Laerdal Medical AS 801002 CPR meter User Manual 004 00031103 CPRmeter II User Guide
Laerdal Medical AS CPR meter 004 00031103 CPRmeter II User Guide
004_00031103 CPRmeter II User Guide
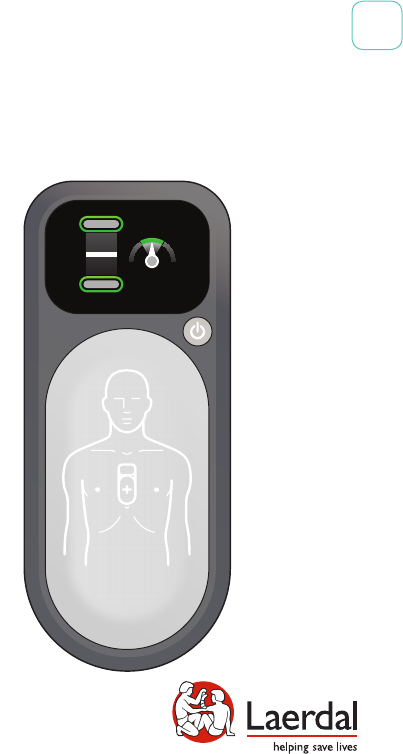
CPRmeter 2
User Guide
EN
www.laerdal.com

Contents
3
Intended Use 4
Indication for Use 4
Important Information 5
Items Included 6
Overview 8
Before Use - Insert Batteries 10
Before Use - Apply Patient Adhesive 11
Getting Started 12
CPRmeter 2 Placement 13
Feedback Display Overview 15
Compression Feedback 16
Depth 16
On Soft Surface 17
Rate 18
Compression Counter 18
Inactivity 19
Debriefing 20
Battery Indicator 22
Maintenance 23
After Each Use 23
Cleaning 24
Disinfection 24
Storing the CPRmeter 2 Between Use 25
Customer Service Indicator 25
Specifications 26
Symbol Glossary 29
Regulatory Information 31
5
Important Information
4
Intended Use
The CPRmeter 2 with Q-CPR® technology is a small, lightweight
device powered by a replaceable battery. The device is intended for
use by responders who have been trained in CPR and use of the
CPRmeter 2.
When attached to the bare chest of a suspected victim of SCA, the
CPRmeter 2 provides real-time feedback on CPR compressions in
accordance with current CPR guidelines. It displays CPR feedback
indicators for depth, release, and rate of chest compressions. It
also counts the number of compressions in a series, and provides
notification of lack of expected CPR activity.
If in doubt about the appropriateness for use, perform CPR without
using the CPRmeter 2.
Indication for Use
The CPRmeter 2 is used as a guide in administering cardiopulmonary
resuscitation (CPR) to a suspected sudden cardiac arrest (SCA)
victim at least 8 years old.
Warning
The CPRmeter 2 is not intended for use on SCA victims under 8 years old.
Note
CPR cannot assure survival, no matter how well it is performed. In
some patients, the underlying problem causing the cardiac arrest is not
survivable despite any available care.
Rx Only (USA)
Caution: Federal law restricts this device to sale by or on the order of
a physician.
67
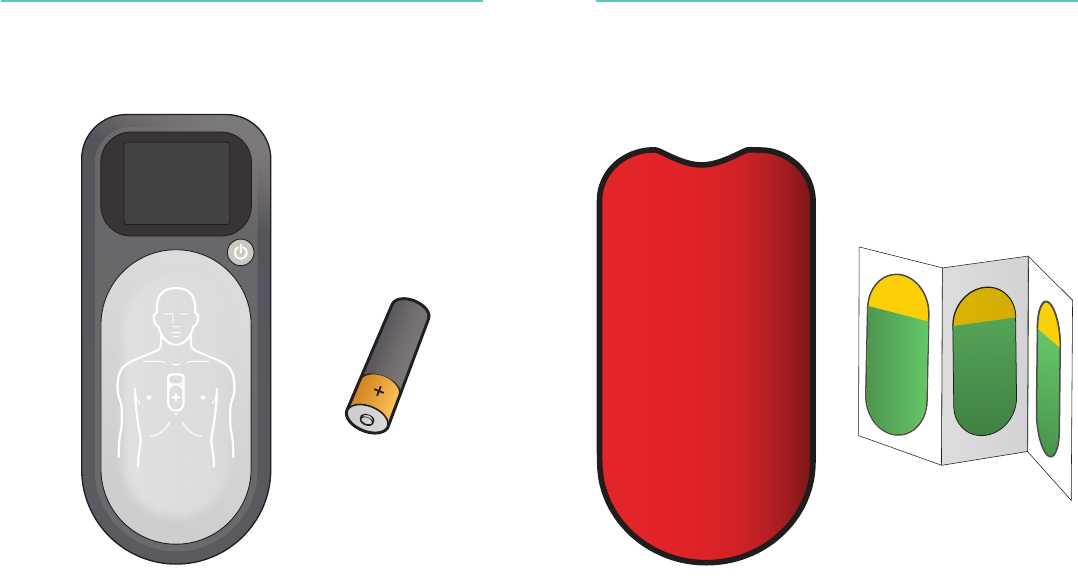
Items Included Items Included
-
A
A
A
X 2
Protective Sleeve
Patient Adhesive
AAA Batteries
CPRmeter 2
89
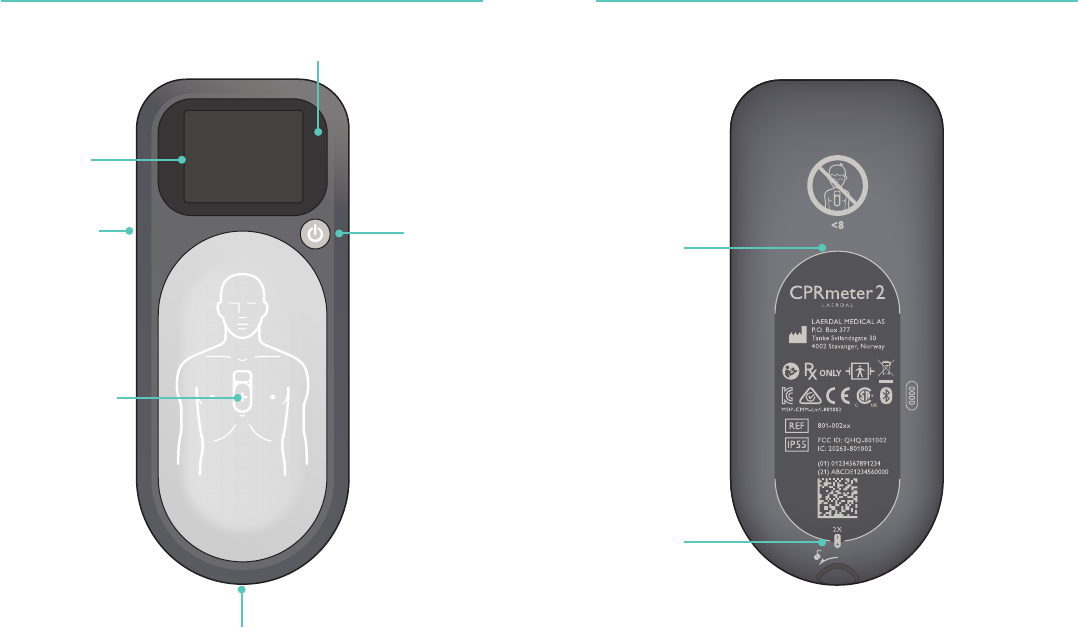
Overview Overview
On/Off
Status Light
Compression
Area
Battery Hatch
Display
Area
Placement guide
for adhesive
Placement guide
for adhesive
Membrane
Por t
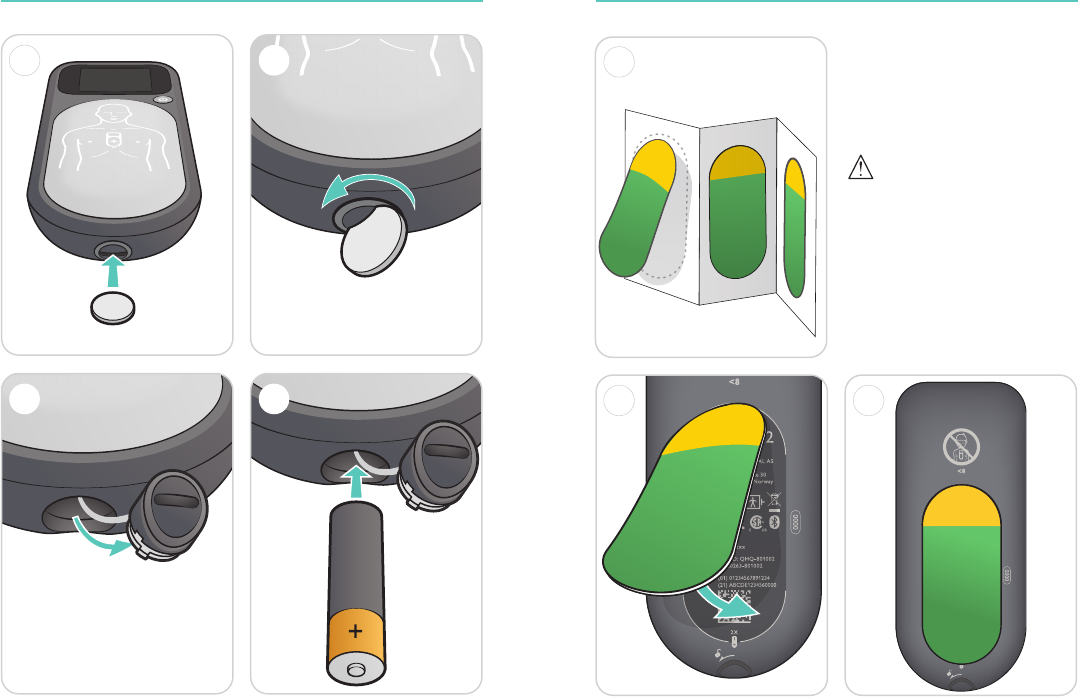
10 11
Before Use - Insert Batteries Before Use - Apply Patient Adhesive
11
x 2
2
4
-
3
1
32
Cautions
• Ensure Patient Adhesives are
within their expiration date.
• Adhesives should be removed
from the device and disposed
of after 2 years.
12 13
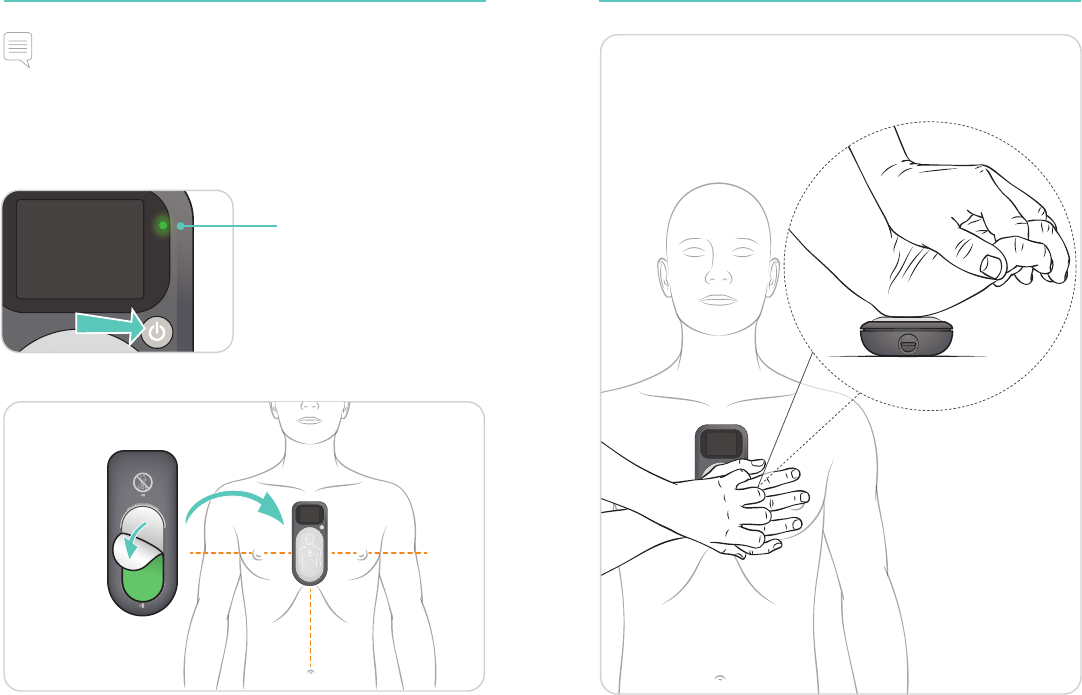
Getting Started CPRmeter 2 Placement
Note
• Remove the device from its protective cover.
• Ensure the patient is on a firm surface.
• Remove clothing from the patient’s chest.
Turn On
Place CPRmeter 2
If needed, wipe
fluids off the chest.
Ensure to use the heel of your hand and apply pressure
to the light grey area.
Begin CPR.
Provide chest
compressions
according to your
CPR protocol.
Status Light turns green
for a few seconds,
when CPRmeter 2 is
turned on.
14 15
CPRmeter 2 Placement Feedback Display Overview
Cautions
• If the CPRmeter 2 moves during use, re-position it to the center of
the chest, as shown.
• If difficulty is encountered in applying the device, do not delay
initiation of CPR. Remove the device and begin compressions.
• If the device's status light is orange and the CPRmeter display is
dark, stop using the CPRmeter and continue CPR. Contact Laerdal
for technical support after the event.
Warnings
• Do not use the device in conjunction with any mechanical or
automated compression device.
• Do not use the device on top of defibrillation pads, unless the
manufacturer of the defibrillator and the defibrillation pads has
explicitly stated that the device can be used in such manner.
• Do not delay CPR. If you experience any problems using the device,
continue CPR without it. If the device appears to be damaged, do
not use.
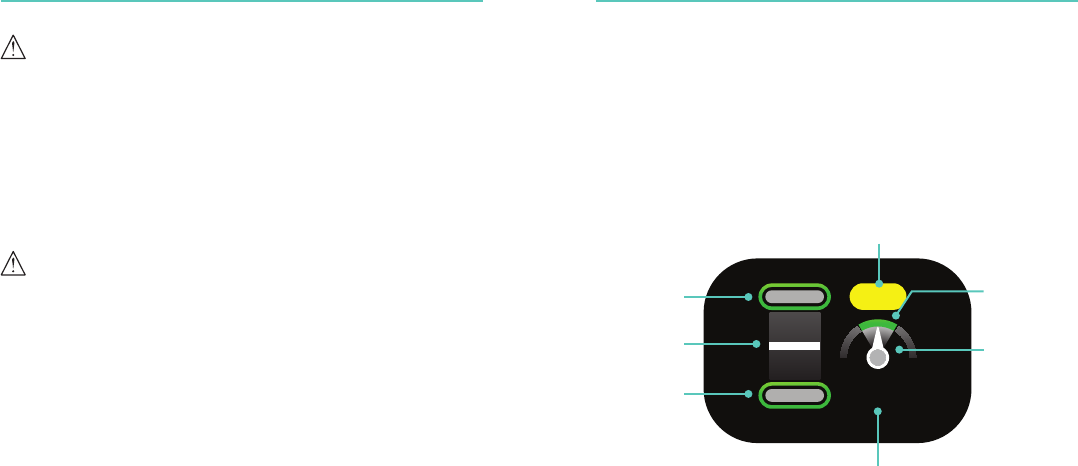
25
0:23
Compression
Release Target
Inactivity Timer
Compression
Depth Indicator Compression
Rate Indicator
Compression
Depth Target
Compression
Rate Target
Compression Counter
16 17
Depth
Release
On Soft Surface
If the CPRmeter 2 detects a compression that exceeds 70 mm (2.75"),
it will show the depth indicator below the target area. If a specific
CPR event requires CPR to be performed on a patient lying on a
mattress, slide a backboard under the patient and compensate for
the mattress softness by ensuring that for each chest compression
the area below the compression depth target lights up.
Warning
When performing CPR on a patient lying on a mattress, a backboard
must be used to limit the amount of compressed depth which is
absorbed by the mattress. Depending on characteristics of the mattress,
backboard and patient, the depth compensation does not guarantee
that the patient chest is compressed by 50 mm (2").
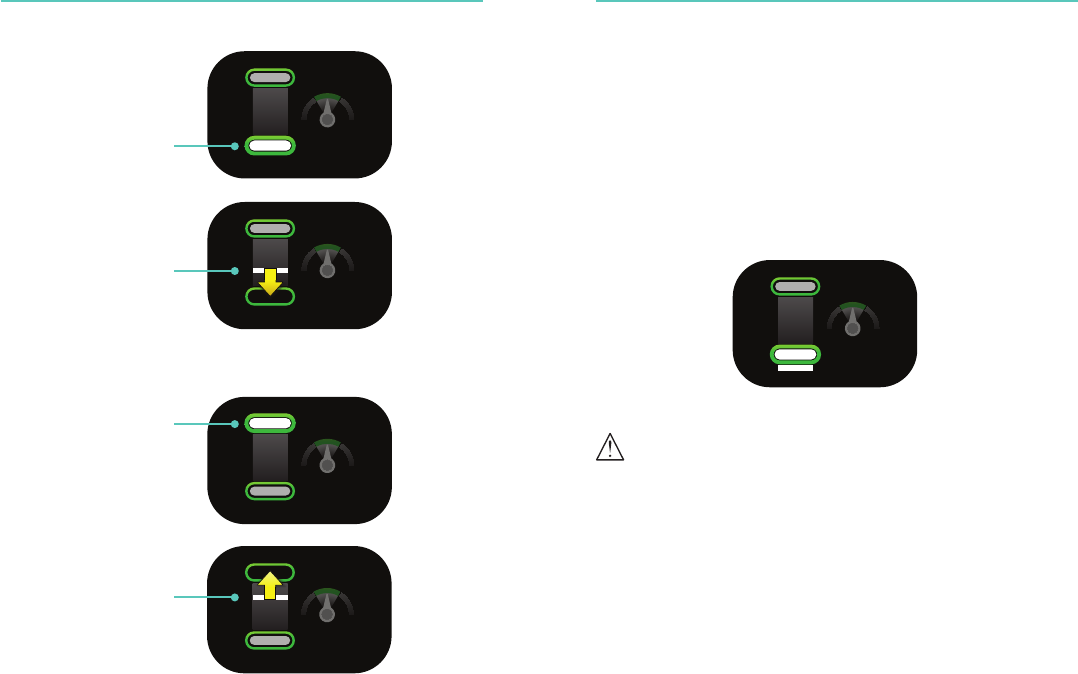
Compression Feedback Compression Feedback
Too shallow
Adequate depth
Adequate release
Incompletely release
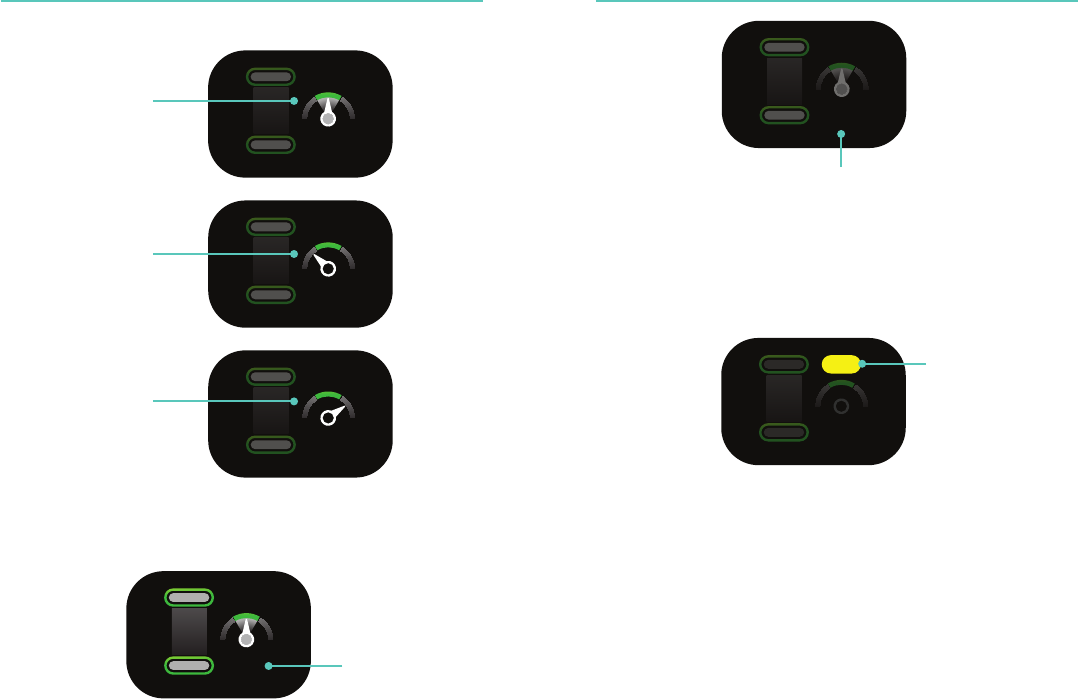
18 19
Compression Feedback Compression Feedback
Rate
Compression Counter
When compressions are started a counter will display grey up to
25 compressions.
Compression
Counter
19
During a cycle of 30 compressions, the counter changes to solid
white between 25 and 30 compressions. Beyond 30 compressions,
the counter digits flash solid white for every tenth compression.
Without a compression the counter is reset after 3 seconds.
Inactivity
• After 3 seconds the CPRmeter 2 displays an inactivity timer
which counts the seconds since the last compression.
• After 20 seconds since the last compression, the inactivity timer
starts flashing.
• After 1 minute, the CPRmeter 2 display fades down to
conserve battery power. The display is restored when a new
compression is delivered.
Inactivity
Timer
0:23
Too slow
Adequate rate
Too fast
25
20 21
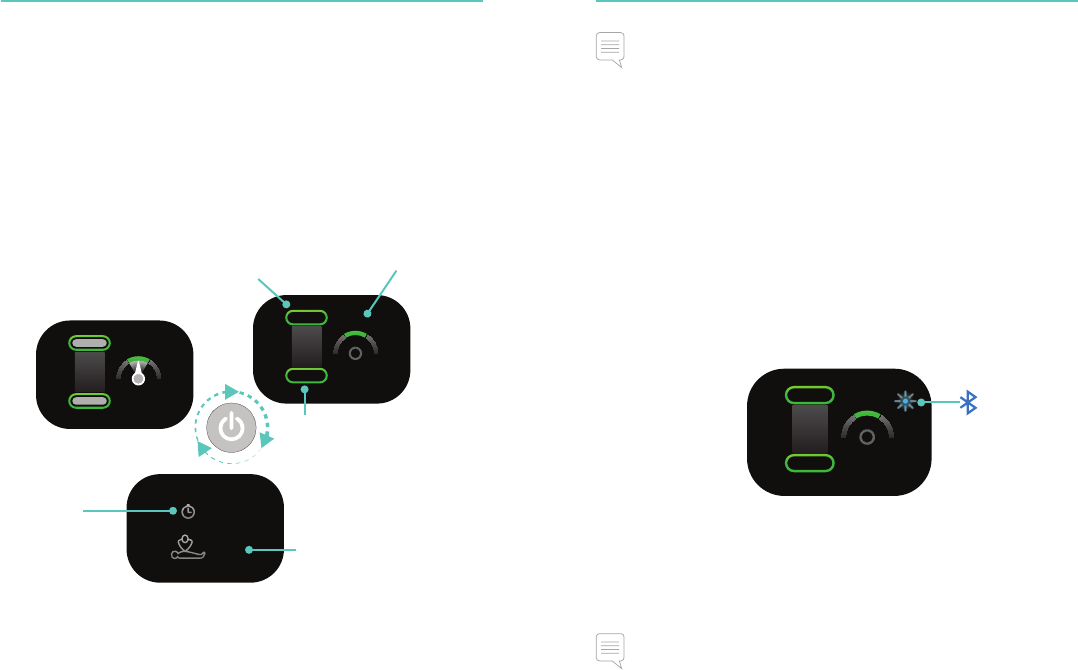
Debriefing Debriefing
Q-CPR® Quick Review
The CPRmeter 2 can display CPR performance statistics for the last
CPR event. When the device is turned on, press the On/Off button
once to activate Q-CPR Quick Review. The statistics are shown
over two displays.
Press the On/Off button once to cycle to the next display.
The CPRmeter 2 reverts to Compression Feedback mode if a
compression is delivered.
98% 70%
96%
24:35
73%
Percentage of
compressions with
adequate release
Percentage of
compressions with
adequate depth
Percentage of
compressions with
adequate rate
% of event
duration where
compressions have
been delivered
Duration of
the CPR event
(minutes: seconds).
Notes
• The CPR event statistics are stored when the CPRmeter 2 is turned
off. When turned on again, the statistics from the last stored CPR
event can be reviewed.
• When the CPRmeter 2 is used in a new CPR event, the Q-CPR
Quick Review will display the current event’s statistics.
• CPR performance statistics are only calculated if at least
10 compressions have been delivered.
Wireless Data Transfer
The CPRmeter 2 has Bluetooth Smart functionality for uploading
complete event data to an external device, like a PC. Bluetooth can
also be used to stream live CPR performance data during training.
To connect a device, go to the MiniEvent Review screen by pushing
the On/Off button. The status light will flash blue indicating that
Bluetooth is on and available for connection. When connected to a
device, the flashing blue light turns steady. The CPRmeter 2 is now
ready to transfer CPR performance data.
Note
Ensure Bluetooth connectivity is disabled during clinical use.
98% 70%
96%
23
Maintenance
22
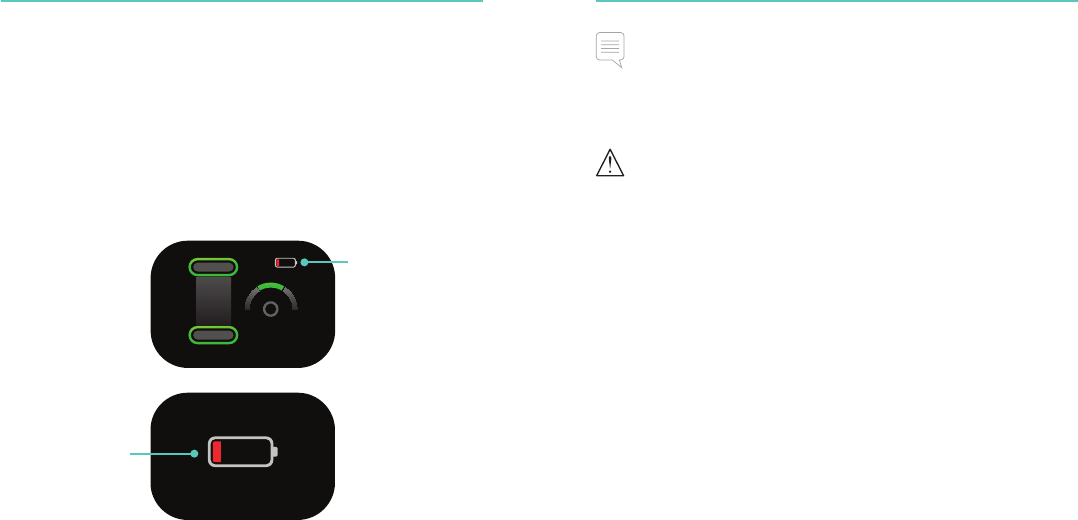
Battery Indicator
22
22
Battery Monitoring
The CPRmeter 2 continuously monitors the power of its battery.
On a routine basis, particularly after periods of non-use, check the
CPRmeter 2 battery status by switching on and checking if the low
battery icon is displayed.
If the remaining power is estimated to be less than that required for
a 30 minute CPR event, the visual indicators signal that the battery
should be replaced before the next use.
Routine Maintenance
1. On a routine basis check the battery status (as described).
2. Replace the battery at least every 2 years.
3. On a routine basis, check that the CPRmeter 2 has a patient
adhesive in place and that the liner remains on it. Replace
the patient adhesive at least every 2 years if it is not used.
A large low-battery
icon appears when
the CPRmeter 2 is
being turned off.
A small low-battery
icon appears when
the CPRmeter 2 is
being turned on.
23
Note
If the remaining battery power during use becomes too low to sustain
further operation, the low-battery icon is shown for 10 seconds and then
the CPRmeter 2 turns itself off.
Warning
Do not interrupt CPR to replace the battery. Continue CPR without
feedback from the CPRmeter 2.
After each Use
After use on a patient, the CPRmeter 2 may be contaminated and
should be handled appropriately.
1. Place the contaminated CPRmeter 2 in a plastic bag until it can
be cleaned (do not insert a contaminated CPRmeter 2 into
its casing).
2. If it is visibly soiled, wipe the CPRmeter 2 with a soft cloth or
paper towel to remove as much contamination as possible.
3. Remove the Patient Adhesive from the back of the CPRmeter 2.
4. Clean the CPRmeter 2 as described under Cleaning and
Disinfection. Proper cleaning is required to achieve disinfection.
5. Check the exterior of the CPRmeter 2 for signs of damage.
Contact Laerdal to arrange for replacement if needed.
6. Apply a new Patient Adhesive to the device as described in
Before Use - Apply Patient Adhesive.
24 25
Maintenance Maintenance
Cleaning
If the CPRmeter 2 has been used in a training situation, it may be
wiped using an alcohol wipe (70% ethanol solution).
If the device has been used in a clinical situation, clean it as follows:
1. Prepare the cleaning solution by mixing (5 ml) of mild
dishwashing liquid in 4 l of warm tap water (40-50 °C)
2. Submerge a small brush (e.g. toothbrush) in the cleaning
solution and scrub the device for a minimum of 2 minutes.
3. If the membrane port is clogged, use the brush to remove
any obstruction.
4. Wipe the exterior with a soft cloth dampened in lukewarm
water (22-40 °C).
Disinfection
1. Disinfect the exterior using a 0.55% solution of ortho-
phthalaldehyde. Spray the solution on to cover all exterior
surfaces, and allow to sit for a minimum of 12 minutes. An
alternative disinfection agent is isopropyl alcohol (70% solution).
If necessary respray to account for evaporation of isopropyl
alcohol.
2. Wipe the exterior with a clean soft cloth dipped in water a
minimum of three times to remove all traces of disinfectant
agent. Allow to dry completely.
Caution
Do not immerse the CPRmeter 2 in water, hold it under running water,
or allow moisture to penetrate it. Do not sterilize the CPRmeter 2.
Storing the CPRmeter 2 between Use
Store the CPRmeter 2 in its protective cover to shield the display
screen from scratches and to protect the patient adhesive from
damage. Ensure that the On/Off button can not be inadvertently
activated during storage.
Customer Service Indicator
If the Customer Service Indicator appears on the CPRmeter 2 at
shutdown, please contact your local Laerdal representative for
further instructions.
26 27
Specifications Specifications
26
CPR Targets
Category Specication
Compression Depth Target > 50 mm ( 2”)
Depth accuracy: ±10%
Compression Release Target Force < 2.5 kg (5.5 lbs)
Force accuracy: ±1.5 kg
(+3.3 lbs, - 3.3 lbs)
Compression Rate Target 100 to 120/min ± 3/min
CPRmeter 2 [REF 801-00233]
The CPRmeter 2 meets the performance requirements of IEC
60601-1, 2nd and 3rd edition.
Category Specication
Dimensions 153 mm x 64 mm x 25 mm (6.0” x 2.5” x 1.0”)
Weight 163 g (5.7 oz) (excluding batteries)
Battery 2 x 1.5V Size AAA (LR03)
Temperature Transport and Storage: -20 °C to 70 °C (-4 °F to 158 °F)
Operation: 0 °C to 40 °C (32 °F to 104 °F)
Sealing: Meets ISO/IEC 60529 class IP55
Product temperature can reach 60 °C (140 °F)
Relative Humidity Transport: 5% to 95%
Storage: 5% to 75%
Operation: 5% to 95%
Atmospheric
Pressure (Atm.p.)
Transport, Storage and Operation:
1014 to 572 mbar (1014 to 572 hPa)
Electromagnetic
Compatiblity
Meets IEC 60601-1-2 and RTCA/DO-160F Section
21 Category M
CPRmeter Patient Adhesives [REF 801-10850]
Category Specication
Dimensions 39 mm x 90 mm (1.5” x 3.5”)
Temperature Storage: -20 °C to 70 °C (-4 °F to 158 °F )
Operation: 0 °C to 50 °C (32 °F to 122 °F)
Relative Humidity Storage: 0% to 75%
Operation: 0% to 95%
Material Foam pad with biocompatible adhesive on each side.
Shelf Life 2 years when applied to the CPRmeter 2 or 4 years
in unopened package. Do not exceed the expiration
date on the packaging.
28
Specifications
Environmental Considerations
Product Information
CPRmeter 2 The CPRmeter 2 contains electronic
components. Dispose of it at an
appropriate recycling facility in
accordance with local regulations.
CPRmeter Patient Adhesive The used Patient Adhesive may be
contaminated with body tissue, fluid,
or blood. Dispose of it as infectious
waste.
29
Symbol Glossary
29
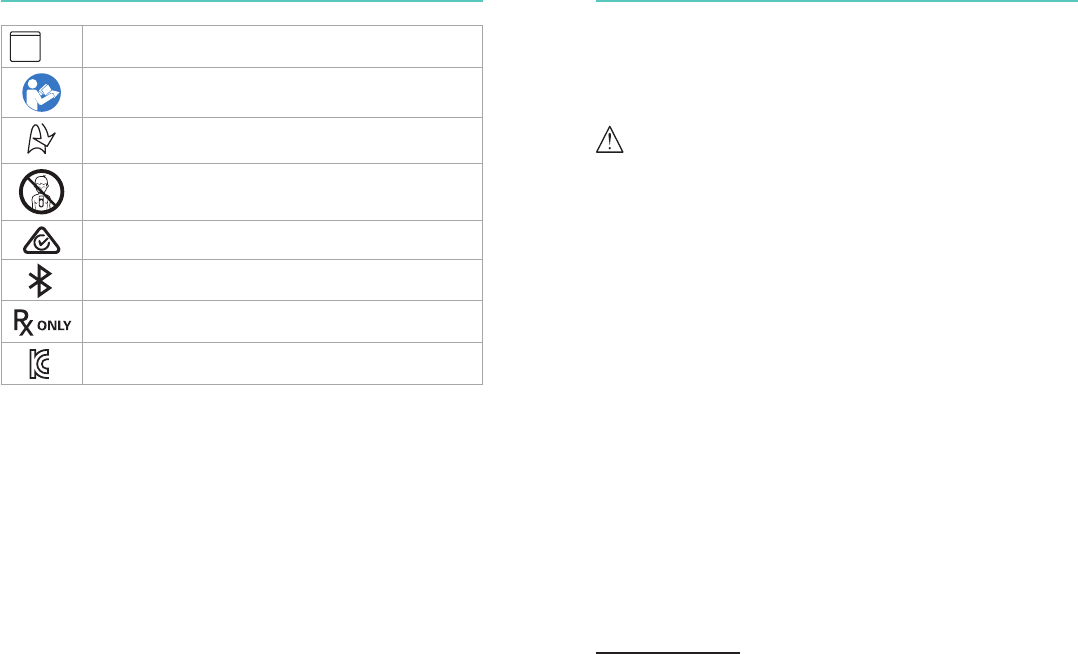
Symbol Denition
The product is in compliance with the essential requirements
of Council Directive 93/42/EEC as amended by Council
Directive 2007/47/EC and Council Directive 1999/5/EC.
Compliance with applicable U.S. and Canadian safety
standards has been certified by Canadian Standards
Association.
These CPRmeter 2 patient adhesives are disposable and are
for single patient use only. Do not re-use. Re-use will lead to
increased risk of cross contamination, and/or degradation of
adhesive performance.
Defibrillation protection. The CPRmeter 2 is defibrillation
protected, type BF patient connection.
Manufacturer
Dispose of in accordance with your country's requirements
Reference order number
IP
55 The CPRmeter meets IEC 60529 class IP55
Expiration date for patient adhesives
LATEX
Not made with natural rubber latex
Warning/Caution symbol
Temperature limitations for transport/storage of the adhesives
31
Regulatory Information
A Warning identifies conditions, hazards, or unsafe practices that can
result in serious personal injury or death.
A Caution identifies conditions, hazards, or unsafe practices that can
result in minor personal injury or damage to the CPRmeter 2.
Warnings
• When the CPRmeter 2 is used together with a defibrillator, make
sure to follow the defibrillator manufacturer’s instructions. Stop
compressions, remove hands from the CPRmeter 2 and remain clear
of all patient contact during defibrillation or when otherwise required, in
accordance with a proper defibrillation protocol.
• The CPRmeter 2 is not intended for use in a moving environment,
such as an ambulance. If used during patient transport, the device
may provide inaccurate feedback. If CPR is indicated in a moving
environment, do not rely on the depth feedback during such conditions.
It is not necessary to remove the device from the patient.
• Do not practice by using the CPRmeter 2 on a person. It may be used
with a training manikin or simply on a compliant surface for practice.
• Properly performed CPR may result in fracturing of the patient’s ribs.1
If rib integrity has been compromised, continue to provide CPR in
accordance with your local protocol.
• Properly performed CPR may result in chest injuries1 e.g. external chest
wall bruising or abrasion.
• Do not rely on CPRmeter 2 feedback during aircraft ascent and
descent, as its accuracy is reduced in such conditions.
1 Black CJ, Busuttil A, Robertson C. Chest wall injuries following
cardiopulmonary resuscitation. Resuscitation. 2004;63:339 –343.
30
Symbol Glossary
30
= # Contains number of CPRmeter 2 patient adhesives shown as
“#.”
Consult Directions for Use
Lift here to peel off the patient adhesive liner and apply to
patient’s bare chest
Not for use on children under 8 years old
Australian RCM mark
Bluetooth symbol
Federal law restricts this device to sale by or on the order of
a physician
KC symbol (for Korea)
32 33
Regulatory Information Regulatory Information
Cautions
• Do not apply the CPRmeter 2 to an open wound or recent incision site.
• The device is designed to be used only with Laerdal-approved accessories
and may perform improperly if non-approved accessories are used. Do
not attempt to modify the device in any way.
• Use only model 801-10850 Patient Adhesives with the CPRmeter 2.
Note
Changes or modifications not expressly approved by Laerdal Medical could
void the user’s authority to operate the equipment.
Recommendation
Responders should receive training, including regular refresher training,
in use of the CPRmeter 2. When training with the device on a CPR
manikin, disable or ignore feedback from the manikin.
WEEE
This appliance is marked according to the European directive 2012/19/EU on
Waste Electrical and Electronic Equipment (WEEE).
By ensuring this product is disposed of correctly, you will help prevent
potential negative consequences for the environment and human health,
which could otherwise be caused by inappropriate waste handling of this
product.
The symbol on the product indicates that this appliance may not be treated
as household waste. Instead it shall be handed over to the applicable
collection point for the recycling of electrical and electronic equipment.
Disposal must be carried out in accordance with local environmental
regulations for waste disposal.
For more detailed information about treatment, recovery and recycling of this
product, please contact your local city office, your household waste disposal
service or the Laerdal representative where you purchased the product.
FCC
Federal Communications Commission Statement and Industry
Canada Statements
This device complies with part 15 of the FCC rules and RSS-210 of the
Industry Canada rules. Operation is subject to the following two conditions:
1. This device may not cause harmful interference, and
2. this device must accept any interference received, including interference
that may cause undesired operation.
Ce dispositif est conforme à la norme CNR-210 d’Industrie Canada
applicable aux appareils radio exempts de licence. Son fonctionnement est
sujet aux deux conditions suivantes:
1. Le dispositif ne doit pas produire de brouillage préjudiciable, et
2. ce dispositif doit accepter tout brouillage reçu, y compris un brouillage
susceptible de provoquer un fonctionnement indésirable.
This equipment has been tested and found to comply with the limits for
a Class B digital device, pursuant to part 15 of the FCC Rules. These limits
are designed to provide reasonable protection against harmful interference
in a residential installation. This equipment generates, uses and can radiate
radio frequency energy and, if not installed and used in accordance with
the instructions, may cause harmful interference to radio communications.
However, there is no guarantee that interference will not occur in a
particular installation. If this equipment does cause harmful interference
to radio or television reception, which can be determined by turning
the equipment off and on, the user is encouraged to try to correct the
interference by one or more of the following measures:
• Reorient or relocate the receiving antenna.
• Increase the separation between the equipment and receiver.
• Consult the dealer or an experienced radio/TV technician for help.
FCC ID: QHQ-801002
IC ID: 20263-801002
The term ”IC” before the equipment certification number only signifies that
the Industry Canada technical specifications were met.
34 35
Regulatory Information Regulatory Information
Electromagnetic Conformity
Guidance and manufacturer’s declaration: The CPRmeter 2 is intended for
use in the electromagnetic environment specified in the tables below.
The user of the CPRmeter 2 should assure that it is used in such an
environment.
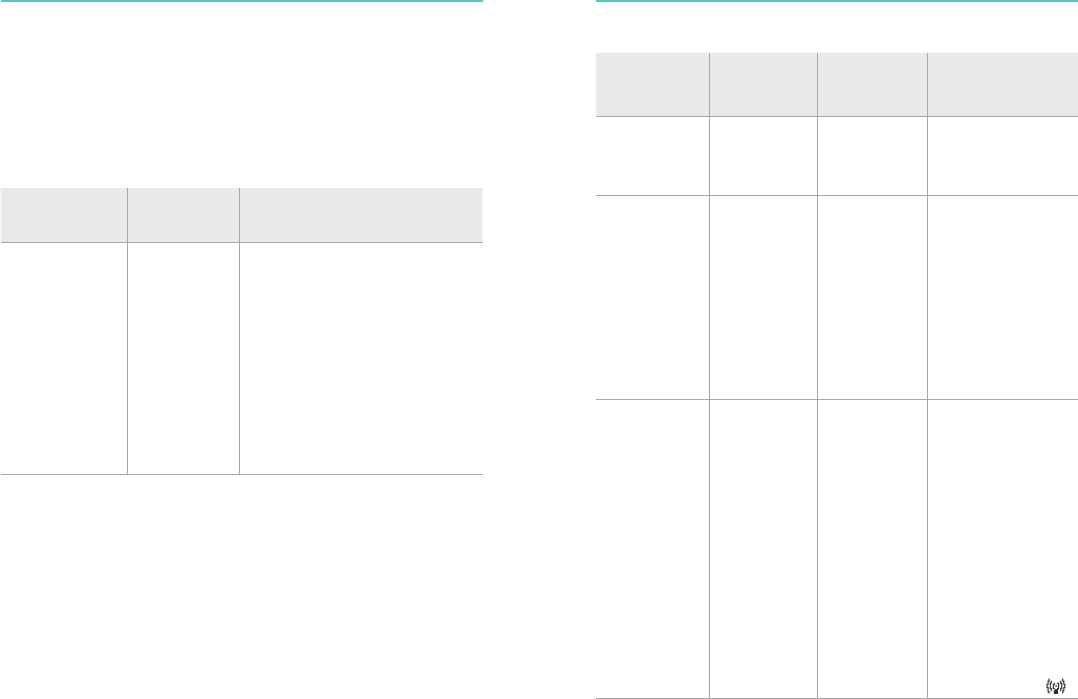
Electromagnetic Emissions
Emissions Test Compliance Electromagnetic Environment
Guidance
RF CISPR 11 Group 1 Class The CPRmeter 2 uses RF energy
only for its internal function.
Therefore, its RF emissions are very
low and are not likely to cause any
interference in nearby electronic
equipment.
The CPRmeter 2 is suitable for
use in all establishments, including
domestic establishments and those
directly connected to the public
low-voltage power supply network
that supplies buildings used for
domestic purposes.
Electromagnetic Immunity
Immunity Test IEC 60601
Test Level
Compliance
Level
Electromagnetic
Environment -
Guidance
Electrostatic
Discharge
(ESD)
IEC 61000-4-2
±6 kV contact
±8 kV air
±6 kV contact
±8 kV air
There are no special
requirements with
respect to electrostatic
discharge.
Power
Frequency
(50/60/400 Hz)
Magnetic Field
IEC 61000-4-8
3 A/m 3 A/m Power frequency
magnetic fields
should be at levels
characteristic of a
typical location in a
typical commercial/
hospital environment.
There are no special
requirements for
non-commercial/
non-hospital
environments.
Radiated RF
IEC 61000-4-3
10 V/m
80 MHz to
2.5 GHz
10 V/m Portable and mobile
RF communications
equipment should
be used no closer
to any part of the
CPRmeter 2, than is
absolutely necessary.
†,‡ The recommended
separation distances
for various transmitters
and the CPRmeter
2 are shown in
the following table.
Interference may
occur in the vicinity
of equipment marked
with the following
symbol:
36 37
Regulatory Information Regulatory Information
† The ISM (industrial, scientific and medical) bands between 150 kHz and 80
MHz are 6,765 MHz to 6,795 MHz; 13,553 MHz to 13,567 MHz; 26,957 MHz
to 27,283 MHz; and 40,660 MHz to 40,700 MHz.
‡ Field strengths from fixed transmitters, such as base stations for radio
(cellular/cordless) telephones and land mobile radios, amateur radio, AM
and FM radio broadcast, and TV broadcast cannot be predicted theoretically
with accuracy. To assess the electromagnetic environment due to fixed RF
transmitters, an electromagnetic site survey should be considered. If the
measured field strength in the location in which the CPRmeter 2 is used
exceeds the applicable RF compliance level above, the CPRmeter 2 should be
observed to verify normal operation. If abnormal performance is observed,
additional measures may be necessary, such as re-orienting or relocating the
CPRmeter 2.
Recommended separation distances between portable and
mobile RF communications equipment and the CPRmeter 2
The CPRmeter 2 is intended for use in an electromagnetic environment in
which radiated RF disturbances are controlled. The customer or the user of
the CPRmeter 2 can help prevent electromagnetic interference by maintaining
a minimum distance between portable and mobile RF communications
equipment (transmitters) and the CPRmeter 2 as recommended below,
according to the maximum output power of the communications equipment.
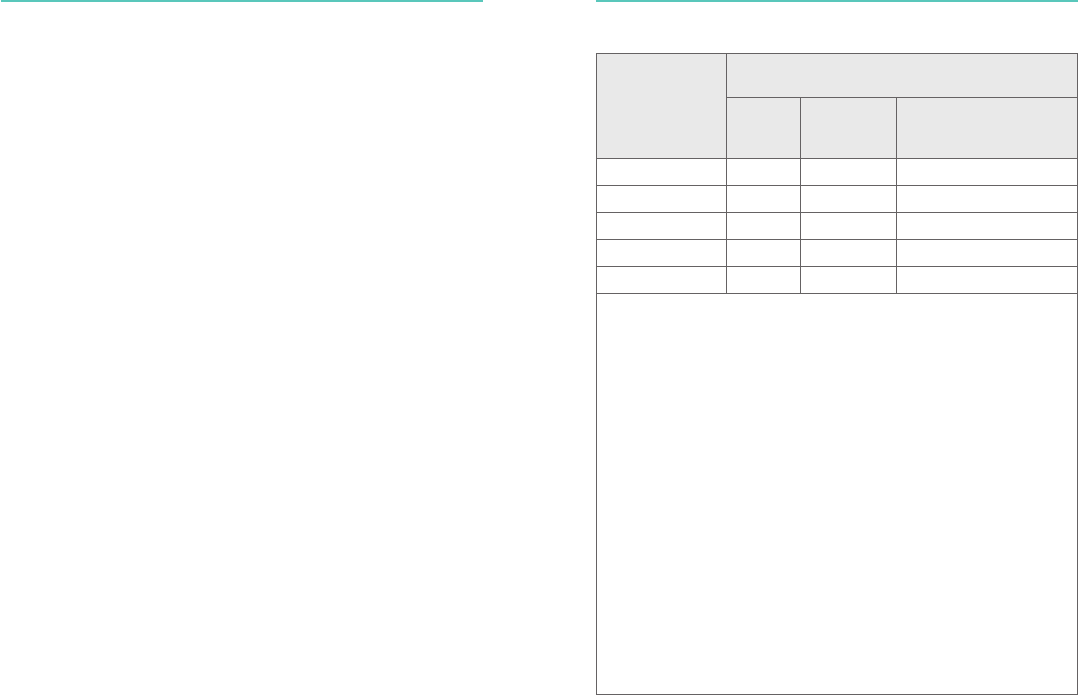
Electromagnetic Emissions
Rated maximum
output power of
transmitter [W]
Separation distance according to frequency of
transmitter [m]
150 kHz
to 80
MHz
80 MHz to
800 MHz
d=1.2√P
800 MHz to 2.5 GHz
d=2.3√P
0.01 NA 0.12 0.23
0.1 NA 0.38 0.73 0,72?
1 NA 1.2 2.3
10 NA 3.8 7.3 7,28?
100 NA 12 23
For transmitters rated at a maximum output power not listed above, the
recommended separation distance d in meters (m) can be determined
using the equation applicable to the frequency of the transmitter, where
P is the maximum output power rating of the transmitter in watts (W)
according to the transmitter manufacturer.
NOTE 1. At 80 MHz and 800 MHz, the separation distance for the higher
frequency range applies.
NOTE 2. The ISM (industrial, scientific and medial) bands between 150 kHz
and 80 MHz are 6,765 MHz to 6,795 MHz; 13,553 MHz to 13, 567 MHz;
26,957 MHz to 27,283 MHz; and 40,660 MHz to 40,700 MHz.
NOTE 3. An additional factor of 10/3 is used in calculating the
recommended separation distance for transmitters in the ISM frequency
bands between 150 kHz and 80 MHz and in the frequency range
80 MHz to 2.5 GHz to decrease the likelihood that mobile/portable
communications equipment could cause interference if it is inadvertently
brought into patient areas.
NOTE 4. These guidelines may not apply in all situations. Electromagnetic
propagation is affected by absorption and reflection from structures, objects
and people
NOTE 5. Transmitters/antennas of this power-level are most likely mounted
on an emergency vehicle chassis. The distances cited here are for open
field. For an external antenna, the separation distance is most likely shorter.

38
Warranty
The Laerdal CPRmeter 2 has a one-year limited Warranty.
Refer to the Laerdal Global Warranty for terms and conditions.
About this edition
The information in this applies to the model 801-00233
CPRmeter 2. This information is subject to change.
The CPRmeter™ with Q-CPR® is protected by U.S. and
International registered patents.The design of CPRmeter™ and
its feedback symbols are protected in several jurisdictions under
international design registrations.
CPRmeter™ and Q-CPR® are trademarks or registered trademark
of Laerdal Medical AS.

20-09504 Rev A
Laerdal® is a registered trademark of Laerdal Medical AS.
© 2015 Laerdal Medical AS. All rights reserved.
P.O. Box 377
Tanke Svilandsgate 30, 4002 Stavanger, Norway
T: (+47) 51511700
Printed in Norway.
www.laerdal.com