29831 Con266117
User Manual: 29831
Open the PDF directly: View PDF ![]() .
.
Page Count: 23

Aciclovir 200mg Tablets
Aciclovir 400mg Tablets
Aciclovir 800mg Tablets
PL 29831/0517
PL 29831/0518
PL 29831/0519
UKPAR
TABLE OF CONTENTS
Lay Summary Page 2
Scientific discussion Page 3
Steps taken for assessment Page 11
Steps taken after authorisation – summary
Page 12
Summary of Product Characteristics Page 13
Product Information Leaflet/Label Page 14
MHRA PAR – Aciclovir 200mg, 400mg and 800mg Tablets (PL 29831/0517-9) - 1 -
MHRA PAR – Aciclovir 200mg, 400mg and 800mg Tablets (PL 29831/0517-9) - 2 -
Aciclovir 200mg Tablets
Aciclovir 400mg Tablets
Aciclovir 800mg Tablets
PL 29831/0517-9
LAY SUMMARY
The Medicine and Healthcare Regulatory Agency (MHRA) granted Wockhardt UK Limited Marketing
Authorisations (licences) for the medicinal products Aciclovir 200mg, 400mg and 800mg Tablets on 25
February 2013. These are prescription-only medicines (POM).
Aciclovir 200mg, 400mg and 800mg Tablets can be used to:
treat herpes and other viral infections caused by the herpes virus (varicella zoster), such as
shingles
prevent recurrent attacks of herpes simplex
help prevent those who have low immune systems from getting herpes infections.
Aciclovir Tablets should not be used to treat herpes simplex virus (HSV) infections in newborns and
babies up to 3 months old or severe HSV infections in children with low resistance to disease.
Aciclovir belongs to a group of medicines called antivirals.
These applications are duplicates of previously granted applications for Aciclovir 200mg, 400mg and
800mg Tablets (PL 29831/0001-3), which were authorised to the Marketing Authorisation Holder
Wockhardt UK Limited on 18 January 2007. Acilovir 200mg, 400mg and 800mg Tablets (PL
29831/0001-3), were originally granted Marketing Authorisations (PL 04543/0392-4) to CD
Pharmaceuticals Limited on 29 June1999. On 18 January 2007, the Marketing Authorisation Holder was
updated by a change of ownership to Wockhardt UK Limited.
No new or unexpected safety concerns arose from these simple applications and it was, therefore, judged
that the benefits of taking Aciclovir 200mg, 400mg and 800mg Tablets outweigh the risks, hence
Marketing Authorisations have been granted.
MHRA PAR – Aciclovir 200mg, 400mg and 800mg Tablets (PL 29831/0517-9) - 3 -
Aciclovir 200mg Tablets
Aciclovir 400mg Tablets
Aciclovir 800mg Tablets
PL 29831/0517-9
SCIENTIFIC DISCUSSION
TABLE OF CONTENTS
Introduction Page 4
Pharmaceutical assessment Page 5
Non-clinical assessment Page 8
Clinical assessment Page 9
Overall conclusions and risk benefit assessment Page 10

MHRA PAR – Aciclovir 200mg, 400mg and 800mg Tablets (PL 29831/0517-9) - 4 -
INTRODUCTION
The Medicine and Healthcare Regulatory Agency (MHRA) granted Wockhardt UK Limited Marketing
Authorisations for the medicinal products Aciclovir 200mg, 400mg and 800mg Tablets on 25 February
2013. These are prescription-only medicines (POM).
Aciclovir belongs to a group of medicines called antivirals and indicated for the following:
treatment of herpes simplex virus (HSV) infections of the skin and mucous
membranes including initial and recurrent genital herpes (excluding neonatal HSV
and severe HSV infections in immunocompromised children)
suppression of recurrent herpes simplex virus infections
prevention of herpes simplex virus infections in immunocompromised patients
treatment of herpes zoster infections.
The antiviral activity of aciclovir is due to intracellular conversion to an active form that inhibits viral
DNA (deoxyribonucleic acid) synthesis and replication by inhibiting the herpes virus DNA polymerase
enzyme as well as being incorporated into viral DNA. Herpes simplex virus type 1 appears to be the
most susceptible, then type 2, followed by varicella zoster virus.
The Epstein-Barr virus and cytomegalovirus are also susceptible to aciclovir to a lesser extent.
Aciclovir has no activity against latent viruses, but there is some evidence that it inhibits latent herpes
simplex virus at an early stage of reactivation.
These applications were submitted as simple abridged applications, according to Article 10(c) of
Directive 2001/83/EC as amended, cross-referring to Aciclovir 200mg, 400mg and 800mg Tablets (PL
29831/0001-3) authorised on 18 January 2007 to the Marketing Authorisation Holder Wockhardt UK
Limited. Acilovir 200mg, 400mg and 800mg Tablets (PL 29831/0001-3), were originally granted
Marketing Authorisations (PL 04543/0392-4) to CD Pharmaceuticals Limited on 29 June1999. On
18 January 2007, the Marketing Authorisation Holder was updated by a change of ownership to
Wockhardt UK Limited
No new data were submitted nor were they necessary for these simple applications, as the data are
identical to those of the previously granted cross-reference products.

MHRA PAR – Aciclovir 200mg, 400mg and 800mg Tablets (PL 29831/0517-9) - 5 -
PHARMACEUTICAL ASSESSMENT
LICENCE NO: PL 29831/0517-9
PROPRIETARY NAME: Aciclovir 200mg, 400mg and 800mg Tablets
ACTIVE(S): Aciclovir
COMPANY NAME: Wockhardt UK Limited
E.C. ARTICLE: Article 10(c) of Directive 2001/83/EC
LEGAL STATUS: POM
1. INTRODUCTION
These are simple, informed consent applications for Aciclovir 200mg, 400mg and 800mg Tablets
submitted under Article 10(c) of Directive 2001/83/EC, as amended. The proposed Marketing
Authorisation Holder is Wockhardt UK Limited, Ash Road North, Wrexham, LL13 9UF, United
Kingdom.
The applications cross-refer to Aciclovir 200mg, 400mg and 800mg Tablets
(PL 29831/0001-3), which were granted Marketing Authorisations to Wockhardt UK Limited on
18 January 2007.
The current applications are considered valid.
2. MARKETING AUTHORISATION APPLICATION FORM
2.1 Name(s)
The proposed names of the products are Aciclovir 200mg, 400mg and 800mg Tablets. The products
have been named in line with current requirements.
2.2 Strength, pharmaceutical form, route of administration, container and pack sizes
The products are for oral administration. Each tablet contains either, 200mg, 400mg or 800mg aciclovir.
Aciclovir 200mg, 400mg and 800mg Tablets are packaged in aluminium blister packs. Aciclovir 200mg
Tablets are available in packs of 25 or 100 tablets. Aciclovir 400mg Tablets are available in packs of 25,
30, 56, 60, 70 or 100 tablets. Aciclovir 800mg Tablets are available in packs of 35 tablets.
The proposed shelf-life (36 months) and storage conditions (Do not store above 25°C) are consistent
with the details registered for the cross-reference products.
2.3 Legal status
On approval, the products will be available as prescription-only medicines (POM).
2.4 Marketing Authorisation Holder/Contact Persons/Company
The Marketing Authorisation Holder is Wockhardt UK Limited, Hillbrow House, Hillbrow Road, Esher,
Surrey, KT10 9NW, United Kingdom.
The Qualified Person (QP) responsible for pharmacovigilance is stated and their CV is included.
2.5 Manufacturers
The proposed manufacturing sites are consistent with those registered for the cross-reference products
and evidence of Good Manufacturing Practice (GMP) compliance has been provided.
2.6 Qualitative and quantitative composition
The proposed compositions are consistent with the details registered for the cross-reference products.
2.7 Manufacturing process

MHRA PAR – Aciclovir 200mg, 400mg and 800mg Tablets (PL 29831/0517-9) - 6 -
The proposed manufacturing process is consistent with the details registered for the cross-reference
products and the maximum batch sizes are stated.
2.8 Finished product/shelf-life specification
The proposed finished product specifications are in line with the details registered for the cross-reference
products.
2.9 Drug substance specification
The proposed drug substance specifications are consistent with the details registered for the cross-
reference products.
European Directorate for the Quality of Medicines (EDQM) Certificates of Suitability for the drug
substance manufacturers has been provided to support the manufacturing and control of the active
substance. These details are in line with those of the reference products.
2.10 TSE Compliance
With the exception of gelatine, lactose and magnesium stearate, none of the excipients contain materials
of animal or human origin. A declaration that gelatine, lactose and magnesium stearate are free from
BSE/TSE risk was provided. This is consistent with the cross-reference products.
None of the excipients are sourced from genetically modified organisms.
2.11 Bioequivalence
No bioequivalence data are required to support these simple abridged informed consent applications, as
the proposed products are manufactured to the same formula utilising the same process as the
cross-reference products Aciclovir 200mg, 400mg and 800mg Tablets
(PL 29831/0001-3).
3. EXPERT REPORTS
The applicant cross-refers to the data for Aciclovir 200mg, 400mg and 800mg tablets (PL 29831/0001-
3), to which they claim identicality. This is acceptable.
4. PRODUCT NAME & APPEARANCE
See 2.1 for details of the proposed product names. The appearance of the products is identical to the
respective cross-reference products.
5. SUMMARY OF PRODUCT CHARACTERISTICS (SmPC)
The proposed SmPCs are consistent with the details registered for the cross-reference products.
6. PATIENT INFORMATION LEAFLET (PIL) AND LABELLING
PIL
The patient information leaflet has been prepared in line with the details registered for the cross-
reference products.
This PIL was submitted to the MHRA along with results of consultations with target patient groups
(‘user testing’), in accordance with Article 59 of Council Directive 2001/83/EC. The results indicate that
the leaflet is well-structured and organised, easy to understand and written in a comprehensive manner.
The test shows that the patients/users are able to act upon the information that it contains.
As the leaflet for the reference products and these products are considered the same, no further user
testing of the leaflet for these products is necessary.
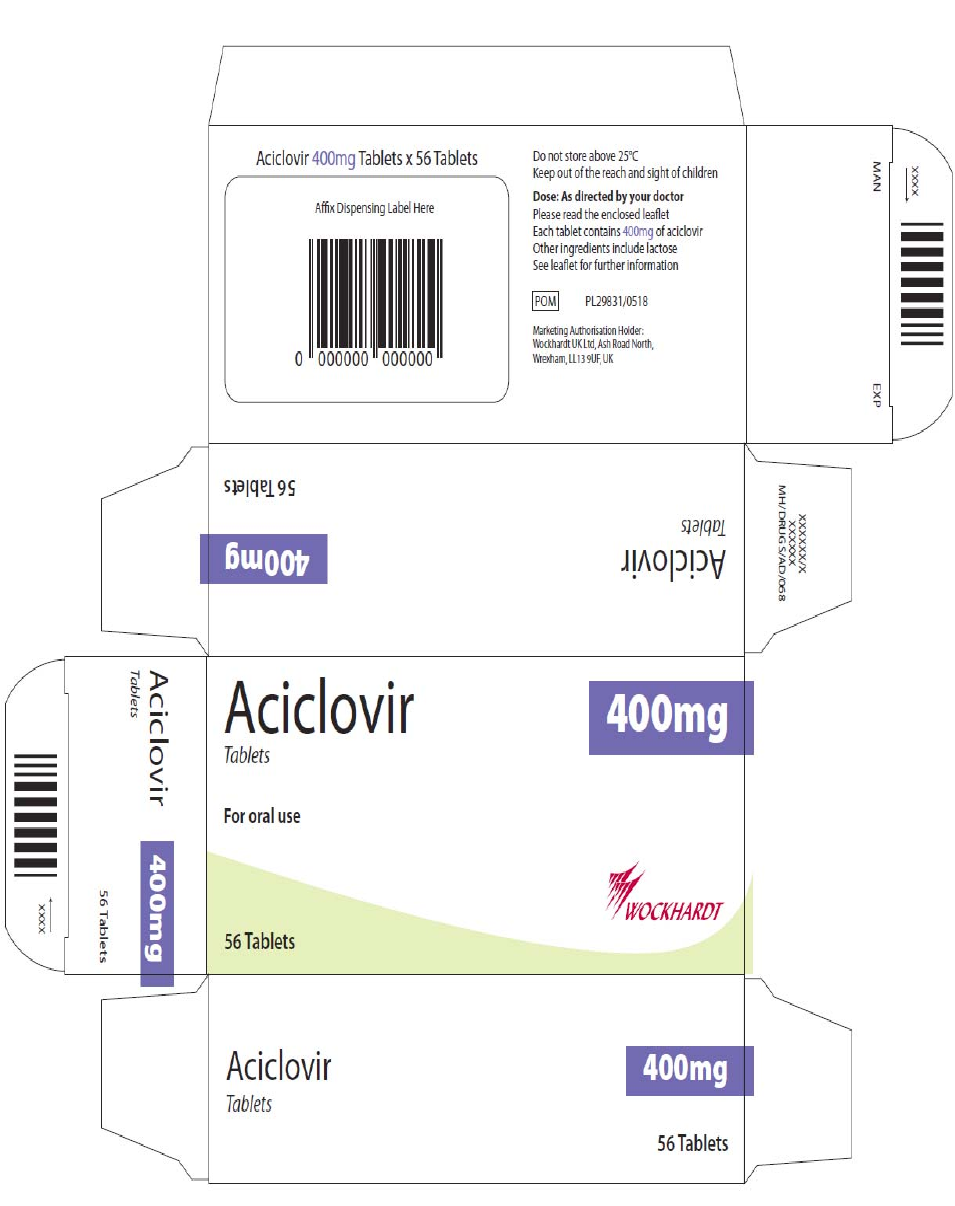
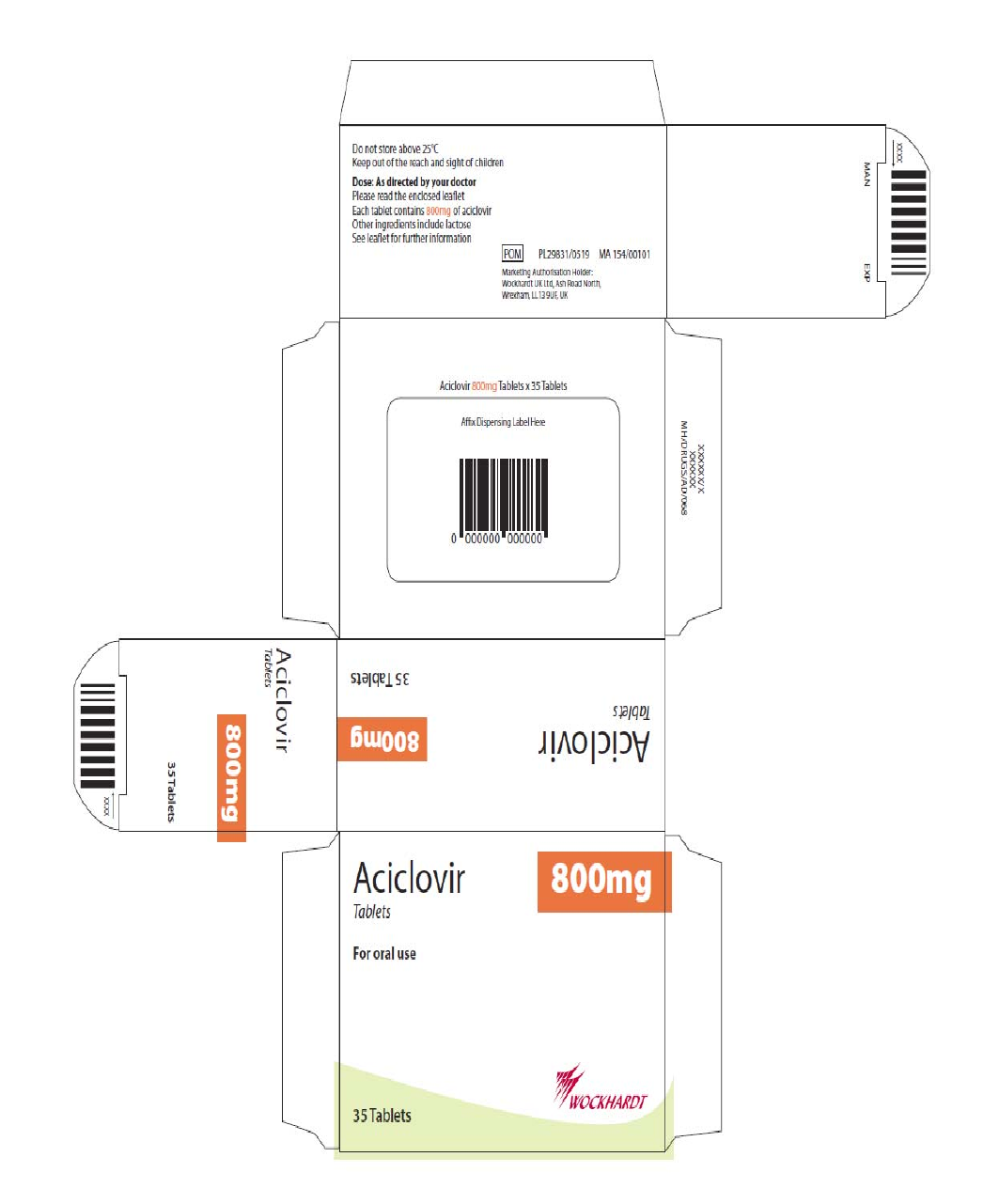
Carton and blister
The proposed artwork is comparable to the artwork registered for the cross-reference products and
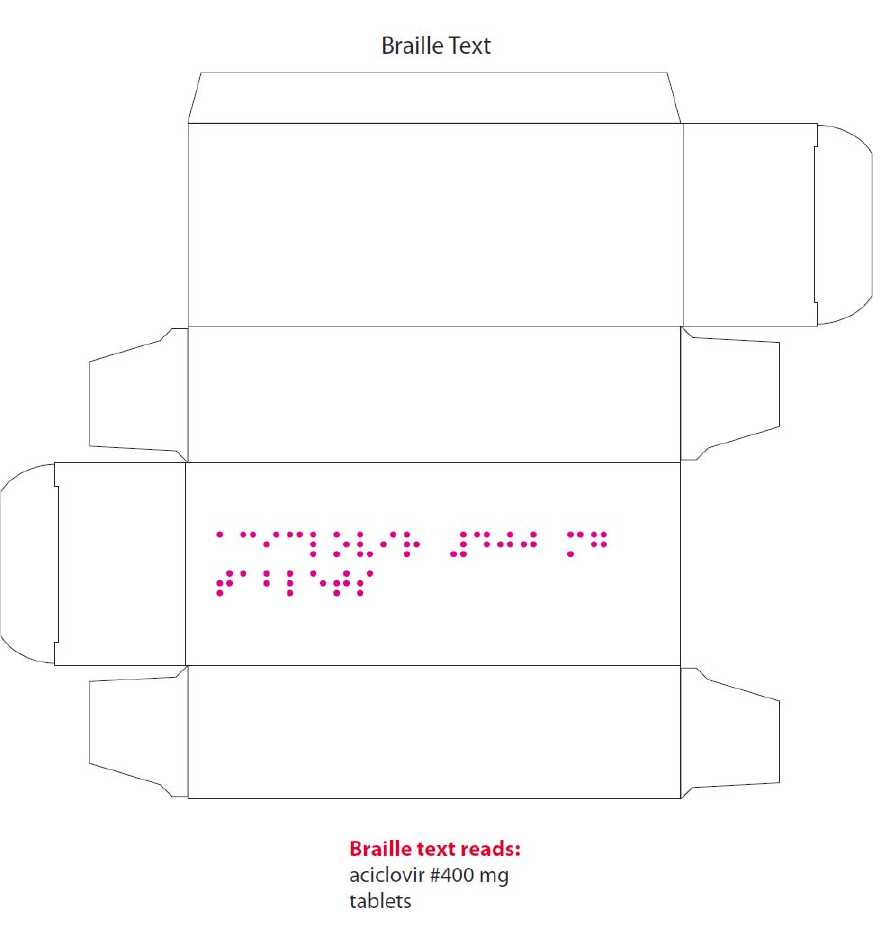
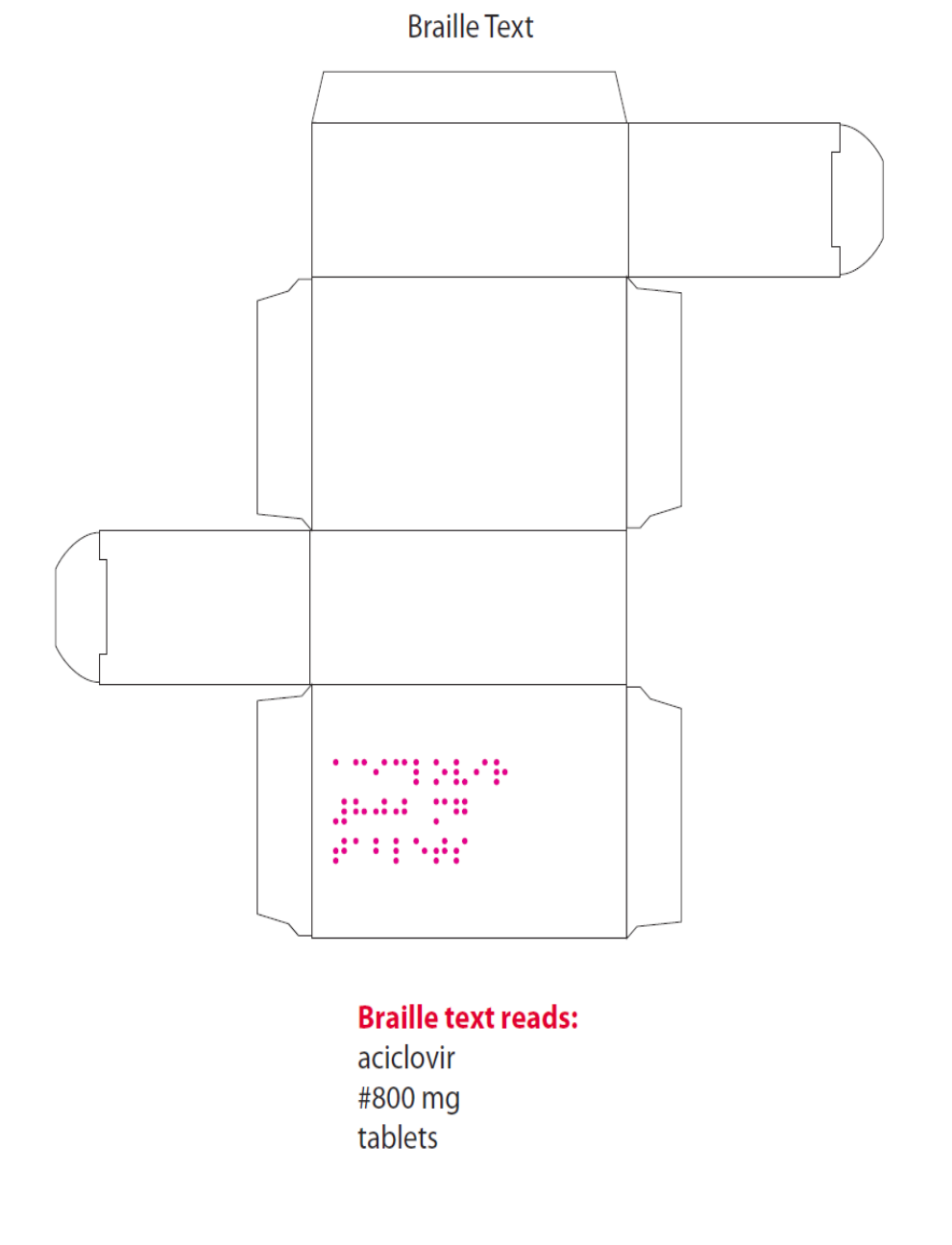
complies with statutory requirements. In line with current legislation, the applicant has also included the
name of the products in Braille on the outer packaging.
MHRA PAR – Aciclovir 200mg, 400mg and 800mg Tablets (PL 29831/0517-9) - 7 -
7. CONCLUSIONS
The data submitted with these applications are acceptable. The grant of Marketing Authorisations is
recommended.

MHRA PAR – Aciclovir 200mg, 400mg and 800mg Tablets (PL 29831/0517-9) - 8 -
NON-CLINICAL ASSESSMENT
No new non-clinical data have been submitted with these applications and none are required for
applications of this type.
The Marketing Authorisation Holder has provided adequate justification for not submitting an
Environment Risk Assessment (ERA). As the applications are for identical versions of an already
authorised reference products, it is not expected that the environmental exposure to aciclovir will
increase following the marketing approval of the products.
The grant of Marketing Authorisations is recommended.

MHRA PAR – Aciclovir 200mg, 400mg and 800mg Tablets (PL 29831/0517-9) - 9 -
CLINICAL ASSESSMENT
No new clinical data have been submitted with these applications and none are required for applications
of this type.
The Marketing Authorisation Holder has provided details of a suitable pharmacovigilance system that
fulfils the requirements and provides adequate evidence that the Marketing Authorisation Holder has the
services of a qualified person responsible for pharmacovigilance and has the necessary means for the
notification of any adverse reaction suspected of occurring either in the Community or in a third country.
The Marketing Authorisation Holder has provided an adequate justification for not submitting a Risk
Management Plan (RMP). As the applications are for identical version of already authorised reference
products, for which safety concerns requiring additional risk minimisation have not been identified, a
risk minimisation system is not considered necessary. The reference products have been in use for many
years and the safety profile of the active ingredient is well-established.
The grant of Marketing Authorisations is recommended.
MHRA PAR – Aciclovir 200mg, 400mg and 800mg Tablets (PL 29831/0517-9) - 10 -
OVERALL CONCLUSION AND BENEFIT/RISK ASSESSMENT
QUALITY
The data for these applications are consistent with those previously assessed for the cross-reference
products and as such have been judged to be satisfactory.
NON-CLINICAL
No new non-clinical data were submitted and none are required for applications of this type.
EFFICACY
These applications are identical to the previously granted applications for Aciclovir 200mg, 400mg and
800mg Tablets (PL 29831/0001-3; Wockhardt UK Limited).
SAFETY
No new or unexpected safety concerns arise from these applications.
PRODUCT LITERATURE
The SmPCs, leaflet and labelling are satisfactory and consistent with those for the cross-reference
products.
BENEFIT/RISK ASSESSMENT
The quality of the products is acceptable and no new non-clinical or clinical safety concerns have been
identified. The applicant’s products are identical to the cross-reference products. Extensive clinical
experience with aciclovir is considered to have demonstrated the therapeutic value of the compound.
The benefit/risk is, therefore, considered to be positive.
MHRA PAR – Aciclovir 200mg, 400mg and 800mg Tablets (PL 29831/0517-9) - 11 -
Aciclovir 200mg Tablets
Aciclovir 400mg Tablets
Aciclovir 800mg Tablets
PL 29831/0517-9
STEPS TAKEN FOR ASSESMENT
1 The MHRA received the marketing authorisation applications on 18 April 2012.
2 Following standard checks and communication with the applicant the MHRA
considered the applications valid on 31 May 2012.
3 Following assessment of the applications the MHRA requested further information on
29 June 2012.
4 The applicant responded to the MHRA’s request, providing further information on
07 August 2012.
5 The applications were determined on 25 February 2013.
MHRA PAR – Aciclovir 200mg, 400mg and 800mg Tablets (PL 29831/0517-9) - 12 -
Aciclovir 200mg Tablets
Aciclovir 400mg Tablets
Aciclovir 800mg Tablets
PL 29831/0517-9
STEPS TAKEN AFTER ASSESSMENT
Date
submitted Application
type Scope Outcome
MHRA PAR – Aciclovir 200mg, 400mg and 800mg Tablets (PL 29831/0517-9) - 13 -
Aciclovir 200mg Tablets
Aciclovir 400mg Tablets
Aciclovir 800mg Tablets
PL 29831/0517-9
SUMMARY OF PRODUCT CHARACTERISTICS
The Summary of Product Characteristics for these products are published on the MHRA website.
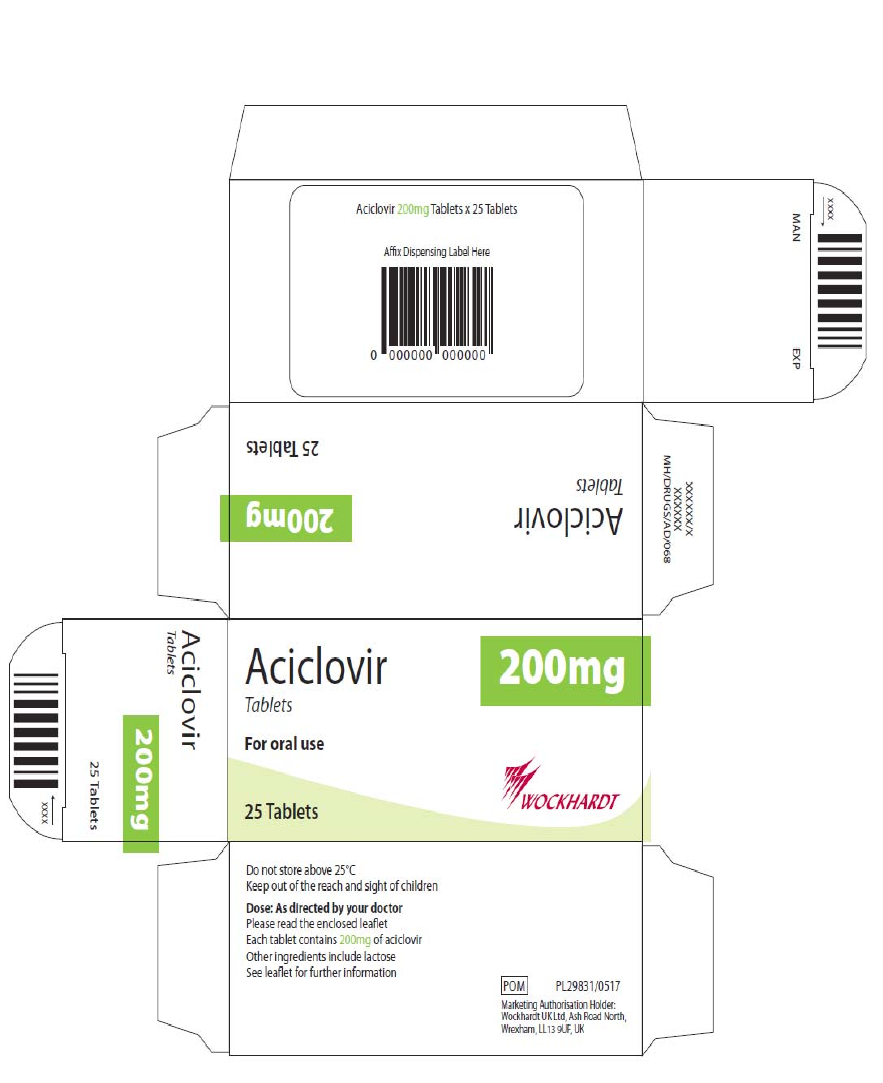
PRODUCT INFORMATION LABEL/LEAFLET
MHRA PAR – Aciclovir 200mg, 400mg and 800mg Tablets (PL 29831/0517-9) - 14 -
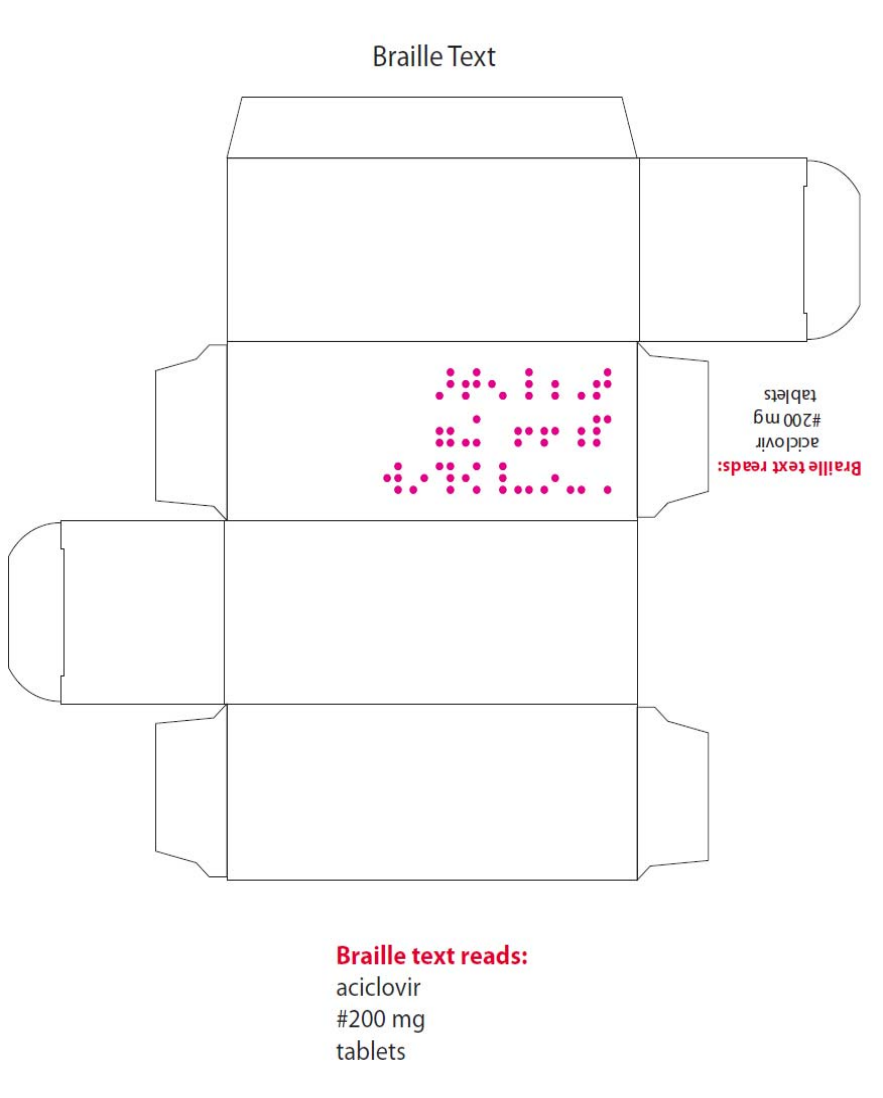
MHRA PAR – Aciclovir 200mg, 400mg and 800mg Tablets (PL 29831/0517-9) - 15 -

MHRA PAR – Aciclovir 200mg, 400mg and 800mg Tablets (PL 29831/0517-9) - 16 -
MHRA PAR – Aciclovir 200mg, 400mg and 800mg Tablets (PL 29831/0517-9) - 17 -
MHRA PAR – Aciclovir 200mg, 400mg and 800mg Tablets (PL 29831/0517-9) - 18 -
MHRA PAR – Aciclovir 200mg, 400mg and 800mg Tablets (PL 29831/0517-9) - 19 -
MHRA PAR – Aciclovir 200mg, 400mg and 800mg Tablets (PL 29831/0517-9) - 20 -
MHRA PAR – Aciclovir 200mg, 400mg and 800mg Tablets (PL 29831/0517-9) - 21 -
MHRA PAR – Aciclovir 200mg, 400mg and 800mg Tablets (PL 29831/0517-9) - 22 - MHRA PAR – Aciclovir 200mg, 400mg and 800mg Tablets (PL 29831/0517-9) - 22 -
MHRA PAR – Aciclovir 200mg, 400mg and 800mg Tablets (PL 29831/0517-9) - 23 -
PRODUCT INFORMATION LEAFLET
The Patient Information Leaflet for these products is published on the MHRA website.