Wear Reduction Technology In Total Knee Arthroplasty 71281763
2015-04-30
: Pdf Wear-Reduction Technology In Total Knee Arthroplasty 71281763 Wear-Reduction_Technology_in_Total_Knee_Arthroplasty_71281763 4 2015 pdf
Open the PDF directly: View PDF ![]() .
.
Page Count: 8

Page – 1
Wear-Reduction Technology in Total Knee Arthroplasty
Steven Haas, MD, MPH 1, Ramprasad Papannagari, MS 2, Mark Morrison, PhD 2, Shilesh Jani, MS 2
1 Hospital for Special Surgery, New York, NY, USA
2 Smith & Nephew, Inc., Orthopaedics, Memphis, TN, USA
Summary
Due to the increasing burden of revision in total knee arthro-
plasty (TKA), sustainable improvements in implant longevity
may require the continued development of advanced bear-
ing materials. The LEGION™ Primary Knee System featuring
VERILAST™ technology is the first device to combine an OX-
INIUM™ Oxidized Zirconium femoral component with a highly
crosslinked ultra-high molecular weight polyethylene (UHM-
WPE) tibial insert to form an advanced TKA bearing. Follow-
ing the review of published volumetric wear rates, this bear-
ing coupling was found to provide the lowest observed wear
of any contemporary TKA device, potentially supporting the
equivalent of 30 years of normal use in vivo. This evidence
supports the assertion that both tibial and femoral bearing
surfaces can significantly affect TKA wear. Moreover, the use
of VERILAST™ technology may reduce long-term revision risk
and support device longevity in younger, more active pa-
tients.
‡Based on in-vitro wear simulation testing, the LEGION Primary Knee System with VERILAST technology is expected to provide
wear performance sufficient for 30 years of actual use under typical conditions. The results of in-vitro wear simulation testing
have not been proven to quantitatively predict clinical wear performance. Also, a reduction in total polyethylene wear volume or
wear rate alone may not result in an improved clinical outcome as wear particle size and morphology are also critical factors in
the evaluation of the potential for wear mediated osteolysis and associated aseptic implant loosening. Particle size and
morphology were not evaluated as part of the testing.
Vol 01, No 01 - December 2010
Bone&JointScience
Our Innovation in Focus
Page – 2
Bone&JointScience Vol 01, No 01 - December 2010
Wear-Reduction Technology in Total Knee Arthroplasty
‡Based on in-vitro wear simulation testing, the LEGION Primary Knee System with VERILAST technology is expected to provide
wear performance sufficient for 30 years of actual use under typical conditions. The results of in-vitro wear simulation testing
have not been proven to quantitatively predict clinical wear performance. Also, a reduction in total polyethylene wear volume or
wear rate alone may not result in an improved clinical outcome as wear particle size and morphology are also critical factors in
the evaluation of the potential for wear mediated osteolysis and associated aseptic implant loosening. Particle size and
morphology were not evaluated as part of the testing.
The Importance of Wear Resistance in TKA
Tibial component wear, attendant osteolysis and loosening
have been identified as the primary causes of long-term fail-
ure in TKA [1–3]. In 1999 alone, 22,000 TKA revision proce-
dures occurred in the United States at an estimated cost of
over $260 million [4]. In 2005, there were 38,300 revisions in
the United States. This number is expected to grow to over
268,000 by 2030 [5]. In order to support optimal patient care
and reduce accelerating healthcare costs, technologies must
be introduced that support improved device longevity. Specifi-
cally, improved tibial and femoral bearing technologies could
limit long-term revision risk in TKA, especially in relatively
young and active patients.
Polyethylene and Wear Performance
The History of UHMWPE
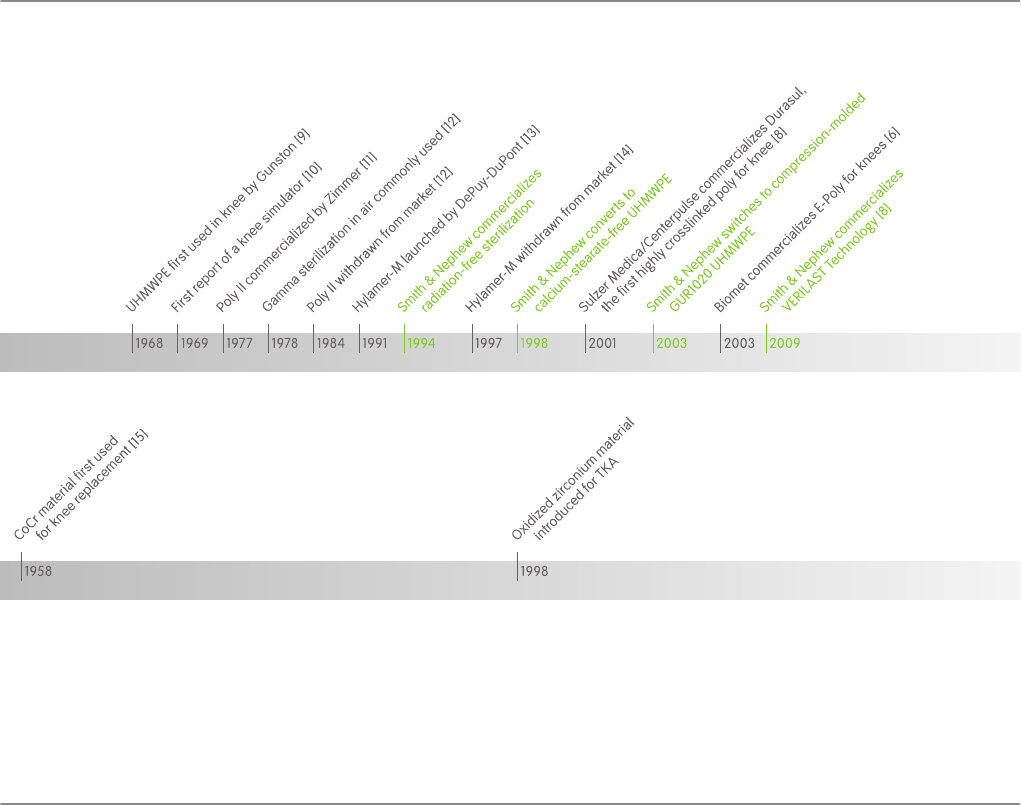
UHMWPE was first utilized in TKA in 1968, setting a standard
for knee replacement that continues today (Figure 1) [6]. More
than 40 years later, every TKA in the world still utilizes a UHM-
WPE tibial bearing. However, polyethylene wear remains a pri-
mary cause of long-term failure [3]. During normal articulation,
millions of microscopic polyethylene wear particles are re-
leased into the tissues surrounding the knee joint. These par-
ticles can cause a cascade of biological responses leading to
osteolysis, aseptic loosening, and eventual revision [7]. In or-
der to address these risks many attempts have been made to
improve polyethylene wear performance, including the unsuc-
cessful introductions of Poly II in 1977 and Hylamer in 1991. In
Figure 1: TKA Milestone Timelines
Page – 3
Bone&JointScience Vol 01, No 01 - December 2010
Wear-Reduction Technology in Total Knee Arthroplasty
‡Based on in-vitro wear simulation testing, the LEGION Primary Knee System with VERILAST technology is expected to provide
wear performance sufficient for 30 years of actual use under typical conditions. The results of in-vitro wear simulation testing
have not been proven to quantitatively predict clinical wear performance. Also, a reduction in total polyethylene wear volume or
wear rate alone may not result in an improved clinical outcome as wear particle size and morphology are also critical factors in
the evaluation of the potential for wear mediated osteolysis and associated aseptic implant loosening. Particle size and
morphology were not evaluated as part of the testing.
mal balance between wear performance and mechanical
properties for TKA [16].
OXINIUM™ Oxidized Zirconium in TKA
Cobalt chrome (CoCr) alloy has served as the standard mate-
rial for femoral components in TKA for more than 40 years
(Figure 1). However, the surfaces of retrieved CoCr femoral
components have been shown to exhibit roughening that can
significantly increase polyethylene wear [17–19]. This evidence
suggests that a femoral bearing surface with improved wear
performance could improve implant longevity.
In contrast to the UHMWPE milestones shown in Figure 1, the
introduction of OXINIUM™ (Smith & Nephew, Inc., Memphis,
TN, USA) Oxidized Zirconium femoral components in 1998 was
the first major TKA bearing advancement on the femoral side
in 40 years. This material was developed to combine the ob-
served wear benefits of ceramics with the toughness of met-
als. The resulting bearing surface is resistant to in-vivo rough-
ening, is less abrasive than CoCr, and has enhanced
biocompatibility, without any risk of catastrophic fracture [20–
25]. Retrieval studies have shown that Oxidized Zirconium
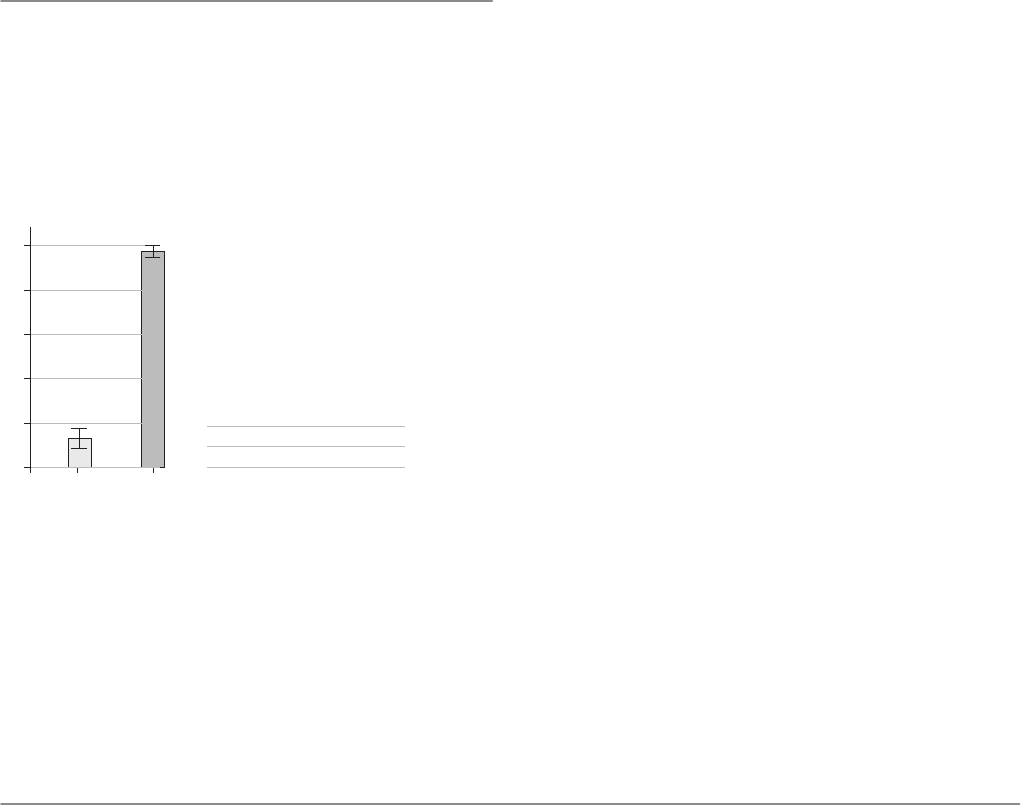
femoral components exhibit minimal scratching. A matched
pair analysis performed at The Hospital for Special Surgery
showed that in vivo femoral scratching was 12 times greater in
the CoCr components compared to Oxidized Zirconium (Fig-
ure 2) [25, 26].
Wear Performance of CoCr and OXINIUM
TKA Bearings
With a CoCr bearing, the only way to significantly improve wear
performance is by increasing the irradiation dose of the poly-
ethylene. However, as previously described, this improved
wear performance must be balanced against unfavorable
changes in mechanical properties. OXINIUM femoral compo-
nents effectively alter the dynamic between irradiation dose,
wear resistance and mechanical properties. Compared to
CoCr, OXINIUM results in less UHMWPE wear at any given ir-
radiation dose, without any sacrifice in mechanical properties
contrast, crosslinked polyethylene has been used since 2001
and has been shown to be highly successful clinically [8].
The Development of Crosslinked UHMWPE
It is well established that the wear resistance of UHMWPE
quickly improves with increased irradiation dose. However,
this gain in wear resistance is attained at the expense of me-
chanical properties. If greater wear resistance is desired, the
UHMWPE can be exposed to a higher radiation dose, but the
mechanical properties will be further decreased. This balance
is particularly important in TKA, where contact stresses are
higher than in total hip arthroplasty (THA). Based on material
and device testing, a highly cross-linked UHMWPE with a ra-
diation dose of 7.5 Mrad (75 kGy) appears to result in an opti-
0.0
1.3
0.4
5.0
2.5
3.8
5.0
OxZr CrCoMo
Significantly less scratching OxZr
p=0.005
Results—Femoral component
Femoral Scratching Pitting Delamination Striations Total
component
OxZr 0.4±0.7 0.3±0.6 0.1±0.3 0.5±0.7 1.6±1.3
CrCoMo 5.0±0 0.1±0.3 1.6±2.3 0±0 9.8±0.5
p 0.44 0.05 0.05 0.005
1.6
9.8
OxZr CoCr
Results—Femoral component
Femoral
component
OxZr 1.6±1.3
CoCr 9.8±0.5
p 0.005
p=0.005
The total average score was significantly lower for the OxZr components (1.6±1.3
vs. 9.8±0.5, p=0.005) [26].
Figure 2: Comparison of wear grades (scratching,
pitting, delaminations, striations) by visual score
for OxZr and CoCr bearing surfaces.
Page – 4
Bone&JointScience Vol 01, No 01 - December 2010
Wear-Reduction Technology in Total Knee Arthroplasty
‡Based on in-vitro wear simulation testing, the LEGION Primary Knee System with VERILAST technology is expected to provide
wear performance sufficient for 30 years of actual use under typical conditions. The results of in-vitro wear simulation testing
have not been proven to quantitatively predict clinical wear performance. Also, a reduction in total polyethylene wear volume or
wear rate alone may not result in an improved clinical outcome as wear particle size and morphology are also critical factors in
the evaluation of the potential for wear mediated osteolysis and associated aseptic implant loosening. Particle size and
morphology were not evaluated as part of the testing.
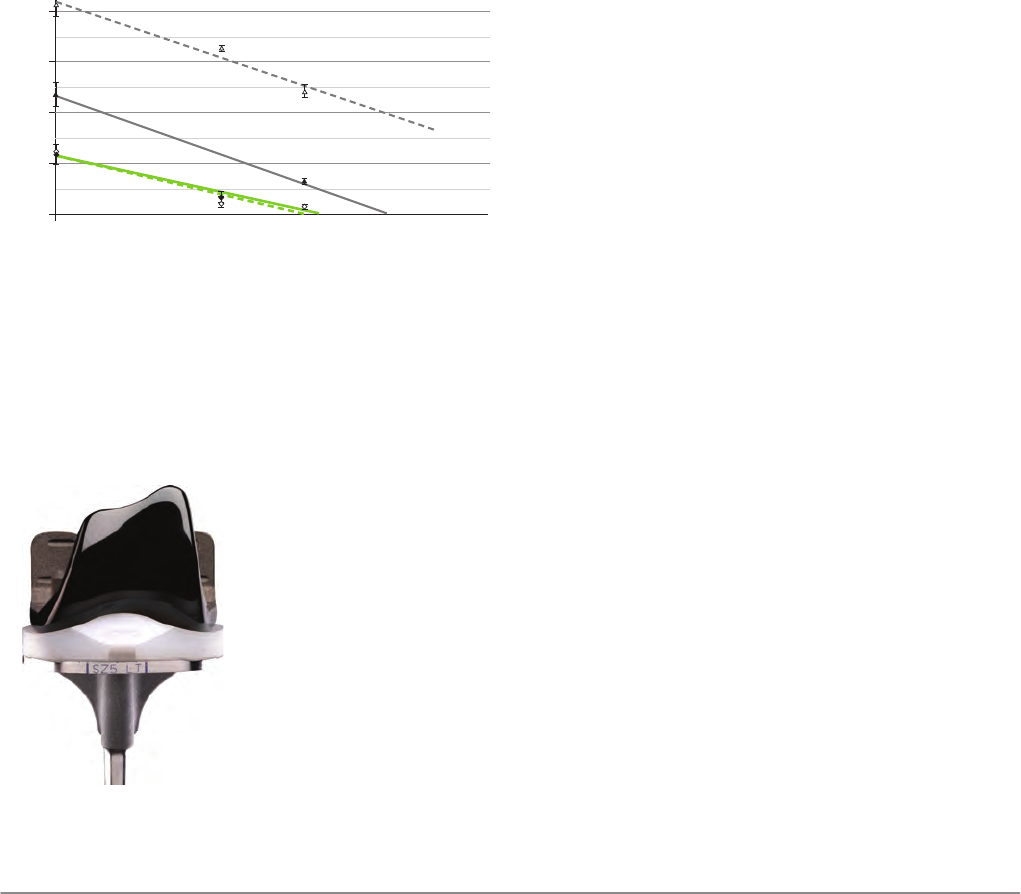
(Figure 3). For example, the wear rate of OXINIUM against a 7.5
Mrad crosslinked UHMWPE is approximately equivalent to that
of CoCr against a 10 Mrad crosslinked UHMWPE with pristine,
new components.
Utilizing an OXINIUM femoral component instead of CoCr pro-
vides a reduction in wear equivalent to an additional 3 Mrad
irradiation dose. In the end, mechanical properties are im-
proved because about 25% less radiation exposure is neces-
sary to achieve the same wear resistance.
The previously described testing conditions represent an ide-
al situation with pristine, new components, featuring highly
polished surfaces. However, the presence of third-body de-
bris such as bone cement, bone chips, or debris shed from
ingrowth surfaces can significantly diminish the gains in wear
resistance provided by crosslinked UHMWPE [27]. Using an in-
vitro tumbling protocol designed to simulate roughening from
third-body debris [28], the polyethylene wear against tumbled
OXINIUM components was compared to the wear produced
by tumbled CoCr femoral components. Results indicated that
the abrasion resistance of OXINIUM appears to prevent
scratching by third-body debris, enabling improved wear re-
sistance (Figure 3).
Muratoglu et al [29] examined the wear of conventional UHM-
WPE and highly crosslinked polyethylene on new and retrieved
CoCr femoral components. Their data indicated that femoral
scratching increases wear in both crosslinked and conven-
tional polyethelene. The increase was over 800% for the
crosslinked polyethylene, but only 266% for conventional
UHMWPE [29]. Based on this data, the scratch-resistant prop-
erties of OXINIUM appear to be especially important in main-
taining the wear resistance of crosslinked polyethylene.
Wear Performance of VERILAST™
The LEGION™ Primary Knee System featuring VERILAST™ tech-
nology (Smith & Nephew, Inc., Memphis, TN USA; Figure 4) is
the first TKA device to combine the advanced wear properties
of 7.5 Mrad highly crosslinked ultra-high molecular weight poly-
ethylene (XLPE) tibial inserts with the superior abrasion resis-
LEGION™ Primary Knee System featuring
VERILAST™ technology (Smith & Nephew, Inc.,
Memphis, TN USA).
Figure 4: LEGION™ Primary Knee
System
Plot of the mean wear rates (± standard deviations) in a knee simulator for
UHMWPE crosslinked to various doses against either CoCr or OXINIUM femoral
components in pristine (solid symbols and lines) and tumbled (open symbols and
dashed lines) conditions.
0 10 30 40
50
20
0 2 6 8 10 124
Wear Rate (mm3 / Mc
ycles)
Radiation Dose (Mrad)
Tumbled
Tumbled CoCr
Tumbled OXINIUM
Pristine CoCr
Pristine OXINIUM
Pristine
Figure 3: Plot of the mean wear rates
Page – 5
Bone&JointScience Vol 01, No 01 - December 2010
Wear-Reduction Technology in Total Knee Arthroplasty
‡Based on in-vitro wear simulation testing, the LEGION Primary Knee System with VERILAST technology is expected to provide
wear performance sufficient for 30 years of actual use under typical conditions. The results of in-vitro wear simulation testing
have not been proven to quantitatively predict clinical wear performance. Also, a reduction in total polyethylene wear volume or
wear rate alone may not result in an improved clinical outcome as wear particle size and morphology are also critical factors in
the evaluation of the potential for wear mediated osteolysis and associated aseptic implant loosening. Particle size and
morphology were not evaluated as part of the testing.
tance of OXINIUM femoral components. This advanced bear-
ing couple could provide improved implant longevity in TKA.
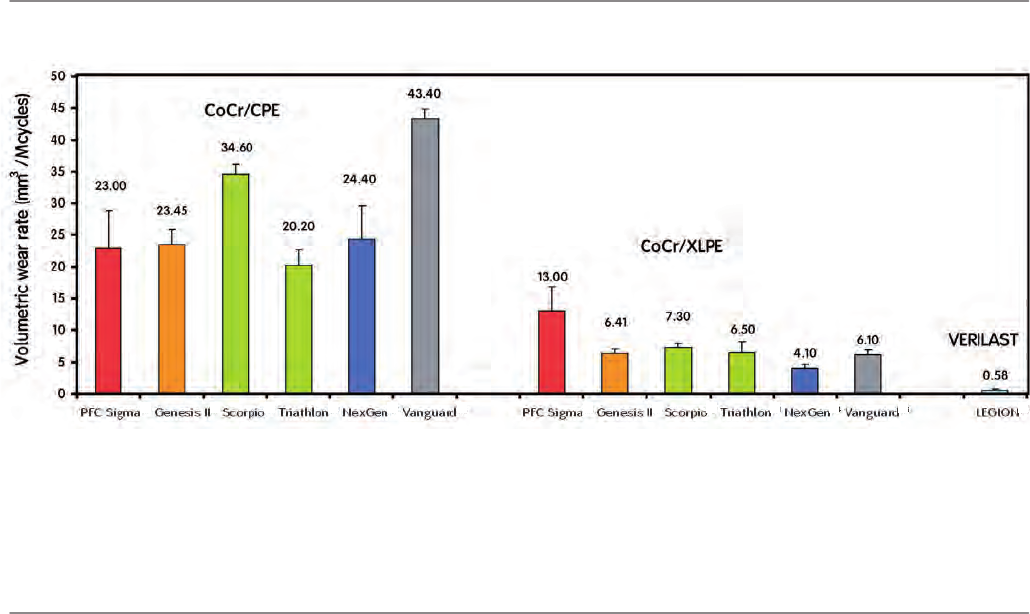
In order to evaluate bearing performance, wear rates from in-
dependent, published studies were compared to wear results
for VERILAST (Figure 5). Volumetric wear rates for CoCr and
conventional UHMWPE (CoCr/CPE) range from 20–43 mm3/
Mcycles. The wear rates for CoCr and crosslinked UHMWPE
(CoCr/XLPE) is significantly less, ranging from 4–13 mm3/Mcy-
cles. In contrast, a wear rate of 0.58 mm3/Mcycles was ob-
served for VERILAST in the 45 Mcycles test.
These results are especially impressive considering the test-
ing protocols that were utilized. The VERILAST bearing was
tested with the kinematically aggressive Leeds protocol [35].
Moreover, the bearing was tested for 45 Mcycles. Simulator
tests reported in the literature are typically conducted for only
5 to 20 Mcycles [30–43].
Some specific examples of reported wear cycles include the
following:
– Crosslinked polyethylene (Prolong) using NexGen CR
(Zimmer, Warsaw, IN) TKR – 20 Mcycles (Popoola et al [39]).
Mean volumetric wear rates (+/- std. dev.) of CoCr against conventional polyethylene (CPE), CoCr against crosslinked polyethylene (XLPE) and OXINIUM against XLPE
(VERILAST) [30-36].
Figure 5: Comparison of mean volumetric wear rates
Volumetric wear rate (mm3 / Mcycles
24.40
4.10
23.00
13.00
43.40
6.10
20.20
6.50
34.60
7.30
0 10 30 40 5020
NexGen PFC Sigma Scorpio Triathlon Vanguard
CoCr/CPE CoCr/XLPEVERILAST
Genesis II
23.45
6.41
0.58
LEGION
VERILAST

Page – 6
Bone&JointScience Vol 01, No 01 - December 2010
Wear-Reduction Technology in Total Knee Arthroplasty
‡Based on in-vitro wear simulation testing, the LEGION Primary Knee System with VERILAST technology is expected to provide
wear performance sufficient for 30 years of actual use under typical conditions. The results of in-vitro wear simulation testing
have not been proven to quantitatively predict clinical wear performance. Also, a reduction in total polyethylene wear volume or
wear rate alone may not result in an improved clinical outcome as wear particle size and morphology are also critical factors in
the evaluation of the potential for wear mediated osteolysis and associated aseptic implant loosening. Particle size and
morphology were not evaluated as part of the testing.
– Crosslinked polyethylene (Durasul) using Natural Knee II
(Zimmer, Warsaw, IN) TKR – 10 Mcycles (Muratoglu
et al [40]).
– Insall-Burstein I (Zimmer, Warsaw, IN) and Kinematic
(Howmedica, Rutherford, NJ)
TKR – up to 11 million cycles (Walker et al [41]).
– Insall-Burstein II (Zimmer, Warsaw, IN) TKR – approx.
11 million cycles (Beaule et al [42])
– JOURNEY (Smith & Nephew, Memphis, TN) TKR – up to
10 million cycles (Ries et al [43])
Conclusion
The amount of volumetric wear observed per million cycles of
testing suggests that the LEGION primary knee coupled with
VERILAST bearing technology may remain viable in vivo for the
equivalent of 30 years of normal use. Moreover, when tested un-
der relatively extreme simulation conditions, this system demon-
strated the lowest wear rate of any contemporary TKA device.
These wear results may be particularly relevant for younger pa-
tient populations. While the longevity of contemporary TKA im-
plants has remained relatively unchanged, the typical patient
has not. Initially, TKA was primarily performed in patients over
the age of 65. However, today an ever increasing number of
patients are having surgery in their 40’s and 50’s [44, 45]. An
estimated device longevity of approximately 15 years may be
sufficient for older populations, but the increased demands of
younger patient groups require an additional 5–15 years of in-
vivo use prior to revision TKA. This demographic shift suggests
that advanced bearing technologies should be adopted to re-
solve an inevitable increase in long-term revision TKA rates.
Page – 7
Bone&JointScience Vol 01, No 01 - December 2010
Wear-Reduction Technology in Total Knee Arthroplasty
References
1. Australian Orthopaedic Association National Joint Replacement Registry.
Annual Report. Adelaide: AOA; 2010. Available at: http://www.dmac.adelaide.edu.
au/aoanjrr/publications.jsp
2. Paxton EW, Inacio M, Slipchencko T, et al. The Kaiser Permanente National Total
Joint Replacement Registry. The Permanente Journal; 12(3): 12–16, 2008.
3. Sharkey PF, Hozack WJ, Rothman RH, et al. Insall Award paper. Why are total knee
arthroplasties failing today? Clin Orthop Relat Res; 404:7–13, 2002.
4. Ingenix. Data Analyst Group. Columbus, OH, Ingenix, 1999.
5. Iorio R, et al. Orthopaedic surgeon workforce and volume assessment for total hip
and knee replacement in the United States: preparing for an epidemic. J Bone Joint
Surg Am; 90(7):1598–1605, 2008.
6. Gunston FH. Polycentric knee arthroplasty: prosthetic simulation of normal knee
movement. J Bone Joint Surg Br; 53(2):272–277, 1971.
7. Archibeck MJ, Jacobs JJ, Roebuck KA, et al. The basic science of periprosthetic
osteolysis. J Bone Joint Surg Am; 82(10):1478–1489, 2001.
8. Kurtz SM. Compendium of highly crosslinked UHMWPEs. UHMWPE Biomaterials
Handbook, Kurtz SM, Ed., Burlington, MA: Academic Press, 2009.
9. Gunston FH. Polycentric knee arthroplasty: Prosthetic simulation of normal knee
movement. J Bone Joint Surg Br; 53(2):272–277, 1971.
10. Freeman MAR, Swanson SAV, Heath JC. Study of the wear of particles produced
from cobalt-chromium-molybdenum-manganese total joint replacement prostheses.
Ann Rheum Dis; 28(Supp 5):29, 1969.
11. Farling G. Human body implant of graphitic carbon fiber reinforced ultra-high
molecular weight polyethylene. U.S. Patent 4,055,862, 1977.
12. Li S. Ultra high molecular weight polyethylene: From Charnley to cross-linked. Oper
Techn Orthop; 11(4):288–295, 2001.
13. Kurtz SM, Muratoglu OK, Evans M, et al. Advances in the processing, sterilization,
and crosslinking of ultra-high molecular weight polyethylene for total joint
arthroplasty. Biomaterials; 20:1659–1688, 1999.
14. Bellare A, Kurtz SM. High pressure crystallized UHMWPEs. UHMWPE Biomaterials
Handbook, Kurtz SM, Ed., Burlington, MA: Academic Press, 2009.
15. Jones GB. Total knee replacement – The Walldius hinge. Clin Orthop Relat Res;
94:50–57, 1973.
16. Asano T, Akagi M, Clarke IC, et al. Dose effects of cross-linking polyethylene for
total knee arthroplasty on wear performance and mechanical properties. J Biomed
Mater Res B; 83B(2):615–622, 2007.
17. Que L, Topoleski LDT, Parks NL. Surface roughness of retrieved CoCrMo alloy
femoral components from PCA artificial total knee joints. J Biomed Mater Res B;
53(1):111–118, 2000.
18. Levesque M, Livingston BJ, Jones WM, et al. Scratches on condyles in normal
functioning total knee arthroplasty. Orthop Res Soc, New Orleans, LA; 247–241,
1998.
19. Fisher J, Firkins P, Reeves EA, et al. The influence of scratches to metallic
counterfaces on the wear of ultra-high molecular weight polyethylene. Proc Inst
Mech Eng [H]; 209(4):263–264, 1995.
20. Poggie RA, Wert J, Mishra A, et al. Friction and wear characterization of UHMWPE
in reciprocating sliding contact with Co-Cr, Ti-6Al-4V, and zirconia implant bearing
surfaces. Wear and Friction of Elastomers, Denton R and Keshavan MK, Eds., West
Conshohocken, PA: ASTM International, 1992.
21. Sebastian M, Roy ME, Whiteside LA, et al. Roughness of retrieved CoCr versus
OxZr femoral knee components. Orthop Res Soc, San Francisco, CA; 1778, 2008.
22. Spector M, Ries M, Bourne RB, et al. Wear performance of ultra-high molecular
weight polyethylene on oxidized zirconium total knee femoral components. J Bone
Joint Surg Am; 83(Supp 2):80–86, 2001.
23. DesJardins JD, Burnikel B, LaBerge M. UHMWPE wear against roughened oxidized
zirconium and CoCr femoral knee components during force-controlled simulation.
Wear; 264(3–4):245–256, 2008.
24. Nasser S, Mott MP, Wooley PH. A prospective comparison of ceramic and oxinium:
TKA femoral components in patients with metal hypersensitivity. AAOS, San Diego,
CA; 437, 2007.
25. Heyse T, Chen D, Kelly N, et al. Matched Pair Total Knee Arthroplasty Retrieval
Analysis: Oxidized Zirconium vs. CoCrMo. The Knee [Epub ahead of print], 2010.
26. Heyse T, Davis J, Haas SB, et al. Retrieval analysis of Femoral Zirconium
Components in TKA: Preliminary Results. J Arthroplasty. 26(3):445-450, 2011.
27. Fisher J, McEwen HM, Tipper JL, et al. Wear, debris, and biologic activity of
cross-linked polyethylene in the knee: benefits and potential concerns. Clin Orthop
Relat Res; 428:114–119, 2004.
28. Widding W, Hines G, Hunter G, et al. Knee simulator protocol for testing of
oxidized zirconium and cobalt chrome femoral components under abrasive
conditions. Orthop Res Soc, Dallas, TX; 1009, 2002.
29. Muratoglu OK, Burroughs BR, Bragdon CR, et al. Knee Simulator Wear of
Polyethylene Tibias Articulating against Explanted Rough Femoral Components. Clin
Ortho Relat Res; 428:108–113, 2004.
30. McEwen HMJ, Barnett PI, Bell CJ, et al. The influence of design, materials and
kinematics on the in vitro wear of total knee replacements. J Biomech; 38(2):357–
365, 2005.
31. Parikh A, Morrison M, Jani S. Wear testing of crosslinked and conventional
UHMWPE against smooth and roughened femoral components. Orthop Res Soc, San
Diego, CA; 0021, 2007.
32. Essner AA, Herrera L, Yau SS, et al. Sequentially crosslinked and annealed
UHMWPE knee wear debris. Orthop Res Soc, Washington D.C.; 71, 2005.
33. Herrera L, Sweetgall J, Essner A, et al. Evaluation of sequentially crosslinked and
annealed wear debris. World Biomater Cong, Amsterdam; 583, 2008.
34. Schaerer C, Mimnaugh K, Popoola O, et al. Wear of UHMWPE tibial inserts under
simulated obese patient conditions. Orthop Res Soc, New Orleans, LA; 2329. 36.
Biomet publication. FDA Cleared Claims for E1 Antioxidant Infused Technology.
http://www.biomet.com/orthopedics/getFile.cfm?id=2657&rt=inline, 2010.
35. Papannagari R, Hines G, Sprague J, et al. Long-term wear performance of an
advanced bearing knee technology. ISTA, Dubai, UAE, 2010.
36. Barnett PI, Fisher J, Auger DD, et al. Comparison of wear in a total knee
replacement under different kinematic conditions. J Mater Sci Mater Med; 12(10–
12):1039–1042, 2001.
37. Haider H, Garvin K. Rotating Platform versus Fixed-bearing Total Knees: An In Vitro
Study of Wear. Clin Orthop Relat Res; 466(11):2677–2685, 2008.
38. Muratoglu OK, Rubash HE, Bragdon CR, et al. Simulated normal gait wear testing
of a highly cross-linked polyethylene tibial insert. J Arthroplasty; 22(3):435–444,
2007.
39. Popoola OO, Yao JQ, Johnson TS, et al. Wear, delamination, and fatigue resistance
of melt-annealed highly crosslinked UHMWPE cruciate-retaining knee inserts under
activities of daily living. J Orthop Res; 28(9):1120–1126, 2010.
40. Muratoglu OK, Bragdon CR, Jasty M, et al. Knee-simulator testing of conventional
and cross-linked polyethylene tibial inserts. J Arthroplasty; 19(7):887–897, 2004.
41. Walker PS. Methodology for long-term wear testing of total knee replacements. Clin
Orthop Relat Res; 372:290–301, 2000.
42. Beaule PE, Campbell PA, Walker PS, et al. Polyethylene wear characteristics in
vivo and in a knee simulator. J Biomed Mater Res A; 60(3):411–419, 2002.
43. Ries M, Victor J, Bellemans J, et al. Effect of guided knee motion and high flexion
TKA on kinematics, implant stresses and wear. AAOS, Chicago, IL; SE33, 2006.
44. D’Apuzzo MR, Hernandez-Polo VH, Sierra RJ. National trends in primary total
knee arthroplasty: A population-based study. AAOS, New Orleans, LA; 681, 2010.
45. Dahl AW, Robertsson O, Lidgren L. Surgical treatment for knee OA in younger
patients. AAOS, New Orleans, LA; P126, 2010.
Page – 8
Great care has been taken to maintain the accuracy of
the information contained in the publication. However,
neither KLEOS, nor the authors can be held responsible
for errors or any consequences arising from the use of
the information contained in this publication. The state-
ments or opinions contained in editorials and articles
in this journal are solely those of the authors thereof
and not of KLEOS. The products, procedures, and thera-
pies described are only to be applied by certified and
trained medical professionals in environments specially
designed for such procedures. No suggested test or
procedure should be carried out unless, in the reader’s
professional judgment, its risk is justified. Because of
rapid advances in the medical sciences, we recom-
mend that independent verification of diagnosis, drugs
dosages, and operating methods should be made be-
fore any action is taken. Although all advertising ma-
terial is expected to conform to ethical (medical) stan-
dards, inclusion in this publication does not constitute
a guarantee or endorsement of the quality or value of
such product or of the claims made of it by its manu-
facturer. Some of the products, names, instruments,
treatments, logos, designs, etc. referred to in this jour-
nal are also protected by patents and trademarks or by
other intellectual property protection laws even though
specific reference to this fact is not always made in
the text. Therefore, the appearance of a name, instru-
ment, etc. without designation as proprietary is not to
be construed as a representation by the publisher that
it is in the public domain. This publication, including all
parts thereof, is legally protected by copyright. Any use,
exploitation or commercialization outside the narrow
limits of copyrights legislation, without the publisher’s
consent, is illegal and liable to prosecution. This applies
in particular to photostat reproduction, copying, scan-
ning or duplication of any kind, translating, prepara-
tion of microfilms and electronic data processing and
storage. Institutions’ subscriptions allow to reproduce
tables of content or prepare lists of articles including
abstracts for internal circulation within the institutions
concerned. Permission of the publisher is required for
resale or distribution outside the institutions. Permis-
sion of the publisher is required for all other derivative
works, including compilations and translations. Permis-
sion of the publisher is required to store or use elec-
tronically any material contained in this journal, includ-
ing any article or part of an article. For inquiries contact
the publisher at the address indicated.
Come and visit us at www.kleos.md
US: Lit.No: 71281763 Rev 0.2
OUS: Lit.No. 2108-e / Ed. 11/10
Produced by the Research and Clinical Departments,
Smith & Nephew Inc.
Published by KLEOS, the medical education service
from Smith & Nephew
Published December 2010
Copyright © 2010 by Smith & Nephew Orthopaedics AG
KLEOS, Oberneuhofstrasse 10d, 6340 Baar, Switzerland
Phone +41 41 766 22 55
kleos@smith-nephew.com
Bone&JointScience is available on the KLEOS website,
www.kleos.md, within “Literature”