Resmed Sleep Screening Tool Apnealink Users Manual 1011262 1 Broch
ApneaLink to the manual cef51901-e13c-4986-94b7-c432afc4cf90
2015-02-06
: Resmed Resmed-Sleep-Screening-Tool-Apnealink-Users-Manual-523808 resmed-sleep-screening-tool-apnealink-users-manual-523808 resmed pdf
Open the PDF directly: View PDF ![]() .
.
Page Count: 4

ApneaLink™
Your link to better sleep health
ApneaLink is a simple, cost-effective
sleep-screening tool that objectively
identifies patients at risk for obstructive
sleep apnea (OSA).

Why use ApneaLink?
Sleep-disordered breathing (SDB) is recognized as a serious
health problem that impacts about 43 million US adults;
however, more than 80% remain undiagnosed and untreated.
1
The ApneaLink connects clinicians to their patients by dramati-
cally increasing the number of identified SDB patients through
primary care and specialty referral sources, thereby routing
more patients for diagnosis to a sleep center.
How does ApneaLink work?
The basic ApneaLink is a single-channel screening device
that uses a simple nasal cannula to record patient breathing.
It automatically analyzes and derives AHI, flow limitation and
snoring and later automatically generates a simple, easy-to-
interpret report with a color-keyed Risk Indicator for the
clinician to review.
With oximetry, the ApneaLink records three channels of
information: respiration, oximetry and pulse.
Simplicity, cost-effectiveness and state-of-the-art technology combine in the ApneaLink,
the ultimate choice for homecare providers, sleep labs and clinicians. The ApneaLink is one of
the few true sleep-screening devices available for obstructive sleep apnea (OSA) patients, pro-
viding the link to an improved quality of life by cost-effectively testing chronic disease patients,
intra-hospital pre-op surgical patients, occupational health patients and other high-risk patients.
The ApneaLink readily transitions patients from referring physicians to sleep centers.
ApneaLink is portable, battery-powered and user-friendly and will streamline any user’s sleep-
screening process. A versatile tool, ApneaLink can be used in the patient’s home in remote
locations (or any location where patients are sleeping).
Three easy steps can link you to more OSA patients and, most importantly, provide them
a better quality of life:
1 Screening – ApneaLink
2 Diagnosis – Polysomnography
3 Treatment – ResMed PAP therapy
ApneaLink without oximetry
The basic ApneaLink is the perfect choice for
those who need a cost-effective, accurate
screening tool. Reliable and simple to use, the basic
ApneaLink offers the following features and benefits:
• Add oximetry at any time, no software changes
required—simply “plug & play”
• Automatically analyzes and derives AHI, AI and HI, flow
limitation and snoring
• Uses one software set for both ApneaLinks (with and
without oximetry)
• Auto-generates a simple, easy-to-interpret one-page report
with a color-keyed Risk Indicator
• Can be used to identify intra-hospital preoperative patients
at risk for SDB, thereby improving overall quality of care
• Enables referral sources to easily and objectively determine
whether to refer a patient for further evaluation
• Cost-effectively screens chronic disease patients, drowsy
drivers and other high-risk individuals
• Improves patient care by providing quicker access to the
treatment care path
• Sensitivity 100%, specificity 87.5% at AHI of 102
1 Young et al. State of the Art, AJRCCM 2002
2 Wang Y et al. Pneumologie 2003
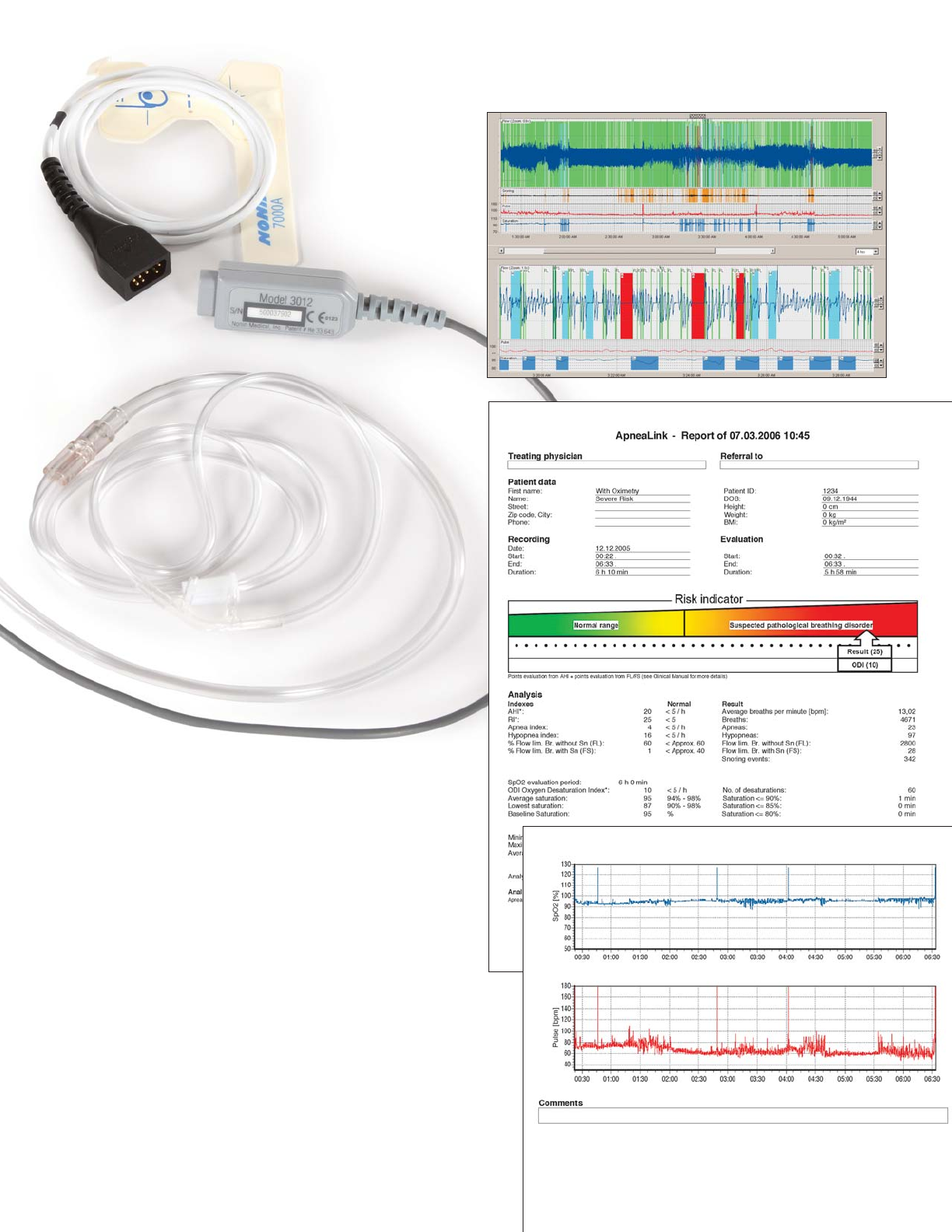
ApneaLink with oximetry
ResMed’s ApneaLink with oximetry is the premium choice in
screening for OSA. In addition to the features and benefits of
the basic ApneaLink device, the pulse oximeter provides two
additional channels of information—pulse and pulse oximetry,
all three channels in a simple “little blue box.” A simple nasal
cannula is used for sensing patient breathing. Oximetry and
pulse are detected using a choice of either reusable or single-
use patient sensors. The original ApneaLink software has been
enhanced to show a two-page patient report with pulse and
oximetry signals, detailed SpO
2
information and waveform data.
The ApneaLink with oximetry is perfect for those who want
more information and are expanding their screening capabilities
to other non-traditional patient areas, such as home oxygen
patients.
ResMed Corp Poway, CA, USA +1 858 746 2400 or 1 800 424 0737 (toll free), ResMed Ltd Bella Vista, NSW, Australia +61 (2) 8884 1000 or 1 800 658 189 (toll free). Offices in Austria, Brazil, Finland, France, Germany, Hong Kong, Japan,
Malaysia, Netherlands, New Zealand, Singapore, Spain, Sweden, Switzerland, United Kingdom (see website for details).
ApneaLink is a trademark of ResMed Ltd. and is registered in U.S. Patent and Trademark Office. ©2006 ResMed. 1011262/1 06 10
Global leaders in sleep and respiratory medicine www.resmed.com
ApneaLink
US and Latin America 22302
Canada 22303
1 Apnealink device 1 Program CD
3 Nasal Cannulas 2 AA Batteries
1 Reusable Belt 1 Carrying Case
1 Quick Software Setup Guide
1 USB download cable
ApneaLink Oximetry Accessories Kit
US and Latin America 22304
Canada 22308
1 Nonin XPOD oximeter
1 Sensor clip
3 Disposable Sensors
TECHNICAL SPECIFICATIONS
APNEALINK AND PULSE OXIMETER
Signal Recording
Breathing sounds
Respiratory flow
Blood oxygen saturation
Pulse
Battery voltage
Sampling Rates for the Channels
Respiratory flow/breathing sounds: 100 Hz
Saturation: 1 Hz
Pulse: 1 Hz
Battery: 1 Hz
Signal Processing
Signal recording: 20 Bit
Signal storage: 16 Bit
Internal Memory
Storage capacity: 15 MB
Recording period: 8 hours minimum
Power Supply to Recorder
2 NiMH rechargeable batteries: Mignon/AA/1.2V/
at least 2.1 Ah or
2 batteries: LR 6/Mignon/AA/1.5V/at least 2.1 Ah
Weight
Recorder (without rechargeable batteries or batteries):
Approximately 50 g (1.8 oz)
Pulse Oximeter: Approximately 30 g (1.1 oz)
Operating Conditions
Temperature: 20°C to 40°C (68°F to 104°F)
Humidity: 10% to 90% RH (non condensing)
Shipment/Storage Conditions*
Temperature: -20°C to +50°C (-4°F to +122°F)
Humidity: 10% to 90% RH
*Recorder without rechargeable batteries or batteries
Operating/Storage Air Pressure
800 hPa to 1060 hPa
Effective Range
Flow sensor: -10 hPa to +10 hPa
SpO2: +/- 2 digits
Pulse: +/- 3 digits
Interfaces
Nasal pressure cannula: Luer connection
Pulse oximeter: 3-pin Binder plug
Computer: Fullspeed USB 1.1
Dimensions
Recorder (length x width x height): 125 x 60 x 30 mm
(4.9" x 2.4" x 1.2")
Pulse oximeter: (length x width x height): 53 x 20 x 15 mm
(2.1" x 0.8" x 0.6")
Electromagnetic Compatibility
Product complies with all applicable electromagnetic compatibility requirements (EMC) according to IEC60601-
1-2, for residential, commercial and light industry environments. For further details, see “Guidance and
Manufacturer’s Declaration – Electromagnetic Emissions and Immunity” as follows.
Guidance and Manufacturer’s Declaration – Electromagnetic Emissions and Immunity
The ApneaLink is intended for use in the electromagnetic environment specified below. The customer or user
of the ApneaLink should ensure that the system is used only in such an environment.
Emissions test Compliance Electromagnetic environment—guidance
RF emissions CISPR11 Group 1 The ApneaLink uses RF energy only for its internal function.
Therefore, its RF emissions are very low and are unlikely to cause
any interference in nearby electronic equipment
RF emissions CISPR11
Harmonic Emissions
IEC 61000-3-2
Voltage fluctuations/
flicker emissions
IEC 61000-3-3
Class B
—
—
The ApneaLink is suitable for use in all establishments, including
domestic establishments and those directly connected to the
public low-voltage network that supplies buildings used for
domestic purposes.
Optional Accessories and Disposables
(US, Latin America and Canada)
Nasal Cannulas (25/pk) 70388
Nasal/Oxygen Cannula (25/pk) 70319
Belt, reusable 629052
Belt, Single Use (24/pk) 70406
Sensor, Oximeter-Single Use 70412
Sensor, Oximeter (Flex Wrap)-Reusable 1431002
Tape, Sensor (for Flex Wrap Sensor), (25/pk) 70276
Sensor, Oximeter (Soft Sensor)-Reusable 70413
ORDERING INFORMATION & PRODUCT CODES
Medical Electrical Equipment needs special precautions with respect to EMC and needs to be installed and
put into service according to EMC information provided in this document.
Warnings: The ApneaLink should not be used adjacent to or stacked with other equipment. If adjacent or
stacked use is necessary, the ApneaLink should be observed to verify normal, operation in the configuration
in which it will be used. The use of accessories other than those specified in this manual is not
recommended. They may result in increased emissions or decreased immunity of the ApneaLink.