Medtronic Monitoring ZLINK2 GSM/ GPRS/ EDGE/ CDMA/ UMTS/HSPA Module User Manual M958549A001
Medtronic Monitoring, Inc. GSM/ GPRS/ EDGE/ CDMA/ UMTS/HSPA Module M958549A001
Users Manual

M958549A001 Rev B Refer to doc # A73903 for Printing
Instructions.
M958549A_fc.fm 3/12/15 2:34 pm
4" x 8" (101 mm x 203 mm)
Medtronic Confidential
mdvtldct_R1.5
SEEQ™
Mobile Cardiac Telemetry (MCT) System
_______________________________________
Draft for Radio Regulatory submission to FCC
Created March 12, 2015
_______________________________________
Instructions for use
Caution: Federal law (USA) restricts this device
to sale by or on the order of a physician.

M958549A001 Rev B Refer to doc # A73903 for Printing
Instructions.
M958549A_fc.fm 3/12/15 2:34 pm
4" x 8" (101 mm x 203 mm)
Medtronic Confidential
mdvtldct_R1.5
The following list includes trademarks or registered trademarks of
Medtronic in the United States and possibly in other countries. All
other trademarks are the property of their respective owners.
Medtronic, SEEQ

SEEQ MCT Instructions for use English 3
M958549A001 Rev B Refer to doc # A73903 for Printing
Instructions.
M958549A_ch.fm 3/12/15 2:34 pm
4" x 8" (101 mm x 203 mm)
Medtronic Confidential
mdvtldct_R1.5
Table of Contents
Getting started 4
Using the SEEQ MCT System during the monitoring period 9
What to do at the end of monitoring 12
Indications for use, contraindications, and precautions 14
Services for physicians 16
Specifications, compliance, and symbols 17
Frequently asked questions 21
Instructions for use
FOLLOW THESE INSTRUCTIONS CAREFULLY AND WATCH
YOUR INSTRUCTIONAL VIDEO BEFORE USING THE SEEQ MCT
SYSTEM.
Medtronic Customer Support USA: 1-877-247-7449
The SEEQ Mobile Cardiac Telemetry (MCT) System is a wearable,
wireless arrhythmia detection system that is used to aid clinicians in
diagnosing suspected cardiac arrhythmias. It consists primarily of the
Wearable Sensor monitoring device and the Transmitter (portable
data transmission device). The SEEQ MCT System, in combination
with interpretation services provided by the Medtronic Monitoring
Center, as well as secure online review of data (for prescribing
physicians only), enables patient- and physician-friendly arrhythmia
detection for up to 30 days at a time.
How the SEEQ MCT System works
Once activated, the Wearable Sensor continuously monitors the
heart and automatically collects ECGs. When rhythm abnormalities
are detected, data is automatically transmitted from the Wearable
Sensor to the Transmitter, which then automatically transmits the
data to the Medtronic Monitoring Center. Patients can also trigger
transmission of ECGs when they experience cardiac symptoms by
using the Patient Trigger Button. Certified cardiographic technicians
at the Medtronic Monitoring Center review received data and
document symptoms reported to the Medtronic Monitoring Center by
patients. Reports prepared by the Medtronic Monitoring Center are
delivered to the prescribing physician and made available to them at
www.medtronic.com to provide them with data for their diagnosis and
identification of various clinical conditions, events and/or trends.
Prescribing physicians may also be contacted by the Medtronic
Monitoring Center directly when arrhythmias that meet pre-defined
criteria are detected.
IMPORTANT:
▪The SEEQ MCT System is not intended to be an alarm or to
alert patients or physicians, and will not summon
emergency response in the event help is needed.
4 English SEEQ MCT Instructions for use
M958549A001 Rev B Refer to doc # A73903 for Printing
Instructions.
M958549A_ch.fm 3/12/15 2:34 pm
4" x 8" (101 mm x 203 mm)
Medtronic Confidential
mdvtldct_R1.5
▪The SEEQ MCT System is not intended to replace direct
communication with healthcare providers. Medtronic does
not provide medical advice to patients. Patients should
communicate with their prescribing physician to obtain
medical advice.
▪Data provided by the system should be used by physicians
along with all other clinical findings and exams to come to
a diagnosis.
▪Patients should talk to their healthcare provider
immediately if there are any concerns or if their condition
changes.
Getting started
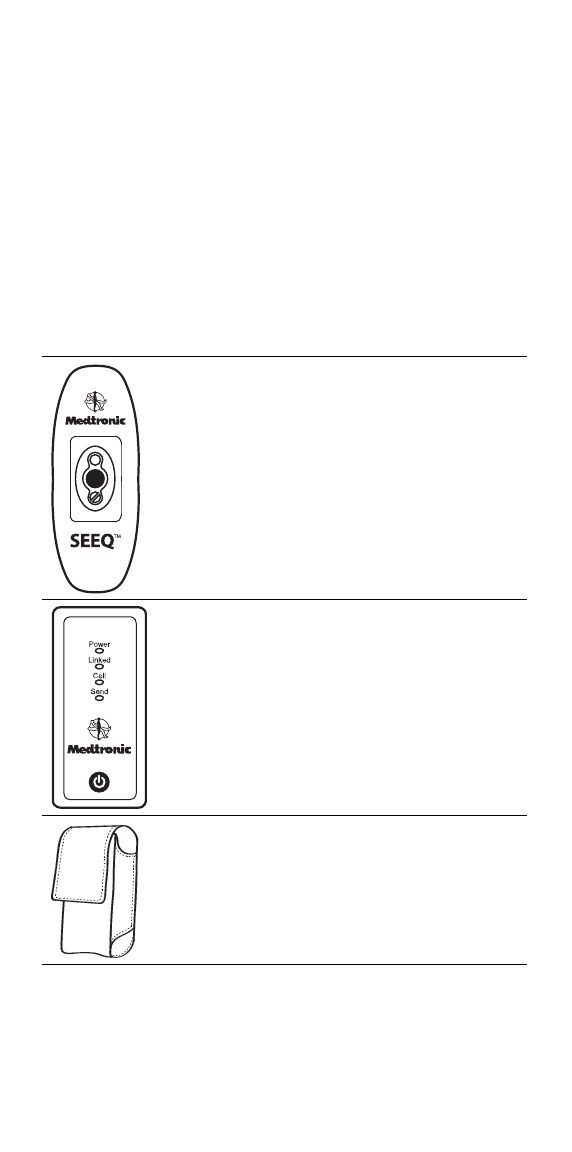
Step 1: Locate the components of the SEEQ MCT System
The Wearable Sensor is a wearable
device that collects and transmits
physiological data. One or more
Wearable Sensors may be included in
the package (inside a foil pouch),
depending on the length of the
prescription.
The Transmitter is a device that
receives data from the Wearable
Sensor and transmits it to Medtronic.
The Transmitter Case is used to
carry the Transmitter.
SEEQ MCT Instructions for use English 5
M958549A001 Rev B Refer to doc # A73903 for Printing
Instructions.
M958549A_ch.fm 3/12/15 2:34 pm
4" x 8" (101 mm x 203 mm)
Medtronic Confidential
mdvtldct_R1.5
Step 2: Charge the Transmitter
▪Connect the Transmitter Charger connector to the Transmitter
and plug it into a standard electrical outlet.
▪If the lights on the Transmitter do not turn on, briefly press the
Power Button for less than 1 second to power-up the
Transmitter.
▪If LED indicators turn ON, the Transmitter has powered up.
▪To fully charge the battery, it is important to keep the
Transmitter connected to wall power for at least 6 hours.
The Transmitter Charger is used to
charge the Transmitter.
The Prep Wipes are used for
cleaning skin before applying the
Wearable Sensor.
Product Literature Literature includes these instructions
and other information for patients.
Power Button
Connector
6 English SEEQ MCT Instructions for use
M958549A001 Rev B Refer to doc # A73903 for Printing
Instructions.
M958549A_ch.fm 3/12/15 2:34 pm
4" x 8" (101 mm x 203 mm)
Medtronic Confidential
mdvtldct_R1.5
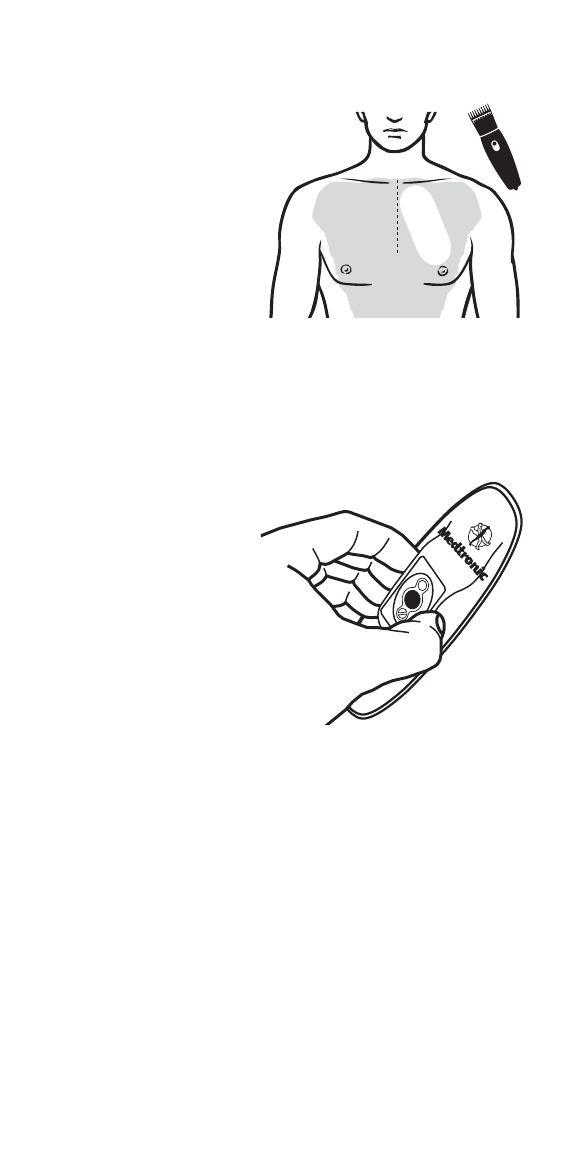
Step 3: Prepare for Wearable Sensor application
IMPORTANT: Monitoring will be affected if hair is not trimmed or
if skin is not cleaned with the Prep Wipe. If this happens, you
may be required to use another Wearable Sensor.
IMPORTANT: Take care not to touch the adhesive gel while you
handle the Wearable Sensor.
•Trim as much hair as
possible from the intended
location (for men) on the upper
left chest, as seen in the
diagram (for example, using an
electric razor or hair trimmer).
Trim an area slightly larger than
the Wearable Sensor.
•Using the Prep Wipe
provided, clean the skin where
the Wearable Sensor will be
applied and allow time to dry
(clean an area slightly larger
than the Wearable Sensor).
• Do not use any creams or
lotions on your skin before
application as this will impact
monitoring.
• Remove the Wearable Sensor
from the foil pouch by tearing
at the notch. If you see any
illuminated lights on the
Wearable Sensor before
application, contact Medtronic
Customer Support at 1-877-
247-7449.
• Grasp the top side of the
Wearable Sensor as seen in
the diagram and turn it over to
view the underside.
• Carefully remove each tab
from the underside of the
Wearable Sensor to expose
the adhesive gel.
SEEQ MCT Instructions for use English 7
M958549A001 Rev B Refer to doc # A73903 for Printing
Instructions.
M958549A_ch.fm 3/12/15 2:34 pm
4" x 8" (101 mm x 203 mm)
Medtronic Confidential
mdvtldct_R1.5
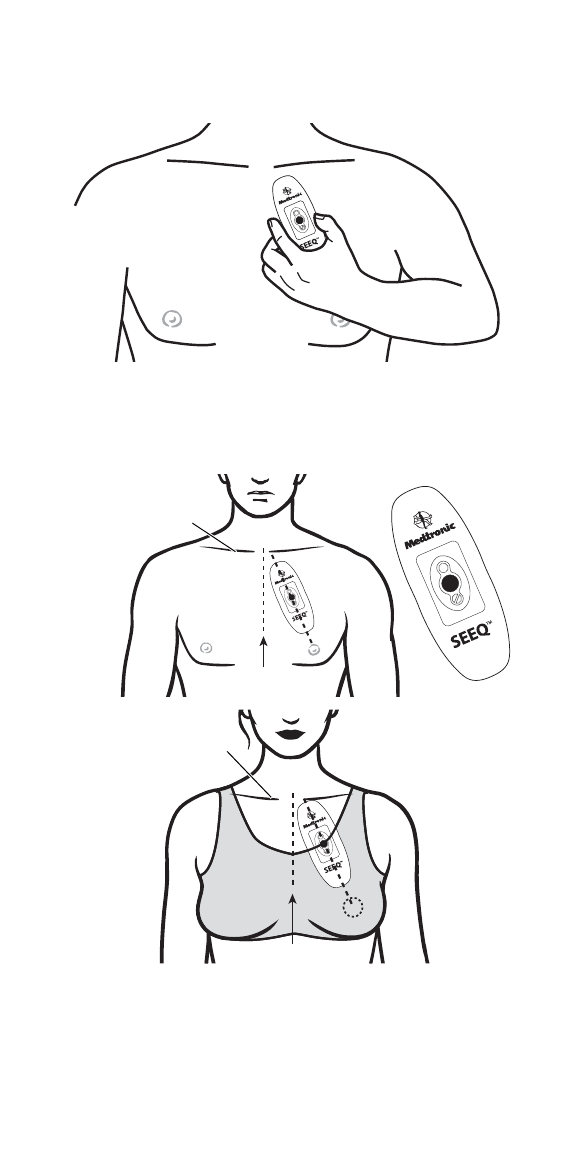
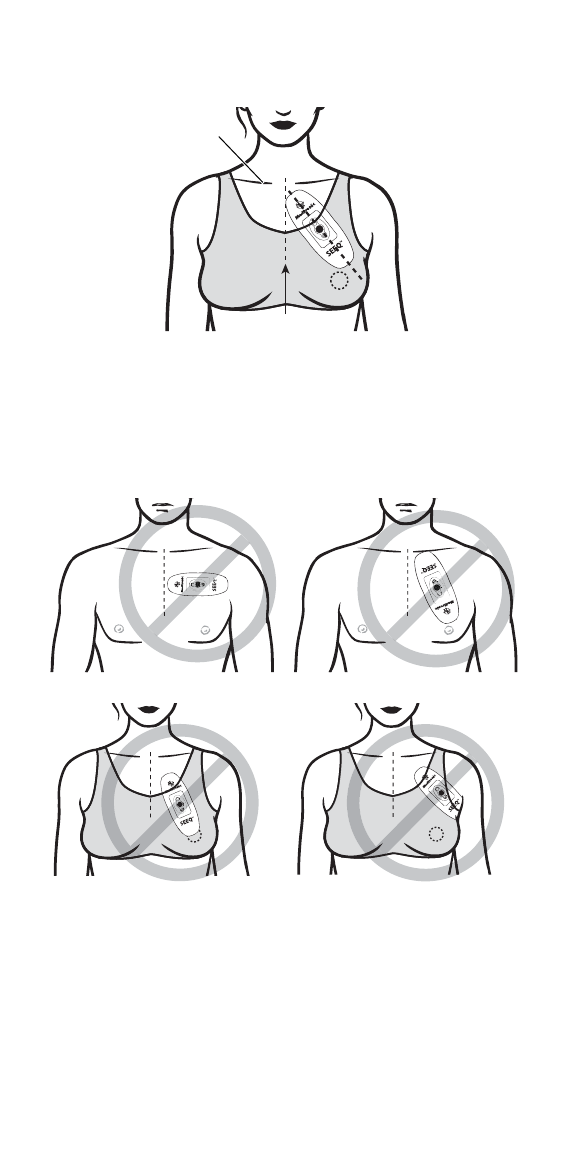
Step 4: Apply the Wearable Sensor to your chest
▪Bring the Wearable Sensor close to your upper left chest,
taking care to hold it only as described in Step 3.
▪As seen in the diagrams below, position the end of the
Wearable Sensor with the Medtronic logo pointing upwards just
below the collarbone and angle the device towards the nipple.
To minimize skin irritation, do not place the Wearable Sensor
over broken or damaged skin.
Male
Collarbone
Sternum
Female
Sternum
Collarbone
8 English SEEQ MCT Instructions for use
M958549A001 Rev B Refer to doc # A73903 for Printing
Instructions.
M958549A_ch.fm 3/12/15 2:34 pm
4" x 8" (101 mm x 203 mm)
Medtronic Confidential
mdvtldct_R1.5
▪Petite patients can angle the Wearable Sensor slightly away
from the nipple and towards the left arm for a comfortable fit, as
seen below.
▪Once applied, use the palm of your hand to firmly apply
pressure across the surface of the Wearable Sensor. Then, use
your fingers to press the edges of the Wearable Sensor onto
your skin.
▪Avoid strenuous motion, activity, or showering for 30 minutes
after Wearable Sensor application.
IMPORTANT: Monitoring will be affected if the Wearable Sensor
is not applied correctly. If this happens, you may be required to
use another Wearable Sensor.
EXAMPLES OF INCORRECT WEARABLE SENSOR APPLICATION
Sternum
Collarbone
Horizontal
Upside down
Applied over the nipple
Applied under the arm

SEEQ MCT Instructions for use English 9
M958549A001 Rev B Refer to doc # A73903 for Printing
Instructions.
M958549A_ch.fm 3/12/15 2:34 pm
4" x 8" (101 mm x 203 mm)
Medtronic Confidential
mdvtldct_R1.5
Step 5: Confirm the Wearable Sensor has activated
Key things to remember
▪Do not dispose of the SEEQ MCT System box or the postal
return label. They will be used to ship the components back to
Medtronic at the end of monitoring.
▪Wear the Wearable Sensor continuously until you see the
following two symbols ( and ) appear on the Wearable
Sensor display. (Refer to the section “When to remove or
replace the Wearable Sensor” on page 11.)
▪You can wear the Wearable Sensor while you shower (it is
water resistant), but do NOT submerge the Wearable Sensor in
water (for example, by swimming or sitting in a hot tub). Avoid
excessive rubbing of the Wearable Sensor while showering.
▪Once applied, do not remove and then reapply or reposition the
Wearable Sensor. If this happens, you may be required to use
another Wearable Sensor (that is, it is meant for one-time use).
▪Keep the Transmitter close to you at all times (within 30 feet
[9 meters]) and charge it daily (for example, each night while
you sleep).
▪Whenever you feel symptoms, push the blue Patient Trigger
Button in the middle of the Wearable Sensor device until you
hear a beep.
Using the SEEQ MCT System during the
monitoring period
What to do when you feel cardiac symptoms
Whenever you feel cardiac symptoms, push the blue Patient Trigger
Button in the middle of the Wearable Sensor device until you hear a
beep.
This will direct the Wearable Sensor to transmit a record of your heart
rhythm (that is, ECG) to your Transmitter, which will then
automatically transmit the information to the Medtronic Monitoring
Center for review by Medtronic technicians.
This information will then be provided to your physician.
• If you see a single blinking green
light within the circle symbol, it
means the Wearable Sensor is
working properly.
• If you DO NOT see this light within
15 minutes of application, please
call Medtronic Customer Support
at 1-877-247-7449.

10 English SEEQ MCT Instructions for use
M958549A001 Rev B Refer to doc # A73903 for Printing
Instructions.
M958549A_ch.fm 3/12/15 2:34 pm
4" x 8" (101 mm x 203 mm)
Medtronic Confidential
mdvtldct_R1.5
IMPORTANT: You may be contacted by a technician from the
Medtronic Monitoring Center to discuss symptoms when the
Patient Trigger Button is used. When you experience any
symptoms, please make note of the following to discuss with a
technician:
▪type of symptoms
▪time of symptoms
▪duration of symptoms
▪what you were doing
How to use the Transmitter
The Transmitter should be kept within 30 feet (9 meters) of you at all
times to allow transmission of data from the Wearable Sensor to the
Transmitter:
▪During the day, carry the Transmitter with you using the
Transmitter Case. The Transmitter must be placed in the case
with the LED lights and switch buttons visible through the clear
plastic cover of the case. If the case is to be carried in close
contact with the body, it must be placed so that the back side is
next to the body and the controls are visible through the clear
plastic cover.
▪At night, keep the Transmitter close to you while you sleep (for
example, on a nightstand).
The Transmitter should be kept ON at all times to allow transmission
of data from the Wearable Sensor to the Transmitter. The Transmitter
may be manually turned OFF by pressing and holding the Power
Button for more than 6 seconds. When a Transmitter is in an OFF
state (no lights are on), it can be turned ON by briefly pressing the
Power Button for less than 1 second. However, please keep the
Transmitter turned ON at all times to allow data transmission.
IMPORTANT: If you are out of range of the Transmitter or
required to turn it off (for example, when on an airplane or if
asked by Medtronic Customer Support), you can continue to
wear the Wearable Sensor and use the Patient Trigger Button to
document symptoms. All of your data will be stored on the
Wearable Sensor and sent to the Transmitter when you are again
within range or when the Transmitter is turned back on.

SEEQ MCT Instructions for use English 11
M958549A001 Rev B Refer to doc # A73903 for Printing
Instructions.
M958549A_ch.fm 3/12/15 2:34 pm
4" x 8" (101 mm x 203 mm)
Medtronic Confidential
mdvtldct_R1.5
How to charge the Transmitter
▪Charge the Transmitter daily (for example, every night while
you sleep).
▪The Transmitter may take up to 6 hours to fully charge.
▪With an adequate charge, the Transmitter can be used for up
to 12 hours before needing to be recharged.
▪The Linked, Cell, and Send lights on the Transmitter are utilized
by Medtronic Customer Support for troubleshooting. Refer to
these lights only if asked by a Medtronic Customer Support
representative.
IMPORTANT: If the battery on the Transmitter runs out, you can
continue to wear the Wearable Sensor and use the Patient
Trigger Button to document symptoms. All of your data will be
stored on the Wearable Sensor and transmitted once the
Transmitter is charged.
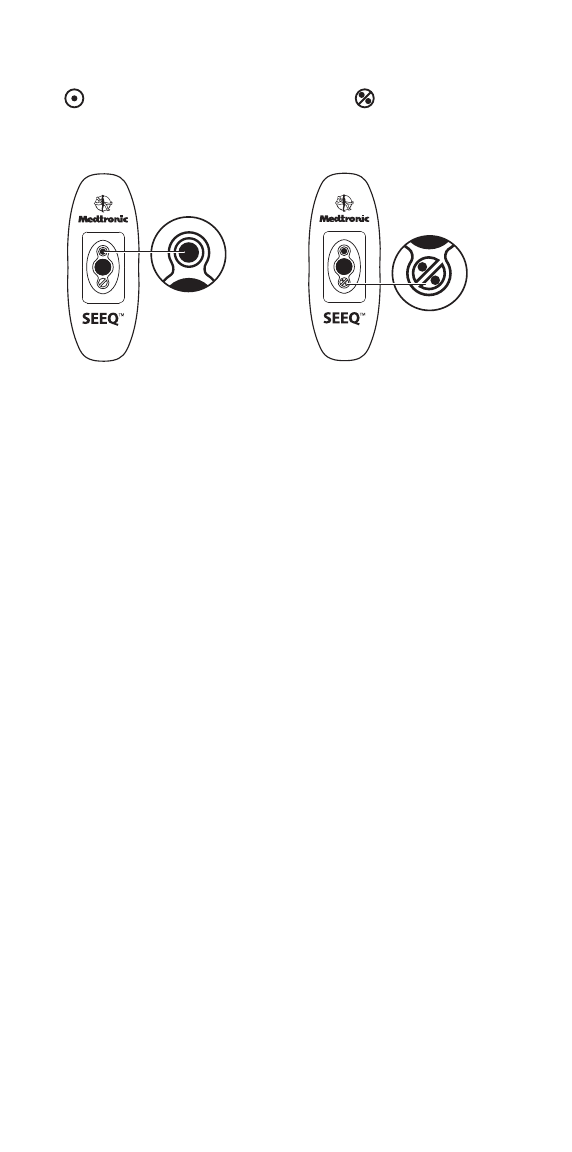
When to remove or replace the Wearable Sensor
Each Wearable Sensor is designed to operate with normal wear and
tear over the course of use for up to 7.5 days. The Wearable Sensor
is also water resistant. You can keep wearing the Wearable Sensor
while you shower, but do not submerge it in water (for example, in a
bath or hot tub).
Depending on the length of your prescription, you may need to wear
more than one Wearable Sensor. As seen in the diagram below, the
Wearable Sensor display will let you know when it should be
removed.
Power light:
Green on: Adequate charge
Yellow on: Low charge
Flashing: Charging required
12 English SEEQ MCT Instructions for use
M958549A001 Rev B Refer to doc # A73903 for Printing
Instructions.
M958549A_ch.fm 3/12/15 2:34 pm
4" x 8" (101 mm x 203 mm)
Medtronic Confidential
mdvtldct_R1.5
IMPORTANT: Each Wearable Sensor should be worn
continuously until the two red lights behind the circle with the
crossed line are seen. This may take up to 7.5 days, but could be
less. Once it has been removed, do not reapply the removed
Wearable Sensor as this will affect monitoring (that is, it is
meant for one-time use only). Please call Medtronic Customer
Support at 1-877-247-7449 if you have any questions about when
to remove the Wearable Sensor.
How to remove the Wearable Sensor
▪Grasp an edge of the Wearable Sensor with one hand and
begin to peel it away from your skin.
▪Using the other hand, slowly and gently push the skin away
from the Wearable Sensor as it is removed.
IMPORTANT: Rapid removal can cause skin irritation. If irritation
persists after Wearable Sensor removal, consult your healthcare
provider for topical treatment options.
What to do with a used Wearable Sensor
▪Do not dispose of used Wearable Sensors. See the following
section for instructions.
▪The Wearable Sensor has a Lithium battery and must not be
disposed of in a fire.
What to do at the end of monitoring
When your prescription is complete, you must return all of your
Wearable Sensors, the Transmitter, the Transmitter Charger, and
the Transmitter Case, to avoid being billed for the value of the
system. Follow the steps below to return the components to
Medtronic:
▪Remove the postal return label provided in the original box.
• If you see a single blinking green
light within the filled circle symbol
, this means the Wearable
Sensor is working properly. You
should continue to wear the
device.
• If you also see two red lights within
the circle with a crossed line
symbol , you should remove
and replace the device with a new
Wearable Sensor (unless your
monitoring period is over).

SEEQ MCT Instructions for use English 13
M958549A001 Rev B Refer to doc # A73903 for Printing
Instructions.
M958549A_ch.fm 3/12/15 2:34 pm
4" x 8" (101 mm x 203 mm)
Medtronic Confidential
mdvtldct_R1.5
▪Place the Wearable Sensors, the Transmitter, the Transmitter
Charger, and the Transmitter Case in the original box.
▪Attach the postal return label to the outside of the package.
▪Place the package in the mail. No additional postage is
required.
IMPORTANT: Please return the components only when your
prescription is complete. Call Medtronic Customer Support at
1-877-247-7449 if you need information about the status of your
prescription.

14 English SEEQ MCT Instructions for use
M958549A001 Rev B Refer to doc # A73903 for Printing
Instructions.
M958549A_ch.fm 3/12/15 2:34 pm
4" x 8" (101 mm x 203 mm)
Medtronic Confidential
mdvtldct_R1.5
Indications for use, contraindications, and
precautions
Indications for use
The SEEQ Mobile Cardiac Telemetry (MCT) System is intended to
continuously measure, record, and periodically transmit physiological
data. The System is indicated for those patients who require
monitoring for the detection of non-lethal cardiac arrhythmias such
as, but not limited to, supraventricular tachycardias (for example,
atrial fibrillation, atrial flutter, paroxysmal SVTs), ventricular ectopy,
bradyarrhythmias, and conduction disorders. The SEEQ MCT
System monitors, derives, and displays the following: ECG, and Heart
Rate.
Description of the system
The SEEQ MCT System consists primarily of the Wearable Sensor
monitoring device and the Transmitter data transmission device.
Once activated, the Wearable Sensor continuously monitors the
heart and automatically collects ECGs. When rhythm abnormalities
are detected, data is automatically transmitted from the Wearable
Sensor to the Transmitter, which then automatically transmits the
data to the Medtronic Monitoring Center. Patients can also trigger
transmission of ECGs when they experience cardiac symptoms by
using the Patient Trigger Button. Certified cardiographic technicians
at the Medtronic Monitoring Center review received data and
document symptoms reported to the Medtronic Monitoring Center by
patients. Reports prepared by the Medtronic Monitoring Center are
delivered to the prescribing physician and made available to them at
www.medtronic.com to provide them with data for their diagnosis and
identification of various clinical conditions, events and/or trends.
Based on the indications, the SEEQ MCT System may be used
for the following:
▪patients who require monitoring for suspected or known,
non-life threatening arrhythmias
▪patients with symptoms such as chest pain, syncope, light-
headedness or near syncope, vertigo, dizziness, fall,
palpitations, transient ischemic episodes, and dyspnea
(shortness of breath) that might be due to cardiac arrhythmias
▪patients with cardiac arrhythmias associated with co-morbid
conditions
▪obtaining correlation of rhythm with symptoms when symptoms
have unknown etiology
▪evaluating possible arrhythmias in a) patients recovering from
cardiovascular or thoracic surgery; b) survivors of myocardial
infarction; c) patients with diagnosed sleep disorder breathing
▪evaluating benefits after initiating or discontinuing
pharmacological therapy (for example, anti-arrhythmic,
beta-blocker, anti-coagulation therapies).
▪assessing the results of an ablation procedure for an
arrhythmia

SEEQ MCT Instructions for use English 15
M958549A001 Rev B Refer to doc # A73903 for Printing
Instructions.
M958549A_ch.fm 3/12/15 2:34 pm
4" x 8" (101 mm x 203 mm)
Medtronic Confidential
mdvtldct_R1.5
▪providing data to guide treatment decisions (for example,
pharmacological or procedural/device-based treatments) and
assessing treatment results in patients with non-life threatening
arrhythmias
Contraindications
▪patients with known allergies or hypersensitivities to adhesives
or hydrogel
▪patients with potentially life-threatening arrhythmias, or who
require inpatient / hospital monitoring
Precautions
▪Minute ventilation sensing on implantable devices should be
disabled for the duration of Wearable Sensor usage.
▪The Wearable Sensor should be removed prior to external
defibrillation or an MRI scan.
▪The Wearable Sensor may cause mild discomfort, skin
irritation, redness, itching, rash, or contact dermatitis in some
individuals. The device should be removed if any pain or
discomfort occurs. If skin irritation or redness persists after the
device has been removed, a topical anti-inflammatory cream
may be applied to the area (in consultation with your health
care provider).
▪The Wearable Sensor is intended for single patient use and
should not be reapplied it if peels off or is removed (that is, it is
meant for one-time use).
▪The Wearable Sensor should not be applied to broken,
damaged, or irritated skin.
▪The Wearable Sensor is water resistant but not waterproof. It
should not be submerged in water (showering is acceptable,
but swimming and submersion bathing are prohibited).
▪The Wearable Sensor should not be disassembled.
▪Do not apply the Wearable Sensor if it appears damaged upon
receipt.
▪No creams or lotions should be applied to the skin immediately
prior to the application of the Wearable Sensor.
▪Store the Wearable Sensor in a cool, dry location. The device
is designed to withstand environmental temperature
fluctuations between -4 °F to 149 °F (-20 °C to 65 °C).
▪The system has not been fully evaluated for use with infants
weighing less than 22 pounds (9.9 kilograms).
▪The system is not designed to detect pacemaker spikes.
Warning
▪No modification of this equipment is allowed.

16 English SEEQ MCT Instructions for use
M958549A001 Rev B Refer to doc # A73903 for Printing
Instructions.
M958549A_ch.fm 3/12/15 2:34 pm
4" x 8" (101 mm x 203 mm)
Medtronic Confidential
mdvtldct_R1.5
Services for physicians
Prescription duration:
After registering with Medtronic, physicians can prescribe the SEEQ
MCT System for up to 30 days at a time. As each Wearable Sensor
is designed to last for up to 7.5 days, prescription lengths greater than
one week will be enabled through the use of more than one Wearable
Sensor.
Clinical reports:
Clinicians can receive clinical reports, including Episode, Daily, and
End of Use Reports, directly from the Medtronic Monitoring Center by
fax and/or email. Clinical data can also be securely reviewed online
at www.medtronic.com. Clinicians may download and/or print clinical
reports, review collected ECGs, and also establish service
preferences. For any questions about online use, please contact
Medtronic Customer Support at 1-877-247-7449.
Notifications:
The Medtronic Monitoring Center may send Episode Reports and
contact prescribing physicians directly when arrhythmias that meet
pre-defined criteria are identified. Contact information and notification
preferences will be established upon registration and can be
updated.
IMPORTANT: Patient data available for transmission during the
monitoring period are updated for physician display upon
detection of a clinical event OR every two hours when no events
are detected, assuming a) the Wearable Sensor is within 30 feet
(9 meters) of Transmitter, b) the Transmitter has been
appropriately installed as specified in “How to use the
Transmitter” and has sufficient power and c) sufficient cellular
coverage for data transmission exists. Analysis of ECGs by the
Medtronic Monitoring Center may also affect the timing of ECG
display. Additional patient data may be available after the end of
monitoring.
SEEQ MCT Instructions for use English 17
M958549A001 Rev B Refer to doc # A73903 for Printing
Instructions.
M958549A_ch.fm 3/12/15 2:34 pm
4" x 8" (101 mm x 203 mm)
Medtronic Confidential
mdvtldct_R1.5
Specifications, compliance, and symbols
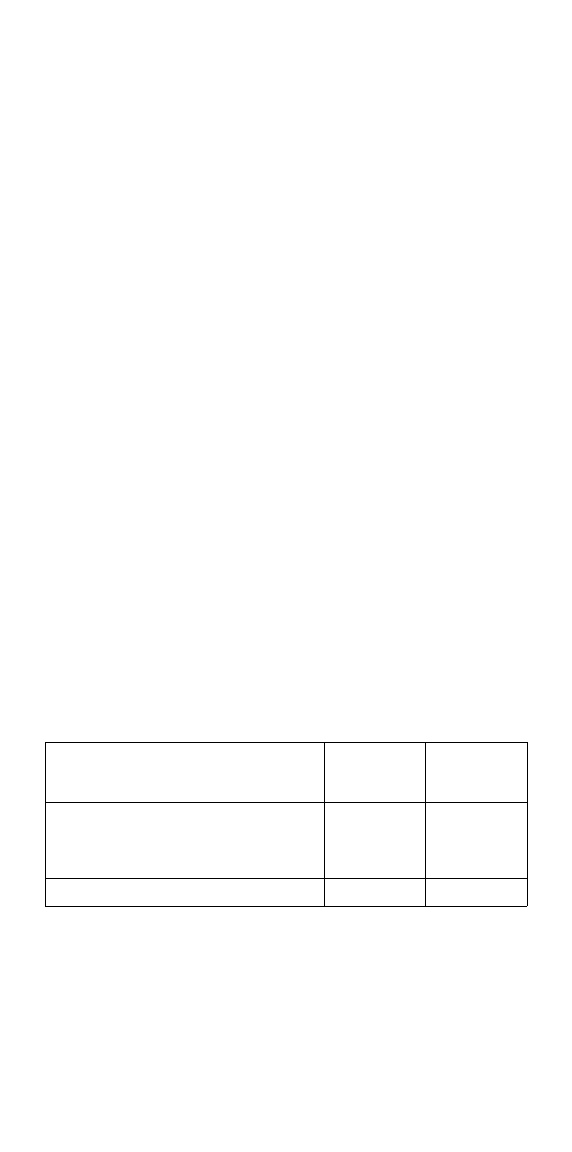
Specifications
The following performance specifications are at 68 °F (20 °C) unless
otherwise stated.
Wearable Sensor Transmitter
Shelf life Refer to Wearable
Sensor pouch label
N/A
Battery Charger power
requirement
N/A 100-240 VAC,
50/60 Hz
Battery life 7.5 days
(180 hours),
non-rechargeable
Provides 12 hours of
function before
recharging
Operating temperature 32 °F to 113 °F
(0 °C to 45 °C)
32 °F to 113 °F
(0 °C to 45 °C)
Maximum temperature of
the applied part
111.2 °F (44 °C) N/A
Storage temperature
(power off)
-4 °F to 149 °F
(-20 °C to 65 °C)
-4 °F to 149 °F
(-20 °C to 65 °C)
Operating humidity 10% to 95% 10% to 95%
Storage humidity 5% to 95% 5% to 95%
Operating atmospheric
pressure
700 hPa to
1060 hPa
700 hPa to
1060 hPa
ECG
Sampling rate
Digital resolution
Input dynamic range
Input offset dynamic range
200 Hz (+/-5%)
16bits
+/-5 mV
+/-300 mV
N/A
Impedance measurements
Peak current injection
RMS current injection
40 uA
29 uA
N/A
Measurement ranges
Heart Rate 25 to 250 bpm N/A
Data storage
Capacity 7.5 days N/A
Weight 1.8 oz / 50 g max 5.3 oz / 150 g max
Communication means Bluetooth between
Wearable Sensor
and Transmitter
Cellular Phone between
Transmitter and Server
18 English SEEQ MCT Instructions for use
M958549A001 Rev B Refer to doc # A73903 for Printing
Instructions.
M958549A_ch.fm 3/12/15 2:34 pm
4" x 8" (101 mm x 203 mm)
Medtronic Confidential
mdvtldct_R1.5
Arrhythmia Detection Algorithms and Automatic ECG Collection
In addition to patient-triggered collection of ECGs using the Patient
Trigger Button, the SEEQ MCT System also uses proprietary
algorithms based on rate, rhythm, and morphology to continuously
analyze rhythm abnormalities and to initiate automatic ECG
transmission. ECGs are automatically transmitted upon detection of
the following conditions:
▪Heart Rate >= 130 bpm
▪Heart Rate <= 40 bpm
▪Pause >= 3 seconds
▪Atrial Fibrillation
▪Ventricular Tachycardia/Ventricular Fibrillation
For example, the detection algorithm of the SEEQ MCT System
detects the peak of each R-wave and calculates the interval between
successive R-waves. The RR intervals are then used to calculate
beat-to-beat heart rate values. RR intervals are also aggregated into
5-minute and 24-hour averages to summarize patient heart rate over
the monitoring period. For Pause detection, the algorithm monitors
the time between successive R-wave peaks. A pause trigger is
activated if an internal timer advances to 3 seconds without R-wave
detection.
In order to provide relevant, exception-based arrhythmia reporting,
the SEEQ MCT System proprietary ECG analysis algorithms
proactively manage redundant reporting of ECGs for a select set of
arrhythmias when persistently detected:
▪Tachycardias with heart rate >= 130 bpm and < 165 bpm
▪Bradycardias with heart rate >= 30 bpm and < 40 bpm
▪Atrial Fibrillation
▪Ventricular Tachycardia < 165 bpm
For these arrhythmias, the Wearable Sensor algorithm detection
sensitivity and positive predictive value results, which are obtained
from the respective databases in strict accordance with EC-57a and
with 0% downtime, are as follows:
Test Rhythm Name Sensitivity
(%)a
aas measured by EC-57 standards testing on 12NOV2013
Positive
Predictive
Value (%)a
QRS Detection (average)
Including all Tachycardias with heart rate >=
130 bpm and < 165 bpm, Bradycardias with
heart rate >=30 bpm and <40 bpm
AHA: 98.95
MIT-BIH:
99.83
AHA: 99.34
MIT-BIH:
99.84
AF Duration (gross) MIT-BIH: 90 MIT-BIH: 85

SEEQ MCT Instructions for use English 19
M958549A001 Rev B Refer to doc # A73903 for Printing
Instructions.
M958549A_ch.fm 3/12/15 2:34 pm
4" x 8" (101 mm x 203 mm)
Medtronic Confidential
mdvtldct_R1.5
Redundant reporting of ECGs for this select set of arrhythmias is
managed as follows:
▪The Wearable Sensor will report no more than two (2) ECGs for
each of these arrhythmias each hour.
▪The Wearable Sensor will wait ten (10) minutes before allowing
a subsequent ECG to be reported for each of these
arrhythmias.
Note: a) ECGs are reported for all Tachycardias with heart rate
>= 165 bpm, all Bradycardias with heart rate < 30 bpm, and all
Pauses >= 3 seconds; b) the Wearable Sensor keeps a complete
count of all arrhythmias that are detected; c) supplemental ECGs are
also reported i) every six (6) hours for prolonged Atrial Fibrillation
episodes and ii) every twenty-four (24) hours, irrespective of the
presence of an arrhythmia.
Transmitter maintenance
Please attempt to keep the Transmitter dust free. If necessary, gently
wipe the Transmitter with a soft dry cloth to clean the surface. The
Transmitter is not waterproof and should be kept dry. This device
does not have user serviceable components inside. Do not
disassemble, crush, puncture, short external contacts or circuits,
dispose of in fire or water, or expose the battery pack to temperatures
higher than 149 ˚F (65 ˚C).
Electromagnetic interference
This equipment complies with International Standard IEC 60601-1-2
for electromagnetic compatibility for medical electrical equipment.
Medical electrical equipment needs special precautions regarding
EMC, and all equipment must be installed and put into service
according to the EMC information provided upon request by calling
Medtronic Customer Support at 1-877-247-7449. Portable and
mobile RF communication equipment can affect nearby medical
electrical equipment.
FCC Compliance Information
Wearable Sensor and Transmitter devices comply with Part 15 of the
Federal Communications Commission (FCC) Rules – Radio
Frequency Devices: Operation is subject to the condition that (1) this
device does not cause harmful interference and (2) this device must
accept any interference received, including interference that may
cause undesired operation. Changes or modifications not expressly
approved by Medtronic could void the user’s authority to operate the
equipment. Note: This equipment has been tested and found to
comply with the limits for a Class B digital device, pursuant to Part 15
of the FCC Rules. These limits are designed to provide reasonable
protection against harmful interference when the equipment is
operated in a residential environment. This equipment generates,
uses, and can radiate radio frequency energy and, if not installed and
used in accordance with the instruction manual, may cause harmful
interference to radio communications.
20 English SEEQ MCT Instructions for use
M958549A001 Rev B Refer to doc # A73903 for Printing
Instructions.
M958549A_ch.fm 3/12/15 2:34 pm
4" x 8" (101 mm x 203 mm)
Medtronic Confidential
mdvtldct_R1.5
Exposure to radio frequency signals
To maintain compliance with FCC RF exposure guidelines when you
carry the Transmitter on your body, use only the Transmitter Case
supplied by Medtronic. Be sure to insert the Transmitter in the case
as instructed in “How to use the Transmitter”. Use of accessories that
are not expressly approved by Medtronic are not approved and might
cause violation of the FCC RF emissions and RF exposure
guidelines.
Specific absorption rate data
The Transmitter meets the U.S. Government requirements for
exposure to radio waves when used as directed in this document.
The Transmitter is a radio transmitter and receiver. It is designed and
manufactured not to exceed the emission limits for exposure to radio
frequency (RF) energy set by the Federal Communications
Commission (FCC) of the U.S. Government when used as directed in
previous sections of this document. These limits are part of
comprehensive guidelines and establish permitted levels of RF
energy for the general population. The exposure standard for
wireless devices employs a unit of measurement known as the
Specific Absorption Rate, or SAR. The SAR limit set by the FCC is
1.6W/kg. Tests for SAR are conducted using standard operating
positions as specified in this document. Before a wireless device
model is available for sale to the public, it must be tested and certified
by the FCC that it does not exceed the limit established by the
government-adopted requirement for safe radio frequency exposure
under the recommendations of the International Commission on Non-
Ionizing Radiation Protection (ICNIRP).
The FCC has granted an Equipment Authorization for this wireless
device model with all reported SAR levels evaluated and is in
compliance with the FCC RF emission guidelines when the
Transmitter is used as directed in this document.
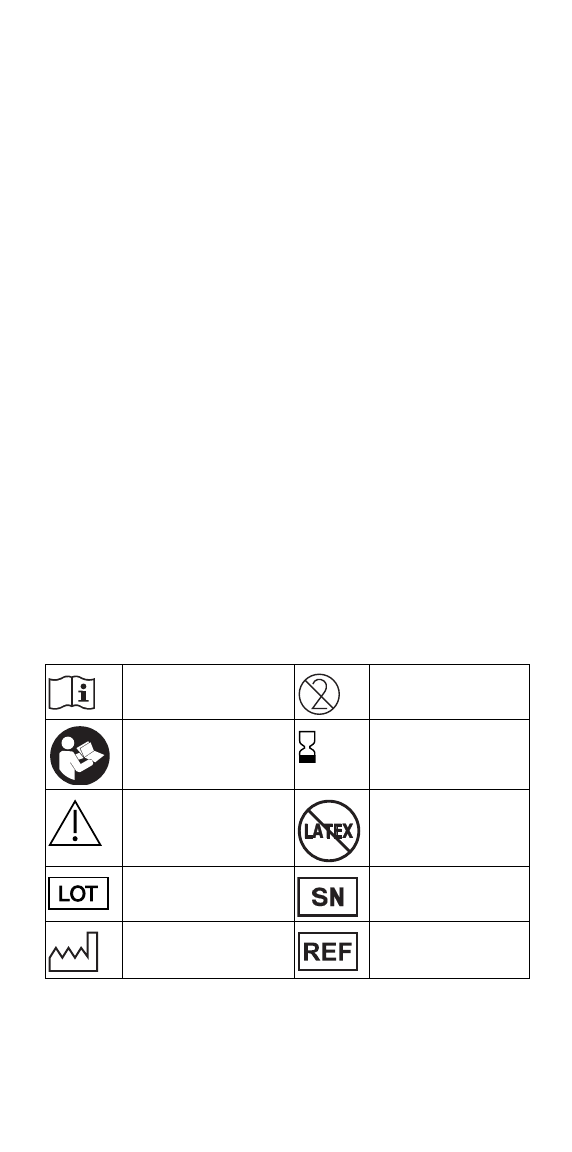
Symbol definitions
Consult instructions for
use
Do not reuse
Follow instructions for use Use-by (year-month) or
(year-month-date)
Caution: consult
accompanying documents
Latex free
Batch number Serial number
Date of manufacture Catalogue number
SEEQ MCT Instructions for use English 21
M958549A001 Rev B Refer to doc # A73903 for Printing
Instructions.
M958549A_ch.fm 3/12/15 2:34 pm
4" x 8" (101 mm x 203 mm)
Medtronic Confidential
mdvtldct_R1.5
Frequently asked questions
What is a Wearable Sensor?
A Wearable Sensor is a patient-worn medical device designed to
comfortably adhere to the skin. The device contains sensors that
collect your ECG waveform (a tool for analyzing the activity of your
heart).
How long will my Wearable Sensor last?
Each Wearable Sensor is designed to last up to 7.5 days. However,
inappropriate Wearable Sensor application, or removal and
reapplication of the Wearable Sensor during use, will affect
monitoring. If this happens, you may be required to use another
Wearable Sensor.
What information is transmitted to my physician?
Your heart activity is monitored 24 hours a day, 7 days a week by the
Wearable Sensor. When unusual heart activity is detected or when
you use the Patient Trigger Button, information is transmitted to the
Medtronic Monitoring Center for review and delivery to your
physician.
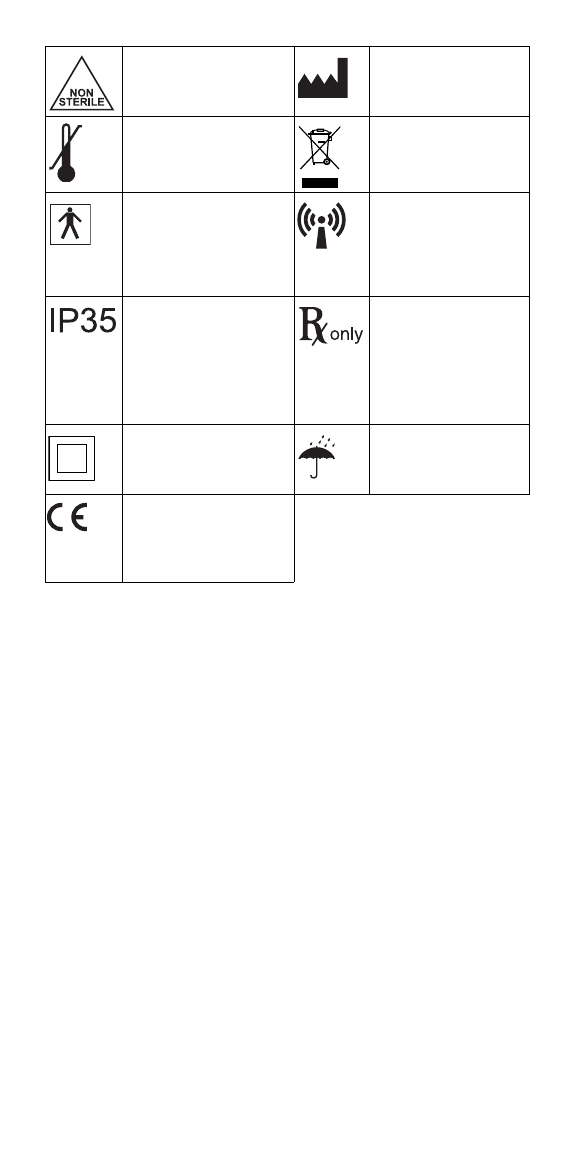
Non-sterile Manufacturer’s name
and address
Temperature limitations Collection of electrical
and electronic
equipment
The entire Wearable
Sensor is a type BF
applied part; Denotes
device is not in direct
contact with cardiac
muscle
Wireless transmission
symbol
Ingress Protection
3 means protection
against objects >=2.5 mm
in diameter (tools)
5 means protection
against water jets
(shower)
Federal (USA) law
restricts this product to
sale by or on the order
of a physician.
Class II equipment Keep dry
Conformité Européenne
(European Conformity).
This symbol means that
the device fully complies
with European Union Acts.

22 English SEEQ MCT Instructions for use
M958549A001 Rev B Refer to doc # A73903 for Printing
Instructions.
M958549A_ch.fm 3/12/15 2:34 pm
4" x 8" (101 mm x 203 mm)
Medtronic Confidential
mdvtldct_R1.5
What do I do when I feel symptoms?
Whenever symptoms occur, trigger the Wearable Sensor to transmit
a record of your heart activity by pushing the blue Patient Trigger
Button located in the middle of the Wearable Sensor until you hear a
beep. An ECG will be transmitted to the Transmitter and then
transmitted to the Medtronic Monitoring Center for review and
delivery to your physician.
How can I report my symptoms?
Technicians from Medtronic may call you to discuss your symptoms.
Please make note of the type of symptoms, the duration of
symptoms, when they happened, and what you were doing so it can
be discussed with a technician. You should contact your physician if
you need medical care.
Can I take a shower while wearing my Wearable Sensor?
Yes, the Wearable Sensor is water resistant so you can shower while
wearing it. However, do NOT submerge the Wearable Sensor in
water by swimming or sitting in a hot tub. In addition, avoid excessive
rubbing of the Wearable Sensor during showering.
Will I need to change the battery in the Transmitter or the
Wearable Sensor?
Battery replacement is not required for the Wearable Sensor and the
Transmitter. However, we recommend that you charge the
Transmitter every night.
How close must I be to the Transmitter to ensure that the data
collected by my Wearable Sensor is transmitted?
Remain within 30 feet (9 meters) of the Transmitter for successful
data transmission.
How often do I need to change the Wearable Sensor device?
When both of the following two symbols ( and ) appear on the
Wearable Sensor display, you should remove the Wearable Sensor.
Please apply a new Wearable Sensor if your monitoring period has
not ended.
What if the Wearable Sensor causes my skin to itch?
If you experience skin irritation while wearing the Wearable Sensor,
speak with your physician.
Should I carry the Transmitter with me when I travel?
Yes, take the Transmitter with you at all times. The Transmitter will
remain charged for up to 12 hours. If you plan to be away for longer
than 12 hours, take the Transmitter Charger with you.
Will I need to notify security screeners about my Wearable
Sensor?
Carry the SEEQ MCT System patient travel card provided as part of
your SEEQ MCT System when traveling or entering high security
areas. Wearable Sensor and Transmitter devices may trigger security
systems, but the devices will not be damaged. If your Wearable
Sensor or Transmitter triggers a security system, simply show the
security personnel this card and tell them you are wearing a medical
device.

SEEQ MCT Instructions for use English 23
M958549A001 Rev B Refer to doc # A73903 for Printing
Instructions.
M958549A_ch.fm 3/12/15 2:34 pm
4" x 8" (101 mm x 203 mm)
Medtronic Confidential
mdvtldct_R1.5
Can I wear a Wearable Sensor through an electronic antitheft
system, such as in a store?
Yes, the Wearable Sensor will not set off antitheft systems and will not
be damaged by them.
Can I use microwave ovens or TV remotes while wearing a
Wearable Sensor?
Yes, it is safe to operate these devices as they will not affect
performance of the Wearable Sensor.
Can I be close to wireless phones, WiFi, or other
electromagnetic devices?
Yes, although some sources of Electromagnetic Interference (EMI)
may temporarily disrupt data transmission.
Can I carry my cellphone while wearing the Wearable Sensor?
Yes, cellphones will not interfere with the Wearable Sensor device.
Will hot or cold environments affect the Wearable Sensor
performance?
The Wearable Sensor provides accurate and reliable performance in
a temperature range of 32 ˚F to 113 ˚F (0 ˚C to 45 ˚C).
Is my medical data protected during transmission?
Your data is transmitted securely to the Medtronic Monitoring Center
and securely stored.

24 English SEEQ MCT Instructions for use
M958549A001 Rev B Refer to doc # A73903 for Printing
Instructions.
M958549A_ch.fm 3/12/15 2:34 pm
4" x 8" (101 mm x 203 mm)
Medtronic Confidential
mdvtldct_R1.5

M958549A001 Rev C Refer to doc # A73903 for Printing
Instructions.
M958549AUS_bc.fm 3/12/15 2:34 pm
4" x 8" (101 mm x 203 mm)
Medtronic Confidential
mdvtldct_R1.5

*M958549A001*
© Medtronic, Inc. 2015
M958549A001C
2015-03-12
M958549A001 Rev C Refer to doc # A73903 for Printing
Instructions.
M958549AUS_bc.fm 3/12/15 2:34 pm
4" x 8" (101 mm x 203 mm)
Medtronic Confidential
mdvtldct_R1.5
Medtronic, Inc.
710 Medtronic Parkway
Minneapolis, MN 55432
USA
www.medtronic.com
+1 763 514 4000
Europe/Middle East/Africa
Medtronic International Trading
Sàrl
Route du Molliau 31
Case Postale 84
CH-1131 Tolochenaz
Switzerland
+41 21 802 7000