08 2470 358..368 CA 2908
User Manual: CA-2908
Open the PDF directly: View PDF ![]() .
.
Page Count: 12
2009;69:358-368. Published online December 31, 2008.Cancer Res
Johanna Chiche, Karine Ilc, Julie Laferrière, et al.
Regulation of the Intracellular pH
Tumor Cell Growth by Counteracting Acidosis through the
Hypoxia-Inducible Carbonic Anhydrase IX and XII Promote
Updated Version 10.1158/0008-5472.CAN-08-2470doi:
Access the most recent version of this article at:
Material
Supplementary http://cancerres.aacrjournals.org/content/suppl/2008/12/30/69.1.358.DC1.html
Access the most recent supplemental material at:
Cited Articles http://cancerres.aacrjournals.org/content/69/1/358.full.html#ref-list-1
This article cites 48 articles, 24 of which you can access for free at:
Citing Articles http://cancerres.aacrjournals.org/content/69/1/358.full.html#related-urls
This article has been cited by 26 HighWire-hosted articles. Access the articles at:
E-mail alerts related to this article or journal.Sign up to receive free email-alerts
Subscriptions
Reprints and .pubs@aacr.orgPublications Department at
To order reprints of this article or to subscribe to the journal, contact the AACR
Permissions .permissions@aacr.orgDepartment at
To request permission to re-use all or part of this article, contact the AACR Publications
American Association for Cancer Research Copyright © 2009 on October 10, 2012cancerres.aacrjournals.orgDownloaded from
DOI:10.1158/0008-5472.CAN-08-2470

Hypoxia-Inducible Carbonic Anhydrase IX and XII Promote Tumor
Cell Growth by Counteracting Acidosis through the Regulation
of the Intracellular pH
Johanna Chiche, Karine Ilc, Julie Laferrie`re, Eric Trottier, Fre´de´ric Dayan, Nathalie M. Mazure,
M. Christiane Brahimi-Horn, and Jacques Pouysse´gur
Institute of Developmental Biology and Cancer Research University of Nice, Centre National de la Recherche Scientifique UMR 6543,
Centre A. Lacassagne, Nice, France
Abstract
Acidosis of the tumor microenvironment is typical of a
malignant phenotype, particularly in hypoxic tumors. All cells
express multiple isoforms of carbonic anhydrase (CA),
enzymes catalyzing the reversible hydration of carbon dioxide
into bicarbonate and protons. Tumor cells express membrane-
bound CAIX and CAXII that are controlled via the hypoxia-
inducible factor (HIF). Despite the recognition that tumor
expression of HIF-1Aand CAIX correlates with poor patient
survival, the role of CAIX and CAXII in tumor growth is not
fully resolved. To understand the advantage that tumor cells
derive from expression of both CAIX and CAXII, we set up
experiments to either force or invalidate the expression of
these enzymes. In hypoxic LS174Tr tumor cells expressing
either one or both CA isoforms, we show that (a) in response
to a ‘‘CO
2
load,’’ both CAs contribute to extracellular
acidification and (b) both contribute to maintain a more
alkaline resting intracellular pH (pH
i
), an action that
preserves ATP levels and cell survival in a range of acidic
outside pH (6.0–6.8) and low bicarbonate medium. In vivo
experiments show that ca9 silencing alone leads to a 40%
reduction in xenograft tumor volume with up-regulation of
ca12 mRNA levels, whereas invalidation of both CAIX and
CAXII gives an impressive 85% reduction. Thus, hypoxia-
induced CAIX and CAXII are major tumor prosurvival pH
i
-
regulating enzymes, and their combined targeting shows that
they hold potential as anticancer targets. [Cancer Res
2009;69(1):358–68]
Introduction
Adaptation of tumor cells to hypoxia and acidosis is a critical
driving force in tumor progression and metastasis (1, 2). Cancer
cells produce a large amount of lactic acid (3), which is generated
through glucose metabolism and inefficient vascular clearing,
resulting in an acidic microenvironment within many solid
tumors (4). Extracellular acidosis represents a threat to cell
survival by modifying the intracellular pH (pH
i
), wherein a 0.1 pH
i
variation can disrupt multiple biological functions, including ATP
production, protein synthesis, cell proliferation, migration, and
apoptosis (5–7). Because numerous intracellular processes require
close regulation of pH
i
, most mammalian cells, particularly
hypoxic tumor cells, have developed key strategies to regulate
their pH
i
. Activation of the hypoxia-inducible factor-1 (HIF-1) in
hypoxia plays a major role in regulating pH homeostasis by
enhancing expression of membrane located transporters,
exchangers, pumps and ecto-enzymes (8). To survive in an acidic
environment, the pH
i
-regulating system of tumor cells actively
extrudes acids via the growth factor–activated Na
+
/H
+
exchanger
1 (NHE-1; refs. 9–12) and the monocarboxylate transporters
(MCT1 and MCT4; ref. 13). We showed previously that NHE-1
plays a key role in tumor development particularly for cells
producing large amounts of lactic acid (14). In the opposite
direction to H
+
extrusion, HCO
3
influx through Na
+
-HCO
3
cotransporters (NBC) and Cl
/HCO
3
exchangers (AE) contributes
to cytoplasmic alkalinization (15–17).
Carbonic anhydrases (CA), which catalyze the reversible
hydration of cell-generated carbon dioxide into protons and
bicarbonate ions, have also been proposed to contribute to
cellular alkalinization (18–20). The direction of the reaction is
dependent on the form, CO
2
or bicarbonate and protons, that
predominates. Mammalian cells express 13 active isoforms of
CAs, with a conserved active site and variable levels of activity,
and 3 inactive isoforms. They differ in their tissue distribution
and cellular localization. The expression of the membrane-
associated CAIX and CAXII is tightly controlled by oxygen levels
in multiple epithelial tumor types (21–23), and CAIX has a higher
extracellular activity than CAXII (23–25). CAIX is highly induced
in an HIF-1–dependent manner (26) and is constitutively
expressed in von Hippel-Lindau (VHL)–defective cells. CAXII is
up-regulated in VHL-defective renal tumors and induced in
hypoxia in tumor cells, but its dependence on HIF is not well
established (22). Whereas tumor expression of HIF-1aand CAIX
correlate with poor patient survival (19, 27), the significance of
CAXII, which lacks the extracellular proteoglycan domain of CAIX
implicated in cell adhesion (28–30), is less obvious (24). CAIX may
be functionally linked to the regulation of the tumor pH, because
it contributes to extracellular acidification (31) and forced CAIX
expression in three-dimensional cultured aggregates influences
pH
i
homeostasis (32). However, direct evidence of the conjugated
roles of CAIX and CAXII in pH
i
regulation in cell lines and in
tumor growth is still missing.
In this study, we show that the hypoxia-inducible CAIX and
CAXII proteins promote cell survival and growth through pH
i
maintenance. We conclude that CAIX and CAXII constitute a
robust pH
i
-regulating system able to confer a tumor growth and
survival advantage on cells exposed to a hypoxic and acidic
microenvironment. Finally, as very often hypothesized, but not
Note: Supplementary data for this article are available at Cancer Research Online
(http://cancerres.aacrjournals.org/).
Requests for reprints: Jacques Pouyssegur, Centre National de la Recherche
Scientifique UMR 6543, 33 Avenue Valombrose, Nice 06189, France. Phone: 33-492-03-
1222; Fax: 33-492-03-1225; E-mail: pouysseg@unice.fr.
I2009 American Association for Cancer Research.
doi:10.1158/0008-5472.CAN-08-2470
Cancer Res 2009; 69: (1). January 1, 2009 358 www.aacrjournals.org
Research Article
American Association for Cancer Research Copyright © 2009 on October 10, 2012cancerres.aacrjournals.orgDownloaded from
DOI:10.1158/0008-5472.CAN-08-2470

shown (23, 24), we validate here that CAIX and CAXII constitute
two new anticancer therapeutic targets.
Materials and Methods
Cell Culture and Hypoxic Exposure
Chinese hamster lung CCL39 fibroblasts (American Type Culture
Collection) and the CCL39-derived mutant PS120 cells, lacking the
amiloride-sensitive Na
+
/H
+
exchanger (6), were maintained in DMEM
(Sigma) supplemented with 7.5% FCS in a humidified atmosphere of 5%
CO
2
, 95% air, or 100% air at 37jC. The colon adenocarcinoma cell line
LS174Tr expressing the tetracycline (Tet) repressor was provided by Dr. van
de Wetering (33) and maintained in DMEM supplemented with 10% FCS.
Other human tumor cell lines were likewise cultured. Incubation in hypoxia
at 1% O
2
was carried out at 37jC in 95% humidity and 5% CO
2
/94% N
2
in a
sealed anaerobic workstation (Ruskinn).
Plasmids
Full-length human ca9 cDNA was obtained from hypoxic HeLa mRNA
extracts by PCR using the following specific primers: forward 5¶-
CGGGGTACCGCCGCCACCATGGCTCCCCTGTGCCCC-3¶and reverse 5¶-
GCTCTAGACTAGGCTCCAGTCTCGGC-3¶.ca9 cDNA was ligated into the
pTREX-A (pcDNA4/TO/myc-His A; Invitrogen) vector (pca9) between the
KpnI and XbaI sites. The short hairpin RNA (shRNA)–ca9 (shca9) was
obtained with oligonucleotide sequences forward 5¶-AGTTAAGCCTAAAT-
CAGAA-3¶and reverse 5¶TTCTGATTTAGGCTTAACT-3¶and inserted into
the pTER vector. The shRNA–hif-1a(shhif-1a)wasobtainedwith
oligonucleotide sequences forward 5¶-CTGATGACCAGCAACTTGA-3¶and
reverse 5¶-TCAAGTTGCTGGTCATCAG-3¶and inserted into the pTER
vector. Lentivirus particles for two independent sequences (1 and 2) of
pLKO.1-Puro Vector shRNA targeting ca12 (ca12
) and nontarget shRNA
(ctl) were from Sigma (TRCN0000116249, TRCN0000116251, and
SHC002V).
Stable Transgenic Cells
CCL39, PS120, and LS174Tr cells were transfected with pca9, whereas
only LS174Tr cells were transfected with shca9 or shhif-1a,using
Polyfectamine (HiPerFect Transfection Reagent, Qiagen) according to the
manufacturer’s instructions. Isolated clones were maintained under zeocin
(500 Ag/mL, Invitrogen). Tet (10 Ag/mL) induces CAIX expression or ca9 or
hif-1asilencing. LS-shca9 cells were also transduced with lentiviral particles
containing shRNA-ca12 (ca12
; Sigma) or nontarget shRNA (ctl) according
to the manufacturer’s instructions and named, respectively, LS-shca9/ca12
and LS-shca9/ctl.
RNA Extraction and Relative and Absolute Real-Time
Quantitative PCR
Total RNA was extracted from cells using the RNA extraction kit (Qiagen)
according to the manufacturer’s instructions. Total RNA (2 Ag) was added to
a20AL reverse transcription–PCR reaction using the Omniscript kit
(Qiagen). The relative expression level of ca9 and ca12 was quantified by
real-time quantitative PCR (qPCR), as reported previously (26). The absolute
quantification of ca9 and ca12 mRNA was obtained by absolute real-time
quantification. A standard curve was prepared using dilutions of the
pTREX-ca9 and pTREX-ca12 vectors from 30 to 3 10
8
copies in triplicate.
The cycle number of ca9 or ca12 amplification of each extract was
compared with the standard curve obtained respectively with pTREX-ca9 or
pTREX-ca12 vectors.
Immunoblotting
Cells were lysed in SDS sample buffer. Proteins (40 Ag) were separated on
7.5% SDS polyacrylamide gels and transferred onto polyvinylidene difluoride
membranes (Millipore). Membranes were blotted with the M75 antibody to
CAIX (Bayer), Hsp90 (Abcam), a polyclonal antibody to recombinant CAXII
(Sigma), and a polyclonal antibody to HIF-1aprepared and validated in our
laboratory (34). Immunoreactive bands were detected with a horseradish
peroxidase (HRP) anti-mouse or anti-rabbit antibody (Promega) by
enhanced chemiluminescence (Amersham Biosciences).
Immunofluorescence
Cells at sparse density were grown on glass coverslips and fixed with 3%
paraformaldehyde for 30 min followed by saturation for 30 min in PBS–2%
gelatin and PBS–1% bovine serum albumin. Cells were then incubated for
1 h with the CAIX antibody without permeabilization, followed by
incubation for 1 h with an anti-mouse Alexa 594–conjugated IgG antibody
(Invitrogen). Cells were mounted onto slides with citifluor and analyzed
with a Leica microscope (objective, 100).
In vitro Determination of CA Activity
Cells incubated in normoxia (PS120-pca9 cells) or hypoxia (to induce CAIX
and CAXII expression in LS174Tr cells) were placed on ice in normoxia, scrapped
into ice-cold PBS, to obtain intact membrane-bound CAIX and CAXII. The cell
suspension was immediately centrifuged and resuspended in a bicarbonate-free
medium (Sigma) buffered at outside pH (pH
o
) 7.4 with 30 mmol/L HEPES. A 0.1
volume of this cell suspension was added to a 3 mmol/L HEPES-buffered
solution (HBS) adjusted to pH
o
8.2 before rapid addition to a CO
2
-saturated
nonbuffered solution and pH determined over time (microelectrode, Schott
Instrument) to monitor the rapid hydration of CO
2
to carbonic acid. For
inhibition of the total CA activity, 100 Amol/L acetazolamide (ACTZ; Sigma) was
added to the cell suspension in normoxia 15 min before the experiment.
Resting pH
i
Measurement
[
14
C]Benzoic acid. The pH
i
was measured using the technique of
distribution of the weak acid [7-
14
C]benzoic acid (Amersham Biosciences)
in intracellular and extracellular spaces for exponentially growing cells (35).
Diisothiocyanatostilbene-2¶,2-disulfonic acid (DIDS; 1 mmol/L, Sigma) was
used as an inhibitor of HCO
3
transporters.
BCECF-AM probe. Exponentially growing cells seeded on glass coverslips
were incubated for 25 min in bicarbonate-free HBS (pH
o
7.4), MES-buffered
solution (MBS; pH
o
6.6), or bicarbonate-buffered solution (BBS; adjusted to
pH
o
7.4). The pH-sensitive fluorescent dye BCECF-AM 1 Amol/L (Sigma) was
then added for 5 min at room temperature. Cells were then quickly washed
with the appropriate HBS, MBS, or BBS solution and transferred to a laminar
flow cell chamber perfused with the same solution. Ratiometric measure-
ment of the fluorescence of 50 randomly selected individual cells per
coverslip was performed in a workstation (Acquacosmos). The pH
i
was
estimated by an in situ two-point calibration (pH
o
6.6–7.6) with perfusion of a
high K
+
buffer solution containing 130 mmol/L KCl, 2 mmol/L CaCl
2
, 1 mmol/
L MgCl
2
, 10 mmol/L glucose in 20 mmol/L HEPES-Tris (or MES-Tris), and
25 Amol/L nigericin to allow pH
i
to equilibrate with the external pH
o
.
DNA Synthesis
Cells (5,000) were plated on 96-well dishes before transfer to a CO
2
-free
incubator for 24 h in HCO
3
free DMEM buffered with 30 mmol/L MES or
HEPES adjusted to different pH
o
(6.2–7.4), supplemented with 10% dialyzed
serum, hypoxanthine (0.1 mmol/L), and UTP (0.1 mmol/L). DNA synthesis
was measured using an ELISA colorimetric kit (Roche Diagnostics) based on
a 2-h incorporation of BrdUrd (20 Amol/L).
ATP Determination
Cells were seeded in DMEM and grown at different pH
o
, as described for
DNA synthesis. ATP levels were measured using a Cell Titer Glo kit (Promega)
according to the manufacture’s instructions. The relative luminescence unit
was normalized to the quantity of protein.
Clonogenicity Assay
Cells (1,000) were seeded onto 60-mm dishes. Once attached, the medium
was replaced by HCO
3
free DMEM buffered at pH
o
6.4 (30 mmol/L MES) or
at pH
o
7.4 (30 mmol/L HEPES), supplemented with 10% dialyzed serum,
hypoxanthine (0.1 mmol/L), and UTP (0.1 mmol/L) for growth in the
absence of CO
2
/HCO
3
and transferred to a CO
2
-free atmosphere for 24 h.
Dishes were then returned to 5% CO
2
in a regular media for 7 d before
staining with Giemsa (Fluka).
Cell Proliferation in Three Dimensions
To grow spheroids, 1,600 cells were seeded in drops in 20 ALof
HCO
3
free DMEM buffered with 30 mmol/L HEPES adjusted to pH
o
7.4
supplemented with 10% FCS. After 12 d, spheroids were collected and cells
CAIX and XII Promote Tumor Growth by Regulating pH
i
www.aacrjournals.org 359 Cancer Res 2009; 69: (1). January 1, 2009
American Association for Cancer Research Copyright © 2009 on October 10, 2012cancerres.aacrjournals.orgDownloaded from
DOI:10.1158/0008-5472.CAN-08-2470
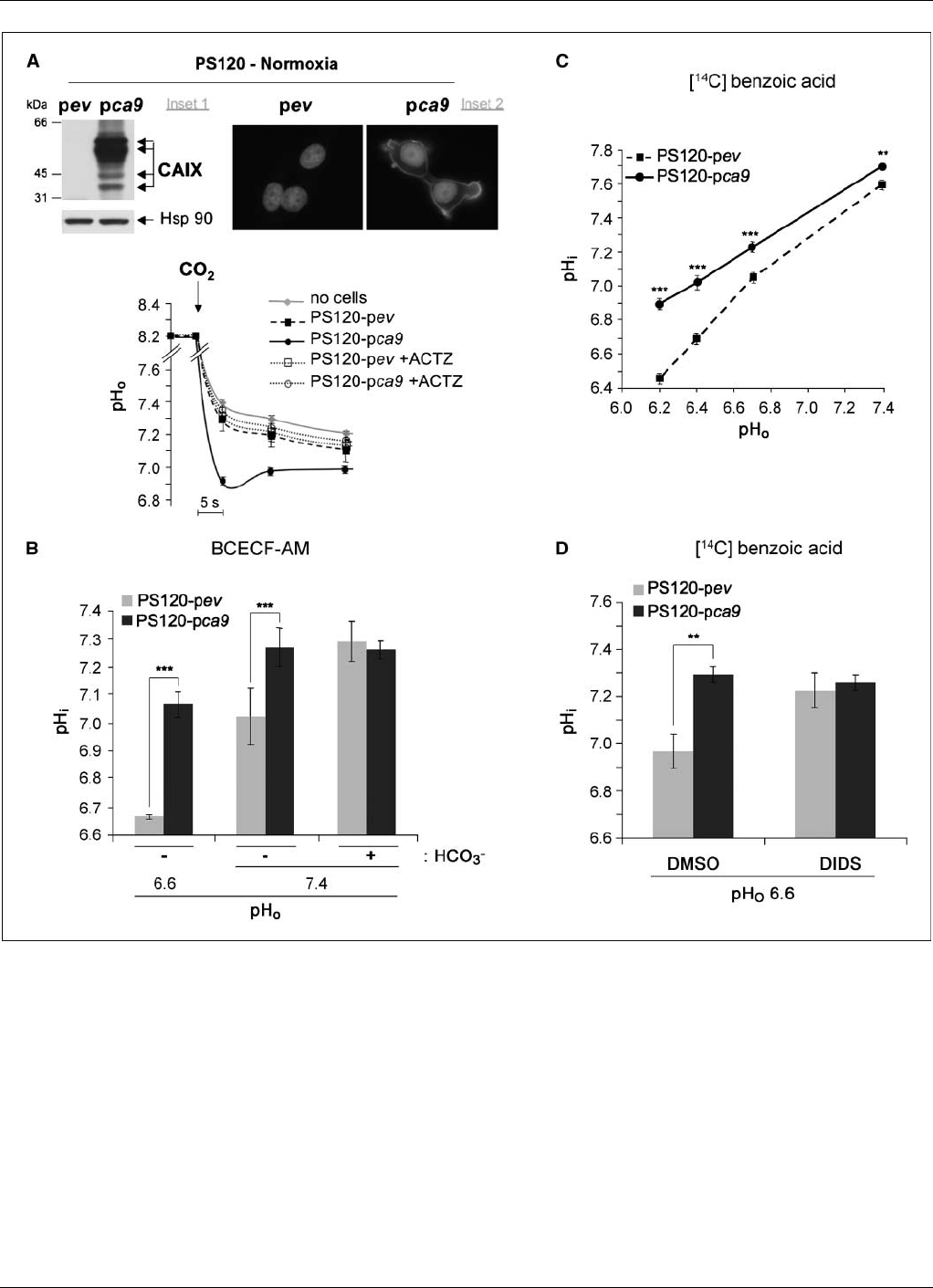
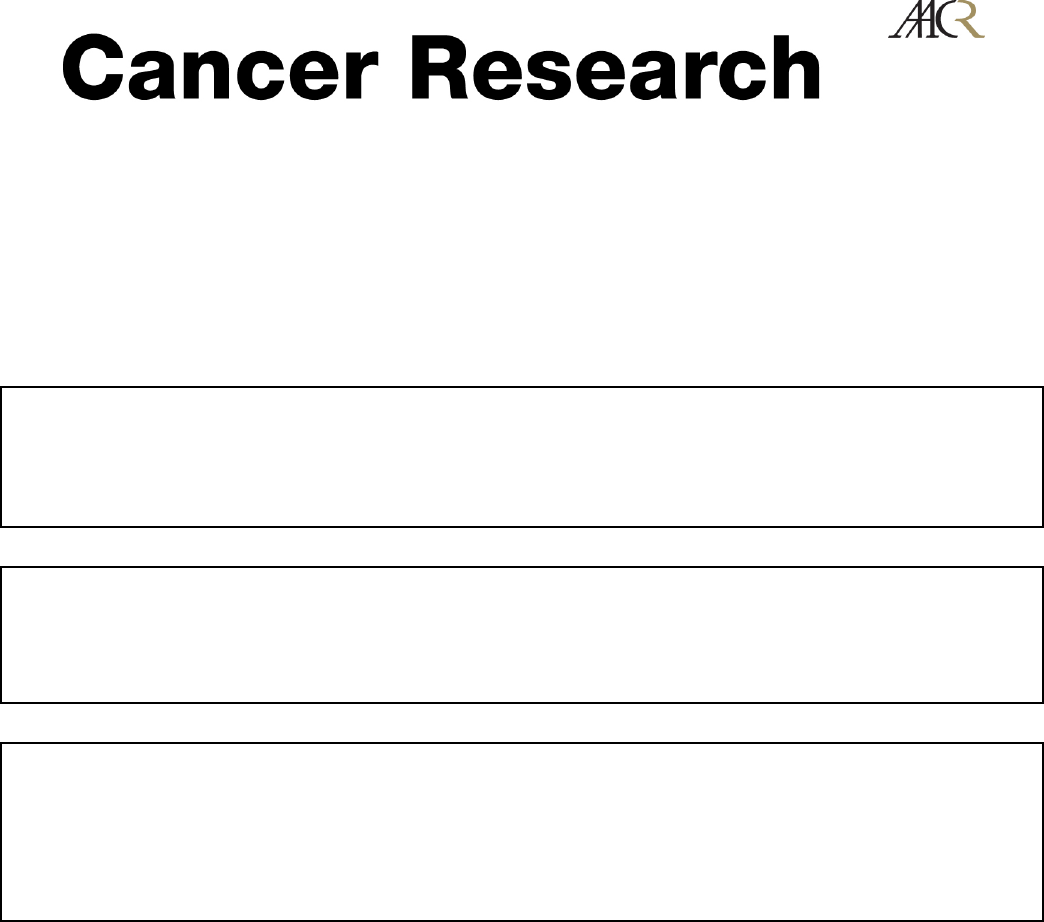
Figure 1. Forced expression of catalytically active CAIX induced extracellular acidification and cytoplasmic alkalinization. A, top, stable expression of human CAIX in
nonneoplastic PS120 fibroblasts lacking the Na
+
/H
+
exchanger (NHE-1). Inset 1, immunoblot to CAIX of lysates of control, empty plasmid vector (PS120-pev ), or
human CAIX stable expressing cells (PS120-pca9). The Hsp90 protein level was used as a loading control. Inset 2, immunofluorescence to CAIX in PS120-pev
and PS120-pca9 cells. Bottom, rate of extracellular acidification in response to a CO
2
load without cells (no cells) or with samples of intact normoxic PS120-pev or
PS120-pca9 cells. The rate of acidification reflects an estimation of the CA activity at the cell surface evaluated with or without a specific inhibitor of CAs (100Amol/L
ACTZ). B, CAIX expression increases the resting pH
i
.pH
i
of PS120-pev and PS120-pca9 cells was determined with pH-sensitive fluorescent dye BCECF-AM.
Histograms indicate the mean basal pH
i
for cells incubated for 30 min in either a 25 mmol/L HCO
3
-buffered solution (+HCO
3
) or HCO
3
–free MES or HBS (-HCO
3
)
adjusted to a pH
o
of 6.6 or 7.4, respectively. Just before their transfer to a laminar flow cell chamber, BCECF-AM was added to the cells for 5 min. Cells were then
perfused with the respective solutions, and a three-point calibration curve was created with high KCl/nigericin solutions to measure pH
i
as a function of the fluorescence
ratio. Significant differences based on the Student’s ttest (***, P< 0.001; n= 200 cells for each PS120-pev and PS120-pca9 cells at pH
o
6.6, n= 3000 cells for pH
o
7.4
with or without HCO
3
). In the absence of HCO
3
, PS120-pca9 cells had a statistically higher resting pH
i
than PS120-pev cells perfused at either a pH
o
of 6.6 or 7.4.
C, CAIX expression increases pH
i
over a range of pH
o
. The pH
i
was determined in PS120-pev and PS120-pca9 cells as a function of pH
o
with [
14
C]benzoic acid.
Exponentially growing cells of two independent clones for PS120-pca9 and PS120-pev cells were equilibrated for 15 min in a nominally HCO
3
free solution with pH
o
varying from 6.2 to 7.4. Cells were then shifted for 15 min to the same solution containing [
14
C]benzoic acid at the specific activity of 1 ACi/mL. pH
i
was calculated as
described under Materials and Methods. Determinations were done in quadruplicate for each condition, and the experiment was repeated at least thrice. Significant
differences are based on the Student’s ttest (**, P< 0.01; ***, P< 0.005). D, DIDS, an inhibitor of HCO
3
transporters, abolishes the differential in CAIX-induced
alkalinization. The steady-state pH
i
of PS120-pev and PS120-pca9 cells was measured with [
14
C]benzoic acid. Exponentially growing cells were incubated for 15 min in
a nominally HCO
3
free MBS at a pH
o
of 6.6 without (DMSO ) or with 1 mmol/L DIDS. Cells were then shifted for 15 min to the same medium containing [
14
C]benzoic
acid at a specific activity of 1 ACi/mL. pH
i
was calculated as described under Materials and Methods. The mean of quadruplicate determinations is given. Significant
differences are based on the Student’s ttest (**, P< 0.01).
Cancer Research
Cancer Res 2009; 69: (1). January 1, 2009 360 www.aacrjournals.org
American Association for Cancer Research Copyright © 2009 on October 10, 2012cancerres.aacrjournals.orgDownloaded from
DOI:10.1158/0008-5472.CAN-08-2470
were dissociated in Accutase (Life Technologies) to determine the number
of individualized living cells.
Nude Mice Tumorigenicity and Immunohistochemistry
Cells (1 10
6
) suspended in 500 AL of serum-free DMEM supplemented
with insulin-transferrin-selenium (Life Technologies) were s.c. injected into
the back of 4-wk-old male athymic mice (Harlan). Animal studies were
conducted according to Centre National de la Recherche Scientifique
institutional guidelines. Food and water were given ad libitum. Doxycycline
(Dox; 750 Ag/mL; Sigma) was given in the drinking water to induce
silencing of hif-1aor ca9. Five mice were used for each experimental
condition. The tumor volume was determined using the formula: (4p/3)
L/2W/2H/2(L, length; W, width; H, height). When the tumor
volume reached f1,500 mm
3
, mice were injected i.p. with hypoxyprobe (60
mg/kg; Chemicon) 4 h before sacrifice. Tumors were collected for RNA,
protein, and immunohistochemical analysis, as described (34). Sections
were incubated with antibodies to hypoxyprobe or CAXII for 1.5 h followed
by incubation with anti-mouse or anti-rabbit IgG-HRP antibodies. Analysis
was performed with a Leica microscope (objective, 20).
Statistical Analysis
The Student’s ttest was used wherein Pvalues of <0.05 were considered
significant.
Results
Forced expression of catalytically active CAIX-induced
extracellular acidification and cytoplasmic alkalinization.
Nonneoplastic Chinese hamster CCL39 lung fibroblasts and
CCL39-derived PS120 mutant cells defective in the Na
+
/
H
+
exchanger, which do not express endogenous CAIX or CAXII
in normoxia or in hypoxia, were selected for expression of
human CAIX and examined for their contribution to pH
i
with
(CCL39 cells) or without (PS120 cells) interference from pH
i
regulation by NHE-1. Stable expression of human CAIX in
normoxia was found to be plasma membrane located in PS120
(Fig. 1A) and CCL39 cells (Supplementary Fig. S1). The level of
expression of transfected human CAIX in these fibroblasts in
Figure 2. CAIX expression promotes survival and growth of PS120 fibroblasts impaired in NHE-1 in vitro in an acidic microenvironment. A, proliferation of PS120-pev
and PS120-pca9 cells (two independent clones for each type) was measured after a 24-h incubation in normoxia in a CO
2
/HCO
3
free microenvironment for media
buffered with either 30 mmol/L MES for a pH
o
of 6.2, 6.4, 6.6, and 6.8 or with 30 mmol/L HEPES for a pH
o
of 7.4 and supplemented with 100 Amol/L hypoxanthine/100
Amol/L uridine/10% dialyzed FCS. A cell proliferation ELISA colorimetric kit, which measures BrdUrd incorporation into newly synthesized DNA, was used. Data
are expressed as an average of three independent experiments performed in triplicate using two independent clones. Significant differences are based on Student’s
ttest (***, P< 0.005). B, quantification of ATP levels in PS120-pev and PS120-pca9 cells (two independent clones for each type) grown in normoxia in a
CO
2
/HCO
3
free environment with varying pH
o
from 6.2 to 7.4 for 24 h using a luciferin/luciferase-based assay, with results expressed as relative luminescence units.
Determinations were done in triplicate, and the entire experiment was done thrice using two independent clones. Significant differences are based on the Student’s ttest
(***, P< 0.005). C, viability assay of PS120-pev and PS120-pca9 cells in normoxia as function of pH
o
.Top, cells (1,000) were seeded in 60-mm dishes. Once
attached, cells were incubated in HCO
3
free media adjusted to pH
o
6.4 or 7.4 for 24 h in a CO
2
-free atmosphere. Fresh medium containing HCO
3
(40 mmol/L) at
pH
o
7.4 was then added to the dishes that were maintained for 10 d in a 5% CO
2
incubator before staining for visualization of the colonies. Bottom, the colony
number per plate was counted by eye for two independent PS120-pca9 and PS120-pev clones. The experiments were repeated thrice using two independent clones.
Student’s ttest (*, P< 0.05).
CAIX and XII Promote Tumor Growth by Regulating pH
i
www.aacrjournals.org 361 Cancer Res 2009; 69: (1). January 1, 2009
American Association for Cancer Research Copyright © 2009 on October 10, 2012cancerres.aacrjournals.orgDownloaded from
DOI:10.1158/0008-5472.CAN-08-2470
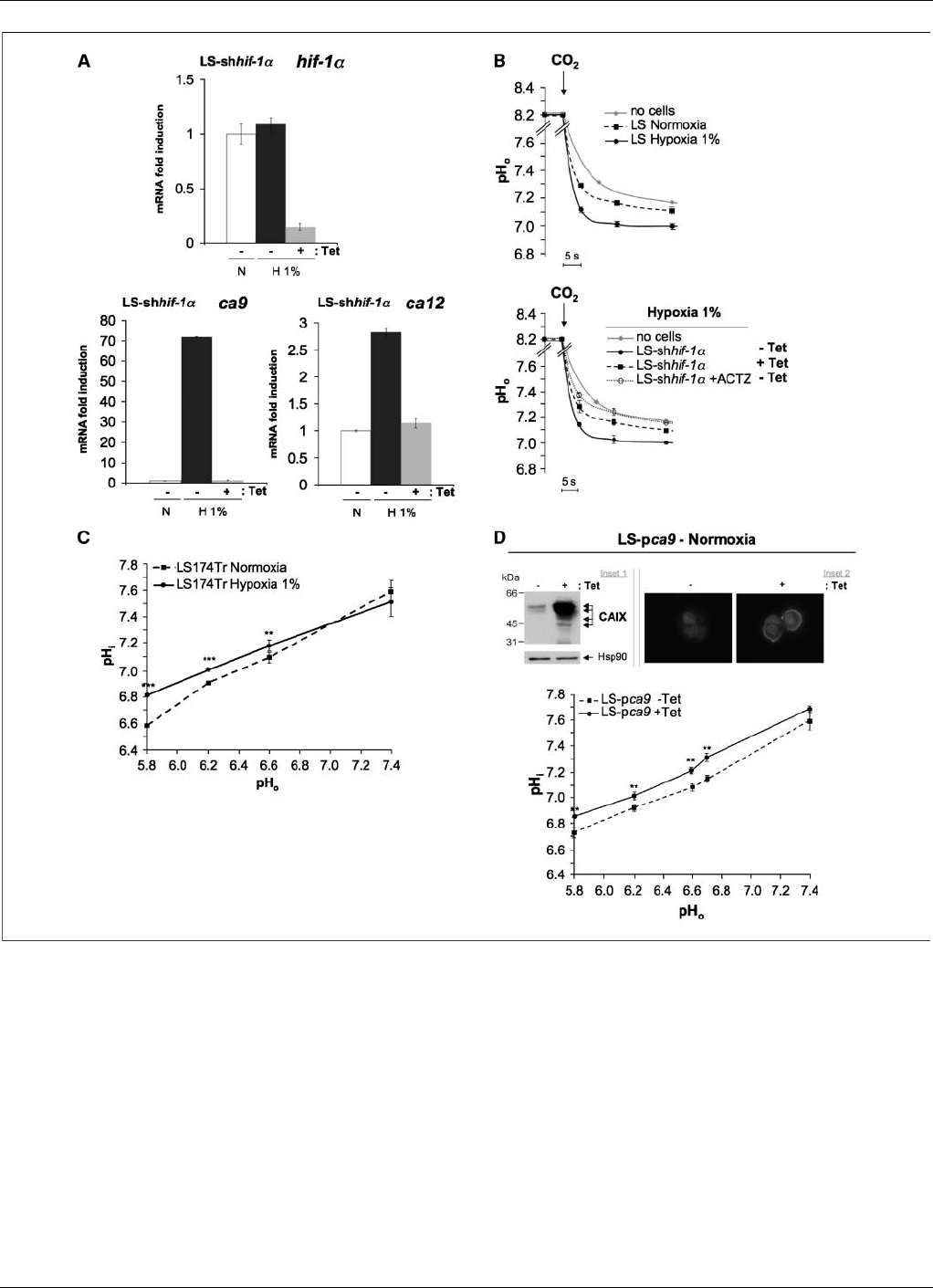
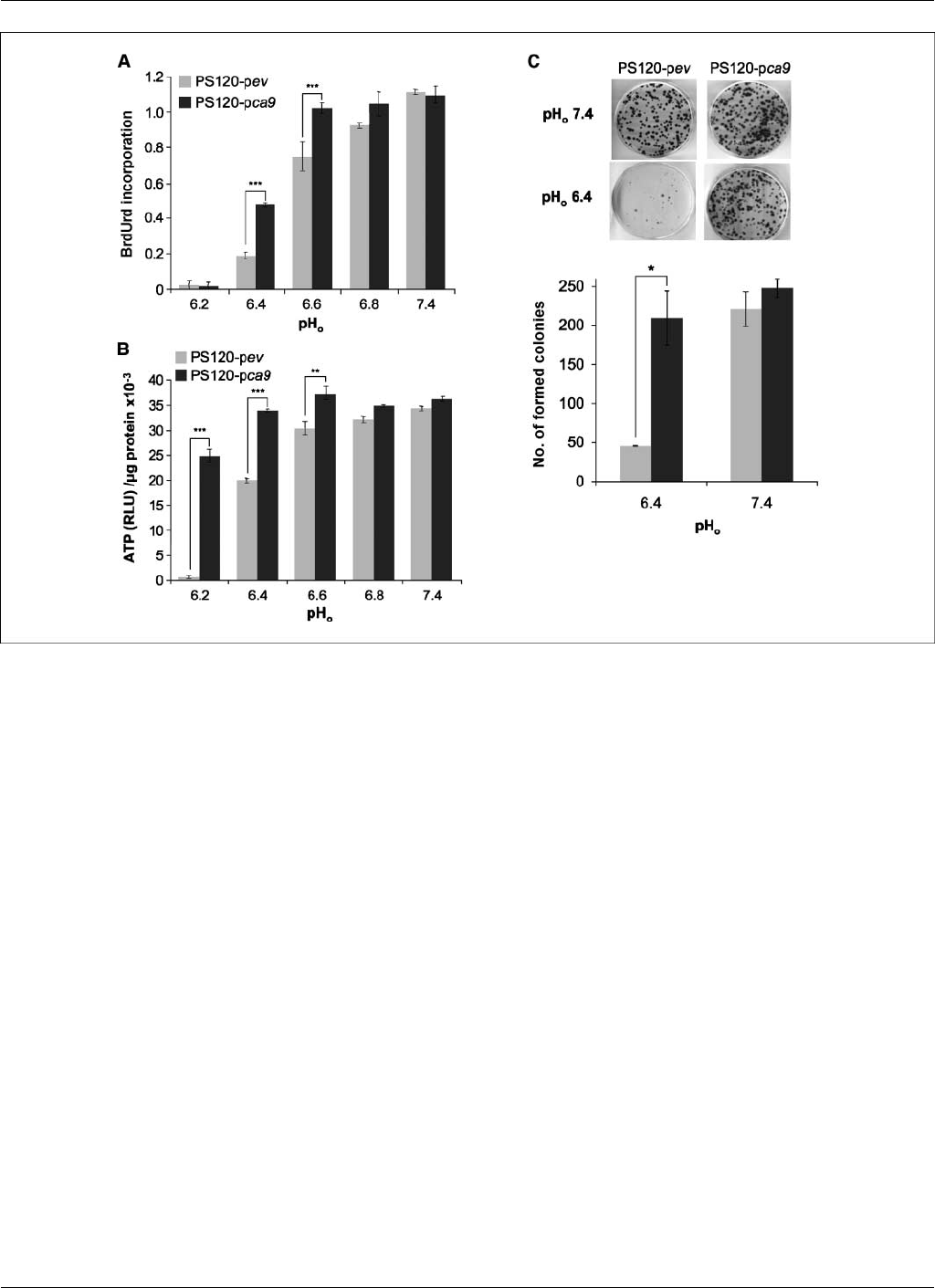
Figure 3. The hypoxia-induced CAIX and CAXII activity contributes to cytoplasmic alkalinization in an acidic microenvironment. A, the genes ca9 and ca12 are
HIF-1–dependent in human colon adenocarcinoma LS174Tr cells. Expression of the mRNA of hif-1a(top ), ca9 , and ca12 (bottom) was determined by real-time qPCR
in the stable LS-shhif-1aclone, in which Tet induces shRNA of hif-1a. Cells were incubated without (Tet ) or with (+Tet ) Tet for 4 d and maintained in either normoxia
21% O
2
(N) or hypoxia 1% O
2
(H1%) for 48 h. Each difference in gene expression was estimated within the limits of a 95% confidence interval. The results are
representative of at least three separate experiments. B, hypoxia increases the extracellular CA activity, as reflected in an increase in the rate of extracellular
acidification compared with normoxic conditions. Top, LS174Tr cells were incubated either in normoxia or hypoxia 1% O
2
for 48 h. The rate of extracellular acidification
in response to a CO
2
load was measured without cells (no cells ) or with intact LS174Tr cells. Bottom, The LS-shhif-1aclone was incubated without (Tet ) or with
(+Tet) Tet to allow for hif-1asilencing for 4 d before incubation in hypoxia 1% O
2
for 48 h without (Tet) or with (+Tet ) Tet. The rate of acidification in response to a
CO
2
load for intact hypoxic LS-shhif-1acells silenced (+Tet ) or not (Tet)forhif-1awas measured. ACTZ (+ACTZ ) at 100 Amol/L was added to a suspension of
hypoxic LS-shhif-1aTet cells for 15 min before CO
2
addition. C, determination of resting pH
i
with [
14
C]benzoic acid as a function of pH
o
in LS174Tr cells exposed to
either normoxia or hypoxia 1% O
2
for 48 h. Exponentially growing LS174Tr cells incubated in normoxia or hypoxia were equilibrated for 15 min in a nominally
HCO
3
free HBS or MBS with a pH
o
varying from 5.8 to 7.4. Cells were incubated for 15 min in the same appropriate equilibration solution containing [
14
C]benzoic acid
at a specific activity of 1 ACi/mL. The pH
i
was calculated as described under Materials and Methods. The experiment was repeated at least thrice, and each point
represents the average of quadruplicates for each experiment (**, P< 0.01; ***, P< 0.005). D, stable Tet-inducible expression of CAIX in LS174Tr cells in normoxia
maintains a higher pH
i
in an acidic and HCO
3
free media. Top, inset 1, immunoblot of inducible expression of CAIX in normoxia in LS174Tr cells (LS-pca9)
without (Tet) or with (+Tet) Tet for 4 d. Total extracts were analyzed by immunoblotting with antibodies against CAIX and Hsp90. The latter was used as a loading
control. Inset 2, immunofluorescence of inducible expression of CAIX expression (LS-pca9 ) in LS174Tr cells in the absence (Tet) or presence (+Tet) of Tet for
4d.Bottom, determination of resting pH
i
with [
14
C]benzoic acid in as a function of pH
o
in Tet-inductible LS-pca9 clone expressing CAIX in normoxia under treatment
for 4 d with (+Tet) or without (Tet ) Tet. Determinations were done in quadruplicate for each condition, and each experiment was repeated at least thrice. Results
represent the average of quadruplicates for each experiment (**, P< 0.01; ***, P< 0.005).
Cancer Research
Cancer Res 2009; 69: (1). January 1, 2009 362 www.aacrjournals.org
American Association for Cancer Research Copyright © 2009 on October 10, 2012cancerres.aacrjournals.orgDownloaded from
DOI:10.1158/0008-5472.CAN-08-2470
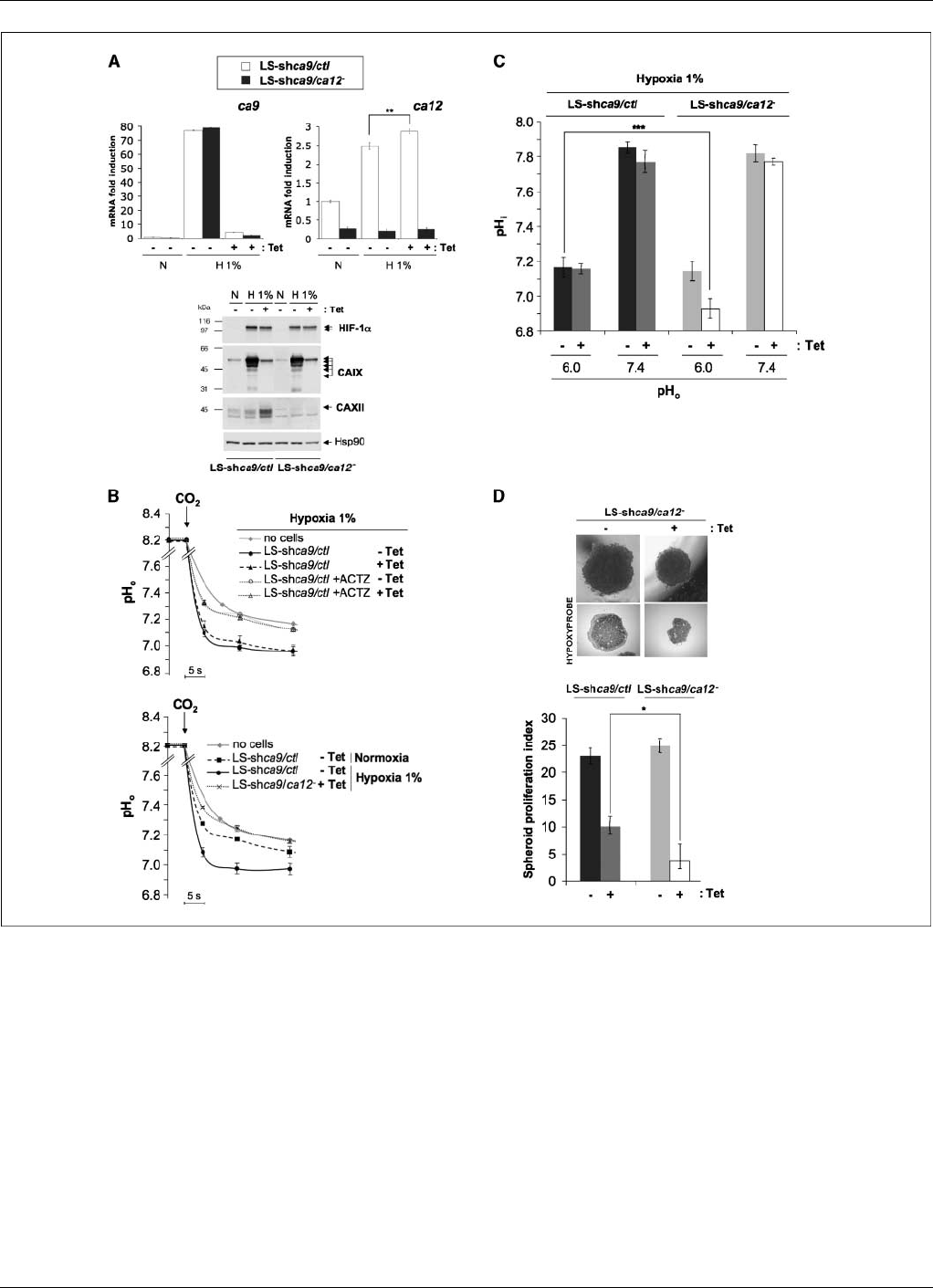
Figure 4. Combined invalidation of CAIX and CAXII reduces pH
i
and spheroids growth. A, silencing of ca9, ca12 , and ca9/ca12 in LS174 cells. Top left, expression of
the mRNA levels of ca9 determined by real-time qPCR. Stable LS-shca9/ctl and LS-shca9/ca12
clones were incubated with (Tet ) or without (+Tet ) Tet for 4 d before
incubation in either normoxia (N) or hypoxia 1% O
2
(H1%) for 48 h. Top right, expression of the mRNA levels of ca12 determined by real-time qPCR under the
conditions described below. LS-shca9/ctl and LS-shca9/ca12
clones were incubated with (Tet ) or without (+Tet ) Tet for 4 d before incubation in either normoxia (N)
or hypoxia 1% O
2
(H1%) for 48 h. The results are representative of at least three separate experiments (**, P< 0.01). Bottom, total extracts were analyzed by
immunoblotting with antibodies against HIF-1a, CAIX, CAXII, and Hsp90. The latter was used as a loading control. B, top, rate of acidification of a medium set at
pH 8.2 in response to the addition of CO
2
incubated without cells (no cells) or with intact LS-shca9/ctl cells previously incubated for 4 d with (Tet ) or without (+Tet )
Tet and exposed in hypoxia 1% O
2
for 48 h. Control LS-shca9/ctl Tet and ca9 silenced LS-shca9/ctl +Tet cells were also treated with 100 Amol/L ACTZ 15 min before
addition of CO
2
.Bottom, rate of acidification in response to a CO
2
load of intact LS-shca9/ctl and LS-shca9/ca12
cells incubated without (Tet) or with (+Tet)Tet
for 4 d and then exposed to either normoxia (N) or hypoxia 1% O
2
(H1%) for 48 h. C, combined ca9 and ca12 invalidation (LS-shca9/ca12
+Tet) diminishes resting
pH
i
in an acidic (pH
o
6.0), MES-buffered, nominally HCO
3
free solution. Stable expressing LS-shca9/ca12
cells were constitutively invalidated for ca12, whereas
exponentially growing Tet-inducible LS174Tr (LS-shca9/ctl and LS-shca9/ca12
) clones were invalidated for ca9 after a 4-d treatment with Tet. Cells were then
incubated in hypoxic incubation 1% O
2
for 48 h. Dishes were then returned to normoxia for resting pH
i
determination in a 20 mmol/L MES-buffered, nominally
HCO
3
free saline/glucose solution adjusted to pH
o
6.0 or in a 20 mmol/L HEPES-buffered, nominally HCO
3
free saline/glucose solution adjusted to pH
o
7.4 (15 min
incubation to reach the equilibrium). For pH
i
determination, cells were shifted for 15 min to the same medium containing [
14
C]benzoic acid at a specific activity 1 ACi/mL.
The pH
i
was calculated as described under Materials and Methods. The experiment was done twice. Each point represents the average of quadruplicates for each
experiment (**, P< 0.01). D, silencing of ca9 and ca12 diminished proliferation of three-dimensional spheroids. Top, an inducible LS174Tr clone invalidated for
ca12 (LS-shca9/ca12
Tet) or both ca9 and ca12 (LS-shca9/ca12
+Tet) with a 4-d Tet treatment (+Tet) were cultured as spheroids in the absence (Tet)or
presence (+Tet) of Tet for 12 d. Hypoxyprobe (pimonidazole) was added to the extracellular media for staining of hypoxic zones 4 h before formaldehyde fixation.
Bottom, spheroids were subjected to Accutase dissociation, and individualized live cells were counted using Trypan blue. The spheroid proliferation index was
calculated as the ratio of the cell number counted at day 12 to the cell number at day 0. Data represent the average of three independent experiments.
CAIX and XII Promote Tumor Growth by Regulating pH
i
www.aacrjournals.org 363 Cancer Res 2009; 69: (1). January 1, 2009
American Association for Cancer Research Copyright © 2009 on October 10, 2012cancerres.aacrjournals.orgDownloaded from
DOI:10.1158/0008-5472.CAN-08-2470
normoxia was comparable with the expression of CAIX in tumor
cells after 48 h in hypoxia 1% O
2
(data not shown). The CA
activity associated with the plasma membrane was determined
by the rapid acidification of a minimally buffered medium in
response to addition of a CO
2
-saturated solution. In the presence
of cell suspensions, the rate and magnitude of acidification was
higher for CAIX-expressing PS120 cells (Fig. 1A) and CAIX-
expressing CCL39 cells (Supplementary Fig. S1) than for control
cells (pev). The CA inhibitor ACTZ reduced the activity of CAIX-
expressing cells to the spontaneous basal level obtained with the
control cells with or without ACTZ. In addition, PS120-pca9 cells
incubated in normoxia or hypoxia (48 h, 1% O
2
) showed the
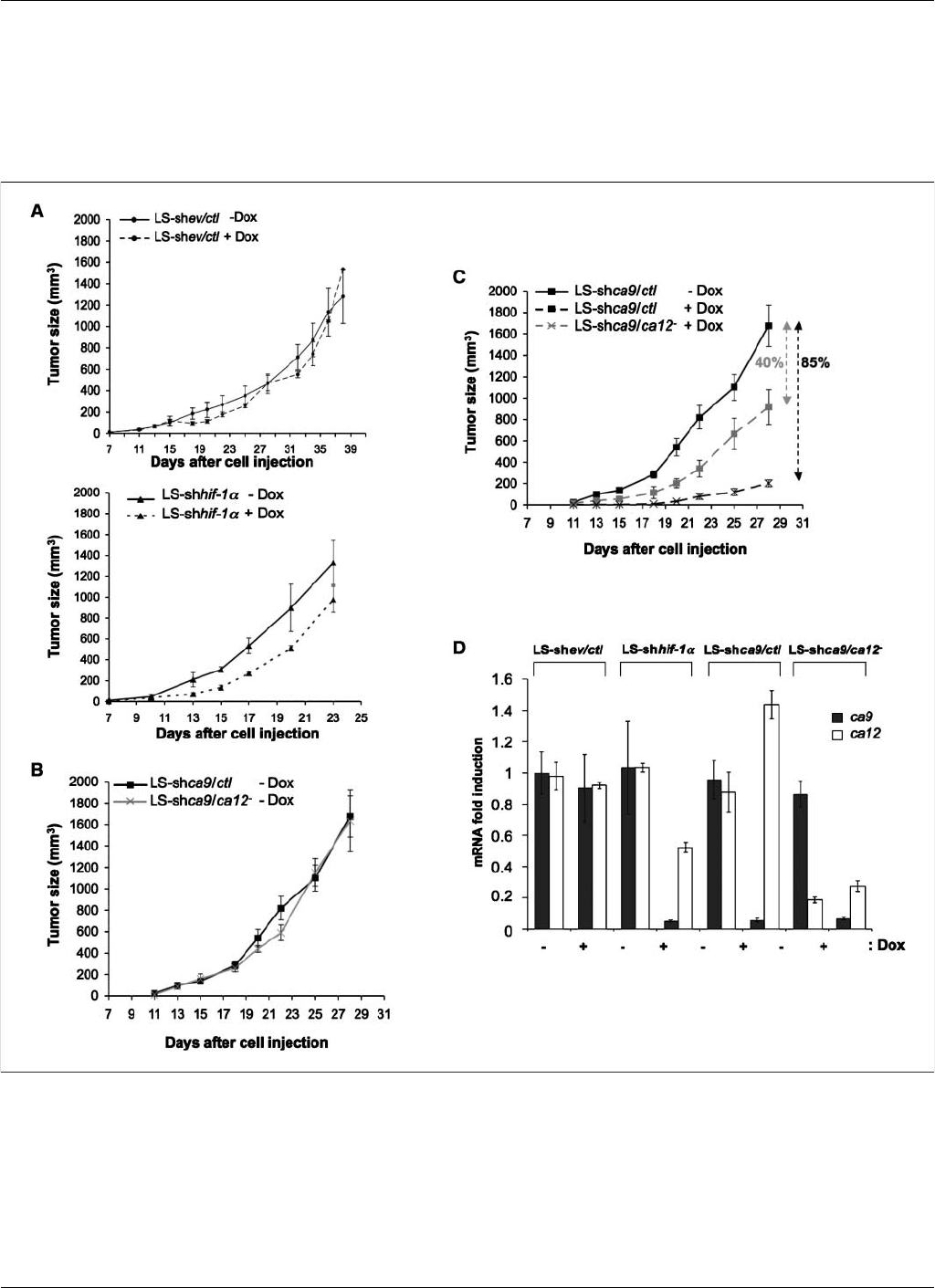
Figure 5. Inducible invalidation of CAIX and CAXII reduces the rate of xenograft tumor growth. Characterization of the growth properties of CAIX-deficient,
CAXII-deficient, and CAIX/CAXII-deficient xenograft tumors. At 4 d before injection of LS-shev/ctl, LS-shhif-1a, LS-shca9/ctl , and LS-shca9/ca12
cells, cells were
incubated without (Tet) or with (+Tet) Tet to silence hif-1a, ca9 or both ca9 and ca12, respectively. For the respective conditions, mice received Dox or not, a
semisynthetic Tet, in the drinking water 4 d before cell injection. In vivo xenograft assays were performed by injecting s.c. into the back of athymic nude mice (1 10
6
)
viable and individual tumor cells LS-shev/ctl Tet, LS-shev/ctl +Tet, LS-shhif-1aTet, LS-shhif-1a+Tet, LS-shca9/ctl Tet, LS-shca9/ctl +Tet, LS-shca9/ca12
Tet, and LS-shca9/ca12
+Tet. Xenograft growth was determined by measuring the tumor volume. A, top, Dox in the drinking water does not affect tumor growth of
control LS-shev/ctl cells; bottom, invalidation of hif-1a(LS-shhif-1a+Tet) showed a slight reduction in tumor growth compared with tumor growth of control cells
(LS-shhif-1a+Tet). B, cells defective for ca12 (LS-shca9/ca12
Tet) formed xenograft tumors at the same rate and of the same size as control cells not silenced for
ca12 (LS-shca9/ctl Tet). C, cells silenced for ca9 (LS-shca9/ctl +Tet) or for both ca9 and ca12 (LS-shca9/ca12
+Tet) grew slower and gave a smaller tumor volume
than control (LS-shca9/ctl Tet) cells. Five mice were studied per condition. In vivo experiments were repeated twice. D, level of mRNA expression of ca9 and ca12 in
the xenograft tumors of different transgenic cells, as determined by real-time qPCR. Results represent an average of four tumors for each condition.
Cancer Research
Cancer Res 2009; 69: (1). January 1, 2009 364 www.aacrjournals.org
American Association for Cancer Research Copyright © 2009 on October 10, 2012cancerres.aacrjournals.orgDownloaded from
DOI:10.1158/0008-5472.CAN-08-2470

same level of activity (data not shown). The resting pH
i
of
PS120-pca9 cells, with the BCECF-AM dye, showed it to be more
alkaline compared with control PS120-pev cells when incubated
in a nominally HCO
3
/CO
2
-free solution set at a pH
o
of 6.6 to
7.4 (Fig. 1B). The difference was more pronounced for a pH
o
of
6.6 (0.4 units) compared with 7.4 (0.15 units). In the presence of
25 mmol/L bicarbonate, no difference in pH
i
was observed
between CAIX-expressing cells and control cells. The intracellular
alkalinization associated with CAIX expression was confirmed in
PS120 (Fig. 1C) and CCL39 (Supplementary Fig. S2) cells with
another technique that uses [
14
C]benzoic acid, indicating that
CAIX expression protects cells against cytoplasmic acidification.
In the presence of endogenous NHE-1 (CCL39 cells), CAIX was
able to restore a more alkaline pH
i
in acidic environments. This
was more marked in fibroblasts impaired in NHE-1 because
CAIX was able to compensate for the lack of NHE-1 in
maintaining pH
i
in acidic environments. The implication of
bicarbonate transport in changes in pH
i
is shown by its
suppression in the presence of the bicarbonate transport
inhibitor DIDS (Fig. 1D). This shows that the function of CAIX
as a pH
i
regulator is revealed only in the absence of added
extracellular bicarbonate.
CAIX-mediated cytoplasmic alkalinization and cell survival
in an acidic environment. To examine if hypoxia-induced CAs
protect cells from extracellular acidosis, we assessed the effect of
forced expression of CAIX in PS120 and CCL39 cells on
proliferation, ATP level, and cell survival. Cell proliferation
determined by BrdUrd incorporation into DNA was significantly
increased in normoxic CAIX-expressing cells at low pH
o
(Fig. 2A
and Supplementary Fig. S3), as was the production of ATP (Fig. 2B
and Supplementary Fig. S4) after 24 h in the absence of CO
2
/HCO
3
.
Cell colony formation was not significantly different when
comparing CAIX-expressing and control cells incubated at a pH
o
of 7.4; however, it was substantially diminished in non–CAIX-
expressing cells at a low pH
o
of 6.4 (Fig. 2Cand Supplementary Fig.
S5). Stable PS120 clones expressing human CAXII were also
obtained and showed similar characteristics to those expressing
CAIX: extracellular acidification, conserved pH
i
regulation, and
increase in cell survival in acidic conditions (data not shown).
However, the CAXII activity was slightly lower compared with that
of CAIX. These results suggest that CAIX and CAXII, by maintaining
a more alkaline resting pH
i
, sustain ATP levels, promoting cell
survival in a bicarbonate-free acidic microenvironment.
Hypoxia-induced CAIX and CAXII activity contributes to
cytoplasmic alkalinization in an acidic microenvironment. A
number of human tumor cell lines showed an increase in the
number of copies of ca9 and ca12 mRNA and CAIX and CAXII
protein expression in response to hypoxia 1% O
2
, including RCC4,
HeLa, A375Tr, A549, and LS174Tr cells (Supplementary Table S1;
Supplementary Fig. S6). To further investigate the contribution of
both CAIX and CAXII, we chose to use the Tet-inducible LS174Tr
human colorectal adenocarcinoma cells to silence hif-1awith
shRNA. In LS-shhif-1acells, comparable levels of expression of
hif-1amRNA were detected in normoxia and hypoxia, confirming
absence of transcriptional regulation of hif-1aby oxygen. In
contrast, incubation of hypoxic LS-shhif-1acells with Tet resulted
in a substantial decrease in the mRNA level of hif-1a(Fig. 3A, top).
Expression of ca9 and ca12 was significantly decreased in hypoxic
LS-shhif-1acells when hif-1awas silenced (+Tet; Fig. 3A, bottom).
The LS174Tr cells endogenously express both membrane-bound
and catalytically active CAs in a HIF-1–dependent manner
(Fig. 3B). Hypoxic induction was associated with significantly
enhanced alkalinization of the resting pH
i
of cells when exposed to
a low pH
o
in the absence of extracellular bicarbonate (Fig. 3C). To
study the contribution of CAIX to pH
i
regulation, hypoxia-induced
CAIX expression was mimicked in LS174Tr cells expressing a basal
level of endogenous CAXII in normoxia (Fig. 3D; Supplementary
Table S1; Supplementary Fig. S7). Forced Tet-inducible expression
of CAIX leads to enhanced alkalinization of the resting pH
i
in an
acidic and bicarbonate-free environment (Fig. 3Dand Supplemen-
tary Fig. S7). Thus, CAIX expression plays a key role in maintaining
the resting pH
i
in an acidic and bicarbonate-limiting environment
in LS174Tr cells.
Combined invalidation of CAIX and CAXII reduces pH
i
and
spheroid growth. LS174Tr cells were selected for Tet-inducible
silencing of ca9 combined (LS-shca9/ca12
) or not (LS-shca9/ctl)
with constitutive silencing of ca12. Tet addition to shca9 cells
(LS-shca9/ctl and LS-shca9/ca12
) resulted in 95% invalidation of
ca9 mRNA (Fig. 4A, top) and protein (Fig. 4A, bottom), whereas 90% of
the ca12 mRNA (Fig. 4A, top) and protein (Fig. 4A, bottom) were
silenced in LS-shca9/ca12
cells. Note that an increase in hypoxia-
inducible expression of the mRNA and protein levels of CAXII was
observed when ca9 was silenced. The copy number of ca9 mRNA is
18-fold lower than that of ca12 in LS-shca9/ctl in normoxia
(Supplementary Table S2), whereas in hypoxia, the number of copies
of ca9 mRNA was only twice higher than that of ca12. Moreover,
when ca9 is suppressed in hypoxia, the number of ca12 mRNA copies
was almost similar to ca9 induced in hypoxia. In addition, when ca9
was silenced in hypoxia, the overall hypoxia-induced CA activity was
unchanged whereas ACTZ showed a marked reduction in acidifica-
tion (Fig. 4B, top). When ca12 was silenced in hypoxia, no reduction
in extracellular acidification occurred (data not shown); however,
combined silencing of ca9 and ca12 in hypoxia (LS-shca9/ca12
+Tet) reduced the CA activity to the basal level (Fig. 4B, bottom).
We then determined the relative contributions of CAIX and CAXII
in hypoxia regarding pH
i
regulation. At a neutral pH
o
of 7.4, silencing
of ca9, ca12, or both together did not affect the resting pH
i
(Fig. 4C).
When cells were exposed to an acidic pH
o
of 6.0, only combined
invalidation of ca9 and ca12 resulted in a significantly lower resting
pH
i
(0.2 units), whereas silencing of either isoform alone had no
effect (Fig. 4C); results were obtained with two independent
sequences targeting ca12 (data not shown for the second sequence).
We then assessed the importance of this pH
i
regulating system in
hypoxia on cells grown as three-dimensional spheroids. In a
bicarbonate-limiting environment, a hypoxic gradient is established
(hypoxyprobe labeling; Fig. 4D,top) and, thus, lactic acid is
produced. When ca9 was silenced (LS-shca9/ctl +Tet) the prolifer-
ation index of hypoxic spheroids diminished compared with control
cells (LS-shca9/ctl Tet) and diminished further when both
isoforms were silenced (LS-shca9/ca12
+Tet; Fig. 4D, bottom).
These results indicate that both CAIX and CAXII play an important
role in the regulation of pH
i
recapitulating the protective effect of
hypoxia in promoting cell survival in an acidic environment.
Inducible invalidation of CAIX and CAXII reduces the rate
of xenograft tumor growth. To investigate the in vivo functional
consequence of CAIX and CAXII expression on tumor growth,
athymic mice were s.c. injected with Tet-inducible LS-shhif-1a,
LS-shca9/ctl,orLS-shca9/ca12
cells. Dox had no significant effect
on tumor growth of control cells (LS-shev/ctl;Fig.5A, top), whereas
Dox silencing of hif-1ashowed a substantial decrease in the size of
tumors (Fig. 5A, bottom). The constitutive silencing of ca12 in an
CAIX and XII Promote Tumor Growth by Regulating pH
i
www.aacrjournals.org 365 Cancer Res 2009; 69: (1). January 1, 2009
American Association for Cancer Research Copyright © 2009 on October 10, 2012cancerres.aacrjournals.orgDownloaded from
DOI:10.1158/0008-5472.CAN-08-2470
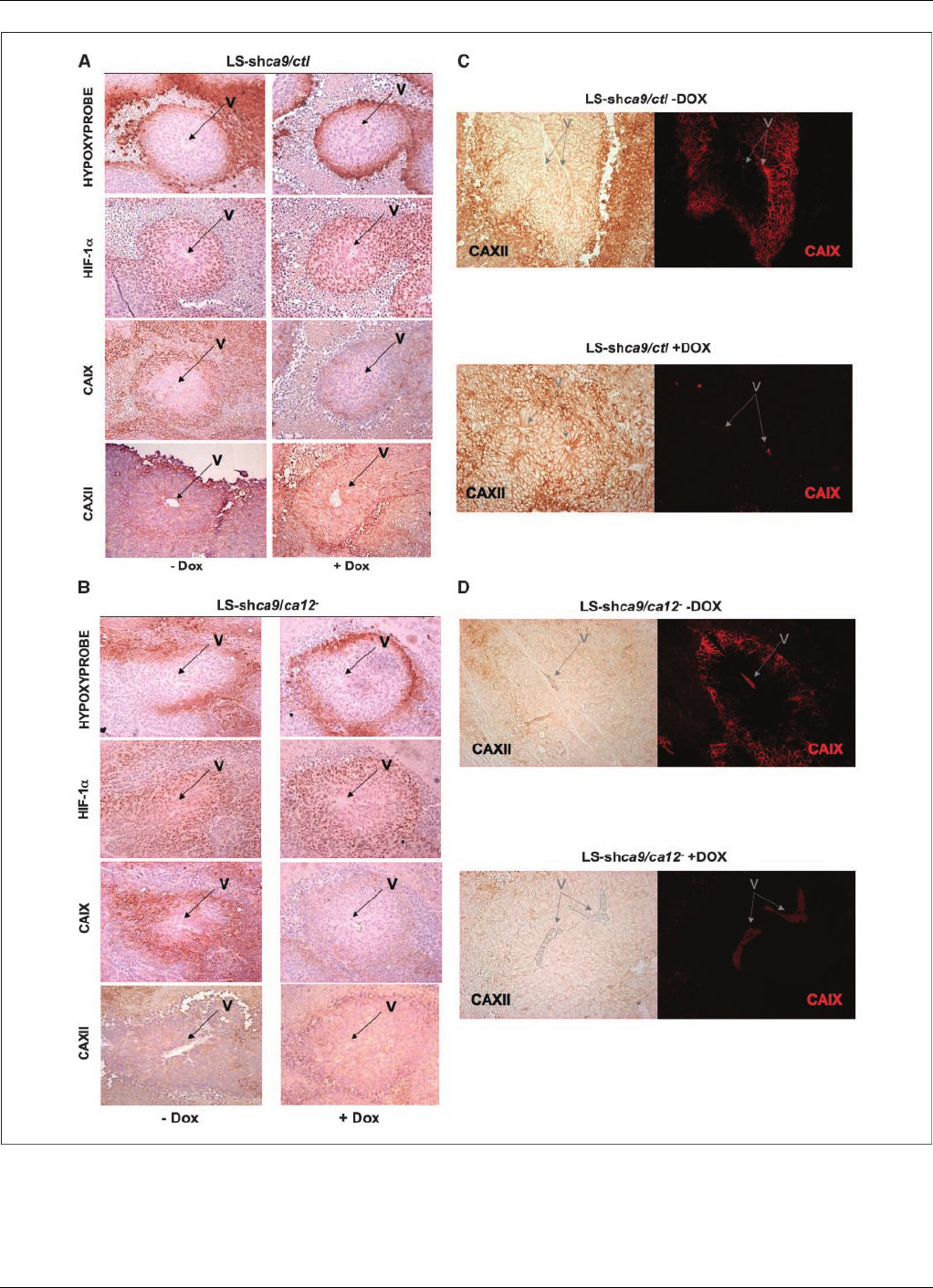
Figure 6. Immunohistologic confirmation of the expression of HIF-1a, CAIX, or CAXII in hypoxic regions of the corresponding tumor xenografts. Mice were injected with
ca9-deficient LS-shca9/ctl cells (A)orca9 - and ca12-deficient LS-shca9/ca12
cells (B) and drinking water supplemented without (Dox ) or with (+Dox ) Dox
was provided to mice. Serial sections of tumors of the same size were stained for hypoxic regions with antibodies to hypoxyprobe (pimonidazole), for nuclear staining
of HIF-1a, or for membrane staining of CAIX or CAXII. Costaining of tumor sections was done first using the immunofluorescence protocol for CAIX followed by
immunohistochemistry for CAXII in the same tumor section of LS-shca9/ctl cells (C) and LS-shca9/ca12
(D). Magnification, 20.V, major blood vessel of the tumor
lobe analyzed in serial sections.
Cancer Research
Cancer Res 2009; 69: (1). January 1, 2009 366 www.aacrjournals.org
American Association for Cancer Research Copyright © 2009 on October 10, 2012cancerres.aacrjournals.orgDownloaded from
DOI:10.1158/0008-5472.CAN-08-2470

endogenous ca9 background (LS-shca9/ca12
Dox) showed
similar tumor growth to that of the control cells (LS-shca9/ctl
Dox; Fig. 5B). However, the silencing of ca9, in the presence of
endogenous ca12 (LS-shca9/ctl +Dox), gave a slight but significant
reduction in tumor growth (40%) compared with control cells (Fig.
5C). Invalidation of both isoforms (LS-shca9/ca12
+Dox) resulted
in a spectacular decrease in tumor size (85%) as a result of slower
cell proliferation (Fig. 5C). Examination of the mRNA levels of ca9
and ca12 in the tumors confirmed almost complete invalidation of
ca9 in the invalidated cell lines when Dox was added (95% for LS-
shhif-1a, 94% for LS-shca9/ctl, and 92% for LS-shca9/ca12
).
Whereas the expression of the ca12 mRNA was diminished by a
half in hif-1ainvalidated cells (Figs. 4Aand 5D), its expression
increased 1.5-fold when ca9 (LS-shca9/ctl +Dox) was silenced
(Fig. 5D). In the LS-shca9/ca12
cell line, the level of ca12 mRNA
was diminished (80%) whereas that of ca9 in the absence of Dox was
not significantly different. These results indicate that the respective
tumor types retain their invalidated phenotype and that the
expression of ca12 responds to the level of expression of ca9,
although the reverse is not the case.
Immunohistochemical and immunofluorescence analysis of
tumor sections from mice injected with LS-ca9/ctl (Fig. 6Aand C)
and LS-ca9/ca12
cells (Fig. 6Band D) showed diminished levels of
CAIX in cells silenced for ca9 and for ca9 plus ca12 in Dox-treated
mice, despite the expression of HIF-1ain hypoxic zones (hypoxyp-
robe staining). CAXII expression is seen in all cells of the tumor
(slightly increased in the perinecrotic area) in contrast to the
expression of CAIX, which correlates with the hypoxic and
perinecrotic regions. It is important to note the increase in CAXII
expression when CAIX is silenced in vivo. Comparison of the
immunohistochemistry of tumor sections of control LS-shev/ctl cells
(Supplementary Fig. S8A) and hif-1asilenced LS-shhif-1acells
(Supplementary Fig. S8B) revealed a significant decrease in HIF-1a,
CAIX, and CAXII in hif-1asilenced cells. For tumor histology, to
insure that the reduced labeling observed for CAIX in ca9/ca12
silenced tumors was indeed due to invalidation and not small-sized
tumors with minimal hypoxic zones, mice were maintained for a
longer time and sacrificed 27 days later than control mice when the
tumors were the same size as for control nonsilenced cells. Thus,
these results show that invalidation of both CAIX and CAXII brings
about a dramatic decrease in tumor xenograft cell growth.
Discussion
Investigation into the implication of hypoxia-inducible CAs in the
regulation of pH has concerned only CAIX and has been restricted
to monolayer or three-dimensional cell cultures (31, 32). During the
preparation of this manuscript, a study showed that ectopically
expressed CAIX in human bladder carcinoma RT112 cells was able
to spatially coordinate pH
i
, but only when cells are cultured as
three-dimensional spheroids (32). In agreement with this report,
CAIX expression in our study had no effect on pH
i
regulation in
isolated cells in a neutral and bicarbonate-buffered medium (25
mmol/L); however, when cells were exposed to a nominally
bicarbonate-free and acidic milieu, CAIX effected on the resting
pH
i
. Here, we conducted in vitro studies in the absence of
extracellular bicarbonate in order not to saturate bicarbonate
transporters at the cell surface. As we showed previously (6, 35), the
presence of a high bicarbonate level (25 mmol/L) totally blunts the
effect of NHE-1 on pH
i
regulation. Thus, we reasoned that, to
investigate the putative contribution of membrane-bound CAIX and
CAXII in pH
i
regulation, it is necessary to operate in nominally
bicarbonate-free solutions exposed only to ambient CO
2
.
We also examined CAIX regulation of pH
i
in fibroblasts impaired
in NHE-1 expression (PS120 cells) to ensure the absence of
interference by this major player in pH
i
homeostasis (12, 36–38).
Nonetheless, the effect of CAIX on pH
i
was also detected in CCL39
fibroblasts and in a human colon adenocarcinoma cell line
LS174Tr expressing endogenous NHE-1. Previous studies showed
that NHE–1–deficient cells fail to grow in a range of acidic pH
o
(6.2–6.8) due to their inability to reach the permissive pH
i
values
required for DNA synthesis and ATP production (6, 7, 39). Forced
CAIX expression in this pH
i
regulation–deficient cell system was
able to restore viability of PS120 cells when exposed to a range of
acidic pH
o
(6.2–6.8). This shows the role of a pH
i
-threshold value
for growth confirming the role of CAIX in pH
i
control.
It is postulated that the mechanism by which membrane-bound
CAs regulate pH
i
occurs through the efficient uptake of HCO
3
locally formed in the ‘‘mouth’’ of CAs through Cl
/HCO
3
exchangers and/or Na
+
/HCO
3
cotransporters, forming tight
functional complexes (40). Although the ‘‘metabolon’’ is an
interesting concept, this notion has been challenged (41–43).
Interaction of the catalytic domain of CAIX with bicarbonate
transporters has also been reported and was shown to increase the
AE exchanger activity (21, 44). Future investigation is under way to
evaluate the key bicarbonate transporters coupled to CAIX and
CAXII in pH
i
regulation in hypoxic tumor cells.
In our study, invalidation of CAIX leads to partial compensa-
tion by up-regulation of CAXII. This may explain the maintenance
of the catalytic activity of ca9 invalidated cells in hypoxia and
suggest that a threshold level of activity is required for cell pH
homeostasis. In the recent study of Swietach and colleagues (32),
overexpression of CAIX down-regulated cytosolic CAII. This result
suggests that different tissues with different expression patterns
of CAs may bring into play different CA isoforms that would
‘‘communicate’’ in a yet unresolved network when confronted
with an acidic stress.
Hypoxic induction may not be the only mechanism by which
CAs regulate pH homeostasis in tumors. Signaling through the
epidermal growth factor pathway by phosphorylation of a
cytoplasmic tyrosine residue of CAIX may either activate CAIX or
enhance its expression by increasing translation of HIF-1a(30). In
addition, phosphorylation activates phosphatidylinositol 3-kinase,
resulting in phosphorylation of Akt and cell survival. The possible
implication of these CAs as signaling molecules, independent of
their function as pH-regulating enzymes, is another important
point that is under investigation and may explain reduced xeno-
graft growth of ca9 silenced cells despite maintenance of CA
catalytic activity.
It has been proposed that an acidic microenvironment promotes
metastasis associated with poor patient survival (4, 45). However,
acidosis may not always favor metastasis (4, 46). When renal
carcinoma cells were treated with ACTZ, their capacity to invade
was diminished (47) but the invasion of carcinoma cells was not
influenced by CAIX in another study (48). Here, we not only show
that CAIX and CAXII promote survival in an acidic environment in
two cell culture systems but extend it in in vivo studies. The
combined silencing of CAIX and CAXII gave a dramatic decrease in
the rate of growth of xenograft tumors that was greater than the
invalidation of HIF-1a, which may reflect the pleotropic action of
HIF-1 on prosurvival and prodeath genes. This may suggest that
the interest being paid to HIF inhibitors as anticancer treatments
CAIX and XII Promote Tumor Growth by Regulating pH
i
www.aacrjournals.org 367 Cancer Res 2009; 69: (1). January 1, 2009
American Association for Cancer Research Copyright © 2009 on October 10, 2012cancerres.aacrjournals.orgDownloaded from
DOI:10.1158/0008-5472.CAN-08-2470

(49, 50) might be better directed to the inhibition of downstream HIF
target gene products, as we recently proposed (1) and, in particular
to CAIX together with CAXII, as novel and potentially efficient drug
approaches.
The present study highlights the role of CAIX and CAXII
expression in pH
i
regulation, a key event controlling cell viability,
and in in vivo tumor growth in a hostile acidic and hypoxic
microenvironment. The results herein call for the development of
specific ‘‘CA-antagonist’’ antibodies or cell impermeable drugs
specifically targeting the membrane-associated and hypoxia-
inducible CAs, taking into consideration the CA isoform profile
of a given tumor type. Such inhibitors are being actively
investigated at the molecular and cellular levels (23, 24) and may
hold promise as effective anticancer treatments.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Received 6/27/2008; revised 10/15/2008; accepted 11/8/2008.
Grant support: LNCC (Equipe labellise´e), ANR, INCA, EUFP7 ‘METOXIA’, and
Canceropoˆle PACA. The laboratory is funded by Centre A. Lacassagne, Centre National
de la Recherche Scientifique, and Institut National de la Sante et de la Recherche
Medicale. J. Laferrie`re was a Research Fellow of the Terry Fox Foundation through an
award from the National Cancer Institute of Canada.
The costs of publication of this article were defrayed in part by the payment of page
charges. This article must therefore be hereby marked advertisement in accordance
with 18 U.S.C. Section 1734 solely to indicate this fact.
We thank Dr. Wakabayashi of the National Cardiovascular Center Research
Institute, Japan, for assistance with pH
i
determination and Drs. Zavada, Pastorekova,
and Pastorek for providing the source of the M75 antibody to CAIX (Bayer).
References
1. Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling
in cancer and approaches to enforce tumour regression.
Nature 2006;441:437–43.
2. Brahimi-Horn MC, Chiche J, Pouyssegur J. Hypoxia
signalling controls metabolic demand. Curr Opin Cell
Biol 2007;19:223–9.
3. Gullino PM, Clark SH, Grantham FH. The interstitial
fluid of solid tumors. Cancer Res 1964;24:780–96.
4. Gatenby RA, Gillies RJ. Why do cancers have high
aerobic glycolysis? Nat Rev Cancer 2004;4:891–9.
5. Roos A, Boron WF. Intracelluar pH. Physiol Rev 1981;
61:296–434.
6. Pouyssegur J, Sardet C, Franchi A, L’Allemain G, Paris S.
A specific mutation abolishing Na+/H+ antiport activity
in hamster fibroblasts precludes growth at neutral and
acidic pH. Proc Natl Acad Sci U S A 1984;81:4833–7.
7. Chambard JC, Pouyssegur J. Intracellular pH controls
growth factor-induced ribosomal protein S6 phosphor-
ylation and protein synthesis in the G0-G1 transition of
fibroblasts. Exp Cell Res 1986;164:282–94.
8. Brahimi-Horn MC, Pouyssegur J. Oxygen, a source of
life and stress. FEBS Lett 2007;581:3582–91.
9. Sardet C, Franchi A, Pouyssegur J. Molecular cloning,
primary structure, and expression of the human growth
factor-activatable Na+/H+ antiporter. Cell 1989;56:
271–80.
10. Wakabayashi S, Shigekawa M, Pouyssegur J. Molec-
ular physiology of vertebrate Na+/H+ exchangers.
Physiol Rev 1997;77:51–74.
11. Cardone RA, Casavola V, Reshkin SJ. The role of
disturbed pH dynamics and the Na+/H+ exchanger in
metastasis. Nat Rev Cancer 2005;5:786–95.
12. Shimoda LA, Fallon M, Pisarcik S, Wang J, Semenza
GL. HIF-1 regulates hypoxic induction of NHE1
expression and alkalinization of intracellular pH in
pulmonary arterial myocytes. Am J Physiol Lung Cell
Mol Physiol 2006;291:L941–9.
13. Ullah MS, Davies AJ, Halestrap AP. The plasma
membrane lactate transporter MCT4, but not MCT1, is
up-regulated by hypoxia through a HIF-1adependent
mechansm. J Biol Chem 2006;281:9030–7.
14. Pouyssegur J, Franchi A, Pages G. pHi, aerobic
glycolysis and vascular endothelial growth factor in
tumour growth. In: Novartis Found Symp; 2001:John
Wiley & Sons, Ltd; 2001. p. 186–96.
15. Alper SL. Molecular physiology of SLC4 anion
exchangers. Exp Physiol 2006;91:153–61.
16. Romero MF, Fulton CM, Boron WF. The SLC4
family of HCO
3
transporters. Pflugers Arch 2004;447:
495–509.
17. Izumi H, Torigoe T, Ishiguchi H, et al. Cellular pH
regulators: potentially promising molecular targets
for cancer chemotherapy. Cancer Treat Rev 2003;29:
541–459.
18. Ivanov S, Liao SY, Ivanova A, et al. Expression of
hypoxia-inducible cell-surface transmembrane carbonic
anhydrases in human cancer. Am J Pathol 2001;158:905–19.
19. Swietach P, Vaughan-Jones RD, Harris AL. Regulation
of tumor pH and the role of carbonic anhydrase 9.
Cancer Metastasis Rev 2007;26:299–310.
20. ThiryA,DogneJM,MasereelB,SupuranCT.
Targeting tumor-associated carbonic anhydrase IX in
cancer therapy. Trends Pharmacol Sci 2006;27:566–73.
21. Ivanov SV, Kuzmin I, Wei MH, et al. Down-regulation
of transmembrane carbonic anhydrases in renal cell
carcinoma cell lines by wild-type von Hippel-Lindau
transgenes. Proc Natl Acad Sci U S A 1998;95:12596–601.
22. Wykoff CC, Beasley NJ, Watson PH, et al. Hypoxia-
inducible expression of tumor-associated carbonic
anhydrases. Cancer Res 2000;60:7075–83.
23. Supuran CT. Carbonic anhydrases: novel therapeutic
applications for inhibitors and activators. Nat Rev Drug
Discov 2008;7:168–81.
24. Pastorekova S, Zatovicova M, Pastorek J. Cancer-
associated carbonic anhydrases and their inhibition.
Curr Pharm Des 2008;14:685–98.
25. Whittington DA, Waheed A, Ulmasov B, et al. Crystal
structure of the dimeric extracellular domain of human
carbonic anhydrase XII, a bitopic membrane protein
overexpressed in certain cancer tumor cells. Proc Natl
Acad Sci U S A 2001;98:9545–50.
26. Dayan F, Roux D, Brahimi-Horn MC, Pouyssegur J,
Mazure NM. The oxygen sensor factor-inhibiting
hypoxia-inducible factor-1 controls expression of dis-
tinct genes through the bifunctional transcriptional
character of hypoxia-inducible factor-1a. Cancer Res
2006;66:3688–98.
27. Hussain SA, Ganesan R, Reynolds G, et al. Hypoxia-
regulated carbonic anhydrase IX expression is associat-
ed with poor survival in patients with invasive breast
cancer. Br J Cancer 2007;96:104–9.
28. Zavada J, Zavadova Z, Pastorek J, Biesova Z, Jezek J,
Velek J. Human tumour-associated cell adhesion protein
MN/CA IX: identification of M75 epitope and of the
region mediating cell adhesion. Br J Cancer 2000;82:
1808–13.
29. Svastova E, Zilka N, Zat’ovicova M, et al. Carbonic
anhydrase IX reduces E-cadherin-mediated adhesion of
MDCK cells via interaction with h-catenin. Exp Cell Res
2003;290:332–45.
30. Dorai T, Sawczuk IS, Pastorek J, Wiernik PH, Dutcher
JP. The role of carbonic anhydrase IX overexpression in
kidney cancer. Eur J Cancer 2005;41:2935–47.
31. Svastova E, Hulikova A, Rafajova M, et al. Hypoxia
activates the capacity of tumor-associated carbonic
anhydrase IX to acidify extracellular pH. FEBS Lett 2004;
577:439–45.
32. Swietach P, Wigfield S, Cobden P, Supuran CT, Harris
AL, Vaughan-Jones RD. Tumor-associated carbonic
anhydrase 9 spatially co-ordinates intracellular pH in
three-dimensional multicellular growths. J Biol Chem
2008;283:20473–83.
33. van de Wetering M, Oving I, Muncan V, et al. Specific
inhibition of gene expression using a stably integrated,
inducible small-interfering-RNA vector. EMBO Rep 2003;
4:609–15.
34. Trastour C, Benizri E, Ettore F, et al. HIF-1aand CA
IX staining in invasive breast carcinomas: prognosis and
treatment outcome. Int J Cancer 2007;120:1451–8.
35. L’Allemain G, Paris S, Pouyssegur J. Growth factor
action and intracellular pH regulation in fibroblasts.
Evidence for a major role of the Na+/H+ antiport. J Biol
Chem 1984;259:5809–15.
36. Cardone RA, Bellizzi A, Busco G, et al. The NHERF1
PDZ2 Domain Regulates PKA-RhoA-p38-mediated
NHE1 Activation and Invasion in Breast Tumor Cells.
Mol Biol Cell 2007;18:1768–80.
37. Counillon L, Pouyssegur J. The expanding family of
eucaryotic Na(+)/H(+) exchangers. J Biol Chem 2000;275:
1–4.
38. Karumanchi SA, Jiang L, Knebelmann B, Stuart-Tilley
AK, Alper SL, Sukhatme VP. VHL tumor suppressor
regulates Cl-/HCO
3
exchange and Na+/H+ exchange
activities in renal carcinoma cells. Physiol Genomics
2001;5:119–28.
39. Pouyssegur J, Franchi A, L’Allemain G, Paris S.
Cytoplasmic pH, a key determinant of growth factor-
induced DNA synthesis in quiescent fibroblasts. FEBS
Lett 1985;190:115–9.
40. Sterling D, Reithmeier RAF, Casey JR. A transport
metabolon. Functional interaction of carbonic anhy-
drase II and chloride/bicarbonate exchangers. J Biol
Chem 2001;276:47886–94.
41. Becker HM, Deitmer JW. Carbonic anhydrase II
increases the activity of the human electronic Na+/
HCO
3
cotransporter. J Biol Chem 2007;282:13508–21.
42. Becker HM, Deitmer JW. Non-enzymatic proton
handling by carbonic anhydrase II during H+-lactate
cotransport via monocarboxylate transporter 1. J Biol
Chem 2008;283:21655–67.
43. Lu J, Daly CM, Parker MD, et al. Effect of human
carbonic anhydrase II on the activity of the human
electogenic Na/HCO3 cotransporter NBCe1-A in Xen-
opus Oocytes. J Biol Chem 2006;281:19241–50.
44. Morgan PE, Pastorekova S, Stuart-Tilley AK, Alper SL,
Casey JR. Interactions of transmembrane carbonic
anhydrase, CAIX, with bicarbonate transporters. Am J
Physiol Cell Physiol 2007;293:C738–48.
45. Walenta S, Mueller-Klieser WF. Lactate: mirror and
motor of tumor malignancy. Semin Radiat Oncol 2004;
14:267–74.
46. Rofstad EK, Mathiesen B, Kindem K, Galappathi K.
Acidic extracellular pH promotes experimental metas-
tasis of human melanoma cells in athymic nude mice.
Cancer Res 2006;66:6699–707.
47. Parkkila S, Rajaniemi H, Parkkila A-K, et al. Carboninc
anhydrase inhibitor suppresses invasion of renal cancer
cells in vitro. Proc Natl Acad Sci U S A 2000;97:2220–4.
48. Robertson N, Potter C, Harris AL. Role of carbonic
anhydrase IX in human tumor cell growth, survival and
invasion. Cancer Res 2004;64:6160–5.
49. Melillo G. Targeting hypoxia cell signaling for cancer
therapy. Cancer Metastasis Rev 2007;26:341–52.
50. Semenza GL. Evaluation of HIF-1 inhibitors as
anticancer agents. Drug Discov Today 2007;12:854–7.
Cancer Research
Cancer Res 2009; 69: (1). January 1, 2009 368 www.aacrjournals.org
American Association for Cancer Research Copyright © 2009 on October 10, 2012cancerres.aacrjournals.orgDownloaded from
DOI:10.1158/0008-5472.CAN-08-2470