WISEWOMAN Program MDE Manual Version 9.04 9 03 Revised
User Manual:
Open the PDF directly: View PDF ![]() .
.
Page Count: 182 [warning: Documents this large are best viewed by clicking the View PDF Link!]
- 1. Introduction
- 2. Administrative MDE Specifications
- Item 0a: MDEver
- Item 1a: StFIPS
- Item 1b: HdANSI
- Item 1c: EnrollSiteID
- Item 1d: ScreenSiteID
- Item 2a: TimePer
- Item 2b: NScreen
- Item 2c: Type
- Item 3a: EncodeID
- 3. Screening and Assessment MDE Specifications
- Item 3b: ResANSI
- Item 3c: ZIP
- Item 3d: MYB
- Item 3e: Latino
- Item 3f: Race1
- Item 3g: Race2
- Item 3h: Education
- Item 3i: Language
- Item 4a: SRHC
- Item 4b: SRHB
- Item 4c: SRD
- Item 4d: SRHA
- Item 5a: HCMeds
- Item 5b: HBPMeds
- Item 5c: DMeds
- Item 5d: HCAdhere
- Item 5e: HBPAdhere
- Item 5f: DAdhere
- Item 6a: BPHome
- Item 6b: BPFreq
- Item 6c: BPSend
- Item 7a: Fruit
- Item 7b: Vegetables
- Item 7c: Fish
- Item 7d: Grains
- Item 7e: Sugar
- Item 7f: SaltWatch
- Item 8a: PAMod
- Item 8b: PAVig
- Item 9a: Smoker
- Item 9b: Sechand
- Item 10a: QOLPH
- Item 10b: QOLMH
- Item 10c: QOLEffect
- Item 11a: Height
- Item 11b: Weight
- Item 11c: Waist
- Item 11d: Hip
- Item 12a: BPDate
- Item 12b: SBP1
- Item 12c: DBP1
- Item 12d: SBP2
- Item 12e: DBP2
- Item 13a: Fast
- Item 14a: TCDate
- Item 14b: TotChol
- Item 14c: HDL
- Item 14d: LDL
- Item 14e: Trigly
- Item 15a: BGDate
- Item 15b: Glucose
- Item 15c: A1C
- Item 16a: BPAlert
- Item 16b: BPDiDate
- Item 16c: BGAlert
- Item 16d: BGDiDate
- 4. Risk Reduction Counseling MDE SPecifications
- Item 17a: RRCDate
- Item 17b: RRCComplete
- Item 17c: RRCNut
- Item 17d: RRCPA
- Item 17e: RRCSmoke
- Item 17f: RRCMedAdhere
- Item 18a: RTCDate
- Item 18b: RTC
- 5. Healthy Behavior SUpport Options MDE Specifications
- Item 19a: RefDate
- Item 20a: LSPHCRec
- Item 20b: Intervention
- Item 20c: LSPHCID
- Item 20d: LSPHCTime
- Item 20e: ContactType
- Item 20f: Setting
- Item 20g: LSPHCComp
- Item 21a: TobResDate
- Item 21b: TobResType
- Item 21c: TResComp
- Appendix A: MDE Submission
- Appendix B: Data Quality and Validation
- Appendix C: Data Analysis and Use
- Appendix D: Technical Assistance Resources
- Appendix E: MDE editionCrosswalk
- Appendix F: Performance Measures
- Appendix G: Examples from American Heart Association’s Life’s Simple 7
- Appendix H: WISEWOMAN Health Risk Assessment MDE Elements
- Appendix I: Submitting records for navigated women
- Appendix J: Other Documents and Communication

OMB Approved
WISEWOMAN
Program
MDE Manual
Edition 9.03 revised
Public reporting burden of this collection of information is estimated to average 24 hours per
program including the time for reviewing instructions, searching existing data sources,
gathering and maintaining the data needed, and completing and reviewing the collection of
information. An agency may not conduct or sponsor, and a person is not required to respond
to a collection of information it displays a currently valid OMB control number. Send comments
regarding this burden estimate or any other aspect of this collection of information, including
suggestions for reducing this burden to CDC/ATSDR Reports, Clearance Officer, 1600 Clifton
Road NE, MS D-74, Atlanta, GA 30333. Attn: PRA (0920-0612). Do not send the completed
form to this address.
OMB Number: 0920-0612
Expiration Date: 12/31/2018
OMB Approved
OMB Approved
WISEWOMAN MDE Manual i August 2017
Edition 9.03 revised
CONTENTS
1. INTRODUCTION .................................................................................................................. 1
2. ADMINISTRATIVE MDE SPECIFICATIONS ........................................................................ 5
a. Summary of Administrative MDEs .................................................................................. 6
b. Administrative MDE Specifications ................................................................................. 7
3. SCREENING AND ASSESSMENT MDE SPECIFICATIONS .............................................. 16
a. Summary of Screening and Assessment MDEs ............................................................ 18
b. Screening and Assessment MDE Specifications ........................................................... 22
4. RISK REDUCTION COUNSELING MDE SPECIFICATIONS ............................................. 84
a. Summary of Risk Reduction Counseling MDEs ............................................................ 85
b. Risk Reduction Counseling MDE Specifications ........................................................... 86
5. HEALTHY BEHAVIOR SUPPORT OPTIONS MDE SPECIFICATIONS ............................ 95
a. Summary of Healthy Behavior Support Options MDEs ................................................. 96
b. Healthy Behavior Support Options MDE Specifications ................................................ 97
APPENDIX A: MDE SUBMISSION ......................................................................................... A-1
Submission Dates ............................................................................................................. A-1
Data Submission Options .................................................................................................. A-1
Direct Data Entry into the MDE Data Management System ............................................... A-1
Bulk Data Submissions ...................................................................................................... A-2
Submission Procedures ..................................................................................................... A-4
Data Confidentiality and Security ....................................................................................... A-4
WISEWOMAN Data Submission Form .............................................................................. A-6
APPENDIX B: DATA QUALITY AND VALIDATION ............................................................... B-1
Validation of Data .............................................................................................................. B-1
Validation Report Format and Contents ............................................................................. B-2
Validation Report Format and Contents ........................................................................... B-14
Data Validation Procedures and Forms ........................................................................... B-14
Error Rate Calculation Method ........................................................................................ B-15
Validation of Data Form ................................................................................................... B-16
Participant ID Change Form ............................................................................................ B-17
Correction to Previous MDE File Form ............................................................................ B-18
APPENDIX C: DATA ANALYSIS AND USE........................................................................... C-1
Data Summary Report Format and Content....................................................................... C-1
Data Use by CDC .............................................................................................................. C-1
Potential Data Use by Funded Programs ........................................................................... C-2
OMB Approved
WISEWOMAN MDE Manual ii August 2017
Edition 9.03 revised
APPENDIX D: TECHNICAL ASSISTANCE RESOURCES ..................................................... D-1
Types of Technical Assistance Available ........................................................................... D-1
Helpdesk for Individualized Technical Assistance Requests .............................................. D-3
APPENDIX E: MDE EDITIONCROSSWALK .......................................................................... E-1
Crosswalk of Changes Between MDE Edition8.2 and 9.00 ................................................ E-1
MDE Items Removed Between MDE Versions 8.2 and 9.00 ............................................ E-10
APPENDIX F: PERFORMANCE MEASURES ........................................................................ F-1
APPENDIX G: EXAMPLES FROM AMERICAN HEART ASSOCIATION’S LIFE’S
SIMPLE 7 ........................................................................................................................ G-1
APPENDIX H: WISEWOMAN HEALTH RISK ASSESSMENT MDE ELEMENTS .................. H-1
APPENDIX I: SUBMITTING RECORDS FOR NAVIGATED WOMEN ..................................... I-1
APPENDIX J: OTHER DOCUMENTS AND COMMUNICATION ............................................ J-1
OMB Approved

OMB Approved
WISEWOMAN MDE Manual 1 August 2017
Edition 9.03 revised
1. INTRODUCTION
This WISEWOMAN MDE Manual was written to provide guidance on the
collection and submission of minimum data elements (MDEs) for the Well-
Integrated Screening and Evaluation for Women Across the Nation
(WISEWOMAN) Program of the Centers for Disease Control and Prevention
(CDC). The Program currently funds 21 grantees across the United States to
improve cardiovascular health among low-income, underinsured, and uninsured
women ages 40 to 64. Grantees are required to collect and report MDEs as part
of standardized data reporting for the WISEWOMAN Program.1 MDEs are used
by CDC and its grantees to describe, monitor, and assess progress and
performance.
This manual is for MDE Edition 9.03 revised, which has been approved for
collection by the Federal Office of Management and Budget (OMB clearance
#0920-0612, expiration 12/31/2018). MDE Edition 9.03 revised pertains to data
collected under the cooperative agreement DP13-1302. Data for the 84 required
MDEs can be separated into several categories: Administrative, Screening and
Assessment, Risk Reduction Counseling, and Healthy Behavior Support Options.
The MDE manual includes information about technical specifications for the MDE
variables included in each of the categories, guidance for their submission, and
conventions for processing the data. Specifications for each MDE include
variable name, format, definition, allowed values, description, and use for
analysis. Please note that the format provided is relevant for data submitted
by grantees for a six-month reporting period and the final analytic file
generated by the data contractor may include a different format. Each
variable is reported for a participant and their values compose the record for a
unique woman. The manual is organized as follows:
• Administrative MDE Specifications. This category includes 9 MDE
variables. It includes data about the grantee program, including its
geography, provider sites, aggregate screenings, and unique IDs of
women for tracking purposes.
• Screening and Assessment MDE Specifications. This category
contains 56 required MDE variables. It includes data about participant
demographics; cardiovascular health status and history; clinical
assessment values; and treatment status.
• Risk Reduction Counseling MDE Specifications. This category
contains 8 required MDE variables. It includes data about the risk
reduction counseling received by participants and their readiness to
change.
1 Throughout this document, capital “Program” refers to the CDC WISEWOMAN Program, and lower-case “program”
refers to the CDC-funded state/tribal programs (grantees).
OMB Approved
WISEWOMAN MDE Manual 2 August 2017
Edition 9.03 revised
• Healthy Behavior Support Options MDE Specifications. This category
contains 11 required MDE variables. It includes data about the Lifestyle
Program/Health Coaching sessions available and received by participants
as well as referrals to community-based tobacco cessation resources.
• Appendix A—MDE Submission. Data are required to be submitted
semiannually. This appendix details important dates for each submission,
grantee options for submission of data, the procedures for each type of
submission option, and data confidentiality and security guidance.
• Appendix B—Data Quality and Validation. To promote high-quality,
consistent data across grantees, several tools are provided for use by
grantees prior to MDE submission and by CDC after submission. This
appendix describes the various validation procedures that grantees can
use prior to submission and that CDC uses to assess data quality. It also
details the format and contents of data quality reports; error and quality
check messages generated from the data validation; the data validation
procedure; and forms. In addition, the method used to calculate error rates
is provided.
• Appendix C—Data Analysis and Use. MDEs have several analytic
purposes for CDC and grantees, including monitoring of program progress
and performance; identification of areas for program improvement, data
quality improvement, and technical assistance; and evaluation of program
effect. This appendix describes the summary report format and the
content produced and provided to grantees after each submission. It also
discusses use of the data by CDC as well as potential ways in which
grantees can use the data.
• Appendix D—Technical Assistance Resources. Several technical
assistance resources are available to support grantees’ MDE data
collection and reporting. This appendix describes the various types of
technical assistance resources that grantees may access, including one-
on-one technical assistance, group trainings, documents, and tools
available on the WISEWOMAN website. It also describes the process for
requesting individual technical assistance and the response process for
CDC and the data contractor.
• Appendix E—MDE Crosswalk. This manual represents Edition 9.03
revised of the WISEWOMAN MDE Manual, and this appendix provides a
crosswalk of key changes between the edition of the manual (8.2) under
the previous cooperative agreement and the current edition. Changes in
Edition 9.03 revised reflect the shift in focus of the WISEWOMAN program
under the new cooperative agreement toward risk reduction, hypertension
control, and clinical-community linkages.
OMB Approved
WISEWOMAN MDE Manual 3 August 2017
Edition 9.03 revised
• Appendix F—Performance Measures. MDEs will be used to calculate
six of seven of the Program’s performance measures. This appendix
provides a list of all Program performance measures, indicating which
ones will be calculated using MDEs.
• Appendix G – Examples from the American Heart Association’s
Life’s Simple 7. This appendix includes a supplemental handout with
examples for MDE items from the American Heart Association’s Life’s
Simple 7.
• Appendix H – WISEWOMAN Health Risk Assessment MDE Elements.
This appendix includes a supplemental handout that outlines the criteria
for a health risk assessment and the associated minimum data elements.
• Appendix I —Other Documents and Communications. This appendix
is for use by grantees that have printed out a hard copy of the manual. It
provides a place to insert data-related documents and communications
that are not part of this manual and may come from CDC or the data
contractor (such as a document containing frequently asked questions).
This manual is a living document that will be updated from time to time. When
changes are made to it, CDC will notify grantees that the updated manual is
available on the WISEWOMAN website [https://partner.cdc.gov/]. Grantees may
choose to download and replace specific pages or sections with changes or
download the entire updated manual.
OMB Approved
OMB Approved
WISEWOMAN MDE Manual 5 August 2017
Edition 9.03 revised
2. ADMINISTRATIVE MDE SPECIFICATIONS
This section provides grantees with the information necessary to support
collection and reporting of administrative MDEs, which must be done according
to the specifications provided in this section of the manual.
These variables provide key contextual information about the structure and
operations of grantee program and are essential for tracking the services
provided through the program. For each participant record, programs must
provide the MDE edition used to collect the data and FIPS/ANSI code of the
program. In addition, for the six-month submission period grantees must report
for each participant the enrollment and screening site, the type of screening
received, and unique participant ID. Missing or invalid values for these variables
will be considered errors.
This section begins with a summary of the 9 required variables (Subsection a)
and then provides the technical specifications for each variable (Subsection b).

OMB Approved
WISEWOMAN MDE Manual 6 August 2017
Edition 9.03 revised
a. Summary of Administrative MDEs
Item
Number Variable
Name Position
Possible
Rounds of
Collection1 Variable Label Type
0a MDEVer 1 1 MDE version Numeric
1a StFIPS 4 1 State/Tribal FIPS code Character
1b HdANSI 6 1 ANSI Geographic code
(provider) Character
1c EnrollSiteID 11 1 Enrollment site ID Character
1d ScreenSiteID 16 1 Screening site ID Numeric
2a TimePer 26 1 Time period of screening Numeric
2b NScreen 27 1 Number of screening cycles
received by the participant Numeric
2c Type 29 2 Type of screening visit Numeric
3a EncodeID 31 1 Unique participant ID number Character
1 Number of times the item may be collected during the screening cycle. For example, for an item
with 2 possible rounds for data collection, a value may be provided at both baseline
screening/rescreening and at follow-up assessment.

OMB Approved
WISEWOMAN MDE Manual 7 August 2017
Edition 9.03 revised
b. Administrative MDE Specifications
Item 0a: MDEver MDE Version
This variable indicates the version of the MDE that was used to collect and report data in the file.
FORMAT Type: Numeric
Item Length: 3
Field Length: 3
Leading Zeros: No
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 1
Valid Range See values; cannot be blank
SOURCE Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening.
VALUES AND
DESCRIPTION 904 MDE version 9.04 MDE version 9.04 should be used to collect and report data
associated with screening visits conducted using this version of the
MDEs
ANALYSIS AND USE To verify the MDE version used to collect and report data the file
OTHER
INFORMATION Guidance
A crosswalk table between version 8.2 and 9.00 is available in Appendix E.
A valid record, at a minimum, includes participants with a valid blood pressure date (12a: BPDate)
and valid values (as described on page 17 of this manual) for the following at baseline and
rescreening visits:
• Month and year of birth (3d);
• Previous cardiovascular disease risk [high cholesterol, hypertension, diabetes, coronary
heart disease/chest pain, heart attack, heart failure, stroke/TIA, vascular disease, or
congenital heart defects (4a-4d)];
• Use of medications to lower cholesterol, blood pressure, or blood sugar (5a – 5c);
• Diet [consumption of fruits, vegetables, fish, whole grains, and beverages with added
sugar (7a-7e)];
• Physical activity [moderate and vigorous physical activity (8a and 8b)];
• Smoking status (9a);
• Biometric screening measures [height and weight (11a and 11b), and first systolic blood
pressure (12b), diastolic blood pressure (12c), total cholesterol (14b), and glucose (15b)
or A1C (15c)]
If a record does not meet these criteria and the program would like CDC to consider including it, the
validation form in Appendix B should be used to valida
te both the record and missing
measurement/health history question.
OMB Approved
WISEWOMAN MDE Manual 8 August 2017
Edition 9.03 revised
Item 1a: StFIPS State/Tribal FIPS Code
This variable indicates the FIPS or tribal program code for the state or tribe where the administration
of the program is located.
FORMAT Type: Character
Item Length: 2
Field Length: 2
Leading Zeros: Yes
Static Field: Yes
Other Format: N/A
Justification: Left
Beginning Position: 4
Valid Range: See values; cannot
be blank
SOURCE National FIPS Code List
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION 01 Alabama (AL) Program administration located in Alabama
06 California (CA) Program administration located in California
08 Colorado (CO)
Program administration located in Colorado
09 Connecticut (CT) Program administration located in Connecticut
17 Illinois (IL) Program administration located in Illinois
18 Indiana (IN)
Program administration located in Indiana
19 Iowa (IA) Program administration located in Iowa
26 Michigan (MI) Program administration located in Michigan
29 Missouri (MO)
Program administration located in Missouri
31 Nebraska (NE) Program administration located in Nebraska
37 North Carolina (NC) Program administration located in North Carolina
41 Oregon (OR) Program administration located in Oregon
42 Pennsylvania (PA) Program administration located in Pennsylvania
44 Rhode Island (RI) Program administration located in Rhode Island
45 South Carolina (SC) Program administration located in South Carolina
49 Utah (UT) Program administration located in Utah
50 Vermont (VT) Program administration located in Vermont
54 West Virginia (WV) Program administration located in West Virginia
55 Wisconsin (WI)
Program administration located in Wisconsin
85 Southeast Alaska Regional Health
Consortium (SEARHC) Program administration located within the tribal
area of SEARHC
92 Southcentral Foundation (SCF) Program administration located within the tribal
area of SCF
ANALYSIS AND USE To calculate the number of women screened by each state or tribal program
To assess the reach of the WISEWOMAN Program nationally and within a particular state or tribe
OTHER
INFORMATION Guidance
The state FIPS codes are the Federal Information Processing Standard codes developed by the
National Institute of Standards and Technology. The tribal program codes are codes assigned by
CDC to be used by tribal programs in lieu of FIPS.
Additional edits
Programs should always record the FIPS code for the state or tribe where their program is located.
This may differ from the FIPS code for the participant’s state or tribe of residence if the participant
resides in a state or tribe different from where the program is located. Any FIPS code that is not the
same as where the program is located will be flagged as an error.
OMB Approved
WISEWOMAN MDE Manual 9 August 2017
Edition 9.03 revised
Item 1b: HdANSI ANSI Geographic Code (Provider)
This indicates the ANSI geographic code of the provider that conducts the WISEWOMAN screening
office visit.
FORMAT Type: Character
Item Length: 5
Field Length: 5
Leading Zeros: Yes
Static Field: No
Other Format: N/A
Justification: Left
Beginning Position: 6
Valid Range: Valid ANSI code
SOURCE National ANSI Code List, Census Bureau
DENOMINATOR
POPULATION The denominator includes all valid screenings
VALUES AND
DESCRIPTION ANSI Geographic Code Five-digit (character) value representing the geographic area of the
provider that conducts the screening office visit
ANALYSIS AND USE To assess whether programs and specific providers are meeting screening goals in targeted
geographic areas
To identify geographic areas where women have access to the WISEWOMAN Program
To provide information for GIS analysis
To assist in identifying areas where there may be potential transportation barriers to accessing
WISEWOMAN services
OTHER INFORMATION ANSI codes are the American National Standards Institute codes, which were developed by the
American National Standards Institute. They are five-digit codes that represent states, counties, and
statistically equivalent areas, along with American Indian and Alaska Native areas.
The first two digits of the provider ANSI geographic code should represent the state of the provider
that conducts the screening office visit, and the last three digits should represent the provider’s
county.
OMB Approved
WISEWOMAN MDE Manual 10 August 2017
Edition 9.03 revised
Item 1c: EnrollSiteID Enrollment Site ID
This variable indicates the site of a woman’s enrollment into the WISEWOMAN Program.
FORMAT Type: Character
Item Length: 5
Field Length: 5
Leading Zeros: N/A
Static Field: Yes
Other Format: N/A
Justification: Left
Beginning Position: 11
Valid Range: Valid ZIP code; cannot
be blank
SOURCE Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION Enrollment Site ID Valid five-digit ZIP code for the person administering enrollment of
participant
ANALYSIS AND USE To identify sites where outreach and enrollment are occurring
To identify sites where the Program is being administered and participants are tracked
To track the number of WISEWOMAN participants enrolled at each WISEWOMAN enrollment site
OTHER
INFORMATION The enrollment site ID should be the ZIP code of the person who enrolls the participant. This may
be the ZIP code for a provider site location if a provider conducts enrollment, or the ZIP code of the
grantee location if the grantee conducts enrollment of the participant.

OMB Approved
WISEWOMAN MDE Manual 11 August 2017
Edition 9.03 revised
Item 1d: ScreenSiteID Screening Site ID
This variable indicates the site where a woman received her WISEWOMAN screening.
FORMAT Type: Numeric
Item Length: 10
Field Length: 10
Leading Zeros: N/A
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 16
Valid Range: Valid code for a
screening site; cannot
be blank
SOURCE National Provider Identifier
DENOMINATOR
POPULATION The denominator includes all valid screenings
VALUES AND
DESCRIPTION Screening Site ID Value representing a National Provider Identifier for the provider who
conducts the screening office visit
ANALYSIS AND USE To identify the geographic locations of sites providing screening services to participants
To track the number of WISEWOMAN participants screened at each WISEWOMAN screening site
To describe differences in participant demographics or other characteristics by screening site
To provide information for GIS analysis
To identify the number of screening providers in a given geographic area
To identify provider pool for assessment of health systems and providers that use clinical systems of
care successful in blood pressure control
OMB Approved
WISEWOMAN MDE Manual 12 August 2017
Edition 9.03 revised
Item 2a: TimePer Time Period of Screening
This variable indicates the 6-month time period of the baseline screening for the participant.
FORMAT Type: Numeric
Item Length: 1
Field Length: 1
Leading Zeros: No
Static Field: Yes
Other Format: N/A
Justification: Right
Beginning Position: 26
Valid Range: See values; cannot be
blank if TYPE is 1
(baseline screening)
SOURCE Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION The denominator includes all valid baseline screenings
VALUES AND
DESCRIPTION 1 6-month period 1 Baseline screening took place between 07/01/13 and 12/31/13
2 6-month period 2 Baseline screening took place between 01/01/14 and 06/30/14
3 6-month period 1 Baseline screening took place between 07/01/14 and 12/31/14
4 6-month period 2 Baseline screening took place between 01/01/15 and 06/30/15
5 6-month period 1 Baseline screening took place between 07/01/15 and 12/31/15
6 6-month period 2 Baseline screening took place between 01/01/16 and 06/30/16
7 6-month period 1 Baseline screening took place between 07/01/16 and 12/31/16
8 6-month period 2
Baseline screening took place between 01/01/17 and 06/30/17
9 6-month period 1 Baseline screening took place between 07/01/17 and 12/31/17
0 6-month period 2 Baseline screening took place between 01/01/18 and 06/30/18
ANALYSIS AND USE To track participants over the course of the cooperative agreement by their baseline screenings
To track the number of unique participants programs have screened
OTHER
INFORMATION Guidance
Time period of screening should be provided for a participant’s baseline screening, only. This field is
used to determine a unique participant for tracking purposes. Time period of baseline screening
should be determined using blood pressure date (12a – BPDate).
Time period of baseline screening should match with the date of baseline screening provided. For
example,
Error: IF TimePer =1 AND first BPDate ≠ 07/01/13 - 12/31/13 AND Type = 1

OMB Approved
WISEWOMAN MDE Manual 13 August 2017
Edition 9.03 revised
Item 2b: NScreen Number of Screening Cycles Received by the Participant
This variable indicates the total number of screening cycles that the participant has received since
the beginning of the cooperative agreement.
FORMAT Type: Numeric
Item Length: 2
Field Length: 2
Leading Zeros: Yes
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 27
Valid Range: Cannot be blank
SOURCE Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION Number of Visits Value representing the number of screening cycles that the participant
has received since the beginning of the cooperative agreement (includes
current screening cycle).
Any values outside 01 to 08 will be flagged for a quality check
ANALYSIS AND USE To track the number of screenings/rescreenings/follow-up assessments after a completed LSP/HC
that the participant has received
OTHER
INFORMATION Guidance
This field should include the number of screening cycles that the participant has received since the
beginning of the cooperative agreement. A screening cycle will include the initial screening contact
for the submission period (baseline screening or rescreening), one or more LSP/HC contacts as
assigned at the screening, and a follow-up assessment contact (that is not also considered a
rescreening) if follow-up occurred during or following completion of the LSP/HC program.
OMB Approved
WISEWOMAN MDE Manual 14 August 2017
Edition 9.03 revised
Item 2c: Type
Type of Screening Visit
This variable indicates whether the record represents a baseline screening visit, a rescreening visit,
or a post-Lifestyle Program (LSP)/Health Coaching (HC) follow-up assessment.
FORMAT
Type: Numeric
Item Length: 1
Field Length: 2
Leading Zeros: No
Static Field:
No
Other Format: N/A
Justification: Right
Beginning Position: 29
See values; cannot be blank if BPDate is valid
SOURCE
Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION
The denominator includes all valid screenings
VALUES AND
1 Screening
Record represents a baseline screening visit
DESCRIPTION
2 Rescreening
Record represents a rescreening visit
3 Follow-up assessment –
LSP/HC complete
Record represents a post-LSP/HC follow-up assessment with a
complete LSP/HC
4 Follow-up assessment –
LSP/HC incomplete Record represents a post-LSP/HC follow-up assessment with an
incomplete LSP/HC
9 No answer recorded No answer recorded
This value will be flagged as an error
ANALYSIS AND USE
To assess the number of unique women served by the WISEWOMAN Program
To track participants screening values over time
To link baseline screenings with rescreenings
To assess participants progress after completion of an LSP/HC
OTHER
INFORMATION Guidance
Screenings, including baseline screenings or rescreenings should,at a minimum, include a valid
blood pressure date (12a: BPDate) and valid values (as described on page 17 of this manual) for
the following:
• Month and year of birth (3d);
• Previous cardiovascular disease risk [high cholesterol, hypertension, diabetes, coronary
heart disease/chest pain, heart attack, heart failure, stroke/TIA, vascular disease, or
congenital heart defects (4a-4d)];
• Use of medications to lower cholesterol, blood pressure, or blood sugar (5a – 5c);
• Diet [consumption of fruits, vegetables, fish, whole grains, and beverages with added
sugar (7a-7e)];
• Physical activity [moderate and vigorous physical activity (8a and 8b)];
• Smoking status (9a);
• Biometric screening measures [height and weight (11a and 11b), and first systolic blood
pressure (12b), diastolic blood pressure (12c), total cholesterol (14b), and glucose (15b)
or A1C (15c)]
Follow-up assessments should include, at minimum, a valid follow-up assessment date, as indicated
by blood pressure date (12a: BPDate), and valid values (as described on page 18 of this manual)
for the following services:
• Use of medications to lower cholesterol, blood pressure, or blood sugar (5a – 5c);
• Blood pressure self-monitoring (6a-6c – for participants with high blood pressure or
previously diagnosed with hypertension (high blood pressure), only);
• Diet [consumption of fruits, vegetables, fish, whole grains, and
beverages with added
sugar (7a-7e)];
• Physical activity [moderate and vigorous physical activity (8a-8b)];
• Smoking or exposure to secondhand smoke (9a-9b);
• Quality of life (10a-10c)
Rescreenings should occur between 11 and 18 months following the previous
screening/rescreening.
Post-LSP/HC follow-up assessments should occur within 4 weeks of LSP/HC completion.
OMB Approved
WISEWOMAN MDE Manual 15 August 2017
Edition 9.03 revised
Item 3a: EncodeID Unique Participant ID Number
This variable indicates a woman’s unique identification number.
FORMAT Type: Character
Item Length: 15
Field Length: 15
Leading Zeros: N/A
Static Field: Yes
Other Format: N/A
Justification: Left
Beginning Position: 31
Valid Range: Cannot be blank
SOURCE Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION Unique Participant ID
Number Value representing the unique identifier for a participant
ANALYSIS AND USE To assess the number of unique women served by the WISEWOMAN Program
To track participants over time
To link baseline screenings with rescreenings
To link screenings with risk reduction counseling, lifestyle programs, health coaching, and
community-based resource referrals
OTHER
INFORMATION Guidance
A participant’s unique ID should not change over time. If it does change, the program should
provide the data contractor and Project Officer with a list of IDs that have changed at the time
of data submission and upload a crosswalk of the previous participant unique IDs to the new
participant unique IDs (see Appendix B).
If a participant’s Social Security number is used as her unique ID, it must be encoded.

OMB Approved
WISEWOMAN MDE Manual 16 August 2017
Edition 9.03 revised
3. SCREENING AND ASSESSMENT MDE SPECIFICATIONS
The purpose of this section is to provide grantees with the information necessary
to support collection and reporting of Screening and Assessment MDEs, which
must be done according to the specifications provided in this section of the
manual. Valid records are determined by MDEs provided under the Screening
and Assessment category.
A valid record, at a minimum, includes participants with a valid blood
pressure date (12a: BPDate) and valid values for the following at baseline
and rescreening visits:2
• Month and year of birth (3d);
• Previous cardiovascular disease risk [high cholesterol, hypertension,
diabetes, coronary heart disease/chest pain, heart attack, heart failure,
stroke/TIA, vascular disease, or congenital heart defects (4a-4d)];
• Use of medications to lower cholesterol, blood pressure, or blood sugar
(5a – 5c);
• Diet [consumption of fruits, vegetables, fish, whole grains, and beverages
with added sugar (7a-7e)];
• Physical activity [moderate and vigorous physical activity (8a and 8b)];
• Smoking status (9a);
• Biometric screening measures [height and weight (11a and 11b), and first
systolic blood pressure (12b), diastolic blood pressure (12c), total
cholesterol (14b), and glucose (15b) or A1C (15c)]
Grantees may report records that do not meet these screening requirements, but
they will not be analyzed in data reports generated by CDC or counted toward
screening goals unless additional documentation is provided.3,4
A Health Risk Assessment should, at a minimum, include:5
2 Values left blank or coded as unable to obtain, refused, missing, or out of range are considered invalid values for the
height variable and the first diastolic and systolic blood pressure measurement variables.
Values are considered invalid for the glucose variable if: (1) participant is fasting and glucose is left blank, coded as
missing, or out of range; or (2) participant is not fasting. In both cases, the record will only be considered valid if the
A1C variable is not left blank, coded as missing, or out of range.
Values left blank, coded as missing, or out of range are considered invalid values for consumption of fruit and
vegetables variables, the weight variable, and the total cholesterol variable.
Values left blank or coded as missing are considered invalid values for health history variables for high cholesterol,
hypertension, diabetes and heart health, medication status variables for cholesterol, blood pressure, and diabetes,
consumption of fish, whole grains, and beverages with added sugar, the smoking status variable, and the moderate
and vigorous exercise variables.
Values left blank are considered invalid values for month and year of birth and blood pressure date.
3 Screening goals are agreed upon between each grantee and CDC. The number of screenings used to assess progress
toward meeting the screening goal is calculated as the number of records meeting minimum screening requirements
(baseline, rescreening, and follow-up assessment after a completed LSP/HC). As part of CDC’s performance
assessment, programs must also provide evidence that they have met or exceeded 95 percent of their screening goal
(performance measure #3).
4 If the program is unable to obtain or the participant refuses to allow measurements for height, weight, first blood
pressure reading or to complete the personal assessment history, the program may choose to submit an explanation
for this situation to be considered as an acceptable screening record. See Appendix B for additional information on this
process.
5 Appendix H provides a summary of the criteria for a health risk assessment and the associated MDEs.

OMB Approved
WISEWOMAN MDE Manual 17 August 2017
Edition 9.03 revised
• Use of medications to lower cholesterol, blood pressure and diabetes (5a-
5c)
• Medication adherence for participants taking medication to lower
cholesterol, blood pressure, or blood sugar (5d-5f)
• Blood pressure self-monitoring [for participants with high blood pressure or
previously diagnosed with hypertension [high blood pressure] only] (6a-6c)
• Diet [consumption of fruits, vegetables, fish, whole grains, beverages with
added sugar, and sodium or salt intake (7a-7f)];
• Physical activity [moderate and vigorous physical activity (8a and 8b)];
• Smoking or exposure to secondhand smoke (9a-9b);
• Quality of life (10a-10c)
Follow-up assessments should include, at minimum, a valid follow-up
assessment date, as indicated by blood pressure date (12a: BPDate), and
valid values for the following services:6
• Use of medications to lower cholesterol, blood pressure, or blood sugar
(5a – 5c);
• Blood pressure self-monitoring (6a-6c – for participants with high blood
pressure or previously diagnosed with hypertension (high blood
pressure), only);
• Diet [consumption of fruits, vegetables, fish, whole grains, and beverages
with added sugar (7a-7e)];
• Physical activity [moderate and vigorous physical activity (8a-8b)];
• Smoking or exposure to secondhand smoke (9a-9b);
• Quality of life (10a-10c)
Grantees may report records that do not meet these follow-up requirements, but
they will not be analyzed in data reports generated by CDC unless additional
documentation is provided.
Below is a summary of the 56 required variables in the Screening and
Assessment file (Subsection a). After the summary, the technical specifications
for each variable are provided (Subsection b).
6 Values left blank, coded as missing, or out of range are considered invalid values for consumption of fruit and
vegetables variables, the secondhand smoking exposure variable, and the quality of life variables.
Values left blank or coded as missing are considered invalid values for the medication status variables for cholesterol,
blood pressure, and diabetes, consumption of fish, whole grains, and beverages with added sugar, the smoking status
variable, the blood pressure self-monitoring variables, the moderate and vigorous exercise variables.

OMB Approved
WISEWOMAN MDE Manual 18 August 2017
Edition 9.03 revised
a. Summary of Screening and Assessment MDEs
Item
Number Variable
Name Position
Possible
Rounds of
Collection 1 Variable Label Type
3b ResANSI 46 1 ANSI geographic code of
residence Character
3c ZIP 51 1 ZIP code of residence Character
3d MYB 56 1 Month and year of birth Numeric
3e Latino 62 1 Hispanic or Latino origin Numeric
3f Race1 63 1 Race: first race Numeric
3g Race2 64 1 Race: second race Numeric
3h Education 65 1 Education (highest grade
completed) Numeric
3i Language 66 1 What is the primary language
spoken in your home? Numeric
4a SRHC 68 2 Do you have high cholesterol? Numeric
4b SRHB 70 2 Do you have hypertension
(high blood pressure)? Numeric
4c SRD 72 2 Do you have diabetes? (either
Type 1 or Type 2) Numeric
4d SRHA 74 2 Have you been diagnosed by
a healthcare provider as
having any of these conditions:
coronary heart disease/chest
pain, heart attack, heart
failure, stroke/transient
ischemic attack (TIA), vascular
disease, or congenital heart
defects?
Numeric
5a HCMeds 76 2 Do you take medication to
lower your cholesterol? Numeric
5b HBPMeds 78 2 Do you take medication to
lower your blood pressure? Numeric
5c DMeds 80 2 Do you take medication to
lower your blood sugar (for
diabetes)?
Numeric
5d HCAdhere 82 2 During the past 7 days, on
how many days did you take
prescribed medication to lower
your cholesterol?
Numeric
5e HBPAdhere 86 2 During the past 7 days, on
how many days did you take
prescribed medication
(including diuretics/water pills)
to lower your blood pressure?
Numeric

OMB Approved
WISEWOMAN MDE Manual 19 August 2017
Edition 9.03 revised
Item
Number Variable
Name Position
Possible
Rounds of
Collection 1 Variable Label Type
5f DAdhere 90 2 During the past 7 days, on
how many days did you take
prescribed medication to lower
blood sugar (for diabetes)?
Numeric
6a BPHome 94 2 Do you measure your blood
pressure at home or using
other calibrated sources?
Numeric
6b BPFreq 100 2 How often do you measure
your blood pressure at home
or using other calibrated
sources?
Numeric
6c BPSend 102 2 Do you regularly share blood
pressure readings with a
health care provider for
feedback?
Numeric
7a Fruit 104 2 How much fruit do you eat in
an average day? Numeric
7b Vegetables 108 2 How many vegetables do you
eat in an average day? Numeric
7c Fish 112 2 Do you eat two servings or
more of fish weekly? Numeric
7d Grains 114 2 Do you eat 3 ounces or more
of whole grains daily? Numeric
7e Sugar 116 2 Do you drink less than 36
ounces (450 calories) of
beverages with added sugars
weekly?
Numeric
7f SaltWatch 118 2 Are you currently watching or
reducing your sodium or salt
intake?
Numeric
8a PAMod 120 2 How much moderate physical
activity do you get in a week? Numeric
8b PAVig 126 2 How much vigorous physical
activity do you get in a week? Numeric
9a Smoker 132 2 Do you smoke? Includes
cigarettes, pipes, or cigars
(smoked tobacco in any form)
Numeric
9b Sechand 134 2 About how many hours a day,
on average, are you in the
same room or vehicle with
another person who is
smoking?
Numeric

OMB Approved
WISEWOMAN MDE Manual 20 August 2017
Edition 9.03 revised
Item
Number Variable
Name Position
Possible
Rounds of
Collection 1 Variable Label Type
10a QOLPH 138 2 Thinking about your physical
health, which includes physical
illness and injury, on how
many days during the past 30
days was your physical health
not good?
Numeric
10b QOLMH 142 2 Thinking about your mental
health, which includes stress,
depression, and problems with
emotions, on how many days
during the past 30 days was
your mental health not good?
Numeric
10c QOLEffect 146 2 During the past 30 days, on
about how many days did poor
physical or mental health keep
you from doing your usual
activities, such as self-care,
work, or recreation?
Numeric
11a Height 150 1 Height, inches Numeric
11b Weight 152 2 Weight, pounds Numeric
11c Waist 158 2 Waist circumference, inches Numeric
11d Hip 162 2 Hip circumference, inches Numeric
12a BPDate 166 2 Blood pressure measurement
date (office visit date) Numeric
12b SBP1 182 2 Systolic blood pressure #1,
mmHg Numeric
12c DBP1 188 2 Diastolic blood pressure #1,
mmHg Numeric
12d SBP2 194 2 Systolic blood pressure #2,
mmHg Numeric
12e DBP2 200 2 Diastolic blood pressure #2,
mmHg Numeric
13a Fast 206 2 Fasting status Numeric
14a TCDate 208 2 Cholesterol measurement date Numeric
14b TotChol 224 2 Total cholesterol (fasting or
nonfasting), mg/dL Numeric
14c HDL 230 2 HDL cholesterol (fasting or
nonfasting), mg/dL Numeric
14d LDL 236 2 LDL cholesterol (fasting only),
mg/dL Numeric
14e Trigly 242 2 Triglycerides (fasting only),
mg/dL Numeric

OMB Approved
WISEWOMAN MDE Manual 21 August 2017
Edition 9.03 revised
Item
Number Variable
Name Position
Possible
Rounds of
Collection 1 Variable Label Type
15a BGDate 250 2 Glucose/A1c measurement
date Numeric
15b Glucose 266 2 Glucose (fasting only), mg/dL Numeric
15c A1C 272 2 A1C percentage Numeric
16a BPAlert 280 2 If average SBP >180 or DBP
>110, what is the status of the
workup?
Numeric
16b BPDiDate 282 2 If average SBP >180 or DBP
>110, workup date Numeric
16c BGAlert 298 2 If GLUCOSE ≤50 or
GLUCOSE ≥250, what is the
status of the workup?
Numeric
16d BGDiDate 300 2 If GLUCOSE ≤50 or
GLUCOSE ≥250, workup
exam date
Numeric
1 Number of times the item may be collected during the screening cycle. For example, for an item
with 2 possible rounds of data collection, a value may be provided at both baseline
screening/rescreening and at follow-up assessment.
OMB Approved
WISEWOMAN MDE Manual 22 August 2017
Edition 9.03 revised
b. Screening and Assessment MDE Specifications
Item 3b: ResANSI ANSI Geographic Code of Residence
This variable indicates the ANSI geographic code of residence of the WISEWOMAN participant.
FORMAT Type: Character
Item Length: 5
Field Length: 5
Leading Zeros: Yes
Static Field: No
Other Format: N/A
Justification: Left
Beginning Position: 46
Valid Range: Valid ANSI code;
cannot be blank
SOURCE
National ANSI Code List
DENOMINATOR
POPULATION
The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION ANSI Geographic Code Value representing the participant’s geographic area of residence
ANALYSIS AND USE To assess whether programs are meeting screening goals in targeted geographic areas
To identify the reach of the WISEWOMAN Program
To assist in identifying areas where there may be potential transportation barriers to accessing
WISEWOMAN services
OTHER
INFORMATION Guidance
ANSI codes are the American National Standards Institute codes, which were developed by the
American National Standards Institute. They are five-digit codes that represent states, counties,
and statistically equivalent areas, along with American Indian and Alaska Native areas.
The first two digits of the participant ANSI geographic code of residence should represent the state
of residence for the participant, and the last three digits should represent the participant’s county of
residence.
Both ANSI geographic area of residence and ZIP code of residence (3c: ZIP) are required. ZIP
code of residence should correspond to the ANSI geographic code of residence, in that the ZIP
code must represent a valid geographic area within the county.
If a participant does not reside in the state where the program is located, the ANSI code from her
actual state of residence should be recorded.
ANSI geographic code of residence should be captured at the first screening visit of the
submission period; if geographic code of residence changes during a submission period, the last
code collected for the submission period should be recorded.

OMB Approved
WISEWOMAN MDE Manual 23 August 2017
Edition 9.03 revised
Item 3c: ZIP ZIP Code of Residence
This variable indicates the participant’s ZIP code of residence.
FORMAT Type: Character
Item Length: 5
Field Length: 5
Leading Zeros: Yes
Static Field: No
Other Format: N/A
Justification: Left
Beginning Position: 51
Valid Range: Valid ZIP code; cannot
be blank
SOURCE National ZIP Code List
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION ZIP Code of Residence Valid five-digit (character) ZIP code
99999 No ZIP code recorded
This value will be flagged as an error
ANALYSIS AND USE To assess whether programs are meeting screening goals in targeted geographic areas
To identify the reach of the WISEWOMAN Program
To identify participant county of residence outside program state boundaries
OTHER
INFORMATION Guidance
Both ANSI geographic code of residence (3b: ResANSI) and ZIP code of residence are required.
ZIP code of residence should correspond to the county code of residence, in that the ZIP code must
represent a valid geographic area within the county.
ZIP code of residence must be recorded regardless of whether or not the woman resides in the
same state as the program. This information will be used in conjunction with geographic code of
residence to identify the area of residence for a woman.
If a participant does not reside in the same state as the program, the ZIP code from her actual state
of residence should be recorded.
ZIP code of residence should be captured at the first screening visit of the submission period; if ZIP
code of residence changes during a submission period, the last code collected for the submission
period should be recorded.
OMB Approved
WISEWOMAN MDE Manual 24 August 2017
Edition 9.03 revised
Item 3d: MYB Month and Year of Birth
This variable indicates the participant’s month and year of birth.
FORMAT Type: Numeric
Item Length: 6
Field Length: 6
Leading Zeros: Yes
Static Field: Yes
Other Format: MMCCYY date
Justification: Right
Beginning Position: 56
Valid Range: Valid date; cannot be blank
SOURCE Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION Month and Year of Birth Month and Year of Birth in MMCCYY format
Example: September 01, 1965 = 091965
ANALYSIS AND USE To estimate the age of the participant; age will be calculated using the month and year of birth and
office visit date (BPDate)
To assist in characterizing the population reached by the WISEWOMAN Program
To provide data element required to determine participant’s cardiovascular risk or risk score
To assess whether the participants are within the Program’s priority age group
OTHER
INFORMATION Guidance
The priority population for the WISEWOMAN Program is women aged 40 to 64. Services provided
to women outside the priority age range will be monitored by CDC.
Month and year of birth at baseline screening or rescreening is required for a record to count as a
valid record. If MYB is blank, the record will not count as a valid record, and the record will not
count toward meeting a program’s screening goal (performance measure #3).
OMB Approved
WISEWOMAN MDE Manual 25 August 2017
Edition 9.03 revised
Item 3e: Latino Hispanic or Latino Origin
This variable indicates whether the participant is of Hispanic or Latino origin.
FORMAT Type: Numeric
Item Length: 1
Field Length: 1
Leading Zeros: No
Static Field: Yes
Other Format: N/A
Justification: Right
Beginning Position: 62
Valid Range: See values; cannot be
blank
SOURCE United States Office of Management and Budget Guidelines
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION 1 Yes Participant reports that she is of Hispanic or Latino origin
2 No Participant reports that she is not of Hispanic or Latino origin
7 Unknown Participant is unsure whether she is of Hispanic or Latino origin
9 No answer recorded Participant has not reported whether she is of Hispanic or Latino origin
This value will be flagged as an error
ANALYSIS AND USE To assess the race/ethnicity of WISEWOMAN participants
To analyze screening, lifestyle programs, and other variables by ethnicity
To assist in characterizing the population reached by the WISEWOMAN Program
To provide data element required to determine participant’s cardiovascular risk or risk score
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
presented to participants. They are provided for funded program use only.
Cross edits
At least one race or Hispanic ethnicity should be reported. An error flag will occur if at least one race
or Hispanic ethnicity is not reported.
Error: LATINO, RACE1-RACE2 all = 9
If a participant is non-Hispanic, she should identify with at least one race. An error flag will occur if a
non-Hispanic participant does not identify with at least one race.
Error: LATINO = 2 AND RACE1-RACE2 all = 9
OMB Approved
WISEWOMAN MDE Manual 26 August 2017
Edition 9.03 revised
Item 3f: Race1 Race: First Race
This variable indicates a race with which the participant identifies.
FORMAT Type: Numeric
Item Length: 1
Field Length: 1
Leading Zeros: No
Static Field: Yes
Other Format: N/A
Justification: Right
Beginning Position: 63
Valid Range: See values; cannot be blank
SOURCE United States Census Bureau; United States Office of Management and Budget Guidelines
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION 1 White Participant identifies White as a race
2 Black or African American Participant identifies Black or African American as a race
3 Asian Participant identifies Asian as a race
4 Native Hawaiian or Other
Pacific Islander Participant identifies Native Hawaiian or Other Pacific Islander as a
race
5 American Indian or Alaska
Native Participant identifies American Indian or Alaska Native as a race
7 Unknown Participant does not know her race or does not identify with any of
the races listed above
If a participant is Hispanic and does not identify a race, this code
should be used
9 No answer recorded Race information is missing for the participant
Any race information gathered should be entered beginning with the
Race1 field
See cross edits related to this value
ANALYSIS AND
USE To assess the race/ethnicity of WISEWOMAN participants
To understand and analyze screening, lifestyle programs, and other variables by race
To assist in characterizing the population reached by the WISEWOMAN Program
To provide data element required to determine participant’s cardiovascular risk or risk score
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
presented to participants. They are provided for funded program use only.
If a participant identifies more than one race, one race is recorded here and other race she identifies is
recorded in the subsequent race field (3g: Race2).
Cross edits
First race should always be recorded unless the participant identifies as Hispanic. In cases where the
participant is Hispanic, first race is permitted to be unknown or not recorded. In all other cases where
first race is unknown or not recorded, this field will be flagged as an error.
Error: RACE1 = 9 AND LATINO ≠ 1
First race should be completed before the other race field. This field will be flagged as an error if it is
unknown or not recorded, while the other race field contain values of ‘1 White,’ ‘2 Black or African
American,’ ‘3 Asian,’ ‘4 Native Hawaiian or other Pacific Islander,’ or ‘5 American Indian or Alaska
Native.
Error: RACE1 = 9 AND RACE2 ≠ 9
Item 3g: Race2 Race: Second Race
This variable indicates a race with which the participant identifies in cases where a participant is
multiracial.
OMB Approved
WISEWOMAN MDE Manual 27 August 2017
Edition 9.03 revised
FORMAT Type: Numeric
Item Length: 1
Field Length: 1
Leading Zeros: No
Static Field: Yes
Other Format: N/A
Justification: Right
Beginning Position: 64
Valid Range: See values; cannot be
blank
SOURCE United States Census Bureau; United States Office of Management and Budget Guidelines
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION 1 White Participant identifies White as a race
Participant who has identified two or more races can have this value
2 Black or African
American Participant identifies Black or African American as a race
Participant who has identified two or more races can have this value
3 Asian Participant identifies Asian as a race
Participant who has identified two or more races can have this value
4 Native Hawaiian or
Other Pacific Islander Participant identifies Native Hawaiian or Other Pacific Islander as a
race
Participant who has identified two or more races can have this value
5 American Indian or
Alaska Native Participant identifies American Indian or Alaska Native as a race
Participant who has identified two or more races can have this value
7 Unknown Participant does not know her race or does not identify with any of
the races listed above
9 No answer recorded If race information is missing for Race2
Participant has not identified any race
Participant has identified one race and does not identify other races
If a participant does not identify a second race, ‘9 No answer
recorded’ should be used for this field and all subsequent race fields
ANALYSIS AND USE To assess the race/ethnicity of WISEWOMAN participants
To understand and analyze screening, lifestyle programs, and other variables by race
To assist in characterizing the population reached by the WISEWOMAN Program
To provide data element required to determine participant’s cardiovascular risk or risk score
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
presented to participants. They are provided for funded program use only.
If a participant identifies two races, one race is recorded in Race1 and a second race is recorded
here.
OMB Approved
WISEWOMAN MDE Manual 28 August 2017
Edition 9.03 revised
Item 3h: Education Education (highest grade completed)
This variable indicates the highest grade the participant completed.
FORMAT Type: Numeric
Item Length: 1
Field Length: 1
Leading Zeros: No
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 65
Valid Range: See values; cannot
be blank
SOURCE CDC Behavioral Risk Factor Surveillance System
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION 1 <9th grade Participant reports that she did not attend high school
2 Some high school Participant reports she attended high school, but did not graduate
3 High school graduate
or equivalent Participant reports that she graduated from high school or has the
equivalent of a high school diploma, and she did not attend any
college or higher education
4 Some college or higher Participant reports that she attended one or more years of college
and/or graduate school (e.g., college graduate, graduate degree)
7 Don’t know/Not sure Participant reports that she does not know the highest grade she
completed
This value will be flagged as a quality check
8 Don’t want to answer Participant does not want to answer the highest grade she completed
This value will be flagged as a quality check
9 No answer recorded Education information is missing for the participant
This value will be flagged as an error
ANALYSIS AND USE To assess the educational attainment of women in the WISEWOMAN population
To understand screening, lifestyle programs , and other variables by education status
To help determine the literacy level needed for materials developed for recruitment, risk reduction
counseling, lifestyle programs, health coaching, and community-based resources
To assist in characterizing the population reached by the WISEWOMAN Program
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
presented to participants. They are provided for funded program use only.

OMB Approved
WISEWOMAN MDE Manual 29 August 2017
Edition 9.03 revised
Item 3i: Language What is the primary language spoken in your home?
This variable indicates the primary language spoken in the participant’s home.
FORMAT Type: Numeric
Item Length: 2
Field Length: 2
Leading Zeros: Yes
Static Field: Yes
Other Format: N/A
Justification: Right
Beginning Position: 66
Valid Range: See values; cannot
be blank
SOURCE
National Survey of Children’s Health
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION 01 English Participant identifies English as the primary language spoken in her
home
02 Spanish Participant identifies Spanish as the primary language spoken in her
home
03 Arabic Participant identifies Arabic as the primary language spoken in her
home
04 Chinese Participant identifies Chinese as the primary language spoken in her
home
05 French Participant identifies French as the primary language spoken in her
home
06 Italian Participant identifies Italian as the primary language spoken in her
home
07 Japanese Participant identifies Japanese as the primary language spoken in
her home
08 Korean Participant identifies Korean as the primary language spoken in her
home
09 Polish Participant identifies Polish as the primary language spoken in her
home
10 Russian Participant identifies Russian as the primary language spoken in her
home
11 Tagalog Participant identifies Tagalog as the primary language spoken in her
home
12 Vietnamese Participant identifies Vietnamese as the primary language spoken in
her home
13 Creole Participant identifies Creole as the primary language spoken in her
home
14 Portuguese Participant identifies Portuguese as the primary language spoken in
her home
15 Hmong Participant identifies Hmong as the primary language spoken in her
home
16 Other Language Participant identifies another language as the primary language
spoken in her home (write-in response)
88 Don’t want to answer Participant does not want to answer the primary language spoken in
her home
This value will be flagged as a quality check
99 No answer recorded Primary language information is missing for the participant
This value will be flagged as an error
OMB Approved
WISEWOMAN MDE Manual 30 August 2017
Edition 9.03 revised
ANALYSIS AND USE To assess the primary language of women in the WISEWOMAN population
To provide context to potential the health literacy issues
To assist in characterizing the population reached by the WISEWOMAN Program
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
presented to participants. They are provided for funded program use only.
OMB Approved
WISEWOMAN MDE Manual 31 August 2017
Edition 9.03 revised
Item 4a: SRHC Do you have high cholesterol?
This variable indicates whether the participant has high cholesterol.
FORMAT Type: Numeric
Item Length: 1
Field Length: 2
Leading Zeros: No
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 68
Valid Range: See values; cannot be blank
if TYPE is 1 or 2 (baseline
screening or rescreening)
SOURCE Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION 1 Yes Participant has high cholesterol
2 No Participant does not have high cholesterol
7 Don’t know/Not sure Participant does not know whether she has high cholesterol
This value will be flagged as a quality check
8 Don’t want to answer Participant does not want to answer whether she has high cholesterol
This value will be flagged as a quality check
9 No answer recorded No answer recorded
This value will be flagged as an error
ANALYSIS AND USE To understand the cardiovascular disease risk factors of individual participants and the overall
WISEWOMAN population
To assess the number of cases of high cholesterol that have been previously diagnosed as
opposed to newly detected cases among the WISEWOMAN population
To assess control of and improvements in cholesterol for newly and previously diagnosed women
To provide data element required to determine participant’s Simple 7 cardiovascular risk score
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
presented to participants. They are provided for funded program use only.
Some programs may have access to participants’ medical charts. In some cases, the medical chart
may show that a participant’s diagnosis for high blood cholesterol is inconsistent with her self-report.
In these instances, if the medical record indicates that she has high blood cholesterol, the program
should recode this field as ‘1 Yes.’
Cholesterol history status at baseline screening or rescreening is required for a record to count as a
valid record. If SRHC is blank or coded as ‘‘9 No answer recorded,’ the record will not count as a
valid record, and the record will not count toward meeting a program’s screening goal (performance
measure #3).
OMB Approved
WISEWOMAN MDE Manual 32 August 2017
Edition 9.03 revised
Item 4b: SRHB Do you have hypertension (high blood pressure)?
This variable indicates whether the participant has hypertension (high blood pressure).
FORMAT Type: Numeric
Item Length: 1
Field Length: 2
Leading Zeros: No
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 70
Valid Range: See values; cannot be blank if
TYPE is 1 or 2 (baseline
screening or rescreening)
SOURCE Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION 1 Yes Participant has hypertension (high blood pressure)
2 No Participant does not have hypertension (high blood pressure)
7 Don’t know/Not sure Participant does not know whether she has hypertension (high blood
pressure)
This value will be flagged as a quality check
8 Don’t want to answer Participant does not want to answer whether she has hypertension
(high blood pressure)
This value will be flagged as a quality check
9 No answer recorded No answer recorded
This value will be flagged as an error
ANALYSIS AND USE To understand the cardiovascular disease risk factors of individual participants and the overall
WISEWOMAN population
To assess the number of cases of hypertension (high blood pressure) that have been previously
diagnosed as opposed to newly detected cases among the WISEWOMAN population
To assess control of and improvements in blood pressure for newly and previously diagnosed
women
To provide data element required to determine participant’s Simple 7 cardiovascular risk score
OTHER
INFORMATION Codes and response options highlighted in gray should not appear on the data collection forms
presented to participants. They are provided for funded program use only.
Some programs may have access to participants’ medical charts. In some cases, the medical chart
may show that a participant’s diagnosis for hypertension is inconsistent with her self-report. In these
instances, if the medical record indicates that she has hypertension, the program should recode this
field as ‘1 Yes.’
Hypertension history status at baseline screening or rescreening is required for a record to count as
a valid record. If SRHB is blank or coded as ‘‘9 No answer recorded,’ the record will not count as a
valid record, and the record will not count toward meeting a program’s screening goal (performance
measure #3).

OMB Approved
WISEWOMAN MDE Manual 33 August 2017
Edition 9.03 revised
Item 4c: SRD Do you have diabetes? (either Type 1 or Type 2)
This variable indicates whether the participant has Type 1 or Type 2 diabetes.
FORMAT Type: Numeric
Item Length: 1
Field Length: 2
Leading Zeros: No
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 72
Valid Range: See values; cannot be blank if
TYPE is 1 or 2 (baseline
screening or rescreening)
SOURCE American Heart Association Life’s Simple 7
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION 1 Yes Participant has Type 1 or Type 2 diabetes
2 No Participant does not have Type 1 or Type 2 diabetes
7 Don’t know/Not sure Participant does not know whether she has Type 1 or Type 2 diabetes
This value will be flagged as a quality check
8 Don’t want to answer Participant does not want to answer whether she has Type 1 or Type
2 diabetes
This value will be flagged as a quality check
9 No answer recorded No answer recorded
This value will be flagged as an error
ANALYSIS AND USE To understand the cardiovascular disease risk factors of individual participants and the overall
WISEWOMAN population
To assess the number of cases of diabetes that have been previously diagnosed as opposed to
newly detected cases among the WISEWOMAN population
To assess control of and improvements in diabetes for newly and previously diagnosed women
To provide data element required to determine participant’s Simple 7 cardiovascular risk score
OTHER INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
presented to participants. They are provided for funded program use only.
Some programs may have access to a participant’s medical chart. In some cases, the medical chart
may show that a participant’s diagnosis for diabetes is inconsistent with her self-report. In these
instances, if the medical record indicates that she has diabetes, the program should recode this field
as ‘1 Yes.’
Diabetes history status at baseline screening or rescreening is required for a record to count as a
valid record. If SRD is blank or coded as ‘‘9 No answer recorded,’ the record will not count as a valid
record, and the record will not count toward meeting a program’s screening goal (performance
measure #3).
OMB Approved
WISEWOMAN MDE Manual 34 August 2017
Edition 9.03 revised
Item 4d: SRHA Have you been diagnosed by a healthcare provider as having any of these conditions:
coronary heart disease/chest pain, heart attack, heart failure, stroke/transient ischemic
attack (TIA), vascular disease, or congenital heart defects?
This variable indicates whether the participant has ever been diagnosed by a healthcare provider as
having coronary heart disease/chest pain, heart attack, heart failure, stroke/TIA, vascular disease,
or congenital heart defects.
FORMAT Type: Numeric
Item Length: 1
Field Length: 2
Leading Zeros: No
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 74
Valid Range: See values; cannot be blank if
TYPE is 1 or 2 (baseline
screening or rescreening)
SOURCE American Heart Association Life’s Simple 7
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION 1 Yes Participant has been diagnosed by a healthcare provider as having
coronary heart disease/chest pain, heart attack, heart failure,
stroke/TIA, vascular disease, or congenital heart defects
2 No Participant has never been diagnosed by a healthcare provider as
having coronary heart disease/chest pain, heart attack, heart failure,
stroke/TIA, vascular disease, or congenital heart defects
7 Don’t know/Not sure Participant does not know whether she has been diagnosed by a
healthcare provider as having coronary heart disease/chest pain,
heart attack, heart failure, stroke/TIA, vascular disease, or congenital
heart defects
This value will be flagged as a quality check
8 Don’t want to answer Participant does not want to answer whether she has been
diagnosed by a healthcare provider as having coronary heart
disease/chest pain, heart attack, heart failure, stroke/TIA, vascular
disease, or congenital heart defects
This value will be flagged as a quality check
9 No answer recorded No answer recorded
This value will be flagged as an error
ANALYSIS AND USE To understand the history of cardiovascular disease among individual participants and the overall
WISEWOMAN population
To assess the number of participants who have been previously diagnosed as having
cardiovascular disease
To provide data element required to determine participant’s Simple 7 cardiovascular risk score
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
presented to participants. They are provided for funded program use only.
Some programs may have access to participants’ medical charts. In some cases, the medical chart
may show that a participant’s diagnosis for heart attack (also called myocardial infarction), angina,
coronary heart disease, or stroke is inconsistent with her self-report. In these instances, if the
medical record indicates that she has had any one of these conditions, the program should recode
this field as ‘1 Yes.’
Heart attack (also called myocardial infarction), angina, coronary heart disease, or stroke history
status at baseline screening or rescreening is required for a record to count as a valid record. If
SRHA is blank or coded as ‘‘9 No answer recorded,’ the record will not count as a valid record, and
the record will not count toward meeting a program’s screening goal (performance measure #3).
OMB Approved
WISEWOMAN MDE Manual 35 August 2017
Edition 9.03 revised
Item 5a: HCMeds
Do you take medication to lower your cholesterol?
This variable indicates whether the participant takes medication to lower her cholesterol.
FORMAT Type: Numeric
Item Length: 1
Field Length: 2
Leading Zeros: No
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 76
Valid Range: See values; cannot be blank
SOURCE American Heart Association Life’s Simple 7
DENOMINATOR
POPULATION
The denominator includes WISEWOMAN participants with high cholesterol or previously diagnosed
with high cholesterol
VALUES AND
DESCRIPTION
1 Yes Participant is taking medication to lower her cholesterol
2 No
Participant is not taking medication to lower her cholesterol
3 No – Could not obtain
medication
Participant is not taking medication to lower her cholesterol because
she could not obtain the medication (e.g., could not obtain due to cost
of medication, could not obtain due to expired prescription, could not
obtain due to problems getting the prescription filled because of lack of
transportation or access to a pharmacy)
5 Not Applicable
This question is not applicable for the patient because she has never
been diagnosed with high cholesterol, either because she does not
have high cholesterol (as assessed with a measurement at screening/
rescreening) or because she reports that she has never been
diagnosed with high cholesterol (as assessed with self-report at
screening/ rescreening).
7 Don’t know/Not sure Participant does not know whether she is taking medication to lower
her cholesterol
This value will be flagged as a quality check
8 Don’t want to answer Participant does not want to answer whether she is taking medication
to lower her cholesterol
This value will be flagged as a quality check
9 No answer recorded No answer recorded
This value will be flagged as an error
ANALYSIS AND USE To understand the cardiovascular disease risk factors of individual participants and the overall
WISEWOMAN population
To assess the number of cases of high cholesterol that have been previously diagnosed as opposed
to newly detected cases among the WISEWOMAN population
To assess the control and management of cholesterol among participants who have high cholesterol
To assist in assessment of adherence to medication for high cholesterol
To provide data element required to determine participant’s Simple 7 cardiovascular risk score
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
presented to participants. They are provided for funded program use only.
If a participant reports that she doesn’t know whether she is taking medication for high cholesterol or
doesn’t want to answer whether she is taking medication for high cholesterol, programs should have
a discussion with her to verify the response.
High cholesterol medication adherance status at baseline screening or rescreening is required for a
record to count as a valid record. If HCMeds is blank or coded as ‘‘9 No answer recorded,’ the record
will not count as a valid record, and the record will not count toward meeting a program’s screening
goal (performance measure #3).
Cross edits
If participant does not have high cholesterol or reports that she has never been diagnosed with high
cholesterol, a response of Not Applicable should be provided. Otherwise, this field will be flagged as
a quality check.
Quality Check: HCMeds ≠ 5
AND
SRHC ≠ 1
AND
TOTCHOL< 240

OMB Approved
WISEWOMAN MDE Manual 36 August 2017
Edition 9.03 revised
Item 5b: HBPMeds
Do you take medication to lower your blood pressure?
This variable indicates whether the participant is taking medication to lower her blood pressure.
FORMAT Type: Numeric
Item Length: 1
Field Length: 2
Leading Zeros: No
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 78
Valid Range: See values; cannot be blank
SOURCE American Heart Association Life’s Simple 7
DENOMINATOR
POPULATION
The denominator includes WISEWOMAN participants with high blood pressure or previously
diagnosed with hypertension (high blood pressure)
VALUES AND
DESCRIPTION
1 Yes Participant is taking medication to lower her blood pressure
2 No
Participant is not taking medication to lower her blood pressure
3 No – Could not obtain
medication
Participant is not taking medication to lower her blood pressure
because she could not obtain the medication (e.g., could not obtain
due to cost of medication, could not obtain due to expired
prescription, could not obtain due to problems getting the prescription
filled because of lack of transportation or access to a pharmacy)
5 Not Applicable
This question is not applicable for the patient because she has never
been diagnosed with high blood pressure, either because she does
not have high blood pressure (as assessed with a measurement at
screening/ rescreening) or because she reports that she has never
been diagnosed with high blood pressure (as assessed with self-
report at screening/ rescreening).
7 Don’t know/Not sure Participant does not know whether she is taking medication to lower
her blood pressure
This value will be flagged as a quality check
8 Don’t want to answer Participant does not want to answer whether she is taking medication
to lower her blood pressure
This value will be flagged as a quality check
9 No answer recorded No answer recorded
This value will be flagged as an error
ANALYSIS AND USE To understand the cardiovascular disease risk factors of individual participants and the overall
WISEWOMAN population
To assess the number of cases of hypertension (high blood pressure) that have been previously
diagnosed as opposed to newly detected cases among the WISEWOMAN population
To assess the control and management of hypertension (high blood pressure) among participants
who have hypertension (high blood pressure)
To assist in assessment of adherence to medication for hypertension (high blood pressure)
To provide data element required to determine participant’s Simple 7 cardiovascular risk score
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
presented to participants. They are provided for funded program use only.
High blood pressure medication adherence status at baseline screening or rescreening is required
for a record to count as a valid record. If HBMeds is blank or coded as ‘‘9 No answer recorded,’ the
record will not count as a valid record, and the record will not count toward meeting a program’s
screening goal (performance measure #3).
Cross edits
If participant does not have high blood pressure or reports that she has never been diagnosed with
high blood pressure (hypertension), a response of Not Applicable should be provided. Otherwise,
this field will be flagged as a quality check.
Quality Check: HBPMeds ≠ 5 AND SRHB ≠ 1 AND
(((SBP1 + SBP2)/2) < 140 AND
((DBP1 + DBP2)/2) < 90)
OMB Approved
WISEWOMAN MDE Manual 37 August 2017
Edition 9.03 revised
Item 5c: DMeds
Do you take medication to lower your blood sugar (for diabetes)?
This variable indicates whether the participant is taking medication to lower her blood sugar for diabetes.
FORMAT Type: Numeric
Item Length: 1
Field Length: 2
Leading Zeros: No
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 80
Valid Range: See values; cannot be blank
SOURCE American Heart Association Life’s Simple 7
DENOMINATOR
POPULATION
The denominator includes WISEWOMAN participants with high levels of blood glucose (fasting) or A1C
or previously diagnosed with diabetes
VALUES AND
DESCRIPTION 1 Yes Participant is taking medication to lower her blood sugar for diabetes
2 No Participant is not taking medication to lower her blood sugar for
diabetes
3 No – Could not obtain
medication
Participant is not taking medication to lower her blood sugar for
diabetes because she could not obtain the medication (e.g., could not
obtain due to cost of medication, could not obtain due to expired
prescription, could not obtain due to problems getting the prescription
filled because of lack of transportation or access to a pharmacy)
5 Not Applicable
This question is not applicable for the patient because she has never
been diagnosed with high blood sugar for diabetes, either because
she does not have high blood sugar or diabetes (as assessed with a
measurement at screening/ rescreening) or because she reports that
she has never been diagnosed with high blood sugar or diabetes (as
assessed with self-report at screening/ rescreening).
7 Don’t know/Not sure Participant does not know whether she is taking medication to lower
her blood sugar for diabetes
This value will be flagged as a quality check
8 Don’t want to answer Participant does not want to answer whether she is taking medication
to lower her blood sugar for diabetes
This value will be flagged as a quality check
9 No answer recorded No answer recorded
This value will be flagged as an error
ANALYSIS AND
USE To understand the cardiovascular disease risk factors of individual participants and the overall
WISEWOMAN population
To assess the number of cases of diabetes that have been previously diagnosed as opposed to newly
detected cases among the WISEWOMAN population
To assess diabetes control and management among participants who have diabetes
To assist in assessment of adherence to medication for diabetes
To provide data element required to determine participant’s Simple 7 cardiovascular risk score
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
presented to participants. They are provided for funded program use only.
If a participant reports that she doesn’t know whether she is taking medication for diabetes or doesn’t
want to answer whether she is taking medication for diabetes, programs should have a discussion with
the participant to verify the response.
Diabetes medication adherence status at baseline screening or rescreening is required for a record to
count as a valid record. If DMeds is blank or coded as ‘‘9 No answer recorded,’ the record will not
count as a valid record, and the record will not count toward meeting a program’s screening goal
(performance measure #3).
Cross edits
If participant does not have high levels of blood glucose (fasting) or A1C or reports that she has never
been diagnosed with diabetes, a response of Not Applicable should be provided. Otherwise, this field
will be flagged as a quality check.
Quality Check: DMeds ≠ 5
AND
SRD ≠ 1
AND
(GLUCOSE < 126)
AND
A1C < 6.5
OMB Approved
WISEWOMAN MDE Manual 38 August 2017
Edition 9.03 revised
Item 5d: HCAdhere During the past 7 days, on how many days did you take prescribed medication to lower your
cholesterol?
This variable indicates the number of days out of the past 7 days, including the day of the
screening, that the participant took prescribed medication to lower her cholesterol.
FORMAT Type: Numeric
Item Length: 2
Field Length: 4
Leading Zeros: Yes
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 82
Valid Range: 00 - 07; cannot be blank
SOURCE Adapted from National Survey of Children’s Health
DENOMINATOR
POPULATION The denominator includes WISEWOMAN participants taking medication to lower cholesterol
VALUES AND
DESCRIPTION Number of days A two-digit (numeric) value indicating the number of days out of the
past 7 days, including the day of the screening, that the participant
took prescribed medication to lower her cholesterol
Any value outside the valid range (00 – 07) will be considered an
error
Example: 2 days = 02
00 None In the past 7 days, including the day of the screening, the participant
did not take prescribed medication to lower her cholesterol
55 Not Applicable This question is not applicable for the patient because she has never
been diagnosed with high cholesterol and/or has indicated that she
does not take medication for high cholesterol
77 Don’t know/Not sure Participant is not sure whether she took prescribed medication to
lower her cholesterol during the past 7 days including the day of the
screening
This value will be flagged as a quality check
88 Don’t want to answer Participant did not want to answer whether she took prescribed
medication to lower her cholesterol during the past 7 days, including
the day of the screening
This value will be flagged as a quality check
99 No answer recorded No answer recorded
This value will be flagged as an error
ANALYSIS AND USE To facilitate assessment of adherence to medication prescribed for high cholesterol
To assist in determining high cholesterol management and control
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
presented to participants. They are provided for funded program use only.
Cross edits
If participant reports that she does not takes medication to lower cholesterol, a response of Not
Applicable should be provided. Otherwise, this field will be flagged as an error.
Error: HCMeds ≠ 1 AND HCAdhere ≠ 55
A valid response should be provided for participants with elevated cholesterol who are taking
medication to lower their cholesterol. Otherwise, this field will be flagged as an error.
Error: HCMeds = 1 AND HCAdhere = 55
OMB Approved
WISEWOMAN MDE Manual 39 August 2017
Edition 9.03 revised
Item 5e: HBPAdhere During the past 7 days, on how many days did you take prescribed medication (including
diuretics/water pills) to lower your blood pressure?
This variable indicates the number of days out of the past 7 days, including the day of the
screening, that the participant took prescribed medication (including diuretics/water pills) to lower
her blood pressure.
FORMAT Type: Numeric
Item Length: 2
Field Length: 4
Leading Zeros: Yes
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 86
Valid Range: 00 - 07; cannot be blank
SOURCE Adapted from National Survey of Children’s Health
DENOMINATOR
POPULATION The denominator includes WISEWOMAN participants taking medication to lower blood pressure
VALUES AND
DESCRIPTION Number of days A two-digit (numeric) value indicating the number of days out of the
past 7 days, including the day of screening, that the participant took
prescribed medication (including diuretics/water pills) to lower her
blood pressure
Any value outside the valid range (00 – 07) will be considered an
error
Example: 2 days = 02
00 None In the past 7 days, including the day of screening, the participant did
not take prescribed medication (including diuretics/water pills) to
lower her blood pressure
55 Not Applicable This question is not applicable for this patient because she has never
been diagnosed with hypertension (high blood pressure) and/or has
indicated that she does not take medication for high blood
pressure/hypertension
77 Don’t know/Not sure Participant is not sure whether she took prescribed medication
(including diuretics/water pills) to lower her blood pressure during the
past seven days including the day of screening
This value will be flagged as a quality check
88 Don’t want to answer
Participant did not want to answer whether she prescribed medication
(including diuretics/water pills) to lower her blood pressure during the
past seven days including the day of screening
This value will be flagged as a quality check
99 No answer recorded No answer recorded
This value will be flagged as an error
ANALYSIS AND USE To facilitate assessment of adherence to medication prescribed for hypertension (high blood
pressure)
To assist in determining hypertension (high blood pressure) prevention, management, and control
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
presented to participants. They are provided for funded program use only.
Cross edits
If participant reports that she does not takes medication to lower blood pressure, a response of Not
Applicable should be provided. Otherwise, this field will be flagged as an error.
Error: HBPMeds ≠ 1 AND HBPAdhere ≠ 55
A valid response should be provided for participants with elevated blood pressure who are taking
medication to lower their blood pressure. Otherwise, this field will be flagged as an error.
Error: HBPMeds = 1 AND HBPAdhere = 55
OMB Approved
WISEWOMAN MDE Manual 40 August 2017
Edition 9.03 revised
Item 5f: DAdhere During the past 7 days, on how many days did you take prescribed medication to lower
blood sugar (for diabetes)?
This variable indicates the number of days out of the past 7 days, including the day of the
screening, that the participant took prescribed medication to lower her blood sugar (for diabetes).
FORMAT Type: Numeric
Item Length: 2
Field Length: 4
Leading Zeros: Yes
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 90
Valid Range: 00 - 07; cannot be blank
SOURCE Adapted from National Survey of Children’s Health
DENOMINATOR
POPULATION The denominator includes WISEWOMAN participants prescribed taking took prescribed medication
to lower blood sugar
VALUES AND
DESCRIPTION Number of days A two-digit (numeric) value indicating the number of days out of the
past 7 days, including the day of screening, that the participant took
prescribed medication to lower her blood sugar (for diabetes)
Any value outside the valid range (00 – 07) will be considered an
error
Example: 2 days = 02
00 None In the past 7 days, including the day of screening, the participant did
not take prescribed medication to lower her blood sugar (for diabetes)
55 Not Applicable This question is not applicable for this patient because she has never
been diagnosed with high blood sugar (for diabetes) and/or has
indicated that she does not take medication for high blood
sugar/diabetes
77 Don’t know/Not sure Participant is not sure whether she took prescribed medication to
lower her blood sugar (for diabetes) during the past seven days
including the day of screening
This value will be flagged as a quality check
88 Don’t want to answer Participant did not want to answer whether she took prescribed
medication to lower her blood sugar (for diabetes) during the past
seven days including the day of screening
This value will be flagged as a quality check
99 No answer recorded No answer recorded
This value will be flagged as an error
ANALYSIS AND USE To facilitate assessment of adherence to medication prescribed for diabetes
To assist in determining diabetes control and management
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
presented to participants. They are provided for funded program use only.
Cross edits
If participant reports that she does not takes medication for diabetes, a response of Not Applicable
should be provided. Otherwise, this field will be flagged as an error.
Error: DMeds ≠ 1 AND DAdhere ≠ 55
A valid response should be provided for participants with who are taking medication for diabetes.
Otherwise, this field will be flagged as an error.
Error: DMeds = 1 AND DAdhere = 55
OMB Approved
WISEWOMAN MDE Manual 41 August 2017
Edition 9.03 revised
Item 6a: BPHome
Do you measure your blood pressure at home or using other calibrated sources?
This variable indicates whether the participant monitors her blood pressure at home or using other
calibrated sources (select all response options that apply).
FORMAT Type: Numeric
Item Length: 3
Field Length: 6
Leading Zeros: Yes
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 94
Valid Range: See values; cannot be blank
SOURCE HealthStyles Survey
DENOMINATOR
POPULATION The denominator includes WISEWOMAN participants with high blood pressure or previously
diagnosed with hypertension (high blood pressure)
VALUES AND
DESCRIPTION
1 Yes Participant reports that she measures her blood pressure at home or
using other calibrated sources
2 No – Was never told to
measure her blood
pressure
Participant reports that she does not measure her blood pressure at
home or using other calibrated sources because she was never told
she should measure her blood pressure
3 No – Doesn’t know how
to measure her blood
pressure
Participant reports that she does not measure her blood pressure at
home or using other calibrated sources because she does not know
how to measure her blood pressure
4 No – Doesn’t have
equipment to measure
her blood pressure
Participant reports that she does not measure her blood pressure at
home or using other calibrated sources because she does not have
access to the required equipment to measure her blood pressure
5 Not Applicable This question is not applicable for the patient because she has never
been diagnosed with hypertension (high blood pressure)
7 Don’t know/Not
sure/Other Participant is not sure whether she measures her blood pressure at
home or using other calibrated sources or provides some other
reason for why she does not measure her blood pressure at home
(for example, participant chooses not to measure her blood at home)
This value will be flagged as a quality check
8 Don’t want to answer Participant did not want to answer whether she measures her blood
pressure at home or using other calibrated sources
This value will be flagged as a quality check
9 No answer recorded No answer recorded
This value will be flagged as an error
ANALYSIS AND USE
To determine self-control and management of hypertension (high blood pressure)
OTHER
INFORMATION
Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
presented to participants. They are provided for funded program use only.
Participants may select more than one of the response options for “2 No – Was never told to
measure her blood pressure,” “3 – Doesn’t know how to measure her blood pressure,” “4 – Doesn’t
have equipment to measure her blood pressure.” If more than one response option is selected,
responses should be listed sequentially. For example, if response options 2 and 3 are selected, a
value of 023 should be entered for this record. If only one response option is selected, grantees
should include two leading zeros before the response values (e.g., ‘1—Yes’ would be coded as 001,
and ‘2—No’ would be coded as 002).
Guidance on blood pressure self-monitoring is available in the Self-Measured Blood Pressure
Monitoring Guide by Million Hearts (Centers for Disease Control and Prevention. Self-Measured
Blood Pressure Monitoring: Action Steps for Public Health Practitioners. Atlanta, GA: Centers for
Disease Control and Prevention, US Dept. of Health and Human Services; 2013.)
Cross edits
A valid response should be provided for participants who have elevated blood pressure or who are
taking medication for hypertension. Otherwise, this field will be flagged as an error.
Error: (SRHB = 1
OR
HBPMeds=1)
AND
BPHome = 5
OMB Approved
WISEWOMAN MDE Manual 42 August 2017
Edition 9.03 revised
Item 6b: BPFreq How often do you measure your blood pressure at home or using other calibrated sources?
This variable indicates how frequently the participant measures her blood pressure at home or using
other calibrated sources.
FORMAT Type: Numeric
Item Length: 1
Field Length: 2
Leading Zeros: No
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 100
Valid Range: See values; cannot be blank
SOURCE HealthStyles Survey
DENOMINATOR
POPULATION The denominator includes WISEWOMAN participants with high blood pressure or previously
diagnosed with hypertension (high blood pressure)
VALUES AND
DESCRIPTION 1 Multiple times per day Participant measures her blood pressure at home or using other
calibrated sources multiple times per day
2 Daily Participant measures her blood pressure at home or using other
calibrated sources once per day
3 A few times per week Participant measures her blood pressure at home or using other
calibrated sources a few times per week
4 Weekly Participant measures her blood pressure at home or using other
calibrated sources once per week
5 Monthly Participant measures her blood pressure at home or using other
calibrated sources once per month
6 Not Applicable This question is not applicable for the patient because she has never
been diagnosed with hypertension (high blood pressure) or does not
monitor her blood pressure at home or using other calibrated sources
7 Don’t know/Not
sure/Other Participant is not sure how frequently she measures her blood
pressure at home or using other calibrated sources
This value will be flagged as a quality check
8 Don’t want to answer Participant did not want to answer how frequently she measures her
blood pressure at home or using other calibrated sources
This value will be flagged as a quality check
9 No answer recorded No answer recorded
This value will be flagged as an error
ANALYSIS AND USE To determine self-control and management of hypertension (high blood pressure)
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
presented to participants. They are provided for funded program use only.
Cross edits
If participant reports that she does not measure her blood pressure at home or using other
calibrated sources, a response of Not Applicable should be provided. Otherwise, this field will be
flagged as an error.
Error: BPHome ≠ 1 AND BPFreq ≠ 6
A valid response should be provided for participants who measure their blood pressure at home or
using other calibrated sources. Otherwise, this field will be flagged as an error.
Error: BPHome = 1 AND BPFreq = 6

OMB Approved
WISEWOMAN MDE Manual 43 August 2017
Edition 9.03 revised
Item 6c: BPSend Do you regularly share blood pressure readings with a health care provider for feedback?
This variable indicates whether the participant shares blood pressure readings taken at home or
using other calibrated sources with a health care provider for feedback almost every time she sees
her provider.
FORMAT Type: Numeric
Item Length: 1
Field Length: 2
Leading Zeros: No
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 102
Valid Range: See values; cannot be blank
SOURCE Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION The denominator includes WISEWOMAN participants with high blood pressure or previously
diagnosed with hypertension (high blood pressure)
VALUES AND
DESCRIPTION 1 Yes Participant reports that she shares blood pressure readings taken at
home or using other calibrated sources with a health care provider for
feedback almost every time she sees her provider
2 No Participant reports that she does not share blood pressure readings
taken at home or using other calibrated sources with a health care
provider for feedback
5 Not Applicable This question is not applicable for the patient because she has never
been diagnosed with hypertension (high blood pressure) or does not
monitor her blood pressure at home or using other calibrated sources
7 Don’t know/Not
sure/Other Participant is not sure whether she shares blood pressure readings
taken at home or using other calibrated sources with a health care
provider for feedback
This value will be flagged as a quality check
8 Don’t want to answer Participant did not want to answer whether she shares blood
pressure readings taken at home or using other calibrated sources
with a health care provider for feedback
This value will be flagged as a quality check
9 No answer recorded No answer recorded
This value will be flagged as an error
ANALYSIS AND USE To determine self-control and management of hypertension (high blood pressure)
To determine whether blood pressure monitoring results are shared with a health care provider for
monitoring of progress
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
presented to participants. They are provided for funded program use only.
Cross edits
If participant reports that she does not measure her blood pressure at home or using other
calibrated sources, a response of Not Applicable should be provided. Otherwise, this field will be
flagged as an error.
Error: BPHome ≠ 1 AND BPSend ≠ 5
A valid response should be provided for participants who measure their blood pressure at home or
using other calibrated sources. Otherwise, this field will be flagged as an error.
Error: BPHome = 1 AND BPSend = 5
OMB Approved
WISEWOMAN MDE Manual 44 August 2017
Edition 9.03 revised
Item 7a: Fruit How much fruit do you eat in an average day?
This variable indicates the amount of fruit the participant consumes in an average day.
FORMAT Type: Numeric
Item Length: 2
Field Length: 4
Leading Zeros: Yes
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 104
Valid Range: 01-50; cannot be blank
SOURCE American Heart Association Life’s Simple 7
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION Number of cups Two-digit (numeric) value representing the number of cups of fruit the
participant consumes in an average day
Any value outside the valid range (01 -50) will be considered an error
Example: 2 cups = 02
00 None Participant does not consume fruit in an average day
88 Don’t want to answer Participant does not want to answer how many cups of fruit she
consumes in an average day
This value will be flagged as a quality check
99 No answer recorded No answer recorded
This value will be flagged as an error
ANALYSIS AND USE To determine the healthy and risky behaviors of individual participants and the overall
WISEWOMAN population
To provide data element required to determine participant’s Simple 7 cardiovascular risk score
OTHER
INFORMATION Guidance
Examples of one cup of fruit from the American Heart Association’s Life’s Simple Seven provided in
Appendix G.
Codes and response options highlighted in gray should not appear on the data collection forms
presented to participants. They are provided for funded program use only.
Average fruit consumption at baseline screening or rescreening is required for a record to count as
a valid record. If Fruit is blank, coded as ‘‘99 No answer recorded,’ or outside of the valid range (1-
50 cups) the record will not count as a valid record, and the record will not count toward meeting a
program’s screening goal (performance measure #3).
OMB Approved
WISEWOMAN MDE Manual 45 August 2017
Edition 9.03 revised
Item 7b: Vegetables How many vegetables do you eat in an average day?
This variable indicates the amount of vegetables the participant consumes in an average day.
FORMAT Type: Numeric
Item Length: 2
Field Length: 4
Leading Zeros: Yes
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 108
Valid Range: 01-15; cannot be blank
SOURCE American Heart Association Life’s Simple 7
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION Number of cups Two-digit (numeric) value representing the number of cups of
vegetables the participant consumes in an average day
Any value outside the valid range (01 – 15) will be considered an
error
Example: 2 cups = 02
00 None Participant does not consume vegetables in an average day
88 Don’t want to answer Participant does not want to answer how many cups of vegetables
she consumes in an average day
This value will be flagged as a quality check
99 No answer recorded No answer recorded
This value will be flagged as an error
ANALYSIS AND USE To determine the healthy and risky behaviors of individual participants and the overall
WISEWOMAN population
To provide data element required to determine participant’s Simple 7 cardiovascular risk score
OTHER
INFORMATION Guidance
Examples of one cup of vegetables from the American Heart Association’s Life’s Simple Seven
provided in Appendix G.
Codes and response options highlighted in gray should not appear on the data collection forms
presented to participants. They are provided for funded program use only.
Average vegetable consumption at baseline screening or rescreening is required for a record to
count as a valid record. If Vegetable is blank, coded as ‘‘99 No answer recorded,’ or outside of the
valid range (1-15 cups) the record will not count as a valid record, and the record will not count
toward meeting a program’s screening goal (performance measure #3).
OMB Approved
WISEWOMAN MDE Manual 46 August 2017
Edition 9.03 revised
Item 7c: Fish Do you eat two servings or more of fish weekly?
This variable indicates whether the participant consumes two servings or more of fish weekly.
FORMAT Type: Numeric
Item Length: 1
Field Length: 2
Leading Zeros: No
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 112
Valid Range: See values; cannot be blank
SOURCE American Heart Association Life’s Simple 7
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION 1 Yes Participant consumes two servings or more of fish weekly
2 No Participant does not consume two servings or more of fish weekly
8 Don’t want to answer Participant does not want to answer whether she consumes two
servings or more of fish weekly
This value will be flagged as a quality check
9 No answer recorded No answer recorded
This value will be flagged as an error
ANALYSIS AND USE To determine the healthy and risky behaviors of individual participants and the overall
WISEWOMAN population
To provide data element required to determine participant’s Simple 7 cardiovascular risk score
OTHER
INFORMATION Examples of two servings of fish from the American Heart Association’s Life’s Simple Seven
provided in Appendix G.
Codes and response options highlighted in gray should not appear on the data collection forms
presented to participants. They are provided for funded program use only.
Average fish consumption at baseline screening or rescreening is required for a record to count as a
valid record. If Fish is blank or coded as ‘‘9 No answer recorded,’ the record will not count as a valid
record, and the record will not count toward meeting a program’s screening goal (performance
measure #3).
OMB Approved
WISEWOMAN MDE Manual 47 August 2017
Edition 9.03 revised
Item 7d: Grains Do you eat 3 ounces or more of whole grains daily?
This variable indicates whether the participant consumes 3 ounces or more of whole grains daily.
FORMAT Type: Numeric
Item Length: 1
Field Length: 2
Leading Zeros: No
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 114
Valid Range: See values; cannot be blank
SOURCE American Heart Association Life’s Simple 7
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION 1 Yes Participant consumes 3 ounces or more of whole grains daily
2 No Participant does not consume 3 ounces or more of whole grains daily
8 Don’t want to answer Participant does not want to answer whether she consumes 3 ounces
or more of whole grains daily
This value will be flagged as a quality check
9 No answer recorded No answer recorded
This value will be flagged as an error
ANALYSIS AND USE To determine the healthy and risky behaviors of individual participants and the overall
WISEWOMAN population
To provide data element required to determine participant’s Simple 7 cardiovascular risk score
OTHER
INFORMATION Examples of 3 ounces of whole grains from the American Heart Association’s Life’s Simple Seven
provided in Appendix G.
Codes and response options highlighted in gray should not appear on the data collection forms
presented to participants. They are provided for funded program use only.
Average whole grain consumption at baseline screening or rescreening is required for a record to
count as a valid record. If Grains is blank or coded as ‘‘9 No answer recorded,’ the record will not
count as a valid record, and the record will not count toward meeting a program’s screening goal
(performance measure #3).
OMB Approved
WISEWOMAN MDE Manual 48 August 2017
Edition 9.03 revised
Item 7e: Sugar Do you drink less than 36 ounces (450 calories) of beverages with added sugars weekly?
This variable indicates whether the participant drinks less than 36 ounces (450 calories) of
beverages with added sugars weekly.
FORMAT Type: Numeric
Item Length: 1
Field Length: 2
Leading Zeros: No
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 116
Valid Range: See values; cannot be blank
SOURCE American Heart Association Life’s Simple 7
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION 1 Yes Participant consumes less than 36 ounces (450 calories) of
beverages with added sugars in an average week
2 No Participant consumes 36 ounces or more (450 calories or more) of
beverages with added sugars in an average week
8 Don’t want to answer Participant does not want to answer whether she consumes less than
36 ounces (450 calories) or more of beverages with added sugars in
an average week
This value will be flagged as a quality check
9 No answer recorded No answer recorded
This value will be flagged as an error
ANALYSIS AND USE To determine the healthy and risky behaviors of individual participants and the overall
WISEWOMAN population
To provide data element required to determine participant’s Simple 7 cardiovascular risk score
OTHER
INFORMATION Examples of 36 ounces of beverages with added sugars from the American Heart Association’s
Life’s Simple Seven provided in Appendix G.
Codes and response options highlighted in gray should not appear on the data collection forms
presented to participants. They are provided for funded program use only.
Average sugar-sweetened beverage consumption at baseline screening or rescreening is required
for a record to count as a valid record. If Sugar is blank or coded as ‘‘9 No answer recorded,’ the
record will not count as a valid record, and the record will not count toward meeting a program’s
screening goal (performance measure #3).
OMB Approved
WISEWOMAN MDE Manual 49 August 2017
Edition 9.03 revised
Item 7f: SaltWatch Are you currently watching or reducing your sodium or salt intake?
This variable indicates whether the participant is currently watching or reducing her sodium or salt
intake.
FORMAT Type: Numeric
Item Length: 1
Field Length: 2
Leading Zeros: No
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 118
Valid Range: See values; cannot be blank
SOURCE CDC Behavioral Risk Factor Surveillance System
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION 1 Yes Participant is currently watching or reducing her sodium or salt intake
2 No Participant is not currently watching or reducing her sodium or salt
intake
8 Don’t want to answer Participant does not want to answer whether she is currently
watching or reducing her sodium or salt intake
This value will be flagged as a quality check
9 No answer recorded No answer recorded
This value will be flagged as an error
ANALYSIS AND USE To determine the healthy and risky behaviors of individual participants and the overall
WISEWOMAN population
OTHER
INFORMATION Codes and response options highlighted in gray should not appear on the data collection forms
presented to participants. They are provided for funded program use only.
OMB Approved
WISEWOMAN MDE Manual 50 August 2017
Edition 9.03 revised
Item 8a: PAMod How much moderate physical activity do you get in a week?
This variable indicates the amount of moderate physical activity the participant gets during an
average week.
FORMAT Type: Numeric
Item Length: 3
Field Length: 6
Leading Zeros: Yes
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 120
Valid Range: 010-850; cannot be blank
SOURCE American Heart Association Life’s Simple 7
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION Number of minutes A three-digit (numeric) value representing the minutes of moderate
physical activity the participant gets during an average week
Any value outside the valid range (010 – 850) will be considered a
quality check
Example: 30 minutes = 030
If the number of minutes of physical activity exceeds 850 minutes,
PAMod should be coded as 850 and the number of minutes of
physical activity should be documented using the Validation of Data
form. See Appendix B for the procedure for validating out-of-range
values.
000 None Participant does not get any moderate physical activity during an
average week
888 Don’t want to answer Participant does not want to answer how much moderate physical
activity she gets during an average week
This value will be flagged as a quality check
999 No answer recorded No answer recorded
This value will be flagged as an error
ANALYSIS AND USE To determine the healthy and risky behaviors of individual participants and the overall
WISEWOMAN population
To provide data element required to determine participant’s Simple 7 cardiovascular risk score
OTHER
INFORMATION Examples of moderate physical activity from the American Heart Association’s Life’s Simple Seven
provided in Appendix G.
Codes and response options highlighted in gray should not appear on the data collection forms
presented to participants. They are provided for funded program use only.
Average moderate physical activity at baseline screening or rescreening is required for a record to
count as a valid record. If PAMod is blank or coded as ‘‘999 No answer recorded,’ the record will
not count as a valid record, and the record will not count toward meeting a program’s screening
goal (performance measure #3).
OMB Approved
WISEWOMAN MDE Manual 51 August 2017
Edition 9.03 revised
Item 8b: PAVig How much vigorous physical activity do you get in a week?
This variable indicates the amount of vigorous physical activity the participant gets during an
average week.
FORMAT Type: Numeric
Item Length: 3
Field Length: 6
Leading Zeros: Yes
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 126
Valid Range: 010-850; cannot be blank
SOURCE American Heart Association Life’s Simple 7
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION Number of minutes A three-digit (numeric) value representing the minutes of vigorous
physical activity the participant gets during an average week
Any value outside the valid range (010 – 850) will be considered a
quality check
Example: 30 minutes = 030
If the number of minutes of physical activity exceeds 850 minutes,
PAVig should be coded as 850 and the number of minutes of
physical activity should be documented using the Validation of Data
form. See Appendix B for the procedure for validating out-of-range
values.
000 None Participant does not get any vigorous physical during an average
week
888 Don’t want to answer Participant does not want to answer how much vigorous physical
activity she gets during an average week
This value will be flagged as a quality check
999 No answer recorded No answer recorded
This value will be flagged as an error
ANALYSIS AND USE To determine the healthy and risky behaviors of individual participants and the overall
WISEWOMAN population
To provide data element required to determine participant’s Simple 7 cardiovascular risk score
OTHER
INFORMATION Examples of vigorous physical activity from the American Heart Association’s Life’s Simple Seven
provided in Appendix G.
Codes and response options highlighted in gray should not appear on the data collection forms
presented to participants. They are provided for funded program use only.
Average vigorous physical activity at baseline screening or rescreening is required for a record to
count as a valid record. If PAVig is blank or coded as ‘‘999 No answer recorded,’ the record will not
count as a valid record, and the record will not count toward meeting a program’s screening goal
(performance measure #3).
OMB Approved
WISEWOMAN MDE Manual 52 August 2017
Edition 9.03 revised
Item 9a: Smoker Do you smoke? Includes cigarettes, pipes, or cigars (smoked tobacco in any form)
This variable indicates whether the participant smokes tobacco in any form, including cigarettes,
pipes, or cigars.
FORMAT Type: Numeric
Item Length: 1
Field Length: 2
Leading Zeros: No
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 132
Valid Range: See values; cannot be blank
SOURCE American Heart Association Life’s Simple 7
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION 1 Current Smoker Participant currently smokes tobacco in any form, including
cigarettes, pipes, or cigars
2 Quit (1-12 months ago) Participant quit smoking tobacco in any form, including cigarettes,
pipes, or cigars, 1 to 12 months ago
3 Quit (More than 12
months ago) Participant quit smoking tobacco in any form, including cigarettes,
pipes, or cigars, more than 12 months ago
4 Never Smoked Participant has never smoked tobacco in any form, including
cigarettes, pipes, or cigars
8 Don’t want to answer Participant does not want to answer whether she smokes tobacco in
any form, including cigarettes, pipes, or cigars
This value will be flagged as a quality check
9 No answer recorded No answer recorded
This value will be flagged as an error
ANALYSIS AND USE To determine the healthy and risky behaviors of individual participants and the overall
WISEWOMAN population
To identify participants who might benefit from smoking cessation counseling and tobacco cessation
resources (quit line and community-based)
To provide data element required to determine participant’s Simple 7 cardiovascular risk score
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
presented to participants. They are provided for funded program use only.
Smoking status at baseline screening or rescreening is required for a record to count as a valid
record. If Smoker is blank or coded as ‘‘9 No answer recorded,’ the record will not count as a valid
record, and the record will not count toward meeting a program’s screening goal (performance
measure #3).
OMB Approved
WISEWOMAN MDE Manual 53 August 2017
Edition 9.03 revised
Item 9b: Sechand About how many hours a day, on average, are you in the same room or vehicle with another
person who is smoking?
This variable indicates how many hours a day, on average, the participant is in the same room or
vehicle as another person who is smoking.
FORMAT Type: Numeric
Item Length: 2
Field Length: 4
Leading Zeros: Yes
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 134
Valid Range: 01-24; cannot be blank
SOURCE
Pregnancy Risk Assessment Monitoring System
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION Number of hours A two-digit (numeric) value indicating the number of hours per day,
on average, the participant is in the same room or vehicle as another
person who is smoking
Any value outside the valid range (01 – 24) will be considered an
error
Example: 2 hours = 02
66 Less than one Participant is in the same room or vehicle with another person who is
smoking less than one hour per day, on average
00 None Participant is never in the same room or vehicle as another person
who is smoking
88 Don’t want to answer Participant does not want to answer the number of hours per day, on
average, that she is in the same room or vehicle as another person
who is smoking
This value will be flagged as a quality check
99 No answer recorded No answer recorded
This value will be flagged as an error
ANALYSIS AND USE To determine the exposure of individual participants and the overall WISEWOMAN population to
risks in the environment
To help assess use of community-based referral resources and risk reduction counseling for those
exposed to secondhand smoke
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
presented to participants. They are provided for funded program use only.
All participants should be asked this question, regardless of their smoking status.
If a participant responds with a value greater than 24 hours, reports that she doesn’t know, or
refuses to answer, a discussion with the participant should be conducted to verify the response.
OMB Approved
WISEWOMAN MDE Manual 54 August 2017
Edition 9.03 revised
Item 10a: QOLPH Thinking about your physical health, which includes physical illness and injury, on how
many days during the past 30 days was your physical health not good?
This variable indicates the number of days during the past 30 days that the participant’s physical
health, including physical illness and injury, was not good.
FORMAT Type: Numeric
Item Length: 2
Field Length: 4
Leading Zeros: Yes
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 138
Valid Range: 00-30; cannot be blank
SOURCE CDC Health-Related Quality of Life Measures
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION Number of days A two-digit (numeric) value representing the number of days during
the past 30 days that the participant’s physical health, including
physical illness and injury, was not good
Any value outside the valid range (00 – 30) will be considered an
error
Example: 2 days = 02
77 Don’t know/Not sure Participant does not know how many days during the past 30 days
that her physical health, including physical illness and injury, was not
good
This value will be flagged as a quality check
88 Don’t want to answer Participant does not want to answer how many days during the past
30 days that her physical health, including physical illness and injury,
was not good
This value will be flagged as a quality check
99 No answer recorded No answer recorded
This value will be flagged as an error
ANALYSIS AND USE To determine the health status of individual participants and the overall WISEWOMAN population
To provide health status information for cost benefit or cost effectiveness analyses
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
presented to participants. They are provided for funded program use only.
OMB Approved
WISEWOMAN MDE Manual 55 August 2017
Edition 9.03 revised
Item 10b: QOLMH Thinking about your mental health, which includes stress, depression, and problems with
emotions, on how many days during the past 30 days was your mental health not good?
This variable indicates the number of days during the past 30 days that the participant’s mental
health, including stress, depression, and problems with emotions, was not good.
FORMAT Type: Numeric
Item Length: 2
Field Length: 4
Leading Zeros: Yes
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 142
Valid Range: 00-30; cannot be blank
SOURCE CDC Health-Related Quality of Life Measures
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION Number of days A two-digit (numeric) value representing the number of days during
the past 30 days that the participant’s mental health, including stress,
depression, and problems with emotions, was not good
Any value outside the valid range (00 – 30) will be considered an
error
Example: 2 days = 02
77 Don’t know/Not sure Participant does not know how many days during the past 30 days
that the participant’s mental health, including stress, depression, and
problems with emotions, was not good
This value will be flagged as a quality check
88 Don’t want to answer Participant does not want to answer how many days during the past
30 days that the participant’s mental health, including stress,
depression, and problems with emotions, was not good
This value will be flagged as a quality check
99 No answer recorded No answer recorded
This value will be flagged as a quality check
ANALYSIS AND USE To determine the health status of individual participants and the overall WISEWOMAN population
To provide health status information for cost benefit or cost effectiveness analyses
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
presented to participants. They are provided for funded program use only.
OMB Approved
WISEWOMAN MDE Manual 56 August 2017
Edition 9.03 revised
Item 10c: QOLEffect During the past 30 days, on about how many days did poor physical or mental health keep
you from doing your usual activities, such as self-care, work, or recreation?
This variable indicates the number of days during the past 30 days that the participant’s poor
physical or mental health kept her from doing her usual activities, such as self-care, work, or
recreation.
FORMAT Type: Numeric
Item Length: 2
Field Length: 4
Leading Zeros: Yes
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 146
Valid Range: 00-30; cannot be blank
SOURCE CDC Health-Related Quality of Life Measures
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION Number of days A two-digit (numeric) value representing the number of days during
the past 30 days that the participant’s poor physical or mental health
kept her from doing her usual activities, such as self-care, work, or
recreation
A value of 00 should be provided for QOLEffect if the participant
indicates that she has not experienced any days of poor physical
health (10a – QOLPH) or poor mental health (10b – QOLMH) during
the past 30 days.
Any value outside the valid range (00 – 30) will be considered an
error
Example: 2 days = 02
77 Don’t know/Not sure Participant does not know how many days during the past 30 days
that the participant’s poor physical or mental health kept her from
doing her usual activities, such as self-care, work, or recreation
This value will be flagged as a quality check
88 Don’t want to answer Participant does not want to answer how many days during the past
30 days that the participant’s poor physical or mental health kept her
from doing her usual activities, such as self-care, work, or recreation
This value will be flagged as a quality check
99 No answer recorded No answer recorded
This value will be flagged as a quality check
ANALYSIS AND USE To determine the health status of individual participants and the overall WISEWOMAN population
To provide health status information for cost benefit or cost effectiveness analyses
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
presented to participants. They are provided for funded program use only.
OMB Approved
WISEWOMAN MDE Manual 57 August 2017
Edition 9.03 revised
Item 11a: Height Height
This variable indicates the participant’s height in inches at baseline screening.
FORMAT Type: Numeric
Item Length: 2
Field Length: 2
Leading Zeros: Yes
Static Field: Yes
Other Format: N/A
Justification: Right
Beginning Position: 150
Valid Range: 48-76; cannot be blank if
TYPE is 1 or 2 (baseline
screening or rescreening)
SOURCE
American Heart Association Life’s Simple 7
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION Height in inches Up to a two-digit (numeric) value representing the participant’s height
at baseline screening
Height values between 48" and 58" or 74" and 76" will be flagged for
quality checks and program verification. See Appendix B for the
procedure for validating out-of-range values. Any values outside 48"-
76" will be considered an error
Example: 62" (5 feet, 2 inches) = 62
77 Unable to obtain Height measurement was attempted, but measurement results were
not obtained. See Appendix B for the procedure for documenting the
reason that the measurement was not obtained
This value will be flagged as an error
88 Client refused Participant refuses to have her height measurement taken
This value will be flagged as an error
99 No measurement
recorded Height measurement was not performed
This value will be flagged as an error
ANALYSIS AND USE To calculate the BMI of WISEWOMAN participants
To understand the cardiovascular disease risk factors of individual participants and the overall
WISEWOMAN population
To provide data element required to determine participant’s Simple 7 cardiovascular risk score
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
completed by the provider. They are provided for funded program use only.
All height measurements should be recorded in inches.
Height measurement at baseline screening or rescreening is required for a record to count as a
valid record. If Height is blank or coded as ‘777 Unable to obtain,’ ‘888 Client refused,’ or ‘999 No
measurement recorded,’ or is outside of the valid range (48-76 inches) the record will not count as a
valid record, and the record will not count toward meeting a program’s screening goal (performance
measure #3).If exceptional circumstances do not allow height measurement, these reasons should
be documented as instructed in Appendix B.
OMB Approved
WISEWOMAN MDE Manual 58 August 2017
Edition 9.03 revised
Item 11b: Weight Weight
This variable indicates the participant’s weight in pounds.
FORMAT Type: Numeric
Item Length: 3
Field Length: 6
Leading Zeros: Yes
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 152
Valid Range: 074-460; cannot be blank if
TYPE is 1 or 2 (baseline
screening or rescreening)
SOURCE
American Heart Association Life’s Simple 7
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION Weight in pounds Up to a three-digit (numeric) value representing the participant’s
weight
Weight values between 74 and 90 lb or 350 and 460 lb will be flagged
for quality checks and program verification. See Appendix B for the
procedure for validating out-of-range values. Any values outside 74-
460 lb will be considered an error
Example: 98 lb = 098
777 Unable to obtain Weight measurement was attempted, but measurement results were
not obtained
This value will be flagged as a quality check. See Appendix B for the
procedure for documenting the reason that the measurement was not
obtained
888 Client refused Participant refuses to have her weight measurement taken
This value will be flagged as a quality check
999 No measurement
recorded Weight measurement was not performed
This value will be flagged as an error
ANALYSIS AND USE To calculate the BMI of WISEWOMAN participants
To understand the cardiovascular disease risk factors of individual participants and the overall
WISEWOMAN population
To provide data element required to determine participant’s Simple 7 cardiovascular risk score
OTHER
INFORMATION Codes and response options highlighted in gray should not appear on the data collection forms
completed by the provider. They are provided for funded program use only.
Weight measurement at baseline screening or rescreening is required for a record to count as a
valid record. If Weight is blank or coded as ‘999 No measurement recorded,’ or is outside of the
valid range (74-460 lbs) the record will not count as a valid record, and the record will not count
toward meeting a program’s screening goal (performance measure #3).If exceptional
circumstances do not allow weight measurement, these reasons should be documented as
instructed in Appendix B.
OMB Approved
WISEWOMAN MDE Manual 59 August 2017
Edition 9.03 revised
Item 11c: Waist Waist Circumference
This variable indicates the participant’s waist circumference in inches.
FORMAT Type: Numeric
Item Length: 2
Field Length: 4
Leading Zeros: No
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 158
Valid Range: 16-71; cannot be blank if
TYPE is 1 or 2 (baseline
screening or rescreening)
SOURCE Not applicable; health screening measurement
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION Waist Circumference in inches Up to a two-digit (numeric) value representing the participant’s
waist circumference in inches
Any value outside the valid range (16 – 71 inches) will be
flagged as a quality check
Example: 30 inches = 30
77 Unable to obtain Waist circumference measurement was attempted, but
measurement results were not obtained
88 Client refused Participant refuses to have her waist circumference
measurement taken
99 No measurement recorded Waist circumference measurement was not performed
ANALYSIS AND USE To determine waist-hip ratio for the participant
To understand the cardiovascular disease risk factors of individual participants and the overall
WISEWOMAN population
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
completed by the provider. They are provided for funded program use only.
OMB Approved
WISEWOMAN MDE Manual 60 August 2017
Edition 9.03 revised
Item 11d: Hip Hip Circumference
This variable indicates the participant’s hip circumference in inches.
FORMAT Type: Numeric
Item Length: 2
Field Length: 4
Leading Zeros: No
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 162
Valid Range: 26-75; cannot be blank if
TYPE is 1 or 2 (baseline
screening or
rescreening)
SOURCE Not applicable; health screening measurement
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION Hip Circumference in inches Up to a two-digit (numeric) value representing the participant’s hip
circumference in inches
Any value outside the valid range (26 – 75 inches) will be flagged
as a quality check
Example: 30 inches = 30
77 Unable to obtain Hip circumference measurement was attempted, but
measurement results were not obtained
88 Client refused Participant refuses to have her hip circumference measurement
taken
99 No measurement
recorded Hip circumference measurement was not performed
ANALYSIS AND USE To determine waist-hip ratio for the participant
To understand the cardiovascular disease risk factors of individual participants and the overall
WISEWOMAN population
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
completed by the provider. They are provided for funded program use only.

OMB Approved
WISEWOMAN MDE Manual 61 August 2017
Edition 9.03 revised
Item 12a: BPDate Blood Pressure Measurement Date (Office Visit Date)
This variable indicates the date of the office visit when a blood pressure measurement is obtained
or the date of the follow-up assessment for a particpant.
FORMAT Type: Numeric
Item Length: 8
Field Length: 16
Leading Zeros: Yes
Static Field: No
Other Format: MMDDCCYY
Justification: Right
Beginning Position: 166
Valid Range: Valid date
SOURCE
Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION Blood pressure
measurement date/Office
visit date
Valid date in MMDDCCYY format
Date of the office visit and when a blood pressure measurement is
obtained or the date of the follow-up assessment for a participant
Example: September 10, 2013 = 09102013
ANALYSIS AND USE To identify the date of the office visit and blood pressure measurements
To facilitate analysis of changes in blood pressure over time
To calculate other service time frames, including time to rescreening, lifestyle program sessions,
lifestyle program/health coaching follow-up assessment, risk reduction counseling sessions, alert
referrals, and labs
OTHER
INFORMATION Guidance
Blood pressure measurement date should be used to indicate the date that blood pressure
measurement was obtained for baseline screening or rescreening, or the date of the follow-up
assessment for a participant.
If a blood pressure measurement was not obtained at the time of the office visit and obtained at a
referral visit within 30 days of the visit, the date of the office visit should be recorded here.
If a blood pressure measurement is attempted but not obtained at the office visit or within 30 days of
the office visit, the date of the office visit date should be recorded here.
Blood pressure measurement date also represents the date of the office visit. As a result, if blood
pressure measurements are marked as being unable to obtain” or refused (SBP1, DBP1, SBP2,
and DBP2 all = 777 or 888), the date of office visit should be entered. An explanation for the
inability to obtain the blood pressure measurements or refusal of blood pressure measurements
should be documented using the validation form in Appendix B.
If BPDate of baseline screening is missing or invalid, the record will not count as a valid record, and
the record will not count toward meeting a program’s screening goal (performance measure #3).
Since all screening measurements and assessments are to be used to determine participation in the
lifestyle programs and health coaching, it is expected that all labs and other screening services will
be completed within as short a time frame as possible. Thirty days is the recommended time frame
in which blood pressure measurements should be done prior to or after the office visit unless
specified by the program’s medical advisory group or medical clinic.
Additional Edits
Since blood pressure measurement date now also represents office visit date, this field should
never be blank. An error flag will occur if blood pressure measurement/office visit date is left blank
or if an invalid date is entered.
Error: BPDATE = . or BPDATE = [invalid]
Blood pressure should have been measured on the current date or earlier.
Error: BPDATE > [current date]
Error: BPDate < July 1, 2013 AND MDE Ver = 900
OMB Approved
WISEWOMAN MDE Manual 62 August 2017
Edition 9.03 revised
Item 12b: SBP1
Systolic Blood Pressure #1
This variable indicates the participant’s first systolic blood pressure reading.
FORMAT
Type: Numeric
Item Length: 3
Field Length: 6
Leading Zeros: Yes
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 182
Valid Range: 074-260; cannot be blank if TYPE is 1 or
2 (baseline screening or rescreening)
SOURCE
American Heart Association Life’s Simple 7
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION
Systolic blood
pressure in
mmHg
A three-
digit (numeric) value representing the participant’s first systolic blood pressure
in mmHg
Systolic blood pressure values between 230 and 260 mmHg will be flagged for quality
checks and program verification. Values outside 74-260 mmHg will be flagged as
errors. See Appendix B for the procedure for validating out-of-range values
If a blood pressure measurement was not obtained at the time of the office visit and
obtained at a referral visit within 30 days of the visit, the blood pressure measurement
from the referral should be recorded here
Example: 90 mmHg = 090
777 Unable to
obtain First systolic blood pressure measurement was attempted, but results were not
obtained due to technical difficulties or errors
See Appendix B for the procedure for documenting the reason that the measurement
could not be obtained
This value will be flagged as an error
888 Client
refused Participant refuses to have her first systolic blood pressure measurement taken
This value will be flagged as an error
999 No
measurement
recorded
First systolic blood pressure measurement was not performed or not recorded
This value will be flagged as an error
ANALYSIS AND
USE
To identify those at increased risk for cardiovascular conditions, including heart attack, heart failure,
stroke, and kidney disease
To identify participants who would benefit from lifestyle programs
To identify participants unaware that they have hypertension (high blood pressure) for referral to medical
management
To determine control and management of blood pressure
To identify participants who require further diagnostic evaluation
To identify hypertension (high blood pressure) risk of the WISEWOMAN population
To provide data element required to determine participant’s Simple 7 cardiovascular risk score
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms completed
by the provider. They are provided for funded program use only.
First systolic blood pressure measurement at baseline screening or rescreening is required for a record to
count as a valid record. If SBP1 is blank or coded as ‘777 Unable to obtain,’ ‘888 Client refused,’ or ‘999
No measurement recorded,’ or is ouside of the valid range (74-260 mmHg) the record will not count as a
valid record, and the record will not count toward meeting a program’s screening goal (performance
measure #3).If exceptional circumstances do not allow a first blood pressure measurement (cases where
first blood pressure measurement is coded as ‘777 Unable to obtain’), these reasons should be
documented as instructed in Appendix B.
Two blood pressures must be taken and reported using proper technique (refer to JNC-7). If more than
two blood pressure readings are taken, the medical director should decide which two to report.
Cross edits
First blood pressure should be recorded before second blood pressure. An error flag will occur if a second
systolic blood pressure measurement is recorded, but a first systolic blood pressure measurement has not
been recorded.
Error: (SBP1 = 777, 888, or 999)
AND (
SBP2 ≠ 777, 888, or 999)
OMB Approved
WISEWOMAN MDE Manual 63 August 2017
Edition 9.03 revised
Item 12c: DBP1 Diastolic Blood Pressure #1
This variable indicates the participant’s first diastolic blood pressure reading.
FORMAT Type: Numeric
Item Length: 3
Field Length: 6
Leading Zeros: Yes
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 188
Valid Range: 002-156; cannot be blank if
TYPE is 1 or 2 (baseline
screening or rescreening)
SOURCE
American Heart Association Life’s Simple 7
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION Diastolic blood pressure
in mmHg A three-digit (numeric) value representing the participant’s diastolic
blood pressure in mmHg
First diastolic blood pressure values between 2-12 mmHg or 122-156
mmHg will be flagged for quality checks and program verification.
Values outside 2-156 mmHg will be considered errors. See Appendix
B for the procedure for validating out-of-range values
If a blood pressure measurement was not obtained at the time of the
office visit and obtained at a referral visit within 30 days of the visit,
the blood pressure measurement from the referral should be
recorded here
Example: 85 mmHg = 085
777 Unable to obtain First diastolic blood pressure measurement was attempted, but
results were not obtained due to technical difficulties or errors
See Appendix B for the procedure for documenting the reason that
the measurement could not be obtained
This value will be flagged as an error
888 Client refused Participant refuses to have her first diastolic blood pressure
measurement taken
This value will be flagged as an error
999 No measurement
recorded First diastolic blood pressure measurement was not performed or not
recorded
This value will be flagged as an error
ANALYSIS AND USE To identify those at increased risk for cardiovascular conditions, including heart attack, heart failure,
stroke, and kidney disease
To identify participants who would benefit from lifestyle programs
To identify participants unaware that they have hypertension(high blood pressure) for referral to
medical management
To determine control and management of blood pressure
To identify participants who require further diagnostic evaluation
To identify hypertension (high blood pressure) risk of the WISEWOMAN population
To provide data element required to determine participant’s Simple 7 cardiovascular risk score

OMB Approved
WISEWOMAN MDE Manual 64 August 2017
Edition 9.03 revised
Item 12c: DBP1 Diastolic Blood Pressure #1
This variable indicates the participant’s first diastolic blood pressure reading.
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
completed by the provider. They are provided for funded program use only.
First diastolic blood pressure measurement at baseline screening or rescreening is required for a
record to count as a valid record. If DBP1 is blank or coded as ‘777 Unable to obtain,’ ‘888 Client
refused,’ or ‘999 No measurement recorded,’ or is ouside of the valid range (2-156 mmHg) the
record will not count as a valid record, and the record will not count toward meeting a program’s
screening goal (performance measure #3).If exceptional circumstances do not allow a first blood
pressure measurement (cases where first blood pressure measurement is coded as ‘777 Unable to
obtain’), these reasons should be documented as instructed in Appendix B.
Two blood pressures must be taken and reported using proper technique (refer to JNC-7). If more
than two blood pressure readings are taken, the medical director should decide which two to report.
Cross edits
First blood pressure should be recorded before second blood pressure. An error flag will occur if a
second diastolic blood pressure measurement is recorded, but a first diastolic blood pressure
measurement has not been recorded.
Error: (DBP1 = 777, 888, or 999) AND (DBP2 ≠ 777, 888, or 999)
If a provider is unable to obtain systolic blood pressure, diastolic blood pressure should also not
have been obtained. An error flag will occur if only one of the first blood pressure measurements is
coded as ’777 Unable to obtain.’
Error: (SBP1 = 777 AND DBP1 ≠ 777) OR (SBP1 ≠ 777 AND DBP1 = 777)
OMB Approved
WISEWOMAN MDE Manual 65 August 2017
Edition 9.03 revised
Item 12d: SBP2 Systolic Blood Pressure #2
This variable indicates the participant’s second systolic blood pressure reading.
FORMAT Type: Numeric
Item Length: 3
Field Length: 6
Leading Zeros: Yes
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 194
Valid Range: 074-260; cannot be blank if
TYPE is 1 or 2 (baseline
screening or rescreening)
SOURCE
American Heart Association Life’s Simple 7
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION Systolic blood pressure
in mmHg A three-digit (numeric) value representing the participant’s second
systolic blood pressure in mmHg
Systolic blood pressure values between 230 and 260 mmHg will be
flagged for quality checks and program verification. Values outside
74-260 mmHg will be flagged as errors. See Appendix B for the
procedure for validating out-of-range values
If a blood pressure measurement was not obtained at the time of the
office visit and obtained at a referral visit within 30 days of the visit,
the blood pressure measurement from the referral should be
recorded here
Example: 90 mmHg = 090
777 Unable to obtain
Second systolic blood pressure measurement was attempted, but
results were not obtained due to technical difficulties or errors
See Appendix B for the procedure for documenting the reason that
the measurement was not obtained
This value will be flagged as an error
888 Client refused Participant refuses to have her second systolic blood pressure
measurement taken
This value will be flagged as an error
999 No measurement
recorded Second systolic blood pressure measurement was not performed or
not recorded
This value will be flagged as an error
ANALYSIS AND USE To identify those at increased risk for cardiovascular conditions, including heart attack, heart failure,
stroke, and kidney disease
To identify participants who would benefit from lifestyle programs
To identify participants unaware that they have hypertension (high blood pressure) for referral to
medical management
To determine control and management of blood pressure among those currently being treated
To identify participants who require further diagnostic evaluation
To identify hypertension (high blood pressure) risk in the WISEWOMAN population
To provide data element required to determine participant’s Simple 7 cardiovascular risk score
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
completed by the provider. They are provided for funded program use only.
Two blood pressures must be taken and reported using proper technique (refer to JNC-7). If more
than two blood pressure readings are taken, the medical director should decide which two to report.

OMB Approved
WISEWOMAN MDE Manual 66 August 2017
Edition 9.03 revised
Item 12e: DBP2 Diastolic Blood Pressure #2
This variable indicates the participant’s second diastolic blood pressure reading.
FORMAT Type: Numeric
Item Length: 3
Field Length: 6
Leading Zeros: Yes
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 200
Valid Range: 002-156; cannot be blank if
TYPE is 1 or 2 (baseline
screening or rescreening)
SOURCE
American Heart Association Life’s Simple 7
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION Diastolic blood pressure
in mmHg A three-digit (numeric) value representing the participant’s diastolic
blood pressure in mmHg
Second diastolic blood pressure values between 2 and 12 mmHg or
122 and 156 mmHg will be flagged for quality checks and program
verification. Values outside 2-156 mmHg will be considered errors.
See Appendix B for the procedure for validating out-of-range values
If a blood pressure measurement was not obtained at the time of the
office visit and obtained at a referral visit within 30 days of the visit,
the blood pressure measurement from the referral should be
recorded here
Example: 85 mmHg = 085
777 Unable to obtain Second diastolic blood pressure measurement was attempted, but
results were not obtained due to technical difficulties or errors
See Appendix B for the procedure for documenting the reason that
the measurement was not obtained
This value will be flagged as an error
888 Client refused Participant refuses to have her second diastolic blood pressure
measurement taken
This value will be flagged as an error
999 No measurement
recorded Second diastolic blood pressure measurement was not performed or
not recorded
This value will be flagged as an error
ANALYSIS AND USE To identify those at increased risk for cardiovascular conditions, including heart attack, heart failure,
stroke, and kidney disease
To identify participants who would benefit from lifestyle programs
To identify participants unaware that they have hypertension (high blood pressure) for referral to
medical management
To determine control and management of blood pressure
To identify participants who require further diagnostic evaluation
To identify hypertension (high blood pressure) risk of the WISEWOMAN population
To provide data element required to determine participant’s Simple 7 cardiovascular risk score
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
completed by the provider. They are provided for funded program use only.
Two blood pressures must be taken and reported using proper technique (refer to JNC-7). If more
than two blood pressure readings are taken, the medical director should decide which two to report.
Cross edits
If a provider is unable to obtain systolic blood pressure, diastolic blood pressure should also not
have been obtained. An error flag will occur if only one of the second blood pressure measurements
is coded as ’777 Unable to obtain.’
Error: (SBP2 = 777 AND DBP2 ≠ 777) OR (SBP2 ≠ 777 AND DBP2 = 777)

OMB Approved
WISEWOMAN MDE Manual 67 August 2017
Edition 9.03 revised
Item 12e: DBP2 Diastolic Blood Pressure #2
This variable indicates the participant’s second diastolic blood pressure reading.
Item 13a: Fast Fasting Status
This variable indicates whether a participant fasted for at least nine hours prior to having blood
drawn for cholesterol or glucose measurements.
FORMAT Type: Numeric
Item Length: 1
Field Length: 2
Leading Zeros: No
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 206
Valid Range: See values; cannot be blank if
TYPE is 1 or 2 (baseline
screening or rescreening)
SOURCE Not applicable; health screening measurement
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION 1 Yes Participant fasted for at least nine hours prior to having blood drawn
2 No Participant did not fast for at least nine hours prior to having blood
drawn
9 No answer recorded No answer recorded
Provider failed to confirm fasting status or no information is available
from the provider
This value should be marked if 14b: TotChol, 14c: HDL, 14d: LDL,
14e: Trigly, and 14b: Glucose all are equal to 999/9999, 777/7777, or
888/8888
This value will be flagged as a quality check
ANALYSIS AND USE To facilitate accurate identification of participants who have high cholesterol, borderline high
cholesterol, diabetes, or pre-diabetes
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
completed by the provider. They are provided for funded program use only.
If a participant reports that she doesn’t know or refuses blood work, programs should have a
discussion with the participant to verify the response.
Cross edits
If not all cholesterol and glucose measurements were obtained because of an inadequate blood
sample or technical difficulties or errors, fasting status should be coded as ‘9 No answer recorded.’
An quality check flag will occur if not all cholesterol and glucose measurements were obtained due
to an inadequate blood sample or technical difficulties or errors and fasting status is not coded as ‘9
No answer recorded.’ This field will also be flagged for a quality check if fasting status is coded as ‘9
No answer recorded’ when at least one cholesterol or glucose measurement is not coded as
‘777/7777 Inadequate blood sample.’
Quality check: (FAST ≠ 9 AND TOTCHOL, HDL, LDL, TRIGLY, GLUCOSE all = 777/7777) OR
(FAST = 9 AND TOTCHOL, HDL, LDL, TRIGLY, GLUCOSE all ≠ 777/7777)
If a participant refused blood work, then fasting status should be coded as ‘9 No answer recorded.’
A quality check flag will occur if cholesterol fasting status is not coded as ‘9 No answer recorded’
when the participant refused blood work for cholesterol measurements.
Quality check: (FAST ≠ 9 AND TOTCHOL, HDL, LDL, TRIGLY, GLUCOSE all = 888/8888) OR
(FAST = 9 AND TOTCHOL, HDL, LDL, TRIGLY, GLUCOSE all ≠ 888/8888)
If no cholesterol measurements were recorded, then cholesterol fasting status should also not be
recorded. A quality check flag will occur if cholesterol fasting status is recorded when no cholesterol
measurements are recorded.
Quality check: FAST ≠ 9 AND TOTCHOL, HDL, LDL, TRIGLY, GLUCOSE all = 999/9999

OMB Approved
WISEWOMAN MDE Manual 68 August 2017
Edition 9.03 revised
Item 12e: DBP2 Diastolic Blood Pressure #2
This variable indicates the participant’s second diastolic blood pressure reading.
Item 14a: TCDate Cholesterol Measurement Date
This variable indicates the date that the cholesterol measurements were taken.
FORMAT Type: Numeric
Item Length: 8
Field Length: 16
Leading Zeros: Yes
Static Field: No
Other Format: MMDDCCYY
Justification: Right
Beginning Position: 208
Valid Range: Valid date; must be blank if
TotChol, and HDL, LDL, and
Trigly all = 888/8888 or
999/9999 ; cannot be blank if
TYPE is 1 (baseline
screening) or 2 (rescreening)
and HDL, LDL, and Trigly all ≠
888/8888 or 999/9999
SOURCE Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION Screening Date Valid date in MMDDCCYY format
The date recorded in this field must be the date that the total and
HDL cholesterol values were taken; total cholesterol and HDL
measurements are minimum requirements for every participant
If a lipid panel is completed as part of the screening process, the
date recorded must be the date that the lipid panel was done
Example: September 10, 2013 = 09102013
ANALYSIS AND USE To determine the date of the cholesterol measurements
To facilitate analysis of changes in control and management of cholesterol over time
OTHER
INFORMATION Cross edits
Cholesterol measurement date should not be blank if there is evidence of an attempt to measure
cholesterol. An error flag will occur if the cholesterol measurement date is blank when there is
evidence of an attempt to measure cholesterol.
Error: TCDATE = . AND (TOTCHOL, HDL, LDL, OR TRIGLY ≠ (888/8888, 999/9999))
Additional edits
Cholesterol should have been measured on the current date or earlier. An error flag will occur if the
cholesterol measurement date is in the future.
Error: TCDATE > [current date]
OMB Approved
WISEWOMAN MDE Manual 69 August 2017
Edition 9.03 revised
Item 14b: TotChol
Total Cholesterol (fasting or nonfasting)
This variable indicates the participant’s total cholesterol level.
FORMAT
Type: Numeric
Item Length: 3
Field Length: 6
Leading Zeros: Yes
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 224
Valid Range: 044-702 mg/dL; cannot be blank if
TYPE is 1 or 2 (baseline screening or
rescreening)
SOURCE
American Heart Association Life’s Simple 7
DENOMINATOR
POPULATION
The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION
Total
cholesterol in
mg/dL
A three-digit (numeric) value representing the participant’s total cholesterol in
mg/dL
Total cholesterol values that are between 44 and 60 mg/dL or 400 and 702 mg/dL
will be flagged for quality checks and program verification. Values outside 44-702
will be considered errors. See Appendix B for the procedure for validating out-of-
range values
Example: 90 mg/dL = 090
777
Inadequate
blood sample
Total cholesterol measurement was attempted, but results were not obtained due
to technical difficulties or errors
This may include issues such as (1) two or more failed venipuncture attempts; (2)
insufficient amount of blood, type of test tube; (3) invalid Cholestech readings due
to very high/low values; (4) sample submitted to laboratory, test not done due to
erroneous or missing laboratory request or other paperwork
See Appendix B for the procedure for documenting the reason that the
measurement was not obtained
This value will be flagged as a quality check
888 Client
refused Participant refuses to have her blood drawn for cholesterol measurements
If the participant refuses to go to the lab, the participant can be considered to have
refused
If the participant does not go to the scheduled lab appointment after follow-up has
been attempted, the participant can be considered to have refused
This value will be flagged as a quality check
999 No
measurement
recorded
No total cholesterol measurement was taken or recorded
This value will be flagged as an error
ANALYSIS AND USE
To identify participants who are unaware that they have high or borderline high cholesterol and need
preventive services or referral to medical management
To determine cholesterol control and management
To assess the percentage of WISEWOMAN participants who have high cholesterol or borderline
high cholesterol
To assess the risk in the WISEWOMAN population for cardiovascular disease
To provide data element required to determine participant’s Simple 7 cardiovascular risk score
OTHER
INFORMATION
Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
completed by the provider. They are provided for funded program use only.
Total cholesterol measurement may be taken as fasting or nonfasting. At a minimum, every
participant must have a total cholesterol and HDL cholesterol (14c: HDL) value recorded. If the
participant was fasting and had a lipid panel completed at the baseline, rescreening, or follow-up
visit, then LDL (14d: LDL) and triglyceride (14e: Trigly) values can also be recorded in addition to
total and HDL cholesterol.
Total cholesterol measurement at baseline screening or rescreening is required for a record to count
as a valid record. If TotChol is blank or coded as ‘999 No measurement recorded,’ or is ouside of
the valid range (044-702 mg/dL) the record will not count as a valid record, and the record will not
count toward meeting a program’s screening goal (performance measure #3).
OMB Approved
WISEWOMAN MDE Manual 70 August 2017
Edition 9.03 revised
Item 14c: HDL
HDL Cholesterol (fasting or nonfasting)
This variable indicates the participant’s HDL cholesterol level.
FORMAT
Type: Numeric
Item Length: 3
Field Length: 6
Leading Zeros: Yes
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 230
Valid Range: 007-196; cannot be blank if
TYPE is 1 or 2 (baseline
screening or rescreening)
SOURCE
Not applicable; health screening measurement
DENOMINATOR
POPULATION
The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION
HDL cholesterol
in mg/dL A three-digit (numeric) value representing the participant’s HDL cholesterol in
mg/dL
HDL cholesterol values that are between 155 and 196 mg/dL will be flagged
for quality checks and program verification. Values outside 007-196 mg/dL will
be considered errors. See Appendix B for the procedure for validating out-of-
range values
Example: 90 mg/dL = 090
777 Inadequate
blood sample HDL cholesterol measurement was attempted, but results were not obtained
due to technical difficulties or errors
This may include issues such as (1) two or more failed venipuncture attempts;
(2) insufficient amount of blood, type of test tube; (3) invalid Cholestech
readings due to very high/low values;(4) sample submitted to laboratory, test
not done due to erroneous or missing laboratory request or other paperwork
See Appendix B for the procedure for documenting the reason that the
measurement was not obtained
This value will be flagged as a quality check
888 Client
refused Participant refuses to have her blood drawn for cholesterol measurements
If the participant refuses to go to the lab, the participant can be considered to
have refused
If the participant does not go to the scheduled lab appointment after follow-up
has been attempted, the participant can be considered to have refused
This value will be flagged as a quality check
999 No
measurement
recorded
No HDL cholesterol measurement was taken or recorded
This value will be flagged as an error
ANALYSIS AND USE
To identify participants who are unaware that they have low HDL cholesterol and need preventive
services or referral to medical management
To assess the percentage of WISEWOMAN participants who have high cholesterol or borderline
high cholesterol
To assess the risk of the WISEWOMAN population for cardiovascular disease
To assist in determining cholesterol control and management
OTHER
INFORMATION
Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
completed by the provider. They are provided for funded program use only.
HDL cholesterol measurement may be taken as fasting or nonfasting. At a minimum, every
participant must have a total cholesterol and HDL cholesterol (14c: HDL) value recorded. If the
participant was fasting and had a lipid panel completed at the baseline, rescreening, or follow-up
visit, then LDL (14d: LDL) and triglyceride (14e: Trigly) values can also be recorded in addition to
total and HDL cholesterol.
In cases where the Cholestech machine indicates a reading of less than 15 mg/dL, the guidance is
to code the participant’s HDL as 015.

OMB Approved
WISEWOMAN MDE Manual 71 August 2017
Edition 9.03 revised
Item 14d: LDL LDL Cholesterol (fasting)
This variable indicates a fasting participant’s fasting LDL cholesterol level.
FORMAT Type: Numeric
Item Length: 3
Field Length: 6
Leading Zeros: Yes
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 236
Valid Range: 020-380; cannot be blank if TYPE is 1 or
2 (baseline screening or rescreening)
SOURCE
Not applicable; health screening measurement
DENOMINATOR
POPULATION
The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION
LDL
cholesterol in
mg/dL
A three-digit (numeric) value representing a fasting participant’s fasting LDL
cholesterol in mg/dL
LDL cholesterol values that are between 344 and 380 mg/dL will be flagged for
quality checks and program verification. Values outside 020-380 mg/dL will be
considered errors. See Appendix B for the procedure for validating out-of-range
values
For nonfasting participants, any value in this field will be flagged for a quality
check
Example: 90 mg/dL = 090
777
Inadequate
blood sample
LDL cholesterol measurement was attempted, but results were not obtained due
to technical difficulties or errors
This may include issues such as (1) two or more failed venipuncture attempts; (2)
insufficient amount of blood, type of test tube; (3) invalid Cholestech readings
due to very high/low values; (4) sample submitted to laboratory, test not done
due to erroneous or missing laboratory request or other paperwork
This
response should be used for participants who were confirmed to be fasting,
but their LDL cholesterol was unable to be obtained
For nonfasting participants, This value will be flagged as a quality check because
all nonfasting participants should have their
LDL cholesterol coded as ‘999 No
measurement recorded’
888 Client
refused Participant refuses to receive a lipid panel that would include LDL measurements
This response should be used for participants who were confirmed to be fasting,
but refused a lipid panel
For nonfasting participants, This value will be flagged as a quality check because
all nonfasting participants should have their LDL cholesterol coded as ‘999 No
measurement recorded’
999 No
measurement
recorded
No LDL cholesterol measurement was taken or recorded
Nonfasting participants should always have this value
ANALYSIS AND USE
To assist in determining cholesterol control and management
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
completed by the provider. They are provided for funded program use only.
LDL cholesterol must be a fasting measurement. At a minimum, every participant must have a total
cholesterol and HDL cholesterol (14c: HDL) value recorded. If the participant was fasting and had a
lipid panel completed at the baseline, rescreening, or follow-up visit, then LDL (14d: LDL) and
triglyceride (14e: Trigly) values can also be recorded in addition to total and HDL cholesterol.
If an LDL measurement is recorded when the participant was not confirmed to be fasting, programs
should check with providers to determine whether the participant actually was fasting.
Cross edits
Providers should only order a lipid panel for participants who confirm that they are fasting.
Participants who were not fasting, do not know their fasting status, or whose fasting status was not
recorded should not have LDL measurements, and should be coded as ‘999 No measurement
recorded.’ Other LDL values for these participants will be flagged for a quality check.
Quality check: (FAST = 2) AND LDL ≠ 999

OMB Approved
WISEWOMAN MDE Manual 72 August 2017
Edition 9.03 revised
Item 14e: Trigly
Triglycerides (fasting)
This variable indicates a fasting participant’s triglycerides measurement.
FORMAT
Type: Numeric
Item Length: 4
Field Length: 8
Leading Zeros: Yes
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 242
Valid Range: 0012-3000; cannot be blank if TYPE is
1 or 2 (baseline screening or
rescreening)
SOURCE
Not applicable; health screening measurement
DENOMINATOR
POPULATION
The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION
Triglycerides
in mg/dL
A four-digit (numeric) value representing a fasting participant’s triglycerides
measurement in mg/dL
Triglycerides values values between 1,000 and 3,000 mg/dL will be flagged for
quality checks and program verification. Values outside 0012-3000 mg/dL will be
considered errors. See Appendix B for the procedure for validating out-of-range
values
For nonfasting participants any value in this field will be flagged for a quality check
Example: 90 mg/dL = 0090
7777
Inadequate
blood sample
Triglycerides measurement was attempted, but results were not obtained due to
technical difficulties or errors
This may include issues such as (1) two or more failed venipuncture attempts; (2)
insufficient amount of blood, type of test tube; (3) invalid Cholestech readings due
to very high/low values; (4) sample submitted to laboratory, test not done due to
erroneous or missing laboratory request or other paperwork
This response should be used for participants who were confirmed to be fasting,
but their triglycerides measurement could not be obtained
For nonfasting
participants, This value will be flagged as a quality check because
all nonfasting participan
ts should have their triglycerides measurement coded as
‘9999 No measurement recorded’
8888 Client
refused Fasting participant refuses to receive a lipid panel that would include triglycerides
measurements
This response should be used for participants who were confirmed to be fasting,
but refused a lipid panel
For nonfasting
participants, This value will be flagged as a quality check because
all nonfasting participants should have their triglycerides measurement coded as
‘9999 No measurement recorded’
9999 No
measurement
recorded
No triglycerides measurement was taken or recorded
Nonfasting participants should always have this value
ANALYSIS AND USE
To assist in determining cholesterol control and management
OTHER
INFORMATION
Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
completed by the provider. They are provided for funded program use only.
Triglycerides must be a fasting measurement. At a minimum, every participant must have a total
cholesterol and HDL cholesterol (14c: HDL) value recorded. If the participant was fasting and had a
lipid panel completed at the baseline, rescreening, or follow-up visit, then LDL (14d: LDL) and
triglyceride (14e: Trigly) values can also be recorded in addition to total and HDL cholesterol.
If a triglyceride measurement is recorded when the participant was not confirmed to be fasting,
programs should check with providers to determine whether the participant actually was fasting.
Cross edits
Providers should only order a lipid panel for participants who confirm that they are fasting.
Participants who were not fasting, do not know their fasting status or whose fasting status was not
recorded should not have triglycerides measurements, and should be coded as ‘9999 No
measurement recorded.’ Other triglycerides values for these participants will be flagged for a quality
check.
Quality check: (FAST = 2) AND TRIGLY ≠ 9999
OMB Approved
WISEWOMAN MDE Manual 73 August 2017
Edition 9.03 revised
Item 15a: BGDate Glucose/A1c Measurement Date
This variable indicates the date that the glucose or A1C measurements were taken.
FORMAT Type: Numeric
Item Length: 8
Field Length: 16
Leading Zeros: Yes
Static Field: No
Other Format: MMDDCCYY
Justification: Right
Beginning Position: 250
Valid Range: Valid date; must be blank if
Glucose and A1C = 888/8888,
999/9999; cannot be blank if
TYPE is 1 (baseline
screening) or 2 (rescreening)
and Glucose and A1C ≠
888/8888 or 999/9999
SOURCE Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION Screening Date Valid date in MMDDCCYY format
Example: September 10, 2013 = 09102013
ANALYSIS AND USE To determine the date of the glucose or A1C measurements
To facilitate analysis of changes in glucose or A1C measurements over time
OTHER
INFORMATION Cross edits
The glucose measurement date should not be blank if there is evidence of an attempt to measure
glucose (15b: Glucose) or A1C (15c: A1C). An error flag will occur if glucose measurement date is
blank when there is evidence of an attempt to measure glucose or A1C.
Error: BGDATE = . AND (GLUCOSE ≠ (888, 999) OR A1C ≠ (8888, 9999))
Additional edits
Glucose should have been measured on the current date or earlier.
Error: BGDATE > [current date]

OMB Approved
WISEWOMAN MDE Manual 74 August 2017
Edition 9.03 revised
Item 15b: Glucose Glucose (fasting)
This variable indicates the participant’s fasting glucose measurement.
FORMAT Type: Numeric
Item Length: 3
Field Length: 6
Leading Zeros: Yes
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 266
Valid Range: 037-571; cannot be blank if
TYPE is 1 or 2 (baseline
screening or rescreening)
SOURCE American Heart Association Life’s Simple 7
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION Total glucose in mg/dL Up to a three-digit (numeric) value representing the participant’s
fasting glucose level in mg/dL
Glucose values that are between 037 and 050 mg/dL or 275 and 571
mg/dL will be flagged for quality checks and program verification.
Values outside 037-571 will be considered errors. See Appendix B for
the procedure for validating out-of-range values
Example: 90 mg/dL = 090
777 Inadequate blood
sample Glucose measurement was attempted, but results were not obtained
due to technical difficulties or errors
See Appendix B for the procedure for documenting the reason that
the measurement was not obtained
This may include issues such as (1) two or more failed venipuncture
attempts; (2) insufficient amount of blood, type of test tube; (3) invalid
Cholestech readings due to very high/low values; (4) sample
submitted to laboratory, test not done due to erroneous or missing
laboratory request or other paperwork
This value will be flagged as a quality check
888 Client refused Participant refuses to have her blood drawn for glucose
measurements
If the participant refuses to go to the lab, the participant can be
considered to have refused
If the participant does not go to the scheduled lab appointment after
follow-up has been attempted, the participant can be considered to
have refused
999 No measurement
recorded No glucose measurement was taken or record
Non-fasting participants should always have this value
ANALYSIS AND USE To identify participants who have pre-diabetes and diabetes
To assist in determining diabetes control and management
To use in conjunction with A1C percentage to accurately assess a participant’s blood glucose
To provide data element required to determine participant’s Simple 7 cardiovascular risk score
To understand the overall rate of diabetes among the WISEWOMAN population

OMB Approved
WISEWOMAN MDE Manual 75 August 2017
Edition 9.03 revised
Item 15b: Glucose Glucose (fasting)
This variable indicates the participant’s fasting glucose measurement.
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection form
completed by the provider. They are provided for funded program use only.
Glucose must be a fasting measurement. Programs may record both a glucose and A1C
measurement for a participant. Having values for both Glucose and A1C will not result in an error.
A1C measurement is never required.
In cases where the Cholestech machine indicates a reading of less than 50 mg/dL, the guidance is
to code the participant’s glucose as 050. This is considered an alert reading and participants should
be seen immediately
Glucose measurement or A1C measurement at baseline screening or rescreening is required for a
record to count as a valid record. Values are considered invalid for the glucose variable if: (1)
participant is fasting and glucose is left blank, coded as missing, or out of range, or (2) participant is
not fasting. In both cases, the record will only be considered valid if the A1C variable is not left
blank, coded as missing, or out of range. Otherwise, the record will not count as a valid record, and
the record will not count toward meeting a program’s screening goal (performance measure #3).
Cross edits
Providers must attempt a glucose or A1C measurement (15c: A1C). An error flag will occur if a
glucose or A1C measurement is not attempted.
Error: GLUCOSE = 999 AND A1C = 9999
Providers should attempt to measure either glucose or A1C (15c: A1C). A quality check flag will
occur if the participant refuses both a glucose and A1C measurement.
Quality check: GLUCOSE = 888 AND AIC = 8888
1Providers should only attempt to measure glucose for fasting participants.
Error: (FAST = 2) AND GLUCOSE ≠ 999
1This edit will not come into effect until January 2015.

OMB Approved
WISEWOMAN MDE Manual 76 August 2017
Edition 9.03 revised
Item 15c: A1C A1C Percentage
This variable indicates the participant’s A1C percentage (if measured).
FORMAT Type: Numeric
Item Length: 4
Field Length: 8
Leading Zeros: Yes
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 272
Valid Range: 02.8-16.2; cannot be blank if
TYPE is 1 or 2 (baseline
screening or rescreening);
decimal points count as part of
the length
SOURCE Not applicable; health screening measurement
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION A1C percentage Numeric value representing the participant’s A1C percentage. A1C
should be reported to one decimal point
If A1C was measured by another provider within the last 3 months,
it is acceptable to input the value if it is available
A1C values between 02.8% and 04.0% or 13.0% and 16.2% will
be flagged for quality checks and program verification. Values
outside 02.8%-16.2% will be considered errors. See Appendix B
for the procedure for validating out-of-range values
Example: 8.5% = 08.5 (where the decimal place counts as part of
the variable length)
7777 Inadequate blood
sample A1C measurement was attempted, but results were not obtained
due to technical difficulties or errors
This value will be flagged as a quality check
8888 Client refused Participant refuses to have an A1C test
If a participant refuses to go to the lab, the participant can be
considered to have refused
If a participant does not go to the scheduled lab appointment after
follow-up has been attempted, the participant can be considered to
have refused
9999 No measurement
recorded No A1C measurement was taken or recorded
ANALYSIS AND USE To identify participants who have diabetes and refer them for medical management
To identify participants who have higher-than-optimal A1C levels and would benefit from
preventive services such as lifestyle programs
To assist in determining diabetes control and management
To assess the cardiovascular disease risk factors in the WISEWOMAN population
To provide data element required to determine participant’s Simple 7 cardiovascular risk score

OMB Approved
WISEWOMAN MDE Manual 77 August 2017
Edition 9.03 revised
Item 15c: A1C A1C Percentage
This variable indicates the participant’s A1C percentage (if measured).
OTHER INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
completed by the provider. They are provided for funded program use only.
Participants with A1C percentage values greater than or equal to 6.5% are considered diabetic.
Participants with A1C percentage values less than 6.5% but greater than or equal to 5.7% are
considered pre-diabetic.
Programs may record both a glucose and A1C measurement for a participant. Having values for
both Glucose and A1C will not result in an error. A1C measurement is never required.
A1C measurement or glucose measurement at baseline screening or rescreening is required for a
record to count as a valid record. If both Glucose and A1C are blank or outside of the valid range
(Glucose: 37-571 mg/dL; A1C: 2.8-16.2%) the record will not count as a valid record, and the
record will not count toward meeting a program’s screening goal (performance measure #3).
Note that WISEWOMAN does not designate an alert value for A1C, because the A1C value itself
is a three-month average and is not accurate enough to identify that an individual’s life is in
imminent danger and requires urgent care.

OMB Approved
WISEWOMAN MDE Manual 78 August 2017
Edition 9.03 revised
Item 16a: BPAlert
If average SBP >180 or DBP >110, what is the status of the workup?
This variable indicates the status of the workup of a participant’s alert level blood pressure.
FORMAT
Type: Numeric
Item Length: 1
Field Length: 2
Leading Zeros: No
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 280
Valid Range: See values; cannot be blank if
TYPE is 1 or 2 (baseline
screening or rescreening)
SOURCE
Not applicable; health screening measurement
DENOMINATOR
POPULATION
Participants who have an alert level blood pressure value are included in the denominator
VALUES AND
DESCRIPTION
1 Workup complete
Workup for participant with an alert level blood pressure reading is
complete
2 Follow-up – workup by
alternate provider Patient intends to see an alternate provider within 7 days
3 Not an alert reading
Participant did not have an alert level blood pressure reading
8 Client refused workup
Participant had an alert level blood pressure reading but refused
workup
9 Workup not completed,
client lost to follow-up Participant had an alert level blood pressure reading but was lost to
follow-up, and workup was not completed
Lost to follow-up is defined as a participant who did not attend her
scheduled workup within three months after a screening visit and
could not be reached to reschedule another appointment
This value will be flagged as an error.
ANALYSIS AND USE To assess whether participants with alert level blood pressure readings are receiving a workup
To assist in determining hypertension (high blood pressure) management, and control
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
completed by the provider. They are provided for funded program use only.
A participant is classified as having an alert blood pressure reading if the average of her two systolic
blood pressure readings (12b: SBP1 and 12d: SBP2) is greater than 180 mmHg or if the average of
her two diastolic blood pressure readings (12c: DBP1 and 12e: DBP2) is greater than 110 mmHg. If
a second blood pressure was not taken, then the first reading should be used to determine whether
a participant has an alert value.
Cross edits
If average systolic or diastolic blood pressure is an alert value, then this field should not be recorded
as a non-alert value. An error flag will occur if a participant with an alert value is recorded as a non-
alert value.
Error: (((SBP1 + SBP2)/2) >180) OR ((DBP1 + DBP2)/2) >110)) AND BPALERT ≠ (1, 2, 8, 9)
If average systolic or diastolic blood pressure is not an alert value, then this field should be coded ‘3
Not an alert reading.’ An error flag will occur if this code is not selected for participants who do not
have an alert value.
Error: (((SBP1 + SBP2)/2) ≤180) AND ((DBP1 + DBP2)/2) ≤110)) AND BPALERT ≠ 3
If first systolic and diastolic blood pressure measurements were not obtained, blood pressure
workup status should be coded ‘3 Not an alert reading’.’ An error flag will occur if this code is not
selected for participants who have no valid blood pressure measurements.
Error: SBP1 = 777, 888, or 999 AND DBP1 = 777, 888, or 999 AND BPALERT ≠ 3
If average systolic or diastolic blood pressure is an alert value, then the blood pressure workup
status should be obtained. An error flag will occur if the alert participant is coded as ‘8 Client refused
workup’ or ‘9 Workup not completed, client lost to follow-up.’
Error: (((SBP1 + SBP2)/2) >180) OR ((DBP1 + DBP2)/2) >110)) AND BPALERT = (8, 9) AND
BPDIDATE = [valid date]

OMB Approved
WISEWOMAN MDE Manual 79 August 2017
Edition 9.03 revised
Item 16b: BPDiDate
If Average SBP >180 or DBP >110, Workup Date
This variable indicates the workup date for a participant with an alert level blood pressure reading.
FORMAT
Type:
Numeric
Item Length: 8
Field Length: 16
Leading Zeros: Yes
Static Field: No
Other Format:
MMDDCCYY
Justification: Right
Beginning Position: 282
Valid Range: Valid date; must be blank if BPAlert=3;
cannot be blank if BPAlert=1, 2, 8, or 9
and TYPE is 1 (baseline screening) or
2 (rescreening)
SOURCE
Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION
Participants who have an alert level blood pressure value are included in the denominator
VALUES AND
DESCRIPTION
Blood Pressure Workup
Date
Valid date in MMDDCCYY format
If follow-up information is provided for this referral, the workup date
can be entered
Example: September 10, 2013 = 09102013
ANALYSIS AND USE To assess whether providers are performing timely workups for participants with alert level blood
pressure values
To determine whether programs are meeting the guideline of workups within one week of the
screening for alert participants
To assist in determining hypertension (high blood pressure) prevention, management, and control
OTHER
INFORMATION
Guidance
A participant is classified as having an alert blood pressure reading if the average of her two systolic
blood pressure readings (12b: SBP1 and 12d: SBP2) is greater than 180 mmHg or if the average of
her two diastolic blood pressure readings (12c: DBP1 and 12e: DBP2) is greater than 110 mmHg. If
a second blood pressure was not taken, then the first reading should be used to determine whether
a participant has an alert value.
Only participants who are coded as having an alert blood pressure reading (15a: BPAlert = ‘1
Workup complete,’ 2 Follow-up – workup by alternate provider,‘ 8 Client refused workup,’ or ‘9
Workup not completed, client lost to follow-up’) can have a blood pressure diagnostic exam date.
If a participant with an alert blood pressure value has a blood pressure workup status (16a: BPAlert)
coded as ‘‘1 Workup complete,’ this field must be completed with the date of the diagnostic exam.
If a participant with an alert blood pressure value has a blood pressure workup status (16a: BPAlert)
coded as ‘‘2 Follow-up – workup by alternate provider,‘ and the program is unable to obtain the date
of her diagnostic exam, this field may be left blank.
If a participant with an alert blood pressure value has a blood pressure workup status (16a: BPAlert)
coded as ‘8 Client refused workup,’ this field should contain the date of refusal as defined by
program protocol.
If a participant with an alert blood pressure value has a blood pressure workup status (16a: BPAlert)
coded as ‘9 Workup not completed, client lost to follow-up,’ this field should contain the date that the
program considered the participant lost to follow-up as defined by program protocol
Cross edits
For participants with an alert blood pressure value who received a complete workup, the diagnostic
exam date should be on or after the blood pressure measurement date.
Error: ((((SBP1 + SBP2)/2) >180) OR ((DBP1 + DBP2)/2) >110) AND BPALERT = 1 AND
BPDIDATE = [valid date] AND BPDATE = [valid date] AND BPDIDATE < BPDATE
A blood pressure diagnostic exam date should not be recorded if average systolic or diastolic blood
pressure is not an alert value. If a blood pressure diagnostic exam date is recorded for a participant
who does not have an alert blood pressure value, this field will be flagged as an error.
Error: ((((SBP1 + SBP2)/2) ≤180) AND ((DBP1 + DBP2)/2) ≤110) AND BPDIDATE = [valid
date]
A blood pressure diagnostic exam date should be recorded only if first systolic or diastolic blood
pressure was obtained. An error flag will occur if a date is recorded when first systolic or diastolic
blood pressure was not obtained.
OMB Approved
WISEWOMAN MDE Manual 80 August 2017
Edition 9.03 revised
Item 16b: BPDiDate
If Average SBP >180 or DBP >110, Workup Date
This variable indicates the workup date for a participant with an alert level blood pressure reading.
OTHER
INFORMATION Guidance (con’t):
Error: SBP1 = 777, 888, or 999 AND DBP1 = 777, 888, or 999 AND BPDIDATE = [valid date]
For participants with an alert blood pressure value who received a complete workup, a blood
pressure diagnostic exam date should not be missing or more than seven days later than the blood
pressure measurement date. An error flag will occur if the diagnostic exam date is missing or more
than seven days after the date that blood pressure measurements were taken.
Error: ((((SBP1 + SBP2)/2) >180) OR ((DBP1 + DBP2)/2) >110) AND BPALERT = 1 AND
((BPDIDATE = [valid date] AND BPDATE = [valid date] AND BPDIDATE - BPDATE >7) OR
BPDIDATE = .)
A blood pressure diagnostic exam date should be recorded for participants with an alert blood
pressure value when the participant intends to see an alternate provider for alert workup. This date
should not be more than seven days after the blood pressure measurement date.
Error: ((((SBP1 + SBP2)/2) >180) OR ((DBP1 + DBP2)/2) >110) AND BPALERT = 2 AND
(BPDIDATE = [valid date] AND BPDATE = [valid date] AND ((BPDIDATE - BPDATE >7) OR
BPDIDATE = . )
A date of refusal should be provided for participants with an alert blood pressure value who refused
a blood pressure workup. An error flag will occur if the date of refusal is not provided.
Error: (((SBP1 + SBP2)/2) > 180 OR ((DBP1 + DBP2)/2) > 110) AND BPALERT = 8 AND
BPDIDATE = .
The date the program considered the participant lost to follow-up should be provided for participants
with an alert blood pressure value who were lost to follow-up for a blood pressure workup. An error
flag will occur if the date of lost to follow-up is not provided.
Error: (((SBP1 + SBP2)/2) > 180 OR ((DBP1 + DBP2)/2) > 110) AND BPALERT = 9 AND
BPDIDATE = .
OMB Approved
WISEWOMAN MDE Manual 81 August 2017
Edition 9.03 revised
Item 16c: BGAlert If GLUCOSE ≤50 or GLUCOSE ≥250, what is the status of the workup?
This variable indicates the status of the workup of a participant’s alert level blood glucose.
FORMAT Type: Numeric
Item Length: 1
Field Length: 2
Leading Zeros: No
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 298
Valid Range: See values; cannot be blank if TYPE is 1
or 2 (baseline screening or rescreening)
SOURCE Not applicable; health screening measurement
DENOMINATOR
POPULATION Participants who have alert level fasting glucose values are included in the denominator.
VALUES AND
DESCRIPTION
1 Workup complete
Workup for participant with an alert level fasting glucose reading is
complete
2 Follow-up – work up by
alternate provider Patient intends to see an alternate provider within 7 days
3 Not an alert reading Participant does not have an alert level fasting glucose reading
8 Client refused workup Participant had an alert level fasting glucose reading but refused
workup
9 Workup not completed,
client lost to follow-up Participant had an alert level fasting glucose reading but was lost to
follow-up, and workup was not completed
Lost to follow-up is defined as a participant who did not attend her
scheduled workup within three months after a screening visit and was
unable to be reached to reschedule another appointment
This value will be flagged as an error.
ANALYSIS AND USE To assess whether participants with alert level blood glucose readings are receiving workup
To assist in determining diabetes prevention, management, and control
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
completed by the provider. They are provided for funded program use only.
A participant is classified as having an alert blood glucose reading if her blood glucose is less than
or equal to 50 mg/dL or greater than or equal to 250 mg/dL. Participants who have been diagnosed
with diabetes as determined by diabetes health history status and/or A1C measurement at
screening will not be classified as having an alert blood glucose reading. If a participant does not
have a valid A1C measurement at screening, her blood glucose will be used to determine her alert
blood glucose status (regardless of diabetes health history status).
Cross edits
If Glucose is an alert value, then this field should not be recorded as a non-alert value. An error flag
will occur if a participant with an alert value is recorded as a non-alert value.
Error: (GLUCOSE ≤50 OR GLUCOSE ≥250) AND ((SRD ≠ 1 AND A1C < 6
.5) OR (A1C = 7777, 8888, 9999)) AND BGALERT ≠ (1, 2, 8, 9)
If Glucose is not an alert value, then this field should be coded ‘3 Not an alert reading.’ An error flag
will occur if this code is not selected for participants who do not have an alert value.
Error: 50< GLUCOSE <250 AND BGALERT ≠ 3
If a glucose measurement is not obtained, glucose workup status should be coded ‘3 Not an alert
reading.’ An error flag will occur if this code is not selected for participants who do not have a
glucose measurement.
Error: GLUCOSE = 777, 888, or 999 AND BGALERT ≠ 3
If Glucose is an alert value, then the glucose workup status should be obtained. An error flag will
occur if the alert participant is coded as ‘8 Client refused workup’ or ‘9 Workup not completed, client
lost to follow-up.
Error: (GLUCOSE ≤50 OR GLUCOSE ≥250) AND ((SRD ≠ 1 AND A1C < 6.5) OR (A1C = 7777,
8888, 9999)) AND BGALERT = (8, 9) AND BGDIDIATE = [valid date]

OMB Approved
WISEWOMAN MDE Manual 82 August 2017
Edition 9.03 revised
Item 16d: BGDiDate If GLUCOSE ≤50 or GLUCOSE ≥250 Workup Exam Date
This variable indicates the workup date for a participant with an alert level fasting blood glucose
reading.
FORMAT Type: Numeric
Item Length: 8
Field Length: 16
Leading Zeros: Yes
Static Field: No
Other Format: MMDDCCYY
Justification: Right
Beginning Position: 300
Valid Range: Valid date; must be blank if
BGAlert=3; cannot be blank if
BGAlert=1, 2, 8, or 9 and TYPE is 1
(baseline screening) or 2
(rescreening)
SOURCE Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION Participants who have alert level fasting glucose values are included in the denominator.
VALUES AND
DESCRIPTION Blood glucose workup
date Valid date in MMDDCCYY format
If follow-up information is provided for this referral, the workup date
can be entered
Example: September 10, 2013 = 09102013
ANALYSIS AND USE To assess whether providers are performing timely workups for participants with alert level fasting
blood glucose values
To determine whether programs are meeting the guideline of workup within one week of the
screening for alert participants
OTHER
INFORMATION Guidance
A participant is classified as having an alert blood glucose reading if her blood glucose is less than
or equal to 50 mg/dL or greater than or equal to 250 mg/dLParticipants who have been diagnosed
with diabetes as determined by diabetes health history status and/or A1C measurement at
screening will not be classified as having an alert blood glucose reading. If a participant does not
have a valid A1C measurement at screening, her blood glucose will be used to determine her alert
blood glucose status (regardless of diabetes health history status).
Only participants who are coded as having an alert blood glucose reading (16c: BGAlert = ‘1
Workup complete,’ ‘2 Follow-up – work up by alternate provider’ ‘8 Client refused workup,’ or ‘9
Workup not completed, client lost to follow-up’) should have a blood glucose diagnostic exam date.
If a participant with an alert blood glucose value has a blood glucose workup status (16c: BGAlert)
coded as ‘‘2 Follow-up – work up by alternate provider’ and the program is unable to obtain the date
of her diagnostic exam, this field may be left blank.
If a participant with an alert blood glucose value has a blood glucose workup status (16c: BGAlert)
coded as ‘8 Client refused workup,’ this field should contain the date of refusal as defined by
program protocol.
If a participant with an alert blood glucose value has a blood glucose workup status (16c: BGAlert)
coded as ‘9 Workup not completed, client lost to follow-up,’ this field should contain the date that the
program considered the participant lost to follow-up as defined by program protocol.
Cross edits
For participants with an alert glucose value who received a complete workup, the diagnostic exam
date should be on or after the glucose measurement date. Otherwise, this field will be flagged as an
error.
Error: (GLUCOSE ≤50 OR GLUCOSE ≥250) AND ((SRD ≠ 1 AND A1C < 6.5) OR (A1C = 7777,
8888, 9999)) AND BGDIDATE = [valid date] AND BGDATE = [valid date] AND BGDIDATE <
BGDATE AND BGALERT = 1
A glucose diagnostic exam date should not be recorded if blood glucose is not an alert value. If a
glucose diagnostic exam date is recorded for a participant who does not have an alert glucose
value, this field will be flagged as an error.
Error: (50< GLUCOSE <250) AND BGDIDATE = [valid date]
OMB Approved
WISEWOMAN MDE Manual 83 August 2017
Edition 9.03 revised
Item 16d: BGDiDate If GLUCOSE ≤50 or GLUCOSE ≥250 Workup Exam Date
This variable indicates the workup date for a participant with an alert level fasting blood glucose
reading.
OTHER
INFORMATION Guidance (con’t):
A glucose diagnostic exam date should only be recorded if blood glucose was obtained. An error
flag will occur if a date is recorded when blood glucose was not obtained.
Error: GLUCOSE = 777, 888, or 999 AND BGDIDATE = [valid date]
For participants with an alert glucose value who received a complete workup, a glucose diagnostic
exam date should not be more than seven days later than the glucose measurement date. An error
flag will occur if the diagnostic exam date is missing or more than seven days after the date that
glucose measurements were taken.
Error: (GLUCOSE ≤50 OR GLUCOSE ≥250) AND ((SRD ≠ 1 AND A1C < 6.5) OR (A1C = 7777,
8888, 9999)) AND BGALERT = 1 AND ((BGDIDATE = [valid date] AND BGDATE = [valid date]
AND BGDIDATE - BGDATE >7) OR BGDIDATE = .)
A glucose diagnostic exam date should be recorded for participants with an alert glucose value
when the participant intends to see an alternate provider for alert workup. This date should not be
more than seven days after the glucose measurement date. An error flag will occur if the glucose
diagnostic exam date is missing or is more than seven days after the glucose measurement date.
Error: (GLUCOSE ≤50 OR GLUCOSE ≥250) AND ((SRD ≠ 1 AND A1C < 6.5) OR (A1C = 7777,
8888, 9999)) AND BGALERT = 2 AND (BGDIDATE = [valid date] AND BGDATE = [valid date]
AND BGDIDATE - BGDATE >7) OR BGDIDATE = .
A date of refusal should be provided for participants with an alert glucose value who refused a
glucose workup. An error flag will occur if the date of refusal is not provided.
Error: (GLUCOSE ≤ 50 OR GLUCOSE ≥250) AND ((SRD ≠ 1 AND A1C < 6.5) OR (A1C =
7777, 8888, 9999)) AND BGALERT = 8 AND BGDIDATE = .
The date the program considered the participant lost to follow-up should be provided for participants
with an alert glucose value who were lost to follow-up for a glucose workup. An error flag will occur if
the date of lost to follow-up is not provided.
Error: (GLUCOSE ≤ 50 OR GLUCOSE ≥250) AND ((SRD ≠ 1 AND A1C < 6.5) OR (A1C = 7777,
8888, 9999)) AND BGALERT = 9 AND BGDIDATE = .

OMB Approved
WISEWOMAN MDE Manual 84 August 2017
Edition 9.03 revised
4. RISK REDUCTION COUNSELING MDE SPECIFICATIONS
This section provides grantees with the information necessary to support
collection and reporting of Risk Reduction Counseling MDEs, which must be
done according to the specifications provided in this section of the manual.
Variables for inclusion are those that can be associated with a participant who
has a valid screening, which at a minimum, is defined as a record with a valid
blood pressure date (12a: BPDate) and valid values at baseline screening and
rescreening for the following:7
• Month and year of birth (3d);
• Previous cardiovascular disease risk [high cholesterol, hypertension,
diabetes, coronary heart disease/chest pain, heart attack, heart failure,
stroke/TIA, vascular disease, or congenital heart defects (4a-4d)];
• Use of medications to lower cholesterol, blood pressure, or blood sugar
(5a – 5c);
• Diet [consumption of fruits, vegetables, fish, whole grains, and beverages
with added sugar (7a-7e)];
• Physical activity [moderate and vigorous physical activity (8a and 8b)];
• Smoking status (9a);
• Biometric screening measures [height and weight (11a and 11b), and first
systolic blood pressure (12b), diastolic blood pressure (12c), total
cholesterol (14b), and glucose (15b) or A1C (15c)]
For risk reduction counseling to be counted and considered in the related
performance measure, it must include a valid risk reduction counseling start and
completion date, participant decided priority area, readiness to change
assessment date, and participant stage of change.8
This section begins with a summary of the 8 required variables (Subsection a)
and then provides the technical specifications for each variable (Subsection b).
7 Valid values for required baseline screening and rescreening items are provided in the footnotes on page 17.
8 Values left blank or coded as unknown are considered invalid values for nutrition priority area and physical activity
priority area if valid date is provided for risk reduction counseling start date.
Values left blank or coded as unknown are considered invalid values for smoking cessation priority area if valid date is
provided for risk reduction counseling start date and the participant is a smoker.
Values left blank or coded as unknown are considered invalid values for hypertension medication adherence priority area
if valid date is provided for risk reduction counseling start date and the participant takes medication for hypertension.
Values left blank are considered invalid values for risk reduction counseling start date.
Values left blank are considered invalid values for risk reduction counseling completion date and readiness to change
assessment date if a valid date is provided for risk reduction counseling start date.
Values of blank or coded as missing are considered invalid values for participant stage of change if a valid date is
provided for readiness to change assessment date.
As part of CDC’s performance assessment, programs must also provide evidence that they have delivered risk reduction
counseling to 100 percent of the women with valid screenings (performance measure #4).

OMB Approved
WISEWOMAN MDE Manual 85 August 2017
Edition 9.03 revised
a. Summary of Risk Reduction Counseling MDEs
Item
Number Variable Name Position
Possible
Rounds of
Collection1 Variable Label Type
17a RRCDate 316 2 Risk reduction counseling
date Numeric
17b RRCComplete 332 2 Risk reduction counseling
completion date Numeric
17c RRCNut 348 2 Participant decided nutrition
is a priority area Numeric
17d RRCPA 350 2 Participant decided physical
activity is a priority area Numeric
17e RRCSmoke 352 2 Participant decided smoking
cessation is a priority area Numeric
17f RRCMedAdhere 354 2 Participant decided
medication adherence for
hypertension (high blood
pressure) is a priority area
Numeric
18a RTCDate 356 2 Readiness to change
assessment date Numeric
18b RTC 372 2 Participant stage of change Numeric
1 Number of times the item may be collected during the screening cycle. For example, for an item
with 2 possible rounds of data collection, a value may be provided at both baseline
screening/rescreening and at follow-up assessment.
OMB Approved
WISEWOMAN MDE Manual 86 August 2017
Edition 9.03 revised
b. Risk Reduction Counseling MDE Specifications
Item 17a: RRCDate Risk Reduction Counseling Date
This variable indicates the date that the initial risk reduction counseling occurred.
FORMAT Type: Numeric
Item Length: 8
Field Length: 16
Leading Zeros: Yes
Static Field: No
Other Format: MMDDCCYY
Justification: Right
Beginning Position: 316
Valid Range: Valid date; cannot be blank if
Type = 1 or 2 (baseline
screening or rescreening)
SOURCE Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION Risk reduction
counseling date Valid date in MMDDCCYY format
Date must occur within the submission period
Example: September 10, 2013 = 09102013
ANALYSIS AND USE To determine the date of the risk reduction counseling
To assess receipt of risk reduction counseling to inform analyses of behavior change
To facilitate analysis of changes in risk reduction counseling provision over time
OTHER INFORMATION Guidance
All participants should receive risk reduction counseling at the time of screening (baseline screening
or rescreening).
To calculate the number of risk reduction counseling sessions per participant, the number of initial
risk reduction counseling dates is counted for each unique participant ID (3a: EncodeID).
Additional edits
Initial risk reduction counseling should have been occured on the current date or earlier. An error
flag will occur if the risk reduction counseling date is in the future.
Error: RRCDate > [current date]
Initial risk reduction counseling date should be recorded if the record represents a baseline
screening visit or a rescreening visit. Otherwise, this field will be flagged as an error.
Error: Type = 1 or 2 AND RRCDate = .
OMB Approved
WISEWOMAN MDE Manual 87 August 2017
Edition 9.03 revised
Item 17b: RRCComplete Risk Reduction Counseling Completion Date
This variable indicates the date that risk reduction counseling was completed.
FORMAT Type: Numeric
Item Length: 8
Field Length: 16
Leading Zeros: Yes
Static Field: No
Other Format: MMDDCCYY
Justification: Right
Beginning Position: 332
Valid Range: Valid date; cannot be
blank if valid date is
provided for RRCDate
SOURCE Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION Risk reduction
counseling follow-up
date
Valid date in MMDDCCYY format
Date must occur within the submission period
Example: September 10, 2013 = 09102013
88888888 Participant
refused further program
contact
Participant refused further program contact
This value will be flagged as a quality check
99999999 Participant
lost to follow-up Provider made three attempts to follow-up with participant but
participant lost to follow-up.
This value will be flagged as a quality check
ANALYSIS AND USE To determine the date of a completed risk reduction counseling session
To facilitate analysis of changes in risk reduction counseling provision over time
OTHER INFORMATION Guidance
If laboratory results are not available at the time of the screening visit to provide risk reduction
counseling, this field should be used to indicate the date on which risk reduction counseling was
completed.
If risk reduction counseling was completed on the same date that it began, the same date
should be recorded for RRCDate and RRCComplete.
Additional edits
Initial risk reduction counseling must be completed on the same date or within 60 days after the
office visit (12a: BPDate). Otherwise, this field will be flagged as a quality check.
Quality Check: RRCComplete ≥ BPDate + 60 days
Initial risk reduction counseling date should have occurred on or before the date that risk
reduction counseling for that session was completed. Otherwise, this field will be flagged as an
error.
Error: RRCComplete < RRCDate
Completion of risk reduction counseling should have been occured on the current date or earlier.
An error flag will occur if the risk reduction counseling completion date is in the future.
Error: RRCComplete > [current date]
Risk reduction counseling completion date should be recorded if initial risk reduction counseling
date was recorded. Otherwise, this field will be flagged as an error.
Error: RRCDate = [valid date] AND RRCComplete = .
OMB Approved
WISEWOMAN MDE Manual 88 August 2017
Edition 9.03 revised
Item 17c: RRCNut Participant Decided Nutrition is a Priority Area
This variable indicates whether the participant decided that nutrition is a priority area after receiving
risk reduction counseling.
FORMAT Type: Numeric
Item Length: 1
Field Length: 2
Leading Zeros: No
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 348
Valid Range: See values; cannot be
blank if valid date is
provided for RRCDate
SOURCE Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants that received risk reduction counseling
VALUES AND
DESCRIPTION 1 Yes Participant decided that nutrition is a priority area
2 No Participant did not decide that nutrition is a priority area
7 Unknown It is unknown whether the participant decided that nutrition is a
priority area
This value will be flagged as an error
ANALYSIS AND USE To determine the number of participants that decided nutrition is a priority area after receiving risk
reduction counseling
To assist in determining participant health education on cardiovascular disease risk factors
To assist in assessments of reduction of risk over time in context of types of counseling received
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
completed by the provider. They are provided for funded program use only.
Additional Edits
Nutrition priority area should be recorded if initial risk reduction counseling date was recorded.
Otherwise, this field will be flagged as an error.
Error: RRCDate = [valid date] AND RRCNut = .
OMB Approved
WISEWOMAN MDE Manual 89 August 2017
Edition 9.03 revised
Item 17d: RRCPA Participant Decided Physical Activity is a Priority Area
This variable indicates whether the participant decided that physical activity is a priority area after
receiving risk reduction counseling.
FORMAT Type: Numeric
Item Length: 1
Field Length: 2
Leading Zeros: No
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 350
Valid Range: See values; cannot be
blank if valid date is
provided for RRCDate
SOURCE Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants that received risk reduction counseling
VALUES AND
DESCRIPTION 1 Yes Participant decided that physical activity is a priority area
2 No Participant did not decide that physical activity is a priority area
7 Unknown It is unknown whether the participant decided that physical activity is
a priority area
This value will be flagged as an error
ANALYSIS AND USE To determine the number of participants that decided physical activity is a priority area after
receiving risk reduction counseling
To assist in determining participant health education on cardiovascular disease risk factors
To assist in assessments of reduction of risk over time in context of types of counseling received
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
completed by the provider. They are provided for funded program use only.
Additional Edits
Physical activity priority area should be recorded if initial risk reduction counseling date was
recorded. Otherwise, this field will be flagged as an error.
Error: RRCDate = [valid date] AND RRCPA = .
OMB Approved
WISEWOMAN MDE Manual 90 August 2017
Edition 9.03 revised
Item 17e: RRCSmoke Participant Decided Smoking Cessation is a Priority Area
This variable indicates whether the participant decided that smoking cessation is a priority area after
receiving risk reduction counseling.
FORMAT Type: Numeric
Item Length: 1
Field Length: 2
Leading Zeros: No
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 352
Valid Range: See values; cannot be
blank if valid date is
provided for RRCDate
and Smoker = 1
SOURCE Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants that have received risk reduction
counseling and are smokers
VALUES AND
DESCRIPTION 1 Yes Participant decided that smoking cessation is a priority area
2 No Participant did not decide that smoking cessation is a priority area
7 Unknown It is unknown whether the participant decided that smoking cessation
is a priority area
This value will be flagged as an error
ANALYSIS AND USE To determine the number of participants that decided smoking cessation is a priority area after
receiving risk reduction counseling
To assist in determining participant health education on cardiovascular disease risk factors
To assist in assessments of reduction of risk over time in context of types of counseling received
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
completed by the provider. They are provided for funded program use only.
Additional Edits
Smoking cessation priority area should be recorded if initial risk reduction counseling date was
recorded and if participant is a smoker. Otherwise, this field will be flagged as an error.
Error: RRCDate = [valid date] AND Smoker = 1 AND RRCSmoke = .
OMB Approved
WISEWOMAN MDE Manual 91 August 2017
Edition 9.03 revised
Item 17f:
RRCMedAdhere Participant Decided Medication Adherence for Hypertension (high blood pressure) is a
Priority Area
This variable indicates whether the participant decided medication adherence for her hypertension
(high blood pressure) is a priority area after receiving risk reduction.
FORMAT Type: Numeric
Item Length: 1
Field Length: 2
Leading Zeros: No
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 354
Valid Range: See values; cannot be
blank if valid date is
provided for RRCDate
and HBPMeds = 1
SOURCE Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants taking medication for hypertension (high
blood pressure) and have received risk reduction counseling
VALUES AND
DESCRIPTION 1 Yes Participant decided that medication adherence for hypertension (high
blood pressure) is a priority area
2 No Participant did not decide that medication adherence for hypertension
(high blood pressure) is a priority area
7 Unknown It is unknown whether the participant decided that medication
adherence for hypertension (high blood pressure) is a priority area
This value will be flagged as an error
ANALYSIS AND USE To determine the number of participants that decided that medication adherence for hypertension
(high blood pressure) is a priority area after receiving risk reduction counseling
To assist in determining hypertension (high blood pressure) prevention, management, and control
To assist in determining participant health education on cardiovascular disease risk factors
To assist in assessments of reduction of risk over time in context of types of counseling received
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
completed by the provider. They are provided for funded program use only.
Additional Edits
Medication adherence for hypertension priority area should be recorded if initial risk reduction
counseling date was recorded and if participant is taking medication for hypertension. Otherwise,
this field will be flagged for a quality check. .
Quality Check: RRCDate = [valid date] AND HBPMeds = 1 AND RRCMEDADHERE = .

OMB Approved
WISEWOMAN MDE Manual 92 August 2017
Edition 9.03 revised
Item 18a: RTCDate Readiness to Change Assessment Date
This variable indicates the date that an assessment of readiness to change occurred.
FORMAT Type: Numeric
Item Length: 8
Field Length: 16
Leading Zeros: Yes
Static Field: No
Other Format: MMDDCCYY
Justification: Right
Beginning Position: 356
Valid Range: Valid date; cannot be
blank if RRCDate date
is valid
SOURCE Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants that received risk reduction counseling
VALUES AND
DESCRIPTION Readiness to change
assessment date Valid date in MMDDCCYY format
Date must occur within the submission period
Example: September 10, 2013 = 09102013
ANALYSIS AND USE To determine the date of the readiness to change assessment
To assist in determining whether the participant has received an assessment of readiness to
change
To facilitate analysis of changes in readiness to change for the participant and for the program over
time
OTHER
INFORMATION Guidance
Readiness to change assessment must be provided to all WISEWOMAN participants at the time of
their risk reduction counseling.
Additional Edits
Readiness to change assessment date should be recorded if initial risk reduction counseling date
was recorded. Otherwise, this field will be flagged as an error.
Error: RRCDate = [valid date] AND RTCDate = .
Readiness to change assessment should have been occured on the current date or earlier. An error
flag will occur if the risk reduction counseling completion date is in the future.
Error: RTCDate > [current date]
OMB Approved
WISEWOMAN MDE Manual 93 August 2017
Edition 9.03 revised
Item 18b: RTC Participant Stage of Change
This variable indicates the participant’s state of change based on a readiness to change
assessment.
FORMAT Type: Numeric
Item Length: 1
Field Length: 2
Leading Zeros: No
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 372
Valid Range: See values; cannot be
blank if valid date is
provided for RTCDate
SOURCE Prochaska, JO.; DiClemente, CC. The transtheroretical approach. In: Norcross, JC; Goldfried, MR.
(eds.) Handbook of psychotherapy integration. 2nd ed. New York: Oxford University Press; 2005. p.
147–171.
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants that received risk reduction counseling
VALUES AND
DESCRIPTION 1 Pre-contemplation Participant has little or no intention to change her behavior in the
foreseeable future
2 Contemplation Participant is thinking about making a change in her behavior
3 Preparation Participant is ready to plan how she will make a change in her
behavior
4 Action Participant is in the process of trying to make a change in her
behavior
5 Maintenance Participant is trying to maintain a change she has made in her
behavior
8 Refused Participant refused to answer readiness to change assessment
questions
This value will be flagged as a quality check
9 No answer recorded No answer was recorded
This value will be flagged as an error if a valid date was provided for
RTCDate
ANALYSIS AND USE To assess participant’s stage of change based on a readiness to change assessment
To facilitate analysis of changes in readiness to change for the participant and for the program over
time
To assist in assessments of participant behavior change outcomes in context of readiness to
change
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
completed by the provider. They are provided for funded program use only.
Readiness to change assessment must be provided to all WISEWOMAN participants at the time of
their risk reduction counseling.
Additional edits
Participant stage of change should be recorded if readiness to change assessment date was
recorded. Otherwise, this field will be flagged as an error.
Error: RTCDate = [valid date] AND RTC = . or 9
OMB Approved
WISEWOMAN MDE Manual 94 August 2017
Edition 9.03 revised

OMB Approved
WISEWOMAN MDE Manual 95 August 2017
Edition 9.03 revised
5. HEALTHY BEHAVIOR SUPPORT OPTIONS MDE SPECIFICATIONS
This section provides grantees with the information necessary to support
collection and reporting of Lifestyle Program/Health Coaching MDEs as well as
referrals to community-based tobacco cessation resources which must be done
according to the specifications provided in this section of the manual.
Variables for inclusion are those that can be associated with a participant who
has a valid screening, which at a minimum, is defined as a record with a valid
blood pressure date (12a: BPDate) and valid values at baseline screening and
rescreening for the following:9
• Month and year of birth (3d);
• Previous cardiovascular disease risk [high cholesterol, hypertension,
diabetes, coronary heart disease/chest pain, heart attack, heart failure,
stroke/TIA, vascular disease, or congenital heart defects (4a-4d)];
• Use of medications to lower cholesterol, blood pressure, or blood sugar
(5a – 5c);
• Diet [consumption of fruits, vegetables, fish, whole grains, and beverages
with added sugar (7a-7e)];
• Physical activity [moderate and vigorous physical activity (8a and 8b)];
• Smoking status (9a);
• Biometric screening measures [height and weight (11a and 11b), and first
systolic blood pressure (12b), diastolic blood pressure (12c), total
cholesterol (14b), and glucose (15b) or A1C (15c)]
An LSP/HC contact is counted if the following MDE variables in a record have
valid values: date of LSP/HC session, LSP/HC ID, and date of referral.10
Grantees may report LSP/HC data that do not meet these requirements, but they
will not be counted as an LSP/HC session, analyzed in data reports generated by
CDC, or counted in the related performance measure unless additional
documentation is provided.
This section begins with a summary of the 11 required variables (Subsection a)
and then provides the technical specifications for each variable (Subsection b).
9 Valid values for required baseline screening and rescreening items are provided in the footnotes on page 17.
10Values left blank or that are not included on the current list of CDC-approved LSP/HC IDs are considered invalid values
for LSP/HC ID if a valid date of LSP/HC session is provided.
Values left blank are considered invalid values for date of LSP/HC session.
As part of CDC’s performance assessment, programs must also provide evidence that 80 percent of the women with valid
screenings and referred to an LSP/HC participate in the program (performance measure #5).

OMB Approved
WISEWOMAN MDE Manual 96 August 2017
Edition 9.03 revised
a. Summary of Healthy Behavior Support Options MDEs
Item
Number Variable
Name Position
Possible
Rounds of
Collection
1 Variable Label Type
19a RefDate 374 2 Lifestyle Program (LSP) /
Health Coaching (HC) referral
date
Numeric
20a LSPHCRec 390 1 Number of Lifestyle Program
(LSP) / Health Coaching (HC)
sessions received by the
participant
Numeric
20b Intervention 392 16 Date of Lifestyle Program
(LSP) / Health Coaching (HC)
session)
Numeric
20c LSPHCID 520 16 Lifestyle Program (LSP) /
Health Coaching (HC) ID Character
20d LSPHCTime 680 16 Length of Lifestyle Program
(LSP) / Health Coaching (HC)
session received by the
participant
Numeric
20e ContactType 728 16 Type of Lifestyle Program
(LSP) / Health Coaching (HC)
session
Numeric
20f Setting 744 16 Setting of Lifestyle Program
(LSP) / Health Coaching (HC)
session
Numeric
20g LSPHCComp 760 16 Completion of Lifestyle
Program (LSP) / Health
Coaching (HC)
Numeric
21a TobResDate 776 3 Date of referral to Tobacco
Cessation Resource Numeric
21b TobResType 800 3 Type of Tobacco Cessation
Resource Numeric
21c TResComp 803 3 Tobacco Cessation activity
completed Numeric
1 Number of times the item may be collected during the screening cycle. For example, for an LSP
curriculum with 16 sessions (or rounds of data collection), a value may be collected and recorded
for each of 16 sessions.
OMB Approved
WISEWOMAN MDE Manual 97 August 2017
Edition 9.03 revised
b. Healthy Behavior Support Options MDE Specifications
Item 19a: RefDate Lifestyle Program (LSP) / Health Coaching (HC) Referral Date
This variable indicates the date that a referral to a LSP/HC occurred.
FORMAT Type: Numeric
Item Length: 8
Field Length: 16
Leading Zeros: Yes
Static Field: No
Other Format: MMDDCCYY
Justification: Right
Beginning Position: 374
Valid Range: Valid date
SOURCE Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION Lifestyle Program/Health
Coaching Referral Date Valid date in MMDDCCYY format
Date must occur within the submission period
Example: September 10, 2013 = 09102013
ANALYSIS AND USE To determine the date of the referral to a LSP/HC
To assist in determining whether the participant has received a referral to a LSP/HC
To assist in determining the number of LSP/HC referrals per participant
To facilitate analysis of changes in LSP/HC referrals over time
OTHER
INFORMATION Guidance
To calculate the number of LSP or HC referrals per participant, the number of LSP/HC referral dates
is counted for each unique participant ID (3a: EncodeID).
Additional edits
Lifestyle program/health coaching referral date should have occured on the current date or earlier.
An error flag will occur if the lifestyle program/health coaching referral date is in the future.
Error: RefDate > [current date]
OMB Approved
WISEWOMAN MDE Manual 98 August 2017
Edition 9.03 revised
Item 20a: LSPHCRec Number of Lifestyle Program (LSP) / Health Coaching (HC) Sessions Received by the
Participant During the Screening Cycle
This variable indicates the number of LSP/HC sessions the participant has received during the
current screening cycle.
FORMAT Type: Numeric
Item Length: 2
Field Length: 2
Leading Zeros: Yes
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 390
Valid Range: Cannot be blank if
RefDate is valid
SOURCE Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION The denominator includes all WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION Number of Sessions Value representing the number of LSP/HC sessions the participant has
received during the current screening cycle
Example: 6 visits = 06
ANALYSIS AND USE To track the number of LSP/HC sessions that the participant has received
To assess LSP/HC sessions in context of types and settings, and readiness to change of a
participant
OTHER
INFORMATION Guidance
The number of LSP sessions the participant has received during the current screening cycle should
be provided in this field. The number of HC sessions the participant has received during the current
screening cycle should be provided in this field. The value reported for this field should include the
sum of LSP sessions AND the sum of HC sessions. A screening cycle should include the initial
screening contact for the submission period (baseline screening or rescreening), one or more
LSP/HC contacts as assigned at the screening, and a follow-up assessment contact (that is not also
considered a rescreening) if follow-up occurred during or following completion of the LSP/HC
program.
During the creation of the analytic file, CDC will check that the number of LSP/HC sessions received
by the participant is equal to the number of unique LSP/HC dates provided during the cooperative
agreement period.
Additional edits
Number of lifestyle programs/health coaching session should be recorded if lifestyle program/health
coaching referral date was recorded. Otherwise, this field will be flagged as an error.
Error: RefDate = [valid date] AND LSPHCRec = .
Number of lifestyle programs/health coaching sessions entered for LSPHCRec should be equal to
the number of lifestyle programs/health coaching session dates provided for the screening cycle.
Otherwise, this field will be flagged for a quality check.
Quality Check: LSPHCRec ≠ COUNT(INTERVENTION)

OMB Approved
WISEWOMAN MDE Manual 99 August 2017
Edition 9.03 revised
Item 20b: Intervention
Date of Lifestyle Program (LSP) / Health Coaching (HC) Session
For LSP/HC records, this variable indicates the date that the LSP/HC session occurred.
FORMAT Type: Numeric
Item Length: 8
Field Length: 128
Leading Zeros: Yes
Static Field: No
Other Format: MMDDCCYY
Justification: Right
Beginning Position: 392
Valid Range: Valid date
SOURCE Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION All LSP/HC sessions among WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION Lifestyle Program/Health
Coaching Session Date Valid date in MMDDCCYY format
Date must occur within the submission period
Example: September 10, 2013 = 09102013
ANALYSIS AND USE To determine the date of the LSP/HC session
To assist in determining whether the participant has received an LSP/HC session
To assist in calculating the number of LSP/HC sessions per participant
To assess whether participants with risk factors receive LSP/HC services
To assess changes in risk profile between participants who participate in the LSP/HC and
participants who do not
OTHER
INFORMATION Guidance
To calculate the number of LSP or HC sessions per participant, the number of LSP/HC session
dates is counted for each unique participant ID (3a: EncodeID).
Cross edits
Date of lifestyle program/health coaching session should have been occured on the current date or
earlier. An error flag will occur if the lifestyle program/health coaching session date is in the future.
Error: Intervention > [current date]
Date of first lifestyle program/health coaching session should be recorded if lifestyle program/health
coaching referral date was recorded. Otherwise, this field will be flagged for a quality check.
Quality Check: RefDate = [valid date] AND Intervention = .
Date of lifestyle program/health coaching referral should be recorded if date of first lifestyle
program/health coaching session was recorded. Otherwise, this field will be flagged as an error.
Error: Intervention = [valid date] AND RefDate = .
OMB Approved
WISEWOMAN MDE Manual 100 August 2017
Edition 9.03 revised
Item 20c: LSPHCID Lifestyle Program (LSP) / Health Coaching (HC) ID
This variable indicates which LSP/HC was used.
FORMAT Type: Character
Item Length: 10
Field Length: 160
Leading Zeros: N/A
Static Field: No
Other Format: N/A
Justification: Left
Beginning Position: 520
Valid Range: Valid code for an
LSP/HC; cannot be
blank if a valid date is
provided for Intervention
SOURCE Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION All LSP/HC sessions among WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION Lifestyle Program ID
Health Coaching ID
Value representing the ID code of the LSP as assigned
Value representing the ID code of the HC as assigned
ANALYSIS AND USE To track the number of WISEWOMAN participants who receive an LSP/HC session from each
WISEWOMAN LSP/HC provider
To describe differences in participant demographics or other characteristics by LSP/HC provider
To identify the number of LSP/HC providers in a given geographic area
OTHER
INFORMATION Guidance
If the participant receives a LSP session, the LSP ID should be provided in this field. If the
participant receives a HC session, a HC ID should be provided in this field.
Additional edits
LSP/HC should be recorded if date of LSP/HC session was recorded. Otherwise, this field will be
flagged as an error.
Error: Intervention = [valid date] AND LSPHCID = .

OMB Approved
WISEWOMAN MDE Manual 101 August 2017
Edition 9.03 revised
Item 20d: LSPHCTime Length of Lifestyle Program (LSP) / Health Coaching (HC) Session Received by the
Participant
This variable indicates the length (in minutes) of the LSP/HC sessions that the participant has
received.
FORMAT Type: Numeric
Item Length: 3
Field Length: 48
Leading Zeros: Yes
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 680
Valid Range: 000-120; cannot be
blank if a valid date is
provided for
Intervention
SOURCE Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION All LSP/HC sessions among WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION Length of Session Value representing the length of the LSP/HC session in minutes
Any value outside the valid range (000-120)will be flagged as quality
checks
Example: 90 minutes = 090
ANALYSIS AND USE To track the length of the LSP/HC sessions that the participant has received
To assess LSP/HC sessions in context of types and settings, and readiness to change of a
participant
OTHER
INFORMATION Guidance
If the participant receives a LSP session, the duration of the LSP session should be provided in this
field. If the participant receives a HC session, the duration of the HC session should be provided in
this field.
Additional edits
Duration of LSP/HC session should be recorded if date of LSP/HC session was recorded.
Otherwise, this field will be flagged as an error.
Error: Intervention = [valid date] AND LSPHCTime = .
OMB Approved
WISEWOMAN MDE Manual 102 August 2017
Edition 9.03 revised
Item 20e: ContactType Type of Lifestyle Program (LSP) / Health Coaching (HC) Session
This variable indicates the type of the LSP/HC session.
FORMAT Type: Numeric
Item Length: 1
Field Length: 16
Leading Zeros: No
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 728
Valid Range: See values; cannot be
blank if a valid date is
provided for
Intervention
SOURCE Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION All LSP/HC contacts among WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION 1 Face-to-face LSP/HC session was completed face-to-face
2 Phone LSP/HC session was completed by phone
3 Smart phone/tablet
Application LSP/HC session was completed with a smart phone or tablet
application session. The program has received evidence that the
application session was completed.
4 Evidence that mailed
materials were opened
and reviewed
LSP/HC session was completed by review of mailed materials. The
program has received evidence that the materials were opened and
reviewed
5 Evidence that
audiotape or DVD as
opened and reviewed
LSP/HC session was completed by review of an audiotape or DVD.
The program has received evidence that the audiotape or DVD was
opened and reviewed
6 Evidence that non-
interactive computer-
based session was
completed
LSP/HC session was completed with a computer-based session that
did not involve an interactive component. The program has received
evidence that the computer-based session was completed
7 Evidence that
interactive computer-
based session was
completed
LSP/HC session was completed with a computer-based session that
involved an interactive component. The program has received
evidence that the interactive computer-based session was
completed.
0 Other Other LSP/HC session type.
9 No answer recorded No answer was recorded
This value will be flagged as an error if a valid date is provided for
Intervention
ANALYSIS AND USE To assess how frequently different types of LSP/HC sessions are being used within and across
programs
To determine whether frequency of LSP/HC session types are consistent with programs’ LSP
models
To assess LSP/HC sessions in context of types and settings, and readiness to change of a
participant
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
completed by the provider. They are provided for funded program use only.
If the participant receives a LSP session, the type of LSP session should be provided in this field. If
the participant receives a HC session, the type of HC session should be provided in this field.
If the receipt of mailed materials, an audiotape, or a DVD is confirmed by a phone call that includes
counseling, the contact type should be coded as ‘2 Phone.’ Together, the mailing of materials and
follow-up call should be considered one HC/LSP contact.
OMB Approved
WISEWOMAN MDE Manual 103 August 2017
Edition 9.03 revised
Item 20e: ContactType Type of Lifestyle Program (LSP) / Health Coaching (HC) Session
This variable indicates the type of the LSP/HC session.
Cross edits
Type of LSP/HC session should be recorded if date of LSP/HC session was recorded. Otherwise,
this field will be flagged as an error.
Error: Intervention = [valid date] AND [ContactType = . OR ContactType = 9]
OMB Approved
WISEWOMAN MDE Manual 104 August 2017
Edition 9.03 revised
Item 20f: Setting Setting of Lifestyle Program (LSP) / Health Coaching (HC) Session
This variable indicates the setting of the LSP/HC session.
FORMAT Type: Numeric
Item Length: 1
Field Length: 16
Leading Zeros: No
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 744
Valid Range: See values; cannot be
blank if a valid date is
provided for
Intervention
SOURCE Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION All LSP/HC contacts among WISEWOMAN participants with a valid baseline screening
VALUES AND
DESCRIPTION 1 Individual LSP/HC contact for the participant occurred as an individual session
2 Group LSP/HC contact for the participant occurred as a group session
3 Combination
LSP/HC contact for the participant occurred as a combination of
individual and group sessions
9 No answer recorded No answer was recorded
This value will be flagged as an error if a valid date is provided for
Intervention
ANALYSIS AND USE To assess how frequently different types of LSP/HC settings are being used within and across
programs
To determine whether the settings of LSP/HC sessions are consistent with programs’ LSP/HC
models
To assess LSP/HC sessions in context of types and settings, and readiness to change of a
participant
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
completed by the provider. They are provided for funded program use only.
If the participant receives a LSP session, the setting of the LSP session should be provided in this
field. If the participant receives a HC session, the setting of the HC session should be provided in
this field.
The setting of each LSP/HC session may vary.
Cross edits
Setting of LSP/HC session should be recorded if date of LSP/HC session was recorded. Otherwise,
this field will be flagged as an error.
Error: Intervention = [valid date] AND [Setting = . OR Setting = 9]
OMB Approved
WISEWOMAN MDE Manual 105 August 2017
Edition 9.03 revised
Item 20g:
LSPHCComp Completion of Lifestyle Program (LSP) / Health Coaching (HC)
This variable indicates if the participant has completed the LSP/HC.
FORMAT Type: Numeric
Item Length: 1
Field Length: 16
Leading Zeros: No
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 760
Valid Range: See values; cannot be
blank if a valid date is
provided for
Intervention
SOURCE Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION All WISEWOMAN participants with a valid baseline screening that received an LSP/HC
VALUES AND
DESCRIPTION 1 Yes – Lifestyle Program/ Health
Coaching is Complete Participant has completed the LSP/HC. An LSP requires
multiple sessions, e.g., multiple sessions in a curriculum
2 No – Lifestyle Program/ Health
Coaching is still in progress Participant’s LSP/HC is still in progress
3 No – Withdrawal/Discontinued Participant has withdrawn from the LSP/HC or discontinued
the LSP/HC
9 No answer recorded No answer was recorded for completion of LSP/HC
This value will be flagged as an error if a valid date is
provided for Intervention
ANALYSIS AND USE To determine whether the participant has completed an LSP/HC
To assist in determining the date of the participant’s final LSP/HC session in the program
To assess changes in risk profile between participants who complete the LSP/HC and participants
who do not
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
completed by the provider. They are provided for funded program use only.
If the participant receives a LSP session, the completion status of the LSP session should be
provided in this field. If the participant receives a HC session, the completion status of the HC
session should be provided in this field.
If a participant receives additional LSP or HC sessions beyond the CDC-approved number of
sessions required for the program to be classified as “Complete”, LSPHCComp should be coded as
1 (Yes – LSP/HC is complete) for each additional session.
Cross edits
Completion status of LSP/HC session should be recorded if date of LSP/HC session was recorded.
Otherwise, this field will be flagged as an error.
Error: Intervention = [valid date] AND [LSPHCComp = . OR LSPHCComp = 9]

OMB Approved
WISEWOMAN MDE Manual 106 August 2017
Edition 9.03 revised
Item 21a: TobResDate
Date of Referral to Tobacco Cessation Resource
This variable indicates the date that the referral to a tobacco cessation resource occurred.
FORMAT Type: Numeric
Item Length: 8
Field Length: 24
Leading Zeros: Yes
Static Field: No
Other Format: MMDDCCYY
Justification: Right
Beginning Position: 776
Valid Range: Valid date; cannot be
blank if RRCSmoke =1
SOURCE Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION WISEWOMAN participants with a valid baseline screening wwho identify themselves as current
smokers
VALUES AND
DESCRIPTION Tobacco Cessation
Resource Referral Date Valid date in MMDDCCYY format
Date must occur within the submission period
Example: September 10, 2013 = 09102013
ANALYSIS AND USE To document the date of a referral to tobacco cessation resource
To assist in tracking receipt of tobacco cessation resource
OTHER
INFORMATION Guidance
To calculate the number of tobacco cessation resources referrals per participant, the number of
tobacco cessation resource referral dates is counted for each unique participant ID (3a: EncodeID).
If a participant is referred to one or more tobacco cessation resources, the date of referral (Item
21a:TobResDate), type of resource the participant was referred to (Item 21b: TobResType), and
completion status for the resource at the end of the screening cycle (Item 21c: TResComp) should
be recorded for each referral. The positions for the type of resource and completion status of
resource for each referral should align with the position of the date of referral. For example, if a
participant is receives two referrals during the screening period, the date of referral, type of
resource, and completion status for the second referral should be provided in the second position
for each item.
Cross edits
Date of referral to tobacco cessation resources should be recorded if participant indicated that
tobacco cessation is a priority area during risk reduction counseling. Otherwise, this field will be
flagged for a quality check.
Quality Check: RRCSmoke = 1 AND TobResDate = .
Date of referral to tobacco cessation resource should have been occured on the current date or
earlier. An error flag will occur if the tobacco cessation resource referral date is in the future.
Error: TobResDate > [current date]
OMB Approved
WISEWOMAN MDE Manual 107 August 2017
Edition 9.03 revised
Item 21b: TobResType Type of Tobacco Cessation Resource
This variable indicates the type of tobacco cessation resource that the participant was referred to.
FORMAT Type: Numeric
Item Length: 1
Field Length: 3
Leading Zeros: No
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 800
Valid Range: See values; cannot be
blank if valid date is
provided for
TobResDate
SOURCE Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION WISEWOMAN participants with a valid baseline screening who identify themselves as current
smokers
VALUES AND
DESCRIPTION 1 Quit line Participant was referred to a proactive tobacco quit line
2 Community-based tobacco
program Participant was referred to a community-based tobacco
program
3 Other tobacco cessation
resources Participant was referred to other tobacco cessation
resources
9 No answer recorded No answer was recorded
This value will be flagged as an error if a valid date is
provided for TobResDate
ANALYSIS AND USE To determine the number of smokers that received a referral to tobacco cessation resource
To determine how frequently different types of tobacco cessation resources are being used within
and across programs
To compare the smoking status at rescreening and follow-up of women who were linked to tobacco
cessation resources versus those who were not
OTHER
INFORMATION Guidance
Codes and response options highlighted in gray should not appear on the data collection forms
completed by the provider. They are provided for funded program use only.
Cross edits
Type of tobacco cessation resource should be recorded if date of tobacco cessation resource
referral was recorded. Otherwise, this field will be flagged as an error.
Error: TobResDate = [valid date] AND [TobResType = . OR TobResType = 9]
OMB Approved
WISEWOMAN MDE Manual 108 August 2017
Edition 9.03 revised
Item 21c: TResComp Tobacco Cessation Activity Completed
This variable indicates whether the participant completed tobacco cessation activity.
FORMAT Type: Numeric
Item Length: 1
Field Length: 3
Leading Zeros: No
Static Field: No
Other Format: N/A
Justification: Right
Beginning Position: 803
Valid Range: See values; cannot be
blank if valid date is
provided for
TobResDate
SOURCE Not applicable; WISEWOMAN-specific variable
DENOMINATOR
POPULATION WISEWOMAN participants with a valid baseline screening who identify themselves as current
smokers
VALUES AND
DESCRIPTION 1 Yes – Completed tobacco
cessation activity Participant completed tobacco cessation activity
2 No – Partially completed
tobacco cessation activity Participant partially completed tobacco cessation activity
3 No – Discontinued from tobacco
cessation activity when reached Participant decided to discontinue from tobacco cessation
counseling when contacted by the tobacco cessation
resource
4 No – Could not reach to conduct
tobacco cessation activity Participant could not be reached when contacted by the
tobacco cessation resource
9 No answer recorded No answer was recorded
This value will be flagged as an error if a valid date is
provided for TobResDate
ANALYSIS AND USE To determine the number of smokers that participated in tobacco cessation activities
To compare the smoking status at rescreening and follow-up of women who were linked to tobacco
cessation resources versus those who were not
OTHER
INFORMATION Guidance
If a participant receives a referral to a tobacco cessation resource but the completion status of the
resource is unknown, TResComp should be coded as 2 (No – Partially completed tobacco
cessation activity) and updated accordingly if the completion status becomes available.
Codes and response options highlighted in gray should not appear on the data collection forms
completed by the provider. They are provided for funded program use only.
Cross edits
Completion of tobacco cessation activity should be recorded if date of tobacco cessation resource
referral was recorded. Otherwise, this field will be flagged as an error.
Error: TobResDate = [valid date] AND [TResComp = . OR TResComp = 9]
APPENDIX A:
MDE SUBMISSION
WISEWOMAN MDE Manual August 2017
Edition 9.03 revised
A-1
This Appendix describes the submission requirements for MDE files, including
those related to timeline, format, procedures, and security. Submissions will not
be processed if grantees fail to follow the guidelines provided below.
Submission Dates
Grantees must submit MDE data semiannually, on April 1 and October 1. The
table below provides the dates for the submissions under FOA DP13-1302. For
this cooperative agreement, grantees are encouraged to submit all data collected
during the cooperative agreement for each submission, including all new data
collecting during the current reporting period. For each submission, grantees also
have the opportunity the make corrections and updates to data submitted for the
previous two reporting periods (the equivalent of one year of data).
Submission
Due Date Reporting Period for New Data Reporting Period for Corrections
to Previous Data
April 1, 2014 Not Applicable Not Applicable
October 1, 2014 Jan 1, 2014–Jun 30, 2014 Not Applicable
April 1, 2015 Jul 1, 2014–Dec 31, 2014 Jan 1, 2014–Jun 30, 2014
October 1, 2015 Jan 1, 2015–Jun 30, 2015 Jan 1, 2014–Dec 31, 2014
April 1, 2016 Jul 1, 2015–Dec 31, 2015 Jul 1, 2014–Jun 30, 2015
October 1, 2016 Jan 1, 2016–Jun 30, 2016 Jan 1, 2015–Dec 31, 2015
April 1, 2017 Jul 1, 2016–Dec 31, 2016 Jul 1, 2015–Jun 30, 2016
October 1, 2017 Jan 1, 2017–Jun 30, 2017 Jan 1, 2016–Dec 31, 2016
After MDE data are submitted, an analytic file is created by the CDC data
contractor. Summary reports are created for each grantee; the format and
content are detailed further in Appendix C—Data Analysis and Use.
Data Submission Options
Grantees may submit data to CDC using one of two options: (1) direct data entry
into the WISEWOMAN Data Management System or (2) submission of bulk data
to the Data Management System.
Direct Data Entry into the MDE Data Management System
Grantees that chose to use the Data Management System for data entry should
refer to the Data Management System Quick Reference Guide, available on the
WISEWOMAN Data Management System website (https://partner.cdc.gov), for
more information about this functionality.
WISEWOMAN MDE Manual August 2017
Edition 9.03 revised
A-2
Bulk Data Submissions
Grantees that chose to submit bulk data, i.e., upload datasets to the MDE Data
Management System, rather than direct entering data into the system must follow
the data, file, and submission conventions described below.
a. Data Conventions
This section provides an overview of the data file format and layout for MDEs for
each screening cycle. It defines data length and position and describes the types
of MDE data. The data conventions described here represent the raw file format
and layout of MDEs that grantees choosing to submit bulk data to CDC must
follow.
• Data Types. There are several data types, including date, geographic,
character, and numeric.
o Dates have the format MMDDCCYY.
MM represents the month and has a range of 01–12; use
leading zeros with months 01–09. If month is missing, month
is blank (as indicated by a period [.] in each blank position).
DD represents the day of the month and has a range of 01–
31; use leading zeros with days 01–09. If day is missing, day
is blank (as indicated by a period [.] in each blank position).
CC represents the century and has a range of 19–20. If
century is missing, century is blank (as indicated by a period
[.] in each blank position).
YY represents the year and has a range of 00–99; use
leading zeros with years 00–09. If year is missing, year is
blank (as indicated by a period [.] in each blank position).
o Geographic data elements are state/tribal FIPS code, ANSI county
code, county of residence, and ZIP code of residence. These are
character variables, and require leading zeros to fill the field length.
o Character data elements are composed of letters of the alphabet,
numbers, and special characters. These are left-justified, and in
cases where the value does not fill the entire field length, extra
spaces in the length should be left blank (as indicated by a period
[.] in each blank position).
o Numeric data elements are composed of numbers, minus signs,
and decimal points. Numeric data elements are right-justified. If
numbers are expected to the right of the decimal, the number of
decimal places required is indicated in the MDE specification. In

WISEWOMAN MDE Manual August 2017
Edition 9.03 revised
A-3
cases where the value does not fill the entire field length, leading
zeros should be used to fill the field length.
• Item Length. Item length represents the number of characters (i.e., letters
of the alphabet, numbers, and special characters) for one entry of the
item.
• Field Length. If the data element may be collected more than one time
during the screening cycle, the field length will allow for multiple entries of
the data element.1 For example, if both a rescreening and a follow-up
screening assessment are provided within the six-month reporting period,
the item length is 8 for the date of the screening and the field length is 16
to allow for recording the two dates. If a participant does not have a follow-
up assessment or has less than 16 LSP sessions during the submission
period, the second round of positions within one or more MDE item fields
may be left blank. A period (.) should be used to indicate each blank
position.
• Static Field. If the field is static, it should not be updated or modified after
the first time the element is recorded. For example, month and year of
birth is considered a static field because it is not expected that a
participant’s date of birth would change over time. However, blood
pressure measurements are not static fields since it could change over
time.
• Beginning Position. Position is the location in the record of a data
element. The length and position of each data element are provided in the
MDE specifications.
b. File Type and Format
Data files must be submitted as fixed-format ASCII text files. For grantees that
choose to submit bulk data to CDC, MDEs must be recorded in the locations
identified in the MDE specifications. Each record in the file should represent data
for one cycle, which should include a unique screening visit (baseline or
rescreening) with all associated activities. The associated activities may include
LSP and/or health coaching (HC) contacts, readiness to change assessments,
and follow-up assessment (if any). The participants’ next valid screening
(rescreening) would then start the next screening cycle. Each data element must
conform to the format and values as specified. Files must include data for the
appropriate time period as shown in the table of submission dates above.
1 A screening cycle will contain a participant’s unique screening visit (baseline or rescreening), any LSP and/or health
coaching (HC) contacts, readiness to change assessments, and a follow-up assessment or follow-up screening (if
any). The next valid screening (rescreening) would then start the participant’s next screening cycle.

WISEWOMAN MDE Manual August 2017
Edition 9.03 revised
A-4
c. File-Naming Convention
File name has the format PPYYMM, where:
• PP is the program abbreviation.
• YYMM is the date of the submission. YY is the two-digit year, and MM is
the month from 01 to 12. Use leading zeros when specifying years and
months between 01 and 09.
An example of a valid file name is NC1404.
Submission Procedures
Data managers for each grantee have been provided with a username and
password to log into the web-based WISEWOMAN Data Management System.
Other grantee staff will be provided with a separate username and password
upon request.
• Grantees directly entering data into the system. Prior to
submission, grantees should check the data entered to make sure all
error and quality check flags are addressed and data are complete.
• Grantees submitting bulk data files. Prior to submission, grantees
should prepare bulk data files as instructed for the relevant period and
run it through the online validation tool to identify errors and quality
checks. These errors and quality checks should be addressed to the
extent possible prior to submission.
All grantees should fill out the submission overview form provided at the
end of this section. Other forms that grantees may submit include data
validation, alert follow-up, and participant unique ID change forms as
described in Appendix B and available through the web-based
WISEWOMAN Data Management System website (https://partner.cdc.gov).
As the data contractor prepares the analytic file after programs’ final
submissions, data issues may be identified for immediate correction. In these
instances, project officers will notify programs that there are data issues for
correction and will follow up with programs about making these corrections. The
project officer will act as a liaison to the data contractor on these issues.
Programs will resubmit corrected data through the WISEWOMAN website and
notify their project officers.
Data Confidentiality and Security
This section describes the data confidentiality and security guidelines for
preparing and submitting MDE data. Data and documents submitted via the
WISEWOMAN website will be encrypted during transmission. Programs must not
WISEWOMAN MDE Manual August 2017
Edition 9.03 revised
A-5
send information that will allow participants to be identified and must use
encoded identifiers and so on to uniquely identify participants’ data. In addition,
data submissions must be de-identified pursuant to the Health Insurance
Portability and Accountability Act of 1996 (HIPAA).
MDE data are “limited data sets” in which all identifying information has been
removed, with the exception of encoded participant ID, county of residence, ZIP
code of residence, birth month and year, Hispanic origin, and race. The
participant ID must not be linked to any other external datasets containing
personal information. Submissions must not include any of the identifiers
stipulated in HIPAA.
Grantees are expected to implement data security procedures that will secure
participant identifying and health information, including those related to back-up,
hardcopy and electronic storage, and transmission. Additional information about
CDC data security procedures will be provided once available.
WISEWOMAN MDE Manual August 2017
Edition 9.03 revised
A-6
WISEWOMAN Data Submission Form
State/Tribal Organization: __________________________________________
Date of Submission: _______________________________________________
Contact Name: ___________________________________________________
Email Address: ___________________________________________________
Telephone Number: _______________________________________________
MDEs Period Covered: _____________________________________________
File Name: _______________________________________________________
Edition of MDE Used to Generate File: _________________________________
Number of Records Submitted: ______________________________________
Validation of Data Form Submitted? (Yes/No): ___________________________
Period Covered: __________________________________________________
Participant ID Change Form Submitted? (Yes/No): _______________________
Period Covered: __________________________________________________
Correction to Previous MDE File Form Submitted? (Yes/No): _______________
Period Covered: __________________________________________________
Additional Comments:
________________________________________________________________
________________________________________________________________
________________________________________________________________
________________________________________________________________
________________________________________________________________
APPENDIX B:
DATA QUALITY AND VALIDATION

WISEWOMAN MDE Manual B-1 August 2017
Edition 9.03 revised
CDC is committed to ensuring that the data submitted are accurate, valid,
reliable, and complete, and provides grantees with several tools to help monitor
and improve data quality. This section describes three items: online validation;
data validation procedures and forms; and the method for calculating error rates.
These items together form a data quality system that allows the identification and
validation/correction of out-of-range values, improbable values, and missing data
(unknown, refused, and not obtained). It also provides an assessment of data
quality through an error rate calculation algorithm.
Validation of Data
Online validation will be available through the WISEWOMAN Data Management
System. Additional information about validation functions will be provided once
available.
CDC distinguishes between errors and quality checks using the following
definitions.
• Errors are out-of-range and missing values for variables that are critical to
assessment of program performance, management, and areas for
improvement. Responses that are not considered programmatically
acceptable may also be defined as errors.
• Quality Checks are values that seem improbable but are still possible;
should be available but are unknown, refused, or unable to be obtained; or
are not required but are missing.1 Responses that may be clinically
problematic may also be highlighted for quality checks as may values that
are programmatically problematic, i.e., values that do not align with
program guidance, such as ages outside of 40-65 years.
Prior to data submission, programs should ensure that their data are validated.
Programs are encouraged to check on the validity of their data multiple times
before the deadline to maximize data quality. Whenever possible, errors should
be corrected and quality check values validated before the data are submitted to
CDC.
As needed, the online validation provided on the web-based WISEWOMAN
website will be updated by the data contractor to reflect any changes in
specifications and to account for nuances discovered about the data. Any
changes will be documented. Revisions to the edits will be noted in revisions of
the WISEWOMAN MDE Manual. The error messages are provided in the
subsections below.
1 Valid values for items used to determine a valid screening record are provided on page 17 of the manual.
WISEWOMAN MDE Manual B-2 August 2017
Edition 9.03 revised
Validation Report Format and Contents
Administrative MDE Error and Quality Check Edit Description
MDE
Item/ Edit
# Status Edit Message
00A_1 Error MDE version is missing
00A_2 Error MDE version is invalid
01A_1 Error State FIPS code is missing
01A_2 Error State FIPS code is invalid
01B_1 Error ANSI geographic code is missing
01C_1 Error Enrollment site ID is missing
01D_1 Error Screening site ID is missing
02A_1 Error Time period of baseline screening is missing
02A_2 Error Time period of baseline screening is invalid
02A_3 Error Time period of baseline screening does not match the date of baseline
screening
02B_1 Error Number of screening cycles received by the participant is missing
02B_2 Quality
check Number of screenings cycles received by the participant is out of range
02C_1 Error Type of screening visit is missing
02C_2 Error Type of screening visit is invalid
03A_1 Error Participant ID is missing
WISEWOMAN MDE Manual B-3 August 2017
Edition 9.03 revised
Screening and Assessment MDE Error and Quality Check Edit Description
MDE Item/
Edit # Status Edit Message
03B_1 Error Geographic code of residence is missing
03C_1 Error ZIP code of residence is missing
03C_2 Error ZIP code of residence is invalid
03D_1 Error Month and year of birth is missing
03E_1 Error Hispanic or Latino indicator is missing
03E_2 Error Hispanic or Latino indicator is invalid
03E_3 Error No race or ethnicity coded
03E_4 Error Patient is non-Hispanic, but all races missing
03F_1 Error First race is missing and participant is non-Hispanic
03F_2 Error First race is invalid
03F_3 Error Patient’s first race is missing while they have other race
information
03G_1 Error Second race is missing
03G_2 Error Second race is invalid
03H_1 Error Education is missing
03H_2 Error Education is invalid
03H_3 Quality
Check Education is unknown/refused
03I_1 Error Primary language is missing
03I_2 Error Primary language is invalid
03I_3 Quality
Check Primary language is refused
04A_1 Error History of high cholesterol is missing
04A_2 Error History of high cholesterol is invalid
04A_3 Quality
Check History of high cholesterol is unknown/refused
04B_1 Error History of high blood pressure is missing
04B_2 Error History of high blood pressure is invalid

WISEWOMAN MDE Manual B-4 August 2017
Edition 9.03 revised
MDE Item/
Edit # Status Edit Message
04B_3 Quality
Check History of high blood pressure is unknown/refused
04C_1 Error History of diabetes is missing
04C_2 Error History of diabetes is invalid
04C_3 Quality
Check History of diabetes is unknown/refused
04D_1 Error History of heart attack is missing
04D_2 Error History of heart attack is invalid
04D_3 Quality
Check History of heart attack is unknown/refused
05A_1 Error Medication for high cholesterol is missing*
05A_2 Error Medication for high cholesterol is invalid
05A_3 Quality
Check Medication status for high cholesterol is unknown/refused
05A_4 Quality
Check Participant does not have high cholesterol and does not have
response of not applicable
05B_1 Error Medication for high blood pressure is missing
05B_2 Error Medication for high blood pressure is invalid
05B_3 Quality
Check Medication status for high blood pressure is unknown/refused
05B_4 Quality
Check Participant does not have high blood pressure and does not have
response of not applicable
05C_1 Error Medication for diabetes is missing*
05C_2 Error Medication for diabetes is invalid
05C_3 Quality
Check Medication status for diabetes is unknown/refused
05C_4 Quality
Check Participant does not have diabetes/high glucose and does not
have a response of not applicable
05D_1 Error High cholesterol medication adherence is missing
05D_2 Error High cholesterol medication adherence is invalid
05D_3 Quality
Check High cholesterol medication adherence is unknown/refused
05D_4 Error Participant not taking cholesterol medication and does not have
response of not applicable
05D_5 Error Participant taking cholesterol medication and has a response of
not applicable
05E_1 Error High blood pressure medication adherence is missing
05E_2 Error High blood pressure medication adherence is invalid
WISEWOMAN MDE Manual B-5 August 2017
Edition 9.03 revised
MDE Item/
Edit # Status Edit Message
05E_3 Quality
Check High blood pressure medication adherence is unknown/refused
05E_4 Error Participant not taking blood pressure medication and does not
have a response of not applicable
05E_5 Error Participant taking blood pressure medication and has a response
of not applicable
05F_1 Error Diabetes medication adherence is missing
05F_2 Error Diabetes medication adherence is invalid
05F_3 Quality
Check Diabetes medication adherence is unknown/refused
05F_4 Error Participant not taking diabetes medication and does not have a
response of not applicable
05F_5 Error Participant taking diabetes medication and has a response of not
applicable
06A_1 Error Blood pressure home measurement is missing
06A_2 Error Blood pressure home measurement is invalid
06A_3 Quality
Check Blood pressure home measurement is unknown/refused
06A_5 Error Participant has high blood pressure and has a response of not
applicable
06B_1 Error Blood pressure home measurement frequency is missing
06B_2 Error Blood pressure home measurement frequency is invalid
06B_3 Quality
Check Blood pressure home measurement frequency is
unknown/refused
06B_4 Error Participant does not measure blood pressure at home and does
not have a response of not applicable
06B_5 Error Participant measures blood pressure at home and has a
response of not applicable
06C_1 Error Blood pressure home measurement feedback status is missing
06C_2 Error Blood pressure home measurement feedback status is invalid
06C_3 Quality
Check Blood pressure home measurement feedback status is
unknown/refused
06C_4 Error Participant does not measure blood pressure at home and does
not have a response of not applicable
06C_5 Error Participant measures blood pressure at home and has a
response of not applicable
07A_1 Error Average fruit consumption is missing
07A_2 Quality
Check Average fruit consumption is refused

WISEWOMAN MDE Manual B-6 August 2017
Edition 9.03 revised
MDE Item/
Edit # Status Edit Message
07A_3 Error Average fruit consumption is out of range
07B_1 Error Average vegetable consumption is missing
07B_2 Quality
Check Average vegetable consumption is refused
07B_3 Error Average vegetable consumption is out of range
07C_1 Error Average fish consumption is missing
07C_2 Error Average fish consumption is invalid
07C_3 Quality
Check Average fish consumption is refused
07D_1 Error Average whole grain consumption is missing
07D_2 Error Average whole grain consumption is invalid
07D_3 Quality
Check Average whole grain consumption is refused
07E_1 Error Average sugar-sweetened beverage consumption is missing
07E_2 Error Average sugar-sweetened beverage consumption is invalid
07E_3 Quality
Check Average sugar-sweetened beverage consumption is refused
07F_1 Error Sodium intake watch status is missing
07F_2 Error Sodium intake watch status is invalid
07F_3 Quality
Check Sodium intake watch status is refused
08A_1 Error Average moderate physical activity is missing
08A_2 Quality
Check Average moderate physical activity is refused
08A_3 Quality
Check Average moderate physical activity is out of range
08B_1 Error Average vigorous physical activity is missing
08B_2 Quality
Check Average vigorous physical activity is refused
08B_3 Quality
Check Average vigorous physical activity is out of range
09A_1 Error Smoking status is missing
09A_2 Error Smoking status is invalid
09A_3 Quality
Check Smoking status is refused
09B_1 Error Secondhand smoke exposure is missing
09B_2 Quality
Check Secondhand smoke exposure is refused
WISEWOMAN MDE Manual B-7 August 2017
Edition 9.03 revised
MDE Item/
Edit # Status Edit Message
09B_3 Error Secondhand smoke exposure is out of range
10A_1 Error Physical health status is missing
10A_2 Quality
Check Physical health status is unknown/refused
10A_3 Error Physical health status is out of range
10B_1 Quality
Check Mental health status is missing
10B_2 Quality
Check Mental health status is unknown/refused
10B_3 Error Mental health status is out of range
10C_1 Quality
Check Mental or physical health effect status is missing
10C_2 Quality
Check Mental or physical health effect status is unknown/refused
10C_3 Error Mental or physical health effect status is out of range
11A_1 Error Height is missing
11A_2 Error Height is out of range
11A_3 Quality
Check Unusually low or high height measurement
11A_4 Error Height could not be obtained
11A_5 Error Height measurement was refused
11B_1 Error Weight is missing
11B_2 Error Weight is out of range
11B_3 Quality
Check Unusually low or high weight measurement
11B_4 Quality
Check Weight could not be obtained
11B_5 Quality
Check Weight measurement was refused
11C_1 Quality
Check Waist circumference is missing
11C_2 Quality
Check Waist circumference is out of range
11D_1 Quality
Check Hip circumference is missing
11D_2 Quality
Check Hip circumference is out of range
12A_1 Error Blood pressure date is blank, missing, or invalid

WISEWOMAN MDE Manual B-8 August 2017
Edition 9.03 revised
MDE Item/
Edit # Status Edit Message
12A_2 Error Blood pressure date occurred after submission date
12A_3 Error Blood pressure date occurred before 7/1/2013
12B_1 Error First systolic blood pressure is missing
12B_2 Error First systolic blood pressure is out of range
12B_3 Error First systolic blood pressure refused
12B_4 Quality
Check Unusually high first systolic blood pressure
12B_5 Error First systolic blood pressure was unable to be obtained
12B_6 Error First systolic blood pressure should be measured before second
12C_1 Error First diastolic blood pressure is missing
12C_2 Error First diastolic blood pressure is out of range
12C_3 Error First diastolic blood pressure refused
12C_4 Quality
Check Unusually high or low first diastolic blood pressure
12C_5 Error First diastolic blood pressure was unable to be obtained
12C_6 Error First diastolic blood pressure should be measured before second
12C_7 Error First systolic or diastolic blood pressure obtained while other one
unobtainable
12D_1 Error Second systolic blood pressure is missing
12D_2 Error Second systolic blood pressure is out of range
12D_3 Error Second systolic blood pressure refused
12D_4 Quality
Check Unusually high second systolic blood pressure
12D_5 Error Second systolic blood pressure was unable to be obtained
12E_1 Error Second diastolic blood pressure is missing
12E_2 Error Second diastolic blood pressure is out of range
12E_3 Error Second diastolic blood pressure refused
12E_4 Quality
Check Unusually low or high second diastolic blood pressure
12E_5 Error Second diastolic blood pressure was unable to be obtained
12E_6 Error Second systolic or diastolic blood pressure obtained while other
one unobtainable
13A_1 Quality
Check Fasting status is missing
13A_2 Error Fasting status is invalid

WISEWOMAN MDE Manual B-9 August 2017
Edition 9.03 revised
MDE Item/
Edit # Status Edit Message
13A_3 Quality
Check Fasting status is not missing and all measurements were refused
13A_4 Quality
Check Fasting status is not missing and all measurements were
unobtainable
13A_5 Quality
Check Fasting status is not missing and all measurements are missing
14A_1 Error Cholesterol date is missing and cholesterol measurement has
been attempted
14A_2 Error Cholesterol date occurred after submission date
14B_1 Error Total cholesterol is missing
14B_2 Error Total cholesterol is out of range
14B_3 Quality
Check Total cholesterol measurement is unusually high or low
14B_4 Quality
Check Total cholesterol measurement is unable to be obtained or
refused
14C_1 Error HDL cholesterol is missing
14C_2 Error HDL cholesterol is out of range
14C_3 Quality
Check HDL measurement is unusually high
14C_4 Quality
Check HDL measurement is unable to be obtained or refused
14D_1 Error LDL cholesterol is missing
14D_2 Error LDL cholesterol is out of range
14D_3 Quality
Check Non-fasting - LDL value should be 999
14D_4 Quality
Check LDL measurement is unusually high
14E_1 Error Triglycerides is missing
14E_2 Error Triglycerides is out of range
14E_3 Quality
Check Non-fasting - triglycerides value should be 9999
14E_4 Quality
Check Triglyceride measurement is unusually high
15A_1 Error Glucose date is missing and glucose measurement has been
attempted
15A_2 Error Glucose date occurred after submission date
15B_1 Error Glucose is missing
15B_2 Error Glucose and A1C are both missing

WISEWOMAN MDE Manual B-10 August 2017
Edition 9.03 revised
MDE Item/
Edit # Status Edit Message
15B_3 Error Glucose is out of range
15B_4 Quality
Check Glucose measurement is usually high or low
15B_5 Quality
Check Glucose measurement is unable to be obtained
15B_6 Quality
Check Glucose and A1C measurements were refused
15B_7 Error Non-fasting - glucose value should be 999
15C_1 Error A1C is missing
15C_2 Error A1C is out of range
15C_3 Quality
Check A1C measurement is unusually high or low
15C_4 Quality
Check A1C measurement is unable to be obtained
16A_1 Error Blood pressure alert status is missing
16A_2 Error Blood pressure alert status is invalid
16A_3 Error Documentation required: Alert BP, workup status coded as non-
alert
16A_4 Error No alert BP, workup status coded as alert
16A_5 Error No BP value, workup status coded as if BP was taken
16A_6 Error Documentation required: refused workup or lost to follow-up
16B_1 Error Alert BP, complete workup, invalid diagnostic exam date
16B_2 Error No alert BP, invalid diagnostic exam date
16B_3 Error No BP value, invalid diagnostic exam date
16B_4 Error Documentation required: BP diagnostic exam for complete
workup missing or not within a week of office visit
16B_5 Error Documentation required: BP workup by alternate provider, exam
date missing or not within a week of office visit
16B_6 Error Documentation required: BP workup refused and date of refusal
missing
16B_7 Error Documentation required: Lost to follow-up and date of lost to
follow-up missing
16C_1 Error Glucose alert is missing
16C_2 Error Glucose alert is invalid
16C_3 Error Documentation required: Alert BG, workup status coded as non-
alert
16C_4 Error No alert BG, workup status coded as alert
WISEWOMAN MDE Manual B-11 August 2017
Edition 9.03 revised
MDE Item/
Edit # Status Edit Message
16C_5 Error No BG value, workup status coded as if BG was taken
16C_6 Error Documentation required: refused workup or lost to follow-up
16D_1 Error Alert BG, complete workup, invalid diagnostic exam date
16D_2 Error No alert BG, invalid diagnostic exam date
16D_3 Error No BG value, invalid diagnostic exam date
16D_4 Error Documentation required: BG diagnostic exam for complete
workup missing or not within a week of office visit
16D_5 Error Documentation required: BG workup by alternate provider, exam
date missing or not within a week of office visit
16D_6 Error Documentation required: BG workup refused and date of refusal
missing
16D_7 Error Documentation required: Lost to follow-up and date of lost to
follow-up missing
WISEWOMAN MDE Manual B-12 August 2017
Edition 9.03 revised
Risk Reduction Counseling MDE Error and Quality Check Edit Description
MDE
Item/
Edit # Status Edit Message
17A_1 Error Initial RRC date is missing and screening type is baseline or rescreening
17A_3 Error Initial RRC date occurred after submission date
17B_1 Error RRC completion date is missing and risk reduction counseling date is valid
17B_2 Error RRC completion date occurs before initial risk reduction counseling date
17B_3 Error RRC completion date occurred after submission date
17B_4 Quality
Check RRC completion date is refused or lost to follow-up
17B_5 Quality
Check RRC was completed on or within 60 days after office visit date
17C_1 Error Nutrition priority area is missing and RRC date is valid
17C_2 Error Nutrition priority area is invalid
17C_3 Error Nutrition priority area is unknown
17D_1 Error Physical activity priority area is missing and RRC date is valid
17D_2 Error Physical activity priority area is invalid
17D_3 Error Physical activity priority area is unknown
17E_1 Error Smoking cessation priority area is missing for a smoker and RRC date is
valid
17E_2 Error Smoking cessation priority area is invalid
17E_3 Error Smoking cessation priority area is unknown
17F_1 Quality
Check Medication adherence priority area missing and RRC date is valid
17F_2 Error Medication adherence for hypertension priority area is invalid
17F_3 Error Medication adherence for hypertension priority area is unknown
18A_1 Error Readiness to change assessment date is missing and RRC date is valid
18A_2 Error Readiness to change assessment date occurred after submission date
18B_1 Error Participant stage of change is missing and readiness to change
assessment date is valid
18B_2 Error Participant stage of change is invalid
18B_3 Quality
Check Participant stage of change is refused
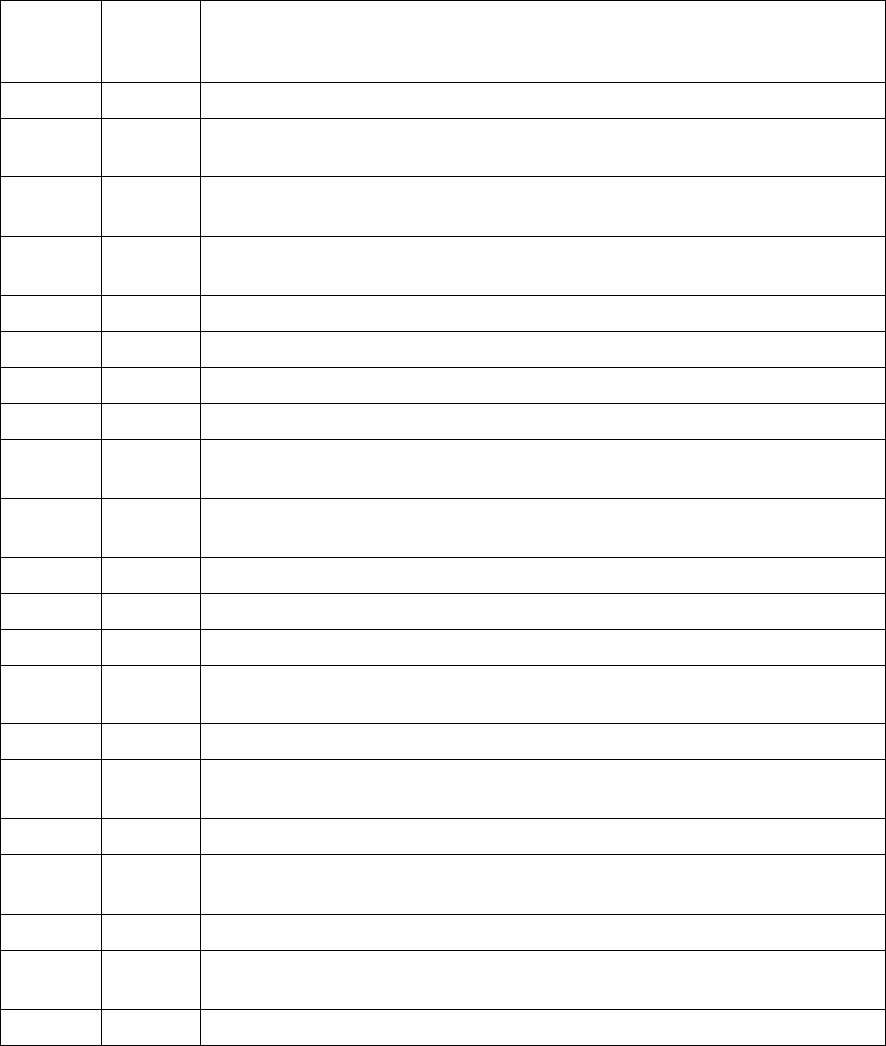
WISEWOMAN MDE Manual B-13 August 2017
Edition 9.03 revised
Healthy Behavior Support Options MDE Error and Quality Check Edit Description
MDE
Item/
Edit # Status Edit Message
19A_1 Error LSP/HC referral date occurred after submission date
20A_1 Error Number of LSP/HC sessions is missing and date of LSP/HC referral is
valid
20A_2 Quality
Check LSPHCRec does not equal number of LSP/HC sessions entered
20B_1 Quality
Check Date of LSP/HC session is missing and date of LSP/HC referral is valid
20B_2 Error LSP/HC date occurred after submission date
20B_3 Error Date of LSP/HC referral is missing and date of LSP/HC session is valid.
20C_1 Error LSP/HC ID is missing and date of LSP/HC session is valid
20D_1 Error Length of LSP/HC session is missing and date of LSP/HC session is valid
20D_2 Quality
check Length of LSP/HC session is out of range
20E_1 Error Contact type of LSP/HC session is missing and date of LSP/HC session is
valid
20E_2 Error Contact type of LSP/HC session is invalid
20F_1 Error Setting of LSP/HC session is missing and date of LSP/HC session is valid
20F_2 Error Setting of LSP/HC session is invalid
20G_1 Error Completion of LSP/HC status is missing and date of LSP/HC session is
valid
20G_2 Error Completion of LSP/HC status is invalid
21A_1 Quality
Check Tobacco cessation resource referral date is missing and smoking
cessation is a priority area
21A_2 Error Tobacco cessation resource referral date occurred after submission date
21B_1 Error Type of tobacco cessation resource is missing and tobacco cessation
resource referral date is valid
21B_2 Error Type of tobacco cessation resource is invalid
21C_1 Error Tobacco cessation activity completion status is missing and tobacco
cessation resource referral date is valid
21C_2 Error Tobacco cessation activity completion status is invalid
WISEWOMAN MDE Manual B-14 August 2017
Edition 9.03 revised
Validation Report Format and Contents
Data Validation Procedures and Forms
Specific response options for some data elements require that grantees provide
information in addition to that in the MDE data files. This section describes the
procedures and forms that can be used to validate or explain values in the MDE
data submitted, to provide explanation for alerts not seen within seven days, and
to notify CDC of changes in participants’ unique IDs.
Validation or Explanation of Values
When quality checks and errors are flagged for values, grantees can confirm
these values to be valid or provide further explanation about them. Values for
validation or explanation fall into the following general categories:
• Out-of-range values. These will be identified as quality checks or errors.
In general, values that are highly unusual will be identified as quality
checks, while values that are nearly impossible or are not a response
option for a categorical field will be identified as errors. For example,
heights less than 48 inches will be flagged as errors. Because such a
height would result in an error for this record, the program might confirm
this height by submitting an attachment to explain the circumstances.
• Responses codes as participant refused. Although participants are
able to refuse any question or clinical service, it may be appropriate to
inform CDC why the program has chosen to include a woman who
refuses basic assessment or screening services as a participant in the
program.
• Other. Other errors or quality checks flagged for which the grantee would
like to provide an explanation.
To validate or provide explanation for a value, grantees should use the form
shown at the end of this Appendix, which will be included in the WISEWOMAN
system. If there are values for validation or explanation, the form should be filled
out through the web-based WISEWOMAN Data Management System at the
time of the MDE submission.
Notification of Participant Unique ID Changes
If the participant unique ID number changes for one or more participants
between submissions, grantees must notify CDC of the change by submitting a
Participant ID Change Form, which details the participant unique IDs affected.
This form can be filled on the web-based WISEWOMAN Data Management
System. CDC and the data contractor will change the woman’s ID in the Data
WISEWOMAN MDE Manual B-15 August 2017
Edition 9.03 revised
Management System. Identifying these changes is critical to accurately link
records between periods and track participant changes over time.
Error Rate Calculation Method
This section provides the method used to calculate error rates. The
WISEWOMAN website will generate a validation report for immediate viewing
after programs submit their data (either through the online validation tool or after
the program indicates that it would like a report generated for direct data entry).
The report contains an error rate calculated for the entire submission. There are
84 variables overall. The error rate is calculated using the following formula:
Error Rate = # of Errors / (# of Records * # of Variables)
Programs can provide explanations for any errors by submitting to CDC the
Validation of Data form shown at the end of this Appendix. The calculation of the
final error rate will be conducted following the final submission and review of
documentation provided by programs.
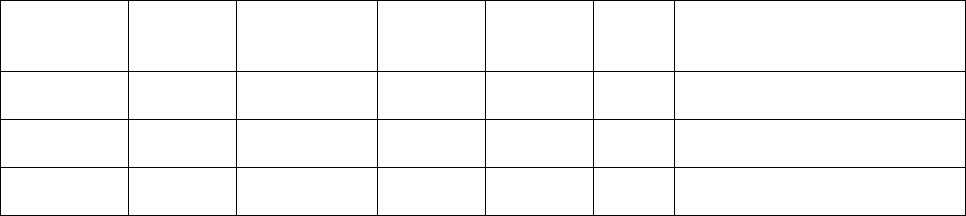
WISEWOMAN MDE Manual B-16 August 2017
Edition 9.03 revised
Validation of Data Form
The Validation of Data Form should be filled out to validate or explain any values
submitted. These values will include mainly those flagged as errors or quality
checks. (See the Validation of Data section above for a list of errors and quality
checks.) However, grantees can use this form to comment on any values in the
MDE data. CDC will review the information provided in this form and consider
these values in the calculation of performance measure #1: Program submits
minimum data elements files on schedule and with no more than a 5% error rate.
Each value in the form (which will be made available on the WISEWOMAN
website) should be reviewed and verified by your program staff. Below are
directions for filling out each column.
• Validation Type. Identify whether the validation or explanation is for an
error (E), quality check (Q), or some other issue (O).
• StFIPS. Provide your state or tribal code as entered for the record to be
validated/explained.
• EncodeID. Provide the participant unique ID number for the record to be
validated/explained.
• BPDate. Provide the BPate for the record to be validated/explained.
• MDE Item #. Provide the MDE item number with the error, quality check,
or other value for validation/explanation.
• Value. Provide the value or code (e.g., numeric value for height, ‘7
unknown’) to be verified/explained.
• Validation/Explanation. Provide an explanation for the value (e.g., review
of hard-copy record, discussion with provider verified value). For ease of
reading longer explanations in Excel, use of wrapped text is encouraged.
Validation
Type
(E, Q, or O)
StFIPS
EncodeID
BPDate
MDE
Item #
Value
Validation/ Explanation
E= error; Q = quality; O = other.
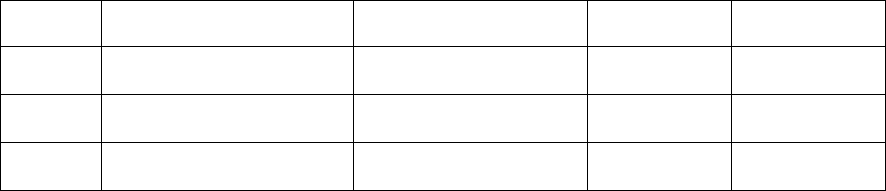
WISEWOMAN MDE Manual B-17 August 2017
Edition 9.03 revised
Participant ID Change Form
The Participant ID Change Form should be filled out when a participant’s Encode
ID has changed after a previous submission. The correct Encode ID for a
participant is needed to link screening and lifestyle intervention records and
calculate rescreening data. Each value in the form (which will be made available
on the WISEWOMAN website) should be reviewed and verified by your program
staff. Below are directions for filling out each column.
• StFIPS. Provide your state or tribal code as entered for the participant with
the new Encode ID.
• Original EncodeID. Provide the original participant unique ID number for
the participant.
• New EncodeID. Provide the new, changed participant unique ID number
for the participant.
• Date of Change. Provide the date that the EncodeIDs were changed.
• Reassigned Date. If the original EncodeID has been reassigned to a new
participant, provide the date of the reassignment here; otherwise, leave
this field blank.
StFIPS
Original EncodeID
New EncodeID
Date of
Change
Reassigned
Date
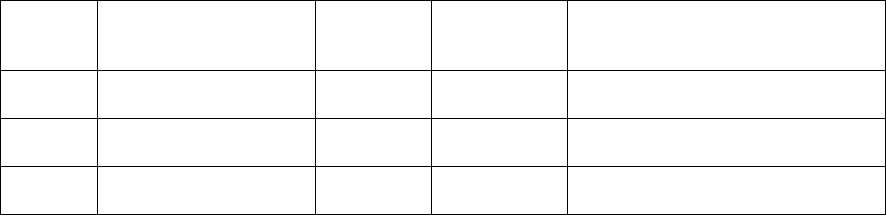
WISEWOMAN MDE Manual B-18 August 2017
Edition 9.03 revised
Correction to Previous MDE File Form
The Correction to Prevoius MDE File Form may be filled out when modifications
have been made to a screening cycle record that had been previously submitted
to CDC. Grantees are not required to submit this form, but may choose to submit
it if they would like to provide an explanation to CDC about updates or
corrections made to previously submitted data.
Each value in the form (which will be made available on the WISEWOMAN
website) should be reviewed and verified by your program staff. Below are
directions for filling out each column.
• StFIPS. Provide your state or tribal code as entered for the participant with
the new Encode ID.
• EncodeID. Provide the original participant unique ID number for the
participant.
• BPDate. Provide the office visit date (BPDate) for the screening (baseline
screening or rescreening) that took place during the screening cycle that
the corrections affect.
• NScreen. Provide the number of screning cycles received by the
particpant (NScreen) as of the screening cycle that the corrections affect.
• Description of Change(s). Provide a summary of the change(s) to the
screening cycle record.
StFIPS
EncodeID
Office
Visit Date
Screening
Cycle
Number
Description of Change(s)
APPENDIX C:
DATA ANALYSIS AND USE
WISEWOMAN MDE Manual C-1 August 2017
Edition 9.03 revised
MDEs provide a rich source of data for the WISEWOMAN Program. CDC and
grantees use MDEs in a variety of ways to monitor and assess progress and
performance. This Appendix describes the data summary report generated with
every submission and other data uses for the MDEs by CDC. It also discusses
potential ways in which grantees can use the data.
Data Summary Report Format and Content
MDE data submissions are used to generate biannual program-specific and
aggregate MDE reports. CDC and grantees use these reports to gauge program
progress in meeting goals and identify areas for improvement. For example, CDC
project officers may use these reports to help identify areas for technical
assistance, and grantees may use them to detect areas where further provider
training is needed. Uses of MDE data are discussed in greater detail in the
subsections below.
Additional information about the data summary report format and content will be
provided once available.
Data Use by CDC
CDC’s use of MDEs can be organized into four main categories: program
progress and performance; program improvement and technical assistance; data
quality improvement; and evaluation. These categories are described below.
• Progress and performance. Overall Program progress and performance
drive justification for Program continuance. MDEs are used to assess
grantee and overall Program progress toward meeting set goals and
performance measures. Overall Program data can also be compared to
other national data to understand potential Program contribution to
meeting national goals for cardiovascular disease. In the WISEWOMAN
Program Guidance and Resource Document, cited uses of MDEs by CDC
include assessing:
o Grantee progress in meeting goals related to screenings
o Prevalence of risk factors in the state/tribal populations served
o Reach of the program to priority populations
o Implementation of risk reduction counseling and LSP/HC in
accordance with CDC WISEWOMAN Program requirements and as
intended
o Grantee progress in meeting performance measures
o Changes in participant outcomes over time
• Program improvement and technical assistance. Program
improvement occurs at two levels: CDC and grantee. MDE data
WISEWOMAN MDE Manual C-2 August 2017
Edition 9.03 revised
aggregated across grantees provide a picture of overall strengths and
weaknesses of the Program, thereby identifying areas where CDC
technical assistance may be needed for all grantees. Individual grantee
data can be viewed alone or compared to that for the overall Program to
assess grantee strengths and areas for improvement. Project officers may
then use grantee MDE data to tailor individualized technical assistance.
• Data quality improvement. Because Program progress and performance
can greatly affect continued support and funding for the Program, having
high-quality data to describe the Program is essential. Analysis of MDEs
can be used to identify the types of data quality issues that exist and
where improvements are needed.
• Evaluation. As distinguished from the monitoring uses bulleted above,
MDEs may contribute to data provided for evaluation. It may provide the
service and outcomes data required for analyses that assess the effect of
the program. It may also help identify areas for further investigation, where
the program is performing better than or not as well as expected.
Potential Data Use by Funded Programs
Grantees use MDEs in a variety of ways to drive program improvement and track
program progress. Below are some examples of MDE use among funded
programs.
• Analysis of provider performance. Grantees have used MDEs to track
the number of screenings and LSP/HC sessions conducted by provider
sites. In addition, some have created program-level performance
measures that they calculate for individual providers.
• Identification of areas for provider trainings. Grantees have used
MDEs to identify areas where provider sites were in need of training or
technical assistance.
• Assessment of performance in comparison to national benchmarks.
Grantees have used MDEs to assess the characteristics and risks of the
population served in comparison to that for their entire state or the nation.
• Assessment of participant changes in risk factors. Grantees have
used MDEs to analyze changes between participants’ baseline screening,
rescreening, and follow-up screening visits.
Grantees interested in receiving technical assistance related to using MDEs as a
data source for program monitoring and evaluation should contact their project
officer.
APPENDIX D:
TECHNICAL ASSISTANCE RESOURCES

WISEWOMAN MDE Manual August 2017
Edition 9.03 revised
D-1
To support grantees in collecting and submitting data, CDC has developed
several strategies and tools to provide technical assistance to grantees. This
appendix describes the various types of technical assistance available to
grantees, the web-based WISEWOMAN Data Management System, the method
for requesting individualized technical assistance, and the technical assistance
Helpdesk.
Types of Technical Assistance Available
Technical assistance available to grantees can be broadly categorized as
individualized technical assistance, group technical assistance, and tools. Below,
specific types of technical assistance/tools within these categories are described.
The table at the end of this subsection summarizes the types of technical
assistance/tools by category, provider, and timeline.
Individualized Technical Assistance
• Data Review Calls. After each MDE submission, summary reports are
generated and reviewed with grantees during a data review call. The
purpose of the calls is to discuss with grantees any programmatic and
quality issues highlighted in the data. As needed, data quality reports and
other materials may also be reviewed.
• Helpdesk Requests. Grantees can request individualized technical
assistance through the Helpdesk (WISEWOMANTA@mathematica-
mpr.com). This type of assistance is tailored to the grantee and the
question. More information is provided in the following subsections of this
appendix, “Requesting Individualized Technical Assistance” and
“Helpdesk for Technical Assistance Requests.”
• Site Visit Consultation. Throughout the year, project officers conduct site
visits to programs. During these visits, project officers provide technical
assistance to their programs on a wide variety of programmatic issues,
including those related to data. If requested by the project officer and/or
program, the data contractor may also accompany the project officer on a
site visit to provide consultation on data issues.
• WISEWOMAN Annual Meeting One-on-One Sessions. In addition to
group presentations at the WISEWOMAN Annual meeting, grantees also
have an opportunity to set up one-on-one meetings with data staff to
answer grantee-specific questions.

WISEWOMAN MDE Manual August 2017
Edition 9.03 revised
D-2
Group Technical Assistance
• WISEWOMAN Annual Meeting Data Sessions. At each annual meeting,
presentations related to data issues are conducted. The topics of these
sessions vary from year to year and are dependent on overall Program
activities and needs at the time.
• All-Program Calls. Data-related issues may be discussed during the
monthly all-program calls. In general, data issues will not be discussed in
depth, as the calls are only an hour long and are designed to cover
multiple topics. Therefore, updates will be provided mainly during all-
program calls, and in-depth data-related discussions or trainings will take
place through other venues.
• Ad Hoc Data Calls and Trainings. Throughout the course of the year,
data issues affecting a majority of or all grantees may be identified, either
through individualized technical assistance or as a result of changes to the
MDE submission process and specifications (e.g., modification of MDE
specifications, added MDE variables). As a result, trainings or group
communications may be needed. If the need for these trainings or group
communications cannot be fulfilled at the annual meeting, ad hoc data
calls and trainings will be held.
Tools
• WISEWOMAN MDE Manual. This manual is a technical assistance tool
for grantees. It provides detailed guidance on the MDE submission
process and MDE specifications, and it will be updated as necessary to
stay current with the data submission and collection requirements.
Grantees can access the current edition on the WISEWOMAN website
(https://partner.cdc.gov).
Edits Documentation. The edits documentation details all the edits
programmed in the validation tool. The documentation provides the coding
used for validation in plain language. It also documents the changes to the
edits from the previous MDE edition. Grantees can access the current
edition on the WISEWOMAN website (https://partner.cdc.gov).
As needed, other tools may be disseminated, such as instructions for using the
web-based WISEWOMAN website. Some tools currently being considered
include a frequently-asked-questions document, a repository for grantee forms,
and a listserv or other interface for grantee communications. All these would be
made available through the WISEWOMAN website.
WISEWOMAN MDE Manual August 2017
Edition 9.03 revised
D-3
Summary of Types of Technical Assistance and Tools Available
TA Type Provider Timeline
Individual
Data review calls Project officers and/or data
contractor Semiannually, after MDE
submission and release of
data summary reports
Helpdesk requests Data contractor As needed
Site visit consultation Project officers and/or data
contractor Annually
Annual meeting one-on-one
session Data contractor Annually
Group
Annual Meeting data sessions CDC staff and data contractor Annually
All-program calls CDC staff and/or data
contractor As needed during monthly
calls
Ad hoc data calls and
trainings Data contractor As needed
Tools*
WISEWOMAN MDE Manual Data contractor Ongoing
Edits documentation Data contractor Ongoing
* Other tools are under development.
Helpdesk for Individualized Technical Assistance Requests
Technical assistance may be requested through by emailing the data contractor
at WISEWOMANTA@mathematica-mpr.com.1 Once a request for technical
assistance related to MDEs is received, Helpdesk will automatically confirm
receipt of the request. For more complicated requests or those requiring project
officer input, responses may take more than 24 hours. Requests received
immediately before or after the data submission period may also take longer to
process, though confirmation of the request will always occur within 24 hours of
its receipt.
All requests are tracked by Helpdesk staff; this is to ensure that follow-up is
completed for all requests and that responses are satisfactory to the requester. In
addition, project officers will be kept abreast of the technical assistance needs of
their programs. The tracking of technical assistance requests by the Helpdesk
and project officers allows CDC to identify common issues to inform Program-
wide technical assistance.
1 Grantees may also choose to telephone individual members of the data contractor team. However, requesting technical
assistance through email or website guarantees that all data contractor team members receive notification of the
request, and therefore requests are more likely to receive a prompt response.
APPENDIX E:
MDE EDITIONCROSSWALK
WISEWOMAN MDE Manual E-1 August 2017
Edition 9.03 revised
Crosswalk of Changes Between MDE Edition8.2 and 9.00
This crosswalk documents changes between MDE 8.2 used in the previous cooperative agreement and MDE 9.00 used in the current cooperative
agreement. Changes to MDE items from MDE 9.02 to MDE 9.04 are noted in the Summary of MDE Manual Updates document that accompanied
this manual.14
MDE 9.00 Items
Comments on Modifications to MDE Item Labels, Values, and Value Descriptions from MDE 8.2 to 9.00a
Item
Number Variable Name
Status
0a MDEver Modified • MDE Version was changed from 8.00 to 9.00.
1a StFIPS Modified • State/Tribal FIPS Code were updated to reflect current grantee states/tribes.
1b HdANSI New
1c EnrollSiteID Modified • EnrollSiteID was modified to capture ZIP code where enrollment was conducted.
1d ScreenSiteID Modified • ScreenSiteID was modified to capture National Provider Identifier (NPI) for provider.
2a TimePer New
2b NScreen New
2c Type New
3a EncodeID No change
3b ResANSI New
3c ZIP No change
14 a Changes to MDE item validation edits from MDE 8.0 to MDE 9.0 are noted in the SQL validation edits spreadsheet. For MDE 9.0, response options 8/88/888
(Don’t want to answer/Client refused) changed to display in grey highlights, indicating that these responses should not appear on data collection forms.
WISEWOMAN MDE Manual E-2 August 2017
Edition 9.03 revised
MDE 9.00 Items
Comments on Modifications to MDE Item Labels, Values, and Value Descriptions from MDE 8.2 to 9.00a
Item
Number Variable Name
Status
3d MYB (previously
DOB) Modified • MDE label changed from “Date of Birth” to “Month and Year of Birth.”
• Response option wording changed from “Date of Birth” to “Month and Year of Birth.”
3e Latino No change
3f Race1 No change
3g Race2 No change
3h Education No change
3i Language New
4a SRHC Modified • MDE label changed from “Have you ever been told by a doctor, nurse, or other health professional that your blood
cholesterol is high?” to “Do you have high cholesterol?”
• Response option 7 changed from “Don’t know” to “Don’t know/Not sure.”
4b SRHB Modified • MDE label changed from “Have you ever been told by a doctor, nurse, or other health professional that you have
high blood pressure?” to “Do you have hypertension (high blood pressure)?”
• Response option 7 changed from “Don’t know” to “Don’t know/Not sure.”
4c SRD Modified • MDE label changed from “Have you ever been told by a doctor, nurse, or other health professional that you have
diabetes?” to “Do you have diabetes? (either Type 1 or Type 2).”
• Modified response option 1 (Yes) description to remove guidance that “This response should include a diagnosis of
diabetes beyond pregnancy.”
• Removed response option 3 (“Yes – Gestational (pregnancy) diabetes only”).
• Response option 7 changed from “Don’t know” to “Don’t know/Not sure.”

WISEWOMAN MDE Manual E-3 August 2017
Edition 9.03 revised
MDE 9.00 Items
Comments on Modifications to MDE Item Labels, Values, and Value Descriptions from MDE 8.2 to 9.00a
Item
Number Variable Name
Status
4d SRHA Modified • MDE label changed from “Has a doctor, nurse, or other health professional ever told you that you had any of the
following: heart attack (also called myocardial infraction), angina, coronary heart disease, or stroke?” to “Have you
been diagnosed by a healthcare provider as having any of these conditions: coronary heart disease/chest pain,
heart attack, heart failure, stroke/transient ischemic attack (TIA), vascular disease, or congenital heart defects?”
• Response option 7 changed from “Don’t know” to “Don’t know/Not sure.”
5a HCMeds Modified • MDE label changed from “Are you taking any medicine prescribed by your doctor, nurse, or other health
professional for your high cholesterol?” to “Do you take medication to lower your cholesterol?”
• Changed coding for response option 3 (No) to 2.
• Added response options 3 (No – Could not obtain medication) and 5 (Not Applicable).
5b HBPMeds Modified • MDE label changed from “Are you taking any medicine prescribed by your doctor, nurse, or other health
professional for your high blood pressure?” to “Do you take medication to lower your blood pressure?”
• Changed coding for response option 3 (No) to 2.
• Added response options 3 (No – Could not obtain medication) and 5 (Not Applicable).
5c DMeds Modified • MDE label changed from “Are you taking any medicine prescribed by your doctor, nurse, or other health
professional for your diabetes?” to “Are you taking medication to lower your blood sugar (for diabetes)?”
• Changed coding for response option 3 (No) to 2.
• Added response options 3 (No – Could not obtain medication) and 5 (Not Applicable).
5d HCAdhere New
5e HBPAdhere New
5f DAdhere New
6a BPHome New
6b BPFreq New
WISEWOMAN MDE Manual E-4 August 2017
Edition 9.03 revised
MDE 9.00 Items
Comments on Modifications to MDE Item Labels, Values, and Value Descriptions from MDE 8.2 to 9.00a
Item
Number Variable Name
Status
6c BPSend New
7a Fruit New
7b Vegetables New
7c Fish New
7d Grains New
7e Sugar New
7f SaltWatch New
8a PAMod New
8b PAVig New
9a Smoker Modified • MDE label changed from “Do you now smoke cigarettes every day, some days, or not at all?” to “Do you smoke?
Includes cigarettes, pipes, or cigars (smoked tobacco in any form).”
• Response option 1 changed from “Every day” to “Current smoker.”
• Response option 2 changed from “Some days” to “Quit (1-12 months ago).”
• Response option 3 changed from “Not at all” to “Quit (more than 12 months ago).”
• Added response option 4 (Never smoked).
• Removed response option 7 (Don’t know/Not sure).

WISEWOMAN MDE Manual E-5 August 2017
Edition 9.03 revised
MDE 9.00 Items
Comments on Modifications to MDE Item Labels, Values, and Value Descriptions from MDE 8.2 to 9.00a
Item
Number Variable Name
Status
9b SecHand Modified • MDE label changed from “Not counting decks, porches, or garages, during the past 7 days, on how many days did
someone other than you smoke tobacco inside your home while you were at home?” to “About how many hours a
day, on average, are you in the same room or vehicle with another person who is smoking?”
• Changed response option from “Number of days” to “Number of hours.”
• Removed response option 77 (Don’t know).
• Added response option 66 “Less than one.”
• Changed coding for response option 0 (None) to 00.
0a QOLPH New
10b QOLMH New
10c QOLEffect New
11a Height Modified • Changed MDE length from 3 to 2.
• Changed valid range from 54-78 inches to 52-76 inches.
• Changed unusually high values from 74-78 inches to 74-76 inches and unusually low values from 54-58 inches to
52-58 inches.
11b Weight Modified • Changed valid range from 75-460 pounds to 74-460 pounds.
• Changed unusually low values from 75-90 pounds to 74-90 pounds.
11c Waist New
11d Hip New
12a BPDate No change
WISEWOMAN MDE Manual E-6 August 2017
Edition 9.03 revised
MDE 9.00 Items
Comments on Modifications to MDE Item Labels, Values, and Value Descriptions from MDE 8.2 to 9.00a
Item
Number Variable Name
Status
12b SBP1 No change
12c DBP1 No change
12d SBP2 No change
12e DBP2 No change
13a Fast (previously
TCFast) Modified • MDE label changed from “Fasting Status for Cholesterol Measurements” to “Fasting status.”
• Removed response options 6 (No cholesterol results available (inadequate blood sample or unable to obtain for
total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides)), 7 (Don’t know), and 8 (Client refused).
14a TCDate No change
14b TotChol No change
14c HDL No change
14d LDL No change
14e Trigly No change
15a BGDate Modified • MDE label changed from “Glucose Measurement Date” to “Glucose/A1C Measurement Date”
15b Glucose Modified • MDE label changed from “Glucose (fasting or nonfasting)” to “Glucose (fasting).”
• Removed response options 666 (Participant has a previous diagnosis of diabetes—glucose reading not necessary),
700 (A1C taken for screening purposes), and 800 (Participant has previous diagnosis of diabetes—A1C measured
by another provider.
15c A1C Modified • Removed response option 6666 (No previous diagnosis of diabetes).

WISEWOMAN MDE Manual E-7 August 2017
Edition 9.03 revised
MDE 9.00 Items
Comments on Modifications to MDE Item Labels, Values, and Value Descriptions from MDE 8.2 to 9.00a
Item
Number Variable Name
Status
16a BPAlert Modified • Removed response options 1 (Workup pending), 3 (Workup not medically indicated, client being treated), and 7 (No
blood pressure value recorded).
• Added response option 2 (Follow-up – workup by alternate provider).
• Changed coding for response option 2 (Workup complete) to 1.
• Changed coding for response option 6 (Not an alert reading) to 3.
16b BPDiDate Modified • MDE label changed from “If Average SBP >180 or DBP >110, Diagnostic Exam Date” to “If Average SBP >180 or
DBP >110, Workup Date.”
• Response option wording changed from “Blood Pressure Diagnostic Exam Date” to “Blood Pressure Workup Date.”
16c BGAlert Modified • MDE label changed from “If GLUCOSE ≤50 or GLUCOSE ≥275, what is the status of the workup?” to “If GLUCOSE
≤50 or GLUCOSE ≥250, what is the status of the workup?”
• Removed response options 1 (Workup pending), 3 (Workup not medically indicated, client being treated), and 7 (7
No blood glucose value recorded).
• Added response option 2 (Follow-up – workup by alternate provider).
• Coding for response option 2 (Workup complete) to 1.
• Coding for response option 6 (Not an alert reading) changed to 3.
16d BGDiDate Modified • MDE label changed from “If GLUCOSE ≤50 or GLUCOSE ≥275 Diagnostic Exam Date” to “If GLUCOSE ≤50 or
GLUCOSE ≥250 Workup Exam Date.”
• Response option wording changed from “Blood glucose diagnostic exam date” to “Blood glucose workup date.”
17a RRCDate New
17b RRCComplete New
17c RRCNut New
17d RRCPA New
WISEWOMAN MDE Manual E-8 August 2017
Edition 9.03 revised
MDE 9.00 Items
Comments on Modifications to MDE Item Labels, Values, and Value Descriptions from MDE 8.2 to 9.00a
Item
Number Variable Name
Status
17e RRCSmoke New
17f RRCMedAdher
e New
18a RTCDate New
18b RTC New
19a RefDate New
20a LSPHCRec New
20b Intervention Modified • MDE label changed from “Date of Lifestyle Intervention (LSI) Session (Date of Referral to a Community-Based
Resource)” to “Date of Lifestyle Program (LSP) / Health Coaching (HC) Session.”
• Response option wording changed from “LSI Session Date (MMDDCCYY)” to “Lifestyle Program/Health Coaching
Session Date (MMDDCCYY).”
20c LSPHCID New
20d LSPHCTime New

WISEWOMAN MDE Manual E-9 August 2017
Edition 9.03 revised
MDE 9.00 Items
Comments on Modifications to MDE Item Labels, Values, and Value Descriptions from MDE 8.2 to 9.00a
Item
Number Variable Name
Status
20e ContactType Modified • MDE label changed from “Type of Lifestyle Intervention (LSI) Contact” to “Type of Lifestyle Program (LSP) / Health
Coaching (HC) Session.”
• Removed response options 6 (Referral to community-based resources with no WISEWOMAN LSI-referral
confirmed), 7 (Referral to community-based resources with no WISEWOMAN LSI-referral not confirmed), and 77
(Unknown).
• Response option 5 wording changed from “Evidence that computer-based session was completed” to “Evidence
that non-interactive computer-based session was completed;” coding for response option 5 (Evidence that
computer-based session was completed) changed to 6.
• Added response options 3 (Smart phone/tablet Application), 7 (Evidence that interactive computer-based session
was completed), 0 (Other), and 99 (No answer recorded).
• Coding for response option 3 (Evidence that mailed materials were opened and reviewed) changed to 4.
• Coding for response option 4 (Evidence that audiotape or DVD was opened and reviewed) changed to 5.
20f Setting Modified • MDE label changed from “Setting of Lifestyle Intervention (LSI) Session” to “Setting of Lifestyle Program (LSP) /
Health Coaching (HC) Session.”
• Removed response option 7 (Unknown).
• Added response options 3 (Combination) and 9 (No answer recorded).
20g LSPHCComp New
21a TobResDate New
21b TobResType New
21c TobResComp New

WISEWOMAN MDE Manual E-10 August 2017
Edition 9.03 revised
MDE Items Removed Between MDE Versions 8.2 and 9.00
Item
Number Variable Name Variable Label
Screening and Assessment MDE Items
1b
HDFips
FIPS County Code (Provider)
2a
NRec
Unique Screening Record ID Number
3b
CntyFIPS
County of Residence
3h
Race3
Race: Third Race
3i
Race4
Race: Fourth Race
3j Race5 Race: Fifth Race
6a FAMHAM Has your father, brother, or son had a stroke or heart attack before age 55?
6b
FAMHAF
Has your mother, sister, or daughter had a stroke or heart attack before age 65?
6c FAMD Has either of your parents, your brother or sister, or your child ever been told by a
doctor, nurse, or other health professional that he or she has diabetes?
12c
BGFast
Fasting Status for Glucose Measurements
13d
TCAlert
If TOTCHOL>400, what is the status of the workup?
13e
TCDiDate
If TOTCHOL>400, Diagnostic Exam Date
Lifestyle Intervention MDE Items
0a
MDEver
MDE Version
1a StFIPS State/Tribal FIPS Code
2a NRec Unique Lifestyle Intervention (LSI) Record ID Number
3a
EncodeID
Unique Participant ID Number
6a
Nutrition
Receive Nutrition Counseling as Part of a Lifestyle Intervention (LSI) Session
6b
NutLink
Linked to Community-Based Nutrition Resources
6c
PA
Received Physical Activity Counseling as Part of LSI Session
6d PALink Linked to Community-Based Physical Activity Resources
6e QuitLine Linked to Proactive Tobacco QuitLine
6f
TobacLink
Linked to Community-Based Tobacco Cessation Resources
6g
TobacCoun
Received Smoking Cessation Counseling During LSI Session
APPENDIX F:
PERFORMANCE MEASURES
WISEWOMAN MDE Manual August 2017
Edition 9.03 revised
F-1
1. Program submits minimum data elements files on schedule and with no more
than a 5% error rate. (Calculated using MDEs)
2. Program has actively engaged with a minimum of two public or private partner
organizations to promote and support environmental changes for increased
physical activity, access to healthy food choices, smoking cessation and
elimination of exposure to secondhand smoke. (Determined from information
provided in the annual performance reports)
3. Program has met or exceeded 95% of its CDC approved screening goals.
Screening goals include baseline and rescreenings (including overscreens),
and follow-up screenings. (Calculated using MDEs)
4. Program delivers risk reduction counseling to 100% of women screened. Risk
reduction counseling includes appropriate referral to health coaching,
community resources or lifestyle programs. (Calculated using MDEs and
annual performance reports)
5. Program follows-up with 100% of women with abnormal blood pressure
values. Follow-up parameters should be determined by WISEWOMAN
guidelines and facility medical protocol. (Calculated using annual
performance reports)
6. Program ensures that 80% of women referred to a lifestyle program or health
coaching participate in the program. Participation is defined as attendance at
a minimum of one lifestyle program or coaching session. (Calculated using
MDEs)
7. Program ensures that 60% of women who participate in a lifestyle program or
health coaching complete the program. Completion is defined as the number
of sessions that the evidence base for the program has determined to be
required for behavior change. (Calculated using MDEs and annual
performance reports)
APPENDIX G:
EXAMPLES FROM AMERICAN HEART ASSOCIATION’S LIFE’S SIMPLE 7
WISEWOMAN MDE Manual G-1 August 2017
Edition 9.03 revised
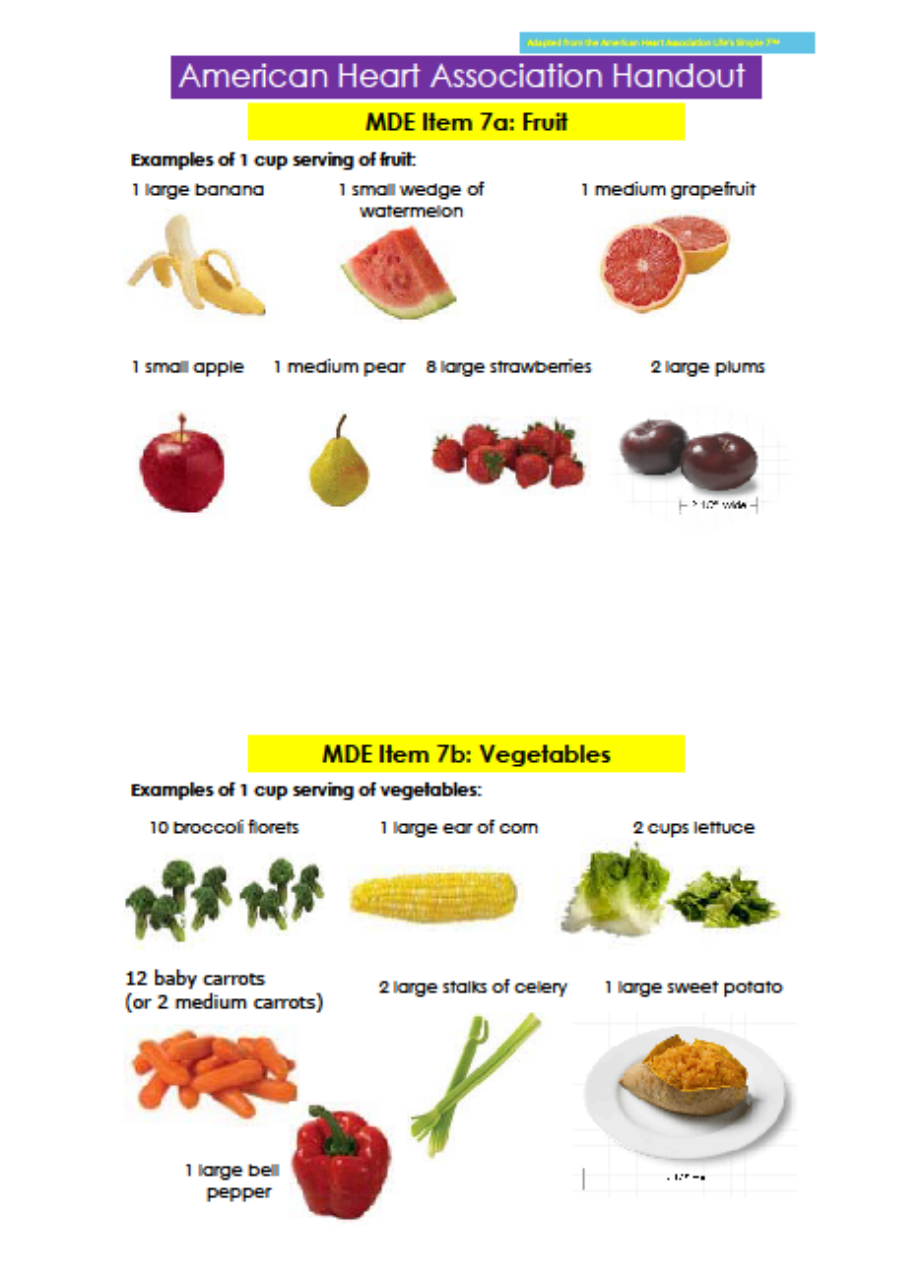
WISEWOMAN MDE Manual G-1 August 2017
Edition 9.03 revised

WISEWOMAN MDE Manual G-2 August 2017
Edition 9.03 revised

WISEWOMAN MDE Manual G-3 August 2017
Edition 9.03 revised

WISEWOMAN MDE Manual G-4 August 2017
Edition 9.03 revised
APPENDIX H:
WISEWOMAN HEALTH RISK ASSESSMENT MDE ELEMENTS
WISEWOMAN MDE Manual H-1 August 2017
Edition 9.03 revised
WISEWOMAN Health Risk Assessment MDE Elements
A health risk assessment is a health questionnaire that provides individuals with
an evaluation of their health risks and quality of life. The information gathered
from the assessments help providers work collaboratively with patients to make
decisions and improve their health.
Cardiovascular health risk assessment must be conducted for each
WISEWOMAN participant during screening visits.1
As health risk assessment results and screening values provide the basis for risk
reduction counseling tailored to each individual, the assessment must be
completed prior to risk reduction counseling. Risk reduction counseling must be
provided to all WISEWOMAN participants face-to-face at the time of their
screening visit.2
The required MDE items for a WISEWOMAN Health Risk Assessment are
listed below:
• Medication Use
o Use of medication to lower cholesterol (5a: HCMeds), blood
pressure (5b: HBPMeds), or blood sugar (5c: DMeds)
• Medication Adherence
o Medication adherence for participants taking medication to lower
cholesterol (5d: HCAdhere), blood pressure (5e: HBPAdhere), or
blood sugar (5f: DAdhere)
• Self-Monitoring Blood Pressure (for participants with high blood
pressure or previously diagnosed with hypertension [high blood pressure]
only)
o Blood pressure self-monitoring (6a-6c: BPHome, BPFreq, BPSend)
• Nutrition
o Consumption of fruits (7a: Fruits), vegetables (7b: Vegetables), fish
(7c: Fish), whole grains (7d: Grains), and beverages with added
sugar (7e: Sugar)
o Sodium or salt intake (7f: SaltWatch)
1 Patients may complete health risk assessment forms in the health care setting at the time of screening or before their
initial visit. If the health risk assessment is completed prior to the screening office visit, the information must be
available to the clinician/counselor and incorporated into risk reduction counseling.
2 Technical Assistance and Guidance Document Version 2 (Page 56).
WISEWOMAN MDE Manual H-2 August 2017
Edition 9.03 revised
• Physical Activity
o Moderate (8a: PAMod) and vigorous physical activity (8b: PAVig)
• Tobacco use and Secondhand Smoke Exposure
o Smoking status (9a: Smoker)
o Exposure to secondhand smoke (9b: Secondhand)
• Quality of Life Indicators (10a-10c: QOLMH, QOLPH, QOLEffect)
In addition to these required MDE items, grantees may choose to use other
health assessment questions that align with their provider clinical practice
protocols, evaluation needs, or other program purposes.
If additional non-MDE questions are used, grantees should use validated
questions and are encouraged to share additional information collected with their
assigned Project Officer.
APPENDIX I:
SUBMITTING RECORDS FOR NAVIGATED WOMEN

WISEWOMAN MDE Manual I-1 August 2017
Edition 9.03 revised
SUBMITTING RECORDS FOR NAVIGATED WOMEN
Starting with the October 2016 submission, grantees have the option to submit
records for navigated women in line with NBCCEDP criteria for navigation.
Navigated women are participants who receive healthy behavior support
services (such as health coaching or lifestyle programs), but whose
cardiovascular screenings are NOT funded* by WISEWOMAN. Submitting data
for navigated women is optional.
The navigated women reporting form should be used to identify records
associated with navigated women. All records for navigated participants included
in the mde submission should be noted in this spreadsheet. This will help
grantees know what percentage of their data and their budget consists of
navigated women.
Instructions:
1. Include records for navigated women in the MDE file due April 1 and
October 1 of each program year. Records for navigated women should
be integrated into the mde file.
2. In addition to the MDE file grantees should complete the WISEWOMAN
Navigated Women Reporting form* excel spreadsheet, which is
availabled in DMS 2.0 under “Documentation”:
a. In the rows provided in the spreadsheet, enter the required fields
for each record for a navigated woman in the MDE file. The
required fields include:
• Grantee FIPS code (1a: StFIPS)
• Unique ID (3a: EncodeID)
• Month and Birth of Year (3d: MYB)
• Blood Pressure Date (12a: BPDate)
b. For each submission, use the excel spreadsheet to report the
complete set of records for navigated women included in the MDE
file. For example, if a grantee includes 10 records for navigated
participants in the October 2016 MDE file, the Navigated Women
Reporting spreadsheet for October 2016 should list 10 records for
navigated women. In April 2017, the spreadsheet should be
cumulative to include all records for navigated women from the
October 2016 reporting period plus the new records for navigated
women from the April 2017 reporting period.
3. Upload the MDE file and Navigated Women Reporting spreadsheet to the
DMS by the MDE submission deadline:

WISEWOMAN MDE Manual I-2 August 2017
Edition 9.03 revised
a. Save the file using the format Navigated_PPYYMM, where PP is
the program abbreviation and YYMM is the date of submission. YY
is the two-digit year and MM is the month from 01 to 12. Use
leading zeros when specifying years and months between 01 and
09 (for example, "Navigated_AL1610").
b. Upload the MDE file and the Navigated Women Reporting
spreadsheet to the DMS by the MDE submission deadline. CDC
will assume that grantees that do NOT submit the Navigated
Women Reporting spreadsheet by the MDE submission deadline
do not have any records associated with navigated women.
4. Contact the WISEWOMAN TA HelpDesk
(WISEWOMANTA@mathematica-mpr.com) with any questions.
*For the purposes of submitting records for navigated women, NOT funded
includes women for whom screening services were reimbursed through an
alternative payment source other than WISEWOMAN. Women screened
using Indian Health Services funds are excluded from this definition.
WISEWOMAN MDE Manual J-1 August 2017
Edition 9.03 revised
APPENDIX J:
OTHER DOCUMENTS AND COMMUNICATION
[INSERT OTHER DOCUMENTS AND COMMUNICATION]