Medtronic 3537 3537 User Manual 2
Medtronic, Inc. 3537 2
Contents
- 1. User Manual 1
- 2. User Manual 2
- 3. User Manual 3
User Manual 2

CONTROLLER
3537
Patient Programming Manual for Test
Stimulation
! USA
Rx only
0123
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
Medtronic
®
and InterStim
®
are trademarks of Medtronic,
Inc., registered in the U.S. and other countries.
! USA
FCC Information
The following is communications regulation information
on the Model 3537 Controller.
FCC ID: LF597745
This device complies with Part 15 of the FCC Rules.
Operation is subject to the following two conditions: (1)
this device may not cause harmful interference and (2)
this device must accept any interference received,
including interference that may cause undesired
operation.
IMPORTANT: Changes or modifications to this
product not authorized by Medtronic, Inc., could
void the FCC Certification and negate your authority
to operate this product.
This device complies with Industry Canada license-
exempt RSS standard(s). Operation is subject to the
following two conditions: (1) this device may not cause
interference, and (2) this device must accept any
interference, including interference that may cause
undesired operation of the device.
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
Label symbols
Explanation of symbols on products and
packaging. Refer to the appropriate product to
see symbols that apply.
Consult instructions for use
Manufacturer
XXX °F
XX °C
-XX °F
-XX °C Temperature limitation
Serial number
Conformité Européenne (European
Conformity). This symbol means that the
device fully complies with MDD 93/42/EEC
(NB 0123) and R&TTE Directive 1999/5/
EC.
EC REP
Authorized representative in the European
community
! USA
For USA audiences only
IEC 60601-1/EN60601-1, Type BF
Equipment
Non-ionizing electromagnetic radiation
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
3
Label symbols
System meets the applicable (CAN/CSA-
C22.2 No. 60601-1) electrical safety
standard requirements.
Do not dispose of this product in the unsorted
municipal waste stream. Dispose of this
product according to local regulations. See
http://recycling.medtronic.com for
instructions on proper disposal of this
product.
Chinese Standard (SJ/T11364-2006) Logo:
Electronic Information Products Pollution
Control Symbol. (The date in this logo means
the environmental protection use period of
the product.)
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
4
Label symbols
Table of contents
Label Symbols 3
Glossary 7
1
Introduction 10
How to use this manual 10
Patient guides 11
2
Using your controller 14
How your controller works 14
Controller screen and keys 15
Unlocking and locking your
controller 17
Unlocking your controller 17
Locking your controller 21
Turning your stimulation on or off 22
Turning your stimulation on 23
Turning your stimulation off 25
Adjusting stimulation 27
Switching stimulation sides (if your clinician
placed two leads) 28
Changing programs (if applicable) 31
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
5
Table of contents
Home screen 34
Checking and replacing batteries 38
Checking the batteries (external
neurostimulator and controller) 38
Replacing the batteries (controller
only) 45
Using the carrying case 46
Always carry your controller 47
3
Troubleshooting 50
Controller screens 50
Warning screens 51
Alert screens 56
Notification screens 61
Possible problems and solutions 64
4
Maintenance and assistance 74
Cleaning and care 74
Safety and technical checks 75
Battery and controller disposal 75
Declaration of conformity 75
Specifications 76
Assistance for the controller 76
Index 78
English 3537
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
6
Table of contents
Glossary
Amplitude - The strength or intensity of an
electrical pulse.
Caution - A statement describing actions that
could result in damage to or improper
functioning of a device.
Clinician - A healthcare professional such as
a doctor or nurse.
Controller - A hand-held device that allows
you to turn your neurostimulator on and off
and check your neurostimulator battery. It is
also used to adjust some of the stimulation
settings.
External neurostimulator (ENS) - See
Neurostimulator.
Lead - A thin wire with protective coating that
has metal electrodes on one end and a
connector on the other.
Neurostimulator - The power source of a
neurostimulation system. It contains the
battery and electronics that control the
stimulation.
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
7
Glossary
Precaution - See Caution.
Program - A specific combination of
stimulation settings assigned to deliver
therapy to a specific site.
Settings - See Stimulation settings.
Stimulation - The delivery of electrical pulses
to a specific site.
Stimulation settings - Refers to all the
features assembled to define the stimulation
you feel. The clinician programs all
stimulation. You can adjust some
stimulation settings within clinician-defined
limits.
Test stimulation - A postoperative multiday
trial period of a patient’s reaction to
stimulation using an external
neurostimulator and implanted leads.
Therapy - Treatment of a disease or condition.
When neurostimulation therapy is
prescribed, a neurostimulation system is
used to deliver stimulation to a specific
site.
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
8
Glossary

1 Introduction
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
How to use this manual
Use this manual during test stimulation. Ask
your clinician to explain anything that is
unclear.
•
A glossary is provided at the beginning of
this manual to describe terms that may be
unfamiliar to you.
•
Chapter 1 "Introduction" describes how to
use this manual, a list of patient guides, and
information on your patient identification
card.
•
Chapter 2 "Using your controller" describes
the controller and how to perform specific
tasks, including turning your stimulation on
and off, adjusting your stimulation,
changing stimulation sides (if applicable),
changing programs (if applicable), and
checking and replacing batteries. This
chapter also describes how to use the
controller carrying case, how to label your
controller, and an overall description of the
Home screen.
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
10
Introduction 1
•
Chapter 3 "Troubleshooting" describes
controller warning and information screens,
how to solve possible problems, and who
to contact if your device is lost or broken.
•
Chapter 4 "Maintenance and assistance"
describes how to care for your controller,
including how to change the batteries, as
well as device specifications and user
assistance.
Please read this entire manual before using
your controller. This manual will help you
understand and use your InterStim system so
you can adjust your stimulation as your needs
change.
Patient guides
In addition to this manual, you should receive
the following documents during test
stimulation:
•
Model 3537 Patient Test Stimulation Quick
Reference Card
•
InterStim Patient Therapy Guide
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
11
Introduction 1
Note: For additional warnings,
precautions, and adverse events related to
InterStim Therapy, refer to the InterStim
Patient Therapy Guide provided by your
clinician.
If you did not receive these documents, contact
your clinician or Medtronic Patient Services.
Refer to the Medtronic contacts at the end of
this manual.
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
12
Introduction 1

2 Using your
controller
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
How your controller works
The controller is used to control and monitor
your external neurostimulator. You will use
your controller to perform the following tasks:
•
Turn your stimulation on or off.
•
Check the external neurostimulator and
controller battery status.
•
Change stimulation settings.
The controller communicates wirelessly with
your external neurostimulator by sending
signals to and receiving signals from the
external neurostimulator. Your clinician has
already set up the controller according to your
specific test stimulation needs.
Note: Make sure to keep your controller with
you at all times in the event that you need to
adjust or turn your stimulation off.
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
14
Using your controller 2
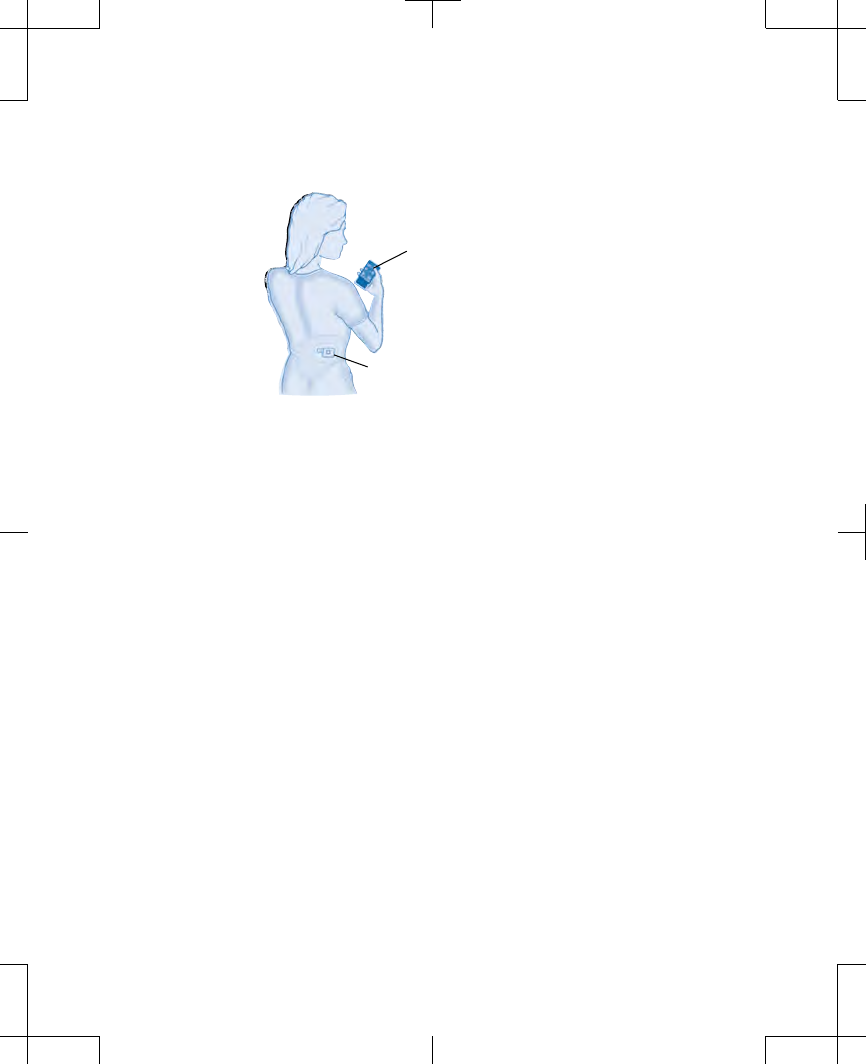
Controller
External neurostimulator
Figure 2.1 A patient using her controller.
Controller screen and keys
Your controller has a touchscreen, a display
screen that reacts to your touch, that allows
you to press buttons that are displayed on the
screen. In addition to these on-screen buttons,
your controller also has a number of keys
(Figure 2.2).
Note: There is a cable port on the bottom of
the controller. This port is not for patient use.
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
15
Using your controller 2
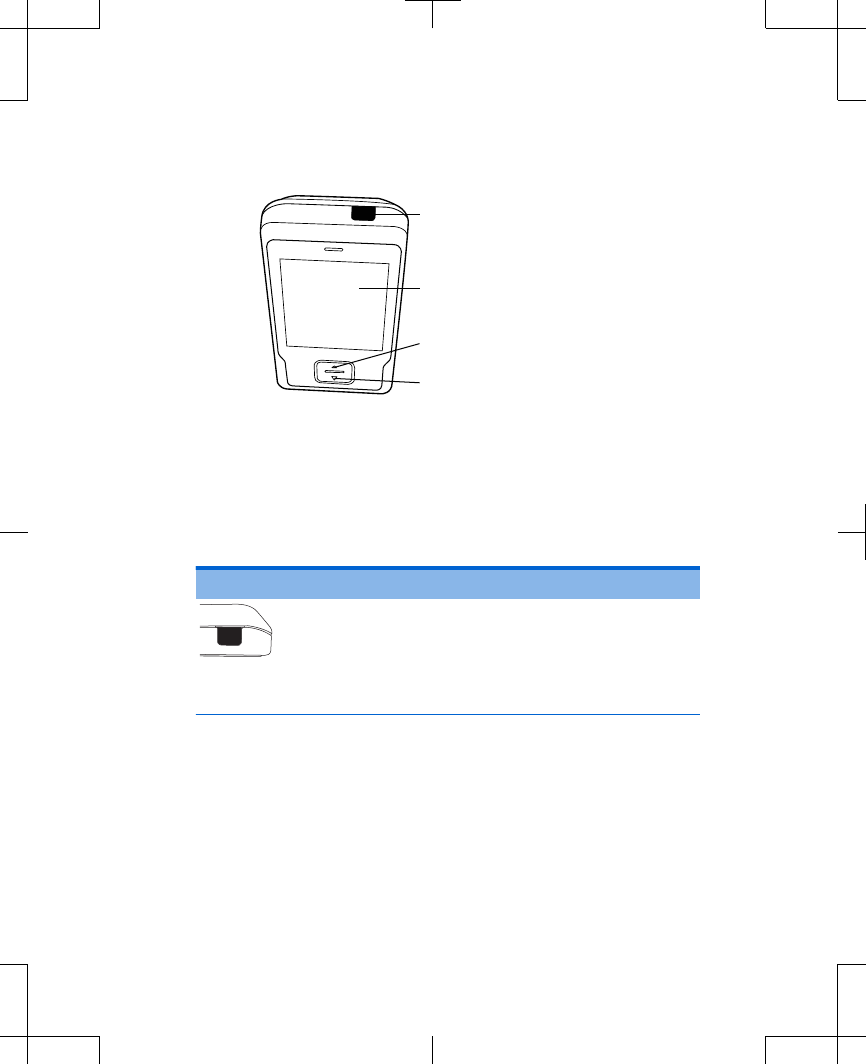
Stimulation On/Off key
Touchscreen
Increase key
Decrease key
Figure 2.2 Controller keys.
See Table 2.1 for a list of these keys and their
functions.
Table 2.1 Controller keys
Key Function
Stimulation
On/Off
•Turns stimulation on or off.
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
16
Using your controller 2
Table 2.1 Controller keys (continued)
Key Function
Increase/
Decrease
•Increases or decreases stimulation
when you are on the Home screen.
•Press and release the Increase/
Decrease key to slowly increase or
decrease the stimulation value (by
0.1). Press and hold to quickly
increase or decrease the stimulation
value (by 0.5).
Unlocking and locking your
controller
Unlocking your controller
When the Increase/Decrease key is pressed,
the Unlock screen appears (Figure 2.3).
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
17
Using your controller 2
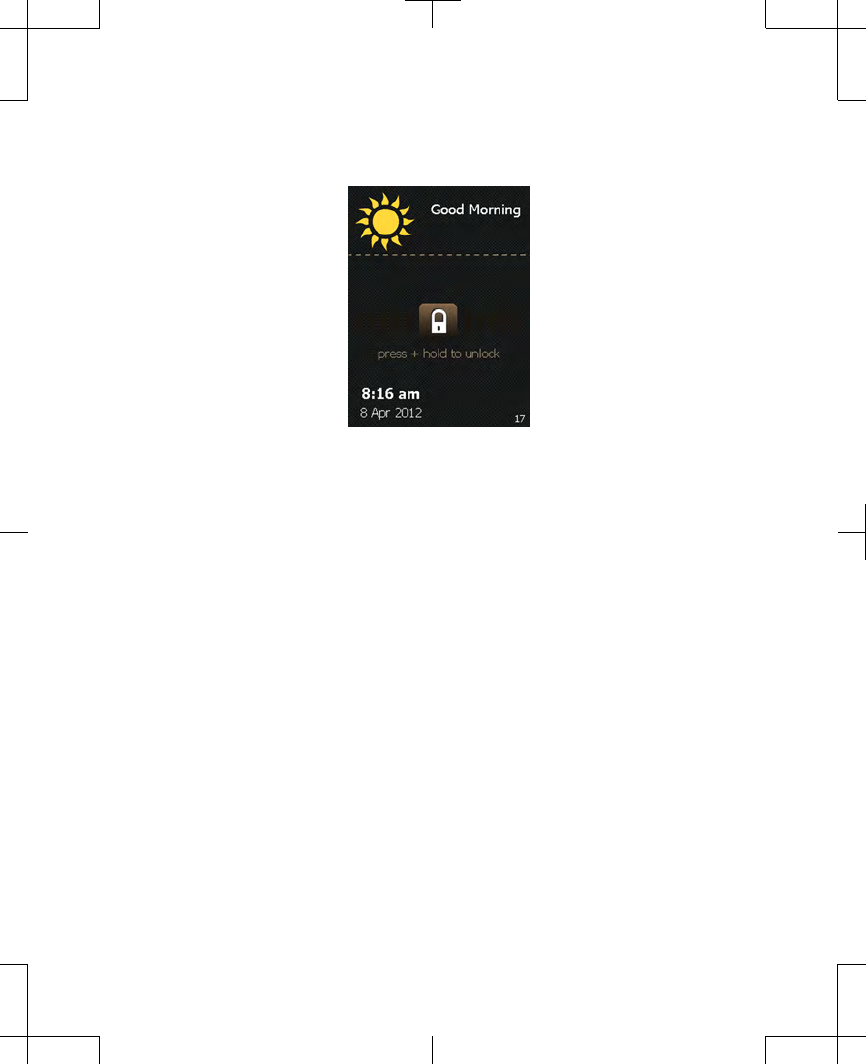
Figure 2.3 The Unlock screen.
Notes:
•
If the controller does not display the Unlock
screen, the controller may not have been
properly initiated. Call your clinician if the
Unlock screen does not appear.
•
When the controller is locked, pressing the
Stimulation On/Off key will provide the
option to bypass the Unlock screen and to
turn stimulation on or off.
1. Press and hold the Lock button on the
Unlock screen.
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
18
Using your controller 2
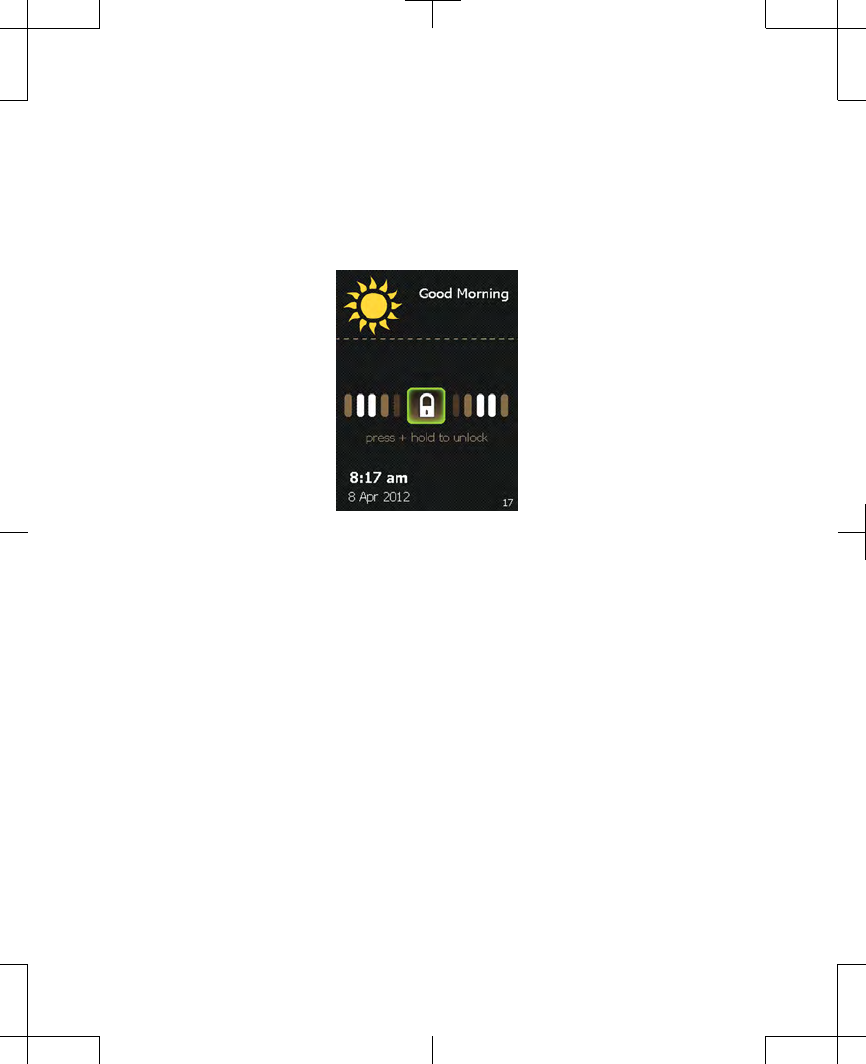
As the Lock button is held, bars appear on
the screen and move toward the Lock
button (Figure 2.4).
Figure 2.4 Unlocking the controller.
2. Stop pressing the screen when the screen
changes to a circle of dots (Figure 2.5). The
controller is searching for your external
neurostimulator.
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
19
Using your controller 2
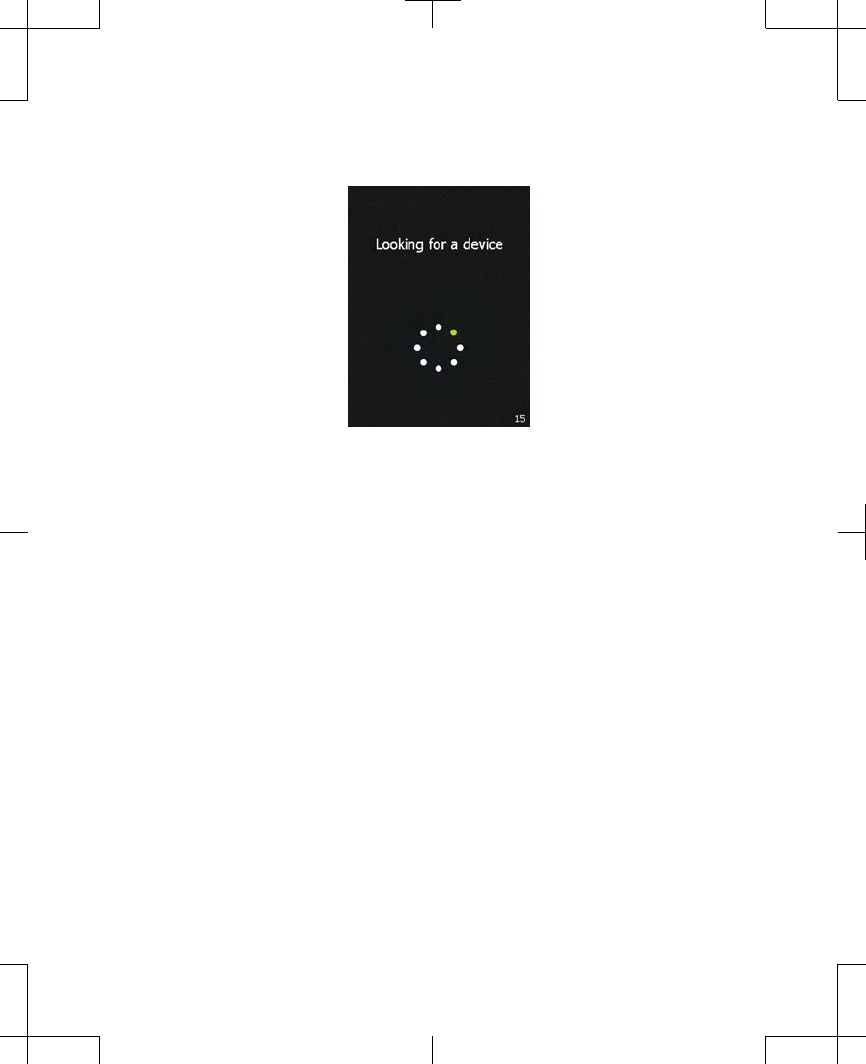
Figure 2.5 The controller searching for your
external neurostimulator.
After unlocking the controller, the first
screen you see is the Home screen
(Figure 2.6).
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
20
Using your controller 2
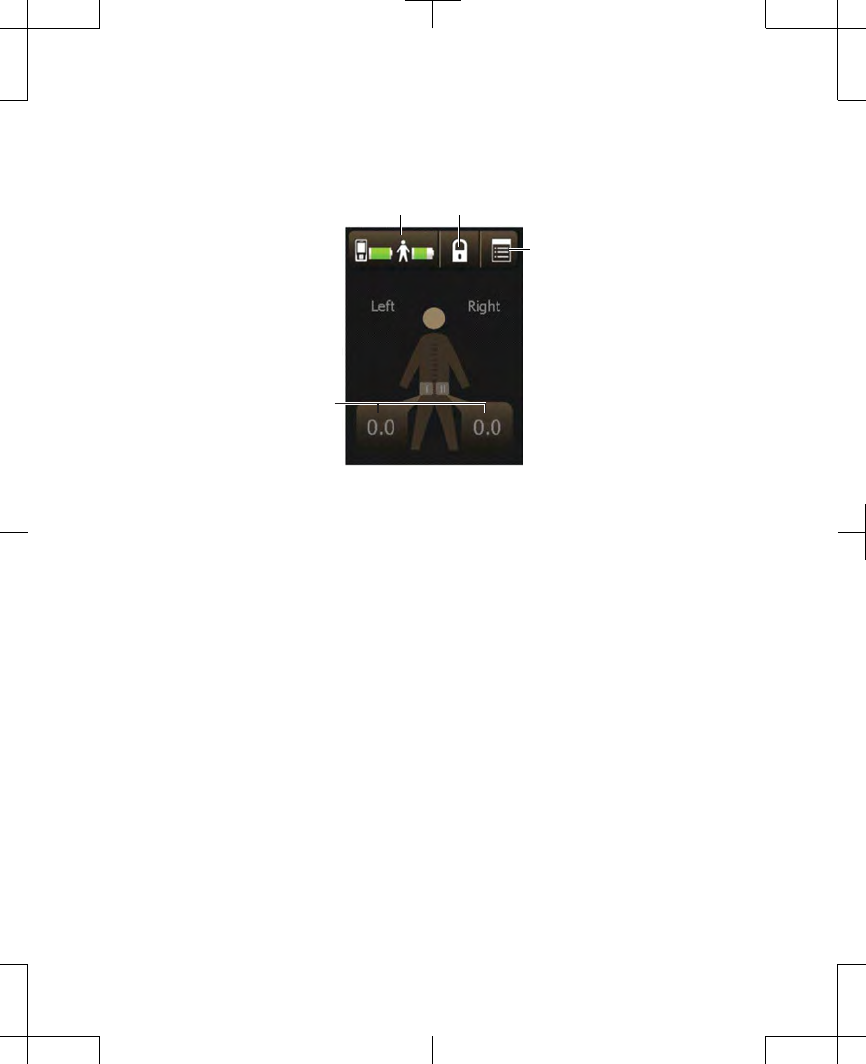
Menu button
Stimulation
value buttons
Battery status button Lock button
Figure 2.6 The Home screen.
If the Home screen does not appear, see
"Possible problems and solutions" on
page 64.
Locking your controller
Your controller can be locked, so if buttons or
keys are accidentally pressed, there will not be
an unexpected change in your stimulation.
Note: The controller screen will dim after 15
seconds of inactivity, and will automatically
lock itself after 2 minutes of inactivity. You can
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
21
Using your controller 2
also press the Lock button to lock the
controller immediately.
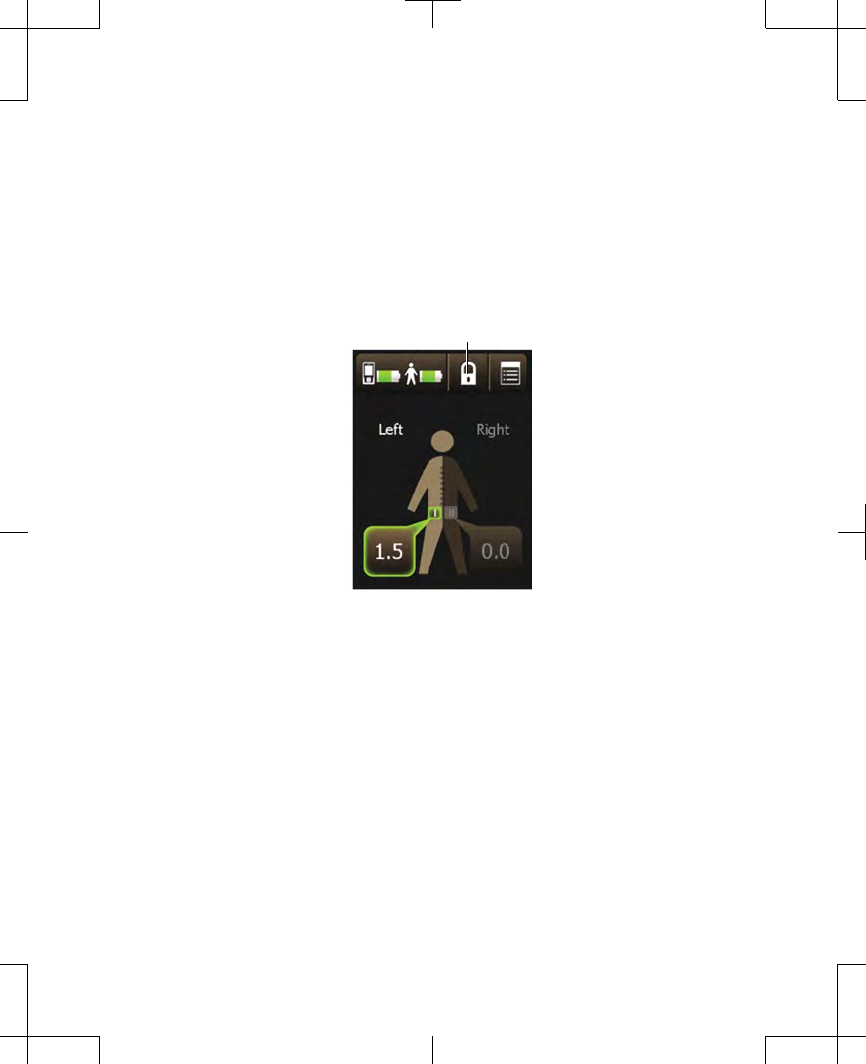
1. From the Home screen, press the Lock
button to lock the controller (Figure 2.7).
Lock button
Figure 2.7 Pressing the Lock button.
Turning your stimulation on or
off
You can turn stimulation on or off at any time.
Follow these steps to turn your stimulation on
or off.
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
22
Using your controller 2
Turning your stimulation on
1. To turn stimulation on, press the
Stimulation On/Off key, which is located
on the top of your controller.
Note: Pressing the Stimulation value
button on the Home screen also allows you
to turn stimulation on.
2. Press the On button to turn stimulation on
(Figure 2.8).
Note: If you do not wish to turn stimulation
on or off, but want to access the Home
screen, press the Go To Unlock button
and unlock your controller.
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
23
Using your controller 2
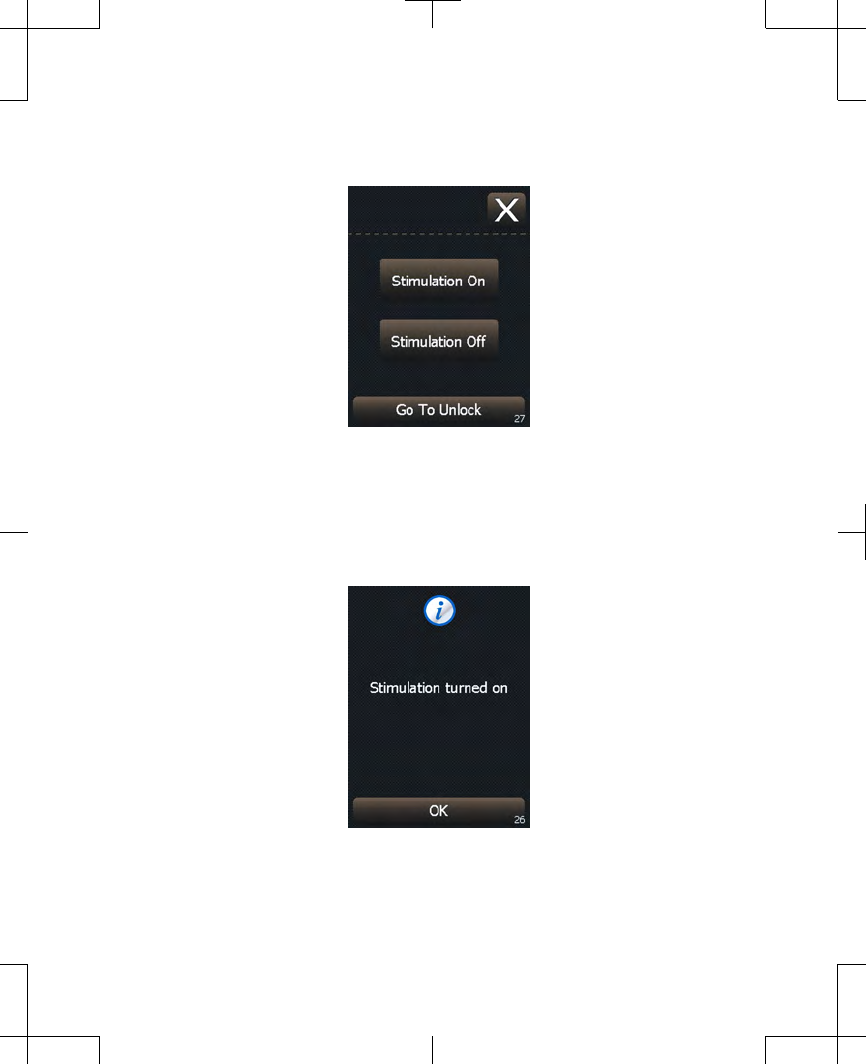
Figure 2.8 Turning stimulation on.
3. A confirmation screen will appear
(Figure 2.9). Press the OK button to
continue to the Home screen.
Figure 2.9 Stimulation turned on.
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
24
Using your controller 2
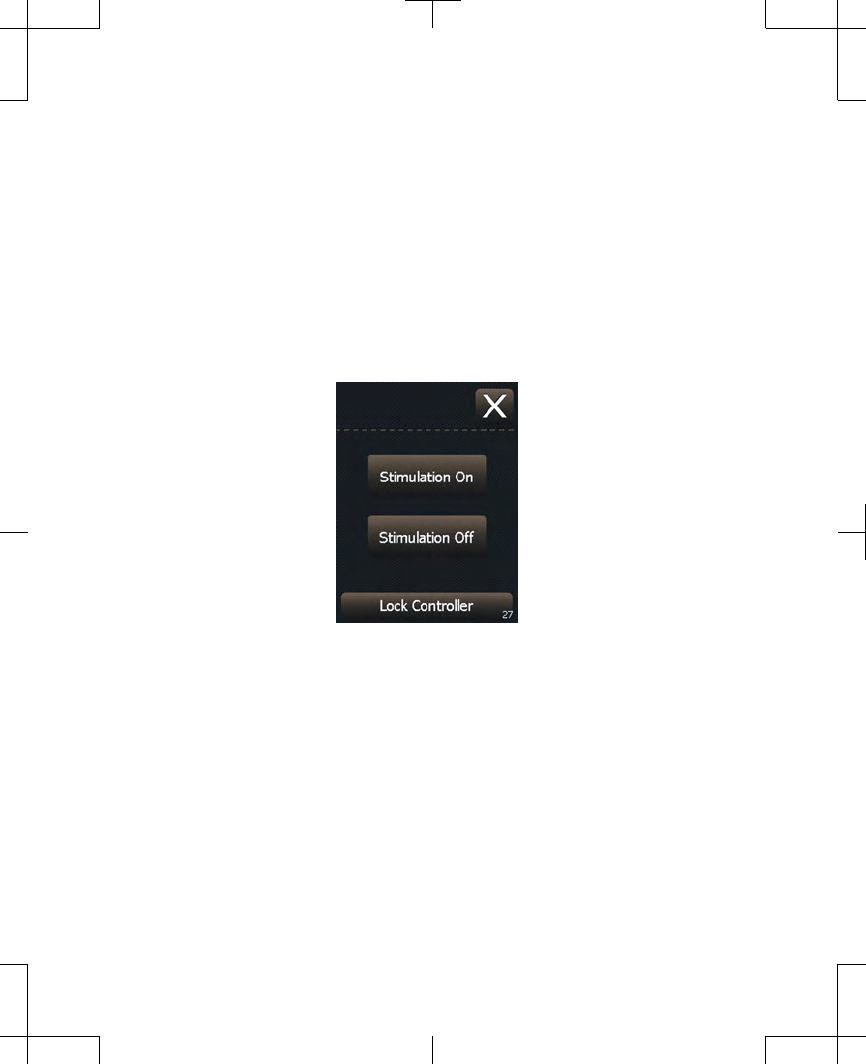
Turning your stimulation off
1. To turn stimulation off, press the
Stimulation On/Off key, which is located
on the top of your controller.
2. Press the Off button to turn stimulation off
(Figure 2.10).
Figure 2.10 Turning stimulation off.
3. If you do not wish to turn stimulation off, but
instead wish to access the Home screen,
press the Go To Unlock button and unlock
your controller.
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
25
Using your controller 2
Note: If your controller was already on, you
will instead have the option to lock the
controller.
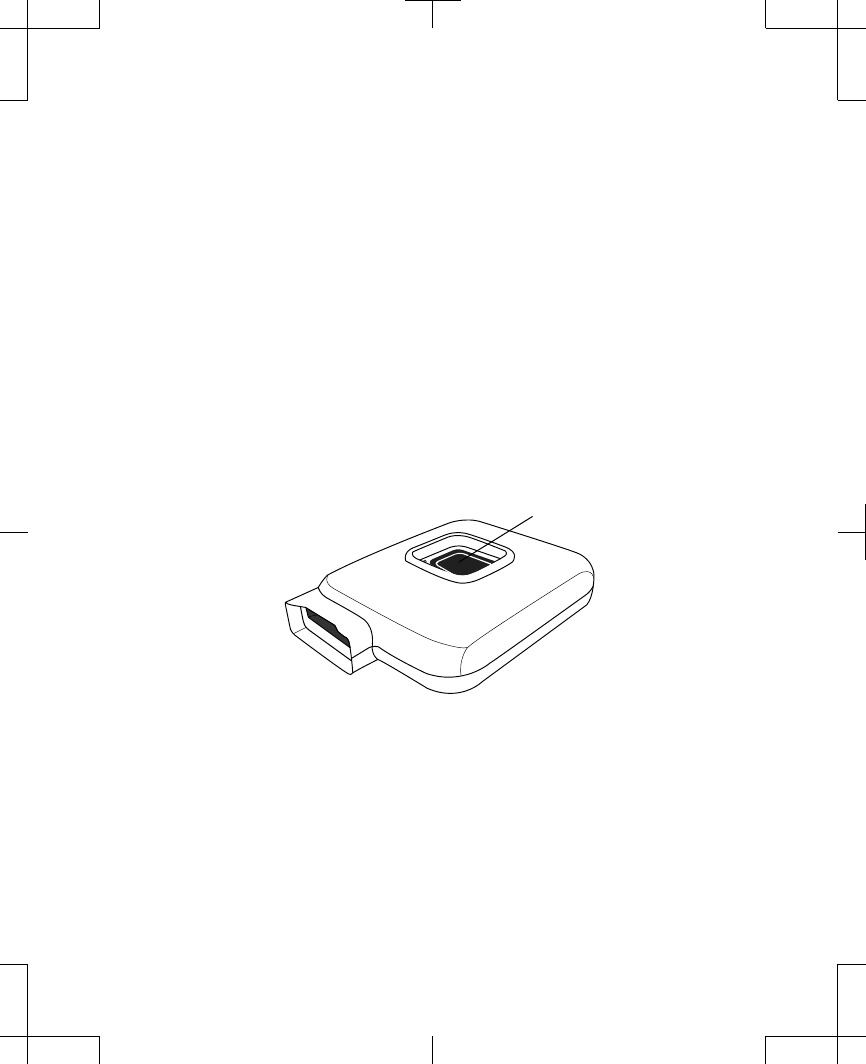
Note: If stimulation is uncomfortable and
you need to turn off your stimulation
immediately, but your controller is
unavailable or not responding, press and
hold the ENS button on the external
neurostimulator for 3 seconds
(Figure 2.11).
ENS button
Figure 2.11 The ENS button on the external
neurostimulator.
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
26
Using your controller 2
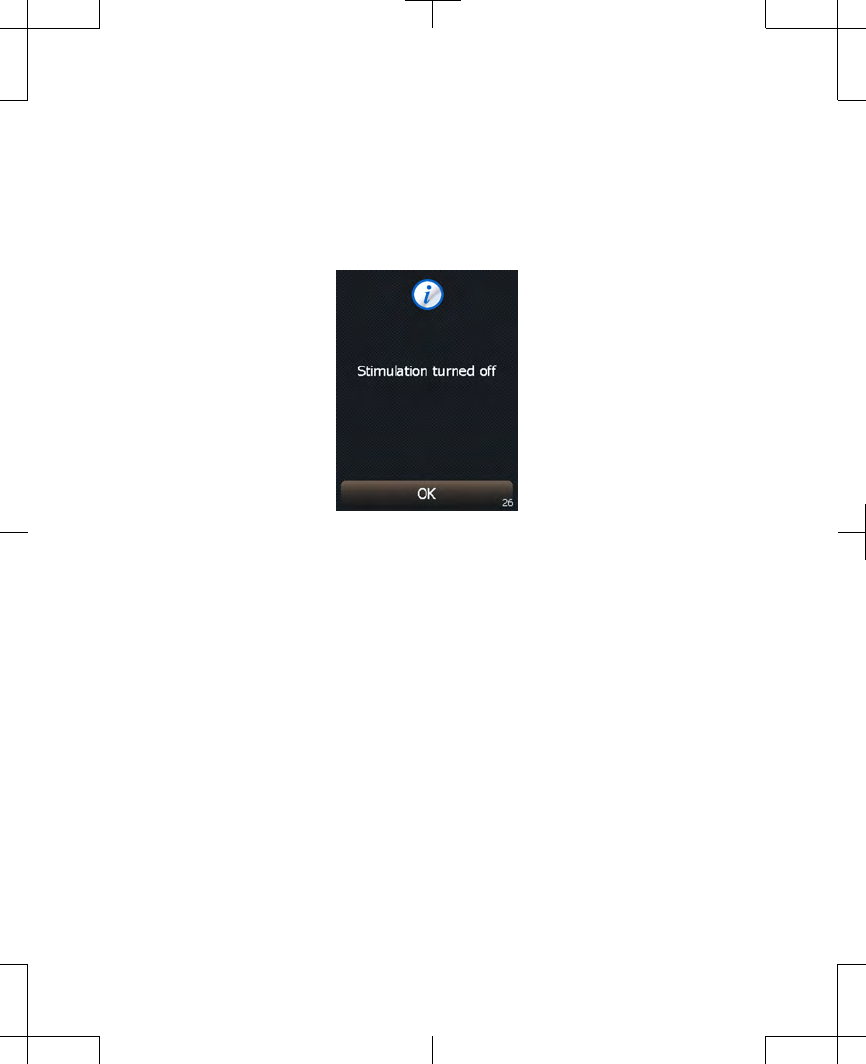
4. A confirmation screen will appear
(Figure 2.12). Press the OK button to
continue to the Home screen.
Figure 2.12 Stimulation turned off.
Adjusting stimulation
Notes:
•
Stimulation needs to be turned on before
adjusting your stimulation. If stimulation is
not on, see "Turning your stimulation on or
off" on page 22.
•
Every time you turn your stimulation on, the
stimulation value starts at zero.
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
27
Using your controller 2
1. From the Home screen, press the Increase
or Decrease key to adjust your stimulation
as advised by your clinician.
–
Press and release the Increase/
Decrease key to slowly increase or
decrease stimulation (by 0.1).
–
Press and hold to quickly increase or
decrease stimulation (by 0.5).
Note: When you press and hold the
Increase key, stimulation will continue to
quickly increase until you release the
button.
2. When finished adjusting stimulation, press
the Lock button ( ) to lock the controller.
Switching stimulation sides (if
your clinician placed two leads)
Notes:
–
Instructions on when to switch
stimulation sides are provided by your
clinician.
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
28
Using your controller 2

–
If your clinician placed only one lead,
switching stimulation sides will not be
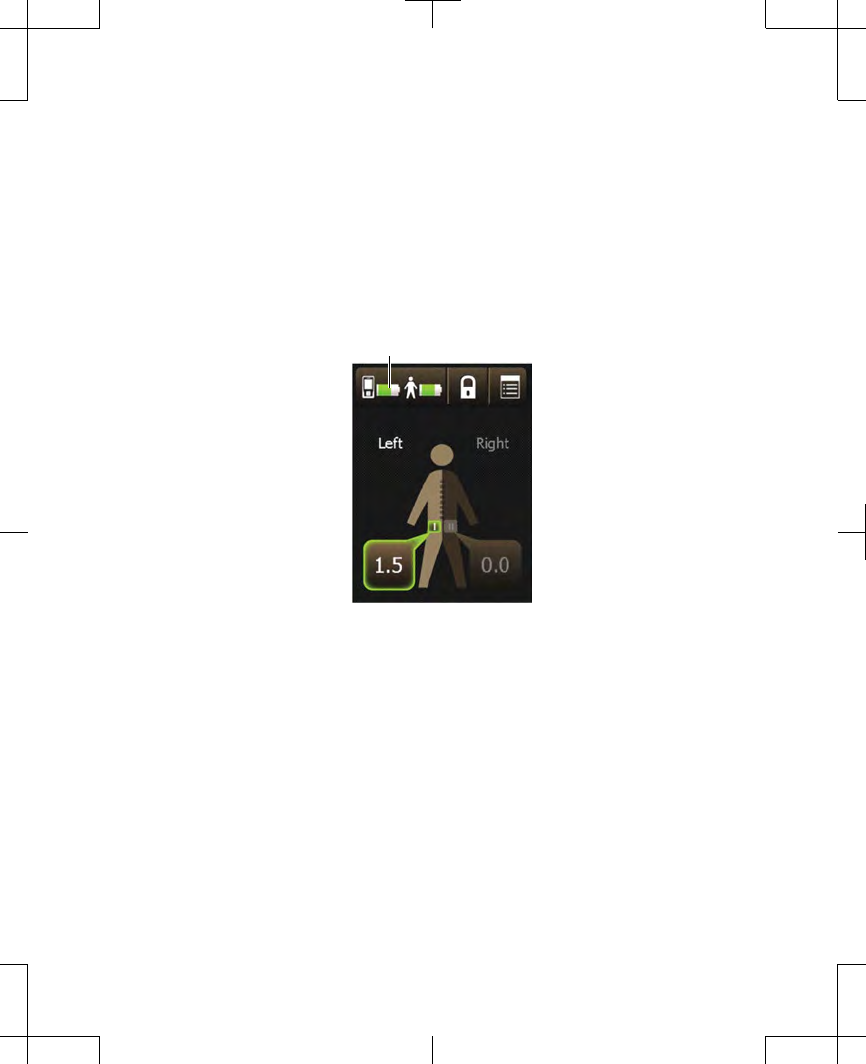
available. Figure 2.13 shows what your
Home screen looks like if you have one
lead, and what your Home screen looks
like if you have two leads.
One lead Two leads
Figure 2.13 Home screens for one lead and for
two leads.
Follow these steps to switch stimulation
sides:
1. Press the Stimulation value button on the
inactive side of the Home screen. A screen
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
29
Using your controller 2
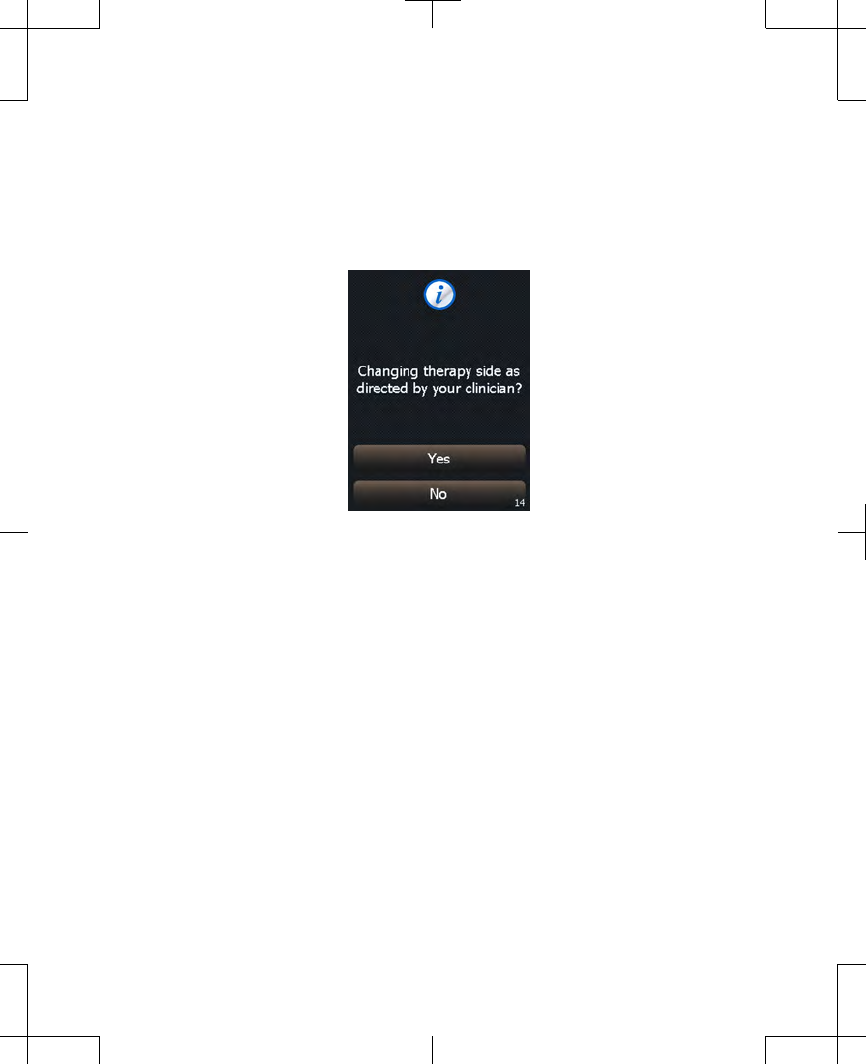
will appear asking you to confirm that you
wish to change stimulation sides
(Figure 2.14).
Figure 2.14 Confirming switch of stimulation
sides.
2. Press the Yes button to confirm switching
stimulation sides.
Note: Pressing the No button cancels the
change and continues stimulation on the
same side.
3. Your stimulation will switch sides, and the
amplitude will be set to zero. Use the
Increase/Decrease key to adjust
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
30
Using your controller 2
stimulation (see "Adjusting stimulation" on
page 27).
Changing programs (if
applicable)
Notes:
•
Instructions on when to change programs,
and which program to select, are provided
by your clinician.
•
Depending on how your clinician set up
your system, you may not be able to
change programs.
1. Press the Menu button ( ) in the top right
corner of the Home screen. A screen
appears with two buttons: Programs and
Clinician Mode (Figure 2.15).
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
31
Using your controller 2
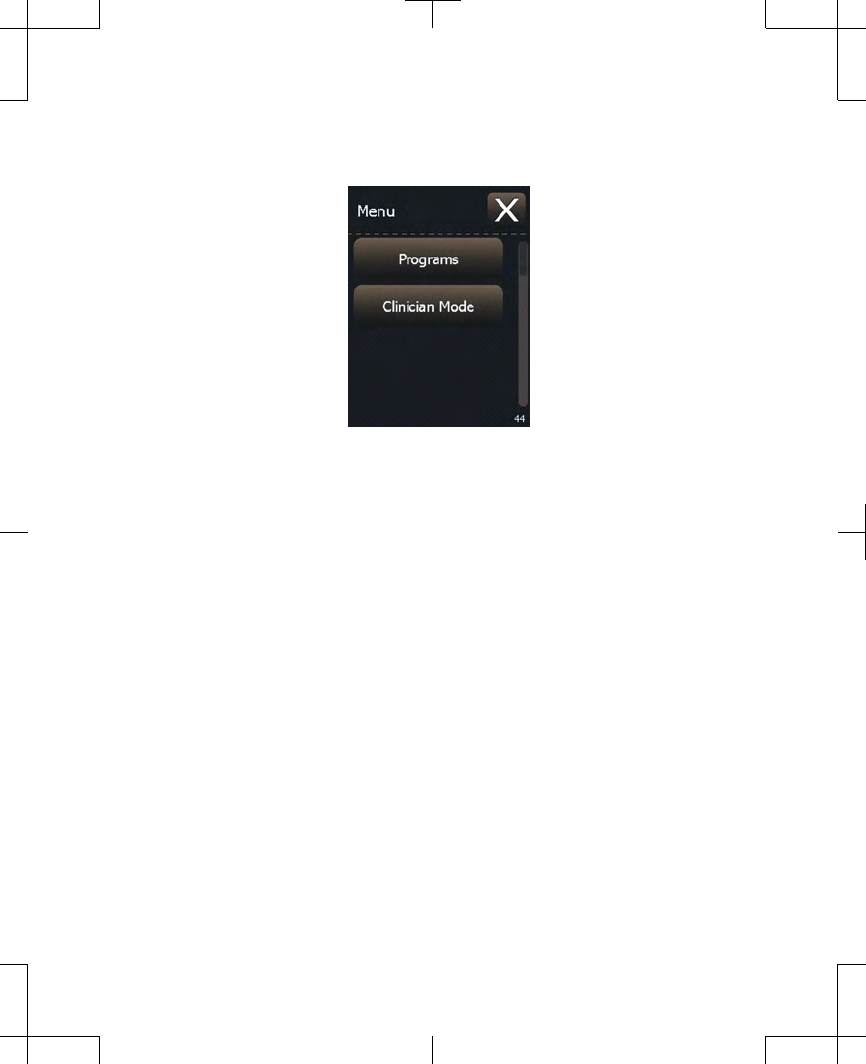
Figure 2.15 The Patient menu.
Note: If your screen does not look like
Figure 2.15 but instead asks you to enter a
code, then your clinician has set up your
system so that changing programs is not
available. Press the Exit button to go back
to the Menu, and then press the Exit button
again to go back to the Home screen.
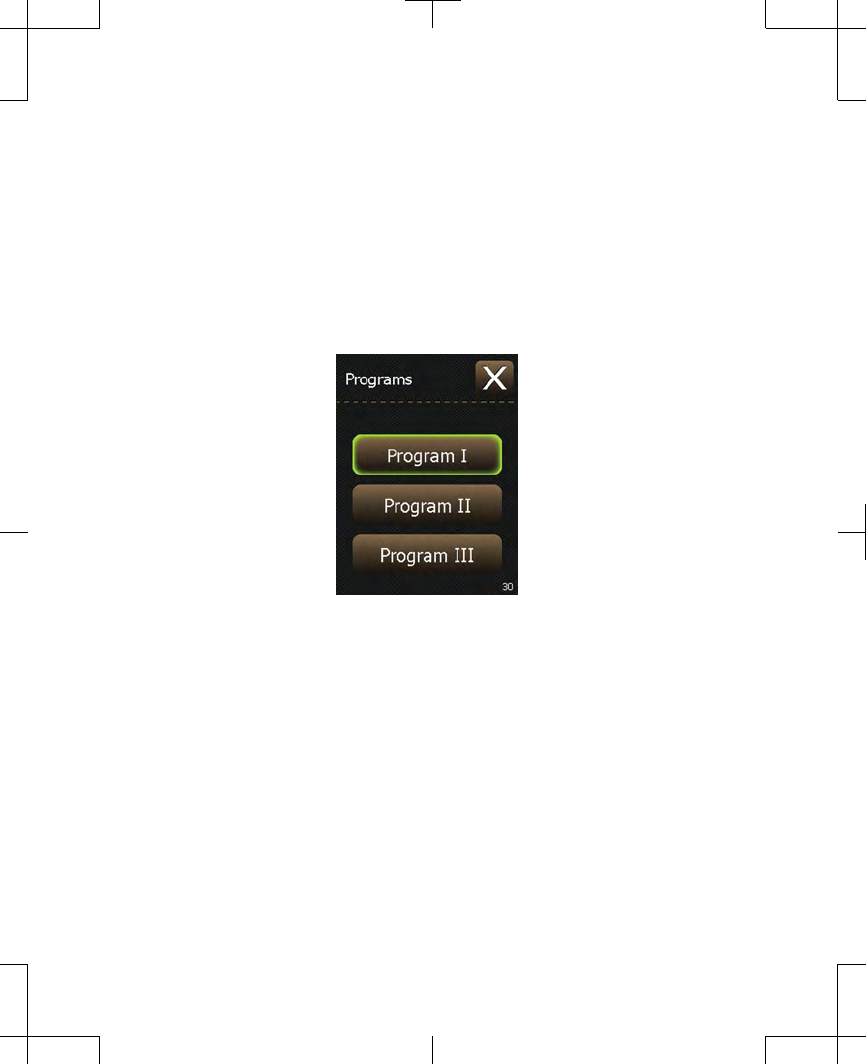
2. Press the Programs button. A screen
appears with three buttons: Program I,
Program II, and Program III (Figure 2.16).
The button outlined in green represents the
program that is currently active.
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
32
Using your controller 2
Note: If only one program is available, your
clinician set up your system so that
changing programs is not possible. Press
the Exit button to return to the Menu. Press
the Exit button again to return to the Home
screen.
Figure 2.16 Selecting a program.
Note: Your system may have been set up
so that one or more programs is not
available. Available program buttons
become highlighted in green when they are
pressed.
3. Select an available program as instructed
by your clinician.
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
33
Using your controller 2
Note: After switching programs, amplitude
automatically decreases to zero.
4. Press the Exit button ( ) to return to the
Menu.
5. Press the Exit button ( ) to return to the
Home screen.
6. Adjust stimulation as advised by your
clinician. See "Adjusting stimulation" on
page 27.
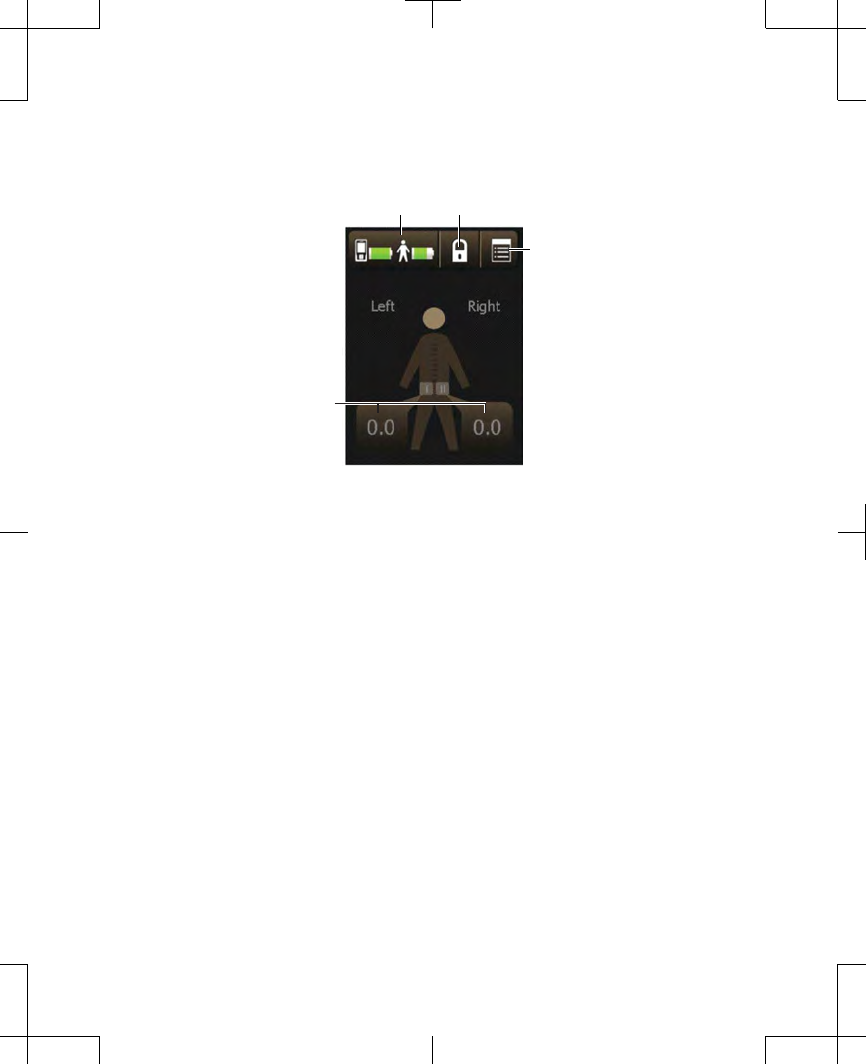
Home screen
The Home screen is the first screen to appear
after unlocking your controller. The Home
screen provides an overview of your
stimulation settings (Figure 2.17).
Note: Your Home screen may look slightly
different than the one shown.
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
34
Using your controller 2
Menu button
Stimulation
value buttons
Battery status button Lock button
Figure 2.17 The Home screen.
Information on the Home screen is split into
two sides: the Left side and the Right side.
Note: It is normal for some patients to be able
to adjust only the left side or only the right side.
This means you have only one lead placed for
test stimulation instead of two leads.
The buttons at the top of the Home screen
allow you to perform the following tasks:
•
Check the battery status of the controller.
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
35
Using your controller 2
•
Check the battery status of the external
neurostimulator.
•
Lock the controller.
•
Access the Programs screen.
Note: Whether you can access the
Programs screen depends on how your
clinician set up your test stimulation
system. Discuss this with your clinician.
Refer to Table 2.2 for more information on
these buttons.
Table 2.2 Home screen buttons
Icons Description
Battery
status button The left icon on this button
displays the controller battery
status. The right icon on this
button displays the external
neurostimulator battery
status.
Press this button to check the
battery status in detail. For
more information on checking
batteries, see "Checking and
replacing batteries" on
page 38.
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
36
Using your controller 2
Table 2.2 Home screen buttons (continued)
Icons Description
Lock button Press this button to lock the
controller.
Menu button Press this button to access the
Patient menu (if applicable).
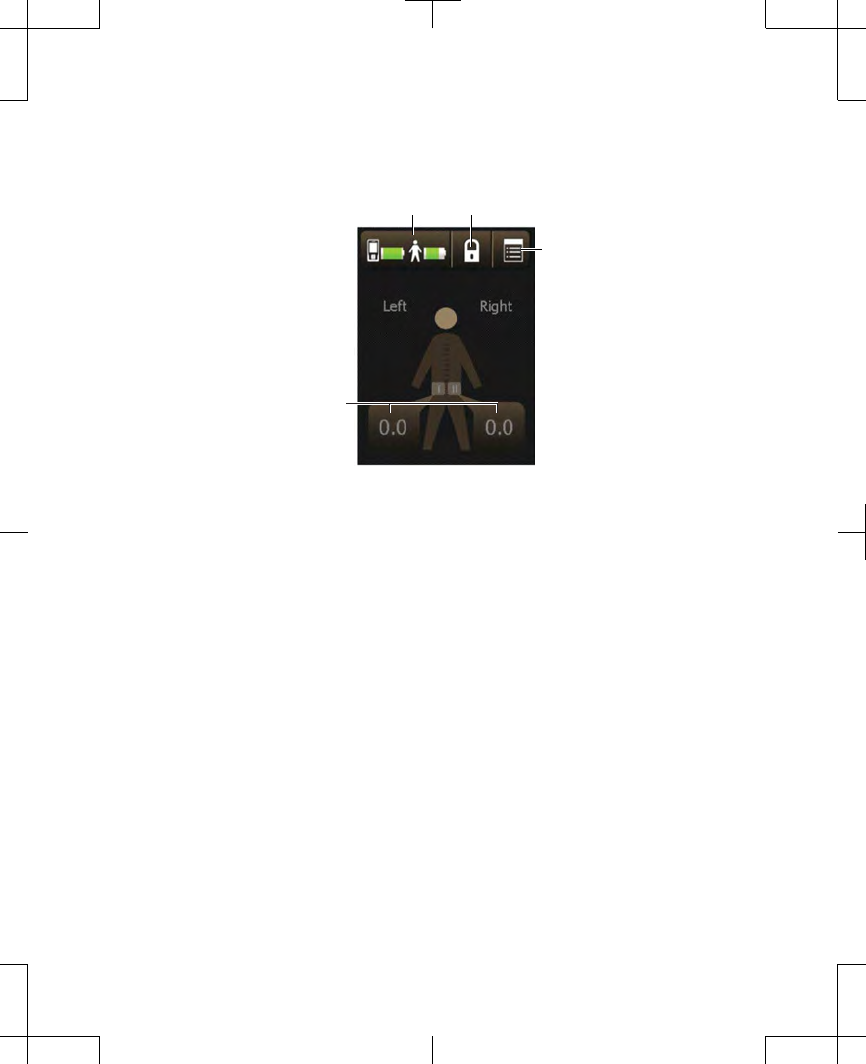
The body figure at the center of the Home
screen indicates which side is receiving
stimulation (if two leads were placed). The
number shown in the Stimulation value
button is the amplitude of that stimulation
(Figure 2.18).
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
37
Using your controller 2
Menu button
Stimulation
value buttons
Battery status button Lock button
Figure 2.18 The Home screen.
Checking and replacing
batteries
Checking the batteries (external
neurostimulator and controller)
Check the status of the batteries in your
external neurostimulator and your controller
every day. You can check the status of the
batteries at any time.
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
38
Using your controller 2
Note: The battery levels of the controller and
the external neurostimulator are shown on the
Battery status button on the Home screen
(Figure 2.19). For further detail about battery
levels, follow the steps below.
Battery status button
Figure 2.19 Viewing the battery status on the
Home screen.
1. From the Home screen, press the Battery
status button. Two battery figures appear
(Figure 2.20).
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
39
Using your controller 2
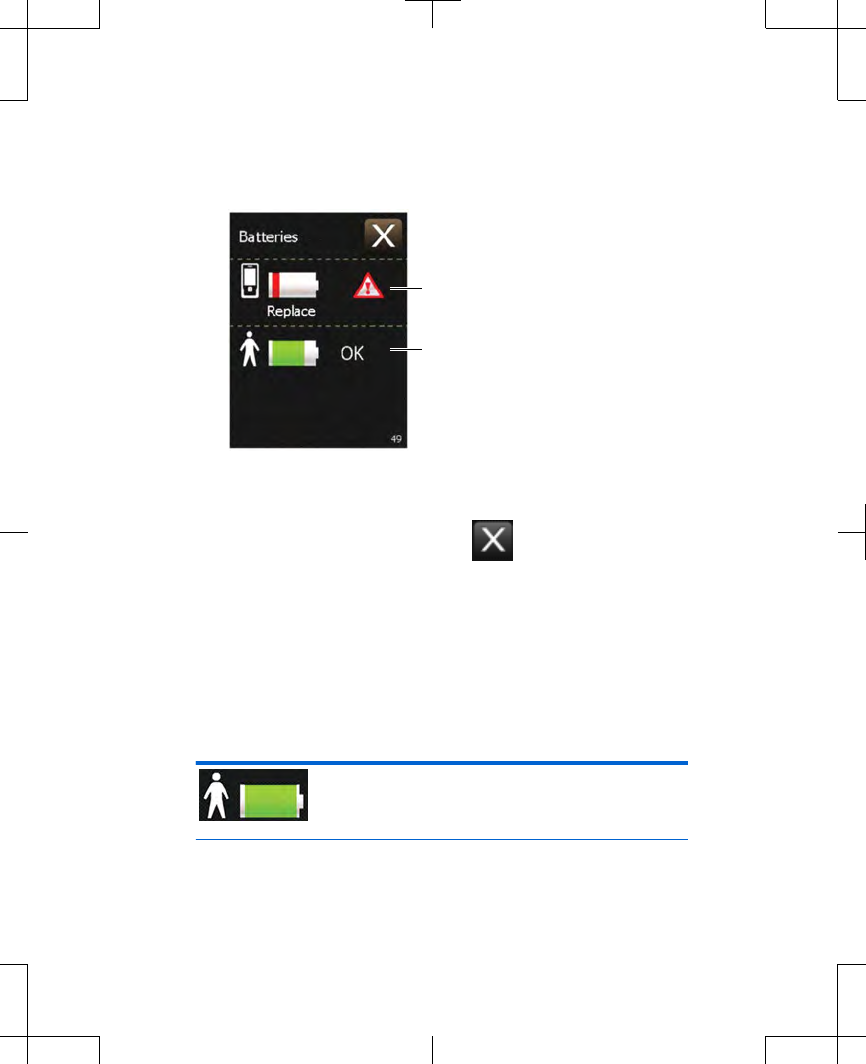
Controller battery status
External neurostimulator
battery status
Figure 2.20 Example of a Battery status screen.
2. Press the Exit button ( ) at the top right
corner of the touchscreen to exit.
Table 2.3 lists several examples of external
neurostimulator battery levels and whether
action is needed.
Table 2.3 Battery level definitions for the
external neurostimulator
External neurostimulator battery icon is
green. Battery level is full. No action is
needed.
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
40
Using your controller 2
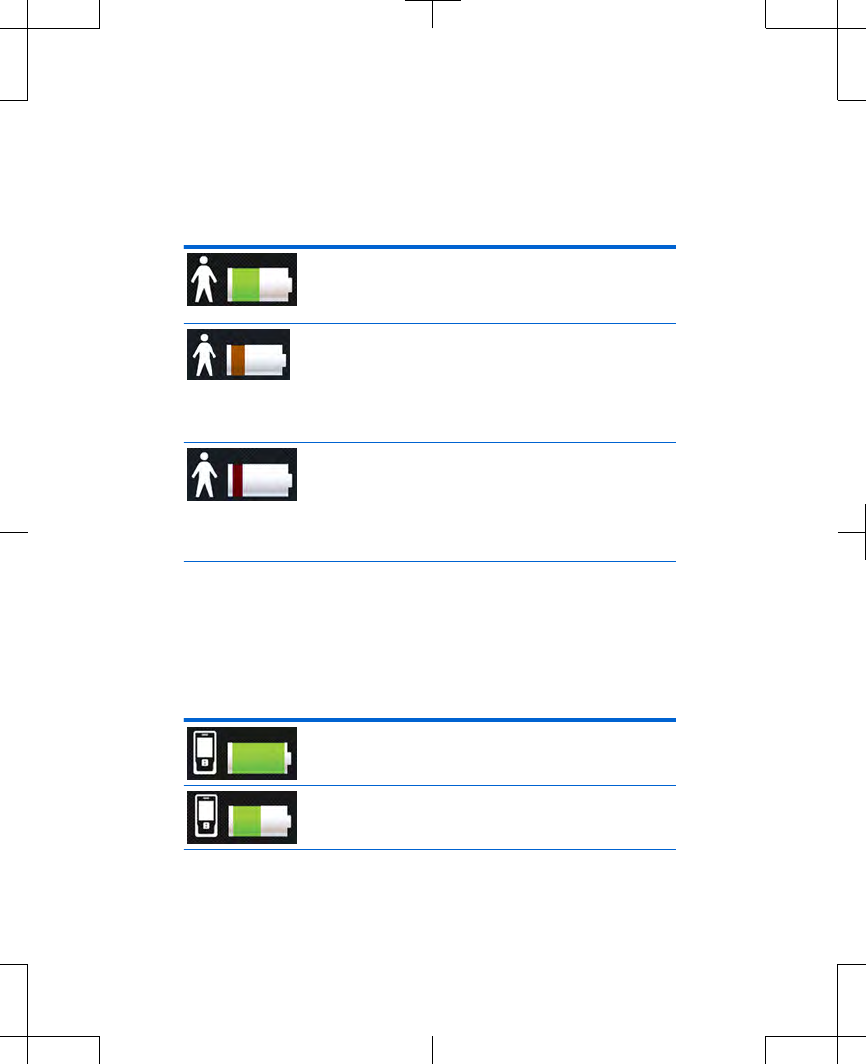
Table 2.3 Battery level definitions for the
external neurostimulator (continued)
External neurostimulator battery icon is
green. Battery level is half full. No
action is needed.
External neurostimulator battery icon is
orange. Stimulation is still being
provided, but the external
neurostimulator batteries need to be
replaced soon. Call your clinician.
External neurostimulator battery icon is
red. Stimulation may no longer be
available. The external neurostimulator
batteries need to be replaced. Call your
clinician.
Table 2.4 lists several examples of controller
battery levels and whether action is needed.
Table 2.4 Battery level definitions for the
controller
Controller battery icon is green. Battery
level is full. No action is needed.
Controller battery icon is green. Battery
level is half full. No action is needed.
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
41
Using your controller 2
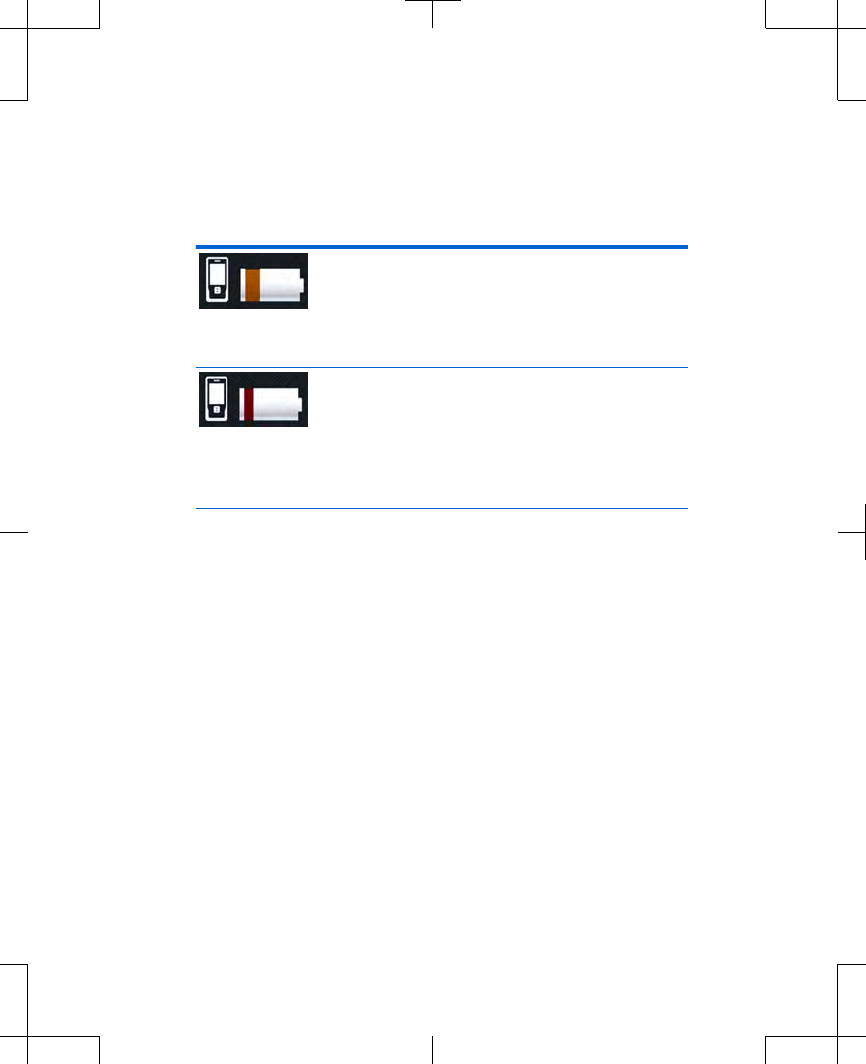
Table 2.4 Battery level definitions for the
controller (continued)
Controller battery icon is orange. The
controller batteries are low. Replace
the controller batteries. See "Replacing
the batteries (controller only)" on
page 45.
Controller battery icon is red. The
controller batteries are nearly depleted
and programming will not be possible
soon. Replace the controller batteries.
See "Replacing the batteries (controller
only)" on page 45.
Table 2.5 lists the warning and alert screens
associated with the batteries for the external
neurostimulator and the controller.
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
42
Using your controller 2
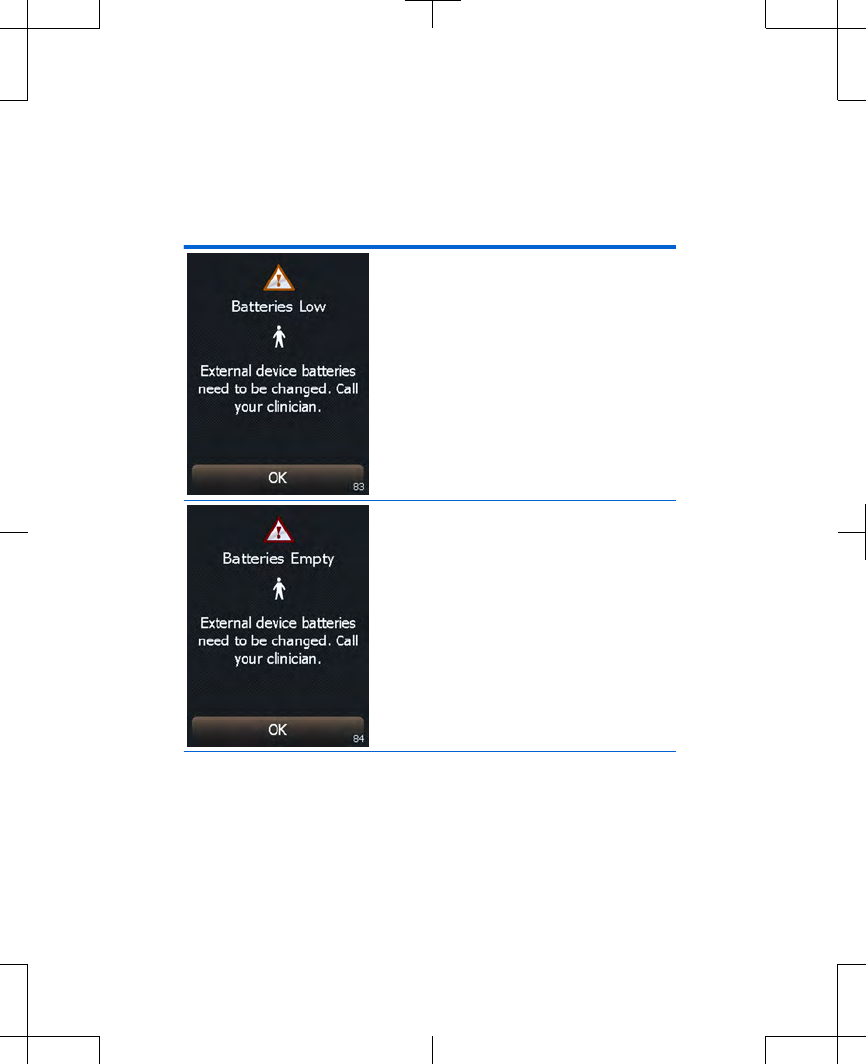
Table 2.5 Battery message screens for the
controller and the external neurostimulator
The external
neurostimulator batteries
are low and stimulation will
not be available soon.
Call your clinician. Press
the OK button to exit this
screen.
Cause: The external
neurostimulator batteries
are depleted and
stimulation is not available.
Action: The external
neurostimulator batteries
need to be replaced. Call
your clinician.
Press the OK button to
exit this screen.
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
43
Using your controller 2
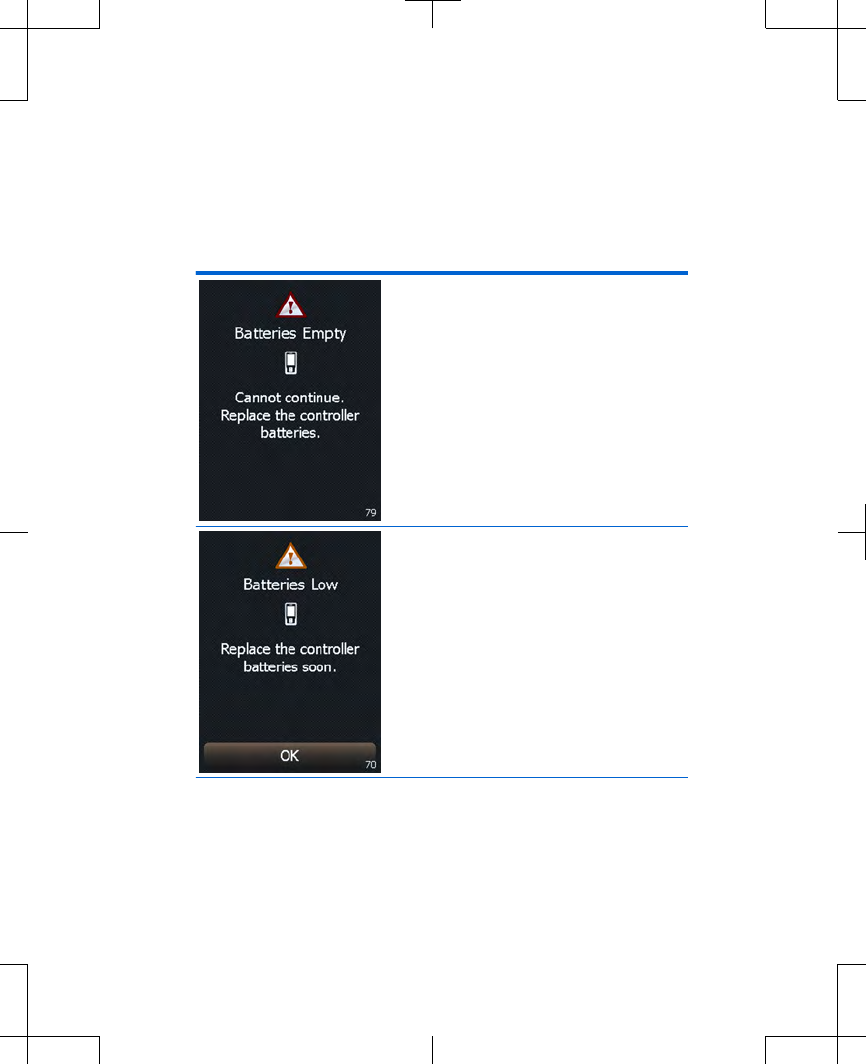
Table 2.5 Battery message screens for the
controller and the external neurostimulator
(continued)
The controller batteries are
depleted. Programming is
not possible.
Replace the controller
batteries now. Refer to
"Replacing the batteries
(controller only)" on
page 45.
The controller batteries are
low. Programming will not
be possible soon.
Replace the controller
batteries now. Refer to
"Replacing the batteries
(controller only)" on
page 45. Press the OK
button to exit this screen.
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
44
Using your controller 2

Replacing the batteries (controller
only)
Note: This section is for replacing the
controller batteries. Do not attempt to replace
the external neurostimulator batteries unless
directed by your clinician.
1. Open the battery compartment cover
(Figure 2.21).
Figure 2.21 Opening the battery cover.
2. Remove the depleted batteries.
3. Insert two new AA alkaline batteries as
shown on the battery compartment label
(Figure 2.22).
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
45
Using your controller 2

Figure 2.22 Inserting new batteries.
4. Close the battery compartment cover.
5. Dispose of old batteries according to local
regulations.
Using the carrying case
The carrying case has a pouch to hold the
controller (Figure 2.23).
The carrying case also has a clip on the back
that can be attached to a belt.
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
46
Using your controller 2
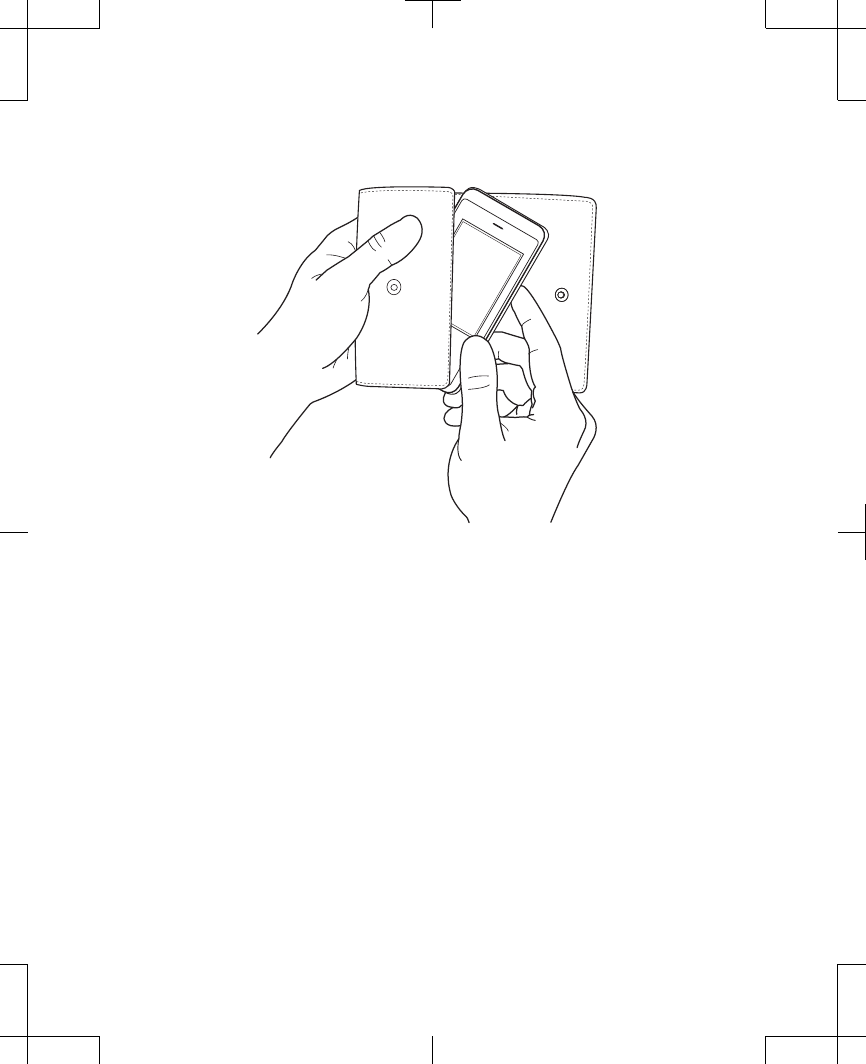
Figure 2.23 Insert the controller into the carrying
case.
Always carry your controller
Because your controller is the only way to
adjust or turn your stimulation on, you should
always carry your controller with you. The
controller is also the recommended way to turn
your stimulation off.
In particular, always bring your controller with
you to follow-up appointments.
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
47
Using your controller 2
In addition, always bring your controller to
appointments with other health care providers.
During certain procedures, you may need to
turn your external neurostimulator off. You
should also bring your InterStim Therapy
Patient Guide. It contains important
information about the InterStim system that
your health care providers should be aware
of.
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
48
Using your controller 2

3
Troubleshooting
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
This chapter will help you solve problems with
your controller.
Note: If a problem is not solved after several
attempts, or if a problem is not described here,
contact your clinician.
Controller screens
The controller displays three different types of
screens: warning screens, alert screens, and
notification screens. These screens provide
you with information about your system, alert
you to a problem with your system, or guide
you during controller use.
•
Warning screens display a red triangle at
the top with an exclamation point ( ).
•
Alert screens display an orange triangle at
the top with an exclamation point ( ).
•
Notification screens display a blue circle at
the top with the letter 'i' ( ).
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
50
Troubleshooting 3
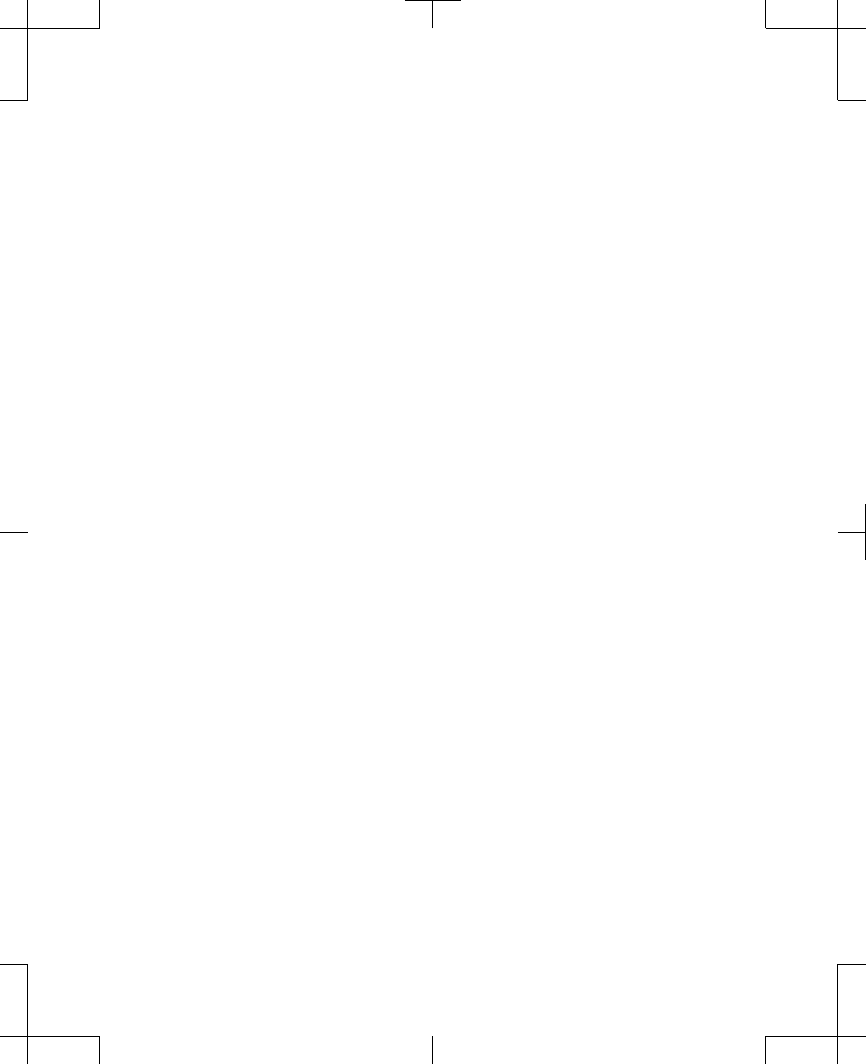
Warning screens
Warning screens indicate a problem with the
controller or the external neurostimulator.
Table 3.1 describes the possible warning
screens and provides instructions (see blue
text) on how to resolve the problem and clear
the screen.
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
51
Troubleshooting 3
Table 3.1 Warning screens
Screen Cause and action
System Problem
Cannot continue.
Please call your
clinician.
Explanation: The system is not
working correctly. Stimulation
may have stopped.
Solution: Write down the
message on the screen and
the screen number in the
bottom right corner, then
follow these steps:
1. Remove and reinsert the
controller batteries, then
retry the action that
caused the error screen to
appear. For instructions
on removing and
inserting the controller
batteries, refer to
"Replacing the batteries
(controller only)" on
page 45.
2. If this does not solve the
problem, call your
clinician.
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
52
Troubleshooting 3
Table 3.1 Warning screens (continued)
Screen Cause and action
Software Problem
Cannot continue.
Please call your
clinician.
Explanation: The software is not
working correctly. Stimulation
may have stopped.
Solution: Write down the
message on the screen and
the screen number in the
bottom right corner, then
follow these steps:
1. Remove and reinsert the
controller batteries, then
retry the action that
caused the error screen to
appear. For instructions
on removing and
inserting the controller
batteries, refer to
"Replacing the batteries
(controller only)" on
page 45.
2. If this does not solve the
problem, call your
clinician.
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
53
Troubleshooting 3
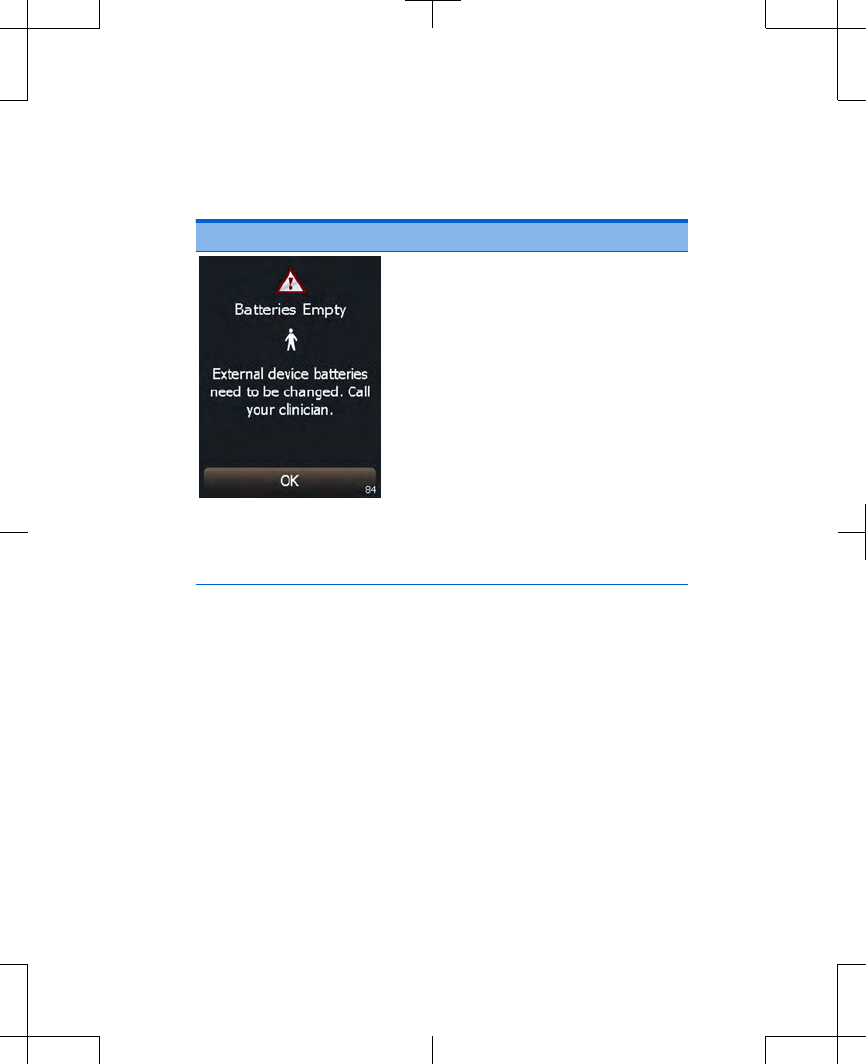
Table 3.1 Warning screens (continued)
Screen Cause and action
Cause: The external
neurostimulator batteries are
depleted and stimulation is not
available.
Action: The external
neurostimulator batteries
need to be replaced. Write
down the message on the
screen and the screen
number in the bottom right
corner, and call your
clinician.
Press the OK button to clear
the screen.
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
54
Troubleshooting 3
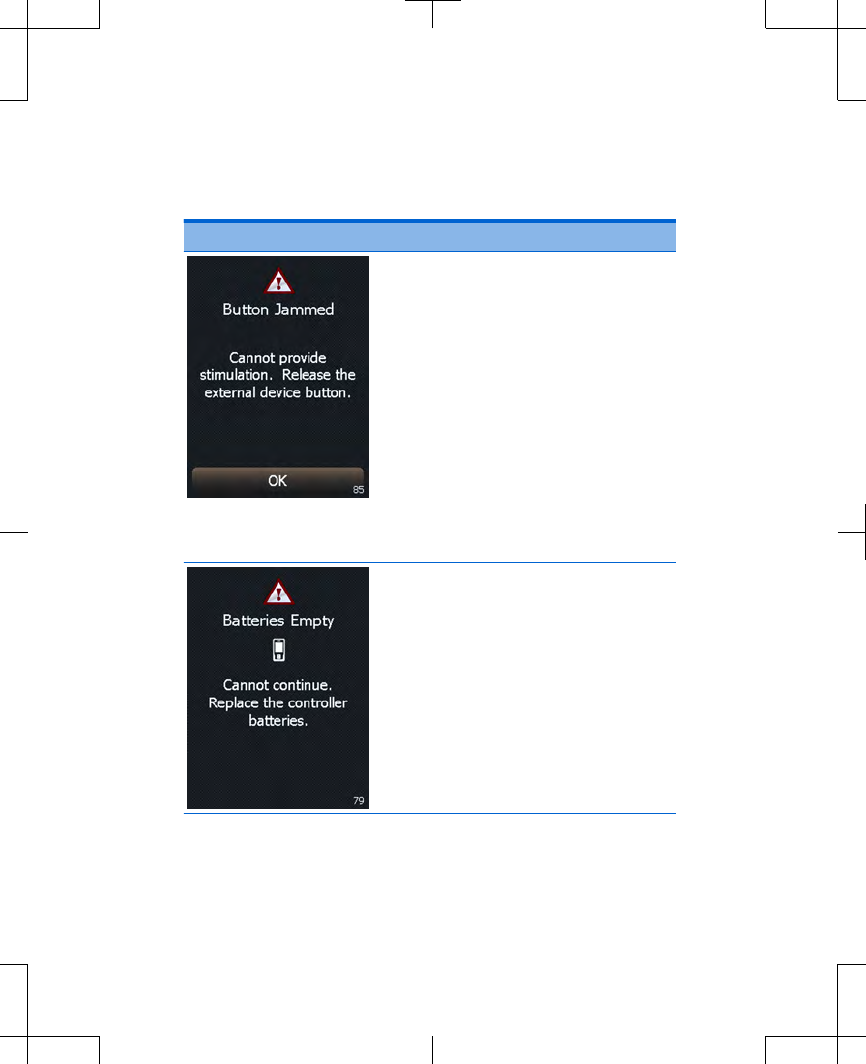
Table 3.1 Warning screens (continued)
Screen Cause and action
Cause: The ENS button on the
external neurostimulator is
stuck in the pressed position.
Action: Press and release the
ENS button, then press the
OK button to clear the
screen.
If this does not solve the
problem, write down the
message on the screen and
the screen number in the
bottom right corner, and call
your clinician.
Explanation: The controller
batteries are depleted.
Programming is not available.
Replace the controller
batteries now. Refer to
"Replacing the batteries
(controller only)" on page 45.
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
55
Troubleshooting 3
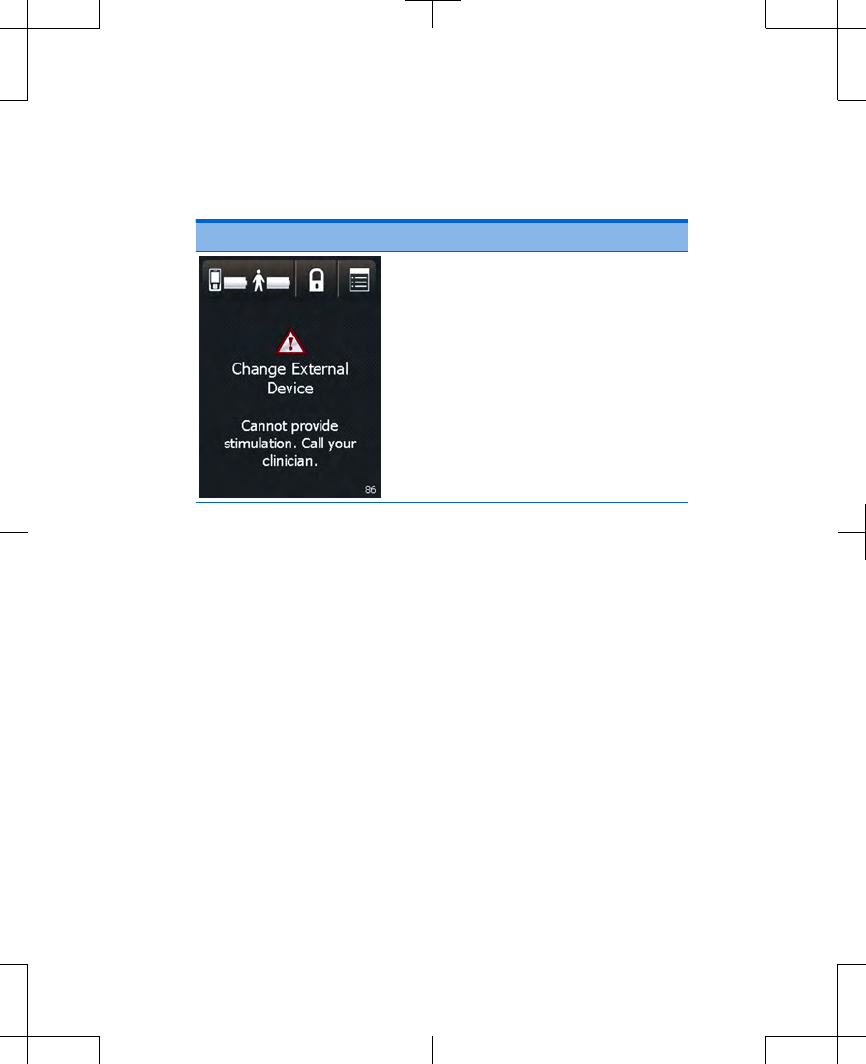
Table 3.1 Warning screens (continued)
Screen Cause and action
Cause: The external
neurostimulator has reached
the end of service. This external
neurostimulator will no longer
provide stimulation.
Action: Write down the
message on the screen and
the screen number in the
bottom right corner, and call
your clinician.
Alert screens
Alert screens indicate a pairing or other
connection problem between the controller
and the external neurostimulator.
Table 3.2 describes the possible alert screens
and provides instructions (see blue text) on
how to resolve the problem and clear the
screen.
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
56
Troubleshooting 3
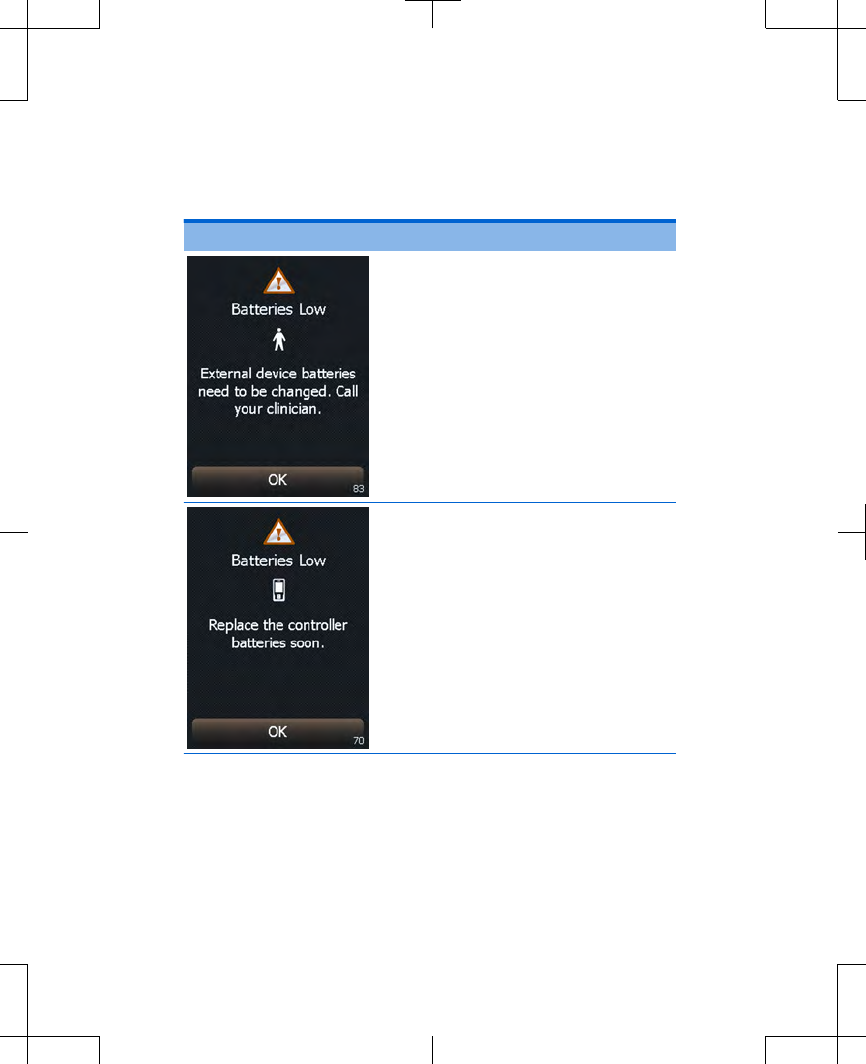
Table 3.2 Alert screens
Screen Cause and action
The external neurostimulator
batteries are low and stimulation
will not be available soon.
Action: Write down the
message on the screen and
the screen number in the
bottom right corner, and call
your clinician. Press the OK
button to exit this screen.
The controller batteries are low.
Programming will not be
possible soon.
Replace the controller
batteries now. Refer to
"Replacing the batteries
(controller only)" on page 45.
Press the OK button to exit
this screen.
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
57
Troubleshooting 3
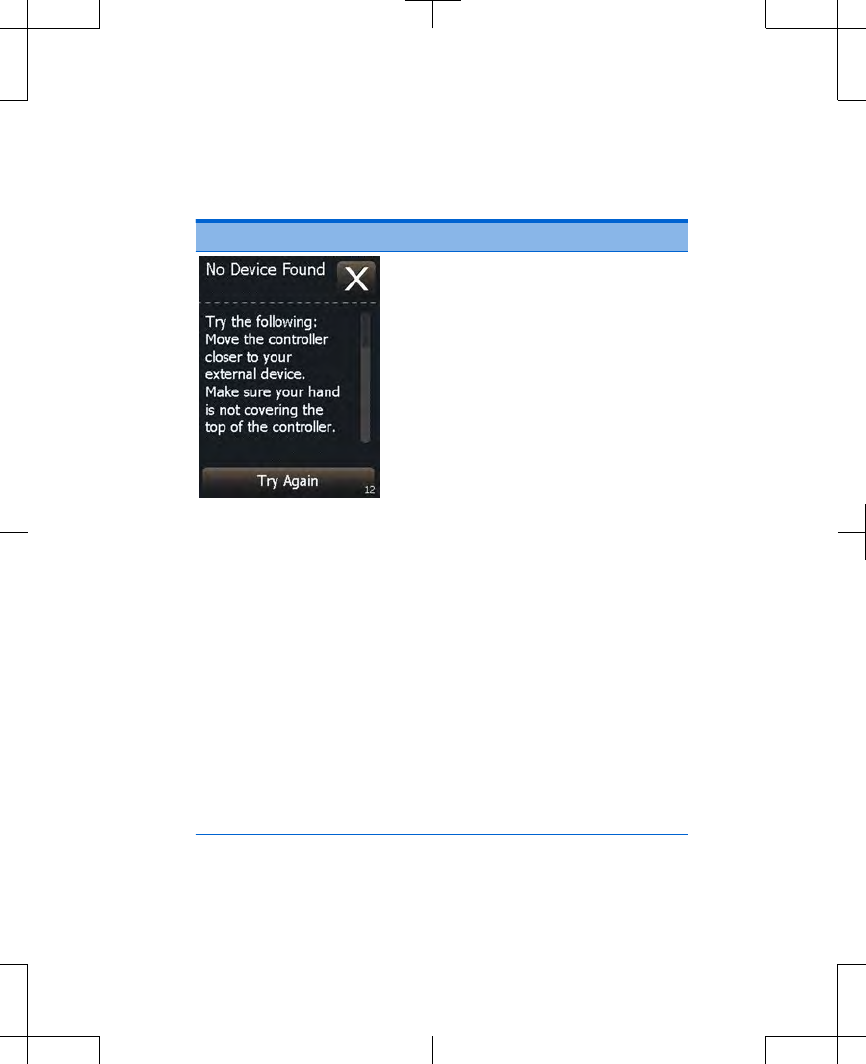
Table 3.2 Alert screens (continued)
Screen Cause and action
Cause: The controller cannot
find the paired external
neurostimulator. The external
neurostimulator may be out of
range or the external
neurostimulator batteries may
be depleted.
Action: Move the controller
closer to the external
neurostimulator and make
sure your hand is not
covering the top of the
controller, then press the Try
Again button.
If this does not solve the
problem, the external
neurostimulator batteries
may need to be replaced.
Write down the message on
the screen and the screen
number in the bottom right
corner, and call your
clinician.
Press the Cancel button to
clear the screen.
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
58
Troubleshooting 3
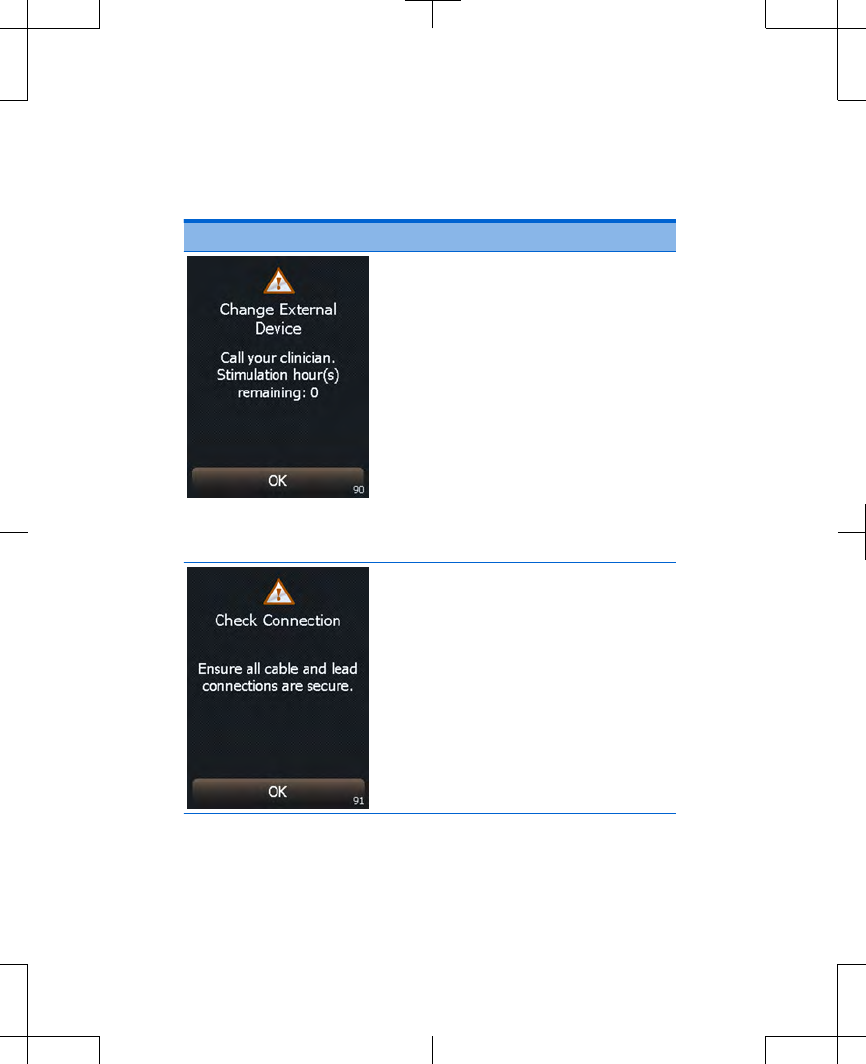
Table 3.2 Alert screens (continued)
Screen Cause and action
Cause: The external
neurostimulator has less than
24 hours of service remaining.
This external neurostimulator
will be unable to provide
stimulation soon.
Write down the message on
the screen and the screen
number in the bottom right
corner, and call your
clinician.
Press the OK button to clear
the screen.
Explanation: The external
neurostimulator has detected
that either a cable or a lead is not
connected.
Solution: Ensure all cable and
lead connections are secure.
Press the OK button to clear
the screen.
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
59
Troubleshooting 3
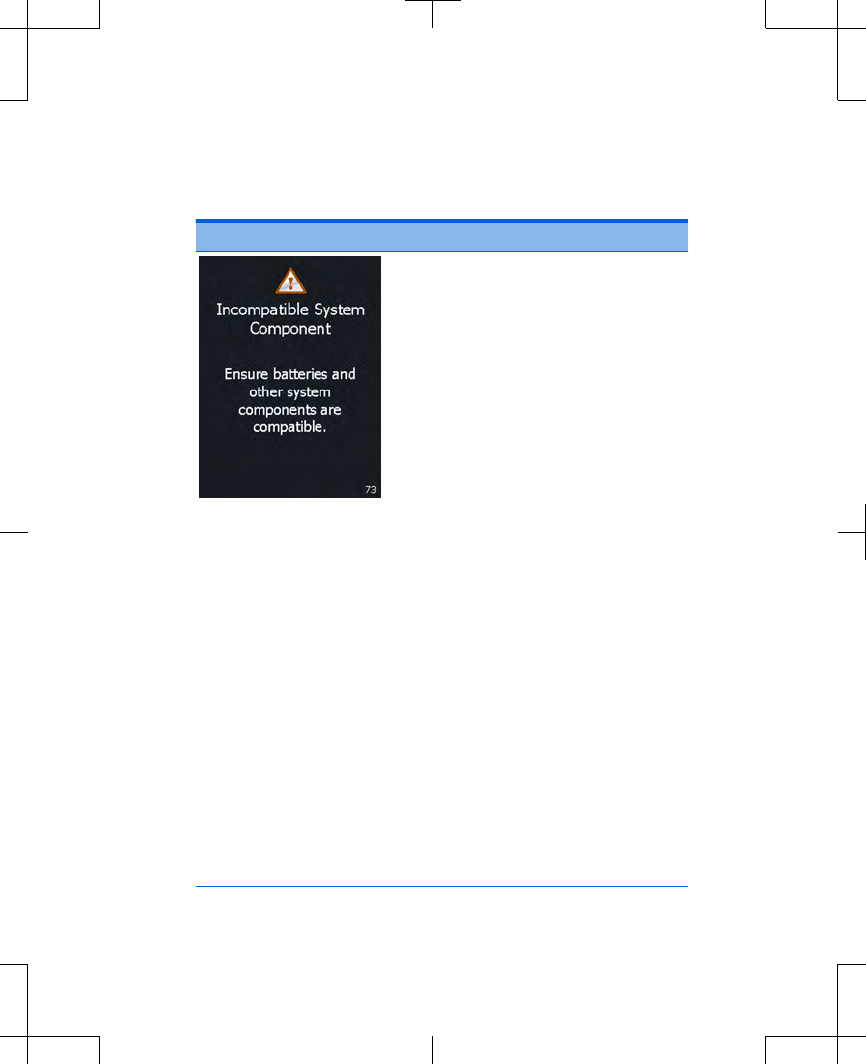
Table 3.2 Alert screens (continued)
Screen Cause and action
Cause: The system has
detected that one or more
components in the system is not
intended for use with your
system.
If you replaced the controller
batteries with new batteries
that were not given to you by
your clinician, remove the
new batteries and use correct
batteries as instructed by
your clinician.
If the issue persists, the
controller batteries may need
to be replaced. For
instructions on replacing the
controller batteries, see
"Replacing the batteries
(controller only)".
If this does not solve the
problem, write down the
message on the screen and
the screen number in the
bottom right corner, and call
your clinician.
Press the OK button to clear
the screen.
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
60
Troubleshooting 3
Notification screens
The information screens provide information
about therapy settings, error conditions, and
battery levels.
Table 3.3 describes the possible information
screens and provides instructions on how to
proceed (see blue text) when these messages
appear.
Table 3.3 Notification screens
Screen Description and action
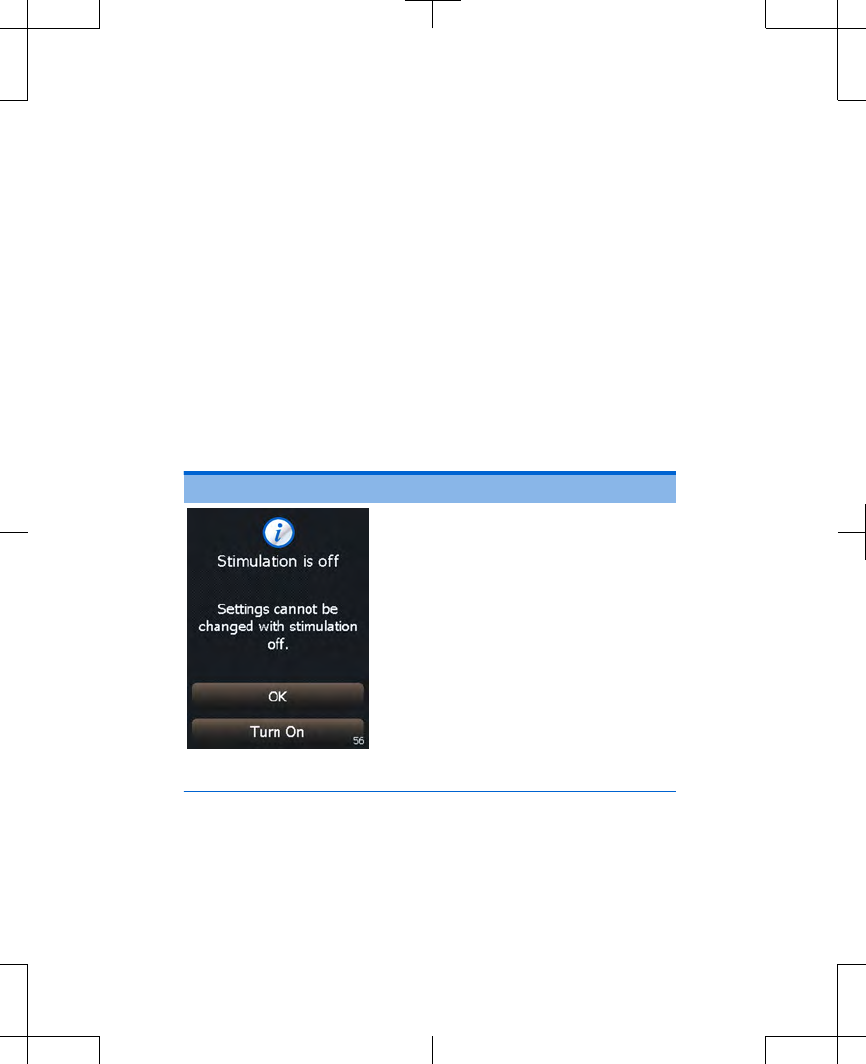
Cause: You tried to adjust your
stimulation when stimulation
was off.
Action: Press the Turn On
button on the controller
screen to turn stimulation
on, then try adjusting
stimulation again.
To keep stimulation off and
not change settings, press
the Cancel button to clear
the screen.
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
61
Troubleshooting 3
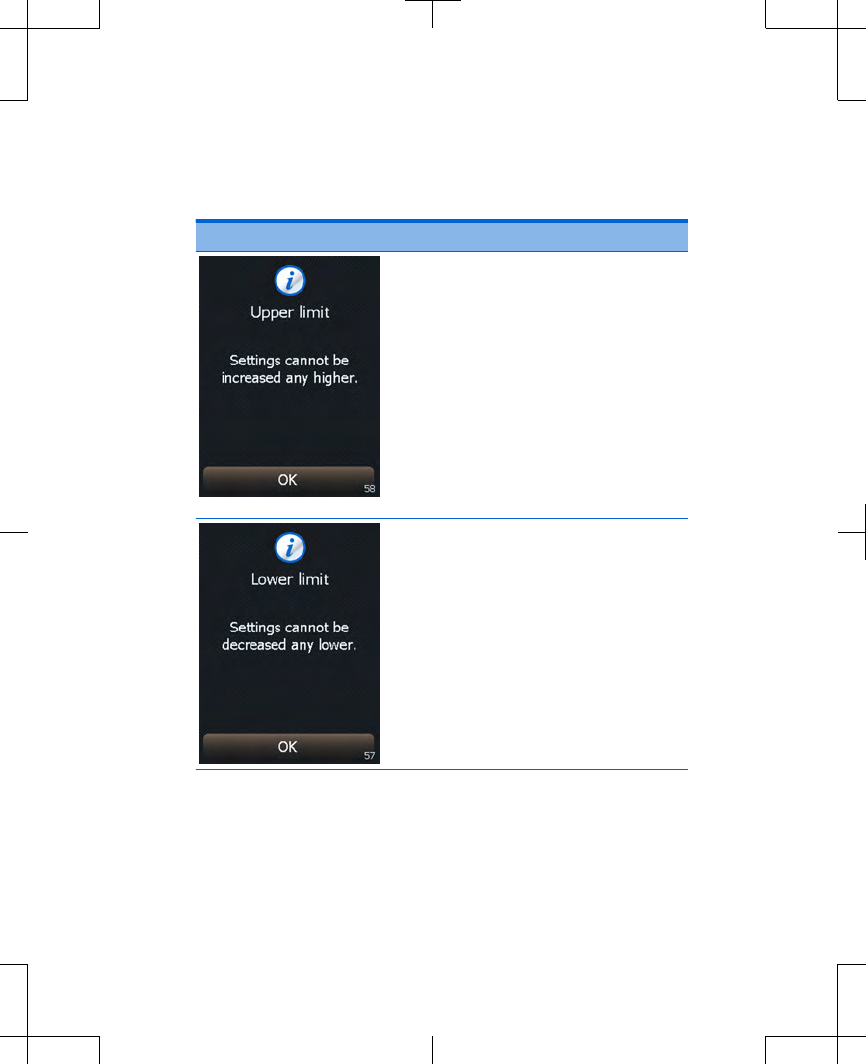
Table 3.3 Notification screens (continued)
Screen Description and action
Cause: You tried increasing
your stimulation above the
highest value allowed. You can
not increase your stimulation
any higher.
Action: Press the OK button
to clear the screen and
return to the highest allowed
setting.
If not receiving symptom
relief, call your clinician.
Cause: You tried decreasing
your stimulation below the
lowest value allowed. You can
not decrease your stimulation
below zero.
Press the OK button to clear
the screen and return to the
lowest allowed setting.
If feeling discomfort, call
your clinician.
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
62
Troubleshooting 3
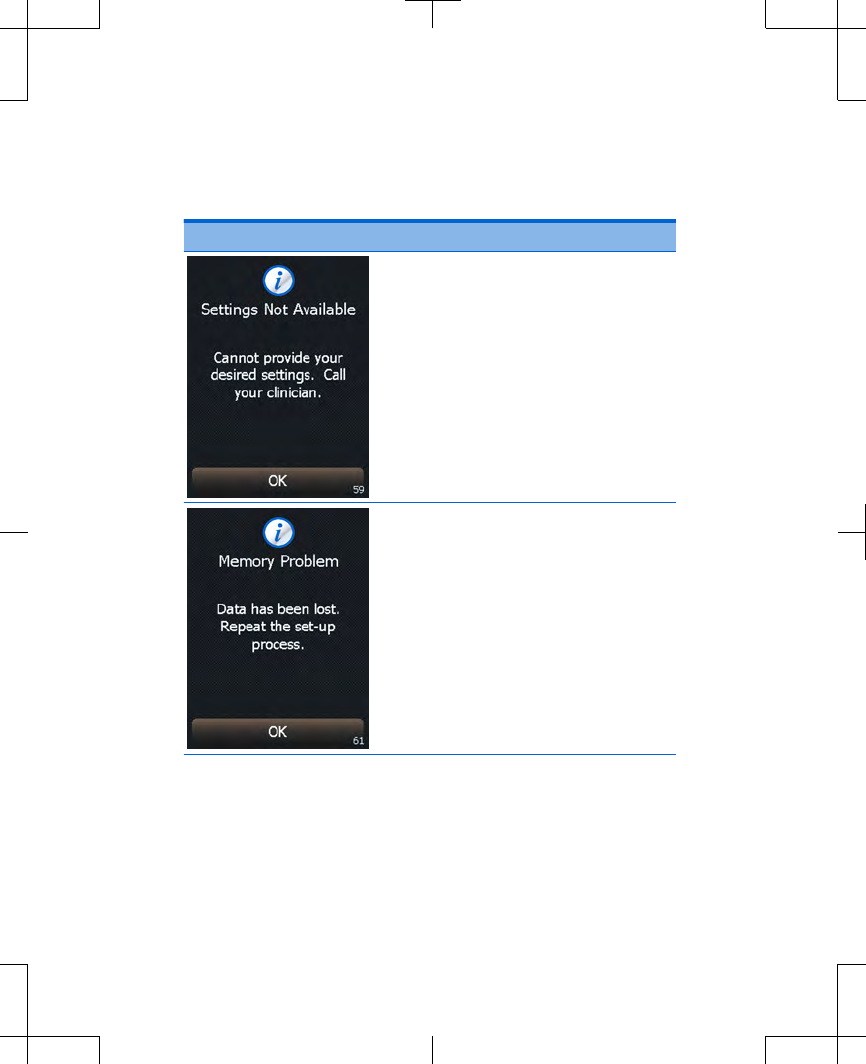
Table 3.3 Notification screens (continued)
Screen Description and action
Cause: Your combined
settings can not currently be
delivered by the external
neurostimulator.
Action: Press the OK button
to clear the screen.
If not receiving symptom
relief, call your clinician.
Cause: Controller settings are
not available.
Action: Write down the
message on the screen and
the screen number in the
bottom right corner, and call
your clinician.
Press the OK button to clear
the screen.
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
63
Troubleshooting 3
Possible problems and
solutions
Table 3.4 will help you solve problems or
identify when to call your clinician. Problems
are described in the left column (bold black
text). The right column lists possible causes of
the problem (plain text) and how to correct the
problem (bold blue text).
Note: If a problem is not solved after several
attempts, or if a problem is not described here,
contact your clinician.
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
64
Troubleshooting 3
Table 3.4 Troubleshooting problems
Problems Causes and actions
Uncomfortable or
Intolerable
stimulation
You are experiencing
side effects from the
stimulation.
1. Use your controller to
turn stimulation down or
off. See "Adjusting
stimulation" on page 27
for instructions on
adjusting stimulation. See
"Turning your stimulation
on or off" on page 22 for
instructions on turning
stimulation off.
2. If your controller is
unavailable or not
responding, press and
hold the ENS button on
the external
neurostimulator for 3
seconds.
3. If not receiving symptom
relief, call your clinician.
Stimulation area
changes
You notice an
unexpected change in
where you feel
stimulation.
Call your clinician.
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
65
Troubleshooting 3
Table 3.4 Troubleshooting problems
(continued)
Problems Causes and actions
Stimulation is too
strong 1. Turn stimulation down or
off.
See "Adjusting
stimulation" on page 27.
2. If this does not work, call
your clinician.
Not receiving
symptom relief
You think that your
stimulation may be
turned off, or that your
stimulation might not be
strong enough.
1. Check that your
stimulation is on, then
increase stimulation as
needed.
2. If this does not solve the
problem, call your
clinician.
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
66
Troubleshooting 3
Table 3.4 Troubleshooting problems
(continued)
Problems Causes and actions
Controller is
unresponsive.
The display screen is
blank when you press a
key.
1. Make sure to press only
one button. The controller
does not respond when
two or more buttons are
pressed at the same
time.
2. If this does not work,
check that the batteries
are inserted correctly as
shown in the battery
compartment.
3. If batteries are inserted
correctly, the batteries
are depleted. Replace the
controller batteries. For
instructions on replacing
controller batteries, see
"Checking and replacing
batteries" on page 38.
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
67
Troubleshooting 3
Table 3.4 Troubleshooting problems
(continued)
Problems Causes and actions
Controller
automatically resets.
Controller screen blinks
and returns to the
Unlock screen.
1. Unlock the controller. The
controller screen may
return to the screen you
were on when the
controller reset.
2. If the controller continues
to reset and you are
unable to program your
stimulation, call your
clinician.
Uncomfortable
tapping sensation
You feel a tapping
sensation that is too
slow or too fast.
1. Turn stimulation down or
off.
2. If this does not solve the
problem, call your
clinician.
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
68
Troubleshooting 3
Table 3.4 Troubleshooting problems
(continued)
Problems Causes and actions
You will be passing
through a theft
detector or security
device.
You will be using
potentially dangerous
equipment.
You will be having a
medical or dental
procedure.
Before engaging in any of
these activities, consult the
InterStim Patient Therapy
Guide for details.
WARNING: Failure to follow
the recommendations in the
InterStim Patient Therapy
Guide may injure you or
damage your InterStim
system.
External defibrillation
You received external
defibrillation.
Turn your stimulation off and
call your clinician.
Controller settings
Controller settings (eg,
brightness) are making
it difficult to adjust your
stimulation.
Call your clinician.
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
69
Troubleshooting 3
Table 3.4 Troubleshooting problems
(continued)
Problems Causes and actions
Dropped controller
Your controller fell or
was dropped.
The controller is designed to
withstand a short drop to a hard
surface and still operate
normally, even if the case is
chipped or nicked.
Turn your stimulation off and
call your clinician.
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
70
Troubleshooting 3
Table 3.4 Troubleshooting problems
(continued)
Problems Causes and actions
Fluid on the controller
Fluid was spilled onto
the controller or the
controller was dropped
into water.
The controller is not waterproof,
and water can damage the
device.
Immediately remove the
controller from the water,
then dry with a soft towel.
Remove the batteries, then
allow the battery
compartment to air dry at
room temperature for 24
hours.
Clean any spills from the
controller with a damp towel.
If the controller does not
work, or if you need to adjust
stimulation while the
controller is drying, call your
clinician.
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
71
Troubleshooting 3
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
72
Troubleshooting 3

4 Maintenance
and assistance
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
Cleaning and care
Follow these guidelines to ensure that the
controller and accessories function properly.
•
Keep the device out of the reach of children
and pets.
•
Use the device only as explained to you by
your clinician or as discussed in this
manual.
•
Handle the device with care. Do not drop,
strike, or step on the device.
•
Do not dismantle or tamper with the
device.
•
Clean the outside of the device with a damp
cloth when necessary. Mild household
cleaners will not damage the device or
labels.
•
The device is not waterproof. Do not allow
moisture to get inside the device.
•
Keep fresh batteries available.
•
Replace low or depleted batteries.
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
74
Maintenance and assistance 4
Safety and technical checks
Periodic safety and technical checks or
periodic maintenance of the controller are not
required. The controller contains no user-
serviceable parts. If repair or service is
needed, contact your clinician or a Medtronic
sales office. Refer to the Medtronic contacts at
the end of this manual.
Battery and controller disposal
Dispose of depleted batteries and worn out
devices according to local requirements.
Return your controller to your clinician.
Declaration of conformity
Medtronic declares that this product is in
conformity with the essential requirements of
Directive 1999/5/EC on Radio and
Telecommunications Terminal Equipment,
and Directive 93/42/EEC on Medical Devices.
For additional information, contact Medtronic.
Refer to the list of Medtronic contacts at the
end of this manual.
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
75
Maintenance and assistance 4
Specifications
Table 4.1 Controller specifications
Item Specification
Power source 2 AA alkaline batteries (non-
rechargeable, LR03)
Operating temperature +9 °C to +43 °C (+49 °F to
+110 °F)
Temperature limitation -30 °C to +57 °C (-22 °F to
+135 °F)
Size Approximately 12.0 cm x 6.0 cm
x 2.4 cm (4.7 in x 2.4 in x 0.9 in)
Weight, including
batteries Approximately 150 g (5.3 oz.)
Battery life 2 months (average) for alkaline
batteries
Mode of operation Continuous
Assistance for the controller
The controller has been designed and tested
to provide trouble-free service. If repair or
service is needed, contact your clinician or a
Medtronic sales office. Refer to the Medtronic
contacts at the end of this manual.
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
76
Maintenance and assistance 4
! USA
For assistance in the US, call Medtronic
Patient Services at: 1-800-510-6735. Patient
Services hours are Monday through Friday, 8
am to 5 pm Central Time.
•
If your controller stops working, first try the
steps in Chapter 3 "Troubleshooting" on
page 49.
Contact your clinician if indicated by the
troubleshooting information or if you need
additional assistance.
•
If you lose your controller, contact your
clinician to order a new controller.
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
77
Maintenance and assistance 4
Index
Adjusting stimulation 28
Assistance 76
Batteries
inserting or replacing 45
Batteries (controller)
checking 38
disposing of 75
Batteries (external neurostimulator)
checking 38
Buttons
home screen 36
Carrying case, controller 46
Changing programs 31
Checking batteries
controller 38
external neurostimulator 38
Controller
checking battery level 38, 41
cleaning and care 74
disposing of 75
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
78
Index
how to use 14
keys 16
locking 22
screens 50
specifications 76
troubleshooting 6768-71
unlocking 18
use 47
Decrease key 17
Disposing of
controller batteries 75
controller 75
Errors
troubleshooting 50
Home screen buttons 36
Home screen 34
Increase key 17
Information screens 61
Keys (controller) 16
Locking the controller 22
Neurostimulator (external)
checking battery level 38, 40
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
79
Index
Problems
troubleshooting 50
Programs
changing 31
Repair 75
Specifications 76
Stimulation On/Off key 16
Stimulation
adjusting 28
changing programs 31
switching sides 28
troubleshooting 66, 69
turning off 22, 25
turning on 22, 23
uncomfortable 65
Switching stimulation sides 28
Troubleshooting 50
Turning stimulation on or off 22
Uncomfortable stimulation
troubleshooting 65
Unlocking the controller 18
Warning screens 51
English 3537 2013-3
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
80
Index
3537 2013-3 English
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
81
Index
Contacts:
Asia: Medtronic International Ltd.
Tel. 02919-1362
Fax 02907-3998
Medtronic Asia Ltd.
Tel. (02)-548-1148
Fax (02)-518-4786
Australia: Medtronic Australasia Pty.
Ltd.
97 Waterloo Road
North Ryde, NSW 2113
Australia
Tel. +61-2-9857-9000
Fax +61-2-9878-5100
Toll free 1-800-668-6700
Austria: Medtronic Österreich GmbH
Tel. 01-240440
Fax 01-24044-100
Belgium: Medtronic Belgium S.A.
Tel. 02-456-0900
Fax 02-460-2667
Canada: Medtronic of Canada Ltd.
Tel. (1-905)-460-3800
Fax (1905)-826-6620
Czech Republic: Medtronic Czechia
s.r.o.
Tel. 2-965-795-80
Fax 2-965-795-89
Denmark: Medtronic Danmark A/S
Tel. 45-32-48-18-00
Fax 45-32-48-18-01
Finland: Medtronic Finland Oy/LTD
Tel. (09)-755-2500
Fax (09)-755-25018
France: Medtronic France S.A.S.
Tel. 01-5538-1700
Fax 01-5538-1800
Germany: Medtronic GmbH
Tel. (02159)-81490
Fax (02159)-8149100
Greece: Medtronic Hellas S.A.
Tel. 210-67-79-099
Fax 210-67-79-399
Hungary: Medtronic Hungária Kft.
Tel. 1-889-06-00
Fax 1-889-06-99
Ireland: Medtronic Ireland Ltd.
Tel. (01)-890-6522
Fax (01)-890-7220
Italy: Medtronic Italia SpA
Tel. 02-241371
Fax 02-241381
Tel. 06-328141
Fax 06-3215812
Japan: Medtronic Japan
Tel. 03-6430-2016
Fax 03-6430-7110
Latin America: Medtronic, Inc.
Tel. (1305)-500-9328
Fax (1786)-709-4244
Norway: Medtronic Norge AS
Tel. 067-10-32-00
Fax 067-10-32-10
Poland: Medtronic Poland Sp. z.o.o.
Tel. (022)-465-69-00
Fax (022)-465-69-17
Portugal: Medtronic Portugal, Lda.
Tel. 21-724-5100
Fax 21-724-5199
Russia: Medtronic Russia
Tel. (8495) 580-7377
Fax (8495) 580-7378
Slovakia Medtronic Slovakia, o.z.
Tel. 0268 206 911
Fax 0268 206 999
Spain: Medtronic Ibérica, S.A.
Tel. 91-625-0400
Fax 91-650-7410
Sweden: Medtronic AB
Tel. 08-568-585-00
Fax 08-568-585-01
Switzerland: Medtronic (Schweiz) AG
Tel. 031-868-0100
Fax 031-868-0199
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3
The Netherlands: Medtronic B.V.
Tel. (045)-566-8000
Fax (045)-566-8668
U.K.: Medtronic U.K. Ltd.
Tel. 01923-212213
Fax 01923-241004
USA: Medtronic, Inc.
Tel. (1763)-505-5000
Fax (1763)-505-1000
Toll-free: (1-800)-328-0810
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3

Manufacturer
Medtronic, Inc.
710 Medtronic Parkway
Minneapolis, MN 55432-5604
USA
www.medtronic.com
Tel. +1-763-505-5000
Fax +1-763-505-1000
Authorized Representative
EC REP
in the European Community
Medtronic B.V.
Earl Bakkenstraat 10
6422 PJ Heerlen
The Netherlands
Tel. +31-45-566-8000
Fax +31-45-566-8668
Europe/Africa/Middle East
Headquarters
Medtronic International Trading Sàrl
Route du Molliau 31
Case Postale 84
CH-1131 Tolochenaz
Switzerland
www.medtronic.eu
Tel. +41-21-802-7000
Fax +41-21-802-7900
Asia-Pacific
Medtronic International Ltd.
Suite 1106-11 16/F, Tower 1, The Gateway
25 Canton Road, Tsimshatsui
Kowloon
Hong Kong
Tel. +852-2919-1362
Fax +852-2907-3998
Contacts for specific countries are listed
inside this cover.
*M943578A001*
© Medtronic, Inc. 2013
All Rights Reserved
M943578A001
Filename Date Time
UC200xxxxxx EN
4.625″ x 6.0″ inches (117 mm x 152 mm)
Medtronic Confidential
PPManual.xsl - PatientProgrammerTemplate.fm
Version: 01-18-2012
M943578A001 Rev X 2013-3