MergedFile B37a7029 E353 4fff 8f53 Feb26d9a4088
2018-02-26
: Pdf B37A7029-E353-4Fff-8F53-Feb26D9A4088 b37a7029-e353-4fff-8f53-feb26d9a4088 2 2018 pdf
Open the PDF directly: View PDF ![]() .
.
Page Count: 26

2/26/2018
1
Naveen Pemmaraju, M.D.
Associate Professor
Department of Leukemia
University of Texas
MD Anderson Cancer Center
Houston, Texas, USA
Introduction to
Myeloproliferative Neoplasms
(MPNs)
Post-ASH 2017 Wrap-Up
•Research support, honorarium, consulting:
–Incyte
–Novartis
–Stemline
–Cellectis
–LFB
–Grant Funding: Affymetrix, Stemline
–Abbvie
–Samus
Disclosures
•Introduction to MPN/MF
•ASH 2017 Wrap-Up: Clinical trials
•Translational Focus: Bench to Bedside and Back to
the Bench
•MPN: Symptom Burden: Why it Matters
Overview/Objectives

2/26/2018
2
“Some Speculations on the
myeloproliferative syndromes”
• “It is possible that these various conditions—
'myeloproliferative disorders'—are all…variable
manifestations of proliferative activity of the bone
marrow cells, perhaps due to a hitherto
undiscovered stimulus.”—William Dameshek, 1951,
Blood
William Dameshek
(1900-1966) www.hematology.org (ASH website)
Premature
death
Natural History of MPNs courtesy Dr Ruben Mesa, MD, Mayo Clinic
CP1266735-1
PV
ET
Early PMF
Leukemic
transformation
Progressive
constitutional
symptoms
Progressive
cytopenias
Progressive
organomegaly/EMH
Overt PMF
Post ET/PV MF
Short term: Vascular
events
Lead time: Typically
years (>10)
to
Time: Variable 3-5
years common
JAK2 V617F
Constitutively active
kinase
Over-signals via STAT,
ERK, MAP kinase,
RAS pathways
Autonomous growth,
cell survival &
differentiation
Slide courtesy of Alison Moliterno, MD, Johns Hopkins
Hospital
JAK2
V617Fpp

2/26/2018
3
CALR
•Chromosome 19p13.3
–Exon 9 of CALR (insertions or deletions)
•Calreticulin= protein Ca++binding fucntion or the
Endoplasmic reticulum
•Also found in nucleus; possible role transcription
regulation
•Klampfel et al NEJM 2013: CALR in 25% pts with
JAK2 negative ET, and in 35% in JAK2 negative MF
Klampfl T et al. N Engl J Med 2013;369:2379-2390.
Cervantes et al., Blood 2009;113:2895-2901
0
.1
.2
.3
.4
.5
.6
.7
.8
.9
1
Probability
024 48 72 96 120 144 168 192 216 240 264 288
Months
95% CI 95% CI 95% CI 95% CI
PMF-PS = 0 PMF-PS = 1 PMF-PS = 2 PMF-PS = 3
Survival by PMF-PS
Heterogeneous clinical outcomes in MF
Slide Courtesy: S. Verstovsek
2/26/2018
4
MF: Risk Stratification:
Poor prognostic variables
•Age >65 y
•Presence of Constitutional symptoms
•Hgb <10
•WBC >25
•Circulating blasts cells ≥1%
•P values were all <0.001
•1054 pts at 7 centers
Cervantes et al, Blood 2009;113:2895-2901
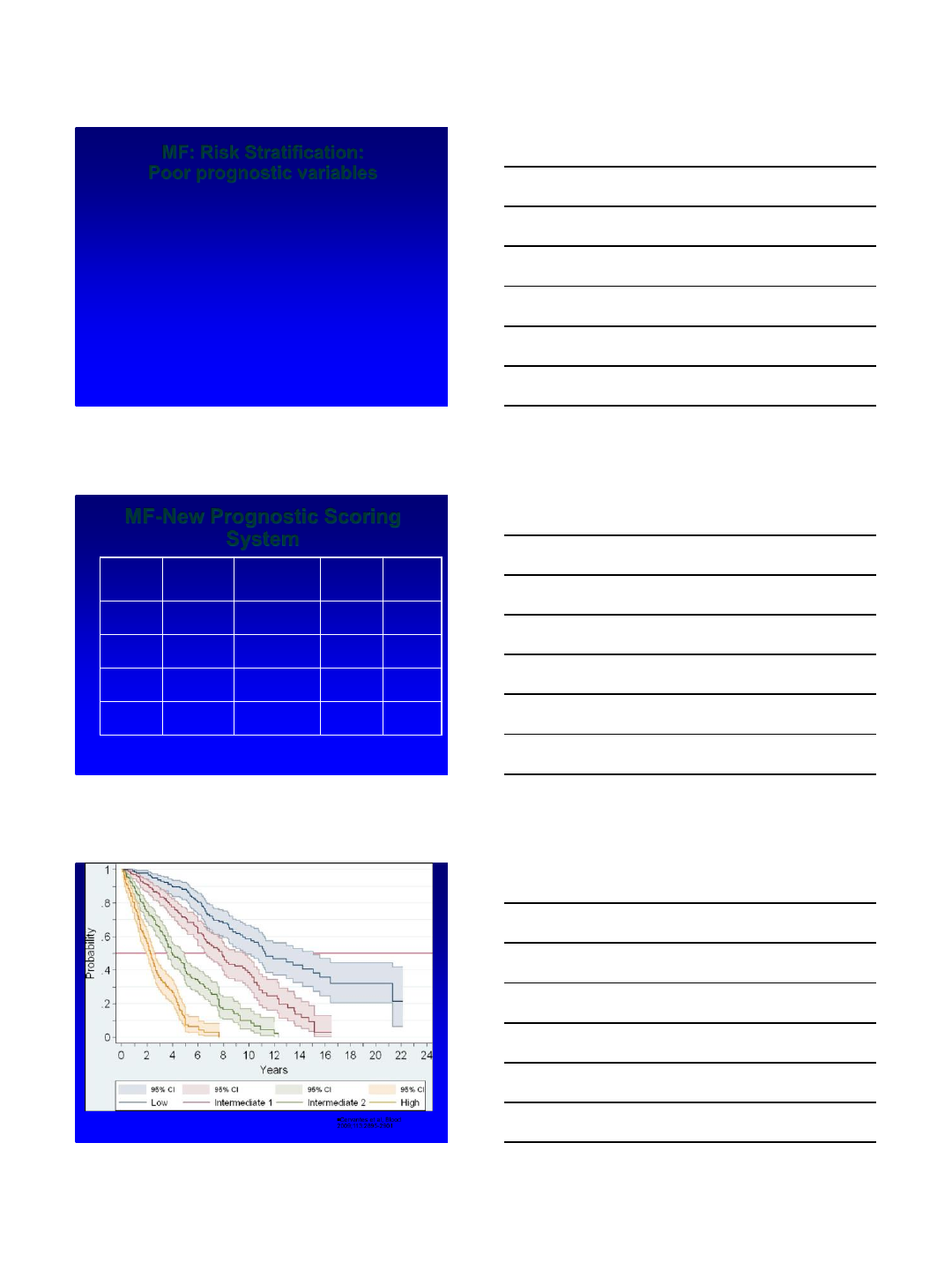
MF-New Prognostic Scoring
System
#factors Patients
%
Med
survival
(months)
Deaths
%
Low 022 135 32
Int-1 1 29 95 50
Int-2 2 28 48 71
High >3 21 27 73
Cervantes et al, Blood
2009;113:2895-2901
Cervantes et al, Blood
2009;113:2895-2901
2/26/2018
5
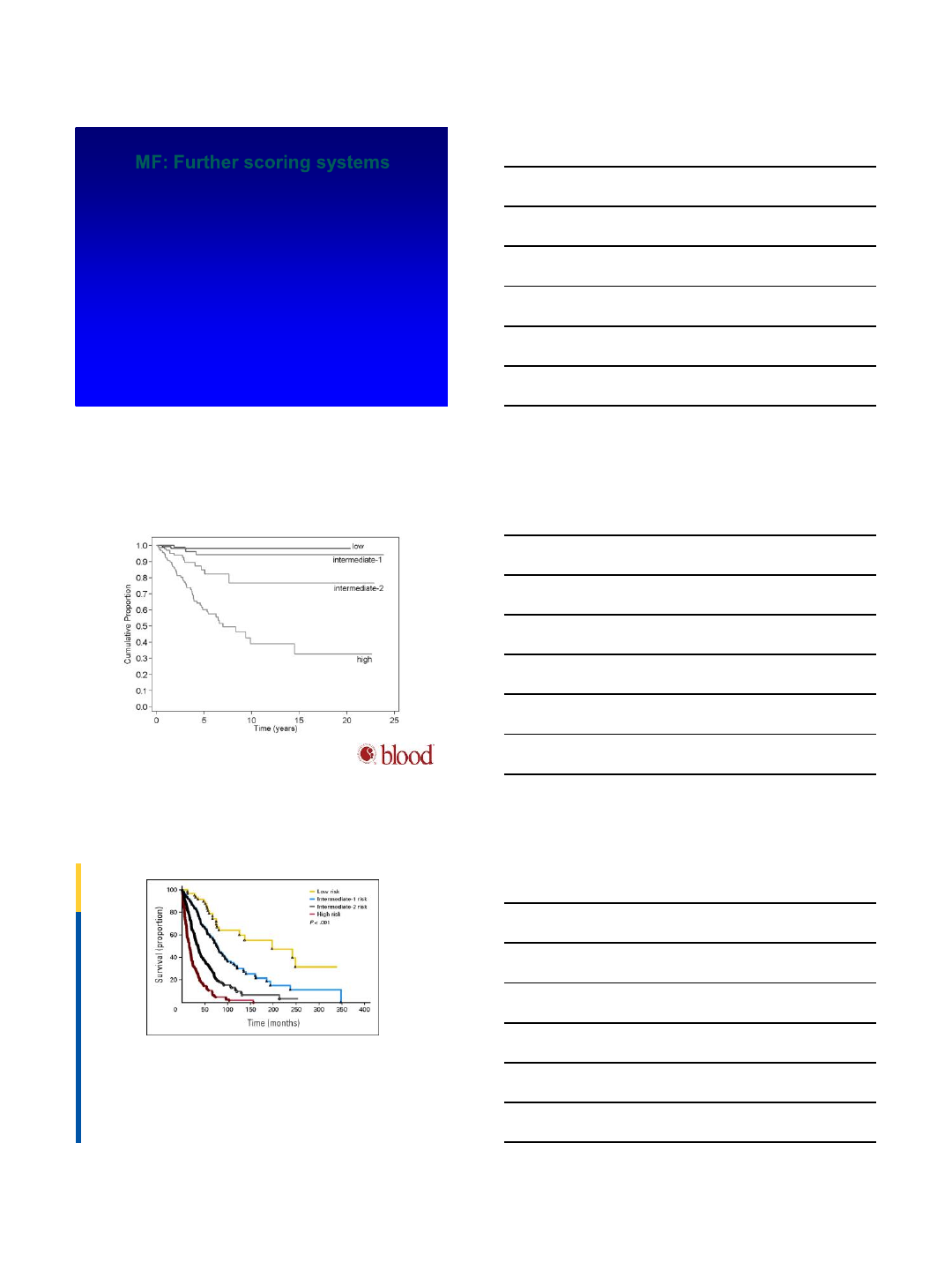
MF: Further scoring systems
•DIPSS (dynamic)—Mayo (Blood 2010;115)
–Modified IPSS to be able to calculate over time: all 1 pt except Hb
(2 points)
–Age >65
–WBC >25K
–Hb <10: 2 points
–Circulating blasts greater than or equal to 1%
–Constitutional sxs
•DIPSS Plus—adds 3 new factors, each 1 point (Mayo, 2011 JCO)
–Unfavorable karyotype
–Plt count <100K
–Transfusion need
Kaplan-Meier estimate of blast phase–free survival in primary myelofibrosis according to the
DIPSS. Risk categories were according to the score obtained anytime during follow-up.
Francesco Passamonti et al. Blood 2010;116:2857-2858
©2010 by American Society of Hematology
Fig 3 Survival data of 793 patients with primary myelofibrosis evaluated at time of their first Mayo Clinic referral and stratified by their Dynamic International Prognostic Scoring System
(DIPSS) + karyotype + platelet count + transfusion status prognostic scores. Low risk, zero adverse points; n = 66; median survival, approximately 185 months. Intermediate-1 risk, one
adverse point; n = 174; median survival, approximately 78 months. Intermediate-2 risk, two or three adverse points; n = 360; median survival, approximately 35 months. High risk, four to
six adverse points; n = 193; median survival, approximately 16 months. Scale for DIPSS: high risk, three adverse points; intermediate-2, two adverse points; intermediate-1, unfavorable
karyotype, platelets < 100 x 109/L, and transfusion need, one adverse point.
Published in: Naseema Gangat; Domenica Caramazza; Rakhee Vaidya; Geeta George; Kebede Begna; Susan Schwager; Daniel Van Dyke; Curtis Hanson; Wenting Wu; Animesh
Pardanani; Francisco Cervantes; Francesco Passamonti; Ayalew Tefferi; JCO 2011, 29, 392-397.
DOI: 10.1200/JCO.2010.32.2446
Copyright © 2010
2/26/2018
6
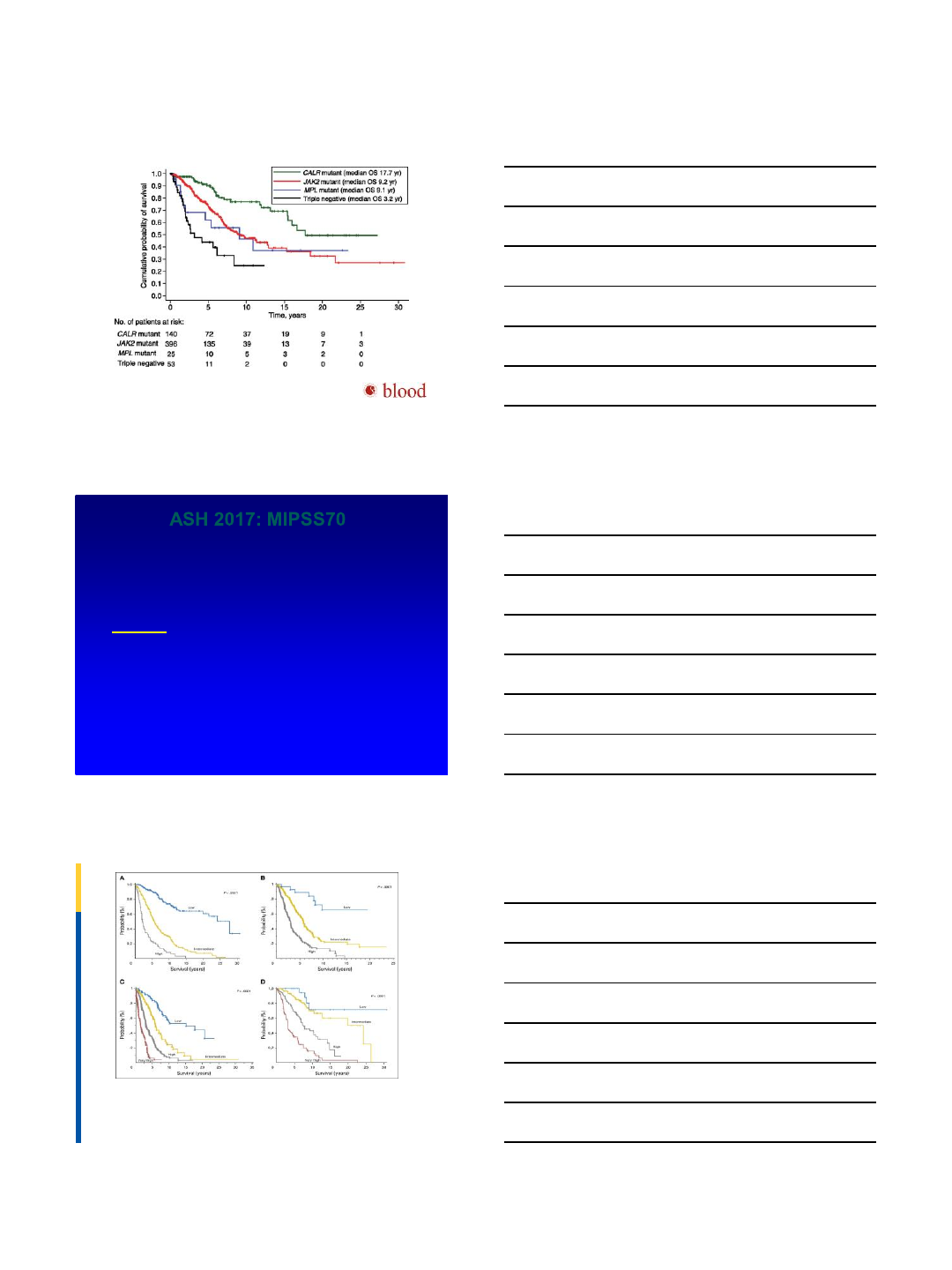
Kaplan-Meier analysis of survival of PMF patients stratified according to their driver mutation.
Elisa Rumi et al. Blood 2014;124:1062-1069
©2014 by American Society of Hematology
ASH 2017: MIPSS70
•ASH 2017: Abstract 200 MIPSS70: Mutation-
Enhanced Prognostic System for Transplant Age
Patients with Primary Myelofibrosis
–Alessandro M. Vannucchi, MD1, et al, ASH 2017
•MVA for OS:
•1)Anemia Hb <10
•2)WBC >25K
•3)plts <100
•4)circulating blasts ≥ 2%
•5)BM fibrosis ≥ 2
•6)Constitutional sxs
•7)absence of CALR Type 1mutation
•8)Presence of HR molecular mutation [ASXL1; EZH2; SRSF2; IDH1/2]
•9)Presence of two or more HR molecular mutations
Guglielmelli P et al JCO
2017; 36: 310-318;
Fig A1. Overall survival (OS) in (A) learning and (B) validation cohorts by the MIPSS70 prognostic scoring system risk classification in all age–inclusive cohorts. OS in (C) learning and (D)
validation cohorts by the MIPSS70-plus prognostic scoring system risk classification in all age–inclusive cohorts. Appendix Table A2 lists details.
Published in: Paola Guglielmelli; Terra L. Lasho; Giada Rotunno; Mythri Mudireddy; Carmela Mannarelli; Maura Nicolosi; Annalisa Pacilli; Animesh Pardanani; Elisa Rumi; Vittorio Rosti;
Curtis A. Hanson; Francesco Mannelli; Rhett P. Ketterling; Naseema Gangat; Alessandro Rambaldi; Francesco Passamonti; Giovanni Barosi; Tiziano Barbui; Mario Cazzola; Alessandro
M. Vannucchi; Ayalew Tefferi; JCO 2018, 36, 310-318.
DOI: 10.1200/JCO.2017.76.4886
Copyright © 2017 American Society of Clinical Oncology

2/26/2018
7
Fig A2. Leukemia-free survival (LFS) in (A) learning and (B) validation cohorts by the MIPSS70 prognostic scoring system risk classification. LFS in (C) learning and (D) validation cohorts
by the MIPSS70-plus prognostic scoring system risk classification. Appendix Table A3 lists details.
Published in: Paola Guglielmelli; Terra L. Lasho; Giada Rotunno; Mythri Mudireddy; Carmela Mannarelli; Maura Nicolosi; Annalisa Pacilli; Animesh Pardanani; Elisa Rumi; Vittorio Rosti;
Curtis A. Hanson; Francesco Mannelli; Rhett P. Ketterling; Naseema Gangat; Alessandro Rambaldi; Francesco Passamonti; Giovanni Barosi; Tiziano Barbui; Mario Cazzola; Alessandro
M. Vannucchi; Ayalew Tefferi; JCO 2018, 36, 310-318.
DOI: 10.1200/JCO.2017.76.4886
Copyright © 2017 American Society of Clinical Oncology
WHO 2016
•New categories/items to note:
•SM: now its own separate myeloid neoplasm outside of
MPN
• Creation of new “pre-fibrotic MF” (ET/MF)
•Lowering of PV Hb threshold
•CALR
•CSF3R
•Fatigue
•Early Satiety
•Abdominal discomfort
•Inactivity
•Concentration problems
•Night Sweats
•Pruritis
•Bone pain
•Fever
•Weight loss
Emmanuel/Mesa JCO 2012, Mesa et al Cancer 2007,
Geyer/Mesa Blood 2014
Symptom Burden in MF: Total Symptom
Score (MPN-TSS)

2/26/2018
8
22
#EBMT16
#MPNSM: An ongoing Twitter
conversation about MPNs
•Inspired by: CTO (based on #hcsm & #btsm) (Katz et al Disease-Specific hashtags
for online communication about cancer care - JCO. 2015;33 suppl abstr 6520); and
for hematology specific influence, #mmsm
•Founder of #MPNSM Twitter community : Naveen Pemmaraju, MD @doctorpemm
–With key co-founders: @mtmdphd, @Vikas_Gupta_1, @mpdrc
•First tweet: @doctorpemm [Aug 2014]but #mpnsm did not really take off as a
regular hashtag until Dec’14-Jan’15: during/after #ASH15 meeting
•As of Sept,13,2015: For #MPNSM, According to @symplur @healthcarehashtags
project: Jan’15-Sept’15
–2013 tweets from 285 participants
–Resulting in: 4,049,415 impressions
•Brings together, in real-time: investigators/researchers, MPN healthcare providers,
patients, advocates, organizations for discussion of basic science, translational, and
clinical topics in MPNs Slide: courtesy Mike Thompson, MD, PhD
Pemmaraju N, e t al Current Hematologic
Malignancy Reports10(4), 413-420. 9/28/15 online
Pemmaraju N, et al Curr Hematol Malig Rep. 2016
Aug 4. [Epub ahead of print]
Thank you
•Please email me npemmaraju@mdanderson.org or
call me 713-792-4956 if you have any questions
•#MPNSM: Twitter/social media
•Thank you to Dr Serge Verstovsek, our chief of
MPNs, research RNs, and MPN team at MDACC

2/26/2018
1
Aaron T. Gerds, MD, MS
Assistant Professor of Medicine
Hematology and Medical Oncology
Leukemia & Myeloid Disorders
Program
@AaronGerds
Beyond single-agent JAK inhibitors:
New therapies and combinations
from ASH 2017
@AaronGerds
Beyond single-agent JAK inhibitors –
ASH 2017
•New drugs Abstract No. Comments
•Givinostat 253/1648 HDACi, CR/PR 86% (ITT, n=30) in PV
•LCL-161 256 Oral Smac Mimetic, ORR 30% in MF
•Glasdegib 258 Modest symptom and spleen reduction in MF
•Sotatercept 255 New “ESA,” activin receptor IIA ligand trap
•Idasanutlin 254 MDM2 inhibitor, in PV/ET, Ph1 study
•Alisertib 1631 Aurora kinase inhibitor
•SL-401 2908 Recombinant IL-3 fused to diphtheria toxin
•Combination therapy
•Pracinostat + ruxolitinib 1632 Modest benefit over single-agent ruxolitinib
•Vismodegib + ruxolitinib 4179 No clear benefit over single-agent ruxolitinib
•Azacitidine + ruxolitinib 1649 AP/BP, ORR 33% (2 of 6 evaluable patients)
•Old is new again!
•Interferons 321/323/320 Front-line and beyond, Ropeginterferon ⍺-2b
•Fedratinib 4197 Wernicke’s exposé from JAKARTA studies
Prithviraj Bose, Naval G. Daver, Naveen Pemmaraju, Elias J. Jabbour, Zeev Estrov, Allison M. Pike,
Julie Huynh-Lu, Madeleine Nguyen-Cao, Xuemei Wang, Lingsha Zhou, Sherry Pierce, Hagop M.
Kantarjian, and Srdan Verstovsek
SOTATERCEPT (ACE-011) ALONE AND WITH
RUXOLITINIB IN PATIENTS WITH MPN-
ASSOCIATED MYELOFIBROSIS (MF) AND ANEMIA
Supported by Celgene Corporation
Slide courtesy of Prithviraj Bose

2/26/2018
2
Phase II Study Design
•MF with Hgb <10 g/dL x ≥ 84 days
•2 cohorts:
•Sotatercept alone q3 wk
•Sotatercept q3 wk in patients on
stable dose of ruxolitinib
• Response (on study x ≥ 84 days):
•Anemic patients: ≥1.5 g/dL ↑ from
baseline x ≥ 84 d
•Tx-dependent patients: transfusion
independence per 2013 IWG-MRT
criteria
Sotatercept
Suragani RN, et al. Nat Med. 2014 Apr;20(4):408-14
Bose P, et al. Blood. 2017 Dec;130(supp1):225
Sotatercept/Luspaterc ept responsive
@AaronGerds
Variable
Value/Category
Sotatercept
(n=24)
Sotatercpt
+ Rux (n=11)
Median age (range)
years
66.5 (47
-84)
68 (57
–84)
Diagnosis
PMF
20
9
Post
-ET/PV MF
4
2
Sex
Male
14
7
Median baseline
hemoglobin (range)
g/dl
7.5 (4.7
–8.7)
7.2 (4.6
–9.1)
Driver mutation
JAK2
16
8
CALR
3
2
MPL
3
1
Triple negative
1,
CALR mutation status unknown in 1
0
Karyotype
Abnormal
8, insufficient metaphases in 1
6
DIPSS category
Intermediate
-2
19
7
High
5
4
Bone marrow fibrosis grade
MF
-2
8
5
MF
-3
16
5
Splenomegaly
Present
13
11
Previously
treated
Yes
19
Median
rux dose (range)
mg PO BID
10 (5
-20)
Bose P, et al. Blood. 2017 Dec;130(supp1):225
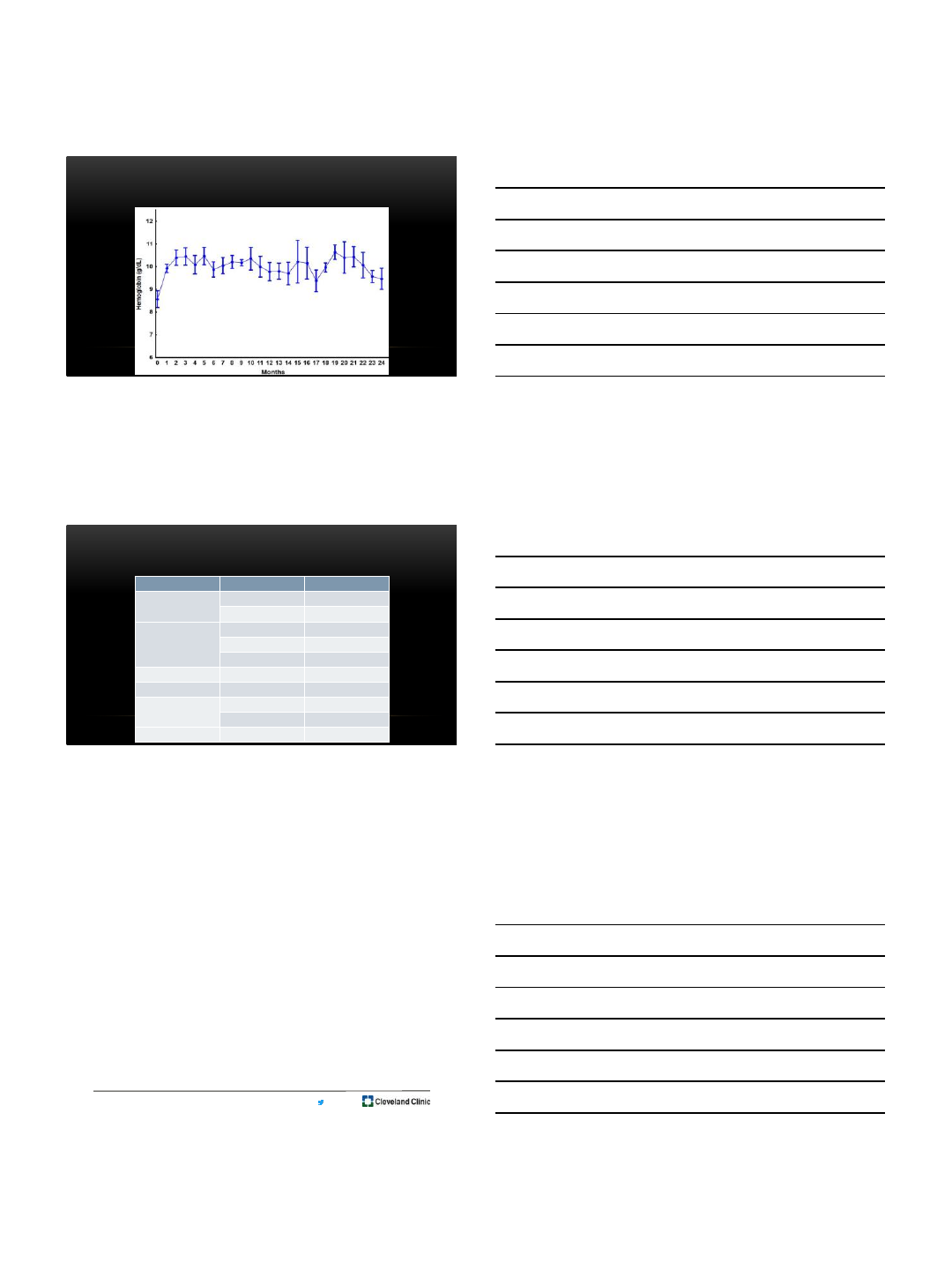
Summary of results
Sotatercept (n=18 evaluable)
•40% response (7/18); 3/11
transfusion-dependent
•Median time to response 7d (1-22d)
•Median response duration 12 (5-24+)
months
•Multiple drug holds in 3 patients due
to Hgb levels ≥11.5 g/dl
Sotatercept + Rux(n=10 evaluable)
•30% response (3/10); 0/4 transfusion-
dependent
• 1.5 g/dl ↑ in Hgb from baseline in 1
additional patient (s/p 3 cycles)
•Responses began at 7, 14 and 140 d
•Response durations of 3+, 4+ and 15+
months
•Multiple drug holds in 1 patient due to
Hgb levels ≥11.5 g/dl
Bose P, et al. Blood. 2017 Dec;130(supp1):225
2/26/2018
3
MEAN HEMOGLOBIN OVER TIME IN RESPONDERS (N=10)
Slide courtesy of
Prithviraj Bose
ADVERSE EVENTS POSSIBLY RELATED TO
SOTATERCEPT (N = 35)
Adverse event
Grade
No. of patients
Hypertension
3
3
2
2
Pain (joints/muscle)
3
1
2
1
1
1
Elevated UMACR
1
2
Limb
edema
1
1
Headache
(in the context
of HTN)
2
1
1
1
Nausea
1
1
Sotatercept in MF
Slide courtesy of
Prithviraj Bose
@AaronGerds
Comclusions
•Sotatercept effective for MPN-associated anemia
•Planned enrollment 60 subjects
•Multi-center phase 2 trial of luspatercept in MF open
•ClinicalTrials.gov Identifier: NCT03194542
Bose P, et al. Blood. 2017 Dec;130(supp1):225
2/26/2018
4
Open Label Phase I Study of Single Agent Oral RG7388
(idasanutlin) in Patients with Polycythemia Vera and
Essential Thrombocythemia
Mascarenhas J1, Lu M1, Virtgaym E1, Kosiorek H 2, Stal M1, Sandy L1, Orellana A1,
Xia L1, Rampal R3, Kremyanskaya M1, Petersen B4, Dueck A 2, Hoffman R1
1Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York, New York
2Mayo Clinic Scottsdale, Scottsdale, Arizona
3Leukemia Service, Memorial Sloan Kettering Cancer Center, New York, New York
4Department of Pathology, Icahn School of Medicine at Mount Sinai, New York, New
York 10029
Slide courtesy of John Mascarenhas
Background: P53/MDM2
Nakatake M et al. Oncogene. (2012); Cassinat and Kiladjian Blood (2012); Shangary and Wang. Clin Cancer
Res. (2008); Lu and Hoffman Oncotarget (2012).
•P53 regulates cell cycle, apoptosis, DNA repair, and senescence
•Wild type P53 seen in chronic phase MPN and mutated P53 in advanced phase
•Down regulation of P53 by MDM2 overexpression
•Promotes proteosomal degradation
•Inhibits P53 transcription
•Inhibits transactivation
•Facilitates export from nucleus
•Nutlins Block the MDM2:P53 interaction and activate the p53 pathway
Slide courtesy of John Mascarenhas
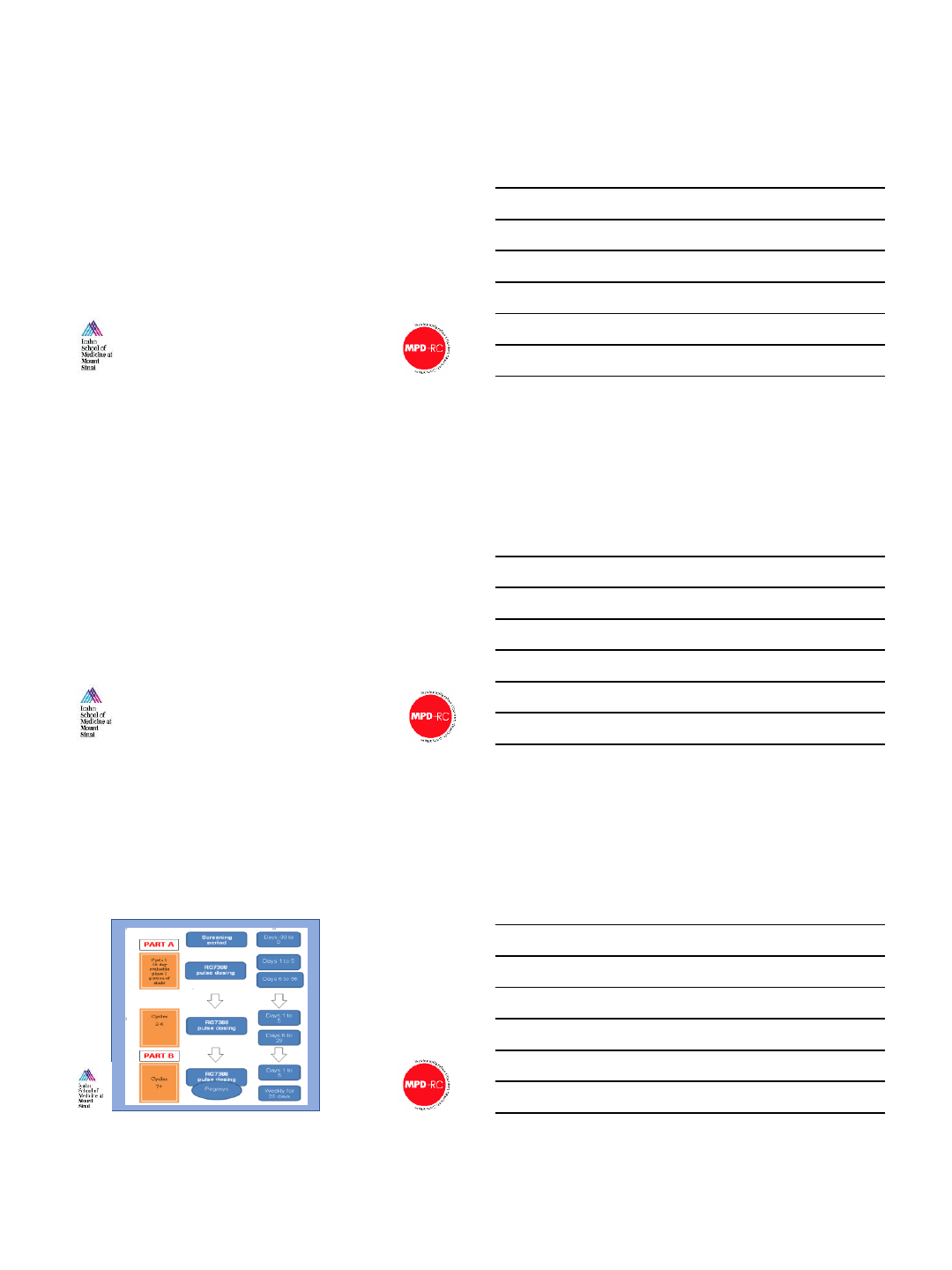
Study Schema and Design
•2 dose cohorts evaluated
•100 mg daily, days 1-5
•150 mg daily, days 1-5
•DLT is defined as:
•non-hematologic AE of
G3+
•hematologic AE of G2+
thrombocytopenia, G3+
neutropenia, or G3+
anemia
•Dosing after cycle 3
dependent on attaining
HCT >42% and/or PLT
>400K at Day 1
Slide courtesy of John
Mascarenhas
2/26/2018
5
Baseline Demographics
100 mg
(N=6)
150 mg
(N=6)
Total
(N=12)
Diagnosis
Essential Thrombocythemia 1 (16.7%) 0 (0.0%) 1 (8.3%)
Polycythemia Vera 5 (83.3%) 6 (100.0%) 11 (91.6%)
Age, median (years)
62 (32-83) 63 (48-68) 63.5 (32-83)
Gender, female
5 (83.3%) 2 (33.3%) 7 (58.3%)
Disease
duration, mos
(prior
to study)
41.6
(14.9-80.1)
65.4
(21.0-154.3)
43.9
(14.9-154.3)
Previous
thrombosis 3 (50.0%) 0 (0.0%) 3 (25.0%)
Prior hydroxyurea therapy
5 (83.3%) 5 (83.3%) 10 (83.3%)
Spleen length by palpation, median (cm)
1.0 (1.0-7.0) 2.5(0.0-18.0) 1.0 (0.0-18.0)
Leukocytes , median (x10
9/L) 10.3
(4.9-15.9)
12.2
(7.4-28.3)
11.3
(4.9-28.3)
Hemoglobin, median (g/
dL)13.4
(12.8-15.6)
13.7
(12.3-14.7)
13.6
(12.3-15.6)
Hematocrit , median
(%) 41.5
(38.3-46.7)
43.0
(40.7-47.8)
42.3
(38.3-47.8)
Platelets median, (x 10
9/L) 443.5
(118.0-1339.0)
412.0
(153.0-700.0)
443.5
(118.0-1339.0)
LDH median, (U/L)
252.0
(184.0-370.0)
252.0
(177.0-616.0)
252.0
(177.0-616.0)
JAK2V617F
Variant Allele Frequency,
median (%)
23.7
(5.3-69.3)
63.7
(6.3-88.6)
40.6
(5.3-88.6)
Slide courtesy of
John Mascarenhas
TEAE occurring in at least 2 patients regardless of attribution
100 mg (n=6) 150 mg (n=6) Total (n=12)
Grade 1/2
Grade 3
Grade 1/2
Grade 3 Any grade
Fatigue
5 (83.3%) 1 (16.7%) 4 (66.7%) 10 (91.7%)
Headache
4 (66.7%) 1 (16.7%) 1 (16.7%) 6 (50%)
Dry skin
2 (33.3%) 2 (33.3%) 4 (33.3%)
Pain
1 (16.7%) 1 (16.7%) 1 (16.7%) 3 (25%)
Arthralgia
3 (50%) 3 (25%)
Dizziness
3 (50%) 3 (25%)
Atrial
fibrillation
2 (33.3%) 2 (16.7%)
Cough
2 (33.3%) 2 (16.7%)
Decreased
appetite
1 (16.7%) 1 (16.7%) 2 (16.7%)
Epistaxis
1 (16.7%) 1 (16.7%) 2 (16.7%)
Flushing
2 (33.3%) 2 (16.7%)
Jaw pain
2 (33.3%) 2 (16.7%)
Oropharyngeal
pain
1 (16.7%) 1 (16.7%) 2 (16.7%)
URI
1 (16.7%) 1 (16.7%) 2 (16.7%)
Weight gain
1 (16.7%) 1 (16.7%) 2 (16.7%)
•3 patients had grade 3 non-
hematologic AE (all at 100 mg)
•Pt #1 –grade 3 fatigue
•Pt #2 –grade 3 headache
•Pt #3 –grade 3 pain
•No grade 4 non-hematologic
adverse events at either dose
level noted
•No hematologic AE of any grade
noted
Slide courtesy of
John Mascarenhas
Focus on Gastrointestinal TEAE
(regardless of attribution)
•No G3/4 GI TEAE
were observed
•GI prophylaxis:
•Ondansetron
•Lorazepam
•Decadron
•Constipation
likely due to 5-
ht3 antagonist
100 mg (n=6) 150 mg (n=6) Total (n=12)
Grade 1/2 Grade 3 Grade 1/2 Grade 3 Any grade
Constipation
6 (100%) 4 (66.7%) 10 (91.7%)
Nausea
5 (83.3%) 4 (66.7%) 9 (75%)
Diarrhea
5 (83.3%) 3 (50%) 8 (66.7%)
Dyspepsia
4 (33.3%) 3 (16.7%) 7 (58.3%)
Abdominal
pain
4 (66.7%) 1 (16.7%) 5 (41.7%)
Anorexia
2 (33.3%) 2 (33.3%) 4 (33%)
Vomiting
3 (50%) 3 (25%)
Abdominal
distension
2 (33.3%) 1 (16.7%) 3 (25%)
Dysgeusia
1 (16.7%) 2 (33.3%) 3 (25%)
Flatulence
1 (16.7%) 1 (16.7%) 2 (16.7%)
Slide courtesy of John Mascarenhas
2/26/2018
6
Responses by 2013 ELN-IWG1criteria
Not
evaluable
(NE)
No
response
(NR)
Partial
Response
(PR)
Complete
Response
(CR)
Overall
Response
(PR+CR)
PART A (n=12)
1
#
4
3*
4
7 (58%)
PART B (n=4)^
1
+
1
1
1
2 (50%)
PART A + PART
B ORR
9 (75%)
1Barosi et al Blood 2013
•# not evaluable due to patient decision to withdraw from study after 4 cycles due to GI toxicity
•*Residual splenomegaly likely due to known portal vein thrombosis, likely a CR (n=1)
•^4 subjects from PART A that had NR continued on to PART B combination idasanutlin + interferon-α
•+not yet completed cycle 7
By 6 cycles of therapy with idasanutlin monotherapy in PART A and combination pegylated interferon-αin PART B
Slide courtesy of John Mascarenhas
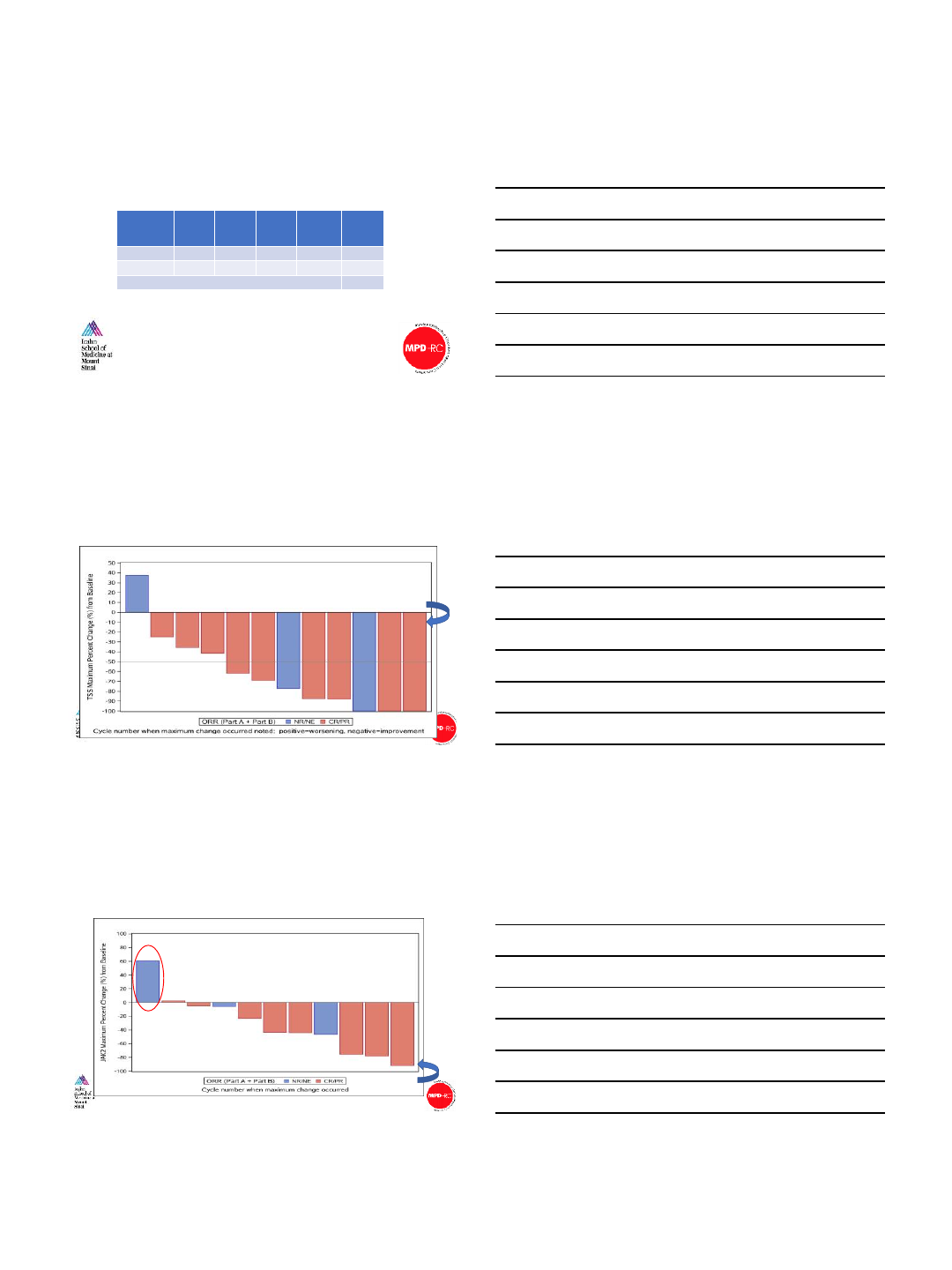
1
2
33
3
4
6
26
2 7 2
888
14 12 42 42 66 50 113 32
Baseline TSS
Maximum Total Symptom Score (TSS) response on study
Slide courtesy of John Mascarenhas
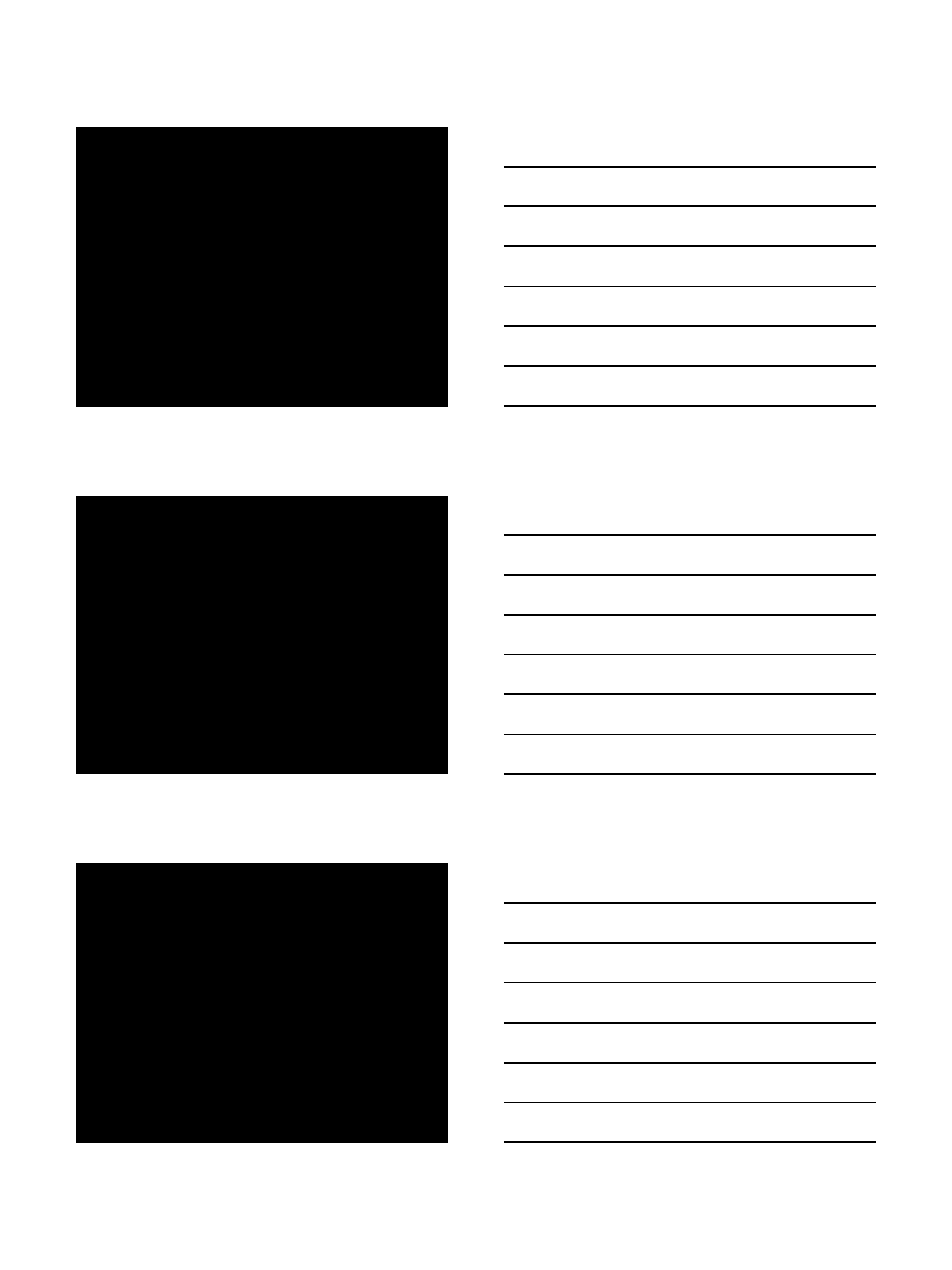
4
78412 39810 6 8
Driver mutation responses with idasanutlin therapy
12 39810 6 8
4
784
Median % reduction -43%
(range -91.9% to +60.3%)
52% 69% 89%
82% 87% 2% 24% 23% 45% 36% 6%
Baseline VAF
Slide courtesy of John Mascarenhas

2/26/2018
7
Pre-treatment X100
Pre-treatment X400
Post-treatment X100
Post-treatment X400
AB
C D
Bone marrow responses with Idasanutlin therapy
Slide courtesy of John Mascarenhas
Conclusions
•Idasanutlin is well tolerated in patients with PV after multiple cycles of exposure and
expected GI toxicity is manageable
•No DLT was observed and 150 mg x 5 days/cycle was chosen to be RPTD
•Idasanutlin may also be safely combined with pegylated IFN to improve upon the
response (PART B)
•On target P53 pathway activation was demonstrated with idasanutlin treatment
•Normalization of the hematologic profile and improvement in symptom burden were
observed with idasanutlin monotherapy and in combination with Pegasys
•Extended treatment-free-periods are possible with idasanutlin therapy
•Bone marrow morphologic and molecular responses were attained with idasanutlin
therapy
•A global, multicenter, single arm phase II trial of idasanutlin in patients with hydroxyurea
resistant/intolerant PV is underway (ClinicalTrials.gov Identifier: NCT03287245)
Slide courtesy of John Mascarenhas
@AaronGerds

2/26/2018
8
@AaronGerds
Fedratinib clinical activity
•JAKARTA-1 (randomized placebo-controlled)
•47% (400mg) and 50% (500mg) of patients with intermediate 2 or high
risk myelofibrosis (MF) had SRV of ≥35% at 24 weeks
•JAKARTA-2 (open-label)
•53% of MF intermediate/high-risk patients who were resistant and 63%
of patients who were intolerant to ruxolitinib had ≥35% reduction in
spleen volume at Week 24
Pardanani A, et al. JAMA Oncol. 2015 Aug;1(5):643-51.
Harrison CN, et al. Lancet Haematol. 2017;4(7):e317-e324.
@AaronGerds
US FDA: Hold it!
•Clinical hold placed on November 15, 2013 as a result of
neurological symptoms, suggestive of Wernicke’s
encephalopathy in 8/877 patients, exposed to fedratinib
@AaronGerds
Thiamine uptake and fedratinib
•Fedratinib IC50 >300 μm against
THTr1 and THTr2
•Clinical Cmax = 3-5 μM at 400 &
500 mg doses, respectively
Thiamine (nmol/L)
Patients (N) Mean Median
End of
fedratinib treatment 161 171 198
Normal range of thiamine levels: 74-224 nmol/L
Harrison CN, et al. Blood. 2017 Dec;130(supp1):4197

2/26/2018
9
@AaronGerds
Harrison CN, et al. Blood. 2017 Dec;130(supp1):4197
@AaronGerds
Summary
•Treatment with fedratinib did not decrease thiamine levels in
patients from the clinical trials
•
•A single confirmed case of WE from 877 treated patients
•2 patients with unconfirmed diagnosis (symptoms and MRI findings
consistent with WE but presence of confounding abnormalities)
•Prevalence of WE in the trials was less than what has been
published for people with MPNs
•Prevalence 0.1%-0.4%
Harrison CN, et al. Blood. 2017 Dec;130(supp1):4197
@AaronGerds
Summary and Conclusions
•New, non-JAK inhibitor agents being developed
•Combination therapy remains burdened with toxicity and limited
additive benefit
•Many challenges remain
•Separate normal biology from pathogenesis
•Spectrum of fitness
•Long time observation until outcome of interest (PV/ET)
• “Ruxolitinib failure” not defined (MF)
•Dealing with cytopenias (MF)

2/26/2018
10
Thanks! Tracy Cinalli, RN
Jacqui Mau, RN
Christine Cooper, RN
Mary Lynn Rush, RN
Rachael Diligente, RN
Andrea Smith, RN
Eric Parsons, RN
Samjhana Bogati, RN
Barbara Paulic, RN, NP
Raychel Berardinelli, RN, NP
Barb Tripp, RN, NP
Alicia Bitterice, RN, NP
Meghan Scully, RN, NP
Becky Habecker, BA
Chante Cavin, BA
Sarah Kaufman, BA
Dennis Kramarz, BA
Ben Pannell, BA
Allison Unger, BA
Abby Statler, MPH
Donna Abounader, BA
Abigail Snow, BA
Justine DeAngelis, BA
Oliovia Kodramaz
Caitlin Swann, PharmD
And Our
Patients!!!
Mikkael Sekeres, MD, MS
Jaroslaw Maciejewski, MD, PhD
Sudipto Mukherjee, MD, PhD
Yogen Saunthararajah, MD
Hetty Carraway, MD, MBA
Anjali Advani, MD
Matt Kalaycio, MD
Ronald Sobecks, MD
Betty Hamilton, MD
Aziz Nazha, MD
John Desamito, MD
@AaronGerds
Leukemia & Myeloid Disorders
Program

2/26/2018
1
Inflammation in MPN
Angela Fleischman M.D. Ph.D.
University of California, Irvine
Feb 26, 2018
Angela Fleischman Disclosures
•Incyte (speakers bureau)
Elevated Inflammatory Cytokines in
Many Hematologic Malignancies
CML
AML MDS
MPN

2/26/2018
2
IFN-γ
(Tyner et al, 2010) (Verstovsek et al, 2010 Slezak et al, 2009,
Boissinot et al, 2010, Tefferi et al 2011)
CD40
IL-2
IL-7
IL-9
IL-6
VEGF
TNF
MIP-1β
MIP-1α
TIMP-1
G-CSF
IL-1α,ß
IL-18
IL-16
ICAM-1
MMP-10
MMP-2
VCAM-1
IFN-α
IL-11
IL-8
Mouse Model MPN patients
Elevated Inflammatory Cytokines in
MPN
IL-10
IL-12
IL-2R
IL-13 IL-15
Both mutant and wild-type cells
produce excessive inflammatory
cytokines in MPN
Wild
-type mutant
Wild
-type
Wild
-type
Kleppe et al, Cancer Discovery 2015
Wild
-type mutant
Wild
-type
mutant
Specific Cytokines Drive Specific
Symptoms in MPN
Geyer et. al. Mediators of Inflammation 2015

2/26/2018
3
Impact of Inflammatory Cytokines and
Chemokines in the MPNs
Symptom
Burden
B2MG
Ferritin
IL-8
Leptin
PAL1
TIMP1
TNF-RII
VCAM1
Disease
Advancement
BMP1 BMP6
BMP7
BMP-
Rcp2
IL-12
TNF-1
IL-8
Leptin
PAL1
TIMP1
TNF-RII
VCAM1
Inferior
Survival
INF
IL12
IL15
IL2RIL8
IP10
TNF-
1
Tefferi et. al. J Clin Oncol. 2011 Apr 1;29(10):1356-63.
Geyer et. al. Mediators of Inflammation 2015. 1-9.
Splenomegaly
HGF
IL1RAMIG
Clonal
expansion/bla
sts
IL8
TNFa
JAK2V617F
INF
IL17A
IL1B
IL8
Stress
hematopoiesis
HSC
exhaustion
Chronic inflammation Exhausts
Blood stem cells
What are methods to control
inflammation?
•Prescription Medications
•Over the counter medications and
supplements
•Stress reduction/mindfulness
•Exercise
•Diet

2/26/2018
4
Inflammation as a Treatment Target in
MPNs
Understanding Our Current Therapies
•Contributes both to:
•Platelet aggregation
inhibition
•Inhibit the activity of
cyclooxygenase which
leads to the formation of
prostaglandins.
Aspirin:
•Alters the inflammatory
pathway and has been
one of the only
therapeutic options which
has been able to alter the
stem cell clone
Interferon: •Initially developed as an anti-
inflammatory in RA
•Associated with reduced
inflammatory markers
(previously described)
•Proposed to be one of the
most powerful non-steroidal
anti-inflammatories available.
Ruxolitinib:
JAK usage by cytokine receptors
From Murray P, Journal of Immunology 2007
JAK inhibitors are anti-inflammatory drugs
JAK inhibitors in development for
MPN
Agent
Company
Activity
Status
Ruxolitinib (INCB18424)
Novartis/Incyte
JAK1/JAK2
FDA
-
approved
Fedratinib (TG101348/SAR302503)
(ON
HOLD; Wernicke’s encephalopathy )
Celgene
JAK2, FLT3
Phase 3
Momelotinib (CYT387)
(ON
HOLD, failed to meet endpoint goals in phase 3
)
Gilead
JAK1/JAK2/
TYK2
Phase 3
Pacritinib (SB1518)
(ON
HOLD, then back to dose-finding)
CTI
BioPharma
JAK2, FLT3,
IRAK1
Phase 3
Lestaurtinib (CEP701)
Cephalon
JAK2/FLT3
Phase 1/2
BMS
-911453
Bristol
-Myers
Squibb
JAK2
Phase 1
NS
-018
Nippon
-Shinyaku
JAK2/
Src
Phase 1/2
AZD1480 (discontinued due to neurotoxicity and
other side effects)
Astra Zeneca
JAK1/JAK2
Phase 1
Gandotinib (LY2784544)
Eli Lily
JAK2 V617F
Phase 1
INCB039110
Incyte
JAK1 (alone)
Phase 2
INCB054329
Incyte
JAK1
Phase 1/2
2/26/2018
5
Rationale for JAK1 inhibitor
•Blockade of inflammatory signaling pathways
that use JAK1 while sparing
myelosuppression attributable to the
inhibition of JAK2-mediated hematopoiesis
•INCB039110 (itacitinib) is a potent and
selective inhibitor of JAK1 with low in
vitro affinity for JAK2 (>20-fold selectivity for
JAK1 over JAK2) and other members of the
JAK family (>100-fold selectivity for JAK1
over JAK3 and TYK2)
Simon two-stage design to assess the efficacy and safety of different
doses of INCB039110
83 patients evaluable for primary endpoint
10 patients in 100 mg twice-daily
42 patients in 200 mg twice-daily
31 patients in 600 mg once-daily cohorts, respectively
Inclusion criteria:
intermediate- or high-risk myelofibrosis
Plt≥50×109/L, Hgb ≥8.0 g/dL, ANC ≥1×109/L
palpable spleen or prior splenectomy
active myelofibrosis-related symptoms
Phase II Open-Label Trial Of INCB039110, A
Selective JAK1 Inhibitor, In Patients With
Myelofibrosis
Mascarenhas et al, Haematologica 2017
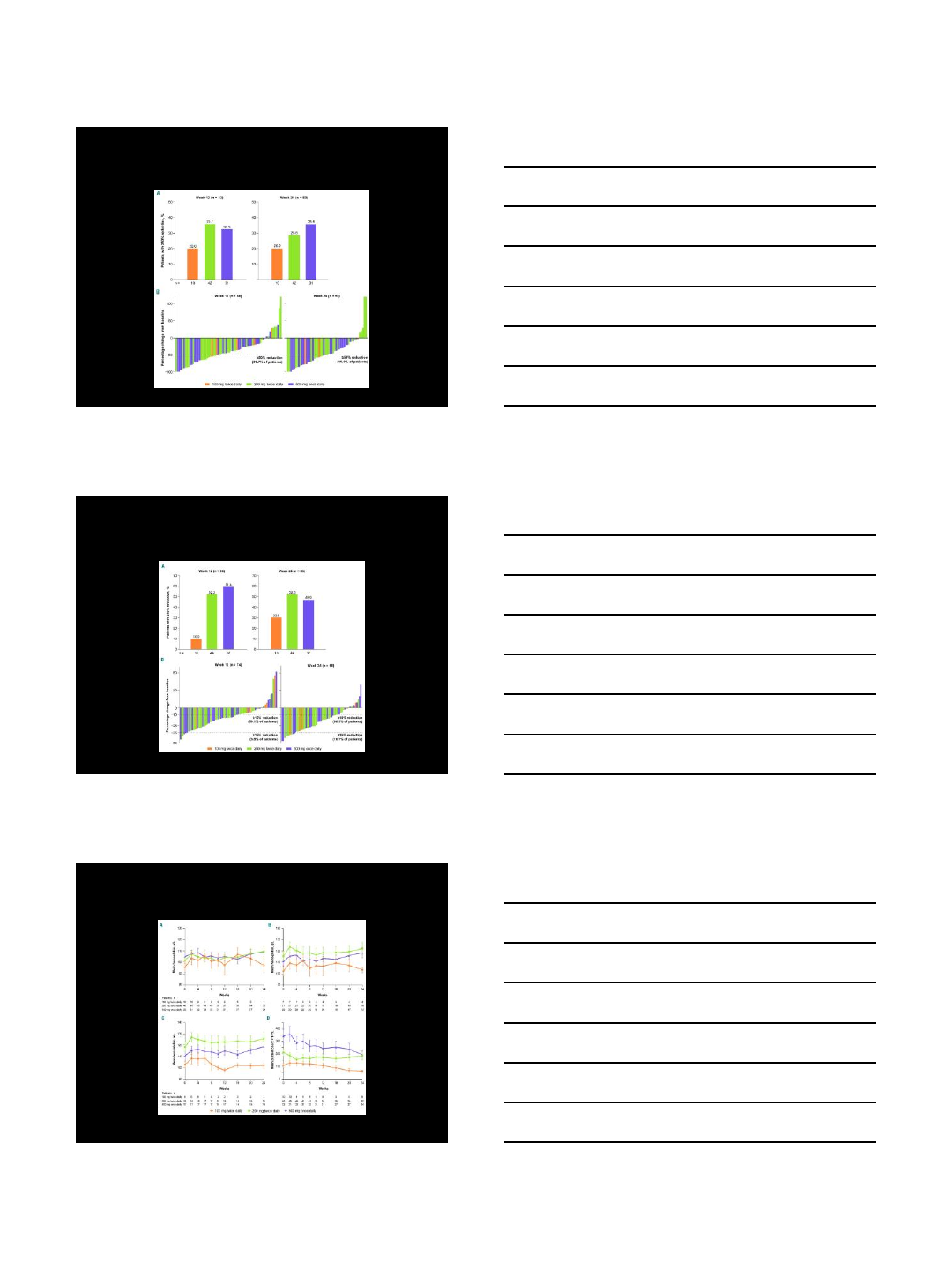
Primary endpoint:
• proportion of patients in each dose group with a ≥50% reduction from
baseline to week 12 in total symptom score (TSS
Secondary endpoints:
• proportion of patients with a ≥50% reduction in TSS from baseline to
week 24
• proportions of patients with a ≥35% reduction in spleen volume from
baseline to weeks 12 and 24
•percentage changes from baseline to weeks 12 and 24 in TSS and
spleen volume
• proportion of patients who exhibited a ≥50% decrease in transfusion
frequency over any 12-week period during the study
Phase II Open-Label Trial Of INCB039110, A
Selective JAK1 Inhibitor, In Patients With
Myelofibrosis
Mascarenhas et al, Haematologica 2017
2/26/2018
6
Treatment Effects on Total
Symptom Score (TSS)
Mascarenhas et al. Haematologica 2017;102:327-335
Treatment Effects on Spleen
Volume
Mascarenhas et al. Haematologica 2017;102:327-335
Effects on Blood Counts
Mascarenhas et al. Haematologica 2017;102:327-335

2/26/2018
7
Impact on plasma cytokines at
week 4
Plasma levels of a
number of key
inflammatory markers,
such as C-reactive
protein, interleukin-6,
interleukin-10, CD40
ligand, RANTES, and
vascular endothelial
growth factor,
decreased in most
patients following 4
weeks of treatment
Mascarenhas et al. Haematologica 2017;102:327-335
Jeoung-Eun Park et al. J Immunol 2009;182:6316-6327
IRAK1 is involved in production of
inflammatory cytokines ligands to IL-1R and
TLRs
Pacritinib is an IRAK1 inhibitor
Agent
Company
Activity
Status
Ruxolitinib (INCB18424)
Novartis/Incyte
JAK1/JAK2
FDA
-
approved
Fedratinib (TG101348/SAR302503)
(ON
HOLD; Wernicke’s encephalopathy )
Celgene
JAK2, FLT3
Phase 3
Momelotinib (CYT387)
(ON
HOLD, failed to meet endpoint goals in phase 3
)
Gilead
JAK1/JAK2/
TYK2
Phase 3
Pacritinib (SB1518)
(ON
HOLD, then back to dose-finding)
CTI
BioPharma
JAK2, FLT3,
IRAK1
Phase 3
Lestaurtinib (CEP701)
Cephalon
JAK2/FLT3
Phase 1/2
BMS
-911453
Bristol
-Myers
Squibb
JAK2
Phase 1
NS
-018
Nippon
-Shinyaku
JAK2/
Src
Phase 1/2
AZD1480 (discontinued due to neurotoxicity and
other side effects)
Astra Zeneca
JAK1/JAK2
Phase 1
Gandotinib (LY2784544)
Eli Lily
JAK2 V617F
Phase 1
INCB039110
Incyte
JAK1 (alone)
Phase 2
INCB054329
Incyte
JAK1
Phase 1/2
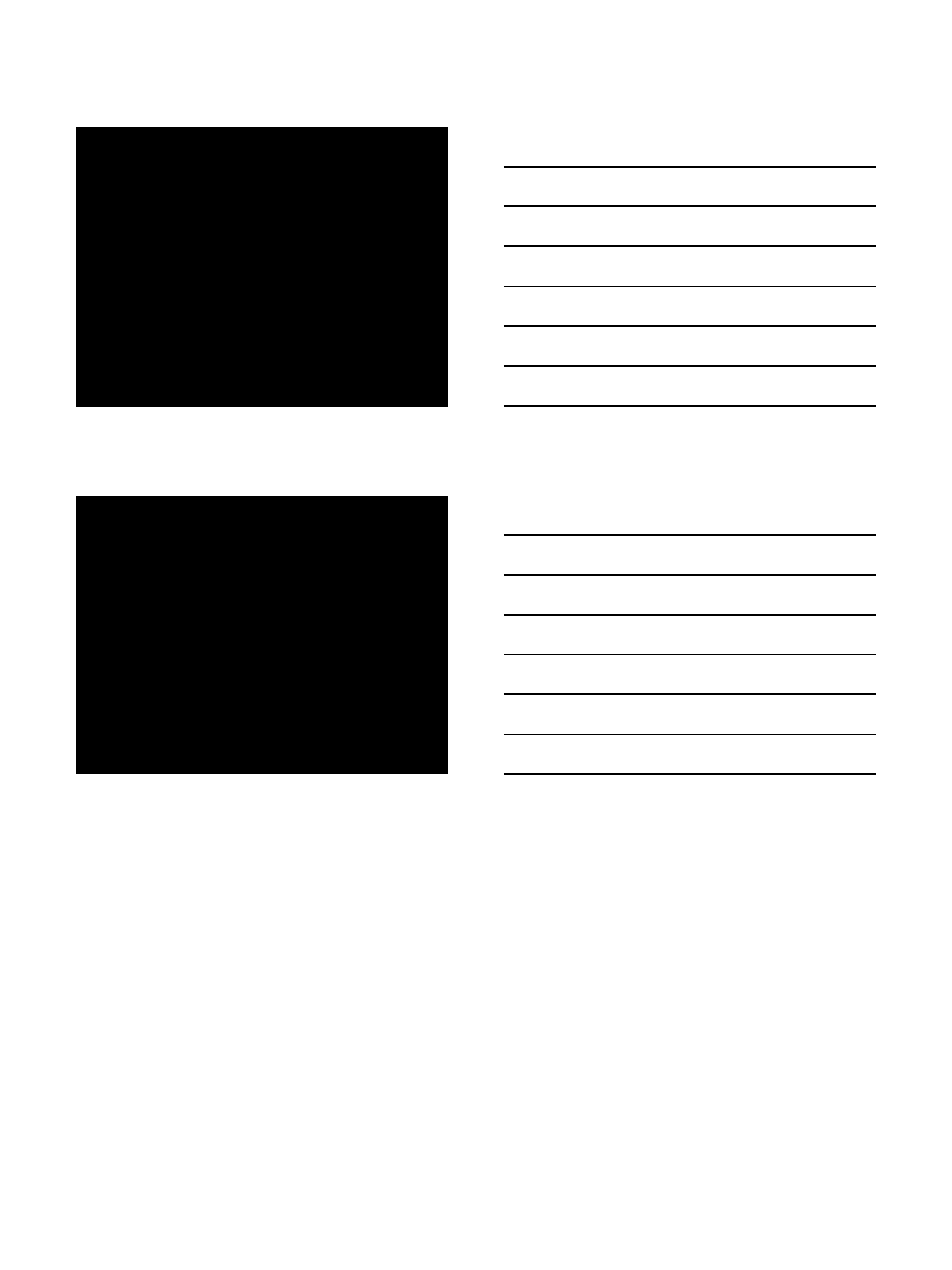
2/26/2018
8
Take Home Points
•Inflammation is high in MPN and drives
symptom burden and potentially disease
progression
•JAK inhibitors reduce inflammation
•Each JAK inhibitor has a unique spectrum
of signaling molecules which it inhibits
Thanks
UC Irvine
Rick Van Etten
Susan O’Brien
Lauren Pinter Brown
Edward Nelson
Deepa Jeyakumar
Elizabeth Brem
Mayo Clinic AZ
Holly Geyer
Amylou Dueck
Jeanne Palmer
Leslie Padrnos
Heidi Kosiorek
Blake Langlais
UT-San Antonio
Robyn Scherber
Ruben Mesa