PainTechnology Medicare Face Notice Bulletin Number User Manual
2013-06-25
User Manual: PainTechnology Medicare Face Notice
Open the PDF directly: View PDF ![]() .
.
Page Count: 11

Disclaimer
This article was prepared as a service to the public and is not intended to grant rights or impose obligations. This article may contain references or links to
statutes, regulations, or other policy materials. The information provided is only intended to be a general summary. It is not intended to take the place of
either the written law or regulations. We encourage readers to review the specific statutes, regulations and other interpretive materials for a full and
accurate statement of their contents. CPT only copyright 2012 American Medical Association.
Page 1 of 11
DEPARTMENT OF HEALTH AND HUMAN SERVICES DEPARTMENT OF HEALTH AND HUMAN SERVICES
Centers for Medicare & Medicaid Services Centers for Medicare & Medicaid Services
REVISED products from the Medicare Learning Network® (MLN)
• “The Durable Medical Equipment, Prosthetics, Orthotics, and Supplies (DMEPOS)
Competitive Bidding Program: Traveling Beneficiary,” Fact Sheet, ICN 904484,
Downloadable only.
MLN Matters® Number: MM8304 Related Change Request (CR) #: CR 8304
Related CR Release Date: May 31, 2013 Effective Date: July 1, 2013
Related CR Transmittal #: R468PI Implementation Date: July 1, 2013
Detailed Written Orders and Face-to-Face Encounters
Provider Types Affected
This MLN Matters® Article is intended for physicians, Physician Assistants (PAs), Nurse Practitioners
(NPs), Clinical Nurse Specialists (CNSs) and suppliers submitting claims to Durable Medical
Equipment Medicare Administrative Contractors (DME MACs) for certain Durable Medical Equipment
(DME) items and services provided to Medicare beneficiaries.
What You Need to Know
This article is based on Change Request (CR) 8304, which instructs DME MACs to implement
requirements, which are effective July 1, 2013, for detailed written orders for face-to-face encounters
conducted by the physician, PA, NP or CNS for certain DME items as defined in 42 CFR 410.38(g).
(That section is available at http://www.gpo.gov/fdsys/pkg/CFR-2011-title42-vol2/pdf/CFR-2011-
title42-vol2-sec410-38.pdf on the Internet.) When a claim for these items is selected for review,

MLN Matters® Number: MM8304 Related Change Request Number: 8304
Disclaimer
This article was prepared as a service to the public and is not intended to grant rights or impose obligations. This article may contain references or links to statutes,
regulations, or other policy materials. The information provided is only intended to be a general summary. It is not intended to take the place of either the written law
or regulations. We encourage readers to review the specific statutes, regulations and other interpretive materials for a full and accurate statement of their contents.
CPT only copyright 2012 American Medical Association.
Page 2 of 11
contractors must deny the claim if the requirements for a face-to-face encounter are not met.
Make
sure that your billing staffs are aware of these requirements.
Background
As a condition for payment, Section 6407 of the Affordable Care Act requires a physician to document
that the physician, PA, NP or CNS has had a face-to-face encounter examination with a beneficiary in
the six (6) months prior to the written order for certain items of DME (the complete list of items is found
in Appendix A at the end of this article). This section does not apply to Power Mobility Devices (PMDs)
as these items are covered under a separate requirement.
This includes encounters conducted via the Centers for Medicare & Medicaid Services (CMS)-
approved use of telehealth (as described in Chapter 15 of the "Medicare Benefit Policy Manual" and
Chapter 12 of the "Medicare Claims Processing Manual"). Those manuals are available at
http://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Internet-Only-Manuals-IOMs.html on
the CMS website.
Note that the date of the written order must not be prior to the date of the face-to-face encounter.
The face-to-face encounter conducted by the physician, PA, NP, or CNS must document that the
beneficiary was evaluated and/or treated for a condition that supports the item(s) of DME ordered.
In the case of a DME ordered by a PA, NP, or CNS, a physician (MD or DO) must document the
occurrence of a face-to-face encounter by signing/co-signing and dating the pertinent portion of the
medical record.
The written order for the DME must include, at a minimum;
1. the beneficiary's name,
2. the item of DME ordered,
3. the prescribing practitioner's National Provider Identifier (NPI),
4. the signature of the ordering practitioner and
5. the date of the order.
Failure to meet any of the above requirements will result in denial of the claim.
Physicians will be provided an additional payment, using code G0454, for signing/co-signing the face-
to-face encounter of the PA/NP/CNS. The physician should not bill the G code when he/she conducts
the face-to-face encounter. Note that the G code may only be paid to the physician one time per
beneficiary per encounter, regardless of the number of covered items documented in the face-to-face
encounter.
CR8304 implements these changes in Chapter 5 of the "Program Integrity Manual" to support 42
Code of Federal Regulations (CFR) 410.38(g) and the revised portion of that manual is attached to
CR8304.

MLN Matters® Number: MM8304 Related Change Request Number: 8304
Page 3 of 11
Additional Information
The official instruction, CR8304, issued to your DME MAC regarding this change, may be viewed at
http://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/Downloads/R468PI.pdf
on the CMS website.
If you have any questions, please contact your DME MAC at their toll-free number, which may be
found at http://www.cms.gov/Research-Statistics-Data-and-Systems/Monitoring-
Programs/provider-compliance-interactive-map/index.html on the CMS website.
Disclaimer
This article was prepared as a service to the public and is not intended to grant rights or impose obligations. This article may contain references or links to statutes,
regulations, or other policy materials. The information provided is only intended to be a general summary. It is not intended to take the place of either the written law
or regulations. We encourage readers to review the specific statutes, regulations and other interpretive materials for a full and accurate statement of their contents.
CPT only copyright 2012 American Medical Association.
MLN Matters® Number: MM8304 Related Change Request Number: 8304
Disclaimer
This article was prepared as a service to the public and is not intended to grant rights or impose obligations. This article may contain references or links to statutes,
regulations, or other policy materials. The information provided is only intended to be a general summary. It is not intended to take the place of either the written law
or regulations. We encourage readers to review the specific statutes, regulations and other interpretive materials for a full and accurate statement of their contents.
CPT only copyright 2012 American Medical Association.
Page 4 of 11
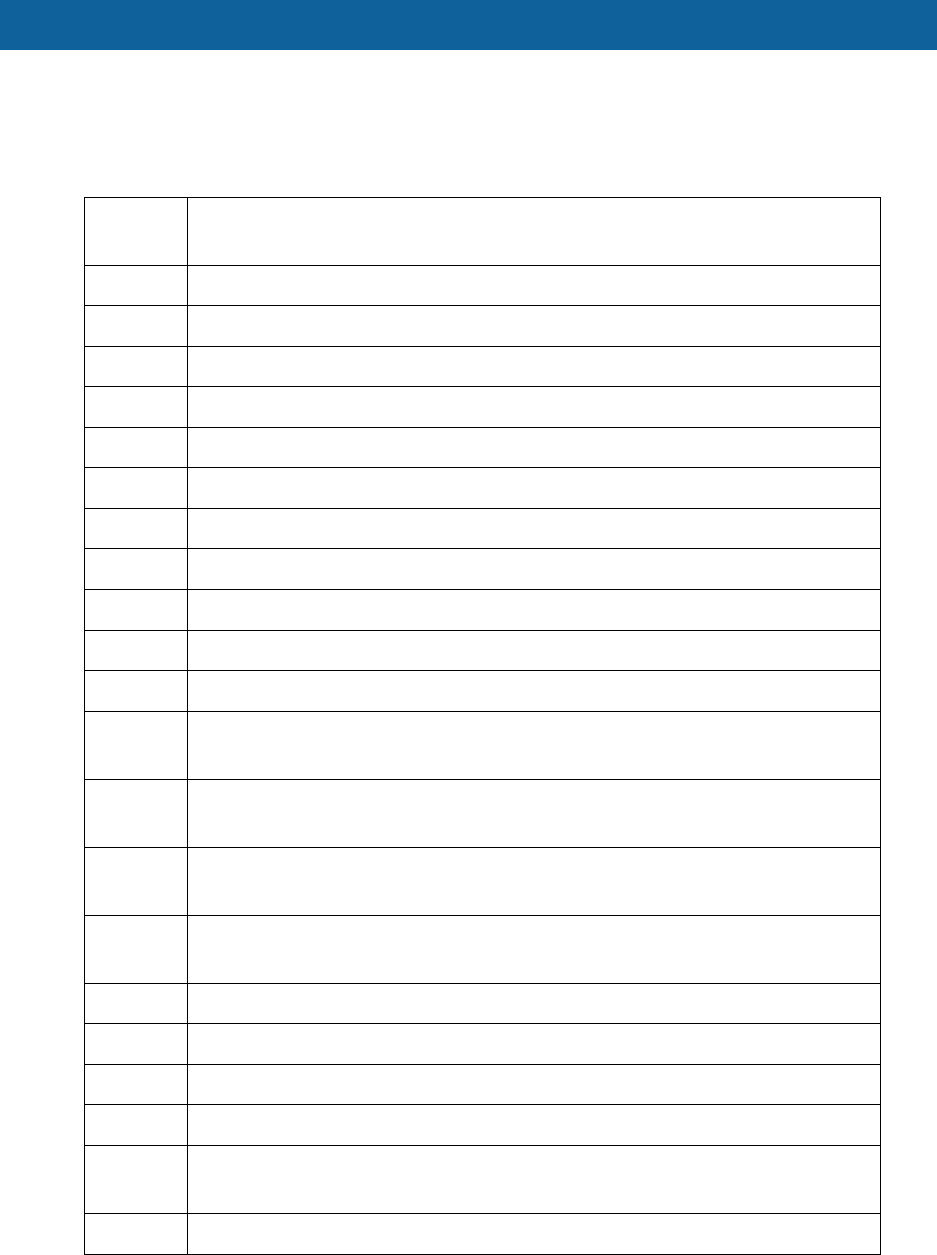
Appendix A
The DME list of Specified Covered Items are as follows, the original list was at 77 FR 44798:
HCPCS
Code Description
E0185 Gel or gel-like pressure mattress pad
E0188 Synthetic sheepskin pad
E0189 Lamb's wool sheepskin pad
E0194 Air fluidized bed
E0197 Air pressure pad for mattress standard length and width
E0198 Water pressure pad for mattress standard length and width
E0199 Dry pressure pad for mattress standard length and width
E0250 Hospital bed fixed height with any type of side rails, mattress
E0251 Hospital bed fixed height with any type side rails without mattress
E0255 Hospital bed variable height with any type side rails with mattress
E0256 Hospital bed variable height with any type side rails without mattress
E0260 Hospital bed semi-electric (Head and foot adjustment) with any type side
rails with mattress
E0261 Hospital bed semi-electric (head and foot adjustment) with any type side rails
without mattress
E0265 Hospital bed total electric (head, foot and height adjustments) with any type
side rails with mattress
E0266 Hospital bed total electric (head, foot and height adjustments) with any type
side rails without mattress
E0290 Hospital bed fixed height without rails with mattress
E0291 Hospital bed fixed height without rail without mattress
E0292 Hospital bed variable height without rail without mattress
E0293 Hospital bed variable height without rail with mattress
E0294 Hospital bed semi-electric (head and foot adjustment) without rail with
mattress
E0295 Hospital bed semi-electric (head and foot adjustment) without rail without
MLN Matters® Number: MM8304 Related Change Request Number: 8304
Disclaimer
This article was prepared as a service to the public and is not intended to grant rights or impose obligations. This article may contain references or links to statutes,
regulations, or other policy materials. The information provided is only intended to be a general summary. It is not intended to take the place of either the written law
or regulations. We encourage readers to review the specific statutes, regulations and other interpretive materials for a full and accurate statement of their contents.
CPT only copyright 2012 American Medical Association.
Page 5 of 11
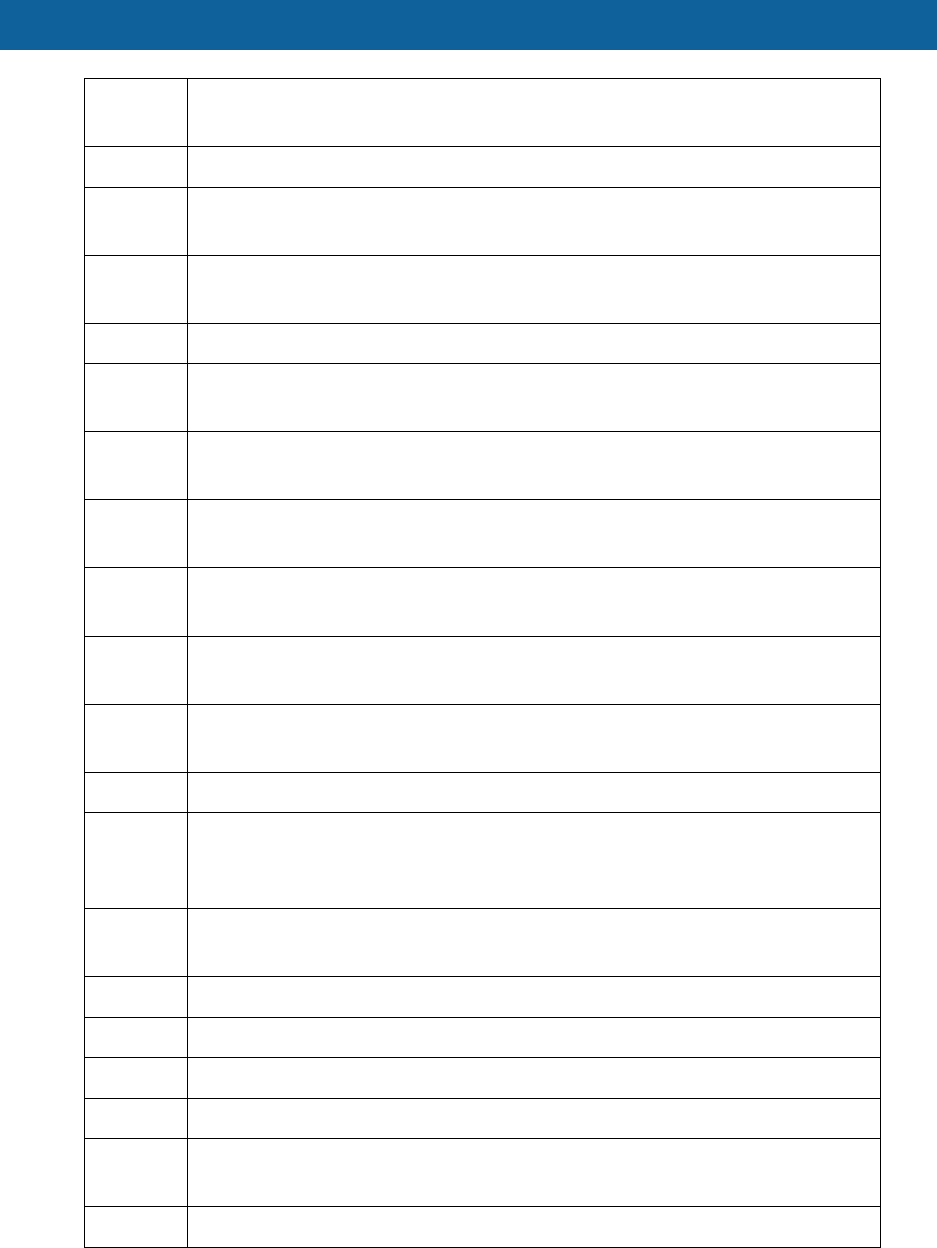
HCPCS
Code Description
mattress
E0296 Hospital bed total electric (head, foot and height adjustments) without rail
with mattress
E0297 Hospital bed total electric (head, foot and height adjustments) without rail
without mattress
E0300 Pediatric crib, hospital grade, fully enclosed
E0301 Hospital bed Heavy Duty extra wide, with weight capacity 350-600 lbs with
any type of rail, without mattress
E0302 Hospital bed Heavy Duty extra wide, with weight capacity greater than 600
lbs with any type of rail, without mattress
E0303 Hospital bed Heavy Duty extra wide, with weight capacity 350-600 lbs with
any type of rail, with mattress
E0304 Hospital bed Heavy Duty extra wide, with weight capacity greater than 600
lbs with any type of rail, with mattress
E0424 Stationary compressed gas Oxygen System rental; includes contents,
regulator, nebulizer, cannula or mask and tubing
E0431 Portable gaseous oxygen system rental includes portable container,
regulator, flowmeter, humidifier, cannula or mask, and tubing
E0433 Portable liquid oxygen system
E0434
Portable liquid oxygen system, rental; includes portable container, supply
reservoir, humidifier, flowmeter, refill adaptor, content gauge, cannula or
mask, and tubing
E0439 Stationary liquid oxygen system rental, includes container, contents,
regulator, flowmeter, humidifier, nebulizer, cannula or mask, and tubing
E0441 Oxygen contents, gaseous (1 months supply)
E0442 Oxygen contents, liquid (1 months supply)
E0443 Portable Oxygen contents, gas (1 months supply)
E0444 Portable oxygen contents, liquid (1 months supply)
E0450 Volume control ventilator without pressure support used with invasive
interface
E0457 Chest shell

MLN Matters® Number: MM8304 Related Change Request Number: 8304
Disclaimer
This article was prepared as a service to the public and is not intended to grant rights or impose obligations. This article may contain references or links to statutes,
regulations, or other policy materials. The information provided is only intended to be a general summary. It is not intended to take the place of either the written law
or regulations. We encourage readers to review the specific statutes, regulations and other interpretive materials for a full and accurate statement of their contents.
CPT only copyright 2012 American Medical Association.
Page 6 of 11
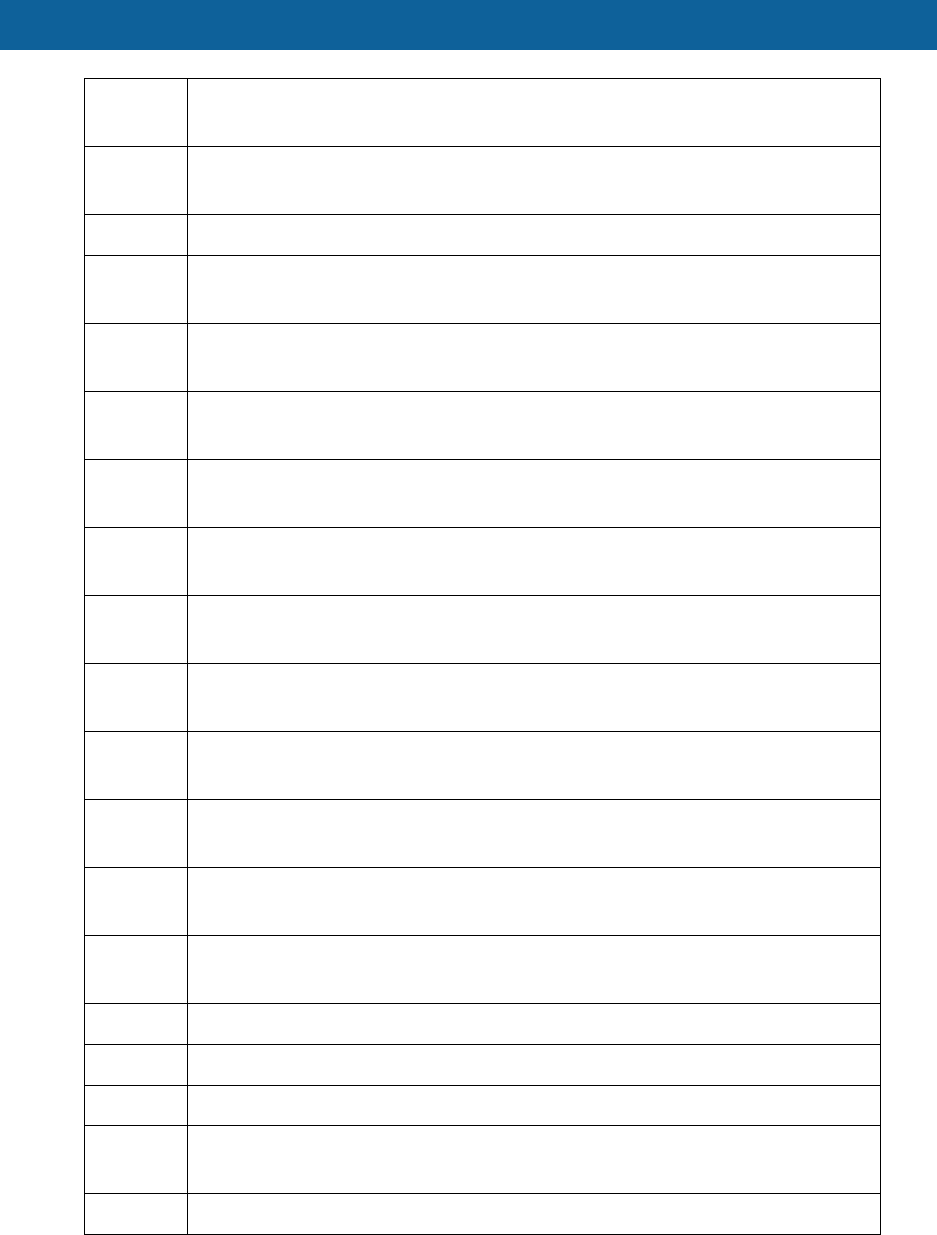
HCPCS
Code Description
E0459 Chest wrap
E0460 Negative pressure ventilator portable or stationary
E0461 Volume control ventilator without pressure support node for a noninvasive
interface
E0462 Rocking bed with or without side rail
E0463 Pressure support ventilator with volume control mode used for invasive
surfaces
E0464 Pressure support vent with volume control mode used for noninvasive
surfaces
E0470 Respiratory Assist Device, bi-level pressure capability, without backup rate
used non-invasive interface
E0471 Respiratory Assist Device, bi-level pressure capability, with backup rate for
a non-invasive interface
E0472 Respiratory Assist Device, bi-level pressure capability, with backup rate for
invasive interface
E0480 Percussor electric/pneumatic home model
E0482 Cough stimulating device, alternating positive and negative airway pressure
E0483 High Frequency chest wall oscillation air pulse generator system
E0484 Oscillatory positive expiratory device, non-electric
E0570 Nebulizer with compressor
E0575 Nebulizer, ultrasonic, large volume
E0580 Nebulizer, durable, glass or autoclavable plastic, bottle type for use with
regulator or flowmeter
E0585 Nebulizer with compressor & heater
E0601 Continuous airway pressure device
E0607 Home blood glucose monitor
E0627 Seat lift mechanism incorporated lift-chair
E0628 Separate Seat lift mechanism for patient owned furniture electric
E0629 Separate seat lift mechanism for patient owned furniture non-electric
MLN Matters® Number: MM8304 Related Change Request Number: 8304
Disclaimer
This article was prepared as a service to the public and is not intended to grant rights or impose obligations. This article may contain references or links to statutes,
regulations, or other policy materials. The information provided is only intended to be a general summary. It is not intended to take the place of either the written law
or regulations. We encourage readers to review the specific statutes, regulations and other interpretive materials for a full and accurate statement of their contents.
CPT only copyright 2012 American Medical Association.
Page 7 of 11
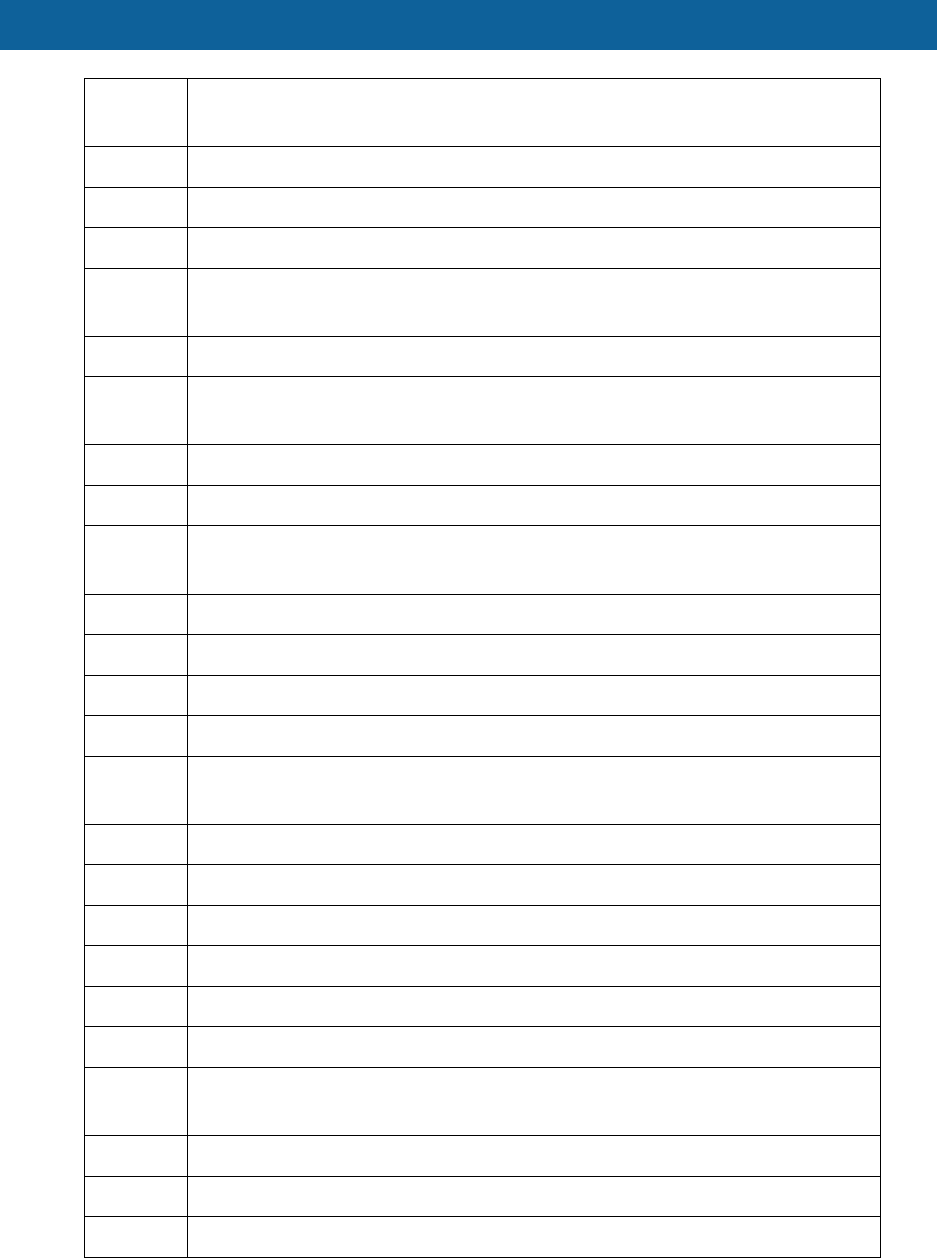
HCPCS
Code Description
E0636 Multi positional patient support system, with integrated lift, patient accessible
controls
E0650 Pneumatic compressor non-segmental home model
E0651 Pneumatic compressor segmental home model without calibrated gradient
pressure
E0652 Pneumatic compressor segmental home model with calibrated gradient
pressure
E0655 Non- segmental pneumatic appliance for use with pneumatic compressor on
half arm
E0656 Non- segmental pneumatic appliance for use with pneumatic compressor on
trunk
E0657 Non- segmental pneumatic appliance for use with pneumatic compressor
chest
E0660 Non- segmental pneumatic appliance for use with pneumatic compressor on
full leg
E0665 Non- segmental pneumatic appliance for use with pneumatic compressor on
full arm
E0666 Non- segmental pneumatic appliance for use with pneumatic compressor on
half leg
E0667 Segmental pneumatic appliance for use with pneumatic compressor on full-
leg
E0668 Segmental pneumatic appliance for use with pneumatic compressor on full
arm
E0669 Segmental pneumatic appliance for use with pneumatic compressor on half
leg
E0671 Segmental gradient pressure pneumatic appliance full leg
E0672 Segmental gradient pressure pneumatic appliance full arm
E0673 Segmental gradient pressure pneumatic appliance half leg
E0675 Pneumatic compression device, high pressure, rapid inflation/deflation cycle,
for arterial insufficiency
E0692 Ultraviolet light therapy system panel treatment 4 foot panel
MLN Matters® Number: MM8304 Related Change Request Number: 8304
Disclaimer
This article was prepared as a service to the public and is not intended to grant rights or impose obligations. This article may contain references or links to statutes,
regulations, or other policy materials. The information provided is only intended to be a general summary. It is not intended to take the place of either the written law
or regulations. We encourage readers to review the specific statutes, regulations and other interpretive materials for a full and accurate statement of their contents.
CPT only copyright 2012 American Medical Association.
Page 8 of 11
HCPCS
Code Description
E0693 Ultraviolet light therapy system panel treatment 6 foot panel
E0694 Ultraviolet multidirectional light therapy system in 6 foot cabinet
E0720 Transcutaneous electrical nerve stimulation, two lead, local stimulation
E0730 Transcutaneous electrical nerve stimulation, four or more leads, for multiple
nerve stimulation
E0731 Form fitting conductive garment for delivery of TENS or NMES
E0740 Incontinence treatment system, Pelvic floor stimulator, monitor, sensor,
and/or trainer
E0744 Neuromuscular stimulator for scoliosis
E0745 Neuromuscular stimulator electric shock unit
E0747 Osteogenesis stimulator, electrical, non-invasive, other than spine
application.
E0748 Osteogenesis stimulator, electrical, non-invasive, spinal application
E0749 Osteogenesis stimulator, electrical, surgically implanted
E0760 Osteogenesis stimulator, low intensity ultrasound, non-invasive
E0762 Transcutaneous electrical joint stimulation system including all accessories
E0764 Functional neuromuscular stimulator, transcutaneous stimulations of
muscles of ambulation with computer controls
E0765 FDA approved nerve stimulator for treatment of nausea & vomiting
E0782 Infusion pumps, implantable, Non-programmable
E0783 Infusion pump, implantable, Programmable
E0784 External ambulatory infusion pump
E0786 Implantable programmable infusion pump, replacement
E0840 Tract frame attach to headboard, cervical traction
E0849 Traction equipment cervical, free-standing stand/frame, pneumatic, applying
traction force to other than mandible
E0850 Traction stand, free standing, cervical traction
E0855 Cervical traction equipment not requiring additional stand or frame
E0856 Cervical traction device, cervical collar with inflatable air bladder
MLN Matters® Number: MM8304 Related Change Request Number: 8304
Disclaimer
This article was prepared as a service to the public and is not intended to grant rights or impose obligations. This article may contain references or links to statutes,
regulations, or other policy materials. The information provided is only intended to be a general summary. It is not intended to take the place of either the written law
or regulations. We encourage readers to review the specific statutes, regulations and other interpretive materials for a full and accurate statement of their contents.
CPT only copyright 2012 American Medical Association.
Page 9 of 11
HCPCS
Code Description
E0958 Manual wheelchair accessory, one-arm drive attachment
E0959 Manual wheelchair accessory-adapter for Amputee
E0960 Manual wheelchair accessory, shoulder harness/strap
E0961 Manual wheelchair accessory wheel lock brake extension handle
E0966 Manual wheelchair accessory, headrest extension
E0967 Manual wheelchair accessory, hand rim with projections
E0968 Commode seat, wheelchair
E0969 Narrowing device wheelchair
E0971 Manual wheelchair accessory anti-tipping device
E0973 Manual wheelchair accessory, adjustable height, detachable armrest
E0974 Manual wheelchair accessory anti-rollback device
E0978 Manual wheelchair accessory positioning belt/safety belt/ pelvic strap
E0980 Manual wheelchair accessory safety vest
E0981 Manual wheelchair accessory Seat upholstery, replacement only
E0982 Manual wheelchair accessory, back upholstery, replacement only
E0983 Manual wheelchair accessory power add on to convert manual wheelchair to
motorized wheelchair, joystick control
E0984 Manual wheelchair accessory power add on to convert manual wheelchair to
motorized wheelchair, Tiller control
E0985 Wheelchair accessory, seat lift mechanism
E0986 Manual wheelchair accessory, push activated power assist
E0990 Manual wheelchair accessory, elevating leg rest
E0992 Manual wheelchair accessory, elevating leg rest solid seat insert
E0994 Arm rest
E1014 Reclining back, addition to pediatric size wheelchair
E1015 Shock absorber for manual wheelchair
E1020 Residual limb support system for wheelchair
E1028 Wheelchair accessory, manual swing away, retractable or removable
MLN Matters® Number: MM8304 Related Change Request Number: 8304
Disclaimer
This article was prepared as a service to the public and is not intended to grant rights or impose obligations. This article may contain references or links to statutes,
regulations, or other policy materials. The information provided is only intended to be a general summary. It is not intended to take the place of either the written law
or regulations. We encourage readers to review the specific statutes, regulations and other interpretive materials for a full and accurate statement of their contents.
CPT only copyright 2012 American Medical Association.
Page 10 of 11
HCPCS
Code Description
mounting hardware for joystick, other control interface or positioning
accessory
E1029 Wheelchair accessory, ventilator tray
E1030 Wheelchair accessory, ventilator tray, gimbaled
E1031 Rollabout chair, any and all types with castors 5" or greater
E1035 Multi-positional patient transfer system with integrated seat operated by care
giver
E1036 Patient transfer system
E1037 Transport chair, pediatric size
E1038 Transport chair, adult size up to 300lb
E1039 Transport chair, adult size heavy duty >300lb
E1161 Manual Adult size wheelchair includes tilt in space
E1227 Special height arm for wheelchair
E1228 Special back height for wheelchair
E1232 Wheelchair, pediatric size, tilt-in-space, folding, adjustable with seating
system
E1233 Wheelchair, pediatric size, tilt-in-space, folding, adjustable without seating
system
E1234 Wheelchair, pediatric size, tilt-in-space, folding, adjustable without seating
system
E1235 Wheelchair, pediatric size, rigid, adjustable, with seating system
E1236 Wheelchair, pediatric size, folding, adjustable, with seating system
E1237 Wheelchair, pediatric size, rigid, adjustable, without seating system
E1238 Wheelchair, pediatric size, folding, adjustable, without seating system
E1296 Special sized wheelchair seat height
E1297 Special sized wheelchair seat depth by upholstery
E1298 Special sized wheelchair seat depth and/or width by construction
E1310 Whirlpool non-portable
E2502 Speech Generating Devices prerecord messages between 8 and 20 Minutes
MLN Matters® Number: MM8304 Related Change Request Number: 8304
Disclaimer
This article was prepared as a service to the public and is not intended to grant rights or impose obligations. This article may contain references or links to statutes,
regulations, or other policy materials. The information provided is only intended to be a general summary. It is not intended to take the place of either the written law
or regulations. We encourage readers to review the specific statutes, regulations and other interpretive materials for a full and accurate statement of their contents.
CPT only copyright 2012 American Medical Association.
Page 11 of 11
HCPCS
Code Description
E2506 Speech Generating Devices prerecord messages over 40 minutes
E2508 Speech Generating Devices message through spelling, manual type
E2510 Speech Generating Devices synthesized with multiple message methods
E2227 Rigid pediatric wheelchair adjustable
K0001 Standard wheelchair
K0002 Standard hemi (low seat) wheelchair
K0003 Lightweight wheelchair
K0004 High strength ltwt wheelchair
K0005 Ultra Lightweight wheelchair
K0006 Heavy duty wheelchair
K0007 Extra heavy duty wheelchair
K0009 Other manual wheelchair/base
K0606 AED garment with electronic analysis
K0730 Controlled dose inhalation drug delivery system