DT 290 Advanstar 201012
User Manual: DT 290
Open the PDF directly: View PDF ![]() .
.
Page Count: 56

Counterfeit drug war
continues 21
®
Voice of the Pharmacist
Voice of the Pharmacist
Study: Post-MI beta blocker
doses are too low 29
December 2010
When does compounding
become manufacturing? 42
VOL. 154 NO. 12
DrugTopics.com
Community and hospital
pharmacists prepare
to steer their course PAGE 22
2.0
CREDIT:
An update on Clostridium diffi cile infection
PAGE 30
Earn CE credit for this activity at DrugTopics.com

© 2010, J M SMITH CORPORATION. QS/1 and NRx are registered trademarks of the J M Smith Corporation.
What’s most important in
outpatient pharmacies?
Keeping patients safe.
The challenges and demands of an outpatient pharmacy can
take away from patient care. QS/1®’s NRx® supports outpatient
pharmacies with easy-to-use processing and multiple inventory
management, including 340B. Our end-to-end pharmacy system
and services work together so you can work with safety and
FRQ¿GHQFH7RSURYLGHJUHDWVHUYLFH7RLPSURYHOLYHVEvery day.
1.800.231.7776
www.qs1.com
Visit us at
ASHP Booth
677

With your support, Amneal has become
the fastest growing generic manufacturer in the U.S.*
Superior quality products all made in the USA
Fully FDA cGMP-compliant manufacturing facilities
Widely recognized Customer Response team
Market-competitive pricing
*IMS Health, National Prescription Audit, June 2010 - Prescriptions of unbranded generics
Copyright © 2010 Amneal Pharmaceuticals, All Rights Reserved - AMN-DT 11.10

2
DRUG TOPICS
December 2010 WWW.DRUGTOPICS.COM
®
EDITORIAL ADVISORY BOARD
Philip P. Burgess, RPh, MBA
Chairman
Community Pharmacy Foundation
Illinois Board of Pharmacy
Chicago, Ill.
James A. Jorgenson
RPh, MS, FASHP
Chief Pharmacy Offi cer, VP
Clarian Health
Indianapolis, Ind.
Gene Memoli Jr., RPh, FASCP
Director
Customer Development, Omnicare
Cheshire, Conn.
David J. Fong, PharmD
Former community chain store
senior pharmacy executive
Danville, Calif.
Frederick S. Mayer, RPh, MPH
President
Pharmacists Planning Service Inc.
San Rafael, Calif.
Jack Rosenberg, PharmD, PhD
Professor and Director
International Drug Information Center
Arnold & Marie Schwartz College
of Pharmacy and Health Sciences
Long Island University, Brooklyn, N.Y.
Christina Medina, PharmD
Manager
Professional and College Relations
CVS Caremark
Hollywood, Fla.
Mary E. Inguanti
RPh, MPH, FASCP
Senior Vice President, Operation
Charter Oak Health Center
Hartford, Conn.
Stephen W. Schondelmeyer
PharmD, PhD
Director, PRIME Institute
College of Pharmacy
University of Minnesota
Minneapolis, Minn.
Perry Cohen, PharmD, FAMCP
The Pharmacy Group LLC
Glastonbury, Conn.
Dennis Burton, BS Pharm, MBA
Bristol, Conn.
Marvin R. Moore, PharmD
Pharmacy manager and co-owner
The Medicine Shoppe/
Pharmacy Solutions Inc.
Two Rivers, Wisc.
EDITORIAL
DIRECTOR OF EDITORIAL Dan Schwartz
EDITOR-IN-CHIEF Julia Talsma
(440) 891-2792 jtalsma@advanstar.com
MANAGING EDITOR Julianne Stein
(440) 826-2834 jstein@advanstar.com
ASSOCIATE EDITOR Christina Phillis
(440) 891-2766 cphillis@advanstar.com
EDITORIAL ASSOCIATE Alicia Hoisington
CONTRIBUTING EDITORS Christine Blank,
Fred Gebhart, Jim Plagakis, RPh,
Gretchen L. Schwenker, PhD
SALES AND MARKETING
PUBLISHER James Granato
(732) 346-3071 jgranato@advanstar.com
ACCOUNT MANAGER Lisa Noble
(732) 346-3060 lnoble@advanstar.com
SALES SUPPORT ADMINISTRATOR Samyu Ganesh
(732) 346-3077 sganesh@advanstar.com
LIST MANAGER Tamara Phillips
(440) 891-2773 / tphillips@advanstar.com
PERMISSIONS AND LICENSING Maureen Cannon
(440) 891-2742 or (800) 225-4569 ext. 2742
Fax: (440) 891-2650 / mcannon@advanstar.com
DISPLAY AND CLASSIFIED ADS Heather Schlosser
(440) 891-2779 hschlosser@advanstar.com
CLASSIFIED RECRUITMENT Joanna Shippoli
800-225-4569 x 2615 jshippoli@advanstar.com
REPRINT SERVICES
(800) 290-5460, ext. 100 / AdvanstarReprints@theYGSgroup.com
(717) 505-9701, ext. 100 (international inquiries)
PRODUCTION
SENIOR PRODUCTION MANAGER Terri Johnstone
(218) 740-6310
CIRCULATION
SUBSCRIPTION CUSTOMER SERVICE / ADDRESS CHANGES
(877) 922-2022 / magazines@superfi ll.com
PO Box 6079, Duluth, MN 55806-6079, USA
CONTACT US
24950 COUNTRY CLUB BLVD., SUITE 200
NORTH OLMSTED, OHIO 44070
MAIN NUMBER: (440) 243-8100
MAIN FAX NUMBER: (440) 891-2735
CUSTOMER SERVICE: (877) 922-2022
CLASSIFIED AND RECRUITMENT SALES:
(800) 225-4569
E-MAIL ADDRESS: DRUGTOPICS@ADVANSTAR.COM
WEBSITE: HTTP://WWW.DRUGTOPICS.COM
Joseph Loggia
CHIEF EXECUTIVE OFFICER
Tom Ehardt
EXECUTIVE VICE PRESIDENT, CHIEF ADMINISTRATIVE OFFICER
Steve Sturm
EXECUTIVE VICE PRESIDENT, CHIEF MARKETING OFFICER
Ted Alpert
EXECUTIVE VICE PRESIDENT, FINANCE, CFO
Georgiann De Cenzo
EXECUTIVE VICE PRESIDENT
Eric Lisman
EXECUTIVE VICE PRESIDENT
J. Vaughn
VICE PRESIDENT, INFORMATION TECHNOLOGY
Francis Heid
VICE PRESIDENT, MEDIA OPERATIONS
Nancy Nugent
VICE PRESIDENT, HUMAN RESOURCES
Ward D. Hewins
VICE PRESIDENT, GENERAL COUNSEL
Danny Phillips
EXECUTIVE VICE PRESIDENT
Chris DeMoulin
EXECUTIVE VICE PRESIDENT
Editorial Mission: Drug Topics, a monthly
news magazine guided by an editorial
advisory board of pharmacy experts,
reports on all phases of community, retail,
and health-system issues and trends. We
offer a forum for the bench pharmacist to
share practical ideas for better pharmacy
management and patient care.

©2010 Mylan Pharmaceuticals Inc. MYNMET003 *Registered trademark of Bristol-Myers Squibb.
Reference: 1. Pelletier AL, Butler AM, Gillies RA, et al. Metformin Stinks, Literally. Ann Intern Med. 2010;152:267-268.
•A blackberry-scented formulation designed to mask the
“fishy smell” associated with some metformin tablets1
•Therapeutically equivalent to Glucophage®*
•Available in 500 mg, 850 mg and 1000 mg tablets
Indication:Metformin hydrochloride tablets are indicated
as an adjunct to diet and exercise to improve glycemic
control in adults and children with type 2 diabetes mellitus.
Adverse reactions:The most common adverse reactions,
reported in > 5% of metformin-treated patients, are:
diarrhea, nausea/vomiting, flatulence, asthenia, indigestion,
abdominal discomfort, and headache.
Contraindications and precautions:
Metformin hydrochloride tablets are contraindicated in
patients with:
1. Renal disease or renal dysfunction (e.g., as suggested
by serum creatinine levels * 1.5 mg/dL [males],
* 1.4 mg/dL [females] or abnormal creatinine clearance)
which may also result from conditions such as
cardiovascular collapse (shock), acute myocardial
infarction, and septicemia.
2. Known hypersensitivity to metformin hydrochloride.
3. Acute or chronic metabolic acidosis, including diabetic
ketoacidosis, with or without coma. Diabetic ketoacidosis
should be treated with insulin.
Metformin should be temporarily discontinued in patients
undergoing radiologic studies involving intravascular
administration of iodinated contrast materials, because
use of such products may result in acute alteration of
renal function.
Before initiation of metformin therapy and at least annually
thereafter, renal function should be assessed and verified
as normal. In patients in whom development of renal
dysfunction is anticipated, renal function should be
assessed more frequently and metformin discontinued
if evidence of renal impairment is present.
WARNINGS: LACTIC ACIDOSIS
Lactic acidosis is a rare but serious, metabolic
complication that can occur because of metformin
accumulation. Reported cases have occurred primarily
in diabetic patients with significant renal insufficiency,
including both intrinsic renal disease and renal
hypoperfusion, often in the setting of multiple
concomitant medical/surgical problems and multiple
concomitant medications. The risk of lactic acidosis
may, therefore, be significantly decreased by regular
monitoring of renal function and by use of the minimum
effective dose. Other conditions that increase the risk
of lactic acidosis include: sepsis, dehydration, excess
alcohol intake, hepatic insufficiency and acute
congestive heart failure.
When lactic acidosis occurs, it is fatal in approximately
50% of cases. The reported incidence of lactic acidosis
in patients receiving metformin is very low
(approximately 0.03 cases/1000 patient-years, with
approximately 0.015 fatal cases/1000 patient years).
The onset is often subtle, accompanied only by
nonspecific symptoms such as malaise, myalgias,
respiratory distress, increasing somnolence and
nonspecific abdominal distress. Laboratory
abnormalities include low pH, increased anion gap,
and elevated blood lactate. If acidosis is suspected,
metformin should be discontinued and the patient
hospitalized immediately.
Metformin treatment should not be initiated in
patients ≥ 80 years of age unless measurement of
creatinine clearance demonstrates that renal function
is not reduced, as these patients are more susceptible
to developing lactic acidosis.
Please see adjacent Brief Summary of Prescribing
Information, including BOXED WARNING with complete
details about lactic acidosis.
Mylan Metformin is
Berry Nice.
A metformin tablet that doesn’t smell like fish.

METFORMIN HYDROCHLORIDE TABLETS, USP
500 mg, 850 mg and 1000 mg
Rx Only
BRIEF SUMMARY: Please see package insert for full prescribing information.
INDICATIONS AND USAGE: Metformin hydrochloride tablets are indicated as an adjunct to diet
and exercise to improve glycemic control in adults and children with type 2 diabetes mellitus.
CONTRAINDICATIONS: Metformin hydrochloride tablets are contraindi cated in patients with:
1. Renal disease or renal dysfunction (e.g., as suggested by serum creatinine levels
≥ 1.5 mg/dL [males], ≥ 1.4 mg/dL [females] or ab normal creatinine clearance)
which may also result from conditions such as cardiovascular collapse (shock),
acute myocardial infarction, and septicemia (see WARNINGS and PRECAUTIONS).
2. Known hypersensitivity to metformin hydrochloride.
3. Acute or chronic metabolic acidosis, including diabetic ketoacidosis, with or without
coma. Diabetic ketoacidosis should be treated with insulin.
Metformin should be temporarily discontinued in patients undergoing radiologic studies
involving intravascular administration of iodinated contrast materials, because use of
such products may result in acute alteration of renal function. (See also PRECAUTIONS.)
WARNINGS:
Lactic Acidosis: Lactic acidosis is a rare, but serious, metabolic complication that
can occur due to metformin accumulation during treatment with metformin; when it
occurs, it is fatal in approximately 50% of cases. Lactic acidosis may also occur in
association with a number of pathophysiologic conditions, including diabetes mellitus,
and whenever there is signifi cant tissue hypoperfusion and hypoxemia. Lactic acidosis
is characterized by elevated blood lactate levels (> 5 mmol/L), decreased blood pH,
electrolyte disturbances with an increased anion gap, and an increased lactate/
pyruvate ratio. When metformin is implicated as the cause of lactic acidosis, metformin
plasma levels > 5 mcg/mL are generally found.
The reported incidence of lactic acidosis in patients receiving metformin
hydrochloride is very low (approximately 0.03 cases/1000 patient-years, with
approximately 0.015 fatal cases/1000 patient-years). In more than 20,000 patient-
years exposure to metformin in clinical trials, there were no reports of lactic acidosis.
Reported cases have occurred primarily in diabetic patients with signifi cant renal
insuffi ciency, including both intrinsic renal disease and renal hypoperfusion, often in the
setting of multiple concomitant medical/surgical problems and multiple concomitant
medications. Patients with congestive heart failure requiring pharmacologic
management, in particular those with unstable or acute congestive heart failure who
are at risk of hypoperfusion and hypoxemia are at increased risk of lactic acidosis.
The risk of lactic acidosis increases with the degree of renal dysfunction and the
patient’s age. The risk of lactic acidosis may, therefore, be signifi cantly decreased by
regular monitoring of renal function in patients taking metformin and by use of the
minimum effective dose of metformin. In particular, treatment of the elderly should be
accompanied by careful monitoring of renal function. Metformin treatment should not
be initiated in patients ≥ 80 years of age unless measurement of creatinine clearance
demonstrates that renal function is not reduced, as these patients are more susceptible
to developing lactic acidosis. In addition, metformin should be promptly withheld in the
presence of any condition associated with hypoxemia, dehydration or sepsis. Because
impaired hepatic function may signifi cantly limit the ability to clear lactate, metformin
should generally be avoided in patients with clinical or laboratory evidence of hepatic
disease. Patients should be cautioned against excessive alcohol intake, either acute or
chronic, when taking metformin, since alcohol potentiates the effects of metformin on
lactate metabolism. In addition, metformin should be temporarily discontinued prior
to any intravascular radiocontrast study and for any surgical procedure (see also
PRECAUTIONS).
The onset of lactic acidosis often is subtle, and accompanied only by nonspecifi c
symptoms such as malaise, myalgias, respira tory distress, increasing somnolence and
nonspecifi c abdominal distress. There may be associated hypothermia, hypotension
and resistant bradyarrhythmias with more marked acidosis. The patient and the
patient’s physician must be aware of the possible importance of such symptoms and
the patient should be instructed to notify the physician immediately if they occur (see
also PRECAUTIONS). Metformin should be withdrawn until the situation is clarifi ed.
Serum electrolytes, ketones, blood glucose and, if indicated, blood pH, lactate levels
and even blood metformin levels may be useful. Once a patient is stabilized on any dose
level of metformin, gastrointestinal symptoms, which are common during initiation of
therapy, are unlikely to be drug-related. Later occurrence of gastrointestinal symptoms
could be due to lactic acidosis or other serious disease.
Levels of fasting venous plasma lactate above the upper limit of normal but less
than 5 mmol/L in patients taking metformin do not necessarily indicate impending
lactic acidosis and may be explainable by other mechanisms, such as poorly controlled
diabetes or obesity, vigorous physical activity or technical problems in sample handling.
(See also PRECAUTIONS.)
Lactic acidosis should be suspected in any diabetic patient with metabolic acidosis
lacking evidence of ketoacidosis (ketonuria and ketonemia).
Lactic acidosis is a medical emergency that must be treated in a hospital setting. In
a patient with lactic acidosis who is taking metformin, the drug should be discontinued
immediately and general supportive measures promptly instituted. Because metformin
hydrochloride is dialyzable (with a clearance of up to 170 mL/min under good
hemodynamic conditions), prompt hemodialysis is recommended to correct the acidosis
and remove the accumulated metformin. Such management often results in prompt
reversal of symptoms and recovery. (See also CONTRAINDICATIONS and PRECAUTIONS.)
PRECAUTIONS: General: Macrovascular Outcomes: There have been no clinical studies
establishing conclusive evidence of macrovascular risk reduction with metformin
hydrochloride tablets or any other anti-diabetic drug.
Monitoring of Renal Function: Metformin is known to be substantially excreted by the
kidney, and the risk of metformin accumulation and lactic acidosis increases with the
degree of impairment of renal function. Thus, patients with serum creatinine levels
above the upper limit of normal for their age should not receive metformin. In patients
with advanced age, metformin should be carefully titrated to establish the minimum dose
for adequate glycemic effect, because aging is associated with reduced renal function. In
elderly patients, particularly those ≥ 80 years of age, renal function should be monitored
regularly and, generally, metformin should not be titrated to the maximum dose (see
WARNINGS and DOSAGE AND ADMINISTRATION in full prescribing information).
Before initiation of metformin therapy and at least annually thereafter, renal function
should be assessed and verifi ed as normal. In patients in whom development of renal
dysfunction is anticipated, renal function should be assessed more frequently and
metformin discontinued if evidence of renal impairment is present.
Use of Concomitant Medications That May Affect Renal Function or Metformin Disposition:
Concomitant medication(s) that may affect renal function or result in signifi cant hemodynamic
change or may interfere with the disposition of metformin, such as cationic drugs that are
eliminated by renal tubular secretion (see PRECAUTIONS: Drug Interactions), should be used
with caution.
Radiologic Studies Involving the Use of Intravascular Iodinated Contrast Materials
(for example, intravenous urogram, intravenous cholangiography, angiography, and
computed tomography (CT) scans with intravascular contrast materials): Intravascular
contrast studies with iodinated materials can lead to acute alteration of renal function
and have been associated with lactic acidosis in patients receiving metformin (see
CONTRAINDICATIONS). Therefore, in patients in whom any such study is planned, metformin
should be temporarily discontinued at the time of or prior to the procedure, and withheld for
48 hours subsequent to the procedure and reinstituted only after renal function has been
reevaluated and found to be normal.
Hypoxic States: Cardiovascular collapse (shock) from whatever cause, acute congestive
heart failure, acute myocardial infarction and other conditions characterized by hypoxemia
have been associated with lactic acidosis and may also cause prerenal azotemia. When such
events occur in patients on metformin therapy, the drug should be promptly discontinued.
Surgical Procedures: Metformin therapy should be temporarily suspended for any surgical
procedure (except minor procedures not associated with restricted intake of food and fl uids)
and should not be restarted until the patient’s oral intake has resumed and renal function
has been evaluated as normal.
Alcohol Intake: Alcohol is known to potentiate the effect of metformin on lactate
metabolism. Patients, therefore, should be warned against excessive alcohol intake, acute
or chronic, while receiving metformin.
Impaired Hepatic Function: Since impaired hepatic function has been associated with
some cases of lactic acidosis, metformin should generally be avoided in patients with
clinical or laboratory evidence of hepatic disease.
Vitamin B12 Levels: In controlled clinical trials of metformin of 29 weeks duration, a
decrease to subnormal levels of previously normal serum vitamin B12 levels, without clinical
manifestations, was observed in approximately 7% of patients. Such decrease, possibly due
to interference with B12 absorption from the B12-intrinsic factor complex, is, however, very
rarely associated with anemia and appears to be rapidly reversible with discontinuation of
metformin or vitamin B12 supplementation. Measurement of hematologic parameters on an
annual basis is advised in patients on metformin and any apparent abnormalities should
be appropriately investigated and managed (see PRECAUTIONS: Laboratory Tests).
Certain individuals (those with inadequate vitamin B12 or calcium intake or absorption)
appear to be predisposed to developing subnormal vitamin B12 levels. In these patients,
routine serum vitamin B12 measurements at 2- to 3-year intervals may be useful.
Change in Clinical Status of Patients with Previously Controlled Type 2 Diabetes: A
patient with type 2 diabetes previously well controlled on metformin who develops laboratory
abnormalities or clinical illness (especially vague and poorly defi ned illness) should be
evaluated promptly for evidence of ketoacidosis or lactic acidosis. Evaluation should
include serum electrolytes and ketones, blood glucose and, if indicated, blood pH, lactate,
pyruvate and metformin levels. If acidosis of either form occurs, metformin must be stopped
immediately and other appropriate corrective measures initiated (see also WARNINGS).
Hypoglycemia: Hypoglycemia does not occur in patients receiving metformin alone
under usual circumstances of use, but could occur when caloric intake is defi cient, when
strenuous exercise is not compensated by caloric supplementation, or during concomitant
use with other glucose-lowering agents (such as sulfonylureas and insulin) or ethanol.
Elderly, debilitated or malnourished patients, and those with adrenal or pituitary insuffi ciency
or alcohol intoxication are particularly susceptible to hypoglycemic effects. Hypoglycemia may be
diffi cult to recognize in the elderly, and in people who are taking beta-adrenergic blocking drugs.
Loss of Control of Blood Glucose: When a patient stabilized on any diabetic regimen is
exposed to stress such as fever, trauma, infection, or surgery, a temporary loss of glycemic
control may occur. At such times, it may be necessary to withhold metformin and temporarily
administer insulin. Metformin may be reinstituted after the acute episode is resolved.
The effectiveness of oral antidiabetic drugs in lowering blood glucose to a targeted level
decreases in many patients over a period of time. This phenomenon, which may be due to
progression of the underlying disease or to diminished responsiveness to the drug, is known
as secondary failure, to distinguish it from primary failure in which the drug is ineffective
during initial therapy. Should secondary failure occur with either metformin or sulfonylurea
monotherapy, combined therapy with metformin and sulfonylurea may result in a response.
Should secondary failure occur with combined metformin/sulfonylurea therapy, it may be
necessary to consider therapeutic alternatives including initiation of insulin therapy.
Information for Patients: Patients should be informed of the potential risks and benefi ts of
metformin and of alternative modes of therapy. They should also be informed about the
importance of adherence to dietary instructions, of a regular exercise program, and of regular
testing of blood glucose, glycosylated hemoglobin, renal function and hematologic parameters.
Page 1 of 2

The risks of lactic acidosis, its symptoms, and conditions that predispose to its
development, as noted in the WARNINGS and PRECAUTIONS sections, should be explained to
patients. Patients should be advised to discontinue metformin immediately and to promptly
notify their health practitioner if unexplained hyperventilation, myalgia, malaise, unusual
somnolence or other nonspecifi c symptoms occur. Once a patient is stabilized on any dose
level of metformin, gastrointestinal symptoms, which are common during initiation of
metformin therapy, are unlikely to be drug related. Later occurrence of gastrointestinal
symptoms could be due to lactic acidosis or other serious disease.
Patients should be counselled against excessive alcohol intake, either acute or chronic,
while receiving metformin.
Metformin hydrochloride tablets alone do not usually cause hypoglycemia, although it
may occur when metformin is used in conjunction with oral sulfonylureas and insulin. When
initiating combination therapy, the risks of hypoglycemia, its symptoms and treatment,
and conditions that predispose to its development should be explained to patients and
responsible family members. (See Patient Information in full prescribing information.)
Laboratory Tests: Response to all diabetic therapies should be monitored by periodic
measurements of fasting blood glucose and glycosylated hemoglobin levels, with a goal
of decreasing these levels toward the normal range. During initial dose titration, fasting
glucose can be used to determine the therapeutic response. Thereafter, both glucose and
glycosylated hemo globin should be monitored. Measurements of glycosylated hemoglobin
may be especially useful for evaluating long-term control (see also DOSAGE AND
ADMINISTRATION in full prescribing information).
Initial and periodic monitoring of hematologic parameters (e.g., hemoglobin/hematocrit
and red blood cell indices) and renal function (serum creatinine) should be performed, at
least on an annual basis. While megaloblastic anemia has rarely been seen with metformin
therapy, if this is suspected, vitamin B12 defi ciency should be excluded.
Drug Interactions (Clinical Evaluation of Drug Interactions Conducted with Metformin):
Glyburide: In a single-dose interaction study in type 2 diabetes patients, coadministration of
metformin and glyburide did not result in any changes in either metformin pharmacokinetics
or pharmacodynamics. Decreases in glyburide AUC and Cmax were observed, but were highly
variable. The single-dose nature of this study and the lack of correlation between glyburide
blood levels and pharmacodynamic effects, makes the clinical signifi cance of this interaction
uncertain (see DOSAGE AND ADMINISTRATION: Concomitant Metformin Hydrochloride Tablet
and Oral Sulfonylurea Therapy in Adult Patients in full prescribing information).
Furosemide: A single-dose, metformin-furosemide drug interaction study in healthy
subjects demonstrated that pharmacokinetic parameters of both compounds were affected
by coadministration. Furosemide increased the metformin plasma and blood Cmax by 22%
and blood AUC by 15%, without any signifi cant change in metformin renal clearance. When
administered with metformin, the Cmax and AUC of furosemide were 31% and 12% smaller,
respectively, than when administered alone, and the terminal half-life was decreased by 32%,
without any signifi cant change in furosemide renal clearance. No information is available
about the interaction of metformin and furosemide when coadministered chronically.
Nifedipine: A single-dose, metformin-nifedipine drug interaction study in normal healthy
volunteers demonstrated that coadministration of nifedipine increased plasma metformin
Cmax and AUC by 20% and 9%, respectively, and increased the amount excreted in the
urine. Tmax and half-life were unaffected. Nifedipine appears to enhance the absorption of
metformin. Metformin had minimal effects on nifedipine.
Cationic Drugs: Cationic drugs (e.g., amiloride, digoxin, morphine, procainamide, quinidine,
quinine, ranitidine, triamterene, trimethoprim, or vancomycin) that are eliminated by
renal tubular secretion theoretically have the potential for interaction with metformin by
competing for common renal tubular transport systems. Such interaction between metformin
and oral cimetidine has been observed in normal healthy volunteers in both single- and
multiple-dose, metformin-cimetidine drug interaction studies, with a 60% increase in peak
metformin plasma and whole blood concentrations and a 40% increase in plasma and
whole blood metformin AUC. There was no change in elimination half-life in the single-dose
study. Metformin had no effect on cimetidine pharmacokinetics. Although such interactions
remain theoretical (except for cimetidine), careful patient monitoring and dose adjustment
of metformin and/or the interfering drug is recommended in patients who are taking cationic
medications that are excreted via the proximal renal tubular secretory system.
Other: Certain drugs tend to produce hyperglycemia and may lead to loss of glycemic
control. These drugs include the thiazides and other diuretics, corticosteroids,
phenothiazines, thyroid products, estrogens, oral contraceptives, phenytoin, nicotinic acid,
sympathomimetics, calcium channel blocking drugs, and isoniazid. When such drugs are
administered to a patient receiving metformin, the patient should be closely observed for
loss of blood glucose control. When such drugs are withdrawn from a patient receiving
metformin, the patient should be observed closely for hypoglycemia.
In healthy volunteers, the pharmacokinetics of metformin and propranolol and metformin
and ibuprofen were not affected when coadministered in single-dose interaction studies.
Metformin is negligibly bound to plasma proteins and is, therefore, less likely to interact
with highly protein-bound drugs such as salicylates, sulfonamides, chloramphenicol, and
probenecid, as compared to the sulfonylureas, which are extensively bound to serum proteins.
Carcinogenesis, Mutagenesis, Impairment of Fertility: Long-term carcinogenicity studies
have been performed in rats (dosing duration of 104 weeks) and mice (dosing duration of
91 weeks) at doses up to and including 900 mg/kg/day and 1500 mg/kg/day, respectively.
These doses are both approximately four times the maximum recommended human daily
dose of 2000 mg based on body surface area comparisons. No evidence of carcinogenicity
with metformin was found in either male or female mice. Similarly, there was no tumorigenic
potential observed with metformin in male rats. There was however, an increased incidence
of benign stromal uterine polyps in female rats treated with 900 mg/kg/day.
There was no evidence of a mutagenic potential of metformin in the following
in vitro tests: Ames test (S. typhimurium), gene mutation test (mouse lymphoma cells),
or chromosomal aberrations test (human lymphocytes). Results in the in vivo mouse
micronucleus test were also negative.
Fertility of male or female rats was unaffected by metformin when administered
at doses as high as 600 mg/kg/day, which is approximately three times the maximum
recommended human daily dose based on body surface area comparisons.
Pregnancy: Teratogenic Effects. Pregnancy Category B: Recent information strongly
suggests that abnormal blood glucose levels during pregnancy are associated with a higher
incidence of congenital abnormalities. Most experts recommend that insulin be used during
pregnancy to maintain blood glucose levels as close to normal as possible. Because animal
reproduction studies are not always predictive of human response, metformin should not
be used during pregnancy unless clearly needed.
There are no adequate and well controlled studies in pregnant women with metformin.
Metformin was not teratogenic in rats and rabbits at doses up to 600 mg/kg/day. This
represents an exposure of about two and six times the maximum recommended human daily
dose of 2000 mg based on body surface area comparisons for rats and rabbits, respectively.
Determination of fetal concentrations demonstrated a partial placental barrier to metformin.
Nursing Mothers: Studies in lactating rats show that metformin is excreted into milk and
reaches levels comparable to those in plasma. Similar studies have not been conducted in
nursing mothers. Because the potential for hypoglycemia in nursing infants may exist, a
decision should be made whether to discontinue nursing or to discontinue the drug, taking
into account the importance of the drug to the mother. If metformin is discontinued, and if
diet alone is inadequate for controlling blood glucose, insulin therapy should be considered.
Pediatric Use: The safety and effectiveness of metformin for the treatment of type 2
diabetes have been established in pediatric patients ages 10 to 16 years (studies have not
been conducted in pediatric patients below the age of 10 years). Use of metformin in this
age group is supported by evidence from adequate and well controlled studies of metformin
in adults with additional data from a controlled clinical study in pediatric patients ages 10
to 16 years with type 2 diabetes, which demonstrated a similar response in glycemic control
to that seen in adults. (See CLINICAL PHARMACOLOGY: Pediatric Clinical Studies in full
prescribing information.) In this study, adverse effects were similar to those described in
adults. (See ADVERSE REACTIONS: Pediatric Patients.) A maximum daily dose of 2000 mg
is recommended. (See DOSAGE AND ADMINISTRATION: Recommended Dosing Schedule:
Pediatrics in full prescribing information.)
Geriatric Use: Controlled clinical studies of metformin did not include suffi cient numbers
of elderly patients to determine whether they respond differently from younger patients,
although other reported clinical experience has not identifi ed differences in responses
between the elderly and younger patients. Metformin is known to be substantially
excreted by the kidney and because the risk of serious adverse reactions to the drug is
greater in patients with impaired renal function, metformin should only be used in
patients with normal renal function (see CONTRAINDICATIONS, WARNINGS, and CLINICAL
PHARMACOLOGY: Pharmacokinetics in full prescribing information). Because aging is
associated with reduced renal function, metformin should be used with caution as age
increases. Care should be taken in dose selection and should be based on careful and
regular monitoring of renal function. Generally, elderly patients should not be titrated to
the maximum dose of metformin (see also WARNINGS and DOSAGE AND ADMINISTRATION
in full prescribing information).
ADVERSE REACTIONS: In a U.S. double-blind clinical study of metformin in patients with
type 2 diabetes, a total of 141 patients received metformin therapy (up to 2550 mg per
day) and 145 patients received placebo. Adverse reactions reported in greater than 5% of
the metformin patients, and that were more common in metformin- than placebo-treated
patients, are listed in Table 1.
Table 1. Most Common Adverse Reactions (> 5%) in a
Placebo-Controlled Clinical Study of Metformin Monotherapy*
Adverse Reaction
Metformin Monotherapy
(n = 141)
Placebo
(n = 145)
% of Patients
Diarrhea 53.2 11.7
Nausea/Vomiting 25.5 8.3
Flatulence 12.1 5.5
Asthenia 9.2 5.5
Indigestion 7.1 4.1
Abdominal Discomfort 6.4 4.8
Headache 5.7 4.8
* Reactions that were more common in metformin- than placebo-treated patients.
Diarrhea led to discontinuation of study medication in 6% of patients treated with
metformin. Additionally, the following adverse reactions were reported in ≥ 1 to ≤ 5%
of metformin patients and were more commonly reported with metformin than placebo:
abnormal stools, hypoglycemia, myalgia, lightheaded, dyspnea, nail disorder, rash, sweating
increased, taste disorder, chest discomfort, chills, fl u syndrome, fl ushing, palpitation.
Pediatric Patients: In clinical trials with metformin in pediatric patients with type 2
diabetes, the profi le of adverse reactions was similar to that observed in adults.
OVERDOSAGE: Overdose of metformin hydrochloride has occurred, including ingestion
of amounts greater than 50 grams. Hypoglycemia was reported in approximately 10%
of cases, but no causal association with metformin hydrochloride has been established.
Lactic acidosis has been reported in approximately 32% of metformin overdose cases
(see WARNINGS). Metformin is dialyzable with a clearance of up to 170 mL/min under
good hemodynamic conditions. Therefore, hemodialysis may be useful for removal of
accumulated drug from patients in whom metformin overdosage is suspected.
Manufactured in India by:
Matrix Laboratories Limited
Secunderabad — 500 003, India
Code No.: MH/DRUGS/25/NKD/89
Manufactured for:
MYLAN®
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
REVISED JANUARY 2010
BS:MX:METB:R1ppt
Page 2 of 2

CONTENTS
C
C
C
C
C
C
C
O
O
O
O
O
O
O
V
V
V
V
V
V
V
E
E
E
E
E
E
E
R
R
R
R
R
R
R
S
S
S
S
S
S
S
T
T
T
T
T
T
T
O
O
O
O
O
O
O
R
R
R
R
R
R
R
Y
Y
Y
Y
Y
Y
Y
DIGITAL EDITION
WWW.DRUGTOPICS.COM
6
DRUG TOPICS
December 2010
Vol. 154 No. 12
December 2010
COUNTER POINTS
9 GUEST EDITORIAL
The MTM challenge:
Grab those crayons
and get on with it
10 LETTERS
Sometimes a
placebo can be a
real lifesaver
13 JP AT LARGE
The 200th column:
Images from a
lifetime in pharmacy
52 VIEWPOINT
“It’s our profession”:
A conversation with JP
ISSUES & TRENDS
14 UPFRONT
New tools for chronic
pain patients and
healthcare providers
16 UPFRONT IN DEPTH
REMS update: Risk
management gets
its third checkup
CHAINS & BUSINESS
21 PROFESSIONAL
The war over
counterfeit drugs
rages on
CLINICAL
29 PACE-MI TRIAL
It’s time to revisit
beta blocker dosage
for MI patients
REGULATORY & LEGAL
42 FDA IN COURT
The deaths of 27
polo ponies touch off
a struggle over the
legal defi nition of
compounding
PRODUCT UPDATES
47 DIGESTIVES
Some resources for
those who dine not
wisely but too well
48 NEW PRODUCTS
Pradaxa: The fi rst new
oral anticoagulant
approved in the United
States in 50 years
CONTINUING
EDUCATION
30 C. DIFFICILE UPDATE
Common risk factors;
treatments and
therapies; interactions
and adverse events;
control and prevention
Drug Topics (ISSN# 0012-6616) is published monthly
and Drug Topics Digital Edition (ISSN# 1937-8157) is
issued every week by Advanstar Communications, Inc.,
131 West First St., Duluth, MN 55806-2065. One-
year subscription rates: $61 in the United States &
Possessions; $109 in Canada and Mexico; all other
countries, $109. Single copies (prepaid only) $10 in
the United States; $10 in Canada and Mexico; all other
countries, $15. Include $6 per copy for U.S. postage
and handling. Periodicals postage paid at Duluth, MN
55806 and additional mailing offi ces. POSTMASTER:
Please send address changes to Drug Topics, P.O.
Box 6079, Duluth, MN 55806-6079. Canadian G.S.T.
number: R-124213133RT001. Publications Mail
Agreement Number 40017597. Printed in the U.S.A.
Subscription inquiries/address changes: toll-free (888)
527-7008, or dial direct (218) 723-9477. ©2010
Advanstar Communications Inc. All rights reserved.
No part of this publication may be reproduced or
transmitted in any form or by any means, electronic
or mechanical including by photocopy, recording, or
information storage and retrieval without permission in
writing from the publisher. Authorization to photocopy
items for internal/educational or personal use, or
the internal/educational or personal use of specifi c
clients is granted by Advanstar Communications Inc. for
libraries and other users registered with the Copyright
Clearance Center, 222 Rosewood Dr. Danvers, MA
01923, 978-750-8400 fax 978-646-8700. For uses
beyond those listed above, please direct your written
request to Permission Dept. fax 440-891-2650 or email:
bgilbert@advanstar.com. Microfi lm or microfi che copies
of issues are available through Advanstar Marketing
Services, (800) 225-4569, Ext. 839. Unsolicited
manuscripts, photographs, art, and other material will
not be returned. Publisher assumes no responsibility
for unsolicited manuscripts, photographs, art, and other
material. Advanstar Communications provides certain
customer contact data (such as customers’ names,
addresses, phone numbers, and e-mail addresses) to
third parties who wish to promote relevant products,
services, and other opportunities that may be of interest
to you. If you do not want Advanstar Communications to
make your contact information available to third parties
for marketing purposes, simply call toll-free (888) 527-
7008 between the hours of 7:30 am and 5 pm CT and
a customer service representative will assist you in
removing your name from Advanstar’s lists. Outside the
U.S., please phone (218) 740-6477.
DRUG TOPICS does not verify any claims or other
information appearing in any of the advertisements
contained in the publication, and cannot take
responsibility for any losses or other damages
incurred by readers in reliance on such content. DRUG
TOPICS welcomes unsolicited articles, manuscripts,
photographs and other materials but cannot be held
responsible for their safekeeping or return.
GETTY IMAGES/COMSTOCK IMAGES
Subscribe to the monthly digital
edition of Drug Topics and receive the
journal electronically with live links.
Go to
http://drugtopics.com/digital
.
9
77% 23%
y
STEADY SAILING FOR NOW
Drug Topics’ 2011 business
outlook survey shows us which way the
wind is blowing as pharmacists plan to
chart their course for the coming year.
WWW.DRUGTOPICS.COM
■ Visit the Drug Topics blog: A new forum for pharmacist voices
(http://drugtopics.com/blog)
■ FDA hears testimony on approval pathway for biosimilars
(http://drugtopics.com/biosimilars)
■ The future of genetics and genomics
(http://drugtopics.com/genomics)
■ Ohio becomes a hub for prescription monitoring
(http://drugtopics.com/hub)
29
30
48
22
DOES REIMBURSEMENT HAVE YOU
DOWN?
FLU SEASON HAVE YOU OUT IN THE
COLD?
OR DO YOU JUST NEED TO BE
HEARD?
E-mail Associate Editor
Christina Phillis at
cphillis@advanstar.com, or
visit the blog on our homepage
(www.drugtopics.com).
Voice your opinions
on Drug Topics’ new blog!
Voice your opinions
on Drug Topics’ new blog!

©2010 Mylan Pharmaceuticals Inc. MYNCLN003
The
Mylan
CLONIDINE TRANSDERMAL SYSTEM is much smaller than the Par
Generic Clonidine patch—closer in size to the brand.1,2 It contains no metal so
it doesn’t need to be removed during an MRI to avoid potential skin burns;1,2
and it has an easy-open tab so there’s no need to cut the pouch open
with scissors like the Par patch.1
The
Mylan
CLONIDINE TRANSDERMAL SYSTEM is designed with patients in mind.
Patch Appeal
Actual Size Actual Size
INDICATION: Clonidine Transdermal System is indicated in the treatment of hypertension. It may be employed alone or concomitantly
with other antihypertensive agents.
IMPORTANT SAFETY INFORMATION: Patients should be instructed not to discontinue therapy without consulting their physician.
Sudden cessation of clonidine treatment has resulted in symptoms such as nervousness, agitation, headache, and confusion accompanied
or followed by a rapid rise in blood pressure and elevated catecholamine plasma concentrations. Such reactions appear to be associated
with higher doses or continuation of concomitant beta-blocker treatment and special caution is therefore advised in these situations.
Rare instances of hypertensive encephalopathy, cerebrovascular accidents and death have been reported after clonidine withdrawal.
When discontinuing therapy with Clonidine, the physician should reduce the dose gradually over 2 to 4 days to avoid withdrawal
symptomatology.
The most frequent adverse reactions to Clonidine are dermatological, including erythema and/or pruritus. Systemic adverse reactions
with transdermal clonidine were mild and included dry mouth, drowsiness, fatigue, headache, and lethargy and sedation.
Please see adjacent Brief Summary of Prescribing Information.
800.RX.MYLAN • www.mylanpharms.com
Small size. No metal. Easy-open packaging.
References: 1. Par Clonidine Transdermal System Prescribing Information. 2. Catapres-TTS Prescribing Information.

CLONIDINE
TRANSDERMAL SYSTEM, USP
BRIEF SUMMARY: Please see package insert for full prescribing information.
INDICATIONS AND USAGE: CLONIDINE TRANSDERMAL SYSTEM, USP is
indicated in the treatment of hypertension. It may be employed alone or
concomitantly with other antihypertensive agents.
CONTRAINDICATIONS: CLONIDINE TRANSDERMAL SYSTEM should not be
used in patients with known hypersensitivity to clonidine or to any other
component of the therapeutic system.
WARNINGS: Withdrawal: Patients should be instructed not to discontinue
therapy without consulting their physician. Sudden cessation of clonidine treat -
ment has, in some cases, resulted in symptoms such as nervousness, agitation,
headache, and confusion accompanied or followed by a rapid rise in blood
pressure and elevated catecholamine concentrations in the plasma. The likelihood
of such reactions to discontinuation of clonidine therapy appears to be greater
after administration of higher doses or continuation of concomitant beta-blocker
treatment and special caution is therefore advised in these situations. Rare
instances of hypertensive encephalopathy, cerebrovascular accidents and death
have been reported after clonidine withdrawal. When discontinuing therapy with
CLONIDINE TRANSDERMAL SYSTEM, the physician should reduce the dose
gradually over 2 to 4 days to avoid withdrawal symptomatology.
An excessive rise in blood pressure following discontinuation of CLONIDINE
TRANSDERMAL SYSTEM therapy can be reversed by administration of oral
clonidine hydrochloride or by intravenous phentolamine. If therapy is to be
discontinued in patients receiving a beta-blocker and clonidine concurrently, the
beta-blocker should be withdrawn several days before the gradual discontinu-
ation of CLONIDINE TRANSDERMAL SYSTEM.
ADVERSE REACTIONS: Clinical trial experience with CLONIDINE TRANSDERMAL
SYSTEM: Most systemic adverse effects during CLONIDINE TRANSDERMAL
SYSTEM therapy have been mild and have tended to diminish with continued
therapy. In a 3-month multiclinic trial of CLONIDINE TRANSDERMAL SYSTEM in
101 hypertensive patients, the systemic adverse reactions were, dry mouth (25
patients) and drowsiness (12), fatigue (6), headache (5), lethargy and sedation (3
each), insomnia, dizziness, impotence/sexual dysfunction, dry throat (2 each) and
constipation, nausea, change in taste and nervousness (1 each).
In the above mentioned 3-month controlled clinical trial, as well as other
uncontrolled clinical trials, the most frequent adverse reactions were dermato-
logical and are described below.
In the 3-month trial, 51 of the 101 patients had localized skin reactions such
as erythema (26 patients) and/or pruritus, particularly after using an adhesive
cover throughout the 7-day dosage interval. Allergic contact sensitization to
CLONIDINE TRANSDERMAL SYSTEM was observed in 5 patients. Other skin
reactions were localized vesiculation (7 patients), hyperpigmentation (5), edema
(3), excoriation (3), burning (3), papules (1), throbbing (1), blanching (1), and a
generalized macular rash (1).
In additional clinical experience, contact dermatitis resulting in treatment discon-
tinuation was observed in 128 of 673 patients (about 19 in 100) after a mean
duration of treatment of 37 weeks. The incidence of contact dermatitis was about
34 in 100 among white women, about 18 in 100 in white men, about 14 in 100 in
black women, and approximately 8 in 100 in black men. Analysis of skin reaction
data showed that the risk of having to discontinue CLONIDINE TRANSDERMAL
SYSTEM treatment because of contact dermatitis was greatest between treatment
weeks 6 and 26, although sensitivity may develop either earlier or later in treatment.
In a large-scale clinical acceptability and safety study by 451 physicians in a
total of 3,539 patients, other allergic reactions were recorded for which a causal
relationship to CLONIDINE TRANSDERMAL SYSTEM was not established:
maculopapular rash (10 cases); urticaria (2 cases); and angioedema of the face
(2 cases), which also affected the tongue in one of the patients.
Marketing Experience with CLONIDINE TRANSDERMAL SYSTEM: The following
adverse reactions have been identified during post-approval use of CLONIDINE
TRANSDERMAL SYSTEM. Because these reactions are reported voluntarily
from a population of uncertain size, it is not always possible to estimate
reliably their frequency or establish a causal relationship to drug exposure.
Decisions to include these reactions in labeling are typically based on one or
more of the following factors: (1) seriousness of the reaction, (2) frequency of
reporting, or (3) strength of causal connection to CLONIDINE TRANSDERMAL
SYSTEM.
Body as a Whole: Fever; malaise; weakness; pallor; and withdrawal syndrome.
Cardiovascular: Congestive heart failure; cerebrovascular accident; electrocar-
diographic abnormalities (i.e., bradycardia, sick sinus syndrome disturbances
and arrhythmias); chest pain; orthostatic symptoms; syncope; increases in
blood pressure; sinus bradycardia and atrioventricular (AV) block with and
without the use of concomitant digitalis; Raynaud’s phenomenon; tachycardia;
bradycardia; and palpitations.
Central and Peripheral Nervous System/Psychiatric: Delirium; mental de -
pression; hallucinations (including visual and auditory); localized numbness;
vivid dreams or nightmares; restlessness; anxiety; agitation; irritability; other
behavioral changes; and drowsiness.
Dermatological: Angioneurotic edema; localized or generalized rash; hives; urticaria;
contact dermatitis; pruritus; alopecia; and localized hypo or hyper pigmentation.
Gastrointestinal: Anorexia and vomiting.
Genitourinary: Difficult micturition; loss of libido; and decreased sexual activity.
Metabolic: Gynecomastia or breast enlargement and weight gain.
Musculoskeletal: Muscle or joint pain; and leg cramps.
Ophthalmological: Blurred vision; burning of the eyes and dryness of the eyes.
Adverse Events Associated with Oral Clonidine Therapy: Most adverse effects
are mild and tend to diminish with continued therapy. The most frequent (which
appear to be dose-related) are dry mouth, occurring in about 40 of 100 patients;
drowsiness, about 33 in 100; dizziness, about 16 in 100; constipation and
sedation, each about 10 in 100. The following less frequent adverse experiences
have also been reported in patients receiving clonidine hydrochloride, USP
tablets, but in many cases patients were receiving concomitant medication and
a causal relationship has not been established.
Body as a Whole: Fatigue, fever, headache, pallor, weakness, and withdrawal
syndrome. Also reported were a weakly positive Coombs’ test and increased
sensitivity to alcohol.
Cardiovascular: Bradycardia, congestive heart failure, electrocardiographic
abnormalities (i.e., sinus node arrest, junctional bradycardia, high degree AV
block and arrhythmias), orthostatic symptoms, palpitations, Raynaud’s
phenomenon, syncope, and tachycardia. Cases of sinus bradycardia and AV
block have been reported, both with and without the use of concomitant digitalis.
Central Nervous System: Agitation, anxiety, delirium, delusional perception,
hallucinations (including visual and auditory), insomnia, mental depression,
nervousness, other behavioral changes, paresthesia, restlessness, sleep disorder,
and vivid dreams or nightmares.
Dermatological: Alopecia, angioneurotic edema, hives, pruritus, rash, and urticaria.
Gastrointestinal: Abdominal pain, anorexia, constipation, hepatitis, malaise, mild
transient abnormalities in liver function tests, nausea, parotitis, pseudo-obstruction
(including colonic pseudo-obstruction), salivary gland pain, and vomiting.
Genitourinary: Decreased sexual activity, difficulty in micturition, erectile
dysfunction, loss of libido, nocturia, and urinary retention.
Hematologic: Thrombocytopenia.
Metabolic: Gynecomastia, transient elevation of blood glucose or serum creatine
phosphokinase, and weight gain.
Musculoskeletal: Leg cramps and muscle or joint pain.
Oro-otolaryngeal: Dryness of the nasal mucosa.
Ophthalmological: Accommodation disorder, blurred vision, burning of the eyes,
decreased lacrimation, and dryness of the eyes.
OVERDOSAGE: Hypertension may develop early and may be followed by
hypo tension, bradycardia, respiratory depression, hypothermia, drowsiness,
de creased or absent reflexes, weakness, irritability and miosis. The frequency of
CNS depression may be higher in children than adults. Large overdoses may
result in reversible cardiac conduction defects or dysrhythmias, apnea, coma
and seizures. Signs and symptoms of overdose generally occur within 30
minutes to 2 hours after exposure. As little as 0.1 mg of clonidine has produced
signs of toxicity in children.
If symptoms of poisoning occur following dermal exposure, remove all
CLONIDINE TRANSDERMAL SYSTEMS. After their removal, the plasma
clonidine levels will persist for about 8 hours, then decline slowly over a period
of several days. Rare cases of CLONIDINE TRANSDERMAL SYSTEM poisoning
due to accidental or deliberate mouthing or ingestion of the patch have been
reported, many of them involving children.
There is no specific antidote for clonidine overdosage. Ipecac syrup-induced
vomiting and gastric lavage would not be expected to remove significant
amounts of clonidine following dermal exposure. If the patch is ingested, whole
bowel irrigation may be considered and the administration of activated charcoal
and/or cathartic may be beneficial. Supportive care may include atropine sulfate
for bradycardia, intravenous fluids and/or vasopressor agents for hypotension
and vasodilators for hypertension. Naloxone may be a useful adjunct for the
management of clonidine-induced respiratory depression, hypotension and/or
coma; blood pressure should be monitored since the administration of naloxone
has occasionally resulted in paradoxical hyper tension. Tolazoline administration
has yielded inconsistent results and is not recommended as first-line therapy.
Dialysis is not likely to significantly en hance the elimination of clonidine.
The largest overdose reported to date, involved a 28-year old male who
ingested 100 mg of clonidine hydrochloride powder. This patient developed hyper-
tension followed by hypotension, bradycardia, apnea, hallucinations, semicoma,
and premature ventricular contractions. The patient fully recovered after intensive
treatment. Plasma clonidine levels were 60 ng/mL after 1 hour, 190 ng/mL after
1.5 hours, 370 ng/mL after 2 hours, and 120 ng/mL after 5.5 and 6.5 hours. In
mice and rats, the oral LD50 of clonidine is 206 and 465 mg/kg, respectively.
MYLAN®
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505
REVISED NOVEMBER 2009
BS:CTS:R9

WWW.DRUGTOPICS.COM
December 2010
DRUG TOPICS
9
DISPENSED AS WRITTEN
Counter Points
I continually receive surveys from pharmacy orga-
nizations, pharmacy practice residents, university
faculty, and others, asking for my opinion on medi-
cation therapy management (MTM). The frustrating thing is
that these surveys focus mainly on barriers.
There is usually a section instructing me to checkmark
all the barriers to providing MTM that I perceive. Then the
survey asks me to rank the barriers according to some or-
der of magnitude. Next I’m asked how I feel about certain
barriers (e.g., “On a scale of 1 to 10, how does the lack of
patient awareness affect the number of MTM services you
provide?”). By the time I get to the last section (which, by
the way, is a blank box asking me to list any other barri-
ers that I may know of, so that they can undoubtedly add
more items to their previous sections), I just want to shout,
“ENOUGH, ALREADY!”
Yes, barriers to providing MTM do exist for pharmacists.
I’m not about to say that providing MTM is as easy (or as
comfortable) as verifying prescriptions, counseling a patient,
or scribbling down a refi ll authorization. But we all have to
fi gure out a way to do it — we should feel an obligation to
do it.
Many community pharmacists are providing MTM in one
form or another, although not enough. The literature is full
of examples of pharmacists successfully implementing MTM
and providing high-level patient care in Asheville Project-
type programs.
• The Pharmacy Society of Wisconsin created an MTM
program that reimburses pharmacists in the state for both
acute interventions and more comprehensive care.
• The federal government recognized the importance of
MTM when it created Medicare Part D.
• Even the medical profession is claiming that pharmacists
are underutilized and need to be part of the team.
Yet it seems to me as if provision of MTM by pharmacists is
more of a niche than the norm right now. This has to change.
If you are a pharmacist in an independent community
pharmacy that is not currently providing MTM, start! If you
don’t know how, pick up the phone and call one of the
thousands who have fi gured it out.
If you are in an upper-level management position in a
chain pharmacy organization that is not allowing pharmacists
time to provide MTM, fi gure out a way! Make it a priority.
The pharmacists working under you need your support. Start
with paying them to be out of the workfl ow 1 hour a week.
Offer them an incentive to provide MTM, and hold them
accountable. Create new ways to make use of technicians.
Think of it as an investment in the future … an advancement
of the profession.
Let’s stop focusing on barriers and listing reasons why we
cannot provide MTM. Years ago (when what is now referred
to as MTM was called pharmaceutical care) a pharmacy pro-
fessor for whom I have a great deal of respect would from time
to time be confronted by a pharmacist or student who was
skeptical about providing high-level patient care. When said
skeptic would rattle off the typical laundry list of issues (lack of
time, lack of tools, lack of space, etc.) that stood in the way, the
professor’s response was “You can provide pharmaceutical care
with a blank sheet of paper and a big fat crayon. Just do it.”
I think it’s time for all of us to start coloring.
Marvin Moore, PharmD, is a community pharmacist in Two
Rivers, Wis., and a Drug Topics board member. He can be
reached at marvmoore4@hotmail.com.
The opinions expressed by guest editorial writers are their own and
do not necessarily represent the views of Drug Topics’ staff or the
staff of Advanstar Communications.
As part of our ongoing initiative to encourage dialogue between
pharmacists working in different environments, each month Drug
Topics presents an editorial by a guest columnist writing on a sub-
ject of his or her choice. Send us your feedback; we look forward to
sharing it in an upcoming issue.
Time to get out the big fat crayon
Marvin Moore, PharmD
I
n
f
Guest editorial

WWW.DRUGTOPICS.COM
Counter Points
10
DRUG TOPICS
December 2010
Context is everything
During my 31 years of pharmacy prac-
tice, I have been asked to fi ll capsules for
the placebo effect. Each time, I did fill
the prescription with the placebo, after I
evaluated the patient’s usage and abuse to
determine that the physician’s choice for
placebo was the correct therapy and in the
best interests of the patient. Most of these
patients were abusing their prescription
drugs. It worked every time, and I never
had a problem with patient or family.
As a Christian pharmacist, I do not
think that deception or a lie is OK unless it
is in the best interests of patient care. I did
not lie to the patients when counseling, as
most of these prescriptions were for refi lls.
Students have not seen this type of
patient in a practical setting and lack the
fi rst-hand knowledge to make this call.
Believe me, when they see the patients
who need this therapy, they will quickly
change their minds.
Mark Barclay, RPh
VENICE, FLA.
Score 1 for placebo ... make that 2
In 1957, I was a brand-new pharmacist
in a New York hospital. An elderly female
patient of the arthritis clinic adjacent to my
pharmacy had no clinical disease in real-
ity but constantly sought treatment. The
rheumatologist, the director, and I con-
ferred and decided to give her a 2-month
supply of saccharin tablets with ad lib re-
fi lls to be taken 1 tid.
This went well for several months, with
the patient reporting good results, until she
came to my window one day, shouting,
and accused us of giving her substandard
medication. The previous night she had
tried to commit suicide by taking the entire
supply — “And look at me: I’m standing
right here in front of you, and I’m alive!”
We sent her for a psych consult. I
didn’t know whether to laugh or to cry.
Irving Gerber, RPh
FAIR LAWN, N.J.
Correction: The Web Exclusive article
“Propofol shortage leads to allergy warnings
about European product” noted in the Table
of Contents of the November 2010 issue stated
that Fresenius Propoven (propofol) 1% con-
tained peanut oil. It does not. The statement
was taken from an inaccurate press release.
The label of the European product contains
the following statement: “...the product con-
tains soybean oil and there are reports of cross
allergies between soybean and peanut oil.”
Drug Topics regrets the error.
Letters
Letters
Placebos and healing
Re: “When to Lie?”
[Regulatory & Legal,
November 2010]: ...Daniel
Moerman, a medical anthro-
pologist and author of dozens
of peer-reviewed articles on
the placebo effect, warns that
to withhold the prescription of
placebos could be considered
unethical in one sense, as they
can be powerfully therapeutic.
He particularly warns against
prohibiting the use of placebos
in clinical trials, in which re-
searchers face the same ethical dilemma as every doctor
and pharmacist.
Robert Speers, MA
BOULDER, COLO.
For more from Robert Speers, including details of a groundbreaking
study mixing placebo prescriptions with prescriptions for specifi c thera-
peutic agents, go to the new DT Blog at www.drugtopics.com.
Printed and e-mailed letters should be brief
and include the writer’s name, address,
daytime phone number, and date of the
issue you are referencing: Editor,
Drug Topics, 24950 Country Club Blvd., Suite
200, North Olmsted, OH 44070-5351.
E-mail address: drugtopics@advanstar.com.
Letters may be edited for length, style,
content, and clarity at our discretion.
We want to hear from you
WWW.DRUGTOPICS.COM
56
DRUG TOPICS
November 2010
Regulatory & Legal
Iteach a course in ethics at Midwest-
ern University College of Pharmacy
in Glendale, Ariz. Recently I present-
ed my students with an ethical problem,
asking them to decide on a correct course
of action. Their answers surprised me so
much that I presented the same problem
to some practicing pharmacists over the
next week. In every case, the answers giv-
en by the students were different from the
responses of the practicing pharmacists.
The problem I set was this. The stu-
dent, now a graduate pharmacist, is
directed by the boss, the pharmacist-
in-charge (PIC), to fi ll a prescription by
emptying the contents of a C III sedative
capsule and replacing them with lactose.
The PIC explains that the doctor is pre-
scribing a placebo and that over the past
few months the pharmacist and physi-
cian have been slowly replacing active
ingredient with inactive ingredient, until
now the capsules contain only lactose.
The new capsules are working as well as
the regular medication worked, and the
patient reports that she is sleeping well
throughout the night.
The PIC then tells the student/pharma-
cist to label the prescription with the name
of the regular C III sedative and to stress,
in counseling the patient, the strength of
the drug and the importance of avoiding
an overdose. In other words, the student
is told to lie to the patient. Then the PIC
departs; he will not return until his regular
shift the following day.
The class assignment was to work
through a decision-tree algorithm
leading to the ultimate decision: What
would the student/pharmacist do when
the patient arrives?
Not one of the students was willing
to fi ll the prescription or counsel the pa-
tient if it meant lying. They came to this
conclusion in spite of the fact that they
had been assigned to watch 2 videos ex-
tolling the necessity and use of placebos.
Intellectually, the students understood
the value of the placebo effect, but some-
how the situation was different when it
became a question of what the students
were willing to do to make the patient
believe in the placebo.
If a pharmacist believes that he or she
cannot participate in a deception, what
are the alternatives?
In this instance, the student/phar-
macist could tell the patient that she will
have to wait to pick up her prescription
until the PIC is on duty.
Would the answer ever be to tell the
patient the truth? In that case, the pa-
tient would learn that her doctor and her
regular pharmacist had been lying to her.
When the same question was pre-
sented to 6 pharmacists who had been
practicing for several years, they gave
the opposite answer. Every one of them
would fi ll the prescription by replacing
the active ingredients with an inert sub-
stance. In addition, all of them would
counsel the patient as if she were receiv-
ing the drug named on the label.
They all understood that the label was
technically wrong and that there was a
risk that the patient would someday dis-
cover the deception. However, all were
strongly motivated to provide the patient
with the full effect of the prescribed med-
ication, even though it was the placebo
effect that had actually been prescribed.
The practicing pharmacists saw no
ethical problem in the concept that some-
times it is okay to lie, that issues are not
always black and white, but can be gray,
and that choices may depend on the con-
squences of the act.
Who is right? Perhaps it is heartening
to fi nd that at some early stage in our ca-
reers, our ethical values are absolute: It is
never okay to lie to a patient. To students,
virtues are pure and ethics are black and
white, with little contrast.
Perhaps it is also good to find that
as we move through life the choices
become more difficult, as we discover
when we deal with real patients and fi nd
that the consequences of our actions can
have a real effect. The 6 older, practicing
pharmacists have all had to make tough
choices; some have actually participated
in placebo treatment.
It would be interesting to ask today’s
students the same question in 20 years.
How would you answer?
These articles are not intended as legal ad-
vice and should not be used as such. When
a legal question arises the pharmacist should
consult with an attorney familiar with
pharmacy law in his or her state.
KEN BAKER consults in the areas of
pharmacy error reduction, communication,
and risk management. He is a pharmacist
and an attorney of counsel with the
Arizona law fi rm Renaud Cook Drury
Mesaros, PA. Contact him at ken@
kenbakerconsulting.com.
ETHICAL PRACTICE
A question of ethics:
When to lie?
Kenneth R. Baker, BS Pharm, JD
The practicing pharma-
cists saw no ethical
problem in the concept
that sometimes it is
okay to lie, that issues
are not always black and
white, but can be gray ...
DOES REIMBURSEMENT HAVE YOU
DOWN?
FLU SEASON HAVE YOU OUT IN THE
COLD?
OR DO YOU JUST NEED TO BE
HEARD?
E-mail Associate Editor
Christina Phillis at
cphillis@advanstar.com, or
visit the blog on our homepage
(www.drugtopics.com).
Voice your opinions
on Drug Topics’ new blog!
Voice your opinions
on Drug Topics’ new blog!

EPIPEN and EPIPEN Jr Auto-Injectors
There is no AB-rated substitute
Help at-risk patients
prepare for a life-threatening
anaphylactic emergency…
* Data on fi le. SDI Health, Physician Disease & Diagnosis Audit, Drug Uses for
Dx Code 9950-Anaphylactic Shock, 1990-2009. www.EpiPen.com
DEY® and the Dey logo are registered trademarks of Dey Pharma, L.P.
EpiPen®, EpiPen 2-Pak®, and EpiPen Jr 2-Pak® are registered trademarks of
Mylan Inc., licensed exclusively to its wholly-owned subsidiary, Dey Pharma, L.P.
©2010. Dey Pharma, L.P. All rights reserved. 09/10 EPI10-1003
#
1
P
R
E
S
C
R
I
B
E
D
A
U
T
O
-
I
N
J
E
C
T
O
R
F
O
R
A
N
A
P
H
Y
L
A
X
I
S
*
YEARS
MORE THAN
Convenient 2-PAK contains
2 Auto-Injectors and a trainer.
• The FDA has not determined a therapeutic equivalent to BX-rated EPIPEN Auto-Injector
• No absolute contraindications to use in life-threatening situations
• EPIPEN Auto-Injector delivers epinephrine with speed and simplicity
• Only EPIPEN Auto-Injector has a unique, user-friendly design with
the added safety of automatic needle protection before and after use
Indications
EpiPen and EpiPen Jr Auto-Injectors (0.3 and 0.15 mg epinephrine) are indicated in the emergency
treatment of type 1 allergic reactions, including anaphylaxis, to allergens, idiopathic and exercise-
induced anaphylaxis, and in patients with a history or increased risk of anaphylactic reactions.
Selection of the appropriate dosage strength is determined according to body weight.
Important Safety Information
EpiPen Auto-Injectors should only be injected into the anterolateral aspect of the thigh. DO NOT
INJECT INTO BUTTOCK, OR INTRAVENOUSLY.
Epinephrine should be used with caution in patients with certain heart diseases, and in patients who
are on drugs that may sensitize the heart to arrhythmias, because it may precipitate or aggravate
angina pectoris and produce ventricular arrhythmias. Adverse reactions include transient moderate
anxiety, apprehensiveness, restlessness, tremor, weakness, dizziness, sweating, palpitations, pallor,
nausea and vomiting, headache, and/or respiratory difficulties.
EpiPen and EpiPen Jr Auto-Injectors are intended for immediate self-administration as emergency
supportive therapy only and are not intended as a substitute for immediate medical or hospital care.
Please see Brief Summary of Prescribing Information on the adjacent page.
EPIPEN carton NDC 49502-500-01
EPIPEN 2-PAK NDC 49502-500-02
EPIPEN Jr carton NDC 49502-501-01
EPIPEN Jr 2-PAK NDC 49502-501-02

EPIPEN® 0.3 mg EPINEPHRINE AUTO-INJECTOR
EPIPEN® Jr 0.15 mg EPINEPHRINE AUTO-INJECTOR
BRIEF SUMMARY. See package insert for full Prescribing Information.
DO NOT REMOVE ACTIVATION CAP UNTIL READY FOR USE.
THIS UNIT CONTAINS NO LATEX.
INDICATIONS AND USAGE: EpiPen® and EpiPen® Jr Auto-Injectors are indicated in the emergency treatment of
allergic reactions (Type I) including anaphylaxis to stinging insects (e.g., order Hymenoptera, which include bees,
wasps, hornets, yellow jackets and fire ants) and biting insects (e.g., triatoma, mosquitos), allergen immunotherapy,
foods, drugs, diagnostic testing substances (e.g., radiocontrast media) and other allergens, as well as idiopathic
anaphylaxis or exercise-induced anaphylaxis. EpiPen® and EpiPen® Jr Auto-Injectors are intended for immediate
administration in patients, who are determined to be at increased risk for anaphylaxis, including individuals
with a history of anaphylactic reactions. Selection of the appropriate dosage strength is determined according to
patient body weight (See DOSAGE AND ADMINISTRATION section of the full Prescribing Information).
Such reactions may occur within minutes after exposure and consist of flushing, apprehension, syncope,
tachycardia, thready or unobtainable pulse associated with a fall in blood pressure, convulsions, vomiting,
diarrhea and abdominal cramps, involuntary voiding, wheezing, dyspnea due to laryngeal spasm, pruritus, rashes,
urticaria or angioedema.
EpiPen® and EpiPen® Jr Auto-Injectors are intended for immediate self-administration as emergency supportive
therapy only and are not a substitute for immediate medical care.
CONTRAINDICATIONS: There are no absolute contraindications to the use of epinephrine in a life-threatening
situation.
WARNINGS: EpiPen® and EpiPen® Jr Auto-Injectors should only be injected into the anterolateral aspect of the
thigh. DO NOT INJECT INTO BUTTOCK. Injection into the buttock may not provide effective treatment of anaphylaxis.
Advise the patient to go immediately to the nearest emergency room for further treatment of anaphylaxis.
Since epinephrine is a strong vasoconstrictor, accidental injection into the digits, hands or feet may
result in loss of blood flow to the affected area. Treatment should be directed at vasodilation in addition to
further treatment of anaphylaxis. (see ADVERSE REACTIONS). Advise the patient to go immediately to the nearest
emergency room and to inform the healthcare provider in the emergency room of the location of the
accidental injection.
DO NOT INJECT INTRAVENOUSLY. Large doses or accidental intravenous injection of epinephrine may
result in cerebral hemorrhage due to sharp rise in blood pressure. Rapidly acting vasodilators can counteract
the marked pressor effects of epinephrine if there is such inadvertent administration.
Epinephrine is the preferred treatment for serious allergic reactions or other emergency situations even though this
product contains sodium metabisulfite, a sulfite that may, in other products, cause allergic-type reactions including
anaphylactic symptoms or life-threatening or less severe asthmatic episodes in certain susceptible persons.
The alternatives to using epinephrine in a life-threatening situation may not be satisfactory. The presence of a sulfite
in this product should not deter administration of the drug for treatment of serious allergic or other emergency
situations even if the patient is sulfite-sensitive.
Epinephrine should be administered with caution in patients who have heart disease, including patients with cardiac
arrhythmias, coronary artery or organic heart disease, or hypertension. In such patients, or in patients who are on
drugs that may sensitize the heart to arrhythmias, e.g., digitalis, diuretics, or anti-arrhythmics, epinephrine may
precipitate or aggravate angina pectoris as well as produce ventricular arrhythmias. It should be recognized that
the presence of these conditions is not a contraindication to epinephrine administration in an acute, life-threatening
situation.
Epinephrine is light sensitive and should be stored in the carrier tube provided. Store at 25°C (77°F); excursions
permitted to 15°C-30°C (59°F-86°F) (See USP Controlled Room Temperature). Do not refrigerate. Before using,
check to make sure the solution in the auto-injector is not discolored. Replace the auto-injector if the solution is
discolored or contains a precipitate.
PRECAUTIONS:
(1) General
EpiPen® and EpiPen® Jr Auto-Injectors are not intended as a substitute for immediate medical care. In conjunction
with the administration of epinephrine, the patient should seek immediate medical or hospital care. More than two
sequential doses of epinephrine should only be administered under direct medical supervision.
Epinephrine is essential for the treatment of anaphylaxis. Patients with a history of severe allergic reactions
(anaphylaxis) to insect stings or bites, foods, drugs, and other allergens as well as idiopathic and exercise-induced
anaphylaxis should be carefully instructed about the circumstances under which epinephrine should be used.
It must be clearly determined that the patient is at risk of future anaphylaxis, since the following risks may
be associated with epinephrine administration (see DOSAGE and ADMINISTRATION section of the full
Prescribing Information).
Epinephrine should be used with caution in patients who have cardiac arrhythmias, coronary artery or
organic heart disease, hypertension, or in patients who are on drugs that may sensitize the heart to
arrhythmias, e.g., digitalis, diuretics, quinidine, or other anti-arrhythmics. In such patients, epinephrine may
precipitate or aggravate angina pectoris as well as produce ventricular arrhythmias. The effects of epinephrine may
be potentiated by tricyclic antidepressants and monoamine oxidase inhibitors.
Some patients may be at greater risk of developing adverse reactions after epinephrine administration. These
include: hyperthyroid individuals, individuals with cardiovascular disease, hypertension, or diabetes, elderly
individuals, pregnant women, pediatric patients under 30 kg (66 lbs.) body weight using EpiPen® Auto-Injector, and
pediatric patients under 15 kg (33 lbs.) body weight using EpiPen® Jr Auto-Injector.
Despite these concerns, epinephrine is essential for the treatment of anaphylaxis. Therefore, patients with these
conditions, and/or any other person who might be in a position to administer EpiPen® or EpiPen® Jr Auto-Injector
to a patient experiencing anaphylaxis should be carefully instructed in regard to the circumstances under which
epinephrine should be used.
(2) Information for Patients
Complete patient information, including dosage, direction for proper administration and precautions can be found
inside each EpiPen®/EpiPen® Jr Auto-Injector carton.
Epinephrine may produce symptoms and signs that include an increase in heart rate, the sensation of a more
forceful heartbeat, palpitations, sweating, nausea and vomiting, difficulty breathing, pallor, dizziness, weakness or
shakiness, headache, apprehension, nervousness, or anxiety. These symptoms and signs usually subside rapidly,
especially with rest, quiet and recumbency. Patients with hypertension or hyperthyroidism may develop more severe
or persistent effects, and patients with coronary artery disease could experience angina. Patients with diabetes may
develop increased blood glucose levels following epinephrine administration. Patients with Parkinson’s disease may
notice a temporary worsening of symptoms.
In case of accidental injection, the patient should be advised to immediately go to the emergency room for
treatment. Since the epinephrine in the EpiPen® Auto-Injector is a strong vasoconstrictor when injected into the digits,
hands or feet, treatment should be directed at vasodilation if there is such an inadvertent administration to these areas.
(see ADVERSE REACTIONS).
(3) Drug Interactions
Patients who receive epinephrine while concomitantly taking cardiac glycosides or diuretics should be observed carefully
for the development of cardiac arrhythmias.
The effects of epinephrine may be potentiated by tricyclic antidepressants, monoamine oxidase inhibitors, levothyroxine
sodium, and certain antihistamines, notably chlorpheniramine, tripelennamine and diphenhydramine.
The cardiostimulating and bronchodilating effects of epinephrine are antagonized by beta-adrenergic blocking
drugs, such as propranolol. The vasoconstricting and hypertensive effects of epinephrine are antagonized by alpha-
adrenergic blocking drugs, such as phentoloamine. Ergot alkaloids may also reverse the pressor effects of
epinephrine.
(4) Carcinogenesis, Mutagenesis, Impairment of Fertility
Epinephrine and other catecholamines have been shown to have mutagenic potential in vitro and to be an oxidative
mutagen in a WP2 bacterial reverse mutation assay. Epinephrine had a moderate degree of mutagenicity, and was
positive in the DNA Repair test with B. subtilis (REC) assay, but was not mutagenic in the Salmonella bacterial reverse
mutation assay. Studies of epinephrine after repeated exposure in animals to evaluate the carcinogenic and mutagenic
potential or the effect on fertility have not been conducted. This should not prevent the use of epinephrine under the
conditions noted under INDICATIONS AND USAGE.
(5) Usage in Pregnancy
Pregnancy Category C: There is no study on the acute effect of epinephrine on pregnancy. Epinephrine has been shown
to have developmental effects when administered subcutaneously in rabbits at a dose of 1.2 mg/kg daily for two to
three days (approximately 30 times the maximum recommended daily subcutaneous or intramuscular dose on a
mg/m2 basis), in mice at a subcutaneous dose of 1 mg/kg daily for 10 days (approximately 7 times the maximum daily
subcutaneous or intramuscular dose on a mg/m2 basis) and in hamsters at a subcutaneous dose of 0.5 mg/kg daily for
4 days (approximately 5 times the maximum recommended daily subcutaneous or intramuscular dose on a mg/m2
basis). These effects were not seen in mice at a subcutaneous dose of 0.5 mg/kg daily for 10 days (approximately
3 times the maximum recommended daily subcutaneous or intramuscular dose on a mg/m2 basis). Although, there
are no adequate and well-controlled studies in pregnant women, epinephrine should be used in pregnancy only if the
potential benefit justifies the potential risk to the fetus.
ADVERSE REACTIONS: Adverse reactions to epinephrine include transient, moderate anxiety; apprehensiveness;
restlessness; tremor; weakness; dizziness; sweating; palpitations; pallor; nausea and vomiting; headache; and/
or respiratory difficulties. These symptoms occur in some persons receiving therapeutic doses of epinephrine, but
are more likely to occur in patients with hypertension or hyperthyroidism. Arrhythmias, including fatal ventricular
fibrillation, have been reported in patients with underlying cardiac disease or certain drugs [see PRECAUTIONS,
Drug Interactions]. Rapid rises in blood pressure have produced cerebral hemorrhage, particularly in elderly
patients with cardiovascular disease. Angina may occur in patients with coronary artery disease. The potential for
epinephrine to produce these types of adverse reactions does not contraindicate its use in an acute life-threatening
allergic reaction.
Accidental injection into the digits, hands or feet may result in loss of blood flow to the affected area (see
WARNINGS). Adverse events experienced as a result of accidental injections may include increased heart rate, local
reactions including injection site pallor, coldness and hypoaesthesia or injury at the injection site resulting in bruising,
bleeding, discoloration, erythema or skeletal injury.
OVERDOSAGE: Epinephrine is rapidly inactivated in the body and treatment following overdose with epinephrine
is primarily supportive. If necessary, pressor effects may be counteracted by rapidly acting vasodilators or alpha-ad-
renergic blocking drugs. If prolonged hypotension follows such measure, it may be necessary to administer another
pressor drug.
Overdosage of epinephrine may produce extremely elevated arterial pressure, which may result in cerebrovascular
hemorrhage, particularly in elderly patients.
Overdosage may also result in pulmonary edema because of peripheral vascular constriction together with cardiac
stimulation. Treatment consists of a rapidly acting alpha-adrenergic blocking drug and/or respiratory support.
Epinephrine overdosage can also cause transient bradycardia followed by tachycardia and these may be
accompanied by potentially fatal cardiac arrhythmias. Premature ventricular contractions may appear within one
minute after injection and may be followed by multifocal ventricular tachycardia (prefibrillation rhythm). Subsidence of
the ventricular effects may be followed by atrial tachycardia and occasionally by atrioventricular block. Treatment of
arrhythmias consists of administration of a beta-blocking drug such as propranolol.
Overdosage sometimes results in extreme pallor and coldness of the skin, metabolic acidosis and kidney failure. Suitable
corrective measures must be taken in such situations.
HOW SUPPLIED: EpiPen® Auto-Injectors (epinephrine injections, USP, 1:1000, 0.3 mL) are available in individual
cartons, NDC 49502-500-01, and as EpiPen 2-Pak®
, NDC 49502-500-02, a pack that contains two EpiPen® Auto-Injectors
(epinephrine injections, USP, 1:1000, 0.3 mL) and one EpiPen® Auto-Injector trainer device.
EpiPen® Jr Auto-Injectors (epinephrine injection, USP, 1:2000, 0.3 mL) are available in individual cartons,
NDC 49502-501-01, and as EpiPen Jr 2-Pak®, NDC 49502-501-02, a pack that contains two EpiPen® Jr Auto-Injectors
(epinephrine injections, USP, 1:2000, 0.3 mL) and one EpiPen® Auto-Injector trainer device.
EpiPen 2-Pak® and EpiPen Jr 2-Pak® also includes an S-clip to clip two cases together.
Store at 25°C (77°F); excursions permitted to 15°C-30°C (59°F-86°F) (See USP Controlled Room Temperature).
Contains no latex. Protect from light.
Rx only.
MANUFACTURED FOR Dey, L.P.,
NAPA, CALIFORNIA, 94558, U.S.A.
by Meridian Medical Technologies, Inc.,
a subsidiary of King Pharmaceuticals®, Inc.,
Columbia, MD 21046, U.S.A.
EpiPen®, EpiPen® Jr, EpiPen 2-Pak®, and EpiPen Jr 2-Pak® are registered trademarks of Mylan, Inc,
licensed exclusively to its wholly-owned affiliate, Dey, L.P. of Napa, California, USA.
03-500-03 (BRS) March 2009
Counter Points
WWW.DRUGTOPICS.COM December 2010
DRUG TOPICS
13
Twenty-plus years and 200 columns
and I am still standing. Pharmacists
have asked for my help, told me their
stories, and lambasted me. So many
moments stand out in memory. Here
are just a few.
January 23, 1989. Publication date
of the fi rst “JP at Large.” My stomach
did fl ips when a woman who suffered
from bipolar disorder told me that she
was going to jump off the Deception Pass
Bridge. Life was just too tough. I just lis-
tened. That’s all she wanted. She prom-
ised to go back on her meds.
1996. A woman asked me how she
could help her mother kill herself. I was
silent. She mentioned
the 180 clonazepam
and 240 APAP/codeine
that had come from
a mail-order outfit.
“My Mom loves mar-
tinis,” she said, giving
me a look. The drugs
plus martinis could
be a deadly cocktail. I
watched the obituaries
for weeks after that.
1990s. I thought I was a jazzman for
a time. I took my troubles down to Madame
Ruth/You know that Gypsy with the gold-
capped tooth/She’s got a shop down at 34th
and Vine/Selling little bottles of/Love Potion
Number Nine.
If you get your juju on, you can see
how that song about Madame Ruth epit-
omizes what we do. Rite-Aid’s got a shop
near a Pennsylvania mine/it sells Viagra and/
cheap red Italian wine.
1998. A store manager accused me of
being unprofessional because I refused to
refi ll an Rx for his friend. I told him to get
a dictionary. I was on the professional train
for the long ride.
Mid-1990s. I did a stealth interview
with the produce manager at a local mar-
ket. I asked him if the OTC famotidine
was any good. Lettuce leaves started
fl ying all over the place. “How the hell
do I know?” He was red-faced from the
cooler. “Ask a pharmacist!”
It was my first justification for a BTC
class of drugs. A month earlier, famoti-
dine had been Rx Only
and too dangerous for
self-use. All of a sudden,
it could be sold at truck
stops. Pathetic oversight
from effete regulators.
Profit rules!
1990. An elderly
man who had been
watching me work
asked, “How long you
been a registered man?”
It was the old-fashioned distinction be-
tween a registered pharmacist and an ap-
prentice. We talked and he told me about
being paid in eggs, 2 dozen every week,
by a cash-poor farm family.
“The people were the best part,” he
said. “I went to weddings and funerals.
Pharmacists don’t seem to have time for
people these days.” 1990 was 2 decades
before the Prescription Mill Red Warning
Timers of 2010.
2004. I did everything I could to shame
a young mother because she had prob-
lems understanding how to dose her baby
with the Prednisolone 15 mg/5 ml syrup.
Then I realized that she did not know
how to read. After that I did everything
I could to help her, including sending her
to the adult reading program at the local
library. Months later, there were tears on
my cheeks when she proudly showed
me the fi rst book she had read by herself:
“Snuggle Piggy and the Magic Blanket.”
A retail pharmacist today
I often wrote about the absurdity of work-
ing in a modern drugstore. You know you
are a pharmacist when you are frequently
referred to as “Hey” and one of the most
common questions you are asked is “Hey,
where are the lawn chairs for $9.99?”
You know you are a pharmacist when
you get home at 10:30 p.m. and your
spouse says, “I fed the kids. You can make
your own dinner.” Your spouse likes your
money but isn’t willing to dance the dance.
Pharmacists often complain that the
profession is in the pits. I invite them to
focus more closely. It’s the job, stupid. The
profession is just fi ne.
JP AT LARGE
Jim Plagakis, RPh
Pharmacy then and now
In 1989, an RPh complained that she was expected to work off the clock after her shift
had ended. I told her that she should not put up with it. I told her that she was a phar-
macist, not a high school dropout stocking shelves. The day I work off the clock will be
the day your company starts paying for a catered deli lunch every day. You’re a professional. You
always have choices. You can be passive, or you can be smart. The company will always take
what it can get.
th d
Jim Plagakis is a community pharmacist
in Galveston, Texas. You can e-mail him
at jpgakis@hotmail.com and cc us at
drugtopics@advanstar.com. You can also
check out his website at jimplagakis.com.
“The people were
the best part,” he
said. “Pharmacists
don’t seem to have
time for people
these days.”

14
DRUG TOPICS
December 2010 WWW.DRUGTOPICS.COM
Up front
Up front
INDUSTRY NEWS & ANALYSIS
Patients with health insurance are abandoning their prescrip-
tions at higher rates than they did a year ago and far more
frequently than they did 5 years ago, according to a recent study
from healthcare data fi rm Wolters Kluwer Pharma Solutions,
Bridgewater, N.J.
From 2006 to 2010, patient abandonment rates at retail
pharmacies have soared 83.6%, according to the Wolters
Kluwers study.
• In the fi rst quarter of 2006, 5% of patients failed to pick
up their brand prescriptions and 2.9% failed to pick up their
generic prescriptions.
• In the fi rst quarter of 2010, 9.4% abandoned their brand
prescriptions and 5.1% abandoned their generics.
“It does seem to be tied to the economic climate right now,
but we will see if it begins to fl atten out or improve [when the
economy improves],” said Mark Spiers, CEO of Wolters Kluwer
Pharma Solutions.
As the United States entered recession in the third quarter
of 2008, rates of prescription abandonment began to rise. “We
really started to see a bump in 2009, which continued into 2010,”
Spiers said.
While prescription abandonment rates stayed the same for ge-
neric medications between the fi rst and second quarter of 2010,
the abandonment rates for brands rose from 9.4% to 9.6%.
Wolters Kluwer also tracks the abandonment rates by cat-
egory of medication and found that adrenergic blocking pre-
scriptions had the highest rate-of-abandonment increase from
2008 to 2009. In 2008, approximately 3% of patients abandoned
adrenergic blocking medications; in 2009, the rate had risen to
approximately 3.9%.
“We have also seen an increase in abandonment of mainte-
nance medications such as those for diabetes and hypertension.
Those are the areas that tend to become more concerning, be-
cause many of the drugs have demonstrated outcomes to prevent
heart attack, stroke, and other conditions that put a greater cost
on the healthcare system,” Spiers said.
Approximately 4.8% of patients abandoned anti-ulcerant
prescriptions in 2009, 4% abandoned diabetes prescriptions,
4.2% abandoned antidepressants, and approximately 3.7%
failed to pick up cholesterol reducers.
— Christine Blank, Contributing Editor
Website for providers, patients, focuses on chronic pain
NEW STUDY
More patients are abandoning prescriptions
Pharmacists working with chronic pain patients have a new
tool. PainSAFE (Pain Safety & Access for Everyone), an initiative
of the American Pain Foundation, has launched the website
www.painsafe.org to educate patients and practitioners about
pain-management therapies and their risks.
In Portland, Ore., at a Bi-Mart
Pharmacy that specializes in
helping patients with chronic pain,
pharmacy staff keeps files on such
patients and “watches for all the
things that go wrong, such as side
effects and errant behavior,” said
Kathy Hahn, PharmD, owner of
the pharmacy and Action Network
Leader of the American Pain
Foundation. “Some of our staff
have become advocates. It’s all
about increasing access and trying to figure how we can work on
the crisis of abusing pain medications,” Hahn said.
PainSAFE organizers agree that the pharmacist’s role
is vital. “Pharmacists play a key role in preventing misuse
and overuse of prescriptions. They are the last gate before
a patient receives their medication,” said Lynn Webster, MD,
FACPM, and an advisor to PainSAFE.
Pharmacists can help prevent misuse of pain medication
by monitoring initial drug doses. “Physicians often start patients
on too high of a dose or increase the dose too rapidly. The
pharmacist can see that and will hopefully contact the physician.
This could save thousands of lives every year,” Webster said.
Pharmacists can also help patients who switch or combine
medications. “Pharmacists need to make sure that patients
understand the higher risks of taking 2 together. There could be a
substantial reduction in deaths immediately,” Webster said.
At the website, simple tips include reminders to lock up
pain medications to prevent misuse by the young or the
elderly. “We have not been good at putting out the information
that pain medications should be locked up,” Hahn said.
— Christine Blank, Contributing Editor
Kathy Hahn
www.painsafe.org: A new tool for chronic pain patients and healthcare providers

GROWTHINNOVAT IONQUALITYAFFORDABILITYImpacting Tomorrow Right Now.
Growth
Expanding product lines
Innovation
Technically complex
products
Affordability
Accessible pharmaceuticals
for all
Quality
The highest standards
for safe and compliant
manufacturing
For more information, please visit www.roxane.com
or call 800.520.1631.
Right Now, Roxane Laboratories is – and always has been – focused on the
needs of our customers and their patients.
125 Years of Impacting
Tomorrow Right Now
years of innovation
16
DRUG TOPICS
December 2010 WWW.DRUGTOPICS.COM
Up front
In Depth
REMS UPDATE
Since the 1999 publication of the
Institute of Medicine (IOM) re-
port “To Err is Human: Build-
ing a Safer Health System,” both public
and private interests have made patient
safety a priority. Pharmacists can play a
critical role in patient safety by ensuring
that correct medications be dispensed
appropriately. But necessary patient
counseling, along with interaction with
physicians and payors, often goes un-
compensated. While recent FDA safety
policies give the agency more authority
to demand post-marketing accountabil-
ity from manufacturers, they present an
increasing burden for pharmacists.
Manufacturer risk reduction
Part of the 2007 FDA Amendment Act
(FDAAA) was the Risk Evaluation and
Mitigation Strategy (REMS) require-
ment for manufacturers, “to ensure
that the benefi ts of a drug or biological
product outweigh its risks.”
FDA now publicly lists more than 160
REMS-approved drugs, detailing for each
product the REMS components, which
may include a medication guide, a com-
munication plan, elements to assure safe
use, and/or an implementation system.
At the Third Annual Risk Manage-
ment and Drug Safety Summit, held
October 18 and 19 in Bethesda, Md.,
attendees and presenters discussed the
latest issues related to risk and safety,
and examined
how REMS and
drug safety have
been faring since
2007.
Annette Stem-
hagen, DrPH,
FISPE, senior vice
president of safety,
epidemiology, reg-
istries, and risk management for Unit-
ed BioSource Corporation, a Summit
sponsor, said that most REMS (70%)
are “a medication-guide-only REMS.”
Pharmacist fact-checking
“The pharmacist has the responsibil-
ity of dispensing the medication guide
along with the prescription, but there’s
a bigger responsibility for the pharma-
cist when there’s a REMS communica-
tion plan,” Stemhagen said, explaining
that when FDA requires a manufac-
turer to provide a com-
munication plan, the
pharmacist will have
to confi rm whether the
patient is eligible to re-
ceive a specifi c product
and whether certain
tests are needed before
the patient can obtain a
drug or drug refi ll, as with Accutane,
which requires a pregnancy test.
Risk management
Before REMS legislation, manufacturers
of Accutane and its generics operated
iPLEDGE, a program designed to re-
duce risk of fetal exposure. On October
22, 2010, FDA approved the iPLEDGE
program as an offi cial REMS. The pro-
gram has continued as before; the only
change was in its offi cial designation.
FDA representative Crystal Rice said
that pharmacists could help commu-
nicate with physicians who are new to
REMS and “help them complete the
needed processes for a particular prod-
uct,” but that pharmacists should not be
expected “to be the primary source of
information regarding the correct pre-
scribing choice for a physician’s patients.”
Summit keynote speaker Peter Pitts,
co-founder and president of the Cen-
ter for Medicine in the Public Interest
(CMPI) and a Summit sponsor, said
that “the REMS issue is part of the larg-
er conversation of postmarket safety.”
He added that he suspects pharmacists
are probably much more knowledgeable
about REMS than are physicians, whose
“awareness of REMS is slim to none.”
Education and technology
FDA is planning a number of projects
to help pharmacists, Rice said, including
a uniform REMS interface for prescrib-
ers, expanded pharmacy
access to REMS, CE for
pharmacists, and more.
“We are researching
the possibilities presented
by today’s technology for
using current or develop-
ing electronic systems to
implement the registra-
tion and prescribing requirements of
REMS,” Rice said.
FDA is also “very involved in ongo-
ing education for pharmacists and other
stakeholders,” Rice said, “to decrease
the burden of the REMS process.”
In her plenary remarks, Summit
presenter Janet Woodcock, MD, Direc-
tor of FDA’s Center for Drug Evaluation
and Research (CDER) said that “CDER
promotes and protects public health by
ensuring that safe and effective drugs
are available to Americans, but ‘safe’
does not mean risk-free, and ‘effective’
does not mean equally for all.”
In other words, Pitts said, the REMS
program “doesn’t tell physicians how to
practice medicine, and it gives the FDA
the route to keep higher-risk drugs on
the market.”
Barbara Hesselgrave is a freelance writer
based in Virginia.
BARBARA HESSELGRAVE
Safety summit: Risk management gets its third checkup
Annette Stemhagen
FDA now
publicly lists
more than 160
REMS-approved
drugs.




WWW.DRUGTOPICS.COM
December 2010
DRUG TOPICS
21
Professional
COUNTERFEIT DRUGS
Gren spoke in October in Washing-
ton, D.C., at Interchange 2010, a con-
ference sponsored by the Partnership
for Safe Medicines, a coalition of indi-
viduals and groups working to protect
consumers from counterfeit or contra-
band medications.
Along with counterfeit medications,
substandard medications are also al-
ways on the rise. These are defi ned as
products made primarily by manufac-
turers who do not comply with regu-
latory standards or U.S. Pharmacopeia
requirements.
Although counterfeit drugs can
contain anything from powdered dry-
wall to highway paint, Gren said, he
believes more counterfeit drugs include
some actual ingredients, making them
effective enough to bring the counter-
feiters repeat business. He called this
one of the most frightening potentials.
Although the counterfeit drug issue
has received more government funding
and attention in recent years, it has not
received nearly enough, Gren told the
meeting, which included the group’s
members, pharmaceutical profession-
als, government offi cials, and others.
Foreign drugs
According to FDA, Gren said, 80% of
active pharmaceutical ingredients (API)
in drugs consumed in the United States
come from other countries, mostly from
nations that don’t have sophisticated reg-
ulatory regimes. An API, he said, “may
come from China, it may be packaged in
the Middle East, it may be sent through
Brazil; ultimately it makes it to the United
States or other parts of the world,” mak-
ing the problem diffi cult to solve.
Diplomatic efforts to address the issue
include dialogues with countries such
as China, India, and Brazil, and include
Department of Commerce initiatives
originated by the U.S.-China Joint Com-
mission on Commerce and Trade. Also,
a series of seminars and other projects
continues under the aegis of Asia-Pacifi c
Economic Cooperation, a forum for the
Pacifi c Rim nations, Gren said.
A growing problem
Another speaker at the Interchange meet-
ing, Nancy Kennedy, senior operations
manager of drug investigations in FDA’s
Offi ce of Criminal Investigations, said that
some years ago imported, unapproved,
and counterfeit drugs came into the Unit-
ed States addressed to individuals or pack-
aged in small quantities. Then quantities
increased, and counterfeits started going
to drop-shippers and distributors working
from online pharmacy operations.
“Now we see quantities of these drugs
coming in and going directly to doctors,
clinics, brick-and-mortar-pharmacies—
and these are for use and distribution
directly to patients,” Kennedy said.
A question of resources
Asked at the meeting about resources
allocated to the counterfeit drug prob-
lem, Kennedy said that for some time
Congress and others have been saying,
“Show us the bodies.” But there may
not be many bodies at one time, she
said, unless an event occurs such as the
mass poisonings that have happened in
other countries.
Currently, she added, counterfeit
drugs are more likely either to cause
injuries or to cause patients to receive
inappropriate doses of prescribed drugs
without their realizing it.
Targeted enforcement
In another session at the partnership’s
forum, FDA Commissioner Margaret
Hamburg said that her agency has now
ranked more than 1,000 active pharma-
ceutical ingredients according to their
vulnerability to economically motivated
adulteration, so that enforcement can be
better targeted.
To jump-start efforts to deal with sup-
ply-chain threats that include counterfeit-
ing, economically motivated adulteration,
diversion, and cargo thefts, Hamburg said,
the agency is creating a new Drug Integ-
rity and Security Program within the Of-
fi ce of Compliance of the FDA’s Center for
Drug Evaluation and Research.
Hamburg also said that FDA is devel-
oping standards for track-and-trace and
authentication systems that would enable
the identifi cation of substandard prescrip-
tion drugs as they move along the supply
chain. The agency will help in efforts to
recall these drugs.
However, asked when the require-
ment that drugs carry a pedigree will
be enforced, as mandated by the 1987
Prescription Drug Marketing Act, Ham-
burg said she could not give a specifi c
answer. That requirement, calling for
wholesale distributors (or others not
defi ned as “authorized distributors”) to
provide a statement of each prior sale,
trade, or purchase of a prescription drug,
has been held up by regulatory and legal
wrangling for 2 decades.
CHAINS & BUSINESS
Kathryn Foxhall
The counterfeiters are winning the global counterfeit drug war, with counterfeit medi-
cations more abundant than ever before, according to Jeffrey Gren, director of the
Offi ce of Health and Consumer Goods in the U.S. Department of Commerce.
Kathryn Foxhall is a healthcare journalist
based in the Washington, D.C., area.
Counterfeit drug war continues,
threatens supply chain
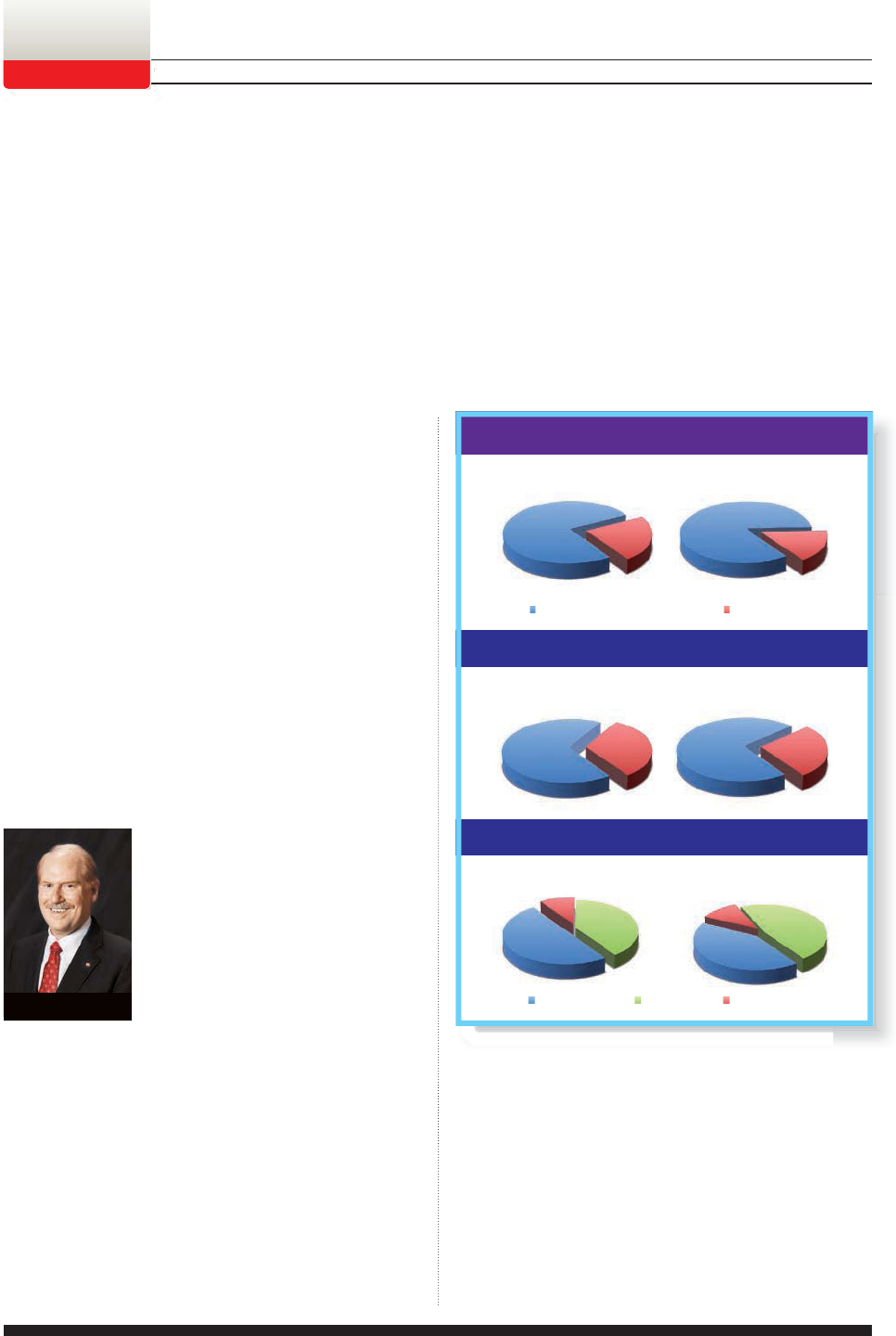
WWW.DRUGTOPICS.COM
22
DRUG TOPICS
December 2010
While the economy in many industries has yet to re-
bound, the pharmacy industry has remained strong
and most pharmacists are hopeful about 2011. How-
ever, optimism isn’t running as high as it was last year, and the
pharmacy community is concerned about how competition,
reimbursement, and healthcare reform will affect the industry.
These are just a few of the conclusions drawn from Drug
Topics’ annual business outlook survey, an online survey con-
ducted in October that received more than 400 responses from
community, hospital, and long-term-care (LTC) pharmacists.
Overall, respondents believe that the business climate
looks positive for 2011. Of the 305 community pharmacists
who responded to the survey, 77% believe that 2010 will
be an excellent, very good, or good business year, and 68%
predict that the trend will continue in 2011.
In addition, of the 96 hospital and LTC pharmacists who
responded, 84% anticipate that 2010 will be an excellent,
very good, or good business year, and 73% believe that will
also be the case in 2011.
“I think there are a lot of very posi-
tive things going on in pharmacy, and
so I think [pharmacists’] optimism is
warranted, but it isn’t going to get any
easier,” said Thomas Menighan, executive
vice president and chief executive offi cer
of the American Pharmacists Associa-
tion (APhA). “Margins are not going to
get larger. People are going to have to be
more effi cient. They are going to have to
fi nd ways to make the services that pharmacists can provide
pay off rather than relying on buy low, sell high.”
Community pharmacists’ fi nancial outlook
Pharmacists’ fi nancial expectations for 2011 are generally
positive, but they aren’t without their concerns.
Nearly half (45%) of community pharmacists surveyed
believe that sales will increase in 2011. For those who antici-
pate rising sales fi gures, the average increase expected is 4.5%.
However, not everyone sees predicted sales in such a
positive light. Survey fi ndings indicate that 11% believe
sales will decrease, and they predict an average decrease
in 2011 of 2.3%.
In addition, 56% anticipate an increase to operating ex-
penses, while 6% expect a decrease. For those who believe
there will be an increase, the average expected increase is
4.1%. For those who anticipate a decrease, the average ex-
pected decrease is 1.2%.
About a third, or 35%, expect net profi ts to increase in
2011 by an average of 2.6%.
Half of community pharmacists believe that in their
pharmacies, pharmacist salaries will increase in 2011, while
40% do not expect an increase. For the 50% who expect an
increase, the average expected increase is 3.6%.
Steady sailing
for now
Drug Topics’ 2011 business outlook survey
Cover Story
Jill Sederstrom
2010: Overall Business View
84%
16%
77% 23%
68% 32% 73% 27%
2011: Overall Business View
Community Pharmacists Hospital/LTC Pharmacists
Community Pharmacists Hospital/LTC Pharmacists
Community Pharmacists Hospital/LTC Pharmacists
C
ommunity Pharmacist
s
50%
40%
10%
2011: Salary Expectations
H
os
pi
ta
l/
LT
C
P
h
armac
i
sts
42%
47%
11%
Positive View Negative View
Salary Increase No Increase
Undecided
Half of community pharmacists expect salaries to increase next year. More than 40%
of hospital and LTC pharmacists believe their salaries will climb in 2011.
Thomas Menighan

WWW.DRUGTOPICS.COM December 2010
DRUG TOPICS
23
Cover Story
BUSINESS OUTLOOK SURVEY
Hospital pharmacists’ fi nancial outlook
Hospital and LTC pharmacists are less optimistic about sales
expectations than are their counterparts in community
pharmacy. According to the results, just 30% of hospital
pharmacists expect an increase in sales in the coming year;
the average increase they anticipate is 3.9%. However, only
6% predict a decrease in sales. For those who expected a
decrease, the average expected decrease was 2.1%.
Most hospital pharmacists surveyed (51%) expect op-
erating expenses to increase in the new year; they predict
an average increase of 5.4%. On the other side of the spec-
trum, 6% of those surveyed believe operating expenses will
decrease and expect an average decrease in 2011 of 0.6%.
Nearly a quarter, or 23%, of hospital pharmacists expect
net profi ts to increase in 2011 by an average increase of 2.5%.
A signifi cant percentage of hospital pharmacists do not be-
lieve a raise is in their future in 2011. According to the survey
results, 47% do not expect a salary increase. However, 42%
do believe that their pharmacies will be giving pharmacists a
raise in 2011. The average increase anticipated is 3%.
Anna Garrett, PharmD, BCPS, man-
ager of outpatient clinical pharmacy
services for Mission Hospital in Ashe-
ville, N.C., believes that if pharmacists
are expecting salary increases now,
those increases will be more in line
with cost-of-living adjustments, rather
than being signifi cant jumps in pay.
“It looks like the days when ev-
erybody is getting the huge raises are
over,” said Garrett, who is joining Drug Topics’ editorial ad-
visory board next month.
Challenges and opportunities
In any given year, the pharmacy industry will face chal-
lenges and opportunities, and 2011 is no exception. In one
section of the survey, pharmacists identifi ed the top 3 posi-
tive and top 3 negative factors that they believe will infl u-
ence the industry in the coming year.
The most frequently cited positive factors expected to
affect business are major brand-name drugs going off patent
(cited by 62% of respondents), an increase of e-prescriptions
(cited by 37% of respondents), and immunization certifi ca-
tion (cited by 36% of respondents).
The most frequently mentioned negative factors affecting
business for 2011 are expected to be the recession (cited by
77% of respondents), low reimbursement from third parties
(cited by 66% of respondents), and mail-order programs
(cited by 63% of respondents).
Charlie Mollien, PharmD, a staff
pharmacist with Meijer Pharmacy, Jen-
ison, Mich., and a Drug Topics Frontline
editorial advisory board member, said
that he is optimistic about the future
of pharmacy, although he does see
some hurdles. While he believes that
pharmacy utilization will continue to
increase, he added that low reimburse-
ment could translate into pharmacists
fi lling more prescriptions for less profi t.
To combat the problem, and to make up profi ts, Mollien
said, pharmacists may need to look into other areas, such
as adopting education programs for smoking cessation or
chronic disease management. For the programs to be suc-
cessful, however, pharmacists would need to be given the
time, staff, and leadership support to run them.
The biggest challenges pharmacists will face in 2011 are
said to be competition from chains offering generics at low
or no cost (cited by 51% of respondents), competition from
mail-order pharmacies (cited by 59%), and state Medicaid
rates and Maximum Allowable Cost (MAC) and Federal
Upper Limit (FUL) programs (cited by 53% of respondents).
To get ahead in the industry, Menighan believes, phar-
macists will need to think creatively and reach out to the
medical community to discuss new changes to healthcare.
“There are opportunities to partner with medicine in col-
laborative ways that are going to benefi t everybody,” he said.
One area that is not expected to be a challenge this year is
the number of pharmacists in the market. While last year more
than half of respondents believed that their states had a short-
age of pharmacists, this year 74% of community pharmacists
and 64% of hospital and LTC pharmacists said that they don’t
believe there is a pharmacist shortage in their states.
Cristina Medina, PharmD, manager
of professional and college relations for
CVS Caremark and a new member of
Drug Topics’ editorial advisory board, said
that although the demand for pharma-
cists varies based on geographic region,
most of the markets are now well staffed.
Medina attributes this change to an
increase in pharmacy programs in the
country and to the struggling economy.
The additional competition in the job market has not
been lost on pharmacy students. Most students realize now
that they may have to spend more time looking for a job,
although Medina doesn’t believe that
the industry has become too diluted.
Anna Garrett
Charlie Mollien
Cristina Medina
Continued on pg. 24

WWW.DRUGTOPICS.COM
24
DRUG TOPICS
December 2010
Cover Story
BUSINESS OUTLOOK SURVEY
“I think there are defi nitely positions. I just think that
in the major metropolitan areas students have to drive out
further or go into different markets, but I think that supply
is right with demand,” she said.
Grading the associations
Pharmacy associations were established to serve the best
interests of pharmacists, but how well they perform can be
a matter of opinion. On the basis of responses to the Drug
Topics survey, it appears that more than 40% of community,
hospital, and LTC pharmacists are satisfi ed with the repre-
sentation they’ve received.
According to the results, 43% of community pharmacists
said they were satisfi ed with their pharmacy associations this
year, while 27% were not and 30% did not know. Similarly,
42% of hospital and LTC pharmacists are satisfi ed with the
way their associations have represented them, while 22%
were not and 37% were not sure.
Mollien, who serves on both the Michigan Pharmacists
Association and the APhA board, believes that associations
can advocate for change that individual pharmacists may
not have the time or power to effect on their own.
“One voice can be lost in a crowd,” he said. “An associa-
tion voice is more likely to be heard.”
Edith Rosato, senior vice president
of pharmacy affairs for the National
Association of Chain Drug Stores
(NACDS) and president of the NACDS
Foundation, credited pharmacy asso-
ciations with ushering in new industry
changes, including the power phar-
macists now have to administer im-
munizations in all 50 states, but, she
said, not all pharmacists are suffi ciently
aware of the accomplishments achieved by associations.
“It’s a fact that a lot of pharmacists out there really don’t
know who their associations are,” she said.
Government programs
Medicare Part D received mixed reviews from community,
hospital, and LTC pharmacists, although community phar-
macists seemed to be more supportive overall of the pro-
gram. According to the survey results, 43% of community
pharmacists said that they believe Medicare Part D has had
a positive impact on their pharmacy, while 23% believe it
has had a negative impact. On the other hand, only 10%
of hospital and LTC pharmacists cited a positive impact on
their pharmacies, while 19% cited a negative impact, and
71% did not know.
Robert J. Greenwood, RPh, is an
independent pharmacy owner in Water-
loo, Iowa, and president of the National
Community Pharmacists Association
(NCPA). When asked about Medicare
Part D, he said that although prescrip-
tion drug plans often offer recipients an
economic incentive to use mail-order
pharmacies, which can present its own
set of challenges, overall he sees the
Medicare Part D program as a positive aspect of business.
“The Medicare D program levels the playing fi eld, [and]
price is not part of the equation anymore,” he said.
Pharmacists also had confl icting feelings about MTM ser-
vices under Medicare Part D.
Drug Topics found that 38% of community pharmacists
reported providing MTM services under Medicare Part D in
2010 and 26% of those who responded to the survey were
paid an average of $1,536.90 for these services.
Furthermore, 17% of hospital and LTC pharmacists
provided MTM services under Medicare Part D, and 6% of
those survey respondents received an average of $1,791.70.
Garrett, who has had success with MTM services, said
that she is able to provide additional counseling to Medi-
care surgical patients during preoperative visits for elective
surgery. The hospital environment gives her more time to
interact with patients and identify possible issues they may
have before surgery.
One reason that MTM services have not been fully em-
braced by pharmacists may be the program’s slow begin-
ning, according to Rosato. She said that when the program
began in 2003, many Medicare participants weren’t eligible
for it. Now, eligibility has increased and, Rosato believes,
MTM services will gain momentum — possibly even be-
yond Medicare.
“One of the things that we’re looking at as a profes-
sion is why not provide MTM services to patients who have
chronic diseases?” she said.
While it’s still unclear what effect the new Affordable
Care Act will have on the industry, Greenwood said, he
thinks the act will give pharmacists greater opportunities to
demonstrate their value in the healthcare arena.
“Patients are going to be seen in a more ambulatory way
than currently, and I see pharmacists being an extension
of primary care delivery from the physician,” he said.
Jill Sederstrom is a freelance journalist based in Overland Park,
Kansas.
Edith Rosato
Robert Greenwood
Continued from pg. 23

For additional information,
visit www.PRADAXAPRO.com
or call 1-866-395-1764.
PRADAXA® is a registered trademark of Boehringer Ingelheim Pharma GmbH & Co. KG and used under license.
COPYRIGHT © 2010 BOEHRINGER INGELHEIM PHARMACEUTICALS, INC. OR ONE OR MORE AFFILIATED COMPANIES. ALL RIGHTS RESERVED. [11/10] PX78720PROF
NOW APPROVED
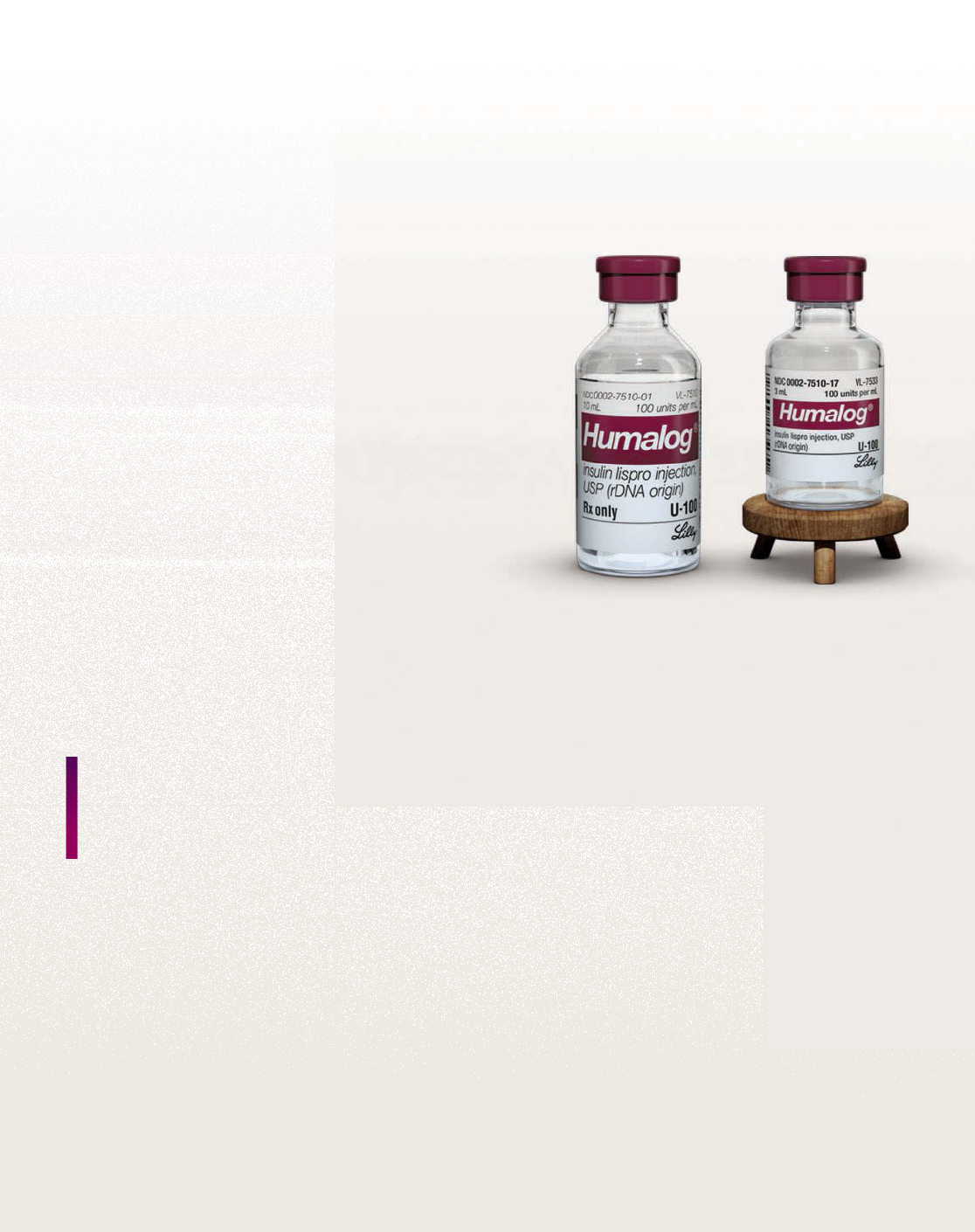
As part of Eli Lilly and Company’s ongoing commitment,
we provide healthcare facilities with a choice of vial sizes.
Humalog® (insulin lispro injection [rDNA origin]),
Humulin® R U-100 (regular insulin human injection, USP
[rDNA origin]), and Humulin® N (NPH human insulin [rDNA
origin] isophane suspension) are available in a smaller vial size.*
The smaller vials are designed to give healthcare facilities
fl exibility when evaluating insulin storage and distribution
(fl oor stock vs individual patient supply), in addition to the
10 mL vial and Humalog® KwikPen™.
• Humalog NDC Number - 0002-7510-17
• Humulin R U-100 NDC Number - 0002-8215-17
• Humulin N NDC Number - 0002-8315-17
* Smaller vials contain 3 mL of insulin in a 5 mL vial.
Humalog Indication
Humalog (insulin lispro injection [rDNA origin]) is for
use in patients with diabetes mellitus for the control of
hyperglycemia. Humalog should be used with longer-
acting insulin, except when used in combination with
sulfonylureas in patients with type 2 diabetes.
Humalog Important Safety Information
Contraindications
Humalog is contraindicated during episodes of hypoglycemia
and in patients sensitive to Humalog or one of its excipients.
Warnings
Humalog differs from regular human insulin by its rapid onset
of action as well as a shorter duration of action. Therefore,
when used as a mealtime insulin, Humalog should be given
within 15 minutes before or immediately after a meal.
Due to the short duration of action of Humalog, patients
with type 1 diabetes also require a longer-acting insulin
to maintain glucose control (except when using an
insulin pump).
Glucose monitoring is recommended for all patients
with diabetes.
The safety and effectiveness of Humalog in patients less
than 3 years of age have not been established. There are
no adequate and well-controlled clinical studies of the use
of Humalog in pregnant or nursing women.
Step up to a range of
insulin delivery options.
HI66337 0810 PRINTED IN USA ©2010, LILLY USA, LLC. ALL RIGHTS RESERVED.
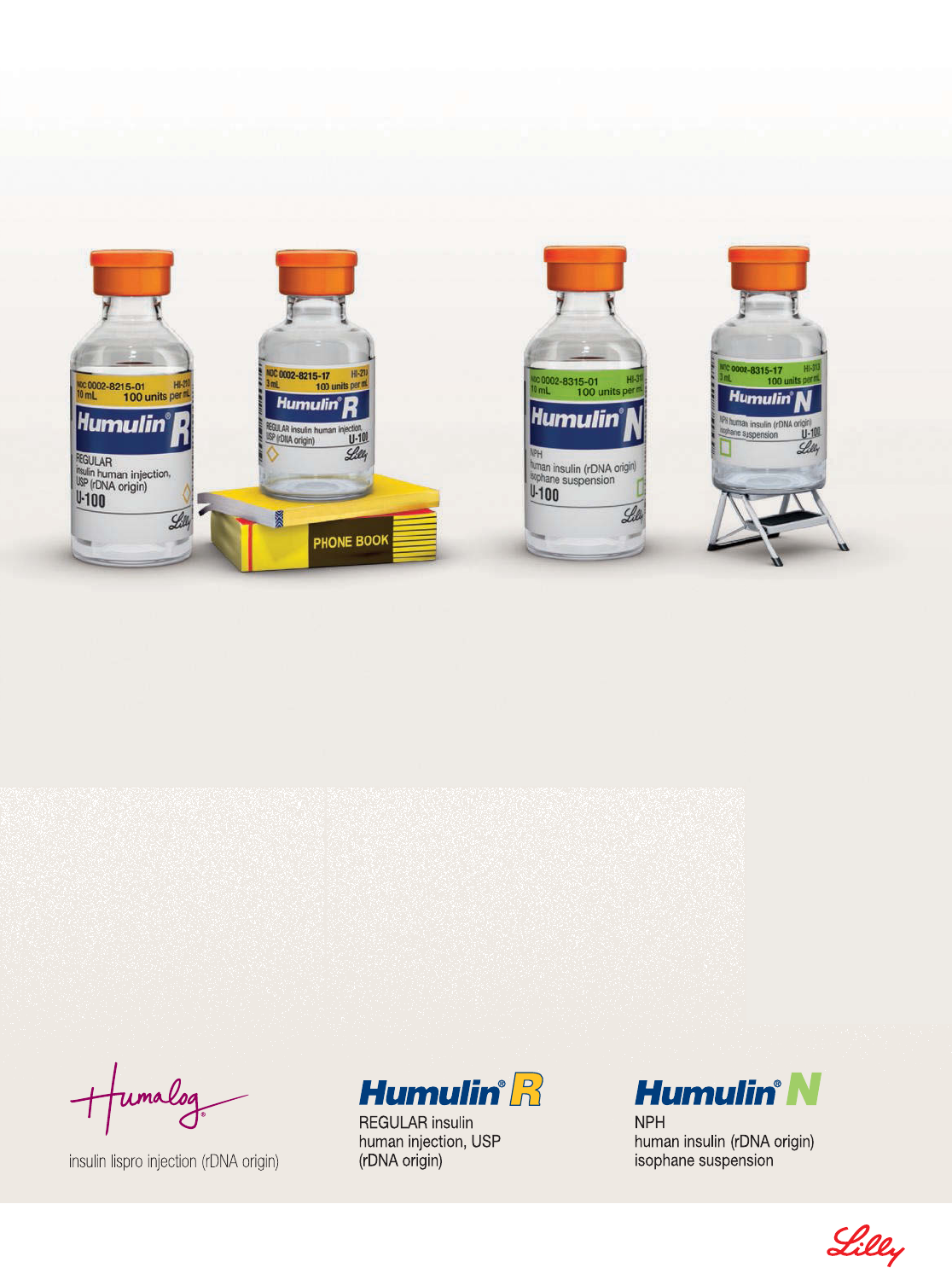
Humalog Important Safety Information, continued
Warnings, continued
Starting or changing insulin therapy should be done
cautiously and only under medical supervision.
Hypoglycemia
Hypoglycemia is the most common adverse effect associated
with insulins, including Humalog. Hypoglycemia can happen
suddenly, and symptoms may be different for each person
and may change from time to time. Severe hypoglycemia
can cause seizures and may be life-threatening.
Other Side Effects
Other potential side effects associated with the use of
insulins include: hypokalemia, weight gain, lipodystrophy,
and hypersensitivity. Systemic allergy is less common,
but may be life-threatening. Because of the difference
Humalog Important Safety Information, continued
Other Side Effects, continued
in action of Humalog, care should be taken in patients
in whom hypoglycemia or hypokalemia may be clinically
relevant (eg, those who are fasting, have autonomic
neuropathy or renal impairment, are using potassium-
lowering drugs, or taking drugs sensitive to serum
potassium level).
Please see reverse side for Brief Summary of full
Prescribing Information.
Please see full user manual that accompanies the pen.
Humalog® and Humalog® KwikPen™ are registered trademarks of Eli Lilly
and Company and are available by prescription only.
Humulin® is a registered trademark of Eli Lilly and Company.
HUMALOG®
INSULIN LISPRO INJECTION (rDNA ORIGIN)
BRIEF SUMMARY: Consult package insert for complete prescribing information.
INDICATIONS AND USAGE: Humalog is an insulin analog that is indicated in the treatment of patients with
diabetes mellitus for the control of hyperglycemia. Humalog has a more rapid onset and a shorter duration of
action than regular human insulin. Therefore, in patients with type 1 diabetes, Humalog should be used in
regimens that include a longer-acting insulin. However, in patients with type 2 diabetes, Humalog may be used
without a longer-acting insulin when used in combination therapy with sulfonylurea agents.
Humalog may be used in an external insulin pump, but should not be diluted or mixed with any other
insulin when used in the pump. Humalog administration in insulin pumps has not been studied in patients
with type 2 diabetes.
CONTRAINDICATIONS: Humalog is contraindicated during episodes of hypoglycemia and in patients sensitive to
Humalog or any of its excipients.
WARNINGS: This human insulin analog differs from regular human insulin by its rapid onset of action as well
as a shorter duration of activity. When used as a mealtime insulin, the dose of Humalog should be given
within 15 minutes before or immediately after the meal. Because of the short duration of action of Humalog,
patients with type 1 diabetes also require a longer-acting insulin to maintain glucose control (except when
using an external insulin pump).
External Insulin Pumps: When used in an external insulin pump, Humalog should not be diluted or mixed
with any other insulin. Patients should carefully read and follow the external insulin pump manufacturer’s
instructions and the “PATIENT INFORMATION” leaflet before using Humalog.
Physicians should carefully evaluate information on external insulin pump use in the Humalog physician
package insert and in the external insulin pump manufacturer’s instructions. If unexplained hyperglycemia or
ketosis occurs during external insulin pump use, prompt identification and correction of the cause is necessary.
The patient may require interim therapy with subcutaneous insulin injections (see PRECAUTIONS, For Patients
Using External Insulin Pumps, and DOSAGE AND ADMINISTRATION).
Hypoglycemia is the most common adverse effect associated with the use of insulins, including Humalog.
As with all insulins, the timing of hypoglycemia may differ among various insulin formulations. Glucose
monitoring is recommended for all patients with diabetes and is particularly important for patients using an
external insulin pump.
Any change of insulin should be made cautiously and only under medical supervision. Changes in insulin
strength, manufacturer, type (eg, regular, NPH, analog), species, or method of manufacture may result in the
need for a change in dosage.
PRECAUTIONS: General—Hypoglycemia and hypokalemia are among the potential clinical adverse effects
associated with the use of all insulins. Because of differences in the action of Humalog and other insulins, care
should be taken in patients in whom such potential side effects might be clinically relevant (eg, patients who are
fasting, have autonomic neuropathy, or are using potassium-lowering drugs or patients taking drugs sensitive to
serum potassium level). Lipodystrophy and hypersensitivity are among other potential clinical adverse effects
associated with the use of all insulins.
As with all insulin preparations, the time course of Humalog action may vary in different individuals or at
different times in the same individual and is dependent on site of injection, blood supply, temperature, and
physical activity.
Adjustment of dosage of any insulin may be necessary if patients change their physical activity or their usual
meal plan. Insulin requirements may be altered during illness, emotional disturbances, or other stress.
Hypoglycemia—As with all insulin preparations, hypoglycemic reactions may be associated with the
administration of Humalog. Rapid changes in serum glucose concentrations may induce symptoms of
hypoglycemia in persons with diabetes, regardless of the glucose value. Early warning symptoms of
hypoglycemia may be different or less pronounced under certain conditions, such as long duration of diabetes,
diabetic nerve disease, use of medications such as beta-blockers, or intensified diabetes control.
Renal Impairment—The requirements for insulin may be reduced in patients with renal impairment.
Hepatic Impairment—Although impaired hepatic function does not affect the absorption or disposition of
Humalog, careful glucose monitoring and dose adjustments of insulin, including Humalog, may be necessary.
Allergy—Local Allergy—As with any insulin therapy, patients may experience redness, swelling, or itching
at the site of injection. These minor reactions usually resolve in a few days to a few weeks. In some instances,
these reactions may be related to factors other than insulin, such as irritants in a skin cleansing agent or poor
injection technique.
Systemic Allergy—Less common, but potentially more serious, is generalized allergy to insulin, which may
cause rash (including pruritus) over the whole body, shortness of breath, wheezing, reduction in blood pressure,
rapid pulse, or sweating. Severe cases of generalized allergy, including anaphylactic reaction, may be life-
threatening. Localized reactions and generalized myalgias have been reported with the use of cresol as an
injectable excipient. In Humalog-controlled clinical trials, pruritus (with or without rash) was seen in 17 patients
receiving Humulin R® (N=2969) and 30 patients receiving Humalog (N=2944) (P=.053).
Antibody Production—In large clinical trials, antibodies that cross-react with human insulin and insulin lispro
were observed in both Humulin R- and Humalog-treatment groups. As expected, the largest increase in the
antibody levels during the 12-month clinical trials was observed with patients new to insulin therapy.
Usage of Humalog in External Insulin Pumps—The infusion set (reservoir syringe, tubing, and catheter),
Disetronic® D-TRON®2,3 or D-TRONplus®2,3 cartridge adapter, and Humalog in the external insulin pump
reservoir should be replaced and a new infusion site selected every 48 hours or less. Humalog in the external
insulin pump should not be exposed to temperatures above 37°C (98.6°F).
In the D-TRON®2,3 or D-TRONplus®2,3 pump, Humalog 3 mL cartridges may be used for up to 7 days. However,
as with other external insulin pumps, the infusion set should be replaced and a new infusion site should be
selected every 48 hours or less.
When used in an external insulin pump, Humalog should not be diluted or mixed with any other insulin (see
INDICATIONS AND USAGE, WARNINGS, PRECAUTIONS, For Patients Using External Insulin Pumps, Mixing of
Insulins, DOSAGE AND ADMINISTRATION, and Storage).
Information for Patients—Patients should be informed of the potential risks and advantages of Humalog and
alternative therapies. Patients should also be informed about the importance of proper insulin storage, injection
technique, timing of dosage, adherence to meal planning, regular physical activity, regular blood glucose
monitoring, periodic hemoglobin A1C testing, recognition and management of hypoglycemia and hyperglycemia,
and periodic assessment for diabetes complications.
Patients should be advised to inform their physician if they are pregnant or intend to become pregnant.
Refer patients to the “PATIENT INFORMATION” leaflet for timing of Humalog dosing (<_15 minutes before or
immediately after a meal), storing insulin, and common adverse effects.
For Patients Using Insulin Pen Delivery Devices: Before starting therapy, patients should read the “PATIENT
INFORMATION” leaflet that accompanies the drug product and the User Manual that accompanies the delivery
device. They should also reread these materials each time the prescription is renewed. Patients should be
instructed on how to properly use the delivery device, prime the Pen to a stream of insulin, and properly dispose
of needles. Patients should be advised not to share their Pens with others.
For Patients Using External Insulin Pumps: Patients using an external infusion pump should be trained in
intensive insulin therapy and in the function of their external insulin pump and pump accessories. Humalog was
tested in the MiniMed®1 Models 506, 507, and 508 insulin pumps using MiniMed®1 Polyfin®1 infusion sets.
Humalog was also tested in the Disetronic®2 H-TRONplus® V100 insulin pump (with plastic 3.15 mL insulin
reservoir), and the Disetronic D-TRON®2,3 and D-TRONplus®2,3 insulin pumps (with Humalog 3 mL cartridges)
using Disetronic Rapid®2 infusion sets.
The infusion set (reservoir syringe, tubing, catheter), D-TRON®2,3 or D-TRONplus®2,3 cartridge adapter,
and Humalog in the external insulin pump reservoir should be replaced, and a new infusion site selected
every 48 hours or less. Humalog in the external pump should not be exposed to temperatures above
37°C (98.6°F).
A Humalog 3 mL cartridge used in the D-TRON®2,3 or D-TRONplus®2,3 pump should be discarded after 7 days,
even if it still contains Humalog. Infusion sites that are erythematous, pruritic, or thickened should be reported to
medical personnel, and a new site selected.
Humalog should not be diluted or mixed with any other insulin when used in an external insulin pump.
Laboratory Tests—As with all insulins, the therapeutic response to Humalog should be monitored by periodic
blood glucose tests. Periodic measurement of hemoglobin A1C is recommended for the monitoring of long-term
glycemic control.
Drug Interactions—Insulin requirements may be increased by medications with hyperglycemic activity, such
as corticosteroids, isoniazid, certain lipid-lowering drugs (eg, niacin), estrogens, oral contraceptives,
phenothiazines, and thyroid replacement therapy (see CLINICAL PHARMACOLOGY).
Insulin requirements may be decreased in the presence of drugs that increase insulin sensitivity or have
hypoglycemic activity, such as oral antidiabetic agents, salicylates, sulfa antibiotics, certain antidepressants
(monoamine oxidase inhibitors), angiotensin-converting-enzyme inhibitors, angiotensin II receptor blocking
agents, beta-adrenergic blockers, inhibitors of pancreatic function (eg, octreotide), and alcohol. Beta-adrenergic
blockers may mask the symptoms of hypoglycemia in some patients.
Mixing of Insulins—Care should be taken when mixing all insulins as a change in peak action may occur.
The American Diabetes Association warns in its Position Statement on Insulin Administration, “On mixing,
physiochemical changes in the mixture may occur (either immediately or over time). As a result, the physiological
response to the insulin mixture may differ from that of the injection of the insulins separately.” Mixing Humalog
with Humulin® N or Humulin® U does not decrease the absorption rate or the total bioavailability of Humalog.
Given alone or mixed with Humulin N, Humalog results in a more rapid absorption and glucose-lowering effect
compared with regular human insulin.
Pregnancy—Teratogenic Effects—Pregnancy Category B—Reproduction studies with insulin lispro have
been performed in pregnant rats and rabbits at parenteral doses up to 4 and 0.3 times, respectively, the average
human dose (40 units/day) based on body surface area. The results have revealed no evidence of impaired
fertility or harm to the fetus due to Humalog. There are, however, no adequate and well-controlled studies with
Humalog in pregnant women. Because animal reproduction studies are not always predictive of human response,
this drug should be used during pregnancy only if clearly needed.
Although there are limited clinical studies of the use of Humalog in pregnancy, published studies with human
insulins suggest that optimizing overall glycemic control, including postprandial control, before conception and
during pregnancy improves fetal outcome. Although the fetal complications of maternal hyperglycemia have been
well documented, fetal toxicity also has been reported with maternal hypoglycemia. Insulin requirements usually
fall during the first trimester and increase during the second and third trimesters. Careful monitoring of the
patient is required throughout pregnancy. During the perinatal period, careful monitoring of infants born to
mothers with diabetes is warranted.
Nursing Mothers—It is unknown whether Humalog is excreted in significant amounts in human milk. Many
drugs, including human insulin, are excreted in human milk. For this reason, caution should be exercised when
Humalog is administered to a nursing woman. Patients with diabetes who are lactating may require adjustments
in Humalog dose, meal plan, or both.
Pediatric Use—In a 9-month, crossover study of prepubescent children (n= 60), aged 3 to 11 years,
comparable glycemic control as measured by A1C was achieved regardless of treatment group: regular human
insulin 30 minutes before meals 8.4%, Humalog immediately before meals 8.4%, and Humalog immediately
after meals 8.5%. In an 8-month, crossover study of adolescents (n=463), aged 9 to 19 years, comparable
glycemic control as measured by A1C was achieved regardless of treatment group: regular human insulin 30 to
45 minutes before meals 8.7% and Humalog immediately before meals 8.7%. The incidence of hypoglycemia
was similar for all 3 treatment regimens. Adjustment of basal insulin may be required. To improve accuracy in
dosing in pediatric patients, a diluent may be used. If the diluent is added directly to the Humalog vial, the shelf
life may be reduced (see DOSAGE AND ADMINISTRATION).
Geriatric Use—Of the total number of subjects (n=2834) in 8 clinical studies of Humalog, 12% (n=338) were
65 years of age or over. The majority of these were patients with type 2 diabetes. A1C values and hypoglycemia
rates did not differ by age. Pharmacokinetic/pharmacodynamic studies to assess the effect of age on the onset
of Humalog action have not been performed.
ADVERSE REACTIONS: Clinical studies comparing Humalog with regular human insulin did not demonstrate a
difference in frequency of adverse events between the 2 treatments.
Adverse events commonly associated with human insulin therapy include the following:
Body as a Whole—allergic reactions (see PRECAUTIONS).
Skin and Appendages—injection site reaction, lipodystrophy, pruritus, rash.
Other—hypoglycemia (see WARNINGS and PRECAUTIONS).
OVERDOSAGE: Hypoglycemia may occur as a result of an excess of insulin relative to food intake, energy
expenditure, or both. Mild episodes of hypoglycemia usually can be treated with oral glucose. Adjustments in
drug dosage, meal patterns, or exercise may be needed. More severe episodes with coma, seizure, or neurologic
impairment may be treated with intramuscular/subcutaneous glucagon or concentrated intravenous glucose.
Sustained carbohydrate intake and observation may be necessary because hypoglycemia may recur after
apparent clinical recovery.
DOSAGE AND ADMINISTRATION: Humalog is intended for subcutaneous administration, including use in select
external insulin pumps (see DOSAGE AND ADMINISTRATION, External Insulin Pumps). Dosage regimens of
Humalog will vary among patients and should be determined by the healthcare provider familiar with the
patient’s metabolic needs, eating habits, and other lifestyle variables. Pharmacokinetic and pharmacodynamic
studies showed Humalog to be equipotent to regular human insulin (ie, one unit of Humalog has the same
glucose-lowering effect as one unit of regular human insulin), but with more rapid activity. The quicker glucose-
lowering effect of Humalog is related to the more rapid absorption rate from subcutaneous tissue. An adjustment
of dose or schedule of basal insulin may be needed when a patient changes from other insulins to Humalog,
particularly to prevent premeal hyperglycemia.
When used as a mealtime insulin, Humalog should be given within 15 minutes before or immediately after a
meal. Regular human insulin is best given 30 to 60 minutes before a meal. To achieve optimal glucose control,
the amount of longer-acting insulin being given may need to be adjusted when using Humalog.
The rate of insulin absorption and consequently the onset of activity are known to be affected by the site of
injection, exercise, and other variables. Humalog was absorbed at a consistently faster rate than regular human
insulin in healthy male volunteers given 0.2 U/kg regular human insulin or Humalog at abdominal, deltoid, or
femoral sites, the 3 sites often used by patients with diabetes. When not mixed in the same syringe with other
insulins, Humalog maintains its rapid onset of action and has less variability in its onset of action among injection
sites compared with regular human insulin (see PRECAUTIONS). After abdominal administration, Humalog
concentrations are higher than those following deltoid or thigh injections. Also, the duration of action of Humalog
is slightly shorter following abdominal injection, compared with deltoid and femoral injections. As with all insulin
preparations, the time course of action of Humalog may vary considerably in different individuals or within the
same individual. Patients must be educated to use proper injection techniques.
Humalog in a vial may be diluted with STERILE DILUENT for Humalog, Humulin N, Humulin R, Humulin 70/30,
and Humulin® R U-500 to a concentration of 1:10 (equivalent to U-10) or 1:2 (equivalent to U-50). Diluted
Humalog may remain in patient use for 28 days when stored at 5°C (41°F) and for 14 days when stored at 30°C
(86°F). Do not dilute Humalog contained in a cartridge or Humalog used in an external insulin pump.
Parenteral drug products should be inspected visually before use whenever the solution and the container
permit. If the solution is cloudy, contains particulate matter, is thickened, or is discolored, the contents must not
be injected. Humalog should not be used after its expiration date. The cartridge containing Humalog is not
designed to allow any other insulin to be mixed in the cartridge or for the cartridge to be refilled with insulin.
External Insulin Pumps—Humalog was tested in MiniMed®1 Models 506, 507, and 508 insulin pumps using
MiniMed®1 Polyfin®1 infusion sets. Humalog was also tested in the Disetronic®2 H-TRONplus® V100 insulin
pump (with plastic 3.15 mL insulin reservoir) and the Disetronic D-TRON®2,3 and D-TRONplus®2,3 pumps (with
Humalog 3 mL cartridges) using Disetronic Rapid®2 infusion sets. Humalog should not be diluted or mixed with
any other insulin when used in an external insulin pump.
HOW SUPPLIED:
Humalog (insulin lispro injection, USP [rDNA origin]) is available in the following package sizes (with each
presentation containing 100 units insulin lispro per mL [U-100]):
10 mL vials NDC 0002-7510-01 (VL-7510)
3 mL vials NDC 0002-7510-17 (VL-7533)
5 x 3 mL cartridges3 NDC 0002-7516-59 (VL-7516)
5 x 3 mL prefilled insulin delivery devices (Pen) NDC 0002-8725-59 (HP-8725)
5 x 3 mL prefilled insulin delivery devices (Humalog® KwikPen™) NDC 0002-8799-59 (HP-8799)
1 MiniMed® and Polyfin® are registered trademarks of MiniMed, Inc.
2
Disetronic®, H-TRONplus®, D-TRON®, and Rapid® are registered trademarks of Roche Diagnostics GMBH.
3 3 mL cartridge is for use in Eli Lilly and Company’s HumaPen® MEMOIR™ and HumaPen® LUXURA™ HD insulin
delivery devices, Owen Mumford, Ltd.’s Autopen® 3 mL insulin delivery device, and Disetronic D-TRON® and
D-TRONplus® pumps. Autopen® is a registered trademark of Owen Mumford, Ltd. HumaPen®,
HumaPen® MEMOIR™ and HumaPen® LUXURA™ HD are trademarks of Eli Lilly and Company.
Other product and company names may be the trademarks of their respective owners.
Storage —Unopened Humalog should be stored in a refrigerator (2° to 8°C [36° to 46°F]), but not in the
freezer. Do not use Humalog if it has been frozen. Unrefrigerated (below 30°C [86°F]) 12 vials, cartridges, Pens,
and KwikPens must be used within 28 days or be discarded, even if they still contain Humalog. Protect from
direct heat and light.
Use in an External Insulin Pump—A Humalog 3mL cartridge used in the D-TRON®2,3 or D-TRONplus®2,3
should be discarded after 7 days, even if it still contains Humalog. Infusion sets, D-TRON®2,3 and D-TRONplus®2,3
cartridge adapters, and Humalog in the external insulin pump reservoir should be discarded every 48 hours
or less.
Literature revised December 7, 2009
KwikPens manufactured by Eli Lilly and Company, Indianapolis, IN 46285, USA.
Pens manufactured by Eli Lilly and Company, Indianapolis, IN 46285, USA or Lilly France,
F-67640 Fegersheim, France.
Vials manufactured by Eli Lilly and Company, Indianapolis, IN 46285, USA or Hospira, Inc.,
Lake Forest, IL 60045, USA or Lilly France, F-67640 Fegersheim, France.
Cartridges manufactured by Lilly France, F-67640 Fegersheim, France for Eli Lilly and Company,
Indianapolis, IN 46285, USA.
www.humalog.com
Copyright © 1996, 2008, Eli Lilly and Company. All rights reserved.

WWW.DRUGTOPICS.COM December 2010
DRUG TOPICS
29
Clinical
PACE-MI TRIAL
“Beta blocker use is better than 93%
at discharge,” said Jeffrey Goldberger, MD,
professor of medicine, Northwestern Uni-
versity Feinberg School of Medicine, Chi-
cago. “We can attribute that to beta block-
ers post-MI becoming a quality measure.
What we’re not doing is ensuring that
they are used at appropriate doses.”
Pace-MI Trial
Dr. Goldberger is lead author of an analysis
of data from the PACE-MI Trial, evaluat-
ing the survival impact of pacemaker-
facilitated beta blocker therapy after MI
in patients who have bradycardia con-
traindications to beta blockers. The study
included a prospective registry of 1,971
consecutive MI patients across 19 centers
admitted with MI between August 2007
and July 2008.
Registry data included beta blocker
dose at discharge, Dr. Goldberger said.
Physicians were queried 3 weeks after dis-
charge for the current beta blocker dose.
Researchers found that while 93.2% of
patients were discharged on a beta blocker,
only 17% of patients were receiving more
than 50% of the recommended dose of
metoprolol (Lopressor, Novartis), carve-
dilol (Coreg, GlaxoSmithKline), atenolol
(Tenormin, AstraZeneca), propranolol (In-
deral, Wyeth), or some other beta blocker.
The right dose
“The question shouldn’t be is the patient
getting a beta blocker or not,” Dr. Gold-
berger told Drug Topics. “The question
should be is the patient getting the right
dose of a beta blocker. For the majority of
patients, the current answer is no.”
Beta blocker dosing is a sticky issue,
said Joseph Saseen, PharmD, FCCP, BCPS,
CLS, professor of
Clinical Pharmacy,
University of Colo-
rado Denver School
of Pharmacy. Much
of the data on ap-
propriate dosing
comes from heart-
failure patients.
Many MI patients
progress to heart
failure, but not all do so. Up-titrating beta
blocker doses may be less important in pa-
tients who do not progress to heart failure.
Older patients may also do well on
lower doses of beta blockers. If the resting
heart rate is about 60, Saseen noted, and
does not spike dramatically during mild
exercise such as walking, the patient is
probably on an appropriate dose.
“This study is a good snapshot of beta
blocker use post-MI, not the full picture,”
he said. “And there is good support for the
notion that the beta blocker dose 3 weeks
after discharge is a good indicator of dos-
age over the next year.”
Short stays, less titration
Patients are traditionally started on a
low dose of beta blocker and titrated
up in the hospital, Dr. Goldberger said.
But with shorter hospital stays, there is
less time for managing dose titration. In
many cases, it simply doesn’t happen.
“Once you take a patient out of the
post-MI setting, people tend to focus on
absolutes like blood pressure and patient
symptoms,” he said. “They just don’t think
about beta blocker dosage. You don’t need
a physician to up the dose. You could just
as easily use a pharmacist to follow up.”
Quality systems
Tweaking existing quality systems can
help, said Mary Andrawis, PharmD,
MPH, director, clinical guidelines and
quality improvement, American So-
ciety of Health-System Pharmacists.
Most health systems evaluate beta
blocker compliance with a simple yes/
no question. Because dosing data are
already captured, it should be possible
to refi ne the qual-
ity measure to ap-
propriate dose, not
just any dose.
“Beta blockers
will be one of the
first and easiest of
these measures to
take,” she said. “Re-
fi ning and evolving
our quality mea-
sures is something we could implement
in lipid dosing, anticoagulation therapy,
and other areas.”
Pharmacy could also play a role in
discharge counseling, Andrawis said. In-
cluding beta blocker dosing as part of the
medication reconciliation process when
patients move into the community is a
reasonable extension of care.
“Part of the discharge process is ensur-
ing that the patient is seen again for ap-
propriate follow-up,” she said. “Checking
a beta blocker dose is exactly what a phar-
macist should be looking for.”
Post-MI beta blocker doses too low
Care for patients with myocardial infarction (MI) may not be as good as quality mea-
sures suggest. New data show that while nearly all MI patients receive beta blockers,
most patients receive suboptimal doses that are never increased.
RX CARE
Fred Gebhart, Contributing Editor
Joseph Saseen
Mary Andrawis
If the resting heart rate
is about 60 and does
not spike dramatically
during mild exercise
such as walking, the
patient is probably on
an appropriate dose.

30
DRUG TOPICS
December 2010 WWW.DRUGTOPICS.COM
Clostridium diffi cile is an anaerobic, gram-positive spore-
forming rod that exists in both a vegetative form
and a resilient spore form. It was recognized as the
cause of antibiotic-associated pseudomembranous colitis in
1978, and it remains the most common cause of healthcare-
associated infectious diarrhea.1,2
C. diffi cile infection (CDI) refers to the presence in patients
of clinical symptoms of disease (usually manifested as diar-
rhea) and either a stool test positive for the presence of C.
diffi cile toxins or fi ndings that reveal pseudomembranous
colitis. CDI ranges in severity from asymptomatic coloniza-
tion to fulminant disease characterized by severe diarrhea
with intestinal complications. CDI is now recognized as a
worldwide public health concern, as it is increasing in inci-
dence, severity of disease, and associated mortality.
Clinical practice guidelines for treating CDI in adults have
recently been updated through a collaboration of the Society
for Healthcare Epidemiology of America (SHEA) and the
Infectious Diseases Society of America (IDSA).3 This article
focuses on these guidelines and reviews the current termi-
nology, etiology, pathogenesis, and treatment recommenda-
tions for CDI.
Epidemiology
Between 1% and 3% of healthy adults and up to 50% of
patients residing in hospitals and nursing homes are colo-
nized with and asymptomatically carry C. diffi cile in their
stool. Interestingly, the rates of colonization are higher in
neonates, with reported ranges between 5% and 70%, al-
though this population is less likely to develop the disease
than adults. It is believed that the lower rates of disease in
neonates are due to the immaturity of their intestinal cells,
which lack receptors for one of the organism’s disease-pro-
ducing toxins.4 C. diffi
cile is currently recognized as the most
common cause of nosocomial infectious diarrhea in nursing
homes, and overall mortality associated with CDI is esti-
mated to be more than 17% in the older adult population.5
CDI occurs in up to 8% of hospitalized patients and
results in an estimated $1.3 billion annually in healthcare
costs in the United States.6,7 As previously mentioned, CDI
is increasing in frequency and severity of disease. During the
1990s, the incidence of CDI in U.S. hospitals was approxi-
mately 30 to 40 cases per 100,000 persons; this number
increased to 84 per 100,000 in 2005. The number of fatal
CDI cases has also increased over this time.
A new strain of C. diffi cile, which has caused disease out-
breaks in the United States, Canada, Europe, and Asia, was
reported by the Centers for Disease Control and Prevention
(CDC) in 2004.8 This hypervirulent strain produces toxins
A and B in quantities 16-fold to 20-fold higher than seen in
other strains, and produces an additional third toxin, as well.
This strain has been referred to by several different names:
“North American pulsed-fi eld gel electrophoresis type 1
(NAP-1)”; “restriction endonuclease pattern BI”; or “PCR
ribotype 027.” It is now commonly referred to as “NAP1/
BI/027.” Overall, the NAP1/BI/027 strain is considered to
be easier to transmit, more virulent, and more diffi cult to
treat than previously identifi ed strains.9
Transmission
Transmission of C. diffi cile occurs via the fecal-oral route,
through ingestion of either the vegetative form or the spore
form of the microorganism. The spore form of C. diffi cile may
survive on inanimate surfaces and is resistant to alcohol
and routine disinfectants.10 Transmission of the microorgan-
ism occurs in healthcare facilities via the fecal-oral route
either directly from person to person (particularly from the
spore-contaminated hands of healthcare workers) or from
a contaminated environment, especially in institutions with
improper hygiene practices, isolation precautions, or envi-
ronmental cleaning practices.11
Pathogenesis
Disease pathogenesis of CDI involves the following factors:
disruption of normal enteric fl ora; colonization with C. dif-
fi cile; production by the microorganism of toxins A and B;
and mucosal injury and infl ammation. In general, disease
progresses from a patient in an uncolonized state becom-
ing colonized with C. difficile to the organism producing
its toxins, ultimately resulting in CDI. The host’s immune
AN ONGOING CE PROGRAM OF THE UNIVERSITY OF FLORIDA
COLLEGE OF PHARMACY AND DRUG TOPICS
Elias B. Chahine, PharmD, BCPS
ASSISTANT PROFESSOR OF PHARMACY PRACTICE
LLOYD L. GREGORY SCHOOL OF PHARMACY
PALM BEACH ATLANTIC UNIVERSITY
WEST PALM BEACH, FLORIDA
Allana J. Sucher, PharmD, BCPS
ASSOCIATE PROFESSOR OF PHARMACY PRACTICE
REGIS UNIVERSITY SCHOOL OF PHARMACY
DENVER, COLORADO
An update on Clostridium
diffi cile infection
GETTY IMAGES/ROB LEWINE
Continuing Education

WWW.DRUGTOPICS.COM Month 2010
DRUG TOPICS
31
EDUCATIONAL OBJECTIVES
EARN CE CREDIT FOR THIS ACTIVITY AT WWW.DRUGTOPICS.COM
CREDIT: 2.0
Goal: To provide pharmacists with the knowledge nec-
essary to appropriately treat and prevent Clostridium
diffi cile infection.
After participating in this activity, pharmacists
should be able to:
● Identify the most common risk factors for
Clostridium diffi cile infection.
● Recommend the most appropriate
therapeutic regimen, including
pharmacologic and nonpharmacologic
agents, for the prevention and treatment of
C. diffi cile infection.
● List common adverse effects and drug
interactions of medications used to treat
C. diffi cile infection.
● Discuss infection-control strategies to
prevent the spread of C. diffi cile.
For questions concerning PRINT CEs, call 352-273-6275.
For questions concerning ONLINE CEs, call 877-252-7711.
To obtain immediate CE credit, take the test online at
www.drugtopics.com. Click on the “Continuing Educa-
tion” box on the Drug Topics home page, which will take
you to the CE site. Log in, find and click on this lesson,
and follow the three simple steps. Test results will be
displayed immediately and you can print the certificate
showing your earned CE credits.
The University of Florida College of Pharmacy is accredited
by the Accreditation Council for Pharmacy Education
as a provider of continuing pharmacy education
ACPE # 0012-9999-10-106-H01-P (K)
This lesson is not valid for CE credit after 12/31/2012.
WWW.DRUGTOPICS.COM
32
DRUG TOPICS
December 2010
Continuing Education
UPDATE: C. DIFFICILE
system helps protect an individual from the progression of
colonization to symptomatic disease. Those who are unable
to produce antitoxin antibodies are more likely to develop
severe disease and experience disease recurrence.1
The spore form of C. diffi cile is resistant to stomach acid
and, once ingested, is able to survive passage through the
stomach into the intestine, where it changes into its active
vegetative form. If normal enteric fl ora are disrupted (as
may occur in the presence of antimicrobial agents), the veg-
etative form can reproduce within intestinal crypts. The bac-
teria then release toxins key to the development of disease.
Toxin A has proinfl ammatory activity, attracting neutrophils
and monocytes, and has enterotoxic activity, loosening tight
junctions between cells that line the colon. This allows the
cytotoxic toxin B to enter into and degrade epithelial cells
of the colon. The effect of both toxins causes colitis and may
cause the formation of pseudomembranes on the intestinal
wall that impair the normal process of reabsorption, ulti-
mately producing watery diarrhea.4
NAP1/BI/027 strains of C. diffi cile have an altered repres-
sor gene, which normally inhibits toxin production. These
isolates produce 16 to 20 times more quantities of toxins A
and B than other strains and also produce a unique third
toxin, referred to as binary toxin, which acts synergisti-
cally with toxins A and B to produce more severe forms
of disease.4
Risk factors
The most important modifi able risk factor for acquiring CDI
is receipt of an antimicrobial agent. Although longer expo-
sure, exposure to multiple agents, or exposure to broader-
spectrum agents may increase a patient’s risk, even a single
dose of an antimicrobial agent increases the risk for C. diffi cile
colonization and symptomatic disease. In addition to use of
antimicrobial agents, there are several other risk factors for
CDI as indicated in Table 1.1,3,7,9,12,13 It is not clear whether
the use of acid-suppressing medications such as histamine-2
blockers or proton pump inhibitors increase a patient’s risk
for CDI; some studies have shown an epidemiologic associa-
tion, but others have found that the association is due to
confounding factors such as severity of illness and duration
of hospitalization. In addition to previously established risk
factors for disease, an increased incidence of CDI has been
reported in otherwise healthy individuals (“community-as-
sociated” infection) and in women during the peripartum
period.1,3,7,9,12,13
Clinical presentation
Although the median incubation period between the in-
gestion of C. diffi cile and the development of CDI is 2 to 3
days, this varies greatly among individuals. Patients may
become symptomatic soon after starting antimicrobial
therapy or several weeks after completion of an antimi-
crobial regimen.1,13
Clinical manifestations of CDI range from asymptom-
atic carriage of the organism to mildly or moderately acute
watery diarrhea, to severe disease characterized by pseudo-
membranous colitis. About 50% of patients will also have
fever, cramping, lower abdominal pain, and leukocytosis.
Complications of severe colitis include sepsis, toxic megaco-
lon (characterized by colonic dilation >6 cm), colonic ileus,
or bowel perforation. Patients who develop toxic megacolon
or an ileus usually have abdominal pain and distension but
may not develop diarrhea.1,12
The updated guidelines include proposed criteria for
the defi nitions of mild or moderate, severe, and compli-
cated CDI. The 3 main criteria that are recommended for
consideration when choosing treatment include age (older
patients may have a decreased immune response to C. dif-
fi cile toxins); peak white blood cell (WBC) count (this may
refl ect the severity of colonic infl ammation and potential
for complications); and elevated serum creatinine level
(this may indicate dehydration or inadequate renal perfu-
sion from severe diarrhea). These criteria for severity of CDI,
based on expert opinion, are included in Table 2.3 Because
treatment recommendations vary according to the patient’s
severity of illness, the clinician should be familiar with these
defi nitions. However, the guidelines acknowledge that these
Risk factors associated with CDI
TABLE 1
Exposure risk factors Host risk factors
Exposure to antimicrobial agents
Duration of hospitalization
GI procedures (including feeding
tubes)
Proximity to patients with CDI
Advanced age
Comorbidities
Compromised immune system
(cancer chemotherapy, other im-
munosuppressive agents, HIV)
Abbreviations: CDI, Clostridium diffi cile infection; GI, gastrointestinal;
HIV, human immunode ciency virus
Source: Refs 1,3,7,9,12,13
Criteria for determining severity of illness
resulting from CDI
TABLE 2
De nition Supportive data
Mild or moderate infection WBC count ≤15,000 cells/μL and
SCr <1.5 times the level prior to
illness
Severe infection WBC count ≥15,000 cells/μL and
SCr >1.5 times the level prior to
illness
Severe, complicated infection Hypotension or shock, ileus,
megacolon
Abbreviations: CDI, Clostridium diffi cile infection; SCr, serum creatinine;
WBC, white blood cell
Source: Ref 3
WWW.DRUGTOPICS.COM December 2010
DRUG TOPICS
33
CONTINUING EDUCATION
criteria may need to be adjusted in the future as further data
become available.
Recurrent CDI is common, with some sources estimating
that up to 50% of patients experience recurrent infection
within 2 weeks after completion of therapy. Mechanisms
of recurrence include relapse of infection from the original
strain of C. diffi cile or re-infection with a new bacterial strain.
Identifi ed risk factors for recurrent CDI include exposure to
antimicrobials during or after initial treatment for CDI and
an impaired immune response against C. diffi cile toxins. A
recently identifi ed potential risk factor for recurrence may be
the use of metronidazole as treatment for CDI, particularly
in patients aged ≥65 years.
Diagnosis
Unless the clinician suspects that a patient has an ileus
due to CDI, it is recommended that only patients with
diarrhea (defi ned as 3 or more unformed stools in ≤24 hr)
be tested for the presence of C. diffi cile. This is because of
the high rate of persons with asymptomatic colonization
with C. diffi cile. As previously stated, the diagnosis of CDI
includes both clinical symptoms of diarrhea and either a
positive stool test for C. diffi cile toxins or the presence of
pseudomembranous colitis.1
In the United States, the most commonly used method
for diagnosis of CDI is an enzyme immunoassay (EIA) test-
ing for C. diffi cile toxins A and B. EIA testing is relatively
easy to use and is associated with low labor costs. In addi-
tion, it has a rapid turnaround time of within 1 day, a high
specifi city, and an estimated sensitivity of 63% to 94%.3
Other testing methods that are more sensitive for detec-
tion of C. diffi cile but are less commonly used, due to higher
cost and slower turnaround time, include a cell cytotoxin
assay (to detect toxin activity in stools) and stool culture.
It is recommended that stool cultures be performed for
molecular typing of strains for the purpose of epidemio-
logic studies. Another testing strategy recently studied is a
2-step testing method that uses EIA detection of glutamate
dehydrogenase (GDH), an enzyme produced by C. diffi cile,
as an initial screening tool. A negative result is considered
negative, while a positive assay requires additional testing
to determine whether the strain of C. diffi cile is toxin-pro-
ducing. This additional testing is performed with the cell
cytotoxin assay or stool culture. The 2-step GDH testing
method has a sensitivity of 85% to 95% and a specifi city
of 89% to 99%.3
Direct visualization of pseudomembranes on the colonic
mucosa via endoscopy or histopathologic examination de-
fi nitively establishes the diagnosis of pseudomembranous
colitis. However, because of its cost, the potential risk of
colonic perforation, and the availability of other tests for
diagnosis, it is usually reserved for times when a rapid
diagnosis is needed, as in the case of a patient with severe
disease, or when other tests are insensitive or the results
are delayed.3,13
Treatment
The goals of treatment for CDI are to stop the production
of toxins, eradicate the microorganism, attenuate signs and
symptoms of the disease, decrease disease recurrence, and
prevent associated morbidity and mortality.
The newly released guidelines recommend treating CDI
on the basis of disease severity.3 In cases of mild CDI, early
discontinuation of a precipitating antimicrobial will resolve
symptoms in 15% to 23% of patients. Most cases, however,
will require antimicrobial therapy directed against C. diffi cile
in addition to routine fl uid and electrolyte replacement as
needed. Defi nitions of disease severity are included in Table
2 (page 32), and adult dosages of antimicrobial agents used
for the treatment of CDI are listed in Table 3.3,14
First episode of CDI. For mild-to-moderate cases of
CDI, metronidazole 500 mg orally 3 times daily for 10 to 14
days is recommended. If the patient has a comorbidity or
drug interaction that prohibits the use of metronidazole, oral
vancomycin may be used instead. For severe cases of CDI,
vancomycin 125 mg orally 4 times daily for 10 to 14 days
is recommended. For severe, complicated cases, vancomy-
cin 500 mg orally or by nasogastric tube in a liquid form 4
times daily plus metronidazole 500 mg intravenously every
8 hours is recommended for 10 to 14 days at the discretion
Abbreviations: CDI, Clostridium diffi cile infection; IV, intravenous;
PO, by mouth; q, every
a Widely used
b No data support its use as monotherapy
c Dosages are for patients with normal renal and hepatic function
Source: Ref 14
Antibiotics Usual dosing regimenc
Vancomycina125 mg PO q 6 hra
250 mg – 500 mg PO q 6 hr
500 mg dissolved in 100 mL nor-
mal saline via retention enemab
q 6 hr
Metronidazolea250 mg PO q 6 hr
500 mg PO q 8 hra
500 mg IV q 8 hr
Nitazoxanide 500 mg PO q 12 hr
Bacitracin 25,000 U PO q 6 hr
Rifaximin 400 mg PO q 12 hr
200 mg PO q 8 hr
Rifampinb300 mg – 600 mg PO q 12 hr
Tigecycline 100 mg IV loading dose, followed
by 50 mg IV q 12 hr
Antimicrobial dosing recommendations
for treatment of CDI
TABLE 3
WWW.DRUGTOPICS.COM
34
DRUG TOPICS
December 2010
Continuing Education
UPDATE: C. DIFFICILE
of the clinician. No evidence supports the routine use of
oral metronidazole and oral vancomycin in combination.
If a patient has a complete ileus, adding vancomycin 500
mg in 100 mL of normal saline administered per rectum
as a retention enema may be considered. For critically ill
patients, colectomy may be considered.3 Vancomycin may
be administered as capsule, or the reconstituted intravenous
formulation may be administered orally after dilution in 1
ounce of water. The intravenous formulation may also be
used to prepare an enema.
Recurrent CDI. The updated guidelines recommend
treating the fi rst recurrence of CDI with the same antibiotic
used for the initial episode as long as the recurrence occurs
at the severity level of the initial episode. The guidelines
recommend against the use of metronidazole beyond the
fi rst recurrence of CDI, because of increased potential for
cumulative neurotoxicity and because stool concentration of
metronidazole tends to wane during recovery. For patients
with a second recurrence of infection, vancomycin should
be given via a tapered or pulsed regimen, such as the fol-
lowing example: at the end of the traditional vancomycin
regimen, continue with vancomycin 125 mg twice daily for
a week, then 125 mg once daily for a week, and 125 mg
every 2 to 3 days for 2 to 8 weeks.3
A closer look at antibiotic treatment options
Vancomycin. Vancomycin is currently the only antibiotic
that has been approved by FDA for the treatment of CDI.
Oral vancomycin achieves high concentrations in the intes-
tinal lumen and has little systemic absorption, which may
explain the rapid response to its use. The administration of 2
g of oral vancomycin daily results in a mean fecal concentra-
tion of 3,100 μg/g of stool, which far exceeds the minimum
inhibitory concentration (MIC) needed to inhibit 90% of
strains of C. diffi cile (MIC90 0.75 – 2 μg/mL).15 Intravenous
vancomycin should never be used for the treatment of CDI,
as subtherapeutic levels reach the gastrointestinal (GI) tract
when given via this route of administration. Higher dosing
of vancomycin is recommended for cases complicated by an
ileus to ensure that adequate therapeutic concentrations of
drug are reached within the lumen of the colon.16
Due to concerns regarding the emergence of vancomy-
cin-resistant enterococci (VRE), the use of vancomycin has
historically been limited to patients with multiple episodes
of CDI, patients intolerant to metronidazole, patients who
had not responded to 2 to 5 days of treatment with metro-
nidazole, or patients who were pregnant or breastfeeding.
The recent emergence of the more toxic epidemic NAP1/
BI/027 strain of C. diffi cile has caused a marked increase in
the use of vancomycin as a fi rst-line antibiotic.
In addition, a recent prospective, randomized, double-
blind, placebo-controlled trial comparing vancomycin to
metronidazole in the treatment of 172 patients with CDI
stratifi ed by disease severity showed that vancomycin was
superior to metronidazole for severe cases.17 The clinical
cure rate was 97% with vancomycin and 76% with met-
ronidazole (P=.02). Severe CDI was defi ned as endoscopic
evidence of pseudomembranous colitis, treatment in the
intensive care unit (ICU), or the presence of 2 or more of
the following conditions within 48 hours of enrollment into
the study: patient aged >60 years; temperature >38.3°C; al-
bumin level <2.5 mg/dL; or WBC count >15,000 cells/mm3.
Factors associated with treatment failure of metronida-
zole in patients with severe disease included a low albumin
level, pseudomembranous colitis, or admission to the ICU. It
is hypothesized that superiority of vancomycin over metro-
nidazole for the treatment of severe disease may be due not
to drug resistance but to decreased blood fl ow to the colon,
and therefore lower local concentrations of metronidazole in
patients with severe disease. For patients who meet criteria
for severe CDI, use of vancomycin for treatment of a fi rst
episode or for disease recurrence is recommended.
Metronidazole. Metronidazole, although not FDA-ap-
proved for treatment of CDI, is widely used for that purpose.
Both oral and intravenous metronidazole achieve suffi cient
concentrations in watery or semiformed stools. The admin-
istration of 1.2 g of oral metronidazole daily results in a
mean fecal concentration of 0.4 to 14.9 μg/g of stool, and
the administration of 1.5 g of intravenous metronidazole
per day results in a mean fecal concentration of 5.1 to 24.2
μg/g of stool. This exceeds the MIC needed to inhibit 90%
of existing strains of C. diffi cile (MIC90 0.2 – 2 μg/mL).
Historically, metronidazole has been preferred to vanco-
mycin because it is less expensive, it is less likely to induce
the development of VRE, and it can be given by IV to patients
who are unable to tolerate oral medications or to those with
an ileus.18,19 Even though a recent study questioned its effi cacy,
oral metronidazole has been proven to be as effi cacious as oral
vancomycin in patients with mild-to-moderate CDI.20
In a recent prospective, randomized, double-blind, pla-
cebo-controlled trial comparing vancomycin to metronida-
zole in the treatment of 172 patients with CDI stratifi ed by
disease severity, the clinical cure rate was 90% with met-
ronidazole and 98% with vancomycin (P=.36).17 However,
when compared to vancomycin, metronidazole is associ-
ated with a slightly delayed response. It is also important for
the clinician to be aware that the NAP1/BI/027 strain of C.
diffi cile may not respond well to metronidazole therapy. In
addition, vancomycin is preferred over metronidazole in pa-
tients with severe episodes of CDI, patients with complicated
courses of CDI, patients with more than one recurrence of
CDI, and patients who are pregnant or breastfeeding.
Nitazoxanide. Nitazoxanide is an antiparasitic agent that
has been shown to be active against C. diffi cile. In a small
prospective, double-blind, randomized controlled trial com-
paring nitazoxanide with vancomycin for the treatment of
50 patients with CDI, the initial response rates were 77%
with nitazoxanide and 74% with vancomycin (95% confi -
dence interval [CI], -24% to 28%) and the sustained response
rates were 89% with nitazoxanide and 78% with vancomy-

WWW.DRUGTOPICS.COM December 2010
DRUG TOPICS
35
CONTINUING EDUCATION
cin (95% CI, -18% to 35%).21 Although results of this small
study suggest that nitazoxanide may be as effective as van-
comycin for the treatment of patients with CDI, the authors
acknowledge that larger studies are needed before defi nitive
conclusions about its place in therapy can be drawn.
In a prospective, randomized, double-blind study com-
paring nitazoxanide with metronidazole for the treatment
of 110 patients with CDI, the initial response rates were
89.5% with nitazoxanide and 82.4% with metronidazole
(95% CI, -7.1% to 25.5%) and the sustained response rates
were 65.8% with nitazoxanide given for 7 days, 74.3% with
nitazoxanide given for 10 days, and 57.6% with metroni-
dazole for 10 days (P=.34).22 The authors concluded that
nitazoxanide is at least as effective as metronidazole in the
treatment of patients with CDI.
In an open-label study, nitazoxanide was also shown to
be effective in 26 (74%) of 35 patients who did not re-
spond to treatment with metronidazole for CDI; however,
7 patients later had recurrent disease yielding an estimated
overall cure rate of 54%.23 Although it is not currently
recommended in the updated guidelines for routine use,
nitazoxanide may represent an alternative option for the
treatment of initial and recurrent CDI.
Bacitracin. Bacitracin, a commonly used topical antibi-
otic for the treatment of staphylococcal infections, has been
shown to be active against C. diffi cile when given in an oral
formulation.24 The injection formulation is extemporaneously
prepared and fl avored to improve palatability. Although par-
enteral bacitracin therapy is associated with renal failure, oral
administration achieves low, nontoxic serum concentrations.
Studies conducted in the 1980s showed that oral bacitracin
was as effective as oral vancomycin in resolving the symp-
toms of CDI, but that it was less effective in eradicating C.
diffi cile and its toxin from the stools of patients.24,25 The fact
that some patients still had detectable toxins in stools did not
affect the number of clinical recurrences in one study.
Rifaximin. Rifaximin, a rifamycin antibiotic with limited
oral bioavailability, is approved by FDA for the treatment of
travelers’ diarrhea and for prophylaxis against hepatic encepha-
lopathy. As it is active in vitro against C. diffi cile and achieves a
high concentration in the colon, rifaximin is currently under
investigation for the treatment of CDI. In a recent uncontrolled
case series, 4 of 6 patients with multiple recurrences of CDI
responded to rifaximin therapy immediately after completing
their last course of vancomycin therapy.26
In another uncontrolled case series, 8 women who each
experienced 4 to 8 episodes of diarrhea due to C. diffi cile were
given a 2-week course of rifaximin therapy immediately
after completing their last course of vancomycin therapy.
Seven of the 8 patients experienced no further recurrence
of diarrhea. However, resistance of C. diffi cile to this agent has
already been reported, so its use is not recommended until
further clinical trials have been performed.27,28
Rifampin. Rifampin has been used as adjunctive ther-
apy with either vancomycin or metronidazole for the treat-
ment of CDI, as it has been shown to have potent in vitro
activity against C. diffi cile and anecdotal reports have shown
success with its use.29 A small prospective, randomized, sin-
gle-blinded study was performed to compare metronidazole
monotherapy versus metronidazole plus rifampin for the
treatment of 39 patients with a primary episode of CDI.29
After 10 days of treatment, no difference was observed
between treatment groups in terms of time to symptom
improvement (6.5 days vs 9 days; P=.74), proportion of pa-
tients with relapse (38% vs 42%; P=1.0), or time to fi rst
relapse (16 days vs 26 days; P=.23). The authors concluded
that rifampin does not have a role for use as routine adjunc-
tive therapy with metronidazole for the treatment of CDI.
Tigecycline. Tigecycline is a parenteral glycylcycline
antibiotic approved by FDA for the treatment of compli-
cated skin and skin-structure infections, complicated intra-
abdominal infections, and community-acquired pneumonia.
Tigecycline is active in vitro against C. diffi cile and has been
reported successful in the treatment of CDI in an uncon-
trolled case series of 4 patients with severe CDI, including
patients with ileus and patients refractory to vancomycin
and metronidazole.30-32 None of the tigecycline-treated pa-
tients relapsed within 3 months of therapy, which makes
tigecycline an attractive potential addition to the arsenal of
antibiotics directed against C. diffi cile.
Antibiotic agents available outside the U.S.
Teicoplanin. Teicoplanin, a glycopeptide similar to vancomy-
cin but not available in the United States, has been shown to
have excellent activity against C. diffi cile and similar effi cacy to
oral vancomycin in prospective randomized studies.33-35
Fusidic acid. Fusidic acid is a commonly used topical
antibiotic in Europe and Canada for the treatment of skin
infections due to gram-positive bacteria. Although oral fu-
sidic acid is effective for the treatment of CDI, its use is as-
sociated with a higher rate of relapse when compared to
vancomycin or metronidazole.35,36
Investigational antibiotic agents
Fidaxomicin. Fidaxomicin, also known by several other
names including OPT-80 or difi micin, is a novel macrocyclic
antibiotic that exhibits potent in vitro activity against C. diffi cile.
It has a narrow spectrum of activity and lacks activity against
Gram-negative bacteria, thus potentially limiting its effect on
GI fl ora. Studies have shown that oral fi daxomicin achieves
high concentrations in stool while maintaining low plasma
concentrations. Preliminary results from phase 2 and 3 clinical
trials involving patients with CDI showed favorable outcomes
for difi micin when compared to oral vancomycin.37
Ramoplanin. Ramoplanin, a novel glycolipodepsipep-
tide nonabsorbable antibiotic, showed a potential benefi t
against C. diffi cile in both in vitro studies and an in vivo ham-
ster model. One study showed that all isolates of C. diffi cile,
independent of their levels of susceptibility to vancomycin
or metronidazole, were susceptible to ramoplanin.38 The
WWW.DRUGTOPICS.COM
36
DRUG TOPICS
December 2010
Continuing Education
UPDATE: C. DIFFICILE
results of a second study suggested that ramoplanin may
be more effective than vancomycin at eradicating C. diffi cile
spores and preventing spore recrudescence.39 The FDA ap-
proved a Special Protocol Assessment non-inferiority trial
against vancomycin for phase 3.
Nonantibiotic treatment options
Anion-binding resins. The anion-binding resins include
cholestyramine and colestipol. Cholestyramine is theoreti-
cally useful for the treatment of CDI because it binds to the
disease-producing toxins secreted by C. diffi cile without alter-
ing GI fl ora. However, although there are some reports of
patients with disease relapses responding to cholestyramine,
not enough clinical evidence supports its routine use for
CDI. In addition, cholestyramine may bind to an oral van-
comycin, thus potentially decreasing its effect. It is impor-
tant for the clinician to note that other medications must be
given 1 hour before or 4 to 6 hours after an anion-binding
resin to maintain therapeutic effi cacy.4,14,40
One randomized, placebo-controlled trial of colestipol in
38 patients with postoperative diarrhea showed no differ-
ence in fecal excretion of C. diffi cile toxins as compared to
placebo. Therefore the use of colestipol for the treatment of
CDI is not justifi ed.41
Toxin-binding polymer. Tolevamer is a nonantimi-
crobial toxin-binding polymer that has been studied for
the treatment of CDI. Although it showed early promise
from in vitro, animal, and phase 2 studies, a randomized,
double-blind, phase 3 clinical trial showed that tolevamer
was less effective than either vancomycin or metronidazole
for CDI.42,43 Interestingly, among patients who did achieve
clinical success with tolevamer, use of this agent was as-
sociated with signifi cantly lower rates of disease recurrence.
Probiotics. Probiotics, which are live microorganisms
that confer a potential health benefi t when administered
in adequate amounts, have been used in practice for the
treatment and prevention of CDI.44 Examples of probiotics
used for this purpose include Bifi dobacterium spp., Lactobacil-
lus rhamnosus GG, and Saccharomyces spp.45 Although some
small studies and meta-analyses have provided data sug-
gesting that prophylactic use of probiotics may decrease the
incidence of CDI, no large, prospective, randomized trials
have been designed to assess the benefi ts of probiotics for
the prevention or treatment of CDI.
One small open-label trial assessing the effect of S. boular-
dii added to oral vancomycin in patients with recurrent CDI
showed that 11 of 13 patients had no further recurrences of
infection with this combination.46
In addition to a lack of data, product dosages of probi-
otics are not standardized, and there have been reports of
fungemia resulting from Saccharomyces spp. and bacteremia
resulting from Lactobacillus spp., especially in patients who
are critically ill or immunocompromised. Thus, the guide-
lines recommend against using probiotics to prevent primary
CDI until further, larger trials are conducted. The guidelines
acknowledge that adjunctive S. boulardii may be useful for
treatment of recurrent CDI, but caution against its use in
those who are immunocompromised or critically ill.
Immunomodulators. Intravenous immunoglobulin
(IVIG) has been used in the treatment of CDI with variable
success.47 IVIG is formed through pooling immunoglobulin
(containing antitoxin A and B antibodies) from donors and
providing this passive immunization to a host who is unable
to form an adequate protective immune response. Because
low levels of IgG antitoxin are associated with increased
disease severity, IVIG may compensate for this failed host
immune response to C. diffi cile toxins.
Although there are no prospective, randomized, con-
trolled clinical trials of IVIG, case reports and case series
suggest that it may be effi cacious for patients with severe or
recurrent CDI.48 There is confl icting evidence, however, as
published retrospective reviews showed no clinical benefi t
with the use of IVIG in patients with severe disease. Ad-
ditional drawbacks to IVIG therapy include its unknown
optimal dose, high cost, limited supply, and adverse effect
profi le. The updated guidelines refer to IVIG as a potential
option for the treatment of recurrent disease.
Recently, a phase 2 randomized, double-blind, placebo-
controlled trial of 2 human monoclonal antibodies against C.
diffi cile toxins A and B was performed.49 This study showed
a signifi cantly decreased rate of recurrence of CDI when
the simultaneously administered monoclonal antibodies
were added to antibiotic agents. No signifi cant difference
was seen in the incidence of adverse effects between the
monoclonal antibody and placebo groups. The ultimate role
of monoclonal antibodies for the treatment of CDI is yet to
be determined.
Corticosteroids. Given that C. diffi cile can cause diarrhea
and colitis from an infl ammatory reaction to its toxins, and
that corticosteroids have been shown to be effective in in-
fl ammatory bowel disease, clinicians may try these agents in
patients with refractory CDI. A preliminary report describing
the use of intravenous methylprednisolone followed by oral
prednisone in a child with severe CDI refractory to treat-
ment with metronidazole and vancomycin suggests that cor-
ticosteroids may be effective.50 The steroid dosing regimen
used in this study was IV methylprednisolone 2 mg/kg/day
in 2 divided doses for 16 days, followed by oral prednisone 2
mg/kg/day in 2 divided doses, with a gradual taper over the
course of 1 month. Further studies are necessary to confi rm
this case-report observation.
Stool transplantation. Fecal bacteriotherapy is defi ned
as the transfer of stool from one person to another. Also
known as fecal transplant, this process seeks to recolonize
the normal bacterial fl ora of the intestines.51 In the largest
retrospective review of patients who received donor stool by
nasogastric tube for recurrent CDI, 15 of 18 patients were
cured, 1 patient experienced a single recurrence, and 2 pa-
tients died of unrelated illnesses.52 No adverse effects were
associated with stool treatment. Several other published re-
WWW.DRUGTOPICS.COM December 2010
DRUG TOPICS
37
CONTINUING EDUCATION
ports have attested to positive results from the use of fecal
bacteriotherapy for recurrent episodes of CDI.53 The updated
guidelines list fecal transplant as a potential option for recur-
rent CDI if the donor is properly screened for potentially
transmissible agents and if the logistical issues of collection
and administration can be adequately addressed.
Vaccines. A C. diffi cile vaccine containing toxoids A and
B is currently under development for prevention of infec-
tion in high-risk individuals. Preliminary results demonstrated
safety and immunogenicity in healthy volunteers as well as
in patients with recurrent CDI.54,55 These results must still be
validated in large randomized controlled trials, because there
is concern that the vaccine may not be effective in those who
are unable to develop an adequate immune response to C.
diffi cile toxins, as is common in patients with recurrent disease.
The vaccine manufacturer is currently recruiting U.S. patients
with CDI for a phase 2 randomized, double-blind, placebo-
controlled dose-ranging study of the vaccine.56
Antiperistaltics. Drugs that inhibit intestinal peristasis,
including loperamide, diphenoxylate, and narcotics, should
generally be avoided in patients with infectious diarrhea. These
agents may hinder the fl ushing of GI bacteria such as C. diffi cile
and its associated toxins. It is hypothesized that these agents
may prolong the mucosal exposure to toxins, possibly predis-
posing patients to complications such as toxic megacolon.57
A recently published literature review challenges this long-
standing hypothesis.58 The authors found that all cases of CDI
treated with an antimotility agent that subsequently developed
complications also failed to receive an appropriate anti-C. dif-
fi cile antimicrobial agent. This review concluded that further
controlled studies are needed to determine the role of antiperi-
staltics (because these agents may assist with faster resolution
of diarrhea) for the treatment of CDI. Until the results of a
prospective clinical trial are available, most practitioners would
withhold antiperistaltic agents from a patient with CDI.
Evaluation of therapeutic outcomes
Effi cacy. When treating patients with CDI, clinicians should
closely monitor them for resolution of signs and symptoms of
infection. A patient’s temperature and WBC count should nor-
malize within 48 to 72 hours of start of effective therapy, and
diarrhea should improve within 48 hours and resolve by day
6 to day 7 of treatment. In light of the risk that infection may
recur, close follow-up of patients is warranted. If a patient’s dis-
ease progresses after the start of treatment, a complication may
be present, and additional or alternative therapeutic options,
including surgery, may need to be considered.12,13
Safety. The common side effects of antimicrobial agents
available in the United States for the treatment of CDI are
listed in Table 4.14 More serious but less commonly encoun-
tered side effects are discussed below.
Although oral vancomycin is not systemically absorbed
to a signifi cant extent in patients with an intact intestinal
mucosa, there may be some systemic absorption when the
intestinal mucosa is severely altered, as is common in severe
CDI. Monitoring for nephrotoxicity is therefore recommend-
ed in patients taking oral vancomycin who have a severely
damaged intestinal mucosa.
Metronidazole carries a black-box carcinogenicity warning
based on animal data. Its prolonged use may cause neuropathy
and seizures. In addition, metronidazole inhibits CYP3A4 and
displaces warfarin from protein-binding sites, thus potentially
increasing a patient’s international normalized ratio (INR) and
risk for bleeding. Close monitoring of INR is highly recom-
mended in patients receiving both metronidazole and warfarin.
Parenteral bacitracin may cause renal failure resulting from
tubular and glomerular necrosis. Although nephrotoxicity is
not expected when bacitracin is used orally, one might consider
monitoring renal function in patients with severe CDI.
Anion-binding resins. When using these as adjunctive
therapy, the clinician should take into account the fact that
these agents have been shown to bind to oral vancomycin
and have the potential to bind to other oral antibiotics and
drugs, and thus compromise their effi cacy.
Tigecycline has the potential to cause permanent dis-
coloration of teeth and is not recommended in pregnant
patients and in patients aged <8 years. Rare cases of pan-
creatitis have also been reported with this agent.
Prevention. CDI often occurs after administration of
antimicrobials, so judicious and appropriate use of antimi-
crobials is extremely important to reduce the risk of CDI.
Implementation of formal antimicrobial stewardship pro-
grams targeted at reducing the frequency and duration of
antimicrobial therapy and restriction of certain high-risk an-
timicrobials such as clindamycin and broad-spectrum agents
have been shown to reduce outbreaks of C. diffi cile.3 Discon-
Abbreviations: CDI, Clostridium diffi cile infection; GI, gastrointestinal
Source: Ref 14
Antibiotics Side effects
Vancomycin Bitter taste, nausea/vomiting,
fever/chills
Metronidazole Metallic taste, GI upset, disulfi -
ram-like reaction with alcohol,
discoloration of urine, confusion
Nitazoxanide Abdominal pain, diarrhea, nau-
sea/vomiting, headache
Bacitracin Nausea/vomiting, hypotension
Rifaximin Headache, dizziness, peripheral
edema, hypersensitivity reactions
Rifampin Red-orange discoloration of body
μ uids, GI upset, increased liver
function tests
Tigecycline Nausea/vomiting, diarrhea,
increased liver function tests
Common side effects of antimicrobials
used to treat CDI
TABLE 4
WWW.DRUGTOPICS.COM
38
DRUG TOPICS
December 2010
Continuing Education
UPDATE: C. DIFFICILE
tinuation of unnecessary acid-suppressing medications may
also be helpful. Prophylactic use of anti-CDI therapy such as
metronidazole or vancomycin, also known as early preven-
tive therapy, has been used in certain patients considered
to be at high risk for the development of disease, despite
the absence of supporting literature for this routine practice.
Administration of probiotics is not recommended to prevent
primary CDI because of lack of supporting evidence and
because of increased risk of bacteremia and fungemia, par-
ticularly in severely ill and immunocompromised patients.3
Close attention to personal hygiene such as the use of a
dedicated commode for each patient, isolation precautions
such as the use of single rooms, barrier precautions such as
the use of gloves and gowns, and appropriate hygiene and
environmental cleaning are also very important in prevent-
ing transmission of healthcare-associated infections, including
CDI. Oral and particularly rectal thermometers should be re-
placed with single-use disposable thermometers to minimize
potential spread of this microorganism.
As mentioned previously, the spore form of C. diffi cile may
survive on inanimate surfaces for long periods of time and is
resistant to alcohol and routine disinfectants. Thus, healthcare
professionals should wash their hands using soap and water
instead of alcohol-based hand rubs. Chlorine-based solutions
such as hypochlorite and isocyanuric acid are preferred for
environmental cleaning over traditional quaternary ammo-
nium-based agents, as the latter are not sporicidal. A recent
study showed that aerosolization of C. diffi cile occurs in patients
with symptomatic CDI.10 Therefore the necessity for isolation
of patients in single rooms as soon as possible after the onset
of diarrhea cannot be overemphasized.
Role of the pharmacist
CDI is an emerging threat to patient safety. Pharmacists play
a vital role in managing the disease by educating patients and
healthcare professionals about risk factors for CDIs. Pharmacists
are in a key position to refer patients with symptoms consistent
with CDI to a clinician for further work-up. Pharmacists are also
well positioned to recommend appropriate antimicrobial treat-
ment regimens, as well as to monitor and counsel patients about
drug therapy to achieve optimal outcomes, while minimizing
side effects and unnecessary toxicity. Pharmacists trained in in-
fectious diseases are essential for the establishment of antimicro-
bial stewardship programs targeted at reducing outbreaks of C.
diffi cile. Finally, pharmacists may also serve on infection control
committees and emphasize the importance of hygiene and en-
vironmental cleaning in reducing transmission of C. diffi cile.
References
1. Poutanen SM, Simor AE. Clostridium diffi cile-associated diarrhea in
adults. CMAJ. 2004;171:51–58.
2. Vasa CV, Glatt AE. Effectiveness and appropriateness of empiric met-
ronidazole for Clostridium diffi cile-associated diarrhea. Am J Gastroenterol.
2003;98:354–358.
3. Cohen SH, Gerding DN, Johnson S, Kelly CP, et al; Society for
Healthcare Epidemiology of America; Infectious Disease Society of
America. Clinical practice guidelines for Clostridium diffi cile infection
in adults: 2010 update by the Society for Healthcare Epidemiology
of America (SHEA) and the Infectious Diseases Society of America
(IDSA). Infect Control Hosp Epidemiol. 2010;31:431–455.
4. McMaster-Baxter NL, Musher DM. Clostridium difficile: Recent
epidemiologic fi ndings and advances in therapy. Pharmacotherapy.
2007;27:1029–1039.
5. Crogan NL, Evans BC. Clostridium diffi cile: An emerging epidemic in
nursing homes. Geriatr Nurs. 2007;28:161–164.
6. Dubberke ER, Wertheimer AI. Review of current literature on the
economic burden of Clostridium diffi cile infection. Infect Control Hosp Epi-
demiol. 2009;30:57–66.
7. Leffl er DA, Lamont JT. Treatment of Clostridium diffi cile-associated
disease. Gastroenterology. 2009;136:1899–1912.
8. Centers for Disease Control and Prevention. Information about the
current epidemic strain of Clostridium diffi cile. http://www.cdc.gov/ncidod/
dhqp/id_CdiffFAQ_newstrain.html. Accessed November 17, 2010.
9. Kelly CP, LaMont JT. Clostridium diffi cile – more diffi cult than ever.
N Engl J Med. 2008;359:1932–1940.
10. Best EL, Fawley WN, Parnell P, Wilcox MH. The potential for air-
borne dispersal of Clostridium diffi cile from symptomatic patients. Clin
Infect Dis. 2010;50:1450–1457.
11. Sunenshine RH, McDonald LC. Clostridium diffi cile-associated dis-
ease: New challenges from an established pathogen. Cleve Clin J Med.
2006;73:187–197.
12. Stanley JD, Burns RP. Clostridium diffi cile and the surgeon. Am Surg.
2010;76:235–244.
13. Efron PA, Mazuski JE. Clostridium diffi cile colitis. Surg Clin North Am.
2009;89:483–500.
14. Lacy CF, Armstrong LL, Goldman MP, Lance LL, eds. Drug Informa-
tion Handbook 2009-2010. 18th ed. Hudson, OH: Lexi-Comp Inc; 2009.
15. Shannon-Lowe J, Matheson NJ, Cooke FJ, Aliyu SH. Preven-
tion and medical management of Clostridium diffi cile infection. BMJ.
2010;340:c1296.
16. Fekety R, Silva J, Kauffman C, Buggy B, Deery HG. Treatment of
antibiotic-associated Clostridium diffi cile colitis with oral vancomycin:
Comparison of two dosage regimens. Am J Med. 1989;86:15–19.
17. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison
of vancomycin and metronidazole for the treatment of Clostridium
diffi cile-associated diarrhea, stratifi ed by disease severity. Clin Infect Dis.
2007;45:302–307.
18. Teasley DG, Gerding DN, Olson MM et al. Prospective randomised
trial of metronidazole versus vancomycin for Clostridium diffi cile-associ-
ated diarrhoea and colitis. Lancet. 1983;2:1043–1046.
19. Johnson S, Peterson LR, Gerding DN. Intravenous metronida-
zole and Clostridium diffi cile-associated diarrhea or colitis. J Infect Dis.
1989;160:1087–1088.
20. Johnson S, Homann SR, Bettin KM et al. Treatment of asymp-
tomatic Clostridium diffi cile carriers (fecal excretors) with vancomycin
or metronidazole. A randomized, placebo-controlled trial. Ann Intern
Med. 1992;117:297–302.
21. Musher DM, Logan N, Bressler AM, Johnson DP, Rossignol JF.
Nitazoxanide versus vancomycin in Clostridium diffi cile infection: a ran-
domized, double-blind study. Clin Infect Dis. 2009;48:e41–e46.

WWW.DRUGTOPICS.COM December 2010
DRUG TOPICS
39
CONTINUING EDUCATION
22. Musher DM, Logan N, Hamill RJ, et al. Nitazoxanide for the treat-
ment of Clostridium diffi cile colitis. Clin Infect Dis. 2006;43:421–427.
23. Musher DM, Logan N, Mehendiratta V et al. Clostridium diffi cile
colitis that fails conventional metronidazole therapy: Response to ni-
tazoxanide. J Antimicrob Chemother. 2007;59:705–710.
24. Dudley MN, McLaughlin JC, Carrington G, et al. Oral bacitracin vs
vancomycin therapy for Clostridium diffi cile-induced diarrhea. A random-
ized double-blind trial. Arch Intern Med. 1986;146:1101–1104.
25. Young GP, Ward PB, Bayley N, et al. Antibiotic-associated colitis
due to Clostridium diffi cile: Double-blind comparison of vancomycin with
bacitracin. Gastroenterology. 1985;89:1038–1045.
26. Johnson S, Schriever C, Patel U, et al. Rifaximin redux: Treatment of
recurrent Clostridium diffi cile infections with rifaximin immediately post-
vancomycin treatment. Anaerobe. 2009;15:290–291.
27. Johnson S, Schriever C, Galang M, Kelly CP, Gerding DN. Interrup-
tion of recurrent Clostridium diffi cile-associated diarrhea episodes by serial
therapy with vancomycin and rifaximin. Clin Infect Dis. 2007;44:846–848.
28. O’Connor JR, Galang MA, Sambol SP, et al. Rifampin and rifaximin
resistance in clinical isolates of Clostridium diffi cile. Antimicrob Agents Che-
mother. 2008;52:2813–2817.
29. Lagrotteria D, Holmes S, Smieja M, Smaill F, Lee C. Prospective,
randomized inpatient study of oral metronidazole versus oral metro-
nidazole and rifampin for treatment of primary episode of Clostridium
diffi cile-associated diarrhea. Clin Infect Dis. 2006;43:547–552.
30. Herpers BL, Vlaminckx B, Burkhardt O, et al. Intravenous tigecycline
as adjunctive or alternative therapy for severe refractory Clostridium dif-
fi cile infection. Clin Infect Dis. 2009;48:1732–1735.
31. Lu CL, Liu CY, Liao CH, et al. Severe and refractory Clostridium diffi cile
infection successfully treated with tigecycline and metronidazole. Int J
Antimicrob Agents. 2010;35:311–312.
32. Hawser SP. Activity of tigecycline against recent European clinical
isolates of Clostridium diffi cile. Int J Antimicrob Agents. 2010;35:97–98.
33. Nelson R. Antibiotic treatment for Clostridium diffi cile-associated diar-
rhea in adults. Cochrane Database Syst Rev. 2007;(3):CD004610.
34. de Lalla F, Nicolin R, Rinaldi E, et al. Prospective study of oral teico-
planin versus oral vancomycin for therapy of pseudomembranous colitis
and Clostridium diffi cile-associated diarrhea. Antimicrob Agents Chemother.
1992;36:2192–2196.
35. Wenisch C, Parschalk B, Hasenhündl M, Hirschl AM, Graninger
W. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic
acid for the treatment of Clostridium diffi cile-associated diarrhea. Clin Infect
Dis. 1996;22:813–818.
36. Wullt M, Odenholt I. A double-blind randomized controlled
trial of fusidic acid and metronidazole for treatment of an initial epi-
sode of Clostridium diffi
cile-associated diarrhea. J Antimicrob Chemother.
2004;54:211–216.
37. Sullivan KM, Spooner LM. Fidaxomicin: A macrocyclic antibiotic
for the management of Clostridium diffi cile infection. Ann Pharmacother.
2010;44:352–359.
38. Peláez T, Alcalá L, Alonso R, et al. In vitro activity of ramoplanin
against Clostridium diffi cile, including strains with reduced susceptibility
to vancomycin or with resistance to metronidazole. Antimicrob Agents
Chemother. 2005;49:1157–1159.
39. Freeman J, Baines SD, Jabes D, Wilcox MH. Comparison of the
effi cacy of ramoplanin and vancomycin in both in vitro and in vivo
models of clindamycin-induced Clostridium diffi cile infection. J Antimicrob
Chemother. 2005;56:717–725.
40. Parmar M. Treating C. diffi cile (author reply). CMAJ. 2005;172:448.
41. Mogg GA, George RH, Youngs D, et al. Randomized controlled trial
of colestipol in antibiotic-associated colitis. Br J Surg. 1982;69:137–139.
42. Baines SD, Freeman J, Wilcox MH. Tolevamer is not effi cacious in
the neutralization of cytotoxin in a human gut model of Clostridium dif-
fi cile infection. Antimicrob Agents Chemother. 2009;53:2202–2204.
43. Louie T, Gerson M, Grimard D, et al. Results of a phase III trial
comparing tolevamer, vancomycin and metronidazole in patients with
Clostridium diffi cile-associated diarrhea (CDAD) [abstract K-425a]. In:
Program and abstracts of the 47th Interscience Conference on Antimi-
crobial Agents and Chemotherapy (ICAAC). Washington, DC: American
Society for Microbiology; 2007:219.
44. Williams NT. Probiotics. Am J Health-Syst Pharm. 2010;67:449–458.
45. Segarra-Newnham M. Probiotics for Clostridium diffi cile-associated
diarrhea: Focus on Lactobacillus rhamnosus GG and Saccharomyces boulardii.
Ann Pharmacother. 2007;41:1212–1221.
46. Surawicz CM, McFarland LV, Elmer G, Chinn J. Treatment of recur-
rent Clostridium diffi cile colitis with vancomycin and Saccharomyces boular-
dii. Am J Gastroenterol. 1989;84:1285–1287.
47. Juang P, Skledar SJ, Zgheib NK, et al. Clinical outcomes of intrave-
nous immune globulin in severe Clostridium diffi cile-associated diarrhea.
Am J Infect Control. 2007;35:131–137.
48. Abougergi MS, Broor A, Cui W, Jaar BG. Intravenous immuno-
globulin for the treatment of severe Clostridium diffi cile colitis: An obser-
vational study and review of the literature. J Hosp Med. 2010;5:E1–E9.
49. Lowy I, Molrine DC, Leav BA, et al. Treatment with monoclonal an-
tibodies against Clostridium difficile toxins. N Engl J Med. 2010;362:197–205.
50. Cavagnaro C, Berezin S, Medow MS. Corticosteroid treatment of
severe, non-responsive Clostridium diffi cile-induced colitis. Arch Dis Child.
2003;88:342–344.
51. Bakken JS. Fecal bacteriotherapy for recurrent Clostridium diffi cile
infection. Anaerobe. 2009;15:285–289.
52. Aas J, Gessert CE, Bakken JS. Recurrent Clostridium diffi cile colitis:
Case series involving 18 patients treated with donor stool administered
via a nasogastric tube. Clin Infect Dis. 2003;36:580–585.
53. You DM, Franzos MA, Holman RP. Successful treatment of fulmi-
nant Clostridium diffi cile infection with fecal bacteriotherapy. Ann Intern
Med. 2008;148:632–633.
54. Sougioultzis S, Kyne L, Drudy D, et al. Clostridium diffi cile toxoid
vaccine in recurrent C. difficile-associated diarrhea. Gastroenterology.
2005;128:764–770.
55. Kotloff K, Wasserman SS, Losonsky GA, et al. Safety and immuno-
genicity of increasing doses of a Clostridium diffi cile toxoid vaccine admin-
istered to healthy adults. Infect Immun. 2001;69:988–995.
56. U.S. National Institutes of Health. Study of a Clostridium diffi cile toxoid
vaccine (ACAM-CDIFF™) in subjects with Clostridium diffi cile infection.
http://clinicaltrials.gov/ct2/show/study/NCT00772343. Accessed Sep-
tember 19, 2010.
57. Gerding D. Antimotility agents for the treatment of Clostridium diffi cile
infection: Is the juice worth the squeeze? Clin Infect Dis. 2009;48:606–608.
58. Koo HL, Koo DC, Musher DM, DuPont HL. Antimotility agents for
the treatment of Clostridium diffi cile diarrhea and colitis. Clin Infect Dis.
2009;48:598–605.

WWW.DRUGTOPICS.COM
40
DRUG TOPICS
December 2010
Continuing Education
TEST QUESTIONS
1. Clostridium dif cile (C. dif cile) is classi ed as an:
a. Aerobic, gram-negative coccus c. Anaerobic, gram-negative rod
b. Aerobic, gram-positive coccus d. Anaerobic, gram-positive rod
2. Which of the following statements is TRUE?
a. Roughly 70% of healthy adults asymptomatically carry C. dif cile in their stool.
b. C. dif cile is more likely to cause disease in neonates than in adults.
c. C. dif cile infections are decreasing in frequency and severity of disease.
d. The newly identi ed NAP1/BI/027 strain of C. dif cile is more virulent
than previously identi ed strains.
3. Which of the following statements correctly identi es the pathogenesis of CDI?
a. If normal enteric fi ora are disrupted, the spore form of the microorganism
reproduces within the intestinal crypts and releases toxins.
b. If normal enteric fi ora are disrupted, the spore form of the microorganism
reproduces within the stomach and degrades its epithelial cells.
c. If normal enteric fi ora are disrupted, the vegetative form of the
microorganism reproduces within intestinal crypts and releases toxins.
d. If normal enteric fi ora are disrupted, the vegetative form of the microorganism
reproduces within the stomach and degrades its epithelial cells.
4. Which of the following is a risk factor for the development of CDI?
a. Genitourinary procedure c. Receipt of cancer chemotherapy
b. Receipt of an antiplatelet agent d. Schizophrenia
5. Which of the following would be classi ed as having severe, complicated CDI?
a. 47-year-old woman with white blood cell (WBC) count = 15,000 and
serum creatinine increase from 1 mg/dL to 1.6 mg/dL
b. 48-year-old woman with WBC = 16,000, serum creatinine increase from
1 mg/dL to 1.5 mg/dL, and ileus
c. 50-year-old man with WBC = 16,000, serum creatinine increase from
1 mg/dL to 1.3 mg/dL, and diabetes mellitus
d. 55-year-old man with WBC = 20,000 and serum creatinine increase from
1 mg/dL to 2 mg/dL
6. What is the most commonly used test for the diagnosis of CDI?
a. Endoscopy to visualize pseudomembranes
b. Enzyme-linked immunoassay for C. dif cile toxins A and B
c. Presence of WBC in stool
d. Stool culture
7. What is the recommended length of treatment of metronidazole for a 58-year-
old man who was recently diagnosed with a mild rst-time case of CDI?
a. 5 to 7 days b. 7 to 10 days c. 10 to 14 days d. 14 to 17 days
8. A 55-year-old man recently diagnosed with CDI has a medical history that includes
alcohol and cocaine abuse. He refuses to undergo alcohol detoxi cation. His pre-
infection laboratory values include: WBC 10,000 cells/μL; serum creatinine (SCr)
0.8 mg/dL; temperature (T) 37.4° C. His laboratory values after C. dif cile include:
WBC 13,500 cells/μL; SCr 1 mg/dL; T 37.6°C. Which of the following treatment
regimens is the MOST appropriate for this patient?
a. Metronidazole 500 mg PO q 8 hours c. Rifaximin 400 mg PO q 12 hours
b. Metronidazole 500 mg IV q 8 hours d. Vancomycin 125 mg PO q 6 hours
9. After receiving antibiotics for healthcare-associated pneumonia, a 73-year-old
woman is diagnosed with a severe CDI. She has no known drug allergies and can
tolerate oral medications. Which treatment regimen would be rst-line for her?
a. Metronidazole 500 mg PO q 8 hours
b. Metronidazole 500 mg IV q 8 hours
c. Rifaximin 400 mg PO q 12 hours
d. Vancomycin 125 mg PO q 6 hours
10. A 70-year-old male patient is transferred from the medical ward to the ICU
for management of severe, complicated CDI associated with hypotension
and acute renal failure. What treatment would you recommend?
a. Metronidazole 500 mg IV q 8 hours plus metronidazole 500 mg PO every 6 hours
b. Metronidazole 500 mg IV q 8 hours plus vancomycin 500 mg PO every 6 hours
c. Rifaximin 400 mg IV q 12 hours plus vancomycin 500 mg PO every 6 hours
d. Vancomycin 1 g IV q 12 hours
11. Which of the following antibiotics has FDA approval for treatment of CDI?
a. Bacitracin b. Metronidazole c. Nitazoxanide d. Vancomycin
12. When used to treat CDI, by which route is vancomycin most commonly given?
a. Intramuscular b. Intravenous c. Oral d. Retention enema
13. Which treatment is recommended for a 30-year-old pregnant woman diagnosed
with CDI after receiving antimicrobial therapy for community-acquired pneumonia?
a. Metronidazole 250 mg PO q 6 hours
b. Metronidazole 500 mg IV q 6 hours
c. Tigecycline 100 mg IV x 1 dose, then 50 mg IV q 12 hours
d. Vancomycin 125 mg PO q 6 hours
14. Which of the following statements is CORRECT?
a. A vaccine to prevent C. dif cile is currently under development.
b. Cholestyramine may be administered at the same time as oral vancomycin.
c. Corticosteroids may be useful because they bind to disease-producing toxins.
d. Probiotics are safe for use in immunocompromised patients.
15. Which of the following agents may prolong mucosal exposure to C. dif cile’s
toxins and predispose patients to toxic megacolon?
a. Cholestyramine c. Loperamide
b. Intravenous immunoglobulin (IVIG) d. Nitazoxanide
16. When treating patients with C. diffi cile infection, a patient’s temperature
should normalize within __________ and diarrhea should resolve within
__________ of effective therapy.
a. 12 hours; 2 days b. 48 hours; 7 days c. 96 hours; 12 days d. 5 days; 17 days
17. Which adjunctive treatment may cause red-orange discoloration of body fi uids?
a. Bacitracin b. Metronidazole c. Nitazoxanide d. Rifampin
18. A 63-year-old man with a history of atrial brillation and diabetes mellitus
takes amiodarone 200 mg PO daily, glipizide 10 mg PO daily, and warfarin
2.5 mg PO daily. Following a recent diagnosis of CDI, he is prescribed oral
metronidazole therapy. Which of the drug interactions below may occur?
a. Metronidazole may decrease amiodarone levels and increase arrhythmia risk.
b. Metronidazole may decrease warfarin levels and increase risk of clot formation.
c. Metronidazole may increase warfarin levels and increase risk of bleeding.
d. Metronidazole does not interact with any of his medications.
19. Which of the following strategies has NOT been shown to control CDI effectively?
a. Antimicrobial stewardship programs
b. Cleaning rectal thermometers with alcohol
c. Isolation precautions
d. Restriction of high-risk antimicrobials
20. Which of the following will reduce the risk of transmission of C. dif cile in a
healthcare facility?
a. The use of alcohol-based hand rubs
b. The use of quaternary ammonium-based agents for cleaning
c. The use of rectal thermometers
d. The use of soap and water to wash hands
Write your answers on the form appearing on page 41 (photocopies of the answer form
are acceptable) or on a separate sheet of paper. Mark the most appropriate answer.
UPDATE: C. DIFFICILE
WWW.DRUGTOPICS.COM December 2010
DRUG TOPICS
41
ANSWER FORM
Are you employed by a chain? If so, which one?
EVALUATION OF CE
CONTINUING EDUCATION
ACPE # 0012-9999-10-106-H01-P (K)
C. Difficile CE Quiz Answers December 2010 Test questions start on page 40
1. a. b. c. d. 5. a. b. c. d. 9. a. b. c. d. 13. a. b. c. d. 17. a. b. c. d.
2. a. b. c. d. 6. a. b. c. d. 10. a. b. c. d. 14. a. b. c. d. 18. a. b. c. d.
3. a. b. c. d. 7. a. b. c. d. 11. a. b. c. d. 15. a. b. c. d. 19. a. b. c. d.
4. a. b. c. d. 8. a. b. c. d. 12. a. b. c. d. 16. a. b. c. d. 20. a. b. c. d.
Not valid for CE credit after 12/31/2012
REGISTRANT INFORMATION
Name:
(Last) (First) (M.I.) Phone
Address: E-mail address:
(Street)
City: State: ZIP:
Florida pharmacists: Please provide your license number
Amount enclosed: □ $6.00 for this lesson
□ $54.00 for any 12 lessons you take
over the next year, starting from the
date you sign up
□ Already series-enrolled for 2010
Submit your check (payable to The University of Florida) and form to:
University of Florida College of Pharmacy
P.O. Box 113195, Gainesville, FL 32611-3195
E-mail address: continuinged@cop.ufl.edu
Fees not refundable or transferable
For questions concerning PRINT CEs, call 352-273-6275.
For questions concerning ONLINE CEs, call 877-252-7711.
2010 CEU CREDIT REQUEST
To obtain immediate CE credit, take the test online at www.drugtopics.com. Just click on the “Continuing Education” box on the
Drug Topics
home page, which will take you to the CE site. Log in, find and click on this lesson, and follow the three simple steps. Test results will be displayed
immediately and you can print the certificate showing your earned CE credits. The cost of the online test for this lesson is $6.
Strongly Agree Agree Disagree Strongly Disagree
1. The objectives listed on page 31 were met.
If not, please describe:
2. The author wrote an article of value.
3. The information was clear and presented well.
4. The learning activity (quiz) was effective.
5. The article content was useful and relevant.
6. The article was educational and not promotional.
Drug Topics is conducting an evaluation of this CE article. Please check the box that best refl ects your
opinion of the evaluation questions. Please keep this evaluation attached to your answer form.
WWW.DRUGTOPICS.COM
42
DRUG TOPICS
December 2010
Regulatory & Legal
A
compounding pharmacy in
Florida, Franck’s Lab, Inc.,
which engages in veterinary
compounding, has been embroiled in
a struggle with the FDA since it com-
pounded a vitamin supplement for ad-
ministration to 21 polo ponies during
the U.S. Open Polo Championships in
April 2009. When they were injected
with the compounded vitamin supple-
ment, all 21 ponies collapsed and died.
As a result, FDA sought to obtain
a preliminary injunction against the
pharmacy to prevent it from engaging
in further veterinary compounding.
FDA makes its case
The agency argued that the pharmacy was
violating a compliance policy guidance
issued by FDA in 2003 for purposes of
clarifying its interpretation of the Animal
Medicinal Drug Use Clarifi cation Act. FDA
asserted that any “compounding from
bulk substances,” such as the Florida phar-
macy had done, “or unapproved drugs
renders the compounded drugs unsafe
as a matter of law, and thus adulterated
in violation of 21 USC § 351(a)(5).” FDA
argued that the bulk drugs constitute new
drugs and therefore require FDA regula-
tory approvals before they may be intro-
duced in interstate commerce.
The pharmacy responds
The pharmacy responded by asserting that
FDA has no authority to ban veterinary
drug compounding from bulk product.
In its defense, the pharmacy stated that
the “use of bulk ingredients to compound
commercially unavailable preparations is a
core part of the traditional pharmacy prac-
tice” which is left for purposes of oversight
to the individual states and not to the fed-
eral government and FDA.
The pharmacy did not dispute that
FDA may have regulatory authority over
the manufacturing of veterinary pharma-
ceuticals if it can show that the pharmacy
is truly engaged in the “manufacturing”
process. But, the pharmacy pointed out,
FDA is not permitted “to override state
law and impose a blanket ban on tradi-
tional pharmacy compounding practices.”
The pharmacy also noted that the leg-
islative history of the Federal Food, Drug,
and Cosmetic Act (FDCA) proves that
Congress intentionally left regulation of
compounding to state regulatory oversight
as part of pharmacy practice, while pre-
serving regulatory oversight of manufac-
turing as a task of the federal government.
Finally, the defendant pharmacy
stated that even if the FDCA provided
FDA with the right to regulate veteri-
nary compounding, FDA would need
to promulgate regulations through for-
mal rule-making procedures that would
include a notice-and-comment period,
rather than through the issuance of an
informal compliance policy guide.
Motion denied
In summary, the judge denied FDA’s mo-
tion for preliminary injunction without
issuing an opinion. However, the court is
currently reviewing the request for per-
manent injunction.
This case illustrates the important
point that legal tension arises when phar-
macy compounding is alleged by FDA to
reach the level of manufacturing. FDA
regulates manufacturing, while state laws
and regulations govern pharmacy com-
pounding. If FDA is able to demonstrate
that a compounding pharmacy was in
fact engaged in manufacturing, then it
will be able to exercise its authority over
the pharmacy, and state regulatory over-
sight would not apply.
Other cases have analyzed this matrix,
and this body of law continues to evolve
as different parties and efforts — including
by the federal government through the
FDA; by state governments and boards
of pharmacy; and through the various
pharmacy practice acts — attempt to draw
the fi ne line whereby compounding and
manufacturing are defi ned.
These articles are not intended as legal ad-
vice and should not be used as such. When a
legal question arises, the pharmacist should
consult an attorney familiar with pharmacy
law in his or her state.
Ned Milenkovich is a member at McDonald
Hopkins, LLC, and chairs its Drug &
Pharmacy Practice Group. He is also a
member of the Illinois State Board of
Pharmacy. Contact him at 312-642-1480 or
at nmilenkovich@mcdonaldhopkins.com.
LEGAL COMPLIANCE
FDA loses preliminary injunction case
against vet-compounding pharmacy
Ned Milenkovich, PharmD, JD
If FDA can demonstrate
that a compounding
pharmacy was in
fact engaged in
manufacturing, then it
will be able to exercise
its authority over the
pharmacy, and state
regulatory oversight
would not apply.

Visit the LEXAPRO website at www.lexapro.com
See the effect of LEXAPRO
Proven effi cacy in MDD in adolescents aged
12 to 17, and in MDD and GAD in adults1-5
In adolescents aged 12 to 17 with
Major Depressive Disorder (MDD)*1
In adults with MDD and Generalized
Anxiety Disorder (GAD)1
WARNING: SUICIDALITY AND ANTIDEPRESSANT DRUGS
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and
young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of
Lexapro or any other antidepressant in a child, adolescent or young adult must balance this risk with the clinical need. Short-term
studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was
a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric
disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy
should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and
caregivers should be advised of the need for close observation and communication with the prescriber. Lexapro is not approved for use
in pediatric patients less than 12 years of age.
Contraindications
• Lexapro is contraindicated in patients taking monoamine oxidase inhibitors (MAOIs). There have been reports of serious, sometimes
fatal, reactions with some cases resembling neuroleptic malignant syndrome (NMS) and serotonin syndrome. Features may include
hyperthermia, rigidity, myoclonus, autonomic instability with possible rapid fl uctuations of vital signs, and mental status changes
that include extreme agitation progressing to delirium and coma. These reactions have also been reported in patients who have
recently discontinued SSRI treatment and have been started on an MAOI. Serotonin
syndrome was reported for two patients who were concomitantly receiving linezolid,
an antibiotic which has MAOI activity. Lexapro should not be used in combination with
an MAOI or within 14 days of discontinuing an MAOI. MAOIs should not be initiated within
14 days of discontinuing Lexapro.
• Lexapro is contraindicated in patients taking pimozide
or with hypersensitivity to escitalopram or citalopram.
Please see additional Important Safety Information on adjacent page.
* LEXAPRO is indicated as an integral part of a total treatment program for MDD.
Drug treatment may not be indicated for all adolescents with this syndrome.
LEXAPRO and citalopram are not the same1,6,7
• LEXAPRO is approved for MDD and GAD1;
citalopram is not approved for GAD.6
• LEXAPRO is approved for MDD in adolescents
aged 12 to 17 years1; citalopram is not.6
There is no generic available for LEXAPRO

Please see accompanying brief summary of Prescribing Information
for LEXAPRO, including Boxed Warning.
Lexapro (escitalopram oxalate) is indicated for the acute and maintenance treatment of major depressive disorder (MDD) in adults
and adolescents aged 12-17 years. Lexapro is also indicated for the acute treatment of generalized anxiety disorder (GAD) in adults.
IMPORTANT SAFETY INFORMATION
Warnings and Precautions
• All patients treated with antidepressants should be monitored appropriately and observed closely for clinical worsening,
suicidality and unusual changes in behavior, especially within the fi rst few months of treatment or when changing the dose.
Consideration should be given to changing the therapeutic regimen, including discontinuing medication, in patients whose
depression is persistently worse, who are experiencing emergent suicidality or symptoms that might be precursors to worsening
depression or suicidality, especially if these symptoms are severe, abrupt in onset, or were not part of the patient’s presenting
symptoms. Families and caregivers of patients treated with antidepressants should be alerted about the need to monitor patients
daily for the emergence of agitation, irritability, unusual changes in behavior, or the emergence of suicidality, and report such
symptoms immediately. Prescriptions for Lexapro should be written for the smallest quantity of tablets, consistent with good
patient management, in order to reduce the risk of overdose.
• A major depressive episode may be the initial presentation of bipolar disorder. In patients at risk for bipolar disorder, treating
such an episode with an antidepressant alone may increase the likelihood of precipitating a mixed/manic episode. Prior to
initiating treatment with an antidepressant, patients should be adequately screened to determine if they are at risk for bipolar
disorder. Lexapro should be used cautiously in patients with a history of mania or seizure disorder. Lexapro is not approved for
use in treating bipolar depression.
• The concomitant use of Lexapro with other SSRIs, SNRIs, triptans, tryptophan, antipsychotics or other dopamine antagonists
is not recommended due to potential development of life-threatening serotonin syndrome or neuroleptic malignant syndrome
(NMS)-like reactions. Reactions have been reported with SNRIs and SSRIs alone, including Lexapro, but particularly with drugs
that impair metabolism of serotonin (including MAOIs). Management of these events should include immediate discontinuation
of Lexapro and the concomitant agent and continued monitoring.
• Patients should be monitored for adverse reactions when discontinuing treatment with Lexapro. During marketing of Lexapro and
other SSRIs and SNRIs, there have been spontaneous reports of adverse events occurring upon discontinuation, including dysphoric
mood, irritability, agitation, dizziness, sensory disturbances (e.g., paresthesias), anxiety, confusion, headache, lethargy, emotional
lability, insomnia and hypomania. A gradual dose reduction rather than abrupt cessation is recommended whenever possible.
• SSRIs and SNRIs have been associated with clinically signifi cant hyponatremia. Elderly patients and patients taking diuretics or
who are otherwise volume-depleted appear to be at a greater risk. Discontinuation of Lexapro should be considered in patients
with symptomatic hyponatremia and appropriate medical intervention should be instituted.
• SSRIs (including Lexapro) and SNRIs may increase the risk of bleeding. Patients should be cautioned that concomitant use of
aspirin, NSAIDs, warfarin or other anticoagulants may add to the risk.
• Patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain
that Lexapro does not affect their ability to engage in such activities.
• Lexapro should be used with caution in patients with severe renal impairment or with diseases or conditions that alter metabolism
or hemodynamic responses. In subjects with hepatic impairment, clearance of racemic citalopram was decreased and plasma
concentrations were increased. The recommended dose of Lexapro in hepatically impaired patients is 10 mg/day.
• For pregnant or nursing mothers, Lexapro should be used only if the potential benefi t justifi es the potential risk to the fetus or child.
Adverse Reactions
• In clinical trials of MDD, the most common adverse reactions in adults treated with Lexapro (approximately 5% or greater
and at least twice the incidence of placebo) were nausea (15% vs 7%), insomnia (9% vs 4%), ejaculation disorder (9%
vs <1%), fatigue (5% vs 2%), somnolence (6% vs 2%), and increased sweating (5% vs 2%). In pediatric patients, the overall
profi le of adverse reactions was similar to that seen in adults; however, the following additional adverse reactions were
reported at an incidence of at least 2% for Lexapro and greater than placebo: back pain, urinary tract infection, vomiting, and
nasal congestion.
• In clinical trials of GAD, the most common adverse reactions in adults treated with Lexapro (approximately 5% or greater and at
least twice the incidence of placebo) were nausea (18% vs 8%), ejaculation disorder (14% vs 2%), insomnia (12% vs 6%), fatigue
(8% vs 2%), decreased libido (7% vs 2%) and anorgasmia (6% vs <1%).
© 2010 Forest Laboratories, Inc. Printed in U.S.A. 41-1017601r 5/10 Visit the LEXAPRO website at www.lexapro.com
There is no generic available
for LEXAPRO
References: 1. LEXAPRO [package insert]. St. Louis, Mo: Forest Pharmaceuticals, Inc.; 2009. 2. Emslie GJ, Ventura D, Korotzer A,
Tourkodimitris S. Escitalopram in the treatment of adolescent depression: a randomized placebo-controlled multisite trial.
J Am Acad Child Adolesc Psychiatry. 2009;48:721-729. 3. Burke WJ, Gergel I, Bose A. Fixed-dose trial of the single isomer SSRI
escitalopram in depressed outpatients. J Clin Psychiatry. 2002;63:331-336. 4. Davidson JRT, Bose A, Korotzer A, Zheng H. Escitalopram
in the treatment of generalized anxiety disorder: double-blind, placebo controlled, fl exible-dose study. Depress Anxiety. 2004;19:23 4-
240. 5. Wade A, Lemming OM, Hedegaard KB. Escitalopram 10 mg/day is effective and well tolerated in a placebo-controlled
study in depression in primary care. Int Clin Psychopharmacol. 2002;17:95-102. 6. Celexa [package insert]. St. Louis, Mo: Forest
Pharmaceuticals, Inc.; 2009. 7. Mork A, Kreilgaard M, Sanchez C. The R-enantiomer of citalopram counteracts escitalopram-induced
increase in extracellular 5-HT in the frontal cortex of freely moving rats. Neuropharmacology. 2003;45:167-173.
LEXAPRO®(escitalopram oxalate) TABLETS/ORAL SOLUTION Rx Only
Brief Summary: For complete details, please see full Prescribing Information for Lexapro.
WARNINGS: SUICIDALITY AND ANTIDEPRESSANT DRUGS
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and
young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of
Lexapro or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term
studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was
a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric
disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on anti depressant
therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior.
Families and caregivers should be advised of the need for close observation and communication with the prescriber. Lexapro is not
approved for use in pediatric patients less than 12 years of age.
[See Warnings and Precautions: Clinical Worsening and Suicide Risk,
Patient Counseling Information: Information for Patients, and Used in Specific Populations: Pediatric Use].
INDICATIONS AND USAGE: Major Depressive Disorder-Lexapro (escitalopram) is indicated for the acute and maintenance treatment of major
depressive disorder in adults and in adolescents 12 to 17 years of age [
see Clinical Studies
]. A major depressive episode (DSM-IV) implies a
prominent and relatively persistent (nearly every day for at least 2 weeks) depressed or dysphoric mood that usually interferes with daily func-
tioning, and includes at least five of the following nine symptoms: depressed mood, loss of interest in usual activities, significant change in weight
and/or appetite, insomnia or hypersomnia, psychomotor agitation or retardation, increased fatigue, feelings of guilt or worthlessness, slowed
thinking or impaired concentration, a suicide attempt or suicidal ideation. Generalized Anxiety Disorder-Lexapro is indicated for the acute
treatment of Generalized Anxiety Disorder (GAD) in adults [
see Clinical Studies
]. Generalized Anxiety Disorder (DSM-IV) is characterized by
excessive anxiety and worry (apprehensive expectation) that is persistent for at least 6 months and which the person finds difficult to control. It
must be associated with at least 3 of the following symptoms: restlessness or feeling keyed up or on edge, being easily fatigued, difficulty
concentrating or mind going blank, irritability, muscle tension, and sleep disturbance.
CONTRAINDICATIONS: Monoamine oxidase inhibitors (MAOIs)-Concomitant use in patients taking monoamine oxidase inhibitors (MAOIs) is
contraindicated [
see Warnings and Precautions
]. Pimozide-Concomitant use in patients taking pimozide is contraindicated [
see Drug
Interactions
]. Hypersensitivity to escitalopram or citalopram-Lexapro is contraindicated in patients with a hypersensitivity to escitalopram or
citalopram or any of the inactive ingredients in Lexapro.
WARNINGS AND PRECAUTIONS: Clinical Worsening and Suicide Risk-Patients with major depressive disorder (MDD), both adult and pedi-
atric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in
behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a
known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There
has been a long-standing concern, however, that antidepressants may have a role in inducing worsening of depression and the emergence of
suicidality in certain patients during the early phases of treatment. Pooled analyses of short-term placebo-controlled trials of antidepressant
drugs (SSRIs and others) showed that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and
young adults (ages 18-24) with major depressive disorder (MDD) and other psychiatric disorders. Short-term studies did not show an increase
in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants com-
pared to placebo in adults aged 65 and older. The pooled analyses of placebo-controlled trials in children and adolescents with MDD, obsessive
compulsive disorder (OCD), or other psychiatric disorders included a total of 24 short-term trials of 9 antidepressant drugs in over 4400 patients.
The pooled analyses of placebo-controlled trials in adults with MDD or other psychiatric disorders included a total of 295 short-term trials
(median duration of 2 months) of 11 antidepressant drugs in over 77,000 patients. There was considerable variation in risk of suicidality among
drugs, but a tendency toward an increase in the younger patients for almost all drugs studied. There were differences in absolute risk of
suicidality across the different indications, with the highest incidence in MDD. The risk differences (drug vs. placebo), however, were relatively
stable within age strata and across indications. These risk differences (drug-placebo difference in the number of cases of suicidality per
1000 patients treated) are provided in Table 1.
TABLE 1
Age Range Drug-Placebo Difference in Number of Cases
of Suicidality per 1000 Patients Treated
Increases Compared to Placebo
<18 14 additional cases
18-24 5 additional cases
Decreases Compared to Placebo
25-64 1 fewer case
65 6 fewer cases
No suicides occurred in any of the pediatric trials. There were suicides in the adult trials, but the number was not sufficient to reach any conclu-
sion about drug effect on suicide. It is unknown whether the suicidality risk extends to longer-term use, i.e., beyond several months. However,
there is substantial evidence from placebo-controlled maintenance trials in adults with depression that the use of antidepressants can delay the
recurrence of depression. All patients being treated with antidepressants for any indication should be monitored appropriately and observed
closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy,
or at times of dose changes, either increases or decreases. The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hos-
tility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania, have been reported in adult and pediatric
patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric.
Although a causal link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal
impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality. Consideration should
be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients whose depression is persistently
worse, or who are experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, especially
if these symptoms are severe, abrupt in onset, or were not part of the patient’s presenting symptoms. If the decision has been made to discon-
tinue treatment, medication should be tapered, as rapidly as is feasible, but with recognition that abrupt discontinuation can be associated with
certain symptoms [
see Dosage and Administration
]
.
Families and caregivers of patients being treated with antidepressants for major depressive
disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of
agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report
such symptoms immediately to health care providers. Such monitoring should include daily observation by families and caregivers [
see also
Patient Counseling Information
]
.
Prescriptions for Lexapro should be written for the smallest quantity of tablets consistent with good patient
management, in order to reduce the risk of overdose. Screening Patients for Bipolar Disorder-A major depressive episode may be the initial
presentation of bipolar disorder. It is generally believed (though not established in controlled trials) that treating such an episode with an anti -
depressant alone may increase the likelihood of precipitation of a mixed/manic episode in patients at risk for bipolar disorder. Whether any of
the symptoms described above represent such a conversion is unknown. However, prior to initiating treatment with an antidepressant, patients
with depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a
detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression. It should be noted that Lexapro is not approved
for use in treating bipolar depression. Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-like Reactions-The development of a
potentially life-threatening serotonin syndrome or Neuroleptic Malignant Syndrome (NMS)-like reactions have been reported with SNRIs and
SSRIs alone, including Lexapro treatment, but particularly with concomitant use of serotonergic drugs (including triptans) with drugs which
impair metabolism of serotonin (including MAOIs), or with antipsychotics or other dopamine antagonists. Serotonin syndrome symptoms may
include mental status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyper -
thermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination) and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea).
Serotonin syndrome, in its most severe form can resemble neuroleptic malignant syndrome, which includes hyperthermia, muscle rigidity, auto-
nomic instability with possible rapid fluctuation of vital signs, and mental status changes. Patients should be monitored for the emergence of
serotonin syndrome or NMS-like signs and symptoms. The concomitant use of Lexapro with MAOIs intended to treat depression is contraindi-
cated. If concomitant treatment of Lexapro with a 5-hydroxytryptamine receptor agonist (triptan) is clinically warranted, careful observation of
the patient is advised, particularly during treatment initiation and dose increases. The concomitant use of Lexapro with serotonin precursors
(such as tryptophan) is not recommended. Treatment with Lexapro and any concomitant serotonergic or antidopaminergic agents, including
antipsychotics, should be discontinued immediately if the above events occur and supportive symptomatic treatment should be initiated.
Discontinuation of Treatment with Lexapro-During marketing of Lexapro and other SSRIs and SNRIs (serotonin and norepinephrine reuptake
inhibitors), there have been spontaneous reports of adverse events occurring upon discontinuation of these drugs, particularly when abrupt,
including the following: dysphoric mood, irritability, agitation, dizziness, sensory disturbances (e.g., paresthesias such as electric shock sensa-
tions), anxiety, confusion, headache, lethargy, emotional lability, insomnia, and hypomania. While these events are generally self-limiting, there
have been reports of serious discontinuation symptoms. Patients should be monitored for these symptoms when discontinuing treatment with
Lexapro. A gradual reduction in the dose rather than abrupt cessation is recommended whenever possible. If intolerable symptoms occur
following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered.
Subsequently, the physician may continue decreasing the dose but at a more gradual rate [
see Dosage and Administration
]. Seizures-Although
anticonvulsant effects of racemic citalopram have been observed in animal studies, Lexapro has not been systematically evaluated in patients
with a seizure disorder. These patients were excluded from clinical studies during the product’s premarketing testing. In clinical trials of Lexapro,
cases of convulsion have been reported in association with Lexapro treatment. Like other drugs effective in the treatment of major depressive
disorder, Lexapro should be introduced with care in patients with a history of seizure disorder. Activation of Mania/Hypomania-In placebo-
controlled trials of Lexapro in major depressive disorder, activation of mania/hypomania was reported in one (0.1%) of 715 patients treated with
Lexapro and in none of the 592 patients treated with placebo. One additional case of hypomania has been reported in association with Lexapro
treatment. Activation of mania/hypomania has also been reported in a small proportion of patients with major affective disorders treated with
racemic citalopram and other marketed drugs effective in the treatment of major depressive disorder. As with all drugs effective in the treatment
of major depressive disorder, Lexapro should be used cautiously in patients with a history of mania. Hyponatremia-Hyponatremia may occur as
a result of treatment with SSRIs and SNRIs, including Lexapro. In many cases, this hyponatremia appears to be the result of the syndrome of
inappropriate antidiuretic hormone secretion (SIADH), and was reversible when Lexapro was discontinued. Cases with serum sodium lower than
110 mmol/L have been reported. Elderly patients may be at greater risk of developing hyponatremia with SSRIs and SNRIs. Also, patients tak-
ing diuretics or who are otherwise volume depleted may be at greater risk [
see Geriatric Use
]. Discontinuation of Lexapro should be considered
in patients with symptomatic hyponatremia and appropriate medical intervention should be instituted. Signs and symptoms of hyponatremia
include headache, difficulty concentrating, memory impairment, confusion, weakness, and unsteadiness, which may lead to falls. Signs and
symptoms associated with more severe and/or acute cases have included hallucination, syncope, seizure, coma, respiratory arrest, and death.
Abnormal Bleeding-SSRIs and SNRIs, including Lexapro, may increase the risk of bleeding events. Concomitant use of aspirin, nonsteroidal
anti-inflammatory drugs, warfarin, and other anticoagulants may add to the risk. Case reports and epidemiological studies (case-control and
cohort design) have demonstrated an association between use of drugs that interfere with serotonin reuptake and the occurrence of gastroin-
testinal bleeding. Bleeding events related to SSRIs and SNRIs use have ranged from ecchymoses, hematomas, epistaxis, and petechiae to
life-threatening hemorrhages. Patients should be cautioned about the risk of bleeding associated with the concomitant use of Lexapro and
NSAIDs, aspirin, or other drugs that affect coagulation. Interference with Cognitive and Motor Performance-In a study in normal volunteers,
Lexapro 10 mg/day did not produce impairment of intellectual function or psychomotor performance. Because any psychoactive drug may impair
judgment, thinking, or motor skills, however, patients should be cautioned about operating hazardous machinery, including automobiles, until
they are reasonably certain that Lexapro therapy does not affect their ability to engage in such activities. Use in Patients with Concomitant
Illness-Clinical experience with Lexapro in patients with certain concomitant systemic illnesses is limited. Caution is advisable in using Lexapro
in patients with diseases or conditions that produce altered metabolism or hemodynamic responses. Lexapro has not been systematically
evaluated in patients with a recent history of myocardial infarction or unstable heart disease. Patients with these diagnoses were generally exclud-
ed from clinical studies during the product’s premarketing testing. In subjects with hepatic impairment, clearance of racemic citalopram was
decreased and plasma concentrations were increased. The recommended dose of Lexapro in hepatically impaired patients is 10 mg/day [
see
Dosage and Administration
]. Because escitalopram is extensively metabolized, excretion of unchanged drug in urine is a minor route of elimina-
tion. Until adequate numbers of patients with severe renal impairment have been evaluated during chronic treatment with Lexapro, however, it
should be used with caution in such patients [
see Dosage and Administration
]. Potential for Interaction with Monoamine Oxidase Inhibitors-
In patients receiving serotonin reuptake inhibitor drugs in combination with a monoamine oxidase inhibitor (MAOI), there have been reports of
serious, sometimes fatal, reactions including hyperthermia, rigidity, myoclonus, autonomic instability with possible rapid fluctuations of vital
signs, and mental status changes that include extreme agitation progressing to delirium and coma. These reactions have also been reported in
patients who have recently discontinued SSRI treatment and have been started on an MAOI. Some cases presented with features resembling
neuroleptic malignant syndrome. Furthermore, limited animal data on the effects of combined use of SSRIs and MAOIs suggest that these drugs
may act synergistically to elevate blood pressure and evoke behavioral excitation. Therefore, it is recommended that Lexapro should not be used
in combination with an MAOI, or within 14 days of discontinuing treatment with an MAOI. Similarly, at least 14 days should be allowed after
stopping Lexapro before starting an MAOI. Serotonin syndrome has been reported in two patients who were concomitantly receiving linezolid,
an antibiotic which is a reversible non-selective MAOI.
ADVERSE REACTIONS: Clinical Trials Experience-Because clinical studies are conducted under widely varying conditions, adverse reaction
rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect
the rates observed in practice. Clinical Trial Data Sources; Pediatrics (6 -17 years)-Adverse events were collected in 576 pediatric patients
(286 Lexapro, 290 placebo) with major depressive disorder in double-blind placebo-controlled studies. Safety and effectiveness of Lexapro in
pediatric patients less than 12 years of age has not been established. Adults-Adverse events information for Lexapro was collected from 715
patients with major depressive disorder who were exposed to escitalopram and from 592 patients who were exposed to placebo in double-blind,
placebo-controlled trials. An additional 284 patients with major depressive disorder were newly exposed to escitalopram in open-label trials. The
adverse event information for Lexapro in patients with GAD was collected from 429 patients exposed to escitalopram and from 427 patients
exposed to placebo in double-blind, placebo-controlled trials. Adverse events during exposure were obtained primarily by general inquiry and
recorded by clinical investigators using terminology of their own choosing. Consequently, it is not possible to provide a meaningful estimate of
the proportion of individuals experiencing adverse events without first grouping similar types of events into a smaller number of standardized
event categories. In the tables and tabulations that follow, standard World Health Organization (WHO) terminology has been used to classify
reported adverse events. The stated frequencies of adverse reactions represent the proportion of individuals who experienced, at least once, a
treatment-emergent adverse event of the type listed. An event was considered treatment-emergent if it occurred for the first time or worsened
while receiving therapy following baseline evaluation. Adverse Events Associated with Discontinuation of Treatment; Major Depressive
Disorder; Pediatrics (6 -17 years)-Adverse events were associated with discontinuation of 3.5% of 286 patients receiving Lexapro and 1% of
290 patients receiving placebo. The most common adverse event (incidence at least 1% for Lexapro and greater than placebo) associated with
discontinuation was insomnia (1% Lexapro, 0% placebo). Adults-Among the 715 depressed patients who received Lexapro in placebo-controlled
trials, 6% discontinued treatment due to an adverse event, as compared to 2% of 592 patients receiving placebo. In two fixed-dose studies,
the rate of discontinuation for adverse events in patients receiving 10 mg/day Lexapro was not significantly different from the rate of discon -
tinuation for adverse events in patients receiving placebo. The rate of discontinuation for adverse events in patients assigned to a fixed dose of
20 mg/day Lexapro was 10%, which was significantly different from the rate of discontinuation for adverse events in patients receiving 10 mg/day
Lexapro (4%) and placebo (3%). Adverse events that were associated with the discontinuation of at least 1% of patients treated with Lexapro,
and for which the rate was at least twice that of placebo, were nausea (2%) and ejaculation disorder (2% of male patients). Generalized Anxiety
Disorder; Adults-Among the 429 GAD patients who received Lexapro 10-20 mg/day in placebo-controlled trials, 8% discontinued treatment due
to an adverse event, as compared to 4% of 427 patients receiving placebo. Adverse events that were associated with the discontinuation of at
least 1% of patients treated with Lexapro, and for which the rate was at least twice the placebo rate, were nausea (2%), insomnia (1%), and
fatigue (1%). Incidence of Adverse Reactions in Placebo-Controlled Clinical Trials; Major Depressive Disorder; Pediatrics (6 -17 years)-The
overall profile of adverse reactions in pediatric patients was generally similar to that seen in adult studies, as shown in Table 2. However, the
following adverse reactions (excluding those which appear in Table 2 and those for which the coded terms were uninformative or misleading)
were reported at an incidence of at least 2% for Lexapro and greater than placebo: back pain, urinary tract infection, vomiting, and nasal
congestion. Adults-The most commonly observed adverse reactions in Lexapro patients (incidence of approximately 5% or greater and approx-
imately twice the incidence in placebo patients) were insomnia, ejaculation disorder (primarily ejaculatory delay), nausea, sweating increased,
fatigue, and somnolence. Table 2 enumerates the incidence, rounded to the nearest percent, of treatment-emergent adverse events that occurred
among 715 depressed patients who received Lexapro at doses ranging from 10 to 20 mg/day in placebo-controlled trials. Events included are
those occurring in 2% or more of patients treated with Lexapro and for which the incidence in patients treated with Lexapro was greater than
the incidence in placebo-treated patients.
TABLE 2
Treatment-Emergent Adverse Reactions Observed with a Frequency of 2%
and Greater Than Placebo for Major Depressive Disorder
Adverse Reaction Lexapro Placebo
(N=715) (N=592)
Autonomic Nervous System Disorders
Dry Mouth 6% 5%
Sweating Increased 5% 2%
Central & Peripheral Nervous System Disorders
Dizziness 5% 3%
Gastrointestinal Disorders
Nausea 15% 7%
Diarrhea 8% 5%
Constipation 3% 1%
Indigestion 3% 1%
Abdominal Pain 2% 1%
General
Influenza-like Symptoms 5% 4%
Fatigue 5% 2%
Psychiatric Disorders
Insomnia 9% 4%
Somnolence 6% 2%
Appetite Decreased 3% 1%
Libido Decreased 3% 1%
Respiratory System Disorders
Rhinitis 5% 4%
Sinusitis 3% 2%
Urogenital
Ejaculation Disorder1,2 9% <1%
Impotence23% <1%
Anorgasmia32% <1%
1Primarily ejaculatory delay.
2Denominator used was for males only (N=225 Lexapro; N=188 placebo).
3Denominator used was for females only (N=490 Lexapro; N=404 placebo).
Generalized Anxiety Disorder; Adults-The most commonly observed adverse reactions in Lexapro patients (incidence of approximately 5% or
greater and approximately twice the incidence in placebo patients) were nausea, ejaculation disorder (primarily ejaculatory delay), insomnia,
fatigue, decreased libido, and anorgasmia. Table 3 enumerates the incidence, rounded to the nearest percent of treatment-emergent adverse
events that occurred among 429 GAD patients who received Lexapro 10 to 20 mg/day in placebo-controlled trials. Events included are those
occurring in 2% or more of patients treated with Lexapro and for which the incidence in patients treated with Lexapro was greater than the
incidence in placebo-treated patients.
TABLE 3
Treatment-Emergent Adverse Reactions Observed with a Frequency of 2%
and Greater Than Placebo for Generalized Anxiety Disorder
Adverse Reactions Lexapro Placebo
(N=429) (N=427)
Autonomic Nervous System Disorders
Dry Mouth 9% 5%
Sweating Increased 4% 1%
Central & Peripheral Nervous System Disorders
Headache 24% 17%
Paresthesia 2% 1%
Gastrointestinal Disorders
Nausea 18% 8%
Diarrhea 8% 6%
Constipation 5% 4%
Indigestion 3% 2%
Vomiting 3% 1%
Abdominal Pain 2% 1%
Flatulence 2% 1%
Toothache 2% 0%
General
Fatigue 8% 2%
Influenza-like Symptoms 5% 4%
Musculoskeletal System Disorder
Neck/Shoulder Pain 3% 1%
Psychiatric Disorders
Somnolence 13% 7%
Insomnia 12% 6%
Libido Decreased 7% 2%
Dreaming Abnormal 3% 2%
Appetite Decreased 3% 1%
Lethargy 3% 1%
TABLE 3
Treatment-Emergent Adverse Reactions Observed with a Frequency of 2%
and Greater Than Placebo for Generalized Anxiety Disorder (continued)
Adverse Reactions Lexapro Placebo
(N=429) (N=427)
Respiratory System Disorders
Yawning 2% 1%
Urogenital
Ejaculation Disorder
1,2
14% 2%
Anorgasmia
3
6% <1%
Menstrual Disorder 2% 1%
1
Primarily ejaculatory delay.
2
Denominator used was for males only (N=182 Lexapro; N=195 placebo).
3
Denominator used was for females only (N=247 Lexapro; N=232 placebo).
Dose Dependency of Adverse Reactions
-The potential dose dependency of common adverse reactions (defined as an incidence rate of 5% in
either the 10 mg or 20 mg Lexapro groups) was examined on the basis of the combined incidence of adverse events in two fixed-dose trials.
The overall incidence rates of adverse events in 10 mg Lexapro-treated patients (66%) was similar to that of the placebo-treated patients (61%),
while the incidence rate in 20 mg/day Lexapro-treated patients was greater (86%). Table 4 shows common adverse reactions that occurred in
the 20 mg/day Lexapro group with an incidence that was approximately twice that of the 10 mg/day Lexapro group and approximately twice that
of the placebo group.
TABLE 4
Incidence of Common Adverse Reactions in Patients with Major
Depressive Disorder
Adverse Reaction Placebo 10 mg/day 20 mg/day
(N=311) Lexapro Lexapro
(N=310) (N=125)
Insomnia 4% 7% 14%
Diarrhea 5% 6% 14%
Dry Mouth 3% 4% 9%
Somnolence 1% 4% 9%
Dizziness 2% 4% 7%
Sweating Increased <1% 3% 8%
Constipation 1% 3% 6%
Fatigue 2% 2% 6%
Indigestion 1% 2% 6%
Male and Female Sexual Dysfunction with SSRIs
-Although changes in sexual desire, sexual performance, and sexual satisfaction often occur
as manifestations of a psychiatric disorder, they may also be a consequence of pharmacologic treatment. In particular, some evidence suggests
that SSRIs can cause such untoward sexual experiences. Reliable estimates of the incidence and severity of untoward experiences involving
sexual desire, performance, and satisfaction are difficult to obtain, however, in part because patients and physicians may be reluctant to discuss
them. Accordingly, estimates of the incidence of untoward sexual experience and performance cited in product labeling are likely to under-
estimate their actual incidence.
TABLE 5
Incidence of Sexual Side Effects in Placebo-Controlled Clinical Trials
Adverse Event Lexapro Placebo
In Males Only
(N=407) (N=383)
Ejaculation Disorder
(primarily ejaculatory delay) 12% 1%
Libido Decreased 6% 2%
Impotence 2% <1%
In Females Only
(N=737) (N=636)
Libido Decreased 3% 1%
Anorgasmia 3% <1%
There are no adequately designed studies examining sexual dysfunction with escitalopram treatment. Priapism has been reported with all SSRIs.
While it is difficult to know the precise risk of sexual dysfunction associated with the use of SSRIs, physicians should routinely inquire about
such possible side effects.
Vital Sign Changes
-Lexapro and placebo groups were compared with respect to (1) mean change from baseline in
vital signs (pulse, systolic blood pressure, and diastolic blood pressure) and (2) the incidence of patients meeting criteria for potentially clinical-
ly significant changes from baseline in these variables. These analyses did not reveal any clinically important changes in vital signs associated
with Lexapro treatment. In addition, a comparison of supine and standing vital sign measures in subjects receiving Lexapro indicated that
Lexapro treatment is not associated with orthostatic changes.
Weight Changes
-Patients treated with Lexapro in controlled trials did not differ
from placebo-treated patients with regard to clinically important change in body weight.
Laboratory Changes
-Lexapro and placebo groups were
compared with respect to (1) mean change from baseline in various serum chemistry, hematology, and urinalysis variables, and (2) the incidence
of patients meeting criteria for potentially clinically significant changes from baseline in these variables. These analyses revealed no clinically
important changes in laboratory test parameters associated with Lexapro treatment.
ECG Changes
-Electrocardiograms from Lexapro (N=625),
racemic citalopram (N=351), and placebo (N=527) groups were compared with respect to (1) mean change from baseline in various ECG
parameters and (2) the incidence of patients meeting criteria for potentially clinically significant changes from baseline in these variables. These
analyses revealed (1) a decrease in heart rate of 2.2 bpm for Lexapro and 2.7 bpm for racemic citalopram, compared to an increase of 0.3 bpm
for placebo and (2) an increase in QTc interval of 3.9 msec for Lexapro and 3.7 msec for racemic citalopram, compared to 0.5 msec for
placebo. Neither Lexapro nor racemic citalopram were associated with the development of clinically significant ECG abnormalities.
Other
Reactions Observed During the Premarketing Evaluation of Lexapro
-Following is a list of treatment-emergent adverse events, as defined in the
introduction to the ADVERSE REACTIONS section, reported by the 1428 patients treated with Lexapro for periods of up to one year in double-
blind or open-label clinical trials during its premarketing evaluation. The listing does not include those events already listed in Tables 2 & 3, those
events for which a drug cause was remote and at a rate less than 1% or lower than placebo, those events which were so general as to be
uninformative, and those events reported only once which did not have a substantial probability of being acutely life threatening. Events are
categorized by body system. Events of major clinical importance are described in the Warnings and Precautions section. Cardiovascular - hyper-
tension, palpitation. Central and Peripheral Nervous System Disorders - light-headed feeling, migraine. Gastrointestinal Disorders - abdominal
cramp, heartburn, gastroenteritis. General - allergy, chest pain, fever, hot flushes, pain in limb. Metabolic and Nutritional Disorders - increased
weight. Musculoskeletal System Disorders - arthralgia, myalgia jaw stiffness. Psychiatric Disorders - appetite increased, concentration impaired,
irritability. Reproductive Disorders/Female - menstrual cramps, menstrual disorder. Respiratory System Disorders - bronchitis, coughing, nasal
congestion, sinus congestion, sinus headache. Skin and Appendages Disorders - rash. Special Senses - vision blurred, tinnitus. Urinary System
Disorders - urinary frequency, urinary tract infection.
Post-Marketing Experience; Adverse Reactions Reported Subsequent to the Marketing
of Escitalopram
-The following additional adverse reactions have been identified from spontaneous reports of escitalopram received worldwide.
These adverse reactions have been chosen for inclusion because of a combination of seriousness, frequency of reporting, or potential causal
connection to escitalopram and have not been listed elsewhere in labeling. However, because these adverse reactions were reported voluntarily
from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug
exposure. These events include: Blood and Lymphatic System Disorders: anemia, agranulocytis, aplastic anemia, hemolytic anemia, idiopathic
thrombocytopenia purpura, leukopenia, thrombocytopenia. Cardiac Disorders: atrial fibrillation, bradycardia, cardiac failure, myocardial
infarction, tachycardia, torsade de pointes, ventricular arrhythmia, ventricular tachycardia. Ear and Labyrinth Disorders: vertigo Endocrine
Disorders: diabetes mellitus, hyperprolactinemia, SIADH. Eye Disorders: diplopia, glaucoma, mydriasis, visual disturbance. Gastrointestinal
Disorders: dysphagia, gastrointestinal hemorrhage, gastroesophageal reflux, pancreatitis, rectal hemorrhage. General Disorders and
Administration Site Conditions: abnormal gait, asthenia, edema, fall, feeling abnormal, malaise. Hepatobiliary Disorders: fulminant hepatitis,
hepatic failure, hepatic necrosis, hepatitis. Immune System Disorders: allergic reaction, anaphylaxis. Investigations: bilirubin increased,
decreased weight, electrocardiogram QT prolongation, hepatic enzymes increased, hypercholesterolemia, INR increased, prothrombin
decreased. Metabolism and Nutrition Disorders: hyperglycemia, hypoglycemia, hypokalemia, hyponatremia. Musculoskeletal and Connective
Tissue Disorders: muscle cramp, muscle stiffness, muscle weakness, rhabdomyolysis. Nervous System Disorders: akathisia, amnesia, ataxia,
choreoathetosis, cerebrovascular accident, dysarthria, dyskinesia, dystonia, extrapyramidal disorders, grand mal seizures (or convulsions),
hypoaesthesia, myoclonus, nystagmus, Parkinsonism, restless legs, seizures, syncope, tardive dyskinesia, tremor. Pregnancy, Puerperium and
Perinatal Conditions: spontaneous abortion. Psychiatric Disorders: acute psychosis, aggression, agitation, anger, anxiety, apathy, completed
suicide, confusion, depersonalization, depression aggravated, delirium, delusion, disorientation, feeling unreal, hallucinations (visual and
auditory), mood swings, nervousness, nightmare, panic reaction, paranoia, restlessness, self-harm or thoughts of self-harm, suicide attempt,
suicidal ideation, suicidal tendency. Renal and Urinary Disorders: acute renal failure, dysuria, urinary retention. Reproductive System and Breast
Disorders: menorrhagia, priapism. Respiratory, Thoracic and Mediastinal Disorders: dyspnea, epistaxis, pulmonary embolism, pulmonary
hypertension of the newborn. Skin and Subcutaneous Tissue Disorders: alopecia, angioedema, dermatitis, ecchymosis, erythema multiforme,
photosensitivity reaction, Stevens Johnson Syndrome, toxic epidermal necrolysis, urticaria. Vascular Disorders: deep vein thrombosis, flushing,
hypertensive crisis, hypotension, orthostatic hypotension, phlebitis, thrombosis.
DRUG INTERACTIONS: Serotonergic Drugs
-Based on the mechanism of action of SNRIs and SSRIs including Lexapro, and the potential
for serotonin syndrome, caution is advised when Lexapro is coadministered with other drugs that may affect the serotonergic neurotransmitter
systems, such as triptans, linezolid (an antibiotic which is a reversible non-selective MAOI), lithium, tramadol, or St. John’s Wort [
see Warnings
and Precautions
]. The concomitant use of Lexapro with other SSRIs, SNRIs or tryptophan is not recommended.
Triptans
-There have been rare
postmarketing reports of serotonin syndrome with use of an SSRI and a triptan. If concomitant treatment of Lexapro with a triptan is clinically
warranted, careful observation of the patient is advised, particularly during treatment initiation and dose increases [
see Warnings and
Precautions
].
CNS Drugs
- Given the primary CNS effects of escitalopram, caution should be used when it is taken in combination with other
centrally acting drugs.
Alcohol
-Although Lexapro did not potentiate the cognitive and motor effects of alcohol in a clinical trial, as with other
psychotropic medications, the use of alcohol by patients taking Lexapro is not recommended.
Monoamine Oxidase Inhibitors (MAOIs)
-[
see
Contraindications and Warnings and Precautions
].
Drugs That Interfere With Hemostasis (NSAIDs, Aspirin, Warfarin, etc.)
-Serotonin release
by platelets plays an important role in hemostasis. Epidemiological studies of the case-control and cohort design that have demonstrated an
association between use of psychotropic drugs that interfere with serotonin reuptake and the occurrence of upper gastrointestinal bleeding have
also shown that concurrent use of an NSAID or aspirin may potentiate the risk of bleeding. Altered anticoagulant effects, including increased
bleeding, have been reported when SSRIs and SNRIs are coadministered with warfarin. Patients receiving warfarin therapy should be carefully
monitored when Lexapro is initiated or discontinued.
Cimetidine
-In subjects who had received 21 days of 40 mg/day racemic citalopram, com-
bined administration of 400 mg/day cimetidine for 8 days resulted in an increase in citalopram AUC and C
max
of 43% and 39%, respectively. The
clinical significance of these findings is unknown.
Digoxin
-In subjects who had received 21 days of 40 mg/day racemic citalopram, combined
administration of citalopram and digoxin (single dose of 1 mg) did not significantly affect the pharmacokinetics of either citalopram or digoxin.
Lithium
-Coadministration of racemic citalopram (40 mg/day for 10 days) and lithium (30 mmol/day for 5 days) had no significant effect on the
pharmacokinetics of citalopram or lithium. Nevertheless, plasma lithium levels should be monitored with appropriate adjustment to the lithium
dose in accordance with standard clinical practice. Because lithium may enhance the serotonergic effects of escitalopram, caution should
be exercised when Lexapro and lithium are coadministered.
Pimozide and Celexa
-In a controlled study, a single dose of pimozide 2 mg co-
administered with racemic citalopram 40 mg given once daily for 11 days was associated with a mean increase in QTc values of approximately
10 msec compared to pimozide given alone. Racemic citalopram did not alter the mean AUC or C
max
of pimozide. The mechanism of this
pharmacodynamic interaction is not known.
Sumatriptan
-There have been rare postmarketing reports describing patients with weakness,
hyperreflexia, and incoordination following the use of an SSRI and sumatriptan. If concomitant treatment with sumatriptan and an SSRI (e.g.,
fluoxetine, fluvoxamine, paroxetine, sertraline, citalopram, escitalopram) is clinically warranted, appropriate observation of the patient is advised.
Theophylline
-Combined administration of racemic citalopram (40 mg/day for 21 days) and the CYP1A2 substrate theophylline (single dose
of 300 mg) did not affect the pharmacokinetics of theophylline. The effect of theophylline on the pharmacokinetics of citalopram was not
evaluated.
Warfarin
-Administration of 40 mg/day racemic citalopram for 21 days did not affect the pharmacokinetics of warfarin, a CYP3A4 sub-
strate. Prothrombin time was increased by 5%, the clinical significance of which is unknown.
Carbamazepine
-Combined administration of
racemic citalopram (40 mg/day for 14 days) and carbamazepine (titrated to 400 mg/day for 35 days) did not significantly affect the pharmaco-
kinetics of carbamazepine, a CYP3A4 substrate. Although trough citalopram plasma levels were unaffected, given the enzyme-inducing
properties of carbamazepine, the possibility that carbamazepine might increase the clearance of escitalopram should be considered if the two
drugs are coadministered.
Triazolam
-Combined administration of racemic citalopram (titrated to 40 mg/day for 28 days) and the CYP3A4
substrate triazolam (single dose of 0.25 mg) did not significantly affect the pharmacokinetics of either citalopram or triazolam.
Ketoconazole
-
Combined administration of racemic citalopram (40 mg) and ketoconazole (200 mg), a potent CYP3A4 inhibitor, decreased the C
max
and AUC of
ketoconazole by 21% and 10%, respectively, and did not significantly affect the pharmacokinetics of citalopram.
Ritonavir
-Combined adminis-
tration of a single dose of ritonavir (600 mg), both a CYP3A4 substrate and a potent inhibitor of CYP3A4, and escitalopram (20 mg) did not affect
the pharmacokinetics of either ritonavir or escitalopram.
CYP3A4 and -2C19 Inhibitors
-
In vitro
studies indicated that CYP3A4 and -2C19 are the
primary enzymes involved in the metabolism of escitalopram. However, coadministration of escitalopram (20 mg) and ritonavir (600 mg), a
potent inhibitor of CYP3A4, did not significantly affect the pharmacokinetics of escitalopram. Because escitalopram is metabolized by multiple
enzyme systems, inhibition of a single enzyme may not appreciably decrease escitalopram clearance.
Drugs Metabolized by Cytochrome
P4502D6
-
In vitro
studies did not reveal an inhibitory effect of escitalopram on CYP2D6. In addition, steady state levels of racemic citalopram
were not significantly different in poor metabolizers and extensive CYP2D6 metabolizers after multiple-dose administration of citalopram,
suggesting that coadministration, with escitalopram, of a drug that inhibits CYP2D6, is unlikely to have clinically significant effects on escitalo-
pram metabolism. However, there are limited
in vivo
data suggesting a modest CYP2D6 inhibitory effect for escitalopram, i.e., coadministration
of escitalopram (20 mg/day for 21 days) with the tricyclic antidepressant desipramine (single dose of 50 mg), a substrate for CYP2D6, resulted
in a 40% increase in C
max
and a 100% increase in AUC of desipramine. The clinical significance of this finding is unknown. Nevertheless, caution
is indicated in the coadministration of escitalopram and drugs metabolized by CYP2D6.
Metoprolol
-Administration of 20 mg/day Lexapro for
21 days in healthy volunteers resulted in a 50% increase in C
max
and 82% increase in AUC of the beta-adrenergic blocker metoprolol (given in a
single dose of 100 mg). Increased metoprolol plasma levels have been associated with decreased cardioselectivity. Coadministration of Lexapro
and metoprolol had no clinically significant effects on blood pressure or heart rate.
Electroconvulsive Therapy (ECT)
-There are no clinical
studies of the combined use of ECT and escitalopram.
USE IN SPECIFIC POPULATIONS: Pregnancy;
Pregnancy Category C
-
In a rat embryo/fetal development study, oral administration of
escitalopram (56, 112, or 150 mg/kg/day) to pregnant animals during the period of organogenesis resulted in decreased fetal body weight and
associated delays in ossification at the two higher doses (approximately 56 times the maximum recommended human dose [MRHD] of
20 mg/day on a body surface area [mg/m
2
] basis). Maternal toxicity (clinical signs and decreased body weight gain and food consumption), mild
at 56 mg/kg/day, was present at all dose levels. The developmental no-effect dose of 56 mg/kg/day is approximately 28 times the MRHD on a
mg/m
2
basis. No teratogenicity was observed at any of the doses tested (as high as 75 times the MRHD on a mg/m
2
basis). When female rats
were treated with escitalopram (6, 12, 24, or 48 mg/kg/day) during pregnancy and through weaning, slightly increased offspring mortality and
growth retardation were noted at 48 mg/kg/day which is approximately 24 times the MRHD on a mg/m
2
basis. Slight maternal toxicity (clinical
signs and decreased body weight gain and food consumption) was seen at this dose. Slightly increased offspring mortality was also seen at
24 mg/kg/day. The no-effect dose was 12 mg/kg/day which is approximately 6 times the MRHD on a mg/m
2
basis. In animal reproduction
studies, racemic citalopram has been shown to have adverse effects on embryo/fetal and postnatal development, including teratogenic effects,
when administered at doses greater than human therapeutic doses. In two rat embryo/fetal development studies, oral administration of racemic
citalopram (32, 56, or 112 mg/kg/day) to pregnant animals during the period of organogenesis resulted in decreased embryo/fetal growth and
survival and an increased incidence of fetal abnormalities (including cardiovascular and skeletal defects) at the high dose. This dose was also
associated with maternal toxicity (clinical signs, decreased body weight gain). The developmental no-effect dose was 56 mg/kg/day. In a rabbit
study, no adverse effects on embryo/fetal development were observed at doses of racemic citalopram of up to 16 mg/kg/day. Thus, teratogenic
effects of racemic citalopram were observed at a maternally toxic dose in the rat and were not observed in the rabbit. When female rats were
treated with racemic citalopram (4.8, 12.8, or 32 mg/kg/day) from late gestation through weaning, increased offspring mortality during the first
4 days after birth and persistent offspring growth retardation were observed at the highest dose. The no-effect dose was 12.8 mg/kg/day. Similar
effects on offspring mortality and growth were seen when dams were treated throughout gestation and early lactation at doses 24 mg/kg/day.
A no-effect dose was not determined in that study. There are no adequate and well-controlled studies in pregnant women; therefore, escitalo-
pram should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Pregnancy-Nonteratogenic Effects
Neonates exposed to Lexapro and other SSRIs or SNRIs, late in the third trimester, have developed complications requiring prolonged hospital-
ization, respiratory support, and tube feeding. Such complications can arise immediately upon delivery. Reported clinical findings have included
respiratory distress, cyanosis, apnea, seizures, temperature instability, feeding difficulty, vomiting, hypoglycemia, hypotonia, hypertonia,
hyperreflexia, tremor, jitteriness, irritability, and constant crying. These features are consistent with either a direct toxic effect of SSRIs and SNRIs
or, possibly, a drug discontinuation syndrome. It should be noted that, in some cases, the clinical picture is consistent with serotonin syndrome
[
see Warnings and Precautions
]. Infants exposed to SSRIs in late pregnancy may have an increased risk for persistent pulmonary hypertension
of the newborn (PPHN). PPHN occurs in 1-2 per 1000 live births in the general population and is associated with substantial neonatal
morbidity and mortality. In a retrospective, case-control study of 377 women whose infants were born with PPHN and 836 women whose infants
were born healthy, the risk for developing PPHN was approximately six-fold higher for infants exposed to SSRIs after the 20th week of gestation
compared to infants who had not been exposed to antidepressants during pregnancy. There is currently no corroborative evidence regarding the
risk for PPHN following exposure to SSRIs in pregnancy; this is the first study that has investigated the potential risk. The study did not include
enough cases with exposure to individual SSRIs to determine if all SSRIs posed similar levels of PPHN risk. When treating a pregnant woman
with Lexapro during the third trimester, the physician should carefully consider both the potential risks and benefits of treatment [
see Dosage
and Administration
]. Physicians should note that in a prospective longitudinal study of 201 women with a history of major depression who were
euthymic at the beginning of pregnancy, women who discontinued antidepressant medication during pregnancy were more likely to experience
a relapse of major depression than women who continued antidepressant medication.
Labor and Delivery
-The effect of Lexapro on labor and
delivery in humans is unknown.
Nursing Mothers
-Escitalopram is excreted in human breast milk. Limited data from women taking 10-20 mg
escitalopram showed that exclusively breast-fed infants receive approximately 3.9% of the maternal weight-adjusted dose of escitalopram and
1.7% of the maternal weight-adjusted dose of desmethylcitalopram. There were two reports of infants experiencing excessive somnolence,
decreased feeding, and weight loss in association with breastfeeding from a racemic citalopram-treated mother; in one case, the infant was
reported to recover completely upon discontinuation of racemic citalopram by its mother and, in the second case, no follow-up information was
available. Caution should be exercised and breastfeeding infants should be observed for adverse reactions when Lexapro is administered to a
nursing woman.
Pediatric Use
-Safety and effectiveness of Lexapro has not been established in pediatric patients (less than 12 years of age) with
Major Depressive Disorder. Safety and effectiveness of Lexapro has been established in adolescents (12 to 17 years of age) for the treatment of
major depressive disorder [
see Clinical Studies
]. Although maintenance efficacy in adolescent patients with Major Depressive Disorder has not
been systematically evaluated, maintenance efficacy can be extrapolated from adult data along with comparisons of escitalopram pharmaco -
kinetic parameters in adults and adolescent patients. Safety and effectiveness of Lexapro has not been established in pediatric patients less than
18 years of age with Generalized Anxiety Disorder.
Geriatric Use
-Approximately 6% of the 1144 patients receiving escitalopram in controlled
trials of Lexapro in major depressive disorder and GAD were 60 years of age or older; elderly patients in these trials received daily doses of
Lexapro between 10 and 20 mg. The number of elderly patients in these trials was insufficient to adequately assess for possible differential
efficacy and safety measures on the basis of age. Nevertheless, greater sensitivity of some elderly individuals to effects of Lexapro cannot
be ruled out. SSRIs and SNRIs, including Lexapro, have been associated with cases of clinically significant hyponatremia in elderly patients,
who may be at greater risk for this adverse event [see
Hyponatremia
]. In two pharmacokinetic studies, escitalopram half-life was increased by
approximately 50% in elderly subjects as compared to young subjects and C
max
was unchanged [
see Clinical Pharmacology
]. 10 mg/day is the
recommended dose for elderly patients [
see Dosage and Administration
]. Of 4422 patients in clinical studies of racemic citalopram, 1357 were
60 and over, 1034 were 65 and over, and 457 were 75 and over. No overall differences in safety or effectiveness were observed between these
subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger
patients, but again, greater sensitivity of some elderly individuals cannot be ruled out.
DRUG ABUSE AND DEPENDENCE: Abuse and Dependence;
Physical and Psychological Dependence-Animal studies suggest that the abuse
liability of racemic citalopram is low. Lexapro has not been systematically studied in humans for its potential for abuse, tolerance, or physical
dependence. The premarketing clinical experience with Lexapro did not reveal any drug-seeking behavior. However, these observations were not
systematic and it is not possible to predict on the basis of this limited experience the extent to which a CNS-active drug will be misused, divert-
ed, and/or abused once marketed. Consequently, physicians should carefully evaluate Lexapro patients for history of drug abuse and follow such
patients closely, observing them for signs of misuse or abuse (e.g., development of tolerance, incrementations of dose, drug-seeking behavior).
OVERDOSAGE: Human Experience
-In clinical trials of escitalopram, there were reports of escitalopram overdose, including overdoses of up
to 600 mg, with no associated fatalities. During the postmarketing evaluation of escitalopram, Lexapro overdoses involving overdoses of over
1000 mg have been reported. As with other SSRIs, a fatal outcome in a patient who has taken an overdose of escitalopram has been rarely
reported. Symptoms most often accompanying escitalopram overdose, alone or in combination with other drugs and/or alcohol, included
convulsions, coma, dizziness, hypotension, insomnia, nausea, vomiting, sinus tachycardia, somnolence, and ECG changes (including QT
prolongation and very rare cases of torsade de pointes). Acute renal failure has been very rarely reported accompanying overdose.
Management
of Overdose
-Establish and maintain an airway to ensure adequate ventilation and oxygenation. Gastric evacuation by lavage and use of
activated charcoal should be considered. Careful observation and cardiac and vital sign monitoring are recommended, along with general
symptomatic and supportive care. Due to the large volume of distribution of escitalopram, forced diuresis, dialysis, hemoperfusion, and exchange
transfusion are unlikely to be of benefit. There are no specific antidotes for Lexapro. In managing overdosage, consider the possibility of
multiple-drug involvement. The physician should consider contacting a poison control center for additional information on the treatment of any
overdose.
Forest Pharmaceuticals, Inc.
Subsidiary of Forest Laboratories, Inc.
St. Louis, MO 63045 USA
Licensed from H. Lundbeck A/S
© 2009 Forest Laboratories, Inc. Rev. 05/09
LEXAPRO®(escitalopram oxalate) TABLETS/ORAL SOLUTION LEXAPRO®(escitalopram oxalate) TABLETS/ORAL SOLUTION
WWW.DRUGTOPICS.COM December 2010
DRUG TOPICS
47
Product Updates
DANA K. CASSELL
FEATURED THIS MONTH: DIGESTIVES
For those who dine not wisely
but too well
OTC
In this season of gustatory indulgence,
it can’t hurt to prepare for the occa-
sional upset stomach. According to re-
ports, more than 100 million Americans
are affected by some form of digestive
disorder, including gas, heartburn, indi-
gestion, vomiting, bloating, constipation,
diarrhea, and worse. Fortunately, OTC
manufacturers have been hard at work
preparing new and improved digestive
and gastrointestinal aids.
PPI
Schering-Plough HealthCare Products has
introduced Zegerid OTC, a proton pump
inhibitor (PPI), the most potent class of
acid-reducing drugs currently available.
The only nonprescription PPI prod-
uct with 2 active ingredients, Zegerid
OTC combines the PPI omeprazole with
sodium bicarbonate, which protects the
medication from being broken down by
acid in the stomach prior to absorption.
Zegerid OTC is approved for use by adults
18 and over for the treatment of frequent
heartburn (defi ned as occurring 2 or more
days a week).
Fiber
Dietary fiber not only aids digestion,
it can help control weight, because it
makes one feel full faster and longer.
From GlaxoSmithKline comes an ad-
dition to its FiberChoice line: a new
strawberry-fl avored Weight Manage-
ment formula containing chromium
picolinate, a naturally occurring com-
pound that aids in metabolism.
Enzymes and probiotics
When the category is digestives and gas-
trointestinal agents, increasingly popular
elements are enzymes and probiotics. En-
zymedica has combined the 2 in its new
Digest Gold + Probiotics, offering “com-
plete digestive care in a single capsule.”
Enzymedica’s Digest Gold is an
enzyme product formulated to assist in
digestion of proteins, fats, carbohydrates,
and fi ber.
Digest Gold + Probiotics combines
that enzyme formula with 500 million
cultures of specially coated probiotics
that release in the alkaline pH of the
lower intestines, guaranteeing potency.
Bayer HealthCare’s Phillips’ Colon
Health now offers a larger, 60-count
package to give customers 2 months of
daily probiotic support. The product’s 3
strains of good bacteria are derived from
the 2 most common groups of good bac-
teria, Lactobacillus and Bifi dobacterium.
According to Bayer, “Scientifi c evi-
dence demonstrates that Lactobacillus
and Bifi dobacterium help relieve occasion-
al gas, constipation, diarrhea, and other
common GI issues while also helping to
support a healthy immune system. Pro-
biotics work to rebalance the bacteria in
your colon to support overall digestive
health and to help defend against occa-
sional digestive symptoms.”
Dana K. Cassell, a frequent contributor to
Drug Topics, lives in North Stratford, N.H.
FiberChoice Weight
Management: The chewable
tablets offer 4g of ber in
each 2-tablet dose.
Zegerid OTC is the only
nonprescription PPI with 2
active ingredients.
Digest Gold + Probiotics
combines digestive
enzymes and probiotics.
PHOTOS COURTESY OF SCHERING-PLOUGH HEALTHCARE PRODUCTS/GLAXO-SMITH-KLINE/ENZYMEDICA

48
DRUG TOPICS
December 2010
Product Updates
New drugs
FDA has approved Warner Chilcott’s
bisphosphonate Atelvia (risedronate so-
dium delayed-release tablets) for the
treatment of postmenopausal osteoporo-
sis. The product is a delayed-release for-
mulation of Actonel, which is approved
to prevent and treat postmenopausal
osteoporosis and glucocorticoid-induced
osteoporosis (in men and women), to
increase bone mass in men with osteo-
porosis, and to treat Paget’s disease of the
bone. (www.wcrx.com/800-521-8813)
Bristol-Myers Squibb’s Baraclude (en-
tecavir tablets), a nucleoside analog, is
approved for the treatment of chronic
hepatitis B (CHB) in adult patients with
decompensated liver disease. Baraclude
is already indicated for the treatment of
CHB infection in adults with evidence of
active viral replication and evidence of ei-
ther persistent elevations in serum ami-
notransferases (ALT or AST) or histologi-
cally active disease. (www.baraclude.
com/800-321-1335)
FDA has approved ISTA’s Bromday [1]
(bromfenac ophthalmic solution 0.09%),
a nonsteroidal anti-infl ammatory drug
(NSAID), for the treatment of postop-
erative infl ammation and reduction of
ocular pain after cataract surgery. (www.
istavision.com/949-788-6000).
Pradaxa [2] (dabigatran etexilate) cap-
sules from Boehringer Ingelheim are ap-
proved to reduce the risk of stroke and
systemic embolism in patients with non-
valvular atrial fi brillation (AFib). An oral
direct thrombin inhibitor, Pradaxa is the
fi rst new oral anticoagulant approved in
the United States in more than 50 years.
(www.pradaxa.com/800-542-6257)
Fera has launched a preservative-free
formulation of Garamycin (gentamicin sul-
fate ophthalmic ointment), indicated for
the treatment of susceptible ocular bacte-
rial infections, including conjunctivis, ker-
atitis, keratoconjunctivitis, corneal ulcers,
blepharitis, blepharoconjunctivitis, acute
meibomianitis, and dacryocystitis. (www.
ferapharma.com/414-434-6604)
New generics
FDA approved Teva’s ANDA to market
lansoprazole, a generic version of Takeda’s
Prevacid SoluTab. The prod-
uct, a proton pump inhibi-
tor, suppresses gastric acid
production and is often
prescribed for use in gastro-
esophageal reflux disease.
(888-838-2872)
Dr. Reddy’s Laborato-
ries will launch lansopra-
zole delayed-release cap-
sules (15 mg and 30 mg),
a bioequivalent generic
version of Prevacid De-
layed-Release Capsules, in
the U.S. market. (www.
drreddys.com)
Tris Pharma announced that FDA
has approved hydrocodone polistirex and
chlorpheniramine polistirex in extended-
release suspension, the fi rst-ever generic
version of Tussionex (UCB Manufactur-
ing). (www.trispharma.com)
OTC
Chestal, a natural cough syrup free of
dextromethorphan, has been made avail-
able by Boiron. A blend of homeopathic
medicines and honey, Chestal helps loos-
en chest congestion for dry and produc-
tive coughs. (www.childrenschestal.
com/800-266-7661)
Eco Lips announces new colors in
its Eco Tints [3] line: Moonstone, Sugar
Plum, and Coralyte. With more than
90% certifi ed organic ingredients, Eco
Tints are free of carmine, lead, gluten,
lanolin, petroleum, hydrogenated oils,
and nanoparticles. (www.ecolips.
com/866-326-5477)
From Smith & Nephew comes No-
Sting Skin Prep spray, a liquid dressing
that leaves a clear, waterproof, and
breathable fi lm barrier for up to 4 days,
protecting skin from irritation due to
digestive secretions, exudate, adhesives,
frictional forces, and skin trauma dur-
ing tape and adhesive removal. (www.
smith-nephew.com/800-876-1261)
Vitalah has introduced Prenatal Oxylent,
a digestible effervescent vitamin formulat-
ed for needs of preconception, pregnancy,
and lactation. It is naturally sweetened
with stevia; no sugar is added and it has
no artifi cial color, fl avor, gluten, lactose, or
caffeine. (www.vitalah.com)
RX CARE
PHOTOS COURTESY OF ISTA/BOEHRINGER INGELHEIM/ECO LIPS
CORPORATE Amneal Pharmaceuticals 1
CLONIDINE Mylan Pharmaceuticals Inc. 7-8
CORPORATE Mylan Pharmaceuticals Inc. CV4
CORPORATE QS/1 Data Systems Inc. CV2
CORPORATE Roxane Laboratories 15
EPI-PEN Dey Pharma LP 11-12
HUMALOG Eli Lilly and Company 26-28
LEXAPRO Forest Laboratories Inc. 43-46
METFORMIN Mylan Pharmaceuticals Inc. 3-5
PRADAXA Boehringer Ingelheim Ltd. 25
RXMEDIC QS/1 Data Systems Inc. CV3
VIMOVO AstraZeneca 17-20
Advertiser Index
WHAT’S NEW
RX & OTC
New products
1
2
3

The Drug Topics’ team wishes you the best this holiday season
and thanks you for your continued support.
A
MONDAY
P
TUESDAY
WEDNESDAY
Y
THURSDAY FRIDAY SATURDAY
SUNDAY
HP
O
MONDAY
L
TUESDAY
WEDNESDAY
D
THURSDAY
A
FRIDAY
Y
SATURDAY
SUNDAY
HI

MARKETPLACE
Products & Services
Brokers
Books/Publications
Who should consider reading this book?
• Any licensed healthcare professional working or wanting to work a second job!
• Any licensed healthcare professional now working for a “staf ng agency” as a second job!
• Any licensed healthcare professional wanting to start their own “staf ng agency”
as a second job!
• Corporate America looking for ways to help mitigate their number one
expense...overtime and labor cost!
• Those providing insight along with integrity looking to network and join an
association to lend their expertise in the health care industry.
A Step
Ahead...
The FIRST eBook of its kind directed toward the Licensed Health Care
professionals working a SECOND JOB!
Benefi ts Design Consultants/Staffi ng
Making Thousands of $$$
at Your SECOND JOB and Keeping More of it
www.TIMEBANDITSRX.com
50
DRUG TOPICS
December 2010 WWW.DRUGTOPICS.COM
For Products and Services Advertising:
Carla Kastanis at (800) 225-4569 x2711
E-mail: ckastanis@advanstar.com
For Recruitment Advertising:
Joanna Shippoli at (800) 225-4569 x2615
E-mail: jshippoli@advanstar.com
MARKETPLACE ADVERTISING
See More Brokers on next page
Continuing Education
PHARMACY VACATION SEMINARS
ACPE ACCREDITED PROVIDER
2010
Las Vegas at Harrah’s
-
December 8
-
10
2011
Boca Raton, The Beach Club
-
January 26
-
28
Las Vegas at Harrah’s
-
February 23
-
25
Caribbean Cruise
-
March 13-20-Allure of the Seas
-
NEW SHIP!
Naples Grande Beach Resort, Florida
-
April 27
-
29
Las Vegas at Planet Hollywood
-
June 8
-
10
Alaska
-
Voyage of the Glaciers
-
June 11
-
18
Antlers Hilton
-
Colorado Springs July 27
-
29
Las Vegas at Harrah’s
-
September 14
-
16
Westin Maui Resort & Spa
-
October 26
-
28
Caribbean Cruise
-
November 6
-
13
Las Vegas at Harrah’s
-
December 7
-
9
New Drug Update DVD
-
Anytime
-
Anywhere!
1-800-940-5860
www.universitylearning.com
CALL FOR FREE BROCHURE
Kirkland, Washington
COMPOUNDING PHARMACY FOR SALE
All cash customers. No insurance plan hassles.
Also DME and cards/gifts.
No weekends. Priced for quick sale.
Contact juanitabaypharmacy@live.com
Business For Sale
How your family reacts in an emergency
can make all the difference. To make
your family disaster plan, contact your
local American Red Cross chapter or
visit www.redcross.org
Make a plan.

Reach thousands of industry professionals every month
Your message could be here!
NAPLEX
®?
www.prontopass.com
For details, please visit our website.
NAPLEX® Review
QUICKCARDS®
with Memoronics®
Need to Pass
Using Memory Techniques of:
Visual
Auditory
Repetition
Self Testing
*
and it’s
Do the NAPLEX®
3 Step
with
1
2
3
plus Posters, Audio CD,
Math Practice, Telephone quizzing,
and Patient Profiles.
www.prudentialcbs.com
Common Mistakes Sellers Make:
■ Not using a licensed broker specializing in pharmacies
■ Not knowing your true current market value
■ Not effectively advertising to reach enough qualified buyers
Call me before sharing information
about your pharmacy with ANY buyer.
Prudential’s proprietary selling process
will enable you to get the highest price for your pharmacy!
SELLING YOUR PHARMACY?
Call me today at 888-808-4RPH (4774)
Daniel J. Lannon, RPh, VP
Senior Pharmacy Broker Consulting Broker Services
www.prudentialcbs.com
Common Mistakes Sellers Make:
■ Not using a licensed broker specializing in pharmacies
■ Not knowing your true current market value
■ Not effectively advertising to reach enough qualified buyers
Call me before sharing information
about your pharmacy with ANY buyer.
Prudential’s proprietary selling process
will enable you to get the highest price for your pharmacy!
SELLING YOUR PHARMACY?
Call me today at 888-808-4RPH (4774)
Daniel J. Lannon, RPh, VP
Senior Pharmacy Broker Consulting Broker Services
EducationBrokers
Legal Services
Pharmacy Technicians
Financing
Pharmacy Owners:
Keep your pharmacy open!
Cash flow problems? Too much short-term debt?
We can access long-term financing and reduce your monthly
debt service. Up to $2 million available per pharmacy!
If you are looking to sell, we can also provide financing to
your buyer of up to $2 million for a terms of 10-25 years.
Visit shbconsultants.com
or call 800-603-2519
WWW.DRUGTOPICS.COM December 2010
DRUG TOPICS
51
ATTN:PHARMACY TECHNICIANS
DISCOVER NPTA...the largest
professional organization
specifically for pharmacy technicians.
Free CE + Advocacy + Resources
Learn more at:
www.pharmacytechnician.org/discover
MARKETPLACE
Products & Services
LEGAL PROBLEMS
With Medicare/Medicaid, Licensing Boards,
Data Bank, DEA? Impaired Status, Compliance,
Business Structuring. Whistle Blower.
Call Kenneth Haber
Over 30 years experience
Former Assistant US Attorney
Former Senior OIG Attorney
301.670.0016 • www.haberslaw.com
No Obligation
WWW.DRUGTOPICS.COM
52
DRUG TOPICS
December 2010
This issue is a milestone for Drug Topics, with the pub-
lication of the 200th column by Jim Plagakis to appear
under the heading “JP at Large.” In this issue (page
13), he takes us on a journey that began in 1989 with the pub-
lication of his fi rst column, about a patient with bipolar disorder
who needed someone to listen. That someone was Jim.
Jim has been listening and counseling as a pharmacist for
almost 45 years. At his fi rst job, in a downtown drugstore that
still had a lunch counter, patients received a coupon for a free
cup of coffee to drink while they waited for
their prescriptions. Jim would join patients
for a sip of coffee and ask whether they had
any questions about their medications. He
called it “stealth counseling in the 1960s.”
Fast-forward to 1993. Jim explained in a
column that he took “3 focused minutes”
to listen to a father’s grief for his 9-year-old
son, who was near death from brain cancer.
He wrote, “I was his sponge. He released the
poison, and I sucked it up. Not required by
law, but I think it’s part of my job, even if
the next person up for an Rx had to wait 3
extra minutes.”
Today counseling on every new prescrip-
tion is mandatory in Texas, where Jim prac-
tices. When the technician rings up the pre-
scriptions, including new ones, the whole process stops. Jim
usually asks the patient whether he has used the prescription
before. If the patient says yes, Jim asks whether he has any
questions. If there are none, Jim sends the patient on his way.
When the prescription is a new one for the patient, Jim either
sticks to the basic facts or counsels in greater depth.
“If the drug really needs extensive counseling, I move the
patient over to the counseling window and we really talk,” he
told Drug Topics. “I have serious issues with benzodiazepines,
because they are a dangerous class of drugs if used for a long
period of time. Patients hardly even know that they are taking
that stuff, because it works so well. However, for most people,
a certain part of their personality is lost to them, and it can
be the best part of their personality — the passion, the anger,
stuff that normal human beings have.”
In fact, Jim recently told one of his patients, a young medical
student who had been taking a benzodiazepine regularly for 6
months, that she was too young to continue with this drug for
so long. He warned her of the risks of terrible withdrawal and
the need to manage the stress of medical school differently.
“This is the kind of extensive counseling
that pharmacists can do, although most
don’t. Younger pharmacists don’t give
themselves permission to do that. I’m older,
so I actually can intrude like that. It helps
a lot to make eye contact and let patients
know that you are serious,” Jim said. “I
love it. It’s the best part of my job.”
Jim also believes in taking the time to
counsel patients about over-the-counter
medications. He works part-time, approx-
imately 2 to 3 days per week, as a relief
pharmacist for a national chain. While
some pharmacists direct all their attention
to the “prescription mill” behind the coun-
ter, Jim’s patients know that if they come
in on Tuesday or Friday, he is accessible and
knowledgeable about the OTC products that look so benign
on the drugstore shelves.
With the need to fi ll ever-growing numbers of prescriptions,
pharmacists may be worried that technicians will take over
their jobs, Jim said. “Qualifi ed technicians will be legally em-
powered to do more of the fi lling tasks. The idea is to allow
the pharmacist more time for counseling and MTM, as well
as the various professional tasks that will defi ne us in the 21st
century. We must, however, be ever watchful. It’s our profes-
sion. We will have it our way.”
Thank you, Jim, for sharing your perspective and insight.
A glimpse into the world of retail
pharmacy through the eyes of JP
Julia Talsma, Editor-in-Chief
T
l
u
hk
)
VIEWPOINT
Final Word
“This is the kind of
extensive counsel-
ing that pharmacists
can do, although
most don’t ... It helps
a lot to make eye
contact and let
patients know that
you are serious. I
love it. It’s the best
part of my job.”
Counts
top-selling drugs, making
hand-counting a thing of the past.
The RxMedic ACS™ counting system makes pinpoint
accuracy affordable for any pharmacy. The ACS
simultaneously counts and pre-counts pills. It’s available
in four standard configurations or can be customized to fit
your individual needs. The ACS includes the added safety of
digitally imaging vial contents for more complete records.
For higher-volume needs, experience fully-automated
dispensing with the RxMedic ADS™ robotic system.
Ready to count less and do more for your patients?
Visit www.rxmedic.com or call 800-882-3819.
© RxMedic ACS and RxMedic ADS are trademarks of RxMedic Systems Inc.
