Breast
Breast breast breast manuals 92e6fb9859634b9ea4ec71deed4f31e2 development tpassets.devicebits.com 3:
2016-06-20
: Pdf Breast breast manuals 21a8a708c03541f4871a3842da3e267a development
Open the PDF directly: View PDF ![]() .
.
Page Count: 202 [warning: Documents this large are best viewed by clicking the View PDF Link!]

NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®)
Breast Cancer
Version 2.2016
Continue
NCCN.org
NCCN Guidelines for Patients® available at www.nccn.org/patients
Version2.2016 05/06/16© National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer Panel Members
* William J. Gradishar, MD/Chair ‡ †
Robert H. Lurie Comprehensive Cancer
Center of Northwestern University
Benjamin O. Anderson, MD/Vice-Chair ¶
Fred Hutchinson Cancer Research
Center/Seattle Cancer Care Alliance
Ron Balassanian, MD ≠
UCSF Helen Diller Family
Comprehensive Cancer Center
Sarah L. Blair, MD ¶
UC San Diego Moores Cancer Center
Harold J. Burstein, MD, PhD †
Dana-Farber/Brigham and Women’s
Cancer Center
Amy Cyr, MD ¶
Siteman Cancer Center at Barnes-
Jewish Hospital and Washington
University School of Medicine
Anthony D. Elias, MD †
University of Colorado Cancer Center
William B. Farrar, MD ¶
The Ohio State University Comprehensive
Cancer Center - James Cancer Hospital
and Solove Research Institute
Andres Forero, MD ‡ †
University of Alabama at Birmingham
Comprehensive Cancer Center
Sharon Hermes Giordano, MD, MPH †
The University of Texas
MD Anderson Cancer Center
Matthew Goetz, MD ‡ †
Mayo Clinic Cancer Center
Lori J. Goldstein, MD †
Fox Chase Cancer Center
Clifford A. Hudis, MD †
Memorial Sloan Kettering Cancer Center
Steven J. Isakoff, MD, PhD †
Massachusetts General Hospital
Cancer Center
P. Kelly Marcom, MD †
Duke Cancer Institute
Ingrid A. Mayer, MD †
Vanderbilt-Ingram Cancer Center
Beryl McCormick, MD §
Memorial Sloan Kettering Cancer Center
Meena Moran, MD §
Yale Cancer Center/
Smilow Cancer Hospital
Sameer A. Patel, MD Ÿ
Fox Chase Cancer Center
Lori J. Pierce, MD §
University of Michigan
Comprehensive Cancer Center
Elizabeth C. Reed, MD † ξ
Fred & Pamela Buffett Cancer Center
Kilian E. Salerno, MD §
Roswell Park Cancer Institute
Lee S. Schwartzberg, MD ‡ †
St. Jude Children’s Research Hospital/
The University of Tennessee
Health Science Center
Karen Lisa Smith, MD, MPH †
The Sidney Kimmel Comprehensive
Cancer Center at Johns Hopkins
Mary Lou Smith, JD, MBA ¥
Research Advocacy Network
Hatem Soliman, MD †
Moftt Cancer Center
George Somlo, MD ‡ ξ †
City of Hope Comprehensive Cancer Center
Melinda Telli, MD †
Stanford Cancer Institute
John H. Ward, MD ‡ †
Huntsman Cancer Institute
at the University of Utah
NCCN Staff
Rashmi Kumar, PhD
Dorothy A. Shead, MS
Continue
NCCN Guidelines Panel Disclosures
† Medical oncology
‡ Hematology/Oncology
¶ Surgical oncology
≠ Pathology
Ÿ Reconstructive surgery
§ Radiation oncology
ξ Bone marrow transplantation
¥ Patient advocacy
* Discussion section writing
committee
*
Version 2.2016 05/06/16© National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
Clinical Trials: NCCN believes that
the best management for any cancer
patient is in a clinical trial.
Participation in clinical trials is
especially encouraged.
To nd clinical trials online at NCCN
Member Institutions, click here:
nccn.org/clinical_trials/physician.html.
NCCN Categories of Evidence and
Consensus: All recommendations
are category 2A unless otherwise
specied.
See NCCN Categories of Evidence
and Consensus.
NCCN Breast Cancer Panel Members
Summary of Guidelines Updates
Noninvasive Breast Cancer:
Lobular Carcinoma In Situ (LCIS-1)
Ductal Carcinoma In Situ (DCIS) Workup and Primary Treatment (DCIS-1)
DCIS Postsurgical Treatment and Surveillance/Follow-up (DCIS-2)
Margin Status in DCIS (DCIS-A)
Invasive Breast Cancer:
Clinical Stage, Workup (BINV-1)
Locoregional Treatment of Clinical Stage l, llA, or llB Disease or T3,N1, M0 (BINV-2)
Systemic Adjuvant Treatment
Hormone Receptor-Positive HER2-Positive Disease (BINV-5)
Hormone Receptor-Positive HER2-Negative Disease (BINV-6)
Hormone Receptor-Negative HER2-Positive Disease (BINV-7)
Hormone Receptor-Negative HER2-Negative Disease (BINV-8)
Favorable Histologies (BINV-9)
Preoperative Systemic Therapy for Operable Breast Cancer: Workup (BINV-10)
Preoperative Systemic Therapy: Breast and Axillary Evaluation (BINV-11)
Preoperative Systemic Therapy: Surgical Treatment (BINV-12)
Preoperative Systemic Therapy: Adjuvant Therapy (BINV-13)
Preoperative Systemic Therapy for Inoperable or Locally Advanced Breast Cancer (Non-Inammatory): Workup (BINV-14)
Preoperative Systemic Therapy for Inoperable or Locally Advanced Breast Cancer (Non-Inammatory) (BINV-15)
Surveillance/Follow-Up (BINV-16)
Recurrent/Stage IV Disease (BINV-17)
Treatment of Recurrence (BINV-18)
Treatment of Stage IV Disease (BINV-19)
ER and/or PR Positive; HER2 Negative or Positive (BINV-20)
ER and PR Negative; or ER and/or PR Positive and Endocrine Refractory; HER2 Negative (BINV-21)
ER and PR Negative; or ER and/or PR Positive and Endocrine Refractory; HER2 Positive (BINV-22)
Follow-Up Therapy for Endocrine Treatment of Recurrent or Stage IV Disease (BINV-23)
Principles of HER2 Testing (BINV-A)
Principles of Dedicated Breast MRI Testing (BINV-B)
Fertility and Birth Control (BINV-C)
Surgical Axillary Staging - Stage l, llA, llB, and IIIA T3, N1, M0 (BINV-D)
Axillary Lymph Node Staging (BINV-E)
Margin Status in Inltrating Carcinoma (BINV-F)
Special Considerations to Breast-Conserving Therapy Requiring Radiation Therapy (BINV-G)
The NCCN Guidelines® are a statement of evidence and consensus of the authors regarding their views of currently accepted approaches to treatment. Any clinician
seeking to apply or consult the NCCN Guidelines is expected to use independent medical judgment in the context of individual clinical circumstances to determine any
patient’s care or treatment. The National Comprehensive Cancer Network® (NCCN®) makes no representations or warranties of any kind regarding their content, use or
application and disclaims any responsibility for their application or use in any way. The NCCN Guidelines are copyrighted by National Comprehensive Cancer Network®.
All rights reserved. The NCCN Guidelines and the illustrations herein may not be reproduced in any form without the express written permission of NCCN. ©2016.
NCCN Guidelines Version 2.2016
Breast Cancer Table of Contents
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Principles of Breast Reconstruction Following Surgery (BINV-H)
Principles of Radiation Therapy (BINV-I)
Adjuvant Endocrine Therapy (BINV-J)
Preoperative/Adjuvant Therapy Regimens (BINV-K)
Principles of Preoperative Systemic Therapy (BINV-L)
Denition of Menopause (BINV-M)
Endocrine Therapy for Recurrent or Stage IV Disease (BINV-N)
Chemotherapy Regimens for Recurrent or Metastatic Breast Cancer (BINV-O)
Principles of Monitoring Metastatic Disease (BINV-P)
Special Considerations:
Phyllodes Tumor (PHYLL-1)
Paget’s Disease (PAGET-1)
Breast Cancer During Pregnancy (PREG-1)
Inammatory Breast Cancer (IBC-1)
Staging (ST-1)
Version 2.2016 05/06/16© National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
UPDATES-1
NCCN Guidelines Version 2.2016
Breast Cancer Updates
Updates in Version 2.2016 of the NCCN Guidelines for Breast Cancer from Version 1.2016 include:
DCIS-1
Modied the rst sentence in footnote "h": "Complete axillary lymph node
dissection should not be performed in the absence of evidence of invasive
cancer or proven axillary metastatic disease in women with apparent pure DCIS
or mammographically detected DCIS with microcalcications."
DCIS-2
• Under "Risk reduction therapy for ipsilateral breast following breast-
conserving surgery" replaced "tamoxifen" with "endocrine therapy" in the
following bullets:
Consider endocrine therapy for 5 years for:
◊Patients treated with breast-conserving therapy (lumpectomy) and
radiation therapyp (category 1), especially for those with ER-positive
DCIS.
◊The benet of endocrine therapy for ER-negative DCIS is uncertain
Version 2.2016 05/06/16© National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Updates in Version 1.2016 of the NCCN Guidelines for Breast Cancer from Version 2.2015 include:
Discussion
The discussion section has been updated to reect changes in the
algorithm.
BINV-1
• Workup, changed "Fertility counseling if premenopausal" to "Counseling
for fertility concerns if premenopausal."
BINV-2
• Claried imaging for systemic staging, removed "or MRI."
BINV-5
• Systemic adjuvant treatment - hormone receptor-positive - HER2-
positive disease, removed footnote "y" stating "Evidence supports that
the magnitude of benet from surgical or radiation ovarian ablation in
premenopausal women with hormone receptor-positive breast cancer
is similar to that achieved with CMF alone. See Adjuvant Endocrine
Therapy (BINV-J) and Preoperative/Adjuvant Therapy Regimens
(BINV-K).
BINV-9
• Moved the following footnote to the algorithm: "If ER-positive, consider
endocrine therapy for risk reduction and to diminish the small risk of
disease recurrence."
BINV-11
• Claried imaging by adding "with ultrasound."
Added new bullets for "Endocrine therapy:"
◊Tamoxifeno for premenopausal patients
◊Tamoxifeno or aromatase inhibitor for postmenopausal patients with some
advantage for aromatase inhibitor therapy in patients <60 years old or with
concerns for thromboembolism
• Modied footnote "o" for consistency with NCCN Guidelines for Breast
Cancer Risk Reduction. The footnote states "CYP2D6 genotype testing is not
recommended in women who are considering tamoxifen."
Footnote "p", changed "tamoxifen" to "endocrine therapy."
BINV-1
• CBC includes platelets, deleted "platelets"
• "Assess for distress" - moved the link to the NCCN Guidelines for Distress
Management from the algorithm to a footnote.
• Listed CBC, liver function tests and alkaline phosphatase as optional based on
signs and symptoms and clinical stage I-IIB, and IIIA (T3,N1,M0).
• Footnote "k" is new, "See NCCN Guidelines for Older Adult Oncology for special
treatment considerations."
BINV-12
• Removed the statement "Endocrine therapy alone with an aromatase inhibitor
(preferred option for postmenopausal women; given along with ovarian
suppression for premenopausal women) or tamoxifen may be considered for
patients with hormone-receptor positive disease]" and linked to BINV-L (also
applies to BINV-15)
• Footnote "jj" claried "imaging studies" by adding (mammogram and/or breast
MRI).
BINV-22
• Changed trastuzumab containing chemotherapy to HER2-targeted
chemotherapy.
BINV-N
• Added "Selective ER modulators."
IBC-1
• Added a new footnote, "The accurate assessment of in-breast tumor or
regional lymph node response to preoperative systemic therapy is difcult,
and should include physical examination and performance of imaging studies
(mammogram and/or breast MRI) that were abnormal at the time of initial
tumor staging. Selection of imaging methods prior to surgery should be
determined by the multidisciplinary team."
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
UPDATES-2
NCCN Guidelines Version 2.2016
Breast Cancer Updates
BINV-10
• Modied the workup for consistency with BINV-1.
• CBC includes platelets, deleted "platelets"
• "Assess for distress" - moved the link to the NCCN Guidelines for Distress
Management from the algorithm to a footnote.
• Additional studies consider: CBC, liver function tests and alkaline
phosphatase as optional based on signs and symptoms and clinical stage
I-IIB, and IIIA (T3,N1,M0).
• If lymph node FNA or core biopsy positive, axilla may be restaged after
preoperative systemic therapy, added
"
(category 2B)."
• Removed bottom branch for "Surgical resection."
BINV-11
• Clinically negative axillary lymph node, changed "should have" to
"consider."
• Clinically positive axillary lymph node, added (category 2B) to "SLNB or
ALND can be performed if axilla is clinically negative."
BINV-12
• Preoperative systemic therapy, modied the statement " [Endocrine therapy
alone with an aromatase inhibitor (preferred option for postmenopausal
women; given along with ovarian suppression for premenopausal
women) or tamoxifen may be considered for patients with hormone-
receptor positive disease]."
• Added a footnote "See Principles of Preoperative Systemic Therapy (BINV-L)."
BINV-13
• Mastectomy and surgical axillary staging ± reconstruction - Added
a footnote. "See Principles of Breast Reconstruction Following Surgery
(BINV-H)."
• Revised footnote "qq": "Axilla may be restaged after preoperative
systemic therapy (category 2B); ALND should be performed if axilla is
clinically positive; SLNB or ALND can be performed if axilla is clinically
negative."
Updates in Version 1.2016 of the NCCN Guidelines for Breast Cancer from Version 2.2015 include:
Version 2.2016 05/06/16© National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
BINV-2
• Following ≥4 positive axillary nodes, added "internal mammary nodes, and
any part of the axillary bed at risk (category 1). Removed "Strongly consider
radiation therapy to internal mammary nodes (category 2B)
• Footnote "q" was deleted. "Radiation therapy should be given to the internal
mammary lymph nodes that are clinically or pathologically positive; otherwise
the treatment to the internal mammary nodes is at the discretion of the treating
radiation oncologist. CT treatment planning should be utilized in all cases where
radiation therapy is delivered to the internal mammary lymph nodes."
• Following 1-3 positive axillary nodes, removed category 2B from radiation
therapy to internal mammary nodes. "Strongly consider radiation therapy to
infraclavicular, supraclavicular area, internal mammary nodes" added "and any
part of the axillary bed at risk."
BINV-3
• Following ≥4 positive axillary nodes, "Postchemotherapy radiation therapy
to chest wall + infraclavicular region, supraclavicular area" added "internal
mammary nodes, and any part of the axillary bed at risk (category 1)." Removed
"Strongly consider radiation therapy to internal mammary nodes (category 2B)
• Following 1-3 positive axillary nodes, "Strongly consider postchemotherapy
radiation therapy to chest wall + infraclavicular region, supraclavicular area,
internal mammary nodes" added "and any part of the axillary bed at risk."
Removed "if radiation therapy is given, strongly consider internal mammary
node radiation therapy (category 2B)."
• Following Negative axillary nodes and tumor >5 cm or margins positive,
"Consider postchemotherapy radiation therapy to chest wall ± infraclavicular
region, ± supraclavicular area" added "± internal mammary nodes, and
any part of the axillary bed at risk.". Removed "Strongly consider radiation
therapy to internal mammary nodes (category 2B)."
BINV-6
• Added a footnote to "Consider 21-gene RT-PCR assay" stating "Other prognostic
multigene assays may be considered to help assess risk of recurrence but have
not been validated to predict response to chemotherapy."
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
BINV-16
• Added a new bullet; "Periodic screening for changes in family history and
referral to genetic counseling as necessary."
• Added a footnote to "Mammography every 12 mo." The new footnote
states "Studies indicate that annual mammograms are the appropriate
frequency for surveillance of breast cancer patients who have had breast-
conserving surgery and radiation therapy with no clear advantage to shorter
interval imaging. Patients should wait 6 to 12 months after the completion
of radiation therapy to begin their annual mammogram surveillance.
Suspicious ndings on physical examination or surveillance imaging might
warrant a shorter interval between mammograms."
• Added a new bullet "Routine imaging of reconstructed breast is not
indicated."
• Added a new bullet "In the absence of clinical signs and symptoms
suggestive of recurrent disease, there is no indication for laboratory or
imaging studies for metastases screening."
• Added healthy diet and limited alcohol intake to the following bullet
"Evidence suggests that active lifestyle, healthy diet, limited alcohol intake,
and achieving and maintaining an ideal body weight (20–25 BMI) may lead to
optimal breast cancer outcomes."
• Revised footnote "tt": "The use of estrogen, progesterone, or selective
estrogen receptor modulators to treat osteoporosis or osteopenia in women
with breast cancer is discouraged. The
use of a bisphosphonate or denosumab is acceptable to maintain or to
improve bone mineral density. Optimal duration of either therapy has not
been established. Duration beyond 3 y is not known. Factors to consider
for duration of anti-osteoporosis therapy include bone mineral density,
response to therapy, and risk factors for continued bone loss or fracture.
Women treated with a bisphosphonate or denosumab should undergo a
dental examination with preventive dentistry prior to the initiation of therapy,
and should take supplemental calcium and vitamin D."
Updates in Version 1.2016 of the NCCN Guidelines for Breast Cancer from Version 2.201 include:
UPDATES-3
NCCN Guidelines Version 2.2016
Breast Cancer Updates
BINV-14
• Listed CBC, liver function tests and alkaline phosphatase under Additional
studies.
• CBC includes platelets, deleted "platelets"
"Assess for distress" - moved the link to the NCCN Guidelines for Distress
Management from the algorithm to a footnote.
BINV-15
• "Preoperative systemic therapy, modied the statement [Endocrine therapy
alone with an aromatase inhibitor (preferred option for postmenopausal
women; given along with ovarian suppression for premenopausal women)
or tamoxifen may be considered for patients with hormone-receptor
positive disease]."
• Added a footnote "See Principles of Preoperative Systemic Therapy (BINV-L)."
• Removed the following footnotes from page BINV-12 and BINV-15, they have been
incorporated into Principles of Preoperative Systemic Therapy (BINV-L):
A number of chemotherapy regimens have activity in the preoperative
setting. In general, those chemotherapy regimens recommended in the
adjuvant setting may be considered in the preoperative setting. See
Preoperative/Adjuvant Chemotherapy (BINV-K). If treated with endocrine
therapy, an aromatase inhibitor is preferred for postmenopausal women.
Patients with HER2-positive tumors should be treated with preoperative
systemic incorporating trastuzumab for at least 9 weeks of preoperative
therapy
See Preoperative/Adjuvant Chemotherapy (BINV-K).
A pertuzumab-containing regimen may be administered preoperatively to
patients with greater than or equal to T2 or greater than or equal to N1,
HER2-positive breast cancer.
Administration of all chemotherapy prior to surgery is preferred.
• Removed "(plus internal mammary nodes if involved, strongly consider
internal mammary nodes if not clinically involved (category 2B)."
• Removed "delayed" from breast reconstruction.
• Removed "consider" from the mastectomy/lumpectomy choice.
• Added "and internal mammary nodes and any part of the axillary bed at
risk."
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
UPDATES-4
NCCN Guidelines Version 2.2016
Breast Cancer Updates
BINV-17
• CBC includes platelets, deleted "platelets"
BINV-18
• Changed page header to "Treatment of Recurrence."
• Simplied the recommendations for "Radiation therapy", by removing
the following text: "to chest wall and supraclavicular and infraclavicular
nodes."
• Added the following footnote " Multidisciplinary approach is especially
important in the management of breast cancer recurrence to consider all
potential treatment options for optimal outcomes."
BINV-19
Changed page header to "Treatment of Stage IV Disease."
BINV-C
• Fertility and birth control, modied the rst bullet: "All premenopausal
patients should be informed about the potential impact of chemotherapy
on fertility and asked about their desire for potential future pregnancies.
Patients who may desire future pregnancies should be referred to
fertility specialists before chemotherapy and/or endocrine therapy,
to discuss the options based on patient specics, disease stage
and biology, (which determine the urgency and type and sequence
of treatment). Timing and duration allowed for fertility preservation,
options inclusive of oocyte and embryo cryopreservation as well as
evolving technologies, and the probability of successful pregnancies
subsequent to completion of breast cancer therapy are also to be
discussed."
BINV-D
• Footnote 2: Removed the last sentence "However, only peritumoral
injections map to the internal mammary lymph node(s).
BINV-E
• Replaced "Sentinel lymph node biopsy is the preferred method of
axillary lymph node staging if there is an experienced sentinel node
team and the patient is an appropriate sentinel lymph node biopsy
candidate (See BINV-D)." with "Sentinel lymph node biopsy should be
performed and is the preferred method of axillary lymph node staging if
the patient is an appropriate sentinel lymph node biopsy candidate."
Updates in Version 1.2016 of the NCCN Guidelines for Breast Cancer from Version 2.2016 include:
BINV-F
Second paragraph, modied the last sentence "A boost to the tumor bed is
recommended in patients at higher risk for recurrence."
BINV-G
• Absolute contraindications: added "Diffusely positive pathologic margins"
and removed "Positive pathologic margin."
• Relative contraindications: added "Positive pathologic margin" and removed
"Diffusely positive pathologic margins."
• Added a link to NCCN Guidelines for Genetic/Familial High-Risk Assessment
Breast and Ovarian.
BINV-H (1 of 2)
• First paragraph, added the following "However, breast reconstruction should
not interfere with the appropriate surgical management of the cancer or
the scope of appropriate surgical treatment for this disease. Coordinating
consultation and surgical treatment with a reconstructive surgeon should be
executed within a reasonable time frame."
• Modied "Oncoplastic techniques for breast conservation can extend breast-
conserving surgical options in situations where the resection by itself would
likely yield an unacceptable cosmetic outcome."
BINV-H (2 of 2)
• Modied the statement "Evidence of nipple involvement such as Paget’s
disease or other nipple discharge associated with malignancy, and/
or imaging ndings suggesting malignant involvment of the nipple or
subareolar tissues is a contraindicates nipple preservation."
BINV-I
• This page has been reorganized and updated.
BINV-J
• Changed tamoxifen for 5 y (category 1) ± ovarian suppression or ablation
(category 2B) to a (category 1).
• Adjuvant endocrine therapy - premenopausal at diagnosis, added "or aromatase
inhibitor for 5y + ovarian suppression or ablation (category 1)." With a new
footnote " Aromatase inhibitor or tamoxifen for 5 y plus ovarian suppression
should be considered, based on SOFT and TEXT clinical trial outcomes, for
premenopausal women at higher risk of recurrence (i.e. young age, high grade
tumor, lymph node involvement, Pagani, NEJM 2014, Prudence, NEJM 2014).
Survival data still pending."
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
Version 2.2016 05/06/16© National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Footnote 4 is new to the page. "A single study (S0226) in women with
hormone receptor-positive breast cancer and no prior chemotherapy,
biological therapy, or endocrine therapy for metastatic disease
demonstrated that the addition of fulvestrant to anastrozole resulted
in prolongation of time to progression. Subset analysis suggested that
patients without prior adjuvant tamoxifen and more than 10 years since
diagnosis experienced the greatest benet. Two studies with similar
design (FACT and SOFEA) demonstrated no advantage in time to
progression with the addition of fulvestrant to anastrozole."
BINV-O
• Other rst-line agents for HER2-positive disease: Trastuzumab alone has
been removed.
• Footnote 4 is new to the page: Trastuzumab may be safely combined
with all non-anthracycline containing preferred and other single agents
listed above for recurrent or metastatic breast cancer.
• Updated reference list.
BINV-P (3 of 3)
• Suggested intervals of follow-up for patients with metastatic disease,
changed the interval for endocrine therapy follow-up from "2-3 mo" to
"1-3 mo."
PHYLL-1
• Revised footnote "a": "FNA or core biopsy may not distinguish a
broadenoma from a phyllodes tumor in some cases. The sensitivity
of core biopsy for the diagnosis of phyllodes tumor is greater than
that of FNA biopsy, but neither core biopsy or FNA biopsy can always
differentiate phyllodes tumors from broadenomas. In cases with
clinical suspicion for phyllodes tumor, excision of the lesion may be
needed for denitive pathologic classication."
PHYLL-2
• Added footnote "a" to "Tissue sampling"
PREG-1
• Changed: Pregnant patient with conrmed breast cancer diagnosis (core
biopsy preferred) To: Pregnant patient with conrmed breast cancer
diagnosis by FNA or core biopsy; No distant metastases on staging.
BINV-I
• This page has been reorganized and updated.
BINV-J
• Changed tamoxifen for 5 y (category 1) ± ovarian suppression or ablation
(category 2B) to a (category 1).
Adjuvant endocrine therapy - premenopausal at diagnosis, added "or aromatase
inhibitor for 5y + ovarian suppression or ablation (category 1)." With a new footnote
" Aromatase inhibitor or tamoxifen for 5 y plus ovarian suppression should be
considered, based on SOFT and TEXT clinical trial outcomes, for premenopausal
women at higher risk of recurrence (i.e. young age, high grade tumor, lymph
node involvement, Pagani, NEJM 2014, Prudence, NEJM 2014).Survival data still
pending."BINV-K (1 of 7)
• Footnote 5 is new to the page. "The regimens listed for HER2-negative disease are all
category 1 (except where indicated) when used in the adjuvant setting."
• Removed FAC/CAF (uorouracil/doxorubicin/cyclophosphamide) and FEC/CEF
(cyclophosphamide/ epirubicin/uorouracil) from the list of regimens for preoperative/
adjuvant chemotherapy.
BINV-K (3 of 7)
• Under the regimen "FAC followed by weekly paclitaxel, changed 6 to 4 cycles.
BINV-K (4, 5, and 6 of 7)
• Replaced cardiac monitoring at baseline, 3, 6, and 9 mo with "Evaluate left ventricular
ejection fraction (LVEF) prior to and during treatment."
• Added the following footnote " The optimal frequency of LVEF assessment during
adjuvant trastuzumab therapy is not known. The FDA label recommends LVEF
measurements prior to initiation of trastuzumab and every 3 mo during therapy."
BINV-L
• New page - Principles of Preoperative Systemic Therapy.
BINV-N
• Modied rst statement, "Premenopausal patients with
hormone receptor- positive
disease should have ovarian ablation/suppression and follow
• Endocrine therapy for recurrent or stage IV disease, added Palbociclib +
fulvestrant (category 1) with the following footnote: "For postmenopausal women
or for premenopausal women receiving ovarian suppression with an LHRH
agonist, with hormone-receptor positive and HER2-negative metastatic breast
cancer that has progressed on endocrine therapy."
UPDATES-5
Updates in Version 1.2016 of the NCCN Guidelines for Breast Cancer from Version 2.2016 include:
NCCN Guidelines Version 2.2016
Breast Cancer Updates
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
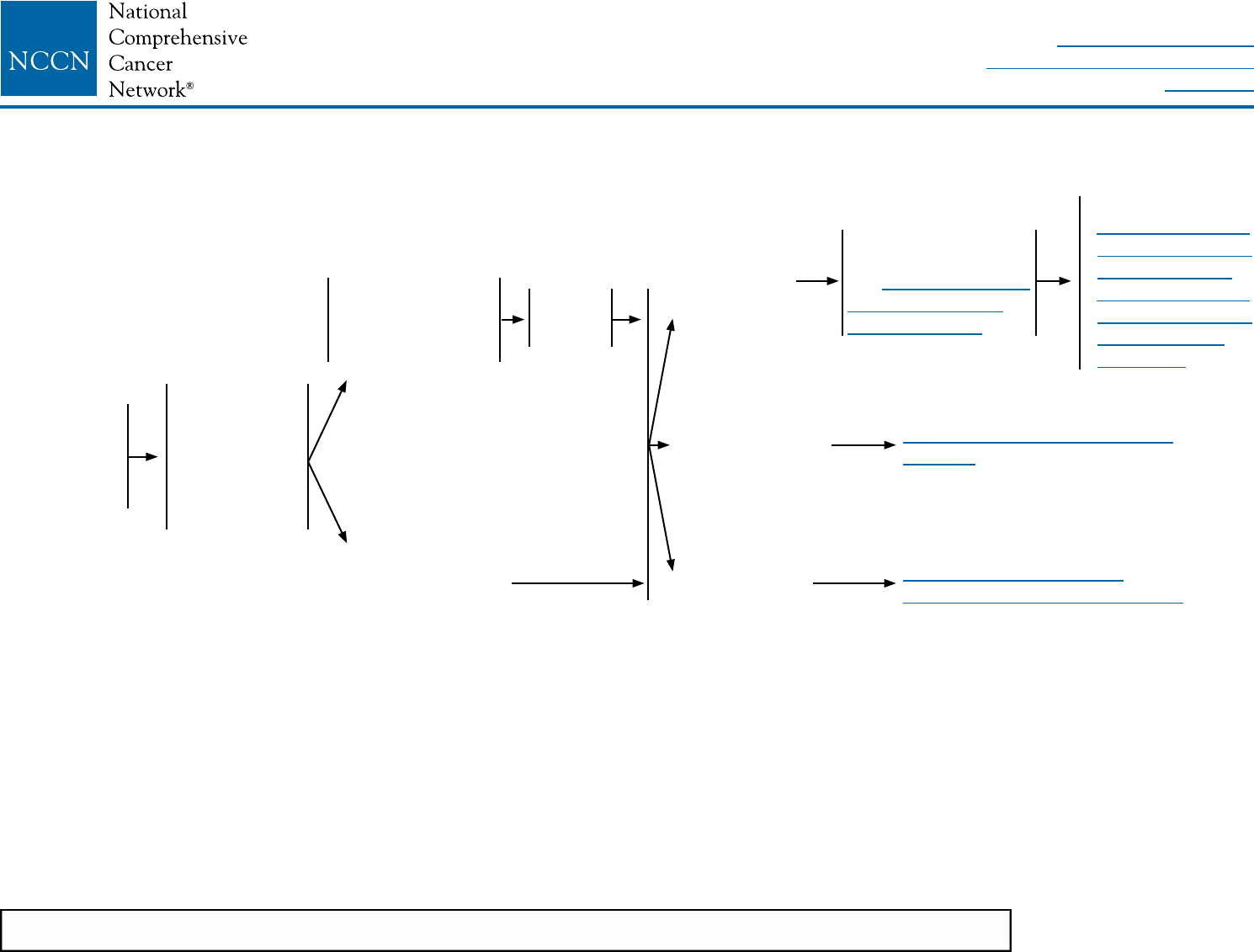
LCIS-1
aLCIS is present on initial biopsy (needle or surgical) or on final excision with or without other proliferative changes (atypical ductal or lobular hyperplasia).
bSome variants of LCIS (pleomorphic LCIS) may have a similar biological behavior to that of DCIS. Clinicians may consider complete excision with negative margins
for pleomorphic LCIS, but outcomes data regarding the efficacy of surgical excision to negative margins are lacking. There are no data to support using radiotherapy
in this setting.
cMultifocal/extensive LCIS involving >4 terminal ductal lobular units on a core biopsy may be associated with increased risk for invasive cancer on surgical excision.
DIAGNOSIS WORKUP RISK REDUCTION SURVEILLANCE
LCIS
identied on
breast biopsy
Stage 0
Tis, N0, M0
• History and
physical
• Diagnostic
bilateral
mammogram
• Pathology
review
Biopsy was core
needle biopsy
(less than surgical
biopsy)a,b
Initial biopsy was
surgical biopsya,b,c
Perform
surgical
excision
LCIS without
other cancer
Ductal carcinoma
in situ (DCIS)
Invasive
breast cancer
Surveillance as per
• NCCN Guidelines
for Breast Cancer
Risk Reduction
• NCCN Guidelines
for Breast Cancer
Screening and
Diagnosis
Counseling regarding
risk reduction,
see NCCN Guidelines
for Breast Cancer
Risk Reduction
See NCCN Guidelines for DCIS
(DCIS-1)
See NCCN Guidelines for
Invasive Breast Cancer (BINV-1)
NCCN Guidelines Version 2.2016
Lobular Carcinoma in Situ (LCIS)
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
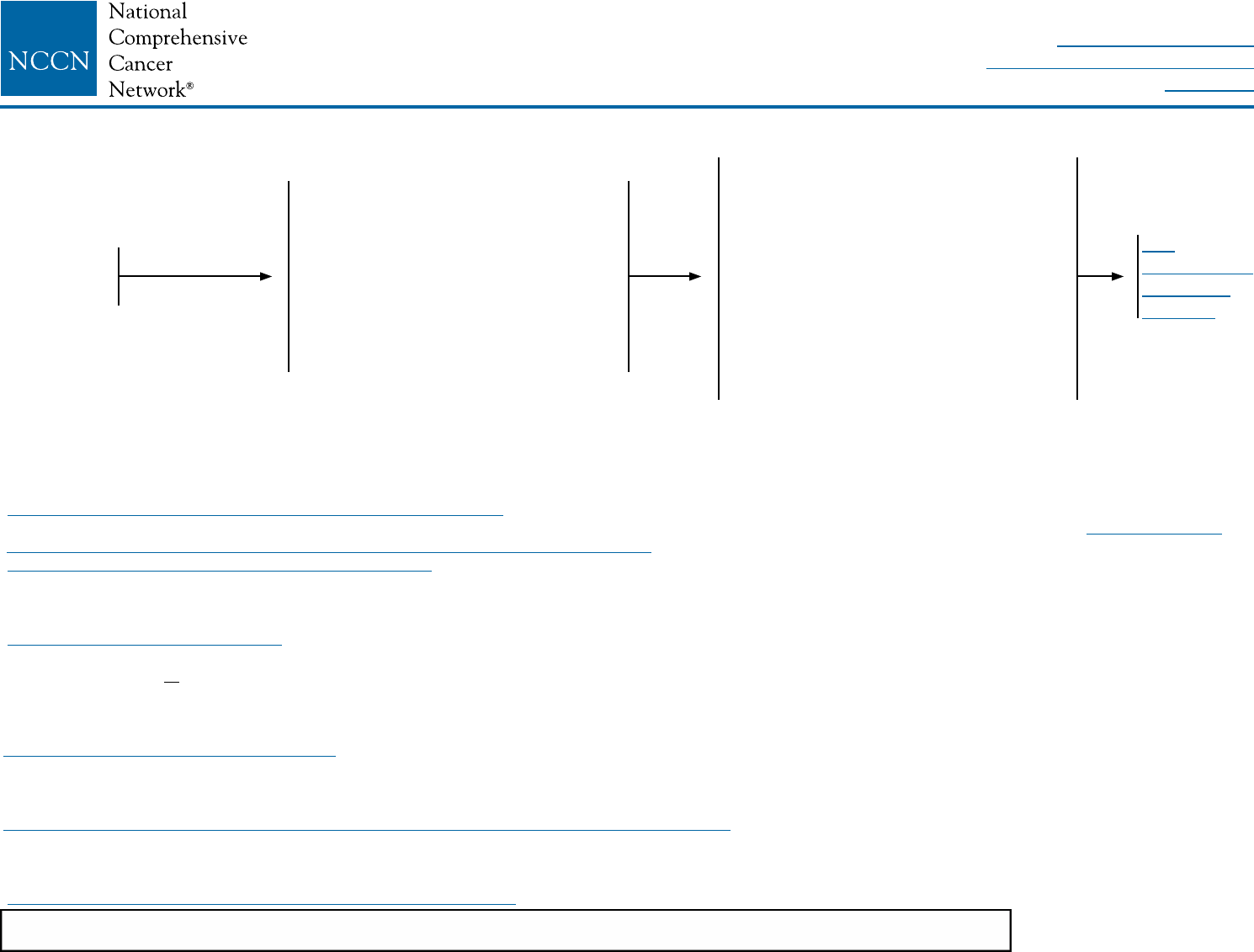
DCIS-1
aSee NCCN Guidelines for Breast Cancer Screening and Diagnosis.
bThe panel endorses the College of American Pathologists Protocol for pathology reporting for all invasive and noninvasive carcinomas of the breast. http://www.cap.org.
cSee NCCN Guidelines for Genetic/Familial High-Risk Assessment: Breast and Ovarian.
dSee Principles of Dedicated Breast MRI Testing (BINV-B).
eThe use of MRI has not been shown to increase likelihood of negative margins or decrease conversion to mastectomy. Data to support improved long-term outcomes are lacking.
fRe-resection(s) may be performed in an effort to obtain negative margins in patients desiring breast-conserving therapy. Patients not amenable to margin-free
lumpectomy should have total mastectomy.
gSee Margin Status in DCIS (DCIS-A).
hComplete axillary lymph node dissection should not be performed in the absence of evidence of invasive cancer or proven axillary metastatic disease in women with
apparent pure DCIS or mammographically detected DCIS with microcalcifications. However, a small proportion of patients with apparent pure DCIS will be found to
have invasive cancer at the time of their definitive surgical procedure. Therefore, the performance of a sentinel lymph node procedure should be strongly considered if
the patient with apparent pure DCIS is to be treated with mastectomy or with excision in an anatomic location compromising the performance of a future sentinel lymph
node procedure.
iSee Principles of Radiation Therapy (BINV-I).
jComplete resection should be documented by analysis of margins and specimen radiography. Post-excision mammography could also be performed whenever
uncertainty about adequacy of excision remains.
kPatients found to have invasive disease at total mastectomy or re-excision should be managed as having stage l or stage ll disease, including lymph node staging.
lSee Special Considerations to Breast-Conserving Therapy Requiring Radiation Therapy (BINV-G).
mWhole-breast radiation therapy following lumpectomy reduces recurrence rates in DCIS by about 50%. Approximately half of the recurrences are invasive and half are DCIS. A
number of factors determine local recurrence risk: palpable mass, larger size, higher grade, close or involved margins, and age <50 years. If the patient and physician view the
individual risk as “low,” some patients may be treated by excision alone. Data evaluating the three local treatments show no differences in patient survival.
nSee Principles of Breast Reconstruction Following Surgery (BINV-H).
DIAGNOSIS WORKUP PRIMARY TREATMENT
DCIS
Stage 0
Tis, N0, M0a
• History and physical exam
• Diagnostic bilateral mammogram
• Pathology reviewb
• Determination of tumor estrogen
receptor (ER) status
• Genetic counseling if patient is
high-risk for hereditary breast
cancerc
• Breast MRId,e (optional)
Lumpectomyf,g without lymph node
surgeryh + whole breast radiation
therapyi,j,k,l,m (category 1)
or
Total mastectomy with or without
sentinel node biopsyh,k ±
reconstructionn
or
Lumpectomyf,g without lymph node
surgeryh without radiation therapyi,k,l,m
(category 2B)
See
Postsurgical
Treatment
(DCIS-2)
NCCN Guidelines Version 2.2016
Ductal Carcinoma in Situ (DCIS)
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
DCIS-2
oCYP2D6 genotype testing is not recommended in women who are considering tamoxifen.
pAvailable data suggest endocrine therapy provides risk reduction in the ipsilateral breast treated with breast conservation and in the contralateral breast in patients
with mastectomy or breast conservation with ER-positive primary tumors. Since a survival advantage has not been demonstrated, individual consideration of risks and
benefits is important (See also NCCN Guidelines for Breast Cancer Risk Reduction).
Risk reduction therapy for ipsilateral breast following
breast-conserving surgery:
• Consider endocrine therapy for 5 years for:
Patients treated with breast-conserving therapy
(lumpectomy) and radiation therapyp (category 1),
especially for those with ER-positive DCIS.
The benet of endocrine therapy for ER-negative DCIS
is uncertain
Patients treated with excision alonep
• Endocrine therapy:
Tamoxifeno for premenopausal patients
Tamoxifeno or aromatase inhibitor for postmenopausal
patients with some advantage for aromatase inhibitor
therapy in patients <60 years old or with concerns for
thromboembolism
Risk reduction therapy for contralateral breast:
• Counseling regarding risk reduction
See NCCN Guidelines for Breast Cancer Risk Reduction
DCIS POSTSURGICAL TREATMENT SURVEILLANCE/FOLLOW-UP
• Interval history and physical exam every 6–12 mo for 5 y,
then annually
• Mammogram every 12 mo (and 6–12 mo postradiation
therapy if breast conserved [category 2B])
• If treated with endocrine therapy, monitor per NCCN
Guidelines for Breast Cancer Risk Reduction
NCCN Guidelines Version 2.2016
Ductal Carcinoma in Situ (DCIS)
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
DCIS-A
MARGIN STATUS IN DCIS
Substantial controversy exists regarding the denition of a negative pathologic margin in DCIS. Controversy arises out of the heterogeneity of
the disease, difculties in distinguishing the spectrum of hyperplastic conditions, anatomic considerations of the location of the margin, and
inadequate prospective data on prognostic factors in DCIS.
Margins greater than 10 mm are widely accepted as negative (but may be excessive and may lead to a less optimal cosmetic outcome).
Margins less than 1 mm are considered inadequate.
With pathologic margins between 1–10 mm, wider margins are generally associated with lower local recurrence rates. However, close surgical
margins (<1 mm) at the broglandular boundary of the breast (chest wall or skin) do not mandate surgical re-excision but can be an indication
for higher boost dose radiation to the involved lumpectomy site (category 2B).
NCCN Guidelines Version 2.2016
Ductal Carcinoma in Situ (DCIS)
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
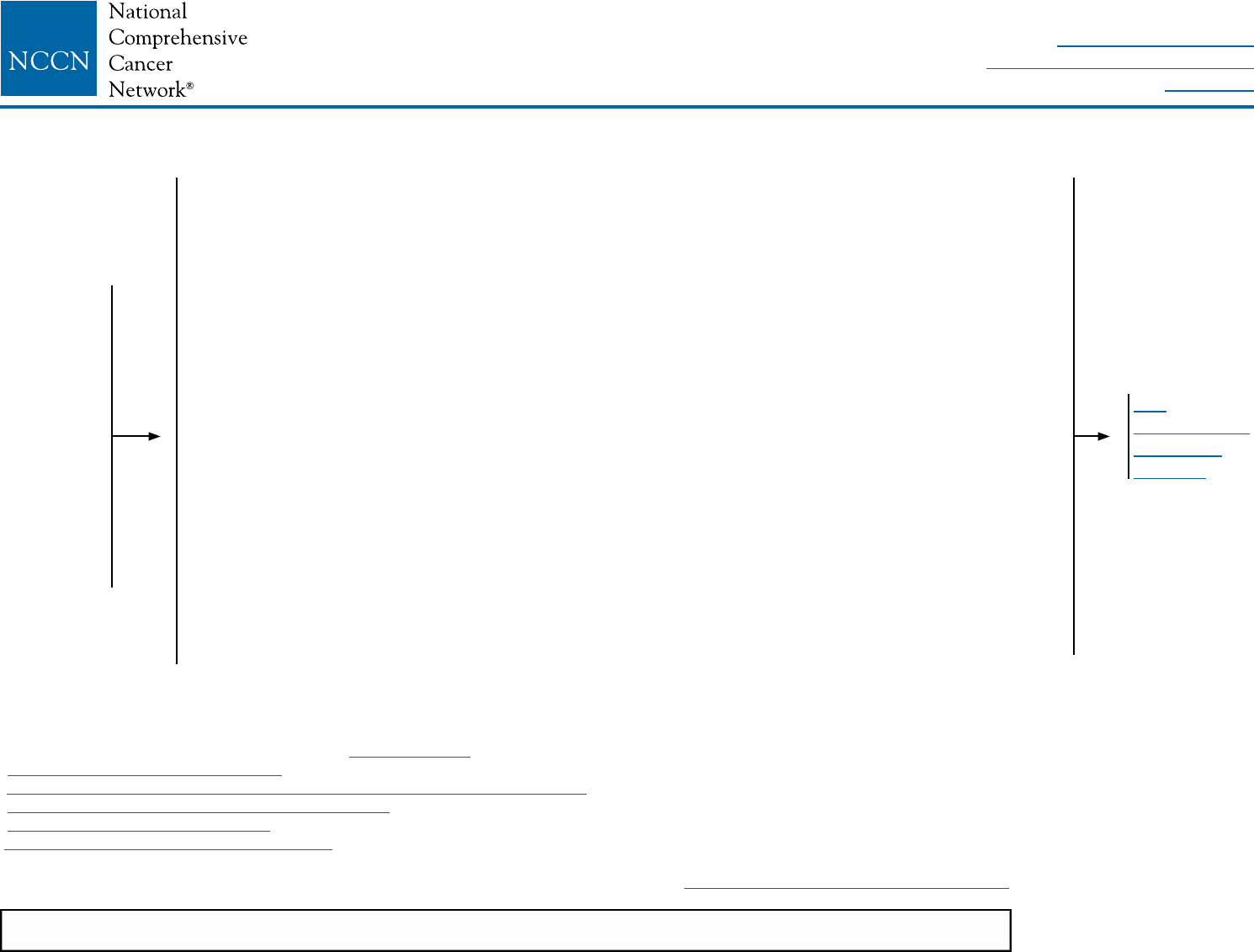
BINV-1
aThe panel endorses the College of American Pathologists Protocol for pathology reporting for all
invasive and noninvasive carcinomas of the breast. http://www.cap.org.
bSee Principles of HER2 Testing (BINV-A).
cSee NCCN Guidelines for Genetic/Familial High-Risk Assessment: Breast and Ovarian.
dSee Principles of Dedicated Breast MRI Testing (BINV-B).
eSee Fertility and Birth Control (BINV-C).
fSee NCCN Guidelines for Distress Management.
gRoutine systemic staging is not indicated for early breast cancer in the absence of symptoms.
hIf FDG PET/CT is performed and clearly indicates bone metastasis, on both the PET and CT
component, bone scan or sodium fluoride PET/CT may not be needed.
CLINICAL
STAGE
WORKUP
Stage I
T1, N0, M0
or
Stage IIA
T0, N1, M0
T1, N1, M0
T2, N0, M0
or
Stage IIB
T2, N1, M0
T3, N0, M0
or
Stage IIIA
T3, N1, M0
• History and physical exam
• Diagnostic bilateral mammogram; ultrasound as necessary
• Pathology reviewa
• Determination of tumor estrogen/progesterone receptor (ER/PR) status and HER2 statusb
• Genetic counseling if patient is high risk for hereditary breast cancerc
• Breast MRId (optional), with special consideration for mammographically occult tumors
• Counseling for fertility concerns if premenopausale
• Assess for distressf
For clinical stage I-IIB, consider additional studies only if directed by signs or symptoms:g
• CBC
• Liver function tests and alkaline phosphatase
• Bone scan indicated if localized bone pain or elevated alkaline phosphatase
• Abdominal ± pelvic diagnostic CT or MRI indicated if elevated alkaline phosphatase, abnormal liver function
tests, abdominal symptoms, or abnormal physical examination of the abdomen or pelvis
• Chest diagnostic CT (if pulmonary symptoms present)
If clinical stage lllA (T3, N1, M0) consider:
• CBC
• Liver function tests and alkaline phosphatase
• Chest diagnostic CT
• Abdominal ± pelvic diagnostic CT or MRI
• Bone scan or sodium uoride PET/CTh (category 2B)
• FDG PET/CTi,j (optional, category 2B)
See
Locoregional
Treatmentk
(BINV-2)
iFDG PET/CT can be performed at the same time as diagnostic CT. The use of PET
or PET/CT scanning is not indicated in the staging of clinical stage I, II, or operable
stage III breast cancer. FDG PET/CT is most helpful in situations where standard
staging studies are equivocal or suspicious, especially in the setting of locally
advanced or metastatic disease.
jFDG PET/CT may also be helpful in identifying unsuspected regional nodal disease
and/or distant metastases in locally advanced breast cancer when used in addition
to standard staging studies.
kSee NCCN Guidelines for Older Adult Oncology for special treatment considerations.
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
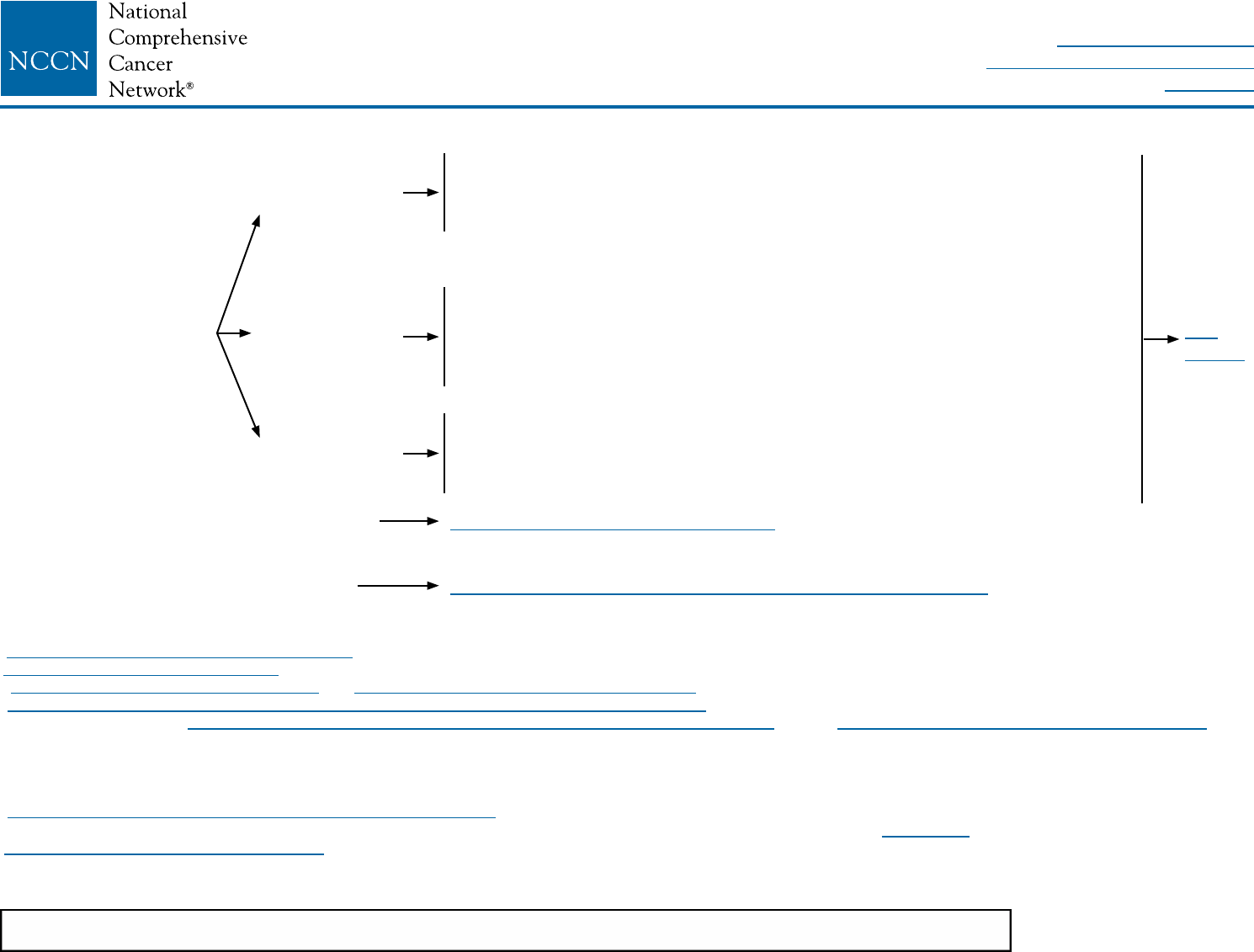
BINV-2
kSee NCCN Guidelines for Older Adult Oncology for special treatment considerations.
lSee Surgical Axillary Staging (BINV-D).
mSee Axillary Lymph Node Staging (BINV-E) and Margin Status in Infiltrating Carcinoma (BINV-F).
nSee Special Considerations to Breast-Conserving Therapy Requiring Radiation Therapy (BINV-G).
oExcept as outlined in the NCCN Guidelines for Genetic/Familial High-Risk Assessment: Breast and Ovarian and the NCCN Guidelines for Breast Cancer Risk Reduction,
prophylactic mastectomy of a breast contralateral to a known unilateral breast cancer is discouraged. When considered, the small benefits from contralateral prophylactic
mastectomy for women with unilateral breast cancer must be balanced with the risk of recurrent disease from the known ipsilateral breast cancer, psychological and social issues
of bilateral mastectomy, and the risks of contralateral mastectomy. The use of a prophylactic mastectomy contralateral to a breast treated with breast-conserving therapy is very
strongly discouraged.
pSee Principles of Breast Reconstruction Following Surgery (BINV-H).
qConsider imaging for systemic staging, including diagnostic CT, bone scan, and optional FDG PET/CT (category 2B) (See BINV-1).
rSee Principles of Radiation Therapy (BINV-I).
sPBI may be administered prior to chemotherapy.
tBreast irradiation may be omitted in patients ≥70 y of age with estrogen-receptor positive, clinically node-negative, T1 tumors who receive adjuvant endocrine therapy
(category 1).
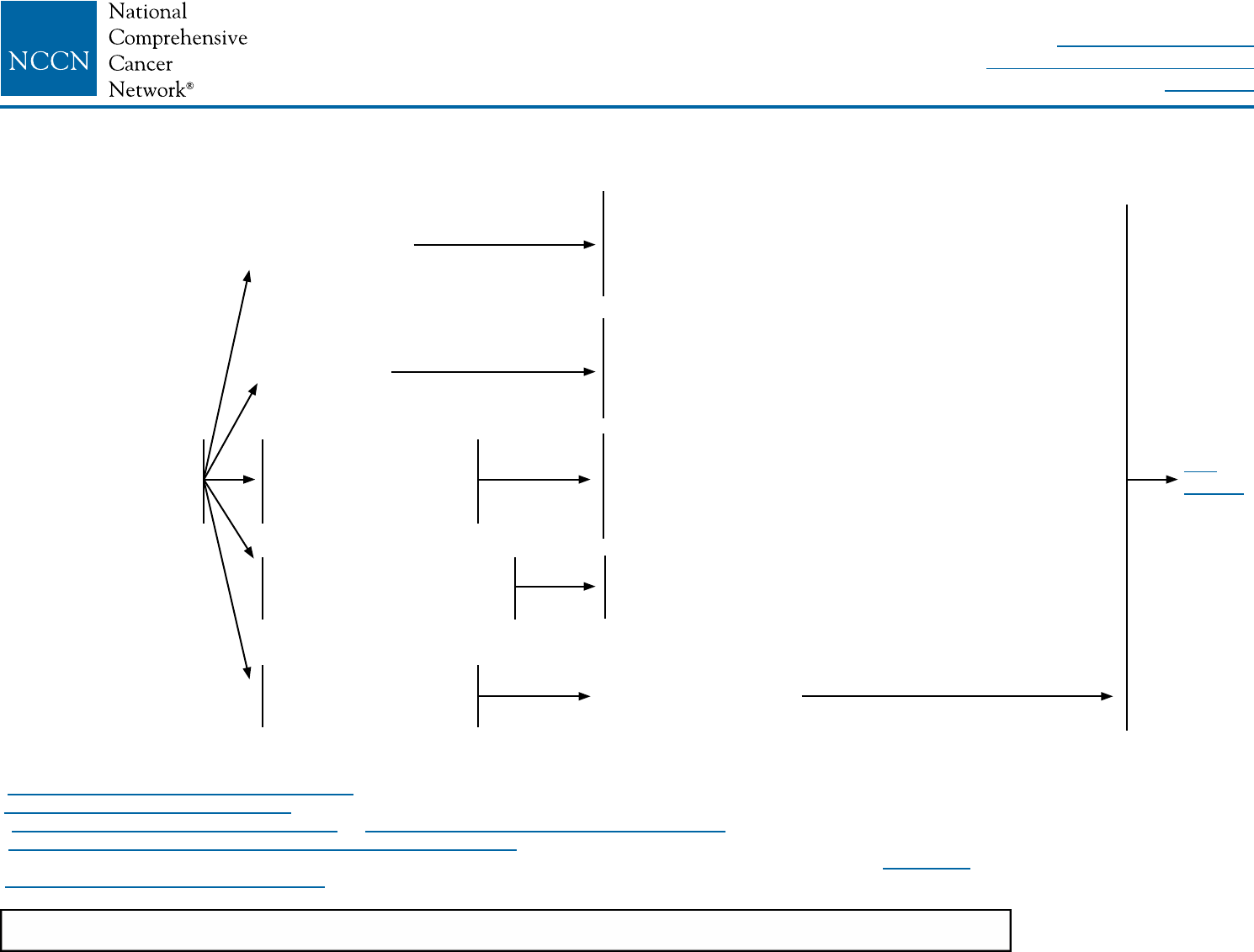
LOCOREGIONAL TREATMENT OF CLINICAL STAGE I, IIA, OR IIB DISEASE OR T3, N1, M0k
Lumpectomy with
surgical axillary staging
(category 1)l,m,n
or
Total mastectomy with surgical axillary
stagingl,m,o (category 1) ± reconstructionp
or
If T2 or T3 and fullls criteria for breast-
conserving therapy except for sizen
≥4 positiveq
axillary nodes
1–3 positive
axillary nodes
Negative
axillary nodes
Radiation therapy to whole breast with or without boostr to tumor bed
(category 1), infraclavicular region, supraclavicular area, internal mammary
nodes, and any part of the axillary bed at risk (category 1). It is common for
radiation therapy to follow chemotherapy when chemotherapy is indicated.
Radiation therapy to whole breast with or without boostr to tumor bed
(category 1). Strongly consider radiation therapy to infraclavicular region,
supraclavicular area, internal mammary nodes, and any part of the axillary
bed at risk. It is common for radiation therapy to follow chemotherapy when
chemotherapy is indicated.
Radiation therapy to whole breast with or without boostr to tumor bed or
consideration of partial breast irradiation (PBI) in selected patients.r,s
It is common for radiation therapy to follow chemotherapy when
chemotherapy is indicated.t
See Locoregional Treatment (BINV-3)
Consider Preoperative Systemic Therapy Guideline (BINV-10)
See
BINV-4
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
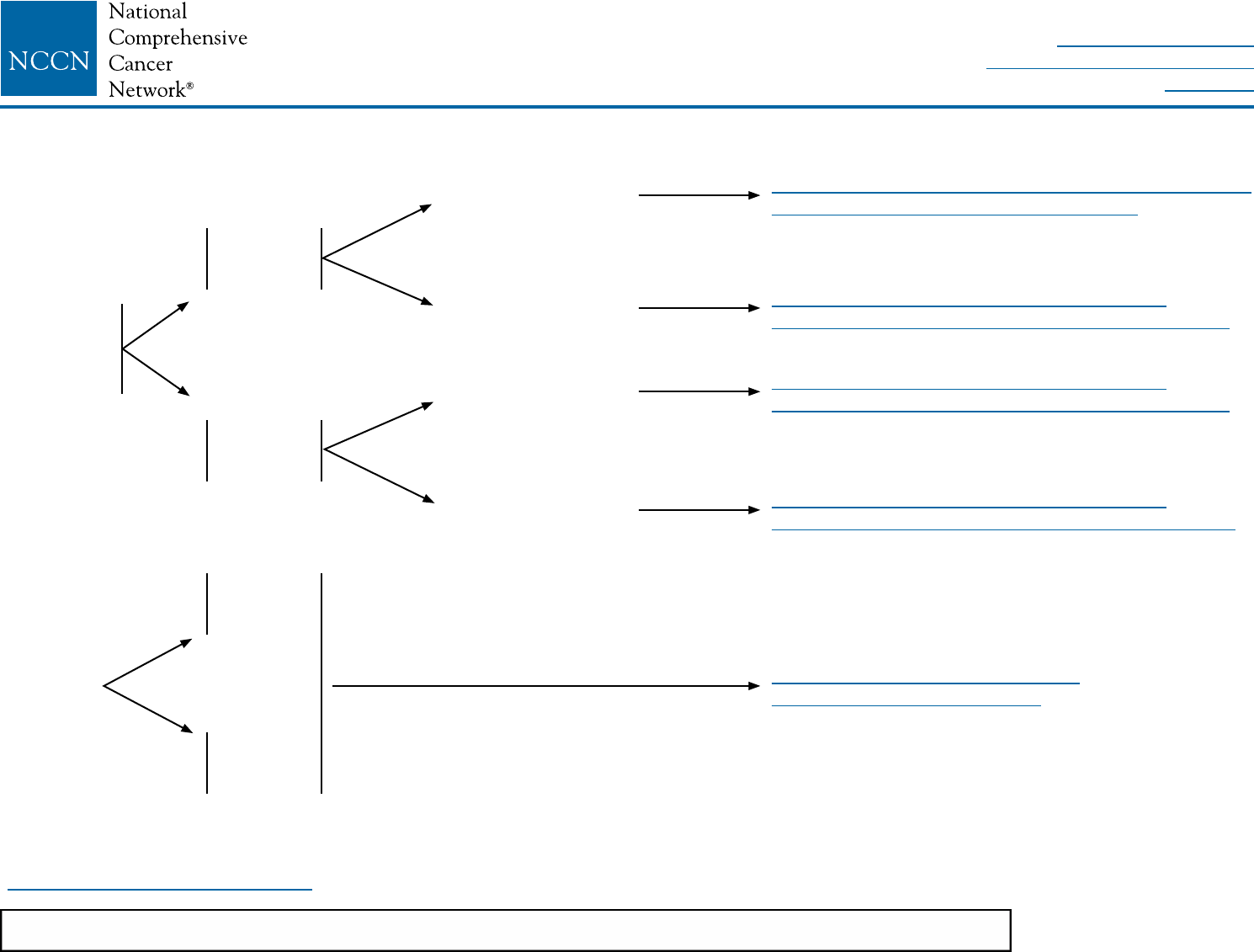
BINV-3
LOCOREGIONAL TREATMENT OF CLINICAL STAGE I, IIA, OR IIB DISEASE OR T3, N1, M0k
kSee NCCN Guidelines for Older Adult Oncology for special treatment considerations.
lSee Surgical Axillary Staging (BINV-D).
mSee Axillary Lymph Node Staging (BINV-E) and Margin Status in Infiltrating Carcinoma (BINV-F).
pSee Principles of Breast Reconstruction Following Surgery (BINV-H).
qConsider imaging for systemic staging, including diagnostic CT, bone scan, and optional FDG PET/CT (category 2B) (See BINV-1).
rSee Principles of Radiation Therapy (BINV-I).
uPostmastectomy radiation therapy may be considered for patients with multiple high-risk recurrence factors.
Total mastectomy
with surgical axillary
stagingl,m (category 1)
± reconstructionp
≥4 positive
axillary nodesq
1–3 positive
axillary nodes
Negative axillary nodes
and tumor >5 cm
or
margins positive
Negative axillary nodes and
tumor ≤5 cm and negative
margins but <1 mm
Negative axillary nodes
and tumor ≤5 cm and
margins ≥1 mm
Radiation therapyr to chest wall + infraclavicular region,
supraclavicular area, internal mammary nodes, and any
part of the axillary bed at risk. (category 1) It is common
for radiation therapy to follow chemotherapy when
chemotherapy is indicated.
Strongly consider radiation therapyr to chest wall +
infraclavicular region, supraclavicular area, internal
mammary nodes, and any part of the axillary bed at risk. It
is common for radiation therapy to follow chemotherapy
when chemotherapy is indicated.
Consider radiation therapyr to chest wall ± infraclavicular
region, ± supraclavicular area, ± internal mammary nodes
and any part of the axillary bed at risk. It is common
for radiation therapy to follow chemotherapy when
chemotherapy is indicated.
Consider radiation therapyr to chest wall. It is common
for radiation therapy to follow chemotherapy when
chemotherapy is indicated.
No radiation therapyu
See
BINV-4
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
BINV-4
bSee Principles of HER2 Testing (BINV-A).
vThis includes medullary and micropapillary subtypes.
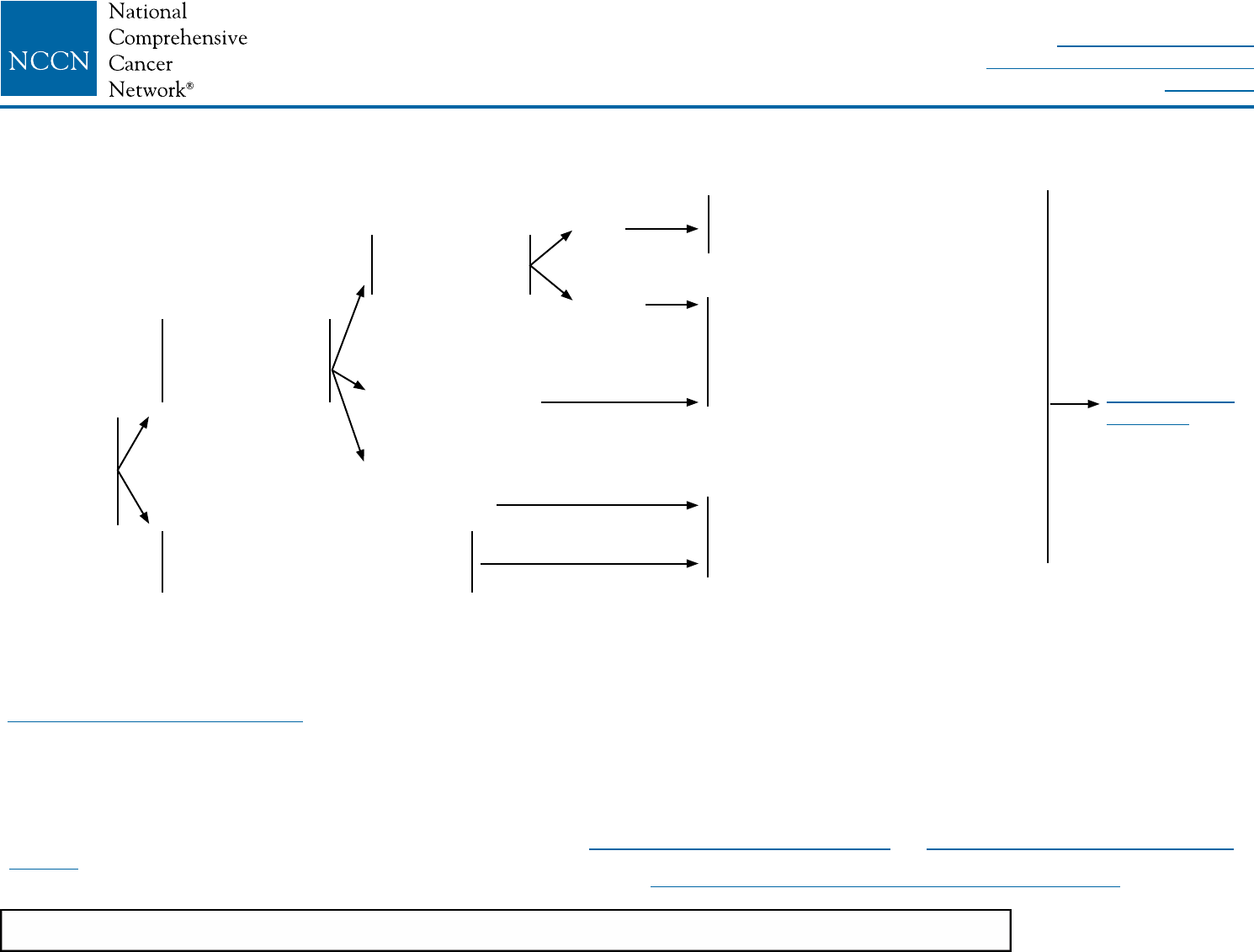
HISTOLOGY HORMONE
RECEPTOR STATUS
HER2 STATUS SYSTEMIC ADJUVANT TREATMENT
• Ductalv
• Lobular
• Mixed
• Metaplastic
• Tubular
• Mucinous
ER positive
and/or
PR positive
ER negative
and
PR negative
ER positive
and/or
PR positive
ER negative
and
PR negative
HER2-positiveb
HER2-negativeb
HER2-positiveb
HER2-negativeb
See Systemic Adjuvant Treatment -
Favorable Histologies (BINV-9)
See Systemic Adjuvant Treatment - Hormone Receptor
Positive - HER2-Positive Disease (BINV-5)
See Systemic Adjuvant Treatment - Hormone
Receptor Positive - HER2-Negative Disease (BINV-6)
See Systemic Adjuvant Treatment - Hormone
Receptor Negative - HER2-Positive Disease (BINV-7)
See Systemic Adjuvant Treatment - Hormone
Receptor Negative - HER2-Negative Disease (BINV-8)
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
BINV-5
bSee Principles of HER2 Testing (BINV-A).
wMixed lobular and ductal carcinoma as well as metaplastic carcinoma should be graded based on the ductal component and treated based on this grading. The metaplastic
or mixed component does not alter prognosis.
xThe prognosis of patients with T1a and T1b tumors that are node negative is uncertain even when HER2 is amplified or overexpressed. This is a population of breast cancer
patients that was not studied in the available randomized trials. The decision for use of trastuzumab therapy in this cohort of patients must balance the known toxicities of
trastuzumab, such as cardiac toxicity, and the uncertain, absolute benefits that may exist with trastuzumab therapy.
zChemotherapy and endocrine therapy used as adjuvant therapy should be given sequentially with endocrine therapy following chemotherapy. Available data suggest that
sequential or concurrent endocrine therapy with radiation therapy is acceptable. See Adjuvant Endocrine Therapy (BINV-J) and Preoperative/Adjuvant Therapy Regimens
(BINV-K).
aaThere are limited data to make chemotherapy recommendations for those >70 y of age. See NCCN Clinical Practice Guidelines for Older Adult Oncology.
bbA pertuzumab-containing regimen can be administered to patients with ≥T2 or ≥N1, HER2-positive, early-stage breast cancer.
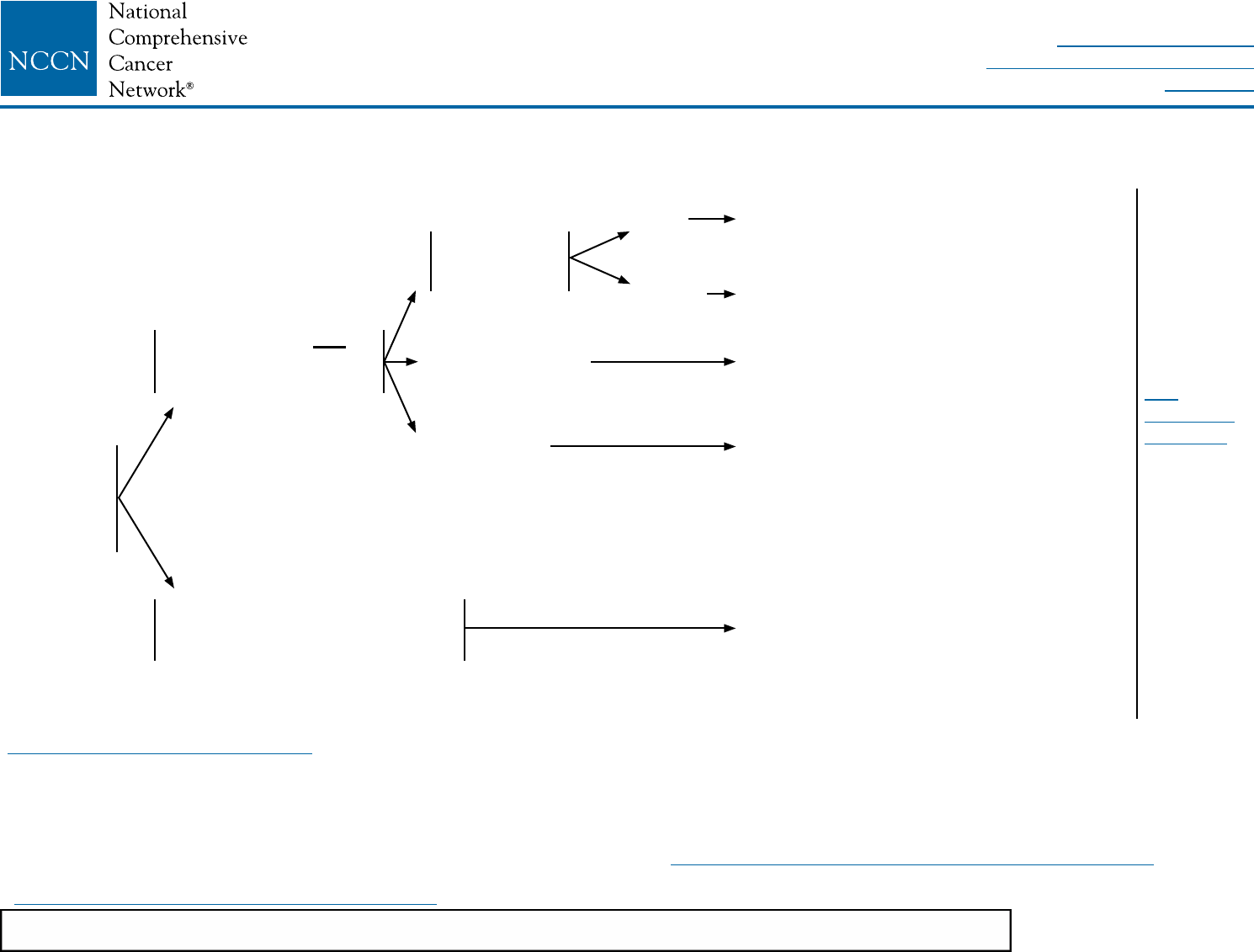
SYSTEMIC ADJUVANT TREATMENT - HORMONE RECEPTOR-POSITIVE - HER2-POSITIVE DISEASEb
Histology:w
• Ductal
• Lobular
• Mixed
• Metaplastic
pT1, pT2, or pT3;
and pN0 or pN1mi
(≤2 mm axillary
node metastasis)
Node positive (one or more
metastases >2 mm to one or more
ipsilateral axillary lymph nodes)
Tumor ≤0.5 cm
including
microinvasive
Tumor 0.6–1.0 cm
Tumor >1 cm
pN0
pN1mi Adjuvant endocrine therapy or
Adjuvant chemotherapyz,aa
with trastuzumabx followed by
endocrine therapy See Follow-Up
(BINV-16)
Consider adjuvant endocrine therapy
± adjuvant chemotherapyz,aa with
trastuzumabx (category 2B)
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Adjuvant chemotherapyz,aa,bb
with trastuzumabx followed by
endocrine therapy (category 1)
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
BINV-6
SYSTEMIC ADJUVANT TREATMENT - HORMONE RECEPTOR-POSITIVE - HER2-NEGATIVE DISEASEb
Histology:w
• Ductal
• Lobular
• Mixed
• Metaplastic
pT1, pT2, or pT3;
and pN0 or pN1mi
(≤2 mm axillary
node metastasis)
Node positive (one or more
metastases >2 mm to one or more
ipsilateral axillary lymph nodes)cc
• Tumor >0.5 cm
pN0
pN1mi
Consider adjuvant endocrine therapyy (category 2B)
Consider
21-gene
RT-PCR
assaydd
Not done
Low
recurrence
score (<18)
Intermediate
recurrence
score (18-30)
High
recurrence
score (≥31)
Adjuvant endocrine therapyy + adjuvant chemotherapyz,aa (category 1)
See
Follow-Up
(BINV-16)
Adjuvant endocrine therapyy
Adjuvant endocrine therapyy
+ adjuvant chemotherapyz,aa
bSee Principles of HER2 Testing (BINV-A).
wMixed lobular and ductal carcinoma as well as metaplastic carcinoma should be graded based on the ductal component and treated based on this grading. The metaplastic or
mixed component does not alter prognosis.
yEvidence supports that the magnitude of benefit from surgical or radiation ovarian ablation in premenopausal women with hormone receptor-positive breast cancer is similar to
that achieved with CMF alone. See Adjuvant Endocrine Therapy (BINV-J) and Preoperative/Adjuvant Therapy Regimens (BINV-K).
zChemotherapy and endocrine therapy used as adjuvant therapy should be given sequentially with endocrine therapy following chemotherapy. Available data suggest that
sequential or concurrent endocrine therapy with radiation therapy is acceptable. See Adjuvant Endocrine Therapy (BINV-J) and Preoperative/AdjuvantTherapy Regimens
(BINV-K).
aaThere are limited data to make chemotherapy recommendations for those >70 y of age. See NCCN Clinical Practice Guidelines for Older Adult Oncology.
ccThe 21-gene RT-PCR assay recurrence score can be considered in select patients with 1–3 involved ipsilateral axillary lymph nodes to guide the addition of combination
chemotherapy to standard hormone therapy. A retrospective analysis of a prospective randomized trial suggests that the test is predictive in this group similar to its
performance in node-negative disease.
ddOther prognostic multigene assays may be considered to help assess risk of recurrence but have not been validated to predict response to chemotherapy.
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Adjuvant endocrine therapyy (category 2B) or
Adjuvant chemotherapyz,aa followed by endocrine
therapyy (category 2B)
Tumor ≤0.5 cm
including
microinvasive
Adjuvant endocrine therapy or
Adjuvant chemotherapyz,aa followed
by endocrine therapyy (category 1)
Adjuvant endocrine therapy or
Adjuvant chemotherapyz,aa
followed by endocrine therapyy
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
BINV-7
bSee Principles of HER2 Testing (BINV-A).
wMixed lobular and ductal carcinoma as well as metaplastic carcinoma should be graded based on the ductal component and treated based on this grading. The
metaplastic or mixed component does not alter prognosis.
xThe prognosis of patients with T1a and T1b tumors that are node negative is uncertain even when HER2 is amplified or overexpressed. This is a population of breast
cancer patients that was not studied in the available randomized trials. The decision for use of trastuzumab therapy in this cohort of patients must balance the known
toxicities of trastuzumab, such as cardiac toxicity, and the uncertain, absolute benefits that may exist with trastuzumab therapy.
aaThere are limited data to make chemotherapy recommendations for those >70 y of age. See NCCN Clinical Practice Guidelines for Older Adult Oncology.
bbA pertuzumab-containing regimen can be administered to patients with ≥T2 or ≥N1, HER2-positive, early-stage breast cancer.
eeSee Preoperative/Adjuvant Therapy Regimens (BINV-K).
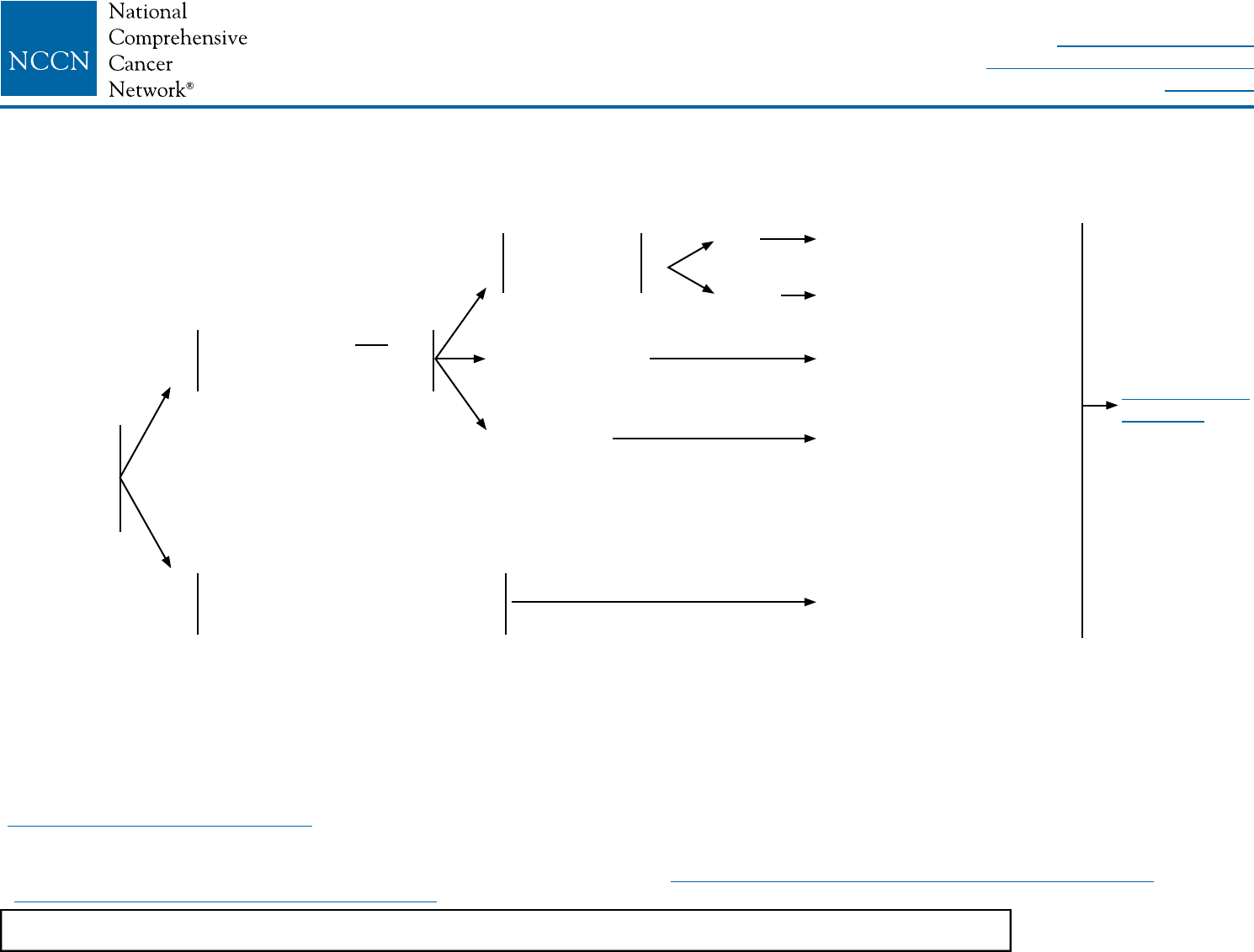
SYSTEMIC ADJUVANT TREATMENT - HORMONE RECEPTOR-NEGATIVE - HER2-POSITIVE DISEASEb
Histology:w
• Ductal
• Lobular
• Mixed
• Metaplastic
pT1, pT2, or pT3; and
pN0 or pN1mi (≤2 mm
axillary node metastasis)
Node positive (one or more
metastases >2 mm to one or more
ipsilateral axillary lymph nodes)
Tumor 0.6–1.0 cm
Tumor >1 cm
Adjuvant chemotherapyaa,bb,ee
with trastuzumab (category 1)
pN0
pN1mi
Consider adjuvant chemotherapyaa,ee
with trastuzumabx (category 2B)
Consider adjuvant chemotherapyaa,ee
with trastuzumabx
Consider adjuvant chemotherapyaa,ee
with trastuzumabx
Adjuvant chemotherapy
aa,bb,ee
with trastuzumab (category 1)
See
Follow-Up
(BINV-16)
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Tumor ≤0.5 cm
including
microinvasive
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
BINV-8
bSee Principles of HER2 Testing (BINV-A).
wMixed lobular and ductal carcinoma as well as metaplastic carcinoma should be graded based on the ductal component and treated based on this grading. The
metaplastic or mixed component does not alter prognosis.
aaThere are limited data to make chemotherapy recommendations for those >70 y of age. See NCCN Clinical Practice Guidelines for Older Adult Oncology.
eeSee Preoperative/Adjuvant Therapy Regimens (BINV-K).
SYSTEMIC ADJUVANT TREATMENT - HORMONE RECEPTOR-NEGATIVE - HER2-NEGATIVE DISEASEb
Histology:w
• Ductal
• Lobular
• Mixed
• Metaplastic
pT1, pT2, or pT3; and pN0
or pN1mi (≤2 mm axillary
node metastasis)
Node positive (one or more
metastases >2 mm to one or more
ipsilateral axillary lymph nodes)
Tumor 0.6–1.0 cm
Tumor >1 cm
pN0
pN1mi
No adjuvant therapy
Consider adjuvant
chemotherapyaa,ee
Consider adjuvant
chemotherapyaa,ee
Adjuvant chemotherapyaa,ee
(category 1)
Adjuvant chemotherapyaa,ee
(category 1)
See Follow-Up
(BINV-16)
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Tumor ≤0.5 cm
including
microinvasive
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
BINV-9
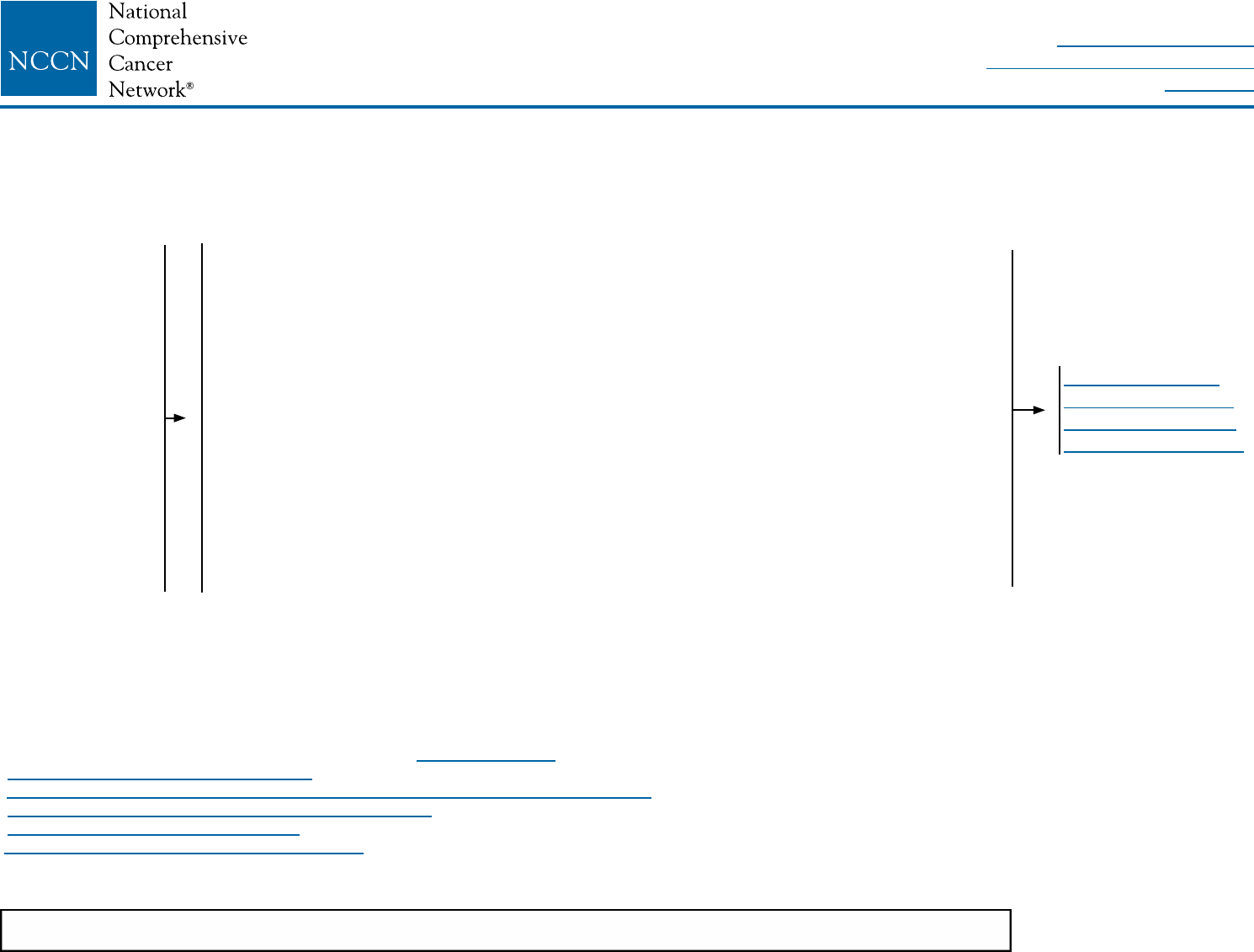
SYSTEMIC ADJUVANT TREATMENT - FAVORABLE HISTOLOGIES
yEvidence supports that the magnitude of benefit from surgical or radiation ovarian ablation in premenopausal women with hormone receptor-positive breast cancer is similar to
that achieved with CMF alone. See Adjuvant Endocrine Therapy (BINV-J) and Preoperative/Adjuvant Therapy Regimens (BINV-K).
zChemotherapy and endocrine therapy used as adjuvant therapy should be given sequentially with endocrine therapy following chemotherapy. Available data suggest
that sequential or concurrent endocrine therapy with radiation therapy is acceptable. See Adjuvant Endocrine Therapy (BINV-J) and Preoperative/Adjuvant Therapy
Regimens (BINV-K).
aaThere are limited data to make chemotherapy recommendations for those >70 y of age. See NCCN Clinical Practice Guidelines for Older Adult Oncology.
Histology:
• Tubular
• Mucinous
ER-positive
and/or
PR-positive
ER-negative
and
PR-negative
pT1, pT2, or pT3;
and pN0 or pN1mi
(≤2 mm axillary
node metastasis)
Node positive (one or more
metastases >2 mm to one or more
ipsilateral axillary lymph nodes)
Repeat determination
of ER/PR status
<1 cm
1–2.9 cm
≥3 cm
ER-positive
and/or
PR-positive
ER-negative
and
PR-negative
Consider adjuvant endocrine
therapy for risk reduction
Consider adjuvant endocrine
therapyy
Adjuvant endocrine therapyy
Adjuvant endocrine therapyy
± adjuvant chemotherapyz,aa
Follow appropriate
pathway above
Treat as usual breast cancer
histology
(See BINV-7 and BINV-8)
See Follow-Up
(BINV-16)
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
BINV-10
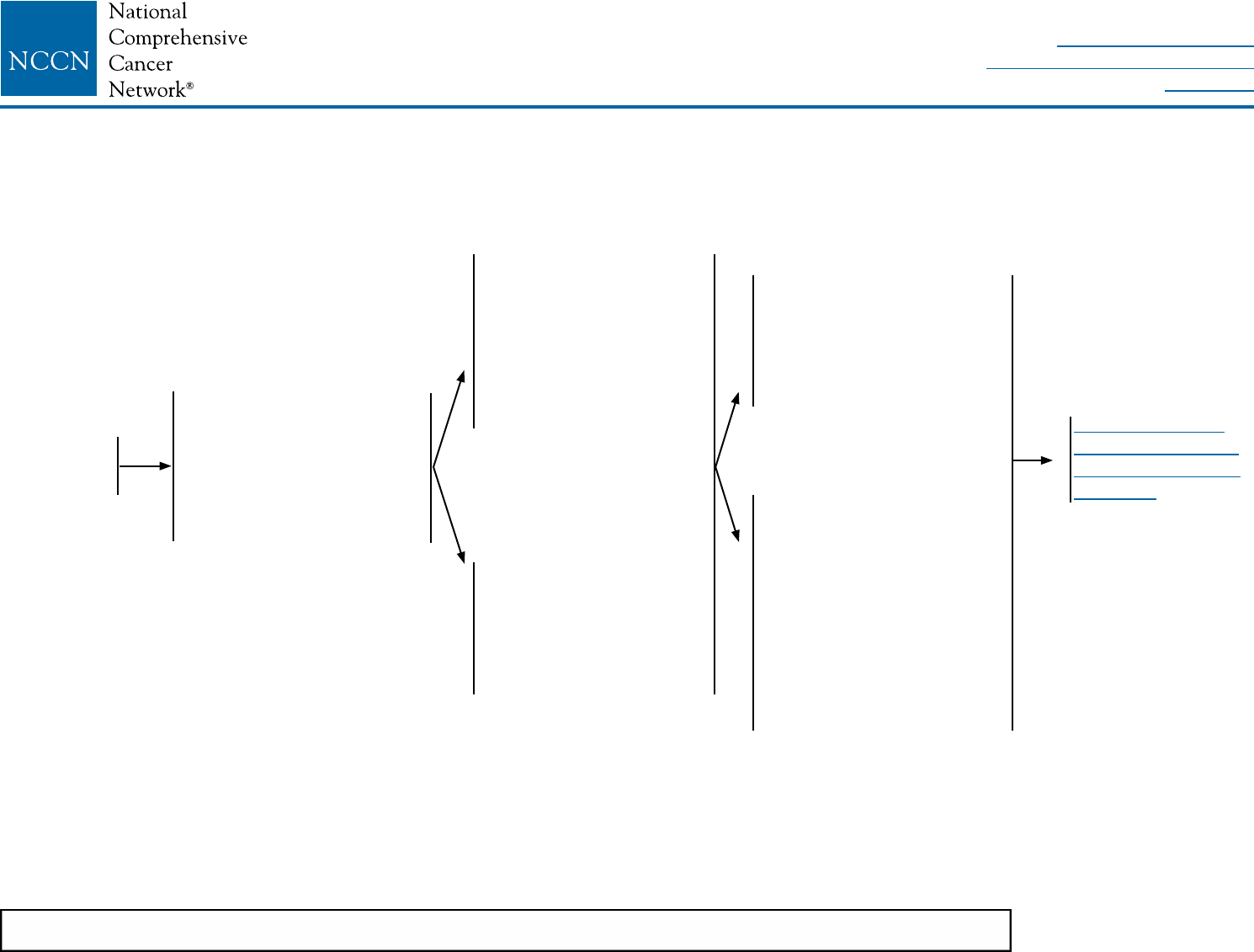
PREOPERATIVE SYSTEMIC THERAPY FOR OPERABLE BREAST CANCER: WORKUP
aThe panel endorses the College of American Pathologists Protocol for pathology reporting for
all invasive and noninvasive carcinomas of the breast. http://www.cap.org.
bSee Principles of HER2 Testing (BINV-A).
cSee NCCN Guidelines for Genetic/Familial High-Risk Assessment: Breast and Ovarian.
dSee Principles of Dedicated Breast MRI Testing (BINV-B).
eSee Fertility and Birth Control (BINV-C).
fSee NCCN Guidelines for Distress Management
gRoutine systemic staging is not indicated for early breast cancer in the absence of symptoms.
hIf FDG PET/CT is performed and clearly indicates bone metastasis, on both the PET and CT
component, bone scan or sodium fluoride PET/CT may not be needed.
iFDG PET/CT can be performed at the same time as diagnostic CT. The
use of PET or PET/CT scanning is not indicated in the staging of clinical
stage I, II, or operable III breast cancer. FDG PET/CT is most helpful in
situations where standard staging studies are equivocal or suspicious,
especially in the setting of locally advanced or metastatic disease.
jFDG PET/CT may also be helpful in identifying unsuspected regional nodal
disease and/or distant metastases in locally advanced breast cancer when
used in addition to standard staging studies.
ffIn cases where breast-conserving surgery may not be possible but
patient will need chemotherapy, preoperative systemic treatment
remains an acceptable option.
CLINICAL
STAGE
WORKUP
Stage IIA
T2, N0, M0
Stage IIB
T2, N1, M0
T3, N0, M0
Stage lllA
T3, N1, M0
and
Fullls criteria for
breast-conserving
surgery except for
tumor sizeff
• History and physical exam
• Diagnostic bilateral mammogram; ultrasound as necessary
• Pathology reviewa
• Determination of tumor ER/PR status and HER2 statusb
• Genetic counseling if patient is high risk for hereditary breast cancerc
Breast MRId (optional), with special consideration for mammographically occult tumors
• Fertility counseling if premenopausale
• Assess for distressf
Additional studies consider:g
• CBC
• Liver function tests and alkaline phosphatase
• Chest diagnostic CT
• Abdominal ± pelvic diagnostic CT or MRI
• Bone scan or sodium uoride PET/CTh (category 2B)
• FDG PET/CTi,j (optional, category 2B)
See Preoperative
Systemic Therapy:
Breast and Axillary
Evaluation (BINV-11)
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
BINV-11
PREOPERATIVE SYSTEMIC THERAPY: BREAST AND AXILLARY EVALUATION
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Core biopsy with placement
of image-detectable
marker(s), if not previously
performed, must be done to
demarcate the tumor bed for
post-chemotherapy surgical
management
Clinically negative axillary
lymph node(s) consider
axillary imaging with
ultrasound; suspicious
nodes should be sampled
by FNA or core biopsy
prior to preoperative
systemic therapygg
Clinically positive axillary
lymph node(s) should
be sampled by FNA or
core biopsy prior to
preoperative systemic
therapygg
See Preoperative
Systemic Therapy:
Surgical Treatment
(BINV-12)
Preoperative
systemic
therapy
ggMarking of sampled axillary nodes with a tattoo or clip should be considered to permit verification that the biopsy-positive lymph node has been removed at the time of
definitive surgery.
hhAmong patients shown to be node-positive prior to preoperative systemic therapy, SLNB has a >10% false-negative rate when performed after preoperative systemic
therapy. This rate can be improved by marking biopsied lymph nodes to document their removal, using dual tracer, and by removing more than 2 sentinel nodes.
If lymph node FNA or core
biopsy negative, sentinel
lymph node biopsy (SLNB)
can be performed before or
after preoperative systemic
therapy
If lymph node FNA or core
biopsy positive, axilla
may be restaged after
preoperative systemic
therapy; axillary lymph node
dissection (ALND) should
be performed if axilla is
clinically positive; SLNB or
ALND can be performed if
axilla is clinically negative
(category 2B)hh
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
BINV-12
SURGICAL TREATMENT
RESPONSEjj
Conrmed progressive disease at any time
Partial response, lumpectomy not possible
Partial response, lumpectomy possible
or
Complete response
Preoperative systemic therapyii
See Mastectomy (BINV-13)
See Lumpectomy (BINV-13)
PREOPERATIVE SYSTEMIC THERAPY: SURGICAL TREATMENT
iiSee Principles of Preoperative Systemic Therapy (BINV-L).
jjThe accurate assessment of in-breast tumor or regional lymph node response to preoperative systemic therapy is difficult, and should include physical examination and
performance of imaging studies (mammogram and/or breast MRI) that were abnormal at the time of initial tumor staging. Selection of imaging methods prior to surgery
should be determined by the multidisciplinary team.
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
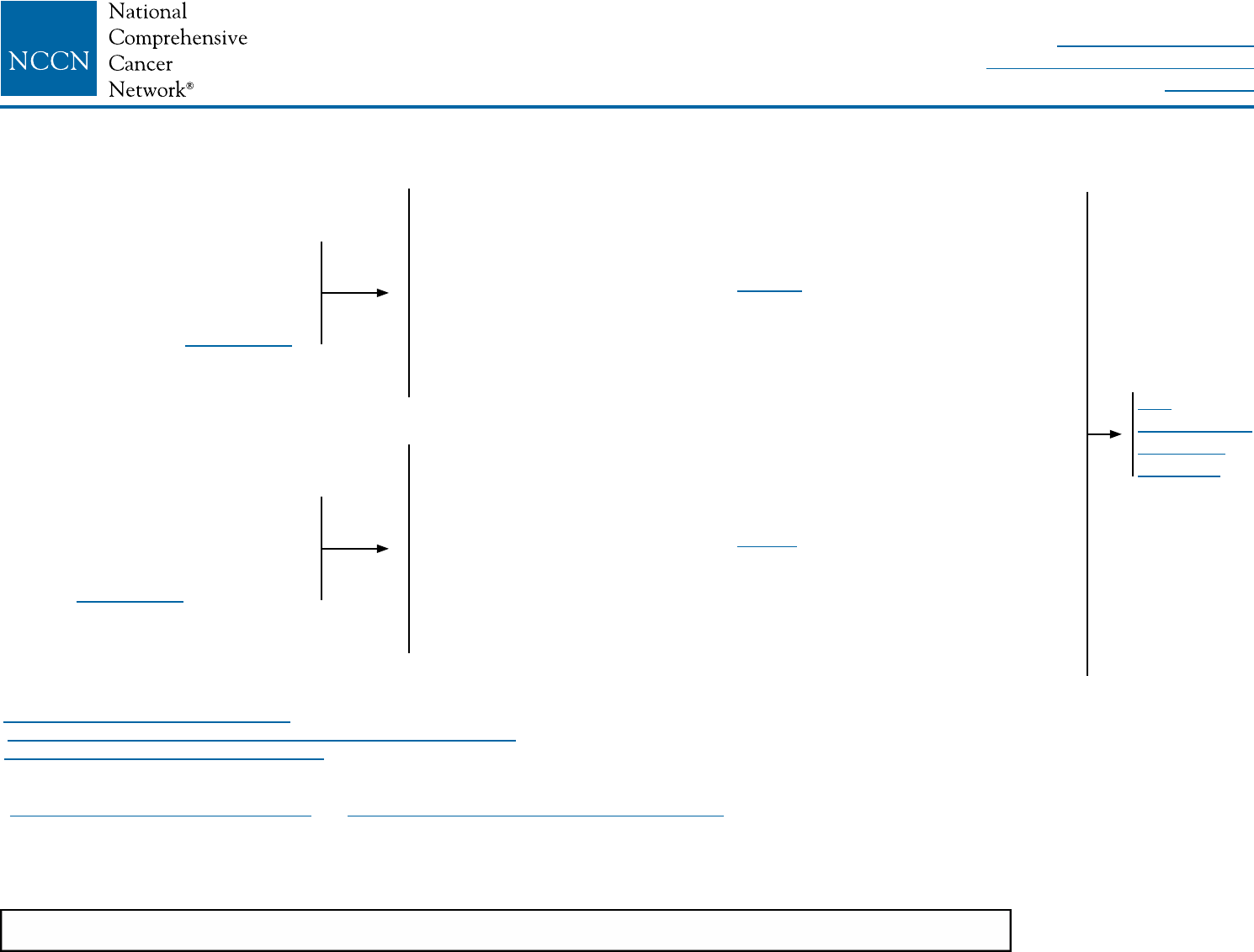
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
BINV-13
SURGICAL TREATMENT ADJUVANT TREATMENT
Mastectomy and surgical axillary
stagingl,kk ± reconstruction.p If
SLNB performed prechemotherapy
and negative ndings, omit axillary
lymph node staging. See BINV-11
Lumpectomy with surgical axillary
staging.l,kk If SLNB performed
prechemotherapy and negative
ndings, omit axillary lymph node
staging. See BINV-11
• Complete planned chemotherapy regimen course if not completed
preoperatively plus endocrine treatment if ER-positive and/or PR-positive
(sequential chemotherapy followed by endocrine therapy).
• Adjuvant radiation therapyr post-mastectomy is based on tumor
characteristics at diagnosis as per BINV-3
and
Endocrine therapy if ER-positive and/or PR-positivez (category 1)
• Complete up to one year of trastuzumab therapy if HER2-positive
(category 1). May be administered concurrently with radiation therapyr
and with endocrine therapy if indicated.
• Complete planned chemotherapy regimen course if not completed
preoperatively plus endocrine treatment if ER-positive and/or PR-positive
(sequential chemotherapy followed by endocrine therapy).
• Adjuvant radiation therapyr post-lumpectomy based on tumor
characteristics at diagnosis as per BINV-2
and
Endocrine therapy if ER-positive and/or PR-positivez (category 1)
• Complete up to one year of trastuzumab therapy if HER2-positive
(category 1). May be administered concurrently with radiation therapyr
and with endocrine therapy if indicated.
See
Surveillance/
Follow-up
(BINV-16)
PREOPERATIVE SYSTEMIC THERAPY: ADJUVANT THERAPY
lSee Surgical Axillary Staging (BINV-D).
pSee Principles of Breast Reconstruction Following Surgery (BINV-H).
rSee Principles of Radiation Therapy (BINV-I).
zChemotherapy and endocrine therapy used as adjuvant therapy should be given sequentially with endocrine therapy following chemotherapy. Available data suggest
that sequential or concurrent endocrine therapy with radiation therapy is acceptable.
See Adjuvant Endocrine Therapy (BINV-J) and Preoperative/Adjuvant Therapy Regimens (BINV-K).
kkAxilla may be restaged after preoperative systemic therapy; ALND should be performed if axilla is clinically positive; SLNB or ALND can be performed if axilla is
clinically negative (category 2B). Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant
chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol 2013;14(7):609-18. Epub 2013/05/21. Boughey JC, Suman VJ, Mittendorf EA, et
al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA
2013;310(14):1455-61. Epub 2013/10/09.
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
BINV-14
CLINICAL STAGE WORKUP
aThe panel endorses the College of American Pathologists Protocol for pathology reporting
for all invasive and noninvasive carcinomas of the breast. http://www.cap.org.
bSee Principles of HER2 Testing (BINV-A).
cSee NCCN Guidelines for Genetic/Familial High-Risk Assessment: Breast and Ovarian.
dSee Principles of Dedicated Breast MRI Testing (BINV-B).
eSee Fertility and Birth Control (BINV-C).
fSee NCCN Guidelines for Distress Management.
hIf FDG PET/CT is performed and clearly indicates bone metastasis, on both the PET
and CT component, bone scan or sodium fluoride PET/CT may not be needed.
iFDG PET/CT can be performed at the same time as diagnostic CT. The use of
PET or PET/CT scanning is not indicated in the staging of clinical stage I, II,
or operable III breast cancer. FDG PET/CT is most helpful in situations where
standard staging studies are equivocal or suspicious, especially in the setting
of locally advanced or metastatic disease.
jFDG PET/CT may also be helpful in identifying unsuspected regional nodal
disease and/or distant metastases in locally advanced breast cancer when
used in addition to standard staging studies.
Stage IIIA
T0, N2, M0
T1, N2, M0
T2, N2, M0
T3, N2, M0
Stage IIIA patients
with T3, N1, M0
disease, see BINV-1
Stage IIIB
T4, N0, M0
T4, N1, M0
T4, N2, M0
Stage lllC
Any T, N3, M0
See Preoperative
Systemic Therapy
For Inoperable or
Locally Advanced
Breast Cancer
(Non-Inammatory)
(BINV-15)
• History and physical exam
• Diagnostic bilateral mammogram; ultrasound as necessary
• Pathology reviewa
• Determination of tumor ER/PR status and HER2 statusb
• Genetic counseling if patient is high risk for hereditary breast cancerc
• Breast MRId (optional), with special consideration for mammographically occult tumors
• Fertility counseling if premenopausale
• Assess for distressf
Additional studies consider:
• CBC
• Liver function tests and alkaline phosphatase
• Chest diagnostic CT
• Abdominal ± pelvic diagnostic CT or MRI
• Bone scan or sodium uoride PET/CTh (category 2B)
• FDG PET/CTi,j (optional, category 2B)
PREOPERATIVE SYSTEMIC THERAPY FOR INOPERABLE OR LOCALLY ADVANCED BREAST CANCER (NON-INFLAMMATORY): WORKUP
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
BINV-15
LOCOREGIONAL TREATMENT ADJUVANT TREATMENT
Total mastectomy + level l/ll axillary
dissection + radiation therapyr to chest
wall, infraclavicular region,
supraclavicular area, internal mammary
nodes, and any part of the axillary bed at
risk ±
breast reconstructionp
or
Lumpectomyll + level l/ll
axillary dissection + radiation therapyr
to whole breast with or without boost
to tumor bed, infraclavicular region,
supraclavicular area, internal mammary
nodes, and any part of the axillary bed at
risk.
Responsejj
No responsejj
Consider additional systemic
chemotherapy and/or preoperative
radiation
• Complete planned
chemotherapy regimen course
if not completed preoperatively
plus endocrine treatment if
ER-positive and/or PR-positive
(sequential chemotherapy
followed by endocrine therapy).
• Complete up to one year of
trastuzumab therapy if HER2-
positive (category 1). May be
administered concurrently with
radiation therapyr and with
endocrine therapy if indicated.
Responsejj - See above pathway
No responsejj Individualized
treatment
See
Surveillance/
Follow-up
(BINV-16)
PREOPERATIVE SYSTEMIC THERAPY FOR INOPERABLE OR LOCALLY ADVANCED BREAST CANCER (NON-INFLAMMATORY)
pSee Principles of Breast Reconstruction Following Surgery (BINV-H).
rSee Principles of Radiation Therapy (BINV-I).
iiSee Principles of Preoperative Systemic Therapy (BINV-L).
jjThe accurate assessment of in-breast tumor or regional lymph node response to preoperative systemic therapy is difficult, and should include physical examination and
performance of imaging studies (mammogram and/or breast MRI) that were abnormal at the time of initial tumor staging. Selection of imaging methods prior to surgery
should be determined by the multidisciplinary team.
llFor patients with skin and/or chest wall involvement (T4 non-inflammatory) prior to preoperative systemic therapy, breast conservation may be performed in carefully
selected patients based on a multidisciplinary assessment of local recurrence risk. In addition to standard contraindications to breast conservation (see BINV-G), exclusion
criteria for breast conservation include: inflammatory (T4d) disease before preoperative systemic therapy and incomplete resolution of skin involvement after preoperative
systemic therapy.
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Preoperative
systemic therapyii
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
BINV-16
mmStudies indicate that annual mammograms are the appropriate frequency for surveillance of breast cancer patients who have had breast-conserving surgery and
radiation therapy with no clear advantage to shorter interval imaging. Patients should wait 6 to 12 months after the completion of radiation therapy to begin their annual
mammogram surveillance. Suspicious findings on physical examination or surveillance imaging might warrant a shorter interval between mammograms.
nnThe use of estrogen, progesterone, or selective estrogen receptor modulators to treat osteoporosis or osteopenia in women with breast cancer is discouraged. The
use of a bisphosphonate or denosumab is acceptable to maintain or to improve bone mineral density. Optimal duration of either therapy has not been established.
Duration beyond 3 y is not known. Factors to consider for duration of anti-osteoporosis therapy include bone mineral density, response to therapy, and risk factors
for continued bone loss or fracture. Women treated with a bisphosphonate or denosumab should undergo a dental examination with preventive dentistry prior to the
initiation of therapy, and should take supplemental calcium and vitamin D.
SURVEILLANCE/FOLLOW-UP
• History and physical exam 1–4 times per year as clinically appropriate for 5 y, then annually
• Periodic screening for changes in family history and referral to genetic counseling as indicated
• Educate, monitor, and refer for lymphedema management
• Mammography every 12 momm
• Routine imaging of reconstructed breast is not indicated
• In the absence of clinical signs and symptoms suggestive of recurrent disease, there is no indication for
laboratory or imaging studies for metastases screening
• Women on tamoxifen: annual gynecologic assessment every 12 mo if uterus present
• Women on an aromatase inhibitor or who experience ovarian failure secondary to treatment should have
monitoring of bone health with a bone mineral density determination at baseline and periodically thereafternn
• Assess and encourage adherence to adjuvant endocrine therapy
• Evidence suggests that active lifestyle, healthy diet, limited alcohol intake, and achieving and maintaining an
ideal body weight (20–25 BMI) may lead to optimal breast cancer outcomes
• See NCCN Guidelines for Survivorship
See Recurrent
Disease
(BINV-17)
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
BINV-17
bSee Principles of HER2 Testing (BINV-A).
cSee NCCN Guidelines for Genetic/Familial High-Risk Assessment: Breast and Ovarian.
hIf FDG PET/CT is performed and clearly indicates bone metastasis, on both the PET and CT component, bone scan or sodium fluoride PET/CT may not be needed.
jFDG PET/CT may also be helpful in identifying unsuspected regional nodal disease and/or distant metastases in locally advanced breast cancer when used in addition
to standard staging studies.
ooFDG PET/CT can be performed at the same time as diagnostic CT. FDG PET/CT is most helpful in situations where standard staging studies are equivocal or
suspicious, especially in the setting of locally advanced or metastatic disease.
ppFalse-negative ER and/or PR determinations occur, and there may be discordance between the ER and/or PR determination between the primary and metastatic
tumor(s). Therefore, endocrine therapy may be considered in patients with non-visceral or asymptomatic visceral tumors, especially in patients with clinical
characteristics predicting for a hormone receptor-positive tumor (eg, long disease-free interval, limited sites of recurrence, indolent disease, older age).
qqIn clinical situations where a biopsy cannot safely be obtained but the clinical evidence is strongly supportive of recurrence, treatment may commence based on the
ER/PR/HER2 status of the primary tumor.
RECURRENT/STAGE IV DISEASE
CLINICAL STAGE WORKUP
Recurrent or
Stage IV disease
• History and physical exam
• CBC
• Liver function tests and alkaline phosphatase
• Chest diagnostic CT
• Abdominal ± pelvic diagnostic CT or MRI
• Brain MRI if suspicious CNS symptoms
• Bone scan or sodium uoride PET/CTh (category 2B)
• FDG PET/CTj,oo (optional, category 2B)
• X-rays of symptomatic bones and long and weight-bearing bones
abnormal on bone scan
• First recurrence of disease should be biopsied
• Determination of tumor ER/PR and HER2 status on metastatic siteb,pp,qq
• Genetic counseling if patient is high risk for hereditary breast cancerc
See Treatment
of Recurrence (BINV-18)
See Treatment of
Stage IV Disease
(BINV-19)
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
BINV-18
rSee Principles of Radiation Therapy (BINV-I).
rrMultidisciplinary approach is especially important in the management of breast cancer recurrence to consider all potential treatment options for optimal outcomes.
ssIn women with a local breast recurrence after breast-conserving surgery who had a prior sentinel node biopsy (SNB), a repeat SNB may be technically possible. The
accuracy of repeat SNB is unproven, and the prognostic significance of repeat SNB after mastectomy is unknown and its use is discouraged.
ttIf not technically resectable, consider systemic therapy to best response, then resect if possible.
uuThe decision to use radiation therapy to treat locoregional recurrence must factor in any prior radiation to the area and the risk of late normal tissue toxicity from the sum of
the prior and planned radiation courses.
vvFor additional information see the Discussion section.
TREATMENT OF RECURRENCE
Local only
recurrencerr
Regional only
or
Local and
regional
recurrencerr
Initial treatment with lumpectomy
+ radiation therapy
Initial treatment with mastectomy + level l/ll
axillary dissection and prior radiation therapy
Initial treatment with mastectomy
and no prior radiation therapy
Axillary recurrence
Supraclavicular recurrence
Internal mammary node recurrence
Total mastectomy
+ axillary lymph node staging if level l/ll
axillary dissection not previously doness
Surgical resection if possiblett
Surgical resection if possiblett +
radiation therapyr
Surgical resection if possiblett
+ radiation therapy if possibler,uu
Radiation therapy if possibler,uu
Radiation therapy if possibler,uu
Consider systemic
therapyvv
See
Adjuvant Endocrine
Therapy (BINV-J)
Preoperative/Adjuvant
Chemotherapy (BINV-K)
Endocrine Therapy for
Recurrent or Stage IV
Disease (BINV-N)
Chemotherapy
Regimens for Recurrent
or Metastatic Breast
Cancer (BINV-O)
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
Systemic
disease or
de novo
stage IVww
Bone disease present
Bone disease not present
Add denosumab,
zoledronic acid, or
pamidronatxx ER and/or PR positive; HER2 negativeb
ER and/or PR positive; HER2 positiveb
ER and PR negative, or ER and/or PR positive
and endocrine refractory; HER2 negativeb
ER/PR negative or ER and/or PR positive
and endocrine refractory; HER2 positiveb
See BINV-20
See BINV-21
See BINV-22
TREATMENT OF STAGE IV DISEASE
bSee Principles of HER2 Testing (BINV-A).
wwThe role and timing of surgical removal of the primary in patients presenting with de novo stage IV disease is the subject of ongoing investigations.
xxDenosumab, zoledronic acid, or pamidronate (all with calcium and vitamin D supplementation) should be given (category 1) in addition to chemotherapy or endocrine
therapy if bone metastasis is present, expected survival is ≥3 months, and renal function is adequate. Patients should undergo a dental examination with preventive
dentistry prior to initiation of this therapy. The optimal schedule for zoledronic acid is monthly x 12, then quarterly.
BINV-19
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/03/16© National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
BINV-20
bSee Principles of HER2 Testing (BINV-A).
yySee Definition of Menopause (BINV-M).
zzLimited studies document a progression-free survival advantage of adding
trastuzumab or lapatinib to aromatase inhibition in postmenopausal patients with
ER-positive, HER2-positive disease. However, no overall survival advantage has
been demonstrated.
aaaSee Endocrine Therapy for Recurrent or Stage IV Disease (BINV-N).
bbbIt is unclear that women presenting at time of initial diagnosis with metastatic
disease will benefit from the performance of palliative local breast surgery and/or
radiation therapy. Generally this palliative local therapy should be considered only
after response to initial systemic therapy.
cccSee Chemotherapy Regimens for Recurrent or Metastatic Breast Cancer (BINV-O).
dddA single study (S0226) in women with hormone receptor-positive breast
cancer and no prior chemotherapy, biological therapy, or endocrine therapy for
metastatic disease demonstrated that the addition of fulvestrant to anastrozole
resulted in prolongation of time to progression. Subset analysis suggested
that patients without prior adjuvant tamoxifen and more than 10 years since
diagnosis experienced the greatest benefit. Two studies with similar design
(FACT and SOFEA) demonstrated no advantage in time to progression with
the addition of fulvestrant to anastrozole.
SYSTEMIC TREATMENT OF RECURRENT OR STAGE IV DISEASE
ER and/or PR POSITIVE; HER2 NEGATIVE OR POSITIVE
ER and/or PR positive;
HER2 negativeb
ER and/or PR positive;
HER2 positiveb,zz
Prior endocrine
therapy within 1 y
No prior endocrine
therapy within 1 y
Premenopausalyy
Postmenopausalyy,zz
Visceral crisis
Premenopausalyy
Postmenopausalyy,zz
Visceral crisis
Ovarian ablation or suppression,
plus endocrine therapy as for
postmenopausal womenaaa,bbb
Consider initial chemotherapyccc
(See BINV-21 and BINV-22)
Ovarian ablation or suppression,
plus endocrine therapy as for
postmenopausal womenaaa,bbb
or
Selective ER modulatorsaaa,bbb
Aromatase inhibitoraaa,bbb,ddd
or
Selective ER modulators or selective
ER down-regulatoraaa
Consider initial chemotherapyccc
(See BINV-21 and BINV-22)
See Follow-up
Therapy For
Endocrine
Treatment of
Recurrent/Stage IV
Disease (BINV-23)
See Follow-up
Therapy For
Endocrine
Treatment of
Recurrent/Stage IV
Disease (BINV-23)
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
BINV-21
bSee Principles of HER2 Testing (BINV-A).
ppFalse-negative ER and/or PR determinations occur, and there may be discordance between the ER and/or PR determination between the primary and metastatic
tumor(s). Therefore, endocrine therapy may be considered in patients with non-visceral or asymptomatic visceral tumors, especially in patients with clinical characteristics
predicting for a hormone receptor-positive tumor (eg, long disease-free interval, limited sites of recurrence, indolent disease, older age).
aaaSee Endocrine Therapy for Recurrent or Stage IV Disease (BINV-N).
cccSee Chemotherapy Regimens for Recurrent or Metastatic Breast Cancer (BINV-O).
eeeSee Principles of Monitoring Metastatic Disease (BINV-P).
SYSTEMIC TREATMENT OF RECURRENT OR STAGE IV DISEASE
ER and PR NEGATIVE; or ER and/or PR POSITIVE and ENDOCRINE REFRACTORY; HER2 NEGATIVE
ER and PR
negative; or ER
and/or PR
positive and
endocrine
refractory; and
HER2 negativeb
Bone or soft
tissue only
or
Asymptomatic
visceral
Yes
No
Consider additional line
of endocrine therapy, if
not endocrine
refractorypp,aaa,eee
or
Chemotherapyccc,eee
Chemotherapyccc,eee
No benet after 3
sequential lines of
chemotherapy
or
ECOG performance
status ≥3
Consider no further
cytotoxic therapy; transition
to palliative care
See NCCN Guidelines for
Palliative Care
and NCCN Guidelines for
Supportive Care
See Endocrine
Therapy (BINV-20)
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
BINV-22
SYSTEMIC TREATMENT OF RECURRENT OR STAGE IV DISEASE
ER and PR NEGATIVE; or ER and/or PR POSITIVE and ENDOCRINE REFRACTORY; and HER2 POSITIVE
ER and PR
negative; or ER
and/or PR
positive and
endocrine
refractory; and
HER2 positiveb
Bone or soft
tissue only
or
Asymptomatic
visceral
Yes
No
Consider endocrine
therapy, if not endocrine
refractorypp,aaa,eee
± HER2-targeted chemotherapy
Pertuzumab +
trastuzumab + taxane
(preferred)ccc,eee
or
Ado-trastuzumab emtansine
(T-DM1) ccc,eee
or
Trastuzumab +
chemotherapy ccc,eee,ggg
See Endocrine Therapy (BINV-20)
HER2-targeted
therapyccc,fff,ggg,hhh
No benet after
3 sequential
lines of targeted
therapy
or
ECOG
performance
status ≥3
Consider no
further
cytotoxic
therapy;
transition to
palliative care
(See NCCN
Guidelines for
Palliative Care)
bSee Principles of HER2 Testing (BINV-A).
ppFalse-negative ER and/or PR determinations occur, and there may be discordance between the ER and/or PR determination between the primary and metastatic
tumor(s). Therefore, endocrine therapy with its low attendant toxicity may be considered in patients with non-visceral or asymptomatic visceral tumors, especially in
patients with clinical characteristics predicting for a hormone receptor-positive tumor (eg, long disease-free interval, limited sites of recurrence, indolent disease, older
age).
aaaSee Endocrine Therapy for Recurrent or Stage IV Disease (BINV-N).
cccSee Chemotherapy Regimens for Recurrent or Metastatic Breast Cancer (BINV-O).
eeeSee Principles of Monitoring Metastatic Disease (BINV-P).
fffContinue HER2-targeted therapy following progression on first-line HER2-targeted chemotherapy for metastatic breast cancer. The optimal duration of trastuzumab in
patients with long-term control of disease is unknown.
gggTrastuzumab given in combination with an anthracycline is associated with significant cardiac toxicity. Concurrent use of trastuzumab and pertuzumab with an
anthracycline should be avoided.
hhhPatients previously treated with chemotherapy plus trastuzumab in the absence of pertuzumab may be considered for one line of therapy including both trastuzumab
plus pertuzumab in combination with or without cytotoxic therapy (such as vinorelbine or taxane). Further research is needed to determine the ideal sequencing
strategy for anti-HER2 therapy.
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
BINV-23
aaaSee Endocrine Therapy for Recurrent or Stage IV Disease (BINV-N).
cccSee Chemotherapy Regimens for Recurrent or Metastatic Breast Cancer (BINV-O).
eeeSee Principles of Monitoring Metastatic Disease (BINV-P).
FOLLOW-UP THERAPY FOR ENDOCRINE TREATMENT OF RECURRENT OR STAGE IV DISEASE
Continue endocrine
therapy until
progressionfff or
unacceptable toxicity
Progressionfff
No clinical benet after 3
sequential endocrine
therapy regimens
or
Symptomatic visceral
disease
Yes
No
Chemotherapyddd
New line of endocrine
therapybbb
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
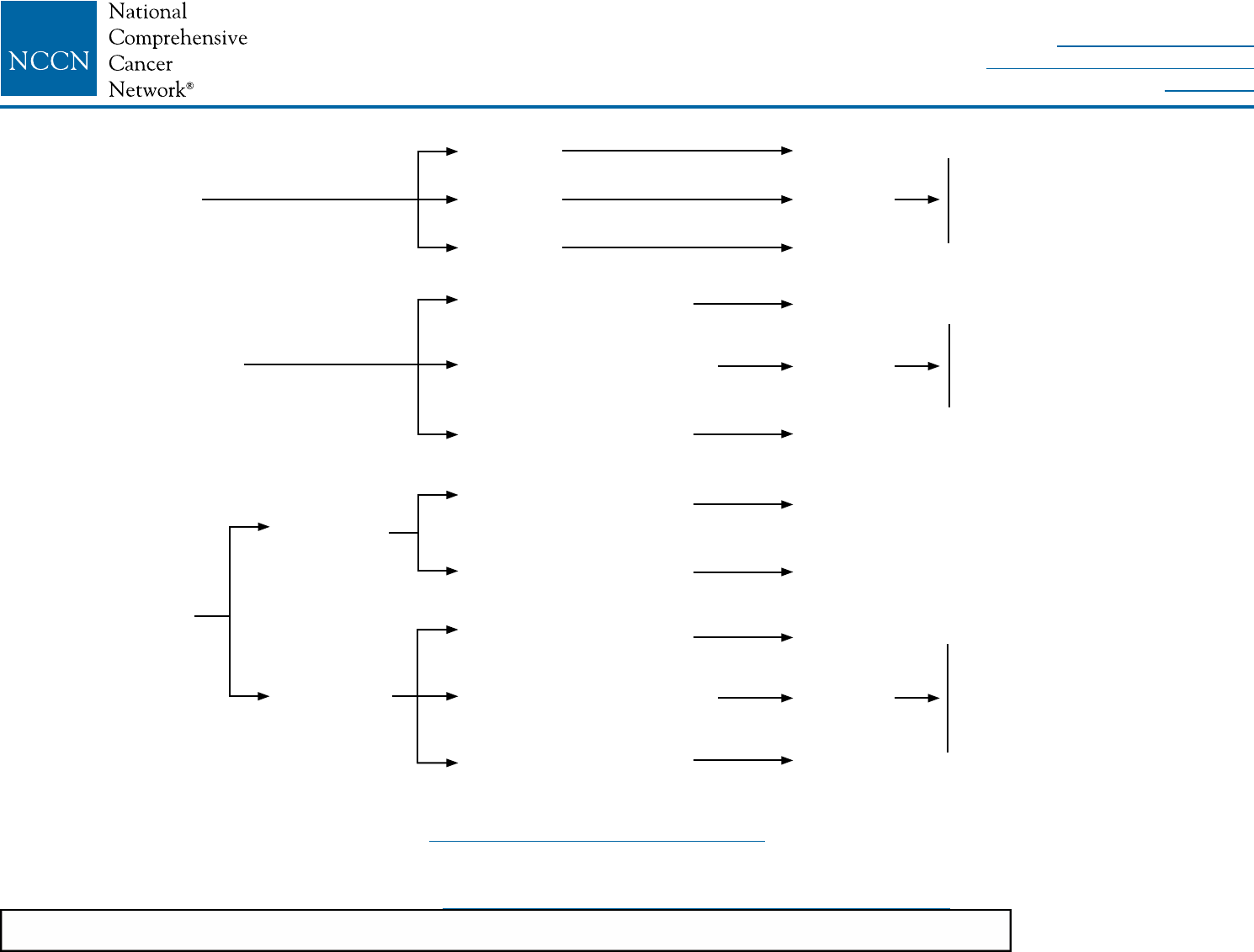
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
BINV-A
PRINCIPLES OF HER2 TESTING1,2
1NCCN Endorses the ASCO/CAP HER2 testing guideline. “Principles of HER2 Testing” modified with permission from Wolff AC, Hammond EH, Hicks DG, et al. Recommendations for
human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update.
J Clin Oncol 2013;31:3997-4013.For additional information, see http://jco.ascopubs.org/content/31/31/3997.full.pdf.
2Laboratory must participate in a quality assurance accreditation program for HER2 testing. Otherwise, tissue specimen should be sent to an accredited laboratory for testing. Health care
systems and providers must cooperate to ensure the highest quality testing.
3Evidence from trastuzumab adjuvant trials show that HER2 testing by ISH or IHC have similar utility to predict clinical benefit from HER2-targeted therapy.
4See ASCO/CAP HER2 Guideline Data Supplement 2E (available at http://www.asco.org/sites/www.asco.org/files/final_her2_testing_ds_10-3-13.pdf) for more information on these rare scenarios.
HER2 testing by
validated IHC assay2,3
HER2 testing by validated
single-probe ISH assay2,3
HER2 testing by
validated dual-probe
ISH assay2,3
HER2/CEP17
ratio ≥2.0
HER2/CEP17
ratio <2.0
IHC 0,1+
IHC 2+
IHC 3+
Average HER2 copy
number <4.0 signals/cell
Average HER2 copy number
≥4.0 and <6.0 signals/cell
Average HER2 copy
number ≥6.0 signals/cell
Average HER2 copy
number <4.0 signals/cell
Average HER2 copy
number ≥4.0 signals/cell
Average HER2 copy
number <4.0 signals/cell
Average HER2 copy number
≥4.0 and <6.0 signals/cell
Average HER2 copy
number ≥6.0 signals/cell
HER2 (-)
Equivocal
result
HER2 (+)
Must reex test with ISH (if same
specimen), or order new test
with IHC or ISH (if new specimen
available).
ISH (-)
Equivocal
result
ISH (+)
ISH (+)4
ISH (+)
ISH (-)
Equivocal
result
ISH (+)
Must reex test with IHC (if same
specimen), test with alternative
ISH chromosome 17 probe, or
order a new test with ISH or IHC
(if new specimen available).
Must reex test with dual-probe
ISH or with IHC (if same specimen),
or order new test with ISH or IHC
(if new specimen available).
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
BINV-B
1Houssami N, Ciatto S, Macaskill P, et al. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in
detection of multifocal and multicentric cancer. J Clin Oncol 2008;26:3248-3258.
PRINCIPLES OF DEDICATED BREAST MRI TESTING
See NCCN Guidelines for Breast Cancer Screening and Diagnosis for indications for screening MRI in women at increased breast cancer risk.
Personnel, Facility, and Equipment
• Breast MRI examinations are performed with IV contrast and should be performed and interpreted by an expert breast imaging team working
in concert with the multidisciplinary treatment team.
• Breast MRI examinations require a dedicated breast coil and breast imaging radiologists familiar with the optimal timing sequences and
other technical details for image interpretation. The imaging center should have the ability to perform MRI-guided needle sampling and/or
image-guided localization of MRI-detected ndings.
Clinical Indications and Applications
• May be used for staging evaluation to dene extent of cancer or presence of multifocal or multicentric cancer in the ipsilateral breast, or as
screening of the contralateral breast cancer at time of initial diagnosis (category 2B). There are no high-level data to demonstrate that the
use of MRI to facilitate local therapy decision-making improves local recurrence or survival.1
• May be helpful for breast cancer evaluation before and after preoperative systemic therapy to dene extent of disease, response to
treatment, and potential for breast-conserving therapy.
• May be useful for identifying primary cancer in women with axillary nodal adenocarcinoma or with Paget’s disease of the nipple with breast
primary not identied on mammography, ultrasound, or physical examination.
• False-positive ndings on breast MRI are common. Surgical decisions should not be based solely on the MRI ndings. Additional tissue
sampling of areas of concern identied by breast MRI is recommended.
• The utility of MRI in follow-up screening of women with prior breast cancer is undened. It should generally be considered only in those
whose lifetime risk of a second primary breast cancer is greater than 20% based on models largely dependent on family history, such as in
those with the risk associated with inherited susceptibility to breast cancer.
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
BINV-C
FERTILITY AND BIRTH CONTROL
See NCCN Guidelines for Adolescent and Young Adult Oncology
• All premenopausal patients should be informed about the potential impact of chemotherapy on fertility and asked about their desire for
potential future pregnancies. Patients who may desire future pregnancies should be referred to fertility specialists before chemotherapy and/
or endocrine therapy to discuss the options based on patient specics, disease stage, and biology (which determine the urgency and type
and sequence of treatment). Timing and duration allowed for fertility preservation, options inclusive of oocyte and embryo cryopreservation
as well as evolving technologies, and the probability of successful pregnancies subsequent to completion of breast cancer therapy are also
to be discussed.
• Although amenorrhea frequently occurs during or after chemotherapy, it appears that the majority of women younger than 35 y resume
menses within 2 y of nishing adjuvant chemotherapy.
• Menses and fertility are not necessarily linked. Absence of regular menses, particularly if the patient is taking tamoxifen, does not
necessarily imply lack of fertility. Conversely, the presence of menses does not guarantee fertility. There are limited data regarding continued
fertility after chemotherapy.
• Patients should not become pregnant during treatment with radiation therapy, chemotherapy, or endocrine therapy.
• Although data are limited, hormone-based birth control is discouraged regardless of the hormone receptor status of the patient's cancer.
• Alternative methods of birth control include intrauterine devices (IUDs), barrier methods, or, for patients with no intent of future pregnancies,
tubal ligation or vasectomy for the partner.
• Randomized trials have shown that ovarian suppression with GnRH agonist therapy administered during adjuvant chemotherapy in
premenopausal women with ER-negative tumors may preserve ovarian function and diminish the likelihood of chemotherapy-induced
amenorrhea.
• Breast feeding following breast-conserving cancer treatment is not contraindicated. However, the quantity and quality of breast milk
produced by the breast conserved may not be sufcient or may be lacking some of the nutrients needed. Breast feeding during active
treatment with chemotherapy and endocrine therapy is not recommended.
• Smaller historical experiences in patients with ER-positive disease have reported conicting results with regard to the protective effect of
GnRH agonist therapy on fertility.
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
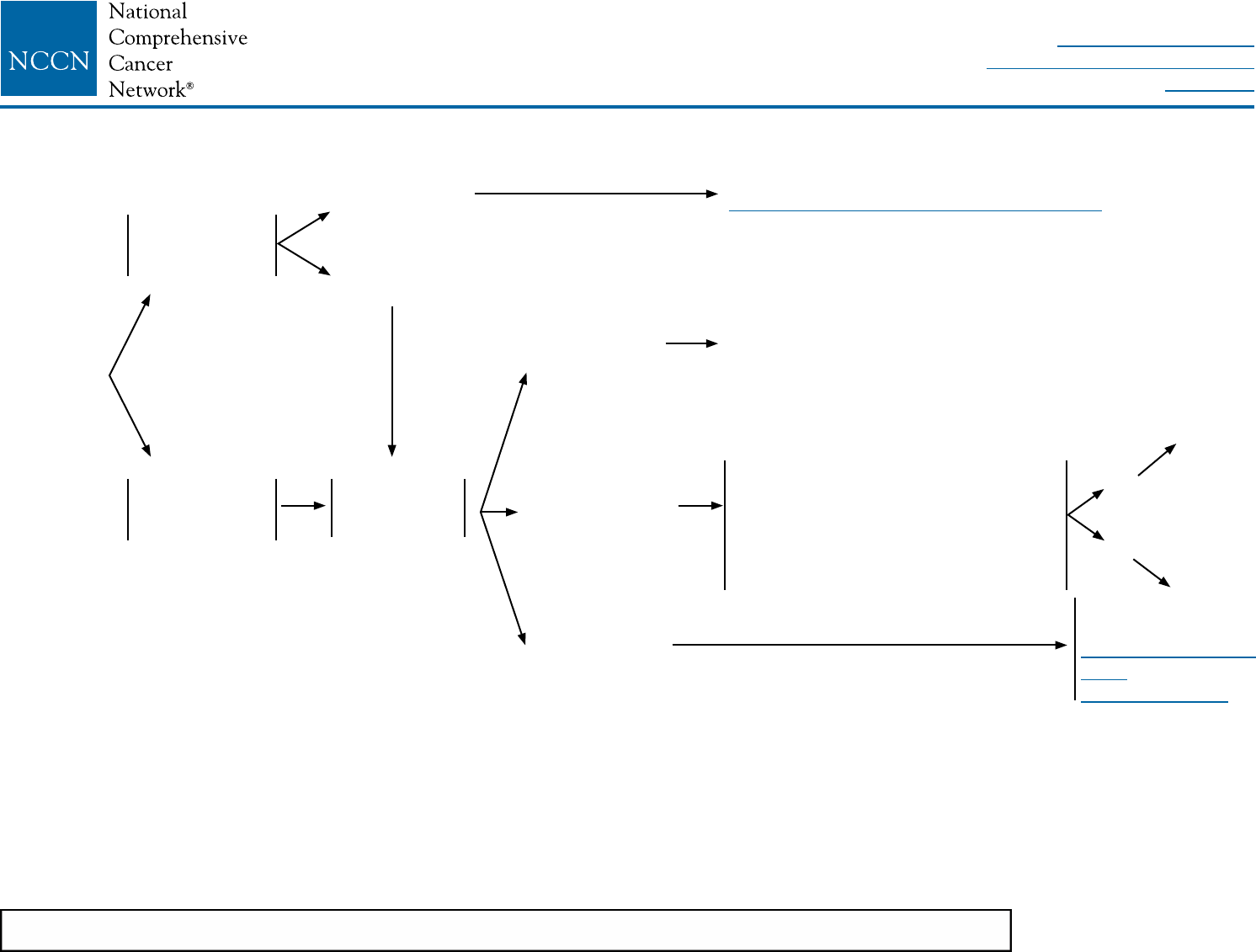
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
BINV-D
1Consider pathologic confirmation of malignancy in clinically positive nodes using ultrasound-guided FNA or core biopsy in determining if a patient needs axillary lymph
node dissection.
2Sentinel lymph node mapping injections may be peritumoral, subareolar, or subdermal.
3Sentinel node involvement is defined by multilevel node sectioning with hematoxylin and eosin (H&E) staining. Cytokeratin immunohistochemistry (IHC) may be used
for equivocal cases on H&E. Routine cytokeratin IHC to define node involvement is not recommended in clinical decision making.
4For patients with clinically negative axillae who are undergoing mastectomy and for whom radiation therapy is planned, axillary radiation may replace axillary dissection
level I/II for regional control of disease.
SURGICAL AXILLARY STAGING - STAGE I, IIA, IIB and lllA T3, N1, M0
Clinical
Stage I, IIA,
IIB and lllA
T3, N1, M0
Clinically node
positive at time
of diagnosis1
Clinically node
negative at time
of diagnosis
Sentinel node
mapping and
excision2,3
Sentinel node
negative3
Sentinel node
positive3
Sentinel node
not identied
Axillary dissection level I/II
See Axillary Lymph Node Staging (BINV-E)
No further axillary surgery (category 1)
Meets ALL of the following criteria:
• T1 or T2 tumor
• 1 or 2 positive sentinel lymph nodes
• Breast-conserving therapy
• Whole-breast RT planned
• No preoperative chemotherapy
Axillary dissection
level I/II4
See Axillary Lymph
Node
Staging (BINV-E)
Yes to all
No
No further axillary
surgery
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
FNA or core
biopsy positive
FNA or core
biopsy negative
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
BINV-E
AXILLARY LYMPH NODE STAGING
SLNB should be performed and is the preferred method of axillary lymph node staging if the patient is an appropriate SLNB candidate
(See BINV-D).
In the absence of denitive data demonstrating superior survival, the performance of axillary staging may be considered optional
in patients who have particularly favorable tumors, patients for whom the selection of adjuvant systemic and/or radiation therapy is
unlikely to be affected, the elderly, or those with serious comorbid conditions.
Level III dissection to the thoracic inlet should be performed only in cases with gross disease in level II and/or lll.
In the absence of gross disease in level II nodes, lymph node dissection should include tissue inferior to the axillary vein from the
latissimus dorsi muscle laterally to the medial border of the pectoralis minor muscle (Level I/II).
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
BINV-F
MARGIN STATUS IN INFILTRATING CARCINOMA
The use of breast-conserving therapy is predicated on achieving a pathologically negative margin of resection. The NCCN Panel accepts the
denition of a negative margin as "No ink on the tumor," from the 2014 Society of Surgical Oncology-American Society for Radiation Oncology
Consensus Guidelines on Margins.1 Cases where there is a positive margin should generally undergo further surgery, either a re-excision to
achieve a negative margin or a mastectomy. If re-excision is technically feasible to allow for breast-conserving therapy, this can be done with
resection of the involved margin guided by the orientation of the initial resection specimen or re-excision of the entire original excision cavity.
It may be reasonable to treat selected cases with breast-conserving therapy with a microscopically focally positive margin in the absence of an
extensive intraductal component (EIC).2 For these patients, the use of a higher radiation boost dose to the tumor bed should be considered.
A boost to the tumor bed is recommended in patients at higher risk for recurrence. Typical doses are 10–16 Gy at 2 Gy/fx.
Margins should be evaluated on all surgical specimens from breast-conserving surgery. Requirements for optimal margin evaluation include:
• Orientation of the surgical specimens
• Description of the gross and microscopic margin status
• Reporting of the distance, orientation, and type of tumor (invasive or DCIS) in relation to the closest margin
1Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving
surgery with whole-breast irradiation in stages I and II invasive breast cancer. J Clin Oncol. 2014 May 10;32(14):1507-15.
2An extensive intraductal component is defined as an infiltrating ductal cancer where greater than 25% of the tumor volume is DCIS and DCIS extends beyond the
invasive cancer into surrounding normal breast parenchyma.
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
BINV-G
1See Margin Status in Infiltrating Carcinoma (BINV-F).
SPECIAL CONSIDERATIONS TO BREAST-CONSERVING THERAPY REQUIRING RADIATION THERAPY
Contraindications for breast-conserving therapy requiring radiation therapy include:
Absolute:
• Radiation therapy during pregnancy
• Diffuse suspicious or malignant-appearing microcalcications
• Widespread disease that cannot be incorporated by local excision through a single incision that achieves negative margins with a
satisfactory cosmetic result
• Diffusely positive pathologic margins1
Relative:
• Prior radiation therapy to the chest wall or breast; knowledge of doses and volumes prescribed is essential.
• Active connective tissue disease involving the skin (especially scleroderma and lupus)
• Tumors >5 cm (category 2B)
• Positive pathologic margin1
• Women with a known or suspected genetic predisposition to breast cancer:
May have an increased risk of ipsilateral breast recurrence or contralateral breast cancer with breast-conserving therapy
Prophylactic bilateral mastectomy for risk reduction may be considered.
(See NCCN Guidelines for Genetic/Familial High-Risk Assessment Breast and Ovarian).
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged. BINV-H
(1 OF 2)
PRINCIPLES OF BREAST RECONSTRUCTION FOLLOWING SURGERY
• Breast reconstruction may be an option for any woman receiving surgical treatment for breast cancer. All women undergoing breast cancer treatment
should be educated about breast reconstructive options as adapted to their individual clinical situation. However, breast reconstruction should not
interfere with the appropriate surgical management of the cancer or the scope of appropriate surgical treatment for this disease. Coordinating consultation
and surgical treatment with a reconstructive surgeon should be executed within a reasonable time frame. The process of breast reconstruction should not
govern the timing or the scope of appropriate surgical treatment for this disease. The availability of or the practicality of breast reconstruction should not
result in the delay or refusal of appropriate surgical intervention.
• An evaluation of the likely cosmetic outcome of lumpectomy should be performed prior to surgery. Oncoplastic techniques for breast conservation can
extend breast-conserving surgical options in situations where the resection by itself would likely yield an unacceptable cosmetic outcome. Application of
these procedures may reduce the need for mastectomy and reduce the chances of secondary surgery for re-excision while minimizing breast deformity.
Patients should be informed of the possibility of positive margins and potential need for secondary surgery, which could include re-excision segmental
resection, or could require mastectomy with or without loss of the nipple. Oncoplastic procedures can be combined with surgery on the contralateral
unaffected breast to minimize long-term asymmetry.
• For mastectomy, the possibility of reconstruction should be discussed and a preoperative evaluation of reconstructive options should be considered.
Surgical options for breast reconstruction following mastectomy include:
Procedures that incorporate breast implants (ie, tissue expander placement followed by implant placement, immediate implant placement)
Procedures that incorporate autologous tissue transplantation (ie, pedicled TRAM ap, fat grafting, various microsurgical aps from the abdomen, back,
buttocks, and thigh)
Procedures that incorporate both breast implants and autologous tissue transplantation (eg, latissimus dorsi aps)
• Breast reconstruction following mastectomy can commence at the same time as mastectomy (“immediate”) or at some time following the completion of
cancer treatment (“delayed”). In many cases, breast reconstruction involves a staged approach requiring more than one procedure such as:
Surgery on the contralateral breast to improve symmetry
Revision surgery involving the breast and/or donor site
Nipple and areola reconstruction and tattoo pigmentation
• As with any mastectomy, there is a risk of local and regional cancer recurrence, and evidence suggests skin-sparing mastectomy is probably equivalent to
standard mastectomy in this regard. Skin-sparing mastectomy should be performed by an experienced breast surgery team that works in a coordinated,
multidisciplinary fashion to guide proper patient selection for skin-sparing mastectomy, determine optimal sequencing of the reconstructive procedure(s)
in relation to adjuvant therapies, and perform a resection that achieves appropriate surgical margins. Post-mastectomy radiation should still be applied in
cases treated by skin-sparing mastectomy following the same selection criteria as for standard mastectomy.
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged. BINV-H
(2 OF 2)
PRINCIPLES OF BREAST RECONSTRUCTION FOLLOWING SURGERY
• Immediate reconstruction is contraindicated in the setting of mastectomy for inammatory breast cancer (IBC) due to the high risk of recurrence,
aggressive nature of the disease, and consequent need to proceed expeditiously to postoperative radiotherapy for local control without any potential
delay. As skin-sparing mastectomy has not yet been demonstrated to be safe for IBC there is also a need to resect currently or previously involved skin at
the time of mastectomy. Thus, there is no advantage to immediate reconstruction in this setting.
• In general, the nipple-areolar complex (NAC) is sacriced with skin-sparing mastectomy for cancer therapy. However, NAC-sparing procedures may
be an option in cancer patients who are carefully selected by experienced multidisciplinary teams. Retrospective data support the use of NAC-sparing
procedures for breast cancer therapy with low nipple-involvement rates and low local-recurrence rates for early-stage, biologically favorable (eg,
Nottingham grade 1 or 2, node-negative, HER2/neu negative, no lymphovascular invasion), invasive cancers and/or DCIS that is peripherally located in the
breast (>2 cm from nipple). Nipple margin assessment is mandatory, and the nipple margin should be clearly designated. Evidence of nipple involvement
such as Paget’s disease or other nipple discharge associated with malignancy, and/or imaging ndings suggesting malignant involvement of the nipple or
subareolar tissues contraindicates nipple preservation.
• In the previously radiated patients, the use of tissue expanders/implants is relatively contraindicated. Tissue expansion of irradiated skin can result in a
signicantly increased risk of capsular contracture, malposition, poor cosmesis, implant exposure, and failed reconstruction. In the setting of previous
radiation, autologous tissue reconstruction is the preferred method of breast reconstruction.
• While noninammatory, locally advanced breast cancer is not an absolute contraindication to immediate reconstruction, post-mastectomy radiation should
still be applied regardless of the reconstruction approach:
When post-mastectomy radiation is required and autologous tissue reconstruction is planned, reconstruction is either delayed until after the completion
of radiation therapy, or it can be initiated at the time of mastectomy with tissue expander placement followed by autologous tissue reconstruction.
While some experienced breast cancer teams have employed protocols in which immediate tissue reconstructions are followed by radiation therapy, it
is generally preferred that the radiation therapy precede the placement of the autologous tissue, because of reported loss in reconstruction cosmesis
(category 2B).
When implant reconstruction is planned in a patient requiring radiation therapy, a staged approach with immediate tissue expander placement followed
by implant placement is preferred. Surgery to exchange the tissue expanders with permanent implants can be performed prior to radiation or after
completion of radiation therapy. Immediate placement of an implant in patients requiring postoperative radiation has an increased rate of capsular
contracture, malposition, poor cosmesis, and implant exposure.
• Reconstruction selection is based on an assessment of cancer treatment, patient body habitus, obesity, smoking history, comorbidities, and patient
concerns. Smoking and obesity increase the risk of complications for all types of breast reconstruction whether with implant or ap. Smoking and obesity
are therefore considered a relative contraindication to breast reconstruction and patients should be made aware of increased rates of wound healing
complications and partial or complete ap failure among smokers and obese patients.
• Women who are not satised with the cosmetic outcome following completion of breast cancer treatment should be offered a plastic surgery consultation.
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
Optimizing Delivery of Individual Therapy:
It is important to individualize radiation therapy planning and delivery. CT-
based treatment planning is encouraged to delineate target volumes and
adjacent organs at risk. Greater target dose homogeneity and sparing of
normal tissues can be accomplished using compensators such as wedges,
forward planning using segments, and intensity-modulated radiation
therapy (IMRT).
Respiratory control techniques including deep inspiration breath-hold and
prone positioning may be used to try to further reduce dose to adjacent
normal tissues, in particular heart and lung. Boost treatment in the setting
of breast conservation can be delivered using enface electrons, photons,
or brachytherapy. Chest wall scar boost when indicated is typically treated
with electrons or photons.
Verication of daily setup consistency is done with weekly imaging. In
certain circumstances, more frequent imaging may be appropriate. Routine
use of daily imaging is not recommended.
Whole Breast Radiation:
Target denition is the breast tissue in entirety. The whole breast should
receive a dose of 46–50 Gy in 23–25 fractions or 40–42.5 Gy in 15–16
fractions (hypofractionation is preferred). All dose schedules are given
5 days per week. A boost to the tumor bed is recommended in patients
at higher risk for recurrence. Typical boost doses are 10–16 Gy in 4–8
fractions.
Chest Wall Radiation (including breast reconstruction):
The target includes the ipsilateral chest wall, mastectomy scar, and drain
sites when indicated. Depending on whether the patient has had breast
reconstruction or not, several techniques using photons and/or electrons
are appropriate. CT-based treatment planning is encouraged in order to
identify lung and heart volumes and minimize exposure of these organs.
Dose is 46–50 Gy in 23–25 fractions to the chest wall +/- scar boost at 2 Gy
per fraction to a total dose of approximately 60 Gy. All dose schedules are
given 5 days per week. Special consideration should be given to the use of
bolus material to ensure that the skin dose is adequate.
Regional Nodal Radiation:
Target delineation is best achieved by the use of CT-based treatment
planning. For the paraclavicular and axillary nodes, prescription depth varies
based on the patient anatomy. For internal mammary node identication,
the internal mammary artery and vein can be used as a surrogate for the
nodal location (as the nodes themselves are not usually visible on planning
imaging). Based on the post-mastectomy radiation randomized studies
and recent trials, radiation therapy of the internal mammary lymph nodes
should be strongly considered when delivering regional nodal irradiation. CT
treatment planning should be utilized when treating the internal mammary
lymph nodal volume to evaluate dose to normal tissues, especially the
heart and lung, and dose constraints respected. Dose is 46–50 Gy in 23–25
fractions to the regional nodal elds. All dose schedules are given 5 days
per week.
Accelerated Partial Breast Irradiation (APBI):
Preliminary studies of APBI suggest that rates of local control in selected
patients with early-stage breast cancer may be comparable to those treated
with standard whole breast RT. However, compared to standard whole
breast radiation, several recent studies document an inferior cosmetic
outcome with APBI. Follow-up is limited and studies are ongoing. Patients
are encouraged to participate in clinical trials. If not trial eligible, per the
consensus statement from the American Society for Radiation Oncology
(ASTRO), patients who may be suitable for APBI are women 60 y and older
who are not carriers of BRCA 1/2 mutation treated with primary surgery for a
unifocal T1N0 ER-positive cancer. Histology should be inltrating ductal or a
favorable ductal subtype and not associated with EIC or LCIS, and margins
should be negative.
34 Gy in 10 fractions delivered twice per day with brachytherapy or 38.5 Gy
in 10 fractions delivered twice per day with external beam photon therapy
is prescribed to the tumor bed. Other fractionation schemes are currently
under investigation.
Preoperative Systemic Therapy:
In patients treated with preoperative systemic therapy, indications for
radiation therapy and treatment elds should be based on the maximum
stage from the pre-therapy clinical stage, pathologic stage, and tumor
characteristics.
BINV-I
PRINCIPLES OF RADIATION THERAPY
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
BINV-J
ADJUVANT ENDOCRINE THERAPY
1See Definition of Menopause (BINV-M).
2Aromatase inhibitor or tamoxifen for 5 y plus ovarian suppression should be considered, based on SOFT and TEXT clinical trial outcomes, for premenopausal women
at higher risk of recurrence (ie, young age, high-grade tumor, lymph node involvement, Pagani, NEJM 2014, Prudence, NEJM 2014).Survival data still pending.
3The panel believes the three selective aromatase inhibitors (ie, anastrozole, letrozole, exemestane) have shown similar anti-tumor efficacy and toxicity profiles in
randomized studies in the adjuvant and preoperative settings. The optimal duration of aromatase inhibitors in adjuvant therapy is uncertain.
4Some SSRIs like fluoxetine and paroxetine decrease the formation of endoxifen, 4-OH tamoxifen, and active metabolites of tamoxifen, and may impact its efficacy.
Caution is advised about coadministration of these drugs with tamoxifen. However, citalopram and venlafaxine appear to have minimal impact on tamoxifen
metabolism. At this time, based on current data the panel recommends against CYP2D6 gene testing for women being considered for tamoxifen therapy.
Coadministration of strong inhibitors of CYP2D6 should be used with caution.
Premenopausal1
at diagnosis
Postmenopausal1
at diagnosis
Tamoxifen4 for 5 y (category 1)
± ovarian suppression or ablation
(category 1)2
or
Aromatase inhibitor3 for 5 y +
ovarian suppression or ablation
(category 1)2
Postmenopausal1
Premenopausal1
Aromatase inhibitor for 5 y3 (category 1)
or
Consider tamoxifen4 for an additional 5 y to
complete 10 y
Consider tamoxifen4 for an additional 5 y to
complete 10 y
or
No further endocrine therapy
Aromatase inhibitor3 for 5 y (category 1)
or
Tamoxifen4 for 2–3 y
or
Aromatase inhibitor3 for 2–3 y (category 1)
Tamoxifen4 for 4.5–6 y
Women with a contraindication to aromatase
inhibitors, who decline aromatase inhibitors, or
who are intolerant of the aromatase inhibitors
Aromatase inhibitor to complete 5 y3 of endocrine
therapy (category 1)
or
Up to 5 y of an aromatase inhibitor3 (category 2B)
Aromatase inhibitor for 5 y3 (category 1)
or
Consider tamoxifen4 for an additional 5 y to
complete 10 y
Tamoxifen4 for 5 y (category 1)
or
Consider tamoxifen4 for up to 10 y
Tamoxifen4 to complete 5 y of endocrine therapy
(category 1)
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged. BINV-K
1 OF 7
PREOPERATIVE/ADJUVANT THERAPY REGIMENS 1,2,3,4
Regimens for HER2-negative disease5
6In patients with HER2-positive and axillary node-positive breast cancer,
trastuzumab should be incorporated into the adjuvant therapy (category 1).
Trastuzumab should also be considered for patients with HER2-positive node-
negative tumors ≥1 cm (category 1).
7Trastuzumab should optimally be given concurrently with paclitaxel as part of
the AC followed by paclitaxel regimen, and should be given for one year total
duration.
8A pertuzumab-containing regimen can be administered to patients with ≥T2 or
≥N1, HER2-positive, early-stage breast cancer preoperatively. Patients who have
not received a pertuzumab-containing regimen can receive adjuvant pertuzumab.
9Trastuzumab given in combination with an anthracycline is associated with
significant cardiac toxicity. Concurrent use of trastuzumab and pertuzumab with
an anthracycline should be avoided.
10Paclitaxel + trastuzumab may be considered for patients with low-risk stage l,
HER2-positive disease, particularly those not eligible for other standard adjuvant
regimens due to comorbidities.
1Retrospective evidence suggests that anthracycline-based chemotherapy
regimens may be superior to non-anthracycline-based regimens in patients with
HER2-positive tumors.
2Randomized clinical trials demonstrate that the addition of a taxane to
anthracycline-based chemotherapy provides an improved outcome.
3CMF and radiation therapy may be given concurrently, or the CMF may be given
first. All other chemotherapy regimens should be given prior to radiotherapy.
4Chemotherapy and endocrine therapy used as adjuvant therapy should be given
sequentially with endocrine therapy following chemotherapy.
5The regimens listed for HER2-negative disease are all category 1 (except where
indicated) when used in the adjuvant setting.
Preferred regimens:
• Dose-dense AC (doxorubicin/cyclophosphamide) followed by
paclitaxel every 2 weeks
• Dose-dense AC (doxorubicin/cyclophosphamide) followed by
weekly paclitaxel
• TC (docetaxel and cyclophosphamide)
Other regimens:
• Dose-dense AC (doxorubicin/cyclophosphamide)
• AC (doxorubicin/cyclophosphamide) every 3 weeks (category 2B)
• CMF (cyclophosphamide/methotrexate/uorouracil)
• AC followed by docetaxel every 3 weeks
• AC followed by weekly paclitaxel
• EC (epirubicin/cyclophosphamide)
• FEC/CEF followed by T
(uorouracil/epirubicin/cyclophosphamide followed by docetaxel) or
(uorouracil/epirubicin/cyclophosphamide followed by weekly paclitaxel)
• FAC followed by T
(uorouracil/doxorubicin/cyclophosphamide followed by weekly paclitaxel)
• TAC (docetaxel/doxorubicin/cyclophosphamide)
Regimens for HER2-positive disease6,7,8
Preferred regimens:
• AC followed by T + trastuzumab ± pertuzumab9
(doxorubicin/cyclophosphamide followed by paclitaxel plus trastuzumab ±
pertuzumab, various schedules)
• TCH (docetaxel/carboplatin/trastuzumab) ± pertuzumab
Other regimens:
• AC followed by docetaxel + trastuzumab ± pertuzumab9
• Docetaxel + cyclophosphamide + trastuzumab
• FEC followed by docetaxel + trastuzumab + pertuzumab9
• FEC followed by paclitaxel + trastuzumab + pertuzumab9
• Paclitaxel + trastuzumab10
• Pertuzumab + trastuzumab + docetaxel followed by FEC9
• Pertuzumab + trastuzumab + paclitaxel followed by FEC9
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged. BINV-K
2 OF 7
DOSING SCHEDULES FOR COMBINATIONS FOR HER2-NEGATIVE DISEASE: PREFERRED REGIMENS
Dose-dense AC followed by paclitaxel chemotherapy1
• Doxorubicin 60 mg/m2 IV day 1
• Cyclophosphamide 600 mg/m2 IV day 1
Cycled every 14 days for 4 cycles.
(All cycles are with myeloid growth factor support)
Followed by:
• Paclitaxel 175 mg/m2 by 3 h IV infusion day 1
Cycled every 14 days for 4 cycles.
(All cycles are with myeloid growth factor support)
Dose-dense AC followed by weekly paclitaxel chemotherapy1
• Doxorubicin 60 mg/m2 IV day 1
• Cyclophosphamide 600 mg/m2 IV day 1
Cycled every 14 days for 4 cycles.
(All cycles are with myeloid growth factor support)
Followed by:
• Paclitaxel 80 mg/m2 by 1 h IV infusion weekly for 12 wks.
TC chemotherapy2
• Docetaxel 75 mg/m2 IV day 1
• Cyclophosphamide 600 mg/m2 IV day 1
Cycled every 21 days for 4 cycles.
(All cycles are with myeloid growth factor support)
OTHER REGIMENS LISTED ON NEXT PAGE
The selection, dosing, and administration of anti-cancer agents and the management of associated toxicities are complex. Modifications of drug dose and schedule and initiation of supportive care interventions are often
necessary because of expected toxicities and individual patient variability, prior treatment, and comorbidity. The optimal delivery of anti-cancer agents therefore requires a health care delivery team experienced in the use
of anti-cancer agents and the management of associated toxicities in patients with cancer.
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged. BINV-K
3 OF 7
DOSING SCHEDULES FOR COMBINATIONS FOR HER2-NEGATIVE DISEASE: OTHER REGIMENS
Dose-dense AC chemotherapy1
• Doxorubicin 60 mg/m2 IV day 1
• Cyclophosphamide 600 mg/m2 IV day 1
Cycled every 14 days for 4 cycles.
(All cycles are with myeloid growth factor support)
AC chemotherapy3
• Doxorubicin 60 mg/m2 IV on day 1
• Cyclophosphamide 600 mg/m2 IV day 1
Cycled every 21 days for 4 cycles.
TAC chemotherapy4
• Docetaxel 75 mg/m2 IV day 1
• Doxorubicin 50 mg/m2 IV day 1
• Cyclophosphamide 500 mg/m2 IV day 1
Cycled every 21 days for 6 cycles.
(All cycles are with myeloid growth factor support)
CMF chemotherapy5
• Cyclophosphamide 100 mg/m2 PO days 1–14
• Methotrexate 40 mg/m2 IV days 1 & 8
• 5-uorouracil 600 mg/m2 IV days 1 & 8
Cycled every 28 days for 6 cycles.
AC followed by docetaxel chemotherapy6
• Doxorubicin 60 mg/m2 IV on day 1
• Cyclophosphamide 600 mg/m2 IV day 1
Cycled every 21 days for 4 cycles.
Followed by:
• Docetaxel 100 mg/m2 IV on day 1
Cycled every 21 days for 4 cycles.
AC followed by weekly paclitaxel7
• Doxorubicin 60 mg/m2 IV day 1
• Cyclophosphamide 600 mg/m2 IV day 1
Cycled every 21 days for 4 cycles.
Followed by:
• Paclitaxel 80 mg/m2 by 1 h IV infusion weekly
for 12 wks.
EC chemotherapy8
• Epirubicin 100 mg/m2 IV day 1
• Cyclophosphamide 830 mg/m2 IV day 1
Cycled every 21 days for 8 cycles.
FEC followed by docetaxel chemotherapy9
• 5-uorouracil 500 mg/m2 IV day 1
• Epirubicin 100 mg/m2 IV day 1
• Cyclophosphamide 500 mg/m2 IV day 1
Cycled every 21 days for 3 cycles.
Followed by:
• Docetaxel 100 mg/m2 IV day 1
Cycled every 21 days for 3 cycles.
FEC followed by weekly paclitaxel10
• 5-uorouracil 600 mg/m2 IV day 1
• Epirubicin 90 mg/m2 IV day 1
• Cyclophosphamide 600 mg/m2 IV day 1
Cycled every 21 days for 4 cycles.
Followed by:
Paclitaxel 100 mg/m2 IV infusion weekly for 8 wks.
FAC followed by weekly paclitaxel
• 5-uorouracil 500 mg/m2 IV days 1 & 8 or days 1 & 4
• Doxorubicin 50 mg/m2 IV day 1
(or by 72-h continuous infusion)
• Cyclophosphamide 500 mg/m2 IV day 1
Cycled every 21 days for 4 cycles.
Followed by:
• Paclitaxel 80 mg/m2 by 1 h IV infusion weekly for
12 wks.
The selection, dosing, and administration of anti-cancer agents and the management of associated toxicities are complex. Modifications of drug dose and schedule and initiation of supportive care interventions are often
necessary because of expected toxicities and individual patient variability, prior treatment, and comorbidity. The optimal delivery of anti-cancer agents therefore requires a health care delivery team experienced in the use
of anti-cancer agents and the management of associated toxicities in patients with cancer.
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged. BINV-K
4 OF 7
DOSING SCHEDULE FOR COMBINATIONS FOR HER2-POSITIVE DISEASE: PREFERRED REGIMENS
AC followed by T chemotherapy with trastuzumab11
• Doxorubicin 60 mg/m2 IV day 1
• Cyclophosphamide 600 mg/m2 IV day 1
Cycled every 21 days for 4 cycles.
Followed by:
Paclitaxel 80 mg/m2 by 1 h IV weekly for 12 wks
With:
• Trastuzumab 4 mg/kg IV with rst dose of paclitaxel
Followed by:
• Trastuzumab 2 mg/kg IV weekly to complete 1 y of treatment. As an
alternative, trastuzumab 6 mg/kg IV every 21 days may be used following
the completion of paclitaxel, and given to complete 1 y of trastuzumab
treatment.
Evaluate left ventricular ejection fraction (LVEF) prior to and during
treatment.*
AC followed by T chemotherapy with trastuzumab + pertuzumab
• Doxorubicin 60 mg/m2 IV day 1
• Cyclophosphamide 600 mg/m2 IV day 1
Cycled every 21 days for 4 cycles.
Followed by:
• Pertuzumab 840 mg IV day 1 followed by 420 mg IV
• Trastuzumab 8 mg/kg IV day 1 followed by 6 mg/kg IV
• Paclitaxel 80 mg/m2 IV days 1, 8, and 15
Cycled every 21 days for 4 cycles
• Trastuzumab 6 mg/kg IV day 1
Cycled every 21 days to complete 1 y of trastuzumab therapy
Evaluate LVEF prior to and during treatment.*
Dose-dense AC followed by paclitaxel chemotherapy with trastuzumab12
• Doxorubicin 60 mg/m2 IV day 1
• Cyclophosphamide 600 mg/m2 IV day 1
Cycled every 14 days for 4 cycles.
Followed by:
• Paclitaxel 175 mg/m2 by 3 h IV infusion day 1
Cycled every 14 days for 4 cycles.
With:
• Trastuzumab 4 mg/kg IV with rst dose of paclitaxel
Followed by:
• Trastuzumab 2 mg/kg IV weekly to complete 1 y of treatment. As an alternative,
trastuzumab 6 mg/kg IV every 21 days may be used following the completion of
paclitaxel, and given to complete 1 y of trastuzumab treatment.
Evaluate LVEF prior to and during treatment.*
TCH chemotherapy13
• Docetaxel 75 mg/m2 IV day 1
• Carboplatin AUC 6 IV day 1
Cycled every 21 days for 6 cycles
With:
• Trastuzumab 4 mg/kg IV wk 1
Followed by:
• Trastuzumab 2 mg/kg IV for 17 wks
Followed by:
• Trastuzumab 6 mg/kg IV every 21 days to complete 1 y of trastuzumab therapy
OR
• Trastuzumab 8 mg/kg IV wk 1
Followed by:
• Trastuzumab 6 mg/kg IV every 21 days to complete 1 y of trastuzumab therapy
Evaluate LVEF prior to and during treatment.*
TCH chemotherapy + pertuzumab14
• Trastuzumab 8 mg/kg IV day 1 followed by 6 mg/kg IV
• Pertuzumab 840 mg IV day 1 followed by 420 mg IV
• Docetaxel 75 mg/m2 IV day 1
• Carboplatin AUC 6 IV day 1
Cycled every 21 days for 6 cycles
Followed by:
• Trastuzumab 6 mg/kg IV every 21 days to complete 1 y of trastuzumab therapy
Evaluate LVEF prior to and during treatment.*
*The optimal frequency of LVEF assessment during adjuvant trastuzumab
therapy is not known. The FDA label recommends LVEF measurements
prior to initiation of trastuzumab and every 3 mo during therapy.
The selection, dosing, and administration of anti-cancer agents and the management of
associated toxicities are complex. Modifications of drug dose and schedule and initiation of
supportive care interventions are often necessary because of expected toxicities and individual
patient variability, prior treatment, and comorbidity. The optimal delivery of anti-cancer agents
therefore requires a health care delivery team experienced in the use of anti-cancer agents and
the management of associated toxicities in patients with cancer.
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged. BINV-K
5 OF 7
DOSING SCHEDULE FOR COMBINATIONS FOR HER2-POSITIVE DISEASE: OTHER REGIMENS
AC followed by docetaxel chemotherapy with trastuzumab13
• Doxorubicin 60 mg/m2 IV day 1
• Cyclophosphamide 600 mg/m2 IV day 1
Cycled every 21 days for 4 cycles
Followed by:
• Docetaxel 100 mg/m2 IV day 1
Cycled every 21 days for 4 cycles
With:
• Trastuzumab 4 mg/kg IV wk 1
Followed by:
• Trastuzumab 2 mg/kg IV weekly for 11 wks
Followed by:
• Trastuzumab 6 mg/kg IV every 21 days to complete 1 y of
trastuzumab therapy
Evaluate LVEF prior to and during treatment.*
AC followed by docetaxel chemotherapy with trastuzumab and pertuzumab
• Doxorubicin 60 mg/m2 IV day 1
• Cyclophosphamide 600 mg/m2 IV day 1
Cycled every 21 days for 4 cycles
Followed by:
• Pertuzumab 840 mg IV day 1 followed by 420 mg IV
• Trastuzumab 8 mg/kg IV day 1 followed by 6 mg/kg IV
• Docetaxel 75–100 mg/m2 IV day 1
Cycled every 21 days for 4 cycles
Followed by:
• Trastuzumab 6 mg/kg IV every 21 days to complete 1 y of
trastuzumab therapy
Evaluate LVEF prior to and during treatment.*
Docetaxel/cyclophosphamide chemotherapy with trastuzumab15
• Docetaxel 75 mg/m2 IV day 1
• Cyclophosphamide 600 mg/m2 IV day 1
Cycled every 21 days for 4 cycles
With:
Trastuzumab 4 mg/kg IV wk 1
Followed by
Trastuzumab 2 mg/kg IV weekly for 11 wks
Followed by
Trastuzumab 6 mg/kg IV every 21 days to complete 1 y of trastuzumab
therapy
OR
• Trastuzumab 8 mg/kg IV wk 1
Followed by:
• Trastuzumab 6 mg/kg IV every 21 days to complete 1 y of trastuzumab
therapy
Evaluate LVEF prior to and during treatment.*
FEC chemotherapy followed by pertuzumab + trastuzumab + docetaxel14
• Fluorouracil 500 mg/m2 IV day 1
• Epirubicin 100 mg/m2 IV day 1
• Cyclophosphamide 600 mg/m2 IV day 1
Cycled every 21 days for 3 cycles
Followed by:
• Pertuzumab 840 mg IV day 1 followed by 420 mg IV
• Trastuzumab 8 mg/kg IV day 1 followed by 6 mg/kg IV
• Docetaxel 75–100 mg/m2 IV day 1
Cycled every 21 days for 3 cycles
Followed by:
• Trastuzumab 6 mg/kg IV every 21 days to complete 1 y of
trastuzumab therapy
Evaluate LVEF prior to and during treatment.*
The selection, dosing, and administration of anti-cancer agents and the management of associated toxicities are complex. Modifications of drug dose and schedule and initiation of supportive care interventions are often
necessary because of expected toxicities and individual patient variability, prior treatment, and comorbidity. The optimal delivery of anti-cancer agents therefore requires a health care delivery team experienced in the use
of anti-cancer agents and the management of associated toxicities in patients with cancer.
*The optimal frequency of LVEF assessment during adjuvant trastuzumab
therapy is not known. The FDA label recommends LVEF measurements
prior to initiation of trastuzumab and every 3 mo during therapy.
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged. BINV-K
6 OF 7
DOSING SCHEDULE FOR COMBINATIONS FOR HER2-POSITIVE DISEASE: OTHER REGIMENS (continued)
FEC chemotherapy followed by pertuzumab + trastuzumab + paclitaxel
• Fluorouracil 500 mg/m2 IV day 1
• Epirubicin 100 mg/m2 IV day 1
• Cyclophosphamide 600 mg/m2 IV day 1
Cycled every 21 days for 3 cycles
Followed by:
• Pertuzumab 840 mg IV day 1 followed by 420 mg IV
• Trastuzumab 8 mg/kg IV day 1 followed by 6 mg/kg IV
• Paclitaxel 80 mg/m2 IV days 1, 8, and 15
Cycled every 21 days for 3 cycles
Followed by:
• Trastuzumab 6 mg/kg IV every 21 days to complete 1 y of trastuzumab
therapy
Evaluate LVEF prior to and during treatment.*
Paclitaxel + trastuzumab16
• Paclitaxel 80 mg/m2 IV weekly for 12 weeks
With:
• Trastuzumab 4 mg/kg IV with rst dose of paclitaxel
Followed by:
• Trastuzumab 2 mg/kg IV weekly to complete 1 y of treatment. As an
alternative, trastuzumab 6 mg/kg IV every 21 days may be used following
the completion of paclitaxel, and given to complete 1 y of trastuzumab
treatment.
Evaluate LVEF prior to and during treatment.*
Pertuzumab + trastuzumab + docetaxel followed by FEC chemotherapy17
Neoadjuvant therapy:
• Pertuzumab 840 mg IV day 1 followed by 420 mg IV
• Trastuzumab 8 mg/kg IV day 1 followed by 6 mg/kg IV
• Docetaxel 75–100 mg/m2 IV day 1
Cycled every 21 days for 4 cycles
Followed by adjuvant therapy
• Fluorouracil 600 mg/m2 IV day 1
• Epirubicin 90 mg/m2 IV day 1
• Cyclophosphamide 600 mg/m2 IV day 1
Cycled every 21 days for 3 cycles
Followed by:
• Trastuzumab 6 mg/kg IV every 21 days to complete 1 y of trastuzumab
therapy
Evaluate LVEF prior to and during treatment.*
Pertuzumab + trastuzumab + paclitaxel followed by FEC chemotherapy
Neoadjuvant therapy:
• Pertuzumab 840 mg IV day 1 followed by 420 mg IV
• Trastuzumab 8 mg/kg IV day 1 followed by 6 mg/kg IV
• Paclitaxel 80 mg/m2 IV days 1, 8, and 15
Cycled every 21 days for 4 cycles
Followed by adjuvant therapy
• Fluorouracil 600 mg/m2 IV day 1
• Epirubicin 90 mg/m2 IV day 1
• Cyclophosphamide 600 mg/m2 IV day 1
Cycled every 21 days for 3 cycles
Followed by:
• Trastuzumab 6 mg/kg IV every 21 days to complete 1 y of trastuzumab
therapy
Evaluate LVEF prior to and during treatment.*
.
The selection, dosing, and administration of anti-cancer agents and the management of associated toxicities are complex. Modifications of drug dose and schedule and initiation of supportive care interventions are often
necessary because of expected toxicities and individual patient variability, prior treatment, and comorbidity. The optimal delivery of anti-cancer agents therefore requires a health care delivery team experienced in the use
of anti-cancer agents and the management of associated toxicities in patients with cancer.
*The optimal frequency of LVEF assessment during adjuvant trastuzumab
therapy is not known. The FDA label recommends LVEF measurements
prior to initiation of trastuzumab and every 3 mo during therapy.
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged. BINV-K
7 OF 7
REFERENCES FOR PREOPERATIVE/ADJUVANT THERAPY REGIMENS
1Citron ML, Berry DA, Cirrincione C, et al: Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination
chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of intergroup trial C9741/cancer and leukemia group B trial
9741. J Clin Oncol 2003;21:1431-1439.
2Jones S, Holmes F, O’Shaughnessey J, et al. Docetaxel with cyclophosphamide is associated with an overall survival benet compared with doxorubicin and
cyclophosphamide: 7-year follow-up of US Oncology Research trial 9735. J Clin Oncol 2009;27:1177-1183.
3Fisher B, Brown AM, Dimitrov NV, et al. Two months of doxorubicin-cyclophosphamide with and without interval reinduction therapy compared with 6 months of
cyclophosphamide, methotrexate, and uorouracil in positive-node breast cancer patients with tamoxifen-nonresponsive tumors: results from the National Surgical
Adjuvant Breast and Bowel Project B-15. J Clin Oncol 1990;8:1483-1496.
4Martin, Pienkowski T, Mackey J, et al: Adjuvant docetaxel for node-positive breast cancer. N Engl J Med 2005;352:22.
5Goldhirsch A, Colleoni M, Coates AS, et al: Adding adjuvant CMF chemotherapy to either radiotherapy or tamoxifen: are all CMFs alike? The International Breast
Cancer Study Group (IBCSG). Ann Oncol 1998;9:489-93.
6von Minckwitz G1, Raab G, Caputo A, et al. Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every
14 days as preoperative treatment in operable breast cancer: the GEPARDUO study of the German Breast Group. J Clin Oncol 2005;23(12):2676-85.
7Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in adjuvant treatment of breast cancer. N Engl J Med 2008;258:1663-1671.
8Piccart MJ, Di Leo A, Beauduin M, et al: Phase III trial comparing two dose levels of epirubicin combined with cyclophosphamide with cyclophosphamide, methotrexate,
and uorouracil in node-positive breast cancer. J Clin Oncol 2001;19:3103-3110.
9Roche H, Fumoleau P, Spielmann M, et al. Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: The FNCLCC
PACS 001 trial. J Clin Oncol 2006; 24:5664-5671.
10Martin M, Rodriguez-Lescure A, Ruiz A, et al: Randomized phase 3 trial of uorouracil, epirubicin, and cyclophosphamide alone or followed by paclitaxel for early
breast cancer. J Natl Cancer Inst 2008;100:805-814.
11Romond EH, Perez EZ, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2 positive breast cancer. N Engl J Med 2005;353:1673-1684.
12Dang C, Fornier M, Sugarman S, et al: The safety of dose-dense doxorubicin and cyclophosphamide followed by paclitaxel with trastuzumab in HER-2/neu over-
expressed/amplied breast cancer. J Clin Oncol 2008;26(8):1216-22.
13Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 2011;365:1273-1283.
14Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free
chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 2013;24:2278-2284.
15Jones SE, Collea R, Paul D, et al. Adjuvant docetaxel and cyclophosphamide plus trastuzumab in patients with HER2-amplied early stage breast cancer: a single-
group, open-label, phase 2 study. Lancet Oncol 2013;14:1121-8.
16Tolaney S, Barry W, Dang C, et al. Adjuvant paclitaxel and trastuzumab for node-negative HER2-positive breast cancer. N Engl J Med 2016;372:134-141.
17Gianni L, Pienkowski T, Im YH, et al. Efcacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inammatory, or early HER2-
positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:25-32.
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged. BINV-L
1 OF 2
PRINCIPLES OF PREOPERATIVE SYSTEMIC THERAPY
• Randomized trials demonstrate similar long-term outcomes when
patients are given the same treatment preoperatively compared with
postoperatively.1
• Preoperative systemic therapy can render surgically inoperable tumors
operable and offers potential benets for patients with operable breast
cancer. Importantly, preoperative systemic therapy can improve rates
of breast conservation therapy eligibility and provides an opportunity
to observe clinical and pathologic response to systemic therapy in an
individual patient.
• Pathologic complete response (pCR) to preoperative systemic therapy is
associated with an extremely favorable disease-free and overall survival,
particularly in situations in which all treatment is given preoperatively.
The correlation between pathologic response and long-term outcome is
strongest for triple-negative breast cancer (TNBC), somewhat less so for
HER2+ disease, and least for ER+ disease.2,3
• A number of chemotherapy regimens have activity in the preoperative
setting. In general, those chemotherapy regimens recommended in the
adjuvant setting may be considered in the preoperative setting. See
Preoperative/Adjuvant Therapy Regimens (BINV-K).
• Endocrine therapy alone (aromatase inhibitor [preferred for
postmenopausal women; given with ovarian suppression for
premenopausal women] or tamoxifen) may be considered for patients with
hormone-receptor positive disease.
• Patients with HER2-positive tumors should be treated with preoperative
systemic therapy incorporating trastuzumab for at least 9 weeks
of preoperative therapy. A pertuzumab-containing regimen may be
administered preoperatively to patients with greater than or equal to T2 or
greater than or equal to N1, HER2-positive early stage breast cancer. See
Preoperative/Adjuvant Therapy Regimens (BINV-K)
• Some studies have reported an increased risk of locoregional
recurrence in patients receiving preoperative systemic
therapy compared with those receiving postoperative adjuvant
systemic therapy.4 This increased risk of locoregional relapse
has been attributed to suboptimal delivery of denitive local
therapy in patients treated in the preoperative setting.
• Not all patients are appropriate candidates for preoperative
systemic therapy. Accurate clinical staging at baseline prior
to initiation of preoperative systemic therapy is critical. See
Preoperative Systemic Therapy: Breast and Axillary Evaluation
(BINV-11)
• When electing preoperative therapy, all treatment should be
given prior to surgery. Tumor response should be routinely
assessed by clinical exam during delivery of preoperative
therapy. Patients with operable breast cancer experiencing
progression of disease during preoperative systemic therapy
should be taken promptly to surgery. Locoregional therapy
principles should be applied in the same manner as in patients
treated with adjuvant systemic therapy.
1Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 2008 Feb 10;26(5):778-85.
2von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 2012
May 20;30(15):1796-804.
3Cortazar P, Zhang L, Untch M, et al.Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014 Jul 12;384(9938):164-72.
4Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005 Feb 2;97(3):188-94.
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged. BINV-L
2 OF 2
Cautions:
• Possible overtreatment with systemic therapy if clinical stage is
overestimated
• Possible undertreatment locoregionally with radiotherapy if
clinical stage is underestimated
• Possibility of disease progression during preoperative systemic
therapy
Candidates for preoperative systemic therapy
• Patients with inoperable breast cancer:
Inammatory breast cancer
Bulky or matted N2 axillary nodes
N3 nodal disease
T4 tumors
• Patients with operable breast cancer:
Large primary tumor relative to breast size in a patient who
desires breast conservation
Non-candidates for preoperative systemic therapy
• Patients with extensive in situ disease when extent of invasive
carcinoma is not well dened
• Patients with a poorly delineated extent of tumor preoperatively
• Patients whose tumors are not palpable or clinically assessable
PRINCIPLES OF PREOPERATIVE SYSTEMIC THERAPY
Known benets of preoperative systemic therapy:
• Facilitates breast conservation
• Can render inoperable tumors operable
• Provides important prognostic information at an individual patient
level based on response to therapy, particularly in patients with triple-
negative and HER2-positive breast cancer
• Allows time for genetic testing
• Allows time to plan breast reconstruction in patients electing
mastectomy
Opportunities:
• May allow sentinel lymph node biopsy alone if a positive axilla is
cleared with therapy
• May provide an opportunity to modify systemic treatment if no
preoperative therapy response or progression of disease
• Might allow for the addition of adjuvant treatments in patients with poor
response
• May allow for smaller radiotherapy ports or less radiotherapy if axillary
nodal disease cleared
• Excellent research platform to test novel therapies and predictive
biomarkers
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
BINV-M
DEFINITION OF MENOPAUSE
Clinical trials in breast cancer have utilized a variety of denitions of menopause. Menopause is generally the permanent cessation of menses,
and as the term is utilized in breast cancer management includes a profound and permanent decrease in ovarian estrogen synthesis. Reasonable
criteria for determining menopause include any of the following:
• Prior bilateral oophorectomy
• Age ≥60 y
• Age <60 y and amenorrheic for 12 or more months in the absence of chemotherapy, tamoxifen, toremifene, or ovarian suppression and
follicle-stimulating hormone (FSH) and estradiol in the postmenopausal range
• If taking tamoxifen or toremifene, and age <60 y, then FSH and plasma estradiol level in postmenopausal ranges
It is not possible to assign menopausal status to women who are receiving an LHRH agonist or antagonist. In women premenopausal at the
beginning of adjuvant chemotherapy, amenorrhea is not a reliable indicator of menopausal status as ovarian function may still be intact or
resume despite anovulation/amenorrhea after chemotherapy. For these women with therapy-induced amenorrhea, oophorectomy or serial
measurement of FSH and/or estradiol are needed to ensure postmenopausal status if the use of aromatase inhibitors is considered as a
component of endocrine therapy.
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
BINV-N
1A combination of exemestane with everolimus can be considered for patients who meet the eligibility criteria for BOLERO-2 (progressed within 12 mo or on non-
steroidal AI, or any time on tamoxifen).
2Palbociclib in combination with letrozole may be considered as a treatment option for first-line therapy for postmenopausal patients with hormone-receptor positive,
HER2-negative metastatic breast cancer.
3For postmenopausal women or for premenopausal women receiving ovarian suppression with an LHRH agonist, with hormone-receptor positive and HER2-negative
metastatic breast cancer that has progressed on endocrine therapy.
4A single study (S0226) in women with hormone receptor-positive breast cancer and no prior chemotherapy, biological therapy, or endocrine therapy for metastatic
disease demonstrated that the addition of fulvestrant to anastrozole resulted in prolongation of time to progression. Subset analysis suggested that patients without
prior adjuvant tamoxifen and more than 10 years since diagnosis experienced the greatest benefit. Two studies with similar design (FACT and SOFEA) demonstrated
no advantage in time to progression with the addition of fulvestrant to anastrozole.
ENDOCRINE THERAPY FOR RECURRENT OR STAGE IV DISEASE
Premenopausal Patients
• Selective ER modulators or ovarian ablation/suppression plus endocrine therapy as for postmenopausal women.
Postmenopausal Patients
• Non-steroidal aromatase inhibitor (anastrozole, letrozole)
• Steroidal aromatase inactivator (exemestane)
• Exemestane + everolimus1
• Palbociclib + letrozole2
• Palbociclib + fulvestrant (category 1)3
• Fulvestrant4
• Tamoxifen or toremifene
• Megestrol acetate
• Fluoxymesterone
• Ethinyl estradiol
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged. BINV-O
1 OF 7
3Trastuzumab given in combination with an anthracycline is associated with significant cardiac
toxicity. Concurrent use of trastuzumab and pertuzumab with an anthracycline should be avoided.
4Trastuzumab may be safely combined with all non-anthracycline containing preferred and other
single agents listed above for recurrent or metastatic breast cancer.
5Patients previously treated with chemotherapy plus trastuzumab in the absence of pertuzumab
in the metastatic setting may be considered for one line of therapy including both trastuzumab
plus pertuzumab in combination with or without cytotoxic therapy (such as vinorelbine or taxane).
Further research is needed to determine the ideal sequencing strategy for anti-HER2 therapy.
CHEMOTHERAPY REGIMENS FOR RECURRENT OR METASTATIC BREAST CANCER1
Preferred single agents:
Anthracyclines
• Doxorubicin
• Pegylated liposomal doxorubicin
Taxanes
• Paclitaxel
Anti-metabolites
• Capecitabine
• Gemcitabine
Other microtubule inhibitors
• Vinorelbine
• Eribulin
Other single agents:
• Cyclophosphamide
• Carboplatin
• Docetaxel
• Albumin-bound paclitaxel
• Cisplatin
• Epirubicin
• Ixabepilone
Chemotherapy combinations:
• CAF/FAC (cyclophosphamide/doxorubicin/uorouracil)
• FEC (uorouracil/epirubicin/cyclophosphamide)
• AC (doxorubicin/cyclophosphamide)
• EC (epirubicin/cyclophosphamide)
• CMF (cyclophosphamide/methotrexate/uorouracil)
• Docetaxel/capecitabine
• GT (gemcitabine/paclitaxel)
• Gemcitabine/carboplatin
• Paclitaxel/bevacizumab2
Preferred rst-line agents for HER2-positive disease:
• Pertuzumab + trastuzumab + docetaxel (category 1)5
• Pertuzumab + trastuzumab + paclitaxel5
Other agents for HER2-positive disease:
• Ado-trastuzumab emtansine (T-DM1)
• Trastuzumab + paclitaxel ± carboplatin
• Trastuzumab + docetaxel
• Trastuzumab + vinorelbine
• Trastuzumab + capecitabine
Agents for trastuzumab-exposed HER2-positive disease:
• Lapatinib + capecitabine
• Trastuzumab + capecitabine
• Trastuzumab + lapatinib (without cytotoxic therapy)
• Trastuzumab + other agents3,4,5
1There is no compelling evidence that combination regimens are
superior to sequential single agents.
2Randomized clinical trials in metastatic breast cancer document
that the addition of bevacizumab to some first- or second-line
chemotherapy agents modestly improves time to progression and
response rates but does not improve overall survival. The time-to-
progression impact may vary among cytotoxic agents and appears
greatest with bevacizumab in combination with weekly paclitaxel.
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged. BINV-O
2 OF 7
DOSING SCHEDULES FOR CHEMOTHERAPY REGIMENS FOR RECURRENT OR METASTATIC BREAST CANCER
Preferred single agents:
Anthracyclines:
Doxorubicin
• 60–75 mg/m2 IV day 1, cycled every 21 days1
or
• 20 mg/m2 IV day 1 weekly2
Pegylated liposomal encapsulated doxorubicin3
• 50 mg/m2 IV day 1
Cycled every 28 days.
Taxanes:
Paclitaxel
• 175 mg/m2 IV day 1
Cycled every 21 days.4
or
• 80 mg/m2 IV day 1 weekly5
Antimetabolites:
Capecitabine6
• 1000–1250 mg/m2 PO twice daily days 1–14
Cycled every 21 days.
Gemcitabine7
• 800–1200 mg/m2 IV days 1, 8, and 15
Cycled every 28 days.
Other microtubule inhibitors:
Vinorelbine8
• 25 mg/m2 IV day 1 weekly
Eribulin9
• 1.4 mg/m2 IV days 1 and 8
Cycled every 21 days.
Other single agents:
Cyclophosphamide10
• 50 mg PO daily on days 1–21
Cycled every 28 days.
Carboplatin11
• AUC 6 IV on day 1
Cycled every 21–28 days.
Docetaxel12,13
• 60–100 mg/m2 IV day 1
Cycled every 21 days.
or
• 35 mg/m2 IV weekly for 6 wks followed by a 2-week rest, then repeat14
Albumin-bound paclitaxel
• 100 mg/m2 or 125 mg/m2 IV days 1, 8, and 15
Cycled every 28 days.15,16
or
• 260 mg/m2 IV
Cycled every 21 days.15
Cisplatin17
• 75 mg/m2 IV on day 1
Cycled every 21 days.
Epirubicin18
• 60–90 mg/m2 IV day 1
Cycled every 21 days.
Ixabepilone19
• 40 mg/m2 IV day 1
Cycled every 21 days.
The selection, dosing, and administration of anti-cancer agents and the management of associated toxicities are complex. Modifications of drug dose and schedule and initiation of supportive care interventions are often
necessary because of expected toxicities and individual patient variability, prior treatment, and comorbidity. The optimal delivery of anti-cancer agents therefore requires a health care delivery team experienced in the use
of anti-cancer agents and the management of associated toxicities in patients with cancer.
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged. BINV-O
3 OF 7
DOSING SCHEDULES FOR CHEMOTHERAPY REGIMENS FOR RECURRENT OR METASTATIC BREAST CANCER
Chemotherapy combinations:
CAF chemotherapy20
• Cyclophosphamide 100 mg/m2 PO days 1–14
• Doxorubicin 30 mg/m2 IV days 1 & 8
• 5-uorouracil 500 mg/m2 IV days 1 & 8
Cycled every 28 days.
FAC chemotherapy21
• 5-uorouracil 500 mg/m2 IV days 1 & 8 or days 1 & 4
• Doxorubicin 50 mg/m2 IV day 1
(or by 72-h continuous infusion)
• Cyclophosphamide 500 mg/m2 IV day 1
Cycled every 21 days.
FEC chemotherapy22
• Cyclophosphamide 400 mg/m2 IV days 1 & 8
• Epirubicin 50 mg/m2 IV days 1 & 8
• 5-uorouracil 500 mg/m2 IV days 1 & 8
Cycled every 28 days.
AC chemotherapy23
• Doxorubicin 60 mg/m2 IV day 1
• Cyclophosphamide 600 mg/m2 IV day 1
Cycled every 21 days.
EC chemotherapy24
• Epirubicin 75 mg/m2 IV day 1
• Cyclophosphamide 600 mg/m2 IV day 1
Cycled every 21 days.
CMF chemotherapy25
• Cyclophosphamide 100 mg/m2 PO days 1–14
• Methotrexate 40 mg/m2 IV days 1 & 8
• 5-uorouracil 600 mg/m2 IV days 1 & 8
Cycled every 28 days.
Docetaxel/capecitabine chemotherapy26
• Docetaxel 75 mg/m2 IV day 1
• Capecitabine 950 mg/m2 PO twice daily days 1–14
Cycled every 21 days.
GT chemotherapy27
• Paclitaxel 175 mg/m2 IV day 1
• Gemcitabine 1250 mg/m2 IV days 1 & 8 (following paclitaxel on day 1)
Cycled every 21 days.
Gemcitabine/carboplatin28
• Gemcitabine 1000 mg/m2 on days 1 & 8
• Carboplatin AUC 2 IV on days 1 & 8
Cycled every 21 days.
Paclitaxel plus bevacizumab29
• Paclitaxel 90 mg/m2 by 1 h IV days 1, 8, & 15
• Bevacizumab 10 mg/kg IV days 1 & 15
Cycled every 28 days.
The selection, dosing, and administration of anti-cancer agents and the management of associated toxicities are complex. Modifications of drug dose and schedule and initiation of supportive care interventions are often
necessary because of expected toxicities and individual patient variability, prior treatment, and comorbidity. The optimal delivery of anti-cancer agents therefore requires a health care delivery team experienced in the use
of anti-cancer agents and the management of associated toxicities in patients with cancer.
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged. BINV-O
4 OF 7
DOSING SCHEDULES FOR CHEMOTHERAPY REGIMENS FOR HER2-POSITIVE RECURRENT OR METASTATIC BREAST CANCER
Preferred rst-line agents for HER2-positive disease:
Pertuzumab + trastuzumab + docetaxel30
• Pertuzumab 840 mg IV day 1 followed by 420 mg IV
• Trastuzumab 8 mg/kg IV day 1 followed by 6 mg/kg IV
• Docetaxel 75–100 mg/m2 IV day 1
Cycled every 21 days.
Pertuzumab + trastuzumab + paclitaxel31
• Pertuzumab 840 mg IV day 1 followed by 420 mg IV cycled every 21 days
• Trastuzumab
4 mg/kg IV day 1 followed by 2 mg/kg IV weekly
or
8 mg/kg IV day 1 followed by 6 mg/kg IV cycled every 21 days33
• Paclitaxel 80 mg/m2 IV day 1 weekly31
or
• Paclitaxel 175 mg/m2 day 1 cycled every 21 days
Other agents for HER2-positive disease:
Ado-trastuzumab emtansine (T-DM1)43
• 3.6 mg/kg IV day 1
Cycled every 21 days.
Paclitaxel/carboplatin + trastuzumab32
• Carboplatin AUC 6 IV day 1
• Paclitaxel 175 mg/m2 IV day 1
Cycled every 21 days.
• Trastuzumab
4 mg/kg IV day 1 followed by 2 mg/kg IV weekly
or
8 mg/kg IV day 1 followed by 6 mg/kg IV every 21 days33
Weekly paclitaxel/carboplatin + trastuzumab34
• Paclitaxel 80 mg/m2 IV days 1, 8, & 15
• Carboplatin AUC 2 IV days 1, 8, & 15
Cycled every 28 days.
• Trastuzumab
4 mg/kg IV day 1 followed by 2 mg/kg IV weekly
or
8 mg/kg IV day 1 followed by 6 mg/kg IV every 21 days33
Trastuzumab + paclitaxel
• Paclitaxel
175 mg/m2 IV day 1 cycled every 21 days35
or
80–90 mg/m2 IV day 1 weekly36
• Trastuzumab
4 mg/kg IV day 1 followed by 2 mg/kg IV weekly
or
8 mg/kg IV day 1 followed by 6 mg/kg IV every 21 days33
Trastuzumab + docetaxel
• Docetaxel
80–100 mg/m2 IV day 1 cycled every 21 days37
or
35 mg/m2 IV days 1, 8, and 15 weekly38
• Trastuzumab
4 mg/kg IV day 1 followed by 2 mg/kg IV weekly
or
8 mg/kg IV day 1 followed by 6 mg/kg IV every 21 days33
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged. BINV-O
5 OF 7
DOSING SCHEDULES FOR CHEMOTHERAPY REGIMENS FOR HER-2 POSITIVE RECURRENT OR METASTATIC BREAST CANCER
Trastuzumab + vinorelbine39
• Vinorelbine
25 mg/m2 IV day 1 weekly
or
30–35 mg/m2 IV days 1 and 8
Cycled every 21 days.
• Trastuzumab
4 mg/kg IV day 1 followed by 2 mg/kg IV weekly
or
8 mg/kg IV day 1 followed by 6 mg/kg IV every 21 days33
Trastuzumab + capecitabine41
• Capecitabine 1000–1250 mg/m2 PO twice daily days 1–14
cycled every 21 days
• Trastuzumab
4 mg/kg IV day 1 followed by 2 mg/kg IV weekly35,42
or
8 mg/kg IV day 1 followed by 6 mg/kg IV every 21 days33
Agents for trastuzumab-exposed HER2-positive disease:
Lapatinib + capecitabine44
• Lapatinib 1250 mg PO daily days 1–21
• Capecitabine 1000 mg/m2 PO twice daily days 1–14
Cycled every 21 days.
Trastuzumab + capecitabine45
• Capecitabine 1000–1250 mg/m2 PO twice daily days 1–14
Cycled every 21 days.
• Trastuzumab
4 mg/kg IV day 1 followed by 2 mg/kg IV weekly35,42
or
8 mg/kg IV day 1 followed by 6 mg/kg IV every 21 days33
Trastuzumab + lapatinib46
• Lapatinib 1000 mg PO daily
• Trastuzumab
4 mg/kg IV day 1 followed by 2 mg/kg IV weekly
or
8 mg/kg IV day 1 followed by 6 mg/kg IV every 21 days33
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged. BINV-O
6 OF 7
1Chan S, Friedrichs K, Noel D, et al. Prospective randomized trial of docetaxel versus doxorubicin in patients with metastatic breast cancer. J Clin Oncol 1999;17:2341-
2354.
2Gasparini G, Dal Fior S, Panizzoni GA, et al. Weekly epirubicin versus doxorubicin as second line therapy in advanced breast cancer. A randomized clinical trial. Am J
Clin Oncol 1991;14:38-44.
3O'Brien ME, Wigler N, Inbar M, et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus
conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol 2004;15:440-449.
4Seidman AD, Tiersten A, Hudis C, et al. Phase II trial of paclitaxel by 3-hour infusion as initial and salvage chemotherapy for metastatic breast cancer. J Clin Oncol
1995;13:2575-2581.
5Perez EA, Vogel CL, Irwin DH, et al. Multicenter phase II trial of weekly paclitaxel in women with metastatic breast cancer. J Clin Oncol 2001;19:4216-4223.
6Bajetta E, Procopio G, Celio L, et al. Safety and efficacy of two different doses of capecitabine in the treatment of advanced breast cancer in older women. J Clin Oncol
2005;23:2155-2161.
7Seidman AD. Gemcitabine as single-agent therapy in the management of advanced breast cancer. Oncology (Williston Park) 2001;15:11-14.
8Zelek L, Barthier S, Riofrio M, et al. Weekly vinorelbine is an effective palliative regimen after failure with anthracyclines and taxanes in metastatic breast carcinoma.
Cancer 2001;92:2267-2272.
9Cortes J, O'Shaughnessy J, Loesch D, et al. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): a
phase 3 open-label randomised study. Lancet 2011;377:914-923.
10Licchetta A, Correale P, Migali C, et al. Oral metronomic chemo-hormonal-therapy of metastatic breast cancer with cyclophosphamide and megestrol acetate. J
Chemother 2010;22(3):201-4.
11Isakoff SJ, Mayer EL, He L, et al. TBCRC009: A multicenter phase II clinical trial of platinum monotherapy with biomarker assessment in metastatic triple-negative
breast cancer. J Clin Oncol 2016 June 10;33(17):1902-9 doi:10.1200/JCO.2014.57.6660.Epub 2016 Apr 6.
12Burris HA, 3rd. Single-agent docetaxel (Taxotere) in randomized phase III trials. Semin Oncol 1999;26:1-6.
13Harvey V, Mouridsen H, Semiglazov V, et al: Phase III trial comparing three doses of docetaxel for second-line treatment of advanced breast cancer. J Clin Oncol
2006;24(31):4963-70.
14Rivera E, Mejia JA, Arun BJ, et al. Phase 3 study comparing the use of docetaxel on an every-3-week versus weekly schedule in the treatment of metastatic breast
cancer. Cancer 2008 Apr 1;112(7):1455-61.
15Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in
women with breast cancer. J Clin Oncol 2005;23:7794-7803.
16Gradishar W, Dimitry K, Sergey C, et al: Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic
breast cancer. J Clin Oncol 2009;27(22):3611-9.
17Silver DP, Richardson AL, Eklund AC, et al. Efficacy of neoadjuvant cisplatin in triple-negative breast cancer. J Clin Oncol 2010;28(7):1145-53.
18Bastholt L, Dalmark M, Gjedde SB, et al. Dose-response relationship of epirubicin in the treatment of postmenopausal patients with metastatic breast cancer: a
randomized study of epirubicin at four different dose levels performed by the Danish Breast Cancer Cooperative Group. J Clin Oncol 1996;14:1146-1155.
19Perez E, Lerzo G, Pivot X, et al. Efficacy and safety of ixabepilone (BMS-247550) in a phase II study of patients with advanced breast cancer resistant to an
anthracycline, a taxane, and capecitabine. J Clin Oncol 2007;25(23):3407-14.
20Bull JM, Tormey DC, Li SH, et al. A randomized comparative trial of adriamycin versus methotrexate in combination drug therapy. Cancer 1978;41:1649-1657.
21Hortobagyi GN, Gutterman JU, Blumenschein GR, et al: Combination chemoimmunotherapy of metastatic breast cancer with 5-fluorouracil, adriamycin,
cyclophosphamide, and BCG. Cancer 1979;43:1225-33.
22Ackland SP, Anton A, Breitbach GP, et al. Dose-intensive epirubicin-based chemotherapy is superior to an intensive intravenous cyclophosphamide, methotrexate, and
fluorouracil regimen in metastatic breast cancer: a randomized multinational study. J Clin Oncol 2001;19:943-953.
23Nabholtz JM, Falkson C, Campos D, et al: Docetaxel and doxorubicin compared with doxorubicin and cyclophosphamide as first-line chemotherapy for metastatic
breast cancer: results of a randomized, multicenter, phase III trial. J Clin Oncol 2003; 21(6): 968-75.
REFERENCES
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged. BINV-O
7 OF 7
24Langley RE, Carmichel J, Jones AL, et al. Phase III trial of epirubicin plus paclitaxel compared with epirubicin plus cyclphosphamide as first-line chemotherapy for
metastatic breast cancer: United Kingdom Cancer Research Institute. J Clin Oncol 2005;23:8322-8330.
25Bonadonna G, Brusamolino E, Valagussa P, et al. Combination chemotherapy as an adjuvant treatment in operable breast cancer. N Engl J Med 1976;294:405-410.
26Mavroudis D, Papakotoulas P, Ardavanis A, et al. Randomized phase III trial comparing docetaxel plus epirubicin versus docetaxel plus capecitabine as first-line
treatment in women with advanced breast cancer. Ann Oncol 21:48(2010).
27Albain KS, Nag S, Calderillo-Ruiz G, et al. Gemcitabine plus paclitaxel versus paclitaxel monotherapy in patients with metastatic breast cancer and prior anthracycline
treatment. J Clin Oncol 2008;26(24):3950-7.
28O’Shaughnessy J, Schwartzberg LS, Danso MA, et al. A randomized phase III study of iniparib (BSI-201) in combination with gemcitabine/carboplatin (G/C) in
metastatic triple-negative breast cancer (TNBC). [abstract]. J Clin Oncol 2011;29 (Suppl_15):Abstract 1007.
29Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 2007;357:2666-2676.
30Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 2012;366:109-119.
31Datko F, D'Andrea G, Dickler M, et al. Phase II study of pertuzumab, trastuzumab, and weekly paclitaxel in patients with metastatic HER2-overexpressing metastatic
breast cancer [abstract]. Cancer Research 2012;72:Abstract P5-18-20.
32Robert N, Leyland-Jones B, Asmar L, et al. Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in
women with HER-2-overexpressing metastatic breast cancer. J Clin Oncol 2006;24:2786-2792.
33Leyland-Jones B, Gelmon K, Ayoub JP, et al. Pharmacokinetics, safety, and efficacy of trastuzumab administered every three weeks in combination with paclitaxel.
J Clin Oncol 2003;21:3965-3971.
34Perez EA, Suman VJ, Rowland KM, et al. Two concurrent phase II trials of paclitaxel/carboplatin/trastuzumab (weekly or every-3-week schedule) as first-line therapy
in women with HER2-overexpressing metastatic breast cancer: NCCTG study 983252. Clin Breast Cancer 2005;6:425-432.
35Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2.
N Engl J Med 2001;344:783-792.
36Seidman A, Berry DA, Cirrincione C, et al. Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab
for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol
9840. J Clin Oncol 2008;26:1642-1649.
37Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human
epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol 2005;23:4265-4274.
38Esteva FJ, Valero V, Booser D, et al. Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. J Clin
Oncol 2002;20:1800-1808.
39Burstein HJ, Keshaviah A, Baron AD, et al. Trastuzumab plus vinorelbine or taxane chemotherapy for HER2-overexpressing metastatic breast cancer: the trastuzumab
and vinorelbine or taxane study. Cancer 2007;110:965-972.
40Andersson M, Lidbrink E, Bjerre K, et al. Phase III randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first-line therapy of
metastatic or locally advanced human epidermal growth factor receptor 2-positive breast cancer: the HERNATA study. J Clin Oncol 2011;29:264-271.
41von Minckwitz G, du Bois A, Schmidt M, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a
german breast group 26/breast international group 03-05 study. J Clin Oncol 2009;27:1999-2006.
42Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-
overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 1999;17:2639-2648.
43Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer [supplementary appendix available online]. N Engl J Med
2012;367:1783-1791.
44Geyer C, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 2006;355:2733-2743.
45Bartsch R, Wenzel C, Altorjai G, et al. Capecitabine and trastuzumab in heavily pretreated metastatic breast cancer. J Clin Oncol 2007;25:3853-3858.
46Blackwell KL, Burstein H, et al. Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory
metastatic breast cancer. J Clin Oncol 2010;28(7):1124-30.
REFERENCES
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged. BINV-P
1 OF 3
1Rising tumor markers (eg, CEA, CA15-3, CA27.29) are concerning for tumor progression, but may also be seen in the setting of responding disease. An isolated
increase in tumor markers should rarely be used to declare progression of disease. Changes in bone lesions are often difficult to assess on plain or cross-sectional
radiology or on bone scan. For these reasons, patient symptoms and serum tumor markers may be more helpful in patients with bone-dominant metastatic disease.
PRINCIPLES OF MONITORING METASTATIC DISEASE
Monitoring of patient symptoms and cancer burden during treatment of metastatic breast cancer is important to determine whether the treatment
is providing benet and that the patient does not have toxicity from an ineffective therapy.
Components of Monitoring:
Monitoring includes periodic assessment of varied combinations of symptoms, physical examination, routine laboratory tests, imaging studies,
and blood biomarkers where appropriate. Results of monitoring are classied as response/continued response to treatment, stable disease,
uncertainty regarding disease status, or progression of disease. The clinician typically must assess and balance multiple different forms of
information to make a determination regarding whether disease is being controlled and the toxicity of treatment is acceptable. Sometimes, this
information may be contradictory.
Denition of Disease Progression:
Unequivocal evidence of progression of disease by one or more of these factors is required to establish progression of disease, either because
of ineffective therapy or acquired resistance of disease to an applied therapy. Progression of disease may be identied through evidence of
growth or worsening of disease at previously known sites of disease and/or of the occurrence of new sites of metastatic disease.
• Findings concerning for progression of disease include:
Worsening symptoms such as pain or dyspnea
Evidence of worsening or new disease on physical examination
Declining performance status
Unexplained weight loss
Increasing alkaline phosphatase, ALT, AST, or bilirubin
Hypercalcemia
New radiographic abnormality or increase in the size of pre-existing radiographic abnormality
New areas of abnormality on functional imaging (eg, bone scan, PET/CT scan)
Increasing tumor markers (eg, CEA, CA15-3, CA27.29)1
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged. BINV-P
2 OF 3
Continued on next page
PRINCIPLES OF MONITORING METASTATIC DISEASE
Use of Objective Criteria for Response/Stability/Progression:
• The most accurate assessments of disease activity typically occur when previously abnormal studies are repeated on a serial and regular
basis. Generally, the same method of assessment should be used over time (eg, an abnormality found on chest CT scan should generally be
monitored with repeat chest CT scans).
• Some non-clinically important variation in measurement of abnormalities by all serial studies is common and expected. Therefore, the use
of objective and widely accepted criteria for response, stability, and progression of disease are encouraged. Such systems include the
Response Evaluation Criteria In Solid Tumors (RECIST) guidelines (Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation
criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-247) and the WHO criteria (Miller AB, Hoogstraten
B, Staquet M, and Winkler A. Reporting results of cancer treatment. Cancer 1981;47:207-214).
• Studies of functional imaging, such as radionuclide bone scans and PET imaging, are particularly challenging when used to assess
response. In the case of bone scans, responding disease may result in a are or increased activity on the scan that may be misinterpreted
as disease progression, especially on the rst follow-up bone scan after initiating a new therapy. PET imaging is challenging because of the
absence of a reproducible, validated, and widely accepted set of standards for disease activity assessment.
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged. BINV-P
3 OF 3
1In patients who have long-term stable disease, the frequency of monitoring can be reduced.
PRINCIPLES OF MONITORING METASTATIC DISEASE
Suggested intervals of follow-up for patients with metastatic disease1
Baseline prior to new
therapy
Chemotherapy Endocrine therapy Restaging if concern for
progression of disease
Symptom assessment Yes Prior to each cycle Every 1–3 months Yes
Physical examination Yes Prior to each cycle Every 1–3 months Yes
Performance status Yes Prior to each cycle Every 1–3 months Yes
Weight Yes Prior to each cycle Every 1–3 months Yes
LFTs, CBC Yes Prior to each cycle Every 1–3 months Yes
CT scan
chest/abd/pelvis
Yes Every 2–4 cycles Every 2–6 months Yes
Bone scan Yes Every 4 cycles Every 4–6 months Yes
PET/CT Optional Unknown Unknown Optional
Tumor markers Optional Optional Optional Optional
Frequency of Monitoring:
The optimal frequency of repeat testing is uncertain, and is primarily based on the monitoring strategies utilized in breast cancer clinical
trials. The frequency of monitoring must balance the need to detect progressive disease, avoid unnecessary toxicity of any ineffective
therapy, resource utilization, and determine cost. The following table is to provide guidance, and should be modied for the individual patient
based on sites of disease, biology of disease, and length of time on treatment. Reassessment of disease activity should be performed in
patients with new or worsening signs or symptoms of disease, regardless of the time interval from previous studies.
NCCN Guidelines Version 2.2016
Invasive Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
PHYLL-1
NCCN Guidelines Version 2.2016
Phyllodes Tumor
CLINICAL PRESENTATION WORKUP FINDINGS TREATMENT
aFNA or core biopsy may not distinguish a fibroadenoma from a phyllodes tumor in some cases. The sensitivity of core biopsy for the diagnosis of phyllodes tumor is
greater than that of FNA biopsy, but neither core biopsy nor FNA biopsy can always differentiate phyllodes tumors from fibroadenomas. In cases with clinical suspicion
for phyllodes tumor, excision of the lesion may be needed for definitive pathologic classification.
bExcisional biopsy includes complete mass removal, but without the intent of obtaining surgical margins.
cWide excision means excision with the intention of obtaining surgical margins ≥1 cm. Narrow surgical margins are associated with heightened local recurrence risk, but
are not an absolute indication for mastectomy when partial mastectomy fails to achieve margin width ≥1 cm.
dThere are no prospective randomized data supporting the use of radiation treatment with phyllodes tumors. However, in the setting where additional recurrence would
create significant morbidity (eg, chest wall recurrence following mastectomy), radiation therapy may be considered following the same principles that are applied to the
treatment of soft tissue sarcoma.
Clinical suspicion of phyllodes
tumor:
• Palpable mass
• Rapid growth
• Large size (>3 cm)
• Imaging with ultrasound
suggestive of broadenoma except
for size and/or history of growth
• History and
physical exam
• Ultrasound
• Mammogram for
women ≥30 y
Excisional
biopsyb
Core needle
biopsya
Phyllodes tumor
includes benign,
borderline, and
malignant
Fibroadenoma
Invasive or
in situ cancer
Fibroadenoma
or indeterminate
Phyllodes tumor
includes benign,
borderline, and
malignant
Invasive or
in situ cancer
Observe
Wide excisionc
without axillary stagingd
See appropriate
guidelines
Excisional
biopsyb
See ndings
above
Wide excisionc
without axillary stagingd
See appropriate
guidelines
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
PHYLL-2
NCCN Guidelines Version 2.2016
Phyllodes Tumor
aFNA or core biopsy may not distinguish a fibroadenoma from a phyllodes tumor in some cases. The sensitivity of core biopsy for the diagnosis of phyllodes tumor is
greater than that of FNA biopsy, but neither core biopsy nor FNA biopsy can always differentiate phyllodes tumors from fibroadenomas. In cases with clinical suspicion
for phyllodes tumor, excision of the lesion may be needed for definitive pathologic classification.
eThere are no prospective randomized data supporting the use of radiation treatment with phyllodes tumors. However, in the setting where additional recurrence would
create significant morbidity (eg, chest wall recurrence following mastectomy), radiation therapy may be considered following the same principles that are applied to the
treatment of soft tissue sarcoma.
PHYLLODES TUMOR RECURRENCE
CLINICAL PRESENTATION WORKUP FINDINGS TREATMENT
Locally recurrent breast
mass following excision
of phyllodes tumor
• History and physical
exam
• Ultrasound
• Mammogram
• Tissue samplinga
(histology preferred)
• Consider chest imaging
No metastatic
disease
Metastatic
disease
Re-excision with wide
margins without axillary
staging
Metastatic disease management following
principles of soft tissue sarcoma
(See NCCN Guidelines for Soft Tissue Sarcoma)
Consider post-
operative radiation
(category 2B)e
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
PAGET-1
NCCN Guidelines Version 2.2016
Paget’s Disease
aNipple or areolar eczema, ulceration, bleeding, or itching.
CLINICAL
PRESENTATION
WORKUP
Clinical suspicion
of Paget’s diseasea
• Clinical breast exam
• Diagnostic bilateral mammogram,
ultrasound as necessary
Examination or imaging
positive for breast lesion
Examination and imaging
negative for breast lesion
See PAGET-2
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
PAGET-2
NCCN Guidelines Version 2.2016
Paget’s Disease
bTo assess the extent of disease or to confirm additional disease, consider MRI. See Principles of Dedicated Breast MRI Testing (BINV-B).
cMastectomy is always an option with any manifestation of Paget’s disease (See Discussion section).
WORKUP TREATMENT
Examination
or imaging
positive for
breast lesion
Examination
and imaging
negative for
breast lesion
Core biopsy of
breast lesion and
full-thickness skin
biopsy of involved
NAC
Full-thickness
skin biopsy of
involved NAC
Breast and NAC
biopsy negative
Clinical follow-up
Re-biopsy if not healing
Breast DCISb
and NAC Paget’s
Breast invasive cancer
and NAC Paget’sb
Breast negative for
cancer and positive
NAC Paget’sb
NAC biopsy positive
for Paget’sb
NAC biopsy
negative for Paget’s
See NCCN Guidelines for Noninvasive Breast
Cancer for DCIS (DCIS-1)
See NCCN Guidelines for Invasive Breast
Cancer (BINV-1)
Clinical follow-up
Re-biopsy if not healing
Appropriate
systemic
adjuvant therapy
as clinically
indicated
See NCCN
Guidelines for
DCIS or Invasive
Breast Cancer
Consider
MRI and
tissue
sampling
Central lumpectomy including NAC
with whole breast radiation therapy
or
Total mastectomyc ± sentinel node
biopsy with or without breast
reconstruction
or
Central lumpectomy including NAC
± sentinel node biopsy without
radiation therapy (category 2B)
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
NCCN Guidelines Version 2.2016
Breast Cancer During Pregnancy
PREG-1
aConsiderations and selection of optimal local therapy and systemic therapy are similar to that recommended in non-pregnancy-associated breast cancer; see other
sections of this guideline. However, the selection and timing of chemotherapy, endocrine therapy, and radiation therapy is different in the pregnant versus non-pregnant
patient (See Discussion section). Chemotherapy should not be administered during the first trimester of pregnancy, and radiation therapy should not be administered
during any trimester of pregnancy. Most experience with chemotherapy during pregnancy for breast cancer is from regimens that utilize various combinations of
doxorubicin, cyclophosphamide, and fluorouracil. Considerations for postpartum chemotherapy are the same as for non-pregnancy-associated breast cancer.
bUse of blue dye is contraindicated in pregnancy; radiolabeled sulfur colloid appears to be safe for sentinel node biopsy in pregnancy.
See Surgical Axillary Staging (BINV-D).
cThere are insufficient safety data to recommend general use of taxanes during pregnancy. However, the use of paclitaxel weekly administration after the first trimester
is acceptable if clinically indicated by disease status. The use of anti-HER2 therapy is contraindicated during pregancy.
CLINICAL PRESENTATION PRIMARY TREATMENTa,b ADJUVANT TREATMENTa,c
Pregnant patient with
conrmed breast
cancer diagnosis by
FNA or core biopsy;
No distant metastases
on staging
1st
trimester
2nd trimester/
Early 3rd trimester
Late 3rd
trimester
Discuss
termination:
Non-
therapeutic
Continuing
pregnancy
Preoperative chemotherapy,a,c
mastectomy, or breast-
conserving surgery + axillary
staginga,b,c postpartum
Begin adjuvant chemotherapy
in 2nd trimestera,c
± Adjuvant radiation therapy
postpartuma
± Adjuvant endocrine therapy
postpartuma
Mastectomy +
axillary staginga,b
Mastectomya or breast-
conserving surgery +
axillary staginga,b
Adjuvant chemotherapya,c
± Adjuvant radiation therapy
postpartuma
± Adjuvant endocrine therapy
postpartuma
± Adjuvant radiation therapy
postpartuma
± Adjuvant endocrine therapy
postpartuma
Adjuvant chemotherapya,c
± Adjuvant radiation therapy
postpartuma
± Adjuvant endocrine therapy
postpartuma
or
Mastectomya or breast-
conserving surgery + axillary
staginga,b,c
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
IBC-1
NCCN Guidelines Version 2.2016
Inammatory Breast Cancer
CLINICAL PRESENTATIONaWORKUP
Clinical
pathologic
diagnosis of
inammatory
breast cancer
(IBC)
Stage T4d, N0-
N3, M0
• History and physical exam by multidisciplinary team
• CBC
• Liver function tests
• Pathology reviewb
• Determination of tumor ER/PR status and
HER2 statusc
• Bilateral diagnostic mammogram, ultrasound as
necessary
• Breast MRI (optional)
• Fertility counseling if premenopausald
• Bone scan or sodium uoride PET/CT (category 2B)e
• Chest/abdominal/pelvic diagnostic CT (category 2B)
• Chest diagnostic CT (if pulmonary symptoms are
present)
• Genetic counseling if patient is high risk for
hereditary breast cancerf
• FDG PET/CT scang,h (category 2B)
Preoperative systemic
therapy,i anthracycline +
taxane (preferred).i If tumor
HER2 positive, HER2-
targeted therapyj
Responsek
No
responsek
aInflammatory breast cancer is a clinical syndrome in women with invasive breast
cancer that is characterized by erythema and edema (peau d'orange) of a third
or more of the skin of the breast. The differential diagnosis includes cellulitis of
the breast or mastitis. Pathologically, a tumor is typically present in the dermal
lymphatics of the involved skin, but dermal lymphatic involvement is neither
required, nor sufficient by itself for a diagnosis of inflammatory breast cancer.
bThe panel endorses the College of American Pathologists Protocol for pathology
reporting for all invasive and noninvasive carcinomas of the breast.
http://www.cap.org.
cSee Principles of HER2 Testing (BINV-A).
dSee Fertility and Birth Control (BINV-C).
eIf FDG PET/CT is performed and clearly indicates bone metastasis on both the PET
and CT component, bone scan or sodium fluoride PET/CT may not be needed.
fSee NCCN Guidelines for Genetic/Familial High-Risk Assessment: Breast and
Ovarian.
gFDG PET/CT can be performed at the same time as diagnostic CT. FDG PET/CT
is most helpful in situations where standard staging studies are equivocal or
suspicious, especially in the setting of locally advanced or metastatic disease.
hFDG PET/CT may also be helpful in identifying unsuspected regional nodal
disease and/or distant metastases in locally advanced breast cancer when
used in addition to standard staging studies.
iSee Preoperative Systemic Therapy/Adjuvant Chemotherapy (BINV-K).
jA pertuzumab-containing regimen may be administered preoperatively to
patients with HER2-positive IBC.
kThe accurate assessment of in-breast tumor or regional lymph node response
to preoperative systemic therapy is difficult, and should include physical
examination and performance of imaging studies (mammogram and/or breast
MRI) that were abnormal at the time of initial tumor staging. Selection of
imaging methods prior to surgery should be determined by the multidisciplinary
team.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
IBC-2
NCCN Guidelines Version 2.2016
Inammatory Breast Cancer
kThe accurate assessment of in-breast tumor or regional lymph node response to preoperative systemic therapy is difficult, and should include physical examination and
performance of imaging studies (mammogram and/or breast MRI) that were abnormal at the time of initial tumor staging. Selection of imaging methods prior to surgery
should be determined by the multidisciplinary team.
lPatients with stage IV or recurrent IBC should be treated according to the guideline for recurrence/stage IV disease (BINV-17 to BINV-22).
mSee Principles of Breast Reconstruction Following Surgery (BINV-H).
nSee Chemotherapy Regimens for Recurrent or Metastatic Breast Cancer (BINV-O).
oSee Principles of Radiation Therapy (BINV-I).
TREATMENTl
Response
No
response
Total mastectomy + level l/ll axillary dissection +
radiation therapy to chest wall and supraclavicular
area (plus internal mammary nodes if involved,
consider internal mammary nodes if not clinically
involved [category 3]) ± delayed breast
reconstructionm
Consider additional systemic
chemotherapyn and/or
preoperative radiation
• Complete planned chemotherapy regimen course
if not completed preoperatively plus endocrine
treatment if ER-positive and/or PR-positive (sequential
chemotherapy followed by endocrine therapy).
• Complete up to one year of HER2-targeted therapy
if HER2 positive (category 1). May be administered
concurrently with radiation therapyo and with
endocrine therapy if indicated.
Responsek
No responsek
See above pathway
Individualized treatment
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
NCCN Guidelines Version 2.2016
Breast Cancer
ST-1
Staging
Table 1
American Joint Committee on Cancer (AJCC)
TNM Staging System For Breast Cancer
Primary Tumor (T) The T classication of the primary tumor is the same
regardless of whether it is based on clinical or pathologic criteria, or both.
Size should be measured to the nearest millimeter. If the tumor size is slightly
less than or greater than a cutoff for a given T classication, it is recommended
that the size be rounded to the millimeter reading that is closest to the cutoff. For
example, a reported size of 1.1 mm is reported as 1 mm, or a size of 2.01 cm
is reported as 2.0 cm. Designation should be made with the subscript “c” or “p”
modier to indicate whether the T classication was determined by clinical (physical
examination or radiologic) or pathologic measurements, respectively. In general,
pathologic determination should take precedence over clinical determination of
T size.
TX Primary tumor cannot be assessed
T0 No evidence of primary tumor
Tis Carcinoma in situ
Tis (DCIS) Ductal carcinoma in situ
Tis (LCIS) Lobular carcinoma in situ
Tis (Paget's) Paget’s disease of the nipple NOT associated with invasive
carcinoma and/or carcinoma in situ (DCIS and/or LCIS) in
the underlying breast parenchyma. Carcinomas in the breast
parenchyma associated with Paget’s disease are categorized
based on the size and characteristics of the parenchymal disease,
although the presence of Paget’s disease should still be noted
T1 Tumor ≤20 mm or less in greatest dimension
T1mi Tumor ≤1 mm in greatest dimension
T1a Tumor >1 mm but ≤5 mm in greatest dimension
T1b Tumor >5 mm but ≤10 mm in greatest dimension
T1c Tumor >10 mm but ≤20 mm in greatest dimension
T2 Tumor >20 mm but ≤50 mm in greatest dimension
T3 Tumor >50 mm in greatest dimension
T4 Tumor of any size with direct extension to the chest wall and/or to
the skin (ulceration or skin nodules).
Note: Invasion of the dermis alone does not qualify as T4
T4a Extension to the chest wall, not including only pectoralis muscle
adherence/invasion
T4b Ulceration and/or ipsilateral satellite nodules and/or edema
(including peau d’orange) of the skin, which do not meet the
criteria for inammatory carcinoma
T4c Both T4a and T4b
T4d Inammatory carcinoma
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
ST-2
Table 1 (continued)
Regional Lymph Nodes (N)
Clinical
NX Regional lymph nodes cannot be assessed (e.g., previously
removed)
N0 No regional lymph node metastasis
N1 Metastases to movable ipsilateral level I, II axillary lymph node(s)
N2 Metastases in ipsilateral level I, II axillary lymph nodes that are
clinically xed or matted; or in clinically detected* ipsilateral
internal mammary nodes in the absence of clinically evident
axillary lymph node metastases
N2a Metastases in ipsilateral level I, II axillary lymph nodes xed to
one another (matted) or to other structures
N2b Metastases only in clinically detected* ipsilateral internal
mammary nodes and in the absence of clinically evident level I, II
axillary lymph node metastases
N3 Metastases in ipsilateral infraclavicular (level III axillary) lymph
node(s) with or without level I, II axillary lymph node involvement;
or in clinically detected* ipsilateral internal mammary lymph
node(s) with clinically evident level I, II axillary lymph node
metastases; or metastases in ipsilateral supraclavicular lymph
node(s) with or without axillary or internal mammary lymph node
involvement
N3a Metastasis in ipsilateral infraclavicular lymph node(s)
N3b Metastasis in ipsilateral internal mammary lymph node(s) and
axillary lymph node(s)
N3c Metastasis in ipsilateral supraclavicular lymph node(s)
*Note: Clinically detected is dened as detected by imaging studies (excluding
lymphoscintigraphy) or by clinical examination and having characteristics highly
suspicious for malignancy or a presumed pathologic macrometastasis based on
ne needle aspiration.
Pathologic (pN)*
pNX Regional lymph nodes cannot be assessed (e.g., previously
removed, or not removed for pathologic study)
pN0 No regional lymph node metastasis histologically
Note: Isolated tumor cell clusters (ITC) are dened as small clusters of cells not
greater than 0.2 mm, or single tumor cells, or a cluster of fewer than 200 cells in
a single histologic cross-section. ITCs may be detected by routine histology or by
immunohistochemical (IHC) methods. Nodes containing only ITCs are excluded
from the total positive node count for purposes of N classication but should be
included in the total number of nodes evaluated.
pN0(i-) No regional lymph node metastasis histologically, negative IHC
pN0(I+) Malignant cells in regional lymph node(s) no greater than 0.2 mm
(detected by H&E or IHC including ITC)
pN0(mol-) No regional lymph node metastases histologically, negative
molecular ndings (RT-PCR)
pN0(mol+) Positive molecular ndings (RT-PCR),** but no regional lymph
node metastases detected by histology or IHC
* Classication is based on axillary lymph node dissection with or without sentinel
lymph node biopsy. Classication based solely on sentinel lymph node biopsy
without subsequent axillary lymph node dissection is designated (sn) for “sentinel
node,” for example, pN0(sn).
** RT-PCR: reverse transcriptase/polymerase chain reaction.
NCCN Guidelines Version 2.2016
Breast Cancer Staging
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
ST-3
Table 1 (continued)
Pathologic (pN) (continued)
pN1 Micrometastases; or metastases in 1–3 axillary lymph nodes;
and/or in internal mammary nodes with metastases detected by
sentinel lymph node biopsy but not clinically detected***
pN1mi Micrometastases (greater than 0.2 mm and/or more than 200 cells,
but none greater than 2.0 mm)
pN1a Metastases in 1–3 axillary lymph nodes, at least one metastasis
greater than 2.0 mm
pN1b Metastases in internal mammary nodes with micrometastases or
macrometastases detected by sentinel lymph node biopsy but
not clinically detected***
pN1c Metastases in 1–3 axillary lymph nodes and in internal mammary
lymph nodes with micrometastases or macrometastases
detected by sentinel lymph node biopsy but not clinically
detected
pN2 Metastases in 4–9 axillary lymph nodes; or in clinically
detected**** internal mammary lymph nodes in the absence
of axillary lymph node metastases
pN2a Metastases in 4–9 axillary lymph nodes (at least one tumor
deposit greater than 2.0 mm)
pN2b Metastases in clinically detected**** internal mammary lymph
nodes in the absence of axillary lymph node metastases
pN3 Metastases in ten or more axillary lymph nodes; or in
infraclavicular (level III axillary) lymph nodes; or in clinically
detected**** ipsilateral internal mammary lymph nodes in the
presence of one or more positive level I, II axillary lymph nodes;
or in more than three axillary lymph nodes and in internal mammary
lymph nodes with micrometastases or macrometastases detected
by sentinel lymph node biopsy but not clinically detected*** ; or in
ipsilateral supraclavicular lymph nodes
pN3a Metastases in ten or more axillary lymph nodes (at least one
tumor deposit greater than 2.0 mm); or metastases to the
infraclavicular (level III axillary lymph) nodes
pN3b Metastases in clinically detected**** ipsilateral internal mammary
lymph nodes in the presence of one or more positive axillary
lymph nodes; or in more than three axillary lymph nodes and
in internal mammary lymph nodes with micrometastases or
macrometastases detected by sentinel lymph node biopsy but
not clinically detected***
pN3c Metastasis in ipsilateral supraclavicular lymph nodes
*** “Not clinically detected” is dened as not detected by imaging studies
(excluding lymphoscintigraphy) or not detected by clinical examination.
**** “Clinically detected” is dened as detected by imaging studies (excluding
lymphoscintigraphy) or by clinical examination and having characteristics
highly suspicious for malignancy or a presumed pathologic macrometastasis
based on ne needle aspiration biopsy with cytologic examination.
Distant Metastasis (M)
M0 No clinical or radiographic evidence of distant metastases
cM0(I+) No clinical or radiographic evidence of distant metastases, but deposits
of molecularly or microscopically detected tumor cells in circulating
blood, bone marrow, or other nonregional nodal tissue that are no larger
than 0.2 mm in a patient without symptoms or signs of metastases
M1 Distant detectable metastases as determined by classic clinical and
radiographic means and/or histologically proven larger than 0.2 mm
NCCN Guidelines Version 2.2016
Breast Cancer Staging
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

NCCN Guidelines Index
Breast Cancer Table of Contents
Discussion
Version 2.2016 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Note: All recommendations are category 2A unless otherwise indicated.
Clinical Trials: NCCN believes that the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
ST-4
Table 1 (continued)
ANATOMIC STAGE/PROGNOSTIC GROUPS
Stage 0 Tis N0 M0
Stage IA T1* N0 M0
Stage IB T0 N1mi M0
T1* N1mi M0
Stage IIA T0 N1** M0
T1* N1** M0
T2 N0 M0
Stage IIB T2 N1 M0
T3 N0 M0
* T1 includes T1mi
** T0 and T1 tumors with nodal micrometastases only are excluded from
Stage IIA and are classied Stage IB.
• M0 includes M0(i+).
• The designation pM0 is not valid; any M0 should be clinical.
• If a patient presents with M1 prior to neoadjuvant systemic therapy, the
stage is considered Stage IV and remains Stage IV regardless of response
to neoadjuvant therapy.
• Stage designation may be changed if postsurgical imaging studies reveal
the presence of distant metastases, provided that the studies are carried
out within 4 months of diagnosis in the absence of disease progression and
provided that the patient has not received neoadjuvant therapy.
• Postneoadjuvant therapy is designated with “yc” or “yp” prex. Of note, no
stage group is assigned if there is a complete pathologic response (CR) to
neoadjuvant therapy, for example, ypT0ypN0cM0.
HISTOLOGIC GRADE (G)
All invasive breast carcinomas should be graded. The Nottingham combined
histologic grade (Elston-Ellis modication of Scarff–Bloom–Richardson
grading system) is recommended.1,2 The grade for a tumor is determined by
assessing morphologic features (tubule formation, nuclear pleomorphism, and
mitotic count), assigning a value of 1 (favorable) to 3 (unfavorable) for each
feature, and adding together the scores for all three categories. A combined
score of 3–5 points is designated as grade 1; a combined score of 6–7 points
is grade 2; a combined score of 8–9 points is grade 3.
HISTOLOGIC GRADE (NOTTINGHAM COMBINED HISTOLOGIC
GRADE IS RECOMMENDED)
GX Grade cannot be assessed
G1 Low combined histologic grade (favorable)
G2 Intermediate combined histologic grade (moderately favorable)
G3 High combined histologic grade (unfavorable)
HISTOPATHOLOGIC TYPE
The histopathologic types are the following:
In situ Carcinomas
NOS (not otherwise specied)
Intraductal
Paget's disease and intraductal
Invasive Carcinomas
NOS
Ductal
Inammatory
Medullary, NOS
Medullary with lymphoid stroma
Mucinous
1Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007
update of recommendations for the use of tumor markers in breast cancer. J Clin
Oncol 2007;25:5287–312.
2Singletary SE, Allred C, Ashley P, et al. Revision of the American Joint Committee
on Cancer staging system for breast cancer. J Clin Oncol 2002;20:3628–36.
Used with the permission of the American Joint Committee on Cancer (AJCC),
Chicago Illinois. The original and primary source for this information is the
AJCC Cancer Staging Manual, Seventh Edition (2010) published by Springer
Science+Business Media, LLC (SBM). (For complete information and data
supporting the staging tables, visit www.cancerstaging.net.) Any citation or quotation
of this material must be credited to the AJCC as its primary source. The inclusion of
this information herein does not authorize any reuse or further distribution without
the expressed, written permission of Springer SBM, on behalf of the AJCC.
Stage IIIA T0 N2 M0
T1* N2 M0
T2 N2 M0
T3 N1 M0
T3 N2 M0
Stage IIIB T4 N0 M0
T4 N1 M0
T4 N2 M0
Stage IIIC Any T N3 M0
Stage IV Any T Any N M1
Papillary (predominantly micropapillary
pattern)
Tubular
Lobular
Paget's disease and inltrating
Undifferentiated
Squamous cell
Adenoid cystic
Secretory
Cribriform
NCCN Guidelines Version 2.2016
Breast Cancer Staging
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-1
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
Discussion
NCCN Categories of Evidence and Consensus
Category 1: Based upon high-level evidence, there is uniform NCCN
consensus that the intervention is appropriate.
Category 2A: Based upon lower-level evidence, there is uniform
NCCN consensus that the intervention is appropriate.
Category 2B: Based upon lower-level evidence, there is NCCN
consensus that the intervention is appropriate.
Category 3: Based upon any level of evidence, there is major NCCN
disagreement that the intervention is appropriate.
All recommendations are category 2A unless otherwise noted.
Table of Contents
Overview ............................................................................................. 2
Literature Search Criteria and Guidelines Update Methodology .......... 2
Staging ............................................................................................... 3
Pathology Assessment ....................................................................... 3
Treatment Approach ........................................................................... 5
Pure Noninvasive Carcinomas (Stage 0) ............................................ 6
Lobular Carcinoma in Situ ............................................................... 6
Ductal Carcinoma in Situ ................................................................. 7
Invasive Breast Cancer ..................................................................... 13
Stage I, IIA, IIB, or III A (T3, N1, M0) ................................................ 13
Workup .......................................................................................... 13
Locoregional Treatment ................................................................ 15
Breast Reconstruction ................................................................... 22
Systemic Therapies (Preoperative and Adjuvant) .......................... 26
Post-Therapy Surveillance and Follow-up ..................................... 45
Stage III Invasive Breast Cancer ....................................................... 45
Staging and Workup ...................................................................... 45
Operable Locally Advanced Breast Cancer ................................... 47
Inoperable Locally Advanced Breast Cancer ................................. 47
Post-Therapy Surveillance and Follow-up for Stage I-III ................ 48
Stage IV Metastatic or Recurrent Breast Cancer .............................. 50
Staging and Workup ...................................................................... 50
Management of Local Disease Only .............................................. 51
Management of Stage IV or Recurrent Metastatic Disease ........... 52
Surgery for Stage IV or Recurrent Metastatic Disease .................. 63
Monitoring Metastatic Disease ...................................................... 64
Special Situations ............................................................................. 65
Paget’s Disease ............................................................................ 65
Phyllodes Tumors of the Breast..................................................... 66
Breast Cancer During Pregnancy .................................................. 67
Inflammatory Breast Cancer .......................................................... 69
Axillary Breast Cancer ................................................................... 72
Summary .......................................................................................... 73
References ....................................................................................... 74
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-2
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
Overview
Breast cancer is the most frequently diagnosed cancer globally and is
the leading cause of cancer-related death in women.1 The American
Cancer Society estimates that 249,260 Americans will be diagnosed
with invasive breast cancer and 40,890will die of the disease in the
United States in 2016.2
Historically, white women have had the highest breast cancer incidence
rates among women aged 40 years and older; however, incidence rates
are converging among white and African American women, particularly
among women aged 50 to 59 years.3 Since 1991, breast cancer
mortality has been declining,4,5 suggesting a benefit from the
combination of early detection and more effective treatment.6
The etiology of the vast majority of breast cancer cases is unknown.
However, numerous risk factors for the disease have been established.
These risk factors include: female gender; increasing patient age; family
history of breast cancer at a young age; early menarche; late
menopause; older age at first live childbirth; prolonged hormone
replacement therapy; previous exposure to therapeutic chest wall
irradiation; benign proliferative breast disease; increased
mammographic breast density; and genetic mutations such as of the
BRCA1/2 genes. However, except for female gender and increasing
patient age, these risk factors are associated with only a minority of
breast cancers. Women with a strong family history of breast cancer
should be evaluated according to the NCCN Guidelines for
Genetic/Familial High-Risk Assessment: Breast and Ovarian. Women at
increased risk for breast cancer (generally those with ≥1.7% 5-year risk
for breast cancer using the Gail model of risk assessment7) may
consider risk reduction strategies (see NCCN Guidelines for Breast
Cancer Risk Reduction).
Proliferative abnormalities of the breast are limited to the lobular and
ductal epithelium. In both the lobular and ductal epithelium, a spectrum
of proliferative abnormalities may be seen, including hyperplasia,
atypical hyperplasia, in situ carcinoma, and invasive carcinoma.8
Approximately 85% to 90% of invasive carcinomas are ductal in origin.9
The invasive ductal carcinomas include unusual variants of breast
cancer, such as mucinous, adenoid cystic, and tubular carcinomas,
which have especially favorable natural histories.
Literature Search Criteria and Guidelines Update
Methodology
Prior to the update of this version of the NCCN Guidelines® for Breast
Cancer, an electronic search of the PubMed database was performed
to obtain key literature in Breast Cancer, published between 06/19/14
and 06/29/15, using the following search terms: Breast Cancer OR
DCIS OR Inflammatory Breast Cancer OR Phyllodes. The PubMed
database was chosen as it remains the most widely used resource for
medical literature and indexes only peer-reviewed biomedical
literature.10
The search results were narrowed by selecting studies in humans
published in English. Results were confined to the following article
types: Clinical Trial, Phase III; Clinical Trial, Phase IV; Guideline;
Randomized Controlled Trial; Meta-Analysis; Systematic Reviews; and
Validation Studies.
The potential relevance of the PubMed search was examined. The data
from key PubMed articles selected by the panel for review during the
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-3
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
Guidelines update meeting as well as articles from additional sources
deemed as relevant to these Guidelines and discussed by the panel
have been included in this version of the Discussion section.
Recommendations for which high-level evidence is lacking are based
on the panel’s review of lower-level evidence and expert opinion.
The complete details of the Development and Update of the NCCN
Guidelines are available on the NCCN webpage.
Staging
All patients with breast cancer should be assigned a clinical stage of
disease, and, if appropriate evaluation is available, a pathologic stage
of disease. The routine use of staging allows for efficient identification of
local treatment options, assists in identifying systemic treatment
options, allows for the comparison of outcome results across institutions
and clinical trials, and provides baseline prognostic information.
Effective January 2010, the AJCC implemented a revision of the 7th
edition of the AJCC Cancer Staging Manual containing important
changes and additions to the TNM staging system for breast cancer.11
This revision differs from the 2003 edition of the AJCC staging manual
by providing more direction relating to the specific methods of clinical
and pathologic tumor measurement; recommending that all invasive
cancers should be assigned a combined histologic tumor grade using
the Elston-Ellis modification of the Scarff-Bloom-Richardson grading
system; providing clarification of the classification of isolated tumor cells
in axillary lymph node (ALN) staging; subdividing stage I into stage IA
and IB based upon the presence or absence of nodal micrometastases
(N0 versus N0mi+); and defining a new category of M0(i+) disease
referring to tumor cells microscopically detectable in bone marrow or
circulating blood or found incidentally in other tissues not exceeding 0.2
mm in patients who have no signs or symptoms of metastasis. This
version of the AJCC staging manual also recommends the collection of
biomarkers such as hormone receptor status (estrogen receptor [ER]
and progesterone receptor [PR]) and human epidermal growth factor
receptor 2 [HER2] status, although these characteristics do not
specifically influence assigned stage of disease.
Pathology Assessment
A central component of the treatment of breast cancer is full knowledge
of extent of disease and biologic features. These factors contribute to
the determination of the stage of disease, assist in the estimation of the
risk that the cancer will recur, and provide information that predicts
response to therapy (eg, ER, PR, HER2). These factors are determined
by examination of excised tissue and are provided in a written pathology
report. Accurate pathology reporting requires communication between
the clinician and the pathologist relating to relevant patient history, prior
breast biopsies, prior irradiation to the chest, pregnancy status,
characteristics of the abnormality biopsied (eg, palpable,
mammographically detected microcalcifications), clinical state of lymph
nodes, presence of inflammatory change or other skin abnormality, and
any prior treatment administered (eg, chemotherapy, radiation therapy).
The specimens should be oriented for the pathologist, and specific
requests for determination of biomarkers should be stated (eg, ER, PR,
and HER2 status). The use of consistent, unambiguous standards for
reporting is strongly encouraged. Data from both national and local
surveys show that as many as 50% of pathology reports for breast
cancer are missing some elements critical to patient management.12,13
Significant omissions include failure to orient and report surgical
margins and failure to report tumor grade consistently.
The College of American Pathologists (CAP) has developed pathology
reporting protocols to promote complete and standardized reporting of
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-4
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
malignant specimens. CAP provides a protocol for each disease site
that includes cancer case summaries (checklists) along with
background documentation. These checklists form the basis for a
synoptic, standardized reporting of pathologic findings. The checklists
are available without charge through the CAP website at www.cap.org.
Consistent, unambiguous, and complete pathology reporting is a
cornerstone of quality breast cancer care, and the NCCN Breast Cancer
Panel endorses the use of the CAP protocols for reporting the
pathologic analysis of all breast cancer specimens.
ER status should be determined for all samples of ductal carcinoma in
situ (DCIS), and ER and PR tumor status should be determined for all
samples of invasive breast cancer. ER and PR tumor status is normally
determined by immunohistochemistry (IHC) testing. Although this
method is considered reliable when performed by experienced
pathology personnel, there have been several reports indicating that the
reliability of ER and PR determinations can vary widely from one
laboratory to another.14-16 These inter-laboratory differences may be
attributable to the diverse methodologies and diverse interpretation
schema used to evaluate tumor hormonal status. An NCCN Task Force
and a panel of ASCO and CAP members have reviewed this topic and
issued recommendations on ER and PR testing in breast cancer.17,18
Breast cancers that have at least 1% of cells staining positive for ER
should be considered ER-positive.17-19
Principles of HER2 Testing
Along with ER and PR, the determination of HER2 tumor status is
recommended for all newly diagnosed invasive breast cancers and for
first recurrences of breast cancer whenever possible. The NCCN Breast
Cancer Panel endorses CAP accreditation for anatomic pathology
laboratories performing HER2 testing.
HER2 status can be assessed by measuring the number of HER2 gene
copies using in situ hybridization (ISH) techniques, or by a
complementary method in which the quantity of HER2 cell surface
receptors is assessed by IHC.20 Assignment of HER2 status based on
mRNA assays or multigene arrays is not recommended. The accuracy
of HER2 assays used in clinical practice is a major concern, and results
from several studies have shown that false-positive21-24 as well as
false-negative21,25 HER2 test results are common. A joint panel from
ASCO and CAP has issued updated HER2 testing guidelines to avoid
such false-positive or false-negative results. These updated guidelines
have been published in the Archives of Pathology & Laboratory
Medicine and ASCO's Journal of Clinical Oncology.26,27 The NCCN
Panel endorses these updated ASCO/CAP recommendations for quality
HER2 testing and has outlined these recommendations in Principles of
HER2 Testing in the NCCN Guidelines for Breast Cancer.
HER2 testing should be performed in laboratories accredited by CAP or
another equivalent authority to carry out such testing. Further, these
laboratories should have standardized HER2 testing procedures in
place, as well as programs to periodically evaluate the proficiency of
personnel performing HER2 testing. HER2 test reports should also
include information on site of tumor, specimen type, histologic type,
fixation method and time, block examined, and details on the HER2
testing method(s) used. Clinicians should be familiar with the
significance of these criteria when making clinical recommendations for
an individual patient.
HER2-Positive Result
Consistent with the ASCO/CAP guidelines, the NCCN Panel considers
either IHC or ISH with either a single or dual probe as an acceptable
method for making an initial determination of HER2 tumor status. Breast
cancer tumors are classified as HER2-positive if they are scored as 3+
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-5
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
by an IHC method defined as uniform membrane staining for HER2 in
10% or more of tumor cells or demonstrate HER2 gene amplification by
an ISH method (single probe, average HER2 copy number ≥6.0
signals/cell; dual probe HER2/CEP17 ratio ≥2.0 with an average HER2
copy number ≥4.0 signals/cell; dual probe HER2/chromosome
enumeration probe (CEP)17 ratio ≥2.0 with an average HER2 copy
number <4.0 signals/cell; and HER2/CEP17 ratio <2.0 with an average
HER2 copy number ≥6.0 signals/cell).
High average copy number of HER2 (≥6.0 signals/cell) is considered
positive regardless of the HER2/CEP17 ratio. The rationale cited by the
joint committee for including rare scenarios such as HER2 positivity
when dual probe HER2/CEP17 ratio is greater than or equal to 2.0 and
average HER2 copy number is less than 4.0 signals/cell is that the first-
generation trials of adjuvant trastuzumab included a small number of
patients with a HER2/CEP17 ratio greater than or equal to 2.0 and
an average HER2 copy number less than 4.0 signals/cell. There is
no trend in these data, suggesting that these patients were not
responsive to trastuzumab and the trastuzumab has a favorable
safety profile.
Equivocal Result
The NCCN Panel agrees with the ASCO/CAP HER2 committee that
results of IHC are equivocal if scored as IHC 2+ “based on
circumferential membrane staining that is incomplete and/or
weak/moderate and within greater than 10% of the invasive tumor cells
or complete and circumferential membrane staining that is intense and
within less than or equal to 10% of the invasive tumor cells.” In such
cases, the panel recommends reflex testing using the ISH method on
the same specimen or repeating tests if a new specimen is available.
Similarly, samples with equivocal results by an ISH assay (for example,
single probe ISH average HER2 copy number ≥4.0 and <6.0
signals/cell; and dual probe HER/CEP17 ratio <2.0 with an average
HER2 copy number ≥4.0 signals/cell) must be confirmed by reflex
testing using the IHC method on the same specimen or repeating tests
if a new specimen is available.
Treatment Approach
The treatment of breast cancer includes the treatment of local disease
with surgery, radiation therapy, or both, and systemic treatment with
chemotherapy, endocrine therapy, biologic therapy, or combinations of
these. The need for and selection of various local or systemic therapies
are based on several prognostic and predictive factors. These factors
include tumor histology, clinical and pathologic characteristics of the
primary tumor, ALN status, tumor hormone receptor (ER/PR) content,
tumor HER2 status, multi-gene testing, presence or absence of
detectable metastatic disease, patient comorbid conditions, patient age,
and menopausal status. One percent of breast cancers occur in men,5
and men with breast cancer should be treated similarly to
postmenopausal women, except that the use of aromatase inhibitors is
ineffective without concomitant suppression of testicular
steroidogenesis.28,29 Patient preference is a major component of the
decision-making process, especially in situations in which survival rates
are equivalent among the available treatment options.
In terms of treatment, breast cancer may be divided into: 1) the pure
noninvasive carcinomas, which include lobular carcinoma in situ (LCIS)
and DCIS (stage 0); 2) operable, locoregional invasive carcinoma with
or without associated noninvasive carcinoma (clinical stage I, stage II,
and some stage IIIA tumors); 3) inoperable locoregional invasive
carcinoma with or without associated noninvasive carcinoma (clinical
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-6
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
stage IIIB, stage IIIC, and some stage IIIA tumors); and 4) metastatic
(stage IV) or recurrent carcinoma.
Pure Noninvasive Carcinomas (Stage 0)
Both LCIS and DCIS may be difficult to distinguish from atypical
hyperplasia or from invasive carcinomas with early invasion.30,31
Therefore, pathology review of all cases is recommended.
Bilateral diagnostic mammography should be performed to identify the
presence of multiple primary tumors and to estimate the extent of the
noninvasive lesion. Genetic counseling is recommended if the patient is
considered to be at high risk for hereditary breast cancer as defined by
the NCCN Guidelines for Genetic/Familial High-Risk Assessment:
Breast and Ovarian. Testing for genetic mutations without formal
genetic counseling is discouraged.
The goal of treatment of pure in situ carcinoma is either preventing the
occurrence of invasive disease or diagnosing the development of an
invasive component when still localized to the breast. Patients with
invasive disease, even if microinvasive, on pathology review or during
re-excision, mastectomy, or ALN staging should be treated according to
the stage-appropriate guideline for invasive carcinoma.
Lobular Carcinoma in Situ
(Stage 0, Tis, N0, M0)
Workup
Recommended workup includes history and physical examination,
diagnostic bilateral mammography, and pathology review.
Controversy exists regarding whether an open surgical excision should
be performed of the region of LCIS diagnosed by core biopsy and that is
not associated with a mammographic structural abnormality or residual
mammographic calcifications. Small retrospective studies have
concluded that excision following the diagnosis of LCIS on core needle
biopsy is not necessary.32-34 Other studies have shown that 17% to 27%
of patients with LCIS diagnosed by core needle biopsy are upgraded to
having invasive cancer or DCIS after larger excisional biopsy.35-39 Based
on core needle biopsies, it may be possible to identify subsets of
patients with LCIS who can be safely spared a surgical excision.34
There are some data of small groups of patients suggesting that LCIS
subtypes, including pleomorphic LCIS and LCIS associated with
necrosis, carry a risk for associated invasive carcinoma similar to DCIS.
Therefore, according to the NCCN Panel, it is reasonable to perform
surgical excision of LCIS found in a core biopsy to exclude an
associated invasive cancer or DCIS. More than 4 foci of LCIS may also
increase the risk for upstaging on surgical biopsy.40 The NCCN Panel
recommends that LCIS of the usual type (involving <4 terminal ductal
lobular units in a single core) found on core biopsy, as a result of routine
screening for calcifications and without imaging discordance, may be
managed by imaging follow-up.
Primary Treatment
Classic LCIS does not require surgical treatment. There is evidence to
support the existence of histologically aggressive variants of LCIS (eg,
“pleomorphic” LCIS), which may have a greater potential than classic
LCIS to develop into invasive lobular carcinoma.41 Clinicians may
consider complete excision with negative margins for pleomorphic LCIS.
However, outcomes data regarding treatment of patients with
pleomorphic LCIS are lacking, due in part to a paucity of histologic
categorization of variants of LCIS. Therefore, recommendations on the
treatment of pleomorphic LCIS as a distinct entity of LCIS have not
been made by the panel (see NCCN Guidelines for Breast Screening
and Diagnosis).
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-7
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
Patients with a confirmed diagnosis of LCIS should be counseled
regarding reducing the risk of developing invasive cancer (see NCCN
Guidelines for Breast Cancer Risk Reduction).
Surveillance
Follow-up of patients with LCIS includes interval history and physical
examinations every 6 to 12 months. Annual diagnostic mammography is
recommended in patients being followed with clinical observation; see
also the NCCN Guidelines for Breast Cancer Screening and Diagnosis.
Patients receiving a risk reduction agent should be monitored as
described in the NCCN Guidelines for Breast Cancer Risk Reduction.
Ductal Carcinoma in Situ
(Stage 0, Tis, N0, M0)
Workup
The recommended workup and staging of DCIS includes: history and
physical examination; bilateral diagnostic mammography; pathology
review; and tumor ER determination. Genetic counseling is
recommended if the patient is considered to be at high risk for
hereditary breast cancer as defined by the NCCN Guidelines for
Genetic/Familial High-Risk Assessment: Breast and Ovarian.
Although HER2 status is of prognostic significance in invasive cancer,
its importance in DCIS has not been elucidated. To date, studies have
either found unclear or weak evidence of HER2 status as a prognostic
indicator in DCIS.42-45 The NCCN Panel concluded that knowing the
HER2 status of DCIS does not alter the management strategy and
routinely should not be determined.
MRI has been prospectively shown to have a sensitivity of up to 98% for
high-grade DCIS.46 In a prospective, observational study, 193 women
with pure DCIS underwent both mammography and MRI imaging
preoperatively; 93 (56%) women were diagnosed by mammography
and 153 (92%) were diagnosed by MRI (P < .0001). Of the 89 women
with high-grade DCIS, 43 (48%) who were not diagnosed by
mammography were diagnosed by MRI alone. Another study evaluated
the role of MRI in determining appropriate candidacy for partial breast
irradiation for women with DCIS. Twenty percent of women with DCIS
were identified as ineligible for partial breast irradiation after a bilateral
breast MRI.47 However, large prospective clinical trials will be necessary
to further investigate the clinical role of MRI for diagnosing DCIS and to
investigate its effect on recurrence rates or mortality. The NCCN Panel
has included breast MRI as optional during the initial workup of DCIS,
noting that the use of MRI has not been shown to increase likelihood of
negative margins or decrease conversion to mastectomy with DCIS.
Primary Treatment
Seemingly pure DCIS on core needle biopsy will be found to be
associated with an invasive cancer on surgical excision in about 25% of
patients.48
For the vast majority of patients with limited disease where negative
margins are achieved with the initial excision or with re-excision,
lumpectomy or total mastectomy are appropriate treatment options.
Patients with DCIS and evidence of widespread disease (ie, disease in
two or more quadrants) on mammography or other imaging, physical
examination, or biopsy require a total mastectomy without lymph node
dissection.
Complete ALN dissection is not recommended in the absence of
evidence of invasive cancer or proven axillary metastatic disease in
patients with apparent pure DCIS, or mammographically detected DCIS
with microcalcifications. However, a small proportion of women with
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-8
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
seemingly pure DCIS on initial biopsy will have invasive breast cancer
at the time of the definitive surgical procedure and thus will ultimately
require ALN staging. In patients with seemingly pure DCIS to be treated
with mastectomy or with excision in an anatomic location (eg, tail of the
breast), which could compromise the performance of a future sentinel
lymph node biopsy (SLNB), an SLNB procedure may be considered.49-52
Reducing Risk of Recurrence:
Many factors impact recurrence risk, including patient age, tumor size,
tumor grade, and margin width. The definition of a negative margin has
not been firmly established in DCIS. There appears to be a consensus
that margins greater than 10 mm are accepted as negative (but may be
excessive and may compromise cosmetic outcome) and margins less
than 1 mm are inadequate, but no uniform consensus exists for margin
status between these values. With pathologic margins between 1 and
10 mm, wider margins are generally associated with lower local
recurrence rates. However, close surgical margins (<1 mm) at the
fibroglandular boundary of the breast (chest wall or skin) do not
mandate surgical re-excision but can be an indication for higher boost
dose radiation to the involved lumpectomy site.
The choice of local treatment does not impact overall disease-related
survival; therefore, the individual patient’s acceptance of the potential
for an increased risk of local recurrence must be considered.
An analysis of specimen margins and specimen radiographs should be
performed to ensure that all mammographically detectable DCIS has
been excised. In addition, a post-excision mammogram should be
considered where appropriate (eg, the mass and/or microcalcifications
are not clearly within the specimen).53
Prospective randomized trials have shown that the addition of whole
breast radiation to a margin-free excision of pure DCIS decreases the
rate of in-breast disease recurrence, but does not affect survival54-60 or
distant metastasis-free survival.61 Whole breast irradiation after
breast-conserving surgery reduces the relative risk of a local failure by
approximately one half.
There is retrospective evidence suggesting that selected patients have
a low risk of in-breast recurrence with excision alone without radiation
therapy to the breast.62-65 For example, in a retrospective review,
10-year disease-free survival (DFS) rates of 186 patients with DCIS
treated with lumpectomy alone were 94% for patients with low-risk DCIS
and 83% for patients with both intermediate- and high-risk DCIS.62
In another retrospective study of 215 patients with DCIS treated with
lumpectomy without radiation therapy, endocrine therapy, or
chemotherapy, the recurrence rate over 8 years was 0%, 21.5%, and
32.1% in patients with low-, intermediate- or high-risk DCIS,
respectively.63
A multi-institutional, nonrandomized, prospective study of selected
patients with low-risk DCIS treated without radiation has also provided
some support for the use of excision without radiation in the treatment
of DCIS.66 At a median follow-up of 6.2 years, the 5-year risk of
ipsilateral breast recurrence was 6.1% (95% confidence interval [CI],
4.1%–8.2%) in the subset of patients with low-/intermediate-grade DCIS
and median tumor size of 6 mm. Margin widths were greater than or
equal to 5 mm in 69.2% and 82.9% of patients in the
low-/intermediate-risk and high-risk arms, respectively, with margin
widths of greater than or equal to 10 mm or no tumor on re-excision
observed in 48.5% and 53.3% of patients in the respective groups.66
Although an acceptably low ipsilateral recurrence rate was observed in
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-9
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
the low-/intermediate-grade arm of the study at 5 years, the 7-year
ipsilateral recurrence rate in this group of patients was considerably
higher (10.5%; 95% CI, 7.5%–13.6%), suggesting that these events
may be delayed but not prevented in this population. Ipsilateral breast
recurrences were approximately equally divided between invasive
breast cancer and DCIS in the low-/intermediate-risk group but only
about one-third of patients with an in-breast recurrence in the high-risk
group had invasive disease.
Another retrospective study reviewed 220 patients with DCIS treated
with breast conservation surgery and radiation. Thirty-six percent
received a radiation boost. At 46 months, none of the 79 patients who
received a radiation boost experienced a local recurrence, whereas 8 of
141 patients who did not receive a boost experienced a local
recurrence.67
More recently, a prospective phase II study followed women with low-
risk DCIS who underwent wide excision alone (without adjuvant
treatment with radiation or tamoxifen). Low-risk criteria for this study
included mammographically detected DCIS, measuring ≤2.5 cm, with a
predominant nuclear grade of 1 or 2, and a margin of ≥1 cm or a
negative re-excision. The cumulative incidence of local recurrence at 10
years was estimated at 15.5%.68
Prospective randomized trials have not been carried out to analyze
whether wider margins can replace the need for radiation therapy for
DCIS. A retrospective series demonstrated that for margin width of 10
mm, radiation had no additional benefit in reducing the already low local
recurrence rate of 4% at the end of 8 years.65 Also, if margin width was
between 1 mm and less than 10 mm, the addition of radiation therapy
led to a non-statistically significant reduction in local recurrence.
However, when margins were less than 1 mm a significant benefit was
seen.65
A meta-analysis of four large multicenter randomized trials confirmed
the results of the individual trials that adding radiation therapy to breast-
conserving surgery for DCIS provides a statistically and clinically
significant reduction in ipsilateral breast events (HR [hazard ratio], 0.49;
95% CI; 0.41–0.58, P < .0000).69
Results from a retrospective study of 445 patients with pure DCIS
treated by excision alone indicated that margin width was the most
important independent predictor of local recurrence, although the trend
for decreasing local recurrence risk with increasing margin width was
most apparent with margins less than 1 mm and greater than or equal
to 10 mm.70
In a meta-analysis of 4660 patients with DCIS treated with
breast-conserving surgery and radiation, a surgical margin of less than
2 mm was associated with increased rates of ipsilateral breast tumor
recurrence compared with margins of 2 mm, although no significant
differences were observed when margins of greater than 2 mm to 5 mm
or greater than 5 mm were compared with 2-mm margins.71 The results
of this study suggest that wide margins (≥2 mm), which can
compromise cosmetic outcome, do not provide additional benefit in the
population of patients with DCIS receiving radiation therapy following
breast-conserving therapy.
A study retrospectively reviewed a database of 2996 patients with DCIS
who underwent breast-conserving surgery to investigate the association
between margin width and recurrence, controlling all other
characteristics.72 Wider margins were significantly associated with a
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-10
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
lower rate of recurrence only in women who did not receive radiation
therapy (P < .0001), but not in those treated with radiation (P = .95).72
After 20 years of follow-up, the SweDCIS trial reported 12% (95% CI,
6.5–17.7) reduction in absolute risk of local recurrence and 37.5%
reduction in relative risk in women who received radiation therapy.73
A recent prospective randomized trial (RTOG 9804) compared radiation
therapy with observation after breast=conserving surgery in a subset of
women with defined, mammographically detected low- or intermediate-
grade DCIS, measuring less than 2.5 cm with margins ≥3 mm.74 At 7
years, the local recurrence rate was 0.9% (95% CI, 0.0%–2.2%) in the
radiation therapy arm versus 6.7% (95% CI, 3.2%–9.6%) in the
observation arm (HR, 0.11; 95% CI, 0.03–0.47; P < .001). In the subset
of patients with good-risk disease features, the local recurrence rate
was low with observation but was decreased significantly with the
addition of radiation therapy.74
The long-term follow-up of the NSABP B-17 showed that at 15 years,
radiation therapy resulted in a 52% reduction of ipsilateral invasive
recurrence compared with excision alone (HR, 0.48; 95% CI, 0.33–0.69,
P < .001).60 However, overall survival (OS) and cumulative all-cause
mortality rate through 15 years was similar between the two groups (HR
for death, 1.08; 95% CI, 0.79–1.48). 60
Similar findings were reported by a large observational study of the
SEER database that included 108,196 patients with DCIS.75 In a
subgroup analysis at 10 years, of 60,000 women treated with breast-
conserving therapy, with or without radiation therapy, although radiation
therapy was associated with a 50% reduction in the risk of ipsilateral
recurrence (adjusted HR, 0.47 [95% CI, 0.42–0.53]; P < .001), breast
cancer-specific mortality was found to be similar (HR, 0.86 [95% CI,
0.67–1.10]; P = .22).75
NCCN Recommendations for Primary Treatment of DCIS
According to the NCCN Panel, primary treatment options for women
with DCIS along with their respective categories of consensus are:
lumpectomy plus whole breast radiation therapy (category 1) as
decreased rates of local recurrence following lumpectomy have been
observed in randomized trials with the addition of whole breast
radiation; total mastectomy, with or without SLNB with or without
reconstruction (category 2A); or lumpectomy alone followed by clinical
observation (category 2B). The option of lumpectomy alone should be
considered only in cases where the patient and the physician view the
individual as having a low risk of disease recurrence. There is no
evidence that OS differs between the three treatment options.
According to the NCCN Panel, complete resection should be
documented by analysis of margins and specimen radiography.
Post-excision mammography should also be performed whenever
uncertainty about adequacy of excision remains. Clips are used to
demarcate the biopsy area because DCIS may be clinically occult and
further surgery may be required pending the margin status review by
pathology.
Women treated with mastectomy are appropriate candidates for breast
reconstruction (see Principles of Breast Reconstruction Following
Surgery in the NCCN Guidelines for Breast Cancer). Contraindications
to breast-conserving therapy with radiation therapy are listed in the
algorithm (see Special Considerations to Breast-Conserving Therapy
Requiring Radiation Therapy in the NCCN Guidelines for Breast
Cancer).
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-11
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
Management of DCIS after Primary Treatment
DCIS falls between atypical ductal hyperplasia and invasive ductal
carcinoma within the spectrum of breast proliferative abnormalities. The
Breast Cancer Prevention Trial performed by National Surgical Adjuvant
Breast and Bowel Project (NSABP) showed a 75% reduction in the
occurrence of invasive breast cancer in patients with atypical ductal
hyperplasia treated with tamoxifen.76,77 These data also showed that
tamoxifen led to a substantial reduction in the risk of developing benign
breast disease.78 The Early Breast Cancer Trialists’ Collaborative
Group (EBCTCG) overview analysis showed that, with 5 years of
tamoxifen therapy, women with ER-positive or receptor-unknown
invasive tumors had a 39% reduction in the annual odds of recurrence
of invasive breast cancer.4
Similarly, the NSABP B-24 trial found a benefit from tamoxifen for
women with DCIS after treatment with breast conservation surgery and
radiation therapy. In that study, women with DCIS who were treated
with breast-conserving therapy were randomized to receive placebo or
tamoxifen. With 13.6 years median follow-up, the women treated with
tamoxifen had a 3.4% absolute reduction in ipsilateral in-breast tumor
recurrence risk (HR, 0.30; 95% CI, 0.21–0.42; P < .001) and a 3.2%
absolute reduction in contralateral breast cancers (HR, 0.68; 95% CI,
0.48–0.95; P = .023).60 The women receiving tamoxifen had a 10-year
cumulative rate of 4.6% for invasive and 5.6% for noninvasive breast
cancers in the ipsilateral breast compared with 7.3% for invasive and
7.2% for noninvasive breast cancers in placebo-treated women. The
cumulative 10-year frequency of invasive and noninvasive breast
cancer in the contralateral breast was 6.9% and 4.7% in the placebo
and tamoxifen groups, respectively. No differences in OS were noted. A
retrospective analysis of ER expression in NSABP B-24 suggests that
increased levels of ER expression predict for tamoxifen benefit in terms
of risk reduction for ipsilateral and contralateral breast cancer
development following breast-conserving therapy.79
A phase III trial for women with excised DCIS randomized subjects in a
2 x 2 fashion to tamoxifen or not and whole breast radiation therapy or
not.59 With 12.7 years of median follow-up, the use of tamoxifen
decreased all new breast events (HR, 0.71; 95% CI, 0.58–0.88; P =
.002). The use of tamoxifen decreased ipsilateral and contralateral
breast events in the subjects not given whole breast radiotherapy
(ipsilateral HR, 0.77; 95% CI, 0.59–0.98; contralateral HR, 0.27; 95%
CI, 0.12–0.59), but not in those receiving whole breast radiotherapy
(ipsilateral HR, 0.93; 95% CI, 0.50–1.75; P = .80; contralateral HR,
0.99; 95% CI, 0.39–2.49; P = 1.0).
In women with ER- and/or PR-positive DCIS treated by wide local
excision with or without breast radiotherapy, a large, randomized,
double-blind, placebo-controlled trial (IBIS-II) compared anastrozole (n
= 1471) with tamoxifen (n = 1509). The results demonstrated non-
inferiority of anastrozole to tamoxifen.80 After a median follow-up of 7.2
years, 67 recurrences were reported with anastrozole versus 77 for
tamoxifen; HR 0.89 [95% CI, 0.64–1.23]. A total 33 deaths were
recorded for anastrozole and 36 for tamoxifen; HR 0.9393 [95% CI,
0.58–1.50, P = .78).80 Although the number of women reporting any
adverse event was similar between anastrozole (1323 women, 91%)
and tamoxifen (1379 women, 93%); the side-effect profiles of the two
drugs were different. There were more fractures, musculoskeletal
events, hypercholesterolemia, and strokes reported with anastrozole
and more muscle spasms, gynecological cancers and symptoms,
vasomotor symptoms, and deep vein thromboses reported with
tamoxifen.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-12
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
The NSABP B-35 study randomly assigned 3,104 postmenopausal
patients to tamoxifen or anastrozole for 5 years. All patients received
breast radiotherapy. Prior to being randomly assigned, patients were
stratified by age—younger or older than age 60. The primary endpoint
was breast cancer–free interval.81 Anastrozole treatment resulted in an
overall statistically significant decrease in breast cancer-free interval
events compared with tamoxifen (HR, 0.73 [95% CI, 0.56–0.96], P =
.0234). The significant difference in breast cancer-free interval between
the two treatments was apparent in the study only after 5 years of
follow-up. The estimated percentage of patients with a 10-year breast
cancer-free interval was 89.1% in the tamoxifen group and 93.1% in the
anastrozole group.81 In addition, anastrozole resulted in further
improvement in breast cancer-free interval, in younger postmenopausal
patients (less than 60 years old). With respect to adverse effects, the
overall incidence of thrombosis or embolism was higher in the tamoxifen
group and the anastrozole group had slightly more cases of arthralgia
and myalgia.81
The results of the IBIS-II and the NSAP-B-35 studies indicate that
anastrozole provides benefit as adjuvant treatment for postmenopausal
women with hormone-receptor-positive DCIS.
NCCN Recommendations for Management of DCIS after Primary
Treatment
According to the NCCN Panel, endocrine therapy, with tamoxifen (for
premenopausal and postmenopausal women) or an aromatase inhibitor
(for postmenopausal women especially those under 60 years of age or
in those with concerns of embolism), may be considered as a strategy
to reduce the risk of ipsilateral breast cancer recurrence in women with
ER-positive DCIS treated with breast-conserving therapy (category 1 for
those undergoing breast-conserving surgery followed by radiation
therapy; category 2A for those undergoing excision alone). The benefit
of endocrine therapy for ER-negative DCIS is not known.
Strategies for reducing the risk of recurrence to the contralateral breast
are described in the NCCN Guidelines for Breast Cancer Risk
Reduction.
Surveillance
According to the NCCN Panel, follow-up of women with DCIS includes
interval history and physical examination every 6 to 12 months for 5
years and then annually, as well as yearly diagnostic mammography. In
patients treated with breast-conserving therapy, the first follow-up
mammogram should be performed 6 to 12 months after the completion
of breast-conserving radiation therapy (category 2B). Patients receiving
risk reduction agents should be monitored as described in the NCCN
Guidelines for Breast Cancer Risk Reduction.
The majority of recurrences of DCIS are in-breast recurrences after
breast-conserving therapy, and recurrences mostly occur close to the
site of prior disease. In those women for whom the initial DCIS was
treated with excision alone, the treatment for a recurrence of DCIS is
similar to that followed previously. In women whom the initial DCIS was
treated with breast-conserving surgery plus radiation therapy,
mastectomy is usually necessary to treat DCIS recurrence. Local
recurrences after mastectomy for DCIS should be treated with wide
local excision with consideration for chest wall irradiation.
Overall, approximately half of the local recurrences after initial treatment
for a pure DCIS are again DCIS, and the others are invasive cancer.
Those with local recurrences that are invasive should receive systemic
treatment as appropriate for a newly diagnosed invasive breast cancer.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-13
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
Invasive Breast Cancer
Stage I, IIA, IIB, or III A (T3, N1, M0)
Workup
The recommended workup of localized invasive breast cancer includes:
history and physical exam; bilateral diagnostic mammography; breast
ultrasonography, if necessary; determination of tumor hormone receptor
status (ER and PR determinations); determination of HER2–receptor
status; and pathology review. Complete blood count (CBC) and liver
function tests (LFTs) have no added benefit in the detection of
underlying metastatic disease in asymptomatic early-stage breast
cancer patients.82 In addition, monitoring of disease relapse with any
tumor markers is not recommended.
Use of MRI is optional and is not universally recommended by experts
in the field. Breast MRI advocates note its high sensitivity for evaluation
of extent of disease, particularly for invasive cancer and in dense
breasts where mammographically occult disease is more likely to elude
preoperative detection. MRI detractors note that MRI has a high
percentage of false-positive findings resulting in further diagnostic
workup in many circumstances including MRI-guided biopsy83-85 MRI
findings tend to overestimate extent of disease86 resulting in increase in
frequency of mastectomies.87-90
MRI findings alone are insufficient to determine whether breast
conservation therapy is optimal as additional tissue sampling is needed
to verify true malignant disease warranting excision. MRI use may
increase mastectomy rates by identifying mammographically occult
disease satellites that would have been adequately treated with post-
lumpectomy radiation had the disease remained undiscovered without
MRI.90
Two prospective randomized studies have examined the utility of pre-
operative MRI in determining disease extent, and neither demonstrated
improvement in rates of post-lumpectomy re-excision.91,92 Retrospective
review of utility MRI showed conflicting outcome results, one with
benefit93 and another without.94 One systematic review85 documented
that breast MRI staging altered surgical treatment in 7.8% to 33.3% of
women,85 however no differences in local recurrence or survival have
yet been demonstrated. In addition, there is no evidence that use of
breast MRI increases rates of margin-negative resection.95,96
If breast MRI imaging is performed, a dedicated breast coil, an imaging
team experienced with breast MRI guided biopsy, and multidisciplinary
treatment team are the standard of care. Clinically positive axillary
nodes and occult primary breast cancer or Paget’s disease of the nipple
with breast primary not identified on mammography, ultrasound, or
physical examination are specific indications for breast MRI imaging.
MRI may also be useful for the evaluation of breast cancer response to
preoperative systemic therapy and to assess the potential for breast-
conserving therapy.
Pathology Assessment: Full knowledge of extent of disease and
biologic features is central to the treatment of breast cancer. Several
factors contribute to the determination of the disease staging,
recurrence risk assessment, and predictive response (ie, ER, PR,
HER2). The excised tissue detailing the written pathology report details
these key factors. The accuracy of pathology reporting requires
communication between the clinician and the pathologist relating
pertinent patient history, prior breast biopsies, prior chest irradiation,
pregnancy status, biopsy characteristics (ie, palpable,
mammographically detected microcalcifications), clinical state of lymph
nodes, presence of inflammatory change or other skin abnormality, and
any prior treatment administered (ie, chemotherapy, radiation therapy).
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-14
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
The specimens should be oriented for the pathologist, and specific
requests for determination of biomarkers should be stated (eg, ER, PR,
and HER2 status). Data from both national and local surveys show that
as many as 50% of pathology reports for breast cancer are missing
some elements critical to patient management.12,13 Significant omissions
include failure to orient and report surgical margins and failure to report
tumor grade consistently. CAP has developed pathology reporting
protocols to promote complete and standardized reporting of malignant
specimens (www.cap.org). The NCCN Breast Cancer Panel endorses
the use of the CAP protocols for reporting the pathologic analysis of all
breast cancer specimens.
Genetic counseling: For patients considered to be at high risk for
hereditary breast cancer as defined by the NCCN Guidelines for
Genetic/Familial High-Risk Assessment: Breast and Ovarian, genetic
counseling is recommended
Distress Assessment: Levels of distress may vary in patients and
should be addressed individually. Psychological distress can be
impacted by body image and other factors. Younger women have
higher rates of psychosocial distress than women diagnosed at older
ages.97-101 The NCCN Breast Cancer Panel recommends accessing for
distress in patients newly diagnosed with breast cancer.
Fertility Counseling: Numerous epidemiologic studies have
demonstrated that child-bearing after treatment for invasive breast
cancer does not increase rates of recurrence or death from breast
cancer.102 The offspring of pregnancies after treatment for breast cancer
do not have an increased rate of birth defects or other serious childhood
illness. However, treatment for breast cancer, especially with cytotoxic
agents, may impair fertility.
Many women, especially those younger than age 35, regain menstrual
function within 2 years of completing chemotherapy.103 Resumption of
menses does not necessarily correlate with fertility, and fertility may be
preserved without menses. All premenopausal patients should be
informed about the potential impact of chemotherapy on fertility and
asked about their desire for potential future pregnancies.
A decision for fertility preservation should include multiple factors such
as patient preference, tumor stage and biology, age of the patient, risk
of premature ovarian failure based on anticipated type and duration of
chemotherapy and/or endocrine therapy, as well as the timing and
duration allowed for fertility preservation.
Several studies report lower rates of fertility discussion among female
patients with cancer104-106 despite the updated ASCO guidelines stating
that patients should not be excluded from consideration for discussion
of fertility preservation for any reason, including parity, prognosis, age,
and socioeconomic status.107 The NCCN Panel recommends that all
women of childbearing potential should have a discussion with their
treating physicians. Patients who desire to bear children after systemic
therapy should be referred to a fertility specialist prior to initiating
systemic (chemotherapy or endocrine) therapy.107-113
Randomized trials have demonstrated that GnRH agonists (such as
goserelin) administered prior to initiating chemotherapy and then
administered concurrently with adjuvant chemotherapy protect against
ovarian failure and reduce the risk of early menopause.114-117 In one trial
goserelin improved the probability of pregnancy from 11% to 21% in
patients with hormone receptor-negative early-stage breast cancer.117
Smaller historical experiences in patients with hormone receptor-
positive disease have conflicting results with respect to the protective
effects of GnRH agonists in fertility preservation.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-15
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
Patients should be informed of all the various modalities available to
minimize gonadal damage and preserve ovarian function and future
fertility. The fertility specialist should discuss specifics of fertility
preservation options inclusive of types of hormonal interventions and
risks involved with ovarian stimulation, embryo or oocyte
cryopreservation, and other investigational options, as well as the
probability of successful gestation and childbirth.118,119
Combining the various modalities for a specific patient may increase the
odds of preservation of future fertility. It is important for fetal safety that
women actively avoid becoming pregnant during breast cancer
treatment. Also see NCCN Guidelines for Adolescent and Young Adult
Oncology.
Additional Workup
The panel has re-iterated that routine systemic imaging is not indicated
for patients with early breast cancer in the absence of signs/symptoms
of metastatic disease.120 These recommendations are based on studies
showing no additional value of these tests in patients with early-stage
disease.121-123 In one study, metastases were identified by bone scan in
5.1%, 5.6%, and 14% of patients with stage I, II, and III disease,
respectively, and no evidence of metastasis was detected by liver
ultrasonography or chest radiography in patients with stage I or II
disease.121 For patients with stage III breast cancer, the prevalence of a
positive liver ultrasound and positive chest x-ray was 6% and 7%,
respectively.121
For patients presenting with disease confined to the breast (stage I to II)
the NCCN Panel does not recommend routine systemic imaging in the
absence of signs or symptoms suspicious for metastatic disease.
According to the panel, additional tests may be considered in patients
who present with locally advanced (T3 N1-3 M0) disease and in those
with signs or symptoms suspicious for metastatic disease.
CBCs and LFTs may be considered if the patient is a candidate for
preoperative systemic therapy, or if otherwise clinically indicated.
Additional tests may be considered only based on the signs and
symptoms.
A chest diagnostic CT is indicated only if pulmonary symptoms (ie,
cough or hemoptysis) are present. Likewise, abdominal imaging using
diagnostic CT or MRI is indicated if the patient has elevated alkaline
phosphatase, abnormal results on LFTs, abdominal symptoms, or
abnormal physical examination of the abdomen or pelvis.
A bone scan is indicated in patients presenting with localized bone pain
or elevated alkaline phosphatase. The use of PET or PET/CT scanning
is not indicated in the staging of clinical stage I, II, or operable III (T3
N1) breast cancer. The recommendation against the use of PET
scanning is supported by the high false-negative rate in the detection of
lesions that are small (<1 cm) and/or low grade, the low sensitivity for
detection of axillary nodal metastases, the low prior probability of these
patients having detectable metastatic disease, and the high rate of
false-positive scans.124-127
FDG PET/CT is most helpful in situations where standard staging
studies are equivocal or suspicious, especially in the setting of locally
advanced or metastatic disease.
Locoregional Treatment
Surgery
In general, patients with early-stage breast cancer undergo primary
surgery (lumpectomy or mastectomy) with or without radiation therapy.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-16
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
Following local treatment, adjuvant systemic therapy may be offered
based on primary tumor characteristics, such as tumor size, grade,
lymph node involvement, ER/PR status, and expression of HER2-
receptor.
Several randomized trials document that mastectomy is equivalent to
breast-conserving therapy (lumpectomy with whole breast irradiation)
with respect to survival as primary breast local treatment for the majority
of women with stage I and stage II breast cancers (category 1).128-132
After surgical resection, a careful histologic assessment of resection
margins is essential. The NCCN Panel notes that benefit of lumpectomy
is predicated on achieving pathologically negative margins after
resection. The NCCN Panel accepts the most recent definition outlined
in the guidelines established by the Society of Surgical Oncology
(SSO)/American Society for Radiation Oncology (ASTRO) of no ink on
a tumor as the standard for negative surgical margins for invasive
cancer (with or without a component of DCIS).133
If margins remain positive after further surgical re-excision(s), then
mastectomy may be required for optimal local disease control.
In order to adequately assess margins following surgery, the panel
recommends that the surgical specimens be directionally oriented and
that the pathologist provide descriptions of the gross and microscopic
margin status and the distance, orientation, and type of tumor (invasive
cancer or pure DCIS) in relation to the closest margin. Marking the
tumor bed with clips facilitates accurate planning of the radiation boost
field, where appropriate. It may be reasonable to treat selected patients
with invasive cancer (without extensive intraductal component) despite
a microscopically focally positive margin with breast conservation
therapy.
Breast-Conserving Therapy (Lumpectomy)
Lumpectomy allows patients to preserve their breast without sacrificing
oncologic outcome. Lumpectomy is contraindicated for patients who are
pregnant and would require radiation during pregnancy; have diffuse
suspicious or malignant-appearing microcalcifications on
mammography; have widespread disease that cannot be incorporated
by local excision through a single incision with a satisfactory cosmetic
result; or have diffusely positive pathologic margins. Relative
contraindications to lumpectomy include previous radiation therapy to
the breast or chest wall; active connective tissue disease involving the
skin (especially scleroderma and lupus), tumors greater than 5 cm
(category 2B), and positive pathologic margins.
Several studies of women with early-stage breast cancer treated with
lumpectomy have identified young age as a significant predictor of an
increased likelihood of ipsilateral breast tumor recurrences after
lumpectomy.134-136 Risk factors, such as a family history of breast cancer
or a genetic predisposition to breast cancer (ie, BRCA1/2 or other
cancer-predisposing mutation), are more likely to exist in the population
of young women with breast cancer, thereby confounding the
independent contributions of age and treatment to clinical outcome.137
Studies have shown that survival outcomes for young women with
breast cancer receiving either lumpectomy or mastectomy are
similar.130,131,138-140 Some recent studies show improved survival141-143 and
fewer post-surgical complications144 with lumpectomy.
Mastectomy
Mastectomy is indicated for patients who are not candidates for
lumpectomy and those who choose to undergo this procedure over
lumpectomy.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-17
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
Only limited data are available on the survival impact of risk-reducing
contralateral mastectomy in women with a unilateral breast cancer.145
Analysis of women included in the SEER database treated with
mastectomy for a unilateral breast cancer from 1998 to 2003 showed
that contralateral mastectomy performed at the time of treatment of a
unilateral cancer was associated with a reduction in breast
cancer-specific mortality only in the population of young women (18–49
years of age) with stage I/II, ER-negative breast cancer (HR, 0.68; 95%
CI, 0.53–0.88; P = .004).146 The 5-year breast cancer survival for this
group was slightly improved with contralateral mastectomy versus
without (88.5% vs. 83.7%, difference = 4.8%).146 These differences
observed in retrospective analysis could be due to selection bias among
patients who chose risk-reducing contralateral mastectomy.147 A
statistical simulation of survival outcomes after risk-reducing
contralateral mastectomy among women with stage I or II breast cancer
with no BRCA mutation found that the absolute 20-year survival benefit
from risk-reducing contralateral mastectomy was less than 1% among
all age, ER status, and cancer stage groups.148 Data from a recent
meta-analysis found no absolute reduction in risk of distant metastases
with risk-reduction mastectomy.149 Furthermore, among patients with
unilateral breast cancer who have an increased familial/genetic risk,
although a decrease in metastatic contralateral breast cancer incidence
was observed in those who received risk-reducing contralateral
mastectomy, no improvement was seen in OS of these patients.149
The panel recommends that women with breast cancer who are less
than or equal to 35 years or premenopausal and carriers of a known
BRCA1/2 mutation consider additional risk reduction strategies following
appropriate risk assessment and counseling (see NCCN Guidelines for
Breast Risk Reduction and NCCN Guidelines for Genetic/Familial
High-Risk Assessment: Breast and Ovarian). This process should
involve multidisciplinary consultations prior to surgery, and should
include a discussion of the risks associated with development of a
contralateral breast cancer as compared with the risks associated with
recurrent disease from the primary cancer. Except as specifically
outlined in these guidelines, risk-reduction mastectomy of a breast
contralateral to a known unilateral breast cancer treated with
mastectomy is discouraged by the panel. The use of a prophylactic
mastectomy contralateral to a breast treated with lumpectomy is very
strongly discouraged in all patients.
The NCCN Panel recommends referring to the NCCN Guidelines for
Older Adult Oncology for special considerations for this population.
Surgical Axillary Staging
The NCCN Guidelines for Breast Cancer include a section for surgical
staging of the axilla for stages I, IIA, IIB, and IIIA (T3 N1 M0) breast
cancer. Pathologic confirmation of malignancy using ultrasound-guided
fine-needle aspiration (FNA)150 or core biopsy must be considered in
patients with clinically positive nodes to determine whether ALN
dissection is needed.
Performance of SLN mapping and resection in the surgical staging of
the clinically negative axilla is recommended and preferred by the panel
for assessment of the pathologic status of the ALNs in patients with
clinical stage I, stage II, and stage IIIA (T3 N1 M0) breast cancer.51,151-159
This recommendation is supported by results of randomized clinical
trials showing decreased arm and shoulder morbidity (ie, pain,
lymphedema, sensory loss) in patients with breast cancer undergoing
SLN biopsy compared with patients undergoing standard ALN
dissection.159,160 No significant differences in the effectiveness of the
SLN procedure or level I and II dissection in determining the presence
or absence of metastases in axillary nodes were seen in these studies.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-18
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
However, not all women are candidates for SLN resection. An
experienced SLN team is mandatory for the use of SLN mapping and
excision.161,162 Women who have clinical stage I or II disease and do not
have immediate access to an experienced SLN team should be referred
to an experienced SLN team for the definitive surgical treatment of the
breast and surgical ALN staging. In addition, potential candidates for
SLN mapping and excision should have clinically negative ALNs at the
time of diagnosis, or a negative core or FNA biopsy of any clinically
suspicious ALN(s). SLNs can be are assessed for the presence of
metastases by both hematoxylin and eosin (H&E) staining and
cytokeratin IHC. The clinical significance of a lymph node that is
negative by H&E staining but positive by cytokeratin IHC is not clear.
Because the historical and clinical trial data on which treatment
decisions are based have relied on H&E staining, the panel does not
recommend routine cytokeratin IHC to define node involvement and
believes that current treatment decisions should be made based solely
on H&E staining. This recommendation is further supported by a
randomized clinical trial (ACOSOG Z0010) for patients with H&E
negative nodes where further examination by cytokeratin IHC was not
associated with improved OS over a median of 6.3 years.163 In the
uncommon situation in which H&E staining is equivocal, reliance on the
results of cytokeratin IHC is appropriate. Multiple attempts have been
made to identify cohorts of women with involved SLNs who have a low
enough risk for non-SLN involvement that complete axillary dissection
might be avoided if the SLN is positive. None of the early studies
identified a low-risk group of patients with positive SLN biopsies but
consistently negative non-sentinel nodes.164-170 A randomized trial
(ACOSOG Z0011) compared SLN resection alone with ALN dissection
in women greater than or equal to 18 years of age with T1/T2 tumors,
fewer than 3 positive SLNs, and undergoing breast-conserving surgery
and whole breast irradiation. In this study, there was no difference in
local recurrence, DFS, or OS between the two treatment groups. Only
ER-negative status, age less than 50, and lack of adjuvant systemic
therapy were associated with decreased OS.171 At a median follow-up of
6.3 years, locoregional recurrences were noted in 4.1% of the ALN
dissection group (n = 420) and 2.8% of the SLN dissection patients (n =
436) (P = .11). Median OS was approximately 92% in each group.172
Therefore, based on these results after SLN mapping and excision, if a
patient has a T1 or T2 tumor with 1 to 2 positive SLNs, did not receive
preoperative systemic therapy, was treated with lumpectomy, and will
receive whole breast radiation, the panel recommends no further
axillary surgery.
The panel recommends level I or II axillary dissection when 1) patients
have clinically positive nodes at the time of diagnosis that is confirmed
by FNA or core biopsy; or 2) sentinel nodes are not identified. For
patients with clinically negative axillae who are undergoing mastectomy
and for whom radiation therapy is planned, the panel notes that axillary
radiation may replace axillary dissection level I/II for regional control of
disease.
Traditional level I and level II evaluation of ALN requires that at least 10
lymph nodes should be provided for pathologic evaluation to accurately
stage the axilla.173,174 ALN should be extended to include level III nodes
only if gross disease is apparent in the level II or III nodes. In the
absence of gross disease in level II nodes, lymph node dissection
should include tissue inferior to the axillary vein from the latissimus
dorsi muscle laterally to the medial border of the pectoralis minor
muscle (level I/II).
Furthermore, according to the panel, without definitive data
demonstrating superior survival with ALN dissection or SLN resection,
these procedures may be considered optional in patients who have
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-19
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
particularly favorable tumors, patients for whom the selection of
adjuvant systemic therapy will not be affected by the results of the
procedure, elderly patients, and patients with serious comorbid
conditions. Women who do not undergo ALN dissection or ALN
irradiation are at increased risk for ipsilateral lymph node recurrence.175
Radiation Therapy
Planning Techniques, Targets, and Doses
It is important to individualize radiation therapy planning and delivery.
CT-based treatment planning is encouraged to delineate target volumes
and adjacent organs at risk. Greater target dose homogeneity and
sparing of normal tissues can be accomplished using compensators
such as wedges, forward planning using segments, and intensity-
modulated radiation therapy (IMRT). Respiratory control techniques
including deep inspiration breath-hold and prone positioning may be
used to try to further reduce dose to adjacent normal tissues,
particularly heart and lung. Boost treatment in the setting of breast
conservation can be delivered using enface electrons, photons, or
brachytherapy. Chest wall scar boost when indicated is typically treated
with electrons or photons. Verification of daily setup consistency is done
with weekly imaging. In certain circumstances, more frequent imaging
may be appropriate. Routine use of daily imaging is not recommended.
Whole Breast Radiation
Whole breast radiation reduces the risk of local recurrence and has
shown to have a beneficial effect on survival.129,132 Randomized trials
have demonstrated decreased in-breast recurrences with an additional
boost dose of radiation (by photons, brachytherapy, or electron beam)
to the tumor bed.176,177 The panel recommends whole breast irradiation
to include breast tissue in its entirety. CT-based treatment planning is
recommended to limit irradiation exposure of the heart and lungs, and to
assure adequate coverage of the breast and lumpectomy site.
For greater homogeneity of target dose and to spare normal tissues
using compensators such as tissue wedges, forward planning using
segments, and IMRT may be used.178,179 Respiratory control techniques
including deep inspiration breath-hold and prone positioning may be
used to try to further reduce dose to adjacent normal tissues,
particularly heart and lung.180 Radiation boost treatment in the setting of
breast conservation can be delivered using enface electrons, photons,
or brachytherapy.
Dose and Fractionation
Four randomized clinical trials have investigated hypofractionated whole
breast radiation schedules (39–42.9 Gy in single fractions of 2.6–3.3
Gy) compared to standard 50 Gy in single fractions of 2 Gy.181-184 The
10-year follow-up data from the START trials185 are consistent with the
10-year results of the Canadian trial,184 which reported that local tumor
control and breast cosmesis were similar with a regimen of 42.5 Gy in
16 fractions over 3.2 weeks compared with 50 Gy in 25 fractions over 5
weeks.184 The START trials reported radiation-related effects to normal
breast tissue such as breast shrinkage, telangiectasia, and breast
edema as less common with the hypofractionated fraction regimen.185
The NCCN Panel recommends whole breast irradiation, a dose of 46 to
50 Gy in 23 to 25 fractions, or a dose of 40 to 42.5 Gy in 15 to 16
fractions. Based on convenience and the data from the START trials,185
the short course of radiation therapy (40–42.5 Gy in 15–16 fractions) is
the NCCN-preferred option for treatment of patients receiving radiation
therapy to the whole breast only. A boost to the tumor bed is
recommended in patients with higher risk characteristics (such as age
<50, high-grade disease, or patients with focally positive margins) in
order to reduce local relapse.177,185-189 Typical boost doses are 10 to 16
Gy in 4 to 8 fractions.
Chest Wall Radiation (Including Breast Reconstruction)
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-20
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
The target includes the ipsilateral chest wall, mastectomy scar, and
drain sites when indicated. Depending on whether the patient has had
breast reconstruction, several techniques using photons and/or
electrons are appropriate. The NCCN Panel recommends a dose of 46
to 50 Gy in 23 to 25 fractions to the chest wall. A boost at the scar with
a dose of 2 Gy per fraction to a total dose of approximately 60 Gy may
be considered in some cases based on risk.
Regional Nodal Irradiation
The NCCN Guidelines include updated recommendations for regional
lymph node irradiation in patients treated with lumpectomy and
mastectomy depending on lymph node involvement (see Principles of
Radiation Therapy in the NCCN Guidelines for Breast Cancer).
Two studies, MA.20 and EORTC 22922/10925, evaluated the addition
of regional nodal irradiation to the internal mammary nodes and the
upper axillary nodes including the supraclavicular region, in addition to
whole breast irradiation or chest wall irradiation after lumpectomy or
mastectomy, respectively. In MA.20, regional recurrences were reduced
from 2.7% with breast irradiation only to 0.7% with the addition of nodal
irradiation.190 The distant recurrences were reduced from 17.3% to
13.4%.190 An improvement in DFS was seen from 77% to 82% at 10
years in those who received regional nodal irradiation compared to
those who did not.190 In EORTC 22922/10925, regional radiation
therapy reduced the incidence of regional recurrences from 4.2% to
2.7% and decreased the rate of distant metastases from 19.6% to
15.9% at a median follow-up of 10.9 years.191
Accelerated Partial Breast Irradiation
Several studies have been reported using accelerated partial breast
irradiation (APBI) rather than whole breast irradiation following complete
surgical excision of in-breast disease. The panel generally views the
use of APBI as investigational, and encourages its use within the
confines of a high-quality, prospective clinical trial.192 For patients who
are not trial eligible, recommendations from ASTRO indicate that APBI
may be suitable in selected patients with early-stage breast cancer and
may be comparable to treatment with standard whole-breast RT.193
Patients who may be suitable for APBI are women 60 years of age and
older who are not carriers of a known BRCA1/2 mutation and who have
been treated with primary surgery for a unifocal stage I, ER-positive
cancer. Tumors should be infiltrating ductal or have a favorable
histology, should not be associated with an extensive intraductal
component or LCIS, and should have negative margins. Thirty-four Gy
in 10 fractions delivered twice per day with brachytherapy or 38.5 Gy in
10 fractions delivered twice per day with external beam photon therapy
to the tumor bed is recommended. Other fractionation schemes are
under investigation. Studies have suggested that the ASTRO
stratification guidelines may not adequately predict ipsilateral breast
tumor recurrences following APBI.194,195 Follow-up is limited and studies
are ongoing.
Radiation Therapy in Patients Receiving Preoperative Systemic
Therapy
The panel recommends that decisions related to administration of
radiation therapy for patients receiving preoperative systemic
chemotherapy should be made based on maximal stage from
pre-chemotherapy tumor characteristics and/or pathological stage,
irrespective of tumor response to preoperative systemic therapy.
Radiation Therapy After Lumpectomy
After lumpectomy, whole breast irradiation is strongly recommended
with or without boost to tumor bed for node-positive disease (category 1
for those with positive nodes; category 2A for those with negative
axillary nodes). This recommendation is supported by the results of a
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-21
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
meta-analysis by the EBCTCG showing reduction in 10-year risk of
recurrence in those who received whole breast irradiation versus those
who did not (19% vs. 35%; RR 0.52; 95% CI, 0.48–0.56).132 In addition,
a significant reduction in 15-year risk of breast cancer death (21% vs.
25%; RR 0.82; 95% CI, 0.75–0.90) was also observed.132
Regional Nodal Irradiation
The reduction in the risk of locoregional and distant recurrence and
improvement in DFS seen in the MA.20 and EORTC 22922/10925 trials
support the importance of regional nodal irradiation after
lumpectomy.190,191 The NCCN Panel strongly recommends irradiation of
infraclavicular and supraclavicular areas, internal mammary nodes, and
any part of the axillary bed that may be suspicious (category 1 for ≥4
positive nodes). Irradiation of the regional nodal area is generally not
recommended by the panel for those with negative axillary nodes.
If adjuvant chemotherapy is indicated after lumpectomy, radiation
should be given after chemotherapy is completed.196,197 This
recommendation is based on results of the “Upfront-Outback” trial in
which patients who had undergone breast-conserving surgery and
axillary dissection were randomly assigned to receive chemotherapy
following radiation therapy or radiation therapy following chemotherapy.
The initial results showed an increased rate of local recurrence in the
group with delayed radiotherapy at a median follow-up of 58 months;197
however, differences in rates of distant or local recurrence were not
statistically significant when the two arms were compared at 135-month
follow-up.196
Radiation Therapy After Lumpectomy in Older Adults
Whole breast irradiation as a component of breast-conserving therapy is
not always necessary in selected women 70 years of age or older. In a
study of women with clinical stage I, ER-positive breast cancer who
were greater than or equal to 70 years of age at diagnosis, patients
were randomized to receive lumpectomy with whole breast radiation or
lumpectomy alone, both with tamoxifen for five years. Locoregional
recurrence rates were 1% in the lumpectomy, radiation, and tamoxifen
arm and 4% in the lumpectomy plus tamoxifen arm. There were no
differences in OS, DFS, or need for mastectomy.198 These results were
confirmed in an updated analysis of this study with a median follow-up
of 12.6 years.199 At 10 years, a statistically significant reduction in
ipsilateral breast recurrences was seen with radiation therapy with 90%
of patients in the lumpectomy and tamoxifen arm compared with 98% in
the lumpectomy plus radiation and tamoxifen arm who were free from
locoregional recurrence.199 Similar results were obtained in other studies
of similar design.200,201 Whether the difference in tumor control is
clinically significant and the patient receives breast radiotherapy should
be individualized based upon discussion between the patient and her
care team.
The NCCN Guidelines allow for the use of lumpectomy (pathologically
negative margin required) plus tamoxifen or an aromatase inhibitor
without breast irradiation in women greater than or equal to 70 years of
age with clinically negative lymph nodes and ER-positive, T1 breast
cancer (category 1).
Radiation Therapy After Mastectomy
Node-Positive Disease: Randomized clinical trials have shown that a
DFS and OS advantage is conferred by the irradiation of chest wall and
regional lymph nodes in women with positive ALNs after mastectomy
and ALN dissection.202-206 In these trials, the ipsilateral chest wall and
the ipsilateral locoregional lymph nodes were irradiated. The results of
EBCTCG meta-analyses207 show that radiotherapy after mastectomy
and axillary node dissection reduced both recurrence and breast cancer
mortality in the women with 1 to 3 positive lymph nodes even when
systemic therapy was administered.191 Based on these studies, the
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-22
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
current guidelines recommend postmastectomy chest wall irradiation in
women with positive ALNs (category 1). Two retrospective analyses
have provided evidence for benefit of radiation therapy for only selected
patients (patients presenting with clinical stage III disease and patients
with four or more positive nodes) receiving preoperative systemic
therapy prior to mastectomy.208,209
Regional Nodal Irradiation
The use of regional nodal irradiation for patients undergoing
mastectomy is supported by a subgroup analysis of studies from the
Danish Breast Cancer Cooperative Group.210 In this analysis, a
substantial survival benefit was associated with postmastectomy
radiation therapy for women with 1 to 3 positive ALNs. In addition, data
from the EORTC 22922/10925 trial supports the role of regional RT in
this population based on the inclusion of patients who had undergone
mastectomy in this study. Based on the above data, the NCCN Panel
recommends irradiation of infraclavicular and supraclavicular areas,
internal mammary nodes, and any part of the axillary bed that may be
suspicious (category 1 for ≥4 positive nodes; 2A for 1–3 positive nodes).
Node-Negative Disease: Features in node-negative tumors that predict
a high rate of local recurrence include primary tumors greater than 5 cm
or positive pathologic margins. Chest wall irradiation is recommended
for these patients.211 Consideration should be given to radiation to the
ipsilateral supraclavicular area and to the ipsilateral internal mammary
lymph nodes, especially in patients with tumors greater than 5 cm, or
positive surgical margins. In patients with tumors less than or equal to 5
cm and negative margins but less than or equal to 1 mm, chest wall
irradiation should be considered.
In patients with negative nodes, tumor less than or equal to 5 cm, and
clear margins (≥1 mm), post-mastectomy radiation therapy is usually
not recommended. However, the panel has noted that it may be
considered only for patients with high risk of recurrence. A retrospective
analysis suggests benefit of post-mastectomy radiation therapy in
reducing risk of recurrence in patients with node-negative disease with
high-risk factors such as close margins, tumors greater than or equal to
2 cm, premenopausal status, and lymphovascular invasion.212 Another
study showed increased risk of locoregional recurrence in women with
node-negative triple-negative breast cancer with tumors less than or
equal to 5 cm.213
Breast Reconstruction
Breast reconstruction may be an option for any woman receiving
surgical treatment for breast cancer. Therefore, all women undergoing
breast cancer treatment should be educated about breast reconstructive
options as adapted to their individual clinical situation and be offered an
opportunity to consult with a reconstructive plastic surgeon. Breast
reconstruction should not interfere with the appropriate surgical
management. This may increase the risk of overall and cancer-related
death, especially in those with late-stage disease.214 Coordinating
consultation and surgical treatment with a reconstructive surgeon
should be executed within a reasonable timeframe.
Several reconstructive approaches are summarized for these patients in
the NCCN Guidelines for Breast Cancer under Principles of Breast
Reconstruction Following Surgery.
The decision regarding type of reconstruction includes patient
preference, body habitus, smoking history, comorbidities, plans for
irradiation, and expertise and experience of the reconstruction team.
Smoking and obesity increase the risk of complications for all types of
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-23
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
breast reconstruction whether with implant or flap.215-219 Smoking and
obesity are therefore considered a relative contraindication to breast
reconstruction by the NCCN Panel. Patients should be informed of
increased rates of wound healing complications and partial or complete
flap failure among smokers and obese patients.
Reconstruction is an optional procedure that does not impact the
probability of recurrence or death, but it is associated with an improved
quality of life for many patients. It is sometimes necessary to perform
surgery on the contralateral breast (ie, breast reduction, implantation) to
achieve optimal symmetry between the ipsilateral reconstructed breast
and the contralateral breast.
Breast Reconstruction After Mastectomy
Mastectomy results in loss of the breast for breastfeeding, loss of
sensation in the skin of the breast and nipple-areolar complex (NAC),
and loss of the breast for cosmetic, body image, and psychosocial
purposes. The loss of the breast for cosmetic, body image, and
psychosocial issues may be partially overcome through the
performance of breast reconstruction with or without reconstruction of
the NAC.
Women undergoing mastectomy should be offered consultation
regarding options and timing of breast reconstruction.
Many factors must be considered in the decision-making about breast
reconstruction. There are several different types of breast
reconstruction that include the use of implants, autogenous tissues, or
both.220-222 Reconstruction with implants can be performed either by
immediate placement of a permanent subpectoral implant or initial
placement of a subpectoral expander implant followed by gradual
expansion of the implant envelope with stretching of the pectoralis
major muscle and overlying skin followed by replacement of the
expander with a permanent implant. A wide variety of implants are
available that contain saline, silicone gel, or a combination of saline and
silicone gel inside a solid silicone envelope.
Autogenous tissue methods of reconstruction use various combinations
of fat, muscle, skin, and vasculature from donor sites (ie, abdomen,
buttock, back) that may be brought to the chest wall with their original
blood supply (pedicle flap) or as free flaps with microvascular
anastomoses to supply blood from the chest wall/thorax.223 Several
procedures using autologous tissue are available including transverse
rectus abdominis myocutaneous flap, latissimus dorsi flap, and gluteus
maximus myocutaneous flap reconstruction.
Composite reconstruction techniques use implants in combination with
autogenous tissue reconstruction to provide volume and symmetry.
Patients with underlying diabetes or who smoke tobacco have
increased rates of complications following autogenous tissue breast
cancer reconstruction, presumably because of underlying microvascular
disease.
Reconstruction can be performed either at the time of the mastectomy
known as “immediate breast reconstruction” and under the same
anesthetic or in a delayed fashion any time, known as “delayed breast
reconstruction.” In many cases, breast reconstruction involves a staged
approach requiring more than one procedure such as surgery on the
contralateral breast to improve symmetry, revision surgery involving the
breast and/or donor site, and/or nipple and areola reconstruction and
tattoo pigmentation.
Plans for post-mastectomy radiation therapy can impact decisions
related to breast reconstruction since there is a significantly increased
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-24
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
risk of implant capsular contracture following irradiation of an implant.
Furthermore, postmastectomy irradiation may have a negative impact
on breast cosmesis when autologous tissue is used in immediate breast
reconstruction, and may interfere with the targeted delivery of radiation
when immediate reconstruction is performed using either autologous
tissue or breast implants.224,225 Some studies, however, have not found a
significant compromise in reconstruction cosmesis after radiation
therapy.226 The preferred approach to breast reconstruction for
irradiated patients was a subject of controversy among the panel. While
some experienced breast cancer teams have employed protocols in
which immediate tissue reconstructions are followed by radiation
therapy, generally radiation therapy is preferred to precede autologous
reconstruction due to the reported loss in reconstruction cosmesis
(category 2B). When implant reconstruction is planned in a post
mastectomy patient requiring radiation therapy, the NCCN Panel prefers
a staged approach with immediate tissue expander placement followed
by implant placement. Immediate placement of an implant in patients
requiring postoperative radiation has an increased rate of capsular
contracture, malposition, poor cosmesis, and implant exposure. Surgery
to exchange the tissue expanders with permanent implants can be
performed prior to radiation or after completion of radiation therapy.
In a previously radiated patient, the use of tissue expanders/implants is
relatively contraindicated.227 Tissue expansion of irradiated skin can
result in a significantly increased risk of capsular contracture,
malposition, poor cosmesis, implant exposure, and failed
reconstruction.228,229 If a patient has previously received radiation therapy
to the breast, autologous tissue reconstruction is the preferred method
of breast reconstruction.
Skin-sparing Mastectomy
Skin-sparing mastectomy procedures are appropriate for some patients
and involve removal of the breast parenchyma including the NAC while
preserving the majority of the original skin envelope, and are followed
by immediate reconstruction with autogenous tissue, a prosthetic
implant, or a composite of autogenous tissue and an implant.
Skin-sparing mastectomy involving preservation of the skin of the NAC
has become the subject of increased attention. Possible advantages of
this procedure include improvements in breast cosmesis, body image,
and nipple sensation following mastectomy, although the impact of this
procedure on these quality-of-life issues has not been well-studied.230-232
There are limited data from surgical series, with short follow-up, that
suggest that performance of NAC-sparing mastectomy in selected
patients is associated with low rates of occult involvement of the NAC
with breast cancer and local disease recurrence.231,233,234 NAC-sparing
procedures may be an option in patients who are carefully selected by
experienced multidisciplinary teams. According to the NCCN Panel,
when considering a NAC-sparing procedure, assessment of nipple
margins is mandatory. Retrospective data support the use of NAC-
sparing procedures for patients with breast cancer with low rates of
nipple involvement and low rates of local recurrence due to early-stage,
biologically favorable (ie, Nottingham grade I or 2, node-negative,
HER2-negative, no lymphovascular invasion) invasive cancers and/or
DCIS that are peripherally located in the breast (>2 cm from
nipple).235,236 Contraindications for nipple preservation include evidence
of nipple involvement such as Paget’s disease or other nipple discharge
associated with malignancy and/or imaging findings suggesting
malignant involvement of nipple and subareolar tissues. Several
prospective trials are underway to evaluate NAC-sparing mastectomy in
the setting of cancer and enrollment in such trials is encouraged.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-25
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
Advantages of a skin-sparing mastectomy procedure include an
improved cosmetic outcome resulting in a reduction in the size of the
mastectomy scar and a more natural breast shape, especially when
autologous tissue is used in reconstruction,237 and the ability to perform
immediate reconstruction. Although no randomized studies have been
performed, results of several mostly retrospective studies have
indicated that the risk of local recurrence is not increased when patients
receiving skin-sparing mastectomies are compared with those
undergoing non-skin–sparing procedures. However, strong selection
biases almost certainly exist in the identification of patients appropriate
for skin-sparing procedures.238-242 Reconstruction of the NAC may also
be performed in a delayed fashion if desired by the patient.
Reconstructed nipples are devoid of sensation. According to the NCCN
Panel, skin-sparing mastectomy should be performed by an
experienced breast surgery team that works in a coordinated,
multidisciplinary fashion to guide proper patient selection for skin-
sparing mastectomy, determine optimal sequencing of the
reconstructive procedure(s) in relation to adjuvant therapies, and
perform a resection that achieves appropriate surgical margins. Post-
mastectomy radiation should still be applied for patients treated by skin-
sparing mastectomy following the same selection criteria as for
standard mastectomy.
Breast Reconstruction After Lumpectomy
Issues related to breast reconstruction also pertain to women who
undergo or have undergone a lumpectomy, particularly in situations
where the surgical defect is large and/or expected to be cosmetically
unsatisfactory. An evaluation of the likely cosmetic outcome of
lumpectomy should be performed prior to surgery. Oncoplastic
techniques for breast conservation can extend breast-conserving
surgical options in situations where the resection by itself would likely
yield an unacceptable cosmetic outcome.243 The evolving field of
oncoplastic surgery includes the use of “volume displacement”
techniques performed in conjunction with a large partial mastectomy.244
Oncoplastic volume displacement procedures combine the removal of
generous regions of breast tissue (typically designed to conform to the
segmentally distributed cancer in the breast) with “mastopexy”
techniques in which remaining breast tissues are shifted together within
the breast envelope to fill the resulting surgical defect and thereby avoid
the creation of significant breast deformity. Volume displacement
techniques are generally performed during the same operative setting
as the breast-conserving lumpectomy by the same surgeon who is
performing the cancer resection.244,245
Advantages of oncoplastic volume displacement techniques are that
they permit the removal of larger regions of breast tissue, thereby
achieving wider surgical margins around the cancer, and at the same
time better preserve the natural shape and appearance of the breast
than do standard breast resections.246
Limitations of oncoplastic volume displacement techniques include lack
of standardization among centers, performance at only a limited number
of sites in the United States, and the possible necessity for subsequent
mastectomy if pathologic margins are positive when further
breast-conserving attempts are deemed impractical or unrealistic.
Nevertheless, the consensus of the panel is that these issues should be
considered prior to surgery for women who are likely to have a surgical
defect that is cosmetically unsatisfactory, and that women who undergo
lumpectomy and are dissatisfied with the cosmetic outcome after
treatment should be offered a consultation with a plastic surgeon to
address the repair of resulting breast defects. Patients should be
informed of the possibility of positive margins and potential need for
secondary surgery, which could include re-excision segmental
resection, or could require mastectomy with or without loss of the
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-26
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
nipple. Oncoplastic procedures can be combined with surgery on the
contralateral unaffected breast to minimize long-term asymmetry.
Finally, decisions regarding breast reconstruction should primarily focus
on treatment of the tumor, and such treatment should not be
compromised.
Systemic Therapies (Preoperative and Adjuvant)
Principles of Preoperative Systemic Therapy
The NCCN Panel has outlined the rationale, appropriate patient
selection, and response assessment for preoperative systemic therapy
in a new section titled, Principles of Preoperative Chemotherapy.
Rationale for Preoperative Chemotherapy
Randomized clinical trials have found no significant differences in long-
term outcomes when systemic chemotherapy is given before or after
surgery.297,298 Historically, a primary advantage of administering
preoperative systemic therapy has been to improve surgical outcomes.
Preoperative systemic therapy can render inoperable tumors resectable
and also downstage patients with operable breast cancer desiring
breast conservation.299 Results from large clinical trials and
retrospective reviews indicate that breast conservation rates are
improved with preoperative systemic therapy.298,300 Clinicians need to
carefully consider the extent of disease in the breast and likelihood of
adequate tumor response before recommending preoperative systemic
therapy to improve the likelihood of successful breast conservation.
In addition, use of preoperative systemic therapy may provide important
prognostic information based on response to therapy. Achieving a
pathologic complete response (pCR) to neoadjuvant therapy is
associated with favorable disease-free and OS in early-stage breast
cancer. The correlation between pathologic response and long-term
outcomes in patients with early-stage breast cancer is strongest for
patients with triple-negative breast cancer, less so for HER2-positive
disease, and least for hormone-positive disease.301-303
Other benefits of preoperative systemic therapy include allowing time
for appropriate genetic testing and for planning breast reconstruction in
patients proceeding with mastectomy. For those with significant residual
disease after standard preoperative systemic therapy, it may provide an
opportunity to identify patients who are candidates for clinical trials of
novel agents in the adjuvant setting. To date, the tailoring of therapy
based on poor response to standard preoperative chemotherapy has
not yet demonstrated improved outcomes. In addition, preoperative
systemic therapy also serves as an excellent research platform to test
novel therapies and predictive biomarkers by providing tumor
specimens and blood samples prior to and during systemic treatment.
Selection of Patients for Preoperative Therapy
Not all patients are appropriate candidates for preoperative systemic
therapy. According to the NCCN Panel, among those with inoperable
breast tumors, preoperative systemic therapy is indicated in women with
locally advanced or inoperable breast cancer including those with
inflammatory breast cancer; those with N2 and N3 regional lymph node
nodal disease; and T4 tumors. In patients with operable breast cancer
who are clear candidates for adjuvant chemotherapy, preoperative
systemic therapy may be considered if a patient desires breast-
conserving surgery but the surgery is not possible due to the size of the
tumor relative to that of the breast, with the hope that this will help
obtain clear surgical margins at final resection. Preoperative systemic
therapy may also be administered in patients with operable tumors if the
patient’s breast cancer subtype is one associated with a high likelihood
of response. When preoperative systemic therapy is used to improve
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-27
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
the likelihood of successful breast conservation, the surgical plan
should consider the possibility that clear surgical margins may not
always be obtained, and a follow-up mastectomy may be required, with
or without breast reconstruction. This consideration is especially
important when oncoplastic breast reduction techniques or contralateral
breast symmetry procedures are added to the breast-conserving
surgery to achieve optimal cosmetic outcomes.
The NCCN Panel cautions that preoperative systemic therapy is not
appropriate for certain patients. Preoperative systemic therapy should
not be offered in patients with extensive in situ disease when the extent
of invasive disease cannot be defined; in patients where the extent of
the tumor is poorly delineated; or in those whose tumors are not
palpable or clinically assessable. The decision to utilize preoperative
therapy should be made in the context of a coordinated and
collaborative multi-disciplinary team.
Preoperative Systemic Therapy Options
Chemotherapy: A number of chemotherapy regimens have activity in
the preoperative setting. According to the NCCN Panel, those regimens
recommended in the adjuvant setting may be considered in the
preoperative setting. In both settings, the underlying aim remains the
same: eradication or control of undiscovered distant metastases.
Endocrine Therapy: Neoadjuvant endocrine therapy alone may be
offered to those with strongly hormone receptor-positive tumors.304-311
According to the NCCN Panel, the endocrine therapy options include an
aromatase inhibitor (with ovarian suppression for premenopausal
women) or tamoxifen. The preferred endocrine therapy option for
postmenopausal women is an aromatase inhibitor.
HER2 Targeted Therapy: For patients with HER2-positive breast
cancer, who are candidates for preoperative systemic therapy,
chemotherapy and trastuzumab-based therapy is recommended.312
Chemotherapy and dual anti-HER2 blockade associated with
trastuzumab plus pertuzumab has shown significant improvements in
the pCR rate when compared with chemotherapy and one anti-HER2
agent in the preoperative setting.313-315 In the Neosphere trial, the
addition of pertuzumab to trastuzumab and docetaxel preoperatively led
to a statistically significant increase in pCR in the breast (16.8%
increase; 95% CI, 3.5–30.1; P = .0141).315 In the TRYPHAENA trial,
preoperative therapy with pertuzumab and trastuzumab given along
with anthracycline-containing or anthracycline-free standard
chemotherapy regimens to patients with operable, locally advanced, or
inflammatory HER2-positive breast cancer showed pCR rates in all
treatment arms ranging from 57% to 66%.316 The mean change in left
ventricular ejection fraction was similar in all treatment arms.316 The
NCCN Panel supports the FDA-approved indication that a pertuzumab-
containing regimen may be administered preoperatively to patients with
greater than or equal to T2, or greater than or equal to N1, HER2-
positive, early-stage breast cancer.
Response Assessment During Preoperative Chemotherapy: The NCCN
panel recommends that tumor response should be routinely assessed
by clinical exam during the delivery of preoperative systemic therapy.
Patients with operable breast cancer experiencing progression of
disease while undergoing preoperative systemic therapy should be
taken promptly to surgery. Imaging during preoperative systemic
therapy should not be done routinely, but may be considered if tumor
progression is suspected. Imaging prior to surgery should be
determined by a multi-disciplinary team.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-28
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
Systemic Adjuvant Therapy
After surgical treatment, adjuvant systemic therapy should be
considered. The decision is often based on individual risk of relapse and
predicted sensitivity to a particular treatment (eg, ER/PR and HER2
status).
The published results of the EBCTCG overview analyses of adjuvant
chemotherapy and tamoxifen show convincing reductions in the odds of
recurrence and death in all age groups for chemotherapy and endocrine
therapy.4,247 Thus, the current guidelines recommend adjuvant therapy
without regard to patient age (category 1). The decision to use systemic
adjuvant therapy requires considering and balancing risk for disease
recurrence with local therapy alone, the magnitude of benefit from
applying adjuvant therapy, toxicity of the therapy, and comorbidity.248,249
The decision-making process requires collaboration between the health
care team and patient.
Estimating Risk of Relapse or Death and Benefits of Systemic
Treatment
Several prognostic factors predict for future recurrence or death from
breast cancer. The strongest prognostic factors are patient age,
comorbidity, tumor size, tumor grade, number of involved ALNs, and
possibly HER2 tumor status. Algorithms have been published
estimating rates of recurrence,248 and a validated, computer-based
model (Adjuvant! Online; www.adjuvantonline.com) is available to
estimate 10-year DFS and OS that incorporates all of the above
prognostic factors except for HER2 tumor status.249,250 These tools aid
the clinician in objectively estimating outcome with local treatment only,
and also assist in estimating the absolute benefits expected from
systemic adjuvant endocrine therapy and chemotherapy. These
estimates may be utilized by the clinician and patient in their shared
decision-making regarding the toxicities and benefits of systemic
adjuvant therapy.251
A determination of the HER2 status of the tumor is recommended for
prognostic purposes for patients with node-negative breast cancer.252
More importantly, HER2 tumor status also provides predictive
information used in selecting optimal adjuvant/neoadjuvant therapy and
in the selection of therapy for recurrent or metastatic disease (category
1). For example, retrospective analyses have demonstrated that
anthracycline-based adjuvant therapy is superior to non-anthracycline–
based adjuvant chemotherapy in patients with HER2-positive tumors,253-
257 and that the dose of doxorubicin may be important in the treatment of
tumors that are HER2-positive.258 Prospective evidence of the predictive
utility of HER2 status in early-stage 259-264 and metastatic breast
cancer265-267 is available for trastuzumab-containing therapies.
Use of DNA microarray technologies to characterize breast cancer has
allowed for development of classification systems of breast cancer by
gene expression profile.268 Five major subtypes of breast cancer have
been identified by DNA microarray gene expression profiling:
ER-positive/HER2-negative (luminal A and luminal B subtypes);
ER-negative/HER2-negative (basal subtype); HER2-positive; and
tumors that have characteristics similar to normal breast tissue.269-271 In
retrospective analyses, these gene expression subtypes are associated
with differing relapse-free survival and OS.
There are many gene-based assays to predict prognosis such as
distant recurrence, local recurrence, or survival.
The 21-gene assay using reverse transcription polymerase chain
reaction (RT-PCR) on RNA isolated from paraffin-embedded breast
cancer tissue is among the best-validated prognostic assays, and there
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-29
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
are data showing that it can predict who is most likely to respond to
systemic chemotherapy.
Studies have shown that the 21-gene assay recurrence score obtained
is predictive of locoregional and distant recurrence for postmenopausal
women treated with tamoxifen or those treated with an aromatase
inhibitor.272-274 Studies have also demonstrated the ability of the
recurrence score to independently predict response to adjuvant
chemotherapy.275-277 Unplanned, retrospective subset analysis from a
single randomized clinical trial in post-menopausal, ALN-positive,
ER-positive breast cancer found that the 21-gene RT-PCR assay may
provide predictive information for chemotherapy benefit in addition to
tamoxifen.275 Patients with a high score in the study benefited from
chemotherapy, whereas patients with a low score did not appear to
benefit from the addition of chemotherapy regardless of the number of
positive lymph nodes.275 Many other multi-gene or multi-gene
expression assay systems have been developed.
The 70-gene signature assay uses microarray technology to analyze
gene expression profile from breast tumor tissue (formalin-fixed,
paraffin-embedded fresh or frozen breast tumor tissue) to help identify
patients with early-stage breast cancer likely to develop distant
metastases.278-284 This assay is approved by the FDA to assist in
assignment of women with ER-positive or ER-negative breast cancer
into a high versus low risk for recurrence, but not for predicting benefit
from adjuvant systemic therapy. The prospective RASTER study
reported that breast cancer patients classified by the 70-gene signature
as low risk (of whom 85% did not receive adjuvant chemotherapy) had
an overall 97% distant recurrence-free interval at five years.285
Another assay with 50 genes identifies intrinsic breast cancer subtypes
(luminal A, luminal B, HER2 enriched and basal-like) in addition to
generating a risk of recurrence (ROR) score that can be used to predict
prognosis among postmenopausal women with hormone-positive breast
cancer. In a retrospective analysis of the ATAC trial,286 the ROR score
obtained using the 50-gene assay in postmenopausal patients treated
with adjuvant tamoxifen or anastrozole was seen to have a continuous
relationship with the risk of distant recurrence at 10 years in node-
negative and node-positive disease. The retrospective analysis also
compared the ROR score obtained using the 50-gene assay with the
recurrence score obtained using the 21-gene assay. Both assays
identified similar percentage of low-risk patients (hormone receptor-
positive, node-negative) with similar risk of recurrence. The ABCSG-8
trial showed that the ROR score provides prognostic information and
predicts the risk of distant recurrence in postmenopausal women with
ER-positive early-stage breast cancer.287 A recent combined analysis of
the ATAC and the ABCSG-8 trials reported ROR score as a strong
predictor of late distant recurrence (greater than 5 years) for patients
with hormone receptor-positive, node-negative disease.288The NCCN
Panel members acknowledge that many assays have been clinically
validated for prediction of prognosis. However, based on the currently
available data, the panel believes that the 21-gene assay has been best
validated for its use as a prognostic test as well as in predicting who is
most likely to respond to systemic chemotherapy.
Patients with a high recurrence score obtained using the 21-gene assay
clearly benefit from chemotherapy, whereas patients with a low score
do not appear to benefit from the addition of chemotherapy regardless
of the number of positive lymph nodes.275 The results from the
prospective TAILORx study support the use of the 21-gene assay to
spare the use of chemotherapy in patients with a low-risk score.289 In
patients with a low-risk score (≤10) at 5 years, the risk of the recurrence
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-30
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
of breast cancer at a distant site was less than 1% and the risk of any
recurrence was less than 2%.289
The additional benefit from adjuvant chemotherapy in addition to
endocrine therapy is currently unclear for patients with intermediate
recurrence score. The long-term follow-up results from the TAILORx
trial clarify the use of chemotherapy in women with hormone-receptor–
positive, HER2-negative, axillary node–negative invasive breast cancer
with mid-range 21-gene assay recurrence score (between 11–25).290
The ongoing RxPONDER trial is evaluating whether adjuvant
chemotherapy is beneficial in patients with hormone receptor-positive,
HER2-negative breast cancer with positive ALNs and a recurrence
score of 25 or less.291
The MINDACT trial is phase III trial comparing the 70-gene signature
with the commonly used clinicopathologic criteria in selecting patients
for adjuvant chemotherapy in breast cancer with 0 to 3 positive
nodes.292 The early results from the MINDACT trial suggest that the 70-
gene signature can help avoid chemotherapy in certain patients
regardless of larger tumor size and nodal status, without compromising
the outcome.293 Among the MINDACT trial patients, if decision on
administering adjuvant chemotherapy was based on clinical
characteristics alone (tumor size and nodal status), 50% would receive
adjuvant chemotherapy; however, only 36% received chemotherapy
using the risk status based on the 70-gene signature—an absolute
reduction of 14% in chemotherapy administration rate.293
Axillary Lymph Node-Negative Tumors
Small tumors (up to 0.5 cm in greatest diameter) that do not involve the
lymph nodes are so favorable that adjuvant systemic therapy is of
minimal incremental benefit and is not recommended as treatment of
the invasive breast cancer. According to the NCCN Panel, endocrine
therapy may be considered to reduce the risk for a second contralateral
breast cancer, especially in those with ER-positive disease. The NSABP
database demonstrated a correlation between the ER status of a new
contralateral breast tumor and the original primary tumor, which
reinforced the notion that endocrine therapy is not an effective strategy
to reduce the risk for contralateral breast cancer in patients diagnosed
with ER-negative tumors.294
Patients with invasive ductal or lobular tumors greater than 0.5 cm in
diameter and no lymph node involvement may be divided into patients
with a low risk of recurrence and those with unfavorable prognostic
features that warrant consideration of adjuvant therapy. Unfavorable
prognostic features include intramammary angiolymphatic invasion,
high nuclear grade, high histologic grade, HER2-positive status, or
hormone receptor-negative status. The use of endocrine therapy and
chemotherapy in these relatively lower risk subsets of women must be
based on balancing the expected absolute risk reduction and the
individual patient’s willingness to experience toxicity to achieve that
incremental risk reduction.
For women with lymph node-negative, hormone receptor-negative
tumors less than or equal to 0.5 cm with micrometastasis (pN1mi) or
tumors 0.6 to 1.0 cm, the NCCN Guidelines suggest considering
adjuvant chemotherapy (category 2A). For tumors greater than 1 cm in
diameter chemotherapy is a category 1 recommendation.
For those with lymph node-negative, hormone receptor-positive breast
cancer tumors greater than 0.5 cm, the panel recommends endocrine
therapy (category 1) with the consideration of chemotherapy.
Incremental benefit of combination chemotherapy in patients with lymph
node-negative, hormone receptor-positive breast cancer may be
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-31
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
relatively small.295 However, chemotherapy should not be withheld from
these patients solely based on ER-positive tumor status.4,295,296 The
panel considers the 21-gene RT-PCR assay an option for these patients
to help estimate likelihood of recurrence and benefit from
chemotherapy. The panel emphasizes that the recurrence score should
be used for decision-making only in the context of other elements of risk
stratification for an individual patient.
Axillary Lymph Node-Positive Tumors
Patients with lymph node-positive disease are most often candidates for
chemotherapy and, if the tumor is hormone receptor-positive, for the
addition of endocrine therapy (category 1). When HER2 is amplified or
over-expressed, HER2-targeted therapy should be incorporated into the
adjuvant chemotherapy. The NCCN Panel has noted in a footnote that
the 21-gene RT-PCR assay recurrence score can be considered in
select patients with 1 to 3 involved ipsilateral ALNs to guide the addition
of combination chemotherapy to standard hormone therapy based on
the retrospective study by Albain et al.275
Stratification for Systemic Adjuvant Therapy
The NCCN Guidelines stratify patients with breast cancer based on their
hormone receptor status and HER2 expression. Patients are then
further stratified based on risk of disease recurrence based on anatomic
and pathologic characteristics (ie, tumor grade, tumor size, ALN status,
angiolymphatic invasion).
Adjuvant Endocrine Therapy
The NCCN Guidelines call for the determination of ER and PR content
in all primary invasive breast cancers.17 Patients with invasive breast
cancers that are ER or PR positive should be considered for adjuvant
endocrine therapy regardless of patient age, lymph node status, or
whether adjuvant chemotherapy is to be administered.317 Selected
studies suggest that HER2-positive breast cancers may be less
sensitive to some endocrine therapies, although other studies have
failed to confirm this finding.255,318-325 A retrospective analysis of tumor
blocks collected in the ATAC trial indicated that HER2 amplification is a
marker of relative endocrine resistance independent of type of
endocrine therapy.326 However, given the favorable toxicity profile of the
available endocrine therapies, the panel recommends the use of
adjuvant endocrine therapy in the majority of women with hormone
receptor-positive breast cancer regardless of menopausal status, age,
or HER2 status of the tumor.
Tamoxifen
The most firmly established adjuvant endocrine therapy is tamoxifen for
both premenopausal and postmenopausal women.4 In women with
ER-positive breast cancer, adjuvant tamoxifen decreases the annual
odds of recurrence by 39% and the annual odds of death by 31%
irrespective of the use of chemotherapy, patient age, menopausal
status, or ALN status.4 In patients receiving both tamoxifen and
chemotherapy, chemotherapy should be given first, followed by
sequential tamoxifen.296 Prospective randomized trials have
demonstrated that 5 years of tamoxifen is more effective than 1 to 2
years of tamoxifen.327,328
The ATLAS trial randomly allocated 12,894 women to continue
tamoxifen up to 10 years or to discontinue tamoxifen (control). The
outcome analyses of 6846 women with ER-positive disease showed
that by extending adjuvant treatment to 10 years, the risk of relapse and
breast cancer-related mortality was reduced.329 The risk of recurrence
during years 5 to 14 was 21.4% for women receiving tamoxifen versus
25.1% for controls (absolute recurrence reduction 3.7%). Patients
receiving tamoxifen beyond 10 years of treatment had a greater
reduction in risk of progression, possibly due to a “carryover effect.” The
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-32
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
reduction in risk of recurrence was 0.90 (95% CI, 0.79–1.02) during 5 to
9 years of tamoxifen treatment and 0.75 (0.62–0.90) after 10 years of
treatment. Furthermore, reduced mortality was apparent after
completion of 10 years of treatment with tamoxifen. With regards to
toxicity, the most important adverse effects noted in all women in the
ATLAS trial after treatment with 10 years of tamoxifen were an
increased risk for endometrial cancer and pulmonary embolism. The
recurrence rate ratio reported for pulmonary embolus was 1.87 (95% CI,
1.13–3.07; P = .01 [including 0.2% mortality in both groups]) and for
endometrial cancer was 1.74 (1.30–2.34, P = .0002). The cumulative
risk for endometrial cancers during 5 to 14 years was 3.1%, with a
mortality of 0.4% associated with endometrial cancer, higher than what
was noted in the control group of patients receiving only 5 years of
therapy (cumulative risk: 1.6%; mortality: 0.2%).329 The results of the
aTTom trial confirm the ATLAS reduction in recurrence and death from
breast cancer.330
In women who are premenopausal at diagnosis, the NCCN Panel
recommends tamoxifen treatment with or without ovarian
suppression/ablation. Ovarian ablation may be accomplished by
surgical oophorectomy or by ovarian irradiation. Ovarian suppression
utilizes luteinizing hormone-releasing hormone (LHRH) agonists that
result in suppression of luteinizing hormone (LH) and release of follicle-
stimulating hormone (FSH) from the pituitary and reduction in ovarian
estrogen production. Available LHRH agonists in the United States
include goserelin and leuprolide and, when used for ovarian
suppression, both agents should be given as monthly injections as the
3-month depots do not reliably suppress estrogen levels in all patients.
The EBCTCG performed a meta-analysis of randomized studies of
ovarian ablation or suppression alone versus no additional systemic
adjuvant therapy for early-stage breast cancer. Analysis of ovarian
suppression versus no adjuvant therapy did not demonstrate significant
reduction in recurrence (HR 0.72; 95% CI, 0.49–1.04) or death (HR
0.82; 95% CI, 0.47–1.43).331 In addition, data on ovarian suppression
with tamoxifen, chemotherapy, or both showed no significant reduction
in reduced recurrence or death.
Studies in premenopausal women of ovarian ablation or suppression
alone versus CMF (cyclophosphamide/methotrexate/fluorouracil)
chemotherapy alone generally demonstrate similar antitumor efficacy in
patients with hormone receptor-positive tumors and superior outcomes
with CMF in patients with hormone receptor-negative tumors.331-339
There is also the suggestion that the benefits of ovarian
suppression/ablation may be greater in the younger premenopausal
group. Studies in premenopausal women of ovarian
ablation/suppression plus tamoxifen versus chemotherapy alone
generally demonstrate no difference in rates of recurrence or
survival.340-342
A large intergroup study in premenopausal women with hormone
receptor-positive, node-positive breast cancer studied adjuvant CAF
(cyclophosphamide/doxorubicin/5-fluorouracil) chemotherapy versus
CAF plus ovarian suppression with goserelin (CAF-Z) versus CAF-Z
plus tamoxifen (CAF-ZT).332 The results demonstrated no improvement
in time to recurrence or OS comparing CAF with CAF-Z. There was
improvement in time to recurrence (HR, 0.73; 95% CI, 0.59–0.90; P <
.01) but not OS with CAF-Z compared with CAF-ZT (HR, 0.91; 95% CI,
0.71–1.15; P = .21). This study did not include a CAF plus tamoxifen
arm, so the contribution of the goserelin to the improved time to
recurrence in the CAF-ZT arm cannot be assessed. The addition of
ovarian suppression/ablation has also been subjected to meta-analysis
by the EBCTCG.340 They identified no statistically significant reduction in
annual rates of recurrence or death with the addition of ovarian
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-33
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
suppression or ablation to chemotherapy in women less than 40 years
or 40 to 49 years of age.
Recent data from the randomized TEXT–SOFT trials evaluating
adjuvant endocrine therapy show that the aromatase inhibitor
exemestane plus ovarian suppression significantly reduces recurrences
as compared with tamoxifen plus ovarian suppression.
In two randomized trials (TEXT and SOFT), premenopausal women
with hormone receptor-positive early-stage breast cancer were
assigned to receive exemestane plus ovarian suppression or tamoxifen
plus ovarian suppression for a period of 5 years.343 Suppression of
ovarian estrogen production was achieved with the use of the
gonadotropin-releasing hormone agonist triptorelin, oophorectomy, or
ovarian irradiation. The DFS was 92.8% in the exemestane plus ovarian
suppression group, as compared with 88.8% in the tamoxifen plus
ovarian suppression group (HR for recurrence, 0.66; 95% CI, 0.55–
0.80; P < .001).343 The OS did not differ significantly between the two
groups (HR for death in the exemestane plus ovarian suppression
group, 1.14; 95% CI, 0.86–1.51; P = .37).343 In the SOFT trial,344
premenopausal women with hormone-receptor breast cancer were
randomized to tamoxifen alone, tamoxifen plus ovarian suppression, or
exemestane plus ovarian suppression for 5 years. In the primary
analysis, tamoxifen plus ovarian suppression was not superior to
tamoxifen alone for DFS. After 67 months of median follow-up, the DFS
rate at 5 years was 86.6% in the tamoxifen–ovarian suppression group
and 84.7% in the tamoxifen alone group (HR 0.83; 95% CI, 0.66–1.04;
P = .10).344 In a subgroup analysis, women at high risk of recurrence,
who received prior chemotherapy, had improved outcomes with ovarian
suppression. Their chance of remaining disease-free at 5 years was
78% with tamoxifen alone, 82.5% with tamoxifen and ovarian
suppression, and 85.7% with exemestane and ovarian suppression.344
In the subgroup of women with no prior chemotherapy, no meaningful
benefit was seen from ovarian suppression, as women who received
tamoxifen alone demonstrated a 95% chance of remaining disease-free
for 5 years.344 The OS data from these trials is still pending because the
overall follow-up is relatively short in the context of endocrine-sensitive
disease.
Based on the results of the SOFT and TEXT trials, the NCCN Panel has
included ovarian suppression plus an aromatase inhibitor for 5 years as
an adjuvant endocrine therapy option for premenopausal women with
hormone-receptor–positive breast cancer at higher risk of recurrence
(eg, young age, high-grade tumor, lymph-node involvement).
Several studies have evaluated aromatase inhibitors in the treatment of
postmenopausal women with early-stage breast cancer. These studies
have utilized the aromatase inhibitors as initial adjuvant therapy, as
sequential therapy following 2 to 3 years of tamoxifen, or as extended
therapy following 4.5 to 6 years of tamoxifen. The aromatase inhibitors
are not active in the treatment of women with functioning ovaries and
should not be used in women whose ovarian function cannot reliably be
assessed owing to treatment-induced amenorrhea. The results from two
prospective, randomized, clinical trials have provided evidence of an OS
benefit for patients with early-stage breast cancer receiving initial
endocrine therapy with tamoxifen followed sequentially by anastrozole
(HR, 0.53; 95% CI, 0.28–0.99; P = .045) or exemestane (HR, 0.83; 95%
CI, 0.69–1.00; P = .05 [excluding patients with ER-negative disease])
when compared with tamoxifen as the only endocrine therapy.345,346 In
addition, the NCIC-CTG MA-17 trial demonstrated a survival advantage
with extended therapy with letrozole compared with placebo in women
with ALN-positive (but not lymph node-negative), ER-positive breast
cancer.347 However, no survival differences have been reported for
patients receiving initial adjuvant therapy with an aromatase inhibitor
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-34
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
versus first-line tamoxifen.348,349 Tamoxifen and aromatase inhibitors
have different side effect profiles. Both contribute to hot flashes and
night sweats and may cause vaginal dryness. Aromatase inhibitors are
more commonly associated with musculoskeletal symptoms,
osteoporosis, and increased rate of bone fracture, while tamoxifen is
associated with an increased risk for uterine cancer and deep venous
thrombosis.
Two studies have examined initial adjuvant endocrine treatment with
either tamoxifen or an aromatase inhibitor. The ATAC trial
demonstrated that anastrozole is superior to tamoxifen or the
combination of tamoxifen and anastrozole in the adjuvant endocrine
therapy of postmenopausal women with hormone receptor-positive
breast cancer.350,351 With a median of 100 months follow-up, results in
5216 postmenopausal women with hormone receptor-positive,
early-stage breast cancer enrolled in the ATAC trial demonstrated fewer
recurrences (HR for DFS, 0.85; 95% CI, 0.76–0.94; P = .003) with
anastrozole compared with tamoxifen.348 No difference in survival has
been observed (HR, 0.90; 95% CI, 0.75–1.07; P = .2). Patients in the
combined tamoxifen and anastrozole group gained no benefit over
those in the tamoxifen group, suggesting a possible deleterious effect
from the weak estrogenic effect of tamoxifen in patients with near
complete elimination of endogenous estrogen levels.351 ATAC trial
sub-protocols show a lesser effect of anastrozole compared with
tamoxifen on endometrial tissue;352 similar effects of anastrozole and
tamoxifen on quality of life, with most patients reporting that overall
quality of life was not significantly impaired;353 a greater loss of bone
mineral density with anastrozole;354 a small pharmacokinetic
interference of anastrozole in the presence of tamoxifen of unclear
significance;355 and no evidence for an interaction between prior
chemotherapy and anastrozole.356
BIG 1-98 is a randomized trial testing the use of tamoxifen alone for 5
years, letrozole alone for 5 years, or tamoxifen for 2 years followed
sequentially by letrozole for 3 years, or letrozole for 2 years followed
sequentially by tamoxifen for 3 years. An early analysis compared
tamoxifen alone versus letrozole alone, including those patients in the
sequential arms during their first 2 years of treatment only.349 With 8010
women included in the analysis, DFS was superior in the
letrozole-treated women (HR, 0.81; 95% CI, 0.70–0.93; log rank P =
.003). No interaction between PR expression and benefit was observed.
No difference in OS was observed. A comparison of the cardiovascular
side effects in the tamoxifen and letrozole arms of the BIG 1-98 trial
showed that the overall incidence of cardiac adverse events was similar
(letrozole, 4.8%; tamoxifen, 4.7%). However, the incidence of grade 3 to
5 cardiac adverse events was significantly higher in the letrozole arm,
and both the overall incidence and incidence of grade 3 to 5
thromboembolic events was significantly higher in the tamoxifen arm.357
In addition, a higher incidence of bone fracture was observed for
women in the letrozole arm compared with those in the tamoxifen arm
(9.5% vs. 6.5%).358 After a longer follow-up (median 71 months) no
significant improvement in DFS was noted with either tamoxifen
followed by letrozole or the reverse sequence as compared with
letrozole alone (HR for tamoxifen followed by letrozole, 1.05; 99% CI,
0.84–1.32; HR for letrozole followed by tamoxifen, 0.96; 99% CI, 0.76–
1.21).359
Five trials have studied the use of tamoxifen for 2 to 3 years followed
sequentially by a third-generation aromatase inhibitor versus continued
tamoxifen in postmenopausal women. The Italian Tamoxifen
Anastrozole (ITA) trial randomized 426 postmenopausal women with
breast cancer who had completed 2 to 3 years of tamoxifen to either
continue tamoxifen or to switch to anastrozole to complete a total of 5
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-35
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
years of endocrine therapy.360 The HR for relapse strongly favored
sequential treatment with anastrozole (HR, 0.35; 95% CI, 0.18–0.68; P
= .001) with a trend towards fewer deaths (P = .10).360 Updated results
from this study show the HR for relapse-free survival as 0.56 (95% CI,
0.35–0.89; P = .01); P value for OS analysis remained at 0.1.361 The IES
trial randomized 4742 postmenopausal women with breast cancer who
had completed a total of 2 to 3 years of tamoxifen to either continue
tamoxifen or to switch to exemestane to complete a total of 5 years of
endocrine therapy.362 The results at a median of 55.7 months of
follow-up demonstrated the superiority of sequential exemestane in
DFS (HR, 0.76; 95% CI, 0.66–0.88; P = .0001) with a significant
difference in OS in only patients with ER-positive tumors (HR, 0.83;
95% CI, 0.69–1.00; log rank P = .05). A prospectively planned,
combined analysis of 3224 patients enrolled in the ABCSG 8 trial and
the Arimidex Nolvadex (ARNO 95) trial has also been reported.363
Patients in this combined analysis had been randomized following 2
years of tamoxifen to complete 5 years of adjuvant tamoxifen or 3 years
of anastrozole. With 28 months of median follow-up available,
event-free survival was superior with crossover to anastrozole (HR,
0.60; 95% CI, 0.44–0.81; P = .0009). No statistically significant
difference in survival has been observed. An analysis of the ARNO 95
trial alone after 58 months of median follow-up demonstrated that
switching from tamoxifen to anastrozole was associated with significant
increases in both DFS (HR, 0.66; 95% CI, 0.44–1.00; P = .049) and OS
(HR, 0.53; 95% CI, 0.28–0.99; P = .045).346 A meta-analysis of ABCSG
8, ARNO 95, and ITA studies showed significant improvement in OS
(HR, 0.71; 95% CI, 0.52-0.98; P = .04) with a switch to anastrozole.364
The TEAM trial compared treatment of exemestane alone versus
sequential therapy of tamoxifen for 2.5 to 3.0 years followed by
exemestane to complete 5 years of hormone therapy.365 At the end of 5
years, 85% of patients in the sequential group versus 86% in the
exemestane group were disease free (HR, 0.97; 95% CI, 0.88–1.08; P
= .60). This is consistent with the data from the BIG 1-98 trial,359 in
which tamoxifen followed by letrozole or the reverse sequence of
letrozole followed by tamoxifen was not associated with significant
differences in efficacy versus letrozole monotherapy after a median
follow-up of 71 months.
Results of the MA-17 trial in 5187 women who had completed 4.5 to 6
years of adjuvant tamoxifen demonstrated that extended therapy with
letrozole provides benefit in postmenopausal women with hormone
receptor-positive, early-stage breast cancer.347,366 At a median follow-up
of 2.5 years, the results showed fewer recurrences or new contralateral
breast cancers with extended letrozole (HR, 0.58; 95% CI, 0.45–0.76; P
< .001). No difference in OS was demonstrated (HR, 0.82; 95% CI,
0.57–1.19; P = .3), although there was a survival advantage in the
subset of patients with ALN-positive disease (HR 0.61; 95% CI, 0.38–
0.98; P = .04). In a separate cohort analysis of the MA-17 trial, the
efficacy of letrozole versus placebo was evaluated after un-blinding of
the study in the 1579 women who had been randomly assigned to
placebo after 4.5 to 6 years of tamoxifen.367,368 The median time since
completion of tamoxifen was 2.8 years. Both DFS and distant DFS were
significantly improved in the group receiving letrozole, thereby providing
some evidence for the efficacy of letrozole in patients who had received
4.5 to 6 years of tamoxifen therapy followed by no endocrine therapy for
an extended period. A formal quality-of-life analysis demonstrated
reasonable preservation of quality of life during extended endocrine
therapy, although women may experience ongoing menopausal
symptoms and loss of bone mineral density.369,370 No data are available
regarding use of aromatase inhibitors for more than 5 years or
long-term toxic effects from extended treatment. In addition, the ATLAS
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-36
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
trial data do not provide clear direction for treatment of postmenopausal
women.371 There are no data available to suggest that an aromatase
inhibitor for 5 years is better for long-term benefit than 10 years of
tamoxifen.
In the extension study of ABCSG trial 6, hormone receptor-positive
postmenopausal patients received 5 years of adjuvant tamoxifen and
were randomized to 3 years of anastrozole or no further therapy.372 At a
median follow-up of 62.3 months, women who received anastrozole (n =
387) were reported to have a statistically significantly reduced risk of
recurrence compared with women who received no further treatment (n
= 469; HR, 0.62; 95% CI, 0.40–0.96; P = .031).372
The differences in design and patient populations among the studies of
the aromatase inhibitors do not allow for the direct comparison of the
results of these studies. A meta-analysis of adjuvant trials of aromatase
inhibitors versus tamoxifen alone versus after 2 or 3 years of tamoxifen
documented lower recurrence rates with the aromatase
inhibitor-containing regimen, with no clear impact on OS.373 It is not
known whether initial, sequential, or extended use of adjuvant
aromatase inhibitors is the optimal strategy.
The optimal duration of aromatase inhibitor treatment is also not known,
nor is the optimal use vis-à-vis chemotherapy established. Further, the
long-term (greater than 5-year) safety and efficacy of these agents are
still under investigation. The various studies are consistent in
demonstrating that the use of a third-generation aromatase inhibitor in
postmenopausal women with hormone receptor-positive breast cancer
lowers the risk of recurrence, including ipsilateral breast tumor
recurrences, contralateral breast cancer, and distant metastatic disease
when used as initial adjuvant therapy, sequential therapy, or extended
therapy. The panel finds no compelling evidence that there is
meaningful efficacy or toxicity differences between the aromatase
inhibitors, anastrozole, letrozole, and exemestane. All three have shown
similar anti-tumor efficacy and toxicity profiles in randomized studies in
the adjuvant settings.
NCCN Recommendations for Adjuvant Endocrine Therapy for
Postmenopausal Women: The NCCN Guidelines for Breast Cancer
recommend the following adjuvant endocrine therapy options for women
with early-stage breast cancer who are postmenopausal at diagnosis:
an aromatase inhibitor as initial adjuvant therapy for 5 years (category
1); and tamoxifen for 2 to 3 years followed by one of the following
options: an aromatase inhibitor to complete 5 years of adjuvant
endocrine therapy (category 1) or 5 years of aromatase inhibitor therapy
(category 2B); or tamoxifen for 4.5 to 6 years followed by 5 years of an
aromatase inhibitor (category 1) or consideration of tamoxifen for up to
10 years. In postmenopausal women, the use of tamoxifen alone for 5
years (category 1) or up to 10 years is limited to those who decline or
who have a contraindication to aromatase inhibitors.
NCCN Recommendations for Adjuvant Endocrine Therapy for
Premenopausal Women: For women premenopausal at diagnosis, the
NCCN Guidelines for Breast Cancer recommend 5 years of tamoxifen
(category 1) with or without ovarian suppression (category 1) or ovarian
suppression plus an aromatase inhibitor for 5 years (category 1).
Women who are premenopausal at diagnosis and who become
amenorrheic with chemotherapy may have continued estrogen
production from the ovaries without menses. Serial assessment of
circulating LH, FSH, and estradiol to assure a true postmenopausal
status is mandatory if this subset of women is to be considered for
therapy with an aromatase inhibitor.374,375
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-37
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
After 5 years of initial endocrine therapy, for women who are
postmenopausal at that time (including those who have become
postmenopausal during the 5 years of tamoxifen therapy), the NCCN
Panel recommends considering extended therapy with an aromatase
inhibitor for up to 5 years (category 1) or based on the data from the
ATLAS trial considering tamoxifen for an additional 5 years. For those
who remain premenopausal after the initial 5 years of tamoxifen, the
panel recommends considering continuing up to 10 years of tamoxifen
therapy.
Response to Adjuvant Endocrine Therapy: The measurement of the
nuclear antigen, Ki-67 by IHC, gives an estimate of the tumor cells in
the proliferative phase (G1, G2, and M phases) of the cell cycle. Studies
have demonstrated the prognostic value of Ki-67 as a biomarker and its
usefulness in predicting response and clinical outcome.376 One small
study suggests that measurement of Ki-67 after short-term exposure to
endocrine treatment may be useful to select patients with tumors
resistant to endocrine therapy and those who may benefit from
additional interventions.377 However, these data require larger analytic
and clinical validation. In addition, standardization of tissue handling
and processing is required to improve the reliability and value of Ki-67
testing. At this time, there is no conclusive evidence that Ki-67 alone,
especially baseline Ki-67 as an individual biomarker, helps to select the
type of endocrine therapy for an individual patient. Therefore, the NCCN
Breast Cancer Panel does not currently recommend assessment of Ki-
67.
The cytochrome P-450 (CYP450) enzyme, CYP2D6, is involved in the
conversion of tamoxifen to endoxifen. Over 100 allelic variants of
CYP2D6 have been reported in the literature.378 Individuals with
wild-type CYP2D6 alleles are classified as extensive metabolizers of
tamoxifen. Those with one or two variant alleles with either reduced
or no activity are designated as intermediate metabolizers and poor
metabolizers, respectively. A large retrospective study of 1325 patients
found that time to disease recurrence was significantly shortened in
poor metabolizers of tamoxifen.379 However, the BIG 1-98 trial reported
on the outcome based on CYP2D6 genotype in a subset of
postmenopausal patients with endocrine-responsive, early invasive
breast cancer.380 The study found no correlation between CYP2D6
allelic status and disease outcome or between CYP2D6 allelic status
and tamoxifen-related adverse effects.380 A genetic analysis of the
ATAC trial found no association between CYP2D6 genotype and clinical
outcomes.381 Given the limited and conflicting evidence at this time,382
the NCCN Breast Cancer Panel does not recommend CYP2D6 testing
as a tool to determine the optimal adjuvant endocrine strategy. This
recommendation is consistent with the ASCO Guidelines.383 When
prescribing a selective serotonin reuptake inhibitor (SSRI), it is
reasonable to avoid potent and intermediate CYP2D6 inhibiting agents,
particularly paroxetine and fluoxetine, if an appropriate alternative
exists.
Adjuvant Cytotoxic Chemotherapy
Several combination chemotherapy regimens are appropriate to
consider when adjuvant cytotoxic chemotherapy is utilized. All adjuvant
chemotherapy regimens listed in the NCCN Guidelines have been
evaluated in phase III clinical trials, and the current version of the
adjuvant chemotherapy guidelines does not distinguish between options
for chemotherapy regimens by ALN status.
The adjuvant chemotherapy guidelines also include specific
representative doses and schedules for the recommended adjuvant
chemotherapy regimens. The regimens have been categorized as
“preferred” or “other.”
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-38
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
The purpose of distinguishing the adjuvant chemotherapy regimens as
preferred and other adjuvant chemotherapy regimens is to convey the
sense of the panel regarding the relative efficacy and toxicity of the
regimens.384 Factors considered by the panel include the efficacy,
toxicity, and treatment schedules of the regimens. Summarized below
are clinical trial results focusing on treatment efficacy.
Preferred Regimens
Regimens listed as preferred include: dose-dense doxorubicin and
cyclophosphamide (AC) with dose-dense sequential paclitaxel;
dose-dense AC followed by sequential weekly paclitaxel; and docetaxel
plus cyclophosphamide (TC).
The results of two randomized trials comparing AC chemotherapy with
or without sequential paclitaxel chemotherapy in women with axillary
node-positive breast cancer suggest improved disease-free rates, and
results from one of the trials showed an improvement in OS, with the
addition of paclitaxel.385,386 On retrospective analysis, the apparent
advantage of the paclitaxel-containing regimen appears greater in
women with ER-negative breast cancers.
A randomized trial evaluated the use of concurrent versus sequential
chemotherapy (doxorubicin followed by paclitaxel followed by
cyclophosphamide vs. doxorubicin plus cyclophosphamide followed by
paclitaxel) given either every 2 weeks with filgrastim support or every 3
weeks. The results show no significant difference between the two
chemotherapy regimens, but demonstrate a 26% reduction in hazard of
recurrence (P = .01) and a 31% reduction in the hazard of death (P =
.013) for the dose-dense regimens.387
The ECOG E1199 study was a four-arm trial that randomized 4950
women to receive AC chemotherapy followed by either paclitaxel or
docetaxel given by either an every-3-week schedule or a weekly
schedule.388-390 At a median 63.8 months of follow-up, no statistically
significant differences in DFS or OS were observed when comparing
paclitaxel to docetaxel or weekly versus every-3-week administration. In
a secondary series of comparisons, weekly paclitaxel was superior to
every-3-week paclitaxel in DFS (HR, 1.27; 95% CI, 1.03–1.57; P = .006)
and OS (HR, 1.32; 95% CI, 1.02–1.72; P = .01), and every-3-week
docetaxel was superior to every-3-week paclitaxel in DFS (HR, 1.23;
95% CI, 1.00–1.52; P = .02) but not in OS.390 Based on these results, as
well as the findings from the CALGB trial 9741 that showed dose-dense
AC followed by paclitaxel every 2 weeks to have a survival benefit when
compared with the regimen of AC followed by every-3-week
paclitaxel,387 the every-3-week paclitaxel regimen has been removed
from the guidelines.
Combination TC was compared with AC chemotherapy in a trial that
randomized 1016 women with stage I to III breast cancer.391 At a
median follow-up of 7 years, overall DFS (81% vs. 75%; HR, 0.74; 95%
CI, 0.56–0.98; P = .033) and OS (87% vs. 82%; HR, 0.69; 95% CI,
0.50–0.97; P = .032) were significantly improved with TC compared with
AC.
Other Regimens
Other regimens included in the guidelines are: AC; epirubicin and
cyclophosphamide (EC); CMF; AC with sequential docetaxel
administered every 3 weeks; AC with sequential weekly paclitaxel;
FEC/CEF followed by docetaxel or weekly paclitaxel; FAC followed by
weekly paclitaxel; and docetaxel, doxorubicin, and cyclophosphamide
(TAC).
The AC regimen for four cycles has been studied in randomized trials,
resulting in relapse-free survival and OS equivalent to CMF
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-39
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
chemotherapy.392,393 No benefit from dose escalation of either
doxorubicin or cyclophosphamide was shown.385,394
Studies of CMF chemotherapy versus no chemotherapy have shown
DFS and OS advantages with CMF chemotherapy.4,395 Studies using
FAC/CAF chemotherapy have shown that the use of full-dose
chemotherapy regimens is important.396 In the EBCTCG overview of
polychemotherapy, comparison of anthracycline-containing regimens
with CMF showed a 12% further reduction in the annual odds of
recurrence (P = .006) and an 11% further reduction in the annual odds
of death (P = .02) with anthracycline-containing regimens.395 Based on
these data, the panel qualified the appropriate chemotherapy regimens
by the statement that anthracycline-containing regimens are preferred
for node-positive patients.
The EBCTCG analysis, however, did not consider the potential
interaction between HER2 tumor status and efficacy of
anthracycline-containing versus CMF chemotherapy regimens.
Retrospective analysis has suggested that the superiority of
anthracycline-containing chemotherapy may be limited to the treatment
of those breast cancers that are HER2-positive.252,254,257,323,397-399 The
retrospective finding across several clinical trials that
anthracycline-based chemotherapy may be more efficacious in patients
whose tumors are HER2-positive has led to a footnote stating that
anthracycline-based chemotherapy may be superior to
non-anthracycline-containing regimens in the adjuvant treatment of
such patients.
A trial compared 2 dose levels of EC chemotherapy with CMF
chemotherapy in women with node-positive breast cancer.400 This study
showed that higher-dose EC chemotherapy was equivalent to CMF
chemotherapy and superior to moderate-dose EC in event-free survival
and OS.
The NSABP B-36 phase III trial data compared six cycles of 5-
fluorouracil, epirubicin, and cyclophosphamide (FEC) with four cycles of
AC, both given every 3 weeks as adjuvant therapy in patients with
node-negative breast cancer. The rationale for the trial was to
determine whether DFS improved with extra cycles of treatments.401
Patient and tumor characteristics were equally distributed between both
arms (<50 years of age: 40%, lumpectomy: 68%, and hormone
positivity: 65%).401 The results reported that DFS after eight years was
not greater for those women who had been on the longer FEC
chemotherapy treatment and that the women on the FEC experienced
greater side effects. Combined grade 3 and 4 toxicities with a significant
difference of 3% or more between AC and FEC arms included fatigue
3.55% versus 8.45%, febrile neutropenia 3.70% versus 9.42%, and
thrombocytopenia 0.74% versus 4.41%, respectively.401 Five deaths
resulted from the toxicity of FEC treatment, compared to the death of
two women on the AC treatment.401
The quality-of-life impact and menstrual history of women on the
NSABP (NRG) B-36 was also investigated in a phase III trial.402 Women
on FEC treatment experienced a worse quality of life at six months and
higher rate of post-chemotherapy amenorrhea.402
Based on the results of the NSABP B-36 trial, the NCCN Panel has now
excluded the FEC/CEF and FAC/CAF regimens as options for adjuvant
therapy.
Two randomized prospective trials of FEC chemotherapy in
ALN-positive breast cancer are available. In one trial, premenopausal
women with node-positive breast cancer were randomized to receive
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-40
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
classic CMF therapy versus FEC chemotherapy using high-dose
epirubicin. Both 10-year relapse-free survival (52% vs. 45%; P = .007)
and OS (62% vs. 58%; P = .085) favored the FEC arm of the trial.403
The second trial compared FEC given intravenously every 3 weeks at 2
dose levels of epirubicin (50 mg/m2 vs. 100 mg/m2) in premenopausal
and postmenopausal women with node-positive breast cancer.
Five-year DFS (55% vs. 66%; P = .03) and OS (65% vs. 76%; P =.007)
both favored the epirubicin 100 mg/m2 arm.404 Another randomized trial
in women with ALN-positive breast cancer compared 6 cycles of FEC
with 3 cycles of FEC followed by 3 cycles of docetaxel.341 Five-year DFS
(78.4% vs. 73.2%; adjusted P = .012) and OS (90.7% vs. 86.7%; P =
.017) were superior with sequential FEC followed by docetaxel.
However, no significant DFS differences were seen in a large
randomized study comparing adjuvant chemotherapy with 4 cycles of
every-3-week FEC followed by 4 cycles of every-3-week docetaxel with
standard anthracycline chemotherapy regimens (eg, FEC or epirubicin
followed by CMF) in women with node-positive or high-risk,
node-negative, operable breast cancer.405
The addition of weekly paclitaxel after FEC was shown to be superior to
FEC alone in a randomized study of 1246 women with early-stage
breast cancer.406 The former regimen was associated with a 23%
reduction in the risk of relapse compared with FEC (HR, 0.77; 95% CI,
0.62–0.95; P = .022), although no significant difference in OS was seen
when the two arms were compared at a median follow-up of 66 months.
The phase III E1199 trial compared patients with node-positive or high-
risk node-negative breast cancer who received 4 cycles of AC every 3
weeks, followed by either paclitaxel or docetaxel, either weekly or every
3 weeks. The 10-year updated results of this trial showed that
incorporation of weekly paclitaxel and docetaxel every 3 weeks was
associated with significant improvements in DFS, and marginal
improvements in OS, compared with paclitaxel given every 3 weeks.
Among patients with triple-negative disease, the10-year DFS rate with
weekly paclitaxel was 69% and the 10-year OS rate was 75%.407
Final results from a randomized trial of TAC versus FAC chemotherapy
in ALN-positive breast cancer demonstrated that TAC is superior to
FAC.408 Estimated 5-year DFS was 75% with TAC and 68% with FAC
(HR, 0.72; 95% CI, 0.59–0.88; P =.001); survival was 87% with TAC
and 81% with FAC (HR, 0.70; 95% CI, 0.53–0.91; P = .008). DFS
favored TAC in both ER-positive and ER-negative tumors. At a median
follow-up of 73 months, results from the 3-arm randomized NSABP
B-30 trial comparing TAC versus AT versus AC followed by docetaxel
(AC followed by T) demonstrated that AC followed by T had a significant
advantage in DFS (HR, 0.83; P = .006) but not in OS (HR, 0.86; P =
.086) when compared with TAC. In addition, both DFS (HR, 0.080; P =
.001) and OS (HR, 0.83; P = .034) were significantly increased when
AC followed by T was compared with AT, with AT demonstrating
non-inferiority compared with TAC.409
Several retrospective studies have evaluated the potential interaction of
chemotherapy benefit and ER status.4,295 These studies assessed the
effect of chemotherapy on the risk of breast cancer recurrence in
patients with ER-positive tumors receiving adjuvant endocrine therapy
when compared with patients with ER-negative tumor status not
undergoing adjuvant endocrine therapy. These analyses suggest that
the benefits of chemotherapy are significantly greater in patients with
ER-negative disease. For example, the results of Berry et al
demonstrated that 22.8% more patients with ER-negative tumors
survived without disease for 5 years if they received chemotherapy; this
benefit was only 7% for patients with ER-positive tumors receiving
chemotherapy.295
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-41
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
For women greater than 70 years of age, the consensus of the panel is
that there are insufficient data to make definitive chemotherapy
recommendations. Although AC or CMF has been shown to be superior
to capecitabine in a randomized trial of women aged greater than or
equal to 65 years with early-stage breast cancer,410 the enrollment in
that study was discontinued early.410 Therefore, there is also a
possibility that AC/CMF is not superior to any chemotherapy in this
cohort. The panel recommends that treatment should be individualized
for women in this age group, with consideration given to comorbid
conditions.
Adjuvant HER2-Targeted Therapy
The panel recommends HER2-targeted therapy in patients with HER2-
positive tumors (see Principles of HER2 Testing in the NCCN
Guidelines for Breast Cancer). Trastuzumab is a humanized
monoclonal antibody with specificity for the extracellular domain of
HER2.411Results of several randomized trials testing trastuzumab as
adjuvant therapy have been reported.259-264,412-414
NSABP B-31 patients with HER2-positive, node-positive breast cancer
were randomly assigned to 4 cycles of AC every 3 weeks followed by
paclitaxel for 4 cycles every 3 weeks or the same regimen with 52
weeks of trastuzumab commencing with paclitaxel. In the NCCTG
N9831 trial, patients with HER2-positive breast cancer that was
node-positive, or node-negative, with primary tumors greater than 1 cm
in size if ER- and PR-negative or greater than 2 cm in size if ER- or
PR-positive, were similarly randomized except that paclitaxel was given
by a low-dose weekly schedule for 12 weeks and a third arm delayed
trastuzumab until the completion of paclitaxel.
The B-31 and NCCTG N9831 trials have been jointly analyzed with the
merged control arms for both trials compared with the merged arms
using trastuzumab begun concurrently with paclitaxel. There were 4045
patients included in the joint analysis performed at 3.9 years median
follow-up. A 48% reduction in the risk of recurrence (HR, 0.52; 95% CI,
0.45–0.60; P < .001) and a 39% reduction in the risk of death (HR, 0.61;
95% CI, 0.50–0.75; log-rank P = .001) were documented.413 Similar
significant effects on DFS were observed when results of the NSABP
B-31 and NCCTG N9831 trials were analyzed separately. Cardiac
toxicity was increased in patients treated with trastuzumab.262,415,416 In
the adjuvant trastuzumab trials, the rates of grade III/IV congestive
heart failure (CHF) or cardiac-related death in patients receiving
treatment regimens containing trastuzumab ranged from 0% (FinHer
trial) to 4.1% (NSABP B-31 trial).259,260,262,264,415,416 The frequency of
cardiac dysfunction appears to be related to both age and baseline left
ventricular ejection fraction. An analysis of data from N9831 showed the
3-year cumulative incidence of CHF or cardiac death to be 0.3%, 2.8%,
and 3.3% in the arms of the trial without trastuzumab, with trastuzumab
following chemotherapy, and with trastuzumab initially combined with
paclitaxel, respectively.415 The acceptable rate of significant cardiac
toxicity observed in the trastuzumab adjuvant trials in part reflects
rigorous monitoring for cardiac dysfunction. Furthermore, concerns
have been raised regarding the long-term cardiac risks associated with
trastuzumab therapy based on results of follow-up evaluations of
cardiac function in patients enrolled in some of these trials.417,418
A third trial (HERA) (N = 5081) tested trastuzumab for 1 or 2 years
compared to none following all local therapy and a variety of standard
chemotherapy regimens in patients with node-positive disease or
node-negative disease with tumor greater than or equal to 1 cm.260 At a
median follow-up of one year, a 46% reduction in the risk of recurrence
was reported in those who received trastuzumab compared with those
who did not (HR, 0.54; 95% CI, 0.43–0.67; P < .0001), there was no
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-42
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
difference in OS, and acceptable cardiac toxicity was reported. The
2-year data indicate that 1 year of trastuzumab therapy is associated
with an OS benefit when compared with observation (HR for risk of
death = 0.66; 95% CI, 0.47–0.91; P = .0115).419 After this initial analysis,
patients randomized to chemotherapy alone were allowed to cross over
to receive trastuzumab. Intent-to-treat analysis including a crossover
patient was reported at 4-year median follow-up.414 The primary
endpoint of DFS continued to be significantly higher in the
trastuzumab-treated group (78.6%) versus the observation group (72.2;
HR, 0.76; 95% CI, 0.66–0.87; P < .0001). At a median follow-up of 8
years, the study reported no significant difference in DFS, a secondary
endpoint, in patients treated with trastuzumab for 2 years compared
with 1 year.261 Therefore, 1 year of adjuvant trastuzumab remains the
current standard of treatment.
The BCIRG 006 study randomized 3222 women with HER2-positive,
node-positive, or high-risk node-negative breast cancer to AC followed
by docetaxel; AC followed by docetaxel plus trastuzumab for one year;
or carboplatin, docetaxel, and trastuzumab for one year.264 At 65-month
follow-up, patients receiving AC followed by docetaxel with trastuzumab
(AC-TH) had an HR for DFS of 0.64 (P < .001) when compared with the
group of patients in the control arm receiving the same chemotherapy
regimen without trastuzumab (AC-T). The HR for DFS was 0.75 (P =
.04) when patients in the carboplatin/docetaxel/ trastuzumab
(TCH)-containing arm were compared to patients in the control arm. No
statistically significant difference in the HR for DFS was observed
between the two trastuzumab-containing arms. An OS advantage was
reported for patients in both trastuzumab-containing arms relative to the
control arm (HR for AC-TH vs. AC-T = 0.63; P = .001; HR for TCH vs.
AC-T = 0.77; P = .04). Cardiac toxicity was significantly lower in the
TCH arm (9.4% patients with >10% relative decline in left ventricular
ejection fraction) compared with the AC-TH arm (18.6%; P < .0001).
CHF was also more frequent with AC-TH than TCH (2% vs. 0.4%; P <
.001). Analysis of this trial by critical clinical event revealed more distant
breast cancer recurrences with TCH (144 vs. 124) but fewer cardiac
events with TCH compared with AC-TH (4 vs. 21).264 In the FinHer trial,
1010 women were randomized to 9 weeks of vinorelbine followed by 3
cycles of FEC chemotherapy versus docetaxel for 3 cycles followed by
3 cycles of FEC chemotherapy.259 Patients (n = 232) with HER2-positive
cancers that were either node-positive or node-negative and greater
than or equal to 2 cm and PR-negative were further randomized to
receive or not receive trastuzumab for 9 weeks during the vinorelbine or
docetaxel portions of the chemotherapy only. With a median follow-up
of 3 years, the addition of trastuzumab was associated with a reduction
in risk of recurrence (HR, 0.42; 95% CI, 0.21–0.83; P = .01). No
statistically significant differences in OS (HR, 0.41; 95% CI, 0.16–1.08;
P = .07) or cardiac toxicity were observed with the addition of
trastuzumab.259 At 5-year follow-up, a comparison of the two arms (ie,
chemotherapy with and without trastuzumab) demonstrated that the
HRs for distant DFS (HR, 0.65; 95% CI, 0.38–1.12; P = .12) and OS
(HR, 0.55; 95% CI, 0.27–1.11; P = .094) were higher relative to those
reported at 3 years.412
All of the adjuvant trials of trastuzumab have demonstrated clinically
significant improvements in DFS, and the combined analysis from the
NSABP B31 and NCCTG N9831 trials, and the HERA trial, showed
significant improvement in OS with the use of trastuzumab in patients
with high-risk, HER2-positive breast cancer. Therefore, regimens from
each of these trials are included as trastuzumab-containing adjuvant
regimen choices in the guideline. The benefits of trastuzumab are
independent of ER status.262,263 In the FNCLCC-PACS-04 trial, 528
women with HER2-positive, node-positive breast cancer were randomly
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-43
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
assigned to receive trastuzumab or observation after completion of
adjuvant anthracycline-based chemotherapy with or without
docetaxel.420 No statistically significant DFS or OS benefit was observed
with the addition of trastuzumab. These results suggest that the
sequential administration of trastuzumab following chemotherapy is not
as efficacious as a schedule involving concomitant chemotherapy and
trastuzumab. The NCCN Guidelines recommend a total of 12 months of
adjuvant trastuzumab as the standard of care. Shorter than 12-month
duration has not been found to be as effective421 and longer than 12
months duration does not have any added benefit; it has been found to
be as effective as the 12 months of trastuzumab therapy.422
Retrospective analyses of low-risk patients with small tumors
demonstrate that in T1a-bN0 breast cancers, HER2 overexpression
added a 15% to 30% risk for recurrence.423-426 These risks rates are
substantially higher than seen among similarly sized HER2-negative
tumors.
A recent single-arm, multicenter trial studied the benefit of trastuzumab-
based chemotherapy in patients with HER2-positive, node-negative
tumors less than or equal to 3 cm. All patients received trastuzumab
and weekly paclitaxel for 12 weeks, followed by completion of a year of
trastuzumab monotherapy.427 Fifty percent of patients enrolled had
tumors less than or equal to 1.0 cm and 9% of patients had tumors that
were between 2 and 3 cm. The endpoint of the study was DFS. The
results presented at the 2013 Annual San Antonio Breast Cancer
Symposium demonstrated that the 3-year DFS rate in the overall
population was 98.7% (95% CI, 97.6–99.8; P < .0001).
Dual anti-HER2 blockade associated with trastuzumab plus lapatinib
and trastuzumab plus pertuzumab has shown significant improvements
in the pCR rate when compared with chemotherapy associated with one
anti-HER2 agent in the neoadjuvant setting.313,314,316
However, in the adjuvant setting, the results of the ALTTO trial failed to
demonstrate a significant improvement in DFS with dual anti-HER2
therapy compared with trastuzumab alone.428 After a median follow-up
of 4.5 years, the DFS rates were 86% for patients who received
trastuzumab alone; 88% for participants treated with trastuzumab and
lapatinib concurrently; and 87% for patients who received trastuzumab
followed by lapatinib.428
NCCN Recommendation for Adjuvant HER2-Targeted Therapy
Based on these studies, the panel has designated use of trastuzumab
with chemotherapy as a category 1 recommendation in patients with
HER2-positive tumors greater than 1 cm.
The NCCN Panel suggests trastuzumab and chemotherapy be used for
women with HER2-positive, node-negative tumors measuring 0.6 to 1.0
cm (ie, T1b) and for smaller tumors that have less than or equal to 2
mm axillary node metastases (pN1mi). Some support for this
recommendation comes from studies showing a higher risk of
recurrence for patients with HER2-positive, node-negative tumors less
than or equal to 1 cm compared to those with HER2-negative tumors of
the same size.423 Ten-year breast cancer-specific survival and 10-year
recurrence-free survival were 85% and 75%, respectively, in women
with tumors characterized as HER2-positive, ER-positive tumors, and
70% and 61%, respectively, in women with HER2-positive, ER-negative
tumors. Two more retrospective studies have also investigated
recurrence-free survival in this patient population. None of the patients
in these two retrospective studies received trastuzumab. In the first
study, 5-year recurrence-free survival rates of 77.1% and 93.7% (P <
.001) were observed for patients with HER2-positive and
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-44
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
HER2-negative T1a-bN0M0 breast tumors, respectively, with no
recurrence-free survival differences seen in the HER2-positive group
when hormonal receptor status was considered.424 In the other
retrospective study of women with small HER2-positive tumors, the risk
of recurrence at 5 years was low (99% [95% CI; 96%–100%] for HER2-
negative disease and 92% [95% CI; 86%–99%] for HER2-positive
disease).429 Subgroup analyses from several of the randomized trials
have shown consistent benefit of trastuzumab irrespective of tumor size
or nodal status.264,430,431
NCCN-Recommended HER-Targeted Regimens
The panel recommends AC followed by paclitaxel with trastuzumab for
1 year commencing with the first dose of paclitaxel as a preferred
HER2-targeting adjuvant regimen. The TCH regimen is also a preferred
regimen, especially for those with risk factors for cardiac toxicity, given
the results of the BCIRG 006 study that demonstrated superior DFS in
patients receiving TCH or AC followed by docetaxel plus trastuzumab
compared with AC followed by docetaxel alone.
Other trastuzumab-containing regimens included in the NCCN
Guidelines are: AC followed by docetaxel and trastuzumab,264 and
docetaxel plus trastuzumab followed by FEC259 (see Preoperative
/Adjuvant Systemic Therapy in NCCN Guidelines for Breast Cancer for
a complete list of regimens).
Considering the unprecedented improvement in OS in the metastatic
setting432 and the significant improvement in pCR seen in the
neoadjuvant setting,314,316 the NCCN Panel considers it reasonable to
incorporate pertuzumab into the above adjuvant regimens, if the patient
did not receive pertuzumab as a part of neoadjuvant therapy. An
ongoing study is evaluating pertuzumab and trastuzumab with standard
chemotherapy regimens in the adjuvant setting.433,434
The NCCN Panel has included paclitaxel and trastuzumab as an option
for patients with low-risk, HER2-positive, stage 1 tumors. This is based
on a trial that studied this combination in 406 patients with small, node-
negative, HER2-positive tumors. The results showed that the 3-year
rate of DFS was 98.7% (95% CI, 97.6–99.8) and the risk of serious toxic
effects with this regimen was low (incidence of heart failure reported
was 0.5%).435
Adjuvant Therapy for Tumors of Favorable Histologies
The guidelines provide systemic treatment recommendations for the
favorable histology of invasive breast cancers, such as tubular and
mucinous cancers, based on tumor size and ALN status. If used, the
treatment options for endocrine therapy, chemotherapy, and
sequencing of treatment with other modalities are similar to those of the
usual histology of breast cancers. The vast majority of tubular breast
cancers are both ER-positive and HER2-negative. Thus, the pathology
evaluation and accuracy of the ER and/or HER2 determination should
be reviewed if a tubular breast cancer is ER-negative and/or
HER2-positive, or if a tumor with an ER- and PR-negative status is
grade 1.17 Should a breast cancer be histologically identified as a
tubular or mucinous breast cancer and be confirmed as ER-negative,
then the tumor should be treated according to the guideline for the
usual histology, ER-negative breast cancers. The panel acknowledges
that prospective data regarding systemic adjuvant therapy of tubular
and mucinous histologies are lacking.
Systemic Therapy for Triple-Negative Breast Cancer
For women with triple-negative breast cancer, several clinical trials
sought to determine whether the addition of carboplatin (alone or in
combination) as neoadjuvant chemotherapy can improve outcomes for
women with triple-negative breast cancer. In the German GeparSixto
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-45
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
trial, 315 patients with triple-negative breast cancer were administered
neoadjuvant therapy consisting of weekly paclitaxel plus non-pegylated
liposomal doxorubicin with bevacizumab and then randomly assigned to
additional treatment with weekly carboplatin.436 The addition of
carboplatin achieved a pCR rate of 59% compared with pCR of 38% in
patients who did not receive carboplatin.436
In the CALGB 40603 randomized phase II trial, 443 patients with stage
II to III triple-negative breast cancer received standard anthracycline-
and taxane-based chemotherapy with or without carboplatin and with or
without bevacizumab. Compared with standard chemotherapy, the
addition of carboplatin resulted in significantly higher pCR rate (54% vs.
41%, OR 1.71).437 The addition of bevacizumab increased the numeric
rate of pCR but was not statistically significant (with bevacizumab, pCR
was 52% [95% CI; 45%–58%] and without bevacizumab pCR was 44%
[95% CI; 38%–51%]; P = .057). In this study,437 as well as in the
GeparSixto study,436 the addition of carboplatin and/or bevacizumab led
to increased rates of adverse events. Neutropenia and
thrombocytopenia were more common with carboplatin. Hypertension
and postsurgical complications were more common with bevacizumab.
Even though the results of randomized trials show improvement in pCR
rates when carboplatin is added to anthracycline- and taxane-based
chemotherapy, the long-term outcomes such as OS or DFS associated
with the incorporation of carboplatin are not yet known. Therefore, at
this time, the NCCN Panel does not recommend addition of carboplatin
to neoadjuvant standard chemotherapy for patients with triple-negative
breast cancer outside a clinical trial setting.
Medullary Carcinoma
Medullary carcinoma is an uncommon variant of infiltrating ductal
carcinoma characterized by high nuclear grade, lymphocytic infiltration,
a pushing tumor border, and the presence of a syncytial growth pattern.
It was previously thought that medullary carcinoma has a lower potential
for metastases and a better prognosis than typical infiltrating ductal
carcinoma. However, the best available evidence suggests that the risk
of metastases equals that of other high-grade carcinomas, even for
cases that meet all the pathologic criteria for typical medullary
carcinoma. Furthermore, typical medullary carcinoma is uncommon,
and there is marked interobserver variation in diagnosing this entity.
Many cases classified as medullary carcinoma do not have all the
pathologic features on subsequent pathologic review. Given these facts,
there is concern that patients may be harmed if a high-grade infiltrating
ductal carcinoma is misclassified as typical medullary carcinoma and
this classification is used as the basis for withholding otherwise
indicated adjuvant systemic therapy. Therefore, the NCCN Panel
believes that including medullary carcinoma with other special histology
cancers that carry a favorable prognosis and often do not require
systemic therapy is not appropriate. The panel recommends that cases
classified as medullary carcinoma be treated as other infiltrating ductal
carcinomas based on tumor size, grade, and lymph node status.
Post-Therapy Surveillance and Follow-up
See page MS-48.
Stage III Invasive Breast Cancer
Staging and Workup
The staging evaluation for most patients with stage III invasive breast
cancer is similar to the one for patients with T3, N1, M0 disease. The
workup includes history and physical exam, a CBC, liver function and
alkaline phosphatase tests, chest imaging, pathology review, and
pre-chemotherapy determination of tumor ER/PR receptor status and
HER2 status. Diagnostic bilateral mammogram and breast ultrasound
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-46
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
should be performed as clinically warranted. Genetic counseling is
recommended if the patient is considered to be at high risk for
hereditary breast cancer as defined by the NCCN Guidelines for
Genetic/Familial High-Risk Assessment: Breast and Ovarian.
The performance of other studies, such as a breast MRI, a bone scan
(category 2B), and abdominal imaging with diagnostic CT (with or
without pelvic CT) or MRI (all category 2A) are optional unless directed
by symptoms or other abnormal study results. PET/CT scan is also
included as an optional additional study (category 2B). Ultrasound is an
alternative when diagnostic CT or MRI is unavailable.
The consensus of the panel is that FDG PET/CT is most helpful in
situations where standard imaging results are equivocal or suspicious.
However, limited studies125,126,438-442 support a potential role for FDG
PET/CT to detect regional node involvement as well as distant
metastases in locally advanced breast cancer, including T3, N1, M0
disease.
A retrospective study comparing bone scan with integrated FDG
PET/CT, in women with stages I–III breast cancer with suspected
metastasis, observed a high concordance (81%) between the two
studies for reporting osseous metastases.443 The NCCN Panel suggests
that bone scan may be omitted if FDG PET/CT results are positive for
bone metastases.
Equivocal or suspicious sites identified by PET/CT scanning should be
biopsied for confirmation whenever possible and if the site of disease
would impact the course of treatment. In the past decade, the advent of
PET/CT scanners has significantly changed the approach to PET
imaging.444 However, the terminology has also created confusion
regarding the nature of the scans obtained from a PET/CT device.
PET/CT scanners have both a PET and CT scanner in the same gantry
that allows precise coregistration of molecular (PET) and anatomic (CT)
imaging. Almost all current clinical PET imaging is performed using
combined PET/CT devices.
In PET/CT tomographs, the CT scanner has a second important role
beyond diagnostic CT scanning.444 For PET applications, the CT scan is
also used for photon attenuation correction and for anatomic localization
of the PET imaging findings. For these tasks, the CT scan is usually
taken without breathholding, to match PET image acquisition, and
typically uses low-dose (non-diagnostic) CT. Radiation exposure for
these non-diagnostic CT scans is lower than for diagnostic CT.
Intravenous contrast is not needed for this task.
PET/CT scanners typically include a high-quality CT device that can
also be used for stand-alone, optimized, and fully diagnostic CT.
Diagnostic CT scans are acquired using breathholding for optimal chest
imaging, and are often performed with intravenous contrast. For fully
diagnostic CT, the CT beam current, and therefore patient radiation
exposure, is considerably higher than for the low-dose CT needed for
PET requirements. Radiation exposures for fully diagnostic CT are often
greater than for the emission (PET) component of the study.
Currently, the approach to clinical PET/CT imaging varies widely across
centers.445 Many centers perform low-dose CT as part of a PET/CT
scan, and perform optimized, fully diagnostic CT only when diagnostic
CT has also been requested in addition to PET/CT. Other centers
combine diagnostic CT scans with PET on all of their PET/CT images.
The CT scans described in the workup section of the guidelines refer to
fully optimized diagnostic CT scans, while the PET or PET/CT scans
refer to scans primarily directed towards the PET component, not
necessarily using diagnostic-quality CT. It is important for referring
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-47
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
physicians to understand the differences between PET/CT performed
primarily for PET imaging and fully optimized CT performed as a
stand-alone diagnostic CT examination.445 It may be convenient to
perform PET/CT and diagnostic CT at the same time.
Operable Locally Advanced Breast Cancer
(Clinical stage T3, N1, M0)
Locally advanced breast cancer describes a subset of invasive breast
cancer where the initial clinical and radiographic evaluation documents
advanced disease confined to the breast and regional lymph nodes.
The AJCC clinical staging system used in these guidelines and for the
determination of operability is recommended, and locally advanced
disease is represented by the stage III category. Patients with stage III
disease may be further divided into: 1) those where an initial surgical
approach is unlikely to successfully remove all disease or to provide
long-term local control; and 2) those with disease where a reasonable
initial surgical approach is likely to achieve pathologically negative
margins and provide long-term local control. Thus, stage IIIA patients
are divided into those who have clinical T3, N1, M0 disease versus
those who have clinical T any, N2, M0 disease, based on evaluation by
a multidisciplinary team.
Postsurgical systemic adjuvant therapy for patients with stage IIIA
breast cancer who do not receive neoadjuvant chemotherapy is similar
to that for patients with stage II disease.
Inoperable Locally Advanced Breast Cancer
(Clinical stage IIIA [except for T3, N1, M0], clinical stage IIIB, or clinical
stage IIIC)
For patients with inoperable, non-inflammatory, locally advanced
disease at presentation, the initial use of anthracycline-based
preoperative systemic therapy with or without a taxane is standard
therapy.446 Patients with locally advanced breast cancer that is
HER2-positive should receive an initial chemotherapy program that
incorporates preoperative trastuzumab and possibly pertuzumab. Local
therapy following a clinical response to preoperative systemic therapy
usually consists of: 1) total mastectomy with level I/II ALN dissection,
with or without delayed breast reconstruction; or 2) lumpectomy and
level I/II axillary dissection.
Both local treatment groups are considered to have sufficient risk of
local recurrence to warrant the use of chest wall (or breast) and
supraclavicular node irradiation. If internal mammary lymph nodes are
involved, they should also be irradiated. Without detected internal
mammary node involvement, consideration may be given to include the
internal mammary lymph nodes in the radiation field (category 2B).
Adjuvant therapy may involve completion of planned chemotherapy
regimen course if not completed preoperatively, followed by endocrine
therapy in patients with hormone receptor-positive disease. Up to one
year of total trastuzumab therapy should be completed if the tumor is
HER2-positive (category 1). Endocrine therapy and trastuzumab can be
administered concurrently with radiation therapy if indicated.
Patients with an inoperable stage III tumor with disease progression
during preoperative systemic therapy should be considered for palliative
breast irradiation in an attempt to enhance local control. In all subsets of
patients, further systemic adjuvant chemotherapy after local therapy is
felt to be standard. Tamoxifen (or an aromatase inhibitor if
postmenopausal) should be added for those with hormone
receptor-positive tumors, and trastuzumab should be given to those with
HER2-positive tumors. Post-treatment follow-up for women with stage
III disease is the same as for women with early-stage invasive breast
cancer.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-48
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
Post-Therapy Surveillance and Follow-up for Stage I-III
Post-therapy follow-up is optimally performed by members of the
treatment team and includes the performance of regular history/physical
examinations every 4 to 6 months for the first 5 years after primary
therapy and annually thereafter. Mammography should be performed
annually.
Regarding frequency of mammograms after breast-conserving surgery
followed by radiation, the NCCN Panel agrees with ASTRO’s “Choosing
Wisely’ list of recommendations released in 2014.447 The
recommendations state that “annual mammograms are the appropriate
frequency for surveillance of breast cancer patients who have had
breast-conserving surgery and radiation therapy with no clear
advantage to shorter interval imaging. Patients should wait 6 to 12
months after the completion of radiation therapy to begin their annual
mammogram surveillance. Suspicious findings on physical examination
or surveillance imaging might warrant a shorter interval between
mammograms.”
The NCCN panel notes that any imaging of reconstructed breast is not
indicated.
According to the NCCN Panel, in the absence of clinical signs and
symptoms suggestive of recurrent disease, laboratory or imaging
studies to screen for metastasis are not necessary. The routine
performance of alkaline phosphatase tests and LFTs are not included in
the guidelines.448-450 In addition, the panel notes no evidence to support
the use of “tumor markers” for breast cancer, and routine bone scans,
CT scans, MRI scans, PET scans, or ultrasound examinations in the
asymptomatic patient provide no advantage in survival or ability to
palliate recurrent disease and are, therefore, not recommended.125,451
The use of breast MRI in follow-up of women with prior breast cancer is
undefined. It may be considered as an option in women with high
lifetime risk (greater than 20% based on models largely dependent on
family history) of developing a second primary breast cancer. Rates of
contralateral breast cancer after either breast-conserving therapy or
mastectomy have been reported to be increased in women with
BRCA1/2 mutations when compared with patients with sporadic breast
cancer.452-454
The panel recommends that women with intact uteri who are taking
adjuvant tamoxifen should have yearly gynecologic assessments and
rapid evaluation of any vaginal spotting that might occur because of the
risk of tamoxifen-associated endometrial carcinoma in postmenopausal
women.455 The performance of routine endometrial biopsy or
ultrasonography in the asymptomatic woman is not recommended.
Neither test has demonstrated utility as a screening test in any
population of women. The vast majority of women with
tamoxifen-associated uterine carcinoma have early vaginal spotting.
If an adjuvant aromatase inhibitor is considered in women with
amenorrhea following treatment, baseline levels of estradiol and
gonadotropin followed by serial monitoring of these hormones should be
performed if endocrine therapy with an aromatase inhibitor is initiated.374
Bilateral oophorectomy assures postmenopausal status in young
women with therapy-induced amenorrhea and may be considered prior
to initiating therapy with an aromatase inhibitor in a young woman.
Symptom management for women on adjuvant endocrine therapies
often requires treatment of hot flashes and the treatment of concurrent
depression. Venlafaxine, a serotonin-norepinephrine reuptake inhibitor
(SNRI) has been studied and is an effective intervention in decreasing
hot flashes.456-459 There is evidence suggesting that concomitant use of
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-49
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
tamoxifen with certain SSRIs (eg, paroxetine, fluoxetine) may decrease
plasma levels of endoxifen, an active metabolite of tamoxifen.460,461
These SSRIs/SNRIs may interfere with the enzymatic conversion of
tamoxifen to endoxifen by inhibiting a particular isoform of CYP2D6.
However, the mild CYP2D6 inhibitors such as citalopram, escitalopram,
sertraline, and venlafaxine appear to have no or only minimal effect on
tamoxifen metabolism.374,462,463
Follow-up also includes assessment of patient adherence to ongoing
medication regimens such as endocrine therapies. Predictors of poor
adherence to medication include the presence of side effects
associated with the medication, and incomplete understanding by the
patient of the benefits associated with regular administration of the
medication.464 The panel recommends the implementation of simple
strategies to enhance patient adherence to endocrine therapy, such as
direct questioning of the patient during office visits, as well as brief,
clear explanations on the value of taking the medication regularly and
the therapeutic importance of longer durations of endocrine therapy.
Lymphedema is a common complication after treatment for breast
cancer. Factors associated with increased risk of lymphedema include
extent of axillary surgery, axillary radiation, infection, and patient
obesity.465,466 The panel recommends educating the patients on
lymphedema, monitoring for lymphedema, and referring for
lymphedema management as needed.
Many young women treated for breast cancer maintain or regain
premenopausal status following treatment for breast cancer. For these
women, the NCCN Panel discourages the use of hormonal birth control
methods, regardless of the hormone receptor status of the tumor.467
Alternative birth control methods are recommended, including
intrauterine devices, barrier methods, and, for those with no intent of
future pregnancy, tubal ligation or vasectomy for the partner.
Breastfeeding during endocrine or chemotherapy treatment is not
recommended by the NCCN Panel because of risks to the infant.
Breastfeeding after breast-conserving treatment for breast cancer is not
contraindicated. However, lactation from an irradiated breast may not
be possible, or may occur only with a diminished capacity.467,468
The panel recommends that women on an adjuvant aromatase inhibitor
or who experience ovarian failure secondary to treatment should have
monitoring of bone health with a bone mineral density determination at
baseline and periodically thereafter. The use of estrogen, progesterone,
or selective ER modulators to treat osteoporosis or osteopenia in
women with breast cancer is discouraged. The use of a bisphosphonate
is generally the preferred intervention to improve bone mineral density.
A single phase 3 study, ABCSG12, demonstrated improved outcomes
with the addition of zoledronic acid in premenopausal women receiving
endocrine therapy with ovarian suppression.469 Use of bisphosphonates
in such patients and in other subgroups remains controversial.
Denosumab has shown to significantly reduce fractures in
postmenopausal women receiving adjuvant therapy aromatase
inhibitors, and improves bone mineral density.470
Optimal duration of bisphosphonate therapy has not been established.
Factors to consider for duration of anti-osteoporosis therapy include
bone mineral density, response to therapy, and risk factors for
continued bone loss or fracture. Women treated with a bisphosphonate
should undergo a dental examination with preventive dentistry prior to
the initiation of therapy, and should take supplemental calcium and
vitamin D.
Evidence suggests that a healthy lifestyle may lead to better breast
cancer outcomes. A nested case control study of 369 women with
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-50
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
ER-positive tumors who developed a second primary breast cancer
compared with 734 matched control patients who did not develop a
second primary tumor showed an association between obesity (body
mass index [BMI] ≥30), smoking, and alcohol consumption and
contralateral breast cancer.471 A prospective study of 1490 women
diagnosed with stage I–III breast cancer showed an association
between high fruit and vegetable consumption, physical activity, and
improved survivorship, regardless of obesity.472 There is emerging
evidence that obesity is associated with poorer outcomes for certain
subtypes of breast cancers. The study by the Women’s Intervention
Nutrition group randomized early-stage breast cancer patients to an
intervention group and a control group. The intervention consisted of
eight one-on-one visits with a registered dietitian who had been trained
on a low-fat eating plan. OS analysis showed no significant difference
between the two study arms (17% for the intervention vs. 13.6%
without); however, subgroup analysis showed that those with ER- and
PR-negative disease who were part of the intervention group saw a
54% improvement in OS.473
The NCCN Panel recommends an active lifestyle and ideal body weight
(BMI 20–25) for optimal overall health and breast cancer outcomes as
there are reports of proven benefits of exercise and active lifestyle
during and after treatment.474-476
For management of issues related to survivorship including late/long-
term effects of cancer and its treatment, see the NCCN Guidelines for
Survivorship.
Stage IV Metastatic or Recurrent Breast Cancer
Staging and Workup
The staging evaluation of women who present with metastatic or
recurrent breast cancer includes history and physical exam; the
performance of a CBC, LFTs, chest diagnostic CT, bone scan, and
radiographs of any long or weight-bearing bones that are painful or
appear abnormal on bone scan; consideration of diagnostic CT of the
abdomen (with or without diagnostic CT of the pelvis) or MRI scan of
the abdomen; and biopsy documentation of first recurrence if possible.
The panel generally discourages the use of sodium fluoride PET or
PET/CT scans for the evaluation of patients with recurrent disease,
except in those situations where other staging studies are equivocal or
suspicious. There is limited evidence (mostly from retrospective studies)
to support the use of PET/CT scanning to guide treatment planning
through determination of the extent of disease in select patients with
recurrent or metastatic disease.125,126,477,478 The panel considers biopsy of
equivocal or suspicious sites to be more likely than PET/CT scanning to
provide accurate staging information in this population of patients.
The consensus of the panel is that FDG PET/CT is optional (category
2B) and most helpful in situations where standard imaging results are
equivocal or suspicious. The NCCN Panel recommends bone scan or
sodium fluoride PET/CT to detect bone metastases (category 2B).
However, if the FDG PET results clearly indicate bone metastasis,
these scans can be omitted.
The NCCN Panel recommends that metastatic disease at presentation
or first recurrence of disease should be biopsied as a part of the workup
for patients with recurrent or stage IV disease. This ensures accurate
determination of metastatic/recurrent disease and tumor histology, and
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-51
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
allows for biomarker determination and selection of appropriate
treatment.
Determination of hormone receptor status (ER and PR) and HER2
status should be repeated in all cases when diagnostic tissue is
obtained. ER and PR assays may be falsely negative or falsely positive,
and there may be discordance between the primary and metastatic
tumors.479,480 The reasons for the discordance may relate to change in
biology of disease, differential effect of prior treatment on clonal
subsets, tumor heterogeneity, or imperfect accuracy and reproducibility
of assays.480 Discordance between the receptor status of primary and
recurrent disease has been reported in a number of studies. The
discordance rates are in the range of 3.4% to 60% for ER-negative to
ER-positive; 7.2% to 31% for ER-positive to ER-negative; and 0.7% to
11% for HER2.481-490
The NCCN Panel recommends that re-testing the receptor status of
recurrent disease be performed, especially in cases when it was
previously unknown, originally negative, or not overexpressed. For
patients with clinical courses consistent with hormone receptor–positive
breast cancer, or with prior positive hormone receptor results, the panel
has noted that a course of endocrine therapy is reasonable, regardless
of whether the receptor assay is repeated or the result of the most
recent hormone receptor assay.
Genetic counseling is recommended if the patient is considered to be at
high risk for hereditary breast cancer, as defined by the NCCN
Guidelines for Genetic/Familial High-Risk Assessment: Breast and
Ovarian.
Management of Local Disease Only
Patients with local recurrence only are divided into 3 groups: those who
had been treated initially by mastectomy alone, those who had been
treated initially by mastectomy plus radiation therapy, and those who
had received breast-conserving therapy.
In one retrospective study of local recurrence patterns in women with
breast cancer who had undergone mastectomy and adjuvant
chemotherapy without radiation therapy, the most common sites of local
recurrence were at the chest wall and the supraclavicular lymph
nodes.491 The recommendations for treatment of the population of
patients experiencing a local recurrence only are supported by analyses
of a combined database of patients from the EORTC 10801 and Danish
Breast Cancer Cooperative Group 82TM trials. The analyses compared
breast-conserving therapy with mastectomy in patients with stage I and
stage II disease. The 133 (approximately 8%) patients experiencing a
local recurrence as an initial event were approximately equally divided
between those who had undergone mastectomy and those who had
received breast-conserving therapy as initial treatment for breast
cancer. Of those in the former group, 51 (76%) were able to undergo
radiation therapy with or without surgery as treatment for local disease
recurrence. No difference in survival emerged between patients
receiving treatment after initial treatment with mastectomy or
breast-conserving therapy; approximately 50% of both groups were
alive at 10-year follow-up.492
According to the NCCN Panel, mastectomy-treated patients should
undergo surgical resection of the local recurrence (if it can be
accomplished without heroic surgery) and involved-field radiation
therapy to the chest wall and supraclavicular area (if the chest wall was
not previously treated or if additional radiation therapy may be safely
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-52
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
administered). The use of surgical resection in this setting implies the
use of limited excision of disease with the goal of obtaining clear
margins of resection. Unresectable chest wall recurrent disease should
be treated with radiation therapy if no prior radiation has been given.
Women with a local recurrence of disease after initial breast-conserving
therapy should undergo a total mastectomy and axillary staging if a
level I/II axillary dissection was not previously performed. Limited data
suggest that a repeat SLN biopsy following local recurrence of disease
may be successfully performed in 80% of women who have previously
undergone breast-conserving therapy and sentinel node biopsy.493 The
consensus of the panel is that the preferred surgical approach for most
women with a local recurrence following breast-conserving therapy and
sentinel node biopsy is mastectomy and a level I/II axillary dissection,
although sentinel node biopsy in lieu of a level I/II axillary dissection can
be considered if prior axillary staging was done by sentinel node biopsy
only.
The results of the CALOR trial found that after complete resection in
patients with isolated locoregional recurrence, adjuvant chemotherapy
improves both DFS and OS.494 After median follow-up of 4.9 years, the
overall DFS was 69% in the chemotherapy group versus 57% in the
group that did not receive chemotherapy (HR = 0.59, P = .046).494 Five-
year OS in all patients in the study was also significantly improved with
chemotherapy (88% vs. 76%, P = .024).494 The benefit of adjuvant
chemotherapy was mostly seen in women with ER-negative disease.
Among women with ER-negative disease, 5-year DFS was 67% versus
35% (HR, 0.32; 95% CI, 0.14–0.73) and in those ER-positive disease,
the 5-year DFS was 70% versus 69% (HR, 0.94; 95% CI, 0.47–1.89).494
According to the NCCN Panel, after local treatment, women with local
recurrences only should be considered for limited duration systemic
chemotherapy or endocrine therapy similar to that outlined in the
adjuvant chemotherapy section. The panel emphasized the importance
of individualizing treatment strategies in patients with a recurrence of
disease limited to a local site.
Management of Stage IV or Recurrent Metastatic Disease
The systemic treatment of breast cancer recurrence or stage IV disease
prolongs survival and enhances quality of life but is not curative.
Therefore, treatments associated with minimal toxicity are preferred.
Thus, the use of the minimally toxic endocrine therapies is preferred to
the use of cytotoxic therapy whenever reasonable.495
Guideline Stratification for Therapy in Systemic Disease
Patients with recurrence of breast cancer or metastatic breast cancer at
diagnosis are initially stratified according to whether bone metastasis is
present. These two patient subsets are then stratified further by tumor
hormone receptor and HER2 status.
Supportive Therapy for Bone Metastases
Treatment targeting osteoclast activity is of value in patients with
metastatic breast cancer in bone to prevent bone fractures, bone pain
requiring radiation therapy, spinal cord compression, and hypercalcemia
(skeletal-related events; SREs).496-498 The bisphosphonates zoledronic
acid or pamidronate have been used for this purpose, and there is
extensive clinical trial support for their efficacy in prevention of SREs
(see section below on Bisphosphonates). Denosumab is a fully human
monoclonal antibody directed against RANK ligand, a mediator of
osteoclast function.499 A single, randomized, active, controlled trial in
metastatic breast cancer showed equivalency and superiority of time to
the occurrence of SRE with denosumab, as compared with zoledronic
acid.498 No study of bisphosphonate or denosumab has demonstrated
an impact on OS in patients with metastatic disease.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-53
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
The bisphosphonates and denosumab are associated with a risk of
development of osteonecrosis of the jaw (ONJ). Poor baseline dental
health or dental procedures during treatment are known risk factors for
ONJ. Thus, a dental examination with preventive dentistry intervention
is recommended prior to treatment with intravenous bisphosphonate or
denosumab, and dental procedures during treatment should be avoided
if at all possible. Additional risk factors for the development of ONJ
include administration of chemotherapy or corticosteroids and poor oral
hygiene with periodontal disease and dental abscess.500
Confirmation of metastatic disease by imaging, including x-ray,
diagnostic CT, or MRI, and initial evaluation of serum calcium,
creatinine, phosphorous, and magnesium levels should be undertaken
prior to the initiation of intravenous bisphosphonate treatment or
subcutaneous denosumab treatment in patients with metastatic
disease. Frequent measurement of calcium, phosphorous, and
magnesium may be prudent since hypophosphatemia and
hypocalcemia have been reported.
Bisphosphonates
An intravenous bisphosphonate (eg, pamidronate, zoledronic acid) in
combination with oral calcium citrate and vitamin D supplementation
should be used in women with bone metastasis, especially if lytic and/or
in weight-bearing bone, if expected survival is 3 months or longer, and if
creatinine levels are below 3.0 mg/dL (category 1).497,501-506
Bisphosphonates are given in addition to chemotherapy or endocrine
therapy. Zoledronic acid may be superior to pamidronate in lytic breast
metastasis.507,508
There are extensive data from randomized trials in support of the use of
bisphosphonates for patients with metastatic disease to bone. The
randomized clinical trial data include the use of zoledronic acid and
pamidronate in the United States and ibandronate and clodronate in
European countries.504,506,508-513 In metastatic bone disease,
bisphosphonate treatment is associated with fewer SREs, fewer
pathologic fractures, and less need for radiation therapy and surgery to
treat bone pain.
The use of bisphosphonates in metastatic disease is a palliative care
measure. No impact on OS has been observed in patients treated with
bisphosphonates. The data indicate that zoledronic acid and
pamidronate may be given on a 3- to 5-week schedule in conjunction
with antineoplastic therapy (ie, endocrine therapy, chemotherapy,
biologic therapy). Recent data from a phase III study showed
zoledronic acid administered once every 12 weeks versus the current
standard of once every four weeks does not compromise efficacy
among women with breast cancer and bone metastases. The SRE rate
was 22% when zoledronic acid was administered every 4 weeks versus
23.2% when administered once every 12 weeks.514
The use of bisphosphonates should be accompanied by calcium and
vitamin D supplementation with daily doses of calcium of 1200 to 1500
mg and vitamin D3 of 400 to 800 IU. Recommended agents for use in
the United States are pamidronate 90 mg intravenously over 2 hours or
zoledronic acid 4 mg intravenously over 15 minutes. The original
studies continued treatment for up to 24 months; however, there are
limited long-term safety data indicating treatment can continue beyond
that time.511,513,515 The risk of renal toxicity necessitates monitoring of
serum creatinine prior to administration of each dose and dose
reduction or discontinuation if renal function is reduced. Current clinical
trial results support the use of bisphosphonates for up to 2 years.
Longer durations of bisphosphonate therapy may provide additional
benefit, but this has not yet been tested in clinical trials.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-54
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
ONJ, a complication of bisphosphonate treatment, has been described.
In a review of more than 16,000 cancer patients, an increased risk for
jaw or facial bone surgery along with an increased risk of being
diagnosed with inflammatory conditions or osteomyelitis of the jaw with
the use of intravenous bisphosphonates was documented. An absolute
risk of 5.48 events per 100 patients treated was seen, with an increase
in risk associated with an increase in cumulative dose of drug.516 It is
recommended that patients should undergo a dental examination with
preventive dentistry prior to initiation of bisphosphonate therapy.
Denosumab
Women with metastatic breast cancer to bone who are candidates for
bisphosphonate therapy may also be considered for treatment with
denosumab (category 1). This recommendation is based on the results
of a single randomized trial comparing denosumab to zoledronic acid.498
All trial patients were recommended to supplement with vitamin D and
calcium. Patients on the experimental arm were given 120 mg of
denosumab injected subcutaneously every 4 weeks plus intravenous
placebo versus the control arm where patients were given an
intravenous infusion of 4 mg of zoledronic acid every 4 weeks, and a
subcutaneous placebo. In this trial with non-inferiority as the primary
endpoint, denosumab was shown to significantly delay time to first SRE
by 18% as compared with zoledronic acid (HR, 0.82; 95% CI, 0.71–
0.95; P < .001 for non-inferiority; P = .01 for superiority) and time to first
and subsequent SREs (rate ratio, 0.77; 95% CI, 0.66–0.89; P = .001).
No difference in time to progression or OS was observed. Adverse
event profiles were similar for the two groups, including incidence of
ONJ, with a reduced risk of renal-related and acute phase adverse
events in the denosumab treatment group. Long-term risks of
denosumab treatment are unknown. The optimal duration of treatment
with denosumab is not known.
Endocrine Therapy for Stage IV or Recurrent Metastatic Disease
Women with recurrent or metastatic disease characterized by tumors
that are ER- and/or PR-positive are appropriate candidates for initial
endocrine therapy.
In premenopausal women without previous exposure to an
antiestrogen, initial treatment is with selective ER modulator alone or
ovarian suppression/ablation plus endocrine therapy as for
postmenopausal women.517 In premenopausal women who received a
prior endocrine therapy within 12 months, the preferred second-line
therapy is ovarian ablation or suppression followed by endocrine
therapy as for postmenopausal women.
Endocrine therapies for recurrent/stage IV disease in postmenopausal
women include nonsteroidal aromatase inhibitors (anastrozole and
letrozole); steroidal aromatase inhibitors (exemestane); serum ER
modulators (tamoxifen or toremifene); ER down-regulators (fulvestrant);
progestin (megestrol acetate); androgens (fluoxymesterone); and
high-dose estrogen (ethinyl estradiol) and recently several new
combination therapies with novel agents have become available such
as exemestane with everolimus, palbociclib in combination with
fulvestrant, and palbociclib with letrozole.
According to some studies, in postmenopausal women, aromatase
inhibitors appear to have superior outcome compared with tamoxifen,
although the differences are modest.518-521 A Cochrane review has also
suggested a survival benefit favoring the aromatase inhibitors over
other endocrine therapies, although the advantage is small.522 A
randomized phase III trial comparing tamoxifen with exemestane as
first-line endocrine therapy for postmenopausal women with metastatic
breast cancer showed no significant differences in progression-free
survival (PFS) or OS between the two arms.520
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-55
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
Fulvestrant appears to be at least as effective as anastrozole in patients
whose disease progressed on previous tamoxifen.523,524 A randomized
phase II study compared anastrozole versus fulvestrant in over 200
patients with advanced breast cancer.525,526 In the initial analysis,
fulvestrant was as effective as anastrozole in terms of overall response
(36.0% vs. 35.5%; odds ratio, 1.02; 95% CI, 0.56 –1.87; P = .947) in
evaluable patients (n = 89 for fulvestrant and n = 93 for anastrozole).525
An improved time to progression was seen with fulvestrant compared to
anastrazole (median time to progression was 23.4 months for
fulvestrant vs. 13.1 months for anastrozole; HR, 0.63; 95% CI, 0.39–
1.00; P = .0496).526 This study used a higher 500 mg loading dose every
2 weeks for 3 doses and then 500 mg monthly.525 The median OS was
observed to be longer in the fulvestrant group than in the anastrozole
group (54.1 months vs. 48.4 months; HR, 0.70; P = .041).527 These
findings are currently being studied in a prospective phase III trial
(ClinicalTrials.gov identifier: NCT01602380).
A phase II study of fulvestrant in postmenopausal women with
advanced breast cancer and disease progression following aromatase
inhibitor therapy documented a partial response rate of 14.3% with an
additional 20.8% of patients achieving stable disease for at least 6
months.528 The clinical benefit rates of exemestane and fulvestrant
observed in a phase III trial of postmenopausal women with hormone
receptor-positive advanced breast cancer who experienced disease
progression on prior nonsteroidal aromatase inhibitor therapy were
comparable (32.2% vs. 31.5%; P = .853).529 In that study, fulvestrant
was administered as a 500 mg loading dose followed by doses of 250
mg on day 14, day 28, and then monthly.
A separate phase III randomized study in postmenopausal women with
metastatic ER-positive breast cancer compared fulvestrant 500 mg
every 2 weeks for 3 doses followed by 500 mg monthly versus
fulvestrant 250 mg monthly. The PFS was superior with the fulvestrant
500 mg regimen (HR, 0.80; 95% CI, 0.68–0.94; P = .006),530 indicating
an increased duration of response with the higher dose of fulvestrant.
The final analyses demonstrated an increase in median OS (4.1
months) and reduced risk of death (19%) with a dose of 500 mg
compared with 250 mg. Median OS was 26.4 versus 22.3 months (HR,
0.81; 95% CI, 0.69–0.96; P = .02).531
Combination endocrine therapy in postmenopausal women with
hormone receptor-positive, previously untreated metastatic breast
cancer has been reported from two studies comparing single-agent
anastrozole versus anastrozole plus fulvestrant.
In one study (FACT), combination endocrine therapy was not superior
to single-agent anastrozole (time to progression HR, 0.99; 95% CI,
0.81–1.20; P = .91).532 In the second study (S0226), PFS (HR, 0.80;
95% CI, 0.68–0.94; stratified log-rank P = .007) and OS (HR, 0.81; 95%
CI, 0.65–1.00; stratified P = .049) were superior with combination
anastrozole plus fulvestrant.533 An unplanned subset analysis in this trial
suggested that patients without prior adjuvant tamoxifen experienced
the greatest benefit. The reason for the divergent outcomes in these two
studies is not known.
A phase III trial studied the effect of fulvestrant alone or in combination
with anastrozole or exemestane in patients with advanced breast
cancer and an acquired non-steroidal aromatase inhibitor-resistant
disease.534 An aromatase inhibitor had been given as adjuvant
treatment to 18% of patients for a median of 27.9 months, and to 82%
of patients for locally advanced/metastatic disease for a median of 19.3
months. Median PFS was 4.8 months, 4.4 months, and 3.4 months for
patients treated with fulvestrant alone, anastrazole plus fulvestrant, and
fulvestrant plus exemestane, respectively. No differences were
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-56
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
observed for overall response rate, clinical benefit rate, and OS. This
trial provides no evidence that adding an aromatase inhibitor to
fulvestrant in patients with non-steroidal aromatase inhibitor-resistant
disease improves the results achieved with fulvestrant alone. In
postmenopausal women who have received previous antiestrogen
therapy and are within one year of antiestrogen exposure, there is
evidence supporting the use of a selective aromatase inhibitor as the
preferred first-line therapy for their recurrent disease.535,536
Palbociclib, a highly selective inhibitor of CDK 4/6 kinase activity, has a
role in treating women with ER-positive metastatic breast cancer in
combination with endocrine therapy. A phase II, open-label,
randomized, multicenter trial evaluated the safety and efficacy of
palbociclib in combination with letrozole versus letrozole alone as first-
line treatment for patients with advanced ER-positive, HER2- negative
breast cancer.537 Median PFS reported was double with the
combination regimen compared to letrozole alone (20.2 months for the
palbociclib plus letrozole group and 10.2 months for the letrozole alone
group; HR, 0.488; 95% CI, 0.319–0.748).537 Grade 3/4 adverse
reactions reported at a higher incidence in the palbociclib plus letrozole
versus letrozole alone group included neutropenia (54% vs. 1%) and
leukopenia (19% vs. 0%). Based on this study, the FDA approved
palbociclib in combination with letrozole for the treatment of
postmenopausal women with ER-positive, HER2-negative advanced
breast cancer as initial endocrine-based therapy for their metastatic
disease.
The phase III trial (PALOMA-3) compared the combination of palbociclib
and fulvestrant to fulvestrant in pre- or post-menopausal hormone
receptor-positive, HER2-negative advanced breast cancer patients,
whose disease progressed on prior endocrine therapy. Pre- or peri-
menopausal patients also received goserelin. The median PFS was 9.2
months for the combination compared to 3.8 months for fulvestrant (HR
0.42, P < .000001) with similar discontinuation rates because of
adverse effects (2.6% and 1.7%, respectively).538 Grade 3/4 adverse
events of palbociclib and fulvestrant were mainly confined to
neutropenia with the same low incidence (0.6%) of febrile neutropenia
in both arms. OS data from this trial are immature.538
The NCCN Panel has included the combination of palbociclib with
letrozole as a first-line endocrine therapy option for postmenopausal
patients with hormone receptor-positive, HER2-negative metastatic
breast cancer. In addition, the recently updated version includes
palbociclib with fulvestrant as a category 1 option for women with
hormone receptor-positive (post-menopausal or premenopausal women
receiving ovarian suppression with an LHRH agonist), HER2-negative
metastatic breast cancer who have progressed on endocrine therapy.
Limited studies document a PFS advantage of adding trastuzumab or
lapatinib to aromatase inhibition in postmenopausal women with
hormone receptor-positive metastatic breast cancer that is HER2-
positive.539,540
Resistance to endocrine therapy in women with hormone
receptor-positive disease is frequent. One mechanism of resistance to
endocrine therapy is activation of the mammalian target of rapamycin
(mTOR) signal transduction pathway. Several randomized studies have
investigated the use of aromatase inhibition in combination with
inhibitors of the mTOR pathway.
A randomized phase II study estimated the efficacy of tamoxifen alone
versus tamoxifen combined with everolimus, an oral inhibitor of mTOR,
in women with hormone receptor-positive, HER2-negative metastatic
breast cancer previously treated with an aromatase inhibitor.541 After a
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-57
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
median follow-up of 13 months, an intent-to-treat analysis showed that
the clinical benefit was 42.1% (95% CI, 29.1–55.9) with tamoxifen alone
and 61.1% (95% CI, 46.9–74.1) with tamoxifen plus everolimus. An
improvement in median time to progression was seen when everolimus
was combined with tamoxifen compared with tamoxifen alone. Median
time to progression was 4.5 months (95% CI, 3.7–8.7) with tamoxifen
alone versus 8.5 months (95% CI, 6.01–13.9) with everolimus and
tamoxifen.541
A phase III trial in postmenopausal women with advanced, hormone
receptor-positive breast cancer with no prior endocrine therapy for
advanced disease, randomized subjects to letrozole with or without the
mTOR inhibitor temsirolimus has been reported.542 In this study, PFS
was not different between the treatment arms (HR, 0.89; 95% CI, 0.75–
1.05; long-rank P = .18).
The results of this trial differ from that of the BOLERO-2 trial (described
below). The reasons for the differences in the outcomes of these two
randomized phase III studies542,543 is uncertain, but may be related to the
issues of patient selection and extent of prior endocrine therapy.
A phase III study (BOLERO-2) randomized postmenopausal women
with hormone receptor-positive advanced breast cancer that had
progressed or recurred during treatment with a nonsteroidal aromatase
inhibitor to exemestane with or without the mTOR inhibitor
everolimus.544 Final results reported after median 18-month follow-up
show that median PFS (by central review) remained significantly longer
with everolimus plus exemestane versus placebo plus exemestane at
11.0 versus 4.1 months, respectively; (HR, 0.38; 95% CI, 0.31–0.48; P
< .0001).543 The adverse events (all grades) that occurred more
frequently in those receiving everolimus included stomatitis, infections,
rash, pneumonitis, and hyperglycemia.543,544 Analysis of safety and
efficacy in the elderly patients enrolled in this trial showed that elderly
patients treated with an everolimus-containing regimen had similar
incidences of these adverse events, but the younger patients had more
on-treatment deaths.545 Based on the evidence from the BOLERO-2
trial, the NCCN Panel has included everolimus plus exemestane as an
option for women who fulfill the entry criteria for BOLERO-2.
Many premenopausal and postmenopausal women with
hormone-responsive breast cancer benefit from sequential use of
endocrine therapies at disease progression. Therefore, women with
breast cancers who respond to an endocrine maneuver with either
shrinkage of the tumor or long-term disease stabilization (clinical
benefit) should receive additional endocrine therapy at disease
progression. After second-line endocrine therapy, little high-level
evidence exists to assist in selecting the optimal sequence of endocrine
therapy. Additional endocrine therapies for second-line and subsequent
therapy are listed in the NCCN Guidelines for Breast Cancer.
Endocrine therapy may be active in patients with negative ER and PR
determinations, especially on the primary tumor and in soft tissue
disease and/or bone-dominant disease.546-548 Endocrine therapy is
associated with relatively low toxicity. Further false-negative
determinations of ER and PR tumor status are not unusual and the
hormone receptor status of primary and metastatic sites of disease may
differ. Therefore, the NCCN Breast Cancer Panel recommends
consideration of a trial of endocrine therapy for patients with disease
characterized as hormone receptor-negative with disease localized to
the bone or soft tissue only or with asymptomatic visceral disease,
irrespective of HER2 tumor status.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-58
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
Cytotoxic Chemotherapy for Stage IV or Recurrent Metastatic Disease
Women with hormone receptor-negative tumors not localized to the
bone or soft tissue only, that are associated with symptomatic visceral
metastasis, or that have hormone receptor-positive tumors that are
refractory to endocrine therapy should receive chemotherapy. A variety
of chemotherapy regimens are felt to be appropriate, as outlined in the
treatment algorithm. Combination chemotherapy generally provides
higher rates of objective response and longer time to progression, in
comparison to single-agent chemotherapy. Combination chemotherapy
is, however, associated with an increase in toxicity and is of little
survival benefit.549-553 Furthermore, administering single agents
sequentially decreases the likelihood that dose reductions will be
needed. Thus, the panel finds little compelling evidence that
combination chemotherapy is superior to sequential single agents.
Standard clinical practice is to continue first-line chemotherapy until
progression. Adverse effects may require dose reduction and cessation
of chemotherapy prior to disease progression. Limited information
suggests that PFS can be prolonged with the use of continuous
chemotherapy versus shorter-course chemotherapy.554,555 Due to the
lack of consistent OS differences, the use of prolonged versus shorter
chemotherapy needs to be weighed against the detrimental effects of
continuous chemotherapy on overall quality of life.
Single cytotoxic agents and combination chemotherapy regimens
recommended by the panel for the treatment of patients with metastatic
disease are listed in the NCCN Guidelines.
Single Agents
Single agents are categorized as either preferred or other single agents
based on a balance of the efficacy, toxicity, and treatment schedules of
the drugs. Among preferred single agents, the panel includes: the
anthracyclines, doxorubicin, epirubicin, and pegylated liposomal
doxorubicin; the taxanes, paclitaxel, docetaxel, and albumin-bound
paclitaxel; anti-metabolites, capecitabine and gemcitabine; and
non-taxane microtubule inhibitors, eribulin and vinorelbine.
Eribulin is a non-taxane microtubule inhibitor used for the treatment of
patients with metastatic breast cancer who have previously received at
least two chemotherapeutic regimens for the treatment of metastatic
disease. Prior therapy should have included an anthracycline and a
taxane in either the adjuvant or metastatic setting. In a phase III trial,
762 patients with metastatic breast cancer were randomized 2:1 to
eribulin or treatment of physicians’ choice. One-year OS was 53.9% for
patients receiving eribulin versus 43.7% for the control arm, and median
OS was 13.12 versus 10.65 months, representing a 19% statistically
significant risk reduction (P = .041). Time to progression was greater
with eribulin 3.7 versus 2.2 months for patients in the control arm (P =
.14).556
Several studies have demonstrated that eribulin is active in metastatic
breast cancer. A large randomized trial of heavily pre-treated patients
with metastatic breast cancer compared treatment with eribulin versus
therapy of physician’s choice. Eribulin demonstrated significant
improvement in OS with 2.5-month prolongation of median OS (median
OS for patients treated with eribulin was 13.1 months compared with
10.6 months for those receiving other treatments. HR, 0.81; 95% CI,
0.66–0.99; P = .041).556
A phase III trial compared eribulin with capecitabine in patients with
metastatic breast cancer. While a survival advantage was observed with
eribulin treatment in all sub-groups of patients, there was a significant
survival advantage observed with eribulin over capecitabine among
patients with HER2-negative (15.9 vs. 13.5 months; HR 0.84; 95% CI
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-59
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
0.72, 0.98; P = .03) and triple-negative (14.4 vs. 9.4 months; HR 0.70;
95% CI 0.55, 0.91; P = .01) breast cancer.557
Among other single agents, the panel includes: cyclophosphamide,
carboplatin, docetaxel, albumin-bound paclitaxel, cisplatin, ixabepilone,
and epirubicin.
Ixabepilone, an epothilone B analogue, is also used for treatment of
recurrent or metastatic breast cancer as a single agent. Use of
ixabepilone as monotherapy has been evaluated in several phase II
trials of women with metastatic breast cancer: in a first-line setting in
patients previously treated with anthracycline chemotherapy 558; in
patients with taxane-resistant metastatic breast cancer559; and in
patients with advanced breast cancer resistant to an anthracycline, a
taxane, and capecitabine.560 In the phase II trials, objective response
rate, median duration of response, and median OS duration were 41.5%
(95% CI, 29.4%–54.4%), 8.2 months (95% CI, 5.7–10.2 months), and
22.0 months (95% CI, 15.6–27.0 months) in the first-line setting;558 12%
(95% CI, 4.7%– 26.5%), 10.4 months, and 7.9 months for the
taxane-resistant patients;559 and 11.5% (95% CI, 6.3%–18.9%), 5.7
months, and 8.6 months for the patients previously treated with an
anthracycline, a taxane, and capecitabine.560 In the study by Perez et
al,560 grade 3/4 treatment-related toxicities included peripheral sensory
neuropathy (14%) and neutropenia (54%).
Combination Regimens
Among combination regimens, the panel includes FAC/CAF; FEC; AC;
EC; CMF; docetaxel, capecitabine; gemcitabine, paclitaxel;
gemcitabine, carboplatin; and paclitaxel, bevacizumab.
A series of trials have sought to define the role for bevacizumab, a
humanized monoclonal antibody against the vascular endothelial
growth factor in the treatment of metastatic breast cancer. The E2100
trial randomized 722 women with recurrent or metastatic breast cancer
to first-line chemotherapy with paclitaxel with or without bevacizumab.561
This trial documented superior PFS (11.8 months vs. 5.9 months; HR
0.60; P <.001) favoring bevacizumab plus paclitaxel compared with
paclitaxel alone. A similar trial enrolled 736 patients who were
randomized to treatment with docetaxel and bevacizumab or docetaxel
and placebo.562 This trial also documented increased PFS in the arm
containing bevacizumab (10.1 months vs. 8.2 months with docetaxel
alone; HR 0.77; P = .006). An additional trial, RIBBON-1, combined
bevacizumab with capecitabine, with a taxane (docetaxel,
nab-paclitaxel), with anthracyclines (FEC, CAF, AC, or EC), or with the
same chemotherapy alone. Results of this trial show a statistically
significant increase in PFS with bevacizumab and capecitabine (8.6
months vs. 5.7 months; HR, 0.69; P < .001) and taxane- or
anthracycline- (9.2 months vs. 8.0 months; HR, 0.64; P < .001)
containing arms.563,564 None of these studies demonstrates an increase
in OS or quality of life when analyzed alone or in a meta-analysis
combining the trials.565 The increase in PFS with bevacizumab is
modest, and appears the greatest in combination with paclitaxel,
especially as reported in an unpublished analysis provided to the
FDA.566
As with endocrine therapy, sequential responses are often observed
with chemotherapy, supporting the use of sequential single agents and
combination chemotherapy regimens. The current guidelines include
doses and schedules of these single agents and combination regimens
for metastatic breast cancer. Failure to achieve a tumor response to 3
sequential chemotherapy regimens or ECOG performance status of 3 or
greater is an indication for supportive therapy only. In this context,
failure to respond to a chemotherapy regimen means the absence of
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-60
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
even a marginal response to the use of a given chemotherapy regimen.
Response to a chemotherapy regimen followed by progression of
disease is not considered a failure to experience response.
Patients with metastatic breast cancer frequently develop many
anatomically localized problems that may benefit from local irradiation,
surgery, or regional chemotherapy (eg, intrathecal methotrexate for
leptomeningeal carcinomatosis).
HER2-Targeted Therapy for Stage IV or Recurrent Metastatic Disease
Patients with tumors that are HER2-positive may derive benefit from
treatment with HER2-targeted therapy. The panel recommends
selecting patients for HER2-targeted therapy if their tumors are either
positive for HER2 by ISH or 3+ by IHC. HER2 testing recommendations
are described in the guideline. Patients with tumors IHC 0 or 1+ for
HER2 or ISH not amplified have very low rates of HER2-targeted
response and HER2-targeted therapy.567 Adequate standardization and
validation of HER2 assays by ISH and IHC used in clinical practice is a
concern, and data suggest that false-positive determinations are
common.22,23,568-570 Recommendations regarding HER2 testing have
been published.568,570
First-Line Regimens for HER2-Positive Tumors
The NCCN Panel has categorized HER2-targeting regimens as either
preferred or other.
Preferred First-Line Regimens
A randomized, double-blind, phase III study compared the efficacy and
safety of pertuzumab in combination with trastuzumab and docetaxel
versus trastuzumab and docetaxel as first-line treatment for
HER2-positive metastatic breast cancer.571 The primary endpoint of the
study was independent assessment of PFS. The secondary endpoints
were PFS assessed by investigator, objective response rate, OS, and
safety. A total of 808 patients were enrolled in this trial.571 The addition
of pertuzumab provided a statistically significant improvement in PFS
compared to trastuzumab plus docetaxel alone. The median
independently assessed PFS was increased by 6.1 months, from 12.4
months in the control group to 18.5 months in the pertuzumab group
(HR for progression or death, 0.62; 95% CI, 0.51– 0.75; P < .001).571 At
a median follow-up of 30 months the results showed a statistically
significant improvement in OS in favor of the pertuzumab-containing
regimen, with a 34% reduction in the risk of death (HR, 0.66; 95% CI,
0.52–0.84; P = .0008). The median OS was 37.6 months in the
non-pertuzumab group and had not yet been reached in the
pertuzumab-containing regimen.432 The most common adverse
reactions reported in the pertuzumab group compared to the control
group were diarrhea, rash, mucosal inflammation, febrile neutropenia,
and dry skin. Peripheral edema and constipation were greater in the
control group.571 Cardiac adverse events or left ventricular systolic
dysfunction were reported slightly more frequently in the control
group.572 Health-related quality of life was not different in the two
treatment groups.573
Phase II trials have also found activity and tolerability for pertuzumab,
pertuzumab with trastuzumab, and for other regimens combining
pertuzumab and trastuzumab together with other active cytotoxics (ie,
paclitaxel, vinorelbine).574,575,576 Phase III trials of pertuzumab plus
chemotherapy without trastuzumab have not been reported.
The NCCN Panel recommends pertuzumab plus trastuzumab in
combination with a taxane as a preferred option for first-line treatment of
patients with HER2-positive metastatic breast cancer. Pertuzumab plus
trastuzumab in combination with docetaxel is an NCCN category 1 and
in combination with paclitaxel is an NCCN category 2A
recommendation.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-61
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
Other First-Line Regimens for HER2-Positive Tumors
First-line trastuzumab in combination with selected
chemotherapeutics266 or as a single agent265,267 is another option for
HER2-positive metastatic breast cancer patients. Randomized trials
demonstrate benefit from adding trastuzumab to other agents including
paclitaxel with or without carboplatin,266,567,577,578 docetaxel,577 and
vinorelbine,577 or as a single agent267 for patients with HER2-positive
disease. In addition, the combination of trastuzumab and capecitabine
has also shown efficacy as a first-line trastuzumab-containing regimen
in this population of patients.579,580 For those patients with hormone
receptor-positive, HER2-positive disease, the panel recommends initial
treatment with endocrine therapy, an approach consistent with most of
these studies. The panel believes the 27% frequency of significant
cardiac dysfunction in patients treated with the combination of
trastuzumab and doxorubicin/cyclophosphamide chemotherapy in the
metastatic setting is too high for use of this combination outside the
confines of a prospective clinical trial.266,580,581
T-DM1 is an antibody-drug conjugate. Through a stable linker, the
HER2-targeting antitumor property of trastuzumab is conjugated with
the cytotoxic activity of the microtubule-inhibitory agent DM1 (derivative
of maytansine).
A randomized, international, multicenter, open-label, phase III study
(EMILIA) evaluated the safety and efficacy of T-DM1 compared with
lapatinib plus capecitabine for HER2-positive patients with locally
advanced breast cancer or metastatic breast cancer previously treated
with trastuzumab and a taxane.582 The primary endpoints of this study
were PFS, OS, and safety. T-DM1 demonstrated a statistically
significant improvement in both primary endpoints of PFS and OS. PFS
(assessed by independent review) was significantly improved with
T-DM1 with median PFS of 9.6 months vs. 6.4 months with lapatinib
plus capecitabine; HR for progression or death from any cause was
0.65 (95% CI, 0.55–0.77; P < .001). At the first interim analysis, T-DM1
also demonstrated significant improvement in OS. The stratified HR for
death from any cause with T-DM1 versus lapatinib plus capecitabine
was 0.62 (95% CI, 0.48–0.81; P = .0005).582 Rates of grade 3 or 4
adverse events were higher with lapatinib plus capecitabine than with
T-DM1 (57% vs. 41%). The incidences of thrombocytopenia and
increased serum aminotransferase levels were higher with T-DM1
(frequency >25%), whereas the incidences of diarrhea, nausea,
vomiting, and palmar-plantar erythrodysesthesia were higher with
lapatinib plus capecitabine.582
In a phase III trial (MARIANNE), 1,095 patients with locally advanced or
metastatic breast cancer were randomized to first-line treatment with T-
DM1 with or without pertuzumab or to treatment with trastuzumab plus a
taxane. The primary endpoints were safety and PFS assessed by
independent review. The PFS for T-DM1 with pertuzumab was found
non-inferior to trastuzumab and a taxane (15.2 and 13.7 months
respectively; HR, 0.87; 97.5% CI, 0.69–1.08; P = .14).583 The PFS for T-
DM1 alone was non-inferior to trastuzumab plus a taxane (14.1 and
13.7, respectively; HR, 0.91; 97.5% CI, 0.73–1.13; P = .31).583 The
incidence of Grade 3–5 adverse events was 54.1%, 45.4%, and 46.2%
in the trastuzumab plus a taxane arm, T-DM1 arm, and T-DM1 plus
pertuzumab arm, respectively. Health-related quality of life was
maintained for a longer duration with a median of 7.7 months for T-DM1
(HR, 0.70; 95% CI, 0.57–0.86) and a median of 9 months for T-DM1
plus pertuzumab (HR, 0.68; 95% CI, 0.55–0.84) compared with a
median of 3.9 months for trastuzumab and a taxane.583
Based on the MARIANNE trial data demonstrating T-DM1 and T-DM1
with pertuzumab being non-inferior, with better QOL compared with
trastuzumab plus taxane and possibly better-tolerated for some
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-62
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
patients, 583 the NCCN Panel included T-DM1 as one of the first-line
options for treatment of patients with HER2-positive metastatic breast
cancer. Pertuzumab, trastuzumab, and a taxane, however, remains the
preferred frontline regimen for HER2-positive metastatic disease based
on data demonstrating improved OS compared to trastuzumab and a
taxane. TDM-1 as first-line therapy should be considered only in those
not suitable for the preferred treatment.
Regimens for Trastuzumab-Exposed HER2-Positive Disease
The NCCN Panel recommends continuation of HER2 blockade for
patients with HER2-positive metastatic breast cancer that progresses
on first-line trastuzumab-containing regimens. This recommendation
also applies to patients who are diagnosed with HER2-positive
metastatic disease after prior exposure to trastuzumab in the adjuvant
setting. Several trials have demonstrated benefit of continuation of
trastuzumab therapy following disease progression on a
trastuzumab-containing regimen.584-586 However, the optimal duration of
trastuzumab in patients with long-term control of disease is unknown.
The NCCN Guidelines include doses and schedules of representative
regimens for use in HER2-positive metastatic breast cancer.
Pertuzumab is active in patients beyond the first-line setting. The results
of a multicenter, open-label, single-arm, phase II study (n = 66) show
that the combination of pertuzumab and trastuzumab is active and well
tolerated in patients with HER2-positive metastatic breast cancer that
has progressed on prior trastuzumab therapy.587 The trial reported an
objective response rate of 24.2% (16 patients out of 66). The overall
median PFS time observed with pertuzumab and trastuzumab
combination was 15.5 months (range, 0.9–17.0 months; 80% CI, 18–31
months.587 The reported median duration of response with the
combination was 5.8 months (range, 2.9–15.3 months).587
To determine whether the clinical benefit seen in the study was from
pertuzumab alone or was a result of the combined effect of pertuzumab
and trastuzumab, a cohort of patients (n = 29) whose disease
progressed during prior trastuzumab-based therapy received
pertuzumab monotherapy until progressive disease or unacceptable
toxicity. Of these, patients with disease progression (n = 17) continued
to receive pertuzumab with the addition of trastuzumab. In the 29
patients who received pertuzumab monotherapy, the objective response
rate and clinical benefit rate reported were 3.4% and 10.3%,
respectively, whereas in the patients who received dual blockade after
progression on pertuzumab, the objective response rate and clinical
benefit rate were 17.6% and 41.2%, respectively.588
According to the NCCN Panel, for patients with disease progression
after treatment with trastuzumab-based therapy without pertuzumab, a
line of therapy containing both trastuzumab plus pertuzumab with or
without a cytotoxic agent (such as vinorelbine or taxane) may be
considered. Further research is needed to determine the ideal
sequencing strategy for anti-HER2 therapy.
The regimen of capecitabine plus lapatinib is also an option for patients
with HER2-positive disease following progression on a
trastuzumab-containing regimen. A phase III study compared lapatinib
plus capecitabine with capecitabine alone in women with advanced or
metastatic breast cancer refractory to trastuzumab in the metastatic
setting and with prior treatment with an anthracycline and a taxane in
either the metastatic or adjuvant setting.589 Time to progression was
increased in the group receiving combination therapy when compared
with the group receiving capecitabine monotherapy (8.4 months vs. 4.4
months; HR, 0.49; 95% CI, 0.34–0.71; P < .001). The patients who
progressed on monotherapy were allowed to cross over to the
combination arm. This resulted in insufficient power to detect significant
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-63
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
differences in OS; an exploratory analysis demonstrated a trend toward
a survival advantage with lapatinib plus capecitabine.590 The analysis
reported a median OS of 75.0 weeks for the combination arm and 64.7
weeks for the monotherapy arm (HR, 0.87; 95% CI, 0.71–1.08; P =
.210).590
Another study of women with metastatic breast cancer showed that
lapatinib in combination with letrozole increased PFS over letrozole
alone in the subset of women with HER2-positive cancer (3.0 months
for letrozole and placebo vs. 8.2 months for letrozole and lapatinib; HR,
0.71; 95% CI, 0.53–0.96; P = .019).539 In addition, results from a phase
III trial in which patients with heavily pretreated metastatic breast cancer
and disease progression on trastuzumab therapy were randomly
assigned to monotherapy with lapatinib or trastuzumab plus lapatinib
showed that PFS was increased from 8.1 weeks to 12 weeks (P = .008)
with the combination.591 The OS analysis data showed that lapatinib
plus trastuzumab improved median survival by 4.5 months, with median
OS of 14 months for the combination therapy and 9.5 months for
lapatinib alone (HR, 0.74; 95% CI, 0.57–0.97; P =.026). 592 This
improvement in OS analysis included patients who were initially
assigned to monotherapy and crossed over to receive combination
therapy at the time of progression.592
Based on the absence of data, the panel does not recommend the
addition of chemotherapy to the trastuzumab and lapatinib combination.
Surgery for Stage IV or Recurrent Metastatic Disease
The primary treatment approach recommended by the NCCN Panel for
women with metastatic breast cancer and an intact primary tumor is
systemic therapy, with consideration of surgery after initial systemic
treatment for those women requiring palliation of symptoms or with
impending complications, such as skin ulceration, bleeding, fungation,
and pain.593 Generally such surgery should be undertaken only if
complete local clearance of tumor may be obtained and if other sites of
disease are not immediately threatening to life. Alternatively, radiation
therapy may be considered as an option to surgery. Often such surgery
requires collaboration between the breast surgeon and the
reconstructive surgeon to provide optimal cancer control and wound
closure.
Retrospective studies suggest a potential survival benefit from complete
excision of the in-breast tumor in select patients with metastatic breast
cancer.594-597 Substantial selection biases exist in all of these studies
and are likely to confound the study results.598,599 Two recent
prospective, randomized studies assessed whether or not surgery on
the primary tumor in the breast is necessary for women who are
diagnosed with metastatic/stage IV breast cancer. The results from both
studies presented at the 2013 Annual San Antonio Breast Cancer
Symposium were similar showing that surgical treatment of primary
tumors in women presenting with stage IV disease does not produce an
increase in OS.600,601
Nevertheless, the panel recognizes the need for randomized clinical
trials that will address the risks and benefits of local therapy for patients
with stage IV disease while eliminating selection biases. Patient
enrollment in such trials is encouraged.
Distant Sites of Recurrence Requiring Consideration of Therapies Local
to the Metastatic Site
Surgery, radiation, or regional chemotherapy (eg, intrathecal
methotrexate) may be indicated as needed for localized clinical
scenarios such as brain metastases, leptomeningeal disease, choroid
metastases, pleural effusion, pericardial effusion, biliary obstruction,
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-64
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
ureteral obstruction, impending pathologic fracture, cord compression,
localized painful bone, or soft-tissue disease.
The guidelines include consideration of the addition of hyperthermia to
irradiation for localized recurrences/metastasis (category 3). There have
been several prospective randomized trials comparing radiation to
radiation plus hyperthermia in the treatment of locally
advanced/recurrent cancers, primarily breast cancer chest wall
recurrences.602,603 While there is heterogeneity among the study results,
a series with strict quality assurance demonstrated a statistically
significant increase in local tumor response and greater duration of local
control with the addition of hyperthermia to radiation compared to
radiation alone.602 No differences in OS have been demonstrated.
Delivery of local hyperthermia is technically demanding and requires
specialized expertise and equipment (eg, the monitoring of
temperatures and management of possible tissue burns). The panel
thus recommends that the use of hyperthermia be limited to treatment
centers with appropriate training, expertise, and equipment. The
addition of hyperthermia generated substantial discussion and
controversy among the panel and is a category 3 recommendation.
Monitoring Metastatic Disease
Monitoring the treatment of metastatic breast cancer involves a wide
array of assessments and the need for the clinician to integrate several
different forms of information to make a determination of the
effectiveness of treatment and the acceptability of toxicity. The
information includes those from direct observations of the patient,
including patient-reported symptoms, performance status, change in
weight, and physical examination; laboratory tests such as alkaline
phosphatase, liver function, blood counts, and calcium; radiographic
imaging; functional imaging; and, where appropriate, tumor biomarkers.
The results of these evaluations generally are classified as response,
continued response to treatment, stable disease, uncertainty regarding
disease status, or progression of disease. The clinician typically must
assess and balance multiple different forms of information to make a
determination regarding whether disease is being controlled and the
toxicity of treatment is acceptable. Sometimes this information may be
contradictory.
The panel recommends using widely accepted criteria for reporting
response, stability, and progression of disease such as the RECIST
criteria604 and the WHO criteria.605 The NCCN Panel also recommends
using the same method of assessment over time. For example, an
abnormality initially found on diagnostic CT scan of the chest should be
monitored with repeat diagnostic CT scans of the chest.
The optimal frequency of testing is uncertain, and is primarily based on
the monitoring strategies utilized in breast cancer clinical trials. The
page titled Principles of Monitoring Metastatic Disease in the algorithm
provides a table outlining general recommendations for the frequency
and type of monitoring as a baseline before initiation of new therapy, for
monitoring the effectiveness of cytotoxic chemotherapy and endocrine
therapy, and as an assessment when there is evidence of disease
progression. The panel has indicated in a footnote that the frequency of
monitoring can be reduced in patients who have long-term stable
disease. These are guidelines and should be modified for the individual
patient using clinical judgment, especially for those with stable or
responding disease for long periods of time.
The clinical use of Circulating Tumor Cells (CTC) in metastatic breast
cancer is not yet included in the NCCN Guidelines for Breast Cancer for
disease assessment and monitoring. Patients with persistently
increased CTC after 3 weeks of first-line chemotherapy have a poor
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-65
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
PFS and OS.606 In spite of its prognostic ability, CTC count has failed to
show a predictive value. A prospective, randomized, phase 3 trial
(SWOG S0500) evaluated the clinical utility of serial enumeration of
CTC in patients with metastatic breast cancer. 606 According to the study
results, switching to an alternative cytotoxic therapy after 3 weeks of
first-line chemotherapy in patients with persistently increased CTC did
not affect either PFS or OS.606
Special Situations
Paget’s Disease
Paget’s disease of the breast is a rare manifestation of breast cancer
characterized by neoplastic cells in the epidermis of the NAC.607 It most
commonly presents with eczema of the areola, bleeding, ulceration, and
itching of the nipple. The diagnosis is often delayed because of the rare
nature of the condition and confusion with other dermatologic
conditions. There is an associated cancer elsewhere in the breast in up
to about 80% to 90% of cases.608-610 The associated cancers are not
necessarily located adjacent to the NAC and may be either DCIS or
invasive cancer.
Women with clinical signs that raise suspicion for Paget’s disease
require a complete history and physical examination and diagnostic
breast imaging. Any breast lesion identified by imaging or examination
should be evaluated according to the NCCN Guidelines for Breast
Screening and Diagnosis. The skin of the NAC should undergo surgical
biopsy, including the full thickness of the epidermis including at least a
portion of any clinically involved NAC. When biopsy of the NAC is
positive for Paget’s disease, breast MRI is recommended to define the
extent of disease and identify additional disease.610,611
There are no category 1 data that specifically address local
management of Paget’s disease. Systemic therapy is based on the
stage and biological characteristics of any underlying cancer, and is
supported by the evidence cited in the relevant stage-specific breast
cancer treatment guidelines.
Management of Paget’s disease has traditionally been total mastectomy
with axillary dissection. Total mastectomy remains a reasonable option
for patients regardless of the absence or presence of an associated
breast cancer.609 Data demonstrate that satisfactory local control may
be achieved with breast-conserving surgery including the excision with
negative margins of any underlying breast cancer along with resection
of the NAC followed by whole breast radiation therapy.612-616 The risk of
ipsilateral breast recurrence after breast-conserving NAC resection and
radiation therapy with or without an associated cancer is similar to that
with breast-conserving surgery and radiation therapy with the typical
invasive or in situ cancer.
For Paget’s disease without an associated cancer (ie, no palpable mass
or imaging abnormality), it is recommended that breast-conserving
surgery consist of removal of the entire NAC with a negative margin of
underlying breast tissue. In cases with an associated cancer elsewhere
in the breast, the surgery includes removal of the NAC with a negative
margin and removal of the peripheral cancer using standard
breast-conserving technique to achieve a negative margin. It is not
necessary to remove the NAC and the peripheral cancer in continuity in
a single surgical specimen or through a single incision. Mastectomy
also remains an appropriate treatment option.
ALN staging is not necessary when breast-conserving therapy is used
to treat Paget’s disease with underlying DCIS without evidence of
invasive cancer following clinical examination, imaging evaluation, and
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-66
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
full-thickness skin biopsy of the involved NAC. In the presence of an
underlying invasive breast cancer treated with breast-conserving
surgery, axillary surgery should be performed according to the Surgical
Axillary Staging outlined in the NCCN Guidelines. In cases treated by
total mastectomy, axillary staging is recommended for patients with
invasive disease and should also be considered for patients with
underlying DCIS without evidence of invasive disease. This is because
the final pathology may reveal an invasive cancer in the mastectomy
specimen and the mastectomy precludes subsequent sentinel node
biopsy. Two retrospective studies have provided evidence for a high
degree of accuracy in the identification of the sentinel node(s) in
patients with Paget’s disease.617,618 Patients treated with breast
conservation should receive whole breast radiation. Extended-field
radiation to regional lymph nodes should be used in cases of an
associated invasive breast cancer with involved lymph nodes as for any
breast cancer as described in the initial sections of the NCCN
Guidelines. A radiation boost should be considered for the site of the
resected NAC and any associated resected cancer site, if applicable.
Women with an associated invasive cancer have substantial risk of
developing metastases. Adjuvant systemic therapy should be
administered according to the stage of the cancer. Women with Paget’s
disease treated with breast conservation and without an associated
cancer or those with associated ER-positive DCIS should consider
tamoxifen for risk reduction. Those with an associated invasive cancer
should receive adjuvant systemic therapy based on the stage and
hormone receptor status.
Phyllodes Tumors of the Breast
(also known as phyllodes tumors, cystosarcoma phyllodes)
Phyllodes tumors of the breast are rare tumors comprised of both
stromal and epithelial elements.619 Phyllodes tumors exist in benign,
borderline, and malignant subtypes, although there is not uniform
agreement on the criteria for assigning subtype or for predicting
biological behavior.620 The subtype of phyllodes tumor appears less
important for risk of recurrence than does the margin of tumor-free
resection achieved by surgical treatment. Diagnosis of phyllodes tumors
prior to excisional biopsy/lumpectomy is uncommon. Phyllodes tumors
occur in an older age distribution than fibroadenoma, a younger age
distribution than the invasive ductal and lobular cancers, and with a
mean age of 40.621 Phyllodes tumors often enlarge rapidly and are
usually painless. Phyllodes tumors often appear on ultrasound and
mammography as fibroadenomas, and FNA cytology and even core
needle biopsy are inadequate to reliably distinguish phyllodes tumors
from fibroadenoma.621 Thus, in the setting of a large or rapidly enlarging
clinical fibroadenoma, excisional biopsy should be considered to
pathologically exclude a phyllodes tumor. Patients with Li-Fraumeni
syndrome (germline TP53 mutation, see NCCN Guidelines for
Genetic/Familial High Risk Assessment) have an increased risk for
phyllodes tumors.622 Local recurrences of phyllodes tumors are the most
common site of recurrence. Most distant recurrences occur in the lung,
and may be solid nodules or thin-walled cavities.
Treatment of phyllodes tumors (which includes benign, borderline, and
malignant subtypes) is with local surgical excision with tumor-free
margins of 1 cm or greater. Lumpectomy or partial mastectomy is the
preferred surgical therapy. Total mastectomy is necessary only if
negative margins cannot be obtained by lumpectomy or partial
mastectomy.623 Since phyllodes tumors rarely metastasize to the ALNs,
surgical axillary staging or ALN dissection is not necessary unless the
lymph nodes are pathologic on clinical examination.624 In those patients
who experience a local recurrence, resection of the recurrence with
wide, tumor-free surgical margins should be performed. Some panel
members recommend local radiation therapy of the remaining breast or
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-67
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
chest wall following resection of a local recurrence, but this
recommendation is controversial (category 2B).625
While the epithelial component of most phyllodes tumors contains ER
(58%) and/or PR (75%),626 endocrine therapy has no proven role in the
treatment of phyllodes tumors. Similarly, there is no evidence that
adjuvant cytotoxic chemotherapy provides benefit in reduction of
recurrences or death. In the rare patient who experiences a systemic
recurrence (usually in the lung), treatment should be as recommended
in the NCCN Guidelines for Soft Tissue Sarcoma.
Breast Cancer During Pregnancy
Breast cancer occurring concurrently with pregnancy is an infrequent
clinical event. In a California registry study, there were 1.3 breast
cancers diagnosed per 10,000 live births.627 Unfortunately, breast
cancer during pregnancy is most often ALN-positive and with larger
primary tumor size. Histologically the tumors are poorly differentiated,
are more frequently ER/PR-negative, and approximately 30% are
HER2-positive.628,629 The diagnosis is often delayed because neither the
patient nor the physician suspects malignancy.
Evaluation of the pregnant patient with suspected breast cancer should
include a physical examination with particular attention to the breast and
regional lymph nodes. Mammogram of the breast with shielding can be
done safely and the accuracy is reported to be greater than 80%.630
Ultrasound of the breast and regional lymph nodes can be used to
assess the extent of disease and also to guide biopsy. Ultrasound has
been reported to be abnormal in up to 100% of breast cancers occurring
during pregnancy.630 Biopsies for cytologic evaluation of a suspicious
breast mass may be done with FNA of the breast and suspicious lymph
nodes. However, the preferred technique is core needle biopsy. This
provides tissue for histologic confirmation of invasive disease as well as
adequate tissue for hormone receptor and HER2 analyses.
Staging assessment of the pregnant patient with breast cancer may be
guided by clinical disease stage. The staging studies should be tailored
to minimize fetal exposure to radiation. For clinically node-negative
T1-T2 tumors, a chest x-ray (with shielding), liver function and renal
function assessment, and a CBC with differential are appropriate. In
patients who have clinically node-positive or T3 breast lesions, in
addition to the aforementioned, an ultrasound of the liver and
consideration of a screening MRI of the thoracic and lumbar spine
without contrast may be employed. The documentation of the presence
of metastases may alter the treatment plan and influence the patient’s
decision regarding maintenance of the pregnancy. Assessment of the
pregnancy should include a maternal fetal medicine consultation and
review of antecedent maternal risks such as hypertension, diabetes,
and complications with prior pregnancies. Documentation of fetal growth
and development and fetal age by means of ultrasonographic
assessment is appropriate. Estimation of the date of the delivery will
help with systemic chemotherapy planning. In addition, maternal fetal
medicine consultation should include counseling regarding maintaining
or terminating pregnancy. Counseling of the pregnant patient with
breast cancer should include a review of the treatment options, which
include mastectomy or breast-conserving surgery as well as the use of
systemic therapy. The most common surgical procedure has been
modified radical mastectomy. However, breast-conserving surgery is
possible if radiation therapy can be delayed to the postpartum period,631
and breast-conserving therapy during pregnancy does not appear to
have a negative impact on survival.631,632 When surgery is performed at
25 weeks of gestation or later, obstetrical and prenatal specialists must
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-68
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
be onsite and immediately available in the event of precipitous delivery
of a viable fetus.
Although there are a limited number of isolated case reports and small
retrospective studies evaluating use of SLN biopsy in pregnant
patients,633,634 the sensitivity and specificity of the procedure has not
been established in this setting. Thus, there are insufficient data on
which to base recommendations for its use in pregnant women.
Decisions related to use of SLN biopsy in pregnancy should be
individualized. A review of the relative and absolute contraindications to
sentinel node biopsy concluded that sentinel node biopsy should not be
offered to pregnant women under 30 weeks gestation.635 There are
limited data with only case reports and estimations of fetal radiation
dose regarding use of radioactive tracer (eg, technetium 99m sulfur
colloid).636-638 Isosulfan blue or methylene blue dye for sentinel node
biopsy procedures is discouraged during pregnancy.
The indications for systemic chemotherapy are the same in the
pregnant patient as in the non-pregnant breast cancer patient, although
chemotherapy should not be administered at any point during the first
trimester of pregnancy. The largest experience in pregnancy has been
with anthracycline and alkylating agent chemotherapy.639,640 Collected
data of chemotherapy exposure in utero indicate that the first trimester
has the greatest risk of fetal malformation.641,642 Fetal malformation risks
in the second and third trimester are approximately 1.3%, not different
than that of fetuses not exposed to chemotherapy during pregnancy. If
systemic therapy is initiated, fetal monitoring prior to each
chemotherapy cycle is appropriate. Chemotherapy during pregnancy
should not be given after week 35 of pregnancy or within 3 weeks of
planned delivery in order to avoid the potential for hematologic
complications during delivery. Data from a single-institution prospective
study indicate that FAC chemotherapy (5-FU 500 mg/m2 IV days 1 and
4, doxorubicin 50 mg/m2 by IV infusion over 72 hours, and
cyclophosphamide 500 mg/m2 IV day 1) may be given with relative
safety during the second and third trimesters of pregnancy.640 As
reported by Gwyn et al, the median gestational age at delivery was 38
weeks, more than 50% of the patients had a vaginal delivery, and there
were no fetal deaths.628 An update of this experience reported on 57
women treated with FAC in the adjuvant or neoadjuvant setting. There
were 57 live births. A survey of parents/guardians reported on the
health of 40 children. There was one child with Down syndrome and two
with congenital abnormalities (club foot, congenital bilateral ureteral
reflux). The children are reported to be healthy and progressing well in
school.640,643 Ondansetron, lorazepam, and dexamethasone can be used
as part of the pre-chemotherapy antiemetic regimen.
There are limited data on the use of taxanes during pregnancy.644-647 If
used, the NCCN Panel recommends weekly administration of paclitaxel
after the first trimester if clinically indicated by disease status. There are
only case reports of trastuzumab use during pregnancy.648-655 The
majority of these case reports indicated oligo- or anhydramnios with
administration of trastuzumab; fetal renal failure occurred in one case. If
trastuzumab is otherwise indicated, it should be administered in the
postpartum period; the panel recommends against its use during
pregnancy.
A single case report of first trimester exposure to lapatinib during
treatment for breast cancer reported an uncomplicated delivery of a
healthy female neonate.656
Endocrine therapy and radiation therapy are contraindicated during
pregnancy. Endocrine therapy and radiation therapy, if indicated, should
thus not be initiated until the postpartum period.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-69
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
Communication between the oncologist and maternal fetal medicine
specialist is essential at every visit and for every treatment decision
point for the patient.
Inflammatory Breast Cancer
Inflammatory breast cancer (IBC) is a rare, aggressive form of breast
cancer estimated to account for 1% to 6% of breast cancer cases in the
United States.657,658 IBC is a clinical diagnosis that requires erythema
and dermal edema (peau d’orange) of a third or more of the skin of the
breast.
IBC is usually hormone receptor-negative and is more frequently
HER2-positive than the usual ductal breast cancers. Studies on gene
expression profiling of IBC have demonstrated that all the subtypes of
IBC exist, but basal and HER2 overexpressed are more frequent.659-662
According to the 7th edition of the AJCC Cancer Staging Manual, IBC is
classified as stage IIIB, stage IIIC, or stage IV breast cancer, depending
on the degree of nodal involvement and whether distant metastases are
present. The primary tumor of IBC is classified as T4d by definition,
even when no mass is specifically apparent in the breast. On
radiographic imaging, findings of skin thickening and, in some cases, an
underlying mass are observed. Despite use of the term “inflammatory,”
the characteristic clinical features of IBC are due to blockage of dermal
lymphatics by tumor emboli. Although a biopsy is required to evaluate
for the presence of cancer in breast tissue and the dermal lymphatics, a
diagnosis of IBC is based on clinical findings, and dermal lymphatic
involvement is neither required, nor sufficient by itself, to assign a
diagnosis of IBC.11,663 The differential diagnosis includes cellulitis of the
breast and mastitis.
In the past, IBC has often been placed under the general heading of
locally advanced breast cancer. There is a growing body of evidence
that IBC patients, when compared with noninflammatory forms of locally
advanced breast cancer, are more likely to have a less favorable
prognosis664-666 and to be younger at the time of disease presentation.667
The NCCN Panel acknowledges that studies focusing on genetic
characterization of IBC are needed to more clearly define IBC as a
disease entity and to optimize treatment.668,669 Nevertheless, current
evidence provides justification for a separate guideline for the workup
and treatment of patients diagnosed with IBC.
StageT4d, N0- N3, M0
Workup
Women with a clinical/pathologic diagnosis of IBC without distant
metastasis (stage T4d, N0-N3, M0) should undergo a thorough staging
evaluation by a multidisciplinary team.
Recommendations for workup include a complete history and physical
examination involving a CBC and platelet count.
A pathology review and pre-chemotherapy determinations of tumor
hormone receptor and HER2 receptor status should be performed.
HER2 has a predictive role in determining which patients with IBC will
benefit from HER2-targeted therapy. The NCCN Panel endorses the
CAP protocol for pathology reporting (www.cap.org) and endorses the
ASCO CAP recommendations for quality control performance of HER2
testing and interpretation of IHC and ISH results.570
Imaging studies help facilitate image-guided biopsy, delineate
locoregional disease, and identify distant metastases. Evaluation of all
women suspected with IBC must include diagnostic bilateral
mammogram, with the addition of ultrasound as necessary. A breast
MRI scan is optional.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-70
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
Evaluations for the presence of distant metastasis in the asymptomatic
patient include LFTs, bone scan or sodium fluoride PET/CT (category
2B), and diagnostic CT imaging of the chest, abdomen, and pelvis
(category 2B; category 2A for diagnostic CT imaging of the chest when
pulmonary symptoms are present).
FDG PET/CT may be most helpful in situations where standard imaging
results are equivocal or suspicious. However, there is limited evidence
suggesting that PET/CT may be a useful adjunct to standard imaging of
IBC due to the increased risk of regional lymph node involvement and
distant spread of disease in this group of patients.125,126,670,671
Nevertheless, equivocal or suspicious sites identified by FDG PET/CT
scanning or other imaging methods should be biopsied for confirmation
of stage IV disease whenever possible. FDG PET/CT is a category 2B
recommendation. The consensus of the panel is that FDG PET/CT can
be performed at the same time as diagnostic CT. If FDG PET and
diagnostic CT are performed and both clearly indicate bone metastases,
bone scan or sodium fluoride PET/CT may not be needed.
Genetic counseling is recommended if the patient is considered to be at
high risk for hereditary breast cancer as defined by the NCCN
Guidelines for Genetic/Familial High-Risk Assessment: Breast and
Ovarian.
Treatment
The treatment of patients with IBC should involve a combined modality
approach657 comprising preoperative systemic therapy followed by
surgery (mastectomy)andradiotherapy.
Preoperative Chemotherapy
There are no large randomized trials evaluating the optimal systemic
treatment of IBC, since it is a rare disease. The systemic therapy
recommendations are based on data from retrospective analyses, small
prospective studies, and data from non-IBC, locally advanced breast
cancer.
The benefit of preoperative systemic therapy followed by mastectomy
over preoperative systemic therapy alone in patients with IBC was
shown in a retrospective analysis in which lower local recurrence rates
and longer disease-specific survival were reported for the combined
modality approach.672 Results from a large retrospective study of
patients with IBC performed over a 20-year period at The University of
Texas M.D. Anderson Cancer Center demonstrated that initial treatment
with doxorubicin-based chemotherapy followed by local therapy (ie,
radiation therapy or mastectomy, or both) and additional postoperative
chemotherapy resulted in a 15-year DFS rate of 28%.673
A retrospective study demonstrated that the addition of a taxane to an
anthracycline-based regimen improved PFS and OS in patients with
ER-negative IBC.674 A systematic review found evidence for an
association between the intensity of preoperative therapy and the
likelihood of a pCR.675 A study of IBC patients, with cytologically
confirmed ALN metastases, treated with anthracycline-based
chemotherapy with or without a taxane indicated that more patients
receiving the anthracycline-taxane combination achieved a pCR
compared with those who received only anthracycline-based therapy. In
addition, patients who had a pCR in the ALNs had superior OS and
DFS compared with those with residual axillary disease.676
The NCCN Panel recommends preoperative systemic therapy with an
anthracycline-based regimen with or without taxanes for the initial
treatment of patients with IBC. The panel also recommends completing
the planned chemotherapy prior to mastectomy. If the chemotherapy
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-71
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
was not completed preoperatively, it should be completed
postoperatively.
Targeted Therapy
All women with hormone receptor-positive IBC are recommended to
receive endocrine therapy sequentially after completing the planned
preoperative systemic therapy.
HER2-positive IBC is associated with a poor prognosis.661,677 For women
with HER2-positive disease, the addition of trastuzumab to primary
systemic chemotherapy is associated with better response rates.678-682 A
prospective study that randomized women with locally advanced breast
cancers, including those with IBC, to neoadjuvant anthracycline-based
chemotherapy with or without trastuzumab for 1 year demonstrated that
the addition of trastuzumab significantly improved the response rate and
event-free survival.678 The NCCN Panel recommends inclusion of
trastuzumab in the chemotherapy regimen and is recommended for
patients with HER2-positive disease.There are no available data to
indicate the optimal duration of trastuzumab, specifically among women
with IBC. However, based on the available data,678 the panel
recommends continuing trastuzumab therapy for up to 1 year.
Results of small phase II trials indicate that other HER2-targeting
agents such as lapatinib and pertuzumab have a clinical benefit in
IBC.314,683 The results of the NEOSPHERE trial that included patients
with IBC showed increased pCR with the pertuzumab-containing
regimens. Therefore, the NCCN Panel has included in a footnote that a
pertuzumab-containing regimen may be administered preoperatively in
patients with HER2-positive IBC.314
Determination of response to neoadjuvant chemotherapy in IBC should
include a combination of physical examination and radiologic
assessment.
Surgery
Patients with a clinical/pathologic diagnosis of IBC should always be
treated with chemotherapy before surgery. It has been known for many
years that surgical treatment as primary treatment of patients with IBC
is associated with poor outcomes.684 SLN dissection is not a reliable
method of assessing ALNs among women with IBC.685 Use of
breast-conserving surgery in patients with IBC has been associated with
poor cosmesis, and limited data suggest that rates of local recurrence
may be higher when compared with mastectomy. Breast-conserving
therapy is not recommended for patients with IBC.
Mastectomy with level I/II ALN dissection is the recommended surgical
procedure recommended by the NCCN Panel for patients who respond
to neoadjuvant chemotherapy. The NCCN Panel has listed delayed
breast reconstruction as an option that can be recommended to women
with IBC who have undergone a modified radical mastectomy.
Reconstruction of the breasts soon after mastectomy may compromise
the post-mastectomy radiation therapy outcomes.686
For patients with IBC who do not respond to preoperative systemic
therapy, mastectomy is not generally recommended. Additional
systemic chemotherapy and/or preoperative radiation should be
considered for these patients. Patients with tumors responding to this
secondary therapy should undergo mastectomy and subsequent
treatment as described above.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-72
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
Radiation
After mastectomy, radiation therapy is recommended after the
completion of the planned chemotherapy.
The probability of locoregional lymph node involvement is high for
women with IBC. To reduce the risk of local recurrence, the panel
recommends radiation therapy to the chest wall and the supraclavicular
region. If the internal mammary lymph node(s) is clinically or
pathologically involved, radiation therapy should include the internal
mammary nodes. If the internal mammary nodes are not clinically or
pathologically involved, then including the internal mammary nodes in
the radiation therapy field is at the discretion of the treating radiation
oncologist (category 3). For HER2-positive disease, trastuzumab may
be administered concomitantly with radiation therapy.
Stage IV or Recurrent IBC
Patients with stage IV or recurrent IBC should be treated according to
the guidelines for recurrence/stage IV breast cancer (See NCCN
Guidelines for Breast Cancer).
Axillary Breast Cancer
Occult breast cancer presenting with axillary metastases is an unusual
presentation that can be a diagnostic and therapeutic challenge.
Evidence to support recommendations on the management of patients
presenting with axillary breast cancer comes from a limited number of
retrospective studies involving small numbers of patients687-689(see also
references therein). Although treatment of women with axillary
metastases from an unknown primary tumor has typically involved
mastectomy and axillary nodal dissection, some of these patients have
also been successfully treated with axillary nodal dissection followed by
radiation therapy.688,689
Patients with a suspected occult primary breast cancer will typically
present to the oncologist after undergoing an initial biopsy: core needle
biopsy (preferred), and/or FNA. Accurate pathologic assessment of the
biopsied material is most important. Therefore, the pathologist must be
consulted to determine whether the available biopsy material is
adequate, or if additional biopsy material is necessary (eg, core needle,
incisional, or excisional biopsy) to provide an accurate and complete
diagnosis.
Workup for Possible Primary Breast Cancer
MRI of the breast can facilitate the identification of occult breast cancer,
and can help select those patients most likely to benefit from
mastectomy.690 For example, in a study of 40 patients with
biopsy-proven breast cancer in the axilla, and a negative or
indeterminate mammogram, MRI identified the primary breast lesion in
70% of the patients.688 In addition, of the 7 patients with a negative MRI
who subsequently underwent ALN dissection and radiation therapy to
the whole breast, no evidence of local recurrence was evident at a
median follow-up of 19 months.
The NCCN Guidelines for Occult Primary Cancer provide guidance on
the diagnosis and initial workup of patients with a suspicious axillary
mass without any signs of a primary tumor. A small subset of these
patients may have a primary cancer in the axillary tail of the breast.
Adenocarcinoma with positive axillary nodes and mediastinal nodes in a
woman is highly suggestive of a breast primary. Adenocarcinoma in the
supraclavicular nodes, chest, peritoneum, retroperitoneum, liver, bone,
or brain could also indicate primary breast cancer in women. The
guidelines suggest the use of a mammogram and breast ultrasound for
such patients.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-73
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
Testing for immunohistochemical markers including ER/PR and HER2
is recommended. Elevated ER/PR levels provide strong evidence for a
breast cancer diagnosis.691 MRI of the breast should be considered for a
patient with histopathologic evidence of breast cancer when
mammography and ultrasound are not adequate to assess the extent of
the disease. MRI may be especially helpful in women with dense breast
tissue, positive axillary nodes, and suspected occult primary breast
tumor or to evaluate the chest wall.692 Breast MRI has been shown to be
useful in identifying the primary site in patients with occult primary
breast cancer and may also facilitate breast conservation in selected
women by allowing for lumpectomy instead of mastectomy.688,693 In one
report, the primary site was identified using MRI in about half of the
women presenting with axillary metastases, irrespective of the breast
density.694
The NCCN Guidelines for Occult Primary Cancer also provide
recommendations for additional workup, including chest and abdominal
CT to evaluate for evidence of distant metastases for patients
diagnosed with adenocarcinoma (or carcinoma not otherwise specified)
of the axillary nodes without evidence of a primary breast lesion. In
particular, breast MRI and ultrasound are recommended. Axillary
ultrasound should also be performed.
Treatment for Possible Primary Breast Cancer
Patients with MRI-positive breast disease should undergo evaluation
with ultrasound or MRI-guided biopsy and receive treatment according
to the clinical stage of the breast cancer. Treatment recommendations
for those with MRI-negative disease are based on nodal status. For
patients with T0, N1, M0 disease, options include mastectomy plus
axillary nodal dissection or axillary nodal dissection plus whole breast
irradiation with or without nodal irradiation. Systemic chemotherapy,
endocrine therapy, or trastuzumab is given according to the
recommendations for stage II or III disease. Neoadjuvant
chemotherapy, trastuzumab, and endocrine therapy should be
considered for patients with T0, N2-N3, M0 disease followed by axillary
nodal dissection and mastectomy as for patients with locally advanced
disease.
Summary
The therapeutic options for patients with noninvasive or invasive breast
cancer are complex and varied. In many situations, the patient and
physician have the responsibility to jointly explore and select the most
appropriate option from among the available alternatives. With few
exceptions, the evaluation, treatment, and follow-up recommendations
in these guidelines are based on the results of past and present clinical
trials. However, there is not a single clinical situation in which the
treatment of breast cancer has been optimized with respect to either
maximizing cure or minimizing toxicity and disfigurement. Therefore,
patient/physician participation in prospective clinical trials allows
patients to not only receive state-of-the-art cancer treatment but also to
contribute to improving the treatment outcomes.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-74
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
References
1. Mohammad H. Forouzanfar KJF, Allyne M. Delossantos, Rafael
Lozano, Alan D. Lopez, Christopher J. L. Murray, Mohsen Naghavi.
Breast and cervical cancer in 187 countries between 1980 and 2010: a
systematic analysis. The Lancet 2011;6736:61351-61352
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J
Clin 2016;66:7-30. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/26742998.
3. DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013.
CA Cancer J Clin 2014;64:52-62. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/24114568.
4. Effects of chemotherapy and hormonal therapy for early breast
cancer on recurrence and 15-year survival: an overview of the
randomised trials. Lancet 2005;365:1687-1717. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15894097.
5. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J
Clin 2015;65:5-29. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/25559415.
6. Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and
adjuvant therapy on mortality from breast cancer. N Engl J Med
2005;353:1784-1792. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16251534.
7. Gail MH, Costantino JP, Bryant J, et al. Weighing the risks and
benefits of tamoxifen treatment for preventing breast cancer. J Natl
Cancer Inst 1999;91:1829-1846. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/10547390.
8. Dupont WD, Page DL. Risk factors for breast cancer in women with
proliferative breast disease. N Engl J Med 1985;312:146-151. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/3965932.
9. Dupont WD, Page DL. Risk factors for breast cancer in women with
proliferative breast disease. N Engl J Med 1985;312:146-151. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/3965932.
10. U.S. National Library of Medicine-Key MEDLINE® Indicators.
Available at: http://www.nlm.nih.gov/bsd/bsd_key.html.
11. Edge SB, Byrd DR, Compton CC, et al., eds. AJCC Cancer Staging
Manual, 7th Edition. New York: Springer; 2010.
12. White J, Morrow M, Moughan J, et al. Compliance with breast-
conservation standards for patients with early-stage breast carcinoma.
Cancer 2003;97:893-904. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/12569588.
13. Wilkinson NW, Shahryarinejad A, Winston JS, et al. Concordance
with breast cancer pathology reporting practice guidelines. J Am Coll
Surg 2003;196:38-43. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/12517547.
14. Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and
predictive factors in breast cancer by immunohistochemical analysis.
Mod Pathol 1998;11:155-168. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/9504686.
15. Rhodes A, Jasani B, Barnes DM, et al. Reliability of
immunohistochemical demonstration of oestrogen receptors in routine
practice: interlaboratory variance in the sensitivity of detection and
evaluation of scoring systems. J Clin Pathol 2000;53:125-130. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/10767828.
16. Rudiger T, Hofler H, Kreipe HH, et al. Quality assurance in
immunohistochemistry: results of an interlaboratory trial involving 172
pathologists. Am J Surg Pathol 2002;26:873-882. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/12131154.
17. Allred DC, Carlson RW, Berry DA, et al. NCCN Task Force Report:
Estrogen receptor and progesterone receptor testing in breast cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-75
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
by immunohistochemistry. J Natl Compr Canc Netw 2009;7 Suppl 6:S1-
S21; quiz S22-23. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19755043.
18. Hammond MEH, Hayes DF, Dowsett M, et al. American Society of
Clinical Oncology/College Of American Pathologists guideline
recommendations for immunohistochemical testing of estrogen and
progesterone receptors in breast cancer. J Clin Oncol 2010;28:2784-
2795. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20404251.
19. Hammond ME. ASCO-CAP guidelines for breast predictive factor
testing: an update. Appl Immunohistochem Mol Morphol 2011;19:499-
500. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22089488.
20. Wang S, Saboorian MH, Frenkel E, et al. Laboratory assessment of
the status of Her-2/neu protein and oncogene in breast cancer
specimens: comparison of immunohistochemistry assay with
fluorescence in situ hybridisation assays. J Clin Pathol 2000;53:374-
381. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10889820.
21. Dybdal N, Leiberman G, Anderson S, et al. Determination of HER2
gene amplification by fluorescence in situ hybridization and
concordance with the clinical trials immunohistochemical assay in
women with metastatic breast cancer evaluated for treatment with
trastuzumab. Breast Cancer Res Treat 2005;93:3-11. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16184453.
22. Paik S, Bryant J, Tan-Chiu E, et al. Real-world performance of
HER2 testing--National Surgical Adjuvant Breast and Bowel Project
experience. J Natl Cancer Inst 2002;94:852-854. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/12048273.
23. Perez EA, Suman VJ, Davidson NE, et al. HER2 testing by local,
central, and reference laboratories in specimens from the North Central
Cancer Treatment Group N9831 intergroup adjuvant trial. J Clin Oncol
2006;24:3032-3038. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16809727.
24. Tubbs RR, Pettay JD, Roche PC, et al. Discrepancies in clinical
laboratory testing of eligibility for trastuzumab therapy: apparent
immunohistochemical false-positives do not get the message. J Clin
Oncol 2001;19:2714-2721. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11352964.
25. Press MF, Sauter G, Bernstein L, et al. Diagnostic evaluation of
HER-2 as a molecular target: an assessment of accuracy and
reproducibility of laboratory testing in large, prospective, randomized
clinical trials. Clin Cancer Res 2005;11:6598-6607. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16166438.
26. Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for
human epidermal growth factor receptor 2 testing in breast cancer:
American Society of Clinical Oncology/College of American Pathologists
clinical practice guideline update. J Clin Oncol 2013;31:3997-4013.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/24101045.
27. Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for
Human Epidermal Growth Factor Receptor 2 testing in breast cancer:
American Society of Clinical Oncology/College of American Pathologists
Clinical Practice Guideline update. Arch Pathol Lab Med 2014;138:241-
256. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24099077.
28. Giordano SH, Buzdar AU, Hortobagyi GN. Breast cancer in men.
Ann Intern Med 2002;137:678-687. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/12379069.
29. Giordano SH, Valero V, Buzdar AU, Hortobagyi GN. Efficacy of
anastrozole in male breast cancer. Am J Clin Oncol 2002;25:235-237.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/12040279.
30. Rosai J. Borderline epithelial lesions of the breast. Am J Surg Pathol
1991;15:209-221. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/1847606.
31. Schnitt SJ, Connolly JL, Tavassoli FA, et al. Interobserver
reproducibility in the diagnosis of ductal proliferative breast lesions
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-76
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
using standardized criteria. Am J Surg Pathol 1992;16:1133-1143.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/1463092.
32. Renshaw AA, Derhagopian RP, Martinez P, Gould EW. Lobular
neoplasia in breast core needle biopsy specimens is associated with a
low risk of ductal carcinoma in situ or invasive carcinoma on
subsequent excision. Am J Clin Pathol 2006;126:310-313. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16891208.
33. Nagi CS, O'Donnell JE, Tismenetsky M, et al. Lobular neoplasia on
core needle biopsy does not require excision. Cancer 2008;112:2152-
2158. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18348299.
34. Hwang H, Barke LD, Mendelson EB, Susnik B. Atypical lobular
hyperplasia and classic lobular carcinoma in situ in core biopsy
specimens: routine excision is not necessary. Mod Pathol
2008;21:1208-1216. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18660792.
35. Elsheikh TM, Silverman JF. Follow-up surgical excision is indicated
when breast core needle biopsies show atypical lobular hyperplasia or
lobular carcinoma in situ: a correlative study of 33 patients with review
of the literature. Am J Surg Pathol 2005;29:534-543. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15767810.
36. Karabakhtsian RG, Johnson R, Sumkin J, Dabbs DJ. The clinical
significance of lobular neoplasia on breast core biopsy. Am J Surg
Pathol 2007;31:717-723. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17460455.
37. O'Neil M, Madan R, Tawfik OW, et al. Lobular carcinoma in
situ/atypical lobular hyperplasia on breast needle biopsies: does it
warrant surgical excisional biopsy? A study of 27 cases. Ann Diagn
Pathol 2010;14:251-255. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/20637429.
38. Foster MC, Helvie MA, Gregory NE, et al. Lobular carcinoma in situ
or atypical lobular hyperplasia at core-needle biopsy: is excisional
biopsy necessary? Radiology 2004;231:813-819. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15105449.
39. Hussain M, Cunnick GH. Management of lobular carcinoma in-situ
and atypical lobular hyperplasia of the breast--a review. Eur J Surg
Oncol 2011;37:279-289. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/21306860.
40. Rendi MH, Dintzis SM, Lehman CD, et al. Lobular in-situ neoplasia
on breast core needle biopsy: imaging indication and pathologic extent
can identify which patients require excisional biopsy. Ann Surg Oncol
2012;19:914-921. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/21861212.
41. Anderson BO, Calhoun KE, Rosen EL. Evolving concepts in the
management of lobular neoplasia. J Natl Compr Canc Netw
2006;4:511-522. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16687097.
42. Kerlikowske K, Molinaro AM, Gauthier ML, et al. Biomarker
expression and risk of subsequent tumors after initial ductal carcinoma
in situ diagnosis. J Natl Cancer Inst 2010;102:627-637. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/20427430.
43. Stackievicz R, Paran H, Bernheim J, et al. Prognostic significance of
HER-2/neu expression in patients with ductal carcinoma in situ. Isr Med
Assoc J 2010;12:290-295. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/20929083.
44. Zhou W, Jirstrom K, Johansson C, et al. Long-term survival of
women with basal-like ductal carcinoma in situ of the breast: a
population-based cohort study. BMC Cancer 2010;10:653. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/21118480.
45. Lari SA, Kuerer HM. Biological markers in DCIS and risk of breast
recurrence: A systematic review. J Cancer 2011;2:232-261. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/21552384.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-77
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
46. Kuhl CK, Schrading S, Bieling HB, et al. MRI for diagnosis of pure
ductal carcinoma in situ: a prospective observational study. Lancet
2007;370:485-492. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17693177.
47. Kowalchik KV, Vallow LA, McDonough M, et al. The role of
preoperative bilateral breast magnetic resonance imaging in patient
selection for partial breast irradiation in ductal carcinoma in situ. Int J
Surg Oncol 2012;2012:206342. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/22655183.
48. Brennan ME, Turner RM, Ciatto S, et al. Ductal carcinoma in situ at
core-needle biopsy: meta-analysis of underestimation and predictors of
invasive breast cancer. Radiology 2011;260:119-128. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/21493791.
49. Cody HS, Van Zee KJ. Point: sentinel lymph node biopsy is
indicated for patients with DCIS. J Natl Compr Canc Netw 2003;1:199-
206. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19768878.
50. Edge SB, Sheldon DG. Counterpoint: sentinel lymph node biopsy is
not indicated for ductal carcinoma in situ. J Natl Compr Canc Netw
2003;1:207-212. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19768879.
51. Lyman GH, Giuliano AE, Somerfield MR, et al. American Society of
Clinical Oncology guideline recommendations for sentinel lymph node
biopsy in early-stage breast cancer. J Clin Oncol 2005;23:7703-7720.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/16157938.
52. Virnig BA, Tuttle TM, Shamliyan T, Kane RL. Ductal carcinoma in
situ of the breast: a systematic review of incidence, treatment, and
outcomes. J Natl Cancer Inst 2010;102:170-178. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/20071685.
53. Waddell BE, Stomper PC, DeFazio JL, et al. Postexcision
mammography is indicated after resection of ductal carcinoma-in-situ of
the breast. Ann Surg Oncol 2000;7:665-668. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11034243.
54. Bijker N, Meijnen P, Peterse JL, et al. Breast-conserving treatment
with or without radiotherapy in ductal carcinoma-in-situ: ten-year results
of European Organisation for Research and Treatment of Cancer
randomized phase III trial 10853--a study by the EORTC Breast Cancer
Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol
2006;24:3381-3387. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16801628.
55. Emdin SO, Granstrand B, Ringberg A, et al. SweDCIS:
Radiotherapy after sector resection for ductal carcinoma in situ of the
breast. Results of a randomised trial in a population offered
mammography screening. Acta Oncol 2006;45:536-543. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16864166.
56. Fisher B, Dignam J, Wolmark N, et al. Lumpectomy and radiation
therapy for the treatment of intraductal breast cancer: findings from
National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol
1998;16:441-452. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/9469327.
57. Houghton J, George WD, Cuzick J, et al. Radiotherapy and
tamoxifen in women with completely excised ductal carcinoma in situ of
the breast in the UK, Australia, and New Zealand: randomised
controlled trial. Lancet 2003;362:95-9102. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/12867108.
58. Julien JP, Bijker N, Fentiman IS, et al. Radiotherapy in breast-
conserving treatment for ductal carcinoma in situ: first results of the
EORTC randomised phase III trial 10853. EORTC Breast Cancer
Cooperative Group and EORTC Radiotherapy Group. Lancet
2000;355:528-533. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/10683002.
59. Cuzick J, Sestak I, Pinder SE, et al. Effect of tamoxifen and
radiotherapy in women with locally excised ductal carcinoma in situ:
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-78
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
long-term results from the UK/ANZ DCIS trial. Lancet Oncol
2011;12:21-29. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/21145284.
60. Wapnir IL, Dignam JJ, Fisher B, et al. Long-term outcomes of
invasive ipsilateral breast tumor recurrences after lumpectomy in
NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl
Cancer Inst 2011;103:478-488. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/21398619.
61. Holmberg L, Garmo H, Granstrand B, et al. Absolute risk reductions
for local recurrence after postoperative radiotherapy after sector
resection for ductal carcinoma in situ of the breast. J Clin Oncol
2008;26:1247-1252. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18250350.
62. Di Saverio S, Catena F, Santini D, et al. 259 Patients with DCIS of
the breast applying USC/Van Nuys prognostic index: a retrospective
review with long term follow up. Breast Cancer Res Treat
2008;109:405-416. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17687650.
63. Gilleard O, Goodman A, Cooper M, et al. The significance of the
Van Nuys prognostic index in the management of ductal carcinoma in
situ. World J Surg Oncol 2008;6:61-61. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18564426.
64. Silverstein MJ, Lagios MD, Craig PH, et al. A prognostic index for
ductal carcinoma in situ of the breast. Cancer 1996;77:2267-2274.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/8635094.
65. Silverstein MJ, Lagios MD, Groshen S, et al. The influence of
margin width on local control of ductal carcinoma in situ of the breast. N
Engl J Med 1999;340:1455-1461. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/10320383.
66. Hughes LL, Wang M, Page DL, et al. Local excision alone without
irradiation for ductal carcinoma in situ of the breast: a trial of the Eastern
Cooperative Oncology Group. J Clin Oncol 2009;27:5319-5324.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/19826126.
67. Wong P, Lambert C, Agnihotram RV, et al. Ductal carcinoma in situ-
the influence of the radiotherapy boost on local control. Int J Radiat
Oncol Biol Phys 2011. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/21664063.
68. Wong JS, Chen YH, Gadd MA, et al. Eight-year update of a
prospective study of wide excision alone for small low- or intermediate-
grade ductal carcinoma in situ (DCIS). Breast Cancer Res Treat
2014;143:343-350. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/24346130.
69. Goodwin A, Parker S, Ghersi D, Wilcken N. Post-operative
radiotherapy for ductal carcinoma in situ of the breast--a systematic
review of the randomised trials. Breast 2009;18:143-149. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19447038.
70. MacDonald HR, Silverstein MJ, Mabry H, et al. Local control in
ductal carcinoma in situ treated by excision alone: incremental benefit of
larger margins. Am J Surg 2005;190:521-525. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16164913.
71. Dunne C, Burke JP, Morrow M, Kell MR. Effect of margin status on
local recurrence after breast conservation and radiation therapy for
ductal carcinoma in situ. J Clin Oncol 2009;27:1615-1620. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19255332.
72. Van Zee KJ, Subhedar P, Olcese C, et al. Relationship between
margin width and recurrence of ductal carcinoma in situ: Analysis of
2996 women treated with breast-conserving surgery for 30 years. Ann
Surg 2015;262:623-631. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/26366541.
73. Warnberg F, Garmo H, Emdin S, et al. Effect of radiotherapy after
breast-conserving surgery for ductal carcinoma in situ: 20 years follow-
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-79
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
up in the randomized SweDCIS Trial. J Clin Oncol 2014;32:3613-3618.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/25311220.
74. McCormick B, Winter K, Hudis C, et al. RTOG 9804: a prospective
randomized trial for good-risk ductal carcinoma in situ comparing
radiotherapy with observation. J Clin Oncol 2015;33:709-715. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/25605856.
75. Narod SA, Iqbal J, Giannakeas V, et al. Breast Cancer Mortality
After a Diagnosis of Ductal Carcinoma In Situ. JAMA Oncol 2015;1:888-
896. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26291673.
76. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the
prevention of breast cancer: current status of the National Surgical
Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst
2005;97:1652-1662. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16288118.
77. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for
prevention of breast cancer: report of the National Surgical Adjuvant
Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998;90:1371-
1388. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9747868.
78. Tan-Chiu E, Wang J, Costantino JP, et al. Effects of tamoxifen on
benign breast disease in women at high risk for breast cancer. J Natl
Cancer Inst 2003;95:302-307. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/12591986.
79. Allred DC, Bryant J, Land S, et al. Estrogen receptor expression as
a predictive marker of the effectiveness of tamoxifen in the treatment of
DCIS: Findings from the NSABP Protocol B-24 [abstract]. Breast
Cancer Res Treat 2002;76(Suppl 1):Abstract A30. Available at:
80. Forbes JF, Sestak I, Howell A, et al. Anastrozole versus tamoxifen
for the prevention of locoregional and contralateral breast cancer in
postmenopausal women with locally excised ductal carcinoma in situ
(IBIS-II DCIS): a double-blind, randomised controlled trial. Lancet 2015.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/26686313.
81. Margolese RG, Cecchini RS, Julian TB, et al. Anastrozole versus
tamoxifen in postmenopausal women with ductal carcinoma in situ
undergoing lumpectomy plus radiotherapy (NSABP B-35): a
randomised, double-blind, phase 3 clinical trial. Lancet 2015. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/26686957.
82. Louie RJ, Tonneson JE, Gowarty M, et al. Complete blood counts,
liver function tests, and chest x-rays as routine screening in early-stage
breast cancer: value added or just cost? Breast Cancer Res Treat 2015.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/26467045.
83. Esserman L. Integration of imaging in the management of breast
cancer. J Clin Oncol 2005;23:1601-1602. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15755961.
84. Gundry KR. The application of breast MRI in staging and screening
for breast cancer. Oncology (Williston Park) 2005;19:159-169. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/15770888.
85. Houssami N, Ciatto S, Macaskill P, et al. Accuracy and surgical
impact of magnetic resonance imaging in breast cancer staging:
systematic review and meta-analysis in detection of multifocal and
multicentric cancer. J Clin Oncol 2008;26:3248-3258. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18474876.
86. Weber JJ, Bellin LS, Milbourn DE, et al. Selective preoperative
magnetic resonance imaging in women with breast cancer: no reduction
in the reoperation rate. Arch Surg 2012;147:834-839. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/22987175.
87. Feigelson HS, James TA, Single RM, et al. Factors associated with
the frequency of initial total mastectomy: results of a multi-institutional
study. J Am Coll Surg 2013;216:966-975. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/23490543.
88. Katipamula R, Degnim AC, Hoskin T, et al. Trends in mastectomy
rates at the Mayo Clinic Rochester: effect of surgical year and
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-80
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
preoperative magnetic resonance imaging. J Clin Oncol 2009;27:4082-
4088. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19636020.
89. Sorbero ME, Dick AW, Beckjord EB, Ahrendt G. Diagnostic breast
magnetic resonance imaging and contralateral prophylactic
mastectomy. Ann Surg Oncol 2009;16:1597-1605. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19330381.
90. Miller BT, Abbott AM, Tuttle TM. The influence of preoperative MRI
on breast cancer treatment. Ann Surg Oncol 2012;19:536-540.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/21751044.
91. Peters NH, van Esser S, van den Bosch MA, et al. Preoperative
MRI and surgical management in patients with nonpalpable breast
cancer: the MONET - randomised controlled trial. Eur J Cancer
2011;47:879-886. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/21195605.
92. Turnbull LW, Brown SR, Olivier C, et al. Multicentre randomised
controlled trial examining the cost-effectiveness of contrast-enhanced
high field magnetic resonance imaging in women with primary breast
cancer scheduled for wide local excision (COMICE). Health Technol
Assess 2010;14:1-182. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/20025837.
93. Fischer U, Zachariae O, Baum F, et al. The influence of
preoperative MRI of the breasts on recurrence rate in patients with
breast cancer. Eur Radiol 2004;14:1725-1731. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15248080.
94. Solin LJ, Orel SG, Hwang W-T, et al. Relationship of breast
magnetic resonance imaging to outcome after breast-conservation
treatment with radiation for women with early-stage invasive breast
carcinoma or ductal carcinoma in situ. J Clin Oncol 2008;26:386-391.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/18202414.
95. Bleicher RJ, Ciocca RM, Egleston BL, et al. Association of routine
pretreatment magnetic resonance imaging with time to surgery,
mastectomy rate, and margin status. J Am Coll Surg 2009;209:180-187;
quiz 294-185. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19632594.
96. Turnbull L, Brown S, Harvey I, et al. Comparative effectiveness of
MRI in breast cancer (COMICE) trial: a randomised controlled trial.
Lancet 2010;375:563-571. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/20159292.
97. Baucom DH, Porter LS, Kirby JS, et al. Psychosocial issues
confronting young women with breast cancer. Breast Dis 2005;23:103-
113. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16823173.
98. Dunn J, Steginga SK. Young women's experience of breast cancer:
defining young and identifying concerns. Psychooncology 2000;9:137-
146. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10767751.
99. Ganz PA, Greendale GA, Petersen L, et al. Breast cancer in
younger women: reproductive and late health effects of treatment. J Clin
Oncol 2003;21:4184-4193. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/14615446.
100. Gorman JR, Bailey S, Pierce JP, Su HI. How do you feel about
fertility and parenthood? The voices of young female cancer survivors. J
Cancer Surviv 2012;6:200-209. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/22179785.
101. Howard-Anderson J, Ganz PA, Bower JE, Stanton AL. Quality of
life, fertility concerns, and behavioral health outcomes in younger breast
cancer survivors: a systematic review. J Natl Cancer Inst 2012;104:386-
405. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22271773.
102. Kranick JA, Schaefer C, Rowell S, et al. Is pregnancy after breast
cancer safe? Breast J 2010;16:404-411. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/20522097.
103. Sukumvanich P, Case LD, Van Zee K, et al. Incidence and time
course of bleeding after long-term amenorrhea after breast cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-81
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
treatment: a prospective study. Cancer 2010;116:3102-3111. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/20564648.
104. Quinn GP, Block RG, Clayman ML, et al. If you did not document
it, it did not happen: rates of documentation of discussion of infertility
risk in adolescent and young adult oncology patients' medical records. J
Oncol Pract 2015;11:137-144. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/25549654.
105. Yee S, Abrol K, McDonald M, et al. Addressing oncofertility needs:
views of female cancer patients in fertility preservation. J Psychosoc
Oncol 2012;30:331-346. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/22571247.
106. Yeomanson DJ, Morgan S, Pacey AA. Discussing fertility
preservation at the time of cancer diagnosis: dissatisfaction of young
females. Pediatr Blood Cancer 2013;60:1996-2000. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/23836521.
107. Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for
patients with cancer: American Society of Clinical Oncology clinical
practice guideline update. J Clin Oncol 2013;31:2500-2510. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/23715580.
108. Cruz MR, Prestes JC, Gimenes DL, Fanelli MF. Fertility
preservation in women with breast cancer undergoing adjuvant
chemotherapy: a systematic review. Fertil Steril 2010;94:138-143.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/19339000.
109. Dunn L, Fox KR. Techniques for fertility preservation in patients
with breast cancer. Curr Opin Obstet Gynecol 2009;21:68-73. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/19125006.
110. Oktem O, Oktay K. Fertility preservation for breast cancer patients.
Semin Reprod Med 2009;27:486-492. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19806518.
111. Redig AJ, Brannigan R, Stryker SJ, et al. Incorporating fertility
preservation into the care of young oncology patients. Cancer
2011;117:4-10. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/21235031.
112. Lee S, Ozkavukcu S, Heytens E, et al. Value of early referral to
fertility preservation in young women with breast cancer. J Clin Oncol
2010;28:4683-4686. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/20876425.
113. Peate M, Meiser B, Friedlander M, et al. It's now or never: fertility-
related knowledge, decision-making preferences, and treatment
intentions in young women with breast cancer--an Australian fertility
decision aid collaborative group study. J Clin Oncol 2011;29:1670-1677.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/21444865.
114. Blumenfeld Z, Evron A. Preserving fertility when choosing
chemotherapy regimens - the role of gonadotropin-releasing hormone
agonists. Expert Opin Pharmacother 2015;16:1009-1020. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/25826240.
115. Del Mastro L, Lambertini M. Temporary Ovarian Suppression With
Gonadotropin-Releasing Hormone Agonist During Chemotherapy for
Fertility Preservation: Toward the End of the Debate? Oncologist
2015;20:1233-1235. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/26463868.
116. Lambertini M, Peccatori FA, Moore HC, Del Mastro L. Reply to the
letter to the editor 'Can ovarian suppression with gonadotropin releasing
hormone analogs (GnRHa) preserve fertility in cancer patients?' by
Rodriguez-Wallberg et al. Ann Oncol 2015. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/26646756.
117. Moore HC, Unger JM, Phillips KA, et al. Goserelin for ovarian
protection during breast-cancer adjuvant chemotherapy. N Engl J Med
2015;372:923-932. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/25738668.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-82
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
118. Moffat R, Guth U. Preserving fertility in patients undergoing
treatment for breast cancer: current perspectives. Breast Cancer (Dove
Med Press) 2014;6:93-101. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/25114587.
119. Oktay K, Turan V, Bedoschi G, et al. Fertility Preservation Success
Subsequent to Concurrent Aromatase Inhibitor Treatment and Ovarian
Stimulation in Women With Breast Cancer. Journal of Clinical Oncology
2015;33:2424-2429. Available at:
http://jco.ascopubs.org/content/33/22/2424.abstract.
120. Baseline staging tests in primary breast cancer: Practice guideline
report # 1-14: Members of the Breast Cancer Disease Site Group.
Available at:
http://www.cancercare.on.ca/common/pages/UserFile.aspx?serverId=6
&path=/File%20Database/CCO%20Files/PEBC/pebc1-14f.pdf
Accessed: May, 2016.
121. Ravaioli A, Pasini G, Polselli A, et al. Staging of breast cancer:
new recommended standard procedure. Breast Cancer Res Treat
2002;72:53-60. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/12000220.
122. Puglisi F, Follador A, Minisini AM, et al. Baseline staging tests after
a new diagnosis of breast cancer: further evidence of their limited
indications. Ann Oncol 2005;16:263-266. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15668281.
123. Brothers JM, Kidwell KM, Brown RK, Henry NL. Incidental
radiologic findings at breast cancer diagnosis and likelihood of disease
recurrence. Breast Cancer Res Treat 2016;155:395-403. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/26797222.
124. Kumar R, Chauhan A, Zhuang H, et al. Clinicopathologic factors
associated with false negative FDG-PET in primary breast cancer.
Breast Cancer Res Treat 2006;98:267-274. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16555126.
125. Podoloff DA, Advani RH, Allred C, et al. NCCN task force report:
positron emission tomography (PET)/computed tomography (CT)
scanning in cancer. J Natl Compr Canc Netw 2007;5 Suppl 1:1-1.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/17509259.
126. Rosen EL, Eubank WB, Mankoff DA. FDG PET, PET/CT, and
breast cancer imaging. Radiographics 2007;27 Suppl 1:S215-229.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/18180228.
127. Wahl RL, Siegel BA, Coleman RE, Gatsonis CG. Prospective
multicenter study of axillary nodal staging by positron emission
tomography in breast cancer: a report of the staging breast cancer with
PET Study Group. J Clin Oncol 2004;22:277-285. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/14722036.
128. Arriagada R, Le MG, Rochard F, Contesso G. Conservative
treatment versus mastectomy in early breast cancer: patterns of failure
with 15 years of follow-up data. Institut Gustave-Roussy Breast Cancer
Group. J Clin Oncol 1996;14:1558-1564. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/8622072.
129. Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of
differences in the extent of surgery for early breast cancer on local
recurrence and 15-year survival: an overview of the randomised trials.
Lancet 2005;366:2087-2106. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16360786.
130. Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a
randomized trial comparing total mastectomy, lumpectomy, and
lumpectomy plus irradiation for the treatment of invasive breast cancer.
N Engl J Med 2002;347:1233-1241. Available at:
http://www.nejm.org/doi/full/10.1056/NEJMoa022152.
131. Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of
a randomized study comparing breast-conserving surgery with radical
mastectomy for early breast cancer. N Engl J Med 2002;347:1227-
1232. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12393819.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-83
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
132. Early Breast Cancer Trialists' Collaborative G, Darby S, McGale P,
et al. Effect of radiotherapy after breast-conserving surgery on 10-year
recurrence and 15-year breast cancer death: meta-analysis of individual
patient data for 10,801 women in 17 randomised trials. Lancet
2011;378:1707-1716. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/22019144.
133. Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical
Oncology-American Society for Radiation Oncology consensus
guideline on margins for breast-conserving surgery with whole-breast
irradiation in stages I and II invasive breast cancer. J Clin Oncol
2014;32:1507-1515. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/24516019.
134. Fourquet A, Campana F, Zafrani B, et al. Prognostic factors of
breast recurrence in the conservative management of early breast
cancer: a 25-year follow-up. Int J Radiat Oncol Biol Phys 1989;17:719-
725. Available at: http://www.ncbi.nlm.nih.gov/pubmed/2777661.
135. Komoike Y, Akiyama F, Iino Y, et al. Ipsilateral breast tumor
recurrence (IBTR) after breast-conserving treatment for early breast
cancer: risk factors and impact on distant metastases. Cancer
2006;106:35-41. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16333848.
136. Zhou P, Gautam S, Recht A. Factors affecting outcome for young
women with early stage invasive breast cancer treated with breast-
conserving therapy. Breast Cancer Res Treat 2007;101:51-57.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/16821084.
137. Golshan M, Miron A, Nixon AJ, et al. The prevalence of germline
BRCA1 and BRCA2 mutations in young women with breast cancer
undergoing breast-conservation therapy. Am J Surg 2006;192:58-62.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/16769276.
138. Kroman N, Holtveg H, Wohlfahrt J, et al. Effect of breast-
conserving therapy versus radical mastectomy on prognosis for young
women with breast carcinoma. Cancer 2004;100:688-693. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/14770422.
139. Blichert-Toft M, Nielsen M, During M, et al. Long-term results of
breast conserving surgery vs. mastectomy for early stage invasive
breast cancer: 20-year follow-up of the Danish randomized DBCG-
82TM protocol. Acta Oncol 2008;47:672-681. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18465335.
140. Litiere S, Werutsky G, Fentiman IS, et al. Breast conserving
therapy versus mastectomy for stage I-II breast cancer: 20 year follow-
up of the EORTC 10801 phase 3 randomised trial. Lancet Oncol
2012;13:412-419. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/22373563.
141. Agarwal S, Pappas L, Neumayer L, et al. Effect of breast
conservation therapy vs mastectomy on disease-specific survival for
early-stage breast cancer. JAMA Surg 2014;149:267-274. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/24429935.
142. Hwang ES, Lichtensztajn DY, Gomez SL, et al. Survival after
lumpectomy and mastectomy for early stage invasive breast cancer: the
effect of age and hormone receptor status. Cancer 2013;119:1402-
1411. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23359049.
143. Hartmann-Johnsen OJ, Karesen R, Schlichting E, Nygard JF.
Survival is better after breast conserving therapy than mastectomy for
early stage breast cancer: A registry-based follow-up study of
Norwegian women Primary operated between 1998 and 2008. Ann
Surg Oncol 2015;22:3836-3845. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/25743325.
144. Chatterjee A, Pyfer B, Czerniecki B, et al. Early postoperative
outcomes in lumpectomy versus simple mastectomy. J Surg Res
2015;198:143-148.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-84
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
145. Recht A. Contralateral prophylactic mastectomy: caveat emptor. J
Clin Oncol 2009;27:1347-1349. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19224834.
146. Bedrosian I, Hu CY, Chang GJ. Population-based study of
contralateral prophylactic mastectomy and survival outcomes of breast
cancer patients. J Natl Cancer Inst 2010;102:401-409. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/20185801.
147. Jatoi I, Parsons HM. Contralateral prophylactic mastectomy and its
association with reduced mortality: evidence for selection bias. Breast
Cancer Res Treat 2014;148:389-396. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/25301088.
148. Portschy PR, Kuntz KM, Tuttle TM. Survival outcomes after
contralateral prophylactic mastectomy: a decision analysis. J Natl
Cancer Inst 2014;106. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/25031308
http://jnci.oxfordjournals.org/content/106/8/dju160.full.pdf.
149. Fayanju OM, Stoll CR, Fowler S, et al. Contralateral prophylactic
mastectomy after unilateral breast cancer: a systematic review and
meta-analysis. Ann Surg 2014;260:1000-1010. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/24950272.
150. Rocha RD, Girardi AR, Pinto RR, de Freitas VA. Axillary
ultrasound and fine-needle aspiration in preoperative staging of axillary
lymph nodes in patients with invasive breast cancer. Radiol Bras
2015;48:345-352. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/26811550.
151. Bass SS, Lyman GH, McCann CR, et al. Lymphatic mapping and
sentinel lymph node biopsy. Breast J 1999;5:288-295. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11348304.
152. Cox CE. Lymphatic mapping in breast cancer: combination
technique. Ann Surg Oncol 2001;8:67S-70S. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11599905.
153. Cox CE, Nguyen K, Gray RJ, et al. Importance of lymphatic
mapping in ductal carcinoma in situ (DCIS): why map DCIS? Am Surg
2001;67:513-519. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11409797.
154. Krag D, Weaver D, Ashikaga T, et al. The sentinel node in breast
cancer--a multicenter validation study. N Engl J Med 1998;339:941-946.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/9753708.
155. Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node
resection compared with conventional axillary-lymph-node dissection in
clinically node-negative patients with breast cancer: overall survival
findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol
2010;11:927-933. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/20863759.
156. Kuehn T, Vogl FD, Helms G, et al. Sentinel-node biopsy for axillary
staging in breast cancer: results from a large prospective German multi-
institutional trial. Eur J Surg Oncol 2004;30:252-259. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15028305.
157. McMasters KM, Giuliano AE, Ross MI, et al. Sentinel-lymph-node
biopsy for breast cancer--not yet the standard of care. N Engl J Med
1998;339:990-995. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/9753717.
158. O'Hea BJ, Hill AD, El-Shirbiny AM, et al. Sentinel lymph node
biopsy in breast cancer: initial experience at Memorial Sloan-Kettering
Cancer Center. J Am Coll Surg 1998;186:423-427. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/9544956.
159. Veronesi U, Paganelli G, Viale G, et al. A randomized comparison
of sentinel-node biopsy with routine axillary dissection in breast cancer.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-85
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
N Engl J Med 2003;349:546-553. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/12904519.
160. Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter
trial of sentinel node biopsy versus standard axillary treatment in
operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst
2006;98:599-609. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16670385.
161. Cox CE, Salud CJ, Cantor A, et al. Learning curves for breast
cancer sentinel lymph node mapping based on surgical volume
analysis. J Am Coll Surg 2001;193:593-600. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11768674.
162. Dupont E, Cox C, Shivers S, et al. Learning curves and breast
cancer lymphatic mapping: institutional volume index. J Surg Res
2001;97:92-96. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11319887.
163. Giuliano AE, Hawes D, Ballman KV, et al. Association of occult
metastases in sentinel lymph nodes and bone marrow with survival
among women with early-stage invasive breast cancer. JAMA
2011;306:385-393. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/21791687.
164. Degnim AC, Reynolds C, Pantvaidya G, et al. Nonsentinel node
metastasis in breast cancer patients: assessment of an existing and a
new predictive nomogram. Am J Surg 2005;190:543-550. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16164917.
165. Houvenaeghel G, Nos C, Giard S, et al. A nomogram predictive of
non-sentinel lymph node involvement in breast cancer patients with a
sentinel lymph node micrometastasis. Eur J Surg Oncol 2009;35:690-
695. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19046847.
166. Katz A, Smith BL, Golshan M, et al. Nomogram for the prediction
of having four or more involved nodes for sentinel lymph node-positive
breast cancer. J Clin Oncol 2008;26:2093-2098. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18445838.
167. Kohrt HE, Olshen RA, Bermas HR, et al. New models and online
calculator for predicting non-sentinel lymph node status in sentinel
lymph node positive breast cancer patients. BMC Cancer 2008;8:66-66.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/18315887.
168. Scow JS, Degnim AC, Hoskin TL, et al. Assessment of the
performance of the Stanford Online Calculator for the prediction of
nonsentinel lymph node metastasis in sentinel lymph node-positive
breast cancer patients. Cancer 2009;115:4064-4070. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19517477.
169. van la Parra RFD, Ernst MF, Bevilacqua JLB, et al. Validation of a
nomogram to predict the risk of nonsentinel lymph node metastases in
breast cancer patients with a positive sentinel node biopsy: validation of
the MSKCC breast nomogram. Ann Surg Oncol 2009;16:1128-1135.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/19252954.
170. Werkoff G, Lambaudie E, Fondrinier E, et al. Prospective
multicenter comparison of models to predict four or more involved
axillary lymph nodes in patients with breast cancer with one to three
metastatic sentinel lymph nodes. J Clin Oncol 2009;27:5707-5712.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/19826125.
171. Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence
after sentinel lymph node dissection with or without axillary dissection in
patients with sentinel lymph node metastases: the American College of
Surgeons Oncology Group Z0011 randomized trial. Ann Surg
2010;252:426-432; discussion 432-423. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/20739842.
172. Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no
axillary dissection in women with invasive breast cancer and sentinel
node metastasis: a randomized clinical trial. JAMA 2011;305:569-575.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/21304082.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-86
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
173. Axelsson CK, Mouridsen HT, Zedeler K. Axillary dissection of level
I and II lymph nodes is important in breast cancer classification. The
Danish Breast Cancer Cooperative Group (DBCG). Eur J Cancer
1992;28A:1415-1418. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/1515262.
174. Kiricuta CI, Tausch J. A mathematical model of axillary lymph node
involvement based on 1446 complete axillary dissections in patients
with breast carcinoma. Cancer 1992;69:2496-2501. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/1568171.
175. Fisher B, Redmond C, Fisher ER, et al. Ten-year results of a
randomized clinical trial comparing radical mastectomy and total
mastectomy with or without radiation. N Engl J Med 1985;312:674-681.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/3883168.
176. Antonini N, Jones H, Horiot JC, et al. Effect of age and radiation
dose on local control after breast conserving treatment: EORTC trial
22881-10882. Radiother Oncol 2007;82:265-271. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17126434.
177. Bartelink H, Horiot JC, Poortmans P, et al. Recurrence rates after
treatment of breast cancer with standard radiotherapy with or without
additional radiation. N Engl J Med 2001;345:1378-1387. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11794170.
178. Pignol JP, Olivotto I, Rakovitch E, et al. A multicenter randomized
trial of breast intensity-modulated radiation therapy to reduce acute
radiation dermatitis. J Clin Oncol 2008;26:2085-2092. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18285602.
179. Mukesh MB, Barnett GC, Wilkinson JS, et al. Randomized
controlled trial of intensity-modulated radiotherapy for early breast
cancer: 5-year results confirm superior overall cosmesis. J Clin Oncol
2013;31:4488-4495. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/24043742.
180. Mulliez T, Veldeman L, van Greveling A, et al. Hypofractionated
whole breast irradiation for patients with large breasts: a randomized
trial comparing prone and supine positions. Radiother Oncol
2013;108:203-208. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/24044803.
181. Group ST, Bentzen SM, Agrawal RK, et al. The UK
Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy
hypofractionation for treatment of early breast cancer: a randomised
trial. Lancet 2008;371:1098-1107. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18355913.
182. Group ST, Bentzen SM, Agrawal RK, et al. The UK
Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy
hypofractionation for treatment of early breast cancer: a randomised
trial. Lancet Oncol 2008;9:331-341. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18356109.
183. Owen JR, Ashton A, Bliss JM, et al. Effect of radiotherapy fraction
size on tumour control in patients with early-stage breast cancer after
local tumour excision: long-term results of a randomised trial. Lancet
Oncol 2006;7:467-471. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16750496.
184. Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of
hypofractionated radiation therapy for breast cancer. N Engl J Med
2010;362:513-520. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/20147717.
185. Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of
Breast Radiotherapy (START) trials of radiotherapy hypofractionation
for treatment of early breast cancer: 10-year follow-up results of two
randomised controlled trials. Lancet Oncol 2013;14:1086-1094.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/24055415.
186. Bartelink H, Horiot JC, Poortmans PM, et al. Impact of a higher
radiation dose on local control and survival in breast-conserving therapy
of early breast cancer: 10-year results of the randomized boost versus
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-87
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
no boost EORTC 22881-10882 trial. J Clin Oncol 2007;25:3259-3265.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/17577015.
187. Romestaing P, Lehingue Y, Carrie C, et al. Role of a 10-Gy boost
in the conservative treatment of early breast cancer: results of a
randomized clinical trial in Lyon, France. J Clin Oncol 1997;15:963-968.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/9060534.
188. Vrieling C, Collette L, Fourquet A, et al. The influence of patient,
tumor and treatment factors on the cosmetic results after breast-
conserving therapy in the EORTC 'boost vs. no boost' trial. EORTC
Radiotherapy and Breast Cancer Cooperative Groups. Radiother Oncol
2000;55:219-232. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/10869738.
189. Jones HA, Antonini N, Hart AA, et al. Impact of pathological
characteristics on local relapse after breast-conserving therapy: a
subgroup analysis of the EORTC boost versus no boost trial. J Clin
Oncol 2009;27:4939-4947. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19720914.
190. Whelan TJ, Olivotto IA, Parulekar WR, et al. Regional nodal
irradiation in early-stage breast cancer. N Engl J Med 2015;373:307-
316. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26200977.
191. Poortmans PM, Collette S, Kirkove C, et al. Internal mammary and
medial supraclavicular irradiation in breast cancer. New England
Journal of Medicine 2015;373:317-327. Available at:
http://www.nejm.org/doi/full/10.1056/NEJMoa1415369.
192. McCormick B. Partial-breast radiation for early staged breast
cancers: hypothesis, existing data, and a planned phase III trial. J Natl
Compr Canc Netw 2005;3:301-307. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16002002.
193. Smith BD, Arthur DW, Buchholz TA, et al. Accelerated partial
breast irradiation consensus statement from the American Society for
Radiation Oncology (ASTRO). Int J Radiat Oncol Biol Phys
2009;74:987-1001. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19545784.
194. Shaitelman SF, Vicini FA, Beitsch P, et al. Five-year outcome of
patients classified using the American Society for Radiation Oncology
consensus statement guidelines for the application of accelerated
partial breast irradiation: an analysis of patients treated on the American
Society of Breast Surgeons MammoSite Registry Trial. Cancer
2010;116:4677-4685. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/20602483.
195. Vicini F, Arthur D, Wazer D, et al. Limitations of the American
Society of Therapeutic Radiology and Oncology consensus panel
guidelines on the use of accelerated partial breast irradiation. Int J
Radiat Oncol Biol Phys 2010. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/20510540.
196. Bellon JR, Come SE, Gelman RS, et al. Sequencing of
chemotherapy and radiation therapy in early-stage breast cancer:
updated results of a prospective randomized trial. J Clin Oncol
2005;23:1934-1940. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15774786.
197. Recht A, Come SE, Henderson IC, et al. The sequencing of
chemotherapy and radiation therapy after conservative surgery for
early-stage breast cancer. N Engl J Med 1996;334:1356-1361.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/8614420.
198. Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus
tamoxifen with or without irradiation in women 70 years of age or older
with early breast cancer. N Engl J Med 2004;351:971-977. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15342805.
199. Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus
tamoxifen with or without irradiation in women age 70 years or older
with early breast cancer: long-term follow-up of CALGB 9343. J Clin
Oncol 2013;31:2382-2387. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/23690420.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-88
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
200. Fyles AW, McCready DR, Manchul LA, et al. Tamoxifen with or
without breast irradiation in women 50 years of age or older with early
breast cancer. N Engl J Med 2004;351:963-970. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15342804.
201. Kunkler IH, Williams LJ, Jack WJ, et al. Breast-conserving surgery
with or without irradiation in women aged 65 years or older with early
breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol
2015;16:266-273. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/25637340.
202. Hellman S. Stopping metastases at their source. N Engl J Med
1997;337:996-997. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/9309106.
203. Overgaard M, Hansen PS, Overgaard J, et al. Postoperative
radiotherapy in high-risk premenopausal women with breast cancer who
receive adjuvant chemotherapy. Danish Breast Cancer Cooperative
Group 82b Trial. N Engl J Med 1997;337:949-955. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/9395428.
204. Overgaard M, Jensen MB, Overgaard J, et al. Postoperative
radiotherapy in high-risk postmenopausal breast-cancer patients given
adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG
82c randomised trial. Lancet 1999;353:1641-1648. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/10335782.
205. Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiation
therapy in patients with high-risk breast cancer receiving adjuvant
chemotherapy: 20-year results of the British Columbia randomized trial.
J Natl Cancer Inst 2005;97:116-126. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15657341.
206. Recht A, Edge SB, Solin LJ, et al. Postmastectomy radiotherapy:
clinical practice guidelines of the American Society of Clinical Oncology.
J Clin Oncol 2001;19:1539-1569. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11230499.
207. Early Breast Cancer Trialists' Collaborative G, McGale P, Taylor C,
et al. Effect of radiotherapy after mastectomy and axillary surgery on
10-year recurrence and 20-year breast cancer mortality: meta-analysis
of individual patient data for 8135 women in 22 randomised trials.
Lancet 2014;383:2127-2135. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/24656685.
208. Huang EH, Tucker SL, Strom EA, et al. Postmastectomy radiation
improves local-regional control and survival for selected patients with
locally advanced breast cancer treated with neoadjuvant chemotherapy
and mastectomy. J Clin Oncol 2004;22:4691-4699. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15570071.
209. McGuire SE, Gonzalez-Angulo AM, Huang EH, et al.
Postmastectomy radiation improves the outcome of patients with locally
advanced breast cancer who achieve a pathologic complete response
to neoadjuvant chemotherapy. Int J Radiat Oncol Biol Phys
2007;68:1004-1009. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17418973.
210. Overgaard M, Nielsen HM, Overgaard J. Is the benefit of
postmastectomy irradiation limited to patients with four or more positive
nodes, as recommended in international consensus reports? A
subgroup analysis of the DBCG 82 b&c randomized trials. Radiother
Oncol 2007;82:247-253. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17306393.
211. Nielsen HM, Overgaard M, Grau C, et al. Study of failure pattern
among high-risk breast cancer patients with or without postmastectomy
radiotherapy in addition to adjuvant systemic therapy: long-term results
from the Danish Breast Cancer Cooperative Group DBCG 82 b and c
randomized studies. J Clin Oncol 2006;24:2268-2275. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16618947.
212. Jagsi R, Raad RA, Goldberg S, et al. Locoregional recurrence
rates and prognostic factors for failure in node-negative patients treated
with mastectomy: implications for postmastectomy radiation. Int J
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-89
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
Radiat Oncol Biol Phys 2005;62:1035-1039. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15990006.
213. Abdulkarim BS, Cuartero J, Hanson J, et al. Increased risk of
locoregional recurrence for women With T1-2N0 triple-negative breast
cancer treated with modified radical mastectomy without adjuvant
radiation therapy compared with breast-conserving therapy. Journal of
Clinical Oncology 2011;29:2852-2858. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/21670451.
214. McLaughlin JM, Anderson RT, Ferketich AK, et al. Effect on
survival of longer intervals between confirmed diagnosis and treatment
initiation among low-income women with breast cancer. J Clin Oncol
2012;30:4493-4500. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/23169521.
215. Liu AS, Kao HK, Reish RG, et al. Postoperative complications in
prosthesis-based breast reconstruction using acellular dermal matrix.
Plast Reconstr Surg 2011;127:1755-1762. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/21228744.
216. McCarthy CM, Mehrara BJ, Riedel E, et al. Predicting
complications following expander/implant breast reconstruction: an
outcomes analysis based on preoperative clinical risk. Plast Reconstr
Surg 2008;121:1886-1892. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18520873.
217. Cowen D, Gross E, Rouannet P, et al. Immediate post-mastectomy
breast reconstruction followed by radiotherapy: risk factors for
complications. Breast Cancer Res Treat 2010;121:627-634. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/20424909.
218. Woerdeman LA, Hage JJ, Hofland MM, Rutgers EJ. A prospective
assessment of surgical risk factors in 400 cases of skin-sparing
mastectomy and immediate breast reconstruction with implants to
establish selection criteria. Plast Reconstr Surg 2007;119:455-463.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/17230076.
219. Antony AK, McCarthy CM, Cordeiro PG, et al. Acellular human
dermis implantation in 153 immediate two-stage tissue expander breast
reconstructions: determining the incidence and significant predictors of
complications. Plast Reconstr Surg 2010;125:1606-1614. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/20517083.
220. Ahmed S, Snelling A, Bains M, Whitworth IH. Breast
reconstruction. BMJ 2005;330:943-948. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15845976.
221. Edlich RF, Winters KL, Faulkner BC, et al. Advances in breast
reconstruction after mastectomy. J Long Term Eff Med Implants
2005;15:197-207. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15777171.
222. Pennington DG. Breast reconstruction after mastectomy: current
state of the art. ANZ J Surg 2005;75:454-458. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15943736.
223. Chang DW. Breast Reconstruction with Microvascular MS-TRAM
and DIEP Flaps. Arch Plast Surg 2012;39:3-10. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/22783484.
224. Kronowitz SJ, Robb GL. Radiation therapy and breast
reconstruction: a critical review of the literature. Plast Reconstr Surg
2009;124:395-408. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19644254.
225. Tran NV, Chang DW, Gupta A, et al. Comparison of immediate
and delayed free TRAM flap breast reconstruction in patients receiving
postmastectomy radiation therapy. Plast Reconstr Surg 2001;108:78-
82. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11420508.
226. Mehta VK, Goffinet D. Postmastectomy radiation therapy after
TRAM flap breast reconstruction. Breast J 2004;10:118-122. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/15009038.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-90
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
227. Berry T, Brooks S, Sydow N, et al. Complication rates of radiation
on tissue expander and autologous tissue breast reconstruction. Ann
Surg Oncol 2010;17 Suppl 3:202-210. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/20853034.
228. Francis SH, Ruberg RL, Stevenson KB, et al. Independent risk
factors for infection in tissue expander breast reconstruction. Plast
Reconstr Surg 2009;124:1790-1796. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19952635.
229. Colwell AS, Damjanovic B, Zahedi B, et al. Retrospective review of
331 consecutive immediate single-stage implant reconstructions with
acellular dermal matrix: indications, complications, trends, and costs.
Plast Reconstr Surg 2011;128:1170-1178. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/22094736.
230. Garcia-Etienne CA, Cody Iii HS, Disa JJ, et al. Nipple-sparing
mastectomy: initial experience at the Memorial Sloan-Kettering Cancer
Center and a comprehensive review of literature. Breast J 2009;15:440-
449. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19496781.
231. Petit JY, Veronesi U, Orecchia R, et al. Nipple sparing mastectomy
with nipple areola intraoperative radiotherapy: one thousand and one
cases of a five years experience at the European institute of oncology of
Milan (EIO). Breast Cancer Res Treat 2009;117:333-338. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19152026.
232. Yueh JH, Houlihan MJ, Slavin SA, et al. Nipple-sparing
mastectomy: evaluation of patient satisfaction, aesthetic results, and
sensation. Ann Plast Surg 2009;62:586-590. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19387167.
233. Chung AP, Sacchini V. Nipple-sparing mastectomy: Where are we
now? Surg Oncol 2008;17:261-266. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18456492.
234. Gerber B, Krause A, Dieterich M, et al. The oncological safety of
skin sparing mastectomy with conservation of the nipple-areola complex
and autologous reconstruction: an extended follow-up study. Ann Surg
2009;249:461-468. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19247035.
235. Mallon P, Feron JG, Couturaud B, et al. The role of nipple-sparing
mastectomy in breast cancer: a comprehensive review of the literature.
Plast Reconstr Surg 2013;131:969-984. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/23629079.
236. Piper M, Peled AW, Foster RD, et al. Total skin-sparing
mastectomy: A aystematic review of oncologic outcomes and
postoperative complications. Ann Plast Surg 2013. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/23486127.
237. Toth BA, Forley BG, Calabria R. Retrospective study of the skin-
sparing mastectomy in breast reconstruction. Plast Reconstr Surg
1999;104:77-84. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/10597677.
238. Carlson GW, Styblo TM, Lyles RH, et al. The use of skin sparing
mastectomy in the treatment of breast cancer: The Emory experience.
Surg Oncol 2003;12:265-269. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/14998566.
239. Downes KJ, Glatt BS, Kanchwala SK, et al. Skin-sparing
mastectomy and immediate reconstruction is an acceptable treatment
option for patients with high-risk breast carcinoma. Cancer
2005;103:906-913. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15651068.
240. Foster RD, Esserman LJ, Anthony JP, et al. Skin-sparing
mastectomy and immediate breast reconstruction: a prospective cohort
study for the treatment of advanced stages of breast carcinoma. Ann
Surg Oncol 2002;9:462-466. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/12052757.
241. Medina-Franco H, Vasconez LO, Fix RJ, et al. Factors associated
with local recurrence after skin-sparing mastectomy and immediate
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-91
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
breast reconstruction for invasive breast cancer. Ann Surg
2002;235:814-819. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/12035037.
242. Newman LA, Kuerer HM, Hunt KK, et al. Presentation, treatment,
and outcome of local recurrence afterskin-sparing mastectomy and
immediate breast reconstruction. Ann Surg Oncol 1998;5:620-626.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/9831111.
243. Clough KB, Kaufman GJ, Nos C, et al. Improving breast cancer
surgery: a classification and quadrant per quadrant atlas for oncoplastic
surgery. Ann Surg Oncol 2010;17:1375-1391. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/20140531.
244. Anderson BO, Masetti R, Silverstein MJ. Oncoplastic approaches
to partial mastectomy: an overview of volume-displacement techniques.
Lancet Oncol 2005;6:145-157. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15737831.
245. Huemer GM, Schrenk P, Moser F, et al. Oncoplastic techniques
allow breast-conserving treatment in centrally located breast cancers.
Plast Reconstr Surg 2007;120:390-398. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17632339.
246. Kaur N, Petit J-Y, Rietjens M, et al. Comparative study of surgical
margins in oncoplastic surgery and quadrantectomy in breast cancer.
Ann Surg Oncol 2005;12:539-545. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15889210.
247. Early Breast Cancer Trialists' Collaborative G, Peto R, Davies C, et
al. Comparisons between different polychemotherapy regimens for early
breast cancer: meta-analyses of long-term outcome among 100,000
women in 123 randomised trials. Lancet 2012;379:432-444. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/22152853.
248. Loprinzi CL, Thome SD. Understanding the utility of adjuvant
systemic therapy for primary breast cancer. J Clin Oncol 2001;19:972-
979. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11181659.
249. Ravdin PM, Siminoff LA, Davis GJ, et al. Computer program to
assist in making decisions about adjuvant therapy for women with early
breast cancer. J Clin Oncol 2001;19:980-991. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11181660.
250. Olivotto IA, Bajdik CD, Ravdin PM, et al. Population-based
validation of the prognostic model ADJUVANT! for early breast cancer.
J Clin Oncol 2005;23:2716-2725. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15837986.
251. Loprinzi CL, Ravdin PM. Decision-making for patients with
resectable breast cancer: individualized decisions for and by patients
and their physicians. J Natl Compr Canc Netw 2003;1:189-196.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/19768877.
252. Cooke T, Reeves J, Lanigan A, Stanton P. HER2 as a prognostic
and predictive marker for breast cancer. Ann Oncol 2001;12 Suppl 1:23-
28. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11521717.
253. Paik S, Bryant J, Park C, et al. erbB-2 and response to doxorubicin
in patients with axillary lymph node-positive, hormone receptor-negative
breast cancer. J Natl Cancer Inst 1998;90:1361-1370. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/9747867.
254. Paik S, Bryant J, Tan-Chiu E, et al. HER2 and choice of adjuvant
chemotherapy for invasive breast cancer: National Surgical Adjuvant
Breast and Bowel Project Protocol B-15. J Natl Cancer Inst
2000;92:1991-1998. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11121461.
255. Piccart MJ, Di Leo A, Hamilton A. HER2. a 'predictive factor' ready
to use in the daily management of breast cancer patients? Eur J Cancer
2000;36:1755-1761. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/10974622.
256. Pritchard KI, Shepherd LE, O'Malley FP, et al. HER2 and
responsiveness of breast cancer to adjuvant chemotherapy. N Engl J
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-92
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
Med 2006;354:2103-2111. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16707747.
257. Thor AD, Berry DA, Budman DR, et al. erbB-2, p53, and efficacy of
adjuvant therapy in lymph node-positive breast cancer. J Natl Cancer
Inst 1998;90:1346-1360. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/9747866.
258. Dressler LG, Berry DA, Broadwater G, et al. Comparison of HER2
status by fluorescence in situ hybridization and immunohistochemistry
to predict benefit from dose escalation of adjuvant doxorubicin-based
therapy in node-positive breast cancer patients. J Clin Oncol
2005;23:4287-4297. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15994142.
259. Joensuu H, Kellokumpu-Lehtinen P, Bono P, et al. Adjuvant
docetaxel or vinorelbine with or without trastuzumab for breast cancer.
N Engl J Med 2006;354:809-820. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16495393.
260. Piccart-Gebhart M, Procter M, Leyland-Jones B, et al.
Trastuzumab after adjuvant chemotherapy in HER2-positive breast
cancer. N Engl J Med 2005;353:1659-1672. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16236737.
261. Goldhirsch A, Piccart-Gebhart M, Procter M, et al. HERA TRIAL: 2
years versus 1 year of trastuzumab after adjuvant chemotherapy in
women with HER2-positive early breast cancer at 8 years of median
follow up. Cancer Research 2012;72:S5-2. Available at:
http://cancerres.aacrjournals.org/cgi/content/meeting_abstract/72/24_M
eetingAbstracts/S5-2.
262. Romond E, Perez E, Bryant J, et al. Trastuzumab plus adjuvant
chemotherapy for operable HER2-positive breast cancer. N Engl J Med
2005;353:1673-1684. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16236738.
263. Romond E, Suman V, Jeong J-H, et al. Trastuzumab plus adjuvant
chemotherapy for HER2-positive breast cancer: Final planned joint
analysis of overall survival (OS) from NSABP B-31 and NCCTG N9831.
Cancer Research 2012;72:S5-5. Available at:
http://cancerres.aacrjournals.org/cgi/content/meeting_abstract/72/24_M
eetingAbstracts/S5-5.
264. Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in
HER2-positive breast cancer. N Engl J Med 2011;365:1273-1283.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/21991949.
265. Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the
efficacy and safety of humanized anti-HER2 monoclonal antibody in
women who have HER2-overexpressing metastatic breast cancer that
has progressed after chemotherapy for metastatic disease. J Clin Oncol
1999;17:2639-2648. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/10561337.
266. Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy
plus a monoclonal antibody against HER2 for metastatic breast cancer
that overexpresses HER2. N Engl J Med 2001;344:783-792. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/11248153.
267. Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of
trastuzumab as a single agent in first-line treatment of HER2-
overexpressing metastatic breast cancer. J Clin Oncol 2002;20:719-
726. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11821453.
268. Jeffrey SS, Lonning PE, Hillner BE. Genomics-based prognosis
and therapeutic prediction in breast cancer. J Natl Compr Canc Netw
2005;3:291-300. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16002001.
269. Perou CM, Jeffrey SS, van de Rijn M, et al. Distinctive gene
expression patterns in human mammary epithelial cells and breast
cancers. Proc Natl Acad Sci U S A 1999;96:9212-9217. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/10430922.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-93
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
270. Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns
of breast carcinomas distinguish tumor subclasses with clinical
implications. Proc Natl Acad Sci U S A 2001;98:10869-10874. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/11553815.
271. Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of
breast tumor subtypes in independent gene expression data sets. Proc
Natl Acad Sci U S A 2003;100:8418-8423. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/12829800.
272. Paik S, Shak S, Tang G, et al. A multigene assay to predict
recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J
Med 2004;351:2817-2826. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15591335.
273. Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant
recurrence using the 21-gene recurrence score in node-negative and
node-positive postmenopausal patients with breast cancer treated with
anastrozole or tamoxifen: a TransATAC study. J Clin Oncol
2010;28:1829-1834. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/20212256.
274. Mamounas EP, Tang G, Fisher B, et al. Association between the
21-gene recurrence score assay and risk of locoregional recurrence in
node-negative, estrogen receptor-positive breast cancer: results from
NSABP B-14 and NSABP B-20. J Clin Oncol 2010;28:1677-1683.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/20065188.
275. Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive
value of the 21-gene recurrence score assay in postmenopausal
women with node-positive, oestrogen-receptor-positive breast cancer
on chemotherapy: a retrospective analysis of a randomised trial. Lancet
Oncol 2010;11:55-65. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/20005174.
276. Paik S, Tang G, Shak S, et al. Gene expression and benefit of
chemotherapy in women with node-negative, estrogen receptor-positive
breast cancer. J Clin Oncol 2006;24:3726-3734. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16720680.
277. Tang G, Shak S, Paik S, et al. Comparison of the prognostic and
predictive utilities of the 21-gene Recurrence Score assay and
Adjuvant! for women with node-negative, ER-positive breast cancer:
results from NSABP B-14 and NSABP B-20. Breast Cancer Res Treat
2011;127:133-142. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/21221771.
278. Glas AM, Floore A, Delahaye LJMJ, et al. Converting a breast
cancer microarray signature into a high-throughput diagnostic test. BMC
Genomics 2006;7:278-278. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17074082.
279. van de Vijver MJ, He YD, van't Veer LJ, et al. A gene-expression
signature as a predictor of survival in breast cancer. N Engl J Med
2002;347:1999-2009. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/12490681.
280. van 't Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression
profiling predicts clinical outcome of breast cancer. Nature
2002;415:530-536. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11823860.
281. Knauer M, Mook S, Rutgers EJ, et al. The predictive value of the
70-gene signature for adjuvant chemotherapy in early breast cancer.
Breast Cancer Res Treat 2010;120:655-661. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/20204499.
282. Kunz G. Use of a genomic test (MammaPrint) in daily clinical
practice to assist in risk stratification of young breast cancer patients.
Arch Gynecol Obstet 2011;283:597-602. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/20383789.
283. Ishitobi M, Goranova TE, Komoike Y, et al. Clinical utility of the 70-
gene MammaPrint profile in a Japanese population. Jpn J Clin Oncol
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-94
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
2010;40:508-512. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/20110242.
284. Mook S, Knauer M, Bueno-de-Mesquita JM, et al. Metastatic
potential of T1 breast cancer can be predicted by the 70-gene
MammaPrint signature. Ann Surg Oncol 2010;17:1406-1413. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/20094918.
285. Drukker CA, Bueno-de-Mesquita JM, Retel VP, et al. A prospective
evaluation of a breast cancer prognosis signature in the observational
RASTER study. Int J Cancer 2013;133:929-936. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/23371464.
286. Dowsett M, Sestak I, Lopez-Knowles E, et al. Comparison of
PAM50 risk of recurrence score with oncotype DX and IHC4 for
predicting risk of distant recurrence after endocrine therapy. J Clin
Oncol 2013;31:2783-2790. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/23816962.
287. Gnant M, Filipits M, Greil R, et al. Predicting distant recurrence in
receptor-positive breast cancer patients with limited clinicopathological
risk: using the PAM50 Risk of Recurrence score in 1478
postmenopausal patients of the ABCSG-8 trial treated with adjuvant
endocrine therapy alone. Ann Oncol 2014;25:339-345. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/24347518.
288. Sestak I, Cuzick J, Dowsett M, et al. Prediction of late distant
recurrence after 5 years of endocrine treatment: a combined analysis of
patients from the Austrian breast and colorectal cancer study group 8
and arimidex, tamoxifen alone or in combination randomized trials using
the PAM50 risk of recurrence score. J Clin Oncol 2015;33:916-922.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/25332252.
289. Sparano JA, Gray RJ, Makower DF, et al. Prospective validation of
a 21-gene expression assay in breast cancer. New England Journal of
Medicine 2015;373:2005-2014. Available at:
http://www.nejm.org/doi/full/10.1056/NEJMoa1510764.
290. Hormone therapy with or without combination chemotherapy in
treating women who have undergone surgery for node-negative breast
cancer (The TAILORx Trial). Clinical Trial ID: NCT00310180. Available
at:
http://clinicaltrials.gov/ct2/show/NCT00310180?term=TAILORx&rank=2.
291. A phase III, randomized clinical trial of standard adjuvant
endocrine therapy +/-chemotherapy in patients with 1-3 positive nodes,
hormone receptor-positive and HER2-negative breast cancer with
recurence score (RS) of 25 or less. RXPONDER: A clinical trial RX for
positive node, endocrine responsive breast cancer. Clincial Trial ID
NCT01272037. Available at:
http://clinicaltrials.gov/show/NCT01272037.
292. MINDACT (Microarray In Node-Negative and 1 to 3 positive lymph
node disease may avoid chemotherapy): A prospective, randomized
study comparing the 70-Gene signature with the common clinical-
pathological criteria in selecting patients for adjuvant chemotherapy in
breast cancer with 0 to 3 positive nodes. Clinical Trial ID:
NCT00433589. Available at:
http://clinicaltrials.gov/ct2/show/NCT00433589?term=NCT00433589&ra
nk=1.
293. Piccart M, Rutgers E, van' t Veer L, et al. Primary analysis of the
EORTC 10041/ BIG 3-04 MINDACT study: a prospective, randomized
study evaluating the clinical utility of the 70-gene signature
(MammaPrint) combined with common clinical-pathological criteria for
selection of patients for adjuvant chemotherapy in breast cancer with 0
to 3 positive nodes [abstract]. In: Proceedings of the 107th Annual
Meeting of the American Association for Cancer Research; 2016 Apr
16-20; New Orleans, Louisiana: AACR; 2016. Abstract CT039 2016.
294. Swain SM, Wilson JW, Mamounas EP, et al. Estrogen receptor
status of primary breast cancer is predictive of estrogen receptor status
of contralateral breast cancer. J Natl Cancer Inst 2004;96:516-523.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/15069113.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-95
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
295. Berry DA, Cirrincione C, Henderson IC, et al. Estrogen-receptor
status and outcomes of modern chemotherapy for patients with node-
positive breast cancer. JAMA 2006;295:1658-1667. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16609087.
296. Albain KS, Barlow WE, Ravdin PM, et al. Adjuvant chemotherapy
and timing of tamoxifen in postmenopausal patients with endocrine-
responsive, node-positive breast cancer: a phase 3, open-label,
randomised controlled trial. Lancet 2009;374:2055-2063. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/20004966.
297. Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant
systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst
2005;97:188-194. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15687361.
298. Rastogi P, Anderson SJ, Bear HD, et al. Preoperative
chemotherapy: updates of National Surgical Adjuvant Breast and Bowel
Project Protocols B-18 and B-27. J Clin Oncol 2008;26:778-785.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/18258986.
299. Gralow JR, Burstein HJ, Wood W, et al. Preoperative therapy in
invasive breast cancer: pathologic assessment and systemic therapy
issues in operable disease. J Clin Oncol 2008;26:814-819. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18258991.
300. Killelea BK, Yang VQ, Mougalian S, et al. Neoadjuvant
chemotherapy for breast cancer increases the rate of breast
conservation: results from the National Cancer Database. J Am Coll
Surg 2015;220:1063-1069. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/25868410.
301. Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant
therapy and long-term survival in patients with triple-negative breast
cancer. J Clin Oncol 2008;26:1275-1281. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18250347.
302. Cortazar P, Zhang L, Untch M, et al. Pathological complete
response and long-term clinical benefit in breast cancer: the CTNeoBC
pooled analysis. Lancet 2014;384:164-172. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/24529560.
303. von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact
of pathologic complete response on prognosis after neoadjuvant
chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol
2012;30:1796-1804. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/22508812.
304. Cataliotti L, Buzdar AU, Noguchi S, et al. Comparison of
anastrozole versus tamoxifen as preoperative therapy in
postmenopausal women with hormone receptor-positive breast cancer:
the Pre-Operative "Arimidex" Compared to Tamoxifen (PROACT) trial.
Cancer 2006;106:2095-2103. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16598749.
305. Smith IE, Dowsett M, Ebbs SR, et al. Neoadjuvant treatment of
postmenopausal breast cancer with anastrozole, tamoxifen, or both in
combination: the Immediate Preoperative Anastrozole, Tamoxifen, or
Combined with Tamoxifen (IMPACT) multicenter double-blind
randomized trial. J Clin Oncol 2005;23:5108-5116. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15998903.
306. Eiermann W, Paepke S, Appfelstaedt J, et al. Preoperative
treatment of postmenopausal breast cancer patients with letrozole: A
randomized double-blind multicenter study. Ann Oncol 2001;12:1527-
1532. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11822750.
307. Ellis MJ, Ma C. Letrozole in the neoadjuvant setting: the P024 trial.
Breast Cancer Res Treat 2007;105 Suppl 1:33-43. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17912634.
308. Ellis MJ, Suman VJ, Hoog J, et al. Randomized phase II
neoadjuvant comparison between letrozole, anastrozole, and
exemestane for postmenopausal women with estrogen receptor-rich
stage 2 to 3 breast cancer: clinical and biomarker outcomes and
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-96
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
predictive value of the baseline PAM50-based intrinsic subtype--
ACOSOG Z1031. J Clin Oncol 2011;29:2342-2349. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/21555689.
309. Masuda N, Sagara Y, Kinoshita T, et al. Neoadjuvant anastrozole
versus tamoxifen in patients receiving goserelin for premenopausal
breast cancer (STAGE): a double-blind, randomised phase 3 trial.
Lancet Oncol 2012;13:345-352. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/22265697.
310. Torrisi R, Bagnardi V, Rotmensz N, et al. Letrozole plus GnRH
analogue as preoperative and adjuvant therapy in premenopausal
women with ER positive locally advanced breast cancer. Breast Cancer
Res Treat 2011;126:431-441. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/21221766.
311. Fontein DB, Charehbili A, Nortier JW, et al. Efficacy of six month
neoadjuvant endocrine therapy in postmenopausal, hormone receptor-
positive breast cancer patients--a phase II trial. Eur J Cancer
2014;50:2190-2200. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/24970786.
312. Petrelli F, Borgonovo K, Cabiddu M, et al. Neoadjuvant
chemotherapy and concomitant trastuzumab in breast cancer: a pooled
analysis of two randomized trials. Anticancer Drugs 2011;22:128-135.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/21218604.
313. Piccart-Gebhart M HA, de Azambuja E, et al. . The association
between event-free survival and pathological complete response to
neoadjuvant lapatinib, trastuzumab or their combination in HER2-
positive breast cancer. Survival follow-up analysis of the NeoALTTO
study (BIG 1-06) [abstract]. SABCS 2013:Abstract S1–01. Available at:
314. Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of
neoadjuvant pertuzumab and trastuzumab in women with locally
advanced, inflammatory, or early HER2-positive breast cancer
(NeoSphere): a randomised multicentre, open-label, phase 2 trial.
Lancet Oncol 2012;13:25-32. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/22153890.
315. Gianni L, Pienkowski T, Im Y-H, et al. Five-year analysis of the
phase II NeoSphere trial evaluating four cycles of neoadjuvant
docetaxel (D) and/or trastuzumab (T) and/or pertuzumab (P). ASCO
Meeting Abstracts 2015;33:505. Available at:
http://meeting.ascopubs.org/cgi/content/abstract/33/15_suppl/505.
316. Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus
trastuzumab in combination with standard neoadjuvant anthracycline-
containing and anthracycline-free chemotherapy regimens in patients
with HER2-positive early breast cancer: a randomized phase II cardiac
safety study (TRYPHAENA). Ann Oncol 2013;24:2278-2284. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/23704196.
317. Tamoxifen for early breast cancer: an overview of the randomised
trials. Early Breast Cancer Trialists' Collaborative Group. Lancet
1998;351:1451-1467. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/9605801.
318. Arpino G, Green SJ, Allred DC, et al. HER-2 amplification, HER-1
expression, and tamoxifen response in estrogen receptor-positive
metastatic breast cancer: a southwest oncology group study. Clin
Cancer Res 2004;10:5670-5676. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15355892.
319. Berry DA, Muss HB, Thor AD, et al. HER-2/neu and p53
expression versus tamoxifen resistance in estrogen receptor-positive,
node-positive breast cancer. J Clin Oncol 2000;18:3471-3479. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/11032587.
320. De Laurentiis M, Arpino G, Massarelli E, et al. A meta-analysis on
the interaction between HER-2 expression and response to endocrine
treatment in advanced breast cancer. Clin Cancer Res 2005;11:4741-
4748. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16000569.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-97
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
321. Eppenberger-Castori S, Kueng W, Benz C, et al. Prognostic and
predictive significance of ErbB-2 breast tumor levels measured by
enzyme immunoassay. J Clin Oncol 2001;19:645-656. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11157014.
322. Knoop AS, Bentzen SM, Nielsen MM, et al. Value of epidermal
growth factor receptor, HER2, p53, and steroid receptors in predicting
the efficacy of tamoxifen in high-risk postmenopausal breast cancer
patients. J Clin Oncol 2001;19:3376-3384. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11454885.
323. Mass R. The role of HER-2 expression in predicting response to
therapy in breast cancer. Semin Oncol 2000;27:46-52. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11236028.
324. Paik S, Shak S, Tang G, et al. Expression of the 21 genes in the
Recurrence Score assay and tamoxifen clinical benefit in the NSABP
study B-14 of node negative, estrogen receptor positive breast cancer
[abstract]. J Clin Oncol 2005;23(Suppl 16):Abstract 510. Available at:
http://meeting.ascopubs.org/cgi/content/abstract/23/16_suppl/510.
325. Pegram MD, Pauletti G, Slamon DJ. HER-2/neu as a predictive
marker of response to breast cancer therapy. Breast Cancer Res Treat
1998;52:65-77. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/10066073.
326. Dowsett M, Allred C, Knox J, et al. Relationship between
quantitative estrogen and progesterone receptor expression and human
epidermal growth factor receptor 2 (HER-2) status with recurrence in
the Arimidex, Tamoxifen, Alone or in Combination trial. J Clin Oncol
2008;26:1059-1065. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18227529.
327. Early Breast Cancer Trialists' Collaborative G, Davies C, Godwin J,
et al. Relevance of breast cancer hormone receptors and other factors
to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of
randomised trials. Lancet 2011;378:771-784. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/21802721.
328. Early Breast Cancer Trialists' Collaborative G. Effects of
chemotherapy and hormonal therapy for early breast cancer on
recurrence and 15-year survival: an overview of the randomised trials.
Lancet 2005;365:1687-1717. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15894097.
329. Davies C, Pan H, Godwin J, et al. Long-term effects of continuing
adjuvant tamoxifen to 10 years versus stopping at 5 years after
diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a
randomised trial. Lancet 2013;381:805-816. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/23219286.
330. Gray R, Rea D, Handley K, et al. aTTom: Long-term effects of
continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in
6,953 women with early breast cancer [Abstract]. J Clin Oncol
2013;31(suppl):Abstract 5. Available at:
331. Cuzick J, Ambroisine L, Davidson N, et al. Use of luteinising-
hormone-releasing hormone agonists as adjuvant treatment in
premenopausal patients with hormone-receptor-positive breast cancer:
a meta-analysis of individual patient data from randomised adjuvant
trials. Lancet 2007;369:1711-1723. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17512856.
332. Davidson NE, O'Neill AM, Vukov AM, et al. Chemoendocrine
therapy for premenopausal women with axillary lymph node-positive,
steroid hormone receptor-positive breast cancer: results from INT 0101
(E5188). J Clin Oncol 2005;23:5973-5982. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16087950.
333. Ejlertsen B, Mouridsen HT, Jensen MB, et al. Similar efficacy for
ovarian ablation compared with cyclophosphamide, methotrexate, and
fluorouracil: from a randomized comparison of premenopausal patients
with node-positive, hormone receptor-positive breast cancer. J Clin
Oncol 2006;24:4956-4962. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17075113.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-98
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
334. Goel S, Sharma R, Hamilton A, Beith J. LHRH agonists for
adjuvant therapy of early breast cancer in premenopausal women.
Cochrane Database Syst Rev 2009:CD004562. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19821328.
335. Kaufmann M, Jonat W, Blamey R, et al. Survival analyses from the
ZEBRA study. goserelin (Zoladex) versus CMF in premenopausal
women with node-positive breast cancer. Eur J Cancer 2003;39:1711-
1717. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12888366.
336. Schmid P, Untch M, Wallwiener D, et al. Cyclophosphamide,
methotrexate and fluorouracil (CMF) versus hormonal ablation with
leuprorelin acetate as adjuvant treatment of node-positive,
premenopausal breast cancer patients: preliminary results of the
TABLE-study (Takeda Adjuvant Breast cancer study with Leuprorelin
Acetate). Anticancer Res 2002;22:2325-2332. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/12174922.
337. Thomson CS, Twelves CJ, Mallon EA, Leake RE. Adjuvant ovarian
ablation vs CMF chemotherapy in premenopausal breast cancer
patients: trial update and impact of immunohistochemical assessment of
ER status. Breast 2002;11:419-429. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/14965706.
338. von Minckwitz G, Graf E, Geberth M, et al. CMF versus goserelin
as adjuvant therapy for node-negative, hormone-receptor-positive
breast cancer in premenopausal patients: a randomised trial (GABG
trial IV-A-93). Eur J Cancer 2006;42:1780-1788. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16765589.
339. Castiglione-Gertsch M, O'Neill A, Price KN, et al. Adjuvant
chemotherapy followed by goserelin versus either modality alone for
premenopausal lymph node-negative breast cancer: a randomized trial.
J Natl Cancer Inst 2003;95:1833-1846. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/14679153.
340. Puhalla S, Brufsky A, Davidson N. Adjuvant endocrine therapy for
premenopausal women with breast cancer. Breast 2009;18 Suppl
3:S122-130. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19914530.
341. Roche H, Fumoleau P, Spielmann M, et al. Sequential adjuvant
epirubicin-based and docetaxel chemotherapy for node-positive breast
cancer patients: the FNCLCC PACS 01 Trial. J Clin Oncol
2006;24:5664-5671. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17116941.
342. Boccardo F, Rubagotti A, Amoroso D, et al. Cyclophosphamide,
methotrexate, and fluorouracil versus tamoxifen plus ovarian
suppression as adjuvant treatment of estrogen receptor-positive pre-
/perimenopausal breast cancer patients: results of the Italian Breast
Cancer Adjuvant Study Group 02 randomized trial.
boccardo@hp380.ist.unige.it. J Clin Oncol 2000;18:2718-2727.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/10894871.
343. Pagani O, Regan MM, Walley BA, et al. Adjuvant exemestane with
ovarian suppression in premenopausal breast cancer. N Engl J Med
2014;371:107-118. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/24881463.
344. Francis PA, Regan MM, Fleming GF, et al. Adjuvant ovarian
suppression in premenopausal breast cancer. N Engl J Med
2015;372:436-446. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/25495490.
345. Coombes RC, Kilburn LS, Snowdon CF, et al. Survival and safety
of exemestane versus tamoxifen after 2-3 years' tamoxifen treatment
(Intergroup Exemestane Study): a randomised controlled trial. Lancet
2007;369:559-570. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17307102.
346. Kaufmann M, Jonat W, Hilfrich J, et al. Improved overall survival in
postmenopausal women with early breast cancer after anastrozole
initiated after treatment with tamoxifen compared with continued
tamoxifen: the ARNO 95 Study. J Clin Oncol 2007;25:2664-2670.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/17563395.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-99
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
347. Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole
following tamoxifen as extended adjuvant therapy in receptor-positive
breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer
Inst 2005;97:1262-1271. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16145047.
348. Forbes JF, Cuzick J, Buzdar A, et al. Effect of anastrozole and
tamoxifen as adjuvant treatment for early-stage breast cancer: 100-
month analysis of the ATAC trial. Lancet Oncol 2008;9:45-53. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/18083636.
349. Thurlimann B, Keshaviah A, Coates AS, et al. A comparison of
letrozole and tamoxifen in postmenopausal women with early breast
cancer. N Engl J Med 2005;353:2747-2757. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16382061.
350. Baum M, Budzar AU, Cuzick J, et al. Anastrozole alone or in
combination with tamoxifen versus tamoxifen alone for adjuvant
treatment of postmenopausal women with early breast cancer: first
results of the ATAC randomised trial. Lancet 2002;359:2131-2139.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/12090977.
351. Howell A, Cuzick J, Baum M, et al. Results of the ATAC (Arimidex,
Tamoxifen, Alone or in Combination) trial after completion of 5 years'
adjuvant treatment for breast cancer. Lancet 2005;365:60-62. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/15639680.
352. Duffy S, Jackson TL, Lansdown M, et al. The ATAC ('Arimidex',
Tamoxifen, Alone or in Combination) adjuvant breast cancer trial: first
results of the endometrial sub-protocol following 2 years of treatment.
Hum Reprod 2006;21:545-553. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16210385.
353. Fallowfield L, Cella D, Cuzick J, et al. Quality of life of
postmenopausal women in the Arimidex, Tamoxifen, Alone or in
Combination (ATAC) Adjuvant Breast Cancer Trial. J Clin Oncol
2004;22:4261-4271. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15514369.
354. Eastell R, Adams JE, Coleman RE, et al. Effect of anastrozole on
bone mineral density: 5-year results from the anastrozole, tamoxifen,
alone or in combination trial 18233230. J Clin Oncol 2008;26:1051-
1057. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18309940.
355. Dowsett M, Cuzick J, Howell A, Jackson I. Pharmacokinetics of
anastrozole and tamoxifen alone, and in combination, during adjuvant
endocrine therapy for early breast cancer in postmenopausal women: a
sub-protocol of the 'Arimidex and tamoxifen alone or in combination'
(ATAC) trial. Br J Cancer 2001;85:317-324. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11487258.
356. Buzdar AU, Guastalla JP, Nabholtz JM, et al. Impact of
chemotherapy regimens prior to endocrine therapy: Results from the
ATAC (anastrozole and tamoxifen, alone or in combination) trial. Cancer
2006;107:472-480. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16804925.
357. Mouridsen H, Keshaviah A, Coates AS, et al. Cardiovascular
adverse events during adjuvant endocrine therapy for early breast
cancer using letrozole or tamoxifen: safety analysis of BIG 1-98 trial. J
Clin Oncol 2007;25:5715-5722. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17998546.
358. Rabaglio M, Sun Z, Price KN, et al. Bone fractures among
postmenopausal patients with endocrine-responsive early breast cancer
treated with 5 years of letrozole or tamoxifen in the BIG 1-98 trial. Ann
Oncol 2009;20:1489-1498. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19474112.
359. Mouridsen H, Giobbie-Hurder A, Goldhirsch A, et al. Letrozole
therapy alone or in sequence with tamoxifen in women with breast
cancer. N Engl J Med 2009;361:766-776. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19692688.
360. Boccardo F, Rubagotti A, Puntoni M, et al. Switching to
anastrozole versus continued tamoxifen treatment of early breast
cancer: preliminary results of the Italian Tamoxifen Anastrozole Trial. J
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-100
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
Clin Oncol 2005;23:5138-5147. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16009955.
361. Boccardo F, Rubagotti A, Guglielmini P, et al. Switching to
anastrozole versus continued tamoxifen treatment of early breast
cancer. Updated results of the Italian tamoxifen anastrozole (ITA) trial.
Ann Oncol 2006;17 Suppl 7:10-14. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16760270.
362. Coombes RC, Hall E, Gibson LJ, et al. A randomized trial of
exemestane after two to three years of tamoxifen therapy in
postmenopausal women with primary breast cancer. N Engl J Med
2004;350:1081-1092. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15014181.
363. Jakesz R, Jonat W, Gnant M, et al. Switching of postmenopausal
women with endocrine-responsive early breast cancer to anastrozole
after 2 years' adjuvant tamoxifen: combined results of ABCSG trial 8
and ARNO 95 trial. Lancet 2005;366:455-462. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16084253.
364. Jonat W, Gnant M, Boccardo F, et al. Effectiveness of switching
from adjuvant tamoxifen to anastrozole in postmenopausal women with
hormone-sensitive early-stage breast cancer: a meta-analysis. Lancet
Oncol 2006;7:991-996. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17138220.
365. van de Velde CJ, Rea D, Seynaeve C, et al. Adjuvant tamoxifen
and exemestane in early breast cancer (TEAM): a randomised phase 3
trial. Lancet 2011;377:321-331. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/21247627.
366. Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole
in postmenopausal women after five years of tamoxifen therapy for
early-stage breast cancer. N Engl J Med 2003;349:1793-1802.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/14551341.
367. Goss PE, Ingle JN, Pater JL, et al. Late extended adjuvant
treatment with letrozole improves outcome in women with early-stage
breast cancer who complete 5 years of tamoxifen. J Clin Oncol
2008;26:1948-1955. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18332475.
368. Ingle JN, Tu D, Pater JL, et al. Intent-to-treat analysis of the
placebo-controlled trial of letrozole for extended adjuvant therapy in
early breast cancer: NCIC CTG MA.17. Ann Oncol 2008;19:877-882.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/18332043.
369. Perez EA, Josse RG, Pritchard KI, et al. Effect of letrozole versus
placebo on bone mineral density in women with primary breast cancer
completing 5 or more years of adjuvant tamoxifen: a companion study
to NCIC CTG MA.17. J Clin Oncol 2006;24:3629-3635. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16822845.
370. Whelan TJ, Goss PE, Ingle JN, et al. Assessment of quality of life
in MA.17: a randomized, placebo-controlled trial of letrozole after 5
years of tamoxifen in postmenopausal women. J Clin Oncol
2005;23:6931-6940. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16157934.
371. Davies C, Pan H, Godwin J, et al. Long-term effects of continuing
adjuvant tamoxifen to 10 years versus stopping at 5 years after
diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a
randomised trial. The Lancet 2012. Available at:
http://linkinghub.elsevier.com/retrieve/pii/S0140673612619631.
372. Jakesz R, Greil R, Gnant M, et al. Extended adjuvant therapy with
anastrozole among postmenopausal breast cancer patients: results
from the randomized Austrian Breast and Colorectal Cancer Study
Group Trial 6a. J Natl Cancer Inst 2007;99:1845-1853. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18073378.
373. Dowsett M, Cuzick J, Ingle J, et al. Meta-analysis of breast cancer
outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-101
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
Clin Oncol 2010;28:509-518. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19949017.
374. Smith IE, Dowsett M, Yap Y-S, et al. Adjuvant aromatase inhibitors
for early breast cancer after chemotherapy-induced amenorrhoea:
caution and suggested guidelines. J Clin Oncol 2006;24:2444-2447.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/16735701.
375. Yu B, Douglas N, Ferin MJ, et al. Changes in markers of ovarian
reserve and endocrine function in young women with breast cancer
undergoing adjuvant chemotherapy. Cancer 2010;116:2099-2105.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/20187091.
376. Dowsett M, Nielsen TO, A'Hern R, et al. Assessment of Ki67 in
Breast Cancer: Recommendations from the International Ki67 in Breast
Cancer Working Group. J Natl Cancer Inst 2011. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/21960707.
377. Dowsett M, Smith IE, Ebbs SR, et al. Prognostic value of Ki67
expression after short-term presurgical endocrine therapy for primary
breast cancer. J Natl Cancer Inst 2007;99:167-170. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17228000.
378. . Available at: http://www.cypalleles.ki.se/.
379. Schroth W, Goetz MP, Hamann U, et al. Association between
CYP2D6 polymorphisms and outcomes among women with early stage
breast cancer treated with tamoxifen. JAMA 2009;302:1429-1436.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/19809024.
380. Leyland-Jones B, Regan M, Bouzyk M, et al. Outcome according
to CYP2D6 genotype among postmenopausal women with endocrine-
responsive early invasive breast cancer randomized in the BIG 1-98 trial
[abstract]. Cancer Res 2010;70(24 Suppl):Abstract nr S1-8. Available
at:
http://cancerres.aacrjournals.org/cgi/content/short/70/24_MeetingAbstra
cts/S1-8.
381. Rae J, Drury S, Hayes D, et al. Lack of correlation between gene
variants in tamoxifen metabolizing enzymes with primary endpoints in
the ATAC trial [abstract]. Cancer Res 2010;70(24 Suppl):Abstract S1-7.
Available at:
http://cancerres.aacrjournals.org/cgi/content/meeting_abstract/70/24_M
eetingAbstracts/S1-7?sid=e2c268c0-3fe1-481b-a9c9-01b32769a3d9.
382. Higgins MJ, Stearns V. Pharmacogenetics of endocrine therapy for
breast cancer. Annu Rev Med 2011;62:281-293. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/21226615.
383. Visvanathan K, Chlebowski RT, Hurley P, et al. American Society
of Clinical Oncology clinical practice guideline update on the use of
pharmacologic interventions including tamoxifen, raloxifene, and
aromatase inhibition for breast cancer risk reduction. J Clin Oncol
2009;27:3235-3258. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19470930.
384. Erban JK, Lau J. On the toxicity of chemotherapy for breast
cancer--the need for vigilance. J Natl Cancer Inst 2006;98:1096-1097.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/16912256.
385. Henderson I, Berry D, Demetri G, et al. Improved outcomes from
adding sequential paclitaxel but not from escalating doxorubicin dose in
an adjuvant chemotherapy regimen for patients with node-positive
primary breast cancer. J Clin Oncol 2003;21:976-983. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/12637460.
386. Mamounas E, Bryant J, Lembersky B, et al. Paclitaxel after
doxorubicin plus cyclophosphamide as adjuvant chemotherapy for
node-positive breast cancer: results from NSABP B-28. J Clin Oncol
2005;23:3686-3696. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15897552.
387. Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-
dense versus conventionally scheduled and sequential versus
concurrent combination chemotherapy as postoperative adjuvant
treatment of node-positive primary breast cancer: first report of
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-102
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin
Oncol 2003;21:1431-1439. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/12668651.
388. Sparano JA, Wang M, Martino S, et al. Phase III study of
doxorubicin-cyclophosphamide followed by paclitaxel or docetaxel given
every 3 weeks or weekly in patients with axillary node positive or high
risk node negative breast cancer [abstract]. San Antonio Breast Cancer
Symposium 2005:Abstract 48. Available at:
389. Sparano JA, Wang M, Martino S, et al. Phase III study of
doxorubicin-cyclophosphamide followed by paclitaxel or docetaxel given
every 3 weeks or weekly in operable breast cancer: Results of
Intergroup Trial E1199 [abstract]. J Clin Oncol 2007;25 (Suppl_18)
Abstract 516. Available at:
http://meeting.ascopubs.org/cgi/content/abstract/25/18_suppl/516.
390. Sparano J, Wang M, Martino S, et al. Weekly paclitaxel in the
adjuvant treatment of breast cancer. N Engl J Med 2008;358:1663-
1671. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18420499.
391. Jones S, Holmes FA, O'Shaughnessy J, et al. Docetaxel with
cyclophosphamide is associated with an overall survival benefit
compared with doxorubicin and cyclophosphamide: 7-year follow-up of
US Oncology Research trial 9735. J Clin Oncol 2009;27:1177-1183.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/19204201.
392. Bang SM, Heo DS, Lee KH, et al. Adjuvant doxorubicin and
cyclophosphamide versus cyclophosphamide, methotrexate, and 5-
fluorouracil chemotherapy in premenopausal women with axillary lymph
node positive breast carcinoma. Cancer 2000;89:2521-2526. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/11135211.
393. Fisher B, Brown AM, Dimitrov NV, et al. Two months of
doxorubicin-cyclophosphamide with and without interval reinduction
therapy compared with 6 months of cyclophosphamide, methotrexate,
and fluorouracil in positive-node breast cancer patients with tamoxifen-
nonresponsive tumors: results from the National Surgical Adjuvant
Breast and Bowel Project B-15. J Clin Oncol 1990;8:1483-1496.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/2202791.
394. Fisher B, Anderson S, Wickerham DL, et al. Increased
intensification and total dose of cyclophosphamide in a doxorubicin-
cyclophosphamide regimen for the treatment of primary breast cancer:
findings from National Surgical Adjuvant Breast and Bowel Project B-22.
J Clin Oncol 1997;15:1858-1869. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/9164196.
395. Polychemotherapy for early breast cancer: an overview of the
randomised trials. Early Breast Cancer Trialists' Collaborative Group.
Lancet 1998;352:930-942. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/9752815.
396. Wood WC, Budman DR, Korzun AH, et al. Dose and dose intensity
of adjuvant chemotherapy for stage II, node-positive breast carcinoma.
N Engl J Med 1994;330:1253-1259. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/8080512.
397. Menard S, Valagussa P, Pilotti S, et al. Response to
cyclophosphamide, methotrexate, and fluorouracil in lymph node-
positive breast cancer according to HER2 overexpression and other
tumor biologic variables. J Clin Oncol 2001;19:329-335. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11208823.
398. Muss HB, Thor AD, Berry DA, et al. c-erbB-2 expression and
response to adjuvant therapy in women with node-positive early breast
cancer. N Engl J Med 1994;330:1260-1266. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/7908410.
399. Watanabe T, Kuranami M, Inoue K, et al. Phase III trial comparing
4-cycle doxorubicin plus cyclophosphamide followed by 4-cycle taxan
with 8-cycle taxan as adjuvant therapy for node-positive breast cancer:
Results of N-SAS-BC02 trial [abstract]. J Clin Oncol 2009;27(Suppl
15):Abstract 516. Available at:
http://meeting.ascopubs.org/cgi/content/abstract/27/15S/516.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-103
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
400. Piccart MJ, Di Leo A, Beauduin M, et al. Phase III trial comparing
two dose levels of epirubicin combined with cyclophosphamide with
cyclophosphamide, methotrexate, and fluorouracil in node-positive
breast cancer. J Clin Oncol 2001;19:3103-3110. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11408507.
401. Samuel JA, Wilson JW, Bandos H, et al. Abstract S3-02: NSABP
B-36: A randomized phase III trial comparing six cycles of 5-fluorouracil
(5-FU), epirubicin, and cyclophosphamide (FEC) to four cycles of
adriamycin and cyclophosphamide (AC) in patients (pts) with node-
negative breast cancer. Cancer Research 2015;75:S3-02. Available at:
http://cancerres.aacrjournals.org/content/75/9_Supplement/S3-
02.abstract.
402. Ganz PA, Wilson JW, Bandos H, et al. Abstract P3-12-01: Impact
of treatment on quality of life (QOL) and menstrual history (MH) in the
NSABP B-36: A randomized phase III trial comparing six cycles of 5-
fluorouracil (5-FU), epirubicin, and cyclophosphamide (FEC) to four
cycles of adriamycin and cyclophosphamide. Cancer Research
2015;75:P3-12-01. Available at:
http://cancerres.aacrjournals.org/content/75/9_Supplement/P3-12-
01.abstract.
403. Levine M, Pritchard K, Bramwell V, et al. Randomized trial
comparing cyclophosphamide, epirubicin, and fluorouracil with
cyclophosphamide, methotrexate, and fluorouracil in premenopausal
women with node-positive breast cancer: update of National Cancer
Institute of Canada Clinical Trials Group Trial MA5. J Clin Oncol
2005;23:5166-5170. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16051958.
404. Benefit of a high-dose epirubicin regimen in adjuvant
chemotherapy for node-positive breast cancer patients with poor
prognostic factors: 5-year follow-up results of French Adjuvant Study
Group 05 Randomized Trial. J Clin Oncol 2001;19:602-611. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/11157009.
405. Ellis P, Barrett-Lee P, Johnson L, et al. Sequential docetaxel as
adjuvant chemotherapy for early breast cancer (TACT): an open-label,
phase III, randomised controlled trial. Lancet 2009;373:1681-1692.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/19447249.
406. Martin M, Rodriguez-Lescure A, Ruiz A, et al. Randomized phase
3 trial of fluorouracil, epirubicin, and cyclophosphamide alone or
followed by Paclitaxel for early breast cancer. J Natl Cancer Inst
2008;100:805-814. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18505968.
407. Sparano JA ZF, Martino S, et al. Ten year update of E1199: Phase
III study of doxorubicin-cyclophosphamide followed by paclitaxel or
docetaxel given every 3 weeks or weekly in patients with axillary node-
positive or high-risk node-negative breast cancer [abstract]. San
Antonio Breast Cancer Symposium. Oral Presentation Abstract S3-03.
2014 Available at:
408. Martin M, Pienkowski T, Mackey J, et al. Adjuvant docetaxel for
node-positive breast cancer. N Engl J Med 2005;352:2302-2313.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/15930421.
409. Swain SM, Jeong J-H, Geyer CE, et al. NSABP B-30: definitive
analysis of patient outcome from a randomized trial evaluating different
schedules and combinations of adjuvant therapy containing
doxorubicin, docetaxel and cyclophosphamide in women with operable,
node-positive breast cancer [abstract]. Cancer Research 2009;69
(Suppl_1):Abstract 75. Available at:
http://cancerres.aacrjournals.org/cgi/content/meeting_abstract/69/2_Me
etingAbstracts/75.
410. Muss HB, Berry DA, Cirrincione CT, et al. Adjuvant chemotherapy
in older women with early-stage breast cancer. N Engl J Med
2009;360:2055-2065. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19439741.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-104
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
411. Burstein HJ. The distinctive nature of HER2-positive breast
cancers. N Engl J Med 2005;353:1652-1654. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16236735.
412. Joensuu H, Bono P, Kataja V, et al. Fluorouracil, epirubicin, and
cyclophosphamide with either docetaxel or vinorelbine, with or without
trastuzumab, as adjuvant treatments of breast cancer: final results of
the FinHer Trial. J Clin Oncol 2009;27:5685-5692. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19884557.
413. Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of
trastuzumab plus adjuvant chemotherapy for operable human epidermal
growth factor receptor 2-positive breast cancer: joint analysis of data
from NCCTG N9831 and NSABP B-31. J Clin Oncol 2011;29:3366-
3373. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21768458.
414. Gianni L, Dafni U, Gelber RD, et al. Treatment with trastuzumab
for 1 year after adjuvant chemotherapy in patients with HER2-positive
early breast cancer: a 4-year follow-up of a randomised controlled trial.
Lancet Oncol 2011;12:236-244. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/21354370.
415. Perez EA, Suman VJ, Davidson NE, et al. Cardiac safety analysis
of doxorubicin and cyclophosphamide followed by paclitaxel with or
without trastuzumab in the North Central Cancer Treatment Group
N9831 adjuvant breast cancer trial. J Clin Oncol 2008;26:1231-1238.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/18250349.
416. Tan-Chiu E, Yothers G, Romond E, et al. Assessment of cardiac
dysfunction in a randomized trial comparing doxorubicin and
cyclophosphamide followed by paclitaxel, with or without trastuzumab
as adjuvant therapy in node-positive, human epidermal growth factor
receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol
2005;23:7811-7819. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16258083.
417. Geyer CE, Jr., Bryant JL, Romond EH, et al. Update of cardiac
dysfunction on NSABP B-31, a randomized trial of sequential
doxorubicin/cyclophosphamide (AC)->paclitaxel (T) vs. AC->T with
trastuzumab (H) [abstract]. J Clin Oncol 2006;24(Suppl 18):Abstract
581. Available at:
http://meeting.ascopubs.org/cgi/content/abstract/24/18_suppl/581.
418. Telli ML, Hunt SA, Carlson RW, Guardino AE. Trastuzumab-
related cardiotoxicity: calling into question the concept of reversibility. J
Clin Oncol 2007;25:3525-3533. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17687157.
419. Smith I, Procter M, Gelber RD, et al. 2-year follow-up of
trastuzumab after adjuvant chemotherapy in HER2-positive breast
cancer: a randomised controlled trial. Lancet 2007;369:29-36. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/17208639.
420. Spielmann M, Roché H, Delozier T, et al. Trastuzumab for patients
with axillary-node-positive breast cancer: results of the FNCLCC-PACS
04 trial. J Clin Oncol 2009;27:6129-6134. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19917839.
421. Pivot X, Romieu G, Debled M, et al. 6 months versus 12 months of
adjuvant trastuzumab for patients with HER2-positive early breast
cancer (PHARE): a randomised phase 3 trial. Lancet Oncol
2013;14:741-748. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/23764181.
422. Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, et al. 2 years versus
1 year of adjuvant trastuzumab for HER2-positive breast cancer
(HERA): an open-label, randomised controlled trial. Lancet
2013;382:1021-1028. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/23871490.
423. Chia S, Norris B, Speers C, et al. Human epidermal growth factor
receptor 2 overexpression as a prognostic factor in a large tissue
microarray series of node-negative breast cancers. J Clin Oncol
2008;26:5697-5704. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19001334.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-105
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
424. Gonzalez-Angulo AM, Litton JK, Broglio KR, et al. High risk of
recurrence for patients with breast cancer who have human epidermal
growth factor receptor 2-positive, node-negative tumors 1 cm or smaller.
J Clin Oncol 2009;27:5700-5706. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19884543.
425. O'Sullivan C, Holmes E, Spielmann M, et al. The prognosis of
small HER2+ breast cancers: A meta-analysis of the randomized
trastuzumab trials [abstract]. San Antonio Breast Cancer Symposium
Meeting Abstracts 2013:Abstract S 6-03 Available at:
426. Zhou Q, Yin W, Du Y, Lu J. For or against adjuvant trastuzumab
for pT1a-bN0M0 breast cancer patients with HER2-positive tumors: A
meta-analysis of published literatures. PLoS One 2014;9:e83646.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/24392090.
427. Tolaney S, Barry W, Dang C, et al. A phase II study of adjuvant
paclitaxel (T) and trastuzumab (H) (APT trial) for node-negative, HER2-
positive breast cancer (BC) [abstract]. San Antonio Breast Symposium
Meeting Abstract 2013:Abstract S 1-04 (Oral Presentation). Available at:
428. Piccart-Gebhart MJ, Holmes AP, Baselga J, et al. First results from
the phase III ALTTO trial (BIG 2-06; NCCTG [Alliance] N063D)
comparing one year of anti-HER2 therapy with lapatinib alone (L),
trastuzumab alone (T), their sequence (T->L), or their combination
(T+L) in the adjuvant treatment of HER2-positive early breast cancer
(EBC). ASCO Meeting Abstracts 2014;32:LBA4. Available at:
http://meeting.ascopubs.org/cgi/content/abstract/32/18_suppl/LBA4.
429. Curigliano G, Viale G, Bagnardi V, et al. Clinical relevance of
HER2 overexpression/amplification in patients with small tumor size and
node-negative breast cancer. J Clin Oncol 2009;27:5693-5699.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/19884553.
430. Perez EA, Romond EH, Suman VJ, et al. Updated results of the
combined analysis of NCCTG N9831 and NSABP B-31 adjuvant
chemotherapy with/without trastuzumab in patients with HER2-positive
breast cancer [abstract]. J Clin Oncol 2007;25(Suppl 18):Abstract 512.
Available at:
http://meeting.ascopubs.org/cgi/content/abstract/25/18_suppl/512.
431. Untch M, Gelber RD, Jackisch C, et al. Estimating the magnitude
of trastuzumab effects within patient subgroups in the HERA trial. Ann
Oncol 2008;19:1090-1096. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18296421.
432. Swain S, Kim S-B, Cortes J, et al. Confirmatory overall survival
(OS) analysis of CLEOPATRA: a randomized, double-blind, placebo-
controlled Phase III study with pertuzumab (P), trastuzumab (T), and
docetaxel (D) in patients (pts) with HER2-positive first-line (1L)
metastatic breast cancer (MBC). Cancer Research 2012;72:P5-18-26.
Available at:
http://cancerres.aacrjournals.org/cgi/content/meeting_abstract/72/24_M
eetingAbstracts/P5-18-26.
433. von MG, Baselga J, Bradbury I, et al. Adjuvant Pertuzumab and
Herceptin IN IniTial TherapY of Breast Cancer: APHINITY (BIG 4–
11/BO25126/TOC4939g) [abstract]. Cancer Res 2011; 71(Suppl 24
):Abstract OT1-02-04. Available at:
434. A randomized multicenter, double-blind, placebo-controlled
comparison of chemotherapy plus trastuzumab plus placebo versus
chemotherapy plus trastuzumab plus pertuzumab as adjuvant therapy
in patients with operable HER2-positive primary breast cancer (Clinical
Trial ID: NCT01358877). Available at:
http://clinicaltrials.gov/ct2/show/NCT01358877.
435. Tolaney SM, Barry WT, Dang CT, et al. Adjuvant paclitaxel and
trastuzumab for node-negative, HER2-positive breast cancer. N Engl J
Med 2015;372:134-141. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/25564897.
436. von Minckwitz G, Schneeweiss A, Loibl S, et al. Neoadjuvant
carboplatin in patients with triple-negative and HER2-positive early
breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial Lancet
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-106
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
Oncol 2014;15:747-756. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/24794243.
437. Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of
carboplatin and/or bevacizumab to neoadjuvant once-per-week
paclitaxel followed by dose-dense doxorubicin and cyclophosphamide
on pathologic complete response rates in stage II to III triple-negative
breast cancer: CALGB 40603 (Alliance) [abstract]. Journal of Clinical
Oncology 2014. Available at:
http://jco.ascopubs.org/content/early/2014/08/01/JCO.2014.57.0572.ab
stract.
438. Aukema TS, Straver ME, Peeters MJ, et al. Detection of extra-
axillary lymph node involvement with FDG PET/CT in patients with
stage II-III breast cancer. Eur J Cancer 2010;46:3205-3210. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/20719497.
439. Fuster D, Duch J, Paredes P, et al. Preoperative staging of large
primary breast cancer with [18F]fluorodeoxyglucose positron emission
tomography/computed tomography compared with conventional
imaging procedures. J Clin Oncol 2008;26:4746-4751. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18695254.
440. Groheux D, Moretti JL, Baillet G, et al. Effect of (18)F-FDG PET/CT
imaging in patients with clinical Stage II and III breast cancer. Int J
Radiat Oncol Biol Phys 2008;71:695-704. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18436392.
441. van der Hoeven JJM, Krak NC, Hoekstra OS, et al. 18F-2-fluoro-2-
deoxy-d-glucose positron emission tomography in staging of locally
advanced breast cancer. J Clin Oncol 2004;22:1253-1259. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15051773.
442. Niikura N, Costelloe CM, Madewell JE, et al. FDG-PET/CT
compared with conventional imaging in the detection of distant
metastases of primary breast cancer. Oncologist 2011;16:1111-1119.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/21765193.
443. Morris PG, Lynch C, Feeney JN, et al. Integrated positron emission
tomography/computed tomography may render bone scintigraphy
unnecessary to investigate suspected metastatic breast cancer. J Clin
Oncol 2010;28:3154-3159. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/20516453.
444. Alessio AM, Kinahan PE, Cheng PM, et al. PET/CT scanner
instrumentation, challenges, and solutions. Radiol Clin North Am
2004;42:1017-1032. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15488555.
445. Wong TZ, Paulson EK, Nelson RC, et al. Practical approach to
diagnostic CT combined with PET. AJR Am J Roentgenol
2007;188:622-629. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17312045.
446. Hortobagyi GN, Singletary SE, Strom EA. Locally advanced breast
cancer. In: Harris JR, Lippman ME, Morrow M, Osborne CK, eds.
Diseases of the Breast. Philadelphia: Lippincott Williams & Wilkins;
2004.
447. https://www.astro.org/Clinical-Practice/Choosing-Wisely/2014-
Choosing-Wisely.aspx Aa. Available at:
448. Impact of follow-up testing on survival and health-related quality of
life in breast cancer patients. A multicenter randomized controlled trial.
The GIVIO Investigators. JAMA 1994;271:1587-1592. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/8182811.
449. Rosselli Del Turco M, Palli D, Cariddi A, et al. Intensive diagnostic
follow-up after treatment of primary breast cancer. A randomized trial.
National Research Council Project on Breast Cancer follow-up. JAMA
1994;271:1593-1597. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/7848404.
450. Smith TJ, Davidson NE, Schapira DV, et al. American Society of
Clinical Oncology 1998 update of recommended breast cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-107
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
surveillance guidelines. J Clin Oncol 1999;17:1080-1082. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/10071303.
451. Bast RC, Ravdin P, Hayes DF, et al. 2000 update of
recommendations for the use of tumor markers in breast and colorectal
cancer: clinical practice guidelines of the American Society of Clinical
Oncology. J Clin Oncol 2001;19:1865-1878. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11251019.
452. Kirova YM, Stoppa-Lyonnet D, Savignoni A, et al. Risk of breast
cancer recurrence and contralateral breast cancer in relation to BRCA1
and BRCA2 mutation status following breast-conserving surgery and
radiotherapy. Eur J Cancer 2005;41:2304-2311. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16140006.
453. Metcalfe K, Lynch HT, Ghadirian P, et al. Contralateral breast
cancer in BRCA1 and BRCA2 mutation carriers. J Clin Oncol
2004;22:2328-2335. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15197194.
454. Pierce LJ, Levin AM, Rebbeck TR, et al. Ten-year multi-
institutional results of breast-conserving surgery and radiotherapy in
BRCA1/2-associated stage I/II breast cancer. J Clin Oncol
2006;24:2437-2443. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16636335.
455. ACOG committee opinion. No. 336: Tamoxifen and uterine cancer.
Obstet Gynecol 2006;107:1475-1478. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16738185.
456. Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in
management of hot flashes in survivors of breast cancer: a randomised
controlled trial. Lancet 2000;356:2059-2063. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11145492.
457. Boekhout AH, Vincent AD, Dalesio OB, et al. Management of hot
flashes in patients who have breast cancer with venlafaxine and
clonidine: a randomized, double-blind, placebo-controlled trial. J Clin
Oncol 2011;29:3862-3868. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/21911720.
458. Kaplan M, Mahon S, Cope D, et al. Putting evidence into practice:
evidence-based interventions for hot flashes resulting from cancer
therapies. Clin J Oncol Nurs 2011;15:149-157. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/21444282.
459. Bordeleau L, Pritchard KI, Loprinzi CL, et al. Multicenter,
randomized, cross-over clinical trial of venlafaxine versus gabapentin
for the management of hot flashes in breast cancer survivors. J Clin
Oncol 2010;28:5147-5152. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/21060031.
460. Garber K. Tamoxifen pharmacogenetics moves closer to reality. J
Natl Cancer Inst 2005;97:412-413. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15770000.
461. Jin Y, Desta Z, Stearns V, et al. CYP2D6 genotype, antidepressant
use, and tamoxifen metabolism during adjuvant breast cancer
treatment. J Natl Cancer Inst 2005;97:30-39. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15632378.
462. Henry NL, Stearns V, Flockhart DA, et al. Drug interactions and
pharmacogenomics in the treatment of breast cancer and depression.
Am J Psychiatry 2008;165:1251-1255. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18829880.
463. Ahern TP, Pedersen L, Cronin-Fenton DP, et al. No increase in
breast cancer recurrence with concurrent use of tamoxifen and some
CYP2D6-inhibiting medications. Cancer Epidemiol Biomarkers Prev
2009;18:2562-2564. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19690182.
464. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med
2005;353:487-497. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16079372.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-108
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
465. Dayes IS, Whelan TJ, Julian JA, et al. Randomized trial of
decongestive lymphatic therapy for the treatment of lymphedema in
women with breast cancer. J Clin Oncol 2013;31:3758-3763. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/24043733.
466. Warren AG, Brorson H, Borud LJ, Slavin SA. Lymphedema: a
comprehensive review. Ann Plast Surg 2007;59:464-472. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17901744.
467. Hickey M, Peate M, Saunders CM, Friedlander M. Breast cancer in
young women and its impact on reproductive function. Human
Reproduction Update 2009;15:323-339. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19174449.
468. Moran MS, Colasanto JM, Haffty BG, et al. Effects of breast-
conserving therapy on lactation after pregnancy. Cancer J 2005;11:399-
403. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16259870.
469. Gnant M, Mlineritsch B, Schippinger W, et al. Endocrine therapy
plus zoledronic acid in premenopausal breast cancer. N Engl J Med
2009;360:679-691. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19213681.
470. Gnant M, Pfeiler G, Dubsky PC, et al. Adjuvant denosumab in
breast cancer (ABCSG-18): a multicentre, randomised, double-blind,
placebo-controlled trial. Lancet 2015;386:433-443. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/26040499.
471. Li CI, Daling JR, Porter PL, et al. Relationship between potentially
modifiable lifestyle factors and risk of second primary contralateral
breast cancer among women diagnosed with estrogen receptor-positive
invasive breast cancer. J Clin Oncol 2009;27:5312-5318. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19738113.
472. Pierce JP, Stefanick ML, Flatt SW, et al. Greater survival after
breast cancer in physically active women with high vegetable-fruit intake
regardless of obesity. J Clin Oncol 2007;25:2345-2351. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17557947.
473. Chlebowski RT BG, et al. . Final survival analysis from the
randomized Women's Intervention Nutrition Study (WINS) evaluating
dietary intervention as adjuvant breast cancer therapy [abstract]. San
Antonio Breast Cancer Symposium 2014;Abstract S5-08. Available at:
474. de Glas NA, Fontein DB, Bastiaannet E, et al. Physical activity and
survival of postmenopausal, hormone receptor-positive breast cancer
patients: results of the Tamoxifen Exemestane Adjuvant Multicenter
Lifestyle study. Cancer 2014;120:2847-2854. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/24840230.
475. Courneya KS, Segal RJ, McKenzie DC, et al. Effects of exercise
during adjuvant chemotherapy on breast cancer outcomes. Med Sci
Sports Exerc 2014;46:1744-1751. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/24633595.
476. Mishra SI, Scherer RW, Snyder C, et al. Exercise interventions on
health-related quality of life for people with cancer during active
treatment. Cochrane Database Syst Rev 2012;8:CD008465. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/22895974.
477. Eubank WB, Mankoff D, Bhattacharya M, et al. Impact of FDG PET
on defining the extent of disease and on the treatment of patients with
recurrent or metastatic breast cancer. AJR Am J Roentgenol
2004;183:479-486. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15269044.
478. Moon DH, Maddahi J, Silverman DH, et al. Accuracy of whole-
body fluorine-18-FDG PET for the detection of recurrent or metastatic
breast carcinoma. J Nucl Med 1998;39:431-435. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/9529287.
479. Arslan C, Sari E, Aksoy S, Altundag K. Variation in hormone
receptor and HER-2 status between primary and metastatic breast
cancer: review of the literature. Expert Opin Ther Targets 2011;15:21-
30. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21105765.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-109
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
480. Pusztai L, Viale G, Kelly CM, Hudis CA. Estrogen and HER-2
receptor discordance between primary breast cancer and metastasis.
Oncologist 2010;15:1164-1168. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/21041379.
481. Bogina G, Bortesi L, Marconi M, et al. Comparison of hormonal
receptor and HER-2 status between breast primary tumours and
relapsing tumours: clinical implications of progesterone receptor loss.
Virchows Arch 2011;459:1-10. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/21643691.
482. Fabi A, Di Benedetto A, Metro G, et al. HER2 protein and gene
variation between primary and metastatic breast cancer: significance
and impact on patient care. Clin Cancer Res 2011;17:2055-2064.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/21307144.
483. Karlsson E, Lindström LS, Wilking U, et al. Discordance in
hormone receptor status in breast cancer during tumor progression
[abstract]. J Clin Oncol 2010;28:(15_Suppl):Abstract 1009. Available at:
http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail
_view&confID=74&abstractID=47385.
484. Sari E, Guler G, Hayran M, et al. Comparative study of the
immunohistochemical detection of hormone receptor status and HER-2
expression in primary and paired recurrent/metastatic lesions of patients
with breast cancer. Med Oncol 2011;28:57-63. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/20099049.
485. Simmons C, Miller N, Geddie W, et al. Does confirmatory tumor
biopsy alter the management of breast cancer patients with distant
metastases? Ann Oncol 2009;20:1499-1504. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19299408.
486. Gong Y, Booser DJ, Sneige N. Comparison of HER-2 status
determined by fluorescence in situ hybridization in primary and
metastatic breast carcinoma. Cancer 2005;103:1763-1769. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15786420.
487. Tapia C, Savic S, Wagner U, et al. HER2 gene status in primary
breast cancers and matched distant metastases. Breast Cancer Res
2007;9. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17511881.
488. Lindstrom LS, Karlsson E, Wilking UM, et al. Clinically used breast
cancer markers such as estrogen receptor, progesterone receptor, and
human epidermal growth factor receptor 2 are unstable throughout
tumor progression. J Clin Oncol 2012;30:2601-2608. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/22711854.
489. Dieci MV, Barbieri E, Piacentini F, et al. Discordance in receptor
status between primary and recurrent breast cancer has a prognostic
impact: a single-institution analysis. Ann Oncol 2013;24:101-108.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/23002281.
490. Aurilio G, Disalvatore D, Pruneri G, et al. A meta-analysis of
oestrogen receptor, progesterone receptor and human epidermal
growth factor receptor 2 discordance between primary breast cancer
and metastases. Eur J Cancer 2014;50:277-289. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/24269135.
491. Katz A, Strom EA, Buchholz TA, et al. Locoregional recurrence
patterns after mastectomy and doxorubicin-based chemotherapy:
implications for postoperative irradiation. J Clin Oncol 2000;18:2817-
2827. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10920129.
492. van Tienhoven G, Voogd AC, Peterse JL, et al. Prognosis after
treatment for loco-regional recurrence after mastectomy or breast
conserving therapy in two randomised trials (EORTC 10801 and DBCG-
82TM). EORTC Breast Cancer Cooperative Group and the Danish
Breast Cancer Cooperative Group. Eur J Cancer 1999;35:32-38.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/10211085.
493. Cox CE, Furman BT, Kiluk JV, et al. Use of reoperative sentinel
lymph node biopsy in breast cancer patients. J Am Coll Surg
2008;207:57-61. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18589362.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-110
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
494. Aebi S, Gelber S, Anderson SJ, et al. Chemotherapy for isolated
locoregional recurrence of breast cancer (CALOR): a randomised trial.
Lancet Oncol 2014;15:156-163. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/24439313.
495. Higgins MJ, Wolff AC. Therapeutic options in the management of
metastatic breast cancer. Oncology (Williston Park) 2008;22:614-623.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/18561551.
496. Gralow JR, Biermann JS, Farooki A, et al. NCCN task force report:
Bone health in cancer care. J Natl Compr Canc Netw 2009;7 Suppl 3:1-
1. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19555589.
497. Hillner BE, Ingle JN, Chlebowski RT, et al. American Society of
Clinical Oncology 2003 update on the role of bisphosphonates and
bone health issues in women with breast cancer. J Clin Oncol
2003;21:4042-4057. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/12963702.
498. Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with
zoledronic acid for the treatment of bone metastases in patients with
advanced breast cancer: A randomized, double-blind study. J Clin
Oncol 2010;28:5132-5139. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/21060033.
499. Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a
cytokine that regulates osteoclast differentiation and activation. Cell
1998;93:165-176. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/9568710.
500. Woo S-B, Hellstein JW, Kalmar JR. Narrative [corrected] review:
bisphosphonates and osteonecrosis of the jaws. Ann Intern Med
2006;144:753-761. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16702591.
501. Ali SM, Esteva FJ, Hortobagyi G, et al. Safety and efficacy of
bisphosphonates beyond 24 months in cancer patients. J Clin Oncol
2001;19:3434-3437. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11454892.
502. Berenson JR, Rosen LS, Howell A, et al. Zoledronic acid reduces
skeletal-related events in patients with osteolytic metastases. Cancer
2001;91:1191-1200. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11283917.
503. Conte PF, Latreille J, Mauriac L, et al. Delay in progression of
bone metastases in breast cancer patients treated with intravenous
pamidronate: results from a multinational randomized controlled trial.
The Aredia Multinational Cooperative Group. J Clin Oncol
1996;14:2552-2559. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/8823335.
504. Hortobagyi GN, Theriault RL, Lipton A, et al. Long-term prevention
of skeletal complications of metastatic breast cancer with pamidronate.
Protocol 19 Aredia Breast Cancer Study Group. J Clin Oncol
1998;16:2038-2044. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/9626201.
505. Theriault RL. The role of bisphosphonates in breast cancer. J Natl
Compr Canc Netw 2003;1:232-241. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19768882.
506. Theriault RL, Lipton A, Hortobagyi GN, et al. Pamidronate reduces
skeletal morbidity in women with advanced breast cancer and lytic bone
lesions: a randomized, placebo-controlled trial. Protocol 18 Aredia
Breast Cancer Study Group. J Clin Oncol 1999;17:846-854. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/10071275.
507. Rosen LS, Gordon D, Kaminski M, et al. Zoledronic acid versus
pamidronate in the treatment of skeletal metastases in patients with
breast cancer or osteolytic lesions of multiple myeloma: a phase III,
double-blind, comparative trial. Cancer J 2001;7:377-387. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11693896.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-111
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
508. Rosen LS, Gordon DH, Dugan W, et al. Zoledronic acid is superior
to pamidronate for the treatment of bone metastases in breast
carcinoma patients with at least one osteolytic lesion. Cancer
2004;100:36-43. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/14692022.
509. Diel IJ, Body JJ, Lichinitser MR, et al. Improved quality of life after
long-term treatment with the bisphosphonate ibandronate in patients
with metastatic bone disease due to breast cancer. Eur J Cancer
2004;40:1704-1712. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15251160.
510. Hortobagyi GN, Theriault RL, Porter L, et al. Efficacy of
pamidronate in reducing skeletal complications in patients with breast
cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer
Study Group. N Engl J Med 1996;335:1785-1791. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/8965890.
511. Lipton A, Theriault RL, Hortobagyi GN, et al. Pamidronate prevents
skeletal complications and is effective palliative treatment in women
with breast carcinoma and osteolytic bone metastases: long term follow-
up of two randomized, placebo-controlled trials. Cancer 2000;88:1082-
1090. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10699899.
512. McLachlan SA, Cameron D, Murray R, et al. Safety of oral
ibandronate in the treatment of bone metastases from breast cancer :
long-term follow-up experience. Clin Drug Investig 2006;26:43-48.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/17163234.
513. Pecherstorfer M, Rivkin S, Body J-J, et al. Long-term safety of
intravenous ibandronic acid for up to 4 years in metastatic breast
cancer: an open-label trial. Clin Drug Investig 2006;26:315-322.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/17163265.
514. Hortobagyi GN, Lipton A, Chew HK, et al. Efficacy and safety of
continued zoledronic acid every 4 weeks versus every 12 weeks in
women with bone metastases from breast cancer: Results of the
OPTIMIZE-2 trial. ASCO Meeting Abstracts 2014;32:LBA9500.
Available at:
http://meeting.ascopubs.org/cgi/content/abstract/32/18_suppl/LBA9500.
515. Rosen LS, Gordon D, Kaminski M, et al. Long-term efficacy and
safety of zoledronic acid compared with pamidronate disodium in the
treatment of skeletal complications in patients with advanced multiple
myeloma or breast carcinoma: a randomized, double-blind, multicenter,
comparative trial. Cancer 2003;98:1735-1744. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/14534891.
516. Wilkinson GS, Kuo Y-F, Freeman JL, Goodwin JS. Intravenous
bisphosphonate therapy and inflammatory conditions or surgery of the
jaw: a population-based analysis. J Natl Cancer Inst 2007;99:1016-
1024. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17596574.
517. Klijn JG, Blamey RW, Boccardo F, et al. Combined tamoxifen and
luteinizing hormone-releasing hormone (LHRH) agonist versus LHRH
agonist alone in premenopausal advanced breast cancer: a meta-
analysis of four randomized trials. J Clin Oncol 2001;19:343-353.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/11208825.
518. Bonneterre J, Thurlimann B, Robertson JF, et al. Anastrozole
versus tamoxifen as first-line therapy for advanced breast cancer in 668
postmenopausal women: results of the Tamoxifen or Arimidex
Randomized Group Efficacy and Tolerability study. J Clin Oncol
2000;18:3748-3757. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11078487.
519. Nabholtz JM, Buzdar A, Pollak M, et al. Anastrozole is superior to
tamoxifen as first-line therapy for advanced breast cancer in
postmenopausal women: results of a North American multicenter
randomized trial. Arimidex Study Group. J Clin Oncol 2000;18:3758-
3767. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11078488.
520. Paridaens RJ, Dirix LY, Beex LV, et al. Phase III study comparing
exemestane with tamoxifen as first-line hormonal treatment of
metastatic breast cancer in postmenopausal women: the European
Organisation for Research and Treatment of Cancer Breast Cancer
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-112
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
Cooperative Group. J Clin Oncol 2008;26:4883-4890. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18794551.
521. Vergote I, Bonneterre J, Thurlimann B, et al. Randomised study of
anastrozole versus tamoxifen as first-line therapy for advanced breast
cancer in postmenopausal women. Eur J Cancer 2000;36 Suppl 4:S84-
85. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11056332.
522. Gibson L, Lawrence D, Dawson C, Bliss J. Aromatase inhibitors for
treatment of advanced breast cancer in postmenopausal women.
Cochrane Database Syst Rev 2009. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19821307.
523. Howell A, Robertson JFR, Quaresma Albano J, et al. Fulvestrant,
formerly ICI 182,780, is as effective as anastrozole in postmenopausal
women with advanced breast cancer progressing after prior endocrine
treatment. J Clin Oncol 2002;20:3396-3403. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/12177099.
524. Osborne CK, Pippen J, Jones SE, et al. Double-blind, randomized
trial comparing the efficacy and tolerability of fulvestrant versus
anastrozole in postmenopausal women with advanced breast cancer
progressing on prior endocrine therapy: results of a North American
trial. J Clin Oncol 2002;20:3386-3395. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/12177098
525. Robertson JF, Llombart-Cussac A, Rolski J, et al. Activity of
fulvestrant 500 mg versus anastrozole 1 mg as first-line treatment for
advanced breast cancer: results from the FIRST study. J Clin Oncol
2009;27:4530-4535. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19704066.
526. Robertson JF, Lindemann JP, Llombart-Cussac A, et al.
Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment
of advanced breast cancer: follow-up analysis from the randomized
'FIRST' study. Breast Cancer Res Treat 2012;136:503-511. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/23065000.
527. Ellis MJ, Llombart-Cussac A, Feltl D, et al. Fulvestrant 500 mg
versus anastrozole 1 mg for the first-Line treatment of advanced breast
cancer: Overall survival analysis from the phase II FIRST study. J Clin
Oncol 2015;33:3781-3787. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/26371134.
528. Ingle JN, Suman VJ, Rowland KM, et al. Fulvestrant in women with
advanced breast cancer after progression on prior aromatase inhibitor
therapy: North Central Cancer Treatment Group Trial N0032. J Clin
Oncol 2006;24:1052-1056. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16505423.
529. Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized
placebo controlled trial of fulvestrant compared with exemestane after
prior nonsteroidal aromatase inhibitor therapy in postmenopausal
women with hormone receptor-positive, advanced breast cancer: results
from EFECT. J Clin Oncol 2008;26:1664-1670. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18316794.
530. Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the
CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant
500 mg in postmenopausal women with estrogen receptor-positive
advanced breast cancer. J Clin Oncol 2010;28:4594-4600. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/20855825.
531. Di Leo A, Jerusalem G, Petruzelka L, et al. Final overall survival:
fulvestrant 500 mg vs 250 mg in the randomized CONFIRM trial. J Natl
Cancer Inst 2014;106:djt337. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/24317176.
532. Bergh J, Jonsson PE, Lidbrink EK, et al. FACT: an open-label
randomized phase III study of fulvestrant and anastrozole in
combination compared with anastrozole alone as first-line therapy for
patients with receptor-positive postmenopausal breast cancer. J Clin
Oncol 2012;30:1919-1925. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/22370325.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-113
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
533. Mehta RS, Barlow WE, Albain KS, et al. Combination anastrozole
and fulvestrant in metastatic breast cancer. N Engl J Med
2012;367:435-444. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/22853014.
534. Johnston SR, Kilburn LS, Ellis P, et al. Fulvestrant plus
anastrozole or placebo versus exemestane alone after progression on
non-steroidal aromatase inhibitors in postmenopausal patients with
hormone-receptor-positive locally advanced or metastatic breast cancer
(SoFEA): a composite, multicentre, phase 3 randomised trial. Lancet
Oncol 2013;14:989-998. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/23902874.
535. Buzdar A, Douma J, Davidson N, et al. Phase III, multicenter,
double-blind, randomized study of letrozole, an aromatase inhibitor, for
advanced breast cancer versus megestrol acetate. J Clin Oncol
2001;19:3357-3366. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11454883.
536. Buzdar AU, Jonat W, Howell A, et al. Anastrozole versus
megestrol acetate in the treatment of postmenopausal women with
advanced breast carcinoma: results of a survival update based on a
combined analysis of data from two mature phase III trials. Arimidex
Study Group. Cancer 1998;83:1142-1152. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/9740079.
537. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6
inhibitor palbociclib in combination with letrozole versus letrozole alone
as first-line treatment of oestrogen receptor-positive, HER2-negative,
advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2
study. Lancet Oncol 2015;16:25-35. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/25524798.
538. Turner NC, Ro J, Andre F, et al. Palbociclib in hormone-receptor-
positive advanced breast cancer. N Engl J Med 2015;373:209-219.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/26030518.
539. Johnston S, Pippen J, Pivot X, et al. Lapatinib combined with
letrozole versus letrozole and placebo as first-line therapy for
postmenopausal hormone receptor-positive metastatic breast cancer. J
Clin Oncol 2009;27:5538-5546. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19786658.
540. Kaufman B, Mackey JR, Clemens MR, et al. Trastuzumab plus
anastrozole versus anastrozole alone for the treatment of
postmenopausal women with human epidermal growth factor receptor
2-positive, hormone receptor-positive metastatic breast cancer: results
from the randomized phase III TAnDEM study. J Clin Oncol
2009;27:5529-5537. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19786670.
541. Bachelot T, Bourgier c, Cropet C, et al. TAMRAD: A GINECO
randomized phase II trial of everolimus in combination with tamoxifen
versus tamoxifen alone in patients (pts) with hormone-receptor positive,
HER2 negative metastatic breast Cancer (MBC) with prior exposure to
aromatase inhibitors (AI) [abstract]. Cancer Res 2010;70(24
Supplement):Abstract: S1-6 Available at:
http://cancerres.aacrjournals.org/cgi/content/meeting_abstract/70/24_M
eetingAbstracts/S1-6.
542. Chow L, Sun Y, Jassem J, et al. Phase 3 study of temsirolimus
with letrozole or letrozole alone in postmenopausal women with locally
advanced or metastatic breast cancer. Breast Cancer Res Treat.
2006;100(Suppl 1):6091. Available at:
543. Yardley DA, Noguchi S, Pritchard KI, et al. Everolimus plus
exemestane in postmenopausal patients with HR(+) breast cancer:
BOLERO-2 final progression-free survival analysis. Adv Ther
2013;30:870-884. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/24158787.
544. Baselga J, Campone M, Piccart M, et al. Everolimus in
postmenopausal hormone-receptor-positive advanced breast cancer. N
Engl J Med 2012;366:520-529. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/22149876.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-114
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
545. Pritchard KI, Burris HA, 3rd, Ito Y, et al. Safety and efficacy of
everolimus with exemestane vs. exemestane alone in elderly patients
with HER2-negative, hormone receptor-positive breast cancer in
BOLERO-2. Clin Breast Cancer 2013;13:421-432 e428. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/24267730.
546. Buzdar A, Jonat W, Howell A, et al. Anastrozole, a potent and
selective aromatase inhibitor, versus megestrol acetate in
postmenopausal women with advanced breast cancer: results of
overview analysis of two phase III trials. Arimidex Study Group. J Clin
Oncol 1996;14:2000-2011. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/8683230.
547. Dombernowsky P, Smith I, Falkson G, et al. Letrozole, a new oral
aromatase inhibitor for advanced breast cancer: double-blind
randomized trial showing a dose effect and improved efficacy and
tolerability compared with megestrol acetate. J Clin Oncol 1998;16:453-
461. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9469328.
548. Lonning PE, Bajetta E, Murray R, et al. Activity of exemestane in
metastatic breast cancer after failure of nonsteroidal aromatase
inhibitors: a phase II trial. J Clin Oncol 2000;18:2234-2244. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/10829043.
549. Albain KS, Nag S, Calderillo-Ruiz G, et al. Global phase III study of
gemcitabine plus paclitaxel (GT) vs. paclitaxel (T) as frontline therapy
for metastatic breast cancer (MBC): First report of overall survival
[Abstract]. J Clin Oncol 2004;22:Abstract 510 Available at:
http://meeting.ascopubs.org/cgi/content/abstract/22/14_suppl/510.
550. Carrick S, Parker S, Wilcken N, et al. Single agent versus
combination chemotherapy for metastatic breast cancer. Cochrane
Database Syst Rev 2005. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15846660.
551. O'Shaughnessy J, Miles D, Vukelja S, et al. Superior survival with
capecitabine plus docetaxel combination therapy in anthracycline-
pretreated patients with advanced breast cancer: phase III trial results. J
Clin Oncol 2002;20:2812-2823. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/12065558.
552. Sledge GW, Neuberg D, Bernardo P, et al. Phase III trial of
doxorubicin, paclitaxel, and the combination of doxorubicin and
paclitaxel as front-line chemotherapy for metastatic breast cancer: an
intergroup trial (E1193). J Clin Oncol 2003;21:588-592. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/12586793.
553. Gennari A, Stockler M, Puntoni M, et al. Duration of chemotherapy
for metastatic breast cancer: a systematic review and meta-analysis of
randomized clinical trials. J Clin Oncol 2011;29:2144-2149. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/21464403.
554. Falkson G, Gelman RS, Pandya KJ, et al. Eastern Cooperative
Oncology Group randomized trials of observation versus maintenance
therapy for patients with metastatic breast cancer in complete remission
following induction treatment. J Clin Oncol 1998;16:1669-1676.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/9586877.
555. Muss HB, Case LD, Richards F, et al. Interrupted versus
continuous chemotherapy in patients with metastatic breast cancer. The
Piedmont Oncology Association. N Engl J Med 1991;325:1342-1348.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/1922236.
556. Cortes J, O'Shaughnessy J, Loesch D, et al. Eribulin monotherapy
versus treatment of physician's choice in patients with metastatic breast
cancer (EMBRACE): a phase 3 open-label randomised study. Lancet
2011;377:914-923. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/21376385.
557. Kaufman PA, Awada A, Twelves C, et al. Phase III open-label
randomized study of eribulin mesylate versus capecitabine in patients
with locally advanced or metastatic breast cancer previously treated
with an anthracycline and a taxane. J Clin Oncol 2015;33:594-601.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/25605862.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-115
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
558. Roche H, Yelle L, Cognetti F, et al. Phase II clinical trial of
ixabepilone (BMS-247550), an epothilone B analog, as first-line therapy
in patients with metastatic breast cancer previously treated with
anthracycline chemotherapy. J Clin Oncol 2007;25:3415-3420.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/17606972.
559. Thomas E, Tabernero J, Fornier M, et al. Phase II clinical trial of
ixabepilone (BMS-247550), an epothilone B analog, in patients with
taxane-resistant metastatic breast cancer. J Clin Oncol 2007;25:3399-
3406. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17606975.
560. Perez EA, Lerzo G, Pivot X, et al. Efficacy and safety of
ixabepilone (BMS-247550) in a phase II study of patients with advanced
breast cancer resistant to an anthracycline, a taxane, and capecitabine.
J Clin Oncol 2007;25:3407-3414. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17606974.
561. Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab
versus paclitaxel alone for metastatic breast cancer. N Engl J Med
2007;357:2666-2676. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18160686.
562. Miles DW, Chan A, Dirix LY, et al. Phase III study of bevacizumab
plus docetaxel compared with placebo plus docetaxel for the first-line
treatment of human epidermal growth factor receptor 2-negative
metastatic breast cancer. J Clin Oncol 2010;28:3239-3247. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/20498403.
563. Robert NJ, Dieras V, Glaspy J, et al. RIBBON-1: Randomized,
double-blind, placebo-controlled, phase III trial of chemotherapy with or
without bevacizumab (B) for first-line treatment of HER2-negative locally
recurrent or metastatic breast cancer (MBC) [abstract]. J Clin Oncol
2009;27(Suppl 15):Abstract 1005. Available at:
http://meeting.ascopubs.org/cgi/content/abstract/27/15S/1005.
564. Robert NJ, Dieras V, Glaspy J, et al. RIBBON-1: randomized,
double-blind, placebo-controlled, phase III trial of chemotherapy with or
without bevacizumab for first-line treatment of human epidermal growth
factor receptor 2-negative, locally recurrent or metastatic breast cancer.
J Clin Oncol 2011;29:1252-1260. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/21383283.
565. O'Shaughnessy J, Miles D, Gray RJ, et al. A meta-analysis of
overall survival data from three randomized trials of bevacizumab (BV)
and first-line chemotherapy as treatment for patients with metastatic
breast cancer (MBC) [abstract]. J Clin Oncol 2010;28(Suppl
15):Abstract 1005. Available at:
http://meeting.ascopubs.org/cgi/content/abstract/28/15_suppl/1005.
566. BRIEFING BOOK, ONCOLOGY DRUGS ADVISORY
COMMITTEE MEETING, AVASTIN (Bevacizumab). Genentech, Inc., A
Member of the Roche Group; 2010. Available at:
http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeeting
Materials/Drugs/OncologicDrugsAdvisoryCommittee/UCM219228.pdf.
567. Seidman A, Berry DA, Cirrincione C, et al. Randomized phase III
trial of weekly compared with every-3-weeks paclitaxel for metastatic
breast cancer, with trastuzumab for all HER-2 overexpressors and
random assignment to trastuzumab or not in HER-2 nonoverexpressors:
final results of Cancer and Leukemia Group B protocol 9840. J Clin
Oncol 2008;26:1642-1649. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18375893.
568. Carlson RW, Moench SJ, Hammond ME, et al. HER2 testing in
breast cancer: NCCN Task Force report and recommendations. J Natl
Compr Canc Netw 2006;4 Suppl 3:1-22. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16813731.
569. Roche PC, Suman VJ, Jenkins RB, et al. Concordance between
local and central laboratory HER2 testing in the breast intergroup trial
N9831. J Natl Cancer Inst 2002;94:855-857. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/12048274.
570. Wolff AC, Hammond MEH, Schwartz JN, et al. American Society of
Clinical Oncology/College of American Pathologists guideline
recommendations for human epidermal growth factor receptor 2 testing
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-116
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
in breast cancer. J Clin Oncol 2007;25:118-145. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17159189.
571. Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab
plus docetaxel for metastatic breast cancer. N Engl J Med
2012;366:109-119. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/22149875.
572. Ewer M, Baselga J, Clark E, et al. Cardiac tolerability of
pertuzumab plus trastuzumab plus docetaxel in patients with HER2-
positive metastatic breast cancer in the CLEOPATRA study [abstract]. J
Clin Oncol 2012;30 (Suppl_15):Abstract 533. Available at:
http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail
_view&confID=114&abstractID=95049.
573. Cortés J, Baselga J, Im Y, et al. Quality of life assessment in
CLEOPATRA, a phase III study combining pertuzumab with
trastuzumab and docetaxel in metastatic breast cancer [abstract]. J Clin
Oncol 2012 30 (Suppl_15) Abstract 598 Available at:
http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail
_view&confID=114&abstractID=95084.
574. Datko F, D'Andrea G, Dickler M, et al. Phase II study of
pertuzumab, trastuzumab, and weekly paclitaxel in patients with
metastatic HER2-overexpressing metastatic breast cancer [abstract].
Cancer Research 2012;72:Abstract P5-18-20. Available at:
http://cancerres.aacrjournals.org/cgi/content/meeting_abstract/72/24_M
eetingAbstracts/P5-18-20.
575. Paclitaxel, trastuzumab, and pertuzumab in the treatment of
metastatic HER2-positive breast cancer (Clinical Trial ID:
NCT01276041). Available at:
http://clinicaltrials.gov/show/NCT01276041. .
576. Perez E, Lopez-Vega J, Mastro L, et al. A combination of
pertuzumab, trastuzumab, and vinorelbine for first-line treatment of
patients with HER2-positive metastatic breast cancer: An open-label,
two-cohort, phase II study (VELVET) [abstract]. J Clin Oncol 2012;30
(Suppl_15):Asbtract TPS653. Available at:
http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail
_view&confID=114&abstractID=93917.
577. Burstein HJ, Keshaviah A, Baron AD, et al. Trastuzumab plus
vinorelbine or taxane chemotherapy for HER2-overexpressing
metastatic breast cancer: the trastuzumab and vinorelbine or taxane
study. Cancer 2007;110:965-972. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17614302.
578. Robert N, Leyland-Jones B, Asmar L, et al. Randomized phase III
study of trastuzumab, paclitaxel, and carboplatin compared with
trastuzumab and paclitaxel in women with HER-2-overexpressing
metastatic breast cancer. J Clin Oncol 2006;24:2786-2792. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16782917.
579. Schaller G, Bangemann N, Weber J, et al. Efficacy and safety of
trastuzumab plus capecitabine in a German multicentre phase II study
of pre-treated metastatic breast cancer [abstract]. J Clin Oncol
2005;23(Suppl 16):Abstract 717. Available at:
http://meeting.ascopubs.org/cgi/content/abstract/23/16_suppl/717.
580. Yamamoto D, Iwase S, Kitamura K, et al. A phase II study of
trastuzumab and capecitabine for patients with HER2-overexpressing
metastatic breast cancer: Japan Breast Cancer Research Network
(JBCRN) 00 Trial. Cancer Chemother Pharmacol 2008;61:509-514.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/17516068.
581. Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the
trastuzumab clinical trials experience. J Clin Oncol 2002;20:1215-1221.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/11870163.
582. Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for
HER2-positive advanced breast cancer. N Engl J Med 2012;367:1783-
1791. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23020162.
583. Ellis PA, Barrios CH, Eiermann W, et al. Phase III, randomized
study of trastuzumab emtansine (T-DM1) {+/-} pertuzumab (P) vs
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-117
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
trastuzumab + taxane (HT) for first-line treatment of HER2-positive
MBC: Primary results from the MARIANNE study. ASCO Meeting
Abstracts 2015;33:507. Available at:
http://meeting.ascopubs.org/cgi/content/abstract/33/15_suppl/507.
584. Bartsch R, Wenzel C, Altorjai G, et al. Capecitabine and
trastuzumab in heavily pretreated metastatic breast cancer. J Clin Oncol
2007;25:3853-3858. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17679724.
585. von Minckwitz G, du Bois A, Schmidt M, et al. Trastuzumab
beyond progression in human epidermal growth factor receptor 2-
positive advanced breast cancer: a german breast group 26/breast
international group 03-05 study. J Clin Oncol 2009;27:1999-2006.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/19289619.
586. Von Minckwitz G, Zielinski C, Maarteense E, et al. Capecitabine
vs. capecitabine + trastuzumab in patients with HER2-positive
metastatic breast cancer progressing during trastuzumab treatment:
The TBP phase III study (GBG 26/BIG 3-05) [abstract]. J Clin Oncol
2008;26(Suppl 15):Abstract 1025. Available at:
http://meeting.ascopubs.org/cgi/content/abstract/26/15_suppl/1025.
587. Baselga J, Gelmon KA, Verma S, et al. Phase II trial of
pertuzumab and trastuzumab in patients with human epidermal growth
factor receptor 2-positive metastatic breast cancer that progressed
during prior trastuzumab therapy. J Clin Oncol 2010;28:1138-1144.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/20124182.
588. Cortes J, Fumoleau P, Bianchi GV, et al. Pertuzumab
monotherapy after trastuzumab-based treatment and subsequent
reintroduction of trastuzumab: activity and tolerability in patients with
advanced human epidermal growth factor receptor 2-positive breast
cancer. J Clin Oncol 2012;30:1594-1600. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/22393084.
589. Geyer C, Forster J, Lindquist D, et al. Lapatinib plus capecitabine
for HER2-positive advanced breast cancer. N Engl J Med
2006;355:2733-2743. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17192538.
590. Cameron D, Casey M, Oliva C, et al. Lapatinib plus capecitabine in
women with HER-2-positive advanced breast cancer: final survival
analysis of a phase III randomized trial. Oncologist 2010;15:924-934.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/20736298.
591. Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study
of lapatinib alone or in combination with trastuzumab in women with
ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin
Oncol 2010;28:1124-1130. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/20124187.
592. Blackwell KL, Burstein HJ, Storniolo AM, et al. Overall survival
benefit with lapatinib in combination with trastuzumab for patients with
human epidermal growth factor receptor 2-positive metastatic breast
cancer: final results from the EGF104900 Study. J Clin Oncol
2012;30:2585-2592. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/22689807.
593. Hortobagyi GN. Multidisciplinary management of advanced primary
and metastatic breast cancer. Cancer 1994;74:416-423. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/8004615.
594. Babiera GV, Rao R, Feng L, et al. Effect of primary tumor
extirpation in breast cancer patients who present with stage IV disease
and an intact primary tumor. Ann Surg Oncol 2006;13:776-782.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/16614878.
595. Khan SA, Stewart AK, Morrow M. Does aggressive local therapy
improve survival in metastatic breast cancer? Surgery 2002;132:620-
626. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12407345.
596. Rao R, Feng L, Kuerer HM, et al. Timing of surgical intervention for
the intact primary in stage IV breast cancer patients. Ann Surg Oncol
2008;15:1696-1702. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18357493.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-118
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
597. Rapiti E, Verkooijen HM, Vlastos G, et al. Complete excision of
primary breast tumor improves survival of patients with metastatic
breast cancer at diagnosis. J Clin Oncol 2006;24:2743-2749. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/16702580.
598. Morrow M, Goldstein L. Surgery of the primary tumor in metastatic
breast cancer: closing the barn door after the horse has bolted? J Clin
Oncol 2006;24:2694-2696. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16702578.
599. Olson JA, Marcom PK. Benefit or bias? The role of surgery to
remove the primary tumor in patients with metastatic breast cancer. Ann
Surg 2008;247:739-740. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18438109.
600. Badwe R, Parmar V, Hawaldar R, et al. Surgical removal of
primary breast tumor and axillary lymph nodes in women with
metastatic breast cancer at first presentation: a randomized controlled
trial. Presented at: 2013 [abstract]. San Antonio Breast Cancer
Symposium 2013:Abstract S2-02. Available at:
601. Soran A, Ozmen V, Ozbas S, et al. Early follow up of a randomized
trial evaluating resection of the primary breast tumor in women
presenting with de novo stage IV breast cancer; Turkish study (protocol
MF07-01) [abstract]. San Antonio Breast Cancer Symposium
2013:Abstract S2-03. Available at:
602. Jones EL, Oleson JR, Prosnitz LR, et al. Randomized trial of
hyperthermia and radiation for superficial tumors. J Clin Oncol
2005;23:3079-3085. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15860867.
603. Vernon CC, Hand JW, Field SB, et al. Radiotherapy with or without
hyperthermia in the treatment of superficial localized breast cancer:
results from five randomized controlled trials. International Collaborative
Hyperthermia Group. Int J Radiat Oncol Biol Phys 1996;35:731-744.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/8690639.
604. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response
evaluation criteria in solid tumours: revised RECIST guideline (version
1.1). Eur J Cancer 2009;45:228-247. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19097774.
605. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results
of cancer treatment. Cancer 1981;47:207-214. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/7459811.
606. Smerage JB, Barlow WE, Hortobagyi GN, et al. Circulating tumor
cells and response to chemotherapy in metastatic breast cancer:
SWOG S0500. J Clin Oncol 2014;32:3483-3489. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/24888818.
607. Sakorafas GH, Blanchard K, Sarr MG, Farley DR. Paget's disease
of the breast. Cancer Treat Rev 2001;27:9-18. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11237774.
608. Kollmorgen DR, Varanasi JS, Edge SB, Carson WE. Paget's
disease of the breast: a 33-year experience. J Am Coll Surg
1998;187:171-177. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/9704964.
609. Marcus E. The management of Paget's disease of the breast. Curr
Treat Options Oncol 2004;5:153-160. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/14990209.
610. Morrogh M, Morris EA, Liberman L, et al. MRI identifies otherwise
occult disease in select patients with Paget disease of the nipple. J Am
Coll Surg 2008;206:316-321. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18222386.
611. Frei KA, Bonel HM, Pelte M-F, et al. Paget disease of the breast:
findings at magnetic resonance imaging and histopathologic correlation.
Invest Radiol 2005;40:363-367. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15905723.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-119
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
612. Bijker N, Rutgers EJ, Duchateau L, et al. Breast-conserving
therapy for Paget disease of the nipple: a prospective European
Organization for Research and Treatment of Cancer study of 61
patients. Cancer 2001;91:472-477. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11169928.
613. Kawase K, Dimaio DJ, Tucker SL, et al. Paget's disease of the
breast: there is a role for breast-conserving therapy. Ann Surg Oncol
2005;12:391-397. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15915373.
614. Marshall JK, Griffith KA, Haffty BG, et al. Conservative
management of Paget disease of the breast with radiotherapy: 10- and
15-year results. Cancer 2003;97:2142-2149. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/12712465.
615. Pierce LJ, Haffty BG, Solin LJ, et al. The conservative
management of Paget's disease of the breast with radiotherapy. Cancer
1997;80:1065-1072. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/9305706.
616. Singh A, Sutton RJ, Baker CB, Sacks NP. Is mastectomy
overtreatment for Paget's disease of the nipple? Breast 1999;8:191-
194. Available at: http://www.ncbi.nlm.nih.gov/pubmed/14731439.
617. Laronga C, Hasson D, Hoover S, et al. Paget's disease in the era
of sentinel lymph node biopsy. Am J Surg 2006;192:481-483. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/16978954.
618. Sukumvanich P, Bentrem DJ, Cody HS, et al. The role of sentinel
lymph node biopsy in Paget's disease of the breast. Ann Surg Oncol
2007;14:1020-1023. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17195914.
619. Telli ML, Horst KC, Guardino AE, et al. Phyllodes tumors of the
breast: natural history, diagnosis, and treatment. J Natl Compr Canc
Netw 2007;5:324-330. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17439760.
620. Anderson BO, Lawton TJ, Lehman CD, Moe RE. Phyllodes
tumors. In: Harris JR, Lippman ME, Morrow M, Osborne CK, eds.
Diseases of the Breast (ed 3rd). Philadelphia: Lippincott Williams &
Wilkins; 2004.
621. Salvadori B, Cusumano F, Del Bo R, et al. Surgical treatment of
phyllodes tumors of the breast. Cancer 1989;63:2532-2536. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/2541890.
622. Birch JM, Alston RD, McNally RJ, et al. Relative frequency and
morphology of cancers in carriers of germline TP53 mutations.
Oncogene 2001;20:4621-4628. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11498785.
623. Chaney AW, Pollack A, McNeese MD, et al. Primary treatment of
cystosarcoma phyllodes of the breast. Cancer 2000;89:1502-1511.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/11013364.
624. Mangi AA, Smith BL, Gadd MA, et al. Surgical management of
phyllodes tumors. Arch Surg 1999;134:487-492. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/10323420.
625. Pandey M, Mathew A, Kattoor J, et al. Malignant phyllodes tumor.
Breast J 2001;7:411-416. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11843853.
626. Tse GMK, Lee CS, Kung FYL, et al. Hormonal receptors
expression in epithelial cells of mammary phyllodes tumors correlates
with pathologic grade of the tumor: a multicenter study of 143 cases.
Am J Clin Pathol 2002;118:522-526. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/12375638.
627. Smith LH, Dalrymple JL, Leiserowitz GS, et al. Obstetrical
deliveries associated with maternal malignancy in California, 1992
through 1997. Am J Obstet Gynecol 2001;184:1504-1512. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11408874.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-120
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
628. Gwyn K, Theriault R. Breast cancer during pregnancy. Oncology
(Williston Park) 2001;15:39-46. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11271981.
629. Middleton LP, Amin M, Gwyn K, et al. Breast carcinoma in
pregnant women: assessment of clinicopathologic and
immunohistochemical features. Cancer 2003;98:1055-1060. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/12942575.
630. Yang WT, Dryden MJ, Gwyn K, et al. Imaging of breast cancer
diagnosed and treated with chemotherapy during pregnancy. Radiology
2006;239:52-60. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16484353.
631. Kuerer HM, Gwyn K, Ames FC, Theriault RL. Conservative surgery
and chemotherapy for breast carcinoma during pregnancy. Surgery
2002;131:108-110. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11812971.
632. Annane K, Bellocq JP, Brettes JP, Mathelin C. Infiltrative breast
cancer during pregnancy and conservative surgery. Fetal Diagn Ther
2005;20:442-444. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16113569.
633. Khera SY, Kiluk JV, Hasson DM, et al. Pregnancy-associated
breast cancer patients can safely undergo lymphatic mapping. Breast J
2008;14:250-254. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18476883.
634. Mondi MM, Cuenca RE, Ollila DW, et al. Sentinel lymph node
biopsy during pregnancy: initial clinical experience. Ann Surg Oncol
2007;14:218-221. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17066225.
635. Filippakis GM, Zografos G. Contraindications of sentinel lymph
node biopsy: are there any really? World J Surg Oncol 2007;5:10.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/17261174.
636. Gentilini O, Cremonesi M, Trifiro G, et al. Safety of sentinel node
biopsy in pregnant patients with breast cancer. Ann Oncol
2004;15:1348-1351. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15319240.
637. Keleher A, Wendt R, Delpassand E, et al. The safety of lymphatic
mapping in pregnant breast cancer patients using Tc-99m sulfur colloid.
Breast J 2004;10:492-495. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15569204.
638. Pandit-Taskar N, Dauer LT, Montgomery L, et al. Organ and fetal
absorbed dose estimates from 99mTc-sulfur colloid lymphoscintigraphy
and sentinel node localization in breast cancer patients. J Nucl Med
2006;47:1202-1208. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16818956.
639. Germann N, Goffinet F, Goldwasser F. Anthracyclines during
pregnancy: embryo-fetal outcome in 160 patients. Ann Oncol
2004;15:146-150. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/14679135.
640. Johnson PH, Gwyn K, Gordon N, et al. The treatment of pregnant
women with breast cancer and the outcomes of the children exposed to
chemotherapy in utero [abstract]. J Clin Oncol 2005;23(Suppl
16):Abstract 540. Available at:
http://meeting.ascopubs.org/cgi/content/abstract/23/16_suppl/540.
641. Doll DC, Ringenberg QS, Yarbro JW. Antineoplastic agents and
pregnancy. Semin Oncol 1989;16:337-346. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/2678485.
642. Ebert U, Loffler H, Kirch W. Cytotoxic therapy and pregnancy.
Pharmacol Ther 1997;74:207-220. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/9336023.
643. Hahn KME, Johnson PH, Gordon N, et al. Treatment of pregnant
breast cancer patients and outcomes of children exposed to
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-121
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
chemotherapy in utero. Cancer 2006;107:1219-1226. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16894524.
644. Gainford MC, Clemons M. Breast cancer in pregnancy: are
taxanes safe? Clinical Oncol (R Coll Radiol) 2006;18:159. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16523825.
645. Garcia-Manero M, Royo MP, Espinos J, et al. Pregnancy
associated breast cancer. Eur J Surg Oncol 2009;35:215-218. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/18550321.
646. Gonzalez-Angulo AM, Walters RS, Carpenter RJ, et al. Paclitaxel
chemotherapy in a pregnant patient with bilateral breast cancer. Clin
Breast Cancer 2004;5:317-319. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15507181.
647. Mir O, Berveiller P, Ropert S, et al. Emerging therapeutic options
for breast cancer chemotherapy during pregnancy. Ann Oncol
2008;19:607-613. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17921242.
648. Bader AA, Schlembach D, Tamussino KF, et al. Anhydramnios
associated with administration of trastuzumab and paclitaxel for
metastatic breast cancer during pregnancy. Lancet Oncol 2007;8:79-81.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/17196514.
649. Fanale MA, Uyei AR, Theriault RL, et al. Treatment of metastatic
breast cancer with trastuzumab and vinorelbine during pregnancy. Clin
Breast Cancer 2005;6:354-356. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16277887.
650. Pant S, Landon MB, Blumenfeld M, et al. Treatment of breast
cancer with trastuzumab during pregnancy. J Clin Oncol 2008;26:1567-
1569. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18349415.
651. Sekar R, Stone PR. Trastuzumab use for metastatic breast cancer
in pregnancy. Obstet Gynecol 2007;110:507-510. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17666645.
652. Shrim A, Garcia-Bournissen F, Maxwell C, et al. Favorable
pregnancy outcome following Trastuzumab (Herceptin) use during
pregnancy--Case report and updated literature review. Reprod Toxicol
2007;23:611-613. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17399946.
653. Waterston AM, Graham J. Effect of Adjuvant Trastuzumab on
Pregnancy. J Clin Oncol 2006;24:321-322. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16401684.
654. Watson WJ. Herceptin (trastuzumab) therapy during pregnancy:
association with reversible anhydramnios. Obstet Gynecol
2005;105:642-643. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15738038.
655. Witzel ID, Müller V, Harps E, et al. Trastuzumab in pregnancy
associated with poor fetal outcome. Ann Oncol 2008;19:191-192.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/18084047.
656. Kelly H, Graham M, Humes E, et al. Delivery of a healthy baby
after first-trimester maternal exposure to lapatinib. Clin Breast Cancer
2006;7:339-341. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17092403.
657. Dawood, S, Cristofanilli M. What progress have we made in
managing inflammatory breast cancer? Oncology 2007;21:673-679.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/17564325
658. Jaiyesimi IA, Buzdar AU, Hortobagyi G. Inflammatory breast
cancer: a review. J Clin Oncol 1992;10:1014-1024. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/1588366.
659. Bertucci F, Finetti P, Rougemont J, et al. Gene expression profiling
identifies molecular subtypes of inflammatory breast cancer. Cancer
Res 2005;65:2170-2178. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15781628.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-122
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
660. Van Laere SJ, Van den Eynden GG, Van der Auwera I, et al.
Identification of cell-of-origin breast tumor subtypes in inflammatory
breast cancer by gene expression profiling. Breast Cancer Res Treat
2006;95:243-255. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16261404.
661. Zell JA, Tsang WY, Taylor TH, et al. Prognostic impact of human
epidermal growth factor-like receptor 2 and hormone receptor status in
inflammatory breast cancer (IBC): analysis of 2,014 IBC patient cases
from the California Cancer Registry. Breast Cancer Res 2009;11:R9.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/19228416.
662. Parton M, Dowsett M, Ashley S, et al. High incidence of HER-2
positivity in inflammatory breast cancer. Breast 2004;13:97-103.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/15019688.
663. Haagensen CD. Inflammatory Carcinoma. Diseases of the Breast.
Philadelphia: WB Saunders; 1956:488-498.
664. Cristofanilli M, Valero V, Buzdar AU, et al. Inflammatory breast
cancer (IBC) and patterns of recurrence: understanding the biology of a
unique disease. Cancer 2007;110:1436-1444. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17694554.
665. Panades M, Olivotto IA, Speers CH, et al. Evolving treatment
strategies for inflammatory breast cancer: a population-based survival
analysis. J Clin Oncol 2005;23:1941-1950. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15774787.
666. Dawood S, Ueno NT, Valero V, et al. Differences in survival among
women with stage III inflammatory and noninflammatory locally
advanced breast cancer appear early: a large population-based study.
Cancer 2011;117:1819-1826. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/21509759.
667. Hance KW, Anderson WF, Devesa SS, et al. Trends in
inflammatory breast carcinoma incidence and survival: the surveillance,
epidemiology, and end results program at the National Cancer Institute.
J Natl Cancer Inst 2005;97:966-975. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15998949.
668. Bleicher RJ, Morrow M. Inflammatory breast cancer: Still poorly
characterized. The Dawood/Cristofanilli article reviewed. Oncology
2007;21:679-680. Available at: http://www.cancernetwork.com/breast-
cancer/content/article/10165/61508.
669. Nguyen DM, Sam K, Tsimelzon A, et al. Molecular heterogeneity of
inflammatory breast cancer: a hyperproliferative phenotype. Clin Cancer
Res 2006;12:5047-5054. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16951220.
670. Carkaci S, Macapinlac HA, Cristofanilli M, et al. Retrospective
study of 18F-FDG PET/CT in the diagnosis of inflammatory breast
cancer: preliminary data. J Nucl Med 2009;50:231-238. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19164229.
671. Chia S, Swain SM, Byrd DR, Mankoff DA. Locally advanced and
inflammatory breast cancer. J Clin Oncol 2008;26:786-790. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18258987.
672. Fleming RY, Asmar L, Buzdar AU, et al. Effectiveness of
mastectomy by response to induction chemotherapy for control in
inflammatory breast carcinoma. Ann Surg Oncol 1997;4:452-461.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/9309333.
673. Ueno NT, Buzdar AU, Singletary SE, et al. Combined-modality
treatment of inflammatory breast carcinoma: twenty years of experience
at M. D. Anderson Cancer Center. Cancer Chemother Pharmacol
1997;40:321-329. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/9225950.
674. Cristofanilli M, Gonzalez-Angulo AM, Buzdar AU, et al. Paclitaxel
improves the prognosis in estrogen receptor negative inflammatory
breast cancer: the M. D. Anderson Cancer Center experience. Clin
Breast Cancer 2004;4:415-419. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/15023242.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.

Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-123
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
675. Kim T, Lau J, Erban J. Lack of uniform diagnostic criteria for
inflammatory breast cancer limits interpretation of treatment outcomes:
a systematic review. Clin Breast Cancer 2006;7:386-395. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17239263.
676. Hennessy BT, Gonzalez-Angulo AM, Hortobagyi GN, et al.
Disease-free and overall survival after pathologic complete disease
remission of cytologically proven inflammatory breast carcinoma axillary
lymph node metastases after primary systemic chemotherapy. Cancer
2006;106:1000-1006. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16444747.
677. Dawood S, Broglio K, Gong Y, et al. Prognostic significance of
HER-2 status in women with inflammatory breast cancer. Cancer
2008;112:1905-1911. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18300243.
678. Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant
chemotherapy with trastuzumab followed by adjuvant trastuzumab
versus neoadjuvant chemotherapy alone, in patients with HER2-positive
locally advanced breast cancer (the NOAH trial): a randomised
controlled superiority trial with a parallel HER2-negative cohort. The
Lancet 2010;375:377-384. Available at:
http://linkinghub.elsevier.com/retrieve/pii/S0140673609619644.
679. Hurley J, Doliny P, Reis I, et al. Docetaxel, cisplatin, and
trastuzumab as primary systemic therapy for human epidermal growth
factor receptor 2-positive locally advanced breast cancer. J Clin Oncol
2006;24:1831-1838. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16549824.
680. Burstein HJ, Harris LN, Gelman R, et al. Preoperative therapy with
trastuzumab and paclitaxel followed by sequential adjuvant
doxorubicin/cyclophosphamide for HER2 overexpressing stage II or III
breast cancer: a pilot study. J Clin Oncol 2003;21:46-53. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/12506169.
681. Limentani SA, Brufsky AM, Erban JK, et al. Phase II study of
neoadjuvant docetaxel, vinorelbine, and trastuzumab followed by
surgery and adjuvant doxorubicin plus cyclophosphamide in women
with human epidermal growth factor receptor 2-overexpressing locally
advanced breast cancer. J Clin Oncol 2007;25:1232-1238. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17296975.
682. Van Pelt AE, Mohsin S, Elledge RM, et al. Neoadjuvant
trastuzumab and docetaxel in breast cancer: preliminary results. Clin
Breast Cancer 2003;4:348-353. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/14715110.
683. Boussen H, Cristofanilli M, Zaks T, et al. Phase II study to evaluate
the efficacy and safety of neoadjuvant lapatinib plus paclitaxel in
patients with inflammatory breast cancer. Journal of Clinical Oncology
2010;28:3248-3255. Available at:
http://jco.ascopubs.org/content/28/20/3248.abstract.
684. Kell MR, Morrow M. Surgical aspects of inflammatory breast
cancer. Breast Dis 2005;22:67-73. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16735788.
685. Stearns V, Ewing CA, Slack R, et al. Sentinel lymphadenectomy
after neoadjuvant chemotherapy for breast cancer may reliably
represent the axilla except for inflammatory breast cancer. Ann Surg
Oncol 2002;9:235-242. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11923129.
686. Motwani SB, Strom EA, Schechter NR, et al. The impact of
immediate breast reconstruction on the technical delivery of
postmastectomy radiotherapy. Int J Radiat Oncol Biol Phys 2006;66:76-
82. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16765534.
687. Blanchard DK, Shetty PB, Hilsenbeck SG, Elledge RM.
Association of surgery with improved survival in stage IV breast cancer
patients. Ann Surg 2008;247:732-738. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18438108.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.
Version 2.2016, 05/06/16 © National Comprehensive Cancer Network, Inc. 2016, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN ® MS-124
NCCN Guidelines Inde
x
Breast Cancer Table of Contents
Discussion
NCCN Guidelines Version 2.2016
Breast Cancer
688. Olson JA, Morris EA, Van Zee KJ, et al. Magnetic resonance
imaging facilitates breast conservation for occult breast cancer. Ann
Surg Oncol 2000;7:411-415. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/10894136.
689. Varadarajan R, Edge SB, Yu J, et al. Prognosis of occult breast
carcinoma presenting as isolated axillary nodal metastasis. Oncology
2006;71:456-459. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/17690561.
690. Schelfout K, Kersschot E, Van Goethem M, et al. Breast MR
imaging in a patient with unilateral axillary lymphadenopathy and
unknown primary malignancy. Eur Radiol 2003;13:2128-2132. Available
at: http://www.ncbi.nlm.nih.gov/pubmed/12928964.
691. Bhatia SK, Saclarides TJ, Witt TR, et al. Hormone receptor studies
in axillary metastases from occult breast cancers. Cancer
1987;59:1170-1172. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/3815292.
692. Bleicher RJ, Morrow M. MRI and breast cancer: role in detection,
diagnosis, and staging. Oncology (Williston Park) 2007;21:1521-1528,
1530; discussion 1530, 1532-1523. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/18077995.
693. Stomper PC, Waddell BE, Edge SB, Klippenstein DL. Breast MRI
in the Evaluation of Patients with Occult Primary Breast Carcinoma.
Breast J 1999;5:230-234. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11348292.
694. Buchanan CL, Morris EA, Dorn PL, et al. Utility of breast magnetic
resonance imaging in patients with occult primary breast cancer. Ann
Surg Oncol 2005;12:1045-1053. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/16244803.
Printed by Jeremy Miller on 6/9/2016 8:03:04 AM. For personal use only. Not approved for distribution. Copyright © 2016 National Comprehensive Cancer Network, Inc., All Rights Reserved.