 sds-4880-4888 en uk
sds-4880-4888 en uk 4880-4888 Sn63Pb37 RA Solder Wire
MG Chemicals UK Limited
Version No: A-1.02 Safety Data Sheet (Conforms to Regulation (EU) No 2015/830)
Issue Date: 09/07/2019 Revision Date: 08/12/2020
L.REACH.GBR.EN
SECTION 1 IDENTIFICATION OF THE SUBSTANCE / MIXTURE AND OF THE COMPANY / UNDERTAKING
1.1. Product Identifier Product name Synonyms
Other means of identification
4880-4888 Sn63Pb37 RA Solder Wire
SDS Code: 4880-4888; 4880-18G, 4884-227G, 4884-454G, 4885-227G, 4885-454G, 4886-227G, 4886-454G, 4887-227G, 4887-454G, 4888-227G, 4888-454G
Not applicable
1.2. Relevant identified uses of the substance or mixture and uses advised against
Relevant identified uses Uses advised against
leaded solder wire FOR INDUSTRIAL USE ONLY
1.3. Details of the supplier of the safety data sheet
Registered company name Address
MG Chemicals UK Limited
Heame House, 23 Bilston Street, Sedgely Dudley DY3 1JA United Kingdom
Telephone Fax
+(44) 1663 362888 Not Available
Website Email
Not Available sales@mgchemicals.com
MG Chemicals (Head office)
9347 - 193 Street Surrey V4N 4E7 British Columbia Canada
+(1) 800-201-8822 +(1) 800-708-9888 www.mgchemicals.com Info@mgchemicals.com
1.4. Emergency telephone number
Association / Organisation
Emergency telephone numbers
Other emergency telephone numbers
Verisk 3E (Access code: 335388) +(44) 20 35147487
+(0) 800 680 0425
SECTION 2 HAZARDS IDENTIFICATION
2.1. Classification of the substance or mixture
Classification according to regulation (EC) No 1272/2008
[CLP] [1]
Legend:
H360 - Reproductive Toxicity Category 1A, H362 - Lactation Effects, H372 - Specific target organ toxicity - repeated exposure Category 1, H351 Carcinogenicity Category 2
1. Classified by Chemwatch; 2. Classification drawn from Regulation (EU) No 1272/2008 - Annex VI
2.2. Label elements
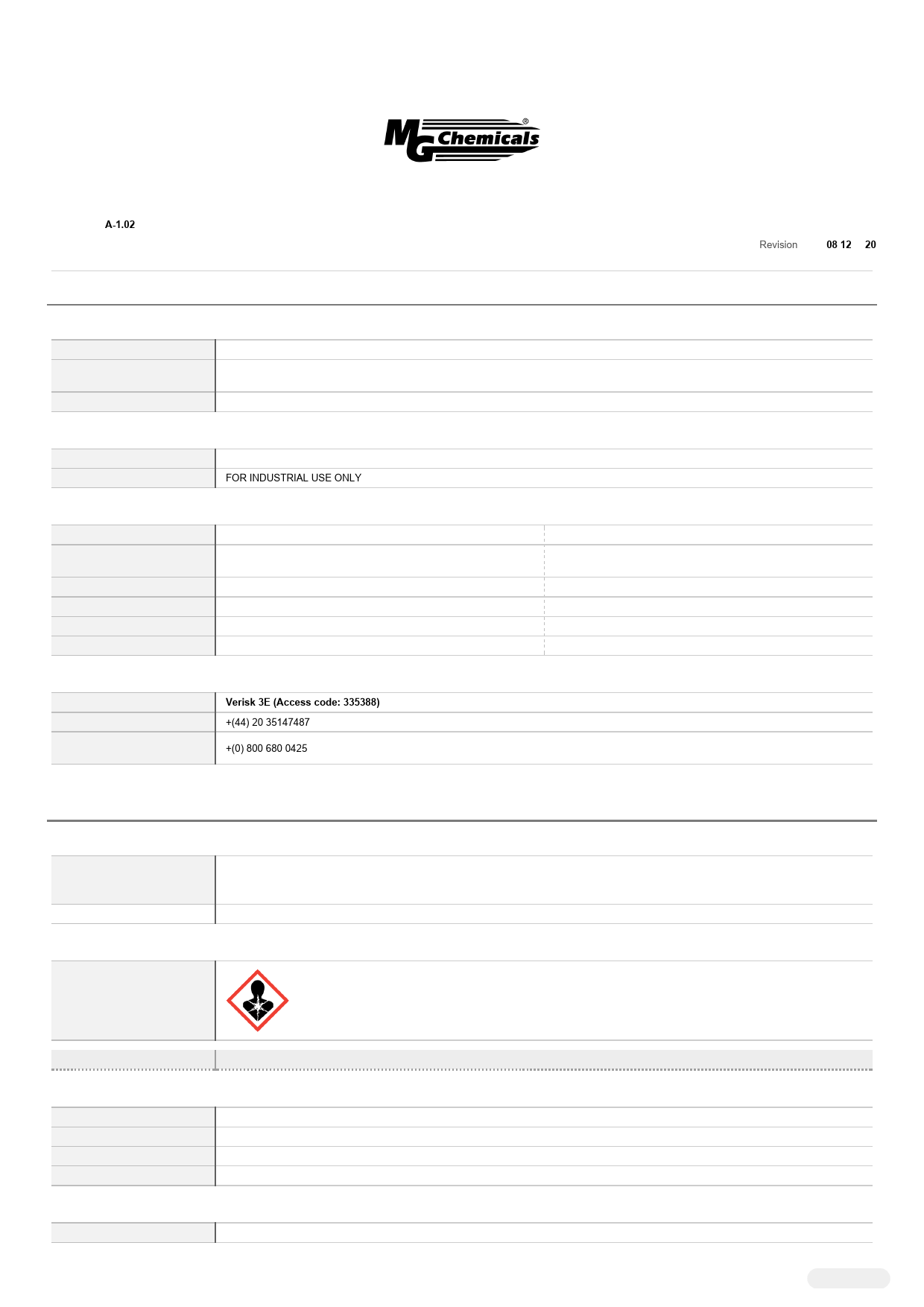
Hazard pictogram(s)
SIGNAL WORD DANGER
Hazard statement(s)
H360 H362 H372 H351
May damage fertility or the unborn child. May cause harm to breast-fed children. Causes damage to organs through prolonged or repeated exposure. Suspected of causing cancer.
Supplementary statement(s) EUH201
Contains lead. Should not be used on surfaces liable to be chewed or sucked by children
Continued...
�Page 2 of 14
4880-4888 Sn63Pb37 RA Solder Wire
Precautionary statement(s) Prevention
P201 P260 P263
Obtain special instructions before use. Do not breathe dust/fume/gas/mist/vapours/spray. Avoid contact during pregnancy and while nursing.
P280 P270
Wear protective gloves/protective clothing/eye protection/face protection. Do not eat, drink or smoke when using this product.
Precautionary statement(s) Response P308+P313 IF exposed or concerned: Get medical advice/ attention. P314 Get medical advice/attention if you feel unwell.
Precautionary statement(s) Storage P405 Store locked up.
Precautionary statement(s) Disposal P501 Dispose of contents/container in accordance with local regulations.
2.3. Other hazards
lead Listed in the European Chemicals Agency (ECHA) Candidate List of Substances of Very High Concern for Authorisation lead Listed in the Europe Regulation (EC) No 1907/2006 - Annex XVII (Restrictions may apply)
SECTION 3 COMPOSITION / INFORMATION ON INGREDIENTS
3.1.Substances See 'Composition on ingredients' in Section 3.2
3.2.Mixtures
1.CAS No 2.EC No 3.Index No 4.REACH No
1.7440-31-5 2.231-141-8 3.Not Available 4.01-2119486474-28-XXXX
1.7439-92-1 2.231-100-4 3.082-013-00-1|082-014-00-7 4.01-2119513221-59XXXX|01-2120762789-33-XXXX
1.65997-05-9 2.500-163-2 3.Not Available 4.01-2119964093-37-XXXX
Legend:
%[weight]
Name
62
tin *
36
lead
Classification according to regulation (EC) No 1272/2008 [CLP] EUH210 [1] Lactation Effects, Reproductive Toxicity Category 1A; H362, H360FD [2]
2
rosin, polymerised
Not Applicable
1. Classified by Chemwatch; 2. Classification drawn from Regulation (EU) No 1272/2008 - Annex VI; 3. Classification drawn from C&L; * EU IOELVs available
SECTION 4 FIRST AID MEASURES
4.1. Description of first aid measures
Eye Contact Skin Contact
If this product comes in contact with the eyes: Wash out immediately with fresh running water. Ensure complete irrigation of the eye by keeping eyelids apart and away from eye and moving the eyelids by occasionally lifting the upper and lower lids. Seek medical attention without delay; if pain persists or recurs seek medical attention. Removal of contact lenses after an eye injury should only be undertaken by skilled personnel. DO NOT attempt to remove particles attached to or embedded in eye . Lay victim down, on stretcher if available and pad BOTH eyes, make sure dressing does not press on the injured eye by placing thick pads under dressing, above and below the eye. Seek urgent medical assistance, or transport to hospital.
If skin or hair contact occurs: Flush skin and hair with running water (and soap if available). Seek medical attention in event of irritation.
In case of burns: Immediately apply cold water to burn either by immersion or wrapping with saturated clean cloth. DO NOT remove or cut away clothing over burnt areas. DO NOT pull away clothing which has adhered to the skin as this can cause further injury. DO NOT break blister or remove solidified material. Quickly cover wound with dressing or clean cloth to help prevent infection and to ease pain. For large burns, sheets, towels or pillow slips are ideal; leave holes for eyes, nose and mouth. DO NOT apply ointments, oils, butter, etc. to a burn under any circumstances. Water may be given in small quantities if the person is conscious. Alcohol is not to be given under any circumstances.
Continued...
�Page 3 of 14
4880-4888 Sn63Pb37 RA Solder Wire
Inhalation Ingestion
Reassure. Treat for shock by keeping the person warm and in a lying position. Seek medical aid and advise medical personnel in advance of the cause and extent of the injury and the estimated time of arrival of the patient.
If dust is inhaled, remove from contaminated area. Encourage patient to blow nose to ensure clear breathing passages. Ask patient to rinse mouth with water but to not drink water. Seek immediate medical attention.
Immediately give a glass of water. First aid is not generally required. If in doubt, contact a Poisons Information Centre or a doctor.
4.2 Most important symptoms and effects, both acute and delayed See Section 11
4.3. Indication of any immediate medical attention and special treatment needed Treat symptomatically.
Copper, magnesium, aluminium, antimony, iron, manganese, nickel, zinc (and their compounds) in welding, brazing, galvanising or smelting operations all give rise to thermally produced particulates of smaller dimension than may be produced if the metals are divided mechanically. Where insufficient ventilation or respiratory protection is available these particulates may produce 'metal fume fever' in workers from an acute or long term exposure.
Onset occurs in 4-6 hours generally on the evening following exposure. Tolerance develops in workers but may be lost over the weekend. (Monday Morning Fever) Pulmonary function tests may indicate reduced lung volumes, small airway obstruction and decreased carbon monoxide diffusing capacity but these abnormalities resolve after several months. Although mildly elevated urinary levels of heavy metal may occur they do not correlate with clinical effects. The general approach to treatment is recognition of the disease, supportive care and prevention of exposure. Seriously symptomatic patients should receive chest x-rays, have arterial blood gases determined and be observed for the development of tracheobronchitis and pulmonary edema.
[Ellenhorn and Barceloux: Medical Toxicology] Gastric acids solubilise lead and its salts and lead absorption occurs in the small bowel. Particles of less than 1 um diameter are substantially absorbed by the alveoli following inhalation. Lead is distributed to the red blood cells and has a half-life of 35 days. It is subsequently redistributed to soft tissue & bone-stores or eliminated. The kidney accounts for 75% of daily lead loss; integumentary and alimentary losses account for the remainder. Neurasthenic symptoms are the most common symptoms of intoxication. Lead toxicity produces a classic motor neuropathy. Acute encephalopathy appears infrequently in adults. Diazepam is the best drug for seizures. Whole-blood lead is the best measure of recent exposure; free erythrocyte protoporphyrin (FEP) provides the best screening for chronic exposure. Obvious clinical symptoms occur in adults when whole-blood lead exceeds 80 ug/dL. British Anti-Lewisite is an effective antidote and enhances faecal and urinary excretion of lead. The onset of action of BAL is about 30 minutes and most of the chelated metal complex is excreted in 4-6 hours, primarily in the bile. Adverse reaction appears in up to 50% of patients given BAL in doses exceeding 5 mg/kg. CaNa2EDTA has also been used alone or in concert with BAL as an antidote. D-penicillamine is the usual oral agent for mobilisation of bone lead; its use in the treatment of lead poisoning remains investigational. 2,3-dimercapto-1-propanesulfonic acid (DMPS) and dimercaptosuccinic acid (DMSA) are water soluble analogues of BAL and their effectiveness is undergoing review. As a rule, stop BAL if lead decreases below 50 ug/dL; stop CaNa2EDTA if blood lead decreases below 40 ug/dL or urinary lead drops below 2 mg/24hrs.
[Ellenhorn & Barceloux: Medical Toxicology]
BIOLOGICAL EXPOSURE INDEX - BEI
These represent the determinants observed in specimens collected from a healthy worker who has been exposed at the Exposure Standard (ES or TLV):
Determinant 1. Lead in blood 2. Lead in urine 3. Zinc protoporphyrin in blood
Index 30 ug/100 ml 150 ug/gm creatinine 250 ug/100 ml erythrocytes OR 100 ug/100 ml blood
Sampling Time Not Critical Not Critical After 1 month exposure
Comments
B B
B: Background levels occur in specimens collected from subjects NOT exposed.
SECTION 5 FIREFIGHTING MEASURES
5.1. Extinguishing media
DO NOT use halogenated fire extinguishing agents. Metal dust fires need to be smothered with sand, inert dry powders. DO NOT USE WATER, CO2 or FOAM.
Use DRY sand, graphite powder, dry sodium chloride based extinguishers, G-1 or Met L-X to smother fire. Confining or smothering material is preferable to applying water as chemical reaction may produce flammable and explosive hydrogen gas. Chemical reaction with CO2 may produce flammable and explosive methane. If impossible to extinguish, withdraw, protect surroundings and allow fire to burn itself out.
5.2. Special hazards arising from the substrate or mixture
Fire Incompatibility
Reacts with acids producing flammable / explosive hydrogen (H2) gas
5.3. Advice for firefighters Fire Fighting
Fire/Explosion Hazard
Alert Fire Brigade and tell them location and nature of hazard. Wear breathing apparatus plus protective gloves in the event of a fire. Prevent, by any means available, spillage from entering drains or water courses. Use fire fighting procedures suitable for surrounding area. DO NOT approach containers suspected to be hot. Cool fire exposed containers with water spray from a protected location. If safe to do so, remove containers from path of fire. Equipment should be thoroughly decontaminated after use.
DO NOT disturb burning dust. Explosion may result if dust is stirred into a cloud, by providing oxygen to a large surface of hot metal. DO NOT use water or foam as generation of explosive hydrogen may result. With the exception of the metals that burn in contact with air or water (for example, sodium), masses of combustible metals do not represent unusual fire risks because they have the ability to conduct heat away from hot spots so efficiently that the heat of combustion cannot be maintained - this means that it will
Continued...
�Page 4 of 14
4880-4888 Sn63Pb37 RA Solder Wire
require a lot of heat to ignite a mass of combustible metal. Generally, metal fire risks exist when sawdust, machine shavings and other metal 'fines' are present. Metal powders, while generally regarded as non-combustible:
May burn when metal is finely divided and energy input is high. May react explosively with water. May be ignited by friction, heat, sparks or flame. May REIGNITE after fire is extinguished. Will burn with intense heat. Note: Metal dust fires are slow moving but intense and difficult to extinguish. Containers may explode on heating. Dusts or fumes may form explosive mixtures with air. Gases generated in fire may be poisonous, corrosive or irritating. Hot or burning metals may react violently upon contact with other materials, such as oxidising agents and extinguishing agents used on fires involving ordinary combustibles or flammable liquids. Temperatures produced by burning metals can be higher than temperatures generated by burning flammable liquids Some metals can continue to burn in carbon dioxide, nitrogen, water, or steam atmospheres in which ordinary combustibles or flammable liquids would be incapable of burning. Ignites spontaneously in air (pyrophoric) and burns with intense heat. May emit poisonous fumes. May emit corrosive fumes. Explosions can occur with coils of foil that have been submerged or partially submerged in water for an extended period of time. Water can penetrate between the layers of foil, react with the aluminum surface and generate heat and hydrogen gas. When the coils are removed from the cooling effects of the water, rapid temperature increases can occur causing steam explosions which result in the rupture of the coils and discharge of debris. Coils of foil may be a potential hazard under the following conditions:
· Coil has been annealed (annealing removes residual oil that could prevent penetration of water · Foil is very thin gauge (5-9 µm thickness which increases surface area) · Coil has been immersed for an extended period of time (several hours or more) · Wetted coil has recently been removed from the cooling effects of the water In such situations, the coils should be isolated (30 meters from any personnel) for at least 72 hours as soon as possible after removal from the water. Coils making crackling sounds or emitting steam should not be approached or transported in commerce. Wetted coils should not be charged into a furnace for remelting until completely dry.
SECTION 6 ACCIDENTAL RELEASE MEASURES
6.1. Personal precautions, protective equipment and emergency procedures See section 8
6.2. Environmental precautions See section 12
6.3. Methods and material for containment and cleaning up
Minor Spills
Clean up waste regularly and abnormal spills immediately. Avoid breathing dust and contact with skin and eyes. Wear protective clothing, gloves, safety glasses and dust respirator. Use dry clean up procedures and avoid generating dust. Vacuum up or sweep up. NOTE: Vacuum cleaner must be fitted with an exhaust micro filter (HEPA type) (consider explosion-proof machines designed to be grounded during storage and use). Dampen with water to prevent dusting before sweeping. Place in suitable containers for disposal.
Major Spills
· Do not use compressed air to remove metal dusts from floors, beams or equipment · Vacuum cleaners, of flame-proof design, should be used to minimise dust accumulation. · Use non-sparking handling equipment, tools and natural bristle brushes. · Provide grounding and bonding where necessary to prevent accumulation of static charges during metal dust handling and transfer operations · Cover and reseal partially empty containers. · Do not allow chips, fines or dusts to contact water, particularly in enclosed areas. If molten: Contain the flow using dry sand or salt flux as a dam. All tooling (e.g., shovels or hand tools) and containers which come in contact with molten metal must be preheated or specially coated, rust free and approved for such use. Allow the spill to cool before remelting scrap. Moderate hazard. CAUTION: Advise personnel in area. Alert Emergency Services and tell them location and nature of hazard. Control personal contact by wearing protective clothing. Prevent, by any means available, spillage from entering drains or water courses. Recover product wherever possible. IF DRY: Use dry clean up procedures and avoid generating dust. Collect residues and place in sealed plastic bags or other containers for disposal. IF WET: Vacuum/shovel up and place in labelled containers for disposal. ALWAYS: Wash area down with large amounts of water and prevent runoff into drains. If contamination of drains or waterways occurs, advise Emergency Services.
6.4. Reference to other sections Personal Protective Equipment advice is contained in Section 8 of the SDS.
SECTION 7 HANDLING AND STORAGE 7.1. Precautions for safe handling
Continued...
�Page 5 of 14
4880-4888 Sn63Pb37 RA Solder Wire
Safe handling
For molten metals: · Molten metal and water can be an explosive combination. The risk is greatest when there is sufficient molten metal to entrap or seal off water. Water and other forms of contamination on or contained in scrap or remelt ingot are known to have caused explosions in melting operations. While the products may have minimal surface roughness and internal voids, there remains the possibility of moisture contamination or entrapment. If confined, even a few drops can lead to violent explosions. · All tooling, containers, molds and ladles, which come in contact with molten metal must be preheated or specially coated, rust free and approved for such use. · Any surfaces that may contact molten metal (e.g. concrete) should be specially coated · Drops of molten metal in water (e.g. from plasma arc cutting), while not normally an explosion hazard, can generate enough flammable hydrogen gas to present an explosion hazard. Vigorous circulation of the water and removal of the particles minimise the hazard.
During melting operations, the following minimum guidelines should be observed: · Inspect all materials prior to furnace charging and completely remove surface contamination such as water, ice, snow, deposits of grease and oil or other surface contamination resulting from weather exposure, shipment, or storage. · Store materials in dry, heated areas with any cracks or cavities pointed downwards. · Preheat and dry large objects adequately before charging in to a furnace containing molten metal. This is typically done by the use of a drying oven or homogenising furnace. The dry cycle should bring the metal temperature of the coldest item of the batch to 200 degree C (400 deg F) and then hold at that temperature for 6 hours.
Fire and explosion protection Other information
Avoid all personal contact, including inhalation. Wear protective clothing when risk of exposure occurs. Use in a well-ventilated area. Prevent concentration in hollows and sumps. DO NOT enter confined spaces until atmosphere has been checked. DO NOT allow material to contact humans, exposed food or food utensils. Avoid contact with incompatible materials. When handling, DO NOT eat, drink or smoke. Keep containers securely sealed when not in use. Avoid physical damage to containers. Always wash hands with soap and water after handling. Work clothes should be laundered separately. Launder contaminated clothing before re-use. Use good occupational work practice. Observe manufacturer's storage and handling recommendations contained within this SDS. Atmosphere should be regularly checked against established exposure standards to ensure safe working conditions are maintained.
See section 5
Store in original containers. Keep containers securely sealed. Store in a cool, dry area protected from environmental extremes. Store away from incompatible materials and foodstuff containers. Protect containers against physical damage and check regularly for leaks. Observe manufacturer's storage and handling recommendations contained within this SDS. For major quantities: Consider storage in bunded areas - ensure storage areas are isolated from sources of community water (including stormwater, ground water, lakes and streams}. Ensure that accidental discharge to air or water is the subject of a contingency disaster management plan; this may require consultation with local authorities.
7.2. Conditions for safe storage, including any incompatibilities
Suitable container
Bulk bags: Reinforced bags required for dense materials. CARE: Packing of high density product in light weight metal or plastic packages may result in container collapse with product release Heavy gauge metal packages / Heavy gauge metal drums
Storage incompatibility
Chips, fines and dust are considerably more reactive in the presence of: Water - slowly generates flammable/explosive hydrogen gas and heat (generation rate is greatly increased with smaller particles (e.g., fines and dusts). Heat - oxidise at a rate dependent upon temperature and particle size. Strong oxidisers - violent reaction with considerable heat generation; an react explosively with nitrates (e.g., ammonium nitrate and fertilizers containing nitrate) when heated or molten. Acids and alkalis - reacts to generate flammable/explosive hydrogen gas; generation rate is greatly increased with smaller particles (e.g., fines and dusts). Halogenated compounds including halogenated fire extinguishing agents, which may react violently with finely divided or molten metals Iron oxide (rust) and other metal oxides (e.g., copper and lead oxides) which may produce a violent thermit reaction, initiated by a weak ignition source, generating considerable heat.. Iron powder and water which may react explosively forming hydrogen gas when heated above 800 degrees C ( 1470 deg F).
Finely divided metals (e.g., powders or wire) may have enough surface oxide to produce thermit reactions/explosions The material is described as an electropositive metal. The activity or electromotive series of metals is a listing of the metals in decreasing order of their reactivity with hydrogen-ion sources such as water and acids. In the reaction with a hydrogen-ion source, the metal is oxidised to a metal ion, and the hydrogen ion is reduced to H2. The ordering of the activity series can be related to the standard reduction potential of a metal cation. The more positive the standard reduction potential of the cation, the more difficult it is to oxidise the metal to a hydrated metal cation and the later that metal falls in the series Three notable groups comprise the series
very electropositive metals electropositive metals electronegative metals Electropositive metals.have electronegativities that fall between 1.4 and 1.9 Cations of these metals generally have standard reduction potentials between 0.0 and -1.6 V They: do not react very readily with water to release hydrogen react with H+ (acids) Electropositive metals do not burn in air as readily as do very electropositive metals. The surfaces of these metals will tarnish in the presence of oxygen forming a protective oxide coating. This coating protects the bulk of the metal against further oxidation (the metal is passivated).
Reaction is reduced in the massive form (sheet, rod, or drop), compared with finely divided forms. The less active metals will not burn in air but: can react exothermically with oxidising acids to form noxious gases. catalyse polymerisation and other reactions, particularly when finely divided react with halogenated hydrocarbons (for example, copper dissolves when heated in carbon tetrachloride), sometimes forming explosive compounds. Elemental metals may react with azo/diazo compounds to form explosive products
Continued...
�Page 6 of 14
4880-4888 Sn63Pb37 RA Solder Wire
7.3. Specific end use(s) See section 1.2
Finely divided metal powders develop pyrophoricity when a critical specific surface area is exceeded; this is ascribed to high heat of oxide formation on exposure to air. Safe handling is possible in relatively low concentrations of oxygen in an inert gas Several pyrophoric metals, stored in glass bottles have ignited when the container is broken on impact. Storage of these materials moist and in metal containers is recommended. The reaction residues from various metal syntheses (involving vacuum evaporation and co-deposition with a ligand) are often pyrophoric
If the surface of the metal is in contact with both oxygen and water, corrosion can occur. In corrosion, the metal acts as an anode and is oxidised.
Many metals may incandesce, react violently, ignite or react explosively upon addition of concentrated nitric acid. Some electropositive metals do not react with nitric acid because they are passivated.
http://www.wou.edu/las/physci/ch412/activity.htm Many metals may incandesce, react violently, ignite or react explosively upon addition of concentrated nitric acid.
SECTION 8 EXPOSURE CONTROLS / PERSONAL PROTECTION
8.1. Control parameters
DERIVED NO EFFECT LEVEL (DNEL) Not Available
PREDICTED NO EFFECT LEVEL (PNEC) Not Available
OCCUPATIONAL EXPOSURE LIMITS (OEL)
INGREDIENT DATA
Source
Ingredient
EU Consolidated List of Indicative
Occupational Exposure Limit
tin
Values (IOELVs)
European Union (EU) Council Directive 98/24/EC on the protection of the health and safety of workers from the risks related to lead chemical agents at work - Annex I: List of Binding Occupational Exposure Limit Values (English)
EMERGENCY LIMITS Ingredient tin lead
Material name Tin Lead
Material name Tin and inorganic tin compounds
Inorganic lead and it's compounds
TEEL-1 6 mg/m3 0.15 mg/m3
Ingredient tin lead rosin, polymerised
MATERIAL DATA
Original IDLH Not Available Not Available Not Available
TWA 2 mg/m3
STEL Not Available
Peak Not Available
Notes Not Available
0,15 mg/m3
Not Available
Not Available
Not Available
TEEL-2 67 mg/m3 120 mg/m3
Revised IDLH Not Available Not Available Not Available
TEEL-3 400 mg/m3 700 mg/m3
A TLV-TWA is recommended so as to minimise the risk of stannosis. The STEL (4.0 mg/m3) has been eliminated (since 1986) so that additional toxicological data and industrial hygiene experience may become available to provide a better base for quantifying on a toxicological basis what the STEL should in fact be. The lead concentration in air is to be maintained so that the lead concentration in workers' blood remains below 0.060 mg/100 g of whole blood. The recommended TLV-TWA has been derived following a review of reports of adverse effects on reproduction, blood-pressure and other end-points of toxicity. A particular focus was an assessment of pre-natal blood lead (PbB) levels and post-natal cognitive levels. The fact that lead is a cumulative toxicant which can produce subtle, persistent and apparently permanent effects in the off-spring of lead exposed women is of particular concern. A current view holds that the identification of the PbB levels, that are protective during a working lifetime, is a necessary prerequisite in the recommendation of the TLV because PbB values, rather than workplace air lead concentrations, are more clearly related to adverse health effects. (see Biological Exposure Index - BEI - in 'Advice to Doctor'.)
8.2. Exposure controls
8.2.1. Appropriate engineering controls
Metal dusts must be collected at the source of generation as they are potentially explosive. Avoid ignition sources. Good housekeeping practices must be maintained. Dust accumulation on the floor, ledges and beams can present a risk of ignition, flame propagation and secondary explosions. Do not use compressed air to remove settled materials from floors, beams or equipment Vacuum cleaners, of flame-proof design, should be used to minimise dust accumulation. Use non-sparking handling equipment, tools and natural bristle brushes. Cover and reseal partially empty containers. Provide grounding and bonding where necessary to prevent accumulation of static charges during metal dust handling and transfer operations. Do not allow chips, fines or dusts to contact water, particularly in enclosed areas. Metal spraying and blasting should, where possible, be conducted in separate rooms. This minimises the risk of supplying oxygen, in the form of metal oxides, to potentially reactive finely divided metals such as aluminium, zinc, magnesium or titanium. Work-shops designed for metal spraying should possess smooth walls and a minimum of obstructions, such as ledges, on which dust accumulation is possible.
Continued...
�Page 7 of 14
4880-4888 Sn63Pb37 RA Solder Wire
Wet scrubbers are preferable to dry dust collectors. Bag or filter-type collectors should be sited outside the workrooms and be fitted with explosion relief doors. Cyclones should be protected against entry of moisture as reactive metal dusts are capable of spontaneous combustion in humid or partially wetted states. Local exhaust systems must be designed to provide a minimum capture velocity at the fume source, away from the worker, of 0.5 metre/sec. Local ventilation and vacuum systems must be designed to handle explosive dusts. Dry vacuum and electrostatic precipitators must not be used, unless specifically approved for use with flammable/ explosive dusts.
Air contaminants generated in the workplace possess varying 'escape' velocities which, in turn, determine the 'capture velocities' of fresh circulating air required to effectively remove the contaminant.
Type of Contaminant: welding, brazing fumes (released at relatively low velocity into moderately still air)
Air Speed: 0.5-1.0 m/s (100-200 f/min.)
Within each range the appropriate value depends on:
Lower end of the range 1: Room air currents minimal or favourable to capture 2: Contaminants of low toxicity or of nuisance value only. 3: Intermittent, low production. 4: Large hood or large air mass in motion
Upper end of the range 1: Disturbing room air currents 2: Contaminants of high toxicity 3: High production, heavy use 4: Small hood-local control only
Simple theory shows that air velocity falls rapidly with distance away from the opening of a simple extraction pipe. Velocity generally decreases with the square of distance from the extraction point (in simple cases). Therefore the air speed at the extraction point should be adjusted, accordingly, after reference to distance from the contaminating source. The air velocity at the extraction fan, for example, should be a minimum of 1-2.5 m/s (200-500 f/min.) for extraction of gases discharged 2 meters distant from the extraction point. Other mechanical considerations, producing performance deficits within the extraction apparatus, make it essential that theoretical air velocities are multiplied by factors of 10 or more when extraction systems are installed or used.
8.2.2. Personal protection
Eye and face protection Skin protection
Hands/feet protection
Safety glasses with side shields. Chemical goggles. Contact lenses may pose a special hazard; soft contact lenses may absorb and concentrate irritants. A written policy document, describing the wearing of lenses or restrictions on use, should be created for each workplace or task. This should include a review of lens absorption and adsorption for the class of chemicals in use and an account of injury experience. Medical and first-aid personnel should be trained in their removal and suitable equipment should be readily available. In the event of chemical exposure, begin eye irrigation immediately and remove contact lens as soon as practicable. Lens should be removed at the first signs of eye redness or irritation - lens should be removed in a clean environment only after workers have washed hands thoroughly. [CDC NIOSH Current Intelligence Bulletin 59], [AS/NZS 1336 or national equivalent]
See Hand protection below
The selection of suitable gloves does not only depend on the material, but also on further marks of quality which vary from manufacturer to manufacturer. Where the chemical is a preparation of several substances, the resistance of the glove material can not be calculated in advance and has therefore to be checked prior to the application. The exact break through time for substances has to be obtained from the manufacturer of the protective gloves and.has to be observed when making a final choice. Personal hygiene is a key element of effective hand care. Gloves must only be worn on clean hands. After using gloves, hands should be washed and dried thoroughly. Application of a non-perfumed moisturiser is recommended. Suitability and durability of glove type is dependent on usage. Important factors in the selection of gloves include:
· frequency and duration of contact, · chemical resistance of glove material, · glove thickness and · dexterity Select gloves tested to a relevant standard (e.g. Europe EN 374, US F739, AS/NZS 2161.1 or national equivalent). · When prolonged or frequently repeated contact may occur, a glove with a protection class of 5 or higher (breakthrough time greater than 240 minutes according to EN 374, AS/NZS 2161.10.1 or national equivalent) is recommended. · When only brief contact is expected, a glove with a protection class of 3 or higher (breakthrough time greater than 60 minutes according to EN 374, AS/NZS 2161.10.1 or national equivalent) is recommended. · Some glove polymer types are less affected by movement and this should be taken into account when considering gloves for long-term use. · Contaminated gloves should be replaced. As defined in ASTM F-739-96 in any application, gloves are rated as: · Excellent when breakthrough time > 480 min · Good when breakthrough time > 20 min · Fair when breakthrough time < 20 min · Poor when glove material degrades For general applications, gloves with a thickness typically greater than 0.35 mm, are recommended. It should be emphasised that glove thickness is not necessarily a good predictor of glove resistance to a specific chemical, as the permeation efficiency of the glove will be dependent on the exact composition of the glove material. Therefore, glove selection should also be based on consideration of the task requirements and knowledge of breakthrough times. Glove thickness may also vary depending on the glove manufacturer, the glove type and the glove model. Therefore, the manufacturers' technical data should always be taken into account to ensure selection of the most appropriate glove for the task. Note: Depending on the activity being conducted, gloves of varying thickness may be required for specific tasks. For example: · Thinner gloves (down to 0.1 mm or less) may be required where a high degree of manual dexterity is needed. However, these gloves are only likely to give short duration protection and would normally be just for single use applications, then disposed of. · Thicker gloves (up to 3 mm or more) may be required where there is a mechanical (as well as a chemical) risk i.e. where there is abrasion or puncture potential Gloves must only be worn on clean hands. After using gloves, hands should be washed and dried thoroughly. Application of a non-perfumed moisturiser is recommended. Protective gloves eg. Leather gloves or gloves with Leather facing
Continued...
�Page 8 of 14
4880-4888 Sn63Pb37 RA Solder Wire
Body protection Other protection
Experience indicates that the following polymers are suitable as glove materials for protection against undissolved, dry solids, where abrasive particles are not present.
polychloroprene. nitrile rubber. butyl rubber. fluorocaoutchouc. polyvinyl chloride. Gloves should be examined for wear and/ or degradation constantly.
See Other protection below
Overalls. P.V.C. apron. Barrier cream. Skin cleansing cream. Eye wash unit.
Respiratory protection Particulate. (AS/NZS 1716 & 1715, EN 143:2000 & 149:001, ANSI Z88 or national equivalent)
Required Minimum Protection Factor up to 10 x ES up to 50 x ES up to 100 x ES
100+ x ES
Half-Face Respirator P1 Air-line* Air-line** -
-
Full-Face Respirator P2 P3 Air-line* Air-line**
Powered Air Respirator PAPR-P1 PAPR-P2 PAPR-P3
* - Negative pressure demand ** - Continuous flow A(All classes) = Organic vapours, B AUS or B1 = Acid gasses, B2 = Acid gas or hydrogen cyanide(HCN), B3 = Acid gas or hydrogen cyanide(HCN), E = Sulfur dioxide(SO2), G = Agricultural chemicals, K = Ammonia(NH3), Hg = Mercury, NO = Oxides of nitrogen, MB = Methyl bromide, AX = Low boiling point organic compounds(below 65 degC)
Respirators may be necessary when engineering and administrative controls do not adequately prevent exposures. The decision to use respiratory protection should be based on professional judgment that takes into account toxicity information, exposure measurement data, and frequency and likelihood of the worker's exposure - ensure users are not subject to high thermal loads which may result in heat stress or distress due to personal protective equipment (powered, positive flow, full face apparatus may be an option). Published occupational exposure limits, where they exist, will assist in determining the adequacy of the selected respiratory protection. These may be government mandated or vendor recommended. Certified respirators will be useful for protecting workers from inhalation of particulates when properly selected and fit tested as part of a complete respiratory protection program. Use approved positive flow mask if significant quantities of dust becomes airborne. Try to avoid creating dust conditions.
8.2.3. Environmental exposure controls See section 12
SECTION 9 PHYSICAL AND CHEMICAL PROPERTIES
9.1. Information on basic physical and chemical properties
Appearance
Silver Grey
Physical state Solid
Odour
Not Available
Odour threshold pH (as supplied) Melting point / freezing point
(°C) Initial boiling point and boiling
range (°C) Flash point (°C) Evaporation rate
Flammability
Not Available Not Available 183
1380 Not Available Not Available Not Available
Upper Explosive Limit (%) Not Available
Lower Explosive Limit (%) Vapour pressure (kPa) Solubility in water Vapour density (Air = 1)
Not Available 0.13 Immiscible Not Available
9.2. Other information Not Available
SECTION 10 STABILITY AND REACTIVITY
Relative density (Water = 1) Partition coefficient n-octanol /
water Auto-ignition temperature (°C)
Decomposition temperature
8.4 Not Available Not Available Not Available
Viscosity (cSt) Not Available
Molecular weight (g/mol) Not Available
Taste Explosive properties Oxidising properties
Surface Tension (dyn/cm or mN/m)
Volatile Component (%vol) Gas group
pH as a solution (1%) VOC g/L
Not Available Not Available Not Available
Not Applicable
Not Available Not Available Not Available Not Available
Continued...
�Page 9 of 14
4880-4888 Sn63Pb37 RA Solder Wire
10.1.Reactivity
10.2. Chemical stability
10.3. Possibility of hazardous reactions
10.4. Conditions to avoid 10.5. Incompatible materials 10.6. Hazardous decomposition
products
See section 7.2 Unstable in the presence of incompatible materials. Product is considered stable. Hazardous polymerisation will not occur.
See section 7.2
See section 7.2 See section 7.2
See section 5.3
SECTION 11 TOXICOLOGICAL INFORMATION
11.1. Information on toxicological effects
The material is not thought to produce adverse health effects or irritation of the respiratory tract (as classified by EC Directives using animal models). Nevertheless, good hygiene practice requires that exposure be kept to a minimum and that suitable control measures be used in an occupational setting. Metals which form part of massive metals and their alloys, are 'locked' into a metal lattice; as a result they are not readily bioavailable following inhalation. Mechanical processing of massive metals (e.g. cutting, grinding) may cause irritation of the upper respiratory tract. Additional health effects from elevated temperature processing (e.g., welding) can cause metal fume fever (nausea, fever, chills, shortness of breath and malaise), reduced ability of the blood to carry oxygen (methaemoglobin) and the accumulation of fluid in the lungs (pulmonary oedema).
Inhaled
Inhalation of freshly formed metal oxide particles sized below 1.5 microns and generally between 0.02 to 0.05 microns may result in 'metal fume fever'. Symptoms may be delayed for up to 12 hours and begin with the sudden onset of thirst, and a sweet, metallic or foul taste in the mouth. Other symptoms include upper respiratory tract irritation accompanied by coughing and a dryness of the mucous membranes, lassitude and a generalised feeling of malaise. Mild to severe headache, nausea, occasional vomiting, fever or chills, exaggerated mental activity, profuse sweating, diarrhoea, excessive urination and prostration may also occur. Tolerance to the fumes develops rapidly, but is quickly lost. All symptoms usually subside within 24-36 hours following removal from exposure. Inhalation of dusts, generated by the material during the course of normal handling, may be damaging to the health of the individual.
Ingestion
Metals which form part of massive metals and their alloys, are 'locked' into a metal lattice; as a result they are not readily bioavailable following ingestion. Secondary processes (e.g. change in pH or intervention by gastrointestinal microorganisms) may allow certain substances to be released in low concentrations. As tin salts (stannous and stannic) are generally poorly absorbed from the gastrointestinal tract. Ingestion of food contaminated with tin may cause transient gastrointestinal disturbances such as nausea, vomiting, diarrhea, fever and headache. Parenteral administration provides a substantial description of tin toxicology. Systemic tin is highly toxic producing diarrhoea, muscle paralysis, twitching and neurological damage. By mouth most tin salts are relatively non-toxic. A number of tin 'food' poisonings, producing vomiting, nausea and diarrhoea, have occurred after ingestion of fruit juices etc. with tin levels above 1400 ppm. This appears to be due to gastric irritation resulting from the activity and astringency of tin compounds, rather than systemic toxicity. Severe growth retardation occurs in rats with dietary stannous salts at levels exceeding 0.3%. The material has NOT been classified by EC Directives or other classification systems as 'harmful by ingestion'. This is because of the lack of corroborating animal or human evidence. The material may still be damaging to the health of the individual, following ingestion, especially where pre-existing organ (e.g liver, kidney) damage is evident. Present definitions of harmful or toxic substances are generally based on doses producing mortality rather than those producing morbidity (disease, ill-health). Gastrointestinal tract discomfort may produce nausea and vomiting. In an occupational setting however, ingestion of insignificant quantities is not thought to be cause for concern.
Skin Contact Eye
Chronic
The material is not thought to produce adverse health effects or skin irritation following contact (as classified by EC Directives using animal models). Nevertheless, good hygiene practice requires that exposure be kept to a minimum and that suitable gloves be used in an occupational setting. Particles and foreign bodies produced by high speed processes may be penetrate the skin. Even after the wound heals persons with retained foreign bodies may experiencing sharp pain with movement or pressure over the site. Discolouration or a visible mass under the epidermis may be obvious. Numbness or tingling ('pins and needles'), with decreased sensation, may be the result of a foreign body pressing against nerves. Persons with diabetes or a history of vascular problems have a higher potential for acquiring an infection Open cuts, abraded or irritated skin should not be exposed to this material
Evidence exists, or practical experience predicts, that the material may cause eye irritation in a substantial number of individuals and/or may produce significant ocular lesions which are present twenty-four hours or more after instillation into the eye(s) of experimental animals. Repeated or prolonged eye contact may cause inflammation characterised by temporary redness (similar to windburn) of the conjunctiva (conjunctivitis); temporary impairment of vision and/or other transient eye damage/ulceration may occur. Contact with the eye, by metal dusts, may produce mechanical abrasion or scratches on the cornea - these injuries usually are minor. However foreign body penetration of the eyeball may produce infection or result in permanent visual damage. High-speed machines (such as drills and saws) can produce white-hot particles of metal that resemble sparks. Any of these white-hot particles can enter the unprotected eye and become embedded deep within it. Foreign bodies that penetrate the inside of the eye can cause infection (endophthalmitis). During the first hours after injury, symptoms of intraocular foreign bodies may be similar to those of corneal abrasions and foreign bodies. However, people with intraocular foreign bodies may also have a noticeable loss of vision. Fluid may leak from the eye, but if the foreign body is small, the leak may be so small that the person is not aware of it. Also, pain may increase after the first several hours Corneal abrasions caused by particles and foreign bodies usually cause pain, tearing, and a feeling that there is something in the eye. They may also cause redness (due to inflamed blood vessels on the surface of the eye) or, occasionally, swelling of the eye and eyelid. Vision may become blurred. Light may be a source of irritation or may cause the muscle that constricts the pupil to undergo a painful spasm. Injuries that penetrate the eye may cause similar symptoms. If a foreign object penetrates the inside of the eye, fluid may leak out.
On the basis, primarily, of animal experiments, concern has been expressed that the material may produce carcinogenic or mutagenic effects; in respect of the available information, however, there presently exists inadequate data for making a satisfactory assessment. Toxic: danger of serious damage to health by prolonged exposure through inhalation, in contact with skin and if swallowed. Serious damage (clear functional disturbance or morphological change which may have toxicological significance) is likely to be caused by repeated or prolonged exposure. As a rule the material produces, or contains a substance which produces severe lesions. Such damage may become apparent following direct application in subchronic (90 day) toxicity studies or following sub-acute (28 day) or chronic (two-year) toxicity tests. There is sufficient evidence to establish a causal relationship between human exposure to the material and impaired fertility
4880-4888 Sn63Pb37 RA Solder Wire
TOXICITY Not Available
IRRITATION Not Available
tin
TOXICITY
IRRITATION
Continued...
�Page 10 of 14
4880-4888 Sn63Pb37 RA Solder Wire
dermal (rat) LD50: >2000 mg/kg[1] Oral (rat) LD50: >2000 mg/kg[1]
TOXICITY dermal (rat) LD50: >2000 mg/kg[1] lead Inhalation (rat) LC50: >5.05 mg/l4 h[1] Oral (rat) LD50: >2000 mg/kg[1]
Eye: no adverse effect observed (not irritating)[1] Skin: no adverse effect observed (not irritating)[1]
IRRITATION Not Available
rosin, polymerised
TOXICITY dermal (rat) LD50: >2000 mg/kg[1] Oral (rat) LD50: >1000 mg/kg[1]
IRRITATION Eye: no adverse effect observed (not irritating)[1] Skin: no adverse effect observed (not irritating)[1]
Legend:
1. Value obtained from Europe ECHA Registered Substances - Acute toxicity 2.* Value obtained from manufacturer's SDS. Unless otherwise specified data extracted from RTECS - Register of Toxic Effect of chemical Substances
LEAD TIN & ROSIN, POLYMERISED
WARNING: Lead is a cumulative poison and has the potential to cause abortion and intellectual impairment to unborn children of pregnant workers. No significant acute toxicological data identified in literature search.
Acute Toxicity Skin Irritation/Corrosion Serious Eye Damage/Irritation
Respiratory or Skin sensitisation Mutagenicity
Carcinogenicity Reproductivity STOT - Single Exposure
STOT - Repeated Exposure
Aspiration Hazard
Legend:
Data either not available or does not fill the criteria for classification Data available to make classification
SECTION 12 ECOLOGICAL INFORMATION
12.1. Toxicity
4880-4888 Sn63Pb37 RA Solder Wire
ENDPOINT Not Available
TEST DURATION (HR) Not Available
SPECIES Not Available
VALUE Not Available
SOURCE Not Available
ENDPOINT
LC50
tin
EC50
EC50
NOEC
TEST DURATION (HR) 96 48 72 72
SPECIES Fish Crustacea Algae or other aquatic plants Algae or other aquatic plants
VALUE >0.0124mg/L 0.00018mg/L 0.009-0.846mg/L 0.001-mg/L
SOURCE 2 5 2 2
ENDPOINT
LC50
EC50 lead
EC50
BCFD
NOEC
TEST DURATION (HR) 96 48 72 8 672
SPECIES Fish Crustacea Algae or other aquatic plants Fish Fish
VALUE 0.001-0.06756mg/L 0.029mg/L 0.0205mg/L 4.324mg/L 0.00003mg/L
SOURCE 2 2 2 4 4
rosin, polymerised
ENDPOINT LC50 EC50 EC50 NOEC
TEST DURATION (HR) 96 48 96 96
SPECIES Fish Crustacea Algae or other aquatic plants Algae or other aquatic plants
VALUE >1-mg/L >2-mg/L 0.031mg/L 0.013mg/L
SOURCE 2 2 2 2
Legend:
Extracted from 1. IUCLID Toxicity Data 2. Europe ECHA Registered Substances - Ecotoxicological Information - Aquatic Toxicity 3. EPIWIN Suite V3.12 (QSAR) - Aquatic Toxicity Data (Estimated) 4. US EPA, Ecotox database - Aquatic Toxicity Data 5. ECETOC Aquatic Hazard Assessment Data 6. NITE (Japan) - Bioconcentration Data 7. METI (Japan) - Bioconcentration Data 8. Vendor Data
May cause long-term adverse effects in the aquatic environment.
Continued...
�Page 11 of 14
4880-4888 Sn63Pb37 RA Solder Wire
Do NOT allow product to come in contact with surface waters or to intertidal areas below the mean high water mark. Do not contaminate water when cleaning equipment or disposing of equipment wash-waters. Wastes resulting from use of the product must be disposed of on site or at approved waste sites. Metal-containing inorganic substances generally have negligible vapour pressure and are not expected to partition to air. Once released to surface waters and moist soils their fate depends on solubility and dissociation in water. Environmental processes (such as oxidation and the presence of acids or bases) may transform insoluble metals to more soluble ionic forms. Microbiological processes may also transform insoluble metals to more soluble forms. Such ionic species may bind to dissolved ligands or sorb to solid particles in aquatic or aqueous media. A significant proportion of dissolved/ sorbed metals will end up in sediments through the settling of suspended particles. The remaining metal ions can then be taken up by aquatic organisms. When released to dry soil most metals will exhibit limited mobility and remain in the upper layer; some will leach locally into ground water and/ or surface water ecosystems when soaked by rain or melt ice. Environmental processes may also be important in changing solubilities. Even though many metals show few toxic effects at physiological pHs, transformation may introduce new or magnified effects. A metal ion is considered infinitely persistent because it cannot degrade further. The current state of science does not allow for an unambiguous interpretation of various measures of bioaccumulation. The counter-ion may also create health and environmental concerns once isolated from the metal. Under normal physiological conditions the counter-ion may be essentially insoluble and may not be bioavailable. Environmental processes may enhance bioavailability.
Lead is primarily an atmospheric pollutant that enters soil and water as fallout, a process determined by physical form and particle size. Lead in the form of alkyls has been introduced to the environment primarily from leaded petrol. These are converted to water-soluble lead compounds of high toxicity and availability to plants. Such compounds easily leach from soil to contaminate water sources close to highways. Lead that has entered the aquatic system from run-off or as fallout of insoluble precipitates is found in sediments. The biological methylation of inorganic lead by lake sediment micro-organisms has been demonstrated although its significance is not entirely clear. Other forms of soluble or insoluble lead may also enter the environment and undergo bioaccumulation through a series of biological incidents. Tin may exist in either divalent (Sn2+) or tetravalent (Sn4+) cationic (positively charged) ions under environmental conditions. Tin(II) dominates in reduced (oxygen-poor) water, and will readily precipitate as a sulfide (SnS) or as a hydroxide (Sn(OH)2) in alkaline water. Tin(IV) readily hydrolyses, and can precipitate as a hydroxide. The solubility product of Sn(OH)4 has been measured at approximately 10 exp(-56) g/L at 25 °C. In general, tin(IV) would be expected to be the only stable ionic species in the weathering cycle. Tin in water may partition to soils and sediments. Cations such as Sn2+ and Sn4+ will generally be adsorbed by soils to some extent, which reduces their mobility. Tin is generally regarded as being relatively immobile in the environment. However, tin may be transported in water if it partitions to suspended sediments, but the significance of this mechanism has not been studied in detail. Transfer coefficients for tin in a soil-plant system were reported to be 0.01-0.1. A bioconcentration factor (BCF) relates the concentration of a chemical in plants and animals to the concentration of the chemical in the medium in which they live. It was estimated that the BCFs of inorganic tin were 100, 1,000, and 3,000 for marine and freshwater plants, invertebrates, and fish, respectively. Marine algae can bioconcentrate tin(IV) ion by a factor of 1,900. Inorganic tin cannot be degraded in the environment, but may undergo oxidation-reduction, ligand exchange, and precipitation reactions. It has been established that inorganic tin can be transformed into organometallic forms by microbial methylation . Inorganic tin may also be converted to stannane (H4Sn) in extremely anaerobic (oxygen-poor) conditions by macroalgae. DO NOT discharge into sewer or waterways.
12.2. Persistence and degradability
Ingredient
Persistence: Water/Soil
No Data available for all ingredients
Persistence: Air No Data available for all ingredients
12.3. Bioaccumulative potential
Ingredient
Bioaccumulation
No Data available for all ingredients
12.4. Mobility in soil Ingredient
Mobility No Data available for all ingredients
12.5.Results of PBT and vPvB assessment
P
Relevant available data
Not Applicable
PBT Criteria fulfilled?
Not Applicable
B Not Applicable Not Applicable
T Not Applicable Not Applicable
12.6. Other adverse effects No data available
SECTION 13 DISPOSAL CONSIDERATIONS
13.1. Waste treatment methods
Product / Packaging disposal
· Recycle wherever possible or consult manufacturer for recycling options. · Consult State Land Waste Management Authority for disposal. Metal scrap recycling operations present a wide variety of hazards, including health hazards associated with chemical exposures and safety hazards associated with material processing operations and the equipment used in these tasks. Many of these metals do not pose any hazard to people who handle objects containing the metal in everyday use. In cases where employees could be exposed to multiple hazardous metals or other hazardous substances at the same time or during the same workday, employers must consider the combined effects of the exposure in determining safe exposure levels. The recycling of scrap metals is associated with illness and injury The most common causes of illness were poisoning (e.g., lead or cadmium poisoning), disorders associated with repeated trauma, skin diseases or disorders, and respiratory conditions due to inhalation of, or other contact with, toxic agents. The most common events or exposures leading to these cases were contact with an object or piece of equipment; overextension; and exposure to a harmful substance. The most common types of these injuries were sprains and strains; heat burns; and cuts, lacerations, and punctures. Any combustible material can burn rapidly when in a finely divided form. If such a dust is suspended in air in the right concentration, under certain conditions, it can become explosible. Even materials that do not burn in larger pieces (such as aluminum or iron), given the proper conditions, can be explosible in dust form. The force from such an explosion can cause employee deaths, injuries, and destruction of entire buildings. Breaking apart large metal pieces may involve the use of gas cutting torch. Classic cutting torches use gas, while other torches use plasma or powder, or even water. Thermal (gas) torches expose employees to sprays of sparks and metal dust particles, to high temperatures, to bright light that could damage eyes (light both inside and outside of the visible spectrum), and to various gases. Materials that require higher temperatures to cut, such as pig iron and heat-resistant alloyed scrap, or materials that conduct heat too well to be cut with thermal torches, such as copper and bronze, may be cut with non-thermal methods such as plasma torches or powder cutting torches. Plasma torches are often used for superconductors of heat or heat-resistant metals, such as alloy steels containing nickel and/or chromium. Plasma torches generate a large amount of smoke and noise, as well as ultraviolet (UV) and infrared(IR) light. Depending on the metal, this smoke could contain
Continued...
�Page 12 of 14
4880-4888 Sn63Pb37 RA Solder Wire
Waste treatment options Sewage disposal options
toxic fumes or dusts. Other hazards common to cutting operations (as well as to welding and brazing) include burns, fires, explosions, electric shock, and heat stress. Even chemicals that are generally not flammable may burn readily when vapourised. Larger scrap metal objects are often broken apart using stationary shears, such as alligator shears used to cut apart short steel for foundries or to cut nonferrous metals. These machines can send small pieces of metal flying. Many scrap metal recycling operations heat scrap pieces to high temperatures to separate different metal components, increase the purity of scrap, bake out non-metal substances, burn off contaminants, remove insulation from wire, or otherwise process the metal scrap. This may be done using furnaces or ovens that use fuel or electrical heating sources. Furnaces generate smoke, dust, and metal fumes, depending on temperature and content. Combustion by-products may include sulfur and nitrogen oxides, and carbon monoxide and carbon dioxide. Organic compounds may be emitted as heating vapourises oil and grease on scraps. In addition, heating or burning of certain plastics (such as plastic-coated wiring) may release phosgene or other hazardous substances. Emissions from fluxing typically include chlorides and fluorides. The highest concentrations of `fugitive' emissions (i.e., gases and vapours that escape from equipment) occur when the lids and doors of a furnace are opened during charging, alloying, and other operations. Chemical processes are also used in a wide range of metal scrap recycling industries as a means to separate scrap into its component metals, to clean scrap metal prior to using physical processes, to remove contaminants (such as paint) from scrap material, or to extract selected metals from a batch of scrap containing many metal types. Chemical processes may include high-temperature chlorination, electrorefining, plating, leaching, chemical separation, dissolution, reduction, or galvanizing. The most probable emissions from these processes include metal fumes and vapours, organic vapours, and acid gases. Other potential hazards may include high amounts of heat, splashing of caustic or other-wise hazardous chemicals, or combustion hazards. The recycling of scrap metals or metals found in e-waste (such as printed circuit boards) may present a significant environmental and human health risk. These may contain heavy metals such as cadmium, cobalt, chrome, copper, nickel, lead and zinc. Roads and premises of nearby public facilities such as a school-yard and outdoor food market have been shown to be adversely impacted by the uncontrolled recycling activity. Heavy metal concentrations, especially lead and copper, in workshop and road dusts were found to be severely enriched, posing potential health risks, especially to children.
· Lead is recycled from solder, cable covering, building construction materials, and residues and drosses from smelter-refinery operations. Employees may be exposed to lead during any of these processes · Recyclers may also encounter lead when working with scraps coated with paints containing lead (especially scraps originating from bridge dismantling and rehabilitation and shipyards). Lead dust can be created by grinding, cutting, drilling, sanding, scraping or blasting surfaces coated with lead paints. Lead fumes can be created by using heat guns or other heating techniques to remove paint from surfaces, or by using heated cutting tools to cut through painted metal. · Employees that encounter lead at work must take precautions so that they do not accidentally take lead dust into their homes through contaminated workplace shoes or clothes. For example, employees must not be allowed to leave the facility wearing the clothes that they wore during their work shift, which may be contaminated with lead dust DO NOT allow wash water from cleaning or process equipment to enter drains. It may be necessary to collect all wash water for treatment before disposal. In all cases disposal to sewer may be subject to local laws and regulations and these should be considered first. Where in doubt contact the responsible authority.
Not Available
Not Available
SECTION 14 TRANSPORT INFORMATION
Land transport (ADR): NOT REGULATED FOR TRANSPORT OF DANGEROUS GOODS
14.1. UN number
Not Applicable
14.2. UN proper shipping name Not Applicable
14.3. Transport hazard class(es)
Class Subrisk
Not Applicable Not Applicable
14.4. Packing group 14.5. Environmental hazard
Not Applicable Not Applicable
14.6. Special precautions for user
Hazard identification (Kemler) Classification code Hazard Label Special provisions Limited quantity Tunnel Restriction Code
Not Applicable Not Applicable Not Applicable Not Applicable Not Applicable Not Applicable
Air transport (ICAO-IATA / DGR): NOT REGULATED FOR TRANSPORT OF DANGEROUS GOODS
14.1. UN number 14.2. UN proper shipping name
Not Applicable Not Applicable
14.3. Transport hazard class(es)
ICAO/IATA Class ICAO / IATA Subrisk ERG Code
Not Applicable Not Applicable Not Applicable
14.4. Packing group 14.5. Environmental hazard
Not Applicable Not Applicable
14.6. Special precautions for user
Special provisions Cargo Only Packing Instructions Cargo Only Maximum Qty / Pack Passenger and Cargo Packing Instructions Passenger and Cargo Maximum Qty / Pack
Not Applicable Not Applicable Not Applicable Not Applicable Not Applicable
Continued...
�Page 13 of 14
4880-4888 Sn63Pb37 RA Solder Wire
Passenger and Cargo Limited Quantity Packing Instructions Passenger and Cargo Limited Maximum Qty / Pack
Not Applicable Not Applicable
Sea transport (IMDG-Code / GGVSee): NOT REGULATED FOR TRANSPORT OF DANGEROUS GOODS
14.1. UN number 14.2. UN proper shipping name
Not Applicable Not Applicable
14.3. Transport hazard class(es)
IMDG Class IMDG Subrisk
Not Applicable Not Applicable
14.4. Packing group 14.5. Environmental hazard
Not Applicable Not Applicable
14.6. Special precautions for user
EMS Number Special provisions Limited Quantities
Not Applicable Not Applicable Not Applicable
Inland waterways transport (ADN): NOT REGULATED FOR TRANSPORT OF DANGEROUS GOODS
14.1. UN number 14.2. UN proper shipping name
Not Applicable Not Applicable
14.3. Transport hazard class(es)
Not Applicable Not Applicable
14.4. Packing group
Not Applicable
14.5. Environmental hazard
Not Applicable
14.6. Special precautions for user
Classification code Special provisions Limited quantity Equipment required Fire cones number
Not Applicable Not Applicable Not Applicable Not Applicable Not Applicable
14.7. Transport in bulk according to Annex II of MARPOL and the IBC code Not Applicable
SECTION 15 REGULATORY INFORMATION
15.1. Safety, health and environmental regulations / legislation specific for the substance or mixture
TIN(7440-31-5) IS FOUND ON THE FOLLOWING REGULATORY LISTS
EU Consolidated List of Indicative Occupational Exposure Limit Values (IOELVs) Europe EC Inventory Europe ECHA Registered Substances - Classification and Labelling - DSD-DPD Europe European Customs Inventory of Chemical Substances
European Chemical Agency (ECHA) Classification & Labelling Inventory - Chemwatch Harmonised classification European Trade Union Confederation (ETUC) Priority List for REACH Authorisation European Union - European Inventory of Existing Commercial Chemical Substances (EINECS)
LEAD(7439-92-1) IS FOUND ON THE FOLLOWING REGULATORY LISTS
EU REACH Regulation (EC) No 1907/2006 - Annex XVII - Restrictions on the manufacture, placing on the market and use of certain dangerous substances, mixtures and articles EU REACH Regulation (EC) No 1907/2006 - Annex XVII (Appendix 12) Restricted substances and maximum concentration limits by weight in homogeneous materials EU REACH Regulation (EC) No 1907/2006 - Annex XVII (Appendix 5) Toxic to reproduction: category 1A (Table 3.1)/category 1 (Table 3.2) EU REACH Regulation (EC) No 1907/2006 - Proposals to identify Substances of Very High Concern: Annex XV reports for commenting by Interested Parties previous consultation Europe AeroSpace and Defence Industries Association of Europe (ASD) REACH Implementation Working Group Priority Declarable Substances List (PDSL) Europe EC Inventory Europe ECHA Registered Substances - Classification and Labelling - DSD-DPD Europe European Chemicals Agency (ECHA) Candidate List of Substances of Very High Concern for Authorisation Europe European Customs Inventory of Chemical Substances
European Chemical Agency (ECHA) Classification & Labelling Inventory - Chemwatch Harmonised classification European Trade Union Confederation (ETUC) Priority List for REACH Authorisation European Union - European Inventory of Existing Commercial Chemical Substances (EINECS) European Union (EU) Annex I to Directive 67/548/EEC on Classification and Labelling of Dangerous Substances - updated by ATP: 31 European Union (EU) Council Directive 98/24/EC on the protection of the health and safety of workers from the risks related to chemical agents at work - Annex I: List of Binding Occupational Exposure Limit Values (English) European Union (EU) Regulation (EC) No 1272/2008 on Classification, Labelling and Packaging of Substances and Mixtures - Annex VI IMO IBC Code Chapter 17: Summary of minimum requirements International Agency for Research on Cancer (IARC) - Agents Classified by the IARC Monographs
ROSIN, POLYMERISED(65997-05-9) IS FOUND ON THE FOLLOWING REGULATORY LISTS
Europe EC Inventory European Chemical Agency (ECHA) Classification & Labelling Inventory - Chemwatch Harmonised classification
European Union (EU) No-Longer Polymers List (NLP) (67/548/EEC)
This safety data sheet is in compliance with the following EU legislation and its adaptations - as far as applicable - : Directives 98/24/EC, - 92/85/EEC, - 94/33/EC, - 2008/98/EC, - 2010/75/EU; Commission Regulation (EU) 2015/830; Regulation (EC) No 1272/2008 as updated through ATPs.
15.2. Chemical safety assessment
Continued...
�Page 14 of 14
4880-4888 Sn63Pb37 RA Solder Wire
No Chemical Safety Assessment has been carried out for this substance/mixture by the supplier.
National Inventory Status National Inventory Australia - AICS Canada - DSL Canada - NDSL China - IECSC Europe - EINEC / ELINCS / NLP Japan - ENCS Korea - KECI New Zealand - NZIoC Philippines - PICCS USA - TSCA Taiwan - TCSI Mexico - INSQ Vietnam - NCI Russia - ARIPS Thailand - TECI
Legend:
Status Yes Yes No (lead; rosin, polymerised; tin) Yes Yes No (lead; tin) Yes Yes Yes Yes Yes No (rosin, polymerised) Yes No (rosin, polymerised) No (lead; rosin, polymerised) Yes = All CAS declared ingredients are on the inventory No = Not determined or one or more ingredients are not on the inventory and are not exempt from listing(see specific ingredients in brackets)
SECTION 16 OTHER INFORMATION
Revision Date Initial Date
16/03/2020 26/07/2017
Full text Risk and Hazard codes H360FD May damage fertility. May damage the unborn child.
SDS Version Summary Version 4.9.1.1.1
Issue Date
09/07/2019
Sections Updated
Acute Health (eye), Acute Health (inhaled), Acute Health (skin), Acute Health (swallowed), Appearance, Chronic Health, Classification, Exposure Standard, Physical Properties, Spills (minor), Synonyms
Other information
Classification of the preparation and its individual components has drawn on official and authoritative sources as well as independent review by the Chemwatch Classification committee using available literature references. The SDS is a Hazard Communication tool and should be used to assist in the Risk Assessment. Many factors determine whether the reported Hazards are Risks in the workplace or other settings. Risks may be determined by reference to Exposures Scenarios. Scale of use, frequency of use and current or available engineering controls must be considered. For detailed advice on Personal Protective Equipment, refer to the following EU CEN Standards: EN 166 Personal eye-protection EN 340 Protective clothing EN 374 Protective gloves against chemicals and micro-organisms EN 13832 Footwear protecting against chemicals EN 133 Respiratory protective devices
Definitions and abbreviations
PCTWA: Permissible Concentration-Time Weighted Average PCSTEL: Permissible Concentration-Short Term Exposure Limit IARC: International Agency for Research on Cancer ACGIH: American Conference of Governmental Industrial Hygienists STEL: Short Term Exposure Limit TEEL: Temporary Emergency Exposure Limit IDLH: Immediately Dangerous to Life or Health Concentrations OSF: Odour Safety Factor NOAEL :No Observed Adverse Effect Level LOAEL: Lowest Observed Adverse Effect Level TLV: Threshold Limit Value LOD: Limit Of Detection OTV: Odour Threshold Value BCF: BioConcentration Factors BEI: Biological Exposure Index
Reason For Change A-1.02 - Added FOR INDUSTRIAL USE ONLY.
end of SDS
�Chemwatch via ABCpdf