Biomedical Systems BRHA02 HANDHELD MOBILE TELEMETRY DEVICE User Manual Operations Manual
Biomedical Systems Corporation HANDHELD MOBILE TELEMETRY DEVICE Operations Manual
Operations Manual
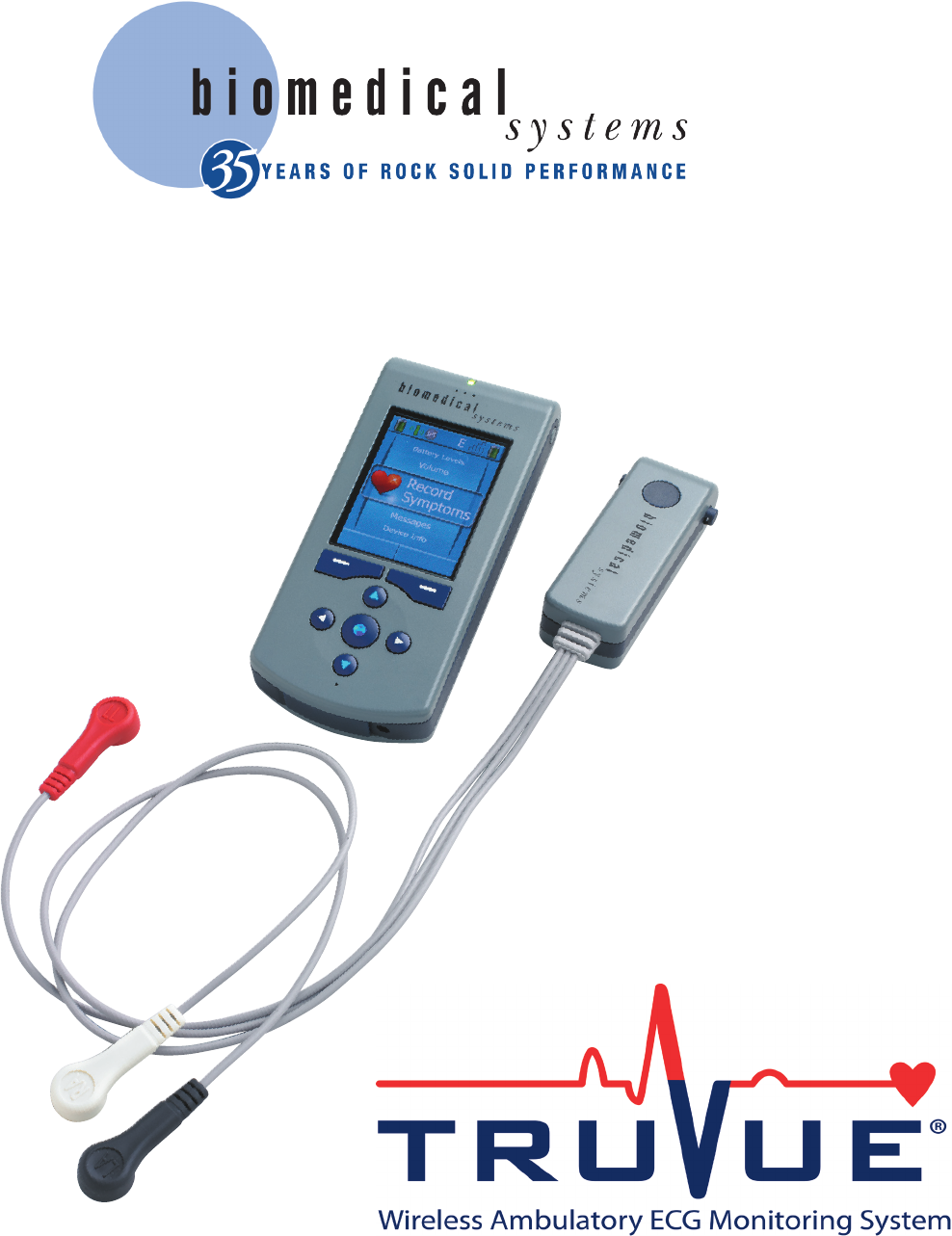
Biomedical Systems
77 Progress Parkway • Saint Louis, MO 63043
www.biomedsys.com/truvue
Toll Free 1-800-877-6334
Physician’s Operation Manual
2

3
TruVue Mobile Telemetry Monitoring System
TABLE OF CONTENTS
Indications for Use ........................................................................................................................4
System Overview ...........................................................................................................................5
Patient Devices ...................................................................................................................................6
Data and Monitoring Center..........................................................................................................7
Service Overview ...........................................................................................................................8
Ordering TruVue .................................................................................................................................8
Initiating Monitoring ........................................................................................................................9
Concluding Monitoring ................................................................................................................ 10
Breaks in Monitoring ..................................................................................................................... 10
Operation and Performance Specications .......................................................................11
Sensor Operation ............................................................................................................................ 11
Sensor Performance Specications ..........................................................................................13
Handheld Operation ...................................................................................................................... 15
Handheld Performance Specications ................................................................................... 24
Algorithm Operation and Performance ................................................................................. 26
Description of Device Symbols ..............................................................................................30
Summary of Caution Statements ..........................................................................................31

PHYSICIAN’S OPERATION MANUAL
TruVue Indications of Use
4
TruVue Indications for Use
Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician.
Indications for Use:
The TruVue® System is intended for use by patients who experience transient events
that may suggest cardiac arrhythmia.
Patients who require monitoring of eect of drugs to control ventricular rate in various
atrial arrhythmias (e.g. atrial brillation).
Patients with symptoms that may be due to cardiac arrhythmias. These may include
but are not limited to symptoms such as: a) dizziness or lightheadedness; b) syncope
of unknown etiology in which arrhythmias are suspected or need to be excluded; and
c) dyspnea (shortness of breath).
Patients recovering from cardiac surgery who are indicated for outpatient arrhythmia
monitoring.
ECG data recorded by the device can be analyzed by other processing systems, such as
the BMS Century Holter system to provide Holter style reports.
Contraindications :
The TruVue System is contraindicated for those patients requiring attended, In-hospital monitoring for life
threatening arrhythmias.
This device complies with part 15 of the FCC Rules. Operation is subject to the following two conditions:
(1) This device may not cause harmful interference, and (2) this device must accept any interference received,
including interference that may cause undesired operation.
NOTICE: Changes or modications to this equipment not expressly approved by
Biomedical Systems may void the FCC authorization to operate this equipment.
Sensor FCC ID: YCVBRSA01
Handheld FCC ID: YCVBRHA01 / YCVBRHA02
Copyright © 2012 Biomedical Systems. All Rights Reserved.
Patent Pending. Biomedical Systems reserves the right to change specications at any time without notice.
5
PHYSICIAN’S OPERATION MANUAL
TruVue System Overview
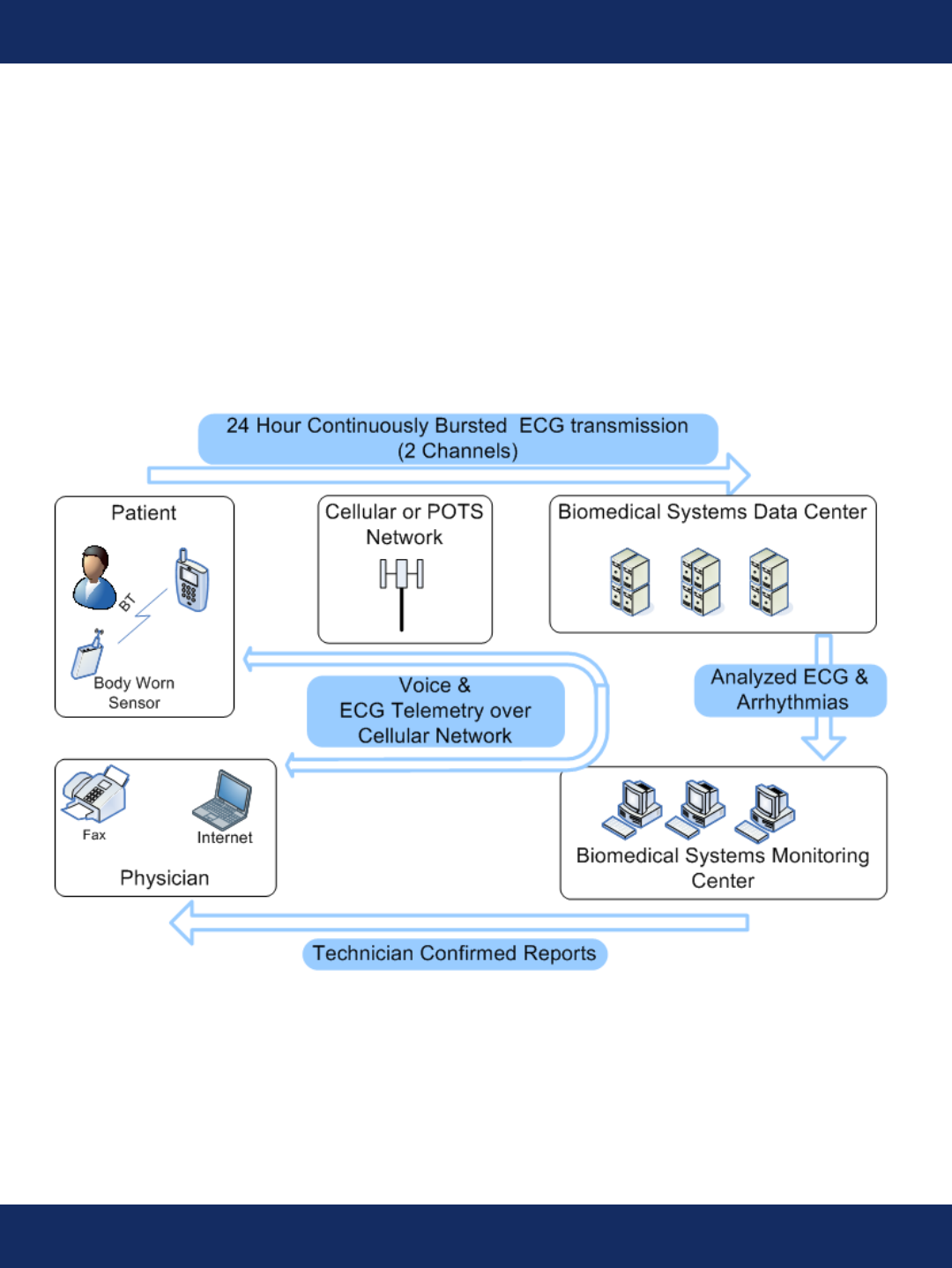
System Overview
The TruVue system is a wireless ECG analysis and monitoring system used for the diagnosis of cardiac
arrhythmia in ambulatory patients. ECG data is acquired from the patient on a body worn sensor,
stored and then transmitted to a data center through a handheld device carried with the patient. No
action is required by the patient to transmit ECG data. At the data center, all ECG is stored and then
analyzed for arrhythmia. Portions of the ECG containing arrhythmic events are transmitted to our
monitoring center for human conrmation before being compiled into a report and transmitted to the
physician. The system also allows for real time 2-way communications of voice and data between the
patient and the monitoring center.
5
TruVue System Overview
System Overview
The TruVue system is a wireless ECG analysis and monitoring system used for the diagnosis of cardiac
arrhythmia in ambulatory patients. ECG data is acquired at the patient on a body worn sensor, stored
and then transmitted to a data center through a handheld device carried with the patient. No action is re-
quired by the patient to transmit ECG data. At the data center, all ECG is stored and then analyzed for
arrhythmia. Portions of the ECG containing arrhythmic events are transmitted to monitoring center for
human confirmation before being compiled into a report and transmitted to the physician. The system
also allows for real time 2-way communications of voice and data between the patient and the monitor-
ing center or physician.
CAUTION: The TruVue system is not an emergency response device. The patient should call 911 and/or
their local emergency medical service if they feel they are having a medical emergency.
Note: The TruVue system does not provide interpretative statements. Interpretation and clinical diag-
nosis is the responsibility of the physician.
PHYSICIAN’S OPERATION MANUAL
6
PHYSICIAN’S OPERATION MANUAL
TruVue System Overview
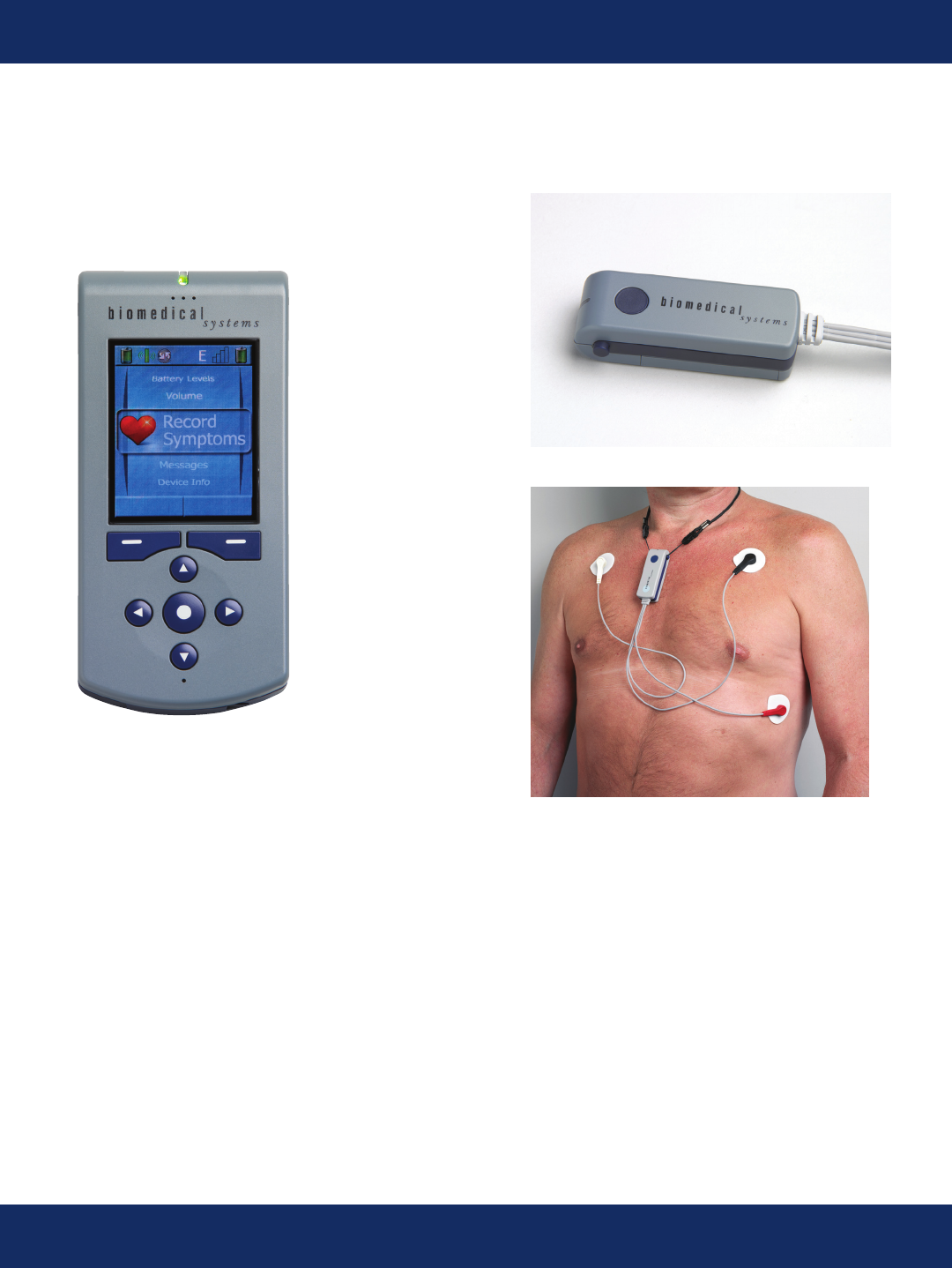
Patient Devices
The patient devices consist of a body worn Sensor, a Handheld device that provides communication
and the patient user interface, and a charger for the Handheld.
The sensor acquires and stores 2 channels of full disclosure ECG data covering the entire monitor-
ing period (up to 30 days). While aquiring ECG data, the sensor also continuously transmits the full
disclosure data to the handheld over a radio link with a range up to approximately 100 feet.
The handheld continuously transmits the full disclosure ECG data over the cellular network to the
24/7 attended BMS monitoring center, where the ECG is analyzed. Any detected arrhythmias are
conrmed by our certied monitoring technicians before being reported to the physician.
If the patient is symptomatic, they can enter their symptoms on the handheld. These symptoms are
immediately transmitted to the monitoring center for review and correlation with the ECG data.
Text messages and voice calls can be placed to the patient handheld any time the device is in
cellular coverage.
TruVue Handheld
TruVue Sensor
Applied Sensor

7
PHYSICIAN’S OPERATION MANUAL
TruVue System Overview
Data and Monitoring Center
Full disclosure ECG data transmitted from the handheld is stored in the BMS monitoring center,
where arrhythmia analysis algorithms analyze for:
• Pause / Asystole
• Tachycardia
• Bradycardia
• Atrial Fibrillation
• Idioventricular Rhythms
• Supraventricular Tachycardia
• Ventricular Tachycardia
• Ventricular Fibrillation
When one of the above arrhythmias is detected, a certied monitoring technician conrms the
arrhythmia and prepares and annotates a sample to be included on a physician report. A report is
sent immediately to the physician if the arrhythmia meets the immediate report criteria specied for
the patient, or sent on a daily summary report per physicians orders.
A daily or weekly summary report is prepared per the prescribing physician’s preference that
can include:
• Heart Rate Trend graph
• Atrial Fibrillation Burden graph
• Samples of any arrhythmias detected, or ECG samples at the high
and low HR if there were no arrhythmias
Reports can be faxed, mailed, and/or viewed and printed on-line. Prior to printing your patient’s
report, you may enter any comments or interpretations on the report.
The TruVue system allows you to view your patient’s monitoring record at any time, including all
reports, samples and full disclosure ECG data since the inception of the monitoring period.

8
PHYSICIAN’S OPERATION MANUAL
TruVue Service Overview
Ordering TruVue
The TruVue Mobile Telemetry system is provided as a service by Biomedical Systems. You or
your staff may order this service for your patients by logging on to our on-line web application,
Global Cardio, and completing the on-line patient enrollment form. If you do not have the Global
Cardio application installed, please contact Biomedical Systems to arrange installation.
NOTE: When ordering TruVue for your patients, all physician orders require the following
information to be provided to Biomedical Systems:
• Patient name (rst, last, and middle initial)
• Patient I.D. and Date of Birth
• Patient demographics (home address, telephone number, cell phone number, etc.)
• Patient primary and secondary insurance information (ID #, group #, address, telephone #)
Upon receipt of an order for the TruVue service Biomedical Systems will:
A) Conrm the insurance coverage for the patient.
B) Contact the patient and conrm the delivery address for the device kit
C) Congure the device for your patient and ship the device kit and all consumables required for
the entire monitoring period.
Please discuss with your patients:
• Reason for ordering mobile telemetry
• Importance of proper hook-up and securing electrodes to skin
• Change sensor battery every 24-hours and charge handheld unit during times of sleep
• Anticipated monitoring duration
• Instruct them to go to the nearest emergency room or call 911 in the event of a life-threatening
Emergency
In addition to discussing the above with your patient, we encourage you to provide the patient with
contact information for Biomedical Systems.
9
Initiating Monitoring
When the patient receives the device, Biomedical Systems will speak with them to walk them
through the hook up and verify the proper operation of the system.
Our certied monitoring technicians will:
A) Conrm the identity of the patient.
B) Review proper device operation with the patient.
C) Instruct the patient on the proper application of electrodes and how to begin monitoring.
D) Take a baseline recording and verify proper operation of the device.
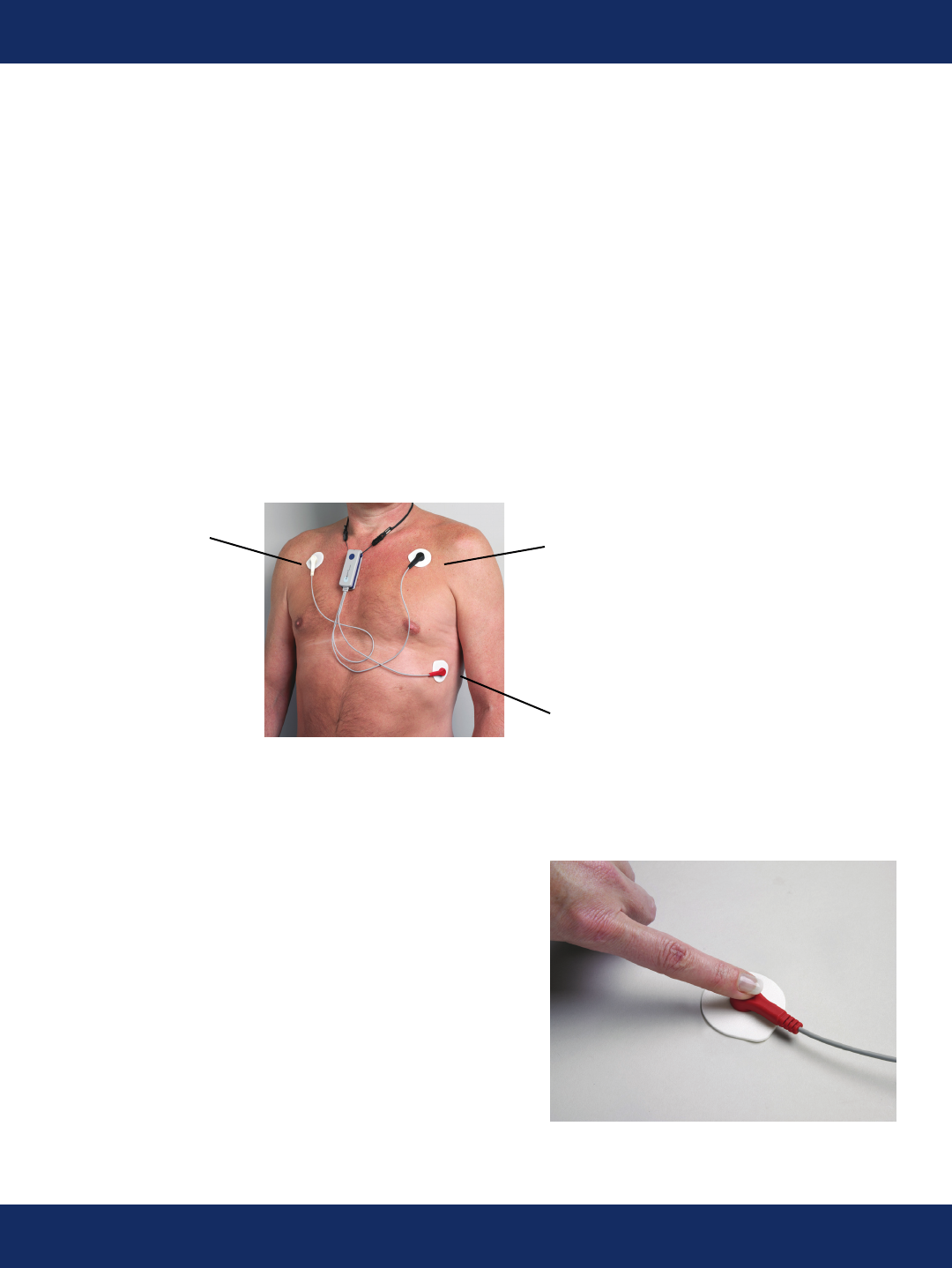
Electrode Site Preparation and Proper Positioning
White Electrode Black Electrode
(RA) (LA)
Red Electrode
Figure 1 (LL)
CAUTION: Shave any hair that is in the area the electrodes are placed.
1. Shave area where electrodes will be
placed (if applicable). Wipe each area with alcohol
in a circular motion and let dry.
(See Figure 1 for electrode placement)
2. Remove the sensor from the box. Snap
each lead wire onto an electrode. (See Figure 2)
3. Remove backing from the
electrode attached to the black
snap and place it on the left
side of your upper chest just
below your clavicle as shown in
Figure 1. Figure 2
PHYSICIAN’S OPERATION MANUAL
TruVue Service Overview

10
PHYSICIAN’S OPERATION MANUAL
TruVue Service Overview
4. Remove backing from the electrode attached to the
red snap and place it on the lower left portion of your
chest as shown in Figure 1.
5. Remove backing from the electrode attached to the
white snap and place it on the right side of your upper
chest just below your clavicle as shown in Figure 1.
CAUTION: Press rmly all around electrode patches to secure them rmly to skin.
Concluding Monitoring
When monitoring is nished, Biomedical Systems will contact the patient and arrange for the device
to be returned to us. Our monitoring staff will prepare a summary report for your review.
If you reach a diagnosis for your patient prior to the end-monitoring date or wish to extend the
monitoring period past the date, please contact the Biomedical Systems monitoring center.
Breaks in Monitoring
The monitoring period can be suspended and resumed later if the patient requires a hospitilization or
a break in service for any other reason, such as out of the country travel. During a monitoring break
you will not receive any daily reports.
Electrode Site Placement and Proper Positioning- Continued

11
PHYSICIAN’S OPERATION MANUAL
Operation and Performance Specications
ECG Acquisition and Storage
The Sensor acquires two channels of ECG through a three wire shielded cable connected to standard
Holter monitoring electrodes. Standard leads II and III of the Einthoven triangle are sampled at 1000
samples per second (SPS) with +/- 40 mV of dynamic range with .05 to 150 Hz band pass. The data is then
ltered and down sampled to 250 (SPS) before being stored on the sensor. The sensor retains up to 30
days of ECG data. ECG data is stored with the patient ID and an error detecting code.
ECG Transmission
The Sensor transmits the acquired ECG data to the handheld over an encrypted Bluetooth link with a
range of up to approximately 100 feet. The range of this link can vary depending on environmental
factors. If the sensor goes out of range of the handheld the patient will be alerted. The handheld and
sensor are paired together prior to providing the kit to the patient and will only communicate with each
other. Neither the sensor or handheld will communicate with other devices (they are “non-discoverable”
and “non-connectable” per the Bluetooth specication). The ECG is protected from data corruption by
an error detecting code that “travels” with ECG data throughout the TruVue system, ensuring that no
corruption of the data occurs during transmission to and storage at the monitoring center.
The Sensor can be placed in “airplane mode” through the handheld user interface. This turns off all
radios so the patient can continue to collect ECG data (but not transmit it to the handheld) in areas where
wireless devices are not allowed. The stored ECG is transmitted to the monitoring center when the radios
are turned back on. If the radios are turned off when the handheld is powered up the patient is prompted
to turn them on again.
User Interface
The Sensor will alert the patient with a speaker tone and a ashing LED when the battery is low, if a lead
falls off, or if the sensor is out of range of the handheld. The patient can silence an alert temporarily by
using the large pushbutton on the sensor if they choose.
Algorithm
When communications between the Handheld and the Biomedical Systems data center are interrupted for
any reason the sensor runs a rhythm analysis algorithm that detects potentially signicant arrhythmias
that have a high heart rate or ventricular rhythm. If a potential arrhythmia is detected then an alert is
presented to the patient that instructs them to move to an area with cellular coverage so the ECG data can
be transmitted to the data center for analysis and conrmation of the rhythm.
Powering the sensor
The sensor is powered by inserting the battery in the battery compartment. It is always on - there is no
separate on/off switch. The patient replaces the battery in the sensor once a day with the supplied lithium
AAA battery.
Sensor Operation

12
PHYSICIAN’S OPERATION MANUAL
Leadset:
The lead wires are permanently attached to the sensor hardware and are not user replaceable.
CAUTION: Do not attempt to remove the lead wires from the sensor.
CAUTION: Inspect the leadwires for any fraying and/or cracking in the insulation prior to use.
Note: BMS will perform this check before providing the equipment to the patient.
Lanyard:
A lanyard (neck strap) is attached to the sensor for the convenience of the patient and to prevent the sensor
and lead wires from dangling. The lanyard can be removed from the sensor if desired.
Battery:
The sensor is powered by a 1.5V AAA lithium battery.
CAUTION: Use only the supplied lithium AAA batteries that are provided with the patient kit.
Do not use rechargeable batteries.
CAUTION: Do not store sensor with the battery in place for extended periods of time. Remove the battery
after each monitoring period.
Cleaning:
The sensor may be cleaned with Isopropyl Alcohol. Do not submerse the sensor in any liquid.
Handling precautions:
To ensure proper operation of the sensor please follow these handling precautions:
CAUTION: Do not drop the sensor or handheld unit.
CAUTION: Do not pull or yank on the sensor lead wires.
CAUTION: Do not expose sensor or handheld to excessive dust or to extreme temperatures.
CAUTION: Do not immerse or otherwise allow any liquid to enter the sensor or handheld.
CAUTION: Do not store the sensor or handheld units in direct sunlight or near corrosive liquids.
CAUTION: Do not allow sensor to get wet.
When Showering or Bathing:
• Remove lead wires attached to sensor from the electrodes.
• Place sensor (attached to lanyard) in a dry secure place.
• Remove electrodes (patches) from skin even if they have already been changed in past 24 hours.
• After showering or bathing, dry skin thoroughly.
Do not apply powder or lotion of any kind to chest area.
• Wipe skin with alcohol in area where electrodes (patches) will be placed.
Replace electrodes (patches) as previously instructed.
Sensor Operation
Operation and Performance Specications
13
PHYSICIAN’S OPERATION MANUAL
Sensor Performance Specications
Standards
The sensor complies with the following medical device standards:
-AAMI EC 38-1998, Ambulatory Electrocardiographs.
-EN60601 -1 Medical electrical equipment, Part 1: General requirements for safety
-EN60601 -1 Medical electrical equipment, Part 1-2: Electromagnetic compatibility
Sensor Performance Specications
Parameter Notes Min. Typ. Max. Unit
Physical:
Length 3.1 in.
Width 1 in.
Thickness .8 in.
Weight With AAA Battery 60 gm
ECG Cable Lengths Dual channel 3 electrode 18 in.
Parameter Test Conditions Min. Typ. Max. Unit
Environmental:
Operating Temperature 0 45 ºC
Storage Temperature -10 60 ºC
Relative Humidity 10 95 %
Shock-Unpackaged Unit 36 in.
Water Resistance Not Water Resistant
Complies with AAMI-EC38
and EN60601-1
Operation and Performance Specications
14
PHYSICIAN’S OPERATION MANUAL
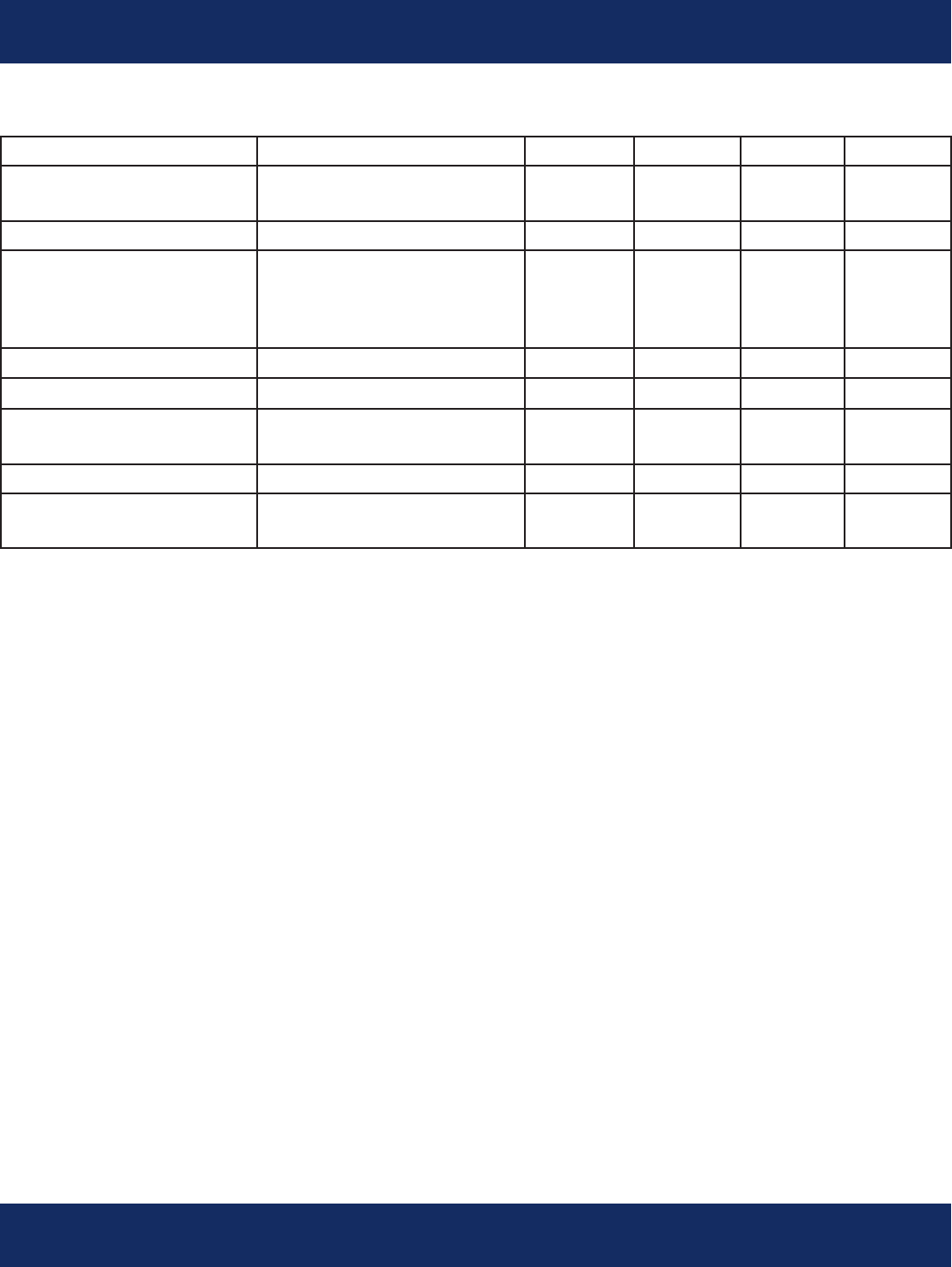
Sensor Performance Specications - Continued
Parameter Test Conditions Min. Typ. Max. Unit
Electrical:
Battery Voltage 1 -AAA Lithium Energizer 0.9 1.5 3.0 Volts
Battery Current At 1.5V Battery Voltage, all 400 mA
circuits turned on
Lithium Battery Lithium-Ion Battery Not User 2.0 3.1 Volts
Voltage Replaceable
VREF Voltage 1.22 1.25 1.0 Volts
Reference
VREFAD Voltage 2.45 2.55 2.60 Volts
Reference
Input Impedence @ 5 Hz 1.0 1.5 1.6 MW
CMRR @ 60 Hz 86 dB
CMR Range AC + DC -1.5 +1.5 Volts
Differential Range AC -40 +40 mV
DC+80 mV AC @ 5 Hz -500 +500 mV
Fast Baseline Reset- After Removing Overloading 0.45 0.5 0.55 Hz
3 db Frequency Signal
Bandwidth +1 dB referenced to 15 0.05 150 Hz
Hz
Low Pass Filter Gain @ 250 Hz -18 -17 dB
Pacemaker Pulse 1 microsecond max pulse rise and fall
Detection times
Pacemaker Pulse 0.2 2.5 msec
Width
Pacemaker Pulse 1.0 250 mV
Amplitude
Communications
Frequency Bluetooth Class 2,3 2.4 gHz
Communications Bluetooth SPP Prole, non 2.2 Ver
Protocol discoverable
Output power 0 dB
User Interface Complies with AAMI-EC38
and EN60601-1
Pushbutton Used for silencing alerts
LED For device alerts
Speaker For device alerts 400-2500 Hz
Complies with AAMI-EC38
and EN60601-1
Operation and Performance Specications

15
PHYSICIAN’S OPERATION MANUAL
Handheld Operation
Communications
The Handheld communicates with the sensor over an encrypted Bluetooth link with a range of up to
approximately 100 feet. The range of this link can vary depending on environmental factors.
If the sensor and handheld lose communication the patient will be notied with a short beep on
the sensor.
The Handheld transmits ECG data to the BMS data center over the cellular network. Transmissions
are bursted with a maximum latency of 2.5 minutes when the Handheld is in coverage on the
network. When out of coverage of the cellular network the handheld commands the sensor to run the
potential arrhythmia detector algorithm (described under the Sensor Operation section).
ECG data is transmitted to the data center without modication and is protected from corruption by
an error detecting code embedded in the ECG data.
Text messages can be sent from the BMS monitoring center for display on the patient’s handheld
device.
The Handheld can also receive a voice call from the monitoring center in the event that monitoring
staff needs to speak with the patient and they cannot be reached at their regular phone numbers.
Only the monitoring center can initiate a voice call, the handheld will only accept incoming calls from
the monitoring center, and the patient cannot initiate an outgoing call.
CAUTION: The handheld is a cellular phone. Follow your implantable device manufacturers
recommendations on the use of cellular phones with your implant.
Operation and Performance Specications

PHYSICIAN’S OPERATION MANUAL
TruVue Indications of Use
16
Handheld Operation
Powering the handheld:
The handheld is powered by an internal rechargeable Lithium Ion battery. The on/off button is
located on the right side of the unit and the handheld is powered on by pressing and holding the
power button for approximately 5 seconds. The provided wall charger charges the battery. The
handheld can be powered on whenever the wall charger is attached, regardless of whether the
battery is depleted or not.
The handheld battery will typically power the handheld 16 hours without recharging. The patient
should leave the handheld attached to the wall charger while they are sleeping.
CAUTION: Do not attempt to replace the handheld battery.
CAUTION: Use only supplied wall charger with the handheld.
Cleaning:
The handheld may be cleaned with Isopropyl Alcohol. Do not submerse the handheld in any liquid.
Handling precautions:
To ensure proper operation of the handheld please follow these handling precautions.
CAUTION: Do not drop the handheld unit.
CAUTION: Always use the carrying case when carrying the handheld on your body.
CAUTION: Do not expose sensor or handheld to excessive dust or to extreme temperatures.
CAUTION: Do not immerse or otherwise allow any liquid to enter the sensor or handheld.
CAUTION: Do not store the sensor or handheld unit in direct sunlight or near corrosive liquids.
Operation and Performance Specications
17
PHYSICIAN’S OPERATION MANUAL
User Interface:
A graphical user interface is incorporated for display of messages to the patient, input of symptoms and
control of the system. A 5-way set of navigation keys and two soft keys are used to navigate the user
interface. An LED illuminates to indicate the status of the device when it is on and to indicate the
charging status when the unit is off . The handheld incorporates a loudspeaker and vibrator for alerting
the patient.
Operating modes:
The TruVue device kit operates in two primary modes, monitoring and pre-monitoring. The unit is
provided to the patient in pre-monitoring mode and is activated into monitoring mode by a BMS
monitoring technician during the hook-up call from the patient. In pre-monitoring mode the patient kit
does not record, store or transmit ECG data.
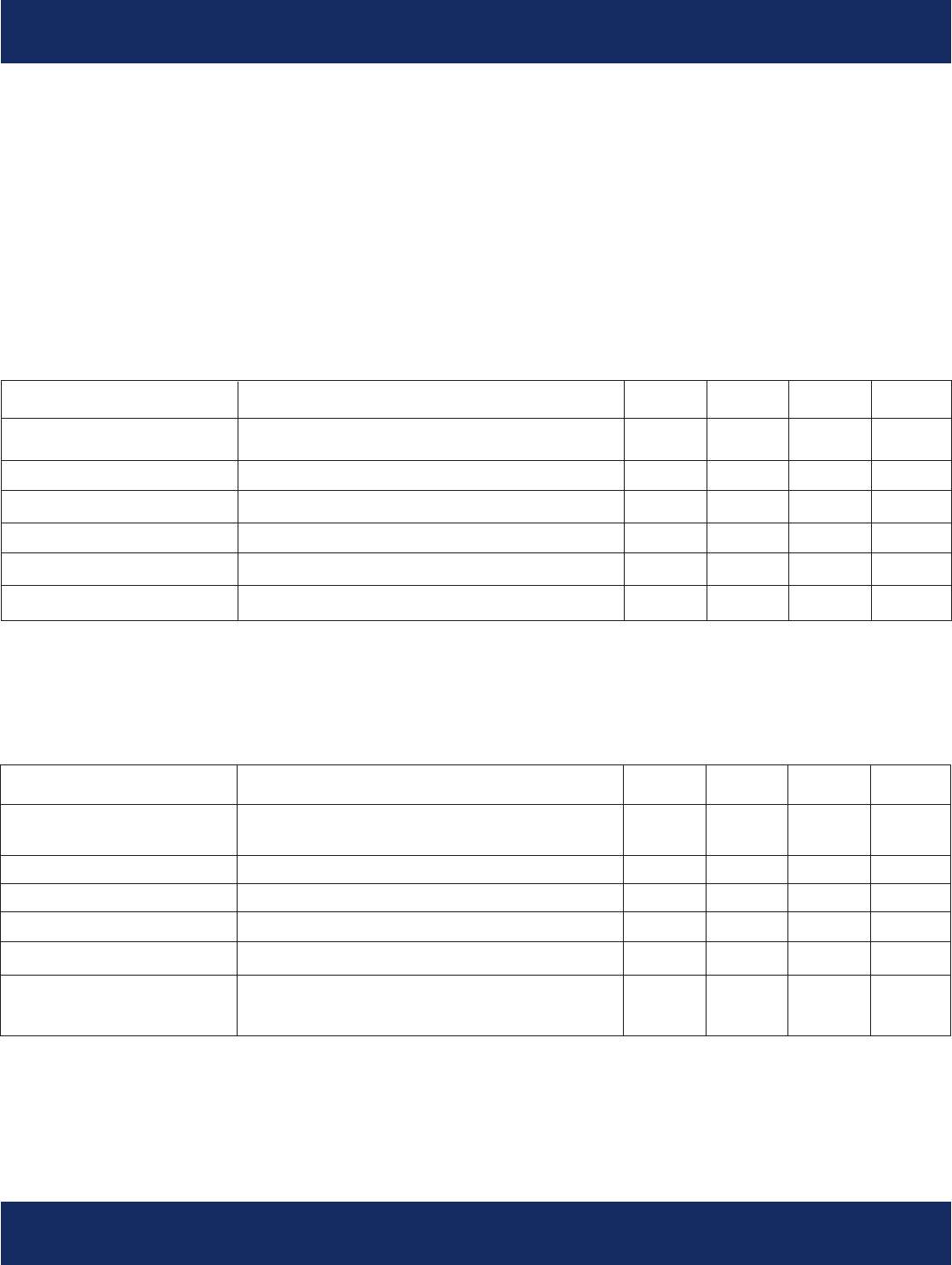
User Interface in Pre-monitoring mode:
When the patient receives the unit and turns it on they will proceed through the following sets of screens
that instruct the patient to call the BMS monitoring center for hook-up instructions:
During the hook-up call, the BMS monitoring center technician will perform the hook-up
(see “Initiating Monitoring” in the “Service Overview” section) and provide the patient with a code that
enables the transition of the unit into monitoring mode. At this point the devices are actively monitoring
the patient.
Operation and Performance Specications

18
User Interface in Monitoring Mode
Main screen
The main screen is the top level screen for the user interface and is displayed when the device is
turned on and whenever the LCD wakes up from power saving mode. It consists of the status bar,
the task wheel and softkey area. The status bar displays various indicators of device operation. The
task wheel scrolls using the up and down arrow keys and allows the patient to select various tasks to
perform by selecting the center key. The softkey area contains two indicators that change depending
on what state or screen the device is in.
Status Bar
Task Wheel
Softkeys
Task Wheel Options
PHYSICIAN’S OPERATION MANUAL
Record symptoms
and activity level
Read Text
messages from
the monitoring
center
Turn wireless
radios on or
o. Place the
device in
“ight mode”
View large
battery level
indicators for
the handheld
and sensor
Adjust the
volume and
vibrator for both
the handheld
and sensor
View device
information
such as serial
number, SW
versions, etc.
Operation and Performance Specications
19
PHYSICIAN’S OPERATION MANUAL
User Interface in Monitoring Mode
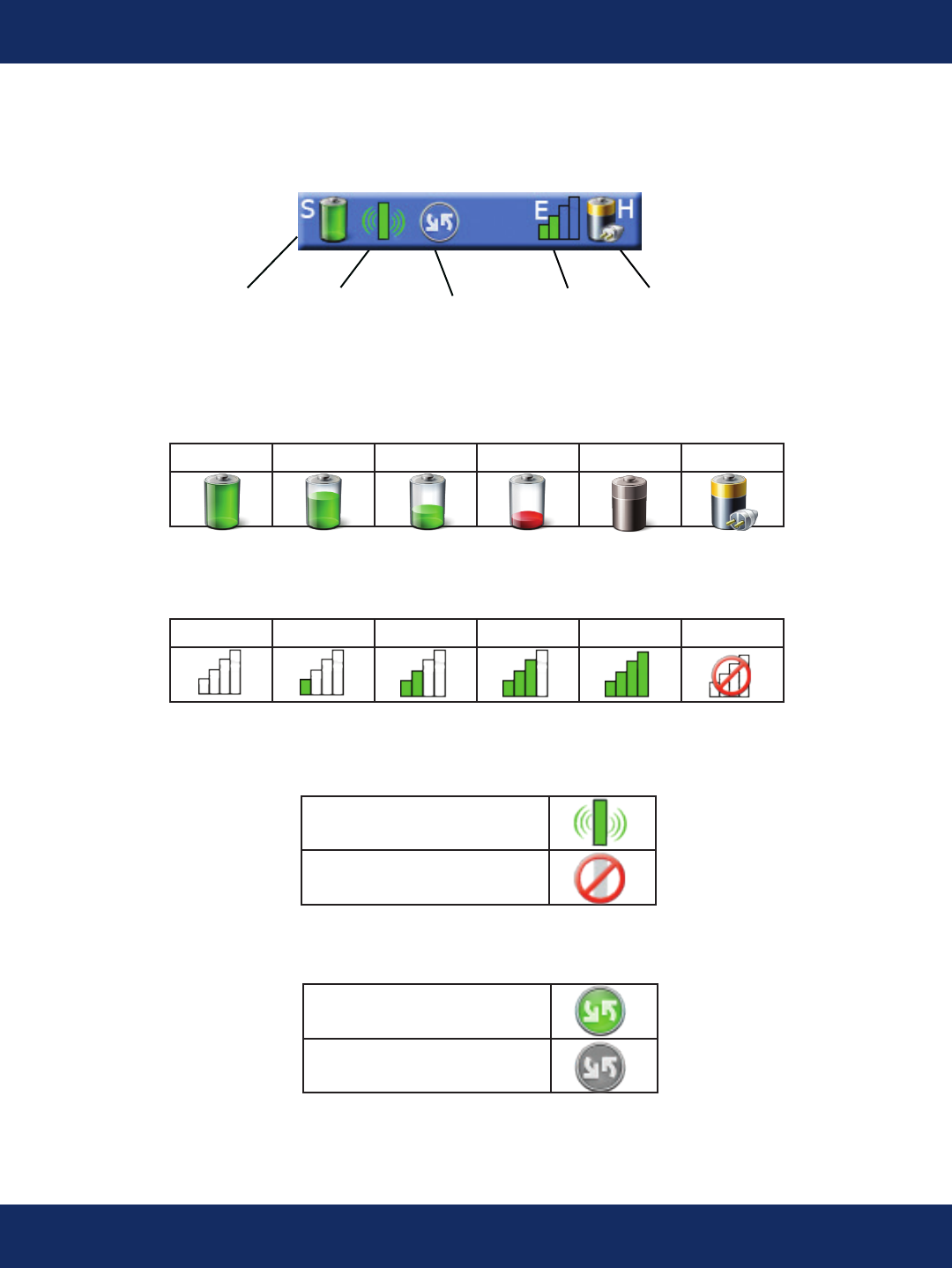
Status Bar Indicators
Upload
Activity
Sensor
Battery
Sensor
Connection
Cell
Strength
Handheld
Battery
Full High Low Empty Absent Charging
Battery Level Icons
Cell Strength Icons
0 1 2 3 4 Cell O
Handheld Only
Sensor Connected
Sensor Disconnected
Sensor Connection
Upload Activity
Upload Inactive
Upload Activity
Operation and Performance Specications
20
PHYSICIAN’S OPERATION MANUAL
User Interface in Monitoring:
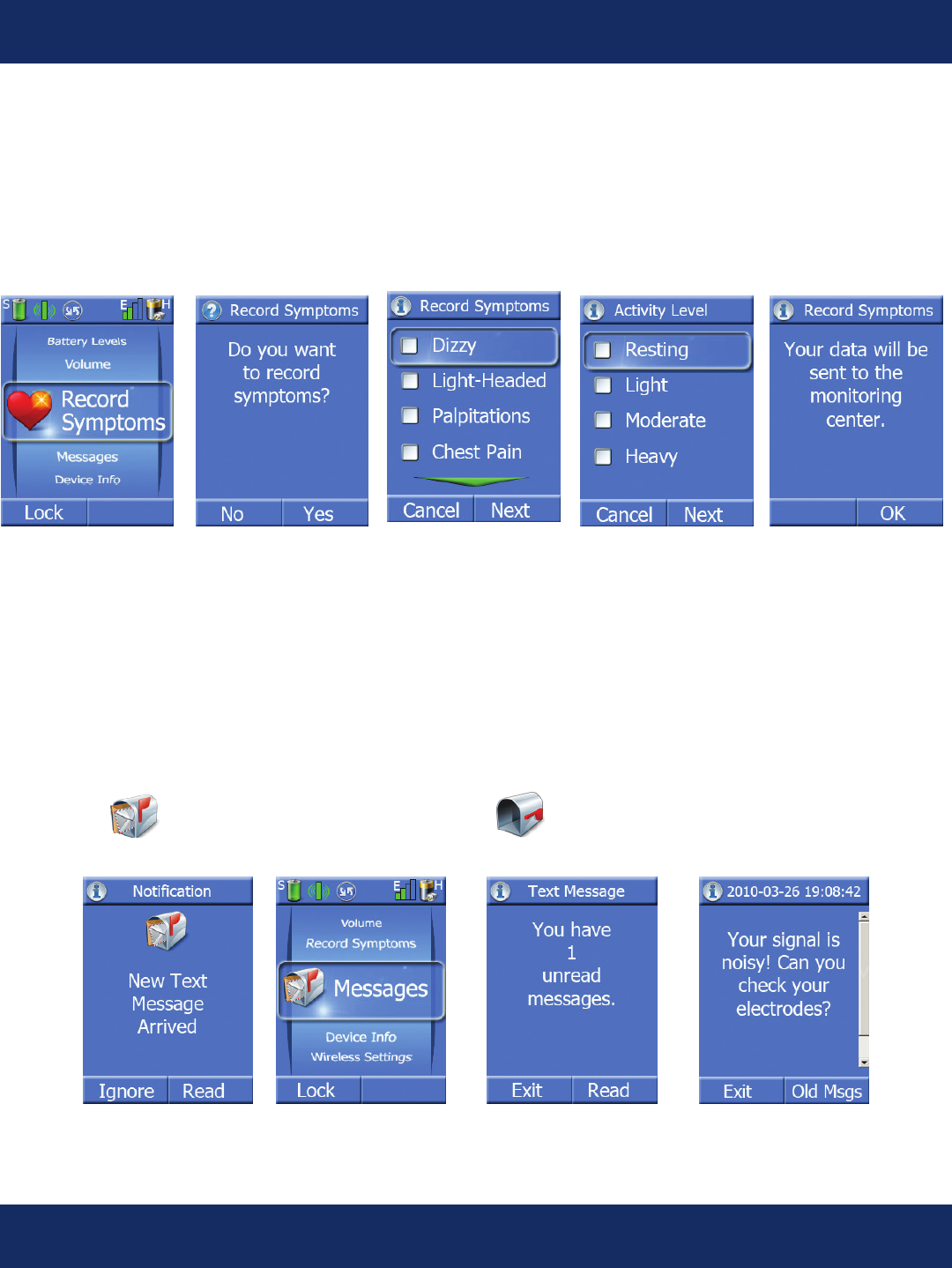
Record Symptoms Screens:
Pressing the center key when the task wheel is on the Record Symptoms task allows the patient to
enter both their current symptoms and their current activity level. This information is uploaded to the
monitoring center and is available for correlation with the patient’s ECG at the time they recorded the
symptom.
Messages Screens:
Pressing the center key when the task wheel is on the Messages task allows the patient to read text
messages sent from the monitoring center. Messages can be entered through GlobalCardio. The
indicator on the main screen changes to indicate that there are unread messages waiting. The
handheld can store up to 4 messages. If a 5th message is received then the earliest read message will
be deleted.
Unread message waiting All messages have been read
Operation and Performance Specications
21
PHYSICIAN’S OPERATION MANUAL
User Interface in Monitoring Mode:
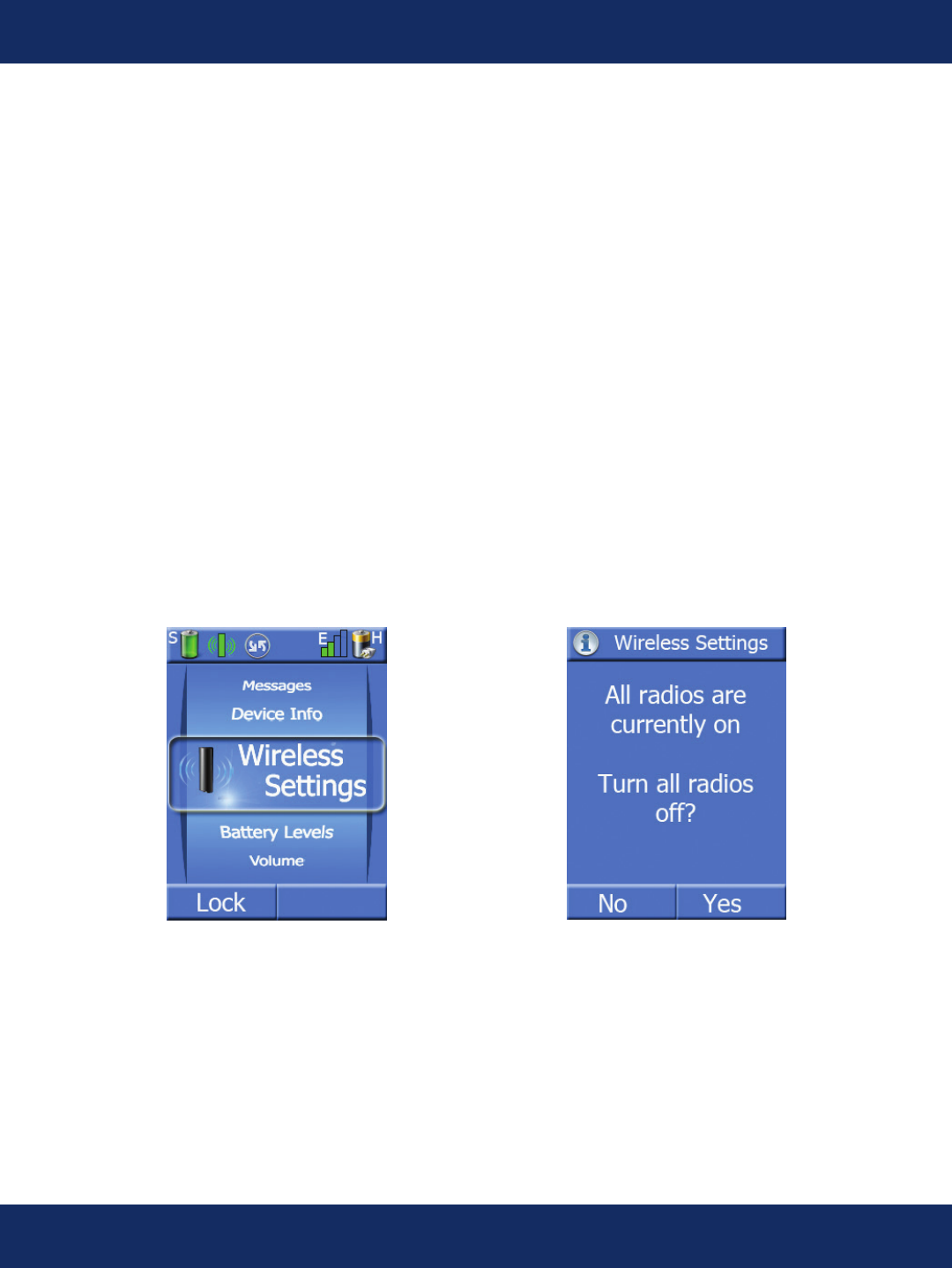
Wireless Settings Screens:
Pressing the center key when the task wheel is on the Wireless Settings task allows the patient to turn
off all wireless radios in the system for a time. This is a useful feature if the patient is on an airplane
or in some other area where cellular phones are not allowed. The sensor will continue to record all
ECG data while the handheld radio is off.
When the radios are turned back on, all stored data will be transmitted to the monitoring center.
When the device is turned back on, the user is always prompted to turn on the radios if the handheld
is in “airplane” mode.
The user is also prompted every two hours to turn the radios on through an alert message.
The potential arrhythmia detection algorithm does not run when the device is in “airplane” mode,
since it is assumed that the patient is unable to transmit any data due to their physical location.
Operation and Performance Specications
22
PHYSICIAN’S OPERATION MANUAL
User Interface in Monitoring Mode:
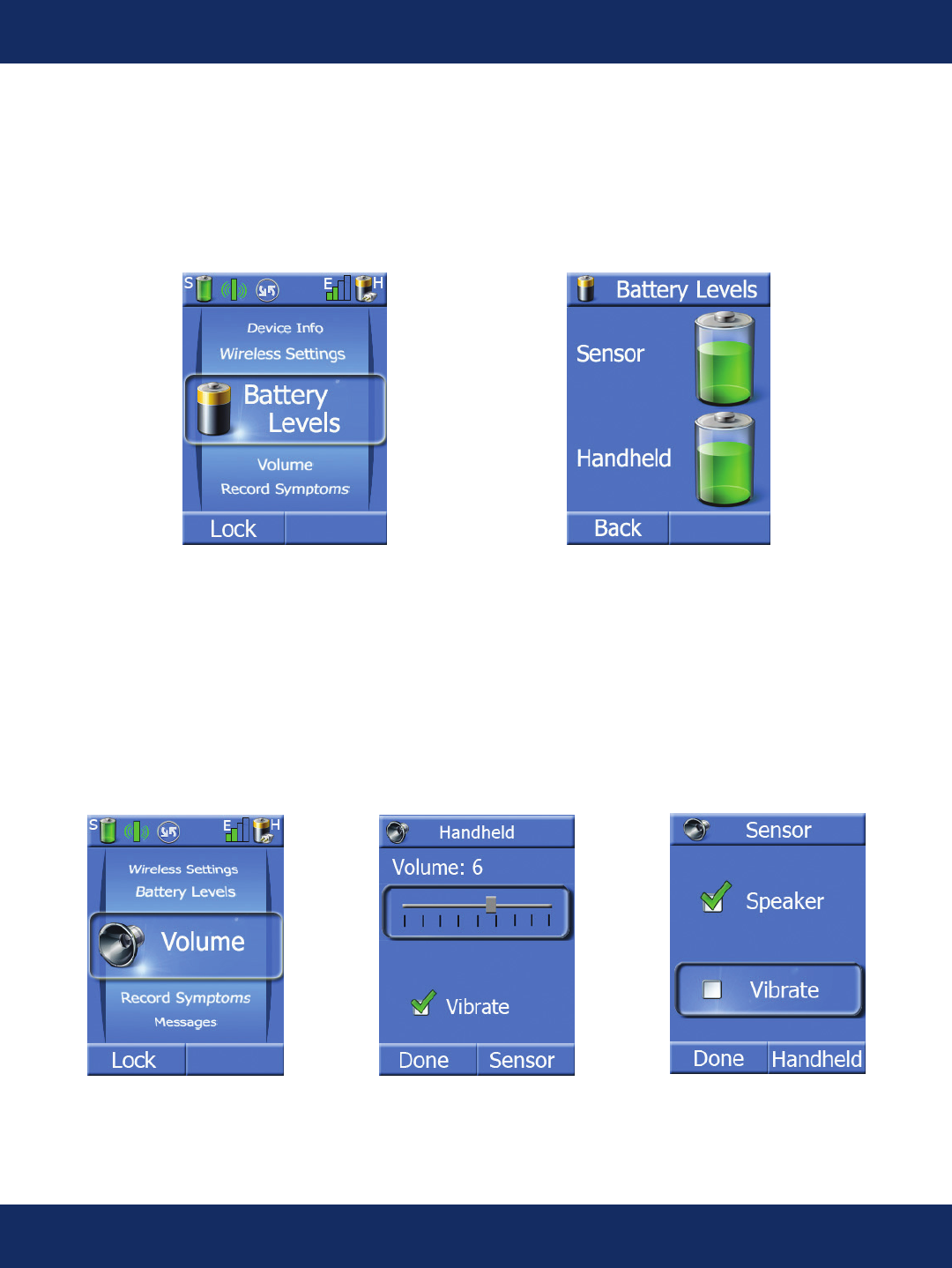
Battery Level Screen:
Pressing the center key when the task wheel is on the Battery Levels task allows the patient to view
the large battery level indicators.
Volume Screens:
Pressing the center key when the task wheel is on the Volume task allows the patient to adjust the
volume and vibrate levels on the handheld. The sensor volume can only be set when the sensor is
connected to the handheld. To set to vibrate only, the volume slider is moved to 0 and the vibrate
indicator is automatically checked. Vibration can also be selected in addition to volume.
Operation and Performance Specications
23
PHYSICIAN’S OPERATION MANUAL
User Interface in Monitoring Mode:
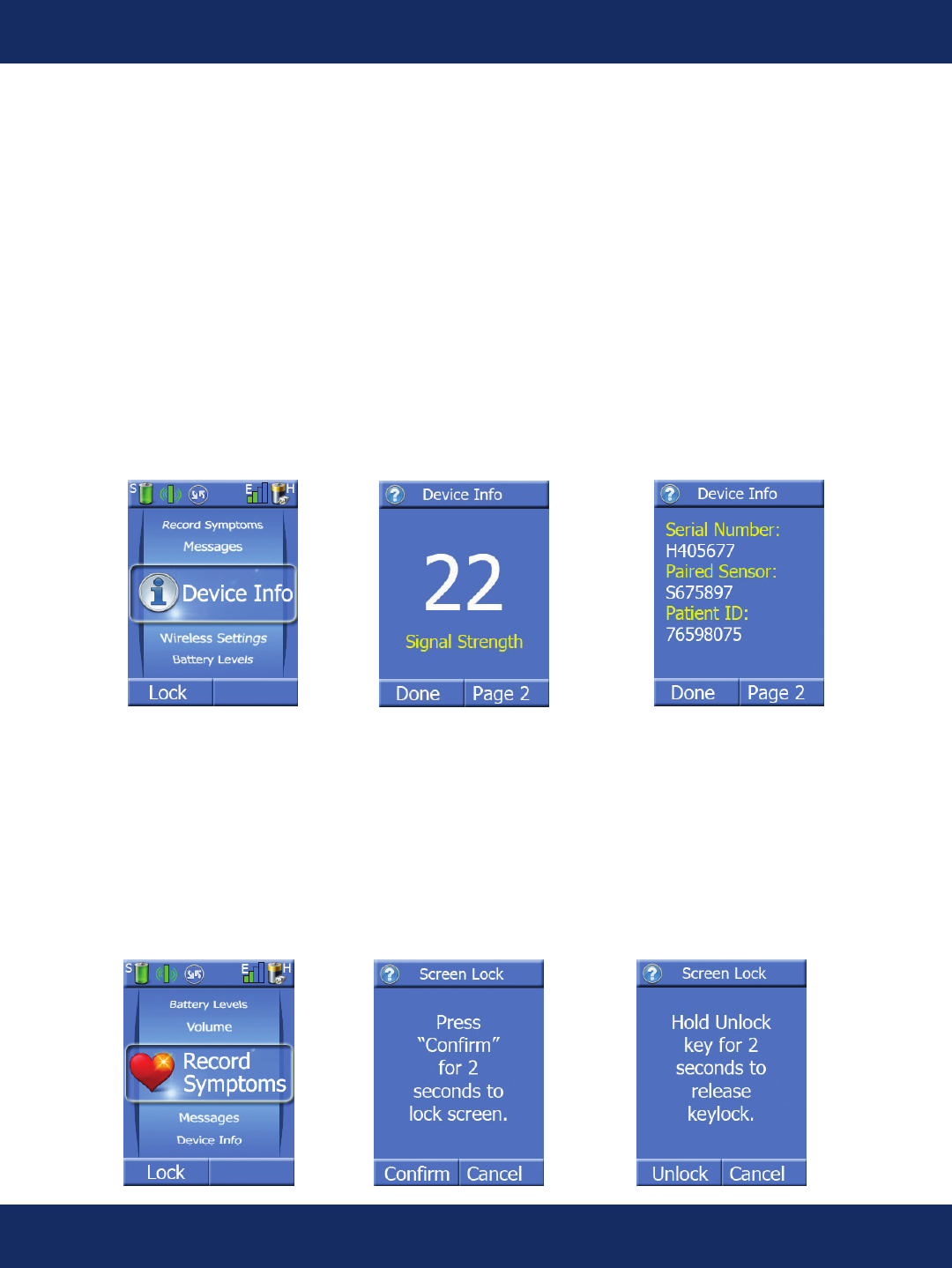
Device Info Screens:
Pressing the center key when the task wheel is on the Device Info task allows the patient to view the
following device information:
• Cellular Network Signal Strength
• Handheld and Sensor Serial Numbers
• Patient ID
• Handheld and Sensor Software Versions
• Wireless Setting (Normal or Off)
Lock/Unlock Screens:
To conserve battery life, there is a lock and unlock screen feature on the Handheld unit. It can be activated
manually, or it will automatically go into lock mode after 2 minutes if you have not pressed a key on the key
pad. If you want to lock the screen manually, press the “Lock” key. Press and hold the Conrm key for 2 seconds
to lock the screen. The patient’s ECG will continue to be sent to our monitoring center for review.
To unlock the keypad, press any key other than the left softkey to display the lock screen and then press the
“Unlock” key.
Operation and Performance Specications
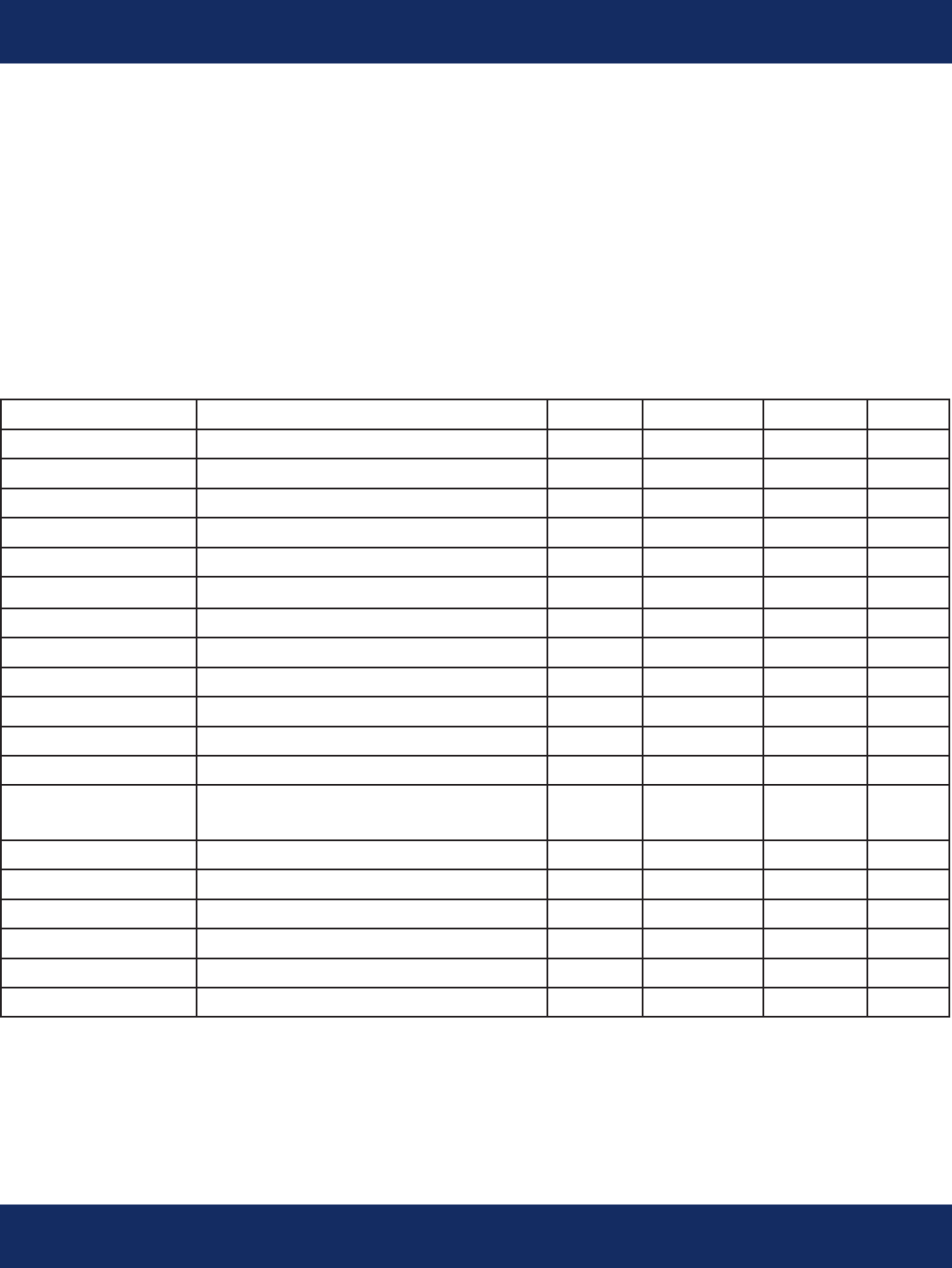
24
PHYSICIAN’S OPERATION MANUAL
Handheld Performance Specications
Standards
The handheld complies with the following medical device standards:
-AAMI EC 38-1998, Ambulatory Electrocardiographs.
-EN60601 -1 Medical electrical equipment, Part 1: General requirements for safety
-EN60601 -1 Medical electrical equipment, Part 1-2: Electromagnetic compatibility
Parameter Notes Min. Typ. Max. Unit
Physical:
Length 5 in.
Width 2.25 in.
Thickness .8 in.
Weight 150 gm
User Interface
Display 240X320 QVGA OLED
Keypad 5 way navigation + 2 softkeys
Receiver/mic For voice call
Loudspeaker For device alerts
Environmental: Complies with AAMI-EC38
and EN60601-1
Operating Temperature
0 45 ºC
Storage Temperature
-10 60 ºC
Relative Humidity
10 95 %
Shock-Unpackaged unit Per AAMI-EC38 36 in.
Shock-Packaged Unit Per AAMI-EC38
Water Resistance IPX 0
Operation and Performance Specications
25
PHYSICIAN’S OPERATION MANUAL
Handheld Performance Specications
Parameter
Lithium Battery Voltage Lithium-Ion Battery Not
User Replaceable
4.2 Volts
Lithium Battery Current 1 Amps
Charger EN60601 Approved direct
plug-in Class II AC adapter
power supply rated 100-
240V~
100 240 Volts
Communications
Cellular EGSM/GPRS/EGPRS
900/1800/850/1900 MHz
Bluetooth Bluetooth Class 1
Communications Protocol Bluetooth SPP Prole , non
discoverable
2.2 Ver
Operation and Performance Specications

26
PHYSICIAN’S OPERATION MANUAL
Algorithm Operation and Performance
Signicant Arrhythmia:
The TruVue arrhythmia detection algorithm continuously processes ECG transmitted from the
patient devices and detects the following rate, rhythm, and morphology based arrhythmias:
When an arrhythmia is detected, it is agged for immediate review by a BMS certied cardiac
technician. The technician conrms the arrhythmia and prepares a report for immediate
transmission if the arrhythmia meets the Signicant Arrhythmia criteria specied by the physician.
Representative Samples, Trend and Arrhythmia Burden:
In addition to detecting signicant arrhythmias, the TruVue analysis algorithm runs an additional
scan of each 24 Hour ECG period. During this second pass, the algorithm collects additional
arrhythmia examples, minimum and maximum HR strips, HR trend data, and
AF burden information.
This data is presented to the monitoring staff for validation and inclusion on the Daily or Weekly
report. It may also be reviewed on-line at any time in Global Cardio.
Representative samples are a set of rhythm strips that represent the patient’s condition for that day.
The algorithm identies the following samples:
1. If no arrhythmia occurred, the algorithm identies the lowest noise, highest heart rate and lowest
noise, lowest heart rate samples.
2. If arrhythmias did occur (for each signicant arrhythmia class dened above) the algorithm
identies the most serious, lowest noise sample.
The HR trend graph presents the patient heart rate represented as a moving average over every
8 beats.
The Atrial Fibrillation burden graph presents the amount of time the patient was in AF with 10
minute resolution. If the patient was in Atrial Fibrillation for over 30 seconds during any 10 minute
period then that period is marked as AF. This graph can selectably be presented for the current day
or for the entire monitoring period to date.
Tachycardia Bradycardia Pause/Asystole
Atrial Fibrillation Idioventricular Rhythm Supraventricular Tachycardia
Ventricular Tachycardia Ventricular Fibrillation
Operation and Performance Specications
27
PHYSICIAN’S OPERATION MANUAL
Algorithm Operation and Performance
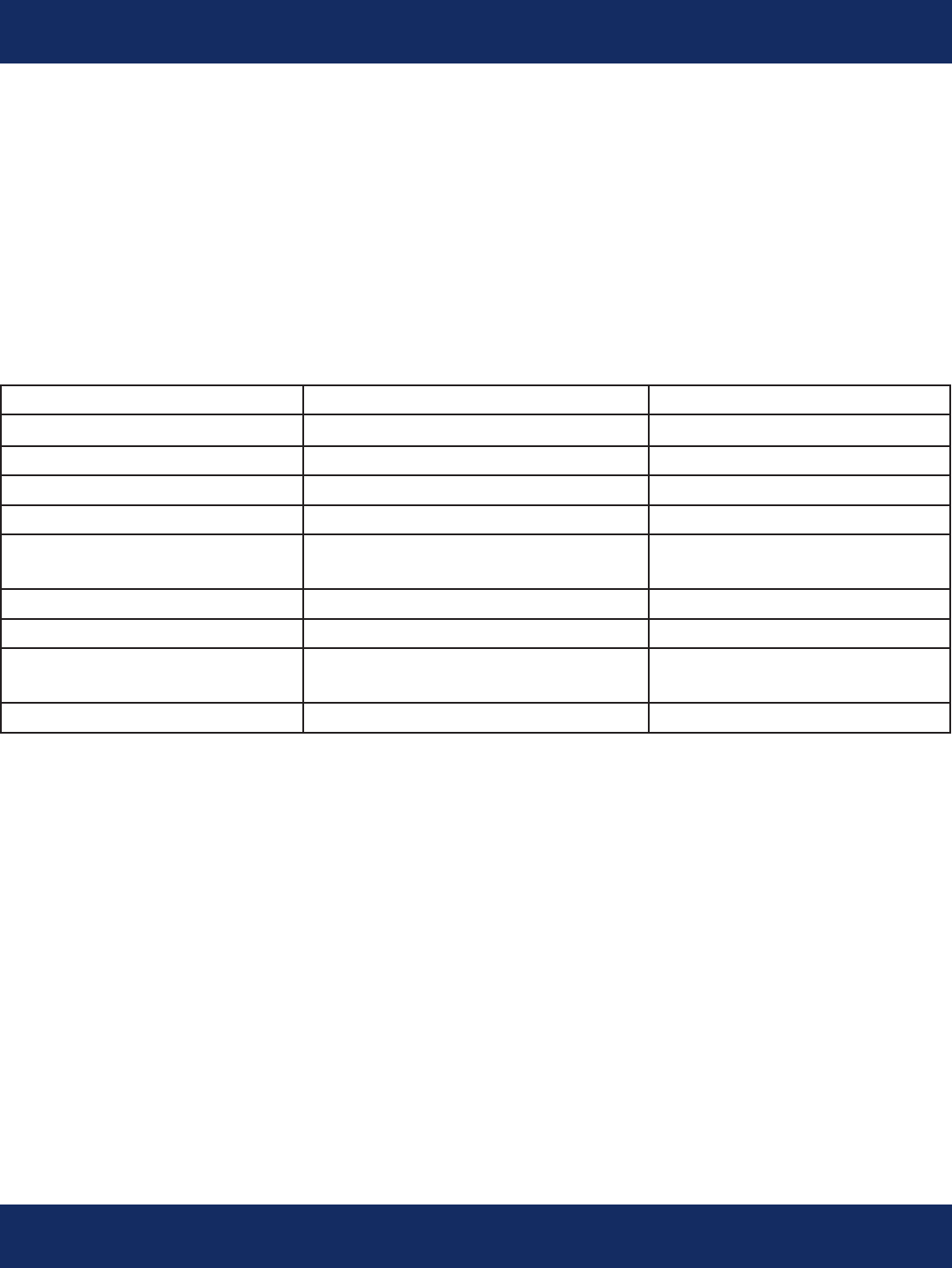
Signicant Arrhythmia Criteria:
Any technician-conrmed serious arrhythmia will be transmitted immediately on a
Signicant Event report by the physician’s preferred method of notication.
The default criteria are:
Arrhythmia Default Criteria Criteria Range
Pause/Asystole > 3 seconds 1-5 seconds
Bradycardia < 40 bpm, >30 sec 20-80 bpm
Tachycardia > 180 bpm, sustained for 15+ beats 120-300 bpm
Supraventricular Tachycardia > 150 bpm, > 30 sec 100-200 bpm, 5-60 sec
Ventricular Tachycardia Rate: > 130 bpm
(4 or more beats)
Rate: 80-150 bpm
Beats: 3-10
Idioventricular Rhythm > 15 beats, HR < dened VT Rate 5-50 beats
Ventricular Fibrillation Always
Atrial Fibrillation First onset for patient
Then rate > 150 or < 40 bpm
1-10 Onsets
Rate: 20-220 bpm
Patient Initiated Always sent
The default criteria above can be used or the physician can specify the criteria to be used as long as it
falls within the criteria range specied above. The criteria can be specied for a particular patient, for
all the physician’s patients, for a particular ofce location or for the entire practice.
Operation and Performance Specications
28
PHYSICIAN’S OPERATION MANUAL
Algorithm Operation and Performance
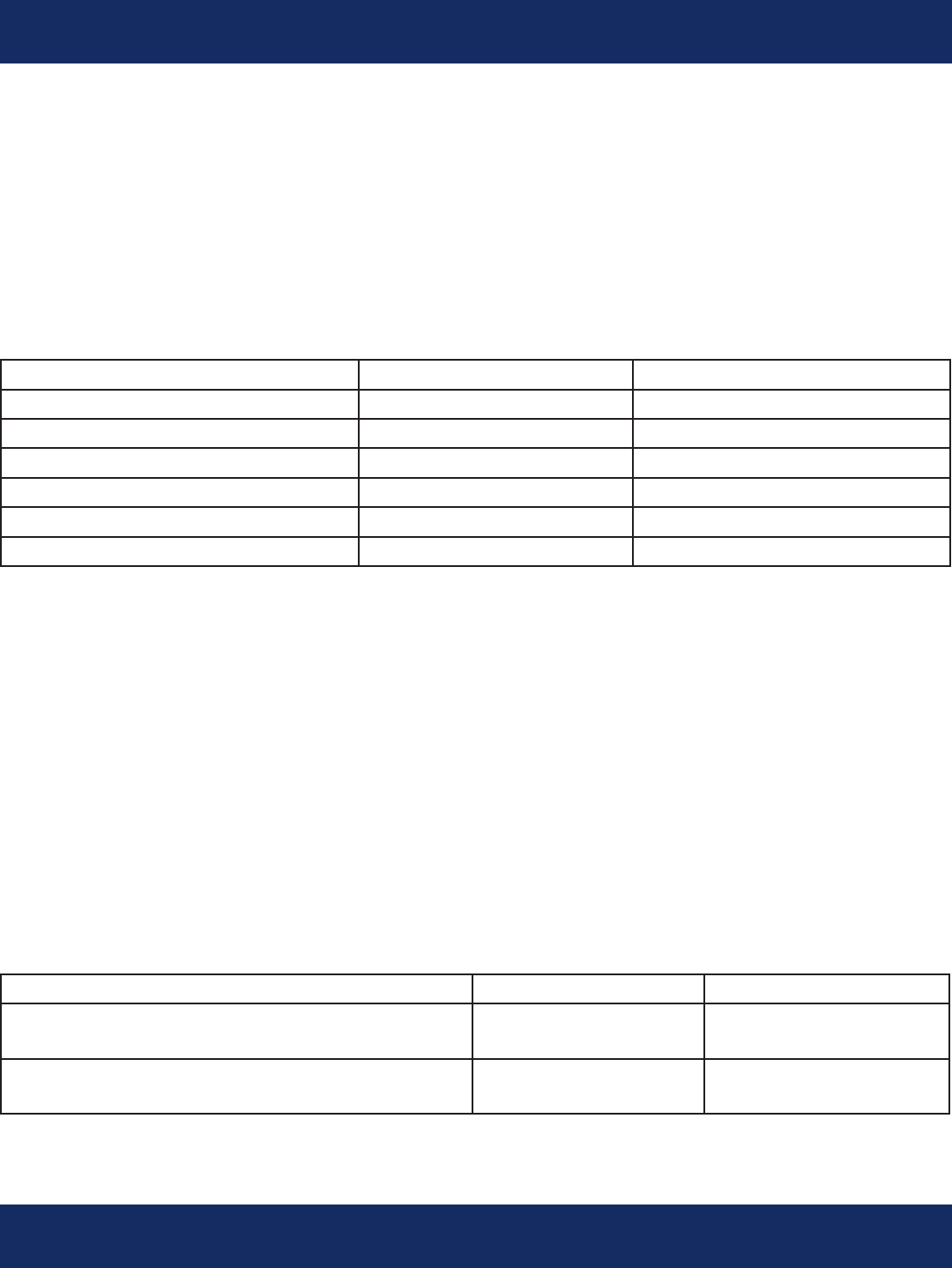
Beat Detection and Classication:
The TruVue algorithm can discriminate between normal and ventricular beat morphologies. For each
beat complex the algorithm determines the R-point for HR calculation.
The beat detection performance (as tested under ANSI/AAMI-EC 57:1998, Testing and Reporting
Performance Results of Cardiac Rhythm and ST Segment Measurement Algorithms) is:
Sensitivity, % Positive Predictivity, %
QRS Detection (MIT DB) 99.91 99.87
QRS Detection (AHA DB) 99.72 99.82
QRS Detection (NST DB) 95.17 87.57
V-morphology Detection (MIT DB) 91.83 93.56
V-morphology Detection (AHA DB) 76.24 92.08
V-morphology (NST-DB) 88.90 46.78
Heart Rate Averaging:
The heart rate is averaged over 8 R-R intervals (HR = 480/duration of 8 consecutive RR intervals in
seconds) and becomes the basis for rate based arrhythmia detection following the beat classication
step. The HR calculation had a mean RMS error of 1.735 as tested per EC-57 on the MIT database.
Atrial Fibrillation Detection Algorithm:
The Atrial brillation algorithm detects the irregularity of R-R intervals and examines the signal for
utter waves. When a certain irregularity is detected, the algorithm performs additional checks to
determine if the underlying rhythm is bigeminy or trigeminy and looks at the presence of utter
waves as a secondary indicator.
Atrial Fibrillation detection performance as tested per EC-57 is:
Sensitivity, % Positive Predictivity, %
Atrial Fibrillation detection - all events
(MIT-DB)
92 100
Atrial Fibrillation detection - events
longer than 30 seconds (MIT-DB)
100 100
Operation and Performance Specications
29
PHYSICIAN’S OPERATION MANUAL
Algorithm Operation and Performance
Ventricular Fibrillation Detection:
The TruVue algorithm can detect VF rhythms with the following performance as tested under EC-57
Sensitivity, % Positive Predictivity, %
Ventricular Fibrillation Detection
(MIT DB)
100 100
Ventricular Fibrillation Detection
(AHA DB)
90 100
Operation and Performance Specications
Ventricular Fibrillation Detection
(CU DB)
97 73
PHYSICIAN’S OPERATION MANUAL
TruVue Indications of Use
30
Description of Device Symbols
This equipment has been tested and found to comply with the limits for a
Class B digital device, pursuant to Part 15 of the FCC Rules.
These limits are designed to provide reasonable protection against harmful
interference in a residential installation. This equipment generates, uses and
can radiate radio frequency energy and, if not installed and used in
accordance with the instructions, may cause harmful interference to radio
communications.
However, there is no guarantee that interference will not occur in a particular
installation. If this equipment does cause harmful interference to radio or
television reception, which can be determined by turning the equipment off and on,
the user is encouraged to try to correct the interference by one or more of the following
measures:
* Reorient or relocate the receiving antenna.
* Increase the separation between the equipment and receiver.
* Connect the equipment into an outlet on a circuit different from that to which the
receiver is connected.
* Consult the dealer or an experienced radio/TV technician for help.
Type BF Electrical Isolation
Read Manual First
DC Current
Radiofrequency radiation exposure information:
For body worn operation, this phone has been tested and meets the FCC RF
exposure guidelines when used with the Biomedical Systems accessories supplied
or designated for this product. Use of other accessories may not ensure compliance
with FCC RF exposure guidelines.

31
PHYSICIAN’S OPERATION MANUAL
Summary of Caution statements:
CAUTION: Do not attempt to remove the lead wires from the sensor.
CAUTION: Inspect the leadwires for any fraying and/or cracking in the insulation prior to use.
CAUTION: Do not drop the sensor or handheld unit.
CAUTION: Always use the carrying case when carrying the handheld on your body.
CAUTION: Do not pull or yank on the sensor lead wires
CAUTION: Do not expose sensor or handheld to excessive dust or to extreme temperatures
CAUTION: Do not immerse or otherwise allow any liquid to enter the sensor or handheld.
CAUTION: Do not get the sensor or handheld wet.
CAUTION: Do not store the sensor or handheld unit in direct sunlight or near corrosive liquids
CAUTION: Do not attempt to replace the handheld battery.
CAUTION: Use only supplied wall charger with the handheld.
CAUTION: The handheld is a cellular phone. Follow your implantable device manufacturers
recommendations on the use of the cellular phones with your implant.
CAUTION: Press rmly all around electrode patches to secure them rmly to skin.
CAUTION: Shave any hair that is in the area the electrodes are placed.
CAUTION: The TruVue system is not an emergency response device. The patient should call 911
and/or their local emergency medical service if they feel they are having a medical emergency.
CAUTION: Federal Law (USA) restricts this device to sale by or on the order of a physician.
Note: The TruVue system does not provide interpretative statements. Interpretation and
clinical diagnosis is the responsibility of the physician.
Summary of Caution Statements

MFG-LB-201.002
Authorized Representative: Tessa De Smet
Biomedical Systems SA/NV
1945 Chausee de Wavre, B1160
Brussels, Belgium
(Belgium) +11 32 2 661 20 70, Fax +11 32 2 661 20 71
0843