SORIN CRM CRTD174 Implantable cardioverter defibrillator User Manual Manual EU INTENSIA CRT D174 U146
SORIN CRM Implantable cardioverter defibrillator Manual EU INTENSIA CRT D174 U146
Manual EU INTENSIA CRT-D174_U146
Implant manual
Implantable cardioverter defibrillator
CRT-D model 174
blank
blank

TABLE OF CONTENTS
1. General description................................................................................................................ 5
2. Indications............................................................................................................................... 6
3. Contraindications....................................................................................................................7
4. Warnings and precautions.....................................................................................................8
4.1. Risks related to medical environment.......................................................................................9
4.2. Sterilization, storage and handling..........................................................................................10
4.3. Implantation and device programming....................................................................................10
4.4. Lead evaluation and lead connection......................................................................................11
4.5. Generator explant and disposal..............................................................................................12
5. Adverse events...................................................................................................................... 13
5.1. MSP study............................................................................................................................... 13
5.2. Potential adverse events.........................................................................................................14
6. Clinical studies...................................................................................................................... 16
6.1. MSP clinical study................................................................................................................... 16
7. Patient selection and treatment...........................................................................................20
7.1. Individualization of treatment..................................................................................................20
7.2. Specific patient populations....................................................................................................21
8. Patient counselling information..........................................................................................22
9. Conformance to standards..................................................................................................23
10. Physician guidelines.............................................................................................................26
10.1. Physician training.................................................................................................................... 26
10.2. Directions for use.................................................................................................................... 26
10.3. Maintaining device quality.......................................................................................................26
11. Patient information............................................................................................................... 28
12. How supplied......................................................................................................................... 29
12.1. Sterility.................................................................................................................................... 29
12.2. Warranty and replacement policy............................................................................................29
13. Device description................................................................................................................ 30
14. Implant procedure................................................................................................................. 32
14.1. Necessary equipment.............................................................................................................32
14.2. Packaging............................................................................................................................... 32
14.3. Optional equipment.................................................................................................................32
14.4. Before opening the package...................................................................................................33
14.5. Prior to implantation................................................................................................................33
14.6. Device placement.................................................................................................................... 33
14.7. Choosing the type of lead.......................................................................................................33
14.8. Shock configuration (+ -> -).....................................................................................................34
14.9. Measurement of thresholds at implant....................................................................................34
14.10.Leads connection.................................................................................................................... 35
14.11. Device implantation................................................................................................................. 36
14.12.Tests and programming...........................................................................................................36
15. Special modes....................................................................................................................... 37
15.1. Safety mode (nominal values).................................................................................................37
15.2. Magnet mode.......................................................................................................................... 37
SORIN – INTENSIA CRT-D 174 – U151A 3

15.3. Response in the presence of interference..............................................................................37
15.4. Detection characteristics in the presence of electromagnetic fields........................................38
15.5. Protection against short-circuits..............................................................................................38
16. Main functions....................................................................................................................... 39
16.1. Automatic lead measurements................................................................................................39
16.2. Atrial tachyarrhythmia management.......................................................................................39
16.3. Ventricular tachyarrhythmia management...............................................................................39
16.4. Pacing..................................................................................................................................... 39
16.5. Sensing................................................................................................................................... 40
16.6. Follow-up function................................................................................................................... 40
16.7. Remote Monitoring function....................................................................................................41
17. Patient follow-up................................................................................................................... 43
17.1. Follow-up recommendations...................................................................................................43
17.2. Holter Function........................................................................................................................ 43
17.3. Recommended Replacement Time (RRT)..............................................................................44
17.4. Explantation............................................................................................................................ 44
17.5. Defibrillator identification.........................................................................................................45
18. Supplemental Information....................................................................................................46
18.1. Adverse events in the SafeR (AAI <> DDD) study..................................................................46
18.2. SafeR (AAI <> DDD) clinical study..........................................................................................47
19. Physical characteristics.......................................................................................................49
19.1. Materials used......................................................................................................................... 49
20. Electrical characteristics......................................................................................................50
20.1. Table of delivered shock energy and voltage..........................................................................50
20.2. Battery..................................................................................................................................... 50
20.3. Longevity................................................................................................................................. 51
21. Programmable parameters...................................................................................................52
21.1. Antibradycardia pacing............................................................................................................52
21.2. Ventricular tachyarrhythmia detection.....................................................................................55
21.3. Ventricular tachyarrhythmia therapies.....................................................................................56
21.4. Remote alerts and warnings...................................................................................................59
22. Non programmable parameters...........................................................................................61
23. Limited warranty................................................................................................................... 62
23.1. Article 1 : Terms of limited warranty........................................................................................62
23.2. Article 2 : Terms of replacement..............................................................................................63
24. Patents................................................................................................................................... 64
25. Explanation of symbols........................................................................................................65
4SORIN – INTENSIA CRT-D 174 – U151A

1. GENERAL DESCRIPTION
1. GENERAL DESCRIPTION
INTENSIA CRT-D 174 is an implantable cardioverter defibrillator for the recognition and
treatment of ventricular tachycardia and fibrillation, with ventricular resynchronization, in
patients with spontaneous or inducible tachyarrhythmias.
INTENSIA CRT-D 174 is equipped with an accelerometer to allow adaptation of pacing to
suit the patient’s activity.
INTENSIA CRT-D 174 is also equipped with the RF wireless technology which enables to
remotely monitor the patients who have the Sorin SMARTVIEW Monitor installed at home.
INTENSIA CRT-D 174 provides high energy shocks (42 J) for enhanced safety, as well as
automatic lead measurements to monitor system integrity.
INTENSIA CRT-D 174 is protected against high-frequency signals emitted by cellular
telephones.
SORIN – INTENSIA CRT-D 174 – U151A 5

2. INDICATIONS
2. INDICATIONS
INTENSIA CRT-D 174 is indicated for ventricular antitachycardia pacing and ventricular
defibrillation for automated treatment of life threatening arrhythmias.
The device is also indicated for the reduction of heart failure symptoms in medically
optimized NYHA Functional Class III and IV patients with left ventricular ejection fraction of
35% or less, and a QRS duration of 150 ms or longer.
6SORIN – INTENSIA CRT-D 174 – U151A

3. CONTRAINDICATIONS
3. CONTRAINDICATIONS
Implantation of INTENSIA CRT-D 174 is contraindicated in patients:
─whose ventricular tachyarrhythmias may have transient or reversible causes such as:
acute myocardial infarction, digitalis intoxication, drowning, electrocution, electrolyte
imbalance, hypoxia, sepsis, or unstable ischemic episodes,
─who present incessant tachyarrhythmia,
─who have an internal pacemaker,
─whose primary disorder is bradyarrhythmias, or atrial tachyarrhythmias.
Dual-chamber and single chamber atrial pacing is contraindicated in patients with chronic
refractory atrial tachyarrhythmias.
SORIN – INTENSIA CRT-D 174 – U151A 7

4. WARNINGS AND PRECAUTIONS
4. WARNINGS AND PRECAUTIONS
The patient should be warned of the potential risks of defibrillator malfunction if he is
exposed to external magnetic, electrical, or electromagnetic signals.
These potential interference sources may cause conversion to inhibited mode (because of
noise detection), erratic delivery of VT or VF therapies, nominal programming, or much more
rarely, irreversible damage to the device’s circuits.
The main sources of high magnitude electromagnetic interference (EMI) are: powerful
radiofrequency equipment (radar), industrial motors and transformers, induction furnaces,
resistance, arc-welding equipment and high power loudspeakers.
Be aware that the changes in the patient’s condition, drug regimen, and other factors may
change the defibrillation threshold (DFT) which may result in non-conversion of the
arrhythmia post-operatively. Successful conversion of ventricular fibrillation or ventricular
tachycardia during arrhythmia conversion testing is no assurance that conversion will occur
post-operatively.
Resuscitation Availability:
Do not perform device testing unless an external defibrillator and medical personnel skilled
in cardiopulmonary resuscitation (CPR) are readily available.
Electrical Isolation:
Do not permit the patient to contact grounded equipment that could produce hazardous
leakage current. Ensuing arrhythmia induction could result in the patient’s death.
Disable the ICD During Handling:
Program Shock Therapy to OFF during surgical implant and explant or post mortem
procedures. The device can deliver a serious high energy shock should accidental contact
be made with the defibrillation electrodes.
Antitheft gates:
Since antitheft devices at the entrance to stores are not subject to any safety standards, it is
advisable to spend as little time as possible in their vicinity.
Airport detection systems:
Since airport detection systems are not subject to any safety standards, it is advisable to
spend as little time as possible in their vicinity.
High voltage power transmission lines:
High voltage power transmission lines may generate enough EMI to interfere with
defibrillator operation if approached too closely.
Communication equipment:
Communication equipment such as microwave transmitters, linear power amplifiers, or high-
power amateur transmitters may generate enough EMI to interfere with defibrillator
operation if approached too closely.
Home appliances:
Home appliances that are in good working order and properly grounded do not usually
produce enough EMI to interfere with defibrillator operation. There are reports of device
8SORIN – INTENSIA CRT-D 174 – U151A

4. WARNINGS AND PRECAUTIONS
disturbances caused by electric hand tools or electric razors used directly over the device
implant site.
4.1. RISKS RELATED TO MEDICAL ENVIRONMENT
It is advisable to carefully monitor defibrillator operation prior to and after any medical
treatment during which an electrical current from an external source passes through the
patient's body.
Magnetic Resonance Imaging:
MRI is strictly contraindicated in cardiac defibrillator patients.
Radiofrequency ablation:
A radiofrequency ablation procedure in a patient with a generator may cause device
malfunction or damage. RF ablation risks may be minimized by:
1. Programming Shock Therapy and ATP to OFF.
2. Avoiding direct contact between the ablation catheter and the implanted lead or generator.
3. Positioning the ground, placing it so that the current pathway does not pass through or
near the device, i.e. place the ground plate under the patient’s buttocks or legs.
4. Having external defibrillation equipment available.
Electrocautery or diathermy device:
Diathermy and electrocautery equipment should not be used. If such devices must be used:
1. Keep the current path and ground plate as far away from the device and the leads as
possible (a minimum of 15 cm [six inches]).
2. Before procedure, disable ATP and shock therapies.
3. During the procedure, keep the electrocautery device as far as possible from the cardiac
defibrillator. Set it at minimum intensity. Use it briefly.
4. After the procedure, check for proper implant function. The device should never be
exposed directly to the diathermy source.
External defibrillation:
INTENSIA CRT-D 174 is protected from external defibrillation shocks.
1. Before external defibrillation, disable ATP and shock therapies.
2. During external defibrillation, it is advisable to avoid placing the defibrillating paddles
directly over the casing or over the leads. The defibrillating paddles should preferably be
placed in an anteroposterior position.
3. Avoid any direct contact between the defibrillation paddles and the conductive parts of the
implanted leads or casing of the implanted device.
4. After external defibrillation, check for proper device function.
Radiation therapy:
Avoid exposure to ionizing radiation. Betatrons are contraindicated. If high doses of radiation
therapy cannot be avoided, the defibrillator should be protected from direct exposure with a
SORIN – INTENSIA CRT-D 174 – U151A 9
CAUTION: Do not tap sharply on the ICD can after implant, because the ICD's sensing
circuits can detect this as P-waves or R-waves, and such oversensing could result in
inappropriate pacing, inhibition, or therapy. Normal activities after implant do not result in
such oversensing.

4. WARNINGS AND PRECAUTIONS
protection shield. ATP and shock therapies should be disabled during exposure and proper
device function should be checked regularly afterwards. Resulting damage may not be
immediately detectable. If irradiation of tissues close to the implantation site is necessary, it
is recommended that the cardiac defibrillator be moved. As a safety measure, an external
defibrillator should be immediately available.
Lithotripsy:
Lithotripsy may permanently damage the device if it is at the focal point of the lithotripsy
beam. If lithotripsy must be used, keep the defibrillator at least 2.5 to 5 cm (1-2 inches) away
from the focal point of the lithotripsy beam.
Diagnostic ultrasound (echography):
The defibrillator is not affected by ultrasound imaging devices.
Scales with body fat monitors and electronic muscle stimulators:
A patient with an implanted INTENSIA CRT-D 174 should not use these devices.
4.2. STERILIZATION, STORAGE AND HANDLING
Resterilization:
Do not resterilize and re-implant explanted ICDs.
"Use Before" Date:
A "Use Before" date is printed on the outer storage package and on the sterile package. Do
not implant the device after this date because the battery may have reduced longevity and
sterility may be affected. It should be returned to Sorin.
If Package is damaged:
Do not use the device or accessories if the packaging is wet, punctured, opened or
damaged because the integrity of the sterile packaging may be compromised. Return the
device to the manufacturer.
Device Storage:
Store the device in a clean area, away from magnets, kits containing magnets, and sources
of electromagnetic interference to avoid device damage. Store the device between 0 - 50 °C
(32 - 122 °F). Temperatures outside the specified range may damage the device.
Equilibration:
Allow the device to reach room temperature before programming or implanting the device
because rapid temperature changes may affect initial device function.
4.3. IMPLANTATION AND DEVICE PROGRAMMING
Use only a Sorin programmer to communicate with the device.
Do not inadvertently position any magnet over the ICD; this suspends tachyarrhythmia
detection and treatment.
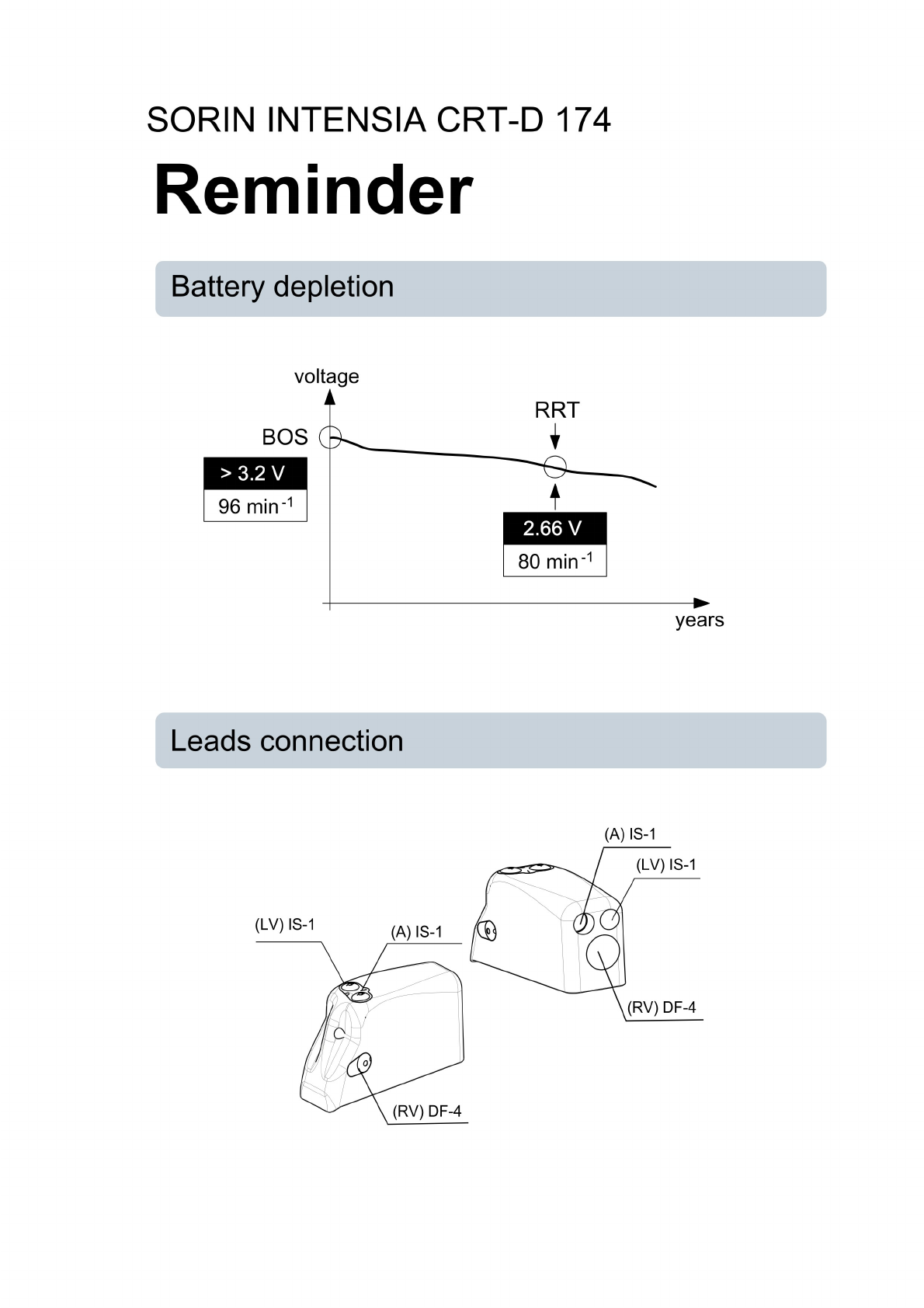
Replace the device when the RRT (Recommended Replacement Time*) point (defined by a
battery voltage of 2.66 ± 0.01 V or a magnet rate lower than or equal to 80 bpm) is reached.
Program device parameters such as sensitivity threshold and VT and VF detection intervals
as specified in the device manuals.
10 SORIN – INTENSIA CRT-D 174 – U151A

4. WARNINGS AND PRECAUTIONS
Lead System:
Do not use a lead system other than those with demonstrated compatibility because
undersensing cardiac activity and failure to deliver necessary therapy may result.
In situations where an ICD and a pacemaker are implanted in the same patient, interaction
testing should be completed. If the interaction between the ICD and the pacemaker cannot
be resolved through repositioning of the leads or reprogramming of either the pacemaker or
the ICD, the pacemaker should not be implanted (or should be explanted if previously
implanted).
Failure to properly insert the torque screwdriver into the perforation at an angle
perpendicular to the connector receptacle may result in damage to the sealing system and
its self-sealing properties.
It is recommended that a security margin of at least 10 J be demonstrated between the
effective shock energy and maximum programmable energy. Carefully confirm that true
ventricular fibrillation has been induced because the DFT for ventricular tachycardia or flutter
may be lower.
The defibrillator should be implanted with the engraved side facing outwards in order to
facilitate telemetric communication with the programming head and to display the
radiographic identification correctly.
*: corresponds to ERI (Elective Replacement Indicator) previously used.
4.4. LEAD EVALUATION AND LEAD CONNECTION
INTENSIA CRT-D 174 has two IS-1, and one DF-4 connector ports.
IS-1 refers to the international standard whereby leads and generators from different
manufacturers are assured a basic fit (ISO 5841-3:2013).
DF-4 refers to the international standard for defibrillation lead connectors (ISO 27186:2010).
Do not use the device with leads which have not been positively tested.
Do not tie a ligature directly to the lead body, tie it too tightly, or otherwise create excessive
strain at the insertion site as this may damage the lead. Use the lead stabilizer to secure the
lead lateral to the venous entry site.
Do not immerse the leads in mineral oil, silicone oil, or any other liquid.
Do not grip the lead with surgical instruments.
Do not use excessive force or surgical instruments to insert a stylet into a lead.
Use ventricular transvenous leads with caution in patients with either a mechanical or
bioprosthetic tricuspid valvular prosthesis.
Use the correct suture sleeve (when needed) for each lead, to immobilize the lead and
protect it against damage from ligatures.
Never implant the system with a lead system that has a measured shock impedance of less
than 30 ohms. A protection circuit in the defibrillator prevents shock delivery when
impedance is too low. If the shock impedance is less than 30 ohms, reposition the lead
system to allow a greater distance between the electrodes.
Do not kink leads. Kinking leads may cause additional stress on the leads, possibly resulting
in lead fracture.
Do not insert a lead connector pin into the connector block without first visually verifying that
the setscrews are sufficiently retracted. Do not tighten the setscrews unless a lead
connector pin is inserted because it could damage the connector block.
SORIN – INTENSIA CRT-D 174 – U151A 11

4. WARNINGS AND PRECAUTIONS
Lead electrodes in contact during a cardioversion or defibrillation therapy will cause current
to bypass the heart, possibly damaging the ICD and the leads. While the ICD is connected
to the leads, make sure that the metal portions of any electrodes do not touch each other.
If a pacing lead is abandoned rather than removed, it must be capped to ensure that it is not
a pathway for currents to or from the heart.
If a thoracotomy is required to place epicardial patches, it should be done during a separate
procedure to reduce the risk of morbidity and mortality.
Do not place the patch lead over nerve tissue as this may cause nerve damage.
Place the patch lead with the conducting coil side facing the heart to ensure delivery of
energy to the heart.
Place the sutures well outside the coil of the patch lead or in the area between the coils to
avoid possible coil fracture.
If countershock is unsuccessful using external paddles, adjust the external paddle position
(e.g., anterior-lateral to anterior-posterior) and be sure that the external paddle is not
positioned over the patch.
Do not fold, alter, or remove any portion of the patch as it may compromise electrode
function or longevity.
If a header port is unused on the generator, the port must be plugged to protect the
generator.
4.5. GENERATOR EXPLANT AND DISPOSAL
Interrogate the device, and program shock therapy off prior to explanting, cleaning or
shipping the device to prevent unwanted shocks.
Return all explanted generators and leads to the manufacturer.
Never incinerate the device due to the potential for explosion. The device must be explanted
before cremation.
12 SORIN – INTENSIA CRT-D 174 – U151A
5. ADVERSE EVENTS
5. ADVERSE EVENTS
Clinical data presented in this section are from the MSP clinical study. INTENSIA CRT-D
174is similar in design and function to the ALTO 2 MSP and OVATIO CRT-D devices. The
data provided are applicable to INTENSIA CRT-D 174.
5.1. MSP STUDY
Sorin conducted an international, multi-center, randomized clinical trial of its cardiac
resynchronization therapy system. Investigators attempted to implant study devices in 190
patients. A total of 182 patients received study devices and had an exposure of over 165
device years. Of those patients, 19 received OVATIO CRT-D, 160 received ALTO 2 MSP,
and 3 received ALTO MSP. The clinical data collected on ALTO MSP, ALTO 2 MSP and
OVATIO CRT-D are applicable to INTENSIA CRT-D 174. The table below summarizes the
adverse events observed for the CRT-D system. No deaths were related to the system.
Event # of Patients % of Patients # of Events Events/100
Device-Years
Deaths not related to the
system
16 8.4 16 0.8
Cardiac arrest 5 2.6 5 0.3
Worsening CHF / CHF
decompensation
3 1.6 3 0.2
Multi-organ dysfunction 2 1.1 2 0.1
Complications related to the
system
28 14.7 35 2.1
Dislodgment or migration 9 4.7 11 0.6
Extracardiac stimulation (e.g.,
phrenic stim)
9 4.7 9 0.5
Complications related to the
implant procedure
18 9.5 21 1.3
Dislodgment or migration 4 2.1 4 0.2
Observations related to the
system
23 12.1 27 1.7
Extracardiac stimulation (e.g.,
phrenic stim)
12 7.9 15 0.8
Observations related to the
implant procedure
24 12.6 28 1.7
Heart block 6 3.2 6 0.3
Extracardiac stimulation (e.g.,
phrenic stim)
3 1.5 5 0.3
Serious adverse events not
related to the system
85 44.7 176 10.8
SORIN – INTENSIA CRT-D 174 – U151A 13

5. ADVERSE EVENTS
Worsening CHF/CHF
decompensation
24 12.6 42 2.1
Atrial fibrillation/flutter 14 7.4 14 0.7
Not Serious events not
related to the system
58 30.5 121 7.4
Pain (in back, arms, chest,
shoulder, groin, head, other)
10 5.3 13 0.7
Worsening CHF/CHF
decompensation
13 6.8 16 0.8
Atrial fibrillation/flutter 7 3.7 8 0.4
Ventricular tachycardia 7 3.7 7 0.4
5.2. POTENTIAL ADVERSE EVENTS
Adverse events (in alphabetical order), including those reported in the previous tables,
associated with ICD systems include:
─Acceleration of arrhythmias (caused by device),
─Air embolism,
─Bleeding,
─Chronic nerve damage,
─Erosion,
─Excessive fibrotic tissue growth,
─Extrusion,
─Fluid accumulation,
─Formation of hematomas or cysts,
─Inappropriate shocks,
─Infection,
─Keloid formation,
─Lead abrasion and fracture,
─Lead migration/dislodgment,
─Myocardial damage,
─Pneumothorax,
─Shunting current or insulating myocardium during defibrillation with internal or external
paddles,
─Potential mortality due to inability to defibrillate or pace,
─Thromboemboli,
─Venous occlusion,
─Venous or cardiac perforation.
Patients susceptible to frequent shocks despite antiarrhythmic medical management may
develop psychological intolerance to an ICD system that may include the following:
─Dependency,
─Depression,
─Fear of premature battery depletion,
─Fear of shocking while conscious,
14 SORIN – INTENSIA CRT-D 174 – U151A

5. ADVERSE EVENTS
─Fear that shocking capability may be lost,
─Imagined shocking (phantom shock).
SORIN – INTENSIA CRT-D 174 – U151A 15

6. CLINICAL STUDIES
6. CLINICAL STUDIES
Clinical data presented in this section are from the MSP clinical study. INTENSIA CRT-D 174
is similar in design and function to the ALTO 2 MSP and OVATIO CRT-D devices. The data
provided are applicable to INTENSIA CRT-D 174.
6.1. MSP CLINICAL STUDY
OVATIO CRT-D and earlier models were evaluated clinically in an international, multi-center,
randomized clinical trial of Sorin’s cardiac resynchronization therapy (CRT-D) system.
Investigators attempted to implant study devices in 190 patients. A total of 182 patients
received study devices and had an exposure of over 165 device years. Of those patients, 19
received OVATIO CRT-D, 160 received ALTO 2 MSP, and 3 received ALTO MSP.
6.1.1 . Objectives
The primary objectives of the study were to demonstrate:
─Greater improvement in a composite endpoint (percent improvement in peak VO2
percent improvement in quality of life) for CRT-D patients than for control patients.
─System complication-free rate ≥ 67 % at six months.
6.1.2 . Methods
Patients were New York Heart Association class III or IV and had one or more indications for
an implantable cardioverter defibrillator (ICD). Patients performed cardiopulmonary exercise
testing at baseline and six-months after randomization. Patients were implanted with a Sorin
ICD with CRT-D, a Situs UW28D left ventricular lead, and commercially available right atrial
and ventricular leads. Routine follow-ups were at pre-discharge, randomization (3-14 days
post-implant), one month, three months, and six months post randomization.
6.1.3 . Results
Improvement in composite endpoint
Patients were included in the analysis if complete (peak VO2 and quality of life) baseline and
six-month data were available.
Number of
patients
contributing to
analysis
Mean percent
improvement in
composite
endpoint for
control group
Mean percent
improvement in
composite
endpoint for
CRT-D group
Percent greater
improvement for
CRT-D group
p-value
132 15.5 % 24.9 % 9.4 % 0.046
16 SORIN – INTENSIA CRT-D 174 – U151A
6. CLINICAL STUDIES
Six-month system complication-free rate
Number of patients
contributing to analysis
Kaplan-Meier six-month
complication-free estimate
One-sided lower 95%
confidence bound for six-
month complication-free
estimate
190 89.5 % 84.1 %
6.1.4 . Absolute Differences in Peak VO2 and QOL
The tables below show the absolute differences between the control and test groups’ peak
VO2 and QOL over the 6 month follow-up period in the clinical trial.
Absolute difference between test and control groups’ change in peak V02 over 6 months
Baseline
Mean ± SD
(range)
6-month
Mean ± SD
(range)
Difference
within group
Difference
between
groups
Change in
Peak VO2
(mL/min/Kg)
Control group
(n=41)
13.39 ± 4.58
(5.02, 24.10)
13.12 ± 3.99
(3.30, 20.70)
- 0.28 1.85
Test group
(n=91)
11.84 ± 3.90
(3.50, 26.3)
13.41 ± 4.28
(6.18, 27.67)
1.57
Absolute difference between test and control groups’ change in QOL score over 6 months
Baseline
Mean ± SD
(range)
6-month
Mean ± SD
(range)
Difference
within group
Difference
between
groups
Change in QOL Control group
(n=41)
47.5 ± 19.29
(9, 90.3)
31.21 ± 23.96
(0, 95)
16.29 1.28
Test group
(n=91)
52.81 ± 21.84
(9, 92)
35.24 ± 23.73
(0, 93)
17.57
The table below presents the percentage of patients in each group who improved,
worsened, or remained unchanged in each element of the composite score and the
composite score itself.
QOL score VO2 Score Composite Score
Control
GROUP
Test GROUP Control
GROUP
Test GROUP Control
GROUP
Test GROUP
% Improved 75.6 74.7 48.8 67.0 62.2 70.9
% Worsened 24.4 25.3 51.2 31.9 37.8 28.6
%
Unchanged
0.0 0.0 0.0 1.1 0.0 0.0
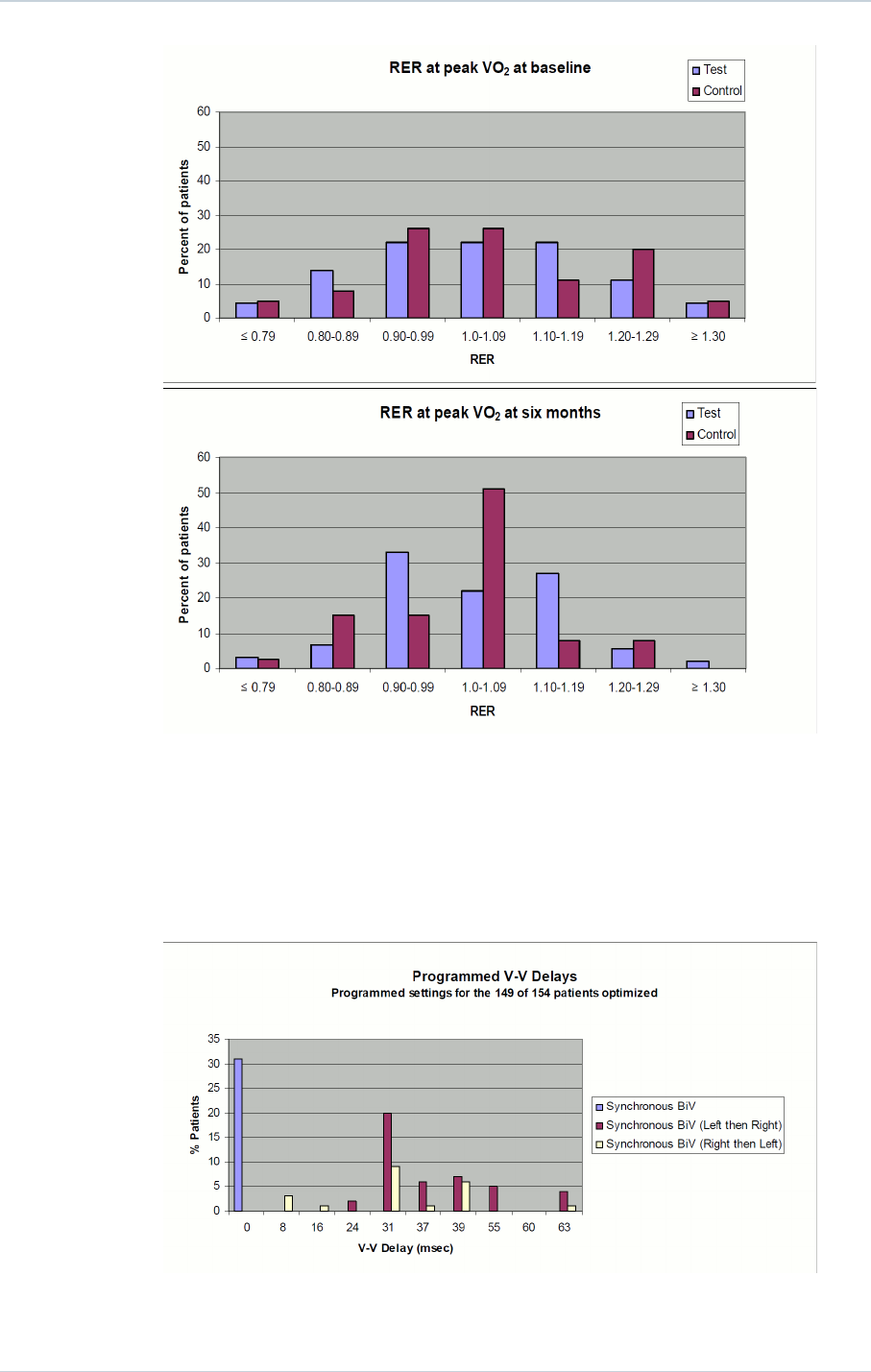
Histograms for Respiratory Exchange Rate (RER) at peak VO2 at baseline and 6 month
follow-up are provided below:
SORIN – INTENSIA CRT-D 174 – U151A 17
6. CLINICAL STUDIES
6.1.5 . Clinical Results V-V timing
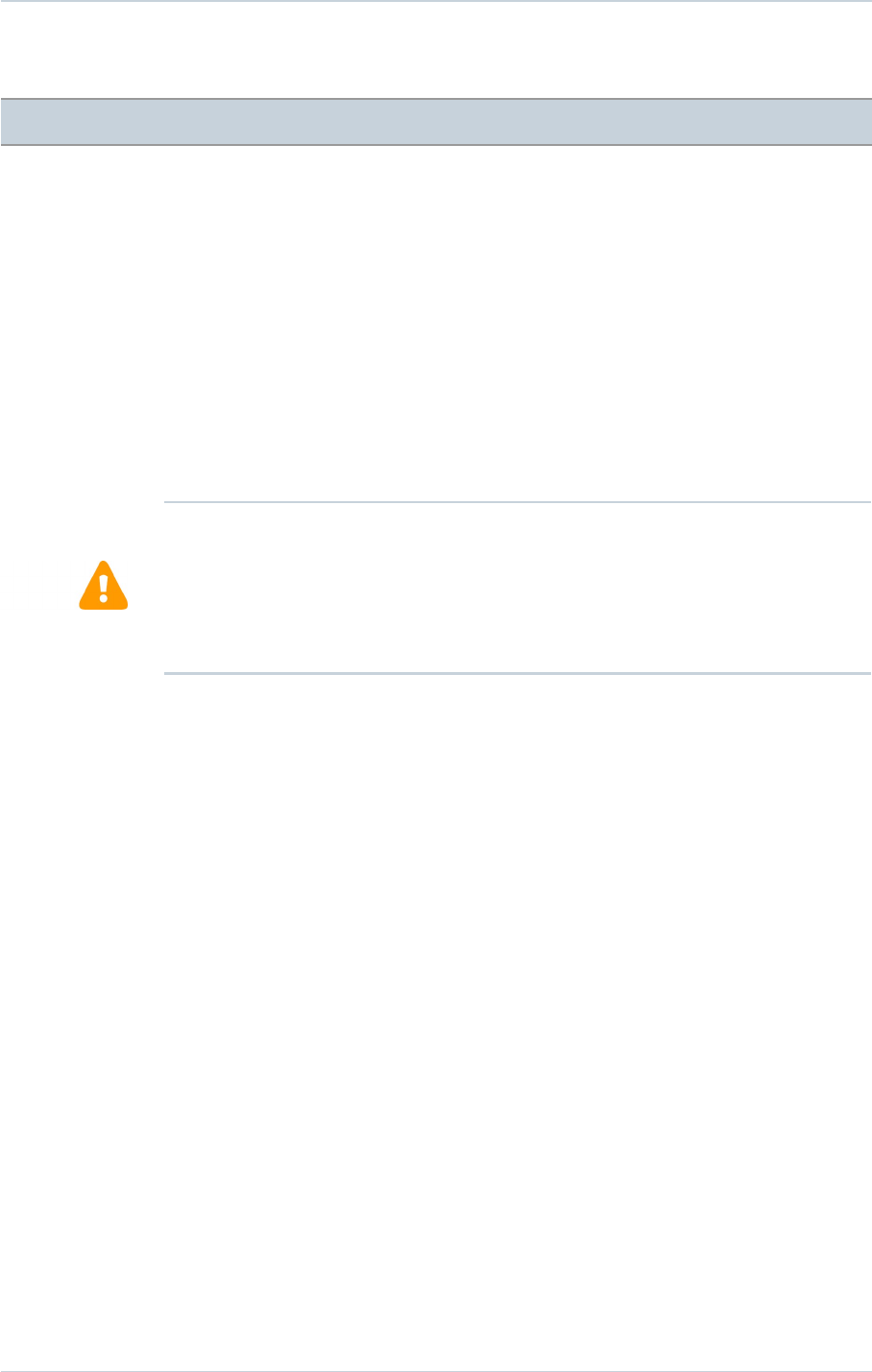
V-V programmable settings were available for the clinical study devices as follows: ALTO
MSP model 617 (not programmable for V-V delay), ALTO 2 MSP model 627 values (0, 31,
39, 47, 55 and 63 ms) and OVATIO CRT-D 6750 values (0 to 63 ms in steps of 8 ms).
The graph below shows the programmed V-V settings at randomization by percentage of
patients programmed to each combination of Synchronous BiV pacing and V-V delay.
The optimization protocol in the clinical study specified that each patient randomized should
undergo echo guided V-V optimization. Per the investigational plan for the MSP Clinical
18 SORIN – INTENSIA CRT-D 174 – U151A

6. CLINICAL STUDIES
Trial, a uniform protocol was used for V-V programming. This protocol required all patients to
undergo echo-guided V-V delay optimization before randomization (2 to 14 days post-
implant). The optimal V-V delay was determined by finding the programmable V-V delay and
ventricular chamber pacing order (RV then LV, or LV then RV) providing the maximum time
velocity integral (TVI or VTI) across the left ventricular outflow tract (LVOT).
Only those patients randomized to the Test arm were required to be programmed per the
optimization protocol for the V-V delay.
Of the 177 patients that presented at randomization, 3 had Model 617 which does not have
V-V programmability hence the inability to optimize. Of the remaining 174 patients, 154
(89%) were tested per the V-V optimization protocol. One hundred forty-nine (149) of the
154 patients who were tested per the V-V optimization protocol were programmed per the
recommended or randomized V-V delay (97%). Thirty-one (31) patients were programmed
to BiV synchronous (V-V delay 0ms), 46 were programmed to Sequential BiV (LV then RV),
22 were programmed to Sequential (RV then LV), and the remaining 50 patients were
randomized to RV only.
A sub-analysis of the composite endpoint comparing the subset of CRT-D patients with
optimized V-V delays vs. the subset of patients that did not undergo V-V delay optimization
demonstrated similar results in both groups. The CRT-D patients who did not undergo V-V
delay optimization showed a smaller improvement in the composite endpoint, although the
sample size did not permit conclusions based on data from this subset.
SORIN – INTENSIA CRT-D 174 – U151A 19
7. PATIENT SELECTION AND TREATMENT
7. PATIENT SELECTION AND TREATMENT
7.1. INDIVIDUALIZATION OF TREATMENT
Exercise stress testing:
If the patient’s condition permits, use exercise stress testing to:
─Determine the maximum rate of the patient’s normal rhythm,
─Identify any supraventricular tachyarrhythmias,
─Identify exercise-induced tachyarrhythmias.
The maximum exercise rate or the presence of supraventricular tachyarrhythmias may
influence selection of programmable parameters. Holter monitoring or other extended ECG
monitoring also may be helpful.
Electrophysiologic (EP) testing:
EP testing may be useful for ICD candidates.
EP testing may identify the classifications and rates of all the ventricular and atrial
arrhythmias, whether spontaneous or during EP testing.
Drug resistant supraventricular tachyarrhythmias (SVTs):
Drug resistant supraventricular tachyarrhythmias (SVTs) may initiate frequent unwanted
device therapy.
A careful choice of programming options is necessary for such patients.
Antiarrhythmic drug therapy:
If the patient is being treated with antiarrhythmic or cardiac drugs, the patient should be on a
maintenance drug dose rather than a loading dose at the time of ICD implantation. If
changes to drug therapy are made, repeated arrhythmia inductions are recommended to
verify ICD detection and conversion. The ICD also may need to be reprogrammed.
Changes in a patient’s antiarrhythmic drug or any other medication that affects the patient’s
normal cardiac rate or conduction can affect the rate of tachyarrhythmias and/or efficacy of
therapy.
Direct any questions regarding the individualization of patient therapy to Sorin’s
representative.
20 SORIN – INTENSIA CRT-D 174 – U151A
CAUTION: To avoid inappropriate therapy during an exercise stress test, do not reprogram
any parameter during the test. When a parameter is reprogrammed, PARAD/PARAD+
algorithm forces acceleration to "ventricular". During conducted sinus tachycardia within the
programmed Tachy zone, the device detects a 1:1 fast rhythm. Assuming that acceleration
was set to ventricular by reprogramming, the device may identify this as a VT, and may
immediately apply the corresponding therapy.

7. PATIENT SELECTION AND TREATMENT
7.2. SPECIFIC PATIENT POPULATIONS
Pregnancy:
If there is a need to image the device, care should be taken to minimize radiation exposure
to the foetus and the mother.
Nursing Mothers:
Although appropriate biocompatibility testing has been conducted for this implant device,
there has been no quantitative assessment of the presence of leachables in breast milk.
Pediatric Patients:
This device has not been studied in patients younger than 18 years of age.
Geriatric Patients:
Most of the patients receiving this device in clinical studies were over the age of 60 years.
Handicapped and Disabled Patients:
Special care is needed in using this device for patients using an electrical wheel chair or
other electrical (external or implanted) devices.
SORIN – INTENSIA CRT-D 174 – U151A 21

8. PATIENT COUNSELLING INFORMATION
8. PATIENT COUNSELLING INFORMATION
The physician should consider the following points in counselling the patient about this
device:
─Persons administering CPR may experience tingling on the patient’s body surface when
the patient’s ICD system delivers a shock.
─Advise patients to carry Sorin ID cards and/or ID bracelets documenting their ICD
system.
22 SORIN – INTENSIA CRT-D 174 – U151A

9. CONFORMANCE TO STANDARDS
9. CONFORMANCE TO STANDARDS
This device was developed in conformance with all or parts of the following standards:
─EN 45502-1: 1998 – Active implantable medical devices. General requirements for
safety, marking and information to be provided by the manufacturer.
─EN 45502-2-1: 2003 – Active implantable medical devices. Part 2-1: Particular
requirements for active implantable medical devices intended to treat bradyarrhythmia
(cardiac pacemakers).
─EN 45502-2-2: 2008 – Active implantable medical devices. Part 2-2: Particular
requirements for active implantable medical devices intended to treat tachyarrhythmia
(includes implantable defibrillators).
─ISO 5841-3: 2013 Low profile connectors (IS1) for implantable pacemakers.
─ISO27186:2010 (DF-4): Active implantable medical devices - Four-pole connector
system for implantable cardiac rhythm management devices — Dimensional and test
requirements.
─ANSI/AAMI PC69: 2007 Active implantable Medical Devices - Electromagnetic
compatibility - EMC test protocols for implantable cardiac pacemakers and implantable
Cardioverter Defibrillators.
─IEC 60601-1-2 (2007): Electromagnetic compatibility - Medical electrical equipment.
General requirements for basic safety and essential performance - Collateral standard.
─EN 50371 (2002): Generic standard to demonstrate the compliance of low power
electronic and electrical apparatus with the basic restrictions related to human exposure
to electromagnetic fields (10 MHz - 300 GHz).
─EN 301 489-1 (v1.8.1) & EN 301 489-27 (v1.1.1): Electromagnetic compatibility and
Radio spectrum Matters (ERM); Electromagnetic Compatibility (EMC) standard for radio
equipment and services - Part 1: Technical Requirements and Part 27: Specific
conditions for Ultra Low Power Active Medical Implants (ULP-AMI) and related
peripheral devices (ULP-AMI-P).
─EN 301 839-1 (v1.3.1) & EN 301 839-2 (v1.2.1): Electromagnetic compatibility and
Radio spectrum Matters (ERM); Short Range Devices (SRD); Ultra Low Power Active
Medical Implants (ULP-AMI) and Peripherals (ULP-AMI-P) operating in the frequency
range 402 MHz to 405 MHz; Part 1: Technical characteristics and test methods and Part
2: Harmonized EN covering essential requirements of Article 3.2 of the R&TTE Directive.
─EN 62311 (2008): Assessment of electronic and electrical equipment related to human
exposure restrictions for electromagnetic fields (0Hz to 300 GHz).
─EN 62209-2 (2010): Human exposure to radio frequency fields from hand-held and
body-mounted wireless communication devices – Human models, instrumentation and
procedures – Part 2: Procedure to determine the specific absorption rate (SAR) for
wireless communication devices used in close proximity to the human body (frequency
range of 30MHz to 6 GHz).
This information should not be used as a basis of comparisons among devices since
different parts of the standards mentioned may have been used.
SORIN – INTENSIA CRT-D 174 – U151A 23
9. CONFORMANCE TO STANDARDS
Sorin declares that this device is in conformity with the essential requirements of Directive
1999/5/EC on Radio and Telecommunications Terminal Equipment, with the mutual
recognition of their conformity (R&TTE).
Federal Communication Commission Interference Statement 47 CFR Section 15.19
and 15.105(b)
The FCC product ID is YSGCRTD174.
This equipment has been tested and found to comply with the limits for a Class B digital
device, pursuant to Part 15 of the FCC Rules. These limits are designed to provide
reasonable protection against harmful interference in a residential installation. This
equipment generates uses and can radiate radio frequency energy and, if not installed and
used in accordance with the instructions, may cause harmful interference to radio
communications. However, there is no guarantee that interference will not occur in a
particular installation.
This device complies with Part 15 of the FCC Rules. Operation is subject to the following
two conditions: (1) This device may not cause harmful interference, and (2) this device must
accept any interference received, including interference that may cause undesired
operation.
FCC Interference Statement 47 CFR Section 15.21 - No Unauthorized Modifications
Identification of the equipment according Section 95.1217(a)
This transmitter is authorized by rule under the Medical Device Radiocommunication Service
(in part 95 of the FCC Rules) and must not cause harmful interference to stations operating
in the 400.150-406.00 MHz band in the Meteorological Aids (i.e., transmitters and receivers
used to communicate weather data), the Meteorological Satellite, or the Earth Exploration
Satellite Services and must accept interference that may be caused by such stations,
including interference that may cause undesired operation. This transmitter shall be used
only in accordance with the FCC Rules governing the Medical Device Radiocommunication
Service. Analog and digital voice communications are prohibited. Although this transmitter
has been approved by the Federal Communications Commission, there is no guarantee that
it will not receive interference or that any particular transmission from this transmitter will be
free from interference.
IC Requirements for Canada
The IC product ID is 10270A-CRT-D174.
This class B digital apparatus meets all requirements of the Canadian Interference- causing
equipment regulations.
This device complies with Industry Canada licence-exempt RSS standard(s). Operation is
subject to the following two conditions: (1) this device may not cause interference, and (2)
this device must accept any interference, including interference that may cause undesired
operation of the device.
Under Industry Canada regulations, this radio transmitter may only operate using an
antenna of a type and maximum (or lesser) gain approved for the transmitter by Industry
Canada. To reduce potential radio interference to other users, the antenna type and its gain
should be so chosen that the equivalent isotropically radiated power (e.i.r.p.) is not more
than that necessary for successful communication.
24 SORIN – INTENSIA CRT-D 174 – U151A
CAUTION: This equipment may not be modified, altered, or changed in any way without
signed written permission from SORIN. Unauthorized modification may void the equipment
authorization from the FCC and will void the SORIN warranty.

9. CONFORMANCE TO STANDARDS
This device may not interfere with stations operating in the 400.150–406.000 MHz band in
the Meteorological Aids, Meteorological Satellite, and Earth Exploration Satellite Services
and must accept any interference received, including interference that may cause undesired
operation.
Cet appareil numérique de la classe B respecte toutes les exigences du règlement sur le
matériel brouilleur du Canada.
Le présent appareil est conforme aux CNR d’Industrie Canada applicables aux appareils
radio exempts de licence. L’exploitation est autorisée aux deux conditions suivantes: (1) il ne
doit pas produire de brouillage, et (2) l’utilisateur du dispositif doit être prêt a accepter tout
brouillage radioélectrique reçu, même si ce brouillage est susceptible de compromettre le
fonctionnement du dispositif.
Conformément à la réglementation d’Industrie Canada, le présent émetteur radio peut
fonctionner avec une antenne d’un type et d’un gain maximal (ou inférieur) approuvé pour
l’émetteur par Industrie Canada. Dans le but de réduire les risques de brouillage
radioélectrique à l’ intention d’autres utilisateurs, il faut choisir le type d’antenne et son gain
de sorte que la puissance isotrope rayonnée équivalente (p.i.r.e.) ne dépasse pas l’intensité
nécessaire à l’établissement d’une communication satisfaisante.
Le présent dispositif ne doit pas causer de brouillage aux stations du service des auxiliaires
de la météorologie, des satellites météorologiques, du service d’exploration de la terre par
satellite, exploitées dans la bande 400,150-406,000 MHz, et il doit accepter tout brouillage
reçu, y compris le brouillage pouvant entraîner un mauvais fonctionnement du dispositif.
SORIN – INTENSIA CRT-D 174 – U151A 25

10. PHYSICIAN GUIDELINES
10. PHYSICIAN GUIDELINES
10.1. PHYSICIAN TRAINING
Physicians should be familiar with sterile pulse generator and left ventricular pacing lead
implant procedures. They must apply these procedures according to professional medical
training and experience.
Physicians should be familiar with follow-up evaluation and management of patients with an
implantable defibrillator (or referral to such a physician).
This training guideline for implantation and follow-up of ICD and CRT-D devices comes from
the Heart Rhythm Society to provide standards for hospital credentialing bodies to help
ensure appropriate patient care and lead to improved patient outcomes. The following is a
summary of requirements for an alternate training pathway for ICD and CRT-D
implantations(1):
─Documentation of current experience: 35 pacemaker implantations per year and 100
implantations over the prior 3 years
─Proctored ICD implantation experience: 10 Implantations, 5 Revisions
─Proctored CRT-D implantation experience: 5 implantations
─Completion of didactic course and/or IBHRE® ExAM
─Monitoring of patient outcomes and complication rates
─Established patient follow-up
─Maintenance of competence: 10 ICD and CRT-D procedures per year, 20 patients per
year in follow-up
(1) Please consult full text of both publications for details. 2004 Heart Rhythm Society Clinical
Competency Statement and the 2005 Addendum on Training Pathways for Implantation of
Cardioverter Defibrillators and Cardiac Resynchronization Devices. Heart Rhythm (2004) 3,
371-375; Heart Rhythm.
10.2. DIRECTIONS FOR USE
ICD operating characteristics should be verified at the time of implantation and recorded in
the patient file. Complete the Patient Registration Form and return it to Sorin, as it provides
necessary information for warranty purposes and patient tracking.
Additional programming instructions can be found by accessing Online Help (click the “?” on
the screen) on the Sorin dedicated programmer. Paper copies of Online Help can be
obtained by contacting your Sorin representative.
10.3. MAINTAINING DEVICE QUALITY
This device is FOR SINGLE USE ONLY. Do not resterilize and reimplant explanted ICDs.
Do not implant the device when:
─It has been dropped on a hard surface because this could have damaged pulse
generator components.
─Its sterility indicator within the inner package is not green, because it might not have
been sterilized.
26 SORIN – INTENSIA CRT-D 174 – U151A

10. PHYSICIAN GUIDELINES
─Its storage package has been pierced or altered, because this could have rendered it
non-sterile.
─It has been stored or transported outside the environmental temperature limits: 32 °F (0
°C) to 122 °F (50 °C) as an electrical reset condition may occur.
─"Use Before" date has expired, because this can adversely affect pulse generator
longevity or sterility.
SORIN – INTENSIA CRT-D 174 – U151A 27

11. PATIENT INFORMATION
11. PATIENT INFORMATION
Information for the patient is available in the patient booklet, contained in the outer storage
package. Additional copies can be obtained by contacting your Sorin representative or on
the Sorin's web site: http://www.sorin.com.
This information should be given to each patient with their first ICD and offered to the patient
on each return visit or as deemed appropriate.
28 SORIN – INTENSIA CRT-D 174 – U151A

12. HOW SUPPLIED
12. HOW SUPPLIED
12.1. STERILITY
The INTENSIA defibrillators are supplied one per package in a sterile package.
12.2. WARRANTY AND REPLACEMENT POLICY
Sorin warrants its defibrillators. Refer to the section "Warranty" for additional information.
Please see the following labelling sections for information concerning the performance of
this device: Indications, Contraindications, Warnings and Precautions, and Adverse Events.
SORIN – INTENSIA CRT-D 174 – U151A 29

13. DEVICE DESCRIPTION
13. DEVICE DESCRIPTION
The INTENSIA CRT-D system includes the model 174 ICD device and programming system.
The programming system includes the Sorin dedicated programmer with the SMARTVIEW
programming software connected to a CPR3 programming head. The programming system
is configured and furnished by Sorin.
The INTENSIA CRT-D 174 can serve as a defibrillation electrode (active housing) with a
total surface area of 76 cm².
The INTENSIA CRT-D 174 is designed to recognize and treat slow or fast VT and VF by
continuously monitoring atrial and ventricular activity to identify persistent ventricular
arrhythmias and to deliver appropriate therapies. INTENSIA CRT-D 174 features the
PARAD/PARAD+ algorithm, which is specifically designed to differentiate ventricular
tachycardias from fast rhythms of supraventricular origin. PARAD/PARAD+ continuously
monitors R-R interval stability, searches for long cycles, assesses the degree of P-R
association, evaluates sudden onset and determines the chamber of arrhythmia
acceleration.
In addition to the advanced detection scheme, INTENSIA CRT-D 174 offers programmable
single, dual or triple-chamber pacing therapy (DDD, DDI, VVI or SafeR (AAI <> DDD)
modes) with or without rate-responsive capabilities (DDDR, DDIR, VVIR, DDD/DDIR and
SafeR-R (AAIR <> DDDR) modes) using an acceleration sensor. An automatic AV delay
algorithm as well as a mode switching function are available.
INTENSIA CRT-D 174 enables an adjustment of the interventricular delay, and provides the
possibility of adapting pacing to each ventricle. The ICD is intended to resynchronize
uncoordinated contraction of the heart by simultaneously or sequentially pacing both
ventricles.
INTENSIA CRT-D 174 offers tiered therapy. Therapies can be programmed independently in
each zone:
─in the Slow VT and VT zones: two ATP programs, up to two shocks with programmable
energy and up to four shocks with maximum energy can be programmed;
─in the VF zone: one ATP program, up to two shocks with programmable energy and up
to four shocks with maximum energy can be programmed.
The ATP can be applied in RV, LV or RV and LV pacing with a VV delay equal to 0 ms. ATP
pacing configuration is independent of ventricular pacing configuration.
When the rhythm changes from one zone to another, the device delivers the therapy
programmed in this zone, starting with the same or more aggressive program for the area.
The ATP program in the VF zone will only be applied if the VT coupling interval is longer
than the programmed fast VT cycle length.
The INTENSIA CRT-D 174 offers biphasic shocks with a maximum stored energy of 42 J.
The shock configuration (electrodes used to apply the shock) can be chosen by
programming one of the following combinations: can and one coil, can and two coils, two
coils only.
Other features are as follows:
─Automatic ventricular sensitivity control
─Non-committed shocks
─Electrophysiological studies (EPS) with real-time markers or electrograms:
30 SORIN – INTENSIA CRT-D 174 – U151A

13. DEVICE DESCRIPTION
─Programmer-controlled VT induction sequences,
─Programmer-controlled VF inductions (30 Hz rapid pacing or shock on T),
─Programmable electrogram vectors (EGM A, EGM V, RVcoil-CAN, SVC-CAN, RVcoil-
SVC, LV bip, LV tip-RV),
─Real-time annotations displayed with the markers and indicating the majority rhythm,
─Manual ATP sequences,
─Manual shocks.
─Rescue shock
─Follow-up tests:
─Pacing lead impedance,
─Coil impedance,
─Capacitor charge time,
─Sensitivity test,
─Pacing threshold tests.
─Data storage:
─Therapy History Report,
─Statistics (pace/sense, therapy, shocks, and battery voltage),
─Up to 14 complete Holter records with event logs, marker channel notation, and
electrogram records.
The connector head has three ports:
─Atrial “IS-1” port: performs atrial bipolar pace/sense,
─LV “IS-1” port: performs left ventricular bipolar pace,
─RV “DF-4” port: performs right ventricular bipolar pace/sense, port for RV/SVC
defibrillation coils.
Both pace/sense ports are compatible with the IS-1 standard and defibrillation ports is
compatible with the DF-4 standard.
Distal lead terminal connections are secured with set-screws accessed via self-sealing
silicone plugs. All lead connections pass through the header into the device via
feedthroughs.
Programming System:
The Sorin programmer is used in conjunction with specific programmer software to
interrogate and program the implanted device at implant and during patient follow-up
procedures.
Remote Monitoring:
INTENSIA CRT-D 174 is also equipped with the RF wireless technology which enables to
remotely monitor the patients who have the Sorin SMARTVIEW Monitor installed at home.
SORIN – INTENSIA CRT-D 174 – U151A 31
14. IMPLANT PROCEDURE
14. IMPLANT PROCEDURE
14.1. NECESSARY EQUIPMENT
Implantation of INTENSIA CRT-D 174 requires the following equipment:
─Sorin dedicated programmer, equipped with the SMARTVIEW software interface and
with the programming head,
─pacing system analyzer, as well as its sterile connecting cables, to evaluate the pacing
and sensing thresholds,
─a complete set of leads with corresponding introducers,
─physiological signal monitor capable of displaying simultaneously the surface ECG and
arterial pressure,
─an external defibrillator with sterile external paddles,
─sterile cover for the telemetry head.
14.2. PACKAGING
14.2.1 . Contents
The INTENSIA CRT-D 174 and its accessories are ethylene oxide sterilized and hermetically
sealed in two-ply clear packaging meeting international requirements.
The sterile packaging contains a defibrillator and one screwdriver.
The non-sterile items contained in the outer storage package are the implant manual, the
ICD Registration Form and its envelope, the patient booklet, the ICD ID card and 12
identification labels.
Once delivered, INTENSIA CRT-D 174 is programmed to as-shipped values that are
different from nominal values (see Chapter “Programmable Parameters” for details).
14.3. OPTIONAL EQUIPMENT
The following equipment may be required during implantation of INTENSIA CRT-D 174:
─an IS-1 insulating plug to close the atrial port
─a DF-4/DF-1 adaptor in case of replacement and use of DF-1 lead
─sterile water to clean traces of blood. Any parts cleaned with sterile water must be
thoroughly dried.
─mineral oil to lubricate if necessary
─a lead cap to isolate a lead which is not used
32 SORIN – INTENSIA CRT-D 174 – U151A
NOTE: In case you’re implanting a DF-4 lead, please verify its compatibility with standard
alligators pin; please refer to the lead user’s manual for more details.
14. IMPLANT PROCEDURE
14.4. BEFORE OPENING THE PACKAGE
Before opening the package, check the "Use Before" date printed on the labels on the box
and on the sterile package. Defibrillators that have not been implanted before that date
should be returned to Sorin.
Interrogate the device:
─if a warning is displayed, do not implant the device and contact your Sorin
representative.
─if magnet rate is lower than 91 min-1, and if the last reforming/charge occurred more
than one week ago, do not implant the device. Otherwise wait for one more week before
checking the magnet rate again.
NOTE : The magnet rate and battery voltage can decrease before the expiration date is
reached. However, the magnet rate should be equal to or higher than 91 min-1 at the time of
implant.
Devices MUST NOT be interrogated and programmed within the vicinity of other devices.
Also check the integrity of the sterile package. The sterility of the contents is no longer
guaranteed if the package has been pierced or altered. If the defibrillator is no longer sterile,
it should be returned in its packaging to Sorin. Any re-sterilization of the unit is at the
discretion of Sorin.
14.5. PRIOR TO IMPLANTATION
Use the programmer to verify the defibrillator can be interrogated before implantation.
Verify all shock therapies are disabled in order to avoid accidental discharge during
implantation.
It is not advisable to program the Smoothing function before implantation, since the
defibrillator may detect noise and pace at a rate higher than the programmed basic rate.
14.6. DEVICE PLACEMENT
The pocket should be prepared in the left pectoral position, either subcutaneously or
submuscularly. Subcutaneous device implantation is recommended for optimal RF
communication efficacy.
Implantation in an abdominal position is not advisable.
In its final position, the defibrillator should be no more than 4 cm below the skin surface.
14.7. CHOOSING THE TYPE OF LEAD
The defibrillator should be connected to:
─one bipolar atrial sensing/pacing lead
─one quadripolar/tripolar right ventricular lead with bipolar sensing/pacing electrodes and
one or two defibrillation coils
─one unipolar or bipolar left ventricular pacing lead.
The choice of leads and their configuration is left to the implanting physician’s judgment.
SORIN – INTENSIA CRT-D 174 – U151A 33
CAUTION: Do not shake or tap sharply on the ICD package with the ICD inside, because
the ICD's sensing circuits can interpret this as P-waves or R-waves and record these as an
arrhythmia episode. If unusual shaking or tapping of the package results in a stored
arrhythmia episode, erase the recording before using the ICD.

14. IMPLANT PROCEDURE
Connectors:
The unipolar and bipolar pacing/sensing connectors are compatible with the IS-1 standard
and the quadripolar connector is compatible with the DF-4 standard (refer to the “Lead
evaluation and lead connection” sub-section in the “Warnings and precautions” section).
14.8. SHOCK CONFIGURATION (+ -> -)
The shock configuration is the energy pathway between the defibrillation electrodes. If an
atrial coil is present, the shock configuration can be programmed for bi-directional shocks.
Programming:
When active case and SVC are both programmed to Yes, the shock configuration can be
programmed to:
─RV to Case (or Case to RV),
─or RV to SVC (or SVC to RV),
─or RV to Case+SVC (or Case+SVC to RV).
RV to Case+SVC RV to Case RV to SVC
The polarity of shock is determined by the parameter itself.
14.9. MEASUREMENT OF THRESHOLDS AT IMPLANT
Pacing and sensing thresholds should be measured at implant.
Pacing thresholds:
Acute thresholds should be lower than 1 V (or 2 mA) for a 0.35 ms pulse width, in both
ventricles and in the atrium.
Sensing thresholds:
For proper right ventricular sensing, the amplitude of the R-wave should be greater than 5
mV.
34 SORIN – INTENSIA CRT-D 174 – U151A
NOTE1: Please note that DF-1 standard compliant lead is not compatible with DF-4
connector. In the opposite, DF-4 standard compliant lead is not compatible with DF-1
connector. In case of defibrillator replacement and use of a DF-1 lead or any other lead type
that require an adaptor for this device, please contact your Sorin representative for any
information on lead / connector compatibility question.
NOTE2: In case no atrial lead is implanted, the atrial port should be plugged with IS-1
insulating plug and a single chamber mode (VVI-VVIR) should be programmed. PARAD and
PARAD+ should not be used.
14. IMPLANT PROCEDURE
For proper atrial sensing, the amplitude of the P-wave should be greater than 2 mV.
Pacing impedance measurements:
Right ventricular, left ventricular and atrial pacing impedances should range from 200 to
3000 ohms (refer to the lead characteristics, especially if high impedance leads are used).
14.10. LEADS CONNECTION
Implant the ventricular leads, then the atrial lead.
Each lead must be connected to the corresponding connector port. The position of each
connector is indicated on the casing.
Tighten only the distal inserts.
To connect each lead, proceed as follows:
1. Clean the lead terminal pins thoroughly, if necessary (device replacement).
2. Lubricate the lead terminal pins with sterile water, if necessary.
3. Do not insert a lead connector pin into the connector block without first visually verifying
that the lead port is not filled with any obstacle.
4. Insert the screwdriver into the pre-inserted screw socket of the appropriate port (in order
to allow excess air to bleed out and to make the insertion of the lead pin easier).
5. Insert the lead pin all the way into the port (check that the pin protrudes beyond the distal
insert).
6. Tighten, check the tightness and ensure the lead pin still protrudes beyond the distal
insert, and did not move.
1. Do not tighten the pre-inserted screws when there is no lead (this could damage the
connector).
2. Do not loosen the screws before inserting the connector (subsequent risk of being unable
to reinsert the screw).
3. When mineral oil or sterile water is used to make lead insertion easier, the screwdriver
should remain inserted into the pre-inserted screw socket when checking the tightness.
As a matter of fact, when the lead port is filled with a liquid, the physics piston effect can
give the feeling the lead is properly tightened.
4. One single set screw is located on the side of the connection header.
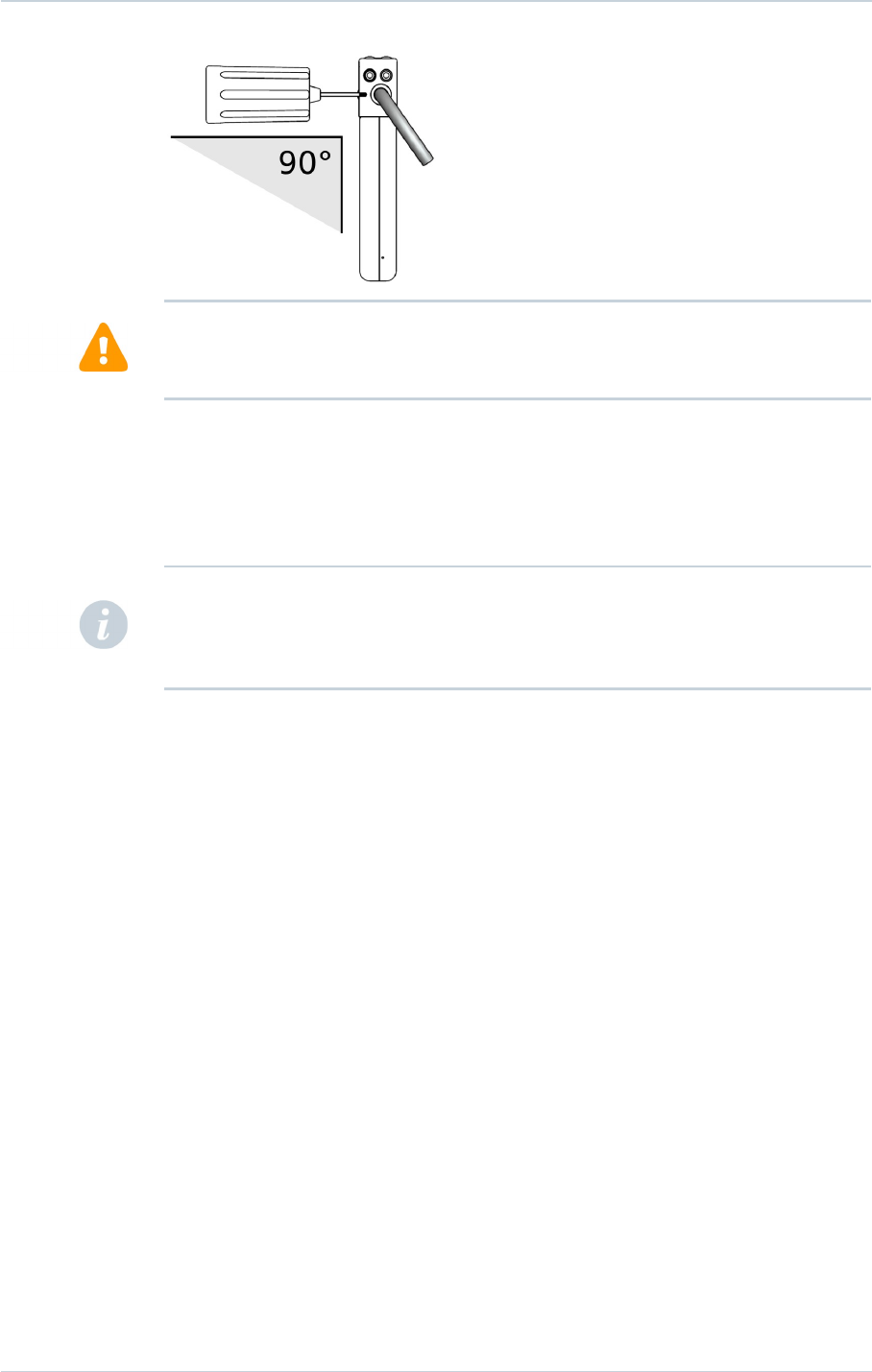
5. Use only the screwdriver provided with the defibrillator. Maintain the screwdriver's shaft
perpendicular to the plane of the defibrillator (see figure below).
6. Removing the screwdriver: to avoid all risk of loosening screws during removal, hold the
screwdriver by its metal part and not by the handle.
SORIN – INTENSIA CRT-D 174 – U151A 35
CAUTION:
CAUTION:
14. IMPLANT PROCEDURE
To ensure full insertion, push the screwdriver's hex tip smoothly into the setscrew until it
reaches the bottom of the hex chamber in the screw, which could be felt as a solid metallic
contact. Do not implant the defibrillator if there is no feeling of solid metallic contact. Do not
implant the defibrillator if the wrench does not click when attempting to tighten the setscrew
on the lead pin.
In the case of an external defibrillation shock delivered to the patient, always check the
programming and functioning of the device, in particular its capacity to deliver shocks.
14.11. DEVICE IMPLANTATION
INTENSIA CRT-D 174 should be implanted with the engraved side facing outwards for
optimal communication with the programming head and radiographic identification.
Carefully wind excess lead and place in a separate pocket to the side of the defibrillator.
It is recommended to not place any excess wire between the can and the heart.
Suture the casing connector to the muscle using the hole provided for this purpose, in order
to avoid potential migration of the device into the pectoral muscle.
14.12. TESTS AND PROGRAMMING
During the implant testing procedure, it is recommended that a security margin of at least 10
J be demonstrated between the effective shock energy and maximum programmable
energy.
Enable shock therapies, then program the defibrillator.
Verify that the defibrillation lead impedance for each shock delivered ranges from 30 to 150
ohms. Check the lead connection if the values are outside these boundaries.
Save the programming data on the programmer’s hard disk and on an external storage
device (if desired).
36 SORIN – INTENSIA CRT-D 174 – U151A
WARNING: Ensure that the screwdriver's tip is fully inserted in the setscrew; otherwise the
screwdriver can damage the setscrew and prevent connection with or disconnection from
the lead.
NOTE: To optimize cardioversion/defibrillation shocks, electrodes must be positioned so that
the electric field between anode(s) and cathode covers the largest myocardial mass. In
normal conditions, the anode and cathode are adequately separated. In case of a short-
circuit, the shock may be aborted to prevent damaging the defibrillator.
15. SPECIAL MODES
15. SPECIAL MODES
15.1. SAFETY MODE (NOMINAL VALUES)
Nominal values may be rapidly restored by pressing the following button on the
programming head or programmer keyboard:
or via the Emergency button on the SMARTVIEW screen.
In safety mode, the defibrillator operates with the parameters underlined in the table of
programmable parameters.
15.2. MAGNET MODE
When the magnet is applied:
─antiarrhythmia functions are inhibited (detection of rhythm disturbances, charging, and
therapy),
─hysteresis, VV delay and AVD paced/sensed offset are set to 0,
─pacing amplitude is set to 6 V,
─pulse width is set to maximum,
─pacing rate is set to the magnet rate,
─the following functions are disabled: ventricular arrhythmia prevention, Mode Switch,
Anti-PMT, Smoothing, Rate Response.
When the magnet is removed:
─the sensor rate is forced to the basic rate,
─arrhythmia detection algorithms and sequential therapies are reinitialized,
─therapies start with the least aggressive program for each area.
The other parameters remain at their programmed value, including the ventricular paced
chamber parameter.
The magnet rate values are as follows:
Magnet rate (bpm) 96 94 91 89 87 85
Magnet period (ms) 625 641 656 672 688 703
Magnet rate (bpm) 83 82 80 78 77
Magnet period (ms) 719 734 750 766 781
15.3. RESPONSE IN THE PRESENCE OF INTERFERENCE
If the defibrillator senses electrical noise at a frequency above 16 Hz, it switches to an
asynchronous mode at the basic rate. The programmed mode is restored as soon as the
noise is no longer detected.
Ventricular pacing is also inhibited by ventricular noise. It can be restored by setting the
parameter V pacing on noise to Yes.
SORIN – INTENSIA CRT-D 174 – U151A 37
15. SPECIAL MODES
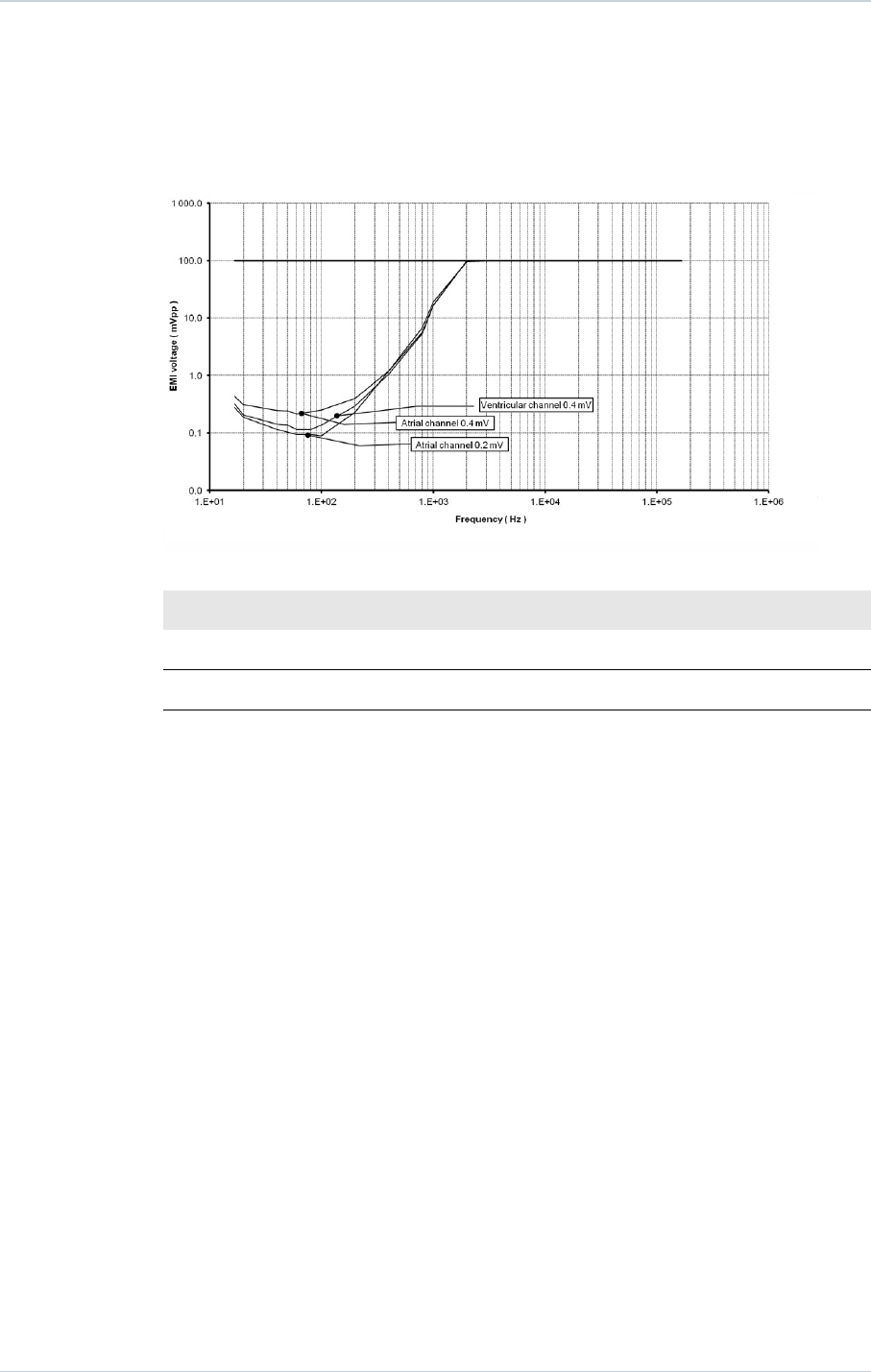
15.4. DETECTION CHARACTERISTICS IN THE PRESENCE OF
ELECTROMAGNETIC FIELDS
Per Clause 27.4 of Standard EN 45502-2-2, detection in the presence of electromagnetic
fields is characterized as follows:
─Differential mode:
─Common mode rejection ratio:
16.6 Hz 50 Hz 60 Hz
Atrial channel ≥ 75 dB 64 dB 62 dB
Ventricular channel ≥ 69 dB ≥ 57 dB ≥ 56 dB
Modulated interference:
For atrial sensitivity setting of 0.2 mV, compliance to the Cenelec standard 45502-2-2 is met
for a maximum test signal amplitude of 8 V for the frequency of 60 MHz. 0.4 mV complies
with the standard for the whole frequency range.
15.5. PROTECTION AGAINST SHORT-CIRCUITS
The defibrillator can undergo a short-circuit if the anode and cathode are not adequately
separated.
In this case, the shock is aborted to prevent damaging the defibrillator and a warning will
indicate that a short circuit (shock impedance < 20 ohms) was detected during the last
shock.
38 SORIN – INTENSIA CRT-D 174 – U151A

16. MAIN FUNCTIONS
16. MAIN FUNCTIONS
16.1. AUTOMATIC LEAD MEASUREMENTS
Automatic pacing lead impedance measurement:
A lead impedance measurement is automatically performed on atrial and ventricular leads
every 6 hours. The daily mean impedance is stored for each chamber.
Automatic coil impedance measurement:
A coil impedance measurement is automatically performed on RV and SVC coils once a
week. The result is stored in the device memory.
16.2. ATRIAL TACHYARRHYTHMIA MANAGEMENT
Mode Switch:
This function is designed to limit the acceleration and variation of ventricular rate in the
presence of atrial arrhythmia.
16.3. VENTRICULAR TACHYARRHYTHMIA MANAGEMENT
Ventricular tachyarrhythmia prevention:
Set of algorithms that can be used to avoid the circumstances of ventricular tachyarrhythmia
onset.
Searching for a long cycle (P And R based Arrhythmia Detection+: PARAD+):
Additional arrhythmia classification criterion to improve identification of atrial fibrillation and
avoid inappropriate shocks.
Fast VT treatment:
Applies detection criteria on fast ventricular tachycardia that are different from those of the
VT zone, as well as different therapies. The fast VT zone is included in the VF zone: its
lower limit is determined by the programmed value for the VF zone and its upper limit by the
programmed value for the fast VT zone.
Polarity alternation on Max shock:
Reverses the programmed polarity of every second shock set at maximum energy. The
number, type, and energy of shocks is independently programmable by detection zone.
16.4. PACING
BTO (Brady Tachy Overlap):
Enables cardiac resynchronization therapy within the slow VT zone to preserve patient
exercise capacity, without affecting detection or treatment of slow VTs.
SORIN – INTENSIA CRT-D 174 – U151A 39

16. MAIN FUNCTIONS
Post-shock mode:
After any automatic shock therapy, the post-shock mode makes it possible to apply a pacing
mode other than the standard antibradycardia pacing mode and/or with different pacing
parameters.
SafeR (AAI <> DDD) mode:
Is intended to minimize deleterious ventricular pacing. The defibrillator functions in AAI
mode, and temporarily switches to DDD mode upon the occurrence of AVB III, AVB II, AVB I
and ventricular pause.
Anti-PMT protection:
Is intended to protect the patient from Pacemaker-Mediated Tachycardia (PMT) without
reducing atrial sensing capability of the device.
16.5. SENSING
Automatic Refractory Periods:
Optimize sensing and make the implant progamming easier. These periods are composed of
a minimal Refractory Period and a triggerable Refractory Period. The duration of the
refractory periods lengthens automatically as needed.
Committed period:
In DDI or DDD modes, the committed period is a non-programmable 95 ms ventricular
relative refractory period that starts with atrial pacing. If a ventricular event is sensed during
the committed period, but outside the blanking period, the ventricle is paced at the end of
the committed period. The committed period prevents inappropriate ventricular inhibition if
crosstalk occurs.
Protection against noise:
Allows the distinction between ventricular noise and ventricular fibrillation. If the device
senses ventricular noise, the ventricular sensitivity is decreased until noise is no longer
detected. Ventricular pacing can be inhibited to avoid a potential paced T-wave.
Automatic sensitivity control:
Optimizes arrhythmia detection and avoids late detection of T-waves and over-detection of
wide QRS waves. The device automatically adjusts the sensitivities based on the ventricular
sensing amplitude. In case of arrhythmia suspicion or after a paced event, the programmed
ventricular sensitivity will be applied. The minimum ventricular sensitivity threshold is 0.4 mV
(minimum programmable value).
16.6. FOLLOW-UP FUNCTION
Storage of memory data:
AIDA+ (Automatic Interpretation for Diagnosis Assistance) software provides access up to 6
months of patient follow-up with day by day data collection, or up to 24 hours with hourly
data collection. Episodes of ventricular tachyarrhythmia are recorded with the programmable
EGM channels: either by selecting up to two traces, or by selecting "Double V" which
enables a one-channel recording that is twice as long.
Alerts / Warnings:
The device routinely performs security self-checks and technical measurements to ensure
system integrity. When system integrity is found to be at risk outside a follow-up, alerts are
40 SORIN – INTENSIA CRT-D 174 – U151A

16. MAIN FUNCTIONS
stored in the device memory. When system integrity is found to be at risk during a follow-up,
the information is managed as a warning (pop-up message) to notify immediately the user.
For example, the following types of event can trigger a warning or an alert: technical
problem during a shock, pacing lead impedance or coil impedance measurements out-of-
range, battery depletion, …
16.7. REMOTE MONITORING FUNCTION
Remote monitoring enables the automatic remote transmission of implant data to the
physician thanks to the wireless Radio Frequency (RF) communication ability of the implant
in order to provide a comprehensive report to the physician about device functioning and
patient cardiac status without having the patient physically in the clinic.
The data is transmitted from the implant and the SMARTVIEW monitor, a small transmitter
placed in the patient home.
Implant data are first transmitted to the SMARTVIEW monitor via RF. Data are then rooted
through the phone line to an internet website. This website is responsible for transforming
the implant data into a comprehensive report that can be consulted by the physician.
16.7.1 . SMARTVIEW Monitor
The SMARTVIEW monitor is a small device equipped with an RF transmission module to
communicate with the implant and a modem to export data through the internet.
The SMARTVIEW monitor is delivered to the patient who has to install it at home. Preferably
the SMARTVIEW monitor will be placed on the nightstand of the patient, as close as
possible to the side of the bed the patient usually sleeps. The SMARTVIEW monitor shall be
connected to the phone line of the patient and the power plug. Regular transmissions are
done during the night when the patient is asleep next to the SMARTVIEW monitor without
any intervention from the patient.
16.7.2 . Transmission trigger
There are 3 different triggers for a remote transmission:
─the remote follow-up transmission is scheduled by the physician to occur regularly
(according to the programming).
─the alert transmission will take place when the implant has recorded an abnormal
event. The list of abnormal event is available in a following paragraph. Alert conditions
are checked daily.
─the on-demand follow-up transmission is triggered by the patient himself through the
use of a specific button on the remote-monitor.
16.7.3 . Data transmitted
The data transmitted are identical to the data available during a standard interrogation with
the dedicated programmer. All counters, histograms, IEGMs and diagnosis available in the
device are transmitted containing (not exhaustive list):
─programmed parameters
─Information on patient and system implanted
─battery status
─lead status (brady leads and defibrillation coils)
─pacing counters and mean heart rate (brady)
SORIN – INTENSIA CRT-D 174 – U151A 41
16. MAIN FUNCTIONS
─atrial and ventricular arrhythmia counters and episodes
─ventricular therapy counters
Data are presented in the form of 2 reports to the physician: the first one contains a
summary of major counters, histograms, warnings and diagnosis. The second one presents
the 3 most important IEGM episodes automatically selected based on the degree of severity
for the patient.
16.7.4 . User website
On the website, the physician is able to:
─consult and schedule the remote follow-ups of their patient
─configure additional ways of being notified of alerts (for instance by SMS, fax or e-mail
─consult, print and export patient reports
16.7.5 . Alert system
The following set of alert trigger can be independently programmed ON/OFF by the
physician using the dedicated programmer and can trigger an alert transmission:
─Low or high impedance (A, RV, LV)
─Abnormal coil impedance (shock lead)
─Low or High shock impedance
─Inefficient high energy shock
─All shocks programmed OFF
─Shock treated VT/VF
─Lack of V pacing in CRT device
─Suspicion of noise on the V lead
─AT/AF occurrence
─Fast V rate during AT/AF
42 SORIN – INTENSIA CRT-D 174 – U151A
WARNING: The use of remote monitoring does not replace regular follow-up. Therefore,
when using remote monitoring, the time period between follow-ups visits may not be
extended.
17. PATIENT FOLLOW-UP
17. PATIENT FOLLOW-UP
17.1. FOLLOW-UP RECOMMENDATIONS
Before the patient is discharged and at each subsequent follow-up visit, it is advisable to:
─check the occurrence of system warnings
─check the battery status,
─check the integrity of the pacing and defibrillation leads,
─check for proper sensing (sensitivity, crosstalk) and pacing ; set the pacing amplitude to
twice the pacing threshold,
─interrogate the implant memories (AIDA+),
─check the efficacy of the therapies delivered,
─keep a printout of programmed parameters, test results, and memory data,
─reset the memory data and statistics.
These operations should be performed by medical personnel in an appropriate care unit,
with resuscitation equipment present.
It is recommended that a routine follow-up examination be done one month after discharge,
and then every three months until the device nears the replacement date.
After a device reset, the magnet rate is equal to 87 bpm; it will be updated within the next 24
hours.
Refer to the online help for a description of displayed warning, and the necessity to contact
Sorin for an evaluation.
Implant software upgrade:
In case a new implant software is downloaded in the device memory through the
programmer, a warning message could be displayed by the programmer to inform the user
and give the proper instructions to follow.
17.2. HOLTER FUNCTION
The Holter records up to 14 tachyarrhythmia episodes as well as the therapy history.
Stored Episodes
INTENSIA CRT-D 174 stores up to 14 episodes (VF, VT, Slow VT, SVT/ST, non-sustained).
For each episode four levels of details are presented:
─Tachogram (to visualize PP and PR intervals)
─Event log for the entire episode:
─PARAD/PARAD+ analysis for each majority,
─Delivered therapies,
SORIN – INTENSIA CRT-D 174 – U151A 43
NOTE: If the last reforming, charge or shock occurred during the week preceding the
interrogation, the last battery value may be still impacted by the event. One week post
event, the battery will recover its steady state value.
17. PATIENT FOLLOW-UP
─Markers: Atrial, ventricular and biventricular markers, sensed, paced and in relative
refractory periods,
─EGM: onset and detection of the arrhythmia, on two therapies, and the return to slow
rhythm by recording electrogram.
Therapy history
For each arrhythmia detection, each therapy delivered (either automatically or during an
electrophysiological study) and at the end of each arrhythmia, INTENSIA CRT-D 174
records the type of majority rhythm, the number of ATP sequences delivered, the energy and
the number of shocks delivered.
17.3. RECOMMENDED REPLACEMENT TIME (RRT)
Recommended Replacement Time (RRT)(1) is controlled by:
─magnet rate equal to 80 ± 1 bpm or
─battery voltage equal to 2.66 V ± 0.01 V
Between the RTT and the EOS (End of Service)(2), INTENSIA CRT-D 174 can still function
for:
─7.4 months (100% atrial and biventricular pacing in DDD mode, 500 ohms, with as-
shipped settings), and deliver 7 shocks at 34 J or
─6.4 months (0% pacing, sensors OFF, one 42 J shock every 2 weeks).
Once the Recommended Replacement Time (RRT) point has been reached, the device
operates normally, except that the charge time increases. Under normal conditions (and
without programmer use) the charge times are as follows:
Shock energy Charge time (sec)
BOS(3) 42 J 10 (± 2)
RTT 42 J 13 (± 3)
(1) Recommended Replacement Time (RRT) corresponds to Elective Replacement
Indicators (ERI) previously used.
(2) End of Service (EOS) corresponds to End of Life (EOL) previously used.
(3) Beginning of Service (BOS) corresponds to Beginning of Life (BOL) previously used.
17.4. EXPLANTATION
The defibrillator should be explanted in the following cases:
─The Recommended Replacement Time (RRT) point is reached
─Confirmed malfunction
─Burial of the patient (for environmental reasons, the local regulation may require the
explantation of the devices containing a battery supply)
─Cremation of the patient (the defibrillator may explode if placed in an incinerator)
The explanted defibrillator should not be reused in another patient.
44 SORIN – INTENSIA CRT-D 174 – U151A
CAUTION: The defibrillator should be replaced as soon as the Recommended Replacement
Time (RRT) point is reached.
17. PATIENT FOLLOW-UP
All explanted defibrillators should be returned to Sorin, carefully cleaned of all traces of
contamination. This may be done by immersing them in an aqueous sodium hypochlorite
containing at least 1% chlorine, followed by rinsing copiously with water.
The defibrillator should be protected against mechanical impact and the temperature
variations that may occur during shipping.
Before explantation, it is advisable to:
─print out all programmed parameters, statistics and Holter function report,
─save Patient data on floppy disk or hard disk,
─disable shock therapies (VT and VF) to avoid any risk of untimely shock.
17.5. DEFIBRILLATOR IDENTIFICATION
The defibrillator can be interrogated and programmed via telemetry, using the programming
head interfaced with the Sorin dedicated programmer.
Position the programming head over the telemetry antenna located in the upper part of the
device, in order to communicate effectively via telemetry (see diagram below).
The device can be non-invasively identified as follows:
1. Take an x-ray to identify the name of the manufacturer and model, printed on the device
(X-ray ID is SDE : S = SORIN ; D = Defibrillator ; E = INTENSIA range).
2. Interrogate the device using the Sorin dedicated programmer. The model and serial
number of the device are automatically displayed. The first figure in the serial number
corresponds to the last figure in the year of manufacture.
SORIN – INTENSIA CRT-D 174 – U151A 45
18. SUPPLEMENTAL INFORMATION
18. SUPPLEMENTAL INFORMATION
Clinical data presented in this section are from the SafeR (AAI <> DDD) clinical study. SafeR
(AAI <> DDD) operation in INTENSIA is similar to that in the Symphony pacemaker. The
data provided are applicable to INTENSIA CRT-D 174.
18.1. ADVERSE EVENTS IN THE SAFER (AAI <> DDD) STUDY
Clinical study of the SafeR (AAI <> DDD) included 45 Symphony 2550 devices implanted in
45 patients. No serious adverse events were device- or feature-related. There were no
deaths in the study.
Table 1: Summary of Symphony safety data during study
Patients Number of events
Number of
patients
% of patients Number of
events
Events per
device year (a)
Deaths 0 0 0 0
Explants 0 0 0 0
Serious
pacemaker
related events
outside the use of
SafeR (AAI <>
DDD)
0000
Non-serious
pacemaker
related events
outside the use of
SafeR (AAI <>
DDD)
0000
Serious events
due to the use of
SafeR (AAI <>
DDD)
0000
Non-serious
events related
due to the use
SafeR (AAI <>
DDD)
13 28.9 15 3.2
Serious non-
pacemaker
related events
6 13.3 9 1.9
Non-serious non-
pacemaker related
events
8 17.8 8 1.7
(a) 4.74 device years
Non-serious events due to the use of SafeR 2 (AAI <> DDD) included: delay in switching on
2nd degree AV block, inappropriate classification of a PAC, disagreement between markers
and recorded EGM, atrial pacing above the maximum rate, recycling on an R-wave in a
46 SORIN – INTENSIA CRT-D 174 – U151A

18. SUPPLEMENTAL INFORMATION
refractory period, and disagreement in the statistics for swuitches to DDD. No patient
symptoms were associated with these events.
18.2. SAFER (AAI <> DDD) CLINICAL STUDY
SafeR (AAI <> DDD) mode in INTENSIA is similar to that in Symphony.
The differences in SafeR (AAI <> DDD) mode between the two devices are:
─To prevent long RR intervals during VT/VF, SafeR (AAI <> DDD) has no effect during
VT/VF therapy, electrophysiologic studies, and post-shock recovery.
─The maximum acceptable AV delay for first degree AV block varies as a function of
pacing rate.
─INTENSIA requires a ventricular sensed event to atrial paced event (RA) interval of at
least 100 ms. Therefore, the device lengthens the atrial escape interval so that it ends at
least 102 ms after the ventricular event.
─During atrial fibrillation episode, pause criterion is fixed to 2s to avoid long bradycardia
episodes in switching to DDD mode.
Despite these differences, the data collected on Symphony devices are applicable to
INTENSIA because the principles of SafeR (AAI <> DDD) operation did not change. The
criteria for switching from AAI to DDD (or vice versa) did not change. The device’s method
for evaluating the presence of AV conduction did not change.
Methods:
All patients were implanted with a Symphony Model 2550 dual-chamber rate-responsive
pacemaker with SafeR (AAI <> DDD) mode. A variety of marketed atrial and ventricular
pacing leads were used. The pacemaker was programmed and interrogated via bi-
directional telemetry using a Sorin dedicated programmer and a CPR3 programming head.
The study’s routine evaluation consisted of enrollment, pre-discharge evaluation, and a
scheduled follow-up visit at one month. At pre-discharge, a 24-hour Holter recording was
performed and pacemaker memory was read. At one month, pacemaker memory was read.
Investigators also documented adverse events.
Patients studied:
A total of 45 patients from 12 centers had Symphony 2550 pacemakers with SafeR (AAI <>
DDD). Of these, 14 (31 %) were female and 31 (69 %) were male. Mean patient age (± SD)
was 74 ± 9 years.
Primary indications for implant were: 1st degree AV block (11.1 %), 2nd degree AV block (6.7
%), 3rd degree AV block (22.2 %), sinus node dysfunction (62.2 %) or other (6.7 %).
Effectiveness results:
To determine the effectiveness of SafeR (AAI <> DDD) mode, the percentage of ventricular
pacing provided over one month was recorded from pacemaker memory.
Thirty-five patients contributed data to evaluate the percentage of ventricular pacing
provided with SafeR (AAI <> DDD). Twenty-nine patients had 1 % or less ventricular pacing
and six patients had a range of 28-97 % ventricular pacing. The graph below shows the
distribution of ventricular pacing observed in patients with and without AV block as a primary
indication for implant.
SORIN – INTENSIA CRT-D 174 – U151A 47

18. SUPPLEMENTAL INFORMATION
The graph shows that many patients programmed to SafeR (AAI <> DDD) had less than 1%
ventricular pacing:
─84 % of patients without AV block at implant.
─63 % of patients with AV block at implant.
In a representative reference group(1) of patients programmed to DDD, none had less than 1
% ventricular pacing and only 10 % had less than 90 % ventricular pacing regardless of AV
block indication at implant.
The actual reduction of ventricular pacing that SafeR (AAI <> DDD) provides in an individual
will depend on the amount of time that the patient spends in AV block. SafeR (AAI <> DDD)
cannot and should not provide any decrease in ventricular pacing while the patient is in AV
block.
(1) Pioger G, Jauvert G, Nitzsché R, Pozzan J, Laure H, Zigelman M, Leny G, Vandrell M,
Ritter P, and Cazeau S. Incidence and predictive factors of atrial fibrillation in paced
patients. PACE, 28, Supp 1: S137-141; January 2005. This was a prospective observational
study of 377 patients with a functionally similar device programmed to DDD. The primary
indications for implant were: AV block (49 %), sinus node disease (16 %), brady-tachy
syndrome (5 %), AV block + sinus node disease (19 %), AV block + brady-tachy syndrome
(6 %), and brady-tachy syndrome + sinus node disease (5 %).
48 SORIN – INTENSIA CRT-D 174 – U151A

19. PHYSICAL CHARACTERISTICS
19. PHYSICAL CHARACTERISTICS
Dimensions 68.4 x 73.4 x 11 mm
Weight 95 g
Volume 38.6 cm3
Active surface area of casing 76 cm2
Connector Atrium: IS-1. Right ventricle: DF-4. Left ventricle: IS-1.
19.1. MATERIALS USED
Active surface area of casing 99% pure titanium
Connectors Polyurethane* and silicone elastomer*
*Medical-grade materials that have undergone "in vitro" and "in vivo" qualifications.
SORIN – INTENSIA CRT-D 174 – U151A 49
20. ELECTRICAL CHARACTERISTICS
20. ELECTRICAL CHARACTERISTICS
Atrial input impedance 80 kilohms ± 30 %
Ventricular input impedance 80 kilohms ± 30 %
D.C. capacitance 148 µF ± 8 %
Capacitor formation No formation required
Rate limit 192 bpm ± 10 bpm
Pacing waveform
Defibrillation waveform
20.1. TABLE OF DELIVERED SHOCK ENERGY AND VOLTAGE
The relationship between stored energies, maximum voltages and delivered energies (at 37
°C, 50 ohm load) for the minimum, low, mean and maximum programmed energy values is
as follows:
Stored energy (J) 0.5 10 20 34 42
V1 (Volt) 75 341 483 631 702
V2 (Volt) 37 173 245 318 353
Delivered E: Phase 1 (J) 0.31 7.0 14.0 23.9 29.6
Delivered E: Phase 2 (J) 0.08 1.8 3.6 6.1 7.5
Delivered E: Total (J) 0.4 8.8 17.6 30.0 37.1
Tolerances are 12% for voltage (25% at 0.5 J) and 30% for energy.
20.2. BATTERY
Manufacturer Greatbatch
Type Quasar High Rate (QHR)
Model GB 2593
Number of batteries 1
50 SORIN – INTENSIA CRT-D 174 – U151A
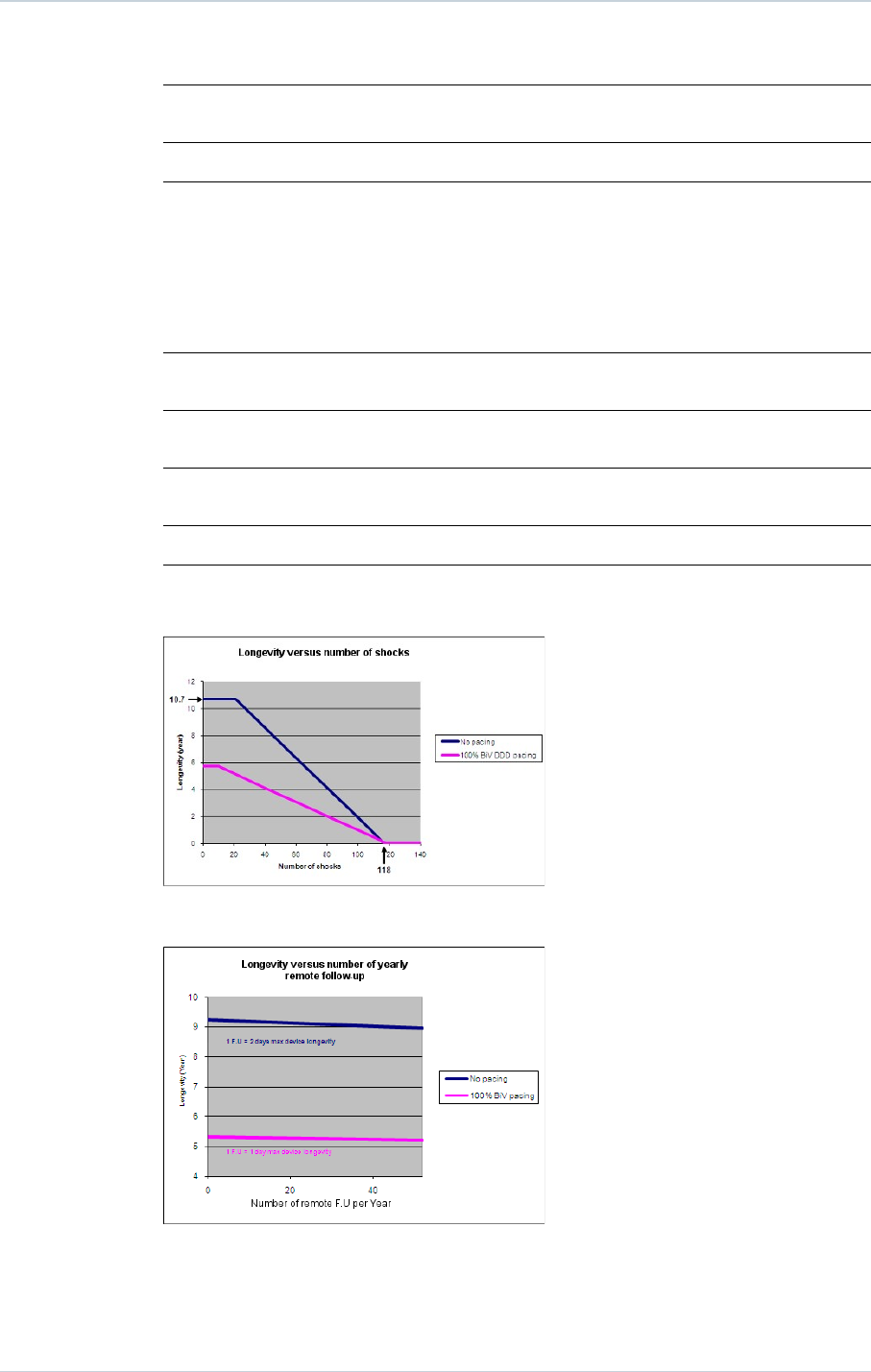
20. ELECTRICAL CHARACTERISTICS
Total capacity 1964 mAh
Usable capacity Between BOS and RRT: 1278 mAh. Between BOS and EOS:
1675 mAh.
Voltage BOS: 3.25 V. RRT: 2.66 V. EOS: 2.5 V.
20.3. LONGEVITY
The longevities mentioned below are calculated by taking into account 6 months storage.
5.1 years Biventricular pacing in DDD mode, 100%, 500 ohm, 3.5 V, 0.35 ms, 60 bpm,
one 42 J shock per quarter, sensor OFF
5.0 years Biventricular pacing in DDD mode, 100%, 500 ohm, 3.5 V, 0.35 ms, 60 bpm,
one 42 J shock per quarter, sensor ON
6.0 years Biventricular pacing in DDD mode, 1% in atrium, 100% in both ventricles, 500
ohm, 3.5 V, 0.35 ms, 60 bpm, one 42 J shock per quarter, sensor OFF
4.2 years Biventricular pacing in DDD mode, 15% in atrium, 100% in both ventricles,
500 ohm, 4.5 V, 0.50 ms, 60 bpm, one 42 J shock per quarter, sensor OFF
9.0 years 0% pacing, one 42 J shock per quarter, sensor OFF
The mean longevity as a function of shocks delivered at maximum energy, with and without
pacing, is as follows:
The mean longevity as a function of yearly remote follow-ups(1), with and without pacing, is
as follows:
(1) An excessive number of remote follow-ups can have a non-negligible impact on device
longevity.
SORIN – INTENSIA CRT-D 174 – U151A 51
21. PROGRAMMABLE PARAMETERS
21. PROGRAMMABLE PARAMETERS
measured at 37 °C under a 500 ohm load
Legend:
Value in bold: "as shipped" value
Underlined value: nominal value
21.1. ANTIBRADYCARDIA PACING
Basic parameters Values
Mode VVI-VVIR-DDD-DDDR-DDD/DDIR-DDI-DDIR-SafeR (AAI
<=> DDD)-SafeR-R (AAIR <=> DDDR)
Basic rate (bpm) (1) From 30 to 90 by steps of 5 ; 60 (± 4 %)
Maximum rate (bpm) From 100 to 145 by steps of 5 ; 120 (± 6 %)
Rate hysteresis (%) 0-5-10-20-35 (± 18 ms)
Rest AV delay (ms) 30-40-45-55-65-70-80-85-95-100-110-115-125-135-140-150-
155-165-170-180-190-195-205-210-220-225-235-250
(± 19 ms)
Exercise AV delay (ms) 30-40-45-55-65-70-80-85-95-100-110-115-125-135-140-150-
155-165-170-180-190-195-205-210-220-225-235-250
(± 19 ms)
AVD Paced/Sensed Offset (ms) 0-10-15-25-30-40-45-55-65-70-80-85-95-100-110-115-125
(± 1 ms)
(1) The corresponding periods are (in ms): 2000-1714-1500-1333-1200-1091-1000-923-857-800-750-
706-667 ms.
Special features Values
Smoothing OFF-Very slow-Slow-Medium-Fast
Mode Switch ON-OFF
Physical activity Very low-Low-Medium-High-Very high
52 SORIN – INTENSIA CRT-D 174 – U151A
21. PROGRAMMABLE PARAMETERS
Pacing/Sensing Values
Atrial sensitivity (mV) (1) From 0.2 to 4 by steps of 0.2 ; 0.4 (± 50 %)
Atrial amplitude (V) (2) 1-1.5-2-2.5-3-3.5-4-4.5-5-6 (± 20 %)
Atrial pulse width (ms) 0.12-0.25-0.35-0.5-0.6-0.75-0.85-1 (± 10 %)
Ventricular sensitivity (mV) (1) From 0.4 to 4 by steps of 0.2 ; 0.4 (± 50 %)
RV amplitude (V) (2) 1-1.5-2-2.5-3-3.5-4-4.5-5-6 (± 20 %)
RV pulse width (ms) 0.12-0.25-0.35-0.5-0.6-0.75-0.85-1 (± 10 %)
LV amplitude (V) (2) 0.25 (± 50 %)
0.5-0.75- (± 30 %)
1-1.25-1.5-1.75-2-2.25-2.5-2.75-3-3.25-3.5-3.75-4-4.25-4.5-
4.75-5-6-7 (± 20 %)
LV pulse width (ms) 0.12-0.25-0.35-0.5-0.6-0.75-0.85-1 (± 10 %)
LV pacing polarity LV bipolar-LV tip to RV ring-LV ring to RV coil
V chambers Right-R+L-L+R
VV delay (ms) 0-8-16-24-32-40-48-56-64 (± 3 ms)
(1) Values are measured using a positive and negative triangular signal of 2/13 ms.
(2) The correlation between the programmed amplitudes, the stored amplitudes and the mid-pulse
delivered amplitudes under a 500 ohm load are given in the following table:
Programmed ampl. (V) 0.25* 0.5* 0.75* 1 1.25* 1.5
Mid-pulse delivered
ampl. (V)
0.28 0.49 0.76 0.97 1.18 1.39
Stored amplitude (V) 0.33 0.57 0.89 1.14 1.38 1.63
Programmed ampl. (V) 1.75* 2 2.25* 2.5 2.75* 3
Mid-pulse delivered
ampl. (V)
1.66 1.79 2.08 2.35 2.56 2.84
Stored amplitude (V) 1.95 2.10 2.44 2.76 3.01 3.33
Programmed ampl. (V) 3.25* 3.5 3.75* 4 4.25* 4.5
Mid-pulse delivered
ampl. (V)
3.05 3.25 3.39 3.58 3.88 4.23
Stored amplitude (V) 3.58 3.82 3.98 4.20 4.55 4.96
Programmed ampl. (V) 4.75* 5 6 7*
Mid-pulse delivered
ampl. (V)
4.36 4.47 5.37 6.26
Stored amplitude (V) 5.12 5.25 6.30 7.35
* For left ventricular amplitude only.
SORIN – INTENSIA CRT-D 174 – U151A 53
21. PROGRAMMABLE PARAMETERS
Ventricular arrhythmia algorithms Values
Atrial pacing on PVC Yes-No
Post extrasystolic pause suppression Yes-No
Acceleration on PVC Yes-No
Max acceleration rate (bpm) From 60 to 145 by steps of 5 ; 100
Post-shock mode Values
Mode OFF-VVI-DDI-DDD
Duration 10s-20s-30s-1min-2min-3min-4min-5min
Basic rate (bpm) From 50 to 90 by steps of 5 ; 60 (± 4 %)
Rest AV delay (ms) 30-40-45-55-65-70-80-85-95-100-110-115-125-135-140-150-
155-165-170-180-190-195-205-210-220-225-235-250
(± 19 ms)
Exercise AV delay (ms) 30-40-45-55-65-70-80-85-95-100-110-115-125-135-140-150-
155-165-170-180-190-195-205-210-220-225-235-250
(± 19 ms)
AVD Paced/Sensed Offset (ms) 0-10-15-25-30-40-45-55-65-70-80-85-95-100-110-115-125
(± 1 ms)
A amplitude (V) 1-1.5-2-2.5-3-3.5-4-4.5-5-6 (± 20 %)
A pulse width (ms) 0.12-0.25-0.35-0.5-0.6-0.75-0.85-1 (± 10 %)
RV amplitude (V) 1-1.5-2-2.5-3-3.5-4-4.5-5-6 (± 20 %)
RV pulse width (ms) 0.12-0.25-0.35-0.5-0.6-0.75-0.85-1 (± 10 %)
LV amplitude (V) 0.25- (± 50 %)
0.5-0.75- (± 30 %)
1-1.25-1.5-1.75-2-2.25-2.5-2.75-3-3.25-3.5-3.75-4-4.25-4.5-
4.75-5-6-7 (± 20 %)
LV pulse width (ms) 0.12-0.25-0.35-0.5-0.6-0.75-0.85-1 (± 10 %)
Refractory periods Values
Atrial refractory period post
ventricular sensing (ms)
45-65-80-95-110-125-140-155 (± 16 ms)
Atrial refractory period post
ventricular pacing (ms)
80-95-110-125-140-155 (± 4 ms)
Sensitivity margins Values
Atrial post pacing/sensing margin
(mV)
From 0 to 1 by steps of 0.2 ; 0.4
Ventricular post pacing margin (mV) From 0 to 2 by steps of 0.2 ; 0.8
54 SORIN – INTENSIA CRT-D 174 – U151A
21. PROGRAMMABLE PARAMETERS
Response to noise Values
Automatic sensitivity on noise ON-OFF
V pacing on noise ON-OFF
SafeR (AAI <=> DDD) parameters Values
AVB I switch Rest+Exercise-Exercise
Long PR: max (ms) From 200 to 500 by steps of 50 ; 450
Long PR: min (ms) From 200 to 500 by steps of 50 ; 250
Max. pause (s) 2-3-4
21.2. VENTRICULAR TACHYARRHYTHMIA DETECTION
Therapy zones Values
Slow VT detection zone (1) Slow VT ON-Slow VT OFF
VT detection zone VT ON-VT OFF
Fast VT / VF detection zone Fast VT+VF ON-VF ON
Slow VT rate (lower limit) (bpm) From 100 to 200 by steps of 5 ; 190
VT rate (lower limit) (bpm) 130-135-140-145-150-155-160-165-170-175-180-185-190-
195-200-210-220-230
VF rate (lower limit) (bpm) 150-155-160-165-170-175-180-185-190-195-200-210-220-
230-240
Fast VT rate (upper limit) (bpm) 155-160-165-170-175-180-185-190-195-200-210-220-230-
240-255
Slow VT persistence (cycles) 4-6-8-12-16-20-30-50-100-200
VT persistence (cycles) 4-6-8-12-16-20-30-50-100-200
VF persistence (cycles) From 4 to 20 by steps of 1 ; 6
(1) The Slow VT zone should be programmed ON only if the VT zone is programmed ON.
SORIN – INTENSIA CRT-D 174 – U151A 55
21. PROGRAMMABLE PARAMETERS
Detection criteria Values
Slow VT and VT detection criteria Rate Only-Stability-Stability+-Stability/Acc-Stability+/Acc-
PARAD-PARAD+
Fast VT detection criteria Rate+Stability-Rate Only
Majority: (X/Y), Y (cycles) 8-12-16
Majority: (X/Y), X (%) 65-70-75-80-90-95-100
Window of RR stability for Slow VT
and VT (ms)
30-45-65-80-95-110-125
Window of RR stability for fast VT
(ms)
30-45-65
Acceleration (%) 6-13-19-25-31-38-44-50
Long cycle persistence extension
(cycles)
From 0 to 16 by steps of 1 ; 10
Long cycle gap (ms) 15-30-45-65-80-95-110-125-140-155-170-190-205
Atrial monitoring Yes-No
21.3. VENTRICULAR TACHYARRHYTHMIA THERAPIES
Common parameters Values
Enable ATP therapy Yes-No
Enable shock therapy Yes-No
ATP pacing chamber Right-Left-R+L
Polarity alternation (42J) Yes-No
Atrial coil (SVC) present Yes-No
Active case Yes-No
Shock configuration (+ --> -) Case to RV-SVC to RV-Case + SVC to RV-RV to Case-RV to
SVC-RV to Case + SVC
SVC exclusion (shock < 15J) Yes-No
56 SORIN – INTENSIA CRT-D 174 – U151A
21. PROGRAMMABLE PARAMETERS
21.3.1 . Therapy parameters in slow VT zone
ATP 1 program Values
ATP program OFF-Burst-Burst+Scan-Ramp-Ramp+Scan
Number of sequences 1-2-3-4-5-6-7-8-9-10-11-12-13-14-15
Cycles in first sequence 1-2-3-4-5-6-7-8-9-10-11-12-13-14-15
Cycles added per sequence 0-1-2-3-4-5-6-7-8-9-10-11-12-13-14-15
Coupling interval (%) 50-55-60-65-70-75-80-85-90-95
Ramp decrement (per cycle) (ms) 0-4-8-12-16-20-30-40-50-60
Scan decrement (per sequence) (ms) 0-4-8-12-16-20-30-40-50-60
Time limit (min) 0.5-1-1.5-2-2.5-3-3.5-4
Minimum cycle length (ms) 95-110-125-140-155-170-190-205-220-235-250-265-280-
295-310
ATP 2 program Values
ATP program OFF-Burst-Burst+Scan-Ramp-Ramp+Scan
Number of sequences 1-2-3-4-5-6-7-8-9-10-11-12-13-14-15
Cycles in first sequence 1-2-3-4-5-6-7-8-9-10-11-12-13-14-15
Cycles added per sequence 0-1-2-3-4-5-6-7-8-9-10-11-12-13-14-15
Coupling interval (%) 50-55-60-65-70-75-80-85-90-95
Ramp decrement (per cycle) (ms) 0-4-8-12-16-20-30-40-50-60
Scan decrement (per sequence) (ms) 0-4-8-12-16-20-30-40-50-60
Time limit (min) 0.5-1-1.5-2-2.5-3-3.5-4
Minimum cycle length (ms) 95-110-125-140-155-170-190-205-220-235-250-265-280-
295-310
Shock program Values
Shock 1 (J) OFF-0.5-0.8-1-1.3-1.5-2-2.5-3-3.5-4-5-6-7-8-9-
10-12-14-16-18-20-22-24-26-28-30-32-34-42
Shock 2 (J) OFF-0.5-0.8-1-1.3-1.5-2-2.5-3-3.5-4-5-6-7-8-9-
10-12-14-16-18-20-22-24-26-28-30-32-34-42
Number of Max. Shock (42 J) OFF-1-2-3-4
SORIN – INTENSIA CRT-D 174 – U151A 57
21. PROGRAMMABLE PARAMETERS
21.3.2 . Therapy parameters in VT zone
ATP 1 program Values
ATP program OFF-Burst-Burst+Scan-Ramp-Ramp+Scan
Number of sequences 1-2-3-4-5-6-7-8-9-10-11-12-13-14-15
Cycles in first sequence 1-2-3-4-5-6-7-8-9-10-11-12-13-14-15
Cycles added per sequence 0-1-2-3-4-5-6-7-8-9-10-11-12-13-14-15
Coupling interval (%) 50-55-60-65-70-75-80-85-90-95
Ramp decrement (per cycle) (ms) 0-4-8-12-16-20-30-40-50-60
Scan decrement (per sequence) (ms) 0-4-8-12-16-20-30-40-50-60
Time limit (min) 0.5-1-1.5-2-2.5-3-3.5-4
Minimum cycle length (ms) 95-110-125-140-155-170-190-205-220-235-250-265-280-
295-310
ATP 2 program Values
ATP program OFF-Burst-Burst+Scan-Ramp-Ramp+Scan
Number of sequences 1-2-3-4-5-6-7-8-9-10-11-12-13-14-15
Cycles in first sequence 1-2-3-4-5-6-7-8-9-10-11-12-13-14-15
Cycles added per sequence 0-1-2-3-4-5-6-7-8-9-10-11-12-13-14-15
Coupling interval (%) 50-55-60-65-70-75-80-85-90-95
Ramp decrement (per cycle) (ms) 0-4-8-12-16-20-30-40-50-60
Scan decrement (per sequence) (ms) 0-4-8-12-16-20-30-40-50-60
Time limit (min) 0.5-1-1.5-2-2.5-3-3.5-4
Minimum cycle length (ms) 95-110-125-140-155-170-190-205-220-235-250-265-280-
295-310
Shock program Values
Shock 1 (J) OFF-0.5-0.8-1-1.3-1.5-2-2.5-3-3.5-4-5-6-7-8-9-
10-12-14-16-18-20-22-24-26-28-30-32-34-42
Shock 2 (J) OFF-0.5-0.8-1-1.3-1.5-2-2.5-3-3.5-4-5-6-7-8-9-
10-12-14-16-18-20-22-24-26-28-30-32-34-42
Number of Max. Shock (42 J) OFF-1-2-3-4
58 SORIN – INTENSIA CRT-D 174 – U151A
21. PROGRAMMABLE PARAMETERS
21.3.3 . Therapy parameters in fast VT / VF zone
ATP 1 program Values
ATP program OFF-Burst-Burst+Scan-Ramp-Ramp+Scan
Number of sequences 1-2-3-4-5-6-7-8-9-10-11-12-13-14-15
Cycles in first sequence 1-2-3-4-5-6-7-8-9-10-11-12-13-14-15
Cycles added per sequence 0-1-2-3-4-5-6-7-8-9-10-11-12-13-14-15
Coupling interval (%) 50-55-60-65-70-75-80-85-90-95
Ramp decrement (per cycle) (ms) 0-4-8-12-16-20-30-40-50-60
Scan decrement (per sequence) (ms) 0-4-8-12-16-20-30-40-50-60
Time limit 10s-20s-30s-1min-1.5min-2min
Minimum cycle length (ms) 95-110-125-140-155-170-190-205-220-235-250-265-280-
295-310
Shock program Values
Shock 1 (J) OFF-0.5-0.8-1-1.3-1.5-2-2.5-3-3.5-4-5-6-7-8-9-
10-12-14-16-18-20-22-24-26-28-30-32-34-42
Shock 2 (J) OFF-0.5-0.8-1-1.3-1.5-2-2.5-3-3.5-4-5-6-7-8-9-
10-12-14-16-18-20-22-24-26-28-30-32-34-42
Number of Max. Shock (42 J) 1-2-3-4
21.4. REMOTE ALERTS AND WARNINGS
The device routinely performs security self-checks and technical measurements to ensure
system integrity. When system integrity is found to be at risk outside a follow-up, alerts are
stored in the device memory. When system integrity is found to be at risk during a follow-up,
the information is managed as a warning (pop-up message) to notify immediately the user.
For example, the following types of event can trigger a warning or an alert: technical
problem during a shock, pacing lead impedance or coil impedance measurements out-of-
range, battery depletion, etc. The Remote tab presents an overview of all the alerts
managed by the device.
General parameters Values
RF communication (1) ON-OFF
Remote alerts (1) ON-OFF
(1) RF and Remote alerts are turned on automatically if Shocks are programmed ON.
When Alerts are programmed "On", the following System Alerts are automatically activated:
─Battery depletion – RRT
─Device reset
─Excessive charge time (>25s)
SORIN – INTENSIA CRT-D 174 – U151A 59
21. PROGRAMMABLE PARAMETERS
─System integrity
Lead Alerts Values
Abnormal A lead impedance ON-OFF
Abnormal A lead low limit (Ohm) 200-250-300-350-400-450-500
Abnormal A lead high limit (Ohm) 1500-1750-2000-2500-3000
Abnormal RV lead impedance ON-OFF
Abnormal RV lead low limit (Ohm) 200-250-300-350-400-450-500
Abnormal RV lead high limit (Ohm) 1500-1750-2000-2500-3000
Abnormal LV lead impedance ON-OFF
Abnormal LV lead low limit (Ohm) 200-250-300-350-400-450-500
Abnormal LV lead high limit (Ohm) 1500-1750-2000-2500-3000
Abnormal RV coil impedance ON-OFF
Abnormal SVC coil impedance ON-OFF
Abnormal Shock impedance (1) ON-OFF
(1) Normal impedance range [20 Ohm-200 Ohm]
Clinical status Values
V oversensing ON-OFF
High AT/AF burden ON-OFF
AT/AF limit (on 24h) (h) 0.5-1-3-6-12-24
Fast V Rate during AT/AF ON-OFF
Fast V Rate limit (bpm) 80-90-100-110-120
Fast V Duration limit (h) 0.5-1-3-6-12-24
Limited % of V pacing in CRT ON-OFF
Limited % of V pacing (%) 50-70-80-85-90-95
Therapy information Values
Shock disabled ON-OFF
Shocks delivered OFF-All shocks-Inefficient shock-Inefficient max shock
60 SORIN – INTENSIA CRT-D 174 – U151A

22. NON PROGRAMMABLE PARAMETERS
22. NON PROGRAMMABLE PARAMETERS
Interval Values
Committed period 95 ms (± 5 ms)
Atrial refractory periods Values
Post atrial sensing 47 ms (± 16 ms)
Post atrial pacing 109 ms (± 4 ms)
Ventricular refractory periods Values
Post ventricular sensing 95 ms (± 16 ms)
Post ventricular pacing 220 ms (± 4 ms)
Post atrial pacing (blanking) 16 ms (± 3 ms)
Tachycardia criteria Values
Window of PR association 63 ms (± 1 ms)
Therapies Values
Waveform (1) Constant tilt (50% - 50%)
Stored energy for the Max. shock 42 J
Pacing amplitude during ATP
therapies
7 V (Actual value at 300 ms: 5.3 V )
Anti-PMT protection Termin
(1) The device has 50% tilt in each phase thus delivers 94% of stored energy. Each phase is limited to
10 ms duration.
SORIN – INTENSIA CRT-D 174 – U151A 61

23. LIMITED WARRANTY
23. LIMITED WARRANTY
The INTENSIA implantable cardioverter defibrillator is the result of highly advanced research
and all components have been selected after exhaustive testing.
Sorin Group Italia S.r.l. (identified as “Sorin” hereafter) guarantees the product INTENSIA
against any damage caused by component failure or production defects during a period of
four years after the implantation date, and Sorin commits itself to replace all INTENSIA
devices according to the terms described in article 1 and described in article 2 of this
section.
Sorin makes no claim that the human body will not react unsuitably to the implantation of the
INTENSIA device, or that failure will never occur.
Sorin does not guarantee the suitability of INTENSIA in defined types of patients; selection
of the device is a medical decision.
Sorin shall not be held liable for any damage indirectly associated with the INTENSIA,
whether as part of normal or abnormal operation, nor damage from its explantation or
replacement.
Sorin does not authorise anyone to modify these limited warranty conditions.
23.1. ARTICLE 1 : TERMS OF LIMITED WARRANTY
1. The INTENSIA implantable cardioverter defibrillator is only guaranteed for the first
implantation.
2. The EURID/Eucomed implant form must be sent to Sorin within 30 days after
implantation.
3. The INTENSIA cardioverter defibrillator must be implanted prior to the use-before date
indicated on the packaging.
4. The limited guarantee only applies to suspect devices returned to the manufacturer,
carefully packed and accompanied by an explantation report duly completed by the
hospital or the doctor and considered defective after analysis by Sorin.
The device must be returned within the 30 days following explantation to Sorin.
Any device returned and replaced under the terms of this limited warranty will become
the exclusive property of Sorin.
Any rights under the terms of this limited warranty will be forfeited if the INTENSIA device
has been opened by anyone other than Sorin.
These rights will also be forfeited if the device has been damaged by carelessness or
accident.
This is the case especially if the device has been exposed to temperatures above 50°C,
to electrical abuse or to mechanical shock, particularly as a result of being dropped.
Consequently, any expert opinion offered by a third party after the device has been
removed also nullifies the guarantee.
5. The limited warranty will be forfeited if it is proven that the device has been misused or
inadequately implanted, against the physicians’manual recommendations of INTENSIA.
6. The limited warranty does not include leads and other accessories used for the
implantation.
7. The replacement terms or conditions described in article 2 include all devices that shall
be replaced within the limited warranty period because of battery depletion, without any
62 SORIN – INTENSIA CRT-D 174 – U151A

23. LIMITED WARRANTY
link to a component failure or a production hazard. The device battery longevity varies
with the type and number of delivered therapies.
8. Legal requirements of jurisdictions where the INTENSIA device is distributed will
supersede any warranty conditions indicated in this manual that conflict with such laws.
23.2. ARTICLE 2 : TERMS OF REPLACEMENT
1. In case of INTENSIA failure because of a component failure, a production defect, or a
conception error, occurring within two-year period starting from the implantation date,
Sorin is committed to:
─replacing free of charge the explanted device by a Sorin device with equivalent
features,
─or issuing a replacement credit equal to the purchase price for the purchase of any
other Sorin replacement device.
2. After a two-year period and up to 4 years after the implantation, Sorin, because of limited
warranty terms, will issue a replacement credit to the buyer of an amount equivalent to
half of the initial purchase price minus prorata temporis during this two-years period.
3. In any case the credit issued by the limited warranty terms cannot exceed the purchase
price of a Sorin replacement device.
SORIN – INTENSIA CRT-D 174 – U151A 63

24. PATENTS
24. PATENTS
The INTENSIA model described in this manual is covered by the following US patents:
5 167 224, 5 226 415, 5 271 394, 5 312 451, 5 325 856, 5 339 820, 5 350 406, 5 411 533, 5
462 060, 5 513 645, 5 545 181, 5 558 097, 5 564 430, 5 591 218, 5 626 619, 5 645 574, 5
674 265, 5 697 960, 5 702 424, 5 702 426, 5 713 928, 5 741 315, 5 776 164, 5 776 165, 5
818 703, 5 836 980, 5 868 793, 5 891 170, 5 891 184, 5 899 931, 5 931 856, 5 935 153, 5
954 660, 5 978 708, 6 181 968, 6 230 058, 6 236 111, 6 251 703, 6 256 206, 6 307 261, 6
337 996, 6 397 105, 6 408 209, 6 487 451, 6 487 452, 6 505 068, 6 532 238, 6 556 866, 6
604 002, 6 622 039, 6 625 491, 6 711 441, 6 738 665, 6 830 548, 6 889 080, 6 898 845, 6
912 421, 6 937 898, 6 975 905, 7 065 402, 7 072 716, 7 076 297, 7 113 826, 7 142 924, 7
164 946, 7 251 526, 7 366 566, 7 400 921, 7 400 922, 7 953 483.
64 SORIN – INTENSIA CRT-D 174 – U151A
25. EXPLANATION OF SYMBOLS
25. EXPLANATION OF SYMBOLS
The symbols on product labelling have the following meaning:
Use by
Date of manufacture
Manufacturer
Serial number
Batch number
For single use only.
Sterilised using ethylene oxide
Temperature limitation
High voltage
Consult instruction for use.
This icon is used to call your attention to a particularly important
point.
This icon alerts you to a hazard that may result in equipment
damage or personal injury. Carefully read the instructions
provided with this icon.
FCC ID : YSGCRTD174
IC : 10270A-CRT-D174
Last revision date of this implant manual: 2013-09
SORIN – INTENSIA CRT-D 174 – U151A 65

Manufactured in Italy by: Distributed by:
Sorin Group Italia S.r.l. Sorin CRM USA, Inc.
Via Crescentino s.n. 14401 West 65th Way
13040 Saluggia (VC) - Italy Arvada, CO 80004 USA
Tel: +39 0161 487095 Tel: 877.663.7674
Facsimile: +39 0161 487524
www.sorin.com
2013-09
U151A
CAUTION: FEDERAL LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN