Sonitus Medical SM03 Hearing System User Manual Users manual
Sonitus Medical Inc. Hearing System Users manual
Contents
- 1. User manual
- 2. Users manual
Users manual

SOUNDBITE™
HEARING SYSTEM
Provider Guide
The treating clinician must review the user manual with the patient as part of the tting process.
Caution: Federal (USA) law restricts this device to sale by or on the order of a licensed
physician, or properly licensed practitioner.

TABLE OF CONTENTS
Indications for Use ................................................................................................................................................ 4
System Description ............................................................................................................................................... 6
Warnings and Precautions .................................................................................................................................... 8
Fitting and Programming ......................................................................................................................................12
Dental Instructions ............................................................................................................................................. 17
Other Information (Clinical Evidence, Cleaning and Charging, Signal Interference) ..................................................................... 19
Technical Description .......................................................................................................................................... 21
PLEASE NOTE:
Instructions for Use are updated periodically. Check www.SonitusMedical.com for the current version of this
document. If an additional paper copy of this Instructions for Use is desired, please contact customer service.
4 5
The SoundBite Hearing System is intended for patients 18 years and older with the following indications:
• Patients with moderately severe, severe, or profound sensorineural hearing loss in one ear and normal hearing in the
other ear (i.e Single-Sided Deafness or SSD). Normal hearing is defined as a pure tone average (PTA) air-conduction
(AC) hearing threshold (measured at 0.5, 1, 2, and 3 kHz) of better than or equal to 25 dB HL
• Patients with conductive hearing loss where the PTA bone conduction hearing threshold (measured at 0.5, 1, 2, and 3
kHz) is better than or equal to 25 dB HL
Additionally, use of SoundBite is intended for patients with:
• At least two contiguous molar or premolar teeth with no untreated tooth decay. Patients with tooth decay present are
to first have restorations before being fitted for SoundBite
• Healthy attachment to those teeth with tooth pockets limited to no more than 5 mm
• No mobile teeth
• Bone loss no greater than a 34% average on the mesial and distal sides of the tooth as measured on X-ray on the
teeth on which the device will be worn
INDICATIONS FOR USE
CONTRAINDICATIONS:
The SoundBite Hearing System and all portions of it are contraindicated for use in an MRI Environment and should
be removed prior to MRI Exposure.
The SoundBite Hearing System should not be used in patients with known hypersensitivity to any of the components
including allergies to polymers.
The SoundBite Hearing System is contraindicated for vulnerable populations such as paraplegics who are unable to
use their hands or others who are unable to comply with the warnings in the product’s labeling.
6 7
The SoundBite Hearing System is a non-surgical and removable bone conduction hearing device designed to transmit
sound through the teeth.
The System consists of the following:
Behind-the-Ear (BTE) microphone unit
The BTE unit is worn on the ear that has the most hearing loss. A small tube connects the
BTE unit to a microphone that is attached to a flexible ear dome and placed in the canal of the
patient’s ear. The microphone location takes advantage of the natural acoustic sound provided
by the shape of the ear. The BTE unit processes sound and transmits the signal wirelessly to the
In-the-Mouth (ITM) device. The BTE unit is removable and rechargeable when docked in the System Charger. When fully
charged, the BTE unit has a minimum of 15 hours of operational life. The BTE can be carried in the small travel case provided.
In-the-Mouth (ITM) hearing device
The ITM device is individually made and placed on the patient’s upper molars on one side. The ITM
device receives the wireless signal from the BTE microphone unit and contains a small component
that converts the signal into tiny vibrations that are sent via the patient’s teeth to the skull and then
to the patient’s inner ear. All components are protected by a watertight seal inside a plastic housing. The ITM device is also
removable and rechargeable when docked in the System Charger. When fully charged, the ITM device has an average of
12 hours of operational life. The ITM can be carried in the small travel case provided.
SYSTEM DESCRIPTION
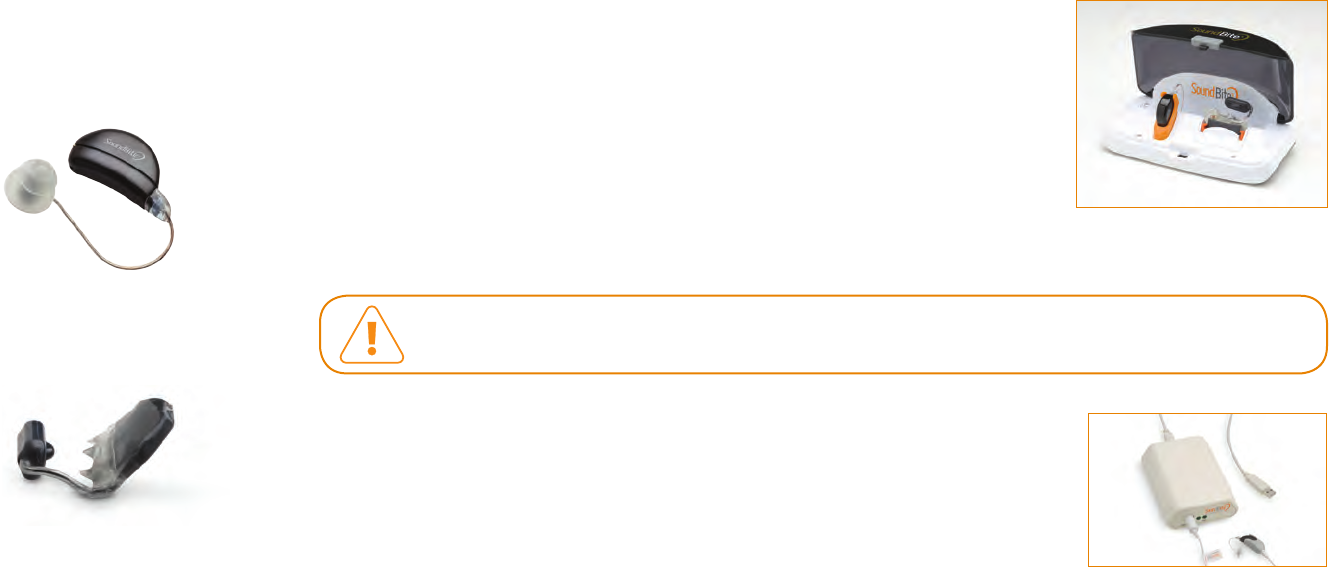
System Charger including power adapter
The Charger has docks to simultaneously recharge the batteries in the ITM device and
BTE unit and is provided with a special AC power adapter for connection to the electrical
outlet. The charger delivers direct contact charging to all components through their gold
contacts. LED lights indicate connection to the power source and individual components,
as well as the charge status of the components. It takes a minimum of 3.5 hours to fully
recharge the ITM device and BTE unit from a completely depleted state.
Use only the system charger and power adapter provided. Other power adapters may look similar but may cause
damage to your SoundBite device.
Programming Software, Box and Cable
Each SoundBite Hearing System is custom programmed for the patient using the
SoundBite programming software provided. Software can be installed onto a computer
with a 32-bit or 64-bit Windows operating system, guided by prompts that will appear
automatically on the screen. For programming, the BTE unit is docked in the boot of the
Programming Cable attached to the Programming Box, which is plugged into the computer via its standard USB port on
the other end. The SoundBite Hearing System is also compatible with programming via the HI-PRO and HI-PRO2 USB.
8 9
WARNINGS (For Users)
• The SoundBite Hearing System and all portions of it are contraindicated for use in an MRI Environment
and should be removed prior to an MRI exam or MRI Exposure. Keep the SoundBite Hearing System
components away from strong magnetic fields.
• If the ITM device is swallowed, seek emergency medical care immediately. Ask the emergency professional to
contact your treatment provider for information regarding the SoundBite device.
• Do not use alcohol or drugs while wearing the device as alcohol and drug use can affect your gagging reflex and
could increase the chance that you could swallow the ITM device.
• If your mouth or the skin in or behind your ear gets sore or irritated, please contact your treatment provider.
• Do not play sports while wearing the device because physical contact with your mouth may damage your teeth
and the device attached to those teeth.
• Be sure that the ITM device unit is dry before placing it in the charger. Do not put a wet device, wet finger, or any
other wet instrument into the charger or charging dock.
• The ITM device and BTE unit components of the SoundBite Hearing System contain batteries that, in some rare
occurrence, could malfunction in a way that may potentially cause discomfort or pain due to electrical current
flow. If you notice any discomfort or pain due to electrical current, immediately remove all components of the
system and contact your treatment provider and Sonitus Medical.
• Keep all components out of the reach of children, pets, or anyone who might swallow them or otherwise
be at risk of being harmed by the device. If any component is swallowed, seek emergency medical
attention immediately.
• Do not allow others to wear or use either component to avoid damage to your device and potential harm to others.
• If the ITM device experiences a hard bite, stop using the system and contact your treatment provider.
• Use only the system charger and power adapter provided. Other power adapters may look similar but may cause
damage to your SoundBite Hearing System.
• The SoundBite Hearing System needs special precautions regarding electromagnetic compatibility (EMC) and
needs to be installed and put into service according to the EMC information provided in the Technical Description.
• Portable and mobile Radio Frequency (RF) communications equipment may affect the performance of your
BTE unit and ITM device.
• The SoundBite Hearing System should not be used adjacent to or stacked with other equipment. If adjacent or
stacked use is necessary, the SoundBite Hearing System should be observed to verify normal operation in the
configuration in which it will be used.
• Do not take the units apart; do not expose them to heat above 140°F (60°C); do not dispose of them in an
incinerator that uses heat to destroy waste.
• Be sure that the Charger is unplugged before cleaning. Be sure that the Charger docks are dry before plugging
Charger back into the wall.

10 11
PRECAUTIONS (For Users)
• Avoid getting the BTE microphone unit very damp, moist or wet. Remove the unit from behind the ear before
showering, bathing, or swimming.
• Do not drop the device as this may cause damage. Be sure to handle the device carefully to keep it in good working
order.
• Protect the devices from very high temperatures. Do not leave any system parts near windows or in a car.
• If you have a history of repeated dizziness and these symptoms occur while wearing the device, you should stop
wearing it and consult with your physician.
• Remove all components of the SoundBite Hearing System before being exposed to X-ray equipment or radiation
machines such as Computer Tomography (CT) scans. These special types of x-ray equipment are not likely to
damage your device but may temporarily affect how well the device works. It is recommended that all components
are removed prior to any such scanning procedures.
• Do not linger in areas where electromagnetic security systems such as anti-theft alarm systems (also known as
electronic article surveillance [EAS]) and metal detector security systems are present and do not lean on such
systems while wearing SoundBite.
• At certain security checkpoints, such as near airport security systems, remove all components of the device and alert
security personnel of the device as necessary.
• Any modifications or changes to your device must be made by a party or agent appointed and approved by Sonitus
Medical, Inc.
• If any component of your system fails to operate or if it is damaged, stop using the device and contact your physician.
• The following precautions should be taken if the ITM device is worn while eating:
• Start slowly by eating softer foods. Consider chewing on the opposite side of your mouth from where the device is
placed.
• Some foods may be difficult to eat because they are too sticky or hard to chew while wearing your device.
• Avoid eating sticky foods and chewing gum because they make it difficult to clean the ITM device and can affect its
function.
• Most food and beverages will not harm the device. But be sure to clean the device thoroughly each time after you
eat to make sure the device works correctly and you maintain good oral health.
• Never use household cleaning products such as bleach to clean your devices as these agents may cause
damage to the device.
• Sonitus Medical makes no claims regarding the use of cell phones with the SoundBite Hearing System.
• SoundBite Hearing Systems may interfere with each other if they are within range of approximately 12 inches or less.
• Some devices, including hand-held computer devices, retail store anti-theft systems and mobile telephones
may cause interference with the SoundBite Hearing System, even if those devices comply with CISPR emission
requirements.
12 13
Prior to your patient’s appointment for tting and programming, create a new patient record in the SoundBite
programming software and gather the recommended supplies listed below.
Device Fitting
ITM FIT
To ensure optimal sound transmission and a comfortable fit, confirm that the ITM device
is properly oriented, with the small side of the device positioned on the outer (cheek side)
tooth surface and the stainless steel wire positioned behind the patient’s back upper
molar, then snap into place.
If the ITM device is loose or uncomfortable, it may not be positioned properly or may need
to be adjusted (See Instructions for Dental Professionals).
FITTING AND PROGRAMMING SOUNDBITE
Device Fitting Device Programming
1. SoundBite Hearing System
(BTE Unit, ITM Device, Charger,
Travel Case)
2. Optional Supplies
(gloves, mouthwash and small
cups, tissues)
Sound Field Testing:
• Calibrated Standard Audiometer
• Calibrated Supraaural (TDH series) or
Circumaural (Sennheiser HDA 200)
Headphones
SoundBite Programming:
• SoundBite Programming Software
• Programming Box (SoundBite or
HI-PRO)
• SoundBite Programming Cable
• External Computer
BTE FIT
Use the microphone sizing tool to select the appropriate microphone tubing size and connect the microphone to the BTE
body by snapping in place. Select the appropriate size dome and connect to the microphone body.
Place the BTE microphone unit on the most impaired ear, taking care to ensure proper placement:
• The BTE unit should rest comfortably behind the patient’s ear.
• The microphone tube and dome should be sized so that the tube provides a good cosmetic
fit and avoids contact with the ear anatomy (e.g. tragus) to the extent possible, with the
dome positioned flush with the opening of the ear canal. Unlike with hearing aids, it is not
necessary to position the SoundBite microphone/dome deep within the ear canal.
Once placed in position on the patient, the BTE and ITM devices will automatically link and begin to transmit sound.
In some cases, it may be necessary to bring the BTE and ITM to within 7 inches to initiate link prior to positioning on
the patient.
14 15
Device Programming
The SoundBite Hearing System is programmed using proprietary software that stores settings on the BTE unit. The
frequency bandwidth of the SoundBite Hearing System spans 250 Hz to 8000 Hz, controlled by settings for 8 discrete
frequency bands. Programming is performed by adjusting the output of the SoundBite Hearing System in each of these
frequency bands so that the patient perceives equivalent sound levels for both ears. This process accounts for any
individual differences in the sound conduction pathway from the tooth to the cochlea.
Program the SoundBite Hearing System in 3 steps:
STEP 1: AIDED THRESHOLD TESTING
In this step, you will establish objectives for device programming and determine what (if any) adjustments are necessary
from factory default settings. After initial device fitting, record Target Thresholds (from a recent audiogram) for the
impaired ear using the Aided Threshold Testing Worksheet.
• Target Thresholds for Single-Sided Deafness patients: Use Thresholds from better ear
• Target Thresholds for Conductive Hearing Loss patients: Use Bone Threshold values of affected ear
In the sound booth, with the SoundBite BTE and ITM in position on the patient, place the audiometer headphones over
the ears. Use the Hughson-Westlake method of testing to obtain Aided Threshold scores for the impaired ear. Record
Aided Threshold values on the Worksheet.
Step 1:
Aided Threshold Testing Step 2: SoundBite
Device Programming Step 3:
Finalize Programming
STEP 2: SOUNDBITE DEVICE PROGRAMMING
Use the Aided Threshold results to customize SoundBite Hearing System settings for optimal
results.
After removing the BTE unit from the patient, dock the BTE in the boot at one end of the
programming cable, with the other end connected to the programming box and computer.
If Aided Threshold Testing values are not within 10dB (without being better than) the Target
Thresholds, adjust the overall gain or the gain in individual frequency bands to program the
device as follows:
Launch programming software and connect BTE unit to the programming cable. Navigate to programming page
and software will link to the BTE unit.
Adjust and save settings: Change gain in individual frequency bands or the overall gain (across all frequencies).
Click “Save to Device” to transfer desired settings to the BTE unit.
Check clinical results of changes: Reposition the BTE unit on your patient in the sound booth and repeat Aided
Threshold Testing with the adjusted settings.
Please note: Adjustments can be completed in as small as 1-decibel increments across frequencies and the Overall Gain
control can be used to add gain across the entire frequency range (Figure 1).
1
2
3

16 17
4
Figure 1
STEP 3: FINAL PROGRAMMING
Confirm final customized settings and add stable gain by activating an advanced feedback-canceling algorithm to reduce
the chances of post-fitting feedback.
Reconnect BTE to programming boot.
Check the “On” box to enable Feedback Canceller. Once enabled, the system will automatically detect and make
adjustments to eliminate feedback within a few seconds.
Click “Save to Device” to save the patient’s configuration.
Click ”Exit To Main Menu” and remove the BTE unit from the programming cable.
5
6
7
7
2
2&6
5
INSTRUCTIONS FOR DENTAL PROFESSIONALS
DENTAL EXAM
A minimum of two contiguous molar or premolar teeth are required to support the ITM device for retention, comfort and
function. The ITM device is made only for the upper teeth.
SoundBite should only be worn on healthy teeth, healthy restorations and healthy gums. All dental disease should be
treated prior to taking impression, and all restorations should be serviceable. X-rays within the last 6 months should be
available and a dental cleaning within the past 6 months is advisable.
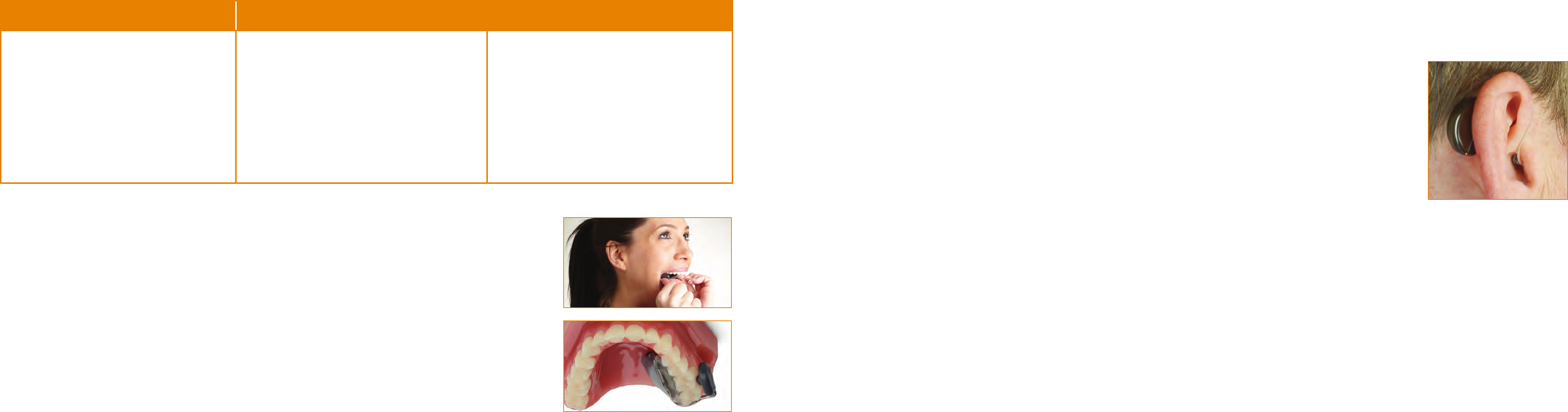
DENTAL MODEL
A full upper model is preferred. However, a quadrant model that captures the midline is also acceptable, using the COE-99
tray. Dental models poured from an alginate impression are preferred.
To return the model to Sonitus Medical, please use provided packaging material. Models should capture dentition as
accurately as possible and be free of defects. Sonitus Medical will inspect each model and will request another model if
one is defective.
Incomplete Quadrant ModelGood Quadrant ModelGood Full Arch Model
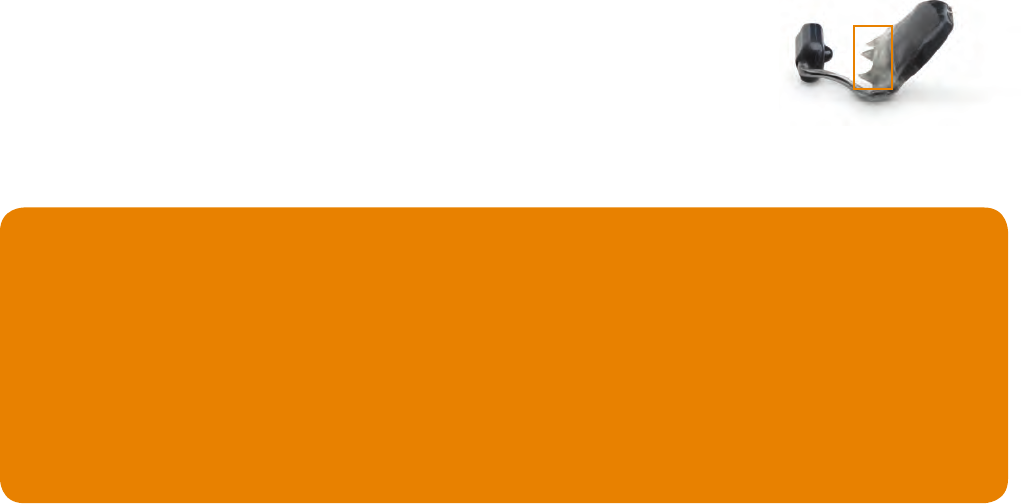
18 19
DEVICE ADJUSTMENT, IF NECESSARY
You must have clear sight into the mouth to evaluate fit and retention. Do not perform any adjustments if you have not
been trained to do so. The ITM device is much like a retainer or partial denture and can be adjusted similarly. Please
note the following:
• DO NOT do any grinding on the metal surfaces or gray surfaces
• DO NOT crimp the metal tube
• The ITM device can be tightened with three prong pliers using very light forces
• Occlusal adjusting can be performed on clear acrylic
Acrylic Section
PLEASE NOTE:
Impressions must be handled and disinfected according to the Center for Disease Control’s (CDC)
recommendation: Guidelines for Infection Control in Dental Health-Care Settings (2003 or latest edition).
The impression should be cleaned before organic soil dries on it and before disinfecting it. Disinfect using an
EPA-registered hospital disinfectant with a tuberculocidal claim, and thoroughly rinsing it after it’s soaked in
the disinfecting solution. Call Sonitus Medical if you have questions about recommended dental materials.
WARNING: The impression must be disinfected according to the CDC guidelines listed above before submission.
OTHER INFORMATION
CLINICAL EVIDENCE
The safety and efficacy of the SoundBite Hearing System have been established in numerous peer-reviewed
publications, which are listed on www.sonitusmedical.com.
CLEANING AND CHARGING
Using the instructions provided with each SoundBite Hearing System User Guide, review cleaning and charging
instructions with your patients. Remind your patients that proper daily care of the SoundBite Hearing System is essential
to ensure it operates well. The ITM device is analogous to a retainer or a partial denture. It is designed for eating and for
wearing during awake hours.
SIGNAL INTERFERENCE
The SoundBite Hearing System contains a wireless link that allows the BTE unit to transmit sound retrieved from the
impaired ear to the ITM device. The system has been tested to applicable regulatory standards, and for interference
with commonly used wireless devices. Should any unusual sounds or other conditions appear, try to reset the devices by
temporarily placing in the charger and then relinking, or recharge the devices if necessary. Refer to the Technical
Description for more detail.
DISPOSAL INSTRUCTIONS
The ITM and BTE contain rechargeable lithium ion batteries. Dispose of them according to local regulations.
20 21
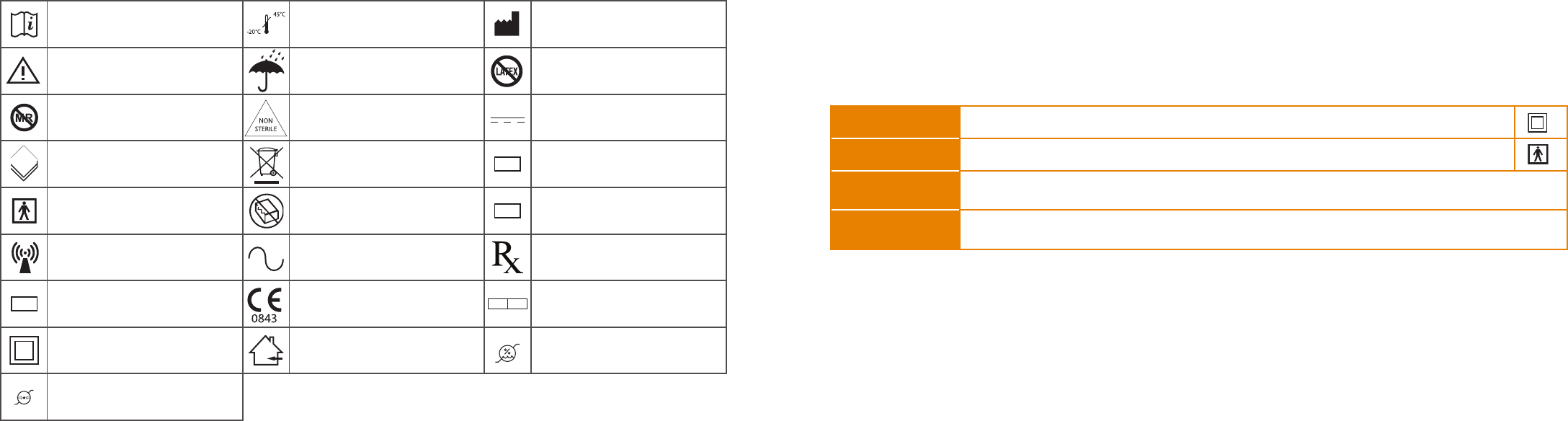
SYMBOLS
Explanation of Symbols Used on Equipment and Accompanying Instructions
Consult Instructions for Use Storage and transport limits Manufactured by
Caution, consult
accompanying documents Keep dry
No Latex
Latex free
Magnetic Resonance (MR)
Unsafe Non-sterile Direct current
Contents of package/box Dispose of in accordance
with local regulations
LOT Lot number
Type BF applied part Do not use if package is
damaged
SN Serial number
Non ionizing radiation Alternating Current Prescription use only
REF Catalog number CE Mark EC REP Authorized Representative
Class II equipment Indoor use only
15%
85%
Storage and transport relative
humidity limits
61 kPA
101 kPA Storage and transport
atmospheric pressure limits
POWER REQUIREMENTS
Power Supply Input: 100 – 240 VAC, 50-60Hz, 0.4A Power Supply Output: 5.0 VDC, 1A
Compatible with: Mepos UE08WCP-050100SPA; Franmar FRM06-S05-Z
WIRELESS TECHNOLOGY
Type: NFMI Frequency: 10.597MHz Receiver BW: 380kHz
Modulation type: CPFSK Channel data rate: 298 kbps Wireless range: 10 inches (25 cm) Effective Radiated Power: <0.1 mW
Quality of Service
The SoundBite Hearing System continuously monitors the audio quality on the incoming audio stream from the BTE and allows for just
0.1% error in the audio output to ensure a high delity audio stream being delivered to the patient. Further the SoundBite Hearing System
ensures a constant delay between the incoming audio from the BTE and the delivered audio to the patient through the ITM.
SOUNDBITE HEARING SYSTEM
TECHNICAL DESCRIPTION
Battery Charger Class II
ITM and BTE Internally Powered Equipment Type BF
ITM The ITM rated IP27 is protected against insertion of ngers and will not be damaged or become unsafe during a
specied test in which is it exposed to immersion up to 1 meter.
BTE The BTE rated IP22 is protected against insertion of ngers and will not be damaged or become unsafe during a
specied test in which it is exposed to vertically or nearly vertically dripping water
DEVICE CLASSIFICATION
22 23
Security
The SoundBite Hearing System wireless technology security features include:
• No patient-specic information is stored inside the BTE or the ITM.
• The wireless link of the SoundBite Hearing System is only in proximity to the head so any intruder to the SoundBite Hearing System is
required to be in close range.
• All audio transmitted across the wireless link is compressed with an ITU G.722 encoding.
RF Exposure
The SoundBite Hearing System has been tested to comply with IEEE C95.1 “IEEE standard for Safety Levels with respect to Human Exposure
to Radio Frequency Electromagnetic elds, 3kHz to 300GHz” to ensure the SoundBite Hearing System does not expose patients to unsafe
levels of radiation.
FCC Compliance Statement
This device complies with part 15 of the FCC Rules. Operation is subject to the following two conditions: (1) This device may not cause
harmful interference, and (2) this device must accept any interference received, including interference that may cause undesired operation.
Changes or modications not expressly approved by Sonitus Medical could void the user’s authority to operate the equipment.
This equipment has been tested and found to comply with the limits for a Class B digital device, pursuant to part 15 of the FCC Rules.
These limits are designed to provide reasonable protection against harmful interference in a residential installation. This equipment
generates, uses and can radiate radio frequency energy and, if not installed and used in accordance with the instructions, may cause
harmful interference to radio communications. However, there is no guarantee that interference will not occur in a particular installation.
If this equipment does cause harmful interference to radio or television reception, which can be determined by turning the equipment off
and on, the user is encouraged to try to correct the interference by one or more of the following measures:
• Reorient or relocate the receiving antenna.
• Increase the separation between the equipment and receiver.
• Connect the equipment into an outlet on a circuit different from that to which the receiver is connected.
• Consult the dealer or an experienced radio/TV technician for help.
Electromagnetic Compatibility Compliance Statement
The following accessories supplied with the SoundBite Hearing System have been tested for electromagnetic emissions compliance.
List of all cables utilized with the SoundBite Hearing System
Cable Type Cable Description Max Cable Length Cable Type Cable Model Number
2-conductor 34AWG
shielded cable BTE Programming Cable 7 feet New England Wire
Technologies 3340
USB Type A to Type B 28AWG
1P + 28AWG 2C, Shielded Programmer USB Cable 6 feet Various Various
Warning: Use of accessories, transducers and cables other than those specied by Sonitus Medical, Inc. may result in
increased EMISSIONS or decreased IMMUNITY of the SoundBite Hearing System.
Emissions Test Compliance Electromagnetic Environment – Guidance
RF emissions CISPR 11 Group 1 The SoundBite Hearing System uses RF energy only for its internal function.
Therefore, its RF emissions are very low and are not likely to cause any
interference in nearby electronic equipment.
RF emissions CISPR 11 Class B The SoundBite Hearing System is suitable for use in all establishments, including
domestic establishments and those directly connected to the public low-voltage
power supply network that supplies buildings used for domestic purposes.
Harmonic emissions IEC 61000-3-2 Class A
Voltage uctuations/icker emissions IEC 61000-3-3 Complies
Guidance and manufacturer’s declaration – electromagnetic emissions
The SoundBite Hearing System is intended for use in the electromagnetic environment specied below.
The customer or user of the SoundBite Hearing System should assure that it is used in such an environment.
2524
Guidance and manufacturer’s declaration - electromagnetic immunity
The SoundBite Hearing System is intended for use in the electromagnetic environment specied below.
The customer or user of the SoundBite Hearing System should assure that it is used in such an environment.
Immunity Test IEC 60601 Test Level Compliance Level Electromagnetic Environment - Guidance
Electrostatic discharge (ESD)
IEC 61000-4-2
+/- 6 kV contact
+/- 8 kV air
+/- 6 kV contact
+/- 8 kV air
Floors should be wood, concrete, or ceramic tile. If
oors are covered with synthetic material, the relative
humidity should be at least 30%.
Electrical fast transient/burst
IEC 61000-4-4
+/- 2 kV for power supply lines
+/- 1 kV for input/output lines
+/- 2 kV for power supply
lines
Mains power quality should be that of a typical
commercial or hospital environment.
Surge IEC 61000-4-5 +/- 1 kV differential mode
+/- 2 kV common mode +/- 1 kV differential mode Mains power quality should be that of a typical
commercial or hospital environment.
Voltage dips, short
interruptions and voltage
variations on power supply
input lines
IEC 61000-4-11
<5% UT (>95% dip in UT)
for 0.5 cycle
40% UT (60% dip in UT)
for 5 cycles
70% UT (30% dip in UT)
for 25 cycles
<5% UT (>95% dip in UT)
for 5 sec
<5% UT (>95% dip in UT)
for 0.5 cycle
40% UT (60% dip in UT)
for 5 cycles
70% UT (30% dip in UT)
for 25 cycles
<5% UT (>95% dip in UT)
for 5 sec
Mains power quality should be that of a typical
commercial or hospital environment. If the user of the
SoundBite System requires continued operation during
power mains interruptions, it is recommended that the
SoundBite System be powered from an uninterruptible
power supply or battery.
(50/60 Hz) magnetic eld
IEC 61000-4-8 3 A/m 3 A/m
Power frequency elds should be at levels characteristic
of a typical location in a typical commercial or hospital
environment.
NOTE: UT is the a.c. mains voltage prior to application of the test level.
Guidance and manufacturer’s declaration – electromagnetic immunity
The SoundBite Hearing System is intended for use in the electromagnetic environment specied below. The customer or user of the
SoundBite Hearing System should assure that it is used in such an environment.
Immunity Test IEC 60601 Test Level Compliance
Level Electromagnetic Environment – Guidance
Portable and mobile RF communications equipment should be used no
closer to any part of the SoundBite Hearing System, including cables,
than the recommended separation distance calculated from the equation
applicable to the frequency of the transmitter.
Recommended separation distance
d
= 1.2√
P
d
= 1.2√
P
80 MHz to 800 MHz
d
= 2.3√
P
800 MHz to 2.5 GHz
Where
P
is the maximum output power rating of the transmitter in watts
(W) according to the transmitter manufacturer and
d
is the recommended
separation distance in meters (m).
Field strengths from xed RF transmitters, as determined by an
electromagnetic site survey,a should be less than the compliance level
in each frequency range.b
Interference may occur in the vicinity of equipment
marked with the following symbol:
NOTE 1: At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reection from structures, objects and people.
a Field strengths from xed transmitters, such as base stations for radio (cellular/cordless) telephones and land-mobile radios, amateur radio, AM and FM radio broadcast and TV
broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to xed RF transmitters, an electromagnetic site survey should be considered.
If the measured eld strength in the location in which the SoundBite Hearing System is used exceeds the applicable RF compliance level above, the SoundBite Hearing System should
be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as reorienting or relocating the SoundBite Hearing System.
b Over the frequency range 150 kHz to 80 MHz, eld strengths should be less than 3 V/m.
Conducted RF IEC 61000-4-6 3 Vrms; 150 kHz to 80 MHz 3 Vrms
Radiated RF IEC 61000-4-3 3 V/m; 80MHz to 2.5 GHz 3 V/m
26
FOLLOW-UP VISITS
TREATING CLINICIAN: A 30-day appointment is recommended to assess how your patient is adapting to the device
and to make device adjustments, if necessary. Once a year, an audiological exam for your patient is recommended.
TREATING DENTIST: A 6-month appointment is recommended for your patient.
OPERATING CONDITIONS
Charger and BTE: 0°C to 40°C (32°F to 104°F) @ 5-95% Rel. Humidity
ITM: 10°C to 40°C (50°F to 104°F) © up to 100% Rel. Humidity
70 kPa to 106 kPa Atmospheric Pressure
STORAGE AND TRANSPORT CONDITIONS
-20°C to 45°C (-4°F to 113°F) © <85% Rel. Humidity
61 kPa to 101 kPa Atmospheric Pressure
WARRANTY
Reasonable care has been taken in the design and manufacture
of this product. Under normal wear conditions, the BTE unit and ITM
device are under warranty for up to 3 years, as specified by each
user’s contract during which time they will be replaced or repaired
for manufacturing defects.
EC REP
EC Representative Contact Information:
Emergo Europe, Molenstraat 15, 2513 BH
The Hague, Netherlands
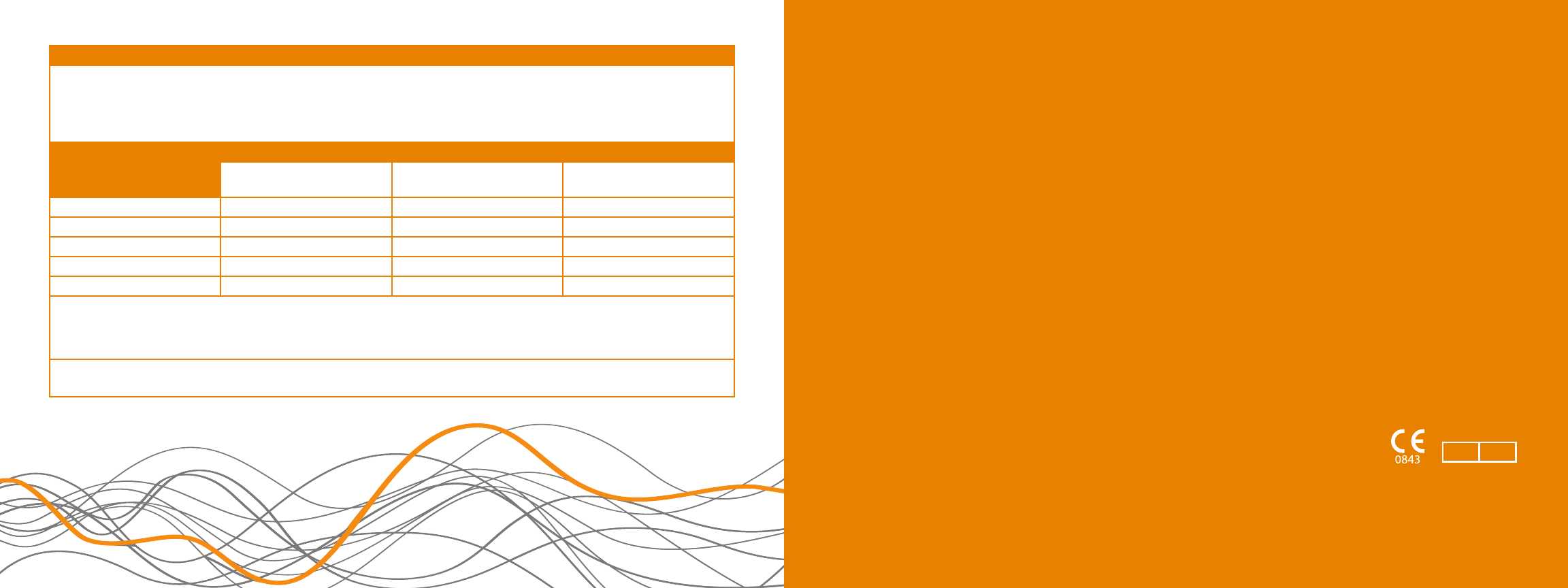
Recommended separation distances between portable and mobile RF communications equipment and the SoundBite Hearing System
The SoundBite Hearing System is intended for use in the electromagnetic environment in which radiated RF disturbances are controlled. The
customer or the user of the SoundBite Hearing System can help prevent electromagnetic interference by maintaining a minimum distance between
portable and mobile RF communications equipment (transmitters) and the SoundBite Hearing System as recommended below, according to the
maximum output power of the communications equipment.
Rated maximum output power
of transmitter
(W)
Separation distance according to frequency of transmitter (m)
150 kHz to 80 MHz
d
= 1.2√
P
80 MHz to 800 MHz
d
= 1.2√
P
800 MHz to 2.5 GHz
d
= 2.3√
P
0.01 0.12 0.12 0.23
0.1 0.37 0.37 0.74
11.17 1.17 2.33
10 3.69 3.69 7.39
100 11.67 11.67 23.33
For transmitters rated at a maximum output power not listed above, the recommended separation distance
d
in meters (m) can be estimated using
the equation applicable to the frequency of the transmitter, where
P
is the maximum output power rating of the transmitter in watts (W) according to
the transmitter manufacturer.
NOTE 1: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reection from structures, objects and people.

Sonitus Medical
1900 Alameda De Las Pulgas, Suite 200
San Mateo, CA 94403 USA
Phone: 866 816 2076
Fax: 650 838 0326
www.sonitusmedical.com
www.soundbitehearing.com
MP 11-0141-B
© Copyright 2014. Sonitus Medical, Inc. All Rights Reserved.
Manufactured By:
This device complies with Industry Canada licence-exempt RSS standard(s). Operation is subject to the
following two conditions: (1) this device may not cause interference, and (2) this device must accept any
interference, including interference that may cause undesired operation of the device.
Cet appareil est conforme avec Industrie Canada exempts de licence standard RSS (s ) . Son
fonctionnement est soumis aux deux conditions suivantes : ( 1 ) cet appareil ne peut pas provoquer
d'interférences , et ( 2) cet appareil doit accepter toute interférence , y compris les interférences qui
peuvent causer un mauvais fonctionnement de l'appareil.
CAN ICES-3 (B)/NMB-3(B)
Draft