Medtronic 97715 Implantable Neurostimulator User Manual
Medtronic, Inc. Implantable Neurostimulator
User Manual
Intellis™ 97715
Rechargeable neurostimulator
Implant manual
! USA
Rx only
2012
Filename Date Time
UC200xxxxxx EN
4.6 x 6 inches (117 mm x 152 mm)
Medtronic Confidential
ImplantManual.xsl - IPGTemplate.fm
Template version: 08-02-2011
M946871A001
2012-06

Filename Date Time
UC200xxxxxx EN
4.6 x 6 inches (117 mm x 152 mm)
Medtronic Confidential
ImplantManual.xsl - IPGTemplate.fm
Template version: 08-02-2011
M946871A001
2012-06
Explanation of symbols on product or package labeling
Refer to the appropriate product for symbols that apply.
Conformité Européenne (European Conformity). This symbol means that the
device fully complies with AIMD Directive 90/385/EEC (NB 0123) and
R&TTE Directive 1999/5/EC.
Warning
Consult instructions for use
Do not reuse
STERILIZE
2
Do not resterilize
Date of manufacture
Manufacturer
Open here
Use by
Serial number
STERILE EO
Sterilized using ethylene oxide
EC REP
Authorized Representative in the European Community
! USA
For USA audiences only
XXX °F
XX °C
-XX °F
-XX °C
Temperature limitation
97715 2012-06 English 1
2012-06
Filename Date Time
UC200xxxxxx EN
4.6 x 6 inches (117 mm x 152 mm)
Medtronic Confidential
ImplantManual.xsl - IPGTemplate.fm
Template version: 08-02-2011
M946871A001

Medtronic
®
and SoftStart/Stop
®
are trademarks of Medtronic, Inc., registered in the U.S. and
other countries.
AdaptiveStim™ and Intellis™ are trademarks of Medtronic, Inc.
2 English 97715 2012-06
2012-06
Filename Date Time
UC200xxxxxx EN
4.6 x 6 inches (117 mm x 152 mm)
Medtronic Confidential
ImplantManual.xsl - IPGTemplate.fm
Template version: 08-02-2011
M946871A001
Table of contents
Description 5
Package contents 5
Patient identification card 5
Device specifications 5
Declaration of conformity 8
Instructions for use 9
Charging the neurostimulator battery 9
Verifying neurostimulator operation 9
Connecting the extension or lead to the neurostimulator 10
Implanting the neurostimulator 11
Checking system integrity 12
Completing the implant procedure 12
Refer to the indications sheet for indications and related information.
Refer to the appropriate information for prescribers booklet for contraindications,
warnings, precautions, adverse events summary, individualization of treatment,
patient selection, use in specific populations, resterilization, and component
disposal.
Refer to System Eligibility, Battery Longevity, Specifications reference manual for
neurostimulator selection, battery longevity calculations and specific
neurostimulator specifications.
Refer to the clinical summary booklet for information on the clinical study
results of the neurostimulation system and individualization of treatment.
97715 2012-06 English 3
2012-06
Filename Date Time
UC200xxxxxx EN
4.6 x 6 inches (117 mm x 152 mm)
Medtronic Confidential
ImplantManual.xsl - IPGTemplate.fm
Template version: 08-02-2011
M946871A001

4 English 97715 2012-06
2012-06
Filename Date Time
UC200xxxxxx EN
4.6 x 6 inches (117 mm x 152 mm)
Medtronic Confidential
ImplantManual.xsl - IPGTemplate.fm
Template version: 08-02-2011
M946871A001

Description
The Medtronic Intellis Model 97715 Neurostimulator is part of a neurostimulation system for
pain therapy.
Package contents
▪
Neurostimulator
▪
Torque wrench
▪
Pocket sizer
▪
Bore plugs (2)
▪
Product literature
▪
Registration form
▪
Patient identification card
▪
Warranty card
Patient identification card
A patient identification card is packaged with this device. Advise the patient to carry the
identification card at all times.
! USA
The patient identification card packaged with the device is temporary; a permanent card
will be mailed to the patient when Medtronic receives the registration form.
The implant registration form registers the device warranties and creates a record of the device
in Medtronic’s implant data system.
Device specifications
The neurostimulator is a multi-programmable, rechargeable device that delivers stimulation
through 1 or more leads. The stimulation settings are stored in programs to target pain areas.
A program is a specific combination of pulse width, rate, and intensity settings acting on a
specific electrode combination (up to 16 electrodes per program). Up to 4 pain areas can be
targeted by programs. When stimulating more than one pain area, the pulses are delivered
sequentially—first a pulse from one program, then a pulse from the next program.
Date, pulse width, intensity, cycling, and electrode polarity for each program within the group
can have different values. Rate, rate limits, pulse width and intensity limits, and ramping for
each program within the group have the same values.
97715 2012-06 English 5
2012-06
Filename Date Time
UC200xxxxxx EN
4.6 x 6 inches (117 mm x 152 mm)
Medtronic Confidential
ImplantManual.xsl - IPGTemplate.fm
Template version: 08-02-2011
M946871A001

Table 1. Operating values for the Intellis Model 97715 Neurostimulator
Programmable parameter Operating values and ranges
a
Number of defined groups 1-3 (optional)
Number of programs per pain
area
1-3
Number of programs 12
Number of pain areas 1-4
Electrode configuration 2-16 electrodes as anode, cathode, or Off
Maximum intensity per
electrode
0-25.5 mA (0.1 mA increment)
Program intensity 0-100 mA
Intensity – limits Enabled or disabled at maximum of 25.5 mA per electrode
Pulse width 60-1000 µs (10-µs increment)
Pulse width – limits
b
Enabled or disabled at maximum of 1000 Hz
Master rate
a
10-1200 Hz (increment: 1 Hz from 10-30 Hz, 5 Hz from
30-250 Hz, 10 Hz from 250-500 Hz, 20 Hz from 500-1000 Hz,
50 Hz from 1000-1200 Hz)
Rate ratio A fraction of the master rate (1/1, 1/2, 1/3, 1/4, 1/5)
Rate limits Enabled or disabled (at maximum of 1200)
a
SoftStart/Stop Off, On: 1, 2, 4, or 8 second ramp duration
Cycling
c
Off, On: 0.1 s-30 min (resolution: 0.1 s from 0.1-1 s, 1 s from 1
s-1 min, 1 min from 1-30 min)
AdaptiveStim Off, On: 7 positions
aRate availability depends on how many pain areas are defined. For example, the maximum rate available in
one defined pain area is 1200 Hz. The maximum rate available if two pain areas are defined is 600 Hz in
each of those two pain areas.
bPulse width limits are not available when AdaptiveStim is enabled.
cCycling is not available for pain areas with AdaptiveStim enabled.
6 English 97715 2012-06
2012-06
Filename Date Time
UC200xxxxxx EN
4.6 x 6 inches (117 mm x 152 mm)
Medtronic Confidential
ImplantManual.xsl - IPGTemplate.fm
Template version: 08-02-2011
M946871A001

Table 2. Physical characteristics of the Intellis Model 97715 Neurostimulator
a
Description Value
Connector type Octapolar, in-line 2.8-mm (0.110-in) spacing
Height 57.0 mm (2.1 in)
Width 47.3 mm (1.9 in)
Thickness
case 6.3 mm (0.2 in)
connector 8.9 mm (0.4 in)
Weight 29.9 g (1.1 oz)
Volume 13.7 cm
3
Battery life 9 years before ERI
Power source Lithium ion rechargeable battery
Storage temperature –18 °C to +52 °C (0 °F to +126 °F)
Serial number model designator
b
NME
Radiopaque identification (ID) code NME
aAll measurements are approximate.
bThe serial number is the model designator followed by a number. The clinician programmer displays the
entire serial number beginning with the model designator.
97715 2012-06 English 7
2012-06
Filename Date Time
UC200xxxxxx EN
4.6 x 6 inches (117 mm x 152 mm)
Medtronic Confidential
ImplantManual.xsl - IPGTemplate.fm
Template version: 08-02-2011
M946871A001
Table 3. Material of components in the Intellis Model 97715 package
Components Material Material contacts
human tissue
Neurostimulator
Case Titanium Yes
Connector block Titanium, polysulfone, silicone
rubber, silicone medical adhesive
Yes
Grommets, seals Silicone rubber Yes
Setscrews Titanium alloy Yes
Adhesive Silicone medical adhesive Yes
Pocket sizer Polypropylene Yes
Bore plug Silicone rubber; stainless steel Yes
Torque wrench
Handle Polyetherimide Yes
Shaft Stainless steel Yes
Declaration of conformity
Medtronic declares that this product is in conformity with the essential requirements of Directive
1999/5/EC on Radio and Telecommunications Terminal Equipment and Directive 90/385/EEC
on Active Implantable Medical Devices.
For additional information, contact the appropriate Medtronic representative listed on the inside
back cover of this manual.
8 English 97715 2012-06
2012-06
Filename Date Time
UC200xxxxxx EN
4.6 x 6 inches (117 mm x 152 mm)
Medtronic Confidential
ImplantManual.xsl - IPGTemplate.fm
Template version: 08-02-2011
M946871A001

Instructions for use
Implanting physicians should be experienced in epidural-access procedures and should be
thoroughly familiar with all product labeling.
w
Warning: DO NOT use the recharger on an unhealed wound. The recharging system
is not sterile, and contact with the wound can cause an infection.
#
Caution: If the neurostimulator is not being used for an extended period of time,
recommend that your patient charge the neurostimulator at least once per year. If the
battery is discharged, stimulation will stop and the neurostimulator may not
communicate with the controller.
#
Caution: Advise patients to charge the neurostimulator when a low battery message is
displayed on the controller in order to maintain uninterrupted therapy from the
neurostimulator. If the battery is discharged, stimulation will stop and the
neurostimulator may not communicate with the controller.
Note: The patient will be able to use the controller and recharger to charge a discharged
battery without causing damage to the battery or the neurostimulator.
#
Cautions:
▪
When using sharp instruments near the neurostimulator, be extremely careful to
avoid nicking or damaging the case, the insulation, or the connector block.
Damaging the neurostimulator may require surgical replacement.
▪
Do not use saline or other ionic fluids at connections, which could result in a short
circuit.
Charging the neurostimulator battery
Check the battery level of the neurostimulator before opening the package, and recharge the
neurostimulator if the battery is low. For charging instructions, refer to the recharging system
user manual. If the patient will be sent home with stimulation on, charge the neurostimulator
in the package before implant.
Verifying neurostimulator operation
Before opening the sterile neurostimulator package, verify that the neurostimulator is operable
by using the clinician programmer to interrogate the neurostimulator and read the
neurostimulator battery charge level. (Refer to the programming guide for instructions on how
to read the battery charge level.)
#
Caution: Do not implant a neurostimulator if it was dropped onto a hard surface from a
height of 30 cm (12 in) or more, because the neurostimulator may be damaged and fail
to operate properly.
Note: The neurostimulator pocket may be flushed with an antibiotic solution; do not submerge
the neurostimulator in fluid.
97715 2012-06 English 9
2012-06
Filename Date Time
UC200xxxxxx EN
4.6 x 6 inches (117 mm x 152 mm)
Medtronic Confidential
ImplantManual.xsl - IPGTemplate.fm
Template version: 08-02-2011
M946871A001
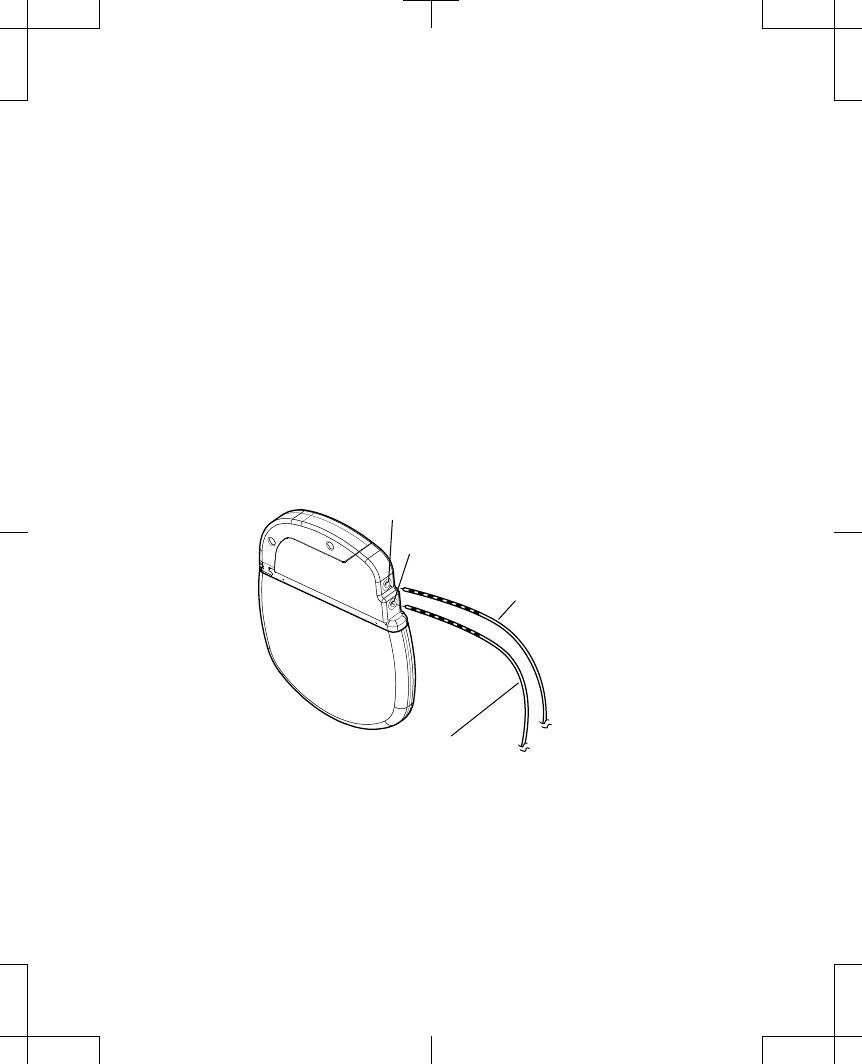
Connecting the extension or lead to the neurostimulator
#
Caution: Before connecting components, wipe off any body fluids and dry all
connections. Fluids in the connection may result in stimulation at the connection site,
intermittent stimulation, or loss of stimulation.
1. Wipe the extension or lead connector pins with sterile gauze. If necessary, use sterile
(United States Pharmacopeia [USP]) water or a nonionic antibiotic solution.
2. Make sure the connector block receptacles are dry and clean.
3. Insert the appropriate extension or lead connector pins into the appropriate
neurostimulator socket until they are seated fully within the connector block (Figure 1).
Notes:
▪
During insertion, some resistance is typical.
▪
To retract the setscrews, insert the torque wrench into the self-sealing grommet and
rotate the setscrews counterclockwise; however, do not remove the setscrews from
the connector block.
#
Caution: Do not insert the extension or lead connector into the connector block if
the setscrews are not sufficiently retracted. If the setscrews are not retracted, the
setscrews may damage the extension or lead and the extension or lead will not be
seated fully into the connector block.
Socket II (Electrodes 8-15)
Extension or Lead 2
Extension or Lead 1
Socket I (Electrodes 0-7)
Figure 1. Insert the extension or lead connector pins fully into the neurostimulator.
10 English 97715 2012-06
2012-06
Filename Date Time
UC200xxxxxx EN
4.6 x 6 inches (117 mm x 152 mm)
Medtronic Confidential
ImplantManual.xsl - IPGTemplate.fm
Template version: 08-02-2011
M946871A001
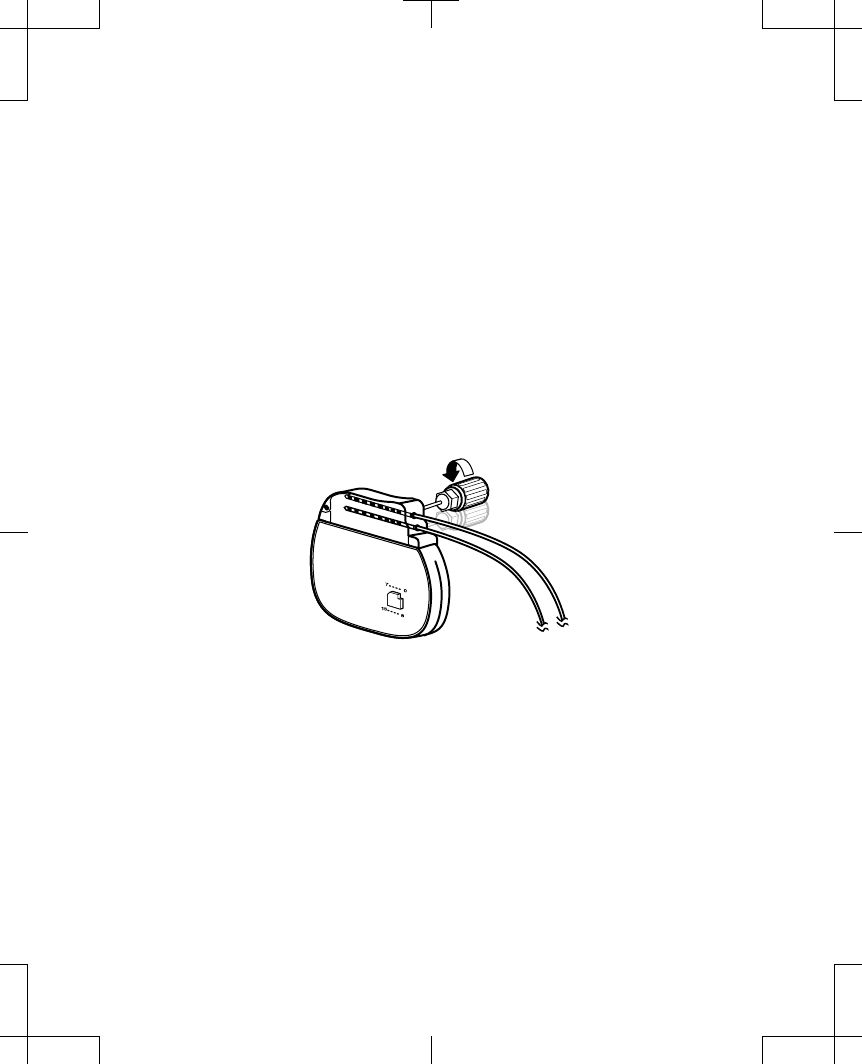
Note: Insert a connector plug (from an accessory kit) into any unused neurostimulator
socket.
4. For each extension, lead, or plug, fully insert the torque wrench (packaged with the
rechargeable neurostimulation system) into each self-sealing grommet of the connector
block and tighten each setscrew (Figure 2).
#
Cautions:
▪
Be sure the torque wrench is fully inserted into the self-sealing grommet. If the
torque wrench is not fully inserted, the setscrew may be damaged, resulting
in intermittent or loss of stimulation.
▪
Before tightening setscrews, ensure that the extension or lead connector pins
are inserted into the connector block to prevent damaging the lead or
extension.
▪
Verify that each leaf of the self-sealing grommet is closed after the torque
wrench is withdrawn. If fluid leaks through a grommet seal that is not fully
closed, the patient may experience shocking, burning, or irritation at the
neurostimulator implant location, or intermittent stimulation, or loss of
stimulation.
Figure 2. Tightening the setscrews in the self-sealing grommet.
Implanting the neurostimulator
1. Place the neurostimulator into the subcutaneous pocket using the pocket sizer if desired
and ensure that the extension or lead is not bent sharply. The neurostimulator can be
implanted and charged with either side facing outward. for optimal recharging, place the
neurostimulator within 1-2 cm (0.8 in.) of the skin surface.
#
Cautions:
▪
Ensure that the neurostimulator is placed no deeper than 3 cm (1.2 in) below
the skin and is parallel to the skin. If the neurostimulator is too deep or is not
parallel to the skin, recharge may be inefficient or unsuccessful.
97715 2012-06 English 11
2012-06
Filename Date Time
UC200xxxxxx EN
4.6 x 6 inches (117 mm x 152 mm)
Medtronic Confidential
ImplantManual.xsl - IPGTemplate.fm
Template version: 08-02-2011
M946871A001
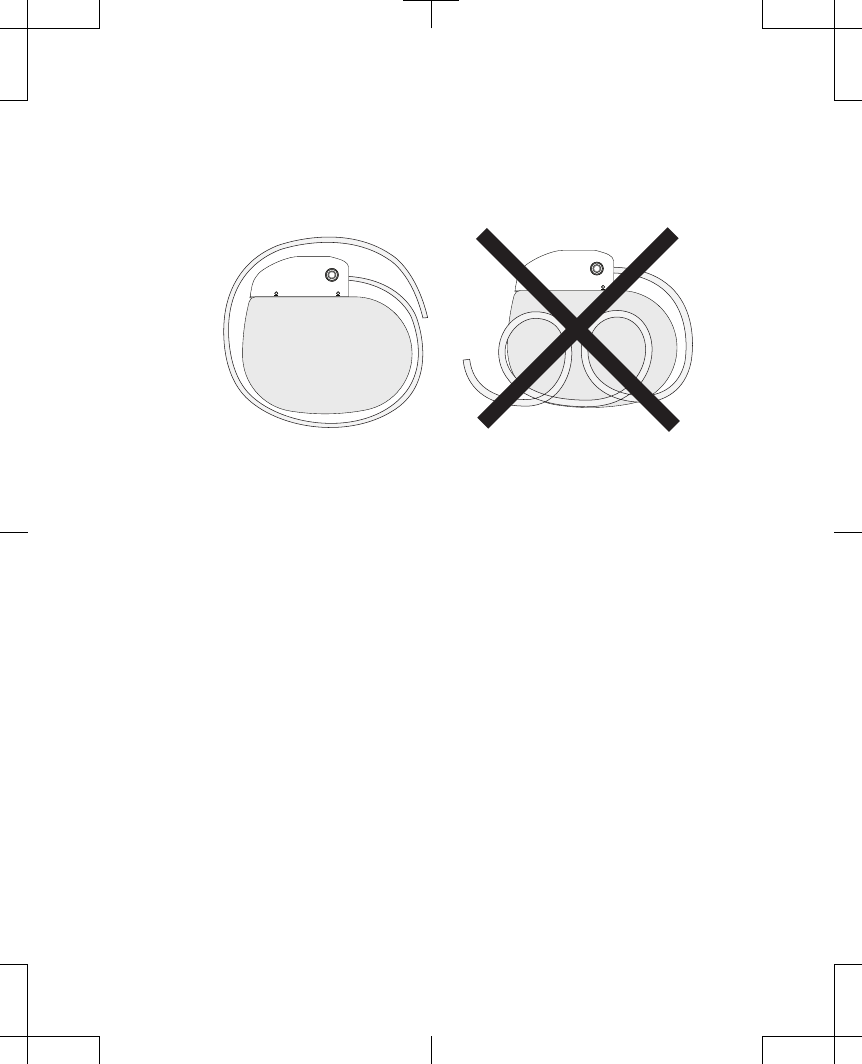
▪
Do not coil excess extension in front of the neurostimulator. Wrap excess
extension or leads around the perimeter (Figure 3) or behind the
neurostimulator to help minimize potential damage during neurostimulator
replacement surgery, help minimize potential kinking of the extension or lead,
and minimize interference with telemetry and recharge operation.
Figure 3. Wrap excess extension around the perimeter of the neurostimulator.
2. Use the suture holes in the connector block to secure the neurostimulator to the muscle
fascia with nonabsorbable silk.
Note: Secure the neurostimulator in the pocket to minimize movement or migration of
the neurostimulator.
Checking system integrity
The connections of the extensions and leads to the neurostimulator can be checked using the
clinician programmer. Refer to the programming guide for detailed programming
instructions.
w
Warning: To use the nonsterile programmer system components in a sterile field, place
a sterile barrier between the patient and system components to prevent infection. Do not
sterilize any components of the programmer system. Sterilization may damage the
components.
1. To ensure proper connection of each extension or lead to the neurostimulator, use the
clinician programmer to perform the lead insertion check.
2. If the lead insertion check results are not acceptable, refer to "Connecting the extension
or lead to the neurostimulator".
Completing the implant procedure
1. Close and dress all incisions.
2. Turn off stimulation before sending your patient home.
3. Ensure that a patient control device is given to the patient.
12 English 97715 2012-06
2012-06
Filename Date Time
UC200xxxxxx EN
4.6 x 6 inches (117 mm x 152 mm)
Medtronic Confidential
ImplantManual.xsl - IPGTemplate.fm
Template version: 08-02-2011
M946871A001

4. Complete the device tracking and patient registration paperwork and return the
documents to Medtronic.
97715 2012-06 English 13
2012-06
Filename Date Time
UC200xxxxxx EN
4.6 x 6 inches (117 mm x 152 mm)
Medtronic Confidential
ImplantManual.xsl - IPGTemplate.fm
Template version: 08-02-2011
M946871A001

Contacts:
Asia:
Medtronic International Ltd.
Tel. 02891-4068
Fax 02591-0313
Medtronic Asia Ltd.
Tel. (02)-548-1148
Fax (02)-518-4786
Australia:
Medtronic Australasia Pty. Ltd.
97 Waterloo Road
North Ryde, NSW 2113
Australia
Tel. +61-2-9857-9000
Fax +61-2-9878-5100
Toll free 1-800-668-6700
Austria:
Medtronic Österreich GmbH
Tel. 01-240440
Fax 01-24044-100
Belgium:
Medtronic Belgium S.A.
Tel. 02-456-0900
Fax 02-460-2667
Canada:
Medtronic of Canada Ltd.
Tel. (1-905)-460-3800
Fax (1905)-826-6620
Czech Republic:
Medtronic Czechia s.r.o.
Tel. 2-965-795-80
Fax 2-965-795-89
Denmark:
Medtronic Danmark A/S
Tel. 45-32-48-18-00
Fax 45-32-48-18-01
Finland:
Medtronic Finland Oy/LTD
Tel. (09)-755-2500
Fax (09)-755-25018
France:
Medtronic France S.A.S.
Tel. 01-5538-1700
Fax 01-5538-1800
Germany:
Medtronic GmbH
Tel. (02159)-81490
Fax (02159)-8149100
Greece:
Medtronic Hellas S.A.
Tel. 210-67-79-099
Fax 210-67-79-399
Hungary:
Medtronic Hungária Kft.
Tel. 1-889-06-00
Fax 1-889-06-99
Ireland:
Medtronic Ireland Ltd.
Tel. (01)-890-6522
Fax (01)-890-7220
Italy:
Medtronic Italia SpA
Tel. 02-241371
Fax 02-241381
Tel. 06-328141
Fax 06-3215812
Japan:
Medtronic Japan
Tel. 3-6430-2016
Fax 3-6430-7110
Latin America:
Medtronic, Inc.
Tel. (1305)-500-9328
Fax (1786)-709-4244
Norway:
Medtronic Norge AS
Tel. 067-10-32-00
Fax 067-10-32-10
Poland:
Medtronic Poland Sp. z.o.o.
Tel. (022)-465-69-00
Fax (022)-465-69-17
Portugal:
Medtronic Portugal, Lda.
Tel. 21-724-5100
Fax 21-724-5199
Russia:
Medtronic Russia
Tel. (8495) 580-7377
Fax (8495) 580-7378
Slovakia
Medtronic Slovakia, o.z.
Tel. 0268 206 911
Fax 0268 206 999
Spain:
Medtronic Ibérica, S.A.
Tel. 91-625-0400
Fax 91-650-7410
Sweden:
Medtronic AB
Tel. 08-568-585-00
Fax 08-568-585-01
Filename Date Time
UC200xxxxxx EN
4.6 x 6 inches (117 mm x 152 mm)
Medtronic Confidential
ImplantManual.xsl - IPGTemplate.fm
Template version: 08-02-2011
2012-06
M946871A001

Switzerland:
Medtronic (Schweiz) AG
Tel. 031-868-0100
Fax 031-868-0199
The Netherlands:
Medtronic B.V.
Tel. (045)-566-8000
Fax (045)-566-8668
U.K.:
Medtronic U.K. Ltd.
Tel. 01923-212213
Fax 01923-241004
USA:
Medtronic, Inc.
Tel. (1763)-505-5000
Fax (1763)-505-1000
Toll-free: (1-800)-328-0810
Filename Date Time
UC200xxxxxx EN
4.6 x 6 inches (117 mm x 152 mm)
Medtronic Confidential
ImplantManual.xsl - IPGTemplate.fm
Template version: 08-02-2011
M946871A001
2012-06

Manufacturer
Medtronic, Inc.
710 Medtronic Parkway,
Minneapolis, MN 55432-5604,
USA.
www.medtronic.com
Tel. +1-763-505-5000
Fax +1-763-505-1000
Authorized Representative
EC REP
in the European Community
Medtronic B.V.
Earl Bakkenstraat 10,
6422 PJ Heerlen,
The Netherlands
Tel. +31-45-566-8000
Fax +31-45-566-8668
Europe/Africa/Middle East
Headquarters
Medtronic International Trading Sàrl
Route du Molliau 31,
Case Postale 84
CH-1131 Tolochenaz,
Switzerland
www.medtronic.eu
Tel. +41-21-802-7000
Fax +41-21-802-7900
Asia-Pacific
Medtronic International Ltd.
Suite 1602 16/F, Manulife Plaza,
The Lee Gardens, 33 Hysan Avenue,
Causeway Bay,
Hong Kong
Tel. +852-2891-4068
Fax +852-2591-0313
Contacts for specific countries are listed inside this cover.
*M946871A001*
© Medtronic, Inc. 2012
All Rights Reserved
M946871A001
Filename Date Time
UC200xxxxxx EN
4.6 x 6 inches (117 mm x 152 mm)
Medtronic Confidential
ImplantManual.xsl - IPGTemplate.fm
Template version: 08-02-2011
2012-06
M946871A001