Fukuda Denshi Co LX7230KM ECG, Respiration and SpO2 Transmitter User Manual
Fukuda Denshi Co Ltd ECG, Respiration and SpO2 Transmitter
User manual
ECG, Respiration and SpO2
Transmitter
LX-7230
Operation Manual
LX-7230N LX-7230KM
● Before using this device, read this operation manual thoroughly.
● Keep this manual near the device for future reference.
CAUTION
Federal Law restricts this device to sale by or on the order of a physician.
CAUTION
Users are advised to periodically contact the FCC or specified frequency
coordinator and determine if other or your transmitter frequencies that
may cause interference.
CAUTION
The manufacturers, installers and users of Wireless Medical Telemetry
System equipment are cautioned that the operation of this equipment
could result in harmful interference to other nearby medical devices.
CAUTION:
• This device for sale by or on the order of a physician.
• The company and product names used in this manual are trademarks or
registered trademarks.
• If this manual has pages missing or out of order, contact Fukuda Denshi
for replacement.
• Only physician or persons instructed by physicians are allowed to use the
equipment.
• The information contained in this document is subject to change without
notice due to improvement in the equipment.
Copyright © 2010 by Fukuda Denshi Co., Ltd.
No part of this document may be copied or transmitted in any form without the prior
written permission of Fukuda Denshi Co., Ltd.
Printed in Japan
Thank you for purchasing this product from Fukuda Denshi.
Before use, read this operation manual thoroughly for correct handling
and operation.
Safety Precautions
Read the “Safety Precautions” thoroughly before use to ensure correct and
safe use of the product.
Make sure to follow the precautions indicated below, as these are important
messages related to safety.
DANGER Failure to follow this message may cause immediate
threat of death or serious injury.
WARNING Failure to follow this message may result in death or
serious injury.
CAUTION Failure to follow this message may cause injury or
failure to the equipment.
NOTE
A note is not related to product safety, but provides
information about the correct use and operating
procedures to prevent incorrect operation and
malfunction of the equipment.
Precaution from Fukuda Denshi
Fukuda Denshi is liable for the safety, reliability, and performance of its device
only if;
Maintenance, modifications, and repairs are carried out by authorized
personnel.
Components are used in accordance with Fukuda Denshi operating
instructions.
If the equipment is used incorrectly and become unusable, Fukuda Denshi is
not liable for the malfunction. Use the equipment only for the purpose specified
in this manual.
i
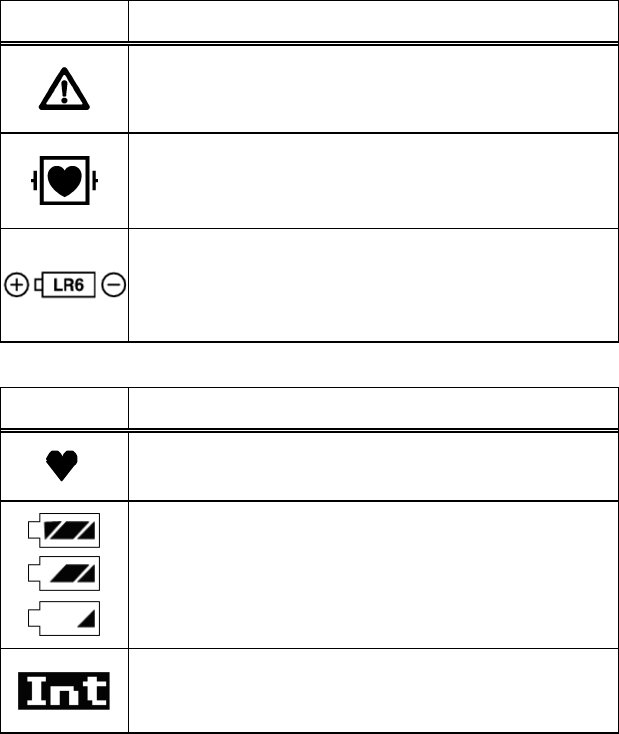
Equipment Symbols
Refer to the following symbols indicated on the LX-7230 for their meanings.
Symbols indicated on the main unit
Symbol Description
Caution; read the operation manual.
Indicates the need to refer to the operation manual before
operation.
Type CF Applied Part with Defibrillation-Proof
Indicates that the degree of protection against electric
shock is Type CF Applied Part with defibrillation-proof.
Indicates the battery type and direction.
Symbols indicated on the LCD screen
Symbol Description
Heart Rate Synchronization Mark:
This mark flashes synchronizing with the heartbeat.
Battery Mark:
Indicates the remaining battery level.
Intermittent SpO2 Mode:
Indicates that the SpO2 Intermittent measurement is
activated.
Displayed only on the LX-7230KM.
ii

Precautions for Safe Operation of Medical Electrical Equipment
Cautions described here are regarding the general instructions for safety use
to the patient and the operator. As for cautions about the LX-7230, please refer
to the following pages.
CAUTION
1. Users should have a thorough knowledge of the operation before
using this equipment.
2. Pay attention to the following when installing or storing the
equipment.
Do not install or store in an area where the equipment will be subject
to splashing water.
Do not install or store in an area where the environmental
conditions, such as atmospheric pressure, temperature, humidity,
ventilation, sunlight, dust, sodium, sulfur, will adversely affect the
system.
Place the equipment on a stable surface where there is no
inclination, vibration, or shock (including during transportation).
Do not install or store in an area where chemicals are stored or
gases are generated.
3. Before operating the equipment, verify the following items.
Check the cable connection and polarity to ensure proper operation
of the equipment.
Ensure that all cables are firmly and safely connected. Especially,
recheck the attachment and connection condition of electrode and
the probe (sensor).
Pay special attention when the equipment is used in conjunction with
other equipment as it may cause erroneous judgment and danger.
Check the remaining battery level.
When replacing the battery, make sure that the battery polarity is
correct. Do not charge the battery.
4. During operation of the equipment, verify the following items.
Do not operate the equipment beyond the time period required for
diagnosis and medical care.
Do not pick up and/or swing the equipment pulling/grabbing the
probe (sensor) or cable part. It may damage the equipment and lead
to measurement error.
Always observe the equipment and patient to ensure safe operation
of the equipment.
If any abnormality is found on the equipment or patient, take
appropriate measures such as ceasing operation of the equipment
and/or detaching the probe (sensor) and/or electrode, in the safest
way for the patient.
Do not allow the patient to come in contact with other equipments.
iii
CAUTION
5. After using the equipment, verify the following items.
Make sure to turn off the power of the equipment.
When unplugging the cables, do not apply excessive force on the
cable and pull from its connector.
Clean the accessories and cables, and keep them together in one
place.
Keep the equipment clean to ensure proper operation for the next
use.
Make sure to remove the batteries if the equipment is not used for a
long time. The leakage from the batteries may damage the device or
an explosion from the batteries may occur.
6. If the equipment is damaged and in need of repair, ensure patient
safety by immediately turning the equipment off and remove the
electrodes and/or probe from the patient. User should not
attempt service. Label the unit “OUT OF ORDER” and contact
Fukuda Denshi representative.
7. Do not remodel the equipment.
8. Maintenance Check
Make sure to periodically check the equipment, and accessories.
Before reusing the equipment that has been left unused for a while,
make sure that the equipment works normally and safely.
9. When using electrosurgical knives or defibrillator with this
equipment, take care of the following.
To prevent burn injury to the patient, verify proper attachment of
patient ground plate, ECG electrode type for the electrosurgical
knives, and the quantity of gel, output energy for the defibrillator.
Also, verify that a proper ground is selected.
Some types of equipment other than the above may cause
accidental hazards to the patient and operator due to the conditions
of the equipment. Read the operation manual attached to each
device and understand the precautionary instructions prior to use.
Non-Explosion Proof
DANGER
Never operate the equipment in the presence of flammable anesthetics,
high concentration of oxygen. It may cause an explosion or fire.
Never operate the equipment inside a hyperbaric chamber. It may
cause an explosion or fire.
Never operate the equipment where flammable gas or fluid such as
anesthetic, oxygen, and hydrogen are used. It may cause an explosion
or fire.
iv
Precautions about Magnetic Resonance Imaging (MRI)
WARNING
Do not operate this equipment in magnetic resonance imaging (MRI)
environments.
When conducting MRI test, remove the electrodes and sensors
connected to the patient (test subject).
The local heating caused by the induced electromotive force may cause
burn injury to the patient (subject). For details, refer to the operation
manual for the MRI testing device.
Electrosurgery Safety
WARNING
When using electrosurgical instrument, make sure the contact between
the patient and the ground plate is secure. If the connection is
incomplete, the patient may suffer a burn at the electrode site.
When using an electrosurgical instrument, it may misidentify noise from
the electrosurgical instrument as a heartbeat or arrhythmia.
Defibrillation Safety
WARNING
Use only the lead cable specified by Fukuda Denshi when defibrillating.
If used by unspecified lead cable, the device may be damaged, resulting
in a safety hazard.
When using the defibrillator, keep away from the electrodes or
medicament applied to the patient chest. If this is not possible, remove
the electrodes or medicament before using it.
If the defibrillator paddles are directly in contact with the electrodes or
medicament, an electrical shock may result from the discharged energy.
When using the defibrillator, do not touch the patient and the metal part
of the device or cables. Electric shock may result from the discharged
energy.
v
Precautions about the Pacemaker
WARNING
Minute ventilation rate-adaptive implantable pacemakers can
occasionally interact with certain cardiac monitoring and diagnostic
equipment, causing the pacemakers to pace at their maximum
programmed rate. The cardiac monitoring and diagnostic equipment
may possibly send wrong information.
If such event occurs, please disconnect the cardiac monitoring and
diagnostic equipment, or follow the procedures described in the
operation manual of the pacemaker.
(For more details, contact FUKUDA DENSHI personnel, your
institution’s professionals, or your pacemaker distributors.)
Reference
“Minute Ventilation Rate-Adaptive Pacemakers”
FDA alerts health professionals that minute ventilation rate-adaptive
implantable pacemakers can occasionally interact with certain cardiac
monitoring and diagnostic equipment, causing pacemakers to pace at their
maximum programmed rate.
[October 14, 1998 (Letter: www .fda.gov/cdrh/safety.html) – FDA]
ECG meter may continue to count the pacemaker rate during
occurrences of cardiac arrest or arrhythmias. Do not rely entirely upon
the ECG meter alarms. Keep pacemaker patients under close
surveillance. Check this manual for disclosure of the pacemaker pulse
rejection capability of this instrument.
Precautions about the LX-7230
WARNING
Do not connect cables not authorized by Fukuda Denshi to any I/O
connector. If done so by mistake, the LX-7230 cannot deliver its
maximum performance and may be damaged, resulting in a safety
hazard.
Do not use this device with multiple patients simultaneously.
CAUTION
Do not pick up and/or swing the LX-7230 pulling/grabbing the probe
(sensor) or cord part. The cable could break or get disconnected from the
LX-7230. And it may hit people or damage other equipment around.
vi
Precautions about Waterproof
CAUTION
Replace the “Battery Compartment Lid” of the LX-7230 regularly to keep
the main unit performance of waterproof. If not regularly replaced, the
quality of the lid will deteriorate and cannot keep the waterproof
performance. For details about the regular replacement, contact your
local Fukuda Denshi service representative.
The lid may be damaged from high impact. If the LX-7230 is dropped or
is subjected to a high impact, make sure that the lid is not damaged.
However, the SpO2 probes (sensors/relay cables) are not waterproof.
Do not take a bath with them, and ensure to be away from liquid.
Do not use the LX-7230 wet. Always wipe the LX-7230 with a soft cloth
and dry it thoroughly before use.
Precautions about ECG
CAUTION
When removing electrodes from the patient, remove them carefully and
slowly. Do not apply excessive force to remove them. Otherwise, it may
damage the skin.
There are some cases when the pacemaker pulse cannot be detected
depending on the pacemaker type, pulse voltage, pulse width, electrode
lead type (unipolar, bipolar), electrode placement, or lead method which
causes the pacemaker pulse amplitude to decrease and disables
pacemaker pulse detection.
If signals similar to a pacemaker pulse are present, such as electric
blanket noise or excessive AC frequency noise, these may be
erroneously detected and displayed as a pacemaker pulse. In this case,
check the condition of the electrodes and ECG lead cable to resolve the
cause or turn off the pacemaker detection setting on the receiving
monitor.
vii
Precautions about SpO2
WARNING
During SpO2 monitoring, always use the probe (sensor)/relay cable
specified by Fukuda Denshi. If any other probe (sensor)/relay cable is
used, a high temperature rise of the probe (sensor) may place the
patient in danger of burns in the worst case.
When the SpO2 probe (sensor) is in a connector-off condition, the SpO2
alarm will not be generated on the receiving monitor. Make sure that the
SpO2 probe (sensor)/ relay cable is securely connected. If the SpO2
waveform/numeric data is not displayed, check the patient’s condition
and pay attention not to miss the connector-off condition.
When measuring the SpO2 of a patient with high fever or peripheral
circulatory insufficiency, check the probe (sensor) attachment
periodically and change the attachment site. The temperature of the
attachment site will rise 2 to 3C due to the sensor heat which may
result in compression necrosis and burn injury.
Even a short duration of attachment may inhibit the blood flow and
generate compression necrosis and burn injury.
When securing the probe (sensor) with tape, do not apply the tape too
tight. At the same time, check the blood flow constantly so that
congestion is not generated at the peripheral. When removing the tape,
remove it slowly with care not to damage the patient’s skin.
CAUTION
For the following case, accurate measurement may not be possible.
Patient with excessive abnormal hemoglobin (COHb, MetHb)
Patient with the pigment injected to the blood
Patient receiving CPR treatment
Placement of SpO2 probe (sensor) on limb with a blood-pressure cuff,
arterial catheter, or intravascular line
When measuring at placement position with venous pulse
Patient with body motion
Patient with small pulse
Excessive body motion (patient’s motion)
Excessive light (direct sunlight, fluorescent, light therapy equipment,
surgical light, infrared heat ramp, etc.)
External colorant such as nail polish
Abnormally low or high hemoglobin concentration
Electrosurgery
Influence of electromagnetic waves from other electronics devices
High-intensity radio waves from cell phone
viii
Precautions about Output Signal
WARNING
Do not use the output signal of the monitor that receives radio wave signal
from the LX-7230 as the trigger signal for IABP, MRI echocardiographic, or
defibrillator for the following reasons.
It may lead to a delay of operating timing due to the delay time of
waveform transmission.
A trigger signal unrelated to the heart rate may be generated due to
the interfusion of spike noise at weak electric field.
Precautions about Accessories and Optional Accessories
WARNING
Use only the accessories, such as ECG Lead cable and SpO2 probe
(sensor)/ relay cable, specified by Fukuda Denshi for the LX-7230.
Otherwise, the LX-7230 cannot deliver its maximum performance and may
be damaged, resulting in a safety hazard.
CAUTION
Do not reuse disposable products.
Store the disposable products properly as mentioned in their user
manuals.
Precautions about Battery
WARNING
Use new "AA" size (“LR06” size) alkaline cell.
Install the battery with the correct polarity.
Do not charge the battery. Any attempt to charge the battery may cause
it to leak or break.
Do not short the (+) and (-) terminals. It may result in exothermic heat
and fire.
Do not throw the battery into fire. It may explode.
ix
Precautions about Disposing of Equipment, Accessories, or
Components
CAUTION
When disposing of the equipment, accessories, or components, use an
industrial waste distributor. Do not dispose of as ordinary waste.
Used disposal items (ECG electrodes, etc.) shall be discarded as
medical waste.
Precautions about Disposing of Battery
CAUTION
Obey the local municipal rule to dispose the used dry cell battery.
Precautions for Use of Medical Telemeter
WARNING
The LX-7230 transmitter must not be co-located or operated in
conjunction with any other antenna or transmitter.
The LX-7230 complies with FCC radiation exposure limits set forth for a
controlled environment and meets the FCC radio frequency (RF)
Exposure Guidelines in Supplement C to OET65. The LX-7230 has very
low levels of RF energy that are deemed to comply without testing of
specific absorption ratio (SAR).
Operation of LX-7230 requires the prior coordination with a frequency
coordinator designated by the FCC for the Wireless Medical Telemetry
Service.
This radio frequency device is susceptible to interference from outside
sources. Interference may prevent the monitoring of patients connected
to this device. If a problem exists, contact your local service
representative.
The LX-7230 transmits vital signs to the receiving monitor using radio
wave signal. Under unstable radio wave signals, the receiving monitor
will not generate any alarms. This situation may miss sudden change in
the patient's condition and may cause a serious accident. Under
unstable radio wave signals, check the patient status consistently under
this situation. To get stable radio wave signals, make sure to have a
proper telemetry installation.
CAUTION
For installation, make sure the following.
The medical institution (hereinafter referred to as the “Institution”) must
decide the telemetry installation plan for the medical department in order
to prevent interference and interference between transmitters (telemetry
x

xi
based on destination country’s radio law). When telemetry has already
been installed and been used, radio format, frequency, and antenna
power are required to be examined to prevent interference.
When using telemetry, which requires zone location, the Institution is to
set up the zones as an operation unit for each transmitter to prevent
electronic interference between telemetry throughout the Institution.
When using telemetry, which requires zone location, display and identify
each prepared zone in the equipment.When laying receiver antenna for
each transmitter, the Institution has to examine the installation so that
electronic interference does not occur.
Based on the above examination result, the Institution should install each
receiver antenna as required.
For management, make sure to follow the precautions below.
The Institution should appoint a person (hereinafter referred to as the
“Overall Manager”) to manage the wireless channels for the whole
Institution.
And when using telemetry, which requires zone location, the Institution
should nominate a person (hereinafter referred to as the “Zone Manager”)
to manage the wireless channels in each zone. However, when using such
telemetry in a local Institution, one person can perform both functions.
The Overall Manager and Zone Manager must be selected from people
who understand the characteristics and functionality of telemetry systems,
and are skilled in operating telemetry.
When installing telemetry, the Overall Manager and the Zone Manager
have to understand the precautions for use of telemetry in advance.
The Overall Manager is responsible for maintenance of wireless channel
and storage and maintenance of telemeter in the overall medical facilities
to give proper instructions to the Zone Manager when using telemetry
needing zone alignment, and to the telemetry user when using telemetry
not-needing zone alignment.
The Overall Manager should create a management log (hereinafter
referred to as the “log”), which contains a list of the management status of
the wireless channels for the whole Institution. When changing a wireless
channel, register it in the log and give proper instructions to the Zone
Manager or to the user.
The Zone Manager assumes responsibility for managing the wireless
channels, storing, and managing telemetry.
The Zone Manager assigns the transmitter to the user, and provides
enough education for use inside the zone.
The telemetry user verifies operation of the transmitter/receiver before
use.
The telemetry user, if using the telemetry in a zone location, follows the
instructions of the Zone Manager for the zone and gives instructions to the
patient if required.
When interference or breakdown occurs in telemetry communication, the
user is required to inform the Zone Manager and the Overall Manager of
the problems. The Zone Manager and Overall Manager are to deal with
the problem properly and/or contact their nearest Fukuda Denshi
representative for service.
Electromagnetic Compatibility
The performance of this device under electromagnetic environment complies
with IEC 60601-1-2 (2007).
Precautions for Safe Operation under Electromagnetic Influence
CAUTION
If any sorts of electromagnetic wave, magnetic field, or static electricity
exist around the device, noise interference or malfunction of the device
may occur. If any unintended malfunction or noise occurs during
monitoring, check the magnetic influence and take appropriate
countermeasures.
The following are examples of the common cause and countermeasures.
●Cellular Phone
The radio wave may cause malfunction to the device.
Cellular phones and radio sets should be turned off in the room
(building) where medical device is located.
●Static Electricity
In a dry environment (room), static electricity is likely to occur. Take
the following countermeasures.
Both operator and patient should remove any static electricity before
entering the room.
Humidify the room.
xii
xiii
EMC Guidance
This equipment complies with IEC 60601-1-2 (2007). However, if portable
transmitter or wireless LAN equipment is used extremely nearby, the
electromagnetic influence may largely exceed the compliance level and may
cause unexpected phenomenon such as noise interference on the waveform,
etc.
Therefore, this equipment should be used in a location specified by each
medical institution. If any unexpected noise interference on the waveform or
failure to the peripheral device occurs, stop using the equipment and follow the
instruction of the technician.
The following is the information relating to EMC (Electromagnetic
Compatibility).
(When using this equipment, verify that it is used within the environment
specified below.)
●Compliance to the Electromagnetic Emissions
The LX-7230 is intended for use in the electromagnetic environment specified
below. The customer or the user of the LX-7230 should assure that it is used in
such an environment.
Emission Test Compliance Electromagnetic Environment –
Guidance
RF Emission
CISPR 11 Group 1
The LX-7230 uses RF energy only for its
internal function. Therefore, its RF
emissions are very low and are not likely
to cause any interference in nearby
electronic equipment.
RF Emission
CISPR 11 Class A
Harmonic Emission
IEC 61000-3-2 N/A
Voltage Limit /
Flicker Emission
IEC 61000-3-3
N/A
This LX-7230 is suitable for use in all
establishments other than domestic and
those directly connected to the public low-
voltage power supply network that
supplies buildings used for domestic
purposes.

xiv
●Compliance to the Electromagnetic Immunity (1)
The LX-7230 is intended for use in the electromagnetic environment specified
below. The customer or the user of the LX-7230 should assure that it is used in
such an environment.
Immunity Test IEC 60601-1-2
Test Level Compliance
Level
Electromagnetic
Environment
Guidance
Electrostatic
Discharge
(ESD)
IEC 61000-4-2
±6kV contact
±8kV air
±6kV contact
±8kV air
Floors should be
wood, concrete or
ceramic tile. If floors
are covered with
synthetic material, the
relative humidity
should be at least
30%.
Electrical fast
transient/burst
IEC 61000-4-4
±2kV:
Power supply lines
±1kV:
Input/output lines
N/A
Surge
IEC 61000-4-5
±1kV:
differential mode
±2kV:
common mode
N/A
Voltage dips,
short
interruptions
and voltage
variations on
power supply
input lines.
IEC 61000-4-11
<5% UT
(>95% dip in UT)
for 0.5 cycle
40% UT
(60% dip in UT)
for 5 cycles
70% UT
(30% dip in UT)
for 25 cycles
<5% UT
(>95% dip in UT)
for 5sec.
N/A
Power
Frequency
(50/60Hz)
Magnetic Field
IEC 61000-4-8
3A/m 3A/m
Power frequency
magnetic fields should
be at levels
characteristic of a
typical location in a
typical commercial or
hospital environment.
Note: UT is the AC mains voltage prior to application of the test level.
●Compliance to the Electromagnetic Immunity (2)
The LX-7230 is intended for use in the electromagnetic environment specified
below. The customer or the user of the LX-7230 should assure that it is used in
such an environment.
Immunity
Test IEC60601-1-2
Test Level Complianc
e Level Electromagnetic Environment
Guidance
Portable and mobile RF
communications equipment should
be used no closer to any part of the
LX-7230, including cables, than the
recommended separation distance
calculated from the equation
applicable to the frequency of the
transmitter.
Recommended Separation Distance
Conducted RF
IEC 61000-4-6
3Vrms
150kHz to 80MHz
3Vrms d = 1.2 P
Radiated RF
IEC 61000-4-3
3V/m
80MHz to 2.5GHz
3V/m d = 1.2 P 80MHz to 800MHz
d = 2.3 P 800MHz to 2.5GHz
Where P is the maximum output
power rating of the transmitter in
watts (W) according to the
transmitter manufacturer and dis the
recommended separation distance in
meters (m).
Field strengths from fixed RF
transmitters, as determined by an
electromagnetic site survey a), should
be less than the compliance level in
each frequency range b).
Interference may occur in the vicinity
of equipment marked with the
following symbol:
Note 1: At 80MHz and 800MHz, the higher frequency range applies.
Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is
affected by absorption and reflection from structures, objects and people.
a) Field strengths from fixed transmitters, such as base stations for radio
(cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM
radio broadcast and TV broadcast cannot be predicted theoretically with accuracy.
To assess the electromagnetic environment due to fixed RF transmitters, an
electromagnetic site survey should be considered. If the measured field strength in
the location in which the LX-7230 is used exceeds the applicable RF compliance
level above, the LX-7230 should be observed to verify normal operation. If
abnormal performance is observed, additional measures may be necessary, such
as re-orienting or relocating the LX-7230.
b) Over the frequency range 150kHz to 80MHz, field strength should be less than
3V/m.
xv
●Recommended Separation Distances between Portable and
Mobile RF Communications Equipment and the LX-7230
The LX-7230 is intended for use in an environment in which radiated RF
disturbances are controlled. The customer or the user of the LX-7230 can help
prevent electromagnetic interference by maintaining a minimum distance
between portable and mobile RF communications equipment (transmitters)
and the LX-7230 as recommended below, according to the maximum output
power of the communications equipment.
Separation Distance according to Frequency of Transmitter (m)
Rated Maximum
Output Power of
Transmitter
(W)
150kHz to 80MHz
d = 1.2 P
80MHz to 800MHz
d = 1.2 P
800MHz to 2.5GHz
d = 2.3 P
0.01 0.12 0.12 0.23
0.1 0.38 0.38 0.73
1 1.2 1.2 2.3
10 3.8 3.8 7.3
100 12 12 23
For transmitters rated at a maximum output power not listed above, the recommended
separation distance d in meters (m) can be determined using the equation applicable to
the frequency of the transmitter, where P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter manufacturer.
Note 1 : At 80MHz and 800MHz, the separation distance for the higher frequency range
applies.
Note 2 : These guidelines may not apply in all situations. Electromagnetic propagation
is affected by absorption and reflection from structures, objects and people.
xvi
xvii
CONTENTS
Safety Precautions..................................................................................... i
Precaution from Fukuda Denshi....................................................... i
Equipment Symbols .........................................................................ii
Precautions for Safe Operation of Medical Electrical Equipment ....iii
Non-Explosion Proof .......................................................................iv
Precautions about Magnetic Resonance Imaging (MRI)................. v
Electrosurgery Safety...................................................................... v
Defibrillation Safety ......................................................................... v
Precautions about the Pacemaker ..................................................vi
Precautions about the LX-7230.......................................................vi
Precautions about Waterproof .......................................................vii
Precautions about ECG .................................................................vii
Precautions about SpO2................................................................ viii
Precautions about Output Signal ....................................................ix
Precautions about Accessories and Optional Accessories .............ix
Precautions about Battery...............................................................ix
Precautions about Disposing of Equipment, Accessories,
or Components ............................................................................... x
Precautions about Disposing of Battery .......................................... x
Precautions for Use of Medical Telemeter ...................................... x
Electromagnetic Compatibility ............................................................xii
Precautions for Safe Operation under Electromagnetic Influence..xii
EMC Guidance.............................................................................. xiii
1. General Description ............................................................................. 1
2. Names of Parts and Their Functions.................................................... 3
3. Preparation .......................................................................................... 5
1) Installing the Batteries ................................................................ 5
2) Operating Power Switch ............................................................. 7
4. ECG Monitoring ................................................................................... 9
■ Connecting the ECG Lead Cable and Electrodes...................... 9
■ Attaching the Electrodes.......................................................... 12
■ Connecting the ECG Lead Cable to the LX-7230 .................... 13
5. Respiration Monitoring ....................................................................... 15
6. SpO2 Monitoring................................................................................. 17
SpO2 Monitoring (LX-7230N) ........................................................ 18
SpO2 Monitoring (LX-7230KM) ..................................................... 35
7. Measurement ..................................................................................... 45
■ Starting Screen........................................................................ 45
■ Waveform Display Screen ....................................................... 45
■ Battery Level Check ................................................................ 46
■ Waveform Display ................................................................... 47
xviii
8. Operation ........................................................................................... 57
■ Changing Setup....................................................................... 57
■ Restarting the LCD display ...................................................... 62
■ Pressing the EVENT button..................................................... 62
9. Other Setting Items ............................................................................ 63
■ Changing the Time Constant ................................................... 63
■ Changing the Detection Sensitivity of the Pacemaker Pulse ... 64
■ Changing the Respiration Detection Signal ON/OFF............... 65
■ Changing the LCD Contrast..................................................... 65
10. Changing the Transmitter Channel and Group ID............................ 67
■ Changing the Transmitter Channel.......................................... 67
■ Changing the Group ID............................................................ 67
11. Troubleshooting ............................................................................... 69
■ List of Displayed messages ..................................................... 69
■ Troubleshooting....................................................................... 72
■ In Case of Dropping the LX-7230 into Water ........................... 74
12. Cleaning and Disinfection ................................................................ 75
■ Cleaning .................................................................................. 75
■ Disinfection .............................................................................. 75
13. Maintenance and Inspection ............................................................ 77
14. Standard and Optional Accessories................................................. 79
■ Standard Accessories.............................................................. 79
■Optional Accessories................................................................. 80
15. Specification..................................................................................... 83
■ Specification ............................................................................ 83
■ Displays................................................................................... 87
■ Details of the “ELECTRODE?” Message................................. 89
■ List of Setup Items................................................................... 90
1
1. General Description
The LX-7230 is a radio telemetry transmitter designed to measure the ECG,
respiration waveform, SpO2 (arterial oxygen saturation), pulse waveform with
two “AA” size (“LR06” size) alkaline batteries.
Information such as ECG measurements, respiration waveform, SpO2
measurements, pulse waveform, battery level, and the conditions of the ECG
electrodes and SpO2 probe (sensor) are displayed on the LCD of the front
panel.
ECG lead selection is available using the two buttons (Enter and ▽) on the
front panel. (In case of using a 3-electrode lead cable or a 5-electrode chest
lead cable)
The LX-7230 can also function as a transmitter to measure only the
ECG/Respiration without SpO2 or to measure only the SpO2 without
ECG/Respiration.
Before using the LX-7230, read also the operation manual of the patient
monitor at the receiving side thoroughly.
There are two types of transmitters depending on the SpO2 Module, which is
built-in as below.
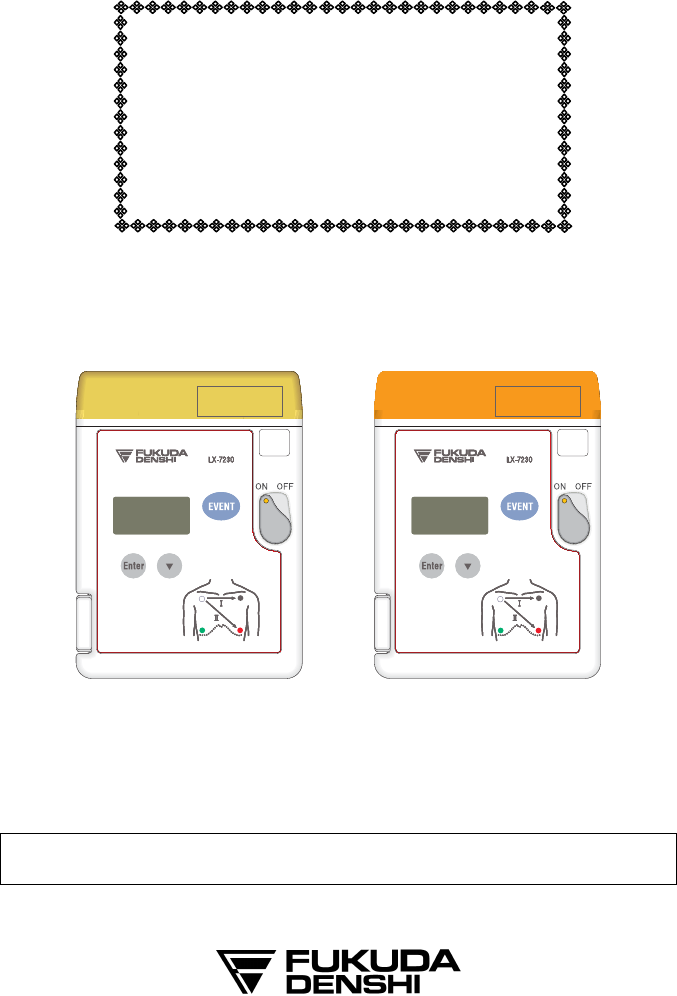
LX-7230N :Built-in Nellcor® SpO2 Module
LX-7230KM :Built-in Konica Minolta® SpO2 Module
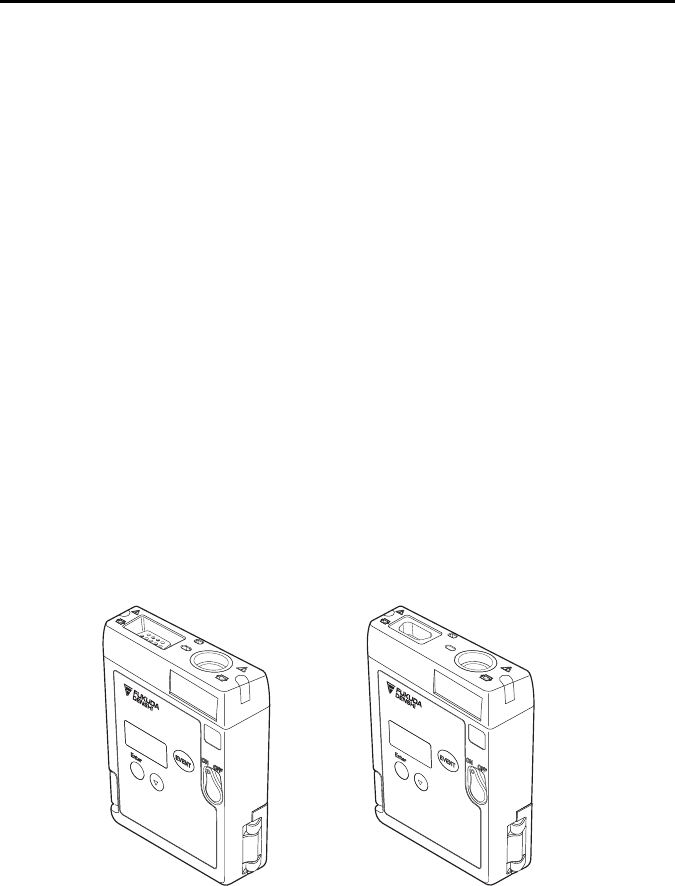
External Appearance
LX-7230N LX-7230KM
1. General Description
2
Blank Page
3
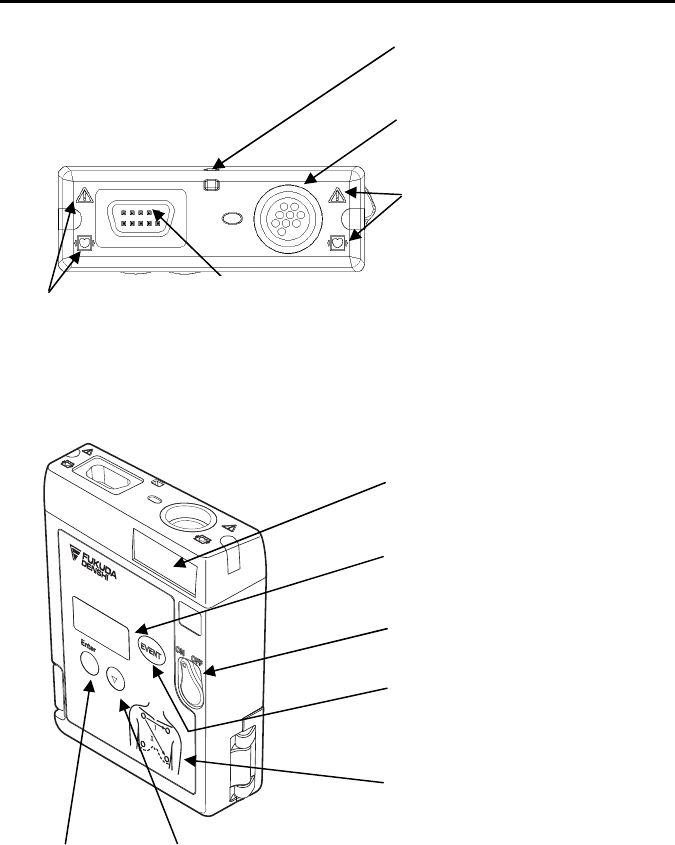
2. Names of Parts and Their Functions
Neck Strap Hole
A
ttaches the neck strap.
ECG/RESP Input Connector
Connects the ECG lead cable to
measure ECG and respiration
waveform.
Refer to “Safety Precautions” in this
manual’s preface.
Refer to “Safety
Precautions” in this
manual’s preface.
SpO2 Input Connector
Connects the SpO2 probe (sensor)/relay
cable.
Channel Number Label
Indicates transmitter channel number.
LCD
Displays measurement waveform and
transmitter information.
Power Switch
Turn ON/OFF the power.
EVENT Button
A
ctivates the function assigned on the
receiving monitor.
Electrode Position Label
Indicates standard ECG electrode
position.
Enter button
Uses for setup.
▽ button
Uses for setup.
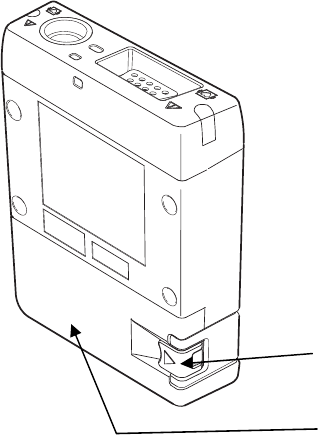
2. Names of Parts and Their Functions
4
Battery Lid Lock Lever
Uses to open/lock the battery
compartment lid.
Battery Compartment Lid
This is the lid for the battery
compartment.
Make sure to close it when the
transmitter is o
p
erated.
5
3. Preparation
1) Installing the Batteries
The LX-7230 functions with two "AA" size (“LR06” size) alkaline batteries.
With new batteries, the LX-7230 is capable of the following operation.
LX-7230N: approximately 2.5 days
LX-7230KM: approximately 5 days
(However, continuous operating time may be shorter than the above
mentioned time depending on the application of the SpO2 probe (sensor).)
WARNING
Unplug the ECG lead cable when the battery compartment lid is
opened. Otherwise, patient leakage current beyond the allowable value
may occur.
Use new "AA" size (“LR06” size) alkaline batteries.
Do not short out the (+) and (-) terminals. It may result in exothermic
heat and fire, the leakage from the batteries may damage the device, or
an explosion from the batteries may occur.
Install the batteries with the correct polarity.
Do not use a disassembled or a damaged battery due to drop or shock.
The leakage from the batteries may damage the device, or an explosion
from the batteries may occur.
Do not use different types of batteries at the same time. The leakage
from the batteries may damage the device, or an explosion from the
batteries may occur.
Remove the exhausted batteries immediately. The leakage from the
batteries may damage the device, or an explosion from the batteries
may occur.
If the transmitter is not in use for a long period of time, remove the
batteries and store the device in an appropriate place. If the batteries
are left in the transmitter for a long period of time, the leakage from the
batteries may damage the device or an explosion from the batteries
may occur.
Make sure to replace the two batteries simultaneously. If a new and
used battery are mixed, a leakage from the batteries may damage the
device or an explosion from the batteries may occur.
CAUTION
Use only alkaline batteries. Other batteries will shorten the continuous
operating time.
Once the power switch is on the OFF position, then open the battery
compartment lid.
Do not replace the batteries with wet hands.
In case of storing the used or unused batteries, make sure that the
terminals are not touching other batteries or metal parts.
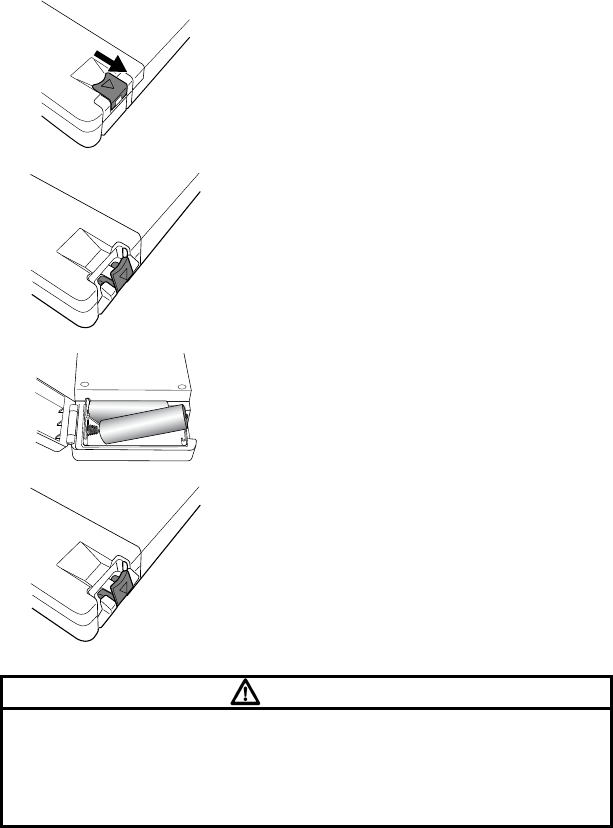
3. Preparation
6
Lift the lock lever to open the battery
compartment lid as shown in the left picture.
Install new batteries according to the polarity
indication inside the battery compartment.
Make sure to first Insert the battery into the
battery compartment from the minus (-)
terminal as shown in the left picture.
Hook the lock lever on the projection from
the body and press it down until it is
horizontal (flat position).
CAUTION
Make sure that any foreign particles, such as hairs, are not held on
the battery compartment lid and dust is not adhered to the edge of the
lid to prevent water entering into the battery compartment area.
Make sure to only turn ON the LX-7230 after closing the battery
compartment lid.
3. Preparation
7
2) Operating Power Switch
Turning the power switch to “ON”
Rotate the power switch to the left until it clicks.
LCD screen turns ON and measurement starts.
Regarding the LCD screen, refer to page 45 (7. Measurement).
The screen automatically turns itself OFF after a minute.
After the power is turned ON, make sure to check the remaining battery level
on the LCD screen.
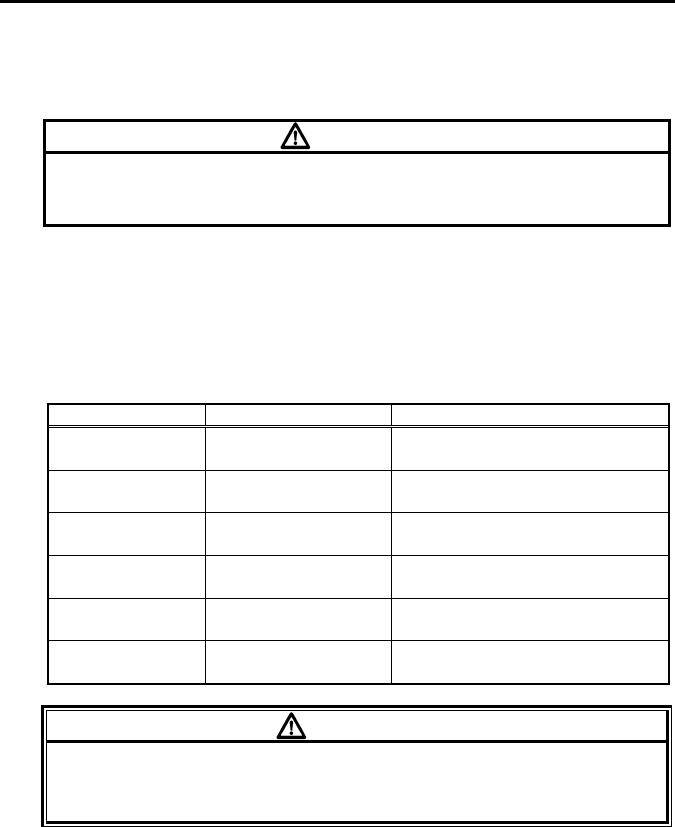
Refer to the following symbol about the remaining battery level.
Battery Symbol Remaining Battery Level
Full
Getting low but still available
Nearly empty;
Replace the battery
The battery level estimation is in case of using alkaline batteries.
Turning the power switch to “OFF”
Rotate the power switch to the right until it clicks.
3. Preparation
8
Blank Page
9
4. ECG Monitoring
When the transmitter is used without the SpO2 probe (sensor), it will measure
only ECG and respiration.
CAUTION
When using the transmitter with only the ECG lead cable, SpO2
measurements on the receiving monitor shall be turned off to prevent an
erroneous alarm.
■ Connecting the ECG Lead Cable and Electrodes
The optional ECG lead cables for LX-7230 are as follows.
ECG Lead Cables
AHA color code:
Item No. Applicable Lead Remark
CMT-01HTH-0.8DA Limb Lead (1CH) 3-electrode Hook Type
(White, Black, Red)
CMT-02HTH-0.8DA Limb Lead (2CH) 4-electrode Hook Type
(White, Black, Red, Green)
CMT-03HTH-0.8DA Limb Lead (1CH)
+Chest (1CH)
5-electrode Hook Type
(White, Black, Red, Green, Brown)
CMT-01FTH-0.8DA Limb Lead (1CH) 3-electrode Clip Type
(White, Black, Red)
CMT-02FTH-0.8DA Limb Lead (2CH) 4-electrode Clip Type
(White, Black, Red, Green)
CMT-03FTH-0.8DA Limb Lead (1CH)
+Chest (1CH)
5-electrode Clip Type
(White, Black, Red, Green, Brown)
WARNING
Use only the specified lead cable from Fukuda Denshi. Otherwise, proper
monitoring may not be performed, and also it may fail defibrillation or cause
a malfunction of the device when the device is used with a defibrillator.

4. ECG Monitoring
10
The relations between the attached electrode positions and lead method are
as follows. Attach the electrodes to monitor proper waveform.
For 3-electrode lead cable
For AHA color code electrode position (No. CMT-01HTH-0.8DA, CMT-01FTH-0.8DA)
Standard Limb leads
Standard Limb leads can be selected from lead I, lead II, or lead III under
the setting of the device.
Refer to “8. Operation ■Changing Setup ●ECG Display Screen (1)
<<Switching Lead>>” in page 57.
Black
(
LA
)
White
(
RA
)
Red
(LL)
For 4-electrode lead cable
For AHA color code electrode position (No. CMT-02HTH-0.8DA, CMT-02FTH-0.8DA)
Standard Limb leads
Two leads measurements, lead I and II are fixed. Lead III, aVR, aVL, and
aVF can be also displayed from the setting on the receiving monitor. For
details, refer to the operation manual of the receiving monitor.
Black
(LA)
White
(RA)
Green
(
RL
)
Red
(LL)
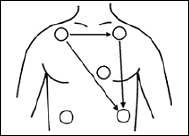
4. ECG Monitoring
11
For 5-electrode (Chest) lead cable
For AHA color code electrode position (No. CMT-03HTH-0.8DA, CMT-03FTH-
0.8DA)
Standard Limb lead
and Chest lead
One limb lead and one chest lead (Brown) measurements are available.
Standard Limb leads can be selected from lead I, lead II, or lead III
under the setting of the device.
The chest lead waveform is measured from the chest lead (Brown)
positioned on the chest.
Refer to “8. Operation ■Changing Setup ●ECG Display Screen (1)
<<Switching Lead>>” in page 57.
Black
(LA)
White
(RA) Brown
(V)
Green
(
RL
)
Red
(LL)
4. ECG Monitoring
12
■ Attaching the Electrodes
CAUTION
Always use the same type of electrodes. If different types of electrodes are
used at the same time, the difference between the polarization potential
from each electrode may interfere with monitoring.
CAUTION
Do not reuse the disposable electrodes. It is intended for single patent use
only.
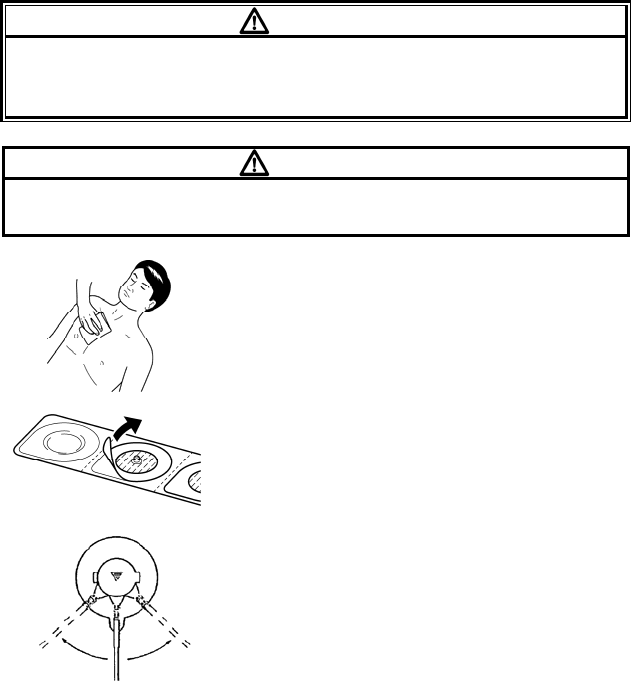
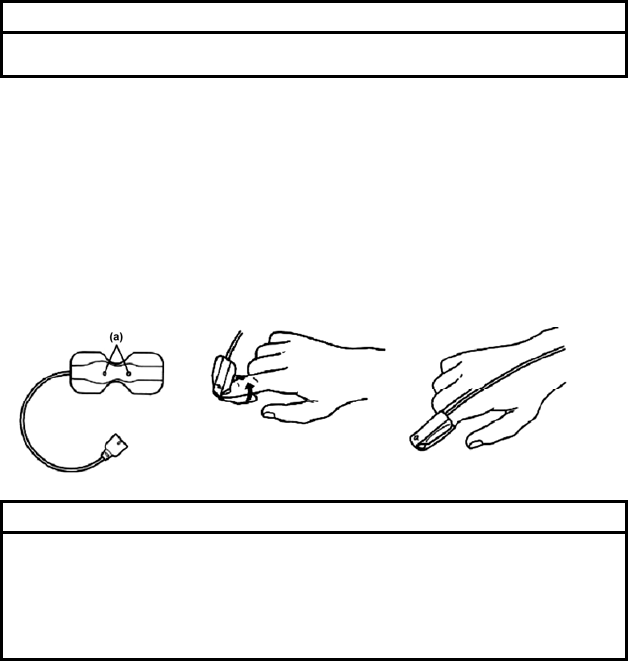
Clean the electrode sites with alcohol wipes
or other skin preparation. If necessary, shave
the electrode sites to remove excessive hair.
Peel off the disposable electrode.
Pay attention not to touch the electrode gel.
Attach the lead cable end to the electrode
(convex part).
Turn right and left to verify that it is securely
attached.
4. ECG Monitoring
13
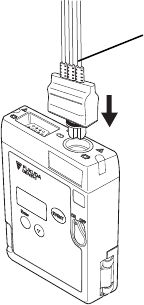
■ Connecting the ECG Lead Cable to the LX-7230
Insert the ECG lead cable firmly into the ECG/RESP input connector matching
the transmitter’s connector guide and the direction of the notched part on the
connector.
ECG Lead cable

4. ECG Monitoring
14
CAUTION
There are some cases when pacemaker pulse cannot be detected
depending on the pacemaker type, pulse voltage, pulse width, electrode
lead type (unipolar, bipolar), electrode placement, or lead method which
causes the pacemaker pulse amplitude to decrease and disables
pacemaker pulse detection.
If signals similar to a pacemaker pulse are present, such as electric
blanket noise or excessive AC frequency noise, these may be
erroneously detected and displayed as a pacemaker pulse. In this case,
check the condition of the electrodes and ECG lead cable to resolve the
cause or turn off the pacemaker detection setting on the receiving
monitor.
Time constant of this device is shorter than Fukuda Denshi monitors
(direct ECG connection). Therefore, there is a difference in the ST
measurement value between them. Pay attention to the difference
when monitoring a patient from a transmitter or a monitor.
When an electrode is attached on the same location for a long time,
some patients may develop skin irritation. Check the patient’s skin
condition periodically and change the electrode position as required.
The indication for continuous use of an electrode is about one day.
Replace the electrode if the skin contact gets loosen due to perspiring,
etc.
Make sure to use new disposable electrodes. Otherwise, the waveform
quality may become poor and it may fail to perform correct monitoring.
When “Check Electrode” message is displayed on the screen of the
receiving monitor or the LCD of this device, check the condition of the
electrodes and ECG lead cable to resolve the cause.
When removing electrodes from the patient, remove them carefully and
slowly. Do not apply excessive force to remove them. Otherwise, it may
damage the skin.
It may not perform a correct measurement due to the attached position
of the electrodes. Attach the electrodes on the patient referring to page
10 and 11 and make sure that the correct waveform is measured on the
LCD.
15
5. Respiration Monitoring
Follow the preparation of “4.ECG Monitoring” to allow the respiration
monitoring.
This respiration monitoring is performed with impedance method.
The ECG electrodes are also used for detecting the respiration. Each lead
cable specifies the electrodes to detect the respiration. For 3-electrode and 5-
electrode (chest) lead cable, the electrodes to detect the respiration are fixed
as follows. Even if lead method is switched, they are no changes.
Lead Cable Color of Electrode
3-electrode White (RA) and Red (LL)
4-electrode White (RA) and Red (LL)
5-electrode (Chest) White (RA) and Red (LL)
WARNING
Minute ventilation rate-adaptive implantable pacemakers can occasionally
interact with certain cardiac monitoring and diagnostic equipment, causing
the pacemakers to pace at their maximum programmed rate. The cardiac
monitoring and diagnostic equipment may possibly send wrong
information.
If such event occurs, please disconnect the cardiac monitoring and
diagnostic equipment, or follow the procedures described in the operation
manual of the pacemaker.
(For more details, contact FUKUDA DENSHI personnel, your institution’s
professionals, or your pacemaker distributors.)
Reference
“Minute Ventilation Rate-Adaptive Pacemakers”
FDA alerts health professionals that minute ventilation rate-adaptive
implantable pacemakers can occasionally interact with certain cardiac
monitoring and diagnostic equipment, causing pacemakers to pace at their
maximum programmed rate.
[October 14, 1998 (Letter: www .fda.gov/cdrh/safety.html) - FDA]
CAUTION
Even if the electrodes are attached on the proper positions for ECG
monitoring, it may not be always the proper ones for respiration
monitoring as well.
When a defibrillator is used during respiration monitoring, a large offset
voltage will be placed on the ECG electrodes, which may cause
interruption of monitoring for a few seconds.
5. Respiration Monitoring
16
Blank Page
17
6. SpO2 Monitoring
When the transmitter is used without the ECG lead cable, it will measure only
SpO2.
WARNING
When the SpO2 probe (sensor) is in a connector-off condition, the SpO2
alarm will not be generated on the receiving monitor. Make sure that the
SpO2 probe (sensor)/ relay cable is securely connected. If the SpO2
waveform/numeric data is not displayed, check the patient’s condition and
pay attention not to miss the connector-off condition.
CAUTION
When using the transmitter with only the SpO2 sensor cable, ECG and
respiration measurements on the receiving monitor shall be turned off to
prevent an erroneous alarm.
There are varieties of SpO2 sensors depending on the purpose and intended
use for LX-7230N and LX-7230KM. Select a proper one and attach it to the
patient.
CAUTION
SpO2 sensor for LX-7230N and SpO2 sensor for LX-7230KM are not
compatible. SpO2 sensors for LX-7230KM cannot be used on the LX-
7230N transmitter; vice-versa. Make sure to use the correct sensor for
operation.
6. SpO2 Monitoring (LX-7230N)
18
SpO2 Monitoring (LX-7230N)
LX-7230N, which has a built-in Nellcor® SpO2 module, is described in this
section.
Regarding the LX-7230KM, which has a built-in Konica Minolta® SpO2 module,
refer to page 35.
< SpO2 Sensors for LX-7230N >
The optional SpO2 sensors available for LX-7230N are as follows.
The following table shows applicable patient and proper site for each SpO2
sensor. Select the proper one depending on the purpose and intended use.
Sensor Types Applicable Patient Applied Site
OxiMax® MAX-I Infant
(weight of 3 to 20Kg) Toe
OxiMax® MAX-P Pediatric
(weight of 10 to 50Kg) Finger
OxiMax® MAX-A/AL Adult
(weight of 30Kg and over) Finger
Durasensor® DS-100A Adult
(weight of 40Kg and over) Finger
OxiMax® MAX-R Adult
(weight of 50Kg and over) Nose
OxiMax® MAX-FAST Adult/Pediatric
(weight of 10Kg and over) Forehead
Adult
(weight of 40Kg and over) Finger
OxiMax® MAX-N Neonate
(weight of less 3Kg) Foot
WARNING
For SpO2 monitoring, always use the sensor specified by Fukuda
Denshi. If any other sensor is used, high temperature rise of the sensor
may place the patient in danger of burns in the worst case.
As with all medical equipment, carefully route cables to reduce the
possibility of patient entanglement and strangulation.
6. SpO2 Monitoring (LX-7230N)
19
CAUTION
SpO2 sensors for LX-7230N cannot be used on the LX-7230KM.
SpO2 sensors are not waterproof. Keep away from liquids.
Do not pick up the device pulling the sensor or cable part. It may get
disconnected from the device and the device may be dropped.
A message is displayed when the SpO2 sensor is disconnected from the
device.
A message is displayed when the device detects that the SpO2 sensor is
disconnected from the patient. Properly attach the SpO2 sensor to the
patient.
Do not reuse the single-use SpO2 sensor. It may cause incorrect
measurements.
Read through the instruction of the SpO2 sensor as well.
CAUTION
The accuracy of SpO2 measurement may be influenced by abnormal
hemoglobin, such as carbon monoxide hemoglobin (COHb) and
methemoglobin (MetHb). It may be also affected by cardiogreen or
intravascular dyes.
In addition, the following case may affect the accuracy of SpO2 and pulse
rate measurement.
Outside light (direct sunlight, fluorescent, light therapy equipment,
surgical light, infrared heat ramp, etc.)
Hypoperfusion
Excessive body motion (patient’s motion)
Pigment injected to the blood for testing
In case of measurement during receiving CPR treatment
Placement of SpO2 sensor on limb with a blood-pressure cuff, arterial
catheter
External colorant such as nail polish
Abnormally low or high hemoglobin concentration
Venous pulse
Electrosurgery
Influence of electromagnetic waves from other electronics
High-intensity radio waves from cell phone
6. SpO2 Monitoring (LX-7230N)
20
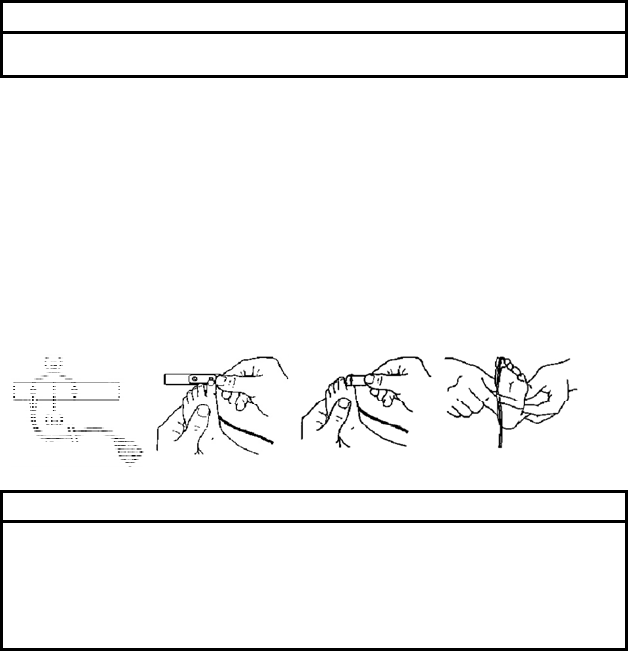
■ Applying the OxiMax® MAX-I sensor
This Nellcor® adhesive sensor, model MAX-I, is indicated for continuous
noninvasive arterial oxygen saturation and pulse rate monitoring and can be
reused on the same patient as long as the adhesive tape attaches without
slippage.
1. Remove the plastic backing from the MAX-I and locate the two transparent
windows on the adhesive side. Windows cover optical components. Note
the corresponding alignment marks (a) on the non-adhesive side and the
dashed line (b) midway between the marks (Figure (1)).
The big toe is the preferred MAX-I location. Alternatively, apply the sensor
to another digit of similar size, for example, the thumb.
NOTE
When selecting the sensor site, priority should be given to an extremity free
of an arterial catheter, blood pressure cuff, or intravascular infusion line.
2. Orient the MAX-I so that the window next to the cable is aligned on the
bottom of the big toe as shown. The cable should extend towards the heel
(Figure (2)).
3. Wrap the MAX-I firmly, but not too tightly around the toe. Windows must
oppose each other for correct measurement (Figure (3)).
4. Wrap any excess tape loosely around the toe. Use additional tape
provided to secure the cable across the bottom of the foot, loosely enough
to maintain good circulation (Figure (4)).
5. Connect the MAX-I into the LX-7230N. Verify proper operation as
described in the operation manual.
(1) (2) (3) (4)
NOTE
If the sensor does not track the pulse reliably, it may be incorrectly
positioned – or the sensor site may be too thick, thin, or deeply pigmented,
or otherwise deeply colored (for example, as a result of externally applied
coloring such as nail polish, dye, or pigmented cream) to permit appropriate
light transmission. If any of these situations occurs, reposition the sensor or
choose an alternate Nellcor sensor to use on a different site.
6. SpO2 Monitoring (LX-7230N)
21
Reapplication
1. The MAX-I can be reused on the same patient as long as the adhesive
tape attaches without slippage.
2. Enclosed adhesive “dots” are provided for reapplication. Place a
transparent dot over each window as shown, and then remove the
protective paper that covers each dot (Figure (5)). The sensor is now ready
to be reapplied to the same patient. For the reapplication, do not remove
the previous adhesive dot, but place the enclosed adhesive dot over it.
(5)
CAUTION
Precautions for Use of adhesive sensor, MAX-I
Do not reuse the sensor on other patients. This is a sterilized product
and it is intended for single patient use only.
Circulation distal on the sensor site should be checked routinely. The
site must be inspected every 8 hours to ensure adhesion, skin
integrity, and correct optical alignment. If skin integrity changes, move
the sensor to another site
Do not use the sensor on patients who exhibit allergic reactions to the
adhesive tape.
Failure to apply the sensor properly may cause incorrect
measurements.
While the sensor is designed to reduce the effects of ambient light,
excessive light may cause inaccurate measurements. In such cases,
cover the sensor with an opaque material.
If the sensor is wrapped too tightly or supplemental tape is applied,
venous pulsations may lead to inaccurate saturation measurements.
Excessive motion may compromise performance. In such cases, try to
keep the patient still, or change the sensor site to one with less
motion.
Intravascular dyes or externally applied coloring such as nail polish,
dye, or pigmented cream may lead to inaccurate measurements.
Do not pull the sensor cable to remove the sensor from the device.
In the event of damage to the sterile packaging, do NOT use. Make
sure to check whether the packaging and product is cracked or
damaged before use. If there is any damage.
Do not immerse in water or cleaning solutions. Do not resterilize.
For additional warnings, cautions or contraindications when using
sensors with Nellcor-compatible device, refer to each SpO2 sensor
instruction manual.
6. SpO2 Monitoring (LX-7230N)
22
■ Applying the OxiMax® MAX-P/ MAX-A/ MAX-AL sensor
This Nellcor® adhesive sensor, model MAX-P/ MAX-A/ MAX-AL, is indicated
for continuous noninvasive arterial oxygen saturation and pulse rate monitoring
and can be reused on the same patient as long as the adhesive tape attaches
without slippage.
1. Remove the plastic backing from the MAX-P/MAX-A/MAX-AL and locate
the transparent windows (a) on the adhesive side. Windows cover optical
components (Figure (1)).
The index finger is the preferred MAX-P/MAX-A/MAX-AL location.
Alternatively, apply the sensor to the small thumb, smaller finger, or big toe.
NOTE
When selecting the sensor site, priority should be given to an extremity free
of an arterial catheter, blood pressure cuff, or intravascular infusion line.
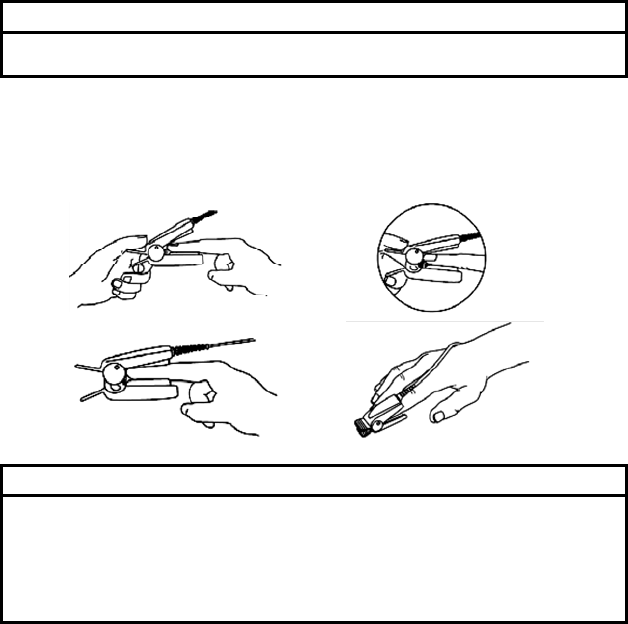
2. Orient the MAX-P/MAX-A/MAX-AL so that the dashed line in the middle of
the sensor is centered on the tip of the finger/toe (Figure (2)). Wrap the
adhesive flaps around the digit. Note that the cable must be positioned on
the top of the hand or foot.
3. Fold the cable end over the top of the finger/toe so that the windows are
directly opposite to each other. Wrap the adhesive securely around both
sides of the digit (Figure (3)).
4. Connect the MAX-P/MAX-A/MAX-AL into the LX-7230N. Verify proper
operation as described in the operation manual.
(1)
(2) (3)
NOTE
If the sensor does not track the pulse reliably, it may be incorrectly
positioned – or the sensor site may be too thick, thin, or deeply pigmented,
or otherwise deeply colored (for example, as a result of externally applied
coloring such as nail polish, dye, or pigmented cream) to permit appropriate
light transmission. If any of these situations occurs, reposition the sensor or
choose an alternate Nellcor sensor to use on a different site.
6. SpO2 Monitoring (LX-7230N)
23
CAUTION
Precautions for Use of adhesive sensors, MAX-P/MAX-A/MAX-AL
Do not reuse the sensor on other patients. This is a sterilized product
and it is intended for single patient use only.
Circulation distal on the sensor site should be checked routinely. The
site must be inspected every 8 hours to ensure adhesion, skin
integrity, and correct optical alignment. If skin integrity changes, move
the sensor to another site
Do not use the sensor on patients who exhibit allergic reactions to the
adhesive tape.
Failure to apply the sensor properly may cause incorrect
measurements.
While the sensor is designed to reduce the effects of ambient light,
excessive light may cause inaccurate measurements. In such cases,
cover the sensor with an opaque material.
If the sensor is wrapped too tightly or supplemental tape is applied,
venous pulsations may lead to inaccurate saturation measurements.
Excessive motion may compromise performance. In such cases, try to
keep the patient still, or change the sensor site to one with less
motion.
Intravascular dyes or externally applied coloring such as nail polish,
dye, or pigmented cream may lead to inaccurate measurements.
Do not pull the sensor cable to remove the sensor from the device.
In the event of damage to the sterile packaging, do NOT use. Make
sure to check whether the packaging and product is cracked or
damaged before use. If there is any damage.
Do not immerse in water or cleaning solutions. Do not resterilize.
For additional warnings, cautions or contraindications when using
sensors with Nellcor-compatible device, refer to each SpO2 sensor
instruction manual.
6. SpO2 Monitoring (LX-7230N)
24
■ Applying
DURASENSOR® DS-100A
This Nellcor® reusable sensor, model DS-100A, is indicated for continuous
noninvasive arterial oxygen saturation and pulse rate monitoring for patients
weighing greater than 40 kg. The DS-100A is contraindicated for use on active
patients or for prolonged use.
1. Place the index finger over the sensor window of the DS-100A with the
finger tip against the stop (Figure (1)).
2. If the fingernail is long, the nail tip will extend over the finger stop (Figure
(2)).
3. Spread open the rear tabs of the sensor to provide even force over the
length of the pads (Figure (3)). If the index finger cannot be positioned
correctly, or is not available, a smaller finger can be used, or use other
OxiMax ® sensor. Do not use the DS-100A on a thumb or toe or across a
child’s hand or foot.
NOTE
When selecting the sensor site, priority should be given to an extremity free
of an arterial catheter, blood pressure cuff, or intravascular infusion line.
4. The sensor should be oriented in such a way that the cable is positioned
along the top of the hand (Figure (4)).
5. Connect the DS-100A into the LX-7230N. Verify proper operation as
described in the operation manual.
(1) (2)
(3) (4)
NOTE
If the sensor does not track the pulse reliably, it may be incorrectly
positioned – or the sensor site may be too thick, thin, or deeply pigmented,
or otherwise deeply colored (for example, as a result of externally applied
coloring such as nail polish, dye, or pigmented cream) to permit appropriate
light transmission. If any of these situations occurs, reposition the sensor or
choose an alternate Nellcor sensor to use on a different site.
6. SpO2 Monitoring (LX-7230N)
25
CAUTION
Precautions for Use of reusable sensors, DS-100A
Do not apply the sensor on the thumb or toe. It may cause incorrect
measurements.
Do not use the sensor for long-term monitoring.
Circulation distal on the sensor site should be checked routinely.
Reusable sensors must be moved to a new site at least every 4 hours.
Because individual skin condition affects the ability of the skin to
tolerate sensor placement, it may be necessary to change the sensor
site more frequently with some patients. If skin integrity changes,
move the sensor to another site. If long-term monitoring is required,
use an OxiMax ® sensor (MAX-A, MAX-AL, or MAX-N).
Failure to apply the sensor properly may cause incorrect
measurements.
While the sensor is designed to reduce the effects of ambient light,
excessive light may cause inaccurate measurements. In such cases,
cover the sensor with an opaque material.
Do not apply tape to secure the sensor in place or to tape it shut; venous
pulsations may lead to inaccurate saturation measurements.
Excessive motion may compromise performance. In such cases, try to
keep the patient still, or change the sensor site to one with less
motion.
Intravascular dyes or externally applied coloring such as nail polish,
dye, or pigmented cream may lead to inaccurate measurements.
Do not pull the sensor cable to remove the sensor from the device.
In the event of damage to the sterile packaging, do NOT use. Make
sure to check whether the packaging and product is cracked or
damaged before use. If there is any damage.
Do not immerse in water or cleaning solutions. Do not resterilize.
For additional warnings, cautions or contraindications when using
sensors with Nellcor-compatible device, refer to each SpO2 sensor
instruction manual.
6. SpO2 Monitoring (LX-7230N)
26
■ Applying the OxiMax® MAX-R sensor
This Nellcor® adhesive sensor, model MAX-R, is indicated for continuous
noninvasive arterial oxygen saturation and pulse rate monitoring. The MAX-R
is designed for use only on the nose. Use this sensor when finger pulsatile flow
is inadequate, or monitoring a finger/toe is not possible.
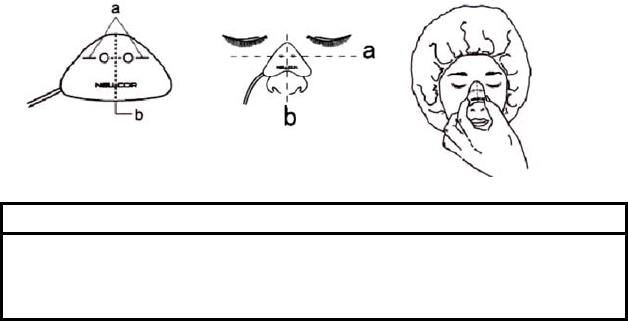
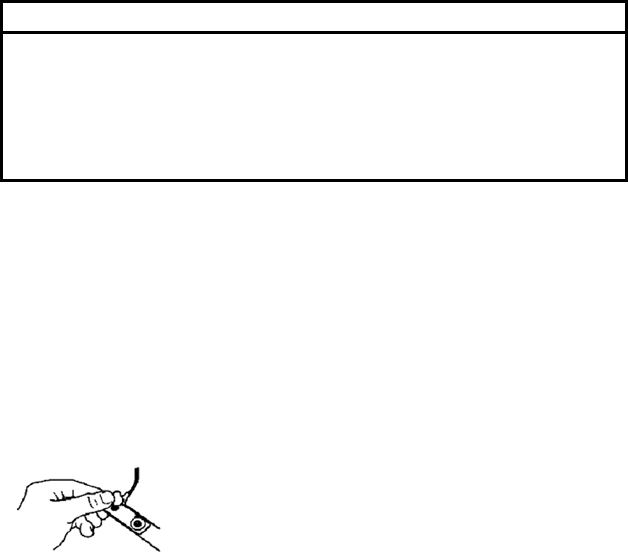
1. Clean the bridge of the patient’s nose with the contents of the enclosed
acetone/alcohol ampule to remove skin oils. Do not allow the
acetone/alcohol solution to get in the patient’s eyes.
2. Remove the plastic backing from the MAX-R and locate the transparent
windows on the adhesive side. Windows cover optical components. Note
the corresponding alignment marks on the non-adhesive side (a) and the
dashed center line (b) midway between the marks (Figure (1)).
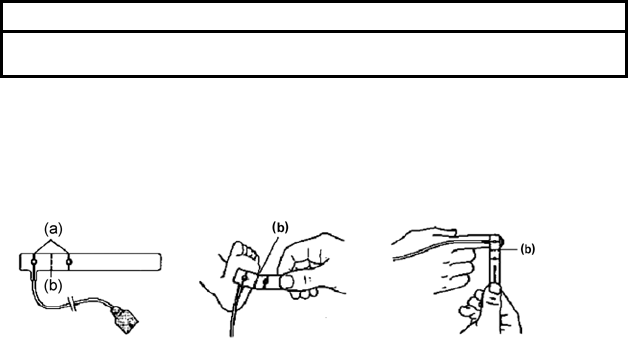
3. Orient the MAX-R so that the dashed line is centered on the nose (a) and
the alignment marks are at the bone-cartilage junction (b). The cable
should extend toward the patient’s right side (Figure (2)).
4. Press the MAX-R firmly onto the nose and hold in place for 10 seconds to
ensure adhesion (Figure (3)). The MAX-R must be secured firmly for
proper operation.
5. As with all medical equipment, carefully route cables to reduce the
possibility of patient entanglement or strangulation.
6. Connect the MAX-R into the LX-7230N. Verify proper operation as
described in the operation manual.
(1) (2)
(3)
NOTE
If the sensor does not track the pulse reliably, it may be incorrectly
positioned – or the sensor site may be too thick, thin, or deeply pigmented
to permit appropriate light transmission. If any of these situations occurs, try
another MAX-R or choose an alternate Nellcor Puritan Bennett sensor.
6. SpO2 Monitoring (LX-7230N)
27
CAUTION
Precautions for Use of adhesive sensor, MAX-R
Do not reuse the sensor on other patients. This is a sterilized product
and it is intended for single patient use only.
Circulation distal on the sensor site should be checked routinely. The
site must be inspected every 8 hours to ensure adhesion, skin
integrity, and correct sensor site. If skin integrity changes, move the
sensor to another site
Do not use the sensor on patients who exhibit allergic reactions to the
adhesive tape.
Do not get the acetone/alcohol cleaning solution in the patient’s eyes.
Failure to apply the sensor properly may cause incorrect
measurements.
While the sensor is designed to reduce the effects of ambient light,
excessive light may cause inaccurate measurements. In such cases,
cover the sensor with an opaque material.
Intravascular dyes or externally applied coloring such as dye or
pigmented cream may lead to inaccurate measurements.
Take care when removing the MAX-R so that the adhesive does not
damage delicate facial tissue.
The MAX-R is not recommended for patients wearing oxygen or
anesthesia masks.
Excessive motion may compromise performance.
Do not pull the sensor cable to remove the sensor from the device.
In the event of damage to the sterile packaging, do NOT use. Make
sure to check whether the packaging and product is cracked or
damaged before use. If there is any damage.
Do not immerse in water or cleaning solutions. Do not resterilize.
For additional warnings, cautions or contraindications when using
sensors with Nellcor-compatible device, refer to each SpO2 sensor
instruction manual.
6. SpO2 Monitoring (LX-7230N)
28
■ Applying the OxiMax® MAX-N sensor
This Nellcor® adhesive sensor, model MAX-N, is indicated for continuous
noninvasive arterial oxygen saturation and pulse rate monitoring and can be
reused on the same patient as long as the adhesive tape attaches without
slippage.
1. Remove the plastic backing from the MAX-N and locate the two
transparent windows on the adhesive side. Windows cover optical
components. Note the corresponding alignment marks (a) on the non-
adhesive side and the dashed line (b) midway between the marks (Figure
(1)).
2. Orient the MAX-N so that the dashed line is on the lateral edge of the site
(a):
Neonates: The preferred site is the foot. Alternatively, use the hand. The
window next to the cable goes on the sole of the foot as shown (Figure (2)).
Adults: The preferred site is the index finger. Alternatively, other fingers
may be used. The window next to the cable goes on the nail side, distal to
the first joint. Do not place on a joint. Note that the cable must be
positioned on the top of the hand (Figure (3)).
NOTE
When selecting a sensor site, priority should be given to an extremity free of
an arterial catheter, blood pressure cuff, or intravascular infusion line.
3. Wrap the MAX-N firmly, but not too tightly around the foot or finger.
Windows must oppose each other.
4. Connect the MAX-N into the LX-7230N. Verify proper operation as
described in the operation manual.
(1)
(2)
(3)
6. SpO2 Monitoring (LX-7230N)
29
NOTE
If the sensor does not track the pulse reliably, it may be incorrectly
positioned - or the sensor site may be excessively wrinkled, or too deeply
pigmented or otherwise deeply colored (for example, as a result of
externally applied coloring such as dye or pigmented cream) to permit
appropriate light transmission. If any of these situations occurs, reposition
the sensor in a different location or choose an alternate Nellcor sensor to
use on a different site.
Reapplication
1. The MAX-A can be reused on the same patient as long as the adhesive
tape attaches without slippage.
2. Enclosed adhesive “dots” are provided for reapplication. Place the
transparent dot over each window as shown, and then remove the
protective paper that covers each dot (Figure (4)). The sensor is now
ready to be reapplied to the same patient. For the reapplication, do not
remove the previous adhesive dot, but place the enclosed adhesive dot
over it.
(4)
6. SpO2 Monitoring (LX-7230N)
30
CAUTION
Precautions for Use of adhesive sensor, MAX-N
Do not reuse the sensor on other patients. This is a sterilized product
and it is intended for single patient use only.
Circulation distal on the sensor site should be checked routinely. The
site must be inspected every 8 hours to ensure adhesion, skin
integrity, and correct optical alignment. If skin integrity changes, move
the sensor to another site.
Do not use the sensor on patients who exhibit allergic reactions to the
adhesive tape.
Failure to apply the sensor properly may cause incorrect
measurements.
While the sensor is designed to reduce the effects of ambient light,
excessive light may cause inaccurate measurements. In such cases,
cover the sensor with an opaque material.
If the sensor is wrapped too tightly or supplemental tape is applied,
venous pulsations may lead to inaccurate saturation measurements.
Excessive motion may compromise performance. In such cases, try to
keep the patient still, or change the sensor site to one with less
motion.
Intravascular dyes or externally applied coloring such as nail polish,
dye, or pigmented cream may lead to inaccurate measurements.
Do not pull the sensor cable to remove the sensor from the device.
In the event of damage to the sterile packaging, do NOT use. Make
sure to check whether the packaging and product is cracked or
damaged before use. If there is any damage.
Do not immerse in water or cleaning solutions. Do not resterilize.
For additional warnings, cautions or contraindications when using
sensors with Nellcor-compatible device, refer to each SpO2 sensor
instruction manual.
6. SpO2 Monitoring (LX-7230N)
31
■ Applying the OXIMAX® MAX-FAST sensor
This is an adhesive sensor, model MAX-FAST, for continuous noninvasive
arterial oxygen saturation and pulse rate monitoring and can be reused on the
same patient as long as the adhesive tape attaches without slippage.
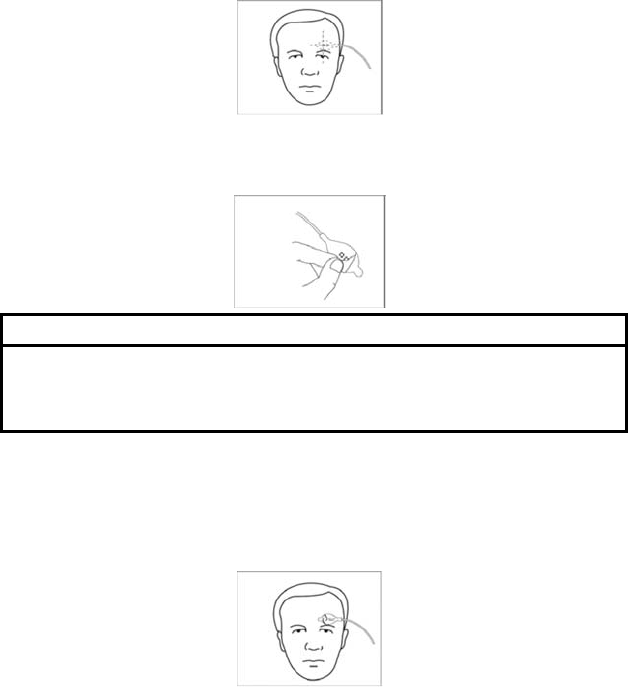
1. Clean the sensor site with an alcohol wipe to remove skin oils. See
illustration for the recommended site. (Figure (1))
(1)
2. Remove the white paper backing to expose the first of three adhesive
pads (Figure (2)). The OXIMAX® MAX-FAST sensor is now ready to be
applied on the patient.
(2)
NOTE
There are three adhesive pads attached to the sensor, each with a pull-tab
for removal. When repositioning the sensor on the same patient, first
expose the new adhesive pad by grasping the tab and peeling off the old
adhesive pad. The sensor is now ready to be reapplied to the patient.
3. Place the sensor onto a flat, hairless portion of the patient’s forehead just
above the left or right eyebrow. If the patient is lying on their side, place
the sensor above the eye on the side of the patient’s head not in contact
with the bed. Press the MAX-FAST sensor firmly in place for 10 seconds,
ensuring that the entire surface area of the adhesive pad makes contact
with the skin (Figure (3)).
(3)
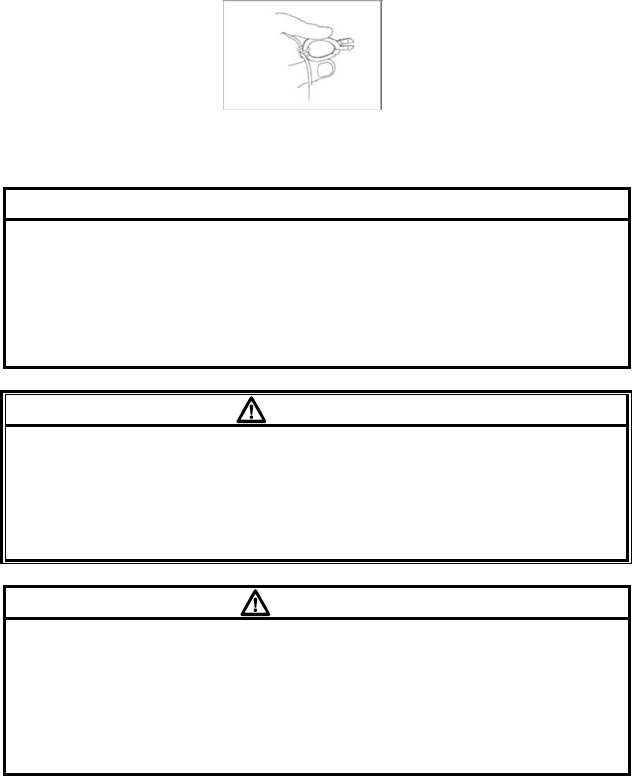
6. SpO2 Monitoring (LX-7230N)
32
4. If desired, the sensor cable can be secured to the patient’s clothing or
other material by using the clip located on the cable. To open, pinch the
sides of the clip; release to close (Figure (4)).
(4)
5. Connect the OXIMAX® MAX-FAST oximetry sensor into the LX-7230N.
Verify proper operation as described in the operation manual.
NOTE
If the sensor does not track the pulse reliably, it may be incorrectly
positioned - or the sensor site may be excessively wrinkled, or too deeply
pigmented or otherwise deeply colored (for example, as a result of
externally applied coloring such as dye or pigmented cream) to permit
appropriate light transmission. If any of these situations occurs, reposition
the sensor in a different location or choose an alternate Nellcor sensor to
use on a different site.
WARNING
Precautions for Use of headband
Do not use headband on children age 24 months and younger.
Do not use headband on children with open fontanelles.
For details, refer to the instruction manual of OXIMAX® MAX-FAST
oximetry sensor.
CAUTION
Precautions for Use of headband
Applying the headband too loose or too tight can cause inaccurate
readings. Make sure the headband applies equal pressure to the entire
sensor. The sensor must be completely covered by the headband.
For details, refer to the instruction manual of OXIMAX® MAX-FAST
oximetry sensor.

6. SpO2 Monitoring (LX-7230N)
33
CAUTION
Precautions for Use of adhesive sensors, MAX-FAST
Do not reuse the sensor on other patients. This is a sterilized product
and it is intended for single patient use only.
Circulation distal on the sensor site should be checked routinely. The
site must be inspected every 12 hours to ensure adhesion, skin
integrity, and correct position. Because individual skin condition
affects the ability of the skin to tolerate sensor placement, it may be
necessary to change the sensor site more frequently with some
patients.
Do not use the OXIMAX® MAX-FAST sensor on patients who exhibit
allergic reactions to the adhesive pad; for patients who perspire
profusely; or under conditions where the patient is in the
Trendelenburg position (head lower than the heart).
Failure to apply the sensor properly may cause incorrect
measurements.
While the sensor is designed to reduce the effects of ambient light,
excessive light may cause inaccurate measurements. In such cases,
cover the sensor with an opaque material.
Do not use tape with the sensor. Use of additional tape or other types
of adhesives may cause skin damage.
Applying the headband too tightly can lead to inaccurate saturation
measurements, or possibly to temporary pressure marks from sensor.
Excessive motion may compromise performance. In such cases, try to
keep the patient still, or change the sensor site to one with less
motion.
For patients in a prone position, venous pooling and/or pulsation may
cause inaccurate SpO2 readings. Use of the headband is advised.
Do not pull the sensor cable to remove the sensor from the device.
In the event of damage to the sterile packaging, do NOT use. Make
sure to check whether the packaging and product is cracked or
damaged before use. If there is any damage.
Do not immerse in water or cleaning solutions. Do not resterilize.
For additional warnings, cautions or contraindications when using
sensors with Nellcor-compatible device, refer to each SpO2 sensor and
headband instruction manual.

6. SpO2 Monitoring (LX-7230N)
34
■ Connecting the Nellcor® SpO2 Sensor to the LX-7230N
1 Insert the SpO2 sensor into the SpO2 input connector on the LX-7230N.
SpO2 Sensor
2 Attach the sensor lock as shown in the following illustration to prevent the
SpO2 sensor to be disconnected.
Sensor Lock
6. SpO2 Monitoring (LX-7230KM)
35
SpO2 Monitoring (LX-7230KM)
LX-7230KM, which has a built-in Konica Minolta® SpO2 module, is described in
this section.
Regarding the LX-7230N, which has a built-in Nellcor® SpO2 module, refer to
page 18.
< SpO2 probes for LX-7230KM >
The optional SpO2 probes available for LX-7230KM are as follows.
The following table shows applicable patient and proper site for each SpO2
probe. Select the proper one depending on the purpose and intended use.
Sensor Types Applicable Patient Applied Site
Finger Clip
SR-5C
Adult and Pediatric
(weight of 30Kg and over) Finger
Spot Check
SP-5C
Adult and Pediatric
(weight of 30Kg and over) Finger
Universal Probe
UD-5C
Adult and Pediatric
(weight of 40Kg and over) Finger
Adult and Pediatric
(weight of 15Kg and over) Finger
Personal Probe
SD-5C(25),
SD-5C(20) Pediatric and Infant
(weight of 5 to 15Kg)
Big toe or the root
of the big toe
WARNING
For SpO2 monitoring, always use the probe/relay cable specified by
Fukuda Denshi. If any other probe/relay cable is used, high temperature
rise of the sensor may place the patient in danger of burns in the worst
case.
As with all medical equipment, carefully route cables to reduce the
possibility of patient entanglement and strangulation.
6. SpO2 Monitor (LX-7230KM)
36
CAUTION
SpO2 probes for LX-7230KM cannot be used on the LX-7230N.
SpO2 probes and relay cable are not waterproof. Keep away from
liquids.
Do not apply excessive force on the sensor or relay cable. Otherwise,
damage to it may result.
Do not pick up the device pulling the probe/relay cable part. It may get
disconnected from the device and the device may be dropped.
A message is displayed when the SpO2 sensor is disconnected from the
device.
A message is displayed when the device detects that the SpO2 probe is
disconnected from the patient. Properly attach the SpO2 probe to the
patient.
Read through the instruction of the SpO2 probe as well.
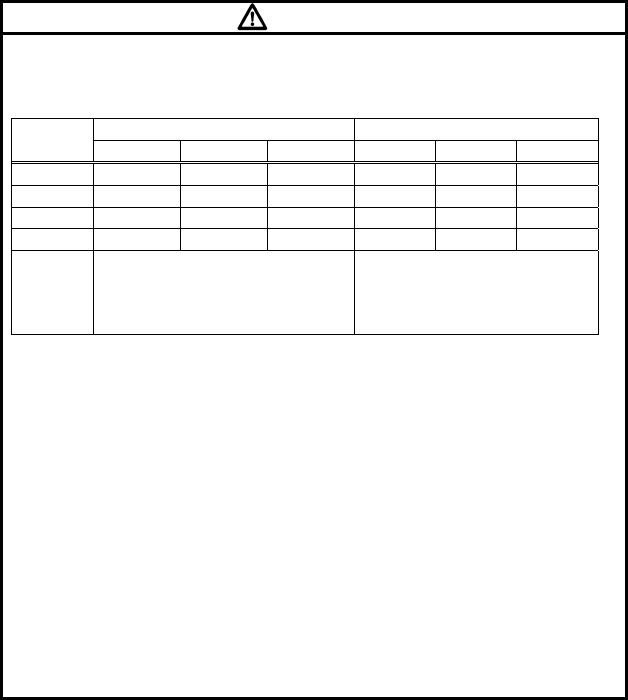
6. SpO2 Monitor (LX-7230KM)
37
CAUTION
The accuracy of the SpO2 measurement may be influenced by abnormal
hemoglobin, such as carbon monoxide hemoglobin (COHb) and
methemoglobin (MetHb). It may be also affected by cardiogreen or
intravascular dyes.
HbCO METHEMOGLOBIN
SpO2 1% 5% 10% 1% 5% 10%
50% -0.1% -0.7% -1.5% 0.2% 1.3% 3.2%
70% -0.1% -0.7% -1.5% -0.6% -2.3% -3.2%
90% -0.2% -0.8% -1.6% -1.5% -6.0% -9.6%
100% -0.2% -0.8% -1.7% -1.8% -7.5% -12.2%
Note The value will be displayed
lower than the actual value.
The value may be
displayed higher than the
actual value when the SpO2
value is around 50%.
The following case may affect the accuracy of SpO2 and pulse rate
measurement.
Outside light (direct sunlight, fluorescent, light therapy equipment,
surgical light, infrared heat ramp, etc.)
Hypoperfusion
Excessive body motion (patient’s motion)
Pigment injected to the blood for testing
In case of measurement during receiving CPR treatment
Placement of SpO2 sensor on limb with a blood-pressure cuff, arterial
catheter
External colorant such as nail polish
Abnormally low or high hemoglobin concentration
Venous pulse
Electrosurgery
Influence of electromagnetic waves from other electronics
High-intensity radio waves from cell phone
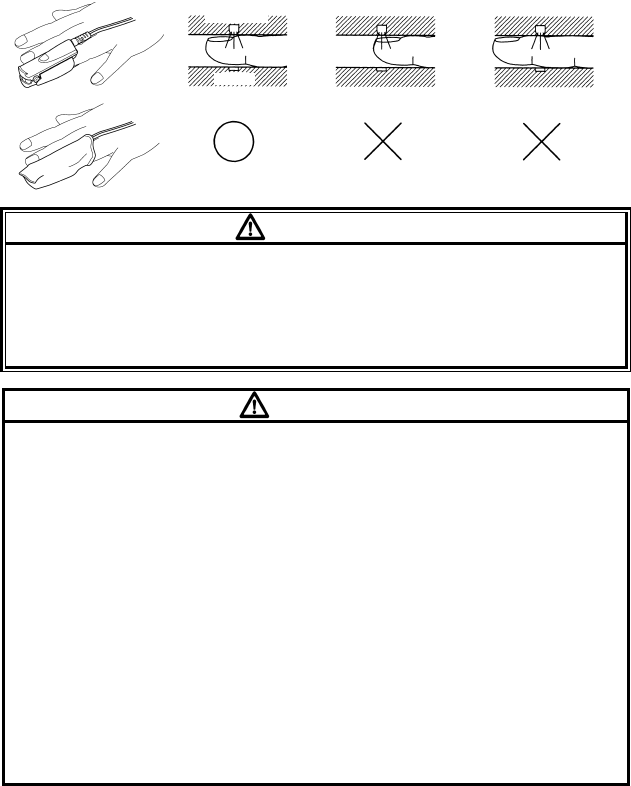
6. SpO2 Monitor (LX-7230KM)
38
■ Applying Finger Clip Probe (SR-5C)
The SR-5C is suitable for middle/long-term measurement, and should be
clipped to the fingertip of an adult/ pediatric who is 30kg or above.
It should be positioned so that the LED (light source) is at the base of the
fingernail, as shown in the figure below. Use the finger mesh cover (FC-M) to
secure the probe. Also, use the finger mesh cover to get better measurement if
it is exposed to high-intensity light.
Light Source
Sensor
Proper attachment Finger is not sufficiently
inserted.
Finger is inserted too far.
Measurement cannot be taken.
WARNING
Precautions for Use of Finger Clip Probe (SR-5C)
If continuously measuring over a long period of time, change the measuring
finger every 8 hours (recommended wearing hours) to prevent low-temperature
burn. Especially for continuous use on patient with peripheral circulatory
disturbance, change the measuring finger more frequently.
Do not use tape to fix the probe. It may cause edema or congestion.
CAUTION
Precautions for Use of Finger Clip Probe (SR-5C)
The probe should be positioned so that the light source is at the base of the
fingernail. Take care not to insert the finger too deep to prevent injury.
When removing the probe, do not pull on the cord.
Cover the probe with the finger mesh cover (FC-M) if it is exposed to high-
intensity light such as direct light and surgical light to prevent measurement error.
The SR-5C is designed for use on finger of an adult/ pediatric who is 30kg and
above. Do not use on other parts.
Before attaching the probe to a patient, clean the probe using a cloth moistened
with sterilizing alcohol.
Remove nail polish before taking measurements to enable stable
measurements.
It may not be possible to take measurements in case of an excessively high
hematocrit value. In such case, reattach the probe to a thinner finger.
Take note that it may not be possible to take measurements in place with
vibrations or while walking.
After attaching the probe, make sure that there is no failure message and the
pulse wave level on the LCD is upper than 2. If not upper than level 2, it may
measure incorrectly. In such case, warm the finger to improve blood circulation.
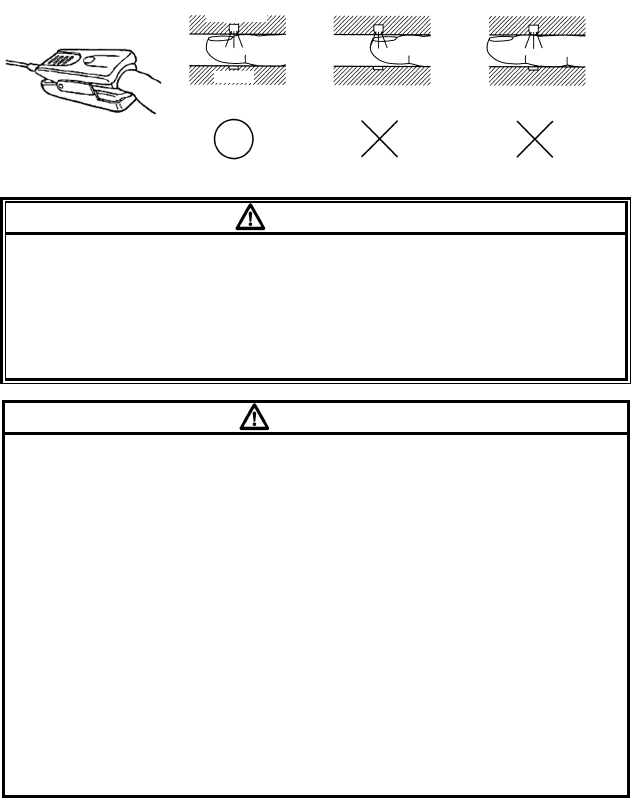
6. SpO2 Monitor (LX-7230KM)
39
■ Applying Spot Check Probe (SP-5C)
The SP-5C is suitable for short-term measurement, and should be clipped to a
fingertip of an adult/ pediatric who is 30kg and above.
It should be positioned so that the LED (light source) is at the base of the
fingernail.
SP-5C
Light Source
Sensor
Proper attachment Finger is not sufficiently
inserted.
Finger is inserted too far.
Measurement cannot be take
n
WARNING
Precautions for Use of Spot Check Probe (SP-5C)
The SP-5C is designed for short-term measurement. If measurements are taken
continuously over a long period of time, change the measuring finger every 2
hours (recommended wearing hours) to prevent low-temperature burn. Especially
for continuous use on patient with peripheral circulatory disturbance, change the
measuring finger more frequently.
Do not use tape to fix the probe. It may cause edema or congestion.
CAUTION
Precautions for Use of Spot Check Probe (SP-5C)
The probe should be positioned so that the light source (LED) is at the base of
the fingernail. Take care not to insert a finger too deep to prevent injury.
When removing the probe, do not pull on the cord.
Cover the probe with a black cover if it is exposed to high-intensity light such as
direct light and surgical light to prevent measurement error.
The SP-5C is designed for use on finger of an adult/ pediatric who is 30kg and
above. Do not use on other parts.
Before attaching the probe to a patient, clean the probe using a cloth moistened
with sterilizing alcohol.
Remove nail polish before taking measurements to enable stable
measurements.
It may not be possible to take measurements in case of an excessively high
hematocrit value. In such case, reattach the probe to a thinner finger.
Take note that it may not be possible to take measurements in place with
vibrations or while walking.
After attaching the probe, make sure that there is no failure message and the
pulse wave level on the LCD is upper than 2. If not upper than level 2, it may
measure incorrectly. In such case, warm the finger to improve blood circulation.
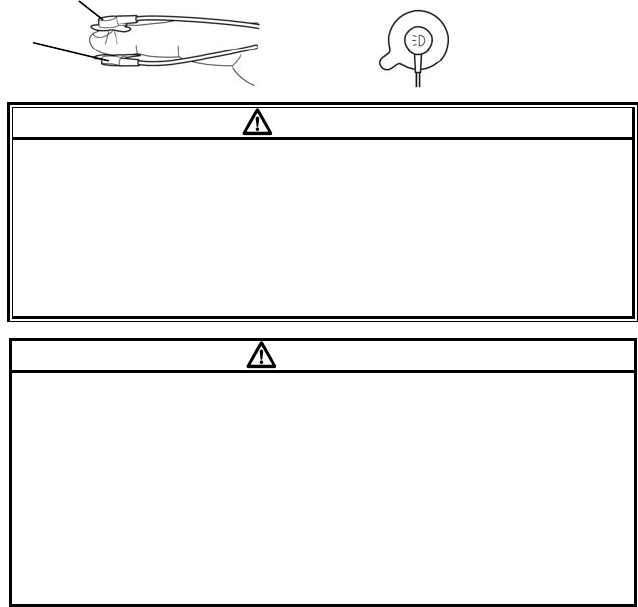
6. SpO2 Monitor (LX-7230KM)
40
■ Applying Universal Probe (UD-5C)
The UD-5C is suitable for long-term measurement, and can be attached to a
fingertip of an adult/ pediatric who is 40kg and above.
1. Fix the probe with tape. Before attaching the probe to a patient, clean the
probe using a cloth moistened with sterilizing alcohol.
2. Make sure that the two pads are aligned.
3. Attach the pad with the LED to the base of the fingernail and the sensor
pad to the opposite side of the finger.
Light source (LED) UD-5C LED mark
Sensor
WARNING
Precautions for Use of Universal Probe (UD-5C)
Although UD-5C is suitable for long-term measurement, change the measuring
site every 8 hours (recommended wearing hours) to prevent rash or a low-
temperature burn. Be especially careful for continuous use on a patient with
peripheral circulatory disturbance by changing the measuring site frequently.
If the use of adhesive tape causes skin irritation, it should be discontinued.
When securing the probe with bandage tape, do not tighten it too hard as this
may cause edema or congestion.
When removing the tape, be careful not to damage the skin.
CAUTION
Precautions for Use of Universal Probe (UD-5C)
When removing the probe, do not pull on the cord, and remove the tape. Do not
apply excessive force to remove the tape. It may break the probe.
Cover the probe with a black cover if it is exposed to high-intensity light such as
direct light and surgical light to prevent measurement error.
Remove nail polish before taking measurements to enable stable
measurements.
It may not be possible to take measurements in case of an excessively high
hematocrit value. In such case, reattach the probe to a thinner finger.
After attaching the probe, make sure that there is no failure message and the
pulse wave level on the LCD is upper than 2. If not upper than level 2, it may
measure incorrectly. In such case, warm the finger to improve blood circulation.
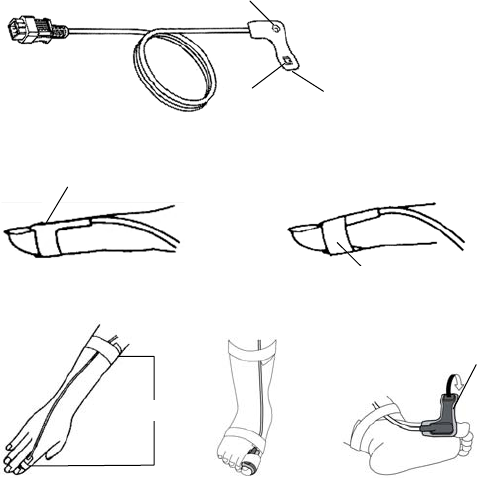
6. SpO2 Monitor (LX-7230KM)
41
■ Applying Personal Probe (SD-5C(20) and SD-5C(25))
The SD-5C (20) and SD-5C (25) are disposable probes to prevent infection.
The probe should be used for only one patient, and should be replaced after
using it for approximately one week.
These probes can be attached to a finger of an adult/pediatric (15kg and
above), or to the big toe or the root of the big toe of a pediatric/ infant (5 to
15kg) by using bandage tape.
1. Before attaching the probe, clean the probe using a cloth moistened with
sterilizing alcohol.
2. When used on finger, position the LED (light source) on the base of the
fingernail, and secure the sensor using bandage tape.
3. When used on the big toe, position the LED (light source) on the base of
the big toenail, and secure the sensor using bandage tape.
Light source (LED)
Sensor Probe pad
Light source (LED)
Bandage tape
Position the LED
on the base of the
big toenail
Bandage tape
6. SpO2 Monitor (LX-7230KM)
42
WARNING
Precautions for Use of Personal Probe (SD-5C(20) and SD-5C(25))
If measurements are taken over a long period of time, change the measuring site
every 8 hours (recommended wearing hours) to prevent rash or low-temperature
burn. Be especially careful for continuous use on a patient with peripheral
circulatory disturbance by changing the measuring site frequently.
If the use of adhesive tape causes skin irritation, it should be discontinued.
When securing the probe with bandage tape, do not tighten it too hard as this may
cause edema or congestion.
When removing the tape, be careful not to damage the skin.
CAUTION
Precautions for Use of Personal Probe (SD-5C(20) and SD-5C(25))
When removing the probe, do not pull on the cord, and remove the tape. Do not
apply excessive force to remove the tape. It may break the probe.
Do not apply excessive force on the LED and sensor; otherwise damage to the
probe may result.
Cover the probe with a black cover if it is exposed to high-intensity light such as
direct light and surgical light to prevent measurement error.
Remove nail polish before taking measurements to enable stable measurements.
It may not be possible to take measurements in case of an excessively high
hematocrit value. In such case, reattach the probe to a thinner finger.
After attaching the probe, make sure that there is no failure message and the pulse
wave level on the LCD is upper than 2. If not upper than level 2, it may measure
incorrectly. In such case, warm the finger to improve blood circulation.
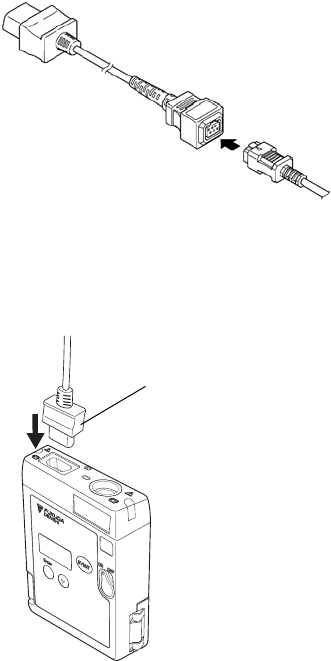
6. SpO2 Monitor (LX-7230KM)
43
■ Connect the SpO2 sensor to the Relay Cable (CI-129)
Connect the SpO2 probe connector to the SpO2 relay cable (CI-129), as shown
in the figure below.
Relay Cable CI-129
SpO2 probe
■ Connecting the Relay Cable (CI-129) to the LX-7230KM
Connect the relay cable (CI-129) to the SpO2 input connector on the LX-
7230KM.
CI-129
6. SpO2 Monitor (LX-7230KM)
44
Blank Page
45
7. Measurement
Turn ON the power and the measurement starts.
■ Starting Screen
When the power is turned ON, the channel number configured on the LX-7230
is displayed at the top of the LCD.
Make sure whether the channel number on the
LCD matches the channel number indicated on
the label of the LX-7230 and the channel number
configured on the receiving monitor.
This screen automatically moves onto the next
waveform display screen.
■ Waveform Display Screen
ECG waveform (1CH when using 3-electrode lead cable, 2CH when using
other lead cable), heart rate, pacemaker marker, respirogram, respiration rate,
pulse wave, SpO2 measurement value, remaining battery level, and various
messages are displayed.
CAUTION
The LX-7230 does not have a diagnostic function. Check the diagnostic
function on the receiving monitor.
The LX-7230 does not have an alarm function. Check the alarm function
on the receiving monitor.
The ECG waveform size and sweep speed settings displayed on the
LCD of the LX-7230 do not interface with the ones displayed on the
screen of the receiving monitor.
The heart rate and respiration rate displayed on the LCD of the LX-7230
may be different from the ones displayed on the receiving monitor.
Because the algorithm of the ECG and respiration rate is different.
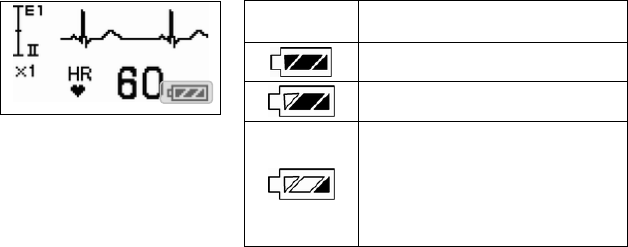
7. Measurement
46
■ Battery Level Check
Check the battery level on the waveform display screen.
Battery
Symbol Remaining Battery Level
Full
Getting low but still available
Nearly empty
Replace the battery.
A message that prompts the
battery check appears on the
screen of the receiving
monitor.
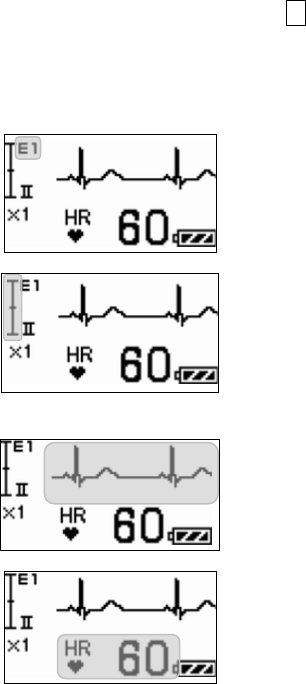
7. Measurement
47
■ Waveform Display
●ECG Display Screen (1)
ECG1 waveform, heart rate, pacemaker marker, remaining battery level, and
electrode check message are displayed.
The LCD display will automatically turn itself OFF after 60 seconds if no
operation is done. To restart the LCD display, refer to page 62.
When the LCD display is active, press the ▽ button to move onto the next
waveform display screen.
【Descriptions of the Screen】
The descriptions of contents displayed on the LCD are as follows.
Indicates ECG 1.
Indicates the scale of the displayed
ECG.
One scale corresponds to 1mV.
In the left illustration, it can display
ECG waveform between -1mV and
+1mV.
Displays the ECG waveform.
Displays the heart rate.
♥ is displayed in synchronization with
the heart rate.
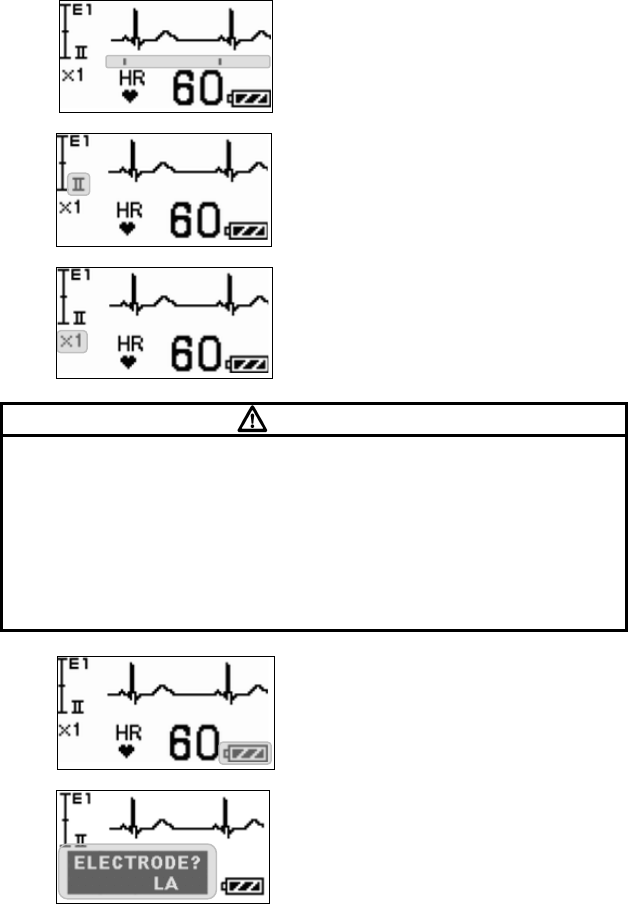
7. Measurement
48
Displays the detection marker when
the pacemaker pulse is detected.
Indicates the measuring lead.
Indicates the ECG waveform size
displayed on the LCD.
CAUTION
The ECG waveform size setting displayed on the LCD does not interact
with the one displayed on the screen of the receiving monitor, because
the LX-7230 cannot transmit the setting information of the waveform
size to the receiving monitor. If the ECG waveform size displayed on the
screen of the receiving monitor is changed, follow the instruction in the
operation manual of the receiving monitor.
In case of the outside of the heart rate range (12 to 300bpm), 0bpm will
be displayed if 11bpm and below is measured and 300bpm will be
displayed If 300bpm and above is measured.
Indicates the remaining battery level.
For details of the battery level, refer to
page 46.
Displays the electrode check
“ELECTRODE?” message appears
when the ECG electrode is detached.
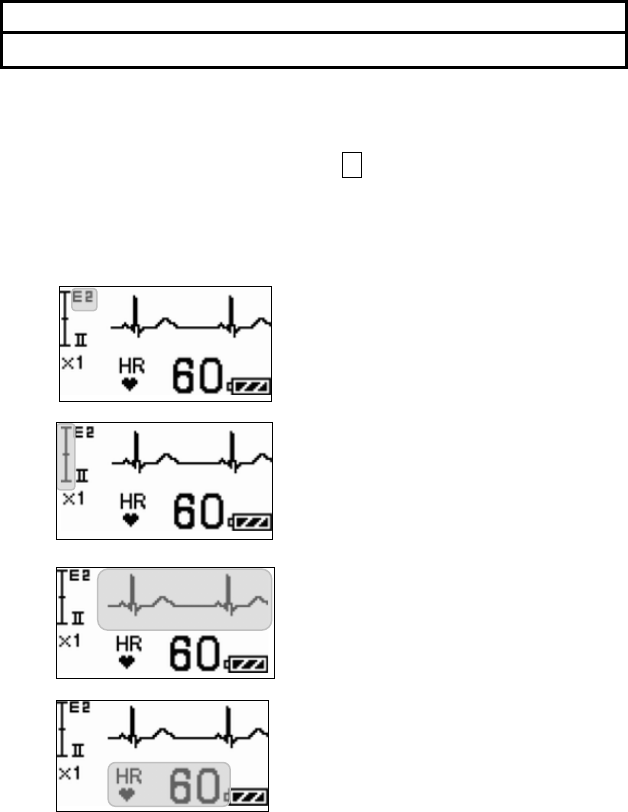
7. Measurement
49
●ECG Display Screen (2)
ECG2 waveform, heart rate, pacemaker marker, remaining battery level, and
electrode check message are displayed.
NOTE
If a 3-electrode lead cable is used, this screen will not appear.
The LCD display will automatically turn itself OFF after 60 seconds if no
operation is done. To restart the LCD display, refer to page 62.
When the LCD display is active, press the ▽ button to move onto the next
waveform display screen.
【Descriptions of the Screen】
The descriptions of contents displayed on the LCD are as follows.
Indicates ECG 2.
Indicates the scale of the displayed
ECG.
One scale corresponds to 1mV.
In the left illustration, it can display ECG
waveform between -1mV and +1mV.
Displays the ECG waveform.
Displays the heart rate.
♥ is displayed in synchronization with
the heart rate.
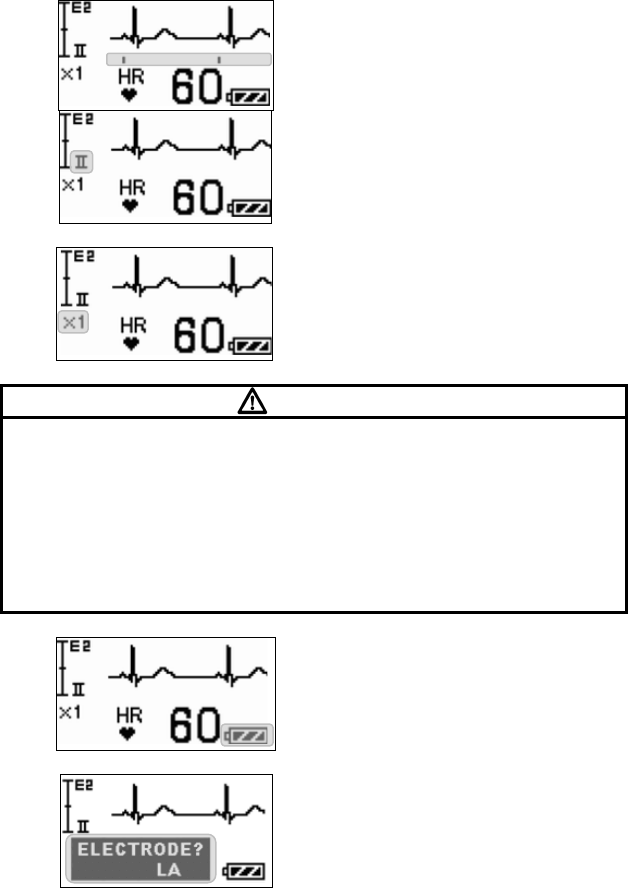
7. Measurement
50
Displays the detection marker when the
pacemaker pulse is detected.
Indicates the measuring lead.
Indicates the ECG waveform size
displayed on the LCD.
CAUTION
The ECG waveform size setting displayed on the LCD of the LX-7230
does not interact with the one displayed on the screen of the receiving
monitor, because the LX-7230 cannot transmit the setting information of
the waveform size to the receiving monitor. If the ECG waveform size
displayed on the screen of the receiving monitor is changed, follow the
instruction in the operation manual of the receiving monitor.
In case of the outside of the heart rate range (12 to 300bpm), 0bpm will
be displayed if 11bpm and below is measured and 300bpm will be
displayed If 300bpm and above is measured.
Indicates the remaining battery level.
For details of the battery level, refer to
page 46.
Displays the electrode check
“ELECTRODE?” message appears
when the ECG electrode is detached.
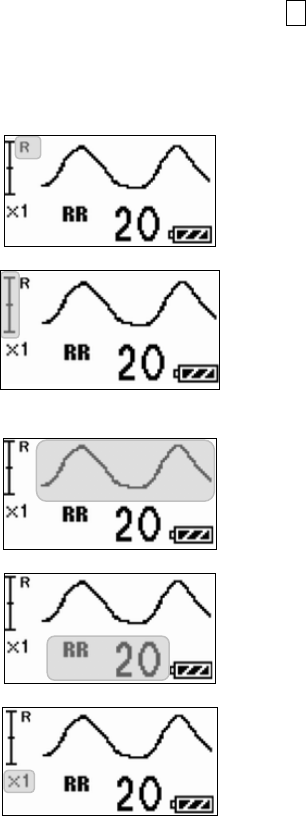
7. Measurement
51
●Respiration Display Screen
Respiration waveform, respiration rate, remaining battery level, and electrode
check message are displayed.
The LCD display will automatically turn itself OFF after 60 seconds if no
operation is done. To restart the LCD display, refer to page 62.
When the LCD display is active, press the ▽ button to move onto the next
waveform display screen.
【Descriptions of the Screen】
The descriptions of contents displayed on the LCD are as follows.
Indicates the respiration waveform
display screen.
Indicates the scale of the displayed
respiration waveform.
One scale corresponds to 1Ω.
In the left illustration, it can display
the change of respiration waveform
between -1Ω and +1Ω.
Displays the respiration waveform.
Displays the respiration rate.
Indicates the respiration waveform
size displayed on the LCD.
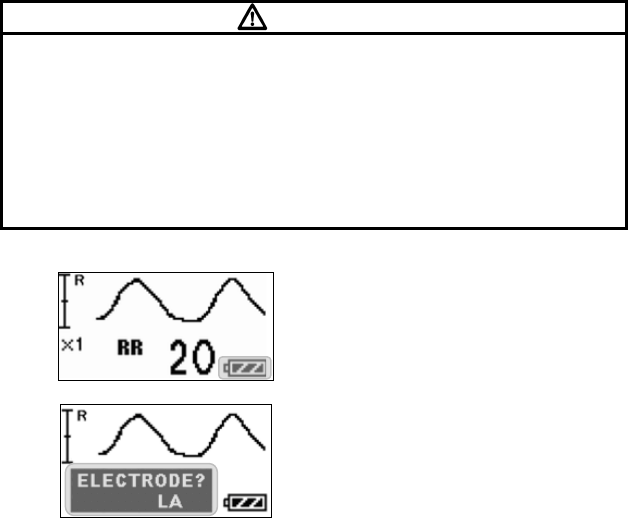
7. Measurement
52
CAUTION
The respiration waveform size setting displayed on the LCD does not
interact with the one displayed on the screen of the receiving monitor,
because the LX-7230 cannot transmit the setting information of the
waveform size to the receiving monitor. If the respiration waveform size
displayed on the screen of the receiving monitor is changed, follow the
instruction in the operation manual of the receiving monitor.
In case of the outside of the respiration rate range (9 to 150Bpm), 0Bpm
will be displayed if 8Bpm and below is measured and 150Bpm will be
displayed If 150Bpm and above is measured.
Indicates the remaining battery level.
For details of the battery level, refer to
page 46.
Displays the electrode check
“ELECTRODE?” message appears
when the ECG electrode is detached.
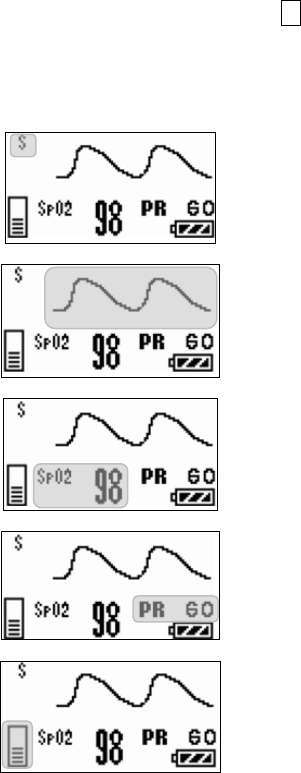
7. Measurement
53
●SpO2 Display Screen
SpO2 display screen depends on the transmitter LX-7230N or LX-7230KM.
For the LX-7230N
Pulse wave, pulse rate, SpO2 measurement value, remaining battery level, and
probe condition are displayed.
The LCD display will automatically turn itself OFF after 60 seconds if no
operation is done. To restart the LCD display, refer to page 62.
When the LCD display is active, press the ▽ button to move onto the next
waveform display screen.
【Descriptions of the Screen】
The descriptions of contents displayed on the LCD are as follows.
Indicates the SpO2 display screen.
Displays the pulse wave.
The waveform size displayed on the
LCD is adjusted automatically.
Displays the SpO2 measurement value.
Displays the pulse rate.
Displays the level meter of the pulse
wave.
Indicates the amplitude level of the
pulse wave in 8 steps.
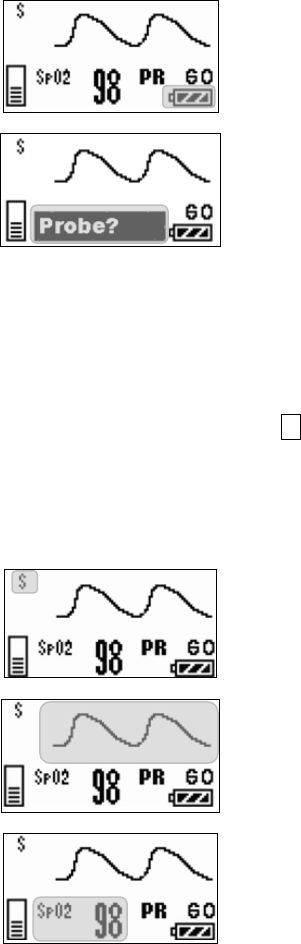
7. Measurement
54
Indicates the remaining battery level.
For details of the battery level, refer to
page 46.
Displays messages such as probe off.
For details of messages, refer to page
87.
For the LX-7230KM
Pulse wave, pulse rate, SpO2 measurement value, remaining battery level, and
probe condition are displayed.
The LCD display will automatically turn itself OFF after 60 seconds if no
operation is done. To restart the LCD display, refer to page 62.
When the LCD display is active, press the ▽ button to move onto the next
waveform display screen.
【Descriptions of the Screen】
The descriptions of contents displayed on the LCD are as follows.
Indicates the SpO2 display screen.
Displays the pulse wave.
The waveform size displayed on LCD
is adjusted automatically.
Displays the SpO2 measurement
value.
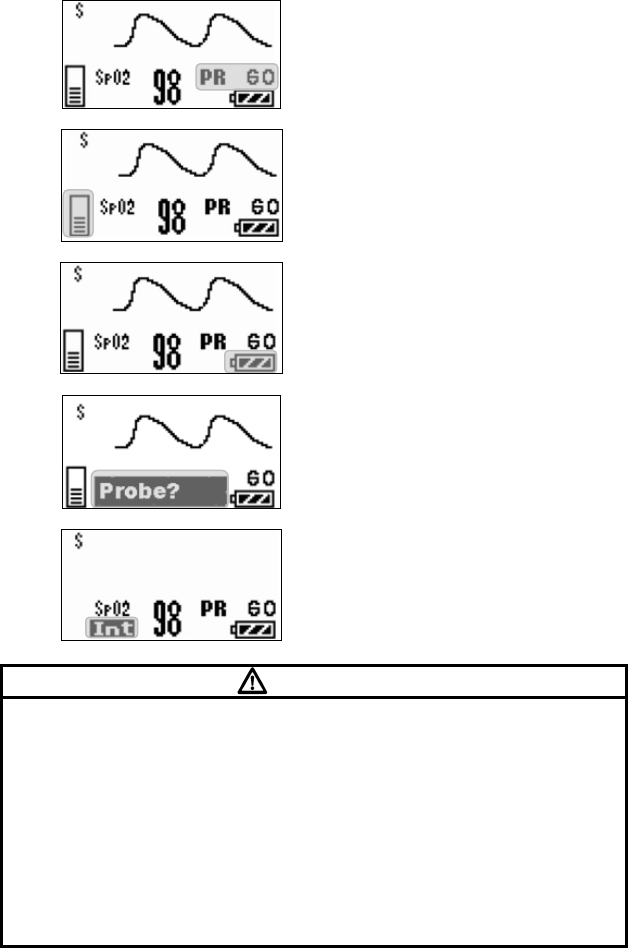
7. Measurement
55
Displays the pulse rate.
Displays the level meter of the pulse
wave.
Indicate the amplitude level of the
pulse wave in 8 steps.
Indicates the remaining battery level.
For details of the battery level, refer to
page 46.
Displays messages such as probe off.
For details of messages, refer to page
87.
Indicates “Int” for the SpO2 intermittent
measurement mode.
For the SpO2 continuous
measurement mode, nothing is
indicated.
CAUTION
In the SpO2 intermittent mode, the SpO2 is measured at 25 seconds
interval. Even when the SpO2 value suddenly changes during this
interval, the LX-7230 and receiving monitor will only show the last SpO2
measurement value. For a patient with the possibility of sudden change,
select SpO2 continuous mode.
Pulse wave and pulse wave level meter are not displayed on the LCD
during the SpO2 intermittent measurement mode. On the screen of the
receiving monitor, pulse wave is not displayed as well.
Make sure that SpO2 is measured correctly, and the SpO2 value and
pulse rate are updated at 25 seconds interval on the LCD or on the
screen of the receiving monitor.
7. Measurement
56
Blank Page
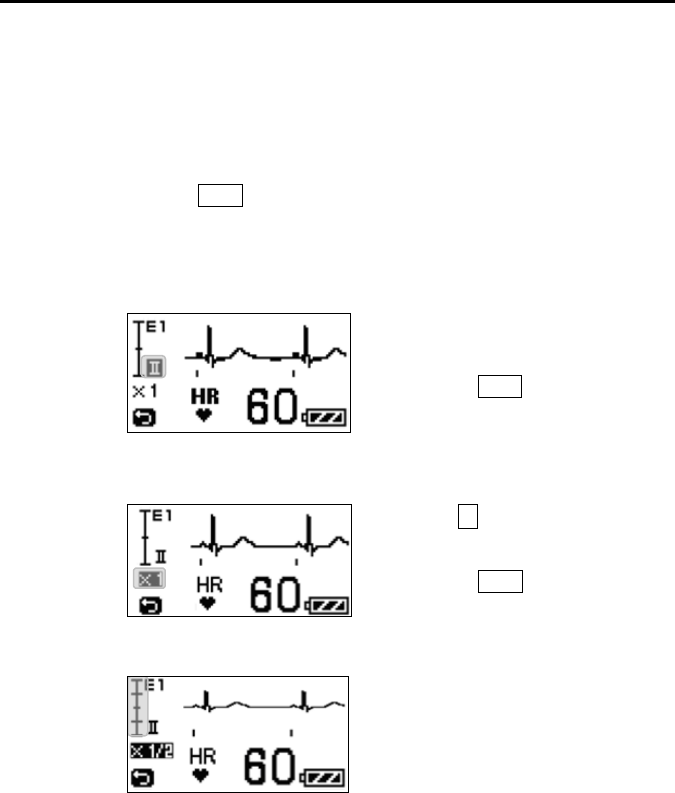
57
8. Operation
■ Changing Setup
●ECG Display Screen (1)
In the ECG display screen (1), the ECG waveform size and lead displayed on
the LCD of the LX-7230 can be changed.
【Setting Method】
How to enter the setup mode:
Press and hold the Enter button for 2 seconds in the ECG display screen (1).
<<Switching Lead>>
Lead of ECG 1 can be switched when 3-electrode lead cable or 5-electrode
(Chest) lead cable is used.
Select an appropriate lead by checking the ECG waveform on the LCD.
The lead indication of ECG 1 is
highlighted.
Pressing the Enter button will
sequentially change the lead of
ECG 1.
Lead I II III I
<<Changing ECG1 Waveform Size on LCD>>
Press the ▽ button to highlight
the size indication of ECG 1.
Pressing the Enter button will
sequentially change the size of
ECG 1.
Size ×1 ×1/2 ×1
When changing the size of the
ECG waveform on the LCD, the
ECG scale will also change.
In the left illustration, it can
display the ECG waveform
between -2mV and +2mV.
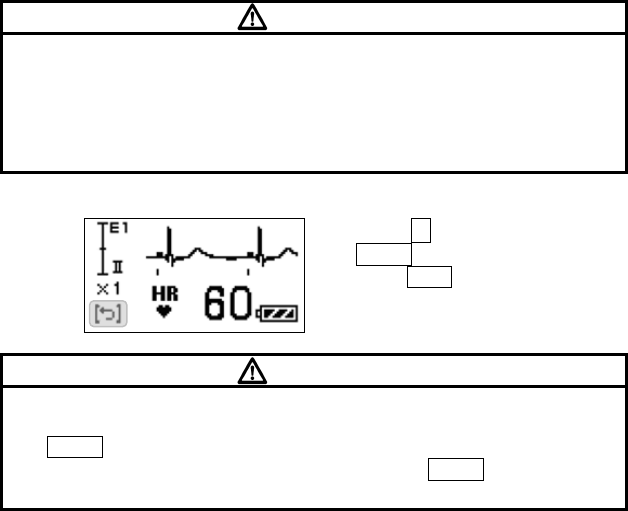
8. Operation
58
CAUTION
The ECG waveform size setting displayed on the LCD of the LX-7230 does
not interact with the one displayed on the screen of the receiving monitor,
because the LX-7230 cannot transmit the setting information of the
waveform size to the receiving monitor. If the ECG waveform size displayed
on the screen of the receiving monitor is changed, follow the instruction in
the operation manual of the receiving monitor.
<< Returning to ECG Display Screen (1) >>
Press the ▽ button to highlight
the Return button.
Press the Enter button to return to
the ECG display screen (1).
CAUTION
Do not operate the LX-7230 with the setup screen open to prevent the
settings to be changed due to an unintended operation. Make sure to press
the Return button to terminate the setup screen. The LCD display will
automatically turn itself OFF after 60 seconds if the Return button is not
pressed.
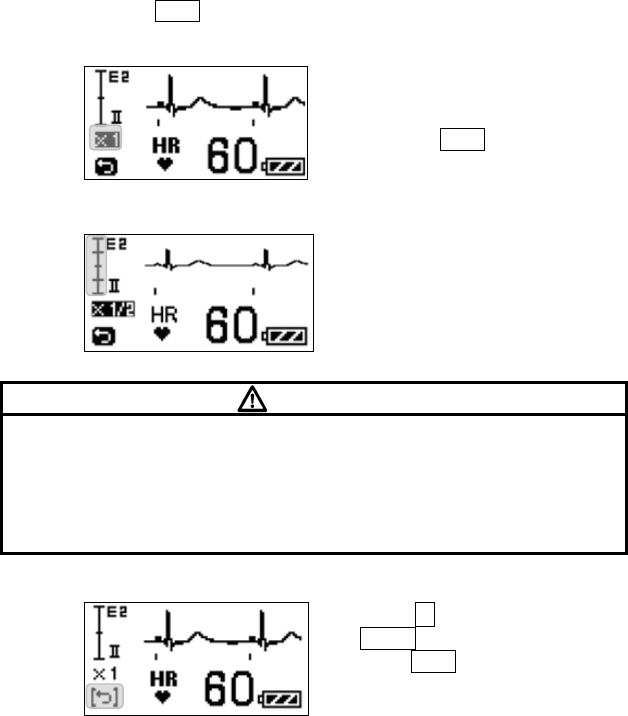
8. Operation
59
●ECG Display Screen (2)
In the ECG display screen (2), the ECG waveform size displayed on the LCD
of the LX-7230 can be changed.
【Setting Method】
How to enter the setup mode:
Press and hold the Enter button for 2 seconds in the ECG display screen (2).
<< Changing ECG2 Waveform Size on LCD >>
The size indication of ECG 2 is
highlighted.
Pressing the Enter button will
sequentially change the size of
ECG 2.
Size ×1 ×1/2 ×1
When changing the size of the
ECG waveform on the LCD, the
ECG scale will also change.
In the left illustration, it can
display ECG waveform between
-2mV and +2mV.
CAUTION
The ECG waveform size setting displayed on the LCD of the LX-7230 does
not interact with the one displayed on the screen of the receiving monitor,
because the LX-7230 cannot transmit the setting information of the
waveform size to the receiving monitor. If the ECG waveform size displayed
on the screen of the receiving monitor is changed, follow the instruction in
the operation manual of the receiving monitor.
<< Returning to ECG display screen (2) >>
Press the ▽ button to highlight
the Return button.
Press the Enter button to return to
the ECG display screen (2).
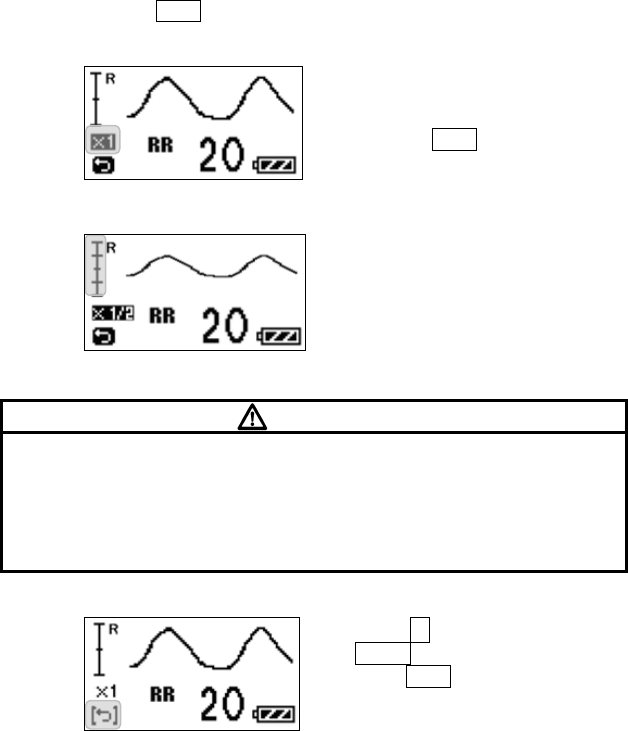
8. Operation
60
●Respiration Display Screen
In the respiration display screen, the respiration waveform size displayed on
the LCD of the LX-7230 can be changed.
【Setting Method】
How to enter the setup mode:
Press and hold the Enter button for 2 seconds in the respiration display screen.
<< Changing Respiration Waveform Size on LCD>>>
The size indication of the
respiration is highlighted.
Pressing the Enter button will
sequentially change the size of
respiration.
Size ×1 ×1/2 ×1
When changing the size of the
respiration waveform on the LCD,
the respiration scale will also
change.
In the left illustration, it can
display the respiration waveform
until 4Ω of change.
CAUTION
The respiration waveform size setting displayed on the LCD of the LX-7230
does not interact with the one displayed on the screen of the receiving
monitor, because the LX-7230 cannot transmit the setting information of the
waveform size to the receiving monitor. If the respiration waveform size
displayed on the screen of the receiving monitor is changed, follow the
instruction in the operation manual of the receiving monitor.
<< Returning to Respiration Display Screen>>
Press the ▽ button to highlight
the Return button.
Press the Enter button to return to
the respiration display screen.
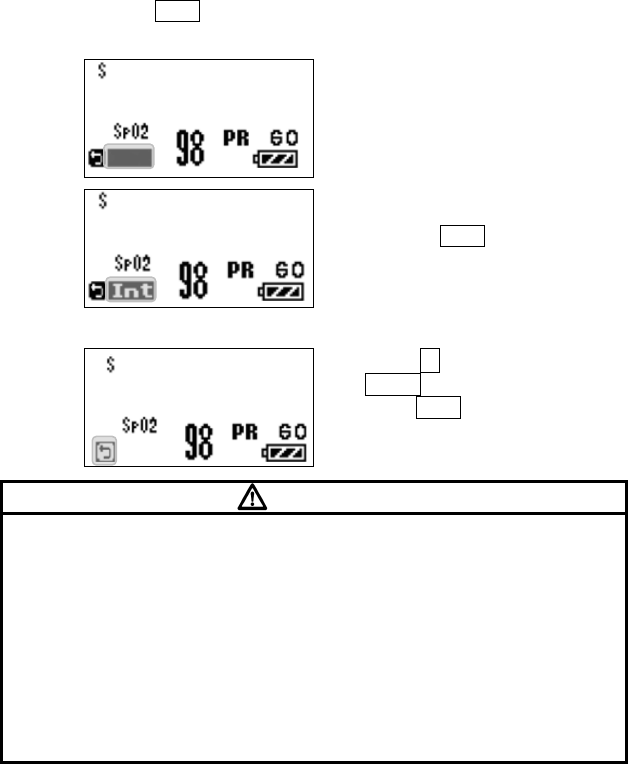
8. Operation
61
●SpO2 Display Screen
SpO2 display screen depends on the transmitter LX-7230N or LX-7230KM.
LX-7230N: LX-7230N has no setup item in the SpO2 display screen.
LX-7230KM: LX-7230KM has a setup item to select the SpO2
measurement mode (continuous or intermittent) in the SpO2
display screen.
【Setting Method】
How to enter the setup mode:
Press and hold the Enter button for 2 seconds in the SpO2 display screen.
<<Changing SpO2 Measurement Mode>>
Indicates the current measurement
mode. (In the SpO2 continuous
measurement mode, nothing is
indicated. In the SpO2 intermittent
measurement mode, “Int” is
indicated.)
Pressing the Enter button will
sequentially change modes.
Mode Continuous intermittent
Continuous
<< Returning to SpO2 display screen>>
Press the ▽ button to highlight
the Return button.
Press the Enter button to return to
the SpO2 display screen.
CAUTION
In the SpO2 intermittent mode, the SpO2 is measured at 25 seconds
interval. Even when the SpO2 value suddenly changes during this
interval, the LX-7230 and receiving monitor will only show the last SpO2
measurement value. For a patient with the possibility of sudden change,
select SpO2 continuous mode.
Pulse wave and pulse wave level meter are not displayed on the LCD
during the SpO2 intermittent measurement mode. On the screen of the
receiving monitor, pulse wave is not displayed as well.
Make sure that SpO2 is measured correctly, and the SpO2 value and
pulse rate are updated at 25 seconds interval on the LCD or on the
screen of the receiving monitor.
8. Operation
62
CAUTION
Do not operate the LX-7230 with the setup screen open to prevent the
settings to be changed due to an unintended operation. Make sure to press
the Return button to terminate the setup screen. The LCD display will
automatically turn itself OFF after 60 seconds if the Return button is not
pressed.
■ Restarting the LCD display
The LCD display will automatically turn itself OFF after 60 seconds if no
operation is done.
Press the Enter button or press and hold the ▽ button to restart the LCD
display.
The starting screen with telemetry channel number appears, and then the
waveform display screen appears.
■ Pressing the EVENT button
Press and hold the EVENT button for 2 seconds to activate the function
assigned on the receiving monitor. The following message appears on the
LCD while the “EVENT” is transmitted.
After the transmission is completed, the starting screen with the telemetry
channel number appears, and then the waveform display screen appears.
“EVENT” operation is available as a remote recording.
For details of the receiving monitor operation and settings related to the
“EVENT” function, refer to the operation manual of the receiving monitor.
63
9. Other Setting Items
The following settings are available for the LX-7230 depending on the use and
condition of the patient. For details of the settings, contact our service
representative.
Items Selection Default Backup
Time Constant 0.4 sec., 0.1 sec. 0.4 sec. Yes
Detection Sensitivity of
Pacemaker Pulse Low, Mid, High Mid Yes
Respiration Detection
Signal ON, OFF ON Yes
LCD Contrast 8 steps 8 Yes
Transmitter Channel
One from the
following channels.
0801 to 0879
0900 to 0979
1000 to 1079
1100 to 1179
1200 to 1279
1300 to 1379
1100 Yes
Group ID One from 00 to 63 00 Yes
■ Changing the Time Constant
The default setting of the time constant is “0.4 seconds”.
If a stable monitoring is difficult with excessive change in the baseline due to
excessive body motion of the patient or an interference noise, such as AC
frequency, by changing the time constant to “0.1 second” the monitoring may
become relatively stable.
For details of the setting change, contact your local Fukuda Denshi service
representative.
CAUTION
When changing the time constant to “0.1 seconds”, the lower frequency
characteristic becomes 1.6Hz ±25%. This setup does not meet IEC
60601-2-27 standard. It may lead to a change in the ECG waveform and
ST measurement value may be especially affected. Fukuda Denshi
recommends “0.4 seconds” setting in normal use.
The LCD screen in normal use does not indicate the selection of time
constant. Make sure to take measures, such as marking on the LX-
7230, to distinguish whether the selection of time constant is changed.
9. Other Setting Items
64
■ Changing the Detection Sensitivity of the Pacemaker Pulse
The default setting of pacemaker pulse detection sensitivity is “Mid”.
The “Mid” setting can detect and reject the following pacemaker pulse
specified in ANSI/AAMI EC13 standard.
Detection/ Rejection of Pacemaker Pulse:
a) Pacemaker Pulse without Over/Undershoot:
Capable to reject pulses of pulse width 0.1 to 2ms,
amplitude ±2 to ±700mV
b) Pacemaker Pulse with Over/Undershoot:
Rejection is not possible.
Fukuda Denshi recommends the “Mid” setting in normal use.
There may be some cases when the pacemaker pulse cannot be detected
depending on the pacemaker type, pulse voltage, pulse width, electrode lead
type (unipolar, bipolar). In this case, change the lead or the position of the
electrodes to be able to detect the pacemaker pulse.
Nonetheless, if the detection is still undetectable, change the setting to “High”
in order to increase the detection sensitivity. So that smaller pacemaker pulse
can be detected. However, the “High” setting may lead to erroneous detection
due to interference noise, such as AC frequency.
If erroneous detections occur due to interference noise, such as AC frequency,
turn OFF the setting of the pacemaker pulse detection in the receiving monitor.
If erroneous detections occur due to interference noise, such as AC frequency,
while monitoring a patient with a pacemaker, and the setting of the pacemaker
pulse detection cannot be turned OFF, replace the electrodes or change the
lead to remove the interference noise, such as AC frequency.
Nonetheless, if erroneous detections still occur, change the setting to “Low” in
order to decrease the detection sensitivity. It makes the LX-7230 less likely to
be interfered by the noise, such as AC frequency.
The “Low” setting decreases the detection sensitivity. Therefore, it cannot
detect the pacemaker pulse specified in ANSI/AAMI EC13 standard.
For details of the setting change, contact your local Fukuda Denshi service
representative.
CAUTION
The LCD screen in normal use does not indicate the setting status of the
pacemaker pulse detection. Make sure to take measures, such as marking
on the LX-7230, to distinguish whether the setting of the pacemaker pulse
detection is changed.
9. Other Setting Items
65
■ Changing the Respiration Detection Signal ON/OFF
The default setting of the respiration detection signal is “ON”.
The respiration waveform can be detected when the setting of the respiration
detection signal is turned “ON”.
WARNING
If the LX-7230 is used with minute ventilation rate-adaptive implantable
pacemaker, the respiration detection signal may cause the pacemaker to
pace at its maximum programmed rate. If such event occurs, change the
setting to “OFF” to prevent an occurrence of erroneous pacing rate.
For details of the setting change, contact your local Fukuda Denshi service
representative.
CAUTION
The respiration waveform cannot be measured if the setting of the
respiration detection signal is turned “OFF”.
Make sure to turn OFF the respiration measurement function on the
receiving monitor to prevent an erroneous detection of the respiration
alarm (on the receiving monitor side).
The LCD screen in normal use does not indicate the setting status of the
respiration detection signal ON/OFF. Make sure to take measures, such
as marking on the LX-7230, to distinguish whether the setting of the
respiration detection signal ON/OFF is changed.
■ Changing the LCD Contrast
The LCD display contrast of the LX-7230 can be changed in 8 steps.
For details of the setting change, contact your local Fukuda Denshi service
representative.
9. Other Setting Items
66
Blank Page
67
10. Changing the Transmitter Channel and Group ID
■ Changing the Transmitter Channel
The LX-7230 is a transmitter of PLL synthesizer type, and its transmitter
channel can be programmed. It can be set up with an arbitrary channel among
the channels assigned by the Telemetry Laws (according to each country).
For details of the setting change, contact your local Fukuda Denshi service
representative.
WARNING
If the transmitter channel is changed, follow the instruction by the
person in charge of the radio telemetry channel in your facility.
Mismanagement may result in a serious accident, such as interference
and mixing up patients.
Replace promptly the new channel label if the transmitter channel has
been changed.
■ Changing the Group ID
The LX-7230 transmits its group ID, which it belongs to, to prevent interference
with neighboring hospital's transmitter.
The receiving monitor checks whether the incoming group ID is the same as
the programmed one that the receiving monitor has. There are 64 group codes
available. The default setting is “00”.
The transmitter group ID can be changed if there is interference with a
neighboring hospital's transmitter,
For details of the setting change, contact your local Fukuda Denshi service
representative.
CAUTION
Possible causes of interference other than radio telemetry from neighboring
hospital's transmitter, are the proximity of cell phone, amateur radio station,
radio taxi, and illegal citizens band, which may be a cause of interference.
In such a case, the situation should be carefully observed to find the cause
of interference.
10. Changing the Transmitter Channel and Group ID
68
Blank Page
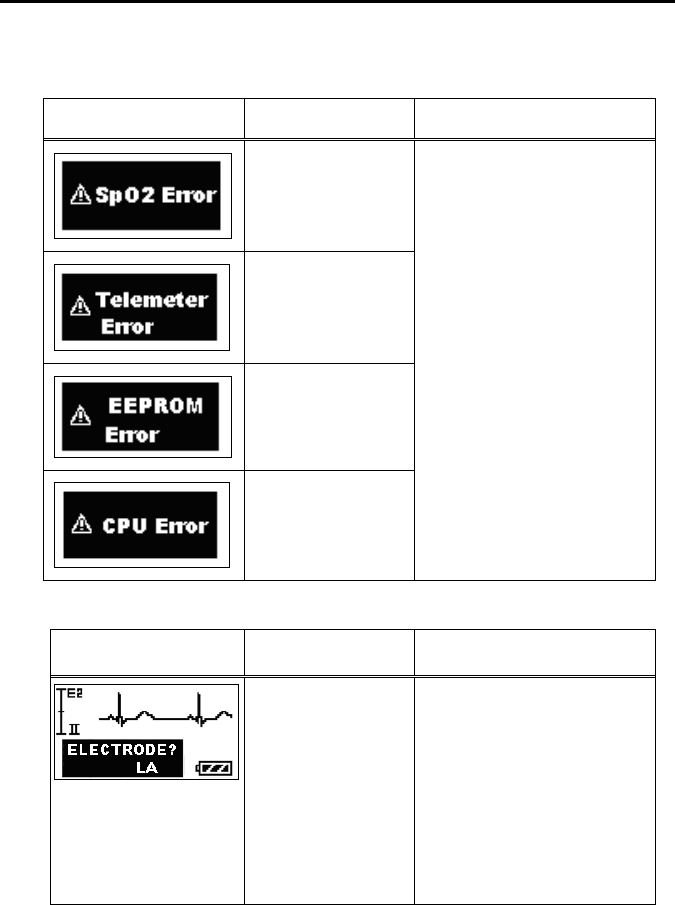
69
11. Troubleshooting
■ List of Displayed messages
Transmitter (main unit)
Message Cause Solution
Faulty SpO2
module.
Failed to transmit
waveform and
value.
Faulty EEPROM.
Failed to initialize
CPU.
Contact your local Fukuda
Denshi service
representative.
ECG
Message Cause Solution
Character string
displayed, such as LA,
depends on the
detached electrode
position.
Electrode is off. Check the electrode
condition.
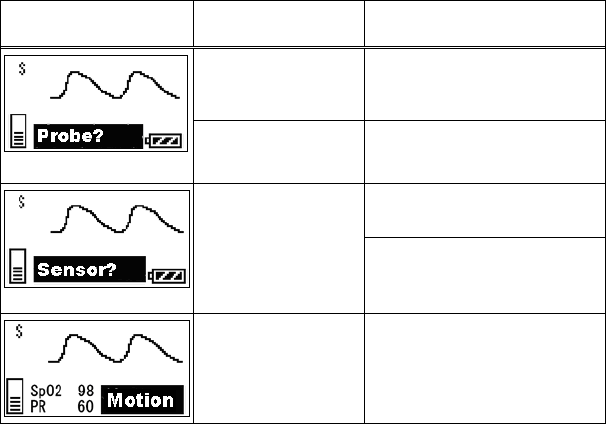
11. Troubleshooting
70
SpO2 (LX-7230N)
Message Cause Solution
Probe is off. Check the attached condition
of the probe.
Faulty Probe. Replace the probe with new
one.
Check the attached condition
of the probe.
SpO2 is not
measured correctly.
Cover the probe with an
opaque material to cut off
the outside light.
Due to excessive
body motion, SpO2
is not measured
correctly
Keep the patient still.
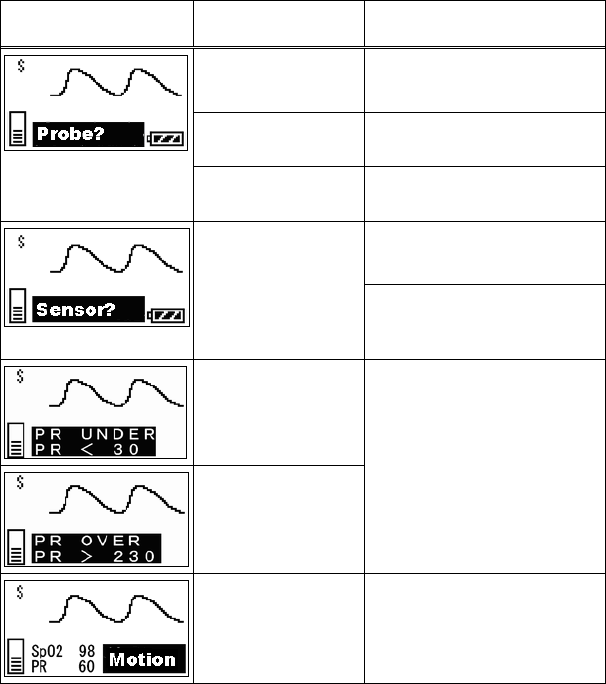
11. Troubleshooting
71
SpO2 (LX-7230KM)
Message Cause Solution
Probe is off. Check the attached condition
of the probe.
Faulty Probe. Replace the probe with new
one.
Unspecified probe
is connected.
Replace the probe with a
specified one.
Check the attached condition
of the probe.
SpO2 is not
measured correctly.
Cover the probe with an
opaque material to cut off
the outside light.
When the pulse rate
is less than 30bpm
(under the
measuring range).
When the pulse rate
is more than
230bpm (over the
measuring range).
Due to excessive
body motion, SpO2
is not measured
correctly
Keep the patient still.
11. Troubleshooting
72
■ Troubleshooting
Make sure of the followings. However, if there is no improvement in the
phenomenon, contact your local Fukuda Denshi service representative.
Transmitter (main unit)
Phenomenon Cause Solution
No battery or wrong
polarity
Install the battery correctly. Nothing is displayed
on the LCD when the
power switch is turned
ON. Battery level is
empty.
Replace the battery with a
new one.
Nothing is displayed
on the receiving
monitor screen.
The channel
number between
the transmitter and
the receiving
monitor do not
match up.
Set the same channel
number for the transmitter
and the receiving monitor.
Same channel
number is already
used.
Make sure to not duplicate
channel numbers.
Follow the instruction by the
person in charge of radio
telemetry channel in your
facility and use the LX-7230
with the correct channel
setting.
Channel
interference
Follow the instruction by the
person in charge of radio
telemetry channel in your
facility and use the LX-7230
with the correct channel
setting.
Transmission problem.
Transmitter failure Contact your local Fukuda
Denshi service
representative.
11. Troubleshooting
73
ECG
Phenomenon Cause Solution
Check the connection
between the lead cable and
the LX-7230.
Lead cable is off.
Check the connection
between the lead cable and
the electrode.
Faulty Lead cable. Replace the ECG cable with
a new one.
Electrode is peeling
off.
Replace the electrode with a
new one.
“ELECTRODE?”
message is displayed.
Polarization
potential of the
electrode is too
high.
Replace the electrode with a
new one.
Electrode gel is dry.
Electrode is peeling
off.
Replace the electrode with a
new one.
Electric blanket is
used.
Cover the electric blanket
with a shield cover.
ECG waveform
contains noise
AC filter setting of
the receiving
monitor is OFF.
Set the AC filter up as ON.
Electrode gel is dry.
Electrode is peeling
off.
Replace the electrode with a
new one.
Respiration waveform
cannot be measured.
The positions of the
electrodes are
improper.
Attach the electrodes where
the respiration waveform can
be measured appropriately.
11. Troubleshooting
74
SpO2
Phenomenon Cause Solution
The probe size is
improper.
Use a probe, which fit
properly.
The probe is
peeling off or is
affected by the
outside light due to
the poor condition
Attach the probe properly
following the instruction.
SpO2 value is
unstable.
Transmitting and
measuring LEDs
sensor are dirty.
Clean both LED sensors
from dirt.
■ In Case of Dropping the LX-7230 into Water
In case of dropping the LX-7230 into water containing disinfectant, pick up the
LX-7230 quickly from it. Rinse it well with running water, and dry it thoroughly
with a soft cloth.
CAUTION
Do not use a drier. The LX-7230 shape may change or be broken.
When the LX-7230 is rinsed with running water, make sure to close the
battery compartment lid.
75
12. Cleaning and Disinfection
The Cleaning and disinfection of the LX-7230, ECG lead cable, and SpO2
probe (sensor) shall be performed as follows.
CAUTION
Do not sterilize the LX-7230, ECG lead cable, and SpO2 probe (sensor) in
any manners, such as radioactive rays, steam, or ethylene oxide.
■ Cleaning
Clean the LX-7230 using a squeezed gauze or an absorbent cotton cloth
dampened with alcohol or a neutral cleanser.
CAUTION
Clean the equipment frequently so stains can be removed easily.
To prevent injury, it is recommended to wear gloves when cleaning the
equipment.
Do not allow any chemical solution to enter the inside of LX-7230 or
connectors.
The LX-7230 cannot be sterilized.
Do not use organic solvents, thinner, toluene and benzene to avoid
damaging the resin case.
Do not polish the housing with abrasive or chemical cleaner.
Use only neutral detergent to clean the housing. Do not use chemical
cloth, scrub brush, abrasive, polishing powder, hot water, volatile solvent
and chemicals (cleanser, thinner, benzine, benzol, and synthetic
detergent for house and furniture), or sharp-edged tools. The surface
resin coating may be damaged, resulting in discoloration, scratches, and
other problems.
■ Disinfection
If there is a possibility of being infected, clean the LX-7230 using a squeezed
gauze or an absorbent cotton cloth dampened with alcohol or invert soap.
CAUTION
Do not immerse the connector parts of the LX-7230 in any chemical
solution to prevent connection failure.
When disinfecting the entire room using a spray solution, pay close
attention not to have liquids get into the LX-7230 or connectors.
12. Cleaning and Disinfection
76
Blank Page
77
13. Maintenance and Inspection
This section explains the daily checks and periodic checks of the LX-7230.
To ensure safety, reliability, and high performance, a “Daily Check” and
“Periodic Check” must be performed. We are not liable for any accident arising
from lack of maintenance.
CAUTION
Do not open the housing or attempt service. The service should be done
by Fukuda Denshi or Fukuda Denshi’s representative.
Do not allow excessive moisture or cleaning agents into the connectors
or inside the equipment.
■ Daily Check
Perform daily checks using the “Daily Check List” on the next page.
■ Periodic Check
Periodic check of medical electronic equipment is mandatory to prevent
failures and accidents and to ensure safety and reliability.
Periodic maintenance may be performed by the medical institution or by a third
party by concluding a “Maintenance Contract”.
For more details, contact your local Fukuda Denshi service representative.
■ Periodic Replacement Parts
The “Battery Compartment Lid (Waterproof)” is the only periodic
replacement part.
To ensure the reliability of waterproof (IPX8) performance of the LX-7230,
replace it once a year.
You may be able to keep using the LX-7230 without periodic replacement of
the lid. However, as it gets older, the reliability of water resistance (IPX8)
performance will not be ensured.
When replacing the lid, contact your local Fukuda Denshi service
representative.
CAUTION
The periodic replacement parts must be replaced at specified period.
13. Maintenance and Inspection
78
Daily Check List
No.
Inspected Date Inspected by Location
Device Type LX-7230N/KM
S/No. Date of Purchase
Items Details Criteria Judgment
Appearance
Visually check for any damage,
cracks, chip, peeled label, and
loosen screw on the housing.
No abnormality should be
found. □OK/ □NG
Visually check for the ring
condition of the battery
compartment lid.
No damage, kink, floating,
and adhesion of dust
should be found.
□OK/ □NG
Battery
Compartment Visually check for the contact
springs, inside the LX-7230, to
the battery and the lock lever of
the battery compartment lid.
No deformation, cracks,
and rust should be found. □OK/ □NG
Power Supply Turn the power ON/OFF to verify
proper switch operation.
With batteries installed,
the LCD should turn ON. □OK/ □NG
ECG Connectors Visually check the connectors of
the cable and the LX-7230.
No damage, chip, and
adhesion of dust should
be found.
□OK/ □NG
ECG Lead cable Visually check each lead for
damages.
No crack and damage
should be found. □OK/ □NG
SpO2
Relay Cable
(LX-7230KM only)
Visually check the cable, and
connector for damages.
No crack, chip and
damage should be found. □OK/ □NG
SpO2 Sensor
(Probe)
Visually check the cable, optical
receiver, LED, and connector for
damages.
No crack, chip, damage,
and adhesion of dust
should be found.
□OK/ □NG
Wireless Channel
Verify whether the transmitting
channel and group ID are the
same with the receiving monitor.
Must match the wireless
channel check list. □OK/ □NG
Transmission
Function
Turn the power ON and make
sure the information is displayed
on the receiving monitor.
Waveforms and values
should be received
without any problem.
□OK/ □NG
Display Function
Turn the power ON and verify
each display condition, such as
SpO2 value and bargraph.
All data should be
properly displayed. □OK/ □NG
Periodic Check Check the date of the previous
periodic check.
Should be within one
year. □OK/ □NG
Comment
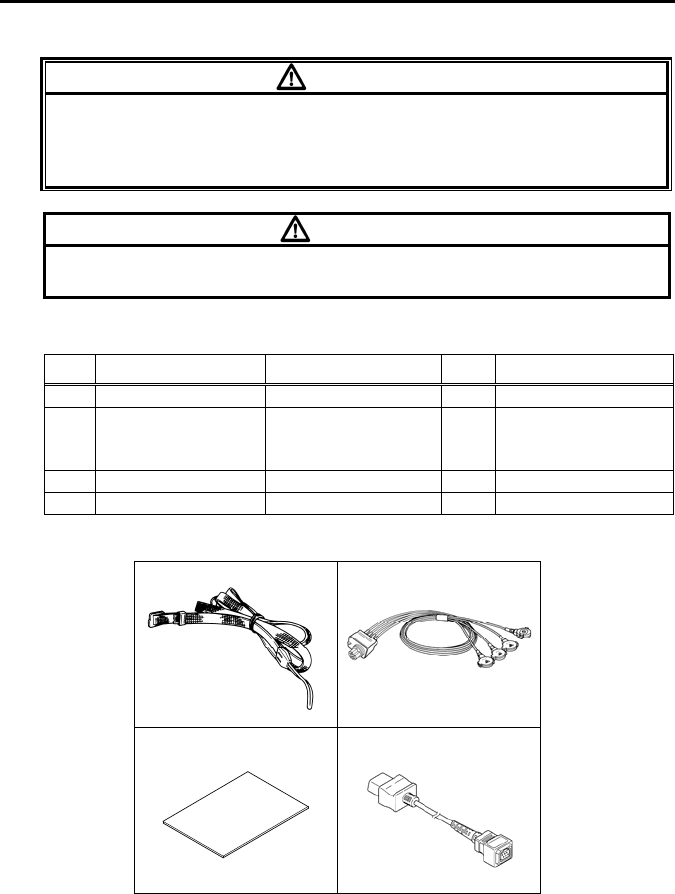
79
14. Standard and Optional Accessories
This section lists the accessories for the LX-7230.
WARNING
Use only the accessories, such as ECG Lead cable and SpO2 probe
(sensor)/ relay cable, specified by Fukuda Denshi for the LX-7230.
Otherwise, the LX-7230 cannot deliver its maximum performance and may
be damaged, resulting in a safety hazard.
CAUTION
For quality improvement, specifications are subject to change without prior
notice.
■ Standard Accessories
No. Item Model Type Q’ty Note
1 Neck Strap OA-311 1
2 4-electrode ECG
lead cable CMT-02HTH-0.8DA 1
AHA color code,
Hook Type, Limb
Lead (2CH)
3 Operation Manual 1
4 SpO2 Relay Cable CI-129 1 LX-7230KM only
1.
2.
3.
4.
This manual
14. Standard and Optional Accessories
80
■Optional Accessories
The following accessories are available as optional for the LX-7230. Purchase
them as required.
●ECG, Impedance Respiration Measurement (For LX-7230N and
LX-7230KM)
AHA color code:
Item Model Type
Note
ECG Hook Type Lead Cable CMT-01HTH-0.8DA 3-electrode (White, Black,
Red),
Limb Lead (1CH)
ECG Hook Type Lead Cable CMT-02HTH-0.8DA 4-electrode (White, Black,
Red, Green),
Limb Lead (2CH)
ECG Hook Type Lead Cable CMT-03HTH-0.8DA
5-electrode (White, Black,
Red, Green, Brown),
Limb Lead (1CH)+Chest
(1CH)
ECG Clip Type Lead Cable CMT-01FTH-0.8DA 3-electrode (White, Black,
Red),
Limb Lead (1CH)
ECG Clip Type Lead Cable CMT-02FTH-0.8DA 4-electrode (White, Black,
Red, Green),
Limb Lead (2CH)
ECG Clip Type Lead Cable CMT-03FTH-0.8DA
5-electrode (White, Black,
Red, Green, Brown),
Limb Lead (1CH)+Chest
(1CH)
14. Standard and Optional Accessories
81
●SpO2 Measurement (For LX-7230N)
Item Model Type Note
Durasensor® DS-100A
Reusable
Adult
(weight of 40Kg and over)
Finger
OxiMax® MAX-P
Single-Use
Pediatric
(weight of 10 to 50Kg)
Finger
OxiMax® MAX-A/AL
Single-Use
Adult
(weight of 30Kg and over)
Finger
OxiMax® MAX-I
Single-Use
Infant
(weight of 3 to 20Kg)
Toe
OxiMax® MAX-R
Single-Use
Adult
(weight of 50Kg and over)
Nose
Single-Use
Adult
(weight of 40Kg and over)
Finger
OxiMax® MAX-N
Neonate
(weight of less 3Kg)
Foot
OxiMax® MAX-FAST
Single-Use
Adult /Pediatric
(weight of 10Kg and over)
Forehead
CAUTION
SpO2 sensors for the LX-7230N cannot be used with the LX-7230KM.
14. Standard and Optional Accessories
82
●SpO2 Measurement (For LX-7230KM)
Item Model Type Note
Finger Clip SR-5C
Reusable
Adult and Pediatric
(weight of 30Kg and over)
Finger
Spot Check SP-5C
Reusable
Adult and Pediatric
(weight of 30Kg and over)
Finger
Universal Probe UD-5C
Semi-Disposable
Adult and Pediatric
(weight of 40Kg and over)
Finger
SD-5C(25)
Semi-Disposable
Adult and Pediatric
(weight of 15Kg and over)
Finger
Personal Probe
SD-5C(20)
Semi-Disposable
Pediatric and Infant
(weight of 5 to 15Kg)
Big toe or the root of the big toe
CAUTION
SpO2 sensors for the LX-7230KM cannot be used with the LX-7230N.
83
15. Specification
■ Specification
CAUTION
For quality improvement, specifications are subject to change without prior
notice.
Standard Specification
Size: 72.0(W) x 98.0(H) x 24.8(D)mm (not including the
protrusion)
Weight: Approximately 190 grams (with batteries)
Transmitting
Waveform:
ECG 1CH or 2CH (selectable from the ECG lead
cable), Respiration waveform, pulse waveform (with
SpO2 value)
ECG Lead cable Type: 3-electrode, 4-electrode, or 5-electrode (Limb+Chest)
lead cable
Automatically detect the type after inserting the lead
cable
Transmitting Status
Data:
Electrode Off, Low Battery, Event Switch, Pacemaker
Detection, Channel ID, 64group Codes, SpO2 Sensor
Off
LCD: Built-in
Waterproof: IPX8
Power Supply: DC: Two 1.5 V “AA” size (“LR06” size) alkaline
batteries
Continuous Operating
Time:
LX-7230N Approximately 2.5 days
LX-7230KM Approximately 5 days
※Continuous operating time is assumed when using new “AA” size (“LR06”
size) Alkaline batteries specified by Fukuda Denshi.
ECG
Numbers of Lead
Electrode:
3-electrode, 4-electrode, or 5-electrode
(Limb+Chest)
Numbers of Input
Channel:
1CH(3-electrode) or 2CH
Accuracy of Sensitivity 10mm /1mV ±1mm
(Display sensitivity on the receiving monitor)
ECG Input Impedance: 5MΩ and above
Maximum Input Voltage: ±5mV and above
Common Mode Rejection
Ratio:
Less than 10mVp-p (95dB and above)
Accuracy of Heart Rate
Measurement:
±10% or ±5bpm, whichever is greater
Display Coverage of
Heart Rate:
0, 12 to 300bpm (1bpm step)
Frequency
Characteristic:
0.5 to 40Hz (within -3dB)
15. Specification
84
Time Constant 0.4 sec ±25%
Switching and available to set 0.1 sec ±25%
Pacemaker Pulse
Detection/ Rejection:
Comply with ANSI/AAMI EC13 Pacemaker pulse
rejection capability
Protection to
Defibrillation:
Meet the requirement of IEC60601-2-27
Respiration (Impedance Method)
Accuracy of Sensitivity: 10mm/1Ω ±2mm
(When standard Impedance is 480Ω.)
Display Coverage of Respiration Rate:0, 9 to 150Bpm
Display Error of Respiration Rate: 3Bpm
Measured Current of Respiration: Below 100μA (at 42kHz)
SpO2
LX-7230N
SpO2 Measurement Range: 1 to 100%
Resolution: 1%
Measurement Accuracy: Accuracy of measurement with SpO2
Probe is as follows.
SpO2 Probe Measurement
Accuracy (±1SD)
OxiMax® MAX-I ±2%
OxiMax® MAX-P ±2%
OxiMax® MAX-A/AL ±2%
Durasensor® DS-100A ±3%
OxiMax® MAX-R ±3.5%
OxiMax® MAX-FAST ±2%
OxiMax® MAX-N ±2%
(When SpO2 is 70 to 100%. Less than 70% is not specified.)
Wavelength: Approx. 660nm (Red light)
Approx. 890nm (Infrared light)
15. Specification
85
LX-7230KM
SpO2 Measurement Range: 0 to 100%
Resolution: 1%
Measurement Accuracy: Accuracy of measurement with SpO2 probe
is as follows.
Measurement Spot Measurement Accuracy
(±1SD)
Finger ±2%
Big toe or the root
of the big toe ±3%
(When SpO2 is 70 to 100%. Less than 70% is not specified.)
Wavelength: 658 to 665nm (Red light), 860 to 925nm
(infrared light)
Power Loss on Probe: 2.0mW
Measurement Reply Time: 12 sec (moving average time)
Measurement Value Update Rate: Continuous mode 1 second
Intermittent mode 25 seconds
NOTE
The SpO2 measurement accuracy is determined based on the values of
the root-mean-square (rms) difference between SpO2 readings of the pulse
oximeter equipment and values of SaO2 determined with a CO-oximeter,
by healthy adult volunteers. The pulse oximeter equipment measurements
are statistically distributed; ±2% measurement accuracy means that only
about two-thirds of pulse oximeter equipment measurements can be
expected to fall within ±2% of the value measured by a CO-oximeter.
Pulse Wave
LX-7230N
Pulse Rate Measurement Range: 20 to 250bpm
Measurement Accuracy ±3bpm
LX-7230KM
Pulse Rate Measurement Range: 30 to 230bpm
Measurement Accuracy ±2bpm (30 to 100bpm)
±2% (100 to 230bpm)
Measurement Response Time: 6 sec. (Moving Average Time)
15. Specification
86
Transmission Method
Modulation Mode: Digital, Frequency shift keying
Frequency: 608 to 614MHz
Oscillation Method: PLL Synthesizer method by crystal control
Channel Spacing: 12.5kHz
Occupied Frequency
Bandwidth:
8.5kHz
RF output power: 1mW ±2dB
Transmitting Antenna: ECG lead cable and/or SpO2 Probe
Safety
General Standard: IEC 60601-1:1988 +A1: 1991 +A2: 1995
(Medical electrical equipment – Part 1: General
requirements for safety)
EMC Standard: IEC 60601-1-2: 2007
(Medical electrical equipment – Part 1: General
requirements for safety – 2. Collateral standard:
Electromagnetic compatibility – Requirements and tests)
The class of
protection
against electric
shock
Internally Powered Equipment
The type of
protection
against electric
shock
Type CF Applied part
Operating Environment
Temperature: 10 to 40C
Humidity: 30 to 85% RH (No condensation)
Vibration/Shock: Comply with IEC60068-2-64:1987, IEC60068-2-32:1975,
IEC60068-2-6:1995
Transport / Storage Environment
Temperature: -10 to 60C
Humidity: 10 to 95% RH (No condensation)
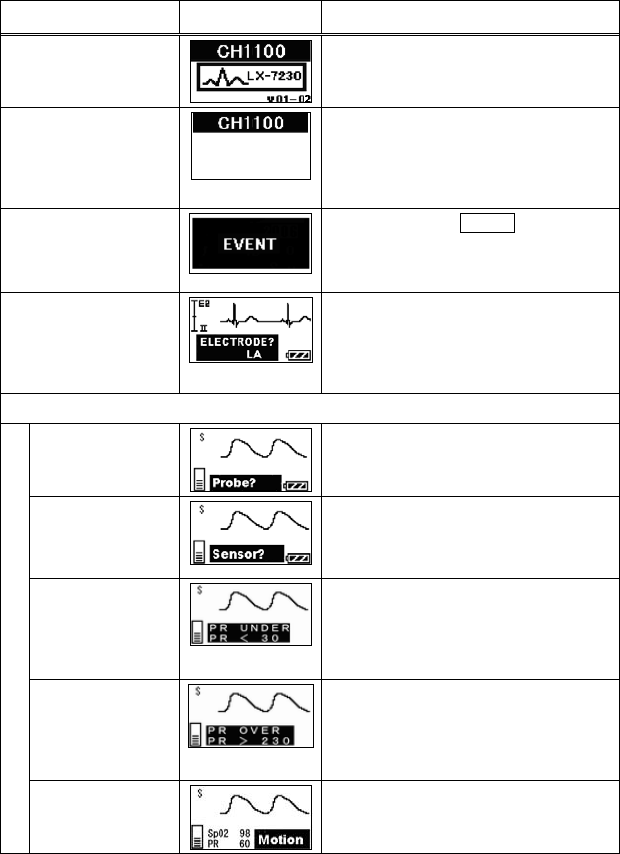
15. Specification
87
■ Displays
The following displays are shown on the LCD of the LX-7230.
Display Description
Starting Screen
Displays after turning on the power.
Automatically moves onto the channel
display screen.
Channel Display
Screen
Displays the transmitter channel after
turning on the power and also when
refreshing the screen.
Automatically move onto the waveform
display screen.
EVENT
Displays when the EVENT button is
pressed.
Automatically move onto the channel
display screen.
ELECTRODE?
Displays when the ECG electrode is
disconnected or the ECG/respiration
waveform cannot be measured normally.
For details about electrode check
message, refer to page 89.
SpO2 measurement Status
Probe? Displays when the SpO2 probe is
disconnected from the device.
Sensor? Displays when the SpO2 probe is off
from the measuring position or SpO2
cannot be measured normally due to
outside light, etc..
PR UNDER
PR<30
Displays when the pulse rate is less than
30bpm, the SpO2 value and pulse rate
cannot be measured.
This display is only available for LX-
7230KM.
PR OVER
PR>230
Displays when the pulse rate is more
than 230bpm, the SpO2 value and pulse
wave rate cannot be measured.
This display is only available for LX-
7230KM.
Motion Displays when the measurement cannot
be executed due to an artifact such as
body motion.
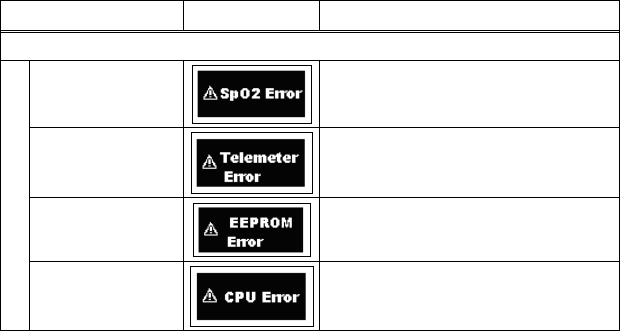
15. Specification
88
Display Description
Error Message
SpO2 Error
Displays when the SpO2 measurement
module is faulty
Telemeter Error
Displays when the transmitter is faulty
EEPROM Error
Displays when the EERPROM is faulty
CPU Error
Displays when the CPU is faulty
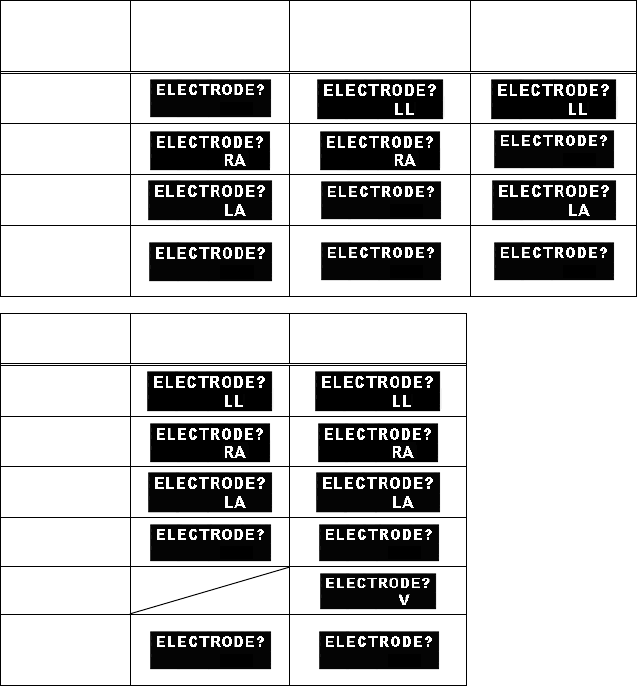
15. Specification
89
■ Details of the “ELECTRODE?” Message
The following “ELECTRODE?” messages are displayed on the LCD depending
on the selected lead cable and lead.
For AHA color code
Check
Position
3-electrode lead
cable
Lead I display
3-electrode lead
cable
Lead II display
3-electrode lead
cable
Lead III display
LL
RA
LA
Several
Position
Simultaneously
Check
Position
4-electrode lead
cable
For 5-electrode
(Chest) lead cable
LL
RA
LA
RL
V
Several
Position
Simultaneously
15. Specification
90
■ List of Setup Items
This section lists the available selection, default setting, and backup status
for each setup item, which is available for the LX-7230.
Items Selection Default Backup
ECG Lead I, II, III II Yes
Display Size of ECG (1) ×1, ×1/2 ×1 Yes
Display Size of ECG (2) ×1, ×1/2 ×1 Yes
Display Size of
Respiration Waveform ×1, ×1/2 ×1 Yes
SpO2 Measurement
mode (LX-7230KM only)
Continuous,
Intermittent Continuous Yes
For details of the following settings, contact our service representative.
Items Selection Default Backup
Time Constant 0.4 sec., 0.1 sec. 0.4 sec. Yes
Detection Sensitivity of
Pacemaker Pulse Low, Mid, High Mid Yes
Respiration Detection
Signal ON, OFF ON Yes
LCD Contrast 8 steps 8 Yes
Transmitter Channel
One from the
following channels.
0801 to 0879
0900 to 0979
1000 to 1079
1100 to 1179
1200 to 1279
1300 to 1379
1100 Yes
Group ID One from 00 to 63 00 Yes

39-4, Hongo 3-chome, Bunkyo-ku, Tokyo, Japan
Phone:+81-3-3815-2121 Fax:+81-3-3814-1222
Printed in Japan 4L0105700 20100805(01)