Bay Pass Manual 2.1
User Manual: Pdf
Open the PDF directly: View PDF ![]() .
.
Page Count: 40
Contents
1 Overview 4
2 Before you start 4
2.1 How to get BayPass?...................... 4
2.2 How to compile BayPass?.................... 5
2.3 Input file format ......................... 6
2.3.1 The genotyping data file [always required] ......... 6
2.3.2 The pool (haploid) size file [only required for Pool–Seq data] 8
2.3.3 The covariate data file [required for the covariate modes] . . 8
2.3.4 The covariance matrix file [optional, required for the AUX
covariate mode] ....................... 9
3 Running BayPass 9
3.1 Overview of the different models available in BayPass .... 9
3.1.1 The core model ...................... 10
3.1.2 The standard covariate model and extensions ..... 11
3.1.3 The auxiliary covariate model .............. 12
3.2 Detailed overview of all the options ............... 15
3.3 Format of the output files .................... 21
4 Miscellaneous R functions 24
4.1 The R function simulate.baypass() ................ 25
4.1.1 Description ........................ 25
4.1.2 Usage ........................... 25
4.1.3 Arguments (in alphabetic order) ............. 25
4.1.4 Values ........................... 27
4.1.5 Examples ......................... 29
4.2 The R function fmd.dist() .................... 29
4.2.1 Description ........................ 29
4.2.2 Usage ........................... 29
4.2.3 Arguments ......................... 29
4.2.4 Values ........................... 30
4.2.5 Example .......................... 30
4.3 The R function geno2YN() .................... 30
4.3.1 Description ........................ 30
4.3.2 Usage ........................... 30
4.3.3 Arguments ......................... 30
4.3.4 Values ........................... 30
4.3.5 Example .......................... 31
2
5 Worked Examples 31
5.1 Cattle allele count data ...................... 31
5.1.1 Analysis under the core model mode: MCMC is run
under the core model ................... 31
5.1.2 Analysis under the IS covariate mode: MCMC is run
under the core model ................... 33
5.1.3 Analysis under the MCMC covariate mode: MCMC is
run under the STD model ................ 34
5.1.4 Analysis under the AUX covariate mode: MCMC is
run under the AUX model ................ 35
5.2 Littorina Pool–Seq read count data ............... 36
6 Credits 36
7 Copyright 37
8 Contact 37
Bibliography 38
Appendix A Comparisons of the computational efficiency of the
different version of BayPass 40
3
1 Overview
The package BayPass is a population genomics software which is primarily
aimed at identifying genetic markers subjected to selection and/or associated
to population-specific covariates (e.g., environmental variables, quantitative
or categorical phenotypic characteristics). The underlying models explicitly
account for (and may estimate) the covariance structure among the popula-
tion allele frequencies that originates from the shared history of the popula-
tions under study. Note that, apart from standard population genetics stud-
ies, BayPass is generic enough to be also suited to the analyses of data from
other kinds of experiments in which the allele frequency covariance structure
is simpler (e.g., experimental evolution). The genetic data typically consists
of allele (when derived from individual genotype calls) or read (when derived
from Pool–Seq experiments) counts at several markers (for now, BayPass is
restricted to the analysis of bi–allelic markers) in several populations. Note
that BayPass can handle missing data (no count available in one or several
populations) which might be helpful in some contexts.
The core BayPass model is based on the Bayenv model which was
proposed by Coop et al. (2010) and G¨
unther and Coop (2013). However,
as detailed in Gautier (2015), in addition to a complete and independant
reprogramming of the core Markov Chain Monte Carlo (MCMC) algorithm,
BayPass allows to monitor most of the parameters and the priors of the
original models and to introduce various extensions (via e.g., the optional
addition of hyper–parameters, the modeling of spatial dependency among
consecutive markers).
BayPass is written in Fortran90. The source code and compilation in-
structions for various platforms (OS X, Windows, Linux) are available. The
executable reads data file(s) supplied by the user, and a number of options
can be passed through the command line. Some R functions are also provided
in the package to facilitate interpretation of the resulting outputs.
This document provides information about how to format the data file,
how to specify the user-defined parameters, and how to interpret the results.
2 Before you start
2.1 How to get BayPass?
Download the archive from http://www1.montpellier.inra.fr/CBGP/software/
baypass/, and extract it from a terminal:
tar -zxvf baypass_2.1.tar.gz
4

2.2 How to compile BayPass?
The source files are to be found in the src subdirectory. BayPass is coded
in Fortran90 and can therefore be compiled for any system supporting a For-
tran90 compiler using the provided Makefile. This Makefile is designed
to work with either the free compiler gfortran1or the commercial ifort
intelrFortran compiler2. As a consequence, using another Fortran90 com-
piler requires modifying the Makefile accordingly. Note also that BayPass
uses OpenMP (http://openmp.org/wp/) to implement multithreading, which
allows parallel calculation on computer systems that have multiple CPUs or
CPUs with multiple cores. Users thus have to make sure that the correspond-
ing libraries are installed (which is usually the case, at Linux OS or following
compiler installation previously described1). The following instructions run
within the src subdirectory allows to compile the code and to produce a
binary:
•using the gfortran free compiler (the command should automatically
produce an executable called g_baypass):
make clean all FC=gfortran
•using the ifort intelrFortran compiler (the command should auto-
matically produce an executable called i_baypass):
make clean all FC=ifort
From my (limited) experience, I would recommend using the ifort intelr
Fortran compiler instead of gfortran. Indeed, although gfortran options
could probably be better adapted to further improve the speed of the exe-
cutable (any feedback about this is welcome), using the options considered
in the Makefile, the ifort executable is more than two times faster than the
gfortran one as illustrated in the comparison below3. An example compar-
ison of the computational performances of the different version and binaries
of the program is illustrated in Table 1.
1If not already installed in your system, binaries are available at https://gcc.gnu.
org/wiki/GFortranBinaries and easy to install for most Windows, Mac and Linux OS
versions (many thanks to Andrew Beckerman for pointing this webpage to me!)
2Intel proposes a free personal non-commercial unsupported version that is es-
sentially identical to the commercial version: https://software.intel.com/en-us/
qualify-for-free-software/academicresearcher
3I however noticed that the ifort executable might sometimes crash in an unpre-
dictable way when reading the command line arguments (for a reason that remains very
obscure to me and that I am trying to figure out!)
5

Under Linux (or MacOS), before the first use, make sure to give appropriate execution
rights to the program. For instance you may run:
chmod +x baypass
In the following, it is assumed that the program was made executable and
accessible in your path. For instance, under Linux, this may be achieved by
copying the executable in a directory declared in the path (e.g., /usr/local/bin)
or by adding the program to the $PATH system variable (using the export
command)
2.3 Input file format
Depending on the type of analyses, different data files might be required by
the program. The following examples of the different input files are available
in the examples directory:
•geno.bta14: this file contains allele count data for 18 French cattle
breeds at 1,394 SNPs mapping to the BTA14 bovine chromosome (see
Gautier (2015) for details).
•bta.pc1: this file contains the SMS (Synthetic Morpholy Score) for the
18 French cattle breeds (see Gautier (2015) for details).
•omega.bta: this file contains the matrix Ωfor the 18 French cat-
tle breeds (b
Ωbpas
BTA) as estimated under the core model from the whole
genome SNP data (see Gautier (2015) for details).
•lsa.geno: this file contains read count data (Pool–Seq data) for 12
populations from the Littorina saxatilis marine snail (Westram et al.,
2014) at 2,500 SNPs randomly chosen among the ones analysed in Gau-
tier (2015) (but including the ca. 150 outlier SNPs identified).
•lsa.poolsize: this file contains the haploid pool sizes of the 12 Litto-
rina saxatilis populations.
•lsa.ecotype: this file contains the code for the ecotype of the 12
Littorina saxatilis populations (−1 for the ”crab” habitat and 1 for the
”wave” habitat).
2.3.1 The genotyping data file [always required]
The genotyping data files contain allele or read count (for PoolSeq experi-
ment) data for each of the nsnp markers assayed in each of the npops popula-
tions sampled. The genotyping data file is simply organised as a matrix with
6

nsnp rows and 2 ∗npop columns. The row field separator is a space. More
precisely, each row corresponds to one marker and the number of columns is
twice the number of populations because each pair of numbers corresponds to
each allele (or read counts for PoolSeq experiment) counts in one population4.
As a schematic example, the genotyping data input file for allele count
data should read as follows:
--- file begins here ---
81 19 86 14 2 98 8 92 32 68 23 77
89 11 81 19 9 91 1 99 27 73 27 73
89 11 91 9 0 0 15 85 77 23 80 20
[...97 more lines...]
--- file ends here ---
In this example, there are 6 populations and 100 SNP markers. At the first
SNP, in the first population, there are 81 copies of the first allele, and 19
copies of the second allele. In the second population, there are 86 copies of
the first allele, and 14 copies of the second allele, etc. Note that both alleles
in the third SNP in the third population have 0 copie. This marker will be
treated as a missing data in the corresponding population. The file named
geno.bta14 in the example directory provides a more realistic example.
Similarly, as a schematic example, the genotyping data input file for allele
count data should read as follows:
--- file begins here ---
71 8 115 0 61 36 51 39 10 91 69 58
82 0 91 0 84 14 24 57 28 80 18 80
93 28 112 30 0 0 0 113 33 68 0 106
[...97 more lines...]
--- file ends here ---
In this example, there are also 6 populations and 100 SNP markers. At
the first SNP, in the first population, there are 71 reads of the first allele, and
8 reads of the second allele. In the second population, there are 115 reads
of the first allele, and 0 read of the second allele, etc. Note that both alleles
in the third SNP in the third population have 0 copie. This marker will be
treated as a missing data in the corresponding population. The file named
lsa.geno in the example directory provides a more realistic example.
4For now, BayPass is restricted to bi–allelic marker
7

For Pool–Seq data to be analyzed properly (i.e. not like allele count data), it is
necessary to provide a file with the (haploid) size of each pool (see 2.3.2).
2.3.2 The pool (haploid) size file [only required for Pool–Seq data]
For Pool–Seq experiment, the haploid size (twice the number of pooled in-
dividuals for diploid species) of each population should be provided. As a
schematic example, the pool (haploid) size file should read as follows:
--- file begins here ---
60 75 100 90 80 50
--- file ends here ---
In this example, there are 6 populations with respective haploid sample
sizes of 60 (first population), 75 (second population), 100 (third population),
90 (fourth population), 80 (fifth population) and 50 (sixth population). The
order of the populations in the pool size file must be the same as in the allele
count (and the covariate) data file(s). The file named lsa.poolsize in the
example directory provides a more realistic example.
2.3.3 The covariate data file [required for the covariate modes]
The values of the covariates (e.g., environmental data, phenotypic traits, etc.)
for the different populations should be provided in a file with the format
exemplified in the following:
--- file begins here ---
150 1500 800 300 200 2500
181.5 172.6 152.3 191.8 154.2 166.8
1 1 0 0 1 1
0.1 0.8 -1.15 1.6 0.02 -0.5
--- file ends here ---
In this example, there are 6 populations (columns) and 4 covariates (row).
The first covariate might be viewed as a typical environmental covariate, like
altitude in meters (the first population is living at ca. 150m above the sea
level, the second at ca. 1,500m, and so on), the second as a quantitative traits
like average population sizes in cm (individuals from the first population are
181.5 cm height on average, individuals from the second population 172.6
cm, and so on), the third covariate as a typical binary trait like presence (1,
for the first, second, fifth and sixth populations) or absence (0, for the thrid
and fourth populations) and the last might be viewed as a synthetic variable
8

like the first principal components of a PCA. The order of the populations
(columns) in the covariate data file must be the same as in the allele count
(and the pool size) data file(s).
The files named bta.pc1 and lsa.ecotype in the example directory pro-
vide alternative real-life examples.
Note that in most cases, it is (strongly) recommended to scale each covariate (so that
ˆµ= 0 and c
σ2= 1 for each covariable). The scalecov option allows to perform this
step automatically prior to analysis, if needed.
2.3.4 The covariance matrix file [optional, required for the AUX co-
variate mode]
For some applications (see below), it might be interesting (e.g., to parallelize
some analyses) or required (when using the AUX covariate mode) to provide
the population covariance matrix Ω. As a schematic example, the covariance
matrix file reads as follows:
--- file begins here ---
0.098053 0.019595 0.032433 -0.029601 -0.024190 -0.029247
0.019595 0.160147 0.018942 -0.027348 -0.039733 -0.039010
0.032433 0.018942 0.149962 -0.054973 -0.058700 -0.057288
-0.029601 0.027348 0.054973 0.187511 0.221914 0.165862
-0.024190 0.039733 0.058700 0.221914 0.562666 0.260231
-0.029247 0.039010 0.057288 0.165862 0.260231 0.219761
--- file ends here ---
In this example, there are 6 populations. Hence, the population covari-
ance matrix is a 6×6 squared symmetric matrix. The order of the populations
(columns and rows) in the matrix Ω should be the same as the columns in
the allele count (and the pool size and the covariate) data file(s). Note that
this file is produced in the appropriate format by the program when running
BayPass under the core model (see 3.3).
The file named omega.bta provides a real-life example.
3 Running BayPass
3.1 Overview of the different models available in Bay-
Pass
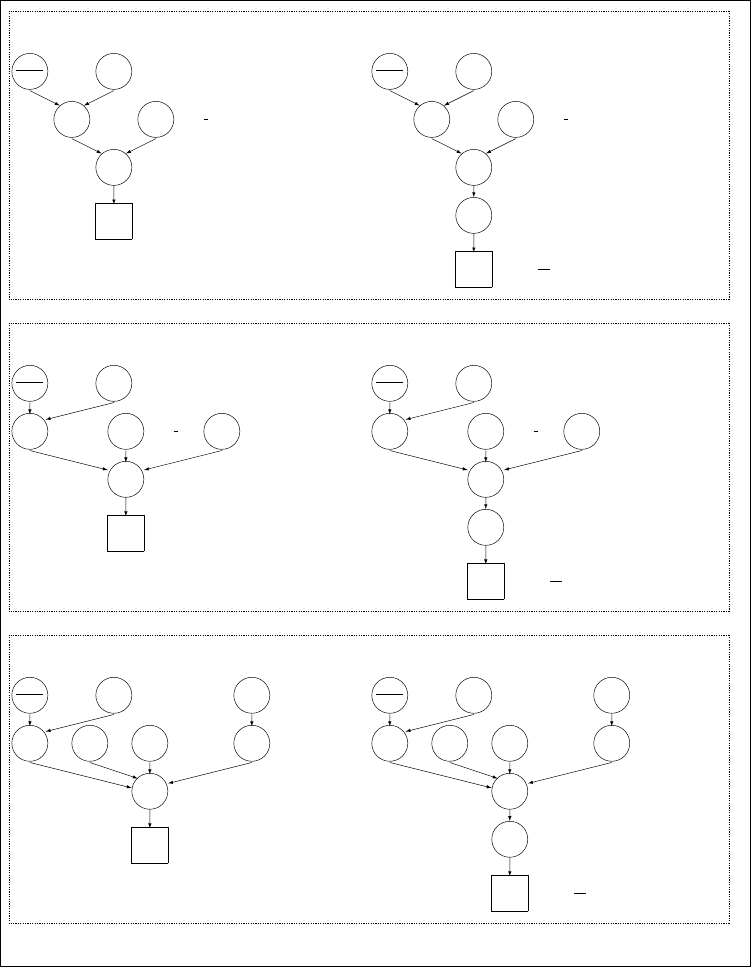
Directed Acyclic Graphs (DAG) of the different family of models are repre-
sented in Figure 1(see Gautier (2015) for details). Briefly, three types of
9

(closely related) models might be investigated using BayPass, considering
either Allele count data (left panel in Figure 1) or Read count data (right
panel in Figure 1) as obtained from Pool–Seq experiments.
3.1.1 The core model
The core model depicted in Figure 1A might be viewed as a generalisation
of the model proposed by Nicholson et al. (2002) and was first proposed by
Coop et al. (2010). This model is the one considered by BayPass when no
covariate data file is provided and is actually nested in the others models.
The main parameter of interest is the (scaled) covariance matrix of pop-
ulation allele frequencies Ωresulting from their (possibly unknown and com-
plex) shared history. Conversely, one might rely on this matrix for demo-
graphic inference. For instance, Ωmight easily be converted (e.g., using
the cov2cor() function in R stats package) into a correlation matrix Σfur-
ther interpreted as a similarity matrix. From this latter matrix, one may
define a distance (dissimilarity) matrix (e.g., dij = 1− | ρij |where dij is
the distance between populations iand jand ρij is the element ij of Σ) to
perform hierarchical clustering5and summarise the history of the popula-
tion as a bifurcating phylogenetic tree (without gene flow). A more complex
demographic inference based on an interpretation the matrix Ω(although
estimated in a less accurate way) in terms of tree with migration has been
recently proposed by Pickrell and Pritchard (2012).
The core model allows to perform genome scan for differentiation (covariate-
free) using the XtX statistics as introduced by G¨
unther and Coop (2013)
which is computed by default in BayPass. The main advantage of this
approach stems is to explicitly account for the covariance structure in pop-
ulation allele frequencies (via estimating Ω) resulting from the demographic
history of the populations. To identify outlier loci (based on the XtX statis-
tics), the R function simulate.baypass() provided in the utils directory
of the package (see 4) allows to simulate data under the inference model (e.g.,
using posterior estimates of Ω and any other hyperparameters) which might
further be analysed to calibrate the neutral XtX distribution (Gautier, 2015).
5For an interesting discussion and examples in R, see http://research.
stowers-institute.org/mcm/efg/R/Visualization/cor-cluster/index.htm
10

In the current implementation of BayPass, the prior distribution for Ωis an Inverse-
Wishart: Ω∼W−1
J(ρIJ, ρ) (where J is the number of populations). By default ρ= 1
(rather than ρ=Jas in Bayenv) which was found as the most reliable value (Gautier,
2015). Similarly, the hyperparameters aπand bπof the prior βdistribution for the
overall (across population) SNP allele frequencies are estimated by default. However,
they might be fixed to aπ=bπ= 1 (as in e.g., Bayenv) using fixpibetapar option
or to any other values using further the betapiprior option (3.2).
3.1.2 The standard covariate model and extensions
The standard covariate model is represented in Figure 1B and is the one
considered by default in BayPasswhen a covariate data file is provided us-
ing efile option (3.2). This model allows to evaluate to which extent the
population covariable(s) kis (linearly) associated to each marker i(which
are assumed independant given Ω) by the introduction of the regression co-
efficients βik (for convenience the indices kfor covariables are dropped in
Figure 1B).
In the current implementation of BayPass, the prior distribution for the βik’s is
Uniform: βik ∼Unif (βmin, βmax). By default, βmin =−0.3 and βmax = 0.3 but
these values might be changed by the user with the minbeta and maxbeta options
respectively (3.2). Note that in Bayenv (Coop et al., 2010), βmin =−0.1 and βmax =
0.1.
The estimation of the βik regression coefficients for each SNP imay be
performed using two different approaches (Gautier, 2015):
•Using an importance sampling estimator (IS) which is the default op-
tion and also allows the computation of Bayes Factor to the compare on
an individual SNP (and covariable) basis the two alternative models,
namely the model with association (βik 6= 0) against the null model
(βik = 0). Bayes Factor (BFis) and βik IS algorithm are inspired from
Coop et al. (2010) and are described in details elsewhere (Gautier,
2015). Note that the IS estimation procedure is based on a numerical
integration that requires the definition of a grid covering the whole sup-
port of the βik prior distribution. In BayPass, the grid consists in nβ
(by default nβ= 201) equidistant points from βmin to βmax (including
the boundaries) leading to a lag between two successive values equal to
βmax−βmin
nβ−1(i.e., 0.003 with default values). Other values for nβmight be
supplied by the user with the nbetagrid option (3.2).
•Using an MCMC algorithm (activated via the covmcmc option). In
this case, the user should provide the matrix Ω(e.g., using posterior
11

estimates available from a previous analysis) and it is recommended to
consider only one covariable at a time (particularly if some covariables
are correlated).
3.1.3 The auxiliary covariate model
The auxiliary covariate model, represented in Figure 1C and activated with
the auxmodel option, is an extension of the previous model (Figure 1B) in-
volving the introduction of a Bayesian (binary) auxiliary variable δik for each
regression coefficient βik (Gautier, 2015). In a similar population genetics
context, this modelling was also proposed by Riebler et al. (2008) to identify
markers subjected to selection in genome-wide scan of adaptive differentia-
tion based on a F–model.
Here, the auxiliary variable actually indicates whether a specific SNP ican
be regarded as associated to a given covariable k(δik = 1) or not (δik = 1).
By looking at the posterior distribution of each auxiliary variable, it is then
straightforward to derive a Bayes Factor (BFmc) to compare both models
while dealing with multiple testing issues (Gautier, 2015). In addition, the
introduction of a Bayesian auxiliary variable makes it easier to account for
spatial dependency among markers. In BayPass, the general form of the δik
prior distribution is indeed that of an 1D Ising model with a parametrization
analogous to the one proposed in a similar context by Duforet-Frebourg et al.
(2014): π(δk)∝Ps1(1 −P)s0eηbis , where δkis the vector of the nsnp auxil-
iary variables for covariable k,s1and s0are the number of SNPs associated
(i.e. with δik = 0) and not associated (i.e. with δik = 0) to the covariable6,
and ηcorresponds to the number of pairs of consecutive markers (neighbors)
that are in the same state at the auxiliary variable7(i.e., δi,k =δi+1,k). The
parameter Pbroadly corresponds to the prior proportion of SNP associated
to the covariable. In the BayPass auxiliary model, Pis assumed a priori
beta distributed: P∼β(aP, bP). By default, aP= 0.02 and bP= 1.98 (this
values might be changed by the user with the auxPbetaprior option) which
amounts to consider that only a small fraction of the SNPs ( aP
aP+bP=1%) are
a priori expected to be associated to the covariable while allowing some un-
certainty on this key parameter (e.g., the prior probability of P >10% being
equal to 0.028 with these parameters). The parameter bis, called the in-
verse temperature in the Ising (and Potts) model literature, determines the
level of spatial homogeneity of the auxiliary variables between neighbors. In
6s1=
nsnp
P
i=1
δik = 1 and s0=
nsnp
P
i=1
δik = 0
7η=P
i∼j
1δik=δjk
12

BayPass, bis = 0 by default implying that auxiliary variables are indepen-
dent (no spatial dependency). Note that bis = 0 amounts to assume the
δik follows a Bernoulli distribution with parameter P. Conversely, bis >0
leads to assume that the δik with similar values tend to cluster according
to the underlying SNP positions (the higher the bis, the higher the level of
spatial homogeneity). In biological terms, SNP associated to a given covari-
able might be expected to cluster due to Linkage Disequilibrium with the
underlying (possibly not genotyped) causal variant(s). In practice, bis = 1 is
commonly used and a value of bis ≤1 is recommended.
In technical terms, the overall parametrisation of the Ising prior assumes no external
field and no weight (as in the so-called compound Ising model) between the neigh-
boring auxiliary variables. In the current implementation of the BayPass auxiliary
covariate model (when bis >0), the information about the distances between SNPs
is therefore not accounted for. Only the relative position of markers are considered.
For the applications where this modeling might be relevant (whole genome scan), this
corresponds to assuming a relative homogeneity in marker spacing as measured by
genetic (rather than physical) distances (which might be unavailable, in practice).
13
A) Basic Model (without covariate)
A.1) Allele count data
aπ
aπ+bπ
∼U(0; 1) aπ+bπ∼Exp(1)
Ω−1∼WJ1
ρIJ, ρ
πi∼β(aπ;bπ)
α?
i
∼NJπi1J;πi(1 −πi)Ω
yij , nij yij ∼Bin min(1,max(0, α?
ij )); nij
A.2) Read count data (Pool–Seq)
aπ
aπ+bπ
∼U(0; 1) aπ+bπ∼Exp(1)
Ω−1∼WJ1
ρIJ, ρ
πi∼β(aπ;bπ)
α?
i
∼NJπi1J;πi(1 −πi)Ω
yij ∼Bin min(1,max(0, α?
ij )); nj
rij , cij rij ∼Bin yij
nj, cij
B) Standard covariate model (STD)
B.1) Allele count data
aπ
aπ+bπ
∼U (0; 1) aπ+bπ∼Exp(1)
πi∼β(aπ;bπ)Ω−1∼WJ1
ρIJ, ρβi∼Uβmin;βmax
α?
i
∼NJπi1J+βiZj;πi(1 −πi)Ω
yij , nij yij ∼Bin min(1,max(0, α?
ij )); nij
B.2) Read count data (Pool–Seq)
aπ
aπ+bπ
∼U (0; 1) aπ+bπ∼Exp(1)
πi∼β(aπ;bπ)Ω−1∼WJ1
ρIJ, ρβi∼Uβmin;βmax
α?
i
∼NJπi1J+βiZj;πi(1 −πi)Ω
yij ∼Bin min(1,max(0, α?
ij )); nj
rij , cij rij ∼Bin yij
nj, cij
C) Auxiliary variable covariate model (AUX)
C.1) Allele count data
aπ
aπ+bπaπ+bπ
πiΩ−1βi∼Uβmin;βmaxδ∼Ising bis;P
P∼βap;bp
α?
i
∼NJπi1J+δiβiZj;πi(1 −πi)Ω
yij , nij yij ∼Bin min(1,max(0, α?
ij )); nij
C.2) Read count data (Pool–Seq)
aπ
aπ+bπaπ+bπ
πiΩ−1βi∼Uβmin;βmaxδ∼Ising bis;P
P∼βap;bp
α?
i
∼NJπi1J+δiβiZj;πi(1 −πi)Ω
yij ∼Bin min(1,max(0, α?
ij )); nj
rij , cij rij ∼Bin yij
nj, cij
Figure 1: Directed Acyclic Graphs of the different hierarchical Bayesian models available
in BayPass (see 3.1). For each model, optional (hyper–)parameters are displayed in
orange.
14

3.2 Detailed overview of all the options
BayPass is a command-line executable. The ASCII hyphen-minus (”-”) is
used to specify options. As specified below, some options take integer or float
values and some options do not. Here is an example call of the program:
baypass -npop 12 -gfile data.geno -efile env.data -outprefix ana1
The full list of the options accepted by BayPass are printed out using the com-
mand: baypass -help as follows:
Version 2.1
Usage: BayPass [options]
Options:
I) General Options:
-help Display the help page
-npop INT Number of populations (always required)
-gfile CHAR Name of the Genotyping Data File (always required)
-efile CHAR Name of the covariate file: activate covariate mode (def="")
-scalecov CHAR Scale covariates (def="")
-poolsizefile CHAR Name of the Pool Size file => activate PoolSeq mode (def="")
-outprefix CHAR Prefix used for the output files (def="")
II) Modeling Options:
-omegafile CHAR Name of the omega matrix file => inactivate estim. of omega (def="")
-rho INT Rho parameter of the Wishart prior on omega (def=1)
-setpibetapar Inactivate estimation of the Pi beta priors parameters
-betapiprior FLOAT2 Pi Beta prior parameters (if -setpibetapar) (def=1.0 1.0)
-minbeta FLOAT Lower beta coef. for the grid (def=-0.3)
-maxbeta FLOAT Upper beta coef. for the grid (def= 0.3)
I.1) IS covariate mode (default covariate mode):
-nbetagrid INT Number of grid points (IS covariate mode) (def=201)
I.2) MCMC covariate mode:
-covmcmc Activate mcmc covariate mode (desactivate estim. of omega)
-auxmodel Activate Auxiliary variable mode to estimate BF
-isingbeta FLOAT Beta (so-called inverse temperature) of the Ising model (def=0.0)
-auxPbetaprior FLOAT2 auxiliary P Beta prior parameters (def=0.02 1.98)
III) MCMC Options:
-nthreads INT Number of threads (def=1)
-nval INT Number of post-burnin and thinned samples to generate (def=1000)
-thin INT Size of the thinning (record one every thin post-burnin sample) (def=25)
-burnin INT Burn-in length (def=5000)
-npilot INT Number of pilot runs (to adjust proposal distributions) (def=20)
-pilotlength INT Pilot run length (def=1000)
-accinf FLOAT Lower target acceptance rate bound (def=0.25)
-accsup FLOAT Upper target acceptance rate bound (def=0.40)
-adjrate FLOAT Adjustement factor (def=1.25)
-d0pi FLOAT Initial delta for the pi all. freq. proposal (def=0.5)
-upalphaalt Alternative update of the pij
-d0pij FLOAT Initial delta for the pij all. freq. proposal (alt. update) (def=0.05)
-d0yij INT Initial delta for the yij all. count (PoolSeq mode) (def=1)
-seed INT Random Number Generator seed (def=5001)
In this menu, each option is followed by the kind of argument (if any)
required (e.g., INT for integer, FLOAT for real, FLOAT2 for a pair of real
number space separated), a brief description of its function, and the default
value.
In the following, we detailed all the options of BayPass:
-help
This option prints out the help menu (see above). Note that this option
is dominating all the other options, i.e. if -help is used in conjunction
15
with any other option of the program, the help menu is displayed. No
argument is required for this option.
-npop
This option (mandatory) gives the number of population considered
in the data set (half the number of column in the genotype data file).
The required argument must be an integer (INT). (e.g., -npop 12 if 12
populations are studied).
-gfile
This option (mandatory) gives the name of the genotyping input file.
See 2.3.1 for a description of the corresponding input file format. The
required argument must be character chain (name of the input file)
without space (e.g., -gfile data.geno if the input file is named ”data.geno”).
-efile
This option gives the name of the covariate input file. See 2.3.3 for a
description of the corresponding input file format. The required argu-
ment must be character chain (name of the input file) without space
(e.g., -gfile data.env if the input file is named ”data.env”).
-scalecov
This option allows to perform scaling of each covariable in the covariate
input file (See 2.3.3). No argument is required for this option. If
activated, an output file named ”covariate.std” containing the scaled
covariables is produced.
-poolsizefile
This option gives the name of the input file containing the haploid sam-
ple size of each population. See 2.3.2 for a description of the correspond-
ing input file format. The required argument must be character chain
(name of the input file) with no space (e.g., -poolsizefile data.poolsize
if the input file is named ”data.poolsize”). Note that this option auto-
matically activates the Pool–Seq mode, i.e., the PoolSeq version of the
different models are considered (as represented in Figures 1A2, B2 and
C2).
-outprefix
16
This option allows to add a prefix to all the output files. The required
argument must be a character chain without space. For instance, if
using -outprefix ana1, the name of all the output files will begin by
”ana1_”. By default, no prefix is added.
-omegafile
This option gives the name of the input file for the population co-
variance matrix (Ωin 3.1.1 and Figure 1). See 2.3.4 for a descrip-
tion of the corresponding input file format. The required argument
must be character chain (name of the input file) with no space (e.g.,
-omegafile matrix.dat if the input file is named matrix.dat). This
option inactivates the estimation of Ωand is mandatory in the co-
variate models involving estimation of the regression coefficients via
MCMC i.e. the standard model (see 3.1.2) with the -covmcmc option
and the auxiliary variable model (see 3.1.3).
-rho
This option allows to specify the value of ρfor the Inverse-Wishart
prior of Ω(see Figure 1and 3.1.1). The required argument must be a
positive integer. By default, -rho 1 (i.e., ρ= 1).
-setpibetapar
This option allows to inactivate estimation of the two (hyper–)parameters
aπand bπof the prior βdistribution for the overall (across population)
SNP allele frequencies (see Figure 1and 3.1.1) (and set them to the val-
ues specified with the -betapiprior option). No argument is required
for this option.
-betapiprior
This option allows to specify the values of the two (hyper–)parameters
aπand bπ(respectively) of the prior βdistribution for the overall
(across population) SNP allele frequencies (see Figure 1and 3.1.1).
The required argument must be two positive real numbers. By default
-betapiprior 1.0 1.0 (i.e., aπ=bπ= 1).
-minbeta
This option allows to specify the lower bound of the Uniform prior
distribution on the regression coefficients (see Figure 1and 3.1.2). The
17
required argument must be a real number (lower than maxbeta defined
below). By default -minbeta -0.3 (i.e., βmin =−0.3).
-maxbeta
This option allows to specify the upper bound of the Uniform prior
distribution on the regression coefficients (see Figure 1and 3.1.2). The
required argument must be a real number (greater than minbeta de-
fined above). By default -maxbeta 0.3 (i.e., βmax = 0.3).
-nthreads
This option gives the number of threads to be used for parallel compu-
tations. By default, -nthreads 1 (i.e., a single core is used hence no
parallelization).
-nval
This option gives the number of post–burn–in (and thinned MCMC)
samples recorded from the posterior distributions of the parameters of
interest. The required argument must be a positive integer. By de-
fault, -nval 1000 (i.e., 1,000 post burn-in and thinned samples are
generated). Note that with default values, the total number of itera-
tions of the MCMC sampler run after the burn-in period is equal to
25,000 (since by default, the thinning rate is equal to 25 , see thin
option)
-thin
This option gives the size of the thinning (i.e., the number of iterations
between any two records from the MCMC). The required argument
must be a positive integer. By default, -thin 25 (i.e., the size of the
thinning is 25).
-burnin
This option gives the length of the burn-in period (i.e., the number
of iterations before the first record from the MCMC). The required
argument must be a positive integer. By default, -burnin 5000 (i.e.,
5,000 iterations are run during the burn–in period).
-npilot
18
This option gives the number of pilot runs (i.e., the number of runs
used to adjust the parameters of the MCMC proposal distributions of
parameters updated through a Metropolis-Hastings algorithm). The
targeted acceptance rates are defined with the accinf and accsup op-
tions (by default, these are set to 0.25 and 0.40 respectively). The
required argument must be a positive integer. By default, -npilot 20
(i.e., 20 pilot runs are performed).
-pilotlength
This option gives the number of iterations of each pilot run (see npilot
option above). The required argument must be a positive integer. By
default, -pilotlength 1000 (i.e., each pilot run consist of 1,000 iter-
ations).
-accinf
This option gives the lower bound of the targeted acceptance rates to
adjust the parameters of the MCMC proposal distributions of parame-
ters updated through a Metropolis-Hastings algorithm, during the pilot
runs. For instance, in the case of a uniform proposal distribution of the
form Unif(x−δ, x +δ) (where xrepresents the current value of the pa-
rameter of interest and δspecifies the size of the support), if acceptance
rates are below this lower bound after a pilot run, then δis increased
(e.g., multiplied by a factor defined with the adjrate parameter and
set to 1.25 by default). The required argument must be a positive
real number (<1 and lower than accsup defined below). By default,
-accinf 0.25 (i.e., acceptance rates should be at least equal to 25%).
-accsup
This option gives the upper bound of the targeted acceptance rates
to adjust the parameters of the MCMC proposal distributions of pa-
rameters updated through a Metropolis-Hastings algorithm, during the
pilot runs. For instance, in the case of a uniform proposal distribution
of the form Unif(x−δ, x +δ) (where xrepresents the current value
of the parameter of interest and δspecifies the size of the support), if
acceptance rates are above this upper bound after a pilot run, then δis
decreased (e.g., divided by a factor defined with the adjrate parameter
and set to 1.25 by default). The required argument must be a positive
real number (≤1 and greater than accinf defined above). By default,
-accinf 0.40 (i.e., acceptance rates should be less than 40%).
19
-adjrate
This option gives the factor used to adjust the parameters of the MCMC
proposal distributions of parameters updated through a Metropolis-
Hastings algorithm, during the pilot runs. For instance, in the case of
a uniform proposal distribution of the form Unif(x−δ, x +δ) (where x
represents the current value of the parameter of interest and δspecifies
the size of the support), if acceptance rates are below (respectively
above) the lower (respectively upper) bound of the targeted regions (as
defined above with the accinf and accsup options) after a pilot run,
then δis multiplied (respectively, divided) by this factor. The required
argument must be a real number >1. By default, -adjrate 1.25.
-d0pi
This option gives the initial value of the δπ, which is half the window
width from which proposal values of the overall SNP allele frequencies
πi(see Figure 1) are drawn uniformly around the current value pij in the
Metroplis–Hastings update. The value of δπis eventually adjusted for
each locus during the pilot runs (see options -npilot,-pilotlength,
-accinf,-accsup and -adjrate). The required argument must be a
positive real number. By default, -d0pi 0.5 (i.e., δp= 0.5).
-upalphaalt
This option activates an alternative Metropolis–Hastings algorithm for
the population SNP allele frequencies αij (see Figure 1). By default,
the proposal is the same as the one described by Coop et al. (2010)
(Appendix A). Briefly, denoting αi. as the vector of allele frequencies
for SNP iin each population, the vector α?cdt
ievaluated in a given
Metropolis–Hastings update is sampled from the following multivariate
Gaussian distribution: α?cdt
i∼MNV (α?
i,Γσ2
i) where Γis obtained
by a Choleski decomposition of the matrix Ω(i.e., Ω=tΓΓ ). The al-
ternative proposal activated with the -upalphaalt option is defined on
a SNP by population basis and is a uniform distribution centred on the
current values of the parameters (i.e., α?cdt
ij ∼Unif(α?ij −λα
ij , α?
ij −λα
ij )).
The algorithm is slower than the default one but may perform better,
in particular when sample sizes are heterogeneous across samples. No
argument is required for this option.
-d0pij
20
This option gives the initial value of the δαused in the proposal distri-
bution of the population SNP allele frequencies αij in the Metroplis–
Hastings updates. Following the notations used above (see -upalphaalt
option), δα=σ2
ifor the default algorithm and δα=λα
ij for the alter-
native algorithm. The value of δαis eventually adjusted for each locus
(and population, in the case of the alternative algorithm) during the pi-
lot runs (see options -npilot,-pilotlength,-accinf,-accsup and
-adjrate). The required argument must be a positive real number.
By default, -d0pij 0.05 (i.e., σ2
i= 0.05 for the default algorithm and
λα
ij = 0.05 for the alternative algorithm).
-d0yij
This option gives, in the Pool–Seq mode, the initial value of the δy
used in the proposal distribution of the population SNP allele count
Yij in the Metroplis–Hastings updates. The value of δyis eventually
adjusted for each locus and each population during the pilot runs (see
options -npilot,-pilotlength,-accinf,-accsup and -adjrate).
The required argument must be a positive integer number lower than
the haploid pool sizes. By default, -d0yij 1 (i.e., δy= 1).
-seed
This option gives the initial seed of the (pseudo-) Random Number
Generator. The required argument must be a positive integer number.
By default, -seed 5001.
3.3 Format of the output files
While running, BayPass printed on the console several information regard-
ing the execution of the analysis (these might be redirected in a log file using
the > log.file unix syntax). At the end of the analysis BayPass produces
several output files which might varied according to the options considered
(see 3.2). The name of these different output files might be preceded by a
prefix as defined with the outprefix options (see 3.2).
In the following, we detailed all the output files that may be generated
by BayPass:
•(outprefix_)summary_pij.out (default mode; e.g., for allele count
data) or (outprefix_)summary_yij_pij.out (Pool–Seq mode; e.g., for
read count data)
21
These files contain for each locus (MRK column) within each popula-
tion (POP column), the mean (M_P column) and the standard deviation
(SD_P column) of the posterior distribution of the α?
ij parameter (see
Figure 1) that is closely related to the frequency of the reference allele
αij = 1 ∧(0 ∨α?
ij ) except that its support is on the real line (hence pos-
sible values <0 or >1). It also contains the posterior mean (M_Pstd
column) and the standard deviation (SD_Pstd column) of the standard-
ized allele frequency αstd (αstd =Γ−1α?). The final adjusted values
of the δα’s, the parameters of the MCMC proposal distributions for
the SNP allele frequencies (see -upalphaalt option) are also reported
in these files (DELTA_P column) together with the post-burn–in final
acceptance rates (ACC_P column). Note that in the default Metropolis–
Hastings algorithm, the α?
ij are updated for each SNP as a vector of
allele frequencies across all populations. Hence, the δαand the accep-
tance rates have same values across all the populations for a given SNP.
In the Pool–Seq mode (i.e., in the (outprefix_)summary_yij_pij.out
file), the columns M_Y,SD_Y,DELTA_Y and ACC_Y similarly report the
posterior mean, the posterior standard-deviation, the δYof the corre-
sponding proposal distributions and the post-burn–in final acceptance
rates for allele counts of each SNP within each population.
•(outprefix_)summary_pi_xtx.out
This file contains for each locus (MRK column), the mean (M_P column)
and the standard deviation (SD_P column) of the posterior distribution
of the (across populations) frequency πiof the SNP reference allele (see
Figure 1). The final adjusted values of the δπ’s, the parameters of the
MCMC proposal distributions are also reported in these files (DELTA_P
column) together with the post-burn–in final acceptance rates (ACC_P
column). In addition, this files contains for each SNP, the posterior
mean (M_XtX column) and standard deviation (SD_XtX column) of the
XtX statistics introduced by G¨
unther and Coop (2013) to identify out-
lier loci in genome–scan of adaptive differentiation (see 3.1.1).
•(outprefix_)summary_lda_omega.out and (outprefix_)mat_omega.out
The (outprefix_)summary_lda_omega.out file contains the posterior
means and posterior standard deviations of each element of the npop ×
npop scaled population allele frequencies covariance matrix Ω(M_omega_ij
and SD_omega_ij columns respectively) as described in Figure 1(see
also 3.1.1), and its inverse Λ=Ω−1(M_lambda_ij and SD_lambda_ij
columns respectively).
22

The (outprefix_)mat_omega.out file contains the posterior means of
the elements of Ωin a matrix format. Note that this file is in the
format required by the -omegafile option of BayPass.
•(outprefix_)summary_beta_params.out (generated with the -estpibetapar
option)
This file contains the posterior mean (Mean column) and standard de-
viation (SD column) of the two parameters (aπand bπ) of the Beta
prior distribution assumed for the (across populations) frequencies of
the SNP reference allele (see Figure 1).
•(outprefix_)summary_betai_reg.out
This file is only produced in the Bayenv–like mode, i.e. the stan-
dard covariate mode (see Figure 1B and 3.1.2) where the estimation
of the Bayes Factor (column BF(dB)) in dB units (i.e., 10 ×log10(BF))
measuring the support of the association of each SNP with each popula-
tion covariable and the corresponding regression coefficients βi(column
Beta_is) are done via an Importance Sampling algorithm (Coop et al.,
2010). In addition, the file also contains the empirical Bayesian P–value
(eBPis) in the log10 scale i.e. eBPis = −log10(1 −2|0.5−Φ(cµβ/cσβ)|)
(where Φ(x) represents the cumulative distribution function for the
standard normal distribution) and thus allowing to evaluate the sup-
port in favour of a non-null regression coefficient (e.g., eBPis >3).
This file contains for each covariable and each SNP, the posterior mean
and standard deviation of the Pearson correlation coefficient (columns
M_Pearson and SD_Pearson respectively) between the scaled allele fre-
quencies f
α?
i=α?
ij
−πi
√π(1−πi)(1..J)
and the given covariable after rotation
of both vectors by Γ−1(see G¨
unther and Coop, 2013) where Γis ob-
tained by a Choleski decomposition of the matrix Ω(i.e., Ω=tΓΓ).
•(outprefix_)summary_betai.out (generated with the -covmcmc op-
tion)
This file is produced in place of the (outprefix_)summary_betai_reg.out
described above when the -covmcmc option is activated (see 3.1.2).
Under the standard model (default), the file contains for each SNP,
the posterior mean cµβ(M_Beta column) and standard deviation cσβ
(SD_Beta column) of the regression coefficient βitogether with the
adjusted δβparameter (DeltaB column) of the proposal distribution
23
and the post-burn–in acceptance rate (AccRateB column). In addition,
the file also contains an approximated Bayesian P–value in the log10
scale (eBPmc) measured as eBPmc = −log10(1 −2|0.5−Φ(cµβ/cσβ)|)
(where Φ(x) represents the cumulative distribution function for the
standard normal distribution) and thus allowing to evaluate the support
in favour of a non-null regression coefficient (e.g., eBPmc >3).
Under the model with auxiliary variables (-auxmodel option, see 3.1.3),
the file contains for each SNP, the posterior mean (M_Beta column)
and standard deviation (SD_Beta column) of the regression coefficient
βi, the posterior mean of the auxiliary variable (M_Delta column) and
the estimate of the Bayes Factor (column BF(dB)) in dB units (i.e.,
10 ×log10(BF)) for comparison of models with (βi6= 0) and without
(βi= 0) correlation with the given covariable.
•(outprefix_)summary_Pdelta.out (covariate model with auxiliary vari-
able, i.e. -auxmodel option, see 3.1.3)
This file contains the posterior mean (M_P column) and standard de-
viation (SD_P column) of the parameter P(see Figure 1C and 3.1.3)
corresponding to the overall proportion of SNP associated to the cor-
responding covariable.
•(outprefix_)covariate.std (generated by the scalecov option)
This file contains the scaled covariables.
•(outprefix_)DIC.out
This files contains the average deviance (bar(D) column), the effective
number of parameters of the models (pD column) and the Deviance
Information Criterion (DIC column) as defined in Spiegelhalter et al.
(2002) and that might be relevant for model comparison purposes. In
addition, the logarithm of the pseudo-marginal likelihood of the model
is also provided (LPML column).
4 Miscellaneous R functions
The baypass_utils.R file in the utils directory contains three R functions
(R Core Team, 2015) (simulate.baypass();fmd.dist() and geno2YN())
that may be helpful to interpret some of the results obtained with BayPass.
To use this functions, one may simply need to source the corresponding
24

files and ensure that the packages mvtnorm (Genz et al., 2015) and geigen
(Hasselman, 2015) have been installed. In addition, although not required by
theses functions, the packages corrplot (Wei, 2013) and ape (Paradis et al.,
2004) might proved useful for the visualisation of the Ωmatrix (see 5).
4.1 The R function simulate.baypass()
4.1.1 Description
The R function simulate.baypass() allows to simulate either allele or read
count data under the core inference model (Figure 1A) and possibly under the
STD covariate model (Figure 1B). It produces several objects and output files
in a format directly appropriate for analyses with BayPass and Bayenv28.
In practice, this function is useful to generate POD for calibration of the XtX
differentiation measure (or any other measures). More broadly, because the
Ωmatrix capture the demographic history of the populations, this function
might also be viewed as an efficient simulator of population genetics data.
4.1.2 Usage
simulate.baypass(omega.mat,nsnp=1000,beta.coef=NA,beta.pi=c(1,1),
pop.trait=0,sample.size=100,pi.maf=0.05,suffix="sim",
remove.fixed.loci=FALSE,coverage=NA)
4.1.3 Arguments (in alphabetic order)
•beta.pi (def=c(1,1))
A two elements vector giving the parameters aπand bπrespectively, for
the Beta distribution of the πi(”ancestral”) allele frequencies.
•beta.coef (def=NA; required for simulation under the STD covariate
model)
A vector giving the values of the regression coefficients (βiin Figure 1)
for the simulated associated SNPs (the number of the simulated asso-
ciated SNPs is equal to the dimension of the vector).
•coverage (def=NA; required to activate simulation of read count data)
8For analyses with Bayenv2, make sure fixed loci have been removed, i.e.,
remove.fixed.loci=TRUE
25
Either a single value or a matrix giving the total read counts. In the
latter case, the vector of total read counts for each simulated SNP
are sampled with replacement from the row of the matrix. It is thus
mandatory that the number of columns of the matrix equals the number
of populations, but no restriction are set for the number of rows. For
instance, if the matrix has only one row, all the SNPs will have the
same read counts within a given population.
•omega.mat (always required)
A positive definite and symmetric matrix of rank npop corresponding
to the covariance matrix of population allele frequencies (Ωin Figure 1)
•nsnp (def=1000)
A single number giving the number of (neutral) SNPs to simulate.
•pi.maf (def=0.05)
A single value giving the MAF threshold on the simulated πi(”an-
cestral”) allele frequencies. In the simulation procedure, the pii’s are
sampled from the Beta distribution with parameters as specified in
the beta.pi argument. For a given SNP i, if pii<pi.maf (resp.
pii>1−pi.maf) then piiis set equal to pi.maf (resp. 1−pi.maf).
Setting pi.maf= 0 inactivates MAF filtering.
•pop.trait (def=0; required for simulation under the STD covariate
model)
A vector of length npop giving each population-specific covariable val-
ues (ordering of the population is assumed to be the same as in the
omega.mat matrix). By default all values are set to 0 (meaning the
associated SNPs behave neutrally irrespective of their values at the
regression coefficients).
•remove.fixed.loci (def=FALSE)
A logical indicating wether or not fixed loci (in the observed simulated
data) should be discarded.
•sample.size (def=100)
If simulating allele count data, either a single value or a matrix giving
the total allele counts (twice the number of genotyped individuals in
26
diploid species). In the latter case, the vector of total allele counts
for each simulated SNP are sampled with replacement from the row of
the matrix. It is thus mandatory that the number of columns of the
matrix equals the number of populations, but no restriction are set for
the number of rows. For instance, if the matrix has only one row, all
the SNPs will have the same allele counts within a given population.
If simulating read count data, either a single value or a vector of length
npop giving the pool haploid sample sizes for each population.
•suffix (def=”sim”)
A character string giving the suffix of the output files generated by the
function.
4.1.4 Values
The function produces an object which is a list with the following compo-
nents:
•omega.sim
A matrix corresponding to the one used for simulation and declared
with the omega.mat argument
•alpha.sim
A matrix with dimension nsnp rows and npop columns giving the sim-
ulated (unbounded) allele frequencies for each simulated SNP within
each population (i.e., α?
ij in Figure 1).
•pi.sim
A vector of length nsnp giving the simulated πi”ancestral” allele fre-
quencies for each simulated SNP.
•N.sim
A matrix with dimension nsnp rows and npop columns giving the total
allele counts for each simulated SNP within each population.
•Y.sim
A matrix with dimension nsnp rows and npop columns giving the al-
lele counts for the reference allele for each simulated SNP within each
population.
27
•N.pool (read count data only)
A matrix with dimension nsnp rows and npop columns giving the total
read counts for each simulated SNP within each population.
•Y.pool (read count data only)
A matrix with dimension nsnp rows and npop columns giving the read
counts for the reference allele for each simulated SNP within each pop-
ulation.
•betacoef.sim (simulation under the STD covariate model only)
A vector of length nsnp giving the regression coefficients of each SNP
used to simulate the data.
In addition, the following output files are printed out (the extension
.suffix is as defined in the suffix argument):
•G.suffix
The allele count data file in BayPass format (see 2.3).
•Gpool.suffix (when simulating read count data)
The read count data file in BayPass format (see 2.3).
•bayenv_freq.suffix
The allele count data file in Bayenv2 format (see 8).
•bayenv_freq_pool.suffix (when simulating read count data)
The read count data file in Bayenv2 format (see 8).
•alpha.suffix
The simulated (unbounded) allele frequencies (nsnp rows and npop
columns) for each simulated SNP within each population (i.e., α?
ij in
Figure 1)
•pi.suffix
The simulated πi”ancestral” allele frequencies for each simulated SNP.
28

•betacoef.suffix (when simulating under the STD covariate model)
The regression coefficients of each SNP used to simulate the data.
•pheno.suffix (when simulating under the STD covariate model)
The covariate data file in BayPass format (see 2.3).
•poolsize.suffix (when simulating read count data)
The haploid pool size data file in BayPass format (see 2.3).
4.1.5 Examples
#source the baypass R functions (check PATH)
source("utils/baypass_utils.R")
#load the bovine covariance matrix
om.bta <- as.matrix(read.table("examples/omega.bta"))
#simulate allele count data for 1000 SNPs
simu.res <- simulate.baypass(omega.mat=om.bta)
#simulate allele count data for 1000 neutral SNPs and
#100 associated SNPs with varying regression coefficients
simu.res <- simulate.baypass(omega.mat=om.bta,beta.coef=runif(100,-0.2,0.2),
pop.trait=rnorm(18))
#simulate read count data for 1000 SNPs
simu.res <- simulate.baypass(omega.mat=om.bta,coverage=50)
4.2 The R function fmd.dist()
4.2.1 Description
This function computes the metric proposed by (F¨
orstner and Moonen, 2003)
to evaluate the distance between two covariance matrices (FMD distance).
4.2.2 Usage
fmd.dist(mat1,mat2)
4.2.3 Arguments
•mat1 and mat2
Two positive-definite (symmetric) matrices
29

4.2.4 Values
The function returns a numeric corresponding to the FMD distance between
the two matrices.
4.2.5 Example
#source the baypass R functions (check PATH)
source("utils/baypass_utils.R")
#load the bovine covariance matrix
om.bta <- as.matrix(read.table("examples/omega.bta"))
#create a dummy diagonal covariance matrix
#this might be obtained from a star-shaped phylogeny with
#branch length (Fst) equal to 0.1
star.bta<-diag(0.1,nrow(om.bta))
#compute the fmd.dist between the two matrices
fmd.dist(om.bta,star.bta)
4.3 The R function geno2YN()
4.3.1 Description
This function reads the allele (or read) count data file in the BayPass format
and extract both the counts for the reference allele and total counts.
4.3.2 Usage
geno2YN(genofile)
4.3.3 Arguments
•genofile
A character string giving the name of the allele (or read) count data
file in the BayPass format.
4.3.4 Values
The function returns two matrices:
•genofile
A character string giving the name of the allele (or read) count data
file in the BayPass format.
30

The function produces an object which is a list containing the two follow-
ing matrices:
•YY
A matrix with nsnp rows and npop columns containing allele or read
counts for the reference allele.
•NN
A matrix with nsnp rows and npop columns containing the total allele
or read counts.
4.3.5 Example
#source the baypass R functions (check PATH)
source("utils/baypass_utils.R")
#load the bovine BTA 14 data
counts.obj <- geno2YN("examples/geno.bta14")
5 Worked Examples
For illustration purposes, different types of analyses of the example files
(see 2.3) are detailed step by step. Users might try to reproduce the cor-
responding examples using the files included in the example directory.
5.1 Cattle allele count data
5.1.1 Analysis under the core model mode: MCMC is run under
the core model
The following command allows to analyse the data under the core model (this
should take ca. 4 min with the i_baypass executable and ca. 7 min with
the g_baypass executable):
baypass -npop 18 -gfile geno.bta14 -outprefix anacore
To visualize the results, one may open an R session and proceed as follows:
require(corrplot) ; require(ape)
#source the baypass R functions (check PATH)
source("utils/baypass_utils.R")
#upload estimate of omega
omega=as.matrix(read.table("anacore_mat_omega.out"))
pop.names=c("AUB","TAR","MON","GAS","BLO","MAN","MAR","LMS","ABO",
"VOS","CHA","PRP","HOL","JER","NOR","BRU","SAL","BPN")
31

dimnames(omega)=list(pop.names,pop.names)
#Compute and visualize the correlation matrix
cor.mat=cov2cor(omega)
corrplot(cor.mat,method="color",mar=c(2,1,2,2)+0.1,
main=expression("Correlation map based on"~hat(Omega)))
#Visualize the correlation matrix as hierarchical clustering tree
bta14.tree=as.phylo(hclust(as.dist(1-cor.mat**2)))
plot(bta14.tree,type="p",
main=expression("Hier. clust. tree based on"~hat(Omega)~"("*d[ij]*"=1-"*rho[ij]*")"))
#Compare the estimate of omega based on the whole genome
#and based on the BTA14 SNPs
wg.omega <- as.matrix(read.table("examples/omega.bta")) #check the PATH
plot(wg.omega,omega) ; abline(a=0,b=1)
fmd.dist(wg.omega,omega)
#Estimates of the XtX differentiation measures
anacore.snp.res=read.table("anacore_summary_pi_xtx.out",h=T)
plot(anacore.snp.res$M_XtX)
One may further wish to calibrate the XtX estimates using a POD sample.
For instance, to produce a (small) POD sample with 1,000 SNPs (continuing
the R example above) using the simulate.baypass() function (see 4):
#get estimates (post. mean) of both the a_pi and b_pi parameters of
#the Pi Beta distribution
pi.beta.coef=read.table("anacore_summary_beta_params.out",h=T)$Mean
#upload the original data to obtain total allele count
bta14.data<-geno2YN("geno.bta14")
#Create the POD
simu.bta<-simulate.baypass(omega.mat=omega,nsnp=1000,sample.size=bta14.data$NN,
beta.pi=pi.beta.coef,pi.maf=0,suffix="btapods")
Then, one may analyse the newly created POD (data file named ”G.btapods”
in the example) giving another prefix for the output files:
baypass -npop 18 -gfile G.btapods -outprefix anapod
Continuing the above example in R, calibration of the XtX and visuali-
sation of the results might be carried out as follows:
#######################################################
#Sanity Check: Compare POD and original data estimates
#######################################################
#get estimate of omega from the POD analysis
pod.omega=as.matrix(read.table("anapod_mat_omega.out"))
plot(pod.omega,omega) ; abline(a=0,b=1)
fmd.dist(pod.omega,omega)
#get estimates (post. mean) of both the a_pi and b_pi parameters of
#the Pi Beta distribution from the POD analysis
pod.pi.beta.coef=read.table("anapod_summary_beta_params.out",h=T)$Mean
plot(pod.pi.beta.coef,pi.beta.coef) ; abline(a=0,b=1)
32

#######################################################
#XtX calibration
#######################################################
#get the pod XtX
pod.xtx=read.table("anapod_summary_pi_xtx.out",h=T)$M_XtX
#compute the 1% threshold
pod.thresh=quantile(pod.xtx,probs=0.99)
#add the thresh to the actual XtX plot
plot(anacore.snp.res$M_XtX)
abline(h=pod.thresh,lty=2)
5.1.2 Analysis under the IS covariate mode: MCMC is run under
the core model
This analysis allows to perform association study (under the STD covariate
model) by estimating for each SNP the Bayes Factor, the empirical Bayesian
P-value (and the underlying regression coefficient) using an Importance Sam-
pling algorithm (Gautier, 2015). As a consequence, the MCMC samples of
the parameters of interest are obtained by running the core model as above
(5.1.1). Hence, if covariables are available, one may rather used this mode as
a default mode. The example below corresponds to an association analysis
with the SMS covariable measured on the 18 French cattle breeds (Gautier,
2015).
baypass -npop 18 -gfile geno.bta14 -efile bta.pc1 -outprefix anacovis
Providing the same seed and the same options have been used, one may
verify that exactly the same estimates for Ω(e.g., files anacovis_mat_omega.out
and anacore_mat_omega.out) and other parameters in common are obtained
than under the previous analysis (5.1.1). Continuing the above example in
R, one may plot the Importance Sampling estimates of the Bayes Factor,
the empirical Bayesian P-value and the underlying regression coefficient as
follows:
covis.snp.res=read.table("anacovis_summary_betai_reg.out",h=T)
graphics.off()
layout(matrix(1:3,3,1))
plot(covis.snp.res$BF.dB.,xlab="SNP",ylab="BFis (in dB)")
plot(covis.snp.res$eBPis,xlab="SNP",ylab="eBPis")
plot(covis.snp.res$Beta_is,xlab="SNP",ylab=expression(beta~"coefficient"))
Recall that in the example only a subset of SNPs mapping to BTA14 are
considered. To improve precision in this example, one may rather provide
the program with a more accurate estimate of the matrix Ωas obtained from
the original study on the complete data sets (with 40 times as many SNPs):
33

baypass -npop 18 -gfile geno.bta14 -efile bta.pc1 \
-omegafile omega.bta -outprefix anacovis2
The resulting Importance Sampling estimates of the Bayes Factor, the
empirical Bayesian P-value and the underlying regression coefficient 9might
be plotted as follows:
covis2.snp.res=read.table("anacovis2_summary_betai_reg.out",h=T)
graphics.off()
layout(matrix(1:3,3,1))
plot(covis2.snp.res$BF.dB.,xlab="SNP",ylab="BFis (in dB)")
plot(covis2.snp.res$eBPis,xlab="SNP",ylab="eBPis")
plot(covis2.snp.res$Beta_is,xlab="SNP",ylab=expression(beta~"coefficient"))
5.1.3 Analysis under the MCMC covariate mode: MCMC is run
under the STD model
This analysis allows to perform association study under the STD covariate
model by estimating the empirical Bayesian P-value and the underlying re-
gression coefficient using parameters values sampled from MCMC run under
the STD model (Gautier, 2015). Although one may also estimate Ωunder
the STD model, this option has been inactivated in BayPass. As a conse-
quence, an estimate of Ω(e.g., as obtained by a first analysis under the core
model of IS covariate mode) must be provided. The example below corre-
sponds to an association analysis with the SMS covariable measured on the
18 French cattle breeds (Gautier, 2015).
baypass -npop 18 -gfile geno.bta14 -efile bta.pc1 \
-covmcmc -omegafile omega.bta -outprefix anacovmcmc
The resulting estimates of the empirical Bayesian P-values, the underly-
ing regression coefficients (posterior mean) and the corrected XtX might be
plotted as follows:
covmcmc.snp.res=read.table("anacovmcmc_summary_betai.out",h=T)
covmcmc.snp.xtx=read.table("anacovmcmc_summary_pi_xtx.out",h=T)$M_XtX
graphics.off()
layout(matrix(1:3,3,1))
plot(covmcmc.snp.res$eBPmc,xlab="SNP",ylab="eBPmc")
plot(covmcmc.snp.res$M_Beta,xlab="SNP",ylab=expression(beta~"coefficient"))
plot(covmcmc.snp.xtx,xlab="SNP",ylab="XtX corrected for SMS")
9Note that one may carry out a calibration of these different measures (as detailed for
the XtX in 5.1.1) by analysing a POD together with the covariables:
baypass -npop 18 -gfile G.pods -efile bta.pc1 -omegafile omega.bta -outprefix podcovis
34

5.1.4 Analysis under the AUX covariate mode: MCMC is run
under the AUX model
This analysis allows to perform association study under the AUX covariate
model by estimating the Bayes Factor (and the underlying regression coef-
ficient) using parameters values sampled from MCMC run under the AUX
model (Gautier, 2015). Although one may also estimate Ωunder the AUX
model, this option has been inactivated in BayPass. As a consequence, an
estimate of Ω(e.g., as obtained by a first analysis under the core model of
IS covariate mode) must be provided. The example below corresponds to
an association analysis with the SMS covariable measured on the 18 French
cattle breeds (Gautier, 2015).
baypass -npop 18 -gfile geno.bta14 -efile bta.pc1 \
-auxmodel -omegafile omega.bta -outprefix anacovaux
The resulting estimates of the Bayes Factor, the underlying regression
coefficients (posterior mean) and the corrected XtX might be plotted as fol-
lows:
covaux.snp.res=read.table("anacovaux_summary_betai.out",h=T)
covaux.snp.xtx=read.table("anacovaux_summary_pi_xtx.out",h=T)$M_XtX
graphics.off()
layout(matrix(1:3,3,1))
plot(covaux.snp.res$BF.dB.,xlab="SNP",ylab="BFmc (in dB)")
plot(covaux.snp.res$M_Beta,xlab="SNP",ylab=expression(beta~"coefficient"))
plot(covaux.snp.xtx,xlab="SNP",ylab="XtX corrected for SMS")
One may further introduce spatial dependency among the SNPs by setting
bis = 1 in the Ising prior (Figure 1C) to refine the associated region:
baypass -npop 18 -gfile geno.bta14 -efile bta.pc1 -auxmodel \
-isingbeta 1.0 -omegafile omega.bta -outprefix anacovauxisb1
The resulting estimates of the posterior mean of the each auxiliary vari-
able δiunder both models (AUX model with no SNP spatial dependency
and AUX model Bayes Factor, the underlying regression coefficients (poste-
rior mean) and the corrected XtX might be plotted as follows:
covauxisb1.snp.res=read.table("anacovauxisb1_summary_betai.out",h=T)
graphics.off()
layout(matrix(1:2,2,1))
plot(covaux.snp.res$M_Delta,xlab="SNP",ylab=expression(delta[i]),main="AUX model")
plot(covauxisb1.snp.res$M_Delta,xlab="SNP",ylab=expression(delta[i]),
main="AUX model with isb=1")
35

5.2 Littorina Pool–Seq read count data
The Littorina Pool–Seq data may be analysed in a similar fashion as the
cattle data above except that one needs to specify the (haploid pool) size file
using the -poolsizefile option to activate the Pool–Seq mode. Because
the haploid pool sizes are relatively large (= 100) in the example, one may
also increase the initial δof the yij proposal distribution (as a rule of thumbs,
one may set it to a fifth of the minimum pool size). Here is an example of
command to run BayPass under the IS covariate mode (MCMC run under
the core model):
i_baypass -npop 12 -gfile lsa.geno -efile lsa.ecotype \
-poolsizefile lsa.poolsize -d0yij 20 -outprefix lsacovis
6 Credits
BayPass makes use of several functions and subroutines that were previously
developed by other authors. These include:
•the Fortran code for the multiple streams MT19937 Mersenne–Twister
(parallel) Random Number Generator was adapted from the subrou-
tines available in the mt_stream_f90-1.11.tar.gz program written by
Ken-Ichi Ishikawa and available under the New BSD License10 at http:
//theo.phys.sci.hiroshima-u.ac.jp/~ishikawa/PRNG/mt_stream_
f90-1.11/).
•Various functions and subroutines for random number generations that
were adapted from the Alan Miller Fortran module random.f90 avail-
able at: http://jblevins.org/mirror/amiller/ available under the
GNU GPL license.
•the Wishart sampler utilities derived from the fortran wishart library
written by John Burkhardt and available at http://people.sc.fsu.
edu/~%20jburkardt/f_src/wishart/wishart.html under the GNU
GPL license.
•the kracken(3f) Fortran module developped by John S. Urban to
parse command line arguments (available at http://home.comcast.
net/~urbanjost/LIBRARY/libCLI/arguments/krackenhelp.html) un-
der the GNU GPL license.
10http://theo.phys.sci.hiroshima-u.ac.jp/~ishikawa/PRNG/mt_stream_f90-1.
11/LICENSE
36
I also wish to thank Renaud Vitalis for providing the L
A
T
EXtemplate for
this manual and Andrew Beckerman for reporting bugs and advices that
helped to improve the program.
7 Copyright
BayPass is a free software under the GPL- and BSD-compatible CeCILL-B
licence (see http://www.cecill.info/licences/Licence_CeCILL-B_V1-en.
html), and c
INRA.
8 Contact
If you have any question, please feel free to contact me. However, I strongly
recommend you read carefully this manual first.
37
Bibliography
Coop, G., D. Witonsky, A. D. Rienzo, and J. K. Pritchard, 2010 Using en-
vironmental correlations to identify loci underlying local adaptation. Ge-
netics 185: 1411–1423.
Duforet-Frebourg, N., E. Bazin, and M. G. B. Blum, 2014 Genome scans for
detecting footprints of local adaptation using a bayesian factor model. Mol
Biol Evol 31: 2483–2495.
F¨
orstner, W., and B. Moonen, 2003 A metric for covariance matrices. In
Geodesy-The Challenge of the 3rd Millennium. Springer Berlin Heidelberg,
299–309.
Gautier, M., 2015 Genome-wide scan for adaptive divergence and association
with population-specific covariates. Genetics 201: 1555–1579.
Genz, A., F. Bretz, T. Miwa, X. Mi, F. Leisch, et al., 2015 mvtnorm: Multi-
variate Normal and t Distributions. R package version 1.0-3.
G¨
unther, T., and G. Coop, 2013 Robust identification of local adaptation
from allele frequencies. Genetics 195: 205–220.
Hasselman, B., 2015 geigen: Calculate Generalized Eigenvalues of a Matrix
Pair. R package 1.5.
Nicholson, G., A. V. Smith, F. Jonsson, O. Gustafsson, K. Stefansson,
et al., 2002 Assessing population differentiation and isolation from single-
nucleotide polymorphism data. J Roy Stat Soc B 64: 695–715.
Paradis, E., J. Claude, and K. Strimmer, 2004 Ape: Analyses of phylogenetics
and evolution in r language. Bioinformatics 20: 289–290.
Pickrell, J. K., and J. K. Pritchard, 2012 Inference of population splits
and mixtures from genome-wide allele frequency data. PLoS Genet 8:
e1002967.
R Core Team, 2015 R: A Language and Environment for Statistical Comput-
ing. R Foundation for Statistical Computing, Vienna, Austria.
Riebler, A., L. Held, and W. Stephan, 2008 Bayesian variable selection for
detecting adaptive genomic differences among populations. Genetics 178:
1817–1829.
38
Spiegelhalter, D. J., N. G. Best, B. P. Carlin, and A. v. d. Linde, 2002
Bayesian measures of model complexity and fit. Journal of the Royal
Statistical Society. Series B (Statistical Methodology) 64: 583–639.
Wei, T., 2013 corrplot: Visualization of a correlation matrix. R package
version 0.73.
Westram, A. M., J. Galindo, M. A. Rosenblad, J. W. Grahame, M. Panova,
et al., 2014 Do the same genes underlie parallel phenotypic divergence in
different littorina saxatilis populations? Mol Ecol 23: 4603–4616.
39

Appendix A Comparisons of the computational
efficiency of the different ver-
sion of BayPass
For illustration purposes, Table 1gives computational times obtained when
running the same example (Pool–Seq) analysis for the Littorina read count
example data set (12 pools, 2,500 SNPs) considered in paragraph 5.2. The
multi-threaded version (baypass 2.0) is compared to the initial non-parallel
version (baypass 1.01) with the same options as in paragraph 5.2 (except
for the number of threads). For both versions, both a gfortran and ifort
compiled binaries were considered. All comparisons were done under the
same standard computer running under Linux Debian 7.1.
Version nthreads gfortran binary ifort binary
BayPass 1.01 1 38 min 59 s 12 min 11 s
BayPass 2.0 1 (default) 39 min 48 s 14 min 54 s
BayPass 2.0 4 (-nthreads 4) 11 min 34 s 5 min 40 s
Table 1: Comparisons of the computational efficiency of different version of BayPass for
the analysis of the Littorina Pool–Seq read count example data set (12 pools, 2,500 SNPs)
considered in paragraph 5.2.
Note that when running on a single core (and for very short runs as
well, not shown), as expected, the baypass 1.01 is slightly more efficient
computationally than the baypass 2.0. Also, the ifort binaries always
outperform the gfortran binaries.
40
