Acelrx Pharmaceuticals ARX2006 Zalviso hand-held patient controlled analgesia (PCA) system User Manual Manual 2 4
AcelRx Pharmaceuticals Inc. Zalviso hand-held patient controlled analgesia (PCA) system Manual 2 4
Contents
- 1. Manual 1/4
- 2. Manual 2/4
- 3. Manual 3/4
- 4. Manual 4/4
Manual 2/4
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 36
Instructions for Use –PL-1678 Rev. K
If you confirm the “Error -System Cannot Be Used”, follow instructions on the
next screen to discontinue the System (see Discontinuation of Therapy, Section
14)and start over with a new Controller, Dispenser and Cartridge.
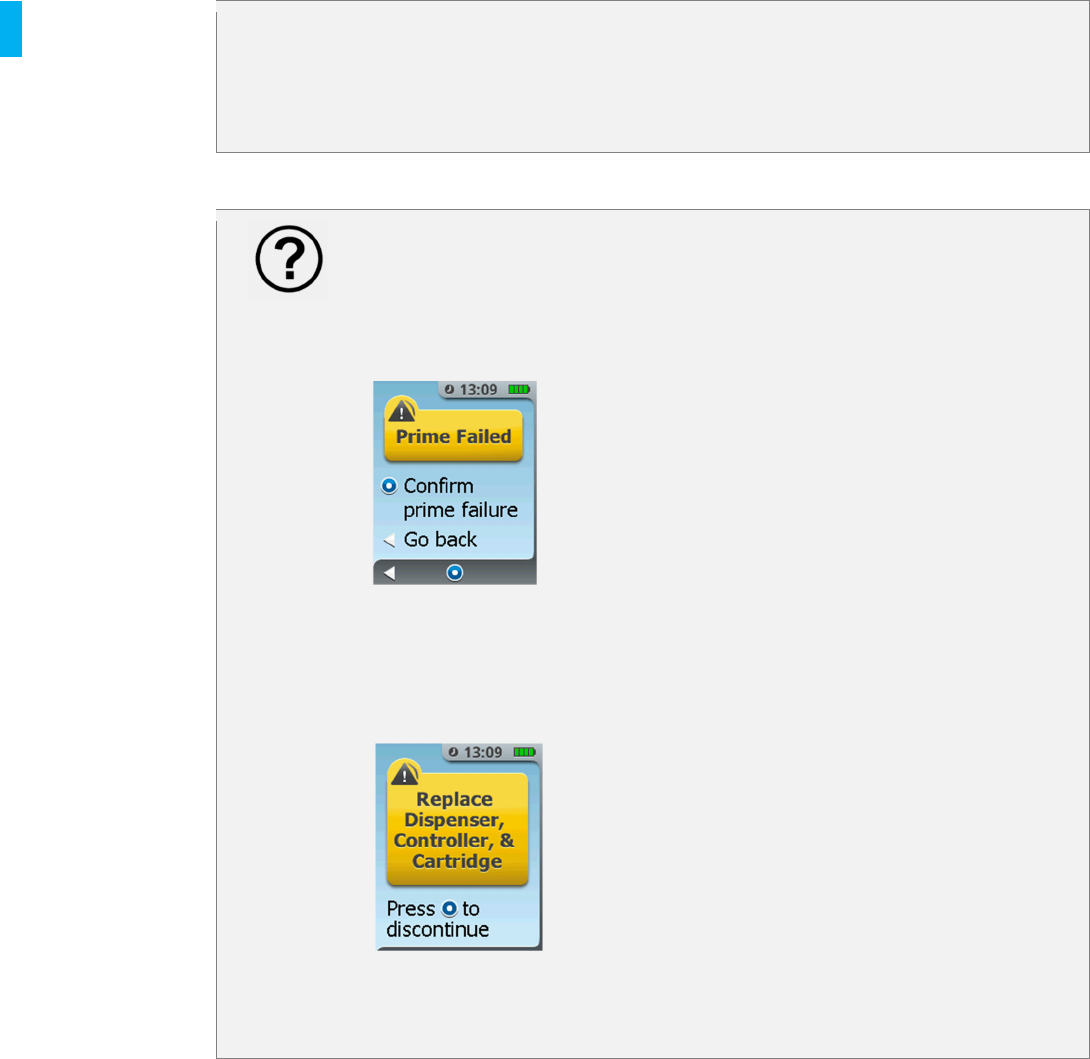
Did you receive the Prime Failed error message?
If any error occurs at this point, follow the on-screen instructions and
discontinue the System (see Discontinuation of Therapy, Section 14)and start
over with a new Controller, Dispenser and Cartridge.
If you confirm the prime failure, follow instructions on the next screen to
discontinue the System and start over with a new Controller, Dispenser and
Cartridge.
Refer to Section 16, Notifications, Alerts, Alarms and Errors, for further
guidance on these notifications.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 37
Instructions for Use –PL-1678 Rev. K
STEP 11 Re-Cap The System
Place the Cap back on the Dispenser before
continuing.
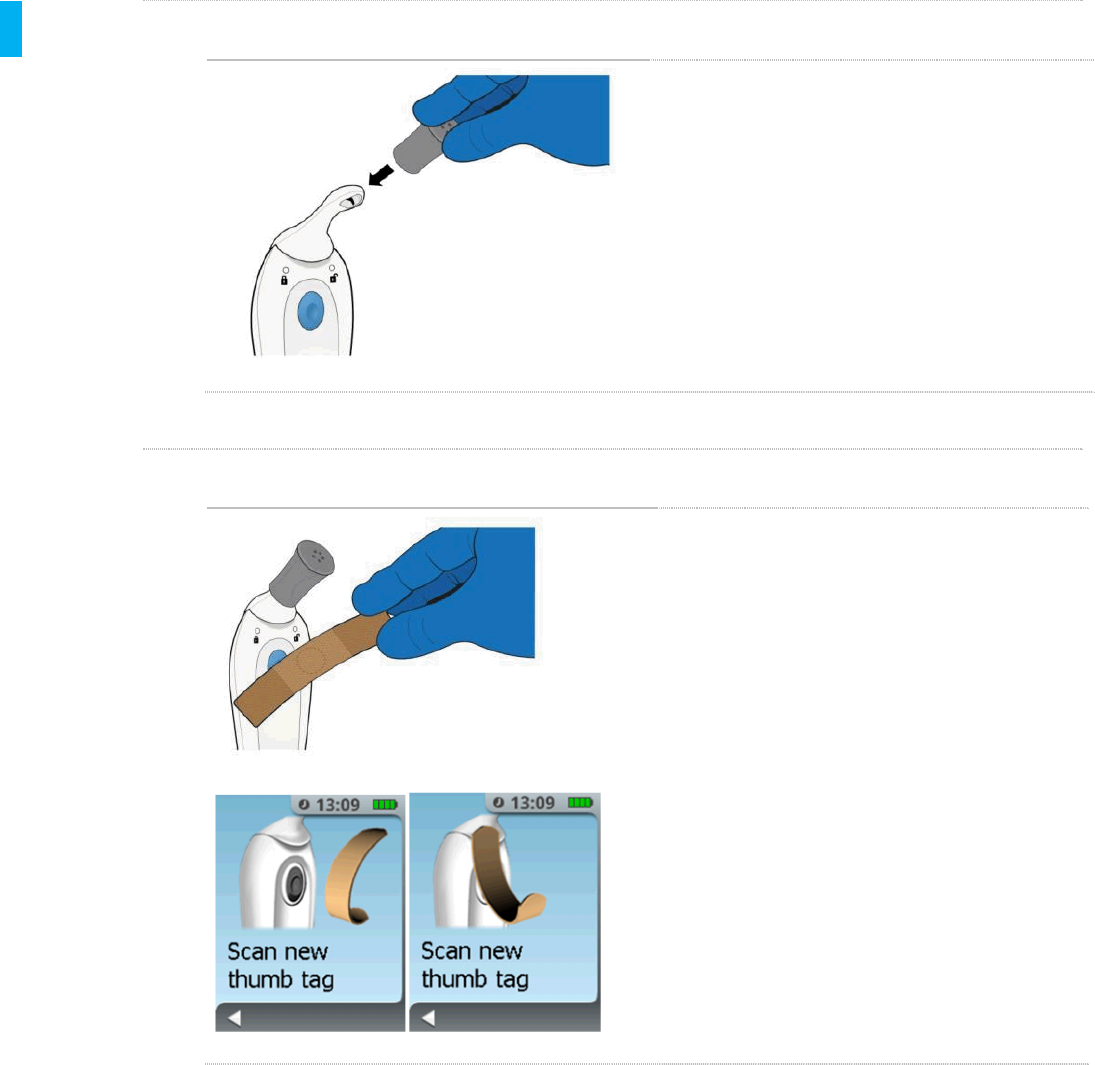
STEP 12 Scan Patient ID Thumb Tag
Scan the new Patient ID Thumb Tag by
touching it to Blue Dose Button on the back
side of the Controller.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 38
Instructions for Use –PL-1678 Rev. K
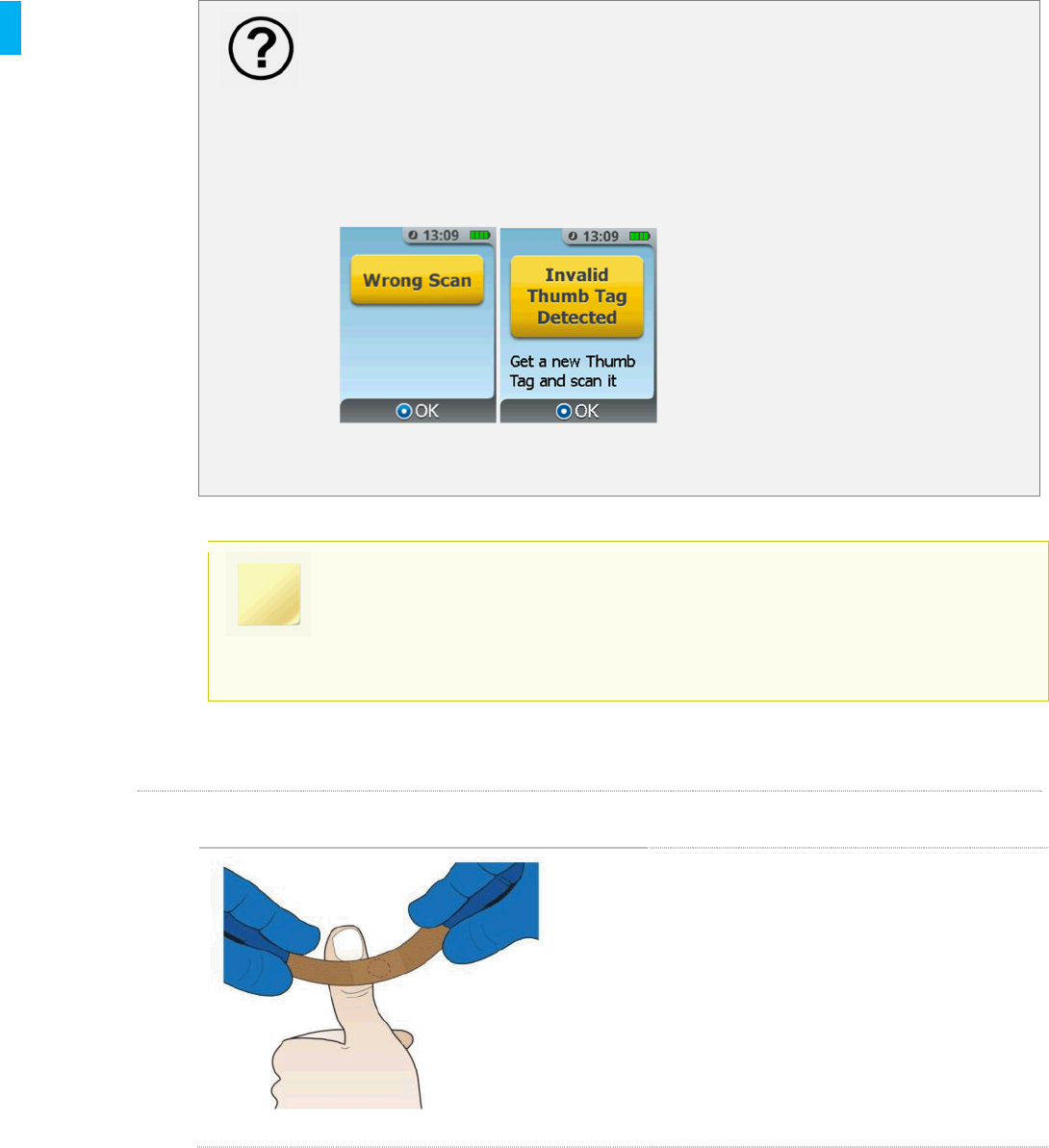
Did you receive the Wrong Scan or Invalid Thumb Tag Detected notification
message?
If one of these notifications occurs at this point, the Patient ID Thumb Tag
scanned is defective or cannot be used by the System. Obtain a new Patient ID
Thumb Tag then press the Enter/Select button and follow the on-screen
instructions to scan the new Patient ID Thumb Tag.
Refer to Section 16, Notifications, Alerts, Alarms and Errors, for further guidance
on these notifications.
NOTE
When the Patient ID Thumb Tag is successfully scanned, you will hear an audible
confirmation tone, and the System will transition to the next screen.
STEP 13 Attach Patient ID Thumb Tag
In preparation for the application of the
Patient ID Thumb Tag, you may clean the
thumb and nail with alcohol wipes and allow
to dry.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 39
Instructions for Use –PL-1678 Rev. K
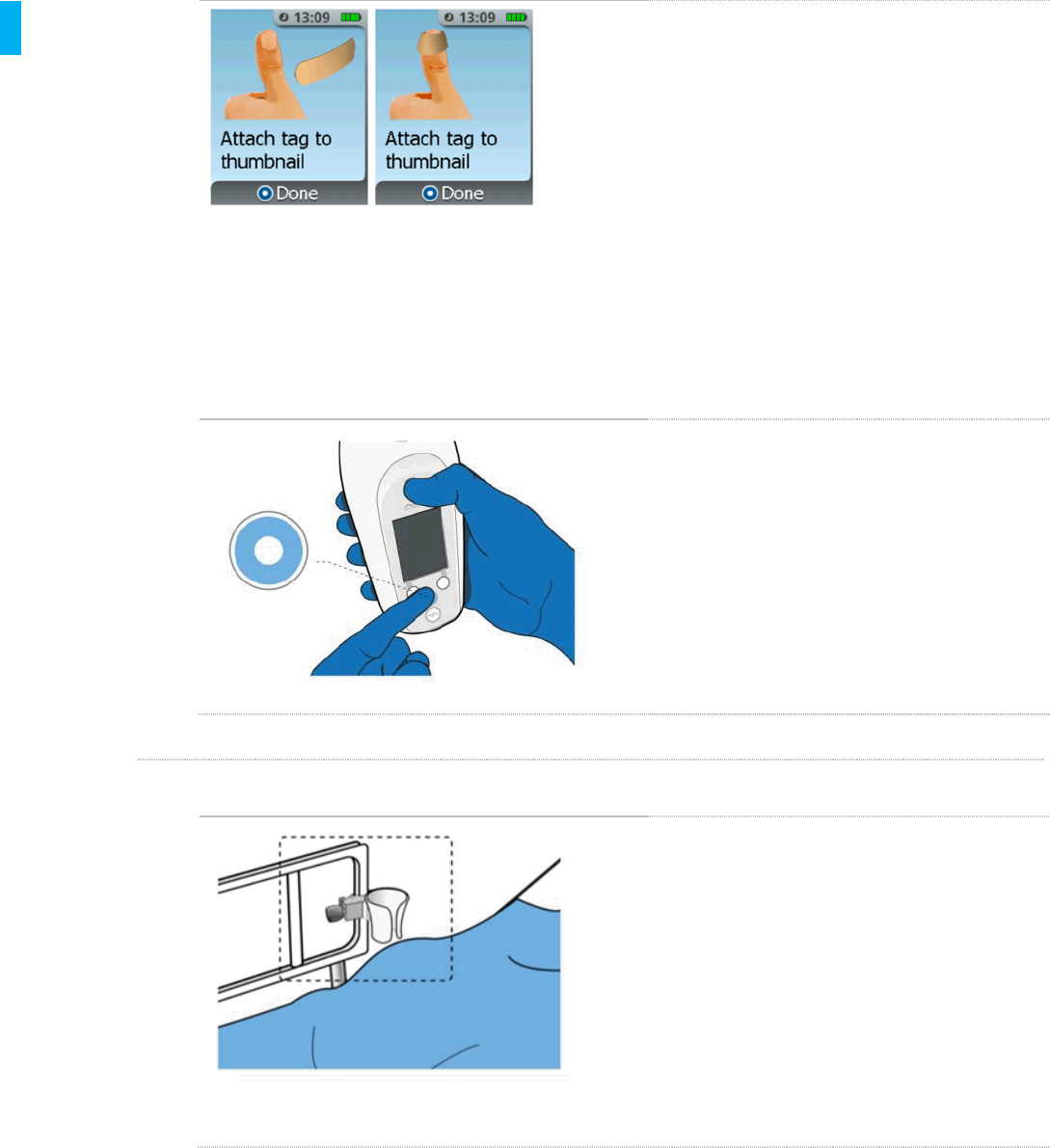
Attach the Patient ID Thumb Tag to the
patient’s thumb. Make sure the center of the
Patient ID Thumb Tag is over the patient’s
thumbnail.
Wrap the Thumb Tag around the thumb
making sure both ends of the Thumb Tag
are adhered to the thumb and not to the
Patient ID Thumb Tag itself.
Confirm the Patient ID Thumb Tag is on the
patient by pressing the Enter/Select
Button on the front of the Controller.
If the Patient ID Thumb Tag is dropped or is
damaged while applying it onto the patient’s
thumb, discard it and continue to complete
the setup of the System for the patient.
After setup is complete, obtain a new Patient
ID Thumb Tag then follow the instructions in
Section 8, Replacing the Patient ID Thumb
Tag.
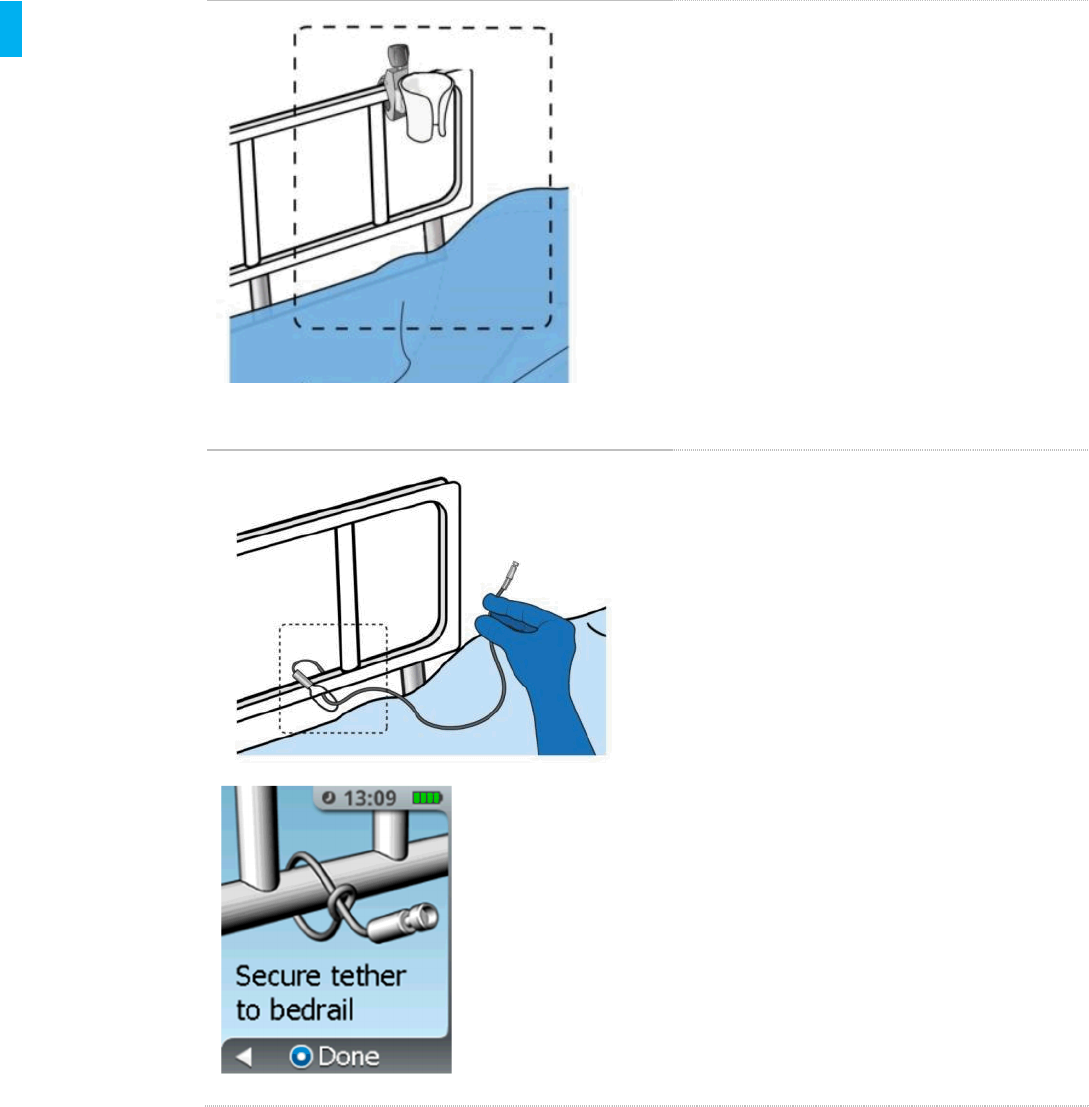
STEP 14 Attach Holster
Example Holster Mounting Position 1
Attach the System Holster with its clamp to
the patient’s bedrail (recommended) or any
other secure object near the patient such
that the System is easily accessible to the
patient.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 40
Instructions for Use –PL-1678 Rev. K
Example Holster Mounting Position 2
Attach the Security Tether securely to the
patient’s bedrail.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 41
Instructions for Use –PL-1678 Rev. K
WARNING
DO NOT attempt to add an extension to the supplied Tether as this may result
in an unsecured System or may present a strangulation hazard. Additionally,
proper use of the Tether requires passing it through the end loop as shown
above. Do not attempt to tie the Tether or use the end loop as a means to
“loop” it over an object; this may result in an unsecure System.
DO NOT attach the Tether to the patient.
Ensure that the Tether does not get tangled with any equipment lines that may
be near the patient, since this could interfere with patient access to therapy.
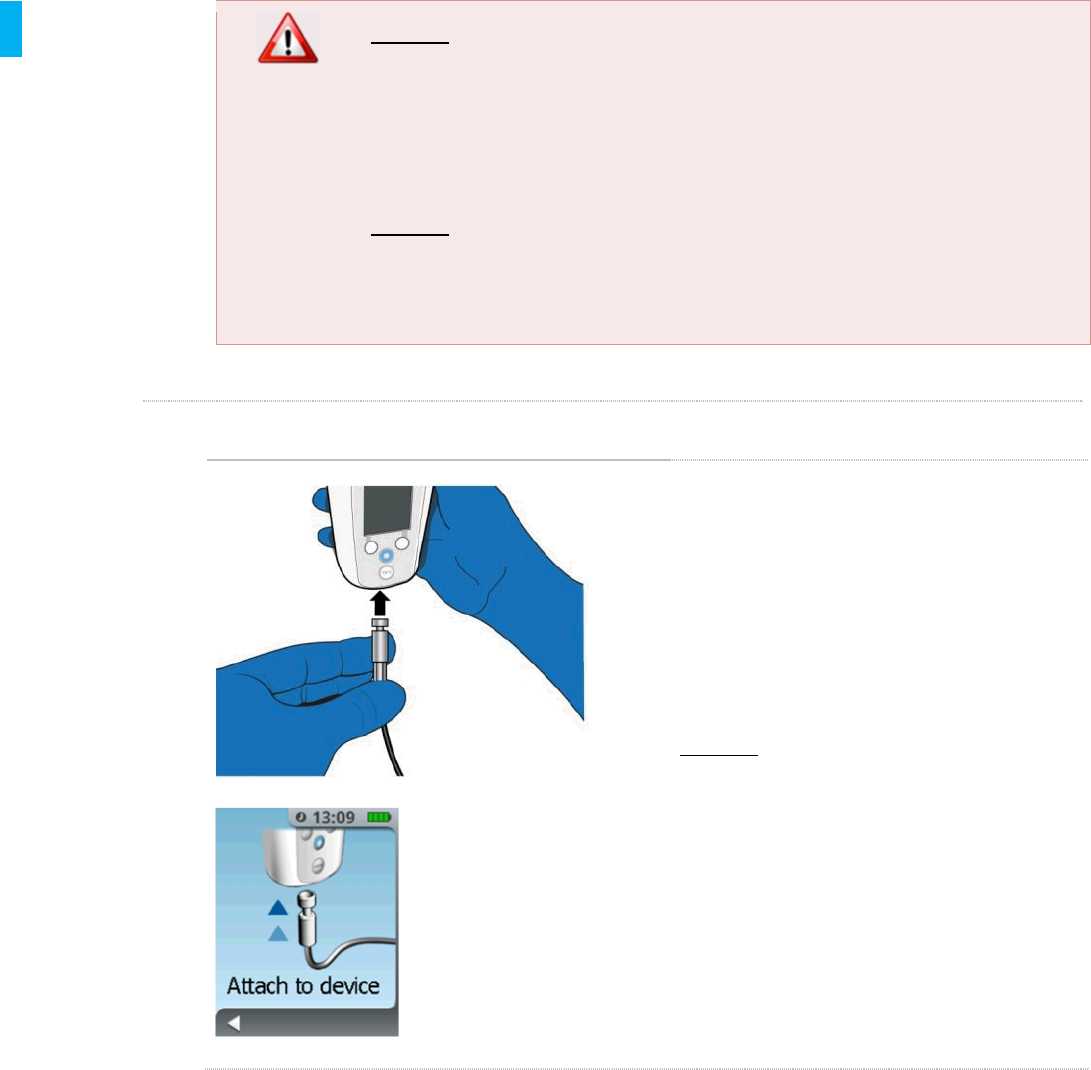
STEP 15 Attach Tether
Attach the other end of the Tether to the
System as shown on the screen; push the
end of the Tether into the Controller until it
stops (a magnet will assist with pulling the
Tether into the System). The System will
sense the Tether and automatically move to
the next sequence in the setup.
DO NOT place anything other than the
Tether into the Tether port.
Printed on: ; Printed by: .

AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 42
Instructions for Use –PL-1678 Rev. K
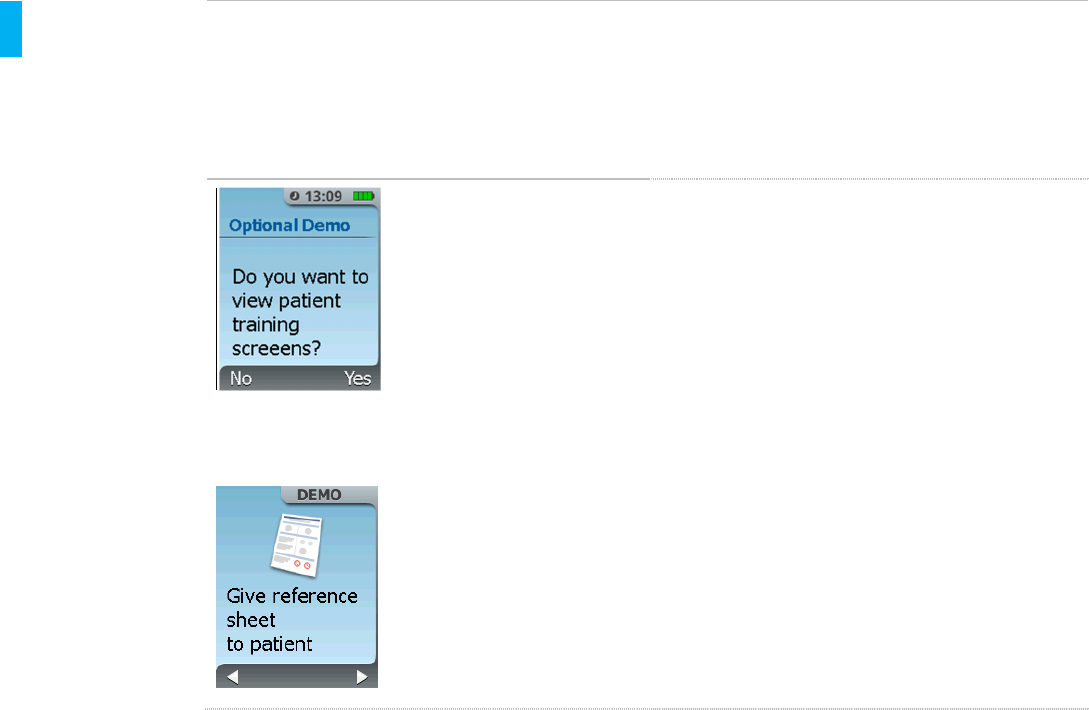
STEP 16 Proceed to Patient Training Screens
After the initial setup,the System will ask
if you want to view the Patient Training
Screens. These are a brief set of
screens that provide cues to the
Healthcare Professional to train the
patient on certain System features.
It is recommended that the Healthcare
Professional always educate and train
patients on the content of these screens
before leaving them with the System to
use on their own. These screens are
accessible anytime during patient use as
well,by using the AAC to access the
menu.
NOTE: No tabletswill be dispensed
during the Patient Training Screens
demonstration.
There will be a flashing DEMO indicator
in the top right hand corner of the screen
throughout the use of the screens to
indicate that the System is not in Patient
mode.
The topics covered in these Training Screens include:
Place the Cap on the System and store the System in the Holster during use
How to identify when a dose is available
Removing the protective Cap before dosing
Holding the System upright during dosing
Placing the Dispenser tip under their tongue
An interactive demonstration of the green Dose Available and blue No Dose Available
indicator lights. It also demonstrates the flashing green light indicator within the Blue
Dose Button when the patient places the thumb with the Patient ID Thumb Tag near it,as
well as the tones that the System provides as feedback to the patient if dosing was
successful or not.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 43
Instructions for Use –PL-1678 Rev. K
Not chewing or swallowing the tablet. Not eating or drinking for 10 minutes after dosing.
Minimize talking for 10 minutes.
How to identify when a dose is not available
Call the nurse if the System is continuously beeping and flashing during use
Provide the patient with the Patient Reference Sheet to supplement patient training
Select YES (Right Button) to continue onto
the next screen to review the Patient Training
Screens.
Select NO (Left Button) to skip these screens
and continue to the next screen which instructs
to provide the patient with the Patient
Reference Sheet (Refer to Attachment 1).
If NO is selected, this screen will appear.
Educate the patient to refer to the Patient
Reference Sheet for basic information on how
to use the System.
Refer to Section 7, Patient Use for further
instructions on patient use.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 44
Instructions for Use –PL-1678 Rev. K
6. Patient Training
Patient Training Screens
The following screens are cues for the Healthcare Professional to train the patient on proper
System use. The screens are not intended to be shown to the patient; rather they are prompts to
the Healthcare Professional to demonstrate System features and train the patient. Some of the
screens enable lights or tone demonstrations, as described below.
NOTE
No tablets will be dispensed during the Patient Training Screens demonstration.
There will be a flashing DEMO indicator in the top right hand corner of the screen
throughout the use of the screens to indicate that the System is not in Patient
mode.
NOTE
In demonstration mode, if the System is left inactive for more than 30 seconds, it
will go into sleep mode. To return to demo mode, use the AAC to access MENU
and return to Demo Mode.
Use the Left and Right Buttons to navigate between the
screens. You can repeat any Patient Training Screen as
needed by pressing the Left Button. The Patient Training
Screens are also accessible through the System Menu any
time after the initial setup as needed.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 45
Instructions for Use –PL-1678 Rev. K
Patient Training Screens
STEP 1 Cap and Store System
Remind the patient to place the Cap on the System after dosing and
store the System in the Holster when not in use. When the patient is
ready to use the System, remove the System from the Holster.
NOTE
It is important to remind the patient to store the System in the Holster when not in
use to minimize the chance of inadvertently dispensing a tablet by accidently
pressing the Dose Button. If this occurs, the patient should call the Healthcare
Professional to ensure the loose tablet is found and disposed of properly.
STEP 2Check Dose Availability
Train the patient that the green Dose Available Light will
illuminate when a dose is available and stay illuminated until a dose
is taken.
Show the patient the green light, located above the unlock icon.
Inform the patient that this light indicates the System is available for
the patient to take a dose.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 46
Instructions for Use –PL-1678 Rev. K
STEP 3 Thumb Tag Required to Dose
Train the patient that only the thumb with the Patient ID Thumb Tag
can be used to press on the Blue Dose Button to receive a dose
of medication.
STEP 4Remove Cap Before Dosing
The patient should be reminded that they must remove the Cap
before dosing, otherwise the tablet will be dispensed into the Cap
and the patient will not receive their dose. Should this occur
accidently, the patient should call the Healthcare Professional to
retrieve and properly dispose of the loose tablet in the Cap. The
patient should request a new Cap if it is dropped or contaminated.
STEP 5Keep System Upright
The patient should be instructed to KEEP THE SYSTEM UPRIGHT
when dosing.
Note: System will still permit dosing if it not perfectly upright.
However, the System will not permit dosing in extreme orientations
(for example inverted), as the tablet may not be delivered to patient
.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 47
Instructions for Use –PL-1678 Rev. K
STEP 6Place Dispenser TipUnder Tongue
The patient should be reminded to first place the Dispenser Tip
UNDER their tongue and THEN press the Blue Dose Button
with their thumb. Do not apply downward pressure on the Dispenser
tip when dosing.
STEP 7 Do Not Touch Dose Button Until the Dispenser tip is under the
Tongue
The patient should be reminded not to touch or press the Blue
Dose Button with their thumb until the dispenser tip is under the
tongue.
STEP 8 Do Not Pull Down When Dosing
The patient should be reminded not to pull down on the dispenser tip
when in the mouth when dosing.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 48
Instructions for Use –PL-1678 Rev. K
STEP 9Simulate Dosing
This demonstration can be repeated multiple times as needed, the System will not dispense any
tablets during this demonstration.
The patient should be reminded not to press the button until
the Dispenser tip is under their tongue, and they should only
remove it after they hear the dose confirmation tone, which will
be heard after pressing the Blue Dose Button, and feel the
motor vibration stop.
Have the patient press the Blue Dose Button to simulate
receiving a dose so the patient can hear the tone that the
System produces to confirm the dose was dispensed. Have
the patient place the System in their mouth and under their
tongue and press the Blue Dose Button to get a feel for
dosing. Tell the patient that the System will not dispense any
tablets during this demonstration.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 49
Instructions for Use –PL-1678 Rev. K
STEP 10 Do Not Chew or Swallow the Tablet, Do Not Eat or Drink for 10 min.
Educate the patient that the tablet should not be chewed or
swallowed, and to not eat or drink for 10 minutes after dosing.
Minimize talking for 10 minutes.
STEP 11 Call a nurse if you drop or find a tablet
The patient should be reminded to call the nurse if they drop a tablet
when dosing or if they find a tablet.
STEP 12 Simulate Dose Lockout
Train the patient on what the System will do when in lockout and
they cannot dispense a dose. The No Dose Available Light
will be illuminated (blue) above the lock icon on the left side of the
System. Train the patient that while the Dose Button can be
pressed, a dose will not be dispensed.
This demonstration can be repeated multiple times as needed.
Educate the patient that the blue light indicates when a dose is not
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 50
Instructions for Use –PL-1678 Rev. K
available. Have the patient press the Blue Dose Button on the
back of the Controller to hear the “no dose” tone that the System
produces to communicate that the patient cannot receive a dose and
the System is in lockout.
STEP 13 Call Nurse
Remind the patient to call the nurse when the System is
continuously beeping and flashing between doses. The System will
continuously beep and flash indicators when this screen is shown.
STEP 14 Patient Reference Sheet
Provide the patient with the Patient Reference Sheet to supplement
patient training. Educate the patient to refer to the Patient
Reference Sheet for basic information on how to use the System.
Please refer to Attachment 1.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 51
Instructions for Use –PL-1678 Rev. K
STEP 15 Confirm Patient Training Complete
After completion of the demonstration with the patient, the System
will confirm that the patient training is complete on the screen with a
message Training Complete –Press OK to continue.
Press the Enter/Select Button.
STEP 16 Confirm Setup
The System will confirm that the setup is complete on the screen
with a message System Ready -Press OK and give to
patient. Press the Enter/Select Button.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 52
Instructions for Use –PL-1678 Rev. K
STEP 17 Dose Available
You will then see a green screen with the message Dose
Available, with the drug name, dosage strength and number of
tabletsremaining. Hand the System to the patient and guide them
through the process of taking their first dose. You should watch the
patient take their first dose to ensure they understand proper System
use.
STEP 18 Provide Additional Verbal Instructions to the Patient
1. Use the System as needed for pain relief.
2. The System is only for the patient to use, not family or friends.
3. The System screen information is for the Healthcare Professional and should not be used
by the patient to determine when to dose.
4. In the event that the System appears to be damaged, the patient should notify the
Healthcare Professional. The Healthcare Professional should replace the damaged
System with a new System as required.
5. The System should not be submerged in liquid, taken into shower, or placed in sink. For
instructions on cleaning the System during patient use, see Section 7, Patient Use.
WARNING
The patient should only dose with a single tablet at the time it is dispensed by
the System. The patient should never dispense tablets into their hands and
take multiple tablets at a later time as this may cause serious injury or death.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 53
Instructions for Use –PL-1678 Rev. K
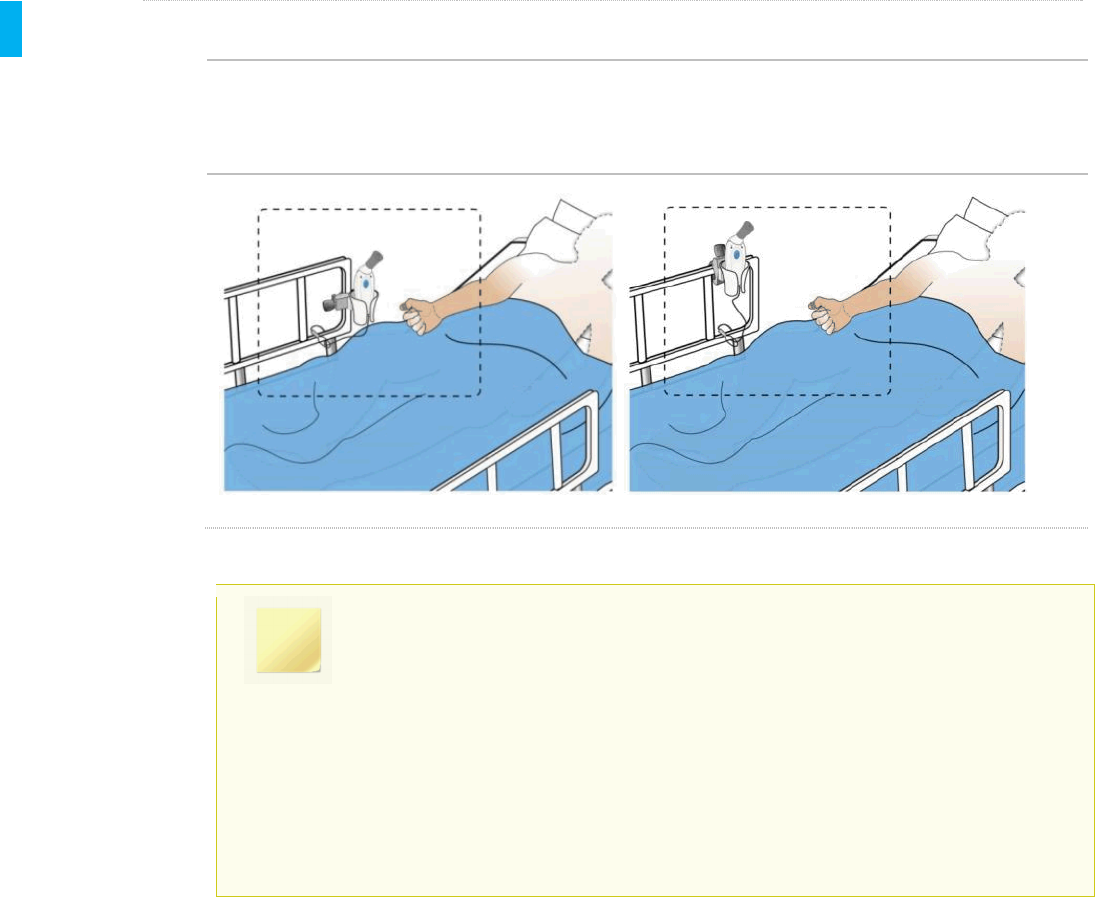
STEP 19 Store System
Before leaving the patient’s room, make sure the System is stored back in the Holster within easy
reach for the patient. The System should always be stored in the Holster when not in use by the
patient or the Healthcare Professional.
NOTE
Discontinuation
Once the patient is finished using the System and has completed their therapy,
you will need to Discontinue the System and handle used components
according to Section 14, Discontinuation of Therapy and Disposition of Used
Components.
System Messages
Refer to Section 7, Patient Use, for a review of tasks and System messages to
be dealt with during the 72-hour use period (or Patient Use).
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 54
Instructions for Use –PL-1678 Rev. K
7. Patient Use
While the patient is using the System the following situations and screens may be encountered:
7.1. How to Take aDose (Dose Available)
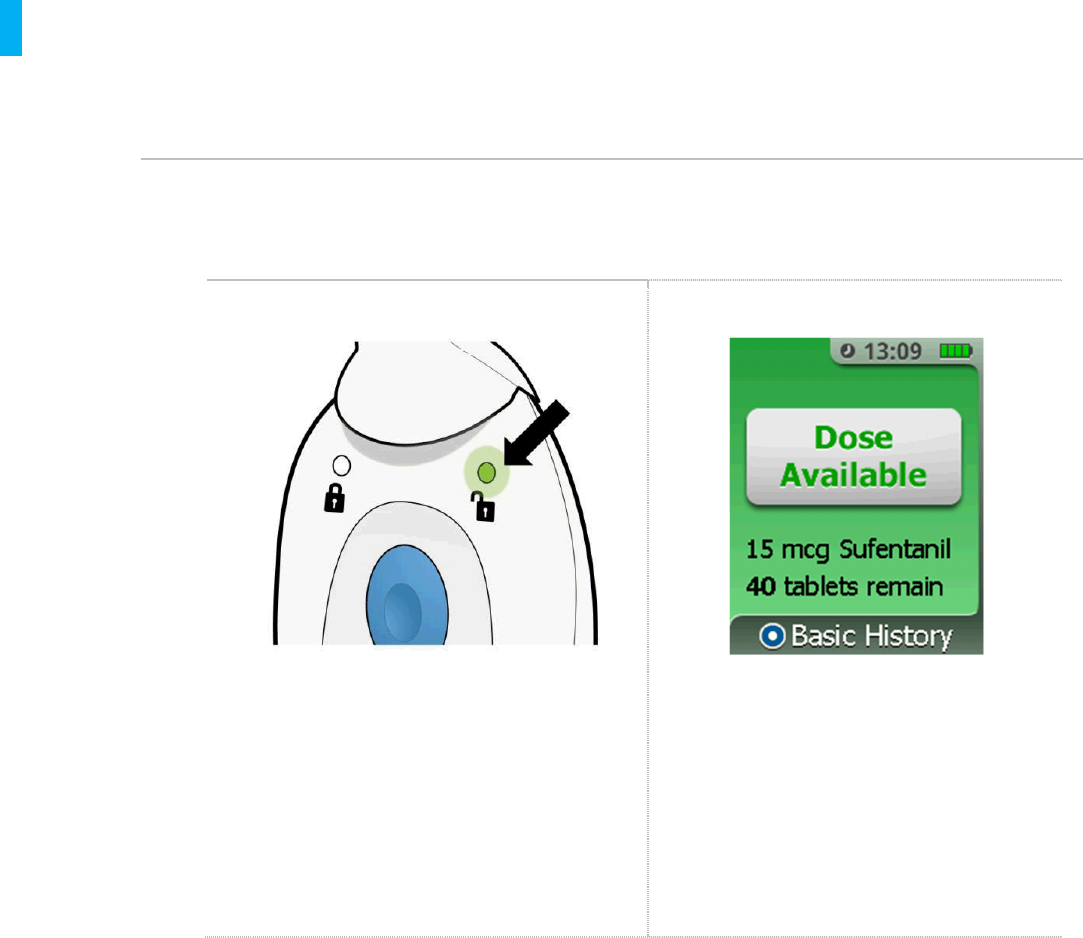
The System has two ways to communicate that a dose is available:
Dose Available Light Dose Available Display
A green light will be visible on the back side of
the Controller (see example above).
If the Enter/Select Button is pressed to
wake up the screen, the front side of the
Controller will display a green screen with
the message Dose Available and list the
drug name, dosage strength and number of
tabletsremaining. In the example above,
the drug is sufentanil, dosage strength is 15
mcg and there are 40 tabletsremaining.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 55
Instructions for Use –PL-1678 Rev. K
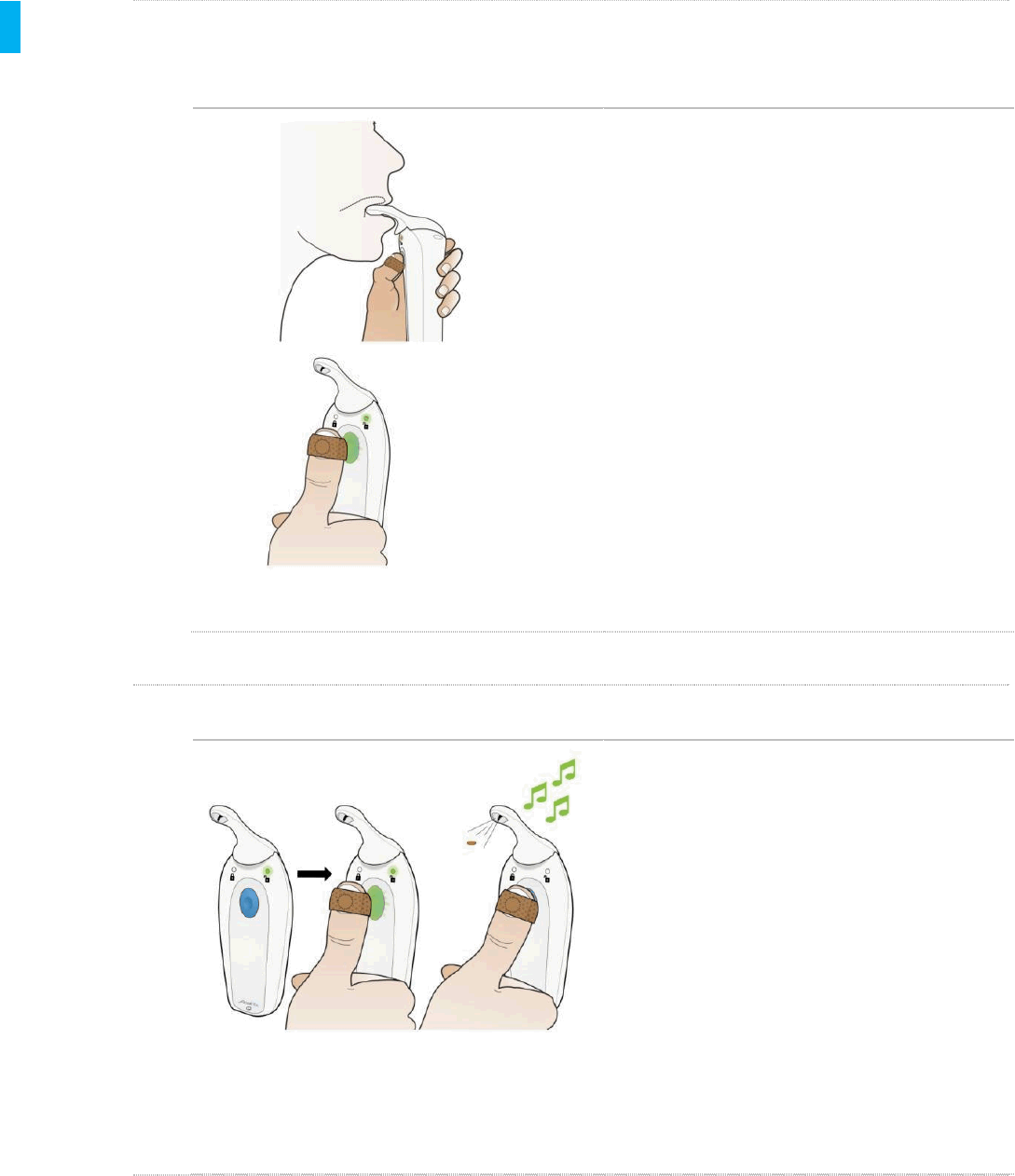
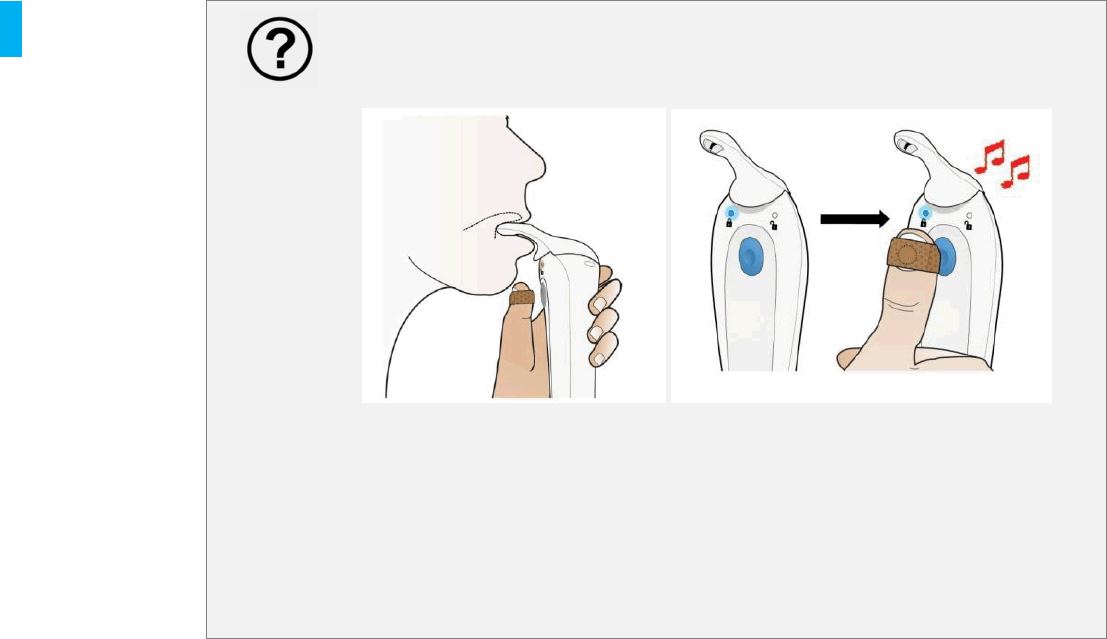
STEP 1 Position Device
When the patient wants to take a dose they should:
Remove the gray Cap.
Hold the System with the same hand as the
Patient ID Thumb Tag.
First place the Dispenser Tip UNDER your
tongue.
The patient should then place their thumb (with
Patient ID Thumb Tag) on the Blue Dose
Button.The Blue Dose Button will flash
green, indicating that the System has detected
the valid Patient ID Thumb Tag and is ready to
dose when the Patient presses the Blue
Dose Button with the thumb..
STEP 2 Dispense Tablet
Press the Blue Dose Button with your
thumb (with Patient ID Thumb Tag).to
dispense the tablet under their tongue.
Do not apply downward pressure on the
Dispenser tip when dosing.
The patient will feel the motor vibration
start.
The System will dispense the tablet.
The motor vibration will stop and an
audible tone will communicate that the
tablet was successfully dispensed.
Once the audible tone is heard, the
patient can remove the System from
their mouth.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 56
Instructions for Use –PL-1678 Rev. K
NOTE
Tablet
The patient should not chew or swallow the tablet and should not eat or drink for
10 minutes. The patient should minimize talking for 10 minutes.
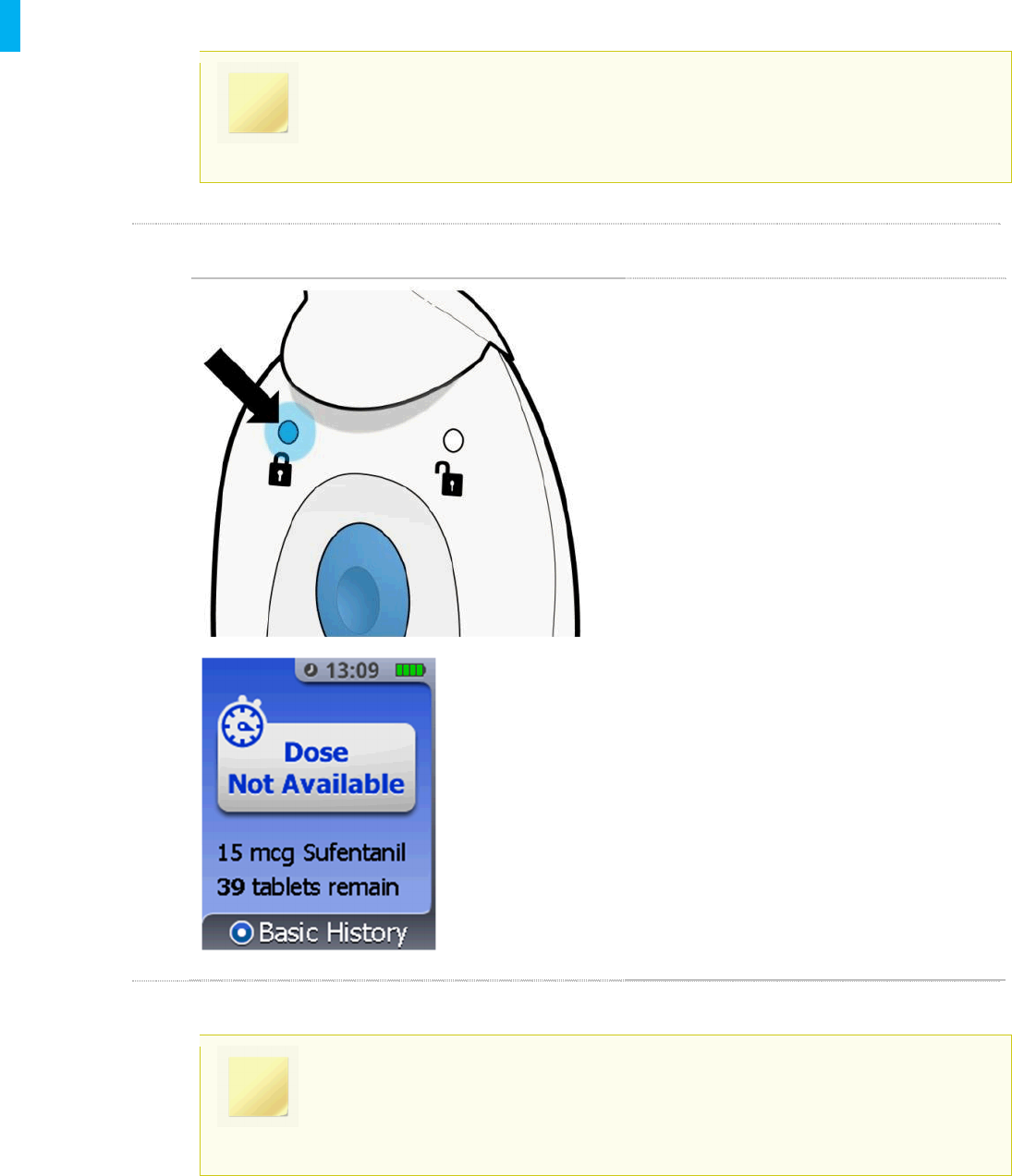
STEP 3 Administer Additional Doses (if necessary)
A blue light will be visible on the back side
of the Controller to communicate that the
System is locked (for 20 minutes), after
which time the patient can receive another
dose if needed.
The number of tabletswill update to reflect
the total number remaining. In the example
on the left there are now 39 tablets
remaining.
Place the Cap back on the Dispenser and
return to the Holster. Refer to the next page
for more information on understanding dose
not available (lockout mode).
NOTE
The System’s display will go to sleep (i.e. display turns off) after 30 seconds of
System inactivity, but the No Dose Available or Dose Available Light will
be lit. To wake up the display (i.e. turn on the display) from sleep, press the
Enter/Select or Menu button.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 57
Instructions for Use –PL-1678 Rev. K
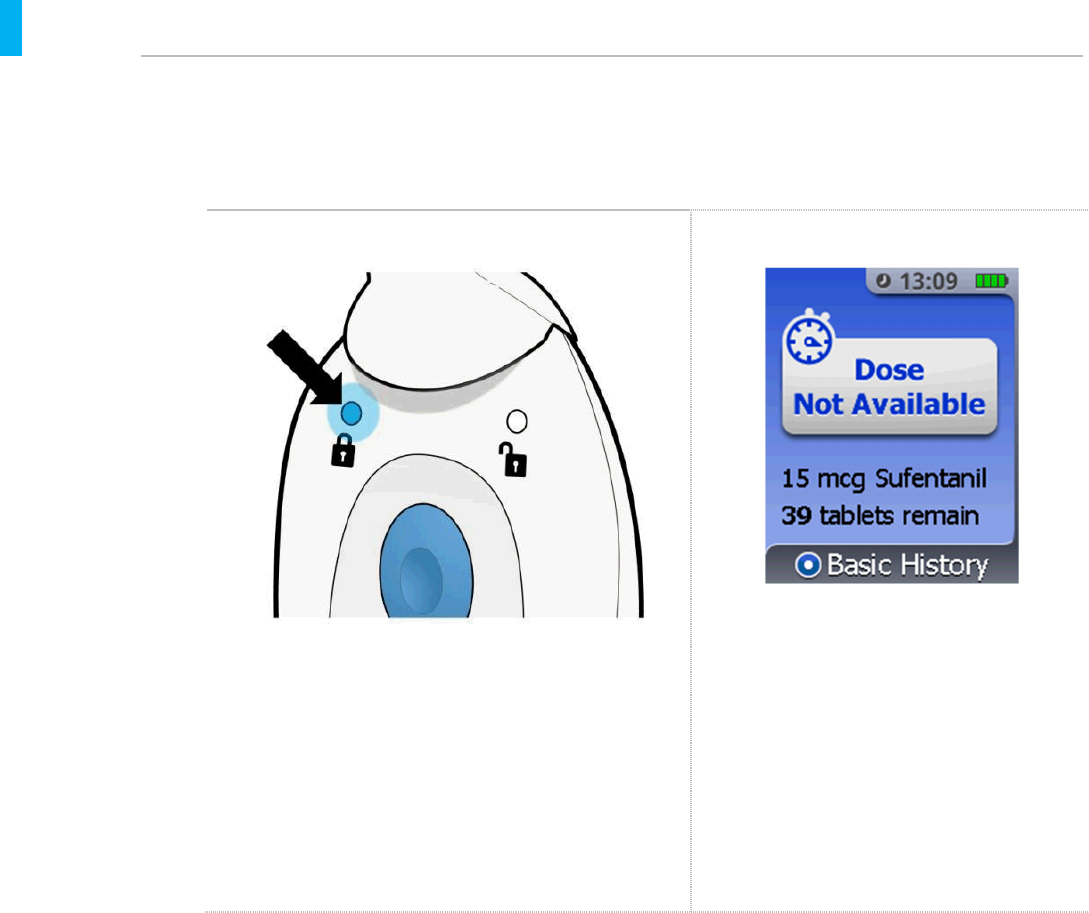
7.2. Understanding Dose not Available (Lockout Mode)
During the 20-minute lockout, the System has two ways to communicate that a dose is not
available:
Controller Light On-Screen Display
A blue light will be visible on the back side of the
Controller (see example above).
If the Enter/Select Button is pressed to
wake up the screen, the front of the
Controller will display a blue screen with
the message Dose Not Available and
list the drug name, dosage strength and
number of tabletsremaining.In the
example below there are now 39 tablets
remaining.
Refer to the next page for what happens if the patient presses the Blue Dose Button during
the lockout period.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 58
Instructions for Use –PL-1678 Rev. K
INFO
What happens if the patient presses the Blue Dose Button during the
lockout period?
If the patient were to press the dose button during the 20-minute lockout,the
System will announce a negative error tone to communicate no dose is available
and the System will record the total number of requests the patient made during
the lockout in the Dose History. The total number of requests can be reviewed
Section 11, Basic Dose History and Detailed History.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 59
Instructions for Use –PL-1678 Rev. K
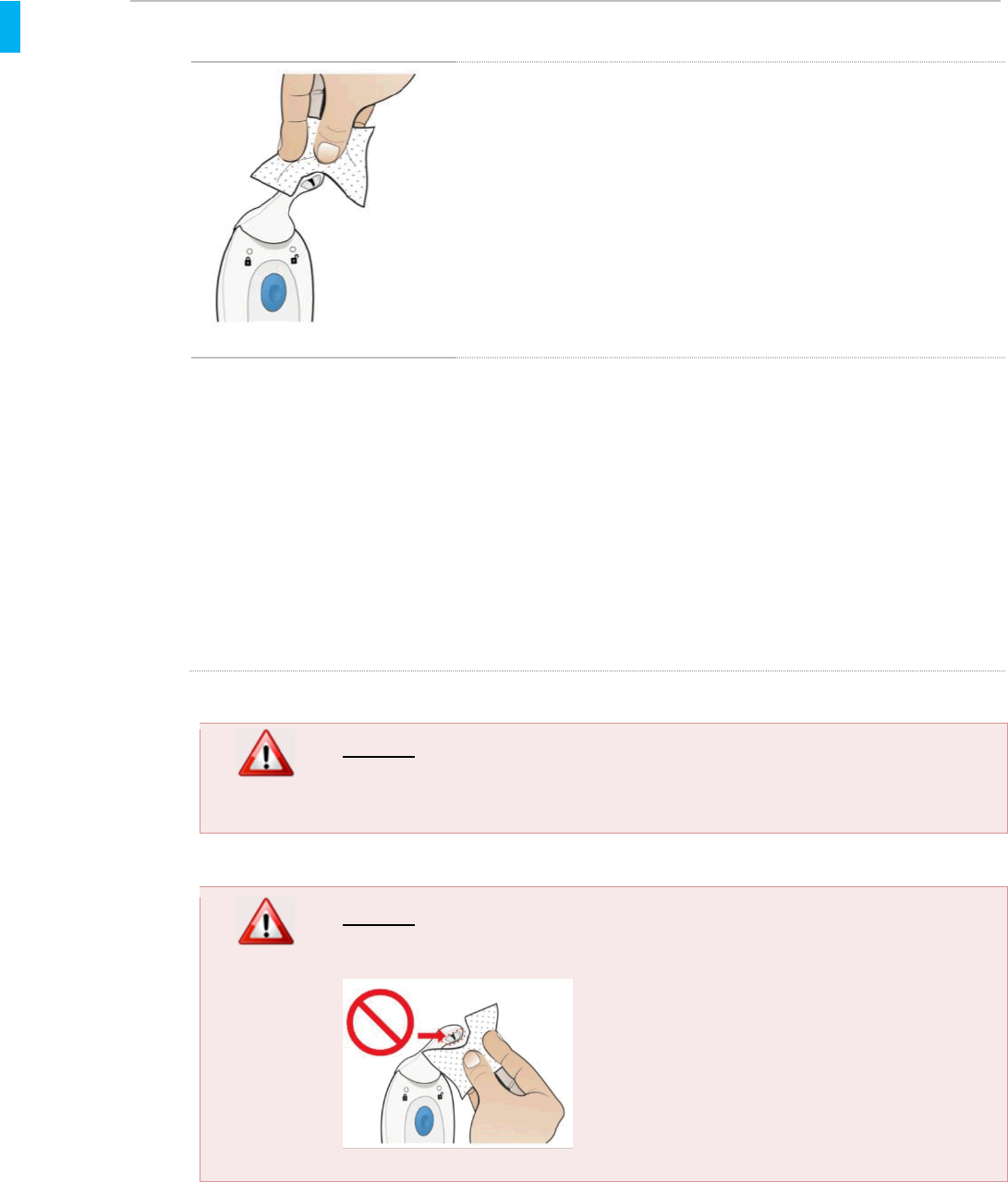
7.3. Cleaning the System During Patient Use
If a patient or Healthcare Professional desires to clean any part
of the System during use, alcohol wipes should be used.Since
the Dispenser is inserted into the patient’s mouth for dosing, the
patient may desire more frequent cleaning of the Dispenser than
other portions of the System, possibly after each dose (though
this is not required; frequency of cleaning is primarily a personal
choice by the patient). Use of the Cap will protect the Dispenser
tip from inadvertent contact by patient guests and Healthcare
Professionals.
If for any reason the patient or Healthcare Professional notices visible contamination, clean the
System as follows:
Wipe as necessary with fresh alcohol wipes until the System appears visually clean.
Do not saturate any part of the System. Wipes should not be excessively wet; squeeze
out excessive liquid from the wipes before use.
Let System dry before next patient use.
The Dispenser, or entire System, should be replaced if the patient or Healthcare Professional is
concerned about severe contamination.
WARNING
DO NOT soak or immerse System in water.
WARNING
DO NOT clean the interior portion of the tip. If interior of tip is visibly
contaminated, replace the Dispenser.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 60
Instructions for Use –PL-1678 Rev. K
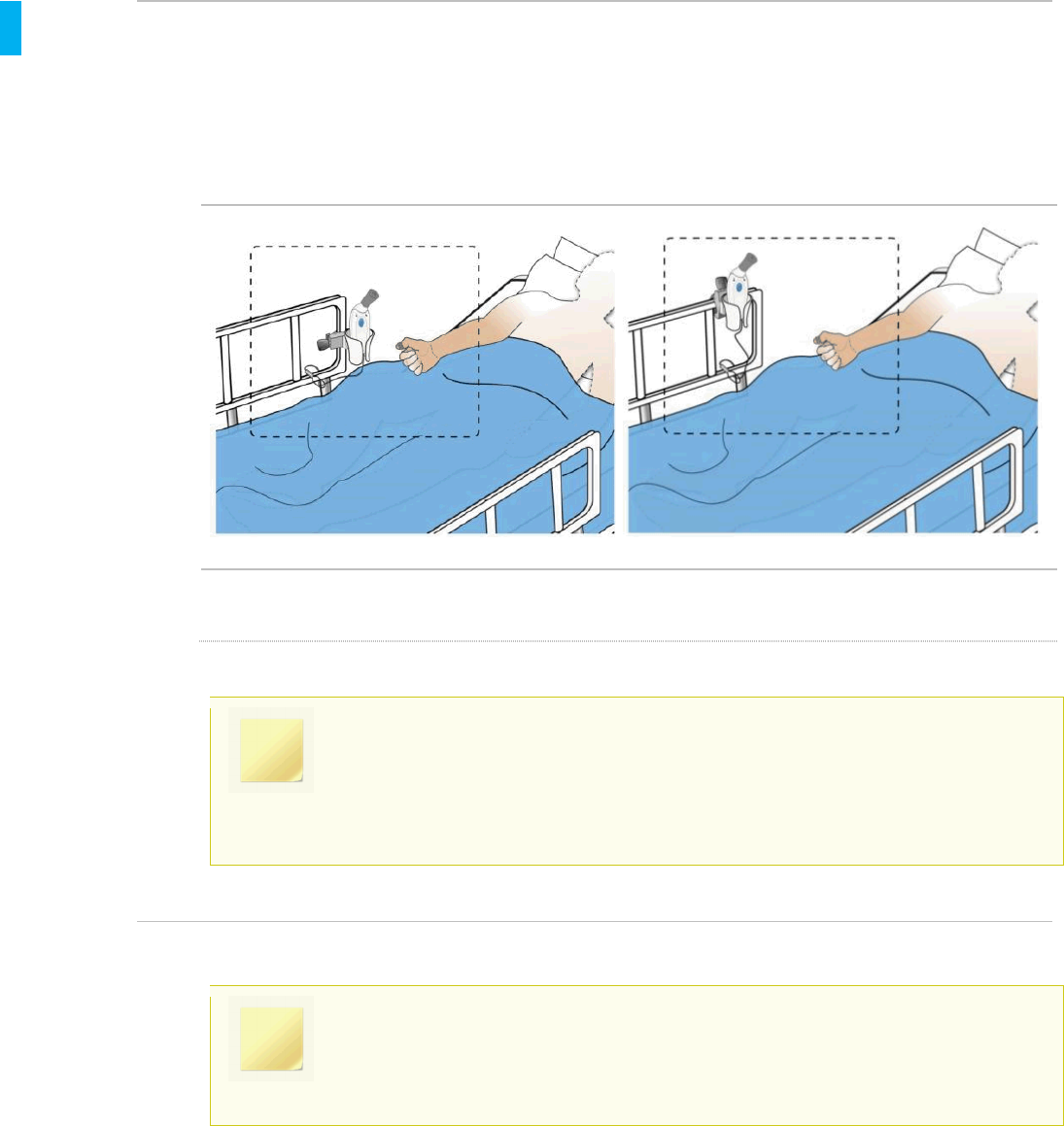
7.4. Storing the System During Patient Use
The System should be stored in the Holster between doses. Use of the Cap is recommended to
avoid inadvertent contact between uses.
Holster Storage Location Examples
NOTE
It is important to remind the patient to store the System in the Holster when not in
use to minimize the chance of inadvertently dispensing a tablet by accidently
pressing the Dose Button. If this occurs, the patient should call the Healthcare
Professional to retrieve and properly dispose of the loose tablet.
7.5. Use of the System
NOTE
Patients who do not appear to be able to appropriately use Zalviso should be
switched to another form of pain management.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 61
Instructions for Use –PL-1678 Rev. K
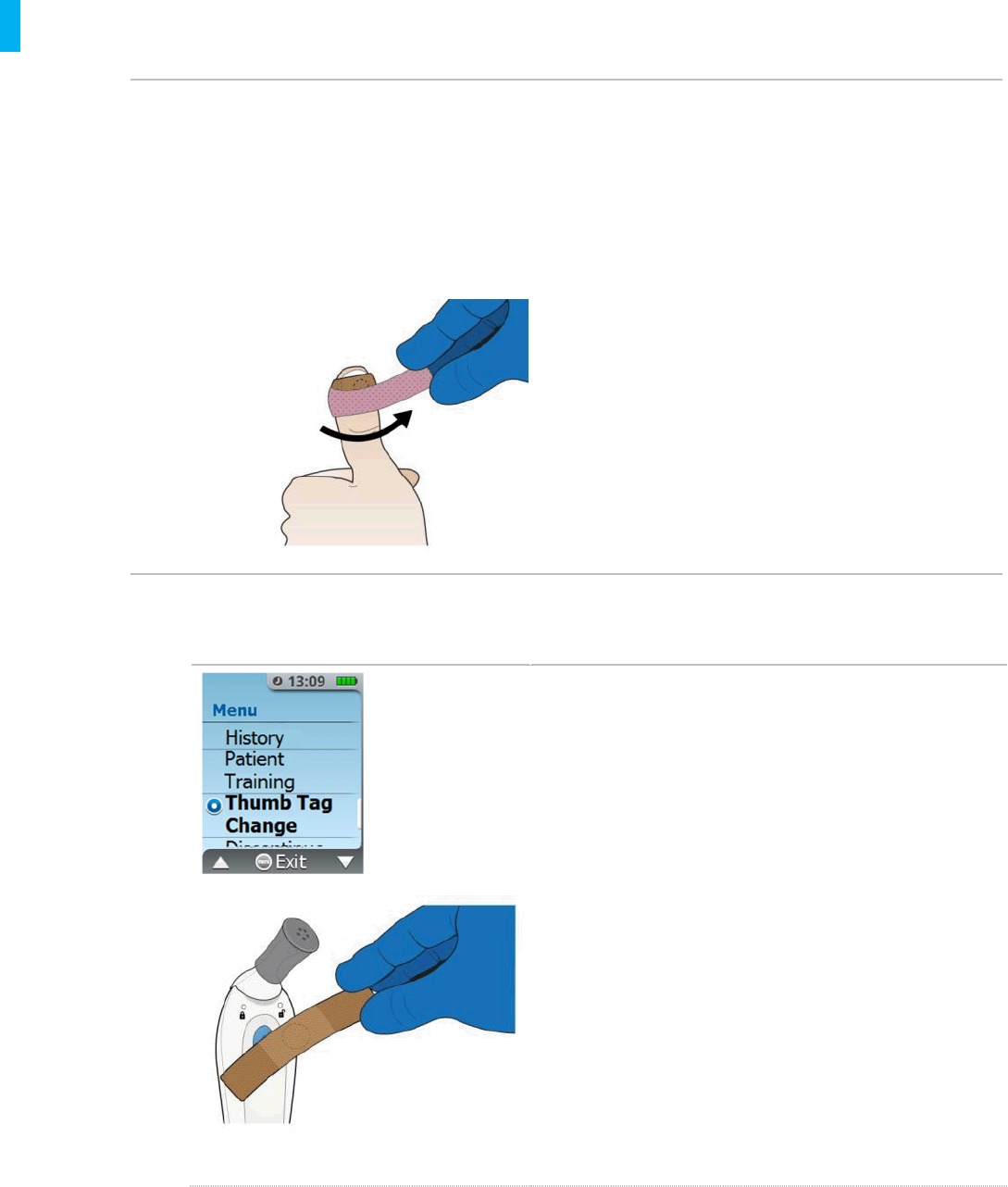
8. Replacing the Patient ID Thumb Tag
8.1. If the Patient ID Thumb Tag is Loose
Secure the Patient ID Thumb Tag in place as needed by readjusting it and making sure
the ends of the Patient ID Thumb Tag are adhered to the thumb and not to itself.
If the Patient ID Thumb Tag continues to be loose, secure it in place by wrapping some
medical tape around the thumb on top of the Patient ID Thumb Tag, keeping the same
orientation.
8.2. If the Patient ID Thumb Tag is Either Lost, Dropped, Defective or
Becomes Non-Functional
Remove and discard used Thumb Tag as
needed
Retrieve a new Patient ID Thumb Tag and an
AAC
Press the Menu button to access the System
Menu, scan the AAC when prompted, then
scroll up or down the menu to Thumb Tag
Change
Select the Thumb Tag Change function
from the menu by pressing the Enter/Select
button.
Follow the screen prompts and affix the new
Patient ID Thumb Tag on the patient’s thumb.
NOTE: When the replacement Patient ID Thumb Tag is
successfully scanned, you will hear an audible
confirmation tone, and the System will transition to the
next screen.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 62
Instructions for Use –PL-1678 Rev. K
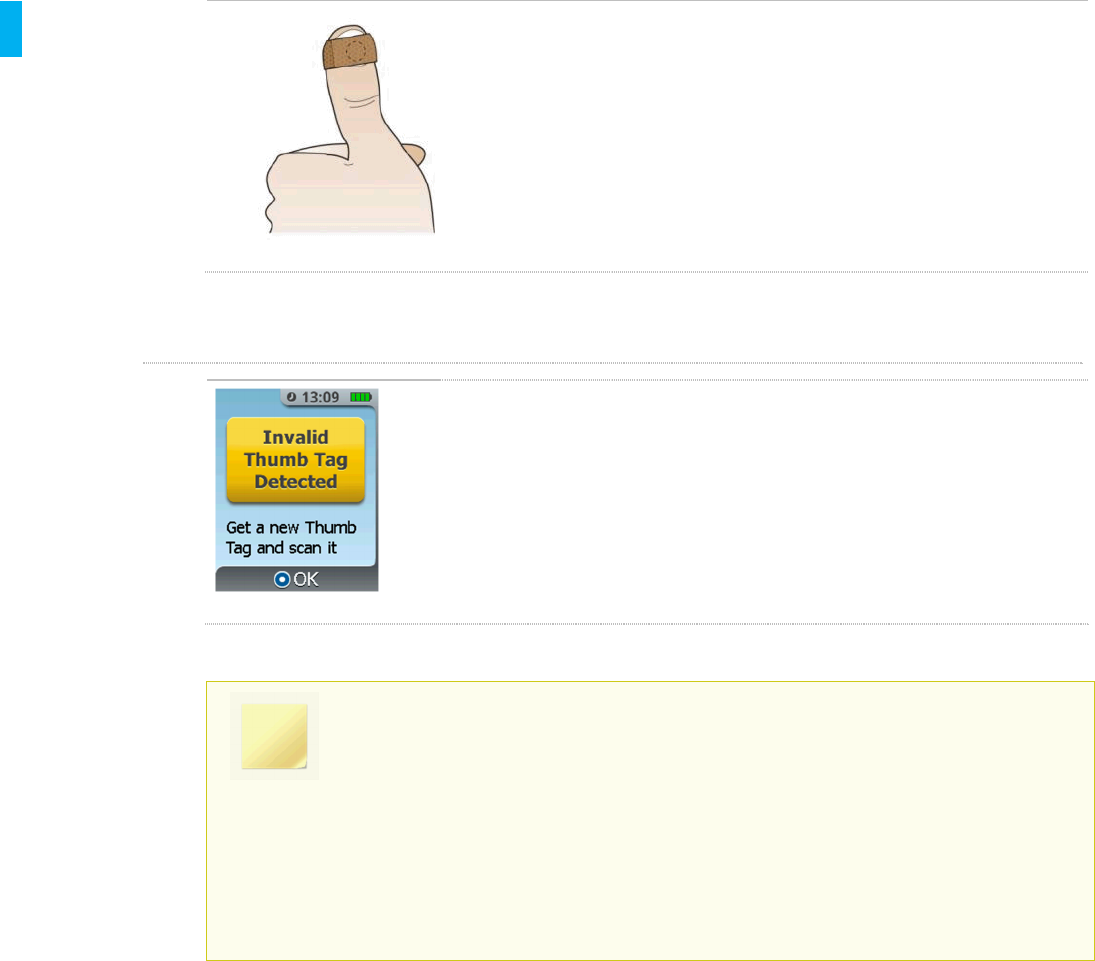
Invalid Patient ID Thumb Tag Prompt
If a previously used Patient ID Thumb Tag was presented to the
System during setup or Patient ID Thumb Tag change, a new Patient
ID Thumb Tag is required
1. Obtain a new Patient ID Thumb Tag.
2. Press the Enter/Select Button.
3. Scan the new Patient ID Thumb Tag.
NOTE
During System use, if the System doesn’t receive any inputs (button presses or
movement), the screen will turn off (sleep) after 30 seconds. To wake the screen,
press the Enter/Select Button or the Menu Button. Upon the screen waking
up, the System will have exited the Thumb Tag Change menu and be in Patient
mode. To return to the Thumb Tag Menu, press the Menu button to access the
System Menu with an AAC, then scroll up or down the menu to Thumb Tag
Change, then select the Thumb Tag Change function from the menu by
pressing the Enter/Select button.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 63
Instructions for Use –PL-1678 Rev. K
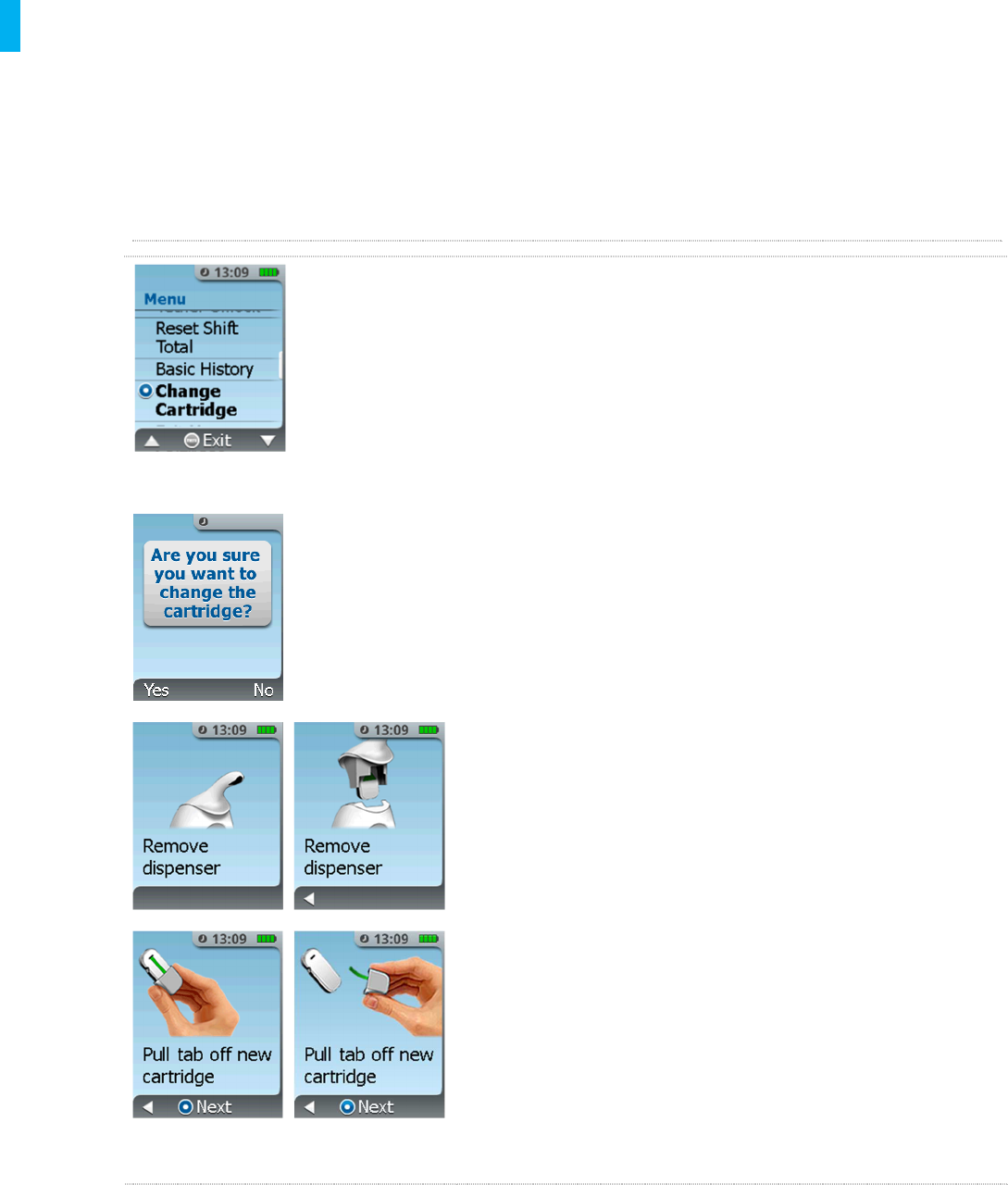
9. Changing a Cartridge
The Cartridge may be changed if the number of tablets is depleted or if the Cartridge is empty.
Follow the screen instructions for changing the Cartridge as shown in the first screen below.
Refer to Section 5, How to Set Up the System for a New Patient, for instructions on how to
prepare the new Cartridge for use with the System.
Changing a Cartridge
1. Retrieve a new Cartridge from the medication storage
system.
2. Retrieve an AAC.
3. Press the MENU button, access the System Menu with
the AAC, and scroll to the Change Cartridge
function.
Note: The System does not allow the re-use of a partially used
Cartridge.
4. Select YES (Left button) if you are sure you want to
change the Cartridge. Selecting YES will unlock the
Dispenser to initiate changing the Cartridge. Select NO
(Right Button) if you do not want to change the Cartridge
and return to the dose status screen.
5. After YES is selected to change the Cartridge, remove
the Dispenser by pulling up on the Dispenser. Remove
and properly dispose of used Cartridge.
6. Obtain a new Drug Cartridge and prepare it for use as
prompted by the screens. Refer to Section 5, Step 6:
Prepare Drug Cartridge. Once Drug Cartridge is
prepared, confirm this step on the screen by pressing
the Enter/Select Button on the Controller.
NOTE: Only a new Drug Cartridge can be used or the System
will display an error or notification.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 64
Instructions for Use –PL-1678 Rev. K
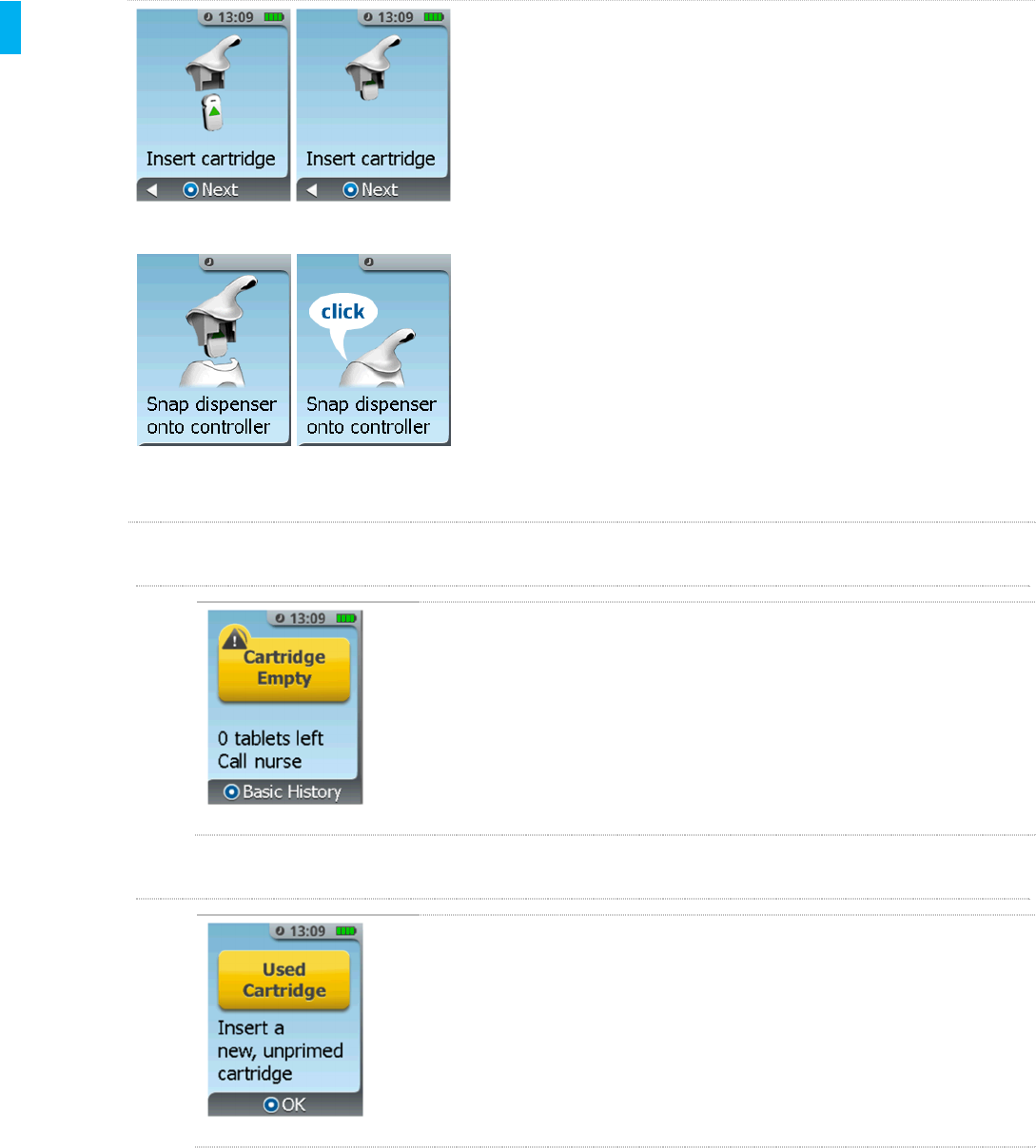
7. Insert the Cartridge into the bottom of the Dispenser
(green arrow on Cartridge label points up). Refer to
Section 5, Step 7: Insert Cartridge. Confirm this step
on the screen by pressing the Enter/Select Button on
the Controller.
8. Snap the Dispenser (with Cartridge attached) onto the
Controller. Refer to Section 5, Step 8: Connect
Dispenser.
9. Remove Cap (refer to Section 5, Step 9).
10. Eject the Priming Cap (refer to Section 5, Step 10).
11. Re-Cap the System (refer to Section 5, Step 11), to
complete the Cartridge change.
Cartridge Empty Prompt
If the last tablet has been dispensed from the Cartridge, the System
will show the Cartridge Empty screen.
Used Cartridge Prompt
If the Used Cartridge screen is displayed after the Dispenser and
Cartridge are snapped into the Controller, the System has detected a
used or defective Cartridge. Retrieve a new Cartridge, press the
Enter/Select button and follow the instructions to prepare and
install the new Cartridge.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 65
Instructions for Use –PL-1678 Rev. K
Basic History Prompt
If the Enter/Select button is pressed instead of the Menu
button, the display will transition to the Basic History screens. If you
want to change the Cartridge press the Menu button to go to the
Main menu and access the System with the AAC. From here you
can change the Cartridge, Discontinue or perform other actions in
the Menu.
NOTE
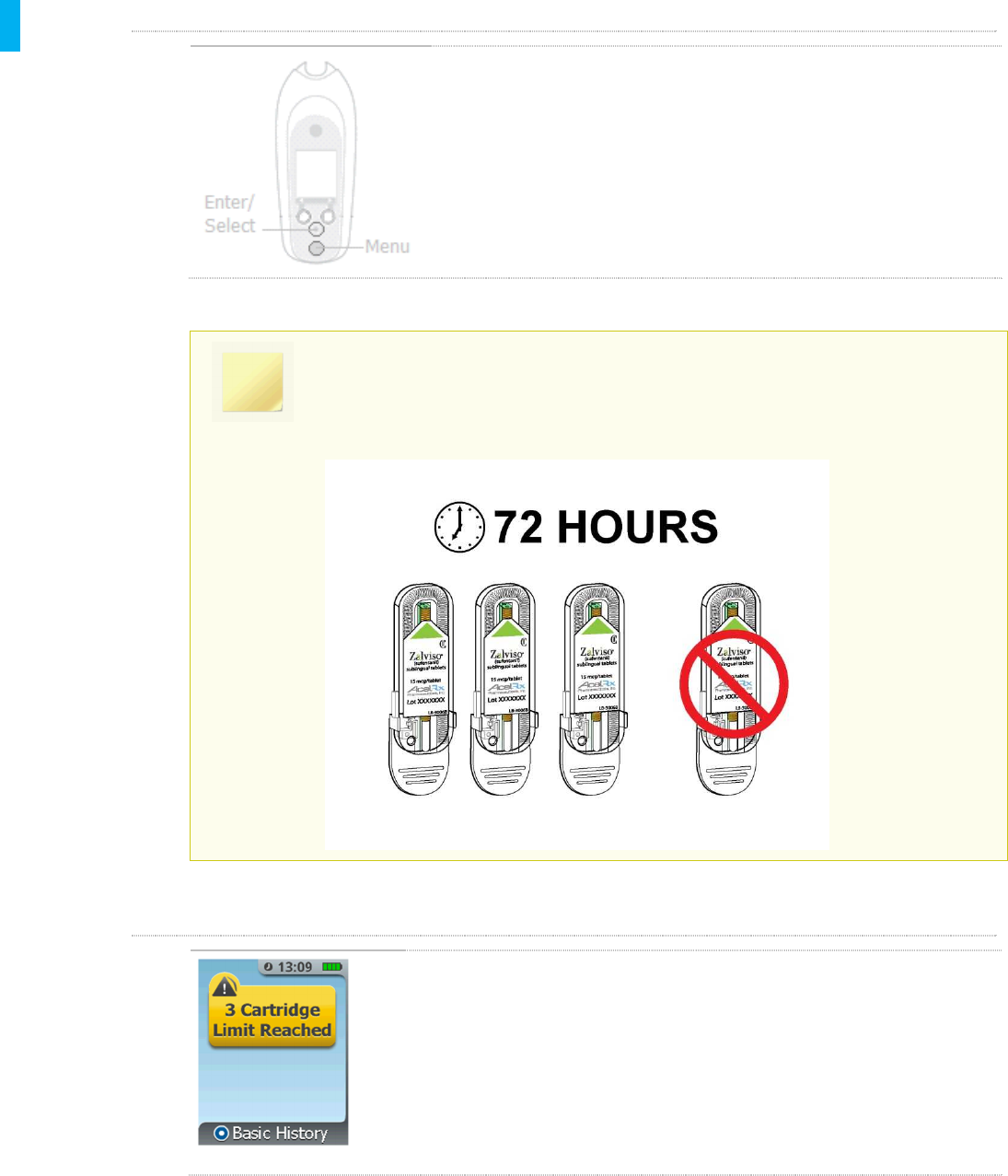
There is a three Cartridge limit that can be used in a 72-hour period with one
System. Additional Cartridge use beyond three Cartridges within 72 hours will
require a new System to be set up.
3 Cartridge Limit Reached
If the 3 Cartridge Limit Reached screen is displayed after Change
Cartridge is selected, the third Cartridge is in use in the System and
changing the Cartridge will not be permitted. Refer to Note above.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 66
Instructions for Use –PL-1678 Rev. K
10. Tether Unlock
STEP 1 Access Tether Unlock Prompt
If for any reason the System needs to be repositioned during patient use,
unlock the Tether from the bottom of the Controller. Examples include
moving the patient to a wheelchair, bedside chair, walker or gurney, in
which case the System can be re-tethered to be in close proximity to the
patient. Ensure that the Tether does not get tangled with any equipment
lines that may be near the patient, since this could interfere with patient
access to therapy. When the patient is being transported, ensure that the
Tether does not get tangled in the wheels which may damage the System
or jam the wheelchair or gurney.
Note: The Security Tether must be reattached in order to continue therapy
and the System will not dispense tablets while the Tether is disconnected.
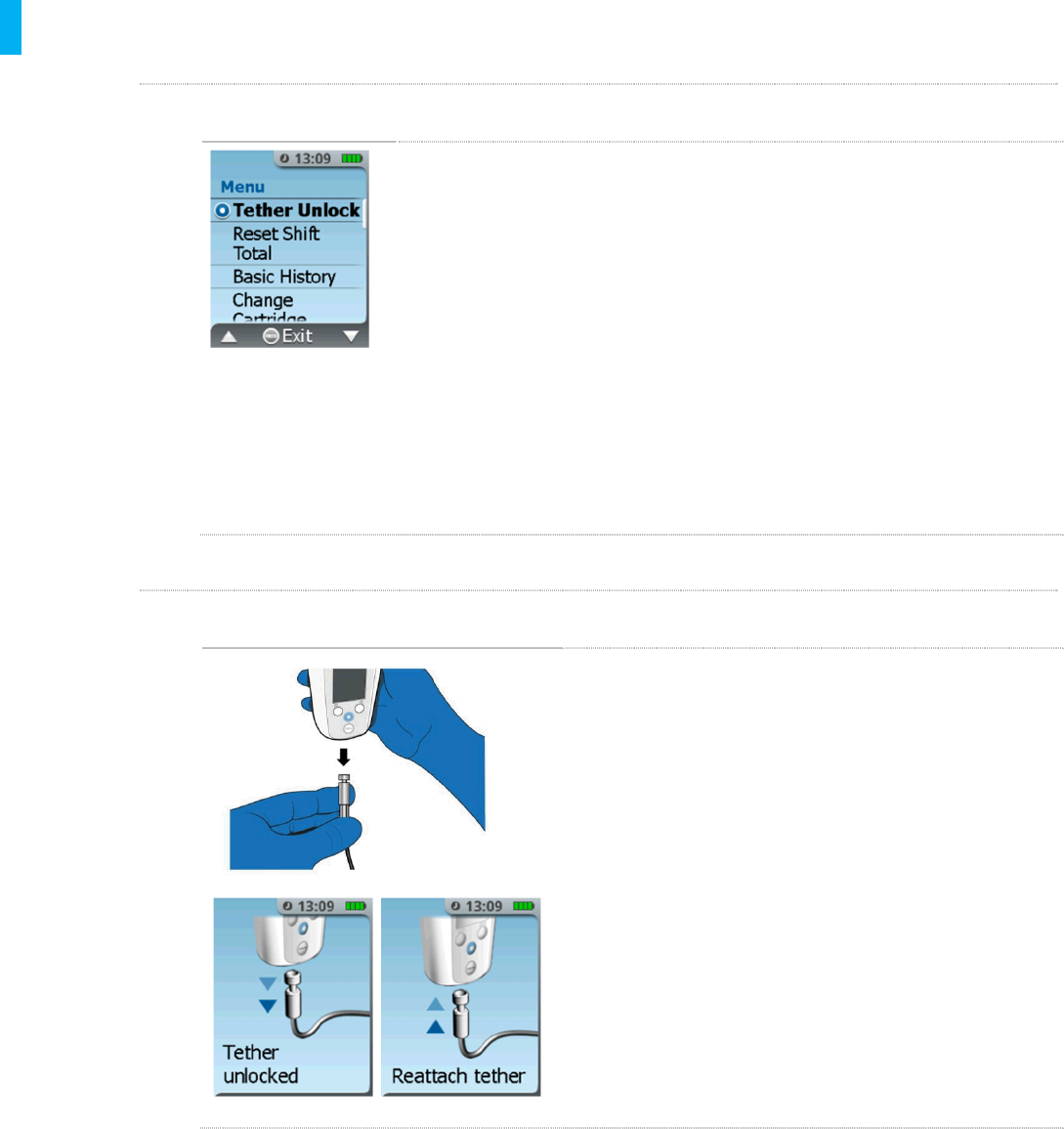
STEP 2 Remove Tether
1. Press the Menu button on the Controller.
2. Touch the Authorized Access Card to the Blue
Dose Button on the back of the Controller to
access the System Menu.
3. Highlight and select the Tether Unlock
function by pressing the Enter/Select Button.
4. The System will release the Tether from the
bottom of the Controller and display that it is
unlocked. Pull down on the Tether to release it
and adjust the location of the System.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 67
Instructions for Use –PL-1678 Rev. K
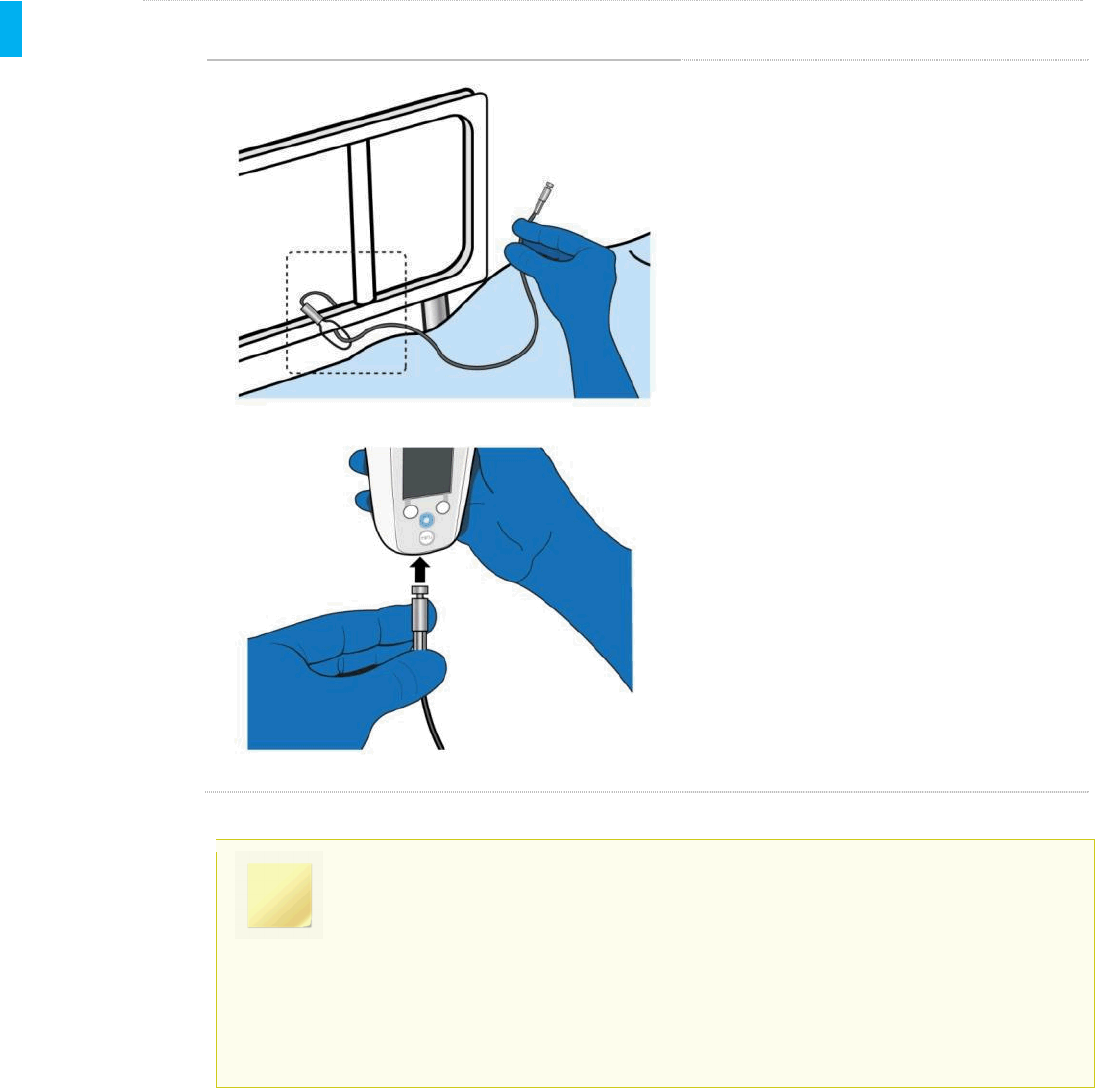
STEP 3 Securely Attach Tether and Attach Holster to New Location
When you are ready to re-secure the
Tether, first loop the Tether around the
bedrail or another object such as a chair,
wheelchair, walker or gurney. Reattach the
Tether into the bottom of the Controller.
The Controller will sense that the Tether
has been inserted and lock automatically.
The Holster should be attached to an object
near the patient to hold the tethered System
(Refer to Section 5, Step 14).
NOTE
If the patient is being transported, the System should be tethered to the
wheelchair or gurney and the System should be placed in the attached Holster.
The patient should only self-administer doses from the System when not in
motion. If the patient attempts to dose while in motion, jostling may cause the
tablet to miss the patient’s sublingual space causing aloose tablet. Should this
occur, the patient should inform the Healthcare Professional so that the loose
tablet can be retrieved and disposed of properly.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 68
Instructions for Use –PL-1678 Rev. K
11. Basic Dose History and Detailed History
11.1. Basic Dose History
The Healthcare Professional can query the System for the basic Dose History of the patient. This
review can be done without the use of the Authorized Access Card. Press the Enter/Select
Button to access the six basic Dose History screens.
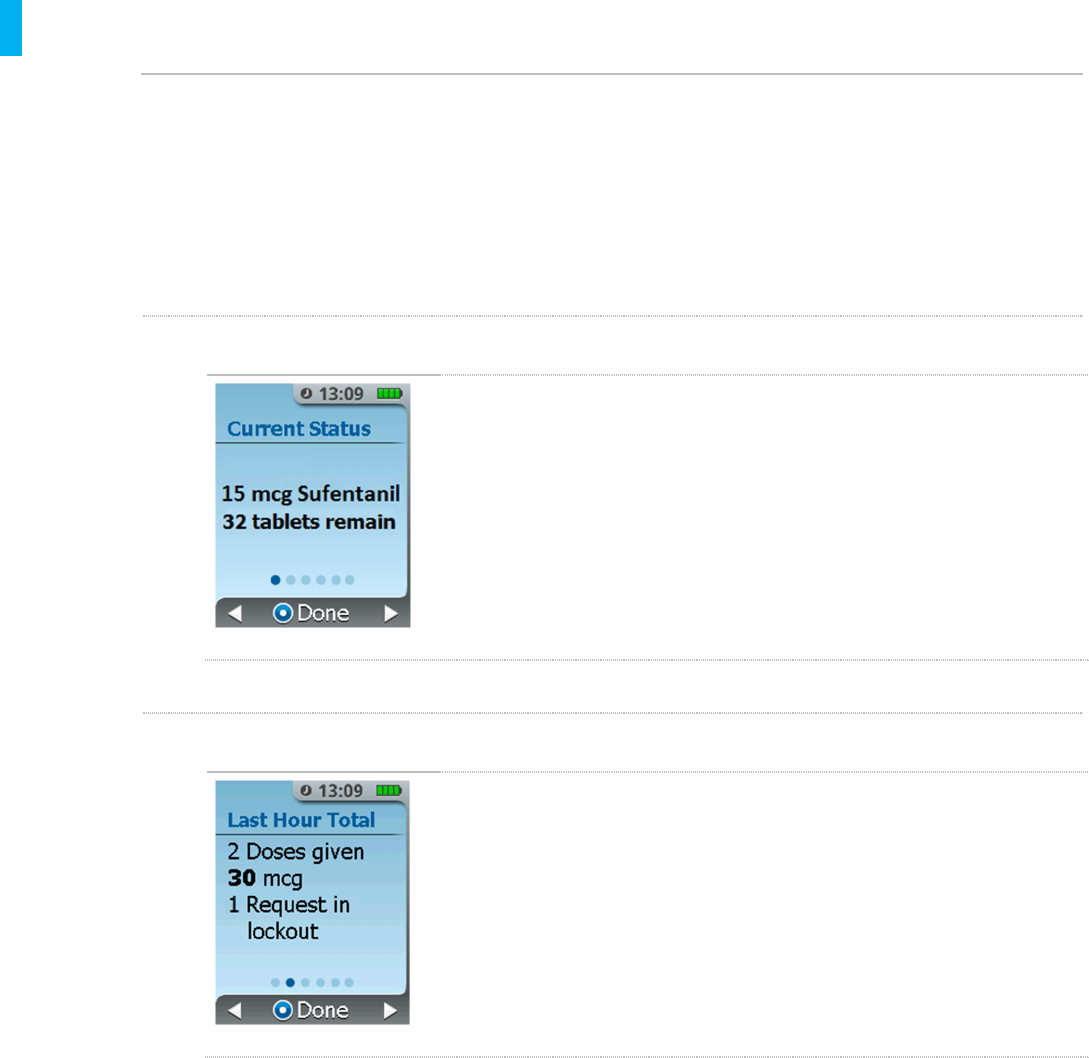
STEP 1 Current Status Screen
When you first access the Dose History screen it displays the
current status. Use the right arrow (press the Right Button)to
review other details, such as the dosing totals for the last hour, dosing
totals since the last shift reset, dosing totals since the System was
setup, and the date/time the shift dosing total was reset.
The first screen displays the drug dosage strength, drug type and the
tablets remaining in the Drug Cartridge.
STEP 2Last Hour Total Screen
The next screen displays the total number of doses dispensed and
total requests in lockout by the patient in the last hour.
Printed on: ; Printed by: .
AcelRx Pharmaceuticals, Inc. –Zalviso™ sufentanil sublingual tablet system 69
Instructions for Use –PL-1678 Rev. K
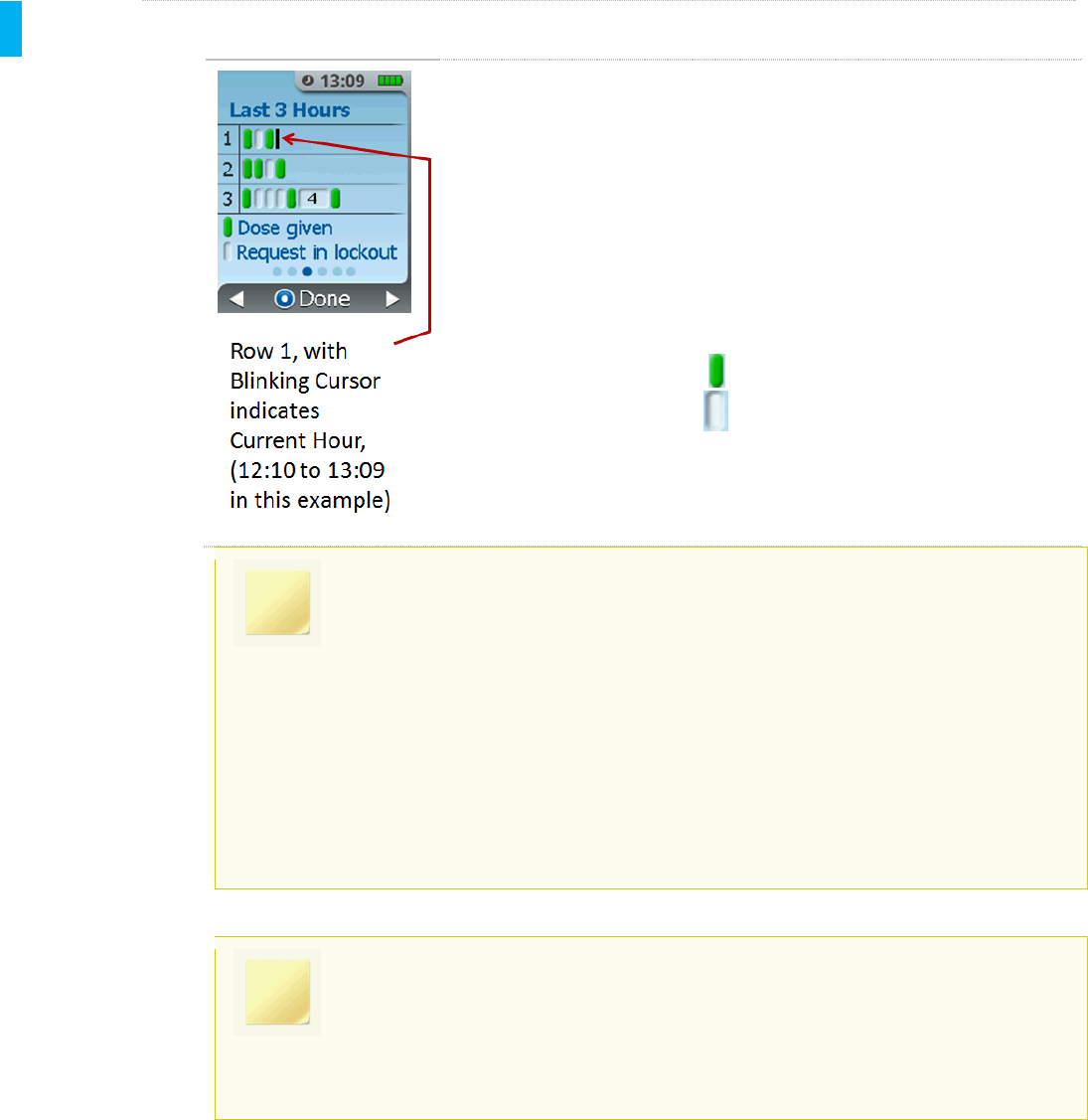
STEP 3 Activity Each Hour Screen
The next screen displays a graphical representation of the dosing
history in the last 3 hours.
Each row represents an hour of time. The current (most recent) hour is
at the top (Row 1). The blinking black “cursor” is an active indicator of
the current hour. In the example at left, Row 1 at the top indicates
dosing history in the current hour, or 12:10 to 13:09.
Within each row is a representation of the doses dispensed
(represented by a green icon ) and requests made during lock out
(represented by a white icon ).
NOTE
If the number of requests made during lockout exceeds 3, the graph will display as
a block which contains the total number of requests during lockout for that time
period (see the white block with “4” in the example above).
Using the example screen above, assuming it is 13:09(1:09PM), the patient’s
dose history is:
Hour 1 (current hour), 12:10 – 13:09: 2 doses given, 1request during
lockout
Hour 2, 11:10 –12:09: 3 dosesgiven, 1request during lockout
Hour 3, 10:10 –11:09: 3 doses given, 7request during lockout
NOTE
The nurse should review the dosing history to look for excessive requests during
lockout and determine the cause.
If a patient does not possess the cognitive ability or manual dexterity to properly
use Zalviso, alternative analgesic therapy should be considered.
Printed on: ; Printed by: .