresses, Uniform resource
locator, World Wide Web sites
Computer databases, See Databases
Computer disks for figure files, 364
Computer file formats
application file definition, 350
compressed files, 62
crystallographic information files, 25, 63,
97–98, 270, 284–286
datument potential, 98
e-mailed files, 61–62
electronic submission of manuscripts,
59–63, 68, 69t
figure preparation, 346, 350–353b, 357b,
361, 363
manuscript templates, 20, 29, 60
Computer file formats—continued
reference management programs, 326
structure preparation, 381
word-processing vs PDF files, 60–61
Computer languages
asterisk use, 211
capitalization, 155
Computer monitors
figure preparation issues, 346, 347, 350, 352
font tips, 357b
Computer platforms
PDF file portability, 61
reference manager programs for, 327
templates for, 29
Computer programs, See Software programs
Computer protocols, capitalization, 156
Computer software, See Software programs
Computer terminology list, 163–168
Computerized title searches, 20
Concentrations
conventions in chemistry, 271–272
square brackets use, 124, 271
“Conclusions” section in scientific papers, 7, 23
Conduct guidelines, See Ethical guidelines
Conference proceedings
description, 17
reference format, 292t, 307–309, 321, 323
standard reporting format, 19
Confidentiality of submitted manuscripts, 9,
12, 15, 16, 73–74
Configurational prefixes, chemical names, 235,
241, 243, 245, 270–271
Conflicts of interest
author responsibility to reveal, 8, 12, 15
in editorial process, 12–13
in review process, 9, 15, 75
Confusable words and phrases, 51–53, 111–112
Conjunctions, See Coordinating conjunctions,
Correlative conjunctions, Subordinating
conjunctions
Conjunctive adverbs, semicolon use, 119–120
Conseil Européen pour la Recherche Nucléaire
(CERN), 89
Constants
defining symbols in text, 211
Greek letter use, 214
italic type use, 212
mathematical abbreviations and symbols,
211, 217–218
as mathematical concept, 210
roman type use, 213
Contents page, hazard footnotes on, 23
Context of research, ethical considerations,
7–9
Continuous-tone art, See Halftone art
Contractions, avoiding, 55
Index ➤ 399
Contracts and copyright, 78, 82
Controlled vocabularies, 97–98
Conventions in chemistry
atoms and molecules, 261–266
bonds, 267
chemical elements and formulas, 257–261
chemical reactions, 272–274
chirality, 270–271
concentration, 271–272
crystallography, 267–270, 284–286
electronic configuration, 256–257
periodic table, 261
physical quantity symbols, 277–283
radicals, 266
reporting analytical data, 274–276
subatomic particles and quanta, 256
Coordinating conjunctions
capitalization in titles and headings, 149
comma use, 113, 114, 115
definition, 47
number of subject, 106
parallelism, 46, 47, 48
Coordination compounds
capitalization examples, 237t
square brackets use, 260
Coordination numbers, 270
Copolymer names, 243
Copy editors (in general)
proofreaders’ marks, 36–39
role, 33
See also Editorial process, Editors
Copyright
basic rights, 79
copyright notice, 79–80, 83
duration of, 81, 82
electronic media, 81, 82, 83
excerpts, 80
fair use, 80–81
font software, 357b, 363
materials not protected by, 78
materials protected by, 77–78, 81–82
misconceptions about, 81–82
ownership, 78, 81–82, 83, 84b, 86
permission request guidelines, 83–86, 366
photographs, 81–82, 361, 366
public domain status, 81, 83
registration, 79–80
reproduced artwork, 29, 83–86, 366
transferring, 32, 33, 78, 82, 86
U.S. government works, 81, 366
U.S. law, 77–82
works made for hire, 78, 82
See also Permissions
Copyright infringement, 80, 82, 357b
Copyright laws, 77–82
Copyright notice, 79–80, 83
Copyright policy, ACS publications, 30, 79b, 82
Copyright registration, 80
Copyright Status Form, ACS, 86
Copyright transfer, 32, 33, 78, 82, 86
Corel Draw (software), 351
“Corporation”, when to abbreviate and when
to spell out, 162
Corrections
to draft manuscripts, 36
to proofs, 34, 36
to published manuscripts, 35
See also Errors entries
Correlative conjunctions
definition, 47
parallelism, 46, 47
Correlative constructions, for parallelism, 46, 47
Correspondence (journal presentation type), 18
Corresponding author
author home page, 62, 63
contact information, 21
role in editorial process, 33, 34
Country names for place of publication, 294,
295, 303, 310
Court cases on copyright, 80, 82
Cover letters for manuscripts, 32, 76
Credit lines
figure captions, 366, 367b
for previously published material, 84b, 85b,
86, 366, 367b
Critical review of manuscripts, See Peer review
Criticism, in scientific writing, 7–8, 14, 16, 74
Cropping of photographs, 352, 360, 362
cross infix in copolymer names, 243
CrossRef application, 317
Crystallographic information files (CIF)
basic principles, 284–285
and datument vocabularies, 97–98
editors for, 286
electronic submission of manuscripts, 63
journal submission requirements, 285–286
as supporting information, 25
validation reports, 63, 286
WWW resources, 284, 286
Crystallography
CIF format, 25, 63, 97–98, 270, 284–286
crystallographic point groups, 269
lattice types, 269
planes and directions in crystals, 267–268
space groups, 269–270
symmetry operations and structural point
groups, 269
CRYSTMET database, 284
CSS (cascading style sheets), 90
Cultivar names, capitalization, 145–146
Curly brackets, combinatorial chemistry rep-
resentation, 246, 251–252
400 ➤ The ACS Style Guide
Curves, plotting in figures, 358–359
Cyan–magenta–yellow–black (CMYK) color
mode, 350, 352, 362
“cyclo” prefix, style for, 236
D in chemical names, 236, 245, 270–271
dA•dT and dG•dC pairings, 244
Dangling modifiers, 45, 109–113
Dashed lines
for figures, 345f, 358
for structures, 378
Dashes, See Em dash, En dash
Data (in general)
CIF format requirements, 284–286
CIF terminology, 284–285
embedding in Excel files, 353b
ethical considerations in presentation, 5
reporting analytical data, 274–276
as source of original figure or table, 84b, 366
as supporting information, 25
and types of artwork, 344, 369
See also Databases, Datuments
“Data” as singular or plural noun, 106
Data entry numbers, reference format, 314,
320, 324
Data sets, reference format, 314–315
Databases
CAS Chemical Registry System, 253–254
crystal structure data sources, 284
data set citation, 314
datuments as alternative, 89
disclosure of research results via, 16
reference collation and management, 326
reference format, 293t, 318, 320, 324
XML display example, 94, 96
Date of access for Internet reference citations,
315, 317–321, 324
Dates
comma use, 118
month abbreviations, 160–161
numeral and word usage, 160, 205
See also Year of publication
Datuments
authoring and editing tools, 98–99
cost-saving potential, 99
documents compared with, 87–89, 96
identifier use, 94
markup language display, 94–96
markup language examples, 90–94
markup languages and the WWW, 89–90
as scientific grid component, 99–100
subsets, 94
validation, 96–97
vocabularies, 97–98
“D.C.”, CASSI spacing exception, 294
Deadline concerns, See Timeliness as issue
Decades, numerals and plural forms, 205
Deceased authors, acknowledgment of, 14
Decimals and decimal point use, 208, 372
Declarative sentences
period use, 118
writing style, 41–42
Definitions used by markup languages, 93,
97–98
Degree of accuracy, numbers style, 208–209
Degree of rotation, crystallographic point
groups, 269
Degree symbol
decimals use, 208
not used with kelvin, 227
spacing, 203, 224
Degrees, academic, See Academic degrees
“Delta (∆)”, spelling out, 155
Demonstrations, citation guidelines, 307, 321
Deoxyribonucleic acid, abbreviations, 244t
Dependent clauses, See Subordinate clauses
Derivations, mathematical, 23, 25
Derivative works and copyright, 78
Descriptors, See Chemical descriptors, Nuclide
descriptors, Stereochemical descriptors
Designation digits for superatoms, 246,
250–251
Determinants
italic type for elements, 212
as mathematical concept, 211
roman type for determinants, 213
Deuterium, representation, 264, 266
Dictionaries
ACS desk references, 127, 135
as CIF component, 97–98, 284
as markup language component, 93, 97
“Different from” in comparisons, 49
Digital Object Identifier (DOI) system, 317,
319
Digital photographs, 360, 361f
See also Photographs
Digits, See Numerals
Dimensions of ACS publications, 354t
Directions in crystals, 267–268
“Discussion” section in scientific papers, 7, 23
Disks for figure files, 364
Display equations
in abstracts, 22
numbering of, 220
punctuation before and after, 221
style and usage guidelines, 211, 218
See also Equations
Displayed chemical reactions, See Chemical
reactions
Dissertations, cited as reference, 292t, 309–
310, 321, 325
Distiller (software), 351
Index ➤ 401
Ditto marks in tables, 372
Division, slash or negative exponent use, 220,
224
DNA, abbreviations, 244t
Document root elements (markup language
component), 91
Document titles, U.S. government publica-
tions, 312
DOI (Digital Object Identifier) system, 317, 319
Dollar sign, spacing, 203
Dot, centered, See Centered dot
Dot, superscript, 266
Dot-matrix printers
avoiding for artwork, 353b, 360
TrueType font format, 357b
Dot size and spacing in halftone art, 347
Dots per inch (dpi), in figure preparation, 346
Dotted lines in figures, 358
Double-blind peer reviews, 71
Double bonds in chemical formulas, 267
Double negatives, avoiding, 45
Double surnames, hyphenation, 139, 152–153
Doubled letters, hyphenation rules, 136
Draft manuscripts, processing of, 27–31
See also Editorial process, Electronic sub-
mission of manuscripts
Drawing programs, 350–352, 363, 378–383
Drawing settings for ACS publications, 381b
Drawings, 347
See also Artwork, Figures, Illustrations,
Structures
DrawIt (software), 379–380, 380b
Drug names
capitalization, 145
in titles, 20
Dublin Core Schema, 90
“Due to”, 51, 111–112
DVDs, cited as references, 293t, 322–323
e (base of natural logarithm)
“exp” vs e, 217
roman type use, 213
“e” for negative electron, 256
“e” or “E” in scientific notation, 210
E prefix in chemical names, 236
“Each”, subject–verb agreement, 107
“Earth”, capitalization, 148
“Ed.” vs “ed.”, 301, 302
EdiKit (software), 60
Editable fonts, description, 357b
Editing services, 75
Edition numbers for books, 301
Editor names in references, 292t, 293t, 300–
302, 320
Editorial computer systems, See Electronic
submission of manuscripts
Editorial lists, See Lists, editorial
Editorial Manager (software), 60, 62, 69t
Editorial process
accepted manuscripts, 33–35
CIF format and text editors, 286
datument potential, 98–99
draft manuscripts, 27–31
editing services, 75
electronic submissions process, 31, 32,
59–69
figure submissions, 343, 346–347, 363–365
final manuscripts, 32–33
proofreaders’ marks, 36–39
reference management programs, 326
review of manuscripts, 31
structure submissions, 381–382
table submissions, 374
Editorial style, See specific aspects, e.g., Capi-
talization
Editors (in general)
CIF validation reports, 286
ethical guidelines, 12–13
proofreaders’ marks, 36–39
role, 33
See also Editorial process
Educational use of copyrighted materials, 80
“Effect” vs “affect” or “impact”, 52
“e.g.”, 117, 160
“Either”, subject–verb agreement, 107
“Either … or”, 47
eJournal Press, editorial systems, 60, 69t
EJPress (software), 60, 62, 69t
Electric charges, indicating, 256
Electricity and magnetism, symbols and SI
units, 278–279
Electrochemistry, symbols and SI units, 279
Electron charges, indicating, 256
Electron shells and subshells, chemical con-
ventions, 256
Electronic bulletin boards, 16
Electronic file formats, See Computer file
formats
Electronic lists and newsgroups, 293t, 322
Electronic mail (e-mail)
manuscript submission via, 31, 59, 61–64
messages cited as references, 293t, 316, 322
permission requests via, 84b
word usage, 50
Electronic mail (e-mail) addresses
for author home pages, 62
for corresponding author, 21
format of, 157
line break guidelines, 157
word choice, 50
Electronic media, See Internet, Web entries,
World Wide Web sites
402 ➤ The ACS Style Guide
Electronic reprints, 35
Electronic source citation
CD-ROMs and DVDs, 293t, 322–323
computer programs, 314, 323–325
data sets, 314
e-mail messages, 293t, 316, 322
examples, 292t–293t
Internet sources, 292t–293t, 314, 316–322
lists and newsgroups, 293t, 322
online books, 293t, 314, 319–320
online periodicals, 292t, 297, 298, 317–319
Web sites, 293t, 314, 320–321
Electronic submission of manuscripts
acceptance of manuscripts, 64
advantages of, 59
author home page, 62, 63, 64, 68
CIF format issues, 63, 284–286
editorial process overview, 30, 31, 32
format and procedural issues, 61–62
preparation of figures, 343, 346–347, 360,
363–365
preparation of materials, 60–61
preparation of structures, 382b
revision of manuscripts, 63–64
software and URLS for selected systems, 60,
65t–67t
submission process, 63
text and image formats for selected systems,
61, 68, 69t
tracking of manuscripts, 62, 63, 64
Element symbols
capitalization, 145, 240–241, 257
concentrations, 271
crystallographic indices, 268
isotope specification, 263–266
in locants and descriptors, 233, 234, 238t, 240
nuclide descriptor specification, 261–263
oxidation number spacing, 262–263
roman type use, 159, 234, 241
spectroscopy data reporting, 276
See also Symbols
Elements, chemical, See Chemical elements
Elements (markup language component),
91–94, 96–97
Elements of determinants and matrices
italic type use, 212
as mathematical concept, 210
Ellipses
in mathematical equations, 219
within quotations, 126
with series items, 127
Elliptical clauses as sentence modifiers, 113
Elsevier software, 379–380, 380b, 381
Em dash
with CASSI abbreviations, 295
for clarity, 126
Em dash—continued
with nonrestrictive clauses, 126
in tables, 372
Embedding
artwork in text, 30, 364, 374
data in Excel files, 353b
font software in files, 357b, 363
En dash
for bonds, 267
between components of mixture, 121, 126,
222, 261
in multiword concepts, 124–125
in phrases used as unit modifiers, 143
with range of three or more items, 117, 125,
209, 289
with reference citation numbers, 117, 209,
289
with surnames, 125, 139
Encapsulated PostScript format, See EPS
format
enCIFer program, 286
Enclosing marks
in mathematical copy, 212, 214–216, 217
in mathematical equations, 219–220
nesting order, 216
with ranges in scientific notation, 209
roman type use, 212
in set notation, 222
with syllabic portion of chemical names, 240
with units of measure in tables, 371
See also Angle brackets, Braces, Parentheses,
Square brackets
Encyclopedias, citation guidelines, 293t,
305–306, 320
End-of-line breaks, See Line breaks
End-of-line hyphenation, chemical names,
233, 241, 247–249
End-of-line punctuation, display equations,
221
EndNote (software), 326–327
Energy levels, indicating, 256
English units system, background of, 228
“enn” in element names, 260
“Ensure” vs “assure” or “insure”, 52
Enumerated lists, 123, 206–207
Enzymes, 155
Eponyms, capitalization, 144
EPRESS (software), 60
EPS format, 61, 351–352, 357b, 363
“eq” or “eqs”, 221
“Equals” vs equal sign, 211
Equations
abbreviation use, 160, 211, 217
in abstracts, 22
citing in text, 221
concentrations in, 271
Index ➤ 403
Equations—continued
definition of variables following, 225
displayed, 22, 211, 218, 220, 221
“Experimental” section contents, 23
identifier use and placement, 220
letters for sequencing, 220
numbering, 206, 220, 272, 377
as part of sentence, 221
running into text, 220
spacing guidelines, 219
See also Structures
Equilibrium reactions, arrows for, 272
Equipment
capitalization of names, 147
identification in text, 22, 23
Errata, publication of, 8, 35
Error analysis, 6
Errors
corrections, 34–35, 36
in manuscript submission, 60
by printers, 34
in references, 24, 30, 31
in research
author obligations, 3–4, 6, 8
editor obligations, 13
peer-review purpose, 72
reader obligations, 9–10
“Erythro”, italicization and capitalization, 145
ESPERE (software), 60
Esters, capitalization examples, 237t
“et al.”, 117–118, 289, 291, 312
Eta (η) in chemical names, 235
“etc.”, 160
Ethers, capitalization examples, 237t
Ethical guidelines
authors, 16
editors, 12–13
purpose of, 11–12
reviewers, 15–16
scientists publishing in popular literature, 16
Ethics in scientific publication
attribution and context concerns, 7–8
author obligations, 3–8, 13–15, 16, 27
authorship determination, 6–7
editor obligations, 12–13
full disclosure of results, 5–6, 8
reader obligations, 9–10
reviewer obligations, 8–9, 15–16, 73, 74, 76
significance and timeliness concerns, 4–5
when to publish, 4–6
European Inventory of Existing Commercial
Chemical Substances, 254
Event names, capitalization, 148
“Ever”, hyphenation in unit modifiers, 141
“Every”, subject–verb agreement, 107–108
Excel (software), 61, 352, 353b
Excerpts, See Extracts (textual)
Excited electronic states, 262
Exclusive rights, copyright ownership, 78
“exp” vs “e”, 217
Experimental details
in scientific papers, 22–23, 25
in tables, 373
Exponents
“exp” vs “e”, 217
negative, for division, 224
scientific notation, 210
after subscripts, 216
Extensible markup language, See XML
Extensible stylesheet language, 90
Extracts (textual)
copyright basics, 80, 83–86
indentation and punctuation, 122
See also Quotations
Extraneous words and phrases
in article titles, 20
examples, 54–55
“f” and “ff” in pagination, 297, 304
Fabrication or falsification of data, 4, 10
Faces, crystallographic notation, 267–269
Fahrenheit, capitalization, 225
Fair use, interpretation, 80–81
Family names (personal), See Surnames
Family names (taxonomic), capitalization,
145–146
Fax, permission requests via, 84b
Federal government publications, See U.S.
government publications
Federal Register, cited as reference, 313
Fences, See Enclosing marks
“Few”, subject–verb agreement, 108
“Fewer” vs “less”, 51
Fictitious names, not to be listed as authors, 14
“Figure”, capitalization, 143, 345
Figure captions, See Captions
Figures
abbreviation use, 225, 365
abstract guidelines, 22
ACS copyright policy, 30
adapted, 84b, 366
citing, 143, 345–346
color use, 344, 345b, 346, 350, 353b
combos, 347, 349f, 352t, 363
credit lines, 366, 367b
data set references, 314
definition, 344
editorial process, 29–30, 363–365
electronic file formats, 350–352
electronic submission of manuscripts, 61
“Experimental” section contents, 23
grayscale line art, 347, 349f, 352, 363
404 ➤ The ACS Style Guide
Figures—continued
halftone art, 347, 348f, 360–362, 363
labeling for editorial processing, 364
line art, 347, 348f, 349f, 352, 354–360, 363
vs lists, 374
numbering, 206, 345, 365
permission requirements, 30, 83–86, 366
photograph reproduction, 360–362, 363
preparing, 343, 346–354, 360
problems to avoid, 358f, 359f, 361f
publication costs, 344
publication medium, 346, 351–352
references in, 289
scanning tips, 353b, 354, 355b, 360, 362
sizing, 352–354, 355
submitting, 343, 346–347, 363–365
as supporting information, 25
symbol use, 353b, 356, 358f, 363, 365
type sizes and fonts, 353b, 356b, 357b, 358f,
360, 363, 365
types of artwork, 29, 347–349
Web-enhanced objects, 25–26
when to use, 344, 370
See also Artwork, Captions, Illustrations,
Photographs, Structures
File formats, See Computer file formats
File names for figure files, 364
File transfer protocol sites
cited as reference, 320
electronic submission of manuscripts,
61–64
Filters, reference manager programs, 326–327
Final manuscripts, processing of, 32–33
See also Editorial process, Electronic sub-
mission of manuscripts
Financial aid, “Acknowledgments” section
contents, 24
Firefox Web browser, markup language
display, 94
First person
as gender-neutral alternative, 58
writing style, 43–44
Five-digit numbers, 207–208
5′ to 3′ direction for nucleotide sequences, 244
FIZ Karlsruhe, ICSD database, 284
Flow charts, 347
“fold” as suffix, 138, 205
Fontisworks (software), 60
Fonts
copyrights, 357b, 363
in figures, 356b, 357b, 358f, 363, 365
in structures, 380
for symbols, 365
Footnotes
abbreviation use in, 160
for charts and schemes, 377, 378
Footnotes—continued
for contributor information, 14
for corresponding author information, 21
for hazard indication, 23
for references, 24, 152
in tables, 371, 372–373
“For” as coordinating conjunction, 47, 113
“For example”, 117, 160
Foreign-language journals, cited as reference,
299
Foreign (non-U.S.) patents, 208, 310–311
Foreign phrases as unit modifiers, 139
Foreign surnames, 139, 152–153
Formal names, foreign, 152–153
Formal tables
definition, 370
submitting, 374
See also Tables
Formats
computer files, See Computer file formats
scientific papers, See Standard format for
scientific papers
Formulas, See Chemical formulas
Four-color format, 350
Four-digit numbers, 207
Fractions
vs decimals, 208
hyphenation, 139
numeral and word usage, 205
spacing when built-up, 219
subject–verb agreement, 108
in subscripts and superscripts, 217
Fragmentation of research reports, avoiding,
13–14
Fraud, ethical considerations, 74
Free radicals, 266
Free style format for computer program cita-
tion, 324–325
Freehand (software), 351–352, 363
Freeware drawing programs, 379–381
“From … to”, 125, 209
FTP sites
cited as reference, 320
electronic submission of manuscripts, 61–64
Full disclosure of research results, 5–6, 8
Full papers, See Journal articles
Functions
italic type use, 212
as mathematical concept, 210
spacing considerations, 214–215, 219
G for general labeling, 265
g infix in copolymer names, 243
Galleys, marking of, See Proofreaders’ marks
and sample markups
“Gamma (γ) ray”, capitalization of “r”, 149
Index ➤ 405
“Gamma” vs γ, 155
Gas state, indicating in reactions, 272
Gender-neutral language, 57–58
General chemistry, symbols and SI units,
279–280
Generic names, 23, 157
Generic structures, in combinatorial chemis-
try, 246, 250–251
Genotypes, italic type use, 154
Genus names
abbreviating vs spelling out, 160
capitalization, 145–146, 150
italic type use, 154
Geographical locations
capitalization, 148
comma use, 118
postal abbreviations, 161–162
See also Place of publication
Geometric lines and points, roman type use,
213, 223
Geometric planes, italic type use, 212, 223
GIF format, 351–352, 357b, 361, 381
Giorgi system, background of, 228
Given names
byline format, 21
foreign name format, 152
hyphenation, 139
See also Author names in references, Author
names in text, Initials, Personal names
Glutamic acid/glutamine mixtures, 245
“Gold book” (IUPAC), 97
Gossett, W., and Student t test, 223
Government, U.S., See U.S. entries
Government publications, cited as reference,
292t, 295, 311–314, 321
graft infix in copolymer names, 243
Grammar overview, 41–50, 105–108
See also specific aspects
Grammatical rank, definition, 46
Grant agencies’ manuscript submission
systems, 60, 65, 67t
Grant numbers, in “Acknowledgments”
section, 24
Graphic artwork, See Artwork, Illustrations
Graphics Interchange Format, See GIF format
Graphs
editorial process, 29
examples, 358f, 359f
types of artwork, 347
when to use, 344
working with, 363
Grayscale line art
definition, 347
example, 349f
file formats, 352
working with, 347, 363
“Greater than” vs “in excess of” or “over”, 51
Greek letters
alphabet, 214
for bonding orbitals, 256
in Boughton system for isotopes, 266
for chemical and physical terms, 155
in locants and descriptors, 233, 235, 238t,
240
in mathematical copy, 214
for subatomic particles, 256
in titles and headings, 150
in unit modifiers, 142
See also specific letters
Greek multiplying prefixes, hyphenation,
136–137
“Green book” (IUPAC), 255
Grid marks in figures, 359
“Group”
as collective noun, 106
lowercased for periodic table references, 261
Group names, isotope position, 265
Group numbers, periodic table references, 261
Grouping of digits in long numbers, 207–208
Grouping words, 50–51
Gunda, Tamas, software reviews by, 380
H in chemical names, 235
η (eta) in chemical names, 235
Halftone art
definition, 347
example, 348f
working with, 347, 360–362, 363
See also Figures, Photographs
Handbooks
description, 18
standard format, 19
Hapticity, η to indicate, 235
Hard copy
checking proofs, 34b, 36
figure printing, 353b, 360
figure submission, 364
manuscript submission, 31
proofreaders’ marks and sample, 36–39
reprint orders, 35
table submission, 374
Hard returns, avoiding in tables, 374
Hazards, identification of, 13, 21, 23
“He”, gender-neutral alternatives, 57, 58
Headings
capitalization, 148–151, 227, 237t–239t
parallelism, 48, 371
Hebrew surnames, 152
Helping verbs, in compound sentences, 108
Helvetica typeface, 356b, 363
Hermann–Mauguin symbols, 269
HighWire Press, editorial systems, 60, 69t
406 ➤ The ACS Style Guide
“His”, gender-neutral alternatives, 57, 58
“Home Page” designation for citations, 320
Host organization information for Web sites,
321
HTML (hypertext markup language), 89–90,
98
“http://”, See Uniform resource locator
HTTP protocol, electronic submission of
manuscripts, 61
Hungarian surnames, 153
Hydrogen, H in chemical names, 235
Hydrogen bonds, representation, 244, 267
Hydrogen ion concentration, negative loga-
rithm of, 271–272
Hydroxide ion concentration, negative loga-
rithm of, 271–272
Hydroxyl group, attachment of, 244
Hyperlinks
reference errors as issue, 24, 30, 31
supporting information availability, 25
Hypertext markup language, 89–90, 98
Hyphenation
ACS desk reference use, 135
in amino acid sequences, 245
in CAS Registry Numbers, 254
with CASSI abbreviations, 295
chemical names at end of line, 233, 241,
247–249
color combinations, 125, 140
compound words, 138–139
e-mail addresses, 157
foreign phrases as unit modifiers, 139
with isotope names or symbols, 264–266
locants and descriptors, 234–236, 240, 241,
270–271
of position numbers, 246
prefixes, 135–137, 152, 240, 247–249
proper nouns and adjectives, 137, 138, 143,
152
spelling recommendations list, 129–133
suffixes, 137–138, 151, 152, 247–249
surnames, 139, 152–153
syllabic portion of chemical names, 233,
240, 241
two-word vs phrasal verbs, 139
unit modifiers, 138, 139–143
with unit of measure, 141–143
Web addresses, 156
“I”, writing style, 43–44
i (imaginary number), 213
“Ibid.” in reference lists, 298, 326
“-ics” ending for nouns, 107
ICSD database, 284
“Idem” in reference lists, 298, 326
“Identical to” and “identical with”, 49
Identification numbers, CAS Registry
Numbers, 91, 94, 253–254
Identifiers in markup languages, 92, 94
Idioms used in comparisons, 49–50
“i.e.”, 117, 160
“II” or “III” in personal names, 117, 291, 301,
309, 312
“Ill”, hyphenation in unit modifiers, 141
Illustrations
adapted, 84b, 366
combinatorial chemistry representation,
251
datument potential, 98
editorial process, 33, 363–365
electronic submission of manuscripts, 60,
61, 69t, 364
handling tips, 364–365
labeling for editorial processing, 362, 364
permission requirements, 83–86, 366
prescreened and rescreened, 362, 363
submitting, 363–365
types of, 29, 347–349
See also Artwork, Figures, Photographs
Illustrator (software), 351–352, 363
Image file formats, See Computer file formats
“Impact” vs “affect” or “effect”, 52
“In”, with titles in reference citations, 300, 301
“In excess of” vs “greater than” or “more than”,
51
“In press” references, 315
“in.”, unit of measure, 118, 223
“Inc.” in publisher name, 303
InChI (International Chemical Identifier), 91,
94, 101–102, 383
“Including”, restrictive and nonrestrictive uses,
116
Indefinite pronouns, subject–verb agreement,
107–108
Independent clauses
definition, 42
semicolon use, 119–120
Independent contractors and copyright, 78
See also Works made for hire and copyright
“Independently”, 55
Index, mathematical concept, 211
Indexes of chemical substances and formulas,
253, 254
Indexing
and abstract content, 21
and byline format, 21
and foreign surname format, 152
and title wording, 20
of U.S. government publications, 312
Web-based search engine use, 383
Indices, crystallographic, 267–268
Infinitive phrases as sentence modifiers, 113
Index ➤ 407
Infinitives
capitalization, 149
as sentence modifiers, 113
split, 43
Informal tables
definition, 370
submitting, 374
See also Tables
Information components, description, 99
Information for authors, See Author guidelines
Information retrieval
abstract content as factor, 21
title wording as factor, 20
See also Databases, Indexing, Literature
searches
Initials
byline format, 21
in e-mail addresses, 157
in foreign names, 152, 153
for genus name, 160
period use and spacing, 119
reference format, 153, 291, 301, 309, 312
Ink cartridges for figure artwork, 353b, 360
Inkjet printers
Excel figure specifications, 353b
TrueType font format, 357b
Inorganic compounds, capitalization exam-
ples, 237t
Inorganic crystal structures, data sources, 284
Inorganic molecules, CIF format requirement,
284
Institutional and organizational names
byline format, 21
capitalization, 147
reference format, 301, 307, 308, 312, 321
See also Company names, Publisher names
Instructions for authors, See Author guidelines
Instrumental diagrams as supporting informa-
tion, 25
“Insure” vs “assure” or “ensure”, 52
Integrity issues, See Ethics entries
Intellectual property and DOI use, 317
See also Copyright, Patents, Permissions
inter infix in copolymer names, 243
Interchange of structure data, 381
Intermediates, in combinatorial chemistry, 251
Intermetallics, crystal structure data sources,
284
International aspects of research and publish-
ing, 59
International Bureau of Weights and Mea-
sures, 255
International Chemical Identifier, See InChI
International CODEN Directory, 340
International Committee on Weights and
Measures, 228
International Organization for Standardiza-
tion (ISO), 255, 276, 294
International System of Units, See SI units
The International System of Units (SI), 255
International Union of Biochemistry and
Molecular Biology, 233
International Union of Crystallography
(IUCr), 63, 284, 286
International Union of Pure and Applied
Chemistry (IUPAC)
centered dot spacing style, 261
chemical conventions, 255
chemical terminology, 97
compound nomenclature, 233
and InChI, 101–102, 383
structure representation standards, 383
unnamed elements, 260
Internet
citation of Internet sources, 292t–293t, 314,
316–322
terminology list, 163–168
See also Electronic source citation, Web
entries, World Wide Web sites
Interviews, punctuation, 122
Introductions in scientific papers, 7, 22
Introductory words and phrases, 48, 114
Investigator integrity issues, See Ethics entries
Iodine symbol, avoiding confusion with
numerals, 262
Ion concentrations, negative logarithms of,
271–272
Ionic charges, indicating, 262, 266
IR spectroscopy, reporting analytical data, 275
“Is” instead of equal sign, 211
ISIS/Draw (software), 379–380, 380b, 382b
ISO, See International Organization for Stan-
dardization
“iso” prefix, style for, 236
Isolation of products and intermediates, 251
Isotopes
“a” or “an” before, 257, 264
labeling, 264–266
mass numbers, 261, 263
modified vs unmodified compounds, 264
substituted compounds, 264, 265
Issue information in references, 296
Italic type
in Boughton system for isotopes, 266
with CASSI abbreviations, 295
for chirality symbols and symmetry site
terms, 270
combinatorial chemistry representation, 246
in copolymer names, 243
in crystallography, 269–270
for definitions, 154
for emphasis, 153
408 ➤ The ACS Style Guide
Italic type—continued
for genotypes, 154
group names indicating isotope position, 265
in locants and descriptors, 233, 234–236
m for molal, 155
in mathematical copy, 160, 212, 223
for orbital axes, 256
plurals of abbreviations ending in, 161
in polycyclic aromatic compounds, 240
reference citation guidelines, 30, 287
references to books, 154, 301, 306
references to computer programs, 323
references to meetings and conferences, 308
references to periodicals, 154, 294, 296,
298–299
references to technical reports and bulletins,
314
references to U.S. government publications,
312
for restriction endonucleases, 155
in subscripts or superscripts, 216, 373
for taxonomic names, 154, 160
for word’s first occurrence, 154
IUCr, See International Union of Crystallography
“ium” in element names, 260
IUPAC, See International Union of Pure and
Applied Chemistry
Japanese surnames, 153
Jargon, avoiding, 20, 54
JMol (software), 379, 380b
Joint Photographic Experts Group format,
351, 352t, 357b
Journal articles (in general)
artwork sizing, 354, 370, 373
CIF format, 285–286
description, 18
standard format, 19
structure representation future, 383
translations, 299
word choice, 50
See also ACS journals and magazines, Man-
uscript entries, Scientific papers
Journal as reference
article titles, 291, 292t
author names, 291, 297–299
CASSI abbreviations, 291, 292–293,
328–339
CASSI sample entry, 340–341
commonly cited journals, 328–339
examples, 292t–293t
foreign-language journals, 299
generic format, 291
multiple parts, sections, and series, 295
repeated information, 297–298
same main title, 295
Journal as reference—continued
special situations, 299
volume information, 295, 296, 297–298
See also Periodical as reference, References
Journal editors, See Editors
Journal of Combinatorial Chemistry, 246, 251
Journal presentations
standard format, 19–20
types of, 18–19
See also ACS journals and magazines, Man-
uscript entries, Scientific papers
Journal titles
foreign-language journals, 299
italic type use, 154
series titles as, 306
JPEG format, 351, 352t, 357b
“Jr.” and “Sr.”, 117, 291, 301, 309, 312
Judicial decisions on copyright, 80, 82
Kappa (κ) in chemical names, 235
“Kappa” vs κ, 155
Kelvin, degree symbol not used with, 227
Ketones, capitalization examples, 237t
Keys to artwork symbols, 356, 365
Keywords
in article titles, 20
for series publications in CASSI, 306
Kinetics, symbols and SI units, 277–278
Kingdom names (taxonomic), capitalization,
145–146
Kirk-Othmer Encyclopedia, cited as reference,
305–306
Korean surnames, 153
L in chemical names, 236, 245, 270–271
Labeling
of axes on illustrations, 225, 359–360
of computer disks, 364
of illustrations for editorial processing, 362,
364
isotopic, 264–266
numeral use, 206, 289, 325, 345, 365
of speculations, 7
See also Numbering
Laboratory reagents, See Reagents
Language issues, See Word usage
Laser printers
Excel figure specifications, 353b
TrueType font format, 357b
Latin multiplying prefixes, hyphenation,
136–137
Latin terms
abbreviations, 158–159
plural forms, 128
roman type use, 154
See also Taxonomic terms
Index ➤ 409
Lattices, crystallography, 268, 269
Laue indices, crystallography, 267
Laws
cited as reference, 313
copyright laws, 77–82
Laymen, word choice for, 16
Legends for artwork symbols, 356, 365
Length of manuscript, limiting, 5, 25, 32
“Less” vs “fewer”, 51
Letters (journal presentation type), 18
“Levels” with plurals of symbols, 211
Libraries, combinatorial, 246, 250–252
Licensing arrangements and copyright, 82
Ligating atom, κ to designate, 235
Ligation sites, 234
“like”, hyphenation as suffix, 138
Limits, mathematical spacing, 219
Line art
definition, 347
example, 348f
file formats, 352
working with, 347, 354–360, 363
See also Figures
Line breaks
e-mail addresses, 157
equations, 220
reaction schemes, 379
Web addresses, 156
Line graphs
color use, 345b
examples, 358f
types of artwork, 347
when to use, 344
working with, 363
Line returns, avoiding hard returns in tables,
374
Line styles
for figures, 345b, 358
for structures, 378
Line width
for figures, 353b, 355, 358, 363
for structures, 378
Linear vs angular formulas, 267, 376
Lines (geometric) in mathematical copy, 213,
223
Liquid state, indicating in reactions, 272
Lists
numbered, 123, 206–207
parallelism, 48
vs tables, 370, 374
See also References, Series items
Lists, editorial
abbreviations, acronyms, and symbols,
169–202
abbreviations not needing definition, 158–
159, 217–218, 260
Lists, editorial—continued
CASSI journal abbreviations, 328–339
chemical name hyphenation, 247–249
computer and Internet terms, 163–168
geometric notation symbols, 223
Greek alphabet, 214
mathematical operators and symbols,
217–218
multiplying prefixes, 136–137, 240
non-SI units, 231t, 232t
physical quantities and symbols, 277–283
plurals, tricky, 128
polymer names, 242
postal abbreviations, 161–162
prefixes not to be hyphenated, 135–136
scientific prefixes, 135–136, 235
set notation symbols, 222
SI and SI-derived units, 229t–230t
spelling and capitalization recommenda-
tions, 129–133
statistical symbols, 223
surnames as modifiers, 152
surnames as units of measure, 152, 225
transport properties, 212
trigonometric and other functions, 213
unit modifiers, 140
words and phrases to avoid, 54–55
Literary vs scientific writing, 28
Literature reviews, See Review articles
Literature searches
author obligation for, 13
reference management programs, 326
“Little”, hyphenation in unit modifiers, 141
Local government publications, cited as refer-
ences, 313–314
Locants
capitalization, 145, 238t–239t, 240
and chirality, 270
hyphenation, 234–236, 240, 241, 270–271
and isotopic labeling, 264–266
for saccharides, 243
style guidelines, 234–236
types of, 233
Logarithms of ion concentrations, 271–272
Loose-leaf services, 314
“-ly” words, hyphenation, 141
m for molal, 155
M for molar, 155, 271
Machine-understandable information and
data, See Datuments, InChI
Macintosh platform
reference manager programs for, 327
templates for, 29
Macromedia software, 351–352, 363
Macromolecular CIF format, 284
410 ➤ The ACS Style Guide
Macromolecular structure data sources, 284
Magazines, See ACS journals and magazines,
Periodical as reference
Magnetism and electricity, symbols and SI
units, 278–279
“Man”, gender-neutral alternatives, 57–58
Manufacturer names, parentheses use, 22, 124
See also Company names
Manuscript Central (software), 60, 62, 69t
Manuscript confidentiality, 9, 12, 15, 16,
73–74
Manuscript format standards, See Standard
format for scientific papers
Manuscript marking by proofreaders, 36–39
Manuscript processing stages
accepted manuscripts, 33–35
draft manuscripts, 27–31
final manuscripts, 32–33
proofreaders’ marks, 36–39
resubmission of manuscripts, 76
review, 31, 71–76
See also Editorial process, Electronic sub-
mission of manuscripts
Manuscript review, See Peer review
Manuscript submission, See Electronic sub-
mission of manuscripts, Manuscript
processing stages
Manuscript templates, See Templates
“Many”, subject–verb agreement, 108
Marking proofs, 34b, 36–39
Markup languages, See Datuments, specific
languages
Mass numbers, 261, 263–264
Mass spectrometry, symbols, 266
Mass spectroscopy, reporting analytical data,
275
Material Safety Data Sheets (MSDSs), 292t,
315
Materials, identification in text, 22
“Materials and Methods” sections, 22–23
Mathematical arguments, See Arguments
(mathematical)
Mathematical concepts, definitions, 210–211
Mathematical constants
as concept, 210
defining symbols in text, 211
Greek letter use, 214
italic type use, 212
roman type use, 213
standard abbreviations and symbols, 211,
217–218
Mathematical derivations, 23, 25
Mathematical derivatives, 219, 220
Mathematical equations, See Equations
Mathematical expressions in superscripts and
subscripts, 217
Mathematical expressions in text
numeral and word usage, 205, 206
punctuation, 124
when to use, 220
Mathematical operators
as concept, 210
link breaks after, 220
roman type use, 212
spacing considerations, 214–215, 217, 219,
222
standard abbreviations and symbols,
217–218
See also Mathematical symbols, Minus sign,
Plus or minus sign (±), Plus sign,
Times sign (×)
Mathematical papers, sentence modifiers in,
112
Mathematical symbols
abbreviations differentiated from, 160,
211
case sensitivity, 159
defining in text, 211
italic type use, 160, 212, 216
not needing definition, 211, 217–218
plural forms, 211
spacing as adjectives, 215
specialized notation, 222–223
See also Abbreviations, Mathematical
operators, Symbols, Units of measure,
specific symbols
Mathematical variables
as concept, 210
definitions after equations, 225
definitions in text, 211
Greek letter use, 214
italic type use, 212
roman type use, 213
spacing between, 215, 219
Mathematics usage and style
abbreviations, 211, 217
boldface type, 213
enclosing marks, 214–216, 217
equations, 218–222
geometric notation, 223
Greek letters, 214
italic type, 160, 212, 223
mathematical concept definitions,
210–211
ratio and mixture notation, 222
roman type, 212–213, 214, 223
script and open-faced letters, 214
set notation, 222
spacing, 214–215
statistics, 223
subscripts and superscripts, 216–217
symbols, 211, 217–218
Index ➤ 411
Matrices
boldface type for matrices, 213
italic type for elements, 212
as mathematical concept, 210, 211
roman type for transposes, 213
MDL molfile format, 382
MDL software, 380b
“Me” vs “myself”, 51
Measure, units of, See Units of measure
Mechanics, symbols and SI units, 280
Meeting and conference presentations
reference format, 56–57, 292t, 307–309,
321, 323
standard reporting format, 19
types of books, 17
Meeting references, “Acknowledgments”
section contents, 24
Melting points, reporting analytical data, 274
Members, in combinatorial chemistry, 250–252
Merriam-Webster’s Collegiate Dictionary,
127, 135
Metadata
datument as scientific grid component,
99–100
definition, 89–90
Metal-organic crystal structures, data sources,
284
Metals, crystal structure data sources, 284
“Methods” as CASSI keyword, 306
“Methods” sections, 22–23
Metric system, background of, 228
Metric units, technical documents usage, 223
Microsoft font format, 357b
Microsoft software
electronic submission of manuscripts, 61
figure prepration, 352, 353b, 363
structure preparation, 379
table preparation, 373
Miller indices, crystallography, 267
Millions, 204, 210
Minus sign
and chirality, 271
for electric charge, 256
for ionic charge, 262, 266
for optical rotation, 270
as stereochemical descriptor, 236
structure preparation, 379
Minutes symbol, spacing, 203, 224
Mirror plane, crystallographic symbols, 269
Misplaced modifiers, 44
Mixtures
amino acid representation, 244
combinatorial chemistry representation,
246, 250–252
notation, 222
punctuation, 121, 126, 222, 261
MKSA system, background of, 228
mmCIF format, 284
Model numbers
capitalization, 147
numerals used as names, 206
parentheses use, 22
Modeling programs as supporting informa-
tion, 25
Modification, isotopic, 264
Modifiers
dangling, 45, 109–112
misplaced, 44
prepositional phrase, 49
sentence, 44–45, 112–113
surnames as, 151
See also Adjectives, Unit modifiers
Mol Draw (software), 380b
Molecules
CIF format requirement, 284
datument potential, 88, 99
electronic configuration, 257
InChI identifiers, 91, 94, 101–102
isotopic substitution, 264
markup languages and the WWW, 91,
92–94, 95f, 96
modeling coordinates as supporting infor-
mation, 25
nuclide descriptors, 261–263
symbols and SI units, 277
molfile format, 382
Monetary values, U.S., 208
Monographs
description, 18
editorial process, 33
standard format, 19
Monosaccharides, abbreviations, 243t
Month abbreviations, 160–161
“Moon”, capitalization, 148
“More than” vs “in excess of” or “over”, 51
“Most”, subject–verb agreement, 108
Movies
still shot permissions, 83
Web-enhanced objects, 25, 26
MSDSs (Material Safety Data Sheets), 292t, 315
“Mu” (µ), spelling out, 155
Multidimensional physical quantities, boldface
type use, 213
Multiple authors
author home page, 62
book categories, 17, 18
byline format, 21
editorial process, 33
ethical issues, 6–7, 14
reference citation format, 289
reference collation, 325
references to books, 300–301
412 ➤ The ACS Style Guide
Multiple authors—continued
references to periodicals, 291
U.S. government publications, 312
See also Authorship
Multiple prefixes, hyphenation, 136
Multiplication
asterisk use, 211
with compound units of measure, 224
spacing guidelines, 215, 219, 224
Multiplication sign (×), 215
Multiplying prefixes
hyphenation, 136–137, 240
numbers style, 207
SI system, 229t
spacing considerations, 226
Multisyllabic words, hyphenation of suffixes,
138
Multiword concepts, en dash use, 124–125
Mutation, designation of, 246
“My”, writing style, 44
myICAAP (software), 60
“Myself” vs “me”, 51
N- vs N-, when to italicize, 235
n before volume number in CASSI, 295
N for normal, roman type use, 155, 271
“Namely”, comma use, 117
Names of persons, See Author names in refer-
ences, Author names in text, Personal names
Namespaces (CML component), 92
Naming of chemical compounds, See Chemi-
cal names, Chemical nomenclature
National Academy of Sciences, 4
National Institute of Standards and Technol-
ogy, 228, 255
National Institutes of Health, 10
National Science Foundation, 10
Negative electrons, indicating, 256
Negative exponents for division, 224
Negative logarithms of ion concentrations,
271–272
Negative numbers, “to” or “through” with,
125, 209
“Neither”, subject–verb agreement, 107
“Neither … nor”, 47
“neo” prefix, style for, 236
Nesting order for enclosing marks, 216
Net plane, crystallographic symbols, 268–269
Newsgroups, electronic, 293t, 322
Newspapers
nonscientific periodicals, 292t, 299
special typefaces, 154
“nil” in element names, 260
NMR spectroscopy
reporting analytical data, 274–275
symbols and SI units, 281
“No one”, subject–verb agreement, 107
“No.” in references, 295, 304
“no.” in unit of measure, 118
“Non”, hyphenation as prefix, 137
Non-English terms, plural forms, 128
Non-native English speakers as writers, 28, 75
Non-SI units, lists, 231t, 232t
“None”, subject–verb agreement, 108
Nonexclusive rights arrangements, 82
Nonhyphenated prefixes, 135–136
Nonrestrictive phrases or clauses
comma use, 44, 109, 116
em dash use, 126
grammar overview, 109
Nonscientific periodicals, citing, 292t, 299
Nontechnical style for numbers, 203, 204, 205
“Nor” as coordinating conjunction, 47, 113, 149
“Not … but”, 47
“Not only … but also”, 47
Notation
ChemSet notation, 246, 251–252
mathematical, 222–223
scientific, 207, 209–210
See also Abbreviations, Symbols
“Note” line in table footnotes, 373
Notes (journal presentation type), 18
Nouns
“-ics” ending, 107
collective, 106–107
hyphenation and proper nouns, 137, 138
phrasal verbs as, 139
possessives, 127
predicate, 108
proper, 137, 138, 143, 152
as unit modifier element, 139–140, 143
Nuclear chemistry, atomic number use, 262
Nuclear reactions, reaction scheme, 273
Nucleic Acid Database, 284
Nucleic acids, abbreviations, 244t
Nucleosides, abbreviations, 244t
Nucleotides, sequence presentation, 244
Nuclide descriptors, atoms and molecules,
261–263
Nuclides, isotopic, 264
Number
of atoms, 263
of the subject, 44, 105–108
“Number” as collective noun, 106
Number–unit of measure combination as
singular, 51
Numbered lists, 123, 206–207
Numbering
of charts, 206, 377
of chemical species in text, 206
of equations and reactions, 206, 220, 272,
377
Index ➤ 413
Numbering—continued
of figures, 206, 345, 365
in nucleotide sequences, 244
of references, 206, 289, 325
of schemes, 206, 346, 378
of structures, 206, 246, 346, 375–377
of tables, 206, 369
Numbers
agency report numbers, 312
atomic numbers, 258t–259t, 260, 262
at beginning of sentence, 204
CAS Registry Numbers, 91, 94, 253–254
charge numbers for ions, 262
chemical concentrations, 271–272
comparison words, 51
coordination numbers, 270
crystallographic point groups, 269
data entry numbers, 314, 320, 324
en dash use, 125, 209
of five or more digits, 207–208
of four or more digits, 207
fractions, 108, 139, 205, 208, 217, 219
government publication numbers, 312
group numbers, 261
hyphenation as unit modifiers, 141–143
large numbers, 204, 207–208, 210
mass numbers, 261, 263–264
model numbers, 22, 147, 206
negative, “to” or “through” with, 125, 209
nontechnical style, 203, 204, 205
numeral and word usage, 203–207
ordinal numbers, 203–204, 205, 308
oxidation numbers, 262–263
parallelism in sentence, 204
patent numbers, 208, 310
position numbers, 246
position of atoms, 263
quantum numbers, 257
retaining parts of in ranges, 209–210
single, in unit modifiers, 142, 143
of species in reactions, 272
species numbers, 272
spelling out vs numerals, 160, 203–205
of standards, 276
style overview, 207–210
unit of measure spacing with, 203, 223–224,
271–272
See also Fractions, Numerals, Reference cita-
tion numbers
Numbers of atoms, subscript use, 263
Numerals
with “a.m.” and “p.m.”, 205
arabic vs roman, 206, 369, 375–378
for artwork labeling, 206, 345, 365
in Boughton system for isotopes, 265–266
in bridged and spiro alicyclic compounds, 236
Numerals—continued
chemical species identification, 206
crystallographic point groups, 269
in dates, 160, 205
decimal point guidelines, 208, 372
designation digits for superatoms, 246,
250–251
ellipses use, 127
en dash use, 125
for equation labeling, 289
expressions used in mathematical sense, 205
hyphenation of prefixes and suffixes, 137,
138
in locants and descriptors, 233, 234, 236,
238t, 240
mathematical use and italic type, 212
as names, 206
numeral and word usage, 203–207
for oxidation numbers, 262–263
in ranges, 125, 204, 209–210, 296
in ratios, 205
for reference labeling, 206, 289, 325
in series, 123, 127, 204, 206–207, 209
for species numbers, 272
spelling out vs numerals, 160, 203–205
See also Numbers, Reference citation
numbers
Numerical roots in element names, 260
“N.Y.”, CASSI spacing exception, 294
o- vs O-, when to capitalize and italicize, 145
O- vs O-, when to italicize, 235
O for products separate, 246, 251, 252
Office of Research Integrity, U.S. Department
of Health and Human Services, 4
Official titles
in bylines, 21
capitalization, 147–148
Oligonucleotides, data sources, 284
Oligosaccharides, representation of, 244
“On the basis of” vs “based on”, 52, 111
Online books, 293t, 314, 319–320
See also Web-based publications
“Online” designation for citations, 318–319
Online information, See Electronic source cita-
tion, Internet, Web entries, World Wide
Web sites
Online periodicals, 292t–293t, 297, 298,
317–319
See also Web-based publications
Online submission of manuscripts, See Elec-
tronic submission of manuscripts
“Only”, placement effects on meaning, 45
Ontologies
datument as scientific grid component, 99
definition, 98
414 ➤ The ACS Style Guide
Open-faced letters, 214
Open Journal Systems, 60
OpenType fonts, 357b
Operators, See Mathematical operators
Optical rotation in chemical names, 270
“Or”
vs “and/or”, 56
as coordinating conjunction, 47, 113, 149
number of subject, 106
slash misused for, 56
Oral presentations
citation guidelines, 307, 321
converted to scientific papers, 57
Orbitals, chemical conventions, 256–257
Order names (taxonomic), capitalization, 146
Ordinal numbers, 203–204, 205, 308
See also Numbers
Organic compounds, capitalization examples,
237t
Organic crystal structures, data sources, 284
Organic groups, common abbreviations for,
260
Organic Syntheses, cited as reference, 305
Organizational names, See Institutional and
organizational names
Orientation of artwork on page, 353b, 364, 370
Original research, reports of, See Scientific
papers
“Our”, writing style, 44
“Over” vs “greater than” or “more than”, 51
Overbar for crystallographic point groups, 269
Overview chapters in proceedings volumes, 17
Oxidation numbers, chemical conventions,
262–263
“p” and “pp” in pagination, 304
Page lengths for ACS publications, 354t
Page number citations
books, 304
data sets, 314
DOI use, 317
numbers style, 208
periodicals, 296–297
U.S. government publications, 312
Page proofs, marking of, See Proofreaders’
marks and sample markups
Paper copy, See Hard copy
“Paper” in references, 304
Paper number, references to meetings and
conferences, 308
Paper stock for figure artwork, 353b
Paragon Plus System, 30, 364, 374, 382b
Paragon System
electronic submission of manuscripts, 60,
62, 69t
submission of artwork, 30, 364, 374, 382b
Paragraphs, numbered, 207
Parallel constructions
numeral and word usage, 204
in tables, 48, 371
writing style, 46–48, 50
Parallel synthesis, description, 252
Parameter settings for ACS publications, 381b
Paraphrasing, ethical considerations, 7
Parent elements (markup language compo-
nent), 91
Parentheses
with abbreviations, 158, 160, 225
in axis labels on figures, 359
with chemical prefixes, 236
with chemical reactions, 272, 377
comma use, 115
with compound units of measure, 224
with credit lines, 367b
with crystallographic indices, 267–268
with enumerations, 123, 206–207
with generic names, 23
for isotopic substitution, 264
with manufacturer names, 22, 124
in mathematical copy, 124, 212, 214–216,
219
in mathematical equations, 219–220
nesting order, 216
for oligosaccharides, 244
with optical rotation indicators, 270
with oxidation numbers, 262–263
in polymer or complex chemical names,
146, 151
with ranges in scientific notation, 209
reference citation guidelines, 30, 124, 209,
287, 289–290
references to books, 305
references to periodicals, 296
with standard deviations or standard errors,
208–209
with syllabic portion of chemical names, 240
Parenthetical expressions
abbreviation use, 160
capitalization in titles and headings, 151
comma use, 117
hyphenation as unit modifiers, 143
parentheses use, 123
Parenthetical sentences, 123
Part number in book references, 304, 306
Participles as unit modifier element, 140, 142,
143
Particles in foreign names, 152–153
Parts of a book, capitalization, 144
Passive voice
avoiding, 42, 54
and dangling modifiers, 109, 110–111
as gender-neutral alternative, 58
Index ➤ 415
Passwords for author home pages, 62, 68
Past participles as unit modifier element, 142
Past tense, 42–43
Patent numbers
citation guidelines, 310
style guidelines, 208
Patents
cited as reference, 292t, 310–311
and publication timing, 5
PDF documents
electronic submission of manuscripts,
60–61, 68
figure preparation, 351, 353b, 357b, 363
font tips, 357b
Peer review
author requests regarding, 13, 15–16, 72, 75
author responsibilities, 75–76
CIF validation reports, 63, 286
double-blind reviews, 71
editorial process, 29, 31, 32
electronic submission of manuscripts, 31,
59, 60, 63, 68
encouragement to new investigators, 76
ethical considerations, 5, 8–9
ethical guidelines, 15–16, 73, 74, 76
of journal presentations, 19
online article regarding, 71
PDF file suitability, 60–61
process of, 72–73
purpose of, 71–72
review forms, 73
reviewer ratings, 73
reviewer responsibilities, 73–74
topic suggestions, 74b
Peer X-Press (software), 60, 62, 69t
People as photographic subjects, 82, 361
“Per” in spelled-out units of measure, 225, 226
per infix in copolymer names, 243
Percent sign, spacing, 203, 224, 271
Period
with abbreviations or acronyms, 118, 119,
223, 294
in bridged and spiro alicyclic compounds,
236
with CASSI abbreviations, 294
as decimal point, 208
in declarative sentences, 118
in e-mail addresses, 157
with ellipses, 126
with “et al.”, 289
with parenthetical sentences, 123
with quotation marks, ACS policy, 122
reference citation guidelines, 289–290
references to books, 301–306
references to periodicals, 291, 294, 297–299
references to theses, 309
Period—continued
references to U.S. government publications,
312–313
with units of measure, 118, 223
in Web addresses, 156
“Periodic table”, capitalization, 261
Periodical as reference
abstracts, 298–299
article titles, 291, 292t
author names, 291, 297–299
CASSI abbreviations, 291, 292–293, 328–339
CASSI sample entry, 340–341
examples, 292t–293t
Federal Register citations, 313
generic format, 291
nonscientific magazines and newspapers,
292t, 299
online periodicals, 292t–293t, 317–319
organization or committee as author, 307
pagination information, 296–297
place of publication, 294, 295
repeated information, 297
special situations, 299
volume information, 295, 296, 297–298
year of publication, 296, 297–298
See also References
Periodical titles, italic type for, 154
Periodicals, ACS, See ACS journals and maga-
zines
Permission request form, ACS, 84b
Permission request guidelines, ACS, 83–86, 366
Permission request guidelines, non-ACS copy-
righted material, 83–86
Permissions
from ACS Copyright Office, 84b
ACS copyright ownership benefits, 86
from ACS Publications Division, 79b, 83, 84b
credit line preferences, 85b, 86, 367b
for e-mail and personal communications,
7, 14, 316
for electronic media, 81, 82, 83
fair use guidelines, 80–81
paperwork submission by author, 32, 33
for photographs of people, 82, 361
for previously published material, 30, 80,
83–86, 366
for unpublished material, 7, 12–13, 16
U.S. government works and public domain,
81
written request necessity, 83–86, 84b, 366
Person, agreement in, 44
Personal communications
author obligation to obtain permission, 7, 14
e-mail citation format, 293t, 316, 322
general citation format, 292t, 316
See also Electronic mail (e-mail)
416 ➤ The ACS Style Guide
Personal criticism, in scientific writing, 7–8,
14, 16, 74
Personal names
byline format, 21
comma use, 117–118
in e-mail addresses, 157
en dash use, 125
foreign name format, 139, 152–153
hyphenation, 139, 152–153
period use, 119
possessives, 127
professional titles with, 21, 147–148,
152
as taxonomic name element, 146
See also Author names in references, Author
names in text, Surnames
Personal opinions, avoiding in writing, 44
pH, style for, 155, 271–272
Phenotypes, roman type use, 154
Phosphate group, attachment of, 244
Photocopying copyrighted material
fair use and restrictions, 80
Photographs
color prints, 362
combos, 347, 349f, 352t, 363
copyright, 81–82, 361, 366
example, 361f
figure preparation, 360–362, 363
file formats, 350–352, 363
handling and labeling, 362, 364–365
of people, 82, 361
permissions, 82, 83, 361, 366
prescreened, 362
scanning tips, 355b, 362
types of artwork, 347
when to use, 344
See also Artwork, Illustrations
Photon frequency, indicating, 272
Photoshop (software), 350–352, 355b
Phrasal adjectives, 149
Phrasal verbs, 139, 149
Phrases, definition, 42
Phrases to avoid, 54–55
Phylum names, capitalization, 145–146
Physical quantities
boldface type use, 213
italic type use, 216
as mathematical concept, 210
non-SI units, 231t
SI-derived units, 229t, 230t
SI units, 228, 229t
symbols, 277–283
Physical terms, Greek letter use, 155
pi (π)
as mathematical constant, 213
for positive proton, 256
Picas as space measure, 353b, 354t, 356b
Pie charts
color use, 345b
types of artwork, 347
when to use, 344
working with, 363
Piece fractions, when to use, 205
See also Fractions
Pixels per inch as measure, See Resolution of
figure files
Place of publication
books, 303
data sets, 314
meetings and conferences, 308
periodicals, 294, 295
theses, 310
U.S. government publications, 312
Plagiarism, ethical considerations, 7, 10, 74
Planck’s constant, indicating, 272
Planes in crystals, 267–269
Planes (geometric) in mathematical copy, 212,
223
Planets, capitalization, 148
Plural forms
of abbreviations, 161
of decades, 205
“eq” or “eqs”, 221
as gender-neutral alternative, 58
of mathematical symbols, 211
of numerals used as names, 206
possessives of, 127
taxonomic names, 146, 154
of trademarks, 157
tricky plurals, 128
of units of measure, 224, 225
Plus or minus sign (±)
with standard error or standard deviation,
208–209
as stereochemical descriptor, 236
Plus sign
vs “and”, 55, 211
with D and L forms, 271
for electric charge, 256
for ionic charge, 262, 266
for optical rotation, 270
as stereochemical descriptor, 236
structure preparation, 379
“Plus” vs “and”, 55
“p.m.”, 205
pOH, definition, 271
See also pH
Point groups, crystallographic, 269
Points as type size measure
in figures, 353b, 355, 356b, 358, 363
in structures, 378
in tables, 373
Index ➤ 417
Points (geometric) in mathematical copy, 213,
223
“poly” prefix, capitalization, 146, 150
Polyatomic molecules, 257
Polycyclic aromatic compounds, 240
Polymer beads, representation of, 251
Polymer chemistry, symbols and SI units, 281
Polymer names
capitalization, 146, 150, 237t, 242–243
copolymers, 243
nucleic acids, 244
roman type use, 242
Pools, in combinatorial libraries, 251–252
Popular literature, ethical guidelines for pub-
lication, 16
Portable Document Files, See PDF documents
Position designations for stereochemical
descriptors, 270
Position numbers after amino acid abbrevia-
tions, 246
Positional prefixes, 145, 235, 238t–239t, 240
Positions
of atoms, 263
isotopic substitution and labeling, 264, 265
of nuclides, 264
of substituents, 246, 250
on sugar residues, 243
Positive protons, indicating, 256
Possessives, tricky, 127
Postal abbreviations, 161–162, 303, 310
Postdoctoral students and peer review, 9, 75
Poster presentations, citation guidelines, 307,
321
PostScript files, 351–352, 353b
PostScript font format, 357b
Powder diffraction analysis, CIF format
requirement, 284
PowerPoint (software), 61, 352, 353b
Powers of 10, 210
ppi as measure, See Resolution of figure files
Predicate adjectives, 143
Predicate nouns, 108
Predicates, compound, 115
Prefixes
to chemical names, 145, 235–236, 240–241,
245, 247–249, 270
to chemical terms, 137
to drug names, 145
hyphenation, 135–137, 152, 240, 247–249
multiplying prefixes, 136–137, 207, 226,
229t, 240
to numerals, 137
to proper nouns and adjectives, 137, 152
Prepositional phrases, with split idioms, 49
Prepositions, capitalization in titles and head-
ings, 149, 150
Preprint servers, citing articles retrieved from,
293t, 317, 319
Preprints
citation format, 308
Prescreened illustrations, 362
Present participles as unit modifier element,
142
Present tense, 43
Presentations, See Journal presentations,
Meeting and conference presentations,
Oral presentations
“Press” in publisher name, 303
Press releases, disclosure of research results, 16
Primed numbering, 244
Primes, spacing considerations, 219
Printed copy, See Hard copy
Printer’s errors, 34
Private communications, See Personal com-
munications
Proceedings volumes
description, 17
reference format, 292t, 307–308, 321, 323
standard reporting format, 19
ProCite (software), 326–327
Production editing, role, 33
See also Editorial process, Editors
Products, in combinatorial chemistry, 246,
251–252
Professional titles
in bylines, 21
capitalization, 147–148
foreign surnames, 152
Programs, computer, See Software programs
“Progress” as CASSI keyword, 306
Pronouns
antecedents of, 45–46, 58
reflexive, 51, 113
Pronunciation, “a” vs “an”, 53, 257, 264
Proofreaders’ marks and sample markups,
36–39
Proofs
checking, 33–35, 36
marking, 34b, 36–39
Proper adjectives, hyphenation, 137, 143, 152
Proper names
adjectives formed from, 144
hyphenation in unit modifiers, 142, 143
hyphenation of prefixes and suffixes, 152
possessives, 127
spelling recommendations, 127
See also Author names in references, Author
names in text, Company names, Per-
sonal names
Proper nouns, hyphenation, 137, 138, 143, 152
Proportionality in artwork, 355, 356, 358f
See also Sizing of artwork
418 ➤ The ACS Style Guide
Protein Data Bank, 284
Proteins, representations of, 154
Proton charges, indicating, 256
Province abbreviations, 161–162
Public domain status, determining, 81, 83
Public pronouncement of research results, 16
Publication information in references, See
Place of publication, Publisher names in
references, Year of publication
Publication number, U.S. government publica-
tions, 312
Publication phase of editorial process, 34–35
Publication volume information in references,
295, 296, 297–298, 303–304, 306
Publicon (software), 99
“Publish or perish” mentality, 5
PublishASAP (software), 60
Publisher names in references
acronym or abbreviation use, 302, 307
books, 302–303
data sets, 314
meetings and conferences, 308
organization or committee as author, 301,
307, 308, 312, 321
U.S. government publications, 312
Publishers’ guidelines, See Author guidelines
Publishers’ manuscript submission systems,
60, 65t–66t, 68, 69t
Publishers’ templates, See Templates
Publishing agency information, U.S. govern-
ment publications, 312
Punctuation
in chemical names, 241
with display equations, 221
e-mail and Web addresses, 156–157
with quotation marks, ACS policy,
121–122
roman type in mathematical copy, 212
unit modifiers with same ending base, 141
See also Colon, Comma, Em dash, En dash,
Period, Semicolon, Slash
Pure and Applied Chemistry, 255
Qualifiers of personal names, 117, 291, 301,
309, 312
Quality vs quantity of research, 5
Quanta, chemical conventions, 256
Quantitative analysis, reporting data, 276
Quantities, Units and Symbols in Physical
Chemistry, 255
Quantities and Units, 255
Quantum numbers, chemical conventions, 257
Quotation marks
ACS policy on, 121–122
square brackets within, 124
use guidelines, 122
Quotations
added material, 124
colon use, 144
comma use, 118
ellipses use, 126
ethical considerations, 7
interviews, 122
quotation marks use, 121–122, 124
spelling recommendations, 127
See also Extracts (textual)
R and R∗ prefixes in chemical names, 236, 270
R for sets of substituents or residues, 246,
250–251
“rad” vs “rd” and “radian”, 225
Radiation, symbols and SI units, 282
Radical sign in running text, 217
Radicals, free and charged species, 266
ran infix in copolymer names, 243
Ranges
en dash use, 117, 125, 209, 289
numeral and word usage, 204, 209–210
pagination information in references, 296,
304
units of measure as final item, 226
Rank, grammatical, 46
Rapid Review (software), 60, 62, 69t
RasMol (helper application), 25
Ratios
isotopic, 265
notation, 222
numeral use, 205
punctuation, 121, 222
spacing considerations, 215, 219
“rd” vs “rad” and “radian”, 225
RDF (Resource Description Framework),
99–100
RDF Site Summary (RSS), 100
Reaction names preceded by element symbols,
145, 234, 240–241
Reactions, See Chemical reactions, Nuclear
reactions, Schemes
Readers, ethical obligations of, 9–10
Reading column in tables, 371, 372t
Reagents
in combinatorial chemistry, 246, 250–252
identification in text, 22, 23
Recasting sentences
for active voice, 42, 54
to avoid beginning with number, 204
for gender-neutral language, 57–58
words and phrases to avoid, 54
“Recently”, 57
Red–green–blue (RGB) color mode, 350, 352,
362
Refereeing of scientific papers, See Peer review
Index ➤ 419
Reference books used by ACS staff, 127, 135
Reference citation numbers
formatting, 30, 287
punctuation, 117, 124, 209, 289
See also Reference numbering
Reference citations in text
abstract guidelines, 22
ACS styles, 287–290
citation manager programs, 326–327
editorial process, 30–31
multiple references by same author, 290
parentheses use, 124
permission requests, 85b, 366
placement in sentence, 290
reporting analytical data, 276
spacing of page numbers, 208
Reference Manager (software), 326–327
Reference manager programs, 326–327
Reference numbering
arabic numeral use, 206
citation guidelines, 289
collating references, 325
See also Reference citation numbers
References
“a” and “b” references, 290, 297–298, 325
abstracts, 298–299, 308, 321
article titles, 291, 292t
author names in, See Author names in refer-
ences
author responsibility, 24, 30, 31, 290
books, 290, 292t–293t, 300–306, 319–320,
323–324
CASSI abbreviations, 291, 292–293, 313,
328–339
CASSI format for abstracts and proceed-
ings, 308
CASSI format for computer programs, 324
CASSI sample entry, 340–341
chapter titles, 301, 306, 312
collating, 290, 325–326
committees as authors, 301, 307
as copyright ownership indicator, 81
data sets, 314–315
editor names, 292t, 293t, 300–302, 320
editorial process, 30–31
electronic sources, 292t–293t, 297, 298,
316–325
errors in, 24, 30, 31
ethical obligations of authors, 7, 13, 14
ethical obligations of reviewers, 15
examples, 292t–293t
foreign surname format, 152
government publications, 292t, 295, 311–
314, 321
in illustrations, 289, 373
management programs for, 326–327
References—continued
Material Safety Data Sheets, 292t, 315
material without publication information,
308–309
meeting and conference proceedings, 56–57,
292t, 307–309, 321, 323
minimum data, 290, 306, 309, 310
miscellaneous information, 305
nonscientific periodicals, 292t, 299
organizations as authors, 301, 307, 308, 312,
321
pagination information, 208, 296–297, 304
patents, 292t, 310–311
periodicals, 290–299, 292t–293t, 317–319
place of publication, 295, 303
placement in manuscript, 24
publisher names in, See Publisher names in
references
repeated information, 290, 297–298
series publications, 292t, 304, 305–306
special situations, 299, 305–306
technical reports and bulletins, 292t, 314, 324
theses, 292t, 309–310, 321, 324
unpublished materials, 308–309, 315–316,
321
volume information, 295, 296, 297–298,
303–304, 306
year of publication, 296, 297–298, 303
See also Book as reference, Journal as refer-
ence, Periodical as reference
“References therein”, use in citations, 297
Reflexive pronouns, 51, 113
RefWorks (software), 326–327
Regions of the country, capitalization, 148
Registered trademark symbol, See Trademark
symbol
Registry Numbers, CAS, 253–254
Regulatory agencies, commercial chemical
inventories of, 254
Rejection of manuscripts
author guidelines, 75
ethical considerations, 12
reasons for, 72, 73, 75–76
“Relative to”, 50
Religious titles in bylines, 21
Repetitive research reports, 5
Report numbers, U.S. government publica-
tions, 312
Reports, technical, 292t, 314, 324
Reports of original research, See Scientific
papers
Reprinting, permissions for, See Permissions
Reprints, for authors, 35
Reproducibility of results
ethical considerations, 5–6, 8, 13
mathematical detail in papers, 23
420 ➤ The ACS Style Guide
Reproduction
artwork, 29, 83–86, 366
photographs, 360–362, 363
See also Permissions
Rescreening of illustrations, 363
Research articles, See Journal articles
Research errors, See Errors
Research ethics issues, See Ethics entries
Research grant agencies’ manuscript submis-
sion systems, 60, 65, 67t
Research reports, See Scientific papers
Residues
in combinatorial chemistry, 246, 250–251
of saccharides, 243, 244
Resolution of figure files
Excel tips, 353b
and file formats, 351–352
graphics from Web sites, 364
photographs and halftone figures, 360–362
and publication medium, 346
scanning tips, 355b
structure graphics, 378, 379, 382b
and type of artwork, 347
Resonance structures, arrows for, 272
Resource Description Framework (RDF),
99–100
“Respectively”, 50, 55
Restriction endonucleases, 155
Restrictive phrases or clauses
comma use, 44, 116–117
grammar overview, 109
writing style, 44
Results integrity issues, See Ethics entries
“Results” section in scientific papers, 7, 23
Review articles
description, 19
standard format, 20, 21
Reviewers of manuscripts
anonymity of, 8, 71–72
author requests regarding, 13, 72, 75
editorial process, 29, 31, 32
electronic submission of manuscripts, 60,
63, 68
ethical considerations, 5, 8–9
ethical guidelines, 15–16, 73, 74, 76
peer-review process, 72–73
responsibilities of, 73–74
selection of, 15, 72–73, 75
See also Peer review
Revising your manuscript, 31, 32b, 63–64,
75–76
RGB color mode, 350, 352, 362
Ribonucleic acid, abbreviations, 244t
Rich Site Summary (RSS), 100
Ring size, structure preparation, 378
RNA, abbreviations, 244t
Roman numerals
vs arabic, 206, 369, 375–378
chemical structure identification, 376–377
for oxidation numbers, 262
Roman type
in abbreviations and symbols, 154–155,
159–160, 212–213, 216, 241, 271–272
for chemical compounds, 259
for chemical element symbols, 159, 234, 241
combinatorial chemistry representation,
246
for electronic configuration, 256
in figures, 356b
in locants and descriptors, 234
in mathematical copy, 212–213, 214, 223
for phenotypes, 154
for polymer names, 242
references to books, 301–304, 306
references to meetings and conferences,
308–309
references to periodicals, 291, 297–299
references to theses, 309
references to U.S. government publications,
312
for restriction endonucleases, 155
in subscripts or superscripts, 216
superatom representation, 246
for syllabic portion of chemical names, 233,
240
for trigonometric and other functions, 219,
220
See also Boldface type, Italic type
Rotation
crystallographic point groups, 269
optical, 270
specific, 274
RSS (RDF Site Summary or Rich Site
Summary), 100
Rules, straddle, 371, 372t
S- vs S-, when to italicize, 235
S and S∗ prefixes in chemical names, 236, 270
s orbitals for polymer bead representation,
251
Saccharides, abbreviations, 243t–244
Safety information, See Chemical safety infor-
mation
Scalar, mathematical concept, 210
Scalar variables, single-letter, 215
Scaling of artwork, See Proportionality in
artwork, Sizing of artwork
Scanning programs, 350–352, 355b
Scanning tips for figures, 353b, 354, 355b, 360,
362
Scatter plots, 347
Scheduling concerns, See Timeliness as issue
Index ➤ 421
Schemas
in CML, 95, 96
in XML, 90–91, 96, 98, 99
“Scheme”, capitalization, 143
Schemes
in abstracts, 22
citing, 143
CML example, 93s
in combinatorial chemistry, 246, 251–252,
252f
definition, 376
editorial process, 29
footnotes, 378
numbering, 206, 346, 378
preparing, 379
titles, 148, 378
types of artwork, 347
when to use, 376, 377f
XML example, 91s
See also Chemical reactions
Schoenflies symbols, crystallography, 269
ScholarOne, editorial systems, 60, 69t
Scientific and technical information exchange
(STIX) fonts, 357b
Scientific method, relation to format of scien-
tific paper, 19
Scientific notation, 207, 209–210
Scientific papers (in general)
book categories, 17–18
figure use, 343, 344
journal presentations, 18–19
standard format, 19–26, 28
structure use, 375
table use, 369
word choice, 50
Scientific publication, ethics in, See Ethical
guidelines, Ethics in scientific publication
Scientific publishers, See Publisher entries
Scientific search services, 72
Scientific vs other writing styles, 16, 28, 50
SciFinder (search service), 72, 254
SciFinder Scholar (search service), 72
Screening (printing process), 347, 362, 363
Script letters, 214
Search engines, Web-based, 99, 383
Search services, 72, 254
Seasons, capitalization, 148
Second person as gender-neutral alternative, 58
Seconds symbol, spacing, 203, 224
“Section”, capitalization, 144
Semantic web concept, 89, 96
See also Datuments
Semantics, in markup languages, 93–94, 96–99
Semicolon
between independent clauses, 119–120
reference citation guidelines, 289
Semicolon—continued
references to books, 301–305
references to meetings and conferences,
308–309
references to patents, 310
references to periodicals, 291, 297–299
references to U.S. government publications,
312–313
with series items, 119
Semiotics, definition, 98
Sentence modifiers, 44–45, 109–113
Sentence structure
comparisons, 48–50
parallelism, 46–48, 50
sentence modifiers, 44–45, 109–113
split infinitives, 43
subject–verb agreement, 43–44, 105–108
verb omissions, 108
verb tenses, 42–43
voice, 42
word order, 45–46
“Separately”, 55
Sequences, nucleotide, 244
Sequential numbering, See Numbering
Serial comma, 114, 115, 119
Serial data sets, 314–315
Serial unit modifiers with same base, 141
“Series”
as CASSI keyword, 306
as collective noun, 306
Series items
comma use, 114, 115, 119
ellipses use, 127
en dash use, 209
enumerations, 123, 206–207
numeral and word usage, 123, 127, 204,
206–207, 209
parallelism, 48
semicolon use, 119
units of measure as final item, 226
Series publications, reference format, 292t,
304, 305–306
Series titles, 20, 305–306
Service mark symbol, 157
Set notation, 222
“Several”, subject–verb agreement, 108
Sexist language, avoiding, 57–58
SGML (Standard Generalized Markup Lan-
guage), 90
Shades of gray, types of artwork, 347
Shading in artwork, 345b, 347, 359f, 363
Shareware, public domain status, 81
SI-derived units, 229t, 230t
SI units
atomic and molecular, 277
base units, 228, 229t
422 ➤ The ACS Style Guide
SI units—continued
capitalization, 228
chemical kinetics, 277–278
electricity and magnetism, 278–279
electrochemistry, 279
general chemistry, 279–280
mechanics, 280
multiplying prefixes, 229t
NMR spectroscopy, 281
polymer chemistry, 281
radiation, 282
space and time, 282
supplementary units, 228, 229t
system background, 228
technical documents usage, 223
thermodynamics, 283
transport properties, 283
Significance of results as publication issue, 4–5
“Similar to” in comparisons, 49
Simple past tense, 43
Simple sentences, definition, 42
Simultaneous manuscript submissions, 5, 14, 16
“Since”, usage guidelines, 46
Single-crystal analysis, CIF format require-
ment, 284
Single letters or numbers in unit modifiers,
142, 143
Sizing of artwork, 352–354, 355, 370, 373
Sketches, 347
See also Artwork, Figures, Illustrations
Slang, avoiding, 54
Slash
between components of mixture, 121, 126,
222, 261
with compound units of measure, 224, 226
for division, 220, 224
misuse for “and” or “or”, 56
in ratios, 121, 222
spacing considerations, 215, 217, 219–220
and spelled-out units of measure, 225, 226
and units of measure in tables, 371
in Web addresses, 156
Small capital letters in chemical names, 233,
236, 238t, 245, 270–271
Small-molecule crystal structures, data
sources, 284
SMILES identifiers, 94
“So” as coordinating conjunction, 47, 113, 149
Software processing of data, See Databases,
Datuments
Software programs
capitalization, 156
and CIF format, 285–286
citation management, 326–327
cited as references, 314, 323–325
combinatorial chemistry representation, 251
Software programs—continued
data set citation format, 314
datument potential, 98–99
editorial process, 29, 30
electronic submission of manuscripts, 60,
65t–67t
figure preparation, 343, 346, 350–352, 354,
355b, 363
font copyrights, 357b
structure preparation, 375, 378–383
table preparation, 373–374
Web-enhanced object viewing, 25
See also specific software
Solid state, indicating in reactions, 272
Solid supports, in combinatorial chemistry,
251
Solidus, See Slash
Soluble supports, in combinatorial chemistry,
251
“Some”, subject–verb agreement, 108
Sound files, Web-enhanced objects, 25
Source file formats, See Computer file formats
“Source” line in table footnotes, 373
“sp.”, capitalization, 146
Space and time, symbols and SI units, 282
Space groups, crystallographic, 269–270
Spacing
in CASSI abbreviations, 294
with centered dots, 215, 219, 261
in chemical reactions, 272, 379
in citations of standards, 276
with compound units of measure, 224
between curves in figures, 358
between display fractions, 219
between element and spectral line, 276
with ellipses, 219
with “exp”, 217
around geometric notation, 223
of indices and element names or symbols,
268
mathematical copy guidelines, 210–223
around mathematical functions and opera-
tors, 214–215, 217, 219, 222
around mathematical variables, 215
in multiplication, 215, 219, 224
in numbers with five or more digits,
207–208
in numbers with four digits, 207
between numerals and units, 203, 223–224,
271–272
of oxidation numbers, 262–263
with percent sign, 203, 224, 271
of periods after initials, 119
of position numbers, 246
with ratio colons, 215, 219
reference citation guidelines, 117, 209, 289
Index ➤ 423
Spacing—continued
references to books, 304
references to periodicals, 296–297
with slashes, 215, 217, 219–220
in standard deviation or standard error,
208–209
Spanish surnames, 153
Spans, See Ranges
Special characters, See Greek letters, Symbols
Special event names, capitalization, 148
Special materials used in research, distribu-
tion of, 8
Special Publication 330, National Institute of
Standards, 255
Special sections, standard format, 24–25
Special typefaces, See Boldface type,
Italic type
Species names
abbreviating vs spelling out, 160
capitalization, 146, 150
italic type use, 154
Species numbers, 272
Specific rotation, reporting analytical data, 274
Spectra, Web-enhanced objects, 25
Spectral characterization data as supporting
information, 25
Spectral information, datument potential, 99
Spectroscopy
data types and representation, 274–276
symbols and SI units, 281
Speculations, labeling in papers, 7
Speeches, converted to scientific papers, 57
Spelling
ACS desk reference use, 127
company names, 127
computer and Internet terms, 163–168
in direct quotations and titles, 127
plurals, 128
possessives, 127
recommended spelling list, 129–133
Spelling out
abbreviations, 158, 160
“equation” in text, 221
fractions, 139
numbers vs numerals, 160, 203–205
taxonomic names, 160
terms in titles, 20
units of measure, 160, 225–226
Spin multiplicity, chemical conventions, 257
Spiro alicyclic compounds, nomenclature, 236
“spiro” prefix, style for, 236
Split idioms, 49
Split infinitives, 43
Sponsors, “Acknowledgments” section con-
tents, 24
“spp.”, capitalization, 146
Square brackets
in bridged and spiro alicyclic compound
names, 236
with concentrations, 124, 271
with coordination entities, 260
with crystallographic indices, 268
isotopic labeling, 264–265
in mathematical copy, 124, 212, 214–216
in mathematical equations, 219–220
nesting order, 216
for oligosaccharides, 244
in polycyclic aromatic compounds, 240
in polymer or complex chemical names, 151
with quotation marks, 124
references to online sources, 315, 318
with syllabic portion of chemical names, 240
Square roots, radicals vs superscripts, 217
“Sr.” and “Jr.”, 117, 291, 301, 309, 312
Stacking (aligning) subscripts and super-
scripts, 269
Staggering subscripts and superscripts
conventions in chemistry, 269
for ionic charge, 262
mathematical copy preferences, 216
Standard deviation or standard error, numbers
style, 208–209
Standard format for scientific papers
abstract, 21–22
acknowledgments, 24
byline and affiliation, 21
conclusions, 23
discussion, 23
editorial process, 28, 29
experimental details or theoretical basis,
22–23
introduction, 22
references, 24
results, 23
special sections, 24–25
summary, 24
supporting information, 23, 25
title, 20
Web-enhanced objects, 25–26
Standard Generalized Markup Language
(SGML), 90
Standards
for chemical symbols and nomenclature,
255
citing in reports, 276
for structure representation, 383
stat infix in copolymer names, 243
State abbreviations
with CASSI abbreviations, 294
U.S. Postal Service, 161–162, 303, 310
State and city names for place of publication,
294, 295, 303, 310
424 ➤ The ACS Style Guide
State government publications, cited as refer-
ences, 313–314
Statistical notation, 223
Stereochemical descriptors
in chemical names, 235, 236, 239t, 240
and chirality, 270
See also Chemical descriptors, Locants
Stereochemical prefixes, 235
Stereoisomer descriptors, 145
“Still”, hyphenation in unit modifiers, 141
STIX fonts, 357b
STM publishing, definition, 27, 90
STN International, 254
Straddle rules, 371, 372t
Structural descriptors, 236, 238t–239t
Structural point groups, crystallography, 269
Structural prefixes, chemical names, 235–236
Structure data for crystals, See Crystallo-
graphic information files (CIF)
Structure numbers in ChemSet notation, 246,
251–252
Structures
in abstracts, 22
CAS Chemical Registry Numbers, 253–254
citing, 376–378
in combinatorial chemistry, 246, 250–252
description, 375
editorial process, 29
font selection, 380
freeware for drawing, 379–381
future of representation, 382–383
grouped in charts, 376, 379
as illustrations, 273
InChI identifiers, 101–102, 383
numbering, 206, 246, 346, 375–377
organic group abbreviations, 260
preparing, 378–381
submitting, 381–382
in tables, 374, 378
types of artwork, 347
unique label possibility, 383
Web-enhanced objects, 25
when to use, 375–376, 377f
See also Artwork, Illustrations
Stub in tables, 371, 372t
Student t test, 223
Subatomic particles, chemical conventions,
256
Subheadings
capitalization, 148
parallelism, 48
Subject–verb agreement, 43–44, 105–108
Submission of manuscripts, See Electronic
submission of manuscripts, Manuscript
processing stages
Submitted materials in reference lists, 315–316
Submitting agents, 62
Subordinate clauses
comma use, 114
definition, 42
as sentence modifiers, 113
Subordinating conjunctions
comma use, 116
in comparisons, 48
in titles and headings, 150
writing style, 46
Subscripts
for atomic numbers, 262
in Boughton system for isotopes, 265–266
with chemical reactions, 273
for electronic configuration, 257
fractions in, 217
“index” definition, 211
for ionic charge, 262
italic vs roman type, 216
in mathematical copy, 160, 215–217
mathematical expressions in, 217
in mathematical symbols, 211
for number of atoms, 263
for orbital axes, 256
plurals of abbreviations ending in, 161
spacing around operators, 215, 219
staggering and stacking with superscripts,
216, 262, 269
in superatom designations, 246, 251
in titles, 20
Subspecies names
capitalization, 146
italic type use, 154
Substituents, in combinatorial chemistry, 246,
250
Substitution, isotopic, 264–266
Subtitles, capitalization, 148
“Such as”, restrictive and nonrestrictive uses,
116
Suffixes
hyphenation, 137–138, 150, 152, 247–249
to proper nouns and adjectives, 152
subject–verb agreement, 107
Sugars
nucleic acid abbreviations, 244t
saccharide abbreviations, 243t, 244
Summaries, scientific paper format, 24
See also Abstracts
“Sun”, capitalization, 148
Superatoms, designation, 246, 250–251
Superlatives, hyphenation in unit modifiers,
141
Superscripts
with chemical reactions, 274
for corresponding author information, 21
for electric charge indicators, 256
Index ➤ 425
Superscripts—continued
for electronic configuration, 256–257
fractions in, 217
“index” definition, 211
for ionic charge, 262, 266
italic vs roman type, 216, 373
for mass number, 261, 263
in mathematical copy, 160, 215–217
mathematical expressions in, 217
in mathematical symbols, 211
misuse in isotopic labeling, 265
for oxidation numbers, 262–263
plurals of abbreviations ending in, 161
vs radical signs, 217
reference citation guidelines, 30, 117, 209,
287
references to periodicals, 298
spacing around operators, 215, 219
staggering and stacking with subscripts,
216, 262, 269
in table footnotes, 373
in titles, 20
Supplements, reference format, 296
Supporting information
datument potential, 98–99
datument validation, 96
electronic submission of manuscripts, 63,
69t, 382b
ethical considerations, 5
for lengthy derivations, 23
manuscript revisions, 32b
standard format, 23, 25
Supports, in combinatorial chemistry, 251
Surnames
byline format, 21
capitalization, 151–152, 153, 225
foreign, 139, 152–153
hyphenation, 139, 152–153
as modifiers, 151
reference collation, 290, 325–326
reference format, 153, 291, 301, 309, 312
as units of measure, 151–152, 225
See also Author names in references, Author
names in text, Personal names
Syllabic portion of chemical names, 233, 240,
241
Symbols
atomic and molecular symbols, 277
capitalization guidelines, 145
case sensitivity, 159
chemical kinetics symbols, 277–278
in chemical reactions, 272
commonly used in chemistry, 169–202
crystallography symbols, 269
electricity and magnetism symbols, 278–279
electrochemistry symbols, 279
Symbols—continued
en dash use, 125, 209
in figures, 353b, 356, 358f, 363, 365
font availability, 365
general chemistry symbols, 279–280
for isotopes, 264–266
isotopic labeling, 264–266
mathematical notation, 222–223
mechanics symbols, 280
NMR spectroscopy symbols, 281
for non-SI units, 231t
nonmathematical symbols, 212
for physical quantities, 277–283
polymer chemistry symbols, 281
for positions of atoms, 263
radiation symbols, 282
reporting analytical data, 274
Schoenflies, 269
for SI-derived units, 229t, 230t
for SI units, 229t
sizing of, 353b, 356
space and time symbols, 282
spacing guidelines, 203
in tables, 370, 371, 373, 374
thermodynamics symbols, 283
in titles, 20
for trademarks, 23, 157
transport properties symbols, 283
See also Abbreviations, Acronyms, Chemical
symbols, Element symbols, Mathemat-
ical symbols, specific symbols
Symmetry operations, crystallography, 269
Symmetry site terms and chirality, 270
Symposia, See Meeting and conference pre-
sentations
“Syn”, italicization and capitalization, 145
Synonymous terms, word choice consider-
ations, 50
Syntax and CIF format, 286
Synthesis, combinatorial, See Combinatorial
chemistry
Systematic chemical names, 22
Système International d’Unités, See SI units
t test, 223
Tab key, avoiding in table preparation, 373
“Table”, capitalization, 143, 369
Table feature of software, 373–374
Tables
abbreviation use, 160, 225, 370, 371, 372,
373
abstract guidelines, 22
adapted, 84b, 366
citing, 143, 369–370
column entry guidelines, 207, 371–374
column heading guidelines, 225, 371, 372t
426 ➤ The ACS Style Guide
Tables—continued
column width guidelines, 370
editorial process, 29–30, 33
“Experimental” section contents, 23
footnotes, 371, 372–373
formal vs informal, 370, 374
vs lists, 370, 374
numbering, 206, 369
parallelism, 48, 371
parts of, 371, 372t
permission requirements, 83–86, 366
preparing, 370–374
references in, 289, 373
repeated entries, 372
row heading guidelines, 371
section heading guidelines, 48
section widths, 371
sizing, 370, 373
standard deviations or standard errors, 209
structures in, 374, 378
submitting, 374
as supporting information, 25
symbol use, 370, 371, 373, 374
titles, 148, 371, 372t
typesetting cost, 370
unit of measure guidelines, 207, 225, 371,
373
when to use, 344b, 369, 370
word-processing software use, 373–374
See also Artwork
Tagged Image File Format, See TIFF format
Tags, markup
described, 89
HTML limitations, 90
“tag soup” problem, 93
See also Datuments
“Taking” as sentence modifier, 112
Tangible medium of expression, definition, 77
Tarred files, 62
Taxonomic terms
abbreviating vs spelling out, 160
capitalization, 145–146, 150
italic type use, 155, 160
Technical editing
datument potential, 98–99
editing services, 75
reference accuracy, 30
role, 33
See also Editorial process, Editors
Technical reports and bulletins, reference
format, 292t, 314, 324
Television commercials, still shot permissions,
83
Telnet sites, cited as reference, 320
Temperature, units of, See Units of temperature
Temperature scale names, capitalization, 225
Templates
editorial process, 29
electronic submission of manuscripts, 60
reference management programs, 326–327
standard format, 20
structure preparation, 380–381
Temple Peer Review Manager (software), 60
Tenses, 42–43
Tensors
boldface type for tensors, 213
definition, 210
italic type for components, 212
Territory abbreviations, 161–162
TeX (software), 61
Text editors and CIF format, 286
Text file formats, See Computer file formats
Text settings for ACS publications, 381b
Thai surnames, 153
“Than” in comparisons, 48
“That”
antecedent clarity, 46
with restrictive clauses, 109
“That is”, 117, 160
“The”
before collective nouns, 106
as gender-neutral alternative, 58
in journal names, 294
in titles, 20, 149, 154
Theoretical papers, standard format, 20, 23
Thermodynamics, symbols and SI units, 283
Theses, cited as reference, 292t, 309–310, 321,
325
“This”, antecedent clarity, 46
Thomson Scientific products, 327
Three-dimensional art, structure preparation,
378
“Through”, vs en dash, 125, 209
Tick marks in figures, 353b
TIFF format, 61, 351–352, 353b, 357b, 363,
381, 382b
Tilde (~) for polyatomic molecules, 257
Time, units of, See Units of time
Time and space, symbols and SI units, 282
Timeliness as issue
ASAP articles, 34, 318–319
for authors, 4–5, 75
checking proofs, 33, 34
datument potential, 99
for editors, 12
and full disclosure, 5–6
and nonstandard formats, 20
for peer reviewers, 9, 16, 73
permission requests, 30, 84–86
and significance of results, 4–5
and word choice, 57
“Times”, nonmathematical sense, 205
Index ➤ 427
Times New Roman typeface, 357b
Times Roman typeface, 356b, 363
Times sign (×), use guidelines, 215
Titles (in references), See Article titles in
periodical citations, Book titles, Chapter
titles, Journal titles
Titles (in text)
abbreviation guidelines, 20, 150, 159, 160
artwork guidelines, 371, 377, 378
capitalization, 148–151, 227, 237t–239t
quotation marks use, 122
spelling recommendations, 127
standard format, 20
trademarks not used in, 157
unnumbered lists in manuscripts, 374
Titles (professional or personal)
in bylines, 21
capitalization, 147–148
foreign surnames, 152
“To”
capitalization in infinitives, 149
vs en dash, 124–125, 209
“To be” verb forms, 51, 111, 143
Toner cartridges for figure artwork, 353b, 360
Top-level elements (markup language compo-
nent), 91
“torr” vs “Torr”, 225
Toxic Substances Control Act Inventory, 254
Trade names vs generic names, 157
Trademark symbol, 23, 157
Trademarks
capitalization, 146, 157
definition, 157
scientific paper format, 23
in titles, 20
use guidelines, 157
“Trans”, italicization and capitalization, 145, 150
Transcripts of speeches, 57
Transfer of copyright, 32, 33, 78, 82, 86
Transitional phrases
comma use, 117
semicolon use, 119–120
Translations, citation formats, 299
Transliteration of foreign names, 153
Transport properties, 212, 215, 283
Transposes of matrices, 213
Tree view of markup languages, 94, 95f
“tri” in element names, 260
Tricky possessives and plurals, 127–128
Trigonometric and other functions
line breaks, 220
roman type use, 213, 220
spacing considerations, 213
Trim size for ACS books, 354t
Triple bonds in chemical formulas, 267
Tritium, representation, 264, 266
Trivial chemical names, 22
TrueType font format, 357b
Truncation in e-mail addresses, 157
Two-letter initials, foreign names, 153
Two-letter variables, italic type use, 212
Two-word compounds, hyphenation, 137, 138
See also Compound words
Two-word concepts, en dash use, 124–125
Two-word verbs, hyphenation, 139
Type and photograph combinations, See
Combos (artwork)
Type size
chemical reactions, 272
figures, 353b, 356b, 357b, 358f, 360, 365
structures, 378
tables, 373
Type weight, definition and examples, 356b
Typefaces, See Boldface type, Fonts, Italic type,
Roman type
U for uniform labeling, 265
“U.K.”
in references, 294, 303, 310
when to abbreviate, 161
Undergraduate students and peer review, 9, 75
Underscore in e-mail addresses, 157
Unhyphenated prefixes, 135–136
Uniform resource identifier (URI), 92
Uniform resource locator (URL)
in Internet reference citations, 315, 317–320
line break guidelines, 156
persistent URLs, 317
WWW address format, 156
WWW history, 89
See also World Wide Web sites
Unit cells, crystallographic types, 269
Unit modifiers
capitalization in titles and headings, 150,
225, 227
definition and types of, 139–140
editorial list, 140
foreign phrases as, 139
hyphenation, 138, 139–143
phrasal verbs as, 139
as predicate adjectives, 143
serial, with same ending base, 141
three or more words, 142–143
Unit of measure–number combination as
singular, 51
United Kingdom
in references, 294, 303, 310
when to abbreviate, 161
United States
commercial chemical inventories, 254
when to abbreviate, 161
See also U.S. entries
428 ➤ The ACS Style Guide
Units of measure
abbreviating vs spelling out, 160, 225–226
axis labels on illustrations, 359
capitalization, 150, 225, 227
case sensitivity, 159
as collective nouns, 107
comparison words, 51
compound, 224, 226, 230t
decimals use, 208
definition not required, 224
discipline-specific preferences, 207
hyphenation of unit modifiers, 141–143
large numbers with, 207
non-SI units list, 231t, 232t
not having abbreviations, 225
numeral and word usage, 203, 204
period use, 118, 223
plural forms, 224, 225
in ranges and series, 226
roman type use, 212
spacing, 203, 223–224, 271–272
spelling out, 160, 225–226
surnames as, 151–152, 225
systems overview, 228
in tables, 207, 225, 371, 373
usage overview, 223–227
See also SI units
Units of temperature
capitalization, 225
degree symbol guidelines, 203, 208, 224, 227
non-SI units lists, 231t
SI units list, 229t
Units of time
decimals use, 208
hyphenation of unit modifiers, 141
minute and second symbol spacing, 203, 224
month abbreviations, 160–161
non-SI units lists, 231t
numeral and word usage, 160, 203, 205
roman type use, 212
seasons capitalization, 149
SI units list, 229t
University name and location in thesis cita-
tion, 310
Unknown quantities
defining symbols in text, 211
italic type use, 212
Unnamed chemical elements, 260
Unprimed numbering, 244
Unpublished materials
ethical obligations
authors, 7
editors, 12–13
reviewers, 9, 16
reference format, 308–309, 315–316, 322
URI (uniform resource identifier), 92
URL, See Uniform resource locator
“U.S.”
in references, 294
when to abbreviate, 161
U.S. Code, cited as reference, 313
U.S. Copyright Office, 79–80, 82
U.S. Department of Health and Human Ser-
vices, Office of Research Integrity, 4
U.S. government authorship, ACS copyright
policy, 81
U.S. Government Printing Office, 311, 312
U.S. government publications
cited as references, 292t, 295, 311–313, 321
Code of Federal Regulations, 313
copyright, 81, 366
Federal Register, 313
U.S. Code, 313
U.S. laws, 313
U.S. laws, cited as reference, 313
U.S. monetary values, 208
U.S. National Institute of Standards and Tech-
nology, 101
U.S. patents
cited as reference, 292t, 310–311
style guidelines, 208
U.S. Postal Service abbreviations, 161–162,
303, 310
U.S. regulatory agencies, 254
“U.S.A.”, CASSI spacing exception, 294
Usage of words, See Word usage
User names for author home pages, 62
Uuencoded files, 62
UV–visible spectroscopy, reporting analytical
data, 276
Valence configurations, 256
Validation of datuments, 96–97
Validation reports for CIF compliance, 63, 286
“Values” with plurals of symbols, 211
Vanadium symbol, avoiding confusion with
numerals, 262
Variables
in combinatorial chemistry, 246, 250
defining, 211, 225, 371
Greek letter use, 214
italic type use, 212
as mathematical concept, 210
roman type use, 213
spacing between, 215, 219
Variety names
capitalization, 146
italic type use, 154
“Variety of” as collective noun, 106
Vectors
boldface type for vectors, 213
Greek letter use, 214
Index ➤ 429
Vectors—continued
italic type for components, 212
as mathematical concept, 210
Verb–subject agreement, 43–44, 105–108
Verb tenses, 42–43
Verbs, phrasal, 139, 150
Verbs, two-word, 139
“Versus”, 50, 124–125, 160
Video
still shot permissions, 83
Web-enhanced objects, 25, 26
Vietnamese surnames, 153
Vocabularies, controlled, 97–98
Voice, See Active voice, Passive voice
“Vols.” vs “vols.” in book citations, 304
Volume number
books, 303–304, 306
meetings and conferences, 308
periodicals, 295, 296, 297–298
“vs”, 160
Water of hydration, centered dot use, 261
“We”, writing style, 43–44
Web-based applets for drawing, 379
Web-based publications
caption preparation, 365
font tips, 357b
illustration preparation, 344, 346, 350–352
reference accuracy, 24, 30, 31
supporting information, 25
See also Electronic source citation, Online
books, Online periodicals
Web-based search engines, 99, 383
Web-based systems
manuscript submission via, 31, 32, 59–69
permission requests via, 83–84
proof return via, 34b
reference management programs, 326–327
supporting information on, 5, 25
Web browsers
electronic submission of manuscripts, 61, 68
markup language display, 94
markup languages and the WWW, 90
Web-enhanced objects (WEOs), 25–26
Web sites, See World Wide Web sites
WebLab Viewer (helper application), 25
Webster’s New World College Dictionary, 127,
135
Webster’s Third New International Dictionary,
127, 135
“Well”, hyphenation in unit modifiers, 141
When to publish, See Timeliness as issue
“Whereas”, usage guidelines, 46
“Whether” vs “Whether or not”, 52–53
“Which”, with nonrestrictive clauses, 109
“While”, usage guidelines, 46
“Who”, with nonrestrictive clauses, 109
“wide”, hyphenation as suffix, 138
“Wife”, gender-neutral alternatives, 58
Windows platform
reference manager programs for, 327
templates for, 29
“With”, alternatives to, 56
Word (software), 61, 352, 363, 373, 379
Word choice, See Word usage
Word definitions, italic type use, 154
Word omissions
in comparisons, 48–49
verbs and auxiliary verbs, 108
Word order
in comparisons, 48–49
sentence construction, 45–46
Word-processing file formats, See Computer
file formats
Word-processing software
electronic submission of manuscripts, 61
figure preparation, 352, 363
reference management programs, 326
structure preparation, 379
table preparation, 373–374
Word-processing templates, See Templates
Word spacing, See Spacing
Word usage
in captions, 365
confusable words and phrases, 51–53,
111–112
gender-neutral language, 57–58
grouping and comparison words, 50–51
and manuscript revisions, 32b
numeral and word usage, 160, 203–207
in scientific vs other writing, 16, 28, 50
in titles, 20
units of measure guidelines, 223–227
words and phrases to avoid, 54–57
Wordiness
in article titles, 20
examples, 54–55
WordPerfect (software), 61, 352, 363, 373
Works made for hire and copyright, 78, 82
World Wide Web
and markup languages, 89–96
URL forms, 156
World Wide Web sites
ACS author guidelines, 29, 343, 381b
ACS ethical guidelines, 11
ACS permission requests, 83
ACS publication home pages, 28b
ACS Publications Division, 86
ASAP articles, 34
author guidelines on, 75
Cambridge Crystallographic Data Centre,
284, 286
430 ➤ The ACS Style Guide
World Wide Web sites—continued
Chemical Abstracts Service, 253
citation of Internet sources, 293t, 314,
320–321
company sites, 127
computer and Internet terms on, 163–168
copyright issues, 79, 81, 82, 83
CRYSTMET database, 284
data set citation format, 314
DOI system, 317
drawing software resources on, 380, 380b
Dublin Core Schema, 90
electronic submission of manuscripts via,
60, 65t–67t
graphics resolution on, 364
ICSD database, 284
International Organization for Standardiza-
tion, 255
International Union of Crystallography,
284, 286
International Union of Pure and Applied
Chemistry, 97, 102, 255, 383
molfile format information on, 381
National Academy of Sciences, 4
National Institute of Standards and Tech-
nology, 228, 255
Nucleic Acid Database, 284
Office of Research Integrity, 4
Organic Syntheses, 305
peer-review information, 71
Protein Data Bank, 284
STIX font downloads on, 357b
structure representation standards on, 383
supporting information on, 25
templates on, 29
XML-related information, 90
Writing process, getting started, 27–28
Writing style
scientific vs other styles, 16, 28, 50
sentence structure guidelines, 41–50, 105–113
Writing style—continued
usage guidelines, 50–58, 111–112, 160,
203–207, 223–227
See also specific aspects
WWW, See World Wide Web entries
X for products mixed, 246, 251, 252
“X-ray”, capitalization, 144, 149
X-ray diffraction spectroscopy, reporting ana-
lytical data, 276
XML (extensible markup language)
datument as scientific grid component,
99
datument potential, 99
datument validation, 96
datument vocabularies, 98
display of, 94–96
examples of, 90–94, 91s
WWW history, 90
XML Query protocol, 99
Xpress Track (software), 60
XSL (extensible stylesheet language), 90
XSLT technology, 94
Year of publication
books, 303
editorial process overview, 30
periodicals, 296, 297–298
theses, 310
U.S. government publications, 312
See also Author–date citations
“Yet” as coordinating conjunction, 47, 113,
149
Z prefix in chemical names, 236
Zero
before decimal point, 208
as electric charge indicator, 256
Zipped files, 62
Zones, crystallographic notation, 268