Patient Reported Outcome Measures Of The Corail Pinnacle Construct 1
2016-12-06
: Pdf Patient Reported Outcome Measures Of The Corail Pinnacle Construct 1 1 Patient_Reported_Outcome_Measures_of_the_Corail_Pinnacle_Construct_1_1 12 2016 pdf
Open the PDF directly: View PDF ![]() .
.
Page Count: 4

1
PATIENT REPORTED OUTCOME MEASURES
OF THE CORAIL®/PINNACLE® HIP CONSTRUCT
John Leopold, MS, Biostatistician | DePuy Synthes Joint Reconstruction
INTRODUCTION
The CORAIL® Hip System was designed to accommodate
a metal or ceramic femoral head, and the PINNACLE®
Modular Acetabular Cup System was designed to
accommodate a polyethylene, or ceramic liner. The
combination of a CORAIL Hip System and a PINNACLE
Acetabular Cup System together with the various head
and liner options comprises a modular system which has
been commercially available for 15 years. The modularity
of this system provides surgeons with options in primary
total hip arthroplasty (THA), which may potentially
reduce the need to revise an otherwise well positioned,
well-fixed stem or cup in a revision procedure.
The purpose of this clinical brief is to present Patient
Reported Outcome Measures (PROMs) from the National
Joint Registry for England, Wales, Northern Ireland and
the Isle of Man (NJR) for primary THA with the CORAIL/
PINNACLE Hip construct and cementless fixation in
articulation constructs which are currently available
(CoC*, CoP, and MoP).
METHODS
The NJR is a large national joint replacement registry
which has been established for the purpose of tracking
the outcomes of total joint replacement procedures.
Historically, survivorship of the implant was the only
outcome tracked by the NJR. However, in recent years
PROMs have become increasingly important in the
monitoring of an implanted device by measuring the
improvement in quality of life. As such, PROMS have
been routinely collected by all providers of publically-
funded care in England since April 2009. The PROMs
questionnaire is administered twice; an initial
questionnaire prior to surgery and then again
approximately six months following the operation.
PROMs collected include the Oxford score for Hips and
Knees, the EQ-5D Index and VAS scores, and Success
and Satisfaction questions. The Oxford Hip Score1 is an
established hip focused quality of life score, while the
EQ-5D2 is a standardized instrument used to measure
general health outcomes, applicable to a wide range of
health conditions and treatments. All EQ-5D Index
scores are calculated using UK specificcoefficients.
The NJR has published class-level PROMs results in their
2013 annual report.3 Additionally, the NJR provides
companies with data on their own products,4 with
which product-level analyses can be conducted. An
analysis of NJR CORAIL/PINNACLE Hip construct data
was performed by DePuy Synthes and compared with
the class-level published results.
Results from the analysis of NJR data include primary
THA procedures with the CORAIL/PINNACLE Hip
construct and cementless fixation in articulation
constructs which are currently available (CoC, CoP, and
MoP) for which both the preoperative and 6 month
Oxford and EQ-5D scores were completed. Similar
search criteria were used for the published class-level
results, with details available in the 2013 Annual Report.
Median and interquartile ranges (IQR) for continuous
scores and frequency counts for categorical questions
are presented and compared to all primary hips due to
the availability of published summaries for all PROMs.
RESULTS
There were a total of 11,576 CORAIL/PINNACLE Hip
constructs for which pre-operative and 6 month PROMs
were complete. These were on surgeries done from
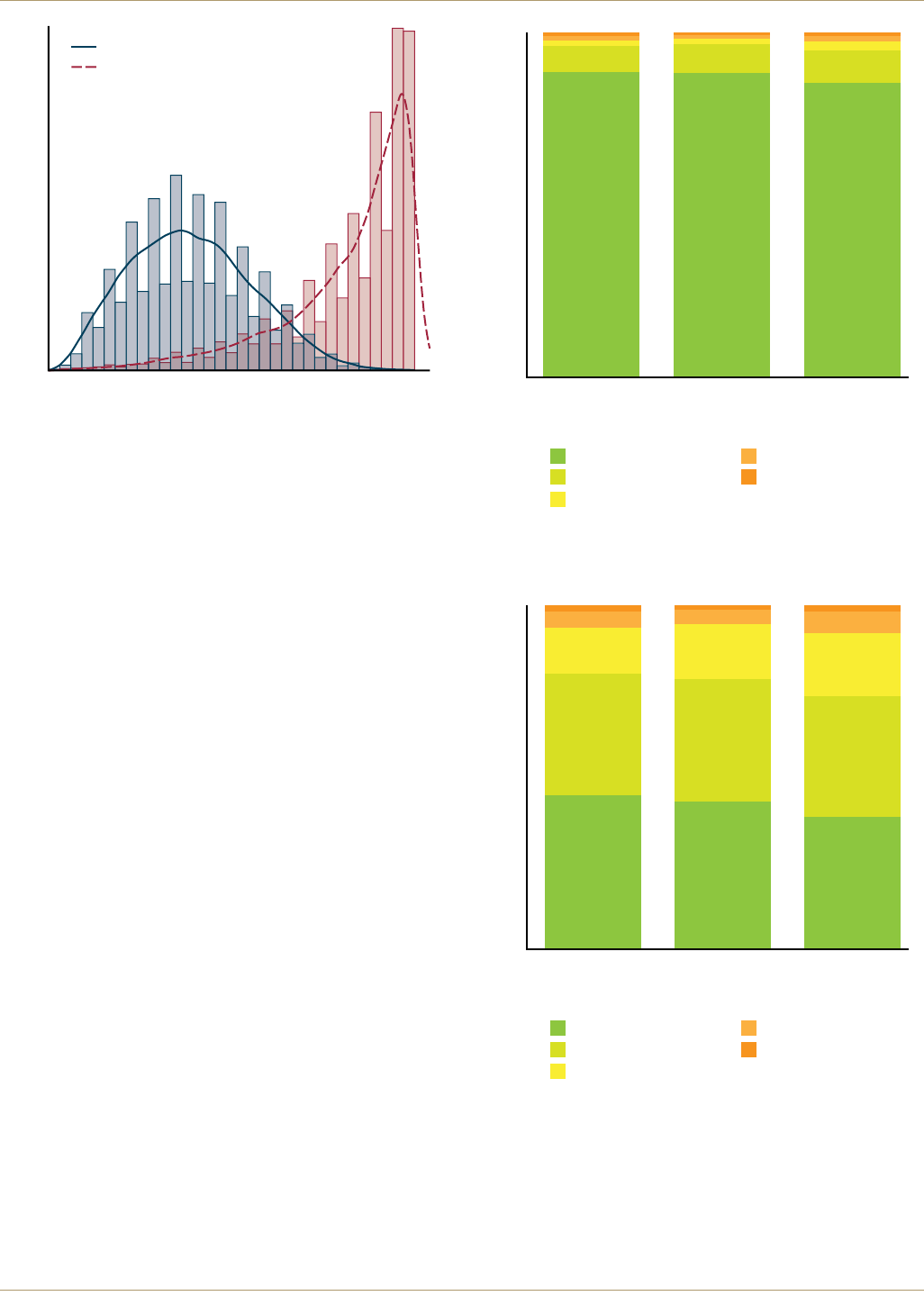
September 2008 through October 2012. The median
pre-operative Oxford hip score was 18, the median 6
month score was 43 with a median improvement of 22
points for CORAIL/PINNACLE Hip constructs. The
median pre-operative score was identical to the class of
all primary hips, with CORAIL/PINNACLE Hip constructs
having a 1 point greater median improvement.4
* THE CORAIL TOTAL HIP SYSTEM IS not approved for use with a
ceramic-on-ceramic system in the United States.
General health improvement, as measured by the EQ-5D
Index and VAS scores, was similar for both the CORAIL/
PINNACLE Hip construct and All Primary Hips. (Table 1)4
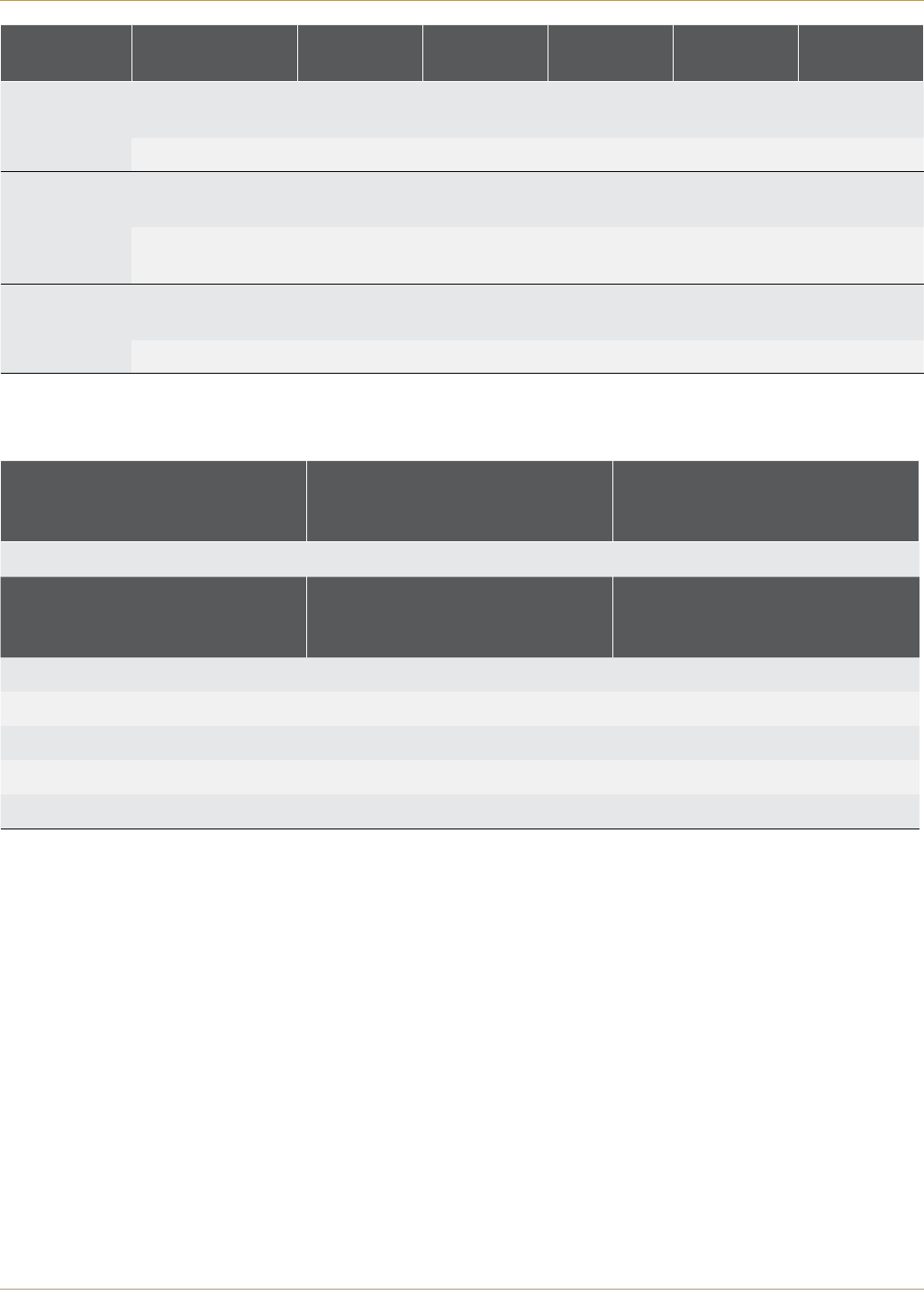
The PROMs also include questions regarding the overall
success and satisfaction of the operation from the
patient’s perspective. Six months following hip
replacement 88.6% of CORAIL/PINNACLE Hip construct
patients reported that their problems with their hip joint
were much better, indicating a very successful
procedure. An excellent or very good result of the
operation was also reported by 79.1% of CORAIL/
PINNACLE Hip construct patients. Success and
satisfaction both compare favorably to All Primary Hips.
(Table 2; Figures 2 and 3)4
2
Figure 1. Pre-Op and 6 Month Oxford Hip Scores for CORAIL/PINNACLE subjects
Oxford Score
01020304
050
0
5
10
15
Percent
Pre-op Oxford Score
6 month Post-op Oxford Score
CORAIL/PINNACLE
(MoP, CoP)
CORAIL/PINNACLE
(CoC*)
All Primary Hips
0
10
90
100
Success (%)
80
70
60
50
40
30
20
Much better
A little better
About the same
A little worse
Much worse
0
10
90
100
Satisfaction (%)
80
70
60
50
40
30
20
CORAIL/PINNACLE
(MoP, CoP)
CORAIL/PINNACLE
(CoC*)
All Primary Hips
Excellent
Very good
Good
Fair
Poor
Figure 2. Overall, how are your problems now, compared to before your operation?
Figure 3. How would you describe the results of your operation?
* THE CORAIL TOTAL HIP SYSTEM IS not approved for use with a
ceramic-on-ceramic system in the United States.
CONCLUSIONS
Based on these data patients are generally happy and
satisfied with their CORAIL/PINNACLE Hip Construct.
Subjects who received a CORAIL/PINNACLE Hip
Construct had a statistically significant median increase
of 22 points on the Oxford hip score. Global health, as
measured by the EQ-5D, also significantly increased. The
6 month Oxford hip and EQ-5D scores were generally
higher than the class of all primary hip replacements in
the UK, although not likely to be statistically significant.
Six months after surgery 88.6% of CORAIL/PINNACLE
Hip Construct patients self-reported problems with their
hip to be much better. This was 3 percentage points
higher than all primary hips.
Six months after surgery 79.1% of CORAIL/PINNACLE
Hip Construct patients reported excellent or very good
levels of satisfaction with their hip replacement
compared to 73.5% of all primary hips.4
3
Measure
(Score Range) Group NPre-Op 6 Month Heath Gain P-value
Oxford Hip
(0 – 48)
CORAIL/PINNACLE
Hip Construct 11,576 18 (13, 24) 43 (37, 47) 22 (15, 28) <0.001
All Primary Hips 92,133 18 (12, 24) 41 (34, 46) 21 (14, 28) <0.001
EQ-5D Index
(-0.59 – 1.00)
CORAIL/PINNACLE
Hip Construct 11,576 0.516
(0.055, 0.691)
0.848
(0.691, 1.00)
0.380
(0.193, 0.700) <0.001
All Primary Hips 83,202 0.516
(0.055, 0.656)
0.796
(0.691, 1.00)
0.380
(0.175, 0.694) <0.001
EQ-5D VAS
(0 – 100)
CORAIL/PINNACLE
Hip Construct 11,576 70 (50, 80) 80 (70, 90) 10 (0, 24) <0.001
All Primary Hips 80,394 70 (50, 80) 80 (69, 90) 9 (-2, 20) <0.001
Table 1. Median (IQR) PROMs for subjects with pre-operative and 6 month scores.
Success (Overall, how are your
problems now, compared to before
your operation?)
CORAIL/PINNACLE
Hip Construct
Frequency (%)
All Primary Hips
Frequency (%)
Much Better 10,251 (88.6%) 78,617 (85.6%)
Satisfaction (How would you
describe the results of your
operation?)
CORAIL/PINNACLE
Hip Construct
Frequency (%)
All Primary Hips
Frequency (%)
Excellent 5,061 (43.7%) 35,313 (38.5%)
Very Good 4,102 (35.4%) 32,147 (35.0%)
Good 1,723 (14.9%) 16,826 (18.3%)
Fair 519 (4.5%) 5,739 (6.3%)
Poor 171 (1.5%) 1,735 (1.9%)
Table 2. Success and Satisfaction Distributions

References
1. Dawson J, Fitzpatrick R, Carr A, Murray D. Questionnaire on the perceptions of subjects about total hip replacement. J Bone Joint
Surg March 1996: 78-B, No 2: 185-190.
2. The EuroQol Group. EuroQol – a new facility for the measurement of health-related quality of life Health Policy (1990;16(3):199-208).
3. National Joint Registry for England, Wales and Northern Ireland, 10th Annual Report, 2013. Available from www.njrreports.org.uk.
4. NJR-NJR data from 1st April 2003 -10th July 2015 on DePuy products supplied for post-marketing surveillance, NJR Centre, 2015.
DePuy Orthopaedics, Inc.
700 Orthopaedic Drive
Warsaw, IN 46582
USA
Tel: +1 (800) 366-8143
Fax: +1 (800) 669-2530
www.depuysynthes.com
© DePuy Synthes 2016. All rights reserved.
DSUS/JRC/0216/1429 10/16
DePuy France S.A.S
7 allée Irène Joliot Curie
CS 30078
69801 SAINT PRIEST Cedex
Tel : +33 (0)4 72 79 27 27
Fax : +33 (0)4 72 79 28 28
DePuy (Ireland)
Loughbeg, Ringaskiddy
Co. Cork
Ireland
Tel: + 353 21 4914 000
Fax: + 353 21 4914 199
DePuy International, Ltd.
St Anthony’s Road
Leeds LS11 8DT
England
Tel: +44 (0) 113 270 0461
Fax: +44 (0) 113 272 4101