Acelrx Pharmaceuticals ARX2006 Zalviso hand-held patient controlled analgesia (PCA) system User Manual Manual 3 4
AcelRx Pharmaceuticals Inc. Zalviso hand-held patient controlled analgesia (PCA) system Manual 3 4
Contents
- 1. Manual 1/4
- 2. Manual 2/4
- 3. Manual 3/4
- 4. Manual 4/4
Manual 3/4
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 70
Instructions for Use –PL-1678 Rev. K
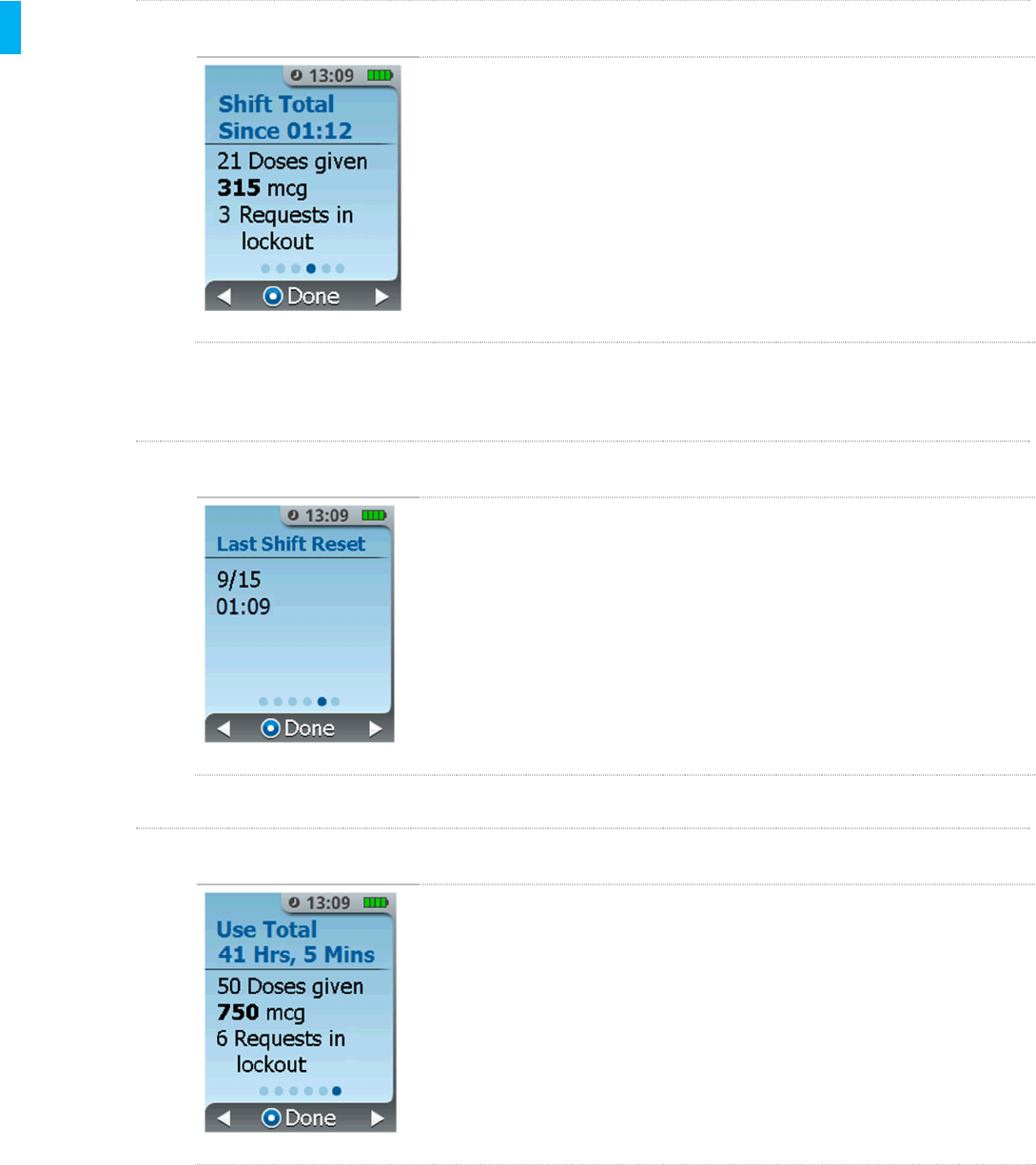
STEP 4 Shift Total Screen
The next screen displays the total number of doses dispensed and
requests made by the patient since the last shift reset.
STEP 5 Last Shift Reset Screen
The next screen displays the date and time the shift dosing total was
last reset.
STEP 6 Use Total Screen
The final screen displays the total number of doses dispensed and
requests made by the patient since the therapy was initiated (since first
dose). The Use Total time displayed is the elapsed time since the first
tablet dosed.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 71
Instructions for Use –PL-1678 Rev. K
11.2. Detailed History
The System stores detailed history as an event log that includes data on:
System power on and power off
Access using the AAC
Patient ID Thumb Tag activation, Drug Cartridge information
System setup
Tether lock and unlock
Access to patient training screens
Quantity of successful doses, total doses delivered, patient attempted doses
Dispenser removed
Cartridge changes
Shift reset
System Discontinue
Remaining battery capacity, battery charger connected or disconnected
System alarm and errors
The event log is stored in the System’s non-volatile memory; therefore, the event log data is
maintained in the System when the System is powered off or if the System experiences an
unexpected power loss. The event log cannot be deleted from the System. When the event log
reaches its storage capacity, the oldest event is erased to create space for new events in a first-
in-first-out process.
Detailed History Access
The Healthcare Professional uses the Authorized Access Card (AAC) to review all events that
have occurred during System use.
STEP 1 Access Detailed History
1. Press the Menu button on the Controller.
2. Touch the AAC to the Blue Dose Button on the back of the
Controller to access the System Menu.
3. Highlight and select the Detailed History function by pressing
the Enter/Select Button.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 72
Instructions for Use –PL-1678 Rev. K
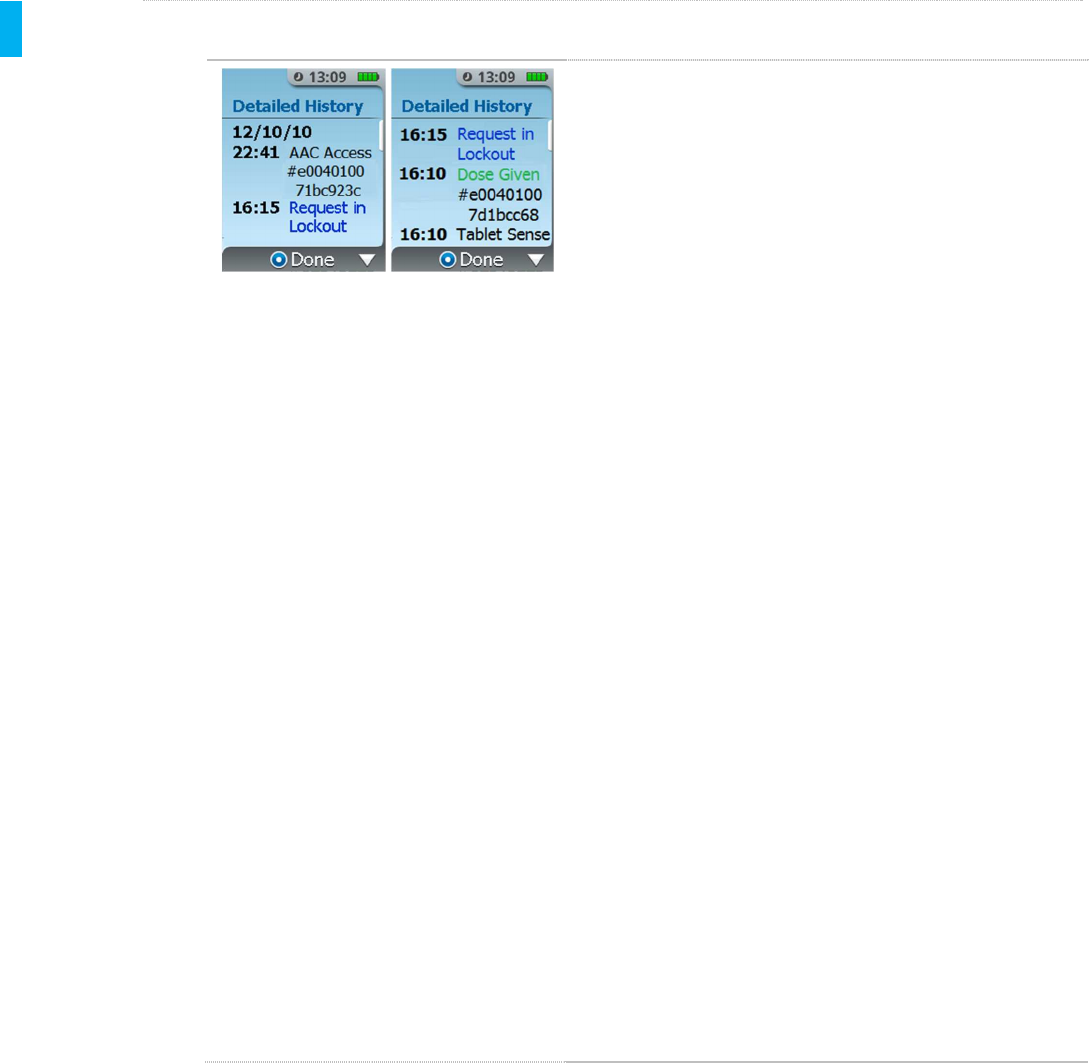
STEP 2 View Detailed History
Each item displays a time stamp and a message of what
event occurred at that time.
If a dose was dispensed this is communicated
as Dose Given in green text followed by the
Patient ID Thumb Tag unique ID number.
If a dose was requested during the lockout
period this is communicated as Requested in
Lockout in blue text.
AAC access
Tether Locked/Unlocked (Tether Connected)
Cartridge changed
Shift Reset
Patient Training Screens Used (Demo Used)
Shift Total and the cumulative dose values
associated with that shift
The full history is presented as a scrollable list. There is
a white scroll bar on the right of the screen to indicate
how much content is available before or after the current
page of content being viewed. The history is presented
with the most recent event on top of the list. As shown
in the above example, doses dispensed are shown as
“Dispensed” in green text and dose requests are
displayed as “Requested” in blue text.
When you are done reviewing the history,select the
Enter/Select Button to return to the System Menu.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 73
Instructions for Use –PL-1678 Rev. K
12. Resetting Shift Total
The Healthcare Professional can reset the cumulative dose count for the shift.
STEP 1 Access Reset Shift with AAC
1. Press the Menu button on the Controller.
2. Touch the AAC to the back of the Controller to access the
System Menu.
3. Highlight and select the Reset Shift Total function and
press the Enter/Select Button.
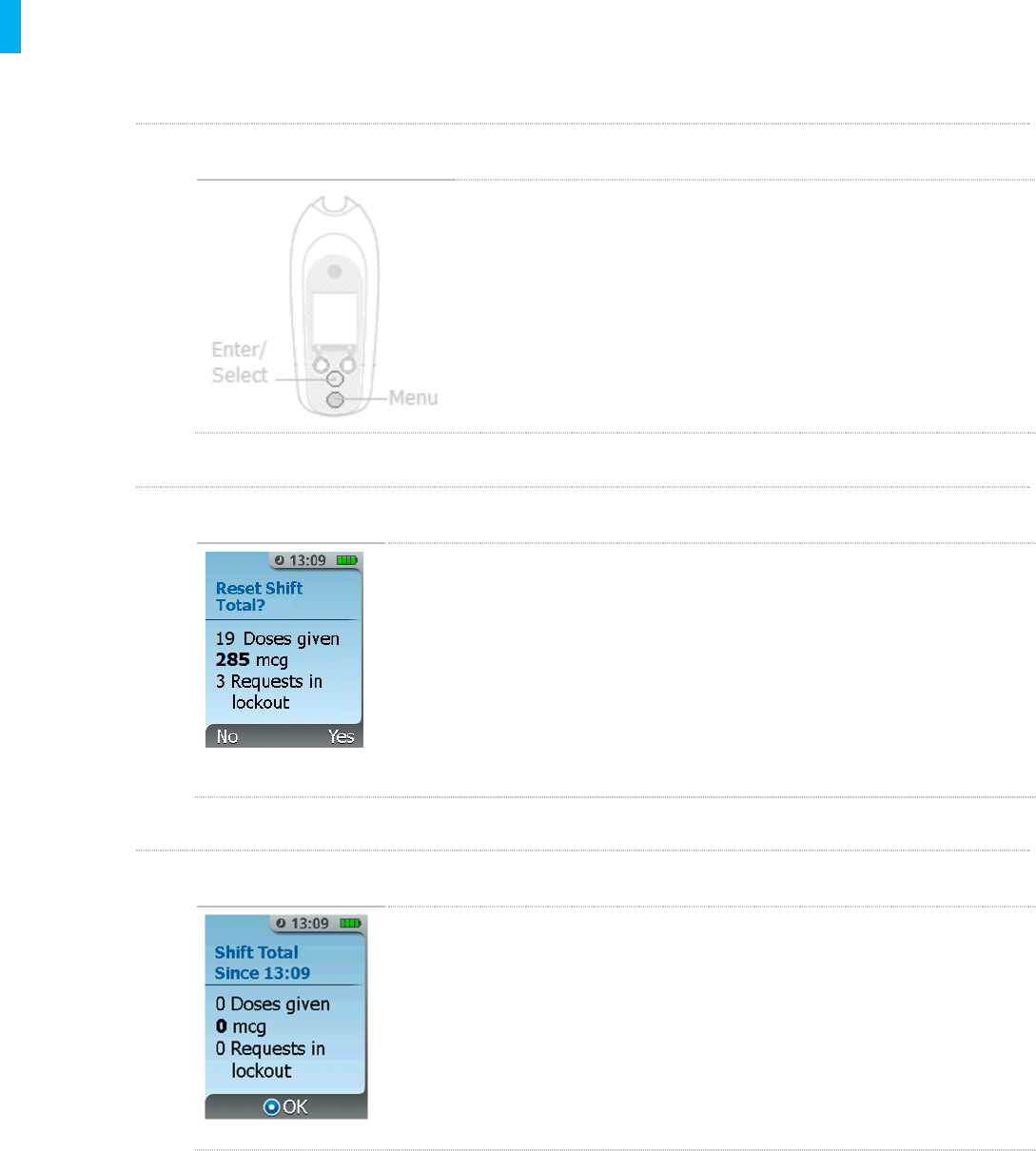
STEP 2 Press Reset Shift
1. The screen will display the cumulative number of tablets
dispensed, the cumulative dose and the total number of requests
since the last total reset.
2. Select YES (Right Button) to reset the shift total. NOTE:This
function cannot be cancelled. Make sure you want to reset the
shift total before selecting “Yes”.
STEP 3Confirm Reset Shift
1. Select NO (Left Button) to return to the previous screen.
2. Once you have selected YES, the shift totals will display 0.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 74
Instructions for Use –PL-1678 Rev. K
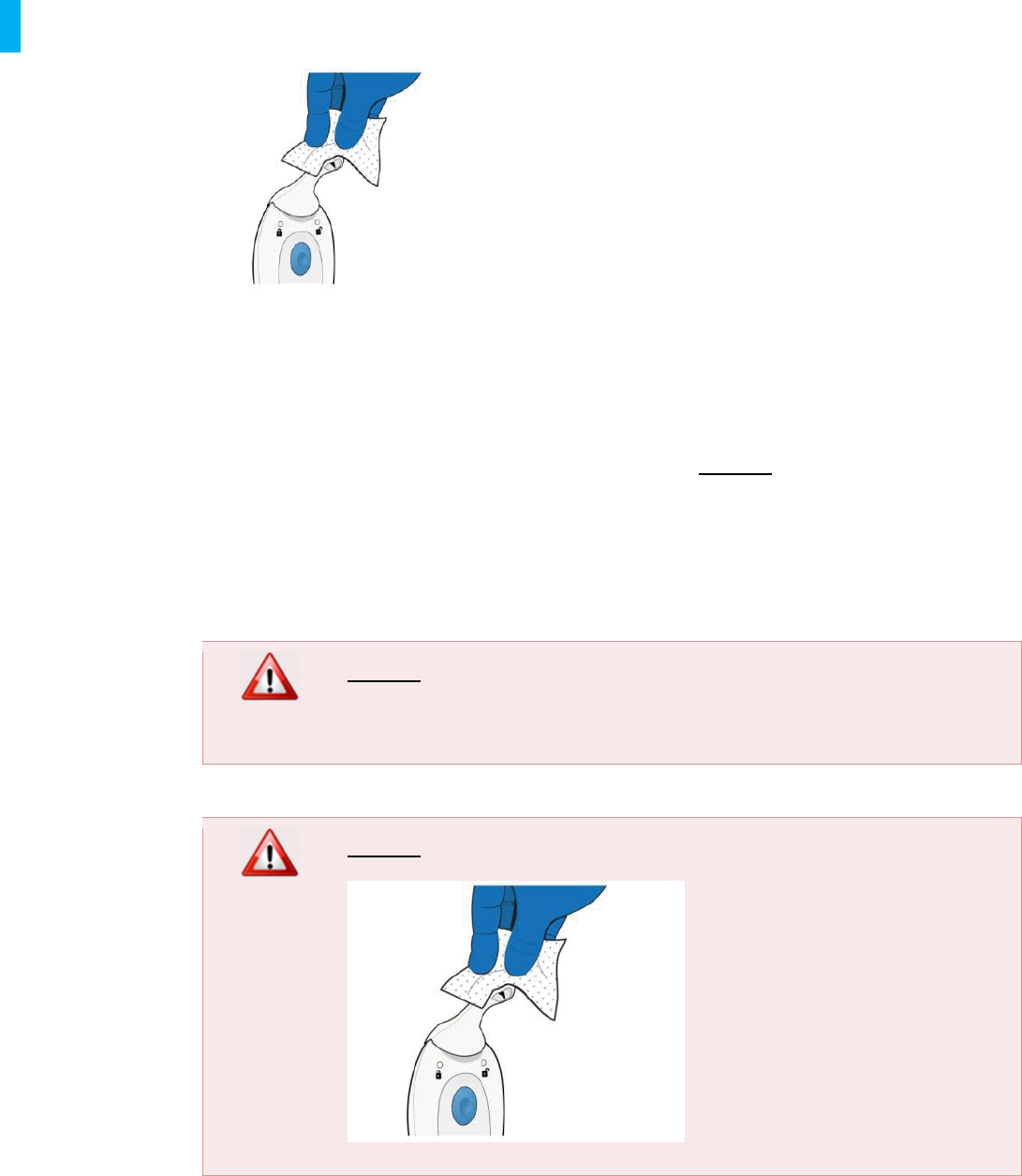
13. Cleaning During Patient Use
The System may need to be cleaned as needed during patient use as described below.
The System including all of its components may be cleaned by the patient or Healthcare
Professional as needed. When cleaning the System,only alcohol wipes should be used.
Wipe as necessary until the System appears visually clean. DO NOT saturate any part of the
System. Wipes should not be excessively wet; squeeze out excessive liquid from the wipes
before use. The System should be replaced if the patient or Healthcare Professional is concerned
about severe contamination.
WARNING
DO NOT soak or immerse System in water.
WARNING
DO NOT clean the interior portion of the Dispenser tip.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 75
Instructions for Use –PL-1678 Rev. K
14. Discontinuation of Therapy, Disposition of
Used Components and Accounting of
Remaining Tablets
When a patient is finished using the System,the Healthcare Professional will need to Discontinue
the System to shut it down and disassemble the used components for disposition. The System
will retain the use history for five patients. Refer to Section 20, Reviewing Former Patient Data,
for details.
Important Safety Message! Due to the risk of accidental exposure and/or overdose,
Zalviso must never be dispensed for outpatient pain management or continued after the
patient is discharged from the hospital. Do not send any Zalviso tablets, Cartridges, or
system components home with any patient upon discharge.
14.1. Discontinuation of Therapy and Disposition of Used Components
To Discontinue,perform the following:
STEP 1 Access ‘Discontinue’ Menu
1. Press the Menu Button near the bottom of the Controller and the
System will prompt you to touch the Authorized Access Card to the
Blue Dose Button on the back of the Controller to access the
System Menu.
2. Scroll to the Discontinue function and select it by pressing the
Enter/Select Button.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 76
Instructions for Use –PL-1678 Rev. K
STEP 2 Confirm ‘Discontinue’
1. The screen will ask if you want to discontinue treatment.
2. Record the number of tablets remaining per hospital
procedures, including any double signatures or witnessing by a
second Healthcare Professional.
3. Press No (Left Button) to return to the previous screen.
4. Press Yes (Right Button) to acknowledge that you would like
to Discontinue the System.NOTE: You will not be able to cancel
this function. Make sure you want to shut down before selecting
Yes.
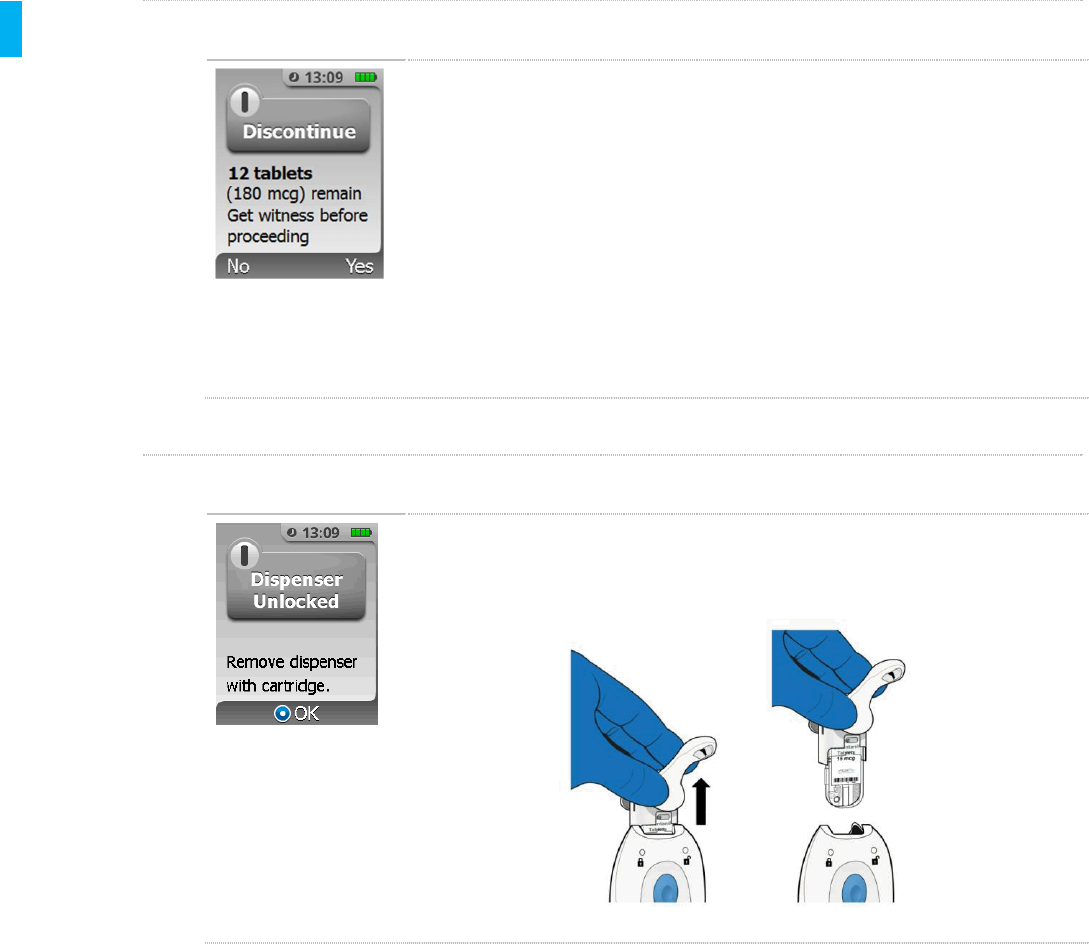
STEP 3 Dispenser Unlocked
The System automatically unlocks the Dispenser and Tether. Remove the
Dispenser with Cartridge from the System. Press the Enter/Select
button to continue.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 77
Instructions for Use –PL-1678 Rev. K
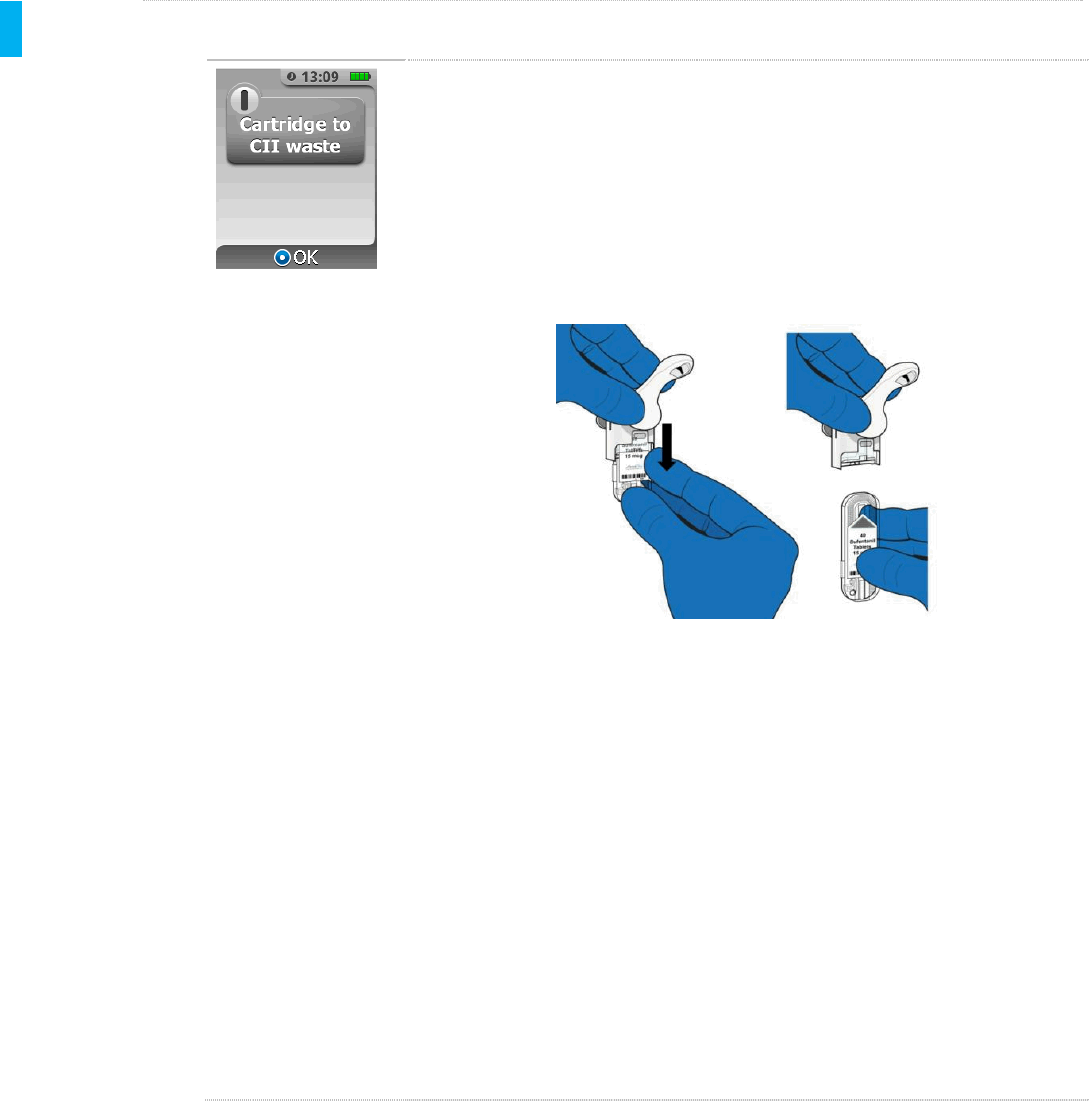
STEP 4Dispose of Cartridge to CII Waste
Remove the Cartridge from the Dispenser by pulling the Cartridge down.
The screen will show a reminder to dispose of the used Cartridge in CII
waste.
Alternatively, the used Drug Cartridge may be returned to the hospital
pharmacy for disposal.
Press the Enter/Select button to continue.
Tools to aid sufentanil tablet accountability include:
The Controller will display the number of tabletsremaining in
the Cartridge at the time of System Discontinuation of therapy.
The Cartridge Label RFID Reader (see Section 24, Use of
Cartridge RFID Label Reader)enables the Healthcare
Professional to scan and display the number of remaining
tablets in used Cartridges, at the time of System
Discontinuation of therapy, as electronically recorded on the
Cartridge RFID Label.
Manual counting of tablets may be performed on used
Cartridges after removal from the System (see Section 14.2,
below). This method can be used if discrepancies or diversion
is suspected to have occurred.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 78
Instructions for Use –PL-1678 Rev. K
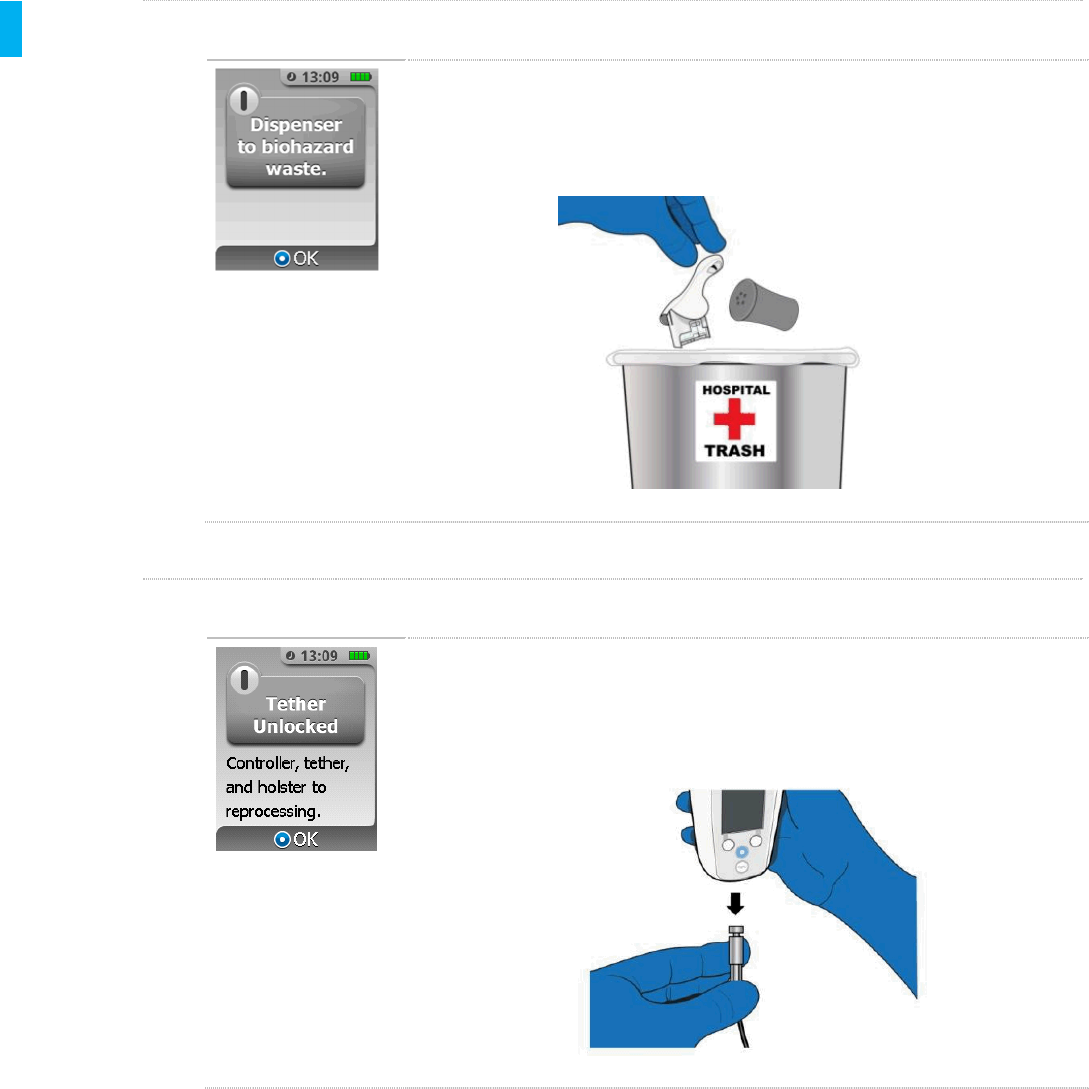
STEP 5Dispose Dispenser to Biohazard Waste
Reminder to dispose the used Dispenser and Cap into biohazard waste
according to institutional procedures. Press the Enter/Select button to
continue.
STEP 6Remove Tether, Send Reusable Components to Reprocessing
Remove the Tether from the bottom of the Controller. Screen shows
reminder to send reusable System components (i.e. Controller, Tether and
Holster) to reprocessing. Press the Enter/Select button to continue.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 79
Instructions for Use –PL-1678 Rev. K
STEP 7Power Off Device
Press the Power Button briefly to shut the System down (screen turns
off). Send reusable System components (Controller, Tether and Holster) to
reprocessing.
WARNINGS
Never re-use the Dispenser or Cap with another patient.
Never attempt to re-use a Cartridge, either for the same patient or another
patient (the System will not allow it).
Never remove remaining tabletsfrom a used Cartridge to dose a patient.
Remove the used Patient ID Thumb Tag and dispose according to
institutional procedures.
Reprocess the Controller, Tether and Holster for use with the next
patient using the instructions described in Section 17, Reprocessing
Instructions.
14.2. Accounting of Remaining Tablets
Since the drug tablets are a Schedule II (CII) substance, accounting of tablets is very important after
Discontinuation of Therapy. There are three methods for remaining tablet accounting:
1. Electronic -Controller Display During Discontinuation of Therapy
2. Electronic -Cartridge Label RFID Reader
3. Manual –Counting Tablets While Still in Cartridge
Method 1 can be used when the Cartridge is still contained within the Controller during
discontinuation.
Method 2 can be used with a stand-alone Cartridge.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 80
Instructions for Use –PL-1678 Rev. K
Method 3 can be used with a stand-alone Cartridge to count the number of tablets remaining
in the Drug Cartridge and confirm the amount of tablets remaining from Methods 1 and 2
above.
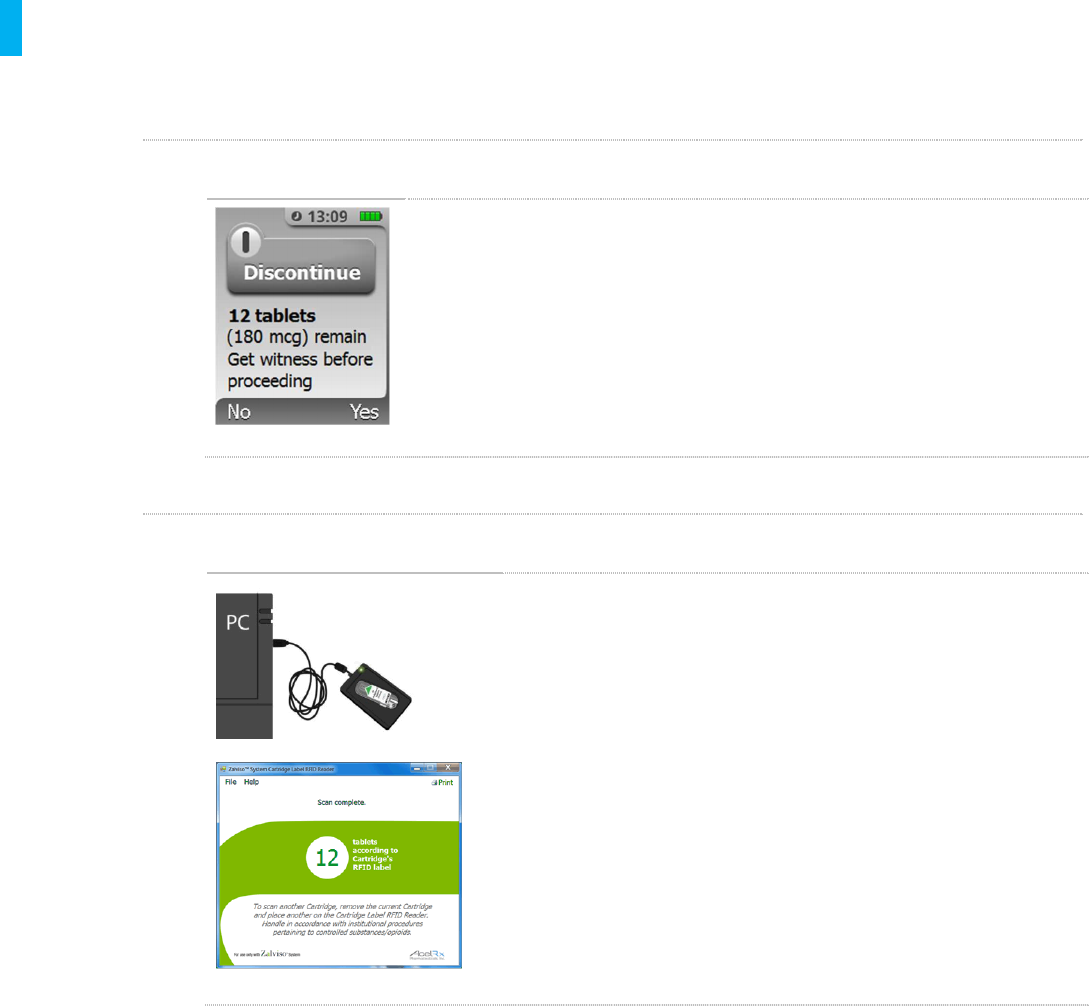
METHOD 1 Electronic –System Display During Discontinuation of Therapy
During Discontinuation of Therapy (Section 14.1)the System displays the
number of tablets remaining. In the example at left, 12 tablets are
remaining. This is the primary method for accounting of remaining tablets.
NOTE: as is typical with CII drug, it is recommended to get a witness at
this point to verify and document the number of remaining tablets.
METHOD 2 Electronic –Cartridge Label RFID Reader
The Cartridge Label RFID Reader (see Section 24, Use of
Cartridge RFID Label Reader)enables the Healthcare
Professional to read and display the number of remaining
tablets in used Cartridges, at the time of System
Discontinuation of therapy, as electronically recorded on the
Cartridge Label RFID. In the example at left, 12 tablets should
be remaining in the used Cartridge.
NOTE: as is typical with CII drug, it is recommended to get a
witness at this point to verify and document the number of
remaining tablets.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 81
Instructions for Use –PL-1678 Rev. K
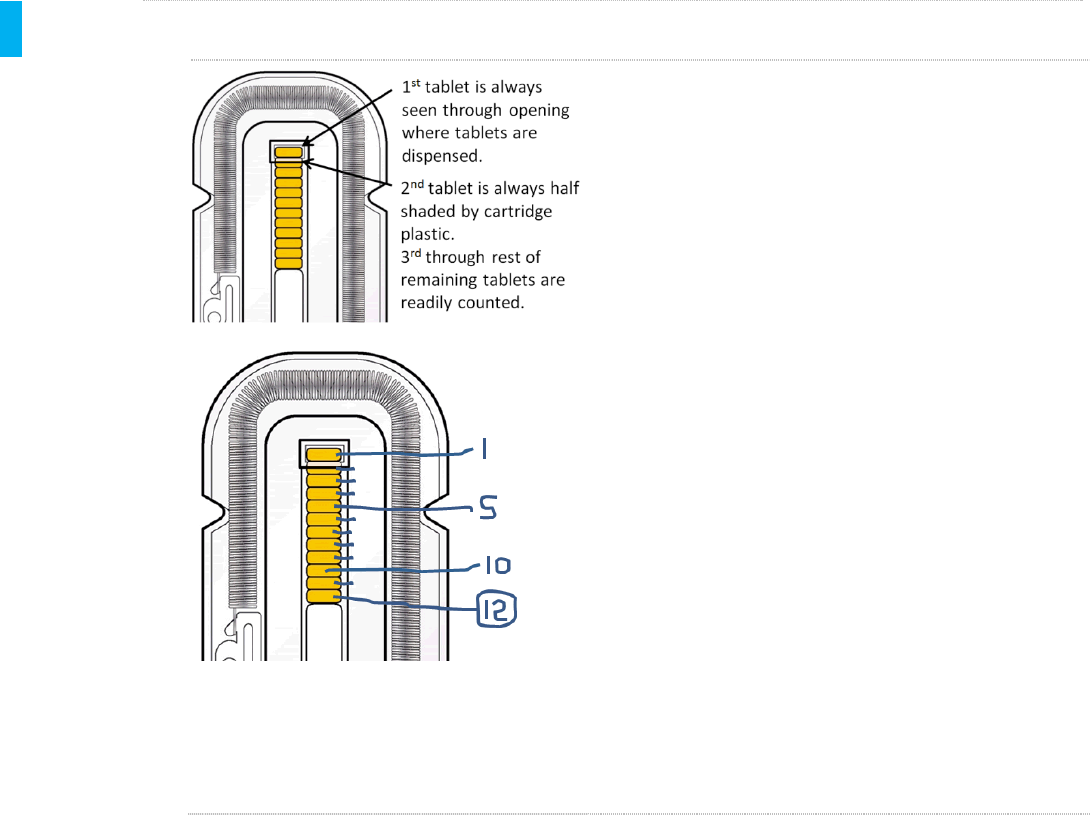
METHOD 3Manual –Counting Tablets While Still In Cartridge
All remaining tablets are visible through the clear
plastic cartridge. The example shown at left has 12
tablets remaining.
To aid counting, magnification is recommended. The
tablets can be magnified using reading glasses, or
enlarging a photo image taken by a smartphone.
A suggested alternative method (when over 10 tablets
are remaining), is to print an enlarged image of the
tablets using the “zoom” feature on a photocopier.
The tablets can then be counted off on the paper
copy with a pen in sets of 5 to facilitate counting the
tablets, as shown in the example at left. In this
example, there are 12 tablets remaining.
NOTE:Regardless of the enlargement method used,
be sure to count the 1st tablet as the one in the
rectangular slot at the top of the cartridge; this slot is
where tablets exit the dispenser during use.
NOTE: as is typical with CII drug, it is recommended
to get a witness at this point to verify and document
the number of remaining tablets.
Printed on: ; Printed by: .
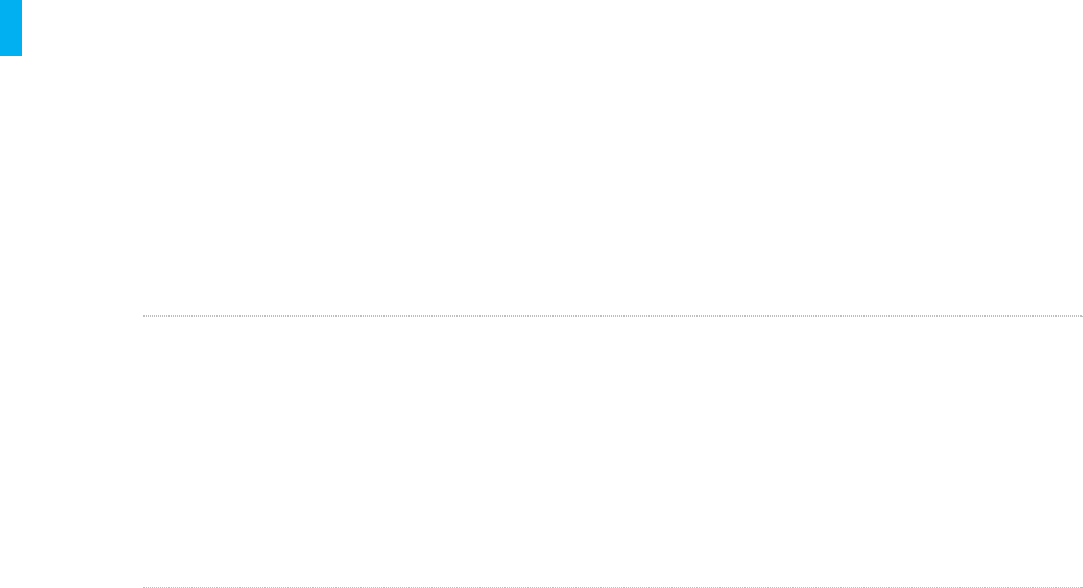
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 82
Instructions for Use –PL-1678 Rev. K
15. Replacing the System
The System will need to be replaced if the 72-hour limit for the System has been reached or when
a low battery condition is observed and the patient still requires therapy.
Replacement of the System may also need to be done by the Healthcare Professional in the
event that the System has become non-functional or experiences an Error that precludes further
use.
To replace the System:
Follow the steps for Discontinuing the System in Section 14, Discontinuation of Therapy
and Disposition of Used Components.
The used System should be removed after the discontinuation process.
Follow the instructions for setting up a new System as described in Section 5, How to Set
Up the System for a New Patient.
To return a non-functional or System with an Error that precludes use:
The used System should be returned to biomedical engineering for reprocessing (Section 17,
Reprocessing Instructions)and diagnostics (Section 26, Diagnostics).
Printed on: ; Printed by: .

AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 83
Instructions for Use –PL-1678 Rev. K
16. Notifications, Alerts, Alarms and Errors
In addition to dosing information,the System will notify the patient and Healthcare Professional of
certain situations:
Notifications (Section 16.1)
Alerts (Section16.2)
Alarms (Section 16.3)
Errors (Section16.2)
Refer to each section below for more details. For more information, please refer to Section 26,
Diagnostics, for Diagnostic test screens and errors.
16.1. Notifications
Notifications are a visual and/or audio signal indicating operating status or a message that may
require action,though there is not an unsafe condition. Notifications are indicated with a yellow
screen without the “!” symbol, and are accompanied by the low level notification tone. The tone
and screen will repeat based on the type of message.
Review the types of notifications below. For each notification the meaning, example of the screen
and the action(s) to take are listed.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 84
Instructions for Use –PL-1678 Rev. K
Notification Type Meaning Action(s) to Take
Low Battery If this screen appears, the battery
capacity is getting low to the point
where it will soon not be able to
continue functioning to delivery
therapy (approximately 2 hours)
If in patient use, System must be removed
and sent for reprocessing and recharging.
Do not connect the Charger to the
sufentanil sublingual tablet system while it
is being used by a patient. Battery
charging is only active when the Controller
is discontinued.
Replace with a new System as needed. If
System is being charged, continue
charging until battery is completely
charged.
Press the Menu Button.
Scan the AAC (notification is silenced at
this point).
Proceed to System Menu.
Proceed to Discontinue and replace with a
new System if required.
Reprocess and charge the Controller
(Sections 17 and 18, respectively).
Four green bars mean the battery
is fully charged.
Two yellow bars means the
battery is running low.
One red bar means the battery is
critically low and the Controller
should be discontinued.
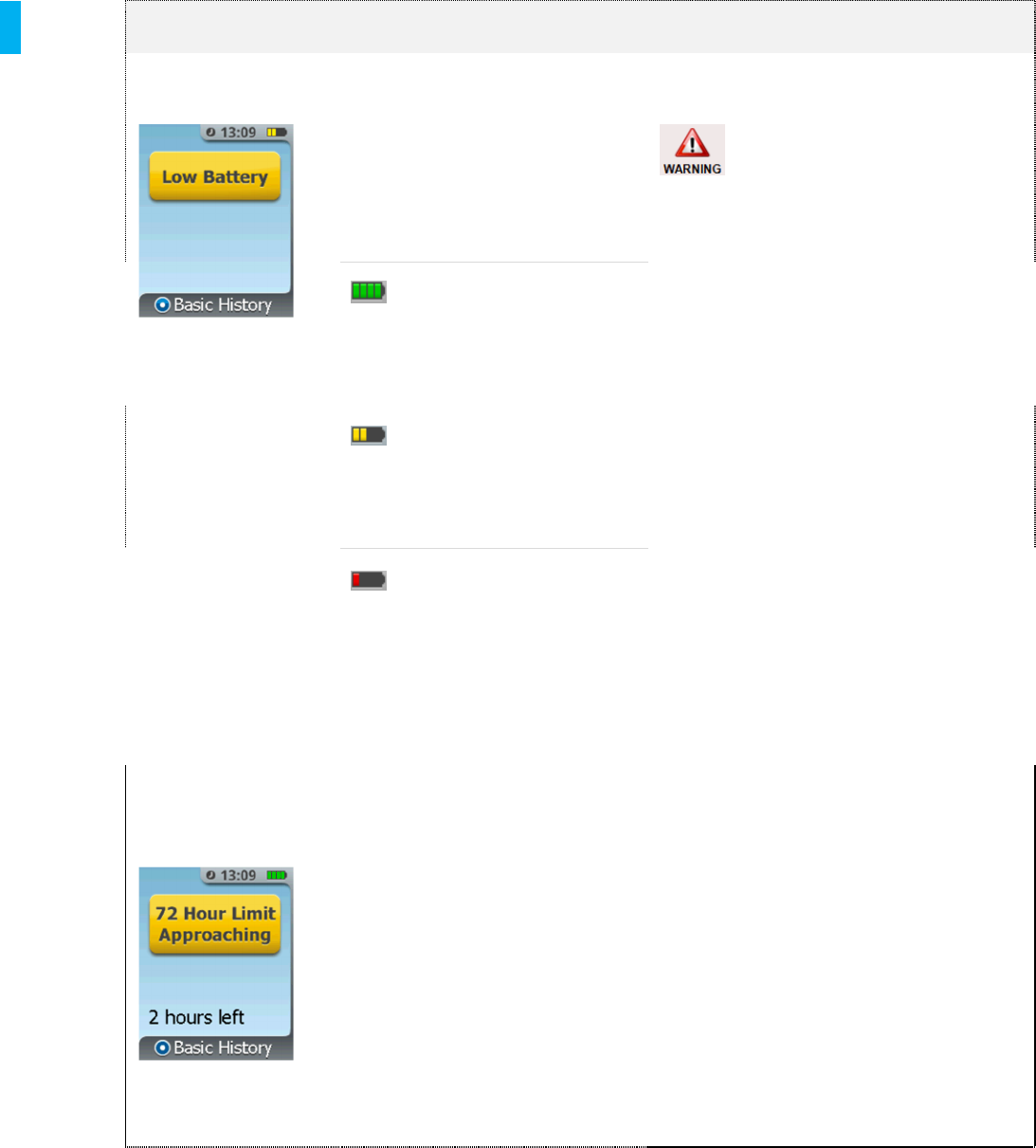
72 Hour Limit
Approaching
The 72 hour time limit is
approaching. The screen will
count down starting at 2 hours, 1
hour and 30 minutes left. This
message will re-appear at set
intervals if the System is not
discontinued.
The System must be removed from patient
use before the time period has expired and
replaced as needed for continued therapy.
Press the Menu Button.
Scan the AAC (notification is silenced at
this point).
Proceed to System Menu.
Scroll to Discontinue and replace with a
new System if required.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 85
Instructions for Use –PL-1678 Rev. K
Notification Type Meaning Action(s) to Take
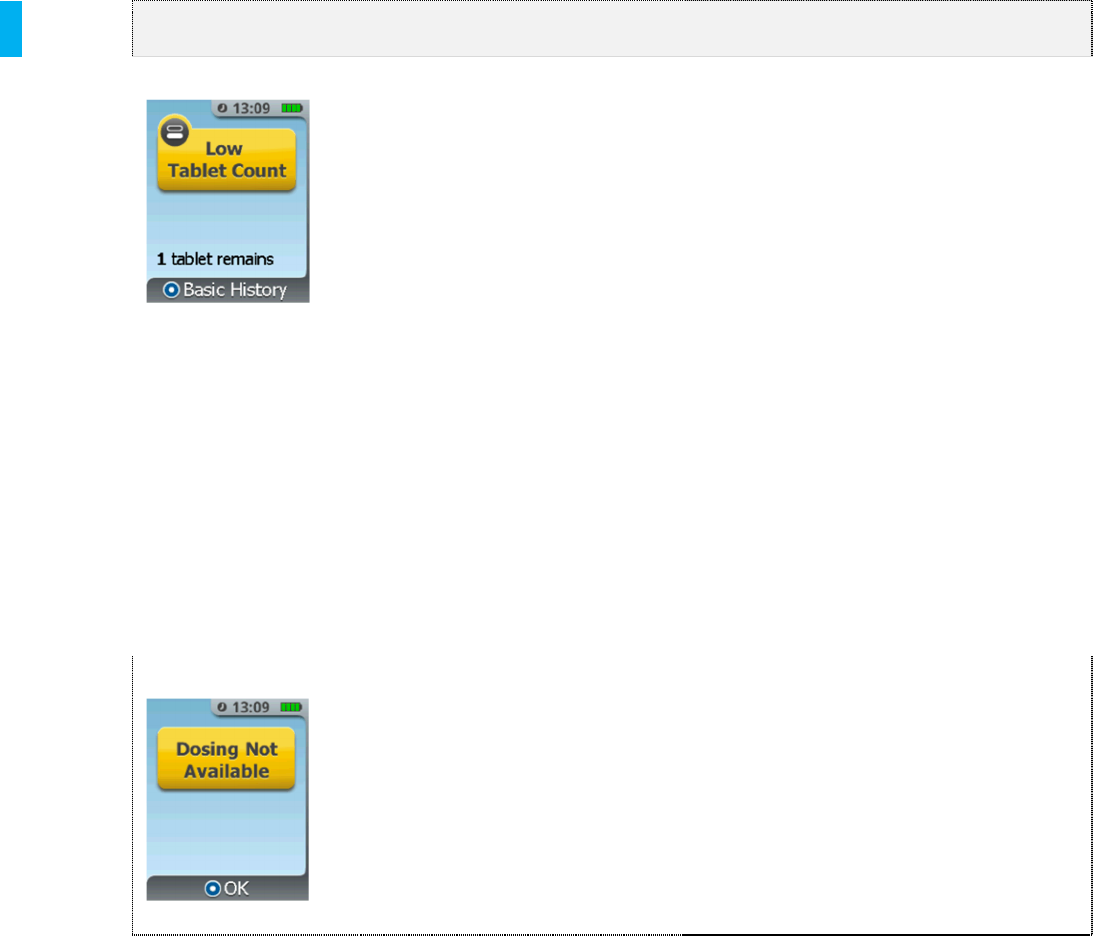
Low Tablet Count Tablet count is getting low. This
screen will start notifying of this
condition when there are 2 tablets
remaining and will re-appear at 1
and 0 tablets remaining.
The Cartridge will need to be replaced
once empty. If the 3 Cartridge limit has
been reached, the System will not permit
use of additional Cartridges. A new
System will be required to continue
therapy.
Press the Menu Button.
Scan the AAC (notification is silenced at
this point).
Proceed to System Menu.
Scroll to Replace Cartridge option and
follow directions.
If 3 Cartridge limit has been reached, refer
to “3 Cartridge Limit Reached” notification
below.
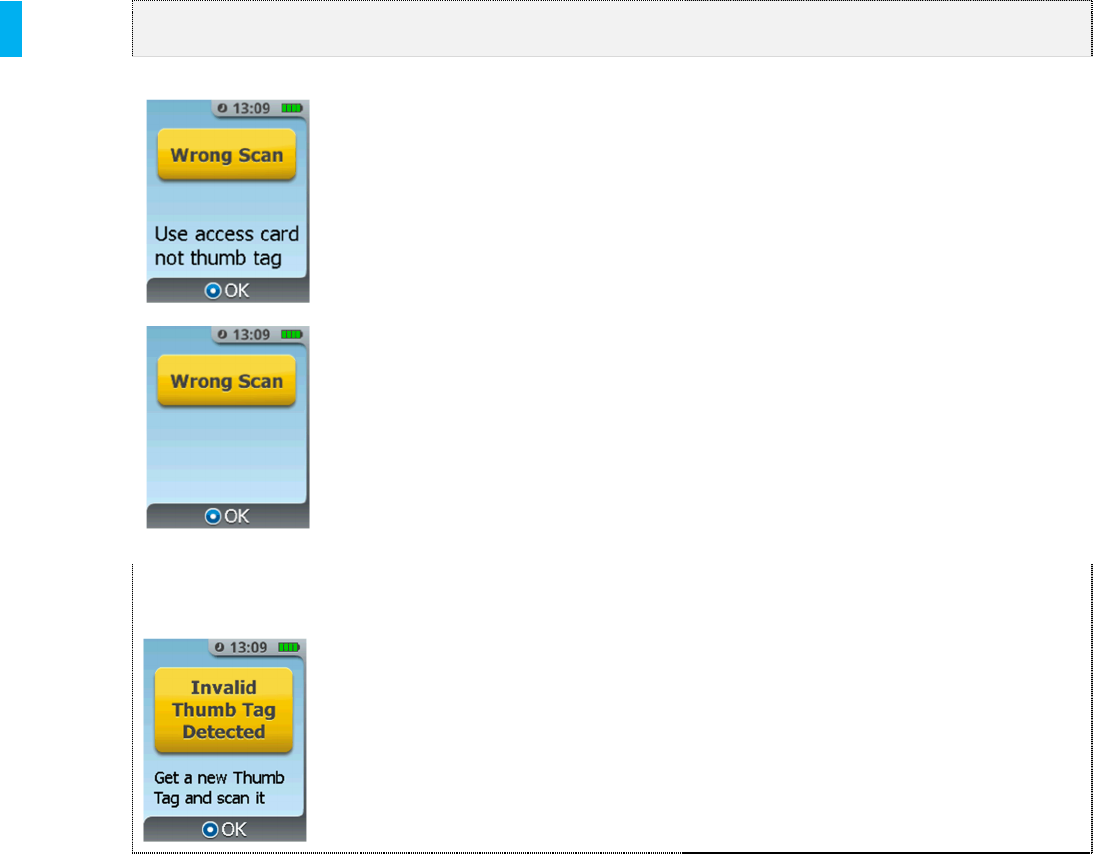
Dosing Not Available The System cannot be returned to
the patient for dosing.
This screen appears when
another condition occurred that
prompted menu access, but
returning to patient mode is not
an option, such as 3 Cartridge
limit, low battery alarm, etc.
The System must be removed from use.
Press the Enter/Select Button to return
to the System Menu.
Scroll to Discontinue and replace with a
new System if required.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 86
Instructions for Use –PL-1678 Rev. K
Notification Type Meaning Action(s) to Take
Wrong ID Depending on the stage of setup,
either the Patient ID Thumb Tag
was scanned instead of the AAC,
or the AAC was scanned instead
of the Patient ID Thumb Tag.
During initial power-up, the AAC must be
scanned. If this Error message is received
at this point, the Patient ID Thumb Tag was
scanned accidentally. Scan the AAC to
correct this problem.
Once in setup, on the Patient ID activation
step, if the AAC was scanned accidentally
this Error message will appear. Scan the
Patient ID Thumb Tag to correct this
problem.
Invalid Patient ID
Thumb Tag
A previously used Patient ID
Thumb Tag was presented to the
System during setup or Patient ID
Thumb Tag change. A new
Patient ID Thumb Tag is required.
1. Obtain a new Patient ID Thumb
Tag.
2. Press the Enter/Select Button.
3. Scan the new Patient ID Thumb
Tag.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 87
Instructions for Use –PL-1678 Rev. K
Notification Type Meaning Action(s) to Take
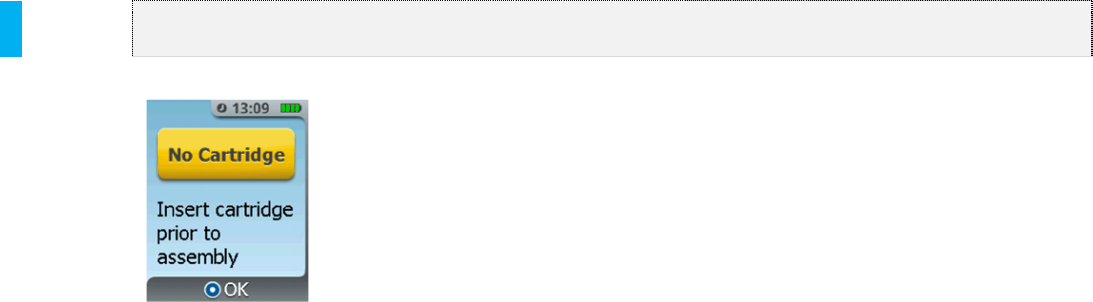
No Cartridge The System was assembled
without a Cartridge.
If the Healthcare Professional determines
that there is no Cartridge in the Dispenser,
a Cartridge should be inserted into the
Dispenser. If the Healthcare Professional
determines that there is a Cartridge
present in the System and yet the error
message shows up on the System, a new
Cartridge should be retrieved and inserted
into the System.
1. Press the Enter/Select Button.
2. Follow the screen prompts to
remove the Dispenser.
3. Insert a new Cartridge, then
proceed with the normal setup
outlined in Section 5.
4. Retain the unused Cartridge for
reconciliation per institutional
procedures governing controlled
substances.
If this condition persists, notify Biomedical
Engineering and set up a new System as
needed.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 88
Instructions for Use –PL-1678 Rev. K
Notification Type Meaning Action(s) to Take
Used Cartridge The Cartridge that was inserted
has been used or tampered with.
Remove the Cartridge from the Dispenser
and replace with a new Cartridge to
continue or begin therapy.
1. Press the Enter/Select
Button.
2. Follow the screen prompts to
remove the Dispenser.
1. Insert a new Cartridge, and then
proceed with the normal setup
outlined in Section 5.
Retain the used Cartridge for reconciliation
per institutional procedures governing CII
opioids.
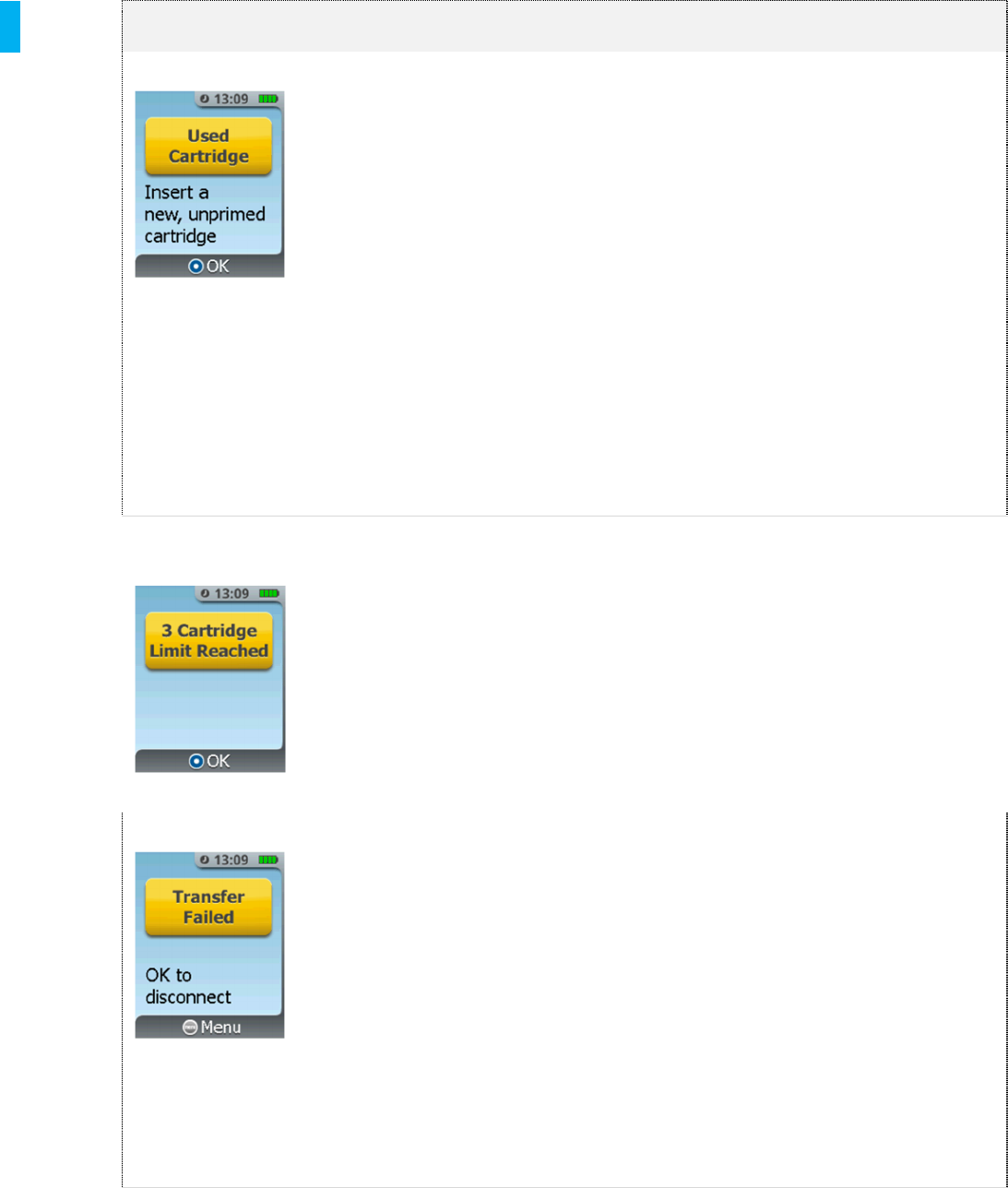
3-Cartridge Limit
Reached
This screen only appears if a
Cartridge change is attempted
while on the 3rd Cartridge or if the
3rd Cartridge is empty.
1. Press the Menu button to return
to the System Menu.
2. Proceed to Discontinue and
replace with a new System if
required.
Transfer Failed Data transfer from the System to
a computer has failed.
Repeat steps for Data Transfer.
2. Disconnect the Data Cable from
the System.
3. Press the menu button to return to
the System Menu.
4. Retry Transfer Data (refer to
Section 19).
If the error continues after replacing the
Data Cable, contact the biomedical
technician or contact the manufacturer to
arrange for the return of the Controller.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 89
Instructions for Use –PL-1678 Rev. K
Notification Type Meaning Action(s) to Take
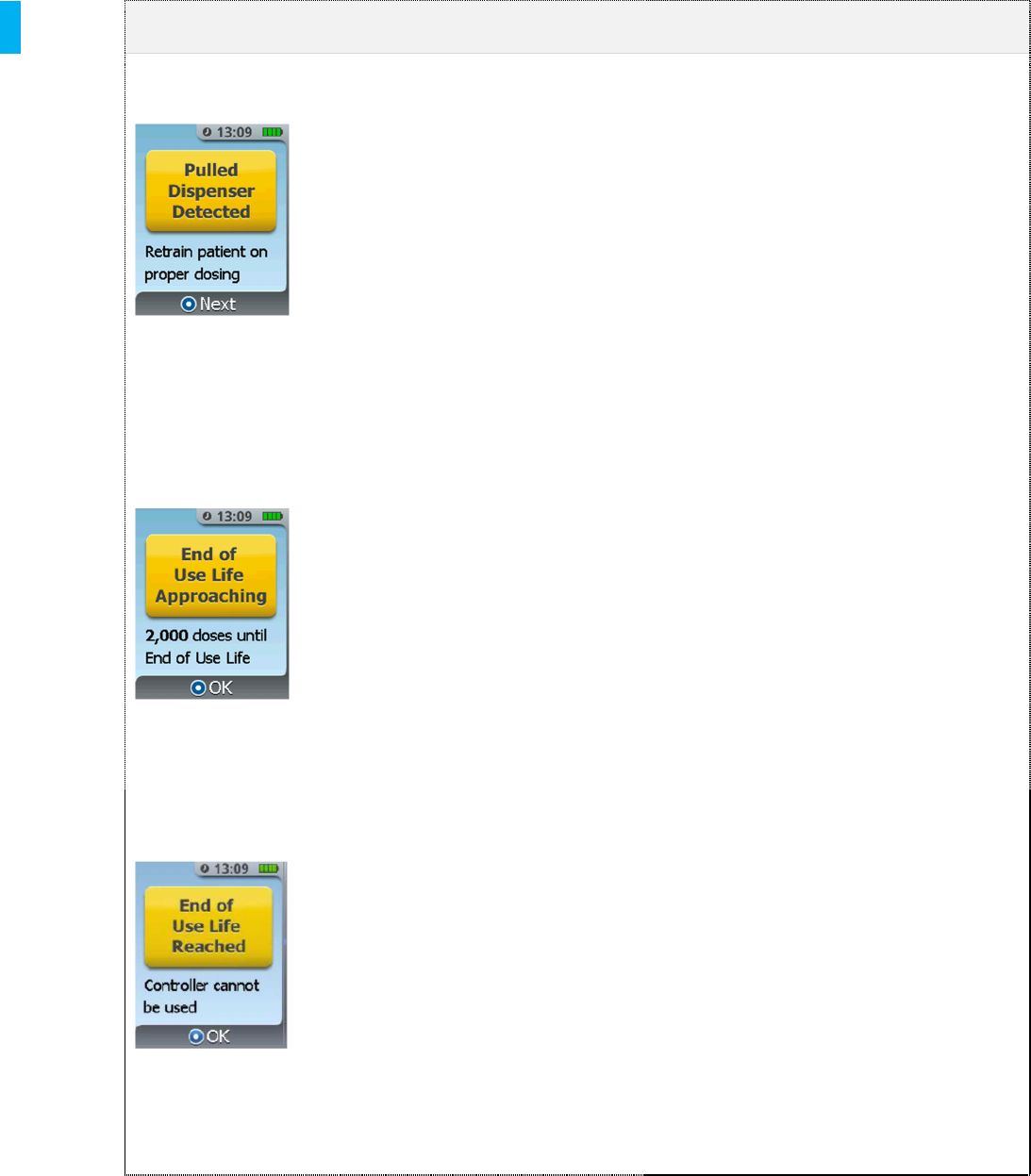
Pulled Dispenser
Detected
Patient is pulling down on
Dispenser when attempting to
dose.
1. Press the Enter/Select
Button.
2. Follow the screen prompts to
remind the patient not to pull down
on the Dispenser when dosing and
observe patient dosing.
3. Two attempts are allowed to retrain
the patient on proper dosing. After
the second attempt, the System
will proceed to “Error –System
Cannot Be Used”. (refer to Section
16.2)
End of Use Life
Approaching
The “End of Use Life
Approaching” screen is displayed
only when the Controller is
connected to a Charger and the
cummulative dose count of the
Controller exceeds 27,000 doses,
or 90% of the Controller 30,000
dose use life. This alert indicates
that the hospital should consider
ordering a new Controller within
the next several months.
1. Press the Enter/Select
Button to continue charging the
Controller.
End of Use Life
Reached
The “End of Use Life Reached”
screen is displayed when the
cummulative dose count exceeds
29,880 doses, or there are less
than 120 doses (3 40-count
cartridges) left until the 30,000
dose limit is reached. The
Controller cannot be used to
setup a new patient, avoiding the
possibility of the end of use life
occuroing during patient use (up
to 3 cartridges).
1. Press the Enter/Select
Button.
2. Follow the screen prompts to
power off the Controller.
3. Get another Controller to setup a
new patient.
4. Dispose of the old Controller per
hospital procedures for battery and
electronic waste.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 90
Instructions for Use –PL-1678 Rev. K
16.2. Alerts and Errors
Alerts are a visual and audio signal indicating the need for immediate action, though there
is not an unsafe condition. These are represented by a yellow screen with an Alert symbol
(see left)in the in the upper-left corner coupled with a repetitive audible alert tone.
System alerts are reviewed below. For each alert; the meaning, example of the screen,
and the action(s) to take are listed. A System error is displayed as an alert.
Alert Type Meaning Action(s) to Take
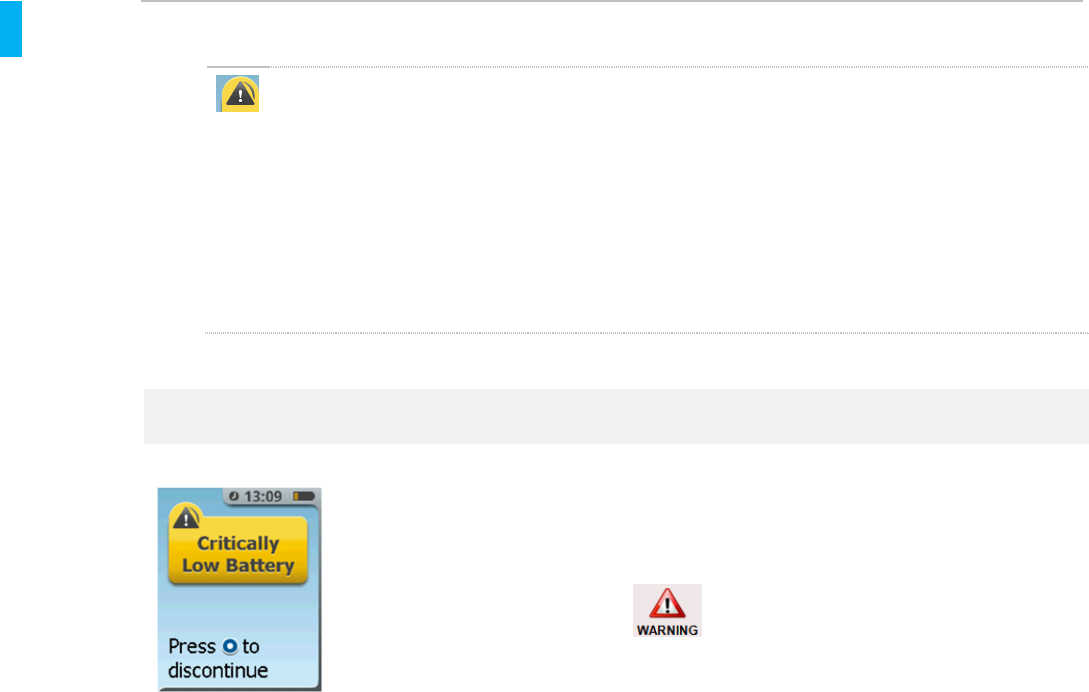
Low Battery If this screen appears with
the “!” warning, the
battery capacity is too low
to continue therapy.
If in patient use, System must be removed,
reprocessed and charged. If System is being
charged, continue charging until battery is
completely charged.
Do not connect the Charger to the
sufentanil sublingual tablet system while it is being
used by a patient. Battery charging is only active
when the Controller is discontinued.
If System is being charged, continue charging until
battery is completely charged
1. Press the Enter/Select Button.
2. Scan the AAC (Alert is silenced at this
point).
3. Record the tablet count if applicable.
4. Scroll to Discontinue the System.
5. Remove both the Dispenser and
Cartridge.
6. Set up a new System as needed.
7. Reprocess and charge the Controller
(Sections 17 and 18, respectively).
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 91
Instructions for Use –PL-1678 Rev. K
Alert Type Meaning Action(s) to Take
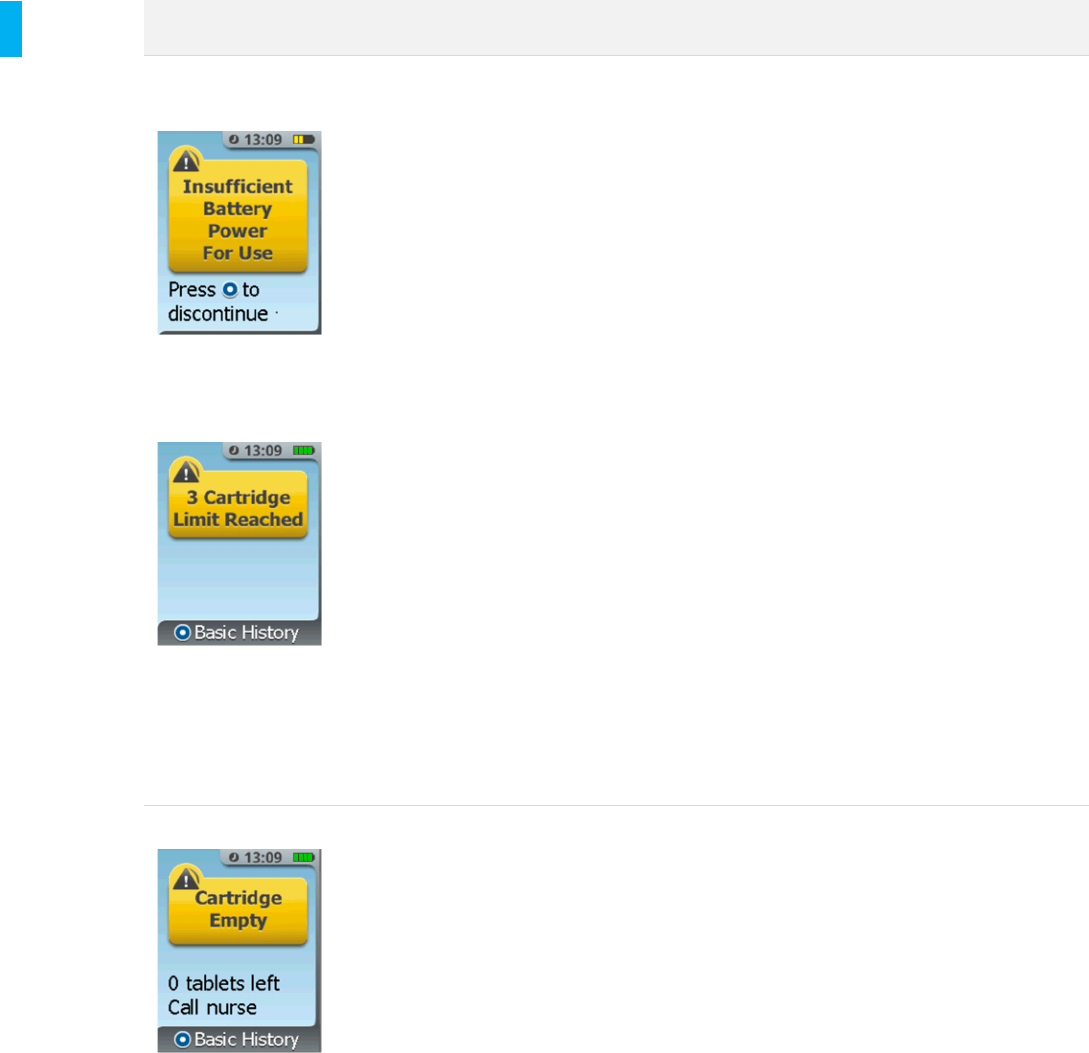
Insufficient Battery
Power for Use
During setup, the System
checks to see if there is
enough power for 72
hours of therapy. If the
System does not have
enough battery power to
be used for therapy, this
alert will be displayed.
The System must be replaced and charged.
1. Press the Enter/Select Button to exit
the setup mode and proceed to the
System Menu or
2. Depending on the stage of setup, press
the Enter/Select Button and proceed
to the Discontinue process.
3. Reprocess and charge the Controller
(Sections 17 and 18, respectively).
3 Cartridge Limit
Reached
The System is factory
programmed to only allow
3 Cartridges to be used
during the 72 hours of
therapy with a single
Controller. This alert
indicates that three
Cartridges have been
used and no more are
allowed with this System.
The System must be removed from use.
1. Press the Menu Button.
2. Scan the AAC (Alert is silenced at this
point).
3. Press the Right Button below the screen
to proceed to the Discontinue process or
press the Left button to proceed to the
System Menu. Going to the System
Menu does not allow exit to patient dosing
mode. Select Discontinue from the
System Menu and proceed to discontinue
the System.
4. Set up a new System as needed.
Empty Cartridge All 40 tabletshave been
dispensed from the
Cartridge.
A new Cartridge must be loaded if continuation of
therapy is desired.
1. Press the Menu Button.
2. Scan the AAC (Alert is silenced at this
point).
If the 3 Cartridge limit has not been reached,
press the Enter/Select Button and proceed to
setup with a new Cartridge as prompted.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 92
Instructions for Use –PL-1678 Rev. K
Alert Type Meaning Action(s) to Take
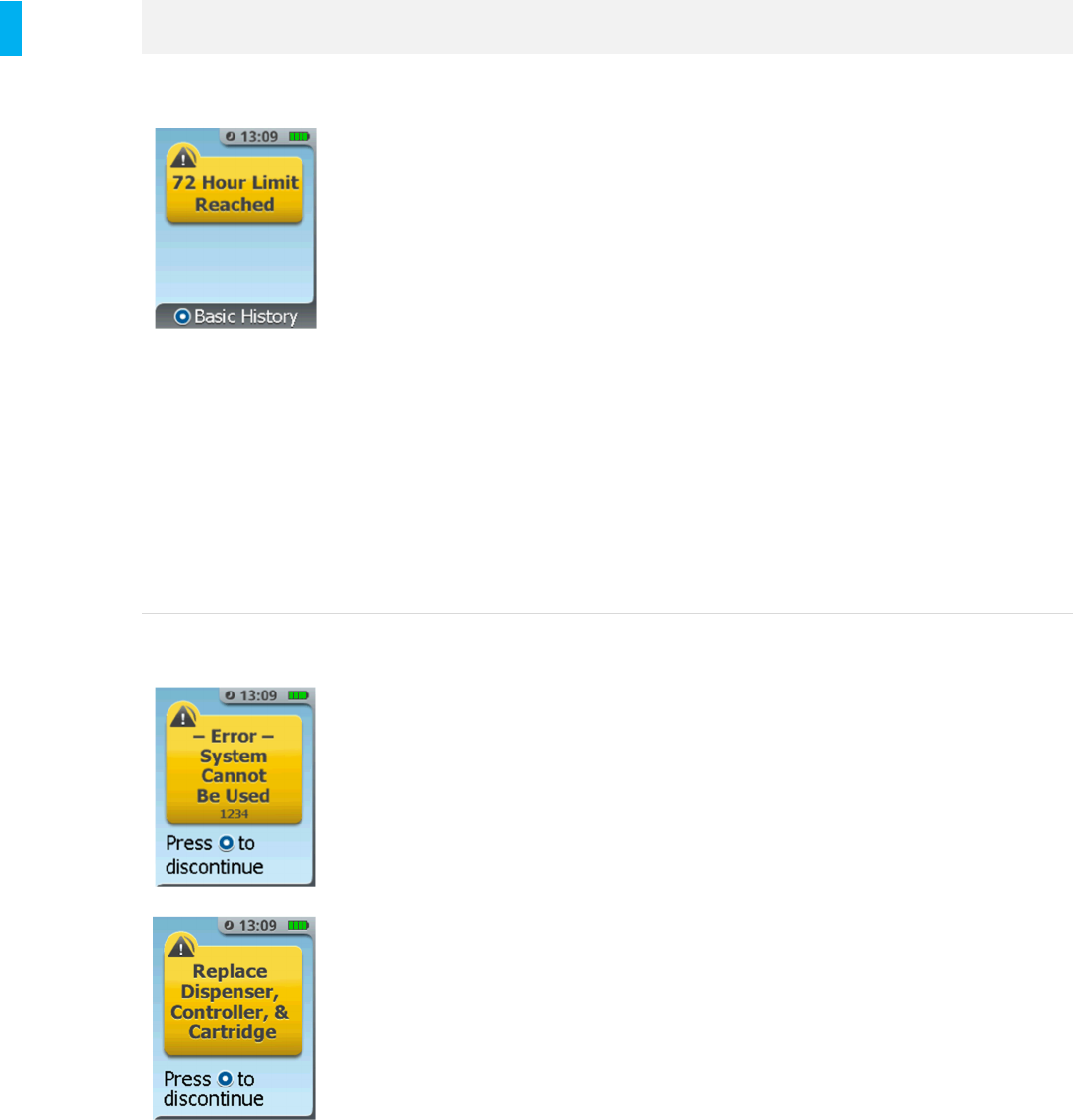
72 Hour Limit
Reached
The System is designed
for 72 hours of use and
the 72 hour time limit has
been reached.
The System must be removed from use.
Press the Menu Button.
Scan the AAC (Alert is silenced at this
point).
Press the Right Button below the screen
to proceed to the Discontinue process OR
press the Left Button to proceed to the
System Menu. If you proceed to the
System Menu, you will not be able to exit
to patient dosing mode, as further patient
dosing is not permitted. Select
Discontinue from the System Menu and
proceed to discontinue the System.
Proceed to Discontinue and replace with a new
System if additional therapy is needed.
Error –System
Cannot Be Used
A System error has
occurred. System Errors
are accompanied by Error
Codes-eg,“Error 301,
Error 302” etc. In the
example at left, the error
code is “1234”
The System must be removed from current patient
use:
1. Press the Enter/Select Button.
2. Scan the AAC (Alert silenced at this
point).
3. Record the tablet count if applicable.
4. Press the Enter/Select Button to
discontinue, or, if the System is
unresponsive, press and hold the Power
Button to power-off the System.
5. Remove both the Dispenser and Cartridge
and dispose according to Section 14.
6. Do not reuse the Controller, Dispenser or
Cartridge for a new setup. Return the
Controller to Biomed for follow up with the
manufacturer as described in the note
below.
7. Set up a new System as needed.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 93
Instructions for Use –PL-1678 Rev. K
NOTE
If any System error occurs, the Biomedical Engineering staff (or HCP) should
call 1-855-ZALVISO to report the error.Based on the type of error and other
system details, the user will be instructed to either reprocess the Controller and
return it to use, or return the Controller to the manufacturer.
Alert Type Meaning Action(s) to Take
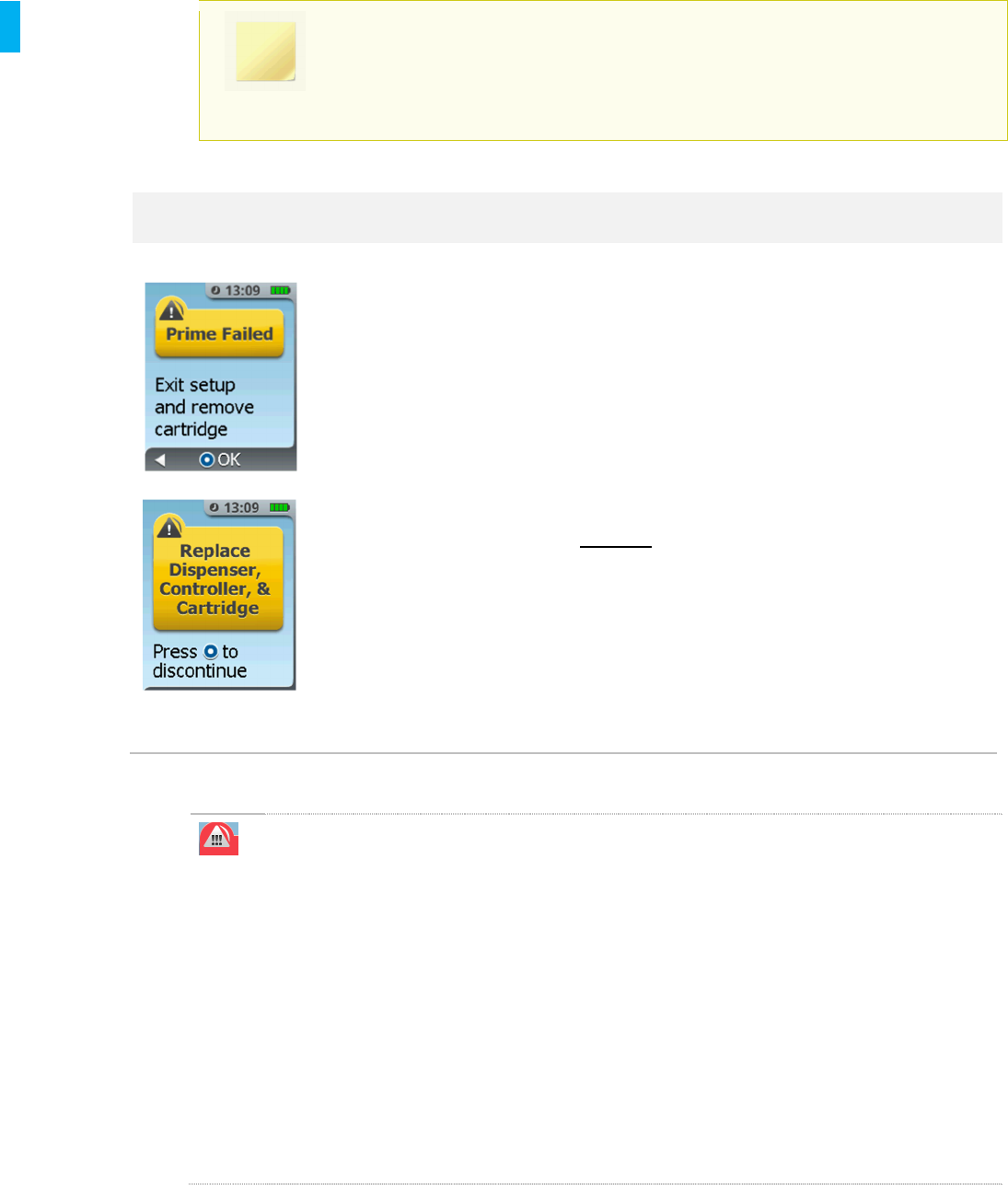
Prime Failed The Priming Cap failed to
dispense due to functional
error or tampered
Cartridge.
The System must be removed from use.
1. Press the Enter/Select Button to
continue and follow instructions on the
screen to discontinue the System.
Remove both the Dispenser and Cartridge
and dispose according to Section 14.
Return the Controller for reprocessing and
charging.
DO NOT reuse the Controller, Dispenser or
Cartridge for a new setup.
Set up a new System as needed.
16.3. Alarm
Alarms are situations where immediate action is required and a potentially unsafe
condition exists. Alarms are indicated by a flashing red screen with an alarm symbol
(see left) in the upper-left corner and a flashing alarm message accompanied by
flashing indicator lights and a repetitive audible high-level alarm tone produced by the
System which repeats until the Healthcare Professional confirms and responds to the
alarm. The audio alarm cannot be silenced and will stop only when System Discontinue
is completed.
The System has only one alarm condition. This high priority alarm condition will occur
only when the Dispenser has been disconnected or pried from the Controller once the
System has been set up. If this alarm situation were to occur during patient use, the
Healthcare Professional should make an assessment as to whether intentional misuse is
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 94
Instructions for Use –PL-1678 Rev. K
suspected or if this condition was caused by an accidental dislodging of the Dispenser.
If an alarm was to occur, the Healthcare Professional should press the Enter/Select
Button and follow the on-screen instructions to Discontinue the System.
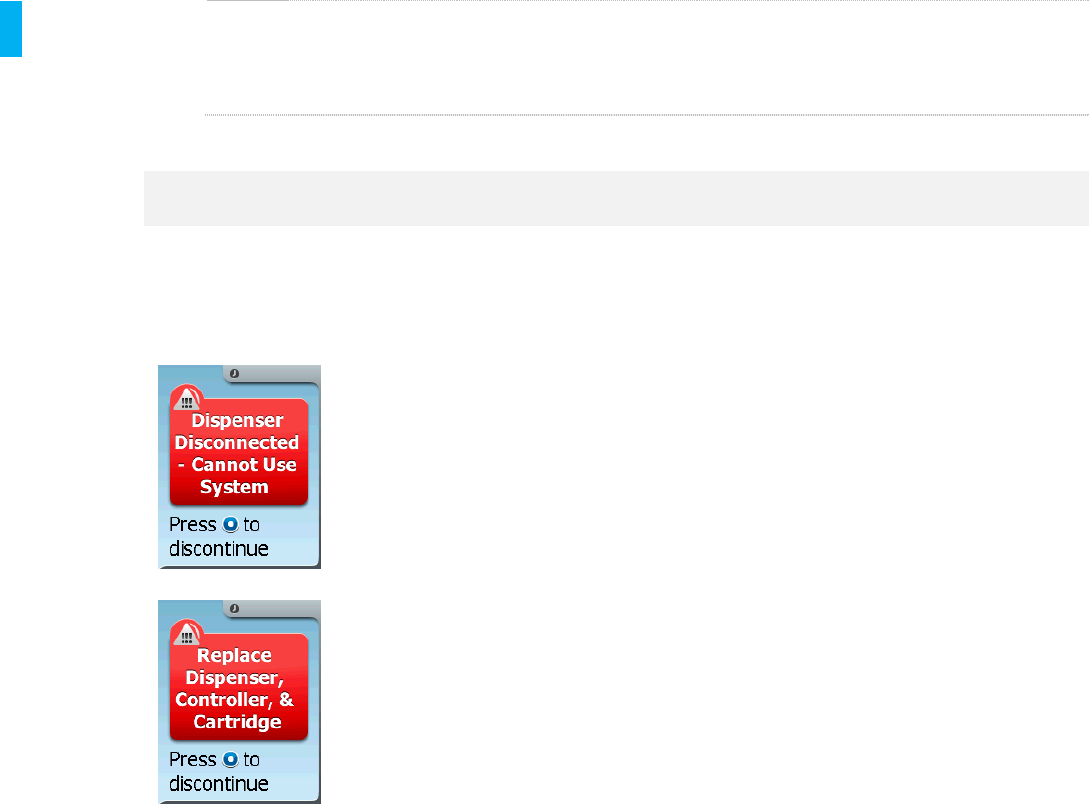
Alarm Type Meaning Action(s) to Take
High Priority Alarm
Dispenser
Disconnected
The Dispenser has
become disconnected.
Press the Enter/Select button to proceed to
Discontinue and replace with a new System
(Replace the Dispenser, Controller and
Cartridge).
Investigate for cause of alarm to determine if
caused intentionally or accidentally.
Note: The “Dispenser Disconnected” alarm
screen flashes five times then the “Replace
Dispenser, Controller, & Cartridge” alarm
screen flashes five times at a periodic rate.
The audio alarm will stop only when
Discontinue is completed.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 95
Instructions for Use –PL-1678 Rev. K
17. Reprocessing Instructions
17.1. Reprocessing of Reusable Components
Reprocessing is required for the reusable,patient contacting parts of the sufentanil sublingual
tablet system, which are the:
Controller
Security Tether (“Tether”)
Holster
These three reusable parts must be reprocessed before the next patient may use the System.
Reprocessing greatly decreases the chance of passing on pathogens from one patient to the
next.
Reprocessing the Cleaning Plug
The Cleaning Plug, used to protect the Charging/Data port during reprocessing of the Controller,
must itself be reprocessed after use to avoid potentially transmitting contamination from one
Controller to the next.
Printed on: ; Printed by: .

AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 96
Instructions for Use –PL-1678 Rev. K
Reprocessing Instructions
Reprocessing consists of two distinct and important steps:
Cleaning –cleaning prevents passing contaminants (e.g., food, human waste, bacteria)
from one patient to the next. More importantly, it removes dirt and contaminants from the
device so that the next step, disinfection, is effective.
Disinfection –disinfection kills pathogens that may come from the patient (e.g., through
touch, blood or other bodily fluids, coughing or sneezing on the device), or may come
from Healthcare Professionals or visitors that may handle the device with unclean hands.
Effective disinfection helps prevent the spread of disease to the next user of the System.
Cleaning and Disinfection Supplies
Sani-Cloth Plus® Germicidal Disposal Cloths
(“Sani-Cloth Plus Wipes”) are recommended for both cleaning and
disinfecting the Controller, Tether and Holster before use by the next
patient, and also for reprocessing the Cleaning Plug. Sani-Cloth Plus
Wipes have been shown to effectively clean and disinfect these reusable
parts. Sani-Cloth Plus Wipes are manufactured by PDI, and are available
through hospital supply stores. These wipes are EPA Registered (Reg.
No. 9480-6) and approved for use on hard patient-contacting surfaces in
many hospitals in the US. These wipes have been proven to be effective,
when the label directions are followed, in killing a broad range of bacteria
and viruses typically found in hospital settings.
NOTE: If an alternative germicidal wipe is used, the hospital must confirm
that the germicidal effectiveness, per the manufacturer, is equivalent to
Sani-Cloth Plus Wipes.
WARNING
It is very important to follow the Sani-Cloth Plus Wipes’ label instructions for
cleaning and disinfecting, which have been incorporated into these
reprocessing instructions. Especially important for disinfection of pathogens is
to keep the component wet for the full recommended “Contact Time” of 5
minutes. Anything less than 5 minutes will not assure effective disinfection of
the System for the next patient.
Printed on: ; Printed by: .

AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 97
Instructions for Use –PL-1678 Rev. K
Pointed Disposable Cleaning Swabs
These should be available for cleaning and disinfecting hard to reach
areas on the Controller, Tether and Holster that may accumulate dirt and
debris during patient use. As described in the reprocessing instructions
below, the swab may be used as a stiffener for a wipe to dig into small
crevices, or may be wetted with a wipe and used alone to clean tight
areas.
(An example of an effective pointed swab is Qosmedix 10222,
“Point/Point Cotton Swab, Paper Handle, 3in”, manufactured and
distributed by Qosina, Edgewood, NY.)
Custom Cleaning Plug
This is provided to protect the Charging/Data port on the bottom of the
Controller during the cleaning step of reprocessing. The Charging/Data
port has metal contacts near the opening that may be damaged by
germicidal solutions, as are in Sani-Cloth Plus wipes. Only the Cleaning
Plug should be used to cover the Charging/Data port during cleaning,
otherwise the Controller port contacts may be damaged.
Clean Gloves
Gloves (Non-latex recommended) should be worn when reprocessing the
Controller, Tether, Holster and Cleaning Plug, as recommended by the
Sani-Cloth Wipe manufacturer. Gloves not only protect hospital
personnel from prolonged contact with the germicidal chemicals in the
wipes, but also protect the device from possible contamination from the
personnel.
Cleaning Brush
A soft bristled brush (e.g., Graham Field Adult Toothbrush, Cat. No.
3395-1, available from Fisher Scientific, Cat. No. 19027438) should be
used.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 98
Instructions for Use –PL-1678 Rev. K
Pre-Treatment of the Reusable Part of the System at Point-of-Use, Prior to
Reprocessing
After discontinuation of patient treatment and the System has been disassembled per the
instructions, the reusable parts of the System (Controller, Tether and Holster) should be sent to
the appropriate reprocessing center within the hospital. If hospital procedures allow, any part that
has excessive contamination at the point-of-use (e.g., has been bled or vomited upon), should be
pre-treated by cleaning at the point of use prior to delivery to the reprocessing area. Pre-
treatment helps prevent the spread of pathogens which may be present on the device as it is
conveyed to the reprocessing area. In addition, pre-treatment will help remove heavy soil from
the device which may otherwise dry, possibly requiring extra effort to thoroughly clean.
If hospital procedures permit, pre-treat any heavily soiled, reusable part at point-of-use by
carefully performing the cleaning as follows:
Pre-Treatment of Excessively Soiled Controller at Point-of-Use
Follow the “Cleaning the Controller” instructions below. Pre-treatment cleaning only requires
gloved hands and Sani-Cloth Plus wipes. If no swabs are available to get into crevices on device,
remove as much contamination as possible at point of use; swabs can be used during actual
reprocessing cleaning.
WARNING
If a Cleaning Plug is not available for pre-treatment, take extra care to avoid the
charging/data port and Tether hole when cleaning the Controller. See other
cautions in the cleaning instructions for the Controller.
Pre-Treatment of Excessively Soiled Tether at Point-of-Use
Follow the “Cleaning the Tether” instructions below.
Pre-Treatment of Excessively Soiled Holster at Point-of-Use
Follow the “Cleaning the Holster” instructions below. NOTE: In normal use, the Holster clamp
metal parts (screw, spring, etc.) are not typically soiled. If the Holster Clamp becomes
excessively soiled, dispose the entire Holster in biohazard waste per hospital instructions.
After pre-treatment at point-of-use, transport components for reprocessing.
Printed on: ; Printed by: .

AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 99
Instructions for Use –PL-1678 Rev. K
17.2. Reprocessing the Controller
WARNING
The Controller contains electronic components. Never spray or submerge the
Controller –use only germicidal wipes. Avoid excessive liquid from the wipes
around holes to avoid damage to internal components.
WARNING
DO NOT contact the Charging/Data port with germicidal wipes –these have
been found to corrode the metal terminals after successive reprocessing. Avoid
wetting the metal contacts of the Charging/Data port, or excessive fluid around
the Tether hole or Charging/Data port. Electronic components are exposed and
just inside the device and may be damaged by excessive moisture.
DO NOT insert a wipe, brush or swab into the Tether port; this may damage the
Controller.
WARNING
Use of the Cleaning Plug is highly recommended. If a Cleaning Plug is not
available, take extra care to avoid the Charging/Data port when cleaning the
Controller.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 100
Instructions for Use –PL-1678 Rev. K
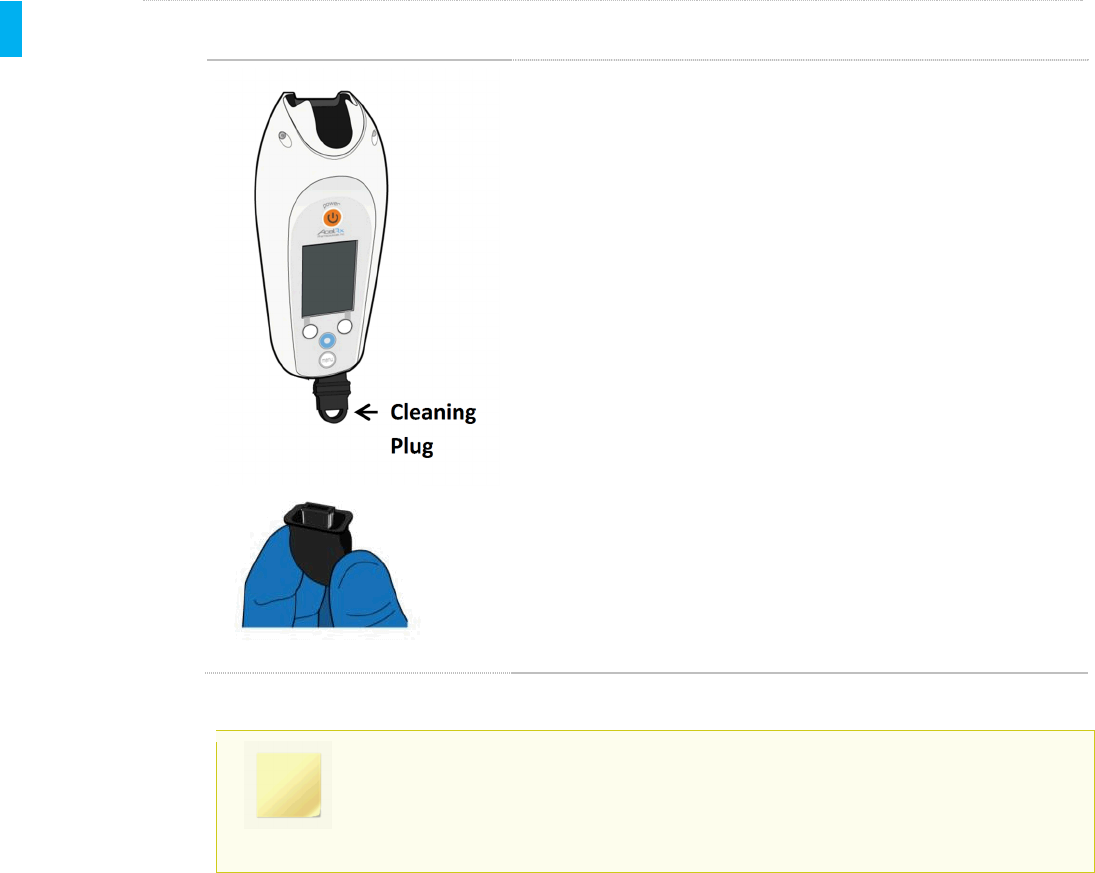
STEP 1 Insert Cleaning Plug
1. Put on a pair of clean gloves.
2. Insert a cleaned and disinfected Cleaning Plug into the
Charging/Data port. The Cleaning Plug should snap
into place, just like the charging and data cables.
3. There is no need to plug the Tether port.
NOTE
Use as many fresh wipes as necessary to reprocess the Controller.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 101
Instructions for Use –PL-1678 Rev. K
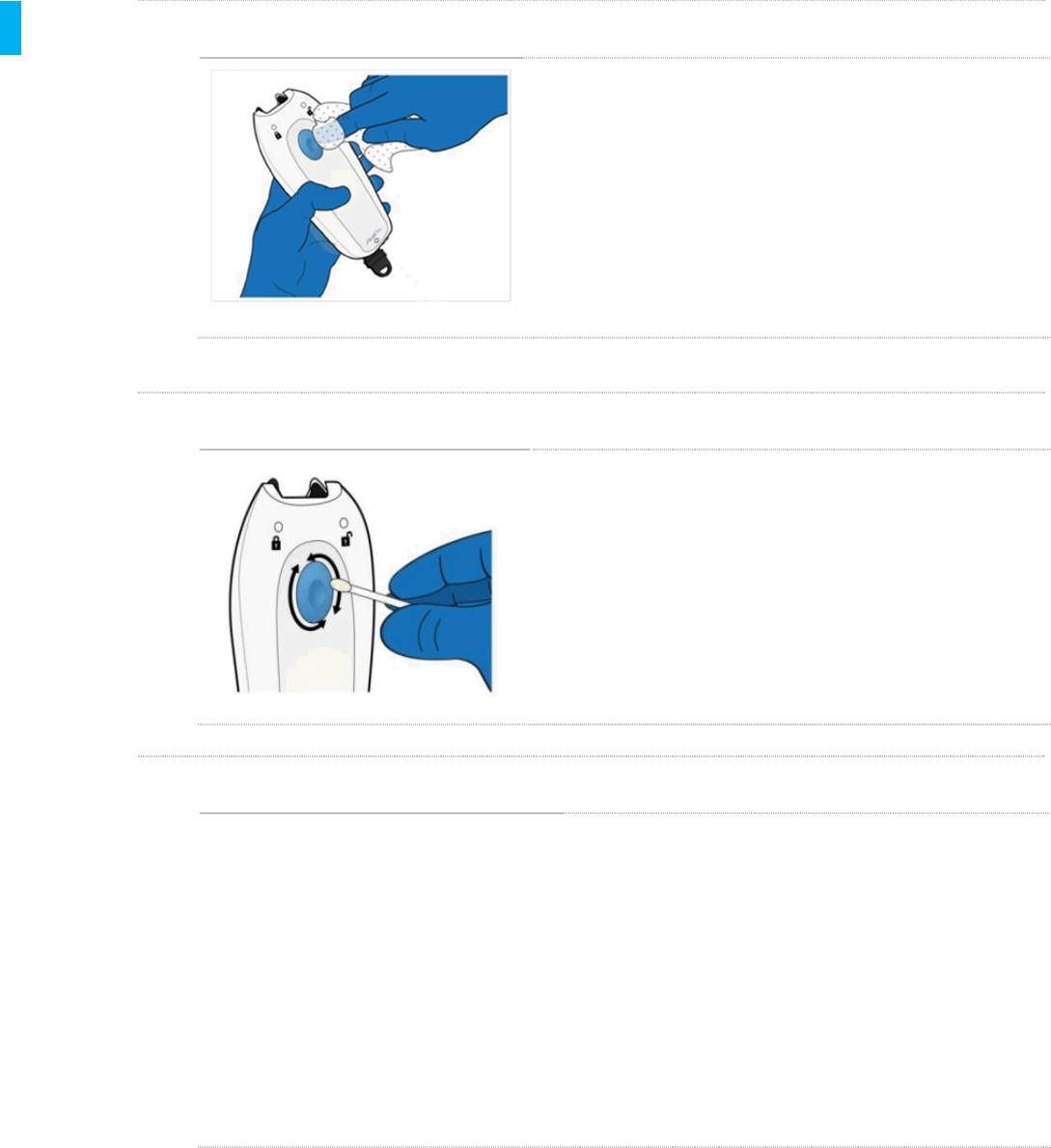
STEP 2 Clean the Controller
Using a fresh Sani-Cloth Plus Wipe, thoroughly clean the
outside surfaces of the Controller, starting with the Dose
Button Side. Remove all visible contamination (“soil”).
Use as many fresh wipes as necessary to clean the outside
surfaces of the Controller.
STEP 3 Clean Dose Button Area
Disinfecting wipes can and should be used generously
around the Dose Button since the Dose Button is
mechanically sealed to the Controller cover to allow
thorough cleaning. Be sure to thoroughly clean the Dose
Button and the area between the shell and button. Use
swabs, moistened with a Sani-Cloth Plus wipe, to get into
crevice around Dose Button.
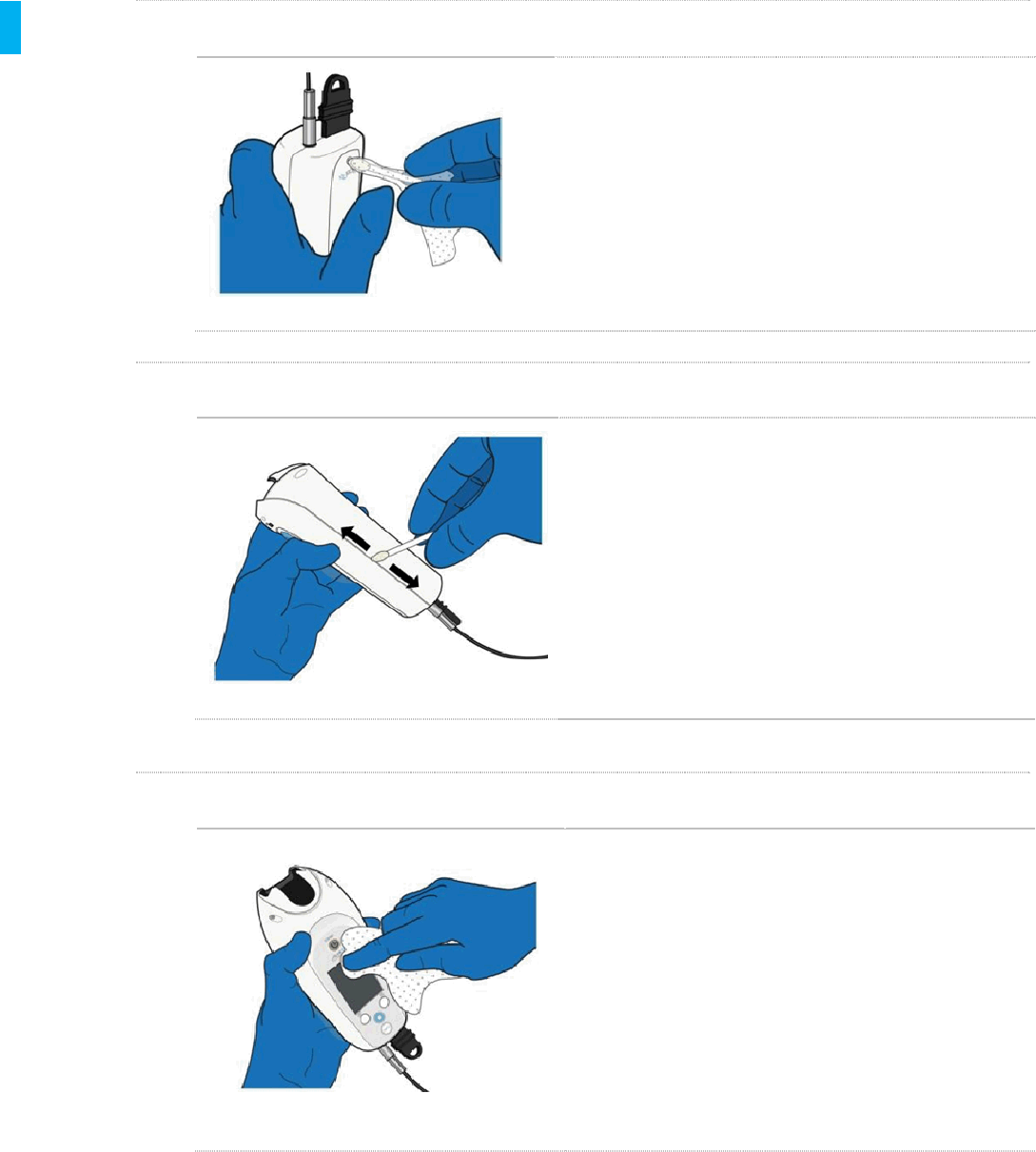
STEP 4 Clean Indicator Lights
Using a Sani-Cloth Plus wipe wrapped around a swab
(or a swab moistened with a wipe), clean the two lights
on the Dose Button side. The black “pocket” in the top
of the Controller (dotted arrow, at left) is covered by
the Dispenser during use and need not be cleaned.
However, if desired, the pocket may be cleaned with
wipes or moistened swabs.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 102
Instructions for Use –PL-1678 Rev. K
STEP 5 Clean Recessed Area
Using a Sani-Cloth Plus wipe wrapped around a swab
(or a swab moistened with a wipe), clean the recessed
area as noted in the figure below on the Dose Button
Side of the Controller.
STEP 6 Clean Seam
Using a swab moistened with a Sani-Cloth Plus Wipe,
or a wipe held tightly over a gloved fingernail,
thoroughly clean the seam that runs around the
Controller.
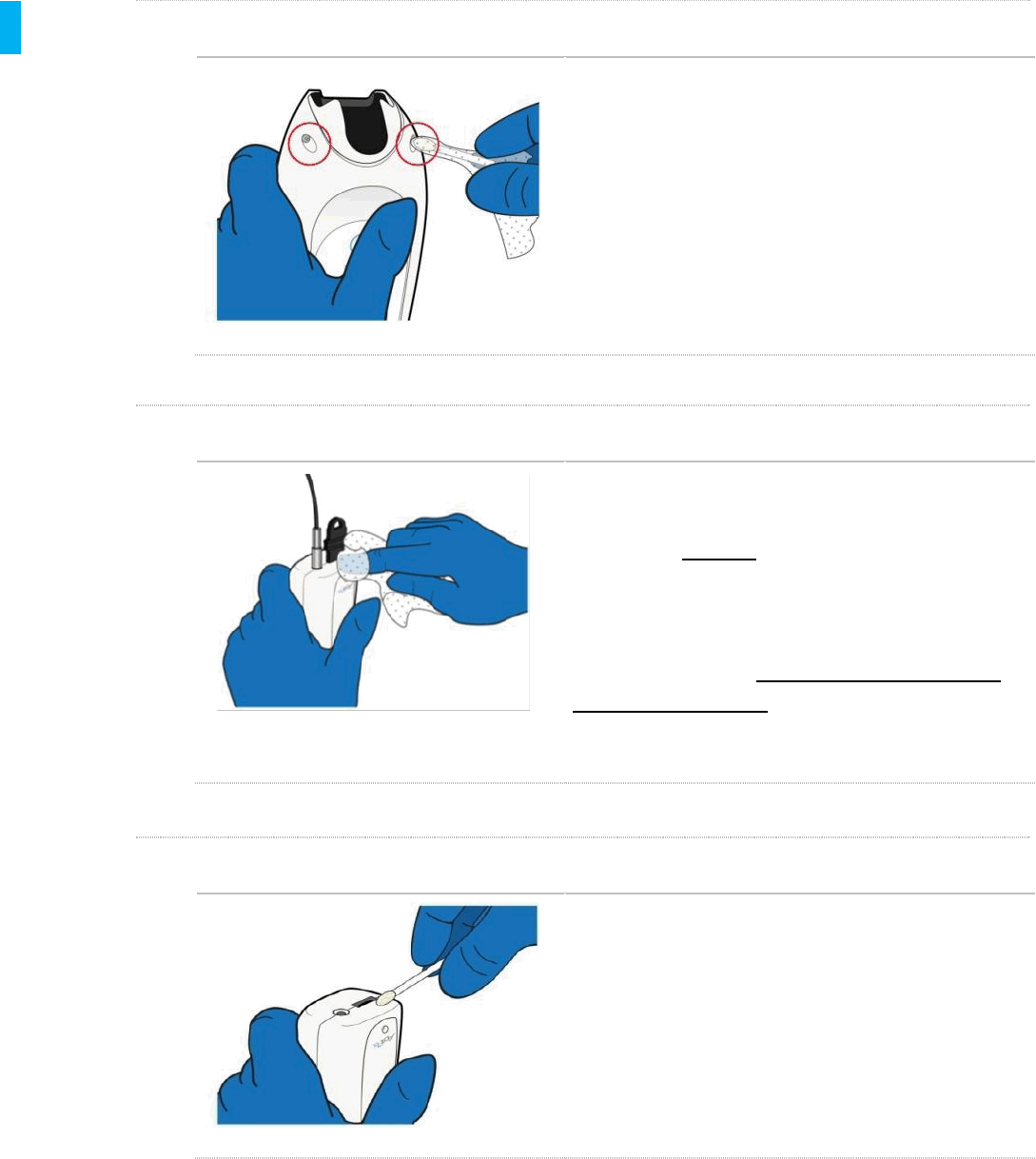
STEP 7Clean Screen Side
Using Sani-Cloth Plus Wipes and swabs, thoroughly
clean the front side (the screen side) of the Controller.
The front side of the Controller has several buttons
and a display which are protected by a sealed plastic
membrane. Use as many wipes and swabs as
needed to thoroughly clean these areas, as well as
the crevice between the Controller shell and plastic
membrane. Remove all visible soil.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 103
Instructions for Use –PL-1678 Rev. K
STEP 8 Clean Recessed Areas on Controller
Using a Sani-Cloth Plus wipe, wrapped around a
swab (or a swab moistened with a wipe), clean the
recessed areas as noted in the figure below on the
front side of the Controller.
STEP 9 Clean Bottom of Controller
Thoroughly clean the bottom of the Controller,
carefully cleaning around the Cleaning Plug and
Tether port. DO NOT insert a wipe, brush or swab
into the Tether port; this may damage the Controller.
After the Controller has been thoroughly cleaned (all
visible soil removed), remove the Cleaning Plug by
squeezing on the sides. The Cleaning Plug may now
be contaminated and should be set aside for
reprocessing.
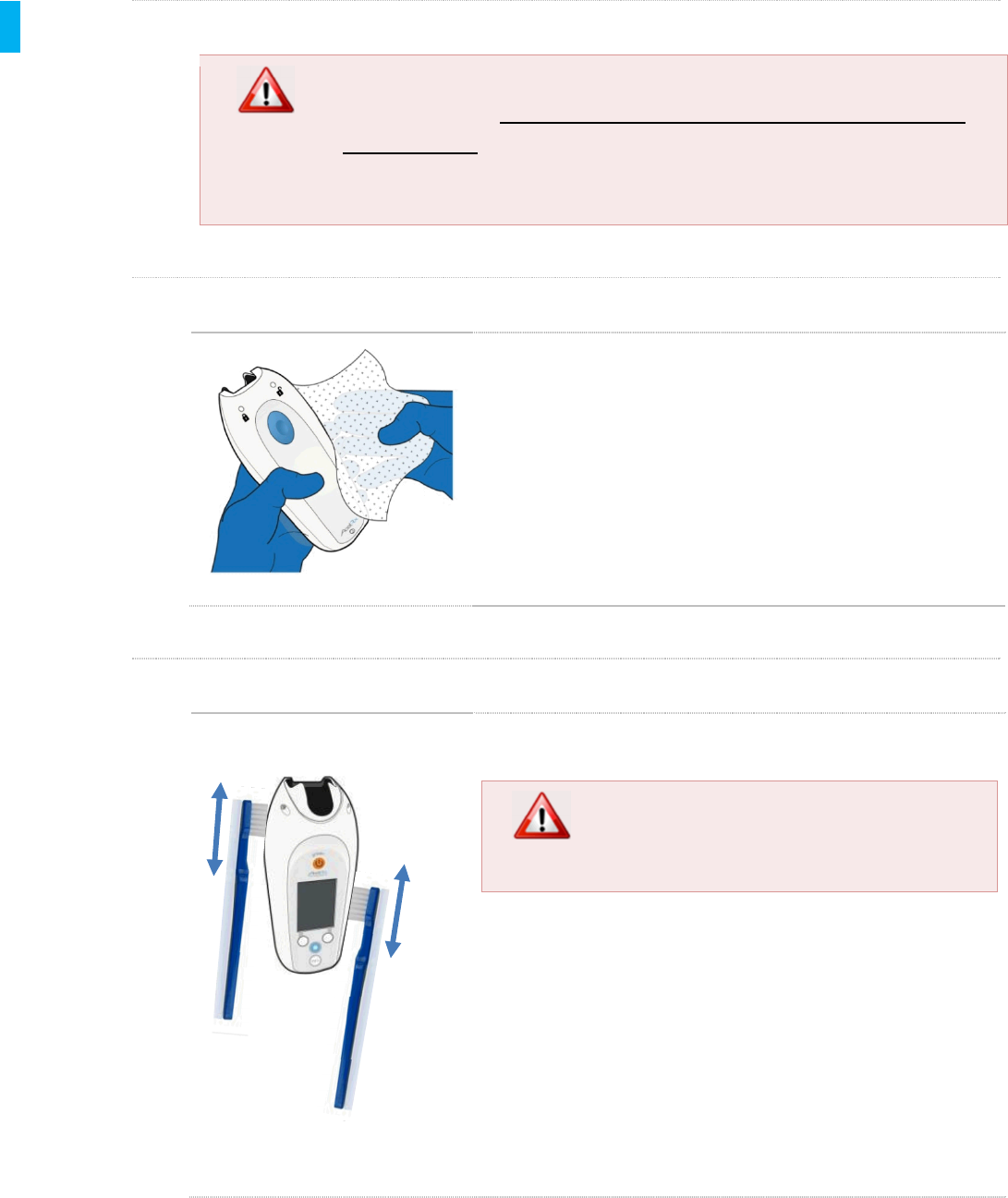
STEP 10 Clean Area Under Cleaning Plug
Using a swab moistened with a Sani-Cloth Plus wipe,
carefully clean the Controller surface around the
bottom of the Controller, avoiding the inside of the
Tether port and Charging/Data Port. Thoroughly
clean the areas exposed after removing the Cleaning
Plug, and areas hard to reach on the outside of the
Controller when the Cleaning Plug was in place.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 104
Instructions for Use –PL-1678 Rev. K
STEP 11 Disinfect the Controller
WARNING
All external surfaces of the Controller, especially crevices and seams should be
thoroughly wetted (being careful to avoid wetting the Charging/Data port
and Tether port). Most surfaces will dry within 2-3 minutes, so multiple
applications of fresh wipes at each location will be necessary to keep the
surfaces wetted for 5 minutes.
STEP 12Thoroughly Wet the Outside of Controller
Using a fresh Sani-Cloth Plus Wipe, thoroughly and vigorously
wet the entire outside surface of the Controller. Use swabs
wrapped in germicidal wipes, or swabs wetted with the wipes
to thoroughly wet the crevices and screw holes on the
Controller.
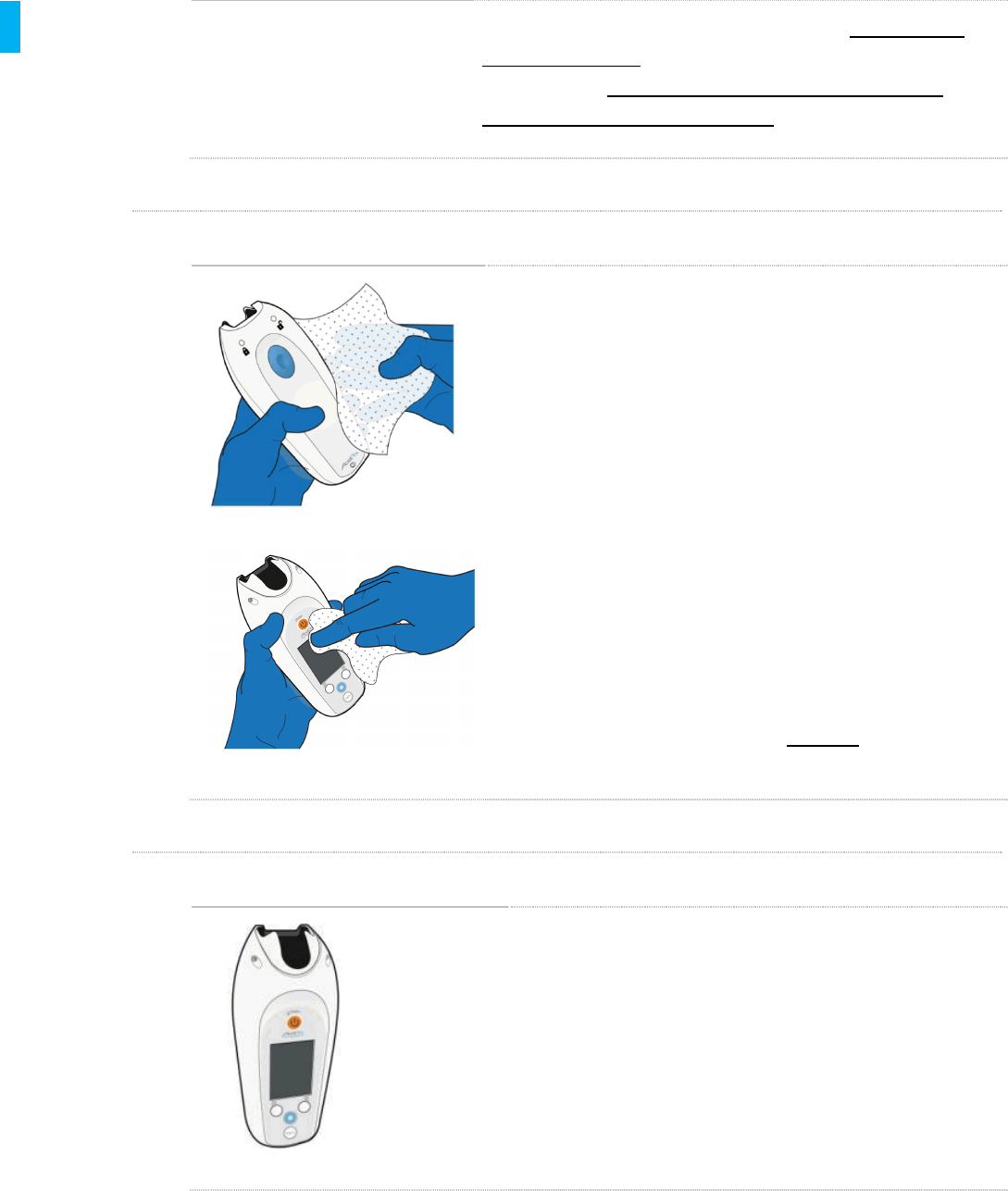
STEP 13 Use the Brush to Disinfect the Seams
WARNING
A soft bristled brush MUST be used on the
seams of the Controller to ensure disinfection
of the seams.
Use a soft bristled brush (e.g., Graham Field Adult Toothbrush,
Cat. No. 3395-1, available from Fisher Scientific, Cat. No.
19027438) to disinfect the seams on the sides of the
Controller.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 105
Instructions for Use –PL-1678 Rev. K
KEEP
WET
5 MINUTES
Keep the surfaces of the Controller wet for a MINIMUM OF
FIVE (5) MINUTES, as recommended by the wipe
manufacturer. The full 5 minute wetting (contact) time is
important to kill resistant pathogens.
STEP 14 (Optional) Disinfect Controller in Two Stages
In order to keep all of the surfaces of the Controller wet for
the minimum 5 minute time, the Controller may be disinfected
in two stages: one with the Dose Button side and left side
seam held generally upward and horizontal, and a second
stage with the Screen side and right side held generally
upward and horizontal. For each stage, the Controller
surfaces, crevices and seams should be wetted for 5 minutes.
The seams and crevices of the Controller (except the
Charging/Data port and Tether port) can withstand thorough
wetting, therefore a Sani-Cloth Plus wipe may be “wrung” in
order to allow liquid disinfectant from the wipe to pool in the
recess and wick into the crevices. A soft-bristled brush
should also be used in the seams and crevices to assure
disinfectant penetrates sufficiently. DO NOT use this method
around the Data/Charging Port or Tether hole.
STEP 15 Air-dry Controller
1. After all Controller surfaces have been kept wetted
for 5 minutes, set the Controller down on a clean
dry surface (previously disinfected with Sani-Cloth
Plus Wipes) and allow to air dry.
2. Controller reprocessing is now complete. After
confirming that the Controller is completely dry,
place the reprocessed Controller into a clean
storage bag.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 106
Instructions for Use –PL-1678 Rev. K
WARNING
Confirm that the Controller is dry before storage. If not completely dry,
condensation within the bag or container may harm internal electronic parts.
17.3. Reprocessing the Controller’s Cleaning Plug
The Cleaning Plug may be reused to reprocess the next Controller, but
should first be cleaned and disinfected, to avoid possible transfer of
contamination from one Controller to the next.
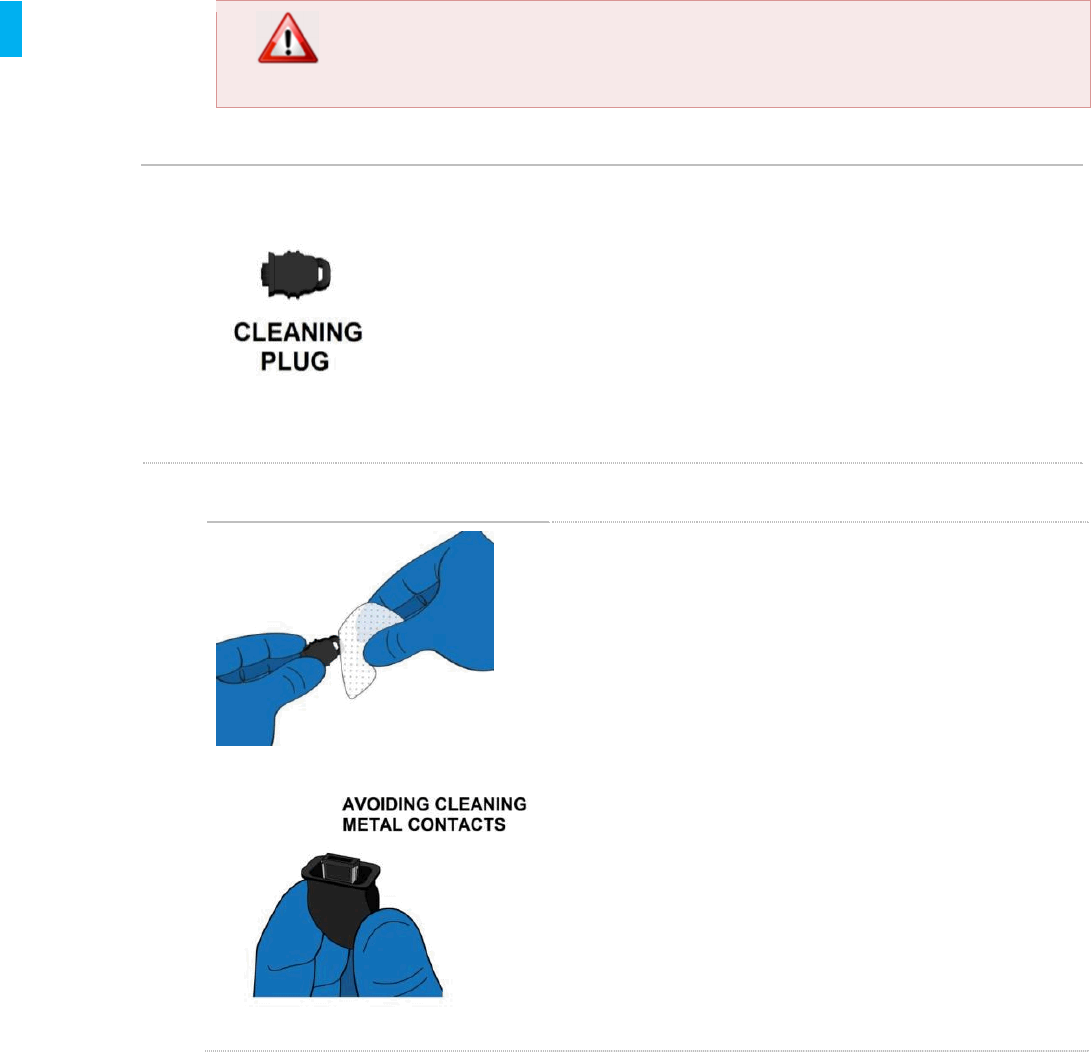
STEP 1 Clean the Cleaning Plug
1. Put on a pair of fresh gloves.
2. Using a fresh Sani-Cloth Plus Wipe, clean off the
outside surface and the inside “lip”, avoiding the
metal contacts inside the Cleaning Plug. Use
additional wipes as necessary to clean the plug
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 107
Instructions for Use –PL-1678 Rev. K
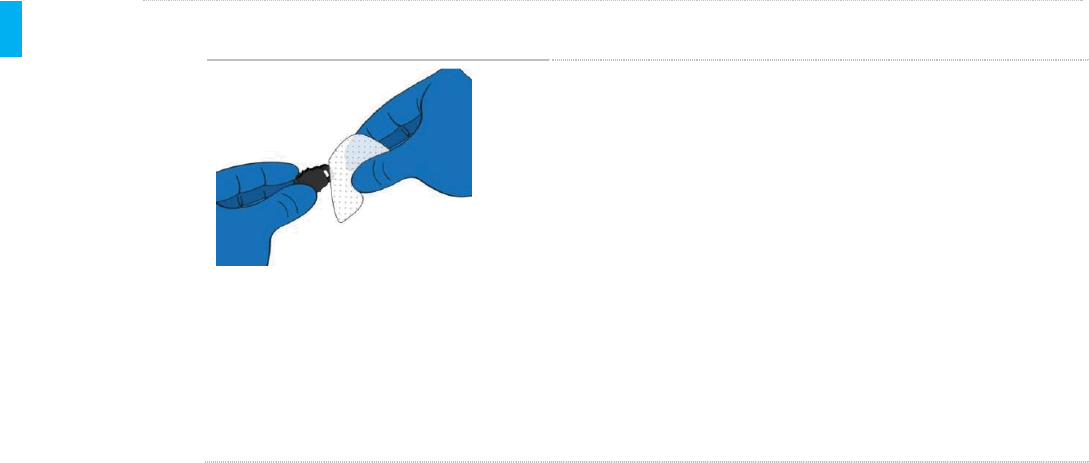
STEP 2 Disinfect the Plug
KEEP WET
5 MINUTES
1. Using a fresh Sani-Cloth Plus Wipe, thoroughly
and vigorously wet the entire outside surface and
inside lip of the Cleaning Plug.
2. Keep the surfaces of the Cleaning Plug wet for a
MINIMUM OF FIVE (5) MINUTES, as
recommended by the wipe manufacturer. The full
5 minute wetting (contact) time is important to kill
resistant pathogens.
3. Allow Cleaning Plug to air dry on a clean dry
surface. After confirming the Cleaning Plug is
completely dry, store in a clean storage bag.
4. Reprocessing of the Cleaning Plug is complete.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 108
Instructions for Use –PL-1678 Rev. K
17.4. Reprocessing the Tether
STEP 1 Clean the Cable
1. Put on a pair of clean gloves.
2. Using a fresh Sani-Cloth Plus Wipe,
thoroughly clean the cable portion of the
Tether. Use as many fresh Sani-Cloth Plus
Wipes as needed to remove all soil from the
cable.
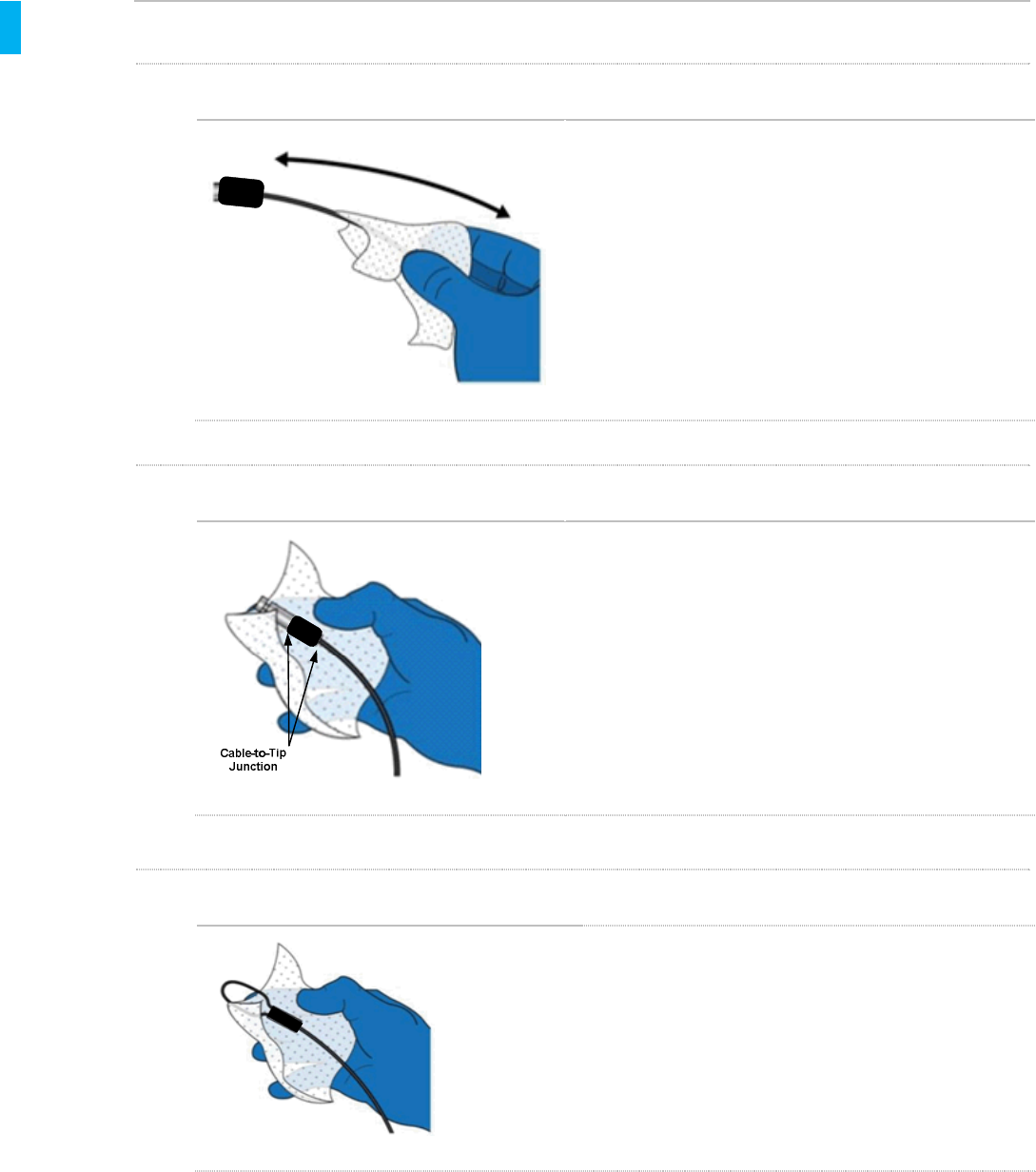
STEP 2 Clean the Tip
Using additional wipes, clean the Tether Tip, the
metal portion shown in the figure at left. Pay special
attention to crevices at the cable-to-tip junction. A
wipe wrapped around a swab, or a swab wetted with
a wipe may be used to thoroughly clean the tip.
STEP 3 Clean the Loop
1. Using a fresh Sani-Cloth Plus Wipe clean
the Tether loop. Remove all visible soil.
2. Now that the Tether has been thoroughly
cleaned, it still needs to be disinfected.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 109
Instructions for Use –PL-1678 Rev. K
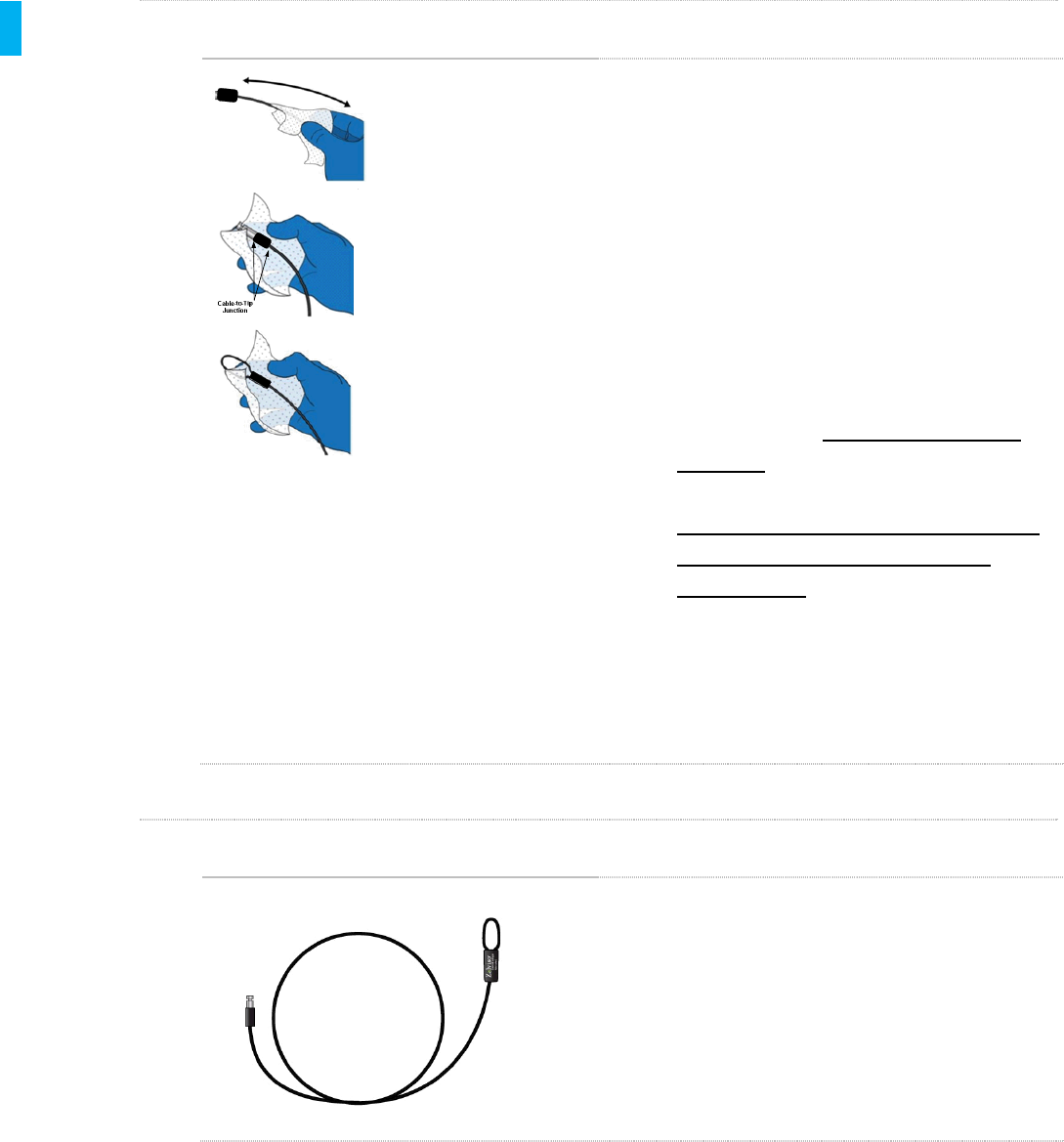
STEP 4 Disinfect the Tether
Wet the Cable,
the Tip, and
the Loop
KEEP WET
5 MINUTES
1. Using a fresh Sani-Cloth Plus Wipe,
thoroughly and vigorously wet the entire
outside surface of the entire Tether,
including the Tether tip, Tether cable and
Tether loop. Use swabs wrapped in
germicidal wipes, or swabs wetted with the
wipes to thoroughly wet the crevices on the
Tether tip, especially the cable-to-tip
junction, and the Tether loop.
2. Allow the surfaces of the entire Tether to
remain wet for a MINIMUM OF FIVE (5)
MINUTES.
3. THE FULL 5 MINUTE WETTING TIME IS
IMPORTANT TO KILL RESISTANT
PATHOGENS.
4. If parts of the Tether dry off before 5
minutes have elapsed, use additional wipes
to keep surfaces of the Tether wetted for
the entire 5 minutes.
STEP 5Air Dry the Tether
1. Set the Tether down on a clean surface
(previously disinfected with Sani-Cloth Plus
Wipes) and allow to air dry. Store the
reprocessed Tether in a clean storage bag.
2. Tether reprocessing is now complete.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 110
Instructions for Use –PL-1678 Rev. K
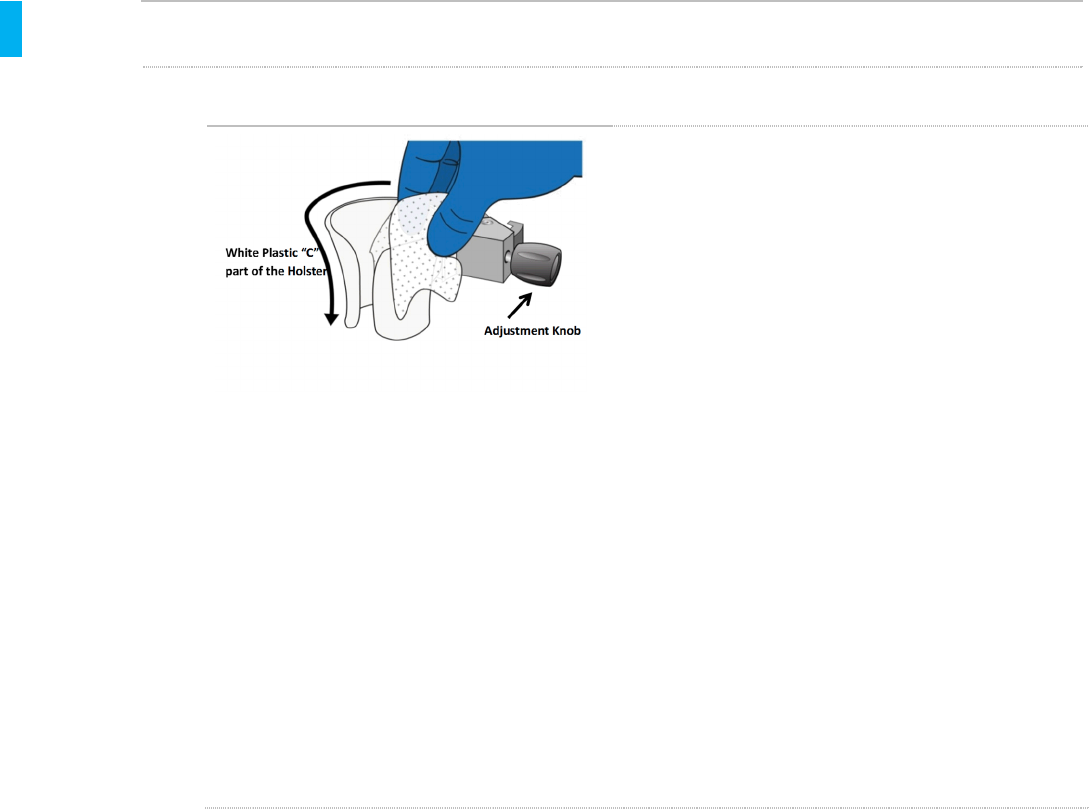
17.5. Reprocessing the Holster
STEP 1 Clean the Holster
1. Put on a pair of clean gloves.
2. Using a fresh Sani-Cloth Plus Wipe,
thoroughly clean the white plastic portion of
the Holster. Use as many fresh Sani-Cloth
Plus Wipes as needed to remove all soil
from this area, both inside and outside the
“C” of the Holster.
3. Use swabs wrapped in germicidal wipes, or
swabs wetted with the wipes to thoroughly
clean the crevices on the “C” of the Holster,
especially at the bottom.
4. Using a fresh Sani-Cloth Plus Wipe,
thoroughly clean the adjustment knob on
the clamp.
5. Now that the Holster has been thoroughly
cleaned, it still needs to be disinfected.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 111
Instructions for Use –PL-1678 Rev. K
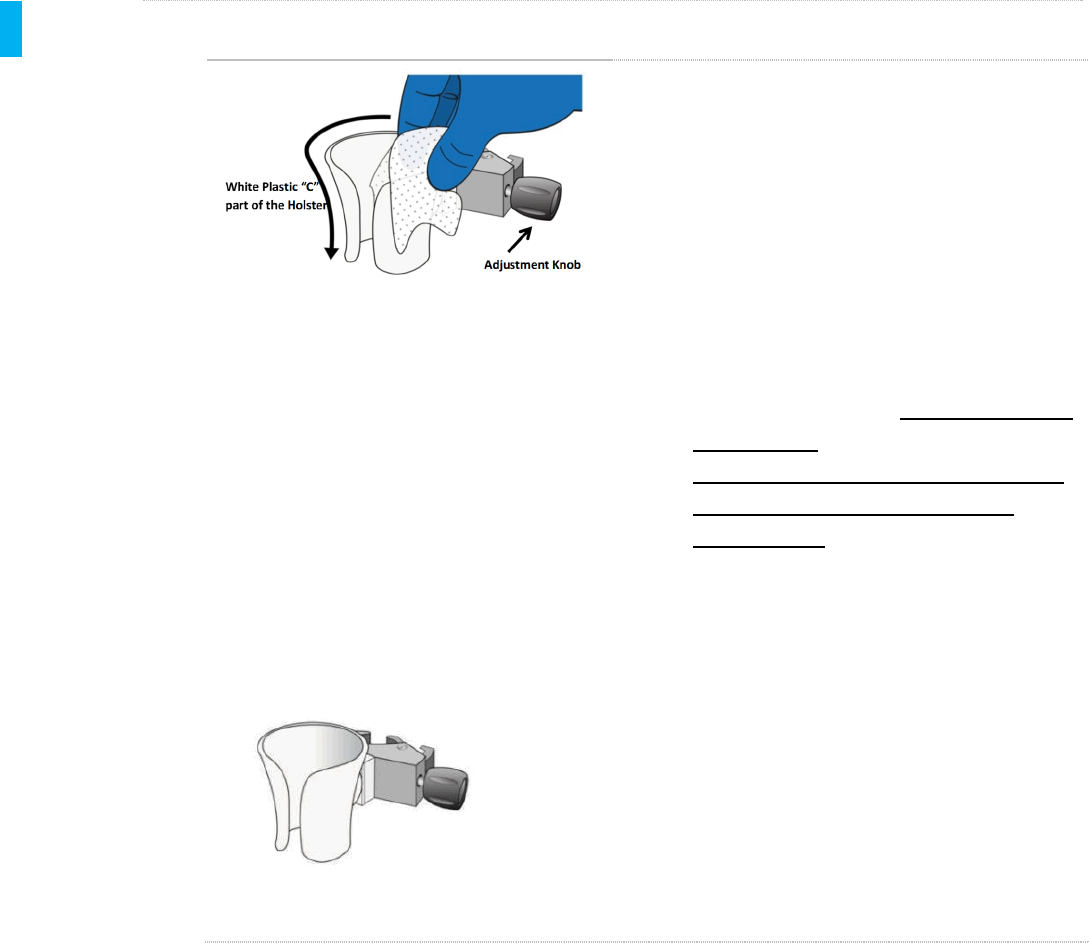
STEP 2 Disinfect the Holster
KEEP WET
5 MINUTES
1. Using a fresh Sani-Cloth Plus Wipe,
thoroughly and vigorously wet all of the
surfaces of the white plastic “C” part of the
Holster and the Adjustment Knob on the
Holster clamp. Use swabs wrapped in
germicidal wipes, or swabs wetted with the
wipes to thoroughly wet crevices on the
surfaces of the white plastic “C” part of the
Holster and the adjustment knob on the
Holster clamp. Keep the surfaces of the
entire Holster wet for a MINIMUM OF FIVE
(5) MINUTES.
2. THE FULL 5 MINUTE WETTING TIME IS
IMPORTANT TO KILL RESISTANT
PATHOGENS.
3. If parts of the Holster dry off before 5
minutes haveelapsed, use additional wipes
to keep all surfaces wetted for the entire 5
minutes.
4. After 5 minutes haveelapsed, set the
Holster down on a clean surface
(previously disinfected with Sani-Cloth Plus
Wipes) and allow to air dry. After
confirming the Holster is completely dry,
store the reprocessed Holster in a clean
storage bag.
5.
Holster reprocessing is now complete.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 112
Instructions for Use –PL-1678 Rev. K
18. Recharging the Controller
WARNING
Use only the Charger specified for the Controller. Use of any other power
supply adapter may damage the Controller or cause personal injury.
WARNING
Do not connect the Charger to the sufentanil sublingual tablet system while it is
being used by a patient. Battery charging is only active when the Controller is
discontinued.
The Controller’s rechargeable battery should be charged for at least 8 hours or until the “Charging
Complete” message appears on the display screen. If the Controller has been used by a patient,
it should always be reprocessed according to instructions in Section 17, Reprocessing
Instructions,before following the charging instructions below.
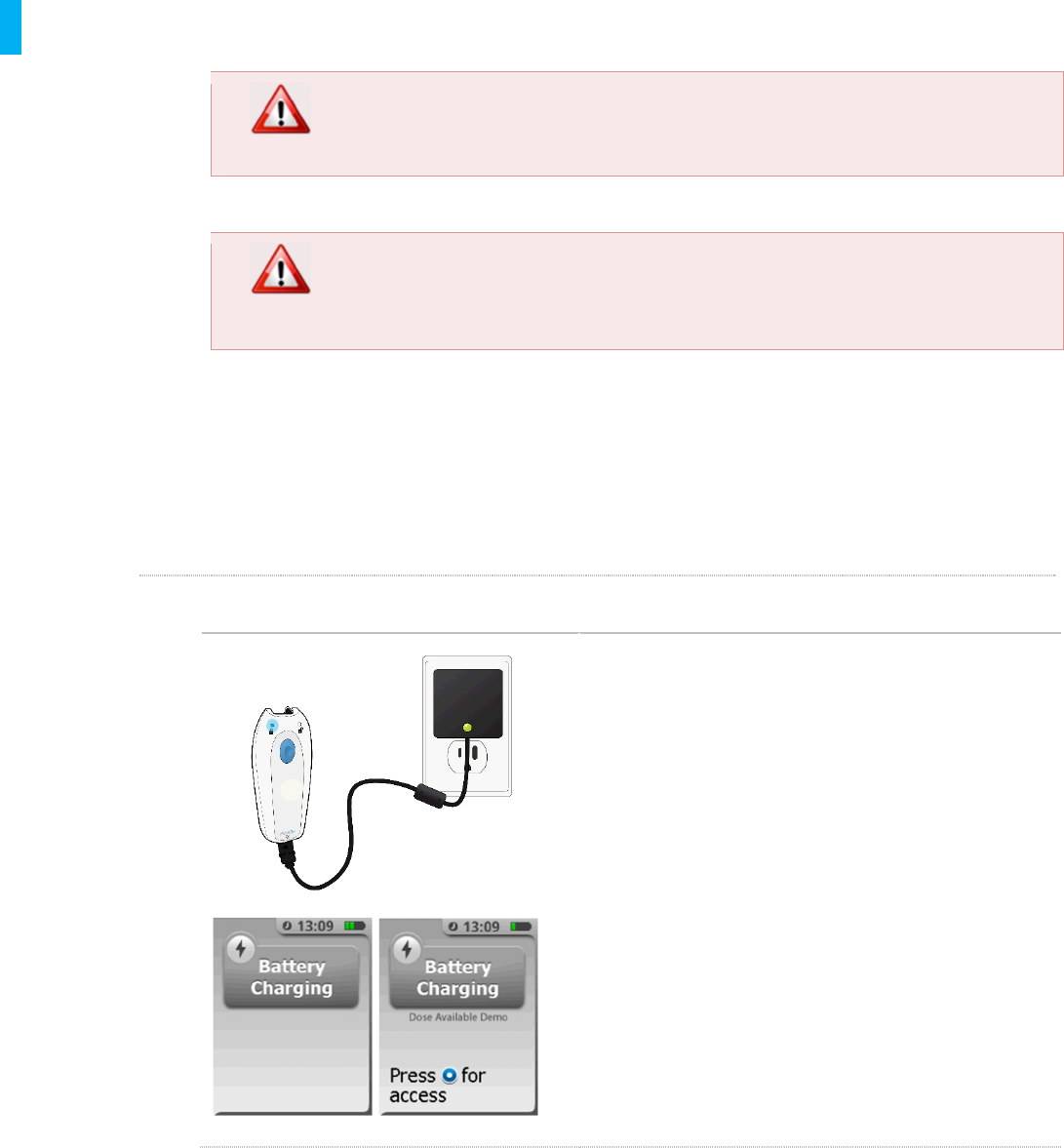
STEP 1 Connect Charger
1. Use the supplied Charger only or damage to
the Controller may occur.
2. Connect the Charger to a 110/120V 60Hz AC
power outlet. The green indicator light on the
Charger should light when charging.
3. Insert the end of the Charger into the
charging port on the Controller being careful
not to insert it into the Tether port. The
Charger has polarization keys and can only
be inserted into the Controller’s charging port
in one orientation. The Charger should insert
and lock into the Controller’s charging port.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 113
Instructions for Use –PL-1678 Rev. K
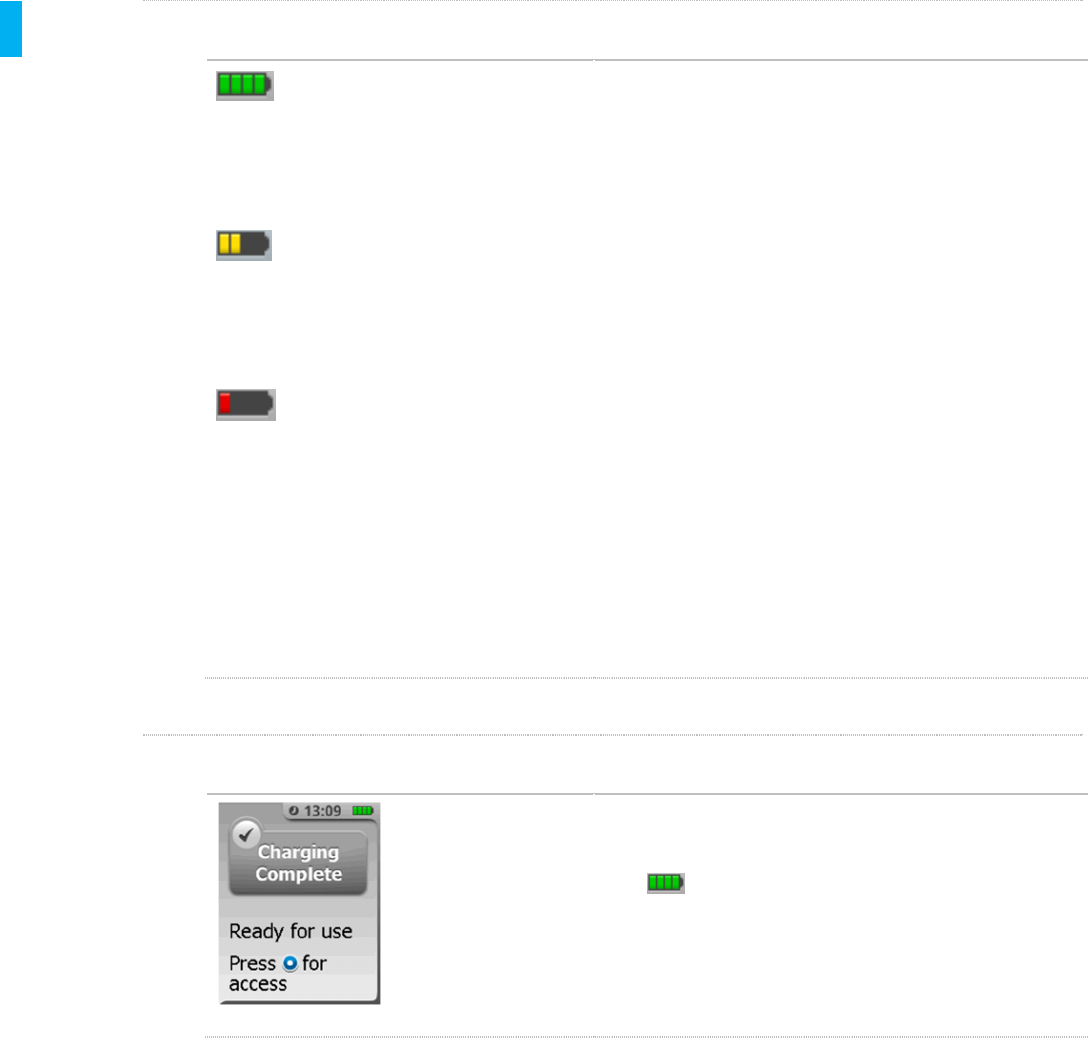
STEP 2 Charge Battery
Fully Charged
Four green bars mean the battery isfully
charged.
Running Low
Two yellow bars means the battery is
running low.
Fatally Low
One red bar means the battery is fatally
low and the Controller should be
charged.
1. While the Controller’s battery is charging, the
display screen will show a “Battery Charging”
message and the battery icon in the upper-
right corner of the screen will animate to
show that it’s charging. On the back of the
Controller the blue No Dose light will turn on
and flash as a redundant indicator that the
Controller is being charged.
2. A battery icon is displayed in the upper-right
corner of the Controller screen and is
composed of 4 bars. The battery icon will
show 4 green bars when it is full and will
display one red bar when it’s fatally low.
3. While charging, the Controller will turn off the
display after 30-seconds of inactivity. Moving
the Controller will turn on the display and
show the charging status.
STEP 3 Confirm Charge Complete
When the battery is done charging the screen will
display a message “Charging Complete” and 4 green
bars will display in the upper-right corner of the
screen to confirm that the battery is fully charged.
The blue LED on the back of the unit will stop flashing
and the green LED will be illuminated.
Printed on: ; Printed by: .