PRECICE Intramedullary Limb Lengthening System De7c96d5 687e 4102 9f5f Ab9828d508ad
2017-07-07
: Pdf De7C96D5-687E-4102-9F5F-Ab9828D508Ad de7c96d5-687e-4102-9f5f-ab9828d508ad 7 2017 pdf
Open the PDF directly: View PDF ![]() .
.
Page Count: 19
- Body of review
- Overview of the market
- Introduction to the device
- Clinical profile & postmarketing findings
- Lengthening in children & adults for leg length discrepancy & stature
- Limb lengthening in children with a new, controllable, internal device
- Precision of the new remote controlled internal lengthening nail
- Internal lengthening device for congenital femoral deficiency & fibular hemimelia
- Precision of the PRECICE internal bone lengthening nail
- How precise is PRECICE compared to ISKD in intramedullary limb lengthening?
- Alternative devices
- Status of the device
- Conclusion
- Expert commentary
- Five-year view
- Financial & competing interests disclosure
PRECICE intramedullary limb
lengthening system
Expert Rev. Med. Devices Early online, 1–19 (2015)
Dror Paley
The Paley Advanced Limb Lengthening
Institute at St. Mary’s Medical Center,
901 45th Street, Kimmel Building,
West Palm Beach, FL 33407, USA
Tel.: +1 561 844 5244
Fax: +1 561 844 5245
drorpaley@gmail.com
The PRECICE
Intramedullary Limb Lengthening System (Ellipse Technologies Inc., CA, USA) is
a remotely controlled, magnetically driven, implantable limb lengthening intramedullary nail
system. It has both CE mark and US FDA clearance for its first- (2011) and second-generation
(2013) implants. It is indicated for the treatment of limb length discrepancy and short stature.
It has been used worldwide in over 1000 cases. Its reported and published results in over
250 cases has been excellent with less pain and lower complication rates than with external
fixation methods or previous implantable nail systems.
KEYWORDS:congenital femoral deficiency .distraction osteogenesis .fibular hemimelia .intramedullary nails .leg
length discrepancy .limb length discrepancy .magnet .noninvasive .PRECICE
Ellipse Technologies (Irvine, CA, USA) devel-
oped the PRECICE nail with a team of sur-
geons (including myself) headed by Dr. Stuart
Green. They used the same mechanism that
they had developed for their spinal growing
rod called ‘the MAGEC System’[1]. There is a
magnetic metal spindle that is connected to a
series of gears (FIGURE 1). The gears are con-
nected to a coupling, which is connected to a
threaded drive shaft. The mechanism is acti-
vated by an external remote control (ERC)
device (FIGURE 2A). The ERC employs two
motor-driven rotating magnets to magnetically
couple to and rotate the magnetic metal
spindle (FIGURE 2B). Facing the ERC in one
direction causes the nail to lengthen, while fac-
ing it the other direction would go in the
reverse (shortening) direction. PRECICE is the
second FDA-cleared implantable lengthening
nail device (July 2011) and the first one to
have bidirectional control (lengthening and
shortening). I had the privilege of implanting
the first PRECICE nail in the USA on
1 December 2011.
The Ilizarov procedure remained the best
option for limb lengthening until the intro-
duction of these new nails in the 1990s. It
was thought that with this new technology
the complications related to limb lengthening
would be greatly diminished. Intramedullary
nail techniques are not without complica-
tions, which may include nonunions, nerve
injuries, nail fractures, joint contractures and
other serious complications [2–10]. External
fixation approaches to limb lengthening
remain the gold standard. Experience with
implantable limb lengthening have made evi-
dent that the complications of limb lengthen-
ing can be divided into two categories, device
related and distraction related. The device-
related complications are very different for
external versus internal lengthening devices.
The high complication rates related to pin
tract infections, joint stiffness and contrac-
tures due to tethering of the muscles by the
pins, and large pin tract scars, do not occur
with implantable devices. The complications
related to distraction are similar between
external and internal lengthening methods.
These complications include premature con-
solidation, delayed and failed bone formation,
nerve stretch injury, muscle contractures and
joint subluxation. The reduced device compli-
cations and even some reduced distraction
complications (e.g., axial deviation) have
made the switch to implantable devices very
attractive and have created a market for these
technologically advanced devices.
Body of review
Overview of the market
The indications for limb lengthening are for
the treatment of limb length discrepancy of
the lower or upper limb and for stature
lengthening. The indications for leg lengthen-
ing are for the treatment of leg length dis-
crepancy or short stature. Unilateral limb
lengthening is used to equalize limb length in
informahealthcare.com 10.1586/17434440.2015.1005604 2015 Informa UK Ltd ISSN 1743-4440 1
Device Profile
Expert Review of Medical Devices Downloaded from informahealthcare.com by 73.46.221.27 on 02/18/15
For personal use only.
the treatment of limb length discrepancy. Bilateral limb length-
ening is used for increasing height and restoring body propor-
tions in patients with dysplasias (e.g., achondroplasia and
hypochondroplasia) and other genetic conditions (e.g., Turner’s
syndrome), for the treatment of height dysphoria in patients
with short stature and for increasing height for cosmetic
reasons.
Leg length discrepancy can be due to congenital, develop-
mental or posttraumatic causes.
Developmental or posttraumatic causes may include growth
plate arrest, malunion, nonunion, bone loss from open frac-
tures, osteomyelitis or tumor. Minor leg length discrepancy is
prevalent, with 23% of the general population possessing a dis-
crepancy of at least 1 cm. The prevalence of leg length discrep-
ancy where a corrective device is required is approximately 1 in
1000. More severe discrepancies such as congenital femoral
deficiency and fibular hemimelia are rare and complex congeni-
tal disorders of the lower limb with an incidence of approxi-
mately one in 50,000 live births for congenital femoral
deficiency [11–13] and between 7.4 and 20 per million live births
for fibular hemimelia [14–16].
Short stature is a common feature of most dysplasias and
musculoskeletal syndromes. Achondroplasia is the most com-
mon dysplasia and occurs in 1:40,000 births. Constitutional
short stature is defined as under the 5th percentile for height
(5% of the population). Height dysphoria is a well-known con-
dition also referred to as height neurosis. It is a very common
condition although no accurate statistics exist as to its preva-
lence. Most patients with height dysphoria are over the 5th
percentile in height. Height dysphoria represents a body image
anxiety disorder, which is generally unresponsive to psychother-
apy. Lengthening for height dysphoria is considered cosmetic
or aesthetic lengthening.
Limb lengthening for limb length discrepancy is a well-
accepted indication for treatment. Nevertheless, its market is
relatively small. Similarly, stature lengthening for the treatment
of dysplasias, although more controversial, is also a well-
accepted indication for treatment, again with a relatively small
market.
Stature lengthening for cosmetic reasons is a very controver-
sial indication for treatment. Nevertheless, there is a growing
demand for such treatment by the population. There are
numerous social network websites that serve to voice the inter-
est and demand for such treatment. As such it is a huge
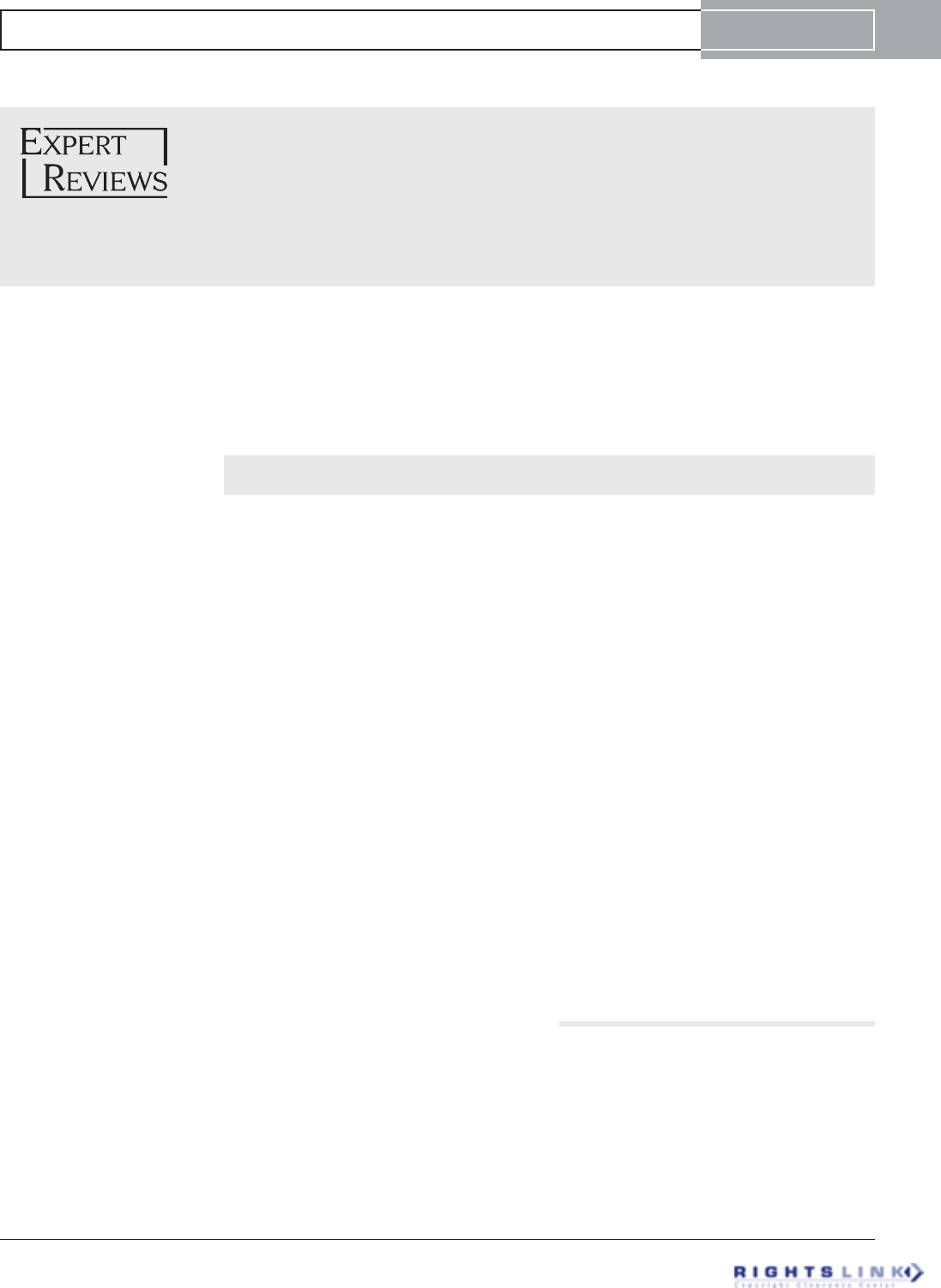
Figure 1. Photograph of tibial nails: PRECICE 1 (P1, left)
and PRECICE 2 (P2, right). Note the three welds in P1 and no
welds in P2.
A
Flux lines
Rotation
S
N
N
S
SN
B
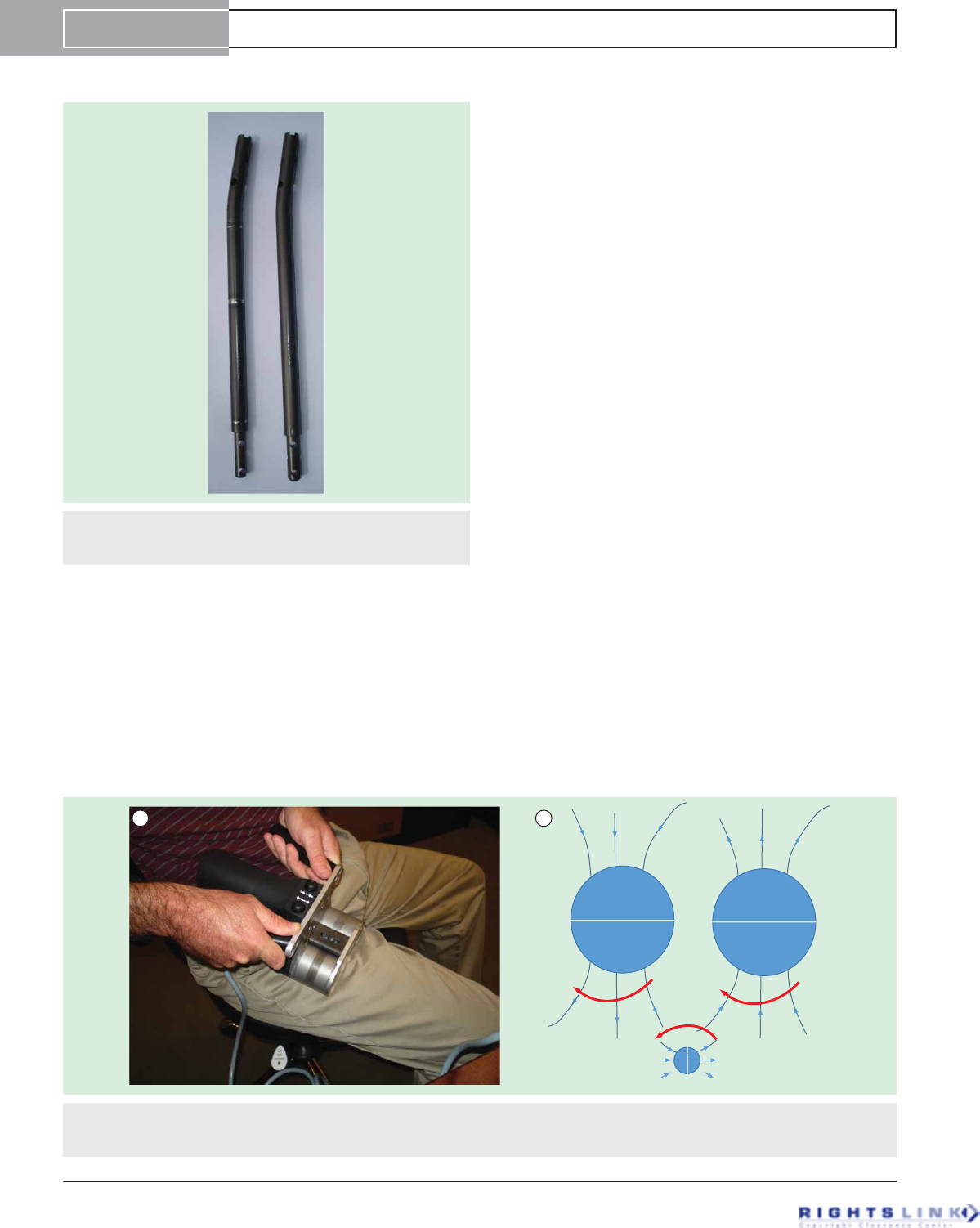
Figure 2. External remote control. (A) Photograph of external remote control device being applied to the thigh for femur lengthening.
(B) Illustration of how rotation of the 2 external remote control magnets (big circles) cause the nail magnet (small circle) to rotate
through their magnetic fields.
Device Profile Paley
doi: 10.1586/17434440.2015.1005604 Expert Rev. Med. Devices
Expert Review of Medical Devices Downloaded from informahealthcare.com by 73.46.221.27 on 02/18/15
For personal use only.

untapped market. A small but growing number of cosmetic
stature lengthening centers have sprung up around the world
to service this demand.
The concept of cosmetic stature lengthening is foreign to
most orthopedic surgeons. Unlike our plastic surgery col-
leagues, we have no tradition of cosmetic or aesthetic surgery.
Orthopedic surgery is founded on the principles of treatment
of pain, disability and deformity. We use our surgery to treat
or prevent pain and disability. Our surgery is funded by reim-
bursement by third-party payers since it is not considered
cosmetic.
With the decreasing reimbursement for surgery, the allure of
a cash-paying cosmetic surgery practice will attract many ortho-
pedic surgeons in the near future. The cosmetic market may
become the largest indication for implantable limb lengthening.
Until now the external fixator has been the gold standard for
limb lengthening. Most orthopedic surgeons shy away from
external fixation and as such it has become the realm of a small
group of orthopedic specialists. The external fixator has also
kept patients away from this treatment. Now that the surgery is
both simpler for the surgeon and less complicated for the
patients, we can anticipate an increase in the number of sur-
geons who take up limb lengthening and patients who are keen
to have it done. The number of implants used for the treatment
of leg length discrepancy and dysplasias will, in the future, be
far outnumbered by the number used for cosmetic stature
lengthening. Needless to say each cosmetic case represents two
implants compared to one for each leg length difference. While
limb length discrepancy (LLD) patients often have staged
lengthening over the course of their childhood, stature patients
often have a second pair of lengthening for their other segments
(e.g., two femurs and then two tibias) still outnumbering the
LLD indications. Unfortunately, the ease of application of the
device is misleading. While the surgery is relatively easy and can
be carried out by most orthopedic surgeons, the postoperative
follow-up and the learning curve for the treatment of
distraction-related problems and complications is quite steep.
Most orthopedic surgeons are not knowledgeable in the postoper-
ative management of the limb lengthening patient. As such we
may anticipate a large number of complications created by sur-
geons knowledgeable in the insertion of nails but unprepared for
the management of the limb lengthening process. Since the use
for stature is so potentially lucrative, this sector of the market may
grow precipitously but may also be subject to significant abuse.
External fixation defines the existing market for limb lengthen-
ing. Implantable lengthening nails are indicated for the same
market currently occupied by limb lengthening external fixators.
The indications for their use are the same: limb length discrep-
ancy and short stature. Implantable nails are currently limited by
anatomy, length, diameter and limited ability to correct defor-
mity especially in children and the short bones of dysplastic
patients. In the USA, the only FDA-approved device was intra-
medullary skeletal distractor (ISKD) (Orthofix Inc, Lewisville,
TX, USA). It was introduced to the market in 2001 and was
finally withdrawn from the US market in 2011. In Europe,
Fitbone, Phenix and Albizzia all on very limited releases were the
only other alternatives. Phenix is now off the market due to the
untimely death of Arnaud Soubieran its inventor. It is due to
resurface (perhaps in 2015) as a product called Novus from
Smith and Nephew Orthopedics (Memphis, TN, USA). Fitbone
(Wittenstein, Igersheim, Germany) is distributed to a small num-
ber of surgeons under the direct approval by Dr. Rainer Baum-
gart. The rationale for approval is supposedly based on
experience and limited by number of centers per country. This
decision seems very arbitrary and as a case in point, I was unable
to receive approval by Dr. Baumgart for its use. Fitbone also has
the distinction of being the most expensive implantable nail. The
modified Albizzia, referred to as the Betz Bone, and the Guichet
nail are only used by these two namesake surgeons and are not
available for commercial distribution. Thus, PRECICE intro-
duced both in the USA and internationally in 2011 has become
the only commercially available remote controlled implantable
limb lengthening device.
Introduction to the device
Device description
PRECICE is a telescopic intramedullary locking nail. The
mechanism has a rare earth magnetic metal spindle that is con-
nected to a series of three planetary gear clusters (FIGURE 3). Each
gear cluster produces a reduction of 1/4 for a total reduction of
1/64 between the magnet and the drive screw. There is a cou-
pling between the gears and the drive screw with a safeguard
Magnet
Gear box
Lead screw
Figure 3. Radiograph of the actuator of PRECICE showing
its different parts. The magnet is connected to a series of three
gears (gear box) that are connected to a coupling which links to
the lead screw. The threaded drive shaft (lead screw) lengthens
or shortens the smaller diameter tube of the nail relative to the
bigger diameter tube.
PRECICE intramedullary limb lengthening system Device Profile
informahealthcare.com doi: 10.1586/17434440.2015.1005604
Expert Review of Medical Devices Downloaded from informahealthcare.com by 73.46.221.27 on 02/18/15
For personal use only.

(like a clutch) built in to avoid breakage in the face of extreme
torque resistance. The mechanism (actuator) is activated by an
ERC device (FIGURE 2A). The ERC employs two motor-driven
rotating magnets to magnetically couple to and rotate the mag-
netic metal spindle (FIGURE 2B). The ERC performs 30 revolutions
per minute. It takes 7 min and 210 revolutions to achieve
1 mm of lengthening. Facing the ERC in one direction causes
the nail to lengthen, while facing it the other direction would
go in the reverse (shortening) direction.
PRECICE nail & locking screws
The first generation of the Ellipse PRECICE Limb Lengthen-
ing System is composed of the PRECICE intramedullary nail,
locking screws, implantation tools and accessories, and an
ERC. The implantable nail is supplied sterile by gradiation
and is available in tibia and femur models with diameters of
10.7 and 12.5 mm, stroke length of 65 mm, overall starting
lengths of 230–355 mm, and distal screw hole patterns to
accommodate a variety of patient anatomies. Likewise, the
locking screws are available in two different diameters and a
variety of lengths from 20 to 75 mm in 5 mm increments.
The first-generation PRECICE (PRECICE 1) is modular
such that the intramedullary limb lengthening actuator compo-
nent had to be assembled in surgery to an extension rod of dif-
ferent length and anatomical shape. The length of the
intramedullary limb lengthening actuator is fixed, while the
extension rods varied in length. The intramedullary limb
lengthening actuator including its rare earth magnet, telescop-
ing lead screw/nut assembly and gearing were housed in a rod
segment that was welded above and below to the rest of the
nail housing. In total, there were three welds in this unit and
one connection to the extension rod via set screw. Extension
rods, locking screws and reusable accessories are supplied non-
sterile and must be sterilized prior to use. The second-
generation Ellipse PRECICE 2 Limb Lengthening System
released in November 2013 is composed of the PRECICE
intramedullary nail, locking screws, implantation tools and
accessories, and an ERC. The modified implantable nail is
supplied sterile by gradiation and is available in tibia and
femur models with diameters of 8.5, 10.7 and 12.5 mm, stroke
lengths of 50 and 80 mm, overall starting lengths of
195–365 mm, and distal screw hole patterns to accommodate
a variety of patient anatomies. Likewise, the PRECICE locking
screws are available in different diameters and a variety of
lengths ranging from 20 to 75 mm. The PRECICE locking
screws and implantation instruments and accessories are sup-
plied nonsterile and must be sterilized prior to use. The PRE-
CICE nail is surgically placed in the intramedullary canal of
the femur or tibia. The femur models offer piriformis, trochan-
teric and retrograde entry models. The metal in contact with
the patient’s body fluids and bone is composed of medical
grade titanium alloy (Ti-6Al-4V). The rare earth magnet and
gears are sealed from body fluid contact.
External remote control
The ERC provides a noninvasive method for precisely distract-
ing the nail at defined intervals. The ERC includes two perma-
nent magnets that are rotated by a motor which is
electronically powered. When the ERC magnets are rotated in
proximity (less than 5.5 cm) to the PRECICE nail, the rare
earth magnet in the nail turns and the distracting rod either
lengthens or shortens the intramedullary nail depending on the
direction of rotation of the ERC magnets. The first use of the
ERC is in the operating room to test the functionality of the
nail mechanism. A 1-mm test distraction is performed and con-
firmed radiographically. Postoperatively after a latency period
ranging from 0 to 7 days, the nail is lengthened daily by the
patient (or their caregivers) according to the daily prescription
set by the treating physician (FIGURE 4).
Implant technique (PRECICE 2)
Surgical technique femur
Step 1: The patient is positioned supine on a radiolucent oper-
ating table. A radiolucent bump (usually a folded towel or
sheet) is placed underneath the ischium on the operative side.
This allows good visualization of the hip on both antero-poste-
rior (AP) and cross table lateral views.
Step 2: Using the image intensifier (fluoroscopy), the tip of
the level of the greater trochanter is marked on the skin. Know-
ing the length of the nail to be used for the surgery, a ruler is
used to mark the distal end of the nail. For retrograde nailing,
measure from 1 cm proximal to the intercondylar notch.
Step 3: The level of the osteotomy is determined by know-
ing the amount of distraction planned. One must plan to end
up with the larger diameter of the nail always engaged on both
sides of the distraction gap at the end of lengthening. Assuming
one wants to have 2 cm of the larger diameter of the nail
engaged, then add 2 cm plus 3 cm of the smaller diameter
nail, which is exposed plus the distraction amount. This total
measured from the distal end of the nail represents the level of
the desired osteotomy that will leave at least 2 cm of the larger
diameter of the nail always engaged in cortical bone on the
opposite side of the distraction gap.
0 mm 1 mm 2 mm
Figure 4. Radiograph before distraction (0 mm) and after
distraction of 1 and 2 mm.
Device Profile Paley
doi: 10.1586/17434440.2015.1005604 Expert Rev. Med. Devices
Expert Review of Medical Devices Downloaded from informahealthcare.com by 73.46.221.27 on 02/18/15
For personal use only.

Step 4: Make a 1-cm incision laterally at the level of the
osteotomy. Drill holes using a 4.8-mm drill bit. I prefer one
entrance and three exit holes, anteromedial, posteromedial and
medial. Then make two more drill holes anterolateral and pos-
terolateral at the level of the other holes. These six holes will serve
to vent the canal from fat emboli and to allow the reamings that
extrude through the holes to auto-bonegraft the osteotomy site.
Step 5: Insert a Steinmann pin into the piriformis fossa for
adults or adolescents with closed growth plates. For patients
with an open proximal femoral growth plate, insert the Stein-
mann pin into the tip of the greater trochanter. For retrograde
nailing, insert the Steinmann pin at the center of the intercon-
dylar notch on the AP and at the distal end of Blumensaat’s
line on the lateral.
Step 6: Expand the starting point with a 10-mm anterior
cruciate ligament reamer.
Step 7: Insert a beaded guide rod down the femur.
Step 8: Ream in 1-mm increments until there is chatter and
then in 0.5-mm increments. Ream to 10.5 mm for the
8.5-mm nail, 12.5 mm for the 10.7-mm nail and to 14.5 mm
for the 12.5-mm nail. For the 8.5-mm nail, ream the upper
part of the bone to 11 mm. For trochanteric entry, it may be
helpful to ream an extra millimeter in the upper part of the
bone to allow lower resistance passage of the nail as it goes
around the bend.
Step 9: Prepare the nail for insertion. With PRECICE 1,
choose and assemble the insertion end type (trochanteric, piri-
formis, retrograde, tibial) and lengths. The mechanism comes
in one length, while the final nail length depends on the length
of the insertion end chosen. With the new PRECICE 2, the
nail is not modular and one must choose the length of the
entire nail in advance.
Step 10: Apply the proximal targeting device and test its
alignment to the screw holes by inserting the drill guides and
drill bits.
Step 11: Place the nail under the beam of the image intensi-
fier to confirm that the mechanism is not predistracted. Save
this image for reference.
Step 12: Remove the beaded guide wire used for reaming as
the nail is not cannulated. Insert the nail into the canal up to
the level of the planned osteotomy (drill holes).
Step 13: Have one assistant lift the foot off the table. Have
the other assistant lift the proximal end of the nail using the
insertion guide. The two assistants are applying an extension
moment to the femur to prevent displacement of the femur
during the osteotomy.
Step 14: Use a sharp 6–8 mm osteotome to osteotomize the
femur through the 1-cm lateral incision. The femur will easily
break through the six drill holes. Listen for the break and once
it occurs withdraw the osteotome. Test that the femur is frac-
tured by moving the femur gently into varus and valgus and
watching it move using live fluoroscopy. Maintain the exten-
sion moment throughout this test.
Step 15: Once the break is confirmed to be complete,
advance the nail by gently hammering on the impactor until
the nail crosses the osteotomy line into the distal (antegrade
nailing) or proximal (retrograde nailing) segment of the femur.
The extension moment is no longer needed to stabilize the
femur. Advance the nail until the upper end is at the level of
the base of the piriformis fossa or just inside the greater tro-
chanter for piriformis and trochanteric nails, respectively. For
retrograde nails, make sure that the end of the nail is inside the
femur at the intercondylar notch.
Step 16: Lock the nail proximally (antegrade) and distally
(retrograde) with two screws using the locking guide. For distal
locking screws (antegrade) and proximal locking screws (retro-
grade), my personal technique preference is to insert a long
1.8-mm wire into the locking hole, followed by a 3.5-mm can-
nulated drill for the 8.5-mm nail, 3.8-mm cannulated drill for
10.7-mm nails and a 4.8-mm cannulated drill for the 12.5-mm
nail. In the 12.5-mm nail, overdrill with a solid 5-mm drill bit
and in the 10.7-mm nail overdrill with a solid 4-mm drill bit.
Step 17: Lock the nail distally with two lateral-medial
screws. Do not use anteroposterior middle screw because it can
act as a stress riser for fracture of the femur.
Step 18: Insert the end cap into the proximal part of the
nail for antegrade nails only.
Step 20: Close all the incisions.
Step 21: Insert the ERC device into a sterile sleeve. Mark
out the level of the magnet on the skin using fluoroscopy.
Apply the ERC directly over the magnetic spindle using the
image intensifier to mark out the magnet. Face the ERC dis-
tally for antegrade nails and proximally for retrograde nails. It
takes 7 min to lengthen the femur 1 mm.
Step 22: Check if the distraction gap is seen radiographically
and compare it to the predistraction space (FIGURE 4). If an objec-
tive increase in space is seen the procedure is completed. If not,
do a second millimeter of distraction to confirm. In the rare
case where the bone does not separate, the nail must be
extracted and tested on the bench and if it does not distract
then replaced with another nail. An incomplete osteotomy can
cause a failure of distraction.
Surgical technique tibia
Step 1: Mark the proximal and distal end of the nail as before.
Step 2: Mark the level of the osteotomy as before. In the
tibia, err on the side of a more proximal osteotomy for better
bone formation.
Step 3: Make a single unicortical drill hole anteriorly at the
level of the tibial osteotomy. A second unicortical drill hole can
be made on the medial surface. Avoid getting into the anterior
and deep posterior compartments. Multiple drill holes are not
made prior to reaming to avoid extrusion of reamings into the
deep posterior and anterior compartments, this minimizing the
risk of a compartment syndrome.
Step 4: Insert a guide wire from the distal fibula to the tibia.
This wire should be inclined from distal on the fibula to proxi-
mal on the tibia. Incise the skin on the tibial side and drill
over the wire with a 3.2-mm drill bit across both bones. Insert
a solid 4.5-mm screw from the tibia to the fibula such that the
PRECICE intramedullary limb lengthening system Device Profile
informahealthcare.com doi: 10.1586/17434440.2015.1005604
Expert Review of Medical Devices Downloaded from informahealthcare.com by 73.46.221.27 on 02/18/15
For personal use only.

head of the screw is on the tibial side. The screw should always
be oriented so that the tibial side is more proximal than the
fibular side.
Step 5: Perform a mid-diaphyseal fibular osteotomy through
a small posterolateral incision between the superficial posterior
and lateral muscle compartments.
Step 6: To get the starting point, insert a Steinmann pin
into the proximal tibia at the level of the joint in line with the
medial tibial spine on the AP and at the joint line on the lat-
eral view. The starting point is usually medial to the
patellar tendon.
Step 7: Ream the tibia in 1-mm increments until there is
chatter and then in 0.5-mm increments as for the femur.
Step 8: After reaming, add multiple drill holes to the
planned osteotomy level in the tibia. Using a sharp osteotome,
complete the osteotomy through the anterior incision in a
percutaneous fashion.
Step 9: Once the osteotomy is confirmed to be complete,
insert and advance the PRECICE tibial nail until the upper
end is below the level of the bone.
Step 10: Drill the first proximal locking screw from antero-
medial to posterolateral aiming for the head of the fibula:
upper locking screw hole on the left and lower locking screw
hole on the right. Measure a locking screw long enough to fix-
ate the tibia to the proximal fibula. Lock the second proximal
locking screw from the anterolateral side into the tibia only. If
the first drill hole and screw misses the fibula, then lock the
fibula with a separate 4.5-mm screw using a wire followed by a
cannulated drill from the tibia to the fibula.
Step 11: Free hand lock two of the three distal screws leav-
ing either the middle or distal one empty.
Step 12: Perform a distraction test of 1 mm using the
ERC (FIGURE 4).
Postoperative procedures
The ERC is placed firmly but comfortably over the area where
the magnet of the PRECICE implant is located. The implant
is distracted to the desired amount as viewed on the ERC dis-
play screen. Radiographs should be obtained every 2 weeks to
monitor the progress of the lengthening and to confirm that
the amount of lengthening performed has been achieved. The
radiographs also monitor the quality of the regenerate. While
1 mm/day is generally recommended, clinical and radiographic
examination may show that lengthening should progress at a
faster or slower pace (FIGURE 4). Unilateral lengthening patients
are instructed on partial weight-bearing restrictions using a
walker and then progressing to crutches. They are taught to
judge weight-bearing using a bathroom scale. Weight-bearing is
restricted to 22 kg (50 lbs) for the 8.5- and 10.7-mm nails and
34 kg (75 lbs) for the 12.5-mm PRECICE 2 nail (P2). For the
original PRECICE (now referred to as P1), the restriction was
always 22 kg (50 lbs). Bilateral lengthening cases, with
12.5-mm P2 devices are taught to walk using a walker and to
place up to 68 kg (150 lbs) when standing on both legs and
34 kg (75 lbs) on each leg during single leg stance of walking.
Similarly, bilateral 8.5- or 10.7-mm P2 cases are taught to
walk with a walker and place up to 45 kg (100 lbs) when
standing on both legs and 22 kg (50 lbs) during single leg
stance of walking. Bilateral cases are also transitioned to
crutches from a walker. All patients are given a wheelchair to
use for longer distances.
Cost–effectiveness
No studies have been performed on the cost–effectiveness of
PRECICE. The PRECICE implant is priced competitively and
comparably with other limb lengthening external fixation devi-
ces such as the Taylor Spatial Frame (Smith and Nephew
Orthopedics, Memphis, TN, USA). The PRECICE device may
even be cheaper than the combination of implants used in
techniques such as Lengthening over Nail, Lengthening and
then Nailing, etc. [17]. Reduction in device- and pin-related
complications should lead to a greater cost–effectiveness of this
device compared to external fixation. For example, external fix-
ation lengthening has a significant rate of unplanned secondary
procedure to treat device-related complications such as deep
pin infections and loosening, axial deviation, muscle contrac-
tures related to transfixation by wires or pins, etc. [18].
Therefore, reduction in unplanned secondary surgery will sig-
nificantly increase the cost–effectiveness of the PRECICE
method.
Clinical profile & postmarketing findings
Lengthening in children & adults for leg length discrepancy &
stature
Paley et al. (2013) conducted a retrospective, single-center study
to report the early results of the PRECICE system, based on
the experience of three surgeons at a single center [19].Institu-
tional Review Board approval was obtained for this study. The
results were first presented at the EPOS Annual Meeting on
April 2013 in Athens, Greece, and then at POSNA in Toronto,
Canada. This study was recently published [20]. The authors
reviewed the results of 48 consecutive patients (65 PRECICE
nails) implanted between 12/1/2011 and 12/4/2012 (all PRE-
CICE 1). The mean age of the patients in this series was
25.6 years (10.3–58.4 years). Lengthening for congenital
discrepancy (FIGURE 5) was performed in 23 patients; mean age
was 18.5 years (10.3–43.7 years) and mean pre-operative
lengthening goal was 4.91 cm (1.5–6.5 cm), while the preopera-
tive mean LLD was 6.27 cm (1.5–18.2 cm). The lengthening
rate was 0.8 mm/day. The goals of lengthening were met in all
except five patients in this group; mean lengthening was 4.5 cm
(0.5–6.5 cm) (FIGURES 6& 7). Lengthening for developmental dis-
crepancy was performed in four patients; mean age was
17.8 years (13–27 years) and mean preoperative lengthening
goal was 3.68 cm (1.5–6.5 cm). The lengthening rate was
1 mm/day. The mean lengthening was 3.68 cm (1.5–6.5 cm).
All patients achieved the preoperative lengthening goal without
any complication. Lengthening for posttraumatic limb length
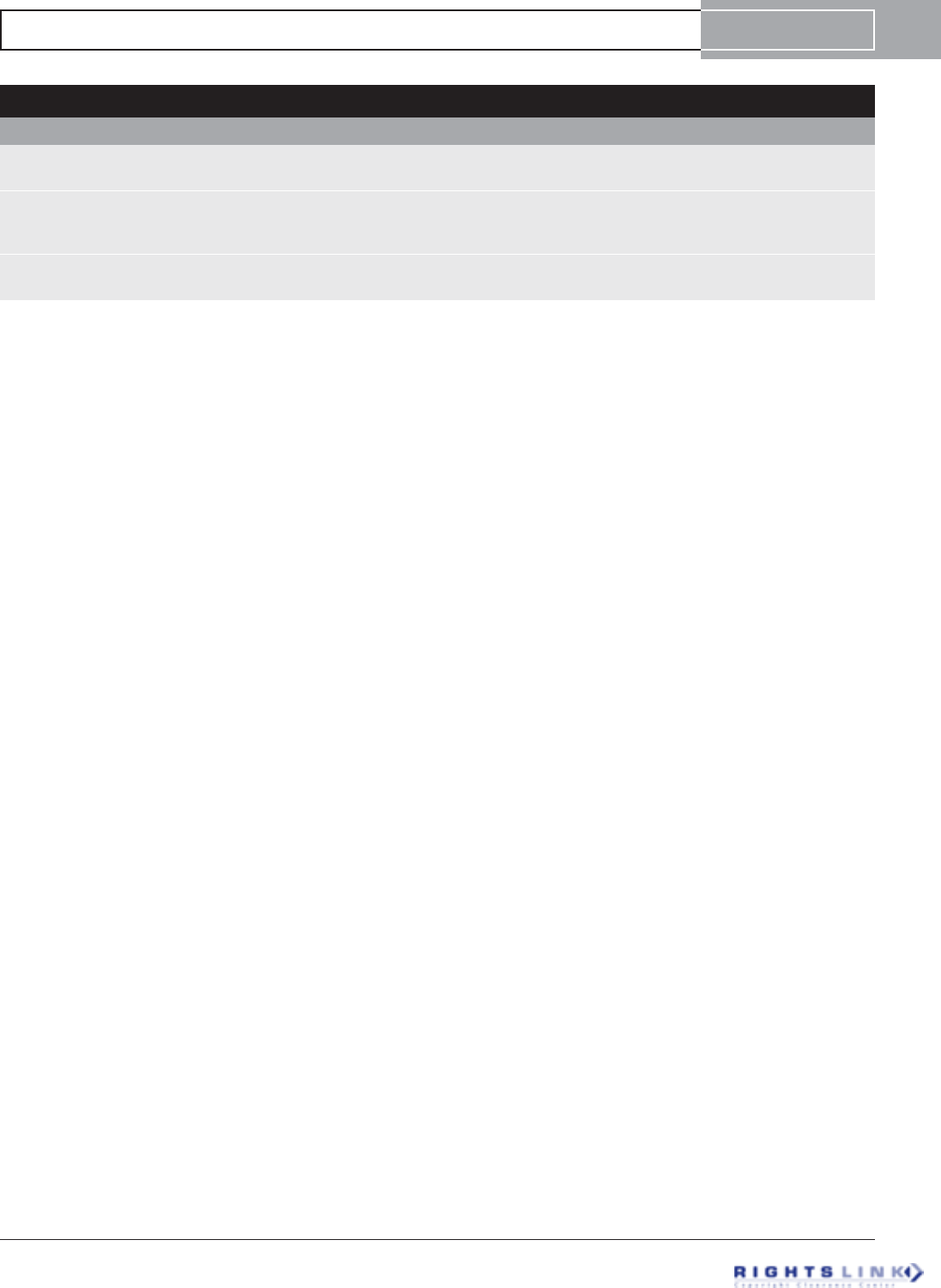
discrepancies (FIGURE 8) was carried out in six patients; mean pre-
operative goal was 3.48 cm (1.7–5.0 cm) and mean age was
Device Profile Paley
doi: 10.1586/17434440.2015.1005604 Expert Rev. Med. Devices
Expert Review of Medical Devices Downloaded from informahealthcare.com by 73.46.221.27 on 02/18/15
For personal use only.
49.0 years (30–58 years). The lengthening rate was 0.93 mm/
day. The mean lengthening was 3.48 cm (1.7–5.0 cm). All
patients achieved the preoperative lengthening goal without any
complication. In addition to this, 15 patients underwent stature
lengthening either for achondroplasia (FIGURE 9) or for cosmetic
reasons (FIGURE 10). Their mean age was 29.7 years (15–48 years),
baseline height was 166.2 cm (150–177 cm) and the preopera-
tive goal of lengthening was 5.64 cm (3.0–6.5 cm). The length-
ening rate was 1 mm/day. The mean lengthening was 4.63 cm
(2.7–6.5 cm). Eight patients electively stopped lengthening
before reaching their preoperative goal due to personal reasons
and not due to medical reasons.
The mean length gained was 4.41 cm (range, 0.5–6.5 cm),
with a mean distraction rate for all nail segments of 0.83 mm/
day (range, 0.50–1.11 mm/day). Three patients required a
bone grafting procedure for failed regenerate (all congenital).
Three nails broke in two patients during the consolidation
phase. All three had ignored the weight-bearing restrictions and
had stopped using crutches. Each was exchanged for a locked
standard intramedullary nail and length was preserved in each
case. Each nail that broke fractured at the proximal (2) or mid-
dle (1) weld of the first-generation PRECICE nail.
Seven nails in six patients ceased to lengthen during the dis-
traction phase. Two of these were due to operator error in
applying the ERC device in the wrong direction, leading to
breakage of the mechanism. These two were replaced and
lengthened uneventfully. In the other five, mechanism breakage
is thought to have occurred due to lengthening against a dense
regenerate or premature consolidation. All nails that ceased to
lengthen were in males with large muscular thighs. The nails
were replaced in four. The mean length achieved was 4.73 cm
(4.1–5.5 cm) in these four nails.
Mean healing time and return to full weight-bearing was
125.3 days (range, 52–262 days). In total, there were
18 unplanned surgeries in 16 patients. The remaining patients
successfully completed treatment without any complication.
The authors concluded that PRECICE demonstrated excellent
rate control and accuracy. The bidirectional feature proved use-
ful in one subject who was acutely shortened 2 cm in conjunc-
tion with treatment of a seroma, followed by resumption of
gradual lengthening 2 weeks later.
Limb lengthening in children with a new, controllable, internal
device
Herzenberg et al. (2013) conducted a retrospective, single-
center study to report preliminary results of the PRECICE sys-
tem in children [21,22]. The results were presented at the EPOS
Annual Meeting on April 2013 in Athens, Greece. The authors
A B C
8 CM
5 CM
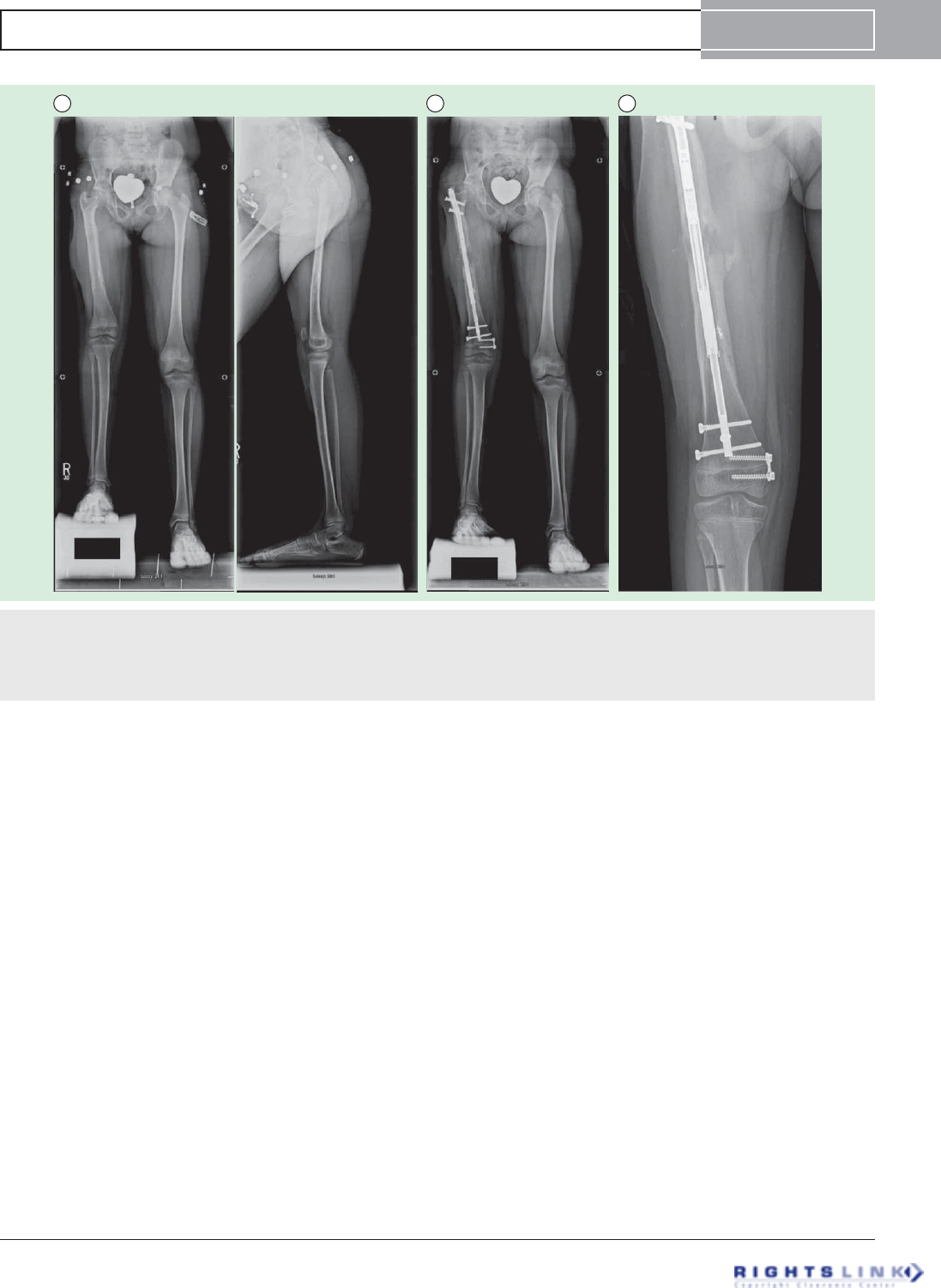
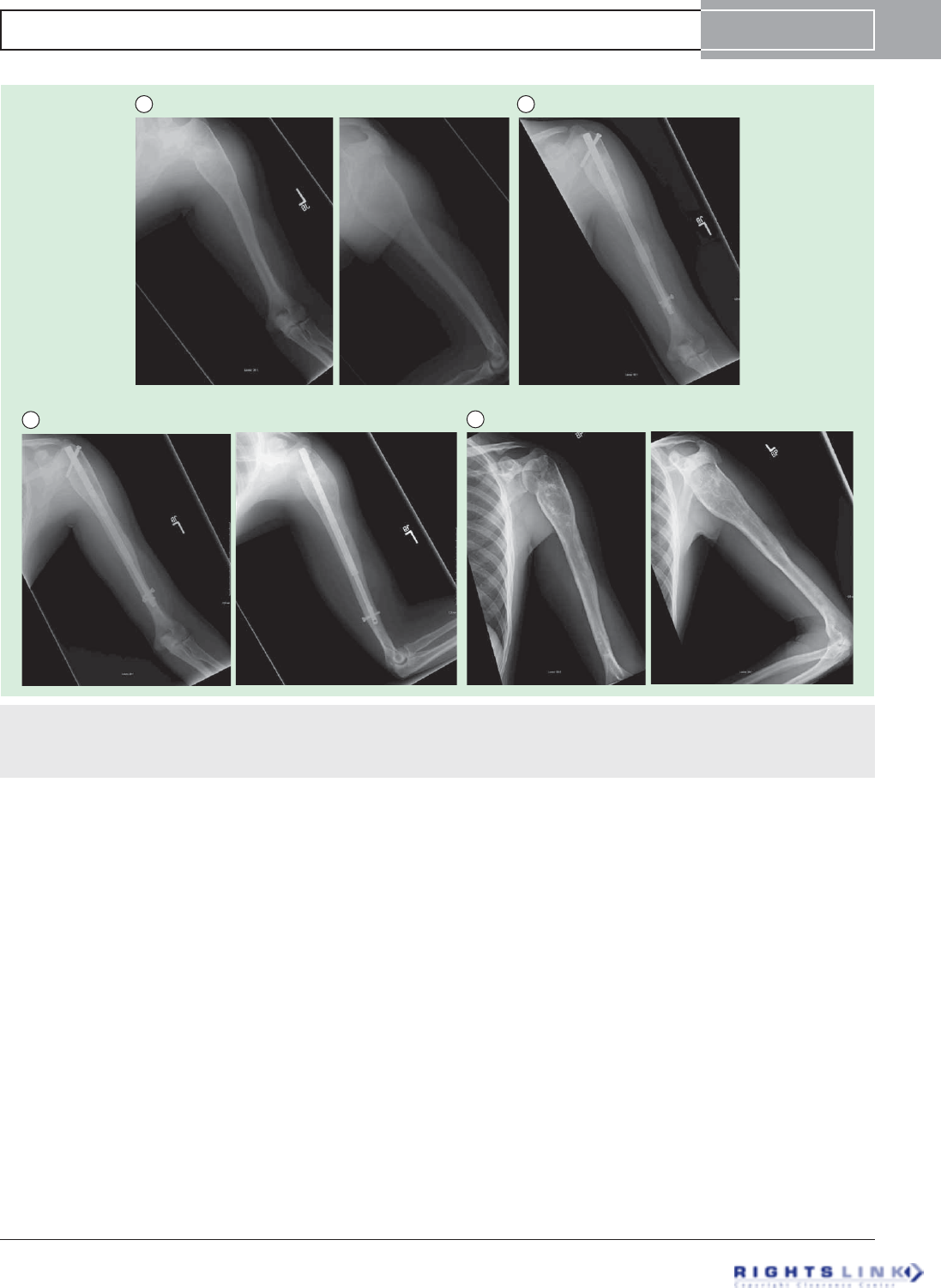
Figure 5. Eight year old girl with congenital femoral deficiency. (A) Preoperative AP and lateral radiographs. She had been
previously treated by a superhip-superknee surgery and has also successfully undergone one external fixator lengthening at age 4. Her
leg length discrepancy was 7 cms. (B) AP radiograph after 5cms of lengthening by Precice2 8.5mms x 245mms, trochanteric entry femo-
ral nail. Her genu valgum was treated at the same time by a hemi-epiphysiodesis plate. (C) This AP radiograph during the consolidation
phase shows the distraction gap filling in with bone one month after stopping distraction.
PRECICE intramedullary limb lengthening system Device Profile
informahealthcare.com doi: 10.1586/17434440.2015.1005604
Expert Review of Medical Devices Downloaded from informahealthcare.com by 73.46.221.27 on 02/18/15
For personal use only.
ABC
DE
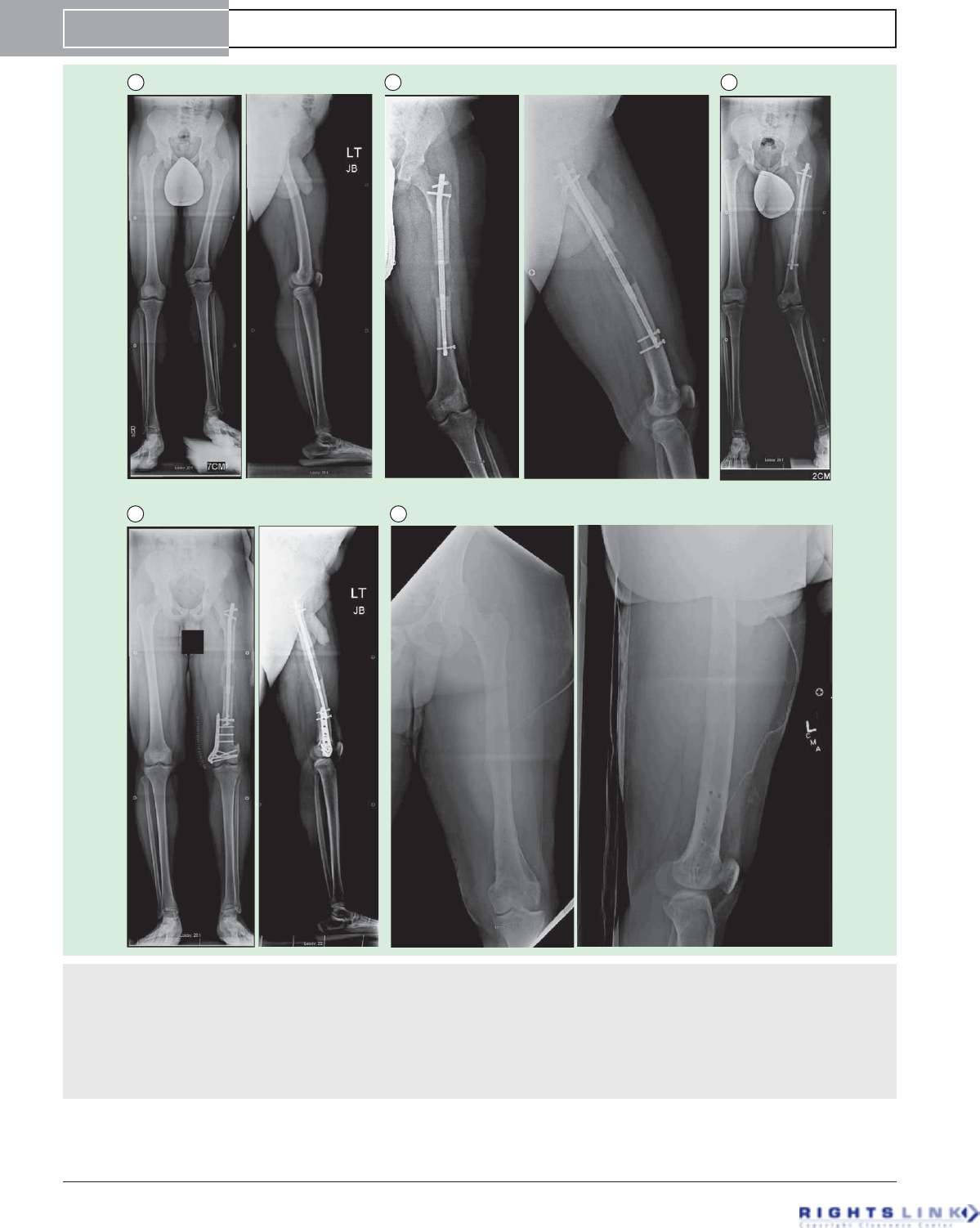
Figure 6. Sixteen year old boy with a leg length discrepancy and valgus deformity secondary to a partial growth arrest of
the distal femur (developmental etiology). (A) Preoperative AP and lateral radiographs (B) AP and lateral radiographs after 6.5cms
lengthening using a 10.7mms trochanteric entry Precice1 tibial nail (we used a tibial nail since at the time the trochanteric entry femoral
nail was unavailable; the current P2 tibial nail has a shorter magnet and would not be a good choice to use but the P1 tibial nail had the
regular length magnet and could be used in the femur). This was our very first Precice case in December 2011. (C) AP radiograph show-
ing the worsened valgus deformity after 6.5cms of lengthening. (D) AP and lateral radiographs after acute correction of the valgus defor-
mity by a medial closing wedge osteotomy with internal fixaton using a locking plate. A peroneal nerve decompression was also done to
minimize the risk of nerve stretch injury. (E) The final AP and lateral radiographs after removal of all of the hardware a year later.
Device Profile Paley
doi: 10.1586/17434440.2015.1005604 Expert Rev. Med. Devices
Expert Review of Medical Devices Downloaded from informahealthcare.com by 73.46.221.27 on 02/18/15
For personal use only.
reviewed 20 children of various etiologies who underwent
implant between January 2012 and March 2013. Mean subject
age was 13.5 years (range, 7–16 years). Mean target lengthen-
ing was 5.2 cm (range, 2.5–6.5 cm). Subjects who underwent
lengthening with external fixation as well as with the PRECICE
system were sent questionnaires and asked to compare
their experience.
At the time of the meeting, 20 of 27 limbs completed
lengthening. Target lengths programmed into the ERC closely
matched radiographic measurements (average of 5.2 vs 5.6 cm,
respectively). Range of motion (ROM) was well maintained
throughout the lengthening and consolidation phases. Of the
16 patients who were completely healed, the average distraction
index was 2.4 cm/month (range, 1.2–3.25 cm/month) and the
average healing index was 1.12 months/cm (range, 0.46–2.0
months/cm). Three complications were observed and resolved:
one prominent screw treated with revision, one peroneal nerve
entrapment treated with decompression and one rotary subluxa-
tion treated with ligament reconstruction. Nine of the 13 chil-
dren who had prior lengthening with external fixation
completed a questionnaire comparing their experience with
internal and external fixation. All reported that PRECICE
allowed for easier physical therapy, better cosmetic results and
a higher rate of overall satisfaction. When asked which device
they would choose if another surgery was needed, all nine
patients said that they would choose the PRECICE system.
The authors concluded that the device is accurate, allows for
good ROM, results in few complications and is an attractive
alternative to external fixation. More patients and long-term
follow-up are still needed.
Precision of the new remote controlled internal lengthening nail
Kirane et al. (2013) conducted a retrospective, single-center
study to evaluate the clinical efficacy of the PRECICE system.
The results were presented at the Hospital for Special Surgery
Research Symposium in June 2013 in New York, NY [23]. The
authors reviewed 10 femur and seven tibia lengthening cases
using the PRECICE system. Medical records were reviewed for
etiology, patient characteristics, surgery details, distraction pro-
cess, bone alignment, adjacent joint ROM and any
A B
CD
Figure 7. Arm shortening (6.5 cms) due to growth arrest from a unicameral bone cyst. (A) Preoperative AP and Lateral
radiographs of left humerus (B) AP radiograph at the end of 6.5cms distraction using a 10.7mms Precice1 femoral piriformis entry nail.
(C) AP and lateral radiographs after three months of consolidation. There is excellent bony bridging. (D) The nail was removed one year
later. The bone and previous cyst are well consolidated.
PRECICE intramedullary limb lengthening system Device Profile
informahealthcare.com doi: 10.1586/17434440.2015.1005604
Expert Review of Medical Devices Downloaded from informahealthcare.com by 73.46.221.27 on 02/18/15
For personal use only.
complications. Distraction distance measurements were done at
every follow-up visit using a calibrated digital radiology system
(PACS, OnePacs LLC, New York, NY, USA).
The results indicated that at 13.5 weeks follow-up (range,
4–30 weeks), the lengthening was 33.65 mm (range,
14–61 mm) with an accuracy of 100.7 ±0.23%. All femur
AB
C D
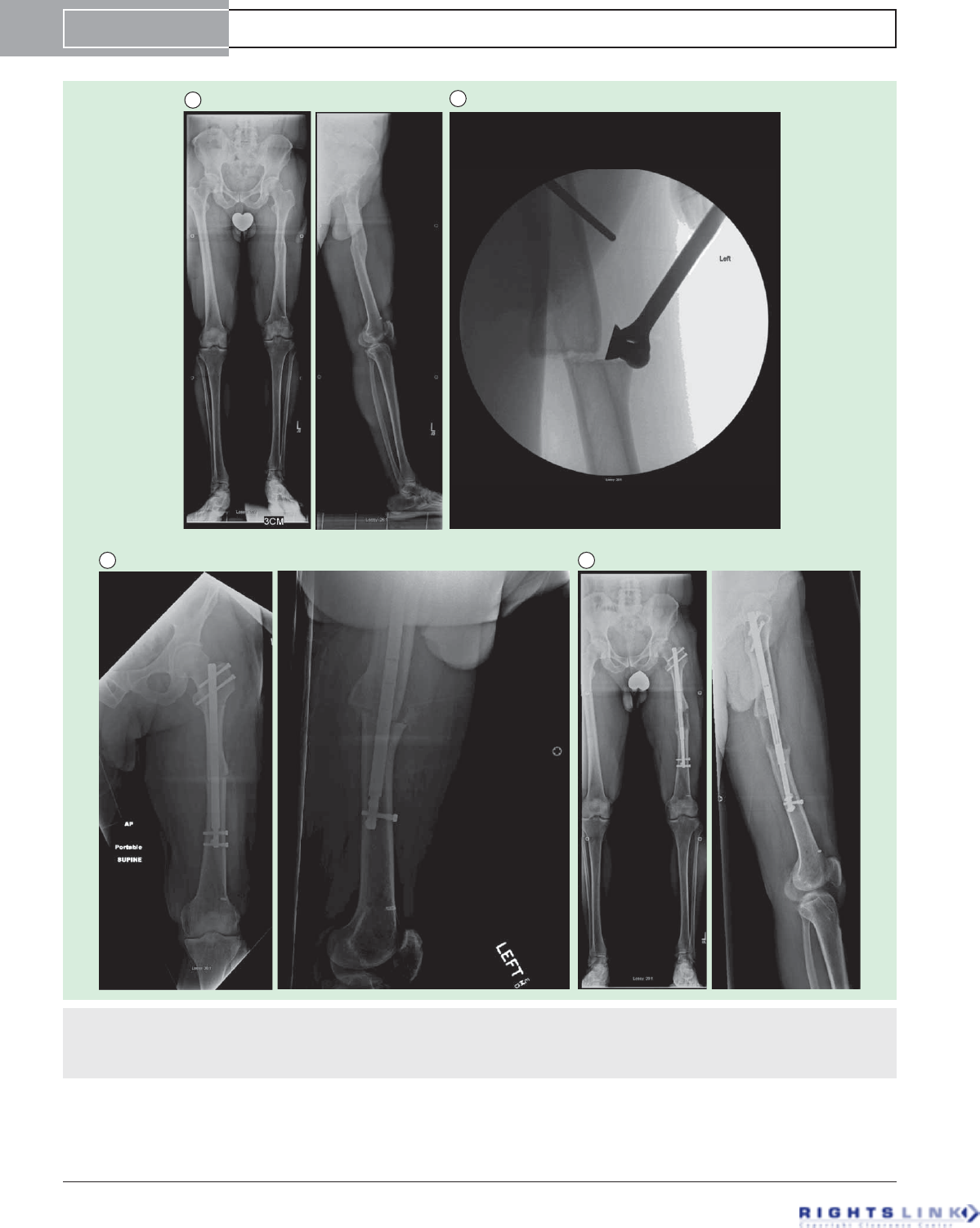
Figure 8. Forty-eight year old man with a symptomatic post-traumatic 5 cms LLD of the femur. (A) Preoperative AP and lateral
radiographs of the femur. (B) Using fixator assistance, the osteotomy was made prior to reaming in order to realign the medullary canals
of the previously bayonet deformity. (C) AP and lateral radiographs after insertion of Precice1 12.5 mms nail. The bayonet deformity is
reduced. (D) AP and lateral radiographs after 5cms of lengthening. Good bone healing is seen. He proceeded to heal completely.
Device Profile Paley
doi: 10.1586/17434440.2015.1005604 Expert Rev. Med. Devices
Expert Review of Medical Devices Downloaded from informahealthcare.com by 73.46.221.27 on 02/18/15
For personal use only.
cases had excellent bone healing, while two tibia cases required
insertion of bone marrow concentrate for delayed bone healing.
There were no implant failures or major complications. The
authors concluded that the new PRECICE internal lengthening
nails have an accuracy of distraction close to 100%. The use of
an external magnetic controller was straightforward and easy to
explain to patients. Notably, there were no implant failures in
this initial series. In several patients, realignment of the preex-
isting deformity was possible through an osteotomy at the apex
of the deformity. Furthermore, the hip, knee and ankle ROM
were well maintained. Iliotibial band release and gastrocnemius
recession were helpful in maintaining knee and ankle ROM,
respectively, during lengthening. Tibia lengthening was associ-
ated with more difficulties than femur lengthening. Addition-
ally, a tendency of varus-procurvatum deformity of the femur
and valgus-procurvatum deformity of the tibia was successfully
prevented by inserting blocking screws into the concavity of
the potential deformity. Lastly, consideration must be given to
the length of the thicker nail segment beyond the osteotomy to
ensure adequate stability and to prevent iatrogenic deformities.
Internal lengthening device for congenital femoral deficiency &
fibular hemimelia
Shabtai et al. (2014) reported a prospective, nonrandomized,
single-center study to evaluate the PRECICE system in terms
of healing index, complications, accuracy of the device’s exter-
nal controller and adjacent-joint ROM [24]. Institutional Review
Board approval was obtained prior to performing any study-
related procedures. Sixty six subjects were enrolled and treated
for congenital limb shortening between January 2012 and May
2013. Of these, 21 were treated using the PRECICE system
and 18 met the eligibility criteria for analysis of the PRECICE
system. Ten females and eight males were enrolled with a
mean age of 19 years. Sixteen femurs and five tibias were
lengthened with a mean of 4.4 cm (range, 2.1–6.5 cm). Mean
distraction index was 1.0 mm/day (range, 0.5–1.8 mm/day).
Healing index complications, device accuracy and ROM
were recorded.
At the time of the publication, 10 of the 21 devices had
been removed. This was typically done 12–24 months after
insertion when the bone was solidly healed on all four cortices.
Mean healing index was 0.91 months/cm (range, 0.2–2.0
months/cm). There were seven complications requiring an
additional unplanned surgery, including one hip flexion con-
tracture, three femurs with delayed healing, one tibia with
delayed healing, one hip subluxation/dislocation and one knee
subluxation. The external controller was accurate as pro-
grammed and actual lengthening amounts were consistent.
ROMs of the hip, knee and ankle were essentially maintained.
The authors concluded that the intramedullary implant of the
A B C D
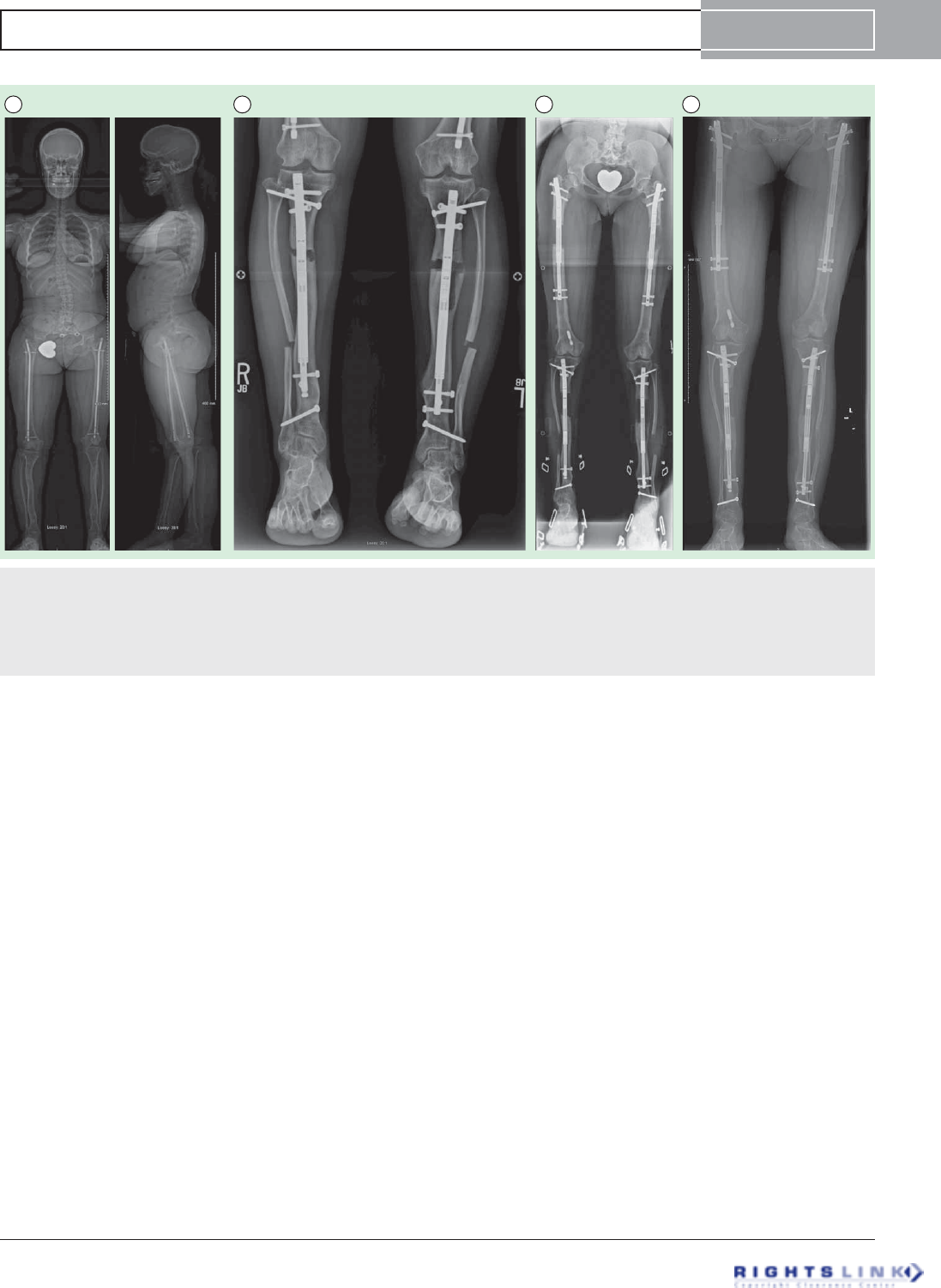
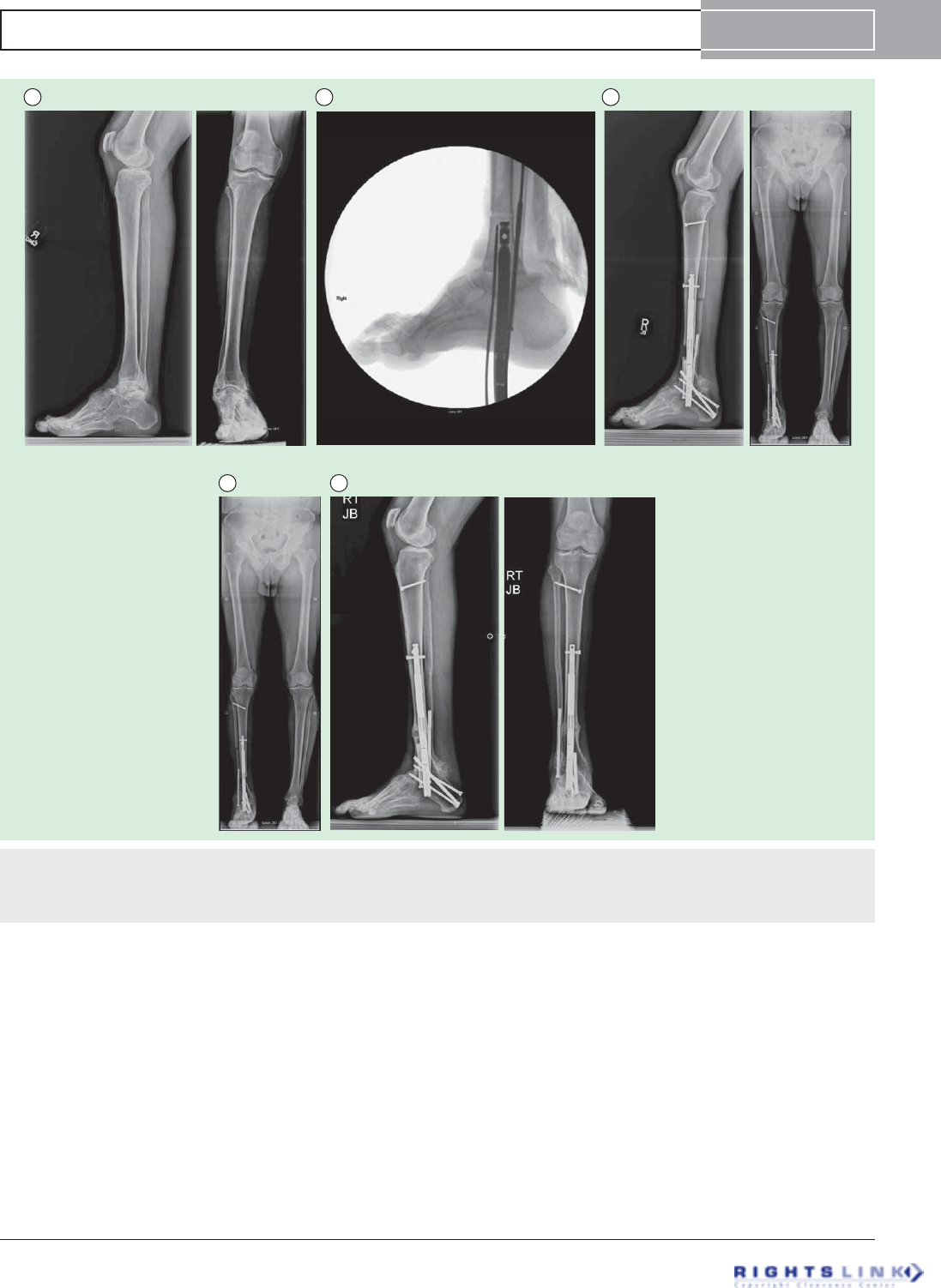
Figure 9. Sixteen year old girl with achondroplasia who had undergone 2 previous lower limb (combined femur and tibia 4
segment lengthening) and one bilateral humeral lengthening. (A) Preoperative standing EOS scan radiographs. (B) Bilateral tibial
lengthening was carried out using Precice1 10.7mms tibial nails. (C) Two weeks later she underwent removal of the previous femoral
nails and insertion of bilateral 10.7 mms Precice1 trochanteric entry nails. Both femurs were lengthened 6.5 cms and tibias 5 cms simul-
taneously. The staggered nail insertion was to avoid reaming all four bones at the same time because of the risk of fat embolism. (D)
The patient is now 62 inches (158 cms) tall.
PRECICE intramedullary limb lengthening system Device Profile
informahealthcare.com doi: 10.1586/17434440.2015.1005604
Expert Review of Medical Devices Downloaded from informahealthcare.com by 73.46.221.27 on 02/18/15
For personal use only.
device allows for satisfactory joint motion during treatment in
most patients. Lengthening was achieved in an accurate, con-
trolled manner and all patients reached their goal length. Com-
plications remain a concern as is the case with all approaches
in this complex patient population.
Precision of the PRECICE internal bone lengthening nail
Kirane et al. (2014) reported a retrospective, single-center
study to evaluate the PRECICE system in terms of accuracy
and precision of distraction, effects on bone alignment,
effects on adjacent-joint ROM, and frequency of implant-
related and non-implant-related complications [25].Twenty
four patients were reviewed whounderwentfemoraland/or
tibial lengthening procedures using the PRECICE nail from
August 2012 to July 2013 for conditions of varied etiology.
At each postoperative visit, the accuracy and precision of dis-
traction, bone alignment, joint ROM and any complications
were recorded. Accuracy reflected how close the measured
lengthening was to the prescribed distraction at each postop-
erative visit, while precision reflected how close the repeated
measurements were to each other over the course of the total
lengthening period. No patients were lost to follow-up.
Minimum follow-up from surgery was 3 weeks (mean
14 weeks; range, 3–29 weeks).
The results indicated that mean total lengthening was
35 mm (range, 14–65 mm), with an accuracy of 96% and a
precision of 86%. All patients achieved target lengthening with
minimal unintentional side effects on bone alignment. The
knee and ankle ROMs were minimally affected. Of the compli-
cations requiring return to the operating room for an addi-
tional surgical procedure, there was one (4%) implant failure
caused by a nonfunctional distraction mechanism and six
(24%) non-implant-related complications, including premature
consolidation in one patient (4%), delayed bone healing in two
(8%), delayed equinius contracture in two (8%) and toe claw-
ing in one (4%). The authors concluded that the PRECICE
system is a valid option to achieve accurate and precise limb
lengthening to treat a variety of conditions with limb shorten-
ing or length discrepancy.
How precise is PRECICE compared to ISKD in intramedullary limb
lengthening?
Schiedel et al. (2014) reported a prospective, nonrandomized,
single-center study to evaluate the reliability and safety of the
A
B
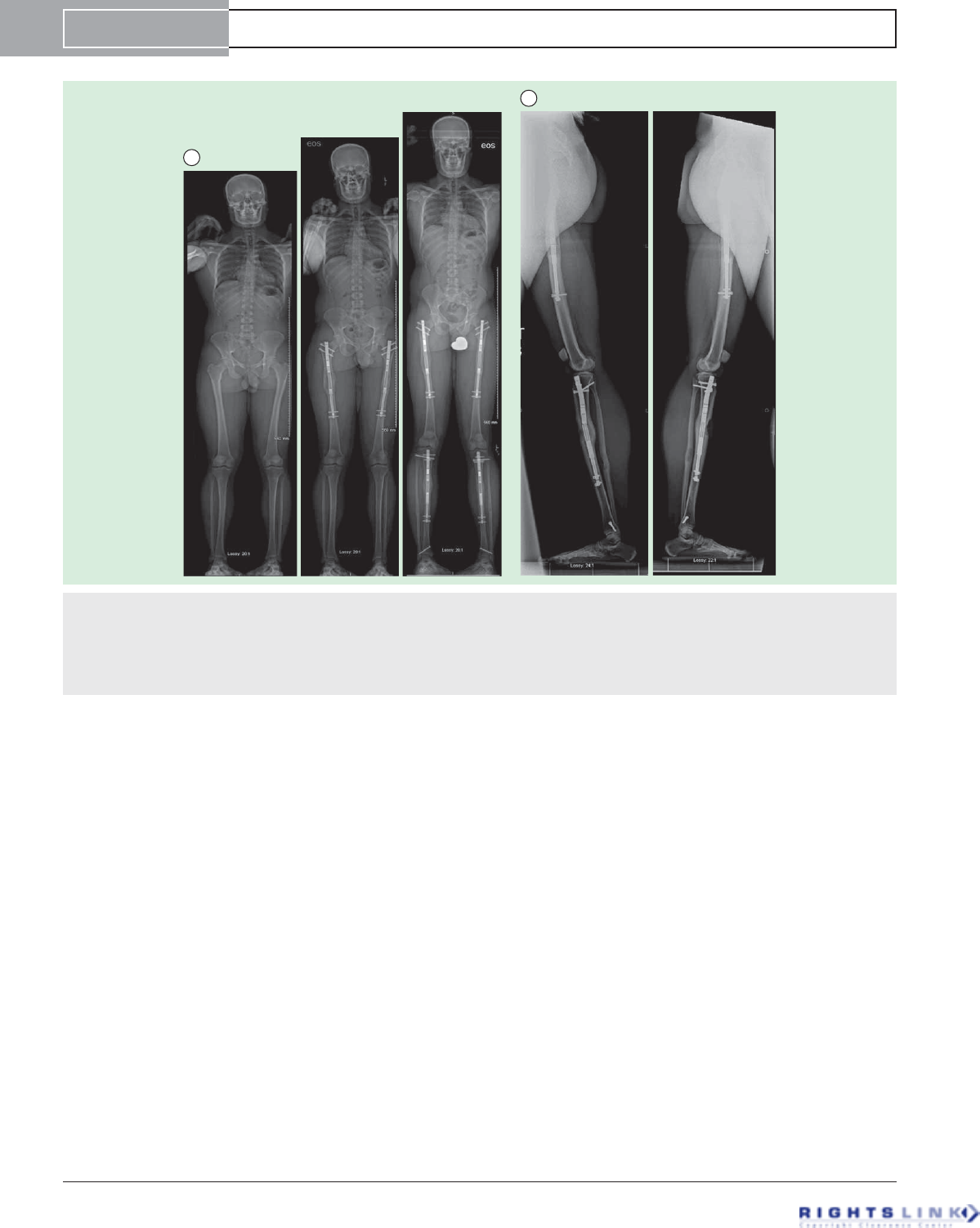
Figure 10. Cosmetic stature lengthening. (A) Three EOS scan AP radiographs (left), after bilateral femoral lengthening (6.5 cms) using
Precice1 12.5 mms piriformis entry nails (middle), and after bilateral tibial lengthening (4.5cms) using Precice1 10.7 mms tibial nails (left).
The total height increase was 11cms. (B) Long lateral radiographs of both femurs and tibias show that there is a deficiency in the ante-
rior bone formation of the left tibia compensated by hypertrophic regenerate bone formation posteriorly. On the right there is a break in
the Precice 1 nail through one of the welds. This break occurred due to delay in regenerate bone formation and resumption of weight-
bearing. Fortunately, the bone healed with minimal procurvatum deformation of the tibia.
Device Profile Paley
doi: 10.1586/17434440.2015.1005604 Expert Rev. Med. Devices
Expert Review of Medical Devices Downloaded from informahealthcare.com by 73.46.221.27 on 02/18/15
For personal use only.
first generation of the PRECICE system [26]. Institutional
Review Board approval was obtained prior to performing any
study-related procedures. Twenty four patients with 26 PRE-
CICE nails were enrolled and implanted between June
2012 and March 2013. Anteroposterior radiographs of the leg
while the patient was standing were obtained before surgery,
every 14 days during lengthening and during or after consoli-
dation. The occurrence of any problems, obstacles and compli-
cations was recorded after lengthening was completed. The
Paley classification was used for comparison with other length-
ening procedures and external lengthening procedures. Prelimi-
nary results were compared with the known difficulties in the
use of mechanical lengthening devices such as ISKD.
The results showed that two nails did not function properly,
yielding 24 of the 26 nails implanted that lengthened over the
desired distance. Lengthening desired was 38 mm and length-
ening obtained was 37 mm. There were two nail breakages,
one occurring in the welding seam and one due to an acciden-
tal fall by the patient during consolidation. In total, 15 prob-
lems, five obstacles and four complications were observed
throughout treatment of the study cohort. The authors con-
cluded that the mean accuracy of lengthening with PRECICE,
97%, is similar to that in the comparable ISKD study
(96%) [26], and that continuing improvements to the system by
the manufacturer, including the recent release of the second-
generation system, will address the issues observed in this
study.
Alternative devices
The use of limb lengthening instruments is a well-known tech-
nique in the treatment of lower limb discrepancies. TABLE 1lists
other implantable limb lengthening devices described in the lit-
erature. The indication/intended use statements are gathered
from the literature and other publically available sources of
information, such as company web sites.
Published reports on Albizzia, ISKD and Fitbone report a
wide variety of problems and complications. Simpson et al.
reported that seven of their 33 (21.2%) ISKD nails were classi-
fied as runaway implants [8]. This meant that the rate of dis-
traction could not be controlled in 21.2% of patients and
exceeded the desired lengthening rate. A total of 15/33
(45.4%) of their nails experienced rate control complications,
with seven lengthening too quickly and another seven being
overly difficult to lengthen. Elsewhere in the literature, we can
find reports of ISKD nails that lengthened at rates much
greater than 1 mm/day or were classified as runaway nails rang-
ing from 9% (1/11, Kubiak) to 18.9% (7/37, Kenawey) to
83.3% (10/12) in the series by Mahboubian et al. (Hankeme-
ier) [27–30]. Wang et al. reports that five of their 16 nails length-
ened uncontrollably, forcing them to ask these patients to
modify their weight-bearing and activity level from week to
week based on the rate of distraction of the nail. If it were dis-
tracting too slowly, they would be asked to increase their
weight-bearing and to become more active, and vice versa
(Wang, Simpson) [31,32]. At best, this was a very imperfect way
of controlling the rate of distraction of ISKD. There are addi-
tional series that further detail runaway nail rates that range
from 9% (Kubiak) to 20% (Paley) [27,33]. The article by the
ISKD’s designer (Cole) reviewed his initial series of 20 nails in
18 patients [34]. They reported lengthening rates of up to
1.7 mm/day, but no mention is made as to how many patients
lengthened at such a rapid rate. In an unpublished study by
Paley, of 350 ISKD lengthenings, distraction rates of up to
5 mm/day were documented.
A large majority of patients with runaway nails went on to
develop poor regenerate or nonunion at the distraction site.
While the article by Cole et al. observed zero nonunions or
patients who required a later bone graft procedure, other
articles document rates of runaway nails requiring additional
surgery in the form of either bone grafting or exchange nailing
that range from 20% (Wang 1/5, Kenawey 5/7 to Simpson
6/7) to 86% [8,28,31].
Certainly, poor regenerate formation/nonunion is not exclu-
sive to intramedullary nails that fail to maintain safe rate con-
trol, but rather this remains a well-known complication for all
limb lengthening procedures. While only 1/5 of the runaway
nails in the article by Wang et al. required later bone grafting,
a total of six out of their 16 ISKDs (37.5%) required an addi-
tional surgery to treat poor regenerate or nonunion [31]. Simp-
son needed to treat only 6/8 (75%) of his runaway nails with
additional surgery, although, a total of 8/33 (24.2%) nails ulti-
mately required this approach [8]. Five of the seven (71.4%)
runaway nails in Kenawey’s series required bone graft and/or
exchange nailing, along with an additional three nails that simi-
larly developed deficient bone healing, for a total of
8/37 (21.6%) [28]. Singh et al. reported that 3/24 (12.5%) of
Table 1. Alternative devices and intended use.
Device Manufacturer Intended use Distraction mechanism Materials Device status
Albizzia/Guichet DePuy
Orthopedics
For limb lengthening of
the tibia and femur
Mechanical telescoping,
gear actuation
Stainless
steel
CE Mark, not approved
in the USA
Fitbone Wittenstein
GmbH
For limb lengthening of
the tibia and femur
Motorized, radiofrequency
powered telescoping, gear
actuation
Titanium
alloy
CE Mark, not approved
in the USA
ISKD Orthofix Inc. For limb lengthening of
the tibia and femur
Mechanical telescoping,
gear actuation
Titanium
alloy
CE Mark, recalled in the
USA
PRECICE intramedullary limb lengthening system Device Profile
informahealthcare.com doi: 10.1586/17434440.2015.1005604
Expert Review of Medical Devices Downloaded from informahealthcare.com by 73.46.221.27 on 02/18/15
For personal use only.

their Fitbone nail segments required later bone grafting, and
Baumgart et al. saw that 1/12 (8.3%) Fitbone segments need
additional surgery to achieve adequate healing [3,34].
Kenawey et al. found a significant association between poor
regenerate and age of patients greater than 30, total lengthening
greater than 4 cm, smoking and a distraction rate greater than
1.5 mm/day [28].
One risk that has been reported to be a predisposing factor
to poor regenerate is a distraction rate greater than 1.5 mm/
day (Kenawey) [28]. This is entirely avoided with the PRECICE
nail. In comparison to the results listed above, Paley et al.
reported that only three of 65 implant segments went on to
develop poor regenerate of nonunion that necessitated an addi-
tional bone grafting surgery.
Another well-reported problem with implantable lengthen-
ing nails is difficulty with distraction. Kubiak et al. attributed
this to impingement and friction secondary to a straight nail
attempting to lengthen a curved femur as well as compressive
forces caused by the soft tissues that are substantial enough to
limit lengthening. This complication is still the most frequent
one with the Albizzia nail. Failure to distract can be related to
wear of the teeth of the internal ratchet gear or due to the
inability of the patient to turn the limb the full 20 degrees
due to pain. Similarly with ISKD, some patients have too
much pain to rotate the femur the 3–9 degrees needed.
Manipulation by the surgeon in the office to manipulation
under epidural or other anesthesia has been used to treat this
problem. Botox injection into the quadriceps to reduce muscle
spasm is also useful. Incidence of failure to distract with
ISKD varies from 0% (Cole) to 64% (Kubiak), of whom 6/
7 of the patients in that series required a return to the operat-
ing room [27,33]. Similarly, the Simpson series had a rate of
24.2% (8/33) of ISKD nails that were difficult to distract,
and 75% (6/8) of those needed a return to the operating
room [8].
Paley et al. reported seven cases of PRECICE with failure to
distract. One bilateral case occurred due to user error. The
others occurred due to resistance from abundant callus and
large thigh musculature. It is likely that the failure of the mech-
anism occurred after repeated lengthening attempts against the
force of a nearly prematurely consolidated bone. Since the
mechanism was changed in PRECICE 1 (May 2013) and since
the introduction of PRECICE 2 which contains the new mech-
anism, there have been no further failures of distraction in over
150 cases [PALEY D, PERS.COMM.].
Mechanical failure of other implantable nails can be
divided into two groups: mechanical failure of the distraction
mechanism and breakage of the integrity of the nail itself. In
the Baumgart et al. series of 12 cases, two patients required
reoperation for failure of mechanism [34]. There were no nail
breakages in this series. In a Fitbone series of 24 nails, two
patients had to have exchange nails to larger diameter Fit-
bone nails as the gears were too weak for distraction. Both
these patients had congenital deformities (Singh et al.)[3].
Another Fitbone cohort of eight patients reported one
mechanism failure and one nail breakage; both were also
congenital etiologies (Krieg et al.)[35]. The ISKD initial series
(20 nails) reported two hardware failures; both nails broke
with patients fully weight-bearing and at the junction of the
proximal and distal components. Design changes were made
in the nail and authors claimed no further breakages. This
further stresses the importance of in vivo analysis of these
devices and appropriate engineering adjustments to improve
product design. No mention of mechanism failure was noted
in this series (Cole et al.)[33]. Another review of 57 ISKD
nails revealed no nail breakages; however, three failures of
the lengthening mechanism occurred. One required an
exchange nail with an examination of the failed nail showing
a jammed ratchet mechanism. The other two nails required
manipulation another anesthesia, One nail acutely lengthened
3 cm instead of 3 mm despite external monitoring, again
illustrating the unpredictability of the ISKD (Kenawey
et al.)[28]. In the largest ISKD series of 242 devices,
15 (6.2%) experienced mechanical failure. Ten of these fail-
ures were nail fractures, two of which were in the same
patient undergoing stature lengthening. Most fractures were
in the male component; however, other areas of nail were
prone to failure as well. The remaining five nails failed at
the lengthening mechanism, two of which failed due to
assembly error (Burghardt et al.)[34]. Ensuring the functional-
ity of the nail during surgery, as in our surgical protocol,
would circumvent these types of complications. In 41 Albizzia
nail insertions, three failures were related to the distraction
mechanism and one to nail fracture, all requiring reoperation
(Guichet et al.)[35].
Among 10 patients (24 nails) with the Fitbone device, two
patients (20%) did not reach the anticipated length due to
restricted knee movement. Both these patients were undergoing
stature lengthening and had femoral and tibial lengthening
(Singh et al.)[3]. In a smaller series (eight patients) using the
same device, they achieved 93% (83–100%) planned length.
However, two of the eight patients were eliminated from this
analysis due to nail failure (Krieg et al.)[35].
Using the ISKD nail lengthening of 33 limbs resulted in
32 achieving desired goals. However, eight patients (eight
limbs) required additional procedures (manipulation, fixator-
assisted) to achieve this due to slow or no progression of dis-
traction (Simpson et al. 2009) [8]. Baumgart’s cohort of
12 patients attained complete length objectives in all patients [2].
Interestingly, all these patients received unilateral lengthening,
which eliminates many factors that may cause premature termi-
nation. Similar to our series most of these terminations were in
the bilateral group. Nevertheless, internal devices in previous
and in our current series seem to have a good track record for
obtaining desired lengths.
Pain is an important consideration with every lengthening
method. Pain is an expected part of lengthening. The degree
of pain does vary between external and internal fixation meth-
ods. Pin sites and pin infections as well as tethering of
muscles and other soft tissues are believed to be a major cause
Device Profile Paley
doi: 10.1586/17434440.2015.1005604 Expert Rev. Med. Devices
Expert Review of Medical Devices Downloaded from informahealthcare.com by 73.46.221.27 on 02/18/15
For personal use only.
of pain during lengthening with external fixation. Since all of
these are absent with implantable devices, the pain is related
to stability, rate of distraction, physical therapy and stretch of
soft tissues. While it is not possible to eliminate stretch, con-
trol of rate and stability of fixation is device dependent. Fric-
tion may also play a part and can be limited by the type of
reamers used (straight vs flexible), amount of overreaming as
well as by the level of osteotomy (at apex of curvature of
femur; leaving as short an amount of nail to drag on the
moving segment).
Pain is a major factor with Albizzia and ISKD. Both of these
devices rotate through the callus. Such rotation leads to friction
and muscle spasm pain. This type of pain has been notably
absent from reports on Fitbone and from the experience
with PRECICE.
Using devices that require no rotation such as the motor-
ized Fitbone, there was minimumtonopainondistraction
(Singh et al.)[3].However,ofthe10patients,whohad
24 implants, only two achieved 60 mm. The rest were
between 27 and 50 mm, with a mean of 40 mm/nail. In
contrast, of the 31 patients using the Albizzia nail, all experi-
enced discomfort or pain during lengthening. Twelve
patients (39%) required readmission to perform racheting
under a general anesthetic (Guichet et al.)[36]. PRECICE
patients seem to have minimal to no pain during
lengthening.
A B C
D E
Figure 11. Fifty five year old man with ankle arthritis and pain. (A) Preoperative AP and lateral radiographs of the tibia, ankle and
foot. (B) Intra-operative fluroscopic picture of a lateral of the foot showing the ankle fusion and retrograde insertion of the first Precice2
in the US November 2013. (C) The distal tibia was lengthened 2.5 cm. (D) The ankle was fused and LLD equalized intentionally leaving a
1 cm discrepancy. (E) AP and lateral radiographs showing union of the ankle fusion and the distraction gap.
PRECICE intramedullary limb lengthening system Device Profile
informahealthcare.com doi: 10.1586/17434440.2015.1005604
Expert Review of Medical Devices Downloaded from informahealthcare.com by 73.46.221.27 on 02/18/15
For personal use only.
Status of the device
The first generation of the PRECICE system was granted
CE Mark in September 2010 and FDA 510(k) clearance in
July 2011 for limb lengthening of the femur or tibia. The
PRECICE 2 system received CE Mark in May 2013 and
FDA 510(k) clearance in October 2013. Commercial use
of the PRECICE system began internationally and in the
USA in 2011. The PRECICE 2 received FDA clearance in
October 2013. Its clinical use in the USA began in November
2013 (FIGURE 11).
PRECICE 2
In 2012, in response to preliminary results of breakage through
the welds and failure to distract in some cases (Paley et al.,
EPOS, POSNA) [20], Ellipse Technologies together with Paley
began a redesign of the nail housing to eliminate the welds as
well as a redesign of the nail mechanism to increase its ability
to distract against resistance (FIGURES 5 & 11). The mechanism
problem was solved first but modifying the coupling between
the drive shaft and the gears. Previously, the powerful torque
strength of the rotating magnet could break this connection if
it met too much resistance. The new mechanism is protected
against this and is now three-times stronger in distraction
strength. The tibial models have been loaded with a smaller
magnet to prevent from overtorquing the magnet due to the
closer proximity of the ERC to the magnet in the tibia. Conse-
quently, PRECICE 2 tibial nails should not be used in the
femur since there would not be enough strength to turn the
smaller magnet with the ERC at larger distances from the mag-
net. The new mechanism had its debut in May 2013 with a
retrofit of all PRECICE 1. The newer mechanism has per-
formed perfectly with no documented cases of failure to dis-
tract to date. At our center alone, over 150 nails with the new
mechanism have been implanted and lengthened successfully
to date.
The elimination of the welds has also greatly strengthened
the nail. Laboratory testing has shown that the weld-less
PRECICE 2 nail is four-times stronger for bending strength
and three-times stronger for axial loading than the original
nail. To date, with over 250 of these PRECICE 2 nails
implanted there have been only two reports of nail breakage.
One was my own patient who was undergoing a congenital
femoral lengthening after a previous knee fusion. This
18-year-old young man chose to ignore all weight-bearing
restrictions after a unilateral 8-cm lengthening. His nail
broke the external shell at the distal telescopic junction.
Angulation occurred without any loss of length. The nail
was successfully exchanged for a trauma locking nail 3 days
after the failure. One European tibial nail broke through the
proximal locking screw hole. After review of that case, it
was evident to me that the nail was inserted too anteriorly
leading to poor support from the surrounding bone. Fur-
thermore, there was a blocking screw inserted at the level of
the break. Since titanium is very notch sensitive, slight
notching of the nail by the blocking screw at the level of
the locking hole with a very proximaltibialosteotomyset
up the nail for failure. Surgeon error is more at fault in this
case. Weight-bearing restrictions are essential to the use of
this nail. They have been increased to 75 lbs during the dis-
traction phase for the 12.5-mm PRECICE 2 from only
50 lbs for the 12.5-mm PRECICE 1. These should be
adjusted downwards when additional factors add to the load
on the nail as is illustrated in the two failure cases. PRE-
CICE 2 seems to have eliminated the two main device fail-
ure mechanisms that were identified with PRECICE 1. The
company has been very responsive and implemented these
changes in record time. One observation made by Paley is
that some nails showed a varus bowing of the nail in some
femoral lengthenings (FIGURE 12). This has not led to any fail-
uretodistractortoanysignificantdeformityorbreakage.
It may be related to the nail being made of titanium. This
is especially in bilateral application where patients may be
weight-bearing more than allowed. One solution would be
changing the material of the nail to a stiffer alloy such as
cobalt-chrome. In 2014 some fragmentation of the end of
the outer tube of the nail has been noted. The end of the
larger diameter of the nail is slotted at four places and
mated and welded to a crown that has 4 ridges to provide
anti-rotation stability to the telescopic nail. The fins
between the slots are the sight of fragmentation of the end
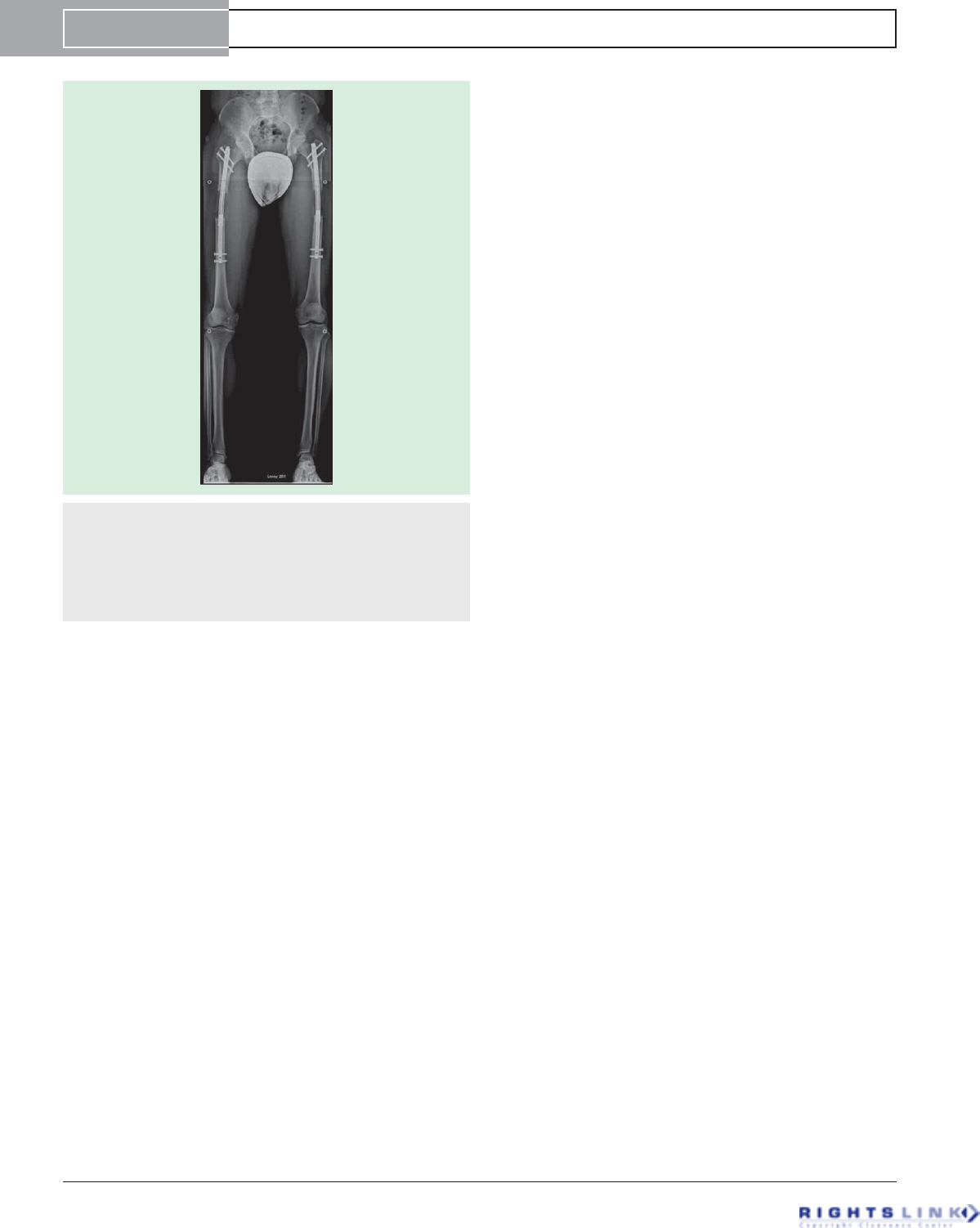
Figure 12. AP radiograph of bilateral femoral lengthening
seen during an 8-cm cosmetic stature lengthening. This
went on to heal uneventfully. The mild varus neutralizes the
valgus deviation that is associated with lengthening along the
anatomic axis. The concern is mostly regarding the risks of
nail breakage. Strict weight-bearing precautions should accom-
pany any bilateral lengthening and especially if bowing is seen.
Device Profile Paley
doi: 10.1586/17434440.2015.1005604 Expert Rev. Med. Devices
Expert Review of Medical Devices Downloaded from informahealthcare.com by 73.46.221.27 on 02/18/15
For personal use only.

of the nail tube. Breakages of this crown and fins have been
identified in some cases including this patient (FIGURE 13).In
two cases it has been linked to breakage of the nail. It is
also contributing to the bowing of the nail. To strengthen
the nail and avoid crown failures, in December 2014,
Ellipse Technologies releasedtheP2.1whichhasamodified
keying feature without ‘thru-slots’or tack welds (FIGURE 13).
Conclusion
PRECICE is the newest generation implantable limb length-
ening device and the first CE-marked and FDA-cleared device
to have both forward and reverse length adjustment capability.
It had demonstrated excellent rate control. This is the most
important factor for achieving good results with any limb
lengthening. The reverse mechanism although not often used
is very helpful when it is needed. It can be used to close
down a distraction gap when there is failed bone formation,
or nerve irritation or palsy. It can also be used to dynamize
the regenerate bone (compression-distraction) when there is
delayed consolidation (accordion maneuver). The reduction in
size of the implant down to 8.5-mm diameter as well as the
reduction in length of the device down to 195 mm makes
PRECICE applicable to pediatric femurs as young as 7 years
of age. The improvement in strength of the nail and the
mechanism have also made it more reliable, allowing for
weight-bearing.
Expert commentary
Implantable limb lengthening is the natural progression for
limb lengthening technology. Since I got involved with limb
lengthening in 1985, we have witnessed an evolution of tech-
niques related directly and indirectly to limb lengthen-
ing [37–39]. The gold standard external fixation device has
advanced with circular fixators being computer controlled
and automated [39], monolateral fixators having hinges and
spanning joints. Meanwhile, the implantable lengthening
devices have struggled to get FDA approval. Early devices
such as Bliskunov, Albizzia and Fitbone developed in Europe
asearlyasthe80sand90shavenevergotFDAclearance.
Therefore, the FDA clearance (510k) of the Orthofix ISKD
in 2001 was a landmark event. Unfortunately, the lack of
rate control and pain issues dampened the initial enthusiasm
with this device. The FDA clearance of PRECICE in
2011 represented the next major milestone for implantable
devices.Theappearanceofthisdeviceonthemarketwill
probably soon be followed by other such devices. PRECICE
A B
C
Figure 13. Photographs of two nails removed from a patient who underwent bilateral femoral lengthening. There is a crown
breakage on the right femur nail with propagation of cracks up from the nail slots (left side of figure) while the crown and slots remain
intact on the nail from the left femur (right side of figure) (A). The P2 nail anti-rotation mechanism uses four female ‘thru-slots’in the
distal end of the proximal nail tube mating with the crown’s four male ridges, and secured with tacking welds after assembly (B). The
new P 2.1 anti-rotation feature is machined into the inner diameter of the distal end of the proximal nail tube without breaking through
to the surface and without requiring welds (C).
PRECICE intramedullary limb lengthening system Device Profile
informahealthcare.com doi: 10.1586/17434440.2015.1005604
Expert Review of Medical Devices Downloaded from informahealthcare.com by 73.46.221.27 on 02/18/15
For personal use only.

however has raised the bar. To compete, a device will have to
demonstrate excellent rate control, forward and reverse capa-
bility, sufficient strength of the mechanism to resist the large
forces of the regenerate bone and musculature, and sufficient
strength to allow partial weight-bearing without breakage of
the implant.
Five-year view
Bliskunov first introduced implantable limb lengthening in
1983 (over 30 years ago). Therefore, the use of implantable
lengthening devices is still initsinfancy.AsPRECICEisthe
first limb lengthening nail to have forward and reverse capa-
bility,itisfairtocallitthefirstsecond-generationimplant-
able limb lengthening device. The first-generation devices
were ones that can only lengthen and distract but not
shorten and compress. Thus far, the indications for implant-
able lengthening have only been for lengthening of long
bones and for distraction of the spine for the treatment of
scoliosis [35]. The gold standard of distraction is the external
fixator. The external fixator is able to apply distraction for
many other indications. These include treatment of bone
defectsbybonetransport,treatmentofdeformitybyasym-
metric distraction, treatment of nonunions by compression-
distraction, etc. The next 5 years will see a plethora of new
remote controlled implantable devices and a wide variety of
drive mechanisms. We anticipate a bone transport nail, a
bone lengthening plate, an angular deformity plate, a trauma
nail that can correct mal-rotation and length, a nonunion
device that can compress-distract, implantable articulated dis-
traction of joints and joint contractures,etc.Currently,size
is a major limiting factor. External fixators or external cable-
driven implants are the only way to apply distraction tech-
nology to smaller bones such as in small children, forearm,
hand and foot, and cranio-maxillo-facial bones. The next
5 years will see miniaturization of remote control implant
technology.
Acknowledgements
The author wishes to thank C Hafner from Ellipse Technologies for his
help in the research and preparation of this manuscript.
Financial & competing interests disclosure
D Paley is a paid consultant for Ellipse Technologies and also receive royalties
on this device. The author has no relevant affiliations or financial involve-
ment with any organization or entity with a financial interest in or financial
conflict with the subject matter or materials discussed in the manuscript.
This includes employment, consultancies, honoraria, stock ownership or
options, expert testimony, grants or patents received or pending, or royalties.
Key issues
.Implantable distraction technology is reliable in producing distraction osteogenesis of bone and distraction histogenesis of soft
tissues.
.PRECICE is the first forward and reverse remote controlled implant for bone lengthening.
.PRECICE has excellent efficacy and rate control and reduces the pain of limb lengthening compared to external fixation.
.PRECICE reduces device-related complications seen with external fixation.
.Miniaturization of diameter and length has made it useable in smaller long bones such as the humerus, small patients such as children
and dwarfism.
.PRECICE is the first second-generation implantable lengthening device.
.Many new applications and devices are expected over the next 5 years.
References
Papers of special note have been highlighted as:
.of interest
.. of considerable interest
1. Cheung KM, Cheung JP, Samartzis D,
et al. Magnetically controlled growing rods
for severe spinal curvature in young
children: a prospective case series. Lancet
2012;379(9830):1967-74
2. Baumgart R, Betz A, Schweiberer L. A fully
implantable motoroized intramedullary nail
for limb lengthening and bone transport.
Clin Orthop Rel Res 1997;343:135-43
3. Singh S, Lahiri A, Iqbal M. The results of
limb lengthening by callus distraction using
an extending intramedullary nail (Fitbone)
in non-traumatic disorders. J Bone Joint
Surg Br 2006;88:938-42
4. Baumgart R, Betz A, Schweiberer L. A fully
implantable motorized intramedullary nail
for limb lengthening and bone transport.
Clin Orthop Rel Res 1997;343:135-43
5. Krieg AH, Speth BM, Foster BK. Leg
lengthening with a motorized nail in
adolescents: an alternative to external
fixators? Clin Orthop Rel Res 1994;14:
352-7
6. Young N, Bell DF, Anthony A. Pediatric
pain patterns during Ilizarov treatment of
limb length discrepancy and angular
deformity. J Pediatr Orthop 1994;14:
352-35
7. Garcia-Ciembrelo E, Olsen B,
Ruiz-Yague M, et al. Ilizarov technique:
result and difficulties. Clin Orthop Rel Res
1992;283:116-23
8. Simpson AH, Shalaby H, Kennan G.
Femoral lengthening with the
intramedullary skeletal kinetic distractor.
J bone Joint Surg Br 2009;91:955-61
9. Sabharwal S, Rozbruch SR. What’s new in
limb lengthening and deformity correction?
J Bone Joint Surg Am 2011;93:2323-232
10. Gross RH. Leg length discrepancy: how
much is too much? Orthopedics 1978;1:
307-10
11. Torode IP, Gillespie R. Classification and
management of congenital abnormalities of
Device Profile Paley
doi: 10.1586/17434440.2015.1005604 Expert Rev. Med. Devices
Expert Review of Medical Devices Downloaded from informahealthcare.com by 73.46.221.27 on 02/18/15
For personal use only.

the femur. J Bone Joint Surg Br 1983;65:
557-68
12. Kalamachi A, Cowell HR, Kim KI.
Congenital deficiency of the femur. J
Pediatr Orthop 1991;11:90-4
13. Pappas AM. Congenital abnormalities of the
femur and related lower extremity
malformations: classification and treatment.
J Pediatr Orthop 1983;3:45-60
14. Boakes JL, Stevens PM, Moseley RF.
Treatment of genu valgus deformity in
congenital absence of the fibula. J Pedatr
Orthop 1991;11:721-4
15. Froster UG, Baird PA. Congenital defects of
lower limbs and associated malformations:
a population based study. Am J Med Genet
1993;45:60-4
16. Rogala EJ, Wynne-Davies R, Littlejohn A,
Gormley J. Congenital limb abnormalities:
frequency and aetiological factors: data from
the Edinburgh Register of the Newborn
(1964-68). J Med Genet 1974;11:221-33
17. Paley D, Herzenberg JE, Paremain G,
Bhave A. Femoral lengthening over an
intramedullary nail. A matched-case
comparison with Ilizarov femoral
lengthening. J Bone Joint Surg Am 1997;
79(10):1464-80
18. Paley D. Problems, obstacles, and
complications of limb lengthening by the
Ilizarov technique. Clin Orthop Rel Res
1990;250:81-104
.This is the first study to classify and
categorize complications of limb
lengthening according to their level of
significance.
19. Paley D, Harris M, Debiparshad K,
Prince D. Limb lengthening by implantable
limb lengthening devices. Techniques in
Orthopaedics 2014;29(2):72-85
.. This is the first and largest clinical series
of PRECICE lengthenings studied and
published. It also presents the technique
as well as the complications well.
20. Harris M, Paley D, Prince D. New
implantable lengthening nail. Presented at:
EPOS Annual Meeting. Athens, Greece.
April 2013
21. Herzenberg JE, Specht SC, Standard SC,
Conway JD. Limb lengthening with a new
internal magnetic device for post-traumatic
injury. Presented at: AAOS/OTA/SOMOS/
ORS Extremity War Injuries VIII: sequelae
of Combat Injuries Research Symposium.
Washington, DC, USA. February 2013
22. Herzenberg JE, Specht SC, Standard SC.
Limb lengthening in children with a new,
controllable, internal device. Presented at:
EPOS Annual Meeting. Athens, Greece.
April 2013
23. Kirane Y, Fragomen A, Rozbruch SR.
Precision of the new remote controlled
internal lengthening nail. Presented at: HSS
Research Symposium. New York, NY. June
2013
.. This study confirms the reliability and
efficacy of the PRECICE mechanism
especially with regard to rate.
24. Shabtai L, Specht SC, Standard SC,
Herzenberg JE. Internal lengthening device
for congenital femoral deficiency and fibular
hemimelia. Clin Orthop Relat Res 2014;
472(12):3860-8
.. This study demonstrates that excellent
results can be achieved with PRECICE
even when lengthening for congenital leg
length discrepancy.
25. Kirane YM, Fragomen AT, Rozbruch SR.
Precision of the PRECICEInternal bone
lengthening nail. Clin Orthop Relat Res
2014;472(12):3869-78
26. Schiedel FM, Vogt B, Tretow HL, et al.
How precise is the PRECICE compared to
ISKD in intramedullary limb lengthening?
Acta Orthop 2014;85(3):293-8
27. Kubiak E, Strauss E, Grant A, et al. Early
complications encountered using a
self-lengthening intramedullary nail for the
correction of limb length inequality. Jt Dis
Relat Surg 2007;18:52-7
28. Kenawey M, Krettek C, Liodakis E, et al.
Insufficient bone regenerate after
intramedullary femoral lengthening: risk
factors and classification system. Clin
Orthop Rel Res 2010;469:264-73
29. Mahboubian S, Seah M, Fragomen AT,
Rozbruch SR. Femoral lengthening with
lengthening over a nail has fewer
complications than intramedullary skeletal
kinetic distraction. Clin Orthop Rel Res
2012;470:1221-31
30. Hankmeier S, Pape HC, Gosling T, et al.
Improved comfort in lower limb
lengthening with the intramedullary skeletal
kinetic distractor. Principles and preliminary
clinical experiences. Arch Orthop Trauma
Surg 2004;124:129-33
31. Wang K, Edwards E. Intramedullary skeletal
kinetic distractor in the treatment of leg
length discrepancy – A review of 16 cases
and analysis of complications. J Orthop
Trauma 2012;26(9):138-44
32. Simpson AH, Kenwright J. Fracture after
distraction osteogenesis. J Bone Joint Surg
Br 2000;82:659-65
33. Burghardt RD, Herzenberg JE, Specht SC,
Paley D. Mechanical failure of the
intramedullary skeletal distractor in limb
lengthening. J Bone Joint Surg Br 2011;93:
639-43
34. Cole JD, Justin D, Kasparis T, et al. The
intramedullary skeletal kinetic distractor
(ISKD) : first clinical results of a new
intramedullary nail for lengthening of the
femur and tibia. Injury 2001;32(suppl 4):
SD129-39
35. Krieg AH, Speth BM, Foster BK. Leg
lengthening with a motorized nail in
adolescents: an alternative to external
fixators? Clin Orthop Rel Res 2008;466:
189-97
36. Guichet JM, Deromedis B, Donnan LT,
et al. Gradual femoral lengthening with the
Albizzia nail. J Bone Joint Surg Am
2003;85:838-48
37. Paley D. Progress in and from Limb
Lengthening. In: Current Progress in
Orthopedics. Tree Life Media, India; 2014
38. Burghardt RD, Paley D, Specht SC,
Herzenberg JE. The effect on mechanical
axis deviation of femoral lengthening with
an intramedullary telescopic nail. J Bone
Joint Surg Br 2012;94(9):1241-5
39. Kirane Y, Fragomen A, Rozbruch R.
Precision of the PRECICE internal bone
lengthening nail. Clin Orthop Relat Res
2014;472(12):3869-78
PRECICE intramedullary limb lengthening system Device Profile
informahealthcare.com doi: 10.1586/17434440.2015.1005604
Expert Review of Medical Devices Downloaded from informahealthcare.com by 73.46.221.27 on 02/18/15
For personal use only.
This article may include discussion of both on and off-label uses of our products. Please note that this study may have limitations
whether it be the study-size, endpoints, lack of randomization, period of follow-up, etc. Discussion in this article may also provide
only anecdotal information. This article may be the result of company-sponsored research or grants provided to physicians who
conducted the research on their own. Some authors may be consultants to Ellipse-Tech, others are not. The reader should
understand these limitations before reading them and critically review all these sources of information.
The FDA-cleared intended use for PRECICE is as follows:
The Ellipse Technologies PRECICE Intramedullary Limb Lengthening System is indicated for limb lengthening of the tibia or femur.
Please see the full prescribing information (IFU) for the contraindications, side effects, warnings and precautions.