Expert Panel Report 3 FO 97 P Asthsumm
User Manual: FO 97 P
Open the PDF directly: View PDF ![]() .
.
Page Count: 74

National Asthma Education
and Prevention Program
Expert Panel Report 3
Guidelines for the
Diagnosis and Management
of Asthma
SUMMARY REPORT 2007

National Asthma Education
and Prevention Program
Expert Panel Report 3
Guidelines for the
Diagnosis and Management
of Asthma
SUMMARY REPORT 2007
NIH Publication Number 08-5846
October 2007

Contents
Acknowledgments iii
Preface ix
Introduction 1
Asthma Definition and Implications for Treatment 9
Definition and Pathophysiology 9
Causes of Asthma 10
Implications for Treatment 10
Diagnosis of Asthma 11
Managing Asthma Long Term 15
Four Components of Asthma Care 15
Component 1: Assessing and Monitoring Asthma Severity and Asthma Control 15
Component 2: Education for a Partnership in Care 18
Component 3: Control of Environmental Factors and Comorbid Conditions That Affect Asthma 23
Allergens and Irritants 23
Comorbid Conditions 25
Component 4: Medications 28
General Mechanisms and Role in Therapy 28
DeliveryDevices for Inhaled Medications 29
Safety Issues for Inhaled Corticosteroids and Long-Acting Beta2-Agonists 29
Inhaled Corticosteroids 29
Inhaled Corticosteroids and Linear Growth in Children 30
Long-Acting Beta2-Agonists 30
Stepwise Approach for Managing Asthma 30
Principles of the Stepwise Approach 30
Stepwise Treatment Recommendations for Different Ages 34
Steps for Children 0–4 Years of Age 34
Steps for Children 5–11 Years of Age 35
Steps for Youths >12 Years of Age and Adults 37
Managing Special Situations 38
Exercise-Induced Bronchospasm 38
Pregnancy 38
Surgery 39
Disparities 39
Managing Exacerbations 53
Classifying Severity 53
Home Management 53
Management in the Urgent or Emergency Care and Hospital Settings 54
For More Information back cover
i
Contents

List of Boxes and Figures
Figure 1. Summary of Recommended Key Clinical Activities for the
Diagnosis and Management of Asthma 4
Figure 2. The Interplay and Interaction Between Airway Inflammation and the
Clinical Symptoms and Pathophysiology of Asthma 9
Figure 3. Suggested Items for Medical History* 13
Figure 4. Sample Patient Self-Assessment Sheet for Followup Visits* 17
Figure 5. Asthma Action Plan—Adult 20
Figure 6. Sample Asthma Action Plan—Child 21
Figure 7. Delivery of Asthma Education by Clinicians During Patient Care Visits 22
Figure 8. Asthma Education Resources 24
Figure 9. How To Control Things That Make Your Asthma Worse 26
Figure 10. Aerosol Delivery Devices 31
Figure 11. Classifying Asthma Severity and Initiating Therapy in Children 40
Figure 12. Assessing Asthma Control and Adjusting Therapy in Children 41
Figure 13. Stepwise Approach for Managing Asthma Long Term in Children,
0–4 Years of Age and 5–11 Years of Age 42
Figure 14. Classifying Asthma Severity and Initiating Treatment in
Youths ≥12 Years of Age and Adults 43
Figure 15. Assessing Asthma Control and Adjusting Therapy in
Youths ≥12 Years of Age and Adults 44
Figure 16. Stepwise Approach for Managing Asthma in Youths ≥12 Years of Age and Adults 45
Figure 17. Usual Dosages for Long-term control Medications* 46
Figure 18. Estimated Comparative Daily Dosages for Inhaled Corticosteroids 49
Figure 19. Usual Dosages for Quick-Relief Medications* 50
Figure 20. Classifying Severity of Asthma Exacerbations in the Urgent or Emergency Care Setting 54
Figure 21. Management of Asthma Exacerbations: Emergency Department and
Hospital-Based Care 55
Figure 22. Dosages of Drugs for Asthma Exacerbations 56
Figure 23a. Emergency Department—Asthma Discharge Plan 59
Figure 23b. Emergency Department—Asthma Discharge Plan: How to Use Your Metered-Dose Inhaler 60
ii Guidelines for the Diagnosis and Management of Asthma

National Asthma Education and Prevention
Program Coordinating Committee
Agency for Healthcare Research and Quality
Denise Dougherty, Ph.D.
Allergy & Asthma Network Mothers of Asthmatics
Nancy Sander
American Academy of Allergy, Asthma, and
Immunology
Michael Schatz, M.D., M.S.
American Academy of Family Physicians
Kurtis S. Elward, M.D., M.P.H., F.A.A.F.P.
American Academy of Pediatrics
GaryS. Rachelefsky, M.D.
American Academy of Physician Assistants
Tera Crisalida, P.A.-C., M.P.A.S.
American Associationfor Respiratory Care
Thomas J. Kallstrom, R.R.T., F.A.A.R.C., AE-C
American College of Allergy, Asthma, and
Immunology
William Storms, M.D.
American College of Chest Physicians
John Mitchell, M.D., F.A.C.P.
American College of Emergency Physicians
Richard M. Nowak, M.D., M.B.A., F.A.C.E.P.
American Lung Association
Noreen M. Clark, Ph.D.
American Medical Association
Paul V. Williams, M.D.
American Nurses Association
Karen Huss, D.N.Sc., R.N., A.P.R.N.B.C., F.A.A.N.,
F.A.A.A.A.I.
American Pharmacists Association
Dennis M. Williams, Pharm.D.
American Public Health Association
Pamela J. Luna, Dr.P.H., M.Ed.
American School Health Association
Lani S. M. Wheeler, M.D., F.A.A.P., F.A.S.H.A.
American Society of Health-System Pharmacists
Kathryn V. Blake, Pharm.D.
American Thoracic Society
Stephen C. Lazarus, M.D.
Asthma and Allergy Foundation of America
Mo Mayrides
Council of Stateand Territorial Epidemiologists
Sarah Lyon-Callo, M.A., M.S.
National Association of School Nurses
Donna Mazyck, R.N., M.S., N.C.S.N.
National Black Nurses Association, Inc.
Susan B. Clark, R.N., M.N.
National Center for Chronic Disease Prevention,
Centers forDisease Control and Prevention (CDC)
Sarah Merkle, M.P.H.
National Center for Environmental Health, CDC
Paul M. Garbe, M.D.
National Center for Health Statistics, CDC
Lara Akinbami, M.D.
National Institute for Occupational Safety and
Health, CDC
Margaret Filios, S.M., R.N.
National Heart, Lung, and Blood Institute
National Institutes of Health (NIH)
Elizabeth Nabel, M.D.
National Heart, Lung, and Blood Institute
NIH, Ad Hoc Committee on Minority Populations
Ruth I. Quartey, Ph.D.
National Institute of Allergy and Infectious Diseases
(NIAID), NIH
Peter J. Gergen, M.D., M.P.H.
iii
Acknowledgements
Acknowledgements

National Institute of Environmental Health Sciences,
NIH
Charles A. Wells, Ph.D.
National Medical Association
Michael Lenoir, M.D.
National Respiratory Training Center
Pamela Steele, M.S.N., C.P.N.P., AE-C
Society for Academic Emergency Medicine
Rita Cydulka, M.D., M.S.
Society for Public Health Education
Judith C. Taylor-Fishwick, M.Sc., AE-C
U.S. Department of Education
Dana Carr
U.S. Environmental Protection Agency
Indoor Environments Division
David Rowson, M.S.
U.S. Environmental Protection Agency
Office of Research and Development
Hillel S. Koren, Ph.D.
U.S. Food and Drug Administration
RobertJ.Meyer,M.D.
ThirdExpert Panel on the Management of Asthma
William W. Busse, M.D., Chair
University ofWisconsin Medical School
Madison, Wisconsin
Homer A. Boushey, M.D.
University of California–San Francisco
San Francisco, California
Carlos A. Camargo, Jr., M.D., Dr.P.H.
Massachusetts General Hospital
Boston, Massachusetts
David Evans, Ph.D., A.E.-C,
Columbia University
New York, New York
Michael B. Foggs, M.D.
Advocate Health Centers
Chicago, Illinois
Susan L. Janson, D.N.Sc., R.N., A.N.P., F.A.A.N.
University of California–San Francisco
San Francisco, California
H. William Kelly, Pharm.D.
University of New Mexico Health Sciences Center
Albuquerque, New Mexico
Robert F. Lemanske, M.D.
University of Wisconsin Hospital and Clinics
Madison, Wisconsin
Fernando D. Martinez, M.D.
University of Arizona Medical Center
Tucson, Arizona
Robert J. Meyer, M.D.
U.S. Food and Drug Administration
Silver Spring, Maryland
Harold S. Nelson, M.D.
National Jewish Medical and Research Center
Denver, Colorado
Thomas A. E. Platts-Mills, M.D., Ph.D.
University of Virginia School of Medicine
Charlottesville, Virginia
Michael Schatz, M.D., M.S.
Kaiser-Permanente–San Diego
San Diego, California
Gail Shapiro, M.D.*
University of Washington
Seattle, Washington
Stuart Stoloff, M.D.
University of Nevada School of Medicine
Carson City, Nevada
Stanley J. Szefler, M.D.
National Jewish Medical and Research Center
Denver, Colorado
Scott T. Weiss, M.D., M.S.
Brigham and Women’s Hospital
Boston, Massachusetts
Barbara P. Yawn, M.D., M.Sc.
Olmstead Medical Center
Rochester, Minnesota
iv Guidelines for the Diagnosis and Management of Asthma

Development of the guidelines was funded by the
NHLBI, NIH. Expert Panel members completed
financial disclosure forms, and the Expert Panel
members disclosed relevant financial interests to each
other prior to their discussions. Expert Panel mem-
bers participated as volunteers and were compensated
only for travel expenses related to the Expert Panel
meetings. Financial disclosure information covering
the 3-year period during which the guidelines were
developed is provided for each Expert Panel member
below.
Dr. Busse has served on the Speakers’ Bureaus of
GlaxoSmithKline, Merck, Novartis, and Pfizer; and on
the Advisory Boards of Altana, Centocor, Dynavax,
Genentech/Novartis, GlaxoSmithKline, Isis, Merck,
Pfizer, Schering, and Wyeth. He has received fund-
ing/grant support for research projects from Astellas,
Centocor, Dynavax, GlaxoSmithKline, Novartis,
and Wyeth. Dr. Busse also has research support from
the NIH.
Dr. Bousheyhas served as a consultant for Altana,
Protein DesignLab, and Sumitomo. He has received
honoraria from Boehringer-Ingelheim, Genentech,
Merck, Novartis, and Sanofi Aventis, and funding/-
grant support for research projects from the NIH.
Dr. Camargohas served on the Speakers’ Bureaus of
AstraZeneca, GlaxoSmithKline, Merck, and Schering
Plough; and as a consultant for AstraZeneca, Critical
Therapeutics, Dey Laboratories, GlaxoSmithKline,
MedImmune, Merck, Novartis, Praxair, Respironics,
Schering Plough, Sepracor, and TEVA. He has
received funding/grant support for research projects
from a variety of Government agencies and not-for-
profit foundations, as well as AstraZeneca, Dey
Laboratories, GlaxoSmithKline, MedImmune, Merck,
Novartis, and Respiromics.
Dr. Evans has received funding/grant support for
research projects from the NHLBI.
Dr. Foggs has served on the Speakers’ Bureaus of
GlaxoSmithKline, Merck, Pfizer, Sepracor, and UCB
Pharma; on the Advisory Boards of Alcon, Altana,
AstraZeneca, Critical Therapeutics, Genentech,
GlaxoSmithKline, and IVAX, and as consultant for
Merck and Sepracor. He has received funding/grant
support for research projects from GlaxoSmithKline.
Dr. Janson has served on the Advisory Board of
Altana, and as a consultant for Merck. She has
received funding/grant support for research projects
from the NHLBI.
Dr. Kelly has served on the Speakers’ Bureaus of
AstraZeneca and GlaxoSmithKline; and on the MAP
Pharmaceuticals Advisory Boards of AstraZeneca,
Merck, Novartis, and Sepracor.
Dr. Lemanske has served on the Speakers’ Bureaus of
GlaxoSmithKline and Merck, and as a consultant for
AstraZeneca, Aventis, GlaxoSmithKline, Merck, and
Novartis. He has received honoraria from Altana,
and funding/grant support for research projects from
the NHLBI and NIAID.
Dr. Martinez has served on the Advisory Board
of Merck and as a consultant for Genentech,
GlaxoSmithKline, and Pfizer. He has received
honoraria from Merck.
Dr. Meyer has no relevant financial interests.
Dr. Nelson has served on the Speakers’ Bureaus of
AstraZeneca, GlaxoSmithKline, Pfizer, and Schering
Plough; and as a consultant for Air Pharma, Altana
Pharma US, Astellas, AstraZeneca, Curalogic,
DeyLaboratories, Dynavax Technologies,
Genentech/Novartis, GlaxoSmithKline, Inflazyme
Pharmaceuticals, MediciNova, Protein Design
Laboratories, Sanofi-Aventis, Schering Plough,
and Wyeth Pharmaceuticals. He has received
funding/grant support for research projects from
Altana, Astellas, AstraZeneca, Behringer, Critical
Therapeutics, Dey Laboratories, Epigenesis,
Genentech, GlaxoSmithKline, IVAX, Medicinova,
Novartis, Sanofi-Aventis, Schering Plough, Sepracor,
TEVA, and Wyeth.
Dr. Platts-Mills has served on the Advisory
Committee of Indoor Biotechnologies. He has
received funding/grant support for a research project
from Pharmacia Diagnostics.
Dr. Schatz has served on the Speakers’ Bureaus of
AstraZeneca, Genentech, GlaxoSmithKline, and
Merck; and as a consultant for GlaxoSmithKline on
an unbranded asthma initiative. He has received
funding/grant support for research projects from
GlaxoSmithKline, Merck, and Sanofi-Aventis.
v
Acknowledgements
*The NAEPP would like to acknowledge the contributions of
Dr. Gail Shapiro, who served on the NAEPP Expert Panels from 1991
until her death in August 2006. She had a passion for improving asthma
care and an unwavering commitment to develop evidence-based recom-
mendations that would offer practical guidance for clinicians and
patients to work together to achieve asthma control.

Dr. Shapiro* served on the Speakers’ Bureaus of
AstraZeneca, Genentech, GlaxoSmithKline, IVAX
Laboratories, Key Pharmaceuticals, Merck, Pfizer
Pharmaceuticals, Schering Corporation, UCB
Pharma, and 3M; and as a consultant for Altana,
AstraZeneca, Dey Laboratories, Genentech/Novartis,
GlaxoSmithKline, ICOS, IVAX Laboratories, Merck,
Sanofi-Aventis, and Sepracor. She received
funding/grant support for research projects from
Abbott, AstraZeneca, Boehringer Ingelheim,
Bristol-Myers-Squibb, Dey Laboratories, Fujisawa
Pharmaceuticals, Genentech, GlaxoSmithKline,
Immunex, Key, Lederle, Lilly Research, MedPointe
Pharmaceuticals, Medtronic Emergency Response
Systems, Merck, Novartis, Pfizer, Pharmaxis, Purdue
Frederick, Sanofi-Aventis, Schering, Sepracor, 3M
Pharmaceuticals, UCB Pharma,
and Upjohn Laboratories.
Dr. Stoloff has served on the Speakers’ Bureaus
of Alcon, Altana, AstraZeneca, Genentech,
GlaxoSmithKline, Novartis, Pfizer, Sanofi Aventis,
and Schering; and as a consultant for Alcon, Altana,
AstraZeneca, Dey, Genentech, GlaxoSmithKline,
Merck, Novartis, Pfizer, Sanofi Aventis, and Schering.
Dr. Szefler has served on the Advisory Boards of
Altana, AstraZeneca, Genentech, GlaxoSmithKline,
Merck, Novartis, and Sanofi Aventis; and as a
consultant for Altana, AstraZeneca, Genentech,
GlaxoSmithKline, Merck, Novartis, and Sanofi
Aventis. He has received funding/grant support
for a research project from Ross.
Dr. Weiss has served on the Advisory Board of
Genentech, and as a consultant for Genentech and
GlaxoSmithKline. He has received funding/grant
support for research projects from GlaxoSmithKline.
Dr. Yawn has served on the Advisory Boards of
Altana, AstraZeneca, Merck, Sanofi Aventis, and
Schering Plough. She has received honoraria from
Pfizerand Schering Plough, and funding/grant
support for research projects from the Agency for
Healthcare Research and Quality, the CDC, the
NHLBI, Merck, and Schering Plough.
Consultant Reviewers
Financial disclosure information covering a
12 month period prior to the review of the
guidelines is provided below for each consultant.
Andrea J. Apter, M.D., M.Sc.
University of Pennsylvania Medical Center
Philadelphia, Pennsylvania
Noreen M. Clark, Ph.D.
University of Michigan School of Public Health
Ann Arbor, Michigan
Anne Fuhlbrigge, M.D., M.S.
Brigham and Women’s Hospital
Boston, Massachusetts
Elliott Israel, M.D.
Brigham and Women’s Hospital
Boston, Massachusetts
Meyer Kattan, M.D.
Mount Sinai Medical Center
New Yor k , New Yo r k
Jerry A. Krishnan. M.D., Ph.D.
The Johns Hopkins School of Medicine
Baltimore, Maryland
James T. Li, M.D., Ph.D., F.A.A.A.A.I.
Mayo Clinic
Rochester, Minnesota
Dennis R. Ownby, M.D.
Medical College of Georgia
Augusta, Georgia
Gary S. Rachelefsky, M.D.
University of California–Los Angeles, School of
Medicine
Los Angeles, California
Brian H. Rowe, M.D., M.Sc., C.C.F.P. (E.M.), F.C.C.P.
University of Alberta Hospital
Edmonton, Alberta, Canada
E. Rand Sutherland, M.D., M.P.H.
National Jewish Medical and Research Center
Denver, Colorado
Sandra R. Wilson, Ph.D.
Palo Alto Medical Foundation
Palo Alto, California
Robert A. Wood, M.D.
The Johns Hopkins School of Medicine
Baltimore, Maryland
vi Guidelines for the Diagnosis and Management of Asthma

Robert Zeiger, M.D.
Kaiser Permanente Medical Center
San Diego, California
Dr. Apter owns stock in Johnson & Johnson. She
has received funding/grant support for research
projects from the NHLBI.
Dr. Clark has no relevant financial interest.
Dr. Fulhlbrigge has served on the Speakers’ Bureau
of GlaxoSmithKline, the Advisory Boards of
GlaxoSmithKline and Merck, the Data Systems
Monitoring Board for a clinical trial sponsored by
Sepracor, and as a consultant for GlaxoSmithKline.
She has received honoraria from GlaxoSmithKline
and Merck, and funding/grant support for a research
project from Boehringer Ingelheim.
Dr. Israel has served on the Speakers’ Bureau of
Genentech and Merck, and as a consultant for
Asthmatx, Critical Therapeutics, Genentech, Merck,
Novartis Pharmaceuticals, Protein Design Labs,
Schering-Plough Company, and Wyeth. He has
received funding/grant support for research projects
from Asthmatx, Boehringer Ingelheim, Centocor,
Genentech, GlaxoSmithKline, and Merck.
Dr. Kattan has served on the Speakers’ Bureau of
AstraZeneca.
Dr. Krishnan has received funding/grant support
for a research project from Hill-Rom, Inc.
Dr. Li has received funding/grant support for
research projects from the American Lung
Association, GlaxoSmithKline, Pharming, and
ZLB Behring.
Dr. Ownby has no relevant financial interest.
Dr. Rachelefsky has served on the Speakers’ Bureaus
of AstraZeneca, GlaxoSmithKline, IVAX, Medpointe,
Merck, and Schering Plough. He has received
honoraria from AstraZeneca, GlaxoSmithKline,
IVAX, Medpointe, Merck, and Schering Plough.
Dr. Rowe has served on the Advisory Boards
of Abbott, AstraZeneca, Boehringer Ingelheim,
and GlaxoSmithKline. He has received honoraria
from Abbott, AstraZeneca, Boehringer Ingelheim,
and GlaxoSmithKline. Hehas received funding/
grant support for research projects from Abbott,
AstraZeneca, Boehringer Ingelheim, GlaxoSmith-
Kline, and Trudell.
Dr. Sutherland has served on the Speakers’ Bureau of
Novartis/Genentech and the Advisory Board of Dey
Laboratories. He has received honoraria from IVAX
and funding/grant support for research projects from
GlaxoSmithKline and the NIH.
Dr. Wilson has served as a consultant for the
Department of Urology, University of California,
San Francisco (UCSF); Asthmatx, Inc.; and the
Stanford UCSF Evidence-Based Practice Center.
She has received funding/grant support for research
projects from the NHLBI and from a subcontract to
Stanford University from Blue Shield Foundation.
Dr. Wood has served on the Speakers’ Bureaus of
Dey Laboratories, GlaxoSmithKline, and Merck; on
the Advisory Board of Dey Laboratories; and as a
consultant to Dey Laboratories. He has received
honoraria from Dey Laboratories, GlaxoSmithKline,
and Merck, and funding/grant support for a research
project from Genentech.
Dr. Zeiger has served on the Data Monitoring Board
of Genentech, Advisory Board of GlaxoSmithKline,
and as a consultant for Aerocrine, AstraZeneca,
and Genentech. He has received honoraria from
AstraZeneca and funding/grant support for a research
project from Sanofi-Aventis.
National Heart, Lung, and Blood Institute
Robinson (Rob) Fulwood, Ph.D., M.S.P.H.
Branch Chief, Enhanced Dissemination and
Utilization Branch
Division for the Application of Research Discoveries
James P. Kiley, Ph.D.
Director
Division of Lung Diseases
Gregory J. Morosco, Ph.D., M.P.H.
Associate Director for Prevention, Education, and
Control
Director
Division for the Application of Research Discoveries
Diana K. Schmidt, M.P.H.
Coordinator
National Asthma Education and Prevention Program
Virginia S. Taggart, M.P.H.
Program Director
Division of Lung Diseases
vii
Acknowledgements

American Institutes for Research
Heather Banks, M.A., M.A.T.
Senior Editor
Patti Louthian
Senior Desktop Publisher
Karen L. Soeken, Ph.D.
Methodologist
Mary Tierney, M.D.
Project Manager
viii Guidelines for the Diagnosis and Management of Asthma

ix
Preface
Preface
The Expert Panel Report 3 (EPR—3) Summary
Report 2007: Guidelines for the Diagnosis and
Management of Asthma was developed by an expert
panel commissioned by the National Asthma
Education and Prevention Program (NAEPP)
Coordinating Committee (CC), coordinated by the
National Heart, Lung, and Blood Institute (NHLBI)
of the National Institutes of Health.
Using the 1997 EPR—2 guidelines and the 2002
update on selected topics as the framework, the
expert panel organized the literature review and
updated recommendations for managing asthma
long term and for managing exacerbations around
four essential components of asthma care, namely:
assessment and monitoring, patient education,
control of factors contributing to asthma severity, and
pharmacologic treatment. Subtopics were developed
foreach of these four broad categories.
The EPR—3 Full Report and the EPR—3 Summary
Report 2007 have been developed under the excellent
leadership of Dr. William Busse, Panel Chair. The
NHLBI is grateful for the tremendous dedication of
time and outstanding work of all the members of the
expert panel, and for the advice from an expert
consultant group in developing this report. Sincere
appreciation is also extended to the NAEPP CC and
the Guidelines Implementation Panel as well as other
stakeholder groups (professional societies, voluntary
health, government, consumer/patient advocacy
organizations, and industry) for their invaluable
comments during the public review period that
helped to enhance the scientific credibility and
practical utility of this document.
Ultimately, the broad change in clinical practice
depends on the influence of local primary care
physicians and other health professionals who not
only provide state-of-the-art care to their patients,
butalso communicate to their peers the importance
of doing the same. The NHLBI and its partners will
forgenew initiatives based on these guidelines to
stimulate adoption of the recommendations at all
levels, but particularly with primary care clinicians at
the community level. We ask for the assistance of
every reader in reaching our ultimate goal: improving
asthma care and the quality of life for every asthma
patient with asthma
Gregory Morosco, Ph.D., M.P.H. James Kiley, Ph.D.
Director Director
Division for the Application of Research Discoveries Division of Lung Diseases
National Heart, Lung, and Blood Institute National Heart, Lung, and Blood Institute

xGuidelines for the Diagnosis and Management of Asthma

More than 22 million Americans have asthma, and
it is one of the most common chronic diseases of
childhood, affecting an estimated 6 million children.
The burden of asthma affects the patients, their
families, and society in terms of lost work and school,
lessened quality of life, and avoidable emergency
department (ED) visits, hospitalizations, and deaths.
Improved scientific understanding of asthma has
led to significant improvements in asthma care, and
the National Asthma Education and Prevention
Program (NAEPP) has been dedicated to translating
these research findings into clinical practice through
publicationand dissemination of clinical practice
guidelines. The first NAEPP guidelines were
published in 1991, and updates were made in 1997,
2002, and now with the current report. Important
gains have been made in reducing morbidity and
mortality rates due to asthma; however, challenges
remain. The NAEPP hopes that the “Expert Panel
Report3: Guidelines for the Diagnosis and
Management of Asthma—Full Report 2007”
(EPR—3: Full Report 2007) will support the efforts
of those who already incorporate best practices and
will help enlist even greater numbers of primary care
clinicians, asthma specialists, health care systems
and providers, and communities to join together in
making quality asthma care available to all people
who have asthma. The goal, simply stated, is to help
people with asthma control their asthma so that they
can be active all day and sleep well at night.
This EPR—3: Summary Report 2007 presents the
key recommendations from the EPR—3: Full Report
2007 (See www.nhlbi.nih.gov/guidelines/asthma/
asthgdln. htm). Detailed recommendations, the levels
of scientific evidence upon which they are based,
citations from the published scientific literature,
discussion of the Expert Panel’s rationale for the
recommendations, and description of methods used
todevelop the report are included in that resource
document. Because EPR—3: Full Report 2007 is
an update of previous NAEPP guidelines, highlights
of major changes in the update are presented below,
and figure 1 presents a summary of recommended
key clinical activities.
1
Introduction
Introduction
2Guidelines for the Diagnosis and Management of Asthma
HIGHLIGHTS OF MAJOR CHANGES IN EPR—3: FULL REPORT 2007
The following are highlights of major changes. Many recommendations were updated or expanded
based on new evidence. See EPR—3: Full Report 2007 for key differences at the beginning of each
section and for a full discussion.
New focus on monitoring asthma control as the goal for asthma therapy and distinguishing between
classifying asthma severity and monitoring asthma control.
■Severity: the intrinsic intensity of the disease process. Assess asthma severity to initiate therapy.
■Control: the degree to which the manifestations of asthma are minimized by therapeutic interventions and the
goals of therapy are met. Assess and monitor asthma control to adjust therapy.
New focus on impairment and risk as the two key domains of severity and control, and multiple
measures for assessment. The domains represent different manifestations of asthma, they may not correlate
with each other, and they may respond differentially to treatment.
■Impairment: frequency and intensity of symptoms and functional limitations the patient is experiencing currently
or has recently experienced.
■Risk: the likelihood of either asthma exacerbations, progressive decline in lung function (or, for children, lung
growth), or risk of adverse effects from medication.
Modifications in the stepwise approach to managing asthma long term.
■Treatment recommendations are presented for three age groups (0–4 years of age, 5–11 years of age, and
youths ≥12 years of age and adults). The course of the disease maychange over time; the relevance of
different measures of impairment or risk and the potential short- and long-term impact of medications may
be age related; and varied levels of scientific evidence are available for these three age groups.
■The stepwise approach expands to six steps to simplify the actions within each step. Previous guidelines had
several progressive actions within different steps; these are now separated into different steps.
■Medications have been repositioned within the six steps of care.
—Inhaled corticosteroids (ICSs) continue as preferred long-term control therapy for all ages.
—Combination of long-acting beta2-agonist (LABA) and ICS is presented as an equally preferred option, with
increasing the dose of ICS in step 3 care, in patients 5 years of age or older. This approach balances the
established beneficial effects of combination therapy in older children and adults with the increased risk for
severe exacerbations, although uncommon, associated with daily use of LABA.
—Omalizumabis recommended for consideration for youths ≥12 years of age who have allergies or for adults
who require step 5 or 6 care (severe asthma). Clinicians who administer omalizumab should be prepared
and equipped to identify and treat anaphylaxis that may occur.

3
Introduction
New emphasis on multifaceted approaches to patient education and to the control of environmental
factors or comorbid conditions that affect asthma.
■Patient education for a partnership is encouraged in expanded settings.
— Patient education should occur at all points of care: clinic settings (offering separate self-management
programs as well as integrating education into every patient visit), Emergency Departments (EDs) and hospitals,
pharmacies, schools and other community settings, and patients’ homes.
—Provider education should encourage clinician and health care systems support of the partnership (e.g.,
through interactive continuing medical education, communication skills training, clinical pathways, and
information system supports for clinical decisionmaking.
■Environmental control includes several strategies:
—Multifaceted approaches to reduce exposures are necessary; single interventions are generally ineffective.
—Consideration of subcutaneous immunotherapy for patients who have allergies at steps 2–4 of care (mild
or moderate persistent asthma) when there is a clear relationship between symptoms and exposure to an
allergen to which the patient is sensitive. Clinicians should be prepared to treat anaphylaxis that may occur.
—Potential benefits to asthma control by treating comorbid conditions that affect asthma.
Modifications to treatment strategies for managing asthma exacerbations. These changes:
■Simplify the classification of severity of exacerbations. For the urgent or emergency care setting: <40 percent
predicted forced expiratoryvolume in 1 second (FEV1)or peak expiratory flow (PEF) indicates severe
exacerbation and potential benefit from use of adjunctive therapies; ≥70 percent predicted FEV1or PEF is a
goal for discharge from the emergency care setting.
■Encourage development of prehospital protocols for emergency medical services to allow administration of
albuterol, oxygen, and, with medical oversight, anticholinergics and oral systemic corticosteroids.
■Modify recommendations on medications:
— Add levalbuterol.
— Add magnesium sulfate or heliox for severe exacerbations unresponsive to initial treatments.
—Emphasize use of oral corticosteroids. Doubling the dose of ICS for home management is not effective.
—Emphasize thatanticholinergics are used in emergency care, not hospital care.
— Add consideration of initiating ICS at discharge.
4Guidelines for the Diagnosis and Management of Asthma
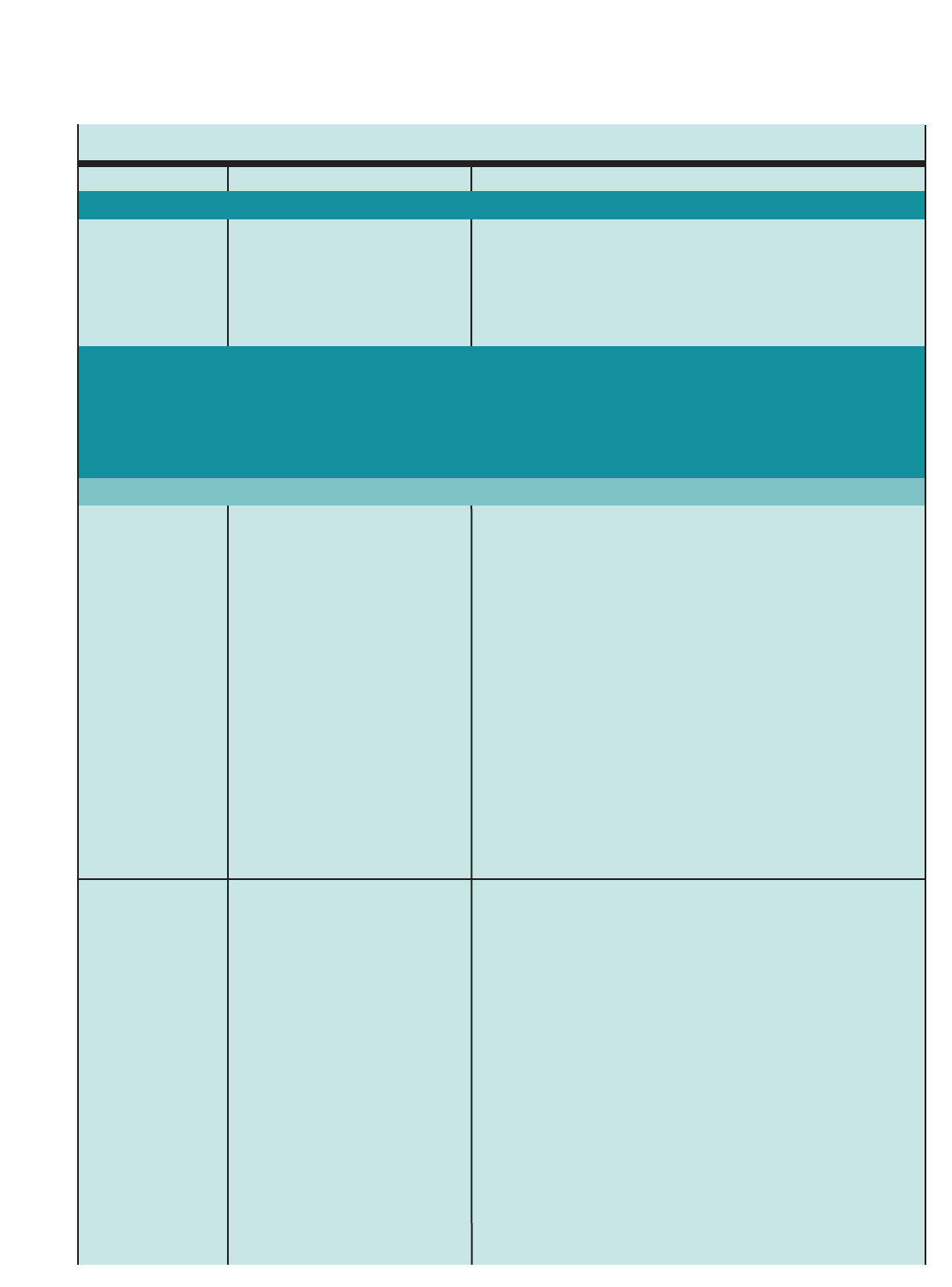
Figure 1. SUMMARY OF RECOMMENDED KEY CLINICAL ACTIVITIES FOR THE DIAGNOSIS AND MANAGEMENT OF ASTHMA
Clinical Issue Key Clinical Activities Action Steps
DIAGNOSIS
Establish asthma diagnosis.
MANAGING ASTHMA
LONG TERM
Assessment and
Monitoring
Four Components of Care
Assess asthma severity to initiate therapy.
Assess asthma control to monitor and
adjust therapy.
Schedule followup care.
Use severity classification chart, assessing both domains of impairment and
risk, to determine initial treatment.
Use asthma control chart, assessing both domains of impairment and risk, to
determine if therapy should be maintained or adjusted (step up if necessary,
step down if possible).
Use multiple measures of impairment and risk: different measures assess
different manifestations of asthma; theymaynot correlate with each other;
and they may respond differently to therapy. Obtain lung function measures by
spirometry at least every 1–2 years, more frequently for not-well-controlled
asthma.
Asthma is highly variable over time, and periodic monitoring is essential. In
general, consider scheduling patients at 2- to 6-week intervals while gaining
control; at 1–6 month intervals, depending on step of care required or duration
of control, to monitor if sufficient control is maintained; at 3-month intervals if
astep down in therapy is anticipated.
Assess asthma control, medication technique, written asthma action plan,
patient adherence and concerns at every visit.
Use medical history and physical examination to determine that symptoms of
recurrent episodes of airflow obstruction are present.
Use spirometry in all patients ≥5years of age to determine that airway
obstruction is at least partially reversible.
Consider alternative causes of airway obstruction.
Goal of asthma therapy is asthma control:
■Reduce impairment (prevent chronic symptoms, require infrequent use of short-acting beta2-agonist
(SABA), maintain (near) normal lung function and normal activity levels).
■Reduce risk (prevent exacerbations, minimize need for emergency care or hospitalization, prevent loss of
lung function, or for children, prevent reduced lung growth, have minimal or no adverse effects of therapy).
Education Provide self-management education. Teach and reinforce:
■Self-monitoring to assess level of asthma control and signs of worsening
asthma (either symptom or peak flow monitoring shows similar benefits for
most patients). Peak flow monitoring may be particularly helpful for patients
who have difficulty perceiving symptoms, a history of severe exacerbations,
or moderate or severe asthma.
■Using written asthma action plan (review differences between long-term
control and quick-relief medication).
■Taking medication correctly (inhaler technique and use of devices).
■Avoiding environmental factors that worsen asthma.
Tailor education to literacy level of patient. Appreciate the potential role of a
patient’scultural beliefs and practices in asthma management.
5
Introduction
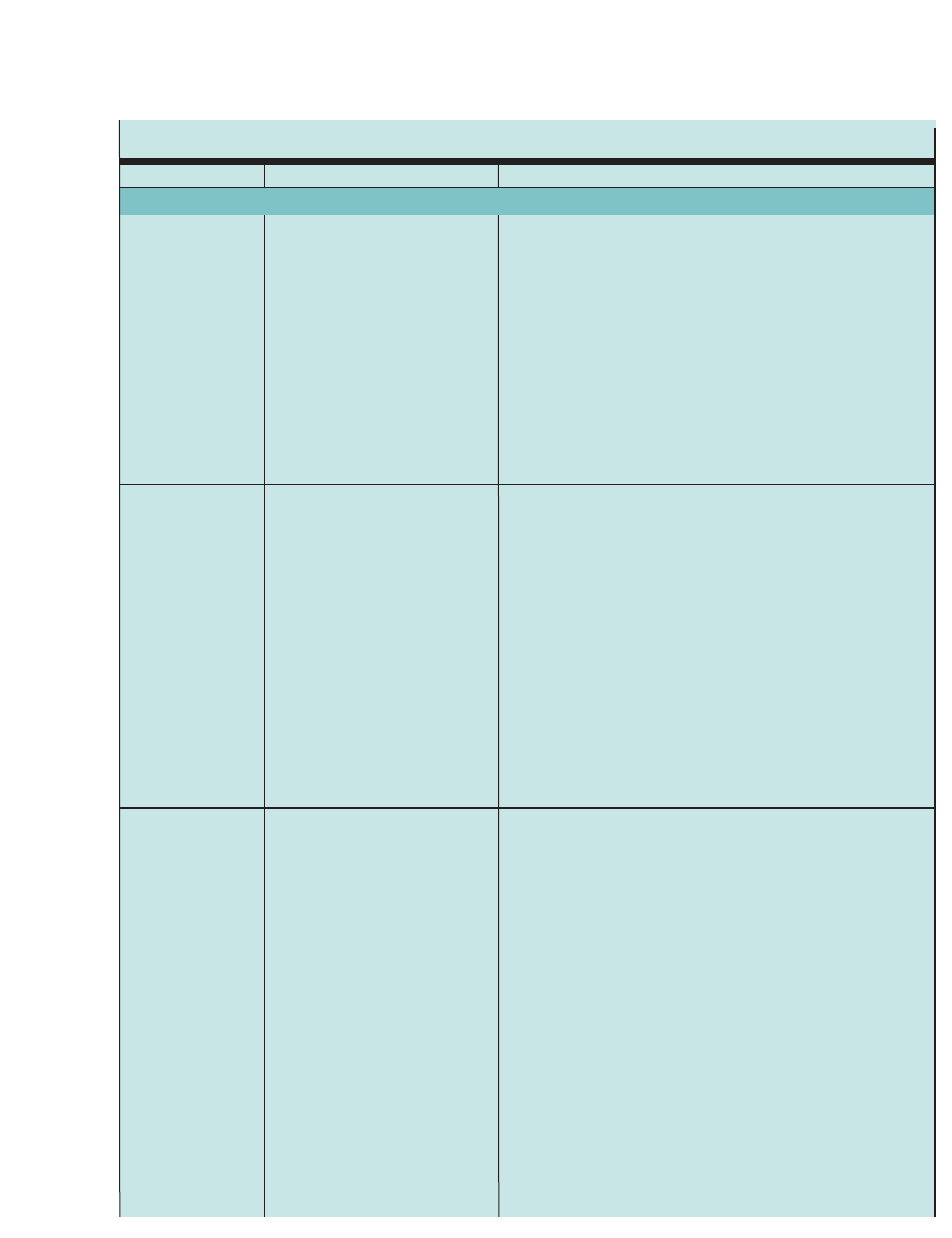
Figure 1. SUMMARY OF RECOMMENDED KEY CLINICAL ACTIVITIES FOR THE DIAGNOSIS AND MANAGEMENT OF ASTHMA (continued)
Clinical Issue Key Clinical Activities Action Steps
Education (continued Develop a written asthma action plan
in partnership with patient.
Integrate education into all points of
care where health professionals
interact with patients.
Agree on treatment goals and address patient concerns.
Provide instructions for (1) daily management (long-term control medication, if
appropriate, and environmental control measures) and (2) managing worsening
asthma (how to adjust medication, and know when to seek medical care).
Involve all members of the health care team in providing/reinforcing education,
including physicians, nurses, pharmacists, respiratory therapists, and asthma
educators.
Encourage education at all points of care: clinics (offering separate self-
management education programs as well as incorporating education into every
patient visit), Emergency Departments and hospitals, pharmacies, schools and
other community settings, and patients’ homes.
Use a variety of educational strategies and methods.
Control Environmental
Factors and Comorbid
conditions
Medications
Recommend measures to control
exposures to allergens and pollutants or
irritants thatmake and asthma worse.
Treat comorbid conditions.
Select medication and delivery
devices to meet patient’s needs and
circumstances.
Determine exposures, history of symptoms in presence of exposures, and
sensitivities (In patients who have persistent asthma, use skin or in vitro testing
to assess sensitivity to perennial indoor allergens.).
Advise patients on ways to reduce exposure to those allergens and pollutants,
or irritants to which the patient is sensitive. Multifaceted approaches are bene-
ficial; single steps alone are generally ineffective. Advise all patients and preg-
nant women to avoid exposure to tobacco smoke.
Consider allergen immunotherapy, by specifically trained personnel, for patients
who have persistent asthma and when there is clear evidence of a relationship
between symptoms and exposure to an allergen to which the patient is sensitive.
Consider especially: allergic bronchopulmonary aspergillosis; gastroesophageal
reflux, obesity, obstructive sleep apnea, rhinitis and sinusitis, and stress or
depression. Recognition and treatment of these conditions may improve
asthma control.
Consider inactivated influenza vaccine for all patients over 6 months of age.
Use stepwise approach (See below.) to identify appropriate treatment options.
Inhaled corticosteroids (ICSs) are the most effective long-term control therapy.
When choosing among treatment options, consider domain of relevance to
the patient (impairment, risk, or both), patient’s history of response to the
medication, and patient’s willingness and ability to use the medication.
Four Components of Care (continued)
6Guidelines for the Diagnosis and Management of Asthma
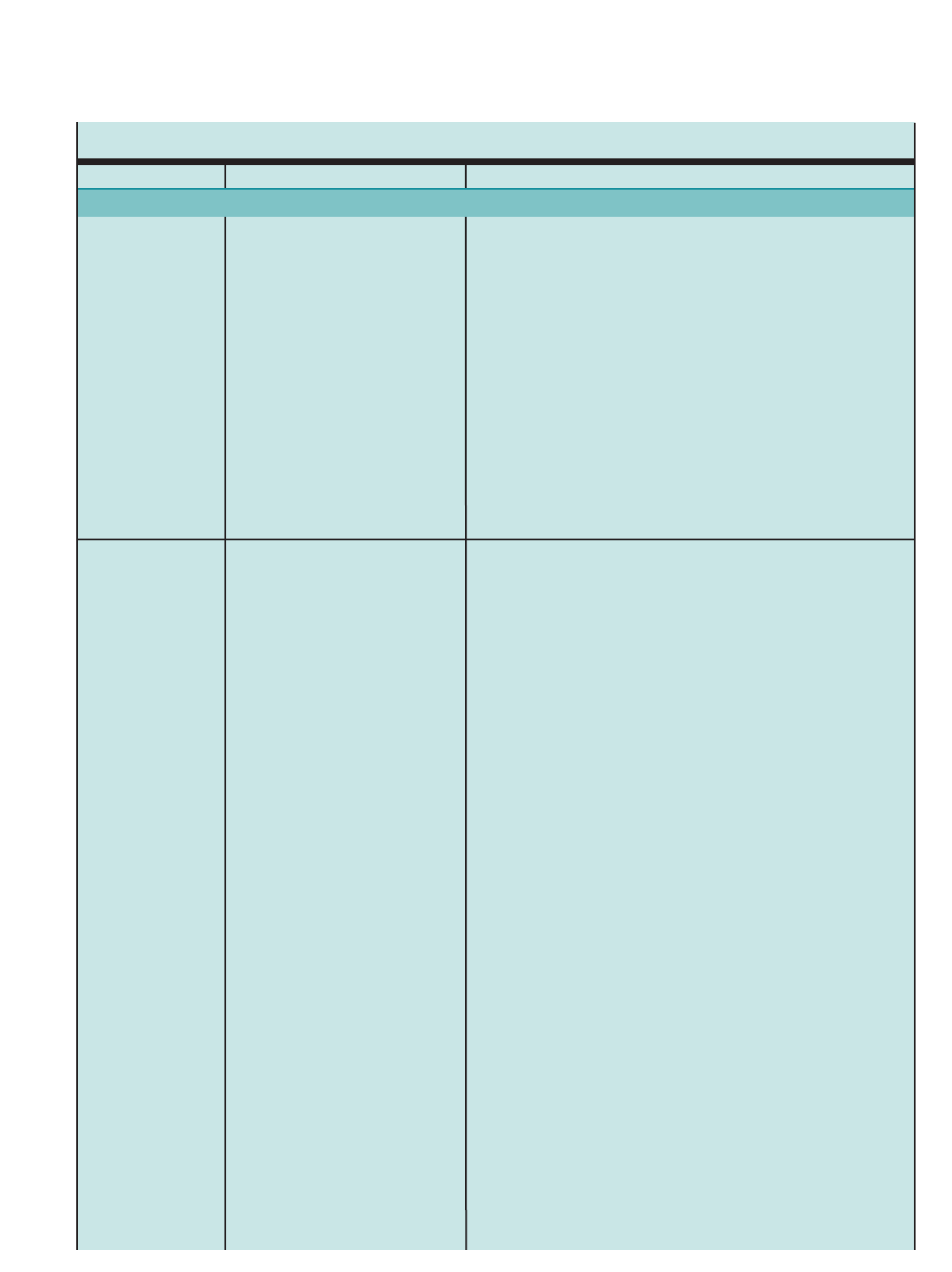
Figure 1. SUMMARY OF RECOMMENDED KEY CLINICAL ACTIVITIES FOR THE DIAGNOSIS AND MANAGEMENT OF ASTHMA (continued)
Clinical Issue Key Clinical Activities Action Steps
General Principles for
All Age Groups
Incorporate four components of care.
Initiate therapy based on asthma severity.
Adjust therapy based on asthma control.
Include medications, patient education, environmental control measures, and
management of comorbidities at each step. Monitor asthma control regularly
(See above, assessment and monitoring.).
For patients not taking long-term control therapy, select treatment step based
on severity (See figures on stepwise approach for different age groups.).
Patients who have persistent asthma require daily long-term control medication.
Once therapy is initiated, monitor the level of asthma control and adjust therapy
accordingly: step up if necessary and step down if possible to identify the
minimum amount of medication required to maintain asthma control.
Refer to an asthma specialist for consultation or comanagment if there are
difficulties achieving or maintaining control; step 4 care or higher is required
(step 3 care or higher for children 0–4 years of age); immunotherapy or
omalizumab is considered; or additional testing is indicated; or if the patient
required 2 bursts of oral systemic corticosticosteroids in the past year or a
hospitalization.
Stepwise Approach
Young children may be at high risk for severe exacerbations, yet have low
levels of impairment between exacerbations. Initiate daily long-term control
therapy for:
■Children who had ≥4episodes of wheezing the past year that lasted
>1 dayand affected sleep AND who have a positive asthma risk profile,
either (1) one of the following: parental history of asthma, physician
diagnosis of atopic dermatitis, or evidence of sensitization to aeroallergens
OR (2) two of the following: sensitization to foods, ≥4percent blood
eosinophilia, or wheezing apart from colds.
Consider initiating dailylong-term control therapy for:
■Children who consistentlyrequire SABA treatment >2 days per week
for >4 weeks.
■Children who have two exacerbations requiring oral systemic
corticosteroids within 6 months.
If no clear and positive response occurs within 4–6 weeks and the
patient’s/caregiver’s medication technique and adherence are satisfactory,
stop the treatment and consider alternative therapies or diagnoses.
If clear benefit is sustained for at least 3 months, consider step down to
evaluate the continued need for daily therapy. Children this age have high
rates of spontaneous remission of symptoms.
Ages 0–4 Years Consider daily long-term control therapy.
Monitor response closely, and
adjust treatment.
7
Introduction
Figure 1. SUMMARY OF RECOMMENDED KEY CLINICAL ACTIVITIES FOR THE DIAGNOSIS AND MANAGEMENT OF ASTHMA (continued)
Clinical Issue Key Clinical Activities Action Steps
Stepwise Approach (continued)
Address child’s concerns, preferences, and school schedule in selecting
treatments.
Encourage students to take a copy of written asthma action plan to school/
afterschool activities.
Treat exercise-induced bronchospasm (EIB) (See below.) Step up daily therapy
if the child has poor endurance or symptoms during normal play activities.
Treatment will not alter underlying progression of the disease, but a step up in
therapy may be required to maintain asthma control.
Address youth’s concerns, preferences, and school schedule in selecting
treatment.
Encourage students to take a copy of written asthma action plan to
school/afterschool activities.
Treat EIB. Step up daily therapy if the child has poor endurance or symptoms
during normal daily activities.
Establish reversibility with a short course of oral systemic corticosteroids.
Consider, for example: calcium and vitamin D supplements for patients who
take ICS and have risk factors for osteoporosis; increased sensitivity to side
effects of bronchodilators with increasing age; increased drug interactions
with theophylline; medications for arthritis (NSAIDs), hypertension, or
glaucoma (beta blockers) may exacerbate asthma.
Ages 5–11 Years Involve child in developing a written
asthma action plan.
Promote physical activity.
Monitor for disease progression and loss
of lung growth.
Involve youths in developing written
asthma action plan.
Promote physical activity.
Assess possible benefit of treatment in
older patients.
Adjust medications to address
coexisting medical conditions common
among older patients.
Treatment strategies to prevent EIB include:
■Long-term control therapy.
■Pretreatment before exercise with SABA, leukotriene receptor antagonists
(LTRAs), cromolyn or nedocromil; frequent or chronic use of long acting
beta2-agonist (LABA) for pretreatment is discouraged, as it may
disguise poorly controlled persistent asthma.
■Warmup period or a mask or scarf over the mouth for cold-induced EIB.
Exercise-Induced
Bronchospasm (EIB)
Prevent EIB
Monitor asthma control during all prenatal visits; asthma worsens in one-third
of women during pregnancy and improves in one-third; medications should
be adjusted accordingly.
It is safer to be treated with asthma medications than to have poorly
controlled asthma. Maintaining lung function is important to ensure oxygen
supply to the fetus.
Albuterol is the preferred SABA. ICS is the preferred long-term control
medication (Budesonide is preferred because more data are available on this
medication during pregnancy.).
Pregnancy Maintain asthma control through
pregnancy.
Assess asthma control prior to surgery. If lung function is not well controlled,
provide medications to improve lung function. A short course of oral systemic
corticosteroids may be necessary.
For patients receiving oral systemic corticosteroids during 6 months prior
to surgery, and for selected patients on high dose ICS, give 100 mg
hydrocortisone every 8 hours intravenously during the surgical period, and
reduce the dose rapidly within 24 hours after surgery.
Surgery Reduce risks for complications during
and after surgery.
Ages 12 and Older

8Guidelines for the Diagnosis and Management of Asthma
Figure 1. SUMMARY OF RECOMMENDED KEY CLINICAL ACTIVITIES FOR THE DIAGNOSIS AND MANAGEMENT OF ASTHMA (continued)
Clinical Issue Key Clinical Activities Action Steps
Managing Exacerbations
Include assessment and monitoring, patient education, environmental control,
and medications.
Instruct patients how to:
■Recognize early signs, symptoms, peak expiratory flow (PEF) measures that
indicate worsening asthma.
■Adjust medications (increase SABA and, in some cases, add oral systemic
corticosteroids) and remove or withdraw from environmental factors
contributing to the exacerbation.
■Monitor response and seek medical care if there is serious deterioration or
lack of response to treatment.
Home Management Incorporate four components of care.
Develop a written asthma action plan.
Treatment strategies include:
■Assessing initial severity by lung function measures (for ages ≥5years)
and symptom and functional assessment
■Supplemental oxygen
■Repetitive or continuous SABA
■Oral systemic corticosteroids
■Monitoring response with serial assessment of lung function measures,
pulse oximetry, and symptoms
■Considering adjunctive treatments magnesium sulfate or heliox in severe
exacerbations (e.g., forced expiratory volume in 1 second (FEV1)or
PEF <40 percent predicted) unresponsive to initial treatment
■Providing atdischarge:
—Medications: SABA, oral systemic corticosteroids; consider
initiating ICS
—Referral to followup care
— An emergency department asthma discharge plan
—Review of inhaler technique and, whenever possible, environmental
control measures
Management in the
Urgent or Emergency
Care Setting
Assess severity.
Treat to relieve hypoxemia and airflow
obstruction; reduce airway inflammation.
Monitor response.
Discharge with medication and patient
education

Definition and Pathophysiology
Asthma is a complex disorder characterized by variable
and recurring symptoms, airflow obstruction, bronchial
hyperresponsiveness, and an underlying inflammation.
The interaction of these features determines the clinical
manifestations and severity of asthma (See figure 2,
“The Interplay and Interaction Between Airway
Inflammation and the Clinical Symptoms and
Pathophysiology of Asthma.”) and the response to
treatment. The working definition of asthma is as
follows:
Asthma is a chronic inflammatory disorder of the airways
in whichmany cells and cellular elements play a role: in
particular, mast cells, eosinophils, neutrophils (especially in
sudden onset, fatal exacerbations, occupational asthma,
and patients who smoke), T lymphocytes, macrophages,
and epithelial cells. In susceptible individuals, this inflam-
mation causes recurrent episodes of coughing (particularly
at night or early in the morning), wheezing, breathlessness,
and chest tightness. These episodes are usually associated
with widespread but variable airflow obstruction that is
often reversible either spontaneously or with treatment.
Airflow limitation is caused by a variety of changes in
the airway, all in influenced by airway inflamation:
■Bronchoconstriction—bronchial smooth muscle
contraction that quickly narrows the airways
in response to exposure to a variety of stimuli,
including allergens or irritants.
■Airway hyperresponsiveness—an exaggerated
bronchoconstrictor response to stimuli.
■Airway edema—as the disease becomes more
persistent and inflammation becomes more
progressive, edema, mucus hypersecretion, and
formation of inspissated mucus plugs further
limit airflow.
Remodeling of airways may occur. Reversibility
of airflowlimitation may be incomplete in some
patients. Persistent changes in airway structure
occur, including sub-basement fibrosis, mucus
hypersecretion, injury to epithelial cells, smooth
muscle hypertrophy, and angiogenesis.
Recent studies provide insights on different phenotypes
of asthma that exist. Different manifestations of
asthma may have specific and varying patterns of
inflammation (e.g., varying intensity, cellular mediator
pattern, and therapeutic response). Further studies
will determine if different treatment approaches
benefit the different patterns of inflammation.
9
Asthma Definition and Implications for Treatment
Figure2. THE INTERPLAY AND INTERACTION BETWEEN
AIRWAY INFLAMMATION AND THE CLINICAL SYMPTOMS AND
PATHOPHYSIOLOGY OF ASTHMA
Inflammation
Airway
Hyperresponsiveness
Clinical Symptoms
Airway
Obstruction
Asthma Definition and Implications
for Treatment

Causes of Asthma
The development of asthma appears to involve the
interplay between host factors (particularly genetics)
and environmental exposures that occur at a crucial
time in the development of the immune system. A
definitive cause of the inflammatory process leading
to asthma has not yet been established.
■Innate immunity. Numerous factors may affect the
balance between Th1-type and Th2- type cytokine
responses in early life and increase the likelihood
that the immune response will downregulate the
Th1 immune response that fights infection and
instead will be dominated by Th2 cells, leading to
the expression of allergic diseases and asthma.
This is known as the “hygiene hypothesis,” which
postulates that certain infections early in life,
exposure to other children (e.g., presence of older
siblingsand early enrollment in childcare, which
have greater likelihood of exposure to respiratory
infection), less frequent use of antibiotics, and
“country living” is associated with a Th1 response
and lower incidence of asthma, whereas the
absence of these factors is associated with a
persistent Th2 response and higher rates of
asthma. Interventions to prevent the onset of
this process (e.g., with probiotics) are under study,
butno recommendations can yet be made.
■Genetics. Asthma has an inheritable component,
butthe genetics involved remain complex. As
the linkage of genetic factors to different asthma
phenotypes becomes clearer, treatment approaches
may become directed to specific patient
phenotypes and genotypes.
■Environmental factors.
— Two major factors are the most important in
the development, persistence, and possibly
the severity of asthma: airborne allergens
(particularly sensitization and exposure to
house-dust miteand Alternaria) and viral
respiratory infections (including respiratory
syncytial virus [RSV] and rhinovirus).
— Other environmental factors are under study:
tobacco smoke (exposure in utero is associated
with an increased risk of wheezing, but it is not
certain this is linked to subsequent development
of asthma), air pollution (ozone and particular
matter) and diet (obesity or low intake of
antioxidants and omega-3 fatty acids). The
association of these factors with the onset of
asthma has not been clearly defined. A number
of clinical trials have investigated dietary and
environmental manipulations, but these
trials have not been sufficiently long term or
conclusive to permit recommendations.
Implications for Treatment
Knowledge of the importance of inflammation to the
central features of asthma continues to expand and
underscores inflammation as a primary target of
treatment. Studies indicate that current therapeutic
approaches are effective in controlling symptoms,
reducing airflow limitation, and preventing
exacerbations, but currently available treatments do
not appear to prevent the progression of asthma in
children. As various phenotypes of asthma are defined
and inflammatory and genetic factors become more
apparent, new therapeutic approaches may be
developed that will allow even greater specificity to
tailor treatment to the individual patient’s needs
and circumstances.
10 Guidelines for the Diagnosis and Management of Asthma
To establish a diagnosis of asthma, the clinician
should determine that symptoms of recurrent
episodes of airflow obstruction or airway
hyperresponsiveness are present; airflow
obstruction is at least partially reversible; and
alternative diagnoses are excluded.
■Episodic symptoms of airflow obstruction or
airway hyperresponsiveness are present.
■Airflow obstruction is at least partially reversible,
measured by spirometry. Reversibility is deter-
mined by an increase in FEV1of >200 mL and ≥12
percent from baseline measure after inhalation of
short-acting beta2-agonist (SABA). Some studies
indicate that an increase of ≥10 percent of the
predicted FEV1after inhalation of a SABA may
have higher likelihood of separating patients
who have asthma from those who have chronic
obstructive pulmonary disease (COPD).
■Alternativediagnoses are excluded. See discussion
below.
Recommended methods to establish the diagnosis
are:
■Detailed medical history. See figure 3, “Suggested
Items for Medical History,” for questions to
include.
■Physical examination may reveal findingsthat
increase the probability of asthma, but the
absence ofthese findingsdoes not rule out
asthma, because the disease is variable and signs
may be absent between episodes. The examination
focuses on:
—upper respiratory tract (increased nasal
secretion, mucosal swelling, and/or nasal polyp;
— chest (sounds of wheezing during normal
breathing or prolonged phase of forced
exhalation, hyperexpansion of the thorax, use
of accessory muscles, appearance of hunched
shoulders, chest deformity); and
— skin (atopic dermatitis, eczema).
■Spirometry can demonstrate obstruction and assess
reversibility in patients ≥5years of age. Patients’
perceptions of airflow obstruction are highly
variable. Spirometry is an essential objective
measure to establish the diagnosis of asthma,
11
Diagnosis of Asthma
KEY SYMPTOM INDICATORS FOR CONSIDERING
ADIAGNOSIS OF ASTHMA
The presence of multiple key indicators increases the
probability of asthma, but spirometry is needed to establish
adiagnosis.
■Wheezing—high-pitched whistling sounds when
breathing out—especially in children. A lack of wheezing
and a normal chest examination do not exclude asthma.
■History of any of the following:
—Cough (worse particularly at night)
—Recurrent wheeze
—Recurrent difficulty in breathing
—Recurrent chest tightness
■Symptoms occur or worsen in the presence of:
—Exercise
—Viral infection
—Inhalant allergens (e.g., animals with fur or hair,
house-dust mites, mold, pollen)
—Irritants (tobacco or wood smoke, airborne chemicals)
—Changes in weather
—Strong emotional expression (laughing or crying hard)
—Stress
—Menstrual cycles
■Symptoms occur or worsen atnight, awakening the patient.
Diagnosis of Asthma
12 Guidelines for the Diagnosis and Management of Asthma
because the medical history and physical
examination are not reliable means of excluding
other diagnoses or of assessing lung status.
Spirometry is generally recommended, rather than
measurements by a peak flow meter, due to wide
variability in peak flow meters and reference values.
Peak flow meters are designed for monitoring, not as
diagnostic tools.
Adifferential diagnosis of asthma should be
considered. Recurrent episodes of cough and
wheezing most often are due to asthma in both
children and adults; however, other significant causes
of airway obstruction leading to wheeze must be
considered both in the initial diagnosis and if there
is no clear response to initial therapy.
■Additional studies are not routinely necessary
but may be useful when considering alternative
diagnoses.
—Additional pulmonary function studies will
help if thereare questions about COPD
(diffusing capacity), a restrictive defect
(measures of lung volumes), or VCD
(evaluation of inspiratory flow-volume loops).
—Bronchoprovocation with methacholine,
histamine, cold air, orexercise challenge may
be useful when asthma is suspected and
spirometry is normal or near normal. For
safety reasons, bronchoprovocation should be
carried out only by a trained individual. A
positive test is diagnostic for airway hyperre
sponsiveness, which is a characteristic feature
of asthma but can also be present in other
conditions. Thus, a positive test is consistent
with asthma, but a negative test may be more
helpful to rule out asthma.
—Chest x ray may be needed to exclude other
diagnoses.
—Biomarkersof inflammation arecurrently
being evaluated for their usefulness in the
diagnosis and assessment of asthma.
Biomarkers include total and differential cell
count and mediator assays in sputum, blood,
urine, and exhaled air.
■Common diagnostic challenges include the
following:
—Cough variant asthma. Coughcan bethe
principal—or only—manifestation of
asthma, especially in young children.
DIFFERENTIAL DIAGNOSTIC POSSIBILITIES FOR ASTHMA
Infants and Children
Upper airway diseases
■Allergic rhinitis and sinusitis
Obstructions involving large airways
■Foreign body in trachea or bronchus
■Vocal cord dysfunction (VCD)
■Vascular rings or laryngeal webs
■Laryngotracheomalacia, tracheal stenosis, or
bronchostenosis
■Enlarged lymph nodes or tumor
Obstructions involving small airways
■Viral bronchiolitis or obliterative bronchiolitis
■Cystic fibrosis
■Bronchopulmonary dysplasia
■Heart disease
Other causes
■Recurrent cough not due to asthma
■Aspiration from swallowing mechanism dysfunction
or gastroesophageal reflux
Adults
■Chronic obstructive pulmonary disease (COPD)
(e.g., chronic bronchitis or emphysema)
■Congestive heart failure
■Pulmonary embolism
■Mechanical obstruction of the airways
(benign and malignant tumors)
■Pulmonaryinfiltration with eosinophilia
■Cough secondaryto drugs (e.g., angiotensin-
converting enzyme [ACE] inhibitors)
■Vocal cord dysfunction (VCD)

13
Diagnosis of Asthma
FIGURE 3. SUGGESTED ITEMS FOR MEDICAL HISTORY*
1. Symptoms
Cough
Wheezing
Shortness of breath
Chest tightness
Sputum production
2. Pattern of symptoms
Perennial, seasonal, or both
Continual, episodic, or both
Onset, duration, frequency (number of days or nights,
per week or month)
Diurnal variations, especially nocturnal and on awakening
in early morning
3. Precipitating and/or aggravating factors
Viral respiratoryinfections
Environmental allergens, indoor (e.g., mold, house-dust
mite, cockroach, animal dander or secretory products)
and outdoor (e.g., pollen)
Characteristics of home including age, location, cooling and
heating system, wood-burning stove, humidifier, carpeting
over concrete, presence of molds or mildew, presense of
pets with fur or hair, characteristics of rooms where
patient spends time (e.g., bedroom and living room with
attention to bedding, floor covering, stuffed furniture)
Smoking (patient and others in home or daycare)
Exercise
Occupational chemicals or allergens
Environmental change (e.g., moving to new home; going on
vacation; and/or alterations in workplace, work processes,
or materials used)
Irritants (e.g., tobacco smoke, strong odors, air pollutants,
occupational chemicals, dusts and particulates, vapors,
gases, and aerosols)
Emotions (e.g., fear, anger, frustration, hard crying or laughing)
Stress (e.g., fear, anger, frustration)
Drugs (e.g., aspirin; and other nonsteroidal anti-inflammatory
drugs, beta-blockers including eye drops, others)
Food, food additives, and preservatives (e.g., sulfites)
Changes in weather, exposure to cold air
Endocrine factors (e.g., menses, pregnancy, thyroid disease)
Comorbid conditions (e.g. sinusitis, rhinitis, gastroesophageal
reflux disease (GERD)
4. Development of disease and treatment
Age of onset and diagnosis
History of early-life injury to airways (e.g., bronchopulmonary
dysplasia, pneumonia, parental smoking)
Progression of disease (better or worse)
Present management and response, including plans for
managing exacerbations
Frequency of using short-acting beta2-agonist (SABA)
Need for oral corticosteroids and frequency of use
5. Family history
History of asthma, allergy, sinusitis, rhinitis, eczema, or
nasal polyps in close relatives
6. Social history
Daycare, workplace, and school characteristics that may
interfere with adherence
Social factors that interfere with adherence, such as
substance abuse
Social support/social networks
Level of education completed
Employment
7. History of exacerbations
Usual prodromal signs and symptoms
Rapidity of onset
Duration
Frequency
Severity (need for urgent care, hospitalization, intensive
care unit (ICU) admission.)
Life-threatening exacerbations (e.g., intubation, intensive care
unit admission)
Number and severity of exacerbations in the past year.
Usual patterns and management (whatworks?)
8. Impact of asthma on patient and family
Episodes of unscheduled care (emergency department (ED),
urgent care, hospitalization)
Number of days missed from school/work
Limitation of activity, especially sports and strenuous work
History of nocturnal awakening
Effect on growth, development, behavior, school or work
performance, and lifestyle
Impact on family routines, activities, or dynamics
Economic impact
9. Assessment of patient’s and family’s perceptions
of disease
Patient’s, parent’s, and spouse’s or partner’s knowledge of
asthma and belief in the chronicity of asthma and in
the efficacy of treatment
Patient’s perception and beliefs regarding use and long-
term effects of medications
Ability of patient and parents, spouse, or partner to cope
with disease
Level of family support and patient’s and parents’,
spouse’s, or partner’scapacity to recognize severity
of an exacerbation
Economic resources
Sociocultural beliefs
Adetailed medical history of the new patient who is known or thought to have asthma should address the following items
*This list does not represent a standardized assessment or diagnostic instrument. The validity and reliability of this list have not been assessed.

Monitoring of PEF or bronchoprovocation may
be helpful. Diagnosis is confirmed by a posi-
tive response to asthma medications.
—VCD can mimic asthma, but it is a distinct
disorder. VCD may coexist with asthma.
Asthma medications typically do little, if any
thing, to relieve VCD symptoms. Variable
flattening of the inspiratory flow loop on
spirometry is strongly suggestive of VCD.
Diagnosis of VCD is from indirect or direct
vocal cord visualization during an episode,
during which the abnormal adduction can be
documented. VCD should be considered in
difficult-to-treat, atypical asthma patients
and in elite athletes who have exercise-related
breathlessness unresponsive to asthma
medication.
—Gastroesophageal reflux disease (GERD),
obstructive sleep apnea (OSA), and allergic
bronchopulmonary aspergillosis (ABPA) may
coexist with asthma and complicate diagnosis.
Seethe sectionon “Comorbid Conditions,” for
further discussion.
—Children ages 0–4 years. Diagnosis in infants
and young children is challenging and is
complicated by the difficultyin obtaining
objective measurements of lung function in
this age group. Caution is needed to avoid
giving young children inappropriate
prolonged asthma therapy. However, it is
important to avoid underdiagnosing asthma,
and thereby missing the opportunity to
treat a child, by using such labels as “wheezy
bronchitis,” “recurrent pneumonia,” or
“reactive airway disease” (RAD). The chronic
airway inflammatory response and structural
changes that are characteristic of asthma can
develop in the preschool years, and appropriate
asthma treatment will reduce morbidity.
■Consider referral to an asthma specialist if signs
and symptoms are atypical, if there are problems
with a differential diagnosis, or if additional
testing is indicated.
14 Guidelines for the Diagnosis and Management of Asthma
Achieving and maintaining asthma control requires
four components of care: assessment and monitoring,
education for a partnership in care, control of envi-
ronmental factors and comorbid conditions that
affect asthma, and medications. A stepwise approach
to asthma management incorporates these four
components, emphasizing that pharmacologic
therapy is initiated based on asthma severity and
adjusted (stepped up or down) based on the level of
asthma control. Special considerations of therapeutic
options within the stepwise approach may be
necessary for situations such as exercise-induced
bronchospasm (EIB), surgery, and pregnancy.
Four Components of Asthma Care
Component 1: Assessing and Monitoring Asthma
Severity and Asthma Control
The functions of assessment and monitoring are
closely linked to the concepts of severity, control, and
responsiveness to treatment:
■Severity: the intrinsic intensity of the disease
process. Severity is most easily and directly measured
in a patient who is not receiving long-term control
therapy. Severity can also be measured, once
asthma control is achieved, by the step of care
(i.e., the amount of medication) required to
maintain control.
■Control: the degree to which the manifestations of
asthma are minimized by therapeutic intervention
and the goals of therapy are met.
■Responsiveness: the ease with which asthma
control is achieved by therapy.
Asthma severity and asthma control include the
domains of current impairment and future risk.
■Impairment: frequency and intensity of symptoms
and functional limitations the patient is currently
experiencing or has recently experienced.
15
Managing Asthma Long Term
Managing Asthma Long Term
GOAL OF THERAPY: CONTROL OF ASTHMA
Reduce Impairment
■Prevent chronic and troublesome symptoms (e.g., coughing or breathlessness in the daytime, in the night, or after exertion).
■Require infrequent use (≤2days a week) of inhaled SABA for quick relief of symptoms (not including prevention of
exercise-induced bronchospasm [EIB]).
■Maintain (near) normal pulmonary function.
■Maintain normal activity levels (including exercise and other physical activity and attendance at school or work).
■Meet patients’ and families’ expectations of and satisfaction with asthma care.
Reduce Risk
■Prevent recurrent exacerbations of asthma and minimize the need for ED visits or hospitalizations.
■Prevent loss of lung function; for children, prevent reduced lung growth.
■Provide optimal pharmacotherapy with minimal or no adverse effects of therapy.

■Risk: the likelihood of either asthma exacerbations,
progressive decline in lung function (or, for
children, reduced lung growth), or risk of adverse
effects from medication.
This distinction emphasizes the multifaceted nature
of asthma and the need to consider separately
asthma’s current, ongoing effects on the present
quality of life and functional capacity and the future
risk of adverse events. The two domains may
respond differentially to treatment. For example,
evidence demonstrates that some patients can
have adequate control of symptoms and minimal
day-to-day impairment, but still be at significant
risk of exacerbations; these patients should be
treated accordingly.
The specific measures used to assess severity and
control are similar: symptoms, use of SABAs for quick
relief of symptoms, limitations to normal activities due
to asthma, pulmonary function, and exacerbations.
Multiple measures are important, because different
measures assess different manifestations of the disease
and maynot correlatewith each other.
The concepts of severity and control are used as
follows for managing asthma:
■Assess severityto initiate therapy. Seesectionon
“Stepwise Approach for Managing Asthma” for
figures on classifying asthma severity and initiating
therapy in different age groups. During a patient’s
initial presentation, if the patient is not currently
taking long-term control medication, asthma severity
is assessed to guide clinical decisions for initiating
the appropriate medication and other therapeutic
interventions.
■Assess control to adjust therapy. See section on
“Stepwise Approach for Managing Asthma” for
figures on assessing asthma control and adjusting
therapyin different age groups. Once therapy is
initiated, the emphasis for clinical management
thereafter is changed to the assessment of asthma
control. The level of asthma control will guide
decisions either to maintain or to adjust therapy
(i.e., step up if necessary, step down if possible).
■For assessing a patient’s overall asthma severity,
once the most optimal asthma control is achieved
and maintained, or for population-based evalua-
tions or clinical research, asthma severity can be
inferredby correlating the level of severity with the
lowest level of treatment required to maintain
control.
However, the emphasis for clinical management is
to assess asthma severity prior to initiating therapy
and then to assess asthma control for monitoring
and adjusting therapy.
For the initial assessment to characterize the patient’s
asthma and guidedecisions for initiating therapy, use
information from the diagnostic evaluation to:
■Classify asthma severity.
■Identify precipitating factors for episodic symp-
toms (e.g., exposure at home, work, daycare, or
school to inhalant allergens or irritants).
■Identify comorbid conditions that may impede
asthma management (e.g., sinusitis, rhinitis, GERD,
OSA, obesity, stress, or depression).
■Assess the patient’s knowledge and skills for
self-management.
Forperiodic monitoring of asthma control to guide
decisions for maintaining or adjusting therapy:
■Instruct patients to monitor their asthma control in
an ongoing manner. All patients should be taught
how to recognize inadequate asthma control.
— Either symptom or peak flow monitoring is
appropriate for most patients; evidence suggests
the benefits are similar.
— Consider daily peak-flow monitoring for
patients who have moderate or severe persistent
asthma, patients who have a history of severe
exacerbations, and patients who poorly perceive
airway obstruction or worsening asthma.
■Monitor asthma control periodically in clinical
visits, because asthma is highly variable over time
andtherapy may need to be adjusted (stepped up
if necessary, stepped down if possible). The
frequency ofmonitoring is a matter of clinical
judgment. In general:
16 Guidelines for the Diagnosis and Management of Asthma
Lowest level
of treatment
required to
maintain
control
(See “Stepwise
Approach for
Managing
Asthma”
for treatment
steps.)
Classification of Asthma Severity When
Asthma Is Well Controlled
Persistent
Intermittent Mild Moderate Severe
Step 1 Step 2 Step 3 Step 5
or or
Step 4 Step 6
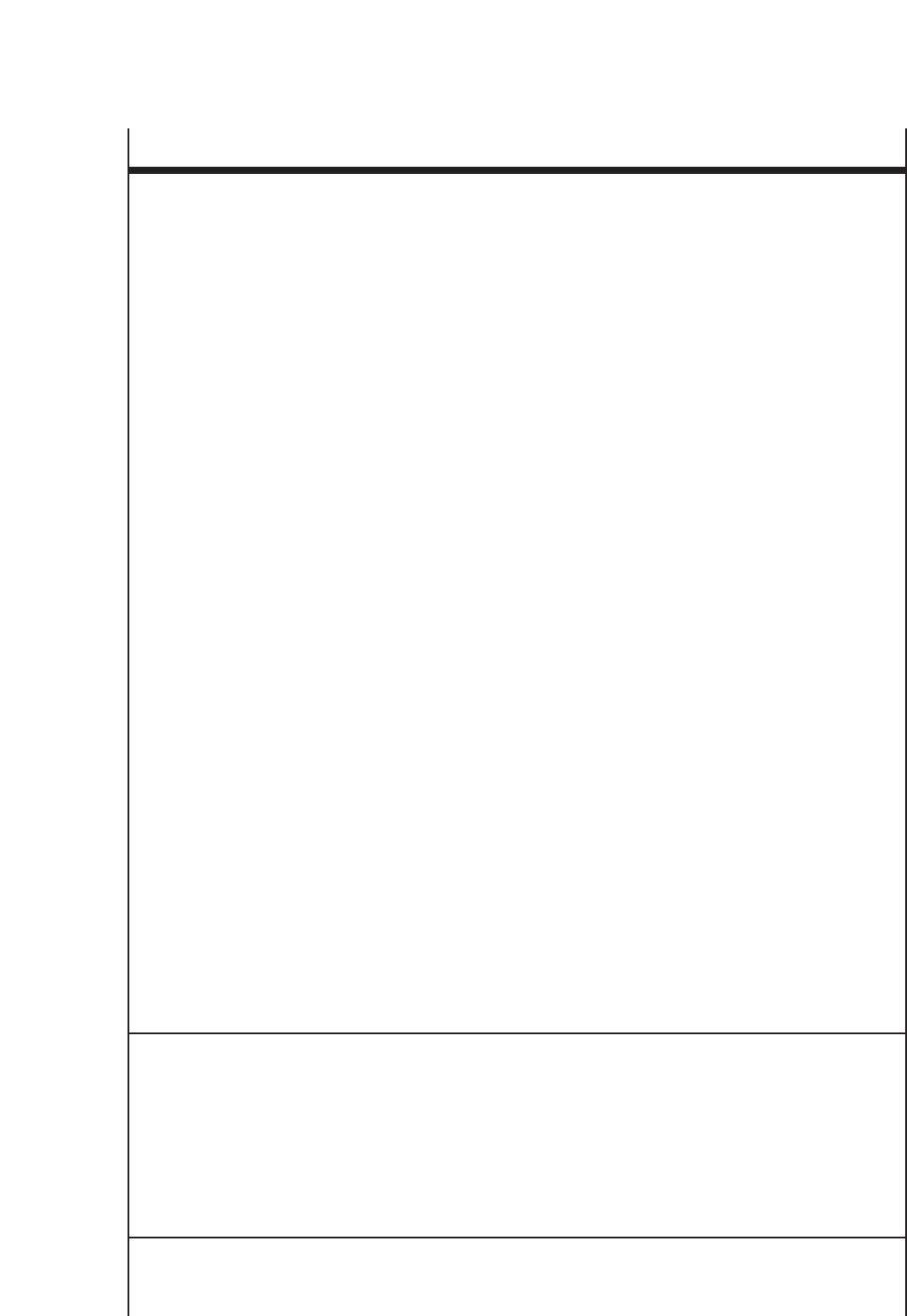
17
Managing Asthma Long Term
FIGURE 4. SAMPLE PATIENT SELF-ASSESSMENT SHEET FOR FOLLOWUP VISITS*
Name:___________________________________ Date:________________________
Your Asthma Control
How many days in the past week have you had chest tightness, cough, shortness of breath, or
wheezing (whistling in your chest)?
_____ 0 _____ 1 _____ 2 _____ 3 _____ 4 _____ 5 _____ 6 _____ 7
How many nights in the past week have you had chest tightness, cough, shortness of breath, or
wheezing (whistling in your chest)?
_____ 0 _____ 1 _____ 2 _____ 3 _____ 4 _____ 5 _____ 6 _____ 7
Do you perform peak flow readings at home? ______ yes ______ no
If yes, did you bring your peak flow chart? ______ yes ______ no
How manydays in the past week has asthma restricted your physical activity?
_____ 0 _____ 1 _____ 2 _____ 3 _____ 4 _____ 5 _____ 6 _____ 7
Have you had anyasthma attacks since your last visit? ______ yes ______ no
Have you had any unscheduled visits to a doctor, including to the emergency department,
since your last visit? ______ yes ______ no
How well controlled is your asthma, in your opinion? ____ very well controlled
____ somewhat controlled
____ not well controlled
Average number of puffs per day of quick-relief
medication (short acting beta2-agonist) ____________________
Taking your medicine
What problems have you had taking your medicine or following your asthma action plan?
Please ask the doctor or nurse to review how you take your medicine.
Your questions
Whatquestions or concerns would you like to discuss with the doctor?
How satisfied are you with your asthma care? ____ very satisfied
____ somewhat satisfied
____ not satisfied
*These questions are examples and do not represent a standardized assessment instrument. Other examples of asthma control questions:
Asthma Control Questionnaire (Juniper); Asthma TherapyAssessment Questionnaire (Volmer); Asthma Control Test (Nathan); Asthma Control
Score (Boulet)
18 Guidelines for the Diagnosis and Management of Asthma
— Schedule visits at 2- to 6-week intervals for
patients who are just starting therapy or who
require a step up in therapy to achieve or
regain asthma control.
— Schedule visits at 1- to 6-month intervals, after
asthma control is achieved, to monitor whether
asthma control is maintained. The interval will
depend on factors such as the duration of asthma
control or the level of treatment required.
— Consider scheduling visits at 3-month intervals
if a step down in therapy is anticipated.
■Assess asthma control, medication technique,
the written asthma action plan, adherence, and
patient concerns at every patient visit. See figure
4for a sample patient self-assessment of overall
asthma control and asthma care.
■Use spirometryto obtain objective measures of lung
function.
—Perform spirometry at the following times:
• Atthe initial assessment.
• After treatment is initiated and symptoms
and PEF have stabilized.
• During periods of progressive or prolonged
loss of asthma control.
• At least every 1–2 years; more frequently
depending on response to therapy.
—Low FEV1indicates current obstruction
(impairment) and risk for future exacerbations
(risk). For children, FEV1/forced vital capacity
(FVC) appears to be a more sensitive measure
of severity and control in the impairment
domain. FEV1is a useful measure of risk for
exacerbations, although it is emphasized that
even children who have normal lung function
experience exacerbations.
■Minimally invasive markers (called biomarkers)
suchas fractionated exhaled nitric oxide (FeNO)
and sputum eosinophils may be useful, but bio
markers require further evaluation before they
can be recommended as clinical tools for routine
management.
Component 2: Education for a Partnership in Care
Apartnership between the clinician and the person
who has asthma (and the caregiver, for children) is
required for effective asthma management. By working
together, an appropriate treatment can be selected, and
the patient can learn self-management skills necessary
to control asthma. Self-management education
improves patient outcomes (e.g., reduced urgent care
visits, hospitalizations, and limitations on activities as
well as improved health status, quality of life, and
perceived control of asthma) and can be cost-effective.
Self-management education is an integral component
of effective asthma care and should be treated as such
by health care providers as well as by health care policies
and reimbursements.
KEY EDUCATIONAL MESSAGES: TEACH AND REINFORCE
AT EVERY OPPORTUNITY
Basic Facts About Asthma
■The contrast between airways of a person who has and a
person who does not have asthma; the role of inflammation.
■What happens to the airways during an asthma attack.
Role of Medications: Understanding the Difference
Between:
■Long-term control medications: prevent symptoms, often by
reducing inflammation. Must be taken daily. Do not expect
them to give quick relief.
■Quick-relief medications: SABAs relax airway muscles to
provide prompt relief of symptoms. Do not expect them to
provide long-term asthma control. Using SABA >2 days
aweek indicates the need for starting or increasing long-
term control medications.
Patient Skills
■Taking medications correctly
— Inhaler technique (demonstrate to the patient and have
the patient return the demonstration).
—Use of devices, as prescribed (e.g., valved holding
chamber (VHC) or spacer,nebulizer).
■Identifying and avoiding environmental exposures that
worsen the patient’s asthma; e.g., allergens, irritants,
tobacco smoke.
■Self-monitoring
—Assess level of asthma control.
— Monitor symptoms and, if prescribed, PEF measures.
—Recognize early signs and symptoms of worsening asthma.
■Using a written asthma action plan to know when and how to:
— Take daily actions to control asthma.
—Adjust medication in response to signs of worsening asthma.
■Seeking medical care as appropriate.

19
Managing Asthma Long Term
Develop an active partnership with the patient and
family by:
■Establishing open communications that consider
cultural and ethnic factors, as well as language and
health care literacy needs, of each patient and family.
■Identifying and addressing patient and family
concerns about asthma and asthma treatment.
■Developing treatment goals and selecting
medications together with the patient and family,
allowing full participation in treatment decision
making.
■Encouraging self-monitoring and self-management
by reviewing at each opportunity the patient’s
reports of asthma symptoms and response to
treatment.
Provide to all patients a written asthma action
plan that includes instructions for both daily
management (long-term control medication, if
appropriate, and environmental control measures)
and actions tomanage worsening asthma (what signs,
symptoms, and PEF measurements (if used) indicate
worsening asthma; what medications to take in
response; what signs and symptoms indicate the need
for immediate medical care). Written asthma action
plans are particularly recommended for patients who
have moderate or severe persistent asthma (i.e.,
requiring treatment at step 4, 5, or 6), a history of
severe exacerbations, or poorly controlled asthma.
See figures 5 and 6 for samples of written asthma
action plans.
Integrate asthma self-management education into
all aspects of asthma care. Asthma self management
requires repetition and reinforcement. It should:
■Begin at the time of diagnosis and continue through
followup care. See figure 7, “Delivery of Asthma
Education byClinicians During Patient Care Visits,”
for a sample of how to incorporate teaching into
routine clinic visits.
■Involve all members of the health care team, including
physicians, nurses, pharmacists, respiratory
therapists, and asthma educators, as well as other
health professionals who come in contact with
asthma patients and their families.
■Occur at all points of care where health care profes-
sionals interact with patients who have asthma.
The strongest evidence supports self-management
education in the clinic setting. Evidence also
supports education provided in patients' homes,
pharmacies, targeted education in EDs and hospi-
tals, and selected programs in schools and other
community sites. Proven community programs
should be considered because of their potential to
reach large numbers of people who have asthma
and encourage “asthma-friendly” support from
their families and community environments.
■Use a variety of educational strategies to reach
people who have varying levels of health literacy
or learning styles. Individual instruction, group
programs, written materials (at a 5th grade reading
level or below), video- or audiotapes, and comput-
er and Internet programs all provide effective
educational opportunities. See figure 8, “Asthma
Education Resources,” for a sample of available
resources.
■Incorporate individualized case/care management
by trained health care professionals for patients
who have poorly controlled asthma and have
recurrent visits tothe emergency department
or hospital. This will provide tailored
self-management education and skills training.
Encourage patients' adherence to the written
asthma actionplan by:
■Choosing treatment that achieves outcomes and
addresses preferences that are important to the
patient, and reminding patients that adherence
will help them achieve the outcomes they want.
■Reviewing with the patient at each visit the success
of the treatment plan to achieve asthma control
and make adjustments as needed.
■Reviewing patients' concerns about their asthma
or treatment at every visit. Inquire about any
difficulties encountered in adhering to the written
asthma action plan.
■Assessing the patient's and family's level of social
support, and encouraging family involvement.
■Tailoring the self-management approach to the
needs and literacy levels of the patient, and
maintaining sensitivity to cultural beliefs and
ethnocultural practices.
Encouragehealth care provider and health care
system support of the therapeutic partnership by:
■Incorporating effective clinician education strategies,

20 Guidelines for the Diagnosis and Management of Asthma
FIGURE 5. SAMPLE ASTHMA ACTION PLAN—ADULT
Adapted and reprinted with permission from the Regional Asthma Management and Prevention (RAMP) Initiative, a program of the Public Health
Institute, to include terms used in the EPR—3: Full Report 2007.
Source: http://www.calasthma.org/uploads/resources/actionplanpdf.pdf; San Francisco Bay Area Regional Asthma Management Plan,
http://www.rampasthma.org

21
Managing Asthma Long Term
FIGURE 6. SAMPLE ASTHMA ACTION PLAN—CHILD
Adapted and reprinted with permission from “The Asthma Action Plan” developed by a committee facilitated by the Regional Asthma
Management and Prevention (RAMP) Initiative, a program of the Public Health Institute.
Source: http://www.calasthma.org/uploads/resources/actionplanpdf.pdf; San Francisco Bay Area Regional Asthma Management Plan,
http://www.rampasthma.org
22 Guidelines for the Diagnosis and Management of Asthma
FIGURE 7. DELIVERY OF ASTHMA EDUCATION BY CLINICIANS DURING PATIENT CARE VISITS
Assessment Questions Information Skills
Focus on:
■Expectations of visit
■Asthma control
■Patients’ goals of treatment
■Medications
■Quality of life
Ask relevant questions
“What worries you most about your asthma?”
“What do you want to accomplish at this visit?”
“What do you want to be able to do that you can’t do
now because of your asthma?”
“What do you expect from treatment?”
“What medicines have you tried?”
“What other questions do you have for me today?”
“Are there things in your environment that make your
asthma worse?”
Recommendations for Initial Visit
Teach in simple language:
■What is asthma? Asthma is a chronic lung disease.
The airways are very sensitive. They become
inflamed and narrow; breathing becomes difficult.
■The definition of asthma control: few daytime symp-
toms, no nighttime awakenings due to asthma, able
to engage in normal activities, normal lung function.
■Asthma treatments: two types of medicines are
needed:
— Long-term control: medications that prevent
symptoms, often by reducing inflammation.
— Quick relief: short-acting bronchodilator relaxes
muscles around airways.
■Bring all medications to every appointment.
■When to seek medical advice. Provide appropriate
telephone number.
Teach or review and demonstrate:
■Inhaler and spacer or valved holding chamber
(VHC) use. Check performance.
■Self-monitoring skills that are tied to a written
asthma action plan:
— Recognize intensity and frequency of asthma
symptoms.
— Review the signs of deterioration and the need
to reevaluate therapy:
sWaking at night or early morning with asthma
sIncreased medication use
sDecreased activity tolerance
■Use of a written asthma action plan (See figures 5
and 6.) that includes instructions for daily
management and for recognizing and handling
worsening asthma.
Focus on:
■Expectations of visit
■Asthma control
■Patient’s goals of treatment
■Medications
■Patient’s treatment preferences
■Quality of life
Ask relevant questions from previous visit and
also ask:
“Whatmedications are you taking?”
“How and when are you taking them?”
“What problems have you had using your
medications?”
“Please show me how you use your inhaled
medications.”
Recommendations for First Followup Visit (2 to 4 Weeks or Sooner as Needed)
Teach in simple language:
■Use of two types of medications.
■Remind patient to bring all medications and the
peak flow meter, if using, to every appointment
for review.
■Self/assessment of asthma control using symptoms
and/or peak flow as a guide.
Teach or review and demonstrate:
■Use of written asthma action plan. Review and
adjust as needed.
■Peak flow monitoring if indicated
■Correct inhaler and spacer or VHC technique.
Focus on:
■Expectations of visit
■Asthma control
■Patients’ goals of treatment
■Medications
■Quality of life
Ask relevant questions from previous visits and
also ask:
“Have you noticed anything in your home, work, or
school
thatmakes your asthma worse?”
“Describe for me how you know when to call your
doctor or go to the hospital for asthma care.”
“What questions do you have about the asthma
action plan?”
“Can we make it easier?”
“Are your medications causing you anyproblems?”
“Have you noticed anything in your environment that
makes your asthma worse?”
“Have you missed anyof your medications?”
Recommendations for Second Followup Visit
Teach in simple language:
■Self-assessment of asthma control, using symptoms
and/or peak flow as a guide.
■Relevant environmental control/avoidance strategies:
— How to identify home, work, or school exposures
thatcan cause or worsen asthma
— How to control house-dust mites, animal
exposures if applicable
— How to avoid cigarette smoke (active and
passive)
■Review all medications.
Teach or review and demonstrate:
■Inhaler/spacer or VHC technique.
■Peak flow monitoring technique.
■Use of written asthma action plan. Review and
adjust as needed.
■Confirm that patient knows what to do if
asthma gets worse
23
Managing Asthma Long Term
such as interactive formats, practice-based
case studies, and multidimensional teaching
approaches that reinforce guideline-based care.
■Providing communication skills training to
clinicians to enhance competence in caring for
all patients, especially multicultural populations.
■Using systems approaches, such as clinical pathways
and clinical information system prompts, to
improve the quality of asthma care and to support
clinical care decisionmaking.
Component 3: Control of Environmental Factors
and Comorbid Conditions That Affect Asthma
If patients who have asthma are exposed to irritants or
inhalant allergens to which they are sensitive, their
asthma symptoms may increase and precipitate an
asthma exacerbation. Substantially reducing exposure
to these factors may reduce inflammation, symptoms,
and need for medication. Several comorbid conditions
can impedeasthma management. Recognition and
treatment of these conditions may improve asthma
control. See questions in figure 3, “Suggested Items
for Medical History,” above, for questions related to
environmental exposures and comorbid conditions.
Allergens and Irritants
Evaluate the potential role of allergens (particularly
inhalant allergens) and irritants.
■Identify allergen and pollutants or irritant
exposures. The most important allergens for both
children and adults appear to be those that are
inhaled.
■For patients who have persistent asthma, use skin
testing or in vitro testing to assess sensitivity to
perennial indoor allergens. Assess the significance
of positive tests in the context of the person’s
history of symptoms when exposed to the allergen.
Advise patients who have asthma to reduce exposure
to allergens and pollutants or irritants to which they
are sensitive.
■Seefigure 9, “How To Control Things That
MakeYour Asthma Worse,” for a sample patient
information sheet.
■Effective allergen avoidance requires a multifaceted,
comprehensive approach; single steps alone are
generally ineffective. Multifaceted allergen-control
education programs provided in the home setting
can help patients reduce exposures to cockroach,
dust-mite, and rodent allergens and, consequently,
improve asthma control.
■Advise patients who have severe persistent asthma,
nasal polyps, or a history of sensitivity to aspirin or
nonsteroidal anti-inflammatory drugs (NSAIDs)
about their risk of severe and even fatal exacerba-
tions from using these drugs.
■Indoor air-cleaning devices (high-efficiency
particulate air [HEPA] and electrostatic precipitating
filters), cannot substitute for more effective
dust-miteand cockroach control measures because
FIGURE 7. DELIVERY OF ASTHMA EDUCATION BY CLINICIANS DURING PATIENT CARE VISITS (continued)
Assessment Questions Information Skills
Focus on:
■Expectations of visit
■Asthma control
■Patients’ goals of treatment
■Medications
■Quality of life
Ask relevant questions from previous visits and
also ask:
“How have you tried to control things that make
your asthma worse?”
“Please show me how you use your inhaled
medication.”
Recommendations for All Subsequent Visits
Teach in simple language:
■Review and reinforce all:
— Educational messages
— Environmental control strategies at home, work,
or school
—Medications
— Self-assessment of asthma control, using
symptoms and/or peak flow as a guide
Teach or review and demonstrate:
■Inhaler/spacer or VHC technique.
■Peak flow monitoring technique, if appropriate.
■Use of written asthma action plan. Review and
adjust as needed.
■Confirm that patient knows what to do if asthma
gets worse.
Sources: Adapted from Guevara et al. 2003; Janson et al. 2003; Powell and Gibson 2003; Wilson et al. 1993.
24 Guidelines for the Diagnosis and Management of Asthma
FIGURE 8. ASTHMA EDUCATION RESOURCES
Allergy & Asthma Network Mothers of Asthmatics 1–800–878–4403
2751 Prosperity Avenue, Suite 150 1–703–641–9595
Fairfax, VA 22030
www.breatherville.org
American Academy of Allergy, Asthma and Immunology 1–414–272–6071
555 East Wells Street, Suite 100
Milwaukee, WI 53202-3823
www.aaaai.org
American Association For Respiratory Care 1–972–243–2272
9125 North MacArthur Boulevard, Suite 100
Irving, TX 75063
www.aarc.org
American College of Allergy, Asthma, and Immunology 1–800–842–7777
85 West Algonquin Road 1–847–427–1200
Suite 550
Arlington Heights, IL 60005
www.Acaai.Org
American Lung Association 1–800–586–4872
61 Broadway
New York, NY 10006
www.lungusa.org
Association of Asthma Educators 1–888–988–7747
1215 Anthony Avenue
Columbia, SC 29201
www.asthmaeducators.org
Asthma and Allergy Foundation of America 1–800–727–8462
1233 20th Street, NW., Suite 402
Washington, DC 20036
www.aafa.org
Centers for Disease Control and Prevention 1–800–311–3435
1600 Clifton Road
Atlanta, GA 30333
Food Allergy & Anaphylaxis Network 1–800–929–4040
11781 Lee Jackson Highway, Suite 160
Fairfax, VA 22033
www.foodallergy.org
National Heart, Lung, and Blood Institute Information Center 1–301–592–8573
P
.O. Box 30105
Bethesda, MD 20824-0105
www.nhlbi.nih.gov
National Jewish Medical and Research Center (Lung Line) 1–800–222–Lung
1400 Jackson Street
Denver, CO 80206
www.njc.org
U.S. Environmental Protection Agency 1–800–490–9198
National Center for Environmental Publications
P.O. Box 42419
Cincinnati, OH 45242-0419
www.airnow.gov

25
Managing Asthma Long Term
these particles do not remain airborne. The devices
can reduce airborne dog and cat allergens, mold
spores, and particulate tobacco smoke; however,
most studies do not show an effect on symptoms or
lung function.
■Use of humidifiers or evaporative (swamp) coolers
is not generally recommended in homes of patients
who are sensitive to dust mites or mold.
Consider subcutaneous allergen immunotherapy for
patients who have persistent asthma when there is
clear evidence of a relationship between symptoms
and exposure to an allergen to which the patient
is sensitive. Evidence is strongest for use of subcuta-
neous immunotherapy for single allergens, particularly
house dust mites, animal dander, and pollen. The
role of allergy in asthma is greater in children than in
adults. If use of allergen immunotherapy is elected,
it should beadministered only in a physician’s office
where facilities and trained personnel are available
to treat any life-threatening reaction that can, but
rarely does, occur.
Consider inactivated influenza vaccination for
patients who have asthma. This vaccine is safe for
administration to children over 6 months of age and
adults, and the Advisory Committee on Immunization
Practices ofthe Centers for Disease Control and
Prevention (CDC) recommends vaccination for per-
sons who have asthma because they are considered to
be at risk forcomplications from influenza. However,
the vaccine should not be given with the expectation
that it will reduce either the frequency or severity of
asthma exacerbations during the influenza season.
Dietary factors have an inconclusive role in asthma.
Food allergenies are rarely an aggravating factor in
asthma. Anexception is that sulfites in foods (e.g.,
shrimp,dried fruit, processed potatoes, beer, and wine)
can precipitate asthma symptoms in people who
aresensitiveto these food items. Furthermore,
individuals who have both food allergy and asthma
are at increased risk for fatal anaphlylactic reactions
tothe food to whichtheyaresensitized.
Comorbid Conditions
Identify and treat comorbid conditions that may
impede asthma management. If these conditions are
treated appropriately, asthma control may improve.
■Allergic Bronchopulmonary Aspergillosis (ABPA)
may be considered in patients who have
asthma and a history of pulmonary infiltrates,
immunoglobulin E (IgE) sensitization to
Aspergillus, and/or are corticosteroid dependent.
Diagnostic criteria include: positive immediate
skin test and elevated serum IgE and/or IgG to
Aspergillus, total serum IgE >417 IU (1,000
ng/mL), and central bronchiectasis. Treatment
is prednisone, initially 0.5 mg per kilogram
with gradual tapering. Azole antifungal agents
as adjunctive therapy may also be helpful.
■Gastroesophageal Reflux (GERD) treatment may
benefit patients who have asthma and complain of
frequent heartburn or pyrosis, particularly those
who have frequent nighttime asthma symptoms.
Even in the absence of suggestive GERD symptoms,
consider evaluation for GERD in patients who
have poorly controlled asthma, especially with
nighttime symptoms. Treatment includes: avoid-
ing heavy meals, fried foods, caffeine, and alcohol;
avoiding food and drink within 3 hours of retiring;
elevating the head of the bed on 6- to 8-inch
blocks; using proton pump inhibitor medication.
■Obese or overweight patients who have asthma
maybe advised that weight loss, in addition to
improving overall health, might also improve
asthma control.
■Obstructive SleepApnea (OSA) may be considered
in patients who have not well controlled asthma,
particularly those who are overweight or obese.
Treatment for OSA is nasal continuous positive air
waypressure (CPAP). However, this treatment may
disrupt the sleep of asthma patients who do not
also have OSA. Accurate diagnosis is important.
■Rhinitis or sinusitis symptoms or diagnosis should
beevaluated in patients who have asthma, because
the interrelationship of the upper and lower
airway suggests that therapy for the upper airway
will improve asthma control. Treatment of
allergic rhinitis includes intranasal corticosteroids,
antihistamine therapy, and the consideration of
immunotherapy. Treatment of sinusitis includes
intranasal corticosteroids and antibiotics. Evidence
is inconclusive regarding the effect on asthma
of sinus surgery in patients who have chronic
rhinosinusitis.
■Stress and depression should be considered in
patients who have asthma that is not well
controlled. Additional education to improve
self-management and coping skills may be helpful.
26 Guidelines for the Diagnosis and Management of Asthma
FIGURE 9. HOW TO CONTROL THINGS THAT MAKE YOUR ASTHMA WORSE
You can help prevent asthma episodes by staying
away from things that make your asthma worse.
This guide suggests many ways to help you do this.
You need to find out what makes your asthma worse.
Some things that make asthma worse for some
people are not a problem for others. You do not
need to do all of the things listed in this guide.
Look at the things listed below. Put a check next to
the ones that you know make your asthma worse,
particularly if you are allergic to these things. Then,
decide with your doctor what steps you will take.
Start with the things in your bedroom that bother your
asthma. Try something simple first.
Tobacco Smoke
■■If you smoke, ask your doctor for ways to help you
quit. Ask family members to quit smoking, too.
■■Do not allow smoking in your home, car or around
you.
■■Be sure no one smokes at a child’s daycare
center or school.
Dust Mites
Many people who have asthma are allergic to dust
mites. Dust mites are like tiny “bugs” you cannot
see that live in cloth or carpet.
Things that will help the most:
■■Encase your mattress in a special dust-mite proof
cover.*
■■Encase your pillow in a special dust-mite proof
cover* or wash the pillow each week in hot water.
Water must be hotter than 130 °F to kill the
mites. Cooler water used with detergent and
bleach can also be effective.
■■Wash the sheets and blankets on your bed each
week in hot water.
Other things that can help:
■■Reduce indoor humidity to or below 60 percent,
ideally 30–50 percent. Dehumidifiers or central air
conditioners can do this.
■■Try not to sleep or lie on cloth-covered cushions
or furniture.
■■Remove carpets from your bedroom and those
laid on concrete, if you can.
■■Keep stuffed toys out of the bed, or wash the
toys weekly in hot water or in cooler water with
detergent and bleach. Placing toys weekly in a
dryer or freezer may help. Prolonged exposure
to dry heat or freezing can kill mites but does not
remove allergen.
*To find out where to get products mentioned in this guide, call:
Asthma and Allergy Foundation of America (800–727–8462)
Allergy & Asthma Network Mothers of Asthmatics (800–878–4403)
American Academy of Allergy, Asthma, and Immunology (800–822–2762)
National Jewish Medical and Research Center (Lung Line) (800–222–5864)
American College of Allergy, Asthma, and Immunology (800–842–7777)
27
Managing Asthma Long Term
Animal Dander
Some people are allergic to the flakes of skin or dried saliva
from animals.
The best thing to do:
■■Keep pets with fur or hair out of your home.
If you can’t keep the pet outdoors, then:
■■Keep the pet out of your bedroom, and keep the
bedroom door closed.
■■Remove carpets and furniture covered with cloth from
your home. If that is not possible, keep the pet out of
the rooms where these are.
Cockroach
Many people with asthma are allergic to the dried
droppings and remains of cockroaches.
■■Keep all food out of your bedroom.
■■Keep food and garbage in closed containers
(Never leave food out).
■■Use poison baits, powders, gels, or paste
(for example, boric acid). You can also use traps.
■■If a spray is used to kill roaches, stay out of the room
until the odor goes away.
Vacuum Cleaning
■■Try to get someone else to vacuum for you once or
twice a week, if you can. Stay out of rooms while they
are being vacuumed and for a short while afterward.
■■If you vacuum, use a dust mask (from a hardware store),
a central cleaner with the collecting bag outside the
home, or a vacuum cleaner with a HEPA filter or a
double-layered bag.*
Indoor Mold
■■Fix leaking faucets, pipes, or other sources of water.
■■Clean moldy surfaces.
■■Dehumidify basements if possible.
Pollen and Outdoor Mold
During your allergy season (when pollen or mold spore
counts are high):
■■Try to keep your windows closed.
■■If possible, stay indoors with windows closed during the
midday and afternoon, if you can. Pollen and some mold
spore counts are highest at that time.
■■Ask your doctor whether you need to take or increase
anti-inflammatory medicine before your allergy season
starts.
Smoke, Strong Odors, and Sprays
■■If possible, do not use a wood-burning stove, kerosene
heater, fireplace, unvented gas stove, or heater.
■■Try to stay away from strong odors and sprays, such as
perfume, talcum powder, hair spray, paints, new carpet,
or particle board.
Exercise or Sports
■■You should be able to be active without symptoms.
See your doctor if you have asthma symptoms when
you are active—such as when you exercise, do sports,
play, or work hard.
■■Ask your doctor about taking medicine before you
exercise to prevent symptoms.
■■Warm up for a period before you exercise.
■■Check the air quality index and try not to work or play
hard outside when the air pollution or pollen levels
(if you are allergic to the pollen) are high.
Other Things That Can Make Asthma Worse
■■Sulfites in foods: Do not drink beer or wine or eat
shrimp, dried fruit, or processed potatoes if they cause
asthma symptoms.
■■Cold air: Cover your nose and mouth with a scarf on
cold or windy days.
■■Other medicines: Tell your doctor about all the
medicines you may take. Include cold medicines, aspirin,
and even eye drops.
Key: HEPA, high-efficiency particulate air

Component 4: Medications
Medications for asthma are categorized into two
general classes: long-term control medication and
quick-relief medication. Selection of medications
includes consideration of the general mechanisms
and role of the medication in therapy, delivery
devices, and safety.
General Mechanisms and Role in Therapy
Long-term control medications are used daily to
achieve and maintain control of persistent asthma.
The most effective are those that attenuate the
underlying inflammation characteristic of asthma.
Long-term control medications include the
following (listed in alphabetical order):
■Corticosteroids are anti-inflammatory medications
that reduce airway hyperresponsiveness, inhibit
inflammatory cell migration and activation, and
block late phase reaction to allergen. Inhaled
Corticosteriods (ICSs) are the most consistently
effective long-term control medication at all steps
of care for persistent asthma, and ICSs improve
asthma control more effectively in both children
and adults than leukotriene receptor antagonists
(LTRAs) or any other single, long-term control
medication do. ICSs reduce impairment and risk
of exacerbations, but ICSs do not appear to alter
the progression or underlying severity of the dis-
ease in children. Short courses of oral systemic
corticosteroids are often used to gain prompt con-
trol of asthma. Oral systemic corticosteroids are
used long term to treat patients who require step 6
care (for severe persistent asthma).
■Cromolyn sodium and nedocromil stabilize mast
cells and interfere with chloride channel function.
They are used as alternative, but not preferred,
medication for patients requiring step 2 care (for
mild persistent asthma). They also can be used as
preventive treatment before exercise or unavoidable
exposure to known allergens.
■Immunomodulators. Omalizumab (anti-IgE) is a
monoclonal antibody that prevents binding of IgE
to the high-affinity receptors on basophils and mast
cells. Omalizumab is used as adjunctive therapy
for patients 12 years of age who have sensitivity to
relevant allergens (e.g., dust mite, cockroach, cat, or
dog) and who require step 5 or 6 care (for severe
persistent asthma). Clinicians who administer
omalizumab should be prepared and equipped to
identify and treat anaphylaxis that may occur.
■Leukotriene modifiers interfere with the pathway
of leukotriene mediators, which are released from
mast cells, eosinophils, and basophils. These med-
ications include LTRAs (montelukast and zafir-
lukast) and a 5-lipoxygenase inhibitor (zileuton).
LTRAs are alternative, but not preferred, therapy for
the treatment of patients who require step 2 care
(for mild persistent asthma). LTRAs also can be
used as adjunctive therapy with ICSs, but for youths
12 years of age and adults, they are not preferred
adjunctive therapy compared to the addition of
LABAs. LTRAs can attenuate EIB. Zileuton can be
used as alternative, but not preferred, adjunctive
therapy in adults; liver function monitoring is
essential.
■LABAs (salmeterol and formoterol) are
inhaled bronchodilators that have a duration of
bronchodilation of at least 12 hours after a
single dose.
— LABAs are not to be used as monotherapy for
long-term control of asthma.
— LABAs are used in combination with ICSs for
long-term control and prevention of symptoms
in moderate or severe persistent asthma (Step 3
care or higher in children ≥5 years of age and
adults and Step 4 care or higher in children 0–4
years of age, although few data are available for
0–4-year-olds.).
— Of the adjunctive therapies available, LABA is
the preferred therapy to combine with ICS in
youths ≥12 years of age and adults.
— A LABA may be used before exercise to prevent
EIB, but duration of action does not exceed
5 hours with chronic, regular use. Frequent
or chronic use before exercise is discouraged,
because this may disguise poorly controlled
persistent asthma. See also the section “Safety
Issues for Inhaled Corticosteroids and Long-
Acting Beta2-Agonists.”
■Methylxanthines. Sustained-release theophylline
is a mild to moderate bronchodilator used as
alternative, not preferred, therapy for step 2 care
(for mild persistent asthma) or as adjunctive therapy
with ICS in patients ≥5 years of age. Theophylline
may have mild anti-inflammatory effects. Monitoring
of serum theophylline concentration is essential.
28 Guidelines for the Diagnosis and Management of Asthma

29
Managing Asthma Long Term
Quick-relief medications are used to treat acute
symptoms and exacerbations. They include the
following (listed in alphabetical order):
■Anticholinergics inhibit muscarinic cholinergic
receptors and reduce intrinsic vagal tone of the air-
way. Ipratropium bromide provides additive benefit
to SABA in moderate or severe exacerbations in the
emergency care setting, not the hospital setting.
Ipratropium bromide may be used as an alternative
bronchodilator for patients who do not tolerate
SABA, although it has not been compared to SABAs.
■SABAs—albuterol, levalbuterol, and pirbuterol—are
bronchodilators that relax smooth muscle. They are
the treatment of choice for relief of acute symptoms
and prevention of EIB. Increasing use of SABA
treatment or the use of SABA >2 days a week for
symptom relief (not prevention of EIB) generally
indicates inadequate asthma control and the need
for initiating or intensifying anti-inflammatory
therapy. Regularly scheduled, daily, chronic use of
SABA is not recommended.
■Systemic corticosteroids. Although not short-
acting, oral systemic corticosteroids are used for
moderate and severe exacerbations in addition to
SABA to speed recovery and to prevent recurrence of
exacerbations.
Complementary and alternative medications
(CAMs) and interventions generally have insuffi-
cient evidence to permit recommendations. Because
as much as one-third of the U.S. population uses com-
plementary alternative healing methods, it is important
to discuss their use with patients.
■Ask patients about all the medications and
interventions they are using. Some cultural
beliefs and practices may be of no harm and can
be integrated into the recommended asthma
management strategies, but it is important to
advise patients that alternative healing methods are
not substitutes for recommended therapeutic
approaches. Clinical trials on safety and efficacy
are limited, and their scientific basis has not been
established.
■Evidence is insufficient to recommend or not
recommend most CAMs or treatments for
asthma. These include chiropractic therapy,
homeopathy and herbal medicine, and breathing
or relaxation techniques. Acupuncture is not
recommended for the treatment of asthma.
■Patients who use herbal treatments for asthma
should be cautioned about the potential for
harmful ingredients and for interactions with
recommended asthma medications.
Delivery Devices for Inhaled Medications
Patients should be instructed in the use of inhaled
medications, and patients’ technique should be
reviewed at every patient visit. The major
advantages of delivering drugs directly into the lungs
via inhalation are that higher concentrations can be
delivered more effectively to the airways and that
systemic side effects are lessened. Inhaled medications,
or aerosols, are available in a variety of devices that
differ in the technique required. See figure 10, “Aerosol
Delivery Devices,” for a summary of issues to consider
for different devices.
Safety Issues for Inhaled Corticosteroids and Long-
Acting Beta2-Agonists
Inhaled Corticosteroids
■ICSs are the preferred long-term control therapy in
children of all ages and adults. In general, ICSs are
well tolerated and safe at the recommended dosages.
■Most benefits of ICS for patients who have mild or
moderate asthma occur at the low- to medium-dose
ranges. Data suggest higher doses may further
reduce the risk of exacerbations. Furthermore,
higher doses are beneficial for patients who have
more severe asthma. The risk of adverse effects
increases with the dose.
■High doses of ICS administered for prolonged
periods of time (e.g., >1 year) have significantly
less potential than oral systemic corticosteroids for
having adverse effects. High doses of ICS used for
prolonged periods of time (e.g., >1 year), particu-
larly in combination with frequent courses of oral
corticosteroids, may be associated with risk of
posterior subcapsular cataracts or reduced bone
density. Slit-lamp eye exam and bone densitometry
may be considered. For adult patients, consider
supplements of calcium and vitamin D, particularly
in perimenopausal women. For children, age-
appropriate dietary intake of calcium and vitamin
D should be reviewed with parents or caregivers.
■To reduce the potential for adverse effects, the
following measures are recommended.
— Advise patients to use spacers or VHCs with
nonbreath-activated metered-dose inhalers

(MDIs) to reduce local side effects. There are
no clinical data on use of spacers with ultrafine
particle hydrofluoroalkane (HFA) MDIs.
— Advise patients to rinse the mouth (rinse and
spit) after inhalation.
— Use the lowest dose of ICS that maintains
asthma control. Evaluate the patient’s inhaler
technique and adherence, as well as environ-
mental control measures, before increasing
the dose.
— Consider adding a LABA, or alternative
adjunctive therapy, to a low or medium dose
of ICS rather than using a higher dose of ICS
to maintain asthma control.
Inhaled Corticosteroids and Linear Growth in Children
■The potential risks of ICSs are well balanced by
their benefits.
■Poorly controlled asthma may delay growth.
Children who have asthma tend to have longer
periods of reduced growth rates before puberty.
■Growth rates are highly variable in children.
Short-term evaluation may not be predictive of
final adult height attained.
■The potential for adverse effects on linear growth
from ICS appear to be dose dependent. In
treatment of children who have mild or moderate
persistent asthma, low-to medium-dose ICS
therapy may be associated with a possible, but
not predictable, adverse effect on linear growth
(approximately 1 cm). The effect on growth
velocity appears to occur in the first several
months of treatment and is generally small and
not progressive. The clinical significance of this
potential systemic effect has yet to be determined.
■In general, the efficacy of ICSs is sufficient to out
weigh any concerns about growth or other systemic
effects. However, ICSs should be titrated to as low
a dose as needed to maintain good control of the
child’s asthma, and children receiving ICSs should
be monitored for changes in growth by using a
stadiometer.
Long-Acting Beta2-Agonists
■The addition of LABA (salmeterol or formoterol)
to the treatment of patients who require more than
low-dose ICS alone to control asthma improves
lung function, decreases symptoms, reduces
exacerbations and use of SABA for quick relief in
most patients to a greater extent than doubling the
dose of ICSs.
■A large clinical trial comparing daily treatment
with salmeterol or placebo added to usual asthma
therapy resulted in an increased risk of asthma-
related deaths in patients treated with salmeterol
(13 deaths among 13,176 patients treated for
28 weeks with salmeterol versus 3 deaths among
13,179 patients treated with placebo). In addition,
increased numbers of severe asthma exacerbations
were noted in the pivotal trials submitted to the
U.S. Food and Drug Administration (FDA) for
formoterol approval, particularly in the arms of the
trials with higher dose formoterol. Thus, the
FDA determined that a Black Box warning was
warranted on all preparations containing a LABA.
■The established beneficial effects of LABA for
the great majority of patients who require more
therapy than low-dose ICS alone to control asthma
(i.e., require step 3 care or higher) should be
weighed against the increased risk for severe
exacerbations, although uncommon, associated
with the daily use of LABAs.
■Daily use of LABA generally should not exceed
100 mcg salmeterol or 24 mcg formoterol.
■It is not currently recommended that LABA be
used for treatment of acute symptoms or
exacerbations.
■LABAs are not to be used as monotherapy for long-
term control. Patients should be instructed not to
stop ICS therapy while taking LABA, even though
their symptoms may significantly improve.
Stepwise Approach for Managing Asthma
Principles of The Stepwise Approach
A stepwise approach to managing asthma is recom-
mended to gain and maintain control of asthma in
both the impairment and risk domains. These
domains may respond differentially to treatment.
For children, see:
Figure 11, “Classifying Asthma Severity and
Initiating Therapy in Children”
30 Guidelines for the Diagnosis and Management of Asthma
31
Managing Asthma Long Term
FIGURE 10. AEROSOL DELIVERY DEVICES
Device/Drugs Population Optimal Technique*
Metered-dose inhaler (MDI)
Beta2-agonists
Corticosteroids
Cromolyn sodium
Anticholinergics
Breath-actuated MDI
Beta2-agonist
≥5 years old
(<5 with spacer or
valved holding
chamber (VHC) or
mask)
≥5 years old
Actuation during a slow (30 L/min
or 3–5 seconds) deep inhalation,
followed by 10-second breathhold.
Under laboratory conditions, open-
mouth technique (holding MDI 2
inches away from open mouth)
enhances delivery to the lung. This
technique, however, has not been
shown to enhance clinical benefit
consistently compared to closed-
mouth technique (inserting MDI
mouthpiece between lips and teeth).
Tight seal around mouthpiece and
slightly more rapid inhalation than
standard MDI (see above) followed
by 10-second breathhold.
Slow inhalation and coordination of actuation during inhalation
may be difficult, particularly in young children and elderly.
Patients may incorrectly stop inhalation at actuation. Deposition
of 50–80 percent of actuated dose in oropharynx. Mouth washing
and spitting is effective in reducing the amount of drug swallowed
and absorbed systemically.
Lung delivery under ideal conditions varies significantly between
MDIs due to differences in formulation (suspension versus solution),
propellant (chlorofluorocarbon [CFC] versus hydrofluoralkane [HFA]),
and valve design. For example, inhaled corticosteroid (ICS) delivery
varies from 5–50 percent.
May be particularly useful for patients unable to coordinate
inhalation and actuation. May also be useful for elderly patients.
Patients may incorrectly stop inhalation at actuation. Cannot be
used with currently available spacer/valved holding chamber
(VHC) devices.
Therapeutic Issues
Dry powder inhaler (DPI)
Beta2-agonists
Corticosteroids
Anticholinergics
≥4 years old Rapid (60 L/min or 1–2 seconds),
deep inhalation. Minimally effective
inspiratory flow is device dependent.
Most children <4 years of age may
not generate sufficient inspiratory
flow to activate the inhaler.
Dose is lost if patient exhales through device after actuating.
Delivery may be greater or lesser than MDI, depending on device
and technique. Delivery is more flow dependent in devices with
highest internal resistance. Rapid inhalation promotes greater
deposition in larger central airways. Mouth washing and spitting
is effective in reducing amount of drug swallowed and absorbed.
Spacer or valved holding
chamber (VHC)
≥4 years old
<4 years old VHC
with face mask
Slow (30 L/min or 3–5 seconds)
deep inhalation, followed by
10-second breathhold immediately
following actuation.
Actuate only once into spacer/VHC
per inhalation.
If face mask is used, it should have
a tight fit and allow 3–5 inhalations
per actuation.
Rinse plastic VHCs once a month
with low concentration of liquid
household dishwashing detergent
(1:5,000 or 1–2 drops per cup of
water) and let drip dry.
Indicated for patients who have difficulty performing adequate
MDI technique.
May be bulky. Simple tubes do not obviate coordinating actuation
and inhalation. The VHCs are preferred.
Face mask allows MDIs to be used with small children. However,
use of a face mask reduces delivery to lungs by 50 percent.
The VHC improves lung delivery and response in patients who
have poor MDI technique.
The effect of a spacer or VHC on output from an MDI depends
on both the MDI and device type; thus data from one combination
should not be extrapolated to all others. Spacers and/or VHCs
decrease oropharyngeal deposition and thus decrease risk of
topical side effects (e.g., thrush).
Spacers will also reduce the potential systemic availability of ICSs
with higher oral absorption. However, spacer/VHCs may increase
systemic availability of ICSs that are poorly absorbed orally by
enhancing delivery to lungs.
No clinical data are available on use of spacers or VHCs with
ultrafine-particle-generated HFA MDIs.
Use anti-static VHCs or rinse plastic non-anti-static VHCs with
dilute household detergents to enhance delivery to lungs and
efficacy. This effect is less pronounced for albuterol MDIs with
HFA propellant than for albuterol MDIs with CFC propellant.
As effective as nebulizer for delivering SABAs and anticholinergics
in mild- to moderate-exacerbations; data in severe exacerbations
are limited.
Figure 12, “Assessing Asthma Control and
Adjusting Therapy in Children”
Figure 13, “Stepwise Approach for Managing
Asthma Long Term in Children, 0–4 Years of Age
and 5–11 Years ofAge”
For adults, see:
Figure 14, “Classifying Asthma Severity and
Initiating Treatment in Youths 12 Years of Age
and Adults”
Figure 15, “Assessing Asthma Control and
Adjusting Therapy in Youths ≥12 Years of Age and
Adults”
Figure 16, “Stepwise Approach for Managing
Asthma in Youths ≥12 Years of Age and Adults”
For medication dosages, see:
Figure 17, “Usual Dosages for Long-Term Control
Medications”
Figure 18, “Estimated Comparative Daily Dosages
for Inhaled Corticosteroids”
Figure 19, “Usual Dosages for Quick-Relief
Medications”
■The stepwise approach incorporates all four
components of care: assessment of severity to
initiate therapy or assessment of control to monitor
and adjust therapy; patient education; environmental
control measures, and management of comorbid
conditions at every step; and selection of medication.
■The type, amount, and scheduling of medication is
determined by the level of asthma severity or
asthma control.
— Therapy is increased (stepped up) as necessary
and decreased (stepped down) when possible.
— Because asthma is a chronic inflammatory
disorder, persistent asthma is most effectively
controlled with daily long-term control
medication directed toward suppressing
inflammation. ICSs are the most consistently
effective anti-inflammatory therapy for all age
groups, at all steps of care for persistent asthma.
— Selection among alternative treatment options
is based on consideration of treatment
effectiveness for the domain of particular
relevance to the patient (impairment, risk, or
both), the individual patient’s history
of previous response to therapies (sensitivity
and responsiveness to different asthma
medications can vary among patients), and the
willingness and ability of the patient and family
to use the medication.
■Once asthma control is achieved, monitoring and
followup are essential, because asthma often varies
over time. A step up in therapy may be needed,
or a step down may be possible, to identify the
minimum medication necessary to maintain
control.
32 Guidelines for the Diagnosis and Management of Asthma
FIGURE 10. AEROSOL DELIVERY DEVICES (continued)
Device/Drugs Population Optimal Technique* Therapeutic Issues
Nebulizer
Beta2-agonists
Corticosteroids
Cromolyn sodium
Anticholinergics
Patients of any age
who cannot use MDI
with VHC and face
mask.
Slow tidal breathing with occasional
deep breaths. Tightly fitting face
mask for those unable to use
mouthpiece.
Using the “blow by” technique (i.e.,
holding the mask or open tube near
the infant’s nose and mouth) is not
appropriate.
Less dependent on patient’s coordination and cooperation.
Delivery method of choice for cromolyn sodium in young children.
May be expensive; time consuming; bulky; output is dependent
on device and operating parameters (fill volume, driving gas flow);
internebulizer and intranebulizer output variances are significant.
Use of a face mask reduces delivery to lungs by 50 percent.
Nebulizers are as effective as MDIs plus VHCs for delivering bron-
chodilators in the ED for mild to moderate exacerbations; data in
severe exacerbations are limited. Choice of delivery system is
dependent on resources, availability, and clinical judgment of the cli-
nician caring for the patient.
Potential for bacterial infections if not cleaned properly.
Key: ED, emergency department; SABAs, inhaled short-acting beta2-agonists
*See figures in component 2—Education for a Partnership in Asthma Care for description of MDI and DPI techniques.

33
Managing Asthma Long Term
The stepwise approach and recommended treat-
ments are meant to assist, not replace, the clinical
decisionmaking necessary to determine the most
appropriate treatment to meet the individual
patient’s needs and circumstances.
Referral to an asthma specialist for consultation or
comanagement is recommended if there are diffi-
culties achieving or maintaining control of asthma,
if the patient required >2 bursts of oral systemic
corticosteriods in 1 year or has an exacerbation
requiring hospitalization, if step 4 care or higher is
required (step 3 care or higher for children 0–4
years of age), if immunotherapy or omalizumab is
considered, or if additional testing is indicated.
To achieve control of asthma, the following
sequence of activities is recommended:
■For patients who are not already taking long-term
control medications, assess asthma severity and
initiate therapy according to the level of severity.
■For patients who are already taking long-term
control medications, assess asthma control and
step up therapy if the patient’s asthma is not well
controlled on current therapy. Before stepping up,
review the patient’s adherence to medications,
inhaler technique, and environmental control
measures.
■Evaluate asthma control in 2–6 weeks (depending
on level of initial severity or control).
— In general, classify the level of asthma control
by the most severe indicator of impairment
or risk.
— The risk domain is usually more strongly
associated with morbidity in young children
than the impairment domain because young
children are often symptom free between
exacerbations.
— If office spirometry suggests worse control than
other measures of impairment, consider fixed
obstruction and reassess the other measures.
If fixed obstruction does not explain the lack of
control, step up therapy, because low FEV1is a
predictor of exacerbations.
— If the history of exacerbations suggests poorer
control than does assessment of impairment,
reassess impairment measures, and consider a
step up in therapy. Review plans for handling
exacerbations and include the use of oral
systemic corticosteroids, especially for patients
who have a history of severe exacerbations.
■If asthma control is not achieved with the above
actions:
— Review the patient’s adherence to medications,
inhaler technique, environmental control
measures (or whether there are new
exposures), and management of comorbid
conditions.
— If adherence and environment control
measures are adequate, then step up one step
(if not well controlled ) or two steps (if very
poorly controlled).
— If an alternative treatment was used initially,
discontinue its use and use the preferred
treatment option before stepping up therapy.
— A short course of oral systemic cortico-
steroids may be considered to gain more
rapid control for patients whose asthma
frequently interrupts sleep or normal daily
activities or who are experiencing an
exacerbation at the time of assessment.
— If lack of control persists, consider alternative
diagnoses before stepping up further.
— If the patient experiences side effects,
consider different treatment options.
To maintain control of asthma, regular followup con-
tact is essential because asthma often varies over time.
■Schedule patient contact at 1- to 6-month intervals;
the interval will depend on such factors as the level
or duration of asthma control and the level of
treatment required.
■Consider a step down in therapy once asthma is
well controlled for at least 3 months. A step down
is necessary to identify the minimum therapy
required to maintain good control. A reduction
in therapy should be gradual and must be closely
monitored. Studies are limited in guiding therapy
reduction. In general, the dose of ICS may be
reduced 25 percent to 50 percent every 3 months
to the lowest possible dose.
■Consider seasonal periods of daily long-term
control therapy for patients who have asthma

symptoms only in relation to certain seasons (e.g.,
seasonal pollens, allergens, or viral respiratory
infections) and who have intermittent asthma the
rest of the year. This approach has not been
rigorously evaluated; close monitoring for 2–6
weeks after therapy is discontinued is essential to
assure sustained asthma control.
Stepwise Treatment Recommendations for
Different Ages
Recommendations for treatments in the different
steps are presented in three different age groups
(0–4 years, 5–11 years, and 12 years and older)
because the course of the disease may change over
time, the relevance of measures of impairment or risk
and the potential short- and long-term impact of
medications may be age related, and varied levels of
scientific evidence are available for the different ages.
Steps for Children 0–4 Years of Age
See figure 13, for recommended treatments in the
different steps and figures 17–19 for recommended
medication dosages. In addition to the general
principles of the stepwise approach, special consider-
ations for this age group include initiating therapy,
selecting among treatment options, and monitoring
response to therapy.
The initiation of daily long-term control therapy in
children ages 0–4 years is recommended as follows:
■It is recommended for reducing impairment and
risk of exacerbations in infants and young children
who had four or more episodes of wheezing in the
past year that lasted more than 1 day and affected
sleep AND who have a positive asthma predictive
index (either (1) one of the following: a parental
history of asthma, a physician’s diagnosis of
atopic dermatitis, or evidence of sensitization
to aeroallergens; OR (2) two of the following:
evidence of sensitization to foods, >4 percent
peripheral blood eosinophilia, or wheezing apart
from colds).
■It should be considered for reducing impairment
in infants and young children who consistently
require symptomatic treatment >2 days per week
for a period of more than 4 weeks.
■It should be considered for reducing risk in infants
and young children who have two exacerbations
requiring systemic corticosteroids within 6 months.
■It may be considered for use only during periods,
or seasons, of previously documented risk (e.g.,
during seasons of viral respiratory infections).
The decision about when to start long-term daily
therapy is difficult. The chronic airway inflammatory
response in asthma can develop in the preschool
years; for example, between 50–80 percent of children
who have asthma developed symptoms before their
fifth birthday. Adequate treatment will reduce the
burden of illness, and underdiagnosis and undertreat-
ment are key problems in this age group. Not all
wheeze and cough are caused by asthma, however,
and caution is needed to avoid giving inappropriate,
prolonged therapy.
Initiating long-term control therapy will depend
on consideration of issues regarding diagnosis
and prognosis.
— Viral respiratory infections are the most
common cause of asthma symptoms in this age
group, and many children who wheeze with
respiratory infections respond well to asthma
therapy even though the diagnosis of asthma is
not clearly established. For children who have
exacerbations with viral infections, exacerba-
tions are often severe (requiring emergency
care or hospitalization), yet the child has
no significant symptoms in between these
exacerbations. These children have a low level
of impairment but a high level of risk.
— Most young children who wheeze with viral
respiratory infection experience a remission
of symptoms by 6 years of age, perhaps due to
growing airway size.
— However, two-thirds of children who have
frequent wheezing AND also have a positive
asthma predictive index (see above) are likely
to have asthma throughout childhood. Early
identification of these children allows appropriate
treatment with environmental control
measures and medication to reduce morbidity.
Select medications with the following considerations
for young children:
■Asthma treatment for young children, especially
infants, has not been studied adequately. Most
recommendations are based on limited data and
extrapolations from studies in older children and
adults. Preferred treatment options are based on
34 Guidelines for the Diagnosis and Management of Asthma

individual drug efficacy studies in this age group;
comparator trials are not available.
■The following long-term control medications are
FDA approved for the following ages in young
children: ICS budesonide nebulizer solution (1–8
years of age); ICS fluticasone dry power inhaler
(DPI) (>4 years of age); LABA salmeterol DPI,
alone or in combination with ICS (>4 years of age);
LTRA montelukast (chewable tablets, 2–6 years of
age; granules, down to 1 year old).
■Several delivery devices are available, and the doses
received may vary considerably among devices and
age groups. In general, children <4 years of age
will have less difficulty with a face mask and either
(1) a nebulizer or (2) an MDI with a VHC. (See
figure 10 above.)
■ICSs are the preferred long-term control medication
for initiating therapy. The benefits of ICSs out
weigh any concerns about potential risks of a small,
nonprogressive reduction in growth velocity or
other possible adverse effects. ICSs, as with all
medications, should be titrated to as low a dose
as needed to maintain control.
■For children whose asthma is not well controlled
on low-dose ICS, few studies are available on
stepup therapy in this age group, and the studies
have mixed findings. Some data on children ≤4
years old and younger show dose-dependent
improvements in the domains of impairment and
risk of exacerbation from taking ICS. Data from
studies on LABA combined with ICS have only
small numbers of 4-year-old children, and these
data show improvement in the impairment but
not risk domain. Adding a noncorticosteroid
long-term control medication to medium-dose
ICS may be considered before increasing the dose
of ICS to high dose to avoid potential risk of side
effects with high doses of medication.
Monitor response to therapy closely, because
treatment of young children is often in the form of
a therapeutic trial.
■If a clear and beneficial response is not obvious
within 4–6 weeks and the patient’s/family’s med-
ication technique and adherence are satisfactory,
treatment should be stopped. Alternative therapies
or alternative diagnoses should be considered.
■If a clear and beneficial response is sustained for
at least 3 months, consider a step down to evalu-
ate the need for continued daily long-term control
therapy. Children in this age group have high rates
of spontaneous remission of symptoms.
Steps for Children 5–11 Years of Age
See figure 13, “Stepwise Approach for Managing
Asthma Long Term in Children, 0–4 Years of Age and
5–11 Years of Age,” for recommended treatments in
different steps and figures 17, 18, and 19 for recom-
mended medication dosages. Special considerations
for this age group include the following:
Promote active participation in physical activities,
exercise, and sports because physical activity is an
essential part of a child’s life. Treatment immediate-
ly before vigorous activity usually prevents EIB
(see section on “Exercise-Induced Bronchospasm”).
However, if the child has poor endurance or has
symptoms during usual play activities, a step up
in therapy is warranted.
Directly involve children ≥10 years of age (and
younger children as appropriate) in developing
their written asthma action plans and reviewing
their adherence. This involvement may help address
developmental issues of emerging independence by
building the children’s confidence, increasing person-
al responsibility, and gaining problem-solving skills.
Encourage parents to take a copy of the written
asthma action plan to the student’s school, or
childcare or extended care setting, or camp.
Consider the following when selecting treatment
options:
■ICSs are the preferred long-term control therapy.
The benefits of ICSs outweigh any concerns about
potential risks of a small, nonprogressive reduction
in growth velocity or other possible adverse effects.
ICSs, as with all medications, should be titrated
to as low a dose as needed to maintain control.
High-quality evidence demonstrates the effective-
ness of ICS in children 5–11 years of age, and
comparator studies demonstrate improved control
with ICS on a range of asthma outcomes compared
to other long-term control medications.
■Step up treatment options for children whose asth-
ma is not well controlled on low-dose ICS have not
been adequately studied or compared in this age
group. The selection will depend on the domain
35
Managing Asthma Long Term
36 Guidelines for the Diagnosis and Management of Asthma
SAMPLE RECORD FOR MONITORING THE RISK DOMAIN IN CHILDREN: RISK OF ASTHMA PROGRESSION
(INCREASED EXACERBATIONS OR NEED FOR DAILY MEDICATION, OR LOSS OF LUNG FUNCTION), AND
POTENTIAL ADVERSE EFFECTS OF CORTICOSTEROID THERAPY
Patient name:
Date
ICS daily dose*
LTRA
LABA
Theophylline
Other
FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; ICS, inhaled corticosteroid; LABA, long-acting beta2agonist; LTRA, leukotriene
receptor antagonist
*Consider ophthalmologic exam and bone density measurement in children using high doses of ICS or multiple courses of oral corticosteroids.
Long-term control medication
Significant exacerbations
Long-term control medication
Potential risk of adverse corticosteroid effects
(as indicated by corticosteroid dose and duration of treatment)
Exacerbations (number/month)
Oral systemic corticosteroids (number/year)*
Hospitalization (number/year)
Prebronchodilator FEV1/FVC
Prebronchodilator FEV1percent predicted
Postbronchodilator FEV1percent predicted
Percent bronchodilator reversibility
Height, cm
Percentile
Plots of growth velocity

of particular relevance (impairment, risk, or both)
and clinician–patient preference.
— For the impairment domain:
• Children who have low lung function and >2
days per week impairment may be better served
by adding a LABA to a low dose of ICS (based
on studies in older children and adults).
• Increasing the dose of ICS to medium dose can
improve symptoms and lung function in those
children who have greater levels of impairment
(based on studies in children).
• One study in children suggests some benefit in
the impairment domain with adding LTRA.
— For the risk domain:
• Studies have not demonstrated that adding
LABA or LTRA reduces exacerbations in children.
Adding LABA has the potential risk of rare
life-threatening or fatal exacerbations.
• Studies in older children and adults show that
increasing the dose of ICS can reduce the risk of
exacerbations, but this may require up to a four-
fold increase in the dose. This dose may increase
the potential risk of systemic effects, although
the risk is small within the medium-dose range.
■The need for step 4 care usually involves children
who have a low level of lung function contributing
to their impairment. The combination of ICS and
LABA is preferred, on the basis of studies in older
children and adults.
■Before maintenance dose of oral corticosteroids is
initiated in step 6, consider a 2-week course of oral
corticosteroids to confirm clinical reversibility,
measured by spirometry, and the possibility of an
effective response to therapy. If the response is poor,
a careful review for other pulmonary conditions or
comorbid conditions should be conducted to ensure
that the primary diagnosis is severe asthma.
Monitor asthma progression. Declines in lung func-
tion or repeated periods of worsening asthma
impairment may indicate a progressive worsening
of the underlying severity of asthma. Although there
is no indication that treatment alters the progression
of the underlying disease in children, adjustments
in treatment may be necessary to maintain asthma
control.
Steps for Youths 12 Years of Age and Adults
See figure 16, “Stepwise Approach for Managing
Asthma in Youths 12 Years of Age and Adults,” for
recommended treatment options in different steps
and figures 18 and 19, for recommended medication
dosages for youths 12 years of age and adults.
Special considerations for this age group include
the following:
For youths:
■Involve adolescents in the development of their
written asthma action plans and reviewing their
adherence.
■Encourage students to take a copy of their plan
to school, after school programs, and camps.
■Encourage adolescents to be physically active.
For older adults:
■Consider a short course of oral systemic
corticosteroids to establish reversibility and the
extent of possible benefit from asthma treatment.
Chronic bronchitis and emphysema may coexist
with asthma.
■Adjust medications as necessary to address
coexisting medical conditions. For example,
consider calcium and vitamin D supplements for
patients who take ICS and have risk factors for
osteoporosis. Consider increased sensitivity to side
effects of bronchodilators, especially tremor and
tachycardia with increasing age, and increased
possibilities for drug interactions with theophylline.
Consider also that NSAIDs prescribed for arthritis
and the beta-blockers prescribed for hypertension
or glaucoma may exacerbate asthma.
■Review the patient’s technique and adherence
in using medications, and make necessary
adjustments. Physical or cognitive impairments
may make proper technique difficult.
Consider the following when selecting treatment
options:
■Recommended treatment for step 3 weighs the
high-quality evidence demonstrating the benefits of
adding LABA to low-dose ICS against the potential
risk of rare life-threatening or fatal exacerbations
with the use of LABA. The selection will depend
on the domain of particular relevance (impair-
ment, risk, or both) and clinician–patient preference.
37
Managing Asthma Long Term

— Adding LABA more consistently results in
improvements in the impairment domain
compared to increasing the dose of ICS.
— If the risk domain is of particular concern, then
a balance of potential risks needs to be considered.
• Adding LABA to low-dose ICS reduces the fre-
quency of exacerbations to a greater extent than
doubling the dose of ICS, but adding LABA has
the potential risk of rare life-threatening or fatal
exacerbations.
• Increasing the dose of ICS can significantly reduce
the risk of exacerbations, but this benefit may
require up to a fourfold increase in the ICS dose.
This dose may increase the potential risk of
systemic effects, although the risk is small within
the medium-dose range.
■Comparator studies demonstrate significantly
greater improvements with adding LABA to ICS
compared to other adjunctive therapies.
■Clinicians who administer omalizumab are advised
to be prepared and equipped for the identification
and treatment of anaphylaxis that may occur, to
observe patients for an appropriate period of time
following each omalizumab injection (the optimal
length of the observation is not established), and to
educate patients about the risks of anaphylaxis and
how to recognize and treat it if it occurs (e.g., using
prescription auto injectors for emergency self
treatment, and seeking immediate medical care).
Managing Special Situations
Patients who have asthma may encounter situations
that will require adjustments to their asthma manage-
ment to keep their asthma under control, such as
EIB, pregnancy, and surgery.
Exercise-Induced Bronchospasm
EIB should be anticipated in all asthma patients. A
history of cough, shortness of breath, chest pain or
tightness, wheezing, or endurance problems during
exercises suggests EIB. An exercise challenge,
in which a 15 percent decrease in PEF or FEV1
(measured before and after exercise at 5-minute inter-
vals for 20–30 minutes) will establish the diagnosis.
An important dimension of adequate asthma control
is a patient’s ability to participate in any activity he or
she chooses without experiencing asthma symptoms.
EIB should not limit either participation or success
in vigorous activities.
Recommended treatments for EIB include:
■Long-term control therapy, if appropriate.
Frequent or severe EIB may indicate the need to
initiate or step up long-term control medications.
■Pretreatment before exercise:
— Inhaled beta2-agonists will prevent EIB for
more than 80 percent of patients. SABA used
shortly before exercise may be helpful for 2–3
hours. LABA can be protective up to 12 hours,
but there is some shortening of the duration of
protection when LABA is used on a daily basis.
Frequent or chronic use of LABA as pretreat-
ment for EIB is discouraged, as it may disguise
poorly controlled persistent asthma.
— LTRAs, with an onset of action generally hours
after administration, can attenuate EIB in up
to 50 percent of patients.
— Cromolyn or nedocromil taken shortly before
exercise is an alternative treatment, but it is
not as effective as SABAs.
— A warmup period before exercise may reduce
the degree of EIB.
— A mask or scarf over the mouth may attenuate
cold-induced EIB.
Pregnancy
Maintaining asthma control during pregnancy is
important for the health and well-being of both the
mother and her baby. Maintaining lung function
is important to ensure oxygen supply to the fetus.
Uncontrolled asthma increases the risk of perinatal
mortality, preeclampsia, preterm birth, and
low-birth-weight infants. It is safer for pregnant
women to be treated with asthma medications than
to have asthma symptoms and exacerbations.
■Monitor the level of asthma control and lung
function during prenatal visits. The course of
asthma improves in one-third of women and
worsens for one-third of women during pregnancy.
Monthly evaluations of asthma will allow the
opportunity to step up therapy if necessary and to
step down therapy if possible.
38 Guidelines for the Diagnosis and Management of Asthma

■Albuterol is the preferred SABA. The most data
related to safety during human pregnancy are
available for abuterol.
■ICSs are the preferred long-term control
medication. Budesonide is the preferred ICS
because more data are available on using budesonide
in pregnant women than are available on other ICSs,
and the data are reassuring. However, no data
indicate that the other ICS preparations are unsafe
during pregnancy.
Surgery
Patients who have asthma are at risk for complica-
tions during and after surgery. These complications
include acute bronchoconstriction triggered by
intubation, hypoxemia and possible hypercapnia,
impaired effectiveness of cough, atelectasis, and
respiratory infection, and, if a history of sensitivity
is present, reactions to latex exposure or some
anesthetic agents.
The following actions are recommended to reduce
the risk of complications during surgery:
■Before surgery, review the level of asthma control,
medication use (especially oral systemic cortico-
steroids within the past 6 months), and pulmonary
function.
■Provide medications before surgery to improve
lung function if lung function is not well
controlled. A short course of oral systemic
corti costeroids may be necessary.
■For patients receiving oral systemic corticosteroids
during the 6 months prior to surgery and for
selected patients on long-term high-dose ICS, give
100 mg hydrocortisone every 8 hours intravenously
during the surgical period, and reduce the dose
rapidly within 24 hours after surgery.
Disparities
Multiple factors contribute to the higher rates of
poorly controlled asthma and asthma deaths among
Blacks and Latinos compared to Whites. These
factors include socioeconomic disparities in access to
quality medical care, underprescription and under-
utilization of long-term control medication, cultural
beliefs and practices about asthma management, and
perhaps biological and pathophysiological differences
that affect the underlying severity of asthma and
response to treatment. Heightened awareness of
disparities and cultural barriers, improving access
to quality care, and improving communication
strategies between clinicians and ethnic or racial
minority patients regarding use of asthma
medications may improve asthma outcomes.
39
Managing Asthma Long Term
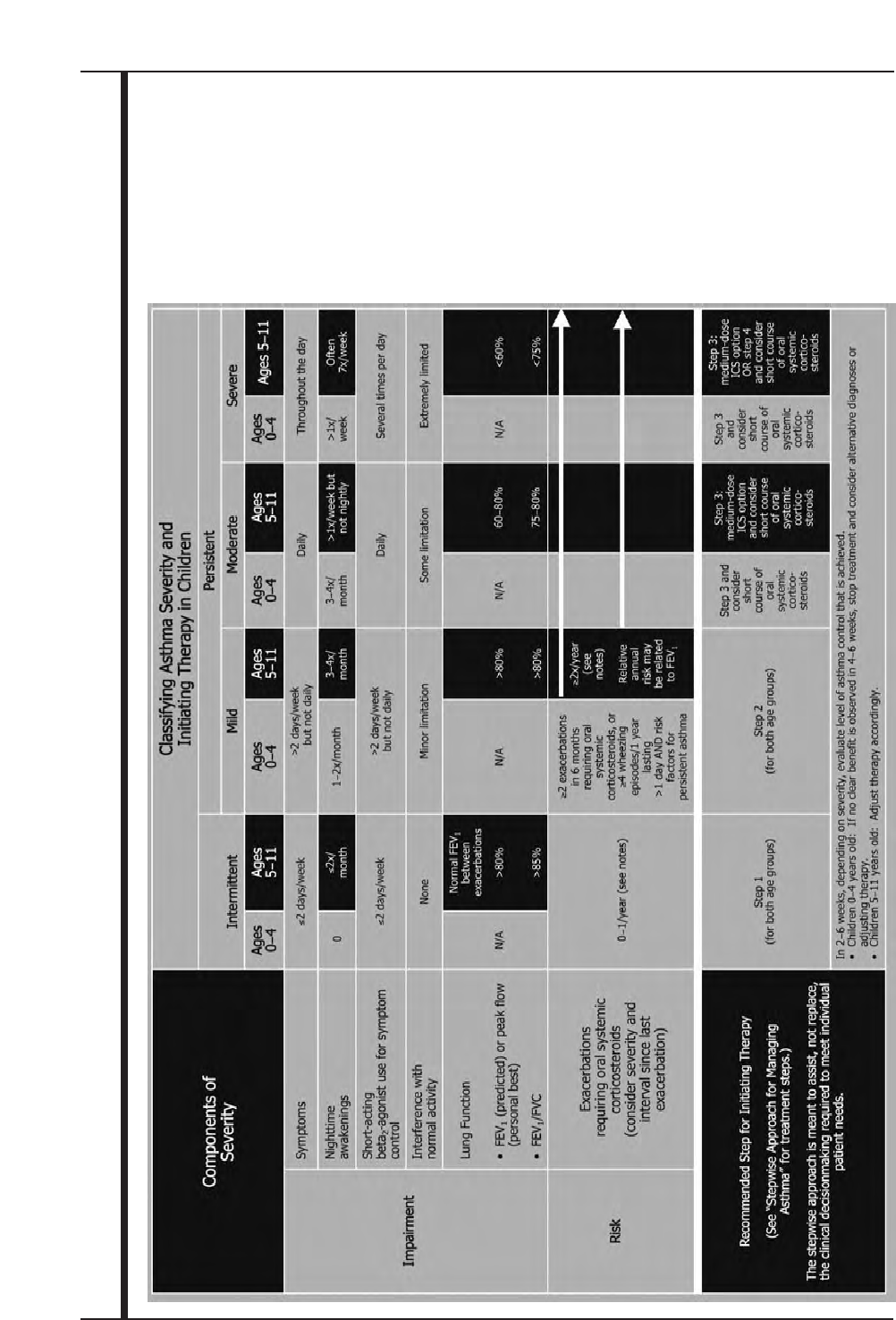
40 Guidelines for the Diagnosis and Management of Asthma
FIGURE 11. CLASSIFYING ASTHMA SEVERITY AND INITIATING THERAPY IN CHILDREN
Key: FEV1, forced expiratory volume
in 1 second; FVC, forced vital capacity;
ICS, inhaled corticosteroids; ICU,
intensive care unit; N/A, not applicable
Notes:
■Level of severity is determined by
both impairment and risk. Assess
impairment domain by caregiver’s
recall of previous 2–4 weeks.
Assign severity to the most severe
category in which any feature
occurs.
■Frequency and severity of exacerba-
tions may fluctuate over time for
patients in any severity category.
At present, there are inadequate
data to correspond frequencies
of exacerbations with different
levels of asthma severity.In general,
more frequent and severe exacerba-
tions (e.g., requiring urgent,
unscheduled care, hospitalization,
or ICU admission) indicate greater
underlying disease severity.For
treatment purposes, patients with ≥2
exacerbations described above may
be considered the same as patients
who have persistent asthma, even in
the absence of impairment levels
consistent with persistent asthma.
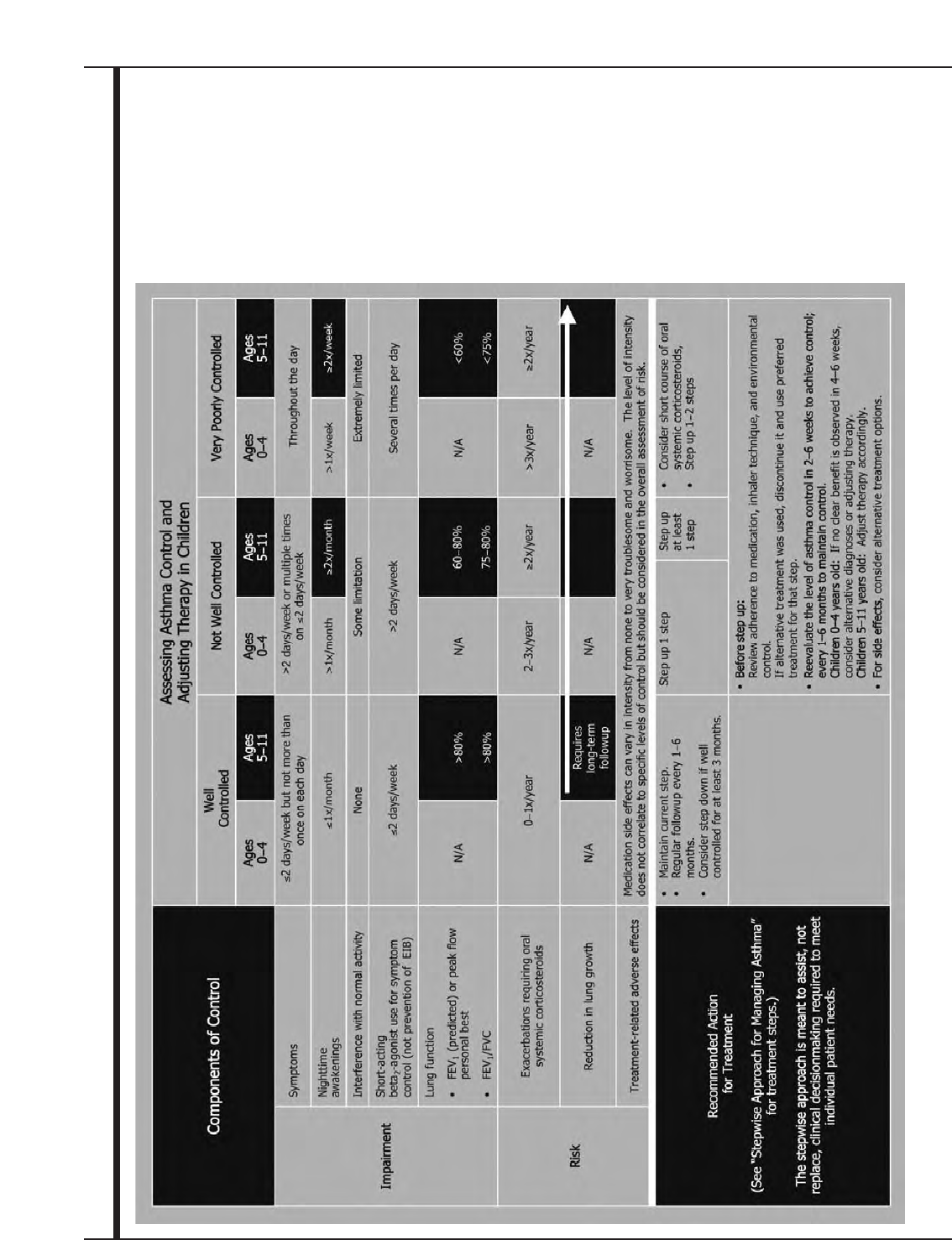
41
Managing Asthma Long Term
FIGURE 12.ASSESSING ASTHMA CONTROL AND ADJUSTING THERAPY IN CHILDREN
Key: EIB, exercise-induced bron-
chospasm, FEV1, forced expiratory
volume in 1 second; FVC, forced vital
capacity; ICU, intensive care unit;
N/A, not applicable
Notes:
■The level of control is based on the
most severe impairment or risk
category. Assess impairment
domain by patient’s or caregiver’s
recall of previous 2–4 weeks.
Symptom assessment for longer
periods should reflect a global
assessment, such as whether
the patient’s asthma is better or
worse since the last visit.
■At present, there are inadequate
data to correspond frequencies of
exacerbations with different levels of
asthma control. In general, more
frequent and intense exacerbations
(e.g., requiring urgent, unscheduled
care, hospitalization, or ICU
admission) indicate poorer
disease control.
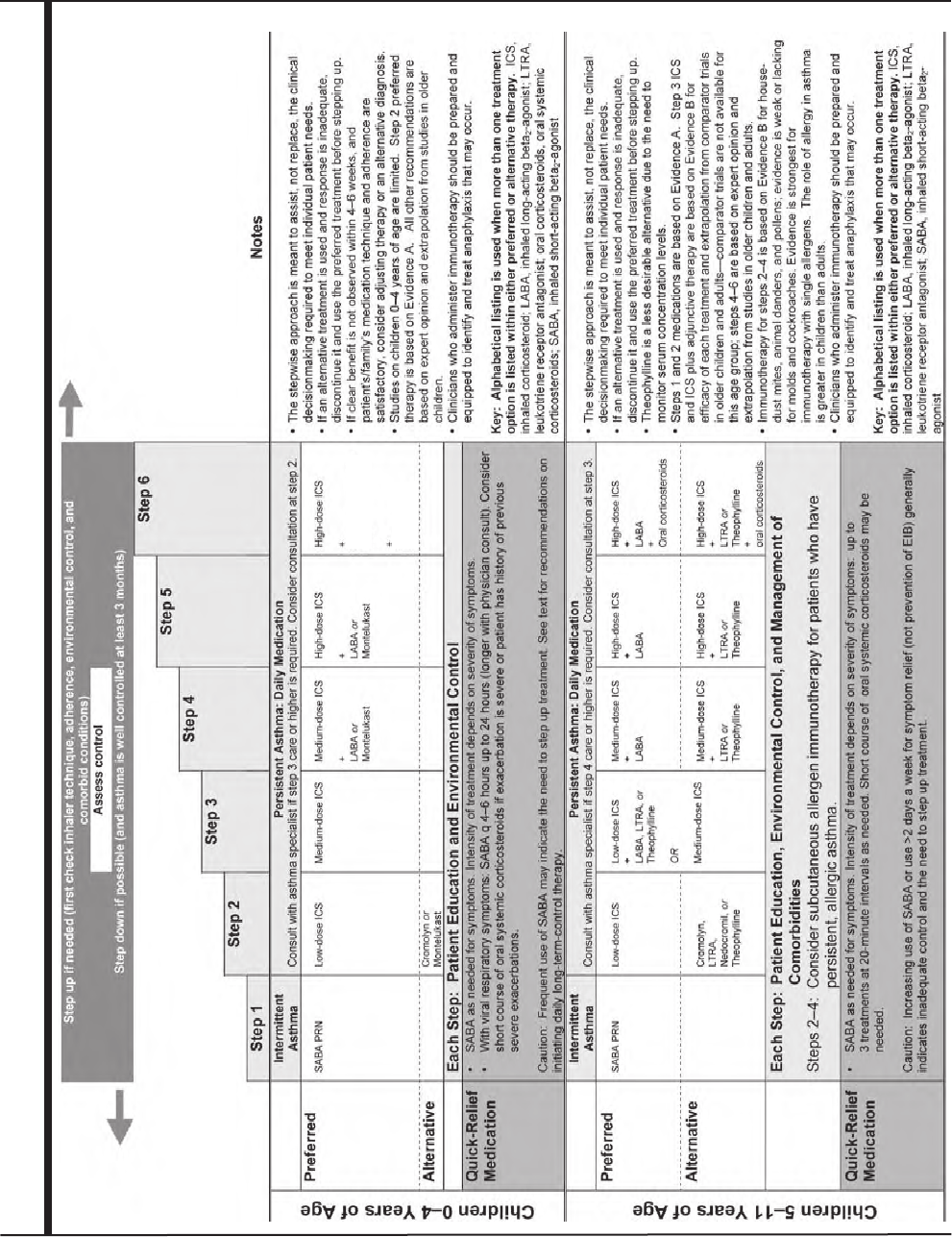
42 Guidelines for the Diagnosis and Management of Asthma
FIGURE 13. STEPWISE APPROACH FOR MANAGING ASTHMA LONG TERM IN CHILDREN, 0–4 YEARS OF AGE AND 5–11 YEARS OF AGE
Oral corticosteriods
ICS
LABA or
Montelukast
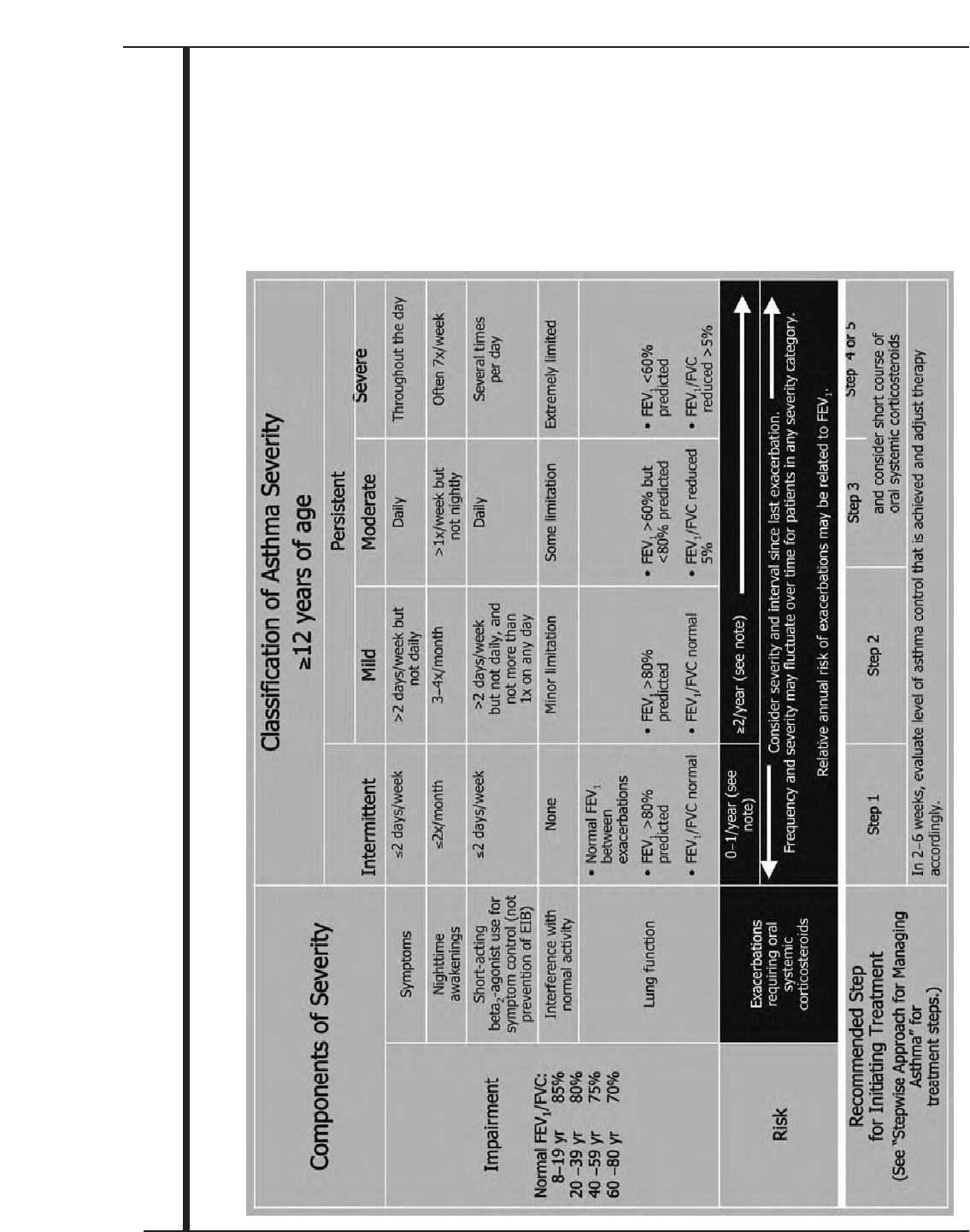
43
Managing Asthma Long Term
FIGURE 14.CLASSIFYING ASTHMA SEVERITY AND INITIATING TREATMENT IN YOUTHS 12 YEARS OF AGE AND ADULTS
Assessing severity and initiating treatment for patients who are not currently taking
long-term control medications
Key: EIB, exercise-induced bron-
chospasm, FEV1, forced expiratory
volume in 1 second; FVC, forced vital
capacity; ICU, intensive care unit
Notes:
•The stepwise approach is meant to
assist, not replace, the clinical
decisionmaking required to meet
individual patient needs.
•Level of severity is determined by
assessment of both impairment and
risk. Assess impairment domain by
patient’s/caregiver’s recall of
previous 2–4 weeks and spirometry.
Assign severity to the most severe
category in which any feature
occurs.
•At present, there are inadequate
data to correspond frequencies of
exacerbations with different levels
of asthma severity.In general, more
frequent and intense exacerbations
(e.g., requiring urgent, unscheduled
care, hospitalization, or ICU
admission) indicate greater
underlying disease severity.For
treatment purposes, patients who
had ≥2 exacerbations requiring oral
systemic corticosteroids in the past
year may be considered the same
as patients who have persistent
asthma, even in the absence of
impairment levels consistent with
persistent asthma.

44 Guidelines for the Diagnosis and Management of Asthma
FIGURE 15. ASSESSING ASTHMA CONTROL AND ADJUSTING THERAPY IN YOUTHS ≥12 YEARS OF AGE AND ADULTS
*ACQ values of 0.76–1.4 are indeterminate regarding
well-controlled asthma.
Key: EIB, exercise-induced bronchospasm; ICU, intensive care
unit
Notes:
•The stepwise approach is meant to assist, not replace,
the clinical decisionmaking required to meet individual
patient needs.
•The level of control is based on the most severe impair-
ment or risk category. Assess impairment domain by
patient’s recall of previous 2–4 weeks and by
spirometry/or peak flow measures. Symptom assessment
for longer periods should reflect a global assessment, such
as inquiring whether the patient’s asthma is better or
worse since the last visit.
•At present, there are inadequate data to correspond fre-
quencies of exacerbations with different levels of asthma
control. In general, more frequent and intense
exacerbations (e.g., requiring urgent, unscheduled care,
hospitalization, or ICU admission) indicate poorer disease
control. For treatment purposes, patients who had ≥2
exacerbations requiring oral systemic corticosteroids in the
past year may be considered the same as patients who
have not-well-controlled asthma, even in the absence of
impairment levels consistent with not-well-controlled asthma.
ATAQ = Asthma Therapy Assessment Questionnaire©
ACQ = Asthma Control Questionnaire©
ACT = Asthma Control Test™
Minimal Important
Difference: 1.0 for the ATAQ; 0.5 for the ACQ; not
determined for the ACT.
Before step up in therapy:
— Review adherence to medication, inhaler technique,
environmental control, and comorbid conditions.
— If an alternative treatment option was used in a step,
discontinue and use the preferred treatment for that step.
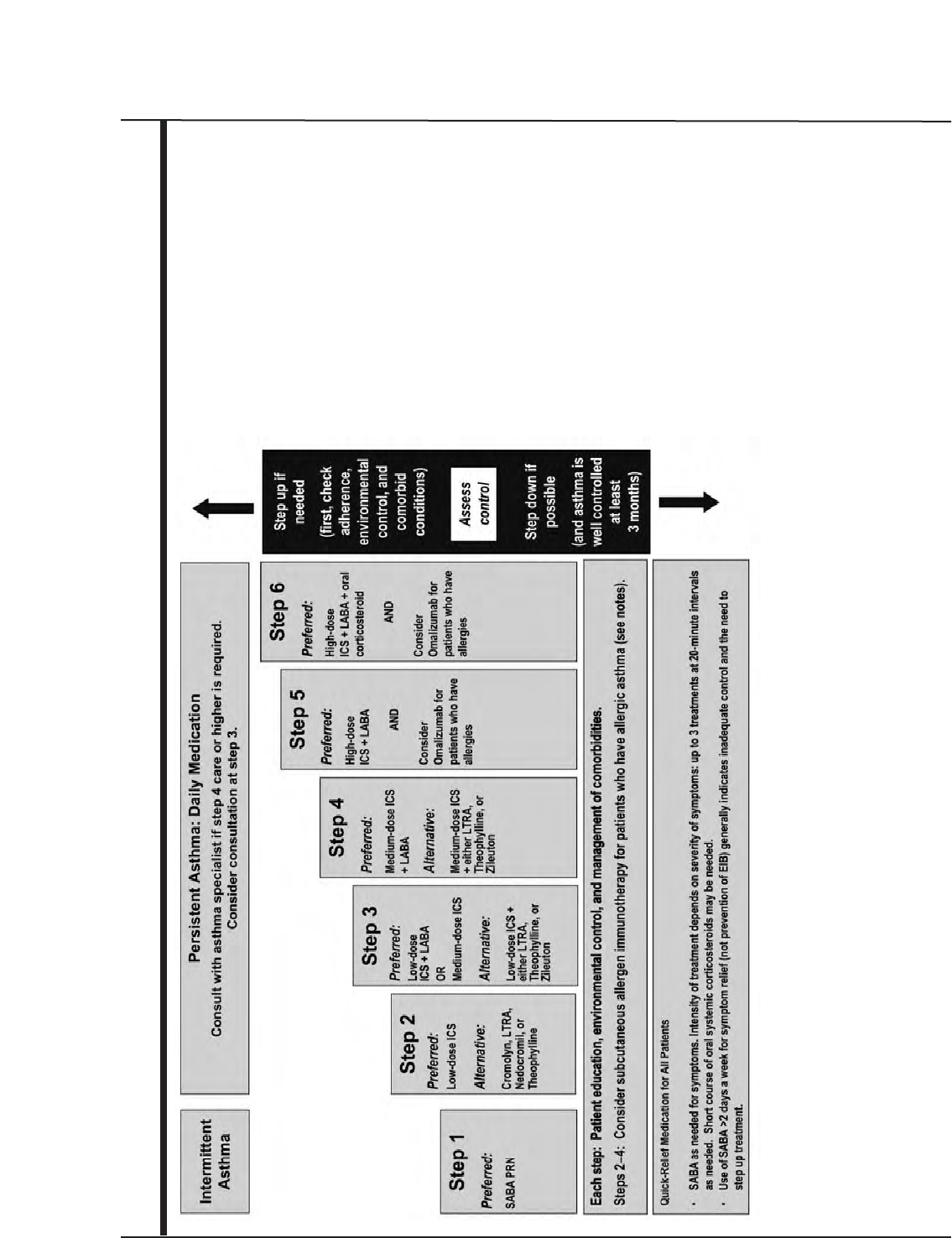
45
Managing Asthma Long Term
FIGURE 16. STEPWISE APPROACH FOR MANAGING ASTHMA IN YOUTHS ≥12 YEARS OF AGE AND ADULTS
Key: Alphabetical order is used when more than one
treatment option is listed within either preferred or
alternative therapy.ICS, inhaled corticosteroid; LABA, long-
acting inhaled beta2-agonist; LTRA, leukotriene receptor
antagonist; SABA, inhaled short-acting beta2-agonist
Notes:
•The stepwise approach is meant to assist, not replace, the
clinical decisionmaking required to meet individual patient
needs.
•If alternative treatment is used and response is inadequate,
discontinue it and use the preferred treatment before
stepping up.
•Zileuton is a less desirable alternative due to limited
studies as adjunctive therapy and the need to monitor
liver function. Theophylline requires monitoring of serum
concentration levels.
•In step 6, before oral corticosteroids are introduced, a trial
of high-dose ICS + LABA + either LTRA, theophylline, or
zileuton may be considered, although this approach has
not been studied in clinical trials.
•Step 1, 2, and 3 preferred therapies are based on Evidence
A; step 3 alternative therapy is based on Evidence A for
LTRA, Evidence B for theophylline, and Evidence D for
zileuton. Step 4 preferred therapy is based on Evidence B,
and alternative therapy is based on Evidence B for LTRA
and theophylline and Evidence D zileuton. Step 5
preferred therapy is based on Evidence B. Step 6 preferred
therapy is based on (EPR—2 1997) and Evidence B for
omalizumab.
•Immunotherapy for steps 2–4 is based on Evidence B for
house-dust mites, animal danders, and pollens; evidence is
weak or lacking for molds and cockroaches. Evidence is
strongest for immunotherapy with single allergens. The role
of allergy in asthma is greater in children than in adults.
•Clinicians who administer immunotherapy or omalizumab
should be prepared and equipped to identify and treat
anaphylaxis that may occur.
46 Guidelines for the Diagnosis and Management of Asthma
FIGURE 17. USUAL DOSAGES FOR LONG-TERM CONTROL MEDICATIONS*
Medication
0–4 Years
of Age
Methylprednisolone
2, 4, 8, 16,
32 mg tablets
Prednisolone
5 mg tablets,
5 mg/5 cc,
15 mg/5 cc
Prednisone
1, 2.5, 5, 10, 20,
50 mg tablets;
5 mg/cc,
5 mg/5 cc
0.25–2 mg/kg
daily in single
dose in a.m. or
qod as needed
for control
Short-course
“burst”: 1–2
mg/kg/day, maxi-
mum 60 mg/day
for 3–10 days
5–11 Years
of Age
0.25–2 mg/kg
daily in single
dose in a.m. or
qod as needed
for control
Short-course
“burst”: 1–2
mg/kg/day, maxi-
mum 60 mg/day
for 3–10 days
≥12 Years of
Age and Adults
7.5–60 mg daily
in a single dose
in a.m. or qod
as needed for
control
Short-course
“burst”: to
achieve control,
40–60 mg per
day as single or
2 divided doses
for 3–10 days
■Short-term use: reversible
abnormalities in glucose metabo-
lism, increased appetite, fluid
retention, weight gain, mood
alteration, hypertension, peptic
ulcer, and rarely aseptic necrosis.
■Long-term use: adrenal axis
suppression, growth suppression,
dermal thinning, hypertension,
diabetes, Cushing’s syndrome,
cataracts, muscle weakness,
and—in rare instances
—impaired immune function.
■Consideration should be given to
coexisting conditions that could
be worsened by systemic corti-
costeroids, such as herpes virus
infections, varicella, tuberculosis,
hypertension, peptic ulcer, dia-
betes mellitus, osteoporosis, and
Strongyloides
Potential Adverse Effects
Inhaled Corticosteroids (See Figure 18, “Estimated Comparative Daily Dosages for ICSs.”)
Key: DPI, dry powder inhaler; EIB, exercise-induced broncospasm; HFA, hydrofluoroalkane; ICS, inhaled corticosteroids; IgE, immunoglobulin E; MDI, metered-dose inhaler;
NA, not available (either not approved, no data available, or safety and efficacy not established for this age group); SABA, short-acting beta2-agonist
*Note: Dosages are provided for those products that have been approved by the U.S. Food and Drug Administration or have sufficient clinical trial safety and efficacy data
in the appropriate age ranges to support their use.
Comments (not all inclusive)
■For long-term treatment of severe
persistent asthma, administer single
dose in a.m. either daily or on alternate
days (alternate-day therapy may
produce less adrenal suppression).
■Short courses or “bursts” are effective
for establishing control when initiating
therapy or during a period of gradual
deterioration.
■There is no evidence that tapering
the dose following improvement in
symptom control and pulmonary function
prevents relapse.
■Children receiving the lower dose
(1 mg/kg/day) experience fewer
behavioral side effects, and it appears
to be equally efficacious.
■For patients unable to tolerate the liquid
preparations, dexamethasone syrup at
0.4 mg/kg/day may be an alternative.
Studies are limited, however, and the
longer duration of activity increases the
risk of adrenal suppression.
Oral Systemic Corticosteroids (Apply to all three corticosteriods.)
Salmeterol
DPI 50 mcg/
blister
Formoterol
DPI 12 mcg/
single-use
capsule
NA
NA
1 blister
q 12 hours
1 capsule
q 12 hours
1 blister
q 12 hours
1 capsule
q 12 hours
■Tachycardia, skeletal muscle
tremor, hypokalemia, prolongation
of QTc interval in overdose.
■A diminished bronchoprotective
effect may occur within 1 week
of chronic therapy. Clinical signif-
icance has not been established.
■Potential risk of uncommon,
severe, life-threatening or fatal
exacerbation; see text for addi-
tional discussion regarding safety
of LABAs.
■Should not be used for acute
symptom relief or exacerbations.
Use only with ICSs.
■Decreased duration of protection against
EIB may occur with redgular use.
■Most children <4 years of age cannot
provide sufficient inspiratory flow for
adequate lung delivery.
■Do not blow into inhaler after dose is
activated.
■Each capsule is for single use only; addi-
tional doses should not be administered
for at least 12 hours.
■Capsules should be used only with the
inhaler and should not be taken orally.
Inhaled Long-Acting Beta2-Agonists (LABAs) (Apply to both LABAs.)
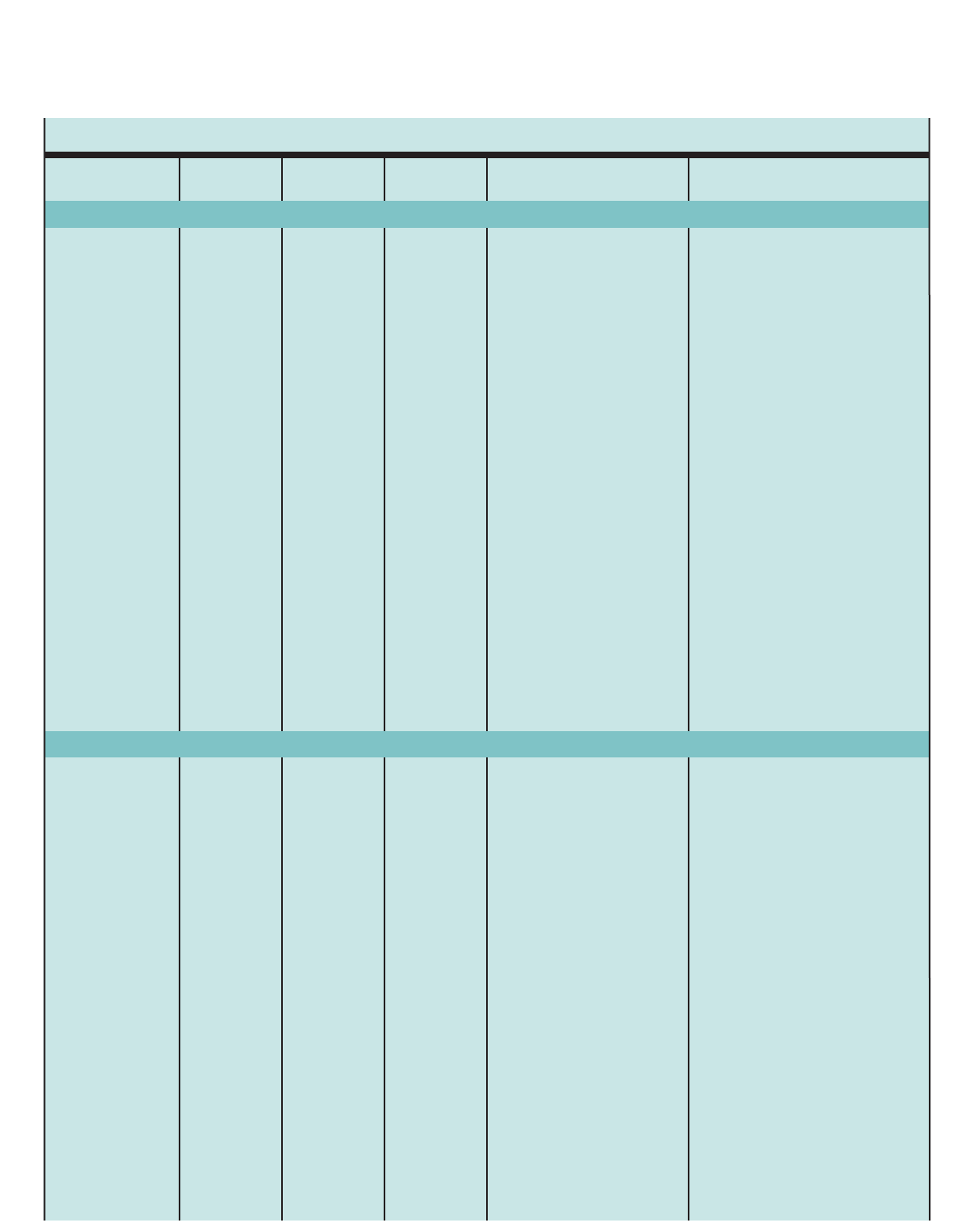
47
Managing Asthma Long Term
FIGURE 17. USUAL DOSAGES FOR LONG-TERM CONTROL MEDICATIONS* (continued)
Medication
0–4 Years
of Age
Fluticasone/Salmeterol
DPI
100 mcg/50 mcg,
250 mcg/50 mcg,
or 500 mcg/
50 mcg
HFA
45 mcg/21 mcg
115 mcg/21 mcg
230 mcg/21 mcg
NA
5–11 Years
of Age
1 inhalation bid,
dose depends on
level of severity
or control
≥12 Years of
Age and Adults
1 inhalation bid;
dose depends on
level of severity
or control
■See notes for ICS and LABA.
Potential Adverse Effects Comments (not all inclusive)
■There have been no clinical trials in
children <4 years of age.
■Most children <4 years of age cannot
provide sufficient inspiratory flow for
adequate lung delivery.
■Do not blow into inhaler after dose is
activated.
■100/50 DPI or 45/21 HFA for patients
who have asthma not controlled on
low- to medium-dose ICS
■250/50 DPI or 115/21 HFA for patients
who have asthma not controlled on
medium to high dose ICS.
Combined Medication
Budesonide/
Formoterol
HFA MDI
80 mcg/4.5 mcg
160mcg/4.5 mcg
NA 2 puffs bid, dose
depends on level
of severity or
control
2 puffs bid; dose
depends on level
of severity or
control
■See notes for ICS and LABA.
Cromolyn
MDI
0.8 mg/puff
Nebulizer
20 mg/ampule
Nedocromil
MDI
1.75 mg/puff
NA
1 ampule qid
NA <2 years of
age
NA <6 years of
age
2 puffs qid
1 ampule qid
2 puffs qid
2 puffs qid
1 ampule qid
2 puffs qid
■Cough and irritation.
■15–20 percent of patients
complain of an unpleasant taste
from nedocromil.
■Safety is the primary advantage
of these
Cromolyn/Nedocromil
■One dose of cromolyn before exercise
or allergen exposure provides effective
prophylaxis for 1–2 hours. Not as
effective as inhaled beta2-agonists for
EIB as SABA.
■4- to 6-week trial of cromolyn or
nedocromil may be needed to determine
maximum benefit.
■Dose by MDI may be inadequate to
affect hyperresponsiveness.
■Once control is achieved, the frequency
of dosing may be reduced.
■There have been no clinical trials in
children <4 years of age.
■Currently approved for use in youths
≥12 years of age. Dose for children
5–12 years of age based on clinical
trials using DPI with slightly different
delivery characteristics.
■80/4.5 for patients who have asthma
not controlled on low- to medium-dose
ICS.
■160/4.5 for patients who have asthma
not controlled on medium- to high-dose
ICS.
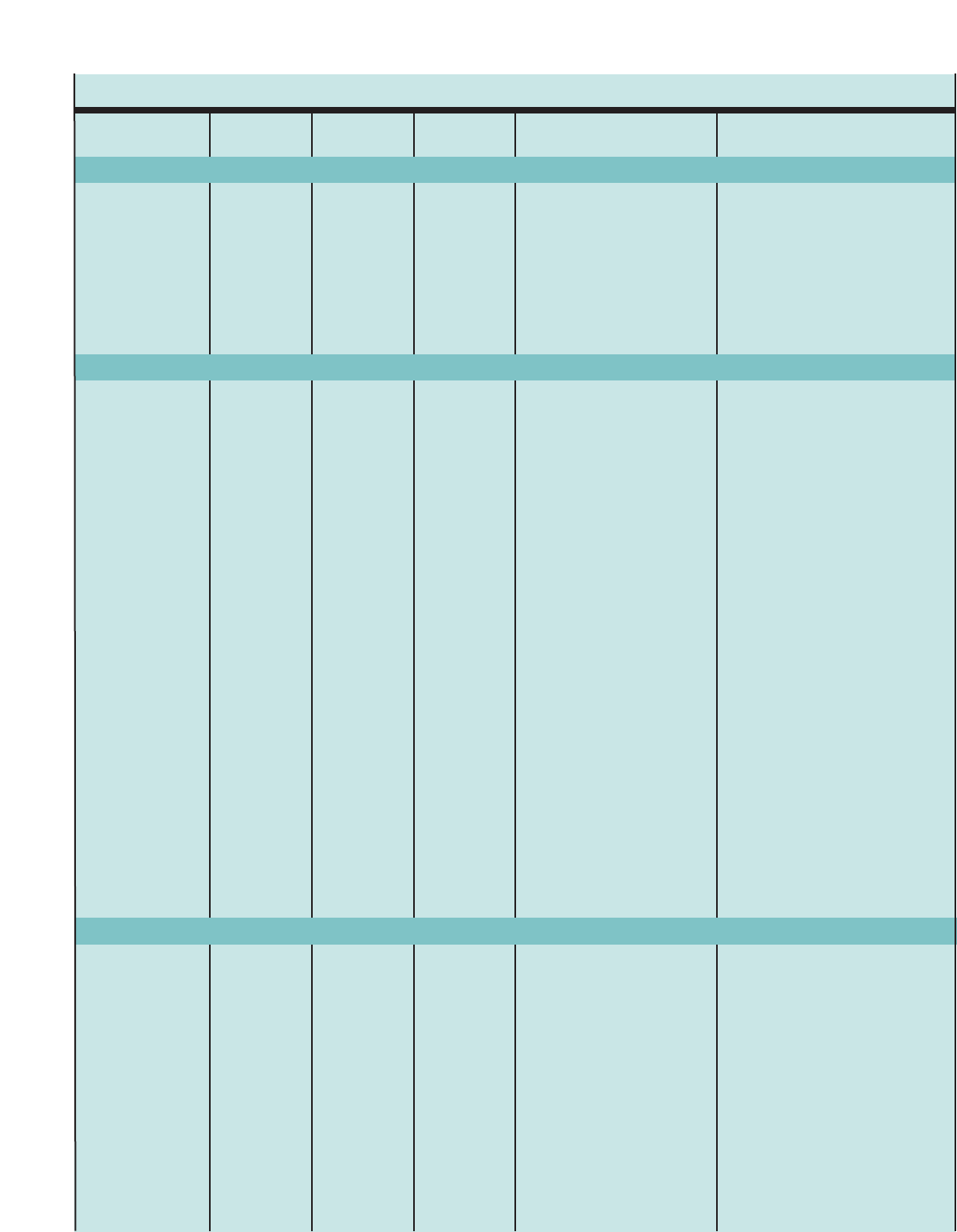
48 Guidelines for the Diagnosis and Management of Asthma
FIGURE 17. USUAL DOSAGES FOR LONG-TERM CONTROL MEDICATIONS* (continued)
Medication
0–4 Years
of Age
5–11 Years
of Age
≥12 Years of
Age and Adults Potential Adverse Effects Comments (not all inclusive)
Omalizumab
(Anti IgE)
Subcutaneous
injection, 150 mg/
1.2 mL following
reconstitution with
1.4 mL sterile
water for injection
NA NA 150–375 mg SC
q 2–4 weeks,
depending on
body weight and
pretreatment
serum IgE level
■Pain and bruising of injection sites
in 5–20 percent of patients.
■Anaphylaxis has been reported in
0.2% of treated patients.
■Malignant neoplasms were
reported in 0.5 percent of patients
compared to 0.2 percent receiving
placebo; relationship to drug is
unclear.
■Do not administer more than 150 mg
per injection site.
■Monitor patients following injections; be
prepared and equipped to identify and
treat anaphylaxis that may occur.
■Whether patients will develop significant
antibody titers to the drug with
long-term administration is unknown.
Leukotriene Receptor
Antagonists (LTRAs)
Montelukast
4 mg or 5 mg
chewable tablet
4 mg granule
packets
10 mg tablet
Zafirlukast
10 mg tablet
20 mg tablet
4 mg qhs
(1–5 years of
age)
NA
5 mg qhs
(6–14 years of
age)
10 mg bid
(7–11 years of
age)
10 mg qhs
40 mg daily
(20 mg tablet
bid)
■No specific adverse effects have
been identified.
■Rare cases of Churg-Strauss
have occurred, but the
association is unclear.
■Postmarketing surveillance has
reported cases of reversible
hepatitis and, rarely, irreversible
hepatic failure resulting in death
and liver transplantation.
Leukotriene Modifiers
■Montelukast exhibits a flat dose-response
curve. Doses >10 mg will not produce
a greater response in adults.
■No more efficacious than placebo in
infants ages 6–24 months.
■As long-term therapy may attenuate
exercise-induced bronchospasm in some
patients, but less effective than ICS therapy.
■For zafirlukast, administration with meals
decreases bioavailability; take at least
1 hour before or 2 hours after meals.
■Zarfirlukast is a microsomal P450 enzyme
inhibitor that can inhibit the metabolism
of warfarin. Doses of these drugs should
be monitored accordingly.
■Monitor hepatic enzymes (ALT). Warn
patients to discontinue use if they
experience signs and symptoms of liver
dysfunction.
Immunomodulators
5-Lipoxygenase
Inhibitor
Zileuton
600 mg tablet
NA NA 2,400 mg daily
(give tablets qid)
■Elevation of liver enzymes has
been reported. Limited case
reports of reversible hepatitis and
hyperbilirubinemia.
■For zileuton, monitor hepatic enzymes (ALT).
■Zileuton is a microsomal P450 enzyme
inhibitor that can inhibit the metabolism
of warfarin and theophylline. Doses
of these drugs should be monitored
accordingly.
Theophylline
Liquids, sustained-
release tablets,
and capsules
Starting dose
10 mg/kg/day;
usual maximum:
■<1 year of
age: 0.2 (age
in weeks) + 5
= mg/kg/day
■≥1 year
of age:
16 mg/kg/day
Starting dose
10 mg/kg/day;
usual maximum:
16 mg/kg/day
Starting dose
10 mg/kg/day up
to 300 mg
maximum; usual
maximum:
800 mg/day
■Dose-related acute toxicities
include tachycardia, nausea and
vomiting, tachyarrhythmias (SVT),
central nervous system stimula-
tion, headache, seizures,
hematemesis, hyperglycemia,
and hypokalemia.
■Adverse effects at usual
therapeutic doses include
insomnia, gastric upset,
aggravation of ulcer or reflux,
increase in hyperactivity in
some children, difficulty in
urination in elderly males
who have prostatism.
Methylxanthines
■Adjust dosage to achieve serum
concentration of 5–15 mcg/mL at steady
state (at least 48 hours on same dosage).
■Due to wide interpatient variability
in theophylline metabolic clearance,
routine serum theophylline level
monitoring is essential.
■Patients should be told to discontinue
if they experience toxicity.
■Various factors (diet, food, febrile illness,
age, smoking, and other medications)
can affect serum concentrations. See
EPR—3 Full Report 2007 and package
inserts for details.
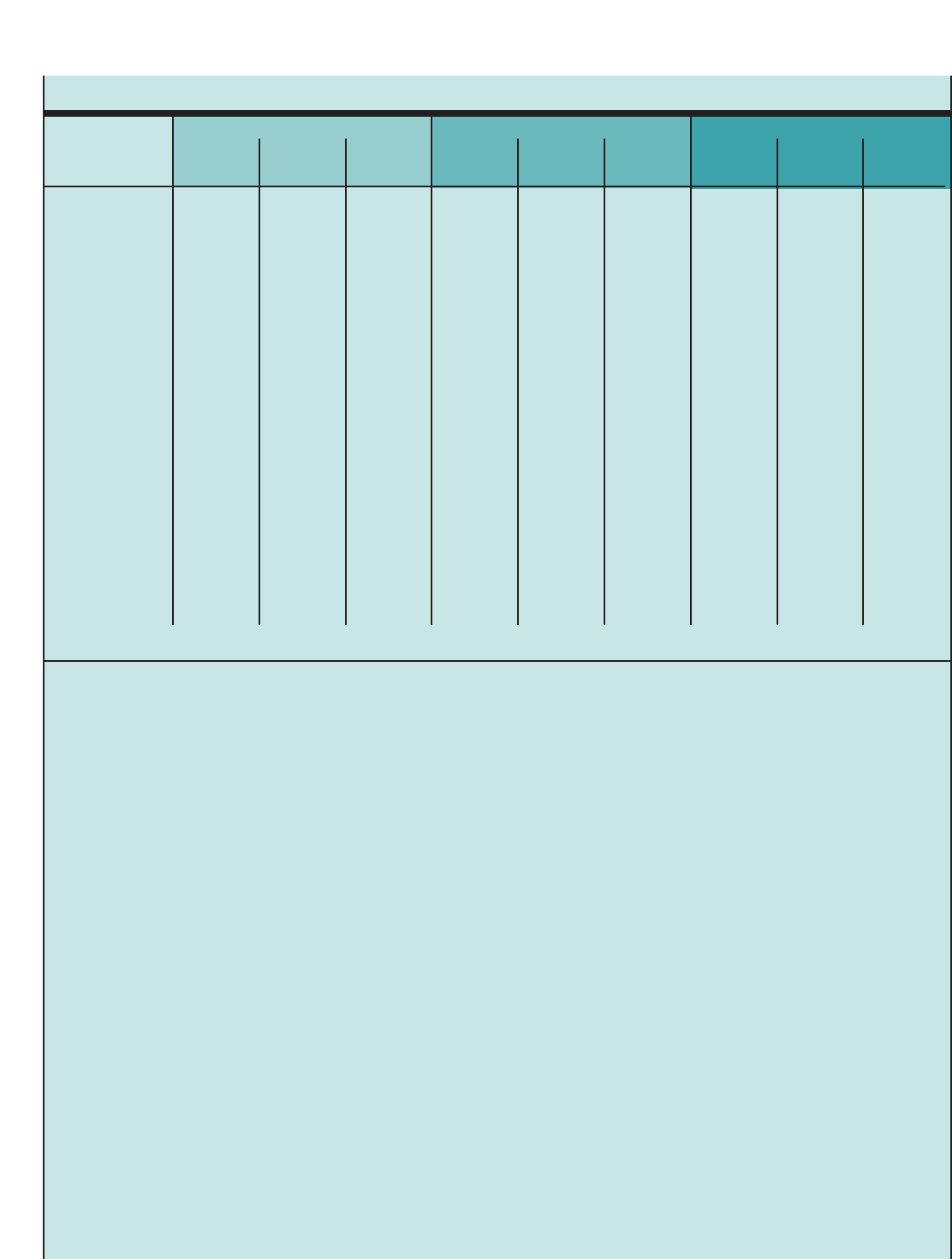
49
Managing Asthma Long Term
FIGURE 18. ESTIMATED COMPARATIVE DAILY DOSAGES FOR INHALED CORTICOSTEROIDS
Beclomethasone HFA
40 or 80 mcg/puff
Budesonide DPI
90, 180, or 200
mcg/inhalation
Budesonide Inhaled
Inhalation suspension
for nebulization
Flunisolide
250 mcg/puff
Flunisolide HFA
80 mcg/puff
Fluticasone
HFA/MDI: 44, 110, or
220 mcg/puff
DPI: 50, 100, or
250 mcg/inhalation
Mometasone DPI
200 mcg/inhalation
Triamcinolone
acetonide
75 mcg/puff
NA
NA
0.25–0.5 mg
NA
NA
176 mcg
NA
NA
NA
80–160 mcg
180–400 mcg
0.5 mg
500–750 mcg
160 mcg
88–176 mcg
100–200 mcg
NA
300–600 mcg
80–240 mcg
180–600 mcg
NA
500–1,000 mcg
320 mcg
88–264 mcg
100–300 mcg
200 mcg
300–750 mcg
NA
NA
>0.5–1.0 mg
NA
NA
>176–352 mcg
NA
NA
NA
Key: DPI, dry power inhaler; HFA, hydrofluoroalkane; MDI, metered-dose inhaler; NA, not available (either not approved, no data available, or safety and efficacy not established for this age group)
>160–320 mcg
>400–800 mcg
1.0 mg
1,000–
1,250 mcg
320 mcg
>176–352 mcg
>200–400 mcg
NA
>600–900 mcg
>240–480 mcg
>600–
1,200 mcg
NA
>1,000–
2,000 mcg
>320–640 mcg
>264–440 mcg
>300–500 mcg
400 mcg
>750–
1,500 mcg
NA
NA
>1.0 mg
NA
NA
>352 mcg
NA
NA
NA
>320 mcg
>800 mcg
2.0 mg
>1,250 mcg
≥640 mcg
>352 mcg
>400 mcg
NA
>900 mcg
Drug
>480 mcg
>1,200 mcg
NA
>2,000 mcg
>640 mcg
>440 mcg
>500 mcg
>400 mcg
>1,500 mcg
Therapeutic Issues:
■The most important determinant of appropriate dosing is the clinician’s judgment of the patient’s response to therapy. The clinician must monitor the patient’s response on several
clinical parameters and adjust the dose accordingly. Once control of asthma is achieved, the dose should be carefully titrated to the minimum dose required to maintain control.
■Preparations are not interchangeable on a mcg or per puff basis. This figure presents estimated comparable daily doses. See EPR—3 Full Report 2007 for full discussion.
■Some doses may be outside package labeling, especially in the high-dose range. Budesonide nebulizer suspension is the only inhaled corticosteroid (ICS) with FDA-approved
labeling for children <4 years of age.
■For children <4 years of age: The safety and efficacy of ICSs in children <1 year has not been established. Children <4 years of age generally require delivery of ICS (budesonide
and fluticasone HFA) through a face mask that should fit snugly over nose and mouth and avoid nebulizing in the eyes. Wash face after each treatment to prevent local corticos-
teroid side effects. For budesonide, the dose may be administered 1–3 times daily. Budesonide suspension is compatible with albuterol, ipratropium, and levalbuterol nebulizer
solutions in the same nebulizer. Use only jet nebulizers, as ultrasonic nebulizers are ineffective for suspensions. For fluticasone HFA, the dose should be divided 2 times daily;
the low dose for children <4 years of age is higher than for children 5–11 years of age due to lower dose delivered with face mask and data on efficacy in young children.
Potential Adverse Effects of Inhaled Corticosteroids:
■Cough, dysphonia, oral thrush (candidiasis).
■Spacer or valved holding chamber with non-breath-actuated MDIs and mouthwashing and spitting after inhalation decrease local side effects.
■A number of the ICSs, including fluticasone, budesonide, and mometasone, are metabolized in the gastrointestinal tract and liver by CYP 3A4 isoenzymes. Potent inhibitors of
CYP 3A4, such as ritonavir and ketoconazole, have the potential for increasing systemic concentrations of these ICSs by increasing oral availability and decreasing systemic
clearance. Some cases of clinically significant Cushing syndrome and secondary adrenal insufficiency have been reported.
■In high doses, systemic effects may occur, although studies are not conclusive, and clinical significance of these effects has not been established (e.g., adrenal suppression,
osteoporosis, skin thinning, and easy bruising). In low-to-medium doses, suppression of growth velocity has been observed in children, but this effect may be transient, and
the clinical significance has not been established.
Child 0–4
Years of Age
Child 5–11
Years of Age
≥12 Years
of Age and
Adults
Low Daily Dose
Child 0–4
Years of Age
Child 5–11
Years of Age
≥12 Years
of Age and
Adults
Medium Daily Dose
Child 0–4
Years of Age
Child 5–11
Years of Age
≥12 Years
of Age and
Adults
High Daily Dose
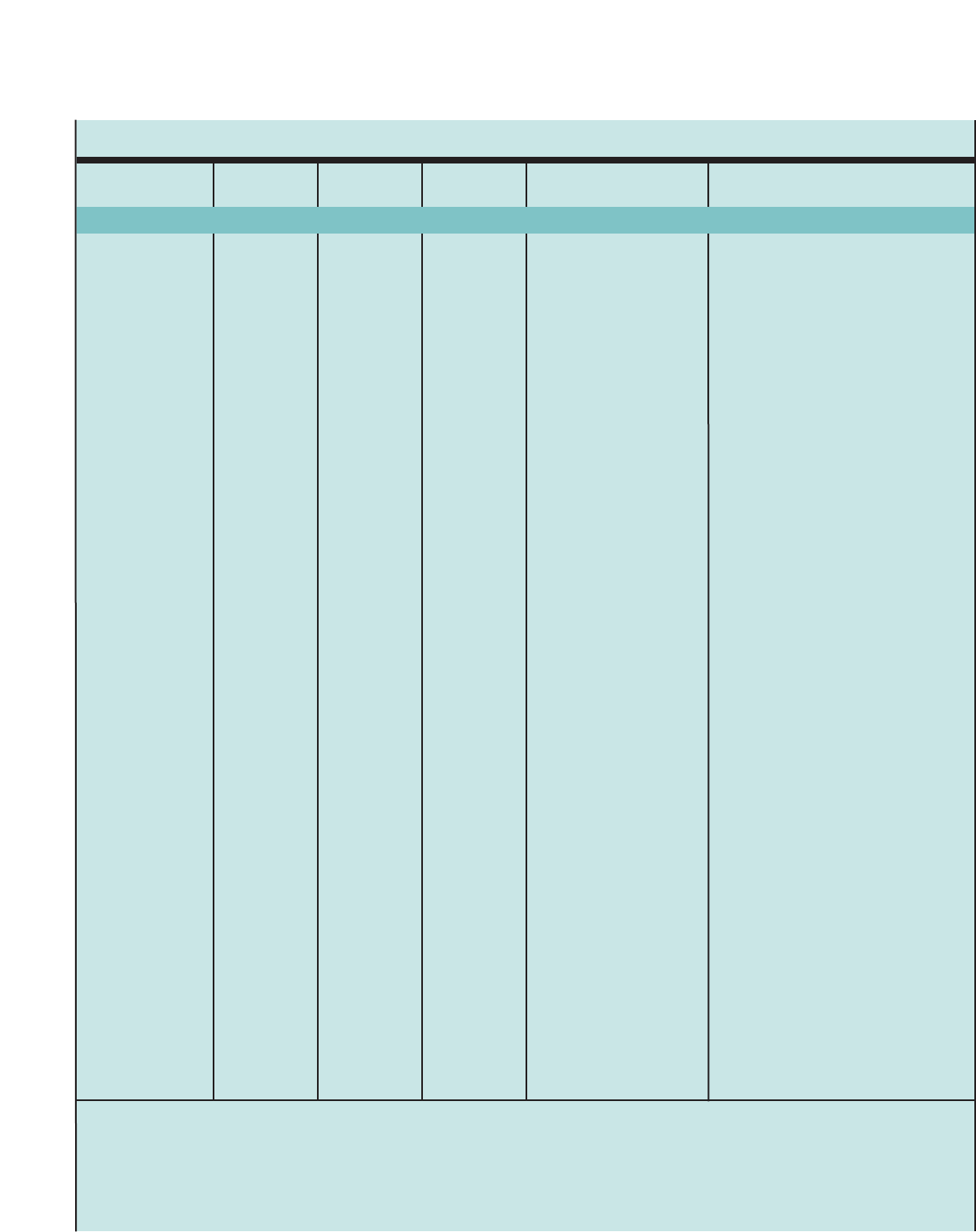
50 Guidelines for the Diagnosis and Management of Asthma
FIGURE 19. USUAL DOSAGES FOR QUICK-RELIEF MEDICATIONS*
Medication
<5 Years
of Age
MDI
Albuterol CFC
90 mcg/puff,
200 puffs/canister
Albuterol HFA
90 mcg/puff,
200 puffs/canister
Levalbuterol HFA
45 mcg/puff,
200 puffs/canister
Pirbuterol CFC
Autohaler
200 mcg/puff,
400 puffs/canister
1–2 puffs
5 minutes before
exercise
2 puffs every
4–6 hours, as
needed for
symptoms
NA <4 years of
age
NA
5–11 Years
of Age
2 puffs
5 minutes before
exercise
2 puffs every
4–6 hours, as
needed for
symptoms
NA
≥12 Years of
Age and Adults
2 puffs
5 minutes before
exercise
2 puffs every
4–6 hours, as
needed for
symptoms
■Tachycardia, skeletal muscle
tremor, hypokalemia,
increased lactic acid,
headache, hyperglycemia.
Inhaled route, in general,
causes few systemic
adverse effects. Patients
with preexisting cardiovas-
cular disease, especially the
elderly, may have adverse
cardiovascular reactions
with inhaled therapy.
Potential Adverse Effects Comments (not all inclusive)
■Drugs of choice for acute bronchospasm.
■Differences in potencies exist, but all
products are essentially comparable on a
puff per puff basis.
■An increasing use or lack of expected effect
indicates diminished control of asthma.
■Not recommended for long-term daily treat-
ment. Regular use exceeding 2 days/week
for symptom control (not prevention of EIB)
indicates the need for additional long-term
control therapy.
■May double usual dose for mild exacerbations.
■For levalbuterol, prime the inhaler by releasing
4 actuations prior to use.
■For HFA: periodically clean HFA actuator, as
drug may plug orifice.
■For autohaler: children <4 years of age
may not generate sufficient inspiratory flow
to activate an auto-inhaler.
■Nonselective agents (i.e., epinephrine,
isoproterenol, metaproterenol) are not recom-
mended due to their potential for excessive
cardiac stimulation, especially in high doses.
Inhaled Short-Acting Beta2-Agonists
Key: CFC, chlorofluorocarbon; ED, emergency department; EIB, exercise-induced bronchospasm; HFA, hydrofluoroalkane; IM, intramuscular; MDI, metered-dose inhaler;
NA, not available (either not approved, no data available, or safety and efficacy not established for this age group); PEF, peak expiratory flor; SABA, short-acting beta2-agonist
*Dosages are provided for those products that have been approved by the U.S. Food and Drug Administration (FDA) or have sufficient clinical trial safety and efficacy data in the
appropriate age ranges to support their use.
Nebulizer solution
Albuterol
0.63 mg/3 mL
1.25 mg/3 mL
2.5 mg/3 mL
5 mg/mL (0.5%)
Levalbuterol
(R-albuterol)
0.31 mg/3 mL
0.63 mg/3 mL
1.25 mg/0.5 mL
1.25 mg/3 mL
0.63–2.5 mg in
3 cc of saline
q 4–6 hours, as
needed
0.31–1.25 mg
in 3 cc
q 4–6 hours, as
needed for symp-
toms
1.25–5 mg in
3 cc of saline
q 4–8 hours, as
needed
0.31–0.63 mg,
q 8 hours,
as needed for
symptoms
1.25–5 mg in
3 cc of saline
q 4–8 hours, as
needed
0.63 mg–
1.25 mg
q 8 hours,
as needed for
symptoms
(Same as with MDI)
(Same as with MDI)
■May mix with cromolyn solution, budesonide
inhalant suspension, or ipratropium solution
for nebulization. May double dose for severe
exacerbations.
■Does not have FDA-approved labeling for
children <6 years of age.
■Compatible with budesonide inhalant
suspension. The product is a sterile-filled
preservative-free unit dose vial.
Dose applies
to Albuterol.
Dose applies
to
Albuterol/and
Levalbuterol.
Dose applies
to all four
SABAs
Apply to all four (SABAs)
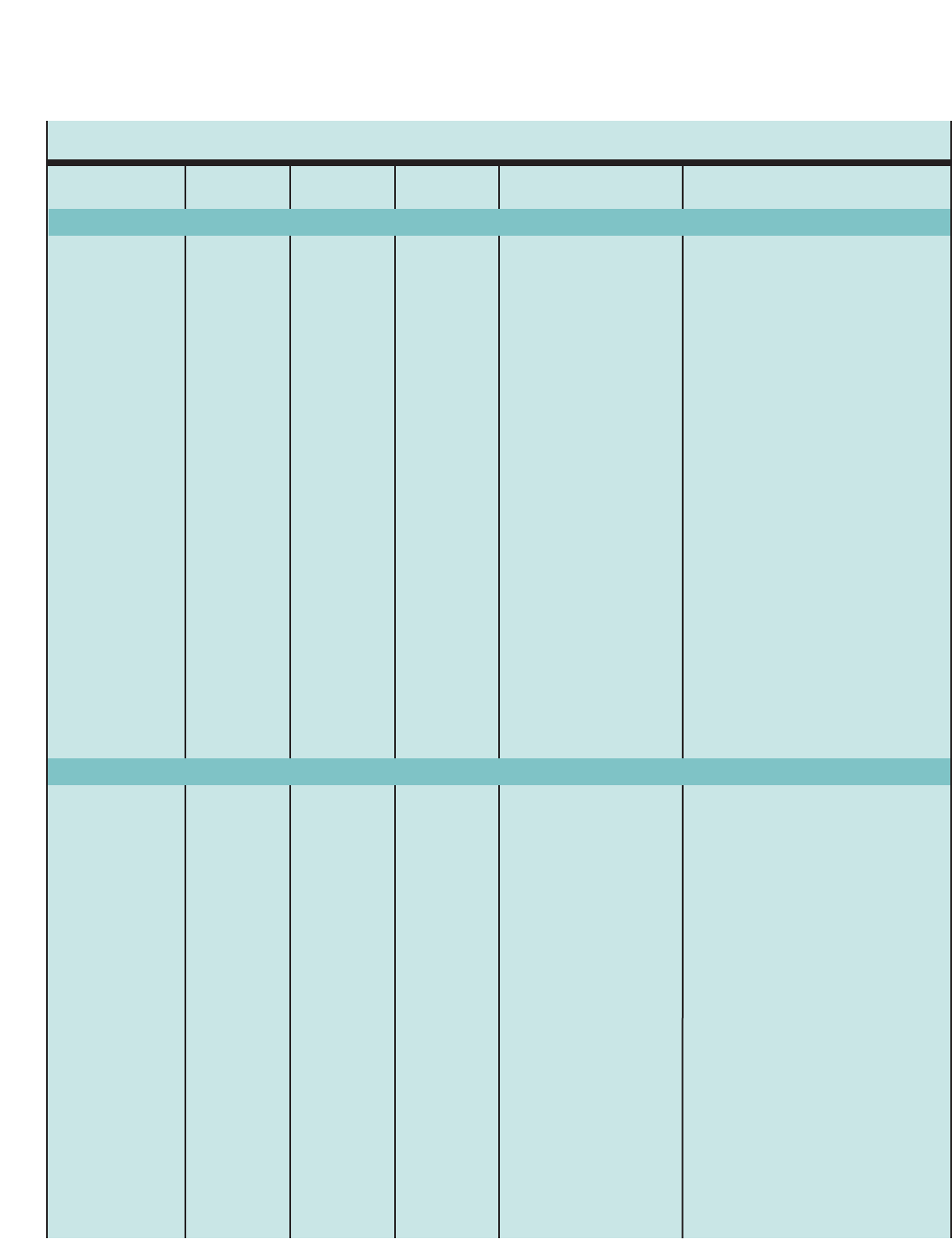
51
Managing Asthma Long Term
FIGURE 19. USUAL DOSAGES FOR QUICK-RELIEF MEDICATIONS* (continued)
Medication
<5 Years
of Age
5–11 Years
of Age
≥12 Years of
Age and Adults Potential Adverse Effects Comments (not all inclusive)
Ipratropium HFA
MDI
17 mcg/puff,
200 puffs/canister
Nebulizer solution
0.25 mg/mL
(0.025%)
Ipratropium with
albuterol
MDI
18 mcg/puff of
ipratropium
bromide and
90 mcg/puff of
albuterol
200 puffs/canister
Nebulizer solution
0.5 mg/3 mL
ipratropium
bromide and
2.5 mg/3 mL
albuterol
NA
NA
NA
NA
NA
NA
NA
NA
2–3 puffs
q 6 hours
0.25 mg
q 6 hours
2–3 puffs
q 6 hours
3 mL
q 4–6 hours
■Drying of mouth and
respiratory secretions,
increased wheezing in
some individuals, blurred
vision if sprayed in eyes.
If used in the ED, produces
less cardiac stimulation
than SABAs.
■Multiple doses in the emergency department
(not hospital) setting provide additive benefit
to SABA.
■Treatment of choice for bronchospasm due
to beta-blocker medication.
■Does not block EIB.
■Reverses only cholinergically mediated
bronchospasm; does not modify reaction
to antigen.
■May be an alternative for patients who
do not tolerate SABA.
■Has not proven to be efficacious as
long-term control therapy for asthma.
■Contains EDTA to prevent discoloration of
the solution. This additive does not induce
bronchospasm.
Anticholinergics
Methylprednisolone
2, 4, 6, 8, 16,
32 mg tablets
Prednisolone
5 mg tablets,
5 mg/5 cc,
15 mg/5 cc
Prednisone
1, 2.5, 5, 10, 20,
50 mg tablets;
5 mg/cc,
5 mg/5 cc
Short course
“burst:”
1–2 mg/kg/
day, maximum
60 mg/day, for
3–10 days
Short course
“burst”:
1-2 mg/kg/day
maximum
60 mg/day
for 3–10 days
Short course
“burst”:
40–60 mg/day
as single or
2 divided doses
for 3–10 days
■Short-term use: reversible
abnormalities in glucose
metabolism, increased
appetite, fluid retention,
weight gain, facial flushing,
mood alteration, hyperten-
sion, peptic ulcer, and
rarely aseptic necrosis.
■Consideration should be
given to coexisting condi-
tions that could be wors-
ened by systemic corticos-
teroids, such as herpes
virus infections, varicella,
tuberculosis, hypertension,
peptic ulcer, diabetes
mellitus, osteoporosis,
and Strongyloides.
(Applies to the first three corticosteroids.)
■Short courses or “bursts” are effective for
establishing control when initiating therapy
or during a period of gradual deterioration.
Action may begin within an hour.
■The burst should be continued until patient
achieves 80 percent PEF personal best or
symptoms resolve. This usually requires
3–10 days but may require longer. There is
no evidence that tapering the dose following
improvement prevents relapse in asthma
exacerbations.
■Other systemic corticosteroids such as
hydrocortisone and dexamethasone given
in equipotent daily doses are likely to be as
effective as prednisolone.
Systemic Corticosteroids
Dosages apply to first three corticosteroids.
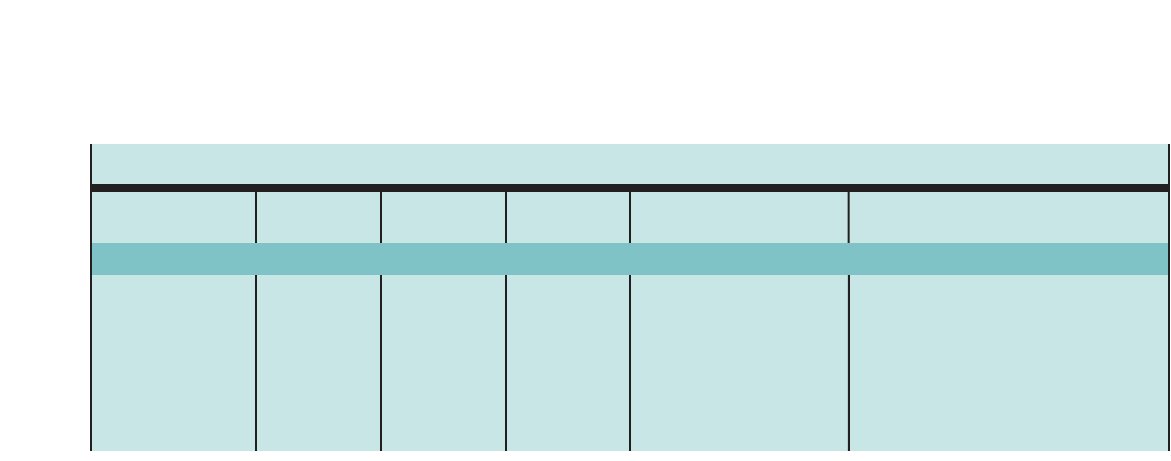
52 Guidelines for the Diagnosis and Management of Asthma
FIGURE 19. USUAL DOSAGES FOR QUICK-RELIEF MEDICATIONS* (continued)
Medication
<5 Years
of Age
5–11 Years
of Age
≥12 Years of
Age and Adults Potential Adverse Effects Comments (not all inclusive)
Repository
injection
(Methylprednisolone
acetate)
40 mg/mL
80 mg/mL
7.5 mg/kg IM
once
240 mg IM once 240 mg IM once ■May be used in place of a short burst of
oral steroids in patients who are vomiting or
if adherence is a problem.
Systemic Corticosteroids (continued)

Asthma exacerbations are acute or subacute episodes
of progressively worsening shortness of breath, cough,
wheezing, and chest tightness, or some combination of
these symptoms. Exacerbations are characterized by
decreases in expiratory airflow; objective measures of
lung function (spirometry or PEF) are more reliable
indicators of severity than symptoms are. Individuals
whose asthma is well controlled with ICSs have
decreased risk of exacerbations. However, these
patients can still be vulnerable to exacerbations, for
example, when they have viral respiratory infections.
Effective management of exacerbations incorporates
the same four components of asthma management
used in managing asthma long term: assessment
and monitoring, patient education, environmental
control, and medications.
Classifying Severity
Do not underestimate the severity of an exacerba-
tion. Severe exacerbations can be life threatening
and can occur in patients at any level of asthma
severity—i.e., intermittent, or mild, moderate, or
severe persistent asthma. See figure 20, “Classifying
Severity of Asthma Exacerbations in the Urgent or
Emergency Care Setting.”
Patients at high risk of asthma-related death require
special attention
—
particularly intensive education,
monitoring, and care. Such patients should be
advised to seek medical care early during an exacer-
bation. Risk factors for asthma-related death include:
■Previous severe exacerbation (e.g., intubation or
ICU admission for asthma)
■Two or more hospitalizations or >3 ED visits in the
past year
■Use of >2 canisters of SABA per month
■Difficulty perceiving airway obstruction or the
severity of worsening asthma
■Low socioeconomic status or inner-city residence
■Illicit drug use
■Major psychosocial problems or psychiatric disease
■Comorbidities, such as cardiovascular disease or
other chronic lung disease
Home Management
Early treatment by the patient at home is the best
strategy for managing asthma exacerbations.
Patients should be instructed how to:
■Use a written asthma action plan that notes when
and how to treat signs of an exacerbation. A peak
flow-based plan may be particularly useful for
patients who have difficulty perceiving airflow
obstruction or have a history of severe
exacerbations.
■Recognize early indicators of an exacerbation,
including worsening PEF.
■Adjust their medications by increasing SABA and,
in some cases, adding a short course of oral
systemic corticosteroids. Doubling the dose of
ICSs is not effective.
■Remove or withdraw from allergens or irritants in
the environment that may contribute to the
exacerbation.
■Monitor response to treatment and promptly
communicate with the clinician about any serious
deterioration in symptoms or PEF or about
decreased responsiveness to SABA treatment,
including decreased duration of effect.
The following home management techniques are
not recommended because no studies demonstrate
their effectiveness and they may delay patients from
obtaining necessary care: drinking large volumes of
liquids; breathing warm, moist air; or using over-the-
counter products, such as antihistamines or cold
remedies. Pursed-lip and other forms of breathing
may help to maintain calm, but these methods do not
improve lung function.
Managing Exacerbations
53
Managing Exacerbations
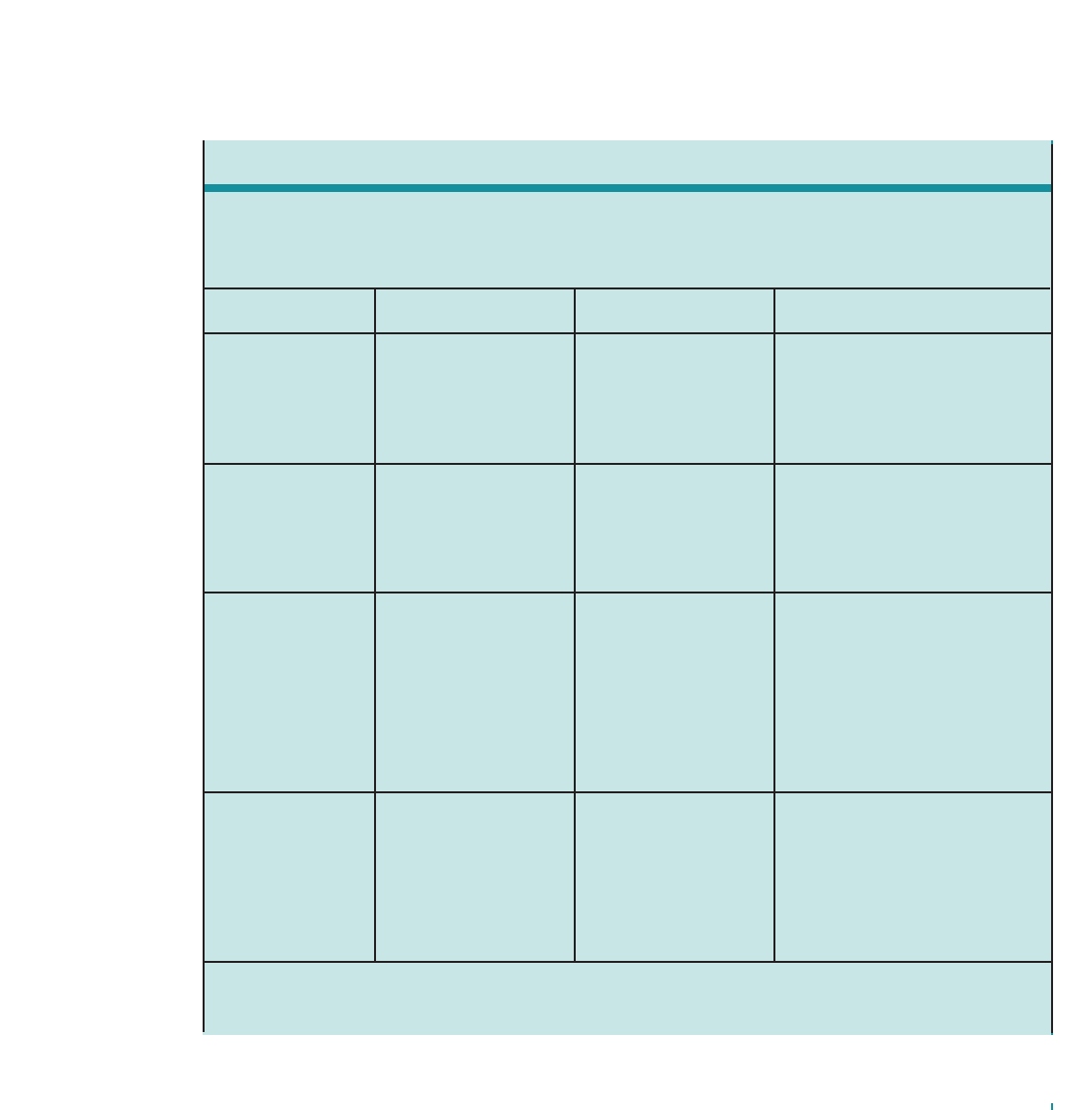
54 Guidelines for the Diagnosis and Management of Asthma
FIGURE 20. CLASSIFYING SEVERITY OF ASTHMA EXACERBATIONS IN THE URGENT OR EMERGENCY CARE SETTING
Symptoms and Signs
Mild
Moderate
Severe
Subset: Life
threatening
Dyspnea only with
activity (assess
tachypnea in young
children)
Initial PEF (or FEV1)
PEF ≥70 percent
predicted or personal best
Clinical Course
■Usually cared for at home
■Prompt relief with inhaled SABA
■Possible short course of oral
systemic corticosteroids
■Usually requires office or ED visit
■Relief from frequent inhaled SABA
■Oral systemic corticosteroids;
some symptoms last for
1–2 days after treatment is begun
■Usually requires ED visit and
likely hospitalization
■Partial relief from frequent
inhaled SABA
■Oral systemic corticosteroids;
some symptoms last for
>3 days after treatment is begun
■Adjunctive therapies are helpful
■Requires ED/hospitalization;
possible ICU
■Minimal or no relief from
frequent inhaled SABA
■Intravenous corticosteroids
■Adjunctive therapies are helpful
Key: ED, emergency department; FEV1, forced expiratory volume in 1 second; ICU, intensive care unit; PEF, peak expiratory flow;
SABA, short-acting beta2-agonist
Note: Patients are instructed to use quick-relief medications if symptoms occur or if PEF drops below 80 percent predicted or personal best. If
PEF is 50–79 percent, the patient should monitor response to quick-relief medication carefully and consider contacting a clinician. If PEF is below
50 percent, immediate medical care is usually required. In the urgent or emergency care setting, the following parameters describe the severity
and likely clinical course of an exacerbation.
Dyspnea interferes with
or limits usual activity
PEF 40–69 percent
predictedor personal
best
PEF <40 percent
predicted or personal best
PEF <25 percent
predicted or personal best
Dyspnea at rest;
interferes with
conversation
Too dyspneic to speak;
perspiring
Management in the Urgent or Emergency Care and
Hospital Settings
Emergency medical services providers should have
prehospital protovols that allow administration of
SABA, supplemental oxygen, and (with appropriate
medical oversight) anticholinergics and oral systemic
corticosteriods to patients who have signs or symp-
toms of an asthma exacerbation.
Treatment strategies for managing moderate or severe
exacerbations in the urgent or emergency care setting
are described below. Also see figure 21 for a detailed
sequence of recommended actions for monitoring
and treatment and figure 22 for dosages of drugs for
asthma exacerbations.
■Administer supplemental oxygen to correct signifi-
cant hypoxemia in moderate or severe exacerbations.
■Administer repetitive or continuous administra-
tion of SABA to reverse airflow obstruction rapidly.
■Administer oral systemic corticosteroids to
decrease airway inflammation in moderate or
severe exacerbations or for patients who fail to
respond promptly and completely to SABA
treatment.
■Monitor response to therapy with serial
assessments.
—For children:

55
Managing Exacerbations
FIGURE 21. MANAGEMENT OF ASTHMA EXACERBATIONS: EMERGENCY DEPARTMENT AND HOSPITAL-BASED CARE
Key: FEV1,forced expiratory volume in 1 second; ICS, inhaled corticosteroid; MDI, metered-dose inhaler; PCO2, partial pressure carbon dioxide;
PEF, peak expiratory flow; SABA, short-acting beta2-agonist; SaO2, oxygen saturation
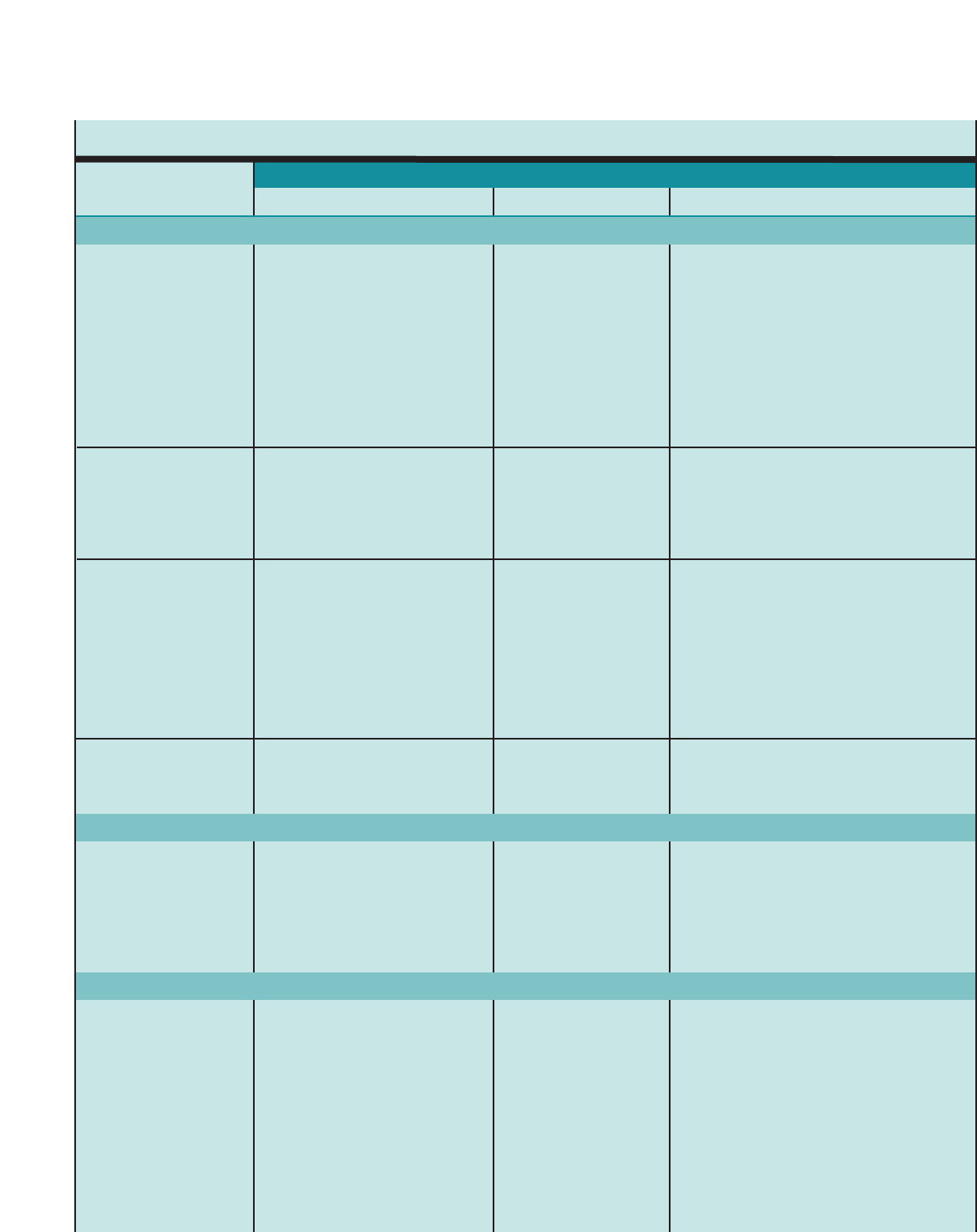
56 Guidelines for the Diagnosis and Management of Asthma
FIGURE 22. DOSAGES OF DRUGS FOR ASTHMA EXACERBATIONS
Medication Child Dose* Adult Dose
Albuterol
Nebulizer solution
(0.63 mg/3 mL,
1.25 mg/3 mL,
2.5 mg/3 mL,
5.0 mg/mL)
MDI
(90 mcg/puff)
Bitolterol
Nebulizer solution
(2 mg/mL)
MDI
(370 mcg/puff)
Levalbuterol
(R-albuterol)
Nebulizer solution
(0.63 mg/3 mL,
1.25 mg/0.5 mL
1.25 mg/3 mL)
MDI
(45 mcg/puff)
0.15 mg/kg (minimum dose
2.5 mg) every 20 minutes for
3 doses then 0.15–0.3 mg/kg up to
10 mg every 1–4 hours as needed, or
0.5 mg/kg/hour by continuous
nebulization.
4–8 puffs every 20 minutes for 3 doses,
then every 1–4 hours inhalation maneu-
ver as needed. Use VHC; add mask in
children <4 years.
See albuterol dose; thought to be half as
potent as albuterol on mg basis.
See albuterol MDI dose.
0.075 mg/kg (minimum dose 1.25 mg)
every 20 minutes for 3 doses, then
0.075–0.15 mg/kg up to 5 mg every
1–4 hours as needed.
See albuterol MDI dose
2.5–5 mg every 20 minutes
for 3 doses, then 2.5–10 mg
every 1–4 hours as
needed, or 10–15 mg/hour
continuously.
4–8 puffs every 20 minutes
up to 4 hours, then every
1–4 hours as needed.
See albuterol dose.
See albuterol MDI dose.
1.25–2.5 mg every
20 minutes for 3 doses,
then 1.25–5 mg every
1–4 hours as needed.
See albuterol MDI dose.
Only selective beta2agonists are recommended.
For optimal delivery, dilute aerosols to minimum of
3 mL at gas flow of 6–8 L/min. Use large volume
nebulizers for continuous administration. May mix
with ipratropium nebulizer solution.
In mild-to-moderate exacerbations, MDI plus VHC is
as effective as nebulized therapy with appropriate
administration technique and coaching by trained
personnel.
Has not been studied in severe asthma exacerbations.
Do not mix with other drugs.
Has not been studied in severe asthma exacerbations.
Levalbuterol administered in one-half the mg dose of
albuterol provides comparable efficacy and safety.
Has not been evaluated by continuous nebulization.
Comments (not all inclusive)
Dosage
Inhaled Short-Acting Beta2-Agonists (SABA)
Epinephrine
1:1,000 (1 mg/mL)
Terbutaline
(1 mg/mL)
0.01 mg/kg up to 0.3–0.5 mg every
20 minutes for 3 doses sq.
0.01 mg/kg every 20 minutes for
3 doses then every 2–6 hours as
needed sq.
0.3–0.5 mg every
20 minutes for 3 doses sq.
0.25 mg every 20 minutes
for 3 doses sq.
No proven advantage of systemic therapy over aerosol.
No proven advantage of systemic therapy over aerosol.
Systemic (Injected) Beta2-Agonists
Ipratropium bromide
Nebulizer solution
(0.25 mg/mL)
MDI
(18 mcg/puff)
0.25–0.5 mg every 20 minutes for
3 doses, then as needed
4–8 puffs every 20 minutes as
needed up to 3 hours
0.5 mg every 20 minutes for
3 doses, then as needed
8 puffs every 20 minutes as
needed up to 3 hours
May mix in same nebulizer with albuterol. Should not
be used as first-line therapy; should be added to
SABA therapy for severe exacerbations. The addition
of ipratropium has not been shown to provide further
benefit once the patient is hospitalized.
Should use with VHC and face mask for children
<4 years. Studies have examined ipratropium bromide
MDI for up to 3 hours.
Anticholinergics
Pirbuterol
MDI
(200 mcg/puff)
See albuterol MDI dose; thought to be
half as potent as albuterol on a mg basis.
See albuterol MDI dose. Has not been studied in severe asthma exacerbations
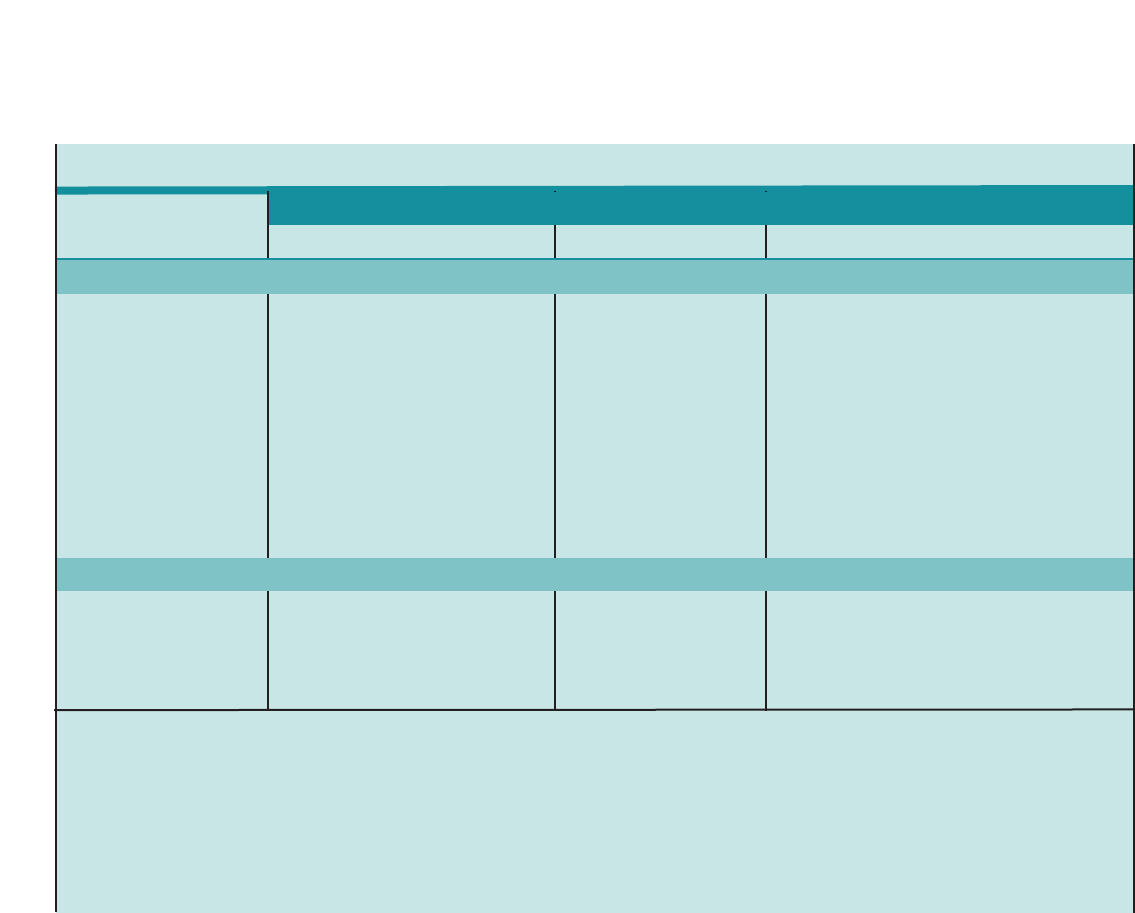
• No single measure is best for assessing severity or
predicting hospital admission.
• Lung function measures (FEV1or PEF) may be
useful for children ≥5 years of age, but these
measures may not be obtainable during an
exacerbation.
• Pulse oximetry may be useful for assessing the
initial severity; a repeated measure of pulse
oximetry of <92–94 percent after 1 hour is
predictive of the need for hospitalization.
• Signs and symptoms scores may be helpful.
Children who have signs and symptoms after 1–2
hours of initial treatment and who continue to
meet the criteria for a moderate or severe
exacerbation have a >84 percent chance of
requiring hospitalization.
— For adults:
• Repeated lung function measures (FEV1or
PEF) at 1 hour and beyond are the strongest
single predictor of hospitalization. Such
measures may not be helpful, or easily obtained,
during severe exacerbations.
• Pulse oximetry is indicated for patients who are
in severe distress, have FEV1or PEF <40 percent
predicted, or are unable to perform lung
function measures. Only repeat assessments
after initial treatment, not a single assessment
upon admission, are useful for predicting the
need for hospitalization.
• Signs and symptoms scores at 1 hour after
initial treatments improve the ability to predict
need for hospitalization. The presence of
drowsiness is a useful predictor of impending
respiratory failure and is reason to consider
immediate transfer to a facility equipped to
offer ventilatory support.
57
Managing Exacerbations
FIGURE 22. DOSAGES OF DRUGS FOR ASTHMA EXACERBATIONS (continued)
Medication Child Dose* Adult Dose Comments (not all inclusive)
Dosage
Ipratropium with albuterol
Nebulizer solution
(Each 3 mL vial
contains 0.5 mg
ipratropium bromide
and 2.5 mg albuterol.)
MDI
(Each puff contains
18 mcg ipratropium
bromide and 90 mcg
of albuterol.)
1.5-3 mL every 20 minutes for 3 doses,
then as needed
4–8 puffs every 20 minutes as needed up
to 3 hours
3 mL every 20 minutes for 3
doses, then as needed
8 puffs every 20 minutes as
needed up to 3 hours
May be used for up to 3 hours in the initial
management of severe exacerbations. The addition
of ipratropium to albuterol has not been shown to
provide further benefit once the patient is hospitalized.
Should use with VHC and face mask for children
<4 years.
Anticholinergics (continued)
Prednisone
Methylprednisolone
Prednisolone
1-2 mg/kg in 2 divided doses (maximum =
60 mg/day) until PEF is 70 percent of
predicted or personal best
40–80 mg/day in 1 or 2
divided doses until PEF
reaches 70 percent of
predicted or personal best
For outpatient “burst,” use 40–60 mg in single or
2 divided doses for total of 5–10 days in adults
(children: 1–2 mg/ kg/day maximum 60 mg/day for
3–10 days).
Systemic Corticosteroids (Apply to all three corticosteriods.)
* Children ≤12 years of age
Key: ED, emergency department; MDI, metered-dose inhaler; PEF, peak expiratory flow, VHC, valved holding chamber
Notes:
•There is no known advantage for higher doses of corticosteroids in severe asthma exacerbations, nor is there any advantage for intravenous administration over oral therapy
provided gastrointestinal transit time or absorption is not impaired.
•The total course of systemic corticosteroids for an asthma exacerbation requiring an ED visit of hospitalization may last from 3 to 10 days. For corticosteroid courses of less than
1 week, there is no need to taper the dose. For slightly longer courses (e.g., up to 10 days), there probably is no need to taper, especially if patients are concurrently taking ICSs.
•ICSs can be started at any point in the treatment of an asthma exacerbation.

■Consider adjunctive treatments, such as
intravenous magnesium sulfate or heliox, in
severe exacerbations, if patients are unresponsive to
the initial treatments listed above (e.g., FEV1or
PEF <40 percent predicted or personal best after
initial treatments).
■Provide the following to prevent relapse of the
exacerbation and recurrence of another
exacerbation:
—Referral to followup asthma care within 1–4
weeks. In addition, encourage the patient to
contact (e.g., by telephone) his/her asthma care
provider during the first 3–5 days after
discharge. A followup visit is essential to
review the patient’s written asthma action plan,
adherence, and environmental control and to
consider a step up in therapy. If appropriate,
consider referral to an asthma self-management
education program.
— An ED asthma discharge plan. See figure 23a, b
“Emergency Department—Asthma Discharge
Plan.”
—Review of inhaler technique whenever possible.
— Consideration of initiating ICS.
■Treatments that are not recommended in the
emergency care or hospital setting include:
methylxanthines, antobiotics (except as needed for
comorbid conditions), aggressive hydration, chest
physical therapy, mucolytics, or sedation. Inhaled
ipratropium bromide is a helpful adjunctive
therapy in the emergency care setting, but does not
provide additional benefit after a patient is
hospitalized for a severe exacerbation.
58 Guidelines for the Diagnosis and Management of Asthma
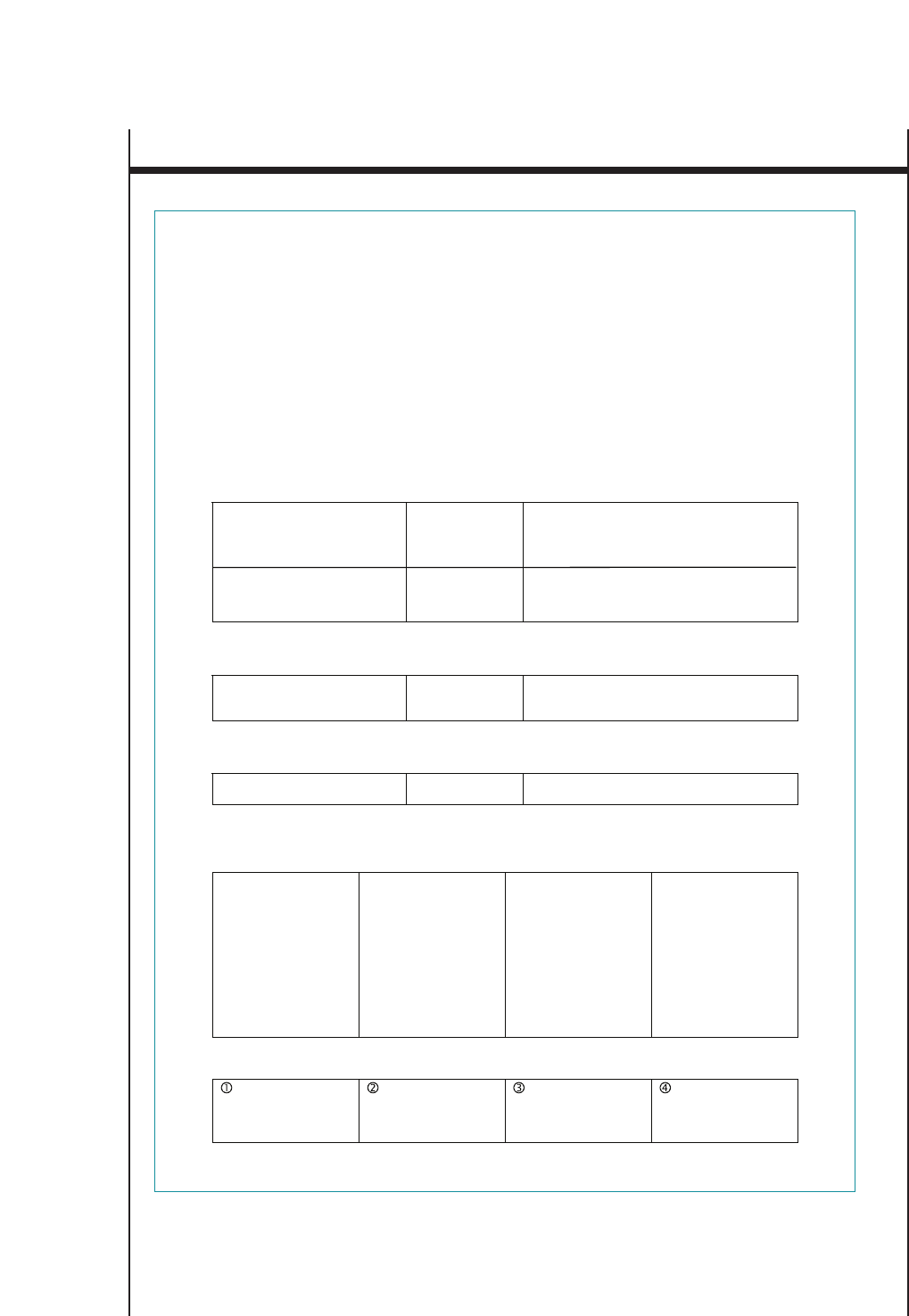
59
Managing Exacerbations
FIGURE 23a. EMERGENCY DEPARTMENT—ASTHMA DISCHARGE PLAN
Reprinted by permission from Carlos Camargo, M.D., Principal Investigator of Agency for Health Care Research and Quality. Grant No.
R13H31094.
Source: Camargo CA Jr, Emond SD, Boulet L, Gibson PG, Kolbe J, Wagner CW, Brenner BE. Emergency Department Asthma Discharge
Plan. Developed at "Asthma Education in the Adult Emergency Department: A Multidisciplinary Consensus Conference," New York
Academy of Medicine, New York, NY; 2001 April 1–5. Boston, MA: Massachusetts General Hospital, 2001. 2 pp.
EMERGENCY DEPARTMENT—ASTHMA DISCHARGE PLAN
Name:
_________________________
was seen by Dr.
________________
on ___/___/___
••Take your prescribed medications as directed—do not delay!
••-term treatment plan.
•
•Even when you feel well, you may need daily medicine to keep your asthma in good
control and prevent attacks.
••Visit your doctor or other health care provider as soon as you can to discuss how to
control your asthma and to develop your own action plan.
Your followup appointment with ________________ is on: ___/___/___.Tel: ____________
YOUR MEDICINE FOR THIS ASTHMA ATTACK IS:
Medication
Amount
Doses per day, for # days
Prednisone/prednisolone
(oral corticosteroid)
Inhaled albuterol
_____ a day for _____ days
Take the entire prescription, even when you
start to feel better.
_____ puffs every 4 to 6 hours if you have
symptoms, for _____days
YOUR DAILY MEDICINE FOR LONG-TERM CONTROL AND PREVENTING ATTACKS IS:
Medication
Amount
Doses per day
Inhaled corticosteroids
YOUR QUICK-RELIEF MEDICINE WHEN YOU HAVE SYMPTOMS IS:
Medication
Amount
Number of doses/day
Inhaled albuterol
ASK YOURSELF 2 TO 3 TIMES PER DAY, EVERY DAY, FOR AT LEAST 1 WEEK:
“How good is my asthma compared to when I left the hospital?”
If you feel much
better:
•Take your daily
long-term control
medicine.
If you feel better, but
still need your quick-
relief inhaler often:
•Take your daily long-
term control
medicine.
•See your doctor as
soon as possible.
If you feel about the
same:
•Use your quick-
relief inhaler.
•Take your daily
long-term control
medicine.
•See your doctor as
soon as possible—
don’t delay.
If you feel worse:
•Use your quick-
relief inhaler.
•Take your daily
long-term control
medicine.
•Immediately go to
the emergency
department or call
9–1–1.
YOUR ASTHMA IS UNDER CONTROL WHEN YOU:
Can be active daily
and sleep through the
night.
Need fewer than 4
doses of quick-relief
medicine in a week.
Are free of
shortness of breath,
wheeze, and cough.
Achieve an
acceptable “peak flow”
(discuss with your
health care provider).
60 Guidelines for the Diagnosis and Management of Asthma
FIGURE 23b. EMERGENCY DEPARTMENT—ASTHMA DISCHARGE PLAN: HOW TO USE YOUR METERED-DOSE INHALER
Clean your inhaler as needed, and know when to replace your inhaler. For instructions, read the package insert or talk to your doctor,
other health care provider, or pharmacist.
Using an inhaler seems simple, but most patients do not use it the right way. When you use your inhaler the wrong
way, less medicine gets to your lungs.
For the next few days, read these steps aloud as you do them or ask someone to read them to you. Ask your doctor,
nurse, other health care provider, or pharmacist to check how well you are using your inhaler.
Use your inhaler in one of the three ways pictured below (A or B are best, but C can be used if you have trouble with
A and B). (Your doctor may give you other types of inhalers.)
Steps for Using Your Inhaler
A. Hold inhaler 1 to 2 inches in
front of your mouth (about
the width of two fingers).
C. Put the inhaler in your
mouth. Do not use for
steroids.
B. Use a spacer/holding
chamber. These come in
many shapes and can be
useful to any patient.
Getting ready 1. Take off the cap and shake the inhaler.
2. Breathe out all the way.
3. Hold your inhaler the way your doctor said (A, B, or C below).
Breathe in slowly 4. As you start breathing in slowly through your mouth, press down on the inhaler one time.
(If you use a holding chamber, first press down on the inhaler. Within5 seconds, begin to
breathe in slowly.)
5. Keep breathing in slowly, as deeply as you can.
Hold your breath 6. Hold your breath as you count to 10 slowly, if you can.
7. For inhaled quick-relief medicine (short-acting beta2agonists), wait about 15–30 seconds
between puffs. There is no need to wait between puffs for other medicines.

For More Information
The National Heart, Lung, and Blood Institute (NHLBI) Health Information Center
(HIC) is a service of the NHLBI of the National Institutes of Health. The NHLBI HIC
provides information to health professionals, patients, and the public about the
HIC treatment, diagnosis, and prevention of heart, lung, and blood
diseases and sleep disorders. For more information, contact:
NHLBI Health Information Center
P.O. Box 30105
Bethesda, MD 20824-0105
Phone: 301-592-8573
TTY: 240-629-3255
Fax: 301-592-8563
Web site: http://www.nhlbi.nih.gov
DISCRIMINATION PROHIBITED: Under provisions of applicable public laws enacted by
Congress since 1964, no person in the United States shall, on the grounds of race,
color, national origin, handicap, or age, be excluded from participation in, be denied
the benefits of, or be subjected to discrimination under any program or activity (or, on
the basis of sex, with respect to any education program and activity) receiving Federal
financial assistance. In addition, Executive Order 11141 prohibits discrimination on
the basis of age by contractors and subcontractors in the performance of Federal
contracts, and Executive Order 11246 States that no federally funded contractor may
discriminate against any employee or applicant for employment because of race,
color, religion, sex, or national origin. Therefore, the National Heart, Lung, and Blood
Institute must be operated in compliance with these laws and Executive Orders.
