Foot And Ankle Biologic Solutions 1
Foot And Ankle Biologic Solutions 1 foot_and_ankle_biologic_solutions_1 foot_and_ankle_biologic_solutions_1 3 2013 pdf 258413772373414384
Foot And Ankle Biologic Solutions foot_and_ankle_biologic_solutions foot_and_ankle_biologic_solutions 3 2013 pdf 258413772373414384
Foot And Ankle Biologic Solutions foot_and_ankle_biologic_solutions foot_and_ankle_biologic_solutions 2 2013 pdf 258413772373414384
2013-02-24
: Pdf Foot And Ankle Biologic Solutions 1 foot_and_ankle_biologic_solutions_1 2 2013 pdf
Open the PDF directly: View PDF ![]() .
.
Page Count: 8

Bone Graft Substitutes
Soft Tissue Repair
Foot & Ankle
Midfoot
Rearfoot
Forefoot
Ankle
Foot & Ankle
Vitoss
Vitoss is the #1 selling Synthetic Bone Gra for one
good reason... IT WORKS.1
Human clinical data supports its ecacy2—over 425,000
implantations worldwide.3
Vitoss BA has a unique porosity, bioactivity, and
chemistry. Literature shows that bioactive glass stimulates
osteoblastic activity and facilitates bone formation in
in-vitro and animal models.4-6 Upon implantation, the
ionic constituents (Si+, Na+, Ca2+) of bioactive glass are
released into the surrounding environment and react with
the bodily uids.7-10 is reaction produces the deposition
of a thin layer of physiologic calcium phosphate at its
surface, favorable for osteoblast attachment.11 is is
commonly referred to as an osteostimulatory eect.4-6, 9, 12-14
In a direct comparison animal study, glass demonstrated
accelerated healing when bioactive glass was added to an
osteoconductive scaold.15
HydroSet
HydroSet is a calcium phosphate cement that converts to
hydroxyapatite, the principle mineral component of bone.
It is injectable, sculptable and fast setting.
HydroSet can be drilled and tapped to accommodate
the placement of provisional hardware during the surgical
procedure.
Specically formulated to set in a wet eld environment,
HydroSet exhibits outstanding wet-eld characteristics.16
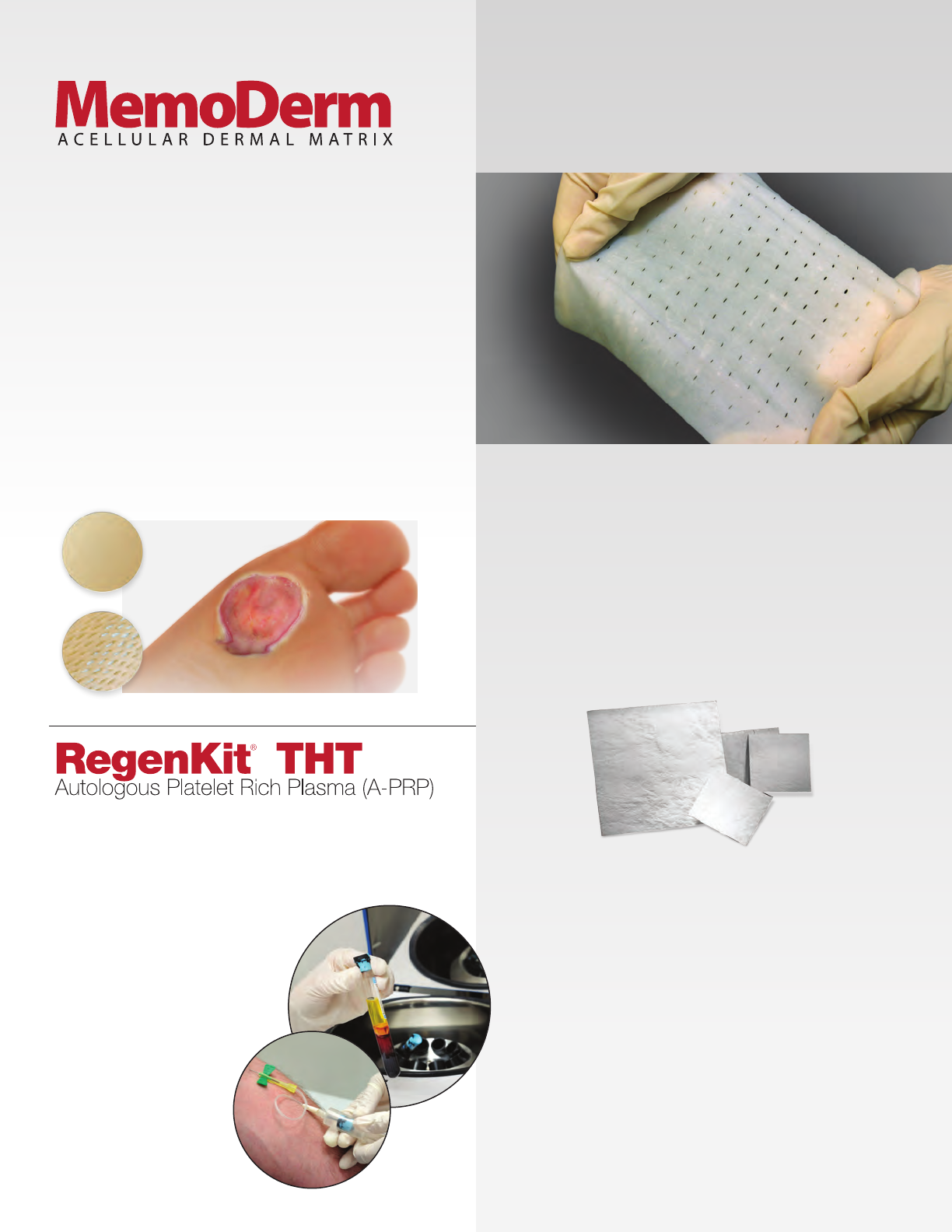
MemoDerm
MemoDerm is a sterile acellular dermal matrix derived from
human allogra skin tissue, specically designed to play a key
role in the revascularization and repopulation of cells.
e unique processing method removes the epidermal layer
and dermal cells, minimizing the potential for an immunogenic
response.
TissueMend
By combining physical properties of strength and thickness
with biologic properties that encourage cell and blood vessel
penetration, the TissueMend collagen matrix is designed to
handle the vigorous demands of tendon repair.
Over time, normal tissue remodeling occurs where TissueMend
is incorporated into the native tissue and ultimately replaced by
natural, healthy, native cells and tissue.
PriMatrix
PriMatrix is an acellular collagen matrix that provides an
environment to support cell repopulation and revascularization
during the wound healing process.
PriMatrix uses a proprietary process designed to remove
cellular components and potentially infectious agents from
raw material, while preserving the biological properties and
structure of native collagen.
PriMatrix can be cut into strips to eciently ll deep or
tunneled wounds.

4 | Bone and Tissue
Foam Pack
Vitoss Foam Pack is a versatile material that is
stable in a uid environment, can soak and hold
bone marrow, and is compression-resistant.
Foam Strip
Vitoss Foam Strip is a compression-resistant, pre-
formed strip that is exible when wet, can soak
and hold bone marrow, and is easily customized
for various graing applications.
Morsels
Vitoss Morsels are an economical way to provide a
quality synthetic product to your patients for large
volume graing applications. Vitoss Morsels oer
a cost comparative option to allogra chips.
Canisters
Vitoss Canisters oer the handling and delivery
of Vitoss Morsels with the use of bone marrow
aspirate or blood. It is a closed system designed
to minimize handling and exposure to potential
contaminants.
Vitoss Products
Vitoss BA features a combination of porosity, structure, bioactivity
and chemistry. As a calcium phosphate that is up is to 90% porous17,
Vitoss’s interconnected porosity allows for 3-D regeneration of bone,
as opposed to creeping substitution. e increased porosity has been
shown to lead to higher fusion rates in an animal study.18
Literature shows that bioactive glass stimulates osteoblastic activity
and facilitates bone formation in in-vitro and animal models.4-6 Upon
implantation, the ionic constituents (Si+, Na+, Ca2+) of bioactive
glass are released into the surrounding environment and react with
the bodily uids.7-10 is reaction produces the deposition of a
thin layer of physiologic calcium phosphate at its surface, favorable
for osteoblast attachment.11 is is commonly referred to as an
osteostimulatory eect.4-6, 9, 12-14 In a direct comparison animal study,
glass demonstrated accelerated healing when bioactive glass was
added to an osteoconductive scaold.15
Chemistry aects the rate of resorption. Bone gras should resorb as
new bone forms in a physiologic time frame. Vitoss is composed of
β-TCP and is stable at physiologic pH. It resorbs during the natural
remodeling process of bone.
Stryker Foot & Ankle | 5
Product Specifications
Chemical Composition
Powder: Dicalcium phosphate dihydrate, Tetracalcium
phosphate and Tri-sodium citrate
Liquid: Sodium phosphate, Polyvinylpyrrolidone and water
Packaging Contains: Bowl of powder, Liquid-lled syringe,
Delivery syringe, Cannula and spatula.
Shelf Life: 1 year Sterilization: Ethelyne oxide and
Gamma irradiation
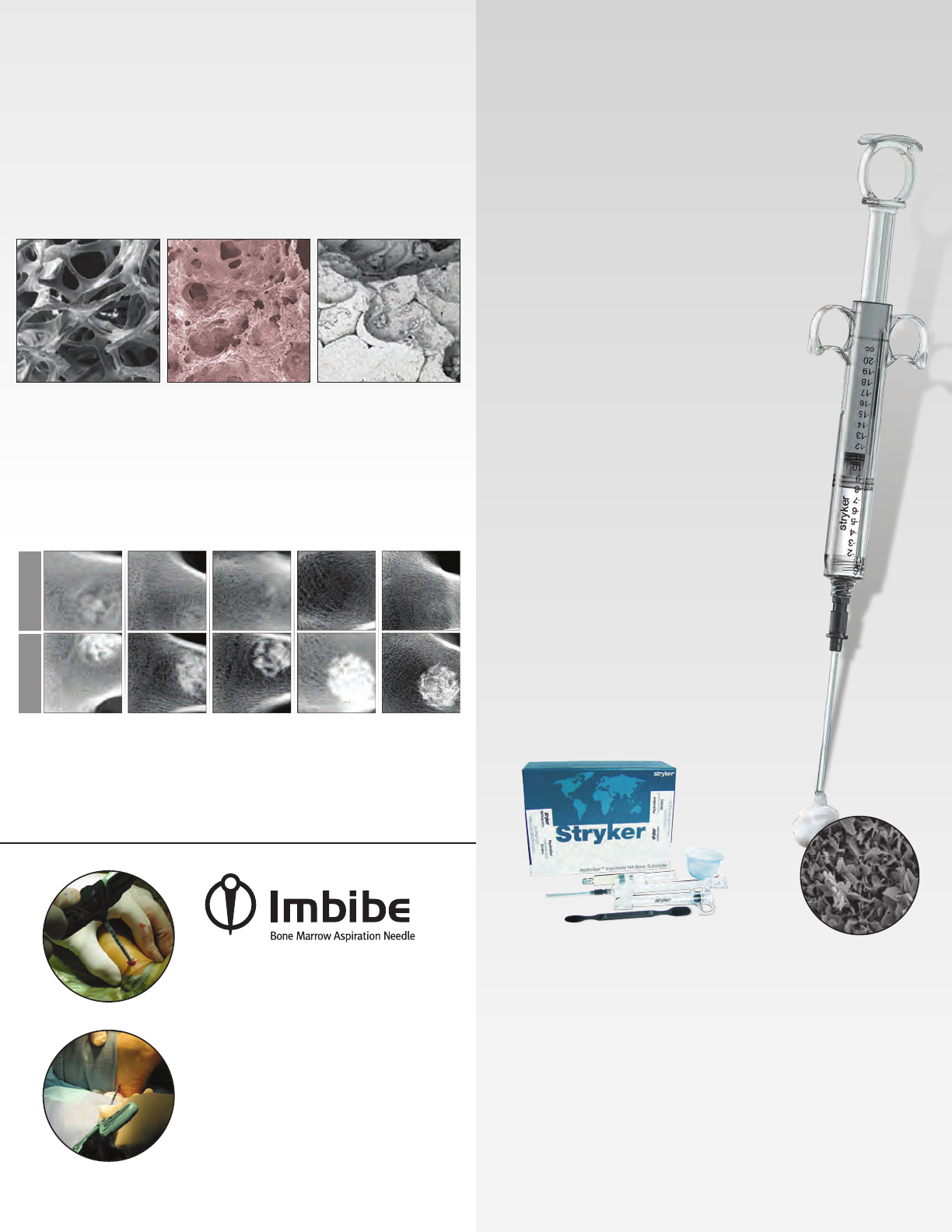
Imbibe is a system of disposable bone
marrow aspiration devices. e Imbibe
bone marrow aspiration needles
provide a minimally invasive way to
harvest bone marrow. e needles
come with both bullet-tip and sharp-
trocar stylets, which are color coded.
Bone marrow aspirate harvested using
Imbibe bone marrow aspirate syringes
can then be used to rehydrate Vitoss.
®
HydroSet is a self-setting calcium phosphate cement
indicated to ll bony voids or gaps of the skeletal
system—extremities, craniofacial, spine, and pelvis.
HydroSet is indicated only for bony voids or gaps that
are not intrinsic to the stability of the bony structure.
HydroSet cured in situ provides an open void/gap
ller than can augment provisional hardware—
K-Wires, plates, screws—to help support bone
fragments during the surgical procedure. e
cured cement acts only as a temporary support
media and is not intended to provide structural
support during the healing process.
HydroSet can be easily implanted via simple
injection or manual application techniques for a
variety of applications. It is specically designed
to set quickly once implanted under normal
physiological conditions. HydroSet does not
release any heat as it sets, preventing potential
thermal injury.
• HydroSetischemicallyformulatedtosetina
wet eld environment,16 eliminating the need
to meticulously dry the operative site prior
to implantation.
• ecompositionofhydroxyapititeclosely
matches that of bone mineral, thus imparting
osteoconductive properties.20
• HydroSetcanbedrilledandtappedto
accommodate the placement of provisional
hardware during the surgical procedure.
3 Weeks 6 Weeks 12 Weeks 24 Weeks 52 Weeks
Bone Vitoss Other Scaffolds
Iliac Crest (PSIS, ASIS)
Calcaneus
Vitoss
Comparison of Vitoss to competitive gra in a canine metaphyseal study
in order to radiologically compare healing at 3, 6, 12, 24, and 52 weeks.
A 10mm x 22mm drill defect was created in the canine proximal
humerus and lled with 2cc of bone gra.19
Materials with interconnected porosity will allow for 3-D regeneration
of bone as opposed to creeping substitution. Vitoss is a highly porous
calcium-phosphate (up to 90% porous).17 Additionally, increased porosity
has been shown to lead to higher fusion rates in an animal model.18
Not All Scaffolds are Created Equal
Porosity
HydroSet®
Injectable HA Bone Substitute
Competitive
Graft
6 | Bone and Tissue
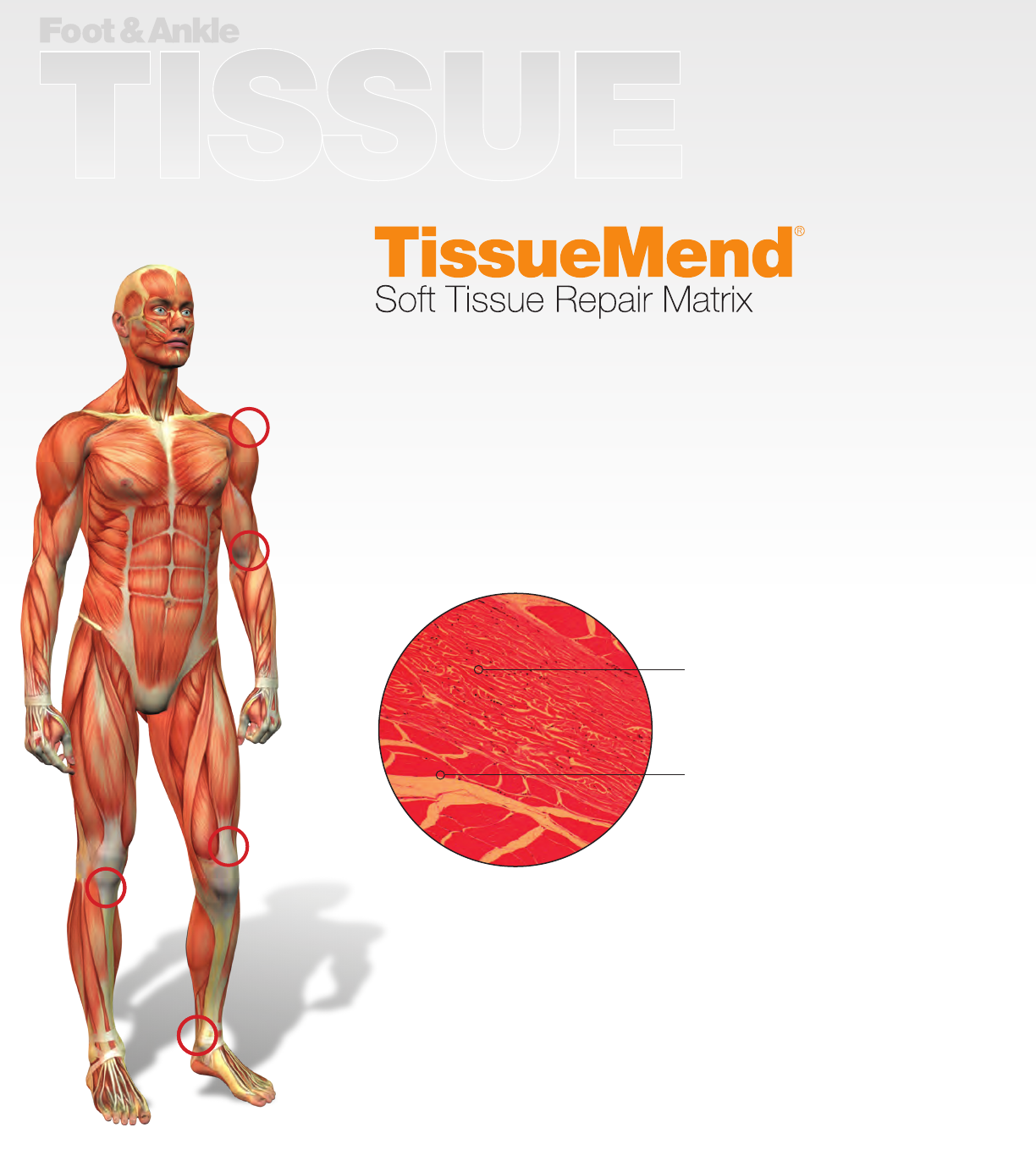
e TissueMend So Tissue Repair Matrix is an acellular, collagen
membrane used to repair and reinforce so tissues where weakness exists.
A unique collagen composition and structure makes TissueMend an
excellent biologically inspired scaold for tissue remodeling. Non-artical
cross-linking helps to provide a medium for normal cellular growth and
maturity, allowing for the development of new, healthy tissue.
When implanted, TissueMend provides an environment where host cells
and supporting vasculature can take up residence and mature. Over time,
normal tissue remodeling occurs where TissueMend is incorporated into the
native tissue and ultimately replaced by native collagen bers.
As a direct result of its technologically advanced production process,
the TissueMend So Tissue Repair Matrix has demonstrated a lack of
inammation and foreign body reaction when implanted into native tissue.21
• Composedof99%pure,non-denaturedcollagen
• Availableasarectangularpatchwithdimensionsof
3cm x 3cm, 5cm x 6cm, or 6cm x 10cm
• Asaresultofitsthickness,externalforcesaredistributed
over a greater surface area
• Canbeeasilycutandtrimmedbothbeforeandaer
gra hydration
• Hydratesinseconds
• Non-crosslinked
• RichinTypeIIICollagen
• Potentialforreducedscarring22
Host fibroblasts have
migrated into and populated
TissueMend beginning the
remodeling process.
Note the lack of foreign body
reaction to TissueMend.
Inflammatory cells are absent
and the implant has not been
encapsulated.
Three month rat intramuscular
biocompatibility evaluation
Stryker Foot & Ankle | 7
PriMatrix
PriMatrix is an acellular collagen matrix derived from fetal bovine
dermis. is biologic matrix helps provide an environment to
support the cell repopulation and revascularization process
essential to wound healing. In addition to non-denatured Type I
collagen, PriMatrix is rich in Type III collagen, which is active in
developing and healing tissues. e product delivers this unique
fetal collagen composition and architecture into the wound bed.
PriMatrix uses a proprietary process designed to remove cellular
components and potentially infectious agents from raw material,
while preserving the biological properties and structure of
native collagen.
PriMatrix is oered in a variety of sizes and congurations; large
and small pieces may be applied. PriMatrix can be cut into strips
to eciently ll deep or tunneled wounds. All congurations
of PriMatrix may be used in conjunction with vacuum-assisted
wound healing systems.
• Availableinbothfenestratedandnon-fenestratedversions
• ree-yearshelflife
• Biocompatibleandcell-friendlywithnoarticial
chemical crosslinking
• Terminallysterilized
Providing an Environment to Manage Wound Healing
MemoDerm Acellular Dermal Matrix is derived from
human allogra skin tissue that is terminally sterilized.
e unique processing method removes the epidermal and
dermal cells, minimizing the potential for an immunogenic
response. e resulting acellular dermal matrix has the
natural histomorphology preserved.
MemoDerm is specically designed to play a key role in the
revascularization and repopulation of cells. e proprietary
gamma irradiation sterilization process does not damage the
matrix. e cellular elements, typically considered
immunogenic, are absent.
• ProprietaryGammaPrecisionDoseSterilizationprocess
provides a Sterility Assurance Level (SAL) of 10-6
• ExcellentTensileStrength
• ExcellentSutureRetentionStrength
• Indicatedfortherepairorreplacementofdamagedor
inadequate integumental tissue. Note - meshed MemoDerm
is to be used for wound care only (burns, ulcers)
e RegenKit THT is designed to be used for the safe and
rapid preparation of autologous platelet-rich plasma (A-PRP)
from a small sample of blood at the patient’s point of care.
Aer the simple 4-step preparation, the A-PRP can be applied
to an orthopedic surgical site, as deemed necessary by clinical
use requirements.
Features and Potential Benets
• Simplicity
• Lowbloodvolumerequired
• Optimumplateletrecovery23
• Consistentisolationof
platelet concentrate
and plasma23
• Leukocytesat
physiological levels23
• Erythrocytedepletion23
Non-Meshed
Meshed
PriMatrix™
Dermal Repair Matrix
®

Joint Replacements
Trauma & Extremities
Craniomaxillofacial
Spine
Biologics
Surgical Products
Neuro & ENT
Interventional Spine
Navigation
Endoscopy
Communications
Imaging
Patient Care & Handling Equipment
EMS Equipment
Distributed by:
Stryker
325 Corporate Drive
Mahwah, NJ 07430
t: 201 831 5000
www.stryker.com
Vitoss is manufactured by:
Stryker Orthobiologics
77 Great Valley Parkway
Malvern, PA 19355
Vitoss
Foam pack - Also available in Vitoss BA2X** / BA*
1.2cc ...........................**2102-2101
*2102-1601, 2102-1401
2.5cc ...........................**2102-2102
*2102-1602, 2102-1402
5cc... ...........................**2102-2105
*2102-1605, 2102-1405
10cc ............................**2102-2110
*2102-1610, 2102-1410
Foam Strip - Also available in Vitoss BA*
25 x 100 x 4mm - 10cc *2102-1500
25 x 100 x 4mm - 10cc.. 2102-1100
25 x 100 x 8mm - 20cc *2102-1520
25 x 100 x 8mm - 20cc.. 2102-1120
25 x 240 x 4mm - 24cc.. 2102-1101
25 x 50 x 4mm - 5cc ..... *2102-1505
25 x 50 x 4mm - 5cc ...... 2102-1105
25 x 50 x 8mm - 10cc ... *2102-1510
25 x 50 x 8mm - 10cc .... 2102-1110
75 x 100 x 4mm - 30cc.. 2102-1130
Morsels
15cc Macro .................... 2102-0020
30cc Macro .................... 2102-0021
1.2cc Blocks .................. 2102-0013
10cc Blocks ................... 2102-0006
30cc Macro (x10) ........... 2102-0131
Canisters
5cc Micro ....................... 2102-0026
5cc Standard ................. 2102-0030
10cc Micro ..................... 2102-0027
10cc Standard ................ 2102-0031
15cc Micro ..................... 2102-0028
15cc Standard ................ 2102-0032
30cc Micro ..................... 2102-0029
30cc Standard ................ 2102-0033
Imbibe
Syringes
10cc .............................. 2105-0010
20cc .............................. 2105-0020
30cc .............................. 2105-0030
Needles
11 gauge x 4in ............... 2090-0027
11 gauge x 6in ............... 2090-0028
8 gauge x 6in ................. 2090-0029
8 gauge x 8in ................. 2090-0047
Fenestrated
8 gauge x 6in ................. 2090-0030
TissueMend
5cm x 6cm .................... 6495-9-001
6cm x 10cm .................. 6495-9-004
3cm x 3cm .................... 6495-9-006
MemoDerm
Non-Meshed
2cm x 4cm (0.33-0.61mm thickness)
HTM0330204
4cm x 8cm (0.80-1.40mm thickness)
HTM0800408
5cm x 5cm (0.80-1.20mm thickness)
HTM0900505
5cm x 5cm (1.00-2.00mm thickness)
HTM1500505
5cm x 10cm (2.00-3.50mm thickness)
HTM2000510
Meshed
4cm x 4cm (0.40-0.80mm thickness)
HTM0400404M
4cm x 8cm (0.40-0.80mm thickness
HTM0400408M
RegenKit THT
RegenKit THT ................ 8495-9-001
Universal Centrifuge ....... 8495-9-010
Balancing Tube 3Pk ....... 8495-9-998
PriMatrix
4cm x 4cm .................... 6495-9-104
6cm x 6cm .................... 6495-9-106
8cm x 8cm .................... 6495-9-108
4cm x 4cm Fenestrated . 6495-9-114
6cm x 6cm Fenestrated . 6495-9-116
8cm x 8cm Fenestrated . 6495-9-118
1. Millennium Research Group: US Markets for Orthopedic
Biomaterials
2. Vitoss Bibliography, P/N 5606-011 7 Rev. 00, 2010.
3. Stryker Orthobiologics Internal Sales Data, May 2012.
4. Hench, L.L., Splinter, R.J., and Allen, W.C., Bonding Mechanisms at
the Interface of Ceramic Prosthetic Materials. Journal of Biomedical
Materials Research, 1971; 2(1): 117-141.
5. Hench, L.L., Paschall, H.A., Direct Chemical Bond of Bioactive
Glass-Ceramic Materials to Bone and Muscle.Journal of Biomedical
Materials Research, 1973; 4: 25-42.
6. Gross, U., e Interface of Various Glasses and Glass Ceramics with a
Bony Implantation Bed. Journal of Biomedical Materials Research,
1985; 19: 251-271.
7. Hench, L.L., e Story of Bioglass. Journal of Materials Science:
Materials in Medicine, 2006 Nov; 17(11): 967-78.
8. Oonishi, H., et al., Particulate Bioglass Compared with
Hydroxyapatite as a Bone Gra Substitute. Clinical Orthopaedics
and Related Research, 1997 Jan; 334: 316-25.
9. Vrouwenvelder, W.C.A., Histological and Biochemical Evaluation of
Osteoblasts Cultured on Bioactive Glass, Hydroxyapatite, Titanium
Alloy, and Stainless Steel. Journal of Biomedical Materials Research,
1993 Apr; 27(4): 465-75.
10. Xynos, I.D., Edgar, A.J., Buttery, L.D.K., Hench, L.L., and Polak, J.M.,
Ionic Products of Bioactive Glass Dissolution Increase Proliferation
of Human Osteoblasts and Induce Insulin-like Growth Factor
II mRNA Expression and Protein Synthesis. Biochemical and
Biophysical Research Communications, 2000 September 24; 276(2):
461-5.
11. Hench, L.L., Polak, J.M., Xynos, I.D., Buttery, L.D.K., Bioactive
Materials to Control Cell Cycle. Materials Research Innovations,
2000; 3(6): 313-323.
12. Sanders, D.M., Hench, L.L., Mechanisms of Glass Corrosion. Journal
of American Ceramic Society. 1973; 56(7): 373-377.
13. Hench, L.L., Characterization of Glass Corrosion and Durability.
Journal of Non-Crystalline Solids, 1975; 19: 27-39.
14. Ogino, M., Hench, L.L., Formation of Calcium Phosphate Films on
Silicate Glasses. Journal of Non-Crystalline Solids, 1980; 38 and 39:
673-678. Spinal Fusion Model. European Spine Journal, 2007; 16:
2215-2224.
15. Havener, M.B., Brown, L.S., Darmoc, M.M., Owsianny, R.S.,
Cline, T.D,, Improvements in Healing with a Bioactive Bone Gra
Substitute in a Canine Metaphyseal Defect. 55th Annual Meeting of
the Orthopaedic Research Society, 2009.
16. 1808.E703
17. Orthovita Test Report P/N 1070-0008R.
18. Motomiya, M., et al., Eect of Hydroxyapatite Porous Characteristics
on Healing Outcomes in Rabbit Posterolateral Spinal Fusion Model.
European Spine Journal, 2007; 16: 2215-2224.
19. Havener, MB, Cline, TD, Darmoc, MM, Brown, LS, Owsiany, R, A
Comparative Study of Synthetic Bone Gra Substitutes in a Canine
Metaphyseal Defect. 54th Annual Meeting of the Orthopaedic
Research Society, 2008.
20. Dikson, K.F., et al. e Use of BoneSource Hydroxyapatite Cement
for Traumatic Metaphyseal Bone Void Filling. J Trauma 2002;
53:1103-1108.
21. Zerris,V., et al., Repair of the dura mater with processed collagen
devices. Journal of Biomedical Materials Research Part B: Applied
Biomaterials, 2007. 83(2): p. 580-8.
22. Larson et al. “Scarless Fetal Wound Healing: A Basic Science
Review” Plastic and Reconstructive Surgery 126(4) 1172-1180,
2010.)
23. Study Report – Regen™ THT Tube Performance Testing, USFDA
510(k) BK090048, May 2010, Data on le at RegenLab, Switzerland
DESCRIPTION ................... PART NUMBER
References:
A surgeon must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a
particular patient. Stryker does not dispense medical advice and recommends that surgeons be trained in the use of any particular product before
usingitinsurgery.•einformationpresentedisintendedtodemonstratethebreadthofStrykerproductoerings.Asurgeonmustalwaysreferto
the package insert, product label and/or instructions for use before using any Stryker product. Products may not be available in all markets because
product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your Stryker representative if you have
questionsabouttheavailabilityofStrykerproductsinyourarea.•Caution:Federallawrestricsthisdevicetosalebyorontheorderofaphysician.
Foradditionalsafetyinformation,pleaseseetheVitossProductInstructionsforUse.•StrykerCorporationoritsdivisionsorothercorporate
aliated entities own, use or have applied for the following trademarks or service marks: HydroSet, Imbibe, MemoDerm, Stryker, Vitoss. All other
trademarks are trademarks of their respective owners or holders. Lit.#NL12-BR-JP-581 Copyright ® 2012 Stryker