Given Imaging SMARTPILL Capsule User Manual SmartPill pH p
Given Imaging Limited Capsule SmartPill pH p
User_manual

Appendix: Electrical Safety
SmartPill® GI Monitoring
System
User Manual
MotiliGI® v3.0
Doc: 111482-01
March 2013

Appendix: Electrical Safety
Copyrights
Text, graphics, logos and images in this manual are the property of Given Imaging and
protected by United States and international copyright laws. This Manual may not be
transferred or reproduced in any form without the written permission of Given
Imaging. Copyright © 2006-2012 Given Imaging. All Rights Reserved.
Trademarks
MotiliGI® and The Measure of GI Health® are registered trademarks, and
SmartPill™ and the SmartPill logo are trademarks of Given Imaging. Such marks are
protected by United States common law, federal and/or international trademark laws
and may not be used in violation of Given Imaging’s rights.
Patents
Certain uses and features of the products referenced herein are protected by one or
more United States and international patents or have pending patent applications.
Limited Warranties
The SmartPill GI Monitoring System
Given Imaging warrants the system* for a period of one (1) year from date of
purchase, and that the components of the system* have been designed, manufactured,
packaged and tested and, if properly used, are free from any defect of workmanship or
materials that would materially and adversely affect their intended use.
If any component of the system* fails during the period of this Limited Warranty for
reasons covered by this Limited Warranty, Given Imaging, at its option, shall replace
the specific failed component.
This Limited Warranty does not cover failure occurring in connection with or arising
out of uses not intended by Given Imaging, misuse, neglect, alteration, repair,
improper installation or improper testing. Without limiting the generality of the
foregoing statement, this Limited Warranty shall be invalidated if any repairs, services
or modifications are made to any of the components by any person not explicitly
authorized by Given Imaging.
Given Imaging is not liable or otherwise responsible for any loss, damage, or expense
arising, directly or indirectly, from the use of the system* or SmartPill capsule. Without
limiting the generality of the foregoing statement, customers are liable for all matters
beyond Given Imaging’s control such as handling, storage, cleaning, misuse, treatment
and diagnosis.

Appendix: Electrical Safety
This Limited Warranty is in lieu of and excludes all other warranties, whether
expressed or implied, including without limitation warranties of merchantability or
fitness.
Extended Warranty Options are available.
* Only the data receiver, docking station and activation fixture of the SmartPill GI
Monitoring System are covered by this Limited Warranty:
The SmartPill Capsule
Given Imaging warrants each Capsule is free from defects in workmanship and
materials until the Capsule’s labeled expiration date.
If Given Imaging verifies capsule failure during the warranty period for reasons
covered by the Limited Warranty, Given Imaging shall replace the failed capsule.
Additional Limitations
The Limited Warranty does not cover software or damages due to misuse, neglect,
alteration, repair, improper installation, set-up, calibration or improper testing.
Given Imaging is not liable for any incidental or consequential loss, damage, or
expense arising, directly or indirectly, from the use of the system or capsule.
Customers are liable for all matters beyond Given Imaging’s control such as handling,
storage, cleaning, misuse, treatment, and diagnosis.
This warranty is in lieu of and excludes all other warranties whether expressed or
implied warranties of merchantability or fitness.
The System Computer
The system computer is covered under the manufacturer’s warranty.
Rx Only
Given Imaging
3950 Shackleford Road, Suite 500 Duluth GA
30096 USA supportUS@givenimaging.com
Given Imaging GmbH
Borsteler Chaussee 47 D-22453
Hamburg, Germany supportEU@givenimaging.com
This device complies with Part 15 of the FCC. Operation is subject to the following two conditions:
1. This device may not cause harmful interference.
2. This device must accept any interference received, including interference that may cause undesired
operation.
Appendix: Electrical Safety
Table of Contents
............................................................................................................................................. 1
Introduction and Components ............................................................................................... 9
Using this Manual ................................................................................................................. 9
System Components ........................................................................................................... 10
SmartPill Capsule Pack ................................................................................................... 10
Capsule Operational Specifications ............................................................................... 10
SmartBar ........................................................................................................................... 10
Data Receiver ................................................................................................................... 11
Docking Station ............................................................................................................... 12
Activation Fixture ........................................................................................................... 13
System Computer and MotiliGI Software ................................................................... 13
Accessories ....................................................................................................................... 14
Use and Care of the System .................................................................................................. 16
Acronyms, Use, and Symbols ............................................................................................ 16
Acronyms ......................................................................................................................... 16
Intended Use/Indications for Use ................................................................................... 16
Contraindications for Use .............................................................................................. 16
Restricted Use .................................................................................................................. 17
Storage .................................................................................................................................. 17
Power Requirements ....................................................................................................... 17
Recycling and Disposal Instructions ............................................................................. 18
Device Markings ................................................................................................................. 18
Data Receiver Display Messages ................................................................................... 19
Risks and Safety .................................................................................................................. 20
Non-Passage .................................................................................................................... 20
Patient-Contacting Materials .......................................................................................... 21
Care, Cleaning and Maintenance ....................................................................................... 21
Data Receiver ................................................................................................................... 21
Docking Station ............................................................................................................... 22
Troubleshooting and Support ........................................................................................... 22
Setting-Up the System ........................................................................................................... 23
Getting Started .................................................................................................................... 23
Appendix: Electrical Safety
Setting up the Computer ................................................................................................ 23
Preparing for a Test ............................................................................................................... 25
Before the Test Day ........................................................................................................... 25
Charging the Data Receiver ........................................................................................... 25
Preparing the Patient ...................................................................................................... 26
During the Office Visit .................................................................................................. 27
Starting a Test ......................................................................................................................... 30
Preparing the System ......................................................................................................... 30
Performing the Test – Test Initiation Wizard ................................................................ 30
Step 1: Connect the Data Receiver ............................................................................... 30
Step 2: Enter Patient Information ................................................................................ 31
Creating Templates: ........................................................................................................ 33
Using Templates ............................................................................................................. 33
Step 3: Assemble Materials ............................................................................................ 34
Step 4: Activate Capsule ................................................................................................. 35
Step 5: Select Capsule ..................................................................................................... 36
Step 6: Enter Pressure Calibration Code ..................................................................... 38
Step 7: Add pH Calibration Buffer ............................................................................... 38
Step 8: Capsule pH Calibration ..................................................................................... 39
Step 9: Ingestion.............................................................................................................. 40
Step 10: Complete Discharge Checklist ....................................................................... 42
Live Monitoring Mode (optional) .................................................................................... 43
Aborting a Test – Deactivating the Capsule ................................................................... 44
Ending a Test ......................................................................................................................... 45
Returning the Data Receiver ............................................................................................. 45
Downloading a Test ........................................................................................................... 45
Post Test Notes ............................................................................................................... 46
Confirming Capsule Exit ............................................................................................... 47
Analyzing the Test ................................................................................................................. 48
Introduction to the Test Analysis Wizard ....................................................................... 48
Analyzing the Test .............................................................................................................. 50
Patient Diary Events ....................................................................................................... 50
Select Capsule Ingestion ................................................................................................ 51
Appendix: Electrical Safety
Gastric Acidity ................................................................................................................. 52
Procedure Deviation – Additional Meal Before 6 Hours .......................................... 53
Select Gastric Emptying ................................................................................................. 55
Magnitude of pH Rise at Emptying .............................................................................. 56
Gastric Pressure Characteristics .................................................................................... 57
Small Bowel pH profile .................................................................................................. 57
Small Bowel Pressure Characteristics ........................................................................... 58
Select Ileo-Cecal Junction .............................................................................................. 59
Colonic Pressure Characteristics ................................................................................... 60
Warning: Data Collected During Capsule Low Voltage ............................................ 61
Select Body Exit .............................................................................................................. 62
Review Your Physiological Markers with MotiliGI’s Markers ...................................... 64
MotiliGI-Computed Capsule Ingestion ........................................................................ 65
MotiliGI-Computed Gastric Emptying ........................................................................ 66
Absence of Gastric Emptying ....................................................................................... 67
Gastric Emptying Statistics ............................................................................................ 68
Gastric Emptying Time Evaluation .............................................................................. 69
MotiliGI-Computed ICJ ................................................................................................. 70
Small Bowel Transit Time Evaluation .......................................................................... 71
MotiliGI-Computed Body Exit ..................................................................................... 72
Absence of Body Exit ..................................................................................................... 73
Colonic Transit Time Evaluation .................................................................................. 74
Test Analysis Review ...................................................................................................... 74
Reading Test Summary Reports........................................................................................ 75
Transit Data Tab ............................................................................................................. 76
Descriptive Data Tab ...................................................................................................... 77
Interpretations Tab ......................................................................................................... 78
Properties Tab ................................................................................................................. 79
Examples of Physiological Markers .................................................................................. 79
Ingestion ........................................................................................................................... 79
Gastric Emptying ............................................................................................................ 80
ICJ ..................................................................................................................................... 81
Body Exit Time ............................................................................................................... 81
Appendix: Electrical Safety
MotiliGI Features................................................................................................................... 83
MotiliGI User Interface ..................................................................................................... 83
Titlebar ............................................................................................................................. 83
Main Menus ..................................................................................................................... 83
Toolbar Icons .................................................................................................................. 83
Graph Area ...................................................................................................................... 85
Data Panels ...................................................................................................................... 85
Time Slider Control ........................................................................................................ 85
Display Tabs .................................................................................................................... 85
Status Bar ......................................................................................................................... 85
MotiliGI Keyboard Shortcuts ....................................................................................... 86
Opening a Test by the Patient’s Name ........................................................................ 86
Opening a Test by the File Name ................................................................................. 87
Closing a Test .................................................................................................................. 87
Exiting MotiliGI.............................................................................................................. 88
Setting General User Preferences ..................................................................................... 88
General Tab ..................................................................................................................... 88
Graph Tab ....................................................................................................................... 89
Report Tab ....................................................................................................................... 90
Creating Report Letterhead ........................................................................................... 91
Files Tab ........................................................................................................................... 92
Setting Preferences Back to Default ............................................................................. 92
Editing Patient Information .............................................................................................. 92
Changing the Graph Display ............................................................................................ 93
Selecting Data Plots ........................................................................................................ 93
Changing the Test View ................................................................................................. 94
Changing Axis Scales ...................................................................................................... 96
Analyzing Data ................................................................................................................... 97
Showing and Hiding Annotations ................................................................................ 97
Editing Patient Diary Events ......................................................................................... 97
Creating New Event Annotations ................................................................................ 98
Editing Event Annotations ............................................................................................ 98
Adding or Changing the Location of Physiological Markers .................................... 99
Appendix: Electrical Safety
Viewing Transit Times ..................................................................................................100
Calculating Statistics ......................................................................................................100
Creating Output ................................................................................................................101
Generating a Report ......................................................................................................101
Exporting Data to Excel ..............................................................................................102
Exporting Images to JPEG or GIF ............................................................................104
System Administrator Tools ...............................................................................................105
Password Management ....................................................................................................105
Logging into the System Computer ............................................................................105
Logging into MotiliGI ..................................................................................................106
MotiliGI Security ..............................................................................................................106
Security Tab ...................................................................................................................106
Backing Up Patient Files ..............................................................................................107
Installing Software ............................................................................................................108
MotiliGI Administrator ................................................................................................108
Installing Software from a CD ....................................................................................109
Installing Software from a File on the System Computer Hard Drive ...................109
Uninstalling MotiliGI ....................................................................................................110
Installing MotiliGI .........................................................................................................110
Appendix: Electrical Safety .................................................................................................112
Index ...................................................................................................................................116
Contacting Given Imaging ..............................................................................................119

Appendix: Electrical Safety
,QWURGXFWLRQDQG&RPSRQHQWV
Using this Manual
Complete user training and read this manual before running the SmartPill
System. This manual contains important safety information and
contraindications.
This manual makes use of 2 special notations: Warning and Caution, the meanings of
which are:
Warning
Potentially hazardous situations which could result in serious adverse reactions (death or serious injury) or
serious safety hazards to users and patients. All warnings are boxed.
Caution
Potentially hazardous situations which could result in minor or moderate injury or damage to the equipment
or other property.

Appendix: Electrical Safety
System Components
SmartPill Capsule Pack
Each capsule pack contains a single-use capsule, calibration buffer, instructions for use
and a patient diary.
The capsule measures pressure, pH, and temperature to determine transit times of the
stomach, small bowel, and colon. Transit times derived by capsule motility procedures
provide alternatives to other tests such as gastric emptying scintigraphy, whole gut
scintigraphy, and radio-opaque markers.
Caution
Do not use a SmartPill capsule if it has been dropped as this may affect function.
Capsule Operational Specifications
Attribute
Specification
Pressure Accuracy
0-99 mmHg ± 5 mmHg
100-350 mmHg ± 10% of applied pressure
Pressure Range
0-350 mmHg
pH Accuracy
± 0.5 pH units
pH Range
1–9 pH units
Temperature Accuracy
± 1°C (between 20 – 42ºC)
Transmission Frequency
434.2 MHz (radiating between 426 – 445 MHz)
Battery Life
Capsule and Data Receiver, >5 days
Weight
4.5 grams
Size
26 x 13mm
SmartBar
The SmartBar is a standardized meal that is ingested immediately before capsule
ingestion. To accurately measure gastric emptying patients must consume a standard
meal immediately before ingesting the capsule.

Appendix: Electrical Safety
Data Receiver
The data receiver records biomedical data sent by the capsule. It is worn by the
patient on a belt clip or a lanyard (around the neck). The data receiver features an
Event button that when pushed places a marker in the electronic data. A patient diary
for recording the time and reason for the event button use is stored on the backside of
the receiver. The data receiver weighs approximately 225g (0.5 lb).
Caution
Use only the SmartPill Docking Station (REF 50100400) to charge and download data from the receiver.
Figure 1
Featu
re
Description
A - Data Display
See Data Receiver Display Modes, Page 19 for more details.
B – Backlight Control
Button
The backlight button turns on a light, enabling the display to be read in low light
conditions. The backlight button is also us
ed to turn the data receiver off. The
backlight button and e
vent button (described below) must be simultaneously
depressed for 5 seconds to turn the data receiver off.
C – Event Button
The Event button turns the data receiver on. Patients press the event button when
engaging in an event (light exercise, eating, going to the bathroom, sleeping,
abdominal discomfort, pain, etc.) or experiencing any symptom which the clinician
believes may affect GI physiology and may be of interest. Pressing the
e
vent button
inserts a marker in the test data record.
D – Belt Clip and
Lanyard
The data receiver is equipped with a belt clip and supplied with a lanyard. The
patient has the choice of clipping the
data receiver on a waist belt or wearing the
data receiver suspended from a lanyard.
E – Patient Diary
The patient diary is to be used by the patient to record events, activities and
symptoms listed in the
patient instruction sheet, and the date and time the events
occurred. The entries in the
patient diary should correspond to the event button
markers inserted into the test data record when the event button is pressed.
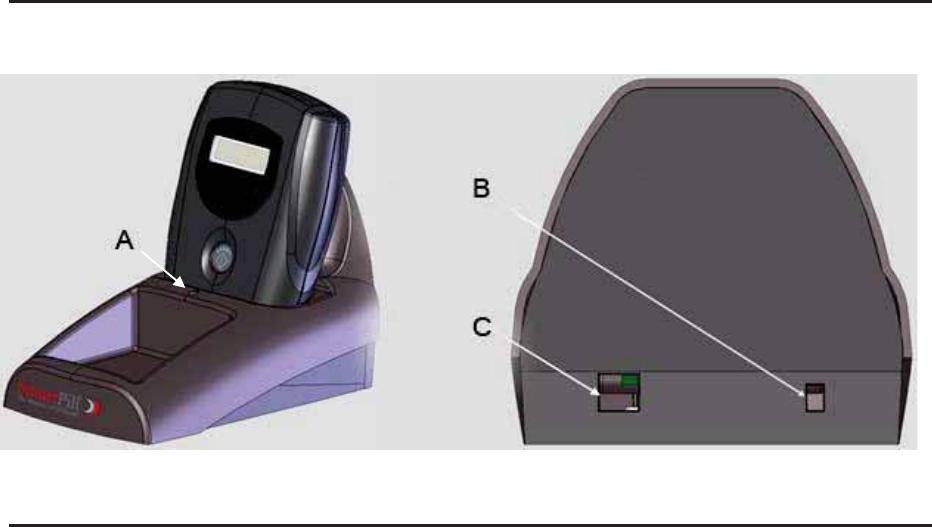
Appendix: Electrical Safety
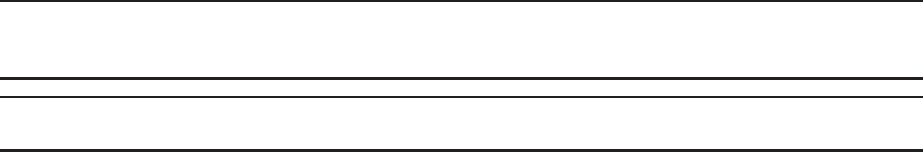
Docking Station
The docking station establishes electronic communication between the data receiver
and the system computer for data download and serves as a charging stand for the data
receiver. The docking station weighs approximately 200 grams (0.45 lbs).
Caution
Use only the Power Supply (REF 30100900) supplied with the SmartPill Docking Station.
Feature
Description
A
– LED Light
Indicates the state of data receiver. See table below.
B
– DC Power Connector
Provides a connection point for the power supply.
C
– USB Connector
Provides a connection point for the USB cable,
connecting the docking station to the system
computer.
Figure 2

Appendix: Electrical Safety
LE
D Color
State
Action Recommended
Red
Charging
Continue charging until the LED turns green.
Yellow
A charging circuit fault has
occurred
Undock and then re-dock the data receiver in the
docking station. If the yellow light persists
contact technical support.
Green
Fully charged
The data receiver is ready for use.
Off
xDocking station is not
connected to an AC power
source
xReceiver is not fully connected
to the docking station
xDocking station or data
receiver are in thermal
shutdown
xConnect the docking station to a source of
AC power.
xUndock and then re-
dock the data receiver in
the docking station.
xContact technical support for assistance.
Activation Fixture
The activation fixture turns the capsule on and off using strong magnets that interact
with the capsule’s internal power switch.
Warning
Individuals with pacemakers should not come within one (1) foot of the SmartPill activation fixture. The
fixture contains strong magnets that could interfere with pacemaker operation.
Warning
Do not store the SmartPill Activation Fixture in the same room with or a room adjacent to MRI equipment.
The fixture contains strong magnets and could become a dangerous projectile.
Caution
Keep the SmartPill Activation Fixture more than two (2) feet from magnetic media and computer monitors.
Caution
Do not store unused capsules within one (1) foot of the SmartPill Activation Fixture. Stray magnetic fields
from the activation fixture may activate the capsule.
System Computer and MotiliGI Software
MotiliGI software comes installed on the system computer. MotiliGI receives and
processes downloaded data from the data receiver, stores test data, provides data
analysis tools, and graphically displays test results. MotiliGI features algorithms that
calculate GET, SBTT, CTT, WGTT, and motility indices of the antrum and
Appendix: Electrical Safety
duodenum. An optical mouse is supplied with the system computer. An electronic
copy of this user manual is included in the software.
Caution
Use MotiliGI only on the system computer supplied with the SmartPill GI Monitoring System. Installing and
operating MotiliGI on another computer is not recommended or supported by SmartPill Corporation.
Caution
Do not use the vertical pipe (|) character in text fields or the software will remove it.
Minimum System Computer Requirements
Manufacturer
Operating System
Dell (preferred), IBM, HP, Toshiba
Any of the following 32-bit Microsoft Windows versions:
xXP Professional Service Pack 3 (recommended)
xWindows Vista Business
xWindows Vista Ultimate
xWindows 7 Professional (recommended)
xWindows 7 Ultimate
System does not work with 64-bit operating systems
Hard Drive
100 megabytes of available space for the MotiliGI application and 10
gigabytes of available space for test files (recommended)
Communication Port
1 open USB port
Peripherals
CD/RW Drive; printer is optional
Processor
Pentium IV 1 Gigahertz (GHz) or higher
Memory
1 gigabyte (GB) of RAM or
Display
1280 x 800 resolution
widescreen aspect ratio (recommended)
96 dpi
32-bit color
Other
Microsoft .NET Framework 1.1 SP 1
Adobe Acrobat 7.0.8 or later
Accessories
Your starter kit includes the acceosories below:
Accessory
Description
Starter Kit Backpack
A protective backpack that holds the system
components.
Belt Clips and Lanyards
Attach to the back of the data receiver.
User Manual
Complete the user training and read this manual
before running the SmartPill System. The user
manual contains important safety information.
USB Cable
Connects the docking station to the system computer.
Power Cord
Powers the docking station
Patient Instruction Sheets
Instruction sheets to send home with the patient
Power Adaptor (non US systems)
Modifies the power supply.

Appendix: Electrical Safety
Warning
Do not connect items to the SmartPill GI Monitoring System that are not part of the system.
Appendix: Electrical Safety
8VHDQG&DUHRIWKH6\VWHP
Acronyms, Use, and Symbols
Acronyms
GET
Gastric emptying time
SBTT
Small bowel transit time
CTT
Colonic transit time
SLBTT
Combined small and large bowel transit
time
WGTT
Whole gut transit time
Intended Use/Indications for Use
The SmartPill GI Monitoring System measures whole gut and regional gut (stomach,
small bowel, and colon) transit times. Measurements of gastrointestinal (GI) tract
transit times are used for evaluating motility disorders.
The system measures pH, pressure, and temperature throughout the GI tract. Pressure
contraction data from the antrum and duodenum can be used to calculate motility
indices.
Suspected disease or
condition to evaluate
Indicated
M
easurement
Use
Gastroparesis
GET
Delayed gastric emptying is implicated
in such disorders as idiopathic and
diabetic gastroparesis and functional
non-ulcer dyspepsia.
Chronic
constipation
CTT
Aids in differentiating slow and normal
transit constipation.
SLBTT
A surrogate measure of colonic transit
in patients with chronic constipation
when CTT alone cannot be
determined.
Caution
Do not use in patients younger than 18 years old.
Contraindications for Use
Do not use in patients with these diseases or conditions:
Appendix: Electrical Safety
xhistory of gastric bezoar
xswallowing disorders
xsuspected or known strictures, fistulas, or physiological/mechanical GI
obstruction
xGI surgery within the past 3 months
xsevere dysphagia to food or pills
xCrohn’s disease or diverticulitis
ximplanted or portable electro-mechanical medical device such as a cardiac
pacemaker, defibrillator or infusion pump
xyounger than 18 years old.
Data transmission from the capsule to the data receiver is influenced by patient BMI.
Significant data dropout can occur in severely obese patients (>40 BMI).
Restricted Use
Caution
The SmartPill GI Monitoring System equipment is not suitable for use in the presence of flammable anesthetic
mixture with air or with oxygen or nitrous oxide.
Not for use with oxygen or oxygen-enriched atmospheres.
Storage
Store SmartPill GI Monitoring System components and capsules at ambient room
temperature (-15–40ºC) and humidity (rH 30–90%).
Caution
Do not expose the capsules to UV light. UV light can permanently damage the pH sensor.
Caution
Do not store capsules within 30cm (1 foot) of the activation fixture. The fixture’s magnetic field could
inadvertently activate the capsules.
Power Requirements
System Computer
110/220 VAC, 50–60 Hz
May require the use of a
power plug adapter
Docking Station
110/220 VAC, 50–60 Hz
May require the use of a
power plug adapter
Capsule
3.1 VDC self-contained
batteries
none
Data Receiver
8.4 VDC, self-contained,
none

Appendix: Electrical Safety
rechargeable batteries
Recycling and Disposal Instructions
xRecycle the data receiver and docking station following the local, regional, and
national regulations for electronic devices.
xThe capsule contains silver oxide batteries. Recycle unused capsules following the
local, regional and national regulations for electronic devices.
xDispose of used capsules following local, regional and national regulations for
disposing of human excrement.
xThe calibration buffer contains sodium citrate, a common food preservative.
Dispose of used buffer following local, regional, and national disposal regulations.
Device Markings
Caution, consult accompanying
documents
Consult directions for use
US FCC compliance
Prescription use only
Recycle. Dispose of properly
Single use only. Do not reuse
Part or catalog number
Manufacturer
Date of manufacture
Use by YYYY-MM
Lot number
Minimum and maximum storage
temperature
Caution: Strong magnet
Warning: Keep away from pacemakers
Appendix: Electrical Safety
BF
Type BF equipment
0123
CE marking and notified body number
Authorized representative
Sufficient for one test
IP57
Ingress protection rating
Serial number
Fragile
Keep dry
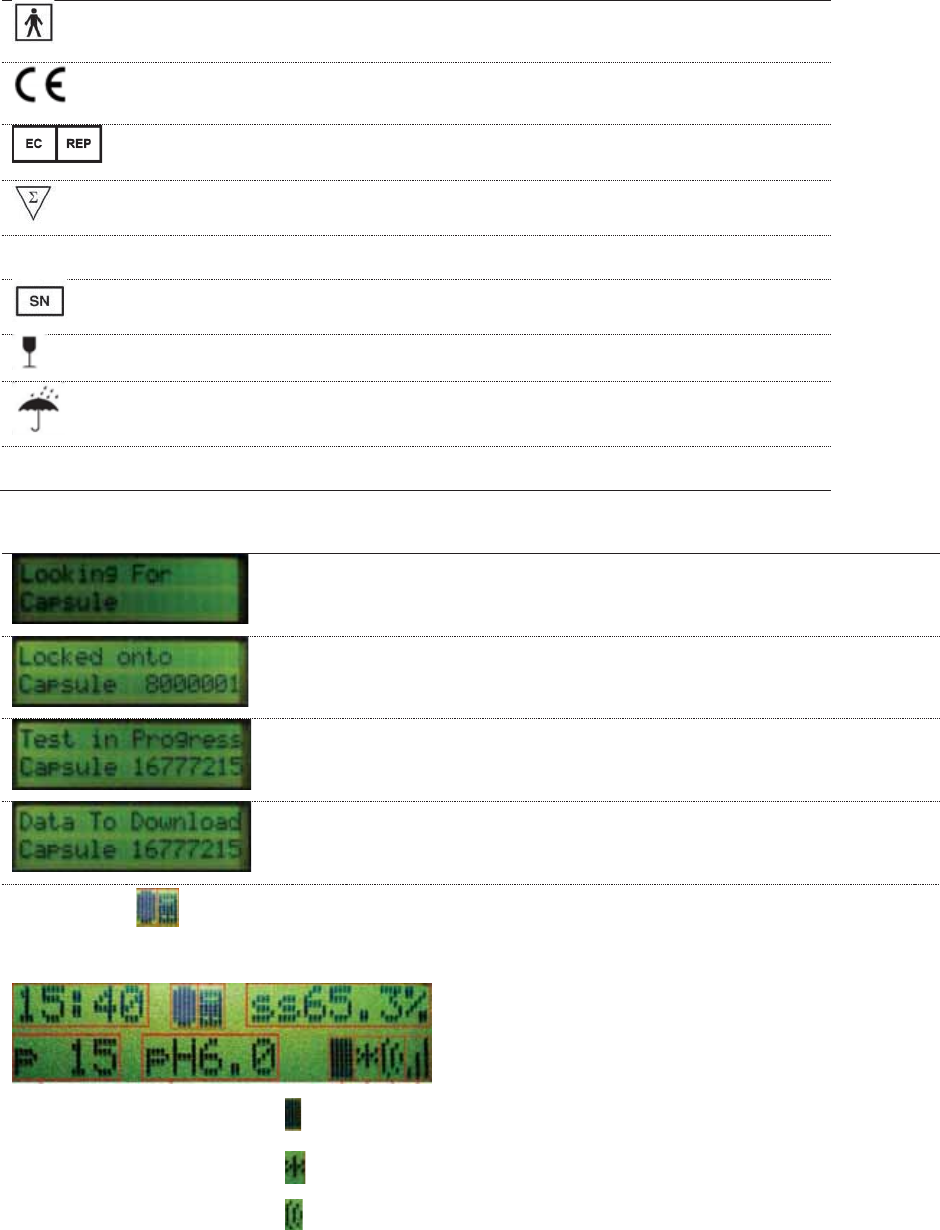
Data Receiver Display Messages
Looking for capsule—appears during test initiation before
the receiver receives
the first data packet from the capsule.
Locked onto capsule—appears after the data receiver
receives first data packet from the capsule.
Test in progress—appears when a test is in progress and
the data receiver is turned off and back on.
Data to download—appears when the data receiver stops
collecting data and has data to be downloaded.
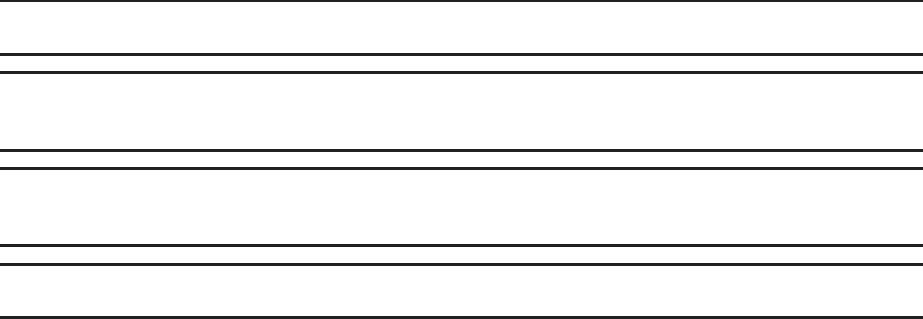
Time
Capsule and data
receiver status icons /
[X] indicates a failure
Signal Strength
Pressure
pH
Indicates the data receiver is writing data from the capsule.
Indicates the event button is pushed.
The capsule’s data was received. / [X] indicates the data was not
received.

Appendix: Electrical Safety
The receiver’s battery life.
1 bar = 1 day of battery life.
3 bars = 3+ days.
Risks and Safety
Warning
This device does not differentiate between slow motility and functional outlet obstruction.
Non-Passage
Risks associated with capsule ingestion and transit are minimal. The primary hazard is
capsule retention. Retention incidence, as determined by a review of published studies
of capsule endoscopy in adults, is estimated as 0.75% in patients without known
stenosis and 21% in patients with known stenosis. Stenosis and strictures can be
complications in inflammatory bowel disease.
If you suspect a delay in passage and the Capsule is located in the stomach, a pro-
motility drug could be administered to assist in emptying the capsule from the
stomach. Alternatively, endoscopy could be performed in order to retrieve the capsule.
If located in the colon, laxative therapy could be administered to facilitate capsule
movement, or a colonoscopy could be performed in order to retrieve the capsule.
Adverse events reported in clinical studies involving the SmartPill are listed below.
Reported Adverse Events in Clinical Study Subjects (n=484)
Number of Events Reported
59
Number not related to the device
33
Number probably not related to the
device
17
Number possibly related to the device
5
Number definitely related to the device
4
Reported Adverse Events in Clinical Practice
In clinical practice since 2007, the company identified 25 events whose circumstances
suggested a potentially reportable event to regulatory authorities. After investigation
and follow up, seven of these events were deemed reportable including three instances
of esophageal retention, one gastric retention and three small bowel retentions.
Surgery was required for resolution in one instance of capsule small bowel retention
that led to identification of a stricture. A bowel prep resolved the second instance of
small bowel retention, and the third resolved with fluids and bed rest. Capsule
retention in the stomach was resolved endoscopically. Two of the retentions in the
esophagus were resolved endoscopically and in the third instance the patient vomited
and then performed a self-applied Heimlick maneuver to expel the capsule. There
Appendix: Electrical Safety
was one additional esophageal retention, eight gastric retentions, four small bowel
retentions and five colonic retentions. These resolved either without intervention or
with endoscopy/colonoscopy.
Patient-Contacting Materials
Patient-contacting materials include polyurethane, a polyurethane-polycarbonate blend,
Teflon coated with polyhema, an ISFET pH sensor, epoxy and UV-cured adhesive.
All patient contacting materials have been tested for biocompatibility and have been
found non-toxic, non-sensitizing, and non-irritating.
The device does not contain natural rubber latex.
Care, Cleaning and Maintenance
Caution
Do not reuse capsules. The capsule is a single-use, disposable item.
Caution
Do not immerse the data receiver in water or other liquids. Immersion damages internal electrical components
resulting in data receiver inoperability.
Caution
Do not immerse the docking station in water or other liquids. Immersion damages internal electrical
components and could result in electrical shock.
Caution
The data receiver, docking station, power supply, and capsule are not user serviceable.
Data Receiver
Clean and disinfect the outside surfaces of the data receiver after each patient use.
1. Turn off data receiver by simultaneously depressing the backlight button and event
button for 5 seconds.
2. Wipe outside surfaces with a cloth dampened (not saturated) with a mild detergent
and water. Suitable detergents include dishwashing detergent (e.g., Joy, Dawn®,
Palmolive®) solutions, or laboratory glassware cleaners such as Alconox®.
3. Wipe dry.
4. Disinfect by wiping outside surfaces with a cloth dampened (not saturated) with
disinfectant. A solution of 10% household bleach can be used as a disinfectant.
5. Wipe dry.
6. Wait at least 5 minutes after cleaning and disinfection before turning the data
receiver on or placing it in the docking station.
7. Conduct a preventive maintenance inspection. Check for cracks or damage. Shake
the data receiver and listen for detached batteries. Do not use the data receiver if
the case is cracked or damaged, or a rattling can be heard.
Appendix: Electrical Safety
Caution
Do not attempt to replace batteries in the data receiver.
Docking Station
1. Unplug docking station from the power supply.
2. Dust the surfaces of the docking station with a soft, dry cloth. Do not use water or
liquids.
3. Clean the gold connector pins with a swab and Isopropyl alcohol.
4. Conduct a preventive maintenance inspection. Check for cracks or damage. Do not
use the docking station if the case is cracked or damaged.
Caution
Do not use the components of the system with any other equipment.
Troubleshooting and Support
Contact technical support if you have a problem setting up or operating the system:
For swift and direct support, please be prepared to answer the following questions:
xWhat version of MotiliGI are you using? To determine what version you are
using:
xSelect Help > About.
xRefer to the version printed on the MotiliGI CD.
xWhat operation or steps did you take before the problem occurred?
xWhat operations or steps did you take after the problem occurred?
xWhat is the exact error message that appeared?
xIf you have trouble with a capsule or other hardware component, have the
component’s lot and serial numbers ready. These numbers are on labels affixed
to the hardware component or packaging.
Appendix: Electrical Safety
6HWWLQJ-8SWKH6\VWHP
Getting Started
1. Remove the data receiver, docking station, activation fixture, USB cable, power
adapter, and power cord from the starter kit case.
2. Unpack the system computer and power supply from its shipping container.
►Connect the computer to a source of AC power.
Setting up the Computer
1. To login to the System Computer, enter the information below.
User Name
Password
sgims1
12345
2. Ensure the system computer has the correct date, time, language and regional
settings.
Changing the System Computer Date, Time, Language and Regional Options
1. Select Start > Control Panel > Clock, Region and Language
2. Change the desired settings.
►Changing certain settings may require you to reboot the system computer.
Launching MotiliGI (for the first time)
1. Open the MotiliGI software:
xClick on the desktop.
xSelect Start > All Programs > SmartPill folder > MotiliGI.
2. When launching MotiliGI for the first time, the MotiliGI License Agreement will
appear. Review and click I Agree. You must accept the terms and conditions in
order to run MotiliGI.
3. The MotiliGI Configuration appears. Enter an identification number or word (e.g.
001 or Main Office) to identify SmartPill tests run at your particular office.
xIf your institution or practice has more than one office, you may wish to use
this feature to identify at which office a specific test was conducted.

Appendix: Electrical Safety
4. Click OK. You will be asked to confirm the clinic identification number you
entered; click Yes to proceed to the First Login Sequence screen.
5. Click OK. The administrator user login screen appears.
6. Create a username and password for the administrator account for MotiliGI. The
username and password must contain a minimum of 5 alphanumeric characters.
7. Verify the password by re-entering it, and click Login. You will be required to
enter this username and password each time you start the program. The MotiliGI
Start-Up screen appears.
Connecting the Docking Station to the System Computer
1. Connect the docking station to an AC power source using the power cord.
2. Connect the docking station to the system computer using the USB cable.
3. Turn the data receiver on by depressing the event button.
4. Dock the data receiver by placing it in the docking station cradle. Push down on
the top of the data receiver to ensure the data receiver is fully docked.
5. Confirm the system computer and docking station are communicating by noting
the presence of the data receiver connected icon in the MotiliGI status bar (the
horizontal bar at the bottom of the MotiliGI user interface).
xIf you observe the receiver communication disabled and test state unknown
icons, either the docking station is not properly connected to the system
computer, or the data receiver is not fully docked.
►Reseat the data receiver in the docking station and ensure the docking station is
connected to both the computer and a power supply. The test state unknown icon will
disappear once the connection is established. If it does not disappear, contact Technical
Support for assistance.
6. Before starting a test, fully charge the Data Receiver. See Chapter 4: Charging the
Data Receiver for instructions.

Appendix: Electrical Safety
3UHSDULQJIRUD7HVW
Before the Test Day
Fully charge the data receiver the day or night before starting a test. Charging can take
up to 5 hours.
Charging the Data Receiver
1. Ensure that the data receiver is off. If any characters appear on the display, turn off
the data receiver by simultaneously pressing the 2 buttons on the front of the unit
for approximately 5 seconds.
Caution
The data receiver must be turned off during charging. Charging the data receiver while turned on results in a
partial charge. You may lose test data.
2. When the back light dims, release both buttons.
3. Plug the docking station into an electrical outlet.
►If necessary, use a power adapter.
4. Firmly push the data receiver into the docking station to initiate charging. The
docking station’s LED turns red.
Figure 3
xAllow up to 5 hours for the data receiver to fully charge.
xThe docking station’s LED turns green when the data receiver is fully charged.
Caution
Charging or recharging the data receiver with a power source other than the SmartPill power supply may
permanently damage the data receiver, docking station, or system computer and will void the product
warranty.

Appendix: Electrical Safety
Preparing the Patient
Before the Office Visit
1. Review these requirements and restrictions with the patient. The test requires
fasting for accurate results.
Schedule
Restriction
24 hours before the test
Do not consume alcohol.
8 hours before the start of the test
Do not eat or drink.
Do not use tobacco.
6 hours after the start of the test
Do not use tobacco.
Do not eat.
Do not sleep.
Do not consume alcohol.
2. Review the use of medications with the patient. Stop medications that alter motility
or gastric pH. Examples of medications are provided below.
xUnless the patient is well stabilized (condition and dose has been stable for 3
months or more), discontinue these medications prior to the start of the test.
Schedule
Type
Examples
7 days before the tests
Proton pump inhibitors
Omeprazole,
Lansoprazole, Nexium
48 Hours
Histamine
2
blockers
Zantac
48 Hours
Motility-altering
medications
Cisapride,
D
omperidone,
M
etoclopramide
(Reglan)
48 Hours
Antiemetics & 5HT3
anatagonists
Zofran, Kytril
48 Hours
Macrolides
Erythromycin,
Z
ithromycin
48 Hours
Anticholinergics
Phenergan, Compazine
48 Hours
5HT4 partial agonists
Zelnorm
24 Hours
Antacids
Maalox, Mylanta,

Appendix: Electrical Safety
Rolaids
xInsulin-dependent diabetic patients must take half of their normal morning dose
of insulin and monitor glucose levels according to normal routines.
3. Review SmartBar ingredients and rule out any food allergies.
xThe SmartBar contains a small amount of gluten.
xThe SmartBar does not contain lactose.
xThe SmartBar does not contain nuts but is manufactured in a plant that
processes nuts.
SmartBar
Ingredients
Granola (Rolled Oats, Evaporated Cane Juice, Expeller Pressed Canola Oil,
Defatted Wheat Germ, Oat Flour, Brown Rice Syrup, Molasses, Salt, Natural
Flavor, Soy Lecithin), Whey Crisp, Rice Syrup, Corn Syrup, Whey Protein Isolate,
Invert Sugar, Puffed Wheat, Apples, Maltodextrin, Sorbitol, Apple Juice
Concentrate, Partially Hydrogenated Veg
etable Oil (Cottonseed, Soybean), Honey,
Natural and Artificial Flavor, Salt, Vanilla.
Nutritional
Composition
66% carbohydrate
3% fiber
(in % of weight)
17% protein
243 kcal
2% fat
If necessary, an egg based meal can be substituted for SmartBar. You must have a
microwave to prepare this meal.
Ingredients
½ cup (120 g) Egg Beaters®,
equivalent to the volume of 2 large
egg whites; 60 kcal
2 slices of bread; 120 kcal
1.5 Tbsp. (30 g) strawberry jam; 75
kcal
½ cup (120 ml) water
Caution
To accurately measure regional gut transit times the patient must consume either SmartBar or the egg based
meal immediately before ingesting the capsule.
During the Office Visit
1. Ensure the patient has adhered to restrictions required before the test.
2. Provide printed instructions (included in starter kit) to the patient. Additional
copies can be purchased.
3. Review the schedule, restrictions, and use of medications with the patient. Inform
the patient that failure to follow these instructions may invalidate the test.
Sche
dule
Restriction
6 hours after ingesting the
capsule
xDo not eat.
xDo not take medication.
Appendix: Electrical Safety
xDo not use tobacco.
During the entire test
xDo not consume alcohol.
x
If you are diabetic, monitor glucose levels and follow your personal
treatment plan. If you are un
sure, contact the doctor who manages your
diabetes.
x
Avoid vigorous exercise such as sit-ups, abdominal crunches, and
prolonged aerobic activity (greater than 15 minutes).
x
Do not wear the data receiver while bathing or showering.
x
Do not use laxatives, bowel cathartics, anti-
diarrhea medications, alcohol
and other drugs or medications that affect motility until after the capsule
passes.
4. Instruct the patient to keep the receiver as close to the abdomen as possible except
while bathing or showering:
xPositioned on a belt
xSuspended by a lanyard
Caution
Instruct patients not to use the lanyard when sleeping
Caution
Eating anything (except limited quantities of water) before the capsule has emptied will delay gastric emptying
of the test meal and invalidate results.
5. Instruct the patient on the use of the patient diary and the data
receiver’s event button. Every time the patient presses event, the
patient must record the time displayed on the data receiver and a brief
description of the event or activity in the diary.
►Patients must press event for all bowel movements.
Pressing event:
xMarks bowel movements which are useful for verifying capsule exit.
xMarks other events and symptoms which may be useful when reviewing test
data.
xAdds an event marker to the electronic data record.
Other events and symptoms you may ask the patient to mark and enter in the
patient diary include:
eating a meal
getting up in the morning; going to bed at night
cramping or pain
nausea
passing gas
vigorous exercise or activity
Resumption of Normal Routine
6. Review these instructions and warnings with the patient.
Appendix: Electrical Safety
xPatients may resume a normal diet 6 hours after swallowing the capsule.
xExcept for restrictions noted on the Patient Instructions Sheets, patients may
resume normal activities when released from your office.
Warning
Magnetic Resonance Imaging (MRI) must not be performed on a patient who has ingested the capsule until
capsule passage is confirmed by the physician’s review of the MotiliGI graph or abdominal x-ray. An MRI test
performed with an ingested capsule may result in damage to the GI tract.
Warning
Instruct the patient to contact your office if he or she experiences acute pain, sudden nausea, or vomiting
beyond his or her typical pattern within 5 days of ingesting the capsule as these symptoms could indicate
bowel obstruction.
Scheduling a Follow-up Office Visit
7. Schedule a visit to return the data receiver 4-5 days (96-120 hours) from the start of
the test.
Appendix: Electrical Safety
6WDUWLQJD7HVW
Preparing the System
Perform these steps immediately before or after a patient’s arrival at the office.
1. Turn on the system computer.
2. Open MotiliGI:
xClick on the desktop.
xSelect Start > All Programs > SmartPill > MotiliGI.
3. If prompted, enter a username and password. Click OK. The main startup screen
appears.
Performing the Test ± Test Initiation Wizard
Step 1: Connect the Data Receiver
Caution
Do not close MotiliGI while operating the Test Initiation Wizard because data can be lost. Complete or
cancel the Wizard first.
Caution
You may cancel the wizard any time. However, a capsule exposed to the pH calibration buffer must be
ingested within 2 hours or discarded.
1. Follow the prompts. Check the results:
Component
Success
Troubleshooting
Docking station’s
LED
Green
xRed
The data receiver may not be fully charged. See Chapter 4
:
Charging the Data Receiver.
xYellow
Undock and re-dock the data receiver in the docking
station. If the yellow light persists, contact SmartPill
Technical Support.
Data receiver
display
The “Looking For
Capsule” m
essage
appears.
xLCD is blank
Turn on the data receiver by pressing the event button.
System
Computer
x2 beeps
xThese icons
appear in the
lower-right
corner of the
screen:
x
xNo beeps
xCheck the computer’s sound level.
xUndock and re-dock the data receiver in
the docking
station. If there are still no beeps, contact Technical
Support.
xThe “Receiver is Not Fully Charged” message appears.
The charge is insufficient for a 5-day test.
Appendix: Electrical Safety
x
xReplace the data receiver with a fully charged receiver.
xAllow additional charging time for the current
receiver.
The data receiver must be turned off during charging. Charging the data receiver while
turned on results in a partial charge. You may lose test data.
2. Click Next >.
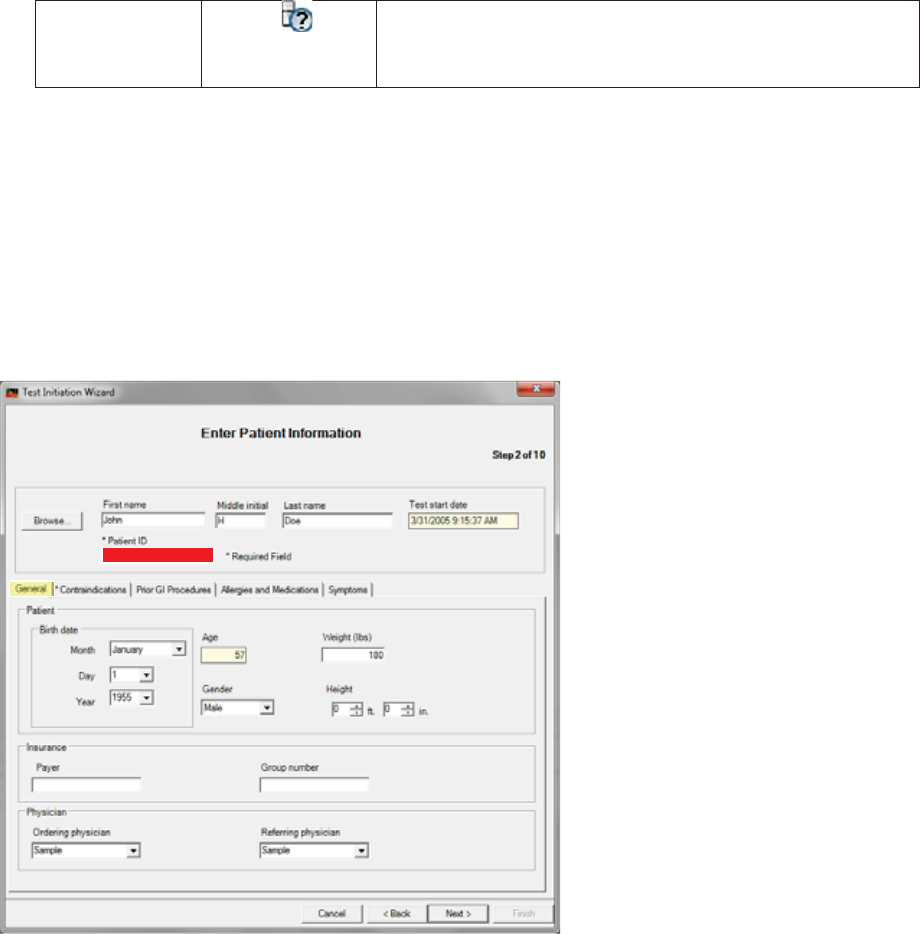
Step 2: Enter Patient Information
Patient information becomes a permanent part of the patient’s MotiliGI record.
General Tab
*indicates a required field
1. Enter the required information.
Contraindications Tab
You must view this screen.
2. Complete the applicable fields.
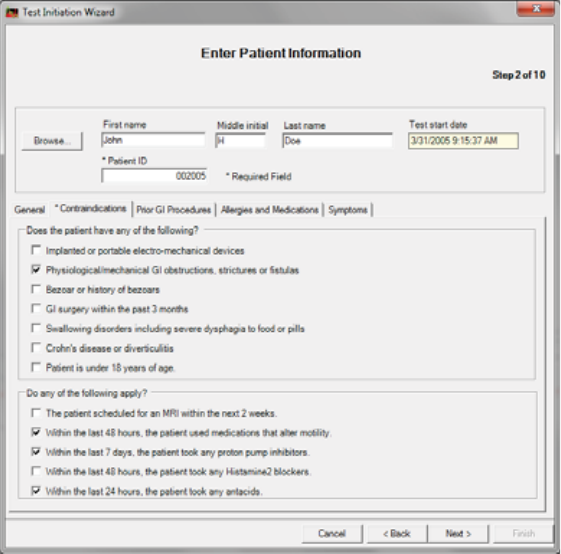
Appendix: Electrical Safety
Depending on your selections, other screens may appear. For example, an override
screen for a contraindication or caution may appear.
x(Optional) Enter notes and click Physician Ok.
x(Optional) Click End Test.
3. Proceed to the optional tabs below or click Next >.
Optional Tabs: Prior GI Procedures, Allergies and Medications, Symptoms
Entering information in these tabs is optional. The symptoms tab includes a selection
of ICD9 and ICD10 codes.
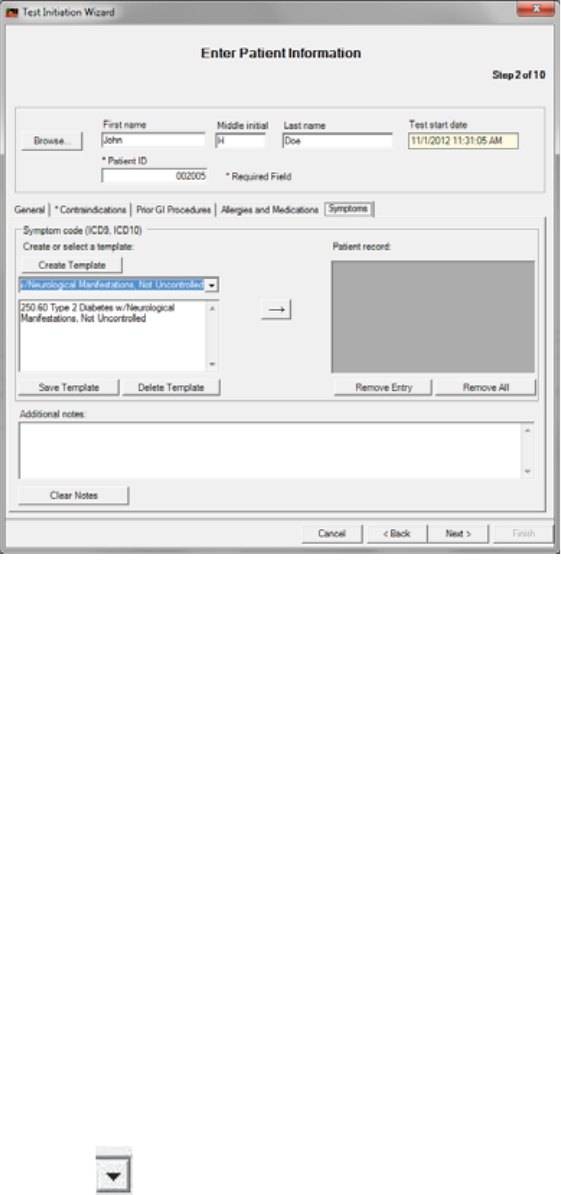
Appendix: Electrical Safety
Creating Templates:
To save time you can create text descriptions that are saved in MotiliGI. These text
templates can be reused from patient to patient. Text descriptions saved this way are
called templates. We recommend creating general templates with blank lines to
indicate where patient specific information can be inserted.
1. Click Create Template.
a. Enter a title for the prior abdominal surgery or implanted device. Examples:
xAppendectomy
xGastric stimulator
b. (Optional) Click in the text field under the title. Enter descriptive details. Examples:
xDate:______________
xName of the surgeon:___________________
2. Click Save Template.
Using Templates
1. Click for saved templates.
2. Select a template. Insert any patient specific information.
3. Click → to move information to the patient record.
►(Optional) To remove information from the patient’s record, select it and click Remove Entry.
Appendix: Electrical Safety
The templates remains in the Template list. To delete the selection, click Delete
Template.
Step 3: Assemble Materials
1. Follow the prompts.
xThe capsule’s instructions for use are in the inside pocket of the capsule pack.
2. Click Next >.

Appendix: Electrical Safety
Step 4: Activate Capsule
1. Follow the prompts.
2.
Figure 4 - Capsule Activation Window
Caution
Once the capsule is activated, keep the capsule at least one (1) foot away from the activation fixture. If the
capsule is kept near the activation fixture, stray magnetic fields from the activation fixture may deactivate the
capsule.
3. Confirm the capsule has activated:
xObserve the data receiver’s display for at least 1 minute. The message “Locked
onto Capsule” and the capsule’s serial number will appear on the data receiver
for approximately 4 seconds.
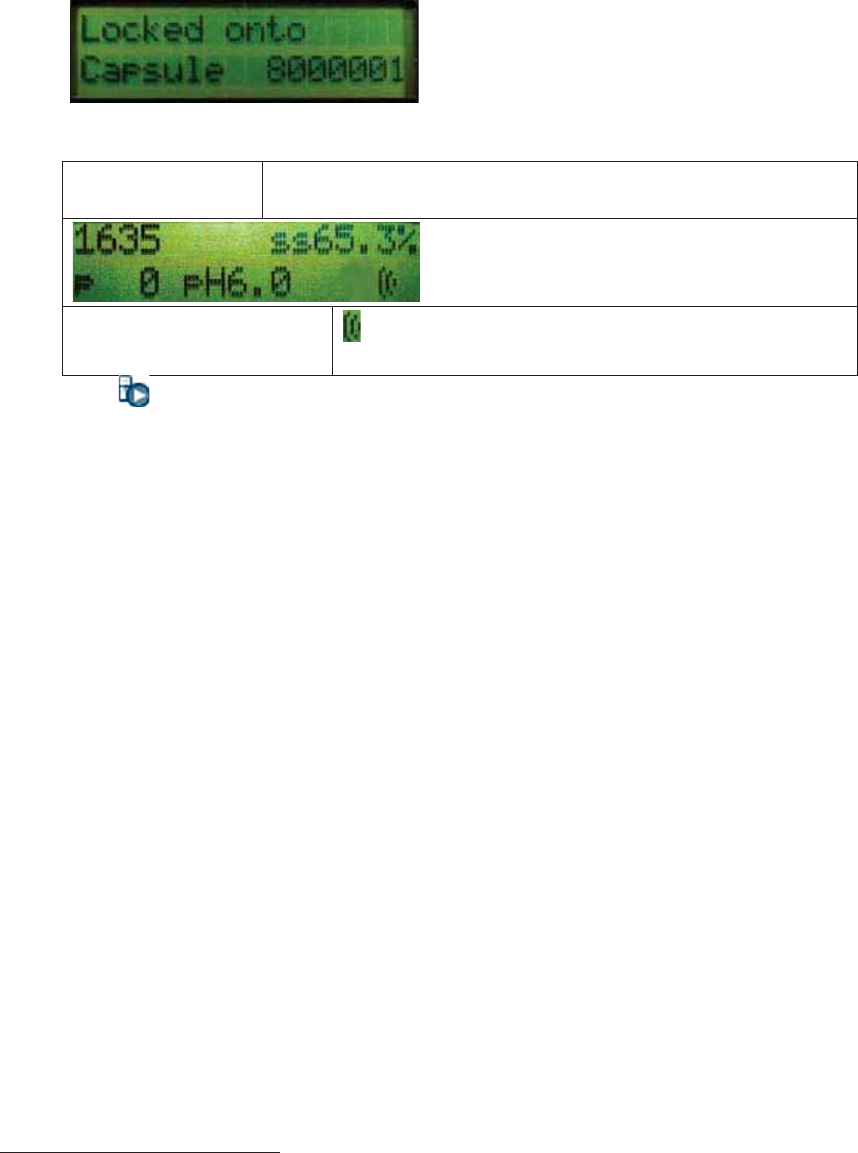
Appendix: Electrical Safety
xThe data receiver’s display then indicates the capsule’s data:
Capsule Serial
number
SS = signal strength
P = pressure
The capsule’s data was received. / [
X] = The data was not
received.
xThe icon appears in the lower-right corner of MotiliGI: .
►If the capsule did not activate, repeat the procedure.
4. Click Next >.
5. If you wish to deactivate the test, see Aborting a Test – Deactivating the Capsule,
Page 44.
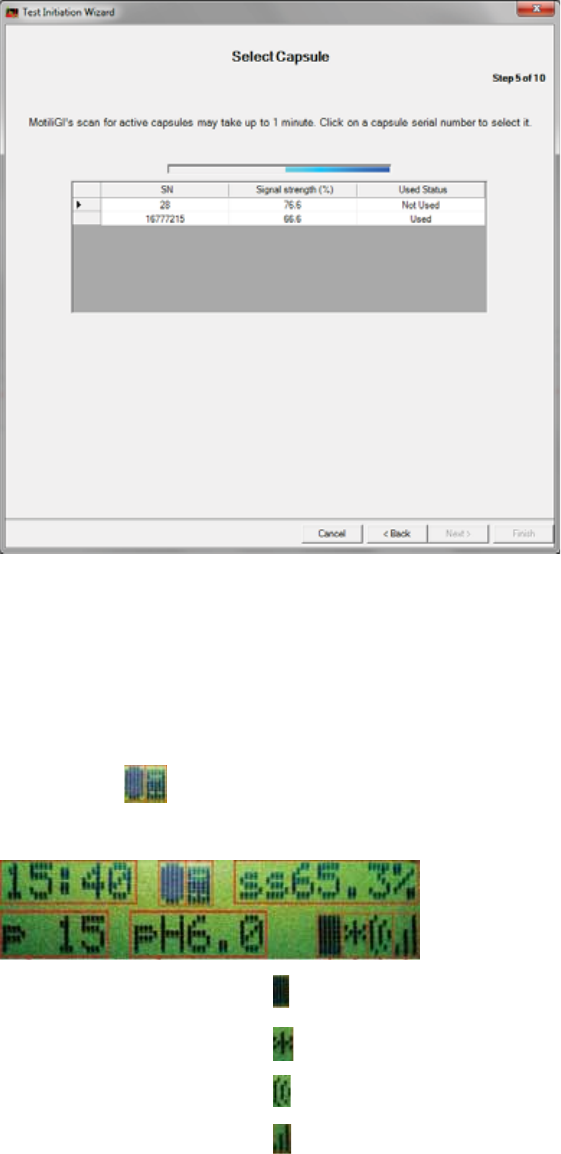
Step 5: Select Capsule
All activated capsules (ingested or not yet ingested) within range of the data receiver
appear1. This process may take up to 1 minute.
1 The data receiver can detect the capsule in open air (not ingested) at distances of up to 40 feet.
Appendix: Electrical Safety
1. To select the capsule you activated, click the row that matches the capsule
container’s serial number. An arrow indicates your selection.
xIf the software does not detect the capsule within a minute, observe the data
receiver’s display to confirm the capsule is active.
xIf the capsule is activated the data receiver displays this data during a test.
Time
Capsule and data
receiver status icons /
[X] indicates a failure
Signal Strength
Pressure
pH
Indicates the data receiver is writing data from the capsule.
Indicates the event button is pushed.
The capsule’s data was received. / [X] = The data was not received.
The receiver’s battery life.
1 bar = 1 day of battery life.
3 bars = 3+ days.
2. Click Next >.
Appendix: Electrical Safety
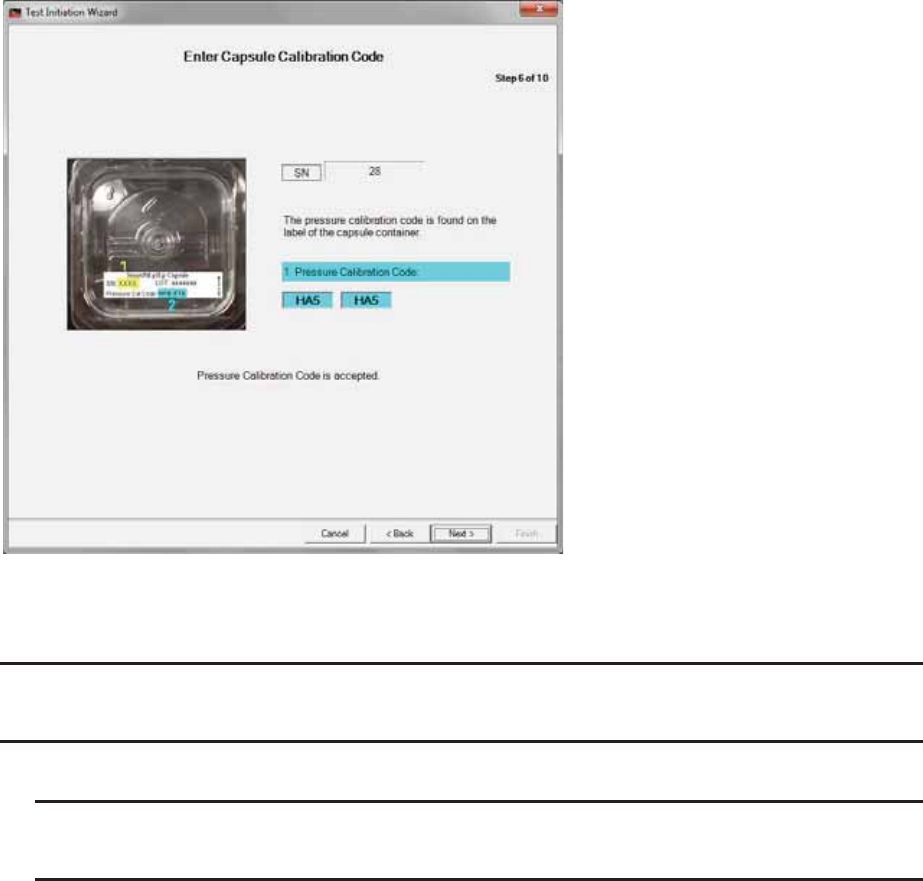
Step 6: Enter Pressure Calibration Code
1. Locate the calibration code found on the lid of the capsule container. Enter the
code.
2. Click Next >.
Step 7: Add pH Calibration Buffer
Caution
Once exposed to buffer, the capsule must be ingested within 2 hours or discarded. Confirm that the patient is
available and prepared to proceed with the test before you begin Step 7.
1. Put gloves on and follow the prompts.
Caution
Wear gloves when handling the capsule. Do not touch the calibration buffer or capsule with your finger as
this could cause an electrostatic charge.
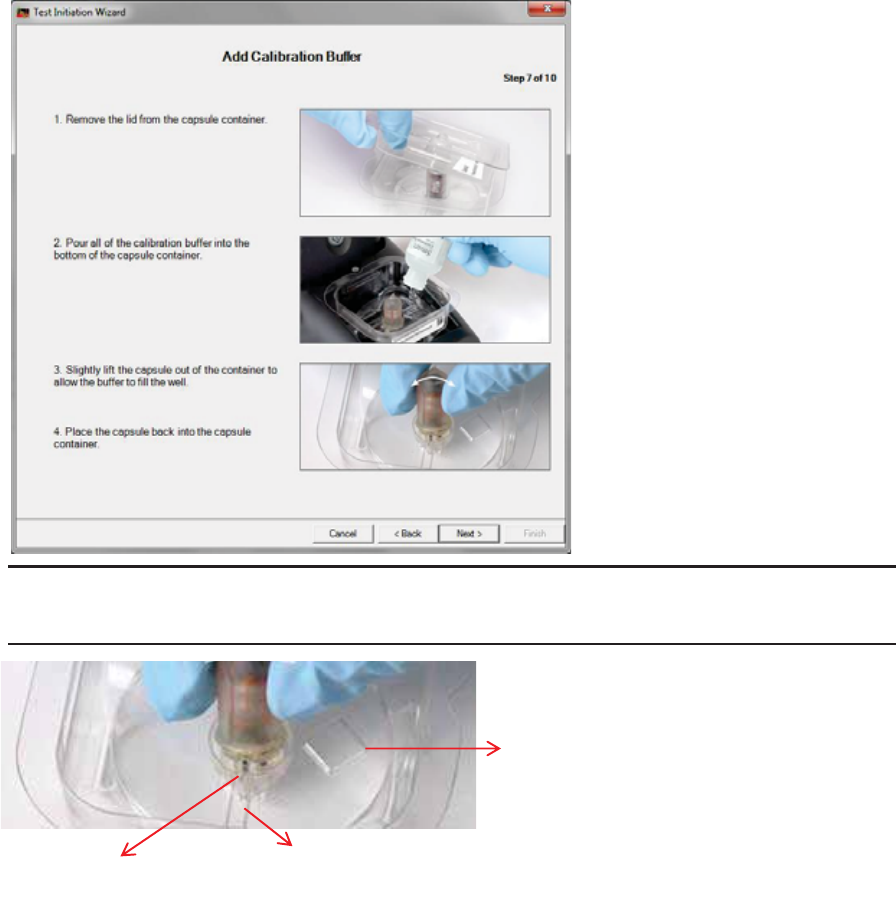
Appendix: Electrical Safety
Caution
The orientation of the capsule in the capsule container is important. When reinserting the capsule, ensure
the pH sensor is in the same orientation relative to the diamond-shaped window of the capsule container.
Figure 5 - Capsule Orientation
A
– Capsule pH sensor
B
– Buffer filling channel
C
– Diamond shaped window
Do not discard the capsule container’s cover. The cover contains information that is
needed if the test cannot take place as planned or assistance from Technical Support is
required.
2. Click Next >.
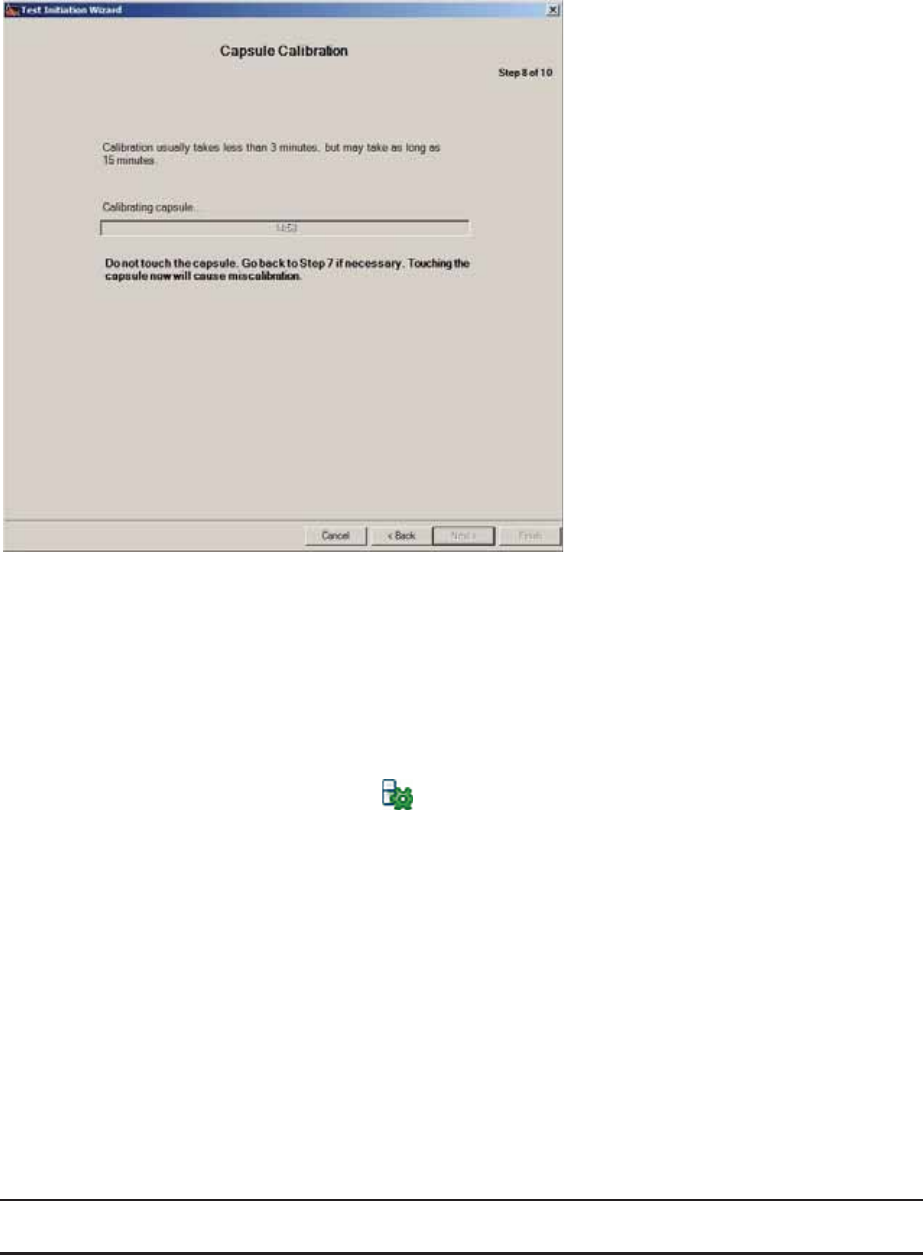
Step 8: Capsule pH Calibration
No action is necessary. The software checks the status of the capsule and data receiver
during this step.
A
B
C
Appendix: Electrical Safety
xCalibration normally takes less than 3 minutes, but may take up to 15 minutes.
xDuring calibration, it is normal for fluctuations in the pH value to occur.
Calibration is complete when all these signals appear:
xThe pH value stabilizes at 6.0.
xThe “pH Calibration Complete” message appears.
xThe MotiliGI status bar icon indicates the capsule is ready for use and the
data receiver is recording information from the capsule.
If the capsule does not calibrate within 15 minutes, this message appears: “pH
Calibration Timeout.”
1. Ensure the capsule is immersed in buffer.
2. Click Back.
3. Click Next. The calibration process reinitiates.
xIf the calibration fails after 2 attempts contact Technical Support.
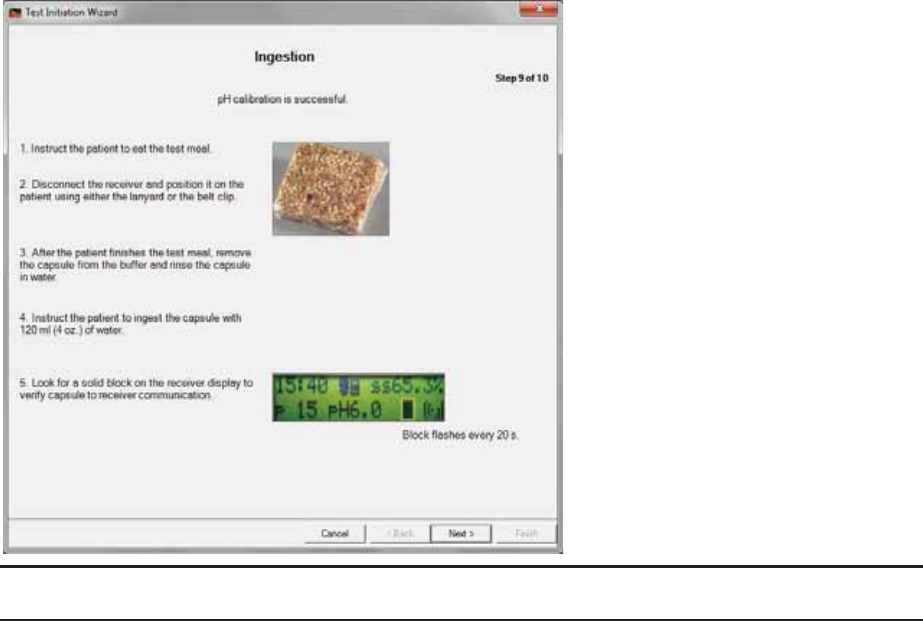
Step 9: Ingestion
1. Follow the prompts. Record the time of completion in the patient diary.
Caution
The meal must be eaten immediately before capsule ingestion.
Appendix: Electrical Safety
Caution
To avoid loss of data during Step 9.2, avoid separating the capsule and data receiver by more than 5 feet.
The data receiver records biomedical data from within the patient’s GI tract. Every
few seconds, the pH and pressure readings update on the data receiver’s display.
►Dispose of the pH calibration buffer following all applicable regulations. The buffer is a solution
of sodium citrate pH 6.0.
2. Click Next >.
Appendix: Electrical Safety
Step 10: Complete Discharge Checklist
1. Review the discharge checklist with the patient. Instruct the patient to wait 3
minutes in the bathroom before flushing the toilet after each bowel movement.
Explain that to confirm body exit, the patient must let the capsule communicate
with the data receiver after bowel movements.
2. Review these warnings:
Warning
Magnetic resonance imaging (MRI) must not be performed on a patient who has ingested the capsule until
capsule passage is confirmed by physician review of the MotiliGI graph or abdominal x-ray. An MRI test
performed with an ingested capsule may result in damage to the GI tract.
Warning
Instruct the patient to contact your office if he or she experiences acute pain, sudden nausea, or vomiting
beyond his or her typical pattern within 5 days of ingesting the capsule as these symptoms could indicate
bowel obstruction.
Warning
If you suspect bowel obstruction, treat consistent with your management of a foreign object causing
obstruction. Consider an abdominal x-ray to determine if the capsule is retained and its location within the
GI tract.
3. Schedule an appointment for the patient to return the data receiver.
The capsule typically passes naturally within 2–5 days after ingestion depending
upon the patient’s condition.
Appendix: Electrical Safety
Guidelines:
Indication for Use
Return Data Receiver After
Measuring GET
24-48 hours
Measuring WGTT
2–5 days
4. Check the box I have completed the patient’s discharge.
5. Click Finish. The Patient Information screen appears.
6. Do any of the following:
xEnter notes regarding the patient’s capsule ingestion.
xClose MotiliGI.
xUse Live Monitoring mode to view live data while the patient is still in the
office.
Live Monitoring Mode (optional)
You may view live data from the capsule while the patient is still in the office.
Prerequisites:
xThe test has begun.
xThe docking station is connected to the system computer.
xThe data receiver is in the docking station.
1. Click or select Action > Live Monitoring. The Live Monitoring control panels
appears on the right.
It may take several minutes for sufficient data to be captured before the MotiliGI
graph begins to show a noticeable progression of pressure, pH, and temperature data
values.
Caution
The patient must remain in close proximity of the docked data receiver during live monitoring. If the patient
moves away from the data receiver, test data may be permanently lost.
xYou may remove the data receiver from the docking station at any time during
live monitoring without data loss if the data receiver is kept near the patient
during the transition from being docked to being worn by the patient. When
you remove the data receiver from the docking station, live monitoring stops.
xThe MotiliGI software cannot save test data displayed during live monitoring,
but the data are stored on the data receiver and are downloaded as part of the
completed test.
2. To exit live monitoring, click Stop on the live monitoring control panel.

Appendix: Electrical Safety
Aborting a Test ± Deactivating the Capsule
Caution
If a test is aborted before the capsule has been exposed to buffer solution, the capsule may be deactivated but
must be used within two (2) hours or discarded.
Caution
If a test is aborted before ingestion and the capsule has not been exposed to buffer solution, the capsule may
be deactivated but must be used within one month or before its expiration date is reached, whichever is
sooner. Otherwise discard the capsule (refer to Recycling and Disposal Instructions, page 18).
1. Place the capsule’s container onto the activation fixture.
2. Align the diamond-shaped window on the capsule’s container with the Off mark
on the activation fixture.
3. Leave the capsule container in place for 5 seconds and then lift straight up and off
the activation fixture.
4. Confirm the capsule is deactivated:
xPlace the capsule container in the docking station’s well and observe the data
receiver’s display. The capsule is off if this icon (lower right) is replaced with
an X. The X may take 1-2 minutes to appear.

Appendix: Electrical Safety
(QGLQJD7HVW
To determine capsule exit, you can observe the data display on the data receiver for at
least 2 minutes. If the data receiver is receiving data from the capsule, this icon
appears every 20–40 seconds, which confirms that the capsule is still in the patient. No
evidence of the icon within the 2-minute observation is presumptive evidence of
capsule exit. Actual passage can only be confirmed clinically by examination of the
graph or physical examination.
Returning the Data Receiver
Following the test, the patient returns the data receiver and patient diary to your office.
A nurse or medical technician can perform these steps.
1. Collect the data receiver.
2. Ask the patient about his or her experience with the SmartPill test. Use MotiliGI’s
Post-Test Notes screen for recording information:
xDid the patient experience any unusual cramping, pain or discomfort?
xAt any time during the test was the patient separated from the data receiver?
xDid the patient observe the capsule in their stool confirming body exit?
3. Collect the patient diary.
►Review the diary’s contents to ensure the patient’s notes are legible and comprehensible.
4. Release the patient.
Downloading a Test
1. Turn on the data receiver.
2. Connect the docking station to a source of AC power and to the system
computer’s USB port.
►Check the data receiver’s battery power. If battery power bars are not visible in the lower-
right corner of the data receiver, turn the data receiver off and charge it for 10 minutes before
downloading.
3. Dock the data receiver in the docking station.
4. Launch the MotiliGI software and log in.
xThe software detects when the data receiver contains test data. A message asks
whether you want to open the patient’s information.
5. Click Yes. The patient’s file opens. The file name appears in MotiliGI’s title bar.
6. Download the data:
Appendix: Electrical Safety
xSelect Action > Download Data.
xClick the toolbar icon.
The Patient Information screen opens.
Post Test Notes
7. In the Procedure Notes tab, click in the post-test notes field to enter notes elicited
from the patient which were captured when the patient returned the data receiver.
xSee Adding and Creating a Template.
8. Click OK. (If you click Cancel, the data receiver continues to record capsule data
and events.)
The Test Download screen appears.
Duration of test
Duration of download (typical)
2 days
<3 minutes
4 days
<5 minutes
MotiliGI protects current test data stored on the data receiver until current test data
is completely downloaded.
When the download is complete the MotiliGI software displays the entire test
graph and the Test Summary screen.
9. Turn the data receiver off and place in the docking station to be recharged.

Appendix: Electrical Safety
Confirming Capsule Exit
Caution
Physicians should confirm capsule exit. Monitor patients until capsule passage is confirmed.
1. Use one of these methods:
xAsk the patient whether the capsule was observed in his or her stool.
xDownload the test. Analyze the MotiliGI graph for evidence of exit: an abrupt
drop in temperature or loss of signal that coincides with a diary entry for a
bowel movement.
xIf you cannot confirm capsule exit cannot using these methods, or suspect a
bowl obstruction, consider an abdominal x-ray and treat consistent with your
management of a foreign object causing obstruction. An abdominal x-ray
determines whether the capsule is retained and its location within the GI tract.
If you suspect a delay in passage and the capsule is in the stomach, consider:
xA pro-motility drug to help empty the capsule from the stomach.
xEndoscopy to retrieve the capsule.
If the capsule is in the colon, consider:
xLaxative therapy to facilitate capsule movement.
xColonoscopy to retrieve the capsule.
Appendix: Electrical Safety
$QDO\]LQJWKH7HVW
The MotiliGI software identifies capsule ingestion, gastric emptying, ICJ, body exit,
and computes GET, SBTT, CTT, SLBTT, WGTT, and motility indices.
You can:
xMatch events with patient diary entries.
xAnalyze test to mark capsule ingestion, gastric emptying, ICJ and body exit.
xCompare the markers you defined with markers identified by the MotiliGI
software.
Introduction to the Test Analysis Wizard
After downloading a test, a message appears asking if you want to analyze the test.
1. Click Yes. The Test Analysis Wizard appears.
You may close the wizard at any time. If you close the wizard before completing
the analysis:
xThe Test Summary screen does not appear.
xThe test report will contain the note “Test data not reviewed by physician.”
xThe test wizard automatically saves.
To re-open the wizard:
xClick the toolbar icon.
xSelect Action > Analyze Test.
The test analysis wizard resumes from your last completed step.

Appendix: Electrical Safety
Figure 6 - Test Analysis Wizard
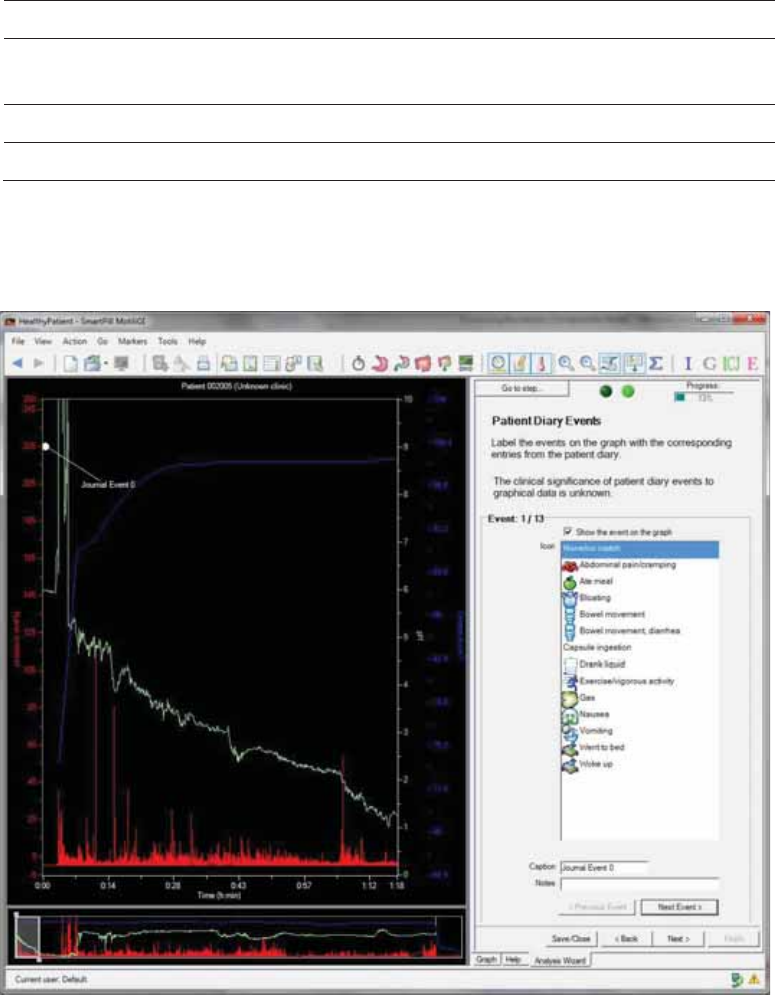
Appendix: Electrical Safety
1
Navigates to any step of the wizard that you have completed
2
Yellow light: You must take action to complete this step.
Green light: You have completed this step.
3
To take an action, click a link.
4
You can enter notes.
Analyzing the Test
Patient Diary Events
The graph displays each event in succession.
1. Match each journal event with a recorded event, based on time, from the diary.
a. Select an event icon, displayed in this step.
b. (Optional ) Enter a caption and notes.
c. Click Next Event to navigate through all events in the graph.
xTo go back to an event click Previous Event.
xIf you cannot find a matching event in the diary:
xSelect None/No Match from the list.
Appendix: Electrical Safety
xHide the event by uncheck the box Show the Event on the Graph.
You can edit this information at any time by using the Point Annotation Viewer or the
Annotation Viewer:
2. Click Next >.
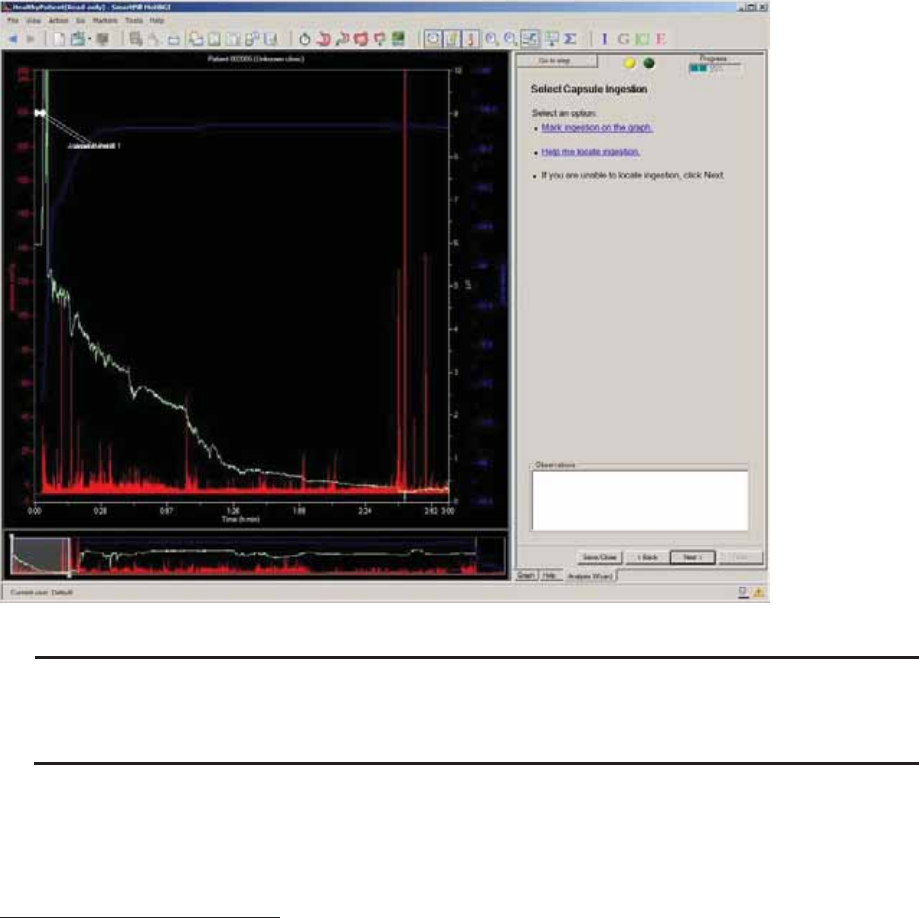
Select Capsule Ingestion
Term
Definition
Ingestion
The point where you observe the temperature beginning to rise to body
temperature.
Often, a pH spike to an off-scale value then a decrease to physiologic pH occurs just
before ingestion due to exposure of the capsule to air.2
1. Click Mark ingestion on the graph
2 Fackler, WK, et. al. Ambulatory gastric pH monitoring: proper probe placement and normal values. Aliment Pharmacol
Ther 2001; 15:1155-1162
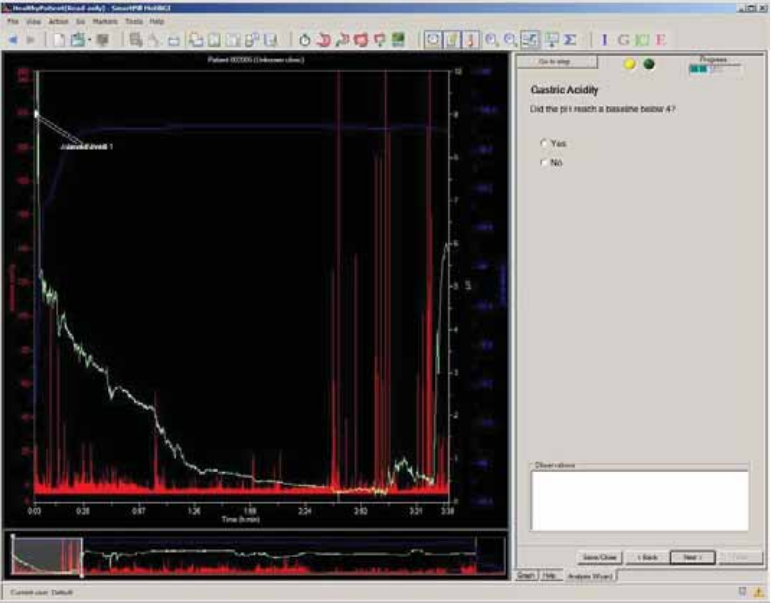
Appendix: Electrical Safety
2. Move the cursor over the graph and click on the location where you believe
ingestion occurred.
3. Click Next >.
Gastric Acidity
A stomach baseline pH below 4 indicates normal acidic conditions. Baselines above 4
may indicate ineffective gastric acid production, buffering due to meals, or medications
that suppress gastric acid.
►Click Next >.
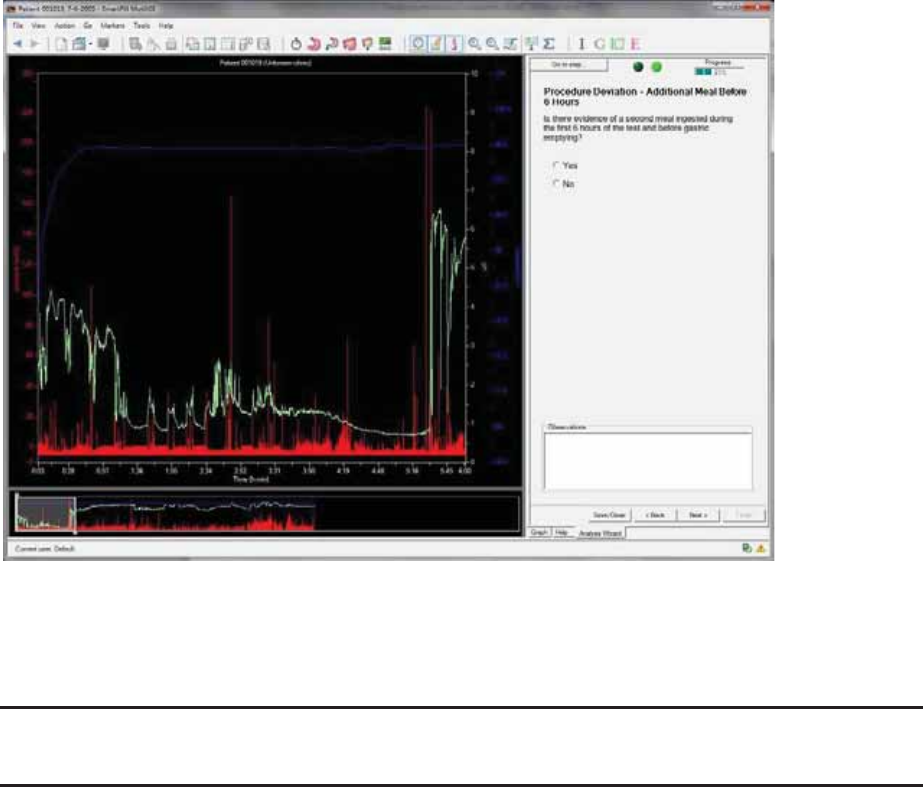
Appendix: Electrical Safety
Procedure Deviation ± Additional Meal Before 6 Hours
Snacks or meals may be recorded in the diary. A rise in pH and drop in temperature
may indicate a meal response in the stomach. In figure above there is no evidence of
an additional meal prior to capsule gastric emptying. See the figure below describing an
ingested liquid meal
Caution
Eating anything (except limited quantities of water) before the capsule has emptied the stomach will delay
gastric emptying and invalidate GET results.
►Click Next >.
Food and liquid intake cause a rise in pH which can be mistaken as gastric emptying.
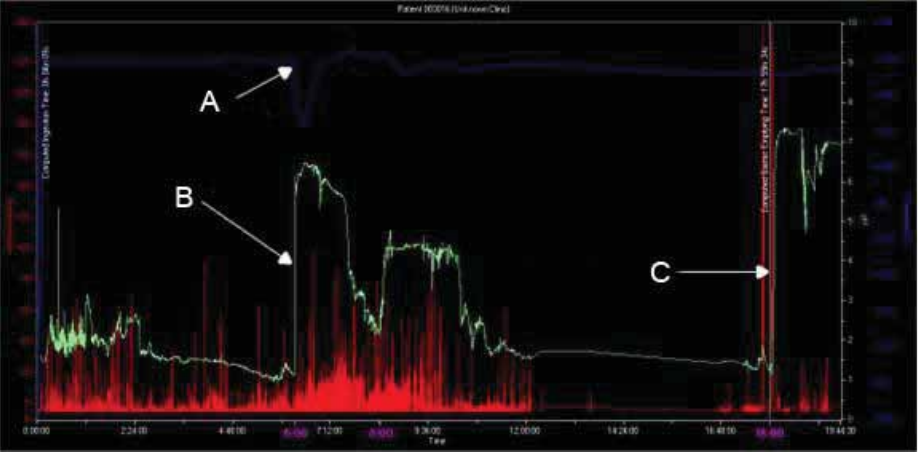
Appendix: Electrical Safety
Figure 7 - Eating a meal
In the figure above, the patient ingested a liquid meal (Ensure) at 6 hours after capsule
ingestion and ate dinner approximately 8 hours after capsule ingestion. The ingestion
of food (Ensure and dinner) caused an abrupt rise in pH (B) and a decrease in
temperature (A).
At approximately 12 hours after capsule ingestion, the pH returned to baseline (below
pH 2) and remained at baseline until the capsule emptied the stomach 18 hours after
ingestion (C).
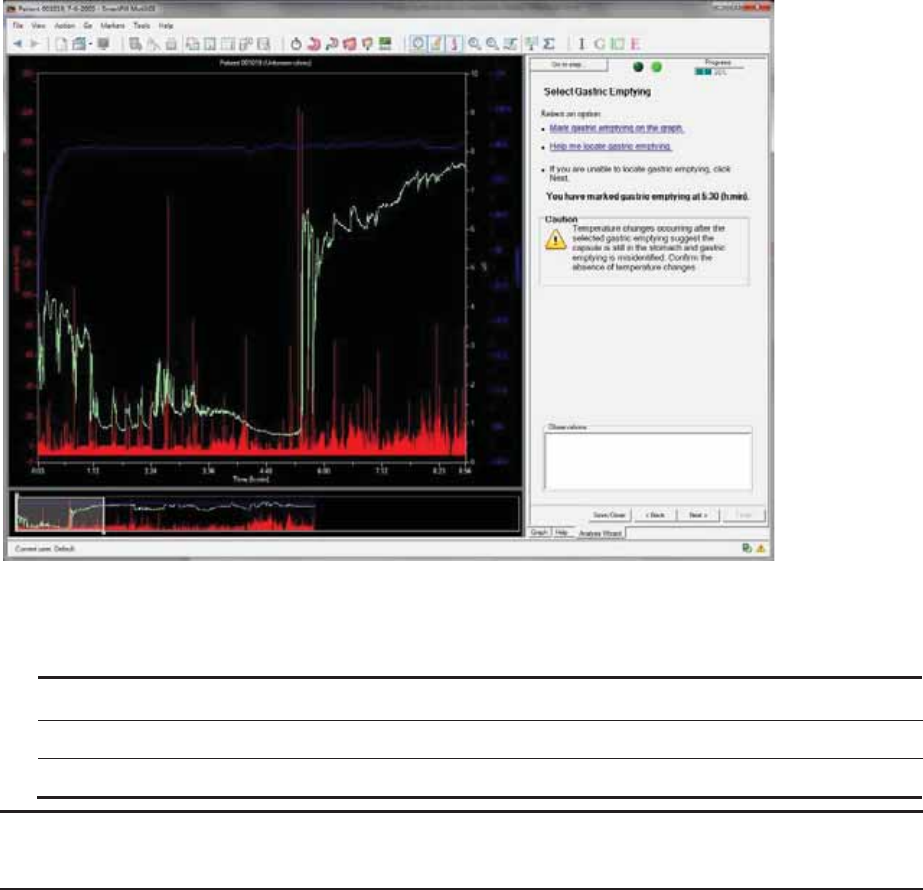
Appendix: Electrical Safety
Select Gastric Emptying
Use GET to evaluate the patient’s gastric emptying function. Generally, an abrupt
increase of 3 or more pH units occurs as the capsule exits the stomach and enters the
duodenum.
Term
Definition
Gastric emptying
The beginning of the first increase in pH that results in a sustained pH rise.
Sustained pH increase
No transient pH drops occur for greater than 10 minutes.
Caution
Medications, therapies, and diseases may alter gastric or small bowel pH. Alterations in gastric and/or small
bowel pH may mask the physiological pH landmark for gastric emptying time.
1. Click Mark gastric emptying on the graph.
2. Move cursor over to the MotiliGI graph and click on the graph where you believe
gastric emptying occurred.
3. Click Next >.
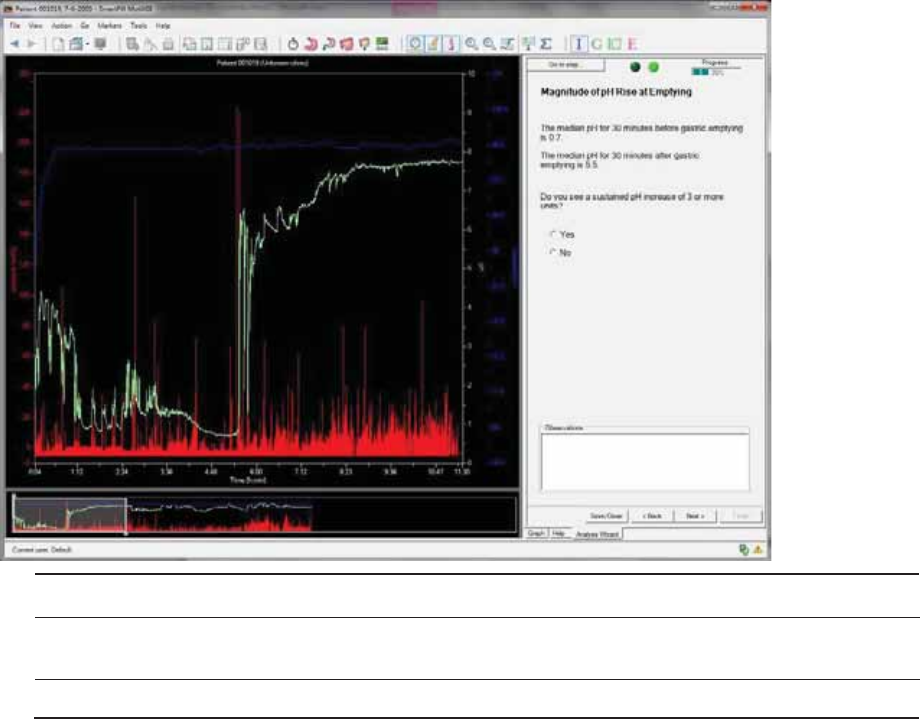
Appendix: Electrical Safety
Magnitude of pH Rise at Emptying
Term
Definition
Duodenal pH baseline
Determined by observing the pH within the first 30 minutes after
gastric emptying.
Sustained pH increase
No transient pH drops occur for greater than 10 minutes.
►Click Next >.
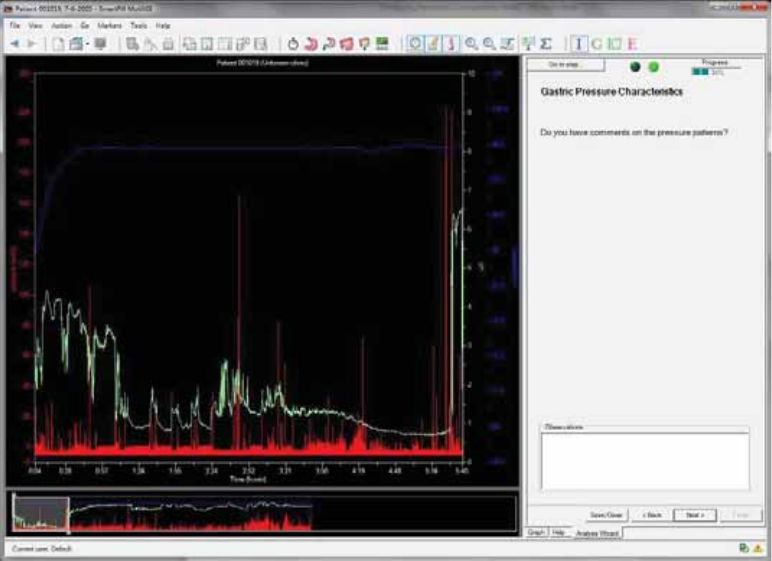
Appendix: Electrical Safety
Gastric Pressure Characteristics
The relevance of SmartPill pressure patterns in the stomach to pressure patterns
obtained using manometry is unknown.
►Click Next >.
Small Bowel pH profile
1. Determine whether you observe a gradually rising pH profile characteristic of the
small bowel.
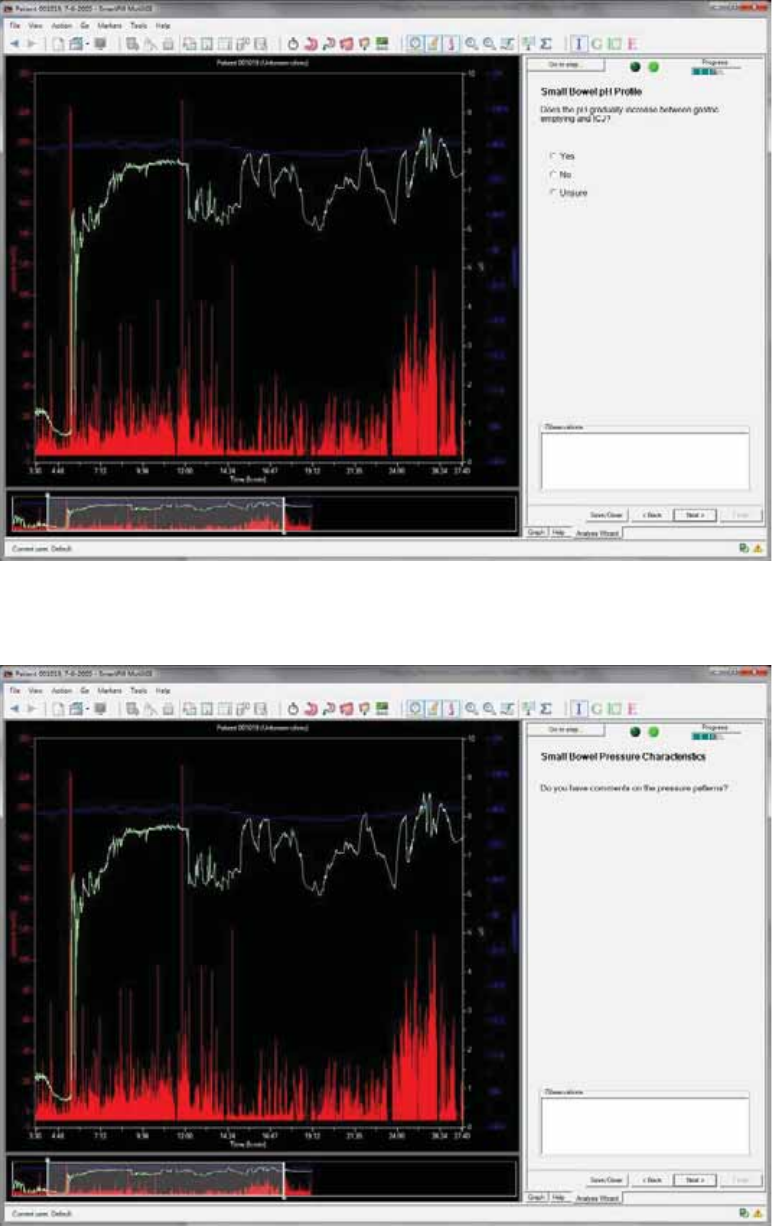
Appendix: Electrical Safety
2. Click Next >.
Small Bowel Pressure Characteristics
The clinical significance of SmartPill pressure patterns in the small bowel is unknown.
►Click Next >.
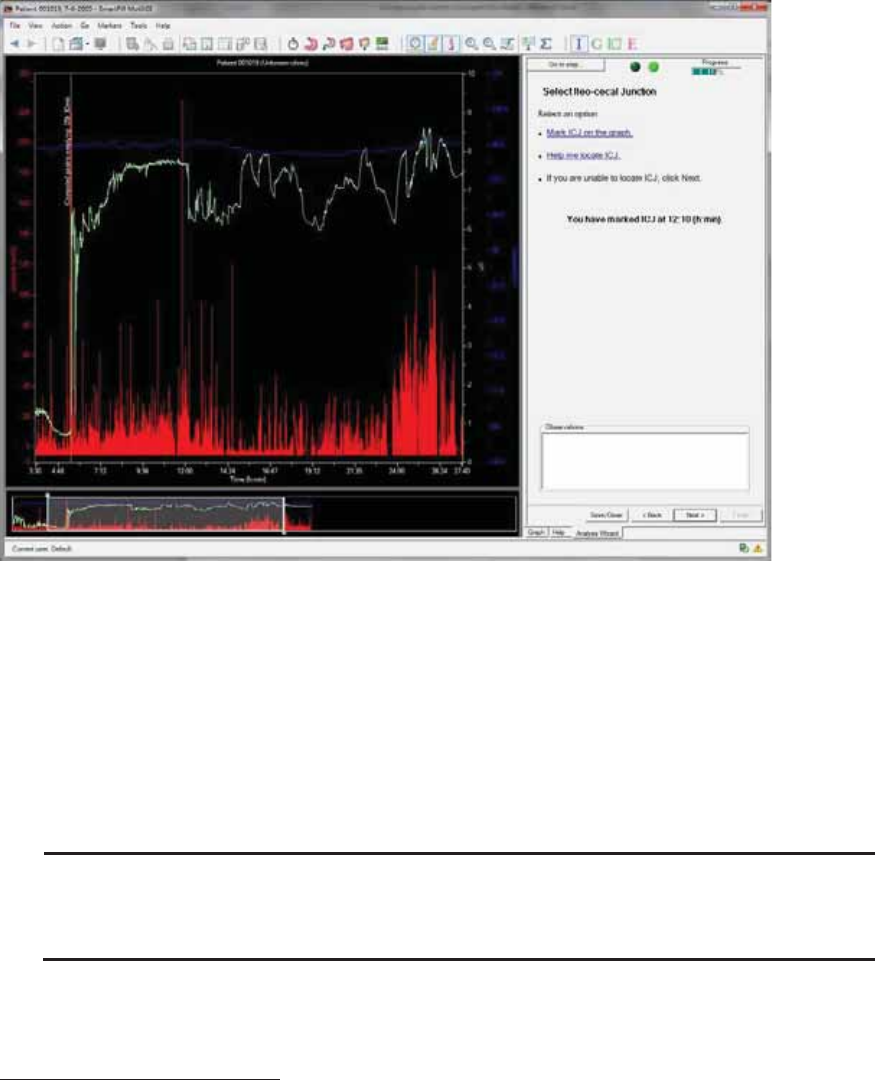
Appendix: Electrical Safety
Select Ileo-Cecal Junction
The standard small bowel pH profile shows a gradual pH rise usually followed by a
plateau of the pH pattern. Transient pH drops may interrupt this profile but returns to
their previous level within 10 minutes.
The pH drop associated with the capsule passing through the ICJ occurs after the
gradual sustained increase in pH through the small bowel. This drop of approximately
0.5–1.0 pH units usually occurs between 2–6 hours after gastric emptying. 3
Before ICJ, the pH rises smoothly and continuously in comparison to a more erratic
profile after ICJ.
Term
Definition
ICJ
Ileo-Cecal Junction, the start of pH decrease of approximately 0.5–1.0
units.
►Click Next >.
3 Zarate N., Scott SM. Accurate localisation of a fall in pH within the ileo-caecal region: validation using a dual
scintigraphic technique. Am J Physiol Gastrointest Liver Physiol 2010; 299 (6): G1276-86.
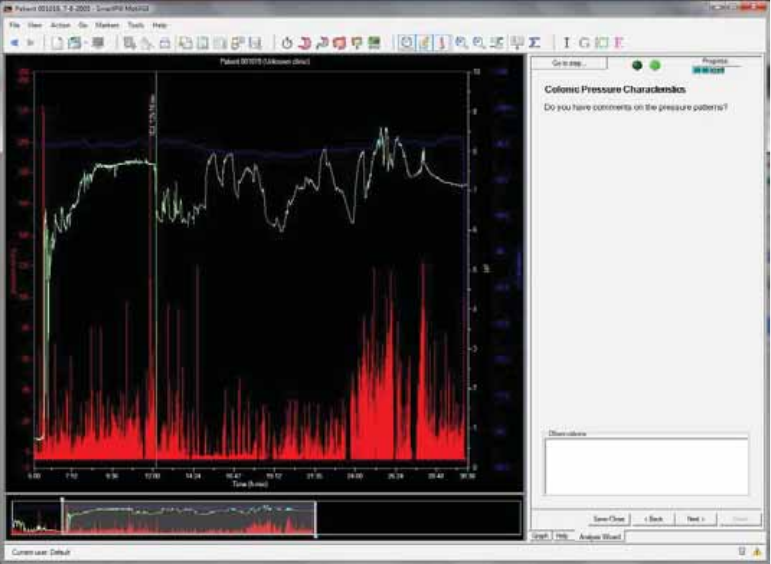
Appendix: Electrical Safety
Colonic Pressure Characteristics
The relevance of SmartPill pressure patterns in the colon to colonic pressure patterns
obtained using manometry is unknown.
►Click Next >.

Appendix: Electrical Safety
Warning: Data Collected During Capsule Low Voltage
If the MotiliGI software detects a low voltage condition in the capsule, a warning
appears.
Caution
When a low voltage warning is present, body exit confirmation cannot be determined solely by temperature
drop. Low voltage may cause loss of signal before capsule exits the body. Whole gut and small/large bowel
transit times are not definitive without the body exit endpoint. Regional transit times are not valid without
start and endpoints.
1. Read and understand the warning.
2. To acknowledge this warning, check the box I Understand.
3. Click Next >.
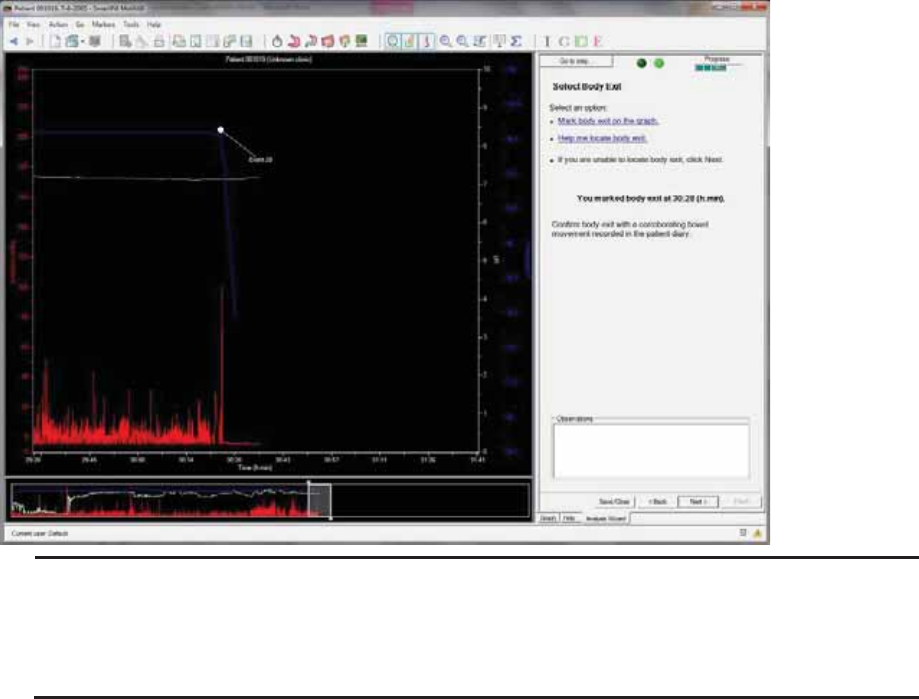
Appendix: Electrical Safety
Select Body Exit
Term
Definition
Body exit
xThe time of the last temperature reading at body temperature, coinciding
with an abrupt loss of capsule data.
xThe time of the last abrupt rise in pressure.
An abrupt loss of signal from the capsule does not reliably indicate body exit. You
must corroborate (to within 30 minutes) body exit with a recorded bowel movement in
the patient diary.
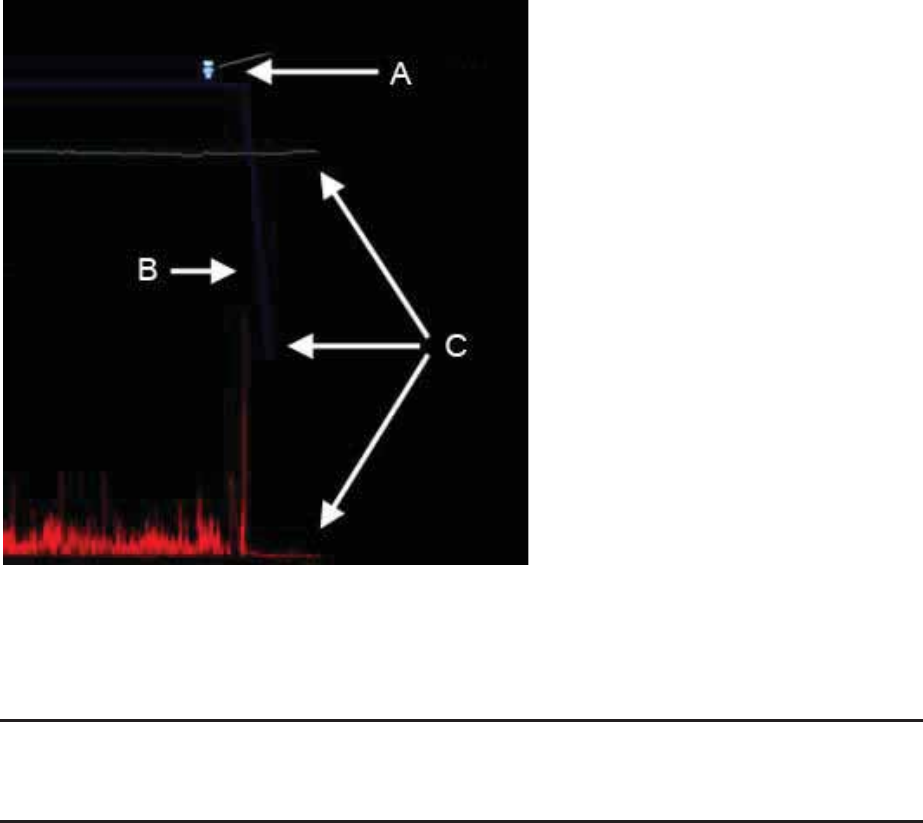
Appendix: Electrical Safety
Figure 8 - Body Exit
The figure above provides an optimal representation of body exit including a bowel
movement event marker on the graph and in the patient diary temperature drop (A), a
decrease in temperature (B) and an abrupt loss of data (C).
Caution
If the diary does not contain a bowel movement entry concomitant with a temperature drop and/or loss of
pressure and pH signal, the patient should be monitored for symptoms of bowel obstruction. If the patient
exhibits symptoms consistent with bowel obstruction, a KUB exam is indicated.
►Click Next >.
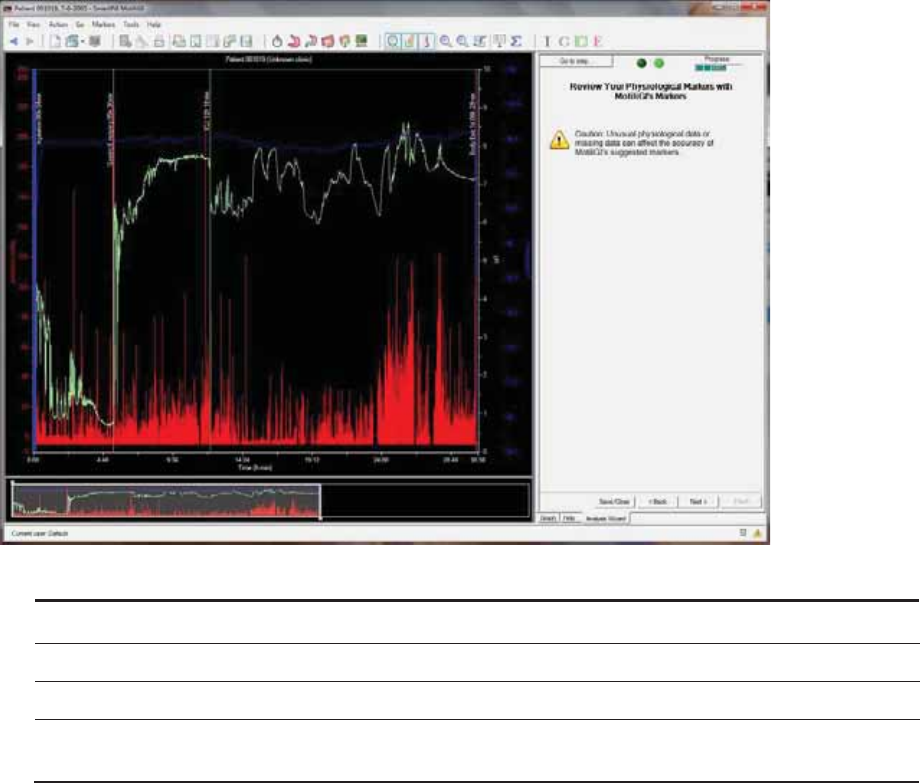
Appendix: Electrical Safety
Review Your Physiological Markers with
MotiliGI¶V0DUNHUV
Term
Description
Computed markers
Dashed vertical line on the graph, labeled “computed.”
Your markers
Solid vertical lines.
Equivalent
Your marker and the computed marker are within 5 minutes of each
other, labeled “equivalent.” The computed time is grayed out.
►Click Next >.
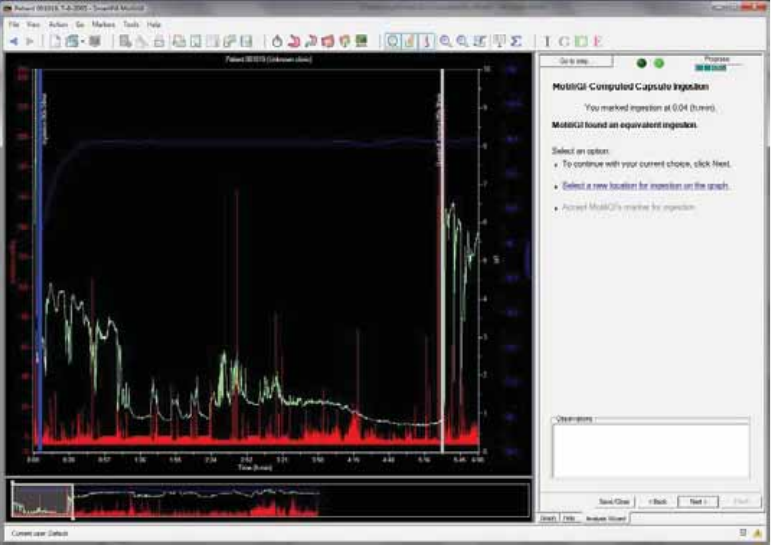
Appendix: Electrical Safety
MotiliGI-Computed Capsule Ingestion
MotiliGI determines ingestion by finding the point at which temperature stabilized at
body temperature.
1. Follow the prompts.
2. Click Next >.
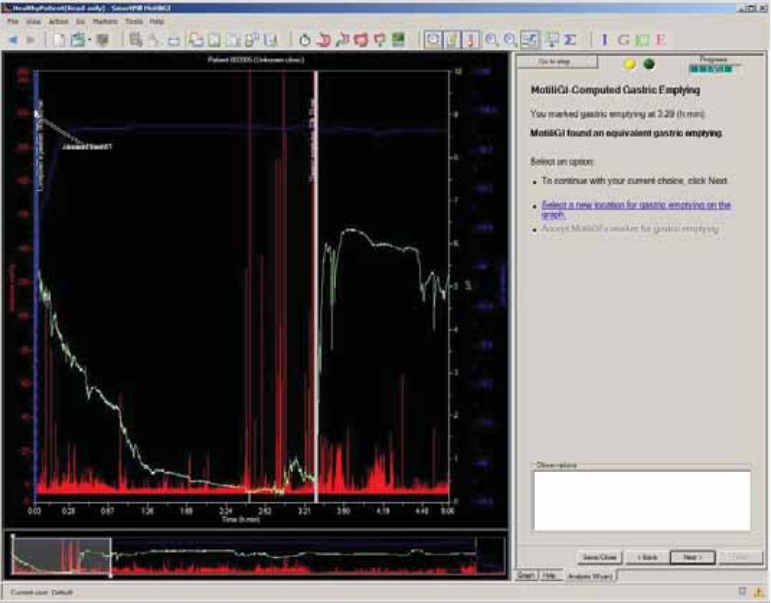
Appendix: Electrical Safety
MotiliGI-Computed Gastric Emptying
MotiliGI selects gastric emptying by finding the beginning of the abrupt, sustained pH
rise. Medications, therapies, and diseases that alter gastric or small bowel pH may
influence the accuracy of computed gastric emptying.
1. Follow the prompts.
2. Click Next >.
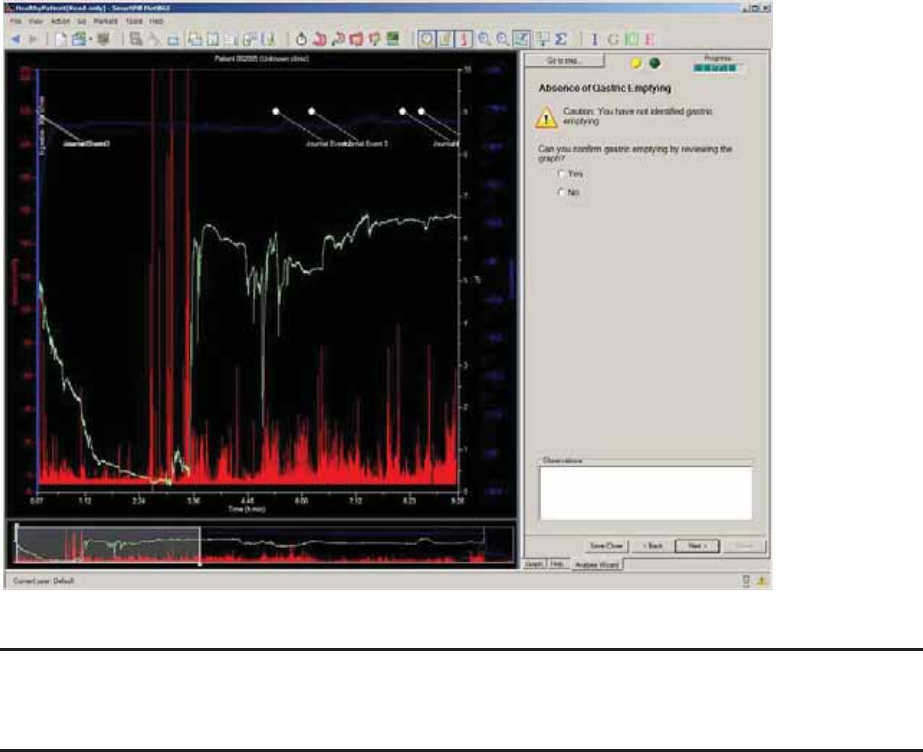
Appendix: Electrical Safety
Absence of Gastric Emptying
If you did not identify gastric emptying, the wizard reminds you of the omission and
asks you to select gastric emptying.
xTo review the MotiliGI computed gastric emptying, click <Back.
Caution
If gastric emptying is not observed, body exit should be confirmed by analysis of data. If body exit cannot be
confirmed, the patient should be monitored for symptoms of bowel obstruction. If the patient exhibits
symptoms consistent with bowel obstruction, a KUB exam is indicated to determine capsule exit or retention.
1. Follow the prompts and select Yes or No.
2. Click Next >.
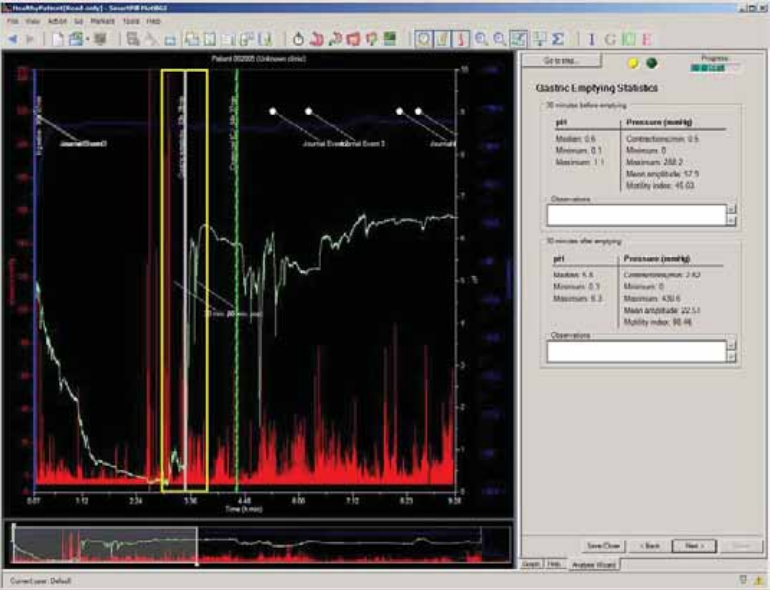
Appendix: Electrical Safety
Gastric Emptying Statistics
This step allows you to enter observations about the gastric emptying statistics.
►Click Next >.
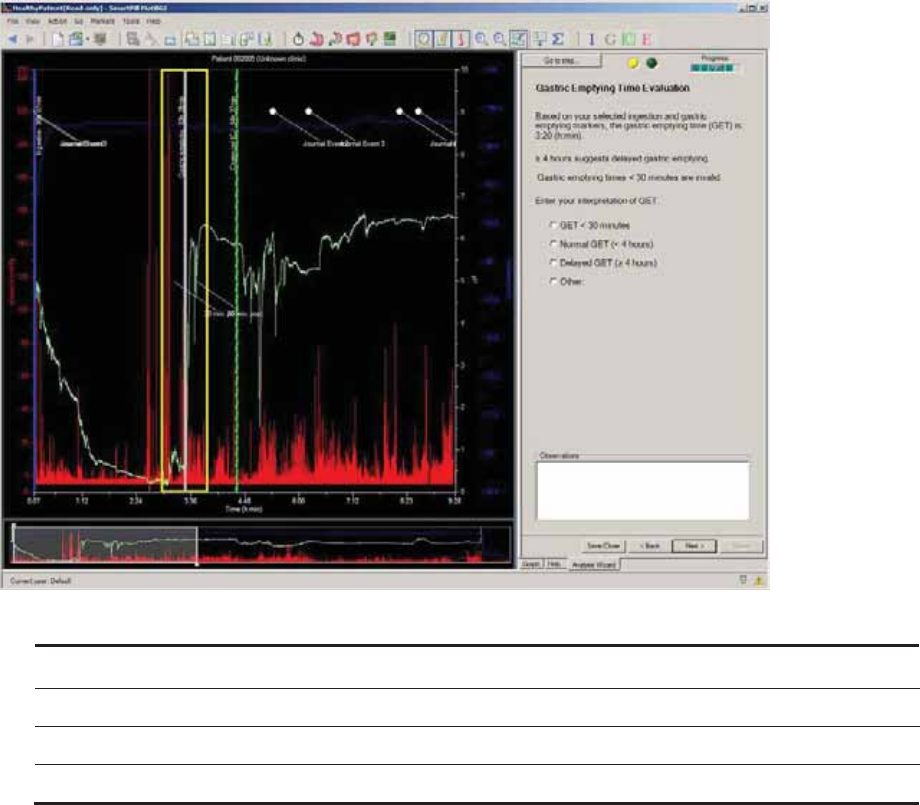
Appendix: Electrical Safety
Gastric Emptying Time Evaluation
Use GET to evaluate the patient’s gastric emptying function.
Term
Definition
Premature gastric emptying
Within 30 minutes of ingestion.
Normal gastric emptying
After 2 hours but before 4 hours of ingestion.
Delayed gastric emptying
After 4 hours of ingestion.
►Click Next >.
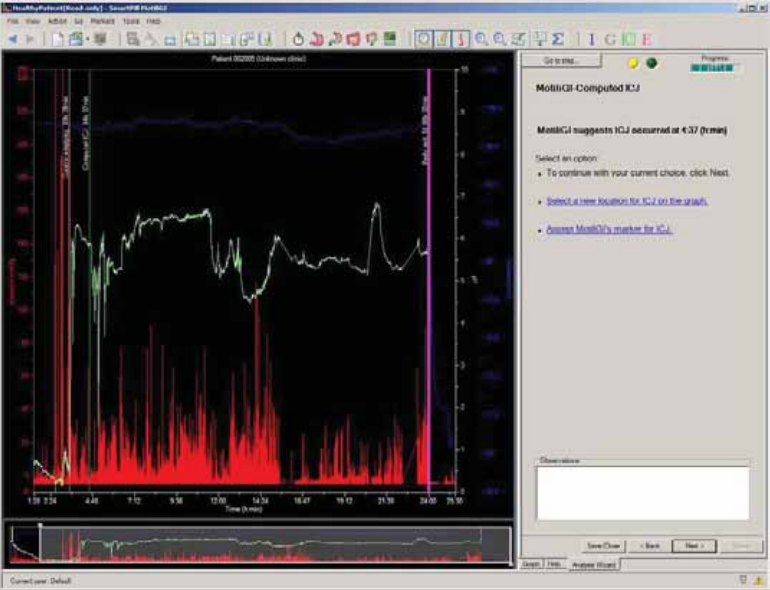
Appendix: Electrical Safety
MotiliGI-Computed ICJ
MotiliGI selects ICJ by looking for the first and largest pH drop between 30 minutes
and 6 hours after gastric emptying.
1. Follow the prompts.
2. Click Next >.
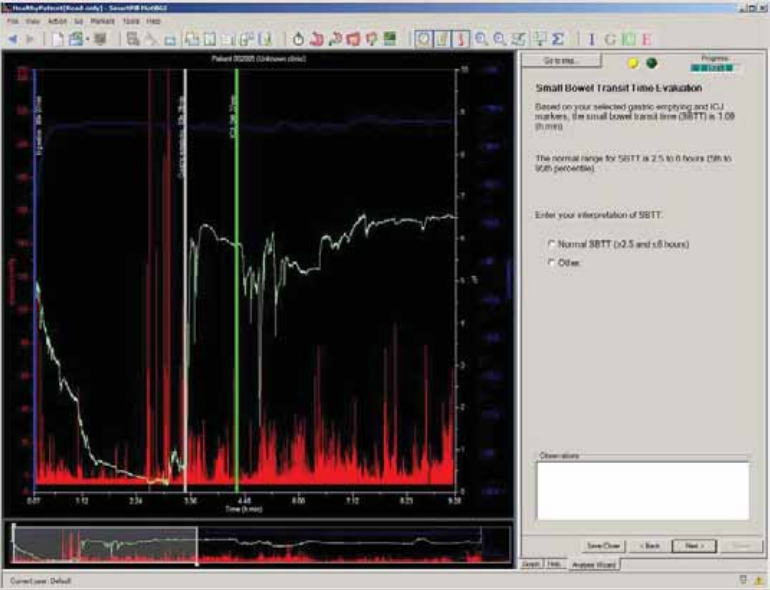
Appendix: Electrical Safety
Small Bowel Transit Time Evaluation
The clinical significance of SmartPill’s transit time in the small bowel is unknown.
►Click Next >.
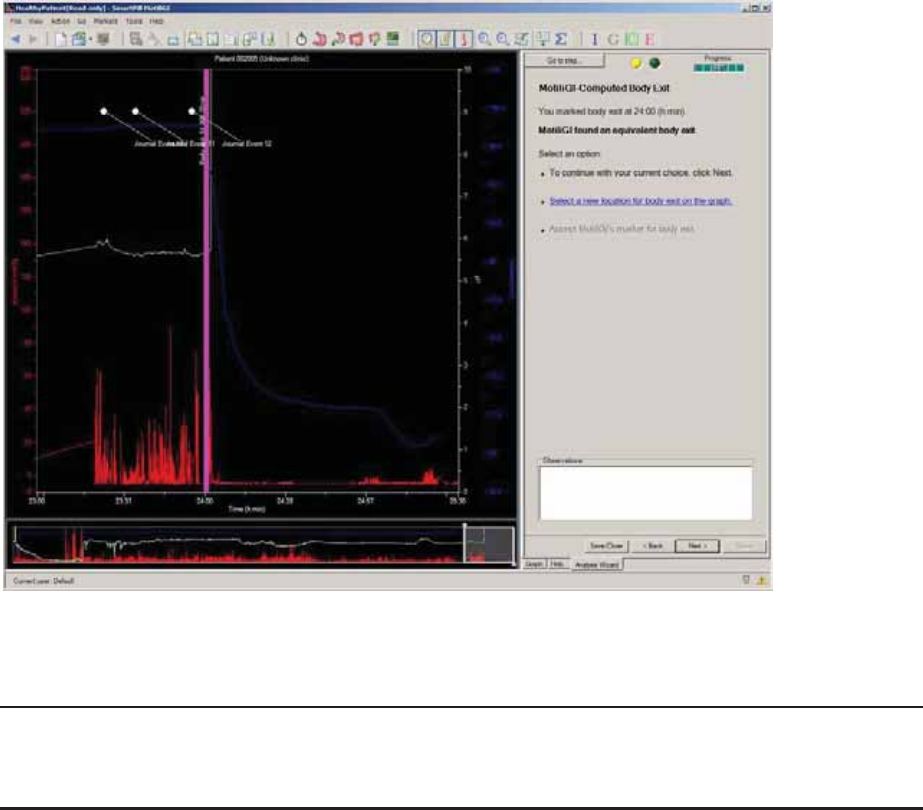
Appendix: Electrical Safety
MotiliGI-Computed Body Exit
MotiliGI selects body exit based on temperature and pressure. An abrupt loss of signal
from the capsule does not reliably indicate body exit. You must corroborate (to within
30 minutes) body exit with a recorded bowel movement in the patient diary.
Caution
If the diary does not contain a bowel movement entry concomitant with a temperature drop and/or loss of
pressure\pH signal, the patient should be monitored for symptoms of bowel obstruction. If the patient
exhibits symptoms consistent with bowel obstruction, a KUB exam is indicated.
1. Follow the prompts.
2. Click Next >.
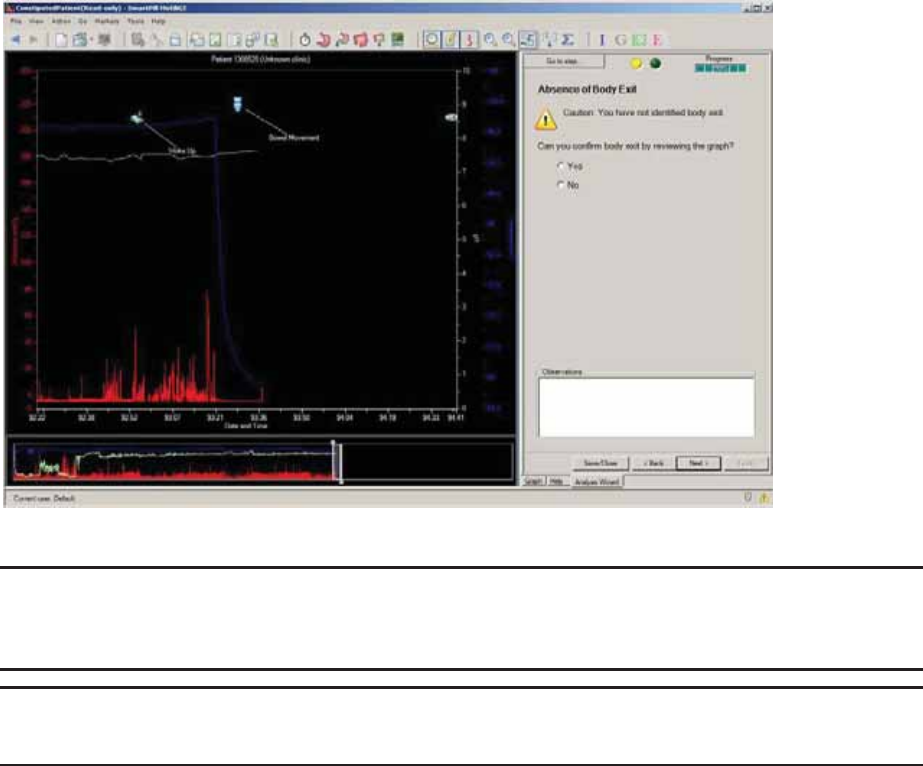
Appendix: Electrical Safety
Absence of Body Exit
If you did not identify body exit, this step appears.
xTo review the computed body exit, click <Back.
Caution
If the diary does not contain a bowel movement entry concomitant with a temperature drop and/or loss of
pressure and pH signal, the patient should be monitored for symptoms of bowel obstruction. If the patient
exhibits symptoms consistent with bowel obstruction, a KUB exam is indicated.
Caution
If MotiliGI displays a low voltage indication on the graph, the validity of data collected during the low voltage
state cannot be determined, including any temperature drops that mimic body exit.
1. Select Yes or No.
2. Click Next >.
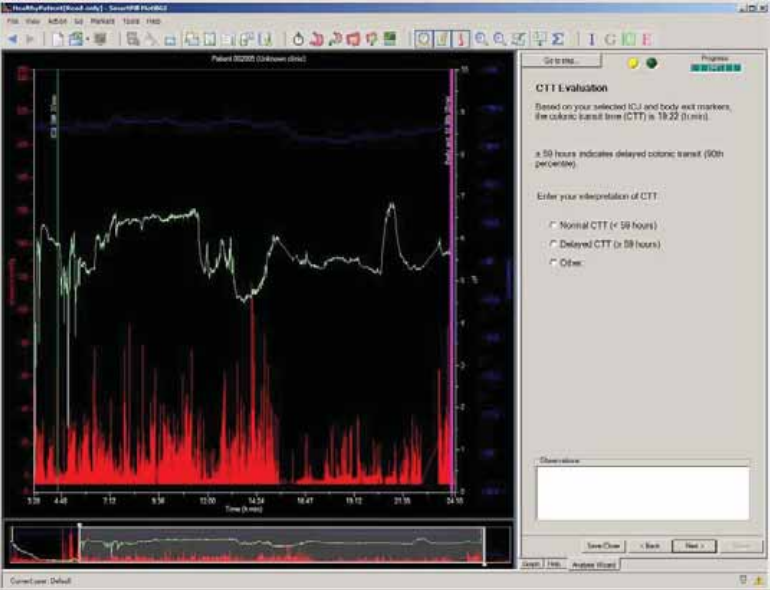
Appendix: Electrical Safety
Colonic Transit Time Evaluation
MotiliGI uses your interpretation of ICJ (colonic entry) and body exit events to
compute the CTT.
►Click Next >.
Test Analysis Review
The analysis is complete.
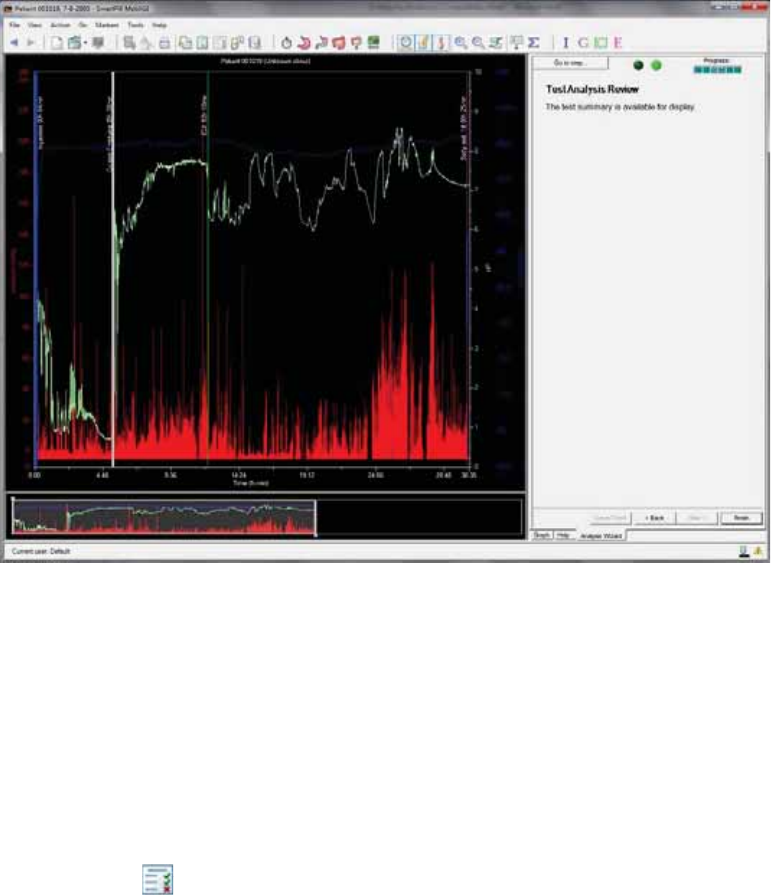
Appendix: Electrical Safety
►Click Finish.
Reading Test Summary Reports
When the test analysis wizard is complete, a summary report of results and descriptive
data appears.
These reports summarize test data, statistics, and observations, and help evaluate
delayed gastric emptying, constipation and gastrointestinal motility.
You can display the test summary reports any time:
xClick the toolbar icon.
xSelect View > Test Summary.
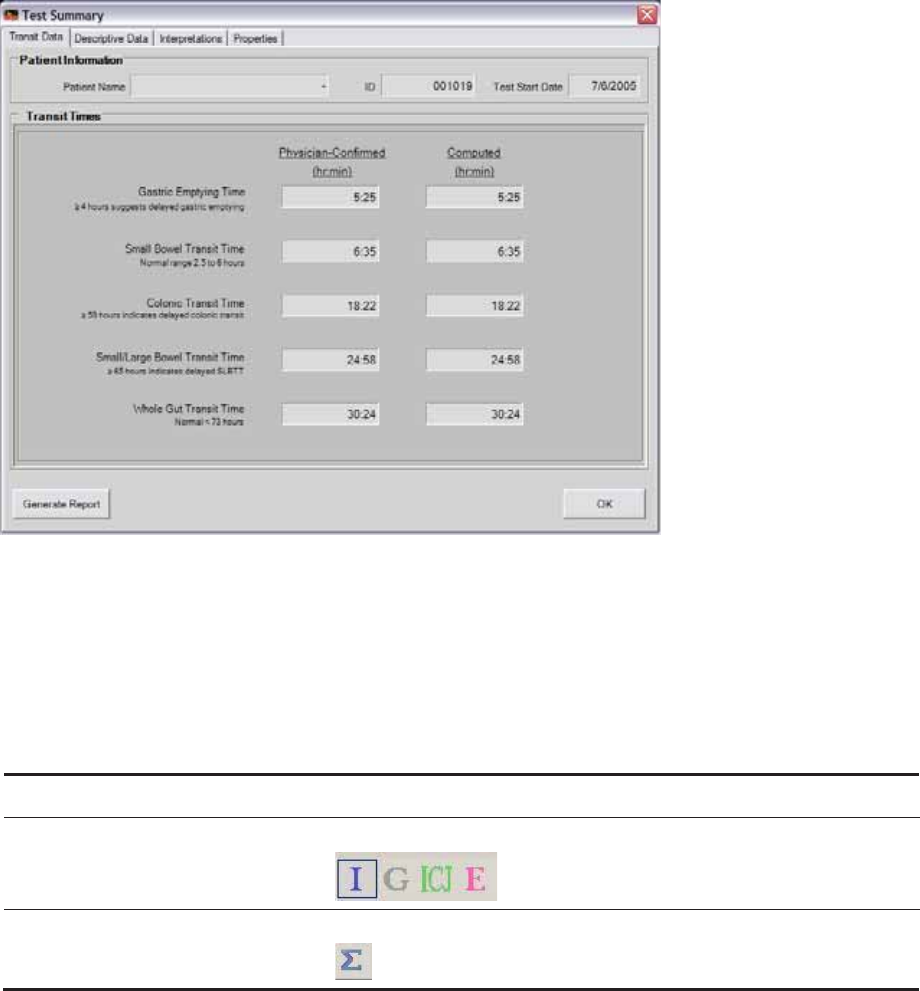
Appendix: Electrical Safety
Transit Data Tab
This tab lets you compare your transit time data to the computed data.
xIf one or more endpoint markers are not defined, n/a (not applicable) appears for
the transit time value.
xCombined small and large bowel transit time (SLBTT) can be used as a surrogate
measure for CTT when CTT cannot be determined.
You can edit the data at any time, and the corresponding reports update accordingly:
Action
Tools to use
For more information
Edit ingestion time, gastric emptying
time a
nd body exit time
Graph marker tools
Chapter 8: MotiliGI Features
Edit or enter motility statistics
Statistics Mode tools
Chapter 8: MotiliGI Features
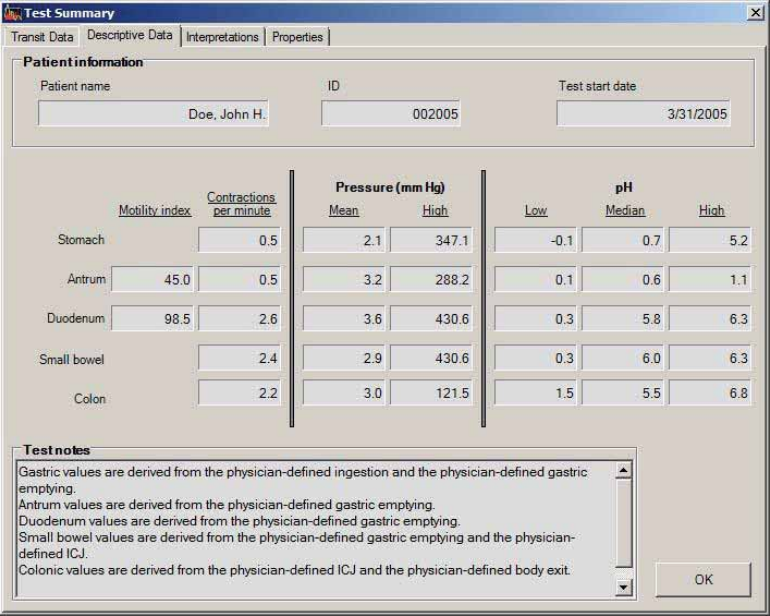
Appendix: Electrical Safety
Descriptive Data Tab
This tab summarizes calculated pressure and pH data points. The clinical significance
of these pressure and pH descriptive statistics is unknown.

Appendix: Electrical Safety
Interpretations Tab
This tab displays notes made during analysis and provides a text section for capturing
post-test observations, conclusions and recommendations. The information entered in
this tab becomes part of the patient’s record.
Alternatively, you can enter interpretations through the Interpretations tab of the
Patient Information screen. Interpretations can be entered before or after the test is
downloaded.
Click to use preconfigured templates to describe test interpretations.
Goal
Action
Create a template
Enter a title in the Template field and a description in the text
field below it. Click Save Template.
Delete a template
Select the title in the Template list. Click Delete Template.
Add the description to the patient’s record
Select the template title. Click .
Remove the description from the patient’s
record
Select the description in the Patient record box. Click Remove
Entry.
Print a description in the final report
Select checkboxes in the Patient record box.
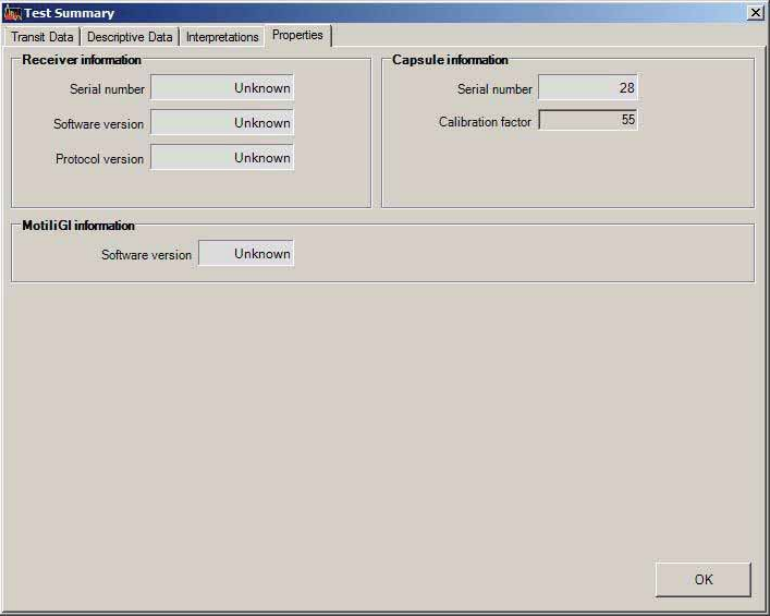
Appendix: Electrical Safety
Properties Tab
This tab displays information about the equipment used for the test.
Examples of Physiological Markers
Ingestion
The ingestion landmark starts the test and is the beginning of GET. MotiliGI
determines the ingestion landmark by finding the point just before temperature
stabilizes to body temperature, as illustrated below. The pH also starts to become
more acidic.
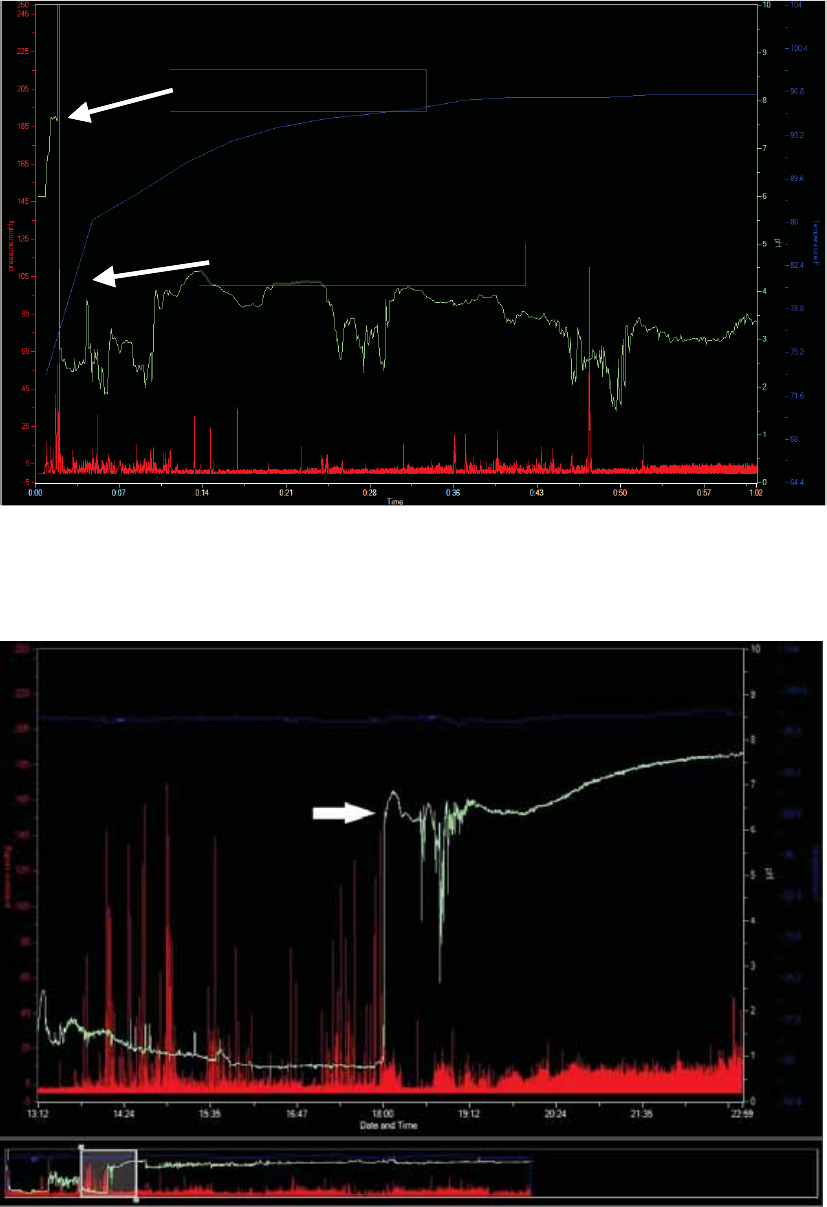
Appendix: Electrical Safety
Figure 9 - Ingestion
Gastric Emptying
Gastric emptying marks the end of GET and the start of SBTT as shown below.
Figure 10 - Gastric Emptying
MotiliGI looks for an abrupt increase of 3 or more pH unit as the capsule exits the
stomach and enters the duodenum.
B
A
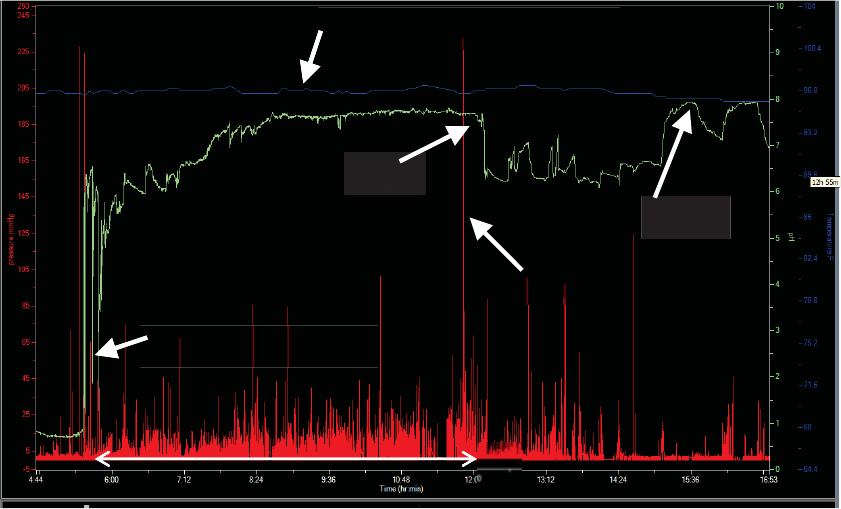
Appendix: Electrical Safety
Factors that may influence gastric emptying include:
xMedications, therapies, and diseases that alter gastric or small bowel pH.
ICJ
ICJ is usually found between 2 to 6 hours after gastric emptying, as seen below. ICJ
marks the transition of the capsule from the small bowel into the colon. ICJ marks the
end of SBTT and the start of CTT.
How to find ICJ:
1. Locate gastric emptying
2. Look for a steady rise in pH followed by a plateau.
3. Look for a 1 to 2 unit pH drop between 2 to 6 hours after gastric emptying
xA high amplitude spike in pressure is often seen near ICJ
xAfter ICJ an erratic pH profile in the colon is characteristic
Figure 11 - ICJ
Body Exit Time
Body exit marks the end of the test as shown below. Body exit marks the exit of the
capsule from the body and the end of WGTT and CTT.
E
B
A
ICJ
D
C
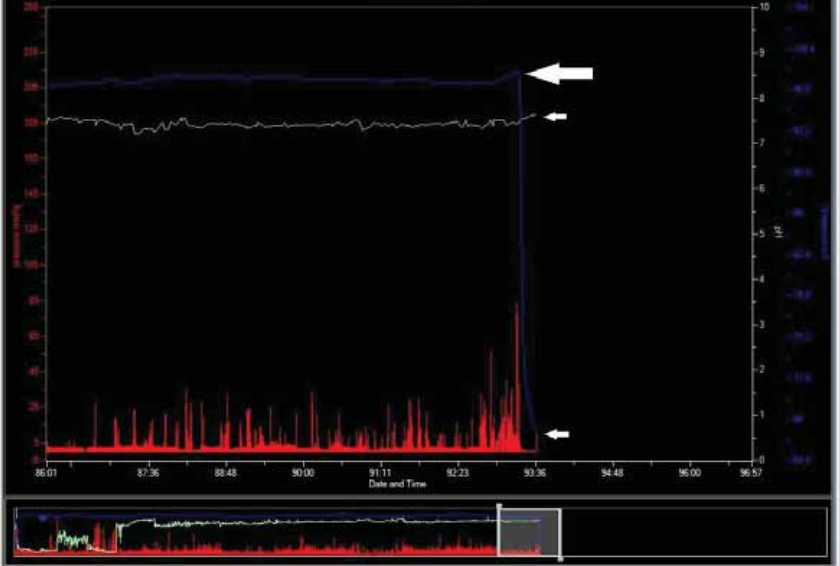
Appendix: Electrical Safety
Figure 12 - Body Exit
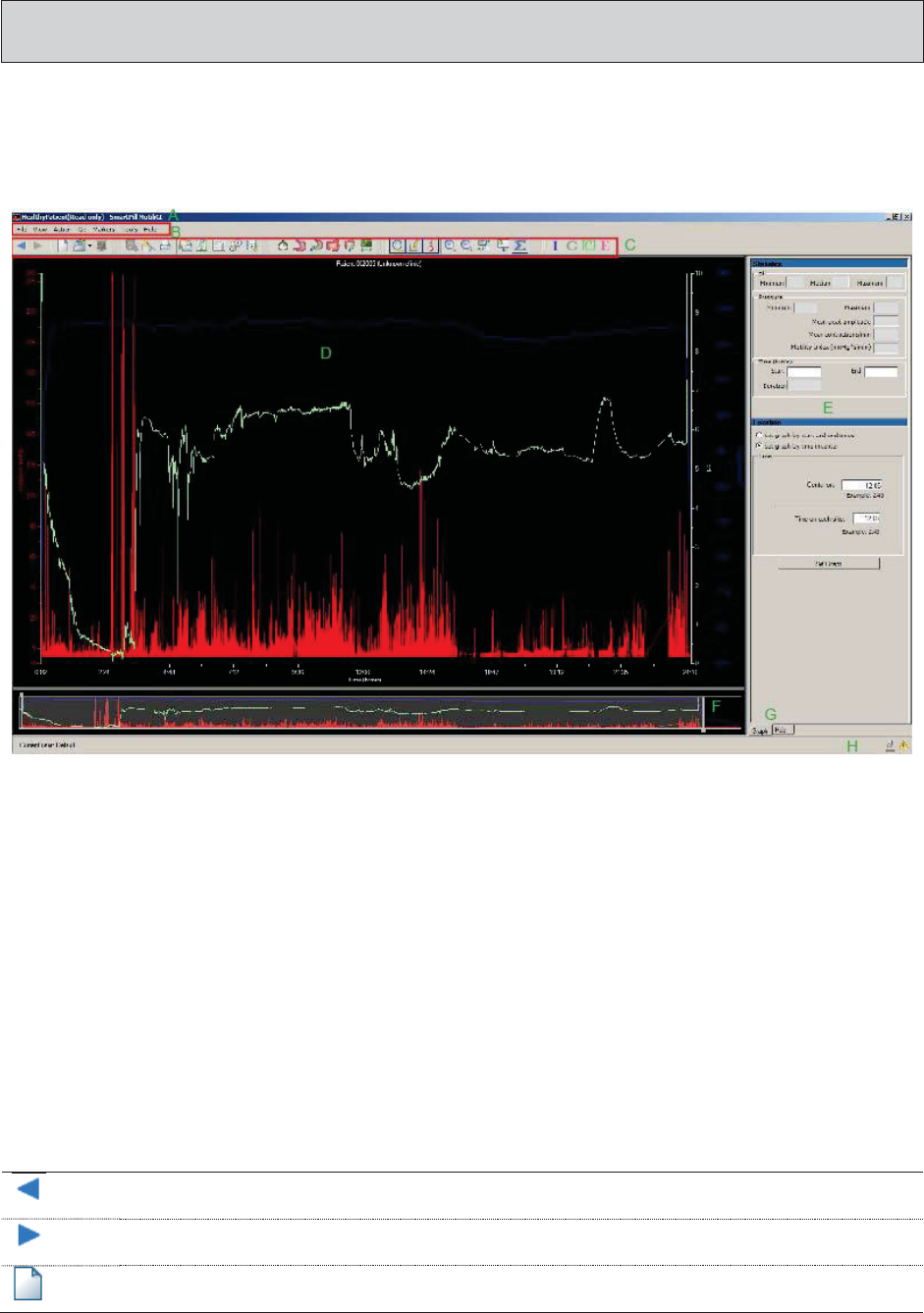
Appendix: Electrical Safety
0RWLOL*,)HDWXUHV
MotiliGI User Interface
The MotiliGI user interface consists of main dropdown menus, toolbar, graph area,
control panels, time slider control and status bar.
Figure 13 - MotiliGI User Interface
Titlebar
Labeled A on Figure 13.
The titlebar displays the filename constructed from the Patient ID and the time and
date of the beginning of the test, for example Patient ID #207516, 9:29:44 AM, 28
March, 2007.
Main Menus
Labeled B on Figure 13.
Toolbar Icons
Labeled C on Figure 13.
Back to Previous View
Forward to Next View
New – Start a new test

Appendix: Electrical Safety
Open – Open a test. You can choose to open by patient name or filename.
Live Monitoring
Download Data
Analyze Test
Print
Print Preview
Generate Report
Test Summary
Patient Info
Annotation Viewer
Display Ingestion
Display Stomach
Display Gastric Emptying
Display Small/Large Bowel
Display Body Exit
Display Entire Test
Show/Hide Pressure
Show/Hide pH
Show/Hide Temperature
Zoom In
Zoom Out
Show/Hide Annotations
Annotations
Statistics
Set Ingestion Marker
Appendix: Electrical Safety
Set Gastric Emptying Marker
Set ICJ Marker
Set Body Exit Marker
Graph Area
Labeled D on Figure 13.
The graph area presents plots of pressure, pH, and temperature data captured
throughout the test, and point and range annotations.
The left Y-axis is the pressure scale, the right Y-axes are pH and temperature scales,
and the X-axis is the elapsed time of the test.
Data Panels
Labeled E on Figure 13.
MotiliGI’s two data panels, positioned to the right of the graph area, provide
information and controls related to test “Statistics” and graph “Location.”
To show or hide the data panel:
►Double-clicking the bar between the graph and the data panels.
Time Slider Control
Labeled F on Figure 13.
The time slider control shows what appears in the main graph in relation to the entire
test.
Display Tabs
Labeled G on Figure 13.
Navigate between the Graph (statistics panel), Help (user manual) and Analysis Wizard
(not pictured).
To show or hide the data panel:
►Double-clicking the bar between the graph and the data panels.
Status Bar
Labeled H on Figure 13.
The status bar displays the username, and data receiver and test status icons. MotiliGI
uses 7 icons to indicate the status of the data receiver and test.
Appendix: Electrical Safety
Data Receiver Status Icons
Icon
Description
Disconnected – No USB connection exists between MotiliGI and the data receiver.
Connected – MotiliGI is communicating with the data receiver.
Test Status Icons
Icon
Descriptio
n
Capsule Not Found – MotiliGI is not receiving data from a capsule and indicates a capsule has
not been activated or the capsule is out of range of the data receiver.
Test Status Unknown – MotiliGI cannot determine the status of the test.
Test Ready to Begin – MotiliGI is properly communicating with the data receiver and the
capsule. This icon appears during capsule calibration.
Test in Progress – The test has begun. The data receiver is recording data from the capsule.
Test Ended – Data collection has stopped. This icon appears during data download.
MotiliGI Keyboard Shortcuts
Keyboard Shortcut
Effect
Control X
Cut text from any form field, copied to the clipboard
Control C
Copy text from any form field to the clipboard
Control V
Paste text from the clipboard to any form field
Tab
Move from one text field to the next
Enter
Press the default button for the current form, panel, or dialog box
2SHQLQJD7HVWE\WKH3DWLHQW¶V1DPH
1. Open a test:
xClick on the toolbar.
xClick File > Open
2. Select By Patient Name. The Find Patient window appears.
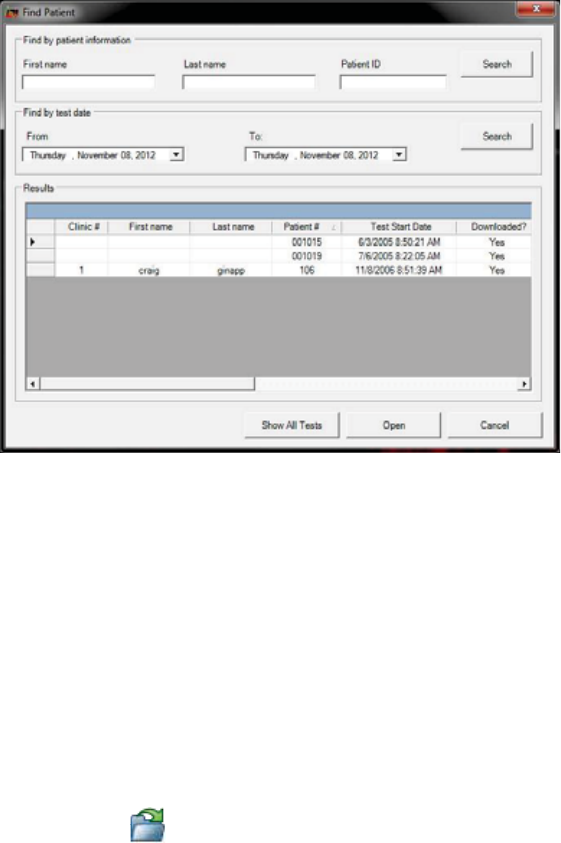
Appendix: Electrical Safety
xTo show all tests in the Results box, click Show All Tests.
xTo reduce the size of the list, enter all or part of the patient’s name or patient ID.
You can also enter a test date or range of dates in the “Find by test date” box.
3. Click Search. MotiliGI displays matching tests in the Results box.
4. Click a row to select the patient’s test.
5. Click Open.
Opening a Test by the File Name
1. Open a test:
xClick on the toolbar.
xClick File > Open
2. Select By Filename. The Open File screen appears.
3. Browse to the test file:
xWindows XP: C:\Documents and Settings\All Users\Application
Data\SmartPill\Tests
xWindows 7: C:\ Program Data\SmartPill\Tests
4. Click Open.
Closing a Test
►Select File > Close.
MotiliGI saves changes to the test when you close.
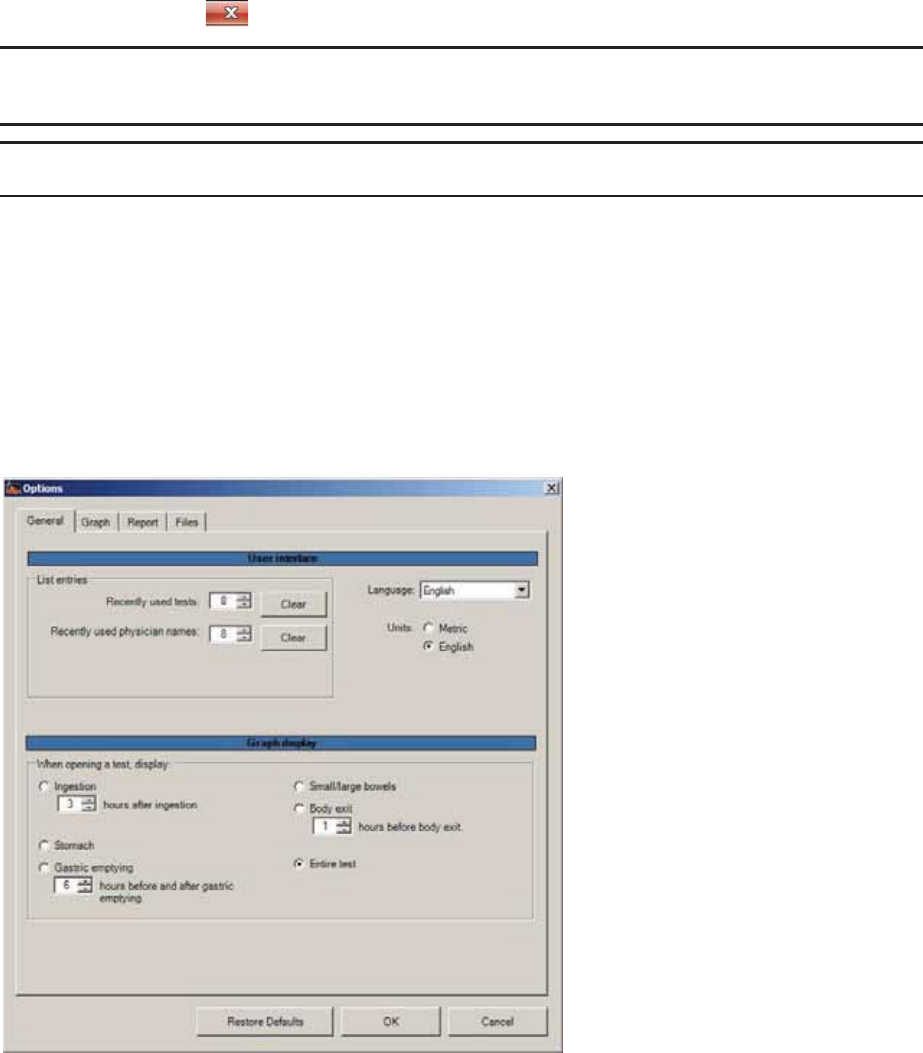
Appendix: Electrical Safety
Exiting MotiliGI
MotiliGI saves changes to the test when you exit. To exit MotiliGI, either:
xSelect File > Exit
xClose using the in the upper right hand corner.
Caution
Do not close MotiliGI while operating the Test Initiation Wizard because data can be lost. Complete or abort
the Wizard first.
Caution
Do not close MotiliGI while downloading a test because data can be lost. Allow the test to fully download.
Setting General User Preferences
Each user can have saved preferences for the General, Graph and File tabs. Report
preferences apply to all users.
To customize the look and feel of the user interface:
►Select Tools > Options.
General Tab
You can select preferences for the User Interface:
xThe number of tests appearing in the File > Recent Tests menu.
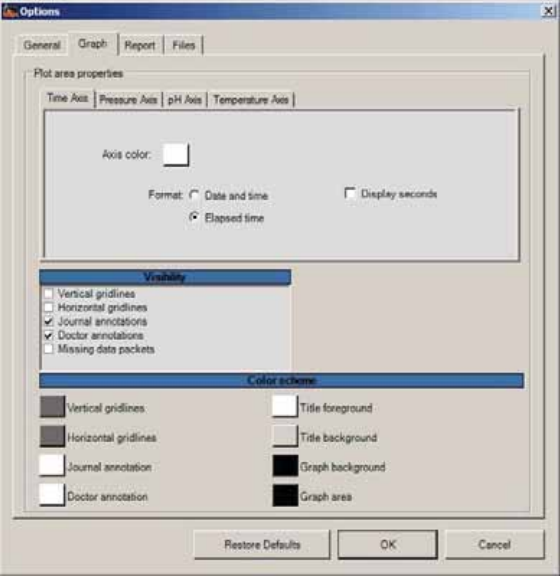
Appendix: Electrical Safety
xThe number of physician’s names appearing in the Ordering and Primary Care
Physician drop down lists on the General tab of the Enter Patient Information
screen.
xThe display language. Changes will take effect when you restart MotiliGI.
xThe unit of measure, English or metric.
You can select preferences for the view displayed when MotiliGI opens a test:
xIngestion and the number of hours displayed after ingestion
xStomach
xGastric emptying and the number of hours displayed before and after gastric
emptying
xSmall/large bowel
xBody exit and the number of hours displayed before body exit
xEntire test
Graph Tab
You can select preferences for the Plot Area Properties for Time, Pressure, pH and
Temperature:
xColor, line style, and point style of data plot
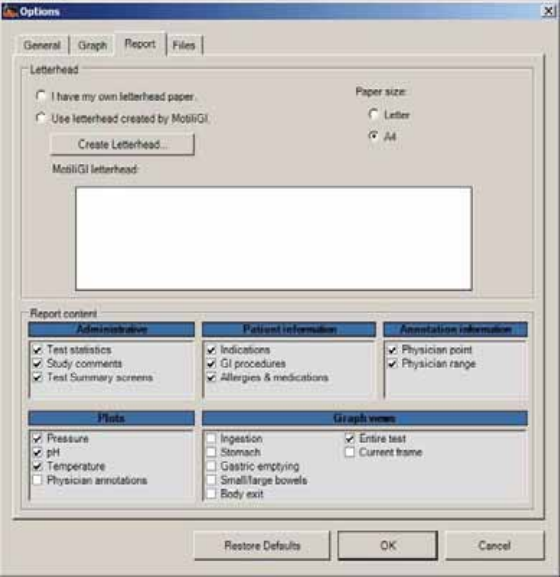
Appendix: Electrical Safety
xShow or hide the plot
xThe ranges, tick values and colors of the X and Y axes
You can select preferences for Visibility:
xShow or hide gridlines, annotations, and missing data packets
You can select preferences for Color Scheme:
xThe color of gridlines, annotations, title text, and background color
Report Tab
You can select an option for printing reports. MotiliGI lets you print reports on your
own letterhead stationery or create a custom letterhead.
You can select report options. You can choose to include the following in the report:
xLetterhead and paper size
xAdministrative information including test statistics, study comments, and screen
prints of the Test Summary screens.
xThe patient’s information.
xThe Annotation information including statistics and notes.
xThe plots to be graphed: pressure, pH, temperature and annotations.
xThe graph views of the test.
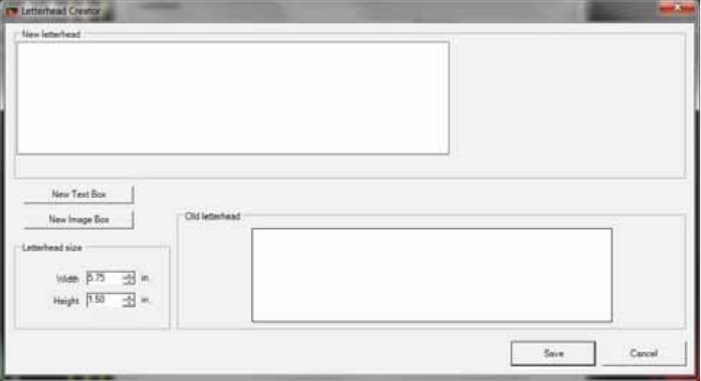
Appendix: Electrical Safety
Creating Report Letterhead
1. Select Tools > Options > Report Tab.
2. Choose paper size.
3. Click Create Letterhead.
xThe Letterhead Creator appears:
Add text to the letterhead
1. Click New Text Box. A text box appears in the new letterhead.
2. Type the desired text.
3. Click and drag the text box to move to a location in the Letterhead Canvas.
xYou can modify the font, resize the text box or delete the text box.
a. Right-click in the text box.
b. Choose desired option.
c. To resize the text box drag your mouse to the desired size and click to apply.
d. Click OK.
4. Click Save.
Add an image to the letterhead
1. Click New Image Box. An image box appears in the new letterhead.
2. Right-click in the image box to select an image.
3. Click Browse for Image... An Open Image screen appears.
4. Find and select a bitmap, JPEG or GIF file.
5. Click OK.
xYou can modify the image appearance, resize or delete the image box.
►Right-click in the image box. Choose desired option.
Appendix: Electrical Safety
xTo resize the image box drag your mouse to the desired size and click to
apply.
6. Click Save.
Files Tab
You can select the target folder to save files to.
xClick Browse to find target folder.
xType in the folder location.
Setting Preferences Back to Default
►Click Restore Defaults to return all tabs and settings to default states. Your language preference
remains the same.
Editing Patient Information
Edit patient information any time after the start of the test. Patient information is
initially entered in the test initiation wizard. You can edit information on all tabs
within the patient information screen.
If you edit the patient ID, MotiliGI updates the electronic test filename, log filename
and the folder containing the files with the new patient ID number and it will ask you
to confirm. If you previously generated a report (WordPad document), your report
filename and patient ID number will not be updated. You will need to generate a new
report.
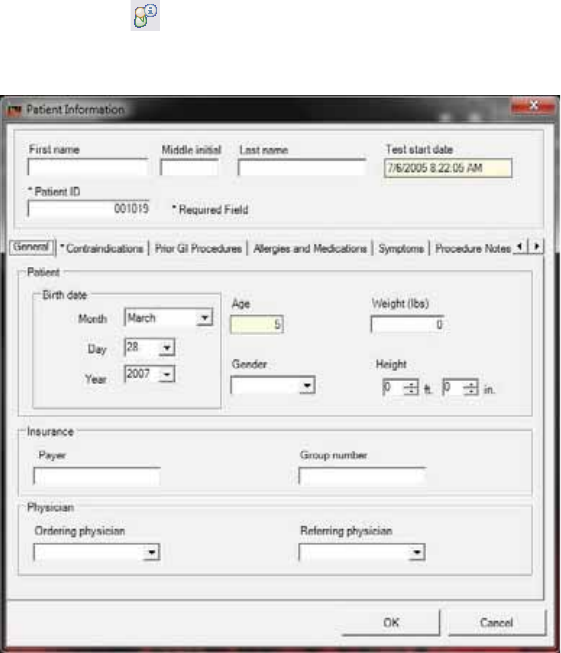
Appendix: Electrical Safety
1. To access the patient information either:
xClick on the toolbar
xClick View > Patient Info.
2. Edit the patient’s information.
3. Click OK.
Changing the Graph Display
Selecting Data Plots
MotiliGI gives you 3 methods to select the data (pressure, pH and temperature)
displayed on the graph:
Method 1 ± Context Menu
xRight-click on the graph and select a data plot. A checkmark indicates the
plot appears on the graph.
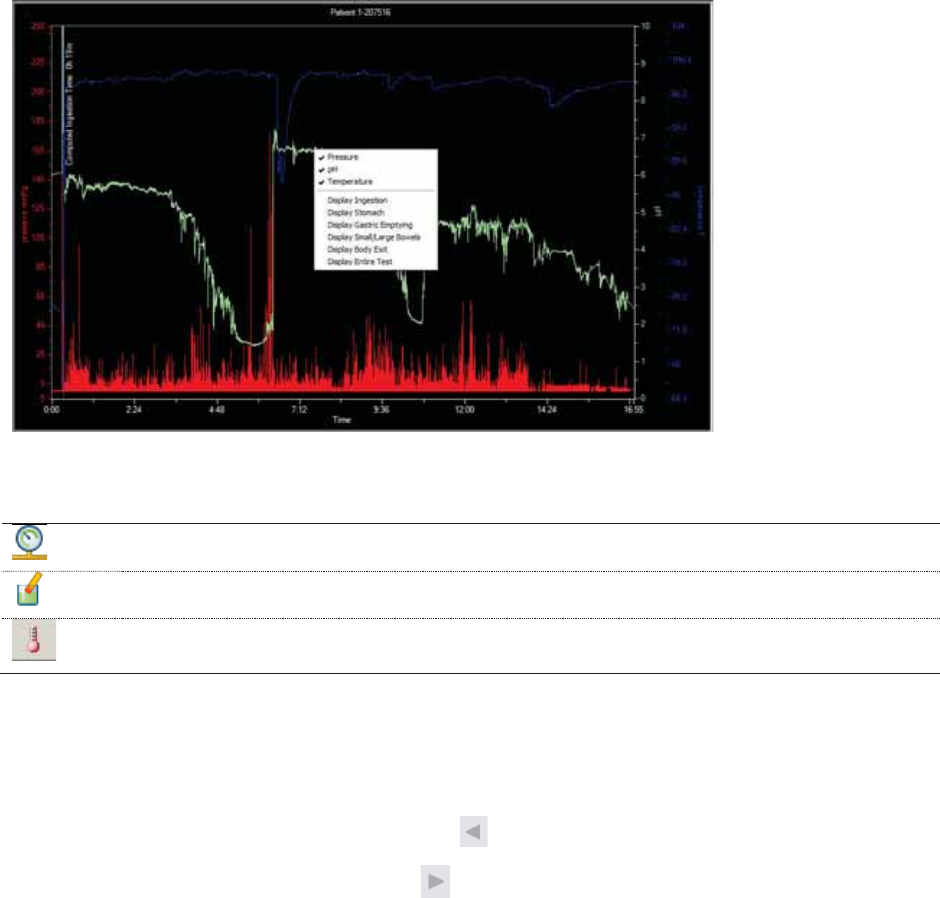
Appendix: Electrical Safety
Method 2 ± Toolbar Icons
►Click on a “Show/Hide” data plot icon.
Show/Hide Pressure
Show/Hide pH
Show/Hide Temperature
Method 3 ± Main Menu
►Click View > Plots. Select a data plot icon to toggle the plot.
Changing the Test View
xTo undo any view operation, click the toolbar icon.
xTo redo a view operation, click the toolbar icon.
MotiliGI prefers the physician-determined marker rather than the computed marker to
define the regions.
MotiliGI gives you several methods to select a test view.
Method 1 ± Context Menu
►Right-click on the graph and select a desired view.
Method 2 ± Main Menu
►Click Go and choose a desired view.
Method 3 ± Toolbar Icons
►Click on a display icon
Appendix: Electrical Safety
Display Ingestion – Test data around the ingestion marker.
Display Stomach – Test data between the ingestion marker and the gastric emptying marker.
Display Gastric Emptying – Test data around the gastric emptying marker
Display Small/Large Bowel – Test data from the gastric emptying marker to the body exit
marker.
Display Body Exit – Test data around the body exit marker.
Display Entire Test – Test data from the ingestion marker to the body exit marker.
Method 4 ± Time Slider Control
The portion of the test shown in the graph area is highlighted in white.
To move the slider:
1. Place your cursor on the slider. The cursor changes to a finger.
2. Click and drag the cursor to the left or right.
3. Release the cursor. The main graph updates.
xYou can also move the slider with the scroll wheel on the mouse.
To zoom with the slider:
1. Place your cursor over the border of the highlighted area. The cursor changes to a
double arrowhead.
2. Click and drag the border to the left or right.
3. Release the cursor. The main graph updates.
Method 5 ± Zoom Controls
To zoom in:
1. From the toolbar, select . The cursor changes to a magnifying glass icon.
2. Select an area to view by:
xClicking on a graph location (This method only zooms in on the X axis)
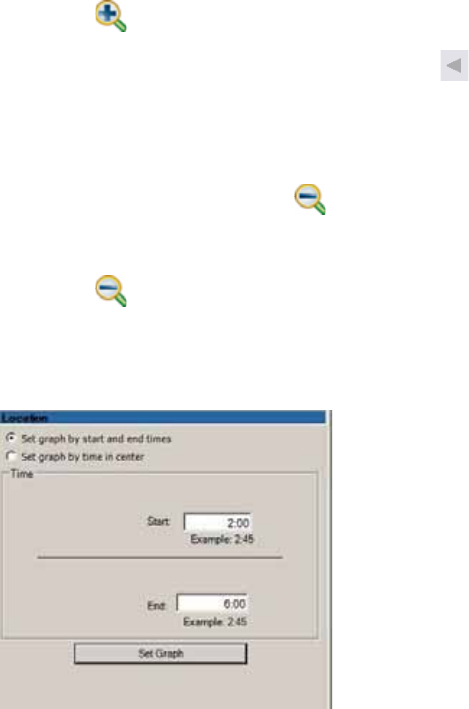
Appendix: Electrical Safety
xClicking and dragging your mouse to select a graph region (This method zooms
in on both the X and Y axis)
3. Click to exit the zoom in mode. The cursor returns to normal.
xTo return to your last view use the icon.
To zoom out:
1. From the toolbar, select . The cursor changes to a magnifying glass icon.
2. Select an area to view by clicking on a graph location.
3. Click to exit zoom out mode. The cursor returns to normal.
Method 6 ± Using the Data Panel: Location
1. Define a window of time by selecting Start and End Times or Time In Center
2. Enter the time you wish to display.
3. Click Set Graph.
Changing Axis Scales
Y Axes
To change the scale of the pressure, pH or temperature axis:
1. Double-click on the desired axis.
2. Enter the new axis scale minimum and maximum values.
3. Click OK.
To restore the scale to the saved user preferences:
1. Double-click on the desired axis.
2. Click Restore Axis Defaults or Restore Defaults for All Axes.
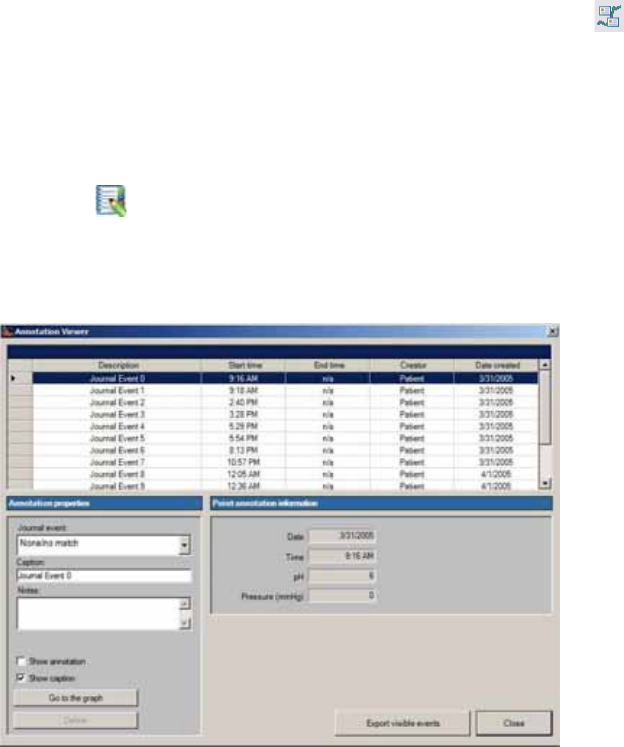
Appendix: Electrical Safety
3. Click OK.
X Axis
To change the time format between calendar date/time and elapsed time either:
xDouble-click on the time axis to toggle between elapsed time or real date/time.
xGo to Tools > Options >Graph > Time Axis
Analyzing Data
Showing and Hiding Annotations
Annotations include patient diary events, range annotations, and point annotations.
Annotations are visible by default.
xTo toggle the visibility of all graph annotations, click on the toolbar.
Editing Patient Diary Events
To view and edit patient diary events
1. Open the Annotation Viewer:
xClick on the toolbar.
xSelect View > Annotation Viewer.
The Annotation Viewer screen appears.
2. Click the event you wish to edit. The event details appear in the annotation
properties box.
3. Select an icon from the Journal Event drop-down list. The Update button appears.
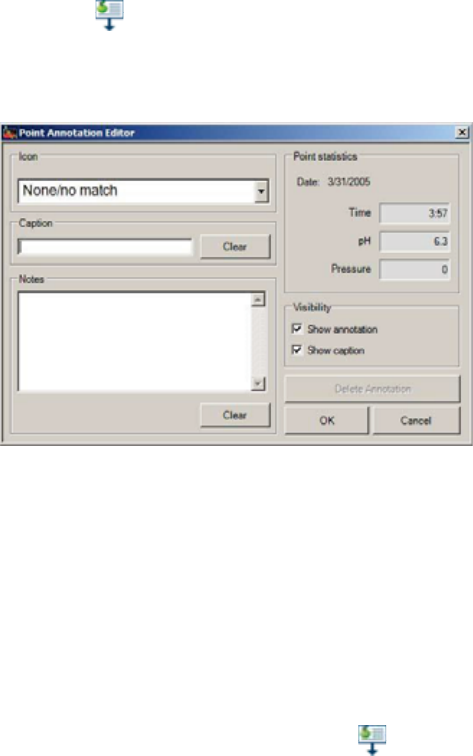
Appendix: Electrical Safety
x(Optional) You can enter text in the Caption box and in the Notes box.
Caption text shows on the graph, while Notes text shows in the Annotation
Viewer and in the printed report.
xYou can click the checkboxes to show or hide the annotation and the caption.
4. Click Update to save changes.
Disregard diary entries and events that do not have a reasonable time correlation.
Creating New Event Annotations
To enter an event that does not appear on the graph:
1. Click on the toolbar. The cursor changes to a similar icon.
2. Click on the graph at the appropriate time to create the annotation. The Point
Annotation Editor appears.
3. Select an icon from the Icon dropdown box.
►(Optional) You can enter text in the Caption box and the Notes box. Caption text shows on
the graph. Notes only show in the Annotation Viewer, Point Annotation Editor and in the
printed report.
xYou can view the time, pH and pressure values at the selected graph point.
xYou can click the checkboxes to show or hide the annotation and the caption.
4. Click OK.
►To exit the Annotation mode, click on the toolbar. The cursor returns to normal.
Editing Event Annotations
Event icons cannot be moved, only deleted or hidden. An event created by the patient
pushing the event button on the data receiver cannot be deleted; it can only be hidden.
Physician-created annotations can be deleted.
7RHGLWDQHYHQWDQQRWDWLRQ¶VLFRQRUWH[W
Double-click the event icon. The Point Annotation Editor appears.
Appendix: Electrical Safety
7RPRYHDQDQQRWDWLRQ¶VFDSWLRQRQWKHJUDSK
1. Click on the toolbar.
2. Move cursor over the caption text. The cursor changes to a crosshair.
3. Click and drag the caption text to a new location.
To delete an annotation:
1. Double-click the event icon. The Point Annotation Editor appears.
2. Click Delete.
Adding or Changing the Location of Physiological Markers
Physiological markers are used to compute transit times. MotiliGI-computed markers
appear on the graph as dotted vertical lines. Physician-defined markers appear on the
graph as solid vertical lines.
Physiological Markers
Marker
Color
Toolbar
Icon
Ingestion - Time the capsule was ingested
Blue
Gastric emptying - Time the capsule left the stomach
Silver
Ileo-cecal junction - Time the capsule entered the colon
Green
Body exit - Time the capsule left the body
Magenta
To add or change the location of a marker:
1. Click the appropriate toolbar icon. The cursor changes to a crosshair.
2. Click the graph to set the marker at a location. The cursor returns to normal.
To clear (delete) a physician-defined marker:
►Select Markers > Clear Defined Markers…
You can choose to clear one or all physician-defined markers.
To hide a marker:
►Select Markers > Show/Hide Markers
You can choose to show/hide one or all physician-defined and MotiliGI-computed
markers.
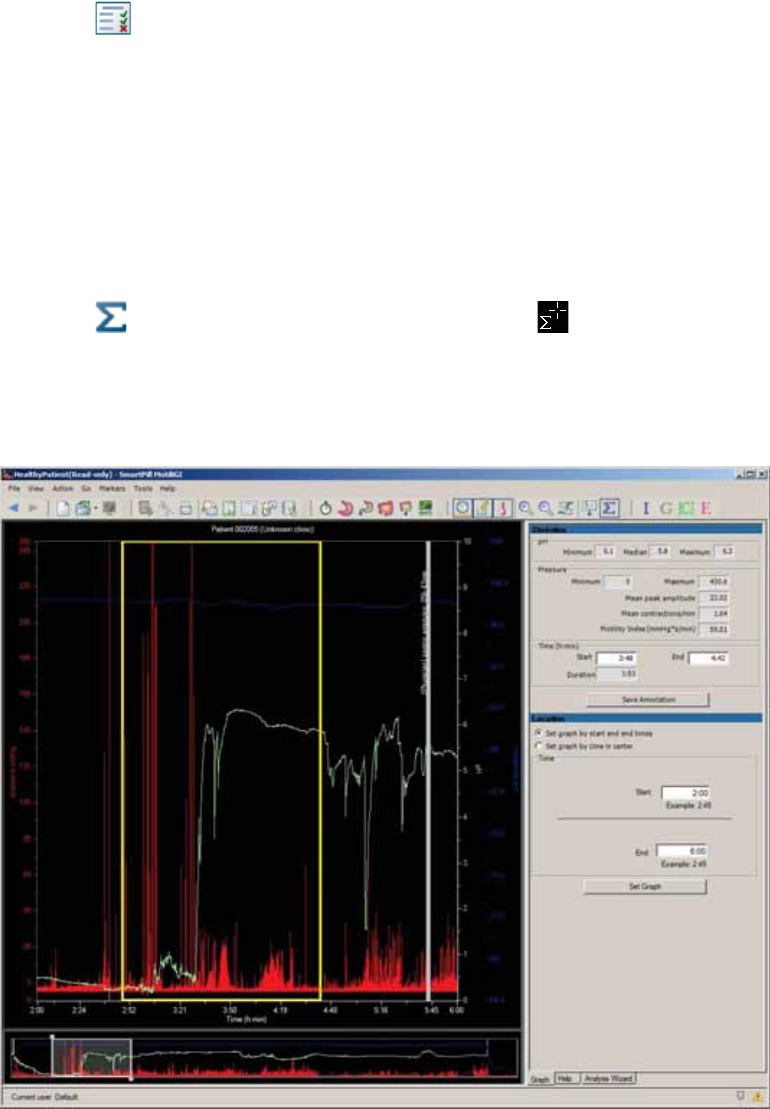
Appendix: Electrical Safety
Viewing Transit Times
xClick on the toolbar.
xSelect View > Test Summary
The Test Summary screen appears. Transit times computed using the physician-
defined markers appear on the left and MotiliGI-computed markers appear on the
right.
Calculating Statistics
To enter the Statistics mode:
1. Click on the toolbar. The cursor changes to .
2. Click and drag to select any area of the graph.
A yellow box appears around the selected area and creates a range. The Statistics
panel displays pressure, pH and temperature statistics for the range.
To save the range as an annotation on the graph:
1. On the statistics panel, click Save Annotation. The Range Annotation Editor
screen appears.
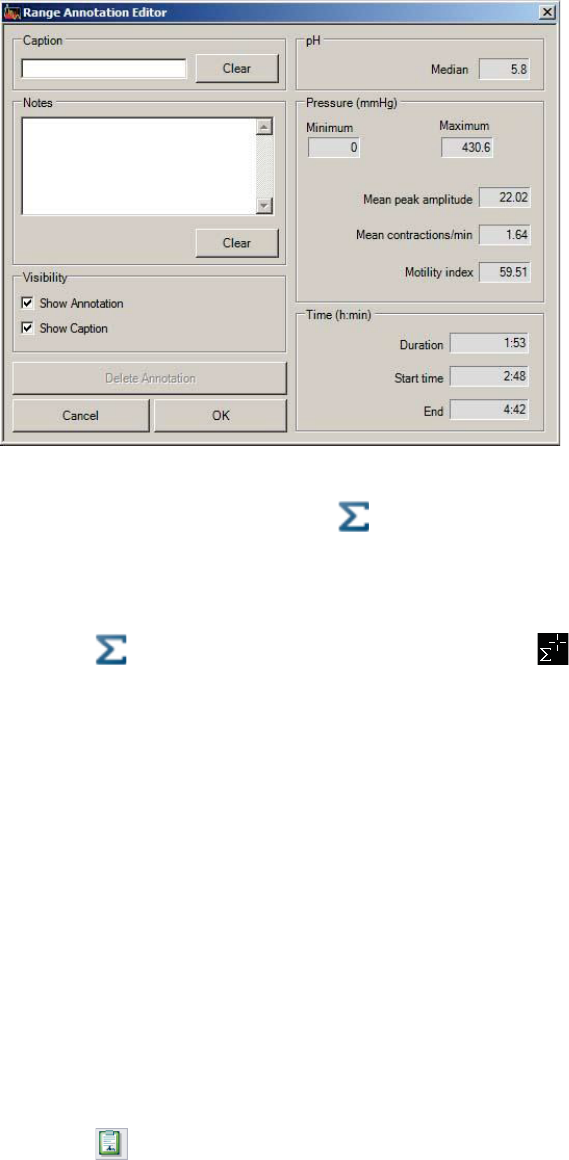
Appendix: Electrical Safety
x(Optional) You can enter text in the Caption and Notes boxes.
xYou can click the checkboxes to show or hide the annotation and the caption.
2. Click OK.
You can create and save multiple range annotations.
►To exit the Statistics mode, Click on the toolbar. The cursor returns to normal.
To edit a range annotation:
1. Click on the toolbar. The cursor changes to .
2. Double-click the border of a range. The Range Annotation Editor screen appears.
3. Edit the annotation.
4. Click OK.
To view the statistics of a range annotation:
Hold the cursor over the yellow border of the range. The statistics appear in the Data
Panel - Statistics.
Creating Output
Generating a Report
1. You can either:
xClick on the toolbar, or

Appendix: Electrical Safety
xSelect Action > Generate Report
The report opens in Microsoft WordPad. MotiliGI immediately saves the report to
the patient’s folder using a default name.
Caution
Edits made to the WordPad report will not be saved to the MotiliGI test.
To save the report:
1. Select File > Save As.
2. Type in a new name in File Name.
3. Click Save.
Saving the Report as a PDF File
1. In WordPad, select File > Print. The Windows Print screen appears.
2. In the Printer Name drop down menu, Select PDFill PDF Editor.
3. Click Print. The Save PDF File As screen appears.
4. Enter a filename
5. Click OK.
Printing the Graph
You can use Print Preview to check the graph before you print it. To preview, either
xSelect File > Print Preview, or
xClick on the toolbar.
To print the graph, either:
xSelect File > Print…
xClick on the toolbar. The Windows Print screen appears. Follow the on-
screen instructions.
Exporting Data to Excel
You can export test data (pH, pressure and temperature) to a text file in comma-
separated value format. You can export data from the current graph view or from the
entire test.
To Export the Current Graph View
1. Select File > Export > Test Data Snapshot. A Save As screen appears.
xMotiliGI creates a folder that contains separate CSV files for pH, pressure and
temperature.
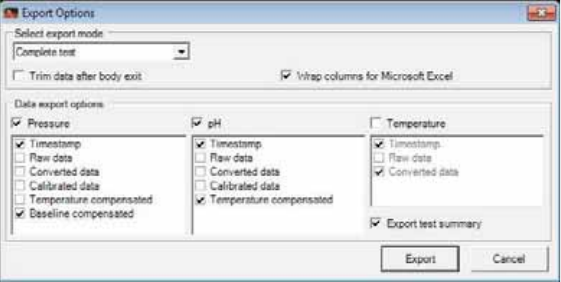
Appendix: Electrical Safety
2. Name the folder.
3. Click Save.
To Export the Entire Test
1. Select File > Export > Complete Test Data. A Save As screen appears
xMotiliGI creates a folder that contains separate CSV files for pH, pressure and
temperature.
2. Name the folder.
3. Click Save.
To Export Individual Data Sets
1. Select File > Export > Customize Export. The Export Options screen appears.
2. Select the desired preferences.
xYou can select to export data sets from the complete test or current view.
xCheck Trim After BET if you do not want to export data after body exit.
xCheck Wrap Columns for Microsoft Excel. This feature wraps data sets larger
than 32000 rows into adjacent columns.
xUnder Data Export Options, select one or more data sets to export.
xUnder each data set, select one or more parameters to export.
xCheck Export Test Summary to export the transit and descriptive data from the
Test Summary Screen.
3. Click Export.
Data Parameter Definitions
xTimestamps are in milliseconds starting at capsule calibration.
xRaw Data is in millivolts.
xConverted Data is raw data multiplied by the sensor’s scale factor.
Appendix: Electrical Safety
Data Set
Units
pH
pH units
Pressure
mmHg
Temperature
°C
xCalibrated Data is converted data adjusted by the calibration point (pH 6,
atmospheric pressure, room temperature).
xTemperature Compensated is the calibrated data adjusted for sensor drift due to
increased temperature.
xBaseline Compensated is temperature compensated data with the baseline adjusted
to 0.
Exporting Images to JPEG or GIF
You can export graphs to an image file in JPEG or GIF format. You can export
images from the current graph view or from the entire test.
To export the image of the current graph view:
1. Select File > Export> Graph Image Snapshot. A Save As screen appears.
2. Name the file.
3. Select file type from the dropdown box.
4. Click Save.
To export the image of the entire test:
1. Select File > Export> Complete Graph Image. A Save As screen appears.
2. Name the file.
3. Select file type from the dropdown box.
4. Click Save.
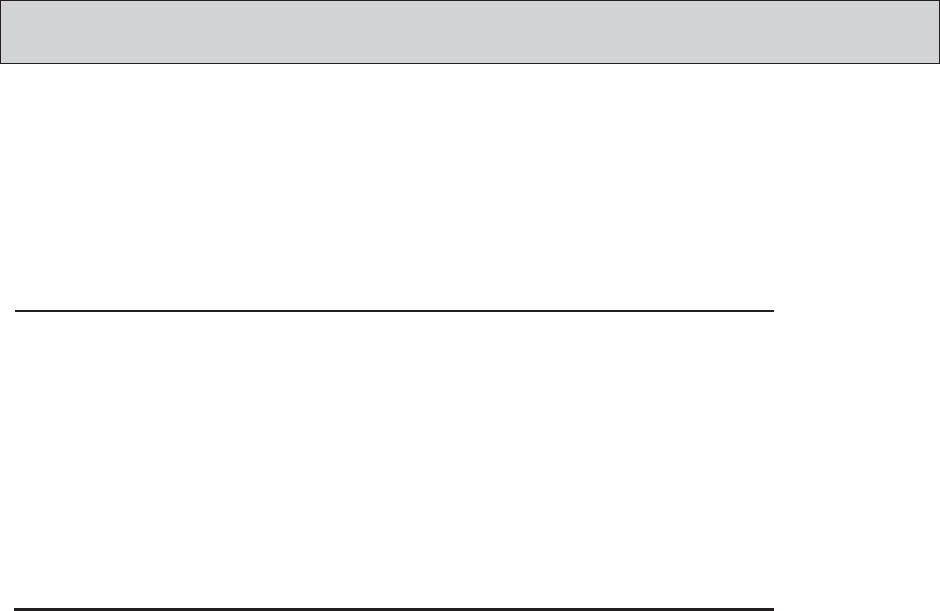
Appendix: Electrical Safety
6\VWHP$GPLQLVWUDWRU7RROV
Password Management
Logging into the System Computer
For security purposes, after 5 unsuccessful attempts to logon, the user will not be
allowed to logon for 15 minutes
Enter the Windows user name and password noted below.
User Name
Password
Privileges
sgims1
12345
Can operate the computer and save documents.
C
an install programs, printers or change
system settings by
using MotiliGI Administrator.
x
To change the user name or password, contact
Technical Support for assistance.
Administrator
Password
supplied by
Technical
Support
Used in emergency situations.
Can install programs, printers or change system settings.
Windows Administrative Logon (IT Administrator use only)
Under normal circumstances, use of this account should not be necessary.
To log in as the Windows administrator:
1. Turn on the SmartPill System Computer
2. Log into the Windows sgims1 account (see system computer above).
3. Go to the Windows Start Menu > SmartPill> MotiliGI Administrator.
4. Enter the MotiliGI Administrator password and click Login.
5. Click Retrieve Key
6. Upon retrieving the key, contact Technical Support to obtain an administrative
password that can only be used once.
After obtaining the password:
7. Go to Start>Log Off
8. Log into Windows. Enter “Administrator” into the username field and the
password provided by SmartPill into the password field.
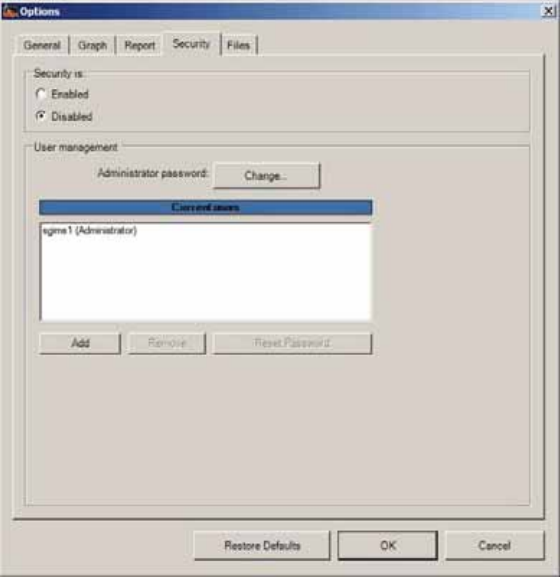
Appendix: Electrical Safety
Logging into MotiliGI
When using MotiliGI for the first time, an administrator account is set up. The
administrator has privileges to set up additional users and, edit user names and
passwords.
Forgotten Password
If a user has forgotten his or her password, the administrator must log into MotiliGI
and reset the user’s password. If the administrator has forgotten his or her username
or password, they must call Technical Support.
xThe Reset Password option resets the selected user’s password to 12345.
MotiliGI Security
Security Tab
You must be logged into MotiliGI as the administrator to view the security tab.
To log in as the administrator:
1. Go to Tools > Login In As…
2. Type in the administrator username and password.
3. Click Log In.
To change the security settings:
►Go to Tools > Options > Security

Appendix: Electrical Safety
Security enabled is the default setting and requires users to log in with their username
and password. Security disabled eliminates the login (user authentication) step. The
user will be logged into the default user account.
Recommendation: Assign a unique username and password to each MotiliGI user.
The username is used as the Technician Name in the printed report.
Adding a User
1. Go to Tools > Options > Security.
2. Under User Management click Add.
3. Under Add User, enter a user name and password (both require a minimum of 5
alpha-numeric characters).
4. Click OK.
The new user will appear in the Current Users box.
Removing a User
►Select the username from the list of current users and click “Remove.” To change a username,
select the user from the list of current users, click “Remove” and then add a new user.
Backing Up Patient Files
1. Verify that MotiliGI is closed (not running).
2. Insert a blank CD into the CD Drive or insert a USB drive into a USB port.
3. Double-click on the Tests icon on the Desktop.
4. Right-click on the patient record folder, click Send To > Compressed (zipped)
Folder.
5. Right-click on the Patient_xxxx.zip file you just created, where xxxx is the patient’s
ID. Click Send To > DVD/CD-RW Drive (D:) or Removable Disk (E:).
6. Click on the toolbar.
7. Right-click on the Log folder, click Send To > Compressed (zipped) Folder.
8. Right-click on the Log.zip file you just created and click Send To > DVD/CD-RW
Drive (D:) or Removable Disk (E:).
If you are writing to a CD:
a. Click on the balloon You have files waiting to be written to the CD.
b. Under the CD Writing Tasks heading, click Write these files to CD.
c. Confirm that the files have been written to the CD by opening the DVD/CD-RW Drive (D:)
in Windows Explorer.
If you are writing to a USB drive:

Appendix: Electrical Safety
a. Confirm the files have been saved by opening the Removable Disk (E:) in Windows
Explorer.
b. Click located on the right side of the Start bar. Select Safely remove the Removable Disk
(E:).
Installing Software
To install third-party software, log into the system computer as the system computer
administrator – or –utilize SmartPill’s MotiliGI Administrator application.
Caution
Exercise caution installing third-party software (e.g. applications, drivers) on the system computer. Installing
third-party software not listed below may increase the potential for software conflicts with MotiliGI.
SmartPill Approved Third
-Party Software
Microsoft Excel
Microsoft Word
Adobe Acrobat Reader
PDFill PDF Editor
Printer drivers
Windows
Accessories
MotiliGI Administrator
MotiliGI Administrator serves two purposes:
xIt provides the means to install a limited set of software.
xIt provides access to the administrative account.
Launching MotiliGI Administrator
1. Turn on the system computer
2. Log into the system computer using the sgims1 account.
3. Go to the Start Menu > SmartPill> MotiliGI Administrator
4. Create a password.
►The password is case sensitive, must be at least five characters long, and must be confirmed. The
MotiliGI Administrator password is independent of MotiliGI and must be entered for each
subsequent running of MotiliGI Administrator.
5. Retype the password and click Login.
Figure 14
After logging in, the MotiliGI Administrator screen will appear.

Appendix: Electrical Safety
Figure 15
Installing Software from a CD
1. Place the CD in the CD-ROM drive and click Update from CD. The contents of
the CD will be displayed in the center box of the main screen.
2. Double click on the file you wish to install.
Installing Software from a File on the System Computer Hard Drive
1. Locate the file you wish to install.
2. Move the file into C:\Program Files\SmartPill\ MotiliGI
Administrator\Update\ Directory
3. Click Update from Folder. The contents of the update directory will be displayed
in the center box of the screen.
Figure 16
4. Double click on the file you wish to install.

Appendix: Electrical Safety
Uninstalling MotiliGI
Caution
If you find it necessary to uninstall MotiliGI, ensure you have backed up and/or saved patient test
files, exported data files and reports (except those that have been saved as PDFs) to another location
on your hard drive or to external media (e.g., auxiliary or external hard drive, CD).
You must be logged into the system computer as the Windows administrator to
uninstall MotiliGI.
1. Backup any data files you have created.
2. Close MotiliGI.
3. Log into the computer as the Windows administrator.
4. Go to the Windows Start Menu > Control Panel > Add/Remove Programs.
5. Locate and select MotiliGI in the programs list, click Add/Remove.
6. Select Yes to confirm removal of MotiliGI.
7. Delete the folder C:\Program Files\SmartPill\ MotiliGI
Administrator\Update\ Directory
Installing MotiliGI
Use MotiliGI Administrator to reinstall or upgrade MotiliGI from a CD.
1. Reboot the system computer and log into the sgims1 Windows account. You must
reboot the computer immediately prior to upgrading MotiliGI to avoid any
problems.
2. Go to Start > All Programs > SmartPill folder > MotiliGI Administrator.
3. Enter the password and click Login.
4. Insert the MotiliGI CD into the CD-ROM drive.
5. Click Update from CD.
Appendix: Electrical Safety
Figure 17
6. Double-click on the MotiliGI folder.
7. Double-click on Setup.exe. Follow all instructions in the installation wizard.
8. When the wizard finishes, close the wizard and close MotiliGI Administrator.
9. Reboot the PC.
If you are installing MotiliGI on a computer other than your system computer (for
reading test data only), you will need to install Adobe Acrobat Reader 7.0 or greater for
MotiliGI to run properly. MotiliGI is only compatible with Windows XP and
Windows 7 32-bit operating systems. MotiliGI is not warranted for use with any
other operating system.
MotiliGI relies on the Microsoft .NET framework version 1.1. If you are installing
MotiliGI on another computer to view test files, run the Windows Update feature of
the Microsoft website (http://update.microsoft.com/windowsupdate) to check for
and install any critical updates to the Microsoft .NET framework before completing
the installation.
The first time the data receiver is connected to the system computer, Windows XP will
acknowledge it has found a new USB device and will load the drivers necessary for it
to communicate with the data receiver. You may see a dialog box pop up asking for
the Windows Administrator username and password. Do not take action – MotiliGI
administrator will automatically fill in the required information after a few seconds and
the installation will continue.

Appendix: Electrical Safety
$SSHQGL[(OHFWULFDO6DIHW\
External equipment intended for connection to signal input, signal output or other
connectors shall comply with relevant IEC standard (e.g. IEC 60950 for IT equipment
and the IEC 60601 series for medical electrical equipment). In addition, all such
combinations – systems – shall comply with the standard IEC 60601-1-1, Safety
requirements for medical electrical systems. Equipment not complying with IEC
60601-1 shall be kept outside the patient environment, as defined in the standard.4Any
person who connects external equipment to signal input, signal output or other
connectors has formed a system and is therefore responsible for the system to comply
with the requirements of IEC 60601-1-1. If in doubt, contact a qualified biomedical
engineer or Given Imaging.
The following tables outline the SmartPill GI Monitoring System’s compliance with
INTERNATIONAL STANDARD, International Electrotechnical Commission (IEC)
IEC 60601-1-2, Medical electrical equipment – Part 1-2: General requirements for
safety – Collateral standard: Electromagnetic compatibility – Requirements and tests.
*XLGDQFHDQGPDQXIDFWXUHU¶VGHFODUDWLRQ± electromagnetic emissions
The SmartPill GI Monitoring System is intended for use in the electromagnetic environment specified
below. The customer or the user of the SmartPill GI Monitoring System assures that it is used in such
an environment.
Emissions test
Compliance
Elec
tromagnetic environment – guidance
RF emissions
CISPR 11
Group 1
The SmartPill GI Monitoring System uses RF energy exclusively
for its internal function. Thus the RF emission is very low and it
is unlikely that nearby electronic devices would be disturbed.
RF emissions
CISPR 11
Class B
The SmartPill GI Monitoring System is suitable for use in all
establishments, including domestic establishments and those
directly connected to the public low
-voltage power supply
network that supplies buildings used for d
omestic purposes.
Harmonic
emissions
IEC 61000-3-2
IEC 61000-3-2
Voltage
fluctuations/flicker
emissions
IEC 61000-3-3
Complies
4 The normal distance is at least 1.5 m from the patient or the patient support.

*XLGDQFHDQGPDQXIDFWXUHU¶VGHFODUDWLRQ± electromagnetic immunity
The SmartPill GI Monitoring System is intended for use in the electromagnetic environment specified below.
The customer or the user of the SmartPill GI Monitoring System should assure that it is used in such an
environment.
Immunity test
IEC 60601 test level
Compliance level
Electromagnetic environment
– guidance
Electrostatic
discharge (ESD)
IEC 61000
-4-2
±6 kV contact
±8 kV air
±6 kV contact
±8 kV air
Floors should be wood, concrete or ceramic
tile. If floors are covered with synthetic
material, the relative humidity should be at
least 30%.
Electrical fast
transient/burst
IEC 61000
-4-4
±2 kV for power
supply lines
±1 kV for
input/output lines
±2 kV on AC
mains
±1 kV on
measurement I/O
lines
Mains power quality should be that of a
typical commercial or hospital environment.
Surge
IEC 61000
-4-5
±1 kV line(s) to line(s)
±2 kV line(s) to earth
±1 kV on Line to
Neutral
±2 kV on Line or
Neutral to
protected earth
Mains power quality should be that of a
typical commercial or hospital environment.
Voltage dips,
short
interruptions and
voltage variations
on power supply
input lines
IEC 61000
-4-11
<5 % U
T
(>95 % dip
in U
T) for 0.5 cycle
40 % U
T
(60 % dip in
U
T) for 5 cycles
70 % U
T
(30 % dip in
U
T) for 25 cycles
<5 % U
T
(>95 % dip
in U
T
) for 5 sec
N/A
N/A
Power frequency
(50/60 Hz)
magnetic field
IEC 61000-4-8
3 A/m
3 A/m @ 50 Hz
and 60 Hz
Power frequency magnetic fields should be at
levels characteristic of a typical location in a
typical commercial or hospital environment.
Note U
T
is the a.c. mains voltage prior to application of the test level.
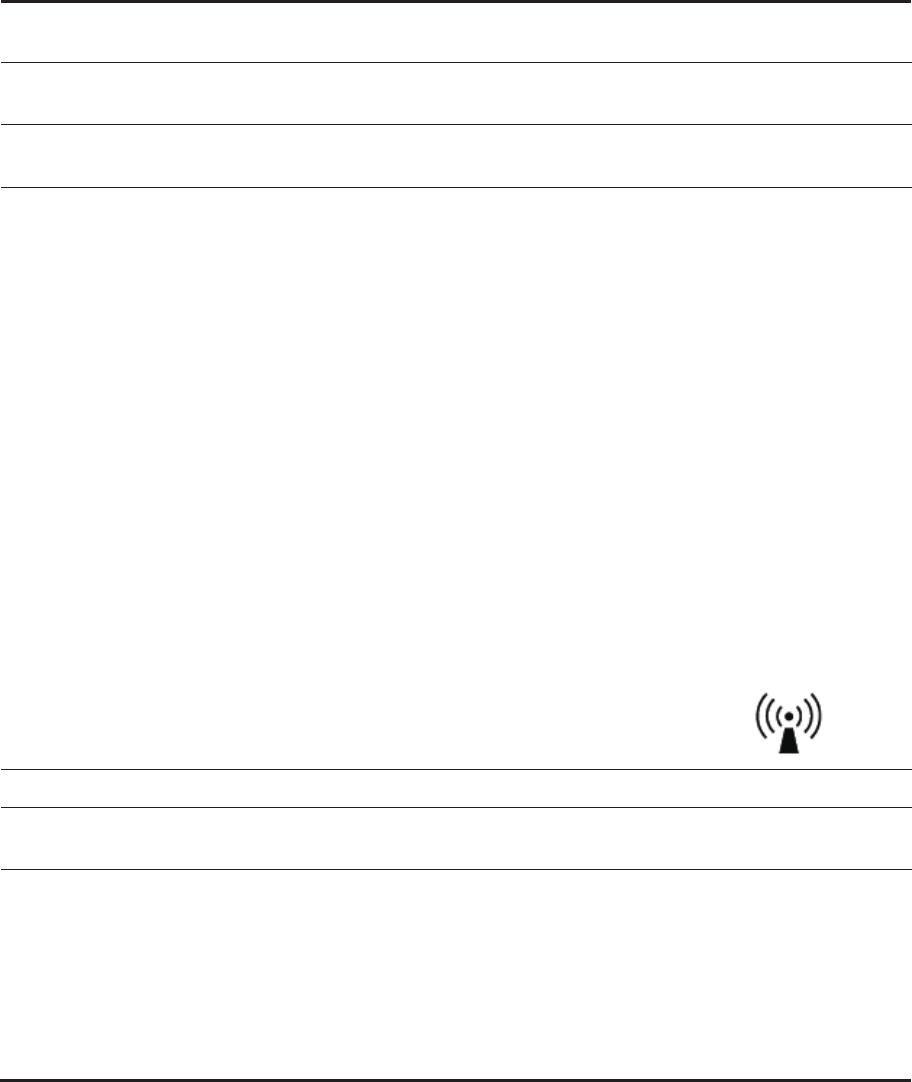
GuidancHDQGPDQXIDFWXUHU¶VGHFODUDWLRQ± electromagnetic immunity
The SmartPill GI Monitoring System is intended for use in the electromagnetic environment specified below.
The user of the SmartPill GI Monitoring System should assure that it is used in such an environment.
Immunity
test
IEC 60601
test level
Compliance
level
Electromagnetic environment
– guidance
Conducted
RF
IEC 61000-
4
-6
Radiated
RF
IEC 61000-
4
-3
3 Vrms
150 kHz to
80 MHz
3 V/m
80 MHz to
2.5 GHz
3 V
3 V/m
Portable and mobile RF communications equipment should be
used no closer to any part of the SmartPill GI Monitoring System,
including cables, than the recommended separation distance
calculated from the equation applicable to the frequency of the
transmitter.
Recommended sepa
ration distance
d = 1.17 √P
d = 1.17 √P 80 MHz to 800 MHz
d = 2.33 √P 800 MHz to 2.5 GHz
where P is the maximum output power rating of the transmitter in
watts (W) according to the transmitter manufacturer and d is the
recommended separation distance in meters (
m).
Field strengths from fixed RF transmitters, as determined by an
electromagnetic site survey.
(a) should be less than the compliance level in
each frequency range.
(b) Interference may occur in the vicinity of
equipment marked with the following symbol:
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reflection from structures, objects and people.
a Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and
land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted
theoretically with accuracy. To assess the electromagnetic environment
due to fixed RF transmitters, an
electromagnetic site survey should be considered. If the measured field strength in the location in which the
SmartPill GI Monitoring System is used exceeds the applicable RF compliance level above, the SmartPill GI
Monito
ring System should be observed to verify normal operation. If abnormal performance is observed,
additional measures may be necessary, such as reorienting or relocating the SmartPill GI Monitoring System.
b Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.

Recommended separation distances between portable and mobile RF
communications equipment and the SmartPill GI Monitoring System
The SmartPill GI Monitoring System is intended for use in an electromagnetic
environment in which radiated RF disturbances are controlled. The customer or the
user of the SmartPill GI Monitoring System can help prevent electromagnetic
interference by maintaining a minimum distance between portable and mobile RF
communications equipment (transmitters) and the SmartPill GI Monitoring System as
recommended below, according to the maximum output power of the
communications equipment.
Rated maximum output
power of transmitter
W
Separation distance according to frequency of transmitter m
150 kHz to 80 MHz
d = 1.17 √P
80 MHz to 800
MHz
d = 1.17 √P
800 MHz to 2,5
GHz
d = 2.33 √P
0,01
0.12
0.12
0.23
0,1
0.37
0.37
0.74
1
1.17
1.17
2.33
10
3.69
3.69
7.38
100
11.67
11.67
23.33
For transmitters rated at a maximum output power not listed above, the
recommended separation distance
d in meters (m) can be estimated using the
equation applicable to the frequency of the transmitter, where
P is the maximum
output power rating of the transmitter in watts (W) according to the transmitter
manufact
urer.
NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency
range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic
propagation is affected by absorption and reflection from structures, objects and
p
eople.

Device Classifications
SmartPill pH.p Capsule
SmartPill Data Receiver
SmartPill Docking Station
Power:
Internally Powered
Equipment
Internally Powered Equipment
Class I Equipment
Electric Shock
Protection:
Type BF Applied Part
Type BF Applied Part
Ordinary Equipment
Harmful Ingress
Water:
Watertight Equipment
(IP57)
Ordinary Equipment
Ordinary Equipment
Equipment not suitable for
use in the presence of
FLAMMABLE
ANAESTHETIC
MIXTURE WITH AIR or
WITH OXYGEN OR
NITROUS OXIDE. Not
for use with o
xygen or
oxygen enriched
atmospheres.
Equipment not suitable for use
in the presence of
FLAMMABLE
ANAESTHETIC MIXTURE
WITH AIR or WITH
OXYGEN OR NITROUS
OXIDE. Not for use with
oxygen or oxygen enriched
atmospheres.
Equipment not suitable for use
in the pr
esence of
FLAMMABLE
ANAESTHETIC MIXTURE
WITH AIR or WITH
OXYGEN OR NITROUS
OXIDE. Not for use with
oxygen or oxygen enriched
atmospheres.
Mode of
Operation:
Intermittent operation
Continuous Operation
Continuous Operation
Connectivity Restrictions
External equipment intended for connection to signal input, signal output or other
connectors shall comply with IEC 60601 series for medical electrical equipment. In
addition, all such combinations – systems – shall comply with the standard IEC 60601-
1-1, Safety requirements for medical electrical systems. Equipment not complying with
IEC 60601-1 shall be kept outside the patient environment, as defined in the
standard.5
Any person who connects external equipment to signal input, signal output or other
connectors has formed a system and is responsible for the system to comply with the
requirements of IEC 60601-1-1. If in doubt, contact a qualified biomedical
professional or the SmartPill Corporation.
Index
5 The normal distance is at least 1.5 m from the patient or the patient support.
Accessories, 6
Activation Fixture, 5
Adverse Events, 13
Body Exit, 63, 82
Capsule
Activation, 32
Confirming Exit, 45
Deactivating, 41
Low Voltage, 62
Operational Specifications, 2
Pack, 2
Power Requirements, 11
Tray Orientation, 36
Capsule Ingestion, 50
Chronic constipation, 9
Colonic transit time, 9
Combined small and large bowel transit
time, 9
Contraindications, 10
CTT. See Colonic transit time
Data Display. See Data Receiver
Display
Data Receiver, 3
Backlight Control Button, 3
Belt Clip and Lanyard, 3
Care, Cleaning and Maintenance, 14
Charging, 21
Display, 12
Event Button, 3
Patient Diary, 3
Power Requirements, 11
Device Markings, 11
Docking Station, 4
Care, Cleaning and Maintenance, 15
Connecting to the System Computer,
18
LED Light, 4
Power Connection, 4
Power Requirements, 10
USB Connector, 4
Exporting Data, 106
Gastric Emptying, 55, 81
Gastric emptying time, 9
Gastroparesis, 9
GET. See Gastric emptying time
Graph Display, 96
ICJ. See Ileo-Cecal Junction
Ileo-Cecal Junction, 60,
Ingestion, 80
Installing Software, 112
Intended Use/Indications for Use, 9
Letterhead
Creating, 93
Live Monitoring, 40
Medications, 22
MotiliGI
Exiting, 90
Installing, 114
Keyboard Shortcuts, 88
Security, 110
Set up, 17
Uninstalling, 114
User Interface, 85
MotiliGI Administrator, 112
MotiliGI Software, 5
Non-Passage, 13
Patient Files
Backing Up, 111
Patient Information
Editing, 95
Patient Preparation, 22
Patient-Contacting Materials, 14
Power Requirements, 10
Recycling and Disposal Instructions, 11
Report
Generating, 105
Risks and Safety, 13
SBTT. See Small bowel transit time
SLBTT. See Combined small and large
bowel transit time
Small bowel transit time, 9
SmartBar, 2, 23
Statistics
Calculating, 103
Storage, 10
System Computer, 5
Date, Time, Language and Regional
Options, 17
Logging in, 109
Power Requirements, 10
Requirements, 6
Set up, 17
Templates
Creating, 30
Using, 30
Test
Analyzing, 49
Downloading, 43
Test Analysis Wizard, 47
Test Initiation Wizard, 27
Test Summary Reports, 76
Troubleshooting and Support, 15
User Preferences, 90
WGTT. See Whole gut transit time
Whole gut transit time, 9
Windows Administrative Logon, 109

Contacting Given Imaging
USA & Headquarters of the Americas
Given Imaging, Inc.
3950 Shackleford Road, Suite 500
Duluth, GA 30096, USA
Phone: 770-662-00870
Toll-Free: 1-800-GIVENGI
Fax: 770-662-0510
USA: supportUS@givenimaging.com
Latin America: supportLA@givenimaging.com
Germany & European Headquarters
Authorized Representative in the EU
Given Imaging GmbH
Borsteler Chaussee 47
Hamburg 22453
Germany
Phone: +49 40 513 3000
Fax: +49 40 4606 9611
supportEU@givenimaging.com
Canada
Given Imaging, Inc. - Canadian Office
2425 Matheson Blvd. E., 8th Floor
Mississauga, ON L4W 5K4
Canada
Phone: 905 361 2830
Toll-Free: 1866 98 GIVEN
Fax: 1 800 786 1967
supportCA@givenimaging.com
France
Given Imaging France S.A.S.
22, Rue Guynemer
78600 Maison-Laffitte
Paris, France
Phone: +33 (0) 1 34 93 80 00
Fax: +33 (0) 1 34 93 80 11
supportFR@givenimaging.com
Australia & New Zealand
Given Imaging Pty Limited
Unit 6A, The Park
5 Talavera Road
North Ryde, NSW 2113, Australia
Phone: +61 2 9889 3944
Fax: +61 2 9889 3955
supportAU@givenimaging.com
Asia
Given Imaging (Asia) Company Limited
27/F. Unit A, Cheuk Nang Plaza
250 Hennessy Road, Wanchai
Hong Kong
Phone: +852 2989 0888
Fax: +852 2989 0899
supportAP@givenimaging.com
Japan
Given Imaging K.K.
2F KDX Kojimachi Bldg.
3-3 Kojimachi, Chiyoda-k
Tokyo 102-0083
Japan
Phone: + 81-3-5214-0588
supportJP@givenimaging.com

This manual and the compilation of the content in this manual, including but not
limited to text, graphics, logos and images, is the exclusive property of Given Imaging
and is protected by United States and international copyright laws. This manual may
not be transferred in any form without permission of Given Imaging.
Copyright © 2006-2013 Given Imaging.
All Rights Reserved.
The SmartPill logo, “The Measure of GI Health” and “MotiliGI,” are trademarks of
Given Imaging. Such marks are protected by United States common law, federal
and/or international trademark laws and may not be used in violation of Given
Imaging’s rights.
Given Imaging
3950 Shackleford Road
Duluth, GA 30096 USA
www.givenimaging.com