Alcon Research NGPFSW1 Centurion Vision System Footswitch User Manual
Alcon Research Ltd. Centurion Vision System Footswitch
User Manual

Operator's Manual
Manufacturer:
Alcon Laboratories, Inc. Alcon Laboratories (UK) Ltd.
6201 South Freeway Frimley Business Park
Fort Worth, Texas 76134-2099 Frimley, Camberley
U.S.A. Surrey, GU16 7SR, United Kingdom
Produced By:
Alcon Research, Ltd.
15800 Alton Parkway
Irvine, California 92618-3818
U.S.A.
Telephone: 949/753-1393
800/832-7827
FAX: 949/753-6614
8065751772, CATALOG NUMBER
905-2150-001 P4, TEXT ONLY
© 2012 Novartis
Directive 93/42/EEC
EC REP

ii 8065751772
Centurion® Vision System Operator's Manual
8065751772
MANUAL REVISION RECORD
DATE REVISION ECN NUMBER AND DESCRIPTION
March 2011 P2 2011XXXX - Preliminary release of Centurion® Vision System
Operator's Manual with catalog number 8065751772, and 905-2150-
001 text (applies to Centurion® Vision System consoles with software
version X.00).
June, 2012 P3 2012XXXX - On page 1.23 changed Maximum Input Current in Table
1-3 from 6A to 10A. On page 1.25 changed amperage on label from
12A to 10A. Text Cover Sheet and pages i & ii also updated.
June, 2012 P4 2012XXXX - On page 1.25 added IPX1 to remote control label. Text
Cover Sheet and pages i & ii also updated.
END USER LICENSE AGREEMENT:
This product contains software licensed from Microsoft Corporation.
* Registered in the U.S. Patent & Trademark Ofce.
** Mackool is a trademark of Richard J. Mackool, M.D.
Cycoloy and Lexan are registered trademarks of Sabic Innovative Plastics IP


vi 8065751772
PREFACE
This operator's manual is your written guide to the Centurion® Vision System and considers all
options available to the customer; therefore, when reading this manual, ignore the options which do
not apply to your specic unit.
Please read the entire manual carefully before operating the instrument. Recommended settings are
given only as guidelines, and are not meant to restrict the surgeon; however, before trying other
settings, the surgeon and support personnel should be experienced with the system and familiar with
the new settings.
NOTE: If an inconsistency exists between the instructions in the operator's manual and the
Directions For Use (DFU) supplied with a consumable pack or accessory, follow the DFU.
Equipment improvement is an on-going process and, as such, changes may be made to the
equipment after this manual is printed.
Pay close attention to Warnings, Cautions, and Notes in this manual. A WARNING! statement is
written to protect individuals from bodily harm. A Caution statement, with the CAUTION heading
centered above the text, is written to protect the instrument from damage. A NOTE: is written to
bring attention to highlighted information.
If you have questions, or want additional information, please contact your local Alcon representative
or the Alcon Technical Services Department at:
Alcon Research, Ltd.
15800 Alton Parkway
Irvine, California 92618
(949) 753-1393
FAX (949) 753-6614
CAUTION: U.S. Federal Law restricts this device to sale by or on the order of a physician.


8065751772 1.3
GENERAL INFORMATION
The Centurion® Vision System is indicated for emulsication, separation, irrigation, and
aspiration of cataracts, residual cortical material and lens epithelial cells, vitreous aspiration
and cutting associated with anterior vitrectomy, bipolar coagulation, and intra-ocular lens
injection. The AutoSert® IOL Injector Handpiece is intended to deliver qualied AcrySof®
intraocular lenses into the eye following cataract removal.
The AutoSert® IOL Injector Handpiece achieves the functionality of injection of intraocular
lenses. The AutoSert
®
IOL Injector Handpiece is indicated for use with AcrySof
®
lenses SN60WF,
SN6AD1, SN6AT3 through SN6AT9, as well as approved AcrySof
®
lenses that are specically
indicated for use with this inserter, as indicated in the approved labeling of those lenses.
The
Centurion® Vision System
, including accessories approved by Alcon, constitutes a complete
surgical system and is intended exclusively for use by licensed ophthalmic surgeons and
their surgical teams. These surgical teams are experienced at conducting phacoemulsication
procedures in a properly maintained surgical environment (qualied personnel, availability of
backup equipment) and are familiar with the operation of the equipment used as outlined in
operator's manuals and directions for use (setup/checkout procedures to be completed before
the surgical procedure; processing of reusable devices; maintenance; etc.).
Patient selection for use with the
Centurion® Vision System
(such as age, ophthalmic
pathology, and other factors) is determined by the surgeon. The general patient age can range
from newborn to geriatric, although there have been studies that have identied the mean age
of patients that underwent cataract surgery was 72.32 yrs - men and 74.89 yrs - women.1
Intended Use Environments
The Centurion
®
Vision System is intended for use in hospitals and ambulatory surgery centers.
Phaco Handpiece Note
Throughout the rest of this manual the CENTURION® OZil® handpiece and the INFINITI®
OZil® handpiece will be referred to as phaco handpieces, unless one or the other must be
referred to exclusively.
Trademark Note
A button, mode, or step labeled OZil®, AutoSert
®
, or UltraChop refers to a display screen
control used with a phaco handpiece, INTREPID® AutoSert
®
IOL injector, or ALCON®
UltraChopper® tip, respectively.
Abbreviation Descriptions
Many of the abbreviations used in this manual and on the Centurion® Vision System are
described in Table 1-x. Icons are identied in Figure 1-x.
Accessory Equipment
Accessory equipment connected to or used with this equipment must be certied according
to the respective IEC Standard (e.g., IEC 60950-1 for data processing equipment, and IEC
60601-1 for medical equipment). Furthermore, all congurations shall comply with clause 16
of IEC 60601-1:2005 (as amended). Anyone connecting additional equipment or otherwise
causing a different system conguration than provided by Alcon is responsible for continued
compliance to the requirements of clause 16 of IEC 60601-1:2005 (as amended). If in doubt,
consult the Technical Services department or your local Alcon representative.
Follow local governing ordinances and recycling plans regarding disposal or recycling of
device components and packaging.
1. “Age and sex prole of patients having cataract surgery between 1986 and 2003”
Philip O'Reilly, FRCSI (Ophth), U. Mahmound, FRCOphth, P. Hayes, FRCOphth, P. Tormey, FRCOphth, S. Beatty, MD.
Journal of Cataract Refractive Surgery 2005; 31:2162-2166
1.4 8065751772
User Information – Environmental Considerations
The equipment that you have purchased requires the use of natural resources for its
production and operation. This equipment may also contain hazardous substances
which could have potential effect on the environment and human health if disposed of
improperly.
In order to avoid the entry of any such substances into our environment, and to promote
natural resource conservation, please install, maintain, and operate the equipment in
accordance with the instructions. Information on the location of hazardous substances,
resource consumption and emissions of the equipment can be found throughout this
Operator's Manual. Please use the appropriate take-back systems. Such take-back systems
reuse or recycle many of the materials in your end-of-life equipment in a benecial way.
Please contact your local Alcon ofce for assistance in take-back options through Alcon
or other providers.
The crossed-bin symbol located on this equipment reminds you to use take-back
systems, while also emphasizing the requirement to collect waste equipment
separately, and not dispose of it as unsorted municipal waste. The Pb notation, if
present, indicates that the labeled device contains greater than 0.004% lead.
If you need more information on the collection, reuse or recycle systems available to
you, please contact your local or regional waste administration, or contact your local
Alcon ofce for more information.
Universal Precautions
Universal precautions shall be observed by all people who come in contact with the
instrument and/or accessories to help prevent their exposure to blood-borne pathogens
and/or other potentially infectious materials. In any circumstance, wherein the exact
status of blood or body uids/tissues encountered are unknown, it shall be uniformly
considered potentially infectious and handled in accordance with OSHA or your own
national guidelines.
EMC Statements
It is important to install and use the equipment in accordance with the instructions in
order to prevent harmful interference with other devices in the vicinity. If this equipment
causes harmful interference to other devices (determined by turning equipment off and
on), the user is encouraged to try to correct interference by one or more of the following
measures:
• Reorient or relocate the other device(s).
• Increase the distance between the equipment.
• Connect this equipment into an outlet on a circuit different from that to which the
other device(s) is connected.
• Consult the manufacturer or your Alcon eld service engineer for help.
Pb
8065751772 1.5
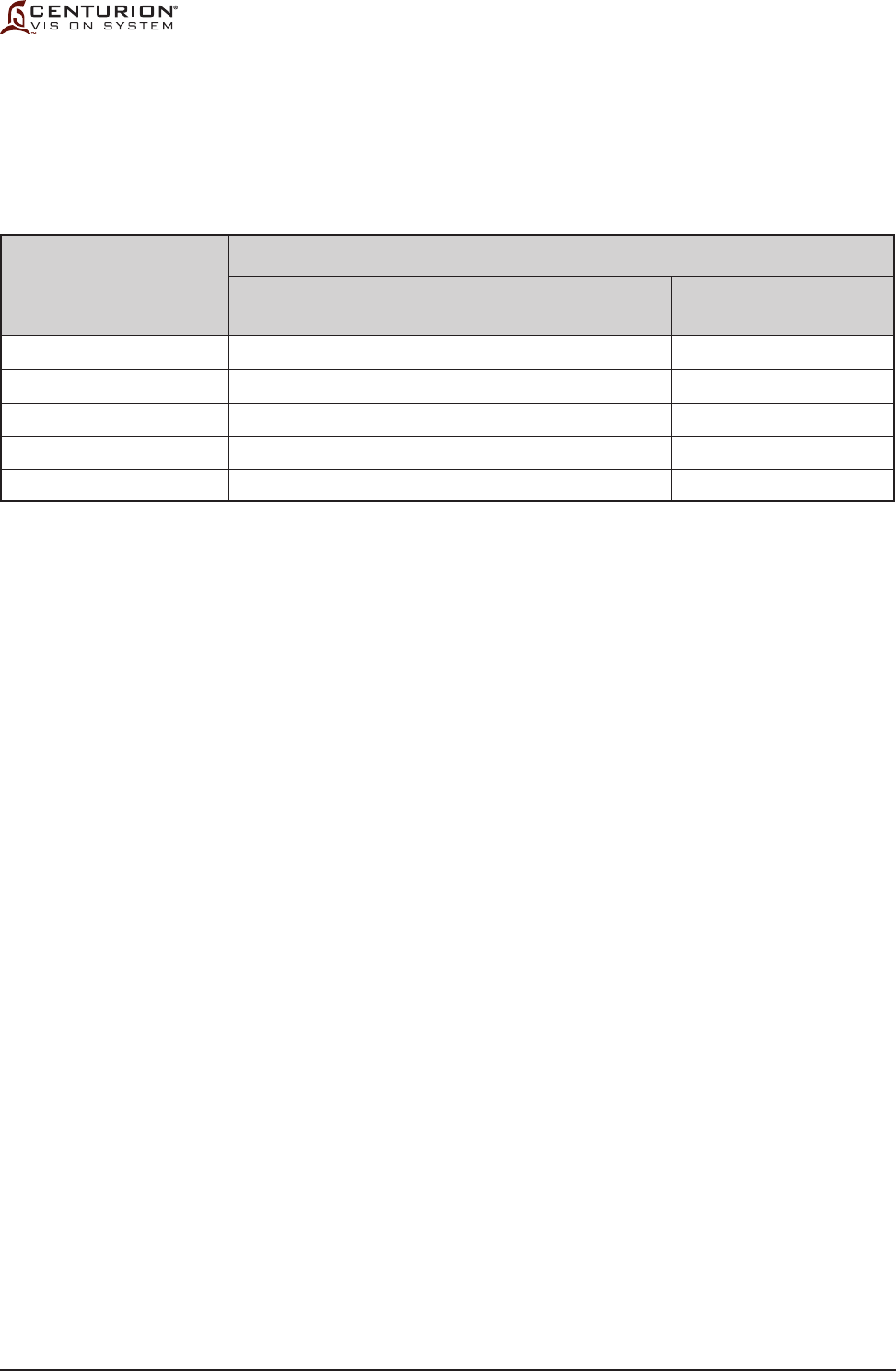
Table 1-1
Guidance and Manufacturer's Declaration - Electromagnetic Emissions - The Centurion®
Vision System is intended for use in the electromagnetic environment specied below. The
customer or the user of the Centurion® Vision System should assure that it is used in such
an environment.
Emissions Test
RF emissions
CISPR 11
RF emissions
CISPR 11
Harmonic emissions
IEC 61000-3-2
Voltage uctuations/
Flicker emissions
IEC 61000-3-3
Electromagnetic Environment-Guidance
The Centurion® Vision System uses RF energy only for its internal function.
Therefore, its RF emissions are very low and are not likely to cause any
interference in nearby electronic equipment.
The Centurion® Vision System is suitable for use in all establishments
other than domestic and those directly connected to a low voltage power
supply network that supplies buildings used for domestic purposes.
The EMC Statement provides guidance on steps to take in case of
electromagnetic interference.
Compliance
Group 1
Class A
Class A
Complies
Users should be aware of known RF sources, such as radio or TV stations and hand-held
or mobile two-way radios, and consider them when installing a medical device or system.
Portable and mobile RF communications equipment such as cellular telephones can affect
medical electrical equipment (see Table 1-3 for recommended separation distances).
Be aware that adding accessories or components, or modifying the medical device or
system, may degrade the EMI performance. Consult with qualied personnel regarding
changes to the system conguration.
WARNINGS!
The use of accessories, transducers, and cables other than those specied, with
the exception of transducers and cables sold by the manufacturer of the system as
replacement parts for internal components, may result in increased emissions or
decreased immunity of the system.
The system should not be used adjacent to, or stacked with, other equipment; and that if
adjacent to or stacked use is necessary, the system should be observed to verify normal
operation in the conguration in which it will be used.
MAGNETIC AND ELECTRICAL INTERFERENCE - Magnetic and electrical elds are
capable of interfering with the proper performance of the device. For this reason make
sure that all external devices operated in the vicinity of the device comply with the
relevant EMC requirements. X-ray equipment, magnetic resonance tomography (MRT),
nuclear magnetic resonance (NMR), or magnetic resonance imaging (MRI) devices are
possible sources of interference as they may emit higher levels of electromagnetic
radiation. See the Magnetic Resonance Unsafe icon in Figure 1-2.

1.6 8065751772
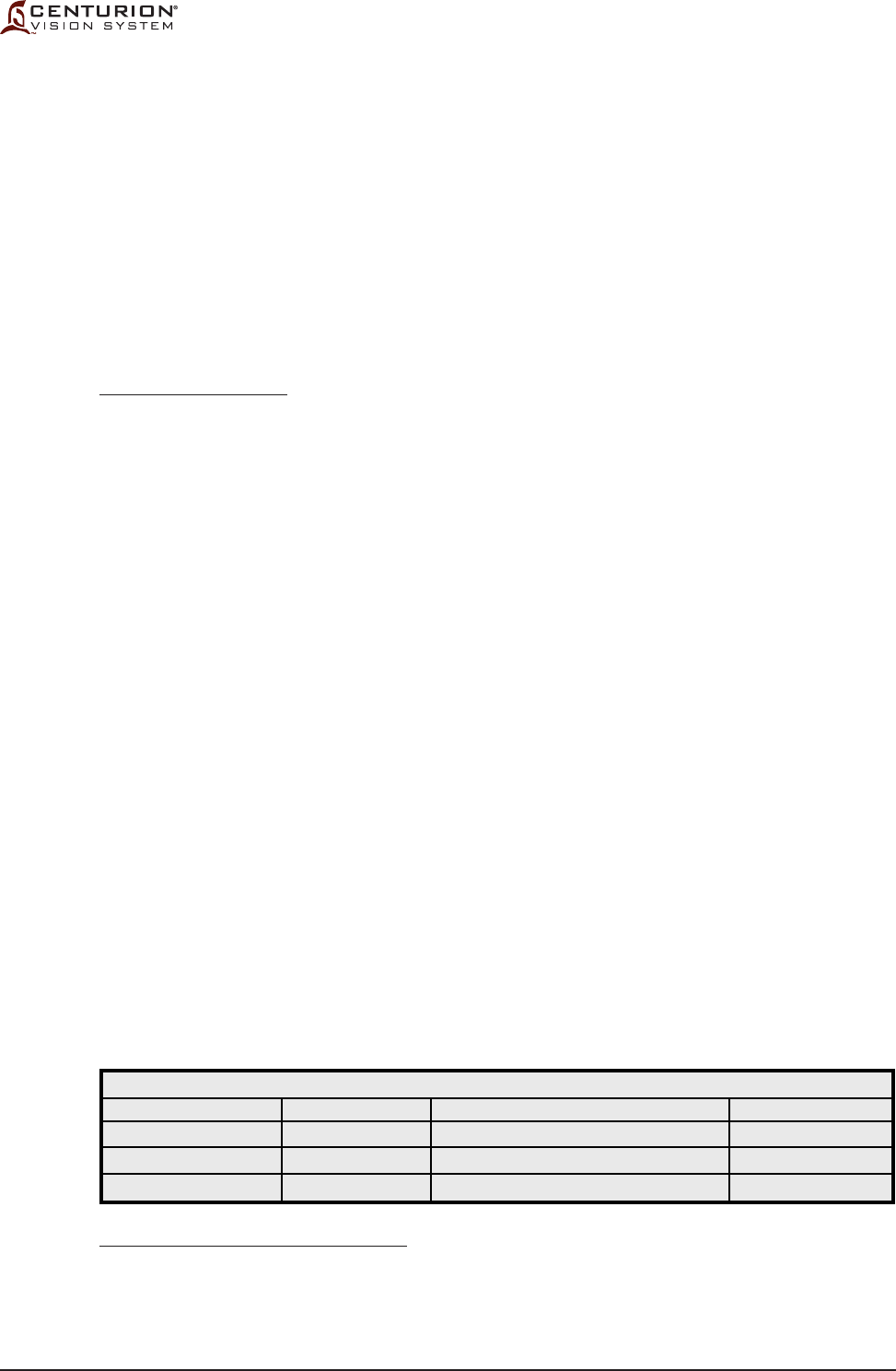
Table 1-2 Guidance and Manufacturer's Declaration - Electromagnetic Immunity - The Centurion® Vision
System is intended for use in the electromagnetic environment specied below. The customer or
the user of the Centurion® Vision System should assure that it is used in such an environment.
Note: UT is the a.c. mains voltage prior to application of the test level.
Note 1: At 80 MHz and 800 MHz, the higher frequency range applies.
Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reection from
structures, objects, and people.
a Field strengths from xed transmitters, such as base stations for radio (cellular/cordless) and land mobile radios, amateur radio, AM and
FM radio broadcast, and TV broadcast cannot be predicted theoretically with accuracy. To access the electromagnetic environment due
to xed RF transmitters, an electromagnetic site survey should be considered. If the measured eld strength in the location in which the
(equipment or system) is used exceeds the applicable RF compliance level above, the (equipment or system) should be observed to
verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as re-orienting or relocating
the Centurion® Vision System.
b Over the frequency range 150 kHz to 80 MHz, eld strengths should be less than 3 V/m.
Immunity Test
Electrostatic discharge
(ESD)
IEC 61000-4-2
Electrical fast
transient/burst
IEC 61000-4-4
Surge
IEC 61000-4-5
Voltage dips, short
interruptions, and
voltage variations on
power supply input
lines
IEC 61000-4-11
Power frequency
(50/60 Hz)
magnetic eld
IEC 61000-4-8
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
IEC 60601 Test Level
• ±6 kV contact
• ±8 kV air
• ±2 kV for power supply
lines
• ±1 kV for input/output
lines
• ±1 kV differential mode
• ±2 kV common mode
• < 5 % UT (> 95 % dip in
UT) for 0.5 cycle
• 40 % UT (60 % dip in
UT) for 5 cycles
• 70 % UT (30 % dip in
UT) for 25 cycles
• < 5 % UT (> 95 % dip in
UT) for 5 s
3 A/m
3 Vrms
150 kHz to 80 MHz
3 V/m
80 MHz to 2.5 GHz
Compliance Level
• ±6 kV contact
• ±8 kV air
• ±2 kV for power supply
lines
• ±1 kV for input/output
lines
• ±1 kV differential mode
• ±2 kV common mode
• < 5 % UT (> 95 % dip in
UT) for 0.5 cycle
• 40 % UT (60 % dip in
UT) for 5 cycles
• 70 % UT (30 % dip in
UT) for 25 cycles
• < 5 % UT (> 95 % dip in
UT) for 5 s
3 A/m
3 Vrms
3 V/m
Electromagnetic Environment-Guidance
Floors should be wood, concrete, or ceramic tile. If oors
are covered with synthetic material, the relative humidity
should be at least 30 %.
Mains power quality should be that of a typical hospital
(including ambulatory surgery center) environment. To avoid
pre-
mature shutdown due to fast transients avoid powering
the Centurion® Vision System on the same branch circuit
with sources that can generate fast transients (inductive
switching; e.g., high current motors).
Mains power quality should be that of a typical hospital
(including ambulatory surgery center) environment.
Mains power quality should be that of a typical hospital
(including ambulatory surgery center) environment.
If the use of the Centurion® Vision System requires
continued operation during power mains interruptions, it
is recommended that the Centurion® Vision System be
powered from an uninterruptible power supply or a battery.
Power frequency magnetic elds should be at levels
characteristic of a typical location in a typical hospital
(including ambulatory surgery center) environment.
Portable and mobile RF communications equipment should
be used no closer to any part of the Centurion® Vision
System, including cables, than the recommended
separation
distance calculated from the equation applicable to the
frequency to the transmitter.
Recommended separation distance:
d = 1.2√P
d = 1.2 √ P 80 MHz to 800 MHz
d = 2.3 √ P 800 MHz to 2.5 GHz
where P is the maximum output power rating to the
transmitter in watts (W) according to the transmitter
manufacturer and d is the recommended separation
distance in meters (m).
Field strength from xed RF transmitters, as determined
by an electromagnetic site surveya, should be less than
the compliance level in each frequency rangeb.
Interference may occur in the vicinity of
equipment marked with following symbol.
8065751772 1.7
Table 1-3 Recommended Separation Distances Between Portable and Mobile RF Communications
Equipment and the Centurion® Vision System - The Centurion® Vision System is intended
for use in an electromagnetic environment in which radiated RF disturbances are controlled.
The customer or the user of the Centurion® Vision System can help prevent electromagnetic
interference by maintaining a minimum distance between portable and mobile RF
communications equipment (transmitters) and the Centurion® Vision System as recommended
below, according to the maximum output power of the communications equipment.
For transmitters rates at a maximum output power not listed above, the recommended separation distance d in meters (m) can
be estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter manufacturer.
Note 1 - At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
Note 2 - These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reection from
structures, objects, and people.
800 MHz to 2.5 GHz
d = 2.3 √ P
0.23
0.73
2.3
7.3
23
80 MHz to 800 MHz
d = 1.2 √ P
0.12
0.38
1.2
3.8
12
150 kHz to 80 MHz
d = 1.2 √ P
0.12
0.38
1.2
3.8
12
Rated maximum output
power of transmitter
(W)
0.01
0.1
1
10
100
Separation distance according to frequency of transmitter
(m)

1.8 8065751772
Equipment Contains Radio Transmitters
• ZigBee Radio Modular (Communication link with Footswitch, HUD and Media Center)
- Frequency or frequency band of transmission: 2.405 – 2.480 GHz
- Type and frequency characteristics of the modulation: OQPSK (Offset quadrature
phase-shift keying)
- The Effective Radiated Power (ERP): 12.91 dBm (19.54 mW)
• Wireless LAN device (Optional)
-
Frequency or frequency band of transmission: 2.412 – 2.484 GHz and 5.180 - 5.700 GHz
- Type and frequency characteristics of the modulation: OFDM, DSSS, CCK, DQPSK,
DBPSK, 64 QAM, 16 QAM
- The Effective Radiated Power (ERP): 17.09 dBm (51.17 mW)
• Wireless Footswitch Charger
- Frequency or frequency band of charging transmission: 50 kHz
- Frequency or frequency band communication transmission: 115 kHz
- Type and frequency characteristics of the modulation: FSK (Frequency Shift Keying)
- The Effective Radiated Power (ERP): -14.89 dBm (53.18 μW)
USA – Federal Communications Commission (FCC)
This device complies with part 15 of the FCC Rules. Operation is subject to the following
two conditions: (1) This device may not cause harmful interference, and (2) this device
must accept any interference received, including interference that may cause undesired
operation.
CAUTION
Change or modications made to this equipment (including antenna) not expressly
approved by Alcon may void the FCC authorization to operate this equipment.
FCC Radiation Exposure Statement
CAUTION
To ensure that the radio transmitter complies with current FCC regulations limiting
both maximum output RF power and human exposure to radio frequency radiation,
a separate distance of at least 20 cm must be maintained between the unit’s antenna
and the body of the user and any nearby persons at all times, and unit’s antenna
must not be co-located or operating in conjunction with any other antenna or
transmitter.
8065751772 1.9
Canada – Industry of Canada (IC)
This device complies with Industry Canada licence-exempt RSS standards. Operation
is subject to the following two conditions: (1) This device may not cause harmful
interference, and (2) this device must accept any interference, including interference that
may cause undesired operation of the device.
Cet appareil est conforme aux normes d’Industrie Canada RSS exemptes de licence.
Son fonctionnement est soumis aux deux conditions suivantes: (1) Cet appareil ne
doit pas provoquer d’interférences nuisibles, et (2) cet appareil doit accepter toute
interférence, y compris les interférences pouvant provoquer un fonctionnement
indésirable de l’appareil.
Transmitter Antenna:
Under Industry Canada regulations, this radio transmitter may only operate using an
antenna of a type and maximum (or lesser) gain approved for the transmitter by Industry
Canada. To reduce potential radio interference to other users, the antenna type and its
gain should be so chosen that the equivalent isotropically radiated power (e.i.r.p.) is not
more than that necessary for successful communication.
Conformément à la réglementation de l’industrie du Canada, cet émetteur de radio
ne peut être utilisé qu’avec un type d’antenne approuvé pour l’émetteur par Industrie
Canada et seulement avec une valeur de gain inferieur ou égale au gain maximum
approuvé par Industrie Canada. Pour réduire les risques potentiels d’interférence
à autrui, le type d’antenne et son gain doivent être choisis de sorte que la puissance
isotrope rayonnée équivalente (p.i.r.e.) ne dépasse pas la valeur qui est nécessaire
pour une communication réussi.
The radio transmitters, contained in the system, have been approved by Industry Canada
to operate with the antenna types listed below with the maximum permissible gain and
required antenna impedance for each antenna type indicated. Antenna types not included
in this list, having a gain greater than the maximum gain indicated for that type, are
strictly prohibited for use with this device.
Les émetteurs radio, contenues dans le système, ont été approuvés par Industrie
Canada pour fonctionner avec les types d’antenne énumérés ci-dessous et ayant un
gain admissible maximal et l’impédance requise pour chaque type d’antenne. Les
types d’antenne non inclus dans cette liste, ou dont le gain est supérieur au gain
maximal indiqué, sont strictement interdits pour l’exploitation de l’émetteur.
Approved Antennas
Part Number Manufacturer Description Min. Separation
WPC25A Taoglas 2.4 GHz Ceramic Patch Antenna 20 cm
GW.71.5153 Taoglas 2.4 / 5.8 GHz Dipole Antenna 20 cm
ANTB98-061A0 Sansei Denki Sleeve antenna 2.4 / 5 GHz 20 cm
Exposure of Humans to RF Fields:
This device complies with the RF exposure limits for humans as called out in RSS-102.
Cet appareil est conforme aux limites d’exposition RF pour les êtres humains comme
elles le sont notiées dans la norme RSS-102.
1.10 8065751772
Table 1-4 Information on the Location of Hazardous Substances in the Centurion® Vision System -
The Centurion® Vision System contains hazardous substances which could have potential
effect on the environment and human health if disposed of improperly.
Material Location Hazardous Substances Contained
Printed Circuit Board Assembly Lead, Polybrominated Biphenyls (PBB)
Other Electrical / Electronic Device Lead, Polybrominated Biphenyls (PBB)
Cable Assembly Lead
Power Supply Lead, Polybrominated Biphenyls (PBB)
Host PC Module Lead, Polybrominated Biphenyls (PBB)
Liquid Crystal Display Lead
Battery Lead, Lithium, Zn/MnO2
IV Pole Assembly Lead, Polybrominated Biphenyls (PBB)
Remote Control Lead
Fluidics Assembly Lead
Pneumatic Assembly Lead
Europe – R&TTE Directive 99/5/EC
This device complies with the requirements of the Council Directive 99/5/EC (R&TTE).
CAUTION
The radio equipment is intended to be used in all EU and AFTA countries. Outdoor
use may be restricted to certain frequencies and/or may require a license for
operation. Contact local Authority for procedure to follow.
NOTE: Combinations of power levels and antennas resulting in a radiated power
of above 100 mW equivalent isotropic radiated power (e.i.r.p) are considered as not
compliant with the above mentioned directive and are not allowed for use within the
European community and countries that have adopted the European R&TTE directive
1999/5/EC.
For more details on legal combinations of power levels and antennas, contact Alcon
Compliance.
Japan
This device complies with ARIB STD-66 Radio Standard in Japan.
8065751772 1.11
WARNINGS AND CAUTIONS
Many of these warnings are stated elsewhere in this manual; however, for easy reference
they are repeated in greater detail here. If additional information is required, please
contact your local Alcon service representative, or the Technical Services Department.
There are no user serviceable components inside the Centurion® Vision System console
or footswitch. Refer all service issues to your factory-trained Alcon service engineer.
WARNINGS!
The Centurion® Vision System battery can only be serviced by a factory-trained Alcon
service engineer. Access by untrained personnel can lead to injury.
A qualied technician must perform a visual inspection of the following components
every twelve months:
• Warning Labels (see section one of this manual)
• Power Cord
• Fuses
In case of a deciency, do not use the system; call Alcon Technical Services.
A qualied technician must check ground continuity and leakage current every twelve
months to ensure they are within the limits of the applicable standards (for example:
EN60601-1/IEC60601-1). Values must be recorded, and if they are above the limits of
the applicable standards, or 50 % above initial measurement, do not use the system;
call Alcon Technical Services.
If the Centurion
®
Vision System is used at the 220 V - 240 V range in the United States
or Canada, it should be used on a center-tapped, 240 V single phase circuit.
Console isolation from mains is achieved through a two pole power switch. Turn OFF
power switch or unplug the power cord from wall outlet to achieve isolation from mains.
• Do not use the Centurion® Vision System near ammable anesthetics.
Do not exceed maximum capacity of drain bag (500 ml). Excessive pressure can result
from exceeding drain bag maximum capacity and potentially result in a hazardous
condition for the patient.
Inadvertent actuation of Prime or Tune while a handpiece is in the eye can create a
hazardous condition that may result in patient injury.
Keep clear of display base when raising display from stored position to prevent skin,
hair, and /or clothing from being trapped at the base.
The maximum allowable load on the instrument tray is 20 lb. (9 kg).
Place the instrument tray in the stored position prior to transportation to avoid a
situation that could cause the system to tip.
Console might overbalance when it is pushed and its wheels are immobilized (blocked).
Route the footswitch cable, power cord and any other cables connected to the
Centurion® Vision System to avoid tripping.
1.12 8065751772
WARNINGS!
Appropriate use of Centurion® Vision System parameters and accessories is
important for successful procedures. Use of low vacuum limits, low ow rates, low
bottle heights, high power settings, extended power usage, power usage during
occlusion conditions (beeping tones), failure to sufciently aspirate viscoelastic
prior to using power, excessively tight incisions, and combinations of the above
actions may result in signicant temperature increases at incision site and inside
the eye, and lead to severe thermal eye tissue damage.
Good clinical practice dictates testing for adequate irrigation, aspiration ow, reux,
and operation as applicable for each handpiece prior to entering eye.
Ensure that the tubings are not occluded during any phase of operation.
If the handpiece test chamber is collapsed after tuning, there is a potential of low
irrigation ow through the handpiece and may result in a uidic imbalance. This, in
turn, may cause a shallowing or collapsing of the anterior chamber.
Avoid setting the patient above the FMS unless PEL is used. Operating with the patient
above the FMS without PEL adjustment will result in a lower irrigation pressure than
indicated on the display, and possible underventing.
Use of BSS® irrigating uid bags other than those approved by Alcon for use in the
active uidics system can result in patient injury or system damage.
Use of appropriate technique and settings is important to minimize fragments and
turbulence.
Do not remove the FMS during the surgical procedure.
In the event of a system error release footswitch to the up position.
Improper handling or removal of dual irrigation handpiece tip from eye may cause
draining of the uidics system.
CAUTIONS
• Modication of the equipment is NOT allowed without prior authorization
from the manufacturer. If this equipment is modied, appropriate inspection
and testing must be conducted to ensure continued safe use of the equipment.
• Avoid spilling BSS® irrigating solution, or moisture of any kind, around the
electrical handpiece connectors.
• Do not spray any liquid (i.e. cleaning solution or water) upward into the
console vents.
• Do not push or pull the unit by the display, the tray, or the IV pole. Wrapping
around the rear and sides of the system is a handle provided for moving the
instrument. The unit should be pulled and not pushed, especially over elevator
and door thresholds.
8065751772 1.13
Handpiece Care
Phaco handpieces are surgical instruments and must be handled with care. The
handpiece tip should not touch any solid object while in operation. Immediately
following surgery the handpiece must be thoroughly cleaned. Be sure handpiece
connector is completely dry before connecting it to console. For cleaning and
sterilization procedures, see the Directions for Use (DFU) supplied with the
handpiece.
WARNINGS!
If in the medical opinion of the physician a patient with a prion related disease
undergoes a high risk procedure, the instrument should be destroyed or be processed
according to local requirements.
Use of a phaco handpiece in the absence of irrigation ow and/or in the presence of
reduced or lost aspiration ow and/or sideways orientation of the Kelman® and OZil® 1 2
tips can cause excessive heating and potential thermal injury to adjacent eye tissues.
Appropriate use of Centurion® Vision System parameters and accessories is important
for successful procedures. Use of low vacuum limits, low ow rates, low bottle
heights, high power settings, extended power usage, power usage during occlusion
conditions (beeping tones), failure to sufciently aspirate viscoelastic prior to using
power, excessively tight incisions, and combinations of the above actions may result
in signicant temperature increases at incision site and inside the eye, and lead to
severe thermal eye tissue damage.
Use of an ultrasonic handpiece other than an OZil® torsional handpiece, or use of a
handpiece repaired without Alcon authorization, is not permitted, and may result in
patient injury, including potential shock hazard to patient and/or operator.
The U/S tips supplied in the Centurion® Vision System pack are only to be used on
an OZil® torsional handpiece. Each U/S tip is intended to be used only once per case,
and then disposed of according to local governing ordinances.
Mismatching U/S tips and infusion sleeves may create potentially hazardous uidic
imbalances.
Directing energy toward non-lens material, such as iris or capsule, may cause
mechanical and/or thermal tissue damage.
Perform visual inspection of accessories for burs or bent tips prior to use.
Use of appropriate technique and settings is important to minimize fragments and
turbulence.
1.14 8065751772
CAUTIONS
Never ultrasonically clean the phaco handpiece; irreparable damage may result.
Prior to sterilization, the phaco handpiece should always have the connector end
cap secured and placed in the sterilization tray. This will prevent damage to the
connectors and handpieces during handling, and especially during autoclaving.
The phaco handpiece and INTREPID®
AutoSert®
IOL Injector must be at room
temperature just before use. Allow the handpiece to air cool for at least 15
minutes after autoclaving; never immerse the handpiece in liquid when hot.
Do not operate the phaco handpiece unless the tip is immersed in BSS® sterile
irrigating solution or distilled water or is in surgical use. Irreparable damage to
the handpiece and tip can result if run dry.
Ensure that test chamber is lled with BSS® sterile irrigating solution before
tuning the phaco handpiece. Tuning a handpiece dry may result in premature tip
failure and breakage.
Quenching a hot handpiece in water can cause damage and will void warranty.
Be sure handpiece is completely dry before connecting it to console. Damage to
handpiece and console may result if plugged in when wet.
Handpiece Tips
Ensure that handpiece tip is fully tightened to the handpiece. If not securely attached,
an error may be generated and/or inadequate tuning will occur. Ensure that the tip is
not too tight so that it can be removed after use.
Use of a tool other than tip wrenches supplied by Alcon may cause damage to the tip
and/or handpiece.
WARNING!
Poor clinical performance will result if tip is not secured tightly to the handpiece.
During any ultrasonic procedure, metal particles may result from inadvertent
touching of the ultrasonic tip with a second instrument. Another potential source
of metal particles resulting from any ultrasonic handpiece may be the result of
ultrasonic energy causing micro abrasion of the ultrasonic tip.
8065751772 1.15
Ultraow® II (I/A) Handpiece
Prior to each procedure inspect the two O-rings where the tip screws onto the
Ultraow® II I/A handpiece. If damaged or missing, replace the o-rings. If in doubt,
contact Alcon's Technical Services Department.
WARNINGS!
Use of non-Alcon surgical reusable or disposable I/A handpieces that do not meet
Alcon surgical specications, or use of an Alcon handpiece not specied for use with
the Centurion® Vision System, may result in a uidic imbalance. This, in turn, may
cause a shallowing or collapsing of the anterior chamber.
Exceeding the recommended level of 100 mmHg (133 hPa) with a 0.5 mm or larger
I/A tip may cause anterior chamber shallowing and/or incarceration or tearing of
posterior capsule.
I/A tips are not to be used with a phaco handpiece.
Recommended Vacuum Range for I/A Tips
It is important that only the proper size I/A tip be used when operating with maximum
vacuum. Only 0.2 mm or 0.3 mm I/A tips should be used with vacuum limits above
100 mmHg (133 hPa). I/A adjustable vacuum range is 0 - 700 mmHg (0 - 933 hPa).
Centurion® Vitrectomy Probe
The vitrectomy probe, a guillotine vitreous cutter, is intended for single use only.
Vitrectomy cutting performance may vary at high altitudes. Consult Alcon Technical
Service for additional information.
WARNINGS!
Do not test or operate vitrectomy probe unless tip of probe is immersed in BSS®
sterile irrigating solution or distilled water or is in surgical use. Irreparable
damage to the probe and tip can result if run dry.
Perform visual inspection of accessories for burs or bent tips prior to use.
Connect pneumatic tubing connectors from vitrectomy probe to console prior to
initiating prime of probe. Initiating prime of the vitrectomy probe, or running the
vitrectomy system, with one or both pneumatic connectors disconnected may cause
the ow of non-sterile air over the sterile eld for a brief moment.
Do not use vitrectomy probes that are not approved for use on Centurion® system.
After lling and testing, and before surgical use, verify that the probe is properly
actuating and aspirating. This may require lowering cut rate to achieve good
visualization. The port should always remain in open position in footpedal position 1.
If cutting port is partially closed while in position 1, replace the probe. Prior to entry
into the eye, and with tip of probe in sterile irrigating solution, the surgeon
should step on the footpedal for visual verication that the probe is cutting:
• If the cutter is observed to not fully close, or does not move when the probe
is actuated, replace the probe.
• If cutting port is partially closed while idle, replace the probe.
• If air bubbles are observed in the aspiration line or exiting the probe tip during
priming, replace the probe.
• If a reduction of cutting capability or vacuum is observed during the surgical
procedure, stop immediately and replace the probe.
1.16 8065751772
INTREPID® AutoSert
®
IOL Injector
CAUTIONS
•
Do not ultrasonically clean the AutoSert
®
IOL Injector connector. Ultrasonic
cleaning will cause irreparable damage.
• Use care when handling
AutoSert
®
IOL Injector, particularly when cleaning. Always
clean handpiece over a surface cushioned with a pad or rubber mat.
• Be sure handpiece cable connector is dry before connecting it to the console.
• Do not disconnect cable connector from Centurion® system console until the IOL
Injector plunger is fully retracted.
• Do not immerse the AutoSert
®
IOL Injector in any uid when the plunger is not
retracted.
• As part of a properly maintained surgical environment, it is recommended that
a backup IOL injector be made available in the event the AutoSert
®
IOL injector
handpiece does not perform as expected.
WARNINGS!
• The AutoSert
®
IOL Injector is non-sterile and must be cleaned and sterilized prior
to, and immediately after, each use.
• Never immerse the AutoSert
®
IOL Injector in liquid after autoclaving; allow it to
air cool for at least 15 minutes. Quenching could result in a potentially hazardous
condition for the patient.
• The AutoSert
®
IOL Injector delivery system is for the implantation of Alcon qualied
AcrySof
®
foldable IOLs. Unqualied lenses shall not be used with the system. See
DFU, or contact your Alcon representative, for qualied lens/cartridge combinations.
• The cartridge/IOL combination listed in the DFU, along with Alcon settings, has been
validated per section 5 of BS EN ISO 11979-3:2006. Appropriate use of AutoSert
®
IOL Injector settings is important for successful IOL implantation. Inappropriate
use of settings may lead to a potentially hazardous condition for the patient.
Aspiration/Vacuum Adjustments
Adjusting aspiration rates or vacuum limits above the preset values may result in
aspiration levels (volumes) exceeding irrigation inow.
WARNING!
Adjusting aspiration rates or vacuum limits above the preset values, or lowering the
IOP or IV pole below the preset values, may cause chamber shallowing or collapse
which may result in patient injury.
8065751772 1.17
Presurgical Check-out Tests
Presurgical check-out tests must be performed as outlined in the Operating Instructions
section. If an Event message is displayed on the front panel, refer to the Troubleshooting
section of this manual. If the problem persists, DO NOT PROCEED.
WARNINGS!
When lling handpiece test chamber, if stream of uid is weak or absent, good
uidics response will be jeopardized. Good clinical practice dictates the testing for
adequate irrigation and aspiration ow prior to entering the eye.
Ensure that tubings are not occluded or pinched during any phase of operation.
Perform visual inspection of accessories for burrs or bent tips prior to use.
IV Pole
WARNINGS!
Keep clear of the IV pole when it is in motion to prevent skin, hair, and/or clothing
from being trapped in the IV pole mechanism. The IV pole moves during power on/
off, priming, and bottle height adjustment.
IV pole rises automatically. To avoid stretching drip chamber tubing, and possibly
pulling drip chamber out of bottle, tubing must hang freely with no interference.
When out of use, remove uid bottle from IV pole and ip bottle hanger into its storage
position to avoid injury.
Empirical numbers for bottle heights are not a replacement for competent surgical
technique. The surgeon should visually and physically monitor intraocular pressure.
Footswitch
If required, the footswitch may be wiped with alcohol, mild soap and water, or any
germicidal solution that is compatible with the plastic parts.
WARNING!
Route the footswitch cable properly to avoid tripping.
CAUTIONS
Do not clean the footswitch using solvents, abrasives, or any cleaner that is not
compatible with plastic parts made of GE Cycoloy CU 6800 and LEXAN 920A.
Damage may result.
Never pick up or move the footswitch by the cable. Dropping or kicking the
footswitch can cause irreparable damage.
1.18 8065751772
Occlusion Tones
Two different occlusion tones (intermittent beeping tones during occlusion) indicate
that the vacuum is near or at its preset limit, and aspiration ow is reduced or stopped
to avoid exceeding the limit. The rst type, the I/A occlusion tone, sounds when
occlusion occurs during aspiration only (in the absence of ultrasonic power). The
I/A occlusion tone is a lower, intermittent single beep. The second type of occlusion
tone, the phaco occlusion tone, is a higher, intermittent double beep, and sounds when
occlusion occurs during application of ultrasonic power.
The I/A occlusion and phaco occlusion tones indicate that the vacuum has reached its
maximum allowed preset value. The I/A occlusion tone can be turned off, while the
phaco occlusion tone cannot be turned off.
WARNINGS!
The phaco occlusion bell indicates no aspiration ow. Use of high U/S settings and/or
prolonged use may lead to thermal injury.
Use of the phaco handpiece in the absence of irrigation ow and/or in the presence
of reduced or lost aspiration ow can cause excessive heating and potential thermal
injury to adjacent eye tissues.
In the event of a persistent loss of aspiration during the application of U/S power,
remove U/S power via footswitch control.
Vacuum Tone
A vacuum tone is provided. The pitch will vary relative to the amount of vacuum. A
high vacuum can indicate that little to no ow is occuring. This tone can be reduced
in volume, but not turned off.
WARNINGS!
A moderate to high vacuum tone may indicate little to no ow is occuring. Use of the
phaco handpiece in the absence of irrigation ow and/or in the presence of reduced
or lost aspiration ow can cause excessive heating and potential thermal injury to
adjacent eye tissues.
Do not exceed maximum capacity of drain bag (500 ml). Excessive pressure can result
from exceeding drain bag maximum capacity and potentially result in a hazardous
condition for the patient.
In the event of a persistent loss of aspiration during the application of U/S power,
remove U/S power via footswitch control.
Cautery, Diathermy, Coagulation Denition
The Centurion® Vision System uses the word “Coagulation” in place of Cautery or
Diathermy, based on the following denition:
Coagulation - Isolated, bipolar, high frequency current supplied to conductors (e.g.
forceps). Current passes between these electrodes, halting bleeding. (Abbreviated
“Coag” in some of the text of this operator’s manual.)
8065751772 1.19
Coagulation Function
Listed below are general precautions to be followed when using the Coagulation function:
• To ensure safe operation of the coagulation function, only approved cables
and accessories must be used (See your Alcon representative). Coagulation
performance can be guaranteed only when using Alcon components or Alcon-
endorsed components.
• To reduce the risk of accidental burns, caution should always be taken when
operating high-frequency surgical equipment.
• Interference produced by the operation of high-frequency surgical equipment may
adversely inuence the operation of other electronic equipment.
• Accessories should be checked regularly; electrode cables should particularly be
checked for possible damage to the insulation.
• Operation of the coagulation step is limited to extraocular uses only.
• The lowest power level in coagulation step should always be selected for the
intended purpose.
• Skin-to-skin contact (for example between the arms and body of the patient)
should be avoided, for example by insertion of dry gauze.
• When HF (high frequency) surgical equipment and physiological monitoring
equipment are used simultaneously on the same patient, any monitoring electrodes
should be placed as far as possible from the surgical electrodes. Needle monitoring
electrodes are not recommended.
• In all cases, monitoring systems incorporating high frequency current-limiting
devices are recommended.
• The cables to the surgical electrodes should be positioned in such a way that
contact with the patient or other leads is avoided.
• Temporarily unused active electrodes should be stored so that they are isolated
from the patient.
• The use of ammable anaesthetics or oxidizing gases such as nitrous oxide (N2O)
and oxygen should be avoided if a surgical procedure is carried out in the region of
the thorax or the head, unless these agents are sucked away.
•
Non-ammable agents should be used for cleaning and disinfection wherever possible.
• Flammable agents used for cleaning or disinfecting, or as solvents of adhesives,
should be allowed to evaporate before the application of HF surgery. Some
materials, for example cotton, wool and gauze, when saturated with oxygen may
be ignited by sparks produced in normal use of the HF surgical equipment.
• Accessories should have a rated voltage equal to or greater than the maximum
coagulation output voltage.
WARNINGS!
Do not use the coagulation function on patients with pacemakers or implanted
debrillatory devices. If electrosurgery is used on patients with implanted cardiac
pacemakers or debrillatory devices or pacemaker electrodes, be aware that
irreparable damage to the pacemaker or debrillatory device and its function
may occur and lead to ventricular brillation. Please check with the pacemaker
or debrillatory device manufacturers for their recommendations.
Failure of the HF surgical equipment (coagulation circuitry) could result in an
unintended increase of output power.
1.20 8065751772
VideOverlay System
WARNINGS!
Do not remove VideOverlay cover; there are no user-serviceable parts inside.
Refer servicing to qualied service personnel.
Do not simultaneously touch the VideOverlay enclosure and the patient.
CAUTIONS
• Do not use multiple portable socket outlets with this system.
• Use only the Alcon-supplied serial cable to connect the Centurion® Vision
System to the VideOverlay System.
8065751772 1.21
Consumable Packs
Consumable items used with the Centurion® Vision System during surgery are
designed to be used once and then discarded, unless labeled otherwise.
All Centurion® packs contain Directions for Use (DFU). It is important to read and
understand the DFU’s prior to use.
In all cases, the instrument setup instructions contained in the manual should be
thoroughly understood prior to using any of the pack congurations.
NOTE: If an inconsistency exists between the instructions in the operator’s
manual and the Directions For Use (DFU) supplied with a consumable pack or
accessory, follow the DFU.
WARNINGS!
Mismatch of consumable components and use of settings not specially adjusted for
a particular combination of consumable components may create a patient hazard.
Do not use packs that have exceeded the expiration date.
Sterile disposable medical devices should not be reused! These components have
been designed for one time use only; do not reuse.
Potential risk from reuse or reprocessing the following products labeled for single
use include:
• Bipolar Coagulation Instruments - Thermal injury or electrical shock caused by a
damaged bipolar instrument, and foreign particle introduction into the eye.
• Fluid Management Components - Fluid path leaks or obstruction resulting in reduced
uidics performance, and foreign particle introduction into the eye.
• Phacoemulsication Tips - Reduced tip cutting performance, presence of tip burrs,
uid path obstruction, and foreign particle introduction into the eye.
• Vitreous Cutting Instruments - Reduced vitreous cutting performance, uid path
obstruction, and foreign particle introduction into the eye.
The equipment used in conjunction with the Alcon disposables constitutes a complete
surgical system. Use of disposables other than Alcon disposables may affect system
performance and create potential hazards, and if it is determined to have contributed
to the malfunction of the equipment under contract, could result in the voidance of
the contract and/or invoicing at prevailing hourly rates.
Perform visual inspection of accessories for burs or bent tips prior to use.
Do not remove the FMS during the procedure.
Do not use any Vitrectomy probes that have not been approved for use on the
Centurion® Vision System.

1.22 8065751772
PRODUCT SERVICE
For product service, please contact Alcon’s Technical Services Department at the
number provided below.
Operators experiencing problems with the system should refer to the Operating
Instructions and Troubleshooting sections of this manual. A problem which persists
should be referred to the Alcon Technical Services Department or your local
authorized service representative.
For optimum performance, it is the user’s responsibility to schedule preventive
maintenance service on the system and its accessories a minimum of one time per
year. Additional preventive maintenance may be required based upon system use.
Alcon’s Field Service Engineers are trained and equipped to provide the highest
quality of workmanship.
Safety performance should be veried by the user (e.g., qualied service personnel) at
least twice a year. Ground resistance, leakage current, and dielectric withstand voltage
must be checked to appropriate national standard.
To avoid unnecessary shipping, please contact your Alcon Technical Services
Department prior to return of any system or accessories. If return of the equipment is
deemed necessary, a Return Material Authorization will be issued with appropriate
shipping instructions.
Alcon Technical Services Department
15800 Alton Parkway
Irvine, California 92618-3818
(800) 832-7827, or (949) 753-1393
8065751772 1.23
LIMITED WARRANTY
Alcon will repair or replace at its option, any system or accompanying accessories
found to be defective in material and/or workmanship for a period of one (1) year
from the date of initial installation. This warranty applies to the original purchaser
of the system, when said system is properly installed, maintained, and operated in
accordance with published instructions.
Alcon shall not be obligated to provide services under this warranty for damage to
or destruction of systems covered where such damage or destruction is a result of
or caused by re or explosion of any origin, riot, civil commotion, aircraft, war, or
any Act of God including, but not limited to lightning, windstorm, hail, ood or an
earthquake.
This warranty does not cover damage resulting from service repair or other alteration
by any person other than an Alcon-authorized service person, and any warranties
provided by Alcon with respect to this equipment shall become void and of no further
force and effect if this equipment is serviced by anyone other than Alcon-authorized
service personnel. In particular, Alcon shall have no obligation to replace, repair or
credit customer’s account for the cost of the equipment, which has been subject to
service or other alteration by persons other than Alcon-authorized service personnel.
The express warranty above is the sole warranty obligation of Alcon, and the
remedy provided above is in lieu of any and all other remedies. There are no other
agreements, guarantees, or warranties – oral or written, expressed or implied –
including , without limitation, warranties of merchantability or tness for a particular
purpose. Alcon shall have no liability whatsoever for any incidental or consequential
damages arising out of any defect, improper use, or unauthorized service or repair.
WARNING!
The consumable products used in conjunction with ALCON® instrument
products constitute a complete surgical system. Use of consumable products
and handpieces other than those manufactured by Alcon may affect system
performance and create potential hazards. If it is determined that consumable
products or handpieces not manufactured by Alcon have contributed to the
malfunction of the equipment during warranty period, service will be provided
at prevailing hourly rates.
8065751772 1.25
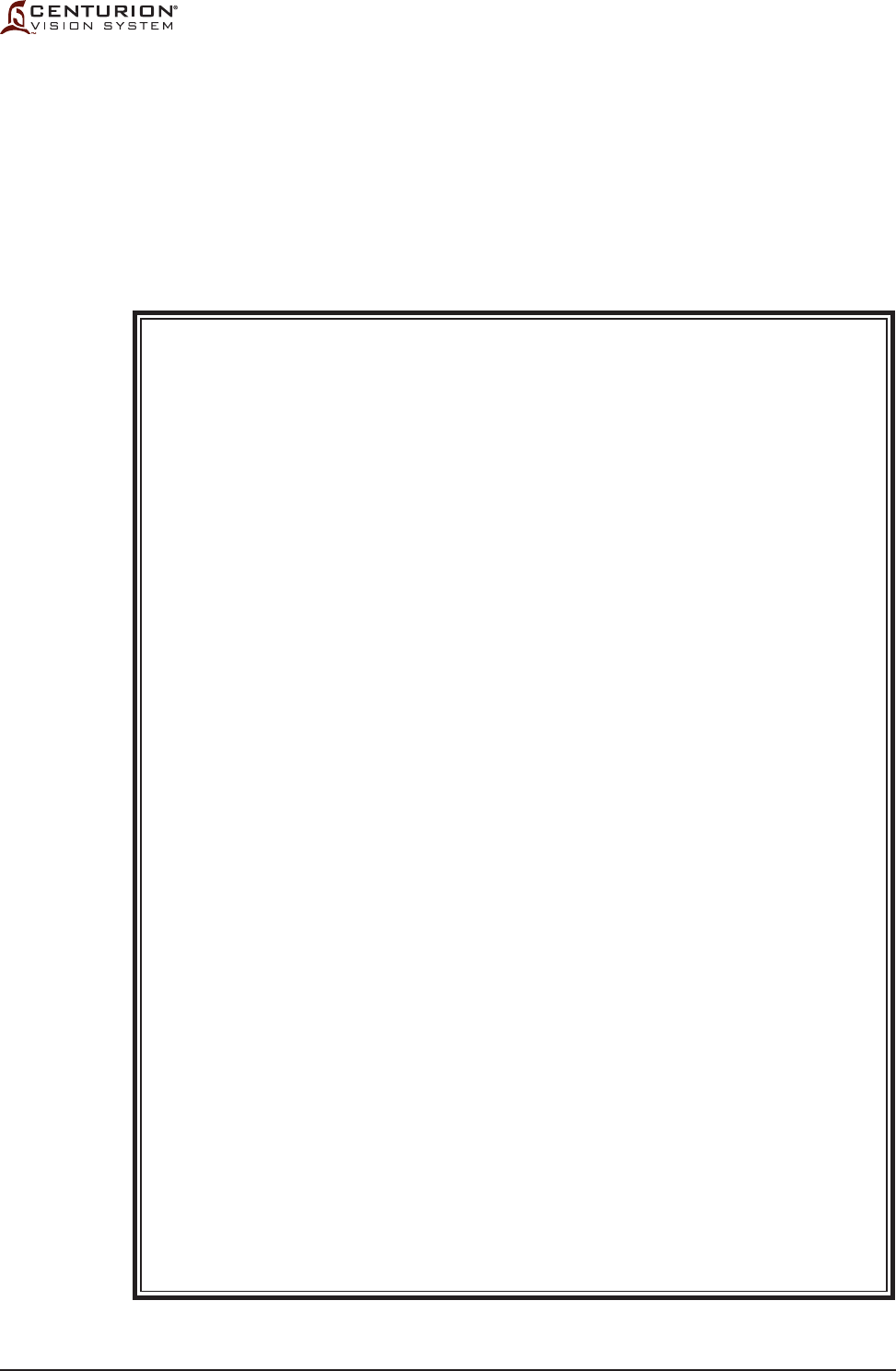
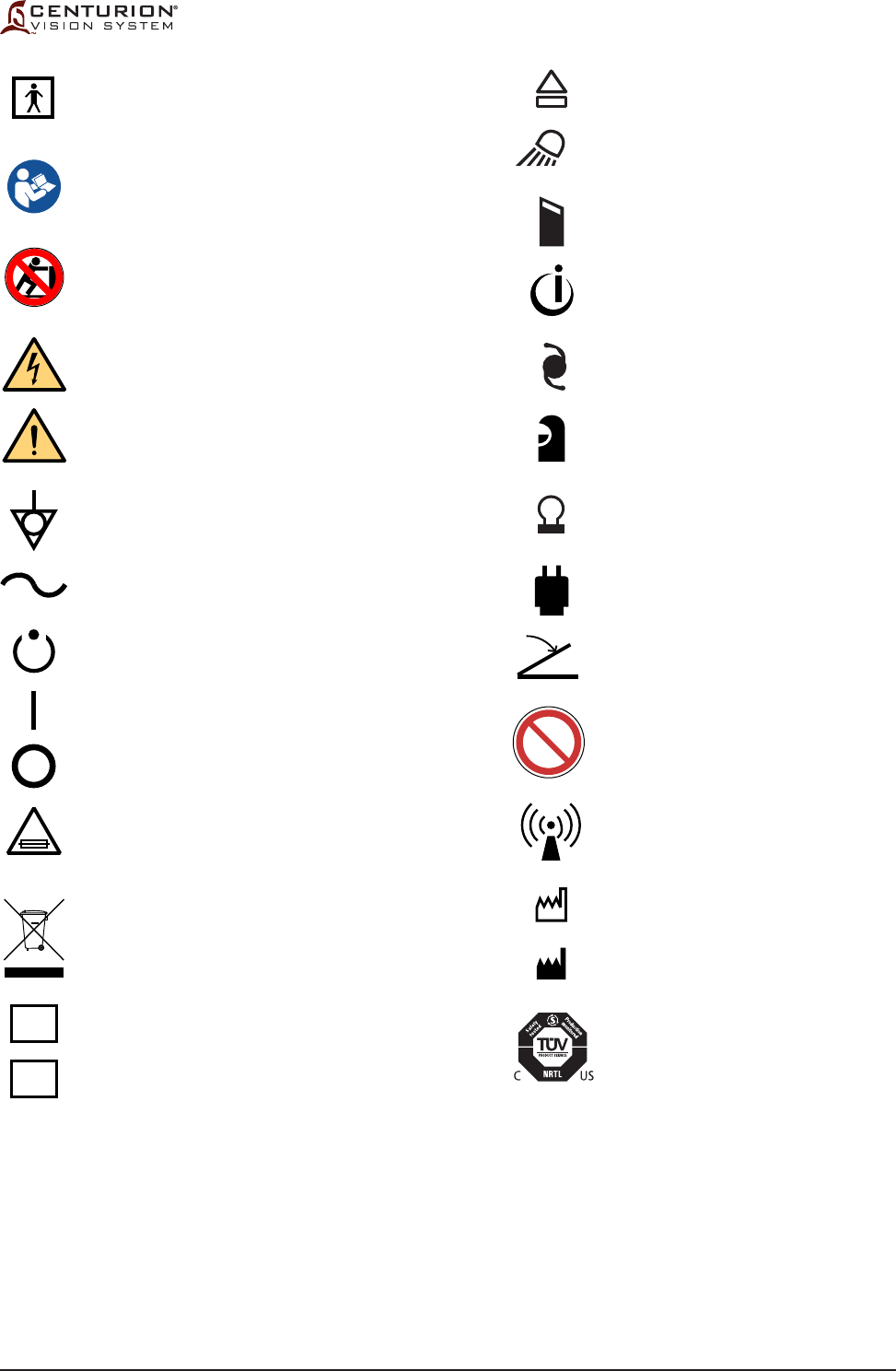
Figure 1-2 ICONS USED WITH CENTURION® VISION SYSTEM - Icons identifying modes, functions,
etc., that are used with the Centurion® Vision System are identied in this chart. The icons shown on
this page are for reference only.
Eject FMS
Lamp to illuminate instrument tray
Connector for
CENTURION® OZil® handpiece
Connector for
INFINITI® OZil® handpiece
Connector for
INTREPID® AutoSert® IOL Injector
Connector for
Vitrectomy probe tubing
Connector for
Autocapsulorhexis handpiece
Connector for
Coagulation handpiece
Connector for
Cabled Footswitch
Magnetic Resonance Unsafe
Non-ionizing electromagnetic radiation
Date of Manufacture
Manufacturer
OSHA recognized NRTL
, TUV SUD
America mark, providing electrical
safety
certification to North American
requirements for Medical Devices.
Type BF equipment, providing both the
attributes of basic insulation and "floated"
isolation.
Follow Instructions for Use
(white figure on blue background)
WARNING: The console might
overbalance when it is pushed and its
wheels are immobilized (blocked)
(black symbol behind lined out red circle)
WARNING: Dangerous Voltage
(black symbols on yellow background)
GENERAL WARNING
(black symbols on yellow background)
Equipotential ground connection
AC Voltage
Power stand-by state
for a part of equipment
ON (POWER)
OFF (POWER)
Fuse Size, Type, and Rating
Use appropriate take-back system
(see Environmental Considerations in this manual)
Pb notation, if present, indicates lead
content greater than 0.004%.
Catalog Number
Serial Number
Pb
SN
REF
T10.0AH/250V
MR
1.26 8065751772
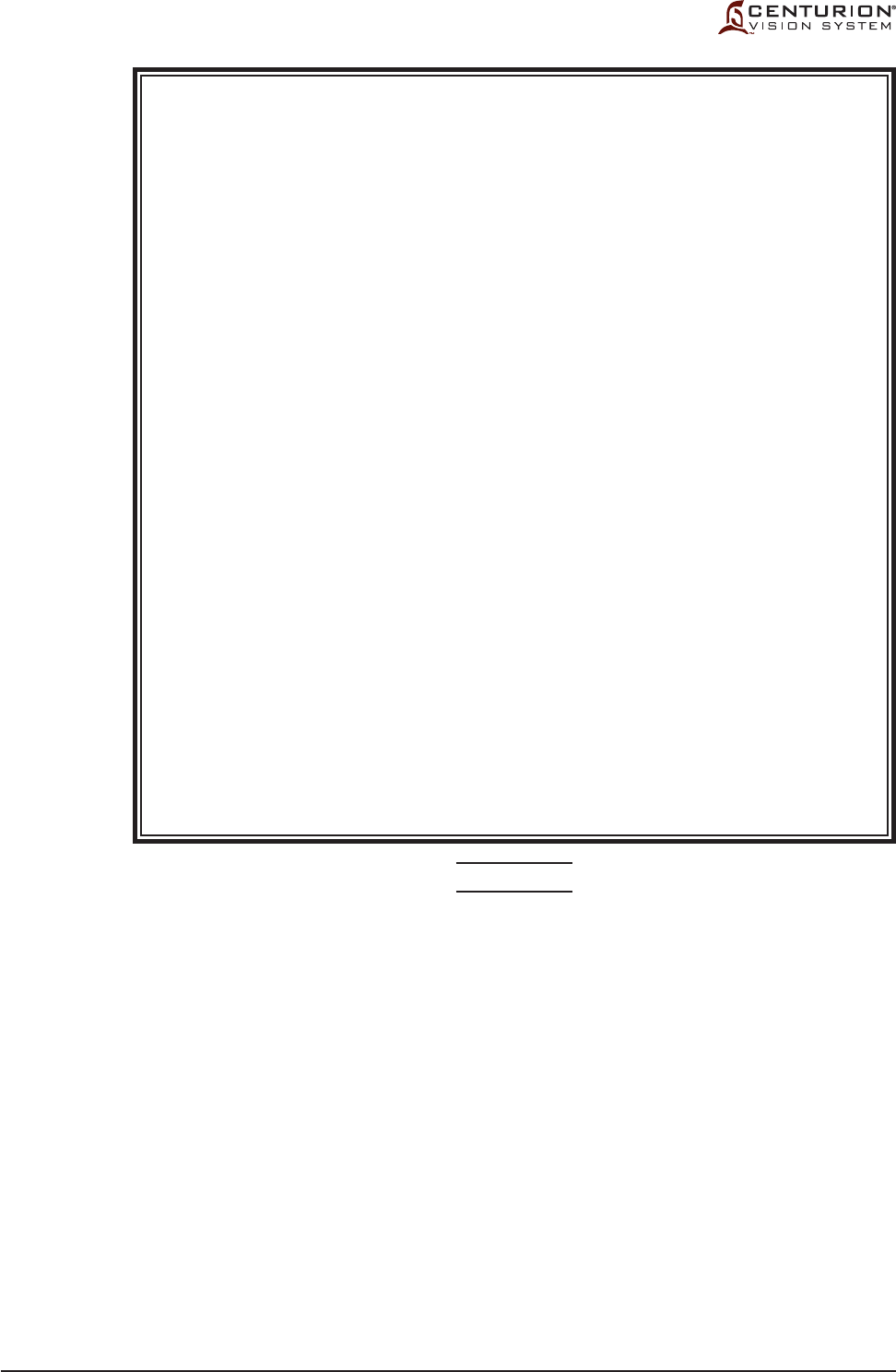
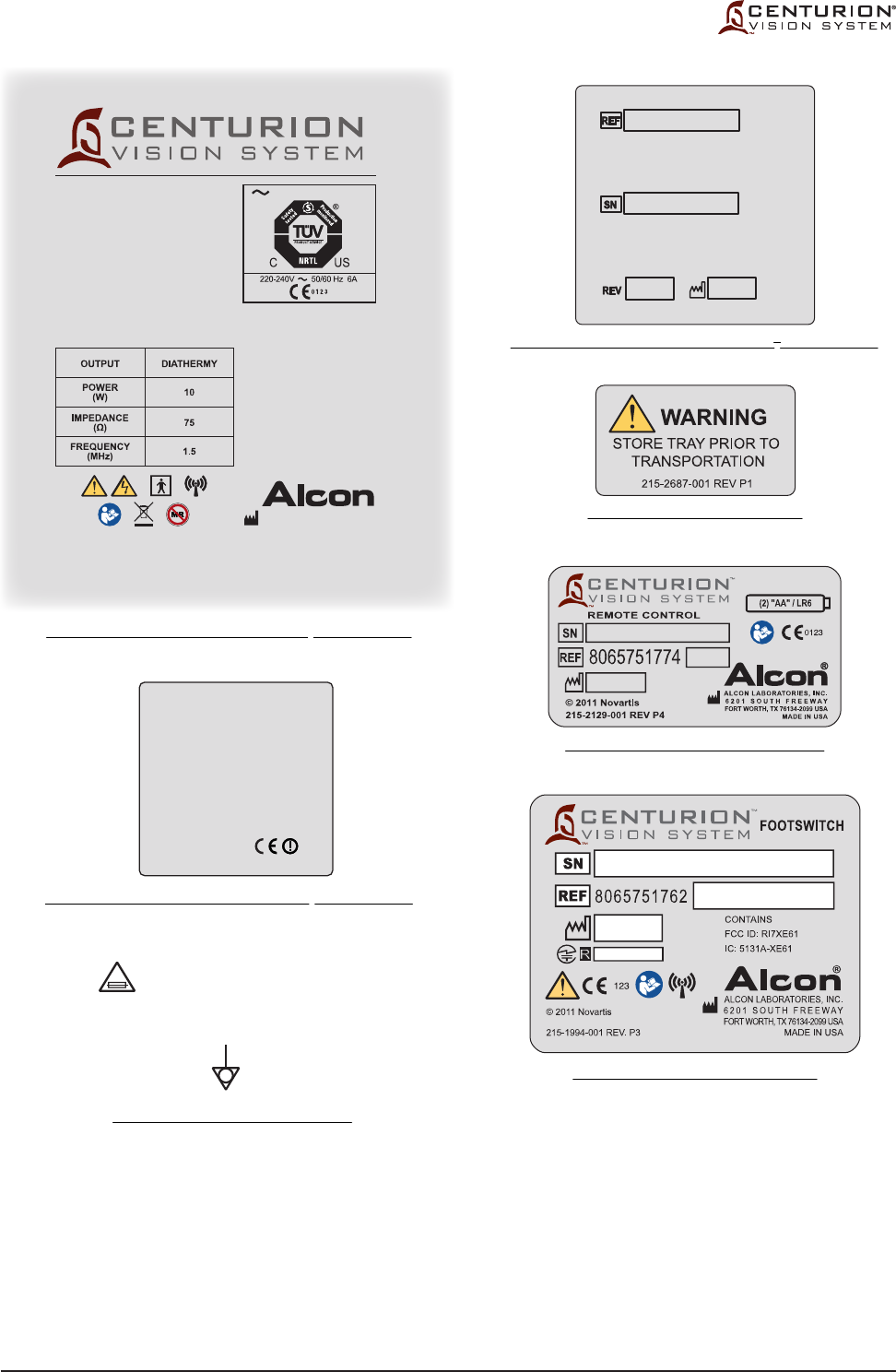
Figure 1-3 LABELING ON CENTURION® VISION SYSTEM - Labels used on the Centurion® Vision
System are illustrated here.
The labels on this page are intended for reference only.
215-2216-001 REV P0
Label printed on rear panel of Centurion® Vision System
Label located on rear panel of Centurion® Vision System
Label printed on power input module
Label located on rear panel of Centurion® Vision System
Label located on back of remote control
Label located on bottom of footswitch
Label located on instrument tray
T10.0AH/250V
WARNING:
FOR CONTINUED
PROTECTION AGAINST RISK OF FIRE,
REPLACE ONLY WITH SAME TYPE
AND RATING OF FUSE.
IPX8
IPX1
®
© 2012 Novartis
220-240V 50/60 Hz 6A
100-120V 50/60 Hz 10A
For applicable patents, please see the ABOUT
screen on the monitor during operation.
ALCON LABORATORIES, INC.
6201 SOUTH FREEWAY
FORT WORTH, TX 76134-2099 USA
MADE IN USA
®
®
DANGER:
DANGER:
CAUTION:
CAUTION:
RISK OF EXPLOSION IF USED IN THE
PRESENCE OF FLAMMABLE
ANESTHETICS.
RISQUE D’EXPLOSION. NE PAS
EMPLOYER EN PRESENCE
D’ANESTHESIQUES INFLAMMABLES.
GROUNDING RELIABILITY CAN ONLY
BE ACHIEVED WHEN EQUIPMENT IS
CONNECTED TO AN EQUIVALENT
RECEPTACLE MARKED HOSPITAL
GRADE.
RISK OF BURNS AND FIRE - DO NOT
USE NEAR CONDUCTIVE MATERIALS.
RENEW ELECTRODE CABLES UPON
EVIDENCE OF DETERIORATION.
This device complies with part 15 of the FCC
rules. Operation is subject to the following two
conditions: (1) This device may not cause
harmful interference, and (2) this device must
accept any interference received, including
interference that may cause undesired
operation
FCC ID: VMCNGPFSW1
IC: 7345A-NGPFSW1
Contains:
FCC ID: RI7XE61
IC: 5131A-XE61
FCC ID: N6C-SDMAN
IC: 4908B-SDMAN
215-2985-001 REV P0

1.28 8065751772
LAST PAGE OF THIS SECTION
THIS PAGE INTENTIONALLY BLANK