Erchonia Medical MSM1-RF MSM1-RF User Manual Myologics O M Manual O M MYO
Erchonia Medical MSM1-RF Myologics O M Manual O M MYO
Contents
- 1. Transmitter design guide
- 2. Operation and maintenance manual
Operation and maintenance manual

OPERATION
AND
MAINTENANCE
MANUAL
Myologics MSM1-RF
i
Acknowledgement & Accreditation
We at Erchonia® Medical, Inc. would like to thank you for purchasing the Myologics
MSM1-RF unit. Our devices are manufactured in accordance to:
Good Manufacturing Practices (GMP), and ISO Quality Standards
INFORMATION TO USER
This device complies with Part 15 of the FCC Rules. Operation is subject to the following two conditions: (1) This device may not cause
harmful interference, and (2) This device must accept any interference received, including interference that may cause undesired
operation.
This equipment has been tested and found to comply with the limits for Class B Digital Device, pursuant to Part 15 of the FCC Rules.
These limits are designed to provide reasonable protection against harmful interference in a residential installation. This equipment
generates and can radiate radio frequency energy and, if not installed and used in accordance with the instructions, may cause harmful
interference to radio communications. However, there is no guarantee that interference will not occur in a particular installation. If this
equipment does cause harmful interference to radio or television reception, which can be determined by turning the equipment off and on,
the user is encouraged to try to correct the interference by one or more of the following measures.
· Reorient or relocate the receiving antenna
· Increase the separation between the equipment and receiver
· Connect the equipment into an outlet on a circuit different from that to which the receiver is connected
· Consult the dealer or an experienced radio/TV technician for help
Any changes or modifications not expressly approved by the party responsible for compliance could void the user’s authority to operate the
equipment.
FCC ID# SP5MSM1-RF
Doc No Issue Date Rev. Level Rev. Date
O&M-Myo 01/20/04 3 08/25/04
O&M-Myo 01/20/04 4 11/29/04
Erchonia® Medical, Inc.
4751 E. Indigo St. Mesa, AZ 85205
Phone 480.633.3129 • Fax 480.545.2784

Table of Contents
Acknowledgement & Accreditation i
SECTION 1
Introduction to Contents 1
The Myologics MSM1-RF 2
Myologics MSM1-RF 2
Battery Charger 2
Storage Case 3
Technical Information 3
Visual Inspection 3
SECTION 2
The Myologics MSM1-RF 4
Description of Apparatus 4
Master Unit 5
Slave Unit 5
Computer Interface Unit 5
Pain Threshold Posts 5
Software Disk 5
Touch Pad and Push Buttons 5
Mechanical Instructions for Use 5
Recharging the battery 7
For Optimal Mechanical 8
Labels 8
SECTION 3
Application / Administration 10
Professional Use Instructions 10
Maintenance & Cleaning 10
Disposal 11
SECTION 4
Warranty Information 12
Limited Warranty 12
Terms and Conditions 12
Point of Contact 13
Warranty Card 13

MYOLOGICS MSM1-RF OPERATION & MAINTENANCE MANUAL
1
CAUTION: FEDERAL LAW RESTRICTS THE USE OF THIS DEVICE BY ORDER OF PHYSICIAN
Introduction to Contents
Identifies and describes each item included in the Myologics
MSM1-RF package.
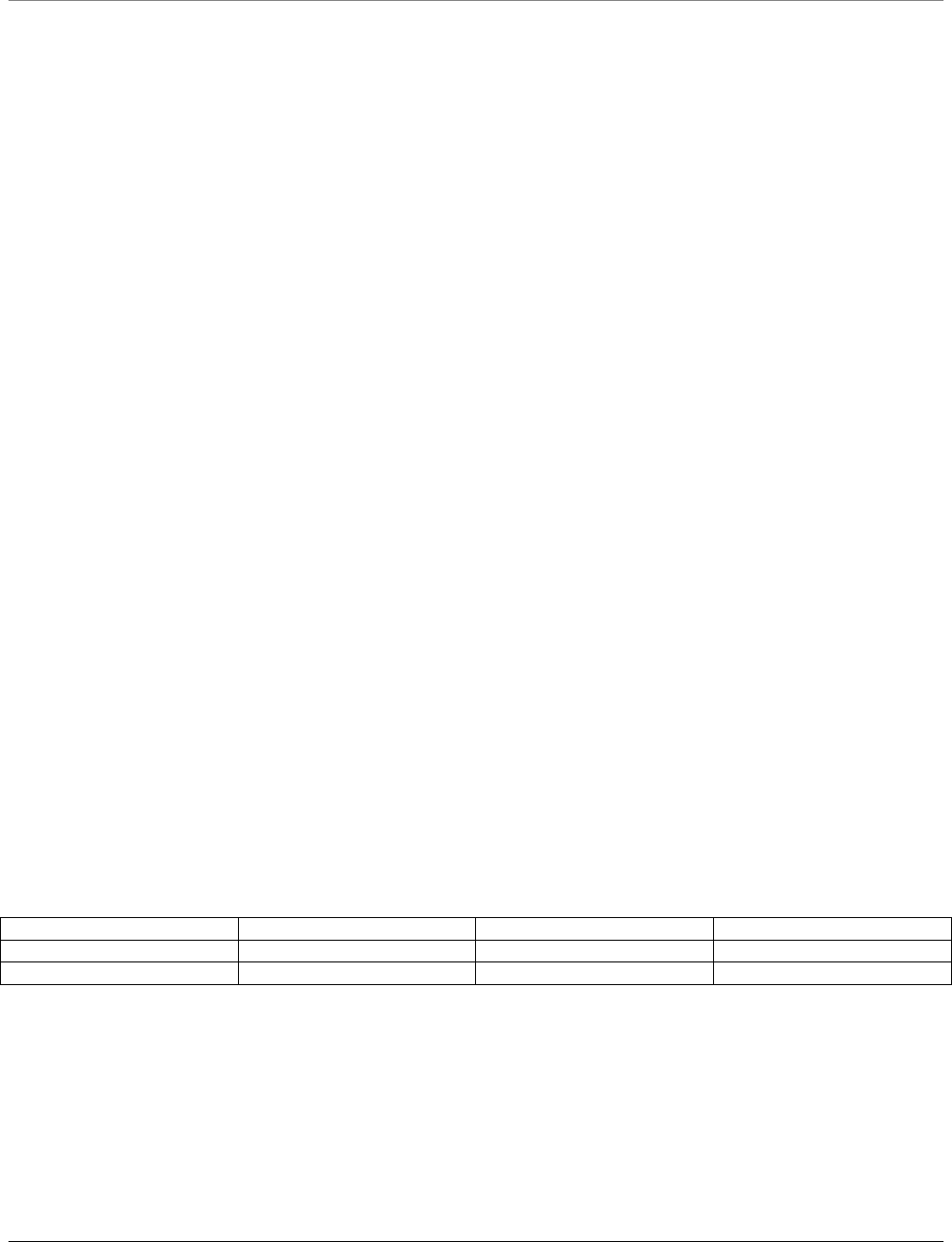
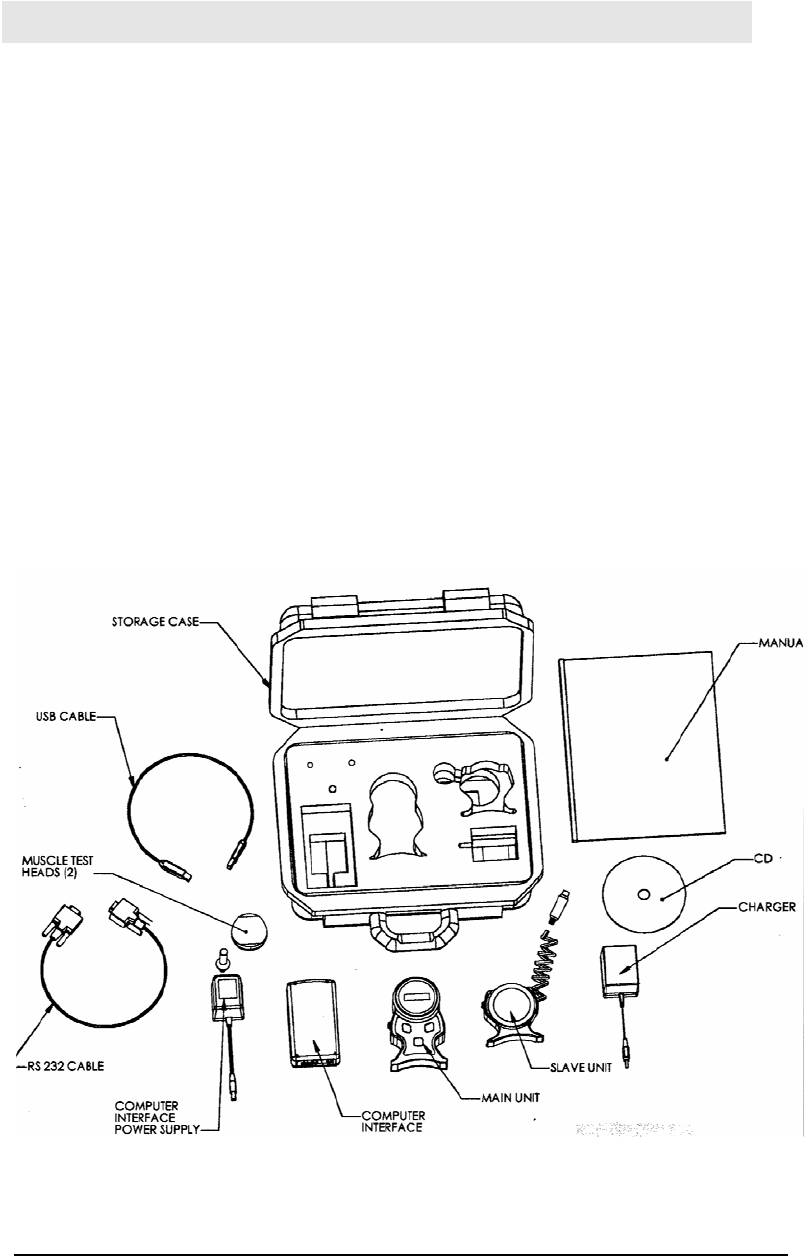
he Myologics MSM1-RF package is made up of the following equipment
items:
• Master Unit
• Slave Unit with attached 6 pin connector
• Computer Interface Unit
• Battery Charger
• USB Cable, RS-232 Cable and 2 AC adapters
• Software CD
• 1 Pain Test Post, 1 Contoured, and 1 Flat Muscle Test Post
In addition to the equipment items, we have included this Operation &
Maintenance manual that contains
• Written instructions for use and care
• Compliance information and label identification
• A warranty agreement and return card
Each Myologics MSM1-RF package is subjected to a thorough Quality Assurance
inspection in order to ensure that you, the physician, receive the highest quality
Sectio
n
1
T
MSM1-RF OPERATION & MAINTENANCE MANUAL
2
product. Through the shipping process marginal loss / or damage may occur.
Please take the time to ensure you have received each item, and on visual
inspection that each component appears to be in good working order, as all items
will be referred to in the following paragraphs and sections.
The Myologic MSM1-RF
The MSM1-RF
The Myologics MSM1-RF
device is an accurate
portable Combination
Force Evaluation and
Range of Motion testing
system. The MSM1-RF is
a portable, self-contained
unit that is battery operated
for ease of use.
• Fits comfortably in the
palm of your hand.
• Provides you with
objective, quantifiable
data from the time-
tested art of “hands-on”
manual muscle and range of motion testing.
• Aids in the differential diagnosis, prognosis and treatment protocols for
neuromuscular and musculoskeletal disorders.
The Battery Charger
The Myologics MSM1-RF contains a unique battery system designed by specification
to provide constant and consistent power, capable of intense use for extended periods,
while yet offering lightweight for portability.
The battery system encompasses both the internal battery component and the power
pack, or battery charger. The internal battery is sealed by the vendor and then encased
within the device housing and can only be replaced by the manufacturer. The battery
component is refreshed by the use of an external charger. The charger is an IEC
60601 certified unit, compliant to CE standards.
The battery component and the charger are a matched set and work in
harmony with each other; therefore, for optimum battery life and performance
use ONLY the supplied battery charger.
MSM1-RF OPERATION & MAINTENANCE MANUAL
3
Storage Case
The Myologic MSM1-RF and its peripheral components are packaged in an
industrial strength molded plastic storage case. This case provides protection to
the device when not in use or during transportation.
Technical Information
Technical documentation required by the physician, in case of necessary reparations,
will be provided by our EU agent. These documents will be supplied once the
manufacturer, working with the EU agent, makes the determination that the requested
documents do not constitute a disclosure of priority or patent protected information
and are a part of the filed and documented technical file.
Visual Inspection
This completes the listing of and description of the components. Once you have
familiarized yourself with each and ensured all are in good working order, proceed
to the next section.

MSM1-RF OPERATION AND MAINTENANCE MANUAL
4
The Myologics MSM1-RF
Detailed description of the Myologics MSM1-RF device including label
identification and operating instructions.
Description of Apparatus
he Myologics MSM1-RF is a 3 piece unit containing a master unit that measures
pain thresholds and communicates to the computer interface unit. A slave unit
which measures range of motion when coupled together with the master unit
via the 6 pin connecting cable. Finally a computer interface unit that is
connected to your computer via the provided USB or RS-232 cable. An internal battery
that is recharged using an external electric source powers the unit. This configuration
offers portability as well as consistency of power.
The Myologics MSM1-RF is manufactured in accordance to the Good Manufacturing
Procedures set forth by the FDA and CE standards and testing. The MSM1-RF device
has been built to an ISO Certified Quality Assurance Program.
Master Unit
The function of the master unit, when used for muscle testing, displays and records
numerically the peak force being applied to the transducer pad. At the completion of
this test the highest force value, or peak force is displayed. The MSM1-RF shows
peak force readings in pounds or kilograms, depending upon what you specified at
purchase. When used in inclinometer mode the unit displays the angle variation of
your start and stop points when your test is complete. All data and numerical values
are displayed to the user on the LCD (Liquid crystal display) which is on the front of
this unit.
Sectio
n
2
T

MSM1-RF OPERATION & MAINTENANCE MANUAL
5
Slave Unit
This unit is connected to the master unit using the 6 pin connector for inclinometer or
range of motion testing. This device measures a static angle in relationship to the
horizontal, vertical or to a determined zero starting point. The MSM1-RF inclinometer
is a gauge that uses a sensor to accurately measure to within 1.0 degrees. When the
inclinometer is moved the sensor will settle to a stable position in 0.1 seconds which
means you can click the button to mark the angle as soon as the patient stops moving.
Computer Interface Unit
This unit is connected to your computer using the provided USB or RS-232 cables.
The computer interface unit receives the data from the master unit using an RF (radio
frequency) transmittal. The data is then sent to the computer to be displayed to the end
user.
Pain Threshold Posts
There are three types of pain threshold posts. A curved transducer pad, for use on
round surfaces such as the arm or lower leg. A flat transducer pad, used on large
surfaces such as the back or upper leg. A digit transducer pad is used on smaller areas
such as fingers and toes.
Software Disk
This disk contains the software necessary to record and display test data to the end
user.
Touch Pad and Push Buttons
The touch pad contains three keys readily displayed to the user which are the
CMMT, CROM, and a Reset buttons. There is one push button switch located on the
master unit and on the slave unit.
Mechanical Instructions for Use
Note: Need to charge master unit 8-12 hours before initial use.
There is no ON/OFF switch therefore the incorporated design is a sleep mode. The
unit will enter SLEEP mode if unused for a period of about five minutes. Pressing
the RESET button on the keypad on the front of the unit will turn the unit back on.

MSM1-RF OPERATION & MAINTENANCE MANUAL
6
As long as the unit is being used and a reading in taken in less than five minutes, the
unit will remain ON and continue to operate. If the unit is idle for 5(five) minutes,
the unit will go to SLEEP but will retain the last used settings.
To operate the system, the computer interface unit must be powered ON (by
plugging the AC adapter with the larger charge port on the end of the cable) into the
unit and the AC adapter inserted into a 110 volt outlet. The computer interface unit
must also be connected to the computer by either the SERIAL communications
cable or the USB interface cable. The appropriate software drivers must have been
previously installed in the computer for either of these cables to operate correctly.
Please refer to the Myologics operation manual for proper installation of the
software and communications drivers. NOTE: That either SERIAL or USB
communications must be set up to use the correct COM port as required in the
OPTIONS screen of the Myologics software.
After the Computer interface unit is powered up and connected to the computer, the
Master unit must be powered ON (by pressing RESET). The Master unit may be
used by itself (Single Site Testing) or in conjunction with the Slave unit (Dual Site
Testing). If dual site testing is desired, the Slave unit must be connected to the
Master unit by plugging the connector on the end of the Slave unit into the
receptacle on the top of the Master unit. The RESET button must be pressed after
the Slave is connected to allow the Master unit to read the Slave. The Slave unit
must be disconnected from the Master unit if single site testing is desired or if
muscle test is being preformed.
At this time if all units are working correctly, the Green LED indicator on the
Computer Interface unit will be flashing and the RED LED indicator may or may
not flash. The RED LED indicator signifies a valid USB signal, so if the USB
operation was chosen, then the RED indicator should also be flashing.
To perform ROM (range of motion) tests, press and release the CROM button on the
keypad on the front of the master unit. The angle of tilt will be displayed on the
LCD screen on the master unit and the same value should be displayed on the
computer screen of the computer running the Myologics software application.
Pressing either of the RESET buttons (RESET on the keypad of the Master unit or
push button on the side of the Slave unit) will reset the unit and display a reading of
ZERO on the LCD screen and the computer monitor. Any tilt of either the Master
unit or the Slave unit (if connected) will be measured and displayed simultaneously
on both of these displays. To store the readings in the computer, press and release
the push button on the side of the Master unit.
To perform Muscle Testing (MT), press and release the CMMT button on the
keypad on the front of the Master unit. NOTE: That the Slave unit must NOT be
connected to the Master at this time. To perform muscle testing, insert the

MSM1-RF OPERATION & MAINTENANCE MANUAL
7
appropriate MT pad into the opening. The reading of the muscle test is displayed in
two (2) locations on the LCD display of the Master unit and also on the Computer
monitor of the computer running the Myologics software application. As force is
applied to the padded end of the MT adapter, the force reading will be displayed in
“real time” on the right side of the LCD screen on the front of the Master unit and
the last reading taken will be displayed on the left side of the LCD screen. The peak
force value is automatically stored on the left side of the LCD screen and in the
computer running the Myologics application. The operator does not need to press
any keys or buttons to store the result. NOTE: That the force applied to the padded
MT adapter must exceed two (2) pounds before the value will register and be
displayed.
After testing is completed, the Master unit will go into SLEEP mode automatically
after five (5) minutes of inactivity. There is no need to place the unit in any
particular state, but the muscle testing adapter pads should be removed from the
Master unit to prevent any unwanted force from being applied to the Muscle Test
unit. This could inadvertently produce unwanted readings on the device which
could prevent if from powering OFF into SLEEP mode as desired.
There is no maintenance required as there are no user serviceable parts in the unit.
Recharging the Battery on the Master Unit
This unit contains a non-replaceable rechargeable battery system that is not
accessible to the user. The batteries must be recharged using the supplied battery
charger/ AC adapter on a periodic basis. This adapter is identifiable by the smaller
charge port.
To recharge the Master Unit:
1. Plug is inserted into the CHARGE PORT on the back of the master unit.
2. Plug the battery charger into any 110-volt electric outlet. Alternatively, if the
device is being used in another country, a special charger, which can be obtained
from Erchonia®, must be used.
3. Leave the charge on for 8-12 hours to ensure a full charge.
4. After heavy use, unit should be charged again for 8 – 12 hours.
5. To check charge status press and hold the CROM button on the keypad which is
on the front of the unit, while holding this button immediately press and release

MSM1-RF OPERATION & MAINTENANCE MANUAL
8
the RESET button on the keypad. NOTE: The reading on the LCD display will
show battery voltage along with the letters BAT. A reading of nine (9) indicates
a full charge, eight (8) indicated over a ¾ charge, seven (7) or below shows the
battery is in need of recharging. If voltage drops below six (6) the unit will not
function and the LCD screen will not display the battery voltage.
The battery is an internal, non-accessible unit, and as such can only be changed by the
manufacturer. There is no risk to the device and or the user for the battery to remain
within the unit when not in use for extended periods. Prior to beginning reuse, after an
extended period of being unused, recharge battery using supplied battery charger.
DO NOT USE ANY CHARGER OTHER THAN MANUFACTURER’S.
DOING SO MANY CAUSE DAMAGE TO THE UNIT AND VOIDS THE
MANUFACTURERS WARRANTY.
For Optimal Mechanical Performance
1. Avoid operating unit with the battery charger connected. The unit will function
with the charger plugged in, however it is not recommended to use it in this
manner, as battery will become weak. This posses no risk to the physician or
patient; however it will shorten the battery life.
2. Do not store next to electronic equipment that emits a radio frequency, as
interference may occur. This posses no risk to the physician or patient however,
it may interfere with normal operation of the device.
Labels
Labels are placed on the unit for two reasons, 1) Compliance to the governing codes and
regulations, and 2) Information for the doctor.
The compliance issues have been concatenated into one label that is located on the body
of the device.

MSM1-RF OPERATION & MAINTENANCE MANUAL
9
All company information is embedded into the device art, (the burgundy and white)
membranes. Company information provides the patient with the manufacture’s name,
address, and telephone number.
Manufacture Information: Distributor Information:
Erchonia® Medical, Inc. Myo-logics
4751 E. Indigo St. 11417 124th Av, NE Ste #102
Mesa, AZ 85205 Kirkland, WA 98033
Phone: 480-633-3129 (800) 768-7253

MSM1-RF OPERATION & MAINTENANCE MANUAL
10
Application / Administration
This section defines instructions for the application of the MSM1-RF, established
protocols and the precautions to be considered during administration.
he Myologics MSM1-RF device is intended for use by healthcare
professionals for treatment of the symptoms associated with pain. Treatment
protocols defined herein, were developed by healthcare professional
knowledgeable with the product.
Professional Use Instructions
The Myologics MSM1-RF device is to be administered externally for accurate combination
force evaluation and range of motion use. Using the approved agency regulations
Maintenance and Cleaning
The Myologics MSM1-RF, if used properly will operate efficiently for years. To ensure
proper care, it is advisable for the physician to perform:
1. Regular visual inspections to ensure there is no external damage other than
normal wear and tear. If during these inspections, you identify an area of
concern, please contact the manufacture to determine if action is required.
2. If you notice a change in the performance of the device, while in the ON
position, please contact the manufacture to determine if action is required.
Sectio
n
3
T

MSM1-RF OPERATION & MAINTENANCE MANUAL
11
3. The internal components should not require any maintenance, however if an
issues arises, which will show itself in the form of altered performance, the
device must be sent to the manufacture.
4. Since the device is a hand held device, periodic cleanings of the exterior surface
is required. To perform this, lightly dampen a cloth with rubbing alcohol and
gentle wipe the exterior surface. Take care not to have the dampened cloth come
into contact with the power port or the rubber “O” rings on the test posts.
5. If during treatment, any part of the device comes in contact with a patient,
perform the same cleaning process as in item (4) four.
Disposal
The Myologics MSM1-RF device is a self-contained unit that emits a radio frequency
and as such creates no byproduct that requires disposal, however the unit itself, when
spent and beyond repair or functional use, should be sent back to the manufacturer for
disposal. This process ensures the proper separation and handling of all the internal parts
and reduces any risk to the user and environment.

ERCHONIA OPERATION AND MAINTENANCE MANUAL
12
Warranty Information
Detailed description of the Terms and Condition for warranty of the
Myologics MSM1-RF device.
Limited Warranty
he Myologics MSM1-RF device is warranted to be free from defect in
material and workmanship for a period of TWO YEAR from the date of
purchase. For warranty to be valid, it is critical that the physician complete
and return the enclosed warranty card. Failure to return warranty card may
adversely impact warranty processing and / or void warranty.
Terms and Conditions
• Shipping required to facilitate warranty repair and or maintenance issues
within the first 90-days, will be paid by manufacturer.
• Shipping required to facilitate warranty repair and or maintenance issues after
90-days, is the financial responsibility of the patient.
• The warranty DOES NOT cover instances involving or damages resulting
from:
− Accident, misuse or abuse
− Lack of responsible care
− Use of unapproved battery charger
− Alteration or disassembly
− Loss of parts
Sectio
n
4
T

MSM1-RF OPERATION & MAINTENANCE MANUAL
13
− Exposure to the elements
− Ingress of liquid
− Exposure to excessive electromagnetic frequency
Point of Contact
If for any reason you are dissatisfied with this product, warranty concerns or questions
regarding proper operation, please call 480.633.3129x116, for immediate assistance.
Warranty Card
Please remove warranty card from pocket below, complete and mail within 90 days of
purchase. Failure to do so may adversely impact manufacture’s ability to successfully
administer warranty.