270562a5 6b9d 42ad 93a4 866482bd38e5
2017-08-11
: Pdf 270562A5-6B9D-42Ad-93A4-866482Bd38E5 270562a5-6b9d-42ad-93a4-866482bd38e5 8 2017 pdf
Open the PDF directly: View PDF ![]() .
.
Page Count: 3
+ + =
mL mL mL mL
EXPAREL Bupivacaine
0.25%
Normal
Saline
Total
120
505020
This case report represents the individual experience of Dr Stan Dysart and is intended to demonstrate his methodology for
using EXPAREL in a specific orthopedic procedure.
Pacira Pharmaceuticals, Inc., recognizes that there are alternative methodologies for administering local anesthetics, as well
as individual patient considerations, when selecting the dose for a specific procedure.
EXPAREL is indicated for administration into the surgical site to produce postsurgical analgesia.
CASE INFORMATION
Physician Name Stan Dysart, MD
Affiliation Pinnacle Orthopaedics/Wellstar Health System
Surgical Case Performed Total knee arthroplasty (TKA)
Inpatient or Outpatient Procedure Inpatient
PROCEDURAL DETAILS
Incision Size 15 cm
Preoperative Analgesics Used AC block—20 mL of 0.25% bupivacaine with epinephrine
Intraoperative Analgesics Used
TIVA general—150-200 mcg/kg/min propofol titrated based on surgical needs;
50-100 mcg fentanyl as needed
Periarticular injection with 20 mL EXPAREL and 50 mL 0.25% bupivacaine
Dose of EXPAREL and
Total Volume Used
The recommended dose of EXPAREL is based on the size of the surgical site, the volume required to cover the area, and individual patient
factors that may impact the safety of an amide local anesthetic. The maximum dose of EXPAREL should not exceed 266 mg.
EXPAREL can be administered undiluted (20 mL) or diluted to increase volume up to a total of 300 mL (final concentration of 0.89 mg/mL
[ie, 1:14 dilution by volume]) with normal (0.9%) saline or lactated Ringer’s solution.
Bupivacaine HCl may be administered immediately before EXPAREL or admixed in the same syringe, as long as the ratio of the milligram
dose of bupivacaine HCl to EXPAREL does not exceed 1:2. Admixing may impact the pharmacokinetic and/or physiochemical properties
of EXPAREL, and this effect is concentration dependent. The toxic effects of these drugs are additive and their administration should
be used with caution, including monitoring for neurological and cardiovascular effects related to toxicity. Other than with bupivacaine,
EXPAREL should not be admixed with other drugs prior to administration.
Please see Important Safety Information on the last page and refer to the accompanying full Prescribing Information for
complete Dosage and Administration information before using EXPAREL.
PATIENT CHARACTERISTICS
Gender Male
Age 81 years
Patient History and Characteristics Patient previously underwent a successful left TKA
Pathology Patient has right knee osteoarthritis and is now undergoing a right TKA
with an ERAS protocol
AC, adductor canal; ERAS, enhanced recovery after surgery; TIVA, total intravenous anesthesia.
Administration Case Report With EXPAREL
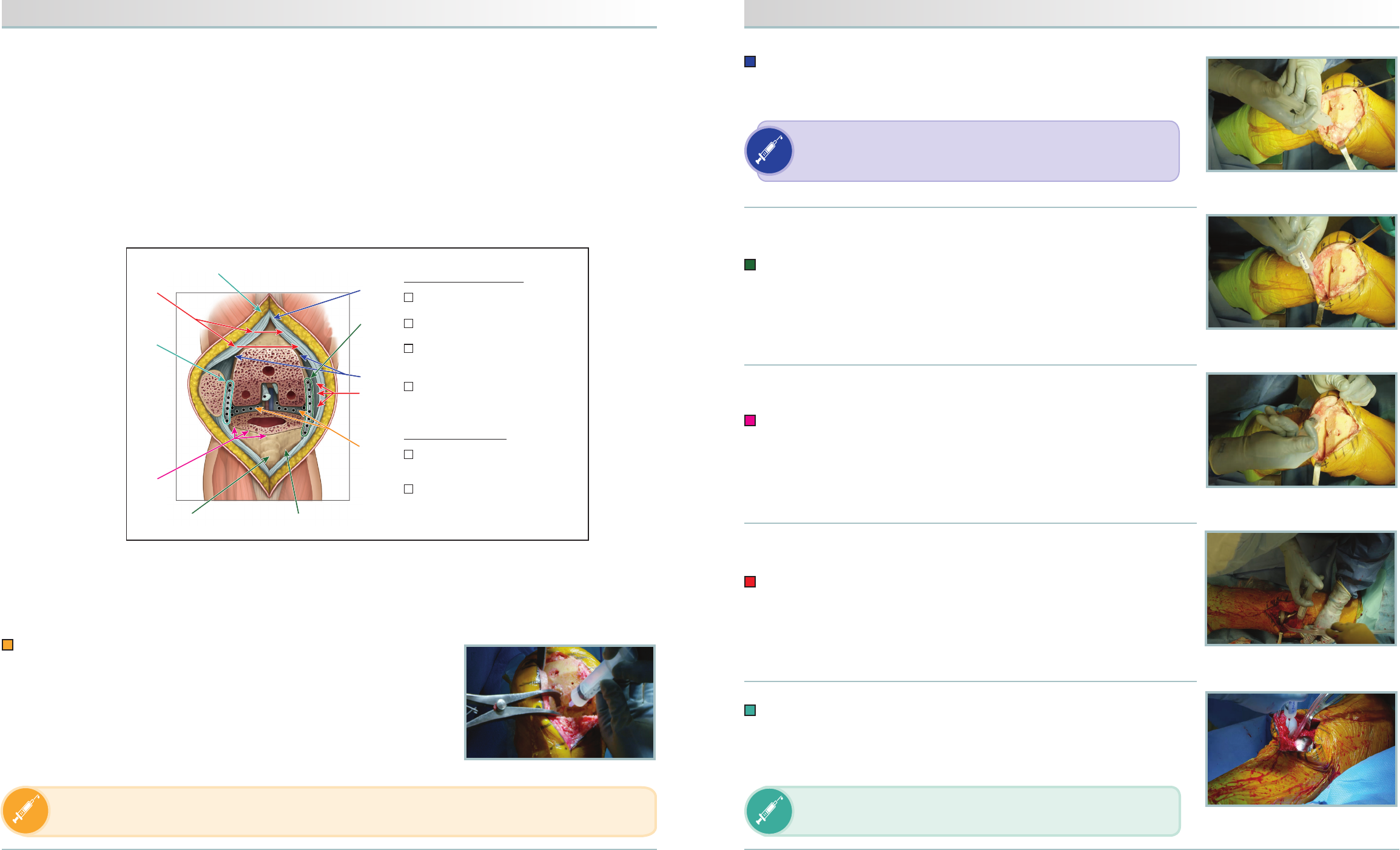
#6
#6
#4
#5
#3
#2
#2
#3#3
#5
#1
PRIOR TO CEMENTATION
■Syringe #1: Posterior capsule
■Syringe #2: Femur
■Syringe #3: Tibia, pes anserinus,
medial collateral ligament (MCL), gutter
■Syringe #4: Circumferential
periosteum
AFTER CEMENTATION
■Syringe #5: Quadriceps tendon,
retinaculum, medial gutter
■Syringe #6: Lateral gutter,
subcutaneous tissue
Syringe #2:
Medial and lateral infiltration of femoral periosteal/synovial tissues and of
suprapatellar tissue with 20 needle sticks of 1 mL to 1.5 mL per injection
Syringe #3:
Injection of fat pad, pes anserinus, MCL, and medial gutter,
saturating the area
Syringe #4:
Medial and lateral infiltration of the circumferential periosteum
of the tibia using 15 to 20 needle sticks
Syringe #5:
Injection of the synovial tissue beneath the quadriceps tendon
and the retinacular tissue medially from the femur to the tibia
Syringe #6:
Injection of the lateral gutter and the lateral retinacular tissue from
the femur to the tibia. Residual volume is used in the subcutaneous tissue
medially and laterally. There will likely be swelling of the tissue from fluid volume
Inject until a noticeable bubble forms. It is normal for there to be
more dramatic swelling in this thick, fibrous layer than when soft
tissue is infiltrated.
INFILTRATION NOTES (cont)
#
INFILTRATION NOTES
Please see Important Safety Information on the last page and refer to the accompanying full Prescribing Information for
complete Dosage and Administration information before using EXPAREL.
ASSESSED THE SIZE OF THE SURGICAL SITE AND DEPTH OF TISSUE, THEN PREPARED INJECTION
MATERIALS ACCORDINGLY
In this procedure, Dr Dysart determined that a total volume of approximately 120 mL would be needed to cover the
surgical site. He expanded 20 mL of EXPAREL with 50 mL of normal saline and admixed this solution with 50 mL of
0.25% bupivacaine. Dr Dysart added bupivacaine to provide short-term local analgesia in the postanesthesia care
unit that overlapped with the long-term local analgesia provided by EXPAREL.
DIVIDED INJECTATE INTO SYRINGES WITH NEEDLE GAUGES APPROPRIATE FOR INFILTRATION (20- TO 25-GAUGE)
AND PLANNED WHICH AREAS TO INFILTRATE WITH EACH INJECTION
For this procedure, Dr Dysart divided the injectate evenly into six 20-mL syringes using a 21-gauge needle and infiltrated
as follows:
FIGURE 4. Circumferential periosteum
of tibia
FIGURE 5. Synovial tissue (quadriceps)
and medial retinacular tissue (femur to tibia)
FIGURE 6. Lateral gutter and retinacular
tissue (femur to tibia)
FIGURE 2. Femoral periosteal/synovial
and suprapatellar tissues
FIGURE 3. Fat pad, pes anserinus, MCL,
and medial gutter
INFILTRATED AFTER THE BONY CUTS WERE PERFORMED
After completing the bony cuts, Dr Dysart inserted a laminar spreader between the cut femur and tibia,
exposing the posterior capsule of the knee. He then proceeded with infiltrating the injectate as follows:
Syringe #1:
Medial and lateral infiltration of the posterior capsule
• Medial infiltration of posterior capsule with approximately 10 needle sticks
to create a field block
• Lateral infiltration of posterior capsule with approximately 10 needle sticks
Before each injection, be sure to aspirate to minimize the risk of intravascular injection. Be sure not to inject
too far laterally, and monitor the volume injected because of the proximity of the peroneal nerve.
Adapted with permission; International Guidelines Center (guidelinecentral.com) —Erin Daniel, illustrator.
FIGURE 1. Posterior capsule
When infiltrating, stay in the tissue to reduce the amount
of extravasation.

Maker of EXPAREL® (bupivacaine liposome injectable suspension)
Maker of EXPAREL
®
(bupivacaine liposome injectable suspension)
Pacira Pharmaceuticals is the maker of EXPAREL®
Important Safety Information
EXPAREL is contraindicated in obstetrical paracervical block anesthesia.
In clinical trials, the most common adverse reactions (incidence ≥10%) following EXPAREL administration were nausea,
constipation, and vomiting.
EXPAREL is not recommended to be used in the following patient population: patients <18 years old and/or
pregnant patients.
Because amide-type local anesthetics, such as bupivacaine, are metabolized by the liver, EXPAREL should be used
cautiously in patients with hepatic disease. Patients with severe hepatic disease, because of their inability to metabolize
local anesthetics normally, are at a greater risk of developing toxic plasma concentrations.
Warnings and Precautions Specific to EXPAREL
EXPAREL is not recommended for the following types or routes of administration: epidural, intrathecal, regional nerve
blocks, or intravascular or intra-articular use.
Non-bupivacaine-based local anesthetics, including lidocaine, may cause an immediate release of bupivacaine from
EXPAREL if administered together locally. The administration of EXPAREL may follow the administration of lidocaine after a
delay of 20 minutes or more. Formulations of bupivacaine other than EXPAREL should not be administered within 96 hours
following administration of EXPAREL.
Warnings and Precautions for Bupivacaine-Containing Products
Central Nervous System (CNS) Reactions: There have been reports of adverse neurologic reactions with the use of
local anesthetics. These include persistent anesthesia and paresthesias. CNS reactions are characterized by excitation
and/or depression.
Cardiovascular System Reactions: Toxic blood concentrations depress cardiac conductivity and excitability which may
lead to dysrhythmias sometimes leading to death.
Allergic Reactions: Allergic-type reactions (eg, anaphylaxis and angioedema) are rare and may occur as a result of
hypersensitivity to the local anesthetic or to other formulation ingredients.
Chondrolysis: There have been reports of chondrolysis (mostly in the shoulder joint) following intra-articular infusion of
local anesthetics, which is an unapproved use.
Disclosure: Dr Dysart is a paid consultant for Pacira Pharmaceuticals, Inc.
PROPER TECHNIQUE IS CRUCIAL FOR ANALGESIC COVERAGE
When infiltrating EXPAREL, Dr Dysart makes sure to infiltrate below the fascia, above the fascia,
and into the subcutaneous tissue using a moving needle technique. With a moving needle
technique, the injections are spread in a rapid and precise fan-like pattern to maximize the
number of injection areas. The tissues are infiltrated as the needle is advanced and withdrawn
to maximize the coverage area. This technique should be systematically and meticulously
repeated with each subsequent injection site, and the next site should overlap
with the prior infiltrated area to maximize effect.
EXPAREL Bupivacaine
INFILTRATION NOTES (cont)
Watch Dr Dysart infiltrate with EXPAREL at www.EXPAREL.com
©2017 Pacira Pharmaceuticals, Inc., Parsippany, NJ 07054 PP-EX-US-2065 01/17